User login
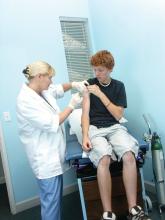
Flu vaccine visits reveal missed opportunities for HPV vaccination
BALTIMORE – according to a study.
“Overall in preventive visits, missed opportunities were much higher for HPV, compared to the other two vaccines” recommended for adolescents, MenACWY (meningococcal conjugate vaccine) and Tdap, Mary Kate Kelly, MPH, of Children’s Hospital of Philadelphia, told attendees at the Pediatric Academic Societies annual meeting. “In order to increase vaccination rates, it’s essential to implement efforts to reduce missed opportunities.”
According to 2018 Centers for Disease Control and Prevention data, Ms. Kelly said, vaccine coverage for the HPV vaccine is approximately 66%, compared with 85% for the MenACWY vaccine and 89% for the Tdap vaccine.
Ms. Kelly and her colleagues investigated how often children or adolescents missed an opportunity to get an HPV vaccine when they received an influenza vaccine during an office visit. This study was part of the larger STOP HPV trial funded by the National Institutes of Health and aimed at implementing evidence-based interventions to reduce missed opportunities for HPV vaccination in primary care.
The researchers retrospectively reviewed EHRs from 2015 to 2018 for 48 pediatric practices across 19 states. All practices were part of the American Academy of Pediatrics’ Pediatric Research in Office Settings (PROS) national pediatric primary care network. The researchers isolated all visits for patients aged 11-17 years who received their flu vaccine and were eligible to receive the HPV vaccine.
The investigators defined a missed opportunity as one in which a patient was due for the HPV vaccine but did not receive one at the visit when they received their flu vaccine.
The study involved 40,129 patients who received the flu vaccine at 52,818 visits when they also were eligible to receive the HPV vaccine. The median age of patients was 12 years old, and 47% were female.
In 68% of visits, the patient could have received an HPV vaccine but did not – even though they were due and eligible for one. The rate was the same for boys and for girls. By contrast, only 38% of visits involved a missed opportunity for the MenACWY vaccines and 39% for the Tdap vaccine.
Rates of missed opportunities for HPV vaccination ranged among individual practices from 22% to 81% of overall visits. Patients were more than twice as likely to miss the opportunity for an HPV vaccine dose if it would have been their first dose – 70% of missed opportunities – versus being a second or third dose, which comprised 30% of missed opportunities (adjusted relative risk, 2.48; P less than .001)).
“However, missed opportunities were also common for subsequent HPV doses when vaccine hesitancy is less likely to be an issue,” Ms. Kelly added.
It also was much more likely that missed opportunities occurred during nurse visits or visits for an acute or chronic condition rather than preventive visits, which made up about half (51%) of all visits analyzed. While 48% of preventive visits involved a missed opportunity, 93% of nurse visits (aRR compared with preventive, 2.18; P less than.001) and 89% of acute or chronic visits (aRR, 2.11; P less than .001) did.
Percentages of missed opportunities were similarly high for the MenACWY and Tdap vaccines at nurse visits and acute/chronic visits, but much lower at preventive visits for the MenACWY (12%) and Tdap (15%) vaccines.
“Increasing simultaneous administration of HPV and other adolescent vaccines with the influenza vaccine may help to improve coverage,” Ms. Kelly concluded.
The study was limited by its use of a convenience sample from practices that were interested in participating and willing to stock the HPV vaccine. Additionally, the researchers could not detect or adjust for EHR errors or inaccurate or incomplete vaccine histories, and they were unable to look at vaccine hesitancy or refusal with the EHRs.
The research was funded by the National Institutes of Health, the U.S. Department of Health & Human Services, and the National Research Network to Improve Children’s Health. The authors reported no relevant financial disclosures.
BALTIMORE – according to a study.
“Overall in preventive visits, missed opportunities were much higher for HPV, compared to the other two vaccines” recommended for adolescents, MenACWY (meningococcal conjugate vaccine) and Tdap, Mary Kate Kelly, MPH, of Children’s Hospital of Philadelphia, told attendees at the Pediatric Academic Societies annual meeting. “In order to increase vaccination rates, it’s essential to implement efforts to reduce missed opportunities.”
According to 2018 Centers for Disease Control and Prevention data, Ms. Kelly said, vaccine coverage for the HPV vaccine is approximately 66%, compared with 85% for the MenACWY vaccine and 89% for the Tdap vaccine.
Ms. Kelly and her colleagues investigated how often children or adolescents missed an opportunity to get an HPV vaccine when they received an influenza vaccine during an office visit. This study was part of the larger STOP HPV trial funded by the National Institutes of Health and aimed at implementing evidence-based interventions to reduce missed opportunities for HPV vaccination in primary care.
The researchers retrospectively reviewed EHRs from 2015 to 2018 for 48 pediatric practices across 19 states. All practices were part of the American Academy of Pediatrics’ Pediatric Research in Office Settings (PROS) national pediatric primary care network. The researchers isolated all visits for patients aged 11-17 years who received their flu vaccine and were eligible to receive the HPV vaccine.
The investigators defined a missed opportunity as one in which a patient was due for the HPV vaccine but did not receive one at the visit when they received their flu vaccine.
The study involved 40,129 patients who received the flu vaccine at 52,818 visits when they also were eligible to receive the HPV vaccine. The median age of patients was 12 years old, and 47% were female.
In 68% of visits, the patient could have received an HPV vaccine but did not – even though they were due and eligible for one. The rate was the same for boys and for girls. By contrast, only 38% of visits involved a missed opportunity for the MenACWY vaccines and 39% for the Tdap vaccine.
Rates of missed opportunities for HPV vaccination ranged among individual practices from 22% to 81% of overall visits. Patients were more than twice as likely to miss the opportunity for an HPV vaccine dose if it would have been their first dose – 70% of missed opportunities – versus being a second or third dose, which comprised 30% of missed opportunities (adjusted relative risk, 2.48; P less than .001)).
“However, missed opportunities were also common for subsequent HPV doses when vaccine hesitancy is less likely to be an issue,” Ms. Kelly added.
It also was much more likely that missed opportunities occurred during nurse visits or visits for an acute or chronic condition rather than preventive visits, which made up about half (51%) of all visits analyzed. While 48% of preventive visits involved a missed opportunity, 93% of nurse visits (aRR compared with preventive, 2.18; P less than.001) and 89% of acute or chronic visits (aRR, 2.11; P less than .001) did.
Percentages of missed opportunities were similarly high for the MenACWY and Tdap vaccines at nurse visits and acute/chronic visits, but much lower at preventive visits for the MenACWY (12%) and Tdap (15%) vaccines.
“Increasing simultaneous administration of HPV and other adolescent vaccines with the influenza vaccine may help to improve coverage,” Ms. Kelly concluded.
The study was limited by its use of a convenience sample from practices that were interested in participating and willing to stock the HPV vaccine. Additionally, the researchers could not detect or adjust for EHR errors or inaccurate or incomplete vaccine histories, and they were unable to look at vaccine hesitancy or refusal with the EHRs.
The research was funded by the National Institutes of Health, the U.S. Department of Health & Human Services, and the National Research Network to Improve Children’s Health. The authors reported no relevant financial disclosures.
BALTIMORE – according to a study.
“Overall in preventive visits, missed opportunities were much higher for HPV, compared to the other two vaccines” recommended for adolescents, MenACWY (meningococcal conjugate vaccine) and Tdap, Mary Kate Kelly, MPH, of Children’s Hospital of Philadelphia, told attendees at the Pediatric Academic Societies annual meeting. “In order to increase vaccination rates, it’s essential to implement efforts to reduce missed opportunities.”
According to 2018 Centers for Disease Control and Prevention data, Ms. Kelly said, vaccine coverage for the HPV vaccine is approximately 66%, compared with 85% for the MenACWY vaccine and 89% for the Tdap vaccine.
Ms. Kelly and her colleagues investigated how often children or adolescents missed an opportunity to get an HPV vaccine when they received an influenza vaccine during an office visit. This study was part of the larger STOP HPV trial funded by the National Institutes of Health and aimed at implementing evidence-based interventions to reduce missed opportunities for HPV vaccination in primary care.
The researchers retrospectively reviewed EHRs from 2015 to 2018 for 48 pediatric practices across 19 states. All practices were part of the American Academy of Pediatrics’ Pediatric Research in Office Settings (PROS) national pediatric primary care network. The researchers isolated all visits for patients aged 11-17 years who received their flu vaccine and were eligible to receive the HPV vaccine.
The investigators defined a missed opportunity as one in which a patient was due for the HPV vaccine but did not receive one at the visit when they received their flu vaccine.
The study involved 40,129 patients who received the flu vaccine at 52,818 visits when they also were eligible to receive the HPV vaccine. The median age of patients was 12 years old, and 47% were female.
In 68% of visits, the patient could have received an HPV vaccine but did not – even though they were due and eligible for one. The rate was the same for boys and for girls. By contrast, only 38% of visits involved a missed opportunity for the MenACWY vaccines and 39% for the Tdap vaccine.
Rates of missed opportunities for HPV vaccination ranged among individual practices from 22% to 81% of overall visits. Patients were more than twice as likely to miss the opportunity for an HPV vaccine dose if it would have been their first dose – 70% of missed opportunities – versus being a second or third dose, which comprised 30% of missed opportunities (adjusted relative risk, 2.48; P less than .001)).
“However, missed opportunities were also common for subsequent HPV doses when vaccine hesitancy is less likely to be an issue,” Ms. Kelly added.
It also was much more likely that missed opportunities occurred during nurse visits or visits for an acute or chronic condition rather than preventive visits, which made up about half (51%) of all visits analyzed. While 48% of preventive visits involved a missed opportunity, 93% of nurse visits (aRR compared with preventive, 2.18; P less than.001) and 89% of acute or chronic visits (aRR, 2.11; P less than .001) did.
Percentages of missed opportunities were similarly high for the MenACWY and Tdap vaccines at nurse visits and acute/chronic visits, but much lower at preventive visits for the MenACWY (12%) and Tdap (15%) vaccines.
“Increasing simultaneous administration of HPV and other adolescent vaccines with the influenza vaccine may help to improve coverage,” Ms. Kelly concluded.
The study was limited by its use of a convenience sample from practices that were interested in participating and willing to stock the HPV vaccine. Additionally, the researchers could not detect or adjust for EHR errors or inaccurate or incomplete vaccine histories, and they were unable to look at vaccine hesitancy or refusal with the EHRs.
The research was funded by the National Institutes of Health, the U.S. Department of Health & Human Services, and the National Research Network to Improve Children’s Health. The authors reported no relevant financial disclosures.
REPORTING FROM PAS 2019
U.S. measles cases climb to over 800 for the year
according to the Centers for Disease Control and Prevention.
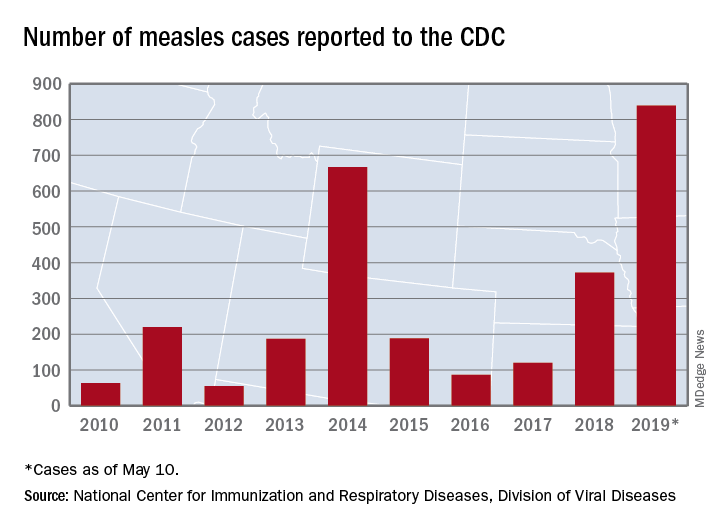
There are 10 states dealing with ongoing outbreaks now that Pennsylvania has been added to the list, the CDC reported May 13. The state has had five cases so far, all in Allegheny County. New York City continued to have the most active outbreak, adding 43 more cases in Brooklyn last week for a total of 410 in the city since the beginning of 2019, NYC Health said.
