User login
Love them or hate them, masks in schools work
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
On March 26, 2022, Hawaii became the last state in the United States to lift its indoor mask mandate. By the time the current school year started, there were essentially no public school mask mandates either.
Whether you viewed the mask as an emblem of stalwart defiance against a rampaging virus, or a scarlet letter emblematic of the overreaches of public policy, you probably aren’t seeing them much anymore.
And yet, the debate about masks still rages. Who was right, who was wrong? Who trusted science, and what does the science even say? If we brought our country into marriage counseling, would we be told it is time to move on? To look forward, not backward? To plan for our bright future together?
Perhaps. But this question isn’t really moot just because masks have largely disappeared in the United States. Variants may emerge that lead to more infection waves – and other pandemics may occur in the future. And so I think it is important to discuss a study that, with quite rigorous analysis, attempts to answer the following question: Did masking in schools lower students’ and teachers’ risk of COVID?
We are talking about this study, appearing in the New England Journal of Medicine. The short version goes like this.
Researchers had access to two important sources of data. One – an accounting of all the teachers and students (more than 300,000 of them) in 79 public, noncharter school districts in Eastern Massachusetts who tested positive for COVID every week. Two – the date that each of those school districts lifted their mask mandates or (in the case of two districts) didn’t.
Right away, I’m sure you’re thinking of potential issues. Districts that kept masks even when the statewide ban was lifted are likely quite a bit different from districts that dropped masks right away. You’re right, of course – hold on to that thought; we’ll get there.
But first – the big question – would districts that kept their masks on longer do better when it comes to the rate of COVID infection?
When everyone was masking, COVID case rates were pretty similar. Statewide mandates are lifted in late February – and most school districts remove their mandates within a few weeks – the black line are the two districts (Boston and Chelsea) where mask mandates remained in place.
Prior to the mask mandate lifting, you see very similar COVID rates in districts that would eventually remove the mandate and those that would not, with a bit of noise around the initial Omicron wave which saw just a huge amount of people get infected.
And then, after the mandate was lifted, separation. Districts that held on to masks longer had lower rates of COVID infection.
In all, over the 15-weeks of the study, there were roughly 12,000 extra cases of COVID in the mask-free school districts, which corresponds to about 35% of the total COVID burden during that time. And, yes, kids do well with COVID – on average. But 12,000 extra cases is enough to translate into a significant number of important clinical outcomes – think hospitalizations and post-COVID syndromes. And of course, maybe most importantly, missed school days. Positive kids were not allowed in class no matter what district they were in.
Okay – I promised we’d address confounders. This was not a cluster-randomized trial, where some school districts had their mandates removed based on the vicissitudes of a virtual coin flip, as much as many of us would have been interested to see that. The decision to remove masks was up to the various school boards – and they had a lot of pressure on them from many different directions. But all we need to worry about is whether any of those things that pressure a school board to keep masks on would ALSO lead to fewer COVID cases. That’s how confounders work, and how you can get false results in a study like this.
And yes – districts that kept the masks on longer were different than those who took them right off. But check out how they were different.
The districts that kept masks on longer had more low-income students. More Black and Latino students. More students per classroom. These are all risk factors that increase the risk of COVID infection. In other words, the confounding here goes in the opposite direction of the results. If anything, these factors should make you more certain that masking works.
The authors also adjusted for other factors – the community transmission of COVID-19, vaccination rates, school district sizes, and so on. No major change in the results.
One concern I addressed to Dr. Ellie Murray, the biostatistician on the study – could districts that removed masks simply have been testing more to compensate, leading to increased capturing of cases?
If anything, the schools that kept masks on were testing more than the schools that took them off – again that would tend to imply that the results are even stronger than what was reported.
Is this a perfect study? Of course not – it’s one study, it’s from one state. And the relatively large effects from keeping masks on for one or 2 weeks require us to really embrace the concept of exponential growth of infections, but, if COVID has taught us anything, it is that small changes in initial conditions can have pretty big effects.
My daughter, who goes to a public school here in Connecticut, unmasked, was home with COVID this past week. She’s fine. But you know what? She missed a week of school. I worked from home to be with her – though I didn’t test positive. And that is a real cost to both of us that I think we need to consider when we consider the value of masks. Yes, they’re annoying – but if they keep kids in school, might they be worth it? Perhaps not for now, as cases aren’t surging. But in the future, be it a particularly concerning variant, or a whole new pandemic, we should not discount the simple, cheap, and apparently beneficial act of wearing masks to decrease transmission.
Dr. Perry Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
On March 26, 2022, Hawaii became the last state in the United States to lift its indoor mask mandate. By the time the current school year started, there were essentially no public school mask mandates either.
Whether you viewed the mask as an emblem of stalwart defiance against a rampaging virus, or a scarlet letter emblematic of the overreaches of public policy, you probably aren’t seeing them much anymore.
And yet, the debate about masks still rages. Who was right, who was wrong? Who trusted science, and what does the science even say? If we brought our country into marriage counseling, would we be told it is time to move on? To look forward, not backward? To plan for our bright future together?
Perhaps. But this question isn’t really moot just because masks have largely disappeared in the United States. Variants may emerge that lead to more infection waves – and other pandemics may occur in the future. And so I think it is important to discuss a study that, with quite rigorous analysis, attempts to answer the following question: Did masking in schools lower students’ and teachers’ risk of COVID?
We are talking about this study, appearing in the New England Journal of Medicine. The short version goes like this.
Researchers had access to two important sources of data. One – an accounting of all the teachers and students (more than 300,000 of them) in 79 public, noncharter school districts in Eastern Massachusetts who tested positive for COVID every week. Two – the date that each of those school districts lifted their mask mandates or (in the case of two districts) didn’t.
Right away, I’m sure you’re thinking of potential issues. Districts that kept masks even when the statewide ban was lifted are likely quite a bit different from districts that dropped masks right away. You’re right, of course – hold on to that thought; we’ll get there.
But first – the big question – would districts that kept their masks on longer do better when it comes to the rate of COVID infection?
When everyone was masking, COVID case rates were pretty similar. Statewide mandates are lifted in late February – and most school districts remove their mandates within a few weeks – the black line are the two districts (Boston and Chelsea) where mask mandates remained in place.
Prior to the mask mandate lifting, you see very similar COVID rates in districts that would eventually remove the mandate and those that would not, with a bit of noise around the initial Omicron wave which saw just a huge amount of people get infected.
And then, after the mandate was lifted, separation. Districts that held on to masks longer had lower rates of COVID infection.
In all, over the 15-weeks of the study, there were roughly 12,000 extra cases of COVID in the mask-free school districts, which corresponds to about 35% of the total COVID burden during that time. And, yes, kids do well with COVID – on average. But 12,000 extra cases is enough to translate into a significant number of important clinical outcomes – think hospitalizations and post-COVID syndromes. And of course, maybe most importantly, missed school days. Positive kids were not allowed in class no matter what district they were in.
Okay – I promised we’d address confounders. This was not a cluster-randomized trial, where some school districts had their mandates removed based on the vicissitudes of a virtual coin flip, as much as many of us would have been interested to see that. The decision to remove masks was up to the various school boards – and they had a lot of pressure on them from many different directions. But all we need to worry about is whether any of those things that pressure a school board to keep masks on would ALSO lead to fewer COVID cases. That’s how confounders work, and how you can get false results in a study like this.
And yes – districts that kept the masks on longer were different than those who took them right off. But check out how they were different.
The districts that kept masks on longer had more low-income students. More Black and Latino students. More students per classroom. These are all risk factors that increase the risk of COVID infection. In other words, the confounding here goes in the opposite direction of the results. If anything, these factors should make you more certain that masking works.
The authors also adjusted for other factors – the community transmission of COVID-19, vaccination rates, school district sizes, and so on. No major change in the results.
One concern I addressed to Dr. Ellie Murray, the biostatistician on the study – could districts that removed masks simply have been testing more to compensate, leading to increased capturing of cases?
If anything, the schools that kept masks on were testing more than the schools that took them off – again that would tend to imply that the results are even stronger than what was reported.
Is this a perfect study? Of course not – it’s one study, it’s from one state. And the relatively large effects from keeping masks on for one or 2 weeks require us to really embrace the concept of exponential growth of infections, but, if COVID has taught us anything, it is that small changes in initial conditions can have pretty big effects.
My daughter, who goes to a public school here in Connecticut, unmasked, was home with COVID this past week. She’s fine. But you know what? She missed a week of school. I worked from home to be with her – though I didn’t test positive. And that is a real cost to both of us that I think we need to consider when we consider the value of masks. Yes, they’re annoying – but if they keep kids in school, might they be worth it? Perhaps not for now, as cases aren’t surging. But in the future, be it a particularly concerning variant, or a whole new pandemic, we should not discount the simple, cheap, and apparently beneficial act of wearing masks to decrease transmission.
Dr. Perry Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
On March 26, 2022, Hawaii became the last state in the United States to lift its indoor mask mandate. By the time the current school year started, there were essentially no public school mask mandates either.
Whether you viewed the mask as an emblem of stalwart defiance against a rampaging virus, or a scarlet letter emblematic of the overreaches of public policy, you probably aren’t seeing them much anymore.
And yet, the debate about masks still rages. Who was right, who was wrong? Who trusted science, and what does the science even say? If we brought our country into marriage counseling, would we be told it is time to move on? To look forward, not backward? To plan for our bright future together?
Perhaps. But this question isn’t really moot just because masks have largely disappeared in the United States. Variants may emerge that lead to more infection waves – and other pandemics may occur in the future. And so I think it is important to discuss a study that, with quite rigorous analysis, attempts to answer the following question: Did masking in schools lower students’ and teachers’ risk of COVID?
We are talking about this study, appearing in the New England Journal of Medicine. The short version goes like this.
Researchers had access to two important sources of data. One – an accounting of all the teachers and students (more than 300,000 of them) in 79 public, noncharter school districts in Eastern Massachusetts who tested positive for COVID every week. Two – the date that each of those school districts lifted their mask mandates or (in the case of two districts) didn’t.
Right away, I’m sure you’re thinking of potential issues. Districts that kept masks even when the statewide ban was lifted are likely quite a bit different from districts that dropped masks right away. You’re right, of course – hold on to that thought; we’ll get there.
But first – the big question – would districts that kept their masks on longer do better when it comes to the rate of COVID infection?
When everyone was masking, COVID case rates were pretty similar. Statewide mandates are lifted in late February – and most school districts remove their mandates within a few weeks – the black line are the two districts (Boston and Chelsea) where mask mandates remained in place.
Prior to the mask mandate lifting, you see very similar COVID rates in districts that would eventually remove the mandate and those that would not, with a bit of noise around the initial Omicron wave which saw just a huge amount of people get infected.
And then, after the mandate was lifted, separation. Districts that held on to masks longer had lower rates of COVID infection.
In all, over the 15-weeks of the study, there were roughly 12,000 extra cases of COVID in the mask-free school districts, which corresponds to about 35% of the total COVID burden during that time. And, yes, kids do well with COVID – on average. But 12,000 extra cases is enough to translate into a significant number of important clinical outcomes – think hospitalizations and post-COVID syndromes. And of course, maybe most importantly, missed school days. Positive kids were not allowed in class no matter what district they were in.
Okay – I promised we’d address confounders. This was not a cluster-randomized trial, where some school districts had their mandates removed based on the vicissitudes of a virtual coin flip, as much as many of us would have been interested to see that. The decision to remove masks was up to the various school boards – and they had a lot of pressure on them from many different directions. But all we need to worry about is whether any of those things that pressure a school board to keep masks on would ALSO lead to fewer COVID cases. That’s how confounders work, and how you can get false results in a study like this.
And yes – districts that kept the masks on longer were different than those who took them right off. But check out how they were different.
The districts that kept masks on longer had more low-income students. More Black and Latino students. More students per classroom. These are all risk factors that increase the risk of COVID infection. In other words, the confounding here goes in the opposite direction of the results. If anything, these factors should make you more certain that masking works.
The authors also adjusted for other factors – the community transmission of COVID-19, vaccination rates, school district sizes, and so on. No major change in the results.
One concern I addressed to Dr. Ellie Murray, the biostatistician on the study – could districts that removed masks simply have been testing more to compensate, leading to increased capturing of cases?
If anything, the schools that kept masks on were testing more than the schools that took them off – again that would tend to imply that the results are even stronger than what was reported.
Is this a perfect study? Of course not – it’s one study, it’s from one state. And the relatively large effects from keeping masks on for one or 2 weeks require us to really embrace the concept of exponential growth of infections, but, if COVID has taught us anything, it is that small changes in initial conditions can have pretty big effects.
My daughter, who goes to a public school here in Connecticut, unmasked, was home with COVID this past week. She’s fine. But you know what? She missed a week of school. I worked from home to be with her – though I didn’t test positive. And that is a real cost to both of us that I think we need to consider when we consider the value of masks. Yes, they’re annoying – but if they keep kids in school, might they be worth it? Perhaps not for now, as cases aren’t surging. But in the future, be it a particularly concerning variant, or a whole new pandemic, we should not discount the simple, cheap, and apparently beneficial act of wearing masks to decrease transmission.
Dr. Perry Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
No benefit of rivaroxaban in COVID outpatients: PREVENT-HD
A new U.S. randomized trial has failed to show benefit of a 35-day course of oral anticoagulation with rivaroxaban for the prevention of thrombotic events in outpatients with symptomatic COVID-19.
The PREVENT-HD trial was presented at the American Heart Association scientific sessions by Gregory Piazza, MD, Brigham and Women’s Hospital, Boston.
“With the caveat that the trial was underpowered to provide a definitive conclusion, these data do not support routine antithrombotic prophylaxis in nonhospitalized patients with symptomatic COVID-19,” Dr. Piazza concluded.
PREVENT-HD is the largest randomized study to look at anticoagulation in nonhospitalized COVID-19 patients and joins a long list of smaller trials that have also shown no benefit with this approach.
However, anticoagulation is recommended in patients who are hospitalized with COVID-19.
Dr. Piazza noted that the issue of anticoagulation in COVID-19 has focused mainly on hospitalized patients, but most COVID-19 cases are treated as outpatients, who are also suspected to be at risk for venous and arterial thrombotic events, especially if they have additional risk factors. Histopathological evidence also suggests that at least part of the deterioration in lung function leading to hospitalization may be attributable to in situ pulmonary artery thrombosis.
The PREVENT-HD trial explored the question of whether early initiation of thromboprophylaxis dosing of rivaroxaban in higher-risk outpatients with COVID-19 may lower the incidence of venous and arterial thrombotic events, reduce in situ pulmonary thrombosis and the worsening of pulmonary function that may lead to hospitalization, and reduce all-cause mortality.
The trial included 1,284 outpatients with a positive test for COVID-19 and who were within 14 days of symptom onset. They also had to have at least one of the following additional risk factors: age over 60 years; prior history of venous thromboembolism (VTE), thrombophilia, coronary artery disease, peripheral artery disease, cardiovascular disease or ischemic stroke, cancer, diabetes, heart failure, obesity (body mass index ≥ 35 kg/m2) or D-dimer > upper limit of normal. Around 35% of the study population had two or more of these risk factors.
Patients were randomized to rivaroxaban 10 mg daily for 35 days or placebo.
The primary efficacy endpoint was time to first occurrence of a composite of symptomatic VTE, myocardial infarction, ischemic stroke, acute limb ischemia, non–central nervous system systemic embolization, all-cause hospitalization, and all-cause mortality up to day 35.
The primary safety endpoint was time to first occurrence of International Society on Thrombosis and Hemostasis (ISTH) critical-site and fatal bleeding.
A modified intention-to-treat analysis (all participants taking at least one dose of study intervention) was also planned.
The trial was stopped early in April this year because of a lower than expected event incidence (3.2%), compared with the planned rate (8.5%), giving a very low likelihood of being able to achieve the required number of events.
Dr. Piazza said reasons contributing to the low event rate included a falling COVID-19 death and hospitalization rate nationwide, and increased use of effective vaccines.
Results of the main intention-to-treat analysis (in 1,284 patients) showed no significant difference in the primary efficacy composite endpoint, which occurred in 3.4% of the rivaroxaban group versus 3.0% of the placebo group.
In the modified intention-to-treat analysis (which included 1,197 patients who actually took at least one dose of the study medication) there was shift in the directionality of the point estimate (rivaroxaban 2.0% vs. placebo 2.7%), which Dr. Piazza said was related to a higher number of patients hospitalized before receiving study drug in the rivaroxaban group. However, the difference was still nonsignificant.
The first major secondary outcome of symptomatic VTE, arterial thrombotic events, and all-cause mortality occurred in 0.3% of rivaroxaban patients versus 1.1% of placebo patients, but this difference did not reach statistical significance.
However, a post hoc exploratory analysis did show a significant reduction in the outcome of symptomatic VTE and arterial thrombotic events.
In terms of safety, there were no fatal critical-site bleeding events, and there was no difference in ISTH major bleeding, which occurred in one patient in the rivaroxaban group versus no patients in the placebo group.
There was, however, a significant increase in nonmajor clinically relevant bleeding with rivaroxaban, which occurred in nine patients (1.5%) versus one patient (0.2%) in the placebo group.
Trivial bleeding was also increased in the rivaroxaban group, occurring in 17 patients (2.8%) versus 5 patients (0.8%) in the placebo group.
Discussant for the study, Renato Lopes, MD, Duke University Medical Center, Durham, N.C., noted that the relationship between COVID-19 and thrombosis has been an important issue since the beginning of the pandemic, with many proposed mechanisms to explain the COVID-19–associated coagulopathy, which is a major cause of death and disability.
While observational data at the beginning of the pandemic suggested patients with COVID-19 might benefit from anticoagulation, looking at all the different randomized trials that have tested anticoagulation in COVID-19 outpatients, there is no treatment effect on the various different primary outcomes in those studies and also no effect on all-cause mortality, Dr. Lopes said.
He pointed out that PREVENT-HD was stopped prematurely with only about one-third of the planned number of patients enrolled, “just like every other outpatient COVID-19 trial.”
He also drew attention to the low rates of vaccination in the trial population, which does not reflect the current vaccination rate in the United States, and said the different direction of the results between the main intention-to-treat and modified intention-to-treat analyses deserve further investigation.
However, Dr. Lopes concluded, “The results of this trial, in line with the body of evidence in this field, do not support the routine use of any antithrombotic therapy for outpatients with COVID-19.”
