User login
New measles outbreaks reported in Los Angeles and El Paso
according to the Centers for Disease Control and Prevention.
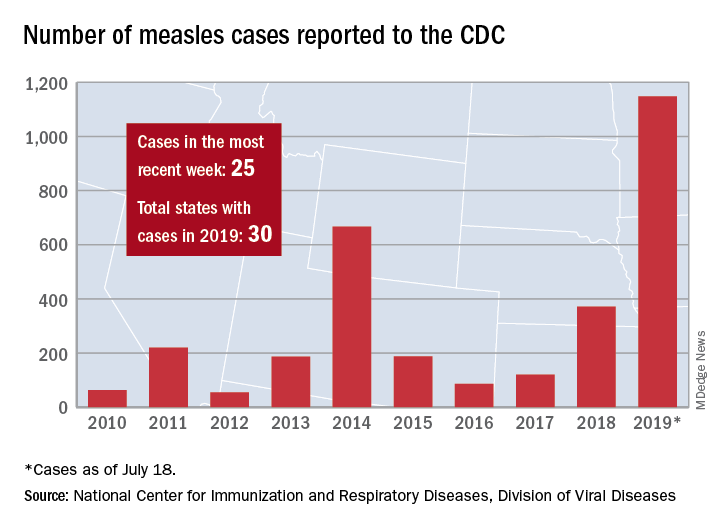
The total number of confirmed cases of measles in the United States is now up to 1,148 for the year, which is 25 more than the previous week, the CDC said on July 22. The highest 1-week total for the year was the 90 cases reported during the week of April 11.
The number of outbreaks is back up to five as California returned to the list after a 1-week absence and El Paso, Tex., made its first appearance of the year. The current outbreak in California – the state’s fifth – is occurring in Los Angeles, which is now up to 16 total cases in 2019. El Paso just reported its fourth case on July 17, and the city’s health department noted that “it had been more than 25 years since El Paso saw its last case of measles before these four recent cases.” Outbreaks also are ongoing in Rockland County, N.Y.; New York City; and three counties in Washington State.
States that joined the ranks of the measles-infected during this most recent reporting week were Alaska and Ohio, which brings the total number to 30 for the year, the CDC said.
The Alaska Department of Health and Social Services said that it “has confirmed a single case of measles in an unvaccinated teenager from the Kenai Peninsula who recently traveled out of state to Arizona via Seattle.” The Ohio case is a “young adult from Stark County [who] recently traveled to a state with confirmed measles cases,” according to the state’s health department.
according to the Centers for Disease Control and Prevention.

The total number of confirmed cases of measles in the United States is now up to 1,148 for the year, which is 25 more than the previous week, the CDC said on July 22. The highest 1-week total for the year was the 90 cases reported during the week of April 11.
The number of outbreaks is back up to five as California returned to the list after a 1-week absence and El Paso, Tex., made its first appearance of the year. The current outbreak in California – the state’s fifth – is occurring in Los Angeles, which is now up to 16 total cases in 2019. El Paso just reported its fourth case on July 17, and the city’s health department noted that “it had been more than 25 years since El Paso saw its last case of measles before these four recent cases.” Outbreaks also are ongoing in Rockland County, N.Y.; New York City; and three counties in Washington State.
States that joined the ranks of the measles-infected during this most recent reporting week were Alaska and Ohio, which brings the total number to 30 for the year, the CDC said.
The Alaska Department of Health and Social Services said that it “has confirmed a single case of measles in an unvaccinated teenager from the Kenai Peninsula who recently traveled out of state to Arizona via Seattle.” The Ohio case is a “young adult from Stark County [who] recently traveled to a state with confirmed measles cases,” according to the state’s health department.
according to the Centers for Disease Control and Prevention.

The total number of confirmed cases of measles in the United States is now up to 1,148 for the year, which is 25 more than the previous week, the CDC said on July 22. The highest 1-week total for the year was the 90 cases reported during the week of April 11.
The number of outbreaks is back up to five as California returned to the list after a 1-week absence and El Paso, Tex., made its first appearance of the year. The current outbreak in California – the state’s fifth – is occurring in Los Angeles, which is now up to 16 total cases in 2019. El Paso just reported its fourth case on July 17, and the city’s health department noted that “it had been more than 25 years since El Paso saw its last case of measles before these four recent cases.” Outbreaks also are ongoing in Rockland County, N.Y.; New York City; and three counties in Washington State.
States that joined the ranks of the measles-infected during this most recent reporting week were Alaska and Ohio, which brings the total number to 30 for the year, the CDC said.
The Alaska Department of Health and Social Services said that it “has confirmed a single case of measles in an unvaccinated teenager from the Kenai Peninsula who recently traveled out of state to Arizona via Seattle.” The Ohio case is a “young adult from Stark County [who] recently traveled to a state with confirmed measles cases,” according to the state’s health department.
Adjuvanted flu vaccine performs better than others in young children
according to an industry-funded synthesis of six studies.
The vaccine “offers significant advances over conventional inactivated influenza vaccines and presents an acceptable safety profile in children 6 months through 5 years of age,” Sanjay S. Patel, PhD, of Novartis Vaccines and Diagnostics, Cambridge, Mass., and associates wrote in the analysis, published in the International Journal of Infectious Diseases. “The noteworthy increases in antibody responses and decreases in influenza cases following vaccination suggest an alternative for use in a population that is heavily impacted by influenza disease.”
Children are, of course, vulnerable to flu. The Centers for Disease Control and Prevention reported that 186 children died of flu during the landmark 2017-2018 flu season. That’s the highest number of pediatric flu deaths since they became a notifiable condition in 2004 (exclusive of the 2009 pandemic, when 358 pediatric deaths were reported from April 15, 2009, to October 2, 2010).The CDC said the vaccine during that flu season had an overall effectiveness level of 40%. According to research of others, however, flu vaccines are less effective in younger children than in adolescents and adults (Vaccine. 2014;32[31]:3886-94; Cochrane Database Syst Rev. 2008. doi: 10.1002/14651858.CD004879.pub3).
Fluad – a MF59-adjuvanted inactivated trivalent seasonal influenza vaccine – is used in adults over 65 in the United States and 29 other countries, and it is approved for children aged 6 months through 23 months in Canada.
Dr. Patel and associates examined the results of six studies – one phase 1b, three phase 2, and two phase 3 – that tested Fluad with or without other vaccines in 11,942 children aged 6 months to 5 years. The studies, mostly multicenter, were conducted in various countries, mainly in Europe and South and Central America, from 2006 to 2012.
In general, children in the intervention groups in the studies received two doses of the Fluad vaccine 4 weeks apart: two 0.25-mL doses for children aged 6-35 months and two 0.5-mL doses for those aged 3 years or older. In most of the studies, parallel control groups received nonadjuvanted trivalent or quadrivalent influenza vaccines.
Most participants (93%-94%) completed the studies. Solicited adverse effects were common in all groups (72% in the Fluad group vs. 67% who received IIV3 vaccines), and generally mild to moderate and resolved in 1-3 days. Unsolicited adverse effects were similar (55% and 62%, respectively) in the two flu vaccine groups. The authors wrote that “these data reflect a safety profile consistent with other licensed inactivated influenza vaccines administered to children.”
As for results, Dr. Patel and colleagues said, “HI [hemagglutination inhibition] antibody responses to both homologous and heterologous influenza strains are higher following vaccination with aIIV3, and this increase in immunogenicity is observed across all age subgroups in children aged 6 months through 5 years, and most profound in the children 6 to 36 months.”
For example, in one of the phase 3 studies when the influenza viruses were antigenically matched (homologous) for A/H1N1 among the children aged 6-35 months seroconversion was 100% for allV3 (Fluad) and 38% for IIV3-1/IIV3-4 (trivalent/quadrivalent flu vaccines); among children aged 3-5 years seroconversion was 100% for allV3 and 82% for IIV3-1/IIV3-4. For AH3N2 homologous among children aged 6-35 months, seroconversion was 98% for allV3 and 44% for IIV3-1/IIV3-4. For the B strain homologous among children aged 6-35 months, seroconversion was 88% for allV3 and 19% for IIV3-1/IIV3-4; among children aged 3-5 years seroconversion for B was 99% for allV3 and 59% for IIV3-1/IIV3-4.
In the same study when the influenza viruses were antigenically mismatched (heterologous) for A/H1N1 among children of all ages 6 months to greater than 72 months, seroconversion was 96% for allV3 (Fluad) and 44% for IIV3-1/IIV3-4; for A/H3N2 it was 98% for allV3 and 49% for IIV3-1/IIV3-4, and for the B strain it was 10% for allV3 and 3% for IIV3-1/IIV3-4.
They added that “in addition, aIIV3 had the fastest onset of immunogenicity and longest persistence of immune response, which has implications for the real-world clinical setting, where the influenza season might start earlier than expected or last longer, and second (follow-up) vaccinations may be missed.”
Dr. Patel and associates said the MF59 adjuvant in Fluad “recruits immune cells (primarily monocytes, macrophages, neutrophils, and dendritic cells) at the site of injection and differentiates them into antigen-presenting cells. With an MF59-adjuvanted vaccine, more antigen is transported from the injection site to the draining lymph node, wherein MF59 leads to T-cell activation and an increased B-cell expansion and a greater number and diversity of antibodies.”
According to goodrx.com, one syringe of Fluad 0.5 mL costs $45-$74 with coupon. The same dose of Fluzone Quadrivalent, a flu vaccine recently approved by the Food and Drug Administration for use in young children aged 6-35 months, costs $31 with coupon.
The study was funded by Novartis Vaccines and Diagnostics and Seqirus (formerly part of Novartis Vaccines and Diagnostics). The study authors disclosed employment by Novartis and Seqirus.
SOURCE: Patel SS et al. Int J Infect Dis. 2019. doi: 10.1016/j.ijid.2019.05.009.
according to an industry-funded synthesis of six studies.
The vaccine “offers significant advances over conventional inactivated influenza vaccines and presents an acceptable safety profile in children 6 months through 5 years of age,” Sanjay S. Patel, PhD, of Novartis Vaccines and Diagnostics, Cambridge, Mass., and associates wrote in the analysis, published in the International Journal of Infectious Diseases. “The noteworthy increases in antibody responses and decreases in influenza cases following vaccination suggest an alternative for use in a population that is heavily impacted by influenza disease.”
