User login
LAIV doesn’t up asthmatic children’s risk of lower respiratory events
, according to an analysis published in Vaccine.
The data corroborate other research indicating that live attenuated influenza vaccine (LAIV) is safe for children with asthma older than 2 years and suggest that the choice of vaccination in this population should be based on effectiveness, according to James D. Nordin, MD, MPH, a clinical researcher at HealthPartners Institute in Minneapolis, and colleagues.
Children and adolescents with asthma have an increased risk of morbidity if they contract influenza. They represent a disproportionate number of pediatric influenza hospitalizations and have been a focus of efforts to vaccinate children against influenza. Since 2003, the inactivated influenza vaccine (IIV) and the LAIV have been available. Research indicates that LAIV is more effective than IIV at preventing culture-confirmed influenza in children. Two studies found an increased risk of wheezing in children who received LAIV, but other studies failed to replicate these findings.
A retrospective cohort study
Dr. Nordin and associates conducted a retrospective observational cohort study to investigate whether use of a guideline recommending LAIV for children aged 2 years and older with asthma increased the risk of lower respiratory events within 21 or 42 days of vaccination, compared with standard guidelines to administer IIV in children with asthma. The investigators drew data from two large medical groups with independent clinical leadership that serve demographically similar populations in Minnesota. One group (the LAIV group) switched its preference for all children from IIV to LAIV in 2010. The control group continued using IIV for children with asthma throughout the study period. Each group operates more than 20 clinics.
The investigators included children and adolescents aged 2-17 years who presented during one or more influenza season from 2007-2008 through 2014-2015. Eligible participants had a diagnosis of asthma or wheezing, received one or more influenza vaccines, had continuous insurance enrollment, and had at least one primary care or asthma related subspecialty encounter. They excluded patients with contraindications for LAIV (e.g., pregnancy, malignancy, and cystic fibrosis) and those with any hospitalization, ED visit, or outpatient encounter for a lower respiratory event in the 42 days before influenza vaccination.
Dr. Nordin and colleagues used a generalized estimating equation regression to estimate the ratio of rate ratios (RORs) comparing events before and after vaccination between the LAIV guideline and control groups. The researchers examined covariates such as age, gender, race or ethnicity, Medicaid insurance for at least 1 month in the previous year, neighborhood poverty, and neighborhood rates of asthma.
No increased risk
The investigators included 4,771 children and 7,851 child-influenza records in their analysis. During the period from 2007 to 2010, there were 2,215 child-influenza records from children and adolescents included from the LAIV group and 735 from the IIV guideline group. From 2010 to 2015, there were 3,767 child-influenza records in children and adolescents from the LAIV group and 1,134 from the IIV guideline group. After the LAIV group adopted the new guideline, the proportion of patients receiving LAIV increased from 23% to 68% in the LAIV group and from 7% to 11% in the control group.
About 88% of lower respiratory events included diagnoses for asthma exacerbations. When the investigators adjusted the data for age, asthma severity, asthma control, race or ethnicity, and Medicaid coverage, they found no increase in lower respiratory events associated with the LAIV guideline. The adjusted ROR was 0.74 for lower respiratory events within 21 days of vaccination and 0.77 for lower respiratory events within 42 days of vaccination. The results were similar when Dr. Nordin and colleagues stratified the data by age group, and including additional covariates did not alter the ROR estimates. In all, 21 hospitalizations occurred within 42 days of influenza vaccination, and the LAIV guideline did not increase the risk for hospitalization.
“Findings from this study are consistent with several recent observational studies of LAIV in children and adolescents with asthma,” said Dr. Nordin and colleagues.
One limitation of the current study was that the data were restricted to the information available in electronic health care or claims records. The researchers therefore were able to observe only medically attended lower respiratory events. Furthermore, the exclusion of asthma management encounters and the classification of asthma severity were based on diagnoses, visits, and medication orders and fills. The estimates thus are prone to misclassification, which may have biased the results. Finally, information on important variables such as daycare attendance, presence of school-age siblings, and exposure to secondhand smoke was not available.
The research was funded by a grant from the National Institute of Allergy and Infectious Diseases. The authors had no relevant financial disclosures.
SOURCE: Nordin JD et al. Vaccine. 2019 Jun 10. doi: 10.1016/j.vaccine.2019.05.081.
, according to an analysis published in Vaccine.
The data corroborate other research indicating that live attenuated influenza vaccine (LAIV) is safe for children with asthma older than 2 years and suggest that the choice of vaccination in this population should be based on effectiveness, according to James D. Nordin, MD, MPH, a clinical researcher at HealthPartners Institute in Minneapolis, and colleagues.
Children and adolescents with asthma have an increased risk of morbidity if they contract influenza. They represent a disproportionate number of pediatric influenza hospitalizations and have been a focus of efforts to vaccinate children against influenza. Since 2003, the inactivated influenza vaccine (IIV) and the LAIV have been available. Research indicates that LAIV is more effective than IIV at preventing culture-confirmed influenza in children. Two studies found an increased risk of wheezing in children who received LAIV, but other studies failed to replicate these findings.
A retrospective cohort study
Dr. Nordin and associates conducted a retrospective observational cohort study to investigate whether use of a guideline recommending LAIV for children aged 2 years and older with asthma increased the risk of lower respiratory events within 21 or 42 days of vaccination, compared with standard guidelines to administer IIV in children with asthma. The investigators drew data from two large medical groups with independent clinical leadership that serve demographically similar populations in Minnesota. One group (the LAIV group) switched its preference for all children from IIV to LAIV in 2010. The control group continued using IIV for children with asthma throughout the study period. Each group operates more than 20 clinics.
The investigators included children and adolescents aged 2-17 years who presented during one or more influenza season from 2007-2008 through 2014-2015. Eligible participants had a diagnosis of asthma or wheezing, received one or more influenza vaccines, had continuous insurance enrollment, and had at least one primary care or asthma related subspecialty encounter. They excluded patients with contraindications for LAIV (e.g., pregnancy, malignancy, and cystic fibrosis) and those with any hospitalization, ED visit, or outpatient encounter for a lower respiratory event in the 42 days before influenza vaccination.
Dr. Nordin and colleagues used a generalized estimating equation regression to estimate the ratio of rate ratios (RORs) comparing events before and after vaccination between the LAIV guideline and control groups. The researchers examined covariates such as age, gender, race or ethnicity, Medicaid insurance for at least 1 month in the previous year, neighborhood poverty, and neighborhood rates of asthma.
No increased risk
The investigators included 4,771 children and 7,851 child-influenza records in their analysis. During the period from 2007 to 2010, there were 2,215 child-influenza records from children and adolescents included from the LAIV group and 735 from the IIV guideline group. From 2010 to 2015, there were 3,767 child-influenza records in children and adolescents from the LAIV group and 1,134 from the IIV guideline group. After the LAIV group adopted the new guideline, the proportion of patients receiving LAIV increased from 23% to 68% in the LAIV group and from 7% to 11% in the control group.
About 88% of lower respiratory events included diagnoses for asthma exacerbations. When the investigators adjusted the data for age, asthma severity, asthma control, race or ethnicity, and Medicaid coverage, they found no increase in lower respiratory events associated with the LAIV guideline. The adjusted ROR was 0.74 for lower respiratory events within 21 days of vaccination and 0.77 for lower respiratory events within 42 days of vaccination. The results were similar when Dr. Nordin and colleagues stratified the data by age group, and including additional covariates did not alter the ROR estimates. In all, 21 hospitalizations occurred within 42 days of influenza vaccination, and the LAIV guideline did not increase the risk for hospitalization.
“Findings from this study are consistent with several recent observational studies of LAIV in children and adolescents with asthma,” said Dr. Nordin and colleagues.
One limitation of the current study was that the data were restricted to the information available in electronic health care or claims records. The researchers therefore were able to observe only medically attended lower respiratory events. Furthermore, the exclusion of asthma management encounters and the classification of asthma severity were based on diagnoses, visits, and medication orders and fills. The estimates thus are prone to misclassification, which may have biased the results. Finally, information on important variables such as daycare attendance, presence of school-age siblings, and exposure to secondhand smoke was not available.
The research was funded by a grant from the National Institute of Allergy and Infectious Diseases. The authors had no relevant financial disclosures.
SOURCE: Nordin JD et al. Vaccine. 2019 Jun 10. doi: 10.1016/j.vaccine.2019.05.081.
, according to an analysis published in Vaccine.
The data corroborate other research indicating that live attenuated influenza vaccine (LAIV) is safe for children with asthma older than 2 years and suggest that the choice of vaccination in this population should be based on effectiveness, according to James D. Nordin, MD, MPH, a clinical researcher at HealthPartners Institute in Minneapolis, and colleagues.
Children and adolescents with asthma have an increased risk of morbidity if they contract influenza. They represent a disproportionate number of pediatric influenza hospitalizations and have been a focus of efforts to vaccinate children against influenza. Since 2003, the inactivated influenza vaccine (IIV) and the LAIV have been available. Research indicates that LAIV is more effective than IIV at preventing culture-confirmed influenza in children. Two studies found an increased risk of wheezing in children who received LAIV, but other studies failed to replicate these findings.
A retrospective cohort study
Dr. Nordin and associates conducted a retrospective observational cohort study to investigate whether use of a guideline recommending LAIV for children aged 2 years and older with asthma increased the risk of lower respiratory events within 21 or 42 days of vaccination, compared with standard guidelines to administer IIV in children with asthma. The investigators drew data from two large medical groups with independent clinical leadership that serve demographically similar populations in Minnesota. One group (the LAIV group) switched its preference for all children from IIV to LAIV in 2010. The control group continued using IIV for children with asthma throughout the study period. Each group operates more than 20 clinics.
The investigators included children and adolescents aged 2-17 years who presented during one or more influenza season from 2007-2008 through 2014-2015. Eligible participants had a diagnosis of asthma or wheezing, received one or more influenza vaccines, had continuous insurance enrollment, and had at least one primary care or asthma related subspecialty encounter. They excluded patients with contraindications for LAIV (e.g., pregnancy, malignancy, and cystic fibrosis) and those with any hospitalization, ED visit, or outpatient encounter for a lower respiratory event in the 42 days before influenza vaccination.
Dr. Nordin and colleagues used a generalized estimating equation regression to estimate the ratio of rate ratios (RORs) comparing events before and after vaccination between the LAIV guideline and control groups. The researchers examined covariates such as age, gender, race or ethnicity, Medicaid insurance for at least 1 month in the previous year, neighborhood poverty, and neighborhood rates of asthma.
No increased risk
The investigators included 4,771 children and 7,851 child-influenza records in their analysis. During the period from 2007 to 2010, there were 2,215 child-influenza records from children and adolescents included from the LAIV group and 735 from the IIV guideline group. From 2010 to 2015, there were 3,767 child-influenza records in children and adolescents from the LAIV group and 1,134 from the IIV guideline group. After the LAIV group adopted the new guideline, the proportion of patients receiving LAIV increased from 23% to 68% in the LAIV group and from 7% to 11% in the control group.
About 88% of lower respiratory events included diagnoses for asthma exacerbations. When the investigators adjusted the data for age, asthma severity, asthma control, race or ethnicity, and Medicaid coverage, they found no increase in lower respiratory events associated with the LAIV guideline. The adjusted ROR was 0.74 for lower respiratory events within 21 days of vaccination and 0.77 for lower respiratory events within 42 days of vaccination. The results were similar when Dr. Nordin and colleagues stratified the data by age group, and including additional covariates did not alter the ROR estimates. In all, 21 hospitalizations occurred within 42 days of influenza vaccination, and the LAIV guideline did not increase the risk for hospitalization.
“Findings from this study are consistent with several recent observational studies of LAIV in children and adolescents with asthma,” said Dr. Nordin and colleagues.
One limitation of the current study was that the data were restricted to the information available in electronic health care or claims records. The researchers therefore were able to observe only medically attended lower respiratory events. Furthermore, the exclusion of asthma management encounters and the classification of asthma severity were based on diagnoses, visits, and medication orders and fills. The estimates thus are prone to misclassification, which may have biased the results. Finally, information on important variables such as daycare attendance, presence of school-age siblings, and exposure to secondhand smoke was not available.
The research was funded by a grant from the National Institute of Allergy and Infectious Diseases. The authors had no relevant financial disclosures.
SOURCE: Nordin JD et al. Vaccine. 2019 Jun 10. doi: 10.1016/j.vaccine.2019.05.081.
FROM VACCINE
Uncomplicated appendicitis can be treated successfully with antibiotics
Clinical question: What is the late recurrence rate for patients with uncomplicated appendicitis treated with antibiotics only?
Background: Short-term results support antibiotic treatment as alternative to surgery for uncomplicated appendicitis. Long-term outcomes have not been assessed.
Study design: Observational follow-up.
Setting: Six hospitals in Finland.
Synopsis: The APPAC trial looked at 530 patients, aged 18-60 years, with CT confirmed acute uncomplicated appendicitis, who were randomized to receive either appendectomy or antibiotics. In this follow-up report, outcomes were assessed by telephone interviews conducted 3-5 years after the initial interventions. Overall, 100 of 256 (39.1%) of the antibiotic group ultimately underwent appendectomy within 5 years. Of those, 70/100 (70%) had their recurrence within 1 year of their initial presentation.
Bottom line: Patients with uncomplicated appendicitis treated with antibiotics have a 39% cumulative 5-year recurrence rate, with most recurrences occurring within the first year.
Citation: Salminem P et al. Five-year follow-up of antibiotic therapy for uncomplicated acute appendicitis in the APPAC Randomized Clinical Trial. JAMA. 2018;320(12):1259-65.
Dr. Asuen is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Clinical question: What is the late recurrence rate for patients with uncomplicated appendicitis treated with antibiotics only?
Background: Short-term results support antibiotic treatment as alternative to surgery for uncomplicated appendicitis. Long-term outcomes have not been assessed.
Study design: Observational follow-up.
Setting: Six hospitals in Finland.
Synopsis: The APPAC trial looked at 530 patients, aged 18-60 years, with CT confirmed acute uncomplicated appendicitis, who were randomized to receive either appendectomy or antibiotics. In this follow-up report, outcomes were assessed by telephone interviews conducted 3-5 years after the initial interventions. Overall, 100 of 256 (39.1%) of the antibiotic group ultimately underwent appendectomy within 5 years. Of those, 70/100 (70%) had their recurrence within 1 year of their initial presentation.
Bottom line: Patients with uncomplicated appendicitis treated with antibiotics have a 39% cumulative 5-year recurrence rate, with most recurrences occurring within the first year.
Citation: Salminem P et al. Five-year follow-up of antibiotic therapy for uncomplicated acute appendicitis in the APPAC Randomized Clinical Trial. JAMA. 2018;320(12):1259-65.
Dr. Asuen is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Clinical question: What is the late recurrence rate for patients with uncomplicated appendicitis treated with antibiotics only?
Background: Short-term results support antibiotic treatment as alternative to surgery for uncomplicated appendicitis. Long-term outcomes have not been assessed.
Study design: Observational follow-up.
Setting: Six hospitals in Finland.
Synopsis: The APPAC trial looked at 530 patients, aged 18-60 years, with CT confirmed acute uncomplicated appendicitis, who were randomized to receive either appendectomy or antibiotics. In this follow-up report, outcomes were assessed by telephone interviews conducted 3-5 years after the initial interventions. Overall, 100 of 256 (39.1%) of the antibiotic group ultimately underwent appendectomy within 5 years. Of those, 70/100 (70%) had their recurrence within 1 year of their initial presentation.
Bottom line: Patients with uncomplicated appendicitis treated with antibiotics have a 39% cumulative 5-year recurrence rate, with most recurrences occurring within the first year.
Citation: Salminem P et al. Five-year follow-up of antibiotic therapy for uncomplicated acute appendicitis in the APPAC Randomized Clinical Trial. JAMA. 2018;320(12):1259-65.
Dr. Asuen is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Can You Put Your Finger on the Diagnosis?
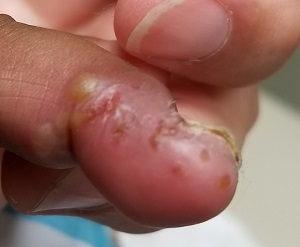
An 8-year-old boy is brought in for evaluation of a collection of blisters on his finger, near the nail. The problem manifested about 6 days ago. The affected area is tender to touch. The child reportedly feels well, with no fever or malaise.
The patient has an extensive personal and family history of atopy. Since birth, he has had dry, sensitive skin and has experienced episodes of eczema, seasonal allergies, and asthma. Three months ago, he was admitted to the hospital with eczema herpeticum and successfully treated with IV acyclovir.
