User login
Consider C. difficile early in children with cancer with GI symptoms
Children with cancer are at increased risk of potentially life-threatening Clostridioides difficile infections (CDI), and Brianna Murphy, DO, reported at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
CDI are characterized by diarrhea, fever, and loss of appetite. The clinical features are caused by the release of toxins A and B by this gram-positive bacterium. In pediatric groups, CDI are a leading cause of antibiotic-associated gastric illness. This in turn can lead to a protracted stay in hospital and increases risk of mortality. The rising incidence in the United States over the last 2 decades prompted Dr. Murphy, a pediatric hematology oncology fellow working at the department of pediatric research at the University of Texas MD Anderson Cancer Center, Houston, to investigate further. A search of the literature found limited information regarding CDI and pediatric oncology patients.
Recognized factors for contracting CDI include the presence of other illnesses, a weakened immune system because of drugs or disease, enteral nutrition, usage of medicines such as proton pump inhibitors which decrease gastric acid production, and classically, treatment with broad spectrum antibiotics.
Dr. Murphy’s study included patients aged 1-18 years, all of whom had a cancer diagnosis and a positive stool culture for C. difficile. Presenting symptoms were three or more loose stools per day or acute onset ileus. The study evaluated data for the years 2000-2017 and included 11,366 children; 207 CDI (0.98%) cases were identified among pediatric oncology patients during the study period. This compares with historical data showing an incidence of 0.14% among hospitalized children in general.
Malignancy data were then subdivided into three groups: hematologic, nonneural solid tumors (NNST), and neural tumors. Hematologic malignancies had a CDI prevalence higher than the average for oncologic patients at 5.4%. Inside this group those suffering with acute myeloid leukemia had a rate of 10.5%. In the NNST and neural tumor groups, CDI rates were lower and closer to the overall average.
Dr. Murphy then looked at her patient population in more detail. Poor clinical outcomes (PCOs) were defined as severe, refractory, recurrent, or multiple infections. Severe CDI included features such as toxic megacolon, gastrointestinal perforation, or need for surgical intervention. Refractory CDI were defined as continuation of symptoms beyond 7 days of appropriate therapy, and recurrent CDI were classed as reinfection within 8 weeks of a previous CDI. Ultimately, 51% of patients in this study died. Patients with severe CDI experienced increased mortality (P = .02). There was no difference shown when looking at the type of cancer, age, gender, or patient ethnicity.
Next, Dr. Murphy looked for associations. Hematologic and biochemical testing identified that elevated creatinine was statistically associated with the likelihood of PCOs, compared with leukocytosis and neutropenia, particularly in the NNST group. Treatment modality also was studied. Here radiation therapy was the only treatment shown to increase PCOs in patients with CDI. One-fifth (22%) of radiation therapy recipients experienced multiple CDI, compared with 12% of the total population.
In commenting on her paper, Louis Bent, MD, from the Netherlands raised the issue of deaths in septic patients. What was the origin of the responsible organism, for example from the GI tract or from central lines, and were patients receiving appropriate antibiotic treatment?
Dr. Kelly responded that sepsis was generally believed to occur as a result of infection with mixed bacterial translocation through the bowel wall, notably Escherichia coli. Patients were usually on a cocktail of antibiotics targeting CDI, but also other infections illustrating the serious nature of the situation.
Dr. Murphy had no financial conflicts of interest to declare.
Children with cancer are at increased risk of potentially life-threatening Clostridioides difficile infections (CDI), and Brianna Murphy, DO, reported at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
CDI are characterized by diarrhea, fever, and loss of appetite. The clinical features are caused by the release of toxins A and B by this gram-positive bacterium. In pediatric groups, CDI are a leading cause of antibiotic-associated gastric illness. This in turn can lead to a protracted stay in hospital and increases risk of mortality. The rising incidence in the United States over the last 2 decades prompted Dr. Murphy, a pediatric hematology oncology fellow working at the department of pediatric research at the University of Texas MD Anderson Cancer Center, Houston, to investigate further. A search of the literature found limited information regarding CDI and pediatric oncology patients.
Recognized factors for contracting CDI include the presence of other illnesses, a weakened immune system because of drugs or disease, enteral nutrition, usage of medicines such as proton pump inhibitors which decrease gastric acid production, and classically, treatment with broad spectrum antibiotics.
Dr. Murphy’s study included patients aged 1-18 years, all of whom had a cancer diagnosis and a positive stool culture for C. difficile. Presenting symptoms were three or more loose stools per day or acute onset ileus. The study evaluated data for the years 2000-2017 and included 11,366 children; 207 CDI (0.98%) cases were identified among pediatric oncology patients during the study period. This compares with historical data showing an incidence of 0.14% among hospitalized children in general.
Malignancy data were then subdivided into three groups: hematologic, nonneural solid tumors (NNST), and neural tumors. Hematologic malignancies had a CDI prevalence higher than the average for oncologic patients at 5.4%. Inside this group those suffering with acute myeloid leukemia had a rate of 10.5%. In the NNST and neural tumor groups, CDI rates were lower and closer to the overall average.
Dr. Murphy then looked at her patient population in more detail. Poor clinical outcomes (PCOs) were defined as severe, refractory, recurrent, or multiple infections. Severe CDI included features such as toxic megacolon, gastrointestinal perforation, or need for surgical intervention. Refractory CDI were defined as continuation of symptoms beyond 7 days of appropriate therapy, and recurrent CDI were classed as reinfection within 8 weeks of a previous CDI. Ultimately, 51% of patients in this study died. Patients with severe CDI experienced increased mortality (P = .02). There was no difference shown when looking at the type of cancer, age, gender, or patient ethnicity.
Next, Dr. Murphy looked for associations. Hematologic and biochemical testing identified that elevated creatinine was statistically associated with the likelihood of PCOs, compared with leukocytosis and neutropenia, particularly in the NNST group. Treatment modality also was studied. Here radiation therapy was the only treatment shown to increase PCOs in patients with CDI. One-fifth (22%) of radiation therapy recipients experienced multiple CDI, compared with 12% of the total population.
In commenting on her paper, Louis Bent, MD, from the Netherlands raised the issue of deaths in septic patients. What was the origin of the responsible organism, for example from the GI tract or from central lines, and were patients receiving appropriate antibiotic treatment?
Dr. Kelly responded that sepsis was generally believed to occur as a result of infection with mixed bacterial translocation through the bowel wall, notably Escherichia coli. Patients were usually on a cocktail of antibiotics targeting CDI, but also other infections illustrating the serious nature of the situation.
Dr. Murphy had no financial conflicts of interest to declare.
Children with cancer are at increased risk of potentially life-threatening Clostridioides difficile infections (CDI), and Brianna Murphy, DO, reported at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
CDI are characterized by diarrhea, fever, and loss of appetite. The clinical features are caused by the release of toxins A and B by this gram-positive bacterium. In pediatric groups, CDI are a leading cause of antibiotic-associated gastric illness. This in turn can lead to a protracted stay in hospital and increases risk of mortality. The rising incidence in the United States over the last 2 decades prompted Dr. Murphy, a pediatric hematology oncology fellow working at the department of pediatric research at the University of Texas MD Anderson Cancer Center, Houston, to investigate further. A search of the literature found limited information regarding CDI and pediatric oncology patients.
Recognized factors for contracting CDI include the presence of other illnesses, a weakened immune system because of drugs or disease, enteral nutrition, usage of medicines such as proton pump inhibitors which decrease gastric acid production, and classically, treatment with broad spectrum antibiotics.
Dr. Murphy’s study included patients aged 1-18 years, all of whom had a cancer diagnosis and a positive stool culture for C. difficile. Presenting symptoms were three or more loose stools per day or acute onset ileus. The study evaluated data for the years 2000-2017 and included 11,366 children; 207 CDI (0.98%) cases were identified among pediatric oncology patients during the study period. This compares with historical data showing an incidence of 0.14% among hospitalized children in general.
Malignancy data were then subdivided into three groups: hematologic, nonneural solid tumors (NNST), and neural tumors. Hematologic malignancies had a CDI prevalence higher than the average for oncologic patients at 5.4%. Inside this group those suffering with acute myeloid leukemia had a rate of 10.5%. In the NNST and neural tumor groups, CDI rates were lower and closer to the overall average.
Dr. Murphy then looked at her patient population in more detail. Poor clinical outcomes (PCOs) were defined as severe, refractory, recurrent, or multiple infections. Severe CDI included features such as toxic megacolon, gastrointestinal perforation, or need for surgical intervention. Refractory CDI were defined as continuation of symptoms beyond 7 days of appropriate therapy, and recurrent CDI were classed as reinfection within 8 weeks of a previous CDI. Ultimately, 51% of patients in this study died. Patients with severe CDI experienced increased mortality (P = .02). There was no difference shown when looking at the type of cancer, age, gender, or patient ethnicity.
Next, Dr. Murphy looked for associations. Hematologic and biochemical testing identified that elevated creatinine was statistically associated with the likelihood of PCOs, compared with leukocytosis and neutropenia, particularly in the NNST group. Treatment modality also was studied. Here radiation therapy was the only treatment shown to increase PCOs in patients with CDI. One-fifth (22%) of radiation therapy recipients experienced multiple CDI, compared with 12% of the total population.
In commenting on her paper, Louis Bent, MD, from the Netherlands raised the issue of deaths in septic patients. What was the origin of the responsible organism, for example from the GI tract or from central lines, and were patients receiving appropriate antibiotic treatment?
Dr. Kelly responded that sepsis was generally believed to occur as a result of infection with mixed bacterial translocation through the bowel wall, notably Escherichia coli. Patients were usually on a cocktail of antibiotics targeting CDI, but also other infections illustrating the serious nature of the situation.
Dr. Murphy had no financial conflicts of interest to declare.
FROM ESPID 2020
Should we use antibiotics to treat sore throats?
The use of antibiotics to treat a sore throat remains contentious, with guidelines from around the world providing contradictory advice. This topic generated a lively debate at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
Lauri Ivaska, MD, of the department of pediatrics and adolescent medicine at Turku (Finland) University Hospital, argued for the use of antibiotics, while Borbála Zsigmond, MD, of Heim Pál Children’s Hospital in Budapest, made the case against their use. Interestingly, this debate occurred against the background of a poll conducted before the debate, which found that only 11% of the audience voted in favor of using antibiotics to treat sore throats.
Both speakers began by exploring their approach to the treatment of a recent clinical case involving a 4-year-old girl presenting with sore throat. Dr. Ivaska stressed the difference between a sore throat, pharyngitis, and tonsillitis: the latter two refer to a physical finding, while the former is a subjective symptom.
International guidelines differ on the subject
The debate moved to discussing the international guidelines for treating pharyngitis and tonsillitis. Dr. Zsigmond believes that these are flawed and unhelpful, arguing that they differ depending on what part of the world a physician is practicing in. For example, the 2012 Infectious Diseases Society of America guidelines recommend using best clinical judgment and then backing this up by testing. If testing proves positive for group A Streptococcus pyogenes (GAS), the physician should universally treat. By comparison, the European Society of Clinical Microbiology and Infectious Diseases Sore Throat Guideline Group focuses on severity rather than the cause of the infection. If the case is deemed to be serious, antibiotics can be prescribed without a positive test.
Sore throat is frequently associated with a common cold. In a recent study, more that 80% of students with an acute viral respiratory tract infection had soreness at the beginning of their illness.
Reporting from his own research, Dr. Ivaska argued that viruses can be detected in almost two-thirds of children with pharyngitis using polymerase chain reaction analysis. He thinks antibiotics should be reserved for those 30%-40% of patients with a confirmed GAS infection. The potential role of Fusobacterium necrophorum was raised, but there is no evidence of the benefits of antibiotic treatment in such cases.
There are diagnostic aids for GAS infection
It was suggested that, instead of concentrating on sore throat, the debate should be about whether to use antibiotics to treat GAS infection. But how can the diagnosis be confirmed simply in a clinical setting? Dr. Ivaska recommended adopting diagnostic aids such as Centor, McIsaac, and FeverPAIN, which award scores for several common disease features – the higher the score, the more likely a patient is to be suffering from a GAS infection.
Dr. Zsigmond also likes scoring symptoms but believes they are often inaccurate, especially in young children. She pointed to a report that examined the use of the Centor tool among 441 children attending a pediatric ED. The authors concluded that the Centor criteria were ineffective in predicting a positive GAS culture in throat swabs taken from symptomatic patients.
When are antibiotics warranted?
It is widely accepted that antibiotics should be avoided for viral infections. Returning to the case described at the start of this debate, Dr. Zsigmond calculated that her patient with a 2-day history of sore throat, elevated temperature, pussy tonsils, and enlarged cervical lymph glands but no cough or rhinitis had a FeverPAIN score of 4-5 and a Centor score of 4, meaning that, according to the European guidelines, she should receive antibiotic treatment. However, viral swabs proved positive for adenovirus.
Dr. Ivaska responded with his recent experiences of a similar case, where a 5-year-old boy had a FeverPAIN score of 4-5 and Centor score of 3. Cultures from his throat were GAS positive, illustrating the problem of differentiating between bacterial and viral infections.
But does a GAS-positive pharyngeal culture necessarily mean that antibiotic treatment is indicated? Dr. Ivaska believes it does, citing the importance of preventing serious complications such as rheumatic fever. Dr. Zsigmind countered by pointing out the low levels of acute rheumatic fever in developed nations. In her own country, Hungary, there has not been a case in the last 30 years. Giving antibiotics for historical reasons cannot, in her view, be justified.
Dr. Ivaska responded that perhaps this is because of early treatment in children with sore throats.
Another complication of tonsillitis is quinsy. Dr. Zsigmond cited a study showing that there is no statistically significant evidence demonstrating that antibiotics prevent quinsy. She attributed this to quinsy appearing quickly, typically within 2 days. Delay in seeking help means that the window to treat is often missed. However, should symptoms present early, there is no statistical evidence that prior antibiotic use can prevent quinsy. Also, given the rarity of this condition, prevention would mean excessive use of antibiotics.
Are there other possible benefits of antibiotic treatment in patients with a sore throat? Dr. Ivaska referred to a Cochrane review that found a shortening in duration of throat soreness and fever. Furthermore, compared with placebo, antibiotics reduced the incidence of suppurative complications such as acute otitis media and sinusitis following a sore throat. Other studies have also pointed to the potential benefits of reduced transmission in families where one member with pharyngitis was GAS positive.
As the debate ended, Dr. Zsigmond reported evidence of global antibiotic overprescribing for sore throat ranging from 53% in Europe to 94% in Australia. She also highlighted risks such as altered gut flora, drug resistance, and rashes.
Robin Marlow from the University of Bristol (England), PhD, MBBS, commented that “one of the most enjoyable parts of the ESPID meeting is hearing different viewpoints rationally explained from across the world. As [antibiotic prescription for a sore throat is] a clinical conundrum that faces pediatricians every day, I thought this debate was a really great example of how, despite our different health care systems and ways of working, we are all striving together to improve children’s health using the best evidence available.”
The presenters had no financial conflicts of interest to declare.
The use of antibiotics to treat a sore throat remains contentious, with guidelines from around the world providing contradictory advice. This topic generated a lively debate at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
Lauri Ivaska, MD, of the department of pediatrics and adolescent medicine at Turku (Finland) University Hospital, argued for the use of antibiotics, while Borbála Zsigmond, MD, of Heim Pál Children’s Hospital in Budapest, made the case against their use. Interestingly, this debate occurred against the background of a poll conducted before the debate, which found that only 11% of the audience voted in favor of using antibiotics to treat sore throats.
Both speakers began by exploring their approach to the treatment of a recent clinical case involving a 4-year-old girl presenting with sore throat. Dr. Ivaska stressed the difference between a sore throat, pharyngitis, and tonsillitis: the latter two refer to a physical finding, while the former is a subjective symptom.
