User login
Microbiome Impacts Vaccine Responses
When infants are born, they have nearly a clean slate with regard to their immune systems. Virtually all their immune cells are naive. They have no immunity memory. Vaccines at birth, and in the first 2 years of life, elicit variable antibody levels and cellular immune responses. Sometimes, this leaves fully vaccinated children unprotected against vaccine-preventable infectious diseases.
Newborns are bombarded at birth with microbes and other antigenic stimuli from the environment; food in the form of breast milk, formula, water; and vaccines, such as hepatitis B and, in other countries, with BCG. At birth, to avoid immunologically-induced injury, immune responses favor immunologic tolerance. However, adaptation must be rapid to avoid life-threatening infections. To navigate the gauntlet of microbe and environmental exposures and vaccines, the neonatal immune system moves through a gradual maturation process toward immune responsivity. The maturation occurs at different rates in different children.
Reassessing Vaccine Responsiveness
Vaccine responsiveness is usually assessed by measuring antibody levels in blood. Until recently, it was thought to be “bad luck” when a child failed to develop protective immunity following vaccination. The bad luck was suggested to involve illness at the time of vaccination, especially illness occurring with fever, and especially common viral infections. But studies proved that notion incorrect. About 10 years ago I became more interested in variability in vaccine responses in the first 2 years of life. In 2016, my laboratory described a specific population of children with specific cellular immune deficiencies that we classified as low vaccine responders (LVRs).1 To preclude the suggestion that low vaccine responses were to be considered normal biological variation, we chose an a priori definition of LVR as those with sub-protective IgG antibody levels to four (≥ 66 %) of six tested vaccines in DTaP-Hib (diphtheria toxoid, tetanus toxoid, pertussis toxoid, pertactin, and filamentous hemagglutinin [DTaP] and Haemophilus influenzae type b polysaccharide capsule [Hib]). Antibody levels were measured at 1 year of age following primary vaccinations at child age 2, 4, and 6 months old. The remaining 89% of children we termed normal vaccine responders (NVRs). We additionally tested antibody responses to viral protein and pneumococcal polysaccharide conjugated antigens (polio serotypes 1, 2, and 3, hepatitis B, and Streptococcus pneumoniae capsular polysaccharides serotypes 6B, 14, and 23F). Responses to these vaccine antigens were similar to the six vaccines (DTaP/Hib) used to define LVR. We and other groups have used alternative definitions of low vaccine responses that rely on statistics.
I recently reviewed the topic of the determinants of vaccine responses in early life, with a focus on the infant microbiome and metabolome: a.) cesarean section versus vaginal delivery, b.) breast versus formula feeding and c.) antibiotic exposure, that impact the immune response2 (Figure). In the review I also discussed how microbiome may serve as natural adjuvants for vaccine responses, how microbiota-derived metabolites influence vaccine responses, and how low vaccine responses in early life may be linked to increased infection susceptibility (Figure).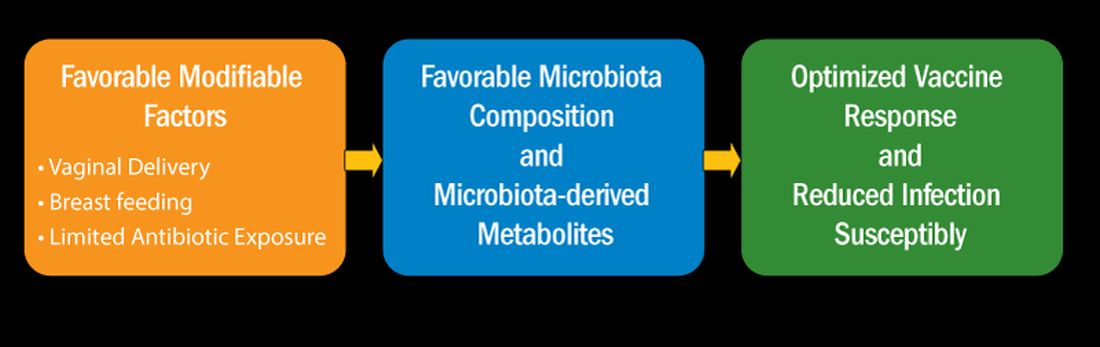
Cesarean section births occur in nearly 30% of newborns. Cesarean section birth has been associated with adverse effects on immune development, including predisposing to infections, allergies, and inflammatory disorders. The association of these adverse outcomes has been linked to lower total microbiome diversity. Fecal microbiome seeding from mother to infant in vaginal-delivered infants results in a more favorable and stable microbiome compared with cesarean-delivered infants. Nasopharyngeal microbiome may also be adversely affected by cesarean delivery. In turn, those microbiome differences can be linked to variation in vaccine responsiveness in infants.
Multiple studies strongly support the notion that breastfeeding has a favorable impact on immune development in early life associated with better vaccine responses, mediated by the microbiome. The mechanism of favorable immune responses to vaccines largely relates to the presence of a specific bacteria species, Bifidobacterium infantis. Breast milk contains human milk oligosaccharides that are not digestible by newborns. B. infantis is a strain of bacteria that utilizes these non-digestible oligosaccharides. Thereby, infants fed breast milk provides B. infantis the essential source of nutrition for its growth and predominance in the newborn gut. Studies have shown that Bifidobacterium spp. abundance in early life is correlated with better immune responses to multiple vaccines. Bifidobacterium spp. abundance has been positively correlated with antibody responses measured after 2 years, linking the microbiome composition to the durability of vaccine-induced immune responses.
Antibiotic exposure in early life may disproportionately damage the newborn and infant microbiome compared with later childhood. The average child receives about three antibiotic courses by the age of 2 years. My lab was among the first to describe the adverse effects of antibiotics on vaccine responses in early life.3 We found that broader spectrum antibiotics had a greater adverse effect on vaccine-induced antibody levels than narrower spectrum antibiotics. Ten-day versus five-day treatment courses had a greater negative effect. Multiple antibiotic courses over time (cumulative antibiotic exposure) was negatively associated with vaccine-induced antibody levels.
Over 11 % of live births worldwide occur preterm. Because bacterial infections are frequent complications of preterm birth, 79 % of very low birthweight and 87 % of extremely low birthweight infants in US NICUs receive antibiotics within 3 days of birth. Recently, my group studied full-term infants at birth and found that exposure to parenteral antibiotics at birth or during the first days of life had an adverse effect on vaccine responses.4
Microbiome Impacts Immunity
How does the microbiome affect immunity, and specifically vaccine responses? Microbial-derived metabolites affect host immunity. Gut bacteria produce short chain fatty acids (SCFAs: acetate, propionate, butyrate) [115]. SCFAs positively influence immunity cells. Vitamin D metabolites are generated by intestinal bacteria and those metabolites positively influence immunity. Secondary bile acids produced by Clostridium spp. are involved in favorable immune responses. Increased levels of phenylpyruvic acid produced by gut and/or nasopharyngeal microbiota correlate with reduced vaccine responses and upregulated metabolome genes that encode for oxidative phosphorylation correlate with increased vaccine responses.
In summary, immune development commences at birth. Impairment in responses to vaccination in children have been linked to disturbance in the microbiome. Cesarean section and absence of breastfeeding are associated with adverse microbiota composition. Antibiotics perturb healthy microbiota development. The microbiota affect immunity in several ways, among them are effects by metabolites generated by the commensals that inhabit the child host. A child who responds poorly to vaccines and has specific immune cell dysfunction caused by problems with the microbiome also displays increased infection proneness. But that is a story for another column, later.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Pichichero ME et al. J Infect Dis. 2016 Jun 15;213(12):2014-2019. doi: 10.1093/infdis/jiw053.
2. Pichichero ME. Cell Immunol. 2023 Nov-Dec:393-394:104777. doi: 10.1016/j.cellimm.2023.104777.
3. Chapman TJ et al. Pediatrics. 2022 May 1;149(5):e2021052061. doi: 10.1542/peds.2021-052061.
4. Shaffer M et al. mSystems. 2023 Oct 26;8(5):e0066123. doi: 10.1128/msystems.00661-23.
When infants are born, they have nearly a clean slate with regard to their immune systems. Virtually all their immune cells are naive. They have no immunity memory. Vaccines at birth, and in the first 2 years of life, elicit variable antibody levels and cellular immune responses. Sometimes, this leaves fully vaccinated children unprotected against vaccine-preventable infectious diseases.
Newborns are bombarded at birth with microbes and other antigenic stimuli from the environment; food in the form of breast milk, formula, water; and vaccines, such as hepatitis B and, in other countries, with BCG. At birth, to avoid immunologically-induced injury, immune responses favor immunologic tolerance. However, adaptation must be rapid to avoid life-threatening infections. To navigate the gauntlet of microbe and environmental exposures and vaccines, the neonatal immune system moves through a gradual maturation process toward immune responsivity. The maturation occurs at different rates in different children.
Reassessing Vaccine Responsiveness
Vaccine responsiveness is usually assessed by measuring antibody levels in blood. Until recently, it was thought to be “bad luck” when a child failed to develop protective immunity following vaccination. The bad luck was suggested to involve illness at the time of vaccination, especially illness occurring with fever, and especially common viral infections. But studies proved that notion incorrect. About 10 years ago I became more interested in variability in vaccine responses in the first 2 years of life. In 2016, my laboratory described a specific population of children with specific cellular immune deficiencies that we classified as low vaccine responders (LVRs).1 To preclude the suggestion that low vaccine responses were to be considered normal biological variation, we chose an a priori definition of LVR as those with sub-protective IgG antibody levels to four (≥ 66 %) of six tested vaccines in DTaP-Hib (diphtheria toxoid, tetanus toxoid, pertussis toxoid, pertactin, and filamentous hemagglutinin [DTaP] and Haemophilus influenzae type b polysaccharide capsule [Hib]). Antibody levels were measured at 1 year of age following primary vaccinations at child age 2, 4, and 6 months old. The remaining 89% of children we termed normal vaccine responders (NVRs). We additionally tested antibody responses to viral protein and pneumococcal polysaccharide conjugated antigens (polio serotypes 1, 2, and 3, hepatitis B, and Streptococcus pneumoniae capsular polysaccharides serotypes 6B, 14, and 23F). Responses to these vaccine antigens were similar to the six vaccines (DTaP/Hib) used to define LVR. We and other groups have used alternative definitions of low vaccine responses that rely on statistics.
I recently reviewed the topic of the determinants of vaccine responses in early life, with a focus on the infant microbiome and metabolome: a.) cesarean section versus vaginal delivery, b.) breast versus formula feeding and c.) antibiotic exposure, that impact the immune response2 (Figure). In the review I also discussed how microbiome may serve as natural adjuvants for vaccine responses, how microbiota-derived metabolites influence vaccine responses, and how low vaccine responses in early life may be linked to increased infection susceptibility (Figure).
Cesarean section births occur in nearly 30% of newborns. Cesarean section birth has been associated with adverse effects on immune development, including predisposing to infections, allergies, and inflammatory disorders. The association of these adverse outcomes has been linked to lower total microbiome diversity. Fecal microbiome seeding from mother to infant in vaginal-delivered infants results in a more favorable and stable microbiome compared with cesarean-delivered infants. Nasopharyngeal microbiome may also be adversely affected by cesarean delivery. In turn, those microbiome differences can be linked to variation in vaccine responsiveness in infants.
Multiple studies strongly support the notion that breastfeeding has a favorable impact on immune development in early life associated with better vaccine responses, mediated by the microbiome. The mechanism of favorable immune responses to vaccines largely relates to the presence of a specific bacteria species, Bifidobacterium infantis. Breast milk contains human milk oligosaccharides that are not digestible by newborns. B. infantis is a strain of bacteria that utilizes these non-digestible oligosaccharides. Thereby, infants fed breast milk provides B. infantis the essential source of nutrition for its growth and predominance in the newborn gut. Studies have shown that Bifidobacterium spp. abundance in early life is correlated with better immune responses to multiple vaccines. Bifidobacterium spp. abundance has been positively correlated with antibody responses measured after 2 years, linking the microbiome composition to the durability of vaccine-induced immune responses.
Antibiotic exposure in early life may disproportionately damage the newborn and infant microbiome compared with later childhood. The average child receives about three antibiotic courses by the age of 2 years. My lab was among the first to describe the adverse effects of antibiotics on vaccine responses in early life.3 We found that broader spectrum antibiotics had a greater adverse effect on vaccine-induced antibody levels than narrower spectrum antibiotics. Ten-day versus five-day treatment courses had a greater negative effect. Multiple antibiotic courses over time (cumulative antibiotic exposure) was negatively associated with vaccine-induced antibody levels.
Over 11 % of live births worldwide occur preterm. Because bacterial infections are frequent complications of preterm birth, 79 % of very low birthweight and 87 % of extremely low birthweight infants in US NICUs receive antibiotics within 3 days of birth. Recently, my group studied full-term infants at birth and found that exposure to parenteral antibiotics at birth or during the first days of life had an adverse effect on vaccine responses.4
Microbiome Impacts Immunity
How does the microbiome affect immunity, and specifically vaccine responses? Microbial-derived metabolites affect host immunity. Gut bacteria produce short chain fatty acids (SCFAs: acetate, propionate, butyrate) [115]. SCFAs positively influence immunity cells. Vitamin D metabolites are generated by intestinal bacteria and those metabolites positively influence immunity. Secondary bile acids produced by Clostridium spp. are involved in favorable immune responses. Increased levels of phenylpyruvic acid produced by gut and/or nasopharyngeal microbiota correlate with reduced vaccine responses and upregulated metabolome genes that encode for oxidative phosphorylation correlate with increased vaccine responses.
In summary, immune development commences at birth. Impairment in responses to vaccination in children have been linked to disturbance in the microbiome. Cesarean section and absence of breastfeeding are associated with adverse microbiota composition. Antibiotics perturb healthy microbiota development. The microbiota affect immunity in several ways, among them are effects by metabolites generated by the commensals that inhabit the child host. A child who responds poorly to vaccines and has specific immune cell dysfunction caused by problems with the microbiome also displays increased infection proneness. But that is a story for another column, later.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Pichichero ME et al. J Infect Dis. 2016 Jun 15;213(12):2014-2019. doi: 10.1093/infdis/jiw053.
2. Pichichero ME. Cell Immunol. 2023 Nov-Dec:393-394:104777. doi: 10.1016/j.cellimm.2023.104777.
3. Chapman TJ et al. Pediatrics. 2022 May 1;149(5):e2021052061. doi: 10.1542/peds.2021-052061.
4. Shaffer M et al. mSystems. 2023 Oct 26;8(5):e0066123. doi: 10.1128/msystems.00661-23.
When infants are born, they have nearly a clean slate with regard to their immune systems. Virtually all their immune cells are naive. They have no immunity memory. Vaccines at birth, and in the first 2 years of life, elicit variable antibody levels and cellular immune responses. Sometimes, this leaves fully vaccinated children unprotected against vaccine-preventable infectious diseases.
Newborns are bombarded at birth with microbes and other antigenic stimuli from the environment; food in the form of breast milk, formula, water; and vaccines, such as hepatitis B and, in other countries, with BCG. At birth, to avoid immunologically-induced injury, immune responses favor immunologic tolerance. However, adaptation must be rapid to avoid life-threatening infections. To navigate the gauntlet of microbe and environmental exposures and vaccines, the neonatal immune system moves through a gradual maturation process toward immune responsivity. The maturation occurs at different rates in different children.
