User login
FDA approves Recarbrio for cUTI, cIAI treatment in adults
The Recarbrio is a three-drug combo injection containing imipenem/cilastatin, an antibiotic previously approved by the FDA, and relebactam, a beta-lactamase inhibitor.
The efficacy of Recarbrio was supported by data on the efficacy of imipenem/cilastatin in the treatment of cUTI and cIAI and by in vitro studies and animal models of infection with treatment by relebactam. The safety was assessed in a pair of clinical studies, one that assessed cUTI patients and another that assessed cIAI patients.
The most common adverse events reported were nausea, diarrhea, headache, fever, and increased liver enzymes. Treatment with Recarbrio is not recommended in patients taking ganciclovir, valproic acid, or divalproex sodium because there is an increased risk of seizures, according to the FDA.
“The FDA remains focused on facilitating the development of safe and effective new antibacterial drugs to give patients more options to fight serious infections. It is important that the use of Recarbrio be reserved for situations when there are limited or no alternative antibacterial drugs for treating a patient’s infection,” Ed Cox, MD, MPH, director for the Office of Antimicrobial Products in FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
The Recarbrio is a three-drug combo injection containing imipenem/cilastatin, an antibiotic previously approved by the FDA, and relebactam, a beta-lactamase inhibitor.
The efficacy of Recarbrio was supported by data on the efficacy of imipenem/cilastatin in the treatment of cUTI and cIAI and by in vitro studies and animal models of infection with treatment by relebactam. The safety was assessed in a pair of clinical studies, one that assessed cUTI patients and another that assessed cIAI patients.
The most common adverse events reported were nausea, diarrhea, headache, fever, and increased liver enzymes. Treatment with Recarbrio is not recommended in patients taking ganciclovir, valproic acid, or divalproex sodium because there is an increased risk of seizures, according to the FDA.
“The FDA remains focused on facilitating the development of safe and effective new antibacterial drugs to give patients more options to fight serious infections. It is important that the use of Recarbrio be reserved for situations when there are limited or no alternative antibacterial drugs for treating a patient’s infection,” Ed Cox, MD, MPH, director for the Office of Antimicrobial Products in FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
The Recarbrio is a three-drug combo injection containing imipenem/cilastatin, an antibiotic previously approved by the FDA, and relebactam, a beta-lactamase inhibitor.
The efficacy of Recarbrio was supported by data on the efficacy of imipenem/cilastatin in the treatment of cUTI and cIAI and by in vitro studies and animal models of infection with treatment by relebactam. The safety was assessed in a pair of clinical studies, one that assessed cUTI patients and another that assessed cIAI patients.
The most common adverse events reported were nausea, diarrhea, headache, fever, and increased liver enzymes. Treatment with Recarbrio is not recommended in patients taking ganciclovir, valproic acid, or divalproex sodium because there is an increased risk of seizures, according to the FDA.
“The FDA remains focused on facilitating the development of safe and effective new antibacterial drugs to give patients more options to fight serious infections. It is important that the use of Recarbrio be reserved for situations when there are limited or no alternative antibacterial drugs for treating a patient’s infection,” Ed Cox, MD, MPH, director for the Office of Antimicrobial Products in FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
Little association found between in utero H1N1 vaccine and 5-year health outcomes
according to Laura K. Walsh of the University of Ottawa and associates.
The investigators conducted a population-based retrospective cohort study from November 2009 to October 2010 of all live births within the province of Ontario. Of the 104,249 eligible live births reported to the Ontario birth registry, 31,295 were exposed to the H1N1 vaccine in utero. After adjustment, there were no significant differences in the women who did and did not receive vaccines during pregnancy, according to the study, published in the BMJ.
After a median follow-up of 5 years, 14% of children received an asthma diagnosis, with a median age at diagnosis of 1.8 years. Children were more likely to receive an asthma diagnosis if their mothers had a preexisting condition or if they were born preterm. At follow-up, 34% of children had at least one upper respiratory tract infection. Sensory disorder, neoplasm, and pediatric complex chronic condition were rare, each occurring in less than 1% of the study cohort (BMJ. 2019 Jul 10. doi: 10.1136/bmj.l4151).
No significant association was found between prenatal exposure to the H1N1 vaccine and upper or lower respiratory infections, otitis media, all infections, neoplasms, sensory disorders, rates of urgent and inpatient health services use, pediatric complex chronic conditions, or mortality. A weak but significant association was observed for asthma (adjusted hazard ratio, 1.05; 95% confidence interval, 1.02-1.09), and a weak inverse association was found for gastrointestinal infections (adjusted incidence rate ratio, 0.94; 95% CI, 0.91-0.98).
“Although we observed a small, but statistically significant, increase in pediatric asthma and a reduction in gastrointestinal infections, we are not aware of any biologic mechanisms to explain these findings. Future studies in different settings and with different influenza vaccine formulations will be important for developing the evidence base on longer-term pediatric outcomes following influenza vaccination during pregnancy,” the investigators concluded.
The study was funded by grants from the Canadian Institutes of Health Research and the Institute for Clinical Evaluative Sciences.
according to Laura K. Walsh of the University of Ottawa and associates.
The investigators conducted a population-based retrospective cohort study from November 2009 to October 2010 of all live births within the province of Ontario. Of the 104,249 eligible live births reported to the Ontario birth registry, 31,295 were exposed to the H1N1 vaccine in utero. After adjustment, there were no significant differences in the women who did and did not receive vaccines during pregnancy, according to the study, published in the BMJ.
After a median follow-up of 5 years, 14% of children received an asthma diagnosis, with a median age at diagnosis of 1.8 years. Children were more likely to receive an asthma diagnosis if their mothers had a preexisting condition or if they were born preterm. At follow-up, 34% of children had at least one upper respiratory tract infection. Sensory disorder, neoplasm, and pediatric complex chronic condition were rare, each occurring in less than 1% of the study cohort (BMJ. 2019 Jul 10. doi: 10.1136/bmj.l4151).
No significant association was found between prenatal exposure to the H1N1 vaccine and upper or lower respiratory infections, otitis media, all infections, neoplasms, sensory disorders, rates of urgent and inpatient health services use, pediatric complex chronic conditions, or mortality. A weak but significant association was observed for asthma (adjusted hazard ratio, 1.05; 95% confidence interval, 1.02-1.09), and a weak inverse association was found for gastrointestinal infections (adjusted incidence rate ratio, 0.94; 95% CI, 0.91-0.98).
“Although we observed a small, but statistically significant, increase in pediatric asthma and a reduction in gastrointestinal infections, we are not aware of any biologic mechanisms to explain these findings. Future studies in different settings and with different influenza vaccine formulations will be important for developing the evidence base on longer-term pediatric outcomes following influenza vaccination during pregnancy,” the investigators concluded.
The study was funded by grants from the Canadian Institutes of Health Research and the Institute for Clinical Evaluative Sciences.
according to Laura K. Walsh of the University of Ottawa and associates.
The investigators conducted a population-based retrospective cohort study from November 2009 to October 2010 of all live births within the province of Ontario. Of the 104,249 eligible live births reported to the Ontario birth registry, 31,295 were exposed to the H1N1 vaccine in utero. After adjustment, there were no significant differences in the women who did and did not receive vaccines during pregnancy, according to the study, published in the BMJ.
After a median follow-up of 5 years, 14% of children received an asthma diagnosis, with a median age at diagnosis of 1.8 years. Children were more likely to receive an asthma diagnosis if their mothers had a preexisting condition or if they were born preterm. At follow-up, 34% of children had at least one upper respiratory tract infection. Sensory disorder, neoplasm, and pediatric complex chronic condition were rare, each occurring in less than 1% of the study cohort (BMJ. 2019 Jul 10. doi: 10.1136/bmj.l4151).
No significant association was found between prenatal exposure to the H1N1 vaccine and upper or lower respiratory infections, otitis media, all infections, neoplasms, sensory disorders, rates of urgent and inpatient health services use, pediatric complex chronic conditions, or mortality. A weak but significant association was observed for asthma (adjusted hazard ratio, 1.05; 95% confidence interval, 1.02-1.09), and a weak inverse association was found for gastrointestinal infections (adjusted incidence rate ratio, 0.94; 95% CI, 0.91-0.98).
“Although we observed a small, but statistically significant, increase in pediatric asthma and a reduction in gastrointestinal infections, we are not aware of any biologic mechanisms to explain these findings. Future studies in different settings and with different influenza vaccine formulations will be important for developing the evidence base on longer-term pediatric outcomes following influenza vaccination during pregnancy,” the investigators concluded.
The study was funded by grants from the Canadian Institutes of Health Research and the Institute for Clinical Evaluative Sciences.
FROM THE BMJ
Measles cases have slowed but not stopped
The United States continues to slowly add new cases of measles to 2019’s postelimination-record total, but California was officially removed from the outbreak list this week, according to the Centers for Disease Control and Prevention.