International guidelines differ on the subject
The debate moved to discussing the international guidelines for treating pharyngitis and tonsillitis. Dr. Zsigmond believes that these are flawed and unhelpful, arguing that they differ depending on what part of the world a physician is practicing in. For example, the 2012 Infectious Diseases Society of America guidelines recommend using best clinical judgment and then backing this up by testing. If testing proves positive for group A Streptococcus pyogenes (GAS), the physician should universally treat. By comparison, the European Society of Clinical Microbiology and Infectious Diseases Sore Throat Guideline Group focuses on severity rather than the cause of the infection. If the case is deemed to be serious, antibiotics can be prescribed without a positive test.
Sore throat is frequently associated with a common cold. In a recent study, more that 80% of students with an acute viral respiratory tract infection had soreness at the beginning of their illness.
Reporting from his own research, Dr. Ivaska argued that viruses can be detected in almost two-thirds of children with pharyngitis using polymerase chain reaction analysis. He thinks antibiotics should be reserved for those 30%-40% of patients with a confirmed GAS infection. The potential role of Fusobacterium necrophorum was raised, but there is no evidence of the benefits of antibiotic treatment in such cases.
There are diagnostic aids for GAS infection
It was suggested that, instead of concentrating on sore throat, the debate should be about whether to use antibiotics to treat GAS infection. But how can the diagnosis be confirmed simply in a clinical setting? Dr. Ivaska recommended adopting diagnostic aids such as Centor, McIsaac, and FeverPAIN, which award scores for several common disease features – the higher the score, the more likely a patient is to be suffering from a GAS infection.
Dr. Zsigmond also likes scoring symptoms but believes they are often inaccurate, especially in young children. She pointed to a report that examined the use of the Centor tool among 441 children attending a pediatric ED. The authors concluded that the Centor criteria were ineffective in predicting a positive GAS culture in throat swabs taken from symptomatic patients.
When are antibiotics warranted?
It is widely accepted that antibiotics should be avoided for viral infections. Returning to the case described at the start of this debate, Dr. Zsigmond calculated that her patient with a 2-day history of sore throat, elevated temperature, pussy tonsils, and enlarged cervical lymph glands but no cough or rhinitis had a FeverPAIN score of 4-5 and a Centor score of 4, meaning that, according to the European guidelines, she should receive antibiotic treatment. However, viral swabs proved positive for adenovirus.
Dr. Ivaska responded with his recent experiences of a similar case, where a 5-year-old boy had a FeverPAIN score of 4-5 and Centor score of 3. Cultures from his throat were GAS positive, illustrating the problem of differentiating between bacterial and viral infections.
But does a GAS-positive pharyngeal culture necessarily mean that antibiotic treatment is indicated? Dr. Ivaska believes it does, citing the importance of preventing serious complications such as rheumatic fever. Dr. Zsigmind countered by pointing out the low levels of acute rheumatic fever in developed nations. In her own country, Hungary, there has not been a case in the last 30 years. Giving antibiotics for historical reasons cannot, in her view, be justified.
Dr. Ivaska responded that perhaps this is because of early treatment in children with sore throats.
Another complication of tonsillitis is quinsy. Dr. Zsigmond cited a study showing that there is no statistically significant evidence demonstrating that antibiotics prevent quinsy. She attributed this to quinsy appearing quickly, typically within 2 days. Delay in seeking help means that the window to treat is often missed. However, should symptoms present early, there is no statistical evidence that prior antibiotic use can prevent quinsy. Also, given the rarity of this condition, prevention would mean excessive use of antibiotics.
Are there other possible benefits of antibiotic treatment in patients with a sore throat? Dr. Ivaska referred to a Cochrane review that found a shortening in duration of throat soreness and fever. Furthermore, compared with placebo, antibiotics reduced the incidence of suppurative complications such as acute otitis media and sinusitis following a sore throat. Other studies have also pointed to the potential benefits of reduced transmission in families where one member with pharyngitis was GAS positive.
As the debate ended, Dr. Zsigmond reported evidence of global antibiotic overprescribing for sore throat ranging from 53% in Europe to 94% in Australia. She also highlighted risks such as altered gut flora, drug resistance, and rashes.
Robin Marlow from the University of Bristol (England), PhD, MBBS, commented that “one of the most enjoyable parts of the ESPID meeting is hearing different viewpoints rationally explained from across the world. As [antibiotic prescription for a sore throat is] a clinical conundrum that faces pediatricians every day, I thought this debate was a really great example of how, despite our different health care systems and ways of working, we are all striving together to improve children’s health using the best evidence available.”
The presenters had no financial conflicts of interest to declare.
The use of antibiotics to treat a sore throat remains contentious, with guidelines from around the world providing contradictory advice. This topic generated a lively debate at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
Lauri Ivaska, MD, of the department of pediatrics and adolescent medicine at Turku (Finland) University Hospital, argued for the use of antibiotics, while Borbála Zsigmond, MD, of Heim Pál Children’s Hospital in Budapest, made the case against their use. Interestingly, this debate occurred against the background of a poll conducted before the debate, which found that only 11% of the audience voted in favor of using antibiotics to treat sore throats.
Both speakers began by exploring their approach to the treatment of a recent clinical case involving a 4-year-old girl presenting with sore throat. Dr. Ivaska stressed the difference between a sore throat, pharyngitis, and tonsillitis: the latter two refer to a physical finding, while the former is a subjective symptom.
International guidelines differ on the subject
The debate moved to discussing the international guidelines for treating pharyngitis and tonsillitis. Dr. Zsigmond believes that these are flawed and unhelpful, arguing that they differ depending on what part of the world a physician is practicing in. For example, the 2012 Infectious Diseases Society of America guidelines recommend using best clinical judgment and then backing this up by testing. If testing proves positive for group A Streptococcus pyogenes (GAS), the physician should universally treat. By comparison, the European Society of Clinical Microbiology and Infectious Diseases Sore Throat Guideline Group focuses on severity rather than the cause of the infection. If the case is deemed to be serious, antibiotics can be prescribed without a positive test.
Sore throat is frequently associated with a common cold. In a recent study, more that 80% of students with an acute viral respiratory tract infection had soreness at the beginning of their illness.
Reporting from his own research, Dr. Ivaska argued that viruses can be detected in almost two-thirds of children with pharyngitis using polymerase chain reaction analysis. He thinks antibiotics should be reserved for those 30%-40% of patients with a confirmed GAS infection. The potential role of Fusobacterium necrophorum was raised, but there is no evidence of the benefits of antibiotic treatment in such cases.
There are diagnostic aids for GAS infection
It was suggested that, instead of concentrating on sore throat, the debate should be about whether to use antibiotics to treat GAS infection. But how can the diagnosis be confirmed simply in a clinical setting? Dr. Ivaska recommended adopting diagnostic aids such as Centor, McIsaac, and FeverPAIN, which award scores for several common disease features – the higher the score, the more likely a patient is to be suffering from a GAS infection.
Dr. Zsigmond also likes scoring symptoms but believes they are often inaccurate, especially in young children. She pointed to a report that examined the use of the Centor tool among 441 children attending a pediatric ED. The authors concluded that the Centor criteria were ineffective in predicting a positive GAS culture in throat swabs taken from symptomatic patients.
When are antibiotics warranted?
It is widely accepted that antibiotics should be avoided for viral infections. Returning to the case described at the start of this debate, Dr. Zsigmond calculated that her patient with a 2-day history of sore throat, elevated temperature, pussy tonsils, and enlarged cervical lymph glands but no cough or rhinitis had a FeverPAIN score of 4-5 and a Centor score of 4, meaning that, according to the European guidelines, she should receive antibiotic treatment. However, viral swabs proved positive for adenovirus.
Dr. Ivaska responded with his recent experiences of a similar case, where a 5-year-old boy had a FeverPAIN score of 4-5 and Centor score of 3. Cultures from his throat were GAS positive, illustrating the problem of differentiating between bacterial and viral infections.
But does a GAS-positive pharyngeal culture necessarily mean that antibiotic treatment is indicated? Dr. Ivaska believes it does, citing the importance of preventing serious complications such as rheumatic fever. Dr. Zsigmind countered by pointing out the low levels of acute rheumatic fever in developed nations. In her own country, Hungary, there has not been a case in the last 30 years. Giving antibiotics for historical reasons cannot, in her view, be justified.
Dr. Ivaska responded that perhaps this is because of early treatment in children with sore throats.
Another complication of tonsillitis is quinsy. Dr. Zsigmond cited a study showing that there is no statistically significant evidence demonstrating that antibiotics prevent quinsy. She attributed this to quinsy appearing quickly, typically within 2 days. Delay in seeking help means that the window to treat is often missed. However, should symptoms present early, there is no statistical evidence that prior antibiotic use can prevent quinsy. Also, given the rarity of this condition, prevention would mean excessive use of antibiotics.
Are there other possible benefits of antibiotic treatment in patients with a sore throat? Dr. Ivaska referred to a Cochrane review that found a shortening in duration of throat soreness and fever. Furthermore, compared with placebo, antibiotics reduced the incidence of suppurative complications such as acute otitis media and sinusitis following a sore throat. Other studies have also pointed to the potential benefits of reduced transmission in families where one member with pharyngitis was GAS positive.
As the debate ended, Dr. Zsigmond reported evidence of global antibiotic overprescribing for sore throat ranging from 53% in Europe to 94% in Australia. She also highlighted risks such as altered gut flora, drug resistance, and rashes.
Robin Marlow from the University of Bristol (England), PhD, MBBS, commented that “one of the most enjoyable parts of the ESPID meeting is hearing different viewpoints rationally explained from across the world. As [antibiotic prescription for a sore throat is] a clinical conundrum that faces pediatricians every day, I thought this debate was a really great example of how, despite our different health care systems and ways of working, we are all striving together to improve children’s health using the best evidence available.”
The presenters had no financial conflicts of interest to declare.
FROM ESPID 2020
Vaccine-preventable infection risk high for pediatric hematopoietic cell transplantation recipients
Vaccine-preventable infections (VPIs) in pediatric hematopoietic cell transplantation (HCT) recipients cause significant morbidity, health care burden, and mortality.
Dana Danino, MD, and colleagues presented their evaluation of the prevalence and epidemiology of pediatric VPI-associated hospitalizations occurring within 5 years post HCT at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
“Pediatric HCT recipients are at increased risk of VPIs, and HCT recipients have poor outcomes from VPIs, compared with the general population,” explained Dr. Danino, of the department of pediatrics, and divisions of infectious diseases and host defense at the Ohio State University, Columbus. “However, the contemporary prevalence, risk factors, morbidity and mortality resulting from VPIs in children post HCT are not well known.”
Their epidemiological study, using the Pediatric Health Information System (PHIS) database, identified all children under 18 years that underwent allogeneic or autologous HCT in an 8-year period. A total of 9,591 unique HCT recipients were identified.
The researchers demonstrated that 7.1% of this cohort were hospitalized for a VPI in the first 5 years post HCT. Dr. Danino explained that 67% of VPI hospitalizations occurred during the first year, at a median of 222 days, and 22% of VPIs occurred during the initial HCT admission.
As to the type of infection, Dr. Danino and colleagues found that, the prevalence of VPI hospitalizations were highest for influenza, followed by varicella and invasive pneumococcal infections. They identified no hospitalizations due to measles or rubella during the study period.
The study findings revealed that the influenza infections occurred a median 231 days post HCT; varicella infections occurred a median 190 days; and invasive pneumococcal infections occurred a median 311 days post HCT.
“When we did a multivariate analysis by time post HCT, we found that age at transplantation, primary immune deficiency as an indication for transplantation, and graft versus host disease were independent predictors of VPIs during the initial HCT admission,” said Dr. Danino.
Children with a VPI who spent longer in hospital were more likely to be admitted to an ICU and have higher mortality, compared with children without a VPI diagnosis.
“VPIs led to longer duration of hospitalization, higher rates of ICU admission, and higher mortality, compared to HCT recipients without VPIs,” Dr. Danino explained. It was not possible in this retrospective study to determine whether increased mortality was VPI related.
These results underline the seriousness of infections in vulnerable children after HCT. Dr. Danino concluded by saying that “efforts to optimize vaccination strategies early post HCT are warranted to decrease VPIs.”
Dr. Danino had nothing to disclose.
Vaccine-preventable infections (VPIs) in pediatric hematopoietic cell transplantation (HCT) recipients cause significant morbidity, health care burden, and mortality.
Dana Danino, MD, and colleagues presented their evaluation of the prevalence and epidemiology of pediatric VPI-associated hospitalizations occurring within 5 years post HCT at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
“Pediatric HCT recipients are at increased risk of VPIs, and HCT recipients have poor outcomes from VPIs, compared with the general population,” explained Dr. Danino, of the department of pediatrics, and divisions of infectious diseases and host defense at the Ohio State University, Columbus. “However, the contemporary prevalence, risk factors, morbidity and mortality resulting from VPIs in children post HCT are not well known.”
Their epidemiological study, using the Pediatric Health Information System (PHIS) database, identified all children under 18 years that underwent allogeneic or autologous HCT in an 8-year period. A total of 9,591 unique HCT recipients were identified.
The researchers demonstrated that 7.1% of this cohort were hospitalized for a VPI in the first 5 years post HCT. Dr. Danino explained that 67% of VPI hospitalizations occurred during the first year, at a median of 222 days, and 22% of VPIs occurred during the initial HCT admission.
As to the type of infection, Dr. Danino and colleagues found that, the prevalence of VPI hospitalizations were highest for influenza, followed by varicella and invasive pneumococcal infections. They identified no hospitalizations due to measles or rubella during the study period.
The study findings revealed that the influenza infections occurred a median 231 days post HCT; varicella infections occurred a median 190 days; and invasive pneumococcal infections occurred a median 311 days post HCT.
“When we did a multivariate analysis by time post HCT, we found that age at transplantation, primary immune deficiency as an indication for transplantation, and graft versus host disease were independent predictors of VPIs during the initial HCT admission,” said Dr. Danino.
Children with a VPI who spent longer in hospital were more likely to be admitted to an ICU and have higher mortality, compared with children without a VPI diagnosis.
“VPIs led to longer duration of hospitalization, higher rates of ICU admission, and higher mortality, compared to HCT recipients without VPIs,” Dr. Danino explained. It was not possible in this retrospective study to determine whether increased mortality was VPI related.
These results underline the seriousness of infections in vulnerable children after HCT. Dr. Danino concluded by saying that “efforts to optimize vaccination strategies early post HCT are warranted to decrease VPIs.”
Dr. Danino had nothing to disclose.
Vaccine-preventable infections (VPIs) in pediatric hematopoietic cell transplantation (HCT) recipients cause significant morbidity, health care burden, and mortality.
Dana Danino, MD, and colleagues presented their evaluation of the prevalence and epidemiology of pediatric VPI-associated hospitalizations occurring within 5 years post HCT at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
“Pediatric HCT recipients are at increased risk of VPIs, and HCT recipients have poor outcomes from VPIs, compared with the general population,” explained Dr. Danino, of the department of pediatrics, and divisions of infectious diseases and host defense at the Ohio State University, Columbus. “However, the contemporary prevalence, risk factors, morbidity and mortality resulting from VPIs in children post HCT are not well known.”
Their epidemiological study, using the Pediatric Health Information System (PHIS) database, identified all children under 18 years that underwent allogeneic or autologous HCT in an 8-year period. A total of 9,591 unique HCT recipients were identified.
The researchers demonstrated that 7.1% of this cohort were hospitalized for a VPI in the first 5 years post HCT. Dr. Danino explained that 67% of VPI hospitalizations occurred during the first year, at a median of 222 days, and 22% of VPIs occurred during the initial HCT admission.