Children are, of course, vulnerable to flu. The Centers for Disease Control and Prevention reported that 186 children died of flu during the landmark 2017-2018 flu season. That’s the highest number of pediatric flu deaths since they became a notifiable condition in 2004 (exclusive of the 2009 pandemic, when 358 pediatric deaths were reported from April 15, 2009, to October 2, 2010).The CDC said the vaccine during that flu season had an overall effectiveness level of 40%. According to research of others, however, flu vaccines are less effective in younger children than in adolescents and adults (Vaccine. 2014;32[31]:3886-94; Cochrane Database Syst Rev. 2008. doi: 10.1002/14651858.CD004879.pub3).
Fluad – a MF59-adjuvanted inactivated trivalent seasonal influenza vaccine – is used in adults over 65 in the United States and 29 other countries, and it is approved for children aged 6 months through 23 months in Canada.
Dr. Patel and associates examined the results of six studies – one phase 1b, three phase 2, and two phase 3 – that tested Fluad with or without other vaccines in 11,942 children aged 6 months to 5 years. The studies, mostly multicenter, were conducted in various countries, mainly in Europe and South and Central America, from 2006 to 2012.
In general, children in the intervention groups in the studies received two doses of the Fluad vaccine 4 weeks apart: two 0.25-mL doses for children aged 6-35 months and two 0.5-mL doses for those aged 3 years or older. In most of the studies, parallel control groups received nonadjuvanted trivalent or quadrivalent influenza vaccines.
Most participants (93%-94%) completed the studies. Solicited adverse effects were common in all groups (72% in the Fluad group vs. 67% who received IIV3 vaccines), and generally mild to moderate and resolved in 1-3 days. Unsolicited adverse effects were similar (55% and 62%, respectively) in the two flu vaccine groups. The authors wrote that “these data reflect a safety profile consistent with other licensed inactivated influenza vaccines administered to children.”
As for results, Dr. Patel and colleagues said, “HI [hemagglutination inhibition] antibody responses to both homologous and heterologous influenza strains are higher following vaccination with aIIV3, and this increase in immunogenicity is observed across all age subgroups in children aged 6 months through 5 years, and most profound in the children 6 to 36 months.”
For example, in one of the phase 3 studies when the influenza viruses were antigenically matched (homologous) for A/H1N1 among the children aged 6-35 months seroconversion was 100% for allV3 (Fluad) and 38% for IIV3-1/IIV3-4 (trivalent/quadrivalent flu vaccines); among children aged 3-5 years seroconversion was 100% for allV3 and 82% for IIV3-1/IIV3-4. For AH3N2 homologous among children aged 6-35 months, seroconversion was 98% for allV3 and 44% for IIV3-1/IIV3-4. For the B strain homologous among children aged 6-35 months, seroconversion was 88% for allV3 and 19% for IIV3-1/IIV3-4; among children aged 3-5 years seroconversion for B was 99% for allV3 and 59% for IIV3-1/IIV3-4.
In the same study when the influenza viruses were antigenically mismatched (heterologous) for A/H1N1 among children of all ages 6 months to greater than 72 months, seroconversion was 96% for allV3 (Fluad) and 44% for IIV3-1/IIV3-4; for A/H3N2 it was 98% for allV3 and 49% for IIV3-1/IIV3-4, and for the B strain it was 10% for allV3 and 3% for IIV3-1/IIV3-4.
They added that “in addition, aIIV3 had the fastest onset of immunogenicity and longest persistence of immune response, which has implications for the real-world clinical setting, where the influenza season might start earlier than expected or last longer, and second (follow-up) vaccinations may be missed.”
Dr. Patel and associates said the MF59 adjuvant in Fluad “recruits immune cells (primarily monocytes, macrophages, neutrophils, and dendritic cells) at the site of injection and differentiates them into antigen-presenting cells. With an MF59-adjuvanted vaccine, more antigen is transported from the injection site to the draining lymph node, wherein MF59 leads to T-cell activation and an increased B-cell expansion and a greater number and diversity of antibodies.”
According to goodrx.com, one syringe of Fluad 0.5 mL costs $45-$74 with coupon. The same dose of Fluzone Quadrivalent, a flu vaccine recently approved by the Food and Drug Administration for use in young children aged 6-35 months, costs $31 with coupon.
The study was funded by Novartis Vaccines and Diagnostics and Seqirus (formerly part of Novartis Vaccines and Diagnostics). The study authors disclosed employment by Novartis and Seqirus.
SOURCE: Patel SS et al. Int J Infect Dis. 2019. doi: 10.1016/j.ijid.2019.05.009.
according to an industry-funded synthesis of six studies.
The vaccine “offers significant advances over conventional inactivated influenza vaccines and presents an acceptable safety profile in children 6 months through 5 years of age,” Sanjay S. Patel, PhD, of Novartis Vaccines and Diagnostics, Cambridge, Mass., and associates wrote in the analysis, published in the International Journal of Infectious Diseases. “The noteworthy increases in antibody responses and decreases in influenza cases following vaccination suggest an alternative for use in a population that is heavily impacted by influenza disease.”
Children are, of course, vulnerable to flu. The Centers for Disease Control and Prevention reported that 186 children died of flu during the landmark 2017-2018 flu season. That’s the highest number of pediatric flu deaths since they became a notifiable condition in 2004 (exclusive of the 2009 pandemic, when 358 pediatric deaths were reported from April 15, 2009, to October 2, 2010).The CDC said the vaccine during that flu season had an overall effectiveness level of 40%. According to research of others, however, flu vaccines are less effective in younger children than in adolescents and adults (Vaccine. 2014;32[31]:3886-94; Cochrane Database Syst Rev. 2008. doi: 10.1002/14651858.CD004879.pub3).
Fluad – a MF59-adjuvanted inactivated trivalent seasonal influenza vaccine – is used in adults over 65 in the United States and 29 other countries, and it is approved for children aged 6 months through 23 months in Canada.
Dr. Patel and associates examined the results of six studies – one phase 1b, three phase 2, and two phase 3 – that tested Fluad with or without other vaccines in 11,942 children aged 6 months to 5 years. The studies, mostly multicenter, were conducted in various countries, mainly in Europe and South and Central America, from 2006 to 2012.
In general, children in the intervention groups in the studies received two doses of the Fluad vaccine 4 weeks apart: two 0.25-mL doses for children aged 6-35 months and two 0.5-mL doses for those aged 3 years or older. In most of the studies, parallel control groups received nonadjuvanted trivalent or quadrivalent influenza vaccines.
Most participants (93%-94%) completed the studies. Solicited adverse effects were common in all groups (72% in the Fluad group vs. 67% who received IIV3 vaccines), and generally mild to moderate and resolved in 1-3 days. Unsolicited adverse effects were similar (55% and 62%, respectively) in the two flu vaccine groups. The authors wrote that “these data reflect a safety profile consistent with other licensed inactivated influenza vaccines administered to children.”
As for results, Dr. Patel and colleagues said, “HI [hemagglutination inhibition] antibody responses to both homologous and heterologous influenza strains are higher following vaccination with aIIV3, and this increase in immunogenicity is observed across all age subgroups in children aged 6 months through 5 years, and most profound in the children 6 to 36 months.”
For example, in one of the phase 3 studies when the influenza viruses were antigenically matched (homologous) for A/H1N1 among the children aged 6-35 months seroconversion was 100% for allV3 (Fluad) and 38% for IIV3-1/IIV3-4 (trivalent/quadrivalent flu vaccines); among children aged 3-5 years seroconversion was 100% for allV3 and 82% for IIV3-1/IIV3-4. For AH3N2 homologous among children aged 6-35 months, seroconversion was 98% for allV3 and 44% for IIV3-1/IIV3-4. For the B strain homologous among children aged 6-35 months, seroconversion was 88% for allV3 and 19% for IIV3-1/IIV3-4; among children aged 3-5 years seroconversion for B was 99% for allV3 and 59% for IIV3-1/IIV3-4.
In the same study when the influenza viruses were antigenically mismatched (heterologous) for A/H1N1 among children of all ages 6 months to greater than 72 months, seroconversion was 96% for allV3 (Fluad) and 44% for IIV3-1/IIV3-4; for A/H3N2 it was 98% for allV3 and 49% for IIV3-1/IIV3-4, and for the B strain it was 10% for allV3 and 3% for IIV3-1/IIV3-4.
They added that “in addition, aIIV3 had the fastest onset of immunogenicity and longest persistence of immune response, which has implications for the real-world clinical setting, where the influenza season might start earlier than expected or last longer, and second (follow-up) vaccinations may be missed.”
Dr. Patel and associates said the MF59 adjuvant in Fluad “recruits immune cells (primarily monocytes, macrophages, neutrophils, and dendritic cells) at the site of injection and differentiates them into antigen-presenting cells. With an MF59-adjuvanted vaccine, more antigen is transported from the injection site to the draining lymph node, wherein MF59 leads to T-cell activation and an increased B-cell expansion and a greater number and diversity of antibodies.”
According to goodrx.com, one syringe of Fluad 0.5 mL costs $45-$74 with coupon. The same dose of Fluzone Quadrivalent, a flu vaccine recently approved by the Food and Drug Administration for use in young children aged 6-35 months, costs $31 with coupon.
The study was funded by Novartis Vaccines and Diagnostics and Seqirus (formerly part of Novartis Vaccines and Diagnostics). The study authors disclosed employment by Novartis and Seqirus.
SOURCE: Patel SS et al. Int J Infect Dis. 2019. doi: 10.1016/j.ijid.2019.05.009.
FROM INTERNATIONAL JOURNAL OF INFECTIOUS DISEASES
Adjuvanted influenza vaccine appears safe for at-risk children
according to a study in the International Journal of Infectious Diseases.
Sanjay S. Patel, PhD, of Novartis Vaccines and Diagnostics, Cambridge, Mass., and colleagues performed a retrospective analysis on an integrated dataset that drew from six randomized clinical trials comparing aIIV3 with nonadjuvanted trivalent inactivated influenza vaccine (IIV3). The dataset comprised 10,794 patients aged 6 months through 5 years, of whom 373 (3%) were deemed at risk of influenza complications after review of their medical history for conditions such as heart disease, asthma, and endocrine disorders.
