User login
Pretreatment Lab Testing for Chronic Skin Diseases Diverges From Guidelines
in a national commercial insurance claims database.
Because of concerns for the potential reactivation of tuberculosis or hepatitis B or C, or for an increased risk for infections, myelosuppression, and hepatoxicity in the wake of immunomodulator use, some medical societies recommend screening patients for hepatitis B, hepatitis C, and tuberculosis before starting these medications, wrote Maria C. Schneeweiss, MD, of Brigham and Women’s Hospital, Boston, Massachusetts, and colleagues.
“Conducting this study was crucial because of the increasing use of systemic immunomodulatory agents for chronic inflammatory skin diseases and the recognized need for pretreatment testing to prevent complications,” coauthor Denys Shay, a PhD candidate in population health sciences at Harvard University, Cambridge, Massachusetts, said in an interview.
“Despite recommendations from professional societies, there was a lack of clarity on how consistently these guidelines were being followed in the United States. This study aimed to fill that gap in knowledge, providing a comprehensive view of current practices and highlighting areas for improvement,” he said.
In the study, published online in JAMA Dermatology, he and his coauthors identified 122,308 adults in the United States with psoriasis, hidradenitis suppurativa, or atopic dermatitis who started an immunomodulatory agent, including methotrexate (28,684 patients), tumor necrosis factor (TNF)–alpha inhibitors (40,965), ustekinumab (12,841), interleukin (IL)-23 inhibitors (6116), IL-17A inhibitors (9799), dupilumab (7787), and apremilast (16,116). The data were from a commercial insurance claims database from December 31, 2002, to December 31, 2020.
The primary outcome was the proportion of patients who underwent recommended screening lab tests including tuberculosis, hepatitis, liver function, complete blood cell counts (CBCs), and lipid panels within 6 months before treatment initiation and during the first 2 years of treatment. The median age of the study population was 49 years, and 52.1% were male.
A CBC was the most common pretreatment test across treatments, performed in 41%-69% of patients before starting treatment. Tuberculosis screening occurred in 11%-59% of patients within 6 months of initiating treatment, and 3%-26% had updated tests after 1 year. Similarly, 13%-41% of patients underwent hepatitis screening prior to treatment.
The highest levels of pretreatment testing occurred for TNF-alpha inhibitors, ustekinumab, IL-17A inhibitors, and IL-23 inhibitors, with similar patterns, while the lowest levels of testing occurred with apremilast and dupilumab.
Testing prevalence before starting apremilast and after a year of treatment was 15%-45% and 9%-36%, respectively. Testing before initiation and a year into treatment with dupilumab was 11%-41% and 3%-25%, respectively.
The findings were limited by several factors including the descriptive design, which does not allow for evaluation of the testing practices, the researchers said.
However, the results show the extent of patients with chronic inflammatory skin diseases (CISDs) who do not undergo pretreatment testing, and research is needed to create testing practices on the basis of recommendations for each agent and incorporating each patient’s history and clinical profile, they concluded.
“The finding that less than 60% of patients received recommended pretreatment testing was initially somewhat surprising,” Shay said in the interview. “However, the context provided by higher rates of baseline testing within the 6-12 months before treatment initiation and the potential for additional testing not captured by the dataset — such as hospital stays — suggests that the gap may not be as large as this estimate,” he said.
“The key message for clinicians is that there are considerable variations in laboratory testing practices with regard to the initiation of systemic immunomodulatory agents in patients with CISDs,” Shay said. “This represents a divergence from existing testing guidelines.”
“Further research is needed to understand the reasons for the variations in pretreatment testing practices and whether this heterogeneity affects patient outcomes,” he added.
Resist Routine Testing
The study findings represent a call to action in the form of ongoing assessment of the safety, clinical utility, and cost-effectiveness of pretreatment testing, wrote Clinton W. Enos, MD, Ana Ormaza Vera, MD, and Abby S. Van Voorhees, MD, of the Department of Dermatology, Eastern Virginia Medical School, Norfolk, Virginia, in an accompanying editorial.
The data in the current study suggesting less frequent laboratory testing compared with current guidelines could stem from a high comfort level with many of the therapies that have been available and in use for many years, they noted. Clinicians’ lack of knowledge of the laboratory screening and monitoring guidelines also may play a role, they said.
However, the authors cautioned against routine checking of laboratory results “without purpose” and without attention to their clinical utility and cost. “A thorough medical history is essential and can serve as a sensitive indicator of which patients are more at risk for diseases such as TB or hepatitis, thereby allowing for more meaningful laboratory screening use,” they said.
Evidence supporting prescreening labs for the spectrum of systemic agents used in dermatology varies considerably, “some trapped in time and carried forward for decades until finally questioned, others rooted in treatment mechanism and clinical data,” Adam Friedman, MD, professor and chief of dermatology at George Washington University, Washington, DC, said in an interview.
The study elucidated the current state of clinical practice, said Friedman, who was not involved with the study. This includes screening even if the label says it is not necessary and letting screening slide when guidelines say otherwise — even if the guidelines are outdated and insurance requires certain metrics prior to approval, he said.
Looking ahead, “we need better consensus and even better communication/education on said guidance,” Dr. Friedman said. “Clear, concise, evidenced-based, and expert-validated guidance to ensure we are meaningfully using medical resources” is what is needed, he added. “It will certainly take a village, and close collaboration between the industry and practitioners is key to success.”
The study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Shay had no financial conflicts to disclose. Lead author Dr. Schneeweiss disclosed grants from UCB Pharma and AbbVie to Brigham and Women’s Hospital outside the submitted work. Other authors disclosed receiving personal fees from Aetion and grants from UCB Pharma and Takeda outside the submitted work; grants from Amarin, Kowa, Novartis, and Pfizer outside the submitted work; and personal fees from Hims & Hers, AbbVie, Sun Pharmaceuticals, Pfizer, Digital Diagnostics, Lilly, Equillium, ASLAN, Boehringer Ingelheim, ACOM, Olaplex, and Legacy Healthcare during the study. No other disclosures were reported.
Editorial author Dr. Enos disclosed serving as an investigator for Amgen and Castle Biosciences and receiving grants from Arcutis Biotherapeutics outside the submitted work. Dr. Van Voorhees disclosed an honorarium outside the submitted work.
Dr. Friedman had no relevant financial conflicts to disclose.
A version of this article appeared on Medscape.com.
in a national commercial insurance claims database.
Because of concerns for the potential reactivation of tuberculosis or hepatitis B or C, or for an increased risk for infections, myelosuppression, and hepatoxicity in the wake of immunomodulator use, some medical societies recommend screening patients for hepatitis B, hepatitis C, and tuberculosis before starting these medications, wrote Maria C. Schneeweiss, MD, of Brigham and Women’s Hospital, Boston, Massachusetts, and colleagues.
“Conducting this study was crucial because of the increasing use of systemic immunomodulatory agents for chronic inflammatory skin diseases and the recognized need for pretreatment testing to prevent complications,” coauthor Denys Shay, a PhD candidate in population health sciences at Harvard University, Cambridge, Massachusetts, said in an interview.
“Despite recommendations from professional societies, there was a lack of clarity on how consistently these guidelines were being followed in the United States. This study aimed to fill that gap in knowledge, providing a comprehensive view of current practices and highlighting areas for improvement,” he said.
In the study, published online in JAMA Dermatology, he and his coauthors identified 122,308 adults in the United States with psoriasis, hidradenitis suppurativa, or atopic dermatitis who started an immunomodulatory agent, including methotrexate (28,684 patients), tumor necrosis factor (TNF)–alpha inhibitors (40,965), ustekinumab (12,841), interleukin (IL)-23 inhibitors (6116), IL-17A inhibitors (9799), dupilumab (7787), and apremilast (16,116). The data were from a commercial insurance claims database from December 31, 2002, to December 31, 2020.
The primary outcome was the proportion of patients who underwent recommended screening lab tests including tuberculosis, hepatitis, liver function, complete blood cell counts (CBCs), and lipid panels within 6 months before treatment initiation and during the first 2 years of treatment. The median age of the study population was 49 years, and 52.1% were male.
A CBC was the most common pretreatment test across treatments, performed in 41%-69% of patients before starting treatment. Tuberculosis screening occurred in 11%-59% of patients within 6 months of initiating treatment, and 3%-26% had updated tests after 1 year. Similarly, 13%-41% of patients underwent hepatitis screening prior to treatment.
The highest levels of pretreatment testing occurred for TNF-alpha inhibitors, ustekinumab, IL-17A inhibitors, and IL-23 inhibitors, with similar patterns, while the lowest levels of testing occurred with apremilast and dupilumab.
Testing prevalence before starting apremilast and after a year of treatment was 15%-45% and 9%-36%, respectively. Testing before initiation and a year into treatment with dupilumab was 11%-41% and 3%-25%, respectively.
The findings were limited by several factors including the descriptive design, which does not allow for evaluation of the testing practices, the researchers said.
However, the results show the extent of patients with chronic inflammatory skin diseases (CISDs) who do not undergo pretreatment testing, and research is needed to create testing practices on the basis of recommendations for each agent and incorporating each patient’s history and clinical profile, they concluded.
“The finding that less than 60% of patients received recommended pretreatment testing was initially somewhat surprising,” Shay said in the interview. “However, the context provided by higher rates of baseline testing within the 6-12 months before treatment initiation and the potential for additional testing not captured by the dataset — such as hospital stays — suggests that the gap may not be as large as this estimate,” he said.
“The key message for clinicians is that there are considerable variations in laboratory testing practices with regard to the initiation of systemic immunomodulatory agents in patients with CISDs,” Shay said. “This represents a divergence from existing testing guidelines.”
“Further research is needed to understand the reasons for the variations in pretreatment testing practices and whether this heterogeneity affects patient outcomes,” he added.
Resist Routine Testing
The study findings represent a call to action in the form of ongoing assessment of the safety, clinical utility, and cost-effectiveness of pretreatment testing, wrote Clinton W. Enos, MD, Ana Ormaza Vera, MD, and Abby S. Van Voorhees, MD, of the Department of Dermatology, Eastern Virginia Medical School, Norfolk, Virginia, in an accompanying editorial.
The data in the current study suggesting less frequent laboratory testing compared with current guidelines could stem from a high comfort level with many of the therapies that have been available and in use for many years, they noted. Clinicians’ lack of knowledge of the laboratory screening and monitoring guidelines also may play a role, they said.
However, the authors cautioned against routine checking of laboratory results “without purpose” and without attention to their clinical utility and cost. “A thorough medical history is essential and can serve as a sensitive indicator of which patients are more at risk for diseases such as TB or hepatitis, thereby allowing for more meaningful laboratory screening use,” they said.
Evidence supporting prescreening labs for the spectrum of systemic agents used in dermatology varies considerably, “some trapped in time and carried forward for decades until finally questioned, others rooted in treatment mechanism and clinical data,” Adam Friedman, MD, professor and chief of dermatology at George Washington University, Washington, DC, said in an interview.
The study elucidated the current state of clinical practice, said Friedman, who was not involved with the study. This includes screening even if the label says it is not necessary and letting screening slide when guidelines say otherwise — even if the guidelines are outdated and insurance requires certain metrics prior to approval, he said.
Looking ahead, “we need better consensus and even better communication/education on said guidance,” Dr. Friedman said. “Clear, concise, evidenced-based, and expert-validated guidance to ensure we are meaningfully using medical resources” is what is needed, he added. “It will certainly take a village, and close collaboration between the industry and practitioners is key to success.”
The study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Shay had no financial conflicts to disclose. Lead author Dr. Schneeweiss disclosed grants from UCB Pharma and AbbVie to Brigham and Women’s Hospital outside the submitted work. Other authors disclosed receiving personal fees from Aetion and grants from UCB Pharma and Takeda outside the submitted work; grants from Amarin, Kowa, Novartis, and Pfizer outside the submitted work; and personal fees from Hims & Hers, AbbVie, Sun Pharmaceuticals, Pfizer, Digital Diagnostics, Lilly, Equillium, ASLAN, Boehringer Ingelheim, ACOM, Olaplex, and Legacy Healthcare during the study. No other disclosures were reported.
Editorial author Dr. Enos disclosed serving as an investigator for Amgen and Castle Biosciences and receiving grants from Arcutis Biotherapeutics outside the submitted work. Dr. Van Voorhees disclosed an honorarium outside the submitted work.
Dr. Friedman had no relevant financial conflicts to disclose.
A version of this article appeared on Medscape.com.
in a national commercial insurance claims database.
Because of concerns for the potential reactivation of tuberculosis or hepatitis B or C, or for an increased risk for infections, myelosuppression, and hepatoxicity in the wake of immunomodulator use, some medical societies recommend screening patients for hepatitis B, hepatitis C, and tuberculosis before starting these medications, wrote Maria C. Schneeweiss, MD, of Brigham and Women’s Hospital, Boston, Massachusetts, and colleagues.
“Conducting this study was crucial because of the increasing use of systemic immunomodulatory agents for chronic inflammatory skin diseases and the recognized need for pretreatment testing to prevent complications,” coauthor Denys Shay, a PhD candidate in population health sciences at Harvard University, Cambridge, Massachusetts, said in an interview.
“Despite recommendations from professional societies, there was a lack of clarity on how consistently these guidelines were being followed in the United States. This study aimed to fill that gap in knowledge, providing a comprehensive view of current practices and highlighting areas for improvement,” he said.
In the study, published online in JAMA Dermatology, he and his coauthors identified 122,308 adults in the United States with psoriasis, hidradenitis suppurativa, or atopic dermatitis who started an immunomodulatory agent, including methotrexate (28,684 patients), tumor necrosis factor (TNF)–alpha inhibitors (40,965), ustekinumab (12,841), interleukin (IL)-23 inhibitors (6116), IL-17A inhibitors (9799), dupilumab (7787), and apremilast (16,116). The data were from a commercial insurance claims database from December 31, 2002, to December 31, 2020.
The primary outcome was the proportion of patients who underwent recommended screening lab tests including tuberculosis, hepatitis, liver function, complete blood cell counts (CBCs), and lipid panels within 6 months before treatment initiation and during the first 2 years of treatment. The median age of the study population was 49 years, and 52.1% were male.
A CBC was the most common pretreatment test across treatments, performed in 41%-69% of patients before starting treatment. Tuberculosis screening occurred in 11%-59% of patients within 6 months of initiating treatment, and 3%-26% had updated tests after 1 year. Similarly, 13%-41% of patients underwent hepatitis screening prior to treatment.
The highest levels of pretreatment testing occurred for TNF-alpha inhibitors, ustekinumab, IL-17A inhibitors, and IL-23 inhibitors, with similar patterns, while the lowest levels of testing occurred with apremilast and dupilumab.
Testing prevalence before starting apremilast and after a year of treatment was 15%-45% and 9%-36%, respectively. Testing before initiation and a year into treatment with dupilumab was 11%-41% and 3%-25%, respectively.
The findings were limited by several factors including the descriptive design, which does not allow for evaluation of the testing practices, the researchers said.
However, the results show the extent of patients with chronic inflammatory skin diseases (CISDs) who do not undergo pretreatment testing, and research is needed to create testing practices on the basis of recommendations for each agent and incorporating each patient’s history and clinical profile, they concluded.
“The finding that less than 60% of patients received recommended pretreatment testing was initially somewhat surprising,” Shay said in the interview. “However, the context provided by higher rates of baseline testing within the 6-12 months before treatment initiation and the potential for additional testing not captured by the dataset — such as hospital stays — suggests that the gap may not be as large as this estimate,” he said.
“The key message for clinicians is that there are considerable variations in laboratory testing practices with regard to the initiation of systemic immunomodulatory agents in patients with CISDs,” Shay said. “This represents a divergence from existing testing guidelines.”
“Further research is needed to understand the reasons for the variations in pretreatment testing practices and whether this heterogeneity affects patient outcomes,” he added.
Resist Routine Testing
The study findings represent a call to action in the form of ongoing assessment of the safety, clinical utility, and cost-effectiveness of pretreatment testing, wrote Clinton W. Enos, MD, Ana Ormaza Vera, MD, and Abby S. Van Voorhees, MD, of the Department of Dermatology, Eastern Virginia Medical School, Norfolk, Virginia, in an accompanying editorial.
The data in the current study suggesting less frequent laboratory testing compared with current guidelines could stem from a high comfort level with many of the therapies that have been available and in use for many years, they noted. Clinicians’ lack of knowledge of the laboratory screening and monitoring guidelines also may play a role, they said.
However, the authors cautioned against routine checking of laboratory results “without purpose” and without attention to their clinical utility and cost. “A thorough medical history is essential and can serve as a sensitive indicator of which patients are more at risk for diseases such as TB or hepatitis, thereby allowing for more meaningful laboratory screening use,” they said.
Evidence supporting prescreening labs for the spectrum of systemic agents used in dermatology varies considerably, “some trapped in time and carried forward for decades until finally questioned, others rooted in treatment mechanism and clinical data,” Adam Friedman, MD, professor and chief of dermatology at George Washington University, Washington, DC, said in an interview.
The study elucidated the current state of clinical practice, said Friedman, who was not involved with the study. This includes screening even if the label says it is not necessary and letting screening slide when guidelines say otherwise — even if the guidelines are outdated and insurance requires certain metrics prior to approval, he said.
Looking ahead, “we need better consensus and even better communication/education on said guidance,” Dr. Friedman said. “Clear, concise, evidenced-based, and expert-validated guidance to ensure we are meaningfully using medical resources” is what is needed, he added. “It will certainly take a village, and close collaboration between the industry and practitioners is key to success.”
The study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Shay had no financial conflicts to disclose. Lead author Dr. Schneeweiss disclosed grants from UCB Pharma and AbbVie to Brigham and Women’s Hospital outside the submitted work. Other authors disclosed receiving personal fees from Aetion and grants from UCB Pharma and Takeda outside the submitted work; grants from Amarin, Kowa, Novartis, and Pfizer outside the submitted work; and personal fees from Hims & Hers, AbbVie, Sun Pharmaceuticals, Pfizer, Digital Diagnostics, Lilly, Equillium, ASLAN, Boehringer Ingelheim, ACOM, Olaplex, and Legacy Healthcare during the study. No other disclosures were reported.
Editorial author Dr. Enos disclosed serving as an investigator for Amgen and Castle Biosciences and receiving grants from Arcutis Biotherapeutics outside the submitted work. Dr. Van Voorhees disclosed an honorarium outside the submitted work.
Dr. Friedman had no relevant financial conflicts to disclose.
A version of this article appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Mixing Paxlovid With Specific Immunosuppressants Risks Serious Adverse Reactions
The Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA) has issued a reminder to healthcare professionals regarding the potential serious adverse reactions associated with Paxlovid when administered in combination with specific immunosuppressants.
These immunosuppressants, encompassing calcineurin inhibitors (tacrolimus and ciclosporin) and mTOR inhibitors (everolimus and sirolimus), possess a narrow safe dosage range. They are recognized for their role in diminishing the activity of the immune system and are typically prescribed for autoimmune conditions and organ transplant recipients.
The highlighted risk arises due to drug-drug interactions, which can compromise the body’s ability to eliminate these medicines effectively.
Paxlovid, also known as nirmatrelvir with ritonavir, is an antiviral medication used to treat COVID-19 in adults who do not require supplemental oxygen and who are at an increased risk of progressing to severe COVID-19. It should be administered as soon as possible after a diagnosis of COVID-19 has been made and within 5 days of symptom onset.
Conditional marketing authorization for Paxlovid was granted across the European Union (EU) on January 28, 2022, and subsequently transitioned to full marketing authorization on February 24, 2023.
Developed by Pfizer, Paxlovid exhibited an 89% reduction in the risk for hospitalization or death among unvaccinated individuals in a phase 2-3 clinical trial. This led the National Institutes of Health to prioritize Paxlovid over other COVID-19 treatments. Subsequent real-world studies have affirmed its effectiveness, even among the vaccinated.
When combining Paxlovid with tacrolimus, ciclosporin, everolimus, or sirolimus, healthcare professionals need to actively monitor their blood levels. This proactive approach is essential to mitigate the risk for drug-drug interactions and potential serious reactions. They should collaborate with a multidisciplinary team of specialists to navigate the complexities of administering these medications concurrently.
Further, Paxlovid must not be coadministered with medications highly reliant on CYP3A liver enzymes for elimination, such as the immunosuppressant voclosporin. When administered together, there is a risk for these drugs interfering with each other’s metabolism, potentially leading to altered blood levels, reduced effectiveness, or an increased risk for adverse reactions.
After a thorough review, PRAC has highlighted potential serious adverse reactions, including fatal cases, due to drug interactions between Paxlovid and specified immunosuppressants. Thus, it issued a direct healthcare professional communication (DHPC) to emphasize the recognized risk for these interactions, as previously outlined in Paxlovid’s product information.
The DHPC for Paxlovid will undergo further evaluation by EMA’s Committee for Medicinal Products for Human Use and, upon adoption, will be disseminated to healthcare professionals. The communication plan will include publication on the DHPCs page and in national registers across EU Member States.
A version of this article appeared on Medscape.com.
The Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA) has issued a reminder to healthcare professionals regarding the potential serious adverse reactions associated with Paxlovid when administered in combination with specific immunosuppressants.
These immunosuppressants, encompassing calcineurin inhibitors (tacrolimus and ciclosporin) and mTOR inhibitors (everolimus and sirolimus), possess a narrow safe dosage range. They are recognized for their role in diminishing the activity of the immune system and are typically prescribed for autoimmune conditions and organ transplant recipients.