As to the type of infection, Dr. Danino and colleagues found that, the prevalence of VPI hospitalizations were highest for influenza, followed by varicella and invasive pneumococcal infections. They identified no hospitalizations due to measles or rubella during the study period.
The study findings revealed that the influenza infections occurred a median 231 days post HCT; varicella infections occurred a median 190 days; and invasive pneumococcal infections occurred a median 311 days post HCT.
“When we did a multivariate analysis by time post HCT, we found that age at transplantation, primary immune deficiency as an indication for transplantation, and graft versus host disease were independent predictors of VPIs during the initial HCT admission,” said Dr. Danino.
Children with a VPI who spent longer in hospital were more likely to be admitted to an ICU and have higher mortality, compared with children without a VPI diagnosis.
“VPIs led to longer duration of hospitalization, higher rates of ICU admission, and higher mortality, compared to HCT recipients without VPIs,” Dr. Danino explained. It was not possible in this retrospective study to determine whether increased mortality was VPI related.
These results underline the seriousness of infections in vulnerable children after HCT. Dr. Danino concluded by saying that “efforts to optimize vaccination strategies early post HCT are warranted to decrease VPIs.”
Dr. Danino had nothing to disclose.
FROM ESPID 2020
COVID-19 vaccines: Safe for immunocompromised patients?
Coronavirus vaccines have become a reality, as they are now being approved and authorized for use in a growing number of countries including the United States. The U.S. Food and Drug Administration has just issued emergency authorization for the use of the COVID-19 vaccine produced by Pfizer and BioNTech. Close behind is the vaccine developed by Moderna, which has also applied to the FDA for emergency authorization.
The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, and the FDA has said that the 95% credible interval for the vaccine efficacy was 90.3%-97.6%. But as with many initial clinical trials, whether for drugs or vaccines, not all populations were represented in the trial cohort, including individuals who are immunocompromised. At the current time, it is largely unknown how safe or effective the vaccine may be in this large population, many of whom are at high risk for serious COVID-19 complications.
At a special session held during the recent annual meeting of the American Society of Hematology, Anthony Fauci, MD, the nation’s leading infectious disease expert, said that individuals with compromised immune systems, whether because of chemotherapy or a bone marrow transplant, should plan to be vaccinated when the opportunity arises.
In response to a question from ASH President Stephanie J. Lee, MD, of the Fred Hutchinson Cancer Center, Seattle, Dr. Fauci emphasized that, despite being excluded from clinical trials, this population should get vaccinated. “I think we should recommend that they get vaccinated,” he said. “I mean, it is clear that, if you are on immunosuppressive agents, history tells us that you’re not going to have as robust a response as if you had an intact immune system that was not being compromised. But some degree of immunity is better than no degree of immunity.”
That does seem to be the consensus among experts who spoke in interviews: that as long as these are not live attenuated vaccines, they hold no specific risk to an immunocompromised patient, other than any factors specific to the individual that could be a contraindication.
“Patients, family members, friends, and work contacts should be encouraged to receive the vaccine,” said William Stohl, MD, PhD, chief of the division of rheumatology at the University of Southern California, Los Angeles. “Clinicians should advise patients to obtain the vaccine sooner rather than later.”
Kevin C. Wang, MD, PhD, of the department of dermatology at Stanford (Calif.) University, agreed. “I am 100% with Dr. Fauci. Everyone should get the vaccine, even if it may not be as effective,” he said. “I would treat it exactly like the flu vaccines that we recommend folks get every year.”
Dr. Wang noted that he couldn’t think of any contraindications unless the immunosuppressed patients have a history of severe allergic reactions to prior vaccinations. “But I would even say patients with history of cancer, upon recommendation of their oncologists, are likely to be suitable candidates for the vaccine,” he added. “I would say clinicians should approach counseling the same way they counsel patients for the flu vaccine, and as far as I know, there are no concerns for systemic drugs commonly used in dermatology patients.”
However, guidance has not yet been issued from either the FDA or the Centers for Disease Control and Prevention regarding the use of the vaccine in immunocompromised individuals. Given the lack of data, the FDA has said that “it will be something that providers will need to consider on an individual basis,” and that individuals should consult with physicians to weigh the potential benefits and potential risks.
The CDC’s Advisory Committee on Immunization Practices has said that clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies. The CDC itself has not yet released its formal guidance on vaccine use.
COVID-19 vaccines
Vaccines typically require years of research and testing before reaching the clinic, but this year researchers embarked on a global effort to develop safe and effective coronavirus vaccines in record time. Both the Pfizer/BioNTech and Moderna vaccines have only a few months of phase 3 clinical trial data, so much remains unknown about them, including their duration of effect and any long-term safety signals. In addition to excluding immunocompromised individuals, the clinical trials did not include children or pregnant women, so data are lacking for several population subgroups.
But these will not be the only vaccines available, as the pipeline is already becoming crowded. U.S. clinical trial data from a vaccine jointly being developed by Oxford-AstraZeneca, could potentially be ready, along with a request for FDA emergency use authorization, by late January 2021.
In addition, China and Russia have released vaccines, and there are currently 61 vaccines being investigated in clinical trials and at least 85 preclinical products under active investigation.
The vaccine candidates are using both conventional and novel mechanisms of action to elicit an immune response in patients. Conventional methods include attenuated inactivated (killed) virus and recombinant viral protein vaccines to develop immunity. Novel approaches include replication-deficient, adenovirus vector-based vaccines that contain the viral protein, and mRNA-based vaccines, such as the Pfizer and Moderna vaccines, that encode for a SARS-CoV-2 spike protein.
“The special vaccine concern for immunocompromised individuals is introduction of a live virus,” Dr. Stohl said. “Neither the Moderna nor Pfizer vaccines are live viruses, so there should be no special contraindication for such individuals.”
Live vaccine should be avoided in immunocompromised patients, and currently, live SARS-CoV-2 vaccines are only being developed in India and Turkey.
It is not unusual for vaccine trials to begin with cohorts that exclude participants with various health conditions, including those who are immunocompromised. These groups are generally then evaluated in phase 4 trials, or postmarketing surveillance. While the precise number of immunosuppressed adults in the United States is not known, the numbers are believed to be rising because of increased life expectancy among immunosuppressed adults as a result of advances in treatment and new and wider indications for therapies that can affect the immune system.
According to data from the 2013 National Health Interview Survey, an estimated 2.7% of U.S. adults are immunosuppressed. This population covers a broad array of health conditions and medical specialties; people living with inflammatory or autoimmune conditions, such as inflammatory rheumatic diseases (rheumatoid arthritis, axial spondyloarthritis, lupus); inflammatory bowel disease (Crohn’s disease and ulcerative colitis); psoriasis; multiple sclerosis; organ transplant recipients; patients undergoing chemotherapy; and life-long immunosuppression attributable to HIV infection.
As the vaccines begin to roll out and become available, how should clinicians advise their patients, in the absence of any clinical trial data?
Risk vs. benefit
Gilaad Kaplan, MD, MPH, a gastroenterologist and professor of medicine at the University of Calgary (Alta.), noted that the inflammatory bowel disease (IBD) community has dealt with tremendous anxiety during the pandemic because many are immunocompromised because of the medications they use to treat their disease.
“For example, many patients with IBD are on biologics like anti-TNF [tumor necrosis factor] therapies, which are also used in other immune-mediated inflammatory diseases such as rheumatoid arthritis,” he said. “Understandably, individuals with IBD on immunosuppressive medications are concerned about the risk of severe complications due to COVID-19.”
The entire IBD community, along with the world, celebrated the announcement that multiple vaccines are protective against SARS-CoV-2, he noted. “Vaccines offer the potential to reduce the spread of COVID-19, allowing society to revert back to normalcy,” Dr. Kaplan said. “Moreover, for vulnerable populations, including those who are immunocompromised, vaccines offer the potential to directly protect them from the morbidity and mortality associated with COVID-19.”
That said, even though the news of vaccines are extremely promising, some cautions must be raised regarding their use in immunocompromised populations, such as persons with IBD. “The current trials, to my knowledge, did not include immunocompromised individuals and thus, we can only extrapolate from what we know from other trials of different vaccines,” he explained. “We know from prior vaccines studies that the immune response following vaccination is less robust in those who are immunocompromised as compared to a healthy control population.”
Dr. Kaplan also pointed to recent reports of allergic reactions that have been reported in healthy individuals. “We don’t know whether side effects, like allergic reactions, may be different in unstudied populations,” he said. “Thus, the medical and scientific community should prioritize clinical studies of safety and effectiveness of COVID-19 vaccines in immunocompromised populations.”
So, what does this mean for an individual with an immune-mediated inflammatory disease like Crohn’s disease or ulcerative colitis who is immunocompromised? Dr. Kaplan explained that it is a balance between the potential harm of being infected with COVID-19 and the uncertainty of receiving a vaccine in an understudied population. For those who are highly susceptible to dying from COVID-19, such as an older adult with IBD, or someone who faces high exposure, such as a health care worker, the potential protection of the vaccine greatly outweighs the uncertainty.
“However, for individuals who are at otherwise lower risk – for example, young and able to work from home – then waiting a few extra months for postmarketing surveillance studies in immunocompromised populations may be a reasonable approach, as long as these individuals are taking great care to avoid infection,” he said.
No waiting needed
Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, feels that the newly approved vaccine should be safe for most of his patients.
“Patients with psoriatic disease should get the mRNA-based COVID-19 vaccine as soon as possible based on eligibility as determined by the CDC and local public health officials,” he said. “It is not a live vaccine, and therefore patients on biologics or other immune-modulating or immune-suppressing treatment can receive it.”
However, the impact of psoriasis treatment on immune response to the mRNA-based vaccines is not known. Dr. Gelfand noted that, extrapolating from the vaccine literature, there is some evidence that methotrexate reduces response to the influenza vaccine. “However, the clinical significance of this finding is not clear,” he said. “Since the mRNA vaccine needs to be taken twice, a few weeks apart, I do not recommend interrupting or delaying treatment for psoriatic disease while undergoing vaccination for COVID-19.”
Given the reports of allergic reactions, he added that it is advisable for patients with a history of life-threatening allergic reactions such as anaphylaxis or who have been advised to carry an epinephrine autoinjector, to talk with their health care provider to determine if COVID-19 vaccination is medically appropriate.
The National Psoriasis Foundation has issued guidance on COVID-19, explained Steven R. Feldman, MD, PhD, professor of dermatology, pathology, and social sciences & health policy at Wake Forest University, Winston-Salem, N.C., who is also a member of the committee that is working on those guidelines and keeping them up to date. “We are in the process of updating the guidelines with information on COVID vaccines,” he said.
He agreed that there are no contraindications for psoriasis patients to receive the vaccine, regardless of whether they are on immunosuppressive treatment, even though definitive data are lacking. “Fortunately, there’s a lot of good data coming out of Italy that patients with psoriasis on biologics do not appear to be at increased risk of getting COVID or of having worse outcomes from COVID,” he said.
Patients are going to ask about the vaccines, and when counseling them, clinicians should discuss the available data, the residual uncertainty, and patients’ concerns should be considered, Dr. Feldman explained. “There may be some concern that steroids and cyclosporine would reduce the effectiveness of vaccines, but there is no concern that any of the drugs would cause increased risk from nonlive vaccines.”
He added that there is evidence that “patients on biologics who receive nonlive vaccines do develop antibody responses and are immunized.”
Boosting efficacy
Even prior to making their announcement, the American College of Rheumatology had said that they would endorse the vaccine for all patients, explained rheumatologist Brett Smith, DO, from Blount Memorial Physicians Group and East Tennessee Children’s Hospital, Alcoa. “The vaccine is safe for all patients, but the problem may be that it’s not as effective,” he said. “But we don’t know that because it hasn’t been tested.”
With other vaccines, biologic medicines are held for 2 weeks before and afterwards, to get the best response. “But some patients don’t want to stop the medication,” Dr. Smith said. “They are afraid that their symptoms will return.”
As for counseling patients as to whether they should receive this vaccine, he explained that he typically doesn’t try to sway patients one way or another until they are really high risk. “When I counsel, it really depends on the individual situation. And for this vaccine, we have to be open to the fact that many people have already made up their mind.”
There are a lot of questions regarding the vaccine. One is the short time frame of development. “Vaccines typically take 6-10 years to come on the market, and this one is now available after a 3-month study,” Dr. Smith said. “Some have already decided that it’s too new for them.”
The process is also new, and patients need to understand that it doesn’t contain an active virus and “you can’t catch coronavirus from it.”
Dr. Smith also explained that, because the vaccine may be less effective in a person using biologic therapies, there is currently no information available on repeat vaccination. “These are all unanswered questions,” he said. “If the antibodies wane in a short time, can we be revaccinated and in what time frame? We just don’t know that yet.”
Marcelo Bonomi, MD, a medical oncologist from The Ohio State University Comprehensive Cancer Center, Columbus, explained that one way to ensure a more optimal response to the vaccine would be to wait until the patient has finished chemotherapy.* “The vaccine can be offered at that time, and in the meantime, they can take other steps to avoid infection,” he said. “If they are very immunosuppressed, it isn’t worth trying to give the vaccine.”
Cancer patients should be encouraged to stay as healthy as possible, and to wear masks and social distance. “It’s a comprehensive approach. Eat healthy, avoid alcohol and tobacco, and exercise. [These things] will help boost the immune system,” Dr. Bonomi said. “Family members should be encouraged to get vaccinated, which will help them avoid infection and exposing the patient.”
Jim Boonyaratanakornkit, MD, PhD, an infectious disease specialist who cares for cancer patients at the Fred Hutchinson Cancer Research Center, agreed. “Giving a vaccine right after a transplant is a futile endeavor,” he said. “We need to wait 6 months to have an immune response.”
He pointed out there may be a continuing higher number of cases, with high levels peaking in Washington in February and March. “Close friends and family should be vaccinated if possible,” he said, “which will help interrupt transmission.”
The vaccines are using new platforms that are totally different, and there is no clear data as to how long the antibodies will persist. “We know that they last for at least 4 months,” said Dr. Boonyaratanakornkit. “We don’t know what level of antibody will protect them from COVID-19 infection. Current studies are being conducted, but we don’t have that information for anyone yet.”
*Correction, 1/7/21: An earlier version of this article misattributed quotes from Dr. Marcelo Bonomi.
Coronavirus vaccines have become a reality, as they are now being approved and authorized for use in a growing number of countries including the United States. The U.S. Food and Drug Administration has just issued emergency authorization for the use of the COVID-19 vaccine produced by Pfizer and BioNTech. Close behind is the vaccine developed by Moderna, which has also applied to the FDA for emergency authorization.
The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, and the FDA has said that the 95% credible interval for the vaccine efficacy was 90.3%-97.6%. But as with many initial clinical trials, whether for drugs or vaccines, not all populations were represented in the trial cohort, including individuals who are immunocompromised. At the current time, it is largely unknown how safe or effective the vaccine may be in this large population, many of whom are at high risk for serious COVID-19 complications.
At a special session held during the recent annual meeting of the American Society of Hematology, Anthony Fauci, MD, the nation’s leading infectious disease expert, said that individuals with compromised immune systems, whether because of chemotherapy or a bone marrow transplant, should plan to be vaccinated when the opportunity arises.
In response to a question from ASH President Stephanie J. Lee, MD, of the Fred Hutchinson Cancer Center, Seattle, Dr. Fauci emphasized that, despite being excluded from clinical trials, this population should get vaccinated. “I think we should recommend that they get vaccinated,” he said. “I mean, it is clear that, if you are on immunosuppressive agents, history tells us that you’re not going to have as robust a response as if you had an intact immune system that was not being compromised. But some degree of immunity is better than no degree of immunity.”
That does seem to be the consensus among experts who spoke in interviews: that as long as these are not live attenuated vaccines, they hold no specific risk to an immunocompromised patient, other than any factors specific to the individual that could be a contraindication.