The rates of solicited adverse events (such as erythema, diarrhea, fever, and localized swelling) were 74% in the aIIV3 group and 73% in the IIV3 group. The rates for any unsolicited adverse events (such as upper respiratory tract infection) for aIIV3 and IIV3 were 54% and 59%, respectively (Int J Infect Dis. 2019. doi: 10.1016/j.ijid.2019.04.023).
One of the six studies included in the dataset randomized 2,655 children for immunogenicity analyses, of whom 103 (4%) were deemed at risk. Hemagglutination inhibition assay geometric mean titers against homologous A/H1N1, A/H3N2, and B strains 21 days after the second of two doses of vaccines were two to three times higher in the aIIV3 than in the IIV3 group, which suggests that aIIV3 is more immunogenic than IIV3. As the investigators noted, this is likely because the adjuvanted vaccine induces a greater magnitude of immune response to the vaccine, something already lower in children than in adults, as well as more breadth of response, meaning the response goes beyond strains included in the vaccines.
The small number of at-risk children in the study poses a limitation on its findings. Dr. Patel and associates said that, regardless, the results of immunogenicity analyses were strong. “Overall, this analysis indicates that aIIV3 has a similar safety profile in young children with underlying medical conditions, consistent with other licensed inactivated influenza vaccines.”
Novartis Vaccines and Diagnostics originally funded the study, but was later acquired by CSL Group and now operates as Seqirus, which continued funding for the study. The authors were employees of one or the other of these companies.
according to a study in the International Journal of Infectious Diseases.
Sanjay S. Patel, PhD, of Novartis Vaccines and Diagnostics, Cambridge, Mass., and colleagues performed a retrospective analysis on an integrated dataset that drew from six randomized clinical trials comparing aIIV3 with nonadjuvanted trivalent inactivated influenza vaccine (IIV3). The dataset comprised 10,794 patients aged 6 months through 5 years, of whom 373 (3%) were deemed at risk of influenza complications after review of their medical history for conditions such as heart disease, asthma, and endocrine disorders.
The rates of solicited adverse events (such as erythema, diarrhea, fever, and localized swelling) were 74% in the aIIV3 group and 73% in the IIV3 group. The rates for any unsolicited adverse events (such as upper respiratory tract infection) for aIIV3 and IIV3 were 54% and 59%, respectively (Int J Infect Dis. 2019. doi: 10.1016/j.ijid.2019.04.023).
One of the six studies included in the dataset randomized 2,655 children for immunogenicity analyses, of whom 103 (4%) were deemed at risk. Hemagglutination inhibition assay geometric mean titers against homologous A/H1N1, A/H3N2, and B strains 21 days after the second of two doses of vaccines were two to three times higher in the aIIV3 than in the IIV3 group, which suggests that aIIV3 is more immunogenic than IIV3. As the investigators noted, this is likely because the adjuvanted vaccine induces a greater magnitude of immune response to the vaccine, something already lower in children than in adults, as well as more breadth of response, meaning the response goes beyond strains included in the vaccines.
The small number of at-risk children in the study poses a limitation on its findings. Dr. Patel and associates said that, regardless, the results of immunogenicity analyses were strong. “Overall, this analysis indicates that aIIV3 has a similar safety profile in young children with underlying medical conditions, consistent with other licensed inactivated influenza vaccines.”
Novartis Vaccines and Diagnostics originally funded the study, but was later acquired by CSL Group and now operates as Seqirus, which continued funding for the study. The authors were employees of one or the other of these companies.
according to a study in the International Journal of Infectious Diseases.
Sanjay S. Patel, PhD, of Novartis Vaccines and Diagnostics, Cambridge, Mass., and colleagues performed a retrospective analysis on an integrated dataset that drew from six randomized clinical trials comparing aIIV3 with nonadjuvanted trivalent inactivated influenza vaccine (IIV3). The dataset comprised 10,794 patients aged 6 months through 5 years, of whom 373 (3%) were deemed at risk of influenza complications after review of their medical history for conditions such as heart disease, asthma, and endocrine disorders.
The rates of solicited adverse events (such as erythema, diarrhea, fever, and localized swelling) were 74% in the aIIV3 group and 73% in the IIV3 group. The rates for any unsolicited adverse events (such as upper respiratory tract infection) for aIIV3 and IIV3 were 54% and 59%, respectively (Int J Infect Dis. 2019. doi: 10.1016/j.ijid.2019.04.023).
One of the six studies included in the dataset randomized 2,655 children for immunogenicity analyses, of whom 103 (4%) were deemed at risk. Hemagglutination inhibition assay geometric mean titers against homologous A/H1N1, A/H3N2, and B strains 21 days after the second of two doses of vaccines were two to three times higher in the aIIV3 than in the IIV3 group, which suggests that aIIV3 is more immunogenic than IIV3. As the investigators noted, this is likely because the adjuvanted vaccine induces a greater magnitude of immune response to the vaccine, something already lower in children than in adults, as well as more breadth of response, meaning the response goes beyond strains included in the vaccines.
The small number of at-risk children in the study poses a limitation on its findings. Dr. Patel and associates said that, regardless, the results of immunogenicity analyses were strong. “Overall, this analysis indicates that aIIV3 has a similar safety profile in young children with underlying medical conditions, consistent with other licensed inactivated influenza vaccines.”
Novartis Vaccines and Diagnostics originally funded the study, but was later acquired by CSL Group and now operates as Seqirus, which continued funding for the study. The authors were employees of one or the other of these companies.
FROM THE INTERNATIONAL JOURNAL OF INFECTIOUS DISEASES
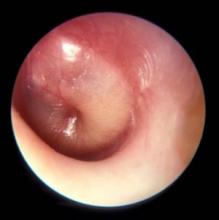
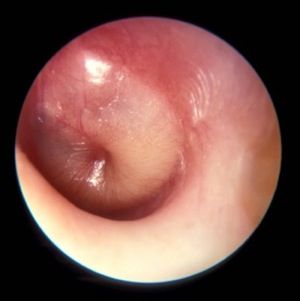
How are your otoscopy skills?
If the name Michael E. Pichichero, MD, is unfamiliar, you haven’t been reading some of the best articles on this website . Dr. Pichichero, an infectious disease specialist at the Research Institute at the Rochester General Hospital in New York, reports in his most recent ID Consult column on new research presented at the June 2019 meeting of the International Society for Otitis Media, including topics such as transtympanic antibiotic delivery, biofilms, probiotics, and biomarkers.
Dr. Pichichero described work he and his colleagues have been doing on the impact of overdiagnosis of acute otitis media (AOM). They found when “validated otoscopists” evaluated children, half as many reached the diagnostic threshold of being labeled “otitis prone” as when community-based pediatricians performed the exams.
Looking around at the colleagues with whom you share patients, do you find that some of them diagnose AOM much more frequently than does the coverage group average? How often do you see a child a day or two after he has been diagnosed with AOM by a colleague and find that the child’s tympanic membranes are transparent and mobile? Do you or your practice group keep track of each provider’s diagnostic tendencies? If these data exists, is there a mechanism for addressing apparent outliers? I suspect that the answer to those last two questions is a firm “No.”
I don’t have the stomach this morning to open those two cans of worms. But certainly Dr. Pichichero’s findings suggest that these are issues that need to be addressed. How the process should proceed in a nonthreatening way is a story for another day. But I’m not sure that involving your community ear, nose, and throat (ENT) specialist as a resource is the best answer. The scenarios in which pediatricians and ENTs perform otoscopies couldn’t be more different. In the pediatrician’s office, the child is generally sick, feverish, and possibly in pain. In the ENT’s office, the acute process has probably passed and the assessment may lean more heavily on history. The child is more likely to accept the exam without resistance, and the findings are not those of AOM but of a chronic process. The fact that Dr. Pichichero has been able to find and train “validated otoscopists” suggests that improving the quality of otoscopy among the physicians in communities like yours and mine is achievable.
How are your otoscopy skills? Do feel comfortable that you can do a good exam and accurately diagnose AOM? When did you acquire that comfort level? Probably not in medical school. More likely as a house officer when you were guided by a more senior house officer who may nor not been a master otoscopist. How would you rate your training? Or were you self-taught? Do you insufflate? Are you a skilled cerumen extractor? Or do you give up after one attempt? Be honest. How is your equipment? Are the bulbs and batteries fresh? Do you find yourself frustrated by an otoscope that is tethered to the wall charger by a cord that ensnarls you, the parent, and the patient? Have you complained to the practice administrators that your otoscopes are inadequate?
These are not minor issues. It is clear that overdiagnosis of AOM happens. It may occur even more often than Dr. Pichichero suggests, but I doubt it is less. Overdiagnosis can result in overtreatment with antibiotics, and the cascade of consequences both for the patient, the community, and the environment. Overdiagnosis can be the first step on the path to unnecessary surgery. It is incumbent on all of us to make sure that our otoscopy skills and those of our colleagues are sharp, that our equipment is well maintained and that we remain abreast of the latest developments in the diagnosis and treatment of AOM.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
If the name Michael E. Pichichero, MD, is unfamiliar, you haven’t been reading some of the best articles on this website . Dr. Pichichero, an infectious disease specialist at the Research Institute at the Rochester General Hospital in New York, reports in his most recent ID Consult column on new research presented at the June 2019 meeting of the International Society for Otitis Media, including topics such as transtympanic antibiotic delivery, biofilms, probiotics, and biomarkers.
Dr. Pichichero described work he and his colleagues have been doing on the impact of overdiagnosis of acute otitis media (AOM). They found when “validated otoscopists” evaluated children, half as many reached the diagnostic threshold of being labeled “otitis prone” as when community-based pediatricians performed the exams.
Looking around at the colleagues with whom you share patients, do you find that some of them diagnose AOM much more frequently than does the coverage group average? How often do you see a child a day or two after he has been diagnosed with AOM by a colleague and find that the child’s tympanic membranes are transparent and mobile? Do you or your practice group keep track of each provider’s diagnostic tendencies? If these data exists, is there a mechanism for addressing apparent outliers? I suspect that the answer to those last two questions is a firm “No.”
