User login
Experts explain the ‘perfect storm’ of rampant RSV and flu
Headlines over the past few weeks are ringing the alarm about earlier and more serious influenza (flu) and respiratory syncytial virus (RSV) outbreaks compared with previous years. Add COVID-19 to the mix and you have a dangerous mash of viruses that have many experts calling for caution and searching for explanations.
RSV and the flu “are certainly getting more attention, and they’re getting more attention for two reasons,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University, Nashville, Tenn.
“The first is that they’re both extraordinarily early. The second is that they’re both out there spreading very, very rapidly,” he told this news organization.
RSV usually follows a seasonal pattern with cases peaking in January and February. Both viruses tend to hit different regions of the country at different times, and that’s not the case in 2022.
“This is particularly striking for RSV, which usually doesn’t affect the entire country simultaneously,” Dr. Schaffner said.
“Yes, RSV is causing many more hospitalizations and earlier than any previously recorded season in the U.S.,” according to figures from the Centers for Disease Control and Prevention on RSV hospitalizations, said Kevin Messacar, MD, PhD, associate professor at the University of Colorado at Denver, Aurora, and a pediatric infectious disease specialist at Children’s Hospital Colorado in Aurora.
Although there could be some increase in diagnoses because of increased awareness, the jump in RSV and flu cases “is a real phenomenon for multiple reasons,” said Peter Chin-Hong, MD, professor in the division of infectious diseases at the University of California, San Francisco.
With fewer COVID-related restrictions, people are moving around more. Also, during fall and winter, people tend to gather indoors. Colder temperatures and lower humidity contribute as well, Dr. Chin-Hong said, because “the droplets are just simply lighter.
“I think those are all factors,” he told this news organization.
Paul Auwaerter, MD, agreed that there are likely multiple causes for the unusual timing and severity of RSV and flu this year.
“Change in behaviors is a leading cause,” said the clinical director for the division of infectious diseases at the Johns Hopkins University, Baltimore. More people returning to the workplace and children going to school without masks are examples, he added.
Less exposure to these three viruses also means there was less immune boosting among existing populations, he said. This can lead to “larger susceptible populations, especially infants and younger children, due to the relative absence of circulating virus in past years.”
A leading theory
Are we paying a price now for people following the edicts from officials to mask up, stand apart, and take other personal and public health precautions during the COVID-19 pandemic?
It’s possible, but that may not be the whole story.
“When it comes to RSV, I think that theory of isolation, social distancing, mask wearing, and not attending schools is a very valid one,” Dr. Schaffner said. “That’s everybody’s favorite [reason].”
He said he is confident that the jump in RSV cases is being driven by previous COVID public health protections. However, he’s “a little more cautious about influenza, in part because influenza is so variable.
“Like people in influenza say, if you’ve seen one influenza season, you’ve seen one influenza season,” Dr. Schaffner said.
“There’s a lot of debate,” he added. “Nobody can say definitively whether the immune deficit or debt is a consequence of not being stimulated and restimulated by the influenza virus over the past two seasons.”
‘A perfect storm’
“Now you kind of have the perfect storm,” Dr. Chin-Hong said. “It’s not a good situation for COVID with the variants that are emerging. For influenza, not having seen a lot of influenza the last 2 years, we’re probably more susceptible to getting infected.”
RSV cases rose during summer 2021, but now the weather is colder, and people are interacting more closely. “And it’s very, very transmissible,” he said.
Dr. Chin-Hong also predicted that “even though we don’t have a lot of COVID now, COVID will probably pick up.”
The rise in RSV was unexpected by some experts. “This early influenza is also a bit of a surprise and may be influenced by the fact that lots of us are going back and seeing each other again close-to-close, face-to-face in many enclosed environments,” Dr. Schaffner said.
He estimated the 2022-2023 flu season started 4-6 weeks early “and it’s taken off like a rocket. It started in the Southeast, quickly went to the Southwest and up the East Coast. Now it’s moving dramatically through the Midwest and will continue. It’s quite sure to hit the West Coast if it isn’t there already.”
A phenomenon by any other name
Some are calling the situation an “immunity debt,” while others dub it an “immunity pause” or an “immunity deficit.” Many physicians and immunologists have taken to social media to push back on the term “immunity debt,” saying it’s a mischaracterization that is being used to vilify COVID precautions, such as masking, social distancing, and other protective measures taken during the pandemic.
“I prefer the term ‘immunity gap’ ... which is more established in the epidemiology literature, especially given the politicization of the term ‘immunity debt’ by folks recently,” Dr. Messacar said.
“To me, the immunity gap is a scientific observation, not a political argument,” he added.
In a July 2022 publication in The Lancet, Dr. Messacar and his colleagues stated that “decreased exposure to endemic viruses created an immunity gap – a group of susceptible individuals who avoided infection and therefore lack pathogen-specific immunity to protect against future infection. Decreases in childhood vaccinations with pandemic disruptions to health care delivery contribute to this immunity gap for vaccine-preventable diseases, such as influenza,measles, and polio.”
The researchers noted that because of isolation during the pandemic, older children and newborns are being exposed to RSV for the first time. Returning to birthday parties, playing with friends, and going to school without masks means “children are being exposed to RSV, and that’s likely the reason that RSV is moving early and very, very substantially through this now expanded pool of susceptible children,” Dr. Schaffner said.
How likely are coinfections?
With peaks in RSV, flu, and COVID-19 cases each predicted in the coming months, how likely is it that someone could get sick with more than one infection at the same time?
Early in the pandemic, coinfection with COVID and the flu was reported in people at some centers on the West Coast, Dr. Auwaerter said. Now, however, “the unpredictable nature of the Omicron subvariants and the potential for further change, along with the never-before-seen significant lessening of influenza over 2 years, leave little for predictability.
“I do think it is less likely, given the extent of immunity now to SARS-CoV-2 in the population,” Dr. Auwaerter said.
“I most worry about viral coinfections ... in people with suppressed immune systems if we have high community rates of the SARS-CoV-2 and influenza circulating this fall and winter,” he added.
Studies during the pandemic suggest that coinfection with the SARS-CoV-2 virus and another respiratory virus were either rare or nonexistent.
Dr. Schaffner said these findings align with his experience at Vanderbilt University, which is part of a CDC-sponsored network that tracks laboratory-confirmed RSV, flu, and COVID cases among people in the hospital. “Coinfections are, at least to date, very unusual.”
There needs to be an asterisk next to that, Dr. Schaffner added. “Looking back over the last 2 years, we’ve had very little influenza, and we’ve had curtailed RSV seasons. So there hasn’t been a whole lot of opportunity for dual infections to occur.
“So this year may be more revelatory as we go forward,” he said.
Future concerns
The future is uncertain, Dr. Messacar and colleagues wrote in The Lancet: “Crucially, the patterns of these returning viral outbreaks have been heterogeneous across locations, populations, and pathogens, making predictions and preparations challenging.”
Dr. Chin-Hong used a horse race analogy to illustrate the situation now and going forward. RSV is the front-running horse, and influenza is running behind but trying to catch up. “And then COVID is the dark horse. It’s trailing the race right now – but all these variants are giving the horse extra supplements.
“And the COVID horse is probably going to be very competitive with the front-runner,” he said.
“We’re just at the beginning of the race right now,” Dr. Chin-Hong said, “so that’s why we’re worried that these three [viruses] will be even more pronounced come later in the year.”
A version of this article first appeared on Medscape.com.
Headlines over the past few weeks are ringing the alarm about earlier and more serious influenza (flu) and respiratory syncytial virus (RSV) outbreaks compared with previous years. Add COVID-19 to the mix and you have a dangerous mash of viruses that have many experts calling for caution and searching for explanations.
RSV and the flu “are certainly getting more attention, and they’re getting more attention for two reasons,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University, Nashville, Tenn.
“The first is that they’re both extraordinarily early. The second is that they’re both out there spreading very, very rapidly,” he told this news organization.
RSV usually follows a seasonal pattern with cases peaking in January and February. Both viruses tend to hit different regions of the country at different times, and that’s not the case in 2022.
“This is particularly striking for RSV, which usually doesn’t affect the entire country simultaneously,” Dr. Schaffner said.
“Yes, RSV is causing many more hospitalizations and earlier than any previously recorded season in the U.S.,” according to figures from the Centers for Disease Control and Prevention on RSV hospitalizations, said Kevin Messacar, MD, PhD, associate professor at the University of Colorado at Denver, Aurora, and a pediatric infectious disease specialist at Children’s Hospital Colorado in Aurora.
Although there could be some increase in diagnoses because of increased awareness, the jump in RSV and flu cases “is a real phenomenon for multiple reasons,” said Peter Chin-Hong, MD, professor in the division of infectious diseases at the University of California, San Francisco.
With fewer COVID-related restrictions, people are moving around more. Also, during fall and winter, people tend to gather indoors. Colder temperatures and lower humidity contribute as well, Dr. Chin-Hong said, because “the droplets are just simply lighter.
“I think those are all factors,” he told this news organization.
Paul Auwaerter, MD, agreed that there are likely multiple causes for the unusual timing and severity of RSV and flu this year.
“Change in behaviors is a leading cause,” said the clinical director for the division of infectious diseases at the Johns Hopkins University, Baltimore. More people returning to the workplace and children going to school without masks are examples, he added.
Less exposure to these three viruses also means there was less immune boosting among existing populations, he said. This can lead to “larger susceptible populations, especially infants and younger children, due to the relative absence of circulating virus in past years.”
A leading theory
Are we paying a price now for people following the edicts from officials to mask up, stand apart, and take other personal and public health precautions during the COVID-19 pandemic?
It’s possible, but that may not be the whole story.
“When it comes to RSV, I think that theory of isolation, social distancing, mask wearing, and not attending schools is a very valid one,” Dr. Schaffner said. “That’s everybody’s favorite [reason].”
He said he is confident that the jump in RSV cases is being driven by previous COVID public health protections. However, he’s “a little more cautious about influenza, in part because influenza is so variable.
“Like people in influenza say, if you’ve seen one influenza season, you’ve seen one influenza season,” Dr. Schaffner said.
“There’s a lot of debate,” he added. “Nobody can say definitively whether the immune deficit or debt is a consequence of not being stimulated and restimulated by the influenza virus over the past two seasons.”
‘A perfect storm’
“Now you kind of have the perfect storm,” Dr. Chin-Hong said. “It’s not a good situation for COVID with the variants that are emerging. For influenza, not having seen a lot of influenza the last 2 years, we’re probably more susceptible to getting infected.”
RSV cases rose during summer 2021, but now the weather is colder, and people are interacting more closely. “And it’s very, very transmissible,” he said.
Dr. Chin-Hong also predicted that “even though we don’t have a lot of COVID now, COVID will probably pick up.”
The rise in RSV was unexpected by some experts. “This early influenza is also a bit of a surprise and may be influenced by the fact that lots of us are going back and seeing each other again close-to-close, face-to-face in many enclosed environments,” Dr. Schaffner said.
He estimated the 2022-2023 flu season started 4-6 weeks early “and it’s taken off like a rocket. It started in the Southeast, quickly went to the Southwest and up the East Coast. Now it’s moving dramatically through the Midwest and will continue. It’s quite sure to hit the West Coast if it isn’t there already.”
A phenomenon by any other name
Some are calling the situation an “immunity debt,” while others dub it an “immunity pause” or an “immunity deficit.” Many physicians and immunologists have taken to social media to push back on the term “immunity debt,” saying it’s a mischaracterization that is being used to vilify COVID precautions, such as masking, social distancing, and other protective measures taken during the pandemic.
“I prefer the term ‘immunity gap’ ... which is more established in the epidemiology literature, especially given the politicization of the term ‘immunity debt’ by folks recently,” Dr. Messacar said.
“To me, the immunity gap is a scientific observation, not a political argument,” he added.
In a July 2022 publication in The Lancet, Dr. Messacar and his colleagues stated that “decreased exposure to endemic viruses created an immunity gap – a group of susceptible individuals who avoided infection and therefore lack pathogen-specific immunity to protect against future infection. Decreases in childhood vaccinations with pandemic disruptions to health care delivery contribute to this immunity gap for vaccine-preventable diseases, such as influenza,measles, and polio.”
The researchers noted that because of isolation during the pandemic, older children and newborns are being exposed to RSV for the first time. Returning to birthday parties, playing with friends, and going to school without masks means “children are being exposed to RSV, and that’s likely the reason that RSV is moving early and very, very substantially through this now expanded pool of susceptible children,” Dr. Schaffner said.
How likely are coinfections?
With peaks in RSV, flu, and COVID-19 cases each predicted in the coming months, how likely is it that someone could get sick with more than one infection at the same time?
Early in the pandemic, coinfection with COVID and the flu was reported in people at some centers on the West Coast, Dr. Auwaerter said. Now, however, “the unpredictable nature of the Omicron subvariants and the potential for further change, along with the never-before-seen significant lessening of influenza over 2 years, leave little for predictability.
“I do think it is less likely, given the extent of immunity now to SARS-CoV-2 in the population,” Dr. Auwaerter said.
“I most worry about viral coinfections ... in people with suppressed immune systems if we have high community rates of the SARS-CoV-2 and influenza circulating this fall and winter,” he added.
Studies during the pandemic suggest that coinfection with the SARS-CoV-2 virus and another respiratory virus were either rare or nonexistent.
Dr. Schaffner said these findings align with his experience at Vanderbilt University, which is part of a CDC-sponsored network that tracks laboratory-confirmed RSV, flu, and COVID cases among people in the hospital. “Coinfections are, at least to date, very unusual.”
There needs to be an asterisk next to that, Dr. Schaffner added. “Looking back over the last 2 years, we’ve had very little influenza, and we’ve had curtailed RSV seasons. So there hasn’t been a whole lot of opportunity for dual infections to occur.
“So this year may be more revelatory as we go forward,” he said.
Future concerns
The future is uncertain, Dr. Messacar and colleagues wrote in The Lancet: “Crucially, the patterns of these returning viral outbreaks have been heterogeneous across locations, populations, and pathogens, making predictions and preparations challenging.”
Dr. Chin-Hong used a horse race analogy to illustrate the situation now and going forward. RSV is the front-running horse, and influenza is running behind but trying to catch up. “And then COVID is the dark horse. It’s trailing the race right now – but all these variants are giving the horse extra supplements.
“And the COVID horse is probably going to be very competitive with the front-runner,” he said.
“We’re just at the beginning of the race right now,” Dr. Chin-Hong said, “so that’s why we’re worried that these three [viruses] will be even more pronounced come later in the year.”
A version of this article first appeared on Medscape.com.
Headlines over the past few weeks are ringing the alarm about earlier and more serious influenza (flu) and respiratory syncytial virus (RSV) outbreaks compared with previous years. Add COVID-19 to the mix and you have a dangerous mash of viruses that have many experts calling for caution and searching for explanations.
RSV and the flu “are certainly getting more attention, and they’re getting more attention for two reasons,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University, Nashville, Tenn.
“The first is that they’re both extraordinarily early. The second is that they’re both out there spreading very, very rapidly,” he told this news organization.
RSV usually follows a seasonal pattern with cases peaking in January and February. Both viruses tend to hit different regions of the country at different times, and that’s not the case in 2022.
“This is particularly striking for RSV, which usually doesn’t affect the entire country simultaneously,” Dr. Schaffner said.
“Yes, RSV is causing many more hospitalizations and earlier than any previously recorded season in the U.S.,” according to figures from the Centers for Disease Control and Prevention on RSV hospitalizations, said Kevin Messacar, MD, PhD, associate professor at the University of Colorado at Denver, Aurora, and a pediatric infectious disease specialist at Children’s Hospital Colorado in Aurora.
Although there could be some increase in diagnoses because of increased awareness, the jump in RSV and flu cases “is a real phenomenon for multiple reasons,” said Peter Chin-Hong, MD, professor in the division of infectious diseases at the University of California, San Francisco.
With fewer COVID-related restrictions, people are moving around more. Also, during fall and winter, people tend to gather indoors. Colder temperatures and lower humidity contribute as well, Dr. Chin-Hong said, because “the droplets are just simply lighter.
“I think those are all factors,” he told this news organization.
Paul Auwaerter, MD, agreed that there are likely multiple causes for the unusual timing and severity of RSV and flu this year.
“Change in behaviors is a leading cause,” said the clinical director for the division of infectious diseases at the Johns Hopkins University, Baltimore. More people returning to the workplace and children going to school without masks are examples, he added.
Less exposure to these three viruses also means there was less immune boosting among existing populations, he said. This can lead to “larger susceptible populations, especially infants and younger children, due to the relative absence of circulating virus in past years.”
A leading theory
Are we paying a price now for people following the edicts from officials to mask up, stand apart, and take other personal and public health precautions during the COVID-19 pandemic?
It’s possible, but that may not be the whole story.
“When it comes to RSV, I think that theory of isolation, social distancing, mask wearing, and not attending schools is a very valid one,” Dr. Schaffner said. “That’s everybody’s favorite [reason].”
He said he is confident that the jump in RSV cases is being driven by previous COVID public health protections. However, he’s “a little more cautious about influenza, in part because influenza is so variable.
“Like people in influenza say, if you’ve seen one influenza season, you’ve seen one influenza season,” Dr. Schaffner said.
“There’s a lot of debate,” he added. “Nobody can say definitively whether the immune deficit or debt is a consequence of not being stimulated and restimulated by the influenza virus over the past two seasons.”
‘A perfect storm’
“Now you kind of have the perfect storm,” Dr. Chin-Hong said. “It’s not a good situation for COVID with the variants that are emerging. For influenza, not having seen a lot of influenza the last 2 years, we’re probably more susceptible to getting infected.”
RSV cases rose during summer 2021, but now the weather is colder, and people are interacting more closely. “And it’s very, very transmissible,” he said.
Dr. Chin-Hong also predicted that “even though we don’t have a lot of COVID now, COVID will probably pick up.”
The rise in RSV was unexpected by some experts. “This early influenza is also a bit of a surprise and may be influenced by the fact that lots of us are going back and seeing each other again close-to-close, face-to-face in many enclosed environments,” Dr. Schaffner said.
He estimated the 2022-2023 flu season started 4-6 weeks early “and it’s taken off like a rocket. It started in the Southeast, quickly went to the Southwest and up the East Coast. Now it’s moving dramatically through the Midwest and will continue. It’s quite sure to hit the West Coast if it isn’t there already.”
A phenomenon by any other name
Some are calling the situation an “immunity debt,” while others dub it an “immunity pause” or an “immunity deficit.” Many physicians and immunologists have taken to social media to push back on the term “immunity debt,” saying it’s a mischaracterization that is being used to vilify COVID precautions, such as masking, social distancing, and other protective measures taken during the pandemic.
“I prefer the term ‘immunity gap’ ... which is more established in the epidemiology literature, especially given the politicization of the term ‘immunity debt’ by folks recently,” Dr. Messacar said.
“To me, the immunity gap is a scientific observation, not a political argument,” he added.
In a July 2022 publication in The Lancet, Dr. Messacar and his colleagues stated that “decreased exposure to endemic viruses created an immunity gap – a group of susceptible individuals who avoided infection and therefore lack pathogen-specific immunity to protect against future infection. Decreases in childhood vaccinations with pandemic disruptions to health care delivery contribute to this immunity gap for vaccine-preventable diseases, such as influenza,measles, and polio.”
The researchers noted that because of isolation during the pandemic, older children and newborns are being exposed to RSV for the first time. Returning to birthday parties, playing with friends, and going to school without masks means “children are being exposed to RSV, and that’s likely the reason that RSV is moving early and very, very substantially through this now expanded pool of susceptible children,” Dr. Schaffner said.
How likely are coinfections?
With peaks in RSV, flu, and COVID-19 cases each predicted in the coming months, how likely is it that someone could get sick with more than one infection at the same time?
Early in the pandemic, coinfection with COVID and the flu was reported in people at some centers on the West Coast, Dr. Auwaerter said. Now, however, “the unpredictable nature of the Omicron subvariants and the potential for further change, along with the never-before-seen significant lessening of influenza over 2 years, leave little for predictability.
“I do think it is less likely, given the extent of immunity now to SARS-CoV-2 in the population,” Dr. Auwaerter said.