The highlighted risk arises due to drug-drug interactions, which can compromise the body’s ability to eliminate these medicines effectively.
Paxlovid, also known as nirmatrelvir with ritonavir, is an antiviral medication used to treat COVID-19 in adults who do not require supplemental oxygen and who are at an increased risk of progressing to severe COVID-19. It should be administered as soon as possible after a diagnosis of COVID-19 has been made and within 5 days of symptom onset.
Conditional marketing authorization for Paxlovid was granted across the European Union (EU) on January 28, 2022, and subsequently transitioned to full marketing authorization on February 24, 2023.
Developed by Pfizer, Paxlovid exhibited an 89% reduction in the risk for hospitalization or death among unvaccinated individuals in a phase 2-3 clinical trial. This led the National Institutes of Health to prioritize Paxlovid over other COVID-19 treatments. Subsequent real-world studies have affirmed its effectiveness, even among the vaccinated.
When combining Paxlovid with tacrolimus, ciclosporin, everolimus, or sirolimus, healthcare professionals need to actively monitor their blood levels. This proactive approach is essential to mitigate the risk for drug-drug interactions and potential serious reactions. They should collaborate with a multidisciplinary team of specialists to navigate the complexities of administering these medications concurrently.
Further, Paxlovid must not be coadministered with medications highly reliant on CYP3A liver enzymes for elimination, such as the immunosuppressant voclosporin. When administered together, there is a risk for these drugs interfering with each other’s metabolism, potentially leading to altered blood levels, reduced effectiveness, or an increased risk for adverse reactions.
After a thorough review, PRAC has highlighted potential serious adverse reactions, including fatal cases, due to drug interactions between Paxlovid and specified immunosuppressants. Thus, it issued a direct healthcare professional communication (DHPC) to emphasize the recognized risk for these interactions, as previously outlined in Paxlovid’s product information.
The DHPC for Paxlovid will undergo further evaluation by EMA’s Committee for Medicinal Products for Human Use and, upon adoption, will be disseminated to healthcare professionals. The communication plan will include publication on the DHPCs page and in national registers across EU Member States.
A version of this article appeared on Medscape.com.
The Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA) has issued a reminder to healthcare professionals regarding the potential serious adverse reactions associated with Paxlovid when administered in combination with specific immunosuppressants.
These immunosuppressants, encompassing calcineurin inhibitors (tacrolimus and ciclosporin) and mTOR inhibitors (everolimus and sirolimus), possess a narrow safe dosage range. They are recognized for their role in diminishing the activity of the immune system and are typically prescribed for autoimmune conditions and organ transplant recipients.
The highlighted risk arises due to drug-drug interactions, which can compromise the body’s ability to eliminate these medicines effectively.
Paxlovid, also known as nirmatrelvir with ritonavir, is an antiviral medication used to treat COVID-19 in adults who do not require supplemental oxygen and who are at an increased risk of progressing to severe COVID-19. It should be administered as soon as possible after a diagnosis of COVID-19 has been made and within 5 days of symptom onset.
Conditional marketing authorization for Paxlovid was granted across the European Union (EU) on January 28, 2022, and subsequently transitioned to full marketing authorization on February 24, 2023.
Developed by Pfizer, Paxlovid exhibited an 89% reduction in the risk for hospitalization or death among unvaccinated individuals in a phase 2-3 clinical trial. This led the National Institutes of Health to prioritize Paxlovid over other COVID-19 treatments. Subsequent real-world studies have affirmed its effectiveness, even among the vaccinated.
When combining Paxlovid with tacrolimus, ciclosporin, everolimus, or sirolimus, healthcare professionals need to actively monitor their blood levels. This proactive approach is essential to mitigate the risk for drug-drug interactions and potential serious reactions. They should collaborate with a multidisciplinary team of specialists to navigate the complexities of administering these medications concurrently.
Further, Paxlovid must not be coadministered with medications highly reliant on CYP3A liver enzymes for elimination, such as the immunosuppressant voclosporin. When administered together, there is a risk for these drugs interfering with each other’s metabolism, potentially leading to altered blood levels, reduced effectiveness, or an increased risk for adverse reactions.
After a thorough review, PRAC has highlighted potential serious adverse reactions, including fatal cases, due to drug interactions between Paxlovid and specified immunosuppressants. Thus, it issued a direct healthcare professional communication (DHPC) to emphasize the recognized risk for these interactions, as previously outlined in Paxlovid’s product information.
The DHPC for Paxlovid will undergo further evaluation by EMA’s Committee for Medicinal Products for Human Use and, upon adoption, will be disseminated to healthcare professionals. The communication plan will include publication on the DHPCs page and in national registers across EU Member States.
A version of this article appeared on Medscape.com.
Management of Tinea Capitis in Children Varies, Survey Finds
TOPLINE:
METHODOLOGY:
- The fungal scalp infection tinea capitis affects an estimated 3%-13% of children.
- While international guidelines exist for the treatment of tinea capitis in infants and children, no such document has been developed in the United States.
- Researchers distributed a survey by email to dermatologists through the and the Society for Pediatric Dermatology in the United States, asking about how they treated and managed pediatric patients with tinea capitis; 56 dermatologists participated.
TAKEAWAY:
- Most respondents (88.2%) said they felt comfortable prescribing oral medications prior to confirmation for those aged 2-18 years ( was the most common choice in 60.4% of cases), compared with 81.6% for those aged 2 months to 2 years ( was the most common treatment choice in 41.5% of cases), and 48.7% for those aged 0-2 months ( was the most common choice in 28.6% of cases).
- When asked what topical medication they would start prior to confirmation, most respondents said shampoo (62.3% for those aged 0-2 months and 75.5% each for those aged 2 months to 2 years and those aged 2-18 years), yet between 11.3% and 13% said they would use none.
- The most common form of confirmatory testing was , followed by potassium hydroxide preparation, trichoscopy, and Wood’s lamp.
- More than half of survey respondents would alter their choice of oral medication based on culture results, but most would not change their topical medication preference.
IN PRACTICE:
“The management of tinea capitis in the United States is currently variable, particularly with the introduction of newer antifungals,” the authors wrote. “Future steps involve establishing evidence-based clinical practice guidelines that consider drug efficacy, safety profiles, and costs.”
SOURCE:
Bernard Cohen, MD, of the Departments of Pediatrics and Dermatology at Johns Hopkins University, Baltimore, Maryland, led the research, which was published in Pediatric Dermatology.
LIMITATIONS:
Lower response rates associated with online surveys and predefined age groups restrict the granularity of responses.
DISCLOSURES:
The authors reported having no financial disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The fungal scalp infection tinea capitis affects an estimated 3%-13% of children.
- While international guidelines exist for the treatment of tinea capitis in infants and children, no such document has been developed in the United States.
- Researchers distributed a survey by email to dermatologists through the and the Society for Pediatric Dermatology in the United States, asking about how they treated and managed pediatric patients with tinea capitis; 56 dermatologists participated.
TAKEAWAY:
- Most respondents (88.2%) said they felt comfortable prescribing oral medications prior to confirmation for those aged 2-18 years ( was the most common choice in 60.4% of cases), compared with 81.6% for those aged 2 months to 2 years ( was the most common treatment choice in 41.5% of cases), and 48.7% for those aged 0-2 months ( was the most common choice in 28.6% of cases).
- When asked what topical medication they would start prior to confirmation, most respondents said shampoo (62.3% for those aged 0-2 months and 75.5% each for those aged 2 months to 2 years and those aged 2-18 years), yet between 11.3% and 13% said they would use none.
- The most common form of confirmatory testing was , followed by potassium hydroxide preparation, trichoscopy, and Wood’s lamp.
- More than half of survey respondents would alter their choice of oral medication based on culture results, but most would not change their topical medication preference.
IN PRACTICE:
“The management of tinea capitis in the United States is currently variable, particularly with the introduction of newer antifungals,” the authors wrote. “Future steps involve establishing evidence-based clinical practice guidelines that consider drug efficacy, safety profiles, and costs.”
SOURCE:
Bernard Cohen, MD, of the Departments of Pediatrics and Dermatology at Johns Hopkins University, Baltimore, Maryland, led the research, which was published in Pediatric Dermatology.
LIMITATIONS:
Lower response rates associated with online surveys and predefined age groups restrict the granularity of responses.
DISCLOSURES:
The authors reported having no financial disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The fungal scalp infection tinea capitis affects an estimated 3%-13% of children.
- While international guidelines exist for the treatment of tinea capitis in infants and children, no such document has been developed in the United States.
- Researchers distributed a survey by email to dermatologists through the and the Society for Pediatric Dermatology in the United States, asking about how they treated and managed pediatric patients with tinea capitis; 56 dermatologists participated.
TAKEAWAY:
- Most respondents (88.2%) said they felt comfortable prescribing oral medications prior to confirmation for those aged 2-18 years ( was the most common choice in 60.4% of cases), compared with 81.6% for those aged 2 months to 2 years ( was the most common treatment choice in 41.5% of cases), and 48.7% for those aged 0-2 months ( was the most common choice in 28.6% of cases).
- When asked what topical medication they would start prior to confirmation, most respondents said shampoo (62.3% for those aged 0-2 months and 75.5% each for those aged 2 months to 2 years and those aged 2-18 years), yet between 11.3% and 13% said they would use none.
- The most common form of confirmatory testing was , followed by potassium hydroxide preparation, trichoscopy, and Wood’s lamp.
- More than half of survey respondents would alter their choice of oral medication based on culture results, but most would not change their topical medication preference.
IN PRACTICE:
“The management of tinea capitis in the United States is currently variable, particularly with the introduction of newer antifungals,” the authors wrote. “Future steps involve establishing evidence-based clinical practice guidelines that consider drug efficacy, safety profiles, and costs.”
SOURCE:
Bernard Cohen, MD, of the Departments of Pediatrics and Dermatology at Johns Hopkins University, Baltimore, Maryland, led the research, which was published in Pediatric Dermatology.
LIMITATIONS:
Lower response rates associated with online surveys and predefined age groups restrict the granularity of responses.
DISCLOSURES:
The authors reported having no financial disclosures.
A version of this article appeared on Medscape.com.
Bivalent Vaccines Protect Even Children Who’ve Had COVID
This transcript has been edited for clarity.
It was only 3 years ago when we called the pathogen we now refer to as the coronavirus “nCOV-19.” It was, in many ways, more descriptive than what we have today. The little “n” there stood for “novel” — and it was really that little “n” that caused us all the trouble.
You see, coronaviruses themselves were not really new to us. Understudied, perhaps, but with four strains running around the globe at any time giving rise to the common cold, these were viruses our bodies understood.
But Instead of acting like a cold, it acted like nothing we had seen before, at least in our lifetime. The story of the pandemic is very much a bildungsroman of our immune systems — a story of how our immunity grew up.
The difference between the start of 2020 and now, when infections with the coronavirus remain common but not as deadly, can be measured in terms of immune education. Some of our immune systems were educated by infection, some by vaccination, and many by both.
When the first vaccines emerged in December 2020, the opportunity to educate our immune systems was still huge. Though, at the time, an estimated 20 million had been infected in the US and 350,000 had died, there was a large population that remained immunologically naive. I was one of them.
If 2020 into early 2021 was the era of immune education, the postvaccine period was the era of the variant. From one COVID strain to two, to five, to innumerable, our immune memory — trained on a specific version of the virus or its spike protein — became imperfect again. Not naive; these variants were not “novel” in the way COVID-19 was novel, but they were different. And different enough to cause infection.
Following the playbook of another virus that loves to come dressed up in different outfits, the flu virus, we find ourselves in the booster era — a world where yearly doses of a vaccine, ideally matched to the variants circulating when the vaccine is given, are the recommendation if not the norm.
But questions remain about the vaccination program, particularly around who should get it. And two populations with big question marks over their heads are (1) people who have already been infected and (2) kids, because their risk for bad outcomes is so much lower.
This week, we finally have some evidence that can shed light on these questions. The study under the spotlight is this one, appearing in JAMA, which tries to analyze the ability of the bivalent vaccine — that’s the second one to come out, around September 2022 — to protect kids from COVID-19.
Now, right off the bat, this was not a randomized trial. The studies that established the viability of the mRNA vaccine platform were; they happened before the vaccine was authorized. But trials of the bivalent vaccine were mostly limited to proving immune response, not protection from disease.
Nevertheless, with some good observational methods and some statistics, we can try to tease out whether bivalent vaccines in kids worked.
The study combines three prospective cohort studies. The details are in the paper, but what you need to know is that the special sauce of these studies was that the kids were tested for COVID-19 on a weekly basis, whether they had symptoms or not. This is critical because asymptomatic infections can transmit COVID-19.
Let’s do the variables of interest. First and foremost, the bivalent vaccine. Some of these kids got the bivalent vaccine, some didn’t. Other key variables include prior vaccination with the monovalent vaccine. Some had been vaccinated with the monovalent vaccine before, some hadn’t. And, of course, prior infection. Some had been infected before (based on either nasal swabs or blood tests).
Let’s focus first on the primary exposure of interest: getting that bivalent vaccine. Again, this was not randomly assigned; kids who got the bivalent vaccine were different from those who did not. In general, they lived in smaller households, they were more likely to be White, less likely to have had a prior COVID infection, and quite a bit more likely to have at least one chronic condition.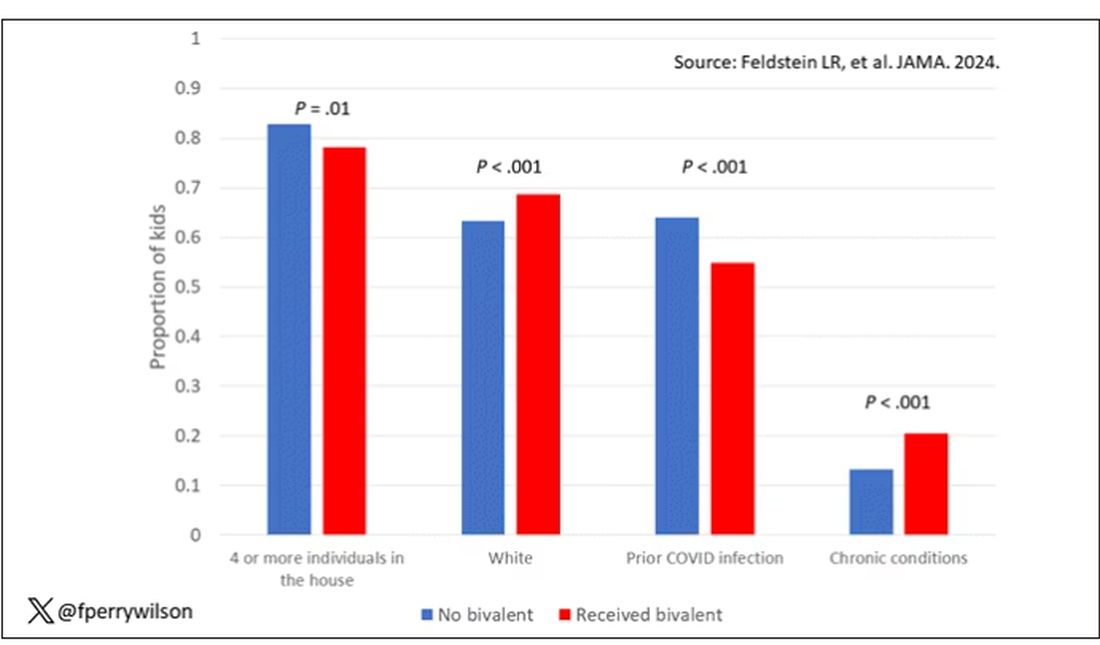
To me, this constellation of factors describes a slightly higher-risk group; it makes sense that they were more likely to get the second vaccine.
Given those factors, what were the rates of COVID infection? After nearly a year of follow-up, around 15% of the kids who hadn’t received the bivalent vaccine got infected compared with 5% of the vaccinated kids. Symptomatic infections represented roughly half of all infections in both groups.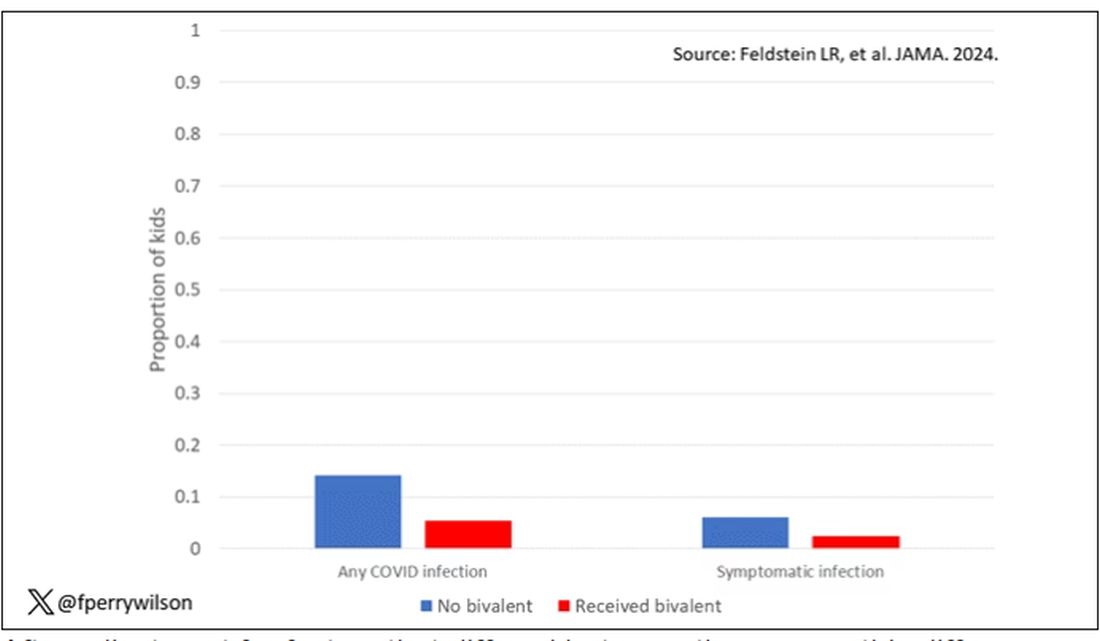
After adjustment for factors that differed between the groups, this difference translated into a vaccine efficacy of about 50% in this population. That’s our first data point. Yes, the bivalent vaccine worked. Not amazingly, of course. But it worked.
What about the kids who had had a prior COVID infection? Somewhat surprisingly, the vaccine was just as effective in this population, despite the fact that their immune systems already had some knowledge of COVID. Ten percent of unvaccinated kids got infected, even though they had been infected before. Just 2.5% of kids who received the bivalent vaccine got infected, suggesting some synergy between prior infection and vaccination.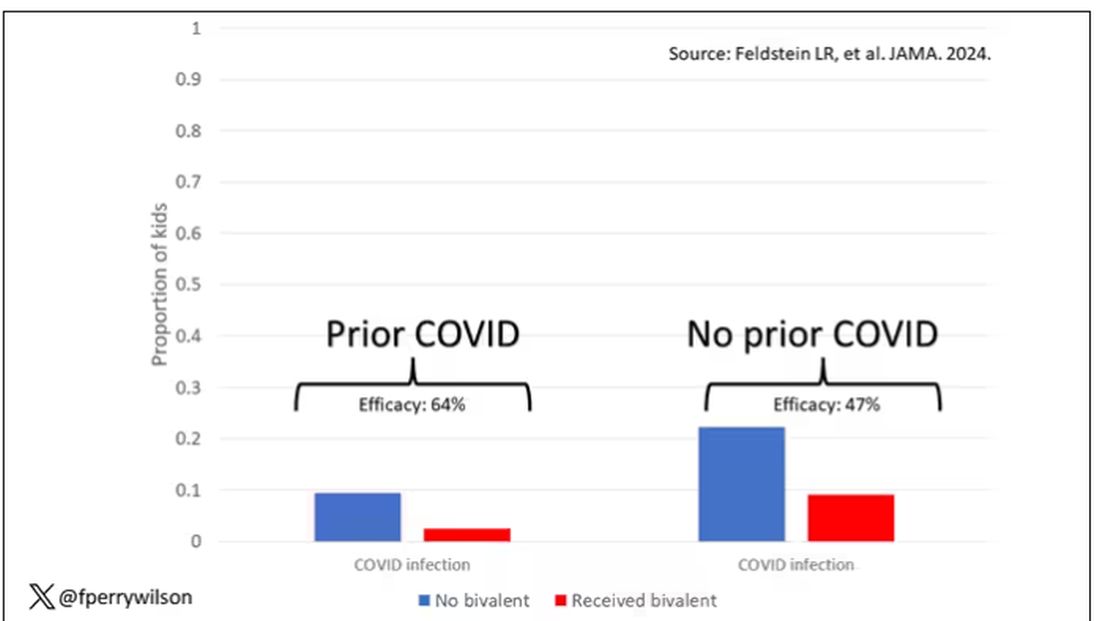
These data suggest that the bivalent vaccine did reduce the risk for COVID infection in kids. All good. But the piece still missing is how severe these infections were. It doesn’t appear that any of the 426 infections documented in this study resulted in hospitalization or death, fortunately. And no data are presented on the incidence of multisystem inflammatory syndrome of children, though given the rarity, I’d be surprised if any of these kids have this either.
So where are we? Well, it seems that the narrative out there that says “the vaccines don’t work” or “the vaccines don’t work if you’ve already been infected” is probably not true. They do work. This study and others in adults show that. If they work to reduce infections, as this study shows, they will also work to reduce deaths. It’s just that death is fortunately so rare in children that the number needed to vaccinate to prevent one death is very large. In that situation, the decision to vaccinate comes down to the risks associated with vaccination. So far, those risk seem very minimal.