Reassessing Vaccine Responsiveness
Vaccine responsiveness is usually assessed by measuring antibody levels in blood. Until recently, it was thought to be “bad luck” when a child failed to develop protective immunity following vaccination. The bad luck was suggested to involve illness at the time of vaccination, especially illness occurring with fever, and especially common viral infections. But studies proved that notion incorrect. About 10 years ago I became more interested in variability in vaccine responses in the first 2 years of life. In 2016, my laboratory described a specific population of children with specific cellular immune deficiencies that we classified as low vaccine responders (LVRs).1 To preclude the suggestion that low vaccine responses were to be considered normal biological variation, we chose an a priori definition of LVR as those with sub-protective IgG antibody levels to four (≥ 66 %) of six tested vaccines in DTaP-Hib (diphtheria toxoid, tetanus toxoid, pertussis toxoid, pertactin, and filamentous hemagglutinin [DTaP] and Haemophilus influenzae type b polysaccharide capsule [Hib]). Antibody levels were measured at 1 year of age following primary vaccinations at child age 2, 4, and 6 months old. The remaining 89% of children we termed normal vaccine responders (NVRs). We additionally tested antibody responses to viral protein and pneumococcal polysaccharide conjugated antigens (polio serotypes 1, 2, and 3, hepatitis B, and Streptococcus pneumoniae capsular polysaccharides serotypes 6B, 14, and 23F). Responses to these vaccine antigens were similar to the six vaccines (DTaP/Hib) used to define LVR. We and other groups have used alternative definitions of low vaccine responses that rely on statistics.
I recently reviewed the topic of the determinants of vaccine responses in early life, with a focus on the infant microbiome and metabolome: a.) cesarean section versus vaginal delivery, b.) breast versus formula feeding and c.) antibiotic exposure, that impact the immune response2 (Figure). In the review I also discussed how microbiome may serve as natural adjuvants for vaccine responses, how microbiota-derived metabolites influence vaccine responses, and how low vaccine responses in early life may be linked to increased infection susceptibility (Figure).
Cesarean section births occur in nearly 30% of newborns. Cesarean section birth has been associated with adverse effects on immune development, including predisposing to infections, allergies, and inflammatory disorders. The association of these adverse outcomes has been linked to lower total microbiome diversity. Fecal microbiome seeding from mother to infant in vaginal-delivered infants results in a more favorable and stable microbiome compared with cesarean-delivered infants. Nasopharyngeal microbiome may also be adversely affected by cesarean delivery. In turn, those microbiome differences can be linked to variation in vaccine responsiveness in infants.
Multiple studies strongly support the notion that breastfeeding has a favorable impact on immune development in early life associated with better vaccine responses, mediated by the microbiome. The mechanism of favorable immune responses to vaccines largely relates to the presence of a specific bacteria species, Bifidobacterium infantis. Breast milk contains human milk oligosaccharides that are not digestible by newborns. B. infantis is a strain of bacteria that utilizes these non-digestible oligosaccharides. Thereby, infants fed breast milk provides B. infantis the essential source of nutrition for its growth and predominance in the newborn gut. Studies have shown that Bifidobacterium spp. abundance in early life is correlated with better immune responses to multiple vaccines. Bifidobacterium spp. abundance has been positively correlated with antibody responses measured after 2 years, linking the microbiome composition to the durability of vaccine-induced immune responses.
Antibiotic exposure in early life may disproportionately damage the newborn and infant microbiome compared with later childhood. The average child receives about three antibiotic courses by the age of 2 years. My lab was among the first to describe the adverse effects of antibiotics on vaccine responses in early life.3 We found that broader spectrum antibiotics had a greater adverse effect on vaccine-induced antibody levels than narrower spectrum antibiotics. Ten-day versus five-day treatment courses had a greater negative effect. Multiple antibiotic courses over time (cumulative antibiotic exposure) was negatively associated with vaccine-induced antibody levels.
Over 11 % of live births worldwide occur preterm. Because bacterial infections are frequent complications of preterm birth, 79 % of very low birthweight and 87 % of extremely low birthweight infants in US NICUs receive antibiotics within 3 days of birth. Recently, my group studied full-term infants at birth and found that exposure to parenteral antibiotics at birth or during the first days of life had an adverse effect on vaccine responses.4
Microbiome Impacts Immunity
How does the microbiome affect immunity, and specifically vaccine responses? Microbial-derived metabolites affect host immunity. Gut bacteria produce short chain fatty acids (SCFAs: acetate, propionate, butyrate) [115]. SCFAs positively influence immunity cells. Vitamin D metabolites are generated by intestinal bacteria and those metabolites positively influence immunity. Secondary bile acids produced by Clostridium spp. are involved in favorable immune responses. Increased levels of phenylpyruvic acid produced by gut and/or nasopharyngeal microbiota correlate with reduced vaccine responses and upregulated metabolome genes that encode for oxidative phosphorylation correlate with increased vaccine responses.
In summary, immune development commences at birth. Impairment in responses to vaccination in children have been linked to disturbance in the microbiome. Cesarean section and absence of breastfeeding are associated with adverse microbiota composition. Antibiotics perturb healthy microbiota development. The microbiota affect immunity in several ways, among them are effects by metabolites generated by the commensals that inhabit the child host. A child who responds poorly to vaccines and has specific immune cell dysfunction caused by problems with the microbiome also displays increased infection proneness. But that is a story for another column, later.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Pichichero ME et al. J Infect Dis. 2016 Jun 15;213(12):2014-2019. doi: 10.1093/infdis/jiw053.
2. Pichichero ME. Cell Immunol. 2023 Nov-Dec:393-394:104777. doi: 10.1016/j.cellimm.2023.104777.
3. Chapman TJ et al. Pediatrics. 2022 May 1;149(5):e2021052061. doi: 10.1542/peds.2021-052061.
4. Shaffer M et al. mSystems. 2023 Oct 26;8(5):e0066123. doi: 10.1128/msystems.00661-23.
HPV Vaccine Shown to Be Highly Effective in Girls Years Later
TOPLINE:
METHODOLOGY:
- Cervical cancer is the fourth most common cancer among women worldwide.
- Programs to provide Cervarix, a bivalent vaccine, began in the United Kingdom in 2007.
- After the initiation of the programs, administering the vaccine became part of routine care for girls starting at age 12 years.
- Researchers collected data in 2020 from 447,845 women born between 1988 and 1996 from the Scottish cervical cancer screening system to assess the efficacy of Cervarix in lowering rates of cervical cancer.
- They correlated the rate of cervical cancer per 100,000 person-years with data on women regarding vaccination status, age when vaccinated, and deprivation in areas like income, housing, and health.
TAKEAWAY:
- No cases of cervical cancer were found among women who were immunized at ages 12 or 13 years, no matter how many doses they received.
- Women who were immunized between ages 14 and 18 years and received three doses had fewer instances of cervical cancer compared with unvaccinated women regardless of deprivation status (3.2 cases per 100,00 women vs 8.4 cases per 100,000).
IN PRACTICE:
“Continued participation in screening and monitoring of outcomes is required, however, to assess the effects of changes in vaccines used and dosage schedules since the start of vaccination in Scotland in 2008 and the longevity of protection the vaccines offer.”
SOURCE:
The study was led by Timothy J. Palmer, PhD, Scottish Clinical Lead for Cervical Screening at Public Health Scotland.
LIMITATIONS:
Only 14,645 women had received just one or two doses, which may have affected the statistical analysis.
DISCLOSURES:
The study was funded by Public Health Scotland. A coauthor reports attending an advisory board meeting for HOLOGIC and Vaccitech. Her institution received research funding or gratis support funding from Cepheid, Euroimmun, GeneFirst, SelfScreen, Hiantis, Seegene, Roche, Hologic, and Vaccitech in the past 3 years.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Cervical cancer is the fourth most common cancer among women worldwide.
- Programs to provide Cervarix, a bivalent vaccine, began in the United Kingdom in 2007.
- After the initiation of the programs, administering the vaccine became part of routine care for girls starting at age 12 years.
- Researchers collected data in 2020 from 447,845 women born between 1988 and 1996 from the Scottish cervical cancer screening system to assess the efficacy of Cervarix in lowering rates of cervical cancer.
- They correlated the rate of cervical cancer per 100,000 person-years with data on women regarding vaccination status, age when vaccinated, and deprivation in areas like income, housing, and health.
TAKEAWAY:
- No cases of cervical cancer were found among women who were immunized at ages 12 or 13 years, no matter how many doses they received.
- Women who were immunized between ages 14 and 18 years and received three doses had fewer instances of cervical cancer compared with unvaccinated women regardless of deprivation status (3.2 cases per 100,00 women vs 8.4 cases per 100,000).
IN PRACTICE:
“Continued participation in screening and monitoring of outcomes is required, however, to assess the effects of changes in vaccines used and dosage schedules since the start of vaccination in Scotland in 2008 and the longevity of protection the vaccines offer.”
SOURCE:
The study was led by Timothy J. Palmer, PhD, Scottish Clinical Lead for Cervical Screening at Public Health Scotland.
LIMITATIONS:
Only 14,645 women had received just one or two doses, which may have affected the statistical analysis.
DISCLOSURES:
The study was funded by Public Health Scotland. A coauthor reports attending an advisory board meeting for HOLOGIC and Vaccitech. Her institution received research funding or gratis support funding from Cepheid, Euroimmun, GeneFirst, SelfScreen, Hiantis, Seegene, Roche, Hologic, and Vaccitech in the past 3 years.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Cervical cancer is the fourth most common cancer among women worldwide.
- Programs to provide Cervarix, a bivalent vaccine, began in the United Kingdom in 2007.
- After the initiation of the programs, administering the vaccine became part of routine care for girls starting at age 12 years.
- Researchers collected data in 2020 from 447,845 women born between 1988 and 1996 from the Scottish cervical cancer screening system to assess the efficacy of Cervarix in lowering rates of cervical cancer.
- They correlated the rate of cervical cancer per 100,000 person-years with data on women regarding vaccination status, age when vaccinated, and deprivation in areas like income, housing, and health.
TAKEAWAY:
- No cases of cervical cancer were found among women who were immunized at ages 12 or 13 years, no matter how many doses they received.
- Women who were immunized between ages 14 and 18 years and received three doses had fewer instances of cervical cancer compared with unvaccinated women regardless of deprivation status (3.2 cases per 100,00 women vs 8.4 cases per 100,000).
IN PRACTICE:
“Continued participation in screening and monitoring of outcomes is required, however, to assess the effects of changes in vaccines used and dosage schedules since the start of vaccination in Scotland in 2008 and the longevity of protection the vaccines offer.”
SOURCE:
The study was led by Timothy J. Palmer, PhD, Scottish Clinical Lead for Cervical Screening at Public Health Scotland.
LIMITATIONS:
Only 14,645 women had received just one or two doses, which may have affected the statistical analysis.
DISCLOSURES:
The study was funded by Public Health Scotland. A coauthor reports attending an advisory board meeting for HOLOGIC and Vaccitech. Her institution received research funding or gratis support funding from Cepheid, Euroimmun, GeneFirst, SelfScreen, Hiantis, Seegene, Roche, Hologic, and Vaccitech in the past 3 years.
A version of this article appeared on Medscape.com.
The Breakthrough Drug Whose Full Promise Remains Unrealized
Celebrating a Decade of Sofosbuvir for Hepatitis C
Prior to 2013, the backbone of hepatitis C virus (HCV) therapy was pegylated interferon (PEG) in combination with ribavirin (RBV). This year-long therapy was associated with significant side effects and abysmal cure rates. Although efficacy improved with the addition of first-generation protease inhibitors, cure rates remained suboptimal and treatment side effects continued to be significant.
Clinicians and patients needed better options and looked to the drug pipeline with hope. However, even among the most optimistic, the idea that HCV therapy could evolve into an all-oral option seemed a relative pipe dream.
The Sofosbuvir Revolution Begins
The Liver Meeting held in 2013 changed everything.
Several presentations featured compelling data with sofosbuvir, a new polymerase inhibitor that, when combined with RBV, offered an all-oral option to patients with genotypes 2 and 3, as well as improved efficacy for patients with genotypes 1, 4, 5, and 6 when it was combined with 12 weeks of PEG/RBV.
However, the glass ceiling of HCV care was truly shattered with the randomized COSMOS trial, a late-breaker abstract that revealed 12-week functional cure rates in patients receiving sofosbuvir in combination with the protease inhibitor simeprevir.
This phase 2a trial in treatment-naive and -experienced genotype 1 patients with and without cirrhosis showed that an all-oral option was not only viable for the most common strain of HCV but was also safe and efficacious, even in difficult-to-treat populations.
On December 6, 2013, the US Food and Drug Administration (FDA) approved sofosbuvir for the treatment of HCV, ushering in a new era of therapy.
Guidelines quickly changed to advocate for both expansive HCV screening and generous treatment. Yet, as this more permissive approach was being recommended, the high price tag and large anticipated volume of those seeking prescriptions were setting off alarms. The drug cost triggered extensive restrictions based on degree of fibrosis, sobriety, and provider type in an effort to prevent immediate healthcare expenditures.
Given its high cost, rules restricting a patient to only one course of sofosbuvir-based therapy also surfaced. Although treatment with first-generation protease inhibitors carried a hefty price of $161,813.49 per sustained virologic response (SVR), compared with $66,000-$100,000 for 12 weeks of all-oral therapy, its uptake was low and limited by side effects and comorbid conditions. All-oral treatment appeared to have few medical barriers, leading payers to find ways to slow utilization. These restrictions are now gradually being eliminated.
Because of high SVR rates and few contraindications to therapy, most patients who gained access to treatment achieved cure. This included patients who had previously not responded to treatment and prioritized those with more advanced disease.
This quickly led to a significant shift in the population in need of treatment. Prior to 2013, many patients with HCV had advanced disease and did not respond to prior treatment options. After uptake of all-oral therapy, individuals in need were typically treatment naive without advanced disease.
This shift also added new psychosocial dimensions, as many of the newly infected individuals were struggling with active substance abuse. HCV treatment providers needed to change, with increasing recruitment of advanced practice providers, primary care physicians, and addiction medication specialists.
Progress, but Far From Reaching Targets
Fast-forward to 2023.
Ten years after FDA approval, 13.2 million individuals infected with HCV have been treated globally, 82% with sofosbuvir-based regimens and most in lower-middle-income countries. This is absolutely cause for celebration, but not complacency.
In 2016, the World Health Assembly adopted a resolution of elimination of viral hepatitis by 2030. The World Health Organization (WHO) defined elimination of HCV as 90% reduction in new cases of infection, 90% diagnosis of those infected, 80% of eligible individuals treated, and 65% reduction of deaths by 2030.
Despite all the success thus far, the CDA Foundation estimates that the WHO elimination targets will not be achieved until after the year 2050. They also note that in 2020, over 50 million individuals were infected with HCV, of which only 20% were diagnosed and 1% annually treated.
The HCV care cascade, by which the patient journeys from screening to cure, is complicated, and a one-size-fits-all solution is not possible. Reflex testing (an automatic transition to HCV polymerase chain reaction [PCR] testing in the lab for those who are HCV antibody positive) has significantly improved diagnosis. However, communicating these results and linking a patient to curative therapy remain significant obstacles.
Models and real-life experience show that multiple strategies can be successful. They include leveraging the electronic medical record, simplified treatment algorithms, test-and-treat strategies (screening high-risk populations with a point-of-care test that allows treatment initiation at the same visit), and co-localizing HCV screening and treatment with addiction services and relinkage programs (finding those who are already diagnosed and linking them to treatment).
In addition, focusing on populations at high risk for HCV infection — such as people who inject drugs, men who have sex with men, and incarcerated individuals — allows for better resource utilization.
Though daunting, HCV elimination is not impossible. There are several examples of success, including in the countries of Georgia and Iceland. Although, comparatively, the United States remains behind the curve, the White House has asked Congress for $11 billion to fund HCV elimination domestically.
As we await action at the national level, clinicians are reminded that there are several things we can do in caring for patients with HCV:
- A one-time HCV screening is recommended in all individuals aged 18 or older, including pregnant people with each pregnancy.
- HCV antibody testing with reflex to PCR should be used as the screening test.