Several of this year’s outbreaks were predicted in an analysis published in the Lancet Infectious Diseases (2019 May 9. doi: 10.1016/S1473-3099(19)30231-2). Investigators identified the 25 counties most likely to experience a measles outbreak in 2019 – a list that includes Queens, N.Y. (adjacent to Brooklyn), Multnomah, Ore. (adjacent to Clark County, Wash., where 71 people were infected earlier this year), and San Mateo, Calif., where 4 cases have been reported.
“We recommend that public health officials and policymakers prioritize monitoring the counties we identify to be at high risk that have not yet reported cases, especially those that lie adjacent to counties with ongoing outbreaks and those that house large international airports,” senior author Lauren Gardner of Johns Hopkins University, Baltimore, said in a written statement.
The outbreak in Clark County was declared over in late April, but Gov. Jay Inslee signed a bill on May 10 that removes the personal/philosophical exemption for the MMR vaccine from the state’s school and child care immunization requirements. “We must step up our leadership to educate the public about the critical role vaccines have in keeping us healthy and safe, and continue working with communities to improve vaccination rates,” Washington State Secretary of Health John Wiesman said in a written statement.
In Oregon, a bill that would eliminate religious and philosophical exemptions to child vaccination requirements passed the state house of representatives by a 35-25 vote and is moving to the senate. Gov. Kate Brown has said that she plans to sign the bill, according to OregonLive.com.
according to the Centers for Disease Control and Prevention.

There are 10 states dealing with ongoing outbreaks now that Pennsylvania has been added to the list, the CDC reported May 13. The state has had five cases so far, all in Allegheny County. New York City continued to have the most active outbreak, adding 43 more cases in Brooklyn last week for a total of 410 in the city since the beginning of 2019, NYC Health said.
Several of this year’s outbreaks were predicted in an analysis published in the Lancet Infectious Diseases (2019 May 9. doi: 10.1016/S1473-3099(19)30231-2). Investigators identified the 25 counties most likely to experience a measles outbreak in 2019 – a list that includes Queens, N.Y. (adjacent to Brooklyn), Multnomah, Ore. (adjacent to Clark County, Wash., where 71 people were infected earlier this year), and San Mateo, Calif., where 4 cases have been reported.
“We recommend that public health officials and policymakers prioritize monitoring the counties we identify to be at high risk that have not yet reported cases, especially those that lie adjacent to counties with ongoing outbreaks and those that house large international airports,” senior author Lauren Gardner of Johns Hopkins University, Baltimore, said in a written statement.
The outbreak in Clark County was declared over in late April, but Gov. Jay Inslee signed a bill on May 10 that removes the personal/philosophical exemption for the MMR vaccine from the state’s school and child care immunization requirements. “We must step up our leadership to educate the public about the critical role vaccines have in keeping us healthy and safe, and continue working with communities to improve vaccination rates,” Washington State Secretary of Health John Wiesman said in a written statement.
In Oregon, a bill that would eliminate religious and philosophical exemptions to child vaccination requirements passed the state house of representatives by a 35-25 vote and is moving to the senate. Gov. Kate Brown has said that she plans to sign the bill, according to OregonLive.com.
according to the Centers for Disease Control and Prevention.

There are 10 states dealing with ongoing outbreaks now that Pennsylvania has been added to the list, the CDC reported May 13. The state has had five cases so far, all in Allegheny County. New York City continued to have the most active outbreak, adding 43 more cases in Brooklyn last week for a total of 410 in the city since the beginning of 2019, NYC Health said.
Several of this year’s outbreaks were predicted in an analysis published in the Lancet Infectious Diseases (2019 May 9. doi: 10.1016/S1473-3099(19)30231-2). Investigators identified the 25 counties most likely to experience a measles outbreak in 2019 – a list that includes Queens, N.Y. (adjacent to Brooklyn), Multnomah, Ore. (adjacent to Clark County, Wash., where 71 people were infected earlier this year), and San Mateo, Calif., where 4 cases have been reported.
“We recommend that public health officials and policymakers prioritize monitoring the counties we identify to be at high risk that have not yet reported cases, especially those that lie adjacent to counties with ongoing outbreaks and those that house large international airports,” senior author Lauren Gardner of Johns Hopkins University, Baltimore, said in a written statement.
The outbreak in Clark County was declared over in late April, but Gov. Jay Inslee signed a bill on May 10 that removes the personal/philosophical exemption for the MMR vaccine from the state’s school and child care immunization requirements. “We must step up our leadership to educate the public about the critical role vaccines have in keeping us healthy and safe, and continue working with communities to improve vaccination rates,” Washington State Secretary of Health John Wiesman said in a written statement.
In Oregon, a bill that would eliminate religious and philosophical exemptions to child vaccination requirements passed the state house of representatives by a 35-25 vote and is moving to the senate. Gov. Kate Brown has said that she plans to sign the bill, according to OregonLive.com.
N.Y. hospitals report near-universal CMV screening when newborns fail hearing tests
BALTIMORE – Over the past 2 years, Northwell Health, a large medical system in the metropolitan New York area, increased cytomegalovirus screening for infants who fail hearing tests from 6.6% to 95% at five of its birth hospitals, according to a presentation at the Pediatric Academic Societies annual meeting.
Three cases of congenital cytomegalovirus (CMV) have been picked up so far. The plan is to roll the program out to all 10 of the system’s birth hospitals, where over 40,000 children are born each year.
“We feel very satisfied and proud” of the progress that’s been made at Northwell in such a short time, said Alia Chauhan, MD, a Northwell pediatrician who presented the findings.
Northwell launched its “Hearing Plus” program in 2017 to catch the infection before infants leave the hospital. Several other health systems around the country have launched similar programs, and a handful of states – including New York – now require CMV screening for infants who fail mandated hearing tests.
The issue is gaining traction because hearing loss is often the only sign of congenital CMV, so it’s a bellwether for infection. Screening children with hearing loss is an easy way to pick it up early, so steps can be taken to prevent problems down the road. As it is, congenital CMV is the leading nongenetic cause of hearing loss in infants, accounting for at least 10% of cases.
The Northwell program kicked off with an education campaign to build consensus among pediatricians, hospitalists, and nurses. A flyer was made about CMV screening for moms whose infants fail hearing tests, printed in both English and Spanish.
Initially, the program used urine PCR [polymerase chain reaction] to screen for CMV, but waiting for infants to produce a sample often delayed discharge, so a switch was soon made to saliva swab PCRs, which take seconds, with urine PCR held in reserve to confirm positive swabs.
To streamline the process, a standing order was added to the electronic records system so nurses could order saliva PCRs without having to get physician approval. “I think [that] was one of the biggest things that’s helped us,” Dr. Chauhan said.
Children who test positive must have urine confirmation within 21 days of birth; most are long gone from the hospital by then and have to be called back in. “We haven’t lost anyone to follow-up, but it can be stressful trying to get someone to come back,” she said.
Six of 449 infants have screened positive on saliva – three were false positives with negative urine screens. Of the three confirmed cases, two infants later turned out to have normal hearing on repeat testing and were otherwise asymptomatic.
These days, Dr. Chauhan said, if children have a positive saliva PCR but later turn out to have normal hearing, and are otherwise free of symptoms with no CMV risk factors, “we are not confirming with urine.”
Dr. Chauhan did not have any disclosures. No funding source was mentioned.
SOURCE: Chauhan A et al. PAS 2019. Abstract 306
BALTIMORE – Over the past 2 years, Northwell Health, a large medical system in the metropolitan New York area, increased cytomegalovirus screening for infants who fail hearing tests from 6.6% to 95% at five of its birth hospitals, according to a presentation at the Pediatric Academic Societies annual meeting.
Three cases of congenital cytomegalovirus (CMV) have been picked up so far. The plan is to roll the program out to all 10 of the system’s birth hospitals, where over 40,000 children are born each year.
“We feel very satisfied and proud” of the progress that’s been made at Northwell in such a short time, said Alia Chauhan, MD, a Northwell pediatrician who presented the findings.
Northwell launched its “Hearing Plus” program in 2017 to catch the infection before infants leave the hospital. Several other health systems around the country have launched similar programs, and a handful of states – including New York – now require CMV screening for infants who fail mandated hearing tests.
The issue is gaining traction because hearing loss is often the only sign of congenital CMV, so it’s a bellwether for infection. Screening children with hearing loss is an easy way to pick it up early, so steps can be taken to prevent problems down the road. As it is, congenital CMV is the leading nongenetic cause of hearing loss in infants, accounting for at least 10% of cases.
The Northwell program kicked off with an education campaign to build consensus among pediatricians, hospitalists, and nurses. A flyer was made about CMV screening for moms whose infants fail hearing tests, printed in both English and Spanish.
Initially, the program used urine PCR [polymerase chain reaction] to screen for CMV, but waiting for infants to produce a sample often delayed discharge, so a switch was soon made to saliva swab PCRs, which take seconds, with urine PCR held in reserve to confirm positive swabs.
To streamline the process, a standing order was added to the electronic records system so nurses could order saliva PCRs without having to get physician approval. “I think [that] was one of the biggest things that’s helped us,” Dr. Chauhan said.
Children who test positive must have urine confirmation within 21 days of birth; most are long gone from the hospital by then and have to be called back in. “We haven’t lost anyone to follow-up, but it can be stressful trying to get someone to come back,” she said.
Six of 449 infants have screened positive on saliva – three were false positives with negative urine screens. Of the three confirmed cases, two infants later turned out to have normal hearing on repeat testing and were otherwise asymptomatic.
These days, Dr. Chauhan said, if children have a positive saliva PCR but later turn out to have normal hearing, and are otherwise free of symptoms with no CMV risk factors, “we are not confirming with urine.”
Dr. Chauhan did not have any disclosures. No funding source was mentioned.
SOURCE: Chauhan A et al. PAS 2019. Abstract 306
BALTIMORE – Over the past 2 years, Northwell Health, a large medical system in the metropolitan New York area, increased cytomegalovirus screening for infants who fail hearing tests from 6.6% to 95% at five of its birth hospitals, according to a presentation at the Pediatric Academic Societies annual meeting.
Three cases of congenital cytomegalovirus (CMV) have been picked up so far. The plan is to roll the program out to all 10 of the system’s birth hospitals, where over 40,000 children are born each year.
“We feel very satisfied and proud” of the progress that’s been made at Northwell in such a short time, said Alia Chauhan, MD, a Northwell pediatrician who presented the findings.
Northwell launched its “Hearing Plus” program in 2017 to catch the infection before infants leave the hospital. Several other health systems around the country have launched similar programs, and a handful of states – including New York – now require CMV screening for infants who fail mandated hearing tests.
The issue is gaining traction because hearing loss is often the only sign of congenital CMV, so it’s a bellwether for infection. Screening children with hearing loss is an easy way to pick it up early, so steps can be taken to prevent problems down the road. As it is, congenital CMV is the leading nongenetic cause of hearing loss in infants, accounting for at least 10% of cases.
The Northwell program kicked off with an education campaign to build consensus among pediatricians, hospitalists, and nurses. A flyer was made about CMV screening for moms whose infants fail hearing tests, printed in both English and Spanish.
Initially, the program used urine PCR [polymerase chain reaction] to screen for CMV, but waiting for infants to produce a sample often delayed discharge, so a switch was soon made to saliva swab PCRs, which take seconds, with urine PCR held in reserve to confirm positive swabs.
To streamline the process, a standing order was added to the electronic records system so nurses could order saliva PCRs without having to get physician approval. “I think [that] was one of the biggest things that’s helped us,” Dr. Chauhan said.
Children who test positive must have urine confirmation within 21 days of birth; most are long gone from the hospital by then and have to be called back in. “We haven’t lost anyone to follow-up, but it can be stressful trying to get someone to come back,” she said.
Six of 449 infants have screened positive on saliva – three were false positives with negative urine screens. Of the three confirmed cases, two infants later turned out to have normal hearing on repeat testing and were otherwise asymptomatic.
These days, Dr. Chauhan said, if children have a positive saliva PCR but later turn out to have normal hearing, and are otherwise free of symptoms with no CMV risk factors, “we are not confirming with urine.”