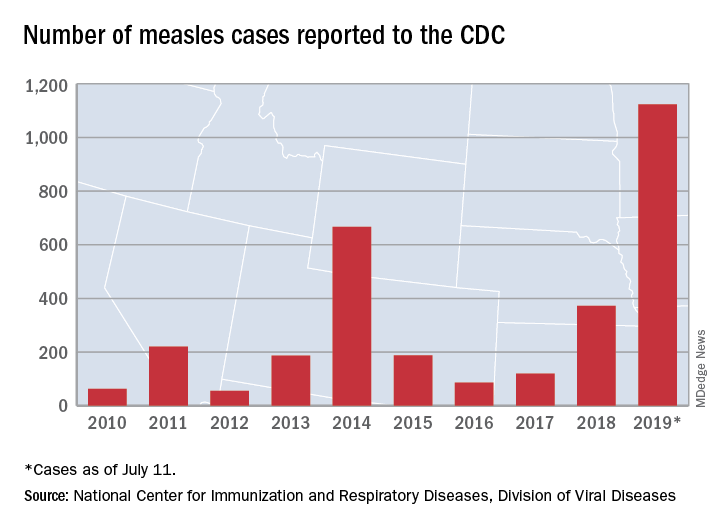
That is the highest number of cases reported since measles was declared eliminated in 2000 and the most in a single year since 1992.
The end of outbreak-related activity in California leaves three locations still dealing with ongoing cases: Rockland County, N.Y.; New York City; and King, Pierce, and Snohomish Counties in Washington, the CDC said.
Those three jurisdictions currently report the following:
- reported four new cases from July 3 to July 11 and is up to 175 cases for the year.
- had one new case from July 1 to July 8 and is now at 564 for the year.
- reported two cases from July 1 to July 10 and is now at 10 for the year (the other two counties have a total of three cases). Clark County in Washington reported 71 cases in an earlier, unrelated outbreak.
The United States continues to slowly add new cases of measles to 2019’s postelimination-record total, but California was officially removed from the outbreak list this week, according to the Centers for Disease Control and Prevention.

That is the highest number of cases reported since measles was declared eliminated in 2000 and the most in a single year since 1992.
The end of outbreak-related activity in California leaves three locations still dealing with ongoing cases: Rockland County, N.Y.; New York City; and King, Pierce, and Snohomish Counties in Washington, the CDC said.
Those three jurisdictions currently report the following:
- reported four new cases from July 3 to July 11 and is up to 175 cases for the year.
- had one new case from July 1 to July 8 and is now at 564 for the year.
- reported two cases from July 1 to July 10 and is now at 10 for the year (the other two counties have a total of three cases). Clark County in Washington reported 71 cases in an earlier, unrelated outbreak.
The United States continues to slowly add new cases of measles to 2019’s postelimination-record total, but California was officially removed from the outbreak list this week, according to the Centers for Disease Control and Prevention.

That is the highest number of cases reported since measles was declared eliminated in 2000 and the most in a single year since 1992.
The end of outbreak-related activity in California leaves three locations still dealing with ongoing cases: Rockland County, N.Y.; New York City; and King, Pierce, and Snohomish Counties in Washington, the CDC said.
Those three jurisdictions currently report the following:
- reported four new cases from July 3 to July 11 and is up to 175 cases for the year.
- had one new case from July 1 to July 8 and is now at 564 for the year.
- reported two cases from July 1 to July 10 and is now at 10 for the year (the other two counties have a total of three cases). Clark County in Washington reported 71 cases in an earlier, unrelated outbreak.
Cellulitis ranks as top reason for skin-related pediatric inpatient admissions
AUSTIN, TEX. – showed.
“Skin conditions significantly affect pediatric inpatients, and dermatologists ought be accessible for consultation to enhance care and costs,” the study’s first author, Marcus L. Elias, said in an interview prior to the annual meeting of the Society for Pediatric Dermatology.
According to Mr. Elias, who is a 4th-year medical student at Rutgers New Jersey Medical School–Newark, few national studies on skin diseases for pediatric inpatients have been published in the medical literature. Earlier this year, researchers examined inpatient dermatologic conditions in patients aged 18 years and older (J Am Acad Dermatol 2019;80[2]:425-32), but Mr. Elias and associates set out to analyze the burden of inpatient pediatric dermatologic conditions on a national basis. “We wanted to see if the same conditions that were hospitalizing adults were also hospitalizing kids,” he said. “We found that this was indeed the case.”
The researchers queried the National Inpatient Sample database for all cases involving patients aged 18 years and younger during 2001-2013. The search yielded a sample of 16,837,857 patients. From this, the researchers analyzed diagnosis-related groups for dermatologic conditions denoting the principal diagnosis at discharge, which left a final sample of 84,090 patients. Frequency and chi-squared tests were used to analyze categorical variables.
More than half of patients (54%) were male, 36% were white, 48% had Medicaid insurance, and 43% had private insurance. Mr. Elias reported that the median length of stay for patients was 2 days and the median cost of care was $6,289.50 for each case. More than three-quarters of pediatric inpatients with dermatologic diagnoses were treated for “cellulitis” (66,147 cases, or 79%), with most cases involving the legs (16,875 cases, or 20%). Other pediatric inpatients were admitted for “minor skin disorder without complications” (5,458 cases, or 7%), and “minor skin disorder with complications” (2,822 cases, or 3%). A total of 64 patients died during the study period. Of these, 31 cases (50%) involved “skin graft and/or debridement of skin ulcer or cellulitis without complications,” the study found.
“We were surprised that the major cause of mortality for our patients was classified as ‘skin graft and/or debridement of skin ulcer or cellulitis without complications,’ as a similar diagnosis-related groupings exist denoting that complications did arise,” Mr. Elias said. “Still, it is not possible for us to determine if the mortality was from the skin graft/debridement or another cause entirely. It is possible that the procedure was without complications, only to have the patient succumb to an ancillary process.”
He acknowledged certain limitations of the study, including the fact that the function of dermatologic consults for hospitalized patients was not examined. “We also cannot draw conclusions as to whether improved outpatient therapy reduces the need for hospitalization,” he said. Mr. Elias reported having no financial disclosures.
AUSTIN, TEX. – showed.
“Skin conditions significantly affect pediatric inpatients, and dermatologists ought be accessible for consultation to enhance care and costs,” the study’s first author, Marcus L. Elias, said in an interview prior to the annual meeting of the Society for Pediatric Dermatology.
According to Mr. Elias, who is a 4th-year medical student at Rutgers New Jersey Medical School–Newark, few national studies on skin diseases for pediatric inpatients have been published in the medical literature. Earlier this year, researchers examined inpatient dermatologic conditions in patients aged 18 years and older (J Am Acad Dermatol 2019;80[2]:425-32), but Mr. Elias and associates set out to analyze the burden of inpatient pediatric dermatologic conditions on a national basis. “We wanted to see if the same conditions that were hospitalizing adults were also hospitalizing kids,” he said. “We found that this was indeed the case.”
The researchers queried the National Inpatient Sample database for all cases involving patients aged 18 years and younger during 2001-2013. The search yielded a sample of 16,837,857 patients. From this, the researchers analyzed diagnosis-related groups for dermatologic conditions denoting the principal diagnosis at discharge, which left a final sample of 84,090 patients. Frequency and chi-squared tests were used to analyze categorical variables.
More than half of patients (54%) were male, 36% were white, 48% had Medicaid insurance, and 43% had private insurance. Mr. Elias reported that the median length of stay for patients was 2 days and the median cost of care was $6,289.50 for each case. More than three-quarters of pediatric inpatients with dermatologic diagnoses were treated for “cellulitis” (66,147 cases, or 79%), with most cases involving the legs (16,875 cases, or 20%). Other pediatric inpatients were admitted for “minor skin disorder without complications” (5,458 cases, or 7%), and “minor skin disorder with complications” (2,822 cases, or 3%). A total of 64 patients died during the study period. Of these, 31 cases (50%) involved “skin graft and/or debridement of skin ulcer or cellulitis without complications,” the study found.
“We were surprised that the major cause of mortality for our patients was classified as ‘skin graft and/or debridement of skin ulcer or cellulitis without complications,’ as a similar diagnosis-related groupings exist denoting that complications did arise,” Mr. Elias said. “Still, it is not possible for us to determine if the mortality was from the skin graft/debridement or another cause entirely. It is possible that the procedure was without complications, only to have the patient succumb to an ancillary process.”
He acknowledged certain limitations of the study, including the fact that the function of dermatologic consults for hospitalized patients was not examined. “We also cannot draw conclusions as to whether improved outpatient therapy reduces the need for hospitalization,” he said. Mr. Elias reported having no financial disclosures.
AUSTIN, TEX. – showed.
“Skin conditions significantly affect pediatric inpatients, and dermatologists ought be accessible for consultation to enhance care and costs,” the study’s first author, Marcus L. Elias, said in an interview prior to the annual meeting of the Society for Pediatric Dermatology.
According to Mr. Elias, who is a 4th-year medical student at Rutgers New Jersey Medical School–Newark, few national studies on skin diseases for pediatric inpatients have been published in the medical literature. Earlier this year, researchers examined inpatient dermatologic conditions in patients aged 18 years and older (J Am Acad Dermatol 2019;80[2]:425-32), but Mr. Elias and associates set out to analyze the burden of inpatient pediatric dermatologic conditions on a national basis. “We wanted to see if the same conditions that were hospitalizing adults were also hospitalizing kids,” he said. “We found that this was indeed the case.”
The researchers queried the National Inpatient Sample database for all cases involving patients aged 18 years and younger during 2001-2013. The search yielded a sample of 16,837,857 patients. From this, the researchers analyzed diagnosis-related groups for dermatologic conditions denoting the principal diagnosis at discharge, which left a final sample of 84,090 patients. Frequency and chi-squared tests were used to analyze categorical variables.