- Pan-genotypic all-oral therapy is recommended for patients with HCV. Cure rates are greater than 95%, and there are few contraindications to treatment.
- Most people are eligible for simplified treatment algorithms that allow minimal on-treatment monitoring.
Without increased screening and linkage to curative therapy, we will not meet the WHO goals for HCV elimination.
Dr. Reau is chief of the hepatology section at Rush University Medical Center in Chicago and a regular contributor to this news organization. She serves as editor of Clinical Liver Disease, a multimedia review journal, and recently as a member of HCVGuidelines.org, a web-based resource from the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America, as well as educational chair of the AASLD hepatitis C special interest group. She continues to have an active role in the hepatology interest group of the World Gastroenterology Organisation and the American Liver Foundation at the regional and national levels. She disclosed ties with AbbVie, Gilead, Arbutus, Intercept, and Salix.
A version of this article appeared on Medscape.com.
Celebrating a Decade of Sofosbuvir for Hepatitis C
Celebrating a Decade of Sofosbuvir for Hepatitis C
Prior to 2013, the backbone of hepatitis C virus (HCV) therapy was pegylated interferon (PEG) in combination with ribavirin (RBV). This year-long therapy was associated with significant side effects and abysmal cure rates. Although efficacy improved with the addition of first-generation protease inhibitors, cure rates remained suboptimal and treatment side effects continued to be significant.
Clinicians and patients needed better options and looked to the drug pipeline with hope. However, even among the most optimistic, the idea that HCV therapy could evolve into an all-oral option seemed a relative pipe dream.
The Sofosbuvir Revolution Begins
The Liver Meeting held in 2013 changed everything.
Several presentations featured compelling data with sofosbuvir, a new polymerase inhibitor that, when combined with RBV, offered an all-oral option to patients with genotypes 2 and 3, as well as improved efficacy for patients with genotypes 1, 4, 5, and 6 when it was combined with 12 weeks of PEG/RBV.
However, the glass ceiling of HCV care was truly shattered with the randomized COSMOS trial, a late-breaker abstract that revealed 12-week functional cure rates in patients receiving sofosbuvir in combination with the protease inhibitor simeprevir.
This phase 2a trial in treatment-naive and -experienced genotype 1 patients with and without cirrhosis showed that an all-oral option was not only viable for the most common strain of HCV but was also safe and efficacious, even in difficult-to-treat populations.
On December 6, 2013, the US Food and Drug Administration (FDA) approved sofosbuvir for the treatment of HCV, ushering in a new era of therapy.
Guidelines quickly changed to advocate for both expansive HCV screening and generous treatment. Yet, as this more permissive approach was being recommended, the high price tag and large anticipated volume of those seeking prescriptions were setting off alarms. The drug cost triggered extensive restrictions based on degree of fibrosis, sobriety, and provider type in an effort to prevent immediate healthcare expenditures.
Given its high cost, rules restricting a patient to only one course of sofosbuvir-based therapy also surfaced. Although treatment with first-generation protease inhibitors carried a hefty price of $161,813.49 per sustained virologic response (SVR), compared with $66,000-$100,000 for 12 weeks of all-oral therapy, its uptake was low and limited by side effects and comorbid conditions. All-oral treatment appeared to have few medical barriers, leading payers to find ways to slow utilization. These restrictions are now gradually being eliminated.
Because of high SVR rates and few contraindications to therapy, most patients who gained access to treatment achieved cure. This included patients who had previously not responded to treatment and prioritized those with more advanced disease.
This quickly led to a significant shift in the population in need of treatment. Prior to 2013, many patients with HCV had advanced disease and did not respond to prior treatment options. After uptake of all-oral therapy, individuals in need were typically treatment naive without advanced disease.
This shift also added new psychosocial dimensions, as many of the newly infected individuals were struggling with active substance abuse. HCV treatment providers needed to change, with increasing recruitment of advanced practice providers, primary care physicians, and addiction medication specialists.
Progress, but Far From Reaching Targets
Fast-forward to 2023.
Ten years after FDA approval, 13.2 million individuals infected with HCV have been treated globally, 82% with sofosbuvir-based regimens and most in lower-middle-income countries. This is absolutely cause for celebration, but not complacency.
In 2016, the World Health Assembly adopted a resolution of elimination of viral hepatitis by 2030. The World Health Organization (WHO) defined elimination of HCV as 90% reduction in new cases of infection, 90% diagnosis of those infected, 80% of eligible individuals treated, and 65% reduction of deaths by 2030.
Despite all the success thus far, the CDA Foundation estimates that the WHO elimination targets will not be achieved until after the year 2050. They also note that in 2020, over 50 million individuals were infected with HCV, of which only 20% were diagnosed and 1% annually treated.
The HCV care cascade, by which the patient journeys from screening to cure, is complicated, and a one-size-fits-all solution is not possible. Reflex testing (an automatic transition to HCV polymerase chain reaction [PCR] testing in the lab for those who are HCV antibody positive) has significantly improved diagnosis. However, communicating these results and linking a patient to curative therapy remain significant obstacles.
Models and real-life experience show that multiple strategies can be successful. They include leveraging the electronic medical record, simplified treatment algorithms, test-and-treat strategies (screening high-risk populations with a point-of-care test that allows treatment initiation at the same visit), and co-localizing HCV screening and treatment with addiction services and relinkage programs (finding those who are already diagnosed and linking them to treatment).
In addition, focusing on populations at high risk for HCV infection — such as people who inject drugs, men who have sex with men, and incarcerated individuals — allows for better resource utilization.
Though daunting, HCV elimination is not impossible. There are several examples of success, including in the countries of Georgia and Iceland. Although, comparatively, the United States remains behind the curve, the White House has asked Congress for $11 billion to fund HCV elimination domestically.
As we await action at the national level, clinicians are reminded that there are several things we can do in caring for patients with HCV:
- A one-time HCV screening is recommended in all individuals aged 18 or older, including pregnant people with each pregnancy.
- HCV antibody testing with reflex to PCR should be used as the screening test.
- Pan-genotypic all-oral therapy is recommended for patients with HCV. Cure rates are greater than 95%, and there are few contraindications to treatment.
- Most people are eligible for simplified treatment algorithms that allow minimal on-treatment monitoring.
Without increased screening and linkage to curative therapy, we will not meet the WHO goals for HCV elimination.
Dr. Reau is chief of the hepatology section at Rush University Medical Center in Chicago and a regular contributor to this news organization. She serves as editor of Clinical Liver Disease, a multimedia review journal, and recently as a member of HCVGuidelines.org, a web-based resource from the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America, as well as educational chair of the AASLD hepatitis C special interest group. She continues to have an active role in the hepatology interest group of the World Gastroenterology Organisation and the American Liver Foundation at the regional and national levels. She disclosed ties with AbbVie, Gilead, Arbutus, Intercept, and Salix.
A version of this article appeared on Medscape.com.
Prior to 2013, the backbone of hepatitis C virus (HCV) therapy was pegylated interferon (PEG) in combination with ribavirin (RBV). This year-long therapy was associated with significant side effects and abysmal cure rates. Although efficacy improved with the addition of first-generation protease inhibitors, cure rates remained suboptimal and treatment side effects continued to be significant.
Clinicians and patients needed better options and looked to the drug pipeline with hope. However, even among the most optimistic, the idea that HCV therapy could evolve into an all-oral option seemed a relative pipe dream.
The Sofosbuvir Revolution Begins
The Liver Meeting held in 2013 changed everything.
Several presentations featured compelling data with sofosbuvir, a new polymerase inhibitor that, when combined with RBV, offered an all-oral option to patients with genotypes 2 and 3, as well as improved efficacy for patients with genotypes 1, 4, 5, and 6 when it was combined with 12 weeks of PEG/RBV.
However, the glass ceiling of HCV care was truly shattered with the randomized COSMOS trial, a late-breaker abstract that revealed 12-week functional cure rates in patients receiving sofosbuvir in combination with the protease inhibitor simeprevir.
This phase 2a trial in treatment-naive and -experienced genotype 1 patients with and without cirrhosis showed that an all-oral option was not only viable for the most common strain of HCV but was also safe and efficacious, even in difficult-to-treat populations.
On December 6, 2013, the US Food and Drug Administration (FDA) approved sofosbuvir for the treatment of HCV, ushering in a new era of therapy.
Guidelines quickly changed to advocate for both expansive HCV screening and generous treatment. Yet, as this more permissive approach was being recommended, the high price tag and large anticipated volume of those seeking prescriptions were setting off alarms. The drug cost triggered extensive restrictions based on degree of fibrosis, sobriety, and provider type in an effort to prevent immediate healthcare expenditures.
Given its high cost, rules restricting a patient to only one course of sofosbuvir-based therapy also surfaced. Although treatment with first-generation protease inhibitors carried a hefty price of $161,813.49 per sustained virologic response (SVR), compared with $66,000-$100,000 for 12 weeks of all-oral therapy, its uptake was low and limited by side effects and comorbid conditions. All-oral treatment appeared to have few medical barriers, leading payers to find ways to slow utilization. These restrictions are now gradually being eliminated.
Because of high SVR rates and few contraindications to therapy, most patients who gained access to treatment achieved cure. This included patients who had previously not responded to treatment and prioritized those with more advanced disease.
This quickly led to a significant shift in the population in need of treatment. Prior to 2013, many patients with HCV had advanced disease and did not respond to prior treatment options. After uptake of all-oral therapy, individuals in need were typically treatment naive without advanced disease.
This shift also added new psychosocial dimensions, as many of the newly infected individuals were struggling with active substance abuse. HCV treatment providers needed to change, with increasing recruitment of advanced practice providers, primary care physicians, and addiction medication specialists.
Progress, but Far From Reaching Targets
Fast-forward to 2023.
Ten years after FDA approval, 13.2 million individuals infected with HCV have been treated globally, 82% with sofosbuvir-based regimens and most in lower-middle-income countries. This is absolutely cause for celebration, but not complacency.
In 2016, the World Health Assembly adopted a resolution of elimination of viral hepatitis by 2030. The World Health Organization (WHO) defined elimination of HCV as 90% reduction in new cases of infection, 90% diagnosis of those infected, 80% of eligible individuals treated, and 65% reduction of deaths by 2030.
Despite all the success thus far, the CDA Foundation estimates that the WHO elimination targets will not be achieved until after the year 2050. They also note that in 2020, over 50 million individuals were infected with HCV, of which only 20% were diagnosed and 1% annually treated.
The HCV care cascade, by which the patient journeys from screening to cure, is complicated, and a one-size-fits-all solution is not possible. Reflex testing (an automatic transition to HCV polymerase chain reaction [PCR] testing in the lab for those who are HCV antibody positive) has significantly improved diagnosis. However, communicating these results and linking a patient to curative therapy remain significant obstacles.
Models and real-life experience show that multiple strategies can be successful. They include leveraging the electronic medical record, simplified treatment algorithms, test-and-treat strategies (screening high-risk populations with a point-of-care test that allows treatment initiation at the same visit), and co-localizing HCV screening and treatment with addiction services and relinkage programs (finding those who are already diagnosed and linking them to treatment).
In addition, focusing on populations at high risk for HCV infection — such as people who inject drugs, men who have sex with men, and incarcerated individuals — allows for better resource utilization.
Though daunting, HCV elimination is not impossible. There are several examples of success, including in the countries of Georgia and Iceland. Although, comparatively, the United States remains behind the curve, the White House has asked Congress for $11 billion to fund HCV elimination domestically.
As we await action at the national level, clinicians are reminded that there are several things we can do in caring for patients with HCV:
- A one-time HCV screening is recommended in all individuals aged 18 or older, including pregnant people with each pregnancy.
- HCV antibody testing with reflex to PCR should be used as the screening test.
- Pan-genotypic all-oral therapy is recommended for patients with HCV. Cure rates are greater than 95%, and there are few contraindications to treatment.
- Most people are eligible for simplified treatment algorithms that allow minimal on-treatment monitoring.
Without increased screening and linkage to curative therapy, we will not meet the WHO goals for HCV elimination.
Dr. Reau is chief of the hepatology section at Rush University Medical Center in Chicago and a regular contributor to this news organization. She serves as editor of Clinical Liver Disease, a multimedia review journal, and recently as a member of HCVGuidelines.org, a web-based resource from the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America, as well as educational chair of the AASLD hepatitis C special interest group. She continues to have an active role in the hepatology interest group of the World Gastroenterology Organisation and the American Liver Foundation at the regional and national levels. She disclosed ties with AbbVie, Gilead, Arbutus, Intercept, and Salix.
A version of this article appeared on Medscape.com.
Five Bold Predictions for Long COVID in 2024
With a number of large-scale clinical trials underway and researchers on the hunt for new therapies, long COVID scientists are hopeful that this is the year patients — and doctors who care for them — will finally see improvements in treating their symptoms.
Here are five bold predictions — all based on encouraging research — that could happen in 2024. At the very least, they are promising signs of progress against a debilitating and frustrating disease.
#1: We’ll gain a better understanding of each long COVID phenotype
This past year, a wide breadth of research began showing that long COVID can be defined by a number of different disease phenotypes that present a range of symptoms.
Researchers identified four clinical phenotypes: Chronic fatigue-like syndrome, headache, and memory loss; respiratory syndrome, which includes cough and difficulty breathing; chronic pain; and neurosensorial syndrome, which causes an altered sense of taste and smell.
Identifying specific diagnostic criteria for each phenotype would lead to better health outcomes for patients instead of treating them as if it were a “one-size-fits-all disease,” said Nisha Viswanathan, MD, director of the long COVID program at UCLA Health, Los Angeles, California.
Ultimately, she hopes that this year her patients will receive treatments based on the type of long COVID they’re personally experiencing, and the symptoms they have, leading to improved health outcomes and more rapid relief.
“Many new medications are focused on different pathways of long COVID, and the challenge becomes which drug is the right drug for each treatment,” said Dr. Viswanathan.
#2: Monoclonal antibodies may change the game
We’re starting to have a better understanding that what’s been called “viral persistence” as a main cause of long COVID may potentially be treated with monoclonal antibodies. These are antibodies produced by cloning unique white blood cells to target the circulating spike proteins in the blood that hang out in viral reservoirs and cause the immune system to react as if it’s still fighting acute COVID-19.
Smaller-scale studies have already shown promising results. A January 2024 study published in The American Journal of Emergency Medicine followed three patients who completely recovered from long COVID after taking monoclonal antibodies. “Remission occurred despite dissimilar past histories, sex, age, and illness duration,” wrote the study authors.
Larger clinical trials are underway at the University of California, San Francisco, California, to test targeted monoclonal antibodies. If the results of the larger study show that monoclonal antibodies are beneficial, then it could be a game changer for a large swath of patients around the world, said David F. Putrino, PhD, who runs the long COVID clinic at Mount Sinai Health System in New York City.
“The idea is that the downstream damage caused by viral persistence will resolve itself once you wipe out the virus,” said Dr. Putrino.
#3: Paxlovid could prove effective for long COVID
The US Food and Drug Administration granted approval for Paxlovid last May for the treatment of mild to moderate COVID-19 in adults at a high risk for severe disease. The medication is made up of two drugs packaged together. The first, nirmatrelvir, works by blocking a key enzyme required for virus replication. The second, ritonavir, is an antiviral that’s been used in patients with HIV and helps boost levels of antivirals in the body.
In a large-scale trial headed up by Dr. Putrino and his team, the oral antiviral is being studied for use in the post-viral stage in patients who test negative for acute COVID-19 but have persisting symptoms of long COVID.
Similar to monoclonal antibodies, the idea is to quell viral persistence. If patients have long COVID because they can’t clear SAR-CoV-2 from their bodies, Paxlovid could help. But unlike monoclonal antibodies that quash the virus, Paxlovid stops the virus from replicating. It’s a different mechanism with the same end goal.