Dr. Chauhan did not have any disclosures. No funding source was mentioned.
SOURCE: Chauhan A et al. PAS 2019. Abstract 306
REPORTING FROM PAS 2019
Key clinical point: A metropolitan N.Y. health system provides a model for how to implement cytomegalovirus screening for infants who fail hearing tests.
Major finding: .
Study details: Pre-post quality improvement project.
Disclosures: The lead investigator had no disclosures. No funding source was mentioned.
Source: Chauhan A et al. PAS 2019. Abstract 306.
HM19: Pediatric clinical conundrums
Atypical symptoms and diagnoses
Presenters
Yemisi Jones, MD; Mirna Giordano, MD
Session title
Pediatric Clinical Conundrums
Session summary
Dr. Mirna Giordano of Columbia University Irving Medical Center, New York, and Dr. Yemisi Jones of Cincinnati Children’s Hospital Medical Center, moderated the Pediatric Clinical Conundrums session at HM19. After reviewing multiple submissions, they invited four trainees to present their interesting cases.
Malignancy or infection? Dr. Jeremy Brown, a resident at the University of Louisville, presented a case of a 15-year-old male with right upper quadrant abdominal pain with associated weight loss and intermittent fevers, over the course of several weeks. CT revealed multiple liver lesions, providing concern for possible malignancy, although liver biopsy proved otherwise, with mostly liquefactive tissue and benign liver parenchyma. After a large infectious work-up ensued, the patient was diagnosed with disseminated Bartonella. He was treated with a 10-day course of azithromycin, and his symptoms resolved.
Leg blisters as an uncommon manifestation of a common childhood disease. Dr. Stefan Mammele, a resident at Kapi’olani Medical Center in Honolulu, and the University of Hawaii, presented a case of an 11-year-old boy with a painful and pruritic rash associated with multiple 5- to 10-mm tense bullae located on the patient’s bilateral lower extremities with extension to the trunk. The patient was also found to have hematuria and proteinuria. The bullae drained both serosanguinous and purulent material. Fluid culture grew group A Streptococcus and skin biopsy confirmed IgA vasculitis. Bullae are a rare characteristic of Henoch Schönlein pupura in children, but are more commonly seen as a disease manifestation in adults. The patient was treated with cefazolin, and his lesions improved over the course of several weeks with resolution of his hematuria by 6 months.
Is she crying blood? Dr. Joshua Price, a resident at Baystate Children’s Hospital in Springfield, Mass., described a 12-year-old female who presented with 7 days of left-sided hemolacria with acute vision loss and unilateral eye pain. This patient did not respond to outpatient topical steroids and antibiotics, as prescribed by ophthalmology. For this reason, she underwent further work-up and imaging. MRI of the head and orbits revealed left maxillary sinus disease. She was treated with antibiotics for acute left maxillary sinusitis and her hemolacria resolved within 24 hours. While the differential diagnosis for hemolacria is broad, rarely acute sinusitis has been reported as a cause in medical literature.
Recurrent bronchiolitis or something more? Dr. Moira Black, a resident at Children’s Memorial Hermann in Houston, presented a case of a 7-month-old female with a history of recurrent admissions for increased work of breathing believed to be secondary to viral bronchiolitis. Her first hospitalization occurred at 7 weeks of age and was complicated by spontaneous pneumothorax requiring chest tube placement. She was again hospitalized at 5 months of age with resolution of her increased work of breathing with high-flow nasal cannula. She presented again at 7 months of age with presumed bronchiolitis, however, she decompensated and required intubation on the 5th day of hospitalization. A bronchoscopy was performed and revealed a significantly narrowed left bronchus at the carina and a blind pouch on the right with notable pulsation of the walls. She underwent further imaging and was ultimately diagnosed with a left pulmonary artery sling. Left pulmonary artery slings are a rare, but potentially fatal anomaly that can present with wheezing, stridor, and recurrent respiratory infections. Patient underwent correction by cardiovascular surgery and has since been doing well.
Key takeaways for HM
• Bartonella is a common cause of fever of unknown origin, and should be considered in unusual presentations of febrile illnesses.
• Bullae in IgA vasculitis are rare in children and do not have prognostic value, but streptococcal infection may be a trigger for IgA vasculitis.
• Hemolacria is an atypical presentation of rare and common diagnoses that should prompt further work-up.
• Acute respiratory distress can be caused by underlying cardiac or vascular anomalies and can be mistaken for common viral illnesses.
Dr. Marsicek is a pediatric hospital medicine fellow at Johns Hopkins All Children’s Hospital, St. Petersburg, Fla. Dr. Wysocka is a pediatric resident at Johns Hopkins All Children’s Hospital.
Atypical symptoms and diagnoses
Atypical symptoms and diagnoses
Presenters
Yemisi Jones, MD; Mirna Giordano, MD
Session title
Pediatric Clinical Conundrums
Session summary
Dr. Mirna Giordano of Columbia University Irving Medical Center, New York, and Dr. Yemisi Jones of Cincinnati Children’s Hospital Medical Center, moderated the Pediatric Clinical Conundrums session at HM19. After reviewing multiple submissions, they invited four trainees to present their interesting cases.
Malignancy or infection? Dr. Jeremy Brown, a resident at the University of Louisville, presented a case of a 15-year-old male with right upper quadrant abdominal pain with associated weight loss and intermittent fevers, over the course of several weeks. CT revealed multiple liver lesions, providing concern for possible malignancy, although liver biopsy proved otherwise, with mostly liquefactive tissue and benign liver parenchyma. After a large infectious work-up ensued, the patient was diagnosed with disseminated Bartonella. He was treated with a 10-day course of azithromycin, and his symptoms resolved.
Leg blisters as an uncommon manifestation of a common childhood disease. Dr. Stefan Mammele, a resident at Kapi’olani Medical Center in Honolulu, and the University of Hawaii, presented a case of an 11-year-old boy with a painful and pruritic rash associated with multiple 5- to 10-mm tense bullae located on the patient’s bilateral lower extremities with extension to the trunk. The patient was also found to have hematuria and proteinuria. The bullae drained both serosanguinous and purulent material. Fluid culture grew group A Streptococcus and skin biopsy confirmed IgA vasculitis. Bullae are a rare characteristic of Henoch Schönlein pupura in children, but are more commonly seen as a disease manifestation in adults. The patient was treated with cefazolin, and his lesions improved over the course of several weeks with resolution of his hematuria by 6 months.
Is she crying blood? Dr. Joshua Price, a resident at Baystate Children’s Hospital in Springfield, Mass., described a 12-year-old female who presented with 7 days of left-sided hemolacria with acute vision loss and unilateral eye pain. This patient did not respond to outpatient topical steroids and antibiotics, as prescribed by ophthalmology. For this reason, she underwent further work-up and imaging. MRI of the head and orbits revealed left maxillary sinus disease. She was treated with antibiotics for acute left maxillary sinusitis and her hemolacria resolved within 24 hours. While the differential diagnosis for hemolacria is broad, rarely acute sinusitis has been reported as a cause in medical literature.
Recurrent bronchiolitis or something more? Dr. Moira Black, a resident at Children’s Memorial Hermann in Houston, presented a case of a 7-month-old female with a history of recurrent admissions for increased work of breathing believed to be secondary to viral bronchiolitis. Her first hospitalization occurred at 7 weeks of age and was complicated by spontaneous pneumothorax requiring chest tube placement. She was again hospitalized at 5 months of age with resolution of her increased work of breathing with high-flow nasal cannula. She presented again at 7 months of age with presumed bronchiolitis, however, she decompensated and required intubation on the 5th day of hospitalization. A bronchoscopy was performed and revealed a significantly narrowed left bronchus at the carina and a blind pouch on the right with notable pulsation of the walls. She underwent further imaging and was ultimately diagnosed with a left pulmonary artery sling. Left pulmonary artery slings are a rare, but potentially fatal anomaly that can present with wheezing, stridor, and recurrent respiratory infections. Patient underwent correction by cardiovascular surgery and has since been doing well.
Key takeaways for HM
• Bartonella is a common cause of fever of unknown origin, and should be considered in unusual presentations of febrile illnesses.
• Bullae in IgA vasculitis are rare in children and do not have prognostic value, but streptococcal infection may be a trigger for IgA vasculitis.
• Hemolacria is an atypical presentation of rare and common diagnoses that should prompt further work-up.
• Acute respiratory distress can be caused by underlying cardiac or vascular anomalies and can be mistaken for common viral illnesses.
Dr. Marsicek is a pediatric hospital medicine fellow at Johns Hopkins All Children’s Hospital, St. Petersburg, Fla. Dr. Wysocka is a pediatric resident at Johns Hopkins All Children’s Hospital.
Presenters
Yemisi Jones, MD; Mirna Giordano, MD
Session title
Pediatric Clinical Conundrums
Session summary
Dr. Mirna Giordano of Columbia University Irving Medical Center, New York, and Dr. Yemisi Jones of Cincinnati Children’s Hospital Medical Center, moderated the Pediatric Clinical Conundrums session at HM19. After reviewing multiple submissions, they invited four trainees to present their interesting cases.
Malignancy or infection? Dr. Jeremy Brown, a resident at the University of Louisville, presented a case of a 15-year-old male with right upper quadrant abdominal pain with associated weight loss and intermittent fevers, over the course of several weeks. CT revealed multiple liver lesions, providing concern for possible malignancy, although liver biopsy proved otherwise, with mostly liquefactive tissue and benign liver parenchyma. After a large infectious work-up ensued, the patient was diagnosed with disseminated Bartonella. He was treated with a 10-day course of azithromycin, and his symptoms resolved.
Leg blisters as an uncommon manifestation of a common childhood disease. Dr. Stefan Mammele, a resident at Kapi’olani Medical Center in Honolulu, and the University of Hawaii, presented a case of an 11-year-old boy with a painful and pruritic rash associated with multiple 5- to 10-mm tense bullae located on the patient’s bilateral lower extremities with extension to the trunk. The patient was also found to have hematuria and proteinuria. The bullae drained both serosanguinous and purulent material. Fluid culture grew group A Streptococcus and skin biopsy confirmed IgA vasculitis. Bullae are a rare characteristic of Henoch Schönlein pupura in children, but are more commonly seen as a disease manifestation in adults. The patient was treated with cefazolin, and his lesions improved over the course of several weeks with resolution of his hematuria by 6 months.
Is she crying blood? Dr. Joshua Price, a resident at Baystate Children’s Hospital in Springfield, Mass., described a 12-year-old female who presented with 7 days of left-sided hemolacria with acute vision loss and unilateral eye pain. This patient did not respond to outpatient topical steroids and antibiotics, as prescribed by ophthalmology. For this reason, she underwent further work-up and imaging. MRI of the head and orbits revealed left maxillary sinus disease. She was treated with antibiotics for acute left maxillary sinusitis and her hemolacria resolved within 24 hours. While the differential diagnosis for hemolacria is broad, rarely acute sinusitis has been reported as a cause in medical literature.
Recurrent bronchiolitis or something more? Dr. Moira Black, a resident at Children’s Memorial Hermann in Houston, presented a case of a 7-month-old female with a history of recurrent admissions for increased work of breathing believed to be secondary to viral bronchiolitis. Her first hospitalization occurred at 7 weeks of age and was complicated by spontaneous pneumothorax requiring chest tube placement. She was again hospitalized at 5 months of age with resolution of her increased work of breathing with high-flow nasal cannula. She presented again at 7 months of age with presumed bronchiolitis, however, she decompensated and required intubation on the 5th day of hospitalization. A bronchoscopy was performed and revealed a significantly narrowed left bronchus at the carina and a blind pouch on the right with notable pulsation of the walls. She underwent further imaging and was ultimately diagnosed with a left pulmonary artery sling. Left pulmonary artery slings are a rare, but potentially fatal anomaly that can present with wheezing, stridor, and recurrent respiratory infections. Patient underwent correction by cardiovascular surgery and has since been doing well.
Key takeaways for HM
• Bartonella is a common cause of fever of unknown origin, and should be considered in unusual presentations of febrile illnesses.