More than half of patients (54%) were male, 36% were white, 48% had Medicaid insurance, and 43% had private insurance. Mr. Elias reported that the median length of stay for patients was 2 days and the median cost of care was $6,289.50 for each case. More than three-quarters of pediatric inpatients with dermatologic diagnoses were treated for “cellulitis” (66,147 cases, or 79%), with most cases involving the legs (16,875 cases, or 20%). Other pediatric inpatients were admitted for “minor skin disorder without complications” (5,458 cases, or 7%), and “minor skin disorder with complications” (2,822 cases, or 3%). A total of 64 patients died during the study period. Of these, 31 cases (50%) involved “skin graft and/or debridement of skin ulcer or cellulitis without complications,” the study found.
“We were surprised that the major cause of mortality for our patients was classified as ‘skin graft and/or debridement of skin ulcer or cellulitis without complications,’ as a similar diagnosis-related groupings exist denoting that complications did arise,” Mr. Elias said. “Still, it is not possible for us to determine if the mortality was from the skin graft/debridement or another cause entirely. It is possible that the procedure was without complications, only to have the patient succumb to an ancillary process.”
He acknowledged certain limitations of the study, including the fact that the function of dermatologic consults for hospitalized patients was not examined. “We also cannot draw conclusions as to whether improved outpatient therapy reduces the need for hospitalization,” he said. Mr. Elias reported having no financial disclosures.
REPORTING FROM SPD 2019
Key clinical point: Cellulitis is the cause of the majority of skin-related pediatric inpatient admissions in the United States.
Major finding: In all, 79% of pediatric inpatients with dermatologic diagnoses were treated for cellulitis.
Study details: An analysis of data from 84,090 patients younger than age 18 in the National Inpatient Sample.
Disclosures: The researchers reported having no financial disclosures.
Patients with COPD at heightened risk for community-acquired pneumonia requiring hospitalization
Patients with chronic obstructive pulmonary disease are at a significantly increased risk for hospitalization for community-acquired pneumonia (CAP), compared with patients without COPD, a large prospective study has found.
Jose Bordon, MD, and colleagues aimed to define incidence and outcomes of COPD patients hospitalized with pneumonia in the city of Louisville, Ky., and to extrapolate the burden of disease in the U.S. population. They conducted a secondary analysis of data from the University of Louisville Pneumonia Study, a prospective population-based cohort study of all hospitalized adults with CAP who were residents in the city of Louisville, Ky., from June 1, 2014, to May 31, 2016.
COPD prevalence in the city of Louisville was derived via data from the 2014 Behavioral Risk Factor Surveillance System (BRFSS) as well as from the 2014 National Health Interview Survey (NHIS). In addition, the researchers analyzed clinical outcomes including time to clinical stability (TCS), length of hospital stay (LOS), and mortality, according to Dr. Bordon, an infectious disease specialist at Providence Health Center, Washington, and colleagues on behalf of the University of Louisville Pneumonia Study Group.
The researchers found an 18-fold greater incidence of community-acquired pneumonia in patients with COPD, compared with non-COPD patients.
A total of 18,246 individuals aged 40 and older with COPD were estimated to live in Louisville, Ky. The researchers found that 3,419 COPD patients were hospitalized due to CAP in Louisville during the 2-year study period. COPD patients, compared with non-COPD patients, were more likely to have a history of heart failure, more ICU admissions, and use of mechanical ventilation, compared with patients without COPD. The two groups had similar pneumonia severity index scores, and 17% received oral steroids prior to admission. COPD patients had more pneumococcal pneumonia, despite receiving pneumococcal vaccine significantly more often than non-COPD patients.
The annual incidence of hospitalized CAP was 9,369 cases per 100,000 COPD patients in the city of Louisville. In the same period, the incidence of CAP in patients without COPD was 509 per 100,000, a more than 18-fold difference.
Although the incidence of CAP in COPD patients was much higher than in those without, the difference didn’t appear to have an impact on clinical outcomes. There were no clinical differences among patients with vs. without COPD in regard to time to reach clinical improvement and time of hospital discharge, and in-hospital mortality was not statistically significantly different between the groups, the authors reported. The mortality of COPD patients during hospitalization, at 30 days, at 6 months, and at 1 year was 5.6% of patients, 11.9%, 24.3%, and 33.0%, respectively vs. 6.6%, 14.2%, 24.2%, and 30.1% in non-COPD patients. However, 1-year all-cause mortality was a significant 25% greater among COPD patients, as might be expected by the progression and effects of the underlying disease.
“[Our] observations mean that nearly 1 in 10 persons with COPD will be hospitalized annually due to CAP. This translates into approximately 500,000 COPD patients hospitalized with CAP every year in the U.S., resulting in a substantial burden of approximately 5 billion U.S. dollars in hospitalization costs,” the researchers stated.
“Modifiable factors associated with CAP such as tobacco smoking and immunizations should be health interventions to prevent the burden of CAP in COPD patients,” even though “pneumococcal vaccination was used more often in the COPD population than in other CAP patients, but pneumococcal pneumonia still occurred at a numerically higher rate,” they noted.
The study was supported by the University of Louisville, Ky., with partial support from Pfizer. The authors reported having no conflicts.
SOURCE: Bordon JM et al. Clin Microbiol Infect. 2019 Jun 26; doi: 10.1016/j.cmi.2019.06.025.
Patients with chronic obstructive pulmonary disease are at a significantly increased risk for hospitalization for community-acquired pneumonia (CAP), compared with patients without COPD, a large prospective study has found.
Jose Bordon, MD, and colleagues aimed to define incidence and outcomes of COPD patients hospitalized with pneumonia in the city of Louisville, Ky., and to extrapolate the burden of disease in the U.S. population. They conducted a secondary analysis of data from the University of Louisville Pneumonia Study, a prospective population-based cohort study of all hospitalized adults with CAP who were residents in the city of Louisville, Ky., from June 1, 2014, to May 31, 2016.
COPD prevalence in the city of Louisville was derived via data from the 2014 Behavioral Risk Factor Surveillance System (BRFSS) as well as from the 2014 National Health Interview Survey (NHIS). In addition, the researchers analyzed clinical outcomes including time to clinical stability (TCS), length of hospital stay (LOS), and mortality, according to Dr. Bordon, an infectious disease specialist at Providence Health Center, Washington, and colleagues on behalf of the University of Louisville Pneumonia Study Group.
The researchers found an 18-fold greater incidence of community-acquired pneumonia in patients with COPD, compared with non-COPD patients.
A total of 18,246 individuals aged 40 and older with COPD were estimated to live in Louisville, Ky. The researchers found that 3,419 COPD patients were hospitalized due to CAP in Louisville during the 2-year study period. COPD patients, compared with non-COPD patients, were more likely to have a history of heart failure, more ICU admissions, and use of mechanical ventilation, compared with patients without COPD. The two groups had similar pneumonia severity index scores, and 17% received oral steroids prior to admission. COPD patients had more pneumococcal pneumonia, despite receiving pneumococcal vaccine significantly more often than non-COPD patients.
The annual incidence of hospitalized CAP was 9,369 cases per 100,000 COPD patients in the city of Louisville. In the same period, the incidence of CAP in patients without COPD was 509 per 100,000, a more than 18-fold difference.
Although the incidence of CAP in COPD patients was much higher than in those without, the difference didn’t appear to have an impact on clinical outcomes. There were no clinical differences among patients with vs. without COPD in regard to time to reach clinical improvement and time of hospital discharge, and in-hospital mortality was not statistically significantly different between the groups, the authors reported. The mortality of COPD patients during hospitalization, at 30 days, at 6 months, and at 1 year was 5.6% of patients, 11.9%, 24.3%, and 33.0%, respectively vs. 6.6%, 14.2%, 24.2%, and 30.1% in non-COPD patients. However, 1-year all-cause mortality was a significant 25% greater among COPD patients, as might be expected by the progression and effects of the underlying disease.
“[Our] observations mean that nearly 1 in 10 persons with COPD will be hospitalized annually due to CAP. This translates into approximately 500,000 COPD patients hospitalized with CAP every year in the U.S., resulting in a substantial burden of approximately 5 billion U.S. dollars in hospitalization costs,” the researchers stated.
“Modifiable factors associated with CAP such as tobacco smoking and immunizations should be health interventions to prevent the burden of CAP in COPD patients,” even though “pneumococcal vaccination was used more often in the COPD population than in other CAP patients, but pneumococcal pneumonia still occurred at a numerically higher rate,” they noted.
The study was supported by the University of Louisville, Ky., with partial support from Pfizer. The authors reported having no conflicts.
SOURCE: Bordon JM et al. Clin Microbiol Infect. 2019 Jun 26; doi: 10.1016/j.cmi.2019.06.025.
Patients with chronic obstructive pulmonary disease are at a significantly increased risk for hospitalization for community-acquired pneumonia (CAP), compared with patients without COPD, a large prospective study has found.
Jose Bordon, MD, and colleagues aimed to define incidence and outcomes of COPD patients hospitalized with pneumonia in the city of Louisville, Ky., and to extrapolate the burden of disease in the U.S. population. They conducted a secondary analysis of data from the University of Louisville Pneumonia Study, a prospective population-based cohort study of all hospitalized adults with CAP who were residents in the city of Louisville, Ky., from June 1, 2014, to May 31, 2016.
COPD prevalence in the city of Louisville was derived via data from the 2014 Behavioral Risk Factor Surveillance System (BRFSS) as well as from the 2014 National Health Interview Survey (NHIS). In addition, the researchers analyzed clinical outcomes including time to clinical stability (TCS), length of hospital stay (LOS), and mortality, according to Dr. Bordon, an infectious disease specialist at Providence Health Center, Washington, and colleagues on behalf of the University of Louisville Pneumonia Study Group.