“Patients, family members, friends, and work contacts should be encouraged to receive the vaccine,” said William Stohl, MD, PhD, chief of the division of rheumatology at the University of Southern California, Los Angeles. “Clinicians should advise patients to obtain the vaccine sooner rather than later.”
Kevin C. Wang, MD, PhD, of the department of dermatology at Stanford (Calif.) University, agreed. “I am 100% with Dr. Fauci. Everyone should get the vaccine, even if it may not be as effective,” he said. “I would treat it exactly like the flu vaccines that we recommend folks get every year.”
Dr. Wang noted that he couldn’t think of any contraindications unless the immunosuppressed patients have a history of severe allergic reactions to prior vaccinations. “But I would even say patients with history of cancer, upon recommendation of their oncologists, are likely to be suitable candidates for the vaccine,” he added. “I would say clinicians should approach counseling the same way they counsel patients for the flu vaccine, and as far as I know, there are no concerns for systemic drugs commonly used in dermatology patients.”
However, guidance has not yet been issued from either the FDA or the Centers for Disease Control and Prevention regarding the use of the vaccine in immunocompromised individuals. Given the lack of data, the FDA has said that “it will be something that providers will need to consider on an individual basis,” and that individuals should consult with physicians to weigh the potential benefits and potential risks.
The CDC’s Advisory Committee on Immunization Practices has said that clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies. The CDC itself has not yet released its formal guidance on vaccine use.
COVID-19 vaccines
Vaccines typically require years of research and testing before reaching the clinic, but this year researchers embarked on a global effort to develop safe and effective coronavirus vaccines in record time. Both the Pfizer/BioNTech and Moderna vaccines have only a few months of phase 3 clinical trial data, so much remains unknown about them, including their duration of effect and any long-term safety signals. In addition to excluding immunocompromised individuals, the clinical trials did not include children or pregnant women, so data are lacking for several population subgroups.
But these will not be the only vaccines available, as the pipeline is already becoming crowded. U.S. clinical trial data from a vaccine jointly being developed by Oxford-AstraZeneca, could potentially be ready, along with a request for FDA emergency use authorization, by late January 2021.
In addition, China and Russia have released vaccines, and there are currently 61 vaccines being investigated in clinical trials and at least 85 preclinical products under active investigation.
The vaccine candidates are using both conventional and novel mechanisms of action to elicit an immune response in patients. Conventional methods include attenuated inactivated (killed) virus and recombinant viral protein vaccines to develop immunity. Novel approaches include replication-deficient, adenovirus vector-based vaccines that contain the viral protein, and mRNA-based vaccines, such as the Pfizer and Moderna vaccines, that encode for a SARS-CoV-2 spike protein.
“The special vaccine concern for immunocompromised individuals is introduction of a live virus,” Dr. Stohl said. “Neither the Moderna nor Pfizer vaccines are live viruses, so there should be no special contraindication for such individuals.”
Live vaccine should be avoided in immunocompromised patients, and currently, live SARS-CoV-2 vaccines are only being developed in India and Turkey.
It is not unusual for vaccine trials to begin with cohorts that exclude participants with various health conditions, including those who are immunocompromised. These groups are generally then evaluated in phase 4 trials, or postmarketing surveillance. While the precise number of immunosuppressed adults in the United States is not known, the numbers are believed to be rising because of increased life expectancy among immunosuppressed adults as a result of advances in treatment and new and wider indications for therapies that can affect the immune system.
According to data from the 2013 National Health Interview Survey, an estimated 2.7% of U.S. adults are immunosuppressed. This population covers a broad array of health conditions and medical specialties; people living with inflammatory or autoimmune conditions, such as inflammatory rheumatic diseases (rheumatoid arthritis, axial spondyloarthritis, lupus); inflammatory bowel disease (Crohn’s disease and ulcerative colitis); psoriasis; multiple sclerosis; organ transplant recipients; patients undergoing chemotherapy; and life-long immunosuppression attributable to HIV infection.
As the vaccines begin to roll out and become available, how should clinicians advise their patients, in the absence of any clinical trial data?
Risk vs. benefit
Gilaad Kaplan, MD, MPH, a gastroenterologist and professor of medicine at the University of Calgary (Alta.), noted that the inflammatory bowel disease (IBD) community has dealt with tremendous anxiety during the pandemic because many are immunocompromised because of the medications they use to treat their disease.
“For example, many patients with IBD are on biologics like anti-TNF [tumor necrosis factor] therapies, which are also used in other immune-mediated inflammatory diseases such as rheumatoid arthritis,” he said. “Understandably, individuals with IBD on immunosuppressive medications are concerned about the risk of severe complications due to COVID-19.”
The entire IBD community, along with the world, celebrated the announcement that multiple vaccines are protective against SARS-CoV-2, he noted. “Vaccines offer the potential to reduce the spread of COVID-19, allowing society to revert back to normalcy,” Dr. Kaplan said. “Moreover, for vulnerable populations, including those who are immunocompromised, vaccines offer the potential to directly protect them from the morbidity and mortality associated with COVID-19.”
That said, even though the news of vaccines are extremely promising, some cautions must be raised regarding their use in immunocompromised populations, such as persons with IBD. “The current trials, to my knowledge, did not include immunocompromised individuals and thus, we can only extrapolate from what we know from other trials of different vaccines,” he explained. “We know from prior vaccines studies that the immune response following vaccination is less robust in those who are immunocompromised as compared to a healthy control population.”
Dr. Kaplan also pointed to recent reports of allergic reactions that have been reported in healthy individuals. “We don’t know whether side effects, like allergic reactions, may be different in unstudied populations,” he said. “Thus, the medical and scientific community should prioritize clinical studies of safety and effectiveness of COVID-19 vaccines in immunocompromised populations.”
So, what does this mean for an individual with an immune-mediated inflammatory disease like Crohn’s disease or ulcerative colitis who is immunocompromised? Dr. Kaplan explained that it is a balance between the potential harm of being infected with COVID-19 and the uncertainty of receiving a vaccine in an understudied population. For those who are highly susceptible to dying from COVID-19, such as an older adult with IBD, or someone who faces high exposure, such as a health care worker, the potential protection of the vaccine greatly outweighs the uncertainty.
“However, for individuals who are at otherwise lower risk – for example, young and able to work from home – then waiting a few extra months for postmarketing surveillance studies in immunocompromised populations may be a reasonable approach, as long as these individuals are taking great care to avoid infection,” he said.
No waiting needed
Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, feels that the newly approved vaccine should be safe for most of his patients.
“Patients with psoriatic disease should get the mRNA-based COVID-19 vaccine as soon as possible based on eligibility as determined by the CDC and local public health officials,” he said. “It is not a live vaccine, and therefore patients on biologics or other immune-modulating or immune-suppressing treatment can receive it.”
However, the impact of psoriasis treatment on immune response to the mRNA-based vaccines is not known. Dr. Gelfand noted that, extrapolating from the vaccine literature, there is some evidence that methotrexate reduces response to the influenza vaccine. “However, the clinical significance of this finding is not clear,” he said. “Since the mRNA vaccine needs to be taken twice, a few weeks apart, I do not recommend interrupting or delaying treatment for psoriatic disease while undergoing vaccination for COVID-19.”
Given the reports of allergic reactions, he added that it is advisable for patients with a history of life-threatening allergic reactions such as anaphylaxis or who have been advised to carry an epinephrine autoinjector, to talk with their health care provider to determine if COVID-19 vaccination is medically appropriate.
The National Psoriasis Foundation has issued guidance on COVID-19, explained Steven R. Feldman, MD, PhD, professor of dermatology, pathology, and social sciences & health policy at Wake Forest University, Winston-Salem, N.C., who is also a member of the committee that is working on those guidelines and keeping them up to date. “We are in the process of updating the guidelines with information on COVID vaccines,” he said.
He agreed that there are no contraindications for psoriasis patients to receive the vaccine, regardless of whether they are on immunosuppressive treatment, even though definitive data are lacking. “Fortunately, there’s a lot of good data coming out of Italy that patients with psoriasis on biologics do not appear to be at increased risk of getting COVID or of having worse outcomes from COVID,” he said.
Patients are going to ask about the vaccines, and when counseling them, clinicians should discuss the available data, the residual uncertainty, and patients’ concerns should be considered, Dr. Feldman explained. “There may be some concern that steroids and cyclosporine would reduce the effectiveness of vaccines, but there is no concern that any of the drugs would cause increased risk from nonlive vaccines.”
He added that there is evidence that “patients on biologics who receive nonlive vaccines do develop antibody responses and are immunized.”
Boosting efficacy
Even prior to making their announcement, the American College of Rheumatology had said that they would endorse the vaccine for all patients, explained rheumatologist Brett Smith, DO, from Blount Memorial Physicians Group and East Tennessee Children’s Hospital, Alcoa. “The vaccine is safe for all patients, but the problem may be that it’s not as effective,” he said. “But we don’t know that because it hasn’t been tested.”
With other vaccines, biologic medicines are held for 2 weeks before and afterwards, to get the best response. “But some patients don’t want to stop the medication,” Dr. Smith said. “They are afraid that their symptoms will return.”
As for counseling patients as to whether they should receive this vaccine, he explained that he typically doesn’t try to sway patients one way or another until they are really high risk. “When I counsel, it really depends on the individual situation. And for this vaccine, we have to be open to the fact that many people have already made up their mind.”
There are a lot of questions regarding the vaccine. One is the short time frame of development. “Vaccines typically take 6-10 years to come on the market, and this one is now available after a 3-month study,” Dr. Smith said. “Some have already decided that it’s too new for them.”
The process is also new, and patients need to understand that it doesn’t contain an active virus and “you can’t catch coronavirus from it.”
Dr. Smith also explained that, because the vaccine may be less effective in a person using biologic therapies, there is currently no information available on repeat vaccination. “These are all unanswered questions,” he said. “If the antibodies wane in a short time, can we be revaccinated and in what time frame? We just don’t know that yet.”
Marcelo Bonomi, MD, a medical oncologist from The Ohio State University Comprehensive Cancer Center, Columbus, explained that one way to ensure a more optimal response to the vaccine would be to wait until the patient has finished chemotherapy.* “The vaccine can be offered at that time, and in the meantime, they can take other steps to avoid infection,” he said. “If they are very immunosuppressed, it isn’t worth trying to give the vaccine.”
Cancer patients should be encouraged to stay as healthy as possible, and to wear masks and social distance. “It’s a comprehensive approach. Eat healthy, avoid alcohol and tobacco, and exercise. [These things] will help boost the immune system,” Dr. Bonomi said. “Family members should be encouraged to get vaccinated, which will help them avoid infection and exposing the patient.”
Jim Boonyaratanakornkit, MD, PhD, an infectious disease specialist who cares for cancer patients at the Fred Hutchinson Cancer Research Center, agreed. “Giving a vaccine right after a transplant is a futile endeavor,” he said. “We need to wait 6 months to have an immune response.”
He pointed out there may be a continuing higher number of cases, with high levels peaking in Washington in February and March. “Close friends and family should be vaccinated if possible,” he said, “which will help interrupt transmission.”
The vaccines are using new platforms that are totally different, and there is no clear data as to how long the antibodies will persist. “We know that they last for at least 4 months,” said Dr. Boonyaratanakornkit. “We don’t know what level of antibody will protect them from COVID-19 infection. Current studies are being conducted, but we don’t have that information for anyone yet.”
*Correction, 1/7/21: An earlier version of this article misattributed quotes from Dr. Marcelo Bonomi.
Coronavirus vaccines have become a reality, as they are now being approved and authorized for use in a growing number of countries including the United States. The U.S. Food and Drug Administration has just issued emergency authorization for the use of the COVID-19 vaccine produced by Pfizer and BioNTech. Close behind is the vaccine developed by Moderna, which has also applied to the FDA for emergency authorization.
The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, and the FDA has said that the 95% credible interval for the vaccine efficacy was 90.3%-97.6%. But as with many initial clinical trials, whether for drugs or vaccines, not all populations were represented in the trial cohort, including individuals who are immunocompromised. At the current time, it is largely unknown how safe or effective the vaccine may be in this large population, many of whom are at high risk for serious COVID-19 complications.
At a special session held during the recent annual meeting of the American Society of Hematology, Anthony Fauci, MD, the nation’s leading infectious disease expert, said that individuals with compromised immune systems, whether because of chemotherapy or a bone marrow transplant, should plan to be vaccinated when the opportunity arises.
In response to a question from ASH President Stephanie J. Lee, MD, of the Fred Hutchinson Cancer Center, Seattle, Dr. Fauci emphasized that, despite being excluded from clinical trials, this population should get vaccinated. “I think we should recommend that they get vaccinated,” he said. “I mean, it is clear that, if you are on immunosuppressive agents, history tells us that you’re not going to have as robust a response as if you had an intact immune system that was not being compromised. But some degree of immunity is better than no degree of immunity.”
That does seem to be the consensus among experts who spoke in interviews: that as long as these are not live attenuated vaccines, they hold no specific risk to an immunocompromised patient, other than any factors specific to the individual that could be a contraindication.
“Patients, family members, friends, and work contacts should be encouraged to receive the vaccine,” said William Stohl, MD, PhD, chief of the division of rheumatology at the University of Southern California, Los Angeles. “Clinicians should advise patients to obtain the vaccine sooner rather than later.”
Kevin C. Wang, MD, PhD, of the department of dermatology at Stanford (Calif.) University, agreed. “I am 100% with Dr. Fauci. Everyone should get the vaccine, even if it may not be as effective,” he said. “I would treat it exactly like the flu vaccines that we recommend folks get every year.”
Dr. Wang noted that he couldn’t think of any contraindications unless the immunosuppressed patients have a history of severe allergic reactions to prior vaccinations. “But I would even say patients with history of cancer, upon recommendation of their oncologists, are likely to be suitable candidates for the vaccine,” he added. “I would say clinicians should approach counseling the same way they counsel patients for the flu vaccine, and as far as I know, there are no concerns for systemic drugs commonly used in dermatology patients.”
However, guidance has not yet been issued from either the FDA or the Centers for Disease Control and Prevention regarding the use of the vaccine in immunocompromised individuals. Given the lack of data, the FDA has said that “it will be something that providers will need to consider on an individual basis,” and that individuals should consult with physicians to weigh the potential benefits and potential risks.
The CDC’s Advisory Committee on Immunization Practices has said that clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies. The CDC itself has not yet released its formal guidance on vaccine use.
COVID-19 vaccines
Vaccines typically require years of research and testing before reaching the clinic, but this year researchers embarked on a global effort to develop safe and effective coronavirus vaccines in record time. Both the Pfizer/BioNTech and Moderna vaccines have only a few months of phase 3 clinical trial data, so much remains unknown about them, including their duration of effect and any long-term safety signals. In addition to excluding immunocompromised individuals, the clinical trials did not include children or pregnant women, so data are lacking for several population subgroups.
But these will not be the only vaccines available, as the pipeline is already becoming crowded. U.S. clinical trial data from a vaccine jointly being developed by Oxford-AstraZeneca, could potentially be ready, along with a request for FDA emergency use authorization, by late January 2021.
In addition, China and Russia have released vaccines, and there are currently 61 vaccines being investigated in clinical trials and at least 85 preclinical products under active investigation.
The vaccine candidates are using both conventional and novel mechanisms of action to elicit an immune response in patients. Conventional methods include attenuated inactivated (killed) virus and recombinant viral protein vaccines to develop immunity. Novel approaches include replication-deficient, adenovirus vector-based vaccines that contain the viral protein, and mRNA-based vaccines, such as the Pfizer and Moderna vaccines, that encode for a SARS-CoV-2 spike protein.
“The special vaccine concern for immunocompromised individuals is introduction of a live virus,” Dr. Stohl said. “Neither the Moderna nor Pfizer vaccines are live viruses, so there should be no special contraindication for such individuals.”