• Bullae in IgA vasculitis are rare in children and do not have prognostic value, but streptococcal infection may be a trigger for IgA vasculitis.
• Hemolacria is an atypical presentation of rare and common diagnoses that should prompt further work-up.
• Acute respiratory distress can be caused by underlying cardiac or vascular anomalies and can be mistaken for common viral illnesses.
Dr. Marsicek is a pediatric hospital medicine fellow at Johns Hopkins All Children’s Hospital, St. Petersburg, Fla. Dr. Wysocka is a pediatric resident at Johns Hopkins All Children’s Hospital.
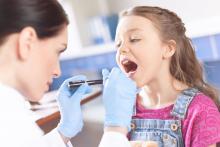
No exudates or fever? Age over 11? Skip strep test
BALTIMORE – In children with pharyngitis, it’s safe to skip group A Streptococcus testing if there are no exudates, children are 11 years or older, and there is either no cervical adenopathy or adenopathy without fever, according to a Boston Children’s Hospital investigation.
The prevalence of group A Streptococcus among children who meet those criteria is 13%, less than the estimated asymptomatic carriage rate of about 15%. Among 67,127 children tested for strep and treated for sore throats in a network of retail health clinics across the United States, 35% fit the profile.
Investigators led by Daniel Shapiro, MD, a pediatrics fellow at Boston Children’s, concluded that “laboratory testing for GAS [group A Streptococcus] might be safely avoided in a large proportion of patients with sore throats. In doing so, we may avoid some of the downstream effects of unnecessary antibiotic use.” Incorporating the rules into EHRs “might help physicians identify patients who are at low risk of GAS pharyngitis.”
The study team tackled a long-standing and vexing problem in general pediatrics: how to distinguish viral from GAS pharyngitis. They often present the same way, so it’s difficult to tell them apart, but important to do so to prevent misuse of antibiotics. Health care providers generally rely on rapid strep tests and other assays to make the call, but they have to be used cautiously, because asymptomatic carriers also will test positive and be at risk for unnecessary treatment, Dr. Shapiro said at the Pediatric Academic Societies annual meeting.
To try to prevent that, the Infectious Disease Society of America (IDSA) recommends against strep testing in children who present with overt viral signs, including cough, rhinorrhea, oral ulcers, and hoarseness (Clin Infect Dis. 2012 Nov 15;55[10]:1279-82).
In a previous study at Boston Children’s ED, however, Dr. Shapiro and his colleagues found that 29% of children with overt viral features were positive for GAS, suggesting that the IDSA guidelines probably go too far (Pediatrics. 2017 May;139[5]. pii: e20163403).
“One might conclude that while it’s a good rule of thumb to avoid testing patients with viral features, some of the patients with viral features really do have GAS pharyngitis, so the recommendation to forgo testing in all these kids needs a little bit of refinement,” he said.
That was the goal of the new study; the team sought to identify viral features that signaled a low risk of GAS pharyngitis and, therefore, no need for testing. Low risk was defined as less than 15%, in keeping with the asymptomatic carriage rate.
The 67,127 patients were aged 3-21 years. Their signs and symptoms were collected at the retail clinics in a standardized form. The subjects had rapid strep tests, with negative results confirmed by DNA probe or culture.
Fifty-four percent had viral features, defined in the study as cough, runny nose, or hoarseness (oral ulcers weren’t collected on the form). The overall prevalence of GAS was 35%, similar to previous studies; 39% of children with no viral features tested positive for GAS versus 26% of children with all three. Exudates and age below 11 years were strongly associated with GAS among patients with viral features.
It turned out that just 23% of children without exudates were GAS positive; the number fell to 15% when limited to children 11 years or older, and to 13% when either no cervical adenopathy or adenopathy without fever were added to the mix.
There was no industry funding, and Dr. Shapiro didn’t have any disclosures.
BALTIMORE – In children with pharyngitis, it’s safe to skip group A Streptococcus testing if there are no exudates, children are 11 years or older, and there is either no cervical adenopathy or adenopathy without fever, according to a Boston Children’s Hospital investigation.
The prevalence of group A Streptococcus among children who meet those criteria is 13%, less than the estimated asymptomatic carriage rate of about 15%. Among 67,127 children tested for strep and treated for sore throats in a network of retail health clinics across the United States, 35% fit the profile.
Investigators led by Daniel Shapiro, MD, a pediatrics fellow at Boston Children’s, concluded that “laboratory testing for GAS [group A Streptococcus] might be safely avoided in a large proportion of patients with sore throats. In doing so, we may avoid some of the downstream effects of unnecessary antibiotic use.” Incorporating the rules into EHRs “might help physicians identify patients who are at low risk of GAS pharyngitis.”
The study team tackled a long-standing and vexing problem in general pediatrics: how to distinguish viral from GAS pharyngitis. They often present the same way, so it’s difficult to tell them apart, but important to do so to prevent misuse of antibiotics. Health care providers generally rely on rapid strep tests and other assays to make the call, but they have to be used cautiously, because asymptomatic carriers also will test positive and be at risk for unnecessary treatment, Dr. Shapiro said at the Pediatric Academic Societies annual meeting.
To try to prevent that, the Infectious Disease Society of America (IDSA) recommends against strep testing in children who present with overt viral signs, including cough, rhinorrhea, oral ulcers, and hoarseness (Clin Infect Dis. 2012 Nov 15;55[10]:1279-82).
In a previous study at Boston Children’s ED, however, Dr. Shapiro and his colleagues found that 29% of children with overt viral features were positive for GAS, suggesting that the IDSA guidelines probably go too far (Pediatrics. 2017 May;139[5]. pii: e20163403).
“One might conclude that while it’s a good rule of thumb to avoid testing patients with viral features, some of the patients with viral features really do have GAS pharyngitis, so the recommendation to forgo testing in all these kids needs a little bit of refinement,” he said.
That was the goal of the new study; the team sought to identify viral features that signaled a low risk of GAS pharyngitis and, therefore, no need for testing. Low risk was defined as less than 15%, in keeping with the asymptomatic carriage rate.
The 67,127 patients were aged 3-21 years. Their signs and symptoms were collected at the retail clinics in a standardized form. The subjects had rapid strep tests, with negative results confirmed by DNA probe or culture.
Fifty-four percent had viral features, defined in the study as cough, runny nose, or hoarseness (oral ulcers weren’t collected on the form). The overall prevalence of GAS was 35%, similar to previous studies; 39% of children with no viral features tested positive for GAS versus 26% of children with all three. Exudates and age below 11 years were strongly associated with GAS among patients with viral features.
It turned out that just 23% of children without exudates were GAS positive; the number fell to 15% when limited to children 11 years or older, and to 13% when either no cervical adenopathy or adenopathy without fever were added to the mix.
There was no industry funding, and Dr. Shapiro didn’t have any disclosures.
BALTIMORE – In children with pharyngitis, it’s safe to skip group A Streptococcus testing if there are no exudates, children are 11 years or older, and there is either no cervical adenopathy or adenopathy without fever, according to a Boston Children’s Hospital investigation.
The prevalence of group A Streptococcus among children who meet those criteria is 13%, less than the estimated asymptomatic carriage rate of about 15%. Among 67,127 children tested for strep and treated for sore throats in a network of retail health clinics across the United States, 35% fit the profile.
Investigators led by Daniel Shapiro, MD, a pediatrics fellow at Boston Children’s, concluded that “laboratory testing for GAS [group A Streptococcus] might be safely avoided in a large proportion of patients with sore throats. In doing so, we may avoid some of the downstream effects of unnecessary antibiotic use.” Incorporating the rules into EHRs “might help physicians identify patients who are at low risk of GAS pharyngitis.”
The study team tackled a long-standing and vexing problem in general pediatrics: how to distinguish viral from GAS pharyngitis. They often present the same way, so it’s difficult to tell them apart, but important to do so to prevent misuse of antibiotics. Health care providers generally rely on rapid strep tests and other assays to make the call, but they have to be used cautiously, because asymptomatic carriers also will test positive and be at risk for unnecessary treatment, Dr. Shapiro said at the Pediatric Academic Societies annual meeting.
To try to prevent that, the Infectious Disease Society of America (IDSA) recommends against strep testing in children who present with overt viral signs, including cough, rhinorrhea, oral ulcers, and hoarseness (Clin Infect Dis. 2012 Nov 15;55[10]:1279-82).
In a previous study at Boston Children’s ED, however, Dr. Shapiro and his colleagues found that 29% of children with overt viral features were positive for GAS, suggesting that the IDSA guidelines probably go too far (Pediatrics. 2017 May;139[5]. pii: e20163403).
“One might conclude that while it’s a good rule of thumb to avoid testing patients with viral features, some of the patients with viral features really do have GAS pharyngitis, so the recommendation to forgo testing in all these kids needs a little bit of refinement,” he said.
That was the goal of the new study; the team sought to identify viral features that signaled a low risk of GAS pharyngitis and, therefore, no need for testing. Low risk was defined as less than 15%, in keeping with the asymptomatic carriage rate.
The 67,127 patients were aged 3-21 years. Their signs and symptoms were collected at the retail clinics in a standardized form. The subjects had rapid strep tests, with negative results confirmed by DNA probe or culture.
Fifty-four percent had viral features, defined in the study as cough, runny nose, or hoarseness (oral ulcers weren’t collected on the form). The overall prevalence of GAS was 35%, similar to previous studies; 39% of children with no viral features tested positive for GAS versus 26% of children with all three. Exudates and age below 11 years were strongly associated with GAS among patients with viral features.
It turned out that just 23% of children without exudates were GAS positive; the number fell to 15% when limited to children 11 years or older, and to 13% when either no cervical adenopathy or adenopathy without fever were added to the mix.
There was no industry funding, and Dr. Shapiro didn’t have any disclosures.
REPORTING FROM PAS 2019
Apremilast and Phototherapy for Treatment of Psoriasis in a Patient With Human Immunodeficiency Virus
To the Editor:
A 50-year old man with Fitzpatrick skin type IV, human immunodeficiency virus (HIV), fatty liver disease, and moderate psoriasis (10% body surface area [BSA] affected) currently treated with clobetasol spray and calcitriol ointment presented with persistent psoriatic lesions on the trunk, arms, legs, and buttocks. His CD4 count was 460 and his HIV RNA count was 48 copies/mL on polymerase chain reaction 2 months prior to the current presentation. He had been undergoing phototherapy 3 times weekly for the last 5 months for treatment of psoriasis.
At the current presentation, he was started on an apremilast starter pack with the dosage titrated from 10 mg to 30 mg over the course of 1 week. He was maintained on a dose of 30 mg twice daily after 1 week and continued clobetasol spray, calcitriol ointment, and phototherapy 3 times weekly with the intent to reduce the frequency after adequate control of psoriasis was achieved. After 3 months of treatment, the affected BSA was 0%. He continued apremilast, and phototherapy was reduced to once weekly. Phototherapy was discontinued after 7 months of concomitant treatment with apremilast after clearance was maintained. It was reinitiated twice weekly after a mild flare (3% BSA affected). After 20 total months of treatment, the patient was no longer able to afford apremilast treatment and presented with a severe psoriasis flare (40% BSA affected). He was switched to acitretin with a plan to apply for apremilast financial assistance programs.
Psoriasis treatment in the HIV population poses a challenge given the immunosuppressed state of these patients, the risk of reactivation of latent infections, and the refractory nature of psoriasis in the setting of HIV. Two of the authors (S.P.R. and J.J.W.) previously reported a case of moderate to severe psoriasis in a patient with HIV and hepatitis C who demonstrated treatment success with apremilast until it was discontinued due to financial implications.1 Currently, apremilast is not widely used to treat psoriasis in the HIV population. The National Psoriasis Foundation 2010 guidelines recommended UV light therapy for treatment of moderate to severe psoriasis in HIV-positive patients, with oral retinoids as the second-line treatment.2 There remains a need for updated guidelines on the use of systemic agents for psoriasis treatment in the HIV population.