The researchers found an 18-fold greater incidence of community-acquired pneumonia in patients with COPD, compared with non-COPD patients.
A total of 18,246 individuals aged 40 and older with COPD were estimated to live in Louisville, Ky. The researchers found that 3,419 COPD patients were hospitalized due to CAP in Louisville during the 2-year study period. COPD patients, compared with non-COPD patients, were more likely to have a history of heart failure, more ICU admissions, and use of mechanical ventilation, compared with patients without COPD. The two groups had similar pneumonia severity index scores, and 17% received oral steroids prior to admission. COPD patients had more pneumococcal pneumonia, despite receiving pneumococcal vaccine significantly more often than non-COPD patients.
The annual incidence of hospitalized CAP was 9,369 cases per 100,000 COPD patients in the city of Louisville. In the same period, the incidence of CAP in patients without COPD was 509 per 100,000, a more than 18-fold difference.
Although the incidence of CAP in COPD patients was much higher than in those without, the difference didn’t appear to have an impact on clinical outcomes. There were no clinical differences among patients with vs. without COPD in regard to time to reach clinical improvement and time of hospital discharge, and in-hospital mortality was not statistically significantly different between the groups, the authors reported. The mortality of COPD patients during hospitalization, at 30 days, at 6 months, and at 1 year was 5.6% of patients, 11.9%, 24.3%, and 33.0%, respectively vs. 6.6%, 14.2%, 24.2%, and 30.1% in non-COPD patients. However, 1-year all-cause mortality was a significant 25% greater among COPD patients, as might be expected by the progression and effects of the underlying disease.
“[Our] observations mean that nearly 1 in 10 persons with COPD will be hospitalized annually due to CAP. This translates into approximately 500,000 COPD patients hospitalized with CAP every year in the U.S., resulting in a substantial burden of approximately 5 billion U.S. dollars in hospitalization costs,” the researchers stated.
“Modifiable factors associated with CAP such as tobacco smoking and immunizations should be health interventions to prevent the burden of CAP in COPD patients,” even though “pneumococcal vaccination was used more often in the COPD population than in other CAP patients, but pneumococcal pneumonia still occurred at a numerically higher rate,” they noted.
The study was supported by the University of Louisville, Ky., with partial support from Pfizer. The authors reported having no conflicts.
SOURCE: Bordon JM et al. Clin Microbiol Infect. 2019 Jun 26; doi: 10.1016/j.cmi.2019.06.025.
FROM CLINICAL MICROBIOLOGY AND INFECTION
Parent education improves pediatric influenza vaccination rates
according to a randomized clinical trial published in Pediatrics.
Vanessa P. Scott, MD, MS, of Columbia University, New York, and colleagues randomized 400 parent-child dyads into any of three arms: receiving a handout based on national data, receiving a handout based on local data, or receiving usual care. This convenience sample was drawn from two pediatric clinics in New York between August 2016 and March 2017.
After adjustment for parents’ education level, the trial found that parents who received either handout were significantly more likely than were those receiving usual care to vaccinate their children by the end of season (75% and 65%, respectively; adjusted odds ratio, 1.68; 95% confidence interval, 1.06-2.67), but the effects of any intervention versus those of usual care on vaccination on day of visit were not statistically significant (59% vs. 53%; aOR, 1.36; 95% CI, 0.89-2.09).The researchers had hoped that using a targeted approach based on local data would increase vaccine receipt, but that was not seen in the results.
They did find that, across all three arms in the trial, baseline parental intent to vaccinate (likely versus unlikely) was associated with vaccination rates: Both vaccination on clinic visit day (70% vs. 22%; aOR, 8.38; 95% CI, 4.85-14.34) and vaccination by end of season (87% vs. 29%; aOR, 18.26; 95% CI, 9.94-33.52) were affected.
Strengths of the study included the randomized, controlled design and assessment of baseline factors, such as intention to vaccinate, to reduce confounding effects. Limitations included use of a convenience sample, which could have introduced selection bias.
One author was an unremunerated coinvestigator of an unrelated trial that received an investigator-initiated grant from the Pfizer Medical Education Group. Two authors were funded by other grants, but no potential conflicts of interests to disclose were indicated by any of the authors in this study.
SOURCE: Scott VP et al. Pediatrics. 2019. doi: 10.1542/peds.2018-2580.
according to a randomized clinical trial published in Pediatrics.
Vanessa P. Scott, MD, MS, of Columbia University, New York, and colleagues randomized 400 parent-child dyads into any of three arms: receiving a handout based on national data, receiving a handout based on local data, or receiving usual care. This convenience sample was drawn from two pediatric clinics in New York between August 2016 and March 2017.
After adjustment for parents’ education level, the trial found that parents who received either handout were significantly more likely than were those receiving usual care to vaccinate their children by the end of season (75% and 65%, respectively; adjusted odds ratio, 1.68; 95% confidence interval, 1.06-2.67), but the effects of any intervention versus those of usual care on vaccination on day of visit were not statistically significant (59% vs. 53%; aOR, 1.36; 95% CI, 0.89-2.09).The researchers had hoped that using a targeted approach based on local data would increase vaccine receipt, but that was not seen in the results.
They did find that, across all three arms in the trial, baseline parental intent to vaccinate (likely versus unlikely) was associated with vaccination rates: Both vaccination on clinic visit day (70% vs. 22%; aOR, 8.38; 95% CI, 4.85-14.34) and vaccination by end of season (87% vs. 29%; aOR, 18.26; 95% CI, 9.94-33.52) were affected.
Strengths of the study included the randomized, controlled design and assessment of baseline factors, such as intention to vaccinate, to reduce confounding effects. Limitations included use of a convenience sample, which could have introduced selection bias.
One author was an unremunerated coinvestigator of an unrelated trial that received an investigator-initiated grant from the Pfizer Medical Education Group. Two authors were funded by other grants, but no potential conflicts of interests to disclose were indicated by any of the authors in this study.
SOURCE: Scott VP et al. Pediatrics. 2019. doi: 10.1542/peds.2018-2580.
according to a randomized clinical trial published in Pediatrics.
Vanessa P. Scott, MD, MS, of Columbia University, New York, and colleagues randomized 400 parent-child dyads into any of three arms: receiving a handout based on national data, receiving a handout based on local data, or receiving usual care. This convenience sample was drawn from two pediatric clinics in New York between August 2016 and March 2017.
After adjustment for parents’ education level, the trial found that parents who received either handout were significantly more likely than were those receiving usual care to vaccinate their children by the end of season (75% and 65%, respectively; adjusted odds ratio, 1.68; 95% confidence interval, 1.06-2.67), but the effects of any intervention versus those of usual care on vaccination on day of visit were not statistically significant (59% vs. 53%; aOR, 1.36; 95% CI, 0.89-2.09).The researchers had hoped that using a targeted approach based on local data would increase vaccine receipt, but that was not seen in the results.
They did find that, across all three arms in the trial, baseline parental intent to vaccinate (likely versus unlikely) was associated with vaccination rates: Both vaccination on clinic visit day (70% vs. 22%; aOR, 8.38; 95% CI, 4.85-14.34) and vaccination by end of season (87% vs. 29%; aOR, 18.26; 95% CI, 9.94-33.52) were affected.
Strengths of the study included the randomized, controlled design and assessment of baseline factors, such as intention to vaccinate, to reduce confounding effects. Limitations included use of a convenience sample, which could have introduced selection bias.
One author was an unremunerated coinvestigator of an unrelated trial that received an investigator-initiated grant from the Pfizer Medical Education Group. Two authors were funded by other grants, but no potential conflicts of interests to disclose were indicated by any of the authors in this study.
SOURCE: Scott VP et al. Pediatrics. 2019. doi: 10.1542/peds.2018-2580.
FROM PEDIATRICS
Teen mothers using long-acting reversible contraception are least likely to use condoms
such as the birth control pill, vaginal ring, contraceptive patch, or injection, according to research in JAMA Pediatrics.
This highlights a need for education to lower the risk of sexually transmitted infections in this population.
“Our finding that less than 30% of sexually active teenage mothers using LARC or non-LARC hormonal methods also reported using condoms suggests the need for enhanced efforts to increase condom use among teenage mothers,” wrote Katherine Kortsmit, PhD, MPH, of the National Center for Chronic Disease Prevention and Health Promotion at the Centers for Disease Control and Prevention, Atlanta, and colleagues.
The researchers performed a cross-sectional analysis of contraceptive use among 5,480 new teenage mothers between 2012 and 2015 who were aged 19 years or younger in the Pregnancy Risk Assessment Monitoring System (PRAMS). Participants were mainly first-time teenage mothers between ages 18 and 19 years (46% non-Hispanic white), current Medicaid users, and reported an unintended pregnancy. Dr. Kortsmit and colleagues monitored use of LARC and non-LARC hormonal methods, including condom use, among participants in PRAMS from 37 different sites.
Among teenage mothers in PRAM, 29% reported using condoms; 18% of mothers using LARC said they also used condoms, compared with 36% of mothers who used non-LARC hormonal methods (adjusted prevalence ratio, 0.50; 95% confidence interval, 0.41-0.60). Participants with IUDs were least likely to report using condoms (15%), compared with participants using implants (22%; aPR, 0.70; 95% CI, 0.51-0.98), participants using the patch, ring, or injection (25%; aPR, 0.61; 95% CI, 0.47-0.79), or the pill (47%; aPR, 0.32; 95% CI, 0.25-0.40).