I don’t have the stomach this morning to open those two cans of worms. But certainly Dr. Pichichero’s findings suggest that these are issues that need to be addressed. How the process should proceed in a nonthreatening way is a story for another day. But I’m not sure that involving your community ear, nose, and throat (ENT) specialist as a resource is the best answer. The scenarios in which pediatricians and ENTs perform otoscopies couldn’t be more different. In the pediatrician’s office, the child is generally sick, feverish, and possibly in pain. In the ENT’s office, the acute process has probably passed and the assessment may lean more heavily on history. The child is more likely to accept the exam without resistance, and the findings are not those of AOM but of a chronic process. The fact that Dr. Pichichero has been able to find and train “validated otoscopists” suggests that improving the quality of otoscopy among the physicians in communities like yours and mine is achievable.
How are your otoscopy skills? Do feel comfortable that you can do a good exam and accurately diagnose AOM? When did you acquire that comfort level? Probably not in medical school. More likely as a house officer when you were guided by a more senior house officer who may nor not been a master otoscopist. How would you rate your training? Or were you self-taught? Do you insufflate? Are you a skilled cerumen extractor? Or do you give up after one attempt? Be honest. How is your equipment? Are the bulbs and batteries fresh? Do you find yourself frustrated by an otoscope that is tethered to the wall charger by a cord that ensnarls you, the parent, and the patient? Have you complained to the practice administrators that your otoscopes are inadequate?
These are not minor issues. It is clear that overdiagnosis of AOM happens. It may occur even more often than Dr. Pichichero suggests, but I doubt it is less. Overdiagnosis can result in overtreatment with antibiotics, and the cascade of consequences both for the patient, the community, and the environment. Overdiagnosis can be the first step on the path to unnecessary surgery. It is incumbent on all of us to make sure that our otoscopy skills and those of our colleagues are sharp, that our equipment is well maintained and that we remain abreast of the latest developments in the diagnosis and treatment of AOM.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
If the name Michael E. Pichichero, MD, is unfamiliar, you haven’t been reading some of the best articles on this website . Dr. Pichichero, an infectious disease specialist at the Research Institute at the Rochester General Hospital in New York, reports in his most recent ID Consult column on new research presented at the June 2019 meeting of the International Society for Otitis Media, including topics such as transtympanic antibiotic delivery, biofilms, probiotics, and biomarkers.
Dr. Pichichero described work he and his colleagues have been doing on the impact of overdiagnosis of acute otitis media (AOM). They found when “validated otoscopists” evaluated children, half as many reached the diagnostic threshold of being labeled “otitis prone” as when community-based pediatricians performed the exams.
Looking around at the colleagues with whom you share patients, do you find that some of them diagnose AOM much more frequently than does the coverage group average? How often do you see a child a day or two after he has been diagnosed with AOM by a colleague and find that the child’s tympanic membranes are transparent and mobile? Do you or your practice group keep track of each provider’s diagnostic tendencies? If these data exists, is there a mechanism for addressing apparent outliers? I suspect that the answer to those last two questions is a firm “No.”
I don’t have the stomach this morning to open those two cans of worms. But certainly Dr. Pichichero’s findings suggest that these are issues that need to be addressed. How the process should proceed in a nonthreatening way is a story for another day. But I’m not sure that involving your community ear, nose, and throat (ENT) specialist as a resource is the best answer. The scenarios in which pediatricians and ENTs perform otoscopies couldn’t be more different. In the pediatrician’s office, the child is generally sick, feverish, and possibly in pain. In the ENT’s office, the acute process has probably passed and the assessment may lean more heavily on history. The child is more likely to accept the exam without resistance, and the findings are not those of AOM but of a chronic process. The fact that Dr. Pichichero has been able to find and train “validated otoscopists” suggests that improving the quality of otoscopy among the physicians in communities like yours and mine is achievable.
How are your otoscopy skills? Do feel comfortable that you can do a good exam and accurately diagnose AOM? When did you acquire that comfort level? Probably not in medical school. More likely as a house officer when you were guided by a more senior house officer who may nor not been a master otoscopist. How would you rate your training? Or were you self-taught? Do you insufflate? Are you a skilled cerumen extractor? Or do you give up after one attempt? Be honest. How is your equipment? Are the bulbs and batteries fresh? Do you find yourself frustrated by an otoscope that is tethered to the wall charger by a cord that ensnarls you, the parent, and the patient? Have you complained to the practice administrators that your otoscopes are inadequate?
These are not minor issues. It is clear that overdiagnosis of AOM happens. It may occur even more often than Dr. Pichichero suggests, but I doubt it is less. Overdiagnosis can result in overtreatment with antibiotics, and the cascade of consequences both for the patient, the community, and the environment. Overdiagnosis can be the first step on the path to unnecessary surgery. It is incumbent on all of us to make sure that our otoscopy skills and those of our colleagues are sharp, that our equipment is well maintained and that we remain abreast of the latest developments in the diagnosis and treatment of AOM.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Small study suggests natural HCV clearance is caused by AR3-antibody response
Individuals who spontaneously cleared their primary hepatitis C virus (HCV) infection or reinfection had significantly more antibodies that recognized multiple HCV genotypes beyond the initial infection, compared with chronically infected individuals, according to a small molecular study of immortalized cultured B cells from patient.
In a study published in the Journal of Hepatology, Sabrina J. Merat of AIMM Therapeutics and colleagues classified patients into two groups based on the outcome of their HCV infection: individuals who became chronically infected (CHRs; n = 5) either after primary infection or after HCV reinfection and individuals who cleared one or more HCV infections and were HCV RNA negative at the end of follow-up (CLs; n = 8). The researchers considered that all CLs who cleared the infection were presumably re-exposed to HCV as they continued injecting drugs for a median of 5.9 years after primary infection. The median follow-up time of individuals after primary HCV infection was 17.5 years.
Although the frequency of total antibodies did not differ between the two groups, the antibodies from CHRs were mainly genotype specific and directed against the genotype of the ongoing infection. Antibodies from CLs showed a much broader reactivity than CHR-derived antibodies, with the absolute number of antibodies recognizing at least three or more genotypes was significantly higher in CLs than in CHRs (13 vs. 0, respectively; P = .03).
In addition, in order to determine which epitopes were being targeted in the CL patients, the researchers tested the antibodies secreted in the B-cell supernatant for binding to E2 alanine mutants in the four epitopes known to be recognized by broadly neutralizing HCV antibodies. They found that the majority of the cross-genotype antibodies (82/113; 73%) were specific for AR3 because they bound to the AR3-specific mutants.
“In chronically infected individuals, AR3-specific antibody responses may be too weak and/or may develop too late to prevent chronic infection. If confirmed, this means that a strong and broadly neutralizing antibody response should be established very early after infection in order to confer protection,” the researchers concluded.
This study was supported by the Virgo consortium, funded by the Dutch government. Sabrina Merat and several coauthors are employees of AIMM Therapeutics, as well as shareholders.
SOURCE: Merat SJ et al. J Hepatol 2019;71:14-24.
Individuals who spontaneously cleared their primary hepatitis C virus (HCV) infection or reinfection had significantly more antibodies that recognized multiple HCV genotypes beyond the initial infection, compared with chronically infected individuals, according to a small molecular study of immortalized cultured B cells from patient.
In a study published in the Journal of Hepatology, Sabrina J. Merat of AIMM Therapeutics and colleagues classified patients into two groups based on the outcome of their HCV infection: individuals who became chronically infected (CHRs; n = 5) either after primary infection or after HCV reinfection and individuals who cleared one or more HCV infections and were HCV RNA negative at the end of follow-up (CLs; n = 8). The researchers considered that all CLs who cleared the infection were presumably re-exposed to HCV as they continued injecting drugs for a median of 5.9 years after primary infection. The median follow-up time of individuals after primary HCV infection was 17.5 years.
Although the frequency of total antibodies did not differ between the two groups, the antibodies from CHRs were mainly genotype specific and directed against the genotype of the ongoing infection. Antibodies from CLs showed a much broader reactivity than CHR-derived antibodies, with the absolute number of antibodies recognizing at least three or more genotypes was significantly higher in CLs than in CHRs (13 vs. 0, respectively; P = .03).
In addition, in order to determine which epitopes were being targeted in the CL patients, the researchers tested the antibodies secreted in the B-cell supernatant for binding to E2 alanine mutants in the four epitopes known to be recognized by broadly neutralizing HCV antibodies. They found that the majority of the cross-genotype antibodies (82/113; 73%) were specific for AR3 because they bound to the AR3-specific mutants.
“In chronically infected individuals, AR3-specific antibody responses may be too weak and/or may develop too late to prevent chronic infection. If confirmed, this means that a strong and broadly neutralizing antibody response should be established very early after infection in order to confer protection,” the researchers concluded.
This study was supported by the Virgo consortium, funded by the Dutch government. Sabrina Merat and several coauthors are employees of AIMM Therapeutics, as well as shareholders.
SOURCE: Merat SJ et al. J Hepatol 2019;71:14-24.
Individuals who spontaneously cleared their primary hepatitis C virus (HCV) infection or reinfection had significantly more antibodies that recognized multiple HCV genotypes beyond the initial infection, compared with chronically infected individuals, according to a small molecular study of immortalized cultured B cells from patient.
In a study published in the Journal of Hepatology, Sabrina J. Merat of AIMM Therapeutics and colleagues classified patients into two groups based on the outcome of their HCV infection: individuals who became chronically infected (CHRs; n = 5) either after primary infection or after HCV reinfection and individuals who cleared one or more HCV infections and were HCV RNA negative at the end of follow-up (CLs; n = 8). The researchers considered that all CLs who cleared the infection were presumably re-exposed to HCV as they continued injecting drugs for a median of 5.9 years after primary infection. The median follow-up time of individuals after primary HCV infection was 17.5 years.
Although the frequency of total antibodies did not differ between the two groups, the antibodies from CHRs were mainly genotype specific and directed against the genotype of the ongoing infection. Antibodies from CLs showed a much broader reactivity than CHR-derived antibodies, with the absolute number of antibodies recognizing at least three or more genotypes was significantly higher in CLs than in CHRs (13 vs. 0, respectively; P = .03).
In addition, in order to determine which epitopes were being targeted in the CL patients, the researchers tested the antibodies secreted in the B-cell supernatant for binding to E2 alanine mutants in the four epitopes known to be recognized by broadly neutralizing HCV antibodies. They found that the majority of the cross-genotype antibodies (82/113; 73%) were specific for AR3 because they bound to the AR3-specific mutants.