Apremilast, a phosphodiesterase 4 inhibitor, is an oral therapy that restores the balance of proinflammatory and anti-inflammatory cytokines by inhibiting inflammatory cytokine (eg, tumor necrosis factor α, IFN-γ, IL-2, IL-12, IL-23) secretion and stimulating anti-inflammatory cytokine (eg, IL-6, IL-10) production. In 2015, the phase 3 ESTEEM 13 and ESTEEM 24 trials demonstrated the efficacy of apremilast 30 mg twice daily for treatment of psoriasis. In both trials, the psoriasis area and severity index 75 response rate at week 16 was significantly
Use of other systemic agents such as tumor necrosis factor α inhibitors and ustekinumab has been reported in HIV-positive patients.5-7 There is no current data on IL-17 and IL-23 inhibitors. Acitretin generally is recommended as a second-line agent in HIV patients given its lack of immunosuppression2; however, methotrexate and cyclosporine should be avoided given the risk of opportunistic infections.8
Apremilast is a promising therapy with a favorable safety profile that should be considered as an adjuvant treatment to first-line agents such as phototherapy in HIV-positive patients. Apremilast has been successfully used in an HIV patient with a concomitant chronic hepatitis C infection.1 Systemic medications such as apremilast should be managed in coordination with infectious disease specialists with close monitoring of CD4 levels and viral loads as well as prophylactic agents.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:e481-e482.
- Menon K, Van Voorhees AS, Bebo BF Jr, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation [published online July 31, 2009]. J Am Acad Dermatol. 2010;62:291-299.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Lindsey SF, Weiss J, Lee ES, et al. Treatment of severe psoriasis and psoriatic arthritis with adalimumab in an HIV-positive patient. J Drugs Dermatol. 2014;13:869-871.
- Saeki H, Ito T, Hayashi M, et al. Successful treatment of ustekinumab in a severe psoriasis patient with human immunodeficiency virus infection. J Eur Acad Dermatol Venereol. 2015;29:1653-1655.
- Paparizos V, Rallis E, Kirsten L, et al. Ustekinumab for the treatment of HIV psoriasis. J Dermatolog Treat. 2012;23:398-399.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections [published online July 11, 2018]. J Am Acad Dermatol. 2019;80:43-53.
To the Editor:
A 50-year old man with Fitzpatrick skin type IV, human immunodeficiency virus (HIV), fatty liver disease, and moderate psoriasis (10% body surface area [BSA] affected) currently treated with clobetasol spray and calcitriol ointment presented with persistent psoriatic lesions on the trunk, arms, legs, and buttocks. His CD4 count was 460 and his HIV RNA count was 48 copies/mL on polymerase chain reaction 2 months prior to the current presentation. He had been undergoing phototherapy 3 times weekly for the last 5 months for treatment of psoriasis.
At the current presentation, he was started on an apremilast starter pack with the dosage titrated from 10 mg to 30 mg over the course of 1 week. He was maintained on a dose of 30 mg twice daily after 1 week and continued clobetasol spray, calcitriol ointment, and phototherapy 3 times weekly with the intent to reduce the frequency after adequate control of psoriasis was achieved. After 3 months of treatment, the affected BSA was 0%. He continued apremilast, and phototherapy was reduced to once weekly. Phototherapy was discontinued after 7 months of concomitant treatment with apremilast after clearance was maintained. It was reinitiated twice weekly after a mild flare (3% BSA affected). After 20 total months of treatment, the patient was no longer able to afford apremilast treatment and presented with a severe psoriasis flare (40% BSA affected). He was switched to acitretin with a plan to apply for apremilast financial assistance programs.
Psoriasis treatment in the HIV population poses a challenge given the immunosuppressed state of these patients, the risk of reactivation of latent infections, and the refractory nature of psoriasis in the setting of HIV. Two of the authors (S.P.R. and J.J.W.) previously reported a case of moderate to severe psoriasis in a patient with HIV and hepatitis C who demonstrated treatment success with apremilast until it was discontinued due to financial implications.1 Currently, apremilast is not widely used to treat psoriasis in the HIV population. The National Psoriasis Foundation 2010 guidelines recommended UV light therapy for treatment of moderate to severe psoriasis in HIV-positive patients, with oral retinoids as the second-line treatment.2 There remains a need for updated guidelines on the use of systemic agents for psoriasis treatment in the HIV population.
Apremilast, a phosphodiesterase 4 inhibitor, is an oral therapy that restores the balance of proinflammatory and anti-inflammatory cytokines by inhibiting inflammatory cytokine (eg, tumor necrosis factor α, IFN-γ, IL-2, IL-12, IL-23) secretion and stimulating anti-inflammatory cytokine (eg, IL-6, IL-10) production. In 2015, the phase 3 ESTEEM 13 and ESTEEM 24 trials demonstrated the efficacy of apremilast 30 mg twice daily for treatment of psoriasis. In both trials, the psoriasis area and severity index 75 response rate at week 16 was significantly
Use of other systemic agents such as tumor necrosis factor α inhibitors and ustekinumab has been reported in HIV-positive patients.5-7 There is no current data on IL-17 and IL-23 inhibitors. Acitretin generally is recommended as a second-line agent in HIV patients given its lack of immunosuppression2; however, methotrexate and cyclosporine should be avoided given the risk of opportunistic infections.8
Apremilast is a promising therapy with a favorable safety profile that should be considered as an adjuvant treatment to first-line agents such as phototherapy in HIV-positive patients. Apremilast has been successfully used in an HIV patient with a concomitant chronic hepatitis C infection.1 Systemic medications such as apremilast should be managed in coordination with infectious disease specialists with close monitoring of CD4 levels and viral loads as well as prophylactic agents.
To the Editor:
A 50-year old man with Fitzpatrick skin type IV, human immunodeficiency virus (HIV), fatty liver disease, and moderate psoriasis (10% body surface area [BSA] affected) currently treated with clobetasol spray and calcitriol ointment presented with persistent psoriatic lesions on the trunk, arms, legs, and buttocks. His CD4 count was 460 and his HIV RNA count was 48 copies/mL on polymerase chain reaction 2 months prior to the current presentation. He had been undergoing phototherapy 3 times weekly for the last 5 months for treatment of psoriasis.
At the current presentation, he was started on an apremilast starter pack with the dosage titrated from 10 mg to 30 mg over the course of 1 week. He was maintained on a dose of 30 mg twice daily after 1 week and continued clobetasol spray, calcitriol ointment, and phototherapy 3 times weekly with the intent to reduce the frequency after adequate control of psoriasis was achieved. After 3 months of treatment, the affected BSA was 0%. He continued apremilast, and phototherapy was reduced to once weekly. Phototherapy was discontinued after 7 months of concomitant treatment with apremilast after clearance was maintained. It was reinitiated twice weekly after a mild flare (3% BSA affected). After 20 total months of treatment, the patient was no longer able to afford apremilast treatment and presented with a severe psoriasis flare (40% BSA affected). He was switched to acitretin with a plan to apply for apremilast financial assistance programs.
Psoriasis treatment in the HIV population poses a challenge given the immunosuppressed state of these patients, the risk of reactivation of latent infections, and the refractory nature of psoriasis in the setting of HIV. Two of the authors (S.P.R. and J.J.W.) previously reported a case of moderate to severe psoriasis in a patient with HIV and hepatitis C who demonstrated treatment success with apremilast until it was discontinued due to financial implications.1 Currently, apremilast is not widely used to treat psoriasis in the HIV population. The National Psoriasis Foundation 2010 guidelines recommended UV light therapy for treatment of moderate to severe psoriasis in HIV-positive patients, with oral retinoids as the second-line treatment.2 There remains a need for updated guidelines on the use of systemic agents for psoriasis treatment in the HIV population.
Apremilast, a phosphodiesterase 4 inhibitor, is an oral therapy that restores the balance of proinflammatory and anti-inflammatory cytokines by inhibiting inflammatory cytokine (eg, tumor necrosis factor α, IFN-γ, IL-2, IL-12, IL-23) secretion and stimulating anti-inflammatory cytokine (eg, IL-6, IL-10) production. In 2015, the phase 3 ESTEEM 13 and ESTEEM 24 trials demonstrated the efficacy of apremilast 30 mg twice daily for treatment of psoriasis. In both trials, the psoriasis area and severity index 75 response rate at week 16 was significantly
Use of other systemic agents such as tumor necrosis factor α inhibitors and ustekinumab has been reported in HIV-positive patients.5-7 There is no current data on IL-17 and IL-23 inhibitors. Acitretin generally is recommended as a second-line agent in HIV patients given its lack of immunosuppression2; however, methotrexate and cyclosporine should be avoided given the risk of opportunistic infections.8
Apremilast is a promising therapy with a favorable safety profile that should be considered as an adjuvant treatment to first-line agents such as phototherapy in HIV-positive patients. Apremilast has been successfully used in an HIV patient with a concomitant chronic hepatitis C infection.1 Systemic medications such as apremilast should be managed in coordination with infectious disease specialists with close monitoring of CD4 levels and viral loads as well as prophylactic agents.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:e481-e482.
- Menon K, Van Voorhees AS, Bebo BF Jr, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation [published online July 31, 2009]. J Am Acad Dermatol. 2010;62:291-299.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Lindsey SF, Weiss J, Lee ES, et al. Treatment of severe psoriasis and psoriatic arthritis with adalimumab in an HIV-positive patient. J Drugs Dermatol. 2014;13:869-871.
- Saeki H, Ito T, Hayashi M, et al. Successful treatment of ustekinumab in a severe psoriasis patient with human immunodeficiency virus infection. J Eur Acad Dermatol Venereol. 2015;29:1653-1655.
- Paparizos V, Rallis E, Kirsten L, et al. Ustekinumab for the treatment of HIV psoriasis. J Dermatolog Treat. 2012;23:398-399.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections [published online July 11, 2018]. J Am Acad Dermatol. 2019;80:43-53.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:e481-e482.
- Menon K, Van Voorhees AS, Bebo BF Jr, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation [published online July 31, 2009]. J Am Acad Dermatol. 2010;62:291-299.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Lindsey SF, Weiss J, Lee ES, et al. Treatment of severe psoriasis and psoriatic arthritis with adalimumab in an HIV-positive patient. J Drugs Dermatol. 2014;13:869-871.
- Saeki H, Ito T, Hayashi M, et al. Successful treatment of ustekinumab in a severe psoriasis patient with human immunodeficiency virus infection. J Eur Acad Dermatol Venereol. 2015;29:1653-1655.
- Paparizos V, Rallis E, Kirsten L, et al. Ustekinumab for the treatment of HIV psoriasis. J Dermatolog Treat. 2012;23:398-399.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections [published online July 11, 2018]. J Am Acad Dermatol. 2019;80:43-53.
Practice Point
- Apremilast may be considered as a first-line therapy in the human immunodeficiency virus population due to decreased immunosuppression.
Rotavirus vaccine had strong protective effect in routine U.K. practice
Oral rotavirus vaccination had a strong protective effect against laboratory-confirmed rotavirus infection in the first 2 years of the U.K. infant immunization program, investigators are reporting.
The estimated effectiveness was 77% for all infants with confirmed infection, and greater than 80% for those under 12 months of age, according to the report. The vaccine did not demonstrate efficacy against all-cause acute gastroenteritis, although this was likely because of high, sustained vaccine coverage coupled with the “substantial impact” of the rotavirus vaccine, wrote investigators led by Sara L. Thomas, MB BS, PhD, of the London School of Hygiene & Tropical Medicine.
Taken together, these findings provide “reassurance” that rotavirus vaccine is effective in a real-world setting and set the stage for future analyses of cost effectiveness, Dr. Thomas and coauthors said in a report on the study appearing in Vaccine: X, the open access mirror journal of Vaccine.
“As data accumulate in the post-vaccination era, more detailed assessment of waning of effectiveness over time can be undertaken, and investigation of rotavirus strain-specific protection,” they wrote.
Oral live-attenuated rotavirus vaccine (Rotarix) was introduced in the U.K. in 2013 as a two-dose schedule at 2 and 3 months of age. Vaccine uptake by the age of 25 weeks was rapid and sustained, exceeding 90%, according to previous reports. Declines in hospital admissions and primary care for all-cause acute gastroenteritis were substantial, associated with an estimated reduction of £12.5 million in health care costs in the first year of the program for children 5 years of age and younger.