“These findings can be used to inform clinician counseling that sexually active teenage mothers have low uptake of condom use combined with more effective contraceptive methods and may need additional counseling on the importance of consistent and correct condom use for the prevention of STIs,” Dr. Kortsmit and associates wrote.
Limitations included the self-reported nature of the study, and lack of information on baseline condom use prior to pregnancy, relationship characteristics, and sexual partners during the postpartum period.
Education on contraceptive methods by clinicians is an important part of an adolescent’s contextualization of the benefits and risks of those methods, especially for women of color and marginalized groups, Andrea J. Hoopes, MD, MPH; and Gina S. Sucato, MD, MPH, wrote in an editorial related to the study by Kortsmit and colleagues.
In particular, these groups have higher rates of unplanned pregnancy, may have a history of being coerced to use contraception, and may be reluctant to discuss their sexual history or contraception use. “Many young women, including teenage mothers, remain at risk for coercion from partners, family members, and health care clinicians, so adopting a stance that ensures autonomy while eliciting unique developmental perspectives is paramount,” they said.
It is critically important to give women access to LARCs that are effective and easily used, and patients have a right to choose the contraception method that best fits their situation. It is through integrated programs, made available by Title X funding, that clinicians may be able to monitor their patients’ sexual, reproductive, and psychological health needs, and have conversations about the importance of contraception and prevention of sexually transmitted infections.
“Future studies should examine specific interventions aimed at promoting all adolescents’ motivations to remain safe and healthy by using condoms consistently and by seeking comprehensive sexual health care services, regardless of contraceptive method,” concluded Dr. Hoopes and Dr. Sucato, of the Adolescent Center at Kaiser Permanente Washington in Seattle. “In addition to receiving counseling about, and access to, condoms, adolescents need to develop the skills to negotiate condom use with partners.”
Dr. Kortsmit received support in the form of an appointment to the Research Participation Program at Centers for Disease Control and Prevention through an interagency agreement. The other authors reported no conflicts of interest.
Dr. Hoopes reported previous grant support from Bayer and the North American Society for Pediatric and Adolescent Gynecology. Dr. Sucato reported previous grant and other research support from Teva.
SOURCE: Kortsmit K et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2019.1136; Hoopes AJ et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2019.1133.
such as the birth control pill, vaginal ring, contraceptive patch, or injection, according to research in JAMA Pediatrics.
This highlights a need for education to lower the risk of sexually transmitted infections in this population.
“Our finding that less than 30% of sexually active teenage mothers using LARC or non-LARC hormonal methods also reported using condoms suggests the need for enhanced efforts to increase condom use among teenage mothers,” wrote Katherine Kortsmit, PhD, MPH, of the National Center for Chronic Disease Prevention and Health Promotion at the Centers for Disease Control and Prevention, Atlanta, and colleagues.
The researchers performed a cross-sectional analysis of contraceptive use among 5,480 new teenage mothers between 2012 and 2015 who were aged 19 years or younger in the Pregnancy Risk Assessment Monitoring System (PRAMS). Participants were mainly first-time teenage mothers between ages 18 and 19 years (46% non-Hispanic white), current Medicaid users, and reported an unintended pregnancy. Dr. Kortsmit and colleagues monitored use of LARC and non-LARC hormonal methods, including condom use, among participants in PRAMS from 37 different sites.
Among teenage mothers in PRAM, 29% reported using condoms; 18% of mothers using LARC said they also used condoms, compared with 36% of mothers who used non-LARC hormonal methods (adjusted prevalence ratio, 0.50; 95% confidence interval, 0.41-0.60). Participants with IUDs were least likely to report using condoms (15%), compared with participants using implants (22%; aPR, 0.70; 95% CI, 0.51-0.98), participants using the patch, ring, or injection (25%; aPR, 0.61; 95% CI, 0.47-0.79), or the pill (47%; aPR, 0.32; 95% CI, 0.25-0.40).
“These findings can be used to inform clinician counseling that sexually active teenage mothers have low uptake of condom use combined with more effective contraceptive methods and may need additional counseling on the importance of consistent and correct condom use for the prevention of STIs,” Dr. Kortsmit and associates wrote.
Limitations included the self-reported nature of the study, and lack of information on baseline condom use prior to pregnancy, relationship characteristics, and sexual partners during the postpartum period.
Education on contraceptive methods by clinicians is an important part of an adolescent’s contextualization of the benefits and risks of those methods, especially for women of color and marginalized groups, Andrea J. Hoopes, MD, MPH; and Gina S. Sucato, MD, MPH, wrote in an editorial related to the study by Kortsmit and colleagues.
In particular, these groups have higher rates of unplanned pregnancy, may have a history of being coerced to use contraception, and may be reluctant to discuss their sexual history or contraception use. “Many young women, including teenage mothers, remain at risk for coercion from partners, family members, and health care clinicians, so adopting a stance that ensures autonomy while eliciting unique developmental perspectives is paramount,” they said.
It is critically important to give women access to LARCs that are effective and easily used, and patients have a right to choose the contraception method that best fits their situation. It is through integrated programs, made available by Title X funding, that clinicians may be able to monitor their patients’ sexual, reproductive, and psychological health needs, and have conversations about the importance of contraception and prevention of sexually transmitted infections.
“Future studies should examine specific interventions aimed at promoting all adolescents’ motivations to remain safe and healthy by using condoms consistently and by seeking comprehensive sexual health care services, regardless of contraceptive method,” concluded Dr. Hoopes and Dr. Sucato, of the Adolescent Center at Kaiser Permanente Washington in Seattle. “In addition to receiving counseling about, and access to, condoms, adolescents need to develop the skills to negotiate condom use with partners.”
Dr. Kortsmit received support in the form of an appointment to the Research Participation Program at Centers for Disease Control and Prevention through an interagency agreement. The other authors reported no conflicts of interest.
Dr. Hoopes reported previous grant support from Bayer and the North American Society for Pediatric and Adolescent Gynecology. Dr. Sucato reported previous grant and other research support from Teva.
SOURCE: Kortsmit K et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2019.1136; Hoopes AJ et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2019.1133.
such as the birth control pill, vaginal ring, contraceptive patch, or injection, according to research in JAMA Pediatrics.
This highlights a need for education to lower the risk of sexually transmitted infections in this population.
“Our finding that less than 30% of sexually active teenage mothers using LARC or non-LARC hormonal methods also reported using condoms suggests the need for enhanced efforts to increase condom use among teenage mothers,” wrote Katherine Kortsmit, PhD, MPH, of the National Center for Chronic Disease Prevention and Health Promotion at the Centers for Disease Control and Prevention, Atlanta, and colleagues.
The researchers performed a cross-sectional analysis of contraceptive use among 5,480 new teenage mothers between 2012 and 2015 who were aged 19 years or younger in the Pregnancy Risk Assessment Monitoring System (PRAMS). Participants were mainly first-time teenage mothers between ages 18 and 19 years (46% non-Hispanic white), current Medicaid users, and reported an unintended pregnancy. Dr. Kortsmit and colleagues monitored use of LARC and non-LARC hormonal methods, including condom use, among participants in PRAMS from 37 different sites.
Among teenage mothers in PRAM, 29% reported using condoms; 18% of mothers using LARC said they also used condoms, compared with 36% of mothers who used non-LARC hormonal methods (adjusted prevalence ratio, 0.50; 95% confidence interval, 0.41-0.60). Participants with IUDs were least likely to report using condoms (15%), compared with participants using implants (22%; aPR, 0.70; 95% CI, 0.51-0.98), participants using the patch, ring, or injection (25%; aPR, 0.61; 95% CI, 0.47-0.79), or the pill (47%; aPR, 0.32; 95% CI, 0.25-0.40).
“These findings can be used to inform clinician counseling that sexually active teenage mothers have low uptake of condom use combined with more effective contraceptive methods and may need additional counseling on the importance of consistent and correct condom use for the prevention of STIs,” Dr. Kortsmit and associates wrote.
Limitations included the self-reported nature of the study, and lack of information on baseline condom use prior to pregnancy, relationship characteristics, and sexual partners during the postpartum period.
Education on contraceptive methods by clinicians is an important part of an adolescent’s contextualization of the benefits and risks of those methods, especially for women of color and marginalized groups, Andrea J. Hoopes, MD, MPH; and Gina S. Sucato, MD, MPH, wrote in an editorial related to the study by Kortsmit and colleagues.
In particular, these groups have higher rates of unplanned pregnancy, may have a history of being coerced to use contraception, and may be reluctant to discuss their sexual history or contraception use. “Many young women, including teenage mothers, remain at risk for coercion from partners, family members, and health care clinicians, so adopting a stance that ensures autonomy while eliciting unique developmental perspectives is paramount,” they said.
It is critically important to give women access to LARCs that are effective and easily used, and patients have a right to choose the contraception method that best fits their situation. It is through integrated programs, made available by Title X funding, that clinicians may be able to monitor their patients’ sexual, reproductive, and psychological health needs, and have conversations about the importance of contraception and prevention of sexually transmitted infections.
“Future studies should examine specific interventions aimed at promoting all adolescents’ motivations to remain safe and healthy by using condoms consistently and by seeking comprehensive sexual health care services, regardless of contraceptive method,” concluded Dr. Hoopes and Dr. Sucato, of the Adolescent Center at Kaiser Permanente Washington in Seattle. “In addition to receiving counseling about, and access to, condoms, adolescents need to develop the skills to negotiate condom use with partners.”