“I most worry about viral coinfections ... in people with suppressed immune systems if we have high community rates of the SARS-CoV-2 and influenza circulating this fall and winter,” he added.
Studies during the pandemic suggest that coinfection with the SARS-CoV-2 virus and another respiratory virus were either rare or nonexistent.
Dr. Schaffner said these findings align with his experience at Vanderbilt University, which is part of a CDC-sponsored network that tracks laboratory-confirmed RSV, flu, and COVID cases among people in the hospital. “Coinfections are, at least to date, very unusual.”
There needs to be an asterisk next to that, Dr. Schaffner added. “Looking back over the last 2 years, we’ve had very little influenza, and we’ve had curtailed RSV seasons. So there hasn’t been a whole lot of opportunity for dual infections to occur.
“So this year may be more revelatory as we go forward,” he said.
Future concerns
The future is uncertain, Dr. Messacar and colleagues wrote in The Lancet: “Crucially, the patterns of these returning viral outbreaks have been heterogeneous across locations, populations, and pathogens, making predictions and preparations challenging.”
Dr. Chin-Hong used a horse race analogy to illustrate the situation now and going forward. RSV is the front-running horse, and influenza is running behind but trying to catch up. “And then COVID is the dark horse. It’s trailing the race right now – but all these variants are giving the horse extra supplements.
“And the COVID horse is probably going to be very competitive with the front-runner,” he said.
“We’re just at the beginning of the race right now,” Dr. Chin-Hong said, “so that’s why we’re worried that these three [viruses] will be even more pronounced come later in the year.”
A version of this article first appeared on Medscape.com.
Hypertension linked to risk of severe COVID
U.K. researchers have established that hypertension is associated with a 22% greater risk of severe COVID-19, with the odds of severe COVID-19 unaffected by medication type.
Hypertension “appears to be one of the commonest comorbidities in COVID-19 patients”, explained the authors of a new study, published in PLOS ONE. The authors highlighted that previous research had shown that hypertension was more prevalent in severe and fatal cases compared with all cases of COVID-19.
They pointed out, however, that whether hypertensive individuals have a higher risk of severe COVID-19, compared with nonhypertensives, and whether the absolute level of systolic blood pressure or the type of antihypertensive medication is related to this risk, remained “unclear.”
To try to answer these questions, the research team, led by University of Cambridge researchers, analyzed data from 16,134 individuals who tested positive for COVID-19 (mean age 65.3 years, 47% male, 90% white), 40% were diagnosed with essential hypertension at the analysis baseline – 22% of whom had developed severe COVID-19.
Systolic blood pressure (SBP) was categorized by 10–mm Hg ranges, starting from < 120 mm Hg up to 180+ mm Hg, with the reference category defined as 120-129 mm Hg, based on data from the SPRINT study, which demonstrated that intensive SBP lowering to below 120 mm Hg, as compared with the traditional threshold of 140 mm Hg, was beneficial. Diastolic blood pressure was categorized by 10–mm Hg ranges, starting from < 60 mm Hg up to 100+ mm Hg with 80-90 mm Hg being the reference category.
In their analyses the researchers adjusted for age, sex, body mass index, ethnicity, smoking status, diabetes status, socioeconomic status, and inflammation (C-reactive protein [CRP]), as these were proposed as potential confounders. To assess the direct effect of hypertension on COVID-19, they also adjusted for intermediate variables, including cardiovascular comorbidities and stroke, on the causal pathway between hypertension and severe COVID-19.
Majority of effect of hypertension on severe COVID-19 was direct
The unadjusted odds ratio of the association between hypertension and severe COVID-19 was 2.33 (95% confidence interval, 2.16-2.51), the authors emphasized. They found that, after adjusting for all confounding variables, hypertension was associated with 22% higher odds of severe COVID-19 (OR, 1.22; 95% CI, 1.12-1.33), compared with normotension.
Individuals with severe COVID-19 were marginally older, more likely to be male, and more deprived, the authors said. “They were also more likely to be hypertensive, compared with individuals without severe COVID-19, and a greater proportion of individuals with severe COVID-19 had cardiovascular comorbidities.”
The majority of the effect of hypertension on development of severe COVID-19 was “direct,” they said. However, a modest proportion of the effect was mediated via cardiovascular comorbidities such as peripheral vascular disease, MI, coronary heart disease, arrhythmias, and stroke. Of note, those with a history of stroke had a 47% higher risk of severe COVID-19 and those with a history of other cardiovascular comorbidities had a 30% higher risk of severe COVID-19, the authors commented.
J-shaped relationship
Of the total of 6,517 (40%) individuals who had a diagnosis of essential hypertension at baseline, 67% were treated (41% with monotherapy, 59% with combination therapy), and 33% were untreated.
There were similar numbers of severe COVID-19 in each medication group: ACE inhibitors, 34%; angiotensin receptor blockers (ARBs), 36%; and “other” medications 34%.
In hypertensive individuals receiving antihypertensive medications, there was a “J-shaped relationship” between the level of blood pressure and risk of severe COVID-19 when using a systolic blood pressure level of 120-129 mm Hg as a reference – 150-159 mm Hg versus 120-129 mm Hg (OR 1.91; 95% CI, 1.44-2.53), > 180+ mm Hg versus 120-129 mm Hg (OR 1.93; 95% CI, 1.06-3.51).
The authors commented that there was no evidence of a higher risk of severe COVID-19 until systolic blood pressure “exceeded 150 mm Hg.”
They said it was an interesting finding that “very well-controlled” systolic blood pressure < 120 mm Hg was associated with a 40% (OR, 1.40; 95% CI, 1.11-1.78) greater odds of severe COVID-19. “This may be due to reverse causality, where low systolic blood pressure levels may indicate poorer health, such that the occurrence of severe COVID-19 may be related to underlying disease rather than the level of SBP per se,” they suggested.
The J-shaped association observed remained after multiple adjustments, including presence of known cardiovascular comorbidities, which suggested a possible “real effect” of low SBP on severe COVID-19, “at least in treated hypertensive individuals.”
Their analyses also identified that, compared with a “normal” diastolic blood pressure (80-90 mm Hg), having a diastolic blood pressure higher than 90 mm Hg was associated with higher odds of severe COVID-19.
The association between hypertension and COVID-19 was “amplified” if the individuals were treated and their BP remained uncontrolled, the authors pointed out.
There did not appear to be any difference in the risk of severe COVID-19 between individuals taking ACE inhibitors and those taking ARBs or other antihypertensive medications, the authors said.
Better understanding of underlying mechanisms needed
Individuals with hypertension who tested positive for COVID-19 had “over twice” the risk of developing severe COVID-19, compared with nonhypertensive individuals, the authors said.
They highlighted that their findings also suggest that there are “further effects” influencing the severity of COVID-19 beyond a “dichotomous” diagnosis of hypertension.
“Individuals with a higher-than-target systolic blood pressure may be less healthy, less active, suffering more severe hypertension, or have developed drug-resistant hypertension, all suggesting that the effects of hypertension have already had detrimental physiological effects on the cardiovascular system, which in turn may offer some explanation for the higher risk of severe COVID-19 with uncontrolled SBP,” they explained.
“Hypertension is an important risk factor for COVID-19,” reiterated the authors, who emphasized that a better understanding of the underlying mechanisms driving this increased risk is warranted in case of “more severe strains or other viruses” in the future.
The authors have declared no competing interests.
A version of this article first appeared on Medscape UK.
U.K. researchers have established that hypertension is associated with a 22% greater risk of severe COVID-19, with the odds of severe COVID-19 unaffected by medication type.
Hypertension “appears to be one of the commonest comorbidities in COVID-19 patients”, explained the authors of a new study, published in PLOS ONE. The authors highlighted that previous research had shown that hypertension was more prevalent in severe and fatal cases compared with all cases of COVID-19.
They pointed out, however, that whether hypertensive individuals have a higher risk of severe COVID-19, compared with nonhypertensives, and whether the absolute level of systolic blood pressure or the type of antihypertensive medication is related to this risk, remained “unclear.”
To try to answer these questions, the research team, led by University of Cambridge researchers, analyzed data from 16,134 individuals who tested positive for COVID-19 (mean age 65.3 years, 47% male, 90% white), 40% were diagnosed with essential hypertension at the analysis baseline – 22% of whom had developed severe COVID-19.
Systolic blood pressure (SBP) was categorized by 10–mm Hg ranges, starting from < 120 mm Hg up to 180+ mm Hg, with the reference category defined as 120-129 mm Hg, based on data from the SPRINT study, which demonstrated that intensive SBP lowering to below 120 mm Hg, as compared with the traditional threshold of 140 mm Hg, was beneficial. Diastolic blood pressure was categorized by 10–mm Hg ranges, starting from < 60 mm Hg up to 100+ mm Hg with 80-90 mm Hg being the reference category.
In their analyses the researchers adjusted for age, sex, body mass index, ethnicity, smoking status, diabetes status, socioeconomic status, and inflammation (C-reactive protein [CRP]), as these were proposed as potential confounders. To assess the direct effect of hypertension on COVID-19, they also adjusted for intermediate variables, including cardiovascular comorbidities and stroke, on the causal pathway between hypertension and severe COVID-19.
Majority of effect of hypertension on severe COVID-19 was direct
The unadjusted odds ratio of the association between hypertension and severe COVID-19 was 2.33 (95% confidence interval, 2.16-2.51), the authors emphasized. They found that, after adjusting for all confounding variables, hypertension was associated with 22% higher odds of severe COVID-19 (OR, 1.22; 95% CI, 1.12-1.33), compared with normotension.
Individuals with severe COVID-19 were marginally older, more likely to be male, and more deprived, the authors said. “They were also more likely to be hypertensive, compared with individuals without severe COVID-19, and a greater proportion of individuals with severe COVID-19 had cardiovascular comorbidities.”
The majority of the effect of hypertension on development of severe COVID-19 was “direct,” they said. However, a modest proportion of the effect was mediated via cardiovascular comorbidities such as peripheral vascular disease, MI, coronary heart disease, arrhythmias, and stroke. Of note, those with a history of stroke had a 47% higher risk of severe COVID-19 and those with a history of other cardiovascular comorbidities had a 30% higher risk of severe COVID-19, the authors commented.
J-shaped relationship
Of the total of 6,517 (40%) individuals who had a diagnosis of essential hypertension at baseline, 67% were treated (41% with monotherapy, 59% with combination therapy), and 33% were untreated.
There were similar numbers of severe COVID-19 in each medication group: ACE inhibitors, 34%; angiotensin receptor blockers (ARBs), 36%; and “other” medications 34%.
In hypertensive individuals receiving antihypertensive medications, there was a “J-shaped relationship” between the level of blood pressure and risk of severe COVID-19 when using a systolic blood pressure level of 120-129 mm Hg as a reference – 150-159 mm Hg versus 120-129 mm Hg (OR 1.91; 95% CI, 1.44-2.53), > 180+ mm Hg versus 120-129 mm Hg (OR 1.93; 95% CI, 1.06-3.51).
The authors commented that there was no evidence of a higher risk of severe COVID-19 until systolic blood pressure “exceeded 150 mm Hg.”
They said it was an interesting finding that “very well-controlled” systolic blood pressure < 120 mm Hg was associated with a 40% (OR, 1.40; 95% CI, 1.11-1.78) greater odds of severe COVID-19. “This may be due to reverse causality, where low systolic blood pressure levels may indicate poorer health, such that the occurrence of severe COVID-19 may be related to underlying disease rather than the level of SBP per se,” they suggested.
The J-shaped association observed remained after multiple adjustments, including presence of known cardiovascular comorbidities, which suggested a possible “real effect” of low SBP on severe COVID-19, “at least in treated hypertensive individuals.”
Their analyses also identified that, compared with a “normal” diastolic blood pressure (80-90 mm Hg), having a diastolic blood pressure higher than 90 mm Hg was associated with higher odds of severe COVID-19.
The association between hypertension and COVID-19 was “amplified” if the individuals were treated and their BP remained uncontrolled, the authors pointed out.
There did not appear to be any difference in the risk of severe COVID-19 between individuals taking ACE inhibitors and those taking ARBs or other antihypertensive medications, the authors said.
Better understanding of underlying mechanisms needed
Individuals with hypertension who tested positive for COVID-19 had “over twice” the risk of developing severe COVID-19, compared with nonhypertensive individuals, the authors said.
They highlighted that their findings also suggest that there are “further effects” influencing the severity of COVID-19 beyond a “dichotomous” diagnosis of hypertension.
“Individuals with a higher-than-target systolic blood pressure may be less healthy, less active, suffering more severe hypertension, or have developed drug-resistant hypertension, all suggesting that the effects of hypertension have already had detrimental physiological effects on the cardiovascular system, which in turn may offer some explanation for the higher risk of severe COVID-19 with uncontrolled SBP,” they explained.
“Hypertension is an important risk factor for COVID-19,” reiterated the authors, who emphasized that a better understanding of the underlying mechanisms driving this increased risk is warranted in case of “more severe strains or other viruses” in the future.
The authors have declared no competing interests.
A version of this article first appeared on Medscape UK.
U.K. researchers have established that hypertension is associated with a 22% greater risk of severe COVID-19, with the odds of severe COVID-19 unaffected by medication type.
Hypertension “appears to be one of the commonest comorbidities in COVID-19 patients”, explained the authors of a new study, published in PLOS ONE. The authors highlighted that previous research had shown that hypertension was more prevalent in severe and fatal cases compared with all cases of COVID-19.
They pointed out, however, that whether hypertensive individuals have a higher risk of severe COVID-19, compared with nonhypertensives, and whether the absolute level of systolic blood pressure or the type of antihypertensive medication is related to this risk, remained “unclear.”
To try to answer these questions, the research team, led by University of Cambridge researchers, analyzed data from 16,134 individuals who tested positive for COVID-19 (mean age 65.3 years, 47% male, 90% white), 40% were diagnosed with essential hypertension at the analysis baseline – 22% of whom had developed severe COVID-19.
Systolic blood pressure (SBP) was categorized by 10–mm Hg ranges, starting from < 120 mm Hg up to 180+ mm Hg, with the reference category defined as 120-129 mm Hg, based on data from the SPRINT study, which demonstrated that intensive SBP lowering to below 120 mm Hg, as compared with the traditional threshold of 140 mm Hg, was beneficial. Diastolic blood pressure was categorized by 10–mm Hg ranges, starting from < 60 mm Hg up to 100+ mm Hg with 80-90 mm Hg being the reference category.
In their analyses the researchers adjusted for age, sex, body mass index, ethnicity, smoking status, diabetes status, socioeconomic status, and inflammation (C-reactive protein [CRP]), as these were proposed as potential confounders. To assess the direct effect of hypertension on COVID-19, they also adjusted for intermediate variables, including cardiovascular comorbidities and stroke, on the causal pathway between hypertension and severe COVID-19.
Majority of effect of hypertension on severe COVID-19 was direct
The unadjusted odds ratio of the association between hypertension and severe COVID-19 was 2.33 (95% confidence interval, 2.16-2.51), the authors emphasized. They found that, after adjusting for all confounding variables, hypertension was associated with 22% higher odds of severe COVID-19 (OR, 1.22; 95% CI, 1.12-1.33), compared with normotension.
Individuals with severe COVID-19 were marginally older, more likely to be male, and more deprived, the authors said. “They were also more likely to be hypertensive, compared with individuals without severe COVID-19, and a greater proportion of individuals with severe COVID-19 had cardiovascular comorbidities.”
The majority of the effect of hypertension on development of severe COVID-19 was “direct,” they said. However, a modest proportion of the effect was mediated via cardiovascular comorbidities such as peripheral vascular disease, MI, coronary heart disease, arrhythmias, and stroke. Of note, those with a history of stroke had a 47% higher risk of severe COVID-19 and those with a history of other cardiovascular comorbidities had a 30% higher risk of severe COVID-19, the authors commented.
J-shaped relationship
Of the total of 6,517 (40%) individuals who had a diagnosis of essential hypertension at baseline, 67% were treated (41% with monotherapy, 59% with combination therapy), and 33% were untreated.
There were similar numbers of severe COVID-19 in each medication group: ACE inhibitors, 34%; angiotensin receptor blockers (ARBs), 36%; and “other” medications 34%.
In hypertensive individuals receiving antihypertensive medications, there was a “J-shaped relationship” between the level of blood pressure and risk of severe COVID-19 when using a systolic blood pressure level of 120-129 mm Hg as a reference – 150-159 mm Hg versus 120-129 mm Hg (OR 1.91; 95% CI, 1.44-2.53), > 180+ mm Hg versus 120-129 mm Hg (OR 1.93; 95% CI, 1.06-3.51).
The authors commented that there was no evidence of a higher risk of severe COVID-19 until systolic blood pressure “exceeded 150 mm Hg.”
They said it was an interesting finding that “very well-controlled” systolic blood pressure < 120 mm Hg was associated with a 40% (OR, 1.40; 95% CI, 1.11-1.78) greater odds of severe COVID-19. “This may be due to reverse causality, where low systolic blood pressure levels may indicate poorer health, such that the occurrence of severe COVID-19 may be related to underlying disease rather than the level of SBP per se,” they suggested.
The J-shaped association observed remained after multiple adjustments, including presence of known cardiovascular comorbidities, which suggested a possible “real effect” of low SBP on severe COVID-19, “at least in treated hypertensive individuals.”
Their analyses also identified that, compared with a “normal” diastolic blood pressure (80-90 mm Hg), having a diastolic blood pressure higher than 90 mm Hg was associated with higher odds of severe COVID-19.
The association between hypertension and COVID-19 was “amplified” if the individuals were treated and their BP remained uncontrolled, the authors pointed out.
There did not appear to be any difference in the risk of severe COVID-19 between individuals taking ACE inhibitors and those taking ARBs or other antihypertensive medications, the authors said.
Better understanding of underlying mechanisms needed
Individuals with hypertension who tested positive for COVID-19 had “over twice” the risk of developing severe COVID-19, compared with nonhypertensive individuals, the authors said.
They highlighted that their findings also suggest that there are “further effects” influencing the severity of COVID-19 beyond a “dichotomous” diagnosis of hypertension.
“Individuals with a higher-than-target systolic blood pressure may be less healthy, less active, suffering more severe hypertension, or have developed drug-resistant hypertension, all suggesting that the effects of hypertension have already had detrimental physiological effects on the cardiovascular system, which in turn may offer some explanation for the higher risk of severe COVID-19 with uncontrolled SBP,” they explained.
“Hypertension is an important risk factor for COVID-19,” reiterated the authors, who emphasized that a better understanding of the underlying mechanisms driving this increased risk is warranted in case of “more severe strains or other viruses” in the future.
The authors have declared no competing interests.
A version of this article first appeared on Medscape UK.
FROM PLOS ONE
HIV: Greater parental involvement needed with young men who have sex with men
“Take it from me, parents just don’t understand.”
Fresh Prince and D.J. Jazzy Jeff penned this lyric roughly 35 years ago, and coincidentally the HIV/AIDS epidemic has also been with us just as long. But the connection between the two may be highly relevant – that is, if you consider how infrequently parents appear (or have the proper tools) to engage with their gay or bisexual sons to prevent and curb HIV infections.
Currently, YMSM between the ages of 13 and 24 are among the most affected by the ongoing HIV epidemic, with CDC estimates suggesting that, in 2020, this group alone represented about 35% of new diagnoses. At the same time, about half of these HIV infections go undiagnosed. Recent data also suggest that care linkage in this group is similar to adults, but only a third of YMSM start antiretroviral therapy and are retained in care, leading to viral suppression rates as low as 12%.
With a goal to change these discouraging numbers, researchers from George Washington University, Washington, and other institutions conducted a randomized controlled pilot study targeting parents of YMSM to improve both the frequency and quality of communication around sexual health and HIV risk, prevention, and testing.
The findings, which were published online in the journal AIDS and Behavior, highlight the observation that parents could be an essential resource for combating the HIV epidemic, but they’re a resource that’s often underutilized. In fact, after participating in an online offering – PATHS (Parents and Adolescents Talking about Healthy Sexuality) – parents reported significantly greater engagement with their sons, especially around discussions focusing on HIV information and condom use.