To assess rotavirus vaccine effectiveness in the public health setting, Dr. Thomas and colleagues conducted a pair of studies: one designed to evaluate vaccine effectiveness against laboratory-confirmed rotavirus infections using laboratory surveillance data for 1,869 children and 1,032 controls and another to estimate vaccine effectiveness against all-cause acute gastroenteritis using electronic health data on 40,723 children.
Stratified by age, the data showed that vaccine effectiveness was 85% in those younger than 12 months, and 54% for older children.
By contrast, they found no evidence that the rotavirus vaccine protected against all-cause acute gastroenteritis in an analysis that adjusted for age and other factors. Analysis also suggested a lack of effectiveness against hospitalized acute gastroenteritis, according to the study authors.
In prelicensure trials, oral live-attenuated rotavirus vaccine in middle- and high-income settings had efficacy against severe rotavirus-confirmed gastroenteritis of greater than 85% and efficacy against severe all-cause gastroenteritis up to 40%, investigators noted.
The lack of vaccine efficacy on all-cause acute gastroenteritis is likely because of “highly effective implementation” of the vaccine program and rapid attainment of coverage, plus high vaccine effectiveness against rotavirus-specific acute gastroenteritis, the investigators said.
“As a result, almost all AGE in the study population in the post-vaccine era was likely to have been due to nonrotavirus organisms or non-infectious causes,” said Dr. Thomas and coauthors.
This highlights the importance of choosing “specific outcomes” to study when vaccine coverage and effectiveness are both high, they concluded.
Funding for this research came from the National Institute for Health Research Health Protection Research Unit in Immunisation at the London School of Hygiene and Tropical Medicine in partnership with Public Health England. The Immunisation and Countermeasures Division of Public Health England provided vaccine manufacturers with postmarketing surveillance reports, according to the article’s disclosure section.
SOURCE: Walker JL et al. Vaccine: X. 2019 Apr 11. doi: 10.1016/j.jvacx.2019.100005.
Oral rotavirus vaccination had a strong protective effect against laboratory-confirmed rotavirus infection in the first 2 years of the U.K. infant immunization program, investigators are reporting.
The estimated effectiveness was 77% for all infants with confirmed infection, and greater than 80% for those under 12 months of age, according to the report. The vaccine did not demonstrate efficacy against all-cause acute gastroenteritis, although this was likely because of high, sustained vaccine coverage coupled with the “substantial impact” of the rotavirus vaccine, wrote investigators led by Sara L. Thomas, MB BS, PhD, of the London School of Hygiene & Tropical Medicine.
Taken together, these findings provide “reassurance” that rotavirus vaccine is effective in a real-world setting and set the stage for future analyses of cost effectiveness, Dr. Thomas and coauthors said in a report on the study appearing in Vaccine: X, the open access mirror journal of Vaccine.
“As data accumulate in the post-vaccination era, more detailed assessment of waning of effectiveness over time can be undertaken, and investigation of rotavirus strain-specific protection,” they wrote.
Oral live-attenuated rotavirus vaccine (Rotarix) was introduced in the U.K. in 2013 as a two-dose schedule at 2 and 3 months of age. Vaccine uptake by the age of 25 weeks was rapid and sustained, exceeding 90%, according to previous reports. Declines in hospital admissions and primary care for all-cause acute gastroenteritis were substantial, associated with an estimated reduction of £12.5 million in health care costs in the first year of the program for children 5 years of age and younger.
To assess rotavirus vaccine effectiveness in the public health setting, Dr. Thomas and colleagues conducted a pair of studies: one designed to evaluate vaccine effectiveness against laboratory-confirmed rotavirus infections using laboratory surveillance data for 1,869 children and 1,032 controls and another to estimate vaccine effectiveness against all-cause acute gastroenteritis using electronic health data on 40,723 children.
Stratified by age, the data showed that vaccine effectiveness was 85% in those younger than 12 months, and 54% for older children.
By contrast, they found no evidence that the rotavirus vaccine protected against all-cause acute gastroenteritis in an analysis that adjusted for age and other factors. Analysis also suggested a lack of effectiveness against hospitalized acute gastroenteritis, according to the study authors.
In prelicensure trials, oral live-attenuated rotavirus vaccine in middle- and high-income settings had efficacy against severe rotavirus-confirmed gastroenteritis of greater than 85% and efficacy against severe all-cause gastroenteritis up to 40%, investigators noted.
The lack of vaccine efficacy on all-cause acute gastroenteritis is likely because of “highly effective implementation” of the vaccine program and rapid attainment of coverage, plus high vaccine effectiveness against rotavirus-specific acute gastroenteritis, the investigators said.
“As a result, almost all AGE in the study population in the post-vaccine era was likely to have been due to nonrotavirus organisms or non-infectious causes,” said Dr. Thomas and coauthors.
This highlights the importance of choosing “specific outcomes” to study when vaccine coverage and effectiveness are both high, they concluded.
Funding for this research came from the National Institute for Health Research Health Protection Research Unit in Immunisation at the London School of Hygiene and Tropical Medicine in partnership with Public Health England. The Immunisation and Countermeasures Division of Public Health England provided vaccine manufacturers with postmarketing surveillance reports, according to the article’s disclosure section.
SOURCE: Walker JL et al. Vaccine: X. 2019 Apr 11. doi: 10.1016/j.jvacx.2019.100005.
Oral rotavirus vaccination had a strong protective effect against laboratory-confirmed rotavirus infection in the first 2 years of the U.K. infant immunization program, investigators are reporting.
The estimated effectiveness was 77% for all infants with confirmed infection, and greater than 80% for those under 12 months of age, according to the report. The vaccine did not demonstrate efficacy against all-cause acute gastroenteritis, although this was likely because of high, sustained vaccine coverage coupled with the “substantial impact” of the rotavirus vaccine, wrote investigators led by Sara L. Thomas, MB BS, PhD, of the London School of Hygiene & Tropical Medicine.
Taken together, these findings provide “reassurance” that rotavirus vaccine is effective in a real-world setting and set the stage for future analyses of cost effectiveness, Dr. Thomas and coauthors said in a report on the study appearing in Vaccine: X, the open access mirror journal of Vaccine.
“As data accumulate in the post-vaccination era, more detailed assessment of waning of effectiveness over time can be undertaken, and investigation of rotavirus strain-specific protection,” they wrote.
Oral live-attenuated rotavirus vaccine (Rotarix) was introduced in the U.K. in 2013 as a two-dose schedule at 2 and 3 months of age. Vaccine uptake by the age of 25 weeks was rapid and sustained, exceeding 90%, according to previous reports. Declines in hospital admissions and primary care for all-cause acute gastroenteritis were substantial, associated with an estimated reduction of £12.5 million in health care costs in the first year of the program for children 5 years of age and younger.
To assess rotavirus vaccine effectiveness in the public health setting, Dr. Thomas and colleagues conducted a pair of studies: one designed to evaluate vaccine effectiveness against laboratory-confirmed rotavirus infections using laboratory surveillance data for 1,869 children and 1,032 controls and another to estimate vaccine effectiveness against all-cause acute gastroenteritis using electronic health data on 40,723 children.
Stratified by age, the data showed that vaccine effectiveness was 85% in those younger than 12 months, and 54% for older children.
By contrast, they found no evidence that the rotavirus vaccine protected against all-cause acute gastroenteritis in an analysis that adjusted for age and other factors. Analysis also suggested a lack of effectiveness against hospitalized acute gastroenteritis, according to the study authors.
In prelicensure trials, oral live-attenuated rotavirus vaccine in middle- and high-income settings had efficacy against severe rotavirus-confirmed gastroenteritis of greater than 85% and efficacy against severe all-cause gastroenteritis up to 40%, investigators noted.
The lack of vaccine efficacy on all-cause acute gastroenteritis is likely because of “highly effective implementation” of the vaccine program and rapid attainment of coverage, plus high vaccine effectiveness against rotavirus-specific acute gastroenteritis, the investigators said.
“As a result, almost all AGE in the study population in the post-vaccine era was likely to have been due to nonrotavirus organisms or non-infectious causes,” said Dr. Thomas and coauthors.
This highlights the importance of choosing “specific outcomes” to study when vaccine coverage and effectiveness are both high, they concluded.
Funding for this research came from the National Institute for Health Research Health Protection Research Unit in Immunisation at the London School of Hygiene and Tropical Medicine in partnership with Public Health England. The Immunisation and Countermeasures Division of Public Health England provided vaccine manufacturers with postmarketing surveillance reports, according to the article’s disclosure section.
SOURCE: Walker JL et al. Vaccine: X. 2019 Apr 11. doi: 10.1016/j.jvacx.2019.100005.
FROM VACCINE
Zero HIV transmission rate when viral load suppressed
according to a new paper published in the Lancet.
The prospective observational PARTNER2 study (Lancet 2019 May 2. doi: 10.1016/S0140-6736[19]304018-0) followed 782 serodiscordant gay couples from 14 European countries, where the HIV-positive partner had to be virally suppressed to below 200 copies of HIV-1 RNA per milliliter, and the couple was engaging in condomless sex without using pre- or postexposure prophylaxis.
After a median follow-up of 2 years, there was not a single case of HIV transmission between couples, representing a transmission rate of zero. The study did record 15 new HIV infections during follow-up but these were all phylogenetically linked to sources other than the HIV-positive partner.
The HIV-positive partners had been on antiretroviral therapy for a median of 4.3 years – most on regimens of three or more drugs – with high levels of adherence. During the follow-up period, 37 HIV-positive partners (5%) reported missing their antiretroviral therapy for more than 4 consecutive days.
“Our results give equivalence of evidence for gay men as for heterosexual couples and indicate that the risk of HIV transmission when HIV viral load is suppressed is effectively zero for both anal and vaginal sex,” wrote Dr. Alison J. Rodger of the Institute for Global Health at University College London, and coauthors. “These findings emphasize the importance of regular monitoring to ensure HIV viral load remains suppressed and supporting HIV-positive people with long-term adherence.”
Around one-quarter of the HIV-negative men and 27% of the HIV-positive men reported a sexually-transmitted infection during follow-up; the most common infections were syphilis, gonorrhea, and chlamydia.
The study noted six additional cases of seroconversion of HIV-negative partners. However, these occurred outside the eligible couple-years of the follow-up period. They were ineligible due to no questionnaire about sexual behavior having been completed by either partner in the couple, a lack of condomless sex between the couple, use of postexposure prophylaxis, or a lack of viral load measurement for the HIV-positive partner in the past year.
The authors did note that the study population was predominantly white and with a median age of 38 years, while most HIV transmission occurs in young people aged under 25 years.
“The results from the PARTNER studies support wider dissemination of the message of the U=U [Undetectable equals Untransmittable] campaign that risk of transmission of HIV in the context of virally suppressive ART is zero,” they wrote.
The study was supported by the National Institute for Health Research, the British HIV Association, the Danish National Research Foundation, ViiV Healthcare, Gilead Sciences, Augustinus Fonden, and A P Møller Fonden. Fourteen authors declared grants, personal fees and other support from the pharmaceutical sector.
SOURCE: Rodger A et al. Lancet 2019, May 2. doi: 10.1016/S0140-6736[19]304018-0.
The results of the PARTNER and PARTNER2 trials show that timely diagnosis and effective treatment can virtually eliminate the risk of HIV transmission. However, access to HIV testing and care is not always easy, and fear, stigma, homophobia, and other forces continue to limit access to HIV treatment. This study also highlights the impact of sexual relations outside the bounds of a couple’s relationship.
While the use of preexposure prophylaxis was a criterion for exclusion from this study, this intervention should also be recognized as an important part of HIV prevention. A recent survey found that men who have sex with men are more likely to trust preexposure prophylaxis for HIV prevention than antiretroviral therapy.
Dr. Myron S Cohen is from the departments of medicine, microbiology, immunology, and epidemiology, University of North Carolina at Chapel Hill, and the UNC Institute for Global Health and Infectious Diseases in Chapel Hill. These comments are adapted from an editorial (Lancet 2019, May 2. doi: 10.1016/S0140-6736[19]30701-9). Dr. Cohen reported advisory board travel fees from Merck and Gilead unrelated to this work.