Dr. Kortsmit received support in the form of an appointment to the Research Participation Program at Centers for Disease Control and Prevention through an interagency agreement. The other authors reported no conflicts of interest.
Dr. Hoopes reported previous grant support from Bayer and the North American Society for Pediatric and Adolescent Gynecology. Dr. Sucato reported previous grant and other research support from Teva.
SOURCE: Kortsmit K et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2019.1136; Hoopes AJ et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2019.1133.
FROM JAMA PEDIATRICS
CDC: Look for early symptoms of acute flaccid myelitis, report suspected cases
the CDC said in a telebriefing.
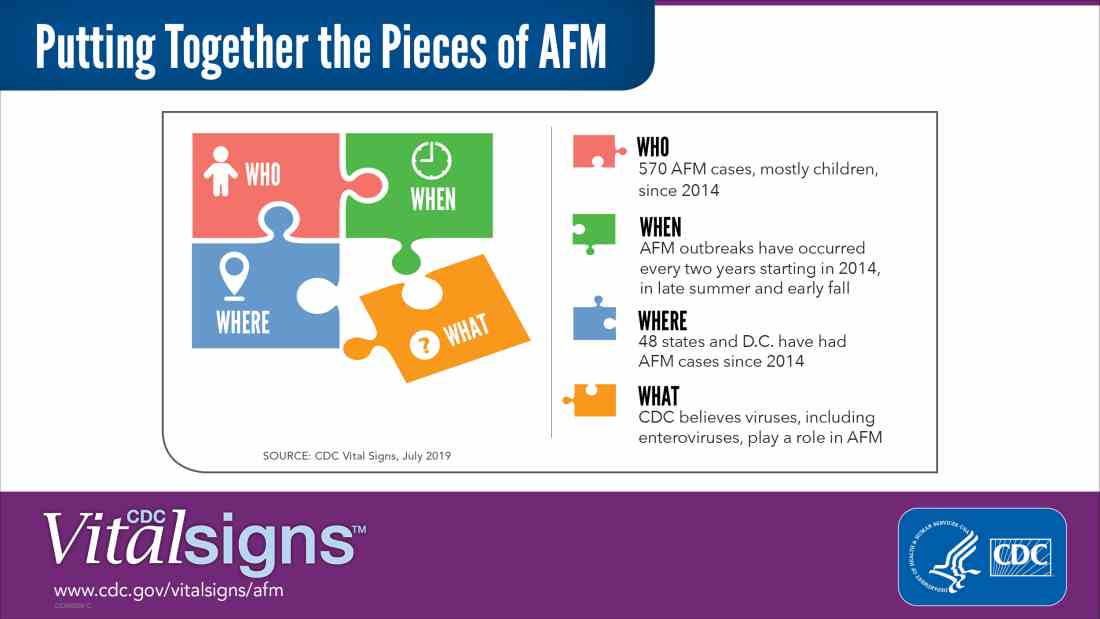
Acute flaccid myelitis (AFM) is defined as acute, flaccid muscle weakness that occurs less than 1 week after a fever or respiratory illness. Viruses, including enterovirus, are believed to play a role in AFM, but the cause still is unknown. The disease appears mostly in children, and the average age of a patient diagnosed with AFM is 5 years.
“Doctors and other clinicians in the United States play a critical role,” Anne Schuchat, MD, principal deputy director of the Centers for Disease Control and Prevention, said in the telebriefing. “We ask for your help with early recognition of patients with AFM symptoms, prompt specimen collection for testing, and immediate reporting of suspected AFM cases to health departments.”
While there is no proven treatment for AFM, early diagnosis is critical to getting patients the best care possible, according to a Vital Signs report released today. This means that clinicians should not wait for the CDC’s case definition before diagnosis, the CDC said.
“When specimens are collected as soon as possible after symptom onset, we have a better chance of understanding the causes of AFM, these recurrent outbreaks, and developing a diagnostic test,” Dr. Schuchat said. “Rapid reporting also helps us to identify and respond to outbreaks early and alert other clinicians and the public.”
AFM appears to follow a seasonal and biennial pattern, with the number of cases increasing mainly in the late summer and early fall. As the season approaches where AFM cases increase, CDC is asking clinicians to look out for patients with suspected AFM so cases can be reported as early as possible.
Since the CDC began tracking AFM, the number of cases has risen every 2 years. In 2018, there were 233 cases in 41 states, the highest number of reported cases since the CDC began tracking AFM following an outbreak in 2014, according to a Vital Signs report. Overall, there have been 570 cases of AFM reported in 48 states and the District of Columbia since 2014.
There is yet to be a confirmatory test for AFM, but clinicians should obtain cerebrospinal fluid, serum, stool and nasopharyngeal swab from patients with suspected AFM as soon as possible, followed by an MRI. AFM has unique MRI features , such as gray matter involvement, that can help distinguish it from other diseases characterized by acute weakness.
In the Vital Signs report, which examined AFM in 2018, 92% of confirmed cases had respiratory symptoms or fever, and 42% of confirmed cases had upper limb involvement. The median time from limb weakness to hospitalization was 1 day, and time from weakness to MRI was 2 days. Cases were reported to the CDC a median of 18 days from onset of limb weakness, but time to reporting ranged between 18 days and 36 days, said Tom Clark, MD, MPH, deputy director of the division of viral diseases at CDC.
“This delay hampers our ability to understand the causes AFM,” he said. “We believe that recognizing AFM early is critical and can lead to better patient management.”
In lieu of a diagnostic test for AFM, clinicians should make management decisions through review of patient symptoms, exam findings, MRI, other test results, and in consulting with neurology experts. The Transverse Myelitis Association also has created a support portal for 24/7 physician consultation in AFM cases.
SOURCE: Lopez A et al. MMWR Morb Mortal Wkly Rep. 2019;68:1-7 .
the CDC said in a telebriefing.

Acute flaccid myelitis (AFM) is defined as acute, flaccid muscle weakness that occurs less than 1 week after a fever or respiratory illness. Viruses, including enterovirus, are believed to play a role in AFM, but the cause still is unknown. The disease appears mostly in children, and the average age of a patient diagnosed with AFM is 5 years.
“Doctors and other clinicians in the United States play a critical role,” Anne Schuchat, MD, principal deputy director of the Centers for Disease Control and Prevention, said in the telebriefing. “We ask for your help with early recognition of patients with AFM symptoms, prompt specimen collection for testing, and immediate reporting of suspected AFM cases to health departments.”
While there is no proven treatment for AFM, early diagnosis is critical to getting patients the best care possible, according to a Vital Signs report released today. This means that clinicians should not wait for the CDC’s case definition before diagnosis, the CDC said.
“When specimens are collected as soon as possible after symptom onset, we have a better chance of understanding the causes of AFM, these recurrent outbreaks, and developing a diagnostic test,” Dr. Schuchat said. “Rapid reporting also helps us to identify and respond to outbreaks early and alert other clinicians and the public.”
AFM appears to follow a seasonal and biennial pattern, with the number of cases increasing mainly in the late summer and early fall. As the season approaches where AFM cases increase, CDC is asking clinicians to look out for patients with suspected AFM so cases can be reported as early as possible.
Since the CDC began tracking AFM, the number of cases has risen every 2 years. In 2018, there were 233 cases in 41 states, the highest number of reported cases since the CDC began tracking AFM following an outbreak in 2014, according to a Vital Signs report. Overall, there have been 570 cases of AFM reported in 48 states and the District of Columbia since 2014.
There is yet to be a confirmatory test for AFM, but clinicians should obtain cerebrospinal fluid, serum, stool and nasopharyngeal swab from patients with suspected AFM as soon as possible, followed by an MRI. AFM has unique MRI features , such as gray matter involvement, that can help distinguish it from other diseases characterized by acute weakness.
In the Vital Signs report, which examined AFM in 2018, 92% of confirmed cases had respiratory symptoms or fever, and 42% of confirmed cases had upper limb involvement. The median time from limb weakness to hospitalization was 1 day, and time from weakness to MRI was 2 days. Cases were reported to the CDC a median of 18 days from onset of limb weakness, but time to reporting ranged between 18 days and 36 days, said Tom Clark, MD, MPH, deputy director of the division of viral diseases at CDC.
“This delay hampers our ability to understand the causes AFM,” he said. “We believe that recognizing AFM early is critical and can lead to better patient management.”
In lieu of a diagnostic test for AFM, clinicians should make management decisions through review of patient symptoms, exam findings, MRI, other test results, and in consulting with neurology experts. The Transverse Myelitis Association also has created a support portal for 24/7 physician consultation in AFM cases.
SOURCE: Lopez A et al. MMWR Morb Mortal Wkly Rep. 2019;68:1-7 .
the CDC said in a telebriefing.

Acute flaccid myelitis (AFM) is defined as acute, flaccid muscle weakness that occurs less than 1 week after a fever or respiratory illness. Viruses, including enterovirus, are believed to play a role in AFM, but the cause still is unknown. The disease appears mostly in children, and the average age of a patient diagnosed with AFM is 5 years.
“Doctors and other clinicians in the United States play a critical role,” Anne Schuchat, MD, principal deputy director of the Centers for Disease Control and Prevention, said in the telebriefing. “We ask for your help with early recognition of patients with AFM symptoms, prompt specimen collection for testing, and immediate reporting of suspected AFM cases to health departments.”
While there is no proven treatment for AFM, early diagnosis is critical to getting patients the best care possible, according to a Vital Signs report released today. This means that clinicians should not wait for the CDC’s case definition before diagnosis, the CDC said.