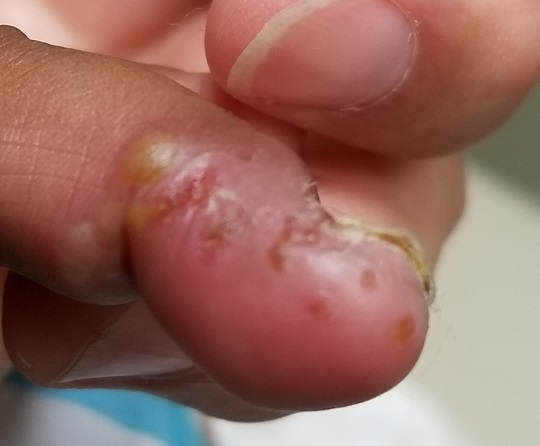
EXAMINATION
A cluster of vesicles is seen in the lateral perionychial area of the left third finger. Very modest erythema surrounds the vesicles, which contain cloudy yellow fluid suggestive of pus. There is a palpable lymph node in the left epitrochlear area.
The child is afebrile and in no distress. Patches of mild eczema are seen on the extremities and trunk.
What’s the diagnosis?
DISCUSSION
The lesion on this child’s finger is a herpetic whitlow. Patients with atopy are often susceptible to all types of skin infections: bacterial, fungal, and viral. In fact, human papillomavirus infection manifesting as multiple warts is not uncommon in this population. Nor is herpes simplex virus (HSV) infection, of which this case represents 1 manifestation.
A culture could have been done to confirm the diagnosis, but that would entail opening a vesicle to collect the fluid and then waiting at least 2 weeks for the results. By then, this whitlow would have long since resolved.
As with all HSV infections in the immunocompetent, treatment with acyclovir must be started in the first 2 to 3 days to have any effect—so such treatment in this case would be useless. If the herpetic whitlow were to recur in the same location, prompt treatment could be initiated, which would likely shorten the disease course and reduce symptoms.
Another HSV infection seen almost exclusively in atopic patients is eczema herpeticum (also known as Kaposi varicelliform eruption). This diffuse infection comprises dozens of tiny papulovesicular lesions, mostly concentrated on the face but often spilling down onto the chest. Patients with Darier disease or seborrheic dermatitis can also acquire it.
TAKE-HOME LEARNING POINTS
- Patients with atopy, especially children, are susceptible to all kinds of skin infections—fungal, bacterial, and viral.
- Herpes simplex virus (HSV) can appear in almost any location, including on fingers, but can also manifest as diffuse papulovesicular lesions on the face and chest of atopic patients.
- The blisters/vesicles of HSV are often pus-filled and usually provoke regional adenopathy.
- If diagnosed early enough, herpetic whitlows can be successfully treated with oral acyclovir; this doesn’t provide a cure but does stop the particular episode.
An 8-year-old boy is brought in for evaluation of a collection of blisters on his finger, near the nail. The problem manifested about 6 days ago. The affected area is tender to touch. The child reportedly feels well, with no fever or malaise.
The patient has an extensive personal and family history of atopy. Since birth, he has had dry, sensitive skin and has experienced episodes of eczema, seasonal allergies, and asthma. Three months ago, he was admitted to the hospital with eczema herpeticum and successfully treated with IV acyclovir.

EXAMINATION
A cluster of vesicles is seen in the lateral perionychial area of the left third finger. Very modest erythema surrounds the vesicles, which contain cloudy yellow fluid suggestive of pus. There is a palpable lymph node in the left epitrochlear area.
The child is afebrile and in no distress. Patches of mild eczema are seen on the extremities and trunk.
What’s the diagnosis?
DISCUSSION
The lesion on this child’s finger is a herpetic whitlow. Patients with atopy are often susceptible to all types of skin infections: bacterial, fungal, and viral. In fact, human papillomavirus infection manifesting as multiple warts is not uncommon in this population. Nor is herpes simplex virus (HSV) infection, of which this case represents 1 manifestation.
A culture could have been done to confirm the diagnosis, but that would entail opening a vesicle to collect the fluid and then waiting at least 2 weeks for the results. By then, this whitlow would have long since resolved.
As with all HSV infections in the immunocompetent, treatment with acyclovir must be started in the first 2 to 3 days to have any effect—so such treatment in this case would be useless. If the herpetic whitlow were to recur in the same location, prompt treatment could be initiated, which would likely shorten the disease course and reduce symptoms.
Another HSV infection seen almost exclusively in atopic patients is eczema herpeticum (also known as Kaposi varicelliform eruption). This diffuse infection comprises dozens of tiny papulovesicular lesions, mostly concentrated on the face but often spilling down onto the chest. Patients with Darier disease or seborrheic dermatitis can also acquire it.
TAKE-HOME LEARNING POINTS
- Patients with atopy, especially children, are susceptible to all kinds of skin infections—fungal, bacterial, and viral.
- Herpes simplex virus (HSV) can appear in almost any location, including on fingers, but can also manifest as diffuse papulovesicular lesions on the face and chest of atopic patients.
- The blisters/vesicles of HSV are often pus-filled and usually provoke regional adenopathy.
- If diagnosed early enough, herpetic whitlows can be successfully treated with oral acyclovir; this doesn’t provide a cure but does stop the particular episode.
An 8-year-old boy is brought in for evaluation of a collection of blisters on his finger, near the nail. The problem manifested about 6 days ago. The affected area is tender to touch. The child reportedly feels well, with no fever or malaise.
The patient has an extensive personal and family history of atopy. Since birth, he has had dry, sensitive skin and has experienced episodes of eczema, seasonal allergies, and asthma. Three months ago, he was admitted to the hospital with eczema herpeticum and successfully treated with IV acyclovir.

EXAMINATION
A cluster of vesicles is seen in the lateral perionychial area of the left third finger. Very modest erythema surrounds the vesicles, which contain cloudy yellow fluid suggestive of pus. There is a palpable lymph node in the left epitrochlear area.
The child is afebrile and in no distress. Patches of mild eczema are seen on the extremities and trunk.
What’s the diagnosis?
DISCUSSION
The lesion on this child’s finger is a herpetic whitlow. Patients with atopy are often susceptible to all types of skin infections: bacterial, fungal, and viral. In fact, human papillomavirus infection manifesting as multiple warts is not uncommon in this population. Nor is herpes simplex virus (HSV) infection, of which this case represents 1 manifestation.
A culture could have been done to confirm the diagnosis, but that would entail opening a vesicle to collect the fluid and then waiting at least 2 weeks for the results. By then, this whitlow would have long since resolved.
As with all HSV infections in the immunocompetent, treatment with acyclovir must be started in the first 2 to 3 days to have any effect—so such treatment in this case would be useless. If the herpetic whitlow were to recur in the same location, prompt treatment could be initiated, which would likely shorten the disease course and reduce symptoms.
Another HSV infection seen almost exclusively in atopic patients is eczema herpeticum (also known as Kaposi varicelliform eruption). This diffuse infection comprises dozens of tiny papulovesicular lesions, mostly concentrated on the face but often spilling down onto the chest. Patients with Darier disease or seborrheic dermatitis can also acquire it.
TAKE-HOME LEARNING POINTS
- Patients with atopy, especially children, are susceptible to all kinds of skin infections—fungal, bacterial, and viral.
- Herpes simplex virus (HSV) can appear in almost any location, including on fingers, but can also manifest as diffuse papulovesicular lesions on the face and chest of atopic patients.
- The blisters/vesicles of HSV are often pus-filled and usually provoke regional adenopathy.
- If diagnosed early enough, herpetic whitlows can be successfully treated with oral acyclovir; this doesn’t provide a cure but does stop the particular episode.
Flu vaccine succeeds in TNF inhibitor users
MADRID – Influenza vaccination is similarly effective for individuals taking a tumor necrosis factor (TNF) inhibitor and healthy controls, but the number needed to vaccinate to prevent one case of influenza for patients taking a TNF inhibitor is much lower, according to data from a study presented at the European Congress of Rheumatology.
The number needed to vaccinate (NNV) to prevent one case of influenza among healthy control patients was 71, compared with an NNV of 10 for patients taking the TNF inhibitor adalimumab (Humira), reported Giovanni Adami, MD, and colleagues at the University of Verona (Italy).
While TNF inhibitors “are known to increase the risk of infection by suppressing the activity of the immune system,” it has not been clear whether the response to vaccination is impaired in patients treated with a TNF inhibitor, Dr. Adami said.
Dr. Adami and colleagues reviewed data from 15,132 adult patients exposed to adalimumab in global rheumatoid arthritis clinical trials and 71,221 healthy controls from clinical trials of influenza vaccines. Overall, the rate of influenza infection was similarly reduced with vaccination in both groups. The rate in healthy individuals went from 2.3% for those unvaccinated to 0.9% for those vaccinated; for TNF inhibitor–treated patients, the rate was 14.4% for those unvaccinated versus 4.5% for those vaccinated.
“It is not surprising that the number needed to vaccinate is dramatically lower in patients treated with immunosuppressors, compared to healthy individuals,” Dr. Adami noted. “As a matter of fact, patients treated with such drugs are at higher risk of infections, namely they have a greater absolute risk of influenza. Nevertheless, [it] is quite surprising that the relative risk reduction is similar between TNF inhibitor–treated patients and healthy controls, meaning that the vaccination is efficacious in both the cohorts.”
The researchers also calculated the cost to prevent one case of influenza, using a cost of approximately 16.5 euro per vaccine. (Dr. Adami also cited an average U.S. cost of about $40/vaccine). Using this method, they estimated a cost for vaccination of 1,174 euro (roughly $1,340) to prevent one influenza infection in the general population, and a cost of about 165 euro (roughly $188) to vaccinate enough people treated with a TNF inhibitor to prevent one infection.
Dr. Adami advised clinicians to remember the low NNV for TNF inhibitor–treated patients with regard to influenza vaccination. “A direct disclosure of the NNV for these patients might help adherence to vaccinations,” he said.
Next steps for research should include extending the real-world effectiveness analysis to other medications and other diseases, such as zoster vaccination in patients treated with Janus kinase inhibitors, Dr. Adami said.
Dr. Adami had no financial conflicts to disclose. Several coauthors disclosed relationships with companies including Abiogen Pharma, Grünenthal, Amgen, Janssen-Cilag, Mundipharma, and Pfizer.
Mitchel L. Zoler contributed to this report.
SOURCE: Adami G et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):192-3. Abstract OP0230, doi: 10.1136/annrheumdis-2019-eular.3088
MADRID – Influenza vaccination is similarly effective for individuals taking a tumor necrosis factor (TNF) inhibitor and healthy controls, but the number needed to vaccinate to prevent one case of influenza for patients taking a TNF inhibitor is much lower, according to data from a study presented at the European Congress of Rheumatology.
The number needed to vaccinate (NNV) to prevent one case of influenza among healthy control patients was 71, compared with an NNV of 10 for patients taking the TNF inhibitor adalimumab (Humira), reported Giovanni Adami, MD, and colleagues at the University of Verona (Italy).
While TNF inhibitors “are known to increase the risk of infection by suppressing the activity of the immune system,” it has not been clear whether the response to vaccination is impaired in patients treated with a TNF inhibitor, Dr. Adami said.
Dr. Adami and colleagues reviewed data from 15,132 adult patients exposed to adalimumab in global rheumatoid arthritis clinical trials and 71,221 healthy controls from clinical trials of influenza vaccines. Overall, the rate of influenza infection was similarly reduced with vaccination in both groups. The rate in healthy individuals went from 2.3% for those unvaccinated to 0.9% for those vaccinated; for TNF inhibitor–treated patients, the rate was 14.4% for those unvaccinated versus 4.5% for those vaccinated.
“It is not surprising that the number needed to vaccinate is dramatically lower in patients treated with immunosuppressors, compared to healthy individuals,” Dr. Adami noted. “As a matter of fact, patients treated with such drugs are at higher risk of infections, namely they have a greater absolute risk of influenza. Nevertheless, [it] is quite surprising that the relative risk reduction is similar between TNF inhibitor–treated patients and healthy controls, meaning that the vaccination is efficacious in both the cohorts.”
The researchers also calculated the cost to prevent one case of influenza, using a cost of approximately 16.5 euro per vaccine. (Dr. Adami also cited an average U.S. cost of about $40/vaccine). Using this method, they estimated a cost for vaccination of 1,174 euro (roughly $1,340) to prevent one influenza infection in the general population, and a cost of about 165 euro (roughly $188) to vaccinate enough people treated with a TNF inhibitor to prevent one infection.
Dr. Adami advised clinicians to remember the low NNV for TNF inhibitor–treated patients with regard to influenza vaccination. “A direct disclosure of the NNV for these patients might help adherence to vaccinations,” he said.
Next steps for research should include extending the real-world effectiveness analysis to other medications and other diseases, such as zoster vaccination in patients treated with Janus kinase inhibitors, Dr. Adami said.
Dr. Adami had no financial conflicts to disclose. Several coauthors disclosed relationships with companies including Abiogen Pharma, Grünenthal, Amgen, Janssen-Cilag, Mundipharma, and Pfizer.
Mitchel L. Zoler contributed to this report.
SOURCE: Adami G et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):192-3. Abstract OP0230, doi: 10.1136/annrheumdis-2019-eular.3088
MADRID – Influenza vaccination is similarly effective for individuals taking a tumor necrosis factor (TNF) inhibitor and healthy controls, but the number needed to vaccinate to prevent one case of influenza for patients taking a TNF inhibitor is much lower, according to data from a study presented at the European Congress of Rheumatology.
The number needed to vaccinate (NNV) to prevent one case of influenza among healthy control patients was 71, compared with an NNV of 10 for patients taking the TNF inhibitor adalimumab (Humira), reported Giovanni Adami, MD, and colleagues at the University of Verona (Italy).
While TNF inhibitors “are known to increase the risk of infection by suppressing the activity of the immune system,” it has not been clear whether the response to vaccination is impaired in patients treated with a TNF inhibitor, Dr. Adami said.
Dr. Adami and colleagues reviewed data from 15,132 adult patients exposed to adalimumab in global rheumatoid arthritis clinical trials and 71,221 healthy controls from clinical trials of influenza vaccines. Overall, the rate of influenza infection was similarly reduced with vaccination in both groups. The rate in healthy individuals went from 2.3% for those unvaccinated to 0.9% for those vaccinated; for TNF inhibitor–treated patients, the rate was 14.4% for those unvaccinated versus 4.5% for those vaccinated.
“It is not surprising that the number needed to vaccinate is dramatically lower in patients treated with immunosuppressors, compared to healthy individuals,” Dr. Adami noted. “As a matter of fact, patients treated with such drugs are at higher risk of infections, namely they have a greater absolute risk of influenza. Nevertheless, [it] is quite surprising that the relative risk reduction is similar between TNF inhibitor–treated patients and healthy controls, meaning that the vaccination is efficacious in both the cohorts.”
The researchers also calculated the cost to prevent one case of influenza, using a cost of approximately 16.5 euro per vaccine. (Dr. Adami also cited an average U.S. cost of about $40/vaccine). Using this method, they estimated a cost for vaccination of 1,174 euro (roughly $1,340) to prevent one influenza infection in the general population, and a cost of about 165 euro (roughly $188) to vaccinate enough people treated with a TNF inhibitor to prevent one infection.
Dr. Adami advised clinicians to remember the low NNV for TNF inhibitor–treated patients with regard to influenza vaccination. “A direct disclosure of the NNV for these patients might help adherence to vaccinations,” he said.
Next steps for research should include extending the real-world effectiveness analysis to other medications and other diseases, such as zoster vaccination in patients treated with Janus kinase inhibitors, Dr. Adami said.
Dr. Adami had no financial conflicts to disclose. Several coauthors disclosed relationships with companies including Abiogen Pharma, Grünenthal, Amgen, Janssen-Cilag, Mundipharma, and Pfizer.
Mitchel L. Zoler contributed to this report.
SOURCE: Adami G et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):192-3. Abstract OP0230, doi: 10.1136/annrheumdis-2019-eular.3088
REPORTING FROM EULAR 2019 CONGRESS
Legislative, educational interventions influenced vaccine status of California kindergartners
After California lawmakers implemented policies to limit and eventually eliminate nonmedical exemptions for childhood vaccinations, the proportion of kindergartners who were not up to date for recommended vaccinations fell from 10% in 2013 to 5% in 2017.
At the same time, the
The findings come from an observational study that used cross-sectional school-entry data from 2000 to 2017 to calculate the rates of kindergartners attending California schools who were not up to date on required vaccinations.
“Large-scale vaccination programs that included school-entry mandates have been essential to maintaining high levels of immunization coverage and low rates of vaccine-preventable diseases,” researchers led by S. Cassandra Pingali, MPH, MS, wrote in JAMA. “However, an increasing number of parents are not vaccinating their children over concerns about potential adverse effects. These parental actions threaten the herd immunity established by decades of high vaccine uptake and increase the potential for disease outbreaks.”