The PREVENT-HD trial was sponsored by Janssen. Dr. Piazza has reported receiving research support from Bristol-Myers Squibb/Pfizer Alliance, Bayer, Janssen, Alexion, Amgen, and Boston Scientific, and consulting fees from Bristol-Myers Squibb/Pfizer Alliance, Boston Scientific, Janssen, NAMSA, Prairie Education and Research Cooperative, Boston Clinical Research Institute, and Amgen.
A version of this article first appeared on Medscape.com.
A new U.S. randomized trial has failed to show benefit of a 35-day course of oral anticoagulation with rivaroxaban for the prevention of thrombotic events in outpatients with symptomatic COVID-19.
The PREVENT-HD trial was presented at the American Heart Association scientific sessions by Gregory Piazza, MD, Brigham and Women’s Hospital, Boston.
“With the caveat that the trial was underpowered to provide a definitive conclusion, these data do not support routine antithrombotic prophylaxis in nonhospitalized patients with symptomatic COVID-19,” Dr. Piazza concluded.
PREVENT-HD is the largest randomized study to look at anticoagulation in nonhospitalized COVID-19 patients and joins a long list of smaller trials that have also shown no benefit with this approach.
However, anticoagulation is recommended in patients who are hospitalized with COVID-19.
Dr. Piazza noted that the issue of anticoagulation in COVID-19 has focused mainly on hospitalized patients, but most COVID-19 cases are treated as outpatients, who are also suspected to be at risk for venous and arterial thrombotic events, especially if they have additional risk factors. Histopathological evidence also suggests that at least part of the deterioration in lung function leading to hospitalization may be attributable to in situ pulmonary artery thrombosis.
The PREVENT-HD trial explored the question of whether early initiation of thromboprophylaxis dosing of rivaroxaban in higher-risk outpatients with COVID-19 may lower the incidence of venous and arterial thrombotic events, reduce in situ pulmonary thrombosis and the worsening of pulmonary function that may lead to hospitalization, and reduce all-cause mortality.
The trial included 1,284 outpatients with a positive test for COVID-19 and who were within 14 days of symptom onset. They also had to have at least one of the following additional risk factors: age over 60 years; prior history of venous thromboembolism (VTE), thrombophilia, coronary artery disease, peripheral artery disease, cardiovascular disease or ischemic stroke, cancer, diabetes, heart failure, obesity (body mass index ≥ 35 kg/m2) or D-dimer > upper limit of normal. Around 35% of the study population had two or more of these risk factors.
Patients were randomized to rivaroxaban 10 mg daily for 35 days or placebo.
The primary efficacy endpoint was time to first occurrence of a composite of symptomatic VTE, myocardial infarction, ischemic stroke, acute limb ischemia, non–central nervous system systemic embolization, all-cause hospitalization, and all-cause mortality up to day 35.
The primary safety endpoint was time to first occurrence of International Society on Thrombosis and Hemostasis (ISTH) critical-site and fatal bleeding.
A modified intention-to-treat analysis (all participants taking at least one dose of study intervention) was also planned.
The trial was stopped early in April this year because of a lower than expected event incidence (3.2%), compared with the planned rate (8.5%), giving a very low likelihood of being able to achieve the required number of events.
Dr. Piazza said reasons contributing to the low event rate included a falling COVID-19 death and hospitalization rate nationwide, and increased use of effective vaccines.
Results of the main intention-to-treat analysis (in 1,284 patients) showed no significant difference in the primary efficacy composite endpoint, which occurred in 3.4% of the rivaroxaban group versus 3.0% of the placebo group.
In the modified intention-to-treat analysis (which included 1,197 patients who actually took at least one dose of the study medication) there was shift in the directionality of the point estimate (rivaroxaban 2.0% vs. placebo 2.7%), which Dr. Piazza said was related to a higher number of patients hospitalized before receiving study drug in the rivaroxaban group. However, the difference was still nonsignificant.
The first major secondary outcome of symptomatic VTE, arterial thrombotic events, and all-cause mortality occurred in 0.3% of rivaroxaban patients versus 1.1% of placebo patients, but this difference did not reach statistical significance.
However, a post hoc exploratory analysis did show a significant reduction in the outcome of symptomatic VTE and arterial thrombotic events.
In terms of safety, there were no fatal critical-site bleeding events, and there was no difference in ISTH major bleeding, which occurred in one patient in the rivaroxaban group versus no patients in the placebo group.
There was, however, a significant increase in nonmajor clinically relevant bleeding with rivaroxaban, which occurred in nine patients (1.5%) versus one patient (0.2%) in the placebo group.
Trivial bleeding was also increased in the rivaroxaban group, occurring in 17 patients (2.8%) versus 5 patients (0.8%) in the placebo group.
Discussant for the study, Renato Lopes, MD, Duke University Medical Center, Durham, N.C., noted that the relationship between COVID-19 and thrombosis has been an important issue since the beginning of the pandemic, with many proposed mechanisms to explain the COVID-19–associated coagulopathy, which is a major cause of death and disability.
While observational data at the beginning of the pandemic suggested patients with COVID-19 might benefit from anticoagulation, looking at all the different randomized trials that have tested anticoagulation in COVID-19 outpatients, there is no treatment effect on the various different primary outcomes in those studies and also no effect on all-cause mortality, Dr. Lopes said.
He pointed out that PREVENT-HD was stopped prematurely with only about one-third of the planned number of patients enrolled, “just like every other outpatient COVID-19 trial.”
He also drew attention to the low rates of vaccination in the trial population, which does not reflect the current vaccination rate in the United States, and said the different direction of the results between the main intention-to-treat and modified intention-to-treat analyses deserve further investigation.
However, Dr. Lopes concluded, “The results of this trial, in line with the body of evidence in this field, do not support the routine use of any antithrombotic therapy for outpatients with COVID-19.”
The PREVENT-HD trial was sponsored by Janssen. Dr. Piazza has reported receiving research support from Bristol-Myers Squibb/Pfizer Alliance, Bayer, Janssen, Alexion, Amgen, and Boston Scientific, and consulting fees from Bristol-Myers Squibb/Pfizer Alliance, Boston Scientific, Janssen, NAMSA, Prairie Education and Research Cooperative, Boston Clinical Research Institute, and Amgen.
A version of this article first appeared on Medscape.com.
A new U.S. randomized trial has failed to show benefit of a 35-day course of oral anticoagulation with rivaroxaban for the prevention of thrombotic events in outpatients with symptomatic COVID-19.
The PREVENT-HD trial was presented at the American Heart Association scientific sessions by Gregory Piazza, MD, Brigham and Women’s Hospital, Boston.
“With the caveat that the trial was underpowered to provide a definitive conclusion, these data do not support routine antithrombotic prophylaxis in nonhospitalized patients with symptomatic COVID-19,” Dr. Piazza concluded.
PREVENT-HD is the largest randomized study to look at anticoagulation in nonhospitalized COVID-19 patients and joins a long list of smaller trials that have also shown no benefit with this approach.
However, anticoagulation is recommended in patients who are hospitalized with COVID-19.
Dr. Piazza noted that the issue of anticoagulation in COVID-19 has focused mainly on hospitalized patients, but most COVID-19 cases are treated as outpatients, who are also suspected to be at risk for venous and arterial thrombotic events, especially if they have additional risk factors. Histopathological evidence also suggests that at least part of the deterioration in lung function leading to hospitalization may be attributable to in situ pulmonary artery thrombosis.
The PREVENT-HD trial explored the question of whether early initiation of thromboprophylaxis dosing of rivaroxaban in higher-risk outpatients with COVID-19 may lower the incidence of venous and arterial thrombotic events, reduce in situ pulmonary thrombosis and the worsening of pulmonary function that may lead to hospitalization, and reduce all-cause mortality.
The trial included 1,284 outpatients with a positive test for COVID-19 and who were within 14 days of symptom onset. They also had to have at least one of the following additional risk factors: age over 60 years; prior history of venous thromboembolism (VTE), thrombophilia, coronary artery disease, peripheral artery disease, cardiovascular disease or ischemic stroke, cancer, diabetes, heart failure, obesity (body mass index ≥ 35 kg/m2) or D-dimer > upper limit of normal. Around 35% of the study population had two or more of these risk factors.
Patients were randomized to rivaroxaban 10 mg daily for 35 days or placebo.
The primary efficacy endpoint was time to first occurrence of a composite of symptomatic VTE, myocardial infarction, ischemic stroke, acute limb ischemia, non–central nervous system systemic embolization, all-cause hospitalization, and all-cause mortality up to day 35.
The primary safety endpoint was time to first occurrence of International Society on Thrombosis and Hemostasis (ISTH) critical-site and fatal bleeding.
A modified intention-to-treat analysis (all participants taking at least one dose of study intervention) was also planned.
The trial was stopped early in April this year because of a lower than expected event incidence (3.2%), compared with the planned rate (8.5%), giving a very low likelihood of being able to achieve the required number of events.
Dr. Piazza said reasons contributing to the low event rate included a falling COVID-19 death and hospitalization rate nationwide, and increased use of effective vaccines.
Results of the main intention-to-treat analysis (in 1,284 patients) showed no significant difference in the primary efficacy composite endpoint, which occurred in 3.4% of the rivaroxaban group versus 3.0% of the placebo group.
In the modified intention-to-treat analysis (which included 1,197 patients who actually took at least one dose of the study medication) there was shift in the directionality of the point estimate (rivaroxaban 2.0% vs. placebo 2.7%), which Dr. Piazza said was related to a higher number of patients hospitalized before receiving study drug in the rivaroxaban group. However, the difference was still nonsignificant.
The first major secondary outcome of symptomatic VTE, arterial thrombotic events, and all-cause mortality occurred in 0.3% of rivaroxaban patients versus 1.1% of placebo patients, but this difference did not reach statistical significance.
However, a post hoc exploratory analysis did show a significant reduction in the outcome of symptomatic VTE and arterial thrombotic events.
In terms of safety, there were no fatal critical-site bleeding events, and there was no difference in ISTH major bleeding, which occurred in one patient in the rivaroxaban group versus no patients in the placebo group.
There was, however, a significant increase in nonmajor clinically relevant bleeding with rivaroxaban, which occurred in nine patients (1.5%) versus one patient (0.2%) in the placebo group.
Trivial bleeding was also increased in the rivaroxaban group, occurring in 17 patients (2.8%) versus 5 patients (0.8%) in the placebo group.
Discussant for the study, Renato Lopes, MD, Duke University Medical Center, Durham, N.C., noted that the relationship between COVID-19 and thrombosis has been an important issue since the beginning of the pandemic, with many proposed mechanisms to explain the COVID-19–associated coagulopathy, which is a major cause of death and disability.
While observational data at the beginning of the pandemic suggested patients with COVID-19 might benefit from anticoagulation, looking at all the different randomized trials that have tested anticoagulation in COVID-19 outpatients, there is no treatment effect on the various different primary outcomes in those studies and also no effect on all-cause mortality, Dr. Lopes said.
He pointed out that PREVENT-HD was stopped prematurely with only about one-third of the planned number of patients enrolled, “just like every other outpatient COVID-19 trial.”
He also drew attention to the low rates of vaccination in the trial population, which does not reflect the current vaccination rate in the United States, and said the different direction of the results between the main intention-to-treat and modified intention-to-treat analyses deserve further investigation.
However, Dr. Lopes concluded, “The results of this trial, in line with the body of evidence in this field, do not support the routine use of any antithrombotic therapy for outpatients with COVID-19.”
The PREVENT-HD trial was sponsored by Janssen. Dr. Piazza has reported receiving research support from Bristol-Myers Squibb/Pfizer Alliance, Bayer, Janssen, Alexion, Amgen, and Boston Scientific, and consulting fees from Bristol-Myers Squibb/Pfizer Alliance, Boston Scientific, Janssen, NAMSA, Prairie Education and Research Cooperative, Boston Clinical Research Institute, and Amgen.
A version of this article first appeared on Medscape.com.
FROM AHA 2022
Children from poorer ZIP codes often untreated for ear infections
Children from socially disadvantaged backgrounds are less likely to be treated for middle ear infections and are likely to experience serious complications from the condition – potentially with lifelong economic consequences – researchers have found.
Problems such as hearing loss and chronic ear infections were more common for children who lived in areas marked by difficult socioeconomic circumstances, according to the researchers, who linked the complications to a lack of adequate treatment in this population.
“We are treating socially disadvantaged kids differently than we are treating more advantaged kids,” said Jason Qian, MD, a resident in otolaryngology and head and neck surgery at Stanford (Calif.) University, who helped conduct the new study. “We have to think about social inequalities so we can ensure all kids are receiving the same level and type of care.”
In the United States, 80% of children will experience otitis media during their lifetime. Untreated ear infections can lead to symptoms ranging from mild discharge from the ear to life-threatening conditions like mastoiditis and intracranial abscesses.
For the new study, published online in JAMA Otolaryngology–Head & Neck Surgery, Dr. Qian and colleagues looked at 4.8 million children with private health insurance across the United States using a database with information on inpatient and outpatient visits and medication use. The researchers identified patients between January 2003 and March 2021 who received treatment for recurrent and suppurative otitis media, those who received tympanostomy tubes, and children who experienced severe complications from undertreated ear infections.
Social disadvantage was assessed using the Social Deprivation Index (SDI), a tool used to measure indicators of poverty throughout the United States based on seven demographic factors including level of educational attainment, the number of single-parent households, the share of people living in overcrowded homes, and other factors.
Every point increase in the SDI score was associated with a 14% lower likelihood of being treated for recurrent ear infections despite having them and a 28% greater chance of being hospitalized for severe ear infections, according to the researchers.
Previous research established that children with government health insurance or no coverage have more difficulty receiving proper treatment for ear infections. Although people with commercial insurance are generally wealthier than those without private coverage, Dr. Qian said, the new data indicate that significant social disparities in care exist even within this group.
Although some studies have found that wealthier children are more likely to develop otitis media, Dr. Qian’s group said that association likely reflects the better access to health care money affords.
“We found that socially disadvantaged children not only have a higher burden of otitis media but are also undertreated both medically and surgically for [ear infections]. Because chronic and complicated forms of otitis media can cause childhood hearing loss, which in turn limits academic and economic potential, undertreatment of [otitis media] in socially disadvantaged populations can contribute to generational cycles of poverty, unemployment, and low pay,” they write.
“The biggest take home is that we are not treating children equitably when it comes to ear infections,” Dr. Qian added. “In order to give children equal access to care, we as health care providers need to find strategies to do better.”
The study was supported by the Stanford Center for Population Health Science Data Core, which is supported by a grant from the National Institutes of Health and internal funding. Dr. Qian has reported receiving grant funding from Merck.
A version of this article first appeared on Medscape.com.
Children from socially disadvantaged backgrounds are less likely to be treated for middle ear infections and are likely to experience serious complications from the condition – potentially with lifelong economic consequences – researchers have found.
Problems such as hearing loss and chronic ear infections were more common for children who lived in areas marked by difficult socioeconomic circumstances, according to the researchers, who linked the complications to a lack of adequate treatment in this population.
“We are treating socially disadvantaged kids differently than we are treating more advantaged kids,” said Jason Qian, MD, a resident in otolaryngology and head and neck surgery at Stanford (Calif.) University, who helped conduct the new study. “We have to think about social inequalities so we can ensure all kids are receiving the same level and type of care.”
In the United States, 80% of children will experience otitis media during their lifetime. Untreated ear infections can lead to symptoms ranging from mild discharge from the ear to life-threatening conditions like mastoiditis and intracranial abscesses.
For the new study, published online in JAMA Otolaryngology–Head & Neck Surgery, Dr. Qian and colleagues looked at 4.8 million children with private health insurance across the United States using a database with information on inpatient and outpatient visits and medication use. The researchers identified patients between January 2003 and March 2021 who received treatment for recurrent and suppurative otitis media, those who received tympanostomy tubes, and children who experienced severe complications from undertreated ear infections.
Social disadvantage was assessed using the Social Deprivation Index (SDI), a tool used to measure indicators of poverty throughout the United States based on seven demographic factors including level of educational attainment, the number of single-parent households, the share of people living in overcrowded homes, and other factors.
Every point increase in the SDI score was associated with a 14% lower likelihood of being treated for recurrent ear infections despite having them and a 28% greater chance of being hospitalized for severe ear infections, according to the researchers.
Previous research established that children with government health insurance or no coverage have more difficulty receiving proper treatment for ear infections. Although people with commercial insurance are generally wealthier than those without private coverage, Dr. Qian said, the new data indicate that significant social disparities in care exist even within this group.
Although some studies have found that wealthier children are more likely to develop otitis media, Dr. Qian’s group said that association likely reflects the better access to health care money affords.
“We found that socially disadvantaged children not only have a higher burden of otitis media but are also undertreated both medically and surgically for [ear infections]. Because chronic and complicated forms of otitis media can cause childhood hearing loss, which in turn limits academic and economic potential, undertreatment of [otitis media] in socially disadvantaged populations can contribute to generational cycles of poverty, unemployment, and low pay,” they write.
“The biggest take home is that we are not treating children equitably when it comes to ear infections,” Dr. Qian added. “In order to give children equal access to care, we as health care providers need to find strategies to do better.”
The study was supported by the Stanford Center for Population Health Science Data Core, which is supported by a grant from the National Institutes of Health and internal funding. Dr. Qian has reported receiving grant funding from Merck.
A version of this article first appeared on Medscape.com.
Children from socially disadvantaged backgrounds are less likely to be treated for middle ear infections and are likely to experience serious complications from the condition – potentially with lifelong economic consequences – researchers have found.
Problems such as hearing loss and chronic ear infections were more common for children who lived in areas marked by difficult socioeconomic circumstances, according to the researchers, who linked the complications to a lack of adequate treatment in this population.
“We are treating socially disadvantaged kids differently than we are treating more advantaged kids,” said Jason Qian, MD, a resident in otolaryngology and head and neck surgery at Stanford (Calif.) University, who helped conduct the new study. “We have to think about social inequalities so we can ensure all kids are receiving the same level and type of care.”
In the United States, 80% of children will experience otitis media during their lifetime. Untreated ear infections can lead to symptoms ranging from mild discharge from the ear to life-threatening conditions like mastoiditis and intracranial abscesses.
For the new study, published online in JAMA Otolaryngology–Head & Neck Surgery, Dr. Qian and colleagues looked at 4.8 million children with private health insurance across the United States using a database with information on inpatient and outpatient visits and medication use. The researchers identified patients between January 2003 and March 2021 who received treatment for recurrent and suppurative otitis media, those who received tympanostomy tubes, and children who experienced severe complications from undertreated ear infections.