“When specimens are collected as soon as possible after symptom onset, we have a better chance of understanding the causes of AFM, these recurrent outbreaks, and developing a diagnostic test,” Dr. Schuchat said. “Rapid reporting also helps us to identify and respond to outbreaks early and alert other clinicians and the public.”
AFM appears to follow a seasonal and biennial pattern, with the number of cases increasing mainly in the late summer and early fall. As the season approaches where AFM cases increase, CDC is asking clinicians to look out for patients with suspected AFM so cases can be reported as early as possible.
Since the CDC began tracking AFM, the number of cases has risen every 2 years. In 2018, there were 233 cases in 41 states, the highest number of reported cases since the CDC began tracking AFM following an outbreak in 2014, according to a Vital Signs report. Overall, there have been 570 cases of AFM reported in 48 states and the District of Columbia since 2014.
There is yet to be a confirmatory test for AFM, but clinicians should obtain cerebrospinal fluid, serum, stool and nasopharyngeal swab from patients with suspected AFM as soon as possible, followed by an MRI. AFM has unique MRI features , such as gray matter involvement, that can help distinguish it from other diseases characterized by acute weakness.
In the Vital Signs report, which examined AFM in 2018, 92% of confirmed cases had respiratory symptoms or fever, and 42% of confirmed cases had upper limb involvement. The median time from limb weakness to hospitalization was 1 day, and time from weakness to MRI was 2 days. Cases were reported to the CDC a median of 18 days from onset of limb weakness, but time to reporting ranged between 18 days and 36 days, said Tom Clark, MD, MPH, deputy director of the division of viral diseases at CDC.
“This delay hampers our ability to understand the causes AFM,” he said. “We believe that recognizing AFM early is critical and can lead to better patient management.”
In lieu of a diagnostic test for AFM, clinicians should make management decisions through review of patient symptoms, exam findings, MRI, other test results, and in consulting with neurology experts. The Transverse Myelitis Association also has created a support portal for 24/7 physician consultation in AFM cases.
SOURCE: Lopez A et al. MMWR Morb Mortal Wkly Rep. 2019;68:1-7 .
NEWS FROM THE FDA/CDC
Recombinant vaccine cut herpes zoster rate in immunocompromised patients
Two doses of recombinant zoster vaccine significantly reduced incidence of herpes zoster in adults who had undergone autologous hematopoietic stem cell transplantation (HSCT), results of a randomized, placebo-controlled trial indicate.
The incidence of herpes zoster was 30 per 1,000 person-years for patients who received the adjuvanted recombinant zoster vaccine (Shingrix) versus 94 per 1,000 person-years for those who received placebo, according to study results.
Recombinant zoster vaccine induced humoral and cellular responses that were strong and occurring at a rate higher than what was seen in the placebo group, said senior author Keith M. Sullivan, MD, of Duke University Medical Center, Durham, N.C., and coauthors, who reported findings on behalf of the Zoster Efficacy Study in Patients Undergoing HSCT (ZOE-HSCT) Study Group.
“The vaccinations were generally well tolerated, and most symptoms were mild and transient and did not substantially deter participants from receiving their second dose,” Dr. Sullivan and colleagues wrote in JAMA.
The risk of herpes zoster is increased for 2-3 years after autologous HSCT because of diminished T-cell immunity, according to the authors.
“Antiviral prophylaxis is commonly administered to patients after HSCT to prevent such complications, but the efficacy depends on adherence to treatment,” they said.
While vaccines could provide long-term protection, immunocompromised individuals receiving live attenuated vaccine would be at increased risk of varicella caused by spread of the vaccine strain, they added.
There have been a few encouraging recent studies of non-live vaccines in this setting, including one large phase 3 trial of a heat-inactivated varicella-zoster virus vaccine that showed patients undergoing autologous HSCT had a 63.8% estimated efficacy in preventing herpes zoster, investigators from that study said in The Lancet (2018 May 26;391[10135]:2116-27).
A phase 1/2a study of the adjuvanted recombinant zoster vaccine in patients undergoing HSCT demonstrated strong humoral and cell-mediated immunity responses, which provided the rationale for studying the vaccine further in the randomized ZOE-HSCT study, according to Dr. Sullivan and coauthors.
Their study included a total of 1,846 adults who had undergone autologous HSCT. They were randomized to receive two doses of the recombinant zoster vaccine, the first at 50-70 days after the procedure and the second 1-2 months later.
Herpes zoster cases were seen in 49 and 136 individuals in the vaccine and placebo groups, respectively, which resulted in overall incidences of 30 and 94 per 1,000 person-years.
The incidence rate ratio of a first episode of herpes zoster was 0.36 for individuals receiving at least one dose, which authors said was equivalent to a vaccine efficacy of 63.7%.
That efficacy rate is “very similar” to the estimated efficacy reported for the heat-inactivated varicella-zoster virus vaccine reported in The Lancet, said Dr. Sullivan and coauthors.
However, the heat-inactivated vaccine achieved that level of protection with a four-dose schedule, including one dose given prior to autologous HSCT.
“An advantage of the short 2-dose posttransplantation schedule is that more patients might complete the vaccination program,” they said in a discussion of the results, noting that 94.7% of the recombinant zoster vaccine recipients completed two doses, compared with 81.9% of recipients who received the heat-inactivated herpes zoster vaccine in the previous report.
The study was funded and sponsored by GlaxoSmithKline Biologicals SA. Dr. Sullivan reported disclosures related to GlaxoSmithKline (GSK), Kiadis Pharmaceutical, Roche Genentech, and the National Institute of Allergy and Infectious Diseases. Coauthors provided disclosures related to GSK, AbbVie, Roche, Gilead, Janssen, Pharmacyclics, Morphosys, Helsinn, Celgene, and others.
SOURCE: Bastidas A et al. JAMA. 2019 July 9. doi: 10.1001/jama.2019.9053.
Two doses of recombinant zoster vaccine significantly reduced incidence of herpes zoster in adults who had undergone autologous hematopoietic stem cell transplantation (HSCT), results of a randomized, placebo-controlled trial indicate.
The incidence of herpes zoster was 30 per 1,000 person-years for patients who received the adjuvanted recombinant zoster vaccine (Shingrix) versus 94 per 1,000 person-years for those who received placebo, according to study results.
Recombinant zoster vaccine induced humoral and cellular responses that were strong and occurring at a rate higher than what was seen in the placebo group, said senior author Keith M. Sullivan, MD, of Duke University Medical Center, Durham, N.C., and coauthors, who reported findings on behalf of the Zoster Efficacy Study in Patients Undergoing HSCT (ZOE-HSCT) Study Group.
“The vaccinations were generally well tolerated, and most symptoms were mild and transient and did not substantially deter participants from receiving their second dose,” Dr. Sullivan and colleagues wrote in JAMA.
The risk of herpes zoster is increased for 2-3 years after autologous HSCT because of diminished T-cell immunity, according to the authors.
“Antiviral prophylaxis is commonly administered to patients after HSCT to prevent such complications, but the efficacy depends on adherence to treatment,” they said.
While vaccines could provide long-term protection, immunocompromised individuals receiving live attenuated vaccine would be at increased risk of varicella caused by spread of the vaccine strain, they added.
There have been a few encouraging recent studies of non-live vaccines in this setting, including one large phase 3 trial of a heat-inactivated varicella-zoster virus vaccine that showed patients undergoing autologous HSCT had a 63.8% estimated efficacy in preventing herpes zoster, investigators from that study said in The Lancet (2018 May 26;391[10135]:2116-27).
A phase 1/2a study of the adjuvanted recombinant zoster vaccine in patients undergoing HSCT demonstrated strong humoral and cell-mediated immunity responses, which provided the rationale for studying the vaccine further in the randomized ZOE-HSCT study, according to Dr. Sullivan and coauthors.
Their study included a total of 1,846 adults who had undergone autologous HSCT. They were randomized to receive two doses of the recombinant zoster vaccine, the first at 50-70 days after the procedure and the second 1-2 months later.
Herpes zoster cases were seen in 49 and 136 individuals in the vaccine and placebo groups, respectively, which resulted in overall incidences of 30 and 94 per 1,000 person-years.
The incidence rate ratio of a first episode of herpes zoster was 0.36 for individuals receiving at least one dose, which authors said was equivalent to a vaccine efficacy of 63.7%.
That efficacy rate is “very similar” to the estimated efficacy reported for the heat-inactivated varicella-zoster virus vaccine reported in The Lancet, said Dr. Sullivan and coauthors.
However, the heat-inactivated vaccine achieved that level of protection with a four-dose schedule, including one dose given prior to autologous HSCT.
“An advantage of the short 2-dose posttransplantation schedule is that more patients might complete the vaccination program,” they said in a discussion of the results, noting that 94.7% of the recombinant zoster vaccine recipients completed two doses, compared with 81.9% of recipients who received the heat-inactivated herpes zoster vaccine in the previous report.
The study was funded and sponsored by GlaxoSmithKline Biologicals SA. Dr. Sullivan reported disclosures related to GlaxoSmithKline (GSK), Kiadis Pharmaceutical, Roche Genentech, and the National Institute of Allergy and Infectious Diseases. Coauthors provided disclosures related to GSK, AbbVie, Roche, Gilead, Janssen, Pharmacyclics, Morphosys, Helsinn, Celgene, and others.
SOURCE: Bastidas A et al. JAMA. 2019 July 9. doi: 10.1001/jama.2019.9053.