It’s been a controversial treatment because it’s life-changing for some patients and ineffective for others. In addition, it can cause a range of side effects such as diarrhea, nausea, vomiting, and an impaired sense of taste. The goal of the trial is to see which patients with long COVID are most likely to benefit from the treatment.
#4: Anti-inflammatories like metformin could prove useful
Many of the inflammatory markers persistent in patients with long COVID were similarly present in patients with autoimmune diseases like rheumatoid arthritis, according to a July 2023 study published in JAMA.
The hope is that anti-inflammatory medications may be used to reduce inflammation causing long COVID symptoms. But drugs used to treat rheumatoid arthritis like abatacept and infliximabcan also have serious side effects, including increased risk for infection, flu-like symptoms, and burning of the skin.
“Powerful anti-inflammatories can change a number of pathways in the immune system,” said Grace McComsey, MD, who leads the long COVID RECOVER study at University Hospitals Health System in Cleveland, Ohio. Anti-inflammatories hold promise but, Dr. McComsey said, “some are more toxic with many side effects, so even if they work, there’s still a question about who should take them.”
Still, other anti-inflammatories that could work don’t have as many side effects. For example, a study published in The Lancet Infectious Diseases found that the diabetes drug metformin reduced a patient’s risk for long COVID up to 40% when the drug was taken during the acute stage.
Metformin, compared to other anti-inflammatories (also known as immune modulators), is an inexpensive and widely available drug with relatively few side effects compared with other medications.
#5: Serotonin levels — and selective serotonin reuptake inhibitors (SSRIs) — may be keys to unlocking long COVID
One of the most groundbreaking studies of the year came last November. A study published in the journal Cell found lower circulating serotonin levels in patents with long COVID than in those who did not have the condition. The study also found that the SSRI fluoxetine improved cognitive function in rat models infected with the virus.
Researchers found that the reduction in serotonin levels was partially caused by the body’s inability to absorb tryptophan, an amino acid that’s a precursor to serotonin. Overactivated blood platelets may also have played a role.
Michael Peluso, MD, an assistant research professor of infectious medicine at the UCSF School of Medicine, San Francisco, California, hopes to take the finding a step further, investigating whether increased serotonin levels in patients with long COVID will lead to improvements in symptoms.
“What we need now is a good clinical trial to see whether altering levels of serotonin in people with long COVID will lead to symptom relief,” Dr. Peluso said last month in an interview with this news organization.
If patients show an improvement in symptoms, then the next step is looking into whether SSRIs boost serotonin levels in patients and, as a result, reduce their symptoms.
A version of this article appeared on Medscape.com.
With a number of large-scale clinical trials underway and researchers on the hunt for new therapies, long COVID scientists are hopeful that this is the year patients — and doctors who care for them — will finally see improvements in treating their symptoms.
Here are five bold predictions — all based on encouraging research — that could happen in 2024. At the very least, they are promising signs of progress against a debilitating and frustrating disease.
#1: We’ll gain a better understanding of each long COVID phenotype
This past year, a wide breadth of research began showing that long COVID can be defined by a number of different disease phenotypes that present a range of symptoms.
Researchers identified four clinical phenotypes: Chronic fatigue-like syndrome, headache, and memory loss; respiratory syndrome, which includes cough and difficulty breathing; chronic pain; and neurosensorial syndrome, which causes an altered sense of taste and smell.
Identifying specific diagnostic criteria for each phenotype would lead to better health outcomes for patients instead of treating them as if it were a “one-size-fits-all disease,” said Nisha Viswanathan, MD, director of the long COVID program at UCLA Health, Los Angeles, California.
Ultimately, she hopes that this year her patients will receive treatments based on the type of long COVID they’re personally experiencing, and the symptoms they have, leading to improved health outcomes and more rapid relief.
“Many new medications are focused on different pathways of long COVID, and the challenge becomes which drug is the right drug for each treatment,” said Dr. Viswanathan.
#2: Monoclonal antibodies may change the game
We’re starting to have a better understanding that what’s been called “viral persistence” as a main cause of long COVID may potentially be treated with monoclonal antibodies. These are antibodies produced by cloning unique white blood cells to target the circulating spike proteins in the blood that hang out in viral reservoirs and cause the immune system to react as if it’s still fighting acute COVID-19.
Smaller-scale studies have already shown promising results. A January 2024 study published in The American Journal of Emergency Medicine followed three patients who completely recovered from long COVID after taking monoclonal antibodies. “Remission occurred despite dissimilar past histories, sex, age, and illness duration,” wrote the study authors.
Larger clinical trials are underway at the University of California, San Francisco, California, to test targeted monoclonal antibodies. If the results of the larger study show that monoclonal antibodies are beneficial, then it could be a game changer for a large swath of patients around the world, said David F. Putrino, PhD, who runs the long COVID clinic at Mount Sinai Health System in New York City.
“The idea is that the downstream damage caused by viral persistence will resolve itself once you wipe out the virus,” said Dr. Putrino.
#3: Paxlovid could prove effective for long COVID
The US Food and Drug Administration granted approval for Paxlovid last May for the treatment of mild to moderate COVID-19 in adults at a high risk for severe disease. The medication is made up of two drugs packaged together. The first, nirmatrelvir, works by blocking a key enzyme required for virus replication. The second, ritonavir, is an antiviral that’s been used in patients with HIV and helps boost levels of antivirals in the body.
In a large-scale trial headed up by Dr. Putrino and his team, the oral antiviral is being studied for use in the post-viral stage in patients who test negative for acute COVID-19 but have persisting symptoms of long COVID.
Similar to monoclonal antibodies, the idea is to quell viral persistence. If patients have long COVID because they can’t clear SAR-CoV-2 from their bodies, Paxlovid could help. But unlike monoclonal antibodies that quash the virus, Paxlovid stops the virus from replicating. It’s a different mechanism with the same end goal.
It’s been a controversial treatment because it’s life-changing for some patients and ineffective for others. In addition, it can cause a range of side effects such as diarrhea, nausea, vomiting, and an impaired sense of taste. The goal of the trial is to see which patients with long COVID are most likely to benefit from the treatment.
#4: Anti-inflammatories like metformin could prove useful
Many of the inflammatory markers persistent in patients with long COVID were similarly present in patients with autoimmune diseases like rheumatoid arthritis, according to a July 2023 study published in JAMA.
The hope is that anti-inflammatory medications may be used to reduce inflammation causing long COVID symptoms. But drugs used to treat rheumatoid arthritis like abatacept and infliximabcan also have serious side effects, including increased risk for infection, flu-like symptoms, and burning of the skin.
“Powerful anti-inflammatories can change a number of pathways in the immune system,” said Grace McComsey, MD, who leads the long COVID RECOVER study at University Hospitals Health System in Cleveland, Ohio. Anti-inflammatories hold promise but, Dr. McComsey said, “some are more toxic with many side effects, so even if they work, there’s still a question about who should take them.”
Still, other anti-inflammatories that could work don’t have as many side effects. For example, a study published in The Lancet Infectious Diseases found that the diabetes drug metformin reduced a patient’s risk for long COVID up to 40% when the drug was taken during the acute stage.
Metformin, compared to other anti-inflammatories (also known as immune modulators), is an inexpensive and widely available drug with relatively few side effects compared with other medications.
#5: Serotonin levels — and selective serotonin reuptake inhibitors (SSRIs) — may be keys to unlocking long COVID
One of the most groundbreaking studies of the year came last November. A study published in the journal Cell found lower circulating serotonin levels in patents with long COVID than in those who did not have the condition. The study also found that the SSRI fluoxetine improved cognitive function in rat models infected with the virus.
Researchers found that the reduction in serotonin levels was partially caused by the body’s inability to absorb tryptophan, an amino acid that’s a precursor to serotonin. Overactivated blood platelets may also have played a role.
Michael Peluso, MD, an assistant research professor of infectious medicine at the UCSF School of Medicine, San Francisco, California, hopes to take the finding a step further, investigating whether increased serotonin levels in patients with long COVID will lead to improvements in symptoms.
“What we need now is a good clinical trial to see whether altering levels of serotonin in people with long COVID will lead to symptom relief,” Dr. Peluso said last month in an interview with this news organization.
If patients show an improvement in symptoms, then the next step is looking into whether SSRIs boost serotonin levels in patients and, as a result, reduce their symptoms.
A version of this article appeared on Medscape.com.
With a number of large-scale clinical trials underway and researchers on the hunt for new therapies, long COVID scientists are hopeful that this is the year patients — and doctors who care for them — will finally see improvements in treating their symptoms.
Here are five bold predictions — all based on encouraging research — that could happen in 2024. At the very least, they are promising signs of progress against a debilitating and frustrating disease.
#1: We’ll gain a better understanding of each long COVID phenotype
This past year, a wide breadth of research began showing that long COVID can be defined by a number of different disease phenotypes that present a range of symptoms.
Researchers identified four clinical phenotypes: Chronic fatigue-like syndrome, headache, and memory loss; respiratory syndrome, which includes cough and difficulty breathing; chronic pain; and neurosensorial syndrome, which causes an altered sense of taste and smell.
Identifying specific diagnostic criteria for each phenotype would lead to better health outcomes for patients instead of treating them as if it were a “one-size-fits-all disease,” said Nisha Viswanathan, MD, director of the long COVID program at UCLA Health, Los Angeles, California.
Ultimately, she hopes that this year her patients will receive treatments based on the type of long COVID they’re personally experiencing, and the symptoms they have, leading to improved health outcomes and more rapid relief.
“Many new medications are focused on different pathways of long COVID, and the challenge becomes which drug is the right drug for each treatment,” said Dr. Viswanathan.
#2: Monoclonal antibodies may change the game
We’re starting to have a better understanding that what’s been called “viral persistence” as a main cause of long COVID may potentially be treated with monoclonal antibodies. These are antibodies produced by cloning unique white blood cells to target the circulating spike proteins in the blood that hang out in viral reservoirs and cause the immune system to react as if it’s still fighting acute COVID-19.
Smaller-scale studies have already shown promising results. A January 2024 study published in The American Journal of Emergency Medicine followed three patients who completely recovered from long COVID after taking monoclonal antibodies. “Remission occurred despite dissimilar past histories, sex, age, and illness duration,” wrote the study authors.
Larger clinical trials are underway at the University of California, San Francisco, California, to test targeted monoclonal antibodies. If the results of the larger study show that monoclonal antibodies are beneficial, then it could be a game changer for a large swath of patients around the world, said David F. Putrino, PhD, who runs the long COVID clinic at Mount Sinai Health System in New York City.
“The idea is that the downstream damage caused by viral persistence will resolve itself once you wipe out the virus,” said Dr. Putrino.
#3: Paxlovid could prove effective for long COVID
The US Food and Drug Administration granted approval for Paxlovid last May for the treatment of mild to moderate COVID-19 in adults at a high risk for severe disease. The medication is made up of two drugs packaged together. The first, nirmatrelvir, works by blocking a key enzyme required for virus replication. The second, ritonavir, is an antiviral that’s been used in patients with HIV and helps boost levels of antivirals in the body.
In a large-scale trial headed up by Dr. Putrino and his team, the oral antiviral is being studied for use in the post-viral stage in patients who test negative for acute COVID-19 but have persisting symptoms of long COVID.
Similar to monoclonal antibodies, the idea is to quell viral persistence. If patients have long COVID because they can’t clear SAR-CoV-2 from their bodies, Paxlovid could help. But unlike monoclonal antibodies that quash the virus, Paxlovid stops the virus from replicating. It’s a different mechanism with the same end goal.
It’s been a controversial treatment because it’s life-changing for some patients and ineffective for others. In addition, it can cause a range of side effects such as diarrhea, nausea, vomiting, and an impaired sense of taste. The goal of the trial is to see which patients with long COVID are most likely to benefit from the treatment.
#4: Anti-inflammatories like metformin could prove useful
Many of the inflammatory markers persistent in patients with long COVID were similarly present in patients with autoimmune diseases like rheumatoid arthritis, according to a July 2023 study published in JAMA.
The hope is that anti-inflammatory medications may be used to reduce inflammation causing long COVID symptoms. But drugs used to treat rheumatoid arthritis like abatacept and infliximabcan also have serious side effects, including increased risk for infection, flu-like symptoms, and burning of the skin.
“Powerful anti-inflammatories can change a number of pathways in the immune system,” said Grace McComsey, MD, who leads the long COVID RECOVER study at University Hospitals Health System in Cleveland, Ohio. Anti-inflammatories hold promise but, Dr. McComsey said, “some are more toxic with many side effects, so even if they work, there’s still a question about who should take them.”
Still, other anti-inflammatories that could work don’t have as many side effects. For example, a study published in The Lancet Infectious Diseases found that the diabetes drug metformin reduced a patient’s risk for long COVID up to 40% when the drug was taken during the acute stage.
Metformin, compared to other anti-inflammatories (also known as immune modulators), is an inexpensive and widely available drug with relatively few side effects compared with other medications.
#5: Serotonin levels — and selective serotonin reuptake inhibitors (SSRIs) — may be keys to unlocking long COVID
One of the most groundbreaking studies of the year came last November. A study published in the journal Cell found lower circulating serotonin levels in patents with long COVID than in those who did not have the condition. The study also found that the SSRI fluoxetine improved cognitive function in rat models infected with the virus.
Researchers found that the reduction in serotonin levels was partially caused by the body’s inability to absorb tryptophan, an amino acid that’s a precursor to serotonin. Overactivated blood platelets may also have played a role.
Michael Peluso, MD, an assistant research professor of infectious medicine at the UCSF School of Medicine, San Francisco, California, hopes to take the finding a step further, investigating whether increased serotonin levels in patients with long COVID will lead to improvements in symptoms.
“What we need now is a good clinical trial to see whether altering levels of serotonin in people with long COVID will lead to symptom relief,” Dr. Peluso said last month in an interview with this news organization.
If patients show an improvement in symptoms, then the next step is looking into whether SSRIs boost serotonin levels in patients and, as a result, reduce their symptoms.
A version of this article appeared on Medscape.com.
DoxyPEP: A New Option to Prevent Sexually Transmitted Infections
Sexually transmitted infections (STIs) continue to have a significant impact on the lives of adolescents and young adults. According to the Centers for Disease Control and Prevention’s (CDC) 2021 surveillance report, rates of gonorrhea and syphilis in the United States were at their highest since the early 1990s. Chlamydia, one of the most common STIs, had a peak rate in 2019, but more recent rates may have been impacted by the COVID-19 pandemic. In 2021, those 15-24 years of age accounted for 58.5% of all chlamydia infections, 40.4% of all gonorrhea infections, and 18.3% of all syphilis infections in the US.
While pediatricians should discuss sexual health and STI screening with all their adolescent and young adult patients, LGBTQ+ youth are disproportionately impacted by STIs. For example, cisgender men who have sex with men have significantly higher rates of HIV, gonorrhea, and syphilis. These disparities are likely related to unequal access to care, systemic homophobia/transphobia, stigma, and differences in sexual networks and are even more pronounced for those with intersectional minoritized identities such as LGBTQ+ youth of color.1
In the past 12 years, there have been significant advances in HIV prevention methods, including the approval and use of pre-exposure prophylaxis or “PrEP” (a medication regimen that is taken on an ongoing basis to provide highly effective protection against HIV infection) and more widespread use of post-exposure prophylaxis or “PEP” (a medication regimen taken for 1 month after a potential exposure to HIV to prevent HIV from establishing an ongoing infection) outside of the medical setting. While these interventions have the potential to decrease rates of HIV infection, they do not prevent any other STIs.2-3
The current strategies to prevent bacterial STIs include discussions of sexual practices, counseling on risk reduction strategies such as decreased number of partners and condom use, routine screening in those at risk every 3-12 months, and timely diagnosis and treatment of infections in patients and their partners to avoid further transmission.4 Given the increasing rates of bacterial STIs (gonorrhea, chlamydia, and syphilis), additional biomedical prevention methods are greatly needed.