Perhaps falling into a flu-like yearly vaccination schedule is not simply the result of old habits dying hard. Maybe it’s actually not a bad idea.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It was only 3 years ago when we called the pathogen we now refer to as the coronavirus “nCOV-19.” It was, in many ways, more descriptive than what we have today. The little “n” there stood for “novel” — and it was really that little “n” that caused us all the trouble.
You see, coronaviruses themselves were not really new to us. Understudied, perhaps, but with four strains running around the globe at any time giving rise to the common cold, these were viruses our bodies understood.
But Instead of acting like a cold, it acted like nothing we had seen before, at least in our lifetime. The story of the pandemic is very much a bildungsroman of our immune systems — a story of how our immunity grew up.
The difference between the start of 2020 and now, when infections with the coronavirus remain common but not as deadly, can be measured in terms of immune education. Some of our immune systems were educated by infection, some by vaccination, and many by both.
When the first vaccines emerged in December 2020, the opportunity to educate our immune systems was still huge. Though, at the time, an estimated 20 million had been infected in the US and 350,000 had died, there was a large population that remained immunologically naive. I was one of them.
If 2020 into early 2021 was the era of immune education, the postvaccine period was the era of the variant. From one COVID strain to two, to five, to innumerable, our immune memory — trained on a specific version of the virus or its spike protein — became imperfect again. Not naive; these variants were not “novel” in the way COVID-19 was novel, but they were different. And different enough to cause infection.
Following the playbook of another virus that loves to come dressed up in different outfits, the flu virus, we find ourselves in the booster era — a world where yearly doses of a vaccine, ideally matched to the variants circulating when the vaccine is given, are the recommendation if not the norm.
But questions remain about the vaccination program, particularly around who should get it. And two populations with big question marks over their heads are (1) people who have already been infected and (2) kids, because their risk for bad outcomes is so much lower.
This week, we finally have some evidence that can shed light on these questions. The study under the spotlight is this one, appearing in JAMA, which tries to analyze the ability of the bivalent vaccine — that’s the second one to come out, around September 2022 — to protect kids from COVID-19.
Now, right off the bat, this was not a randomized trial. The studies that established the viability of the mRNA vaccine platform were; they happened before the vaccine was authorized. But trials of the bivalent vaccine were mostly limited to proving immune response, not protection from disease.
Nevertheless, with some good observational methods and some statistics, we can try to tease out whether bivalent vaccines in kids worked.
The study combines three prospective cohort studies. The details are in the paper, but what you need to know is that the special sauce of these studies was that the kids were tested for COVID-19 on a weekly basis, whether they had symptoms or not. This is critical because asymptomatic infections can transmit COVID-19.
Let’s do the variables of interest. First and foremost, the bivalent vaccine. Some of these kids got the bivalent vaccine, some didn’t. Other key variables include prior vaccination with the monovalent vaccine. Some had been vaccinated with the monovalent vaccine before, some hadn’t. And, of course, prior infection. Some had been infected before (based on either nasal swabs or blood tests).
Let’s focus first on the primary exposure of interest: getting that bivalent vaccine. Again, this was not randomly assigned; kids who got the bivalent vaccine were different from those who did not. In general, they lived in smaller households, they were more likely to be White, less likely to have had a prior COVID infection, and quite a bit more likely to have at least one chronic condition.
To me, this constellation of factors describes a slightly higher-risk group; it makes sense that they were more likely to get the second vaccine.
Given those factors, what were the rates of COVID infection? After nearly a year of follow-up, around 15% of the kids who hadn’t received the bivalent vaccine got infected compared with 5% of the vaccinated kids. Symptomatic infections represented roughly half of all infections in both groups.
After adjustment for factors that differed between the groups, this difference translated into a vaccine efficacy of about 50% in this population. That’s our first data point. Yes, the bivalent vaccine worked. Not amazingly, of course. But it worked.
What about the kids who had had a prior COVID infection? Somewhat surprisingly, the vaccine was just as effective in this population, despite the fact that their immune systems already had some knowledge of COVID. Ten percent of unvaccinated kids got infected, even though they had been infected before. Just 2.5% of kids who received the bivalent vaccine got infected, suggesting some synergy between prior infection and vaccination.
These data suggest that the bivalent vaccine did reduce the risk for COVID infection in kids. All good. But the piece still missing is how severe these infections were. It doesn’t appear that any of the 426 infections documented in this study resulted in hospitalization or death, fortunately. And no data are presented on the incidence of multisystem inflammatory syndrome of children, though given the rarity, I’d be surprised if any of these kids have this either.
So where are we? Well, it seems that the narrative out there that says “the vaccines don’t work” or “the vaccines don’t work if you’ve already been infected” is probably not true. They do work. This study and others in adults show that. If they work to reduce infections, as this study shows, they will also work to reduce deaths. It’s just that death is fortunately so rare in children that the number needed to vaccinate to prevent one death is very large. In that situation, the decision to vaccinate comes down to the risks associated with vaccination. So far, those risk seem very minimal.
Perhaps falling into a flu-like yearly vaccination schedule is not simply the result of old habits dying hard. Maybe it’s actually not a bad idea.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It was only 3 years ago when we called the pathogen we now refer to as the coronavirus “nCOV-19.” It was, in many ways, more descriptive than what we have today. The little “n” there stood for “novel” — and it was really that little “n” that caused us all the trouble.
You see, coronaviruses themselves were not really new to us. Understudied, perhaps, but with four strains running around the globe at any time giving rise to the common cold, these were viruses our bodies understood.
But Instead of acting like a cold, it acted like nothing we had seen before, at least in our lifetime. The story of the pandemic is very much a bildungsroman of our immune systems — a story of how our immunity grew up.
The difference between the start of 2020 and now, when infections with the coronavirus remain common but not as deadly, can be measured in terms of immune education. Some of our immune systems were educated by infection, some by vaccination, and many by both.
When the first vaccines emerged in December 2020, the opportunity to educate our immune systems was still huge. Though, at the time, an estimated 20 million had been infected in the US and 350,000 had died, there was a large population that remained immunologically naive. I was one of them.
If 2020 into early 2021 was the era of immune education, the postvaccine period was the era of the variant. From one COVID strain to two, to five, to innumerable, our immune memory — trained on a specific version of the virus or its spike protein — became imperfect again. Not naive; these variants were not “novel” in the way COVID-19 was novel, but they were different. And different enough to cause infection.
Following the playbook of another virus that loves to come dressed up in different outfits, the flu virus, we find ourselves in the booster era — a world where yearly doses of a vaccine, ideally matched to the variants circulating when the vaccine is given, are the recommendation if not the norm.
But questions remain about the vaccination program, particularly around who should get it. And two populations with big question marks over their heads are (1) people who have already been infected and (2) kids, because their risk for bad outcomes is so much lower.
This week, we finally have some evidence that can shed light on these questions. The study under the spotlight is this one, appearing in JAMA, which tries to analyze the ability of the bivalent vaccine — that’s the second one to come out, around September 2022 — to protect kids from COVID-19.
Now, right off the bat, this was not a randomized trial. The studies that established the viability of the mRNA vaccine platform were; they happened before the vaccine was authorized. But trials of the bivalent vaccine were mostly limited to proving immune response, not protection from disease.
Nevertheless, with some good observational methods and some statistics, we can try to tease out whether bivalent vaccines in kids worked.
The study combines three prospective cohort studies. The details are in the paper, but what you need to know is that the special sauce of these studies was that the kids were tested for COVID-19 on a weekly basis, whether they had symptoms or not. This is critical because asymptomatic infections can transmit COVID-19.
Let’s do the variables of interest. First and foremost, the bivalent vaccine. Some of these kids got the bivalent vaccine, some didn’t. Other key variables include prior vaccination with the monovalent vaccine. Some had been vaccinated with the monovalent vaccine before, some hadn’t. And, of course, prior infection. Some had been infected before (based on either nasal swabs or blood tests).
Let’s focus first on the primary exposure of interest: getting that bivalent vaccine. Again, this was not randomly assigned; kids who got the bivalent vaccine were different from those who did not. In general, they lived in smaller households, they were more likely to be White, less likely to have had a prior COVID infection, and quite a bit more likely to have at least one chronic condition.
To me, this constellation of factors describes a slightly higher-risk group; it makes sense that they were more likely to get the second vaccine.
Given those factors, what were the rates of COVID infection? After nearly a year of follow-up, around 15% of the kids who hadn’t received the bivalent vaccine got infected compared with 5% of the vaccinated kids. Symptomatic infections represented roughly half of all infections in both groups.
After adjustment for factors that differed between the groups, this difference translated into a vaccine efficacy of about 50% in this population. That’s our first data point. Yes, the bivalent vaccine worked. Not amazingly, of course. But it worked.
What about the kids who had had a prior COVID infection? Somewhat surprisingly, the vaccine was just as effective in this population, despite the fact that their immune systems already had some knowledge of COVID. Ten percent of unvaccinated kids got infected, even though they had been infected before. Just 2.5% of kids who received the bivalent vaccine got infected, suggesting some synergy between prior infection and vaccination.
These data suggest that the bivalent vaccine did reduce the risk for COVID infection in kids. All good. But the piece still missing is how severe these infections were. It doesn’t appear that any of the 426 infections documented in this study resulted in hospitalization or death, fortunately. And no data are presented on the incidence of multisystem inflammatory syndrome of children, though given the rarity, I’d be surprised if any of these kids have this either.
So where are we? Well, it seems that the narrative out there that says “the vaccines don’t work” or “the vaccines don’t work if you’ve already been infected” is probably not true. They do work. This study and others in adults show that. If they work to reduce infections, as this study shows, they will also work to reduce deaths. It’s just that death is fortunately so rare in children that the number needed to vaccinate to prevent one death is very large. In that situation, the decision to vaccinate comes down to the risks associated with vaccination. So far, those risk seem very minimal.
Perhaps falling into a flu-like yearly vaccination schedule is not simply the result of old habits dying hard. Maybe it’s actually not a bad idea.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
SARS-CoV-2 a Possible Trigger for Achalasia
TOPLINE:
METHODOLOGY:
- The etiology of achalasia is unclear. Studies have suggested an immune reaction to viral infections, including SARS-CoV-2, as a potential cause.
- Researchers studied four adults who developed achalasia within 5 months of SARS-CoV-2 infection (group 1), six with longstanding achalasia predating SARS-CoV-2 infection (group 2), and two with longstanding achalasia with no known SARS-CoV-2 infection (group 3).
- They tested for the presence of SARS-CoV-2 nucleocapsid (N) and spike (S) proteins, as well as inflammatory markers, in esophageal muscle tissue isolated from the participants.
TAKEAWAY:
- Group 1 patients (confirmed or suspected post–COVID-19 achalasia) had the highest levels of the N protein in all four cases and higher levels of the S protein in the two confirmed cases. No N or S protein was detected in group 3.
- The presence of mRNA for SARS-CoV-2 N protein correlated with a significant increase in the inflammatory markers of NOD-like receptor family pyrin domain-containing 3 and tumor necrosis factor. There were no differences in interleukin 18 in groups 1 and 2.
- The S protein was detected in all muscle tissue samples from group 1. It was also detected in some (but not all) samples from group 2 and to a much lesser degree. The presence of S protein was irrespective of the SARS-CoV-2 vaccination status.
IN PRACTICE:
“Our findings not only show the continued presence of SARS-CoV-2 proteins in esophageal muscle tissue isolated from subjects with achalasia post infection, but they further correlate this with the presence of a sustained inflammatory response,” the authors wrote.
SOURCE:
The study, with first author Salih Samo, MD, MS, Division of Gastroenterology, Hepatology, and Motility, University of Kansas School of Medicine, Kansas City, Kansas, was published online on January 24, 2024, in the American Journal of Gastroenterology.
LIMITATIONS:
The sample size was small, and it was not known which SARS-CoV-2 variant each patient had. The study cannot definitively confirm that SARS-CoV-2 is causative for achalasia.
DISCLOSURES:
The study had no specific funding. Samo reported relationships with Castle Biosciences, Sanofi, Evoke, and EndoGastric Solutions.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The etiology of achalasia is unclear. Studies have suggested an immune reaction to viral infections, including SARS-CoV-2, as a potential cause.
- Researchers studied four adults who developed achalasia within 5 months of SARS-CoV-2 infection (group 1), six with longstanding achalasia predating SARS-CoV-2 infection (group 2), and two with longstanding achalasia with no known SARS-CoV-2 infection (group 3).
- They tested for the presence of SARS-CoV-2 nucleocapsid (N) and spike (S) proteins, as well as inflammatory markers, in esophageal muscle tissue isolated from the participants.
TAKEAWAY:
- Group 1 patients (confirmed or suspected post–COVID-19 achalasia) had the highest levels of the N protein in all four cases and higher levels of the S protein in the two confirmed cases. No N or S protein was detected in group 3.
- The presence of mRNA for SARS-CoV-2 N protein correlated with a significant increase in the inflammatory markers of NOD-like receptor family pyrin domain-containing 3 and tumor necrosis factor. There were no differences in interleukin 18 in groups 1 and 2.
- The S protein was detected in all muscle tissue samples from group 1. It was also detected in some (but not all) samples from group 2 and to a much lesser degree. The presence of S protein was irrespective of the SARS-CoV-2 vaccination status.
IN PRACTICE:
“Our findings not only show the continued presence of SARS-CoV-2 proteins in esophageal muscle tissue isolated from subjects with achalasia post infection, but they further correlate this with the presence of a sustained inflammatory response,” the authors wrote.
SOURCE:
The study, with first author Salih Samo, MD, MS, Division of Gastroenterology, Hepatology, and Motility, University of Kansas School of Medicine, Kansas City, Kansas, was published online on January 24, 2024, in the American Journal of Gastroenterology.
LIMITATIONS:
The sample size was small, and it was not known which SARS-CoV-2 variant each patient had. The study cannot definitively confirm that SARS-CoV-2 is causative for achalasia.
DISCLOSURES:
The study had no specific funding. Samo reported relationships with Castle Biosciences, Sanofi, Evoke, and EndoGastric Solutions.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The etiology of achalasia is unclear. Studies have suggested an immune reaction to viral infections, including SARS-CoV-2, as a potential cause.
- Researchers studied four adults who developed achalasia within 5 months of SARS-CoV-2 infection (group 1), six with longstanding achalasia predating SARS-CoV-2 infection (group 2), and two with longstanding achalasia with no known SARS-CoV-2 infection (group 3).
- They tested for the presence of SARS-CoV-2 nucleocapsid (N) and spike (S) proteins, as well as inflammatory markers, in esophageal muscle tissue isolated from the participants.
TAKEAWAY:
- Group 1 patients (confirmed or suspected post–COVID-19 achalasia) had the highest levels of the N protein in all four cases and higher levels of the S protein in the two confirmed cases. No N or S protein was detected in group 3.
- The presence of mRNA for SARS-CoV-2 N protein correlated with a significant increase in the inflammatory markers of NOD-like receptor family pyrin domain-containing 3 and tumor necrosis factor. There were no differences in interleukin 18 in groups 1 and 2.
- The S protein was detected in all muscle tissue samples from group 1. It was also detected in some (but not all) samples from group 2 and to a much lesser degree. The presence of S protein was irrespective of the SARS-CoV-2 vaccination status.
IN PRACTICE:
“Our findings not only show the continued presence of SARS-CoV-2 proteins in esophageal muscle tissue isolated from subjects with achalasia post infection, but they further correlate this with the presence of a sustained inflammatory response,” the authors wrote.
SOURCE:
The study, with first author Salih Samo, MD, MS, Division of Gastroenterology, Hepatology, and Motility, University of Kansas School of Medicine, Kansas City, Kansas, was published online on January 24, 2024, in the American Journal of Gastroenterology.
LIMITATIONS:
The sample size was small, and it was not known which SARS-CoV-2 variant each patient had. The study cannot definitively confirm that SARS-CoV-2 is causative for achalasia.
DISCLOSURES:
The study had no specific funding. Samo reported relationships with Castle Biosciences, Sanofi, Evoke, and EndoGastric Solutions.
A version of this article appeared on Medscape.com.
Nonepidemic Kaposi Sarcoma: A Case of a Rare Epidemiologic Subtype
To the Editor:
Kaposi sarcoma (KS) is a rare angioproliferative disorder associated with human herpesvirus 8 (HHV-8) infection.1 There are 4 main recognized epidemiologic forms of KS: classic, endemic, epidemic, and iatrogenic (Table). Nonepidemic KS is a recently described rare fifth type of KS that occurs in a subset of patients who do not fit the other classifications—HIV-negative patients without detectable cellular or humoral immune deficiency. This subset has been described as clinically similar to classic KS with limited disease but occurring in younger men.2,3 We describe a case of nonepidemic KS in a Middle Eastern heterosexual immunocompetent man.
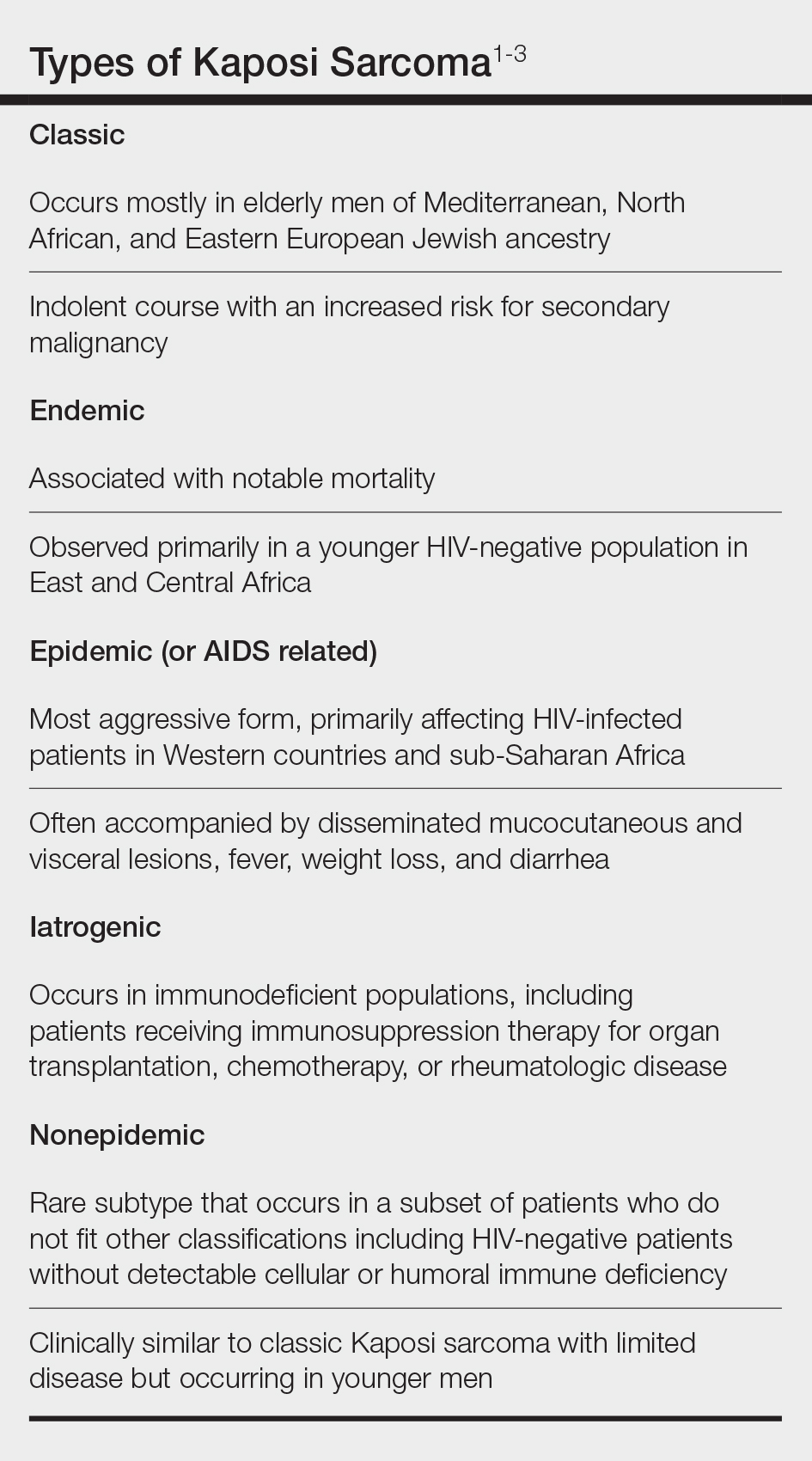
A 30-year-old man presented for evaluation of a growth on the nose of 3 months’ duration. The patient reported being otherwise healthy and was not taking long-term medications. He denied a history of malignancy, organ transplant, or immunosuppressive therapy. He was born in Syria and lived in Thailand for several years prior to moving to the United States. HIV testing 6 months prior to presentation was negative. He denied fever, chills, lymphadenopathy, shortness of breath, hemoptysis, melena, hematochezia, and intravenous drug use.