“From what we know from the research, parents are uncomfortable talking about sex; they’re not great at talking about it. But when they do and do it effectively, those kids seem to have better health outcomes,” lead author David Huebner, PhD, MPH, associate professor of prevention and community health at George Washington University, said in an interview.
“The goal was to get parents to deliver more messages and engage in more behaviors with their sons that we think are likely to help their sons stay healthy,” he said.
For the pilot study, Huebner and his team recruited 61 parents (95% of whom were mothers) with predominantly high school-aged cisgender sons (median, 16.7-17 years) who had come out as gay or bisexual at least a month prior, whose HIV status was negative or unknown, and who were living at home.
The interventions were strictly parent focused, Dr. Huebner said, noting that the only interaction with the kids involved independent surveys at the start and end of the study that explored parental behavior and engagement.
For the study, parental participants were stratified by son’s age (13-17 or 18-22 years) and then randomly assigned to participate in a web-accessible PATHS intervention (intervention group) or view a 35-minute, documentary-style film that encouraged acceptance of lesbian, gay, or bisexual children (control group),
Parents assigned to the intervention group were asked to engage in their own time with six modules that explored the importance of communication, HIV information, using and acquiring condoms, HIV testing, and as follow-up, a “to-do” list encouraging selection of how they would follow up with their sons about the content. They were also offered the option to participate in supplemental modules on pre-exposure prophylaxis (PrEP), anal intercourse, and what to do if a child tested positive for HIV.
“The intervention ... showed strong evidence of being effective at changing the parent behaviors that we hoped to change,” Dr. Huebner explained.
“We got independent reports from parents and kids that showed the same thing: parents were more likely to communicate with their sons about HIV in the 3 months after the intervention and were more likely to help their sons get access to condoms,” he said.
Both of these findings were significant, with parents in the experimental group being almost 10 times more likely to share HIV information with their sons (odds ratio, 9.50; 95% confidence interval, 1.02-39.99; P < .05) and five times more likely to teach proper condom use (OR, 5.04; 95% CI 1.56-12.46; P < .05), compared with parents receiving the placebo.
“It’s very promising that the initial signals from their intervention do show that parents facilitating the acquisition of information for young men who have sex with men really works,” said Dalmacio Dennis Flores, PhD, ACRN, an assistant professor of nursing in family and community health at the University of Pennsylvania, Philadelphia. He was not directly involved in the study.
“On the outcomes that matter for us, such as HIV prevention or getting tested, they were able to document that parents receiving guidance on how to have these conversations does result in youth outcomes – something that has been lacking in the literature specifically for this population up until today,” Dr. Flores told this news organization.
Overall, parents engaging in the PATHS intervention showed improvements in skills, attitudes, and behavioral intention toward engagement with their sons, including assisting with HIV testing. However, what about parental involvement in these types of dialogues with children who have not yet come out to their parents?
Dr. Flores said that, although Dr. Huebner’s work is pivotal for families where the child’s sexual orientation is known to parents, there is value in inclusive sex communication for all youth, regardless of how they identify (that is, out of the closet, closeted, straight, or those who are questioning their identity), especially since younger generations of LGBTQ youth are coming out at earlier ages, compared with previous generations.
It’s not just parents. Clinicians also have critical roles to play in helping bridge the sex-talk communication gaps between parents and adolescents and young adult children.
“In my work, I’ve found that more clinicians are willing to broach this within the discussion with dyads, with parents and adolescents in the room,” said Dr. Flores.
And he added: “If clinicians signal that there’s no such thing as too early to have these conversations or that issues such as consent, safety, and sexting are all okay to talk about because these are the current realities of young people, then parents can feel that they’re empowered to broach or sustain these conversations.”
Importantly, Dr. Huebner and associates are currently recruiting larger numbers of families for a new, yearlong trial that will not only examine parental behavior changes but also whether these changes translate into improvements in their child’s sexual health and/or competency. Interested families can learn more about the study and sign up to receive updates at www.parentwithlove.org.
Dr. Huebner and Dr. Flores reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“Take it from me, parents just don’t understand.”
Fresh Prince and D.J. Jazzy Jeff penned this lyric roughly 35 years ago, and coincidentally the HIV/AIDS epidemic has also been with us just as long. But the connection between the two may be highly relevant – that is, if you consider how infrequently parents appear (or have the proper tools) to engage with their gay or bisexual sons to prevent and curb HIV infections.
Currently, YMSM between the ages of 13 and 24 are among the most affected by the ongoing HIV epidemic, with CDC estimates suggesting that, in 2020, this group alone represented about 35% of new diagnoses. At the same time, about half of these HIV infections go undiagnosed. Recent data also suggest that care linkage in this group is similar to adults, but only a third of YMSM start antiretroviral therapy and are retained in care, leading to viral suppression rates as low as 12%.
With a goal to change these discouraging numbers, researchers from George Washington University, Washington, and other institutions conducted a randomized controlled pilot study targeting parents of YMSM to improve both the frequency and quality of communication around sexual health and HIV risk, prevention, and testing.
The findings, which were published online in the journal AIDS and Behavior, highlight the observation that parents could be an essential resource for combating the HIV epidemic, but they’re a resource that’s often underutilized. In fact, after participating in an online offering – PATHS (Parents and Adolescents Talking about Healthy Sexuality) – parents reported significantly greater engagement with their sons, especially around discussions focusing on HIV information and condom use.
“From what we know from the research, parents are uncomfortable talking about sex; they’re not great at talking about it. But when they do and do it effectively, those kids seem to have better health outcomes,” lead author David Huebner, PhD, MPH, associate professor of prevention and community health at George Washington University, said in an interview.
“The goal was to get parents to deliver more messages and engage in more behaviors with their sons that we think are likely to help their sons stay healthy,” he said.
For the pilot study, Huebner and his team recruited 61 parents (95% of whom were mothers) with predominantly high school-aged cisgender sons (median, 16.7-17 years) who had come out as gay or bisexual at least a month prior, whose HIV status was negative or unknown, and who were living at home.
The interventions were strictly parent focused, Dr. Huebner said, noting that the only interaction with the kids involved independent surveys at the start and end of the study that explored parental behavior and engagement.
For the study, parental participants were stratified by son’s age (13-17 or 18-22 years) and then randomly assigned to participate in a web-accessible PATHS intervention (intervention group) or view a 35-minute, documentary-style film that encouraged acceptance of lesbian, gay, or bisexual children (control group),
Parents assigned to the intervention group were asked to engage in their own time with six modules that explored the importance of communication, HIV information, using and acquiring condoms, HIV testing, and as follow-up, a “to-do” list encouraging selection of how they would follow up with their sons about the content. They were also offered the option to participate in supplemental modules on pre-exposure prophylaxis (PrEP), anal intercourse, and what to do if a child tested positive for HIV.
“The intervention ... showed strong evidence of being effective at changing the parent behaviors that we hoped to change,” Dr. Huebner explained.
“We got independent reports from parents and kids that showed the same thing: parents were more likely to communicate with their sons about HIV in the 3 months after the intervention and were more likely to help their sons get access to condoms,” he said.
Both of these findings were significant, with parents in the experimental group being almost 10 times more likely to share HIV information with their sons (odds ratio, 9.50; 95% confidence interval, 1.02-39.99; P < .05) and five times more likely to teach proper condom use (OR, 5.04; 95% CI 1.56-12.46; P < .05), compared with parents receiving the placebo.
“It’s very promising that the initial signals from their intervention do show that parents facilitating the acquisition of information for young men who have sex with men really works,” said Dalmacio Dennis Flores, PhD, ACRN, an assistant professor of nursing in family and community health at the University of Pennsylvania, Philadelphia. He was not directly involved in the study.
“On the outcomes that matter for us, such as HIV prevention or getting tested, they were able to document that parents receiving guidance on how to have these conversations does result in youth outcomes – something that has been lacking in the literature specifically for this population up until today,” Dr. Flores told this news organization.
Overall, parents engaging in the PATHS intervention showed improvements in skills, attitudes, and behavioral intention toward engagement with their sons, including assisting with HIV testing. However, what about parental involvement in these types of dialogues with children who have not yet come out to their parents?
Dr. Flores said that, although Dr. Huebner’s work is pivotal for families where the child’s sexual orientation is known to parents, there is value in inclusive sex communication for all youth, regardless of how they identify (that is, out of the closet, closeted, straight, or those who are questioning their identity), especially since younger generations of LGBTQ youth are coming out at earlier ages, compared with previous generations.
It’s not just parents. Clinicians also have critical roles to play in helping bridge the sex-talk communication gaps between parents and adolescents and young adult children.
“In my work, I’ve found that more clinicians are willing to broach this within the discussion with dyads, with parents and adolescents in the room,” said Dr. Flores.
And he added: “If clinicians signal that there’s no such thing as too early to have these conversations or that issues such as consent, safety, and sexting are all okay to talk about because these are the current realities of young people, then parents can feel that they’re empowered to broach or sustain these conversations.”
Importantly, Dr. Huebner and associates are currently recruiting larger numbers of families for a new, yearlong trial that will not only examine parental behavior changes but also whether these changes translate into improvements in their child’s sexual health and/or competency. Interested families can learn more about the study and sign up to receive updates at www.parentwithlove.org.
Dr. Huebner and Dr. Flores reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“Take it from me, parents just don’t understand.”
Fresh Prince and D.J. Jazzy Jeff penned this lyric roughly 35 years ago, and coincidentally the HIV/AIDS epidemic has also been with us just as long. But the connection between the two may be highly relevant – that is, if you consider how infrequently parents appear (or have the proper tools) to engage with their gay or bisexual sons to prevent and curb HIV infections.
Currently, YMSM between the ages of 13 and 24 are among the most affected by the ongoing HIV epidemic, with CDC estimates suggesting that, in 2020, this group alone represented about 35% of new diagnoses. At the same time, about half of these HIV infections go undiagnosed. Recent data also suggest that care linkage in this group is similar to adults, but only a third of YMSM start antiretroviral therapy and are retained in care, leading to viral suppression rates as low as 12%.
With a goal to change these discouraging numbers, researchers from George Washington University, Washington, and other institutions conducted a randomized controlled pilot study targeting parents of YMSM to improve both the frequency and quality of communication around sexual health and HIV risk, prevention, and testing.
The findings, which were published online in the journal AIDS and Behavior, highlight the observation that parents could be an essential resource for combating the HIV epidemic, but they’re a resource that’s often underutilized. In fact, after participating in an online offering – PATHS (Parents and Adolescents Talking about Healthy Sexuality) – parents reported significantly greater engagement with their sons, especially around discussions focusing on HIV information and condom use.
“From what we know from the research, parents are uncomfortable talking about sex; they’re not great at talking about it. But when they do and do it effectively, those kids seem to have better health outcomes,” lead author David Huebner, PhD, MPH, associate professor of prevention and community health at George Washington University, said in an interview.
“The goal was to get parents to deliver more messages and engage in more behaviors with their sons that we think are likely to help their sons stay healthy,” he said.
For the pilot study, Huebner and his team recruited 61 parents (95% of whom were mothers) with predominantly high school-aged cisgender sons (median, 16.7-17 years) who had come out as gay or bisexual at least a month prior, whose HIV status was negative or unknown, and who were living at home.
The interventions were strictly parent focused, Dr. Huebner said, noting that the only interaction with the kids involved independent surveys at the start and end of the study that explored parental behavior and engagement.
For the study, parental participants were stratified by son’s age (13-17 or 18-22 years) and then randomly assigned to participate in a web-accessible PATHS intervention (intervention group) or view a 35-minute, documentary-style film that encouraged acceptance of lesbian, gay, or bisexual children (control group),
Parents assigned to the intervention group were asked to engage in their own time with six modules that explored the importance of communication, HIV information, using and acquiring condoms, HIV testing, and as follow-up, a “to-do” list encouraging selection of how they would follow up with their sons about the content. They were also offered the option to participate in supplemental modules on pre-exposure prophylaxis (PrEP), anal intercourse, and what to do if a child tested positive for HIV.
“The intervention ... showed strong evidence of being effective at changing the parent behaviors that we hoped to change,” Dr. Huebner explained.
“We got independent reports from parents and kids that showed the same thing: parents were more likely to communicate with their sons about HIV in the 3 months after the intervention and were more likely to help their sons get access to condoms,” he said.
Both of these findings were significant, with parents in the experimental group being almost 10 times more likely to share HIV information with their sons (odds ratio, 9.50; 95% confidence interval, 1.02-39.99; P < .05) and five times more likely to teach proper condom use (OR, 5.04; 95% CI 1.56-12.46; P < .05), compared with parents receiving the placebo.
“It’s very promising that the initial signals from their intervention do show that parents facilitating the acquisition of information for young men who have sex with men really works,” said Dalmacio Dennis Flores, PhD, ACRN, an assistant professor of nursing in family and community health at the University of Pennsylvania, Philadelphia. He was not directly involved in the study.
“On the outcomes that matter for us, such as HIV prevention or getting tested, they were able to document that parents receiving guidance on how to have these conversations does result in youth outcomes – something that has been lacking in the literature specifically for this population up until today,” Dr. Flores told this news organization.
Overall, parents engaging in the PATHS intervention showed improvements in skills, attitudes, and behavioral intention toward engagement with their sons, including assisting with HIV testing. However, what about parental involvement in these types of dialogues with children who have not yet come out to their parents?
Dr. Flores said that, although Dr. Huebner’s work is pivotal for families where the child’s sexual orientation is known to parents, there is value in inclusive sex communication for all youth, regardless of how they identify (that is, out of the closet, closeted, straight, or those who are questioning their identity), especially since younger generations of LGBTQ youth are coming out at earlier ages, compared with previous generations.
It’s not just parents. Clinicians also have critical roles to play in helping bridge the sex-talk communication gaps between parents and adolescents and young adult children.
“In my work, I’ve found that more clinicians are willing to broach this within the discussion with dyads, with parents and adolescents in the room,” said Dr. Flores.
And he added: “If clinicians signal that there’s no such thing as too early to have these conversations or that issues such as consent, safety, and sexting are all okay to talk about because these are the current realities of young people, then parents can feel that they’re empowered to broach or sustain these conversations.”
Importantly, Dr. Huebner and associates are currently recruiting larger numbers of families for a new, yearlong trial that will not only examine parental behavior changes but also whether these changes translate into improvements in their child’s sexual health and/or competency. Interested families can learn more about the study and sign up to receive updates at www.parentwithlove.org.
Dr. Huebner and Dr. Flores reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AIDS AND BEHAVIOR
Bepirovirsen: Is a ‘functional cure’ for HBV on the horizon?
Treatment with bepirovirsen led to sustained clearance of hepatitis B surface antigen (HBsAg) and hepatitis B virus (HBV) DNA for 24 weeks after the end of treatment for adults with chronic HBV in the phase 2b B-Clear study.
The study results were presented at the annual meeting of the American Association for the Study of Liver Diseases and were simultaneously published in The New England Journal of Medicine.
Currently, nucleoside/nucleotide analogue (NA) therapy is the recommended first-line therapy for patients with chronic HBV because it can inhibit viral replication.
However, fewer than 5% of patients have HBsAg loss after 12 months of NA therapy, which underscores the need for therapies that can achieve a “functional” cure, largely defined as sustained, undetectable levels of HBV DNA and HBsAg in the blood, with or without generation of protective antibodies against HBsAg, the researchers noted.
Bepirovirsen is a potential first-in-class antisense oligonucleotide that targets all HBV messenger RNA and acts to decrease levels of viral proteins.
The phase 2b B-Clear study enrolled 457 patients with chronic HBV; 227 were receiving NA therapy, and 230 were not.
Participants were randomly assigned to receive weekly subcutaneous injections of bepirovirsen 300 mg for 24 weeks; bepirovirsen 300 mg for 12 weeks, then 150 mg for 12 weeks; bepirovirsen 300 mg for 12 weeks, then placebo for 12 weeks; or placebo for 12 weeks, then bepirovirsen 300 mg for 12 weeks (groups 1, 2, 3, and 4, respectively).
The composite primary outcome was HBsAg level below the limit of detection and HBV DNA level below the limit of quantification maintained for 24 weeks after the end of bepirovirsen treatment, without newly initiated antiviral medication.
Bepirovirsen 300 mg weekly for 24 weeks (group 1) led to HBsAg and HBV DNA loss in 9% of patients receiving NA therapy and 10% of patients not receiving NA treatment, which was sustained for 24 weeks after the last dose.
For groups 2, 3, and 4, HBsAg and HBV DNA loss occurred in 9%, 3%, and 0%, respectively, of patients receiving NA therapy and 6%, 1%, and 0%, respectively, of patients not receiving NA treatment.
Patients with low baseline HBsAg levels (< 1,000 IU/mL) responded best to treatment with bepirovirsen. Among patients who received bepirovirsen 300 mg weekly for 24 weeks, the primary outcome was achieved by 16% of patients taking NA therapy and by 25% of patients not taking NA therapy.
Although a “relatively low percentage” of patients overall achieved the primary outcome, the study “indicates the possibility of enhanced efficacy with the selection of patients according to baseline characteristics (low HBsAg level at baseline), with combination therapies, or both,” the researchers wrote.
Adverse events with bepirovirsen included injection-site reactions, pyrexia, fatigue, and increased alanine aminotransferase (ALT) levels. Increases in ALT levels, which were more common in those not receiving NA therapy than in those receiving NA therapy (41% vs. 17%), led to two serious adverse events.
On the basis of phase 2b data, GlaxoSmithKline (GSK) plans to advance bepirovirsen into phase 3 development, according to a news release.
Further pursuit of bepirovirsen therapy is “certainly warranted, with the use of a dose of 300 mg per week for at least 24 weeks; indeed, the duration of therapy might be dictated best by HBsAg levels at baseline,” Jay H. Hoofnagle, MD, director of the liver disease research branch at the National Institute of Diabetes and Digestive and Kidney Diseases, wrote in an editorial in the New England Journal of Medicine.
Several critical questions remain, including whether HBsAg negativity will persist beyond 24 weeks, wrote Dr. Hoofnagle, who was not involved in the study.
It’s a question GSK is addressing in the B-Sure trial, which will follow participants for an additional 33 months, the study noted.
Other questions include when NA therapy can be safely stopped, what other factors predict response, and whether RNA therapy–induced loss of HBsAg materially improves long-term outcomes, Dr. Hoofnagle wrote.
“Bepirovirsen is just one RNA-based HBV therapy now being pursued. Several other antisense RNAs as well as the more malleable small interfering RNA molecules (‘-sirans’) are currently in early-phase clinical trials. A new era in the control of hepatitis B may be at hand with these most modern of therapies for this most ancient disease,” Dr. Hoofnagle noted.
The B-Clear study was supported by GSK. Several authors have disclosed relationships with the company. A complete list of author disclosures is available with the original article. Dr. Hoofnagle has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treatment with bepirovirsen led to sustained clearance of hepatitis B surface antigen (HBsAg) and hepatitis B virus (HBV) DNA for 24 weeks after the end of treatment for adults with chronic HBV in the phase 2b B-Clear study.
The study results were presented at the annual meeting of the American Association for the Study of Liver Diseases and were simultaneously published in The New England Journal of Medicine.
Currently, nucleoside/nucleotide analogue (NA) therapy is the recommended first-line therapy for patients with chronic HBV because it can inhibit viral replication.
However, fewer than 5% of patients have HBsAg loss after 12 months of NA therapy, which underscores the need for therapies that can achieve a “functional” cure, largely defined as sustained, undetectable levels of HBV DNA and HBsAg in the blood, with or without generation of protective antibodies against HBsAg, the researchers noted.
Bepirovirsen is a potential first-in-class antisense oligonucleotide that targets all HBV messenger RNA and acts to decrease levels of viral proteins.
The phase 2b B-Clear study enrolled 457 patients with chronic HBV; 227 were receiving NA therapy, and 230 were not.
Participants were randomly assigned to receive weekly subcutaneous injections of bepirovirsen 300 mg for 24 weeks; bepirovirsen 300 mg for 12 weeks, then 150 mg for 12 weeks; bepirovirsen 300 mg for 12 weeks, then placebo for 12 weeks; or placebo for 12 weeks, then bepirovirsen 300 mg for 12 weeks (groups 1, 2, 3, and 4, respectively).