“In chronically infected individuals, AR3-specific antibody responses may be too weak and/or may develop too late to prevent chronic infection. If confirmed, this means that a strong and broadly neutralizing antibody response should be established very early after infection in order to confer protection,” the researchers concluded.
This study was supported by the Virgo consortium, funded by the Dutch government. Sabrina Merat and several coauthors are employees of AIMM Therapeutics, as well as shareholders.
SOURCE: Merat SJ et al. J Hepatol 2019;71:14-24.
FROM THE JOURNAL OF HEPATOLOGY
New oral polio vaccine is noninferior to currently licensed vaccine
according to Khalequ Zaman, MBBS, PhD, of the International Center for Diarrheal Disease Research in Dhaka, Bangladesh, and associates.
In the first part of the observer-blind, randomized, controlled study, 40 patients aged 5-6 years received either the new vaccine (BBio bOPV) or the licensed vaccine (SII bOPV). In the second part, 1,080 patients aged 6-8 weeks received either BBio bOPV or SII bOPV at age 6, 10, and 14 weeks. Blood samples were taken to assess neutralizing antibody responses against poliovirus types 1 and 3, and safety also was assessed.
In the first part of the study, 12 adverse events were reported, none of which were serious and none of which were related to the vaccines. All participants demonstrated seroprotective titers against both poliovirus types 1 month after vaccination.
In the second part, more than 96% of infants demonstrated seroprotection and seroconversion against both poliovirus types. Geometric mean titers were equivalent in both groups. A total of 387 participants had at least one adverse event, and 18 serious adverse events were reported. None of these were related to the vaccines.
“The BBio bOPV has been proven safe and immunogenic in the target infant population in Bangladesh. As the use of bOPV is expected to be continued until at least 2022, availability of new bOPV bulk manufacturer will be helpful in securing adequate supplies of bOPV for global demand in the polio endgame strategy,” the investigators concluded.
The study was funded by Bilthoven Biologicals. Four coauthors are employed by Bilthoven, manufacturer of the study vaccine; two others are employed by the Serum Institute of India, which provided the control vaccine.
SOURCE: Zaman K et al. Vaccine. 2019 Jun 22. doi: 10.1016/j.vaccine.2019.06.048.
according to Khalequ Zaman, MBBS, PhD, of the International Center for Diarrheal Disease Research in Dhaka, Bangladesh, and associates.
In the first part of the observer-blind, randomized, controlled study, 40 patients aged 5-6 years received either the new vaccine (BBio bOPV) or the licensed vaccine (SII bOPV). In the second part, 1,080 patients aged 6-8 weeks received either BBio bOPV or SII bOPV at age 6, 10, and 14 weeks. Blood samples were taken to assess neutralizing antibody responses against poliovirus types 1 and 3, and safety also was assessed.
In the first part of the study, 12 adverse events were reported, none of which were serious and none of which were related to the vaccines. All participants demonstrated seroprotective titers against both poliovirus types 1 month after vaccination.
In the second part, more than 96% of infants demonstrated seroprotection and seroconversion against both poliovirus types. Geometric mean titers were equivalent in both groups. A total of 387 participants had at least one adverse event, and 18 serious adverse events were reported. None of these were related to the vaccines.
“The BBio bOPV has been proven safe and immunogenic in the target infant population in Bangladesh. As the use of bOPV is expected to be continued until at least 2022, availability of new bOPV bulk manufacturer will be helpful in securing adequate supplies of bOPV for global demand in the polio endgame strategy,” the investigators concluded.
The study was funded by Bilthoven Biologicals. Four coauthors are employed by Bilthoven, manufacturer of the study vaccine; two others are employed by the Serum Institute of India, which provided the control vaccine.
SOURCE: Zaman K et al. Vaccine. 2019 Jun 22. doi: 10.1016/j.vaccine.2019.06.048.
according to Khalequ Zaman, MBBS, PhD, of the International Center for Diarrheal Disease Research in Dhaka, Bangladesh, and associates.
In the first part of the observer-blind, randomized, controlled study, 40 patients aged 5-6 years received either the new vaccine (BBio bOPV) or the licensed vaccine (SII bOPV). In the second part, 1,080 patients aged 6-8 weeks received either BBio bOPV or SII bOPV at age 6, 10, and 14 weeks. Blood samples were taken to assess neutralizing antibody responses against poliovirus types 1 and 3, and safety also was assessed.
In the first part of the study, 12 adverse events were reported, none of which were serious and none of which were related to the vaccines. All participants demonstrated seroprotective titers against both poliovirus types 1 month after vaccination.
In the second part, more than 96% of infants demonstrated seroprotection and seroconversion against both poliovirus types. Geometric mean titers were equivalent in both groups. A total of 387 participants had at least one adverse event, and 18 serious adverse events were reported. None of these were related to the vaccines.
“The BBio bOPV has been proven safe and immunogenic in the target infant population in Bangladesh. As the use of bOPV is expected to be continued until at least 2022, availability of new bOPV bulk manufacturer will be helpful in securing adequate supplies of bOPV for global demand in the polio endgame strategy,” the investigators concluded.
The study was funded by Bilthoven Biologicals. Four coauthors are employed by Bilthoven, manufacturer of the study vaccine; two others are employed by the Serum Institute of India, which provided the control vaccine.
SOURCE: Zaman K et al. Vaccine. 2019 Jun 22. doi: 10.1016/j.vaccine.2019.06.048.
FROM VACCINE
Increased daily water intake benefits premenopausal women with recurrent UTIs
Background: Acute cystitis is a common condition in women and associated with morbidity. A commonly recommended preventative measure is increased oral hydration, but there is limited evidence to support this claim.
Study design: Open-label, randomized, controlled study.
Setting: Clinical research center based in Sofia, Bulgaria.
Synopsis: A 12-month trial done at a clinical research center including healthy women with recurrent cystitis who drank less than 1.5 L of fluid daily. One group was instructed to drink 1.5 L of water/day in addition to their normal fluid intake, and the other was advised not to drink any additional fluid. The mean number of cystitis episodes in the intervention group was 1.7 (95% confidence interval, 1.5-1.8), compared with 3.2 (95% CI, 3.0-3.4) in the control group, which was a statistically significant difference of 1.5 (95% CI, 1.2-1.8; P less than .01).
Though antibiotic prophylaxis is more effective at reducing cystitis, increased daily water intake is a safe and inexpensive method to prevent cystitis without increasing exposure to antimicrobial therapy. This study did rely on information obtained from phone calls with patients. It is also an open-label study design in which patients were not blinded to their assigned group. This would be less of an issue if episodes of cystitis were confirmed with culture. Another limitation of this study is that it included only ambulatory patients and excluded patients with pyelonephritis, so it may be less applicable to our hospitalized patients.
Bottom line: This study shows a benefit in recurrent cystitis by increased water intake in premenopausal women.
Citation: Hooton TM et al. Effect of increased daily water intake in premenopausal women with recurrent urinary tract infections. JAMA Intern Med. 2018 Nov;178(11):1509-15.
Dr. Astik is medical director, clinical documentation, at Northwestern University Feinberg School of Medicine and a hospitalist at Northwestern Memorial Hospital, both in Chicago.
Background: Acute cystitis is a common condition in women and associated with morbidity. A commonly recommended preventative measure is increased oral hydration, but there is limited evidence to support this claim.
Study design: Open-label, randomized, controlled study.
Setting: Clinical research center based in Sofia, Bulgaria.
Synopsis: A 12-month trial done at a clinical research center including healthy women with recurrent cystitis who drank less than 1.5 L of fluid daily. One group was instructed to drink 1.5 L of water/day in addition to their normal fluid intake, and the other was advised not to drink any additional fluid. The mean number of cystitis episodes in the intervention group was 1.7 (95% confidence interval, 1.5-1.8), compared with 3.2 (95% CI, 3.0-3.4) in the control group, which was a statistically significant difference of 1.5 (95% CI, 1.2-1.8; P less than .01).
Though antibiotic prophylaxis is more effective at reducing cystitis, increased daily water intake is a safe and inexpensive method to prevent cystitis without increasing exposure to antimicrobial therapy. This study did rely on information obtained from phone calls with patients. It is also an open-label study design in which patients were not blinded to their assigned group. This would be less of an issue if episodes of cystitis were confirmed with culture. Another limitation of this study is that it included only ambulatory patients and excluded patients with pyelonephritis, so it may be less applicable to our hospitalized patients.
Bottom line: This study shows a benefit in recurrent cystitis by increased water intake in premenopausal women.
Citation: Hooton TM et al. Effect of increased daily water intake in premenopausal women with recurrent urinary tract infections. JAMA Intern Med. 2018 Nov;178(11):1509-15.
Dr. Astik is medical director, clinical documentation, at Northwestern University Feinberg School of Medicine and a hospitalist at Northwestern Memorial Hospital, both in Chicago.
Background: Acute cystitis is a common condition in women and associated with morbidity. A commonly recommended preventative measure is increased oral hydration, but there is limited evidence to support this claim.
Study design: Open-label, randomized, controlled study.
Setting: Clinical research center based in Sofia, Bulgaria.
Synopsis: A 12-month trial done at a clinical research center including healthy women with recurrent cystitis who drank less than 1.5 L of fluid daily. One group was instructed to drink 1.5 L of water/day in addition to their normal fluid intake, and the other was advised not to drink any additional fluid. The mean number of cystitis episodes in the intervention group was 1.7 (95% confidence interval, 1.5-1.8), compared with 3.2 (95% CI, 3.0-3.4) in the control group, which was a statistically significant difference of 1.5 (95% CI, 1.2-1.8; P less than .01).
Though antibiotic prophylaxis is more effective at reducing cystitis, increased daily water intake is a safe and inexpensive method to prevent cystitis without increasing exposure to antimicrobial therapy. This study did rely on information obtained from phone calls with patients. It is also an open-label study design in which patients were not blinded to their assigned group. This would be less of an issue if episodes of cystitis were confirmed with culture. Another limitation of this study is that it included only ambulatory patients and excluded patients with pyelonephritis, so it may be less applicable to our hospitalized patients.
Bottom line: This study shows a benefit in recurrent cystitis by increased water intake in premenopausal women.
Citation: Hooton TM et al. Effect of increased daily water intake in premenopausal women with recurrent urinary tract infections. JAMA Intern Med. 2018 Nov;178(11):1509-15.