Ms. Pingali, of the department of epidemiology at Emory University, Atlanta, and colleagues conducted an observational analysis of California kindergartners who were not up to date on one or more of the required vaccinations during the course of three interventions implemented in the state. The first was Assembly Bill 2109 (AB 2109), which was passed in 2014. It required parents to show proof they had discussed the risks of not vaccinating their children with a health care practitioner before they obtained a personal belief exemption. The second intervention was a campaign carried out in 2015 by the California Department of Public Health and local health departments, designed to educate school staff on the proper application of the conditional admission criteria, which allowed students additional time to catch up on vaccination. The third intervention was the implementation of Senate bill 277 (SB 277), which banned all personal belief exemptions.
Between 2000 and 2017, the researchers reported that the yearly mean kindergarten enrollment in California was 517,962 and the mean number of schools was 7,278. Over this time, the yearly rate of students without up-to-date vaccination status rose from 8% during 2000 to 10% during 2013, before decreasing to 5% during 2017. Ms. Pingali and associates also found that average percentage chance of any within-school contact for a student without up-to-date vaccination status with another student with the same status was 19% during 2000, and increased steadily to 26% during 2014, the first year of AB 2109. The values decreased to 3% (the first year of SB 277), before increasing slightly to 5% during 2017.
“Across the interventions, the percentage of kindergartners attending schools with an up-to-date vaccination status percentage that was greater than the herd immunity threshold also increased for various vaccine-preventable diseases,” the researchers wrote. “Overall, the results suggest that the risk of disease outbreak via potential contact among susceptible children decreased over the course of the interventions.”
The way Matthew M. Davis, MD and Seema K. Shah, JD, see it, the current outbreak of measles in the United States is rooted in the failure of parents to vaccinate their children against the disease based on their beliefs rather than medical contraindications.
“The public health implications of such decisions are amplified because parents who share belief systems about childhood vaccinations tend to congregate socially and residentially, thereby forming clusters of unvaccinated children who are at elevated health risks when exposed to vaccine-preventable diseases,” the authors wrote in an accompanying editorial.
While the study reported by Pingali et al. did not measure actual outbreaks of disease, “reductions in children’s risk of contracting measles are a promising outcome in California resulting from policy changes,” wrote Dr. Davis and Ms. Shah, both of Northwestern University, Chicago (JAMA. 2019;322[1]:33-4). “Yet, because of the ease of domestic and international travel, the mobile nature of young families, and the inability of all states to implement this approach, changes made in each state for nonmedical exemptions may not ensure sufficiently high protection against measles for children across all jurisdictions in the United States. Although states have historically made their own decisions about vaccination exemptions linked to day care or school entry because states exercise primary authority over educational matters, childhood vaccination is a national matter in many respects.”
The best way to remedy the current system failure regarding measles vaccination, they continued, may be to adopt a unified national approach to prohibit nonmedical exemptions. They pointed to the fact that the United States previously achieved virtual eradication of measles as recently as 2000. “Following that achievement, state-level policy changes relaxed immunization requirements and set the stage for progressively larger outbreaks in the United States in recent years. Such system failures result when the products, processes, and people (including the public) that comprise systems do not function or behave in ways that protect health optimally.”
The study was supported by a grant from the National Institutes of Health. One coauthor reported having received consulting fees from Merck and grants from Pfizer and Walgreens. Another reported receiving grants from Pfizer, Merck, GlaxoSmithKline, Sanofi Pasteur, Protein Science, Dynavax, and MedImmune. The remaining coauthors reported no relevant financial disclosures.
The editorialists reported having no financial disclosures.
SOURCE: Pingali SC et al. JAMA. 2019 Jul 2. doi: 10.1001/jama.2019.7924.
After California lawmakers implemented policies to limit and eventually eliminate nonmedical exemptions for childhood vaccinations, the proportion of kindergartners who were not up to date for recommended vaccinations fell from 10% in 2013 to 5% in 2017.
At the same time, the
The findings come from an observational study that used cross-sectional school-entry data from 2000 to 2017 to calculate the rates of kindergartners attending California schools who were not up to date on required vaccinations.
“Large-scale vaccination programs that included school-entry mandates have been essential to maintaining high levels of immunization coverage and low rates of vaccine-preventable diseases,” researchers led by S. Cassandra Pingali, MPH, MS, wrote in JAMA. “However, an increasing number of parents are not vaccinating their children over concerns about potential adverse effects. These parental actions threaten the herd immunity established by decades of high vaccine uptake and increase the potential for disease outbreaks.”
Ms. Pingali, of the department of epidemiology at Emory University, Atlanta, and colleagues conducted an observational analysis of California kindergartners who were not up to date on one or more of the required vaccinations during the course of three interventions implemented in the state. The first was Assembly Bill 2109 (AB 2109), which was passed in 2014. It required parents to show proof they had discussed the risks of not vaccinating their children with a health care practitioner before they obtained a personal belief exemption. The second intervention was a campaign carried out in 2015 by the California Department of Public Health and local health departments, designed to educate school staff on the proper application of the conditional admission criteria, which allowed students additional time to catch up on vaccination. The third intervention was the implementation of Senate bill 277 (SB 277), which banned all personal belief exemptions.
Between 2000 and 2017, the researchers reported that the yearly mean kindergarten enrollment in California was 517,962 and the mean number of schools was 7,278. Over this time, the yearly rate of students without up-to-date vaccination status rose from 8% during 2000 to 10% during 2013, before decreasing to 5% during 2017. Ms. Pingali and associates also found that average percentage chance of any within-school contact for a student without up-to-date vaccination status with another student with the same status was 19% during 2000, and increased steadily to 26% during 2014, the first year of AB 2109. The values decreased to 3% (the first year of SB 277), before increasing slightly to 5% during 2017.
“Across the interventions, the percentage of kindergartners attending schools with an up-to-date vaccination status percentage that was greater than the herd immunity threshold also increased for various vaccine-preventable diseases,” the researchers wrote. “Overall, the results suggest that the risk of disease outbreak via potential contact among susceptible children decreased over the course of the interventions.”
The way Matthew M. Davis, MD and Seema K. Shah, JD, see it, the current outbreak of measles in the United States is rooted in the failure of parents to vaccinate their children against the disease based on their beliefs rather than medical contraindications.
“The public health implications of such decisions are amplified because parents who share belief systems about childhood vaccinations tend to congregate socially and residentially, thereby forming clusters of unvaccinated children who are at elevated health risks when exposed to vaccine-preventable diseases,” the authors wrote in an accompanying editorial.
While the study reported by Pingali et al. did not measure actual outbreaks of disease, “reductions in children’s risk of contracting measles are a promising outcome in California resulting from policy changes,” wrote Dr. Davis and Ms. Shah, both of Northwestern University, Chicago (JAMA. 2019;322[1]:33-4). “Yet, because of the ease of domestic and international travel, the mobile nature of young families, and the inability of all states to implement this approach, changes made in each state for nonmedical exemptions may not ensure sufficiently high protection against measles for children across all jurisdictions in the United States. Although states have historically made their own decisions about vaccination exemptions linked to day care or school entry because states exercise primary authority over educational matters, childhood vaccination is a national matter in many respects.”
The best way to remedy the current system failure regarding measles vaccination, they continued, may be to adopt a unified national approach to prohibit nonmedical exemptions. They pointed to the fact that the United States previously achieved virtual eradication of measles as recently as 2000. “Following that achievement, state-level policy changes relaxed immunization requirements and set the stage for progressively larger outbreaks in the United States in recent years. Such system failures result when the products, processes, and people (including the public) that comprise systems do not function or behave in ways that protect health optimally.”
The study was supported by a grant from the National Institutes of Health. One coauthor reported having received consulting fees from Merck and grants from Pfizer and Walgreens. Another reported receiving grants from Pfizer, Merck, GlaxoSmithKline, Sanofi Pasteur, Protein Science, Dynavax, and MedImmune. The remaining coauthors reported no relevant financial disclosures.
The editorialists reported having no financial disclosures.
SOURCE: Pingali SC et al. JAMA. 2019 Jul 2. doi: 10.1001/jama.2019.7924.
After California lawmakers implemented policies to limit and eventually eliminate nonmedical exemptions for childhood vaccinations, the proportion of kindergartners who were not up to date for recommended vaccinations fell from 10% in 2013 to 5% in 2017.
At the same time, the
The findings come from an observational study that used cross-sectional school-entry data from 2000 to 2017 to calculate the rates of kindergartners attending California schools who were not up to date on required vaccinations.
“Large-scale vaccination programs that included school-entry mandates have been essential to maintaining high levels of immunization coverage and low rates of vaccine-preventable diseases,” researchers led by S. Cassandra Pingali, MPH, MS, wrote in JAMA. “However, an increasing number of parents are not vaccinating their children over concerns about potential adverse effects. These parental actions threaten the herd immunity established by decades of high vaccine uptake and increase the potential for disease outbreaks.”
Ms. Pingali, of the department of epidemiology at Emory University, Atlanta, and colleagues conducted an observational analysis of California kindergartners who were not up to date on one or more of the required vaccinations during the course of three interventions implemented in the state. The first was Assembly Bill 2109 (AB 2109), which was passed in 2014. It required parents to show proof they had discussed the risks of not vaccinating their children with a health care practitioner before they obtained a personal belief exemption. The second intervention was a campaign carried out in 2015 by the California Department of Public Health and local health departments, designed to educate school staff on the proper application of the conditional admission criteria, which allowed students additional time to catch up on vaccination. The third intervention was the implementation of Senate bill 277 (SB 277), which banned all personal belief exemptions.
Between 2000 and 2017, the researchers reported that the yearly mean kindergarten enrollment in California was 517,962 and the mean number of schools was 7,278. Over this time, the yearly rate of students without up-to-date vaccination status rose from 8% during 2000 to 10% during 2013, before decreasing to 5% during 2017. Ms. Pingali and associates also found that average percentage chance of any within-school contact for a student without up-to-date vaccination status with another student with the same status was 19% during 2000, and increased steadily to 26% during 2014, the first year of AB 2109. The values decreased to 3% (the first year of SB 277), before increasing slightly to 5% during 2017.
“Across the interventions, the percentage of kindergartners attending schools with an up-to-date vaccination status percentage that was greater than the herd immunity threshold also increased for various vaccine-preventable diseases,” the researchers wrote. “Overall, the results suggest that the risk of disease outbreak via potential contact among susceptible children decreased over the course of the interventions.”
The way Matthew M. Davis, MD and Seema K. Shah, JD, see it, the current outbreak of measles in the United States is rooted in the failure of parents to vaccinate their children against the disease based on their beliefs rather than medical contraindications.
“The public health implications of such decisions are amplified because parents who share belief systems about childhood vaccinations tend to congregate socially and residentially, thereby forming clusters of unvaccinated children who are at elevated health risks when exposed to vaccine-preventable diseases,” the authors wrote in an accompanying editorial.
While the study reported by Pingali et al. did not measure actual outbreaks of disease, “reductions in children’s risk of contracting measles are a promising outcome in California resulting from policy changes,” wrote Dr. Davis and Ms. Shah, both of Northwestern University, Chicago (JAMA. 2019;322[1]:33-4). “Yet, because of the ease of domestic and international travel, the mobile nature of young families, and the inability of all states to implement this approach, changes made in each state for nonmedical exemptions may not ensure sufficiently high protection against measles for children across all jurisdictions in the United States. Although states have historically made their own decisions about vaccination exemptions linked to day care or school entry because states exercise primary authority over educational matters, childhood vaccination is a national matter in many respects.”
The best way to remedy the current system failure regarding measles vaccination, they continued, may be to adopt a unified national approach to prohibit nonmedical exemptions. They pointed to the fact that the United States previously achieved virtual eradication of measles as recently as 2000. “Following that achievement, state-level policy changes relaxed immunization requirements and set the stage for progressively larger outbreaks in the United States in recent years. Such system failures result when the products, processes, and people (including the public) that comprise systems do not function or behave in ways that protect health optimally.”
The study was supported by a grant from the National Institutes of Health. One coauthor reported having received consulting fees from Merck and grants from Pfizer and Walgreens. Another reported receiving grants from Pfizer, Merck, GlaxoSmithKline, Sanofi Pasteur, Protein Science, Dynavax, and MedImmune. The remaining coauthors reported no relevant financial disclosures.
The editorialists reported having no financial disclosures.
SOURCE: Pingali SC et al. JAMA. 2019 Jul 2. doi: 10.1001/jama.2019.7924.
FROM JAMA
New research in otitis media
New research was presented at the International Society for Otitis Media meeting in June 2019, which I attended. I would like to share a selection of new findings from the many presentations.
Transtympanic antibiotic delivery
Topical therapy has been used to treat only otitis externa and acute otitis media (AOM) with ear discharge. Giving antibiotics through the tympanic membrane could mitigate many of the concerns about antibiotic use driving antibiotic resistance of bacteria among children. Up to now, using antibiotics in the ear canal to treat AOM has not been considered because the tympanic membrane is highly impermeable to the transtympanic diffusion of any drugs. However, in recent years, a number of different drug delivery systems have been developed, and in some cases, animal studies have shown that noninvasive transtympanic delivery is possible so that drugs can reach high concentrations in the middle ear without damage. Nanovesicles and nanoliposomes that contain antibiotics and are small enough to pass through the eardrum have been developed and tested in animal models; these show promise. Ototopical administration of a drug called vinpocetine that was repurposed has been tested in mice and shown to reduce inflammation and mucus production in the middle ear during otitis media.
Biofilms
Antibiotic treatment failure can occur in AOM for several reasons. The treatment of choice, amoxicillin, for example may fail to achieve an adequate concentration because of poor absorption in the gastrointestinal tract or poor penetration into the middle ear. Or, the antibiotic chosen may not be effective because of resistance of the strain causing the infection. Another explanation, especially in recurrent AOM and chronic AOM, could be the presence of biofilms. Biofilms are multicellular bacterial communities incorporated in a polymeric, plasticlike matrix in which pathogens are protected from antibiotic activity. The biofilm provides a physical barrier to antibiotic penetration, and bacteria can persist in the middle ear and periodically cause a new AOM. If AOM persists or becomes a more chronic otitis media with effusion, the “glue ear” causes an environment in the middle ear that is low in oxygen. A low-oxygen environment is favorable to biofilms. Also one might expect that middle ear pus would have a low pH, but actual measurements show the pH is highly alkaline. Species of Haemophilus influenzae have been identified as more virulent when in an alkaline pH or the alkaline pH makes the H. influenzae persist better in the middle ear, perhaps in a biofilm. To eliminate biofilms and improve antibiotic efficacy, a vaccine against a protein expressed by H. influenzae has been developed. Antibodies against this protein have been shown to disrupt and prevent the formation of biofilms in an animal model.
Probiotics
The normal bacteria that live in the nasopharynx of children with recurrent AOM is now known to differ from that of children who experience infrequent AOM or remain AOM-free throughout childhood. The use of oral pre- and probiotics for AOM prophylaxis remains debated because the results of studies are conflicting and frequently show no effect. So the idea of using prebiotics or probiotics to create a favorable “microbiome” of the nose is under investigation. Two species of bacteria that are gathering the most attention are Corynebacterium species (a few types in particular) and a bacteria called Dolosigranulum pigrum. Delivery of the commensal species would be as a nose spray.
Vaccines
The use of pneumococcal conjugate vaccines (PCVs) has reduced the frequency of AOM caused by Streptococcus pneumoniae. PCVs are not as effective against AOM as they are against invasive pneumococcal disease, but they still help a lot. However, because there are now at least 96 different serotypes of the pneumococcus based on different capsular types, we see a pattern of replacement of disease-causing strains by new strains within a few years of introduction of a new formulation. We started with 7 serotypes (Prevnar 7) in year 2000, and it was replaced by the current formulation with 13 serotypes (Prevnar 13) in 2010. Replacements have occurred again so vaccine companies are making new formulations for the future that include more serotypes, up to 20 serotypes. But, technically and feasibility-wise there is a limit to making such vaccines. A vaccine based on killed unencapsulated bacteria has been tested for safety and immunogenicity in young children. There is no test so far for prevention of AOM. Another type of vaccine based on proteins expressed by the pneumococcus that could be vaccine targets was tested in American Navajo children, and it failed to be as efficacious as hoped.
Biomarkers.
Due to recurrent AOM or persistent otitis media with effusion, about 15% of children in the United States receive tympanostomy tubes. Among those who receive tubes, about 20% go on to receive a second set of tubes, often with adenotonsillectomy. To find a biomarker that could identify children likely to require a second set of tubes, the fluid in the middle ear was tested when a first set of tubes were inserted. If bacteria were detected by polymerase chain reaction (PCR) testing or if a profile of specific inflammatory cytokines was measured, those results could be used to predict a high likelihood for a second set of tubes.