The composite primary outcome was HBsAg level below the limit of detection and HBV DNA level below the limit of quantification maintained for 24 weeks after the end of bepirovirsen treatment, without newly initiated antiviral medication.
Bepirovirsen 300 mg weekly for 24 weeks (group 1) led to HBsAg and HBV DNA loss in 9% of patients receiving NA therapy and 10% of patients not receiving NA treatment, which was sustained for 24 weeks after the last dose.
For groups 2, 3, and 4, HBsAg and HBV DNA loss occurred in 9%, 3%, and 0%, respectively, of patients receiving NA therapy and 6%, 1%, and 0%, respectively, of patients not receiving NA treatment.
Patients with low baseline HBsAg levels (< 1,000 IU/mL) responded best to treatment with bepirovirsen. Among patients who received bepirovirsen 300 mg weekly for 24 weeks, the primary outcome was achieved by 16% of patients taking NA therapy and by 25% of patients not taking NA therapy.
Although a “relatively low percentage” of patients overall achieved the primary outcome, the study “indicates the possibility of enhanced efficacy with the selection of patients according to baseline characteristics (low HBsAg level at baseline), with combination therapies, or both,” the researchers wrote.
Adverse events with bepirovirsen included injection-site reactions, pyrexia, fatigue, and increased alanine aminotransferase (ALT) levels. Increases in ALT levels, which were more common in those not receiving NA therapy than in those receiving NA therapy (41% vs. 17%), led to two serious adverse events.
On the basis of phase 2b data, GlaxoSmithKline (GSK) plans to advance bepirovirsen into phase 3 development, according to a news release.
Further pursuit of bepirovirsen therapy is “certainly warranted, with the use of a dose of 300 mg per week for at least 24 weeks; indeed, the duration of therapy might be dictated best by HBsAg levels at baseline,” Jay H. Hoofnagle, MD, director of the liver disease research branch at the National Institute of Diabetes and Digestive and Kidney Diseases, wrote in an editorial in the New England Journal of Medicine.
Several critical questions remain, including whether HBsAg negativity will persist beyond 24 weeks, wrote Dr. Hoofnagle, who was not involved in the study.
It’s a question GSK is addressing in the B-Sure trial, which will follow participants for an additional 33 months, the study noted.
Other questions include when NA therapy can be safely stopped, what other factors predict response, and whether RNA therapy–induced loss of HBsAg materially improves long-term outcomes, Dr. Hoofnagle wrote.
“Bepirovirsen is just one RNA-based HBV therapy now being pursued. Several other antisense RNAs as well as the more malleable small interfering RNA molecules (‘-sirans’) are currently in early-phase clinical trials. A new era in the control of hepatitis B may be at hand with these most modern of therapies for this most ancient disease,” Dr. Hoofnagle noted.
The B-Clear study was supported by GSK. Several authors have disclosed relationships with the company. A complete list of author disclosures is available with the original article. Dr. Hoofnagle has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treatment with bepirovirsen led to sustained clearance of hepatitis B surface antigen (HBsAg) and hepatitis B virus (HBV) DNA for 24 weeks after the end of treatment for adults with chronic HBV in the phase 2b B-Clear study.
The study results were presented at the annual meeting of the American Association for the Study of Liver Diseases and were simultaneously published in The New England Journal of Medicine.
Currently, nucleoside/nucleotide analogue (NA) therapy is the recommended first-line therapy for patients with chronic HBV because it can inhibit viral replication.
However, fewer than 5% of patients have HBsAg loss after 12 months of NA therapy, which underscores the need for therapies that can achieve a “functional” cure, largely defined as sustained, undetectable levels of HBV DNA and HBsAg in the blood, with or without generation of protective antibodies against HBsAg, the researchers noted.
Bepirovirsen is a potential first-in-class antisense oligonucleotide that targets all HBV messenger RNA and acts to decrease levels of viral proteins.
The phase 2b B-Clear study enrolled 457 patients with chronic HBV; 227 were receiving NA therapy, and 230 were not.
Participants were randomly assigned to receive weekly subcutaneous injections of bepirovirsen 300 mg for 24 weeks; bepirovirsen 300 mg for 12 weeks, then 150 mg for 12 weeks; bepirovirsen 300 mg for 12 weeks, then placebo for 12 weeks; or placebo for 12 weeks, then bepirovirsen 300 mg for 12 weeks (groups 1, 2, 3, and 4, respectively).
The composite primary outcome was HBsAg level below the limit of detection and HBV DNA level below the limit of quantification maintained for 24 weeks after the end of bepirovirsen treatment, without newly initiated antiviral medication.
Bepirovirsen 300 mg weekly for 24 weeks (group 1) led to HBsAg and HBV DNA loss in 9% of patients receiving NA therapy and 10% of patients not receiving NA treatment, which was sustained for 24 weeks after the last dose.
For groups 2, 3, and 4, HBsAg and HBV DNA loss occurred in 9%, 3%, and 0%, respectively, of patients receiving NA therapy and 6%, 1%, and 0%, respectively, of patients not receiving NA treatment.
Patients with low baseline HBsAg levels (< 1,000 IU/mL) responded best to treatment with bepirovirsen. Among patients who received bepirovirsen 300 mg weekly for 24 weeks, the primary outcome was achieved by 16% of patients taking NA therapy and by 25% of patients not taking NA therapy.
Although a “relatively low percentage” of patients overall achieved the primary outcome, the study “indicates the possibility of enhanced efficacy with the selection of patients according to baseline characteristics (low HBsAg level at baseline), with combination therapies, or both,” the researchers wrote.
Adverse events with bepirovirsen included injection-site reactions, pyrexia, fatigue, and increased alanine aminotransferase (ALT) levels. Increases in ALT levels, which were more common in those not receiving NA therapy than in those receiving NA therapy (41% vs. 17%), led to two serious adverse events.
On the basis of phase 2b data, GlaxoSmithKline (GSK) plans to advance bepirovirsen into phase 3 development, according to a news release.
Further pursuit of bepirovirsen therapy is “certainly warranted, with the use of a dose of 300 mg per week for at least 24 weeks; indeed, the duration of therapy might be dictated best by HBsAg levels at baseline,” Jay H. Hoofnagle, MD, director of the liver disease research branch at the National Institute of Diabetes and Digestive and Kidney Diseases, wrote in an editorial in the New England Journal of Medicine.
Several critical questions remain, including whether HBsAg negativity will persist beyond 24 weeks, wrote Dr. Hoofnagle, who was not involved in the study.
It’s a question GSK is addressing in the B-Sure trial, which will follow participants for an additional 33 months, the study noted.
Other questions include when NA therapy can be safely stopped, what other factors predict response, and whether RNA therapy–induced loss of HBsAg materially improves long-term outcomes, Dr. Hoofnagle wrote.
“Bepirovirsen is just one RNA-based HBV therapy now being pursued. Several other antisense RNAs as well as the more malleable small interfering RNA molecules (‘-sirans’) are currently in early-phase clinical trials. A new era in the control of hepatitis B may be at hand with these most modern of therapies for this most ancient disease,” Dr. Hoofnagle noted.
The B-Clear study was supported by GSK. Several authors have disclosed relationships with the company. A complete list of author disclosures is available with the original article. Dr. Hoofnagle has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LIVER MEETING
Flu vaccination associated with reduced stroke risk
The risk of stroke was about 23% lower in the 6 months following a flu shot, regardless of the patient’s age, sex, or underlying health conditions.
“There is an established link between upper respiratory infection and both heart attack and stroke. This has been very salient in the past few years throughout the COVID-19 pandemic,” study author Jessalyn Holodinsky, PhD, a stroke epidemiologist and postdoctoral fellow in clinical neurosciences at the University of Calgary (Alta.) told this news organization.
“It is also known that the flu shot can reduce risk of heart attack and hospitalization for those with heart disease,” she said. “Given both of these [observations], we thought it prudent to study whether there is a link between vaccination for influenza and stroke.”
The study was published in the Lancet Public Health.
Large effect size
The investigators analyzed administrative data from 2009 through 2018 from the Alberta Health Care Insurance Plan, which covers all residents of Alberta. The province provides free seasonal influenza vaccines to residents under the insurance plan.
The research team looked for stroke events such as acute ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage, and transient ischemic attack. They then analyzed the risk of stroke events among those with or without a flu shot in the previous 6 months. They accounted for multiple factors, including age, sex, income, location, and factors related to stroke risk, such as anticoagulant use, atrial fibrillation, chronic obstructive pulmonary disease, diabetes, and hypertension.
Among the 4.1 million adults included in the researchers’ analysis, about 1.8 million (43%) received at least one vaccination during the study period. Nearly 97,000 people received a flu vaccine in each year they were in the study, including 29,288 who received a shot in all 10 flu seasons included in the study.
About 38,000 stroke events were recorded, including about 34,000 (90%) first stroke events. Among the 10% of strokes that were recurrent events, the maximum number of stroke events in one person was nine.
Overall, patients who received at least one influenza vaccine were more likely to be older, be women, and have higher rates of comorbidities. The vaccinated group had a slightly higher proportion of people who lived in urban areas, but the income levels were similar between the vaccinated and unvaccinated groups.
The crude incidence of stroke was higher among people who had ever received an influenza vaccination, at 1.25%, compared with 0.52% among those who hadn’t been vaccinated. However, after adjusting for age, sex, underlying conditions, and socioeconomic status, recent flu vaccination (that is, in the previous 6 months) was associated with a 23% reduced risk of stroke.
The significant reduction in risk applied to all stroke types, particularly acute ischemic stroke and intracerebral hemorrhage. In addition, influenza vaccination was associated with a reduced risk across all ages and risk profiles, except patients without hypertension.
“What we were most surprised by was the sheer magnitude of the effect and that it existed across different adult age groups, for both sexes, and for those with and without risk factors for stroke,” said Dr. Holodinsky.
Vaccination was associated with a larger reduction in stroke risk in men than in women, perhaps because unvaccinated men had a significantly higher baseline risk for stroke than unvaccinated women, the study authors write.
Promoting cardiovascular health
In addition, vaccination was associated with a greater relative reduction in stroke risk in younger age groups, lower income groups, and those with diabetes, chronic obstructive pulmonary disease, and anticoagulant use.
Among 2.4 million people observed for the entire study period, vaccination protection increased with the number of vaccines received. People who were vaccinated serially each year had a significantly lower risk of stroke than those who received one shot.
Dr. Holodinsky and colleagues are conducting additional research into influenza vaccination, including stroke risk in children. They’re also investigating whether the reduced risk applies to other vaccinations for respiratory illnesses, such as COVID-19 and pneumonia.
“We hope that this added effect of vaccination encourages more adults to receive the flu shot,” she said. “One day, vaccinations might be considered a key pillar of cardiovascular health, along with diet, exercise, control of hypertension and high cholesterol, and smoking cessation.”
Future research should also investigate the reasons why adults – particularly people at high risk with underlying conditions – don’t receive recommended influenza vaccines, the study authors wrote.
‘Call to action’
Bahar Behrouzi, an MD-PhD candidate focused on clinical epidemiology at the Institute of Health Policy, Management, and Evaluation, University of Toronto, said: “There are a variety of observational studies around the world that show that flu vaccine uptake is low among the general population and high-risk persons. In studying these questions, our hope is that we can continue to build confidence in viral respiratory vaccines like the influenza vaccine by continuing to generate rigorous evidence with the latest data.”
Ms. Behrouzi, who wasn’t involved with this study, has researched influenza vaccination and cardiovascular risk. She and her colleagues have found that flu vaccines were associated with a 34% lower risk of major adverse cardiovascular events, including a 45% reduced risk among patients with recent acute coronary syndrome.
“The broader public health message is for people to advocate for themselves and get the seasonal flu vaccine, especially if they are part of an at-risk group,” she said. “In our studies, we have positioned this message as a call to action not only for the public, but also for health care professionals – particularly specialists such as cardiologists or neurologists – to encourage or remind them to engage in conversation about the broad benefits of vaccination beyond just preventing or reducing the severity of flu infection.”
The study was conducted without outside funding. Dr. Holodinsky and Ms. Behrouzi have reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
The risk of stroke was about 23% lower in the 6 months following a flu shot, regardless of the patient’s age, sex, or underlying health conditions.
“There is an established link between upper respiratory infection and both heart attack and stroke. This has been very salient in the past few years throughout the COVID-19 pandemic,” study author Jessalyn Holodinsky, PhD, a stroke epidemiologist and postdoctoral fellow in clinical neurosciences at the University of Calgary (Alta.) told this news organization.
“It is also known that the flu shot can reduce risk of heart attack and hospitalization for those with heart disease,” she said. “Given both of these [observations], we thought it prudent to study whether there is a link between vaccination for influenza and stroke.”
The study was published in the Lancet Public Health.
Large effect size
The investigators analyzed administrative data from 2009 through 2018 from the Alberta Health Care Insurance Plan, which covers all residents of Alberta. The province provides free seasonal influenza vaccines to residents under the insurance plan.
The research team looked for stroke events such as acute ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage, and transient ischemic attack. They then analyzed the risk of stroke events among those with or without a flu shot in the previous 6 months. They accounted for multiple factors, including age, sex, income, location, and factors related to stroke risk, such as anticoagulant use, atrial fibrillation, chronic obstructive pulmonary disease, diabetes, and hypertension.
Among the 4.1 million adults included in the researchers’ analysis, about 1.8 million (43%) received at least one vaccination during the study period. Nearly 97,000 people received a flu vaccine in each year they were in the study, including 29,288 who received a shot in all 10 flu seasons included in the study.
About 38,000 stroke events were recorded, including about 34,000 (90%) first stroke events. Among the 10% of strokes that were recurrent events, the maximum number of stroke events in one person was nine.
Overall, patients who received at least one influenza vaccine were more likely to be older, be women, and have higher rates of comorbidities. The vaccinated group had a slightly higher proportion of people who lived in urban areas, but the income levels were similar between the vaccinated and unvaccinated groups.
The crude incidence of stroke was higher among people who had ever received an influenza vaccination, at 1.25%, compared with 0.52% among those who hadn’t been vaccinated. However, after adjusting for age, sex, underlying conditions, and socioeconomic status, recent flu vaccination (that is, in the previous 6 months) was associated with a 23% reduced risk of stroke.
The significant reduction in risk applied to all stroke types, particularly acute ischemic stroke and intracerebral hemorrhage. In addition, influenza vaccination was associated with a reduced risk across all ages and risk profiles, except patients without hypertension.
“What we were most surprised by was the sheer magnitude of the effect and that it existed across different adult age groups, for both sexes, and for those with and without risk factors for stroke,” said Dr. Holodinsky.
Vaccination was associated with a larger reduction in stroke risk in men than in women, perhaps because unvaccinated men had a significantly higher baseline risk for stroke than unvaccinated women, the study authors write.
Promoting cardiovascular health
In addition, vaccination was associated with a greater relative reduction in stroke risk in younger age groups, lower income groups, and those with diabetes, chronic obstructive pulmonary disease, and anticoagulant use.
Among 2.4 million people observed for the entire study period, vaccination protection increased with the number of vaccines received. People who were vaccinated serially each year had a significantly lower risk of stroke than those who received one shot.
Dr. Holodinsky and colleagues are conducting additional research into influenza vaccination, including stroke risk in children. They’re also investigating whether the reduced risk applies to other vaccinations for respiratory illnesses, such as COVID-19 and pneumonia.
“We hope that this added effect of vaccination encourages more adults to receive the flu shot,” she said. “One day, vaccinations might be considered a key pillar of cardiovascular health, along with diet, exercise, control of hypertension and high cholesterol, and smoking cessation.”
Future research should also investigate the reasons why adults – particularly people at high risk with underlying conditions – don’t receive recommended influenza vaccines, the study authors wrote.
‘Call to action’
Bahar Behrouzi, an MD-PhD candidate focused on clinical epidemiology at the Institute of Health Policy, Management, and Evaluation, University of Toronto, said: “There are a variety of observational studies around the world that show that flu vaccine uptake is low among the general population and high-risk persons. In studying these questions, our hope is that we can continue to build confidence in viral respiratory vaccines like the influenza vaccine by continuing to generate rigorous evidence with the latest data.”
Ms. Behrouzi, who wasn’t involved with this study, has researched influenza vaccination and cardiovascular risk. She and her colleagues have found that flu vaccines were associated with a 34% lower risk of major adverse cardiovascular events, including a 45% reduced risk among patients with recent acute coronary syndrome.
“The broader public health message is for people to advocate for themselves and get the seasonal flu vaccine, especially if they are part of an at-risk group,” she said. “In our studies, we have positioned this message as a call to action not only for the public, but also for health care professionals – particularly specialists such as cardiologists or neurologists – to encourage or remind them to engage in conversation about the broad benefits of vaccination beyond just preventing or reducing the severity of flu infection.”
The study was conducted without outside funding. Dr. Holodinsky and Ms. Behrouzi have reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
The risk of stroke was about 23% lower in the 6 months following a flu shot, regardless of the patient’s age, sex, or underlying health conditions.
“There is an established link between upper respiratory infection and both heart attack and stroke. This has been very salient in the past few years throughout the COVID-19 pandemic,” study author Jessalyn Holodinsky, PhD, a stroke epidemiologist and postdoctoral fellow in clinical neurosciences at the University of Calgary (Alta.) told this news organization.
“It is also known that the flu shot can reduce risk of heart attack and hospitalization for those with heart disease,” she said. “Given both of these [observations], we thought it prudent to study whether there is a link between vaccination for influenza and stroke.”
The study was published in the Lancet Public Health.
Large effect size
The investigators analyzed administrative data from 2009 through 2018 from the Alberta Health Care Insurance Plan, which covers all residents of Alberta. The province provides free seasonal influenza vaccines to residents under the insurance plan.
The research team looked for stroke events such as acute ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage, and transient ischemic attack. They then analyzed the risk of stroke events among those with or without a flu shot in the previous 6 months. They accounted for multiple factors, including age, sex, income, location, and factors related to stroke risk, such as anticoagulant use, atrial fibrillation, chronic obstructive pulmonary disease, diabetes, and hypertension.
Among the 4.1 million adults included in the researchers’ analysis, about 1.8 million (43%) received at least one vaccination during the study period. Nearly 97,000 people received a flu vaccine in each year they were in the study, including 29,288 who received a shot in all 10 flu seasons included in the study.
About 38,000 stroke events were recorded, including about 34,000 (90%) first stroke events. Among the 10% of strokes that were recurrent events, the maximum number of stroke events in one person was nine.
Overall, patients who received at least one influenza vaccine were more likely to be older, be women, and have higher rates of comorbidities. The vaccinated group had a slightly higher proportion of people who lived in urban areas, but the income levels were similar between the vaccinated and unvaccinated groups.
The crude incidence of stroke was higher among people who had ever received an influenza vaccination, at 1.25%, compared with 0.52% among those who hadn’t been vaccinated. However, after adjusting for age, sex, underlying conditions, and socioeconomic status, recent flu vaccination (that is, in the previous 6 months) was associated with a 23% reduced risk of stroke.
The significant reduction in risk applied to all stroke types, particularly acute ischemic stroke and intracerebral hemorrhage. In addition, influenza vaccination was associated with a reduced risk across all ages and risk profiles, except patients without hypertension.
“What we were most surprised by was the sheer magnitude of the effect and that it existed across different adult age groups, for both sexes, and for those with and without risk factors for stroke,” said Dr. Holodinsky.
Vaccination was associated with a larger reduction in stroke risk in men than in women, perhaps because unvaccinated men had a significantly higher baseline risk for stroke than unvaccinated women, the study authors write.
Promoting cardiovascular health
In addition, vaccination was associated with a greater relative reduction in stroke risk in younger age groups, lower income groups, and those with diabetes, chronic obstructive pulmonary disease, and anticoagulant use.
Among 2.4 million people observed for the entire study period, vaccination protection increased with the number of vaccines received. People who were vaccinated serially each year had a significantly lower risk of stroke than those who received one shot.
Dr. Holodinsky and colleagues are conducting additional research into influenza vaccination, including stroke risk in children. They’re also investigating whether the reduced risk applies to other vaccinations for respiratory illnesses, such as COVID-19 and pneumonia.