Doxycycline as post-exposure prophylaxis
Doxycycline is a tetracycline antibiotic effective against a wide range of bacteria and is commonly used in the treatment of acne, skin infections, Lyme disease, and STIs, and can also be used in the treatment and prevention of malaria.5 For STIs, doxycycline is currently the recommended treatment for chlamydia and is also an alternate therapy for syphilis in nonpregnant patients when penicillin is not accessible or in those with severe penicillin allergy.4 This medication is often well tolerated with the most common side effects including gastrointestinal irritation and photosensitivity. Doxycycline is contraindicated in pregnancy due to its potential impact on tooth and bone development.5
Given its general tolerability and activity against bacterial STIs, new and emerging studies have examined doxycycline as post-exposure prophylaxis (DoxyPEP: one dose of doxycycline 200 mg PO within 72 hours after unprotected sex) in an attempt to decrease the incidence of new bacterial STIs in populations at risk. Three of the studies cited in the preliminary CDC DoxyPEP guidelines were conducted in cisgender men who have sex with men and transgender women either living with HIV or taking PrEP for HIV prevention. All three studies demonstrated significantly lower risk of chlamydia and syphilis, while two of the studies also showed a significantly lower risk of gonorrhea. One additional study was conducted in cisgender women in Kenya. This study did not show any statistically significant difference in the risk of chlamydia or gonorrhea in the intervention group, but may have been limited by low adherence to the DoxyPEP regimen. There were no serious adverse events reported in any of the studies attributed to doxycycline.6
Avoiding antibiotic resistance
With the increased use of antibiotics, attention must always be paid to the potential for increasing antibiotic resistance. The preliminary CDC DoxyPEP guidelines outline mixed results in the DoxyPEP studies that had limited follow-up timeframes, making it difficult to draw conclusions: “Current data suggest overall benefit of the use of doxycycline PEP, but potential risks related to the development of resistance and impacts on the microbiome will need to be closely monitored after implementation of these guidelines.” Official guideline recommendations from the CDC regarding DoxyPEP are currently pending after a period of public comment on the preliminary drafted guidelines.6 However, the New York State Department of Health AIDS Institute released guidelines for DoxyPEP in September 2023 and several large urban public health departments have also issued their own guidance that largely align with the preliminary CDC guidelines.7
Recommendations currently emphasize that the goal is to allow for implementation of DoxyPEP in those who would benefit the most from the intervention (i.e., cisgender men who have sex with men and transgender women with a history of at least one bacterial STI in the last 12 months with ongoing risk of infection), while also minimizing antibiotic use. It should also be considered for those without a recent infection who have increased likelihood of exposure to bacterial STIs. DoxyPEP is likely to be effective in other populations (e.g., cisgender women, cisgender men who have sex with women, transgender men), but data are currently limited, and the risk/benefit ratio may be different in these populations. The recommended dose for DoxyPEP is doxycycline 200 mg once as soon as possible (within 72 hours) of unprotected oral, vaginal, or anal sex with a maximum of one dose every 24 hours. For those being prescribed DoxyPEP, gonorrhea and chlamydia screening of all anatomic sites of exposure (urine sample or frontal swab, throat swab, and/or rectal swab) should be conducted at baseline and then every 3-6 months in addition to blood testing for syphilis and HIV if indicated.6-7
Another option in our toolkit
DoxyPEP should be viewed as one more option in our toolkit of sexual health services alongside risk reduction strategies (e.g., open discussions with partners, decreased number of partners, and condom use), routine STI screening and treatment, PrEP and PEP for HIV prevention, and pregnancy prevention. Not all of these tools will be relevant to each individual and discussions around sexual health should be patient-centered and focused on their own personal goals. As pediatricians, we should provide guidance to all adolescents and young adults on options to improve their sexual health and empower them to embrace their bodily autonomy.
Dr. Warus is in the division of adolescent and young adult medicine at Children’s Hospital of Los Angeles, where he specializes in care for transgender and gender-nonconforming youth, and LGBTQ health for youth. He is an assistant professor of pediatrics at USC.
Resources
CDC – Sexually Transmitted Infection Treatment Guidelines, 2021
CDC – [Preliminary] Guidelines for the Use of Doxycycline Post-Exposure Prophylaxis for Bacterial Sexually Transmitted Infection (STI) Prevention
New York State Department of Health AIDS Institute – Doxycycline Post-Exposure Prophylaxis to Prevent Bacterial Sexually Transmitted Infections
Los Angeles County Department of Public Health – DoxyPEP for STI Prevention
References
1. Division of STD Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2021: National Overview of STDs, 2021. Last Reviewed May 16, 2023.
2. Centers for Disease Control and Prevention: US Public Health Service: Preexposure Prophylaxis for the Prevention of HIV Infection in the United States – 2021 Update: A Clinical Practice Guideline. Published 2021.
3. Centers for Disease Control and Prevention: US Department of Health and Human Services: Updated Guidelines for Antiretroviral Postexposure Prophylaxis After Sexual, Injection Drug Use, or Other Nonoccupational Exposure to HIV – United States, 2016. Published 2016. .
4. Workowski KA et al. Sexually Transmitted Infection Treatment Guidelines, 2021. MMWR Recomm Rep. 2021;70(4):2-10.
5. Patel RS and Parmar M. Doxycycline Hyclate. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Last Updated May 22, 2023.
6. Centers for Disease Control and Prevention. [Preliminary] Guidelines for the Use of Doxycycline Post-Exposure Prophylaxis for Bacterial Sexually Transmitted Infection (STI) Prevention. Published Oct 1, 2023.
7. DiMarco DE, et al. Doxycycline Post-Exposure Prophylaxis to Prevent Bacterial Sexually Transmitted Infections. New York State Department of Health AIDS Institute. Published September 25, 2023.
Sexually transmitted infections (STIs) continue to have a significant impact on the lives of adolescents and young adults. According to the Centers for Disease Control and Prevention’s (CDC) 2021 surveillance report, rates of gonorrhea and syphilis in the United States were at their highest since the early 1990s. Chlamydia, one of the most common STIs, had a peak rate in 2019, but more recent rates may have been impacted by the COVID-19 pandemic. In 2021, those 15-24 years of age accounted for 58.5% of all chlamydia infections, 40.4% of all gonorrhea infections, and 18.3% of all syphilis infections in the US.
While pediatricians should discuss sexual health and STI screening with all their adolescent and young adult patients, LGBTQ+ youth are disproportionately impacted by STIs. For example, cisgender men who have sex with men have significantly higher rates of HIV, gonorrhea, and syphilis. These disparities are likely related to unequal access to care, systemic homophobia/transphobia, stigma, and differences in sexual networks and are even more pronounced for those with intersectional minoritized identities such as LGBTQ+ youth of color.1
In the past 12 years, there have been significant advances in HIV prevention methods, including the approval and use of pre-exposure prophylaxis or “PrEP” (a medication regimen that is taken on an ongoing basis to provide highly effective protection against HIV infection) and more widespread use of post-exposure prophylaxis or “PEP” (a medication regimen taken for 1 month after a potential exposure to HIV to prevent HIV from establishing an ongoing infection) outside of the medical setting. While these interventions have the potential to decrease rates of HIV infection, they do not prevent any other STIs.2-3
The current strategies to prevent bacterial STIs include discussions of sexual practices, counseling on risk reduction strategies such as decreased number of partners and condom use, routine screening in those at risk every 3-12 months, and timely diagnosis and treatment of infections in patients and their partners to avoid further transmission.4 Given the increasing rates of bacterial STIs (gonorrhea, chlamydia, and syphilis), additional biomedical prevention methods are greatly needed.
Doxycycline as post-exposure prophylaxis
Doxycycline is a tetracycline antibiotic effective against a wide range of bacteria and is commonly used in the treatment of acne, skin infections, Lyme disease, and STIs, and can also be used in the treatment and prevention of malaria.5 For STIs, doxycycline is currently the recommended treatment for chlamydia and is also an alternate therapy for syphilis in nonpregnant patients when penicillin is not accessible or in those with severe penicillin allergy.4 This medication is often well tolerated with the most common side effects including gastrointestinal irritation and photosensitivity. Doxycycline is contraindicated in pregnancy due to its potential impact on tooth and bone development.5
Given its general tolerability and activity against bacterial STIs, new and emerging studies have examined doxycycline as post-exposure prophylaxis (DoxyPEP: one dose of doxycycline 200 mg PO within 72 hours after unprotected sex) in an attempt to decrease the incidence of new bacterial STIs in populations at risk. Three of the studies cited in the preliminary CDC DoxyPEP guidelines were conducted in cisgender men who have sex with men and transgender women either living with HIV or taking PrEP for HIV prevention. All three studies demonstrated significantly lower risk of chlamydia and syphilis, while two of the studies also showed a significantly lower risk of gonorrhea. One additional study was conducted in cisgender women in Kenya. This study did not show any statistically significant difference in the risk of chlamydia or gonorrhea in the intervention group, but may have been limited by low adherence to the DoxyPEP regimen. There were no serious adverse events reported in any of the studies attributed to doxycycline.6
Avoiding antibiotic resistance
With the increased use of antibiotics, attention must always be paid to the potential for increasing antibiotic resistance. The preliminary CDC DoxyPEP guidelines outline mixed results in the DoxyPEP studies that had limited follow-up timeframes, making it difficult to draw conclusions: “Current data suggest overall benefit of the use of doxycycline PEP, but potential risks related to the development of resistance and impacts on the microbiome will need to be closely monitored after implementation of these guidelines.” Official guideline recommendations from the CDC regarding DoxyPEP are currently pending after a period of public comment on the preliminary drafted guidelines.6 However, the New York State Department of Health AIDS Institute released guidelines for DoxyPEP in September 2023 and several large urban public health departments have also issued their own guidance that largely align with the preliminary CDC guidelines.7
Recommendations currently emphasize that the goal is to allow for implementation of DoxyPEP in those who would benefit the most from the intervention (i.e., cisgender men who have sex with men and transgender women with a history of at least one bacterial STI in the last 12 months with ongoing risk of infection), while also minimizing antibiotic use. It should also be considered for those without a recent infection who have increased likelihood of exposure to bacterial STIs. DoxyPEP is likely to be effective in other populations (e.g., cisgender women, cisgender men who have sex with women, transgender men), but data are currently limited, and the risk/benefit ratio may be different in these populations. The recommended dose for DoxyPEP is doxycycline 200 mg once as soon as possible (within 72 hours) of unprotected oral, vaginal, or anal sex with a maximum of one dose every 24 hours. For those being prescribed DoxyPEP, gonorrhea and chlamydia screening of all anatomic sites of exposure (urine sample or frontal swab, throat swab, and/or rectal swab) should be conducted at baseline and then every 3-6 months in addition to blood testing for syphilis and HIV if indicated.6-7
Another option in our toolkit
DoxyPEP should be viewed as one more option in our toolkit of sexual health services alongside risk reduction strategies (e.g., open discussions with partners, decreased number of partners, and condom use), routine STI screening and treatment, PrEP and PEP for HIV prevention, and pregnancy prevention. Not all of these tools will be relevant to each individual and discussions around sexual health should be patient-centered and focused on their own personal goals. As pediatricians, we should provide guidance to all adolescents and young adults on options to improve their sexual health and empower them to embrace their bodily autonomy.
Dr. Warus is in the division of adolescent and young adult medicine at Children’s Hospital of Los Angeles, where he specializes in care for transgender and gender-nonconforming youth, and LGBTQ health for youth. He is an assistant professor of pediatrics at USC.
Resources
CDC – Sexually Transmitted Infection Treatment Guidelines, 2021
CDC – [Preliminary] Guidelines for the Use of Doxycycline Post-Exposure Prophylaxis for Bacterial Sexually Transmitted Infection (STI) Prevention
New York State Department of Health AIDS Institute – Doxycycline Post-Exposure Prophylaxis to Prevent Bacterial Sexually Transmitted Infections
Los Angeles County Department of Public Health – DoxyPEP for STI Prevention
References
1. Division of STD Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2021: National Overview of STDs, 2021. Last Reviewed May 16, 2023.
2. Centers for Disease Control and Prevention: US Public Health Service: Preexposure Prophylaxis for the Prevention of HIV Infection in the United States – 2021 Update: A Clinical Practice Guideline. Published 2021.
3. Centers for Disease Control and Prevention: US Department of Health and Human Services: Updated Guidelines for Antiretroviral Postexposure Prophylaxis After Sexual, Injection Drug Use, or Other Nonoccupational Exposure to HIV – United States, 2016. Published 2016. .
4. Workowski KA et al. Sexually Transmitted Infection Treatment Guidelines, 2021. MMWR Recomm Rep. 2021;70(4):2-10.
5. Patel RS and Parmar M. Doxycycline Hyclate. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Last Updated May 22, 2023.
6. Centers for Disease Control and Prevention. [Preliminary] Guidelines for the Use of Doxycycline Post-Exposure Prophylaxis for Bacterial Sexually Transmitted Infection (STI) Prevention. Published Oct 1, 2023.
7. DiMarco DE, et al. Doxycycline Post-Exposure Prophylaxis to Prevent Bacterial Sexually Transmitted Infections. New York State Department of Health AIDS Institute. Published September 25, 2023.
Sexually transmitted infections (STIs) continue to have a significant impact on the lives of adolescents and young adults. According to the Centers for Disease Control and Prevention’s (CDC) 2021 surveillance report, rates of gonorrhea and syphilis in the United States were at their highest since the early 1990s. Chlamydia, one of the most common STIs, had a peak rate in 2019, but more recent rates may have been impacted by the COVID-19 pandemic. In 2021, those 15-24 years of age accounted for 58.5% of all chlamydia infections, 40.4% of all gonorrhea infections, and 18.3% of all syphilis infections in the US.
While pediatricians should discuss sexual health and STI screening with all their adolescent and young adult patients, LGBTQ+ youth are disproportionately impacted by STIs. For example, cisgender men who have sex with men have significantly higher rates of HIV, gonorrhea, and syphilis. These disparities are likely related to unequal access to care, systemic homophobia/transphobia, stigma, and differences in sexual networks and are even more pronounced for those with intersectional minoritized identities such as LGBTQ+ youth of color.1
In the past 12 years, there have been significant advances in HIV prevention methods, including the approval and use of pre-exposure prophylaxis or “PrEP” (a medication regimen that is taken on an ongoing basis to provide highly effective protection against HIV infection) and more widespread use of post-exposure prophylaxis or “PEP” (a medication regimen taken for 1 month after a potential exposure to HIV to prevent HIV from establishing an ongoing infection) outside of the medical setting. While these interventions have the potential to decrease rates of HIV infection, they do not prevent any other STIs.2-3
The current strategies to prevent bacterial STIs include discussions of sexual practices, counseling on risk reduction strategies such as decreased number of partners and condom use, routine screening in those at risk every 3-12 months, and timely diagnosis and treatment of infections in patients and their partners to avoid further transmission.4 Given the increasing rates of bacterial STIs (gonorrhea, chlamydia, and syphilis), additional biomedical prevention methods are greatly needed.