Overdiagnosis
Diagnosis of AOM is challenging in young children, in whom it most frequently occurs. The ear canal is typically about 3 mm wide, the child struggles during the examination, and diagnostic skills are not taught in training, resulting in a high overdiagnosis rate. I presented data that suggest too many children who are not truly otitis prone have been classified as otitis prone based on incorrect clinical diagnosis. My colleagues and I found that 30% of children reach the threshold of three episodes of AOM in 6 months or four within a year when diagnosed by community pediatricians, similar to many other studies. Validated otoscopists (trained by experts with diagnosis definitively proven as at least 85% accurate using tympanocentesis) classify 15% of children as otitis prone – half as many. If tympanocentesis is used to prove middle ear fluid has bacterial pathogens (about 95% yield a bacterial otopathogen using culture and PCR), then about 10% of children are classified as otitis prone – one-third as many. This suggests that children clinically diagnosed by community-based pediatricians are overdiagnosed with AOM, perhaps three times more often than true. And that leads to overuse of antibiotics and referrals for tympanostomy tube surgery more often than should occur. So we need to improve diagnostic methods beyond otoscopy. New types of imaging for the eardrum and middle ear using novel technologies are in early clinical trials.
Immunity
The notion that young children get AOM because of Eustachian tube dysfunction in their early years of life (horizontal anatomy) may be true, but there is more to the story. After 10 years of work, the scientists in my research group have shown that children in the first 3 years of life can have an immune system that is suppressed – it is poorly responsive to pathogens and routine pediatric vaccines. Many features resemble a neonatal immune system, beginning life with a suppressed immune system or being in cytokine storm from birth. We introduced the term “prolonged neonatal-like immune profile (PNIP)” to give a general description of the immune responses we have found in otitis-prone children. They outgrow this. So the immune maturation is delayed but not permanent. It is mostly resolved by age 3 years. We found problems in both innate and adaptive immunity. It may be that the main explanation for recurrent AOM in the first years of life is PNIP. Scientists from Australia also reported immunity problems in Aboriginal children and they are very otitis prone, often progressing to chronic suppurative otitis media. Animal model studies of AOM show inadequate innate and adaptive immunity importantly contribute to the infection as well.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Email him at [email protected].
New research was presented at the International Society for Otitis Media meeting in June 2019, which I attended. I would like to share a selection of new findings from the many presentations.
Transtympanic antibiotic delivery
Topical therapy has been used to treat only otitis externa and acute otitis media (AOM) with ear discharge. Giving antibiotics through the tympanic membrane could mitigate many of the concerns about antibiotic use driving antibiotic resistance of bacteria among children. Up to now, using antibiotics in the ear canal to treat AOM has not been considered because the tympanic membrane is highly impermeable to the transtympanic diffusion of any drugs. However, in recent years, a number of different drug delivery systems have been developed, and in some cases, animal studies have shown that noninvasive transtympanic delivery is possible so that drugs can reach high concentrations in the middle ear without damage. Nanovesicles and nanoliposomes that contain antibiotics and are small enough to pass through the eardrum have been developed and tested in animal models; these show promise. Ototopical administration of a drug called vinpocetine that was repurposed has been tested in mice and shown to reduce inflammation and mucus production in the middle ear during otitis media.
Biofilms
Antibiotic treatment failure can occur in AOM for several reasons. The treatment of choice, amoxicillin, for example may fail to achieve an adequate concentration because of poor absorption in the gastrointestinal tract or poor penetration into the middle ear. Or, the antibiotic chosen may not be effective because of resistance of the strain causing the infection. Another explanation, especially in recurrent AOM and chronic AOM, could be the presence of biofilms. Biofilms are multicellular bacterial communities incorporated in a polymeric, plasticlike matrix in which pathogens are protected from antibiotic activity. The biofilm provides a physical barrier to antibiotic penetration, and bacteria can persist in the middle ear and periodically cause a new AOM. If AOM persists or becomes a more chronic otitis media with effusion, the “glue ear” causes an environment in the middle ear that is low in oxygen. A low-oxygen environment is favorable to biofilms. Also one might expect that middle ear pus would have a low pH, but actual measurements show the pH is highly alkaline. Species of Haemophilus influenzae have been identified as more virulent when in an alkaline pH or the alkaline pH makes the H. influenzae persist better in the middle ear, perhaps in a biofilm. To eliminate biofilms and improve antibiotic efficacy, a vaccine against a protein expressed by H. influenzae has been developed. Antibodies against this protein have been shown to disrupt and prevent the formation of biofilms in an animal model.
Probiotics
The normal bacteria that live in the nasopharynx of children with recurrent AOM is now known to differ from that of children who experience infrequent AOM or remain AOM-free throughout childhood. The use of oral pre- and probiotics for AOM prophylaxis remains debated because the results of studies are conflicting and frequently show no effect. So the idea of using prebiotics or probiotics to create a favorable “microbiome” of the nose is under investigation. Two species of bacteria that are gathering the most attention are Corynebacterium species (a few types in particular) and a bacteria called Dolosigranulum pigrum. Delivery of the commensal species would be as a nose spray.
Vaccines
The use of pneumococcal conjugate vaccines (PCVs) has reduced the frequency of AOM caused by Streptococcus pneumoniae. PCVs are not as effective against AOM as they are against invasive pneumococcal disease, but they still help a lot. However, because there are now at least 96 different serotypes of the pneumococcus based on different capsular types, we see a pattern of replacement of disease-causing strains by new strains within a few years of introduction of a new formulation. We started with 7 serotypes (Prevnar 7) in year 2000, and it was replaced by the current formulation with 13 serotypes (Prevnar 13) in 2010. Replacements have occurred again so vaccine companies are making new formulations for the future that include more serotypes, up to 20 serotypes. But, technically and feasibility-wise there is a limit to making such vaccines. A vaccine based on killed unencapsulated bacteria has been tested for safety and immunogenicity in young children. There is no test so far for prevention of AOM. Another type of vaccine based on proteins expressed by the pneumococcus that could be vaccine targets was tested in American Navajo children, and it failed to be as efficacious as hoped.
Biomarkers.
Due to recurrent AOM or persistent otitis media with effusion, about 15% of children in the United States receive tympanostomy tubes. Among those who receive tubes, about 20% go on to receive a second set of tubes, often with adenotonsillectomy. To find a biomarker that could identify children likely to require a second set of tubes, the fluid in the middle ear was tested when a first set of tubes were inserted. If bacteria were detected by polymerase chain reaction (PCR) testing or if a profile of specific inflammatory cytokines was measured, those results could be used to predict a high likelihood for a second set of tubes.
Overdiagnosis
Diagnosis of AOM is challenging in young children, in whom it most frequently occurs. The ear canal is typically about 3 mm wide, the child struggles during the examination, and diagnostic skills are not taught in training, resulting in a high overdiagnosis rate. I presented data that suggest too many children who are not truly otitis prone have been classified as otitis prone based on incorrect clinical diagnosis. My colleagues and I found that 30% of children reach the threshold of three episodes of AOM in 6 months or four within a year when diagnosed by community pediatricians, similar to many other studies. Validated otoscopists (trained by experts with diagnosis definitively proven as at least 85% accurate using tympanocentesis) classify 15% of children as otitis prone – half as many. If tympanocentesis is used to prove middle ear fluid has bacterial pathogens (about 95% yield a bacterial otopathogen using culture and PCR), then about 10% of children are classified as otitis prone – one-third as many. This suggests that children clinically diagnosed by community-based pediatricians are overdiagnosed with AOM, perhaps three times more often than true. And that leads to overuse of antibiotics and referrals for tympanostomy tube surgery more often than should occur. So we need to improve diagnostic methods beyond otoscopy. New types of imaging for the eardrum and middle ear using novel technologies are in early clinical trials.
Immunity
The notion that young children get AOM because of Eustachian tube dysfunction in their early years of life (horizontal anatomy) may be true, but there is more to the story. After 10 years of work, the scientists in my research group have shown that children in the first 3 years of life can have an immune system that is suppressed – it is poorly responsive to pathogens and routine pediatric vaccines. Many features resemble a neonatal immune system, beginning life with a suppressed immune system or being in cytokine storm from birth. We introduced the term “prolonged neonatal-like immune profile (PNIP)” to give a general description of the immune responses we have found in otitis-prone children. They outgrow this. So the immune maturation is delayed but not permanent. It is mostly resolved by age 3 years. We found problems in both innate and adaptive immunity. It may be that the main explanation for recurrent AOM in the first years of life is PNIP. Scientists from Australia also reported immunity problems in Aboriginal children and they are very otitis prone, often progressing to chronic suppurative otitis media. Animal model studies of AOM show inadequate innate and adaptive immunity importantly contribute to the infection as well.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Email him at [email protected].
New research was presented at the International Society for Otitis Media meeting in June 2019, which I attended. I would like to share a selection of new findings from the many presentations.
Transtympanic antibiotic delivery
Topical therapy has been used to treat only otitis externa and acute otitis media (AOM) with ear discharge. Giving antibiotics through the tympanic membrane could mitigate many of the concerns about antibiotic use driving antibiotic resistance of bacteria among children. Up to now, using antibiotics in the ear canal to treat AOM has not been considered because the tympanic membrane is highly impermeable to the transtympanic diffusion of any drugs. However, in recent years, a number of different drug delivery systems have been developed, and in some cases, animal studies have shown that noninvasive transtympanic delivery is possible so that drugs can reach high concentrations in the middle ear without damage. Nanovesicles and nanoliposomes that contain antibiotics and are small enough to pass through the eardrum have been developed and tested in animal models; these show promise. Ototopical administration of a drug called vinpocetine that was repurposed has been tested in mice and shown to reduce inflammation and mucus production in the middle ear during otitis media.
Biofilms
Antibiotic treatment failure can occur in AOM for several reasons. The treatment of choice, amoxicillin, for example may fail to achieve an adequate concentration because of poor absorption in the gastrointestinal tract or poor penetration into the middle ear. Or, the antibiotic chosen may not be effective because of resistance of the strain causing the infection. Another explanation, especially in recurrent AOM and chronic AOM, could be the presence of biofilms. Biofilms are multicellular bacterial communities incorporated in a polymeric, plasticlike matrix in which pathogens are protected from antibiotic activity. The biofilm provides a physical barrier to antibiotic penetration, and bacteria can persist in the middle ear and periodically cause a new AOM. If AOM persists or becomes a more chronic otitis media with effusion, the “glue ear” causes an environment in the middle ear that is low in oxygen. A low-oxygen environment is favorable to biofilms. Also one might expect that middle ear pus would have a low pH, but actual measurements show the pH is highly alkaline. Species of Haemophilus influenzae have been identified as more virulent when in an alkaline pH or the alkaline pH makes the H. influenzae persist better in the middle ear, perhaps in a biofilm. To eliminate biofilms and improve antibiotic efficacy, a vaccine against a protein expressed by H. influenzae has been developed. Antibodies against this protein have been shown to disrupt and prevent the formation of biofilms in an animal model.
Probiotics
The normal bacteria that live in the nasopharynx of children with recurrent AOM is now known to differ from that of children who experience infrequent AOM or remain AOM-free throughout childhood. The use of oral pre- and probiotics for AOM prophylaxis remains debated because the results of studies are conflicting and frequently show no effect. So the idea of using prebiotics or probiotics to create a favorable “microbiome” of the nose is under investigation. Two species of bacteria that are gathering the most attention are Corynebacterium species (a few types in particular) and a bacteria called Dolosigranulum pigrum. Delivery of the commensal species would be as a nose spray.
Vaccines
The use of pneumococcal conjugate vaccines (PCVs) has reduced the frequency of AOM caused by Streptococcus pneumoniae. PCVs are not as effective against AOM as they are against invasive pneumococcal disease, but they still help a lot. However, because there are now at least 96 different serotypes of the pneumococcus based on different capsular types, we see a pattern of replacement of disease-causing strains by new strains within a few years of introduction of a new formulation. We started with 7 serotypes (Prevnar 7) in year 2000, and it was replaced by the current formulation with 13 serotypes (Prevnar 13) in 2010. Replacements have occurred again so vaccine companies are making new formulations for the future that include more serotypes, up to 20 serotypes. But, technically and feasibility-wise there is a limit to making such vaccines. A vaccine based on killed unencapsulated bacteria has been tested for safety and immunogenicity in young children. There is no test so far for prevention of AOM. Another type of vaccine based on proteins expressed by the pneumococcus that could be vaccine targets was tested in American Navajo children, and it failed to be as efficacious as hoped.
Biomarkers.
Due to recurrent AOM or persistent otitis media with effusion, about 15% of children in the United States receive tympanostomy tubes. Among those who receive tubes, about 20% go on to receive a second set of tubes, often with adenotonsillectomy. To find a biomarker that could identify children likely to require a second set of tubes, the fluid in the middle ear was tested when a first set of tubes were inserted. If bacteria were detected by polymerase chain reaction (PCR) testing or if a profile of specific inflammatory cytokines was measured, those results could be used to predict a high likelihood for a second set of tubes.
Overdiagnosis
Diagnosis of AOM is challenging in young children, in whom it most frequently occurs. The ear canal is typically about 3 mm wide, the child struggles during the examination, and diagnostic skills are not taught in training, resulting in a high overdiagnosis rate. I presented data that suggest too many children who are not truly otitis prone have been classified as otitis prone based on incorrect clinical diagnosis. My colleagues and I found that 30% of children reach the threshold of three episodes of AOM in 6 months or four within a year when diagnosed by community pediatricians, similar to many other studies. Validated otoscopists (trained by experts with diagnosis definitively proven as at least 85% accurate using tympanocentesis) classify 15% of children as otitis prone – half as many. If tympanocentesis is used to prove middle ear fluid has bacterial pathogens (about 95% yield a bacterial otopathogen using culture and PCR), then about 10% of children are classified as otitis prone – one-third as many. This suggests that children clinically diagnosed by community-based pediatricians are overdiagnosed with AOM, perhaps three times more often than true. And that leads to overuse of antibiotics and referrals for tympanostomy tube surgery more often than should occur. So we need to improve diagnostic methods beyond otoscopy. New types of imaging for the eardrum and middle ear using novel technologies are in early clinical trials.
Immunity
The notion that young children get AOM because of Eustachian tube dysfunction in their early years of life (horizontal anatomy) may be true, but there is more to the story. After 10 years of work, the scientists in my research group have shown that children in the first 3 years of life can have an immune system that is suppressed – it is poorly responsive to pathogens and routine pediatric vaccines. Many features resemble a neonatal immune system, beginning life with a suppressed immune system or being in cytokine storm from birth. We introduced the term “prolonged neonatal-like immune profile (PNIP)” to give a general description of the immune responses we have found in otitis-prone children. They outgrow this. So the immune maturation is delayed but not permanent. It is mostly resolved by age 3 years. We found problems in both innate and adaptive immunity. It may be that the main explanation for recurrent AOM in the first years of life is PNIP. Scientists from Australia also reported immunity problems in Aboriginal children and they are very otitis prone, often progressing to chronic suppurative otitis media. Animal model studies of AOM show inadequate innate and adaptive immunity importantly contribute to the infection as well.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Email him at [email protected].
Do patients on biologic drugs for rheumatic disease need PCP prophylaxis?
Pneumocystis jirovecii (previously carinii) pneumonia (PCP) is rare in patients taking biologic response modifiers for rheumatic disease.1–10 However, prophylaxis should be considered in patients who have granulomatosis with polyangiitis or underlying pulmonary disease, or who are concomitantly receiving glucocorticoids in high doses. There is some risk of adverse reactions to the prophylactic medicine.1,11–21 Until clear guidelines are available, the decision to initiate PCP prophylaxis and the choice of agent should be individualized.
THE BURDEN OF PCP
In a meta-analysis23 of 867 patients who developed PCP and did not have HIV infection, 20.1% had autoimmune or chronic inflammatory disease and the rest were transplant recipients or had malignancies. The mortality rate was 30.6%.
PHARMACOLOGIC RISK FACTORS FOR PCP
Treatment with glucocorticoids
Treatment with glucocorticoids is an important risk factor for PCP, independent of biologic therapy.
Calero-Bernal et al11 reported on 128 patients with non-HIV PCP, of whom 114 (89%) had received a glucocorticoid for more than 4 weeks, and 98 (76%) were currently receiving one. The mean daily dose was equivalent to 27.73 mg of prednisone per day in those on glucocorticoids only, and 21.34 mg in those receiving glucocorticoids in combination with other immunosuppressants.
Park et al,12 in a retrospective study of Korean patients treated for rheumatic disease with high-dose glucocorticoids (≥ 30 mg/day of prednisone or equivalent for more than 4 weeks), reported an incidence rate of PCP of 2.37 per 100 patient-years in those not on prophylaxis.
Other studies13,14 have also found a prednisone dose greater than 15 to 20 mg per day for more than 4 weeks or concomitant use of 2 or more disease-modifying antirheumatic drugs to be a significant risk factor.13,14
Tumor necrosis factor alpha antagonists
A US Food and Drug Administration review1 of voluntary reports of adverse drug events estimated the incidence of PCP to be 2.3 per 100,000 patient-years with infliximab and 1.6 per 100,000 patient-years with etanercept. In most cases, other immunosuppressants were used concomitantly.1
Postmarketing surveillance2 of 5,000 patients with rheumatoid arthritis showed an incidence of suspected PCP of 0.4% within the first 6 months of starting infliximab therapy.