“We hope that this added effect of vaccination encourages more adults to receive the flu shot,” she said. “One day, vaccinations might be considered a key pillar of cardiovascular health, along with diet, exercise, control of hypertension and high cholesterol, and smoking cessation.”
Future research should also investigate the reasons why adults – particularly people at high risk with underlying conditions – don’t receive recommended influenza vaccines, the study authors wrote.
‘Call to action’
Bahar Behrouzi, an MD-PhD candidate focused on clinical epidemiology at the Institute of Health Policy, Management, and Evaluation, University of Toronto, said: “There are a variety of observational studies around the world that show that flu vaccine uptake is low among the general population and high-risk persons. In studying these questions, our hope is that we can continue to build confidence in viral respiratory vaccines like the influenza vaccine by continuing to generate rigorous evidence with the latest data.”
Ms. Behrouzi, who wasn’t involved with this study, has researched influenza vaccination and cardiovascular risk. She and her colleagues have found that flu vaccines were associated with a 34% lower risk of major adverse cardiovascular events, including a 45% reduced risk among patients with recent acute coronary syndrome.
“The broader public health message is for people to advocate for themselves and get the seasonal flu vaccine, especially if they are part of an at-risk group,” she said. “In our studies, we have positioned this message as a call to action not only for the public, but also for health care professionals – particularly specialists such as cardiologists or neurologists – to encourage or remind them to engage in conversation about the broad benefits of vaccination beyond just preventing or reducing the severity of flu infection.”
The study was conducted without outside funding. Dr. Holodinsky and Ms. Behrouzi have reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM LANCET PUBLIC HEALTH
Don’t let amoxicillin shortage go to waste, antibiotic stewards say
Some experts are encouraging clinicians to see the amoxicillin shortage through pink-colored glasses.
The ongoing shortage, which was first reported in October and was prompted by a surge in demand linked in part to influenza and respiratory syncytial virus (RSV), could be an opportunity for clinicians to refine their prescribing practices and avoid unnecessary and potentially harmful orders for the medication, they say.
Antibiotics are often prescribed to patients who do not need them. In many cases, patients’ symptoms are caused by viral infections, not bacteria, so antibiotics do not help.
Even when symptoms resolve after a patient takes an antibiotic, the drug may have had nothing to do with their improvement.
Time to double-down on assessment; use antibiotics only when needed,” Jason Gallagher, PharmD, of Temple University School of Pharmacy in Philadelphia, posted on Twitter.
When antibiotics are not helping, they still may cause harm. Treatment with antibiotics entails risks for antibiotic resistance, infection with Clostridioides difficile, and side effects, such as rashes and – as Dr. Gallagher noted – diarrhea.
“They say ‘never let a good shortage go to waste,’ ” Michael Cosimini, MD, a pediatrician at Oregon Health & Science University, Portland, tweeted about the lack of amoxicillin in October.
Dr. Cosimini offered his thoughts about “improving our amoxicillin prescribing patterns” in pediatrics and encouraged colleagues to do so.
For example, he highlighted guidelines that state that antimicrobial therapy is not routinely required for preschool-aged children with community-acquired pneumonia (CAP) because most cases are caused by viral pathogens.
And trials show that when antibiotics are used for CAP, a shorter treatment duration, such as 5 days, rather than the standard 7-10 days, can be sufficient.
“As physicians, a shortage like this is an opportunity to do our best in the short term, as well as reflect on our current practice and make changes for the better in the long run,” Dr. Cosimini told this news organization.
Amoxicillin is the most commonly prescribed antibiotic in the outpatient setting and is the first choice among antimicrobial agents for common infections, such as otitis media, strep throat, and pneumonia, he said. “We use it frequently, so even small changes could go a long way to improve our prescribing practice,” Dr. Cosimini said.
Inappropriate antibiotic prescribing may be common
A 2021 statement on antibiotic stewardship from the American Academy of Pediatrics (AAP) declared that while antibiotics have saved countless lives, they can also cause harm and are frequently used inappropriately.
“One in five pediatric ambulatory visits result in an antibiotic prescription, accounting for nearly 50 million antibiotic prescriptions annually in the United States, at least half of which are considered inappropriate. [Acute respiratory tract infections] account for more than two-thirds of antibiotic prescriptions for children, at least one-third of which are unnecessary,” according to the society.
Outpatient antibiotic stewardship efforts could focus on clinical encounters in which the medications could be avoided altogether, the AAP suggested.
“Examples include antibiotic prescribing for nonspecific upper respiratory infection, bronchiolitis, acute bronchitis, asthma exacerbation, or conjunctivitis,” the group said.
Given the epidemiology of bacterial infections seen in ambulatory care settings that warrant antibiotic therapy, researchers conservatively estimate “that antibiotic prescribing could be safely reduced by 30%,” the statement noted.
That said, treatment decisions are not always clear cut.
“Certain infections in children, such as ear infections and lung infections, can be caused by viruses, bacteria, or both at the same time,” Dr. Cosimini said. “As such, it is very difficult to know which children benefit from which antibiotics.”
Watching, waiting, vaccinating
Pediatricians know that many children with ear infections will get better without antibiotics. “Parents should know that their doctor may suggest watching an ear infection without antibiotics, as is the recommendation from the AAP,” Dr. Cosimini said.
Data indicate that doctors are not following this practice as often as they could be, he said.
When antibiotic treatment is needed during the shortage, agents other than amoxicillin suspension can be used.
“Even though amoxicillin suspension is our go-to antibiotic for many infections, there are effective alternative options,” Dr. Cosimini said. “Children’s Hospital of Philadelphia has a good list for doctors looking for alternatives.”
Another approach to reducing the use of antibiotics in the future involves preventing infections through vaccination.
Research shows that routine childhood vaccines may have averted millions of respiratory and ear infections. And because bacterial infections can follow viral infections, the annual flu vaccine and COVID-19 vaccines “are also great tools to reduce antibiotic use,” Dr. Cosimini said.
A turn to more toxic options?
The shortage of amoxicillin oral powder for suspension was reported by the Food and Drug Administration and the American Society of Health-System Pharmacists (ASHP) in October.
On Nov. 4, the Society of Infectious Diseases Pharmacists (SIDP) issued a statement on the amoxicillin shortage, noting that increased demand for the drug coincided with a surge in respiratory viral infections, including RSV and influenza, among children.
“Though supportive care is the mainstay of treatment for viral infections, antibiotics may be indicated for the treatment of superimposed bacterial infections, including pneumonia and acute otitis media,” the SIDP statement said. “While alternative antibiotics may be available depending on the indication, many have a broader spectrum of activity, increased toxicity, and excess cost relative to amoxicillin. Furthermore, it is anticipated alternatives may soon become in short supply as well, given increased usage.”
SIDP “encourages the judicious use of antibiotics” and supports watch-and-wait strategies and the use of the shortest effective duration of therapy when appropriate.
Michael Ganio, PharmD, senior director of pharmacy practice and quality for ASHP, monitors around 250 drug shortages at any given time.
The amoxicillin shortage, while not “overly worrisome,” stands out because of how widely the drug is used and the fact that the shortage appears to have been sparked by an increase in demand rather than supply chain or manufacturing quality problems that more typically lead to shortages, he said.
Unlike some other shortages, the amoxicillin shortfall largely does not involve disrupting a medication regimen that someone was already receiving, and substitutions should be available.
“That said, it’s very, very disruptive to parents or a caregiver when you have a sick child who needs an antibiotic and it’s not available,” Dr. Ganio said.
Can a poster change practice?
In an unrelated move, the U.S. Agency for Healthcare Research and Quality published new resources and strategies to reduce inappropriate antibiotic use in ambulatory care settings.
One of the tools is a poster that doctors can print and hang in their offices. It states: “We commit to only prescribing antibiotics when they will help you. Taking antibiotics when you do not need them will NOT make you better. You will still feel sick, and the antibiotic may give you a skin rash, diarrhea, or a yeast infection.”
Jeffrey A. Linder, MD, MPH, a general internist and researcher at Northwestern University in Chicago, helped develop some of the approaches to improve prescribing practices in primary care.
Dr. Linder explained on a recent episode of the Freakonomics, M.D. podcast that the poster can be key.
One reason clinicians may prescribe antibiotics inappropriately is because they assume – perhaps erroneously – that patients want and expect them. By addressing the issue up front by displaying the poster, they may be able to “short-circuit” that type of thinking.
A minority of patients do expect antibiotics. “But the vast majority of patients are thinking, ‘I don’t feel well, I want to know what’s going on, and I want to know how to feel better and what’s going to happen.’ ”
For their part, patients can tell their doctors that they want an antibiotic only if they really need it, Dr. Linder said.
A version of this article first appeared on Medscape.com.
Some experts are encouraging clinicians to see the amoxicillin shortage through pink-colored glasses.
The ongoing shortage, which was first reported in October and was prompted by a surge in demand linked in part to influenza and respiratory syncytial virus (RSV), could be an opportunity for clinicians to refine their prescribing practices and avoid unnecessary and potentially harmful orders for the medication, they say.
Antibiotics are often prescribed to patients who do not need them. In many cases, patients’ symptoms are caused by viral infections, not bacteria, so antibiotics do not help.
Even when symptoms resolve after a patient takes an antibiotic, the drug may have had nothing to do with their improvement.
Time to double-down on assessment; use antibiotics only when needed,” Jason Gallagher, PharmD, of Temple University School of Pharmacy in Philadelphia, posted on Twitter.
When antibiotics are not helping, they still may cause harm. Treatment with antibiotics entails risks for antibiotic resistance, infection with Clostridioides difficile, and side effects, such as rashes and – as Dr. Gallagher noted – diarrhea.
“They say ‘never let a good shortage go to waste,’ ” Michael Cosimini, MD, a pediatrician at Oregon Health & Science University, Portland, tweeted about the lack of amoxicillin in October.
Dr. Cosimini offered his thoughts about “improving our amoxicillin prescribing patterns” in pediatrics and encouraged colleagues to do so.
For example, he highlighted guidelines that state that antimicrobial therapy is not routinely required for preschool-aged children with community-acquired pneumonia (CAP) because most cases are caused by viral pathogens.
And trials show that when antibiotics are used for CAP, a shorter treatment duration, such as 5 days, rather than the standard 7-10 days, can be sufficient.
“As physicians, a shortage like this is an opportunity to do our best in the short term, as well as reflect on our current practice and make changes for the better in the long run,” Dr. Cosimini told this news organization.
Amoxicillin is the most commonly prescribed antibiotic in the outpatient setting and is the first choice among antimicrobial agents for common infections, such as otitis media, strep throat, and pneumonia, he said. “We use it frequently, so even small changes could go a long way to improve our prescribing practice,” Dr. Cosimini said.
Inappropriate antibiotic prescribing may be common
A 2021 statement on antibiotic stewardship from the American Academy of Pediatrics (AAP) declared that while antibiotics have saved countless lives, they can also cause harm and are frequently used inappropriately.
“One in five pediatric ambulatory visits result in an antibiotic prescription, accounting for nearly 50 million antibiotic prescriptions annually in the United States, at least half of which are considered inappropriate. [Acute respiratory tract infections] account for more than two-thirds of antibiotic prescriptions for children, at least one-third of which are unnecessary,” according to the society.
Outpatient antibiotic stewardship efforts could focus on clinical encounters in which the medications could be avoided altogether, the AAP suggested.
“Examples include antibiotic prescribing for nonspecific upper respiratory infection, bronchiolitis, acute bronchitis, asthma exacerbation, or conjunctivitis,” the group said.
Given the epidemiology of bacterial infections seen in ambulatory care settings that warrant antibiotic therapy, researchers conservatively estimate “that antibiotic prescribing could be safely reduced by 30%,” the statement noted.
That said, treatment decisions are not always clear cut.
“Certain infections in children, such as ear infections and lung infections, can be caused by viruses, bacteria, or both at the same time,” Dr. Cosimini said. “As such, it is very difficult to know which children benefit from which antibiotics.”
Watching, waiting, vaccinating
Pediatricians know that many children with ear infections will get better without antibiotics. “Parents should know that their doctor may suggest watching an ear infection without antibiotics, as is the recommendation from the AAP,” Dr. Cosimini said.
Data indicate that doctors are not following this practice as often as they could be, he said.
When antibiotic treatment is needed during the shortage, agents other than amoxicillin suspension can be used.
“Even though amoxicillin suspension is our go-to antibiotic for many infections, there are effective alternative options,” Dr. Cosimini said. “Children’s Hospital of Philadelphia has a good list for doctors looking for alternatives.”
Another approach to reducing the use of antibiotics in the future involves preventing infections through vaccination.
Research shows that routine childhood vaccines may have averted millions of respiratory and ear infections. And because bacterial infections can follow viral infections, the annual flu vaccine and COVID-19 vaccines “are also great tools to reduce antibiotic use,” Dr. Cosimini said.
A turn to more toxic options?
The shortage of amoxicillin oral powder for suspension was reported by the Food and Drug Administration and the American Society of Health-System Pharmacists (ASHP) in October.
On Nov. 4, the Society of Infectious Diseases Pharmacists (SIDP) issued a statement on the amoxicillin shortage, noting that increased demand for the drug coincided with a surge in respiratory viral infections, including RSV and influenza, among children.
“Though supportive care is the mainstay of treatment for viral infections, antibiotics may be indicated for the treatment of superimposed bacterial infections, including pneumonia and acute otitis media,” the SIDP statement said. “While alternative antibiotics may be available depending on the indication, many have a broader spectrum of activity, increased toxicity, and excess cost relative to amoxicillin. Furthermore, it is anticipated alternatives may soon become in short supply as well, given increased usage.”
SIDP “encourages the judicious use of antibiotics” and supports watch-and-wait strategies and the use of the shortest effective duration of therapy when appropriate.
Michael Ganio, PharmD, senior director of pharmacy practice and quality for ASHP, monitors around 250 drug shortages at any given time.
The amoxicillin shortage, while not “overly worrisome,” stands out because of how widely the drug is used and the fact that the shortage appears to have been sparked by an increase in demand rather than supply chain or manufacturing quality problems that more typically lead to shortages, he said.
Unlike some other shortages, the amoxicillin shortfall largely does not involve disrupting a medication regimen that someone was already receiving, and substitutions should be available.
“That said, it’s very, very disruptive to parents or a caregiver when you have a sick child who needs an antibiotic and it’s not available,” Dr. Ganio said.
Can a poster change practice?
In an unrelated move, the U.S. Agency for Healthcare Research and Quality published new resources and strategies to reduce inappropriate antibiotic use in ambulatory care settings.
One of the tools is a poster that doctors can print and hang in their offices. It states: “We commit to only prescribing antibiotics when they will help you. Taking antibiotics when you do not need them will NOT make you better. You will still feel sick, and the antibiotic may give you a skin rash, diarrhea, or a yeast infection.”
Jeffrey A. Linder, MD, MPH, a general internist and researcher at Northwestern University in Chicago, helped develop some of the approaches to improve prescribing practices in primary care.
Dr. Linder explained on a recent episode of the Freakonomics, M.D. podcast that the poster can be key.
One reason clinicians may prescribe antibiotics inappropriately is because they assume – perhaps erroneously – that patients want and expect them. By addressing the issue up front by displaying the poster, they may be able to “short-circuit” that type of thinking.
A minority of patients do expect antibiotics. “But the vast majority of patients are thinking, ‘I don’t feel well, I want to know what’s going on, and I want to know how to feel better and what’s going to happen.’ ”
For their part, patients can tell their doctors that they want an antibiotic only if they really need it, Dr. Linder said.
A version of this article first appeared on Medscape.com.
Some experts are encouraging clinicians to see the amoxicillin shortage through pink-colored glasses.
The ongoing shortage, which was first reported in October and was prompted by a surge in demand linked in part to influenza and respiratory syncytial virus (RSV), could be an opportunity for clinicians to refine their prescribing practices and avoid unnecessary and potentially harmful orders for the medication, they say.
Antibiotics are often prescribed to patients who do not need them. In many cases, patients’ symptoms are caused by viral infections, not bacteria, so antibiotics do not help.
Even when symptoms resolve after a patient takes an antibiotic, the drug may have had nothing to do with their improvement.
Time to double-down on assessment; use antibiotics only when needed,” Jason Gallagher, PharmD, of Temple University School of Pharmacy in Philadelphia, posted on Twitter.
When antibiotics are not helping, they still may cause harm. Treatment with antibiotics entails risks for antibiotic resistance, infection with Clostridioides difficile, and side effects, such as rashes and – as Dr. Gallagher noted – diarrhea.
“They say ‘never let a good shortage go to waste,’ ” Michael Cosimini, MD, a pediatrician at Oregon Health & Science University, Portland, tweeted about the lack of amoxicillin in October.
Dr. Cosimini offered his thoughts about “improving our amoxicillin prescribing patterns” in pediatrics and encouraged colleagues to do so.
For example, he highlighted guidelines that state that antimicrobial therapy is not routinely required for preschool-aged children with community-acquired pneumonia (CAP) because most cases are caused by viral pathogens.
And trials show that when antibiotics are used for CAP, a shorter treatment duration, such as 5 days, rather than the standard 7-10 days, can be sufficient.
“As physicians, a shortage like this is an opportunity to do our best in the short term, as well as reflect on our current practice and make changes for the better in the long run,” Dr. Cosimini told this news organization.
Amoxicillin is the most commonly prescribed antibiotic in the outpatient setting and is the first choice among antimicrobial agents for common infections, such as otitis media, strep throat, and pneumonia, he said. “We use it frequently, so even small changes could go a long way to improve our prescribing practice,” Dr. Cosimini said.
Inappropriate antibiotic prescribing may be common
A 2021 statement on antibiotic stewardship from the American Academy of Pediatrics (AAP) declared that while antibiotics have saved countless lives, they can also cause harm and are frequently used inappropriately.
“One in five pediatric ambulatory visits result in an antibiotic prescription, accounting for nearly 50 million antibiotic prescriptions annually in the United States, at least half of which are considered inappropriate. [Acute respiratory tract infections] account for more than two-thirds of antibiotic prescriptions for children, at least one-third of which are unnecessary,” according to the society.
Outpatient antibiotic stewardship efforts could focus on clinical encounters in which the medications could be avoided altogether, the AAP suggested.
“Examples include antibiotic prescribing for nonspecific upper respiratory infection, bronchiolitis, acute bronchitis, asthma exacerbation, or conjunctivitis,” the group said.
Given the epidemiology of bacterial infections seen in ambulatory care settings that warrant antibiotic therapy, researchers conservatively estimate “that antibiotic prescribing could be safely reduced by 30%,” the statement noted.
That said, treatment decisions are not always clear cut.
“Certain infections in children, such as ear infections and lung infections, can be caused by viruses, bacteria, or both at the same time,” Dr. Cosimini said. “As such, it is very difficult to know which children benefit from which antibiotics.”
Watching, waiting, vaccinating
Pediatricians know that many children with ear infections will get better without antibiotics. “Parents should know that their doctor may suggest watching an ear infection without antibiotics, as is the recommendation from the AAP,” Dr. Cosimini said.
Data indicate that doctors are not following this practice as often as they could be, he said.
When antibiotic treatment is needed during the shortage, agents other than amoxicillin suspension can be used.
“Even though amoxicillin suspension is our go-to antibiotic for many infections, there are effective alternative options,” Dr. Cosimini said. “Children’s Hospital of Philadelphia has a good list for doctors looking for alternatives.”
Another approach to reducing the use of antibiotics in the future involves preventing infections through vaccination.
Research shows that routine childhood vaccines may have averted millions of respiratory and ear infections. And because bacterial infections can follow viral infections, the annual flu vaccine and COVID-19 vaccines “are also great tools to reduce antibiotic use,” Dr. Cosimini said.
A turn to more toxic options?
The shortage of amoxicillin oral powder for suspension was reported by the Food and Drug Administration and the American Society of Health-System Pharmacists (ASHP) in October.
On Nov. 4, the Society of Infectious Diseases Pharmacists (SIDP) issued a statement on the amoxicillin shortage, noting that increased demand for the drug coincided with a surge in respiratory viral infections, including RSV and influenza, among children.
