User login
HCV-infected people who inject drugs also have substantial alcohol use
Curing hepatitis C virus (HCV) infection without addressing the high rate of alcohol use disorder in many patients may undermine the benefits of treatment to long-term liver health, according to the results of a large cohort study.
Because excess alcohol use is known to accelerate liver disease progression, researchers Risha Irvin, MD, and her colleagues from Johns Hopkins University, Baltimore, examined the prevalence of alcohol use in HCV-infected people who inject drugs (PWID). Their study examined the prevalence and associated correlates of alcohol use (Addictive Behaviors 2019;96:56-61).
They followed a large cohort of 1,623 HCV-antibody positive PWID from 2005 to 2013 from the AIDS Linked to the Intravenous Experience (ALIVE) study. They characterized alcohol use with the Alcohol Use Disorders Identification Test (AUDIT-C) questionnaire. Multivariable logistic regression with generalized estimated equations was used to examine sociodemographic, clinical, and substance use correlates of alcohol use.
At baseline, the median age was 47 years, 67% were men, 81% were black, and 34% were HIV positive. The majority (60%) reported injection drug use in the prior 6 months, while 46% reported noninjection cocaine or heroin, 31% reported street-acquired prescription drugs, and 22% reported marijuana use in the same time period. According to the AUDIT-C results, 41% of the patients reported no alcohol use, 21% reported moderate alcohol use, and 38% reported heavy alcohol use at their baseline visit.
The factors that were significantly associated with heavy alcohol use included male sex, black race, income of $5,000 or less, a Center for Epidemiologic Studies Depression Scale (range 0-60) score of 23 or greater, being homeless, being incarcerated, marijuana use, use of street-acquired prescription drugs, noninjection cocaine/heroin, injection drug use, and cigarette smoking. In a model that included the composite summary variable for substance use intensity, one drug type (adjusted odds ratio, 1.92), two drug types (AOR, 2.93), and three drug types (AOR, 3.65) were significantly associated with heavy alcohol use.
“While clinicians are undoubtedly concerned about any level of alcohol use in the setting of HCV infection due to the acceleration of liver fibrosis, there is particular concern for individuals with heavy alcohol use and their increased risk for cirrhosis and liver failure even after HCV cure. Without intervention, alcohol use will persist after HCV is cured with the potential to undermine the benefit of HCV cure. Therefore, our data point to the need to invest in and develop programs that effectively address alcohol use and co-occurring substance use in this population of PWID with HCV,” the researchers concluded.
The study was supported by the U.S. National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, and the National Institute on Alcohol Abuse and Alcoholism. The authors declared that they had no conflicts.
SOURCE: Irvin R et al. Addictive Behaviors. 2019;96:56-61.
Curing hepatitis C virus (HCV) infection without addressing the high rate of alcohol use disorder in many patients may undermine the benefits of treatment to long-term liver health, according to the results of a large cohort study.
Because excess alcohol use is known to accelerate liver disease progression, researchers Risha Irvin, MD, and her colleagues from Johns Hopkins University, Baltimore, examined the prevalence of alcohol use in HCV-infected people who inject drugs (PWID). Their study examined the prevalence and associated correlates of alcohol use (Addictive Behaviors 2019;96:56-61).
They followed a large cohort of 1,623 HCV-antibody positive PWID from 2005 to 2013 from the AIDS Linked to the Intravenous Experience (ALIVE) study. They characterized alcohol use with the Alcohol Use Disorders Identification Test (AUDIT-C) questionnaire. Multivariable logistic regression with generalized estimated equations was used to examine sociodemographic, clinical, and substance use correlates of alcohol use.
At baseline, the median age was 47 years, 67% were men, 81% were black, and 34% were HIV positive. The majority (60%) reported injection drug use in the prior 6 months, while 46% reported noninjection cocaine or heroin, 31% reported street-acquired prescription drugs, and 22% reported marijuana use in the same time period. According to the AUDIT-C results, 41% of the patients reported no alcohol use, 21% reported moderate alcohol use, and 38% reported heavy alcohol use at their baseline visit.
The factors that were significantly associated with heavy alcohol use included male sex, black race, income of $5,000 or less, a Center for Epidemiologic Studies Depression Scale (range 0-60) score of 23 or greater, being homeless, being incarcerated, marijuana use, use of street-acquired prescription drugs, noninjection cocaine/heroin, injection drug use, and cigarette smoking. In a model that included the composite summary variable for substance use intensity, one drug type (adjusted odds ratio, 1.92), two drug types (AOR, 2.93), and three drug types (AOR, 3.65) were significantly associated with heavy alcohol use.
“While clinicians are undoubtedly concerned about any level of alcohol use in the setting of HCV infection due to the acceleration of liver fibrosis, there is particular concern for individuals with heavy alcohol use and their increased risk for cirrhosis and liver failure even after HCV cure. Without intervention, alcohol use will persist after HCV is cured with the potential to undermine the benefit of HCV cure. Therefore, our data point to the need to invest in and develop programs that effectively address alcohol use and co-occurring substance use in this population of PWID with HCV,” the researchers concluded.
The study was supported by the U.S. National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, and the National Institute on Alcohol Abuse and Alcoholism. The authors declared that they had no conflicts.
SOURCE: Irvin R et al. Addictive Behaviors. 2019;96:56-61.
Curing hepatitis C virus (HCV) infection without addressing the high rate of alcohol use disorder in many patients may undermine the benefits of treatment to long-term liver health, according to the results of a large cohort study.
Because excess alcohol use is known to accelerate liver disease progression, researchers Risha Irvin, MD, and her colleagues from Johns Hopkins University, Baltimore, examined the prevalence of alcohol use in HCV-infected people who inject drugs (PWID). Their study examined the prevalence and associated correlates of alcohol use (Addictive Behaviors 2019;96:56-61).
They followed a large cohort of 1,623 HCV-antibody positive PWID from 2005 to 2013 from the AIDS Linked to the Intravenous Experience (ALIVE) study. They characterized alcohol use with the Alcohol Use Disorders Identification Test (AUDIT-C) questionnaire. Multivariable logistic regression with generalized estimated equations was used to examine sociodemographic, clinical, and substance use correlates of alcohol use.
At baseline, the median age was 47 years, 67% were men, 81% were black, and 34% were HIV positive. The majority (60%) reported injection drug use in the prior 6 months, while 46% reported noninjection cocaine or heroin, 31% reported street-acquired prescription drugs, and 22% reported marijuana use in the same time period. According to the AUDIT-C results, 41% of the patients reported no alcohol use, 21% reported moderate alcohol use, and 38% reported heavy alcohol use at their baseline visit.
The factors that were significantly associated with heavy alcohol use included male sex, black race, income of $5,000 or less, a Center for Epidemiologic Studies Depression Scale (range 0-60) score of 23 or greater, being homeless, being incarcerated, marijuana use, use of street-acquired prescription drugs, noninjection cocaine/heroin, injection drug use, and cigarette smoking. In a model that included the composite summary variable for substance use intensity, one drug type (adjusted odds ratio, 1.92), two drug types (AOR, 2.93), and three drug types (AOR, 3.65) were significantly associated with heavy alcohol use.
“While clinicians are undoubtedly concerned about any level of alcohol use in the setting of HCV infection due to the acceleration of liver fibrosis, there is particular concern for individuals with heavy alcohol use and their increased risk for cirrhosis and liver failure even after HCV cure. Without intervention, alcohol use will persist after HCV is cured with the potential to undermine the benefit of HCV cure. Therefore, our data point to the need to invest in and develop programs that effectively address alcohol use and co-occurring substance use in this population of PWID with HCV,” the researchers concluded.
The study was supported by the U.S. National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, and the National Institute on Alcohol Abuse and Alcoholism. The authors declared that they had no conflicts.
SOURCE: Irvin R et al. Addictive Behaviors. 2019;96:56-61.
FROM ADDICTIVE BEHAVIORS
Too many blood cultures ordered for pediatric SSTIs
SEATTLE – Blood cultures were ordered for over half of pediatric skin infection encounters across 38 children’s hospitals, with rates varying from about 20% to 80% between hospitals, according to a review of almost 50,000 encounters in the Pediatric Health Information System database.
It was a surprising finding, because current guidelines from the Infectious Diseases Society of America do not recommend blood cultures as part of the routine evaluation of uncomplicated pediatric skin and soft-tissue infections (SSTIs), meaning infections in children who are otherwise healthy without neutropenia or other complicating factors.
Just 0.6% of the cultures were positive in the review, and it’s likely some of those were caused by contamination. After adjustment for demographics, complex chronic conditions, and severity of illness, culture draws were associated with a 20% increase in hospital length of stay (LOS), hospital costs, and 30-day readmission rates.
“Our data provide more evidence that [routine] blood cultures for children with SSTI represents low-value practice and should be avoided,” said lead investigator John Stephens, MD, a pediatrics professor and hospitalist at the University of North Carolina at Chapel Hill.
Dr. Stephens became curious about how common the practice was across hospitals after he and a friend penned an article about the issue for the Journal of Hospital Medicine’s “Things We Do for No Reason” series. The single-center studies they reviewed showed similarly high rates of both testing and negative cultures (J Hosp Med. 2018 Jul;13[7]:496-9).
Dr. Stephens and his team queried the Pediatric Health Information System database for encounters in children aged 2 months to 18 years with the diagnostic code 383, “cellulitis and other skin infections,” from 2012 to 2017, during which time “there really wasn’t a change” in IDSA guidance, he noted. Transfers, encounters with ICU care, and immunocompromised children were excluded.
Hospital admissions were included in the review if they had an additional code for erysipelas, cellulitis, impetigo, or other localized skin infection. The rate of positive cultures was inferred from subsequent codes for bacteremia or septicemia.
Across 49,291 encounters, the median rate of blood culture for skin infection was 51.6%, with tremendous variation between hospitals. With blood cultures, the hospital LOS was about 1.9 days, the hospital cost was $4,030, and the 30-day readmission rate was 1.3%. Without cultures, LOS was 1.6 days, the cost was $3,291, and the readmission rate was 1%.
Although infrequent, it’s likely that positive cultures triggered additional work-up, time in the hospital, and other measures, which might help account for the increase in LOS and costs.
As for why blood testing was so common, especially in some hospitals, “I think it’s just institutional culture. No amount of clinical variation in patient population could explain” a 20%-80% “variation across hospitals. It’s really just ingrained habits,” Dr. Stephens said at Pediatric Hospital Medicine.
“The rate of positive blood culture was really low, and the association was for higher cost and utilization. I think this really reinforces the IDSA guidelines. We need to focus on quality improvement efforts to do this better,” he said, noting that he hopes to do so at his own institution.
“I’d also like to know more on the positives. In the single center studies, we know more than half of them are contaminants. Often, there’s more contamination than true positives,” he said at the meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Instead of routine blood culture, Dr. Stephens recommended in his article to send pus for a Gram stain and culture and sensitivity, while noting that blood cultures remain reasonable for complicated infections, immunocompromised patients, and neonates.
There was no external funding, and Dr. Stephens didn’t report any disclosures.
SEATTLE – Blood cultures were ordered for over half of pediatric skin infection encounters across 38 children’s hospitals, with rates varying from about 20% to 80% between hospitals, according to a review of almost 50,000 encounters in the Pediatric Health Information System database.
It was a surprising finding, because current guidelines from the Infectious Diseases Society of America do not recommend blood cultures as part of the routine evaluation of uncomplicated pediatric skin and soft-tissue infections (SSTIs), meaning infections in children who are otherwise healthy without neutropenia or other complicating factors.
Just 0.6% of the cultures were positive in the review, and it’s likely some of those were caused by contamination. After adjustment for demographics, complex chronic conditions, and severity of illness, culture draws were associated with a 20% increase in hospital length of stay (LOS), hospital costs, and 30-day readmission rates.
“Our data provide more evidence that [routine] blood cultures for children with SSTI represents low-value practice and should be avoided,” said lead investigator John Stephens, MD, a pediatrics professor and hospitalist at the University of North Carolina at Chapel Hill.
Dr. Stephens became curious about how common the practice was across hospitals after he and a friend penned an article about the issue for the Journal of Hospital Medicine’s “Things We Do for No Reason” series. The single-center studies they reviewed showed similarly high rates of both testing and negative cultures (J Hosp Med. 2018 Jul;13[7]:496-9).
Dr. Stephens and his team queried the Pediatric Health Information System database for encounters in children aged 2 months to 18 years with the diagnostic code 383, “cellulitis and other skin infections,” from 2012 to 2017, during which time “there really wasn’t a change” in IDSA guidance, he noted. Transfers, encounters with ICU care, and immunocompromised children were excluded.
Hospital admissions were included in the review if they had an additional code for erysipelas, cellulitis, impetigo, or other localized skin infection. The rate of positive cultures was inferred from subsequent codes for bacteremia or septicemia.
Across 49,291 encounters, the median rate of blood culture for skin infection was 51.6%, with tremendous variation between hospitals. With blood cultures, the hospital LOS was about 1.9 days, the hospital cost was $4,030, and the 30-day readmission rate was 1.3%. Without cultures, LOS was 1.6 days, the cost was $3,291, and the readmission rate was 1%.
Although infrequent, it’s likely that positive cultures triggered additional work-up, time in the hospital, and other measures, which might help account for the increase in LOS and costs.
As for why blood testing was so common, especially in some hospitals, “I think it’s just institutional culture. No amount of clinical variation in patient population could explain” a 20%-80% “variation across hospitals. It’s really just ingrained habits,” Dr. Stephens said at Pediatric Hospital Medicine.
“The rate of positive blood culture was really low, and the association was for higher cost and utilization. I think this really reinforces the IDSA guidelines. We need to focus on quality improvement efforts to do this better,” he said, noting that he hopes to do so at his own institution.
“I’d also like to know more on the positives. In the single center studies, we know more than half of them are contaminants. Often, there’s more contamination than true positives,” he said at the meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Instead of routine blood culture, Dr. Stephens recommended in his article to send pus for a Gram stain and culture and sensitivity, while noting that blood cultures remain reasonable for complicated infections, immunocompromised patients, and neonates.
There was no external funding, and Dr. Stephens didn’t report any disclosures.
SEATTLE – Blood cultures were ordered for over half of pediatric skin infection encounters across 38 children’s hospitals, with rates varying from about 20% to 80% between hospitals, according to a review of almost 50,000 encounters in the Pediatric Health Information System database.
It was a surprising finding, because current guidelines from the Infectious Diseases Society of America do not recommend blood cultures as part of the routine evaluation of uncomplicated pediatric skin and soft-tissue infections (SSTIs), meaning infections in children who are otherwise healthy without neutropenia or other complicating factors.
Just 0.6% of the cultures were positive in the review, and it’s likely some of those were caused by contamination. After adjustment for demographics, complex chronic conditions, and severity of illness, culture draws were associated with a 20% increase in hospital length of stay (LOS), hospital costs, and 30-day readmission rates.
“Our data provide more evidence that [routine] blood cultures for children with SSTI represents low-value practice and should be avoided,” said lead investigator John Stephens, MD, a pediatrics professor and hospitalist at the University of North Carolina at Chapel Hill.
Dr. Stephens became curious about how common the practice was across hospitals after he and a friend penned an article about the issue for the Journal of Hospital Medicine’s “Things We Do for No Reason” series. The single-center studies they reviewed showed similarly high rates of both testing and negative cultures (J Hosp Med. 2018 Jul;13[7]:496-9).
Dr. Stephens and his team queried the Pediatric Health Information System database for encounters in children aged 2 months to 18 years with the diagnostic code 383, “cellulitis and other skin infections,” from 2012 to 2017, during which time “there really wasn’t a change” in IDSA guidance, he noted. Transfers, encounters with ICU care, and immunocompromised children were excluded.
Hospital admissions were included in the review if they had an additional code for erysipelas, cellulitis, impetigo, or other localized skin infection. The rate of positive cultures was inferred from subsequent codes for bacteremia or septicemia.
Across 49,291 encounters, the median rate of blood culture for skin infection was 51.6%, with tremendous variation between hospitals. With blood cultures, the hospital LOS was about 1.9 days, the hospital cost was $4,030, and the 30-day readmission rate was 1.3%. Without cultures, LOS was 1.6 days, the cost was $3,291, and the readmission rate was 1%.
Although infrequent, it’s likely that positive cultures triggered additional work-up, time in the hospital, and other measures, which might help account for the increase in LOS and costs.
As for why blood testing was so common, especially in some hospitals, “I think it’s just institutional culture. No amount of clinical variation in patient population could explain” a 20%-80% “variation across hospitals. It’s really just ingrained habits,” Dr. Stephens said at Pediatric Hospital Medicine.
“The rate of positive blood culture was really low, and the association was for higher cost and utilization. I think this really reinforces the IDSA guidelines. We need to focus on quality improvement efforts to do this better,” he said, noting that he hopes to do so at his own institution.
“I’d also like to know more on the positives. In the single center studies, we know more than half of them are contaminants. Often, there’s more contamination than true positives,” he said at the meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Instead of routine blood culture, Dr. Stephens recommended in his article to send pus for a Gram stain and culture and sensitivity, while noting that blood cultures remain reasonable for complicated infections, immunocompromised patients, and neonates.
There was no external funding, and Dr. Stephens didn’t report any disclosures.
REPORTING FROM PHM 2019
DRC Ebola epidemic continues unabated despite international response
, “currently the outbreak continues at the same pace, so we don’t see evidence of slowing,” according to Henry Walke, MD, director of the Division of Preparedness and Emerging Infections and Incident Manager, 2018 CDC Ebola Response, Centers for Disease Control and Prevention.
He added that new cases of Ebola have been seen in Goma, which is outside the initial outbreak area. Goma is the largest city in the eastern part of the DRC and a major trading port.
Dr. Walke made his remarks in a telephone media briefing Aug. 1 by the U. S. Department of Health and Human Services outlining the current state of the U.S. response to the outbreak.
He described the efforts of the CDC to provide support to the DRC both from Atlanta and in the field. These efforts included support for vaccination activities in DRC’s North Kivu and Ituri provinces for the population and for at-risk health-care workers in the DRC and neighboring countries. In addition, the United States is involved in the testing of experimental therapeutics and vaccines in the DRC in an effort to aid in this and future outbreaks.
“There are no cases of Ebola in the United States,” said Dr. Walke, and the CDC believes the risk to the United States from the outbreak is low. He cited the limited number of travelers from DRC. “There [are] about 16,000 from the DRC to the U.S. on an annual basis, and only about 100 from Goma itself. There aren’t direct flights and we have at the Goma airport both entry and exit screening.”
According to a World Health Organization report, this Ebola outbreak is the second deadliest on record and has killed 1,750 people out of around 2,518 confirmed cases as of July 23.
Efforts to control the epidemic are severely hampered by civil unrest in the area, public mistrust of the government and health care workers, and a comparative lack of international aid compared to previous Ebola outbreaks.
, “currently the outbreak continues at the same pace, so we don’t see evidence of slowing,” according to Henry Walke, MD, director of the Division of Preparedness and Emerging Infections and Incident Manager, 2018 CDC Ebola Response, Centers for Disease Control and Prevention.
He added that new cases of Ebola have been seen in Goma, which is outside the initial outbreak area. Goma is the largest city in the eastern part of the DRC and a major trading port.
Dr. Walke made his remarks in a telephone media briefing Aug. 1 by the U. S. Department of Health and Human Services outlining the current state of the U.S. response to the outbreak.
He described the efforts of the CDC to provide support to the DRC both from Atlanta and in the field. These efforts included support for vaccination activities in DRC’s North Kivu and Ituri provinces for the population and for at-risk health-care workers in the DRC and neighboring countries. In addition, the United States is involved in the testing of experimental therapeutics and vaccines in the DRC in an effort to aid in this and future outbreaks.
“There are no cases of Ebola in the United States,” said Dr. Walke, and the CDC believes the risk to the United States from the outbreak is low. He cited the limited number of travelers from DRC. “There [are] about 16,000 from the DRC to the U.S. on an annual basis, and only about 100 from Goma itself. There aren’t direct flights and we have at the Goma airport both entry and exit screening.”
According to a World Health Organization report, this Ebola outbreak is the second deadliest on record and has killed 1,750 people out of around 2,518 confirmed cases as of July 23.
Efforts to control the epidemic are severely hampered by civil unrest in the area, public mistrust of the government and health care workers, and a comparative lack of international aid compared to previous Ebola outbreaks.
, “currently the outbreak continues at the same pace, so we don’t see evidence of slowing,” according to Henry Walke, MD, director of the Division of Preparedness and Emerging Infections and Incident Manager, 2018 CDC Ebola Response, Centers for Disease Control and Prevention.
He added that new cases of Ebola have been seen in Goma, which is outside the initial outbreak area. Goma is the largest city in the eastern part of the DRC and a major trading port.
Dr. Walke made his remarks in a telephone media briefing Aug. 1 by the U. S. Department of Health and Human Services outlining the current state of the U.S. response to the outbreak.
He described the efforts of the CDC to provide support to the DRC both from Atlanta and in the field. These efforts included support for vaccination activities in DRC’s North Kivu and Ituri provinces for the population and for at-risk health-care workers in the DRC and neighboring countries. In addition, the United States is involved in the testing of experimental therapeutics and vaccines in the DRC in an effort to aid in this and future outbreaks.
“There are no cases of Ebola in the United States,” said Dr. Walke, and the CDC believes the risk to the United States from the outbreak is low. He cited the limited number of travelers from DRC. “There [are] about 16,000 from the DRC to the U.S. on an annual basis, and only about 100 from Goma itself. There aren’t direct flights and we have at the Goma airport both entry and exit screening.”
According to a World Health Organization report, this Ebola outbreak is the second deadliest on record and has killed 1,750 people out of around 2,518 confirmed cases as of July 23.
Efforts to control the epidemic are severely hampered by civil unrest in the area, public mistrust of the government and health care workers, and a comparative lack of international aid compared to previous Ebola outbreaks.
REPORTING FROM A MEDIA BRIEFING BY HHS
Decreasing Treatment of Asymptomatic Bacteriuria: An Interprofessional Approach to Antibiotic Stewardship
From the Mayo Clinic, Rochester, MN.
Abstract
- Objective: Asymptomatic bacteriuria (ASB) denotes asymptomatic carriage of bacteria within the urinary tract and does not require treatment in most patient populations. Unnecessary antimicrobial treatment has several consequences, including promotion of antimicrobial resistance, potential for medication adverse effects, and risk for Clostridiodes difficile infection. The aim of this quality improvement effort was to decrease both the unnecessary ordering of urine culture studies and unnecessary treatment of ASB.
- Methods: This is a single-center study of patients who received care on 3 internal medicine units at a large, academic medical center. We sought to determine the impact of information technology and educational interventions to decrease both inappropriate urine culture ordering and treatment of ASB. Data from included patients were collected over 3 1-month time periods: baseline, post-information technology intervention, and post-educational intervention.
- Results: There was a reduction in the percentage of patients who received antibiotics for ASB in the post-education intervention period as compared to baseline (35% vs 42%). The proportion of total urine cultures ordered by internal medicine clinicians did not change after an information technology intervention to redesign the computerized physician order entry screen for urine cultures.
- Conclusion: Educational interventions are effective ways to reduce rates of inappropriate treatment of ASB in patients admitted to internal medicine services.
Keywords: asymptomatic bacteriuria, UTI, information technology, education, quality.
Asymptomatic bacteriuria (ASB) is a common condition in which bacteria are recovered from a urine culture (UC) in patients without symptoms suggestive of urinary tract infection (UTI), with no pathologic consequences to most patients who are not treated.1,2 Patients with ASB do not exhibit symptoms of a UTI such as dysuria, increased frequency of urination, increased urgency, suprapubic tenderness, or costovertebral pain. Treatment with antibiotics is not indicated for most patients with ASB.1,3 According to the Infectious Diseases Society of America (IDSA), screening for bacteriuria and treatment for positive results is only indicated during pregnancy and prior to urologic procedures with anticipated breach of the mucosal lining.1
An estimated 20% to 52% of patients in hospital settings receive inappropriate treatment with antibiotics for ASB.4 Unnecessary prescribing of antibiotics has several negative consequences, including increased rates of antibiotic resistance, Clostridioides difficile infection, and medication adverse events, as well as increased health care costs.2,5 Antimicrobial stewardship programs to improve judicious use of antimicrobials are paramount to reducing these consequences, and their importance is heightened with recent requirements for antimicrobial stewardship put forth by The Joint Commission and the Centers for Medicare & Medicaid Services.6,7
A previous review of UC and antimicrobial use in patients for purposes of quality improvement at our institution over a 2-month period showed that of 59 patients with positive UCs, 47 patients (80%) did not have documented symptoms of a UTI. Of these 47 patients with ASB, 29 (61.7%) received antimicrobial treatment unnecessarily (unpublished data). We convened a group of clinicians and nonclinicians representing the areas of infectious disease, pharmacy, microbiology, statistics, and hospital internal medicine (IM) to examine the unnecessary treatment of ASB in our institution. Our objective was to address 2 antimicrobial stewardship issues: inappropriate UC ordering and unnecessary use of antibiotics to treat ASB. Our aim was to reduce the inappropriate ordering of UCs and to reduce treatment of ASB.
Methods
Setting
The study was conducted on 3 IM nursing units with a total of 83 beds at a large tertiary care academic medical center in the midwestern United States, and was approved by the organization’s Institutional Review Board.
Participants
We included all non-pregnant patients aged 18 years or older who received care from an IM primary service. These patients were admitted directly to an IM team through the emergency department (ED) or transferred to an IM team after an initial stay in the intensive care unit.
Data Source
Microbiology laboratory reports generated from the electronic health record were used to identify all patients with a collected UC sample who received care from an IM service prior to discharge. Urine samples were collected by midstream catch or catheterization. Data on urine Gram stain and urine dipstick were not included. Henceforth, the phrase “urine culture order” indicates that a UC was both ordered and performed. Data reports were generated for the month of August 2016 to determine the baseline number of UCs ordered. Charts of patients with positive UCs were reviewed to determine if antibiotics were started for the positive UC and whether the patient had signs or symptoms consistent with a UTI. If antibiotics were started in the absence of signs or symptoms to support a UTI, the patient was determined to have been unnecessarily treated for ASB. Reports were then generated for the month after each intervention was implemented, with the same chart review undertaken for positive UCs. Bacteriuria was defined in our study as the presence of microbial growth greater than 10,000 CFU/mL in UC.
Interventions
Initial analysis by our study group determined that lack of electronic clinical decision support (CDS) at the point of care and provider knowledge gaps in interpreting positive UCs were the 2 main contributors to unnecessary UC orders and unnecessary treatment of positive UCs, respectively. We reviewed the work of other groups who reported interventions to decrease treatment of ASB, ranging from educational presentations to pocket cards and treatment algorithms.8-13 We hypothesized that there would be a decrease in UC orders with CDS embedded in the computerized order entry screen, and that we would decrease unnecessary treatment of positive UCs by educating clinicians on indications for appropriate antibiotic prescribing in the setting of a positive UC.
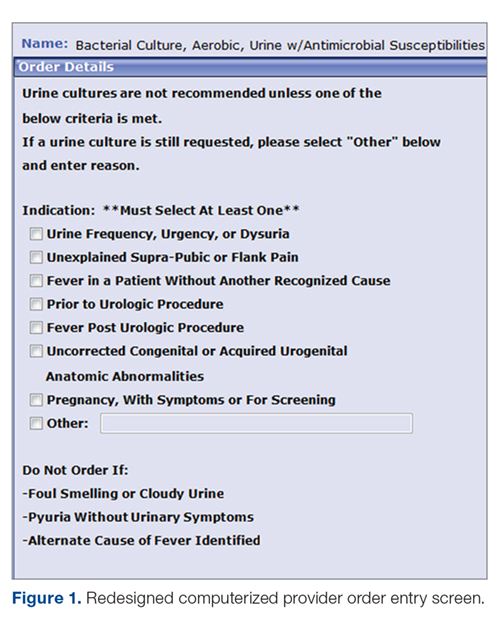
Information technology intervention. The first intervention implemented involved redesign of the UC ordering screen in the computerized physician order entry (CPOE) system. This intervention went live hospital-wide, including the IM floors, intensive care units, and all other areas except the ED, on February 1, 2017 (Figure 1). The ordering screen required the prescriber to select from a list of appropriate indications for ordering a UC, including urine frequency, urgency, or dysuria; unexplained suprapubic or flank pain; fever in patients without another recognized cause; screening obtained prior to urologic procedure; or screening during pregnancy. An additional message advised prescribers to avoid ordering the culture if the patient had malodorous or cloudy urine, pyuria without urinary symptoms, or had an alternative cause of fever. Before we implemented the information technology (IT) intervention, there had been no specific point-of-care guidance on UC ordering.
Educational intervention. The second intervention, driven by clinical pharmacists, involved active and passive education of prescribers specifically designed to address unnecessary treatment of ASB. The IT intervention with CDS for UC ordering remained live. Presentations designed by the study group summarizing the appropriate indications for ordering a UC, distinguishing ASB from UTI, and discouraging treatment of ASB were delivered via a variety of routes by clinical pharmacists to nurses, nurse practitioners, physician assistants, pharmacists, medical residents, and staff physicians providing care to patients on the 3 IM units over a 1-month period in March 2017. The presentations contained the same basic content, but the information was delivered to target each specific audience group.
Medical residents received a 10-minute live presentation during a conference. Nurse practitioners, physician assistants, and staff physicians received a presentation via email, and highlights of the presentation were delivered by clinical pharmacists at their respective monthly group meetings. A handout was presented to nursing staff at nursing huddles, and presentation slides were distributed by email. Educational posters were posted in the medical resident workrooms, nursing breakrooms, and staff bathrooms on the units.