Doxycycline as post-exposure prophylaxis
Doxycycline is a tetracycline antibiotic effective against a wide range of bacteria and is commonly used in the treatment of acne, skin infections, Lyme disease, and STIs, and can also be used in the treatment and prevention of malaria.5 For STIs, doxycycline is currently the recommended treatment for chlamydia and is also an alternate therapy for syphilis in nonpregnant patients when penicillin is not accessible or in those with severe penicillin allergy.4 This medication is often well tolerated with the most common side effects including gastrointestinal irritation and photosensitivity. Doxycycline is contraindicated in pregnancy due to its potential impact on tooth and bone development.5
Given its general tolerability and activity against bacterial STIs, new and emerging studies have examined doxycycline as post-exposure prophylaxis (DoxyPEP: one dose of doxycycline 200 mg PO within 72 hours after unprotected sex) in an attempt to decrease the incidence of new bacterial STIs in populations at risk. Three of the studies cited in the preliminary CDC DoxyPEP guidelines were conducted in cisgender men who have sex with men and transgender women either living with HIV or taking PrEP for HIV prevention. All three studies demonstrated significantly lower risk of chlamydia and syphilis, while two of the studies also showed a significantly lower risk of gonorrhea. One additional study was conducted in cisgender women in Kenya. This study did not show any statistically significant difference in the risk of chlamydia or gonorrhea in the intervention group, but may have been limited by low adherence to the DoxyPEP regimen. There were no serious adverse events reported in any of the studies attributed to doxycycline.6
Avoiding antibiotic resistance
With the increased use of antibiotics, attention must always be paid to the potential for increasing antibiotic resistance. The preliminary CDC DoxyPEP guidelines outline mixed results in the DoxyPEP studies that had limited follow-up timeframes, making it difficult to draw conclusions: “Current data suggest overall benefit of the use of doxycycline PEP, but potential risks related to the development of resistance and impacts on the microbiome will need to be closely monitored after implementation of these guidelines.” Official guideline recommendations from the CDC regarding DoxyPEP are currently pending after a period of public comment on the preliminary drafted guidelines.6 However, the New York State Department of Health AIDS Institute released guidelines for DoxyPEP in September 2023 and several large urban public health departments have also issued their own guidance that largely align with the preliminary CDC guidelines.7
Recommendations currently emphasize that the goal is to allow for implementation of DoxyPEP in those who would benefit the most from the intervention (i.e., cisgender men who have sex with men and transgender women with a history of at least one bacterial STI in the last 12 months with ongoing risk of infection), while also minimizing antibiotic use. It should also be considered for those without a recent infection who have increased likelihood of exposure to bacterial STIs. DoxyPEP is likely to be effective in other populations (e.g., cisgender women, cisgender men who have sex with women, transgender men), but data are currently limited, and the risk/benefit ratio may be different in these populations. The recommended dose for DoxyPEP is doxycycline 200 mg once as soon as possible (within 72 hours) of unprotected oral, vaginal, or anal sex with a maximum of one dose every 24 hours. For those being prescribed DoxyPEP, gonorrhea and chlamydia screening of all anatomic sites of exposure (urine sample or frontal swab, throat swab, and/or rectal swab) should be conducted at baseline and then every 3-6 months in addition to blood testing for syphilis and HIV if indicated.6-7
Another option in our toolkit
DoxyPEP should be viewed as one more option in our toolkit of sexual health services alongside risk reduction strategies (e.g., open discussions with partners, decreased number of partners, and condom use), routine STI screening and treatment, PrEP and PEP for HIV prevention, and pregnancy prevention. Not all of these tools will be relevant to each individual and discussions around sexual health should be patient-centered and focused on their own personal goals. As pediatricians, we should provide guidance to all adolescents and young adults on options to improve their sexual health and empower them to embrace their bodily autonomy.
Dr. Warus is in the division of adolescent and young adult medicine at Children’s Hospital of Los Angeles, where he specializes in care for transgender and gender-nonconforming youth, and LGBTQ health for youth. He is an assistant professor of pediatrics at USC.
Resources
CDC – Sexually Transmitted Infection Treatment Guidelines, 2021
CDC – [Preliminary] Guidelines for the Use of Doxycycline Post-Exposure Prophylaxis for Bacterial Sexually Transmitted Infection (STI) Prevention
New York State Department of Health AIDS Institute – Doxycycline Post-Exposure Prophylaxis to Prevent Bacterial Sexually Transmitted Infections
Los Angeles County Department of Public Health – DoxyPEP for STI Prevention
References
1. Division of STD Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2021: National Overview of STDs, 2021. Last Reviewed May 16, 2023.
2. Centers for Disease Control and Prevention: US Public Health Service: Preexposure Prophylaxis for the Prevention of HIV Infection in the United States – 2021 Update: A Clinical Practice Guideline. Published 2021.
3. Centers for Disease Control and Prevention: US Department of Health and Human Services: Updated Guidelines for Antiretroviral Postexposure Prophylaxis After Sexual, Injection Drug Use, or Other Nonoccupational Exposure to HIV – United States, 2016. Published 2016. .
4. Workowski KA et al. Sexually Transmitted Infection Treatment Guidelines, 2021. MMWR Recomm Rep. 2021;70(4):2-10.
5. Patel RS and Parmar M. Doxycycline Hyclate. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Last Updated May 22, 2023.
6. Centers for Disease Control and Prevention. [Preliminary] Guidelines for the Use of Doxycycline Post-Exposure Prophylaxis for Bacterial Sexually Transmitted Infection (STI) Prevention. Published Oct 1, 2023.
7. DiMarco DE, et al. Doxycycline Post-Exposure Prophylaxis to Prevent Bacterial Sexually Transmitted Infections. New York State Department of Health AIDS Institute. Published September 25, 2023.
Are You Unwittingly Aiding the Rise of Superfungi?
Unnecessary or incorrect use of topical antifungal medications is driving the spread of fungal infections like ringworm, which are becoming more difficult to treat, according to a January 11 study published in Morbidity and Mortality Weekly Report.
If a patient’s condition is not caused by a fungus but is treated as such, treatment will be ineffective.
such as clotrimazole or combinations of antifungals and corticosteroids. And because many topical treatments are also available over-the-counter, doctors should advise patients about how to use them correctly.
“In the last few years, there have been many antifungal resistant cases of tinea corporisand onychomycosisreported,” or ringworm and finger or toenail infections, respectively, said Shari Lipner, MD, PhD, a dermatologist at Weill Cornell Medicine in New York, and an author of the study.
Many of these cases originated in South Asia and have also been reported in Europe and Canada. In 2023, the first cases of a new strain of antifungal-resistant ringworm were reported in the United States. This species, Trichophyton indotineae, does not respond to topical medications, requiring oral treatment instead.
“It’s really a serious problem and a huge public health concern,” Dr. Lipner said.
For the new study, Dr. Lipner and colleagues examined prescription patterns from 2021 Medicare Part D claims of topical antifungals. They report that 6.5 million topical antifungal prescriptions were filled that year, some of which included steroids in the formulation. Primary care clinicians wrote 40% of these prescriptions, the most for any clinician group. The estimate is almost certainly an undercount of topical antifungal use because the database did not include over-the-counter purchases or data from other insurance payers.
The number of prescriptions equate to 1 in every 8 Medicare Part D beneficiary receiving an antifungal, the researchers reported.
“If I think about the patients that come into my office, I’m certainly not giving an antifungal to 1 in 8 of them, and I see a lot of fungal infections,” Dr. Lipner said. The findings suggest to Dr. Lipner that some clinicians are diagnosing ringworm by eyesight alone rather than confirming the diagnosis with techniques such as microscopy, fungal culture testing, or polymerase chain reaction testing.
Sometimes what looks like ringworm may actually be eczema, in which case, the topical antifungal would not be appropriate, according to Avrom Caplan, MD, a dermatologist at NYU Langone Health in New York.
“If you’re prescribing something to somebody that they don’t need, you’re basically exposing them to the side effects without the benefit,” Dr. Caplan, who was not part of the study, said.
Dr. Caplan, who reported the first cases of ringworm that only responded to oral medications in the United States, stressed that topical treatments work fine for many ringworm cases today. But if indiscriminate prescribing spurs the development of more resilient fungi, more situations may arise in which only oral medications work in the future, Dr. Caplan said. In addition, oral medications are inherently more demanding on a patient than something they can rub on their skin, Dr. Caplan added.
“We hope that physicians will really think hard about this study and change their practices if they’re not confirming the diagnosis,” Dr. Lipner said.
Dr. Lipner and Dr. Caplan report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Unnecessary or incorrect use of topical antifungal medications is driving the spread of fungal infections like ringworm, which are becoming more difficult to treat, according to a January 11 study published in Morbidity and Mortality Weekly Report.
If a patient’s condition is not caused by a fungus but is treated as such, treatment will be ineffective.
such as clotrimazole or combinations of antifungals and corticosteroids. And because many topical treatments are also available over-the-counter, doctors should advise patients about how to use them correctly.
“In the last few years, there have been many antifungal resistant cases of tinea corporisand onychomycosisreported,” or ringworm and finger or toenail infections, respectively, said Shari Lipner, MD, PhD, a dermatologist at Weill Cornell Medicine in New York, and an author of the study.
Many of these cases originated in South Asia and have also been reported in Europe and Canada. In 2023, the first cases of a new strain of antifungal-resistant ringworm were reported in the United States. This species, Trichophyton indotineae, does not respond to topical medications, requiring oral treatment instead.
“It’s really a serious problem and a huge public health concern,” Dr. Lipner said.
For the new study, Dr. Lipner and colleagues examined prescription patterns from 2021 Medicare Part D claims of topical antifungals. They report that 6.5 million topical antifungal prescriptions were filled that year, some of which included steroids in the formulation. Primary care clinicians wrote 40% of these prescriptions, the most for any clinician group. The estimate is almost certainly an undercount of topical antifungal use because the database did not include over-the-counter purchases or data from other insurance payers.
The number of prescriptions equate to 1 in every 8 Medicare Part D beneficiary receiving an antifungal, the researchers reported.
“If I think about the patients that come into my office, I’m certainly not giving an antifungal to 1 in 8 of them, and I see a lot of fungal infections,” Dr. Lipner said. The findings suggest to Dr. Lipner that some clinicians are diagnosing ringworm by eyesight alone rather than confirming the diagnosis with techniques such as microscopy, fungal culture testing, or polymerase chain reaction testing.
Sometimes what looks like ringworm may actually be eczema, in which case, the topical antifungal would not be appropriate, according to Avrom Caplan, MD, a dermatologist at NYU Langone Health in New York.
“If you’re prescribing something to somebody that they don’t need, you’re basically exposing them to the side effects without the benefit,” Dr. Caplan, who was not part of the study, said.
Dr. Caplan, who reported the first cases of ringworm that only responded to oral medications in the United States, stressed that topical treatments work fine for many ringworm cases today. But if indiscriminate prescribing spurs the development of more resilient fungi, more situations may arise in which only oral medications work in the future, Dr. Caplan said. In addition, oral medications are inherently more demanding on a patient than something they can rub on their skin, Dr. Caplan added.
“We hope that physicians will really think hard about this study and change their practices if they’re not confirming the diagnosis,” Dr. Lipner said.
Dr. Lipner and Dr. Caplan report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Unnecessary or incorrect use of topical antifungal medications is driving the spread of fungal infections like ringworm, which are becoming more difficult to treat, according to a January 11 study published in Morbidity and Mortality Weekly Report.
If a patient’s condition is not caused by a fungus but is treated as such, treatment will be ineffective.
such as clotrimazole or combinations of antifungals and corticosteroids. And because many topical treatments are also available over-the-counter, doctors should advise patients about how to use them correctly.
“In the last few years, there have been many antifungal resistant cases of tinea corporisand onychomycosisreported,” or ringworm and finger or toenail infections, respectively, said Shari Lipner, MD, PhD, a dermatologist at Weill Cornell Medicine in New York, and an author of the study.
Many of these cases originated in South Asia and have also been reported in Europe and Canada. In 2023, the first cases of a new strain of antifungal-resistant ringworm were reported in the United States. This species, Trichophyton indotineae, does not respond to topical medications, requiring oral treatment instead.
“It’s really a serious problem and a huge public health concern,” Dr. Lipner said.
For the new study, Dr. Lipner and colleagues examined prescription patterns from 2021 Medicare Part D claims of topical antifungals. They report that 6.5 million topical antifungal prescriptions were filled that year, some of which included steroids in the formulation. Primary care clinicians wrote 40% of these prescriptions, the most for any clinician group. The estimate is almost certainly an undercount of topical antifungal use because the database did not include over-the-counter purchases or data from other insurance payers.
The number of prescriptions equate to 1 in every 8 Medicare Part D beneficiary receiving an antifungal, the researchers reported.
“If I think about the patients that come into my office, I’m certainly not giving an antifungal to 1 in 8 of them, and I see a lot of fungal infections,” Dr. Lipner said. The findings suggest to Dr. Lipner that some clinicians are diagnosing ringworm by eyesight alone rather than confirming the diagnosis with techniques such as microscopy, fungal culture testing, or polymerase chain reaction testing.
Sometimes what looks like ringworm may actually be eczema, in which case, the topical antifungal would not be appropriate, according to Avrom Caplan, MD, a dermatologist at NYU Langone Health in New York.
“If you’re prescribing something to somebody that they don’t need, you’re basically exposing them to the side effects without the benefit,” Dr. Caplan, who was not part of the study, said.
Dr. Caplan, who reported the first cases of ringworm that only responded to oral medications in the United States, stressed that topical treatments work fine for many ringworm cases today. But if indiscriminate prescribing spurs the development of more resilient fungi, more situations may arise in which only oral medications work in the future, Dr. Caplan said. In addition, oral medications are inherently more demanding on a patient than something they can rub on their skin, Dr. Caplan added.
“We hope that physicians will really think hard about this study and change their practices if they’re not confirming the diagnosis,” Dr. Lipner said.
Dr. Lipner and Dr. Caplan report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Toothbrushing in Hospital Reduces Infections and Death
Daily toothbrushing is associated with a reduced incidence of hospital-acquired pneumonia (HAP), especially in patients on mechanical ventilation. This practice also is associated with lower intensive care unit (ICU) mortality, shorter ICU admissions, and shorter ventilator dependency. These are the findings of a meta-analysis published in JAMA Internal Medicine. Hospital policies must reassess the importance of oral hygiene even, or perhaps especially, in situations in which attention is focused elsewhere.
Oral Microbiota and Lungs
HAP largely results from the aspiration of microorganisms present in the oral cavity. In fact, the oral microbiota comprises an estimated 700 species of bacteria, fungi, viruses, and protozoa. There is a known link between oral health and the development of pneumonia, and rigorous oral hygiene is part of the recommendations for preventing HAP. But the methods that should be used for ensuring good hygiene haven’t been determined. The use of chlorhexidine-based mouthwash is debated because there is no evidence that it prevents pneumonia and because some studies have suggested a link between chlorhexidine and higher mortality rates.
Yet, guidelines have focused very little on brushing as a measure for preventing hospital-acquired infections, meaning that every hospital has its own way of doing things.
What Data Show
Selina Ehrenzeller, MD, and Michael Klompas, MD, MPH, of the department of population medicine at Harvard Medical School, Boston, conducted a systematic literature analysis to identify randomized clinical studies in which daily toothbrushing was shown to affect the risk for HAP in adult hospital inpatients. Fifteen studies met the inclusion criteria and were used for the meta-analysis. The effective population size was 2786 patients.
Daily toothbrushing was associated with a 33% lower risk for HAP (relative risk [RR], 0.67) and a 29% lower risk for ICU mortality (RR, 0.81). Reduction in pneumonia incidence was significant for patients receiving invasive mechanical ventilation (RR, 0.68) but not for patients who were not receiving invasive mechanical ventilation. Toothbrushing for patients in the ICU was associated with fewer days of mechanical ventilation (mean difference, −1.24 days) and a shorter ICU length of stay (mean difference, −1.78 days). Brushing twice a day vs more frequent intervals was associated with similar effect estimates. No differences were seen in duration of stay in various ICU subdepartments and in the use of antibiotics that were linked to daily toothbrushing.