“Though supportive care is the mainstay of treatment for viral infections, antibiotics may be indicated for the treatment of superimposed bacterial infections, including pneumonia and acute otitis media,” the SIDP statement said. “While alternative antibiotics may be available depending on the indication, many have a broader spectrum of activity, increased toxicity, and excess cost relative to amoxicillin. Furthermore, it is anticipated alternatives may soon become in short supply as well, given increased usage.”
SIDP “encourages the judicious use of antibiotics” and supports watch-and-wait strategies and the use of the shortest effective duration of therapy when appropriate.
Michael Ganio, PharmD, senior director of pharmacy practice and quality for ASHP, monitors around 250 drug shortages at any given time.
The amoxicillin shortage, while not “overly worrisome,” stands out because of how widely the drug is used and the fact that the shortage appears to have been sparked by an increase in demand rather than supply chain or manufacturing quality problems that more typically lead to shortages, he said.
Unlike some other shortages, the amoxicillin shortfall largely does not involve disrupting a medication regimen that someone was already receiving, and substitutions should be available.
“That said, it’s very, very disruptive to parents or a caregiver when you have a sick child who needs an antibiotic and it’s not available,” Dr. Ganio said.
Can a poster change practice?
In an unrelated move, the U.S. Agency for Healthcare Research and Quality published new resources and strategies to reduce inappropriate antibiotic use in ambulatory care settings.
One of the tools is a poster that doctors can print and hang in their offices. It states: “We commit to only prescribing antibiotics when they will help you. Taking antibiotics when you do not need them will NOT make you better. You will still feel sick, and the antibiotic may give you a skin rash, diarrhea, or a yeast infection.”
Jeffrey A. Linder, MD, MPH, a general internist and researcher at Northwestern University in Chicago, helped develop some of the approaches to improve prescribing practices in primary care.
Dr. Linder explained on a recent episode of the Freakonomics, M.D. podcast that the poster can be key.
One reason clinicians may prescribe antibiotics inappropriately is because they assume – perhaps erroneously – that patients want and expect them. By addressing the issue up front by displaying the poster, they may be able to “short-circuit” that type of thinking.
A minority of patients do expect antibiotics. “But the vast majority of patients are thinking, ‘I don’t feel well, I want to know what’s going on, and I want to know how to feel better and what’s going to happen.’ ”
For their part, patients can tell their doctors that they want an antibiotic only if they really need it, Dr. Linder said.
A version of this article first appeared on Medscape.com.
‘A huge deal’: Millions have long COVID, and more are expected
with symptoms that have lasted 3 months or longer, according to the latest U.S. government survey done in October. More than a quarter say their condition is severe enough to significantly limit their day-to-day activities – yet the problem is only barely starting to get the attention of employers, the health care system, and policymakers.
With no cure or treatment in sight, long COVID is already burdening not only the health care system, but also the economy – and that burden is set to grow. Many experts worry about the possible long-term ripple effects, from increased spending on medical care costs to lost wages due to not being able to work, as well as the policy implications that come with addressing these issues.
“At this point, anyone who’s looking at this seriously would say this is a huge deal,” says senior Brookings Institution fellow Katie Bach, the author of a study that analyzed long COVID’s impact on the labor market.
“We need a real concerted focus on treating these people, which means both research and the clinical side, and figuring out how to build a labor market that is more inclusive of people with disabilities,” she said.
It’s not only that many people are affected. It’s that they are often affected for months and possibly even years.
The U.S. government figures suggest more than 18 million people could have symptoms of long COVID right now. The latest Household Pulse Survey by the Census Bureau and the National Center for Health Statistics takes data from 41,415 people.
A preprint of a study by researchers from City University of New York, posted on medRxiv in September and based on a similar population survey done between June 30 and July 2, drew comparable results. The study has not been peer reviewed.
More than 7% of all those who answered said they had long COVID at the time of the survey, which the researchers said corresponded to approximately 18.5 million U.S. adults. The same study found that a quarter of those, or an estimated 4.7 million adults, said their daily activities were impacted “a lot.”
This can translate into pain not only for the patients, but for governments and employers, too.
In high-income countries around the world, government surveys and other studies are shedding light on the extent to which post-COVID-19 symptoms – commonly known as long COVID – are affecting populations. While results vary, they generally fall within similar ranges.
The World Health Organization estimates that between 10% and 20% of those with COVID-19 go on to have an array of medium- to long-term post-COVID-19 symptoms that range from mild to debilitating. The U.S. Government Accountability Office puts that estimate at 10% to 30%; one of the latest studies published at the end of October in The Journal of the American Medical Association found that 15% of U.S. adults who had tested positive for COVID-19 reported current long COVID symptoms. Elsewhere, a study from the Netherlands published in The Lancet in August found that one in eight COVID-19 cases, or 12.7%, were likely to become long COVID.
“It’s very clear that the condition is devastating people’s lives and livelihoods,” WHO Director-General Tedros Adhanom Ghebreyesus wrote in an article for The Guardian newspaper in October.
“The world has already lost a significant number of the workforce to illness, death, fatigue, unplanned retirement due to an increase in long-term disability, which not only impacts the health system, but is a hit to the overarching economy … the impact of long COVID for all countries is very serious and needs immediate and sustained action equivalent to its scale.”
Global snapshot: Lasting symptoms, impact on activities
Patients describe a spectrum of persistent issues, with extreme fatigue, brain fog or cognitive problems, and shortness of breath among the most common complaints. Many also have manageable symptoms that worsen significantly after even mild physical or mental exertion.
Women appear almost twice as likely as men to get long COVID. Many patients have other medical conditions and disabilities that make them more vulnerable to the condition. Those who face greater obstacles accessing health care due to discrimination or socioeconomic inequity are at higher risk as well.
While many are older, a large number are also in their prime working age. The Census Bureau data show that people ages 40-49 are more likely than any other group to get long COVID, which has broader implications for labor markets and the global economy. Already, experts have estimated that long COVID is likely to cost the U.S. trillions of dollars and affect multiple industries.
“Whether they’re in the financial world, the medical system, lawyers, they’re telling me they’re sitting at the computer screen and they’re unable to process the data,” said Zachary Schwartz, MD, medical director for Vancouver General Hospital’s Post-COVID-19 Recovery Clinic.
“That is what’s most distressing for people, in that they’re not working, they’re not making money, and they don’t know when, or if, they’re going to get better.”
Nearly a third of respondents in the Census Bureau’s Household Pulse Survey who said they have had COVID-19 reported symptoms that lasted 3 months or longer. People between the ages of 30 and 59 were the most affected, with about 32% reporting symptoms. Across the entire adult U.S. population, the survey found that 1 in 7 adults have had long COVID at some point during the pandemic, with about 1 in 18 saying it limited their activity to some degree, and 1 in 50 saying they have faced “a lot” of limits on their activities. Any way these numbers are dissected, long COVID has impacted a large swath of the population.
Yet research into the causes and possible treatments of long COVID is just getting underway.
“The amount of energy and time devoted to it is way, way less than it should, given how many people are likely affected,” said David Cutler, PhD, professor of economics at Harvard University, Cambridge, Mass., who has written about the economic cost of long COVID. “We’re way, way underdoing it here. And I think that’s really a terrible thing.”
Population surveys and studies from around the world show that long COVID lives up to its name, with people reporting serious symptoms for months on end.
In October, Statistics Canada and the Public Health Agency of Canada published early results from a questionnaire done between spring and summer 2022 that found just under 15% of adults who had a confirmed or suspected case of COVID-19 went on to have new or continuing symptoms 3 or more months later. Nearly half, or 47.3%, dealt with symptoms that lasted a year or more. More than one in five said their symptoms “often or always” limited their day-to-day activities, which included routine tasks such as preparing meals, doing errands and chores, and basic functions such as personal care and moving around in their homes.
Nearly three-quarters of workers or students said they missed an average of 20 days of work or school.
“We haven’t yet been able to determine exactly when symptoms resolve,” said Rainu Kaushal, MD, the senior associate dean for clinical research at Weill Cornell Medicine in New York. She is co-leading a national study on long COVID in adults and children, funded by the National Institutes of Health RECOVER Initiative.
“But there does seem to be, for many of the milder symptoms, resolution at about 4-6 weeks. There seems to be a second point of resolution around 6 months for certain symptoms, and then some symptoms do seem to be permanent, and those tend to be patients who have underlying conditions,” she said.
Reducing the risk
Given all the data so far, experts recommend urgent policy changes to help people with long COVID.
“The population needs to be prepared, that understanding long COVID is going to be a very long and difficult process,” said Alexander Charney, MD, PhD, associate professor and the lead principal investigator of the RECOVER adult cohort at Icahn School of Medicine at Mount Sinai in New York. He said the government can do a great deal to help, including setting up a network of connected clinics treating long COVID, standardizing best practices, and sharing information.
“That would go a long way towards making sure that every person feels like they’re not too far away from a clinic where they can get treated for this particular condition,” he said.
But the only known way to prevent long COVID is to prevent COVID-19 infections in the first place, experts say. That means equitable access to tests, therapeutics, and vaccines.
“I will say that avoiding COVID remains the best treatment in the arsenal right now,” said Dr. Kaushal. This means masking, avoiding crowded places with poor ventilation and high exposure risk, and being up to date on vaccinations, she said.
A number of papers – including a large U.K. study published in May 2022, another one from July, and the JAMA study from October – all suggest that vaccinations can help reduce the risk of long COVID.
“I am absolutely of the belief that vaccination has reduced the incidence and overall amount of long COVID … [and is] still by far the best thing the public can do,” said Dr. Schwartz.
A version of this article first appeared on WebMD.com.
with symptoms that have lasted 3 months or longer, according to the latest U.S. government survey done in October. More than a quarter say their condition is severe enough to significantly limit their day-to-day activities – yet the problem is only barely starting to get the attention of employers, the health care system, and policymakers.
With no cure or treatment in sight, long COVID is already burdening not only the health care system, but also the economy – and that burden is set to grow. Many experts worry about the possible long-term ripple effects, from increased spending on medical care costs to lost wages due to not being able to work, as well as the policy implications that come with addressing these issues.
“At this point, anyone who’s looking at this seriously would say this is a huge deal,” says senior Brookings Institution fellow Katie Bach, the author of a study that analyzed long COVID’s impact on the labor market.
“We need a real concerted focus on treating these people, which means both research and the clinical side, and figuring out how to build a labor market that is more inclusive of people with disabilities,” she said.
It’s not only that many people are affected. It’s that they are often affected for months and possibly even years.
The U.S. government figures suggest more than 18 million people could have symptoms of long COVID right now. The latest Household Pulse Survey by the Census Bureau and the National Center for Health Statistics takes data from 41,415 people.
A preprint of a study by researchers from City University of New York, posted on medRxiv in September and based on a similar population survey done between June 30 and July 2, drew comparable results. The study has not been peer reviewed.
More than 7% of all those who answered said they had long COVID at the time of the survey, which the researchers said corresponded to approximately 18.5 million U.S. adults. The same study found that a quarter of those, or an estimated 4.7 million adults, said their daily activities were impacted “a lot.”
This can translate into pain not only for the patients, but for governments and employers, too.
In high-income countries around the world, government surveys and other studies are shedding light on the extent to which post-COVID-19 symptoms – commonly known as long COVID – are affecting populations. While results vary, they generally fall within similar ranges.
The World Health Organization estimates that between 10% and 20% of those with COVID-19 go on to have an array of medium- to long-term post-COVID-19 symptoms that range from mild to debilitating. The U.S. Government Accountability Office puts that estimate at 10% to 30%; one of the latest studies published at the end of October in The Journal of the American Medical Association found that 15% of U.S. adults who had tested positive for COVID-19 reported current long COVID symptoms. Elsewhere, a study from the Netherlands published in The Lancet in August found that one in eight COVID-19 cases, or 12.7%, were likely to become long COVID.
“It’s very clear that the condition is devastating people’s lives and livelihoods,” WHO Director-General Tedros Adhanom Ghebreyesus wrote in an article for The Guardian newspaper in October.
“The world has already lost a significant number of the workforce to illness, death, fatigue, unplanned retirement due to an increase in long-term disability, which not only impacts the health system, but is a hit to the overarching economy … the impact of long COVID for all countries is very serious and needs immediate and sustained action equivalent to its scale.”
Global snapshot: Lasting symptoms, impact on activities
Patients describe a spectrum of persistent issues, with extreme fatigue, brain fog or cognitive problems, and shortness of breath among the most common complaints. Many also have manageable symptoms that worsen significantly after even mild physical or mental exertion.
Women appear almost twice as likely as men to get long COVID. Many patients have other medical conditions and disabilities that make them more vulnerable to the condition. Those who face greater obstacles accessing health care due to discrimination or socioeconomic inequity are at higher risk as well.
While many are older, a large number are also in their prime working age. The Census Bureau data show that people ages 40-49 are more likely than any other group to get long COVID, which has broader implications for labor markets and the global economy. Already, experts have estimated that long COVID is likely to cost the U.S. trillions of dollars and affect multiple industries.
“Whether they’re in the financial world, the medical system, lawyers, they’re telling me they’re sitting at the computer screen and they’re unable to process the data,” said Zachary Schwartz, MD, medical director for Vancouver General Hospital’s Post-COVID-19 Recovery Clinic.
“That is what’s most distressing for people, in that they’re not working, they’re not making money, and they don’t know when, or if, they’re going to get better.”
Nearly a third of respondents in the Census Bureau’s Household Pulse Survey who said they have had COVID-19 reported symptoms that lasted 3 months or longer. People between the ages of 30 and 59 were the most affected, with about 32% reporting symptoms. Across the entire adult U.S. population, the survey found that 1 in 7 adults have had long COVID at some point during the pandemic, with about 1 in 18 saying it limited their activity to some degree, and 1 in 50 saying they have faced “a lot” of limits on their activities. Any way these numbers are dissected, long COVID has impacted a large swath of the population.
Yet research into the causes and possible treatments of long COVID is just getting underway.
“The amount of energy and time devoted to it is way, way less than it should, given how many people are likely affected,” said David Cutler, PhD, professor of economics at Harvard University, Cambridge, Mass., who has written about the economic cost of long COVID. “We’re way, way underdoing it here. And I think that’s really a terrible thing.”
Population surveys and studies from around the world show that long COVID lives up to its name, with people reporting serious symptoms for months on end.
In October, Statistics Canada and the Public Health Agency of Canada published early results from a questionnaire done between spring and summer 2022 that found just under 15% of adults who had a confirmed or suspected case of COVID-19 went on to have new or continuing symptoms 3 or more months later. Nearly half, or 47.3%, dealt with symptoms that lasted a year or more. More than one in five said their symptoms “often or always” limited their day-to-day activities, which included routine tasks such as preparing meals, doing errands and chores, and basic functions such as personal care and moving around in their homes.
Nearly three-quarters of workers or students said they missed an average of 20 days of work or school.
“We haven’t yet been able to determine exactly when symptoms resolve,” said Rainu Kaushal, MD, the senior associate dean for clinical research at Weill Cornell Medicine in New York. She is co-leading a national study on long COVID in adults and children, funded by the National Institutes of Health RECOVER Initiative.
“But there does seem to be, for many of the milder symptoms, resolution at about 4-6 weeks. There seems to be a second point of resolution around 6 months for certain symptoms, and then some symptoms do seem to be permanent, and those tend to be patients who have underlying conditions,” she said.
Reducing the risk
Given all the data so far, experts recommend urgent policy changes to help people with long COVID.
“The population needs to be prepared, that understanding long COVID is going to be a very long and difficult process,” said Alexander Charney, MD, PhD, associate professor and the lead principal investigator of the RECOVER adult cohort at Icahn School of Medicine at Mount Sinai in New York. He said the government can do a great deal to help, including setting up a network of connected clinics treating long COVID, standardizing best practices, and sharing information.
“That would go a long way towards making sure that every person feels like they’re not too far away from a clinic where they can get treated for this particular condition,” he said.
But the only known way to prevent long COVID is to prevent COVID-19 infections in the first place, experts say. That means equitable access to tests, therapeutics, and vaccines.
“I will say that avoiding COVID remains the best treatment in the arsenal right now,” said Dr. Kaushal. This means masking, avoiding crowded places with poor ventilation and high exposure risk, and being up to date on vaccinations, she said.
A number of papers – including a large U.K. study published in May 2022, another one from July, and the JAMA study from October – all suggest that vaccinations can help reduce the risk of long COVID.
“I am absolutely of the belief that vaccination has reduced the incidence and overall amount of long COVID … [and is] still by far the best thing the public can do,” said Dr. Schwartz.
A version of this article first appeared on WebMD.com.
with symptoms that have lasted 3 months or longer, according to the latest U.S. government survey done in October. More than a quarter say their condition is severe enough to significantly limit their day-to-day activities – yet the problem is only barely starting to get the attention of employers, the health care system, and policymakers.
With no cure or treatment in sight, long COVID is already burdening not only the health care system, but also the economy – and that burden is set to grow. Many experts worry about the possible long-term ripple effects, from increased spending on medical care costs to lost wages due to not being able to work, as well as the policy implications that come with addressing these issues.
“At this point, anyone who’s looking at this seriously would say this is a huge deal,” says senior Brookings Institution fellow Katie Bach, the author of a study that analyzed long COVID’s impact on the labor market.
“We need a real concerted focus on treating these people, which means both research and the clinical side, and figuring out how to build a labor market that is more inclusive of people with disabilities,” she said.
It’s not only that many people are affected. It’s that they are often affected for months and possibly even years.
The U.S. government figures suggest more than 18 million people could have symptoms of long COVID right now. The latest Household Pulse Survey by the Census Bureau and the National Center for Health Statistics takes data from 41,415 people.
A preprint of a study by researchers from City University of New York, posted on medRxiv in September and based on a similar population survey done between June 30 and July 2, drew comparable results. The study has not been peer reviewed.
More than 7% of all those who answered said they had long COVID at the time of the survey, which the researchers said corresponded to approximately 18.5 million U.S. adults. The same study found that a quarter of those, or an estimated 4.7 million adults, said their daily activities were impacted “a lot.”
This can translate into pain not only for the patients, but for governments and employers, too.
In high-income countries around the world, government surveys and other studies are shedding light on the extent to which post-COVID-19 symptoms – commonly known as long COVID – are affecting populations. While results vary, they generally fall within similar ranges.
The World Health Organization estimates that between 10% and 20% of those with COVID-19 go on to have an array of medium- to long-term post-COVID-19 symptoms that range from mild to debilitating. The U.S. Government Accountability Office puts that estimate at 10% to 30%; one of the latest studies published at the end of October in The Journal of the American Medical Association found that 15% of U.S. adults who had tested positive for COVID-19 reported current long COVID symptoms. Elsewhere, a study from the Netherlands published in The Lancet in August found that one in eight COVID-19 cases, or 12.7%, were likely to become long COVID.
“It’s very clear that the condition is devastating people’s lives and livelihoods,” WHO Director-General Tedros Adhanom Ghebreyesus wrote in an article for The Guardian newspaper in October.
“The world has already lost a significant number of the workforce to illness, death, fatigue, unplanned retirement due to an increase in long-term disability, which not only impacts the health system, but is a hit to the overarching economy … the impact of long COVID for all countries is very serious and needs immediate and sustained action equivalent to its scale.”
Global snapshot: Lasting symptoms, impact on activities
Patients describe a spectrum of persistent issues, with extreme fatigue, brain fog or cognitive problems, and shortness of breath among the most common complaints. Many also have manageable symptoms that worsen significantly after even mild physical or mental exertion.
Women appear almost twice as likely as men to get long COVID. Many patients have other medical conditions and disabilities that make them more vulnerable to the condition. Those who face greater obstacles accessing health care due to discrimination or socioeconomic inequity are at higher risk as well.
While many are older, a large number are also in their prime working age. The Census Bureau data show that people ages 40-49 are more likely than any other group to get long COVID, which has broader implications for labor markets and the global economy. Already, experts have estimated that long COVID is likely to cost the U.S. trillions of dollars and affect multiple industries.