Social disadvantage was assessed using the Social Deprivation Index (SDI), a tool used to measure indicators of poverty throughout the United States based on seven demographic factors including level of educational attainment, the number of single-parent households, the share of people living in overcrowded homes, and other factors.
Every point increase in the SDI score was associated with a 14% lower likelihood of being treated for recurrent ear infections despite having them and a 28% greater chance of being hospitalized for severe ear infections, according to the researchers.
Previous research established that children with government health insurance or no coverage have more difficulty receiving proper treatment for ear infections. Although people with commercial insurance are generally wealthier than those without private coverage, Dr. Qian said, the new data indicate that significant social disparities in care exist even within this group.
Although some studies have found that wealthier children are more likely to develop otitis media, Dr. Qian’s group said that association likely reflects the better access to health care money affords.
“We found that socially disadvantaged children not only have a higher burden of otitis media but are also undertreated both medically and surgically for [ear infections]. Because chronic and complicated forms of otitis media can cause childhood hearing loss, which in turn limits academic and economic potential, undertreatment of [otitis media] in socially disadvantaged populations can contribute to generational cycles of poverty, unemployment, and low pay,” they write.
“The biggest take home is that we are not treating children equitably when it comes to ear infections,” Dr. Qian added. “In order to give children equal access to care, we as health care providers need to find strategies to do better.”
The study was supported by the Stanford Center for Population Health Science Data Core, which is supported by a grant from the National Institutes of Health and internal funding. Dr. Qian has reported receiving grant funding from Merck.
A version of this article first appeared on Medscape.com.
Monkeypox in children appears rare and relatively mild
Monkeypox virus infections in children and adolescents in the United States are rare, and young patients with known infections have all recovered, according to a study from the Centers for Disease Control and Prevention.
In addition, evidence suggests that secondary transmission in schools or childcare facilities may be unlikely.
The study was the first comprehensive study on the impact of monkeypox on children during the 2022 outbreak, according to a statement emailed to this news organization from the California Department of Public Health, one of the state health departments that partnered with the CDC to share information.
News of low infection rates and relatively mild disease was welcome to clinicians, who had braced for severe findings on the basis of sparse prior data, according to Peter Chin-Hong, MD, a professor of medicine and an infectious diseases physician at the University of California, San Francisco.
“We were on heightened alert that kids may do poorly,” said Dr. Chin-Hong, who was not involved in the study but who cared for monkeypox patients during the outbreak. “I think this study is reassuring.
“The other silver lining about it is that most of the kids got infected in the household setting from ways that you would expect them to get [infected],” Dr. Chin-Hong said in an interview.
However, Black and Hispanic children were more likely to contract the disease, underscoring troubling inequities.
“Early on, individuals of color were much less likely to be able to successfully access vaccination,” said first author Ian Hennessee, PhD, MPH, an epidemic intelligence service officer with the CDC and a member of the Special Case Investigation Unit of the Multinational Monkeypox Response Team at the CDC. “We think those kinds of structural inequities really trickled down towards the children and adolescents that have been affected by this outbreak.”
The study was published in Morbidity and Mortality Weekly Report.
A nationwide look at the data
The researchers discussed 83 children and adolescents with monkeypox who came to the CDC’s attention between May 17 and Sept. 24, 2022.
The 83 cases represent 0.3% of the 25,038 reported monkeypox cases in the United States over that period. Of the 28 children aged 12 years or younger, 18 (64%) were boys. Sixteen children were younger than 4 years.
Exposure data were available for 20 (71%) of those aged 0-12. In that group, 19 were exposed at home; 17 cases were due to routine skin-to-skin contact with a household caregiver; and one case was suspected to be caused by fomites (such as a shared towel). Exposure information was unavailable for the remaining case.
Most of the children experienced lesions on the trunk. No lesions were anogenital. Two patients in the youngest age group were hospitalized because of widespread rash that involved the eyelids, and a patient in the 5- to 12-year-old group was hospitalized because of periorbital cellulitis and conjunctivitis.
Among those aged 13-17, there were 55 cases. Of these patients, 89% were boys. Exposure data were available for 35 (64%). In 32 of these patients, the infection occurred from presumed sexual contact. Twenty-three of those adolescents reported male-to-male sexual contact. No case was found to be connected with sexual abuse.
Lesions in the adolescents were mostly truncal or anogenital. Six in this group were hospitalized, and all of them recovered. One adolescent was found to be HIV positive.
Black and Hispanic children accounted for 47% and 35% of all cases, respectively.
Eleven percent of all the children and adolescents were hospitalized, and none received intensive care.
Treatments, when given, included the antiviral drug tecovirimat, intravenous vaccinia immune globulin, and topical trifluridine. There were no deaths.
Ten symptomatic patients attended school or daycare. Among these patients, no secondary transmissions were found to have occurred. Some contacts were offered the JYNNEOS monkeypox vaccine as postexposure prophylaxis.
Limitations of the study included potentially overlooked cases. Data were collected through routine surveillance, children frequently experience rashes, and access to testing has been a challenge, Dr. Hennessee explained.
In addition, data on exposure characteristics were missing for some children.
Inequities and the risks of being judged
The outbreak in the United States has eased in recent months. However, though uncommon in children, monkeypox has affected some racial groups disproportionately.
“Especially in the later course of the outbreak, the majority of cases were among Black and Hispanic individuals,” said co-author Rachel E. Harold, MD, an infectious diseases specialist and supervisory medical officer with the District of Columbia Department of Health’s HIV/AIDS, Hepatitis, STDs, and TB Administration.
“Unfortunately, the pediatric cases do reflect the outbreak overall,” she told this news organization.
Dr. Harold noted there have been efforts in D.C. and other jurisdictions, as well as by the White House monkeypox response team, to reach populations at greatest risk and that they were “really trying to make vaccine available to people of color.”
Vaccination clinics often popped up in unexpected locations at short notice, and that made it hard for some people to get to them, Dr. Chin-Hong pointed out.
Another factor was “the public aspect of accessing diagnostics and vaccines and the way that that’s linked to potential judgment or sexual risk,” he added.
“Not everybody’s out,” Dr. Chin-Hong said, referring to members of the LGBTQ community. “In many communities of color, going to get a test or going to get a vaccine essentially means that you’re out.”
For clinicians who suspect monkeypox in a child, Dr. Harold suggests keeping a broad differential diagnosis, looking for an epidemiologic link, and contacting the CDC for assistance. Infected children should be encouraged to avoid touching their own eyes or mucous membranes, she added.
In addition, she said, tecovirimat is a reasonable treatment and is well tolerated by pediatric monkeypox patients with eczema, an underlying condition that could lead to severe disease.
For infected caregivers, Dr. Hennessee said, measures to prevent infecting children at home include isolation, contact precautions, and in some cases, postexposure prophylaxis via vaccination.
For sexually active adolescents, he advised that clinicians offer vaccination, education on sexual health, and testing for HIV and other sexually transmitted infections.
“It’s important to remember that adolescents may be sexually active, and clinicians should do a thorough and nonjudgmental sexual history,” Dr. Harold added. “That is always true, but especially if there is concern for [monkeypox].”
Dr. Hennessee, Dr. Chin-Hong, and Dr. Harold have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Monkeypox virus infections in children and adolescents in the United States are rare, and young patients with known infections have all recovered, according to a study from the Centers for Disease Control and Prevention.
In addition, evidence suggests that secondary transmission in schools or childcare facilities may be unlikely.
The study was the first comprehensive study on the impact of monkeypox on children during the 2022 outbreak, according to a statement emailed to this news organization from the California Department of Public Health, one of the state health departments that partnered with the CDC to share information.
News of low infection rates and relatively mild disease was welcome to clinicians, who had braced for severe findings on the basis of sparse prior data, according to Peter Chin-Hong, MD, a professor of medicine and an infectious diseases physician at the University of California, San Francisco.
“We were on heightened alert that kids may do poorly,” said Dr. Chin-Hong, who was not involved in the study but who cared for monkeypox patients during the outbreak. “I think this study is reassuring.
“The other silver lining about it is that most of the kids got infected in the household setting from ways that you would expect them to get [infected],” Dr. Chin-Hong said in an interview.
However, Black and Hispanic children were more likely to contract the disease, underscoring troubling inequities.
“Early on, individuals of color were much less likely to be able to successfully access vaccination,” said first author Ian Hennessee, PhD, MPH, an epidemic intelligence service officer with the CDC and a member of the Special Case Investigation Unit of the Multinational Monkeypox Response Team at the CDC. “We think those kinds of structural inequities really trickled down towards the children and adolescents that have been affected by this outbreak.”
The study was published in Morbidity and Mortality Weekly Report.
A nationwide look at the data
The researchers discussed 83 children and adolescents with monkeypox who came to the CDC’s attention between May 17 and Sept. 24, 2022.
The 83 cases represent 0.3% of the 25,038 reported monkeypox cases in the United States over that period. Of the 28 children aged 12 years or younger, 18 (64%) were boys. Sixteen children were younger than 4 years.
Exposure data were available for 20 (71%) of those aged 0-12. In that group, 19 were exposed at home; 17 cases were due to routine skin-to-skin contact with a household caregiver; and one case was suspected to be caused by fomites (such as a shared towel). Exposure information was unavailable for the remaining case.
Most of the children experienced lesions on the trunk. No lesions were anogenital. Two patients in the youngest age group were hospitalized because of widespread rash that involved the eyelids, and a patient in the 5- to 12-year-old group was hospitalized because of periorbital cellulitis and conjunctivitis.
Among those aged 13-17, there were 55 cases. Of these patients, 89% were boys. Exposure data were available for 35 (64%). In 32 of these patients, the infection occurred from presumed sexual contact. Twenty-three of those adolescents reported male-to-male sexual contact. No case was found to be connected with sexual abuse.
Lesions in the adolescents were mostly truncal or anogenital. Six in this group were hospitalized, and all of them recovered. One adolescent was found to be HIV positive.
Black and Hispanic children accounted for 47% and 35% of all cases, respectively.
Eleven percent of all the children and adolescents were hospitalized, and none received intensive care.
Treatments, when given, included the antiviral drug tecovirimat, intravenous vaccinia immune globulin, and topical trifluridine. There were no deaths.
Ten symptomatic patients attended school or daycare. Among these patients, no secondary transmissions were found to have occurred. Some contacts were offered the JYNNEOS monkeypox vaccine as postexposure prophylaxis.
Limitations of the study included potentially overlooked cases. Data were collected through routine surveillance, children frequently experience rashes, and access to testing has been a challenge, Dr. Hennessee explained.
In addition, data on exposure characteristics were missing for some children.
Inequities and the risks of being judged
The outbreak in the United States has eased in recent months. However, though uncommon in children, monkeypox has affected some racial groups disproportionately.
“Especially in the later course of the outbreak, the majority of cases were among Black and Hispanic individuals,” said co-author Rachel E. Harold, MD, an infectious diseases specialist and supervisory medical officer with the District of Columbia Department of Health’s HIV/AIDS, Hepatitis, STDs, and TB Administration.
“Unfortunately, the pediatric cases do reflect the outbreak overall,” she told this news organization.
Dr. Harold noted there have been efforts in D.C. and other jurisdictions, as well as by the White House monkeypox response team, to reach populations at greatest risk and that they were “really trying to make vaccine available to people of color.”
Vaccination clinics often popped up in unexpected locations at short notice, and that made it hard for some people to get to them, Dr. Chin-Hong pointed out.
Another factor was “the public aspect of accessing diagnostics and vaccines and the way that that’s linked to potential judgment or sexual risk,” he added.
“Not everybody’s out,” Dr. Chin-Hong said, referring to members of the LGBTQ community. “In many communities of color, going to get a test or going to get a vaccine essentially means that you’re out.”
For clinicians who suspect monkeypox in a child, Dr. Harold suggests keeping a broad differential diagnosis, looking for an epidemiologic link, and contacting the CDC for assistance. Infected children should be encouraged to avoid touching their own eyes or mucous membranes, she added.
In addition, she said, tecovirimat is a reasonable treatment and is well tolerated by pediatric monkeypox patients with eczema, an underlying condition that could lead to severe disease.
For infected caregivers, Dr. Hennessee said, measures to prevent infecting children at home include isolation, contact precautions, and in some cases, postexposure prophylaxis via vaccination.
For sexually active adolescents, he advised that clinicians offer vaccination, education on sexual health, and testing for HIV and other sexually transmitted infections.
“It’s important to remember that adolescents may be sexually active, and clinicians should do a thorough and nonjudgmental sexual history,” Dr. Harold added. “That is always true, but especially if there is concern for [monkeypox].”
Dr. Hennessee, Dr. Chin-Hong, and Dr. Harold have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Monkeypox virus infections in children and adolescents in the United States are rare, and young patients with known infections have all recovered, according to a study from the Centers for Disease Control and Prevention.
In addition, evidence suggests that secondary transmission in schools or childcare facilities may be unlikely.
The study was the first comprehensive study on the impact of monkeypox on children during the 2022 outbreak, according to a statement emailed to this news organization from the California Department of Public Health, one of the state health departments that partnered with the CDC to share information.
News of low infection rates and relatively mild disease was welcome to clinicians, who had braced for severe findings on the basis of sparse prior data, according to Peter Chin-Hong, MD, a professor of medicine and an infectious diseases physician at the University of California, San Francisco.
“We were on heightened alert that kids may do poorly,” said Dr. Chin-Hong, who was not involved in the study but who cared for monkeypox patients during the outbreak. “I think this study is reassuring.
“The other silver lining about it is that most of the kids got infected in the household setting from ways that you would expect them to get [infected],” Dr. Chin-Hong said in an interview.
However, Black and Hispanic children were more likely to contract the disease, underscoring troubling inequities.
“Early on, individuals of color were much less likely to be able to successfully access vaccination,” said first author Ian Hennessee, PhD, MPH, an epidemic intelligence service officer with the CDC and a member of the Special Case Investigation Unit of the Multinational Monkeypox Response Team at the CDC. “We think those kinds of structural inequities really trickled down towards the children and adolescents that have been affected by this outbreak.”
The study was published in Morbidity and Mortality Weekly Report.
A nationwide look at the data
The researchers discussed 83 children and adolescents with monkeypox who came to the CDC’s attention between May 17 and Sept. 24, 2022.
The 83 cases represent 0.3% of the 25,038 reported monkeypox cases in the United States over that period. Of the 28 children aged 12 years or younger, 18 (64%) were boys. Sixteen children were younger than 4 years.
Exposure data were available for 20 (71%) of those aged 0-12. In that group, 19 were exposed at home; 17 cases were due to routine skin-to-skin contact with a household caregiver; and one case was suspected to be caused by fomites (such as a shared towel). Exposure information was unavailable for the remaining case.
Most of the children experienced lesions on the trunk. No lesions were anogenital. Two patients in the youngest age group were hospitalized because of widespread rash that involved the eyelids, and a patient in the 5- to 12-year-old group was hospitalized because of periorbital cellulitis and conjunctivitis.
Among those aged 13-17, there were 55 cases. Of these patients, 89% were boys. Exposure data were available for 35 (64%). In 32 of these patients, the infection occurred from presumed sexual contact. Twenty-three of those adolescents reported male-to-male sexual contact. No case was found to be connected with sexual abuse.
Lesions in the adolescents were mostly truncal or anogenital. Six in this group were hospitalized, and all of them recovered. One adolescent was found to be HIV positive.
Black and Hispanic children accounted for 47% and 35% of all cases, respectively.
Eleven percent of all the children and adolescents were hospitalized, and none received intensive care.
Treatments, when given, included the antiviral drug tecovirimat, intravenous vaccinia immune globulin, and topical trifluridine. There were no deaths.
Ten symptomatic patients attended school or daycare. Among these patients, no secondary transmissions were found to have occurred. Some contacts were offered the JYNNEOS monkeypox vaccine as postexposure prophylaxis.
Limitations of the study included potentially overlooked cases. Data were collected through routine surveillance, children frequently experience rashes, and access to testing has been a challenge, Dr. Hennessee explained.
In addition, data on exposure characteristics were missing for some children.
Inequities and the risks of being judged
The outbreak in the United States has eased in recent months. However, though uncommon in children, monkeypox has affected some racial groups disproportionately.
“Especially in the later course of the outbreak, the majority of cases were among Black and Hispanic individuals,” said co-author Rachel E. Harold, MD, an infectious diseases specialist and supervisory medical officer with the District of Columbia Department of Health’s HIV/AIDS, Hepatitis, STDs, and TB Administration.
“Unfortunately, the pediatric cases do reflect the outbreak overall,” she told this news organization.
Dr. Harold noted there have been efforts in D.C. and other jurisdictions, as well as by the White House monkeypox response team, to reach populations at greatest risk and that they were “really trying to make vaccine available to people of color.”
Vaccination clinics often popped up in unexpected locations at short notice, and that made it hard for some people to get to them, Dr. Chin-Hong pointed out.
Another factor was “the public aspect of accessing diagnostics and vaccines and the way that that’s linked to potential judgment or sexual risk,” he added.
“Not everybody’s out,” Dr. Chin-Hong said, referring to members of the LGBTQ community. “In many communities of color, going to get a test or going to get a vaccine essentially means that you’re out.”
For clinicians who suspect monkeypox in a child, Dr. Harold suggests keeping a broad differential diagnosis, looking for an epidemiologic link, and contacting the CDC for assistance. Infected children should be encouraged to avoid touching their own eyes or mucous membranes, she added.
In addition, she said, tecovirimat is a reasonable treatment and is well tolerated by pediatric monkeypox patients with eczema, an underlying condition that could lead to severe disease.
For infected caregivers, Dr. Hennessee said, measures to prevent infecting children at home include isolation, contact precautions, and in some cases, postexposure prophylaxis via vaccination.
For sexually active adolescents, he advised that clinicians offer vaccination, education on sexual health, and testing for HIV and other sexually transmitted infections.
“It’s important to remember that adolescents may be sexually active, and clinicians should do a thorough and nonjudgmental sexual history,” Dr. Harold added. “That is always true, but especially if there is concern for [monkeypox].”