Expert Opinion
“This study represents an exciting contribution to infection prevention and reinforces the notion that routine toothbrushing is an essential component of standard of care in ventilated patients,” Rupak Datta, MD, PhD, assistant professor of infectious diseases at Yale University in New Haven, Connecticut, and specialist in antimicrobial resistance in hospital settings, wrote in a commentary on the study. According to Dr. Datta, there is still uncertainty regarding the importance of this practice in preventing nonventilator-HAP, as the investigators could identify only two studies with nonventilated patients that met inclusion criteria. Other studies will be needed to help standardize toothbrushing in hospital patients admitted in general. “As the literature on HAP evolves,” concluded Dr. Datta, “oral hygiene may take on an indispensable role, similar to hand washing, in preventing and controlling hospital-acquired infections.”
This article was translated from Univadis Italy, which is part of the Medscape Professional Network. A version of this article appeared on Medscape.com.
Daily toothbrushing is associated with a reduced incidence of hospital-acquired pneumonia (HAP), especially in patients on mechanical ventilation. This practice also is associated with lower intensive care unit (ICU) mortality, shorter ICU admissions, and shorter ventilator dependency. These are the findings of a meta-analysis published in JAMA Internal Medicine. Hospital policies must reassess the importance of oral hygiene even, or perhaps especially, in situations in which attention is focused elsewhere.
Oral Microbiota and Lungs
HAP largely results from the aspiration of microorganisms present in the oral cavity. In fact, the oral microbiota comprises an estimated 700 species of bacteria, fungi, viruses, and protozoa. There is a known link between oral health and the development of pneumonia, and rigorous oral hygiene is part of the recommendations for preventing HAP. But the methods that should be used for ensuring good hygiene haven’t been determined. The use of chlorhexidine-based mouthwash is debated because there is no evidence that it prevents pneumonia and because some studies have suggested a link between chlorhexidine and higher mortality rates.
Yet, guidelines have focused very little on brushing as a measure for preventing hospital-acquired infections, meaning that every hospital has its own way of doing things.
What Data Show
Selina Ehrenzeller, MD, and Michael Klompas, MD, MPH, of the department of population medicine at Harvard Medical School, Boston, conducted a systematic literature analysis to identify randomized clinical studies in which daily toothbrushing was shown to affect the risk for HAP in adult hospital inpatients. Fifteen studies met the inclusion criteria and were used for the meta-analysis. The effective population size was 2786 patients.
Daily toothbrushing was associated with a 33% lower risk for HAP (relative risk [RR], 0.67) and a 29% lower risk for ICU mortality (RR, 0.81). Reduction in pneumonia incidence was significant for patients receiving invasive mechanical ventilation (RR, 0.68) but not for patients who were not receiving invasive mechanical ventilation. Toothbrushing for patients in the ICU was associated with fewer days of mechanical ventilation (mean difference, −1.24 days) and a shorter ICU length of stay (mean difference, −1.78 days). Brushing twice a day vs more frequent intervals was associated with similar effect estimates. No differences were seen in duration of stay in various ICU subdepartments and in the use of antibiotics that were linked to daily toothbrushing.
Expert Opinion
“This study represents an exciting contribution to infection prevention and reinforces the notion that routine toothbrushing is an essential component of standard of care in ventilated patients,” Rupak Datta, MD, PhD, assistant professor of infectious diseases at Yale University in New Haven, Connecticut, and specialist in antimicrobial resistance in hospital settings, wrote in a commentary on the study. According to Dr. Datta, there is still uncertainty regarding the importance of this practice in preventing nonventilator-HAP, as the investigators could identify only two studies with nonventilated patients that met inclusion criteria. Other studies will be needed to help standardize toothbrushing in hospital patients admitted in general. “As the literature on HAP evolves,” concluded Dr. Datta, “oral hygiene may take on an indispensable role, similar to hand washing, in preventing and controlling hospital-acquired infections.”
This article was translated from Univadis Italy, which is part of the Medscape Professional Network. A version of this article appeared on Medscape.com.
Daily toothbrushing is associated with a reduced incidence of hospital-acquired pneumonia (HAP), especially in patients on mechanical ventilation. This practice also is associated with lower intensive care unit (ICU) mortality, shorter ICU admissions, and shorter ventilator dependency. These are the findings of a meta-analysis published in JAMA Internal Medicine. Hospital policies must reassess the importance of oral hygiene even, or perhaps especially, in situations in which attention is focused elsewhere.
Oral Microbiota and Lungs
HAP largely results from the aspiration of microorganisms present in the oral cavity. In fact, the oral microbiota comprises an estimated 700 species of bacteria, fungi, viruses, and protozoa. There is a known link between oral health and the development of pneumonia, and rigorous oral hygiene is part of the recommendations for preventing HAP. But the methods that should be used for ensuring good hygiene haven’t been determined. The use of chlorhexidine-based mouthwash is debated because there is no evidence that it prevents pneumonia and because some studies have suggested a link between chlorhexidine and higher mortality rates.
Yet, guidelines have focused very little on brushing as a measure for preventing hospital-acquired infections, meaning that every hospital has its own way of doing things.
What Data Show
Selina Ehrenzeller, MD, and Michael Klompas, MD, MPH, of the department of population medicine at Harvard Medical School, Boston, conducted a systematic literature analysis to identify randomized clinical studies in which daily toothbrushing was shown to affect the risk for HAP in adult hospital inpatients. Fifteen studies met the inclusion criteria and were used for the meta-analysis. The effective population size was 2786 patients.
Daily toothbrushing was associated with a 33% lower risk for HAP (relative risk [RR], 0.67) and a 29% lower risk for ICU mortality (RR, 0.81). Reduction in pneumonia incidence was significant for patients receiving invasive mechanical ventilation (RR, 0.68) but not for patients who were not receiving invasive mechanical ventilation. Toothbrushing for patients in the ICU was associated with fewer days of mechanical ventilation (mean difference, −1.24 days) and a shorter ICU length of stay (mean difference, −1.78 days). Brushing twice a day vs more frequent intervals was associated with similar effect estimates. No differences were seen in duration of stay in various ICU subdepartments and in the use of antibiotics that were linked to daily toothbrushing.
Expert Opinion
“This study represents an exciting contribution to infection prevention and reinforces the notion that routine toothbrushing is an essential component of standard of care in ventilated patients,” Rupak Datta, MD, PhD, assistant professor of infectious diseases at Yale University in New Haven, Connecticut, and specialist in antimicrobial resistance in hospital settings, wrote in a commentary on the study. According to Dr. Datta, there is still uncertainty regarding the importance of this practice in preventing nonventilator-HAP, as the investigators could identify only two studies with nonventilated patients that met inclusion criteria. Other studies will be needed to help standardize toothbrushing in hospital patients admitted in general. “As the literature on HAP evolves,” concluded Dr. Datta, “oral hygiene may take on an indispensable role, similar to hand washing, in preventing and controlling hospital-acquired infections.”
This article was translated from Univadis Italy, which is part of the Medscape Professional Network. A version of this article appeared on Medscape.com.
Shingles Vaccine Offers 4 Years of Protection
Two doses of the recombinant zoster vaccine (RZV) are effective against herpes zoster (HZ) for 4 years after vaccination, according to a new study published in Annals of Internal Medicine.
Findings from the prospective cohort study showed that people who received two doses of the vaccine, regardless of when they received their second dose, experienced 79% vaccine effectiveness (VE) during the first year, with effectiveness decreasing to 73% by year 4. By contrast, the rate of effectiveness during the first year was 70% for people who received a single dose, falling to 52% effectiveness by year 4.
The findings also showed that the rate of effectiveness was 65% for those taking corticosteroids.
The study was conducted between 2018 and 2022 using data from the Vaccine Safety Datalink, a collaboration between the US Centers for Disease Control and Prevention (CDC) and nine healthcare systems across the country.
Researchers evaluated the incidence of HZ, as determined by a diagnosis and prescription for antiviral medication within 7 days of diagnosis, and monitored RZV status over time.
The findings may quell fears that waiting too long for the second dose reduces the effectiveness of the herpes vaccine, according to Nicola Klein, MD, PhD, director of the Vaccine Study Center at Kaiser Permanente in Oakland, California, who led the study.
The long-term efficacy of the vaccine is especially important because older adults are now living much longer than in previous years, according to Alexandra Tien, MD, a family physician at Medical Associates of Rhode Island in Providence.
“People live these days into their 80s and even 90s,” Dr. Tien said. “That’s a large number of years to need protection for, so it’s really important to have a long-lasting vaccine.”
The CDC currently recommends two doses of RZV separated by 2-6 months for patients aged 50 years and older. Adults older than 19 years who are immunocompromised should receive two doses of RZV separated by 1-2 months, the agency said.
According to Dr. Klein, research does not show whether VE for RZV wanes after 4 years. But interim findings from another study following people in clinical trials found VE levels remained high after 7 years.
The risk for HZ increases with age, reaching a lifetime risk of 50% among adults aged 85 years. Complications like postherpetic neuralgia (PHN) — characterized by long-term tingling, numbness, and disabling pain at the site of the rash — can interfere with the quality of life and ability to function in older adults. The CDC estimates that up to 18% of people with shingles experience PHN, and the risk increases with age.
Just like with any other vaccine, patients sometimes have concerns about the potential side effects of RZV, said Dr. Tien. But those effects, such as muscle pain, nausea, and fever, are mild compared to shingles.
“I always tell patients, with any vaccine, immunization is one of the biggest bangs for your buck in healthcare because you’re preventing a problem,” Dr. Tien said.
This study was funded by the CDC through contracts with participating sites. Study authors reported no disclosures. Dr. Tien reported no disclosures.
A version of this article appeared on Medscape.com.
Two doses of the recombinant zoster vaccine (RZV) are effective against herpes zoster (HZ) for 4 years after vaccination, according to a new study published in Annals of Internal Medicine.
Findings from the prospective cohort study showed that people who received two doses of the vaccine, regardless of when they received their second dose, experienced 79% vaccine effectiveness (VE) during the first year, with effectiveness decreasing to 73% by year 4. By contrast, the rate of effectiveness during the first year was 70% for people who received a single dose, falling to 52% effectiveness by year 4.
The findings also showed that the rate of effectiveness was 65% for those taking corticosteroids.
The study was conducted between 2018 and 2022 using data from the Vaccine Safety Datalink, a collaboration between the US Centers for Disease Control and Prevention (CDC) and nine healthcare systems across the country.
Researchers evaluated the incidence of HZ, as determined by a diagnosis and prescription for antiviral medication within 7 days of diagnosis, and monitored RZV status over time.
The findings may quell fears that waiting too long for the second dose reduces the effectiveness of the herpes vaccine, according to Nicola Klein, MD, PhD, director of the Vaccine Study Center at Kaiser Permanente in Oakland, California, who led the study.
The long-term efficacy of the vaccine is especially important because older adults are now living much longer than in previous years, according to Alexandra Tien, MD, a family physician at Medical Associates of Rhode Island in Providence.
“People live these days into their 80s and even 90s,” Dr. Tien said. “That’s a large number of years to need protection for, so it’s really important to have a long-lasting vaccine.”
The CDC currently recommends two doses of RZV separated by 2-6 months for patients aged 50 years and older. Adults older than 19 years who are immunocompromised should receive two doses of RZV separated by 1-2 months, the agency said.
According to Dr. Klein, research does not show whether VE for RZV wanes after 4 years. But interim findings from another study following people in clinical trials found VE levels remained high after 7 years.
The risk for HZ increases with age, reaching a lifetime risk of 50% among adults aged 85 years. Complications like postherpetic neuralgia (PHN) — characterized by long-term tingling, numbness, and disabling pain at the site of the rash — can interfere with the quality of life and ability to function in older adults. The CDC estimates that up to 18% of people with shingles experience PHN, and the risk increases with age.
Just like with any other vaccine, patients sometimes have concerns about the potential side effects of RZV, said Dr. Tien. But those effects, such as muscle pain, nausea, and fever, are mild compared to shingles.
“I always tell patients, with any vaccine, immunization is one of the biggest bangs for your buck in healthcare because you’re preventing a problem,” Dr. Tien said.
This study was funded by the CDC through contracts with participating sites. Study authors reported no disclosures. Dr. Tien reported no disclosures.
A version of this article appeared on Medscape.com.
Two doses of the recombinant zoster vaccine (RZV) are effective against herpes zoster (HZ) for 4 years after vaccination, according to a new study published in Annals of Internal Medicine.
Findings from the prospective cohort study showed that people who received two doses of the vaccine, regardless of when they received their second dose, experienced 79% vaccine effectiveness (VE) during the first year, with effectiveness decreasing to 73% by year 4. By contrast, the rate of effectiveness during the first year was 70% for people who received a single dose, falling to 52% effectiveness by year 4.
The findings also showed that the rate of effectiveness was 65% for those taking corticosteroids.
The study was conducted between 2018 and 2022 using data from the Vaccine Safety Datalink, a collaboration between the US Centers for Disease Control and Prevention (CDC) and nine healthcare systems across the country.
Researchers evaluated the incidence of HZ, as determined by a diagnosis and prescription for antiviral medication within 7 days of diagnosis, and monitored RZV status over time.
The findings may quell fears that waiting too long for the second dose reduces the effectiveness of the herpes vaccine, according to Nicola Klein, MD, PhD, director of the Vaccine Study Center at Kaiser Permanente in Oakland, California, who led the study.
The long-term efficacy of the vaccine is especially important because older adults are now living much longer than in previous years, according to Alexandra Tien, MD, a family physician at Medical Associates of Rhode Island in Providence.
“People live these days into their 80s and even 90s,” Dr. Tien said. “That’s a large number of years to need protection for, so it’s really important to have a long-lasting vaccine.”
The CDC currently recommends two doses of RZV separated by 2-6 months for patients aged 50 years and older. Adults older than 19 years who are immunocompromised should receive two doses of RZV separated by 1-2 months, the agency said.
According to Dr. Klein, research does not show whether VE for RZV wanes after 4 years. But interim findings from another study following people in clinical trials found VE levels remained high after 7 years.
The risk for HZ increases with age, reaching a lifetime risk of 50% among adults aged 85 years. Complications like postherpetic neuralgia (PHN) — characterized by long-term tingling, numbness, and disabling pain at the site of the rash — can interfere with the quality of life and ability to function in older adults. The CDC estimates that up to 18% of people with shingles experience PHN, and the risk increases with age.
Just like with any other vaccine, patients sometimes have concerns about the potential side effects of RZV, said Dr. Tien. But those effects, such as muscle pain, nausea, and fever, are mild compared to shingles.
“I always tell patients, with any vaccine, immunization is one of the biggest bangs for your buck in healthcare because you’re preventing a problem,” Dr. Tien said.
This study was funded by the CDC through contracts with participating sites. Study authors reported no disclosures. Dr. Tien reported no disclosures.
A version of this article appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
More Evidence Suggests That ‘Long Flu’ Is a Thing
You may have never heard of it, but you may have had it. More evidence points to “long flu” being a real phenomenon, with a large study showing symptoms persist at least 4 weeks or more after some people are hospitalized for the flu.
Researchers compared long flu to long COVID-19 and found long flu happened less often and was less severe overall. This difference could be because the flu mostly affects the lungs whereas COVID can affect any number of organ systems in the body.
The investigators were surprised that both long flu and long COVID were linked to a greater burden of health loss, compared to either initial infection.
“I think COVID and long COVID made us realize that infections have long-term consequences, and often the toll of those long-term consequences is much larger than the toll of acute disease,” said Ziyad Al-Aly, MD, senior author of the study and chief of research and development at the VA St. Louis Health Care System.
“I know, having studied long COVID for the past 4 years, I should not be surprised. But I am in awe of what these infections can do to the long-term health of affected individuals,” said Dr. Al-Aly, who is also a clinical epidemiologist at Washington University in St. Louis.
Dr. Al-Aly and colleagues Yan Xie, PhD, and Taeyoung Choi, MS, analyzed US Department of Veterans Affairs medical records. They compared 81,280 people hospitalized with COVID to 10,985 people hospitalized with the flu before the COVID pandemic. They checked up to 18 months after initial infections to see who developed long flu or long COVID symptoms.