“Whether they’re in the financial world, the medical system, lawyers, they’re telling me they’re sitting at the computer screen and they’re unable to process the data,” said Zachary Schwartz, MD, medical director for Vancouver General Hospital’s Post-COVID-19 Recovery Clinic.
“That is what’s most distressing for people, in that they’re not working, they’re not making money, and they don’t know when, or if, they’re going to get better.”
Nearly a third of respondents in the Census Bureau’s Household Pulse Survey who said they have had COVID-19 reported symptoms that lasted 3 months or longer. People between the ages of 30 and 59 were the most affected, with about 32% reporting symptoms. Across the entire adult U.S. population, the survey found that 1 in 7 adults have had long COVID at some point during the pandemic, with about 1 in 18 saying it limited their activity to some degree, and 1 in 50 saying they have faced “a lot” of limits on their activities. Any way these numbers are dissected, long COVID has impacted a large swath of the population.
Yet research into the causes and possible treatments of long COVID is just getting underway.
“The amount of energy and time devoted to it is way, way less than it should, given how many people are likely affected,” said David Cutler, PhD, professor of economics at Harvard University, Cambridge, Mass., who has written about the economic cost of long COVID. “We’re way, way underdoing it here. And I think that’s really a terrible thing.”
Population surveys and studies from around the world show that long COVID lives up to its name, with people reporting serious symptoms for months on end.
In October, Statistics Canada and the Public Health Agency of Canada published early results from a questionnaire done between spring and summer 2022 that found just under 15% of adults who had a confirmed or suspected case of COVID-19 went on to have new or continuing symptoms 3 or more months later. Nearly half, or 47.3%, dealt with symptoms that lasted a year or more. More than one in five said their symptoms “often or always” limited their day-to-day activities, which included routine tasks such as preparing meals, doing errands and chores, and basic functions such as personal care and moving around in their homes.
Nearly three-quarters of workers or students said they missed an average of 20 days of work or school.
“We haven’t yet been able to determine exactly when symptoms resolve,” said Rainu Kaushal, MD, the senior associate dean for clinical research at Weill Cornell Medicine in New York. She is co-leading a national study on long COVID in adults and children, funded by the National Institutes of Health RECOVER Initiative.
“But there does seem to be, for many of the milder symptoms, resolution at about 4-6 weeks. There seems to be a second point of resolution around 6 months for certain symptoms, and then some symptoms do seem to be permanent, and those tend to be patients who have underlying conditions,” she said.
Reducing the risk
Given all the data so far, experts recommend urgent policy changes to help people with long COVID.
“The population needs to be prepared, that understanding long COVID is going to be a very long and difficult process,” said Alexander Charney, MD, PhD, associate professor and the lead principal investigator of the RECOVER adult cohort at Icahn School of Medicine at Mount Sinai in New York. He said the government can do a great deal to help, including setting up a network of connected clinics treating long COVID, standardizing best practices, and sharing information.
“That would go a long way towards making sure that every person feels like they’re not too far away from a clinic where they can get treated for this particular condition,” he said.
But the only known way to prevent long COVID is to prevent COVID-19 infections in the first place, experts say. That means equitable access to tests, therapeutics, and vaccines.
“I will say that avoiding COVID remains the best treatment in the arsenal right now,” said Dr. Kaushal. This means masking, avoiding crowded places with poor ventilation and high exposure risk, and being up to date on vaccinations, she said.
A number of papers – including a large U.K. study published in May 2022, another one from July, and the JAMA study from October – all suggest that vaccinations can help reduce the risk of long COVID.
“I am absolutely of the belief that vaccination has reduced the incidence and overall amount of long COVID … [and is] still by far the best thing the public can do,” said Dr. Schwartz.
A version of this article first appeared on WebMD.com.
Severe pediatric oral mucositis
A 12-YEAR-OLD BOY presented to the hospital with a 2-day history of fever, cough, and painful blisters on swollen lips. On examination, he had multiple tense blisters with clear fluid on the buccal mucosa and inner lips (FIGURE 1A), as well as multiple discrete ulcers on his posterior pharynx. The patient had no other skin, eye, or urogenital involvement, but he was dehydrated. Respiratory examination was unremarkable. A complete blood count and metabolic panel were normal, as was a C-reactive protein (CRP) test (0.8 mg/L).
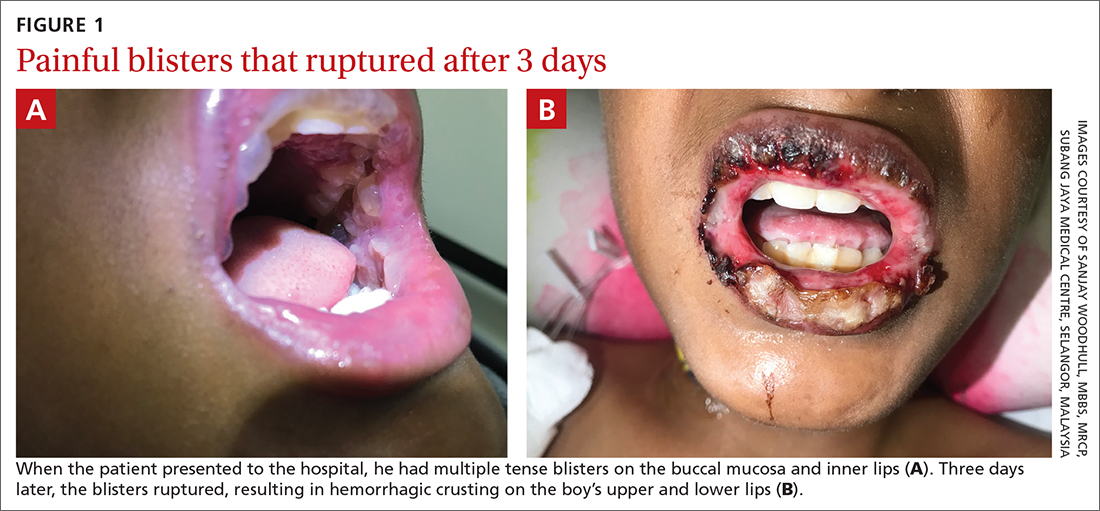
The preliminary diagnosis was primary herpetic gingivostomatitis, and treatment was initiated with intravenous (IV) acyclovir (10 mg/kg every 8 hours), IV fluids, and topical lidocaine gel and topical steroids for analgesia. However, the patient’s fever persisted over the next 4 days, with his temperature fluctuating between 101.3 °F and 104 °F, and he had a worsening productive cough. The blisters ruptured on Day 6 of illness, leaving hemorrhagic crusting on his lips (FIGURE 1B). Herpes simplex virus types 1 and 2 and polymerase chain reaction (PCR) testing were negative.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Mycoplasma pneumoniae–induced rash and mucositis
Further follow-up on Day 6 of illness revealed bibasilar crepitations along with an elevated CRP level of 40.5 mg/L and a positive mycoplasma antibody serology (titer > 1:1280; normal, < 1:80). The patient was given a diagnosis of pneumonia (due to infection with Mycoplasma pneumoniae) and M pneumoniae–induced rash and mucositis (MIRM).
MIRM was first proposed as a distinct clinical entity in 2015 to distinguish it from Stevens-Johnson syndrome and erythema multiforme.1 MIRM is seen more commonly in children and young adults, with a male preponderance.1
A small longitudinal study found that approximately 22.7% of children who have M pneumoniae infections present with mucocutaneous lesions, and of those cases, 6.8% are MIRM.2 Chlamydia pneumoniae is another potential causal organism of mucositis resembling MIRM.3
Pathogenesis. The commonly accepted mechanism of MIRM is an immune response triggered by a distant infection. This leads to tissue damage via polyclonal B cell proliferation and subsequent immune complex deposition, complement activation, and cytokine overproduction. Molecular mimicry between M pneumoniae P1-adhesion molecules and keratinocyte antigens may also contribute to this pathway.
3 criteria to make the diagnosis
Canavan et al1 have proposed the following criteria for the diagnosis of MIRM:
- Clinical symptoms, such as fever and cough, and laboratory findings of M pneumoniae infection (elevated M pneumoniae immunoglobulin M antibodies, positive cultures or PCR for M pneumoniae from oropharyngeal samples or bullae, and/or serial cold agglutinins) AND
- a rash to the mucosa that usually affects ≥ 2 sites (although rare cases may have fewer than 2 mucosal sites involved) AND
- skin detachment of less than 10% of the body surface area.
Continue to: The 3 variants of MIRM include...
The 3 variants of MIRM include:
- Classic MIRM has evidence of all 3 diagnostic criteria plus a nonmucosal rash, such as vesiculobullous lesions (77%), scattered target lesions (48%), papules (14%), macules (12%), and morbilliform eruptions (9%).4
- MIRM sine rash includes all 3 criteria but there is no significant cutaneous, nonmucosal rash. There may be “few fleeting morbilliform lesions or a few vesicles.”4
- Severe MIRM includes the first 2 criteria listed, but the cutaneous rash is extensive, with widespread nonmucosal blisters or flat atypical target lesions.4
Our patient had definitive clinical symptoms, laboratory evidence, and severe oral mucositis without significant cutaneous rash, thereby fulfilling the criteria for a diagnosis of MIRM sine rash variant.
These skin conditions were considered in the differential
The differential diagnosis for sudden onset of severe oral mucosal blisters in children includes herpes gingivostomatitis; hand, foot, and mouth disease
Herpes gingivostomatitis would involve numerous ulcerations of the oral mucosa and tongue, as well as gum hypertrophy.
Hand, foot, and mouth disease is characterized by
Continue to: Erythema multiforme
Erythema multiforme appears as cutaneous target lesions on the limbs that spread in a centripetal manner following herpes simplex virus infection.
SJS/TEN manifests with severe mucositis and is commonly triggered by medications (eg, sulphonamides, beta-lactams, nonsteroidal anti-inflammatory drugs, and antiepileptics).
With antibiotics, the prognosis is good
There are no established guidelines for the treatment of MIRM. Antibiotics and supportive care are universally accepted. Immunosuppressive therapy (eg, systemic steroids) is frequently used in patients with MIRM who have extensive mucosal involvement, in an attempt to decrease inflammation and pain; however, evidence for such an approach is lacking. The hyperimmune reactions of the host to M pneumoniae infection include cytokine overproduction and T-cell activation, which promote both pulmonary and extrapulmonary manifestations. This forms the basis of immunosuppressive therapy, such as systemic corticosteroids, IV immunoglobulin, and cyclosporin A, particularly when MIRM is associated with pneumonia caused by infection with M pneumoniae.1,5,6
The overall prognosis of MIRM is good. Recurrence has been reported in up to 8% of cases, the treatment of which remains the same. Mucocutaneous and ocular sequelae (oral or genital synechiae, corneal ulcerations, dry eyes, loss of eye lashes) have been reported in less than 9% of patients.1 Other rare reported complications following the occurrence of MIRM include persistent cutaneous lesions, B cell lymphopenia, and restrictive lung disease or chronic obliterative bronchitis.
Our patient was started on IV ceftriaxone (50 mg/kg/d), azithromycin (10 mg/kg/d on the first day, then 5 mg/kg/d on the subsequent 5 days), and methylprednisolone (3 mg/kg/d) on Day 6 of illness. Within 3 days, there was marked improvement of mucositis and respiratory symptoms with resolution of fever. He was discharged on Day 10. At his outpatient follow-up 2 weeks later, the patient had made a complete recovery.
1. Canavan TN, Mathes EF, Frieden I, et al. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol 2015;72:239-245. doi: 10.1016/j.jaad.2014.06.026
2. Sauteur PMM, Theiler M, Buettcher M, et al. Frequency and clinical presentation of mucocutaneous disease due to mycoplasma pneumoniae infection in children with community-acquired pneumonia. JAMA Dermatol. 2020;156:144-150. doi: 10.1001/jamadermatol.2019.3602
3. Mayor-Ibarguren A, Feito-Rodriguez M, González-Ramos J, et al. Mucositis secondary to chlamydia pneumoniae infection: expanding the mycoplasma pneumoniae-induced rash and mucositis concept. Pediatr Dermatol 2017;34:465-472. doi: 10.1111/pde.13140
4. Frantz GF, McAninch SA. Mycoplasma mucositis. StatPearls [Internet]. Updated August 8, 2022. Accessed November 1, 2022. www.ncbi.nlm.nih.gov/books/NBK525960/
5. Yang EA, Kang HM, Rhim JW, et al. Early corticosteroid therapy for Mycoplasma pneumoniae pneumonia irrespective of used antibiotics in children. J Clin Med. 2019;8:726. doi: 10.3390/jcm8050726
6. Li HOY, Colantonio S, Ramien ML. Treatment of Mycoplasma pneumoniae-induced rash and mucositis with cyclosporine. J Cutan Med Surg. 2019;23:608-612. doi: 10.1177/1203475419874444
A 12-YEAR-OLD BOY presented to the hospital with a 2-day history of fever, cough, and painful blisters on swollen lips. On examination, he had multiple tense blisters with clear fluid on the buccal mucosa and inner lips (FIGURE 1A), as well as multiple discrete ulcers on his posterior pharynx. The patient had no other skin, eye, or urogenital involvement, but he was dehydrated. Respiratory examination was unremarkable. A complete blood count and metabolic panel were normal, as was a C-reactive protein (CRP) test (0.8 mg/L).

The preliminary diagnosis was primary herpetic gingivostomatitis, and treatment was initiated with intravenous (IV) acyclovir (10 mg/kg every 8 hours), IV fluids, and topical lidocaine gel and topical steroids for analgesia. However, the patient’s fever persisted over the next 4 days, with his temperature fluctuating between 101.3 °F and 104 °F, and he had a worsening productive cough. The blisters ruptured on Day 6 of illness, leaving hemorrhagic crusting on his lips (FIGURE 1B). Herpes simplex virus types 1 and 2 and polymerase chain reaction (PCR) testing were negative.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Mycoplasma pneumoniae–induced rash and mucositis
Further follow-up on Day 6 of illness revealed bibasilar crepitations along with an elevated CRP level of 40.5 mg/L and a positive mycoplasma antibody serology (titer > 1:1280; normal, < 1:80). The patient was given a diagnosis of pneumonia (due to infection with Mycoplasma pneumoniae) and M pneumoniae–induced rash and mucositis (MIRM).
MIRM was first proposed as a distinct clinical entity in 2015 to distinguish it from Stevens-Johnson syndrome and erythema multiforme.1 MIRM is seen more commonly in children and young adults, with a male preponderance.1
A small longitudinal study found that approximately 22.7% of children who have M pneumoniae infections present with mucocutaneous lesions, and of those cases, 6.8% are MIRM.2 Chlamydia pneumoniae is another potential causal organism of mucositis resembling MIRM.3
Pathogenesis. The commonly accepted mechanism of MIRM is an immune response triggered by a distant infection. This leads to tissue damage via polyclonal B cell proliferation and subsequent immune complex deposition, complement activation, and cytokine overproduction. Molecular mimicry between M pneumoniae P1-adhesion molecules and keratinocyte antigens may also contribute to this pathway.
3 criteria to make the diagnosis
Canavan et al1 have proposed the following criteria for the diagnosis of MIRM:
- Clinical symptoms, such as fever and cough, and laboratory findings of M pneumoniae infection (elevated M pneumoniae immunoglobulin M antibodies, positive cultures or PCR for M pneumoniae from oropharyngeal samples or bullae, and/or serial cold agglutinins) AND
- a rash to the mucosa that usually affects ≥ 2 sites (although rare cases may have fewer than 2 mucosal sites involved) AND
- skin detachment of less than 10% of the body surface area.
Continue to: The 3 variants of MIRM include...
The 3 variants of MIRM include:
- Classic MIRM has evidence of all 3 diagnostic criteria plus a nonmucosal rash, such as vesiculobullous lesions (77%), scattered target lesions (48%), papules (14%), macules (12%), and morbilliform eruptions (9%).4
- MIRM sine rash includes all 3 criteria but there is no significant cutaneous, nonmucosal rash. There may be “few fleeting morbilliform lesions or a few vesicles.”4
- Severe MIRM includes the first 2 criteria listed, but the cutaneous rash is extensive, with widespread nonmucosal blisters or flat atypical target lesions.4
Our patient had definitive clinical symptoms, laboratory evidence, and severe oral mucositis without significant cutaneous rash, thereby fulfilling the criteria for a diagnosis of MIRM sine rash variant.
These skin conditions were considered in the differential
The differential diagnosis for sudden onset of severe oral mucosal blisters in children includes herpes gingivostomatitis; hand, foot, and mouth disease
Herpes gingivostomatitis would involve numerous ulcerations of the oral mucosa and tongue, as well as gum hypertrophy.
Hand, foot, and mouth disease is characterized by
Continue to: Erythema multiforme
Erythema multiforme appears as cutaneous target lesions on the limbs that spread in a centripetal manner following herpes simplex virus infection.
SJS/TEN manifests with severe mucositis and is commonly triggered by medications (eg, sulphonamides, beta-lactams, nonsteroidal anti-inflammatory drugs, and antiepileptics).
With antibiotics, the prognosis is good
There are no established guidelines for the treatment of MIRM. Antibiotics and supportive care are universally accepted. Immunosuppressive therapy (eg, systemic steroids) is frequently used in patients with MIRM who have extensive mucosal involvement, in an attempt to decrease inflammation and pain; however, evidence for such an approach is lacking. The hyperimmune reactions of the host to M pneumoniae infection include cytokine overproduction and T-cell activation, which promote both pulmonary and extrapulmonary manifestations. This forms the basis of immunosuppressive therapy, such as systemic corticosteroids, IV immunoglobulin, and cyclosporin A, particularly when MIRM is associated with pneumonia caused by infection with M pneumoniae.1,5,6
The overall prognosis of MIRM is good. Recurrence has been reported in up to 8% of cases, the treatment of which remains the same. Mucocutaneous and ocular sequelae (oral or genital synechiae, corneal ulcerations, dry eyes, loss of eye lashes) have been reported in less than 9% of patients.1 Other rare reported complications following the occurrence of MIRM include persistent cutaneous lesions, B cell lymphopenia, and restrictive lung disease or chronic obliterative bronchitis.
Our patient was started on IV ceftriaxone (50 mg/kg/d), azithromycin (10 mg/kg/d on the first day, then 5 mg/kg/d on the subsequent 5 days), and methylprednisolone (3 mg/kg/d) on Day 6 of illness. Within 3 days, there was marked improvement of mucositis and respiratory symptoms with resolution of fever. He was discharged on Day 10. At his outpatient follow-up 2 weeks later, the patient had made a complete recovery.
A 12-YEAR-OLD BOY presented to the hospital with a 2-day history of fever, cough, and painful blisters on swollen lips. On examination, he had multiple tense blisters with clear fluid on the buccal mucosa and inner lips (FIGURE 1A), as well as multiple discrete ulcers on his posterior pharynx. The patient had no other skin, eye, or urogenital involvement, but he was dehydrated. Respiratory examination was unremarkable. A complete blood count and metabolic panel were normal, as was a C-reactive protein (CRP) test (0.8 mg/L).

The preliminary diagnosis was primary herpetic gingivostomatitis, and treatment was initiated with intravenous (IV) acyclovir (10 mg/kg every 8 hours), IV fluids, and topical lidocaine gel and topical steroids for analgesia. However, the patient’s fever persisted over the next 4 days, with his temperature fluctuating between 101.3 °F and 104 °F, and he had a worsening productive cough. The blisters ruptured on Day 6 of illness, leaving hemorrhagic crusting on his lips (FIGURE 1B). Herpes simplex virus types 1 and 2 and polymerase chain reaction (PCR) testing were negative.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Mycoplasma pneumoniae–induced rash and mucositis
Further follow-up on Day 6 of illness revealed bibasilar crepitations along with an elevated CRP level of 40.5 mg/L and a positive mycoplasma antibody serology (titer > 1:1280; normal, < 1:80). The patient was given a diagnosis of pneumonia (due to infection with Mycoplasma pneumoniae) and M pneumoniae–induced rash and mucositis (MIRM).