Dr. Hennessee, Dr. Chin-Hong, and Dr. Harold have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
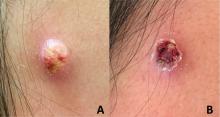
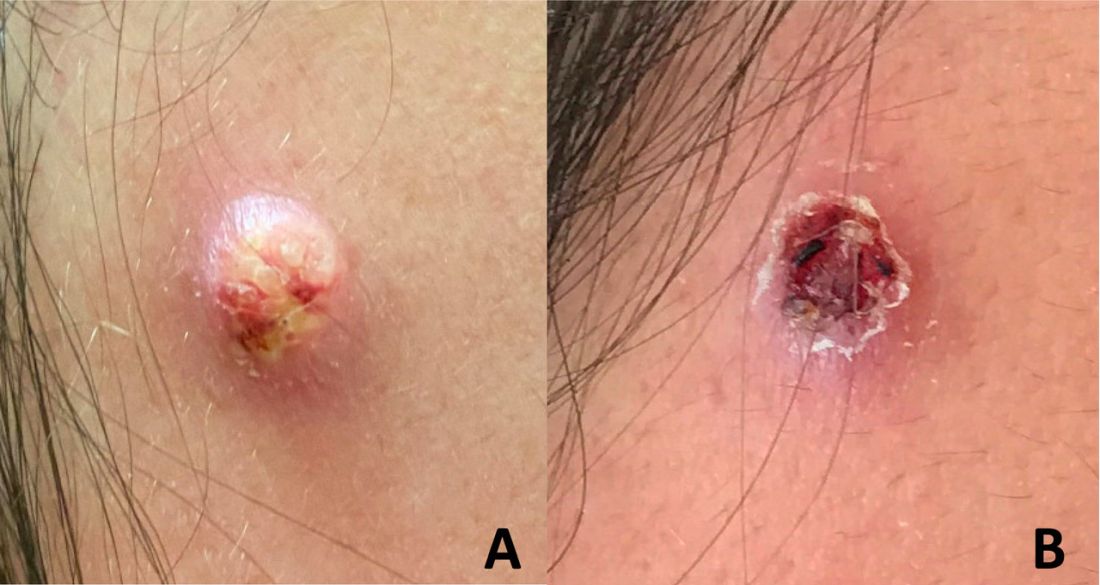
An adolescent male presents with an eroded bump on the temple
The correct answer is (D), molluscum contagiosum. Upon surgical excision, the pathology indicated the lesion was consistent with molluscum contagiosum.
Molluscum contagiosum is a benign skin disorder caused by a pox virus and is frequently seen in children. This disease is transmitted primarily through direct skin contact with an infected individual.1 Contaminated fomites have been suggested as another source of infection.2 The typical lesion appears dome-shaped, round, and pinkish-purple in color.1 The incubation period ranges from 2 weeks to 6 months and is typically self-limited in immunocompetent hosts; however, in immunocompromised persons, molluscum contagiosum lesions may present atypically such that they are larger in size and/or resemble malignancies, such as basal cell carcinoma or keratoacanthoma (for single lesions), or other infectious diseases, such as cryptococcosis and histoplasmosis (for more numerous lesions).3,4 A giant atypical molluscum contagiosum is rarely seen in healthy individuals.
What’s on the differential?
The recent episode of bleeding raises concern for other neoplastic processes of the skin including squamous cell carcinoma or basal cell carcinoma as well as cutaneous metastatic rhabdoid tumor, given the patient’s history.
Eruptive keratoacanthomas are also reported in patients taking nivolumab, an anti-PD-1 immunotherapy, which the patient has received for treatment of his recurrent metastatic rhabdoid tumor.5 More common entities such as a pyogenic granuloma or verruca are also included on the differential. The initial presentation of the lesion, however, is more consistent with the pearly umbilicated papules associated with molluscum contagiosum.
Comments from Dr. Eichenfield
This is a very hard diagnosis to make with the clinical findings and history.
Molluscum contagiosum infections are common, but with this patient’s medical history, biopsy and excision with pathologic examination was an appropriate approach to make a certain diagnosis.
Ms. Moyal is a research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego.
References
1. Brown J et al. Int J Dermatol. 2006 Feb;45(2):93-9.
2. Hanson D and Diven DG. Dermatol Online J. 2003 Mar;9(2).
3. Badri T and Gandhi GR. Molluscum contagiosum. 2022. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing.
4. Schwartz JJ and Myskowski PL. J Am Acad Dermatol. 1992 Oct 1;27(4):583-8.
5. Antonov NK et al. JAAD Case Rep. 2019 Apr 5;5(4):342-5.
The correct answer is (D), molluscum contagiosum. Upon surgical excision, the pathology indicated the lesion was consistent with molluscum contagiosum.
Molluscum contagiosum is a benign skin disorder caused by a pox virus and is frequently seen in children. This disease is transmitted primarily through direct skin contact with an infected individual.1 Contaminated fomites have been suggested as another source of infection.2 The typical lesion appears dome-shaped, round, and pinkish-purple in color.1 The incubation period ranges from 2 weeks to 6 months and is typically self-limited in immunocompetent hosts; however, in immunocompromised persons, molluscum contagiosum lesions may present atypically such that they are larger in size and/or resemble malignancies, such as basal cell carcinoma or keratoacanthoma (for single lesions), or other infectious diseases, such as cryptococcosis and histoplasmosis (for more numerous lesions).3,4 A giant atypical molluscum contagiosum is rarely seen in healthy individuals.
What’s on the differential?
The recent episode of bleeding raises concern for other neoplastic processes of the skin including squamous cell carcinoma or basal cell carcinoma as well as cutaneous metastatic rhabdoid tumor, given the patient’s history.
Eruptive keratoacanthomas are also reported in patients taking nivolumab, an anti-PD-1 immunotherapy, which the patient has received for treatment of his recurrent metastatic rhabdoid tumor.5 More common entities such as a pyogenic granuloma or verruca are also included on the differential. The initial presentation of the lesion, however, is more consistent with the pearly umbilicated papules associated with molluscum contagiosum.
Comments from Dr. Eichenfield
This is a very hard diagnosis to make with the clinical findings and history.
Molluscum contagiosum infections are common, but with this patient’s medical history, biopsy and excision with pathologic examination was an appropriate approach to make a certain diagnosis.
Ms. Moyal is a research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego.
References
1. Brown J et al. Int J Dermatol. 2006 Feb;45(2):93-9.
2. Hanson D and Diven DG. Dermatol Online J. 2003 Mar;9(2).
3. Badri T and Gandhi GR. Molluscum contagiosum. 2022. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing.
4. Schwartz JJ and Myskowski PL. J Am Acad Dermatol. 1992 Oct 1;27(4):583-8.
5. Antonov NK et al. JAAD Case Rep. 2019 Apr 5;5(4):342-5.
The correct answer is (D), molluscum contagiosum. Upon surgical excision, the pathology indicated the lesion was consistent with molluscum contagiosum.
Molluscum contagiosum is a benign skin disorder caused by a pox virus and is frequently seen in children. This disease is transmitted primarily through direct skin contact with an infected individual.1 Contaminated fomites have been suggested as another source of infection.2 The typical lesion appears dome-shaped, round, and pinkish-purple in color.1 The incubation period ranges from 2 weeks to 6 months and is typically self-limited in immunocompetent hosts; however, in immunocompromised persons, molluscum contagiosum lesions may present atypically such that they are larger in size and/or resemble malignancies, such as basal cell carcinoma or keratoacanthoma (for single lesions), or other infectious diseases, such as cryptococcosis and histoplasmosis (for more numerous lesions).3,4 A giant atypical molluscum contagiosum is rarely seen in healthy individuals.
What’s on the differential?
The recent episode of bleeding raises concern for other neoplastic processes of the skin including squamous cell carcinoma or basal cell carcinoma as well as cutaneous metastatic rhabdoid tumor, given the patient’s history.
Eruptive keratoacanthomas are also reported in patients taking nivolumab, an anti-PD-1 immunotherapy, which the patient has received for treatment of his recurrent metastatic rhabdoid tumor.5 More common entities such as a pyogenic granuloma or verruca are also included on the differential. The initial presentation of the lesion, however, is more consistent with the pearly umbilicated papules associated with molluscum contagiosum.
Comments from Dr. Eichenfield
This is a very hard diagnosis to make with the clinical findings and history.
Molluscum contagiosum infections are common, but with this patient’s medical history, biopsy and excision with pathologic examination was an appropriate approach to make a certain diagnosis.
Ms. Moyal is a research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego.
References
1. Brown J et al. Int J Dermatol. 2006 Feb;45(2):93-9.
2. Hanson D and Diven DG. Dermatol Online J. 2003 Mar;9(2).
3. Badri T and Gandhi GR. Molluscum contagiosum. 2022. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing.
4. Schwartz JJ and Myskowski PL. J Am Acad Dermatol. 1992 Oct 1;27(4):583-8.
5. Antonov NK et al. JAAD Case Rep. 2019 Apr 5;5(4):342-5.
More children should be getting flu vaccines
Cold and flu season came early in 2022.
On Nov. 4, 2022, the Centers for Disease Control and Prevention issued a Health Alert Network Health Advisory about early, elevated respiratory disease incidence caused by multiple viruses other than SARS-CoV-2.
Interseasonal spread of respiratory syncytial virus has continued in 2022, with RSV-associated hospitalizations increasing in the late spring and continuing throughout the summer and into the fall. In October, some regions of the country were seeing RSV activity near the peak seasonal levels typically observed in December and January.
Cases of severe respiratory infection in children who tested positive for rhinovirus or enterovirus spiked in August; further testing confirmed the presence of EV-D68 in some children. Rhinovirus and enterovirus continue to circulate and are isolated in hospitalized children with respiratory illness.
In some parts of the country, influenza cases have rapidly increased ahead of what we normally anticipate. According to preliminary estimates from the CDC, between Oct. 1 and Oct. 22, 880,000 people were sickened with flu, 420,000 people visited a health care provider for flu illness, and 6,900 people were hospitalized for flu. The cumulative hospitalization rate is higher than observed at this time of year in every previous flu season since 2010-2011. Hospitalization rates are highest in children aged 0-4 years and adults 65 years and older.
Of course, this report came as no surprise to pediatric health care providers. Many children’s hospitals had been operating at or over capacity for weeks. While a systematic assessment of the surge on children’s hospitals has not been published, anecdotally, hospitals from around the country have described record emergency department visits and inpatient census numbers. Some have set up tents or other temporary facilities to see ambulatory patients and have canceled elective surgeries because of a lack of beds.
There is no quick or easy solution to stem the tide of RSV-related or enterovirus/rhinovirus admissions, but many flu-related hospitalizations are vaccine preventable. Unfortunately, too few children are receiving influenza vaccine. As of the week ending Oct. 15, only about 22.1% of eligible children had been immunized. The American Academy of Pediatrics and the CDC recommend that all children are vaccinated, preferably by the end of October so they have time to develop immunity before influenza starts circulating. As it stands now, the majority of the nation’s children are facing a flu season without the benefits of vaccine.
There is still time to take steps to prevent this flu season from becoming one of the worst in recent memory. A strong provider recommendation for influenza vaccine is consistently associated with higher rates of vaccine acceptance. We need to recommend influenza vaccine to all eligible patients at every visit and in every setting. It will help if we can say it like we mean it. Some of us are tired of debating the merits of COVID-19 vaccine with families and may be leery of additional debates about flu. Some of us may just be tired, as many practices have already expanded office hours to care for the influx of kids with respiratory illness. On the heels of two atypical flu seasons, a few of us may be quietly complacent about the importance of flu vaccines for children.
Anyone in need of a little motivation should check out a paper recently published in Clinical Infectious Diseases that reinforces the value of flu vaccine, even in a year when there is a poor match between the vaccine and circulating viruses.
The 2019-2020 flu season was a bad flu season for children. Two antigenically drifted influenza viruses predominated and cases of influenza soared, resulting in the largest influenza epidemic in children in the United States since 1992. Pediatric Intensive Care Influenza Study investigators used a test-negative design to estimate the effectiveness of influenza vaccine in preventing critical and life-threatening influenza in children during that season. The good news: vaccination reduced the risk of critical influenza by 78% against H1N1pdm09 viruses that were well-matched to vaccine and by 47% against mismatched viruses. Vaccination was estimated to be 75% protective against antigenically drifted B-Victoria viruses. Overall vaccine effectiveness against critical illness from any influenza virus was 63% (95% confidence interval, 38%-78%).
While it might be tempting to attribute suboptimal immunization rates to vaccine hesitancy, ready availability remains an issue for some families. We need to eliminate barriers to access. While the AAP continues to emphasize immunization in the medical home, especially for the youngest infants, the 2022 policy statement suggests that vaccinating children in schools, pharmacies, and other nontraditional settings could improve immunization rates. To the extent feasible, we need to work with partners to support community-based initiatives and promote these to families who struggle to make it into the office.
Improving access is just one potential way to reduce health disparities related to influenza and influenza vaccination. Over 10 influenza seasons, higher rates of influenza-associated hospitalizations and intensive care unit admissions were observed in Black, Hispanic, and American Indian/Alaska Native people. These disparities were highest in children aged younger than 4 years and influenza-associated in-hospital deaths were three- to fourfold higher in Black, Hispanic, and Asian/Pacific Islander children, compared with White children. The reason for the disparities isn’t completely clear but increasing immunization rates may be part of the solution. During the 2020-2021 influenza season, flu immunization rates in Black children (51.6%) were lower than those seen in White (57.4%) and Hispanic children (58.9%).
The AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023, highlight a variety of evidence-based strategies to increase influenza immunization rates. These may provide a little inspiration for clinicians looking to try a new approach. If you wish to share your experience with increasing influenza immunization rates in your practice setting, please email me at [email protected].
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead.
Cold and flu season came early in 2022.
On Nov. 4, 2022, the Centers for Disease Control and Prevention issued a Health Alert Network Health Advisory about early, elevated respiratory disease incidence caused by multiple viruses other than SARS-CoV-2.
Interseasonal spread of respiratory syncytial virus has continued in 2022, with RSV-associated hospitalizations increasing in the late spring and continuing throughout the summer and into the fall. In October, some regions of the country were seeing RSV activity near the peak seasonal levels typically observed in December and January.
Cases of severe respiratory infection in children who tested positive for rhinovirus or enterovirus spiked in August; further testing confirmed the presence of EV-D68 in some children. Rhinovirus and enterovirus continue to circulate and are isolated in hospitalized children with respiratory illness.
In some parts of the country, influenza cases have rapidly increased ahead of what we normally anticipate. According to preliminary estimates from the CDC, between Oct. 1 and Oct. 22, 880,000 people were sickened with flu, 420,000 people visited a health care provider for flu illness, and 6,900 people were hospitalized for flu. The cumulative hospitalization rate is higher than observed at this time of year in every previous flu season since 2010-2011. Hospitalization rates are highest in children aged 0-4 years and adults 65 years and older.
Of course, this report came as no surprise to pediatric health care providers. Many children’s hospitals had been operating at or over capacity for weeks. While a systematic assessment of the surge on children’s hospitals has not been published, anecdotally, hospitals from around the country have described record emergency department visits and inpatient census numbers. Some have set up tents or other temporary facilities to see ambulatory patients and have canceled elective surgeries because of a lack of beds.
There is no quick or easy solution to stem the tide of RSV-related or enterovirus/rhinovirus admissions, but many flu-related hospitalizations are vaccine preventable. Unfortunately, too few children are receiving influenza vaccine. As of the week ending Oct. 15, only about 22.1% of eligible children had been immunized. The American Academy of Pediatrics and the CDC recommend that all children are vaccinated, preferably by the end of October so they have time to develop immunity before influenza starts circulating. As it stands now, the majority of the nation’s children are facing a flu season without the benefits of vaccine.
There is still time to take steps to prevent this flu season from becoming one of the worst in recent memory. A strong provider recommendation for influenza vaccine is consistently associated with higher rates of vaccine acceptance. We need to recommend influenza vaccine to all eligible patients at every visit and in every setting. It will help if we can say it like we mean it. Some of us are tired of debating the merits of COVID-19 vaccine with families and may be leery of additional debates about flu. Some of us may just be tired, as many practices have already expanded office hours to care for the influx of kids with respiratory illness. On the heels of two atypical flu seasons, a few of us may be quietly complacent about the importance of flu vaccines for children.
Anyone in need of a little motivation should check out a paper recently published in Clinical Infectious Diseases that reinforces the value of flu vaccine, even in a year when there is a poor match between the vaccine and circulating viruses.
The 2019-2020 flu season was a bad flu season for children. Two antigenically drifted influenza viruses predominated and cases of influenza soared, resulting in the largest influenza epidemic in children in the United States since 1992. Pediatric Intensive Care Influenza Study investigators used a test-negative design to estimate the effectiveness of influenza vaccine in preventing critical and life-threatening influenza in children during that season. The good news: vaccination reduced the risk of critical influenza by 78% against H1N1pdm09 viruses that were well-matched to vaccine and by 47% against mismatched viruses. Vaccination was estimated to be 75% protective against antigenically drifted B-Victoria viruses. Overall vaccine effectiveness against critical illness from any influenza virus was 63% (95% confidence interval, 38%-78%).
While it might be tempting to attribute suboptimal immunization rates to vaccine hesitancy, ready availability remains an issue for some families. We need to eliminate barriers to access. While the AAP continues to emphasize immunization in the medical home, especially for the youngest infants, the 2022 policy statement suggests that vaccinating children in schools, pharmacies, and other nontraditional settings could improve immunization rates. To the extent feasible, we need to work with partners to support community-based initiatives and promote these to families who struggle to make it into the office.
Improving access is just one potential way to reduce health disparities related to influenza and influenza vaccination. Over 10 influenza seasons, higher rates of influenza-associated hospitalizations and intensive care unit admissions were observed in Black, Hispanic, and American Indian/Alaska Native people. These disparities were highest in children aged younger than 4 years and influenza-associated in-hospital deaths were three- to fourfold higher in Black, Hispanic, and Asian/Pacific Islander children, compared with White children. The reason for the disparities isn’t completely clear but increasing immunization rates may be part of the solution. During the 2020-2021 influenza season, flu immunization rates in Black children (51.6%) were lower than those seen in White (57.4%) and Hispanic children (58.9%).
The AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023, highlight a variety of evidence-based strategies to increase influenza immunization rates. These may provide a little inspiration for clinicians looking to try a new approach. If you wish to share your experience with increasing influenza immunization rates in your practice setting, please email me at [email protected].
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead.
Cold and flu season came early in 2022.
On Nov. 4, 2022, the Centers for Disease Control and Prevention issued a Health Alert Network Health Advisory about early, elevated respiratory disease incidence caused by multiple viruses other than SARS-CoV-2.
Interseasonal spread of respiratory syncytial virus has continued in 2022, with RSV-associated hospitalizations increasing in the late spring and continuing throughout the summer and into the fall. In October, some regions of the country were seeing RSV activity near the peak seasonal levels typically observed in December and January.