Live vaccine should be avoided in immunocompromised patients, and currently, live SARS-CoV-2 vaccines are only being developed in India and Turkey.
It is not unusual for vaccine trials to begin with cohorts that exclude participants with various health conditions, including those who are immunocompromised. These groups are generally then evaluated in phase 4 trials, or postmarketing surveillance. While the precise number of immunosuppressed adults in the United States is not known, the numbers are believed to be rising because of increased life expectancy among immunosuppressed adults as a result of advances in treatment and new and wider indications for therapies that can affect the immune system.
According to data from the 2013 National Health Interview Survey, an estimated 2.7% of U.S. adults are immunosuppressed. This population covers a broad array of health conditions and medical specialties; people living with inflammatory or autoimmune conditions, such as inflammatory rheumatic diseases (rheumatoid arthritis, axial spondyloarthritis, lupus); inflammatory bowel disease (Crohn’s disease and ulcerative colitis); psoriasis; multiple sclerosis; organ transplant recipients; patients undergoing chemotherapy; and life-long immunosuppression attributable to HIV infection.
As the vaccines begin to roll out and become available, how should clinicians advise their patients, in the absence of any clinical trial data?
Risk vs. benefit
Gilaad Kaplan, MD, MPH, a gastroenterologist and professor of medicine at the University of Calgary (Alta.), noted that the inflammatory bowel disease (IBD) community has dealt with tremendous anxiety during the pandemic because many are immunocompromised because of the medications they use to treat their disease.
“For example, many patients with IBD are on biologics like anti-TNF [tumor necrosis factor] therapies, which are also used in other immune-mediated inflammatory diseases such as rheumatoid arthritis,” he said. “Understandably, individuals with IBD on immunosuppressive medications are concerned about the risk of severe complications due to COVID-19.”
The entire IBD community, along with the world, celebrated the announcement that multiple vaccines are protective against SARS-CoV-2, he noted. “Vaccines offer the potential to reduce the spread of COVID-19, allowing society to revert back to normalcy,” Dr. Kaplan said. “Moreover, for vulnerable populations, including those who are immunocompromised, vaccines offer the potential to directly protect them from the morbidity and mortality associated with COVID-19.”
That said, even though the news of vaccines are extremely promising, some cautions must be raised regarding their use in immunocompromised populations, such as persons with IBD. “The current trials, to my knowledge, did not include immunocompromised individuals and thus, we can only extrapolate from what we know from other trials of different vaccines,” he explained. “We know from prior vaccines studies that the immune response following vaccination is less robust in those who are immunocompromised as compared to a healthy control population.”
Dr. Kaplan also pointed to recent reports of allergic reactions that have been reported in healthy individuals. “We don’t know whether side effects, like allergic reactions, may be different in unstudied populations,” he said. “Thus, the medical and scientific community should prioritize clinical studies of safety and effectiveness of COVID-19 vaccines in immunocompromised populations.”
So, what does this mean for an individual with an immune-mediated inflammatory disease like Crohn’s disease or ulcerative colitis who is immunocompromised? Dr. Kaplan explained that it is a balance between the potential harm of being infected with COVID-19 and the uncertainty of receiving a vaccine in an understudied population. For those who are highly susceptible to dying from COVID-19, such as an older adult with IBD, or someone who faces high exposure, such as a health care worker, the potential protection of the vaccine greatly outweighs the uncertainty.
“However, for individuals who are at otherwise lower risk – for example, young and able to work from home – then waiting a few extra months for postmarketing surveillance studies in immunocompromised populations may be a reasonable approach, as long as these individuals are taking great care to avoid infection,” he said.
No waiting needed
Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, feels that the newly approved vaccine should be safe for most of his patients.
“Patients with psoriatic disease should get the mRNA-based COVID-19 vaccine as soon as possible based on eligibility as determined by the CDC and local public health officials,” he said. “It is not a live vaccine, and therefore patients on biologics or other immune-modulating or immune-suppressing treatment can receive it.”
However, the impact of psoriasis treatment on immune response to the mRNA-based vaccines is not known. Dr. Gelfand noted that, extrapolating from the vaccine literature, there is some evidence that methotrexate reduces response to the influenza vaccine. “However, the clinical significance of this finding is not clear,” he said. “Since the mRNA vaccine needs to be taken twice, a few weeks apart, I do not recommend interrupting or delaying treatment for psoriatic disease while undergoing vaccination for COVID-19.”
Given the reports of allergic reactions, he added that it is advisable for patients with a history of life-threatening allergic reactions such as anaphylaxis or who have been advised to carry an epinephrine autoinjector, to talk with their health care provider to determine if COVID-19 vaccination is medically appropriate.
The National Psoriasis Foundation has issued guidance on COVID-19, explained Steven R. Feldman, MD, PhD, professor of dermatology, pathology, and social sciences & health policy at Wake Forest University, Winston-Salem, N.C., who is also a member of the committee that is working on those guidelines and keeping them up to date. “We are in the process of updating the guidelines with information on COVID vaccines,” he said.
He agreed that there are no contraindications for psoriasis patients to receive the vaccine, regardless of whether they are on immunosuppressive treatment, even though definitive data are lacking. “Fortunately, there’s a lot of good data coming out of Italy that patients with psoriasis on biologics do not appear to be at increased risk of getting COVID or of having worse outcomes from COVID,” he said.
Patients are going to ask about the vaccines, and when counseling them, clinicians should discuss the available data, the residual uncertainty, and patients’ concerns should be considered, Dr. Feldman explained. “There may be some concern that steroids and cyclosporine would reduce the effectiveness of vaccines, but there is no concern that any of the drugs would cause increased risk from nonlive vaccines.”
He added that there is evidence that “patients on biologics who receive nonlive vaccines do develop antibody responses and are immunized.”
Boosting efficacy
Even prior to making their announcement, the American College of Rheumatology had said that they would endorse the vaccine for all patients, explained rheumatologist Brett Smith, DO, from Blount Memorial Physicians Group and East Tennessee Children’s Hospital, Alcoa. “The vaccine is safe for all patients, but the problem may be that it’s not as effective,” he said. “But we don’t know that because it hasn’t been tested.”
With other vaccines, biologic medicines are held for 2 weeks before and afterwards, to get the best response. “But some patients don’t want to stop the medication,” Dr. Smith said. “They are afraid that their symptoms will return.”
As for counseling patients as to whether they should receive this vaccine, he explained that he typically doesn’t try to sway patients one way or another until they are really high risk. “When I counsel, it really depends on the individual situation. And for this vaccine, we have to be open to the fact that many people have already made up their mind.”
There are a lot of questions regarding the vaccine. One is the short time frame of development. “Vaccines typically take 6-10 years to come on the market, and this one is now available after a 3-month study,” Dr. Smith said. “Some have already decided that it’s too new for them.”
The process is also new, and patients need to understand that it doesn’t contain an active virus and “you can’t catch coronavirus from it.”
Dr. Smith also explained that, because the vaccine may be less effective in a person using biologic therapies, there is currently no information available on repeat vaccination. “These are all unanswered questions,” he said. “If the antibodies wane in a short time, can we be revaccinated and in what time frame? We just don’t know that yet.”
Marcelo Bonomi, MD, a medical oncologist from The Ohio State University Comprehensive Cancer Center, Columbus, explained that one way to ensure a more optimal response to the vaccine would be to wait until the patient has finished chemotherapy.* “The vaccine can be offered at that time, and in the meantime, they can take other steps to avoid infection,” he said. “If they are very immunosuppressed, it isn’t worth trying to give the vaccine.”
Cancer patients should be encouraged to stay as healthy as possible, and to wear masks and social distance. “It’s a comprehensive approach. Eat healthy, avoid alcohol and tobacco, and exercise. [These things] will help boost the immune system,” Dr. Bonomi said. “Family members should be encouraged to get vaccinated, which will help them avoid infection and exposing the patient.”
Jim Boonyaratanakornkit, MD, PhD, an infectious disease specialist who cares for cancer patients at the Fred Hutchinson Cancer Research Center, agreed. “Giving a vaccine right after a transplant is a futile endeavor,” he said. “We need to wait 6 months to have an immune response.”
He pointed out there may be a continuing higher number of cases, with high levels peaking in Washington in February and March. “Close friends and family should be vaccinated if possible,” he said, “which will help interrupt transmission.”
The vaccines are using new platforms that are totally different, and there is no clear data as to how long the antibodies will persist. “We know that they last for at least 4 months,” said Dr. Boonyaratanakornkit. “We don’t know what level of antibody will protect them from COVID-19 infection. Current studies are being conducted, but we don’t have that information for anyone yet.”
*Correction, 1/7/21: An earlier version of this article misattributed quotes from Dr. Marcelo Bonomi.
Parents favored virtual learning over in-person school attendance
Parents of school-aged children were generally more comfortable with full-time virtual learning in schools in the fall of 2020, compared with full-capacity in-person attendance, according to a survey conducted in July.
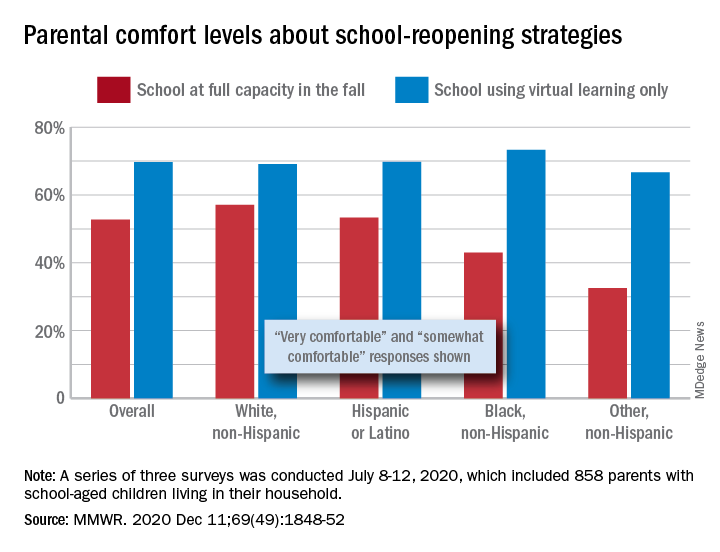
Those of racial/ethnic minorities, however, “were less likely to feel that schools should reopen for all students and were more concerned about” several aspects of in-person instruction than were White parents, Leah K. Gilbert, MD, and associates at the Centers for Disease Control and Prevention’s COVID-19 Response Team said in the Morbidity and Mortality Weekly Report.
A slim majority, just under 53% of the 858 parents surveyed, said that they were very or somewhat comfortable with their children returning to schools that were reopening at full capacity, while almost 70% said they were very/somewhat comfortable with schools going exclusively with virtual learning, the investigators reported.
The question about full-capacity attendance in particular showed considerable variation by race and ethnicity, with 57% of White parents saying they were very/somewhat comfortable, versus 53% of Hispanic or Latino parents, 43% of Black parents, and 32.5% of parents of other races/ethnicities (American Indian/Alaska Native, Asian, or multiracial).
Comfort levels were closer regarding virtual learning: Parents of other races/ethnicities were lowest at 67% and Black parents were highest at 73%. When asked about schools reopening at 50% capacity and 50% virtual learning, Black parents were again lowest at 58% with strong or moderate comfort and White parents were highest at 68%, Dr. Gilbert and associates said.
“Although the majority of parent respondents had concerns about both school reopening for in-person instruction and virtual learning, the perceived risk for SARS-CoV-2 infection and poor health outcomes might account for the differences in parental attitudes and concerns by race and ethnicity,” they wrote.
SOURCE: Gilbert LK et al. MMWR. 2020 Dec 11;69(49):1848-52.
Parents of school-aged children were generally more comfortable with full-time virtual learning in schools in the fall of 2020, compared with full-capacity in-person attendance, according to a survey conducted in July.

Those of racial/ethnic minorities, however, “were less likely to feel that schools should reopen for all students and were more concerned about” several aspects of in-person instruction than were White parents, Leah K. Gilbert, MD, and associates at the Centers for Disease Control and Prevention’s COVID-19 Response Team said in the Morbidity and Mortality Weekly Report.
A slim majority, just under 53% of the 858 parents surveyed, said that they were very or somewhat comfortable with their children returning to schools that were reopening at full capacity, while almost 70% said they were very/somewhat comfortable with schools going exclusively with virtual learning, the investigators reported.
The question about full-capacity attendance in particular showed considerable variation by race and ethnicity, with 57% of White parents saying they were very/somewhat comfortable, versus 53% of Hispanic or Latino parents, 43% of Black parents, and 32.5% of parents of other races/ethnicities (American Indian/Alaska Native, Asian, or multiracial).
Comfort levels were closer regarding virtual learning: Parents of other races/ethnicities were lowest at 67% and Black parents were highest at 73%. When asked about schools reopening at 50% capacity and 50% virtual learning, Black parents were again lowest at 58% with strong or moderate comfort and White parents were highest at 68%, Dr. Gilbert and associates said.
“Although the majority of parent respondents had concerns about both school reopening for in-person instruction and virtual learning, the perceived risk for SARS-CoV-2 infection and poor health outcomes might account for the differences in parental attitudes and concerns by race and ethnicity,” they wrote.
SOURCE: Gilbert LK et al. MMWR. 2020 Dec 11;69(49):1848-52.
Parents of school-aged children were generally more comfortable with full-time virtual learning in schools in the fall of 2020, compared with full-capacity in-person attendance, according to a survey conducted in July.

Those of racial/ethnic minorities, however, “were less likely to feel that schools should reopen for all students and were more concerned about” several aspects of in-person instruction than were White parents, Leah K. Gilbert, MD, and associates at the Centers for Disease Control and Prevention’s COVID-19 Response Team said in the Morbidity and Mortality Weekly Report.
A slim majority, just under 53% of the 858 parents surveyed, said that they were very or somewhat comfortable with their children returning to schools that were reopening at full capacity, while almost 70% said they were very/somewhat comfortable with schools going exclusively with virtual learning, the investigators reported.
The question about full-capacity attendance in particular showed considerable variation by race and ethnicity, with 57% of White parents saying they were very/somewhat comfortable, versus 53% of Hispanic or Latino parents, 43% of Black parents, and 32.5% of parents of other races/ethnicities (American Indian/Alaska Native, Asian, or multiracial).
Comfort levels were closer regarding virtual learning: Parents of other races/ethnicities were lowest at 67% and Black parents were highest at 73%. When asked about schools reopening at 50% capacity and 50% virtual learning, Black parents were again lowest at 58% with strong or moderate comfort and White parents were highest at 68%, Dr. Gilbert and associates said.
“Although the majority of parent respondents had concerns about both school reopening for in-person instruction and virtual learning, the perceived risk for SARS-CoV-2 infection and poor health outcomes might account for the differences in parental attitudes and concerns by race and ethnicity,” they wrote.
SOURCE: Gilbert LK et al. MMWR. 2020 Dec 11;69(49):1848-52.
FROM MMWR
Beware a pair of dermatologic emergencies in children
in a presentation at MedscapeLive’s virtual Women’s & Pediatric Dermatology Seminar.
Eczema herpeticum is a condition in which a herpes simplex virus (HSV-1 or HSV-2) is superimposed over preexisting eczema. “The infection may be primary and sustained from a close contact or result in some of our older patients from reactivation and spread through autoinoculation,” said Dr. Hightower, of Rady Children’s Hospital and the University of California, both in San Diego.
Signs, he said, include acute worsening of atopic dermatitis with new-onset vesicles, pustules, and “punched-out” hemorrhagic crusted erosions. “Presentation ranges from mild to transient to life threatening.”
Potential complications include meningitis, encephalitis, hepatitis, and chronic conjunctivitis. “That’s why immediate ophthalmological evaluation is needed when there’s involvement on the face near the eye,” he said.