Dr. Astik is medical director, clinical documentation, at Northwestern University Feinberg School of Medicine and a hospitalist at Northwestern Memorial Hospital, both in Chicago.
Ebola outbreak: WHO/OCHA call for more aid, better security
The continuing outbreak of Ebola in the Democratic Republic of the Congo (DRC) was the subject of a special United Nations high-level event organized by the World Health Organization (WHO) and the United Nations Office for the Coordination of Humanitarian Affairs (OCHA). It was comoderated by Tedros Adhanom Ghebreyesus, Director-General of the World Health Organization, and Mark Lowcock, the UN Under-Secretary-General for Humanitarian Affairs and Emergency Relief Coordinator.

The DRC Ebola outbreak has drawn continuing concern and was highlighted by an even greater feeling of urgency as “on the eve of the conference,” according to one speaker.
That same infected individual – a priest arriving by bus to the city from an affected area – died of the disease the day after the conference concluded, according to the DRC authorities.
In his opening remarks, Mr. Lowcock stressed the importance of coordinating international efforts with the on-the-ground responses being carried out under the direction of Oly Ilunga Kalenga, MD, the DRC’s Minister of Public Health, who was also present and spoke at the meeting. Dr. Kalenga resigned his post on July 22, 2019.*
Mr. Lowcock stressed three points in particular that make for unique changes to the current response, compared with the earlier outbreak of 2014-2016.
First, in the previous outbreak in West Africa, “we didn’t have the vaccine and we didn’t have some of the successful treatments” that are currently available. Furthermore, more than 160,000 people have now been vaccinated and “the vaccine has a high degree of effectiveness.” This is an asset compared to the previous situation, he stated.
In his second point, he warned that the outbreak in the DRC “is taking place in an insecure and complex area with multiple armed groups present and large-scale preexisting humanitarian needs. Special interests distort the context. A history of disaffection with national authorities and foreigners generates distrust and makes the response more complicated. And one manifestation of that is attacks against health facilities and health care workers.” He added that “two more of our colleagues, trying to be part of the solution,” were killed in the past few days before the meeting. “Therefore, security for the response is of absolutely paramount importance, and we are trying to strengthen the way the UN family supports the government’s own security.”
The third major difference from the West Africa outbreak, Mr. Lowcock pointed out, was the issue of money. There was more than $2 billion in international support available for that earlier response. However, “what we have available for us in the DRC is just a small fraction of that. Donors released funds early on ... but much more is needed.” He warned that the cost of reaching zero cases must not be underestimated, and that the fourth strategic response plan for this outbreak, currently under development, “will be budgeted at a much higher level than the previous three plans, and that’s because it’s our assessment that we need a bigger, more comprehensive response if we’re to get to zero cases than we’ve had hitherto.”
In fact, he said, “unless there is a big scale up in the response, we’re unlikely to get to zero cases.”
The meeting also featured speakers outlining more local aspects of the response and discussing how international workers were coordinating more and more with local authorities and health practitioners in order to deliver health care on the ground while attempting to avoid the distrust created in the past, while still ensuring security for foreign personnel.
Commenting on the issue of security, Rory Stewart, the United Kingdom’s Secretary of State for International Development, described how a major DRC Ebola treatment center was attacked and burned by military insurgents, but is now rebuilt. He said that, while there have been some improvements and reasons to be hopeful, “this isn’t a moment for complacency; [the situation] is literally on the knife-edge.” He added: “If you go into that treatment center now, you will see that, although there are very good medical procedures, there are really, really worrying security procedures. The entire protection for the medical staff consists of a small square of sandbags about the height of this table [he raised his hand just above the standard conference table he was sitting at], behind which the doctors and nurses are supposed to hide if armed men get into the compound.”
In his presentation, David Gressly, the UN Emergency Ebola Response Coordinator (speaking by video from the DRC), stressed the need to cooperate with local authorities and to build local trust, stating that “the UN is putting together a tight, disciplined, coordinated system for rapid response and operational adjustments so that we can shift from chasing the disease to getting ahead of it. We need to quickly detect cases ... that have moved into areas of risk to stop the transmission early.”
Matshidiso Moeti, MD, WHO Regional Director for Africa, added in her presentation: “We’ve identified nine high-risk countries. Among those, Burundi, South Sudan, and Uganda face the highest risk and require our concerted and continued efforts.” She said that more than 10,000 health care workers in areas of high risk have so far been vaccinated against the disease.
In his concluding statement, Dr. Kalenga described the current state of affairs in his country with a modicum of hope. “A community that has been told it has a case of Ebola is a community that is traumatized by the very announcement of this epidemic. With time, the community has learned to face up to this epidemic differently. In some villages we are given a very different welcome than before. ... The villagers ask: ‘What should we do to make sure the Ebola case is the only one? The first and the last one?’ Throughout this epidemic, we have seen the people become more aware, and a certain acceptance of the very difficult and lethal diagnosis. ... So we have seen that work in the community has been maturing and bearing fruit.”
However, he pointed out, “there is a whole debate around the area of vaccinations, and we do need to close down this debate. At this point in time, we have a vaccine that is highly effective, a vaccine that is accepted by the population, after whole periods of mistrust. So we’ve come to a point in time when the population is accepting a vaccine, a vaccine that works, so we decided to no longer open the debate on vaccines and vaccination. ....We don’t want contradictory messages going out here, we don’t want different schemes going out. ...We have an effective weapon, we have an effective molecule. Let’s focus on that. Let’s all go in the same direction,” he concluded.
On July 11, an announcement by DRC officials stated that Merck’s rVSV-ZEBOV would be the only vaccine that will be used during the current Ebola outbreak in North Kivu and Ituri provinces, and that no other clinical vaccine trials to be allowed in the country so as not to confuse the population.
In that same announcement, the DRC reported that, since the beginning of the epidemic, the cumulative number of Ebola cases was 2,451, of which 2,357 were confirmed and 94 probable. There were 1,647 deaths (1,553 confirmed and 94 probable) and 683 people who survived. An additional 364 suspected cases were under investigation.
*Updated Aug. 1, 2019.
SOURCE: United Nations WHO/OCHA Webcast and Media Stakeout. July 15, 2019.
The continuing outbreak of Ebola in the Democratic Republic of the Congo (DRC) was the subject of a special United Nations high-level event organized by the World Health Organization (WHO) and the United Nations Office for the Coordination of Humanitarian Affairs (OCHA). It was comoderated by Tedros Adhanom Ghebreyesus, Director-General of the World Health Organization, and Mark Lowcock, the UN Under-Secretary-General for Humanitarian Affairs and Emergency Relief Coordinator.

The DRC Ebola outbreak has drawn continuing concern and was highlighted by an even greater feeling of urgency as “on the eve of the conference,” according to one speaker.
That same infected individual – a priest arriving by bus to the city from an affected area – died of the disease the day after the conference concluded, according to the DRC authorities.
In his opening remarks, Mr. Lowcock stressed the importance of coordinating international efforts with the on-the-ground responses being carried out under the direction of Oly Ilunga Kalenga, MD, the DRC’s Minister of Public Health, who was also present and spoke at the meeting. Dr. Kalenga resigned his post on July 22, 2019.*
Mr. Lowcock stressed three points in particular that make for unique changes to the current response, compared with the earlier outbreak of 2014-2016.
First, in the previous outbreak in West Africa, “we didn’t have the vaccine and we didn’t have some of the successful treatments” that are currently available. Furthermore, more than 160,000 people have now been vaccinated and “the vaccine has a high degree of effectiveness.” This is an asset compared to the previous situation, he stated.
In his second point, he warned that the outbreak in the DRC “is taking place in an insecure and complex area with multiple armed groups present and large-scale preexisting humanitarian needs. Special interests distort the context. A history of disaffection with national authorities and foreigners generates distrust and makes the response more complicated. And one manifestation of that is attacks against health facilities and health care workers.” He added that “two more of our colleagues, trying to be part of the solution,” were killed in the past few days before the meeting. “Therefore, security for the response is of absolutely paramount importance, and we are trying to strengthen the way the UN family supports the government’s own security.”
The third major difference from the West Africa outbreak, Mr. Lowcock pointed out, was the issue of money. There was more than $2 billion in international support available for that earlier response. However, “what we have available for us in the DRC is just a small fraction of that. Donors released funds early on ... but much more is needed.” He warned that the cost of reaching zero cases must not be underestimated, and that the fourth strategic response plan for this outbreak, currently under development, “will be budgeted at a much higher level than the previous three plans, and that’s because it’s our assessment that we need a bigger, more comprehensive response if we’re to get to zero cases than we’ve had hitherto.”
In fact, he said, “unless there is a big scale up in the response, we’re unlikely to get to zero cases.”
The meeting also featured speakers outlining more local aspects of the response and discussing how international workers were coordinating more and more with local authorities and health practitioners in order to deliver health care on the ground while attempting to avoid the distrust created in the past, while still ensuring security for foreign personnel.
Commenting on the issue of security, Rory Stewart, the United Kingdom’s Secretary of State for International Development, described how a major DRC Ebola treatment center was attacked and burned by military insurgents, but is now rebuilt. He said that, while there have been some improvements and reasons to be hopeful, “this isn’t a moment for complacency; [the situation] is literally on the knife-edge.” He added: “If you go into that treatment center now, you will see that, although there are very good medical procedures, there are really, really worrying security procedures. The entire protection for the medical staff consists of a small square of sandbags about the height of this table [he raised his hand just above the standard conference table he was sitting at], behind which the doctors and nurses are supposed to hide if armed men get into the compound.”
In his presentation, David Gressly, the UN Emergency Ebola Response Coordinator (speaking by video from the DRC), stressed the need to cooperate with local authorities and to build local trust, stating that “the UN is putting together a tight, disciplined, coordinated system for rapid response and operational adjustments so that we can shift from chasing the disease to getting ahead of it. We need to quickly detect cases ... that have moved into areas of risk to stop the transmission early.”
Matshidiso Moeti, MD, WHO Regional Director for Africa, added in her presentation: “We’ve identified nine high-risk countries. Among those, Burundi, South Sudan, and Uganda face the highest risk and require our concerted and continued efforts.” She said that more than 10,000 health care workers in areas of high risk have so far been vaccinated against the disease.