Cases of severe respiratory infection in children who tested positive for rhinovirus or enterovirus spiked in August; further testing confirmed the presence of EV-D68 in some children. Rhinovirus and enterovirus continue to circulate and are isolated in hospitalized children with respiratory illness.
In some parts of the country, influenza cases have rapidly increased ahead of what we normally anticipate. According to preliminary estimates from the CDC, between Oct. 1 and Oct. 22, 880,000 people were sickened with flu, 420,000 people visited a health care provider for flu illness, and 6,900 people were hospitalized for flu. The cumulative hospitalization rate is higher than observed at this time of year in every previous flu season since 2010-2011. Hospitalization rates are highest in children aged 0-4 years and adults 65 years and older.
Of course, this report came as no surprise to pediatric health care providers. Many children’s hospitals had been operating at or over capacity for weeks. While a systematic assessment of the surge on children’s hospitals has not been published, anecdotally, hospitals from around the country have described record emergency department visits and inpatient census numbers. Some have set up tents or other temporary facilities to see ambulatory patients and have canceled elective surgeries because of a lack of beds.
There is no quick or easy solution to stem the tide of RSV-related or enterovirus/rhinovirus admissions, but many flu-related hospitalizations are vaccine preventable. Unfortunately, too few children are receiving influenza vaccine. As of the week ending Oct. 15, only about 22.1% of eligible children had been immunized. The American Academy of Pediatrics and the CDC recommend that all children are vaccinated, preferably by the end of October so they have time to develop immunity before influenza starts circulating. As it stands now, the majority of the nation’s children are facing a flu season without the benefits of vaccine.
There is still time to take steps to prevent this flu season from becoming one of the worst in recent memory. A strong provider recommendation for influenza vaccine is consistently associated with higher rates of vaccine acceptance. We need to recommend influenza vaccine to all eligible patients at every visit and in every setting. It will help if we can say it like we mean it. Some of us are tired of debating the merits of COVID-19 vaccine with families and may be leery of additional debates about flu. Some of us may just be tired, as many practices have already expanded office hours to care for the influx of kids with respiratory illness. On the heels of two atypical flu seasons, a few of us may be quietly complacent about the importance of flu vaccines for children.
Anyone in need of a little motivation should check out a paper recently published in Clinical Infectious Diseases that reinforces the value of flu vaccine, even in a year when there is a poor match between the vaccine and circulating viruses.
The 2019-2020 flu season was a bad flu season for children. Two antigenically drifted influenza viruses predominated and cases of influenza soared, resulting in the largest influenza epidemic in children in the United States since 1992. Pediatric Intensive Care Influenza Study investigators used a test-negative design to estimate the effectiveness of influenza vaccine in preventing critical and life-threatening influenza in children during that season. The good news: vaccination reduced the risk of critical influenza by 78% against H1N1pdm09 viruses that were well-matched to vaccine and by 47% against mismatched viruses. Vaccination was estimated to be 75% protective against antigenically drifted B-Victoria viruses. Overall vaccine effectiveness against critical illness from any influenza virus was 63% (95% confidence interval, 38%-78%).
While it might be tempting to attribute suboptimal immunization rates to vaccine hesitancy, ready availability remains an issue for some families. We need to eliminate barriers to access. While the AAP continues to emphasize immunization in the medical home, especially for the youngest infants, the 2022 policy statement suggests that vaccinating children in schools, pharmacies, and other nontraditional settings could improve immunization rates. To the extent feasible, we need to work with partners to support community-based initiatives and promote these to families who struggle to make it into the office.
Improving access is just one potential way to reduce health disparities related to influenza and influenza vaccination. Over 10 influenza seasons, higher rates of influenza-associated hospitalizations and intensive care unit admissions were observed in Black, Hispanic, and American Indian/Alaska Native people. These disparities were highest in children aged younger than 4 years and influenza-associated in-hospital deaths were three- to fourfold higher in Black, Hispanic, and Asian/Pacific Islander children, compared with White children. The reason for the disparities isn’t completely clear but increasing immunization rates may be part of the solution. During the 2020-2021 influenza season, flu immunization rates in Black children (51.6%) were lower than those seen in White (57.4%) and Hispanic children (58.9%).
The AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023, highlight a variety of evidence-based strategies to increase influenza immunization rates. These may provide a little inspiration for clinicians looking to try a new approach. If you wish to share your experience with increasing influenza immunization rates in your practice setting, please email me at [email protected].
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead.
In patients with untreated AIDS, monkeypox can be life-threatening
Monkeypox, though often mild, may be severe and even fatal in immunocompromised individuals, particularly those with untreated AIDS, according to a Centers for Disease Control and Prevention study in Morbidity and Mortality Weekly Report.
The study described a group of patients recently treated for severe monkeypox. The majority were Black, HIV positive, and not receiving treatment. Many were also facing homelessness.
The authors urged HIV testing for all sexually active individuals with suspected monkeypox. Early or prolonged monkeypox treatment may be necessary, they concluded.
Coauthor John T. Brooks, MD, called the study “a real call to action.”
“If we want to reduce cases of severe monkeypox, we need to reduce the number of persons with HIV who are undiagnosed and not treated,” said Dr. Brooks, a medical epidemiologist who is chief medical officer of CDC›s multinational monkeypox response. Dr. Brooks also leads the epidemiology research team in CDC’s division of HIV/AIDS prevention.
noted Richard Silvera, MD, MPH, CPH, who is associate program director of the infectious diseases fellowship and assistant professor of medicine (infectious diseases) at the Icahn School of Medicine at Mount Sinai, New York. He was not involved with the study.
“These patients really have not been served by the health care system,” Dr. Silvera said. “Monkeypox is just really taking advantage of that.”
How severe monkeypox can manifest
The authors reported on 57 adults hospitalized with severe monkeypox between Aug. 10 and Sept. 10, 2022, for whose care the providers sought CDC consultation.
The vast majority (95%) were men, their median age was 34 years, and 68% were Black. Nearly one in four were homeless (23%).
Overall, 47 (82%) were HIV positive, of whom just 4 had been receiving antiretroviral therapy (ART). Of 43 for whom CD4 counts were known, 71% had fewer than 50 CD4 cells/mm3.
Clinical signs included severe skin lesions in all patients and severe mucosal lesions in 68%. Other affected organ systems included lungs (21%), eyes (21%), and central nervous system (7%).
Treatments included oral or intravenous tecovirimat (93% and 65%, respectively), vaccinia immune globulin intravenous (VIGIV, 51%), and cidofovir (23%).
Nearly 1 in 3 patients (30%) received care in an ICU; 12 died (21%). Monkeypox was considered the cause or a contributing factor in five of the deaths and not a factor in one death; the remaining six deaths are under investigation.
Case studies
The report included details of three representative cases of the CDC consultations.
One was a Hispanic man in his 20s with a fever of 102.8° F, a rash including eschars, oral lesions, neck mass, and cervical lymphadenopathy. He had tested positive for monkeypox as an outpatient and upon admission was found to be HIV positive, with a CD4 count of 79 cells/mm3. He experienced a severe and ultimately fatal clinical course that included intubation, refractory hypotension, seizures, renal failure, and cardiac arrest. An autopsy revealed diffuse organ necrosis plus orthopoxvirus and cytomegalovirus.
The second was a Black man in his 30s with untreated AIDS and diffuse rash. He was tested and treated for gonorrhea, chlamydia, and syphilis before phimosis and urinary retention led to admission and a monkeypox diagnosis 4 weeks after his rash began. He was discharged with oral tecovirimat, but his skin lesions developed necrosis and he was readmitted twice, each time with new lesions. His clinical course included methicillin-resistant Staphylococcus aureus bacteremia, atrial fibrillation, eye and ear involvement, a suprapubic catheter, and progressive necrosis of his lesions. As of the CDC report, he was receiving ART and intravenous tecovirimat.
The third patient, a White man in his 40s with untreated AIDS, presented with diffuse rash. He was promptly diagnosed with monkeypox and admitted for pain control. He was discharged with oral tecovirimat and ART, but homelessness and food insecurity jeopardized the absorption of his tecovirimat (which depends on a full fatty meal), and the lesions worsened. Despite readmission and aggressive medical treatment, the patient required finger debridement and a toe amputation. After discharge, he was again readmitted for lesions and pain and, at report publication, remained hospitalized, taking oral tecovirimat and ART.
The patients in the study may not be typical of severe monkeypox cases, wrote the authors reported. Deaths after the study period were not counted.
Fewer cases, some severe
As of Nov. 7, the CDC has confirmed 28,709 monkeypox cases. These have trended downward since August. Most people with recent diagnoses are men who are gay, bisexual, same gender loving, or who have sex with men, and most are Black, according to Brooks.
Dr. Brooks urges clinicians to report suspected monkeypox cases – especially severe ones – to their health departments.
“We don’t have a good bead on exactly how many severe cases there are in the States because of complexities in our surveillance systems,” Dr. Brooks said.
For patients with suspected or confirmed monkeypox, Brooks recommends testing for sexually transmitted infections, including HIV if status is unknown. Patients with HIV should receive prompt ART. For those at risk for severe disease, the authors recommend early treatment for suspected monkeypox, even before results are back. Some patients may benefit from tecovirimat courses lasting beyond 14 days, plus additional antivirals (cidofovir or brincidofovir) and/or VIGIV.
“With severe cases, clinicians may want to consider the value of more than one drug to attack the virus at different stages of its replication cycle,” Dr. Brooks said.
Inequities matter
The authors called on providers to engage communities burdened by HIV and to ensure access to not only monkeypox vaccination, diagnosis, and treatment but also sustained HIV care.
Dr. Silvera added that providers need to tailor care plans to patients’ social determinants of health. For example, he explained, inpatient care for monkeypox could be appropriate for some patients facing homelessness and food insecurity – even if they are able to take tecovirimat orally.
He recommends tapping others’ expertise: “Our social work colleagues are well versed in this.”
“I don’t think these clinicians failed these patients. ... I think everyone made all the right choices medically,” Dr. Silvera added. “I think that the system failed these patients – and we as clinicians are part of those systems. So we also have the power to change those systems. And I think we just need to start opening our eyes to that and [start] to work together towards that goal to take better care of our patients.”
Dr. Brooks reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Monkeypox, though often mild, may be severe and even fatal in immunocompromised individuals, particularly those with untreated AIDS, according to a Centers for Disease Control and Prevention study in Morbidity and Mortality Weekly Report.
The study described a group of patients recently treated for severe monkeypox. The majority were Black, HIV positive, and not receiving treatment. Many were also facing homelessness.
The authors urged HIV testing for all sexually active individuals with suspected monkeypox. Early or prolonged monkeypox treatment may be necessary, they concluded.
Coauthor John T. Brooks, MD, called the study “a real call to action.”
“If we want to reduce cases of severe monkeypox, we need to reduce the number of persons with HIV who are undiagnosed and not treated,” said Dr. Brooks, a medical epidemiologist who is chief medical officer of CDC›s multinational monkeypox response. Dr. Brooks also leads the epidemiology research team in CDC’s division of HIV/AIDS prevention.
noted Richard Silvera, MD, MPH, CPH, who is associate program director of the infectious diseases fellowship and assistant professor of medicine (infectious diseases) at the Icahn School of Medicine at Mount Sinai, New York. He was not involved with the study.
“These patients really have not been served by the health care system,” Dr. Silvera said. “Monkeypox is just really taking advantage of that.”
How severe monkeypox can manifest
The authors reported on 57 adults hospitalized with severe monkeypox between Aug. 10 and Sept. 10, 2022, for whose care the providers sought CDC consultation.
The vast majority (95%) were men, their median age was 34 years, and 68% were Black. Nearly one in four were homeless (23%).
Overall, 47 (82%) were HIV positive, of whom just 4 had been receiving antiretroviral therapy (ART). Of 43 for whom CD4 counts were known, 71% had fewer than 50 CD4 cells/mm3.
Clinical signs included severe skin lesions in all patients and severe mucosal lesions in 68%. Other affected organ systems included lungs (21%), eyes (21%), and central nervous system (7%).
Treatments included oral or intravenous tecovirimat (93% and 65%, respectively), vaccinia immune globulin intravenous (VIGIV, 51%), and cidofovir (23%).
Nearly 1 in 3 patients (30%) received care in an ICU; 12 died (21%). Monkeypox was considered the cause or a contributing factor in five of the deaths and not a factor in one death; the remaining six deaths are under investigation.
Case studies
The report included details of three representative cases of the CDC consultations.
One was a Hispanic man in his 20s with a fever of 102.8° F, a rash including eschars, oral lesions, neck mass, and cervical lymphadenopathy. He had tested positive for monkeypox as an outpatient and upon admission was found to be HIV positive, with a CD4 count of 79 cells/mm3. He experienced a severe and ultimately fatal clinical course that included intubation, refractory hypotension, seizures, renal failure, and cardiac arrest. An autopsy revealed diffuse organ necrosis plus orthopoxvirus and cytomegalovirus.
The second was a Black man in his 30s with untreated AIDS and diffuse rash. He was tested and treated for gonorrhea, chlamydia, and syphilis before phimosis and urinary retention led to admission and a monkeypox diagnosis 4 weeks after his rash began. He was discharged with oral tecovirimat, but his skin lesions developed necrosis and he was readmitted twice, each time with new lesions. His clinical course included methicillin-resistant Staphylococcus aureus bacteremia, atrial fibrillation, eye and ear involvement, a suprapubic catheter, and progressive necrosis of his lesions. As of the CDC report, he was receiving ART and intravenous tecovirimat.
The third patient, a White man in his 40s with untreated AIDS, presented with diffuse rash. He was promptly diagnosed with monkeypox and admitted for pain control. He was discharged with oral tecovirimat and ART, but homelessness and food insecurity jeopardized the absorption of his tecovirimat (which depends on a full fatty meal), and the lesions worsened. Despite readmission and aggressive medical treatment, the patient required finger debridement and a toe amputation. After discharge, he was again readmitted for lesions and pain and, at report publication, remained hospitalized, taking oral tecovirimat and ART.
The patients in the study may not be typical of severe monkeypox cases, wrote the authors reported. Deaths after the study period were not counted.
Fewer cases, some severe
As of Nov. 7, the CDC has confirmed 28,709 monkeypox cases. These have trended downward since August. Most people with recent diagnoses are men who are gay, bisexual, same gender loving, or who have sex with men, and most are Black, according to Brooks.
Dr. Brooks urges clinicians to report suspected monkeypox cases – especially severe ones – to their health departments.
“We don’t have a good bead on exactly how many severe cases there are in the States because of complexities in our surveillance systems,” Dr. Brooks said.
For patients with suspected or confirmed monkeypox, Brooks recommends testing for sexually transmitted infections, including HIV if status is unknown. Patients with HIV should receive prompt ART. For those at risk for severe disease, the authors recommend early treatment for suspected monkeypox, even before results are back. Some patients may benefit from tecovirimat courses lasting beyond 14 days, plus additional antivirals (cidofovir or brincidofovir) and/or VIGIV.
“With severe cases, clinicians may want to consider the value of more than one drug to attack the virus at different stages of its replication cycle,” Dr. Brooks said.
Inequities matter
The authors called on providers to engage communities burdened by HIV and to ensure access to not only monkeypox vaccination, diagnosis, and treatment but also sustained HIV care.
Dr. Silvera added that providers need to tailor care plans to patients’ social determinants of health. For example, he explained, inpatient care for monkeypox could be appropriate for some patients facing homelessness and food insecurity – even if they are able to take tecovirimat orally.
He recommends tapping others’ expertise: “Our social work colleagues are well versed in this.”
“I don’t think these clinicians failed these patients. ... I think everyone made all the right choices medically,” Dr. Silvera added. “I think that the system failed these patients – and we as clinicians are part of those systems. So we also have the power to change those systems. And I think we just need to start opening our eyes to that and [start] to work together towards that goal to take better care of our patients.”
Dr. Brooks reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Monkeypox, though often mild, may be severe and even fatal in immunocompromised individuals, particularly those with untreated AIDS, according to a Centers for Disease Control and Prevention study in Morbidity and Mortality Weekly Report.
The study described a group of patients recently treated for severe monkeypox. The majority were Black, HIV positive, and not receiving treatment. Many were also facing homelessness.
The authors urged HIV testing for all sexually active individuals with suspected monkeypox. Early or prolonged monkeypox treatment may be necessary, they concluded.
Coauthor John T. Brooks, MD, called the study “a real call to action.”
“If we want to reduce cases of severe monkeypox, we need to reduce the number of persons with HIV who are undiagnosed and not treated,” said Dr. Brooks, a medical epidemiologist who is chief medical officer of CDC›s multinational monkeypox response. Dr. Brooks also leads the epidemiology research team in CDC’s division of HIV/AIDS prevention.
noted Richard Silvera, MD, MPH, CPH, who is associate program director of the infectious diseases fellowship and assistant professor of medicine (infectious diseases) at the Icahn School of Medicine at Mount Sinai, New York. He was not involved with the study.
“These patients really have not been served by the health care system,” Dr. Silvera said. “Monkeypox is just really taking advantage of that.”
How severe monkeypox can manifest
The authors reported on 57 adults hospitalized with severe monkeypox between Aug. 10 and Sept. 10, 2022, for whose care the providers sought CDC consultation.
The vast majority (95%) were men, their median age was 34 years, and 68% were Black. Nearly one in four were homeless (23%).
Overall, 47 (82%) were HIV positive, of whom just 4 had been receiving antiretroviral therapy (ART). Of 43 for whom CD4 counts were known, 71% had fewer than 50 CD4 cells/mm3.
Clinical signs included severe skin lesions in all patients and severe mucosal lesions in 68%. Other affected organ systems included lungs (21%), eyes (21%), and central nervous system (7%).
Treatments included oral or intravenous tecovirimat (93% and 65%, respectively), vaccinia immune globulin intravenous (VIGIV, 51%), and cidofovir (23%).
Nearly 1 in 3 patients (30%) received care in an ICU; 12 died (21%). Monkeypox was considered the cause or a contributing factor in five of the deaths and not a factor in one death; the remaining six deaths are under investigation.
Case studies
The report included details of three representative cases of the CDC consultations.
One was a Hispanic man in his 20s with a fever of 102.8° F, a rash including eschars, oral lesions, neck mass, and cervical lymphadenopathy. He had tested positive for monkeypox as an outpatient and upon admission was found to be HIV positive, with a CD4 count of 79 cells/mm3. He experienced a severe and ultimately fatal clinical course that included intubation, refractory hypotension, seizures, renal failure, and cardiac arrest. An autopsy revealed diffuse organ necrosis plus orthopoxvirus and cytomegalovirus.