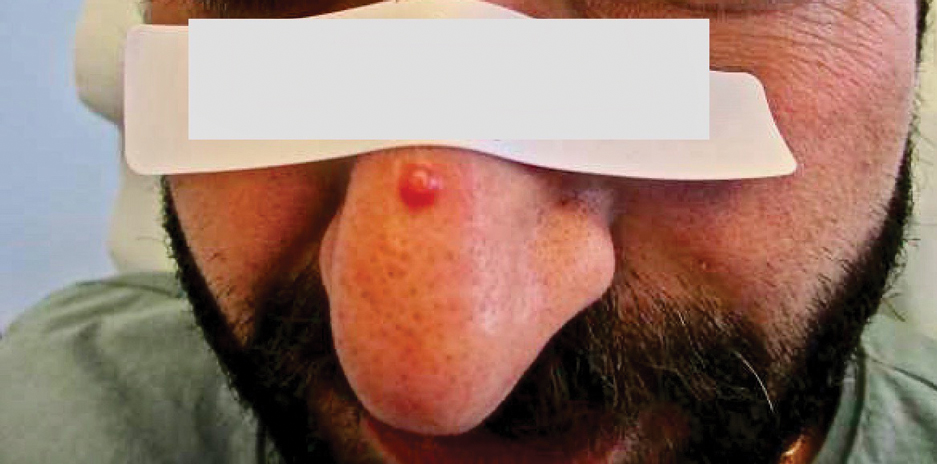
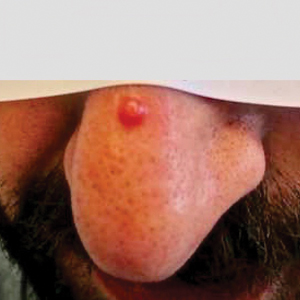
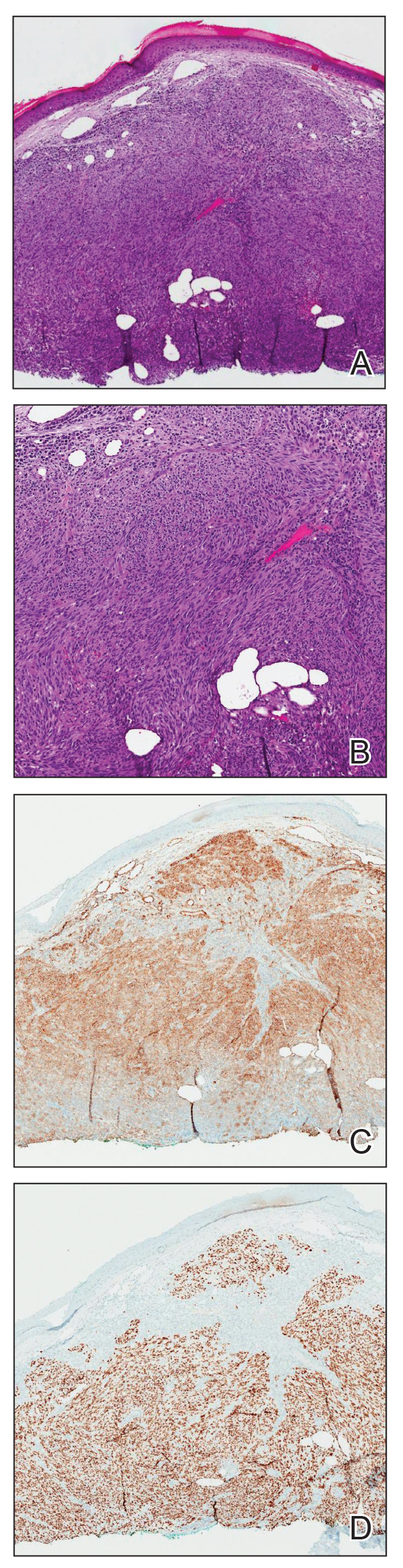
Physical examination revealed a solitary shiny, 7-mm, pink-red papule on the nasal dorsum (Figure 1). No other skin or mucosal lesions were identified. There was no cervical, axillary, or inguinal lymphadenopathy. A laboratory workup consisting of serum immunoglobulins and serum protein electrophoresis was unremarkable. Tests for HIV-1 and HIV-2 as well as human T-lymphotropic virus 1 and 2 were negative. The CD4 and CD8 counts were within reference range. Histopathology of a shave biopsy revealed a dermal spindle cell proliferation arranged in short intersecting fascicles and admixed with plasma cells and occasional mitotic figures. Immunohistochemistry showed that the spindle cells stained positive for CD34, CD31, and HHV-8 (Figure 2). The lesion resolved after treatment with cryotherapy. Repeat HIV testing 3 months later was negative. No recurrence or new lesions were identified at 3-month follow-up.
Similar to the other subtypes of KS, the nonepidemic form is dependent on HHV-8 infection, which is more commonly transmitted via saliva and sexual contact.3,4 After infecting endothelial cells, HHV-8 is believed to activate the mammalian target of rapamycin and nuclear factor κB pathways, resulting in aberrant cellular differentiation and neoangiogenesis through upregulation of vascular endothelial growth factor and basic fibroblast growth factor.2,4 Similar to what is seen with other herpesviruses, HHV-8 infection typically is lifelong due to the virus’s ability to establish latency within human B cells and endothelial cells as well as undergo sporadic bouts of lytic reactivation during its life cycle.4
Nonepidemic KS resembles other variants clinically, manifesting as erythematous or violaceous, painless, nonblanchable macules, papules, and nodules.1 Early lesions often are asymptomatic and can manifest as pigmented macules or small papules that vary from pale pink to vivid purple. Nodules also can occur and be exophytic and ulcerated with bleeding.1 Secondary lymphoproliferative disorders including Castleman disease and lymphoma have been reported.2,5
In contrast to other types of KS in which pulmonary or gastrointestinal tract lesions can develop with hemoptysis or hematochezia, mucocutaneous and visceral lesions rarely are reported in nonepidemic KS.3 Lymphedema, a feature associated with endemic KS, is notably absent in nonepidemic KS.1,3
The differential diagnosis applicable to all KS subtypes includes other vascular lesions such as angiomatosis and angiosarcoma. Histopathologic analysis is critical to differentiate KS from these conditions; visual diagnosis alone has only an 80% positive predictive value for KS.4 The histopathologic presentation of KS is a vascular proliferation in the dermis accompanied by an increased number of vessels without an endothelial cell lining.4 Spindle cell proliferation also is a common feature and is considered to be the KS tumor cell. Immunostaining for HHV-8 antigen as well as for CD31 and CD34 can be used to confirm the diagnosis.4
The management and prognosis of KS depends on the epidemiologic subtype. Classic and nonepidemic KS generally are indolent with a good prognosis. Periodic follow-up is recommended because of an increased risk for secondary malignancy such as lymphoma. The treatment of epidemic KS is highly active antiretroviral therapy. Similarly, reduction of immunosuppression is warranted for iatrogenic KS. For all types, cutaneous lesions can be treated with local excision, cryosurgery, radiation, chemotherapy, intralesional vincristine, or a topical agent such as imiquimod or alitretinoin.6
- Hinojosa T, Lewis DJ, Liu M, et al. Nonepidemic Kaposi sarcoma: a recently proposed category. J Am Acad Dermatol. 2017;3:441-443. doi: 10.1016/j.jdcr.2017.04.012
- Heymann WR. Nonepidemic Kaposi sarcoma: the fifth dimension. Dermatology World Insights and Inquiries. Published October 16, 2019. Accessed January 30, 2024. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/october/nonepidemic-kaposi-sarcoma
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542. doi: 10.1111/ijd.14080
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:9. doi:10.1038/s41572-019-0060-9
- Vecerek N, Truong A, Turner R, et al. Nonepidemic Kaposi’s sarcoma: an underrecognized subtype in HIV-negative patients. J Am Acad Dermatol. 2019;81(suppl 1):AB247. doi:10.1016/j.jaad.2019.09.1096
- Schneider JW, Dittmer DP. Diagnosis and treatment of Kaposi sarcoma. Am J Clin Dermatol. 2017;18:529-539. doi:10.1007/s40257-017-0270-4
To the Editor:
Kaposi sarcoma (KS) is a rare angioproliferative disorder associated with human herpesvirus 8 (HHV-8) infection.1 There are 4 main recognized epidemiologic forms of KS: classic, endemic, epidemic, and iatrogenic (Table). Nonepidemic KS is a recently described rare fifth type of KS that occurs in a subset of patients who do not fit the other classifications—HIV-negative patients without detectable cellular or humoral immune deficiency. This subset has been described as clinically similar to classic KS with limited disease but occurring in younger men.2,3 We describe a case of nonepidemic KS in a Middle Eastern heterosexual immunocompetent man.

A 30-year-old man presented for evaluation of a growth on the nose of 3 months’ duration. The patient reported being otherwise healthy and was not taking long-term medications. He denied a history of malignancy, organ transplant, or immunosuppressive therapy. He was born in Syria and lived in Thailand for several years prior to moving to the United States. HIV testing 6 months prior to presentation was negative. He denied fever, chills, lymphadenopathy, shortness of breath, hemoptysis, melena, hematochezia, and intravenous drug use.

Physical examination revealed a solitary shiny, 7-mm, pink-red papule on the nasal dorsum (Figure 1). No other skin or mucosal lesions were identified. There was no cervical, axillary, or inguinal lymphadenopathy. A laboratory workup consisting of serum immunoglobulins and serum protein electrophoresis was unremarkable. Tests for HIV-1 and HIV-2 as well as human T-lymphotropic virus 1 and 2 were negative. The CD4 and CD8 counts were within reference range. Histopathology of a shave biopsy revealed a dermal spindle cell proliferation arranged in short intersecting fascicles and admixed with plasma cells and occasional mitotic figures. Immunohistochemistry showed that the spindle cells stained positive for CD34, CD31, and HHV-8 (Figure 2). The lesion resolved after treatment with cryotherapy. Repeat HIV testing 3 months later was negative. No recurrence or new lesions were identified at 3-month follow-up.

Similar to the other subtypes of KS, the nonepidemic form is dependent on HHV-8 infection, which is more commonly transmitted via saliva and sexual contact.3,4 After infecting endothelial cells, HHV-8 is believed to activate the mammalian target of rapamycin and nuclear factor κB pathways, resulting in aberrant cellular differentiation and neoangiogenesis through upregulation of vascular endothelial growth factor and basic fibroblast growth factor.2,4 Similar to what is seen with other herpesviruses, HHV-8 infection typically is lifelong due to the virus’s ability to establish latency within human B cells and endothelial cells as well as undergo sporadic bouts of lytic reactivation during its life cycle.4
Nonepidemic KS resembles other variants clinically, manifesting as erythematous or violaceous, painless, nonblanchable macules, papules, and nodules.1 Early lesions often are asymptomatic and can manifest as pigmented macules or small papules that vary from pale pink to vivid purple. Nodules also can occur and be exophytic and ulcerated with bleeding.1 Secondary lymphoproliferative disorders including Castleman disease and lymphoma have been reported.2,5
In contrast to other types of KS in which pulmonary or gastrointestinal tract lesions can develop with hemoptysis or hematochezia, mucocutaneous and visceral lesions rarely are reported in nonepidemic KS.3 Lymphedema, a feature associated with endemic KS, is notably absent in nonepidemic KS.1,3
The differential diagnosis applicable to all KS subtypes includes other vascular lesions such as angiomatosis and angiosarcoma. Histopathologic analysis is critical to differentiate KS from these conditions; visual diagnosis alone has only an 80% positive predictive value for KS.4 The histopathologic presentation of KS is a vascular proliferation in the dermis accompanied by an increased number of vessels without an endothelial cell lining.4 Spindle cell proliferation also is a common feature and is considered to be the KS tumor cell. Immunostaining for HHV-8 antigen as well as for CD31 and CD34 can be used to confirm the diagnosis.4
The management and prognosis of KS depends on the epidemiologic subtype. Classic and nonepidemic KS generally are indolent with a good prognosis. Periodic follow-up is recommended because of an increased risk for secondary malignancy such as lymphoma. The treatment of epidemic KS is highly active antiretroviral therapy. Similarly, reduction of immunosuppression is warranted for iatrogenic KS. For all types, cutaneous lesions can be treated with local excision, cryosurgery, radiation, chemotherapy, intralesional vincristine, or a topical agent such as imiquimod or alitretinoin.6
To the Editor:
Kaposi sarcoma (KS) is a rare angioproliferative disorder associated with human herpesvirus 8 (HHV-8) infection.1 There are 4 main recognized epidemiologic forms of KS: classic, endemic, epidemic, and iatrogenic (Table). Nonepidemic KS is a recently described rare fifth type of KS that occurs in a subset of patients who do not fit the other classifications—HIV-negative patients without detectable cellular or humoral immune deficiency. This subset has been described as clinically similar to classic KS with limited disease but occurring in younger men.2,3 We describe a case of nonepidemic KS in a Middle Eastern heterosexual immunocompetent man.

A 30-year-old man presented for evaluation of a growth on the nose of 3 months’ duration. The patient reported being otherwise healthy and was not taking long-term medications. He denied a history of malignancy, organ transplant, or immunosuppressive therapy. He was born in Syria and lived in Thailand for several years prior to moving to the United States. HIV testing 6 months prior to presentation was negative. He denied fever, chills, lymphadenopathy, shortness of breath, hemoptysis, melena, hematochezia, and intravenous drug use.

Physical examination revealed a solitary shiny, 7-mm, pink-red papule on the nasal dorsum (Figure 1). No other skin or mucosal lesions were identified. There was no cervical, axillary, or inguinal lymphadenopathy. A laboratory workup consisting of serum immunoglobulins and serum protein electrophoresis was unremarkable. Tests for HIV-1 and HIV-2 as well as human T-lymphotropic virus 1 and 2 were negative. The CD4 and CD8 counts were within reference range. Histopathology of a shave biopsy revealed a dermal spindle cell proliferation arranged in short intersecting fascicles and admixed with plasma cells and occasional mitotic figures. Immunohistochemistry showed that the spindle cells stained positive for CD34, CD31, and HHV-8 (Figure 2). The lesion resolved after treatment with cryotherapy. Repeat HIV testing 3 months later was negative. No recurrence or new lesions were identified at 3-month follow-up.

Similar to the other subtypes of KS, the nonepidemic form is dependent on HHV-8 infection, which is more commonly transmitted via saliva and sexual contact.3,4 After infecting endothelial cells, HHV-8 is believed to activate the mammalian target of rapamycin and nuclear factor κB pathways, resulting in aberrant cellular differentiation and neoangiogenesis through upregulation of vascular endothelial growth factor and basic fibroblast growth factor.2,4 Similar to what is seen with other herpesviruses, HHV-8 infection typically is lifelong due to the virus’s ability to establish latency within human B cells and endothelial cells as well as undergo sporadic bouts of lytic reactivation during its life cycle.4
Nonepidemic KS resembles other variants clinically, manifesting as erythematous or violaceous, painless, nonblanchable macules, papules, and nodules.1 Early lesions often are asymptomatic and can manifest as pigmented macules or small papules that vary from pale pink to vivid purple. Nodules also can occur and be exophytic and ulcerated with bleeding.1 Secondary lymphoproliferative disorders including Castleman disease and lymphoma have been reported.2,5
In contrast to other types of KS in which pulmonary or gastrointestinal tract lesions can develop with hemoptysis or hematochezia, mucocutaneous and visceral lesions rarely are reported in nonepidemic KS.3 Lymphedema, a feature associated with endemic KS, is notably absent in nonepidemic KS.1,3
The differential diagnosis applicable to all KS subtypes includes other vascular lesions such as angiomatosis and angiosarcoma. Histopathologic analysis is critical to differentiate KS from these conditions; visual diagnosis alone has only an 80% positive predictive value for KS.4 The histopathologic presentation of KS is a vascular proliferation in the dermis accompanied by an increased number of vessels without an endothelial cell lining.4 Spindle cell proliferation also is a common feature and is considered to be the KS tumor cell. Immunostaining for HHV-8 antigen as well as for CD31 and CD34 can be used to confirm the diagnosis.4
The management and prognosis of KS depends on the epidemiologic subtype. Classic and nonepidemic KS generally are indolent with a good prognosis. Periodic follow-up is recommended because of an increased risk for secondary malignancy such as lymphoma. The treatment of epidemic KS is highly active antiretroviral therapy. Similarly, reduction of immunosuppression is warranted for iatrogenic KS. For all types, cutaneous lesions can be treated with local excision, cryosurgery, radiation, chemotherapy, intralesional vincristine, or a topical agent such as imiquimod or alitretinoin.6
- Hinojosa T, Lewis DJ, Liu M, et al. Nonepidemic Kaposi sarcoma: a recently proposed category. J Am Acad Dermatol. 2017;3:441-443. doi: 10.1016/j.jdcr.2017.04.012
- Heymann WR. Nonepidemic Kaposi sarcoma: the fifth dimension. Dermatology World Insights and Inquiries. Published October 16, 2019. Accessed January 30, 2024. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/october/nonepidemic-kaposi-sarcoma
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542. doi: 10.1111/ijd.14080
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:9. doi:10.1038/s41572-019-0060-9
- Vecerek N, Truong A, Turner R, et al. Nonepidemic Kaposi’s sarcoma: an underrecognized subtype in HIV-negative patients. J Am Acad Dermatol. 2019;81(suppl 1):AB247. doi:10.1016/j.jaad.2019.09.1096
- Schneider JW, Dittmer DP. Diagnosis and treatment of Kaposi sarcoma. Am J Clin Dermatol. 2017;18:529-539. doi:10.1007/s40257-017-0270-4
- Hinojosa T, Lewis DJ, Liu M, et al. Nonepidemic Kaposi sarcoma: a recently proposed category. J Am Acad Dermatol. 2017;3:441-443. doi: 10.1016/j.jdcr.2017.04.012
- Heymann WR. Nonepidemic Kaposi sarcoma: the fifth dimension. Dermatology World Insights and Inquiries. Published October 16, 2019. Accessed January 30, 2024. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/october/nonepidemic-kaposi-sarcoma
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542. doi: 10.1111/ijd.14080
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:9. doi:10.1038/s41572-019-0060-9
- Vecerek N, Truong A, Turner R, et al. Nonepidemic Kaposi’s sarcoma: an underrecognized subtype in HIV-negative patients. J Am Acad Dermatol. 2019;81(suppl 1):AB247. doi:10.1016/j.jaad.2019.09.1096
- Schneider JW, Dittmer DP. Diagnosis and treatment of Kaposi sarcoma. Am J Clin Dermatol. 2017;18:529-539. doi:10.1007/s40257-017-0270-4
Practice Points
- Nonepidemic Kaposi sarcoma (KS) is a recently described fifth subtype of the disease that typically occurs in younger men who are HIV-negative without detectable cellular or humoral immune deficiency.
- The cutaneous manifestations of nonepidemic KS are similar to those of classic KS, except that disease extent is limited and the prognosis is favorable in nonepidemic KS.
- Dermatologists should consider KS when a patient presents with clinically representative findings, even in the absence of typical risk factors such as immunosuppression.
Europe Needs to Get on Top of Its Measles Outbreak
“Measles should be a memory, not a present risk,” Quique Bassat, MBBS, PhD, director general of the Barcelona Institute of Global Health, told this news organization.
That is certainly not the case right now in some parts of Europe.
“What we are seeing currently is an almost 45-fold rise in measles cases in the WHO European Region,” Siddhartha Datta, MD, European regional advisor on vaccine-preventable diseases and immunization for the WHO, told this news organization. “In 2022, there were 940 cases, and in 2023 till November, it was around 42,000 plus. Between 2020 and 2022, we have seen 1.8 million children who have missed their measles vaccine doses.”
Lapses in Vaccinations
The overriding reason for the resurgence of measles is a backslide in vaccination coverage during the COVID-19 pandemic.
“During the COVID pandemic, we had a 5% decrease in coverage for most of the vaccines, and we are still seeing the consequences,” explained Dr. Bassat. “Measles is the perfect example of when you have a small drop of coverage you get outbreaks, as it’s extremely infectious and complicated to control.”
Reported national coverage with the first dose of measles-containing vaccine in the European Region fell from 96% in 2019 to 93% in 2022. Second-dose coverage fell from 92% in 2019 to 91% in 2022.
“You need to have 95% of the population vaccinated if you want herd immunity,” Dr. Bassat said.
Variation Across Europe
The WHO European Region comprises 53 countries, including Russia and some countries in central Asia. Its figures show Kazakhstan had the most recorded cases of measles last year, at more than 13,000, followed by the Russian Federation.
Romania declared a national epidemic in December 2023. Dr. Datta said there have also been outbreaks in Austria and France.
The UK Health Security Agency declared a major incident in January 2024 because of a surge in cases. From October 2023 to January 2024, there were 347 lab-confirmed cases of measles in England, with 127 of these confirmed in January. The West Midlands is an area of particular concern.
“It was not as though everything was rosy before COVID,” said Dr. Datta. “We saw wide variation in the coverage rates before the pandemic. Some countries weren’t doing as well. More particularly between some communities or municipalities, there were wide variations, and COVID-19 exacerbated the inequities in coverage. What we are seeing now is a combination of gaps before and after the pandemic, so it’s a compound problem.”
Belgium has also seen a measles resurgence, but not as many cases as the year before the pandemic. Laura Cornelissen, MD, works at the Belgian Public Health Institute, Sciensano, where she leads a team working on vaccine-preventable diseases.
She told this news organization: “We did observe a significant rise in cases and several clusters in 2023, compared to the very low numbers that were observed during the COVID-19 years. Preliminary figures indicate 85 measles cases for Belgium in 2023, leading to at least 26 hospitalizations. This is compared with eight cases for 2022, seven in 2021, and 47 in 2020; but 480 cases in the pre-pandemic year 2019.”
Sabrina Bacci, MD, head of vaccine-preventable diseases and immunization at the European Centre of Disease Control, told this news organization: “There have been a high number of cases in Romania and smaller outbreaks in other countries. However, there are a number of European countries which haven’t seen measles. Even though we have this variation between the different European countries, the tools to respond to outbreaks are the same.”