The study was published online in The Lancet Infectious Diseases.
It’s an interesting study, said Aaron E. Glatt, MD, chairman of the Department of Medicine and a hospital epidemiologist at Mount Sinai South Nassau in Oceanside, NY, who was not part of the research.
“There is a concern with many viruses that you can have long-term consequences,” said Dr. Glatt, who is also a fellow of the Infectious Diseases Society of America. He said the possibility of long-term symptoms with the flu is not new, “but it’s nice to have more data.”
People hospitalized with COVID had a 50% higher risk of death during the study period than people hospitalized with the flu. Put another way, for every 100 people admitted to the hospital with COVID, about eight more died than those hospitalized with the flu over the following 18 months. Hospital admissions and admissions to the intensive care unit were also higher in the long COVID group — 20 more people and nine more people, respectively, for every 100 people admitted to the hospital with COVID.
More research is needed, Dr. Glatt said. “With many of these viruses, we don’t understand what they do to the body.” A prospective study to see if antiviral treatments make a difference, for example, would be useful, he noted.
Dr. Al-Aly and colleagues would like to do more studies.
“We need to more deeply understand how and why acute infections cause long-term illness,” he said, noting that he also wants to investigate ways to prevent and treat the long-term effects.
“Much remains to be done, and we are deeply committed to doing our best to develop those answers.”
A version of this article first appeared on WebMD.com.
You may have never heard of it, but you may have had it. More evidence points to “long flu” being a real phenomenon, with a large study showing symptoms persist at least 4 weeks or more after some people are hospitalized for the flu.
Researchers compared long flu to long COVID-19 and found long flu happened less often and was less severe overall. This difference could be because the flu mostly affects the lungs whereas COVID can affect any number of organ systems in the body.
The investigators were surprised that both long flu and long COVID were linked to a greater burden of health loss, compared to either initial infection.
“I think COVID and long COVID made us realize that infections have long-term consequences, and often the toll of those long-term consequences is much larger than the toll of acute disease,” said Ziyad Al-Aly, MD, senior author of the study and chief of research and development at the VA St. Louis Health Care System.
“I know, having studied long COVID for the past 4 years, I should not be surprised. But I am in awe of what these infections can do to the long-term health of affected individuals,” said Dr. Al-Aly, who is also a clinical epidemiologist at Washington University in St. Louis.
Dr. Al-Aly and colleagues Yan Xie, PhD, and Taeyoung Choi, MS, analyzed US Department of Veterans Affairs medical records. They compared 81,280 people hospitalized with COVID to 10,985 people hospitalized with the flu before the COVID pandemic. They checked up to 18 months after initial infections to see who developed long flu or long COVID symptoms.
The study was published online in The Lancet Infectious Diseases.
It’s an interesting study, said Aaron E. Glatt, MD, chairman of the Department of Medicine and a hospital epidemiologist at Mount Sinai South Nassau in Oceanside, NY, who was not part of the research.
“There is a concern with many viruses that you can have long-term consequences,” said Dr. Glatt, who is also a fellow of the Infectious Diseases Society of America. He said the possibility of long-term symptoms with the flu is not new, “but it’s nice to have more data.”
People hospitalized with COVID had a 50% higher risk of death during the study period than people hospitalized with the flu. Put another way, for every 100 people admitted to the hospital with COVID, about eight more died than those hospitalized with the flu over the following 18 months. Hospital admissions and admissions to the intensive care unit were also higher in the long COVID group — 20 more people and nine more people, respectively, for every 100 people admitted to the hospital with COVID.
More research is needed, Dr. Glatt said. “With many of these viruses, we don’t understand what they do to the body.” A prospective study to see if antiviral treatments make a difference, for example, would be useful, he noted.
Dr. Al-Aly and colleagues would like to do more studies.
“We need to more deeply understand how and why acute infections cause long-term illness,” he said, noting that he also wants to investigate ways to prevent and treat the long-term effects.
“Much remains to be done, and we are deeply committed to doing our best to develop those answers.”
A version of this article first appeared on WebMD.com.
You may have never heard of it, but you may have had it. More evidence points to “long flu” being a real phenomenon, with a large study showing symptoms persist at least 4 weeks or more after some people are hospitalized for the flu.
Researchers compared long flu to long COVID-19 and found long flu happened less often and was less severe overall. This difference could be because the flu mostly affects the lungs whereas COVID can affect any number of organ systems in the body.
The investigators were surprised that both long flu and long COVID were linked to a greater burden of health loss, compared to either initial infection.
“I think COVID and long COVID made us realize that infections have long-term consequences, and often the toll of those long-term consequences is much larger than the toll of acute disease,” said Ziyad Al-Aly, MD, senior author of the study and chief of research and development at the VA St. Louis Health Care System.
“I know, having studied long COVID for the past 4 years, I should not be surprised. But I am in awe of what these infections can do to the long-term health of affected individuals,” said Dr. Al-Aly, who is also a clinical epidemiologist at Washington University in St. Louis.
Dr. Al-Aly and colleagues Yan Xie, PhD, and Taeyoung Choi, MS, analyzed US Department of Veterans Affairs medical records. They compared 81,280 people hospitalized with COVID to 10,985 people hospitalized with the flu before the COVID pandemic. They checked up to 18 months after initial infections to see who developed long flu or long COVID symptoms.
The study was published online in The Lancet Infectious Diseases.
It’s an interesting study, said Aaron E. Glatt, MD, chairman of the Department of Medicine and a hospital epidemiologist at Mount Sinai South Nassau in Oceanside, NY, who was not part of the research.
“There is a concern with many viruses that you can have long-term consequences,” said Dr. Glatt, who is also a fellow of the Infectious Diseases Society of America. He said the possibility of long-term symptoms with the flu is not new, “but it’s nice to have more data.”
People hospitalized with COVID had a 50% higher risk of death during the study period than people hospitalized with the flu. Put another way, for every 100 people admitted to the hospital with COVID, about eight more died than those hospitalized with the flu over the following 18 months. Hospital admissions and admissions to the intensive care unit were also higher in the long COVID group — 20 more people and nine more people, respectively, for every 100 people admitted to the hospital with COVID.
More research is needed, Dr. Glatt said. “With many of these viruses, we don’t understand what they do to the body.” A prospective study to see if antiviral treatments make a difference, for example, would be useful, he noted.
Dr. Al-Aly and colleagues would like to do more studies.
“We need to more deeply understand how and why acute infections cause long-term illness,” he said, noting that he also wants to investigate ways to prevent and treat the long-term effects.
“Much remains to be done, and we are deeply committed to doing our best to develop those answers.”
A version of this article first appeared on WebMD.com.
FROM THE LANCET INFECTIOUS DISEASES
Musculoskeletal Symptoms Often Misattributed to Prior Tick Bites
Non–Lyme disease, tick-borne illnesses — such as spotted fever group rickettsiosis (SFGR), ehrlichiosis, and alpha-gal syndrome (AGS) — are emerging public health threats, but whether prior tick exposures are responsible for long-term complications, such as musculoskeletal symptoms or osteoarthritis, has been unclear.
Many patients attribute their nonspecific long-term symptoms, such as musculoskeletal pain, to previous illnesses from tick bites, note authors of a study published in JAMA Network Open. But the researchers, led by Diana L. Zychowski, MD, MPH, with the Division of Infectious Diseases at the University of North Carolina at Chapel Hill, found that Ehrlichia or Rickettsia seropositivity was not associated with chronic musculoskeletal symptoms, though they write that “further investigation into the pathogenesis of [alpha-gal] syndrome is needed.”
Tick-Borne Illness Cases Multiplying
Cases of tick-borne illness (TBD) in the United States have multiplied in recent years. More than 50,000 cases of TBD in the United States were reported in 2019, which doubled the number of cases over the previous 2 decades, the authors note.
Most of the cases are Lyme disease, but others — including SFGR and ehrlichiosis — represent an important public health threat, especially in southeastern states, the authors write. Cases of ehrlichiosis, for example, transmitted by the lone star tick, soared more than 10-fold since 2000.
The goal of this study was to evaluate whether there was an association between prior exposure to TBDs endemic to the southeastern United States and chronic musculoskeletal symptoms and radiographic measures of osteoarthritis.
Researchers analyzed 488 blood samples from the fourth visit (2017-2018) of the Johnston County Osteoarthritis (JoCo OA) project, an ongoing population-based study in Johnston County, North Carolina. JoCo OA participants include noninstitutionalized White and Black Johnston County residents 45 years old or older with osteoarthritis.
They measured seroprevalence of Rickettsia- and Ehrlichia-specific immunoglobulin G (IgG) as well as alpha-gal immunoglobulin E (IgE) in patient samples. Only alpha-gal IgE was linked in the study with knee pain, aching, or stiffness. Antibodies to Rickettsia, Ehrlichia, and alpha-gal were not associated with radiographic, symptomatic knee osteoarthritis.
“To our knowledge,” the authors write, “this study was the first population-based seroprevalence study of SFGR, Ehrlichia, and [alpha]-gal.”
The study also found a high prevalence of TBD exposure in the cohort. More than a third (36.5%) had either an alpha-gal IgE level greater than 0.1 IU/mL, a positive test for SFGR IgG antibodies, or a positive test for Ehrlichia IgG antibodies.
Given that not every tick carries an infectious pathogen, the findings show human-tick interactions are common, they write.
“These findings suggest that substantial investment is required to examine the pathogenesis of these TBDs and interventions to reduce human-tick interactions,” the authors conclude.
This study was funded by a Creativity Hub Award from the University of North Carolina Office of the Vice Chancellor for Research. The JoCo OA project is supported in part by grants from the Association of Schools of Public Health/Centers for Disease Control and Prevention (CDC); and grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Authors reported grants from the National Institutes of Health, the CDC, and several pharmaceutical companies.
Non–Lyme disease, tick-borne illnesses — such as spotted fever group rickettsiosis (SFGR), ehrlichiosis, and alpha-gal syndrome (AGS) — are emerging public health threats, but whether prior tick exposures are responsible for long-term complications, such as musculoskeletal symptoms or osteoarthritis, has been unclear.
Many patients attribute their nonspecific long-term symptoms, such as musculoskeletal pain, to previous illnesses from tick bites, note authors of a study published in JAMA Network Open. But the researchers, led by Diana L. Zychowski, MD, MPH, with the Division of Infectious Diseases at the University of North Carolina at Chapel Hill, found that Ehrlichia or Rickettsia seropositivity was not associated with chronic musculoskeletal symptoms, though they write that “further investigation into the pathogenesis of [alpha-gal] syndrome is needed.”
Tick-Borne Illness Cases Multiplying
Cases of tick-borne illness (TBD) in the United States have multiplied in recent years. More than 50,000 cases of TBD in the United States were reported in 2019, which doubled the number of cases over the previous 2 decades, the authors note.
Most of the cases are Lyme disease, but others — including SFGR and ehrlichiosis — represent an important public health threat, especially in southeastern states, the authors write. Cases of ehrlichiosis, for example, transmitted by the lone star tick, soared more than 10-fold since 2000.
The goal of this study was to evaluate whether there was an association between prior exposure to TBDs endemic to the southeastern United States and chronic musculoskeletal symptoms and radiographic measures of osteoarthritis.
Researchers analyzed 488 blood samples from the fourth visit (2017-2018) of the Johnston County Osteoarthritis (JoCo OA) project, an ongoing population-based study in Johnston County, North Carolina. JoCo OA participants include noninstitutionalized White and Black Johnston County residents 45 years old or older with osteoarthritis.
They measured seroprevalence of Rickettsia- and Ehrlichia-specific immunoglobulin G (IgG) as well as alpha-gal immunoglobulin E (IgE) in patient samples. Only alpha-gal IgE was linked in the study with knee pain, aching, or stiffness. Antibodies to Rickettsia, Ehrlichia, and alpha-gal were not associated with radiographic, symptomatic knee osteoarthritis.
“To our knowledge,” the authors write, “this study was the first population-based seroprevalence study of SFGR, Ehrlichia, and [alpha]-gal.”
The study also found a high prevalence of TBD exposure in the cohort. More than a third (36.5%) had either an alpha-gal IgE level greater than 0.1 IU/mL, a positive test for SFGR IgG antibodies, or a positive test for Ehrlichia IgG antibodies.
Given that not every tick carries an infectious pathogen, the findings show human-tick interactions are common, they write.
“These findings suggest that substantial investment is required to examine the pathogenesis of these TBDs and interventions to reduce human-tick interactions,” the authors conclude.
This study was funded by a Creativity Hub Award from the University of North Carolina Office of the Vice Chancellor for Research. The JoCo OA project is supported in part by grants from the Association of Schools of Public Health/Centers for Disease Control and Prevention (CDC); and grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Authors reported grants from the National Institutes of Health, the CDC, and several pharmaceutical companies.
Non–Lyme disease, tick-borne illnesses — such as spotted fever group rickettsiosis (SFGR), ehrlichiosis, and alpha-gal syndrome (AGS) — are emerging public health threats, but whether prior tick exposures are responsible for long-term complications, such as musculoskeletal symptoms or osteoarthritis, has been unclear.
Many patients attribute their nonspecific long-term symptoms, such as musculoskeletal pain, to previous illnesses from tick bites, note authors of a study published in JAMA Network Open. But the researchers, led by Diana L. Zychowski, MD, MPH, with the Division of Infectious Diseases at the University of North Carolina at Chapel Hill, found that Ehrlichia or Rickettsia seropositivity was not associated with chronic musculoskeletal symptoms, though they write that “further investigation into the pathogenesis of [alpha-gal] syndrome is needed.”
Tick-Borne Illness Cases Multiplying
Cases of tick-borne illness (TBD) in the United States have multiplied in recent years. More than 50,000 cases of TBD in the United States were reported in 2019, which doubled the number of cases over the previous 2 decades, the authors note.
Most of the cases are Lyme disease, but others — including SFGR and ehrlichiosis — represent an important public health threat, especially in southeastern states, the authors write. Cases of ehrlichiosis, for example, transmitted by the lone star tick, soared more than 10-fold since 2000.
The goal of this study was to evaluate whether there was an association between prior exposure to TBDs endemic to the southeastern United States and chronic musculoskeletal symptoms and radiographic measures of osteoarthritis.
Researchers analyzed 488 blood samples from the fourth visit (2017-2018) of the Johnston County Osteoarthritis (JoCo OA) project, an ongoing population-based study in Johnston County, North Carolina. JoCo OA participants include noninstitutionalized White and Black Johnston County residents 45 years old or older with osteoarthritis.
They measured seroprevalence of Rickettsia- and Ehrlichia-specific immunoglobulin G (IgG) as well as alpha-gal immunoglobulin E (IgE) in patient samples. Only alpha-gal IgE was linked in the study with knee pain, aching, or stiffness. Antibodies to Rickettsia, Ehrlichia, and alpha-gal were not associated with radiographic, symptomatic knee osteoarthritis.
“To our knowledge,” the authors write, “this study was the first population-based seroprevalence study of SFGR, Ehrlichia, and [alpha]-gal.”
The study also found a high prevalence of TBD exposure in the cohort. More than a third (36.5%) had either an alpha-gal IgE level greater than 0.1 IU/mL, a positive test for SFGR IgG antibodies, or a positive test for Ehrlichia IgG antibodies.
Given that not every tick carries an infectious pathogen, the findings show human-tick interactions are common, they write.
“These findings suggest that substantial investment is required to examine the pathogenesis of these TBDs and interventions to reduce human-tick interactions,” the authors conclude.
This study was funded by a Creativity Hub Award from the University of North Carolina Office of the Vice Chancellor for Research. The JoCo OA project is supported in part by grants from the Association of Schools of Public Health/Centers for Disease Control and Prevention (CDC); and grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Authors reported grants from the National Institutes of Health, the CDC, and several pharmaceutical companies.
FROM JAMA NETWORK OPEN