The results of the PARTNER and PARTNER2 trials show that timely diagnosis and effective treatment can virtually eliminate the risk of HIV transmission. However, access to HIV testing and care is not always easy, and fear, stigma, homophobia, and other forces continue to limit access to HIV treatment. This study also highlights the impact of sexual relations outside the bounds of a couple’s relationship.
While the use of preexposure prophylaxis was a criterion for exclusion from this study, this intervention should also be recognized as an important part of HIV prevention. A recent survey found that men who have sex with men are more likely to trust preexposure prophylaxis for HIV prevention than antiretroviral therapy.
Dr. Myron S Cohen is from the departments of medicine, microbiology, immunology, and epidemiology, University of North Carolina at Chapel Hill, and the UNC Institute for Global Health and Infectious Diseases in Chapel Hill. These comments are adapted from an editorial (Lancet 2019, May 2. doi: 10.1016/S0140-6736[19]30701-9). Dr. Cohen reported advisory board travel fees from Merck and Gilead unrelated to this work.
The results of the PARTNER and PARTNER2 trials show that timely diagnosis and effective treatment can virtually eliminate the risk of HIV transmission. However, access to HIV testing and care is not always easy, and fear, stigma, homophobia, and other forces continue to limit access to HIV treatment. This study also highlights the impact of sexual relations outside the bounds of a couple’s relationship.
While the use of preexposure prophylaxis was a criterion for exclusion from this study, this intervention should also be recognized as an important part of HIV prevention. A recent survey found that men who have sex with men are more likely to trust preexposure prophylaxis for HIV prevention than antiretroviral therapy.
Dr. Myron S Cohen is from the departments of medicine, microbiology, immunology, and epidemiology, University of North Carolina at Chapel Hill, and the UNC Institute for Global Health and Infectious Diseases in Chapel Hill. These comments are adapted from an editorial (Lancet 2019, May 2. doi: 10.1016/S0140-6736[19]30701-9). Dr. Cohen reported advisory board travel fees from Merck and Gilead unrelated to this work.
according to a new paper published in the Lancet.
The prospective observational PARTNER2 study (Lancet 2019 May 2. doi: 10.1016/S0140-6736[19]304018-0) followed 782 serodiscordant gay couples from 14 European countries, where the HIV-positive partner had to be virally suppressed to below 200 copies of HIV-1 RNA per milliliter, and the couple was engaging in condomless sex without using pre- or postexposure prophylaxis.
After a median follow-up of 2 years, there was not a single case of HIV transmission between couples, representing a transmission rate of zero. The study did record 15 new HIV infections during follow-up but these were all phylogenetically linked to sources other than the HIV-positive partner.
The HIV-positive partners had been on antiretroviral therapy for a median of 4.3 years – most on regimens of three or more drugs – with high levels of adherence. During the follow-up period, 37 HIV-positive partners (5%) reported missing their antiretroviral therapy for more than 4 consecutive days.
“Our results give equivalence of evidence for gay men as for heterosexual couples and indicate that the risk of HIV transmission when HIV viral load is suppressed is effectively zero for both anal and vaginal sex,” wrote Dr. Alison J. Rodger of the Institute for Global Health at University College London, and coauthors. “These findings emphasize the importance of regular monitoring to ensure HIV viral load remains suppressed and supporting HIV-positive people with long-term adherence.”
Around one-quarter of the HIV-negative men and 27% of the HIV-positive men reported a sexually-transmitted infection during follow-up; the most common infections were syphilis, gonorrhea, and chlamydia.
The study noted six additional cases of seroconversion of HIV-negative partners. However, these occurred outside the eligible couple-years of the follow-up period. They were ineligible due to no questionnaire about sexual behavior having been completed by either partner in the couple, a lack of condomless sex between the couple, use of postexposure prophylaxis, or a lack of viral load measurement for the HIV-positive partner in the past year.
The authors did note that the study population was predominantly white and with a median age of 38 years, while most HIV transmission occurs in young people aged under 25 years.
“The results from the PARTNER studies support wider dissemination of the message of the U=U [Undetectable equals Untransmittable] campaign that risk of transmission of HIV in the context of virally suppressive ART is zero,” they wrote.
The study was supported by the National Institute for Health Research, the British HIV Association, the Danish National Research Foundation, ViiV Healthcare, Gilead Sciences, Augustinus Fonden, and A P Møller Fonden. Fourteen authors declared grants, personal fees and other support from the pharmaceutical sector.
SOURCE: Rodger A et al. Lancet 2019, May 2. doi: 10.1016/S0140-6736[19]304018-0.
according to a new paper published in the Lancet.
The prospective observational PARTNER2 study (Lancet 2019 May 2. doi: 10.1016/S0140-6736[19]304018-0) followed 782 serodiscordant gay couples from 14 European countries, where the HIV-positive partner had to be virally suppressed to below 200 copies of HIV-1 RNA per milliliter, and the couple was engaging in condomless sex without using pre- or postexposure prophylaxis.
After a median follow-up of 2 years, there was not a single case of HIV transmission between couples, representing a transmission rate of zero. The study did record 15 new HIV infections during follow-up but these were all phylogenetically linked to sources other than the HIV-positive partner.
The HIV-positive partners had been on antiretroviral therapy for a median of 4.3 years – most on regimens of three or more drugs – with high levels of adherence. During the follow-up period, 37 HIV-positive partners (5%) reported missing their antiretroviral therapy for more than 4 consecutive days.
“Our results give equivalence of evidence for gay men as for heterosexual couples and indicate that the risk of HIV transmission when HIV viral load is suppressed is effectively zero for both anal and vaginal sex,” wrote Dr. Alison J. Rodger of the Institute for Global Health at University College London, and coauthors. “These findings emphasize the importance of regular monitoring to ensure HIV viral load remains suppressed and supporting HIV-positive people with long-term adherence.”
Around one-quarter of the HIV-negative men and 27% of the HIV-positive men reported a sexually-transmitted infection during follow-up; the most common infections were syphilis, gonorrhea, and chlamydia.
The study noted six additional cases of seroconversion of HIV-negative partners. However, these occurred outside the eligible couple-years of the follow-up period. They were ineligible due to no questionnaire about sexual behavior having been completed by either partner in the couple, a lack of condomless sex between the couple, use of postexposure prophylaxis, or a lack of viral load measurement for the HIV-positive partner in the past year.
The authors did note that the study population was predominantly white and with a median age of 38 years, while most HIV transmission occurs in young people aged under 25 years.
“The results from the PARTNER studies support wider dissemination of the message of the U=U [Undetectable equals Untransmittable] campaign that risk of transmission of HIV in the context of virally suppressive ART is zero,” they wrote.
The study was supported by the National Institute for Health Research, the British HIV Association, the Danish National Research Foundation, ViiV Healthcare, Gilead Sciences, Augustinus Fonden, and A P Møller Fonden. Fourteen authors declared grants, personal fees and other support from the pharmaceutical sector.
SOURCE: Rodger A et al. Lancet 2019, May 2. doi: 10.1016/S0140-6736[19]304018-0.
FROM THE LANCET
DOPPS participation associated with lower HCV rates in dialysis patients
Dialysis patients are commonly infected with hepatitis C virus (HCV), and such infections are associated with increased morbidity and mortality. A team of international researchers assessed trends in the prevalence, incidence, and risk factors for HCV infection among more than 82,000 dialysis patients as defined by a documented diagnosis or antibody positivity using the Dialysis Outcomes and Practice Patterns Study.
They found that overall, among prevalent hemodialysis patients, HCV prevalence was nearly 10% during 2012-2015. The prevalence ranged from a low of 4% in Belgium to as high as 20% in the Middle East, with intermediate prevalence in China, Japan, Italy, Spain, and Russia. However, the prevalence of HCV decreased over time in most countries participating in more than one phase of DOPPS, and prevalence was around 5% among patients who had initiated dialysis within less than 4 months.
The incidence of . Although most units reported no seroconversions, 10% of units experienced three or more cases over a median of 1.1 years.
The researchers also found that high HCV prevalence in the hemodialysis unit was a powerful facility-level risk factor for seroconversion, but the use of isolation stations for HCV-positive patients was not associated with significantly lower seroconversion rates.
“Overall, despite a trend toward lower HCV prevalence among hemodialysis patients, the prevalence of HCV infection remains higher than in the general population,” wrote Michel Jadoul, MD, Cliniques universitaires Saint-Luc, Université Catholique de Louvain, Brussels, and colleagues.
Their report, sponsored by Merck, appeared in Kidney International. A number of the authors reported being speakers or consultants for a variety of pharmaceutical companies; two of the authors are employees of Merck. Support for the ongoing DOPPS Program is provided without restriction on publications.
SOURCE: Jadoul M et al. Kidney Int. 2019;95:939-47.
Dialysis patients are commonly infected with hepatitis C virus (HCV), and such infections are associated with increased morbidity and mortality. A team of international researchers assessed trends in the prevalence, incidence, and risk factors for HCV infection among more than 82,000 dialysis patients as defined by a documented diagnosis or antibody positivity using the Dialysis Outcomes and Practice Patterns Study.

They found that overall, among prevalent hemodialysis patients, HCV prevalence was nearly 10% during 2012-2015. The prevalence ranged from a low of 4% in Belgium to as high as 20% in the Middle East, with intermediate prevalence in China, Japan, Italy, Spain, and Russia. However, the prevalence of HCV decreased over time in most countries participating in more than one phase of DOPPS, and prevalence was around 5% among patients who had initiated dialysis within less than 4 months.
The incidence of . Although most units reported no seroconversions, 10% of units experienced three or more cases over a median of 1.1 years.
The researchers also found that high HCV prevalence in the hemodialysis unit was a powerful facility-level risk factor for seroconversion, but the use of isolation stations for HCV-positive patients was not associated with significantly lower seroconversion rates.
“Overall, despite a trend toward lower HCV prevalence among hemodialysis patients, the prevalence of HCV infection remains higher than in the general population,” wrote Michel Jadoul, MD, Cliniques universitaires Saint-Luc, Université Catholique de Louvain, Brussels, and colleagues.
Their report, sponsored by Merck, appeared in Kidney International. A number of the authors reported being speakers or consultants for a variety of pharmaceutical companies; two of the authors are employees of Merck. Support for the ongoing DOPPS Program is provided without restriction on publications.
SOURCE: Jadoul M et al. Kidney Int. 2019;95:939-47.
Dialysis patients are commonly infected with hepatitis C virus (HCV), and such infections are associated with increased morbidity and mortality. A team of international researchers assessed trends in the prevalence, incidence, and risk factors for HCV infection among more than 82,000 dialysis patients as defined by a documented diagnosis or antibody positivity using the Dialysis Outcomes and Practice Patterns Study.

They found that overall, among prevalent hemodialysis patients, HCV prevalence was nearly 10% during 2012-2015. The prevalence ranged from a low of 4% in Belgium to as high as 20% in the Middle East, with intermediate prevalence in China, Japan, Italy, Spain, and Russia. However, the prevalence of HCV decreased over time in most countries participating in more than one phase of DOPPS, and prevalence was around 5% among patients who had initiated dialysis within less than 4 months.
The incidence of . Although most units reported no seroconversions, 10% of units experienced three or more cases over a median of 1.1 years.
The researchers also found that high HCV prevalence in the hemodialysis unit was a powerful facility-level risk factor for seroconversion, but the use of isolation stations for HCV-positive patients was not associated with significantly lower seroconversion rates.
“Overall, despite a trend toward lower HCV prevalence among hemodialysis patients, the prevalence of HCV infection remains higher than in the general population,” wrote Michel Jadoul, MD, Cliniques universitaires Saint-Luc, Université Catholique de Louvain, Brussels, and colleagues.
Their report, sponsored by Merck, appeared in Kidney International. A number of the authors reported being speakers or consultants for a variety of pharmaceutical companies; two of the authors are employees of Merck. Support for the ongoing DOPPS Program is provided without restriction on publications.
SOURCE: Jadoul M et al. Kidney Int. 2019;95:939-47.