Komano et al,15 in a case-control study of patients with rheumatoid arthritis treated with infliximab, reported that all 21 patients with PCP were also on methotrexate (median dosage 8 mg per week) and prednisolone (median dosage 7.5 mg per day).
PCP has also been reported after adalimumab use in combination with prednisone, azathioprine, and methotrexate, as well as with certolizumab, golimumab, tocilizumab, abatacept, and rituximab.3–6,24–26
Rituximab
Calero-Bernal et al11 reported that 23% of patients with non-HIV PCP who were receiving immunosuppressant drugs were on rituximab.
Alexandre et al16 performed a retrospective review of 11 cases of PCP complicating rituximab therapy for autoimmune disease, in which 10 (91%) of the patients were also on corticosteroids, with a median dosage of 30 mg of prednisone daily. A literature review of an additional 18 cases revealed similar findings.
PATIENT RISK FACTORS FOR PCP
Pulmonary disease, age, other factors
Komano et al,15 in their study of patients with rheumatoid arthritis treated with infliximab, found that 10 (48%) of 21 patients with PCP had preexisting pulmonary disease, compared with 11 (10.8%) of 102 patients without PCP (P < .001). Patients with PCP were older (mean age 64 vs 54, P < .001), were on higher median doses of prednisolone per day (7.5 vs 5 mg, P = .001), and had lower median serum immunoglobulin G (IgG) levels (944 vs 1,394 mg/dL, P < .001).15
Tadros et al13 performed a case-control study that also showed that patients with autoimmune disease who developed PCP had lower lymphocyte counts than controls on admission. Other risk factors included low CD4 counts and age older than 50.
Li et al17 found that patients with autoimmune or inflammatory disease with PCP were more likely to have low CD3, CD4, and CD8 cell counts, as well as albumin levels less than 28 g/L. They therefore suggested that lymphocyte subtyping may be a useful tool to guide PCP prophylaxis.
Granulomatosis with polyangiitis
Patients with granulomatosis with polyangiitis have a significantly higher incidence of PCP than patients with other connective tissue diseases.
Ward and Donald18 reviewed 223 cases of PCP in patients with connective tissue disease. The highest frequency (89 cases per 10,000 hospitalizations per year) was in patients with granulomatosis with polyangiitis, followed by 65 per 10,000 hospitalizations per year for patients with polyarteritis nodosa. The lowest frequency was in rheumatoid arthritis patients, at 2 per 10,000 hospitalizations per year. In decreasing order, diseases with significant associations with PCP were:
- Polyarteritis nodosa (odds ratio [OR] 10.20, 95% confidence interval [CI] 5.69–18.29)
- Granulomatosis with polyangiitis (OR 7.81, 95% CI 4.71–13.05)
- Inflammatory myopathy (OR 4.44, 95% CI 2.67–7.38)
- Systemic lupus erythematosus (OR 2.52, 95% CI 1.66–3.82).
Vallabhaneni and Chiller,26 in a meta-analysis including rheumatoid arthritis patients on biologics, did not find an increased risk of PCP (OR 1.77, 95% CI 0.42–7.47).
Park et al12 found that the highest incidences of PCP were in patients with granulomatosis with polyangiitis, microscopic polyangiitis, and systemic sclerosis. For systemic sclerosis, the main reason for giving high-dose glucocorticoids was interstitial lung disease.
Other studies19,20,28 also found an association with coexisting pulmonary disease in patients with rheumatoid arthritis.
CURRENT GUIDELINES
There are guidelines for primary and secondary prophylaxis of PCP in HIV-positive patients with CD4 counts less than 200/mm3 or a history of acquired immunodeficiency syndrome (AIDS)-defining illness.27 Additionally, patients with a CD4 cell percentage less than 14% should be considered for prophylaxis.27
Unfortunately, there are no guidelines for prophylaxis in patients taking immunosuppressants for rheumatic disease.
The recommended regimen for PCP prophylaxis in HIV-infected patients is trimethoprim-sulfamethoxazole, 1 double-strength or 1 single-strength tablet daily. Alternative regimens include 1 double-strength tablet 3 times per week, dapsone, aerosolized pentamidine, and atovaquone.27
There are also guidelines for prophylaxis in kidney transplant recipients, as well as for patients with hematologic malignancies and solid-organ malignancies, particularly those on chemotherapeutic agents and the T-cell-depleting agent alemtuzumab.29–31
Italian clinical practice guidelines for the use of tumor necrosis factor antagonists in inflammatory bowel disease recommend consideration of PCP prophylaxis in patients who are also on other immunosuppressants, particularly high-dose glucocorticoids.32
Prophylaxis has been shown to increase life expectancy and quality-adjusted life-years and to reduce cost for patients on immunosuppressive therapy for granulomatosis with polyangiitis.21 The European Society of Clinical Microbiology and Infectious Diseases recently produced consensus statements recommending PCP prophylaxis for patients on rituximab with other concomitant immunosuppressants such as the equivalent of prednisone 20 mg daily for more than 4 weeks.33 Prophylaxis was not recommended for other biologic therapies.34,35
THE RISKS OF PROPHYLAXIS
The risk of PCP should be weighed against the risk of prophylaxis in patients with rheumatic disease. Adverse reactions to sulfonamide antibiotics including disease flares have been reported in patients with systemic lupus erythematosus.36,37 Other studies have found no increased risk of flares in patients taking trimethoprim-sulfamethoxazole for PCP prophylaxis.12,38 A retrospective analysis of patients with vasculitis found no increased risk of combining methotrexate and trimethoprim-sulfamethoxazole.39
KEY POINTS
- PCP is an opportunistic infection with a high risk of death.
- PCP has been reported with biologics used as immunomodulators in rheumatic disease.
- PCP prophylaxis should be considered in patients at high risk of PCP, such as those who have granulomatosis with polyangiitis, underlying pulmonary disease or who are concomitantly taking glucocorticoids.
- US Food and Drug Administration. Safety update on TNF-alpha antagonists: infliximab and etanercept.https://wayback.archive-it.org/7993/20180127041103/https://www.fda.gov/ohrms/dockets/ac/01/briefing/3779b2_01_cber_safety_revision2.htm. Accessed May 3, 2019.
- Takeuchi T, Tatsuki Y, Nogami Y, et al. Postmarketing surveillance of the safety profile of infliximab in 5000 Japanese patients with rheumatoid arthritis. Ann Rheum Dis 2008; 67(2):189–194. doi:10.1136/ard.2007.072967
- Koike T, Harigai M, Ishiguro N, et al. Safety and effectiveness of adalimumab in Japanese rheumatoid arthritis patients: postmarketing surveillance report of the first 3,000 patients. Mod Rheumatol 2012; 22(4):498–508. doi:10.1007/s10165-011-0541-5
- Bykerk V, Cush J, Winthrop K, et al. Update on the safety profile of certolizumab pegol in rheumatoid arthritis: an integrated analysis from clinical trials. Ann Rheum Dis 2015; 74(1):96–103. doi:10.1136/annrheumdis-2013-203660
- Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of tocilizumab for rheumatoid arthritis in Japan: interim analysis of 3881 patients. Ann Rheum Dis 2011; 70(12):2148–2151. doi:10.1136/ard.2011.151092
- Harigai M, Ishiguro N, Inokuma S, et al. Postmarketing surveillance of the safety and effectiveness of abatacept in Japanese patients with rheumatoid arthritis. Mod Rheumatol 2016; 26(4):491–498. doi:10.3109/14397595.2015.1123211
- Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of the safety and effectiveness of etanercept in Japan. J Rheumatol 2009; 36(5):898–906. doi:10.3899/jrheum.080791
- Grubbs JA, Baddley JW. Pneumocystis jirovecii pneumonia in patients receiving tumor-necrosis-factor-inhibitor therapy: implications for chemoprophylaxis. Curr Rheumatol Rep 2014; 16(10):445. doi:10.1007/s11926-014-0445-4
- US Food and Drug Administration. FDA adverse event reporting system (FAERS) public dashboard. www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm070093.htm. Accessed May 3, 2019.
- Rutherford AI, Patarata E, Subesinghe S, Hyrich KL, Galloway JB. Opportunistic infections in rheumatoid arthritis patients exposed to biologic therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology (Oxford) 2018; 57(6):997–1001. doi:10.1093/rheumatology/key023
- Calero-Bernal ML, Martin-Garrido I, Donazar-Ezcurra M, Limper AH, Carmona EM. Intermittent courses of corticosteroids also present a risk for Pneumocystis pneumonia in non-HIV patients. Can Respir J 2016; 2016:2464791. doi:10.1155/2016/2464791
- Park JW, Curtis JR, Moon J, Song YW, Kim S, Lee EB. Prophylactic effect of trimethoprim-sulfamethoxazole for pneumocystis pneumonia in patients with rheumatic diseases exposed to prolonged high-dose glucocorticoids. Ann Rheum Dis 2018; 77(5):644–649. doi:10.1136/annrheumdis-2017-211796
- Tadros S, Teichtahl AJ, Ciciriello S, Wicks IP. Pneumocystis jirovecii pneumonia in systemic autoimmune rheumatic disease: a case-control study. Semin Arthritis Rheum 2017; 46(6):804–809. doi:10.1016/j.semarthrit.2016.09.009
- Demoruelle MK, Kahr A, Verilhac K, Deane K, Fischer A, West S. Recent-onset systemic lupus erythematosus complicated by acute respiratory failure. Arthritis Care Res (Hoboken) 2013; 65(2):314–323. doi:10.1002/acr.21857
- Komano Y, Harigai M, Koike R, et al. Pneumocystis jiroveci pneumonia in patients with rheumatoid arthritis treated with infliximab: a retrospective review and case-control study of 21 patients. Arthritis Rheum 2009; 61(3):305–312. doi:10.1002/art.24283
- Alexandre K, Ingen-Housz-Oro S, Versini M, Sailler L, Benhamou Y. Pneumocystis jirovecii pneumonia in patients treated with rituximab for systemic diseases: report of 11 cases and review of the literature. Eur J Intern Med 2018; 50:e23–e24. doi:10.1016/j.ejim.2017.11.014
- Li Y, Ghannoum M, Deng C, et al. Pneumocystis pneumonia in patients with inflammatory or autoimmune diseases: usefulness of lymphocyte subtyping. Int J Infect Dis 2017; 57:108–115. doi:10.1016/j.ijid.2017.02.010
- Ward MM, Donald F. Pneumocystis carinii pneumonia in patients with connective tissue diseases: the role of hospital experience in diagnosis and mortality. Arthritis Rheum 1999; 42(4):780–789. doi:10.1002/1529-0131(199904)42:4<780::AID-ANR23>3.0.CO;2-M
- Katsuyama T, Saito K, Kubo S, Nawata M, Tanaka Y. Prophylaxis for Pneumocystis pneumonia in patients with rheumatoid arthritis treated with biologics, based on risk factors found in a retrospective study. Arthritis Res Ther 2014; 16(1):R43. doi:10.1186/ar4472
- Tanaka M, Sakai R, Koike R, et al. Pneumocystis jirovecii pneumonia associated with etanercept treatment in patients with rheumatoid arthritis: a retrospective review of 15 cases and analysis of risk factors. Mod Rheumatol 2012; 22(6):849–858. doi:10.1007/s10165-012-0615-z
- Chung JB, Armstrong K, Schwartz JS, Albert D. Cost-effectiveness of prophylaxis against Pneumocystis carinii pneumonia in patients with Wegener’s granulomatosis undergoing immunosuppressive therapy. Arthritis Rheum 2000; 43(8):1841–1848. doi:10.1002/1529-0131(200008)43:8<1841::AID-ANR21>3.0.CO;2-Q
- Selmi C, Generali E, Massarotti M, Bianchi G, Scire CA. New treatments for inflammatory rheumatic disease. Immunol Res 2014; 60(2–3):277–288. doi:10.1007/s12026-014-8565-5
- Liu Y, Su L, Jiang SJ, Qu H. Risk factors for mortality from Pneumocystis carinii pneumonia (PCP) in non-HIV patients: a meta-analysis. Oncotarget 2017; 8(35):59729–59739. doi:10.18632/oncotarget.19927
- Desales AL, Mendez-Navarro J, Méndez-Tovar LJ, et al. Pneumocystosis in a patient with Crohn's disease treated with combination therapy with adalimumab. J Crohns Colitis 2012; 6(4):483–487. doi:10.1016/j.crohns.2011.10.012
- Kalyoncu U, Karadag O, Akdogan A, et al. Pneumocystis carinii pneumonia in a rheumatoid arthritis patient treated with adalimumab. Scand J Infect Dis 2007; 39(5):475–478. doi:10.1080/00365540601071867
- Vallabhaneni S, Chiller TM. Fungal infections and new biologic therapies. Curr Rheumatol Rep 2016; 18(5):29. doi:10.1007/s11926-016-0572-1
- Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. www.aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed May 3, 2019.
- Kourbeti IS, Ziakas PD, Mylonakis E. Biologic therapies in rheumatoid arthritis and the risk of opportunistic infections: a meta-analysis. Clin Infect Dis 2014; 58(12):1649–1657. doi:10.1093/cid/ciu185
- Bia M, Adey DB, Bloom RD, Chan L, Kulkarni S, Tomlanovich S. KDOQI US commentary on the 2009 KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Kidney Dis 2010; 56(2):189–218. doi:10.1053/j.ajkd.2010.04.010
- Baden LR, Swaminathan S, Angarone M, et al. Prevention and treatment of cancer-related infections, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2016; 14(7):882–913. pmid:27407129
- Cooley L, Dendle C, Wolf J, et al. Consensus guidelines for diagnosis, prophylaxis and management of Pneumocystis jirovecii pneumonia in patients with haematological and solid malignancies, 2014. Intern Med J 2014; 44(12b):1350–1363. doi:10.1111/imj.12599
- Orlando A, Armuzzi A, Papi C, et al; Italian Society of Gastroenterology; Italian Group for the study of Inflammatory Bowel Disease. The Italian Society of Gastroenterology (SIGE) and the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) clinical practice guidelines: the use of tumor necrosis factor-alpha antagonist therapy in inflammatory bowel disease. Dig Liver Dis 2011; 43(1):1–20. doi:10.1016/j.dld.2010.07.010
- Mikulska M, Lanini S, Gudiol C, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (agents targeting lymphoid cells surface antigens [I]: CD19, CD20 and CD52). Clin Microbiol Infect 2018; 24(suppl 2):S71–S82. doi:10.1016/j.cmi.2018.02.003
- Baddley J, Cantini F, Goletti D, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (soluble immune effector molecules [I]: anti-tumor necrosis factor-alpha agents). Clin Microbiol Infect 2018; 24(suppl 2):S10–S20. doi:10.1016/j.cmi.2017.12.025
- Winthrop K, Mariette X, Silva J, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (soluble immune effector molecules [II]: agents targeting interleukins, immunoglobulins and complement factors). Clin Microbiol Infect 2018; 24(suppl 2):S21–S40. doi:10.1016/j.cmi.2018.02.002
- Petri M, Allbritton J. Antibiotic allergy in systemic lupus erythematosus: a case-control study. J Rheumatol 1992; 19(2):265–269. pmid:1629825
- Pope J, Jerome D, Fenlon D, Krizova A, Ouimet J. Frequency of adverse drug reactions in patients with systemic lupus erythematosus. J Rheumatol 2003; 30(3):480–484. pmid:12610805
- Vananuvat P, Suwannalai P, Sungkanuparph S, Limsuwan T, Ngamjanyaporn P, Janwityanujit S. Primary prophylaxis for Pneumocystis jirovecii pneumonia in patients with connective tissue diseases. Semin Arthritis Rheum 2011; 41(3):497–502. doi:10.1016/j.semarthrit.2011.05.004
- Tamaki H, Butler R, Langford C. Abstract Number: 1755: Safety of methotrexate and low-dose trimethoprim-sulfamethoxazole in patients with ANCA-associated vasculitis. www.acrabstracts.org/abstract/safety-of-methotrexate-and-low-dose-trimethoprim-sulfamethoxazole-in-patients-with-anca-associated-vasculitis. Accessed May 3, 2019.
Pneumocystis jirovecii (previously carinii) pneumonia (PCP) is rare in patients taking biologic response modifiers for rheumatic disease.1–10 However, prophylaxis should be considered in patients who have granulomatosis with polyangiitis or underlying pulmonary disease, or who are concomitantly receiving glucocorticoids in high doses. There is some risk of adverse reactions to the prophylactic medicine.1,11–21 Until clear guidelines are available, the decision to initiate PCP prophylaxis and the choice of agent should be individualized.
THE BURDEN OF PCP
In a meta-analysis23 of 867 patients who developed PCP and did not have HIV infection, 20.1% had autoimmune or chronic inflammatory disease and the rest were transplant recipients or had malignancies. The mortality rate was 30.6%.