Outcome Measurements
The endpoints of interest were the percentage of patients with positive UCs unnecessarily treated for ASB before and after each intervention and the number of UCs ordered at baseline and after implementation of each intervention. Counterbalance measures assessed included the incidence of UTI, pyelonephritis, or urosepsis within 7 days of positive UC for patients who did not receive antibiotic treatment for ASB.
Results
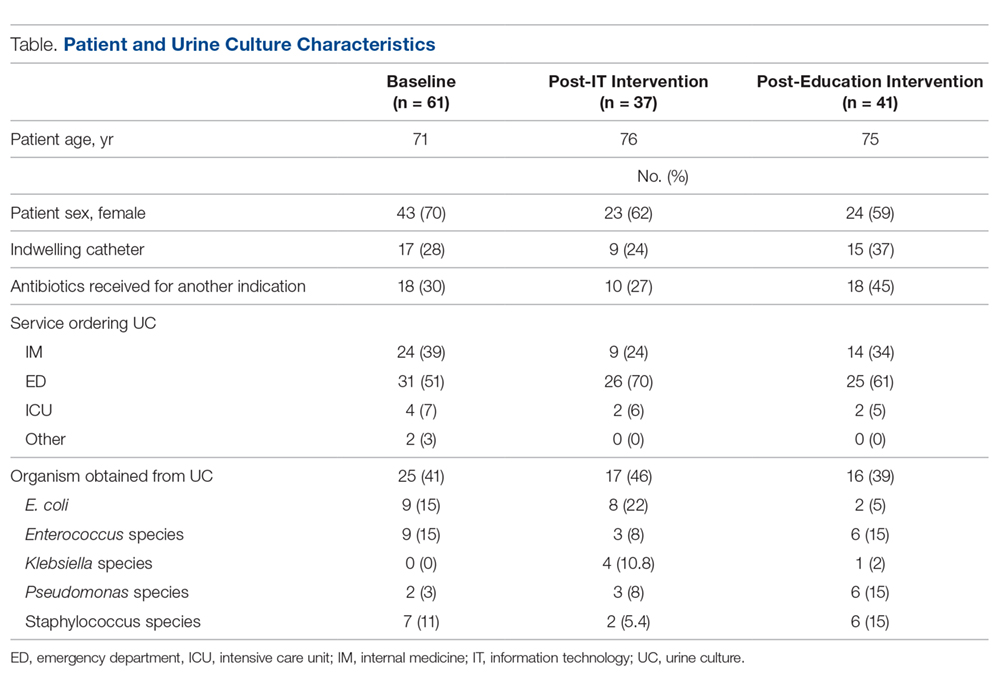
Data from a total of 270 cultures were examined from IM nursing units. A total of 117 UCs were ordered during the baseline period before interventions were implemented. For a period of 1 month following activation of the IT intervention, 73 UCs were ordered. For a period of 1 month following the educational interventions, 80 UCs were ordered. Of these, 61 (52%) UCs were positive at baseline, 37 (51%) after the IT intervention, and 41 (51%) after the educational intervention. Patient characteristics were similar between the 3 groups (Table); 64.7% of patients were female in their early to mid-seventies. The majority of UCs were ordered by providers in the ED in all 3 periods examined (51%-70%). The percentage of patients who received antibiotics prior to UC for another indication (including bacteriuria) in the baseline, post-IT intervention, and post-education intervention groups were 30%, 27%, and 45%, respectively.
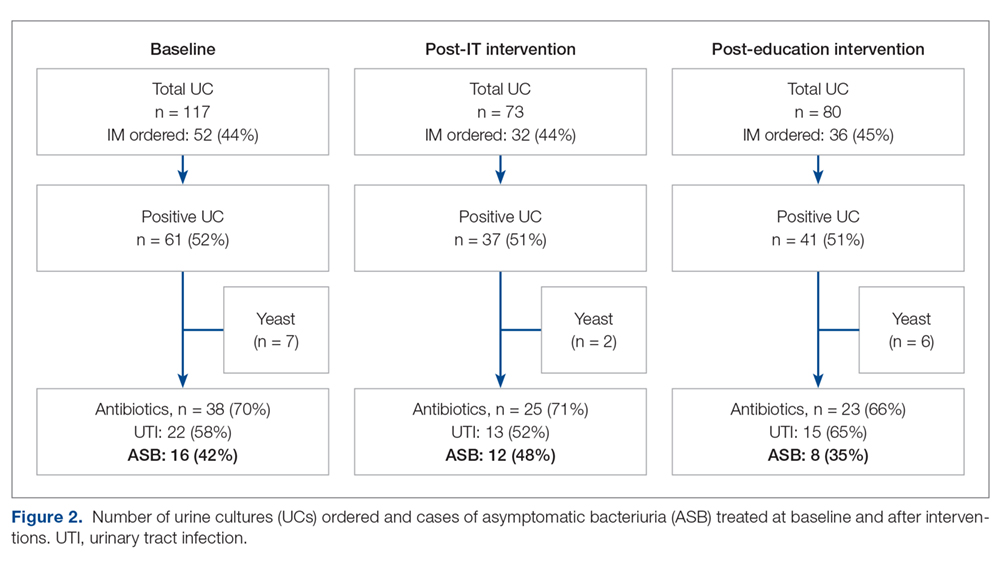
The study outcomes are summarized in Figure 2. Among patients with positive cultures, there was not a reduction in inappropriate treatment of ASB compared to baseline after the IT intervention (48% vs 42%). Following the education intervention, there was a reduction in unnecessary ASB treatment as compared both to baseline (35% vs 42%) and to post-IT intervention (35% vs 48%). There was no difference between the 3 study periods in the percentage of total UCs ordered by IM clinicians. The counterbalance measure showed that 1 patient who did not receive antibiotics within 7 days of a positive UC developed pyelonephritis, UTI, or sepsis due to a UTI in each intervention group.
Discussion
The results of this study demonstrate the role of multimodal interventions in antimicrobial stewardship and add to the growing body of evidence supporting the work of antimicrobial stewardship programs. Our multidisciplinary study group and multipronged intervention follow recent guideline recommendations for antimicrobial stewardship program interventions against unnecessary treatment of ASB.14 Initial analysis by our study group determined lack of CDS at the point of care and provider knowledge gaps in interpreting positive UCs as the 2 main contributors to unnecessary UC orders and unnecessary treatment of positive UCs in our local practice culture. The IT component of our intervention was intended to provide CDS for ordering UCs, and the education component focused on informing clinicians’ treatment decisions for positive UCs.
It has been suggested that the type of stewardship intervention that is most effective fits the specific needs and resources of an institution.14,15 And although the IDSA does not recommend education as a stand-alone intervention,16 we found it to be an effective intervention for our clinicians in our work environment. However, since the CPOE guidance was in place during the educational study periods, it is possible that the effect was due to a combination of these 2 approaches. Our pre-intervention ASB treatment rates were consistent with a recent meta-analysis in which the rate of inappropriate treatment of ASB was 45%.17 This meta-analysis found educational and organizational interventions led to a mean absolute risk reduction of 33%. After the education intervention, we saw a 7% decrease in unnecessary treatment of ASB compared to baseline, and a 13% decrease compared to the month just prior to the educational intervention.
Lessons learned from our work included how clear review of local processes can inform quality improvement interventions. For instance, we initially hypothesized that IM clinicians would benefit from point-of-care CDS guidance, but such guidance used alone without educational interventions was not supported by the results. We also determined that the majority of UCs from patients on general medicine units were ordered by ED providers. This revealed an opportunity to implement similar interventions in the ED, as this was the initial point of contact for many of these patients.
As with any clinical intervention, the anticipated benefits should be weighed against potential harm. Using counterbalance measures, we found there was minimal risk in the occurrence of UTI, pyelonephritis, or sepsis if clinicians avoided treating ASB. This finding is consistent with IDSA guideline recommendations and other studies that suggest that withholding treatment for asymptomatic bacteriuria does not lead to worse outcomes.1
This study has several limitations. Data were obtained through review of the electronic health record and therefore documentation may be incomplete. Also, antimicrobials for empiric coverage or treatment for other infections (eg, pneumonia, sepsis) may have confounded our results, as empirical antimicrobials were given to 27% to 45% of patients prior to UC. This was a quality improvement project carried out over defined time intervals, and thus our sample size was limited and not adequately powered to show statistical significance. Additionally, given the bundling of interventions, it is difficult to determine the impact of each intervention independently. Although CDS for UC ordering may not have influenced ordering, it is possible that the IT intervention raised awareness of ASB and influenced treatment practices.
Conclusion
Our work supports the principles of antibiotic stewardship as brought forth by IDSA.16 This work was the effort of a multidisciplinary team, which aligns with recommendations by Daniel and colleagues, published after our study had ended, for reducing overtreatment of ASB.14 Additionally, our study results provided valuable information for our institution. Although improvements in management of ASB were modest, the success of provider education and identification of other work areas and clinicians to target for future intervention were helpful in consideration of further studies. This work will also aid us in developing an expected effect size for future studies. We plan to provide ongoing education for IM providers as well as education in the ED to target providers who make first contact with patients admitted to inpatient services. In addition, the CPOE UC ordering screen message will continue to be used hospital-wide and will be expanded to the ED ordering system. Our interventions, experiences, and challenges may be used by other institutions to design effective antimicrobial stewardship interventions directed towards reducing rates of inappropriate ASB treatment.
Corresponding author: Prasanna P. Narayanan, PharmD, 200 First Street SW, Rochester, MN 55905; [email protected].
Financial disclosures: None.
1. Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68:e83–75.
2. Trautner BW, Grigoryan L, Petersen NJ, et al. Effectiveness of an antimicrobial stewardship approach for urinary catheter-associated asymptomatic bacteriuria. JAMA Intern Med. 2015;175:1120-1127.
3. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309-332.
4. Trautner BW. Asymptomatic bacteriuria: when the treatment is worse than the disease. Nat Rev Urol. 2011;9:85-93.
5. Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096.
6. The Joint Commission. Prepublication Requirements: New antimicrobial stewardship standard. Jun 22, 2016. www.jointcommission.org/assets/1/6/HAP-CAH_Antimicrobial_Prepub.pdf. Accessed January 24, 2019.
7. Federal Register. Medicare and Medicaid Programs; Hospital and Critical Access Hospital (CAH) Changes to Promote Innovation, Flexibility, and Improvement in Patient Care.Centers for Medicare & Medicaid Services. June 16, 2016. CMS-3295-P
8. Hartley SE, Kuhn L, Valley S, et al. Evaluating a hospitalist-based intervention to decrease unnecessary antimicrobial use in patients with asymptomatic bacteriuria. Infect Control Hosp Epidemiol. 2016;37:1044-1051.
9. Pavese P, Saurel N, Labarere J, et al. Does an educational session with an infectious diseases physician reduce the use of inappropriate antibiotic therapy for inpatients with positive urine culture results? A controlled before-and-after study. Infect Control Hosp Epidemiol. 2009;30:596-599.
10. Kelley D, Aaronson P, Poon E, et al. Evaluation of an antimicrobial stewardship approach to minimize overuse of antibiotics in patients with asymptomatic bacteriuria. Infect Control Hosp Epidemiol. 2014;35:193-195.
11. Chowdhury F, Sarkar K, Branche A, et al. Preventing the inappropriate treatment of asymptomatic bacteriuria at a community teaching hospital. J Community Hosp Intern Med Perspect. 2012;2.
12. Bonnal C, Baune B, Mion M, et al. Bacteriuria in a geriatric hospital: impact of an antibiotic improvement program. J Am Med Dir Assoc. 2008;9:605-609.
13. Linares LA, Thornton DJ, Strymish J, et al. Electronic memorandum decreases unnecessary antimicrobial use for asymptomatic bacteriuria and culture-negative pyuria. Infect Control Hosp Epidemiol. 2011;32:644-648.
14. Daniel M, Keller S, Mozafarihashjin M, et al. An implementation guide to reducing overtreatment of asymptomatic bacteriuria. JAMA Intern Med. 2018;178:271-276.
15. Redwood R, Knobloch MJ, Pellegrini DC, et al. Reducing unnecessary culturing: a systems approach to evaluating urine culture ordering and collection practices among nurses in two acute care settings. Antimicrob Resist Infect Control. 2018;7:4.
16. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:e51–e7.
17. Flokas ME, Andreatos N, Alevizakos M, et al. Inappropriate management of asymptomatic patients with positive urine cultures: a systematic review and meta-analysis. Open Forum Infect Dis. 2017;4:1-10.
From the Mayo Clinic, Rochester, MN.
Abstract
- Objective: Asymptomatic bacteriuria (ASB) denotes asymptomatic carriage of bacteria within the urinary tract and does not require treatment in most patient populations. Unnecessary antimicrobial treatment has several consequences, including promotion of antimicrobial resistance, potential for medication adverse effects, and risk for Clostridiodes difficile infection. The aim of this quality improvement effort was to decrease both the unnecessary ordering of urine culture studies and unnecessary treatment of ASB.
- Methods: This is a single-center study of patients who received care on 3 internal medicine units at a large, academic medical center. We sought to determine the impact of information technology and educational interventions to decrease both inappropriate urine culture ordering and treatment of ASB. Data from included patients were collected over 3 1-month time periods: baseline, post-information technology intervention, and post-educational intervention.
- Results: There was a reduction in the percentage of patients who received antibiotics for ASB in the post-education intervention period as compared to baseline (35% vs 42%). The proportion of total urine cultures ordered by internal medicine clinicians did not change after an information technology intervention to redesign the computerized physician order entry screen for urine cultures.
- Conclusion: Educational interventions are effective ways to reduce rates of inappropriate treatment of ASB in patients admitted to internal medicine services.
Keywords: asymptomatic bacteriuria, UTI, information technology, education, quality.
Asymptomatic bacteriuria (ASB) is a common condition in which bacteria are recovered from a urine culture (UC) in patients without symptoms suggestive of urinary tract infection (UTI), with no pathologic consequences to most patients who are not treated.1,2 Patients with ASB do not exhibit symptoms of a UTI such as dysuria, increased frequency of urination, increased urgency, suprapubic tenderness, or costovertebral pain. Treatment with antibiotics is not indicated for most patients with ASB.1,3 According to the Infectious Diseases Society of America (IDSA), screening for bacteriuria and treatment for positive results is only indicated during pregnancy and prior to urologic procedures with anticipated breach of the mucosal lining.1
An estimated 20% to 52% of patients in hospital settings receive inappropriate treatment with antibiotics for ASB.4 Unnecessary prescribing of antibiotics has several negative consequences, including increased rates of antibiotic resistance, Clostridioides difficile infection, and medication adverse events, as well as increased health care costs.2,5 Antimicrobial stewardship programs to improve judicious use of antimicrobials are paramount to reducing these consequences, and their importance is heightened with recent requirements for antimicrobial stewardship put forth by The Joint Commission and the Centers for Medicare & Medicaid Services.6,7
A previous review of UC and antimicrobial use in patients for purposes of quality improvement at our institution over a 2-month period showed that of 59 patients with positive UCs, 47 patients (80%) did not have documented symptoms of a UTI. Of these 47 patients with ASB, 29 (61.7%) received antimicrobial treatment unnecessarily (unpublished data). We convened a group of clinicians and nonclinicians representing the areas of infectious disease, pharmacy, microbiology, statistics, and hospital internal medicine (IM) to examine the unnecessary treatment of ASB in our institution. Our objective was to address 2 antimicrobial stewardship issues: inappropriate UC ordering and unnecessary use of antibiotics to treat ASB. Our aim was to reduce the inappropriate ordering of UCs and to reduce treatment of ASB.
Methods
Setting
The study was conducted on 3 IM nursing units with a total of 83 beds at a large tertiary care academic medical center in the midwestern United States, and was approved by the organization’s Institutional Review Board.
Participants
We included all non-pregnant patients aged 18 years or older who received care from an IM primary service. These patients were admitted directly to an IM team through the emergency department (ED) or transferred to an IM team after an initial stay in the intensive care unit.
Data Source
Microbiology laboratory reports generated from the electronic health record were used to identify all patients with a collected UC sample who received care from an IM service prior to discharge. Urine samples were collected by midstream catch or catheterization. Data on urine Gram stain and urine dipstick were not included. Henceforth, the phrase “urine culture order” indicates that a UC was both ordered and performed. Data reports were generated for the month of August 2016 to determine the baseline number of UCs ordered. Charts of patients with positive UCs were reviewed to determine if antibiotics were started for the positive UC and whether the patient had signs or symptoms consistent with a UTI. If antibiotics were started in the absence of signs or symptoms to support a UTI, the patient was determined to have been unnecessarily treated for ASB. Reports were then generated for the month after each intervention was implemented, with the same chart review undertaken for positive UCs. Bacteriuria was defined in our study as the presence of microbial growth greater than 10,000 CFU/mL in UC.
Interventions
Initial analysis by our study group determined that lack of electronic clinical decision support (CDS) at the point of care and provider knowledge gaps in interpreting positive UCs were the 2 main contributors to unnecessary UC orders and unnecessary treatment of positive UCs, respectively. We reviewed the work of other groups who reported interventions to decrease treatment of ASB, ranging from educational presentations to pocket cards and treatment algorithms.8-13 We hypothesized that there would be a decrease in UC orders with CDS embedded in the computerized order entry screen, and that we would decrease unnecessary treatment of positive UCs by educating clinicians on indications for appropriate antibiotic prescribing in the setting of a positive UC.
Information technology intervention. The first intervention implemented involved redesign of the UC ordering screen in the computerized physician order entry (CPOE) system. This intervention went live hospital-wide, including the IM floors, intensive care units, and all other areas except the ED, on February 1, 2017 (Figure 1). The ordering screen required the prescriber to select from a list of appropriate indications for ordering a UC, including urine frequency, urgency, or dysuria; unexplained suprapubic or flank pain; fever in patients without another recognized cause; screening obtained prior to urologic procedure; or screening during pregnancy. An additional message advised prescribers to avoid ordering the culture if the patient had malodorous or cloudy urine, pyuria without urinary symptoms, or had an alternative cause of fever. Before we implemented the information technology (IT) intervention, there had been no specific point-of-care guidance on UC ordering.

Educational intervention. The second intervention, driven by clinical pharmacists, involved active and passive education of prescribers specifically designed to address unnecessary treatment of ASB. The IT intervention with CDS for UC ordering remained live. Presentations designed by the study group summarizing the appropriate indications for ordering a UC, distinguishing ASB from UTI, and discouraging treatment of ASB were delivered via a variety of routes by clinical pharmacists to nurses, nurse practitioners, physician assistants, pharmacists, medical residents, and staff physicians providing care to patients on the 3 IM units over a 1-month period in March 2017. The presentations contained the same basic content, but the information was delivered to target each specific audience group.
Medical residents received a 10-minute live presentation during a conference. Nurse practitioners, physician assistants, and staff physicians received a presentation via email, and highlights of the presentation were delivered by clinical pharmacists at their respective monthly group meetings. A handout was presented to nursing staff at nursing huddles, and presentation slides were distributed by email. Educational posters were posted in the medical resident workrooms, nursing breakrooms, and staff bathrooms on the units.
Outcome Measurements
The endpoints of interest were the percentage of patients with positive UCs unnecessarily treated for ASB before and after each intervention and the number of UCs ordered at baseline and after implementation of each intervention. Counterbalance measures assessed included the incidence of UTI, pyelonephritis, or urosepsis within 7 days of positive UC for patients who did not receive antibiotic treatment for ASB.
Results
Data from a total of 270 cultures were examined from IM nursing units. A total of 117 UCs were ordered during the baseline period before interventions were implemented. For a period of 1 month following activation of the IT intervention, 73 UCs were ordered. For a period of 1 month following the educational interventions, 80 UCs were ordered. Of these, 61 (52%) UCs were positive at baseline, 37 (51%) after the IT intervention, and 41 (51%) after the educational intervention. Patient characteristics were similar between the 3 groups (Table); 64.7% of patients were female in their early to mid-seventies. The majority of UCs were ordered by providers in the ED in all 3 periods examined (51%-70%). The percentage of patients who received antibiotics prior to UC for another indication (including bacteriuria) in the baseline, post-IT intervention, and post-education intervention groups were 30%, 27%, and 45%, respectively.

The study outcomes are summarized in Figure 2. Among patients with positive cultures, there was not a reduction in inappropriate treatment of ASB compared to baseline after the IT intervention (48% vs 42%). Following the education intervention, there was a reduction in unnecessary ASB treatment as compared both to baseline (35% vs 42%) and to post-IT intervention (35% vs 48%). There was no difference between the 3 study periods in the percentage of total UCs ordered by IM clinicians. The counterbalance measure showed that 1 patient who did not receive antibiotics within 7 days of a positive UC developed pyelonephritis, UTI, or sepsis due to a UTI in each intervention group.

Discussion
The results of this study demonstrate the role of multimodal interventions in antimicrobial stewardship and add to the growing body of evidence supporting the work of antimicrobial stewardship programs. Our multidisciplinary study group and multipronged intervention follow recent guideline recommendations for antimicrobial stewardship program interventions against unnecessary treatment of ASB.14 Initial analysis by our study group determined lack of CDS at the point of care and provider knowledge gaps in interpreting positive UCs as the 2 main contributors to unnecessary UC orders and unnecessary treatment of positive UCs in our local practice culture. The IT component of our intervention was intended to provide CDS for ordering UCs, and the education component focused on informing clinicians’ treatment decisions for positive UCs.
It has been suggested that the type of stewardship intervention that is most effective fits the specific needs and resources of an institution.14,15 And although the IDSA does not recommend education as a stand-alone intervention,16 we found it to be an effective intervention for our clinicians in our work environment. However, since the CPOE guidance was in place during the educational study periods, it is possible that the effect was due to a combination of these 2 approaches. Our pre-intervention ASB treatment rates were consistent with a recent meta-analysis in which the rate of inappropriate treatment of ASB was 45%.17 This meta-analysis found educational and organizational interventions led to a mean absolute risk reduction of 33%. After the education intervention, we saw a 7% decrease in unnecessary treatment of ASB compared to baseline, and a 13% decrease compared to the month just prior to the educational intervention.
Lessons learned from our work included how clear review of local processes can inform quality improvement interventions. For instance, we initially hypothesized that IM clinicians would benefit from point-of-care CDS guidance, but such guidance used alone without educational interventions was not supported by the results. We also determined that the majority of UCs from patients on general medicine units were ordered by ED providers. This revealed an opportunity to implement similar interventions in the ED, as this was the initial point of contact for many of these patients.
As with any clinical intervention, the anticipated benefits should be weighed against potential harm. Using counterbalance measures, we found there was minimal risk in the occurrence of UTI, pyelonephritis, or sepsis if clinicians avoided treating ASB. This finding is consistent with IDSA guideline recommendations and other studies that suggest that withholding treatment for asymptomatic bacteriuria does not lead to worse outcomes.1
This study has several limitations. Data were obtained through review of the electronic health record and therefore documentation may be incomplete. Also, antimicrobials for empiric coverage or treatment for other infections (eg, pneumonia, sepsis) may have confounded our results, as empirical antimicrobials were given to 27% to 45% of patients prior to UC. This was a quality improvement project carried out over defined time intervals, and thus our sample size was limited and not adequately powered to show statistical significance. Additionally, given the bundling of interventions, it is difficult to determine the impact of each intervention independently. Although CDS for UC ordering may not have influenced ordering, it is possible that the IT intervention raised awareness of ASB and influenced treatment practices.
Conclusion
Our work supports the principles of antibiotic stewardship as brought forth by IDSA.16 This work was the effort of a multidisciplinary team, which aligns with recommendations by Daniel and colleagues, published after our study had ended, for reducing overtreatment of ASB.14 Additionally, our study results provided valuable information for our institution. Although improvements in management of ASB were modest, the success of provider education and identification of other work areas and clinicians to target for future intervention were helpful in consideration of further studies. This work will also aid us in developing an expected effect size for future studies. We plan to provide ongoing education for IM providers as well as education in the ED to target providers who make first contact with patients admitted to inpatient services. In addition, the CPOE UC ordering screen message will continue to be used hospital-wide and will be expanded to the ED ordering system. Our interventions, experiences, and challenges may be used by other institutions to design effective antimicrobial stewardship interventions directed towards reducing rates of inappropriate ASB treatment.
Corresponding author: Prasanna P. Narayanan, PharmD, 200 First Street SW, Rochester, MN 55905; [email protected].
Financial disclosures: None.
From the Mayo Clinic, Rochester, MN.
Abstract
- Objective: Asymptomatic bacteriuria (ASB) denotes asymptomatic carriage of bacteria within the urinary tract and does not require treatment in most patient populations. Unnecessary antimicrobial treatment has several consequences, including promotion of antimicrobial resistance, potential for medication adverse effects, and risk for Clostridiodes difficile infection. The aim of this quality improvement effort was to decrease both the unnecessary ordering of urine culture studies and unnecessary treatment of ASB.
- Methods: This is a single-center study of patients who received care on 3 internal medicine units at a large, academic medical center. We sought to determine the impact of information technology and educational interventions to decrease both inappropriate urine culture ordering and treatment of ASB. Data from included patients were collected over 3 1-month time periods: baseline, post-information technology intervention, and post-educational intervention.
- Results: There was a reduction in the percentage of patients who received antibiotics for ASB in the post-education intervention period as compared to baseline (35% vs 42%). The proportion of total urine cultures ordered by internal medicine clinicians did not change after an information technology intervention to redesign the computerized physician order entry screen for urine cultures.
- Conclusion: Educational interventions are effective ways to reduce rates of inappropriate treatment of ASB in patients admitted to internal medicine services.
Keywords: asymptomatic bacteriuria, UTI, information technology, education, quality.
Asymptomatic bacteriuria (ASB) is a common condition in which bacteria are recovered from a urine culture (UC) in patients without symptoms suggestive of urinary tract infection (UTI), with no pathologic consequences to most patients who are not treated.1,2 Patients with ASB do not exhibit symptoms of a UTI such as dysuria, increased frequency of urination, increased urgency, suprapubic tenderness, or costovertebral pain. Treatment with antibiotics is not indicated for most patients with ASB.1,3 According to the Infectious Diseases Society of America (IDSA), screening for bacteriuria and treatment for positive results is only indicated during pregnancy and prior to urologic procedures with anticipated breach of the mucosal lining.1
An estimated 20% to 52% of patients in hospital settings receive inappropriate treatment with antibiotics for ASB.4 Unnecessary prescribing of antibiotics has several negative consequences, including increased rates of antibiotic resistance, Clostridioides difficile infection, and medication adverse events, as well as increased health care costs.2,5 Antimicrobial stewardship programs to improve judicious use of antimicrobials are paramount to reducing these consequences, and their importance is heightened with recent requirements for antimicrobial stewardship put forth by The Joint Commission and the Centers for Medicare & Medicaid Services.6,7
A previous review of UC and antimicrobial use in patients for purposes of quality improvement at our institution over a 2-month period showed that of 59 patients with positive UCs, 47 patients (80%) did not have documented symptoms of a UTI. Of these 47 patients with ASB, 29 (61.7%) received antimicrobial treatment unnecessarily (unpublished data). We convened a group of clinicians and nonclinicians representing the areas of infectious disease, pharmacy, microbiology, statistics, and hospital internal medicine (IM) to examine the unnecessary treatment of ASB in our institution. Our objective was to address 2 antimicrobial stewardship issues: inappropriate UC ordering and unnecessary use of antibiotics to treat ASB. Our aim was to reduce the inappropriate ordering of UCs and to reduce treatment of ASB.
Methods
Setting
The study was conducted on 3 IM nursing units with a total of 83 beds at a large tertiary care academic medical center in the midwestern United States, and was approved by the organization’s Institutional Review Board.
Participants
We included all non-pregnant patients aged 18 years or older who received care from an IM primary service. These patients were admitted directly to an IM team through the emergency department (ED) or transferred to an IM team after an initial stay in the intensive care unit.
Data Source
Microbiology laboratory reports generated from the electronic health record were used to identify all patients with a collected UC sample who received care from an IM service prior to discharge. Urine samples were collected by midstream catch or catheterization. Data on urine Gram stain and urine dipstick were not included. Henceforth, the phrase “urine culture order” indicates that a UC was both ordered and performed. Data reports were generated for the month of August 2016 to determine the baseline number of UCs ordered. Charts of patients with positive UCs were reviewed to determine if antibiotics were started for the positive UC and whether the patient had signs or symptoms consistent with a UTI. If antibiotics were started in the absence of signs or symptoms to support a UTI, the patient was determined to have been unnecessarily treated for ASB. Reports were then generated for the month after each intervention was implemented, with the same chart review undertaken for positive UCs. Bacteriuria was defined in our study as the presence of microbial growth greater than 10,000 CFU/mL in UC.
Interventions
Initial analysis by our study group determined that lack of electronic clinical decision support (CDS) at the point of care and provider knowledge gaps in interpreting positive UCs were the 2 main contributors to unnecessary UC orders and unnecessary treatment of positive UCs, respectively. We reviewed the work of other groups who reported interventions to decrease treatment of ASB, ranging from educational presentations to pocket cards and treatment algorithms.8-13 We hypothesized that there would be a decrease in UC orders with CDS embedded in the computerized order entry screen, and that we would decrease unnecessary treatment of positive UCs by educating clinicians on indications for appropriate antibiotic prescribing in the setting of a positive UC.
Information technology intervention. The first intervention implemented involved redesign of the UC ordering screen in the computerized physician order entry (CPOE) system. This intervention went live hospital-wide, including the IM floors, intensive care units, and all other areas except the ED, on February 1, 2017 (Figure 1). The ordering screen required the prescriber to select from a list of appropriate indications for ordering a UC, including urine frequency, urgency, or dysuria; unexplained suprapubic or flank pain; fever in patients without another recognized cause; screening obtained prior to urologic procedure; or screening during pregnancy. An additional message advised prescribers to avoid ordering the culture if the patient had malodorous or cloudy urine, pyuria without urinary symptoms, or had an alternative cause of fever. Before we implemented the information technology (IT) intervention, there had been no specific point-of-care guidance on UC ordering.