Vaccine Hesitance
Vaccine hesitance or even refusal is on the rise in Europe and elsewhere in the world.
“We can see from behavioral insights that, during COVID, people’s trust on vaccines, healthcare systems, and the government in general has gone down,” said Dr. Datta. “There had been skepticism before about the MMR jab causing autism, which was proved wrong, but vaccine skepticism shown throughout COVID is now showing its head in routine vaccine systems.”
The rise of so-called anti-vaxxers and associated fake conspiracy theories, including a mistrust of Big Pharma, hasn’t been helpful for encouraging essential childhood vaccination uptake, like measles, mumps, and rubella (MMR).
But the MMR vaccine backslide does not only originate in the pandemic.
Vanessa Saliba, consultant epidemiologist at the UK Health Security Agency, said: “MMR vaccine coverage has been falling for the last decade, with 1 out of 10 children starting school in England not protected.”
It could be that some people have religious concerns about the use of pork gelatin as a stabilizer in MMR vaccines. An alternative vaccine that does not contain pork gelatin can be requested.
Doctors and others in healthcare have a pivotal role to play when it comes to getting on top of the surges and educating patients, according to Dr. Bacci. “Healthcare professionals are the most precious resource we have, as they are the ones on the frontline explaining the importance of vaccination to their patients. It’s a very important dialogue.”
Clinics and Catch-Up Campaigns
Intensified routine immunization clinics and catch-up campaigns have been established in countries across Europe where they are needed.
Countries with large outbreaks are carrying out case investigations, identifying and vaccinating susceptible contacts, and generally raising awareness and implementing outbreak response immunization.
“Countries are really making good efforts and are systematically catching up the children who have missed their doses in the last 2 years. But the recovery to the 2019 levels has been slow, and more efforts and energy [need] to be put into this. We understand healthcare systems are stretched out from COVID, but this is not the time to lower our guard,” Dr. Datta said.
“Some countries are more proactive than others,” added Dr. Bassat. “Measles is an example of a disease where you typically organize catch-up campaigns. Measles has one of the highest reproductive numbers, as in the absence of preventive measures one infected person infects 14-16 others.”
All countries, even if they haven’t yet experienced measles outbreaks, are being urged by European healthcare authorities to look at potential immunity gaps and address them immediately.
When Will It Get Back to Normal?
“Measles was a disease that was targeted for elimination, but because of these outbreaks, we are seeing it almost everywhere again. We need to be careful and get on top of this,” warned Dr. Bassat.
Dr. Datta said it’s up to member states, decision-makers, healthcare leaders, and parents to come together to raise the immunity profiles of the European population. “Vaccination is a shared responsibility. The tools are effective. We just need to be ahead of the virus, and that is the challenge.”
Dr. Bacci added, “We have to remember we are entering the spring, which is a season when, traditionally, the disease can spread more easily, and it can find its way when people are susceptible. The vaccine is the tool that can help, and we have to act now and make sure it’s offered on time.”
A version of this article appeared on Medscape.com.
“Measles should be a memory, not a present risk,” Quique Bassat, MBBS, PhD, director general of the Barcelona Institute of Global Health, told this news organization.
That is certainly not the case right now in some parts of Europe.
“What we are seeing currently is an almost 45-fold rise in measles cases in the WHO European Region,” Siddhartha Datta, MD, European regional advisor on vaccine-preventable diseases and immunization for the WHO, told this news organization. “In 2022, there were 940 cases, and in 2023 till November, it was around 42,000 plus. Between 2020 and 2022, we have seen 1.8 million children who have missed their measles vaccine doses.”
Lapses in Vaccinations
The overriding reason for the resurgence of measles is a backslide in vaccination coverage during the COVID-19 pandemic.
“During the COVID pandemic, we had a 5% decrease in coverage for most of the vaccines, and we are still seeing the consequences,” explained Dr. Bassat. “Measles is the perfect example of when you have a small drop of coverage you get outbreaks, as it’s extremely infectious and complicated to control.”
Reported national coverage with the first dose of measles-containing vaccine in the European Region fell from 96% in 2019 to 93% in 2022. Second-dose coverage fell from 92% in 2019 to 91% in 2022.
“You need to have 95% of the population vaccinated if you want herd immunity,” Dr. Bassat said.
Variation Across Europe
The WHO European Region comprises 53 countries, including Russia and some countries in central Asia. Its figures show Kazakhstan had the most recorded cases of measles last year, at more than 13,000, followed by the Russian Federation.
Romania declared a national epidemic in December 2023. Dr. Datta said there have also been outbreaks in Austria and France.
The UK Health Security Agency declared a major incident in January 2024 because of a surge in cases. From October 2023 to January 2024, there were 347 lab-confirmed cases of measles in England, with 127 of these confirmed in January. The West Midlands is an area of particular concern.
“It was not as though everything was rosy before COVID,” said Dr. Datta. “We saw wide variation in the coverage rates before the pandemic. Some countries weren’t doing as well. More particularly between some communities or municipalities, there were wide variations, and COVID-19 exacerbated the inequities in coverage. What we are seeing now is a combination of gaps before and after the pandemic, so it’s a compound problem.”
Belgium has also seen a measles resurgence, but not as many cases as the year before the pandemic. Laura Cornelissen, MD, works at the Belgian Public Health Institute, Sciensano, where she leads a team working on vaccine-preventable diseases.
She told this news organization: “We did observe a significant rise in cases and several clusters in 2023, compared to the very low numbers that were observed during the COVID-19 years. Preliminary figures indicate 85 measles cases for Belgium in 2023, leading to at least 26 hospitalizations. This is compared with eight cases for 2022, seven in 2021, and 47 in 2020; but 480 cases in the pre-pandemic year 2019.”
Sabrina Bacci, MD, head of vaccine-preventable diseases and immunization at the European Centre of Disease Control, told this news organization: “There have been a high number of cases in Romania and smaller outbreaks in other countries. However, there are a number of European countries which haven’t seen measles. Even though we have this variation between the different European countries, the tools to respond to outbreaks are the same.”
Vaccine Hesitance
Vaccine hesitance or even refusal is on the rise in Europe and elsewhere in the world.
“We can see from behavioral insights that, during COVID, people’s trust on vaccines, healthcare systems, and the government in general has gone down,” said Dr. Datta. “There had been skepticism before about the MMR jab causing autism, which was proved wrong, but vaccine skepticism shown throughout COVID is now showing its head in routine vaccine systems.”
The rise of so-called anti-vaxxers and associated fake conspiracy theories, including a mistrust of Big Pharma, hasn’t been helpful for encouraging essential childhood vaccination uptake, like measles, mumps, and rubella (MMR).
But the MMR vaccine backslide does not only originate in the pandemic.
Vanessa Saliba, consultant epidemiologist at the UK Health Security Agency, said: “MMR vaccine coverage has been falling for the last decade, with 1 out of 10 children starting school in England not protected.”
It could be that some people have religious concerns about the use of pork gelatin as a stabilizer in MMR vaccines. An alternative vaccine that does not contain pork gelatin can be requested.
Doctors and others in healthcare have a pivotal role to play when it comes to getting on top of the surges and educating patients, according to Dr. Bacci. “Healthcare professionals are the most precious resource we have, as they are the ones on the frontline explaining the importance of vaccination to their patients. It’s a very important dialogue.”
Clinics and Catch-Up Campaigns
Intensified routine immunization clinics and catch-up campaigns have been established in countries across Europe where they are needed.
Countries with large outbreaks are carrying out case investigations, identifying and vaccinating susceptible contacts, and generally raising awareness and implementing outbreak response immunization.
“Countries are really making good efforts and are systematically catching up the children who have missed their doses in the last 2 years. But the recovery to the 2019 levels has been slow, and more efforts and energy [need] to be put into this. We understand healthcare systems are stretched out from COVID, but this is not the time to lower our guard,” Dr. Datta said.
“Some countries are more proactive than others,” added Dr. Bassat. “Measles is an example of a disease where you typically organize catch-up campaigns. Measles has one of the highest reproductive numbers, as in the absence of preventive measures one infected person infects 14-16 others.”
All countries, even if they haven’t yet experienced measles outbreaks, are being urged by European healthcare authorities to look at potential immunity gaps and address them immediately.
When Will It Get Back to Normal?
“Measles was a disease that was targeted for elimination, but because of these outbreaks, we are seeing it almost everywhere again. We need to be careful and get on top of this,” warned Dr. Bassat.
Dr. Datta said it’s up to member states, decision-makers, healthcare leaders, and parents to come together to raise the immunity profiles of the European population. “Vaccination is a shared responsibility. The tools are effective. We just need to be ahead of the virus, and that is the challenge.”
Dr. Bacci added, “We have to remember we are entering the spring, which is a season when, traditionally, the disease can spread more easily, and it can find its way when people are susceptible. The vaccine is the tool that can help, and we have to act now and make sure it’s offered on time.”
A version of this article appeared on Medscape.com.
“Measles should be a memory, not a present risk,” Quique Bassat, MBBS, PhD, director general of the Barcelona Institute of Global Health, told this news organization.
That is certainly not the case right now in some parts of Europe.
“What we are seeing currently is an almost 45-fold rise in measles cases in the WHO European Region,” Siddhartha Datta, MD, European regional advisor on vaccine-preventable diseases and immunization for the WHO, told this news organization. “In 2022, there were 940 cases, and in 2023 till November, it was around 42,000 plus. Between 2020 and 2022, we have seen 1.8 million children who have missed their measles vaccine doses.”
Lapses in Vaccinations
The overriding reason for the resurgence of measles is a backslide in vaccination coverage during the COVID-19 pandemic.
“During the COVID pandemic, we had a 5% decrease in coverage for most of the vaccines, and we are still seeing the consequences,” explained Dr. Bassat. “Measles is the perfect example of when you have a small drop of coverage you get outbreaks, as it’s extremely infectious and complicated to control.”
Reported national coverage with the first dose of measles-containing vaccine in the European Region fell from 96% in 2019 to 93% in 2022. Second-dose coverage fell from 92% in 2019 to 91% in 2022.
“You need to have 95% of the population vaccinated if you want herd immunity,” Dr. Bassat said.
Variation Across Europe
The WHO European Region comprises 53 countries, including Russia and some countries in central Asia. Its figures show Kazakhstan had the most recorded cases of measles last year, at more than 13,000, followed by the Russian Federation.
Romania declared a national epidemic in December 2023. Dr. Datta said there have also been outbreaks in Austria and France.
The UK Health Security Agency declared a major incident in January 2024 because of a surge in cases. From October 2023 to January 2024, there were 347 lab-confirmed cases of measles in England, with 127 of these confirmed in January. The West Midlands is an area of particular concern.
“It was not as though everything was rosy before COVID,” said Dr. Datta. “We saw wide variation in the coverage rates before the pandemic. Some countries weren’t doing as well. More particularly between some communities or municipalities, there were wide variations, and COVID-19 exacerbated the inequities in coverage. What we are seeing now is a combination of gaps before and after the pandemic, so it’s a compound problem.”
Belgium has also seen a measles resurgence, but not as many cases as the year before the pandemic. Laura Cornelissen, MD, works at the Belgian Public Health Institute, Sciensano, where she leads a team working on vaccine-preventable diseases.
She told this news organization: “We did observe a significant rise in cases and several clusters in 2023, compared to the very low numbers that were observed during the COVID-19 years. Preliminary figures indicate 85 measles cases for Belgium in 2023, leading to at least 26 hospitalizations. This is compared with eight cases for 2022, seven in 2021, and 47 in 2020; but 480 cases in the pre-pandemic year 2019.”
Sabrina Bacci, MD, head of vaccine-preventable diseases and immunization at the European Centre of Disease Control, told this news organization: “There have been a high number of cases in Romania and smaller outbreaks in other countries. However, there are a number of European countries which haven’t seen measles. Even though we have this variation between the different European countries, the tools to respond to outbreaks are the same.”
Vaccine Hesitance
Vaccine hesitance or even refusal is on the rise in Europe and elsewhere in the world.
“We can see from behavioral insights that, during COVID, people’s trust on vaccines, healthcare systems, and the government in general has gone down,” said Dr. Datta. “There had been skepticism before about the MMR jab causing autism, which was proved wrong, but vaccine skepticism shown throughout COVID is now showing its head in routine vaccine systems.”
The rise of so-called anti-vaxxers and associated fake conspiracy theories, including a mistrust of Big Pharma, hasn’t been helpful for encouraging essential childhood vaccination uptake, like measles, mumps, and rubella (MMR).
But the MMR vaccine backslide does not only originate in the pandemic.
Vanessa Saliba, consultant epidemiologist at the UK Health Security Agency, said: “MMR vaccine coverage has been falling for the last decade, with 1 out of 10 children starting school in England not protected.”
It could be that some people have religious concerns about the use of pork gelatin as a stabilizer in MMR vaccines. An alternative vaccine that does not contain pork gelatin can be requested.
Doctors and others in healthcare have a pivotal role to play when it comes to getting on top of the surges and educating patients, according to Dr. Bacci. “Healthcare professionals are the most precious resource we have, as they are the ones on the frontline explaining the importance of vaccination to their patients. It’s a very important dialogue.”
Clinics and Catch-Up Campaigns
Intensified routine immunization clinics and catch-up campaigns have been established in countries across Europe where they are needed.
Countries with large outbreaks are carrying out case investigations, identifying and vaccinating susceptible contacts, and generally raising awareness and implementing outbreak response immunization.
“Countries are really making good efforts and are systematically catching up the children who have missed their doses in the last 2 years. But the recovery to the 2019 levels has been slow, and more efforts and energy [need] to be put into this. We understand healthcare systems are stretched out from COVID, but this is not the time to lower our guard,” Dr. Datta said.
“Some countries are more proactive than others,” added Dr. Bassat. “Measles is an example of a disease where you typically organize catch-up campaigns. Measles has one of the highest reproductive numbers, as in the absence of preventive measures one infected person infects 14-16 others.”
All countries, even if they haven’t yet experienced measles outbreaks, are being urged by European healthcare authorities to look at potential immunity gaps and address them immediately.
When Will It Get Back to Normal?
“Measles was a disease that was targeted for elimination, but because of these outbreaks, we are seeing it almost everywhere again. We need to be careful and get on top of this,” warned Dr. Bassat.
Dr. Datta said it’s up to member states, decision-makers, healthcare leaders, and parents to come together to raise the immunity profiles of the European population. “Vaccination is a shared responsibility. The tools are effective. We just need to be ahead of the virus, and that is the challenge.”
Dr. Bacci added, “We have to remember we are entering the spring, which is a season when, traditionally, the disease can spread more easily, and it can find its way when people are susceptible. The vaccine is the tool that can help, and we have to act now and make sure it’s offered on time.”
A version of this article appeared on Medscape.com.
Bivalent COVID Vaccine Protected Children, Adolescents
Children and adolescents ages 5-17 who received a bivalent COVID-19 mRNA vaccine were less likely to become infected with SARS-CoV-2 compared with those who were unvaccinated or received only the monovalent COVID-19 vaccine, according to new data published February 6 in JAMA.
“All eligible children and adolescents should remain up to date with recommended COVID-19 vaccinations,” wrote the authors, led by Leora R. Feldstein, PhD, with the US Centers for Disease Control and Prevention (CDC) in Atlanta.
By the end of 2023, at least 911 youths ages 5-17 had died from COVID-related causes.
Researchers found that compared with participants who did not receive the COVID-19 vaccine or got monovalent-only doses 180 days or more before, the adjusted vaccine effectiveness of a bivalent COVID-19 vaccine dose against SARS-CoV-2 infection was 51.3% (95% confidence interval [CI], 23.6%-71.9%) 7-60 days after vaccination. Relative effectiveness was 62.4% (95% CI, 38.5%-81.1%) 61-150 days after vaccination. The researchers said the confidence intervals were wide because of the small sample size.
The information can help inform public health strategies, the authors noted, especially as new variants emerge.
Bivalent Dose Recommended in Fall of 2022
Bivalent mRNA COVID vaccines were recommended in the United States for children and adolescents ages 12 years or older on Sept. 1, 2022, and for children ages 5-11 on Oct. 12, 2022, when Omicron BA.4/5 types were the predominant circulating variant.
The study included 2,959 participants who completed periodic surveys (answering questions on demographics, household details, chronic medical conditions, and COVID-19 symptoms) and submitted weekly self-collected nasal swabs (whether or not they had symptoms). Those in the study submitted additional nasal swabs if they developed any symptoms.
Median adherence to weekly upper respiratory specimen swabbing was high throughout the study period at 93.8%.
Data from Sept. 4, 2022, to Jan. 31, 2023, were combined from three prospective US cohort studies at six sites. In addition to the surveys, researchers used information from state immunization information systems and electronic medical records.
Most of the Infected Were Unvaccinated or Had Monovalent Vax
Of the 426 participants (14.4% of the combined cohorts) infected with SARS-CoV-2, 383 (89.9%) were either unvaccinated or received monovalent vaccine doses only.
Calculations were adjusted for age, sex, race, ethnicity, health conditions, prior SARS-CoV-2 infections, geographic location, proportion of circulating variants by site, and local virus prevalence.
Participants living in Oregon, for example, had the highest uptake of bivalent COVID-19 vaccine (56.2%), whereas those in Texas had the lowest (2.4%). Participants reporting Hispanic ethnicity had lower bivalent uptake (17.1%) compared with non-Hispanic participants of all races (27.1%).
Of the 2,207 participants who did not receive a bivalent dose, 24.2% were unvaccinated and 1,672 (75.8%) received at least 1 monovalent dose.
The researchers said they saw no sign of waning effectiveness 61-150 days (the limit for this analysis) after receipt of the bivalent COVID-19 vaccine.
They wrote that continuation of the cohorts will allow study of waning patterns, which could help inform vaccine recommendations.
Among the limitations of the study are that testing methods and the COVID-19 symptoms surveyed varied among the three cohorts, so there may be some differences in defining infection or symptomatic COVID. In addition, the researchers were not able to account for the social vulnerability index and immunocompromised status, which could have affected vaccine uptake and risk of SARS-CoV-2 infection.
This study was supported by the National Center for Immunization and Respiratory Diseases, US Centers for Disease Control and Prevention, and by the National Institute of Allergy and Infectious Diseases. Coauthor Dr. Caban-Martinez reported grants from the Florida Firefighter Cancer Initiative and the Florida Department of Health. Coauthors Dr. Chu, Dr. Englund, Dr. Martin, and Dr. Monto reported receiving personal fees or grants from multiple pharmaceutical companies. Dr. Hegmann reported being the editor of the American College of Occupational and Environmental Medicine practice guidelines. Coauthor Dr. Gaglani reported serving as cochair of the infectious diseases and immunization committee and the respiratory syncytial virus task force lead for the Texas Pediatric Society and the Texas Chapter of the American Academy of Pediatrics. No other disclosures were reported.
Children and adolescents ages 5-17 who received a bivalent COVID-19 mRNA vaccine were less likely to become infected with SARS-CoV-2 compared with those who were unvaccinated or received only the monovalent COVID-19 vaccine, according to new data published February 6 in JAMA.
“All eligible children and adolescents should remain up to date with recommended COVID-19 vaccinations,” wrote the authors, led by Leora R. Feldstein, PhD, with the US Centers for Disease Control and Prevention (CDC) in Atlanta.
By the end of 2023, at least 911 youths ages 5-17 had died from COVID-related causes.
Researchers found that compared with participants who did not receive the COVID-19 vaccine or got monovalent-only doses 180 days or more before, the adjusted vaccine effectiveness of a bivalent COVID-19 vaccine dose against SARS-CoV-2 infection was 51.3% (95% confidence interval [CI], 23.6%-71.9%) 7-60 days after vaccination. Relative effectiveness was 62.4% (95% CI, 38.5%-81.1%) 61-150 days after vaccination. The researchers said the confidence intervals were wide because of the small sample size.
The information can help inform public health strategies, the authors noted, especially as new variants emerge.
Bivalent Dose Recommended in Fall of 2022
Bivalent mRNA COVID vaccines were recommended in the United States for children and adolescents ages 12 years or older on Sept. 1, 2022, and for children ages 5-11 on Oct. 12, 2022, when Omicron BA.4/5 types were the predominant circulating variant.
The study included 2,959 participants who completed periodic surveys (answering questions on demographics, household details, chronic medical conditions, and COVID-19 symptoms) and submitted weekly self-collected nasal swabs (whether or not they had symptoms). Those in the study submitted additional nasal swabs if they developed any symptoms.
Median adherence to weekly upper respiratory specimen swabbing was high throughout the study period at 93.8%.
Data from Sept. 4, 2022, to Jan. 31, 2023, were combined from three prospective US cohort studies at six sites. In addition to the surveys, researchers used information from state immunization information systems and electronic medical records.
Most of the Infected Were Unvaccinated or Had Monovalent Vax
Of the 426 participants (14.4% of the combined cohorts) infected with SARS-CoV-2, 383 (89.9%) were either unvaccinated or received monovalent vaccine doses only.
Calculations were adjusted for age, sex, race, ethnicity, health conditions, prior SARS-CoV-2 infections, geographic location, proportion of circulating variants by site, and local virus prevalence.
Participants living in Oregon, for example, had the highest uptake of bivalent COVID-19 vaccine (56.2%), whereas those in Texas had the lowest (2.4%). Participants reporting Hispanic ethnicity had lower bivalent uptake (17.1%) compared with non-Hispanic participants of all races (27.1%).