FROM KIDNEY INTERNATIONAL
Part 1: A Disturbing Trend
While reviewing some epidemiology data for a lecture recently, I couldn’t believe my eyes: The numbers indicated an increase in sexually transmitted infections (STIs) among older Americans. Filled with doubt about the accuracy, I decided to research further. My first stop was a PA colleague who works with a mobile urgent care company that specializes in retirement communities—and she confirmed that she has witnessed this “trend”!
The fundamental public health concern for older Americans is, of course, long-term illness, disability, and dependency on others. However, experts on aging agree that since the last century, disability rates among those older than 65 have declined, as have the number of seniors living in nursing homes. Suffice it to say, the good news is that Americans are living longer—the bad news, they are doing so with an increased risk for cardiovascular disease and malignant neoplasms. The other downside is that seniors have increased risk for infectious diseases.1
Healthy People 2020 continues to recognize HIV and other STIs as problems in the United States and to promote efforts to reduce them. Unfortunately, prevention strategies for older adults in primary care settings are often not aimed at these diseases. More broadly, sexual behaviors tend to be discussed less with this population.2
The disturbing climb in STIs among older Americans is part of a more momentous national trend that the CDC says must be tackled. Overall rates of STIs in 2016 were the highest ever recorded in a single year.3 And although STI rates are highest among people ages 15 to 24, the upsurge among older Americans is larger than it is for the rest of the US population. According to the CDC, in 2016, there were 82,938 cases of gonorrhea, syphilis, and chlamydia reported among Americans ages 45 and older—about a 20% percent increase from 2015 and continuing a trend of annual increases since at least 2012.3 The infographic shows the rates for individual STIs.
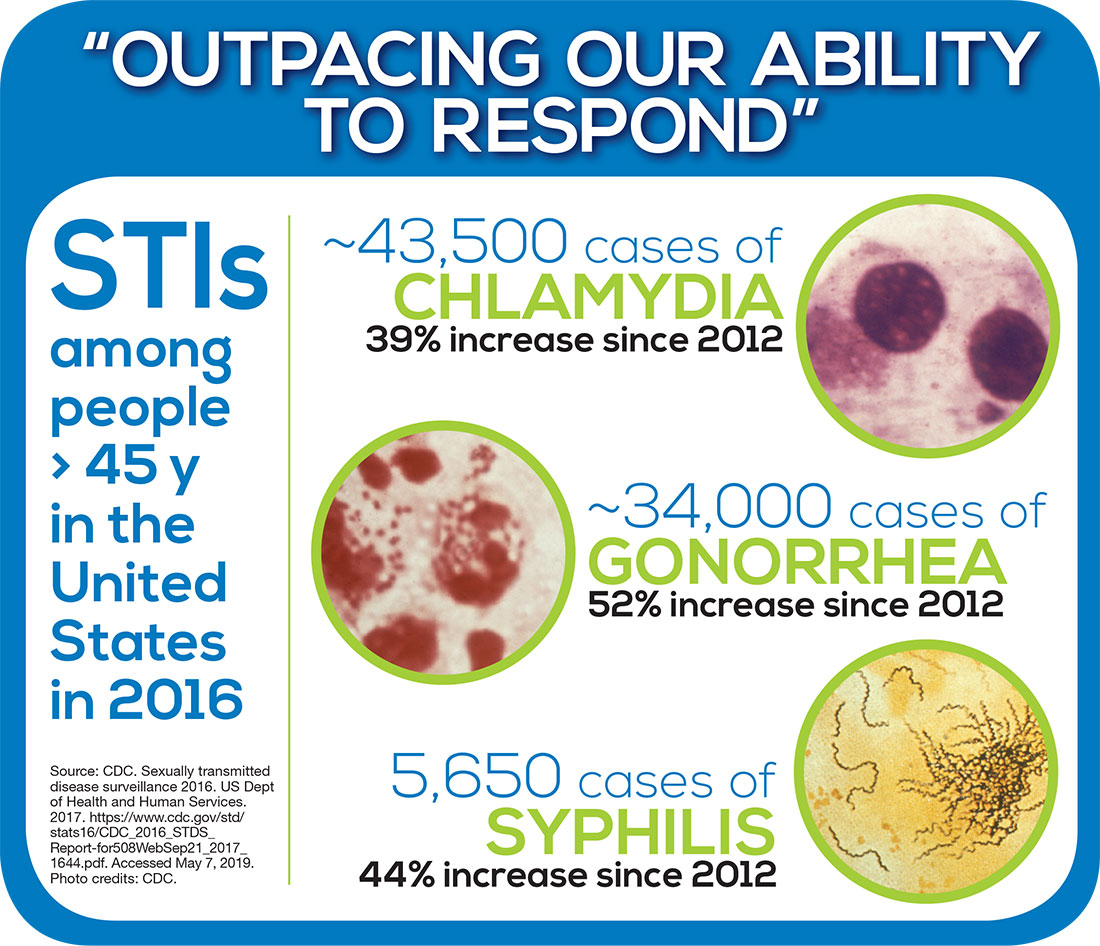
The CDC notes that STIs put people “at risk for severe, lifelong health outcomes like chronic pain, severe reproductive health complications, and HIV" particularly if left untreated.4 Jonathan Mermin, MD, Director of the CDC National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention has described STIs as “a persistent enemy, growing in number and outpacing our ability to respond.”5
Over the next 3 weeks, we will explore this public health issue—starting next week with the big question: Why is this trend occurring? In the meantime, feel free to share your thoughts with me at [email protected]. See you next Thursday!
1. Schneider M. Introduction to Public Health. 5th ed. Burlington, MA: Jones and Bartlett Learning; 2017.
2. Department of Health and Human Services Office of Disease Prevention and Health Promotion. Healthy People 2020: Sexually transmitted diseases. www.healthypeople.gov/2020/topics-objectives/topic/sexually-transmitted-diseases. Accessed May 6, 2019.
3. CDC. 2016 Sexually Transmitted Diseases Surveillance. www.cdc.gov/std/stats16/default.htm. Accessed May 6, 2019.
4. CDC. Fact sheet: reported STDs in the United States, 2017. www.cdc.gov/nchhstp/newsroom/docs/factsheets/std-trends-508.pdf. Accessed May 6, 2019.
5. CDC National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. STDs at record high, indicating urgent need for prevention [press release]. September 26, 2017. www.cdc.gov/nchhstp/newsroom/2017/std-surveillance-report-2016-press-release.html. Accessed May 6, 2019.
While reviewing some epidemiology data for a lecture recently, I couldn’t believe my eyes: The numbers indicated an increase in sexually transmitted infections (STIs) among older Americans. Filled with doubt about the accuracy, I decided to research further. My first stop was a PA colleague who works with a mobile urgent care company that specializes in retirement communities—and she confirmed that she has witnessed this “trend”!
The fundamental public health concern for older Americans is, of course, long-term illness, disability, and dependency on others. However, experts on aging agree that since the last century, disability rates among those older than 65 have declined, as have the number of seniors living in nursing homes. Suffice it to say, the good news is that Americans are living longer—the bad news, they are doing so with an increased risk for cardiovascular disease and malignant neoplasms. The other downside is that seniors have increased risk for infectious diseases.1
Healthy People 2020 continues to recognize HIV and other STIs as problems in the United States and to promote efforts to reduce them. Unfortunately, prevention strategies for older adults in primary care settings are often not aimed at these diseases. More broadly, sexual behaviors tend to be discussed less with this population.2
The disturbing climb in STIs among older Americans is part of a more momentous national trend that the CDC says must be tackled. Overall rates of STIs in 2016 were the highest ever recorded in a single year.3 And although STI rates are highest among people ages 15 to 24, the upsurge among older Americans is larger than it is for the rest of the US population. According to the CDC, in 2016, there were 82,938 cases of gonorrhea, syphilis, and chlamydia reported among Americans ages 45 and older—about a 20% percent increase from 2015 and continuing a trend of annual increases since at least 2012.3 The infographic shows the rates for individual STIs.

The CDC notes that STIs put people “at risk for severe, lifelong health outcomes like chronic pain, severe reproductive health complications, and HIV" particularly if left untreated.4 Jonathan Mermin, MD, Director of the CDC National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention has described STIs as “a persistent enemy, growing in number and outpacing our ability to respond.”5
Over the next 3 weeks, we will explore this public health issue—starting next week with the big question: Why is this trend occurring? In the meantime, feel free to share your thoughts with me at [email protected]. See you next Thursday!
While reviewing some epidemiology data for a lecture recently, I couldn’t believe my eyes: The numbers indicated an increase in sexually transmitted infections (STIs) among older Americans. Filled with doubt about the accuracy, I decided to research further. My first stop was a PA colleague who works with a mobile urgent care company that specializes in retirement communities—and she confirmed that she has witnessed this “trend”!
The fundamental public health concern for older Americans is, of course, long-term illness, disability, and dependency on others. However, experts on aging agree that since the last century, disability rates among those older than 65 have declined, as have the number of seniors living in nursing homes. Suffice it to say, the good news is that Americans are living longer—the bad news, they are doing so with an increased risk for cardiovascular disease and malignant neoplasms. The other downside is that seniors have increased risk for infectious diseases.1
Healthy People 2020 continues to recognize HIV and other STIs as problems in the United States and to promote efforts to reduce them. Unfortunately, prevention strategies for older adults in primary care settings are often not aimed at these diseases. More broadly, sexual behaviors tend to be discussed less with this population.2
The disturbing climb in STIs among older Americans is part of a more momentous national trend that the CDC says must be tackled. Overall rates of STIs in 2016 were the highest ever recorded in a single year.3 And although STI rates are highest among people ages 15 to 24, the upsurge among older Americans is larger than it is for the rest of the US population. According to the CDC, in 2016, there were 82,938 cases of gonorrhea, syphilis, and chlamydia reported among Americans ages 45 and older—about a 20% percent increase from 2015 and continuing a trend of annual increases since at least 2012.3 The infographic shows the rates for individual STIs.

The CDC notes that STIs put people “at risk for severe, lifelong health outcomes like chronic pain, severe reproductive health complications, and HIV" particularly if left untreated.4 Jonathan Mermin, MD, Director of the CDC National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention has described STIs as “a persistent enemy, growing in number and outpacing our ability to respond.”5
Over the next 3 weeks, we will explore this public health issue—starting next week with the big question: Why is this trend occurring? In the meantime, feel free to share your thoughts with me at [email protected]. See you next Thursday!
1. Schneider M. Introduction to Public Health. 5th ed. Burlington, MA: Jones and Bartlett Learning; 2017.
2. Department of Health and Human Services Office of Disease Prevention and Health Promotion. Healthy People 2020: Sexually transmitted diseases. www.healthypeople.gov/2020/topics-objectives/topic/sexually-transmitted-diseases. Accessed May 6, 2019.
3. CDC. 2016 Sexually Transmitted Diseases Surveillance. www.cdc.gov/std/stats16/default.htm. Accessed May 6, 2019.
4. CDC. Fact sheet: reported STDs in the United States, 2017. www.cdc.gov/nchhstp/newsroom/docs/factsheets/std-trends-508.pdf. Accessed May 6, 2019.
5. CDC National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. STDs at record high, indicating urgent need for prevention [press release]. September 26, 2017. www.cdc.gov/nchhstp/newsroom/2017/std-surveillance-report-2016-press-release.html. Accessed May 6, 2019.
1. Schneider M. Introduction to Public Health. 5th ed. Burlington, MA: Jones and Bartlett Learning; 2017.
2. Department of Health and Human Services Office of Disease Prevention and Health Promotion. Healthy People 2020: Sexually transmitted diseases. www.healthypeople.gov/2020/topics-objectives/topic/sexually-transmitted-diseases. Accessed May 6, 2019.
3. CDC. 2016 Sexually Transmitted Diseases Surveillance. www.cdc.gov/std/stats16/default.htm. Accessed May 6, 2019.
4. CDC. Fact sheet: reported STDs in the United States, 2017. www.cdc.gov/nchhstp/newsroom/docs/factsheets/std-trends-508.pdf. Accessed May 6, 2019.
5. CDC National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. STDs at record high, indicating urgent need for prevention [press release]. September 26, 2017. www.cdc.gov/nchhstp/newsroom/2017/std-surveillance-report-2016-press-release.html. Accessed May 6, 2019.