Educational intervention. The second intervention, driven by clinical pharmacists, involved active and passive education of prescribers specifically designed to address unnecessary treatment of ASB. The IT intervention with CDS for UC ordering remained live. Presentations designed by the study group summarizing the appropriate indications for ordering a UC, distinguishing ASB from UTI, and discouraging treatment of ASB were delivered via a variety of routes by clinical pharmacists to nurses, nurse practitioners, physician assistants, pharmacists, medical residents, and staff physicians providing care to patients on the 3 IM units over a 1-month period in March 2017. The presentations contained the same basic content, but the information was delivered to target each specific audience group.
Medical residents received a 10-minute live presentation during a conference. Nurse practitioners, physician assistants, and staff physicians received a presentation via email, and highlights of the presentation were delivered by clinical pharmacists at their respective monthly group meetings. A handout was presented to nursing staff at nursing huddles, and presentation slides were distributed by email. Educational posters were posted in the medical resident workrooms, nursing breakrooms, and staff bathrooms on the units.
Outcome Measurements
The endpoints of interest were the percentage of patients with positive UCs unnecessarily treated for ASB before and after each intervention and the number of UCs ordered at baseline and after implementation of each intervention. Counterbalance measures assessed included the incidence of UTI, pyelonephritis, or urosepsis within 7 days of positive UC for patients who did not receive antibiotic treatment for ASB.
Results
Data from a total of 270 cultures were examined from IM nursing units. A total of 117 UCs were ordered during the baseline period before interventions were implemented. For a period of 1 month following activation of the IT intervention, 73 UCs were ordered. For a period of 1 month following the educational interventions, 80 UCs were ordered. Of these, 61 (52%) UCs were positive at baseline, 37 (51%) after the IT intervention, and 41 (51%) after the educational intervention. Patient characteristics were similar between the 3 groups (Table); 64.7% of patients were female in their early to mid-seventies. The majority of UCs were ordered by providers in the ED in all 3 periods examined (51%-70%). The percentage of patients who received antibiotics prior to UC for another indication (including bacteriuria) in the baseline, post-IT intervention, and post-education intervention groups were 30%, 27%, and 45%, respectively.

The study outcomes are summarized in Figure 2. Among patients with positive cultures, there was not a reduction in inappropriate treatment of ASB compared to baseline after the IT intervention (48% vs 42%). Following the education intervention, there was a reduction in unnecessary ASB treatment as compared both to baseline (35% vs 42%) and to post-IT intervention (35% vs 48%). There was no difference between the 3 study periods in the percentage of total UCs ordered by IM clinicians. The counterbalance measure showed that 1 patient who did not receive antibiotics within 7 days of a positive UC developed pyelonephritis, UTI, or sepsis due to a UTI in each intervention group.

Discussion
The results of this study demonstrate the role of multimodal interventions in antimicrobial stewardship and add to the growing body of evidence supporting the work of antimicrobial stewardship programs. Our multidisciplinary study group and multipronged intervention follow recent guideline recommendations for antimicrobial stewardship program interventions against unnecessary treatment of ASB.14 Initial analysis by our study group determined lack of CDS at the point of care and provider knowledge gaps in interpreting positive UCs as the 2 main contributors to unnecessary UC orders and unnecessary treatment of positive UCs in our local practice culture. The IT component of our intervention was intended to provide CDS for ordering UCs, and the education component focused on informing clinicians’ treatment decisions for positive UCs.
It has been suggested that the type of stewardship intervention that is most effective fits the specific needs and resources of an institution.14,15 And although the IDSA does not recommend education as a stand-alone intervention,16 we found it to be an effective intervention for our clinicians in our work environment. However, since the CPOE guidance was in place during the educational study periods, it is possible that the effect was due to a combination of these 2 approaches. Our pre-intervention ASB treatment rates were consistent with a recent meta-analysis in which the rate of inappropriate treatment of ASB was 45%.17 This meta-analysis found educational and organizational interventions led to a mean absolute risk reduction of 33%. After the education intervention, we saw a 7% decrease in unnecessary treatment of ASB compared to baseline, and a 13% decrease compared to the month just prior to the educational intervention.
Lessons learned from our work included how clear review of local processes can inform quality improvement interventions. For instance, we initially hypothesized that IM clinicians would benefit from point-of-care CDS guidance, but such guidance used alone without educational interventions was not supported by the results. We also determined that the majority of UCs from patients on general medicine units were ordered by ED providers. This revealed an opportunity to implement similar interventions in the ED, as this was the initial point of contact for many of these patients.
As with any clinical intervention, the anticipated benefits should be weighed against potential harm. Using counterbalance measures, we found there was minimal risk in the occurrence of UTI, pyelonephritis, or sepsis if clinicians avoided treating ASB. This finding is consistent with IDSA guideline recommendations and other studies that suggest that withholding treatment for asymptomatic bacteriuria does not lead to worse outcomes.1
This study has several limitations. Data were obtained through review of the electronic health record and therefore documentation may be incomplete. Also, antimicrobials for empiric coverage or treatment for other infections (eg, pneumonia, sepsis) may have confounded our results, as empirical antimicrobials were given to 27% to 45% of patients prior to UC. This was a quality improvement project carried out over defined time intervals, and thus our sample size was limited and not adequately powered to show statistical significance. Additionally, given the bundling of interventions, it is difficult to determine the impact of each intervention independently. Although CDS for UC ordering may not have influenced ordering, it is possible that the IT intervention raised awareness of ASB and influenced treatment practices.
Conclusion
Our work supports the principles of antibiotic stewardship as brought forth by IDSA.16 This work was the effort of a multidisciplinary team, which aligns with recommendations by Daniel and colleagues, published after our study had ended, for reducing overtreatment of ASB.14 Additionally, our study results provided valuable information for our institution. Although improvements in management of ASB were modest, the success of provider education and identification of other work areas and clinicians to target for future intervention were helpful in consideration of further studies. This work will also aid us in developing an expected effect size for future studies. We plan to provide ongoing education for IM providers as well as education in the ED to target providers who make first contact with patients admitted to inpatient services. In addition, the CPOE UC ordering screen message will continue to be used hospital-wide and will be expanded to the ED ordering system. Our interventions, experiences, and challenges may be used by other institutions to design effective antimicrobial stewardship interventions directed towards reducing rates of inappropriate ASB treatment.
Corresponding author: Prasanna P. Narayanan, PharmD, 200 First Street SW, Rochester, MN 55905; [email protected].
Financial disclosures: None.
1. Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68:e83–75.
2. Trautner BW, Grigoryan L, Petersen NJ, et al. Effectiveness of an antimicrobial stewardship approach for urinary catheter-associated asymptomatic bacteriuria. JAMA Intern Med. 2015;175:1120-1127.
3. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309-332.
4. Trautner BW. Asymptomatic bacteriuria: when the treatment is worse than the disease. Nat Rev Urol. 2011;9:85-93.
5. Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096.
6. The Joint Commission. Prepublication Requirements: New antimicrobial stewardship standard. Jun 22, 2016. www.jointcommission.org/assets/1/6/HAP-CAH_Antimicrobial_Prepub.pdf. Accessed January 24, 2019.
7. Federal Register. Medicare and Medicaid Programs; Hospital and Critical Access Hospital (CAH) Changes to Promote Innovation, Flexibility, and Improvement in Patient Care.Centers for Medicare & Medicaid Services. June 16, 2016. CMS-3295-P
8. Hartley SE, Kuhn L, Valley S, et al. Evaluating a hospitalist-based intervention to decrease unnecessary antimicrobial use in patients with asymptomatic bacteriuria. Infect Control Hosp Epidemiol. 2016;37:1044-1051.
9. Pavese P, Saurel N, Labarere J, et al. Does an educational session with an infectious diseases physician reduce the use of inappropriate antibiotic therapy for inpatients with positive urine culture results? A controlled before-and-after study. Infect Control Hosp Epidemiol. 2009;30:596-599.
10. Kelley D, Aaronson P, Poon E, et al. Evaluation of an antimicrobial stewardship approach to minimize overuse of antibiotics in patients with asymptomatic bacteriuria. Infect Control Hosp Epidemiol. 2014;35:193-195.
11. Chowdhury F, Sarkar K, Branche A, et al. Preventing the inappropriate treatment of asymptomatic bacteriuria at a community teaching hospital. J Community Hosp Intern Med Perspect. 2012;2.
12. Bonnal C, Baune B, Mion M, et al. Bacteriuria in a geriatric hospital: impact of an antibiotic improvement program. J Am Med Dir Assoc. 2008;9:605-609.
13. Linares LA, Thornton DJ, Strymish J, et al. Electronic memorandum decreases unnecessary antimicrobial use for asymptomatic bacteriuria and culture-negative pyuria. Infect Control Hosp Epidemiol. 2011;32:644-648.
14. Daniel M, Keller S, Mozafarihashjin M, et al. An implementation guide to reducing overtreatment of asymptomatic bacteriuria. JAMA Intern Med. 2018;178:271-276.
15. Redwood R, Knobloch MJ, Pellegrini DC, et al. Reducing unnecessary culturing: a systems approach to evaluating urine culture ordering and collection practices among nurses in two acute care settings. Antimicrob Resist Infect Control. 2018;7:4.
16. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:e51–e7.
17. Flokas ME, Andreatos N, Alevizakos M, et al. Inappropriate management of asymptomatic patients with positive urine cultures: a systematic review and meta-analysis. Open Forum Infect Dis. 2017;4:1-10.
1. Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68:e83–75.
2. Trautner BW, Grigoryan L, Petersen NJ, et al. Effectiveness of an antimicrobial stewardship approach for urinary catheter-associated asymptomatic bacteriuria. JAMA Intern Med. 2015;175:1120-1127.
3. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309-332.
4. Trautner BW. Asymptomatic bacteriuria: when the treatment is worse than the disease. Nat Rev Urol. 2011;9:85-93.
5. Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096.
6. The Joint Commission. Prepublication Requirements: New antimicrobial stewardship standard. Jun 22, 2016. www.jointcommission.org/assets/1/6/HAP-CAH_Antimicrobial_Prepub.pdf. Accessed January 24, 2019.
7. Federal Register. Medicare and Medicaid Programs; Hospital and Critical Access Hospital (CAH) Changes to Promote Innovation, Flexibility, and Improvement in Patient Care.Centers for Medicare & Medicaid Services. June 16, 2016. CMS-3295-P
8. Hartley SE, Kuhn L, Valley S, et al. Evaluating a hospitalist-based intervention to decrease unnecessary antimicrobial use in patients with asymptomatic bacteriuria. Infect Control Hosp Epidemiol. 2016;37:1044-1051.
9. Pavese P, Saurel N, Labarere J, et al. Does an educational session with an infectious diseases physician reduce the use of inappropriate antibiotic therapy for inpatients with positive urine culture results? A controlled before-and-after study. Infect Control Hosp Epidemiol. 2009;30:596-599.
10. Kelley D, Aaronson P, Poon E, et al. Evaluation of an antimicrobial stewardship approach to minimize overuse of antibiotics in patients with asymptomatic bacteriuria. Infect Control Hosp Epidemiol. 2014;35:193-195.
11. Chowdhury F, Sarkar K, Branche A, et al. Preventing the inappropriate treatment of asymptomatic bacteriuria at a community teaching hospital. J Community Hosp Intern Med Perspect. 2012;2.
12. Bonnal C, Baune B, Mion M, et al. Bacteriuria in a geriatric hospital: impact of an antibiotic improvement program. J Am Med Dir Assoc. 2008;9:605-609.
13. Linares LA, Thornton DJ, Strymish J, et al. Electronic memorandum decreases unnecessary antimicrobial use for asymptomatic bacteriuria and culture-negative pyuria. Infect Control Hosp Epidemiol. 2011;32:644-648.
14. Daniel M, Keller S, Mozafarihashjin M, et al. An implementation guide to reducing overtreatment of asymptomatic bacteriuria. JAMA Intern Med. 2018;178:271-276.
15. Redwood R, Knobloch MJ, Pellegrini DC, et al. Reducing unnecessary culturing: a systems approach to evaluating urine culture ordering and collection practices among nurses in two acute care settings. Antimicrob Resist Infect Control. 2018;7:4.
16. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:e51–e7.
17. Flokas ME, Andreatos N, Alevizakos M, et al. Inappropriate management of asymptomatic patients with positive urine cultures: a systematic review and meta-analysis. Open Forum Infect Dis. 2017;4:1-10.
Sinusitis treatment depends on classification, duration of symptoms
ORLANDO – according to a speaker at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
The major signs and symptoms of sinusitis are pressure and pain on the anterior side of the face or in a localized headache, nasal obstruction, and pus observed at exam that is clouded or colored. Patients may also present with a feeling of facial congestion or fullness, nasal discharge, and fever, noted Brian Bizik, MS, PA-C, from Asthma & Allergy of Idaho and Nevada. The condition can present as acute (up to 4 weeks), subacute (4-12 weeks, with resolution of symptoms), chronic (12 weeks or more), and recurrent acute chronic sinusitis. Most cases of sinusitis are accompanied with contiguous nasal mucosa inflammation, and therefore the term rhinosinusitis is preferred.
To diagnose sinusitis, “you want patients to tell you where they’re hurting, and where their pressure is,” Mr. Bizik said, noting that he instructs patients to “point with one finger and tell me how you feel without using the word ‘sinus.’ ” Clinicians should ask whether a patient’s pain is continuous or cyclic, if they have bad breath even after brushing their teeth, if they have a chronic cough as opposed to postnasal drip, whether they have pain when they chew or walk, and if they feel like they are always tired.
According to guidelines from the Infectious Diseases Society of America, if symptoms last longer than 10 days and patients have a fever above 39° C (102.2° F), it is more likely bacterial rather than viral. Another sign of bacterial infection is when patients get better after a few days before worsening again later, said Mr. Bizik. In patients where clinicians suspect bacterial infection, the IDSA recommends amoxicillin/clavulanate over amoxicillin alone because some acute bacterial rhinosinusitis could be Haemophilus influenzae, and up to 30% of these infections can produce beta-lactamase. Patients with an amoxicillin allergy should take doxycycline, which is the only currently recommended antibiotic for patients with acute bacterial rhinosinusitis.
In general, clinicians should treat acute bacterial rhinosinusitis based on whether the patient has the most severe disease, said Mr. Bizik. “Use those three criteria: fever, symptoms longer than 10 days, purulence, and feeling lousy. If you find these people are in the high-risk group, [the guidelines] recommend antibiotic treatment.”
In addition to antibiotics, patients can likely benefit from use of topical corticosteroids such as mometasone, fluticasone, flunisolide, and beclomethasone. “It comes down to simply what you like and what works well for you,” he said. With regard to oral steroids, patients with severe pain can benefit from medication like prednisone. Finally, decongestants and relief with sinus irrigation treatments like Neti pots can help relieve symptoms and promote healthy mucosal function.
On the other hand, sinusitis with a viral origin tends to have “light” flu symptoms that do not worsen over time and almost always resolve within 10 days. “If they fit the viral mold, we’re going to do everything the same [as bacterial sinusitis]; just skip the antibiotics,” he said.
In patients with chronic rhinosinusitis (CRS), the symptoms persist over a longer period of time. CRS has a large number of associated conditions, such as allergic rhinitis and gastroesophageal reflux, as well as environmental factors like cigarette smoke, viral illness, and rebound rhinitis. If a patient’s CRS is caused by allergies, treating the allergies aggressively will improve CRS symptoms. “If they have an allergic component, you really have to have a reason not to put them on montelukast. I would encourage you to do that,” said Mr. Bizik. “Cetirizine and montelukast at bedtime works very well. They’re cheap, effective, generic, and nonsteroidal.”
Other methods for treating symptoms of CRS include saline irrigation to increase mucociliary flow rates, high doses of mucolytics, and first- and second-generation antihistamines, which can take up to 10 days to see the full effect. “I have a 10-day reminder, and I call them on day 11,” said Mr. Bizik. “If they stick with it, they say it really did help. It’s a great way to avoid antibiotics.”
Intranasal corticosteroids are also effective first-line therapies for CRS. However, technique is important when using these medications. In his presentation, Mr. Bizik described the “opposite-hand” technique he teaches to patients to reduce some of the side effects patients experience when using intranasal corticosteroids, including nosebleeds.
“You insert it in the nose, you go in all the way until you just feel your fingers touching your nose, and you point it towards the earlobe so the left nostril goes to the left earlobe [and vice versa], and you just spray,” once or twice a day depending on indication, he said. “Using those consistently, when you do this, the flower smell is less, it doesn’t bother you, less goes down your throat, and it’s very effective.”
Dr. Bizik reports being a speaking and consultant for Grifols, Boehringer Ingelheim, Meda Pharmaceuticals, and an advisory board member for Circassia Pharmaceuticals.
Global Academy for Medical Education and this news organization are owned by the same parent company.
ORLANDO – according to a speaker at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
The major signs and symptoms of sinusitis are pressure and pain on the anterior side of the face or in a localized headache, nasal obstruction, and pus observed at exam that is clouded or colored. Patients may also present with a feeling of facial congestion or fullness, nasal discharge, and fever, noted Brian Bizik, MS, PA-C, from Asthma & Allergy of Idaho and Nevada. The condition can present as acute (up to 4 weeks), subacute (4-12 weeks, with resolution of symptoms), chronic (12 weeks or more), and recurrent acute chronic sinusitis. Most cases of sinusitis are accompanied with contiguous nasal mucosa inflammation, and therefore the term rhinosinusitis is preferred.
To diagnose sinusitis, “you want patients to tell you where they’re hurting, and where their pressure is,” Mr. Bizik said, noting that he instructs patients to “point with one finger and tell me how you feel without using the word ‘sinus.’ ” Clinicians should ask whether a patient’s pain is continuous or cyclic, if they have bad breath even after brushing their teeth, if they have a chronic cough as opposed to postnasal drip, whether they have pain when they chew or walk, and if they feel like they are always tired.
According to guidelines from the Infectious Diseases Society of America, if symptoms last longer than 10 days and patients have a fever above 39° C (102.2° F), it is more likely bacterial rather than viral. Another sign of bacterial infection is when patients get better after a few days before worsening again later, said Mr. Bizik. In patients where clinicians suspect bacterial infection, the IDSA recommends amoxicillin/clavulanate over amoxicillin alone because some acute bacterial rhinosinusitis could be Haemophilus influenzae, and up to 30% of these infections can produce beta-lactamase. Patients with an amoxicillin allergy should take doxycycline, which is the only currently recommended antibiotic for patients with acute bacterial rhinosinusitis.
In general, clinicians should treat acute bacterial rhinosinusitis based on whether the patient has the most severe disease, said Mr. Bizik. “Use those three criteria: fever, symptoms longer than 10 days, purulence, and feeling lousy. If you find these people are in the high-risk group, [the guidelines] recommend antibiotic treatment.”
In addition to antibiotics, patients can likely benefit from use of topical corticosteroids such as mometasone, fluticasone, flunisolide, and beclomethasone. “It comes down to simply what you like and what works well for you,” he said. With regard to oral steroids, patients with severe pain can benefit from medication like prednisone. Finally, decongestants and relief with sinus irrigation treatments like Neti pots can help relieve symptoms and promote healthy mucosal function.
On the other hand, sinusitis with a viral origin tends to have “light” flu symptoms that do not worsen over time and almost always resolve within 10 days. “If they fit the viral mold, we’re going to do everything the same [as bacterial sinusitis]; just skip the antibiotics,” he said.
In patients with chronic rhinosinusitis (CRS), the symptoms persist over a longer period of time. CRS has a large number of associated conditions, such as allergic rhinitis and gastroesophageal reflux, as well as environmental factors like cigarette smoke, viral illness, and rebound rhinitis. If a patient’s CRS is caused by allergies, treating the allergies aggressively will improve CRS symptoms. “If they have an allergic component, you really have to have a reason not to put them on montelukast. I would encourage you to do that,” said Mr. Bizik. “Cetirizine and montelukast at bedtime works very well. They’re cheap, effective, generic, and nonsteroidal.”
Other methods for treating symptoms of CRS include saline irrigation to increase mucociliary flow rates, high doses of mucolytics, and first- and second-generation antihistamines, which can take up to 10 days to see the full effect. “I have a 10-day reminder, and I call them on day 11,” said Mr. Bizik. “If they stick with it, they say it really did help. It’s a great way to avoid antibiotics.”
Intranasal corticosteroids are also effective first-line therapies for CRS. However, technique is important when using these medications. In his presentation, Mr. Bizik described the “opposite-hand” technique he teaches to patients to reduce some of the side effects patients experience when using intranasal corticosteroids, including nosebleeds.
“You insert it in the nose, you go in all the way until you just feel your fingers touching your nose, and you point it towards the earlobe so the left nostril goes to the left earlobe [and vice versa], and you just spray,” once or twice a day depending on indication, he said. “Using those consistently, when you do this, the flower smell is less, it doesn’t bother you, less goes down your throat, and it’s very effective.”
Dr. Bizik reports being a speaking and consultant for Grifols, Boehringer Ingelheim, Meda Pharmaceuticals, and an advisory board member for Circassia Pharmaceuticals.
Global Academy for Medical Education and this news organization are owned by the same parent company.
ORLANDO – according to a speaker at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
The major signs and symptoms of sinusitis are pressure and pain on the anterior side of the face or in a localized headache, nasal obstruction, and pus observed at exam that is clouded or colored. Patients may also present with a feeling of facial congestion or fullness, nasal discharge, and fever, noted Brian Bizik, MS, PA-C, from Asthma & Allergy of Idaho and Nevada. The condition can present as acute (up to 4 weeks), subacute (4-12 weeks, with resolution of symptoms), chronic (12 weeks or more), and recurrent acute chronic sinusitis. Most cases of sinusitis are accompanied with contiguous nasal mucosa inflammation, and therefore the term rhinosinusitis is preferred.
To diagnose sinusitis, “you want patients to tell you where they’re hurting, and where their pressure is,” Mr. Bizik said, noting that he instructs patients to “point with one finger and tell me how you feel without using the word ‘sinus.’ ” Clinicians should ask whether a patient’s pain is continuous or cyclic, if they have bad breath even after brushing their teeth, if they have a chronic cough as opposed to postnasal drip, whether they have pain when they chew or walk, and if they feel like they are always tired.
According to guidelines from the Infectious Diseases Society of America, if symptoms last longer than 10 days and patients have a fever above 39° C (102.2° F), it is more likely bacterial rather than viral. Another sign of bacterial infection is when patients get better after a few days before worsening again later, said Mr. Bizik. In patients where clinicians suspect bacterial infection, the IDSA recommends amoxicillin/clavulanate over amoxicillin alone because some acute bacterial rhinosinusitis could be Haemophilus influenzae, and up to 30% of these infections can produce beta-lactamase. Patients with an amoxicillin allergy should take doxycycline, which is the only currently recommended antibiotic for patients with acute bacterial rhinosinusitis.
In general, clinicians should treat acute bacterial rhinosinusitis based on whether the patient has the most severe disease, said Mr. Bizik. “Use those three criteria: fever, symptoms longer than 10 days, purulence, and feeling lousy. If you find these people are in the high-risk group, [the guidelines] recommend antibiotic treatment.”
In addition to antibiotics, patients can likely benefit from use of topical corticosteroids such as mometasone, fluticasone, flunisolide, and beclomethasone. “It comes down to simply what you like and what works well for you,” he said. With regard to oral steroids, patients with severe pain can benefit from medication like prednisone. Finally, decongestants and relief with sinus irrigation treatments like Neti pots can help relieve symptoms and promote healthy mucosal function.
On the other hand, sinusitis with a viral origin tends to have “light” flu symptoms that do not worsen over time and almost always resolve within 10 days. “If they fit the viral mold, we’re going to do everything the same [as bacterial sinusitis]; just skip the antibiotics,” he said.
In patients with chronic rhinosinusitis (CRS), the symptoms persist over a longer period of time. CRS has a large number of associated conditions, such as allergic rhinitis and gastroesophageal reflux, as well as environmental factors like cigarette smoke, viral illness, and rebound rhinitis. If a patient’s CRS is caused by allergies, treating the allergies aggressively will improve CRS symptoms. “If they have an allergic component, you really have to have a reason not to put them on montelukast. I would encourage you to do that,” said Mr. Bizik. “Cetirizine and montelukast at bedtime works very well. They’re cheap, effective, generic, and nonsteroidal.”
Other methods for treating symptoms of CRS include saline irrigation to increase mucociliary flow rates, high doses of mucolytics, and first- and second-generation antihistamines, which can take up to 10 days to see the full effect. “I have a 10-day reminder, and I call them on day 11,” said Mr. Bizik. “If they stick with it, they say it really did help. It’s a great way to avoid antibiotics.”
Intranasal corticosteroids are also effective first-line therapies for CRS. However, technique is important when using these medications. In his presentation, Mr. Bizik described the “opposite-hand” technique he teaches to patients to reduce some of the side effects patients experience when using intranasal corticosteroids, including nosebleeds.
“You insert it in the nose, you go in all the way until you just feel your fingers touching your nose, and you point it towards the earlobe so the left nostril goes to the left earlobe [and vice versa], and you just spray,” once or twice a day depending on indication, he said. “Using those consistently, when you do this, the flower smell is less, it doesn’t bother you, less goes down your throat, and it’s very effective.”
Dr. Bizik reports being a speaking and consultant for Grifols, Boehringer Ingelheim, Meda Pharmaceuticals, and an advisory board member for Circassia Pharmaceuticals.
Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM CARPS 2019
Infective endocarditis: Beyond the usual tests
Prompt diagnois of infective endocarditis is critical. Potential consequences of missed or delayed diagnosis, including heart failure, stroke, intracardiac abscess, conduction delays, prosthesis dysfunction, and cerebral emboli, are often catastrophic. Echocardiography is the test used most frequently to evaluate for infective endocarditis, but it misses the diagnosis in almost one-third of cases, and even more often if the patient has a prosthetic valve.
But now, several sophisticated imaging tests are available that complement echocardiography in diagnosing and assessing infective endocarditis; these include 4-dimensional computed tomography (4D CT), fluorodeoxyglucose positron emission tomography (FDG-PET), and leukocyte scintigraphy. These tests have greatly improved our ability not only to diagnose infective endocarditis, but also to determine the extent and spread of infection, and they aid in perioperative assessment. Abnormal findings on these tests have been incorporated into the European Society of Cardiology’s 2015 modified diagnostic criteria for infective endocarditis.1
This article details the indications, advantages, and limitations of the various imaging tests for diagnosing and evaluating infective endocarditis (Table 1).
INFECTIVE ENDOCARDITIS IS DIFFICULT TO DIAGNOSE AND TREAT
Infective endocarditis is difficult to diagnose and treat. Clinical and imaging clues can be subtle, and the diagnosis requires a high level of suspicion and visualization of cardiac structures.
Further, the incidence of infective endocarditis is on the rise in the United States, particularly in women and young adults, likely due to intravenous drug use.2,3
ECHOCARDIOGRAPHY HAS AN IMPORTANT ROLE, BUT IS LIMITED
Echocardiography remains the most commonly performed study for diagnosing infective endocarditis, as it is fast, widely accessible, and less expensive than other imaging tests.
Transthoracic echocardiography (TTE) is often the first choice for testing. However, its sensitivity is only about 70% for detecting vegetations on native valves and 50% for detecting vegetations on prosthetic valves.1 It is inherently constrained by the limited number of views by which a comprehensive external evaluation of the heart can be achieved. Using a 2-dimensional instrument to view a 3-dimensional object is difficult, and depending on several factors, it can be hard to see vegetations and abscesses that are associated with infective endocarditis. Further, TTE is impeded by obesity and by hyperinflated lungs from obstructive pulmonary disease or mechanical ventilation. It has poor sensitivity for detecting small vegetations and for detecting vegetations and paravalvular complications in patients who have a prosthetic valve or a cardiac implanted electronic device.
Transesophageal echocardiography (TEE) is the recommended first-line imaging test for patients with prosthetic valves and no contraindications to the test. Otherwise, it should be done after TTE if the results of TTE are negative but clinical suspicion for infective endocarditis remains high (eg, because the patient uses intravenous drugs). But although TEE has a higher sensitivity than TTE (up to 96% for vegetations on native valves and 92% for those on prosthetic valves, if performed by an experienced sonographer), it can still miss infective endocarditis. Also, TEE does not provide a significant advantage over TTE in patients who have a cardiac implanted electronic device.1,4,5
Regardless of whether TTE or TEE is used, they are estimated to miss up to 30% of cases of infective endocarditis and its sequelae.4 False-negative findings are likelier in patients who have preexisting severe valvular lesions, prosthetic valves, cardiac implanted electronic devices, small vegetations, or abscesses, or if a vegetation has already broken free and embolized. Furthermore, distinguishing between vegetations and thrombi, cardiac tumors, and myxomatous changes using echocardiography is difficult.
CARDIAC CT
For patients who have inconclusive results on echocardiography, contraindications to TEE, or poor sonic windows, cardiac CT can be an excellent alternative. It is especially useful in the setting of a prosthetic valve.
Synchronized (“gated”) with the patient’s heart rate and rhythm, CT machines can acquire images during diastole, reducing motion artifact, and can create 3D images of the heart. In addition, newer machines can acquire several images at different points in the heart cycle to add a fourth dimension—time. The resulting 4D images play like short video loops of the beating heart and allow noninvasive assessment of cardiac anatomy with remarkable detail and resolution.