As for management and care, “where I have concern for HSV patients, I get HSV [polymerase chain reaction] as well as a bacterial culture,” he said. But even before the results are available, empiric treatment with acyclovir can be appropriate. “It’s got to be systemic for these kids with severe involvement,” he said, and they should also be started on medication for staphylococci and streptococci.
During his presentation, Dr. Hightower also highlighted staphylococcal scalded skin syndrome. Patients with the disease commonly have concurrent skin pain (which can appear to be fussiness), fever, irritability, malaise, and poor feeding. Examination may reveal widespread erythema with accentuation at folds/peeling at hands and large sheets of superficial peeling scale with diffuse erythema.
Widespread skin involvement “results not from the presence of staph throughout the skin, but the exotoxin that it produces that becomes systemic,” he said. “Clinical diagnosis is supported by presence of S. aureus on bacterial culture, but the presence of staph is not necessary to make the diagnosis. When in doubt, histopathology is helpful. But again, it’s not necessary to make the diagnosis.”
Cases can be managed with a first- or second-generation cephalosporin, he said. Alternative therapies include antistaphylococcus penicillinase-resistant penicillins (oxacillin or nafcillin) or vancomycin.
While Dr. Hightower doesn’t use clindamycin in these patients, he said it’s an option that some dermatologists consider because of its antistaphylococcus activity. “Historically, people thought it may decrease exotoxin production. The big concern if you are going to use clindamycin is that there are high rates of community resistance,” he said. “So you want to be careful that you know your resistance patterns wherever you are. Follow up on culture to make sure that you have adequate coverage for the bug that the kiddo in front of you has.”
Dr. Hightower reported no relevant disclosures. MedscapeLive and this news organization are owned by the same parent company.
in a presentation at MedscapeLive’s virtual Women’s & Pediatric Dermatology Seminar.
Eczema herpeticum is a condition in which a herpes simplex virus (HSV-1 or HSV-2) is superimposed over preexisting eczema. “The infection may be primary and sustained from a close contact or result in some of our older patients from reactivation and spread through autoinoculation,” said Dr. Hightower, of Rady Children’s Hospital and the University of California, both in San Diego.
Signs, he said, include acute worsening of atopic dermatitis with new-onset vesicles, pustules, and “punched-out” hemorrhagic crusted erosions. “Presentation ranges from mild to transient to life threatening.”
Potential complications include meningitis, encephalitis, hepatitis, and chronic conjunctivitis. “That’s why immediate ophthalmological evaluation is needed when there’s involvement on the face near the eye,” he said.
As for management and care, “where I have concern for HSV patients, I get HSV [polymerase chain reaction] as well as a bacterial culture,” he said. But even before the results are available, empiric treatment with acyclovir can be appropriate. “It’s got to be systemic for these kids with severe involvement,” he said, and they should also be started on medication for staphylococci and streptococci.
During his presentation, Dr. Hightower also highlighted staphylococcal scalded skin syndrome. Patients with the disease commonly have concurrent skin pain (which can appear to be fussiness), fever, irritability, malaise, and poor feeding. Examination may reveal widespread erythema with accentuation at folds/peeling at hands and large sheets of superficial peeling scale with diffuse erythema.
Widespread skin involvement “results not from the presence of staph throughout the skin, but the exotoxin that it produces that becomes systemic,” he said. “Clinical diagnosis is supported by presence of S. aureus on bacterial culture, but the presence of staph is not necessary to make the diagnosis. When in doubt, histopathology is helpful. But again, it’s not necessary to make the diagnosis.”
Cases can be managed with a first- or second-generation cephalosporin, he said. Alternative therapies include antistaphylococcus penicillinase-resistant penicillins (oxacillin or nafcillin) or vancomycin.
While Dr. Hightower doesn’t use clindamycin in these patients, he said it’s an option that some dermatologists consider because of its antistaphylococcus activity. “Historically, people thought it may decrease exotoxin production. The big concern if you are going to use clindamycin is that there are high rates of community resistance,” he said. “So you want to be careful that you know your resistance patterns wherever you are. Follow up on culture to make sure that you have adequate coverage for the bug that the kiddo in front of you has.”
Dr. Hightower reported no relevant disclosures. MedscapeLive and this news organization are owned by the same parent company.
in a presentation at MedscapeLive’s virtual Women’s & Pediatric Dermatology Seminar.
Eczema herpeticum is a condition in which a herpes simplex virus (HSV-1 or HSV-2) is superimposed over preexisting eczema. “The infection may be primary and sustained from a close contact or result in some of our older patients from reactivation and spread through autoinoculation,” said Dr. Hightower, of Rady Children’s Hospital and the University of California, both in San Diego.
Signs, he said, include acute worsening of atopic dermatitis with new-onset vesicles, pustules, and “punched-out” hemorrhagic crusted erosions. “Presentation ranges from mild to transient to life threatening.”
Potential complications include meningitis, encephalitis, hepatitis, and chronic conjunctivitis. “That’s why immediate ophthalmological evaluation is needed when there’s involvement on the face near the eye,” he said.
As for management and care, “where I have concern for HSV patients, I get HSV [polymerase chain reaction] as well as a bacterial culture,” he said. But even before the results are available, empiric treatment with acyclovir can be appropriate. “It’s got to be systemic for these kids with severe involvement,” he said, and they should also be started on medication for staphylococci and streptococci.
During his presentation, Dr. Hightower also highlighted staphylococcal scalded skin syndrome. Patients with the disease commonly have concurrent skin pain (which can appear to be fussiness), fever, irritability, malaise, and poor feeding. Examination may reveal widespread erythema with accentuation at folds/peeling at hands and large sheets of superficial peeling scale with diffuse erythema.
Widespread skin involvement “results not from the presence of staph throughout the skin, but the exotoxin that it produces that becomes systemic,” he said. “Clinical diagnosis is supported by presence of S. aureus on bacterial culture, but the presence of staph is not necessary to make the diagnosis. When in doubt, histopathology is helpful. But again, it’s not necessary to make the diagnosis.”
Cases can be managed with a first- or second-generation cephalosporin, he said. Alternative therapies include antistaphylococcus penicillinase-resistant penicillins (oxacillin or nafcillin) or vancomycin.
While Dr. Hightower doesn’t use clindamycin in these patients, he said it’s an option that some dermatologists consider because of its antistaphylococcus activity. “Historically, people thought it may decrease exotoxin production. The big concern if you are going to use clindamycin is that there are high rates of community resistance,” he said. “So you want to be careful that you know your resistance patterns wherever you are. Follow up on culture to make sure that you have adequate coverage for the bug that the kiddo in front of you has.”
Dr. Hightower reported no relevant disclosures. MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Coronavirus has infected over 2% of U.S. children
After last week’s ever-so-slightly positive news, the COVID-19 numbers in children have gone back to their old ways.
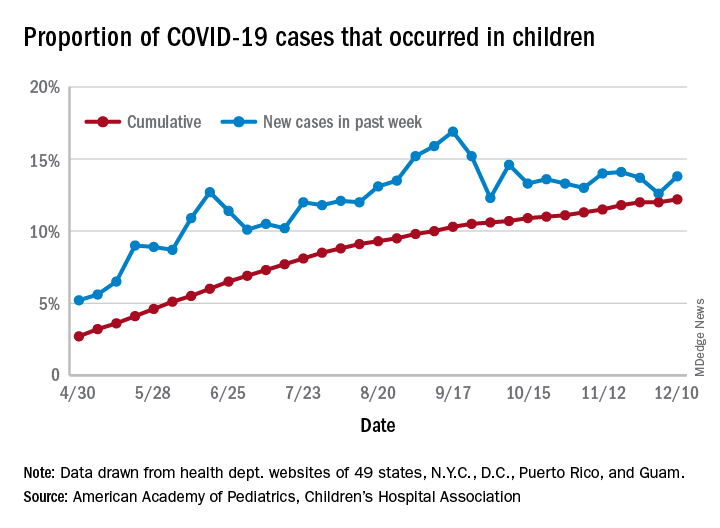
For the week ending Dec. 10, there were 178,823 new COVID-19 cases reported in U.S. children, the highest weekly total yet during the pandemic. The number of new cases had dropped the week before after setting a new high of almost 154,000 during the last full week of November, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
A new weekly high has been seen in 9 of the last 10 weeks, during which time the weekly total of child cases has gone from just over 40,000 (week ending Oct. 8) to almost 179,000, the two organizations said.
and that 2.1% of all children (2,179 per 100,000) in the United States have been infected with the coronavirus, the AAP and CHA said in their weekly report, which includes health department data from 49 states (New York does not report age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
The cumulative proportion of 12.2% has been exceeded in 27 states, as well as Puerto Rico and Guam, with the highest coming in Wyoming (21.3%), South Carolina (18.1%), and Tennessee (18.1%) and the lowest in Florida (6.7%, but the state uses an age range of 0-14 years) and New Jersey (7.6%), the AAP/CHA data show.
In a separate statement, AAP president Sally Goza, MD, welcomed the approval of the Pfizer-BioNTech COVID-19 vaccine but noted that the “virus is at unprecedented levels in nearly every community in the U.S., and in many areas, our health care system is terribly overburdened. The vaccine will not solve this overnight. I urge everyone to continue to practice social distancing, and wear masks or cloth face coverings, and get a flu shot, so we can protect the people we care about.”
Dr. Goza continued: “We applaud Pfizer-BioNTech for including children ages 12 through 17 in their clinical trials and we look forward to learning more about the data from children aged 12-15. We also want to acknowledge the discussion during the committee meeting on including 16- to 17-year-olds in the EUA [emergency-use authorization]. We believe that discussion underscores the need to keep expanding these trials to the pediatric population so we can collect robust data on this age group.”
[email protected]
After last week’s ever-so-slightly positive news, the COVID-19 numbers in children have gone back to their old ways.

For the week ending Dec. 10, there were 178,823 new COVID-19 cases reported in U.S. children, the highest weekly total yet during the pandemic. The number of new cases had dropped the week before after setting a new high of almost 154,000 during the last full week of November, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
A new weekly high has been seen in 9 of the last 10 weeks, during which time the weekly total of child cases has gone from just over 40,000 (week ending Oct. 8) to almost 179,000, the two organizations said.
and that 2.1% of all children (2,179 per 100,000) in the United States have been infected with the coronavirus, the AAP and CHA said in their weekly report, which includes health department data from 49 states (New York does not report age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
The cumulative proportion of 12.2% has been exceeded in 27 states, as well as Puerto Rico and Guam, with the highest coming in Wyoming (21.3%), South Carolina (18.1%), and Tennessee (18.1%) and the lowest in Florida (6.7%, but the state uses an age range of 0-14 years) and New Jersey (7.6%), the AAP/CHA data show.
In a separate statement, AAP president Sally Goza, MD, welcomed the approval of the Pfizer-BioNTech COVID-19 vaccine but noted that the “virus is at unprecedented levels in nearly every community in the U.S., and in many areas, our health care system is terribly overburdened. The vaccine will not solve this overnight. I urge everyone to continue to practice social distancing, and wear masks or cloth face coverings, and get a flu shot, so we can protect the people we care about.”
Dr. Goza continued: “We applaud Pfizer-BioNTech for including children ages 12 through 17 in their clinical trials and we look forward to learning more about the data from children aged 12-15. We also want to acknowledge the discussion during the committee meeting on including 16- to 17-year-olds in the EUA [emergency-use authorization]. We believe that discussion underscores the need to keep expanding these trials to the pediatric population so we can collect robust data on this age group.”
[email protected]
After last week’s ever-so-slightly positive news, the COVID-19 numbers in children have gone back to their old ways.

For the week ending Dec. 10, there were 178,823 new COVID-19 cases reported in U.S. children, the highest weekly total yet during the pandemic. The number of new cases had dropped the week before after setting a new high of almost 154,000 during the last full week of November, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
A new weekly high has been seen in 9 of the last 10 weeks, during which time the weekly total of child cases has gone from just over 40,000 (week ending Oct. 8) to almost 179,000, the two organizations said.
and that 2.1% of all children (2,179 per 100,000) in the United States have been infected with the coronavirus, the AAP and CHA said in their weekly report, which includes health department data from 49 states (New York does not report age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
The cumulative proportion of 12.2% has been exceeded in 27 states, as well as Puerto Rico and Guam, with the highest coming in Wyoming (21.3%), South Carolina (18.1%), and Tennessee (18.1%) and the lowest in Florida (6.7%, but the state uses an age range of 0-14 years) and New Jersey (7.6%), the AAP/CHA data show.
In a separate statement, AAP president Sally Goza, MD, welcomed the approval of the Pfizer-BioNTech COVID-19 vaccine but noted that the “virus is at unprecedented levels in nearly every community in the U.S., and in many areas, our health care system is terribly overburdened. The vaccine will not solve this overnight. I urge everyone to continue to practice social distancing, and wear masks or cloth face coverings, and get a flu shot, so we can protect the people we care about.”
Dr. Goza continued: “We applaud Pfizer-BioNTech for including children ages 12 through 17 in their clinical trials and we look forward to learning more about the data from children aged 12-15. We also want to acknowledge the discussion during the committee meeting on including 16- to 17-year-olds in the EUA [emergency-use authorization]. We believe that discussion underscores the need to keep expanding these trials to the pediatric population so we can collect robust data on this age group.”
[email protected]
A shot in the arm
As the COVID-19 vaccine candidates have begun to roll off the production lines into the distribution networks by the millions, media coverage almost universally includes a still photo or video of someone receiving an injection. Ever observant, a retired lawyer friend of mine who learned to give shots when he was in the Army and again more recently while taking a wilderness survival course emailed me his concerns about what he felt were examples of poor injection technique. Included in his commentary was an Internet link in which a physician, who I suspect may have been a pediatrician, demonstrated what the physician considered proper intramuscular injection technique, which included a single-handed aspiration prior to giving the injection allowing the free hand to stabilize the patient’s – in this case a child’s – arm during the entire process.
I replied to my friend that I too was often troubled by what I considered to be poor injection technique. But, I said the physician in the link touting his improved technique was misguided. My understanding has been that unless the injection site is in the gluteus, there is no need aspirate prior to an intramuscular vaccine injection because the risk of intravascular injection is so small. I then confirmed this by reviewing the Centers for Disease Control and Prevention’s Vaccine Recommendations and Guidelines of the Advisory Committee on Immunization Practices, which was updated in June 2019. Included in those recommendations was the observation that the vaccine administrator does not need to wear gloves unless he or she has open lesions or is at risk from contacting the recipient’s body fluids.
Like many of the technical skills one learns in training, giving intramuscular injections is probably an example of the “see one, do one, teach one” mantra. But in the case of giving shots, I don’t recall any teaching. Do you? It was more “see a dozen and get on with it.” Or maybe you trained in an environment in which nurses gave all the injections. I hope not.
When it comes to giving immunizations to children, the art is in entering into that encounter with a calm, matter-of-fact attitude and body language, hiding the needle, firmly restraining the child, and moving quickly and smoothly. Aspirating and glove donning merely add to the drama and waste time. But how did I learn that art? No one taught me. Like many clinical skills, I watched scores of nurses and physicians, mentally logging in their tricks and mistakes that would help me craft my style.
I always felt and still feel that providing immunizations was per hour spent, the most valuable investment of my time. Doing the injecting myself was both the most efficient way to provide the service, and also emphasized the importance that I placed on the immunization. In the process of my 40-plus–year career, that included several hundred thousand patient encounters in which I gave innumerable injections. And, I egotistically assumed that I was good at it because many infants never cried, and a few children said, “That didn’t hurt.” I suspect you can make the same claim.
Injecting millions of adults with a COVID-19 vaccine, on the other hand, is a piece of cake because restraining the recipient shouldn’t factor into the scenario. However, I wonder who is going to administer all those millions of injections and who is going to train them? How many of the trainers are aware of the CDC-ACIP guidelines? Or, are they going to fall back on old techniques that lack evidence support?