PHARMACOLOGIC RISK FACTORS FOR PCP
Treatment with glucocorticoids
Treatment with glucocorticoids is an important risk factor for PCP, independent of biologic therapy.
Calero-Bernal et al11 reported on 128 patients with non-HIV PCP, of whom 114 (89%) had received a glucocorticoid for more than 4 weeks, and 98 (76%) were currently receiving one. The mean daily dose was equivalent to 27.73 mg of prednisone per day in those on glucocorticoids only, and 21.34 mg in those receiving glucocorticoids in combination with other immunosuppressants.
Park et al,12 in a retrospective study of Korean patients treated for rheumatic disease with high-dose glucocorticoids (≥ 30 mg/day of prednisone or equivalent for more than 4 weeks), reported an incidence rate of PCP of 2.37 per 100 patient-years in those not on prophylaxis.
Other studies13,14 have also found a prednisone dose greater than 15 to 20 mg per day for more than 4 weeks or concomitant use of 2 or more disease-modifying antirheumatic drugs to be a significant risk factor.13,14
Tumor necrosis factor alpha antagonists
A US Food and Drug Administration review1 of voluntary reports of adverse drug events estimated the incidence of PCP to be 2.3 per 100,000 patient-years with infliximab and 1.6 per 100,000 patient-years with etanercept. In most cases, other immunosuppressants were used concomitantly.1
Postmarketing surveillance2 of 5,000 patients with rheumatoid arthritis showed an incidence of suspected PCP of 0.4% within the first 6 months of starting infliximab therapy.
Komano et al,15 in a case-control study of patients with rheumatoid arthritis treated with infliximab, reported that all 21 patients with PCP were also on methotrexate (median dosage 8 mg per week) and prednisolone (median dosage 7.5 mg per day).
PCP has also been reported after adalimumab use in combination with prednisone, azathioprine, and methotrexate, as well as with certolizumab, golimumab, tocilizumab, abatacept, and rituximab.3–6,24–26
Rituximab
Calero-Bernal et al11 reported that 23% of patients with non-HIV PCP who were receiving immunosuppressant drugs were on rituximab.
Alexandre et al16 performed a retrospective review of 11 cases of PCP complicating rituximab therapy for autoimmune disease, in which 10 (91%) of the patients were also on corticosteroids, with a median dosage of 30 mg of prednisone daily. A literature review of an additional 18 cases revealed similar findings.
PATIENT RISK FACTORS FOR PCP
Pulmonary disease, age, other factors
Komano et al,15 in their study of patients with rheumatoid arthritis treated with infliximab, found that 10 (48%) of 21 patients with PCP had preexisting pulmonary disease, compared with 11 (10.8%) of 102 patients without PCP (P < .001). Patients with PCP were older (mean age 64 vs 54, P < .001), were on higher median doses of prednisolone per day (7.5 vs 5 mg, P = .001), and had lower median serum immunoglobulin G (IgG) levels (944 vs 1,394 mg/dL, P < .001).15
Tadros et al13 performed a case-control study that also showed that patients with autoimmune disease who developed PCP had lower lymphocyte counts than controls on admission. Other risk factors included low CD4 counts and age older than 50.
Li et al17 found that patients with autoimmune or inflammatory disease with PCP were more likely to have low CD3, CD4, and CD8 cell counts, as well as albumin levels less than 28 g/L. They therefore suggested that lymphocyte subtyping may be a useful tool to guide PCP prophylaxis.
Granulomatosis with polyangiitis
Patients with granulomatosis with polyangiitis have a significantly higher incidence of PCP than patients with other connective tissue diseases.
Ward and Donald18 reviewed 223 cases of PCP in patients with connective tissue disease. The highest frequency (89 cases per 10,000 hospitalizations per year) was in patients with granulomatosis with polyangiitis, followed by 65 per 10,000 hospitalizations per year for patients with polyarteritis nodosa. The lowest frequency was in rheumatoid arthritis patients, at 2 per 10,000 hospitalizations per year. In decreasing order, diseases with significant associations with PCP were:
- Polyarteritis nodosa (odds ratio [OR] 10.20, 95% confidence interval [CI] 5.69–18.29)
- Granulomatosis with polyangiitis (OR 7.81, 95% CI 4.71–13.05)
- Inflammatory myopathy (OR 4.44, 95% CI 2.67–7.38)
- Systemic lupus erythematosus (OR 2.52, 95% CI 1.66–3.82).
Vallabhaneni and Chiller,26 in a meta-analysis including rheumatoid arthritis patients on biologics, did not find an increased risk of PCP (OR 1.77, 95% CI 0.42–7.47).
Park et al12 found that the highest incidences of PCP were in patients with granulomatosis with polyangiitis, microscopic polyangiitis, and systemic sclerosis. For systemic sclerosis, the main reason for giving high-dose glucocorticoids was interstitial lung disease.
Other studies19,20,28 also found an association with coexisting pulmonary disease in patients with rheumatoid arthritis.
CURRENT GUIDELINES
There are guidelines for primary and secondary prophylaxis of PCP in HIV-positive patients with CD4 counts less than 200/mm3 or a history of acquired immunodeficiency syndrome (AIDS)-defining illness.27 Additionally, patients with a CD4 cell percentage less than 14% should be considered for prophylaxis.27
Unfortunately, there are no guidelines for prophylaxis in patients taking immunosuppressants for rheumatic disease.
The recommended regimen for PCP prophylaxis in HIV-infected patients is trimethoprim-sulfamethoxazole, 1 double-strength or 1 single-strength tablet daily. Alternative regimens include 1 double-strength tablet 3 times per week, dapsone, aerosolized pentamidine, and atovaquone.27
There are also guidelines for prophylaxis in kidney transplant recipients, as well as for patients with hematologic malignancies and solid-organ malignancies, particularly those on chemotherapeutic agents and the T-cell-depleting agent alemtuzumab.29–31
Italian clinical practice guidelines for the use of tumor necrosis factor antagonists in inflammatory bowel disease recommend consideration of PCP prophylaxis in patients who are also on other immunosuppressants, particularly high-dose glucocorticoids.32
Prophylaxis has been shown to increase life expectancy and quality-adjusted life-years and to reduce cost for patients on immunosuppressive therapy for granulomatosis with polyangiitis.21 The European Society of Clinical Microbiology and Infectious Diseases recently produced consensus statements recommending PCP prophylaxis for patients on rituximab with other concomitant immunosuppressants such as the equivalent of prednisone 20 mg daily for more than 4 weeks.33 Prophylaxis was not recommended for other biologic therapies.34,35
THE RISKS OF PROPHYLAXIS
The risk of PCP should be weighed against the risk of prophylaxis in patients with rheumatic disease. Adverse reactions to sulfonamide antibiotics including disease flares have been reported in patients with systemic lupus erythematosus.36,37 Other studies have found no increased risk of flares in patients taking trimethoprim-sulfamethoxazole for PCP prophylaxis.12,38 A retrospective analysis of patients with vasculitis found no increased risk of combining methotrexate and trimethoprim-sulfamethoxazole.39
KEY POINTS
- PCP is an opportunistic infection with a high risk of death.
- PCP has been reported with biologics used as immunomodulators in rheumatic disease.
- PCP prophylaxis should be considered in patients at high risk of PCP, such as those who have granulomatosis with polyangiitis, underlying pulmonary disease or who are concomitantly taking glucocorticoids.
Pneumocystis jirovecii (previously carinii) pneumonia (PCP) is rare in patients taking biologic response modifiers for rheumatic disease.1–10 However, prophylaxis should be considered in patients who have granulomatosis with polyangiitis or underlying pulmonary disease, or who are concomitantly receiving glucocorticoids in high doses. There is some risk of adverse reactions to the prophylactic medicine.1,11–21 Until clear guidelines are available, the decision to initiate PCP prophylaxis and the choice of agent should be individualized.
THE BURDEN OF PCP
In a meta-analysis23 of 867 patients who developed PCP and did not have HIV infection, 20.1% had autoimmune or chronic inflammatory disease and the rest were transplant recipients or had malignancies. The mortality rate was 30.6%.
PHARMACOLOGIC RISK FACTORS FOR PCP
Treatment with glucocorticoids
Treatment with glucocorticoids is an important risk factor for PCP, independent of biologic therapy.
Calero-Bernal et al11 reported on 128 patients with non-HIV PCP, of whom 114 (89%) had received a glucocorticoid for more than 4 weeks, and 98 (76%) were currently receiving one. The mean daily dose was equivalent to 27.73 mg of prednisone per day in those on glucocorticoids only, and 21.34 mg in those receiving glucocorticoids in combination with other immunosuppressants.
Park et al,12 in a retrospective study of Korean patients treated for rheumatic disease with high-dose glucocorticoids (≥ 30 mg/day of prednisone or equivalent for more than 4 weeks), reported an incidence rate of PCP of 2.37 per 100 patient-years in those not on prophylaxis.
Other studies13,14 have also found a prednisone dose greater than 15 to 20 mg per day for more than 4 weeks or concomitant use of 2 or more disease-modifying antirheumatic drugs to be a significant risk factor.13,14
Tumor necrosis factor alpha antagonists
A US Food and Drug Administration review1 of voluntary reports of adverse drug events estimated the incidence of PCP to be 2.3 per 100,000 patient-years with infliximab and 1.6 per 100,000 patient-years with etanercept. In most cases, other immunosuppressants were used concomitantly.1
Postmarketing surveillance2 of 5,000 patients with rheumatoid arthritis showed an incidence of suspected PCP of 0.4% within the first 6 months of starting infliximab therapy.
Komano et al,15 in a case-control study of patients with rheumatoid arthritis treated with infliximab, reported that all 21 patients with PCP were also on methotrexate (median dosage 8 mg per week) and prednisolone (median dosage 7.5 mg per day).
PCP has also been reported after adalimumab use in combination with prednisone, azathioprine, and methotrexate, as well as with certolizumab, golimumab, tocilizumab, abatacept, and rituximab.3–6,24–26
Rituximab
Calero-Bernal et al11 reported that 23% of patients with non-HIV PCP who were receiving immunosuppressant drugs were on rituximab.
Alexandre et al16 performed a retrospective review of 11 cases of PCP complicating rituximab therapy for autoimmune disease, in which 10 (91%) of the patients were also on corticosteroids, with a median dosage of 30 mg of prednisone daily. A literature review of an additional 18 cases revealed similar findings.
PATIENT RISK FACTORS FOR PCP
Pulmonary disease, age, other factors
Komano et al,15 in their study of patients with rheumatoid arthritis treated with infliximab, found that 10 (48%) of 21 patients with PCP had preexisting pulmonary disease, compared with 11 (10.8%) of 102 patients without PCP (P < .001). Patients with PCP were older (mean age 64 vs 54, P < .001), were on higher median doses of prednisolone per day (7.5 vs 5 mg, P = .001), and had lower median serum immunoglobulin G (IgG) levels (944 vs 1,394 mg/dL, P < .001).15
Tadros et al13 performed a case-control study that also showed that patients with autoimmune disease who developed PCP had lower lymphocyte counts than controls on admission. Other risk factors included low CD4 counts and age older than 50.
Li et al17 found that patients with autoimmune or inflammatory disease with PCP were more likely to have low CD3, CD4, and CD8 cell counts, as well as albumin levels less than 28 g/L. They therefore suggested that lymphocyte subtyping may be a useful tool to guide PCP prophylaxis.
Granulomatosis with polyangiitis
Patients with granulomatosis with polyangiitis have a significantly higher incidence of PCP than patients with other connective tissue diseases.
Ward and Donald18 reviewed 223 cases of PCP in patients with connective tissue disease. The highest frequency (89 cases per 10,000 hospitalizations per year) was in patients with granulomatosis with polyangiitis, followed by 65 per 10,000 hospitalizations per year for patients with polyarteritis nodosa. The lowest frequency was in rheumatoid arthritis patients, at 2 per 10,000 hospitalizations per year. In decreasing order, diseases with significant associations with PCP were:
- Polyarteritis nodosa (odds ratio [OR] 10.20, 95% confidence interval [CI] 5.69–18.29)
- Granulomatosis with polyangiitis (OR 7.81, 95% CI 4.71–13.05)
- Inflammatory myopathy (OR 4.44, 95% CI 2.67–7.38)
- Systemic lupus erythematosus (OR 2.52, 95% CI 1.66–3.82).
Vallabhaneni and Chiller,26 in a meta-analysis including rheumatoid arthritis patients on biologics, did not find an increased risk of PCP (OR 1.77, 95% CI 0.42–7.47).
Park et al12 found that the highest incidences of PCP were in patients with granulomatosis with polyangiitis, microscopic polyangiitis, and systemic sclerosis. For systemic sclerosis, the main reason for giving high-dose glucocorticoids was interstitial lung disease.
Other studies19,20,28 also found an association with coexisting pulmonary disease in patients with rheumatoid arthritis.
CURRENT GUIDELINES
There are guidelines for primary and secondary prophylaxis of PCP in HIV-positive patients with CD4 counts less than 200/mm3 or a history of acquired immunodeficiency syndrome (AIDS)-defining illness.27 Additionally, patients with a CD4 cell percentage less than 14% should be considered for prophylaxis.27
Unfortunately, there are no guidelines for prophylaxis in patients taking immunosuppressants for rheumatic disease.
The recommended regimen for PCP prophylaxis in HIV-infected patients is trimethoprim-sulfamethoxazole, 1 double-strength or 1 single-strength tablet daily. Alternative regimens include 1 double-strength tablet 3 times per week, dapsone, aerosolized pentamidine, and atovaquone.27
There are also guidelines for prophylaxis in kidney transplant recipients, as well as for patients with hematologic malignancies and solid-organ malignancies, particularly those on chemotherapeutic agents and the T-cell-depleting agent alemtuzumab.29–31
Italian clinical practice guidelines for the use of tumor necrosis factor antagonists in inflammatory bowel disease recommend consideration of PCP prophylaxis in patients who are also on other immunosuppressants, particularly high-dose glucocorticoids.32
Prophylaxis has been shown to increase life expectancy and quality-adjusted life-years and to reduce cost for patients on immunosuppressive therapy for granulomatosis with polyangiitis.21 The European Society of Clinical Microbiology and Infectious Diseases recently produced consensus statements recommending PCP prophylaxis for patients on rituximab with other concomitant immunosuppressants such as the equivalent of prednisone 20 mg daily for more than 4 weeks.33 Prophylaxis was not recommended for other biologic therapies.34,35
THE RISKS OF PROPHYLAXIS
The risk of PCP should be weighed against the risk of prophylaxis in patients with rheumatic disease. Adverse reactions to sulfonamide antibiotics including disease flares have been reported in patients with systemic lupus erythematosus.36,37 Other studies have found no increased risk of flares in patients taking trimethoprim-sulfamethoxazole for PCP prophylaxis.12,38 A retrospective analysis of patients with vasculitis found no increased risk of combining methotrexate and trimethoprim-sulfamethoxazole.39
KEY POINTS
- PCP is an opportunistic infection with a high risk of death.
- PCP has been reported with biologics used as immunomodulators in rheumatic disease.
- PCP prophylaxis should be considered in patients at high risk of PCP, such as those who have granulomatosis with polyangiitis, underlying pulmonary disease or who are concomitantly taking glucocorticoids.
- US Food and Drug Administration. Safety update on TNF-alpha antagonists: infliximab and etanercept.https://wayback.archive-it.org/7993/20180127041103/https://www.fda.gov/ohrms/dockets/ac/01/briefing/3779b2_01_cber_safety_revision2.htm. Accessed May 3, 2019.
- Takeuchi T, Tatsuki Y, Nogami Y, et al. Postmarketing surveillance of the safety profile of infliximab in 5000 Japanese patients with rheumatoid arthritis. Ann Rheum Dis 2008; 67(2):189–194. doi:10.1136/ard.2007.072967
- Koike T, Harigai M, Ishiguro N, et al. Safety and effectiveness of adalimumab in Japanese rheumatoid arthritis patients: postmarketing surveillance report of the first 3,000 patients. Mod Rheumatol 2012; 22(4):498–508. doi:10.1007/s10165-011-0541-5
- Bykerk V, Cush J, Winthrop K, et al. Update on the safety profile of certolizumab pegol in rheumatoid arthritis: an integrated analysis from clinical trials. Ann Rheum Dis 2015; 74(1):96–103. doi:10.1136/annrheumdis-2013-203660
- Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of tocilizumab for rheumatoid arthritis in Japan: interim analysis of 3881 patients. Ann Rheum Dis 2011; 70(12):2148–2151. doi:10.1136/ard.2011.151092
- Harigai M, Ishiguro N, Inokuma S, et al. Postmarketing surveillance of the safety and effectiveness of abatacept in Japanese patients with rheumatoid arthritis. Mod Rheumatol 2016; 26(4):491–498. doi:10.3109/14397595.2015.1123211
- Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of the safety and effectiveness of etanercept in Japan. J Rheumatol 2009; 36(5):898–906. doi:10.3899/jrheum.080791
- Grubbs JA, Baddley JW. Pneumocystis jirovecii pneumonia in patients receiving tumor-necrosis-factor-inhibitor therapy: implications for chemoprophylaxis. Curr Rheumatol Rep 2014; 16(10):445. doi:10.1007/s11926-014-0445-4
- US Food and Drug Administration. FDA adverse event reporting system (FAERS) public dashboard. www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm070093.htm. Accessed May 3, 2019.