4D CT is increasingly being used in infective endocarditis, and growing evidence indicates that its accuracy is similar to that of TEE in the preoperative evaluation of patients with aortic prosthetic valve endocarditis.6 In a study of 28 patients, complementary use of CT angiography led to a change in treatment strategy in 7 (25%) compared with routine clinical workup.7 Several studies have found no difference between 4D CT and preoperative TEE in detecting pseudoaneurysm, abscess, or valve dehiscence. TEE and 4D CT also have similar sensitivities for detecting infective endocarditis in native and prosthetic valves.8,9
Coupled with CT angiography, 4D CT is also an excellent noninvasive way to perioperatively evaluate the coronary arteries without the risks associated with catheterization in those requiring nonemergency surgery (Figure 1A, B, and C).
4D CT performs well for detecting abscess and pseudoaneurysm but has slightly lower sensitivity for vegetations than TEE (91% vs 99%).9
Gated CT, PET, or both may be useful in cases of suspected prosthetic aortic valve endocarditis when TEE is negative. Pseudoaneurysms are not well visualized with TEE, and the atrial mitral curtain area is often thickened on TEE in cases of aortic prosthetic valve infective endocarditis that do not definitely involve abscesses. Gated CT and PET show this area better.8 This information is important in cases in which a surgeon may be unconvinced that the patient has prosthetic valve endocarditis.
Limitations of 4D cardiac CT
4D CT with or without angiography has limitations. It requires a wide-volume scanner and an experienced reader.
Patients with irregular heart rhythms or uncontrolled tachycardia pose technical problems for image acquisition. Cardiac CT is typically gated (ie, images are obtained within a defined time period) to acquire images during diastole. Ideally, images are acquired when the heart is in mid to late diastole, a time of minimal cardiac motion, so that motion artifact is minimized. To estimate the timing of image acquisition, the cardiac cycle must be predictable, and its duration should be as long as possible. Tachycardia or irregular rhythms such as frequent ectopic beats or atrial fibrillation make acquisition timing difficult, and thus make it nearly impossible to accurately obtain images when the heart is at minimum motion, limiting assessment of cardiac structures or the coronary tree.4,10
Extensive coronary calcification can hinder assessment of the coronary tree by CT coronary angiography.
Contrast exposure may limit the use of CT in some patients (eg, those with contrast allergies or renal dysfunction). However, modern scanners allow for much smaller contrast boluses without decreasing sensitivity.
4D CT involves radiation exposure, especially when done with angiography, although modern scanners have greatly reduced exposure. The average radiation dose in CT coronary angiography is 2.9 to 5.9 mSv11 compared with 7 mSv in diagnostic cardiac catheterization (without angioplasty or stenting) or 16 mSv in routine CT of the abdomen and pelvis with contrast.12,13 In view of the morbidity and mortality risks associated with infective endocarditis, especially if the diagnosis is delayed, this small radiation exposure may be justifiable.
Bottom line for cardiac CT
4D CT is an excellent alternative to echocardiography for select patients. Clinicians should strongly consider this study in the following situations:
- Patients with a prosthetic valve
- Patients who are strongly suspected of having infective endocarditis but who have a poor sonic window on TTE or TEE, as can occur with chronic obstructive lung disease, morbid obesity, or previous thoracic or cardiovascular surgery
- Patients who meet clinical indications for TEE, such as having a prosthetic valve or a high suspicion for native valve infective endocarditis with negative TTE, but who have contraindications to TEE
- As an alternative to TEE for preoperative evaluation in patients with known infective endocarditis.
Patients with tachycardia or irregular heart rhythms are not good candidates for this test.
FDG-PET AND LEUKOCYTE SCINTIGRAPHY
FDG-PET and leukocyte scintigraphy are other options for diagnosing infective endocarditis and determining the presence and extent of intra- and extracardiac infection. They are more sensitive than echocardiography for detecting infection of cardiac implanted electronic devices such as ventricular assist devices, pacemakers, implanted cardiac defibrillators, and cardiac resynchronization therapy devices.14–16
The utility of FDG-PET is founded on the uptake of 18F-fluorodeoxyglucose by cells, with higher uptake taking place in cells with higher metabolic activity (such as in areas of inflammation). Similarly, leukocyte scintigraphy relies on the use of radiolabeled leukocytes (ie, leukocytes previously extracted from the patient, labelled, and re-introduced into the patient) to allow for localization of inflamed tissue.
The most significant contribution of FDG-PET may be the ability to detect infective endocarditis early, when echocardiography is initially negative. When abnormal FDG uptake was included in the modified Duke criteria, it increased the sensitivity to 97% for detecting infective endocarditis on admission, leading some to propose its incorporation as a major criterion.17 In patients with prosthetic valves and suspected infective endocarditis, FDG-PET was found in one study to have a sensitivity of up to 91% and a specificity of up to 95%.18
Both FDG-PET and leukocyte scintigraphy have a high sensitivity, specificity, and negative predictive value for cardiac implanted electronic device infection, and should be strongly considered in patients in whom it is suspected but who have negative or inconclusive findings on echocardiography.14,15
In addition, a common conundrum faced by clinicians with use of echocardiography is the difficulty of differentiating thrombus from infected vegetation on valves or device lead wires. Some evidence indicates that FDG-PET may help to discriminate between vegetation and thrombus, although more rigorous studies are needed before its use for that purpose can be recommended.19
Limitations of nuclear studies
Both FDG-PET and leukocyte scintigraphy perform poorly for detecting native-valve infective endocarditis. In a study in which 90% of the patients had native-valve infective endocarditis according to the Duke criteria, FDG-PET had a specificity of 93% but a sensitivity of only 39%.20
Both studies can be cumbersome, laborious, and time-consuming for patients. FDG-PET requires a fasting or glucose-restricted diet before testing, and the test itself can be complicated by development of hyperglycemia, although this is rare.
While FDG-PET is most effective in detecting infections of prosthetic valves and cardiac implanted electronic devices, the results can be falsely positive in patients with a history of recent cardiac surgery (due to ongoing tissue healing), as well as maladies other than infective endocarditis that lead to inflammation, such as vasculitis or malignancy. Similarly, for unclear reasons, leukocyte scintigraphy can yield false-negative results in patients with enterococcal or candidal infective endocarditis.21
FDG-PET and leukocyte scintigraphy are more expensive than TEE and cardiac CT22 and are not widely available.
Both tests entail radiation exposure, with the average dose ranging from 7 to 14 mSv. However, this is less than the average amount acquired during percutaneous coronary intervention (16 mSv), and overlaps with the amount in chest CT with contrast when assessing for pulmonary embolism (7 to 9 mSv). Lower doses are possible with optimized protocols.12,13,15,23
Bottom line for nuclear studies
FDG-PET and leukocyte scintigraphy are especially useful for patients with a prosthetic valve or cardiac implanted electronic device. However, limitations must be kept in mind.
A suggested algorithm for testing with nuclear imaging is shown in Figure 2.1,4
CEREBRAL MAGNETIC RESONANCE IMAGING
Cerebral magnetic resonance imaging (MRI) is more sensitive than cerebral CT for detecting emboli in the brain. According to American Heart Association guidelines, cerebral MRI should be done in patients with known or suspected infective endocarditis and neurologic impairment, defined as headaches, meningeal symptoms, or neurologic deficits. It is also often used in neurologically asymptomatic patients with infective endocarditis who have indications for valve surgery to assess for mycotic aneurysms, which are associated with increased intracranial bleeding during surgery.
MRI use in other asymptomatic patients remains controversial.24 In cases with high clinical suspicion for infective endocarditis and no findings on echocardiography, cerebral MRI can increase the sensitivity of the Duke criteria by adding a minor criterion. Some have argued that, in patients with definite infective endocarditis, detecting silent cerebral complications can lead to management changes. However, more studies are needed to determine if there is indeed a group of neurologically asymptomatic infective endocarditis patients for whom cerebral MRI leads to improved outcomes.
Limitations of cerebral MRI
Cerebral MRI cannot be used in patients with non-MRI-compatible implanted hardware.
Gadolinium, the contrast agent typically used, can cause nephrogenic systemic fibrosis in patients who have poor renal function. This rare but serious adverse effect is characterized by irreversible systemic fibrosis affecting skin, muscles, and even visceral tissue such as lungs. The American College of Radiology allows for gadolinium use in patients without acute kidney injury and patients with stable chronic kidney disease with a glomerular filtration rate of at least 30 mL/min/1.73 m2. Its use should be avoided in patients with renal failure on replacement therapy, with advanced chronic kidney disease (glomerular filtration rate < 30 mL/min/1.73 m2), or with acute kidney injury, even if they do not need renal replacement therapy.25
Concerns have also been raised about gadolinium retention in the brain, even in patients with normal renal function.26–28 Thus far, no conclusive clinical adverse effects of retention have been found, although more study is warranted. Nevertheless, the US Food and Drug Administration now requires a black-box warning about this possibility and advises clinicians to counsel patients appropriately.
Bottom line on cerebral MRI
Cerebral MRI should be obtained when a patient presents with definite or possible infective endocarditis with neurologic impairment, such as new headaches, meningismus, or focal neurologic deficits. Routine brain MRI in patients with confirmed infective endocarditis without neurologic symptoms, or those without definite infective endocarditis, is discouraged.
CARDIAC MRI
Cardiac MRI, typically obtained with gadolinium contrast, allows for better 3D assessment of cardiac structures and morphology than echocardiography or CT, and can detect infiltrative cardiac disease, myopericarditis, and much more. It is increasingly used in the field of structural cardiology, but its role for evaluating infective endocarditis remains unclear.
Cardiac MRI does not appear to be better than echocardiography for diagnosing infective endocarditis. However, it may prove helpful in the evaluation of patients known to have infective endocarditis but who cannot be properly evaluated for disease extent because of poor image quality on echocardiography and contraindications to CT.1,29 Its role is limited in patients with cardiac implanted electronic devices, as most devices are incompatible with MRI use, although newer devices obviate this concern. But even for devices that are MRI-compatible, results are diminished due to an eclipsing effect, wherein the device parts can make it hard to see structures clearly because the “brightness” basically eclipses the surrounding area.4
Concerns regarding use of gadolinium as described above need also be considered.
The role of cardiac MRI in diagnosing and managing infective endocarditis may evolve, but at present, the 2017 American College of Cardiology and American Heart Association appropriate-use criteria discourage its use for these purposes.16
Bottom line for cardiac MRI
Cardiac MRI to evaluate a patient for suspected infective endocarditis is not recommended due to lack of superiority compared with echocardiography or CT, and the risk of nephrogenic systemic fibrosis from gadolinium in patients with renal compromise.
- Habib G, Lancellotti P, Antunes MJ, et al; ESC Scientific Document Group. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Durante-Mangoni E, Bradley S, Selton-Suty C, et al; International Collaboration on Endocarditis Prospective Cohort Study Group. Current features of infective endocarditis in elderly patients: results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch Intern Med 2008; 168(19):2095–2103. doi:10.1001/archinte.168.19.2095
- Wurcel AG, Anderson JE, Chui KK, et al. Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis 2016; 3(3):ofw157. doi:10.1093/ofid/ofw157
- Gomes A, Glaudemans AW, Touw DJ, et al. Diagnostic value of imaging in infective endocarditis: a systematic review. Lancet Infect Dis 2017; 17(1):e1–e14. doi:10.1016/S1473-3099(16)30141-4
- Cahill TJ, Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol 2017; 69(3):325–344. doi:10.1016/j.jacc.2016.10.066
- Fagman E, Perrotta S, Bech-Hanssen O, et al. ECG-gated computed tomography: a new role for patients with suspected aortic prosthetic valve endocarditis. Eur Radiol 2012; 22(11):2407–2414. doi:10.1007/s00330-012-2491-5
- Habets J, Tanis W, van Herwerden LA, et al. Cardiac computed tomography angiography results in diagnostic and therapeutic change in prosthetic heart valve endocarditis. Int J Cardiovasc Imaging 2014; 30(2):377–387. doi:10.1007/s10554-013-0335-2
- Koneru S, Huang SS, Oldan J, et al. Role of preoperative cardiac CT in the evaluation of infective endocarditis: comparison with transesophageal echocardiography and surgical findings. Cardiovasc Diagn Ther 2018; 8(4):439–449. doi:10.21037/cdt.2018.07.07
- Koo HJ, Yang DH, Kang J, et al. Demonstration of infective endocarditis by cardiac CT and transoesophageal echocardiography: comparison with intra-operative findings. Eur Heart J Cardiovasc Imaging 2018; 19(2):199–207. doi:10.1093/ehjci/jex010
- Feuchtner GM, Stolzmann P, Dichtl W, et al. Multislice computed tomography in infective endocarditis: comparison with transesophageal echocardiography and intraoperative findings. J Am Coll Cardiol 2009; 53(5):436–444. doi:10.1016/j.jacc.2008.01.077
- Castellano IA, Nicol ED, Bull RK, Roobottom CA, Williams MC, Harden SP. A prospective national survey of coronary CT angiography radiation doses in the United Kingdom. J Cardiovasc Comput Tomogr 2017; 11(4):268–273. doi:10.1016/j.jcct.2017.05.002
- Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 2008; 248(1):254–263. doi:10.1148/radiol.2481071451
- Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 2009; 169(22):2078–2086. doi:10.1001/archinternmed.2009.427
- Ploux S, Riviere A, Amraoui S, et al. Positron emission tomography in patients with suspected pacing system infections may play a critical role in difficult cases. Heart Rhythm 2011; 8(9):1478–1481. doi:10.1016/j.hrthm.2011.03.062
- Sarrazin J, Philippon F, Tessier M, et al. Usefulness of fluorine-18 positron emission tomography/computed tomography for identification of cardiovascular implantable electronic device infections. J Am Coll Cardiol 2012; 59(18):1616–1625. doi:10.1016/j.jacc.2011.11.059
- Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P; Rating Panel Members; Appropriate Use Criteria Task Force. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2017 Appropriate use criteria for multimodality imaging in valvular heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Nucl Cardiol 2017; 24(6):2043–2063. doi:10.1007/s12350-017-1070-1
- Saby L, Laas O, Habib G, et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol 2013; 61(23):2374–2382. doi:10.1016/j.jacc.2013.01.092
- Swart LE, Gomes A, Scholtens AM, et al. Improving the diagnostic performance of 18F-fluorodeoxyglucose positron-emission tomography/computed tomography in prosthetic heart valve endocarditis. Circulation 2018; 138(14):1412–1427. doi:10.1161/CIRCULATIONAHA.118.035032
- Graziosi M, Nanni C, Lorenzini M, et al. Role of 18F-FDG PET/CT in the diagnosis of infective endocarditis in patients with an implanted cardiac device: a prospective study. Eur J Nucl Med Mol Imaging 2014; 41(8):1617–1623. doi:10.1007/s00259-014-2773-z
- Kouijzer IJ, Vos FJ, Janssen MJ, van Dijk AP, Oyen WJ, Bleeker-Rovers CP. The value of 18F-FDG PET/CT in diagnosing infectious endocarditis. Eur J Nucl Med Mol Imaging 2013; 40(7):1102–1107. doi:10.1007/s00259-013-2376-0
- Wong D, Rubinshtein R, Keynan Y. Alternative cardiac imaging modalities to echocardiography for the diagnosis of infective endocarditis. Am J Cardiol 2016; 118(9):1410–1418. doi:10.1016/j.amjcard.2016.07.053
- Vos FJ, Bleeker-Rovers CP, Kullberg BJ, Adang EM, Oyen WJ. Cost-effectiveness of routine (18)F-FDG PET/CT in high-risk patients with gram-positive bacteremia. J Nucl Med 2011; 52(11):1673–1678. doi:10.2967/jnumed.111.089714
- McCollough CH, Bushberg JT, Fletcher JG, Eckel LJ. Answers to common questions about the use and safety of CT scans. Mayo Clin Proc 2015; 90(10):1380–1392. doi:10.1016/j.mayocp.2015.07.011
- Duval X, Iung B, Klein I, et al; IMAGE (Resonance Magnetic Imaging at the Acute Phase of Endocarditis) Study Group. Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis: a prospective study. Ann Intern Med 2010; 152(8):497–504, W175. doi:10.7326/0003-4819-152-8-201004200-00006
- ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media: 2018. www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf. Accessed July 19, 2019.
- Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015; 276(1):228–232. doi:10.1148/radiol.2015142690
- McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015; 275(3):772–782. doi:10.1148/radiol.15150025
- Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014; 270(3):834–841. doi:10.1148/radiol.13131669
- Expert Panel on Pediatric Imaging; Hayes LL, Palasis S, Bartel TB, et al. ACR appropriateness criteria headache-child. J Am Coll Radiol 2018; 15(5S):S78–S90. doi:10.1016/j.jacr.2018.03.017
Prompt diagnois of infective endocarditis is critical. Potential consequences of missed or delayed diagnosis, including heart failure, stroke, intracardiac abscess, conduction delays, prosthesis dysfunction, and cerebral emboli, are often catastrophic. Echocardiography is the test used most frequently to evaluate for infective endocarditis, but it misses the diagnosis in almost one-third of cases, and even more often if the patient has a prosthetic valve.
But now, several sophisticated imaging tests are available that complement echocardiography in diagnosing and assessing infective endocarditis; these include 4-dimensional computed tomography (4D CT), fluorodeoxyglucose positron emission tomography (FDG-PET), and leukocyte scintigraphy. These tests have greatly improved our ability not only to diagnose infective endocarditis, but also to determine the extent and spread of infection, and they aid in perioperative assessment. Abnormal findings on these tests have been incorporated into the European Society of Cardiology’s 2015 modified diagnostic criteria for infective endocarditis.1
This article details the indications, advantages, and limitations of the various imaging tests for diagnosing and evaluating infective endocarditis (Table 1).
INFECTIVE ENDOCARDITIS IS DIFFICULT TO DIAGNOSE AND TREAT
Infective endocarditis is difficult to diagnose and treat. Clinical and imaging clues can be subtle, and the diagnosis requires a high level of suspicion and visualization of cardiac structures.
Further, the incidence of infective endocarditis is on the rise in the United States, particularly in women and young adults, likely due to intravenous drug use.2,3
ECHOCARDIOGRAPHY HAS AN IMPORTANT ROLE, BUT IS LIMITED
Echocardiography remains the most commonly performed study for diagnosing infective endocarditis, as it is fast, widely accessible, and less expensive than other imaging tests.
Transthoracic echocardiography (TTE) is often the first choice for testing. However, its sensitivity is only about 70% for detecting vegetations on native valves and 50% for detecting vegetations on prosthetic valves.1 It is inherently constrained by the limited number of views by which a comprehensive external evaluation of the heart can be achieved. Using a 2-dimensional instrument to view a 3-dimensional object is difficult, and depending on several factors, it can be hard to see vegetations and abscesses that are associated with infective endocarditis. Further, TTE is impeded by obesity and by hyperinflated lungs from obstructive pulmonary disease or mechanical ventilation. It has poor sensitivity for detecting small vegetations and for detecting vegetations and paravalvular complications in patients who have a prosthetic valve or a cardiac implanted electronic device.
Transesophageal echocardiography (TEE) is the recommended first-line imaging test for patients with prosthetic valves and no contraindications to the test. Otherwise, it should be done after TTE if the results of TTE are negative but clinical suspicion for infective endocarditis remains high (eg, because the patient uses intravenous drugs). But although TEE has a higher sensitivity than TTE (up to 96% for vegetations on native valves and 92% for those on prosthetic valves, if performed by an experienced sonographer), it can still miss infective endocarditis. Also, TEE does not provide a significant advantage over TTE in patients who have a cardiac implanted electronic device.1,4,5
Regardless of whether TTE or TEE is used, they are estimated to miss up to 30% of cases of infective endocarditis and its sequelae.4 False-negative findings are likelier in patients who have preexisting severe valvular lesions, prosthetic valves, cardiac implanted electronic devices, small vegetations, or abscesses, or if a vegetation has already broken free and embolized. Furthermore, distinguishing between vegetations and thrombi, cardiac tumors, and myxomatous changes using echocardiography is difficult.
CARDIAC CT
For patients who have inconclusive results on echocardiography, contraindications to TEE, or poor sonic windows, cardiac CT can be an excellent alternative. It is especially useful in the setting of a prosthetic valve.
Synchronized (“gated”) with the patient’s heart rate and rhythm, CT machines can acquire images during diastole, reducing motion artifact, and can create 3D images of the heart. In addition, newer machines can acquire several images at different points in the heart cycle to add a fourth dimension—time. The resulting 4D images play like short video loops of the beating heart and allow noninvasive assessment of cardiac anatomy with remarkable detail and resolution.
4D CT is increasingly being used in infective endocarditis, and growing evidence indicates that its accuracy is similar to that of TEE in the preoperative evaluation of patients with aortic prosthetic valve endocarditis.6 In a study of 28 patients, complementary use of CT angiography led to a change in treatment strategy in 7 (25%) compared with routine clinical workup.7 Several studies have found no difference between 4D CT and preoperative TEE in detecting pseudoaneurysm, abscess, or valve dehiscence. TEE and 4D CT also have similar sensitivities for detecting infective endocarditis in native and prosthetic valves.8,9
Coupled with CT angiography, 4D CT is also an excellent noninvasive way to perioperatively evaluate the coronary arteries without the risks associated with catheterization in those requiring nonemergency surgery (Figure 1A, B, and C).
4D CT performs well for detecting abscess and pseudoaneurysm but has slightly lower sensitivity for vegetations than TEE (91% vs 99%).9
Gated CT, PET, or both may be useful in cases of suspected prosthetic aortic valve endocarditis when TEE is negative. Pseudoaneurysms are not well visualized with TEE, and the atrial mitral curtain area is often thickened on TEE in cases of aortic prosthetic valve infective endocarditis that do not definitely involve abscesses. Gated CT and PET show this area better.8 This information is important in cases in which a surgeon may be unconvinced that the patient has prosthetic valve endocarditis.
Limitations of 4D cardiac CT
4D CT with or without angiography has limitations. It requires a wide-volume scanner and an experienced reader.
Patients with irregular heart rhythms or uncontrolled tachycardia pose technical problems for image acquisition. Cardiac CT is typically gated (ie, images are obtained within a defined time period) to acquire images during diastole. Ideally, images are acquired when the heart is in mid to late diastole, a time of minimal cardiac motion, so that motion artifact is minimized. To estimate the timing of image acquisition, the cardiac cycle must be predictable, and its duration should be as long as possible. Tachycardia or irregular rhythms such as frequent ectopic beats or atrial fibrillation make acquisition timing difficult, and thus make it nearly impossible to accurately obtain images when the heart is at minimum motion, limiting assessment of cardiac structures or the coronary tree.4,10
Extensive coronary calcification can hinder assessment of the coronary tree by CT coronary angiography.
Contrast exposure may limit the use of CT in some patients (eg, those with contrast allergies or renal dysfunction). However, modern scanners allow for much smaller contrast boluses without decreasing sensitivity.
4D CT involves radiation exposure, especially when done with angiography, although modern scanners have greatly reduced exposure. The average radiation dose in CT coronary angiography is 2.9 to 5.9 mSv11 compared with 7 mSv in diagnostic cardiac catheterization (without angioplasty or stenting) or 16 mSv in routine CT of the abdomen and pelvis with contrast.12,13 In view of the morbidity and mortality risks associated with infective endocarditis, especially if the diagnosis is delayed, this small radiation exposure may be justifiable.
Bottom line for cardiac CT
4D CT is an excellent alternative to echocardiography for select patients. Clinicians should strongly consider this study in the following situations:
- Patients with a prosthetic valve
- Patients who are strongly suspected of having infective endocarditis but who have a poor sonic window on TTE or TEE, as can occur with chronic obstructive lung disease, morbid obesity, or previous thoracic or cardiovascular surgery
- Patients who meet clinical indications for TEE, such as having a prosthetic valve or a high suspicion for native valve infective endocarditis with negative TTE, but who have contraindications to TEE
- As an alternative to TEE for preoperative evaluation in patients with known infective endocarditis.
Patients with tachycardia or irregular heart rhythms are not good candidates for this test.
FDG-PET AND LEUKOCYTE SCINTIGRAPHY
FDG-PET and leukocyte scintigraphy are other options for diagnosing infective endocarditis and determining the presence and extent of intra- and extracardiac infection. They are more sensitive than echocardiography for detecting infection of cardiac implanted electronic devices such as ventricular assist devices, pacemakers, implanted cardiac defibrillators, and cardiac resynchronization therapy devices.14–16
The utility of FDG-PET is founded on the uptake of 18F-fluorodeoxyglucose by cells, with higher uptake taking place in cells with higher metabolic activity (such as in areas of inflammation). Similarly, leukocyte scintigraphy relies on the use of radiolabeled leukocytes (ie, leukocytes previously extracted from the patient, labelled, and re-introduced into the patient) to allow for localization of inflamed tissue.
The most significant contribution of FDG-PET may be the ability to detect infective endocarditis early, when echocardiography is initially negative. When abnormal FDG uptake was included in the modified Duke criteria, it increased the sensitivity to 97% for detecting infective endocarditis on admission, leading some to propose its incorporation as a major criterion.17 In patients with prosthetic valves and suspected infective endocarditis, FDG-PET was found in one study to have a sensitivity of up to 91% and a specificity of up to 95%.18
Both FDG-PET and leukocyte scintigraphy have a high sensitivity, specificity, and negative predictive value for cardiac implanted electronic device infection, and should be strongly considered in patients in whom it is suspected but who have negative or inconclusive findings on echocardiography.14,15
In addition, a common conundrum faced by clinicians with use of echocardiography is the difficulty of differentiating thrombus from infected vegetation on valves or device lead wires. Some evidence indicates that FDG-PET may help to discriminate between vegetation and thrombus, although more rigorous studies are needed before its use for that purpose can be recommended.19
Limitations of nuclear studies
Both FDG-PET and leukocyte scintigraphy perform poorly for detecting native-valve infective endocarditis. In a study in which 90% of the patients had native-valve infective endocarditis according to the Duke criteria, FDG-PET had a specificity of 93% but a sensitivity of only 39%.20
Both studies can be cumbersome, laborious, and time-consuming for patients. FDG-PET requires a fasting or glucose-restricted diet before testing, and the test itself can be complicated by development of hyperglycemia, although this is rare.
While FDG-PET is most effective in detecting infections of prosthetic valves and cardiac implanted electronic devices, the results can be falsely positive in patients with a history of recent cardiac surgery (due to ongoing tissue healing), as well as maladies other than infective endocarditis that lead to inflammation, such as vasculitis or malignancy. Similarly, for unclear reasons, leukocyte scintigraphy can yield false-negative results in patients with enterococcal or candidal infective endocarditis.21
FDG-PET and leukocyte scintigraphy are more expensive than TEE and cardiac CT22 and are not widely available.
Both tests entail radiation exposure, with the average dose ranging from 7 to 14 mSv. However, this is less than the average amount acquired during percutaneous coronary intervention (16 mSv), and overlaps with the amount in chest CT with contrast when assessing for pulmonary embolism (7 to 9 mSv). Lower doses are possible with optimized protocols.12,13,15,23
Bottom line for nuclear studies
FDG-PET and leukocyte scintigraphy are especially useful for patients with a prosthetic valve or cardiac implanted electronic device. However, limitations must be kept in mind.
A suggested algorithm for testing with nuclear imaging is shown in Figure 2.1,4
CEREBRAL MAGNETIC RESONANCE IMAGING
Cerebral magnetic resonance imaging (MRI) is more sensitive than cerebral CT for detecting emboli in the brain. According to American Heart Association guidelines, cerebral MRI should be done in patients with known or suspected infective endocarditis and neurologic impairment, defined as headaches, meningeal symptoms, or neurologic deficits. It is also often used in neurologically asymptomatic patients with infective endocarditis who have indications for valve surgery to assess for mycotic aneurysms, which are associated with increased intracranial bleeding during surgery.
MRI use in other asymptomatic patients remains controversial.24 In cases with high clinical suspicion for infective endocarditis and no findings on echocardiography, cerebral MRI can increase the sensitivity of the Duke criteria by adding a minor criterion. Some have argued that, in patients with definite infective endocarditis, detecting silent cerebral complications can lead to management changes. However, more studies are needed to determine if there is indeed a group of neurologically asymptomatic infective endocarditis patients for whom cerebral MRI leads to improved outcomes.
Limitations of cerebral MRI
Cerebral MRI cannot be used in patients with non-MRI-compatible implanted hardware.
Gadolinium, the contrast agent typically used, can cause nephrogenic systemic fibrosis in patients who have poor renal function. This rare but serious adverse effect is characterized by irreversible systemic fibrosis affecting skin, muscles, and even visceral tissue such as lungs. The American College of Radiology allows for gadolinium use in patients without acute kidney injury and patients with stable chronic kidney disease with a glomerular filtration rate of at least 30 mL/min/1.73 m2. Its use should be avoided in patients with renal failure on replacement therapy, with advanced chronic kidney disease (glomerular filtration rate < 30 mL/min/1.73 m2), or with acute kidney injury, even if they do not need renal replacement therapy.25
Concerns have also been raised about gadolinium retention in the brain, even in patients with normal renal function.26–28 Thus far, no conclusive clinical adverse effects of retention have been found, although more study is warranted. Nevertheless, the US Food and Drug Administration now requires a black-box warning about this possibility and advises clinicians to counsel patients appropriately.
Bottom line on cerebral MRI
Cerebral MRI should be obtained when a patient presents with definite or possible infective endocarditis with neurologic impairment, such as new headaches, meningismus, or focal neurologic deficits. Routine brain MRI in patients with confirmed infective endocarditis without neurologic symptoms, or those without definite infective endocarditis, is discouraged.
CARDIAC MRI
Cardiac MRI, typically obtained with gadolinium contrast, allows for better 3D assessment of cardiac structures and morphology than echocardiography or CT, and can detect infiltrative cardiac disease, myopericarditis, and much more. It is increasingly used in the field of structural cardiology, but its role for evaluating infective endocarditis remains unclear.