From the efficiency standpoint, it probably doesn’t make much difference. The injection takes but a few seconds. Filling out the paperwork and waiting for the recipient to figure out how to expose his or her deltoid can take fifty times that long.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
As the COVID-19 vaccine candidates have begun to roll off the production lines into the distribution networks by the millions, media coverage almost universally includes a still photo or video of someone receiving an injection. Ever observant, a retired lawyer friend of mine who learned to give shots when he was in the Army and again more recently while taking a wilderness survival course emailed me his concerns about what he felt were examples of poor injection technique. Included in his commentary was an Internet link in which a physician, who I suspect may have been a pediatrician, demonstrated what the physician considered proper intramuscular injection technique, which included a single-handed aspiration prior to giving the injection allowing the free hand to stabilize the patient’s – in this case a child’s – arm during the entire process.
I replied to my friend that I too was often troubled by what I considered to be poor injection technique. But, I said the physician in the link touting his improved technique was misguided. My understanding has been that unless the injection site is in the gluteus, there is no need aspirate prior to an intramuscular vaccine injection because the risk of intravascular injection is so small. I then confirmed this by reviewing the Centers for Disease Control and Prevention’s Vaccine Recommendations and Guidelines of the Advisory Committee on Immunization Practices, which was updated in June 2019. Included in those recommendations was the observation that the vaccine administrator does not need to wear gloves unless he or she has open lesions or is at risk from contacting the recipient’s body fluids.
Like many of the technical skills one learns in training, giving intramuscular injections is probably an example of the “see one, do one, teach one” mantra. But in the case of giving shots, I don’t recall any teaching. Do you? It was more “see a dozen and get on with it.” Or maybe you trained in an environment in which nurses gave all the injections. I hope not.
When it comes to giving immunizations to children, the art is in entering into that encounter with a calm, matter-of-fact attitude and body language, hiding the needle, firmly restraining the child, and moving quickly and smoothly. Aspirating and glove donning merely add to the drama and waste time. But how did I learn that art? No one taught me. Like many clinical skills, I watched scores of nurses and physicians, mentally logging in their tricks and mistakes that would help me craft my style.
I always felt and still feel that providing immunizations was per hour spent, the most valuable investment of my time. Doing the injecting myself was both the most efficient way to provide the service, and also emphasized the importance that I placed on the immunization. In the process of my 40-plus–year career, that included several hundred thousand patient encounters in which I gave innumerable injections. And, I egotistically assumed that I was good at it because many infants never cried, and a few children said, “That didn’t hurt.” I suspect you can make the same claim.
Injecting millions of adults with a COVID-19 vaccine, on the other hand, is a piece of cake because restraining the recipient shouldn’t factor into the scenario. However, I wonder who is going to administer all those millions of injections and who is going to train them? How many of the trainers are aware of the CDC-ACIP guidelines? Or, are they going to fall back on old techniques that lack evidence support?
From the efficiency standpoint, it probably doesn’t make much difference. The injection takes but a few seconds. Filling out the paperwork and waiting for the recipient to figure out how to expose his or her deltoid can take fifty times that long.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
As the COVID-19 vaccine candidates have begun to roll off the production lines into the distribution networks by the millions, media coverage almost universally includes a still photo or video of someone receiving an injection. Ever observant, a retired lawyer friend of mine who learned to give shots when he was in the Army and again more recently while taking a wilderness survival course emailed me his concerns about what he felt were examples of poor injection technique. Included in his commentary was an Internet link in which a physician, who I suspect may have been a pediatrician, demonstrated what the physician considered proper intramuscular injection technique, which included a single-handed aspiration prior to giving the injection allowing the free hand to stabilize the patient’s – in this case a child’s – arm during the entire process.
I replied to my friend that I too was often troubled by what I considered to be poor injection technique. But, I said the physician in the link touting his improved technique was misguided. My understanding has been that unless the injection site is in the gluteus, there is no need aspirate prior to an intramuscular vaccine injection because the risk of intravascular injection is so small. I then confirmed this by reviewing the Centers for Disease Control and Prevention’s Vaccine Recommendations and Guidelines of the Advisory Committee on Immunization Practices, which was updated in June 2019. Included in those recommendations was the observation that the vaccine administrator does not need to wear gloves unless he or she has open lesions or is at risk from contacting the recipient’s body fluids.
Like many of the technical skills one learns in training, giving intramuscular injections is probably an example of the “see one, do one, teach one” mantra. But in the case of giving shots, I don’t recall any teaching. Do you? It was more “see a dozen and get on with it.” Or maybe you trained in an environment in which nurses gave all the injections. I hope not.
When it comes to giving immunizations to children, the art is in entering into that encounter with a calm, matter-of-fact attitude and body language, hiding the needle, firmly restraining the child, and moving quickly and smoothly. Aspirating and glove donning merely add to the drama and waste time. But how did I learn that art? No one taught me. Like many clinical skills, I watched scores of nurses and physicians, mentally logging in their tricks and mistakes that would help me craft my style.
I always felt and still feel that providing immunizations was per hour spent, the most valuable investment of my time. Doing the injecting myself was both the most efficient way to provide the service, and also emphasized the importance that I placed on the immunization. In the process of my 40-plus–year career, that included several hundred thousand patient encounters in which I gave innumerable injections. And, I egotistically assumed that I was good at it because many infants never cried, and a few children said, “That didn’t hurt.” I suspect you can make the same claim.
Injecting millions of adults with a COVID-19 vaccine, on the other hand, is a piece of cake because restraining the recipient shouldn’t factor into the scenario. However, I wonder who is going to administer all those millions of injections and who is going to train them? How many of the trainers are aware of the CDC-ACIP guidelines? Or, are they going to fall back on old techniques that lack evidence support?
From the efficiency standpoint, it probably doesn’t make much difference. The injection takes but a few seconds. Filling out the paperwork and waiting for the recipient to figure out how to expose his or her deltoid can take fifty times that long.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Understanding messenger RNA and other SARS-CoV-2 vaccines
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.
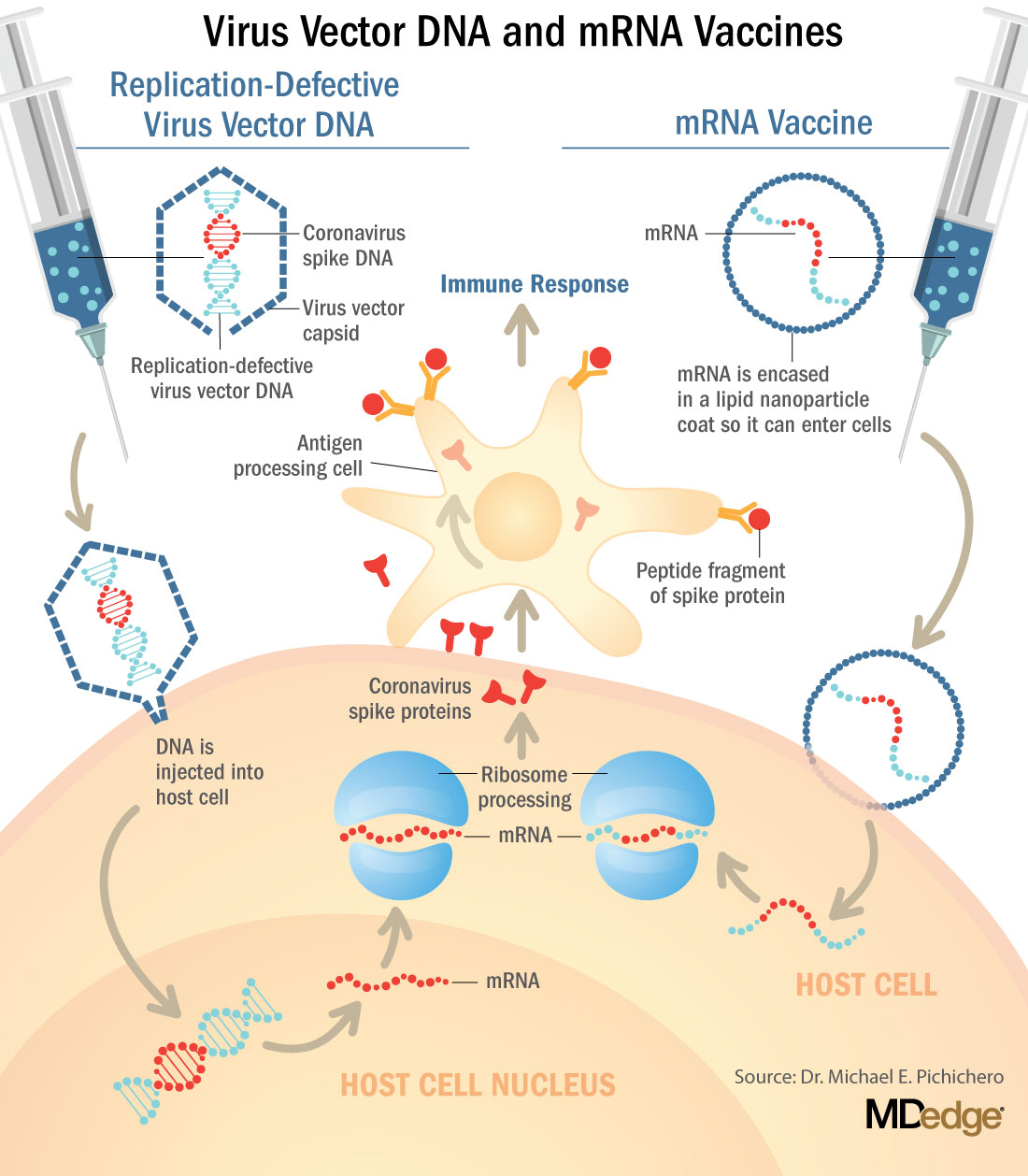
These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at [email protected].
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.

These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at [email protected].
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.

These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at [email protected].
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
USPSTF update on sexually transmitted infections
In August 2020, the US Preventive Services Task Force published an update of its recommendation on preventing sexually transmitted infections (STIs) with behavioral counseling interventions.1
Whom to counsel. The USPSTF continues to recommend behavioral counseling for all sexually active adolescents and for adults at increased risk for STIs. Adults at increased risk include those who have been diagnosed with an STI in the past year, those with multiple sex partners or a sex partner at high risk for an STI, those not using condoms consistently, and those belonging to populations with high prevalence rates of STIs. These populations with high prevalence rates include1
- individuals seeking care at STI clinics,
- sexual and gender minorities, and
- those who are positive for human immunodeficiency virus (HIV), use injection drugs, exchange sex for drugs or money, or have recently been in a correctional facility.
Features of effective counseling. The Task Force recommends that primary care clinicians provide behavioral counseling or refer to counseling services or suggest media-based interventions. The most effective counseling interventions are those that span more than 120 minutes over several sessions. But the Task Force also states that counseling lasting about 30 minutes in a single session can also be effective. Counseling should include information about common STIs and their modes of transmission; encouragement in the use of safer sex practices; and training in proper condom use, how to communicate with partners about safer sex practices, and problem-solving. Various approaches to this counseling can be found at https://uspreventiveservicestaskforce.org/uspstf/recommendation/sexually-transmitted-infections-behavioral-counseling.
This updated recommendation is timely because most STIs in the United States have been increasing in incidence for the past decade or longer.2 Per 100,000 population, the total number of chlamydia cases since 2000 has risen from 251.4 to 539.9 (115%);gonorrhea cases since 2009 have risen from 98.1 to 179.1 (83%).3 And since 2000, the total number of reported syphilis cases per 100,000 has risen from 2.1 to 10.8 (414%).3
Chlamydia affects primarily those ages 15 to 24 years, with highest rates occurring in females (FIGURE 1).2 Gonorrhea affects women and men fairly evenly with slightly higher rates in men; the highest rates are seen in those ages 20 to 29 (FIGURE 2).2 Syphilis predominantly affects men who have sex with men, and the highest rates are in those ages 20 to 34 (FIGURE 3).2 In contrast to these upward trends, the number of HIV cases diagnosed has been relatively steady, with a slight downward trend over the past decade.4Other STIs that can be prevented through behavioral counseling include herpes simplex, human papillomavirus (HPV), hepatitis B virus (HBV) and trichomonas vaginalis.
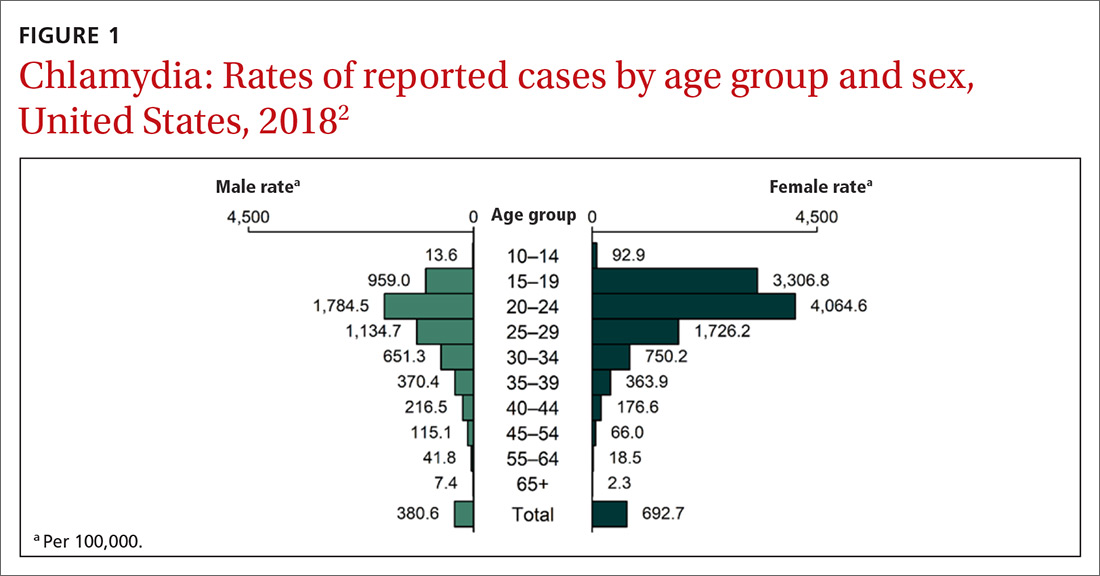
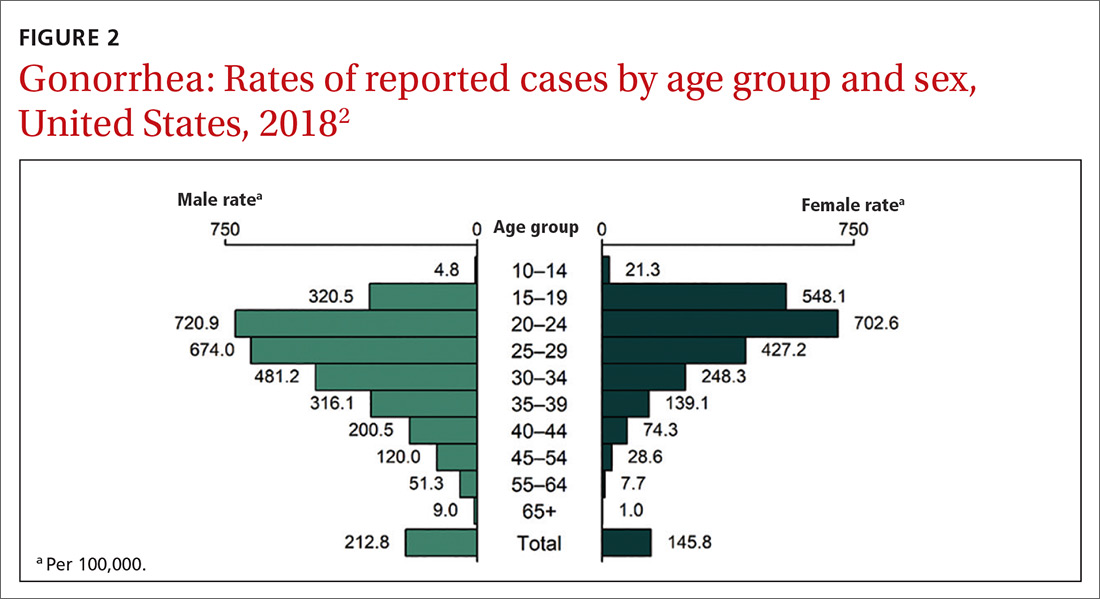
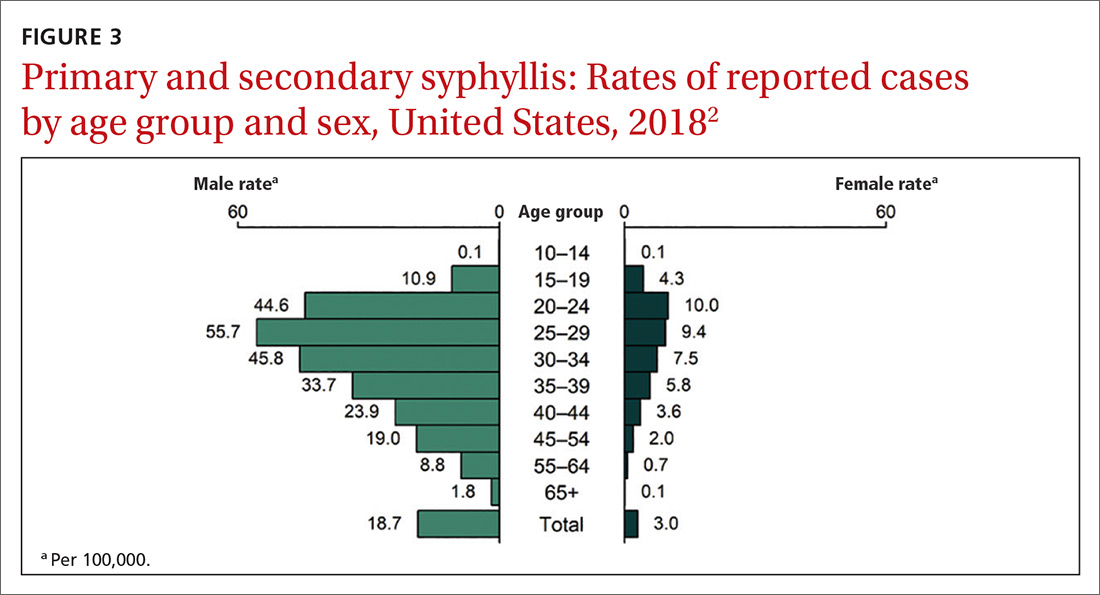
Continue to: How to integrate STI preventioninto the primary care encounter
How to integrate STI preventioninto the primary care encounter
A key resource for learning to recognize the signs and symptoms of STIs, to correctly diagnose them, and to treat them according to CDC guidelines can be found at www.cdc.gov/std/tg2015/default.htm.5 Equally important is to integrate the prevention of STIs into the clinical routine by using a 4-step approach: risk assessment, risk reduction (counseling and chemoprevention), screening, and vaccination.