In his concluding statement, Dr. Kalenga described the current state of affairs in his country with a modicum of hope. “A community that has been told it has a case of Ebola is a community that is traumatized by the very announcement of this epidemic. With time, the community has learned to face up to this epidemic differently. In some villages we are given a very different welcome than before. ... The villagers ask: ‘What should we do to make sure the Ebola case is the only one? The first and the last one?’ Throughout this epidemic, we have seen the people become more aware, and a certain acceptance of the very difficult and lethal diagnosis. ... So we have seen that work in the community has been maturing and bearing fruit.”
However, he pointed out, “there is a whole debate around the area of vaccinations, and we do need to close down this debate. At this point in time, we have a vaccine that is highly effective, a vaccine that is accepted by the population, after whole periods of mistrust. So we’ve come to a point in time when the population is accepting a vaccine, a vaccine that works, so we decided to no longer open the debate on vaccines and vaccination. ....We don’t want contradictory messages going out here, we don’t want different schemes going out. ...We have an effective weapon, we have an effective molecule. Let’s focus on that. Let’s all go in the same direction,” he concluded.
On July 11, an announcement by DRC officials stated that Merck’s rVSV-ZEBOV would be the only vaccine that will be used during the current Ebola outbreak in North Kivu and Ituri provinces, and that no other clinical vaccine trials to be allowed in the country so as not to confuse the population.
In that same announcement, the DRC reported that, since the beginning of the epidemic, the cumulative number of Ebola cases was 2,451, of which 2,357 were confirmed and 94 probable. There were 1,647 deaths (1,553 confirmed and 94 probable) and 683 people who survived. An additional 364 suspected cases were under investigation.
*Updated Aug. 1, 2019.
SOURCE: United Nations WHO/OCHA Webcast and Media Stakeout. July 15, 2019.
The continuing outbreak of Ebola in the Democratic Republic of the Congo (DRC) was the subject of a special United Nations high-level event organized by the World Health Organization (WHO) and the United Nations Office for the Coordination of Humanitarian Affairs (OCHA). It was comoderated by Tedros Adhanom Ghebreyesus, Director-General of the World Health Organization, and Mark Lowcock, the UN Under-Secretary-General for Humanitarian Affairs and Emergency Relief Coordinator.

The DRC Ebola outbreak has drawn continuing concern and was highlighted by an even greater feeling of urgency as “on the eve of the conference,” according to one speaker.
That same infected individual – a priest arriving by bus to the city from an affected area – died of the disease the day after the conference concluded, according to the DRC authorities.
In his opening remarks, Mr. Lowcock stressed the importance of coordinating international efforts with the on-the-ground responses being carried out under the direction of Oly Ilunga Kalenga, MD, the DRC’s Minister of Public Health, who was also present and spoke at the meeting. Dr. Kalenga resigned his post on July 22, 2019.*
Mr. Lowcock stressed three points in particular that make for unique changes to the current response, compared with the earlier outbreak of 2014-2016.
First, in the previous outbreak in West Africa, “we didn’t have the vaccine and we didn’t have some of the successful treatments” that are currently available. Furthermore, more than 160,000 people have now been vaccinated and “the vaccine has a high degree of effectiveness.” This is an asset compared to the previous situation, he stated.
In his second point, he warned that the outbreak in the DRC “is taking place in an insecure and complex area with multiple armed groups present and large-scale preexisting humanitarian needs. Special interests distort the context. A history of disaffection with national authorities and foreigners generates distrust and makes the response more complicated. And one manifestation of that is attacks against health facilities and health care workers.” He added that “two more of our colleagues, trying to be part of the solution,” were killed in the past few days before the meeting. “Therefore, security for the response is of absolutely paramount importance, and we are trying to strengthen the way the UN family supports the government’s own security.”
The third major difference from the West Africa outbreak, Mr. Lowcock pointed out, was the issue of money. There was more than $2 billion in international support available for that earlier response. However, “what we have available for us in the DRC is just a small fraction of that. Donors released funds early on ... but much more is needed.” He warned that the cost of reaching zero cases must not be underestimated, and that the fourth strategic response plan for this outbreak, currently under development, “will be budgeted at a much higher level than the previous three plans, and that’s because it’s our assessment that we need a bigger, more comprehensive response if we’re to get to zero cases than we’ve had hitherto.”
In fact, he said, “unless there is a big scale up in the response, we’re unlikely to get to zero cases.”
The meeting also featured speakers outlining more local aspects of the response and discussing how international workers were coordinating more and more with local authorities and health practitioners in order to deliver health care on the ground while attempting to avoid the distrust created in the past, while still ensuring security for foreign personnel.
Commenting on the issue of security, Rory Stewart, the United Kingdom’s Secretary of State for International Development, described how a major DRC Ebola treatment center was attacked and burned by military insurgents, but is now rebuilt. He said that, while there have been some improvements and reasons to be hopeful, “this isn’t a moment for complacency; [the situation] is literally on the knife-edge.” He added: “If you go into that treatment center now, you will see that, although there are very good medical procedures, there are really, really worrying security procedures. The entire protection for the medical staff consists of a small square of sandbags about the height of this table [he raised his hand just above the standard conference table he was sitting at], behind which the doctors and nurses are supposed to hide if armed men get into the compound.”
In his presentation, David Gressly, the UN Emergency Ebola Response Coordinator (speaking by video from the DRC), stressed the need to cooperate with local authorities and to build local trust, stating that “the UN is putting together a tight, disciplined, coordinated system for rapid response and operational adjustments so that we can shift from chasing the disease to getting ahead of it. We need to quickly detect cases ... that have moved into areas of risk to stop the transmission early.”
Matshidiso Moeti, MD, WHO Regional Director for Africa, added in her presentation: “We’ve identified nine high-risk countries. Among those, Burundi, South Sudan, and Uganda face the highest risk and require our concerted and continued efforts.” She said that more than 10,000 health care workers in areas of high risk have so far been vaccinated against the disease.
In his concluding statement, Dr. Kalenga described the current state of affairs in his country with a modicum of hope. “A community that has been told it has a case of Ebola is a community that is traumatized by the very announcement of this epidemic. With time, the community has learned to face up to this epidemic differently. In some villages we are given a very different welcome than before. ... The villagers ask: ‘What should we do to make sure the Ebola case is the only one? The first and the last one?’ Throughout this epidemic, we have seen the people become more aware, and a certain acceptance of the very difficult and lethal diagnosis. ... So we have seen that work in the community has been maturing and bearing fruit.”
However, he pointed out, “there is a whole debate around the area of vaccinations, and we do need to close down this debate. At this point in time, we have a vaccine that is highly effective, a vaccine that is accepted by the population, after whole periods of mistrust. So we’ve come to a point in time when the population is accepting a vaccine, a vaccine that works, so we decided to no longer open the debate on vaccines and vaccination. ....We don’t want contradictory messages going out here, we don’t want different schemes going out. ...We have an effective weapon, we have an effective molecule. Let’s focus on that. Let’s all go in the same direction,” he concluded.
On July 11, an announcement by DRC officials stated that Merck’s rVSV-ZEBOV would be the only vaccine that will be used during the current Ebola outbreak in North Kivu and Ituri provinces, and that no other clinical vaccine trials to be allowed in the country so as not to confuse the population.
In that same announcement, the DRC reported that, since the beginning of the epidemic, the cumulative number of Ebola cases was 2,451, of which 2,357 were confirmed and 94 probable. There were 1,647 deaths (1,553 confirmed and 94 probable) and 683 people who survived. An additional 364 suspected cases were under investigation.
*Updated Aug. 1, 2019.
SOURCE: United Nations WHO/OCHA Webcast and Media Stakeout. July 15, 2019.
REPORTING FROM A UN MEETING LIVE WEBCAST
Rash on elbows and hands
Based on the target lesions with central epithelial disruption, the FP diagnosed erythema multiforme (EM) in this patient. He also diagnosed genital herpes simplex in the crusting stage and suspected that it was the inciting event for the EM.
EM is considered a hypersensitivity reaction that is often secondary to infections or medications. Herpes simplex viruses (HSVI and HSV2) are the most common causative agents and have been implicated in at least 60% of cases.
The patient did not know that she had genital herpes simplex but remembered having sores in the genital area that preceded the similar rash a year earlier. This was a case of recurrent EM responding to recurrent herpes simplex.
The patient suspected that she had genital herpes simplex on and off for the past 10 years but lacked health insurance, so she never visited a doctor for treatment. She recently obtained health insurance and was willing to be tested for other sexually transmitted diseases. She also wanted to have a test done to confirm this was herpes simplex. The FP explained that it was unlikely that an antiviral agent would help the current case of herpes simplex and the associated EM because of the late timing, but antiviral medication could help to prevent further outbreaks and decrease transmission to her husband.
The patient was enthusiastic to start valacyclovir 500 mg/d for prophylaxis. For symptomatic relief, the FP prescribed a 30-g tube of 0.1% triamcinolone appointment and instructed the patient to apply it to the EM twice daily. The patient was sent for blood tests for syphilis and HIV; fortunately, both came back negative. The herpes simplex PCR test was positive for HSV2.
On a 1-week follow-up visit, the skin lesions were resolving, and the patient had no further symptoms. The patient said she understood that she should refrain from sexual intercourse if she developed lesions while on prophylaxis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Milana C, Smith M. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1161-1168.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

Based on the target lesions with central epithelial disruption, the FP diagnosed erythema multiforme (EM) in this patient. He also diagnosed genital herpes simplex in the crusting stage and suspected that it was the inciting event for the EM.
EM is considered a hypersensitivity reaction that is often secondary to infections or medications. Herpes simplex viruses (HSVI and HSV2) are the most common causative agents and have been implicated in at least 60% of cases.
The patient did not know that she had genital herpes simplex but remembered having sores in the genital area that preceded the similar rash a year earlier. This was a case of recurrent EM responding to recurrent herpes simplex.
The patient suspected that she had genital herpes simplex on and off for the past 10 years but lacked health insurance, so she never visited a doctor for treatment. She recently obtained health insurance and was willing to be tested for other sexually transmitted diseases. She also wanted to have a test done to confirm this was herpes simplex. The FP explained that it was unlikely that an antiviral agent would help the current case of herpes simplex and the associated EM because of the late timing, but antiviral medication could help to prevent further outbreaks and decrease transmission to her husband.