The second was a Black man in his 30s with untreated AIDS and diffuse rash. He was tested and treated for gonorrhea, chlamydia, and syphilis before phimosis and urinary retention led to admission and a monkeypox diagnosis 4 weeks after his rash began. He was discharged with oral tecovirimat, but his skin lesions developed necrosis and he was readmitted twice, each time with new lesions. His clinical course included methicillin-resistant Staphylococcus aureus bacteremia, atrial fibrillation, eye and ear involvement, a suprapubic catheter, and progressive necrosis of his lesions. As of the CDC report, he was receiving ART and intravenous tecovirimat.
The third patient, a White man in his 40s with untreated AIDS, presented with diffuse rash. He was promptly diagnosed with monkeypox and admitted for pain control. He was discharged with oral tecovirimat and ART, but homelessness and food insecurity jeopardized the absorption of his tecovirimat (which depends on a full fatty meal), and the lesions worsened. Despite readmission and aggressive medical treatment, the patient required finger debridement and a toe amputation. After discharge, he was again readmitted for lesions and pain and, at report publication, remained hospitalized, taking oral tecovirimat and ART.
The patients in the study may not be typical of severe monkeypox cases, wrote the authors reported. Deaths after the study period were not counted.
Fewer cases, some severe
As of Nov. 7, the CDC has confirmed 28,709 monkeypox cases. These have trended downward since August. Most people with recent diagnoses are men who are gay, bisexual, same gender loving, or who have sex with men, and most are Black, according to Brooks.
Dr. Brooks urges clinicians to report suspected monkeypox cases – especially severe ones – to their health departments.
“We don’t have a good bead on exactly how many severe cases there are in the States because of complexities in our surveillance systems,” Dr. Brooks said.
For patients with suspected or confirmed monkeypox, Brooks recommends testing for sexually transmitted infections, including HIV if status is unknown. Patients with HIV should receive prompt ART. For those at risk for severe disease, the authors recommend early treatment for suspected monkeypox, even before results are back. Some patients may benefit from tecovirimat courses lasting beyond 14 days, plus additional antivirals (cidofovir or brincidofovir) and/or VIGIV.
“With severe cases, clinicians may want to consider the value of more than one drug to attack the virus at different stages of its replication cycle,” Dr. Brooks said.
Inequities matter
The authors called on providers to engage communities burdened by HIV and to ensure access to not only monkeypox vaccination, diagnosis, and treatment but also sustained HIV care.
Dr. Silvera added that providers need to tailor care plans to patients’ social determinants of health. For example, he explained, inpatient care for monkeypox could be appropriate for some patients facing homelessness and food insecurity – even if they are able to take tecovirimat orally.
He recommends tapping others’ expertise: “Our social work colleagues are well versed in this.”
“I don’t think these clinicians failed these patients. ... I think everyone made all the right choices medically,” Dr. Silvera added. “I think that the system failed these patients – and we as clinicians are part of those systems. So we also have the power to change those systems. And I think we just need to start opening our eyes to that and [start] to work together towards that goal to take better care of our patients.”
Dr. Brooks reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM THE MMWR
A Patient Presenting With Shortness of Breath, Fever, and Eosinophilia
A 70-year-old veteran with a history notable for type 2 diabetes mellitus, complicated by peripheral neuropathy and bilateral foot ulceration, and previous pulmonary tuberculosis (treated in June 2013) presented to an outside medical facility with bilateral worsening foot pain, swelling, and drainage of preexisting ulcers. He received a diagnosis of bilateral fifth toe osteomyelitis and was discharged with a 6-week course of IV daptomycin 600 mg (8 mg/kg) and ertapenem 1 g/d. At discharge, the patient was in stable condition. Follow-up was done by our outpatient parenteral antimicrobial therapy (OPAT) team, which consists of an infectious disease pharmacist and the physician director of antimicrobial stewardship who monitor veterans receiving outpatient IV antibiotic therapy.1
Three weeks later as part of the regular OPAT surveillance, the patient reported via telephone that his foot osteomyelitis was stable, but he had a 101 °F fever and a new cough. He was instructed to come to the emergency department (ED) immediately. On arrival,
- What is your diagnosis?
- How would you treat this patient?
In the ED, the patient was given a provisional diagnosis of multifocal bacterial pneumonia and was admitted to the hospital for further management. His outpatient regimen of IV daptomycin and ertapenem was adjusted to IV vancomycin and meropenem. The infectious disease service was consulted within 24 hours of admission, and based on the new onset chest infiltrates, therapy with daptomycin and notable peripheral blood eosinophilia, a presumptive diagnosis of daptomycin-related acute eosinophilic pneumonia was made. A medication list review yielded no other potential etiologic agents for drug-related eosinophilia, and the patient did not have any remote or recent pertinent travel history concerning for parasitic disease.
The patient was treated with oral prednisone 40 mg (0.5 mg/kg) daily and the daptomycin was not restarted. Within 24 hours, the patient’s fevers, oxygen requirements, and cough subsided. Laboratory values
Discussion
Daptomycin is a commonly used cyclic lipopeptide IV antibiotic with broad activity against gram-positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE). Daptomycin has emerged as a convenient alternative for infections typically treated with IV vancomycin: shorter infusion time (2-30 minutes vs 60-180 minutes), daily administration, and less need for dose adjustments. A recent survey reported higher satisfaction and less disruption in patients receiving daptomycin compared with vancomycin.2 The main daptomycin-specific adverse effect (AE) that warrants close monitoring is elevated creatine kinase (CK) levels and skeletal muscle breakdown (reversible after holding medication).3 Other rarely reported AEs include drug reaction with eosinophilia and systemic symptoms (DRESS), acute eosinophilic pneumonitis, hepatitis, and peripheral neuropathy.4-6 Consequently, weekly monitoring for this drug should include symptom inquiry for cough and muscle pain, and laboratory testing with CBC with differential, comprehensive metabolic panel (CMP), and CK.
Daptomycin-induced eosinophilic pneumonia has been described in several case reports and in a recent study, the frequency of this event was almost 5% in those receiving long-term daptomycin therapy.7 The most common symptoms include dyspnea, fever, infiltrates/opacities on chest imaging, and peripheral eosinophilia. It is theorized that the chemical structure of daptomycin causes immune-mediated pulmonary epithelial cell injury with eosinophils, resulting in increased peripheral eosinophilia.3 Risk factors that have been identified for daptomycin-induced eosinophilia include age > 70 years; the presence of comorbidities of heart and pulmonary disease; duration of daptomycin beyond 2 weeks; and cumulative doses over 10 g. Average onset of illness from initiation of daptomycin has been reported to be about 3 weeks.7,8 The diagnosis of daptomycin-induced eosinophilic pneumonitis is made on several criteria per the FDA. These include exposure to daptomycin, fever, dyspnea with oxygen requirement, new infiltrates on imaging, bronchoalveolar lavage with > 25% eosinophils, and last, clinical improvement on removal of the drug.9 However, as bronchoscopy is an invasive diagnostic modality, it is not always performed or necessary as seen in this case. Furthermore, not all patients will have peripheral eosinophilia, with only 77% of patients having that finding in a systematic review.10 Taken together, the overall true incidence of daptomycin-induced eosinophilia may be underestimated. Treatment involves discontinuation of the daptomycin and initiation of steroids. In a review of 35 cases, the majority did receive systemic steroids, usually 60 to 125 mg of IV methylprednisolone every 6 hours, which was converted to oral steroids and tapered over 2 to 6 weeks.10 However, all patients including those who did not receive steroids had symptom improvement or complete resolution, highlighting that prompt discontinuation of daptomycin is the most crucial intervention.
Conclusions
As home IV antibiotic therapy becomes increasingly used to facilitate shorter lengths of stay in hospitals and enable more patients to receive their infectious disease care at home, the general practitioner must be aware of the potential AEs of commonly used IV antibiotics. While acute cutaneous reactions and disturbances in renal and liver function are commonly recognized entities of adverse drug reactions, symptoms of fever and cough are more likely to be interpreted as acute viral or bacterial respiratory infections. A high index of clinical suspicion is needed for eosinophilic pneumonitis secondary to daptomycin. A simple and readily available test, such as a CBC with differential may facilitate the identification of this potentially serious AE, allowing prompt discontinuation of the drug.
1. Kent M, Kouma M, Jodlowski T, Cutrell JB. 755. Outpatient parenteral antimicrobial therapy program evaluation within a large Veterans Affairs healthcare system. Open Forum Infect Dis. 2019;6(suppl 2):S337. Published 2019 Oct 23. doi:10.1093/ofid/ofz360.823
2. Wu KH, Sakoulas G, Geriak M. Vancomycin or daptomycin for outpatient parenteral antibiotic therapy: does it make a difference in patient satisfaction? Open Forum Infect Dis. 2021;8(8):ofab418. Published 2021 Aug 30. doi:10.1093/ofid/ofab418
3. Gonzalez-Ruiz A, Seaton RA, Hamed K. Daptomycin: an evidence-based review of its role in the treatment of gram-positive infections. Infect Drug Resist. 2016;9:47-58. Published 2016 Apr 15. doi:10.2147/IDR.S99046
4. Sharifzadeh S, Mohammadpour AH, Tavanaee A, Elyasi S. Antibacterial antibiotic-induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: a literature review. Eur J Clin Pharmacol. 2021;77(3):275-289. doi:10.1007/s00228-020-03005-9
5. Mo Y, Nehring F, Jung AH, Housman ST. Possible hepatotoxicity associated with daptomycin: a case report and literature review. J Pharm Pract. 2016;29(3):253-256. doi:10.1177/0897190015625403
6. Villaverde Piñeiro L, Rabuñal Rey R, García Sabina A, Monte Secades R, García Pais MJ. Paralysis of the external popliteal sciatic nerve associated with daptomycin administration. J Clin Pharm Ther. 2018;43(4):578-580. doi:10.1111/jcpt.12666
7. Soldevila-Boixader L, Villanueva B, Ulldemolins M, et al. Risk factors of daptomycin-induced eosinophilic pneumonia in a population with osteoarticular infection. Antibiotics (Basel). 2021;10(4):446. Published 2021 Apr 16. doi:10.3390/antibiotics10040446
8. Kumar S, Acosta-Sanchez I, Rajagopalan N. Daptomycin-induced acute eosinophilic pneumonia. Cureus. 2018;10(6):e2899. Published 2018 Jun 30. doi:10.7759/cureus.2899
9. Center for Drug Evaluation and Research. Eosinophilic pneumonia associated with the use of cubicin. U.S. Food and Drug Administration. Updated August 3, 2017. Accessed October 10, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-eosinophilic-pneumonia-associated-use-cubicin-daptomycin
10. Uppal P, LaPlante KL, Gaitanis MM, Jankowich MD, Ward KE. Daptomycin-induced eosinophilic pneumonia—a systematic review. Antimicrob Resist Infect Control. 2016;5:55. Published 2016 Dec 12. doi:10.1186/s13756-016-0158-8
A 70-year-old veteran with a history notable for type 2 diabetes mellitus, complicated by peripheral neuropathy and bilateral foot ulceration, and previous pulmonary tuberculosis (treated in June 2013) presented to an outside medical facility with bilateral worsening foot pain, swelling, and drainage of preexisting ulcers. He received a diagnosis of bilateral fifth toe osteomyelitis and was discharged with a 6-week course of IV daptomycin 600 mg (8 mg/kg) and ertapenem 1 g/d. At discharge, the patient was in stable condition. Follow-up was done by our outpatient parenteral antimicrobial therapy (OPAT) team, which consists of an infectious disease pharmacist and the physician director of antimicrobial stewardship who monitor veterans receiving outpatient IV antibiotic therapy.1
Three weeks later as part of the regular OPAT surveillance, the patient reported via telephone that his foot osteomyelitis was stable, but he had a 101 °F fever and a new cough. He was instructed to come to the emergency department (ED) immediately. On arrival,
- What is your diagnosis?
- How would you treat this patient?

In the ED, the patient was given a provisional diagnosis of multifocal bacterial pneumonia and was admitted to the hospital for further management. His outpatient regimen of IV daptomycin and ertapenem was adjusted to IV vancomycin and meropenem. The infectious disease service was consulted within 24 hours of admission, and based on the new onset chest infiltrates, therapy with daptomycin and notable peripheral blood eosinophilia, a presumptive diagnosis of daptomycin-related acute eosinophilic pneumonia was made. A medication list review yielded no other potential etiologic agents for drug-related eosinophilia, and the patient did not have any remote or recent pertinent travel history concerning for parasitic disease.
The patient was treated with oral prednisone 40 mg (0.5 mg/kg) daily and the daptomycin was not restarted. Within 24 hours, the patient’s fevers, oxygen requirements, and cough subsided. Laboratory values
Discussion
Daptomycin is a commonly used cyclic lipopeptide IV antibiotic with broad activity against gram-positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE). Daptomycin has emerged as a convenient alternative for infections typically treated with IV vancomycin: shorter infusion time (2-30 minutes vs 60-180 minutes), daily administration, and less need for dose adjustments. A recent survey reported higher satisfaction and less disruption in patients receiving daptomycin compared with vancomycin.2 The main daptomycin-specific adverse effect (AE) that warrants close monitoring is elevated creatine kinase (CK) levels and skeletal muscle breakdown (reversible after holding medication).3 Other rarely reported AEs include drug reaction with eosinophilia and systemic symptoms (DRESS), acute eosinophilic pneumonitis, hepatitis, and peripheral neuropathy.4-6 Consequently, weekly monitoring for this drug should include symptom inquiry for cough and muscle pain, and laboratory testing with CBC with differential, comprehensive metabolic panel (CMP), and CK.
Daptomycin-induced eosinophilic pneumonia has been described in several case reports and in a recent study, the frequency of this event was almost 5% in those receiving long-term daptomycin therapy.7 The most common symptoms include dyspnea, fever, infiltrates/opacities on chest imaging, and peripheral eosinophilia. It is theorized that the chemical structure of daptomycin causes immune-mediated pulmonary epithelial cell injury with eosinophils, resulting in increased peripheral eosinophilia.3 Risk factors that have been identified for daptomycin-induced eosinophilia include age > 70 years; the presence of comorbidities of heart and pulmonary disease; duration of daptomycin beyond 2 weeks; and cumulative doses over 10 g. Average onset of illness from initiation of daptomycin has been reported to be about 3 weeks.7,8 The diagnosis of daptomycin-induced eosinophilic pneumonitis is made on several criteria per the FDA. These include exposure to daptomycin, fever, dyspnea with oxygen requirement, new infiltrates on imaging, bronchoalveolar lavage with > 25% eosinophils, and last, clinical improvement on removal of the drug.9 However, as bronchoscopy is an invasive diagnostic modality, it is not always performed or necessary as seen in this case. Furthermore, not all patients will have peripheral eosinophilia, with only 77% of patients having that finding in a systematic review.10 Taken together, the overall true incidence of daptomycin-induced eosinophilia may be underestimated. Treatment involves discontinuation of the daptomycin and initiation of steroids. In a review of 35 cases, the majority did receive systemic steroids, usually 60 to 125 mg of IV methylprednisolone every 6 hours, which was converted to oral steroids and tapered over 2 to 6 weeks.10 However, all patients including those who did not receive steroids had symptom improvement or complete resolution, highlighting that prompt discontinuation of daptomycin is the most crucial intervention.
Conclusions
As home IV antibiotic therapy becomes increasingly used to facilitate shorter lengths of stay in hospitals and enable more patients to receive their infectious disease care at home, the general practitioner must be aware of the potential AEs of commonly used IV antibiotics. While acute cutaneous reactions and disturbances in renal and liver function are commonly recognized entities of adverse drug reactions, symptoms of fever and cough are more likely to be interpreted as acute viral or bacterial respiratory infections. A high index of clinical suspicion is needed for eosinophilic pneumonitis secondary to daptomycin. A simple and readily available test, such as a CBC with differential may facilitate the identification of this potentially serious AE, allowing prompt discontinuation of the drug.
A 70-year-old veteran with a history notable for type 2 diabetes mellitus, complicated by peripheral neuropathy and bilateral foot ulceration, and previous pulmonary tuberculosis (treated in June 2013) presented to an outside medical facility with bilateral worsening foot pain, swelling, and drainage of preexisting ulcers. He received a diagnosis of bilateral fifth toe osteomyelitis and was discharged with a 6-week course of IV daptomycin 600 mg (8 mg/kg) and ertapenem 1 g/d. At discharge, the patient was in stable condition. Follow-up was done by our outpatient parenteral antimicrobial therapy (OPAT) team, which consists of an infectious disease pharmacist and the physician director of antimicrobial stewardship who monitor veterans receiving outpatient IV antibiotic therapy.1
Three weeks later as part of the regular OPAT surveillance, the patient reported via telephone that his foot osteomyelitis was stable, but he had a 101 °F fever and a new cough. He was instructed to come to the emergency department (ED) immediately. On arrival,
- What is your diagnosis?
- How would you treat this patient?

In the ED, the patient was given a provisional diagnosis of multifocal bacterial pneumonia and was admitted to the hospital for further management. His outpatient regimen of IV daptomycin and ertapenem was adjusted to IV vancomycin and meropenem. The infectious disease service was consulted within 24 hours of admission, and based on the new onset chest infiltrates, therapy with daptomycin and notable peripheral blood eosinophilia, a presumptive diagnosis of daptomycin-related acute eosinophilic pneumonia was made. A medication list review yielded no other potential etiologic agents for drug-related eosinophilia, and the patient did not have any remote or recent pertinent travel history concerning for parasitic disease.
The patient was treated with oral prednisone 40 mg (0.5 mg/kg) daily and the daptomycin was not restarted. Within 24 hours, the patient’s fevers, oxygen requirements, and cough subsided. Laboratory values
Discussion
Daptomycin is a commonly used cyclic lipopeptide IV antibiotic with broad activity against gram-positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE). Daptomycin has emerged as a convenient alternative for infections typically treated with IV vancomycin: shorter infusion time (2-30 minutes vs 60-180 minutes), daily administration, and less need for dose adjustments. A recent survey reported higher satisfaction and less disruption in patients receiving daptomycin compared with vancomycin.2 The main daptomycin-specific adverse effect (AE) that warrants close monitoring is elevated creatine kinase (CK) levels and skeletal muscle breakdown (reversible after holding medication).3 Other rarely reported AEs include drug reaction with eosinophilia and systemic symptoms (DRESS), acute eosinophilic pneumonitis, hepatitis, and peripheral neuropathy.4-6 Consequently, weekly monitoring for this drug should include symptom inquiry for cough and muscle pain, and laboratory testing with CBC with differential, comprehensive metabolic panel (CMP), and CK.