- Rutherford AI, Patarata E, Subesinghe S, Hyrich KL, Galloway JB. Opportunistic infections in rheumatoid arthritis patients exposed to biologic therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology (Oxford) 2018; 57(6):997–1001. doi:10.1093/rheumatology/key023
- Calero-Bernal ML, Martin-Garrido I, Donazar-Ezcurra M, Limper AH, Carmona EM. Intermittent courses of corticosteroids also present a risk for Pneumocystis pneumonia in non-HIV patients. Can Respir J 2016; 2016:2464791. doi:10.1155/2016/2464791
- Park JW, Curtis JR, Moon J, Song YW, Kim S, Lee EB. Prophylactic effect of trimethoprim-sulfamethoxazole for pneumocystis pneumonia in patients with rheumatic diseases exposed to prolonged high-dose glucocorticoids. Ann Rheum Dis 2018; 77(5):644–649. doi:10.1136/annrheumdis-2017-211796
- Tadros S, Teichtahl AJ, Ciciriello S, Wicks IP. Pneumocystis jirovecii pneumonia in systemic autoimmune rheumatic disease: a case-control study. Semin Arthritis Rheum 2017; 46(6):804–809. doi:10.1016/j.semarthrit.2016.09.009
- Demoruelle MK, Kahr A, Verilhac K, Deane K, Fischer A, West S. Recent-onset systemic lupus erythematosus complicated by acute respiratory failure. Arthritis Care Res (Hoboken) 2013; 65(2):314–323. doi:10.1002/acr.21857
- Komano Y, Harigai M, Koike R, et al. Pneumocystis jiroveci pneumonia in patients with rheumatoid arthritis treated with infliximab: a retrospective review and case-control study of 21 patients. Arthritis Rheum 2009; 61(3):305–312. doi:10.1002/art.24283
- Alexandre K, Ingen-Housz-Oro S, Versini M, Sailler L, Benhamou Y. Pneumocystis jirovecii pneumonia in patients treated with rituximab for systemic diseases: report of 11 cases and review of the literature. Eur J Intern Med 2018; 50:e23–e24. doi:10.1016/j.ejim.2017.11.014
- Li Y, Ghannoum M, Deng C, et al. Pneumocystis pneumonia in patients with inflammatory or autoimmune diseases: usefulness of lymphocyte subtyping. Int J Infect Dis 2017; 57:108–115. doi:10.1016/j.ijid.2017.02.010
- Ward MM, Donald F. Pneumocystis carinii pneumonia in patients with connective tissue diseases: the role of hospital experience in diagnosis and mortality. Arthritis Rheum 1999; 42(4):780–789. doi:10.1002/1529-0131(199904)42:4<780::AID-ANR23>3.0.CO;2-M
- Katsuyama T, Saito K, Kubo S, Nawata M, Tanaka Y. Prophylaxis for Pneumocystis pneumonia in patients with rheumatoid arthritis treated with biologics, based on risk factors found in a retrospective study. Arthritis Res Ther 2014; 16(1):R43. doi:10.1186/ar4472
- Tanaka M, Sakai R, Koike R, et al. Pneumocystis jirovecii pneumonia associated with etanercept treatment in patients with rheumatoid arthritis: a retrospective review of 15 cases and analysis of risk factors. Mod Rheumatol 2012; 22(6):849–858. doi:10.1007/s10165-012-0615-z
- Chung JB, Armstrong K, Schwartz JS, Albert D. Cost-effectiveness of prophylaxis against Pneumocystis carinii pneumonia in patients with Wegener’s granulomatosis undergoing immunosuppressive therapy. Arthritis Rheum 2000; 43(8):1841–1848. doi:10.1002/1529-0131(200008)43:8<1841::AID-ANR21>3.0.CO;2-Q
- Selmi C, Generali E, Massarotti M, Bianchi G, Scire CA. New treatments for inflammatory rheumatic disease. Immunol Res 2014; 60(2–3):277–288. doi:10.1007/s12026-014-8565-5
- Liu Y, Su L, Jiang SJ, Qu H. Risk factors for mortality from Pneumocystis carinii pneumonia (PCP) in non-HIV patients: a meta-analysis. Oncotarget 2017; 8(35):59729–59739. doi:10.18632/oncotarget.19927
- Desales AL, Mendez-Navarro J, Méndez-Tovar LJ, et al. Pneumocystosis in a patient with Crohn's disease treated with combination therapy with adalimumab. J Crohns Colitis 2012; 6(4):483–487. doi:10.1016/j.crohns.2011.10.012
- Kalyoncu U, Karadag O, Akdogan A, et al. Pneumocystis carinii pneumonia in a rheumatoid arthritis patient treated with adalimumab. Scand J Infect Dis 2007; 39(5):475–478. doi:10.1080/00365540601071867
- Vallabhaneni S, Chiller TM. Fungal infections and new biologic therapies. Curr Rheumatol Rep 2016; 18(5):29. doi:10.1007/s11926-016-0572-1
- Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. www.aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed May 3, 2019.
- Kourbeti IS, Ziakas PD, Mylonakis E. Biologic therapies in rheumatoid arthritis and the risk of opportunistic infections: a meta-analysis. Clin Infect Dis 2014; 58(12):1649–1657. doi:10.1093/cid/ciu185
- Bia M, Adey DB, Bloom RD, Chan L, Kulkarni S, Tomlanovich S. KDOQI US commentary on the 2009 KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Kidney Dis 2010; 56(2):189–218. doi:10.1053/j.ajkd.2010.04.010
- Baden LR, Swaminathan S, Angarone M, et al. Prevention and treatment of cancer-related infections, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2016; 14(7):882–913. pmid:27407129
- Cooley L, Dendle C, Wolf J, et al. Consensus guidelines for diagnosis, prophylaxis and management of Pneumocystis jirovecii pneumonia in patients with haematological and solid malignancies, 2014. Intern Med J 2014; 44(12b):1350–1363. doi:10.1111/imj.12599
- Orlando A, Armuzzi A, Papi C, et al; Italian Society of Gastroenterology; Italian Group for the study of Inflammatory Bowel Disease. The Italian Society of Gastroenterology (SIGE) and the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) clinical practice guidelines: the use of tumor necrosis factor-alpha antagonist therapy in inflammatory bowel disease. Dig Liver Dis 2011; 43(1):1–20. doi:10.1016/j.dld.2010.07.010
- Mikulska M, Lanini S, Gudiol C, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (agents targeting lymphoid cells surface antigens [I]: CD19, CD20 and CD52). Clin Microbiol Infect 2018; 24(suppl 2):S71–S82. doi:10.1016/j.cmi.2018.02.003
- Baddley J, Cantini F, Goletti D, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (soluble immune effector molecules [I]: anti-tumor necrosis factor-alpha agents). Clin Microbiol Infect 2018; 24(suppl 2):S10–S20. doi:10.1016/j.cmi.2017.12.025
- Winthrop K, Mariette X, Silva J, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (soluble immune effector molecules [II]: agents targeting interleukins, immunoglobulins and complement factors). Clin Microbiol Infect 2018; 24(suppl 2):S21–S40. doi:10.1016/j.cmi.2018.02.002
- Petri M, Allbritton J. Antibiotic allergy in systemic lupus erythematosus: a case-control study. J Rheumatol 1992; 19(2):265–269. pmid:1629825
- Pope J, Jerome D, Fenlon D, Krizova A, Ouimet J. Frequency of adverse drug reactions in patients with systemic lupus erythematosus. J Rheumatol 2003; 30(3):480–484. pmid:12610805
- Vananuvat P, Suwannalai P, Sungkanuparph S, Limsuwan T, Ngamjanyaporn P, Janwityanujit S. Primary prophylaxis for Pneumocystis jirovecii pneumonia in patients with connective tissue diseases. Semin Arthritis Rheum 2011; 41(3):497–502. doi:10.1016/j.semarthrit.2011.05.004
- Tamaki H, Butler R, Langford C. Abstract Number: 1755: Safety of methotrexate and low-dose trimethoprim-sulfamethoxazole in patients with ANCA-associated vasculitis. www.acrabstracts.org/abstract/safety-of-methotrexate-and-low-dose-trimethoprim-sulfamethoxazole-in-patients-with-anca-associated-vasculitis. Accessed May 3, 2019.
- US Food and Drug Administration. Safety update on TNF-alpha antagonists: infliximab and etanercept.https://wayback.archive-it.org/7993/20180127041103/https://www.fda.gov/ohrms/dockets/ac/01/briefing/3779b2_01_cber_safety_revision2.htm. Accessed May 3, 2019.
- Takeuchi T, Tatsuki Y, Nogami Y, et al. Postmarketing surveillance of the safety profile of infliximab in 5000 Japanese patients with rheumatoid arthritis. Ann Rheum Dis 2008; 67(2):189–194. doi:10.1136/ard.2007.072967
- Koike T, Harigai M, Ishiguro N, et al. Safety and effectiveness of adalimumab in Japanese rheumatoid arthritis patients: postmarketing surveillance report of the first 3,000 patients. Mod Rheumatol 2012; 22(4):498–508. doi:10.1007/s10165-011-0541-5
- Bykerk V, Cush J, Winthrop K, et al. Update on the safety profile of certolizumab pegol in rheumatoid arthritis: an integrated analysis from clinical trials. Ann Rheum Dis 2015; 74(1):96–103. doi:10.1136/annrheumdis-2013-203660
- Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of tocilizumab for rheumatoid arthritis in Japan: interim analysis of 3881 patients. Ann Rheum Dis 2011; 70(12):2148–2151. doi:10.1136/ard.2011.151092
- Harigai M, Ishiguro N, Inokuma S, et al. Postmarketing surveillance of the safety and effectiveness of abatacept in Japanese patients with rheumatoid arthritis. Mod Rheumatol 2016; 26(4):491–498. doi:10.3109/14397595.2015.1123211
- Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of the safety and effectiveness of etanercept in Japan. J Rheumatol 2009; 36(5):898–906. doi:10.3899/jrheum.080791
- Grubbs JA, Baddley JW. Pneumocystis jirovecii pneumonia in patients receiving tumor-necrosis-factor-inhibitor therapy: implications for chemoprophylaxis. Curr Rheumatol Rep 2014; 16(10):445. doi:10.1007/s11926-014-0445-4
- US Food and Drug Administration. FDA adverse event reporting system (FAERS) public dashboard. www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm070093.htm. Accessed May 3, 2019.
- Rutherford AI, Patarata E, Subesinghe S, Hyrich KL, Galloway JB. Opportunistic infections in rheumatoid arthritis patients exposed to biologic therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology (Oxford) 2018; 57(6):997–1001. doi:10.1093/rheumatology/key023
- Calero-Bernal ML, Martin-Garrido I, Donazar-Ezcurra M, Limper AH, Carmona EM. Intermittent courses of corticosteroids also present a risk for Pneumocystis pneumonia in non-HIV patients. Can Respir J 2016; 2016:2464791. doi:10.1155/2016/2464791
- Park JW, Curtis JR, Moon J, Song YW, Kim S, Lee EB. Prophylactic effect of trimethoprim-sulfamethoxazole for pneumocystis pneumonia in patients with rheumatic diseases exposed to prolonged high-dose glucocorticoids. Ann Rheum Dis 2018; 77(5):644–649. doi:10.1136/annrheumdis-2017-211796
- Tadros S, Teichtahl AJ, Ciciriello S, Wicks IP. Pneumocystis jirovecii pneumonia in systemic autoimmune rheumatic disease: a case-control study. Semin Arthritis Rheum 2017; 46(6):804–809. doi:10.1016/j.semarthrit.2016.09.009
- Demoruelle MK, Kahr A, Verilhac K, Deane K, Fischer A, West S. Recent-onset systemic lupus erythematosus complicated by acute respiratory failure. Arthritis Care Res (Hoboken) 2013; 65(2):314–323. doi:10.1002/acr.21857
- Komano Y, Harigai M, Koike R, et al. Pneumocystis jiroveci pneumonia in patients with rheumatoid arthritis treated with infliximab: a retrospective review and case-control study of 21 patients. Arthritis Rheum 2009; 61(3):305–312. doi:10.1002/art.24283
- Alexandre K, Ingen-Housz-Oro S, Versini M, Sailler L, Benhamou Y. Pneumocystis jirovecii pneumonia in patients treated with rituximab for systemic diseases: report of 11 cases and review of the literature. Eur J Intern Med 2018; 50:e23–e24. doi:10.1016/j.ejim.2017.11.014
- Li Y, Ghannoum M, Deng C, et al. Pneumocystis pneumonia in patients with inflammatory or autoimmune diseases: usefulness of lymphocyte subtyping. Int J Infect Dis 2017; 57:108–115. doi:10.1016/j.ijid.2017.02.010
- Ward MM, Donald F. Pneumocystis carinii pneumonia in patients with connective tissue diseases: the role of hospital experience in diagnosis and mortality. Arthritis Rheum 1999; 42(4):780–789. doi:10.1002/1529-0131(199904)42:4<780::AID-ANR23>3.0.CO;2-M
- Katsuyama T, Saito K, Kubo S, Nawata M, Tanaka Y. Prophylaxis for Pneumocystis pneumonia in patients with rheumatoid arthritis treated with biologics, based on risk factors found in a retrospective study. Arthritis Res Ther 2014; 16(1):R43. doi:10.1186/ar4472
- Tanaka M, Sakai R, Koike R, et al. Pneumocystis jirovecii pneumonia associated with etanercept treatment in patients with rheumatoid arthritis: a retrospective review of 15 cases and analysis of risk factors. Mod Rheumatol 2012; 22(6):849–858. doi:10.1007/s10165-012-0615-z
- Chung JB, Armstrong K, Schwartz JS, Albert D. Cost-effectiveness of prophylaxis against Pneumocystis carinii pneumonia in patients with Wegener’s granulomatosis undergoing immunosuppressive therapy. Arthritis Rheum 2000; 43(8):1841–1848. doi:10.1002/1529-0131(200008)43:8<1841::AID-ANR21>3.0.CO;2-Q
- Selmi C, Generali E, Massarotti M, Bianchi G, Scire CA. New treatments for inflammatory rheumatic disease. Immunol Res 2014; 60(2–3):277–288. doi:10.1007/s12026-014-8565-5
- Liu Y, Su L, Jiang SJ, Qu H. Risk factors for mortality from Pneumocystis carinii pneumonia (PCP) in non-HIV patients: a meta-analysis. Oncotarget 2017; 8(35):59729–59739. doi:10.18632/oncotarget.19927
- Desales AL, Mendez-Navarro J, Méndez-Tovar LJ, et al. Pneumocystosis in a patient with Crohn's disease treated with combination therapy with adalimumab. J Crohns Colitis 2012; 6(4):483–487. doi:10.1016/j.crohns.2011.10.012
- Kalyoncu U, Karadag O, Akdogan A, et al. Pneumocystis carinii pneumonia in a rheumatoid arthritis patient treated with adalimumab. Scand J Infect Dis 2007; 39(5):475–478. doi:10.1080/00365540601071867
- Vallabhaneni S, Chiller TM. Fungal infections and new biologic therapies. Curr Rheumatol Rep 2016; 18(5):29. doi:10.1007/s11926-016-0572-1
- Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. www.aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed May 3, 2019.