Cardiac MRI does not appear to be better than echocardiography for diagnosing infective endocarditis. However, it may prove helpful in the evaluation of patients known to have infective endocarditis but who cannot be properly evaluated for disease extent because of poor image quality on echocardiography and contraindications to CT.1,29 Its role is limited in patients with cardiac implanted electronic devices, as most devices are incompatible with MRI use, although newer devices obviate this concern. But even for devices that are MRI-compatible, results are diminished due to an eclipsing effect, wherein the device parts can make it hard to see structures clearly because the “brightness” basically eclipses the surrounding area.4
Concerns regarding use of gadolinium as described above need also be considered.
The role of cardiac MRI in diagnosing and managing infective endocarditis may evolve, but at present, the 2017 American College of Cardiology and American Heart Association appropriate-use criteria discourage its use for these purposes.16
Bottom line for cardiac MRI
Cardiac MRI to evaluate a patient for suspected infective endocarditis is not recommended due to lack of superiority compared with echocardiography or CT, and the risk of nephrogenic systemic fibrosis from gadolinium in patients with renal compromise.
Prompt diagnois of infective endocarditis is critical. Potential consequences of missed or delayed diagnosis, including heart failure, stroke, intracardiac abscess, conduction delays, prosthesis dysfunction, and cerebral emboli, are often catastrophic. Echocardiography is the test used most frequently to evaluate for infective endocarditis, but it misses the diagnosis in almost one-third of cases, and even more often if the patient has a prosthetic valve.
But now, several sophisticated imaging tests are available that complement echocardiography in diagnosing and assessing infective endocarditis; these include 4-dimensional computed tomography (4D CT), fluorodeoxyglucose positron emission tomography (FDG-PET), and leukocyte scintigraphy. These tests have greatly improved our ability not only to diagnose infective endocarditis, but also to determine the extent and spread of infection, and they aid in perioperative assessment. Abnormal findings on these tests have been incorporated into the European Society of Cardiology’s 2015 modified diagnostic criteria for infective endocarditis.1
This article details the indications, advantages, and limitations of the various imaging tests for diagnosing and evaluating infective endocarditis (Table 1).
INFECTIVE ENDOCARDITIS IS DIFFICULT TO DIAGNOSE AND TREAT
Infective endocarditis is difficult to diagnose and treat. Clinical and imaging clues can be subtle, and the diagnosis requires a high level of suspicion and visualization of cardiac structures.
Further, the incidence of infective endocarditis is on the rise in the United States, particularly in women and young adults, likely due to intravenous drug use.2,3
ECHOCARDIOGRAPHY HAS AN IMPORTANT ROLE, BUT IS LIMITED
Echocardiography remains the most commonly performed study for diagnosing infective endocarditis, as it is fast, widely accessible, and less expensive than other imaging tests.
Transthoracic echocardiography (TTE) is often the first choice for testing. However, its sensitivity is only about 70% for detecting vegetations on native valves and 50% for detecting vegetations on prosthetic valves.1 It is inherently constrained by the limited number of views by which a comprehensive external evaluation of the heart can be achieved. Using a 2-dimensional instrument to view a 3-dimensional object is difficult, and depending on several factors, it can be hard to see vegetations and abscesses that are associated with infective endocarditis. Further, TTE is impeded by obesity and by hyperinflated lungs from obstructive pulmonary disease or mechanical ventilation. It has poor sensitivity for detecting small vegetations and for detecting vegetations and paravalvular complications in patients who have a prosthetic valve or a cardiac implanted electronic device.
Transesophageal echocardiography (TEE) is the recommended first-line imaging test for patients with prosthetic valves and no contraindications to the test. Otherwise, it should be done after TTE if the results of TTE are negative but clinical suspicion for infective endocarditis remains high (eg, because the patient uses intravenous drugs). But although TEE has a higher sensitivity than TTE (up to 96% for vegetations on native valves and 92% for those on prosthetic valves, if performed by an experienced sonographer), it can still miss infective endocarditis. Also, TEE does not provide a significant advantage over TTE in patients who have a cardiac implanted electronic device.1,4,5
Regardless of whether TTE or TEE is used, they are estimated to miss up to 30% of cases of infective endocarditis and its sequelae.4 False-negative findings are likelier in patients who have preexisting severe valvular lesions, prosthetic valves, cardiac implanted electronic devices, small vegetations, or abscesses, or if a vegetation has already broken free and embolized. Furthermore, distinguishing between vegetations and thrombi, cardiac tumors, and myxomatous changes using echocardiography is difficult.
CARDIAC CT
For patients who have inconclusive results on echocardiography, contraindications to TEE, or poor sonic windows, cardiac CT can be an excellent alternative. It is especially useful in the setting of a prosthetic valve.
Synchronized (“gated”) with the patient’s heart rate and rhythm, CT machines can acquire images during diastole, reducing motion artifact, and can create 3D images of the heart. In addition, newer machines can acquire several images at different points in the heart cycle to add a fourth dimension—time. The resulting 4D images play like short video loops of the beating heart and allow noninvasive assessment of cardiac anatomy with remarkable detail and resolution.
4D CT is increasingly being used in infective endocarditis, and growing evidence indicates that its accuracy is similar to that of TEE in the preoperative evaluation of patients with aortic prosthetic valve endocarditis.6 In a study of 28 patients, complementary use of CT angiography led to a change in treatment strategy in 7 (25%) compared with routine clinical workup.7 Several studies have found no difference between 4D CT and preoperative TEE in detecting pseudoaneurysm, abscess, or valve dehiscence. TEE and 4D CT also have similar sensitivities for detecting infective endocarditis in native and prosthetic valves.8,9
Coupled with CT angiography, 4D CT is also an excellent noninvasive way to perioperatively evaluate the coronary arteries without the risks associated with catheterization in those requiring nonemergency surgery (Figure 1A, B, and C).
4D CT performs well for detecting abscess and pseudoaneurysm but has slightly lower sensitivity for vegetations than TEE (91% vs 99%).9
Gated CT, PET, or both may be useful in cases of suspected prosthetic aortic valve endocarditis when TEE is negative. Pseudoaneurysms are not well visualized with TEE, and the atrial mitral curtain area is often thickened on TEE in cases of aortic prosthetic valve infective endocarditis that do not definitely involve abscesses. Gated CT and PET show this area better.8 This information is important in cases in which a surgeon may be unconvinced that the patient has prosthetic valve endocarditis.
Limitations of 4D cardiac CT
4D CT with or without angiography has limitations. It requires a wide-volume scanner and an experienced reader.
Patients with irregular heart rhythms or uncontrolled tachycardia pose technical problems for image acquisition. Cardiac CT is typically gated (ie, images are obtained within a defined time period) to acquire images during diastole. Ideally, images are acquired when the heart is in mid to late diastole, a time of minimal cardiac motion, so that motion artifact is minimized. To estimate the timing of image acquisition, the cardiac cycle must be predictable, and its duration should be as long as possible. Tachycardia or irregular rhythms such as frequent ectopic beats or atrial fibrillation make acquisition timing difficult, and thus make it nearly impossible to accurately obtain images when the heart is at minimum motion, limiting assessment of cardiac structures or the coronary tree.4,10
Extensive coronary calcification can hinder assessment of the coronary tree by CT coronary angiography.
Contrast exposure may limit the use of CT in some patients (eg, those with contrast allergies or renal dysfunction). However, modern scanners allow for much smaller contrast boluses without decreasing sensitivity.
4D CT involves radiation exposure, especially when done with angiography, although modern scanners have greatly reduced exposure. The average radiation dose in CT coronary angiography is 2.9 to 5.9 mSv11 compared with 7 mSv in diagnostic cardiac catheterization (without angioplasty or stenting) or 16 mSv in routine CT of the abdomen and pelvis with contrast.12,13 In view of the morbidity and mortality risks associated with infective endocarditis, especially if the diagnosis is delayed, this small radiation exposure may be justifiable.
Bottom line for cardiac CT
4D CT is an excellent alternative to echocardiography for select patients. Clinicians should strongly consider this study in the following situations:
- Patients with a prosthetic valve
- Patients who are strongly suspected of having infective endocarditis but who have a poor sonic window on TTE or TEE, as can occur with chronic obstructive lung disease, morbid obesity, or previous thoracic or cardiovascular surgery
- Patients who meet clinical indications for TEE, such as having a prosthetic valve or a high suspicion for native valve infective endocarditis with negative TTE, but who have contraindications to TEE
- As an alternative to TEE for preoperative evaluation in patients with known infective endocarditis.
Patients with tachycardia or irregular heart rhythms are not good candidates for this test.
FDG-PET AND LEUKOCYTE SCINTIGRAPHY
FDG-PET and leukocyte scintigraphy are other options for diagnosing infective endocarditis and determining the presence and extent of intra- and extracardiac infection. They are more sensitive than echocardiography for detecting infection of cardiac implanted electronic devices such as ventricular assist devices, pacemakers, implanted cardiac defibrillators, and cardiac resynchronization therapy devices.14–16
The utility of FDG-PET is founded on the uptake of 18F-fluorodeoxyglucose by cells, with higher uptake taking place in cells with higher metabolic activity (such as in areas of inflammation). Similarly, leukocyte scintigraphy relies on the use of radiolabeled leukocytes (ie, leukocytes previously extracted from the patient, labelled, and re-introduced into the patient) to allow for localization of inflamed tissue.
The most significant contribution of FDG-PET may be the ability to detect infective endocarditis early, when echocardiography is initially negative. When abnormal FDG uptake was included in the modified Duke criteria, it increased the sensitivity to 97% for detecting infective endocarditis on admission, leading some to propose its incorporation as a major criterion.17 In patients with prosthetic valves and suspected infective endocarditis, FDG-PET was found in one study to have a sensitivity of up to 91% and a specificity of up to 95%.18
Both FDG-PET and leukocyte scintigraphy have a high sensitivity, specificity, and negative predictive value for cardiac implanted electronic device infection, and should be strongly considered in patients in whom it is suspected but who have negative or inconclusive findings on echocardiography.14,15
In addition, a common conundrum faced by clinicians with use of echocardiography is the difficulty of differentiating thrombus from infected vegetation on valves or device lead wires. Some evidence indicates that FDG-PET may help to discriminate between vegetation and thrombus, although more rigorous studies are needed before its use for that purpose can be recommended.19
Limitations of nuclear studies
Both FDG-PET and leukocyte scintigraphy perform poorly for detecting native-valve infective endocarditis. In a study in which 90% of the patients had native-valve infective endocarditis according to the Duke criteria, FDG-PET had a specificity of 93% but a sensitivity of only 39%.20
Both studies can be cumbersome, laborious, and time-consuming for patients. FDG-PET requires a fasting or glucose-restricted diet before testing, and the test itself can be complicated by development of hyperglycemia, although this is rare.
While FDG-PET is most effective in detecting infections of prosthetic valves and cardiac implanted electronic devices, the results can be falsely positive in patients with a history of recent cardiac surgery (due to ongoing tissue healing), as well as maladies other than infective endocarditis that lead to inflammation, such as vasculitis or malignancy. Similarly, for unclear reasons, leukocyte scintigraphy can yield false-negative results in patients with enterococcal or candidal infective endocarditis.21
FDG-PET and leukocyte scintigraphy are more expensive than TEE and cardiac CT22 and are not widely available.
Both tests entail radiation exposure, with the average dose ranging from 7 to 14 mSv. However, this is less than the average amount acquired during percutaneous coronary intervention (16 mSv), and overlaps with the amount in chest CT with contrast when assessing for pulmonary embolism (7 to 9 mSv). Lower doses are possible with optimized protocols.12,13,15,23
Bottom line for nuclear studies
FDG-PET and leukocyte scintigraphy are especially useful for patients with a prosthetic valve or cardiac implanted electronic device. However, limitations must be kept in mind.
A suggested algorithm for testing with nuclear imaging is shown in Figure 2.1,4
CEREBRAL MAGNETIC RESONANCE IMAGING
Cerebral magnetic resonance imaging (MRI) is more sensitive than cerebral CT for detecting emboli in the brain. According to American Heart Association guidelines, cerebral MRI should be done in patients with known or suspected infective endocarditis and neurologic impairment, defined as headaches, meningeal symptoms, or neurologic deficits. It is also often used in neurologically asymptomatic patients with infective endocarditis who have indications for valve surgery to assess for mycotic aneurysms, which are associated with increased intracranial bleeding during surgery.
MRI use in other asymptomatic patients remains controversial.24 In cases with high clinical suspicion for infective endocarditis and no findings on echocardiography, cerebral MRI can increase the sensitivity of the Duke criteria by adding a minor criterion. Some have argued that, in patients with definite infective endocarditis, detecting silent cerebral complications can lead to management changes. However, more studies are needed to determine if there is indeed a group of neurologically asymptomatic infective endocarditis patients for whom cerebral MRI leads to improved outcomes.
Limitations of cerebral MRI
Cerebral MRI cannot be used in patients with non-MRI-compatible implanted hardware.
Gadolinium, the contrast agent typically used, can cause nephrogenic systemic fibrosis in patients who have poor renal function. This rare but serious adverse effect is characterized by irreversible systemic fibrosis affecting skin, muscles, and even visceral tissue such as lungs. The American College of Radiology allows for gadolinium use in patients without acute kidney injury and patients with stable chronic kidney disease with a glomerular filtration rate of at least 30 mL/min/1.73 m2. Its use should be avoided in patients with renal failure on replacement therapy, with advanced chronic kidney disease (glomerular filtration rate < 30 mL/min/1.73 m2), or with acute kidney injury, even if they do not need renal replacement therapy.25
Concerns have also been raised about gadolinium retention in the brain, even in patients with normal renal function.26–28 Thus far, no conclusive clinical adverse effects of retention have been found, although more study is warranted. Nevertheless, the US Food and Drug Administration now requires a black-box warning about this possibility and advises clinicians to counsel patients appropriately.
Bottom line on cerebral MRI
Cerebral MRI should be obtained when a patient presents with definite or possible infective endocarditis with neurologic impairment, such as new headaches, meningismus, or focal neurologic deficits. Routine brain MRI in patients with confirmed infective endocarditis without neurologic symptoms, or those without definite infective endocarditis, is discouraged.
CARDIAC MRI
Cardiac MRI, typically obtained with gadolinium contrast, allows for better 3D assessment of cardiac structures and morphology than echocardiography or CT, and can detect infiltrative cardiac disease, myopericarditis, and much more. It is increasingly used in the field of structural cardiology, but its role for evaluating infective endocarditis remains unclear.
Cardiac MRI does not appear to be better than echocardiography for diagnosing infective endocarditis. However, it may prove helpful in the evaluation of patients known to have infective endocarditis but who cannot be properly evaluated for disease extent because of poor image quality on echocardiography and contraindications to CT.1,29 Its role is limited in patients with cardiac implanted electronic devices, as most devices are incompatible with MRI use, although newer devices obviate this concern. But even for devices that are MRI-compatible, results are diminished due to an eclipsing effect, wherein the device parts can make it hard to see structures clearly because the “brightness” basically eclipses the surrounding area.4
Concerns regarding use of gadolinium as described above need also be considered.
The role of cardiac MRI in diagnosing and managing infective endocarditis may evolve, but at present, the 2017 American College of Cardiology and American Heart Association appropriate-use criteria discourage its use for these purposes.16
Bottom line for cardiac MRI
Cardiac MRI to evaluate a patient for suspected infective endocarditis is not recommended due to lack of superiority compared with echocardiography or CT, and the risk of nephrogenic systemic fibrosis from gadolinium in patients with renal compromise.
- Habib G, Lancellotti P, Antunes MJ, et al; ESC Scientific Document Group. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Durante-Mangoni E, Bradley S, Selton-Suty C, et al; International Collaboration on Endocarditis Prospective Cohort Study Group. Current features of infective endocarditis in elderly patients: results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch Intern Med 2008; 168(19):2095–2103. doi:10.1001/archinte.168.19.2095
- Wurcel AG, Anderson JE, Chui KK, et al. Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis 2016; 3(3):ofw157. doi:10.1093/ofid/ofw157
- Gomes A, Glaudemans AW, Touw DJ, et al. Diagnostic value of imaging in infective endocarditis: a systematic review. Lancet Infect Dis 2017; 17(1):e1–e14. doi:10.1016/S1473-3099(16)30141-4
- Cahill TJ, Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol 2017; 69(3):325–344. doi:10.1016/j.jacc.2016.10.066
- Fagman E, Perrotta S, Bech-Hanssen O, et al. ECG-gated computed tomography: a new role for patients with suspected aortic prosthetic valve endocarditis. Eur Radiol 2012; 22(11):2407–2414. doi:10.1007/s00330-012-2491-5
- Habets J, Tanis W, van Herwerden LA, et al. Cardiac computed tomography angiography results in diagnostic and therapeutic change in prosthetic heart valve endocarditis. Int J Cardiovasc Imaging 2014; 30(2):377–387. doi:10.1007/s10554-013-0335-2
- Koneru S, Huang SS, Oldan J, et al. Role of preoperative cardiac CT in the evaluation of infective endocarditis: comparison with transesophageal echocardiography and surgical findings. Cardiovasc Diagn Ther 2018; 8(4):439–449. doi:10.21037/cdt.2018.07.07
- Koo HJ, Yang DH, Kang J, et al. Demonstration of infective endocarditis by cardiac CT and transoesophageal echocardiography: comparison with intra-operative findings. Eur Heart J Cardiovasc Imaging 2018; 19(2):199–207. doi:10.1093/ehjci/jex010
- Feuchtner GM, Stolzmann P, Dichtl W, et al. Multislice computed tomography in infective endocarditis: comparison with transesophageal echocardiography and intraoperative findings. J Am Coll Cardiol 2009; 53(5):436–444. doi:10.1016/j.jacc.2008.01.077
- Castellano IA, Nicol ED, Bull RK, Roobottom CA, Williams MC, Harden SP. A prospective national survey of coronary CT angiography radiation doses in the United Kingdom. J Cardiovasc Comput Tomogr 2017; 11(4):268–273. doi:10.1016/j.jcct.2017.05.002
- Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 2008; 248(1):254–263. doi:10.1148/radiol.2481071451
- Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 2009; 169(22):2078–2086. doi:10.1001/archinternmed.2009.427
- Ploux S, Riviere A, Amraoui S, et al. Positron emission tomography in patients with suspected pacing system infections may play a critical role in difficult cases. Heart Rhythm 2011; 8(9):1478–1481. doi:10.1016/j.hrthm.2011.03.062
- Sarrazin J, Philippon F, Tessier M, et al. Usefulness of fluorine-18 positron emission tomography/computed tomography for identification of cardiovascular implantable electronic device infections. J Am Coll Cardiol 2012; 59(18):1616–1625. doi:10.1016/j.jacc.2011.11.059
- Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P; Rating Panel Members; Appropriate Use Criteria Task Force. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2017 Appropriate use criteria for multimodality imaging in valvular heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Nucl Cardiol 2017; 24(6):2043–2063. doi:10.1007/s12350-017-1070-1
- Saby L, Laas O, Habib G, et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol 2013; 61(23):2374–2382. doi:10.1016/j.jacc.2013.01.092
- Swart LE, Gomes A, Scholtens AM, et al. Improving the diagnostic performance of 18F-fluorodeoxyglucose positron-emission tomography/computed tomography in prosthetic heart valve endocarditis. Circulation 2018; 138(14):1412–1427. doi:10.1161/CIRCULATIONAHA.118.035032
- Graziosi M, Nanni C, Lorenzini M, et al. Role of 18F-FDG PET/CT in the diagnosis of infective endocarditis in patients with an implanted cardiac device: a prospective study. Eur J Nucl Med Mol Imaging 2014; 41(8):1617–1623. doi:10.1007/s00259-014-2773-z
- Kouijzer IJ, Vos FJ, Janssen MJ, van Dijk AP, Oyen WJ, Bleeker-Rovers CP. The value of 18F-FDG PET/CT in diagnosing infectious endocarditis. Eur J Nucl Med Mol Imaging 2013; 40(7):1102–1107. doi:10.1007/s00259-013-2376-0
- Wong D, Rubinshtein R, Keynan Y. Alternative cardiac imaging modalities to echocardiography for the diagnosis of infective endocarditis. Am J Cardiol 2016; 118(9):1410–1418. doi:10.1016/j.amjcard.2016.07.053
- Vos FJ, Bleeker-Rovers CP, Kullberg BJ, Adang EM, Oyen WJ. Cost-effectiveness of routine (18)F-FDG PET/CT in high-risk patients with gram-positive bacteremia. J Nucl Med 2011; 52(11):1673–1678. doi:10.2967/jnumed.111.089714
- McCollough CH, Bushberg JT, Fletcher JG, Eckel LJ. Answers to common questions about the use and safety of CT scans. Mayo Clin Proc 2015; 90(10):1380–1392. doi:10.1016/j.mayocp.2015.07.011
- Duval X, Iung B, Klein I, et al; IMAGE (Resonance Magnetic Imaging at the Acute Phase of Endocarditis) Study Group. Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis: a prospective study. Ann Intern Med 2010; 152(8):497–504, W175. doi:10.7326/0003-4819-152-8-201004200-00006
- ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media: 2018. www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf. Accessed July 19, 2019.
- Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015; 276(1):228–232. doi:10.1148/radiol.2015142690
- McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015; 275(3):772–782. doi:10.1148/radiol.15150025
- Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014; 270(3):834–841. doi:10.1148/radiol.13131669
- Expert Panel on Pediatric Imaging; Hayes LL, Palasis S, Bartel TB, et al. ACR appropriateness criteria headache-child. J Am Coll Radiol 2018; 15(5S):S78–S90. doi:10.1016/j.jacr.2018.03.017
- Habib G, Lancellotti P, Antunes MJ, et al; ESC Scientific Document Group. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Durante-Mangoni E, Bradley S, Selton-Suty C, et al; International Collaboration on Endocarditis Prospective Cohort Study Group. Current features of infective endocarditis in elderly patients: results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch Intern Med 2008; 168(19):2095–2103. doi:10.1001/archinte.168.19.2095
- Wurcel AG, Anderson JE, Chui KK, et al. Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis 2016; 3(3):ofw157. doi:10.1093/ofid/ofw157
- Gomes A, Glaudemans AW, Touw DJ, et al. Diagnostic value of imaging in infective endocarditis: a systematic review. Lancet Infect Dis 2017; 17(1):e1–e14. doi:10.1016/S1473-3099(16)30141-4
- Cahill TJ, Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol 2017; 69(3):325–344. doi:10.1016/j.jacc.2016.10.066
- Fagman E, Perrotta S, Bech-Hanssen O, et al. ECG-gated computed tomography: a new role for patients with suspected aortic prosthetic valve endocarditis. Eur Radiol 2012; 22(11):2407–2414. doi:10.1007/s00330-012-2491-5
- Habets J, Tanis W, van Herwerden LA, et al. Cardiac computed tomography angiography results in diagnostic and therapeutic change in prosthetic heart valve endocarditis. Int J Cardiovasc Imaging 2014; 30(2):377–387. doi:10.1007/s10554-013-0335-2
- Koneru S, Huang SS, Oldan J, et al. Role of preoperative cardiac CT in the evaluation of infective endocarditis: comparison with transesophageal echocardiography and surgical findings. Cardiovasc Diagn Ther 2018; 8(4):439–449. doi:10.21037/cdt.2018.07.07
- Koo HJ, Yang DH, Kang J, et al. Demonstration of infective endocarditis by cardiac CT and transoesophageal echocardiography: comparison with intra-operative findings. Eur Heart J Cardiovasc Imaging 2018; 19(2):199–207. doi:10.1093/ehjci/jex010
- Feuchtner GM, Stolzmann P, Dichtl W, et al. Multislice computed tomography in infective endocarditis: comparison with transesophageal echocardiography and intraoperative findings. J Am Coll Cardiol 2009; 53(5):436–444. doi:10.1016/j.jacc.2008.01.077
- Castellano IA, Nicol ED, Bull RK, Roobottom CA, Williams MC, Harden SP. A prospective national survey of coronary CT angiography radiation doses in the United Kingdom. J Cardiovasc Comput Tomogr 2017; 11(4):268–273. doi:10.1016/j.jcct.2017.05.002
- Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 2008; 248(1):254–263. doi:10.1148/radiol.2481071451
- Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 2009; 169(22):2078–2086. doi:10.1001/archinternmed.2009.427
- Ploux S, Riviere A, Amraoui S, et al. Positron emission tomography in patients with suspected pacing system infections may play a critical role in difficult cases. Heart Rhythm 2011; 8(9):1478–1481. doi:10.1016/j.hrthm.2011.03.062
- Sarrazin J, Philippon F, Tessier M, et al. Usefulness of fluorine-18 positron emission tomography/computed tomography for identification of cardiovascular implantable electronic device infections. J Am Coll Cardiol 2012; 59(18):1616–1625. doi:10.1016/j.jacc.2011.11.059
- Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P; Rating Panel Members; Appropriate Use Criteria Task Force. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2017 Appropriate use criteria for multimodality imaging in valvular heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Nucl Cardiol 2017; 24(6):2043–2063. doi:10.1007/s12350-017-1070-1
- Saby L, Laas O, Habib G, et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol 2013; 61(23):2374–2382. doi:10.1016/j.jacc.2013.01.092
- Swart LE, Gomes A, Scholtens AM, et al. Improving the diagnostic performance of 18F-fluorodeoxyglucose positron-emission tomography/computed tomography in prosthetic heart valve endocarditis. Circulation 2018; 138(14):1412–1427. doi:10.1161/CIRCULATIONAHA.118.035032
- Graziosi M, Nanni C, Lorenzini M, et al. Role of 18F-FDG PET/CT in the diagnosis of infective endocarditis in patients with an implanted cardiac device: a prospective study. Eur J Nucl Med Mol Imaging 2014; 41(8):1617–1623. doi:10.1007/s00259-014-2773-z
- Kouijzer IJ, Vos FJ, Janssen MJ, van Dijk AP, Oyen WJ, Bleeker-Rovers CP. The value of 18F-FDG PET/CT in diagnosing infectious endocarditis. Eur J Nucl Med Mol Imaging 2013; 40(7):1102–1107. doi:10.1007/s00259-013-2376-0
- Wong D, Rubinshtein R, Keynan Y. Alternative cardiac imaging modalities to echocardiography for the diagnosis of infective endocarditis. Am J Cardiol 2016; 118(9):1410–1418. doi:10.1016/j.amjcard.2016.07.053
- Vos FJ, Bleeker-Rovers CP, Kullberg BJ, Adang EM, Oyen WJ. Cost-effectiveness of routine (18)F-FDG PET/CT in high-risk patients with gram-positive bacteremia. J Nucl Med 2011; 52(11):1673–1678. doi:10.2967/jnumed.111.089714
- McCollough CH, Bushberg JT, Fletcher JG, Eckel LJ. Answers to common questions about the use and safety of CT scans. Mayo Clin Proc 2015; 90(10):1380–1392. doi:10.1016/j.mayocp.2015.07.011
- Duval X, Iung B, Klein I, et al; IMAGE (Resonance Magnetic Imaging at the Acute Phase of Endocarditis) Study Group. Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis: a prospective study. Ann Intern Med 2010; 152(8):497–504, W175. doi:10.7326/0003-4819-152-8-201004200-00006
- ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media: 2018. www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf. Accessed July 19, 2019.
- Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015; 276(1):228–232. doi:10.1148/radiol.2015142690
- McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015; 275(3):772–782. doi:10.1148/radiol.15150025
- Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014; 270(3):834–841. doi:10.1148/radiol.13131669
- Expert Panel on Pediatric Imaging; Hayes LL, Palasis S, Bartel TB, et al. ACR appropriateness criteria headache-child. J Am Coll Radiol 2018; 15(5S):S78–S90. doi:10.1016/j.jacr.2018.03.017
KEY POINTS
- Echocardiography can produce false-negative results in native-valve infective endocarditis and is even less sensitive in patients with a prosthetic valve or cardiac implanted electronic device.
- 4D CT is a reasonable alternative to transesophageal echocardiography. It can also be used as a second test if echocardiography is inconclusive. Coupled with angiography, it also provides a noninvasive method to evaluate coronary arteries perioperatively.
- Nuclear imaging tests—FDG-PET and leukocyte scintigraphy—increase the sensitivity of the Duke criteria for diagnosing infective endocarditis. They should be considered for evaluating suspected infective endocarditis in all patients who have a prosthetic valve or cardiac implanted electronic device, and whenever echocardiography is inconclusive and clinical suspicion remains high.
Deciding when a picture is worth a thousand words and several thousand dollars
In a study from the University of Pennsylvania,2 Sedrak et al surveyed residents about their lab test ordering practices. Almost all responders recognized that they ordered “unnecessary tests.” The authors of the paper probed to understand why, and strikingly, the more common responses were the same that my resident peers and I would have given 4 decades ago: the culture of the system (“We don’t want to miss anything or be asked on rounds for data that hadn’t been checked”), the lack of transparency of cost of the tests, and the lack of role-modeling by teaching staff. There has been hope that the last of these would be resolved by increased visibility of subspecialists in hospital medicine, well-versed in the nuances of system-based practice. And the Society of Hospital Medicine, along with the American College of Physicians and others, has pushed hard to promote choosing wisely when ordering diagnostic studies. But we have a way to go.