Daptomycin-induced eosinophilic pneumonia has been described in several case reports and in a recent study, the frequency of this event was almost 5% in those receiving long-term daptomycin therapy.7 The most common symptoms include dyspnea, fever, infiltrates/opacities on chest imaging, and peripheral eosinophilia. It is theorized that the chemical structure of daptomycin causes immune-mediated pulmonary epithelial cell injury with eosinophils, resulting in increased peripheral eosinophilia.3 Risk factors that have been identified for daptomycin-induced eosinophilia include age > 70 years; the presence of comorbidities of heart and pulmonary disease; duration of daptomycin beyond 2 weeks; and cumulative doses over 10 g. Average onset of illness from initiation of daptomycin has been reported to be about 3 weeks.7,8 The diagnosis of daptomycin-induced eosinophilic pneumonitis is made on several criteria per the FDA. These include exposure to daptomycin, fever, dyspnea with oxygen requirement, new infiltrates on imaging, bronchoalveolar lavage with > 25% eosinophils, and last, clinical improvement on removal of the drug.9 However, as bronchoscopy is an invasive diagnostic modality, it is not always performed or necessary as seen in this case. Furthermore, not all patients will have peripheral eosinophilia, with only 77% of patients having that finding in a systematic review.10 Taken together, the overall true incidence of daptomycin-induced eosinophilia may be underestimated. Treatment involves discontinuation of the daptomycin and initiation of steroids. In a review of 35 cases, the majority did receive systemic steroids, usually 60 to 125 mg of IV methylprednisolone every 6 hours, which was converted to oral steroids and tapered over 2 to 6 weeks.10 However, all patients including those who did not receive steroids had symptom improvement or complete resolution, highlighting that prompt discontinuation of daptomycin is the most crucial intervention.
Conclusions
As home IV antibiotic therapy becomes increasingly used to facilitate shorter lengths of stay in hospitals and enable more patients to receive their infectious disease care at home, the general practitioner must be aware of the potential AEs of commonly used IV antibiotics. While acute cutaneous reactions and disturbances in renal and liver function are commonly recognized entities of adverse drug reactions, symptoms of fever and cough are more likely to be interpreted as acute viral or bacterial respiratory infections. A high index of clinical suspicion is needed for eosinophilic pneumonitis secondary to daptomycin. A simple and readily available test, such as a CBC with differential may facilitate the identification of this potentially serious AE, allowing prompt discontinuation of the drug.
1. Kent M, Kouma M, Jodlowski T, Cutrell JB. 755. Outpatient parenteral antimicrobial therapy program evaluation within a large Veterans Affairs healthcare system. Open Forum Infect Dis. 2019;6(suppl 2):S337. Published 2019 Oct 23. doi:10.1093/ofid/ofz360.823
2. Wu KH, Sakoulas G, Geriak M. Vancomycin or daptomycin for outpatient parenteral antibiotic therapy: does it make a difference in patient satisfaction? Open Forum Infect Dis. 2021;8(8):ofab418. Published 2021 Aug 30. doi:10.1093/ofid/ofab418
3. Gonzalez-Ruiz A, Seaton RA, Hamed K. Daptomycin: an evidence-based review of its role in the treatment of gram-positive infections. Infect Drug Resist. 2016;9:47-58. Published 2016 Apr 15. doi:10.2147/IDR.S99046
4. Sharifzadeh S, Mohammadpour AH, Tavanaee A, Elyasi S. Antibacterial antibiotic-induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: a literature review. Eur J Clin Pharmacol. 2021;77(3):275-289. doi:10.1007/s00228-020-03005-9
5. Mo Y, Nehring F, Jung AH, Housman ST. Possible hepatotoxicity associated with daptomycin: a case report and literature review. J Pharm Pract. 2016;29(3):253-256. doi:10.1177/0897190015625403
6. Villaverde Piñeiro L, Rabuñal Rey R, García Sabina A, Monte Secades R, García Pais MJ. Paralysis of the external popliteal sciatic nerve associated with daptomycin administration. J Clin Pharm Ther. 2018;43(4):578-580. doi:10.1111/jcpt.12666
7. Soldevila-Boixader L, Villanueva B, Ulldemolins M, et al. Risk factors of daptomycin-induced eosinophilic pneumonia in a population with osteoarticular infection. Antibiotics (Basel). 2021;10(4):446. Published 2021 Apr 16. doi:10.3390/antibiotics10040446
8. Kumar S, Acosta-Sanchez I, Rajagopalan N. Daptomycin-induced acute eosinophilic pneumonia. Cureus. 2018;10(6):e2899. Published 2018 Jun 30. doi:10.7759/cureus.2899
9. Center for Drug Evaluation and Research. Eosinophilic pneumonia associated with the use of cubicin. U.S. Food and Drug Administration. Updated August 3, 2017. Accessed October 10, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-eosinophilic-pneumonia-associated-use-cubicin-daptomycin
10. Uppal P, LaPlante KL, Gaitanis MM, Jankowich MD, Ward KE. Daptomycin-induced eosinophilic pneumonia—a systematic review. Antimicrob Resist Infect Control. 2016;5:55. Published 2016 Dec 12. doi:10.1186/s13756-016-0158-8
1. Kent M, Kouma M, Jodlowski T, Cutrell JB. 755. Outpatient parenteral antimicrobial therapy program evaluation within a large Veterans Affairs healthcare system. Open Forum Infect Dis. 2019;6(suppl 2):S337. Published 2019 Oct 23. doi:10.1093/ofid/ofz360.823
2. Wu KH, Sakoulas G, Geriak M. Vancomycin or daptomycin for outpatient parenteral antibiotic therapy: does it make a difference in patient satisfaction? Open Forum Infect Dis. 2021;8(8):ofab418. Published 2021 Aug 30. doi:10.1093/ofid/ofab418
3. Gonzalez-Ruiz A, Seaton RA, Hamed K. Daptomycin: an evidence-based review of its role in the treatment of gram-positive infections. Infect Drug Resist. 2016;9:47-58. Published 2016 Apr 15. doi:10.2147/IDR.S99046
4. Sharifzadeh S, Mohammadpour AH, Tavanaee A, Elyasi S. Antibacterial antibiotic-induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: a literature review. Eur J Clin Pharmacol. 2021;77(3):275-289. doi:10.1007/s00228-020-03005-9
5. Mo Y, Nehring F, Jung AH, Housman ST. Possible hepatotoxicity associated with daptomycin: a case report and literature review. J Pharm Pract. 2016;29(3):253-256. doi:10.1177/0897190015625403
6. Villaverde Piñeiro L, Rabuñal Rey R, García Sabina A, Monte Secades R, García Pais MJ. Paralysis of the external popliteal sciatic nerve associated with daptomycin administration. J Clin Pharm Ther. 2018;43(4):578-580. doi:10.1111/jcpt.12666
7. Soldevila-Boixader L, Villanueva B, Ulldemolins M, et al. Risk factors of daptomycin-induced eosinophilic pneumonia in a population with osteoarticular infection. Antibiotics (Basel). 2021;10(4):446. Published 2021 Apr 16. doi:10.3390/antibiotics10040446
8. Kumar S, Acosta-Sanchez I, Rajagopalan N. Daptomycin-induced acute eosinophilic pneumonia. Cureus. 2018;10(6):e2899. Published 2018 Jun 30. doi:10.7759/cureus.2899
9. Center for Drug Evaluation and Research. Eosinophilic pneumonia associated with the use of cubicin. U.S. Food and Drug Administration. Updated August 3, 2017. Accessed October 10, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-eosinophilic-pneumonia-associated-use-cubicin-daptomycin
10. Uppal P, LaPlante KL, Gaitanis MM, Jankowich MD, Ward KE. Daptomycin-induced eosinophilic pneumonia—a systematic review. Antimicrob Resist Infect Control. 2016;5:55. Published 2016 Dec 12. doi:10.1186/s13756-016-0158-8
Medicaid coverage of HPV vaccine in adults: Implications in dermatology
, according to the authors of a review of Medicaid policies across all 50 states.
The human papillomavirus (HPV) vaccine is approved for people aged 9-45 years, for preventing genital, cervical, anal, and oropharyngeal cancers, and genital warts. And the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices recommends routine vaccination with the HPV vaccine for individuals aged 9-26 years, with “shared clinical decision-making” recommended for vaccination of those aged 27-45 years, wrote Nathaniel Goldman of New York Medical College, Valhalla, and coauthors, from the University of Missouri–Kansas City and Harvard Medical School, Boston.
A total of 33 states offered formal statewide Medicaid coverage policies that were accessible online or through the state’s Medicaid office. Another 11 states provided coverage through Medicaid managed care organizations, and 4 states had HPV vaccination as part of their formal Medicaid adult vaccination programs.
Overall, 43 states covered HPV vaccination through age 45 years with no need for prior authorization, and another 4 states (Ohio, Maine, Nebraska, and New York) provided coverage with prior authorization for adults older than 26 years.
The study findings were limited by the use of Medicaid coverage only, the researchers noted. Consequently, patients eligible for HPV vaccination who are uninsured or have other types of insurance may face additional barriers in the form of high costs, given that the current retail price is $250-$350 per shot for the three-shot series, the researchers noted.
However, the results suggest that Medicaid coverage for HPV vaccination may inform dermatologists’ recommendations for patients at increased risk, they said. More research is needed to “better identify dermatology patients at risk for new HPV infection and ways to improve vaccination rates in these vulnerable individuals,” they added.
Vaccine discussions are important in dermatology
“Dermatologists care for patients who may be an increased risk of vaccine-preventable illnesses, either from a skin disease or a dermatology medication,” corresponding author Megan H. Noe, MD, a dermatologist at Brigham and Women’s Hospital, and assistant professor of dermatology, Harvard Medical School, Boston, said in an interview. “Over the last several years, we have seen that all physicians, whether they provide vaccinations or not, can play an important role in discussing vaccines with their patients,” she said.
“Vaccines can be cost-prohibitive for patients without insurance coverage, so we hope that dermatologists will be more likely to recommend the HPV vaccine to patients 27-45 years of age if they know that it is likely covered by insurance,” Dr. Noe noted.
However, “time may be a barrier for many dermatologists who have many important things to discuss with patients during their appointments,” she said. “We are currently working on developing educational information to help facilitate this conversation,” she added.
Looking ahead, she said that “additional research is necessary to create vaccine guidelines specific to dermatology patients and dermatology medications, so we can provide clear recommendations to our patients and ensure appropriate insurance coverage for all necessary vaccines.”
Vaccine discussions
“I think it’s great that many Medicaid plans are covering HPV vaccination,” said Karl Saardi, MD, of the department of dermatology, George Washington University, Washington, who was asked to comment on the study. “I routinely recommend [vaccination] for patients who have viral warts, since it does lead to improvement in some cases,” Dr. Saardi, who was not involved in the current study, said in an interview. “Although we don’t have the HPV vaccines in our clinic for administration, my experience has been that patients are very open to discussing it with their primary care doctors.”
Although the upper age range continues to rise, “I think getting younger people vaccinated will also prove to be important,” said Dr. Saardi, director of the inpatient dermatology service at the George Washington University Hospital.
The point made in the current study about the importance of HPV vaccination in patients with hidradenitis suppurativa is also crucial, he added. “Since chronic skin inflammation in hidradenitis drives squamous cell carcinoma, reducing the impact of HPV on such cancers makes perfect sense.”
The study received no outside funding. Dr. Noe disclosed grants from Boehringer Ingelheim unrelated to the current study. Dr. Saardi had no financial conflicts to disclose.
, according to the authors of a review of Medicaid policies across all 50 states.
The human papillomavirus (HPV) vaccine is approved for people aged 9-45 years, for preventing genital, cervical, anal, and oropharyngeal cancers, and genital warts. And the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices recommends routine vaccination with the HPV vaccine for individuals aged 9-26 years, with “shared clinical decision-making” recommended for vaccination of those aged 27-45 years, wrote Nathaniel Goldman of New York Medical College, Valhalla, and coauthors, from the University of Missouri–Kansas City and Harvard Medical School, Boston.
A total of 33 states offered formal statewide Medicaid coverage policies that were accessible online or through the state’s Medicaid office. Another 11 states provided coverage through Medicaid managed care organizations, and 4 states had HPV vaccination as part of their formal Medicaid adult vaccination programs.
Overall, 43 states covered HPV vaccination through age 45 years with no need for prior authorization, and another 4 states (Ohio, Maine, Nebraska, and New York) provided coverage with prior authorization for adults older than 26 years.
The study findings were limited by the use of Medicaid coverage only, the researchers noted. Consequently, patients eligible for HPV vaccination who are uninsured or have other types of insurance may face additional barriers in the form of high costs, given that the current retail price is $250-$350 per shot for the three-shot series, the researchers noted.
However, the results suggest that Medicaid coverage for HPV vaccination may inform dermatologists’ recommendations for patients at increased risk, they said. More research is needed to “better identify dermatology patients at risk for new HPV infection and ways to improve vaccination rates in these vulnerable individuals,” they added.
Vaccine discussions are important in dermatology
“Dermatologists care for patients who may be an increased risk of vaccine-preventable illnesses, either from a skin disease or a dermatology medication,” corresponding author Megan H. Noe, MD, a dermatologist at Brigham and Women’s Hospital, and assistant professor of dermatology, Harvard Medical School, Boston, said in an interview. “Over the last several years, we have seen that all physicians, whether they provide vaccinations or not, can play an important role in discussing vaccines with their patients,” she said.
“Vaccines can be cost-prohibitive for patients without insurance coverage, so we hope that dermatologists will be more likely to recommend the HPV vaccine to patients 27-45 years of age if they know that it is likely covered by insurance,” Dr. Noe noted.
However, “time may be a barrier for many dermatologists who have many important things to discuss with patients during their appointments,” she said. “We are currently working on developing educational information to help facilitate this conversation,” she added.
Looking ahead, she said that “additional research is necessary to create vaccine guidelines specific to dermatology patients and dermatology medications, so we can provide clear recommendations to our patients and ensure appropriate insurance coverage for all necessary vaccines.”
Vaccine discussions
“I think it’s great that many Medicaid plans are covering HPV vaccination,” said Karl Saardi, MD, of the department of dermatology, George Washington University, Washington, who was asked to comment on the study. “I routinely recommend [vaccination] for patients who have viral warts, since it does lead to improvement in some cases,” Dr. Saardi, who was not involved in the current study, said in an interview. “Although we don’t have the HPV vaccines in our clinic for administration, my experience has been that patients are very open to discussing it with their primary care doctors.”
Although the upper age range continues to rise, “I think getting younger people vaccinated will also prove to be important,” said Dr. Saardi, director of the inpatient dermatology service at the George Washington University Hospital.
The point made in the current study about the importance of HPV vaccination in patients with hidradenitis suppurativa is also crucial, he added. “Since chronic skin inflammation in hidradenitis drives squamous cell carcinoma, reducing the impact of HPV on such cancers makes perfect sense.”
The study received no outside funding. Dr. Noe disclosed grants from Boehringer Ingelheim unrelated to the current study. Dr. Saardi had no financial conflicts to disclose.
, according to the authors of a review of Medicaid policies across all 50 states.
The human papillomavirus (HPV) vaccine is approved for people aged 9-45 years, for preventing genital, cervical, anal, and oropharyngeal cancers, and genital warts. And the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices recommends routine vaccination with the HPV vaccine for individuals aged 9-26 years, with “shared clinical decision-making” recommended for vaccination of those aged 27-45 years, wrote Nathaniel Goldman of New York Medical College, Valhalla, and coauthors, from the University of Missouri–Kansas City and Harvard Medical School, Boston.
A total of 33 states offered formal statewide Medicaid coverage policies that were accessible online or through the state’s Medicaid office. Another 11 states provided coverage through Medicaid managed care organizations, and 4 states had HPV vaccination as part of their formal Medicaid adult vaccination programs.
Overall, 43 states covered HPV vaccination through age 45 years with no need for prior authorization, and another 4 states (Ohio, Maine, Nebraska, and New York) provided coverage with prior authorization for adults older than 26 years.
The study findings were limited by the use of Medicaid coverage only, the researchers noted. Consequently, patients eligible for HPV vaccination who are uninsured or have other types of insurance may face additional barriers in the form of high costs, given that the current retail price is $250-$350 per shot for the three-shot series, the researchers noted.
However, the results suggest that Medicaid coverage for HPV vaccination may inform dermatologists’ recommendations for patients at increased risk, they said. More research is needed to “better identify dermatology patients at risk for new HPV infection and ways to improve vaccination rates in these vulnerable individuals,” they added.
Vaccine discussions are important in dermatology
“Dermatologists care for patients who may be an increased risk of vaccine-preventable illnesses, either from a skin disease or a dermatology medication,” corresponding author Megan H. Noe, MD, a dermatologist at Brigham and Women’s Hospital, and assistant professor of dermatology, Harvard Medical School, Boston, said in an interview. “Over the last several years, we have seen that all physicians, whether they provide vaccinations or not, can play an important role in discussing vaccines with their patients,” she said.
“Vaccines can be cost-prohibitive for patients without insurance coverage, so we hope that dermatologists will be more likely to recommend the HPV vaccine to patients 27-45 years of age if they know that it is likely covered by insurance,” Dr. Noe noted.
However, “time may be a barrier for many dermatologists who have many important things to discuss with patients during their appointments,” she said. “We are currently working on developing educational information to help facilitate this conversation,” she added.
Looking ahead, she said that “additional research is necessary to create vaccine guidelines specific to dermatology patients and dermatology medications, so we can provide clear recommendations to our patients and ensure appropriate insurance coverage for all necessary vaccines.”
Vaccine discussions
“I think it’s great that many Medicaid plans are covering HPV vaccination,” said Karl Saardi, MD, of the department of dermatology, George Washington University, Washington, who was asked to comment on the study. “I routinely recommend [vaccination] for patients who have viral warts, since it does lead to improvement in some cases,” Dr. Saardi, who was not involved in the current study, said in an interview. “Although we don’t have the HPV vaccines in our clinic for administration, my experience has been that patients are very open to discussing it with their primary care doctors.”
Although the upper age range continues to rise, “I think getting younger people vaccinated will also prove to be important,” said Dr. Saardi, director of the inpatient dermatology service at the George Washington University Hospital.