MIRM was first proposed as a distinct clinical entity in 2015 to distinguish it from Stevens-Johnson syndrome and erythema multiforme.1 MIRM is seen more commonly in children and young adults, with a male preponderance.1
A small longitudinal study found that approximately 22.7% of children who have M pneumoniae infections present with mucocutaneous lesions, and of those cases, 6.8% are MIRM.2 Chlamydia pneumoniae is another potential causal organism of mucositis resembling MIRM.3
Pathogenesis. The commonly accepted mechanism of MIRM is an immune response triggered by a distant infection. This leads to tissue damage via polyclonal B cell proliferation and subsequent immune complex deposition, complement activation, and cytokine overproduction. Molecular mimicry between M pneumoniae P1-adhesion molecules and keratinocyte antigens may also contribute to this pathway.
3 criteria to make the diagnosis
Canavan et al1 have proposed the following criteria for the diagnosis of MIRM:
- Clinical symptoms, such as fever and cough, and laboratory findings of M pneumoniae infection (elevated M pneumoniae immunoglobulin M antibodies, positive cultures or PCR for M pneumoniae from oropharyngeal samples or bullae, and/or serial cold agglutinins) AND
- a rash to the mucosa that usually affects ≥ 2 sites (although rare cases may have fewer than 2 mucosal sites involved) AND
- skin detachment of less than 10% of the body surface area.
Continue to: The 3 variants of MIRM include...
The 3 variants of MIRM include:
- Classic MIRM has evidence of all 3 diagnostic criteria plus a nonmucosal rash, such as vesiculobullous lesions (77%), scattered target lesions (48%), papules (14%), macules (12%), and morbilliform eruptions (9%).4
- MIRM sine rash includes all 3 criteria but there is no significant cutaneous, nonmucosal rash. There may be “few fleeting morbilliform lesions or a few vesicles.”4
- Severe MIRM includes the first 2 criteria listed, but the cutaneous rash is extensive, with widespread nonmucosal blisters or flat atypical target lesions.4
Our patient had definitive clinical symptoms, laboratory evidence, and severe oral mucositis without significant cutaneous rash, thereby fulfilling the criteria for a diagnosis of MIRM sine rash variant.
These skin conditions were considered in the differential
The differential diagnosis for sudden onset of severe oral mucosal blisters in children includes herpes gingivostomatitis; hand, foot, and mouth disease
Herpes gingivostomatitis would involve numerous ulcerations of the oral mucosa and tongue, as well as gum hypertrophy.
Hand, foot, and mouth disease is characterized by
Continue to: Erythema multiforme
Erythema multiforme appears as cutaneous target lesions on the limbs that spread in a centripetal manner following herpes simplex virus infection.
SJS/TEN manifests with severe mucositis and is commonly triggered by medications (eg, sulphonamides, beta-lactams, nonsteroidal anti-inflammatory drugs, and antiepileptics).
With antibiotics, the prognosis is good
There are no established guidelines for the treatment of MIRM. Antibiotics and supportive care are universally accepted. Immunosuppressive therapy (eg, systemic steroids) is frequently used in patients with MIRM who have extensive mucosal involvement, in an attempt to decrease inflammation and pain; however, evidence for such an approach is lacking. The hyperimmune reactions of the host to M pneumoniae infection include cytokine overproduction and T-cell activation, which promote both pulmonary and extrapulmonary manifestations. This forms the basis of immunosuppressive therapy, such as systemic corticosteroids, IV immunoglobulin, and cyclosporin A, particularly when MIRM is associated with pneumonia caused by infection with M pneumoniae.1,5,6
The overall prognosis of MIRM is good. Recurrence has been reported in up to 8% of cases, the treatment of which remains the same. Mucocutaneous and ocular sequelae (oral or genital synechiae, corneal ulcerations, dry eyes, loss of eye lashes) have been reported in less than 9% of patients.1 Other rare reported complications following the occurrence of MIRM include persistent cutaneous lesions, B cell lymphopenia, and restrictive lung disease or chronic obliterative bronchitis.
Our patient was started on IV ceftriaxone (50 mg/kg/d), azithromycin (10 mg/kg/d on the first day, then 5 mg/kg/d on the subsequent 5 days), and methylprednisolone (3 mg/kg/d) on Day 6 of illness. Within 3 days, there was marked improvement of mucositis and respiratory symptoms with resolution of fever. He was discharged on Day 10. At his outpatient follow-up 2 weeks later, the patient had made a complete recovery.
1. Canavan TN, Mathes EF, Frieden I, et al. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol 2015;72:239-245. doi: 10.1016/j.jaad.2014.06.026
2. Sauteur PMM, Theiler M, Buettcher M, et al. Frequency and clinical presentation of mucocutaneous disease due to mycoplasma pneumoniae infection in children with community-acquired pneumonia. JAMA Dermatol. 2020;156:144-150. doi: 10.1001/jamadermatol.2019.3602
3. Mayor-Ibarguren A, Feito-Rodriguez M, González-Ramos J, et al. Mucositis secondary to chlamydia pneumoniae infection: expanding the mycoplasma pneumoniae-induced rash and mucositis concept. Pediatr Dermatol 2017;34:465-472. doi: 10.1111/pde.13140
4. Frantz GF, McAninch SA. Mycoplasma mucositis. StatPearls [Internet]. Updated August 8, 2022. Accessed November 1, 2022. www.ncbi.nlm.nih.gov/books/NBK525960/
5. Yang EA, Kang HM, Rhim JW, et al. Early corticosteroid therapy for Mycoplasma pneumoniae pneumonia irrespective of used antibiotics in children. J Clin Med. 2019;8:726. doi: 10.3390/jcm8050726
6. Li HOY, Colantonio S, Ramien ML. Treatment of Mycoplasma pneumoniae-induced rash and mucositis with cyclosporine. J Cutan Med Surg. 2019;23:608-612. doi: 10.1177/1203475419874444
1. Canavan TN, Mathes EF, Frieden I, et al. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol 2015;72:239-245. doi: 10.1016/j.jaad.2014.06.026
2. Sauteur PMM, Theiler M, Buettcher M, et al. Frequency and clinical presentation of mucocutaneous disease due to mycoplasma pneumoniae infection in children with community-acquired pneumonia. JAMA Dermatol. 2020;156:144-150. doi: 10.1001/jamadermatol.2019.3602
3. Mayor-Ibarguren A, Feito-Rodriguez M, González-Ramos J, et al. Mucositis secondary to chlamydia pneumoniae infection: expanding the mycoplasma pneumoniae-induced rash and mucositis concept. Pediatr Dermatol 2017;34:465-472. doi: 10.1111/pde.13140
4. Frantz GF, McAninch SA. Mycoplasma mucositis. StatPearls [Internet]. Updated August 8, 2022. Accessed November 1, 2022. www.ncbi.nlm.nih.gov/books/NBK525960/
5. Yang EA, Kang HM, Rhim JW, et al. Early corticosteroid therapy for Mycoplasma pneumoniae pneumonia irrespective of used antibiotics in children. J Clin Med. 2019;8:726. doi: 10.3390/jcm8050726
6. Li HOY, Colantonio S, Ramien ML. Treatment of Mycoplasma pneumoniae-induced rash and mucositis with cyclosporine. J Cutan Med Surg. 2019;23:608-612. doi: 10.1177/1203475419874444
RSV causes 1 in 50 deaths in children under age 5: European study
But RSV – formally known as respiratory syncytial virus – is also a problem in high-income nations. In those countries, 1 in 56 otherwise healthy babies are hospitalized with RSV during their first year of life, said the study, which was published in the Lancet Respiratory Medicine.
Researchers looked at the health records of 9,154 infants born between July 1, 2017, and July 31, 2020, who were treated at health centers across Europe. Previous studies have concentrated on babies with preexisting conditions, but this one looked at otherwise healthy children, researchers said.
“This is the lowest-risk baby who is being hospitalized for this, so really, numbers are really much higher than I think some people would have guessed,” said study coauthor Louis Bont, MD, a professor of pediatric infectious diseases at Wilhelmina Children’s Hospital at University Medical Center Utrecht in the Netherlands, according to CNN. He is also chairman of the ReSViNET foundation, which aims to reduce RSV infection globally.
The study said more than 97% of deaths from RSV occur in low-income and middle-income countries. The study concluded that “maternal vaccination and passive [immunization] could have a profound impact on the RSV burden.”
In developed nations, children who get RSV usually survive because they have access to ventilators and other health care equipment. Still, just being treated for RSV can have long-range negative effects on a child’s health, Kristina Deeter, MD, chair of pediatrics at the University of Nevada, Reno, told CNN.
“Whether that is just traumatic psychosocial, emotional issues after hospitalization or even having more vulnerable lungs – you can develop asthma later on, for instance, if you’ve had a really severe infection at a young age – it can damage your lungs permanently,” she said of the study. “It’s still an important virus in our world and something that we really focus on.”
The Lancet study was published days after the CDC warned public health officials that respiratory viruses, including RSV, are surging among children across the country.
A version of this article first appeared on WebMD.com.
But RSV – formally known as respiratory syncytial virus – is also a problem in high-income nations. In those countries, 1 in 56 otherwise healthy babies are hospitalized with RSV during their first year of life, said the study, which was published in the Lancet Respiratory Medicine.
Researchers looked at the health records of 9,154 infants born between July 1, 2017, and July 31, 2020, who were treated at health centers across Europe. Previous studies have concentrated on babies with preexisting conditions, but this one looked at otherwise healthy children, researchers said.
“This is the lowest-risk baby who is being hospitalized for this, so really, numbers are really much higher than I think some people would have guessed,” said study coauthor Louis Bont, MD, a professor of pediatric infectious diseases at Wilhelmina Children’s Hospital at University Medical Center Utrecht in the Netherlands, according to CNN. He is also chairman of the ReSViNET foundation, which aims to reduce RSV infection globally.
The study said more than 97% of deaths from RSV occur in low-income and middle-income countries. The study concluded that “maternal vaccination and passive [immunization] could have a profound impact on the RSV burden.”
In developed nations, children who get RSV usually survive because they have access to ventilators and other health care equipment. Still, just being treated for RSV can have long-range negative effects on a child’s health, Kristina Deeter, MD, chair of pediatrics at the University of Nevada, Reno, told CNN.
“Whether that is just traumatic psychosocial, emotional issues after hospitalization or even having more vulnerable lungs – you can develop asthma later on, for instance, if you’ve had a really severe infection at a young age – it can damage your lungs permanently,” she said of the study. “It’s still an important virus in our world and something that we really focus on.”
The Lancet study was published days after the CDC warned public health officials that respiratory viruses, including RSV, are surging among children across the country.
A version of this article first appeared on WebMD.com.
But RSV – formally known as respiratory syncytial virus – is also a problem in high-income nations. In those countries, 1 in 56 otherwise healthy babies are hospitalized with RSV during their first year of life, said the study, which was published in the Lancet Respiratory Medicine.
Researchers looked at the health records of 9,154 infants born between July 1, 2017, and July 31, 2020, who were treated at health centers across Europe. Previous studies have concentrated on babies with preexisting conditions, but this one looked at otherwise healthy children, researchers said.
“This is the lowest-risk baby who is being hospitalized for this, so really, numbers are really much higher than I think some people would have guessed,” said study coauthor Louis Bont, MD, a professor of pediatric infectious diseases at Wilhelmina Children’s Hospital at University Medical Center Utrecht in the Netherlands, according to CNN. He is also chairman of the ReSViNET foundation, which aims to reduce RSV infection globally.
The study said more than 97% of deaths from RSV occur in low-income and middle-income countries. The study concluded that “maternal vaccination and passive [immunization] could have a profound impact on the RSV burden.”
In developed nations, children who get RSV usually survive because they have access to ventilators and other health care equipment. Still, just being treated for RSV can have long-range negative effects on a child’s health, Kristina Deeter, MD, chair of pediatrics at the University of Nevada, Reno, told CNN.
“Whether that is just traumatic psychosocial, emotional issues after hospitalization or even having more vulnerable lungs – you can develop asthma later on, for instance, if you’ve had a really severe infection at a young age – it can damage your lungs permanently,” she said of the study. “It’s still an important virus in our world and something that we really focus on.”
The Lancet study was published days after the CDC warned public health officials that respiratory viruses, including RSV, are surging among children across the country.
A version of this article first appeared on WebMD.com.
FROM LANCET RESPIRATORY MEDICINE
Children and COVID: Weekly cases continue to hold fairly steady
The incidence of new COVID-19 cases in children seems to have stabilized as the national count remained under 30,000 for the fifth consecutive week, but hospitalization data may indicate some possible turbulence.
Just over 28,000 pediatric cases were reported during the week of Nov. 4-10, a drop of 5.4% from the previous week, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report involving data from state and territorial health departments, several of which are no longer updating their websites.
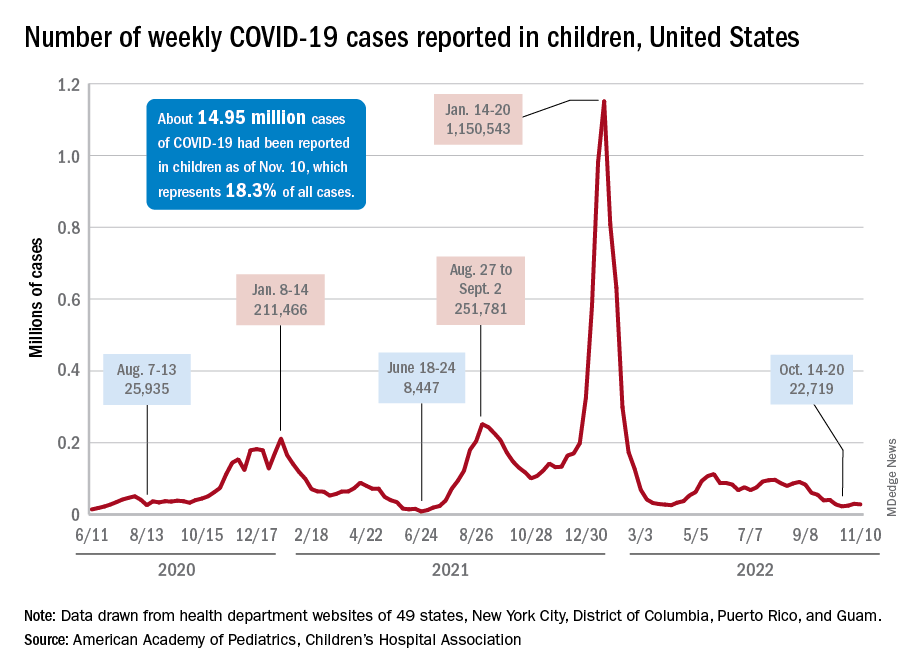
The stability in weekly cases, however, comes in contrast to a very recent and considerable increase in new hospital admissions of children aged 0-17 years with confirmed COVID-19. That rate, which was 0.18 hospitalizations per 100,000 population on Nov. 7 and 0.19 per 100,000 on Nov. 8 and 9, jumped all the way to 0.34 on Nov. 10 and 0.48 on Nov. 11, according to data from the Centers for Disease Control and Prevention. That is the highest rate since the closing days of the Omicron surge in February.
The rate for Nov. 12, the most recent one available, was down slightly to 0.47 admissions per 100,000. There doesn’t seem to be any evidence in the CDC’s data of a similar sudden increase in new hospitalizations among any other age group, and no age group, including children, shows any sign of a recent increase in emergency department visits with diagnosed COVID. (The CDC has not yet responded to our inquiry about this development.)
The two most recent 7-day averages for new admissions in children aged 0-17 show a small increase, but they cover the periods of Oct. 15 to Oct. 31, when there were 126 admissions per day, and Nov. 1 to Nov. 7, when the average went up to 133 per day, the CDC said on its COVID Data Tracker.
The CDC does not publish a weekly count of new COVID cases, but its latest data on the rate of incident cases seem to agree with the AAP/CHA figures: A gradual decline in all age groups, including children, since the beginning of September.
Vaccinations, on the other hand, bucked their recent trend and increased in the last week. About 43,000 children under age 5 years received their initial dose of COVID vaccine during Nov. 3-9, compared with 30,000 and 33,000 the 2 previous weeks, while 5- to 11-year-olds hit their highest weekly mark (31,000) since late August and 12- to 17-year-olds had their biggest week (27,000) since mid-August, the AAP reported based on CDC data.
The incidence of new COVID-19 cases in children seems to have stabilized as the national count remained under 30,000 for the fifth consecutive week, but hospitalization data may indicate some possible turbulence.
Just over 28,000 pediatric cases were reported during the week of Nov. 4-10, a drop of 5.4% from the previous week, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report involving data from state and territorial health departments, several of which are no longer updating their websites.

The stability in weekly cases, however, comes in contrast to a very recent and considerable increase in new hospital admissions of children aged 0-17 years with confirmed COVID-19. That rate, which was 0.18 hospitalizations per 100,000 population on Nov. 7 and 0.19 per 100,000 on Nov. 8 and 9, jumped all the way to 0.34 on Nov. 10 and 0.48 on Nov. 11, according to data from the Centers for Disease Control and Prevention. That is the highest rate since the closing days of the Omicron surge in February.
The rate for Nov. 12, the most recent one available, was down slightly to 0.47 admissions per 100,000. There doesn’t seem to be any evidence in the CDC’s data of a similar sudden increase in new hospitalizations among any other age group, and no age group, including children, shows any sign of a recent increase in emergency department visits with diagnosed COVID. (The CDC has not yet responded to our inquiry about this development.)
The two most recent 7-day averages for new admissions in children aged 0-17 show a small increase, but they cover the periods of Oct. 15 to Oct. 31, when there were 126 admissions per day, and Nov. 1 to Nov. 7, when the average went up to 133 per day, the CDC said on its COVID Data Tracker.
The CDC does not publish a weekly count of new COVID cases, but its latest data on the rate of incident cases seem to agree with the AAP/CHA figures: A gradual decline in all age groups, including children, since the beginning of September.
Vaccinations, on the other hand, bucked their recent trend and increased in the last week. About 43,000 children under age 5 years received their initial dose of COVID vaccine during Nov. 3-9, compared with 30,000 and 33,000 the 2 previous weeks, while 5- to 11-year-olds hit their highest weekly mark (31,000) since late August and 12- to 17-year-olds had their biggest week (27,000) since mid-August, the AAP reported based on CDC data.
The incidence of new COVID-19 cases in children seems to have stabilized as the national count remained under 30,000 for the fifth consecutive week, but hospitalization data may indicate some possible turbulence.
Just over 28,000 pediatric cases were reported during the week of Nov. 4-10, a drop of 5.4% from the previous week, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report involving data from state and territorial health departments, several of which are no longer updating their websites.

The stability in weekly cases, however, comes in contrast to a very recent and considerable increase in new hospital admissions of children aged 0-17 years with confirmed COVID-19. That rate, which was 0.18 hospitalizations per 100,000 population on Nov. 7 and 0.19 per 100,000 on Nov. 8 and 9, jumped all the way to 0.34 on Nov. 10 and 0.48 on Nov. 11, according to data from the Centers for Disease Control and Prevention. That is the highest rate since the closing days of the Omicron surge in February.
The rate for Nov. 12, the most recent one available, was down slightly to 0.47 admissions per 100,000. There doesn’t seem to be any evidence in the CDC’s data of a similar sudden increase in new hospitalizations among any other age group, and no age group, including children, shows any sign of a recent increase in emergency department visits with diagnosed COVID. (The CDC has not yet responded to our inquiry about this development.)
The two most recent 7-day averages for new admissions in children aged 0-17 show a small increase, but they cover the periods of Oct. 15 to Oct. 31, when there were 126 admissions per day, and Nov. 1 to Nov. 7, when the average went up to 133 per day, the CDC said on its COVID Data Tracker.
The CDC does not publish a weekly count of new COVID cases, but its latest data on the rate of incident cases seem to agree with the AAP/CHA figures: A gradual decline in all age groups, including children, since the beginning of September.
Vaccinations, on the other hand, bucked their recent trend and increased in the last week. About 43,000 children under age 5 years received their initial dose of COVID vaccine during Nov. 3-9, compared with 30,000 and 33,000 the 2 previous weeks, while 5- to 11-year-olds hit their highest weekly mark (31,000) since late August and 12- to 17-year-olds had their biggest week (27,000) since mid-August, the AAP reported based on CDC data.