- Kourbeti IS, Ziakas PD, Mylonakis E. Biologic therapies in rheumatoid arthritis and the risk of opportunistic infections: a meta-analysis. Clin Infect Dis 2014; 58(12):1649–1657. doi:10.1093/cid/ciu185
- Bia M, Adey DB, Bloom RD, Chan L, Kulkarni S, Tomlanovich S. KDOQI US commentary on the 2009 KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Kidney Dis 2010; 56(2):189–218. doi:10.1053/j.ajkd.2010.04.010
- Baden LR, Swaminathan S, Angarone M, et al. Prevention and treatment of cancer-related infections, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2016; 14(7):882–913. pmid:27407129
- Cooley L, Dendle C, Wolf J, et al. Consensus guidelines for diagnosis, prophylaxis and management of Pneumocystis jirovecii pneumonia in patients with haematological and solid malignancies, 2014. Intern Med J 2014; 44(12b):1350–1363. doi:10.1111/imj.12599
- Orlando A, Armuzzi A, Papi C, et al; Italian Society of Gastroenterology; Italian Group for the study of Inflammatory Bowel Disease. The Italian Society of Gastroenterology (SIGE) and the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) clinical practice guidelines: the use of tumor necrosis factor-alpha antagonist therapy in inflammatory bowel disease. Dig Liver Dis 2011; 43(1):1–20. doi:10.1016/j.dld.2010.07.010
- Mikulska M, Lanini S, Gudiol C, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (agents targeting lymphoid cells surface antigens [I]: CD19, CD20 and CD52). Clin Microbiol Infect 2018; 24(suppl 2):S71–S82. doi:10.1016/j.cmi.2018.02.003
- Baddley J, Cantini F, Goletti D, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (soluble immune effector molecules [I]: anti-tumor necrosis factor-alpha agents). Clin Microbiol Infect 2018; 24(suppl 2):S10–S20. doi:10.1016/j.cmi.2017.12.025
- Winthrop K, Mariette X, Silva J, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (soluble immune effector molecules [II]: agents targeting interleukins, immunoglobulins and complement factors). Clin Microbiol Infect 2018; 24(suppl 2):S21–S40. doi:10.1016/j.cmi.2018.02.002
- Petri M, Allbritton J. Antibiotic allergy in systemic lupus erythematosus: a case-control study. J Rheumatol 1992; 19(2):265–269. pmid:1629825
- Pope J, Jerome D, Fenlon D, Krizova A, Ouimet J. Frequency of adverse drug reactions in patients with systemic lupus erythematosus. J Rheumatol 2003; 30(3):480–484. pmid:12610805
- Vananuvat P, Suwannalai P, Sungkanuparph S, Limsuwan T, Ngamjanyaporn P, Janwityanujit S. Primary prophylaxis for Pneumocystis jirovecii pneumonia in patients with connective tissue diseases. Semin Arthritis Rheum 2011; 41(3):497–502. doi:10.1016/j.semarthrit.2011.05.004
- Tamaki H, Butler R, Langford C. Abstract Number: 1755: Safety of methotrexate and low-dose trimethoprim-sulfamethoxazole in patients with ANCA-associated vasculitis. www.acrabstracts.org/abstract/safety-of-methotrexate-and-low-dose-trimethoprim-sulfamethoxazole-in-patients-with-anca-associated-vasculitis. Accessed May 3, 2019.
Click for Credit: Roux-en-Y for diabetes; Exercise & fall prevention; more
Here are 5 articles from the July issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Cloud of inconsistency hangs over cannabis data
To take the posttest, go to: https://bit.ly/2NfjaDS
Expires February 6, 2020
2. Roux-en-Y achieves diabetes remission in majority of patients
To take the posttest, go to: https://bit.ly/2x9hLnE
Expires February 6, 2020
3. Socioeconomic status, race found to impact CPAP compliance
To take the posttest, go to: https://bit.ly/2RBpLa9
Expires February 8, 2020
4. Exercise type matters for fall prevention among elderly
To take the posttest, go to: https://bit.ly/2X26OUh
Expires February 12, 2020
5. Adult HIV patients should receive standard vaccinations, with caveats
To take the posttest, go to: https://bit.ly/2X1S7LV
Expires February 12, 2020
Here are 5 articles from the July issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Cloud of inconsistency hangs over cannabis data
To take the posttest, go to: https://bit.ly/2NfjaDS
Expires February 6, 2020
2. Roux-en-Y achieves diabetes remission in majority of patients
To take the posttest, go to: https://bit.ly/2x9hLnE
Expires February 6, 2020
3. Socioeconomic status, race found to impact CPAP compliance
To take the posttest, go to: https://bit.ly/2RBpLa9
Expires February 8, 2020
4. Exercise type matters for fall prevention among elderly
To take the posttest, go to: https://bit.ly/2X26OUh
Expires February 12, 2020
5. Adult HIV patients should receive standard vaccinations, with caveats
To take the posttest, go to: https://bit.ly/2X1S7LV
Expires February 12, 2020
Here are 5 articles from the July issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Cloud of inconsistency hangs over cannabis data
To take the posttest, go to: https://bit.ly/2NfjaDS
Expires February 6, 2020
2. Roux-en-Y achieves diabetes remission in majority of patients
To take the posttest, go to: https://bit.ly/2x9hLnE
Expires February 6, 2020
3. Socioeconomic status, race found to impact CPAP compliance
To take the posttest, go to: https://bit.ly/2RBpLa9
Expires February 8, 2020
4. Exercise type matters for fall prevention among elderly
To take the posttest, go to: https://bit.ly/2X26OUh
Expires February 12, 2020
5. Adult HIV patients should receive standard vaccinations, with caveats
To take the posttest, go to: https://bit.ly/2X1S7LV
Expires February 12, 2020
ACIP approves meningococcal booster for persons at increased risk
according to the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The committee voted unanimously in favor of a booster dose of MenB vaccine 1 year after completion of the primary series, with additional boosters every 2-3 years “for as long as risk remains” for high-risk persons, including microbiologists and persons with complement deficiency, complement inhibitor use, or asplenia.
The committee also voted unanimously in favor of a one-time MenB booster for individuals aged 10 years and older who are at least a year beyond completion of a MenB primary series and deemed at increased risk by public health officials in an outbreak situation.
In addition, “a booster dose interval of 6 months or more may be considered by public health officials depending on the specific outbreak, vaccine strategy, and projected duration of elevated risk” according to the language, which was included in the unanimously approved statement “Meningococcal Vaccination: Recommendations of The Advisory Committee on Immunization Practices.”
The updated statement on meningococcal vaccination was developed in 2019 “to consolidate all existing ACIP recommendations for MenACWY and MenB vaccines in a single document,” said Sarah Mbaeyi, MD, of the CDC’s National Center for Immunization and Respiratory Diseases, who presented immunogenicity data and the proposed recommendations.
The statement includes the recommendation of a MenB primary series for individuals aged 16-23 years based on shared clinical decision making. Kelly Moore, MD, of Vanderbilt University, Nashville, Tenn., noted the importance of ongoing data collection, and said clinicians must make clear to patients that, “if they want protection, they need the booster.”
Approximately 7% of serogroup B cases in the United States are related to disease outbreaks, mainly among college students, Dr. Mbaeyi said. All 13 universities that experienced outbreaks between 2013 and 2019 have implemented a MenB primary series, and one university has implemented an off-label booster program.
The work group concluded that a MenB booster dose is necessary to sustain protection against serogroup B disease in persons at increased risk during an outbreak, and that the potential benefits outweighed the harms given the seriousness of meningococcal disease.
Paul Hunter, MD, of the City of Milwaukee Health Department, noted that “the booster recommendation gives more flexibility” in an outbreak response.
The committee also voted unanimously to approve the Vaccines for Children resolution for the meningococcal vaccine that updates language to align with the new recommendations.
The ACIP members had no financial conflicts to disclose.
according to the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The committee voted unanimously in favor of a booster dose of MenB vaccine 1 year after completion of the primary series, with additional boosters every 2-3 years “for as long as risk remains” for high-risk persons, including microbiologists and persons with complement deficiency, complement inhibitor use, or asplenia.
The committee also voted unanimously in favor of a one-time MenB booster for individuals aged 10 years and older who are at least a year beyond completion of a MenB primary series and deemed at increased risk by public health officials in an outbreak situation.
In addition, “a booster dose interval of 6 months or more may be considered by public health officials depending on the specific outbreak, vaccine strategy, and projected duration of elevated risk” according to the language, which was included in the unanimously approved statement “Meningococcal Vaccination: Recommendations of The Advisory Committee on Immunization Practices.”
The updated statement on meningococcal vaccination was developed in 2019 “to consolidate all existing ACIP recommendations for MenACWY and MenB vaccines in a single document,” said Sarah Mbaeyi, MD, of the CDC’s National Center for Immunization and Respiratory Diseases, who presented immunogenicity data and the proposed recommendations.
The statement includes the recommendation of a MenB primary series for individuals aged 16-23 years based on shared clinical decision making. Kelly Moore, MD, of Vanderbilt University, Nashville, Tenn., noted the importance of ongoing data collection, and said clinicians must make clear to patients that, “if they want protection, they need the booster.”
Approximately 7% of serogroup B cases in the United States are related to disease outbreaks, mainly among college students, Dr. Mbaeyi said. All 13 universities that experienced outbreaks between 2013 and 2019 have implemented a MenB primary series, and one university has implemented an off-label booster program.
The work group concluded that a MenB booster dose is necessary to sustain protection against serogroup B disease in persons at increased risk during an outbreak, and that the potential benefits outweighed the harms given the seriousness of meningococcal disease.
Paul Hunter, MD, of the City of Milwaukee Health Department, noted that “the booster recommendation gives more flexibility” in an outbreak response.
The committee also voted unanimously to approve the Vaccines for Children resolution for the meningococcal vaccine that updates language to align with the new recommendations.
The ACIP members had no financial conflicts to disclose.
according to the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The committee voted unanimously in favor of a booster dose of MenB vaccine 1 year after completion of the primary series, with additional boosters every 2-3 years “for as long as risk remains” for high-risk persons, including microbiologists and persons with complement deficiency, complement inhibitor use, or asplenia.
The committee also voted unanimously in favor of a one-time MenB booster for individuals aged 10 years and older who are at least a year beyond completion of a MenB primary series and deemed at increased risk by public health officials in an outbreak situation.
In addition, “a booster dose interval of 6 months or more may be considered by public health officials depending on the specific outbreak, vaccine strategy, and projected duration of elevated risk” according to the language, which was included in the unanimously approved statement “Meningococcal Vaccination: Recommendations of The Advisory Committee on Immunization Practices.”
The updated statement on meningococcal vaccination was developed in 2019 “to consolidate all existing ACIP recommendations for MenACWY and MenB vaccines in a single document,” said Sarah Mbaeyi, MD, of the CDC’s National Center for Immunization and Respiratory Diseases, who presented immunogenicity data and the proposed recommendations.
The statement includes the recommendation of a MenB primary series for individuals aged 16-23 years based on shared clinical decision making. Kelly Moore, MD, of Vanderbilt University, Nashville, Tenn., noted the importance of ongoing data collection, and said clinicians must make clear to patients that, “if they want protection, they need the booster.”
Approximately 7% of serogroup B cases in the United States are related to disease outbreaks, mainly among college students, Dr. Mbaeyi said. All 13 universities that experienced outbreaks between 2013 and 2019 have implemented a MenB primary series, and one university has implemented an off-label booster program.
The work group concluded that a MenB booster dose is necessary to sustain protection against serogroup B disease in persons at increased risk during an outbreak, and that the potential benefits outweighed the harms given the seriousness of meningococcal disease.
Paul Hunter, MD, of the City of Milwaukee Health Department, noted that “the booster recommendation gives more flexibility” in an outbreak response.
The committee also voted unanimously to approve the Vaccines for Children resolution for the meningococcal vaccine that updates language to align with the new recommendations.
The ACIP members had no financial conflicts to disclose.
REPORTING FROM AN ACIP MEETING
Cryptosporidiosis infections spike during summer swim season
Outbreaks of cryptosporidiosis increased in the United States by an average of 13% each year between 2009 and 2017, based on data from the Centers for Disease Control and Prevention.
In a study published in the CDC’s Morbidity and Mortality Weekly Report, researchers reviewed data from 444 reported outbreaks submitted to the CDC’s National Outbreak Reporting System totaling 7,465 cases, including 287 hospitalizations and one death.
The outbreaks during this period were most commonly associated with pools and water parks (35%), exposure to cattle (15%), and child care settings (13%). Another 3% of outbreaks were associated with drinking unpasteurized milk or apple cider. An outbreak was defined as two or more cases linked to a common source.
The profuse, watery diarrhea associated with infection from the cryptosporidium parasite can last for 3 weeks in healthy individuals and can cause life-threatening malnutrition in the immunocompromised, wrote Radhika Gharpure, DVM, of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, and colleagues.
The overall number of outbreaks peaked during July and August each year; the number associated with pools and water parks peaked between June and August, the number associated with cattle peaked between March and May, and the number associated with child care settings peaked between July and September.
The results were limited by several factors including likely underestimation of the number of outbreaks, the use of multipathogen testing panels that could have inflated the number of outbreaks, and the variation in the ability of jurisdictions to detect, investigate, and report outbreaks, the researchers noted. CryptoNet, a molecularly-based surveillance system, has shown potential to track disease transmission, they said.
However, primary prevention is important to prevent the spread of disease, and strategies include refraining from swimming when one has diarrhea and for 2 weeks after resolution of diarrhea, not sending children to child care when they have diarrhea, and washing hands thoroughly after contact with animals, the researchers said.
“If a cryptosporidiosis outbreak occurs, substantial decontamination measures are needed, including hyperchlorinating public treated recreational water venues (e.g., swimming pools at a hotel, apartment complex, or water park) and using hydrogen peroxide to disinfect surfaces in child care settings to inactivate Cryptosporidium oocysts,” they emphasized.
The researchers had no financial conflicts to disclose.
SOURCE: Gharpure R et al. MMWR. 2019 June 28. 68:568-72.
Outbreaks of cryptosporidiosis increased in the United States by an average of 13% each year between 2009 and 2017, based on data from the Centers for Disease Control and Prevention.
In a study published in the CDC’s Morbidity and Mortality Weekly Report, researchers reviewed data from 444 reported outbreaks submitted to the CDC’s National Outbreak Reporting System totaling 7,465 cases, including 287 hospitalizations and one death.
The outbreaks during this period were most commonly associated with pools and water parks (35%), exposure to cattle (15%), and child care settings (13%). Another 3% of outbreaks were associated with drinking unpasteurized milk or apple cider. An outbreak was defined as two or more cases linked to a common source.
The profuse, watery diarrhea associated with infection from the cryptosporidium parasite can last for 3 weeks in healthy individuals and can cause life-threatening malnutrition in the immunocompromised, wrote Radhika Gharpure, DVM, of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, and colleagues.
The overall number of outbreaks peaked during July and August each year; the number associated with pools and water parks peaked between June and August, the number associated with cattle peaked between March and May, and the number associated with child care settings peaked between July and September.
The results were limited by several factors including likely underestimation of the number of outbreaks, the use of multipathogen testing panels that could have inflated the number of outbreaks, and the variation in the ability of jurisdictions to detect, investigate, and report outbreaks, the researchers noted. CryptoNet, a molecularly-based surveillance system, has shown potential to track disease transmission, they said.
However, primary prevention is important to prevent the spread of disease, and strategies include refraining from swimming when one has diarrhea and for 2 weeks after resolution of diarrhea, not sending children to child care when they have diarrhea, and washing hands thoroughly after contact with animals, the researchers said.
“If a cryptosporidiosis outbreak occurs, substantial decontamination measures are needed, including hyperchlorinating public treated recreational water venues (e.g., swimming pools at a hotel, apartment complex, or water park) and using hydrogen peroxide to disinfect surfaces in child care settings to inactivate Cryptosporidium oocysts,” they emphasized.
The researchers had no financial conflicts to disclose.
SOURCE: Gharpure R et al. MMWR. 2019 June 28. 68:568-72.
Outbreaks of cryptosporidiosis increased in the United States by an average of 13% each year between 2009 and 2017, based on data from the Centers for Disease Control and Prevention.
In a study published in the CDC’s Morbidity and Mortality Weekly Report, researchers reviewed data from 444 reported outbreaks submitted to the CDC’s National Outbreak Reporting System totaling 7,465 cases, including 287 hospitalizations and one death.
The outbreaks during this period were most commonly associated with pools and water parks (35%), exposure to cattle (15%), and child care settings (13%). Another 3% of outbreaks were associated with drinking unpasteurized milk or apple cider. An outbreak was defined as two or more cases linked to a common source.
The profuse, watery diarrhea associated with infection from the cryptosporidium parasite can last for 3 weeks in healthy individuals and can cause life-threatening malnutrition in the immunocompromised, wrote Radhika Gharpure, DVM, of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, and colleagues.
The overall number of outbreaks peaked during July and August each year; the number associated with pools and water parks peaked between June and August, the number associated with cattle peaked between March and May, and the number associated with child care settings peaked between July and September.
The results were limited by several factors including likely underestimation of the number of outbreaks, the use of multipathogen testing panels that could have inflated the number of outbreaks, and the variation in the ability of jurisdictions to detect, investigate, and report outbreaks, the researchers noted. CryptoNet, a molecularly-based surveillance system, has shown potential to track disease transmission, they said.
However, primary prevention is important to prevent the spread of disease, and strategies include refraining from swimming when one has diarrhea and for 2 weeks after resolution of diarrhea, not sending children to child care when they have diarrhea, and washing hands thoroughly after contact with animals, the researchers said.
“If a cryptosporidiosis outbreak occurs, substantial decontamination measures are needed, including hyperchlorinating public treated recreational water venues (e.g., swimming pools at a hotel, apartment complex, or water park) and using hydrogen peroxide to disinfect surfaces in child care settings to inactivate Cryptosporidium oocysts,” they emphasized.
The researchers had no financial conflicts to disclose.
SOURCE: Gharpure R et al. MMWR. 2019 June 28. 68:568-72.
FROM MMWR