Of the 2,207 participants who did not receive a bivalent dose, 24.2% were unvaccinated and 1,672 (75.8%) received at least 1 monovalent dose.
The researchers said they saw no sign of waning effectiveness 61-150 days (the limit for this analysis) after receipt of the bivalent COVID-19 vaccine.
They wrote that continuation of the cohorts will allow study of waning patterns, which could help inform vaccine recommendations.
Among the limitations of the study are that testing methods and the COVID-19 symptoms surveyed varied among the three cohorts, so there may be some differences in defining infection or symptomatic COVID. In addition, the researchers were not able to account for the social vulnerability index and immunocompromised status, which could have affected vaccine uptake and risk of SARS-CoV-2 infection.
This study was supported by the National Center for Immunization and Respiratory Diseases, US Centers for Disease Control and Prevention, and by the National Institute of Allergy and Infectious Diseases. Coauthor Dr. Caban-Martinez reported grants from the Florida Firefighter Cancer Initiative and the Florida Department of Health. Coauthors Dr. Chu, Dr. Englund, Dr. Martin, and Dr. Monto reported receiving personal fees or grants from multiple pharmaceutical companies. Dr. Hegmann reported being the editor of the American College of Occupational and Environmental Medicine practice guidelines. Coauthor Dr. Gaglani reported serving as cochair of the infectious diseases and immunization committee and the respiratory syncytial virus task force lead for the Texas Pediatric Society and the Texas Chapter of the American Academy of Pediatrics. No other disclosures were reported.
Children and adolescents ages 5-17 who received a bivalent COVID-19 mRNA vaccine were less likely to become infected with SARS-CoV-2 compared with those who were unvaccinated or received only the monovalent COVID-19 vaccine, according to new data published February 6 in JAMA.
“All eligible children and adolescents should remain up to date with recommended COVID-19 vaccinations,” wrote the authors, led by Leora R. Feldstein, PhD, with the US Centers for Disease Control and Prevention (CDC) in Atlanta.
By the end of 2023, at least 911 youths ages 5-17 had died from COVID-related causes.
Researchers found that compared with participants who did not receive the COVID-19 vaccine or got monovalent-only doses 180 days or more before, the adjusted vaccine effectiveness of a bivalent COVID-19 vaccine dose against SARS-CoV-2 infection was 51.3% (95% confidence interval [CI], 23.6%-71.9%) 7-60 days after vaccination. Relative effectiveness was 62.4% (95% CI, 38.5%-81.1%) 61-150 days after vaccination. The researchers said the confidence intervals were wide because of the small sample size.
The information can help inform public health strategies, the authors noted, especially as new variants emerge.
Bivalent Dose Recommended in Fall of 2022
Bivalent mRNA COVID vaccines were recommended in the United States for children and adolescents ages 12 years or older on Sept. 1, 2022, and for children ages 5-11 on Oct. 12, 2022, when Omicron BA.4/5 types were the predominant circulating variant.
The study included 2,959 participants who completed periodic surveys (answering questions on demographics, household details, chronic medical conditions, and COVID-19 symptoms) and submitted weekly self-collected nasal swabs (whether or not they had symptoms). Those in the study submitted additional nasal swabs if they developed any symptoms.
Median adherence to weekly upper respiratory specimen swabbing was high throughout the study period at 93.8%.
Data from Sept. 4, 2022, to Jan. 31, 2023, were combined from three prospective US cohort studies at six sites. In addition to the surveys, researchers used information from state immunization information systems and electronic medical records.
Most of the Infected Were Unvaccinated or Had Monovalent Vax
Of the 426 participants (14.4% of the combined cohorts) infected with SARS-CoV-2, 383 (89.9%) were either unvaccinated or received monovalent vaccine doses only.
Calculations were adjusted for age, sex, race, ethnicity, health conditions, prior SARS-CoV-2 infections, geographic location, proportion of circulating variants by site, and local virus prevalence.
Participants living in Oregon, for example, had the highest uptake of bivalent COVID-19 vaccine (56.2%), whereas those in Texas had the lowest (2.4%). Participants reporting Hispanic ethnicity had lower bivalent uptake (17.1%) compared with non-Hispanic participants of all races (27.1%).
Of the 2,207 participants who did not receive a bivalent dose, 24.2% were unvaccinated and 1,672 (75.8%) received at least 1 monovalent dose.
The researchers said they saw no sign of waning effectiveness 61-150 days (the limit for this analysis) after receipt of the bivalent COVID-19 vaccine.
They wrote that continuation of the cohorts will allow study of waning patterns, which could help inform vaccine recommendations.
Among the limitations of the study are that testing methods and the COVID-19 symptoms surveyed varied among the three cohorts, so there may be some differences in defining infection or symptomatic COVID. In addition, the researchers were not able to account for the social vulnerability index and immunocompromised status, which could have affected vaccine uptake and risk of SARS-CoV-2 infection.
This study was supported by the National Center for Immunization and Respiratory Diseases, US Centers for Disease Control and Prevention, and by the National Institute of Allergy and Infectious Diseases. Coauthor Dr. Caban-Martinez reported grants from the Florida Firefighter Cancer Initiative and the Florida Department of Health. Coauthors Dr. Chu, Dr. Englund, Dr. Martin, and Dr. Monto reported receiving personal fees or grants from multiple pharmaceutical companies. Dr. Hegmann reported being the editor of the American College of Occupational and Environmental Medicine practice guidelines. Coauthor Dr. Gaglani reported serving as cochair of the infectious diseases and immunization committee and the respiratory syncytial virus task force lead for the Texas Pediatric Society and the Texas Chapter of the American Academy of Pediatrics. No other disclosures were reported.
FROM JAMA
What’s Eating You? Rhipicephalus Ticks Revisited
Characteristics
Rhipicephalus ticks belong to the Ixodidae family of hard-bodied ticks. They are large and teardrop shaped with an inornate scutum (hard dorsal plate) and relatively short mouthparts attached at a hexagonal basis capitulum (base of the head to which mouthparts are attached)(Figure).1 Widely spaced eyes and festoons also are present. The first pair of coxae—attachment base for the first pair of legs—are characteristically bifid; males have a pair of sclerotized adanal plates on the ventral surface adjacent to the anus as well as accessory adanal shields.2 Rhipicephalus (formerly Boophilus) microplus (the so-called cattle tick) is a newly added species; it lacks posterior festoons, and the anal groove is absent.3

Almost all Rhipicephalus ticks, except for R microplus, are 3-host ticks in which a single blood meal is consumed from a vertebrate host at each active life stage—larva, nymph, and adult—to complete development.4,5 In contrast to most ixodid ticks, which are exophilic (living outside of human habitation), the Rhipicephalus sanguineus sensu lato species (the brown dog tick) is highly endophilic (adapted to indoor living) and often can be found hidden in cracks and crevices of walls in homes and peridomestic structures.6 It is predominately monotropic (all developmental stages feed on the same host species) and has a strong host preference for dogs, though it occasionally feeds on other hosts (eg, humans).7 Although most common in tropical and subtropical climates, they can be found anywhere there are dogs due to their ability to colonize indoor dwellings.8 In contrast, R microplus ticks have a predilection for cattle and livestock rather than humans, posing a notable concern to livestock worldwide. Infestation results in transmission of disease-causing pathogens, such as Babesia and Anaplasma species, which costs the cattle industry billions of dollars annually.9
Clinical Manifestations and Treatment
Tick bites usually manifest as intensely pruritic, erythematous papules at the site of tick attachment due to a local type IV hypersensitivity reaction to antigens in the tick’s saliva. This reaction can be long-lasting. In addition to pruritic papules following a bite, an attached tick can be mistaken for a skin neoplasm or nevus. Given that ticks are small, especially during the larval stage, dermoscopy may be helpful in making a diagnosis.10 Symptomatic relief usually can be achieved with topical antipruritics or oral antihistamines.
Of public health concern, brown dog ticks are important vectors of Rickettsia rickettsii (the causative organism of Rocky Mountain spotted fever [RMSF]) in the Western hemisphere, and Rickettsia conorii (the causative organism of Mediterranean spotted fever [MSF][also known as Boutonneuse fever]) in the Eastern hemisphere.11 Bites by ticks carrying rickettsial disease classically manifest with early symptoms of fever, headache, and myalgia, followed by a rash or by a localized eschar or tache noire (a black, necrotic, scabbed lesion) that represents direct endothelial invasion and vascular damage by Rickettsia.12 Rocky Mountain spotted fever and MSF are more prevalent during summer, likely due, in part, to the combination of increased outdoor activity and a higher rate of tick-questing (host-seeking) behavior in warmer climates.4,7
Rocky Mountain Spotted Fever—Dermacentor variabilis is the primary vector of RMSF in the southeastern United States; Dermacentor andersoni is the major vector of RMSF in Rocky Mountain states. Rhipicephalus sanguineus sensu lato is an important vector of RMSF in the southwestern United States, Mexico, and Central America.11,13
Early symptoms of RMSF are nonspecific and can include fever, headache, arthralgia, myalgia, and malaise. Gastrointestinal tract symptoms (eg, nausea, vomiting, anorexia) may occur; notable abdominal pain occurs in some patients, particularly children. A characteristic petechial rash occurs in as many as 90% of patients, typically at the third to fifth day of illness, and classically begins on the wrists and ankles, with progression to the palms and soles before spreading centripetally to the arms, legs, and trunk.14 An eschar at the inoculation site is uncommon in RMSF; when present, it is more suggestive of MSF.15
The classic triad of fever, headache, and rash is present in 3% of patients during the first 3 days after a tick bite and in 60% to 70% within 2 weeks.16 A rash often is absent when patients first seek medical attention and may not develop (absent in 9% to 12% of cases; so-called spotless RMSF). Therefore, absence of rash should not be a reason to withhold treatment.16 Empiric treatment with doxycycline should be started promptly for all suspected cases of RMSF because of the rapid progression of disease and an increased risk for morbidity and mortality with delayed diagnosis.
Patients do not become antibody positive until 7 to 10 days after symptoms begin; therefore, treatment should not be delayed while awaiting serologic test results. The case fatality rate in the United States is estimated to be 5% to 10% overall and as high as 40% to 50% among patients who are not treated until day 8 or 9 of illness.17
Cutaneous complications include skin necrosis and gangrene due to continuous tissue damage in severe cases.16 Severe infection also may manifest with signs of multiorgan system damage, including altered mental status, cerebral edema, meningismus, transient deafness, myocarditis, pulmonary hemorrhage and edema, conjunctivitis, retinal abnormalities, and acute renal failure.14,16 Risk factors for more severe illness include delayed treatment, age 40 years or older or younger than 10 years, and underlying medical conditions such as alcoholic liver disease and glucose-6-phosphate dehydrogenase deficiency. However, even some healthy young patients die of this disease.17
Mediterranean Spotted Fever—Rhipicephalus sanguineus sensu lato is the primary vector of MSF, which is prevalent in areas adjacent to the Mediterranean Sea, including southern Europe, Africa, and Central Asia; Sicily is the most highly affected region.18 Findings with MSF are nearly identical to those of RMSF, except that tache noire is more common, present in as many as 70% of cases at the site of the inoculating tick bite, and MSF typically follows a less severe clinical course.12 Similar to other rickettsial diseases, the pathogenesis of MSF involves direct injury to vascular endothelial cells, causing a vasculitis that is responsible for the clinical abnormalities observed.
Patients with severe MSF experience complications similar to severe RMSF, including neurologic manifestations and multiorgan damage.18 Risk factors include advanced age, immunocompromised state, cardiac disease, chronic alcoholism, diabetes mellitus, glucose-6-phosphate dehydrogenase deficiency, respiratory insufficiency, and delayed treatment.18
Treatment—For all spotted fever group rickettsial infections, doxycycline is the treatment of choice for all patients, including children and pregnant women. Treatment should be started without delay; recommended dosages are 100 mg twice daily for children weighing more than 45 kg and adults, and 2.2 mg/kg twice daily for children weighing 45 kg or less.12
Rhipicephalus tick bites rarely can result in paralysis; however, Dermacentor ticks are responsible for most cases of tick-related paralysis in North America. Other pathogens proven or reputed to be transmitted by Rhipicephalus sanguineus sensu lato with zoonotic potential include but are not limited to Rickettsia massiliae, Coxiella burnetti, Anaplasma platys, Leishmania infantum, and Crimean-Congo hemorrhagic fever virus (Nairovirus).19
Environmental Treatment and Prevention
The most effective way to prevent tick-borne illness is avoidance of tick bites. Primary prevention methods include vector control, use of repellents (eg, N,N-diethyl-meta-toluamide [DEET]), picaridin, permethrin), avoidance of areas with a high tick burden, use of protective clothing, and detection and removal of ticks as soon as possible.
Environmental and veterinary controls also are important methods of tick-bite prevention. A veterinarian can recommend a variety of agents for dogs and cats that prevent attachment of ticks. Environmental controls include synthetic or natural product-based chemical acaricides and nonchemical methods, such as landscape management (eg, sealing cracks and crevices in homes and controlling tall grasses, weeds, and leaf debris) to minimize potential tick habitat.20 Secondary prevention includes antibiotics for prophylaxis or for treatment of tick-borne disease, when indicated.
Numerous tick repellents are available commercially; others are being studied. DEET, the most widely used topical repellent, has a broad spectrum of activity against many tick species.21 In addition, DEET has a well-known safety and toxicity profile, with rare adverse effects, and is safe for use in pregnant women and children older than 2 years. Alternative repellents, such as those containing picaridin, ethyl butylacetylaminopropionate (IR3535 [Merck]), oil of lemon eucalyptus, and 2-undecanone can be effective; some show efficacy comparable to that of DEET.22 Permethrin, a synthetic pyrethroid, is a highly efficacious tick repellent and insecticide, especially when used in conjunction with a topical repellent such as DEET. Unlike topically applied repellents, permethrin spray is applied to fabric (eg, clothing, shoes, bed nets, camping gear), not to skin.
Indiscriminate use of acaricides worldwide has led to increasing selection of acaricide resistance in Rhipicephalus tick species, which is especially true with the use of acaricides in controlling R microplus livestock infestations; several tick populations now show resistance to all major classes of these compounds.23-25 For that reason, there has been an increasing effort to develop new chemical and nonchemical approaches to tick control that are more environmentally sustainable and strategies to minimize development and progression of resistance such as rotation of acaricides; reducing the frequency of their application; use of pesticide mixtures, synergists, or both; and increasing use of nonacaricidal methods of control.26
Prompt removal of ticks is important for preventing the transmission of tick-borne disease. Proper removal involves rubbing the tick in a circular motion with a moist gauze pad or using fine-tipped tweezers to grasp the tick as close to the skin surface as possible and pulling upward with a steady pressure.17,27 It is important not to jerk, twist, squeeze, smash, or burn the tick, as this can result in insufficient removal of mouthparts or spread contaminated tick fluids to mucous membranes, increasing the risk for infection. Application of petroleum jelly or nail polish to aid in tick removal have not been shown to be effective and are not recommended.16,28
- Dantas-Torres F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol. 2008;152:173-185. doi:10.1016/j.vetpar.2007.12.030
- Madder M, Fourie JJ, Schetters TPM. Arachnida, Metastigmata, Ixodidae (except Ixodes holocyclus). In: Marchiondo AA, Cruthers LR, Fourie JJ, eds. Parasiticide Screening: In Vitro and In Vivo Tests With Relevant Parasite Rearing and Host Infection/Infestation Methods. Volume 1. Elsevier Academic Press; 2019:19-20.
- Burger TD, Shao R, Barker SC. Phylogenetic analysis of mitochondrial genome sequences indicates that the cattle tick, Rhipicephalus (Boophilus) microplus, contains a cryptic species. Mol Phylogenet Evol. 2014;76:241-253. doi:10.1016/j.ympev.2014.03.017
- Gray J, Dantas-Torres F, Estrada-Peña A, et al. Systematics and ecology of the brown dog tick, Rhipicephalus sanguineus. Ticks Tick Borne Dis. 2013;4:171-180. doi:10.1016/j.ttbdis.2012.12.003
- Tian Y, Lord CC, Kaufman PE. Brown dog tick, Rhipicephalus Sanguineus Latrielle (Arachnida: Acari: Ixodidae): EENY-221/IN378. EDIS. March 26, 2020. Accessed January 3, 2024. https://doi.org/10.32473/edis-in378-2020
- Saleh MN, Allen KE, Lineberry MW, et al. Ticks infesting dogs and cats in North America: biology, geographic distribution, and pathogen transmission. Vet Parasitol. 2021;294:109392. doi:10.1016/j.vetpar.2021.109392
- Dantas-Torres F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit Vectors. 2010;3:26. doi:10.1186/1756-3305-3-26
- Dryden MW, Payne PA. Biology and control of ticks infesting dogs and cats in North America. Vet Ther. 2004;5:139-154.
- Nyangiwe N, Yawa M, Muchenje V. Driving forces for changes in geographic range of cattle ticks (Acari: Ixodidae) in Africa: a Review. S Afr J Anim Sci. 2018;48:829. doi:10.4314/sajas.v48i5.4
- Ramot Y, Zlotogorski A, Mumcuoglu KY. Brown dog tick (Rhipicephalus sanguineus) infestation of the penis detected by dermoscopy. Int J Dermatol. 2012;51:1402-1403. doi:10.1111/j.1365-4632.2010.04756.x
- Tucker NSG, Weeks ENI, Beati L, et al. Prevalence and distribution of pathogen infection and permethrin resistance in tropical and temperate populations of Rhipicephalus sanguineus s.l. collected worldwide. Med Vet Entomol. 2021;35:147-157. doi:10.1111/mve.12479
- McClain MT, Sexton DJ, Hall KK, eds. Other spotted fever group rickettsial infections. UpToDate. Updated October 10, 2022. Accessed January 3, 2024. https://www.uptodate.com/contents/other-spotted-fever-group-rickettsial-infections
- Ribeiro CM, Carvalho JLB, Bastos PAS, et al. Prevalence of Rickettsia rickettsii in ticks: systematic review and meta-analysis. Vector Borne Zoonotic Dis. 2021;21:557-565. doi:10.1089/vbz.2021.0004
- Pace EJ, O’Reilly M. Tickborne diseases: diagnosis and management. Am Fam Physician. 2020;101:530-540.
- Patterson JW. Weedon’s Skin Pathology. 5th ed. Elsevier; 2020.
- Dantas-Torres F. Rocky Mountain spotted fever. Lancet Infect Dis. 2007;7:724-732. doi:10.1016/S1473-3099(07)70261-X
- Biggs HM, Behravesh CB, Bradley KK, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep. 2016;65:1-44. doi:10.15585/mmwr.rr6502a1
- Rossio R, Conalbi V, Castagna V, et al. Mediterranean spotted fever and hearing impairment: a rare complication. Int J Infect Dis. 2015;35:34-36. doi:10.1016/j.ijid.2015.04.005
- Dantas-Torres F, Otranto D. Further thoughts on the taxonomy and vector role of Rhipicephalus sanguineus group ticks. Vet Parasitol. 2015;208:9-13. doi:10.1016/j.vetpar.2014.12.014
- Eisen RJ, Kugeler KJ, Eisen L, et al. Tick-borne zoonoses in the United States: persistent and emerging threats to human health. ILAR J. 2017;58:319-335. doi:10.1093/ilar/ilx005
- Nguyen QD, Vu MN, Hebert AA. Insect repellents: an updated review for the clinician. J Am Acad Dermatol. 2018;88:123-130. doi:10.1016/j.jaad.2018.10.053
- Pages F, Dautel H, Duvallet G, et al. Tick repellents for human use: prevention of tick bites and tick-borne diseases. Vector Borne Zoonotic Dis. 2014;14:85-93. doi:10.1089/vbz.2013.1410
- Rodriguez-Vivas RI, Alonso-Díaz MA, et al. Prevalence and potential risk factors for organophosphate and pyrethroid resistance in Boophilus microplus ticks on cattle ranches from the State of Yucatan, Mexico. Vet Parasitol. 2006;136:335-342. doi:10.1016/j.vetpar.2005.05.069
- Rodríguez-Vivas RI, Rodríguez-Arevalo F, Alonso-Díaz MA, et al. Prevalence and potential risk factors for amitraz resistance in Boophilus microplus ticks in cattle farms in the State of Yucatan, Mexico. Prev Vet Med. 2006;75:280-286. doi:10.1016/j.prevetmed.2006.04.001
- Perez-Cogollo LC, Rodriguez-Vivas RI, Ramirez-Cruz GT, et al. First report of the cattle tick Rhipicephalus microplus resistant to ivermectin in Mexico. Vet Parasitol. 2010;168:165-169. doi:10.1016/j.vetpar.2009.10.021
- Rodriguez-Vivas RI, Jonsson NN, Bhushan C. Strategies for the control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitol Res.2018;117:3-29. doi:10.1007/s00436-017-5677-6
- Centers for Disease Control and Prevention. Tick removal. Updated May 13, 2022. Accessed January 3, 2024. https://www.cdc.gov/ticks/removing_a_tick.html
- Diaz JH. Chemical and plant-based insect repellents: efficacy, safety, and toxicity. Wilderness Environ Med. 2016;27:153-163. doi:10.1016/j.wem.2015.11.007
Characteristics
Rhipicephalus ticks belong to the Ixodidae family of hard-bodied ticks. They are large and teardrop shaped with an inornate scutum (hard dorsal plate) and relatively short mouthparts attached at a hexagonal basis capitulum (base of the head to which mouthparts are attached)(Figure).1 Widely spaced eyes and festoons also are present. The first pair of coxae—attachment base for the first pair of legs—are characteristically bifid; males have a pair of sclerotized adanal plates on the ventral surface adjacent to the anus as well as accessory adanal shields.2 Rhipicephalus (formerly Boophilus) microplus (the so-called cattle tick) is a newly added species; it lacks posterior festoons, and the anal groove is absent.3

Almost all Rhipicephalus ticks, except for R microplus, are 3-host ticks in which a single blood meal is consumed from a vertebrate host at each active life stage—larva, nymph, and adult—to complete development.4,5 In contrast to most ixodid ticks, which are exophilic (living outside of human habitation), the Rhipicephalus sanguineus sensu lato species (the brown dog tick) is highly endophilic (adapted to indoor living) and often can be found hidden in cracks and crevices of walls in homes and peridomestic structures.6 It is predominately monotropic (all developmental stages feed on the same host species) and has a strong host preference for dogs, though it occasionally feeds on other hosts (eg, humans).7 Although most common in tropical and subtropical climates, they can be found anywhere there are dogs due to their ability to colonize indoor dwellings.8 In contrast, R microplus ticks have a predilection for cattle and livestock rather than humans, posing a notable concern to livestock worldwide. Infestation results in transmission of disease-causing pathogens, such as Babesia and Anaplasma species, which costs the cattle industry billions of dollars annually.9
Clinical Manifestations and Treatment
Tick bites usually manifest as intensely pruritic, erythematous papules at the site of tick attachment due to a local type IV hypersensitivity reaction to antigens in the tick’s saliva. This reaction can be long-lasting. In addition to pruritic papules following a bite, an attached tick can be mistaken for a skin neoplasm or nevus. Given that ticks are small, especially during the larval stage, dermoscopy may be helpful in making a diagnosis.10 Symptomatic relief usually can be achieved with topical antipruritics or oral antihistamines.