Two doses of recombinant zoster vaccine significantly reduced incidence of herpes zoster in adults who had undergone autologous hematopoietic stem cell transplantation (HSCT), results of a randomized, placebo-controlled trial indicate.
The incidence of herpes zoster was 30 per 1,000 person-years for patients who received the adjuvanted recombinant zoster vaccine (Shingrix) versus 94 per 1,000 person-years for those who received placebo, according to study results.
Recombinant zoster vaccine induced humoral and cellular responses that were strong and occurring at a rate higher than what was seen in the placebo group, said senior author Keith M. Sullivan, MD, of Duke University Medical Center, Durham, N.C., and coauthors, who reported findings on behalf of the Zoster Efficacy Study in Patients Undergoing HSCT (ZOE-HSCT) Study Group.
“The vaccinations were generally well tolerated, and most symptoms were mild and transient and did not substantially deter participants from receiving their second dose,” Dr. Sullivan and colleagues wrote in JAMA.
The risk of herpes zoster is increased for 2-3 years after autologous HSCT because of diminished T-cell immunity, according to the authors.
“Antiviral prophylaxis is commonly administered to patients after HSCT to prevent such complications, but the efficacy depends on adherence to treatment,” they said.
While vaccines could provide long-term protection, immunocompromised individuals receiving live attenuated vaccine would be at increased risk of varicella caused by spread of the vaccine strain, they added.
There have been a few encouraging recent studies of non-live vaccines in this setting, including one large phase 3 trial of a heat-inactivated varicella-zoster virus vaccine that showed patients undergoing autologous HSCT had a 63.8% estimated efficacy in preventing herpes zoster, investigators from that study said in The Lancet (2018 May 26;391[10135]:2116-27).
A phase 1/2a study of the adjuvanted recombinant zoster vaccine in patients undergoing HSCT demonstrated strong humoral and cell-mediated immunity responses, which provided the rationale for studying the vaccine further in the randomized ZOE-HSCT study, according to Dr. Sullivan and coauthors.
Their study included a total of 1,846 adults who had undergone autologous HSCT. They were randomized to receive two doses of the recombinant zoster vaccine, the first at 50-70 days after the procedure and the second 1-2 months later.
Herpes zoster cases were seen in 49 and 136 individuals in the vaccine and placebo groups, respectively, which resulted in overall incidences of 30 and 94 per 1,000 person-years.
The incidence rate ratio of a first episode of herpes zoster was 0.36 for individuals receiving at least one dose, which authors said was equivalent to a vaccine efficacy of 63.7%.
That efficacy rate is “very similar” to the estimated efficacy reported for the heat-inactivated varicella-zoster virus vaccine reported in The Lancet, said Dr. Sullivan and coauthors.
However, the heat-inactivated vaccine achieved that level of protection with a four-dose schedule, including one dose given prior to autologous HSCT.
“An advantage of the short 2-dose posttransplantation schedule is that more patients might complete the vaccination program,” they said in a discussion of the results, noting that 94.7% of the recombinant zoster vaccine recipients completed two doses, compared with 81.9% of recipients who received the heat-inactivated herpes zoster vaccine in the previous report.
The study was funded and sponsored by GlaxoSmithKline Biologicals SA. Dr. Sullivan reported disclosures related to GlaxoSmithKline (GSK), Kiadis Pharmaceutical, Roche Genentech, and the National Institute of Allergy and Infectious Diseases. Coauthors provided disclosures related to GSK, AbbVie, Roche, Gilead, Janssen, Pharmacyclics, Morphosys, Helsinn, Celgene, and others.
SOURCE: Bastidas A et al. JAMA. 2019 July 9. doi: 10.1001/jama.2019.9053.
FROM JAMA
Key clinical point: Two doses of recombinant zoster vaccine significantly reduced incidence of herpes zoster versus placebo in adults who had undergone autologous hematopoietic stem cell transplantation (HSCT).
Major finding: Herpes zoster cases were seen in 49 and 136 individuals in the vaccine and placebo groups, respectively, resulting in overall incidences of 30 and 94 per 1,000 person-years.
Study details: A randomized clinical trial (ZOE-HSCT) including 1,846 adults who had undergone autologous HSCT.
Disclosures: The study was funded and sponsored by GlaxoSmithKline Biologicals SA. Study authors reported disclosures related to GlaxoSmithKline, Kiadis Pharmaceutical, Roche Genentech, AbbVie, Roche, Gilead, Janssen, Pharmacyclics, Morphosys, Helsinn, Celgene, and others.
Source: Bastidas A et al. JAMA. 2019 July 9. doi: 10.1001/jama.2019.9053.
Acquired MMR immunity doesn’t last to age 1 year
according to results of a study in Vaccine.
María José Cilleruelo, PhD, of Hospital Universitario Puerta de Hierro in Majadahonda, Spain, and colleagues showed that, although most infants acquire some protective antibodies against MMR from their mothers during gestation, most have lost this protection as early as 3 months of age. This single-center, observational, prospective study was conducted between October 2013 and December 2014, and it began with 146 mother-child pairs, with 99 remaining in follow-up at 3 months, 77 at 6 months, 63 at 9 months, and 30 at 12 months. For measles, 88% of newborns were seropositive, but only 19% were at 3 months; for mumps, 70% of newborns were seropositive, but only 11% were at 3 months; and for rubella, 91% of newborns were seropositive, but only 13% were at 3 months. No infants were seropositive for mumps or rubella at 9 months, and only 2% were for measles. No infants were seropositive for any of these viruses by 12 months of age.
The investigators noted that, given Spain (where the study was conducted) is a country that gives the first MMR vaccine at 12 months of life, these declining titers can leave most infants vulnerable to those viruses before then.
“We suggest that it may be worth considering administering the first dose of MMR vaccine before 12 months of age,” the investigators concluded, although they advised studies be undertaken into the efficacy and safety of administration of that vaccine in infants younger than 12 months. They noted that the biggest limitation of this study was the high percentage of loss to follow-up, which limited the statistical power to make comparisons.
The study was funded by the Fondo de Investigación Sanitaria, and one of the authors was funded by CIBER de Epidemiología y Salud Pública. The authors declared that there are no conflicts of interest.
SOURCE: Cilleruelo MJ et al. Vaccine. 2019;37:4164-71.
according to results of a study in Vaccine.
María José Cilleruelo, PhD, of Hospital Universitario Puerta de Hierro in Majadahonda, Spain, and colleagues showed that, although most infants acquire some protective antibodies against MMR from their mothers during gestation, most have lost this protection as early as 3 months of age. This single-center, observational, prospective study was conducted between October 2013 and December 2014, and it began with 146 mother-child pairs, with 99 remaining in follow-up at 3 months, 77 at 6 months, 63 at 9 months, and 30 at 12 months. For measles, 88% of newborns were seropositive, but only 19% were at 3 months; for mumps, 70% of newborns were seropositive, but only 11% were at 3 months; and for rubella, 91% of newborns were seropositive, but only 13% were at 3 months. No infants were seropositive for mumps or rubella at 9 months, and only 2% were for measles. No infants were seropositive for any of these viruses by 12 months of age.
The investigators noted that, given Spain (where the study was conducted) is a country that gives the first MMR vaccine at 12 months of life, these declining titers can leave most infants vulnerable to those viruses before then.
“We suggest that it may be worth considering administering the first dose of MMR vaccine before 12 months of age,” the investigators concluded, although they advised studies be undertaken into the efficacy and safety of administration of that vaccine in infants younger than 12 months. They noted that the biggest limitation of this study was the high percentage of loss to follow-up, which limited the statistical power to make comparisons.
The study was funded by the Fondo de Investigación Sanitaria, and one of the authors was funded by CIBER de Epidemiología y Salud Pública. The authors declared that there are no conflicts of interest.
SOURCE: Cilleruelo MJ et al. Vaccine. 2019;37:4164-71.
according to results of a study in Vaccine.
María José Cilleruelo, PhD, of Hospital Universitario Puerta de Hierro in Majadahonda, Spain, and colleagues showed that, although most infants acquire some protective antibodies against MMR from their mothers during gestation, most have lost this protection as early as 3 months of age. This single-center, observational, prospective study was conducted between October 2013 and December 2014, and it began with 146 mother-child pairs, with 99 remaining in follow-up at 3 months, 77 at 6 months, 63 at 9 months, and 30 at 12 months. For measles, 88% of newborns were seropositive, but only 19% were at 3 months; for mumps, 70% of newborns were seropositive, but only 11% were at 3 months; and for rubella, 91% of newborns were seropositive, but only 13% were at 3 months. No infants were seropositive for mumps or rubella at 9 months, and only 2% were for measles. No infants were seropositive for any of these viruses by 12 months of age.
The investigators noted that, given Spain (where the study was conducted) is a country that gives the first MMR vaccine at 12 months of life, these declining titers can leave most infants vulnerable to those viruses before then.
“We suggest that it may be worth considering administering the first dose of MMR vaccine before 12 months of age,” the investigators concluded, although they advised studies be undertaken into the efficacy and safety of administration of that vaccine in infants younger than 12 months. They noted that the biggest limitation of this study was the high percentage of loss to follow-up, which limited the statistical power to make comparisons.
The study was funded by the Fondo de Investigación Sanitaria, and one of the authors was funded by CIBER de Epidemiología y Salud Pública. The authors declared that there are no conflicts of interest.
SOURCE: Cilleruelo MJ et al. Vaccine. 2019;37:4164-71.
FROM VACCINE