The patient was enthusiastic to start valacyclovir 500 mg/d for prophylaxis. For symptomatic relief, the FP prescribed a 30-g tube of 0.1% triamcinolone appointment and instructed the patient to apply it to the EM twice daily. The patient was sent for blood tests for syphilis and HIV; fortunately, both came back negative. The herpes simplex PCR test was positive for HSV2.
On a 1-week follow-up visit, the skin lesions were resolving, and the patient had no further symptoms. The patient said she understood that she should refrain from sexual intercourse if she developed lesions while on prophylaxis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Milana C, Smith M. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1161-1168.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

Based on the target lesions with central epithelial disruption, the FP diagnosed erythema multiforme (EM) in this patient. He also diagnosed genital herpes simplex in the crusting stage and suspected that it was the inciting event for the EM.
EM is considered a hypersensitivity reaction that is often secondary to infections or medications. Herpes simplex viruses (HSVI and HSV2) are the most common causative agents and have been implicated in at least 60% of cases.
The patient did not know that she had genital herpes simplex but remembered having sores in the genital area that preceded the similar rash a year earlier. This was a case of recurrent EM responding to recurrent herpes simplex.
The patient suspected that she had genital herpes simplex on and off for the past 10 years but lacked health insurance, so she never visited a doctor for treatment. She recently obtained health insurance and was willing to be tested for other sexually transmitted diseases. She also wanted to have a test done to confirm this was herpes simplex. The FP explained that it was unlikely that an antiviral agent would help the current case of herpes simplex and the associated EM because of the late timing, but antiviral medication could help to prevent further outbreaks and decrease transmission to her husband.
The patient was enthusiastic to start valacyclovir 500 mg/d for prophylaxis. For symptomatic relief, the FP prescribed a 30-g tube of 0.1% triamcinolone appointment and instructed the patient to apply it to the EM twice daily. The patient was sent for blood tests for syphilis and HIV; fortunately, both came back negative. The herpes simplex PCR test was positive for HSV2.
On a 1-week follow-up visit, the skin lesions were resolving, and the patient had no further symptoms. The patient said she understood that she should refrain from sexual intercourse if she developed lesions while on prophylaxis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Milana C, Smith M. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1161-1168.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
New scale could measure vaccine hesitancy in developing countries
By measuring parents’ attitudes regarding disease salience and community benefit, high scores on a four-item scale was associated with fivefold greater likelihood of not fully vaccinating their children, according to a study published in the Pediatric Infectious Disease Journal.
Mohammad Tahir Yousafzai, MPH, of Aga Khan University, Karachi, Pakistan, and colleagues developed a larger 14-item scale to measure parental attitudes and surveyed 901 households in the Sindh province of Pakistan during 2014. Part of this scale was a short 4-item subscale focusing on disease salience and community benefit, whereas the remaining 10 items form another subscale that measures parents’ perceptions and concerns regarding vaccines directly. The items are presented as 1-5 Likert scales, and scoring higher represents holding more negative attitudes regarding vaccines.
Of the 901 households surveyed, 25% of children were fully vaccinated, which meant children received all primary vaccines up to 14 weeks of age, and 54% were partially vaccinated, which meant at least one of those primary vaccines had been missed. The remaining 21% were unvaccinated.
High scores on the full 14-item scale showed some correlation with no vaccination versus partial or full vaccination (odds ratio, 3.05; 95% confidence interval, 1.75-5.31); the association disappeared after adjustment for children’s age and gender. The subscales performed better after adjustment: The adjusted ORs were 1.52 (95% CI, 1.05-2.21) for the longer subscale and 5.21 (95% CI, 3.60-7.55) for the shorter subscale. The data also showed high association between high scores on the shorter subscale and the likelihood of no or only partial vaccination (aOR, 9.65; 95% CI, 4.81-19.37).
The researchers noted that most similar scales used in developed countries are longer (10 or more items) and have lower internal consistency. This four-item scale, on the other hand, may be especially useful among lower-income populations and those in developing areas that have lower literacy rates.
One of the limitations of the study was that it was conducted only in Pakistan and not multiple developing countries; the researchers acknowledged this could limit generalizability. Another limitation is that the study size was too small for subdomain analysis; the researchers wrote that, although a lack of such analysis is unfortunate, it shouldn’t hamper the shorter subscale’s usability. A further limitation is that there was little variability across Likert scores – mostly answers at the extreme ends rather than a mix. This suggests that interviewees may not have understood how Likert scales work and therefore may not have answered accurately, noted the researchers, who employed a visual chart, trained interviewers, and field monitoring to mitigate this possibility.
“Measurement of the parental attitudes toward childhood vaccination is very important for the appropriate planning of strategies for increasing vaccine coverage and for monitoring,” they wrote.
The study was sponsored by Gavi, The Vaccine Alliance. The authors had no funding or conflicts of interest to disclose.
SOURCE: Yousafzai MT et al. Pediatr Infect Dis J. 2019 Jul;38(7):e143-8.
By measuring parents’ attitudes regarding disease salience and community benefit, high scores on a four-item scale was associated with fivefold greater likelihood of not fully vaccinating their children, according to a study published in the Pediatric Infectious Disease Journal.
Mohammad Tahir Yousafzai, MPH, of Aga Khan University, Karachi, Pakistan, and colleagues developed a larger 14-item scale to measure parental attitudes and surveyed 901 households in the Sindh province of Pakistan during 2014. Part of this scale was a short 4-item subscale focusing on disease salience and community benefit, whereas the remaining 10 items form another subscale that measures parents’ perceptions and concerns regarding vaccines directly. The items are presented as 1-5 Likert scales, and scoring higher represents holding more negative attitudes regarding vaccines.
Of the 901 households surveyed, 25% of children were fully vaccinated, which meant children received all primary vaccines up to 14 weeks of age, and 54% were partially vaccinated, which meant at least one of those primary vaccines had been missed. The remaining 21% were unvaccinated.
High scores on the full 14-item scale showed some correlation with no vaccination versus partial or full vaccination (odds ratio, 3.05; 95% confidence interval, 1.75-5.31); the association disappeared after adjustment for children’s age and gender. The subscales performed better after adjustment: The adjusted ORs were 1.52 (95% CI, 1.05-2.21) for the longer subscale and 5.21 (95% CI, 3.60-7.55) for the shorter subscale. The data also showed high association between high scores on the shorter subscale and the likelihood of no or only partial vaccination (aOR, 9.65; 95% CI, 4.81-19.37).
The researchers noted that most similar scales used in developed countries are longer (10 or more items) and have lower internal consistency. This four-item scale, on the other hand, may be especially useful among lower-income populations and those in developing areas that have lower literacy rates.
One of the limitations of the study was that it was conducted only in Pakistan and not multiple developing countries; the researchers acknowledged this could limit generalizability. Another limitation is that the study size was too small for subdomain analysis; the researchers wrote that, although a lack of such analysis is unfortunate, it shouldn’t hamper the shorter subscale’s usability. A further limitation is that there was little variability across Likert scores – mostly answers at the extreme ends rather than a mix. This suggests that interviewees may not have understood how Likert scales work and therefore may not have answered accurately, noted the researchers, who employed a visual chart, trained interviewers, and field monitoring to mitigate this possibility.
“Measurement of the parental attitudes toward childhood vaccination is very important for the appropriate planning of strategies for increasing vaccine coverage and for monitoring,” they wrote.
The study was sponsored by Gavi, The Vaccine Alliance. The authors had no funding or conflicts of interest to disclose.
SOURCE: Yousafzai MT et al. Pediatr Infect Dis J. 2019 Jul;38(7):e143-8.
By measuring parents’ attitudes regarding disease salience and community benefit, high scores on a four-item scale was associated with fivefold greater likelihood of not fully vaccinating their children, according to a study published in the Pediatric Infectious Disease Journal.
Mohammad Tahir Yousafzai, MPH, of Aga Khan University, Karachi, Pakistan, and colleagues developed a larger 14-item scale to measure parental attitudes and surveyed 901 households in the Sindh province of Pakistan during 2014. Part of this scale was a short 4-item subscale focusing on disease salience and community benefit, whereas the remaining 10 items form another subscale that measures parents’ perceptions and concerns regarding vaccines directly. The items are presented as 1-5 Likert scales, and scoring higher represents holding more negative attitudes regarding vaccines.
Of the 901 households surveyed, 25% of children were fully vaccinated, which meant children received all primary vaccines up to 14 weeks of age, and 54% were partially vaccinated, which meant at least one of those primary vaccines had been missed. The remaining 21% were unvaccinated.
High scores on the full 14-item scale showed some correlation with no vaccination versus partial or full vaccination (odds ratio, 3.05; 95% confidence interval, 1.75-5.31); the association disappeared after adjustment for children’s age and gender. The subscales performed better after adjustment: The adjusted ORs were 1.52 (95% CI, 1.05-2.21) for the longer subscale and 5.21 (95% CI, 3.60-7.55) for the shorter subscale. The data also showed high association between high scores on the shorter subscale and the likelihood of no or only partial vaccination (aOR, 9.65; 95% CI, 4.81-19.37).
The researchers noted that most similar scales used in developed countries are longer (10 or more items) and have lower internal consistency. This four-item scale, on the other hand, may be especially useful among lower-income populations and those in developing areas that have lower literacy rates.
One of the limitations of the study was that it was conducted only in Pakistan and not multiple developing countries; the researchers acknowledged this could limit generalizability. Another limitation is that the study size was too small for subdomain analysis; the researchers wrote that, although a lack of such analysis is unfortunate, it shouldn’t hamper the shorter subscale’s usability. A further limitation is that there was little variability across Likert scores – mostly answers at the extreme ends rather than a mix. This suggests that interviewees may not have understood how Likert scales work and therefore may not have answered accurately, noted the researchers, who employed a visual chart, trained interviewers, and field monitoring to mitigate this possibility.
“Measurement of the parental attitudes toward childhood vaccination is very important for the appropriate planning of strategies for increasing vaccine coverage and for monitoring,” they wrote.
The study was sponsored by Gavi, The Vaccine Alliance. The authors had no funding or conflicts of interest to disclose.
SOURCE: Yousafzai MT et al. Pediatr Infect Dis J. 2019 Jul;38(7):e143-8.
FROM THE PEDIATRIC INFECTIOUS DISEASE JOURNAL