Of public health concern, brown dog ticks are important vectors of Rickettsia rickettsii (the causative organism of Rocky Mountain spotted fever [RMSF]) in the Western hemisphere, and Rickettsia conorii (the causative organism of Mediterranean spotted fever [MSF][also known as Boutonneuse fever]) in the Eastern hemisphere.11 Bites by ticks carrying rickettsial disease classically manifest with early symptoms of fever, headache, and myalgia, followed by a rash or by a localized eschar or tache noire (a black, necrotic, scabbed lesion) that represents direct endothelial invasion and vascular damage by Rickettsia.12 Rocky Mountain spotted fever and MSF are more prevalent during summer, likely due, in part, to the combination of increased outdoor activity and a higher rate of tick-questing (host-seeking) behavior in warmer climates.4,7
Rocky Mountain Spotted Fever—Dermacentor variabilis is the primary vector of RMSF in the southeastern United States; Dermacentor andersoni is the major vector of RMSF in Rocky Mountain states. Rhipicephalus sanguineus sensu lato is an important vector of RMSF in the southwestern United States, Mexico, and Central America.11,13
Early symptoms of RMSF are nonspecific and can include fever, headache, arthralgia, myalgia, and malaise. Gastrointestinal tract symptoms (eg, nausea, vomiting, anorexia) may occur; notable abdominal pain occurs in some patients, particularly children. A characteristic petechial rash occurs in as many as 90% of patients, typically at the third to fifth day of illness, and classically begins on the wrists and ankles, with progression to the palms and soles before spreading centripetally to the arms, legs, and trunk.14 An eschar at the inoculation site is uncommon in RMSF; when present, it is more suggestive of MSF.15
The classic triad of fever, headache, and rash is present in 3% of patients during the first 3 days after a tick bite and in 60% to 70% within 2 weeks.16 A rash often is absent when patients first seek medical attention and may not develop (absent in 9% to 12% of cases; so-called spotless RMSF). Therefore, absence of rash should not be a reason to withhold treatment.16 Empiric treatment with doxycycline should be started promptly for all suspected cases of RMSF because of the rapid progression of disease and an increased risk for morbidity and mortality with delayed diagnosis.
Patients do not become antibody positive until 7 to 10 days after symptoms begin; therefore, treatment should not be delayed while awaiting serologic test results. The case fatality rate in the United States is estimated to be 5% to 10% overall and as high as 40% to 50% among patients who are not treated until day 8 or 9 of illness.17
Cutaneous complications include skin necrosis and gangrene due to continuous tissue damage in severe cases.16 Severe infection also may manifest with signs of multiorgan system damage, including altered mental status, cerebral edema, meningismus, transient deafness, myocarditis, pulmonary hemorrhage and edema, conjunctivitis, retinal abnormalities, and acute renal failure.14,16 Risk factors for more severe illness include delayed treatment, age 40 years or older or younger than 10 years, and underlying medical conditions such as alcoholic liver disease and glucose-6-phosphate dehydrogenase deficiency. However, even some healthy young patients die of this disease.17
Mediterranean Spotted Fever—Rhipicephalus sanguineus sensu lato is the primary vector of MSF, which is prevalent in areas adjacent to the Mediterranean Sea, including southern Europe, Africa, and Central Asia; Sicily is the most highly affected region.18 Findings with MSF are nearly identical to those of RMSF, except that tache noire is more common, present in as many as 70% of cases at the site of the inoculating tick bite, and MSF typically follows a less severe clinical course.12 Similar to other rickettsial diseases, the pathogenesis of MSF involves direct injury to vascular endothelial cells, causing a vasculitis that is responsible for the clinical abnormalities observed.
Patients with severe MSF experience complications similar to severe RMSF, including neurologic manifestations and multiorgan damage.18 Risk factors include advanced age, immunocompromised state, cardiac disease, chronic alcoholism, diabetes mellitus, glucose-6-phosphate dehydrogenase deficiency, respiratory insufficiency, and delayed treatment.18
Treatment—For all spotted fever group rickettsial infections, doxycycline is the treatment of choice for all patients, including children and pregnant women. Treatment should be started without delay; recommended dosages are 100 mg twice daily for children weighing more than 45 kg and adults, and 2.2 mg/kg twice daily for children weighing 45 kg or less.12
Rhipicephalus tick bites rarely can result in paralysis; however, Dermacentor ticks are responsible for most cases of tick-related paralysis in North America. Other pathogens proven or reputed to be transmitted by Rhipicephalus sanguineus sensu lato with zoonotic potential include but are not limited to Rickettsia massiliae, Coxiella burnetti, Anaplasma platys, Leishmania infantum, and Crimean-Congo hemorrhagic fever virus (Nairovirus).19
Environmental Treatment and Prevention
The most effective way to prevent tick-borne illness is avoidance of tick bites. Primary prevention methods include vector control, use of repellents (eg, N,N-diethyl-meta-toluamide [DEET]), picaridin, permethrin), avoidance of areas with a high tick burden, use of protective clothing, and detection and removal of ticks as soon as possible.
Environmental and veterinary controls also are important methods of tick-bite prevention. A veterinarian can recommend a variety of agents for dogs and cats that prevent attachment of ticks. Environmental controls include synthetic or natural product-based chemical acaricides and nonchemical methods, such as landscape management (eg, sealing cracks and crevices in homes and controlling tall grasses, weeds, and leaf debris) to minimize potential tick habitat.20 Secondary prevention includes antibiotics for prophylaxis or for treatment of tick-borne disease, when indicated.
Numerous tick repellents are available commercially; others are being studied. DEET, the most widely used topical repellent, has a broad spectrum of activity against many tick species.21 In addition, DEET has a well-known safety and toxicity profile, with rare adverse effects, and is safe for use in pregnant women and children older than 2 years. Alternative repellents, such as those containing picaridin, ethyl butylacetylaminopropionate (IR3535 [Merck]), oil of lemon eucalyptus, and 2-undecanone can be effective; some show efficacy comparable to that of DEET.22 Permethrin, a synthetic pyrethroid, is a highly efficacious tick repellent and insecticide, especially when used in conjunction with a topical repellent such as DEET. Unlike topically applied repellents, permethrin spray is applied to fabric (eg, clothing, shoes, bed nets, camping gear), not to skin.
Indiscriminate use of acaricides worldwide has led to increasing selection of acaricide resistance in Rhipicephalus tick species, which is especially true with the use of acaricides in controlling R microplus livestock infestations; several tick populations now show resistance to all major classes of these compounds.23-25 For that reason, there has been an increasing effort to develop new chemical and nonchemical approaches to tick control that are more environmentally sustainable and strategies to minimize development and progression of resistance such as rotation of acaricides; reducing the frequency of their application; use of pesticide mixtures, synergists, or both; and increasing use of nonacaricidal methods of control.26
Prompt removal of ticks is important for preventing the transmission of tick-borne disease. Proper removal involves rubbing the tick in a circular motion with a moist gauze pad or using fine-tipped tweezers to grasp the tick as close to the skin surface as possible and pulling upward with a steady pressure.17,27 It is important not to jerk, twist, squeeze, smash, or burn the tick, as this can result in insufficient removal of mouthparts or spread contaminated tick fluids to mucous membranes, increasing the risk for infection. Application of petroleum jelly or nail polish to aid in tick removal have not been shown to be effective and are not recommended.16,28
Characteristics
Rhipicephalus ticks belong to the Ixodidae family of hard-bodied ticks. They are large and teardrop shaped with an inornate scutum (hard dorsal plate) and relatively short mouthparts attached at a hexagonal basis capitulum (base of the head to which mouthparts are attached)(Figure).1 Widely spaced eyes and festoons also are present. The first pair of coxae—attachment base for the first pair of legs—are characteristically bifid; males have a pair of sclerotized adanal plates on the ventral surface adjacent to the anus as well as accessory adanal shields.2 Rhipicephalus (formerly Boophilus) microplus (the so-called cattle tick) is a newly added species; it lacks posterior festoons, and the anal groove is absent.3

Almost all Rhipicephalus ticks, except for R microplus, are 3-host ticks in which a single blood meal is consumed from a vertebrate host at each active life stage—larva, nymph, and adult—to complete development.4,5 In contrast to most ixodid ticks, which are exophilic (living outside of human habitation), the Rhipicephalus sanguineus sensu lato species (the brown dog tick) is highly endophilic (adapted to indoor living) and often can be found hidden in cracks and crevices of walls in homes and peridomestic structures.6 It is predominately monotropic (all developmental stages feed on the same host species) and has a strong host preference for dogs, though it occasionally feeds on other hosts (eg, humans).7 Although most common in tropical and subtropical climates, they can be found anywhere there are dogs due to their ability to colonize indoor dwellings.8 In contrast, R microplus ticks have a predilection for cattle and livestock rather than humans, posing a notable concern to livestock worldwide. Infestation results in transmission of disease-causing pathogens, such as Babesia and Anaplasma species, which costs the cattle industry billions of dollars annually.9
Clinical Manifestations and Treatment
Tick bites usually manifest as intensely pruritic, erythematous papules at the site of tick attachment due to a local type IV hypersensitivity reaction to antigens in the tick’s saliva. This reaction can be long-lasting. In addition to pruritic papules following a bite, an attached tick can be mistaken for a skin neoplasm or nevus. Given that ticks are small, especially during the larval stage, dermoscopy may be helpful in making a diagnosis.10 Symptomatic relief usually can be achieved with topical antipruritics or oral antihistamines.
Of public health concern, brown dog ticks are important vectors of Rickettsia rickettsii (the causative organism of Rocky Mountain spotted fever [RMSF]) in the Western hemisphere, and Rickettsia conorii (the causative organism of Mediterranean spotted fever [MSF][also known as Boutonneuse fever]) in the Eastern hemisphere.11 Bites by ticks carrying rickettsial disease classically manifest with early symptoms of fever, headache, and myalgia, followed by a rash or by a localized eschar or tache noire (a black, necrotic, scabbed lesion) that represents direct endothelial invasion and vascular damage by Rickettsia.12 Rocky Mountain spotted fever and MSF are more prevalent during summer, likely due, in part, to the combination of increased outdoor activity and a higher rate of tick-questing (host-seeking) behavior in warmer climates.4,7
Rocky Mountain Spotted Fever—Dermacentor variabilis is the primary vector of RMSF in the southeastern United States; Dermacentor andersoni is the major vector of RMSF in Rocky Mountain states. Rhipicephalus sanguineus sensu lato is an important vector of RMSF in the southwestern United States, Mexico, and Central America.11,13
Early symptoms of RMSF are nonspecific and can include fever, headache, arthralgia, myalgia, and malaise. Gastrointestinal tract symptoms (eg, nausea, vomiting, anorexia) may occur; notable abdominal pain occurs in some patients, particularly children. A characteristic petechial rash occurs in as many as 90% of patients, typically at the third to fifth day of illness, and classically begins on the wrists and ankles, with progression to the palms and soles before spreading centripetally to the arms, legs, and trunk.14 An eschar at the inoculation site is uncommon in RMSF; when present, it is more suggestive of MSF.15
The classic triad of fever, headache, and rash is present in 3% of patients during the first 3 days after a tick bite and in 60% to 70% within 2 weeks.16 A rash often is absent when patients first seek medical attention and may not develop (absent in 9% to 12% of cases; so-called spotless RMSF). Therefore, absence of rash should not be a reason to withhold treatment.16 Empiric treatment with doxycycline should be started promptly for all suspected cases of RMSF because of the rapid progression of disease and an increased risk for morbidity and mortality with delayed diagnosis.
Patients do not become antibody positive until 7 to 10 days after symptoms begin; therefore, treatment should not be delayed while awaiting serologic test results. The case fatality rate in the United States is estimated to be 5% to 10% overall and as high as 40% to 50% among patients who are not treated until day 8 or 9 of illness.17
Cutaneous complications include skin necrosis and gangrene due to continuous tissue damage in severe cases.16 Severe infection also may manifest with signs of multiorgan system damage, including altered mental status, cerebral edema, meningismus, transient deafness, myocarditis, pulmonary hemorrhage and edema, conjunctivitis, retinal abnormalities, and acute renal failure.14,16 Risk factors for more severe illness include delayed treatment, age 40 years or older or younger than 10 years, and underlying medical conditions such as alcoholic liver disease and glucose-6-phosphate dehydrogenase deficiency. However, even some healthy young patients die of this disease.17
Mediterranean Spotted Fever—Rhipicephalus sanguineus sensu lato is the primary vector of MSF, which is prevalent in areas adjacent to the Mediterranean Sea, including southern Europe, Africa, and Central Asia; Sicily is the most highly affected region.18 Findings with MSF are nearly identical to those of RMSF, except that tache noire is more common, present in as many as 70% of cases at the site of the inoculating tick bite, and MSF typically follows a less severe clinical course.12 Similar to other rickettsial diseases, the pathogenesis of MSF involves direct injury to vascular endothelial cells, causing a vasculitis that is responsible for the clinical abnormalities observed.
Patients with severe MSF experience complications similar to severe RMSF, including neurologic manifestations and multiorgan damage.18 Risk factors include advanced age, immunocompromised state, cardiac disease, chronic alcoholism, diabetes mellitus, glucose-6-phosphate dehydrogenase deficiency, respiratory insufficiency, and delayed treatment.18
Treatment—For all spotted fever group rickettsial infections, doxycycline is the treatment of choice for all patients, including children and pregnant women. Treatment should be started without delay; recommended dosages are 100 mg twice daily for children weighing more than 45 kg and adults, and 2.2 mg/kg twice daily for children weighing 45 kg or less.12
Rhipicephalus tick bites rarely can result in paralysis; however, Dermacentor ticks are responsible for most cases of tick-related paralysis in North America. Other pathogens proven or reputed to be transmitted by Rhipicephalus sanguineus sensu lato with zoonotic potential include but are not limited to Rickettsia massiliae, Coxiella burnetti, Anaplasma platys, Leishmania infantum, and Crimean-Congo hemorrhagic fever virus (Nairovirus).19
Environmental Treatment and Prevention
The most effective way to prevent tick-borne illness is avoidance of tick bites. Primary prevention methods include vector control, use of repellents (eg, N,N-diethyl-meta-toluamide [DEET]), picaridin, permethrin), avoidance of areas with a high tick burden, use of protective clothing, and detection and removal of ticks as soon as possible.
Environmental and veterinary controls also are important methods of tick-bite prevention. A veterinarian can recommend a variety of agents for dogs and cats that prevent attachment of ticks. Environmental controls include synthetic or natural product-based chemical acaricides and nonchemical methods, such as landscape management (eg, sealing cracks and crevices in homes and controlling tall grasses, weeds, and leaf debris) to minimize potential tick habitat.20 Secondary prevention includes antibiotics for prophylaxis or for treatment of tick-borne disease, when indicated.
Numerous tick repellents are available commercially; others are being studied. DEET, the most widely used topical repellent, has a broad spectrum of activity against many tick species.21 In addition, DEET has a well-known safety and toxicity profile, with rare adverse effects, and is safe for use in pregnant women and children older than 2 years. Alternative repellents, such as those containing picaridin, ethyl butylacetylaminopropionate (IR3535 [Merck]), oil of lemon eucalyptus, and 2-undecanone can be effective; some show efficacy comparable to that of DEET.22 Permethrin, a synthetic pyrethroid, is a highly efficacious tick repellent and insecticide, especially when used in conjunction with a topical repellent such as DEET. Unlike topically applied repellents, permethrin spray is applied to fabric (eg, clothing, shoes, bed nets, camping gear), not to skin.
Indiscriminate use of acaricides worldwide has led to increasing selection of acaricide resistance in Rhipicephalus tick species, which is especially true with the use of acaricides in controlling R microplus livestock infestations; several tick populations now show resistance to all major classes of these compounds.23-25 For that reason, there has been an increasing effort to develop new chemical and nonchemical approaches to tick control that are more environmentally sustainable and strategies to minimize development and progression of resistance such as rotation of acaricides; reducing the frequency of their application; use of pesticide mixtures, synergists, or both; and increasing use of nonacaricidal methods of control.26
Prompt removal of ticks is important for preventing the transmission of tick-borne disease. Proper removal involves rubbing the tick in a circular motion with a moist gauze pad or using fine-tipped tweezers to grasp the tick as close to the skin surface as possible and pulling upward with a steady pressure.17,27 It is important not to jerk, twist, squeeze, smash, or burn the tick, as this can result in insufficient removal of mouthparts or spread contaminated tick fluids to mucous membranes, increasing the risk for infection. Application of petroleum jelly or nail polish to aid in tick removal have not been shown to be effective and are not recommended.16,28
- Dantas-Torres F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol. 2008;152:173-185. doi:10.1016/j.vetpar.2007.12.030
- Madder M, Fourie JJ, Schetters TPM. Arachnida, Metastigmata, Ixodidae (except Ixodes holocyclus). In: Marchiondo AA, Cruthers LR, Fourie JJ, eds. Parasiticide Screening: In Vitro and In Vivo Tests With Relevant Parasite Rearing and Host Infection/Infestation Methods. Volume 1. Elsevier Academic Press; 2019:19-20.
- Burger TD, Shao R, Barker SC. Phylogenetic analysis of mitochondrial genome sequences indicates that the cattle tick, Rhipicephalus (Boophilus) microplus, contains a cryptic species. Mol Phylogenet Evol. 2014;76:241-253. doi:10.1016/j.ympev.2014.03.017
- Gray J, Dantas-Torres F, Estrada-Peña A, et al. Systematics and ecology of the brown dog tick, Rhipicephalus sanguineus. Ticks Tick Borne Dis. 2013;4:171-180. doi:10.1016/j.ttbdis.2012.12.003
- Tian Y, Lord CC, Kaufman PE. Brown dog tick, Rhipicephalus Sanguineus Latrielle (Arachnida: Acari: Ixodidae): EENY-221/IN378. EDIS. March 26, 2020. Accessed January 3, 2024. https://doi.org/10.32473/edis-in378-2020
- Saleh MN, Allen KE, Lineberry MW, et al. Ticks infesting dogs and cats in North America: biology, geographic distribution, and pathogen transmission. Vet Parasitol. 2021;294:109392. doi:10.1016/j.vetpar.2021.109392
- Dantas-Torres F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit Vectors. 2010;3:26. doi:10.1186/1756-3305-3-26
- Dryden MW, Payne PA. Biology and control of ticks infesting dogs and cats in North America. Vet Ther. 2004;5:139-154.
- Nyangiwe N, Yawa M, Muchenje V. Driving forces for changes in geographic range of cattle ticks (Acari: Ixodidae) in Africa: a Review. S Afr J Anim Sci. 2018;48:829. doi:10.4314/sajas.v48i5.4
- Ramot Y, Zlotogorski A, Mumcuoglu KY. Brown dog tick (Rhipicephalus sanguineus) infestation of the penis detected by dermoscopy. Int J Dermatol. 2012;51:1402-1403. doi:10.1111/j.1365-4632.2010.04756.x
- Tucker NSG, Weeks ENI, Beati L, et al. Prevalence and distribution of pathogen infection and permethrin resistance in tropical and temperate populations of Rhipicephalus sanguineus s.l. collected worldwide. Med Vet Entomol. 2021;35:147-157. doi:10.1111/mve.12479
- McClain MT, Sexton DJ, Hall KK, eds. Other spotted fever group rickettsial infections. UpToDate. Updated October 10, 2022. Accessed January 3, 2024. https://www.uptodate.com/contents/other-spotted-fever-group-rickettsial-infections
- Ribeiro CM, Carvalho JLB, Bastos PAS, et al. Prevalence of Rickettsia rickettsii in ticks: systematic review and meta-analysis. Vector Borne Zoonotic Dis. 2021;21:557-565. doi:10.1089/vbz.2021.0004
- Pace EJ, O’Reilly M. Tickborne diseases: diagnosis and management. Am Fam Physician. 2020;101:530-540.