Lab tests represent a small fraction of healthcare costs. Imaging tests, especially advanced and complex imaging studies, comprise a far greater fraction of healthcare costs. And here is the challenge: developers of new imaging modalities are now able to design and refine specific tests that are good enough to become the gold standard for diagnosis and staging of specific diseases—great for clinical care, bad for cost savings. One need only review a few new guidelines or clinical research protocols to appreciate the successful integration of these tests into clinical practice. Some tests are supplanting the need for aggressive biopsies, angiography, or a series of alternative imaging tests. This is potentially good for patients, but many of these tests are strikingly expensive and are being adopted for use prior to full vetting of their utility and limitations in large clinical studies; the cost of the tests can be an impediment to conducting a series of clinical studies that include appropriate patient subsets. The increasingly proposed use of positron emission tomography in patients with suspected malignancy, inflammation, or infection is a great example of a useful test that we are still learning how best to interpret in several conditions.
In this issue of the Journal, two testing scenarios are discussed. Lacy et al address the question of when patients with pyelonephritis should receive imaging studies. There are data to guide this decision process, but as noted in the study by Sedrak et al,2 there are forces at work that challenge the clinician to bypass the rational guidelines—not the least of which are the desire for efficiency (don’t take the chance that the test may be required later and delay discharge from the hospital or observation area) and greater surety in the clinical diagnosis. Although fear of litigation was not high on Sedrak’s list of reasons for ordering more “unnecessary” tests, I posit that a decrease in the confidence placed on clinical diagnosis drives a significant amount of imaging, in conjunction with the desire for shorter hospital stays.
The second paper, by Mgbojikwe et al, relates to the issue of which advanced technology should be ordered, and when. They review the limitations of traditional (echocardiographic) diagnosis and staging of infective endocarditis, and discuss the strengths and limitations of several advanced imaging tools in the setting of suspected or known infectious endocarditis. I suspect that in most medical centers the decisions to utilize these tests will rest with the infectious disease, cardiology, and cardiothoracic surgery consultants. But it is worth being aware of how the diagnostic and staging strategies are evolving, and of the limitations to these studies.
We have come a long way from diagnosing bacterial endocarditis with a valve abscess on the basis of finding changing murmurs, a Roth spot, a palpable spleen tip, new conduction abnormalities on the ECG, and documented daily afternoon fevers. Performing that physical examination is cheap but not highly reproducible. The new testing algorithms are not cheap but, hopefully, will offer superior sensitivity and specificity. Used correctly—and we likely have a way to go to learn what that means—these pictures may well be worth the cost.
Although someone still has to suspect the diagnosis of endocarditis.
- Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA 2018; 319(10):1024–1039. doi:10.1001/jama.2018.1150
- Sedrak MS, Patel MS, Ziemba JB, et al. Residents’ self-report on why they order perceived unnecessary inpatient laboratory tests. J Hosp Med 2016; 11(12):869–872. doi:10.1002/jhm.2645
In a study from the University of Pennsylvania,2 Sedrak et al surveyed residents about their lab test ordering practices. Almost all responders recognized that they ordered “unnecessary tests.” The authors of the paper probed to understand why, and strikingly, the more common responses were the same that my resident peers and I would have given 4 decades ago: the culture of the system (“We don’t want to miss anything or be asked on rounds for data that hadn’t been checked”), the lack of transparency of cost of the tests, and the lack of role-modeling by teaching staff. There has been hope that the last of these would be resolved by increased visibility of subspecialists in hospital medicine, well-versed in the nuances of system-based practice. And the Society of Hospital Medicine, along with the American College of Physicians and others, has pushed hard to promote choosing wisely when ordering diagnostic studies. But we have a way to go.
Lab tests represent a small fraction of healthcare costs. Imaging tests, especially advanced and complex imaging studies, comprise a far greater fraction of healthcare costs. And here is the challenge: developers of new imaging modalities are now able to design and refine specific tests that are good enough to become the gold standard for diagnosis and staging of specific diseases—great for clinical care, bad for cost savings. One need only review a few new guidelines or clinical research protocols to appreciate the successful integration of these tests into clinical practice. Some tests are supplanting the need for aggressive biopsies, angiography, or a series of alternative imaging tests. This is potentially good for patients, but many of these tests are strikingly expensive and are being adopted for use prior to full vetting of their utility and limitations in large clinical studies; the cost of the tests can be an impediment to conducting a series of clinical studies that include appropriate patient subsets. The increasingly proposed use of positron emission tomography in patients with suspected malignancy, inflammation, or infection is a great example of a useful test that we are still learning how best to interpret in several conditions.
In this issue of the Journal, two testing scenarios are discussed. Lacy et al address the question of when patients with pyelonephritis should receive imaging studies. There are data to guide this decision process, but as noted in the study by Sedrak et al,2 there are forces at work that challenge the clinician to bypass the rational guidelines—not the least of which are the desire for efficiency (don’t take the chance that the test may be required later and delay discharge from the hospital or observation area) and greater surety in the clinical diagnosis. Although fear of litigation was not high on Sedrak’s list of reasons for ordering more “unnecessary” tests, I posit that a decrease in the confidence placed on clinical diagnosis drives a significant amount of imaging, in conjunction with the desire for shorter hospital stays.
The second paper, by Mgbojikwe et al, relates to the issue of which advanced technology should be ordered, and when. They review the limitations of traditional (echocardiographic) diagnosis and staging of infective endocarditis, and discuss the strengths and limitations of several advanced imaging tools in the setting of suspected or known infectious endocarditis. I suspect that in most medical centers the decisions to utilize these tests will rest with the infectious disease, cardiology, and cardiothoracic surgery consultants. But it is worth being aware of how the diagnostic and staging strategies are evolving, and of the limitations to these studies.
We have come a long way from diagnosing bacterial endocarditis with a valve abscess on the basis of finding changing murmurs, a Roth spot, a palpable spleen tip, new conduction abnormalities on the ECG, and documented daily afternoon fevers. Performing that physical examination is cheap but not highly reproducible. The new testing algorithms are not cheap but, hopefully, will offer superior sensitivity and specificity. Used correctly—and we likely have a way to go to learn what that means—these pictures may well be worth the cost.
Although someone still has to suspect the diagnosis of endocarditis.
In a study from the University of Pennsylvania,2 Sedrak et al surveyed residents about their lab test ordering practices. Almost all responders recognized that they ordered “unnecessary tests.” The authors of the paper probed to understand why, and strikingly, the more common responses were the same that my resident peers and I would have given 4 decades ago: the culture of the system (“We don’t want to miss anything or be asked on rounds for data that hadn’t been checked”), the lack of transparency of cost of the tests, and the lack of role-modeling by teaching staff. There has been hope that the last of these would be resolved by increased visibility of subspecialists in hospital medicine, well-versed in the nuances of system-based practice. And the Society of Hospital Medicine, along with the American College of Physicians and others, has pushed hard to promote choosing wisely when ordering diagnostic studies. But we have a way to go.
Lab tests represent a small fraction of healthcare costs. Imaging tests, especially advanced and complex imaging studies, comprise a far greater fraction of healthcare costs. And here is the challenge: developers of new imaging modalities are now able to design and refine specific tests that are good enough to become the gold standard for diagnosis and staging of specific diseases—great for clinical care, bad for cost savings. One need only review a few new guidelines or clinical research protocols to appreciate the successful integration of these tests into clinical practice. Some tests are supplanting the need for aggressive biopsies, angiography, or a series of alternative imaging tests. This is potentially good for patients, but many of these tests are strikingly expensive and are being adopted for use prior to full vetting of their utility and limitations in large clinical studies; the cost of the tests can be an impediment to conducting a series of clinical studies that include appropriate patient subsets. The increasingly proposed use of positron emission tomography in patients with suspected malignancy, inflammation, or infection is a great example of a useful test that we are still learning how best to interpret in several conditions.
In this issue of the Journal, two testing scenarios are discussed. Lacy et al address the question of when patients with pyelonephritis should receive imaging studies. There are data to guide this decision process, but as noted in the study by Sedrak et al,2 there are forces at work that challenge the clinician to bypass the rational guidelines—not the least of which are the desire for efficiency (don’t take the chance that the test may be required later and delay discharge from the hospital or observation area) and greater surety in the clinical diagnosis. Although fear of litigation was not high on Sedrak’s list of reasons for ordering more “unnecessary” tests, I posit that a decrease in the confidence placed on clinical diagnosis drives a significant amount of imaging, in conjunction with the desire for shorter hospital stays.
The second paper, by Mgbojikwe et al, relates to the issue of which advanced technology should be ordered, and when. They review the limitations of traditional (echocardiographic) diagnosis and staging of infective endocarditis, and discuss the strengths and limitations of several advanced imaging tools in the setting of suspected or known infectious endocarditis. I suspect that in most medical centers the decisions to utilize these tests will rest with the infectious disease, cardiology, and cardiothoracic surgery consultants. But it is worth being aware of how the diagnostic and staging strategies are evolving, and of the limitations to these studies.
We have come a long way from diagnosing bacterial endocarditis with a valve abscess on the basis of finding changing murmurs, a Roth spot, a palpable spleen tip, new conduction abnormalities on the ECG, and documented daily afternoon fevers. Performing that physical examination is cheap but not highly reproducible. The new testing algorithms are not cheap but, hopefully, will offer superior sensitivity and specificity. Used correctly—and we likely have a way to go to learn what that means—these pictures may well be worth the cost.
Although someone still has to suspect the diagnosis of endocarditis.
- Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA 2018; 319(10):1024–1039. doi:10.1001/jama.2018.1150
- Sedrak MS, Patel MS, Ziemba JB, et al. Residents’ self-report on why they order perceived unnecessary inpatient laboratory tests. J Hosp Med 2016; 11(12):869–872. doi:10.1002/jhm.2645
- Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA 2018; 319(10):1024–1039. doi:10.1001/jama.2018.1150
- Sedrak MS, Patel MS, Ziemba JB, et al. Residents’ self-report on why they order perceived unnecessary inpatient laboratory tests. J Hosp Med 2016; 11(12):869–872. doi:10.1002/jhm.2645
When does acute pyelonephritis require imaging?
A previously healthy 44-year-old woman presents to the emergency department with 1 day of fever, flank pain, dysuria, and persistent nausea and vomiting. Her temperature is 38.7°C (101.7°F), heart rate 102 beats per minute, and blood pressure 120/70 mm Hg. She has costovertebral angle tenderness. Laboratory testing reveals mild leukocytosis and a normal serum creatinine level; urinalysis shows leukocytes, as well as leukocyte esterase and nitrites. She has no personal or family history of nephrolithiasis. Urine cultures are obtained, and she is started on intravenous antibiotics and intravenous hydration to treat pyelonephritis.
Is imaging indicated at this point? And if so, which study is recommended?
KEY FEATURES
Acute pyelonephritis, infection of the renal parenchyma and collecting system, most often results from an ascending infection of the lower urinary tract. It is estimated to account for 250,000 office visits and 200,000 hospital admissions each year in the United States.1
Lower urinary tract symptoms such as urinary frequency, urgency, and dysuria accompanied by fever, nausea, vomiting, and flank pain raise suspicion for acute pyelonephritis. Flank pain is a key, nearly universal feature of upper urinary tract infection in patients without diabetes, though it may be absent in up to 50% of patients with diabetes.2
Additional findings include costovertebral angle tenderness on physical examination and leukocytosis, pyuria, and bacteriuria on laboratory studies.
PREDICTING THE NEED FOR EARLY IMAGING
Though guidelines state that imaging is inappropriate in most patients with pyelonephritis,2–4 it is nevertheless often done for diagnosis or identification of complications, which have been reported in more than two-thirds of patients.2–4
Acute pyelonephritis is generally classified as complicated or uncomplicated, though different definitions exist with regard to these classifications. The American College of Radiology’s Appropriateness Criteria2 consider patients with diabetes, immune compromise, a history of urolithiasis, or anatomic abnormality to be at highest risk for complications, and therefore recommend early imaging to assess for hydronephrosis, pyonephrosis, emphysematous pyelonephritis, and intrinsic or perinephric abscess.2
A clinical rule for predicting the need for imaging in acute pyelonephritis was developed and validated in an emergency department population in the Netherlands.3 The study suggested that restricting early imaging to patients with a history of urolithiasis, a urine pH of 7.0 or higher, or renal insufficiency—defined as a glomerular filtration rate (GFR) of 40 mL/min/1.73m2 or lower as estimated by the Modification of Diet in Renal Disease formula—would provide a negative predictive value of 94% to 100% for detection of an urgent urologic disorder (pyonephrosis, renal abscess, or urolithiasis). This high negative predictive value highlights that an absence of these signs and symptoms can safely identify patients who do not need renal imaging.
The positive predictive value was less useful, as only 5% to 23% of patients who had at least 1 risk factor went on to have urgent urologic risk factors.3
Implementation of this prediction rule would have resulted in a relative reduction in imaging of 40% and an absolute reduction of 28%. Of note, use of reduced GFR in this prediction rule is not clearly validated for patients with chronic kidney disease, as the previous GFR for most patients in this study was unknown.3
Based on these data, initial imaging is recommended in patients with diabetes, immune compromise, a history of urolithiasis, anatomic abnormality, a urine pH 7.0 or higher, or a GFR 40 mL/min or lower in a patient with no history of significant renal dysfunction. Early imaging would also be reasonable in patients with a complex clinical presentation, early recurrence of symptoms after treatment, clinical decompensation, or critical illness.
TREATMENT FAILURE
In a retrospective review of 62 patients hospitalized for acute renal infection, Soulen et al5 found that the most reliable indicator of complicated acute pyelonephritis was the persistence of fever and leukocytosis at 72 hours. And another small prospective study of patients with uncomplicated pyelonephritis reported a time to defervescence of no more than 4 days.6
In accordance with the Appropriateness Criteria2 and based on the best available evidence, imaging is recommended in all patients who remain febrile or have persistent leukocytosis after 72 hours of antibiotic therapy. In such cases, there should be high suspicion for a complication requiring treatment.
OPTIONS FOR IMAGING
Computed tomography
Computed tomography (CT) of the abdomen and pelvis with contrast is considered the study of choice in complicated acute pyelonephritis. CT can detect focal parenchymal abnormalities, emphysematous changes, and anatomic anomalies, and can also define the extent of disease. It can also detect perinephric fluid collections and abscesses that necessitate a change in management.2,5
A retrospective study in 2017 found that contrast-enhanced CT done without the usual noncontrast and excretory phases had an accuracy of 90% to 92% for pyelonephritis and 96% to 99% for urolithiasis, suggesting that reduction in radiation exposure through use of only the contrast-enhanced phase of CT imaging may be reasonable.7
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is increasingly acknowledged as effective in the evaluation of renal pathology, including the diagnosis of pyelonephritis; but it lacks the level of evidence that CT provides for detecting renal abscesses, calculi, and emphysematous pyelonephritis.2,8,9
Though it is more costly and time-consuming than CT with contrast enhancement, MRI is nevertheless the imaging study of choice if iodinated contrast or ionizing radiation must be avoided.
MRI typically involves a precontrast phase and a gadolinium contrast-enhanced phase, though there are data to support diffusion-weighted MRI when exposure to gadolinium poses a risk to the patient, such as in pregnancy or renal impairment (particularly when the estimated GFR is < 30 mL/min/1.73 m2).10
Ultrasonography
Conventional ultrasonography is appealing due to its relatively low cost, its availability and portability, and the lack of radiation and contrast exposure. It is most helpful in detecting hydronephrosis and pyonephrosis rather than intrarenal or perinephric abscess.2,9
Color and power Doppler ultrasonography may improve testing characteristics but not to the level of CT; in one study, sensitivity for detection of pyelonephritis was 33.3% with ultrasonography vs 81.0% with CT.11
Recent studies of ultrasonography with contrast enhancement show promising results,2 and it may ultimately prove to have a similar efficacy with lower risk for patients, but this has not been validated in large studies, and its availability remains limited.
Ultrasonography should be considered for patients in whom obstruction (with resulting hydronephrosis or pyonephrosis) is a primary concern, particularly when contrast exposure or radiation is contraindicated and MRI is unavailable.2
Abdominal radiography
While emphysematous pyelonephritis or a large staghorn calculus may be seen on abdominal radiography, it is not recommended for the assessment of complications in acute pyelonephritis because it lacks sensitivity.2
RETURN TO THE CASE SCENARIO
The patient in our case scenario meets the clinical criteria for uncomplicated pyelonephritis and is therefore not a candidate for imaging. Intravenous antibiotics should be started and should lead to rapid improvement in her condition.
Acknowledgment: The authors would like to thank Dr. Lisa Blacklock for her review of the radiology section of this paper.
- Foxman B, Klemstine KL, Brown PD. Acute pyelonephritis in US hospitals in 1997: hospitalization and in-hospital mortality. Ann Epidemiol 2003; 13(2):144–150. pmid:12559674
- Expert Panel on Urologic Imaging: Nikolaidis P, Dogra VS, Goldfarb S, et al. ACR appropriateness criteria acute pyelonephritis. J Am Coll Radiol 2018; 15(11S):S232–S239. doi:10.1016/j.jacr.2018.09.011
- van Nieuwkoop C, Hoppe BP, Bonten TN, et al. Predicting the need for radiologic imaging in adults with febrile urinary tract infection. Clin Infect Dis 2010; 51(11):1266–1272. doi:10.1086/657071
- Kim Y, Seo MR, Kim SJ, et al. Usefulness of blood cultures and radiologic imaging studies in the management of patients with community-acquired acute pyelonephritis. Infect Chemother 2017; 49(1):22–30. doi:10.3947/ic.2017.49.1.22
- Soulen MC, Fishman EK, Goldman SM, Gatewood OM. Bacterial renal infection: role of CT. Radiology 1989; 171(3):703–707. doi:10.1148/radiology.171.3.2655002
- June CH, Browning MD, Smith LP, et al. Ultrasonography and computed tomography in severe urinary tract infection. Arch Intern Med 1985; 145(5):841–845. pmid:3888134
- Taniguchi LS, Torres US, Souza SM, Torres LR, D’Ippolito G. Are the unenhanced and excretory CT phases necessary for the evaluation of acute pyelonephritis? Acta Radiol 2017; 58(5):634–640. doi:10.1177/0284185116665424
- Rathod SB, Kumbhar SS, Nanivadekar A, Aman K. Role of diffusion-weighted MRI in acute pyelonephritis: a prospective study. Acta Radiol 2015; 56(2):244–249. doi:10.1177/0284185114520862
- Stunell H, Buckley O, Feeney J, Geoghegan T, Browne RF, Torreggiani WC. Imaging of acute pyelonephritis in the adult. Eur Radiol 2007; 17(7):1820–1828.
- American College of Radiology. ACR Manual on Contrast Media. www.acr.org/clinical-resources/contrast-manual. Accessed June 19, 2019.
- Yoo JM, Koh JS, Han CH, et al. Diagnosing acute pyelonephritis with CT, Tc-DMSA SPECT, and Doppler ultrasound: a comparative study. Korean J Urol 2010; 51(4):260–265. doi:10.4111/kju.2010.51.4.260
A previously healthy 44-year-old woman presents to the emergency department with 1 day of fever, flank pain, dysuria, and persistent nausea and vomiting. Her temperature is 38.7°C (101.7°F), heart rate 102 beats per minute, and blood pressure 120/70 mm Hg. She has costovertebral angle tenderness. Laboratory testing reveals mild leukocytosis and a normal serum creatinine level; urinalysis shows leukocytes, as well as leukocyte esterase and nitrites. She has no personal or family history of nephrolithiasis. Urine cultures are obtained, and she is started on intravenous antibiotics and intravenous hydration to treat pyelonephritis.
Is imaging indicated at this point? And if so, which study is recommended?
KEY FEATURES
Acute pyelonephritis, infection of the renal parenchyma and collecting system, most often results from an ascending infection of the lower urinary tract. It is estimated to account for 250,000 office visits and 200,000 hospital admissions each year in the United States.1
Lower urinary tract symptoms such as urinary frequency, urgency, and dysuria accompanied by fever, nausea, vomiting, and flank pain raise suspicion for acute pyelonephritis. Flank pain is a key, nearly universal feature of upper urinary tract infection in patients without diabetes, though it may be absent in up to 50% of patients with diabetes.2
Additional findings include costovertebral angle tenderness on physical examination and leukocytosis, pyuria, and bacteriuria on laboratory studies.
PREDICTING THE NEED FOR EARLY IMAGING
Though guidelines state that imaging is inappropriate in most patients with pyelonephritis,2–4 it is nevertheless often done for diagnosis or identification of complications, which have been reported in more than two-thirds of patients.2–4
Acute pyelonephritis is generally classified as complicated or uncomplicated, though different definitions exist with regard to these classifications. The American College of Radiology’s Appropriateness Criteria2 consider patients with diabetes, immune compromise, a history of urolithiasis, or anatomic abnormality to be at highest risk for complications, and therefore recommend early imaging to assess for hydronephrosis, pyonephrosis, emphysematous pyelonephritis, and intrinsic or perinephric abscess.2
A clinical rule for predicting the need for imaging in acute pyelonephritis was developed and validated in an emergency department population in the Netherlands.3 The study suggested that restricting early imaging to patients with a history of urolithiasis, a urine pH of 7.0 or higher, or renal insufficiency—defined as a glomerular filtration rate (GFR) of 40 mL/min/1.73m2 or lower as estimated by the Modification of Diet in Renal Disease formula—would provide a negative predictive value of 94% to 100% for detection of an urgent urologic disorder (pyonephrosis, renal abscess, or urolithiasis). This high negative predictive value highlights that an absence of these signs and symptoms can safely identify patients who do not need renal imaging.
The positive predictive value was less useful, as only 5% to 23% of patients who had at least 1 risk factor went on to have urgent urologic risk factors.3
Implementation of this prediction rule would have resulted in a relative reduction in imaging of 40% and an absolute reduction of 28%. Of note, use of reduced GFR in this prediction rule is not clearly validated for patients with chronic kidney disease, as the previous GFR for most patients in this study was unknown.3
Based on these data, initial imaging is recommended in patients with diabetes, immune compromise, a history of urolithiasis, anatomic abnormality, a urine pH 7.0 or higher, or a GFR 40 mL/min or lower in a patient with no history of significant renal dysfunction. Early imaging would also be reasonable in patients with a complex clinical presentation, early recurrence of symptoms after treatment, clinical decompensation, or critical illness.
TREATMENT FAILURE
In a retrospective review of 62 patients hospitalized for acute renal infection, Soulen et al5 found that the most reliable indicator of complicated acute pyelonephritis was the persistence of fever and leukocytosis at 72 hours. And another small prospective study of patients with uncomplicated pyelonephritis reported a time to defervescence of no more than 4 days.6
In accordance with the Appropriateness Criteria2 and based on the best available evidence, imaging is recommended in all patients who remain febrile or have persistent leukocytosis after 72 hours of antibiotic therapy. In such cases, there should be high suspicion for a complication requiring treatment.
OPTIONS FOR IMAGING
Computed tomography
Computed tomography (CT) of the abdomen and pelvis with contrast is considered the study of choice in complicated acute pyelonephritis. CT can detect focal parenchymal abnormalities, emphysematous changes, and anatomic anomalies, and can also define the extent of disease. It can also detect perinephric fluid collections and abscesses that necessitate a change in management.2,5
A retrospective study in 2017 found that contrast-enhanced CT done without the usual noncontrast and excretory phases had an accuracy of 90% to 92% for pyelonephritis and 96% to 99% for urolithiasis, suggesting that reduction in radiation exposure through use of only the contrast-enhanced phase of CT imaging may be reasonable.7
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is increasingly acknowledged as effective in the evaluation of renal pathology, including the diagnosis of pyelonephritis; but it lacks the level of evidence that CT provides for detecting renal abscesses, calculi, and emphysematous pyelonephritis.2,8,9
Though it is more costly and time-consuming than CT with contrast enhancement, MRI is nevertheless the imaging study of choice if iodinated contrast or ionizing radiation must be avoided.
MRI typically involves a precontrast phase and a gadolinium contrast-enhanced phase, though there are data to support diffusion-weighted MRI when exposure to gadolinium poses a risk to the patient, such as in pregnancy or renal impairment (particularly when the estimated GFR is < 30 mL/min/1.73 m2).10
Ultrasonography
Conventional ultrasonography is appealing due to its relatively low cost, its availability and portability, and the lack of radiation and contrast exposure. It is most helpful in detecting hydronephrosis and pyonephrosis rather than intrarenal or perinephric abscess.2,9
Color and power Doppler ultrasonography may improve testing characteristics but not to the level of CT; in one study, sensitivity for detection of pyelonephritis was 33.3% with ultrasonography vs 81.0% with CT.11
Recent studies of ultrasonography with contrast enhancement show promising results,2 and it may ultimately prove to have a similar efficacy with lower risk for patients, but this has not been validated in large studies, and its availability remains limited.
Ultrasonography should be considered for patients in whom obstruction (with resulting hydronephrosis or pyonephrosis) is a primary concern, particularly when contrast exposure or radiation is contraindicated and MRI is unavailable.2
Abdominal radiography
While emphysematous pyelonephritis or a large staghorn calculus may be seen on abdominal radiography, it is not recommended for the assessment of complications in acute pyelonephritis because it lacks sensitivity.2
RETURN TO THE CASE SCENARIO
The patient in our case scenario meets the clinical criteria for uncomplicated pyelonephritis and is therefore not a candidate for imaging. Intravenous antibiotics should be started and should lead to rapid improvement in her condition.
Acknowledgment: The authors would like to thank Dr. Lisa Blacklock for her review of the radiology section of this paper.
A previously healthy 44-year-old woman presents to the emergency department with 1 day of fever, flank pain, dysuria, and persistent nausea and vomiting. Her temperature is 38.7°C (101.7°F), heart rate 102 beats per minute, and blood pressure 120/70 mm Hg. She has costovertebral angle tenderness. Laboratory testing reveals mild leukocytosis and a normal serum creatinine level; urinalysis shows leukocytes, as well as leukocyte esterase and nitrites. She has no personal or family history of nephrolithiasis. Urine cultures are obtained, and she is started on intravenous antibiotics and intravenous hydration to treat pyelonephritis.
Is imaging indicated at this point? And if so, which study is recommended?
KEY FEATURES
Acute pyelonephritis, infection of the renal parenchyma and collecting system, most often results from an ascending infection of the lower urinary tract. It is estimated to account for 250,000 office visits and 200,000 hospital admissions each year in the United States.1
Lower urinary tract symptoms such as urinary frequency, urgency, and dysuria accompanied by fever, nausea, vomiting, and flank pain raise suspicion for acute pyelonephritis. Flank pain is a key, nearly universal feature of upper urinary tract infection in patients without diabetes, though it may be absent in up to 50% of patients with diabetes.2
Additional findings include costovertebral angle tenderness on physical examination and leukocytosis, pyuria, and bacteriuria on laboratory studies.
PREDICTING THE NEED FOR EARLY IMAGING
Though guidelines state that imaging is inappropriate in most patients with pyelonephritis,2–4 it is nevertheless often done for diagnosis or identification of complications, which have been reported in more than two-thirds of patients.2–4
Acute pyelonephritis is generally classified as complicated or uncomplicated, though different definitions exist with regard to these classifications. The American College of Radiology’s Appropriateness Criteria2 consider patients with diabetes, immune compromise, a history of urolithiasis, or anatomic abnormality to be at highest risk for complications, and therefore recommend early imaging to assess for hydronephrosis, pyonephrosis, emphysematous pyelonephritis, and intrinsic or perinephric abscess.2
A clinical rule for predicting the need for imaging in acute pyelonephritis was developed and validated in an emergency department population in the Netherlands.3 The study suggested that restricting early imaging to patients with a history of urolithiasis, a urine pH of 7.0 or higher, or renal insufficiency—defined as a glomerular filtration rate (GFR) of 40 mL/min/1.73m2 or lower as estimated by the Modification of Diet in Renal Disease formula—would provide a negative predictive value of 94% to 100% for detection of an urgent urologic disorder (pyonephrosis, renal abscess, or urolithiasis). This high negative predictive value highlights that an absence of these signs and symptoms can safely identify patients who do not need renal imaging.
The positive predictive value was less useful, as only 5% to 23% of patients who had at least 1 risk factor went on to have urgent urologic risk factors.3
Implementation of this prediction rule would have resulted in a relative reduction in imaging of 40% and an absolute reduction of 28%. Of note, use of reduced GFR in this prediction rule is not clearly validated for patients with chronic kidney disease, as the previous GFR for most patients in this study was unknown.3
Based on these data, initial imaging is recommended in patients with diabetes, immune compromise, a history of urolithiasis, anatomic abnormality, a urine pH 7.0 or higher, or a GFR 40 mL/min or lower in a patient with no history of significant renal dysfunction. Early imaging would also be reasonable in patients with a complex clinical presentation, early recurrence of symptoms after treatment, clinical decompensation, or critical illness.
TREATMENT FAILURE
In a retrospective review of 62 patients hospitalized for acute renal infection, Soulen et al5 found that the most reliable indicator of complicated acute pyelonephritis was the persistence of fever and leukocytosis at 72 hours. And another small prospective study of patients with uncomplicated pyelonephritis reported a time to defervescence of no more than 4 days.6
In accordance with the Appropriateness Criteria2 and based on the best available evidence, imaging is recommended in all patients who remain febrile or have persistent leukocytosis after 72 hours of antibiotic therapy. In such cases, there should be high suspicion for a complication requiring treatment.
OPTIONS FOR IMAGING
Computed tomography
Computed tomography (CT) of the abdomen and pelvis with contrast is considered the study of choice in complicated acute pyelonephritis. CT can detect focal parenchymal abnormalities, emphysematous changes, and anatomic anomalies, and can also define the extent of disease. It can also detect perinephric fluid collections and abscesses that necessitate a change in management.2,5
A retrospective study in 2017 found that contrast-enhanced CT done without the usual noncontrast and excretory phases had an accuracy of 90% to 92% for pyelonephritis and 96% to 99% for urolithiasis, suggesting that reduction in radiation exposure through use of only the contrast-enhanced phase of CT imaging may be reasonable.7
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is increasingly acknowledged as effective in the evaluation of renal pathology, including the diagnosis of pyelonephritis; but it lacks the level of evidence that CT provides for detecting renal abscesses, calculi, and emphysematous pyelonephritis.2,8,9
Though it is more costly and time-consuming than CT with contrast enhancement, MRI is nevertheless the imaging study of choice if iodinated contrast or ionizing radiation must be avoided.