The point made in the current study about the importance of HPV vaccination in patients with hidradenitis suppurativa is also crucial, he added. “Since chronic skin inflammation in hidradenitis drives squamous cell carcinoma, reducing the impact of HPV on such cancers makes perfect sense.”
The study received no outside funding. Dr. Noe disclosed grants from Boehringer Ingelheim unrelated to the current study. Dr. Saardi had no financial conflicts to disclose.
FROM JAMA DERMATOLOGY
Leukocytoclastic Vasculitis Masquerading as Chronic ITP
Idiopathic thrombocytopenic purpura (ITP) is an immune-mediated acquired condition affecting both adults and children.1 Acute ITP is the most common form, which happens in the presence of a precipitant, leading to a drop in platelet counts. However, chronic ITP can occur when all the causes that might precipitate thrombocytopenia have been ruled out, and it is persistent for ≥ 12 months.2 Its presence can mask other diseases that exhibit somewhat similar signs and symptoms. We present a case of a patient presenting with chronic ITP with diffuse rash and was later diagnosed with idiopathic leukocytoclastic vasculitis (LCV).
Case Presentation
A 79-year-old presented to the hospital with 2-day history of a rash. The rash was purpureal and petechial and located on the trunk and bilateral upper and lower extremities. The rash was associated with itchiness and pain in the wrists, ankles, and small joints of the hands. The patient reported no changes in medication or diet,
The patient mentioned that at the time of diagnosis the platelet count was about 90,000 but had been fluctuating between 50 and 60,000 recently. The patient also reported no history of gum bleeding, nosebleeds, hemoptysis, hematemesis, or any miscarriages. She also had difficulty voiding for 2 to 3 days but no dysuria, frequency, urgency, or incontinence.
Laboratory results were significant for 57,000/µL platelet count (normal range, 150,000-450,000), elevated d-dimer (6.07), < 6 mg/dL C4 (normal range, 88-201). Hemoglobin level, coagulation panel, hemolytic panel, and fibrinogen level results were unremarkable. The hepatitis panel, Lyme disease, and HIV test were negative. The peripheral blood smear showed moderate thrombocytopenia, mild monocytosis, and borderline normochromic normocytic anemia without schistocytes. The autoimmune panel to evaluate thrombocytopenia showed platelet antibody against glycoprotein (GP) IIb/IIIa, GP Ib/Ix, GP Ia/IIa, suggestive toward a diagnosis of chronic idiopathic ITP. However, the skin biopsy of the rash was indicative of LCV.
An autoimmune panel for vasculitis, including antinuclear antibody and antidouble-stranded DNA, was negative. While in the hospital, the patient completed the course of ciprofloxacin for the UTI, the rash started to fade without any intervention, and the platelet count improved to 69,000/µL. The patient was discharged after 3 days with the recommendation to follow up with her hematologist.
Discussion
LCV is a small vessel vasculitis of the dermal capillaries and venules. Histologically, LCV is characterized by fibrinoid necrosis of the vessel wall with frequent neutrophils, nuclear dust, and extravasated erythrocytes.3
Although a thorough evaluation is recommended to determine etiology, about 50% of cases are idiopathic. The most common precipitants are acute infection or a new medication. Postinfectious LCV is most commonly seen after streptococcal upper respiratory tract infection. Among other infectious triggers, Mycobacterium, Staphylococcus aureus, chlamydia, Neisseria, HIV, hepatitis B, hepatitis C, and syphilis are noteworthy. Foods, autoimmune disease, collagen vascular disease, and malignancy are also associated with LCV.4
In our patient we could not find any specific identifiable triggers. However, the presence of a UTI as a precipitating factor cannot be ruled out.5 Moreover, the patient received ciprofloxacin and there have been several case reports of LCV associated with use of a fluroquinolone.6 Nevertheless, in the presence of chronic ITP, which also is an auto-immune condition, an idiopathic cause seemed a reasonable explanation for the patient’s etiopathogenesis.
The cutaneous manifestations of LCV may appear about 1 to 3 weeks after the triggering event if present. The major clinical findings include palpable purpura and/or petechiae that are nonblanching. These findings can easily be confused with other diagnoses especially in the presence of a similar preexisting diagnosis. For example, our patient already had chronic ITP, and in such circumstances, a diagnosis of superimposed LCV can be easily missed without a thorough investigation. Extracutaneous manifestations with LCV are less common. Systemic symptoms may include low-grade fevers, malaise, weight loss, myalgia, and arthralgia. These findings have been noted in about 30% of affected patients, with arthralgia the most common manifestation.7 Our patient also presented with pain involving multiple joints.
The mainstay of diagnosis for LCV is a skin biopsy with direct immunofluorescence. However, a workup for an underlying condition should be considered based on clinical suspicion. If a secondary cause is found, management should target treating the underlying cause, including withdrawal of the offending drug, treatment or control of the underlying infection, malignancy, or connective tissue disease. Most cases of idiopathic cutaneous LCV resolve with supportive measures, including leg elevation, rest, compression stockings, and antihistamines. In resistant cases, a 4- to 6-week tapering dose of corticosteroids and immunosuppressive steroid-sparing agents may be needed.8
Conclusions
Although most cases of LCV are mild and resolve without intervention, many cases go undiagnosed due to a delay in performing a biopsy. However, we should always look for the root cause of a patient’s condition to rule out underlying contributing conditions. Differentiating LCV from any other preexisting condition presenting similarly is important.
1. Gaurav K, Keith RM. Immune thrombocytopenia. Hematol Oncol Clin North Am. 2013;27(3): 495-520. doi:10.1016/j.hoc.2013.03.001
2. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386-2393.
3. James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. Saunders/Elsevier; 2011.
4. Einhorn J, Levis JT. Dermatologic diagnosis: leukocytoclastic vasculitis. Perm J. 2015;19(3):77-78. doi:10.7812/TPP/15-001
5. The role of infectious agents in the pathogenesis of vasculitis. Nicolò P, Carlo S. Best Pract Res Clin Rheumatol. 2008;22(5):897-911. doi:10.7812/TPP/15-001
6. Maunz G, Conzett T, Zimmerli W. Cutaneous vasculitis associated with fluoroquinolones. Infection. 2009;37(5):466-468. doi:10.1007/s15010-009-8437-4
7. Baigrie D, Goyal A, Crane J.C. Leukocytoclastic vasculitis. StatPearls [internet]. Updated May 8, 2022. Accessed October 10, 2022. https://www.ncbi.nlm.nih.gov/books/NBK482159
8. Micheletti RG, Pagnoux C. Management of cutaneous vasculitis. Presse Med. 2020; 49(3):104033. doi:10.1016/j.lpm.2020.104033
Idiopathic thrombocytopenic purpura (ITP) is an immune-mediated acquired condition affecting both adults and children.1 Acute ITP is the most common form, which happens in the presence of a precipitant, leading to a drop in platelet counts. However, chronic ITP can occur when all the causes that might precipitate thrombocytopenia have been ruled out, and it is persistent for ≥ 12 months.2 Its presence can mask other diseases that exhibit somewhat similar signs and symptoms. We present a case of a patient presenting with chronic ITP with diffuse rash and was later diagnosed with idiopathic leukocytoclastic vasculitis (LCV).
Case Presentation
A 79-year-old presented to the hospital with 2-day history of a rash. The rash was purpureal and petechial and located on the trunk and bilateral upper and lower extremities. The rash was associated with itchiness and pain in the wrists, ankles, and small joints of the hands. The patient reported no changes in medication or diet,
The patient mentioned that at the time of diagnosis the platelet count was about 90,000 but had been fluctuating between 50 and 60,000 recently. The patient also reported no history of gum bleeding, nosebleeds, hemoptysis, hematemesis, or any miscarriages. She also had difficulty voiding for 2 to 3 days but no dysuria, frequency, urgency, or incontinence.
Laboratory results were significant for 57,000/µL platelet count (normal range, 150,000-450,000), elevated d-dimer (6.07), < 6 mg/dL C4 (normal range, 88-201). Hemoglobin level, coagulation panel, hemolytic panel, and fibrinogen level results were unremarkable. The hepatitis panel, Lyme disease, and HIV test were negative. The peripheral blood smear showed moderate thrombocytopenia, mild monocytosis, and borderline normochromic normocytic anemia without schistocytes. The autoimmune panel to evaluate thrombocytopenia showed platelet antibody against glycoprotein (GP) IIb/IIIa, GP Ib/Ix, GP Ia/IIa, suggestive toward a diagnosis of chronic idiopathic ITP. However, the skin biopsy of the rash was indicative of LCV.
An autoimmune panel for vasculitis, including antinuclear antibody and antidouble-stranded DNA, was negative. While in the hospital, the patient completed the course of ciprofloxacin for the UTI, the rash started to fade without any intervention, and the platelet count improved to 69,000/µL. The patient was discharged after 3 days with the recommendation to follow up with her hematologist.
Discussion
LCV is a small vessel vasculitis of the dermal capillaries and venules. Histologically, LCV is characterized by fibrinoid necrosis of the vessel wall with frequent neutrophils, nuclear dust, and extravasated erythrocytes.3
Although a thorough evaluation is recommended to determine etiology, about 50% of cases are idiopathic. The most common precipitants are acute infection or a new medication. Postinfectious LCV is most commonly seen after streptococcal upper respiratory tract infection. Among other infectious triggers, Mycobacterium, Staphylococcus aureus, chlamydia, Neisseria, HIV, hepatitis B, hepatitis C, and syphilis are noteworthy. Foods, autoimmune disease, collagen vascular disease, and malignancy are also associated with LCV.4
In our patient we could not find any specific identifiable triggers. However, the presence of a UTI as a precipitating factor cannot be ruled out.5 Moreover, the patient received ciprofloxacin and there have been several case reports of LCV associated with use of a fluroquinolone.6 Nevertheless, in the presence of chronic ITP, which also is an auto-immune condition, an idiopathic cause seemed a reasonable explanation for the patient’s etiopathogenesis.
The cutaneous manifestations of LCV may appear about 1 to 3 weeks after the triggering event if present. The major clinical findings include palpable purpura and/or petechiae that are nonblanching. These findings can easily be confused with other diagnoses especially in the presence of a similar preexisting diagnosis. For example, our patient already had chronic ITP, and in such circumstances, a diagnosis of superimposed LCV can be easily missed without a thorough investigation. Extracutaneous manifestations with LCV are less common. Systemic symptoms may include low-grade fevers, malaise, weight loss, myalgia, and arthralgia. These findings have been noted in about 30% of affected patients, with arthralgia the most common manifestation.7 Our patient also presented with pain involving multiple joints.
The mainstay of diagnosis for LCV is a skin biopsy with direct immunofluorescence. However, a workup for an underlying condition should be considered based on clinical suspicion. If a secondary cause is found, management should target treating the underlying cause, including withdrawal of the offending drug, treatment or control of the underlying infection, malignancy, or connective tissue disease. Most cases of idiopathic cutaneous LCV resolve with supportive measures, including leg elevation, rest, compression stockings, and antihistamines. In resistant cases, a 4- to 6-week tapering dose of corticosteroids and immunosuppressive steroid-sparing agents may be needed.8
Conclusions
Although most cases of LCV are mild and resolve without intervention, many cases go undiagnosed due to a delay in performing a biopsy. However, we should always look for the root cause of a patient’s condition to rule out underlying contributing conditions. Differentiating LCV from any other preexisting condition presenting similarly is important.
Idiopathic thrombocytopenic purpura (ITP) is an immune-mediated acquired condition affecting both adults and children.1 Acute ITP is the most common form, which happens in the presence of a precipitant, leading to a drop in platelet counts. However, chronic ITP can occur when all the causes that might precipitate thrombocytopenia have been ruled out, and it is persistent for ≥ 12 months.2 Its presence can mask other diseases that exhibit somewhat similar signs and symptoms. We present a case of a patient presenting with chronic ITP with diffuse rash and was later diagnosed with idiopathic leukocytoclastic vasculitis (LCV).
Case Presentation
A 79-year-old presented to the hospital with 2-day history of a rash. The rash was purpureal and petechial and located on the trunk and bilateral upper and lower extremities. The rash was associated with itchiness and pain in the wrists, ankles, and small joints of the hands. The patient reported no changes in medication or diet,
The patient mentioned that at the time of diagnosis the platelet count was about 90,000 but had been fluctuating between 50 and 60,000 recently. The patient also reported no history of gum bleeding, nosebleeds, hemoptysis, hematemesis, or any miscarriages. She also had difficulty voiding for 2 to 3 days but no dysuria, frequency, urgency, or incontinence.
Laboratory results were significant for 57,000/µL platelet count (normal range, 150,000-450,000), elevated d-dimer (6.07), < 6 mg/dL C4 (normal range, 88-201). Hemoglobin level, coagulation panel, hemolytic panel, and fibrinogen level results were unremarkable. The hepatitis panel, Lyme disease, and HIV test were negative. The peripheral blood smear showed moderate thrombocytopenia, mild monocytosis, and borderline normochromic normocytic anemia without schistocytes. The autoimmune panel to evaluate thrombocytopenia showed platelet antibody against glycoprotein (GP) IIb/IIIa, GP Ib/Ix, GP Ia/IIa, suggestive toward a diagnosis of chronic idiopathic ITP. However, the skin biopsy of the rash was indicative of LCV.
An autoimmune panel for vasculitis, including antinuclear antibody and antidouble-stranded DNA, was negative. While in the hospital, the patient completed the course of ciprofloxacin for the UTI, the rash started to fade without any intervention, and the platelet count improved to 69,000/µL. The patient was discharged after 3 days with the recommendation to follow up with her hematologist.
Discussion
LCV is a small vessel vasculitis of the dermal capillaries and venules. Histologically, LCV is characterized by fibrinoid necrosis of the vessel wall with frequent neutrophils, nuclear dust, and extravasated erythrocytes.3
Although a thorough evaluation is recommended to determine etiology, about 50% of cases are idiopathic. The most common precipitants are acute infection or a new medication. Postinfectious LCV is most commonly seen after streptococcal upper respiratory tract infection. Among other infectious triggers, Mycobacterium, Staphylococcus aureus, chlamydia, Neisseria, HIV, hepatitis B, hepatitis C, and syphilis are noteworthy. Foods, autoimmune disease, collagen vascular disease, and malignancy are also associated with LCV.4
In our patient we could not find any specific identifiable triggers. However, the presence of a UTI as a precipitating factor cannot be ruled out.5 Moreover, the patient received ciprofloxacin and there have been several case reports of LCV associated with use of a fluroquinolone.6 Nevertheless, in the presence of chronic ITP, which also is an auto-immune condition, an idiopathic cause seemed a reasonable explanation for the patient’s etiopathogenesis.
The cutaneous manifestations of LCV may appear about 1 to 3 weeks after the triggering event if present. The major clinical findings include palpable purpura and/or petechiae that are nonblanching. These findings can easily be confused with other diagnoses especially in the presence of a similar preexisting diagnosis. For example, our patient already had chronic ITP, and in such circumstances, a diagnosis of superimposed LCV can be easily missed without a thorough investigation. Extracutaneous manifestations with LCV are less common. Systemic symptoms may include low-grade fevers, malaise, weight loss, myalgia, and arthralgia. These findings have been noted in about 30% of affected patients, with arthralgia the most common manifestation.7 Our patient also presented with pain involving multiple joints.
The mainstay of diagnosis for LCV is a skin biopsy with direct immunofluorescence. However, a workup for an underlying condition should be considered based on clinical suspicion. If a secondary cause is found, management should target treating the underlying cause, including withdrawal of the offending drug, treatment or control of the underlying infection, malignancy, or connective tissue disease. Most cases of idiopathic cutaneous LCV resolve with supportive measures, including leg elevation, rest, compression stockings, and antihistamines. In resistant cases, a 4- to 6-week tapering dose of corticosteroids and immunosuppressive steroid-sparing agents may be needed.8
Conclusions
Although most cases of LCV are mild and resolve without intervention, many cases go undiagnosed due to a delay in performing a biopsy. However, we should always look for the root cause of a patient’s condition to rule out underlying contributing conditions. Differentiating LCV from any other preexisting condition presenting similarly is important.
1. Gaurav K, Keith RM. Immune thrombocytopenia. Hematol Oncol Clin North Am. 2013;27(3): 495-520. doi:10.1016/j.hoc.2013.03.001
2. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386-2393.
3. James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. Saunders/Elsevier; 2011.
4. Einhorn J, Levis JT. Dermatologic diagnosis: leukocytoclastic vasculitis. Perm J. 2015;19(3):77-78. doi:10.7812/TPP/15-001
5. The role of infectious agents in the pathogenesis of vasculitis. Nicolò P, Carlo S. Best Pract Res Clin Rheumatol. 2008;22(5):897-911. doi:10.7812/TPP/15-001
6. Maunz G, Conzett T, Zimmerli W. Cutaneous vasculitis associated with fluoroquinolones. Infection. 2009;37(5):466-468. doi:10.1007/s15010-009-8437-4
7. Baigrie D, Goyal A, Crane J.C. Leukocytoclastic vasculitis. StatPearls [internet]. Updated May 8, 2022. Accessed October 10, 2022. https://www.ncbi.nlm.nih.gov/books/NBK482159
8. Micheletti RG, Pagnoux C. Management of cutaneous vasculitis. Presse Med. 2020; 49(3):104033. doi:10.1016/j.lpm.2020.104033
1. Gaurav K, Keith RM. Immune thrombocytopenia. Hematol Oncol Clin North Am. 2013;27(3): 495-520. doi:10.1016/j.hoc.2013.03.001
2. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386-2393.
3. James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. Saunders/Elsevier; 2011.
4. Einhorn J, Levis JT. Dermatologic diagnosis: leukocytoclastic vasculitis. Perm J. 2015;19(3):77-78. doi:10.7812/TPP/15-001
5. The role of infectious agents in the pathogenesis of vasculitis. Nicolò P, Carlo S. Best Pract Res Clin Rheumatol. 2008;22(5):897-911. doi:10.7812/TPP/15-001
6. Maunz G, Conzett T, Zimmerli W. Cutaneous vasculitis associated with fluoroquinolones. Infection. 2009;37(5):466-468. doi:10.1007/s15010-009-8437-4
7. Baigrie D, Goyal A, Crane J.C. Leukocytoclastic vasculitis. StatPearls [internet]. Updated May 8, 2022. Accessed October 10, 2022. https://www.ncbi.nlm.nih.gov/books/NBK482159
8. Micheletti RG, Pagnoux C. Management of cutaneous vasculitis. Presse Med. 2020; 49(3):104033. doi:10.1016/j.lpm.2020.104033