- Patterson JW. Weedon’s Skin Pathology. 5th ed. Elsevier; 2020.
- Dantas-Torres F. Rocky Mountain spotted fever. Lancet Infect Dis. 2007;7:724-732. doi:10.1016/S1473-3099(07)70261-X
- Biggs HM, Behravesh CB, Bradley KK, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep. 2016;65:1-44. doi:10.15585/mmwr.rr6502a1
- Rossio R, Conalbi V, Castagna V, et al. Mediterranean spotted fever and hearing impairment: a rare complication. Int J Infect Dis. 2015;35:34-36. doi:10.1016/j.ijid.2015.04.005
- Dantas-Torres F, Otranto D. Further thoughts on the taxonomy and vector role of Rhipicephalus sanguineus group ticks. Vet Parasitol. 2015;208:9-13. doi:10.1016/j.vetpar.2014.12.014
- Eisen RJ, Kugeler KJ, Eisen L, et al. Tick-borne zoonoses in the United States: persistent and emerging threats to human health. ILAR J. 2017;58:319-335. doi:10.1093/ilar/ilx005
- Nguyen QD, Vu MN, Hebert AA. Insect repellents: an updated review for the clinician. J Am Acad Dermatol. 2018;88:123-130. doi:10.1016/j.jaad.2018.10.053
- Pages F, Dautel H, Duvallet G, et al. Tick repellents for human use: prevention of tick bites and tick-borne diseases. Vector Borne Zoonotic Dis. 2014;14:85-93. doi:10.1089/vbz.2013.1410
- Rodriguez-Vivas RI, Alonso-Díaz MA, et al. Prevalence and potential risk factors for organophosphate and pyrethroid resistance in Boophilus microplus ticks on cattle ranches from the State of Yucatan, Mexico. Vet Parasitol. 2006;136:335-342. doi:10.1016/j.vetpar.2005.05.069
- Rodríguez-Vivas RI, Rodríguez-Arevalo F, Alonso-Díaz MA, et al. Prevalence and potential risk factors for amitraz resistance in Boophilus microplus ticks in cattle farms in the State of Yucatan, Mexico. Prev Vet Med. 2006;75:280-286. doi:10.1016/j.prevetmed.2006.04.001
- Perez-Cogollo LC, Rodriguez-Vivas RI, Ramirez-Cruz GT, et al. First report of the cattle tick Rhipicephalus microplus resistant to ivermectin in Mexico. Vet Parasitol. 2010;168:165-169. doi:10.1016/j.vetpar.2009.10.021
- Rodriguez-Vivas RI, Jonsson NN, Bhushan C. Strategies for the control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitol Res.2018;117:3-29. doi:10.1007/s00436-017-5677-6
- Centers for Disease Control and Prevention. Tick removal. Updated May 13, 2022. Accessed January 3, 2024. https://www.cdc.gov/ticks/removing_a_tick.html
- Diaz JH. Chemical and plant-based insect repellents: efficacy, safety, and toxicity. Wilderness Environ Med. 2016;27:153-163. doi:10.1016/j.wem.2015.11.007
- Dantas-Torres F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol. 2008;152:173-185. doi:10.1016/j.vetpar.2007.12.030
- Madder M, Fourie JJ, Schetters TPM. Arachnida, Metastigmata, Ixodidae (except Ixodes holocyclus). In: Marchiondo AA, Cruthers LR, Fourie JJ, eds. Parasiticide Screening: In Vitro and In Vivo Tests With Relevant Parasite Rearing and Host Infection/Infestation Methods. Volume 1. Elsevier Academic Press; 2019:19-20.
- Burger TD, Shao R, Barker SC. Phylogenetic analysis of mitochondrial genome sequences indicates that the cattle tick, Rhipicephalus (Boophilus) microplus, contains a cryptic species. Mol Phylogenet Evol. 2014;76:241-253. doi:10.1016/j.ympev.2014.03.017
- Gray J, Dantas-Torres F, Estrada-Peña A, et al. Systematics and ecology of the brown dog tick, Rhipicephalus sanguineus. Ticks Tick Borne Dis. 2013;4:171-180. doi:10.1016/j.ttbdis.2012.12.003
- Tian Y, Lord CC, Kaufman PE. Brown dog tick, Rhipicephalus Sanguineus Latrielle (Arachnida: Acari: Ixodidae): EENY-221/IN378. EDIS. March 26, 2020. Accessed January 3, 2024. https://doi.org/10.32473/edis-in378-2020
- Saleh MN, Allen KE, Lineberry MW, et al. Ticks infesting dogs and cats in North America: biology, geographic distribution, and pathogen transmission. Vet Parasitol. 2021;294:109392. doi:10.1016/j.vetpar.2021.109392
- Dantas-Torres F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit Vectors. 2010;3:26. doi:10.1186/1756-3305-3-26
- Dryden MW, Payne PA. Biology and control of ticks infesting dogs and cats in North America. Vet Ther. 2004;5:139-154.
- Nyangiwe N, Yawa M, Muchenje V. Driving forces for changes in geographic range of cattle ticks (Acari: Ixodidae) in Africa: a Review. S Afr J Anim Sci. 2018;48:829. doi:10.4314/sajas.v48i5.4
- Ramot Y, Zlotogorski A, Mumcuoglu KY. Brown dog tick (Rhipicephalus sanguineus) infestation of the penis detected by dermoscopy. Int J Dermatol. 2012;51:1402-1403. doi:10.1111/j.1365-4632.2010.04756.x
- Tucker NSG, Weeks ENI, Beati L, et al. Prevalence and distribution of pathogen infection and permethrin resistance in tropical and temperate populations of Rhipicephalus sanguineus s.l. collected worldwide. Med Vet Entomol. 2021;35:147-157. doi:10.1111/mve.12479
- McClain MT, Sexton DJ, Hall KK, eds. Other spotted fever group rickettsial infections. UpToDate. Updated October 10, 2022. Accessed January 3, 2024. https://www.uptodate.com/contents/other-spotted-fever-group-rickettsial-infections
- Ribeiro CM, Carvalho JLB, Bastos PAS, et al. Prevalence of Rickettsia rickettsii in ticks: systematic review and meta-analysis. Vector Borne Zoonotic Dis. 2021;21:557-565. doi:10.1089/vbz.2021.0004
- Pace EJ, O’Reilly M. Tickborne diseases: diagnosis and management. Am Fam Physician. 2020;101:530-540.
- Patterson JW. Weedon’s Skin Pathology. 5th ed. Elsevier; 2020.
- Dantas-Torres F. Rocky Mountain spotted fever. Lancet Infect Dis. 2007;7:724-732. doi:10.1016/S1473-3099(07)70261-X
- Biggs HM, Behravesh CB, Bradley KK, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep. 2016;65:1-44. doi:10.15585/mmwr.rr6502a1
- Rossio R, Conalbi V, Castagna V, et al. Mediterranean spotted fever and hearing impairment: a rare complication. Int J Infect Dis. 2015;35:34-36. doi:10.1016/j.ijid.2015.04.005
- Dantas-Torres F, Otranto D. Further thoughts on the taxonomy and vector role of Rhipicephalus sanguineus group ticks. Vet Parasitol. 2015;208:9-13. doi:10.1016/j.vetpar.2014.12.014
- Eisen RJ, Kugeler KJ, Eisen L, et al. Tick-borne zoonoses in the United States: persistent and emerging threats to human health. ILAR J. 2017;58:319-335. doi:10.1093/ilar/ilx005
- Nguyen QD, Vu MN, Hebert AA. Insect repellents: an updated review for the clinician. J Am Acad Dermatol. 2018;88:123-130. doi:10.1016/j.jaad.2018.10.053
- Pages F, Dautel H, Duvallet G, et al. Tick repellents for human use: prevention of tick bites and tick-borne diseases. Vector Borne Zoonotic Dis. 2014;14:85-93. doi:10.1089/vbz.2013.1410
- Rodriguez-Vivas RI, Alonso-Díaz MA, et al. Prevalence and potential risk factors for organophosphate and pyrethroid resistance in Boophilus microplus ticks on cattle ranches from the State of Yucatan, Mexico. Vet Parasitol. 2006;136:335-342. doi:10.1016/j.vetpar.2005.05.069
- Rodríguez-Vivas RI, Rodríguez-Arevalo F, Alonso-Díaz MA, et al. Prevalence and potential risk factors for amitraz resistance in Boophilus microplus ticks in cattle farms in the State of Yucatan, Mexico. Prev Vet Med. 2006;75:280-286. doi:10.1016/j.prevetmed.2006.04.001
- Perez-Cogollo LC, Rodriguez-Vivas RI, Ramirez-Cruz GT, et al. First report of the cattle tick Rhipicephalus microplus resistant to ivermectin in Mexico. Vet Parasitol. 2010;168:165-169. doi:10.1016/j.vetpar.2009.10.021
- Rodriguez-Vivas RI, Jonsson NN, Bhushan C. Strategies for the control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitol Res.2018;117:3-29. doi:10.1007/s00436-017-5677-6
- Centers for Disease Control and Prevention. Tick removal. Updated May 13, 2022. Accessed January 3, 2024. https://www.cdc.gov/ticks/removing_a_tick.html
- Diaz JH. Chemical and plant-based insect repellents: efficacy, safety, and toxicity. Wilderness Environ Med. 2016;27:153-163. doi:10.1016/j.wem.2015.11.007
PRACTICE POINTS
- Rhipicephalus ticks are vectors of a variety of diseases, including the rickettsial diseases Rocky Mountain spotted fever and Mediterranean spotted fever.
- Presenting symptoms of a tick bite include intensely pruritic, erythematous papules and nodules at the site of tick attachment.
- If rickettsial disease is suspected, treatment with doxycycline should be initiated immediately; do not delay treatment to await results of confirmatory tests or because of the absence of a rash.
- Primary methods of prevention of tick-borne disease include repellents, protective clothing, vector control, and prompt removal of the tick.
Respiratory Virus Surge: Diagnosing COVID-19 vs RSV, Flu
Amid the current wave of winter respiratory virus cases, influenza (types A and B) leads the way with the highest number of emergency room visits, followed closely by COVID-19, thanks to the JN.1 variant, and respiratory syncytial virus (RSV). With various similarities and differences in disease presentations, how challenging is it for physician’s to distinguish between, diagnose, and treat COVID-19 vs RSV and influenza?
While these three respiratory viruses often have similar presentations, you may often find that patients with COVID-19 experience more fever, dry cough, and labored breathing, according to Cyrus Munguti, MD, assistant professor of medicine at KU Medical Center and hospitalist at Wesley Medical Center, Wichita, Kansas.
“COVID-19 patients tend to have trouble breathing because the alveoli are affected and get inflammation and fluid accumulating in the lungs, and they end up having little to no oxygen,” said Dr. Munguti. “When we check their vital signs, patients with COVID tend to have hypoxemia [meaning saturations are less than 88% or 90% depending on the guidelines you follow].”
Patients with RSV and influenza tend to have more upper respiratory symptoms, like runny nose, sternutation — which later can progress to a cough in the upper airways, Dr. Munguti said. Unlike with COVID-19, patients with RSV and influenza — generally until they are very sick — often do not experience hypoxemia.
Inflammation in the airways can form as a result of all three viruses. Furthermore, bacteria that live in these airways could lead to a secondary bacterial infection in the upper respiratory and lower respiratory tracts — which could then cause pneumonia, Dr. Munguti said.
Another note: , according to Panagis Galiatsatos, MD, pulmonologist and associate professor at Johns Hopkins Medicine. “The Alpha through Delta variants really were a lot more lung tissue invading,” Dr. Galiatsatos said. “With the COVID-19 Omicron family — its capabilities are similar to what flu and RSV have done over the years. It’s more airway-invading.”
It’s critical to understand that diagnosing these diseases based on symptoms alone can be quite fickle, according to Dr. Galiatsatos. Objective tests, either at home or in a laboratory, are preferred. This is largely because disease presentation can depend on the host factor that the virus enters into, said Dr. Galiatsatos. For example, virus symptoms may look different for a patient with asthma and for someone with heart disease.
With children being among the most vulnerable for severe respiratory illness, testing and treatment are paramount and can be quite accurate in seasons where respiratory viruses thrive, according to Stan Spinner, MD, chief medical officer at Texas Children’s Pediatrics and Urgent Care. “When individuals are tested for either of these conditions when the prevalence in the community is low, we tend to see false positive results.”
Texas Children’s Pediatrics and Urgent Care’s 12 sites offer COVID-19 and influenza antigen tests that have results ready in around 10 minutes. RSV testing, on the other hand, is limited to around half of the Texas Children’s Pediatrics and none of the urgent care locations, as the test can only be administered through a nasal swab conducted by a physician. As there is no specific treatment or therapy for RSV, the benefits of RSV testing can actually be quite low — often leading to frustrated parents regarding next steps after diagnosis.
“There are a number of respiratory viruses that may present with similar symptoms as RSV, and some of these viruses may even lead to much of the same adverse outcomes as the RSV virus,” Dr. Galiatsatos said. “Consequently, our physicians need to help parents understand this and give them guidance as to when to seek medical attention for worsening symptoms.”
There are two new RSV immunizations to treat certain demographics of patients, Dr. Spinner added. One is an RSV vaccine for infants under 8 months old, though there is limited supply. There is also an RSV vaccine available for pregnant women (between 32 and 36 weeks gestation) that has proved to be effective in fending off RSV infections in newborns up to 6 months old.
Physicians should remain diligent in stressing to patients that vaccinations against COVID-19 and influenza play a key role in keeping their families safe during seasons of staggering respiratory infections.
“These vaccines are extremely safe, and while they may not always prevent infection, these vaccines are extremely effective in preventing more serious consequences, such as hospitalization or death,” Dr. Galiatsatos said.
A version of this article appeared on Medscape.com.
Amid the current wave of winter respiratory virus cases, influenza (types A and B) leads the way with the highest number of emergency room visits, followed closely by COVID-19, thanks to the JN.1 variant, and respiratory syncytial virus (RSV). With various similarities and differences in disease presentations, how challenging is it for physician’s to distinguish between, diagnose, and treat COVID-19 vs RSV and influenza?
While these three respiratory viruses often have similar presentations, you may often find that patients with COVID-19 experience more fever, dry cough, and labored breathing, according to Cyrus Munguti, MD, assistant professor of medicine at KU Medical Center and hospitalist at Wesley Medical Center, Wichita, Kansas.
“COVID-19 patients tend to have trouble breathing because the alveoli are affected and get inflammation and fluid accumulating in the lungs, and they end up having little to no oxygen,” said Dr. Munguti. “When we check their vital signs, patients with COVID tend to have hypoxemia [meaning saturations are less than 88% or 90% depending on the guidelines you follow].”
Patients with RSV and influenza tend to have more upper respiratory symptoms, like runny nose, sternutation — which later can progress to a cough in the upper airways, Dr. Munguti said. Unlike with COVID-19, patients with RSV and influenza — generally until they are very sick — often do not experience hypoxemia.
Inflammation in the airways can form as a result of all three viruses. Furthermore, bacteria that live in these airways could lead to a secondary bacterial infection in the upper respiratory and lower respiratory tracts — which could then cause pneumonia, Dr. Munguti said.
Another note: , according to Panagis Galiatsatos, MD, pulmonologist and associate professor at Johns Hopkins Medicine. “The Alpha through Delta variants really were a lot more lung tissue invading,” Dr. Galiatsatos said. “With the COVID-19 Omicron family — its capabilities are similar to what flu and RSV have done over the years. It’s more airway-invading.”
It’s critical to understand that diagnosing these diseases based on symptoms alone can be quite fickle, according to Dr. Galiatsatos. Objective tests, either at home or in a laboratory, are preferred. This is largely because disease presentation can depend on the host factor that the virus enters into, said Dr. Galiatsatos. For example, virus symptoms may look different for a patient with asthma and for someone with heart disease.
With children being among the most vulnerable for severe respiratory illness, testing and treatment are paramount and can be quite accurate in seasons where respiratory viruses thrive, according to Stan Spinner, MD, chief medical officer at Texas Children’s Pediatrics and Urgent Care. “When individuals are tested for either of these conditions when the prevalence in the community is low, we tend to see false positive results.”
Texas Children’s Pediatrics and Urgent Care’s 12 sites offer COVID-19 and influenza antigen tests that have results ready in around 10 minutes. RSV testing, on the other hand, is limited to around half of the Texas Children’s Pediatrics and none of the urgent care locations, as the test can only be administered through a nasal swab conducted by a physician. As there is no specific treatment or therapy for RSV, the benefits of RSV testing can actually be quite low — often leading to frustrated parents regarding next steps after diagnosis.
“There are a number of respiratory viruses that may present with similar symptoms as RSV, and some of these viruses may even lead to much of the same adverse outcomes as the RSV virus,” Dr. Galiatsatos said. “Consequently, our physicians need to help parents understand this and give them guidance as to when to seek medical attention for worsening symptoms.”
There are two new RSV immunizations to treat certain demographics of patients, Dr. Spinner added. One is an RSV vaccine for infants under 8 months old, though there is limited supply. There is also an RSV vaccine available for pregnant women (between 32 and 36 weeks gestation) that has proved to be effective in fending off RSV infections in newborns up to 6 months old.
Physicians should remain diligent in stressing to patients that vaccinations against COVID-19 and influenza play a key role in keeping their families safe during seasons of staggering respiratory infections.
“These vaccines are extremely safe, and while they may not always prevent infection, these vaccines are extremely effective in preventing more serious consequences, such as hospitalization or death,” Dr. Galiatsatos said.
A version of this article appeared on Medscape.com.
Amid the current wave of winter respiratory virus cases, influenza (types A and B) leads the way with the highest number of emergency room visits, followed closely by COVID-19, thanks to the JN.1 variant, and respiratory syncytial virus (RSV). With various similarities and differences in disease presentations, how challenging is it for physician’s to distinguish between, diagnose, and treat COVID-19 vs RSV and influenza?
While these three respiratory viruses often have similar presentations, you may often find that patients with COVID-19 experience more fever, dry cough, and labored breathing, according to Cyrus Munguti, MD, assistant professor of medicine at KU Medical Center and hospitalist at Wesley Medical Center, Wichita, Kansas.
“COVID-19 patients tend to have trouble breathing because the alveoli are affected and get inflammation and fluid accumulating in the lungs, and they end up having little to no oxygen,” said Dr. Munguti. “When we check their vital signs, patients with COVID tend to have hypoxemia [meaning saturations are less than 88% or 90% depending on the guidelines you follow].”
Patients with RSV and influenza tend to have more upper respiratory symptoms, like runny nose, sternutation — which later can progress to a cough in the upper airways, Dr. Munguti said. Unlike with COVID-19, patients with RSV and influenza — generally until they are very sick — often do not experience hypoxemia.
Inflammation in the airways can form as a result of all three viruses. Furthermore, bacteria that live in these airways could lead to a secondary bacterial infection in the upper respiratory and lower respiratory tracts — which could then cause pneumonia, Dr. Munguti said.
Another note: , according to Panagis Galiatsatos, MD, pulmonologist and associate professor at Johns Hopkins Medicine. “The Alpha through Delta variants really were a lot more lung tissue invading,” Dr. Galiatsatos said. “With the COVID-19 Omicron family — its capabilities are similar to what flu and RSV have done over the years. It’s more airway-invading.”
It’s critical to understand that diagnosing these diseases based on symptoms alone can be quite fickle, according to Dr. Galiatsatos. Objective tests, either at home or in a laboratory, are preferred. This is largely because disease presentation can depend on the host factor that the virus enters into, said Dr. Galiatsatos. For example, virus symptoms may look different for a patient with asthma and for someone with heart disease.
With children being among the most vulnerable for severe respiratory illness, testing and treatment are paramount and can be quite accurate in seasons where respiratory viruses thrive, according to Stan Spinner, MD, chief medical officer at Texas Children’s Pediatrics and Urgent Care. “When individuals are tested for either of these conditions when the prevalence in the community is low, we tend to see false positive results.”
Texas Children’s Pediatrics and Urgent Care’s 12 sites offer COVID-19 and influenza antigen tests that have results ready in around 10 minutes. RSV testing, on the other hand, is limited to around half of the Texas Children’s Pediatrics and none of the urgent care locations, as the test can only be administered through a nasal swab conducted by a physician. As there is no specific treatment or therapy for RSV, the benefits of RSV testing can actually be quite low — often leading to frustrated parents regarding next steps after diagnosis.
“There are a number of respiratory viruses that may present with similar symptoms as RSV, and some of these viruses may even lead to much of the same adverse outcomes as the RSV virus,” Dr. Galiatsatos said. “Consequently, our physicians need to help parents understand this and give them guidance as to when to seek medical attention for worsening symptoms.”
There are two new RSV immunizations to treat certain demographics of patients, Dr. Spinner added. One is an RSV vaccine for infants under 8 months old, though there is limited supply. There is also an RSV vaccine available for pregnant women (between 32 and 36 weeks gestation) that has proved to be effective in fending off RSV infections in newborns up to 6 months old.
Physicians should remain diligent in stressing to patients that vaccinations against COVID-19 and influenza play a key role in keeping their families safe during seasons of staggering respiratory infections.
“These vaccines are extremely safe, and while they may not always prevent infection, these vaccines are extremely effective in preventing more serious consequences, such as hospitalization or death,” Dr. Galiatsatos said.
A version of this article appeared on Medscape.com.