MRI typically involves a precontrast phase and a gadolinium contrast-enhanced phase, though there are data to support diffusion-weighted MRI when exposure to gadolinium poses a risk to the patient, such as in pregnancy or renal impairment (particularly when the estimated GFR is < 30 mL/min/1.73 m2).10
Ultrasonography
Conventional ultrasonography is appealing due to its relatively low cost, its availability and portability, and the lack of radiation and contrast exposure. It is most helpful in detecting hydronephrosis and pyonephrosis rather than intrarenal or perinephric abscess.2,9
Color and power Doppler ultrasonography may improve testing characteristics but not to the level of CT; in one study, sensitivity for detection of pyelonephritis was 33.3% with ultrasonography vs 81.0% with CT.11
Recent studies of ultrasonography with contrast enhancement show promising results,2 and it may ultimately prove to have a similar efficacy with lower risk for patients, but this has not been validated in large studies, and its availability remains limited.
Ultrasonography should be considered for patients in whom obstruction (with resulting hydronephrosis or pyonephrosis) is a primary concern, particularly when contrast exposure or radiation is contraindicated and MRI is unavailable.2
Abdominal radiography
While emphysematous pyelonephritis or a large staghorn calculus may be seen on abdominal radiography, it is not recommended for the assessment of complications in acute pyelonephritis because it lacks sensitivity.2
RETURN TO THE CASE SCENARIO
The patient in our case scenario meets the clinical criteria for uncomplicated pyelonephritis and is therefore not a candidate for imaging. Intravenous antibiotics should be started and should lead to rapid improvement in her condition.
Acknowledgment: The authors would like to thank Dr. Lisa Blacklock for her review of the radiology section of this paper.
- Foxman B, Klemstine KL, Brown PD. Acute pyelonephritis in US hospitals in 1997: hospitalization and in-hospital mortality. Ann Epidemiol 2003; 13(2):144–150. pmid:12559674
- Expert Panel on Urologic Imaging: Nikolaidis P, Dogra VS, Goldfarb S, et al. ACR appropriateness criteria acute pyelonephritis. J Am Coll Radiol 2018; 15(11S):S232–S239. doi:10.1016/j.jacr.2018.09.011
- van Nieuwkoop C, Hoppe BP, Bonten TN, et al. Predicting the need for radiologic imaging in adults with febrile urinary tract infection. Clin Infect Dis 2010; 51(11):1266–1272. doi:10.1086/657071
- Kim Y, Seo MR, Kim SJ, et al. Usefulness of blood cultures and radiologic imaging studies in the management of patients with community-acquired acute pyelonephritis. Infect Chemother 2017; 49(1):22–30. doi:10.3947/ic.2017.49.1.22
- Soulen MC, Fishman EK, Goldman SM, Gatewood OM. Bacterial renal infection: role of CT. Radiology 1989; 171(3):703–707. doi:10.1148/radiology.171.3.2655002
- June CH, Browning MD, Smith LP, et al. Ultrasonography and computed tomography in severe urinary tract infection. Arch Intern Med 1985; 145(5):841–845. pmid:3888134
- Taniguchi LS, Torres US, Souza SM, Torres LR, D’Ippolito G. Are the unenhanced and excretory CT phases necessary for the evaluation of acute pyelonephritis? Acta Radiol 2017; 58(5):634–640. doi:10.1177/0284185116665424
- Rathod SB, Kumbhar SS, Nanivadekar A, Aman K. Role of diffusion-weighted MRI in acute pyelonephritis: a prospective study. Acta Radiol 2015; 56(2):244–249. doi:10.1177/0284185114520862
- Stunell H, Buckley O, Feeney J, Geoghegan T, Browne RF, Torreggiani WC. Imaging of acute pyelonephritis in the adult. Eur Radiol 2007; 17(7):1820–1828.
- American College of Radiology. ACR Manual on Contrast Media. www.acr.org/clinical-resources/contrast-manual. Accessed June 19, 2019.
- Yoo JM, Koh JS, Han CH, et al. Diagnosing acute pyelonephritis with CT, Tc-DMSA SPECT, and Doppler ultrasound: a comparative study. Korean J Urol 2010; 51(4):260–265. doi:10.4111/kju.2010.51.4.260
- Foxman B, Klemstine KL, Brown PD. Acute pyelonephritis in US hospitals in 1997: hospitalization and in-hospital mortality. Ann Epidemiol 2003; 13(2):144–150. pmid:12559674
- Expert Panel on Urologic Imaging: Nikolaidis P, Dogra VS, Goldfarb S, et al. ACR appropriateness criteria acute pyelonephritis. J Am Coll Radiol 2018; 15(11S):S232–S239. doi:10.1016/j.jacr.2018.09.011
- van Nieuwkoop C, Hoppe BP, Bonten TN, et al. Predicting the need for radiologic imaging in adults with febrile urinary tract infection. Clin Infect Dis 2010; 51(11):1266–1272. doi:10.1086/657071
- Kim Y, Seo MR, Kim SJ, et al. Usefulness of blood cultures and radiologic imaging studies in the management of patients with community-acquired acute pyelonephritis. Infect Chemother 2017; 49(1):22–30. doi:10.3947/ic.2017.49.1.22
- Soulen MC, Fishman EK, Goldman SM, Gatewood OM. Bacterial renal infection: role of CT. Radiology 1989; 171(3):703–707. doi:10.1148/radiology.171.3.2655002
- June CH, Browning MD, Smith LP, et al. Ultrasonography and computed tomography in severe urinary tract infection. Arch Intern Med 1985; 145(5):841–845. pmid:3888134
- Taniguchi LS, Torres US, Souza SM, Torres LR, D’Ippolito G. Are the unenhanced and excretory CT phases necessary for the evaluation of acute pyelonephritis? Acta Radiol 2017; 58(5):634–640. doi:10.1177/0284185116665424
- Rathod SB, Kumbhar SS, Nanivadekar A, Aman K. Role of diffusion-weighted MRI in acute pyelonephritis: a prospective study. Acta Radiol 2015; 56(2):244–249. doi:10.1177/0284185114520862
- Stunell H, Buckley O, Feeney J, Geoghegan T, Browne RF, Torreggiani WC. Imaging of acute pyelonephritis in the adult. Eur Radiol 2007; 17(7):1820–1828.
- American College of Radiology. ACR Manual on Contrast Media. www.acr.org/clinical-resources/contrast-manual. Accessed June 19, 2019.
- Yoo JM, Koh JS, Han CH, et al. Diagnosing acute pyelonephritis with CT, Tc-DMSA SPECT, and Doppler ultrasound: a comparative study. Korean J Urol 2010; 51(4):260–265. doi:10.4111/kju.2010.51.4.260
Do probiotics reduce C diff risk in hospitalized patients?
ILLUSTRATIVE CASE
A 68-year-old woman is admitted to the hospital with a diagnosis of community-acquired pneumonia. Should you add probiotics to her antibiotic regimen to prevent infection with Clostridium difficile?
Clostridium difficile infection (CDI) leads to significant morbidity, mortality, and treatment failures. In 2011, it culminated in a cost of $4.8 billion and 29,000 deaths.2,3 Risk factors for infection include antibiotic use, hospitalization, older age, and medical comorbidities.2 Probiotics have been proposed as one way to prevent CDI.
While several systematic reviews have demonstrated efficacy for probiotics in the prevention of CDI,4-6 guidelines from the American College of Gastroenterology and the Society for Healthcare Epidemiology of America did not incorporate a recommendation for the use of probiotics in their CDI prevention strategy.7,8
The PLACIDE trial studied the use of probiotics in inpatients ages ≥ 65 years receiving either oral or parenteral antibiotics and found no difference in the incidence of CDI in those who received probiotics vs those who did not.9 Even though the PLACIDE trial was the largest, high-quality, randomized controlled trial (RCT) on the use of probiotics to prevent CDI, it had a lower incidence of CDI than was assumed in the power calculations. Additionally, previous systematic reviews did not always follow the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and did not focus specifically on hospitalized patients, who are at higher risk for CDI.
Given the conflicting and poor evidence and recommendations, an additional systematic review and meta-analysis was performed following PRISMA guidelines and focusing on studies conducted only on hospitalized adults.
STUDY SUMMARY
Probiotics prevent CDI in hospitalized patients receiving antibiotics
This meta-analysis of 19 RCTs evaluated the efficacy of probiotics for the prevention of CDI in 6261 adult hospitalized patients taking antibiotics. All patients were ≥ 18 years (mean age 68-69 years) and received antibiotics orally, intravenously, or via both routes for any medical indication.
Trials were included if the intervention was for CDI prevention and if the probiotics used were 1 or a combination of 4 strains (Lactobacillus, Saccharomyces, Bifidobacterium, Streptococcus). Probiotic doses ranged from 4 billion to 900 billion colony-forming u/day and were started from 1 to 7 days after first antibiotic dose. Duration of probiotic use was either fixed at between 14 and 21 days or varied based on the duration of antibiotics (extending 3-14 days after the last antibiotic dose).
Continue to: Control groups received...
Control groups received matching placebo in all trials but 2; those 2 used usual care of no probiotics as the control. Common patient exclusions were pregnancy, immune system compromise, intensive care, a prosthetic heart valve, and pre-existing gastrointestinal disorders.
The risk for CDI was lower in the probiotic group (range 0%-11%) than in the control group (0%-40%) with no heterogeneity (I2 = 0.0%; P = .56) when the data were pooled from all 19 studies (relative risk [RR] = 0.42; 95% confidence interval [CI], 0.30-0.57). The median incidence of CDI in the control groups from all studies was 4%, which yielded a number needed to treat (NNT) of 43 (95% CI, 36-58).
The researchers examined the NNT at varying incidence rates. If the incidence of CDI was 1.2%, the NNT to prevent 1 case of CDI was 144, and if the incidence was 7.4%, the NNT was 23. Compared with control groups, there was a significant reduction in CDI if probiotics were started within 1 to 2 days of antibiotic initiation (RR = 0.32; 95% CI, 0.22-0.48), but not if they were started at 3 to 7 days (RR = 0.70; 95% CI, 0.40-1.2). There was no significant difference in adverse events (ie, cramping, nausea, fever, soft stools, flatulence, taste disturbance) between probiotic and control groups (14% vs 16%; P = .35).
WHAT’S NEW
Probiotics provide added benefit if taken sooner rather than later
This high-quality meta-analysis shows that administration of probiotics to hospitalized patients—particularly when started within 1 to 2 days of initiating antibiotic therapy—can prevent CDI.
CAVEATS
Findings do not apply to all patients; specific recommendations are lacking
Findings from this meta-analysis do not apply to patients who have an immunocompromising condition, are pregnant, have a prosthetic heart valve, have a pre-existing gastrointestinal disorder (eg, irritable bowel disease, pancreatitis), or require intensive care. In addition, specific recommendations as to the optimal probiotic species, dose, formulation, and duration of use cannot be made based on this meta-analysis. Lastly, findings from this study do not apply to patients treated with antibiotics in the ambulatory care setting.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Lack of “medication” status leads to limited availability in hospitals
The largest barrier to giving probiotics to hospitalized adult patients is the availability of probiotics on local hospital formularies. Probiotics are not technically a medication; they are not regulated or approved by the US Food and Drug Administration and thus, insurance coverage and availability for inpatient use are limited. Lastly, US cost-effectiveness data are lacking, although such data would likely be favorable given the high costs associated with treatment of CDI.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152:1889-1900.e9.
2. Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis. 2015;60(Suppl 2):S66-S71.
3. Lessa FC, Winston LG, McDonald LC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:2369-2370.
4. Goldenberg JZ, Yap C, Lytvyn L. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
5. Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016:22:27-37.
6. Johnston BC, Goldenberg JZ, Guyatt GH. Probiotics for the prevention of Clostridium difficile–associated diarrhea. In response. Ann Intern Med. 2013;158:706-707.
7. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498.
8. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
9. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
ILLUSTRATIVE CASE
A 68-year-old woman is admitted to the hospital with a diagnosis of community-acquired pneumonia. Should you add probiotics to her antibiotic regimen to prevent infection with Clostridium difficile?
Clostridium difficile infection (CDI) leads to significant morbidity, mortality, and treatment failures. In 2011, it culminated in a cost of $4.8 billion and 29,000 deaths.2,3 Risk factors for infection include antibiotic use, hospitalization, older age, and medical comorbidities.2 Probiotics have been proposed as one way to prevent CDI.
While several systematic reviews have demonstrated efficacy for probiotics in the prevention of CDI,4-6 guidelines from the American College of Gastroenterology and the Society for Healthcare Epidemiology of America did not incorporate a recommendation for the use of probiotics in their CDI prevention strategy.7,8
The PLACIDE trial studied the use of probiotics in inpatients ages ≥ 65 years receiving either oral or parenteral antibiotics and found no difference in the incidence of CDI in those who received probiotics vs those who did not.9 Even though the PLACIDE trial was the largest, high-quality, randomized controlled trial (RCT) on the use of probiotics to prevent CDI, it had a lower incidence of CDI than was assumed in the power calculations. Additionally, previous systematic reviews did not always follow the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and did not focus specifically on hospitalized patients, who are at higher risk for CDI.
Given the conflicting and poor evidence and recommendations, an additional systematic review and meta-analysis was performed following PRISMA guidelines and focusing on studies conducted only on hospitalized adults.
STUDY SUMMARY
Probiotics prevent CDI in hospitalized patients receiving antibiotics
This meta-analysis of 19 RCTs evaluated the efficacy of probiotics for the prevention of CDI in 6261 adult hospitalized patients taking antibiotics. All patients were ≥ 18 years (mean age 68-69 years) and received antibiotics orally, intravenously, or via both routes for any medical indication.
Trials were included if the intervention was for CDI prevention and if the probiotics used were 1 or a combination of 4 strains (Lactobacillus, Saccharomyces, Bifidobacterium, Streptococcus). Probiotic doses ranged from 4 billion to 900 billion colony-forming u/day and were started from 1 to 7 days after first antibiotic dose. Duration of probiotic use was either fixed at between 14 and 21 days or varied based on the duration of antibiotics (extending 3-14 days after the last antibiotic dose).
Continue to: Control groups received...
Control groups received matching placebo in all trials but 2; those 2 used usual care of no probiotics as the control. Common patient exclusions were pregnancy, immune system compromise, intensive care, a prosthetic heart valve, and pre-existing gastrointestinal disorders.
The risk for CDI was lower in the probiotic group (range 0%-11%) than in the control group (0%-40%) with no heterogeneity (I2 = 0.0%; P = .56) when the data were pooled from all 19 studies (relative risk [RR] = 0.42; 95% confidence interval [CI], 0.30-0.57). The median incidence of CDI in the control groups from all studies was 4%, which yielded a number needed to treat (NNT) of 43 (95% CI, 36-58).
The researchers examined the NNT at varying incidence rates. If the incidence of CDI was 1.2%, the NNT to prevent 1 case of CDI was 144, and if the incidence was 7.4%, the NNT was 23. Compared with control groups, there was a significant reduction in CDI if probiotics were started within 1 to 2 days of antibiotic initiation (RR = 0.32; 95% CI, 0.22-0.48), but not if they were started at 3 to 7 days (RR = 0.70; 95% CI, 0.40-1.2). There was no significant difference in adverse events (ie, cramping, nausea, fever, soft stools, flatulence, taste disturbance) between probiotic and control groups (14% vs 16%; P = .35).
WHAT’S NEW
Probiotics provide added benefit if taken sooner rather than later
This high-quality meta-analysis shows that administration of probiotics to hospitalized patients—particularly when started within 1 to 2 days of initiating antibiotic therapy—can prevent CDI.
CAVEATS
Findings do not apply to all patients; specific recommendations are lacking
Findings from this meta-analysis do not apply to patients who have an immunocompromising condition, are pregnant, have a prosthetic heart valve, have a pre-existing gastrointestinal disorder (eg, irritable bowel disease, pancreatitis), or require intensive care. In addition, specific recommendations as to the optimal probiotic species, dose, formulation, and duration of use cannot be made based on this meta-analysis. Lastly, findings from this study do not apply to patients treated with antibiotics in the ambulatory care setting.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Lack of “medication” status leads to limited availability in hospitals
The largest barrier to giving probiotics to hospitalized adult patients is the availability of probiotics on local hospital formularies. Probiotics are not technically a medication; they are not regulated or approved by the US Food and Drug Administration and thus, insurance coverage and availability for inpatient use are limited. Lastly, US cost-effectiveness data are lacking, although such data would likely be favorable given the high costs associated with treatment of CDI.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 68-year-old woman is admitted to the hospital with a diagnosis of community-acquired pneumonia. Should you add probiotics to her antibiotic regimen to prevent infection with Clostridium difficile?
Clostridium difficile infection (CDI) leads to significant morbidity, mortality, and treatment failures. In 2011, it culminated in a cost of $4.8 billion and 29,000 deaths.2,3 Risk factors for infection include antibiotic use, hospitalization, older age, and medical comorbidities.2 Probiotics have been proposed as one way to prevent CDI.
While several systematic reviews have demonstrated efficacy for probiotics in the prevention of CDI,4-6 guidelines from the American College of Gastroenterology and the Society for Healthcare Epidemiology of America did not incorporate a recommendation for the use of probiotics in their CDI prevention strategy.7,8
The PLACIDE trial studied the use of probiotics in inpatients ages ≥ 65 years receiving either oral or parenteral antibiotics and found no difference in the incidence of CDI in those who received probiotics vs those who did not.9 Even though the PLACIDE trial was the largest, high-quality, randomized controlled trial (RCT) on the use of probiotics to prevent CDI, it had a lower incidence of CDI than was assumed in the power calculations. Additionally, previous systematic reviews did not always follow the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and did not focus specifically on hospitalized patients, who are at higher risk for CDI.
Given the conflicting and poor evidence and recommendations, an additional systematic review and meta-analysis was performed following PRISMA guidelines and focusing on studies conducted only on hospitalized adults.
STUDY SUMMARY
Probiotics prevent CDI in hospitalized patients receiving antibiotics
This meta-analysis of 19 RCTs evaluated the efficacy of probiotics for the prevention of CDI in 6261 adult hospitalized patients taking antibiotics. All patients were ≥ 18 years (mean age 68-69 years) and received antibiotics orally, intravenously, or via both routes for any medical indication.
Trials were included if the intervention was for CDI prevention and if the probiotics used were 1 or a combination of 4 strains (Lactobacillus, Saccharomyces, Bifidobacterium, Streptococcus). Probiotic doses ranged from 4 billion to 900 billion colony-forming u/day and were started from 1 to 7 days after first antibiotic dose. Duration of probiotic use was either fixed at between 14 and 21 days or varied based on the duration of antibiotics (extending 3-14 days after the last antibiotic dose).
Continue to: Control groups received...
Control groups received matching placebo in all trials but 2; those 2 used usual care of no probiotics as the control. Common patient exclusions were pregnancy, immune system compromise, intensive care, a prosthetic heart valve, and pre-existing gastrointestinal disorders.
The risk for CDI was lower in the probiotic group (range 0%-11%) than in the control group (0%-40%) with no heterogeneity (I2 = 0.0%; P = .56) when the data were pooled from all 19 studies (relative risk [RR] = 0.42; 95% confidence interval [CI], 0.30-0.57). The median incidence of CDI in the control groups from all studies was 4%, which yielded a number needed to treat (NNT) of 43 (95% CI, 36-58).
The researchers examined the NNT at varying incidence rates. If the incidence of CDI was 1.2%, the NNT to prevent 1 case of CDI was 144, and if the incidence was 7.4%, the NNT was 23. Compared with control groups, there was a significant reduction in CDI if probiotics were started within 1 to 2 days of antibiotic initiation (RR = 0.32; 95% CI, 0.22-0.48), but not if they were started at 3 to 7 days (RR = 0.70; 95% CI, 0.40-1.2). There was no significant difference in adverse events (ie, cramping, nausea, fever, soft stools, flatulence, taste disturbance) between probiotic and control groups (14% vs 16%; P = .35).
WHAT’S NEW
Probiotics provide added benefit if taken sooner rather than later
This high-quality meta-analysis shows that administration of probiotics to hospitalized patients—particularly when started within 1 to 2 days of initiating antibiotic therapy—can prevent CDI.
CAVEATS
Findings do not apply to all patients; specific recommendations are lacking
Findings from this meta-analysis do not apply to patients who have an immunocompromising condition, are pregnant, have a prosthetic heart valve, have a pre-existing gastrointestinal disorder (eg, irritable bowel disease, pancreatitis), or require intensive care. In addition, specific recommendations as to the optimal probiotic species, dose, formulation, and duration of use cannot be made based on this meta-analysis. Lastly, findings from this study do not apply to patients treated with antibiotics in the ambulatory care setting.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Lack of “medication” status leads to limited availability in hospitals
The largest barrier to giving probiotics to hospitalized adult patients is the availability of probiotics on local hospital formularies. Probiotics are not technically a medication; they are not regulated or approved by the US Food and Drug Administration and thus, insurance coverage and availability for inpatient use are limited. Lastly, US cost-effectiveness data are lacking, although such data would likely be favorable given the high costs associated with treatment of CDI.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152:1889-1900.e9.
2. Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis. 2015;60(Suppl 2):S66-S71.
3. Lessa FC, Winston LG, McDonald LC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:2369-2370.
4. Goldenberg JZ, Yap C, Lytvyn L. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
5. Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016:22:27-37.
6. Johnston BC, Goldenberg JZ, Guyatt GH. Probiotics for the prevention of Clostridium difficile–associated diarrhea. In response. Ann Intern Med. 2013;158:706-707.
7. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498.
8. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
9. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
1. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152:1889-1900.e9.
2. Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis. 2015;60(Suppl 2):S66-S71.
3. Lessa FC, Winston LG, McDonald LC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:2369-2370.
4. Goldenberg JZ, Yap C, Lytvyn L. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
5. Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016:22:27-37.
6. Johnston BC, Goldenberg JZ, Guyatt GH. Probiotics for the prevention of Clostridium difficile–associated diarrhea. In response. Ann Intern Med. 2013;158:706-707.
7. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498.
8. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
9. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
PRACTICE CHANGER
Start probiotics within 1 to 2 days of starting antibiotics in hospitalized patients to reduce the risk of Clostridium difficile infection.1
STRENGTH OF RECOMMENDATION
A: Based on a meta-analysis of randomized controlled trials.
Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152:1889-1900. e9.
Facts to help you keep pace with the vaccine conversation
The current increase in measles cases in the United States has sharpened the focus on antivaccine activities. While the percentage of US children who are fully vaccinated remains high (≥ 94%), the number of un- or undervaccinated children has been growing1 because of nonmedical exemptions from school vaccine requirements due to concerns about vaccine safety and an underappreciation of the benefits of vaccines. Family physicians need to be conversant with several important aspects of this matter, including the magnitude of benefits provided by childhood vaccines, as well as the systems already in place for
- assessing vaccine effectiveness and safety,
- making recommendations on the use of vaccines,
- monitoring safety after vaccine approval, and
- compensating those affected by rare but serious vaccine-related adverse events (AEs).
Familiarity with these issues will allow for informed discussions with parents who are vaccine hesitant and with those who have read or heard inaccurate information.
The benefits of vaccines are indisputable
In 1999, the Centers for Disease Control and Prevention (CDC) published a list of 9 selected childhood infectious diseases and compared their incidences before and after immunization was available.2 Each of these infections causes morbidity, sequelae, and mortality at predictable rates depending on the infectious agent. The comparisons were dramatic: Measles, with a baseline annual morbidity of 503,282 cases, fell to just 89 cases; poliomyelitis decreased from 16,316 to 0; and Haemophilus influenzae type b declined from 20,000 to 54. In a 2014 analysis, the CDC stated that “among 78.6 million children born during 1994–2013, routine childhood immunization was estimated to prevent 322 million illnesses (averaging 4.1 illnesses per child) and 21 million hospitalizations (0.27 per child) over the course of their lifetimes and avert 732,000 premature deaths from vaccine-preventable illnesses” (TABLE).3
It is not unusual to hear a vaccine opponent say that childhood infectious diseases are not serious and that it is better for a child to contract the infection and let the immune system fight it naturally. Measles is often used as an example. This argument ignores some important aspects of vaccine benefits.
It is true in the United States that the average child who contracts measles will recover from it and not suffer immediate or long-term effects. However, it is also true that measles has a hospitalization rate of about 20% and a death rate of between 1/500 and 1/1000 cases.4 Mortality is much higher in developing countries. Prior to widespread use of measles vaccine, hundreds of thousands of cases of measles occurred each year. That translated into hundreds of preventable child deaths per year. An individual case does not tell the full story about the public health impact of infectious illnesses.
In addition, there are often unappreciated sequelae from child infections, such as shingles occurring years after resolution of a chickenpox infection. There are also societal consequences of child infections, such as deafness from congenital rubella and intergenerational transfer of infectious agents to family members at risk for serious consequences (influenza from a child to a grandparent). Finally, infected children pose a risk to those who cannot be vaccinated because of immune deficiencies and other medical conditions.
A multilayered US system monitors vaccine safety
Responsibility for assuring the safety of vaccines lies with the US Food and Drug Administration (FDA) Center for Biologics Evaluation and Research and with the CDC’s Immunization Safety Office (ISO). The FDA is responsible for the initial assessment of the effectiveness and safety of new vaccines and for ongoing monitoring of the manufacturing facilities where vaccines are produced. After FDA approval, safety is monitored using a multilayered system that includes the Vaccine Adverse Event Reporting System (VAERS), the Vaccine Safety Datalink (VSD) system, the Clinical Immunization Safety Assessment (CISA) Project, and periodic reviews by the National Academy of Medicine (NAM), previously the Institute of Medicine. In addition, there is a large number of studies published each year by the nation’s—and world’s—medical research community on vaccine effectiveness and safety.
Continue to: VAERS
VAERS (https://vaers.hhs.gov/) is a passive reporting system that allows patients, physicians, and other health care providers to record suspected vaccine-related adverse events.5 It was created in 1990 and is run by the FDA and the CDC. It is not intended to be a comprehensive or definitive list of proven vaccine-related harms. As a passive reporting system, it is subject to both over- and underreporting, and the data from it are often misinterpreted and used incorrectly by vaccine opponents—eg, wrongly declaring that VAERS reports of possible AEs are proven cases. It provides a sentinel system that is monitored for indications of possible serious AEs linked to a particular vaccine. When a suspected interaction is detected, it is investigated by the VSD system.
VSD is a collaboration of the CDC’s ISO and 8 geographically distributed health care organizations with complete electronic patient medical information on their members. VSD conducts studies when a question about vaccine safety arises, when new vaccines are licensed, or when there are new vaccine recommendations. A description of VSD sites, the research methods used, and a list of publications describing study results can be found at https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vsd/index.html#organizations. If the VSD system finds a link between serious AEs and a particular vaccine, this association is reported to the Advisory Committee on Immunization Practices (ACIP) for consideration in changing recommendations regarding that vaccine. This happens only rarely.
CISA was established in 2001 as a network of vaccine safety experts at 7 academic medical centers who collaborate with the CDC’s ISO. CISA conducts studies on specific questions related to vaccine safety and provides a consultation service to clinicians and researchers who have questions about vaccine safety. A description of the CISA sites, past publications on vaccine safety, and ongoing research priorities can be found at https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/cisa/index.html.
NAM (https://nam.edu/) conducts periodic reviews of vaccine safety and vaccine-caused AEs. The most recent was published in 2012 and looked at possible AEs of 8 vaccines containing 12 different antigens.6 The literature search for this review found more than 12,000 articles, which speaks to the volume of scientific work on vaccine safety. These NAM reports document the rarity of severe AEs to vaccines and are used with other information to construct the table for the Vaccine Injury Compensation Program (VICP), which is described below.
Are vaccines killing children?
Vaccine opponents frequently claim that vaccines cause much more harm than is documented, including the deaths of children. A vaccine opponent made this claim in my state (Arizona) at a legislative committee hearing even though our state child mortality review committee has been investigating all child deaths for decades and has never attributed a death to a vaccine.
Continue to: One study conducted...
One study conducted using the VSD system from January 1, 2005, to December 31, 2011, identified 1100 deaths occurring within 12 months of any vaccination among 2,189,504 VSD enrollees ages 9 to 26 years.7 They found that the risk of death in this age group was not increased during the 30 days after vaccination, and no deaths were found to be causally associated with vaccination. Deaths among children do occur and, due to the number of vaccines administered, some deaths will occur within a short time period after a vaccine. This temporal association does not prove the death was vaccine-caused, but vaccine opponents have claimed that it does.
The vaccine injury compensation system
In 1986, the federal government established a no-fault system—the National Vaccine Injury Compensation Program (VICP)—to compensate those who suffer a serious AE from a vaccine covered by the program. This system is administered by the Health Resources and Services Administration (HRSA) in the Department of Health and Human Services (DHHS). HRSA maintains a table of proven AEs of specific vaccines, based in part on the NAM report mentioned earlier. Petitions for compensation—with proof of an AE following the administration of a vaccine that is included on the HRSA table—are accepted and remunerated if the AE lasted > 6 months or resulted in hospitalization. Petitions that allege AEs following administration of a vaccine not included on the table are nevertheless reviewed by the staff of HRSA, who can still recommend compensation based on the medical evidence. If HRSA declines the petition, the petitioner can appeal the case in the US Court of Federal Claims, which makes the final decision on a petition’s validity and, if warranted, the type and amount of compensation.
From 2006 to 2017, > 3.4 billion doses of vaccines covered by VICP were distributed in the United States.8 During this period, 6293 petitions were adjudicated by the court; 4311 were compensated.8 For every 1 million doses of vaccine distributed, 1 individual was compensated. Seventy percent of these compensations were awarded to petitioners despite a lack of clear evidence that the patient’s condition was caused by a vaccine.8 The rate of compensation for conditions proven to be caused by a vaccine was 1/3.33 million.8
The VICP pays for attorney fees, in some cases even if the petition is denied, but does not allow contingency fees. Since the beginning of the program, more than $4 billion has been awarded.8 The program is funded by a 75-cent tax on each vaccine antigen. Because serious AEs are so rare, the trust fund established to administer the VICP finances has a surplus of about $6 billion.
The Advisory Committee on Immunization Practices
After a vaccine is approved for use by the FDA, ACIP makes recommendations for its use in the US civilian population.9,10 ACIP, created in 1964, was chartered as a federal advisory committee to provide expert external advice to the Director of the CDC and the Secretary of DHHS on the use of vaccines
Continue to: As an official...
As an official federal advisory committee governed by the Federal Advisory Committee Act, ACIP operates under strict requirements for public notification of meetings, allowing for written and oral public comment at its meetings, and timely publication of minutes. ACIP meeting minutes are posted soon after each meeting, along with draft recommendations. ACIP meeting agendas and slide presentations are available on the ACIP Web site (https://www.cdc.gov/vaccines/acip/index.html).
ACIP consists of 15 members serving overlapping 4-year terms, appointed by the Secretary of DHHS from a list of candidates proposed by the CDC. One member is a consumer representative; the other members have expertise in vaccinology, immunology, pediatrics, internal medicine, infectious diseases, preventive medicine, and public health. In the CDC, staff support for ACIP is provided by the National Center for Immunization and Respiratory Diseases, Office of Infectious Diseases.
ACIP holds 2-day meetings 3 times a year. Much of the work occurs between meetings, by work groups via phone conferences. Work groups are chaired by an ACIP member and staffed by one or more CDC programmatic, content-expert professionals. Membership of the work groups consists of at least 2 ACIP members, representatives from relevant professional clinical and public health organizations, and other individuals with specific expertise. Work groups propose recommendations to ACIP, which can adopt, revise, or reject them.
When formulating recommendations for a particular vaccine, ACIP considers the burden of disease prevented, the effectiveness and safety of the vaccine, cost effectiveness, and practical and logistical issues of implementing recommendations. ACIP also receives frequent reports from ISO regarding the safety of vaccines previously approved. Since 2011, ACIP has used a standardized, modified GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system to assess the evidence regarding effectiveness and safety of new vaccines and an evidence-to-recommendation framework to transparently explain how it arrives at recommendations.11,12
We can recommend vaccines with confidence
In the United States, we have a secure supply of safe vaccines, a transparent method of making vaccine recommendations, a robust system to monitor vaccine safety, and an efficient system to compensate those who experience a rare, serious adverse reaction to a vaccine. The US public health system has achieved a marked reduction in morbidity and mortality from childhood infectious diseases, mostly because of vaccines. Many people today have not experienced or seen children with these once-common childhood infections and may not appreciate the seriousness of childhood infectious diseases or the full value of vaccines. As family physicians, we can help address this problem and recommend vaccines to our patients with confidence.
1. Mellerson JL, Maxwell CB, Knighton CL, et al. Vaccine coverage for selected vaccines and exemption rates among children in kindergarten—United States, 2017-18 school year. MMWR Morb Mortal Wkly Rep. 2018;67:1115-1122.
2. CDC. Ten great public health achievements—United States, 1900-1999. MMWR Morb Mortal Wkly Rep. 1999;48:241-243.
3. Whitney CG, Zhou F, Singleton J, et al. Benefits from immunization during the Vaccines for Children Program era—United States, 1994-2013. MMWR Morb Mortal Wkly Rep. 2014;63:352-355.
4. CDC. Complications of measles. https://www.cdc.gov/measles/symptoms/complications.html. Accessed July 16, 2019.
5. Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33:4398-4405.
6. IOM (Institute of Medicine). Adverse Effects of Vaccines: Evidence and Causality. Washington, DC: The National Academies Press; 2012.
7. McCarthy NL, Gee J, Sukumaran L, et al. Vaccination and 30-day mortality risk in children, adolescents, and young adults. Pediatrics. 2016;137:1-8.
8. HRSA. Data and Statistics. https://www.hrsa.gov/sites/default/files/hrsa/vaccine-compensation/data/monthly-stats-may-2019.pdf. Accessed July 16, 2019.
9. Pickering LK, Orenstein WA, Sun W, et al. FDA licensure of and ACIP recommendations for vaccines. Vaccine. 2017;37:5027-5036.
10. Smith JC, Snider DE, Pickering LK. Immunization policy development in the United States: the role of the Advisory Committee on Immunization Practices. Ann Intern Med. 2009;150:45-49.
11. Ahmed F, Temte JL, Campos-Outcalt D, et al; for the ACIP Evidence Based Recommendations Work Group (EBRWG). Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the U.S. Centers for Disease Control and Prevention (CDC). Vaccine. 2011;29:9171-9176.
12. Lee G, Carr W. Updated framework for development of evidence-based recommendations by the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018:76:1271-1272.
The current increase in measles cases in the United States has sharpened the focus on antivaccine activities. While the percentage of US children who are fully vaccinated remains high (≥ 94%), the number of un- or undervaccinated children has been growing1 because of nonmedical exemptions from school vaccine requirements due to concerns about vaccine safety and an underappreciation of the benefits of vaccines. Family physicians need to be conversant with several important aspects of this matter, including the magnitude of benefits provided by childhood vaccines, as well as the systems already in place for
- assessing vaccine effectiveness and safety,
- making recommendations on the use of vaccines,
- monitoring safety after vaccine approval, and
- compensating those affected by rare but serious vaccine-related adverse events (AEs).
Familiarity with these issues will allow for informed discussions with parents who are vaccine hesitant and with those who have read or heard inaccurate information.
The benefits of vaccines are indisputable
In 1999, the Centers for Disease Control and Prevention (CDC) published a list of 9 selected childhood infectious diseases and compared their incidences before and after immunization was available.2 Each of these infections causes morbidity, sequelae, and mortality at predictable rates depending on the infectious agent. The comparisons were dramatic: Measles, with a baseline annual morbidity of 503,282 cases, fell to just 89 cases; poliomyelitis decreased from 16,316 to 0; and Haemophilus influenzae type b declined from 20,000 to 54. In a 2014 analysis, the CDC stated that “among 78.6 million children born during 1994–2013, routine childhood immunization was estimated to prevent 322 million illnesses (averaging 4.1 illnesses per child) and 21 million hospitalizations (0.27 per child) over the course of their lifetimes and avert 732,000 premature deaths from vaccine-preventable illnesses” (TABLE).3

It is not unusual to hear a vaccine opponent say that childhood infectious diseases are not serious and that it is better for a child to contract the infection and let the immune system fight it naturally. Measles is often used as an example. This argument ignores some important aspects of vaccine benefits.
It is true in the United States that the average child who contracts measles will recover from it and not suffer immediate or long-term effects. However, it is also true that measles has a hospitalization rate of about 20% and a death rate of between 1/500 and 1/1000 cases.4 Mortality is much higher in developing countries. Prior to widespread use of measles vaccine, hundreds of thousands of cases of measles occurred each year. That translated into hundreds of preventable child deaths per year. An individual case does not tell the full story about the public health impact of infectious illnesses.
In addition, there are often unappreciated sequelae from child infections, such as shingles occurring years after resolution of a chickenpox infection. There are also societal consequences of child infections, such as deafness from congenital rubella and intergenerational transfer of infectious agents to family members at risk for serious consequences (influenza from a child to a grandparent). Finally, infected children pose a risk to those who cannot be vaccinated because of immune deficiencies and other medical conditions.
A multilayered US system monitors vaccine safety
Responsibility for assuring the safety of vaccines lies with the US Food and Drug Administration (FDA) Center for Biologics Evaluation and Research and with the CDC’s Immunization Safety Office (ISO). The FDA is responsible for the initial assessment of the effectiveness and safety of new vaccines and for ongoing monitoring of the manufacturing facilities where vaccines are produced. After FDA approval, safety is monitored using a multilayered system that includes the Vaccine Adverse Event Reporting System (VAERS), the Vaccine Safety Datalink (VSD) system, the Clinical Immunization Safety Assessment (CISA) Project, and periodic reviews by the National Academy of Medicine (NAM), previously the Institute of Medicine. In addition, there is a large number of studies published each year by the nation’s—and world’s—medical research community on vaccine effectiveness and safety.
Continue to: VAERS
VAERS (https://vaers.hhs.gov/) is a passive reporting system that allows patients, physicians, and other health care providers to record suspected vaccine-related adverse events.5 It was created in 1990 and is run by the FDA and the CDC. It is not intended to be a comprehensive or definitive list of proven vaccine-related harms. As a passive reporting system, it is subject to both over- and underreporting, and the data from it are often misinterpreted and used incorrectly by vaccine opponents—eg, wrongly declaring that VAERS reports of possible AEs are proven cases. It provides a sentinel system that is monitored for indications of possible serious AEs linked to a particular vaccine. When a suspected interaction is detected, it is investigated by the VSD system.
VSD is a collaboration of the CDC’s ISO and 8 geographically distributed health care organizations with complete electronic patient medical information on their members. VSD conducts studies when a question about vaccine safety arises, when new vaccines are licensed, or when there are new vaccine recommendations. A description of VSD sites, the research methods used, and a list of publications describing study results can be found at https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vsd/index.html#organizations. If the VSD system finds a link between serious AEs and a particular vaccine, this association is reported to the Advisory Committee on Immunization Practices (ACIP) for consideration in changing recommendations regarding that vaccine. This happens only rarely.
CISA was established in 2001 as a network of vaccine safety experts at 7 academic medical centers who collaborate with the CDC’s ISO. CISA conducts studies on specific questions related to vaccine safety and provides a consultation service to clinicians and researchers who have questions about vaccine safety. A description of the CISA sites, past publications on vaccine safety, and ongoing research priorities can be found at https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/cisa/index.html.
NAM (https://nam.edu/) conducts periodic reviews of vaccine safety and vaccine-caused AEs. The most recent was published in 2012 and looked at possible AEs of 8 vaccines containing 12 different antigens.6 The literature search for this review found more than 12,000 articles, which speaks to the volume of scientific work on vaccine safety. These NAM reports document the rarity of severe AEs to vaccines and are used with other information to construct the table for the Vaccine Injury Compensation Program (VICP), which is described below.
Are vaccines killing children?
Vaccine opponents frequently claim that vaccines cause much more harm than is documented, including the deaths of children. A vaccine opponent made this claim in my state (Arizona) at a legislative committee hearing even though our state child mortality review committee has been investigating all child deaths for decades and has never attributed a death to a vaccine.
Continue to: One study conducted...
One study conducted using the VSD system from January 1, 2005, to December 31, 2011, identified 1100 deaths occurring within 12 months of any vaccination among 2,189,504 VSD enrollees ages 9 to 26 years.7 They found that the risk of death in this age group was not increased during the 30 days after vaccination, and no deaths were found to be causally associated with vaccination. Deaths among children do occur and, due to the number of vaccines administered, some deaths will occur within a short time period after a vaccine. This temporal association does not prove the death was vaccine-caused, but vaccine opponents have claimed that it does.
The vaccine injury compensation system
In 1986, the federal government established a no-fault system—the National Vaccine Injury Compensation Program (VICP)—to compensate those who suffer a serious AE from a vaccine covered by the program. This system is administered by the Health Resources and Services Administration (HRSA) in the Department of Health and Human Services (DHHS). HRSA maintains a table of proven AEs of specific vaccines, based in part on the NAM report mentioned earlier. Petitions for compensation—with proof of an AE following the administration of a vaccine that is included on the HRSA table—are accepted and remunerated if the AE lasted > 6 months or resulted in hospitalization. Petitions that allege AEs following administration of a vaccine not included on the table are nevertheless reviewed by the staff of HRSA, who can still recommend compensation based on the medical evidence. If HRSA declines the petition, the petitioner can appeal the case in the US Court of Federal Claims, which makes the final decision on a petition’s validity and, if warranted, the type and amount of compensation.
From 2006 to 2017, > 3.4 billion doses of vaccines covered by VICP were distributed in the United States.8 During this period, 6293 petitions were adjudicated by the court; 4311 were compensated.8 For every 1 million doses of vaccine distributed, 1 individual was compensated. Seventy percent of these compensations were awarded to petitioners despite a lack of clear evidence that the patient’s condition was caused by a vaccine.8 The rate of compensation for conditions proven to be caused by a vaccine was 1/3.33 million.8
The VICP pays for attorney fees, in some cases even if the petition is denied, but does not allow contingency fees. Since the beginning of the program, more than $4 billion has been awarded.8 The program is funded by a 75-cent tax on each vaccine antigen. Because serious AEs are so rare, the trust fund established to administer the VICP finances has a surplus of about $6 billion.
The Advisory Committee on Immunization Practices
After a vaccine is approved for use by the FDA, ACIP makes recommendations for its use in the US civilian population.9,10 ACIP, created in 1964, was chartered as a federal advisory committee to provide expert external advice to the Director of the CDC and the Secretary of DHHS on the use of vaccines
Continue to: As an official...
As an official federal advisory committee governed by the Federal Advisory Committee Act, ACIP operates under strict requirements for public notification of meetings, allowing for written and oral public comment at its meetings, and timely publication of minutes. ACIP meeting minutes are posted soon after each meeting, along with draft recommendations. ACIP meeting agendas and slide presentations are available on the ACIP Web site (https://www.cdc.gov/vaccines/acip/index.html).
ACIP consists of 15 members serving overlapping 4-year terms, appointed by the Secretary of DHHS from a list of candidates proposed by the CDC. One member is a consumer representative; the other members have expertise in vaccinology, immunology, pediatrics, internal medicine, infectious diseases, preventive medicine, and public health. In the CDC, staff support for ACIP is provided by the National Center for Immunization and Respiratory Diseases, Office of Infectious Diseases.
ACIP holds 2-day meetings 3 times a year. Much of the work occurs between meetings, by work groups via phone conferences. Work groups are chaired by an ACIP member and staffed by one or more CDC programmatic, content-expert professionals. Membership of the work groups consists of at least 2 ACIP members, representatives from relevant professional clinical and public health organizations, and other individuals with specific expertise. Work groups propose recommendations to ACIP, which can adopt, revise, or reject them.
When formulating recommendations for a particular vaccine, ACIP considers the burden of disease prevented, the effectiveness and safety of the vaccine, cost effectiveness, and practical and logistical issues of implementing recommendations. ACIP also receives frequent reports from ISO regarding the safety of vaccines previously approved. Since 2011, ACIP has used a standardized, modified GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system to assess the evidence regarding effectiveness and safety of new vaccines and an evidence-to-recommendation framework to transparently explain how it arrives at recommendations.11,12
We can recommend vaccines with confidence
In the United States, we have a secure supply of safe vaccines, a transparent method of making vaccine recommendations, a robust system to monitor vaccine safety, and an efficient system to compensate those who experience a rare, serious adverse reaction to a vaccine. The US public health system has achieved a marked reduction in morbidity and mortality from childhood infectious diseases, mostly because of vaccines. Many people today have not experienced or seen children with these once-common childhood infections and may not appreciate the seriousness of childhood infectious diseases or the full value of vaccines. As family physicians, we can help address this problem and recommend vaccines to our patients with confidence.
The current increase in measles cases in the United States has sharpened the focus on antivaccine activities. While the percentage of US children who are fully vaccinated remains high (≥ 94%), the number of un- or undervaccinated children has been growing1 because of nonmedical exemptions from school vaccine requirements due to concerns about vaccine safety and an underappreciation of the benefits of vaccines. Family physicians need to be conversant with several important aspects of this matter, including the magnitude of benefits provided by childhood vaccines, as well as the systems already in place for
- assessing vaccine effectiveness and safety,
- making recommendations on the use of vaccines,
- monitoring safety after vaccine approval, and
- compensating those affected by rare but serious vaccine-related adverse events (AEs).
Familiarity with these issues will allow for informed discussions with parents who are vaccine hesitant and with those who have read or heard inaccurate information.
The benefits of vaccines are indisputable
In 1999, the Centers for Disease Control and Prevention (CDC) published a list of 9 selected childhood infectious diseases and compared their incidences before and after immunization was available.2 Each of these infections causes morbidity, sequelae, and mortality at predictable rates depending on the infectious agent. The comparisons were dramatic: Measles, with a baseline annual morbidity of 503,282 cases, fell to just 89 cases; poliomyelitis decreased from 16,316 to 0; and Haemophilus influenzae type b declined from 20,000 to 54. In a 2014 analysis, the CDC stated that “among 78.6 million children born during 1994–2013, routine childhood immunization was estimated to prevent 322 million illnesses (averaging 4.1 illnesses per child) and 21 million hospitalizations (0.27 per child) over the course of their lifetimes and avert 732,000 premature deaths from vaccine-preventable illnesses” (TABLE).3

It is not unusual to hear a vaccine opponent say that childhood infectious diseases are not serious and that it is better for a child to contract the infection and let the immune system fight it naturally. Measles is often used as an example. This argument ignores some important aspects of vaccine benefits.
It is true in the United States that the average child who contracts measles will recover from it and not suffer immediate or long-term effects. However, it is also true that measles has a hospitalization rate of about 20% and a death rate of between 1/500 and 1/1000 cases.4 Mortality is much higher in developing countries. Prior to widespread use of measles vaccine, hundreds of thousands of cases of measles occurred each year. That translated into hundreds of preventable child deaths per year. An individual case does not tell the full story about the public health impact of infectious illnesses.
In addition, there are often unappreciated sequelae from child infections, such as shingles occurring years after resolution of a chickenpox infection. There are also societal consequences of child infections, such as deafness from congenital rubella and intergenerational transfer of infectious agents to family members at risk for serious consequences (influenza from a child to a grandparent). Finally, infected children pose a risk to those who cannot be vaccinated because of immune deficiencies and other medical conditions.
A multilayered US system monitors vaccine safety
Responsibility for assuring the safety of vaccines lies with the US Food and Drug Administration (FDA) Center for Biologics Evaluation and Research and with the CDC’s Immunization Safety Office (ISO). The FDA is responsible for the initial assessment of the effectiveness and safety of new vaccines and for ongoing monitoring of the manufacturing facilities where vaccines are produced. After FDA approval, safety is monitored using a multilayered system that includes the Vaccine Adverse Event Reporting System (VAERS), the Vaccine Safety Datalink (VSD) system, the Clinical Immunization Safety Assessment (CISA) Project, and periodic reviews by the National Academy of Medicine (NAM), previously the Institute of Medicine. In addition, there is a large number of studies published each year by the nation’s—and world’s—medical research community on vaccine effectiveness and safety.
Continue to: VAERS
VAERS (https://vaers.hhs.gov/) is a passive reporting system that allows patients, physicians, and other health care providers to record suspected vaccine-related adverse events.5 It was created in 1990 and is run by the FDA and the CDC. It is not intended to be a comprehensive or definitive list of proven vaccine-related harms. As a passive reporting system, it is subject to both over- and underreporting, and the data from it are often misinterpreted and used incorrectly by vaccine opponents—eg, wrongly declaring that VAERS reports of possible AEs are proven cases. It provides a sentinel system that is monitored for indications of possible serious AEs linked to a particular vaccine. When a suspected interaction is detected, it is investigated by the VSD system.
VSD is a collaboration of the CDC’s ISO and 8 geographically distributed health care organizations with complete electronic patient medical information on their members. VSD conducts studies when a question about vaccine safety arises, when new vaccines are licensed, or when there are new vaccine recommendations. A description of VSD sites, the research methods used, and a list of publications describing study results can be found at https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vsd/index.html#organizations. If the VSD system finds a link between serious AEs and a particular vaccine, this association is reported to the Advisory Committee on Immunization Practices (ACIP) for consideration in changing recommendations regarding that vaccine. This happens only rarely.
CISA was established in 2001 as a network of vaccine safety experts at 7 academic medical centers who collaborate with the CDC’s ISO. CISA conducts studies on specific questions related to vaccine safety and provides a consultation service to clinicians and researchers who have questions about vaccine safety. A description of the CISA sites, past publications on vaccine safety, and ongoing research priorities can be found at https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/cisa/index.html.
NAM (https://nam.edu/) conducts periodic reviews of vaccine safety and vaccine-caused AEs. The most recent was published in 2012 and looked at possible AEs of 8 vaccines containing 12 different antigens.6 The literature search for this review found more than 12,000 articles, which speaks to the volume of scientific work on vaccine safety. These NAM reports document the rarity of severe AEs to vaccines and are used with other information to construct the table for the Vaccine Injury Compensation Program (VICP), which is described below.
Are vaccines killing children?
Vaccine opponents frequently claim that vaccines cause much more harm than is documented, including the deaths of children. A vaccine opponent made this claim in my state (Arizona) at a legislative committee hearing even though our state child mortality review committee has been investigating all child deaths for decades and has never attributed a death to a vaccine.
Continue to: One study conducted...
One study conducted using the VSD system from January 1, 2005, to December 31, 2011, identified 1100 deaths occurring within 12 months of any vaccination among 2,189,504 VSD enrollees ages 9 to 26 years.7 They found that the risk of death in this age group was not increased during the 30 days after vaccination, and no deaths were found to be causally associated with vaccination. Deaths among children do occur and, due to the number of vaccines administered, some deaths will occur within a short time period after a vaccine. This temporal association does not prove the death was vaccine-caused, but vaccine opponents have claimed that it does.
The vaccine injury compensation system
In 1986, the federal government established a no-fault system—the National Vaccine Injury Compensation Program (VICP)—to compensate those who suffer a serious AE from a vaccine covered by the program. This system is administered by the Health Resources and Services Administration (HRSA) in the Department of Health and Human Services (DHHS). HRSA maintains a table of proven AEs of specific vaccines, based in part on the NAM report mentioned earlier. Petitions for compensation—with proof of an AE following the administration of a vaccine that is included on the HRSA table—are accepted and remunerated if the AE lasted > 6 months or resulted in hospitalization. Petitions that allege AEs following administration of a vaccine not included on the table are nevertheless reviewed by the staff of HRSA, who can still recommend compensation based on the medical evidence. If HRSA declines the petition, the petitioner can appeal the case in the US Court of Federal Claims, which makes the final decision on a petition’s validity and, if warranted, the type and amount of compensation.
From 2006 to 2017, > 3.4 billion doses of vaccines covered by VICP were distributed in the United States.8 During this period, 6293 petitions were adjudicated by the court; 4311 were compensated.8 For every 1 million doses of vaccine distributed, 1 individual was compensated. Seventy percent of these compensations were awarded to petitioners despite a lack of clear evidence that the patient’s condition was caused by a vaccine.8 The rate of compensation for conditions proven to be caused by a vaccine was 1/3.33 million.8
The VICP pays for attorney fees, in some cases even if the petition is denied, but does not allow contingency fees. Since the beginning of the program, more than $4 billion has been awarded.8 The program is funded by a 75-cent tax on each vaccine antigen. Because serious AEs are so rare, the trust fund established to administer the VICP finances has a surplus of about $6 billion.
The Advisory Committee on Immunization Practices
After a vaccine is approved for use by the FDA, ACIP makes recommendations for its use in the US civilian population.9,10 ACIP, created in 1964, was chartered as a federal advisory committee to provide expert external advice to the Director of the CDC and the Secretary of DHHS on the use of vaccines
Continue to: As an official...
As an official federal advisory committee governed by the Federal Advisory Committee Act, ACIP operates under strict requirements for public notification of meetings, allowing for written and oral public comment at its meetings, and timely publication of minutes. ACIP meeting minutes are posted soon after each meeting, along with draft recommendations. ACIP meeting agendas and slide presentations are available on the ACIP Web site (https://www.cdc.gov/vaccines/acip/index.html).
ACIP consists of 15 members serving overlapping 4-year terms, appointed by the Secretary of DHHS from a list of candidates proposed by the CDC. One member is a consumer representative; the other members have expertise in vaccinology, immunology, pediatrics, internal medicine, infectious diseases, preventive medicine, and public health. In the CDC, staff support for ACIP is provided by the National Center for Immunization and Respiratory Diseases, Office of Infectious Diseases.
ACIP holds 2-day meetings 3 times a year. Much of the work occurs between meetings, by work groups via phone conferences. Work groups are chaired by an ACIP member and staffed by one or more CDC programmatic, content-expert professionals. Membership of the work groups consists of at least 2 ACIP members, representatives from relevant professional clinical and public health organizations, and other individuals with specific expertise. Work groups propose recommendations to ACIP, which can adopt, revise, or reject them.
When formulating recommendations for a particular vaccine, ACIP considers the burden of disease prevented, the effectiveness and safety of the vaccine, cost effectiveness, and practical and logistical issues of implementing recommendations. ACIP also receives frequent reports from ISO regarding the safety of vaccines previously approved. Since 2011, ACIP has used a standardized, modified GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system to assess the evidence regarding effectiveness and safety of new vaccines and an evidence-to-recommendation framework to transparently explain how it arrives at recommendations.11,12
We can recommend vaccines with confidence
In the United States, we have a secure supply of safe vaccines, a transparent method of making vaccine recommendations, a robust system to monitor vaccine safety, and an efficient system to compensate those who experience a rare, serious adverse reaction to a vaccine. The US public health system has achieved a marked reduction in morbidity and mortality from childhood infectious diseases, mostly because of vaccines. Many people today have not experienced or seen children with these once-common childhood infections and may not appreciate the seriousness of childhood infectious diseases or the full value of vaccines. As family physicians, we can help address this problem and recommend vaccines to our patients with confidence.
1. Mellerson JL, Maxwell CB, Knighton CL, et al. Vaccine coverage for selected vaccines and exemption rates among children in kindergarten—United States, 2017-18 school year. MMWR Morb Mortal Wkly Rep. 2018;67:1115-1122.
2. CDC. Ten great public health achievements—United States, 1900-1999. MMWR Morb Mortal Wkly Rep. 1999;48:241-243.
3. Whitney CG, Zhou F, Singleton J, et al. Benefits from immunization during the Vaccines for Children Program era—United States, 1994-2013. MMWR Morb Mortal Wkly Rep. 2014;63:352-355.
4. CDC. Complications of measles. https://www.cdc.gov/measles/symptoms/complications.html. Accessed July 16, 2019.
5. Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33:4398-4405.
6. IOM (Institute of Medicine). Adverse Effects of Vaccines: Evidence and Causality. Washington, DC: The National Academies Press; 2012.
7. McCarthy NL, Gee J, Sukumaran L, et al. Vaccination and 30-day mortality risk in children, adolescents, and young adults. Pediatrics. 2016;137:1-8.
8. HRSA. Data and Statistics. https://www.hrsa.gov/sites/default/files/hrsa/vaccine-compensation/data/monthly-stats-may-2019.pdf. Accessed July 16, 2019.
9. Pickering LK, Orenstein WA, Sun W, et al. FDA licensure of and ACIP recommendations for vaccines. Vaccine. 2017;37:5027-5036.
10. Smith JC, Snider DE, Pickering LK. Immunization policy development in the United States: the role of the Advisory Committee on Immunization Practices. Ann Intern Med. 2009;150:45-49.
11. Ahmed F, Temte JL, Campos-Outcalt D, et al; for the ACIP Evidence Based Recommendations Work Group (EBRWG). Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the U.S. Centers for Disease Control and Prevention (CDC). Vaccine. 2011;29:9171-9176.
12. Lee G, Carr W. Updated framework for development of evidence-based recommendations by the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018:76:1271-1272.
1. Mellerson JL, Maxwell CB, Knighton CL, et al. Vaccine coverage for selected vaccines and exemption rates among children in kindergarten—United States, 2017-18 school year. MMWR Morb Mortal Wkly Rep. 2018;67:1115-1122.
2. CDC. Ten great public health achievements—United States, 1900-1999. MMWR Morb Mortal Wkly Rep. 1999;48:241-243.
3. Whitney CG, Zhou F, Singleton J, et al. Benefits from immunization during the Vaccines for Children Program era—United States, 1994-2013. MMWR Morb Mortal Wkly Rep. 2014;63:352-355.
4. CDC. Complications of measles. https://www.cdc.gov/measles/symptoms/complications.html. Accessed July 16, 2019.
5. Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33:4398-4405.
6. IOM (Institute of Medicine). Adverse Effects of Vaccines: Evidence and Causality. Washington, DC: The National Academies Press; 2012.
7. McCarthy NL, Gee J, Sukumaran L, et al. Vaccination and 30-day mortality risk in children, adolescents, and young adults. Pediatrics. 2016;137:1-8.
8. HRSA. Data and Statistics. https://www.hrsa.gov/sites/default/files/hrsa/vaccine-compensation/data/monthly-stats-may-2019.pdf. Accessed July 16, 2019.
9. Pickering LK, Orenstein WA, Sun W, et al. FDA licensure of and ACIP recommendations for vaccines. Vaccine. 2017;37:5027-5036.
10. Smith JC, Snider DE, Pickering LK. Immunization policy development in the United States: the role of the Advisory Committee on Immunization Practices. Ann Intern Med. 2009;150:45-49.
11. Ahmed F, Temte JL, Campos-Outcalt D, et al; for the ACIP Evidence Based Recommendations Work Group (EBRWG). Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the U.S. Centers for Disease Control and Prevention (CDC). Vaccine. 2011;29:9171-9176.
12. Lee G, Carr W. Updated framework for development of evidence-based recommendations by the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018:76:1271-1272.