Risk assessment. The first step in prevention is taking a sexual history to accurately assess a patient’s risk for STIs. The CDC provides a tool (www.cdc.gov/std/products/provider-pocket-guides.htm) that can assist in gathering information in a nonjudgmental fashion about 5 Ps: partners, practices, protection from STIs, past history of STIs, and prevention of pregnancy.
Risk reduction. Following STI risk assessment, recommend risk-reduction interventions, as appropriate. Notable in the new Task Force recommendation are behavioral counseling methods that work. Additionally, when needed, pre-exposure prophylaxis with effective antiretroviral agents can be offered to those at high risk of HIV.6
Screening. Task Force recommendations for STI screening are described in the TABLE.7-12 Screening for HIV, chlamydia, gonorrhea, syphilis, and HBV are also recommended for pregnant women. And, although pregnant women are not specifically mentioned in the recommendation on chlamydia screening, it is reasonable to include it in prenatal care testing for STIs.
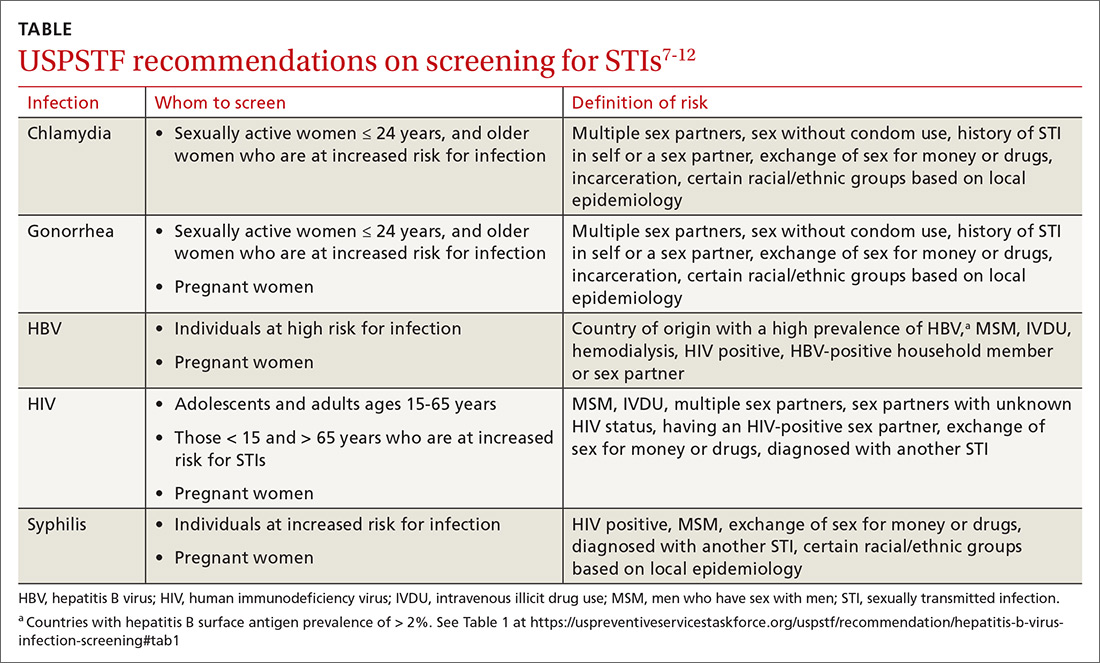
The Task Force has made an “I” statement regarding screening for gonorrhea and chlamydia in males. This does not mean that screening should be avoided, but only that there is insufficient evidence to support a firm statement regarding the harms and benefits in males. Keep in mind that this applies to asymptomatic males, and that testing and preventive treatment are warranted after documented exposure to either infection.
The Task Force recommends against screening for genital herpes, including in pregnant women, because of a lack of evidence of benefit from such screening, the high rate of false-positive tests, and the potential to cause anxiety and harm to personal relationships.
Continue to: Although hepatitis C virus...
Although hepatitis C virus (HCV) is transmitted mainly through intravenous drug use, it can also be transmitted sexually. The Task Force recommends screening for HCV in all adults ages 18 to 79 years.13
Vaccination. Two STIs can be prevented by immunizations: HPV and HBV. The current recommendations by the Advisory Committee on Immunization Practices (ACIP) are to vaccinate all infants with HBV vaccine and all unvaccinated children and adolescents through age 18.14 Unvaccinated adults who are at risk for HBV infection, including those at risk through sexual practices, should also be vaccinated.14
ACIP recommends routine HPV vaccination at age 11 or 12 years, but it can be started as early as 9 years.15 Catch-up vaccination is recommended for males and females through age 26 years.15 The vaccine is approved for use in individuals ages 27 through 45 years, but ACIP has not recommended it for routine use in this age group, and has instead recommended shared clinical decision-making to evaluate whether there is potential individual benefit from the vaccine.15
Public health implications
All STIs are reportable to local or state health departments. This is important for tracking community infection trends and, if resources are available, for contact notification and testing. In most jurisdictions, local health department resources are limited and contact tracing may be restricted to syphilis and HIV infections. When this is the case, it is especially important to instruct patients in whom STIs have been detected to notify their recent sex partners and advise them to be tested or preventively treated.
Expedited partner therapy (EPT)—providing treatment for exposed sexual contacts without a clinical encounter—is allowed in some states and is a tool that can prevent re-infection in the treated patient and suppress spread in the community. This is most useful for partners of those with gonorrhea, chlamydia, or trichomonas. The CDC has published guidance on how to implement EPT in a clinical setting if state law allows it.16
1. Henderson JT, Senger CA, Henninger M, et al. Behavioral counseling interventions to prevent sexually transmitted infections. JAMA. 2020;324:682-699.
2. CDC. Sexually transmitted disease surveillance, 2018. www.cdc.gov/std/stats18/slides.htm. Accessed November 25, 2020.
3. CDC. Sexually transmitted disease surveillance 2018. www.cdc.gov/std/stats18/tables/1.htm. Accessed November 25, 2020.
4. CDC. Estimated HIV incidence and prevalence in the United States (2010-2018). www.cdc.gov/hiv/pdf/library/slidesets/cdc-hiv-surveillance-epidemiology-2018.pdf. Accessed November 25, 2020.
5. CDC. 2015 sexually transmitted disease treatment guidelines. www.cdc.gov/std/tg2015/default.htm. Accessed November 25, 2020.
6. USPSTF. Prevention of human immunodeficiency (HIV) infection: pre-exposure prophylaxis. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis. Accessed November 25, 2020.
7. LeFevre ML, U.S. Preventive Services Task Force. Screening for chlamydia and gonorrhea: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:902-910. 8. USPSTF. Syphilis infection in nonpregnant adults and adolescents: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/syphilis-infection-in-nonpregnant-adults-and-adolescents. Accessed November 25, 2020.
9. Curry SJ, Krist AH, Owens DK, et al. Screening for syphilis in pregnant women: US Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2018;320:911-917.
10. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for HIV infection: US Preventive Services Task Force recommendation statement. JAMA. 2019;321:2326-2336.
11. USPSTF. US Preventive Services Task Force issues draft recommendation statement on screening for hepatitis B virus infection in adolescents and adults. www.uspreventiveservicestaskforce.org/uspstf/sites/default/files/file/supporting_documents/hepatitis-b-nonpregnant-adults-draft-rs-bulletin.pdf. Accessed November 25, 2020.
12. Owens DK, Davidson KW, Krist AH, et al. Screening for Hepatitis B Virus Infection in Pregnant Women: US Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2019;322:349-354.
13. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening. Accessed November 25, 2020. 14. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67;1-31.
15. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
16. CDC. Expedited partner therapy in the management of sexually transmitted diseases. www.cdc.gov/std/treatment/eptfinalreport2006.pdf. Accessed November 25, 2020.
In August 2020, the US Preventive Services Task Force published an update of its recommendation on preventing sexually transmitted infections (STIs) with behavioral counseling interventions.1
Whom to counsel. The USPSTF continues to recommend behavioral counseling for all sexually active adolescents and for adults at increased risk for STIs. Adults at increased risk include those who have been diagnosed with an STI in the past year, those with multiple sex partners or a sex partner at high risk for an STI, those not using condoms consistently, and those belonging to populations with high prevalence rates of STIs. These populations with high prevalence rates include1
- individuals seeking care at STI clinics,
- sexual and gender minorities, and
- those who are positive for human immunodeficiency virus (HIV), use injection drugs, exchange sex for drugs or money, or have recently been in a correctional facility.
Features of effective counseling. The Task Force recommends that primary care clinicians provide behavioral counseling or refer to counseling services or suggest media-based interventions. The most effective counseling interventions are those that span more than 120 minutes over several sessions. But the Task Force also states that counseling lasting about 30 minutes in a single session can also be effective. Counseling should include information about common STIs and their modes of transmission; encouragement in the use of safer sex practices; and training in proper condom use, how to communicate with partners about safer sex practices, and problem-solving. Various approaches to this counseling can be found at https://uspreventiveservicestaskforce.org/uspstf/recommendation/sexually-transmitted-infections-behavioral-counseling.
This updated recommendation is timely because most STIs in the United States have been increasing in incidence for the past decade or longer.2 Per 100,000 population, the total number of chlamydia cases since 2000 has risen from 251.4 to 539.9 (115%);gonorrhea cases since 2009 have risen from 98.1 to 179.1 (83%).3 And since 2000, the total number of reported syphilis cases per 100,000 has risen from 2.1 to 10.8 (414%).3
Chlamydia affects primarily those ages 15 to 24 years, with highest rates occurring in females (FIGURE 1).2 Gonorrhea affects women and men fairly evenly with slightly higher rates in men; the highest rates are seen in those ages 20 to 29 (FIGURE 2).2 Syphilis predominantly affects men who have sex with men, and the highest rates are in those ages 20 to 34 (FIGURE 3).2 In contrast to these upward trends, the number of HIV cases diagnosed has been relatively steady, with a slight downward trend over the past decade.4Other STIs that can be prevented through behavioral counseling include herpes simplex, human papillomavirus (HPV), hepatitis B virus (HBV) and trichomonas vaginalis.



Continue to: How to integrate STI preventioninto the primary care encounter
How to integrate STI preventioninto the primary care encounter
A key resource for learning to recognize the signs and symptoms of STIs, to correctly diagnose them, and to treat them according to CDC guidelines can be found at www.cdc.gov/std/tg2015/default.htm.5 Equally important is to integrate the prevention of STIs into the clinical routine by using a 4-step approach: risk assessment, risk reduction (counseling and chemoprevention), screening, and vaccination.
Risk assessment. The first step in prevention is taking a sexual history to accurately assess a patient’s risk for STIs. The CDC provides a tool (www.cdc.gov/std/products/provider-pocket-guides.htm) that can assist in gathering information in a nonjudgmental fashion about 5 Ps: partners, practices, protection from STIs, past history of STIs, and prevention of pregnancy.
Risk reduction. Following STI risk assessment, recommend risk-reduction interventions, as appropriate. Notable in the new Task Force recommendation are behavioral counseling methods that work. Additionally, when needed, pre-exposure prophylaxis with effective antiretroviral agents can be offered to those at high risk of HIV.6
Screening. Task Force recommendations for STI screening are described in the TABLE.7-12 Screening for HIV, chlamydia, gonorrhea, syphilis, and HBV are also recommended for pregnant women. And, although pregnant women are not specifically mentioned in the recommendation on chlamydia screening, it is reasonable to include it in prenatal care testing for STIs.

The Task Force has made an “I” statement regarding screening for gonorrhea and chlamydia in males. This does not mean that screening should be avoided, but only that there is insufficient evidence to support a firm statement regarding the harms and benefits in males. Keep in mind that this applies to asymptomatic males, and that testing and preventive treatment are warranted after documented exposure to either infection.
The Task Force recommends against screening for genital herpes, including in pregnant women, because of a lack of evidence of benefit from such screening, the high rate of false-positive tests, and the potential to cause anxiety and harm to personal relationships.
Continue to: Although hepatitis C virus...
Although hepatitis C virus (HCV) is transmitted mainly through intravenous drug use, it can also be transmitted sexually. The Task Force recommends screening for HCV in all adults ages 18 to 79 years.13
Vaccination. Two STIs can be prevented by immunizations: HPV and HBV. The current recommendations by the Advisory Committee on Immunization Practices (ACIP) are to vaccinate all infants with HBV vaccine and all unvaccinated children and adolescents through age 18.14 Unvaccinated adults who are at risk for HBV infection, including those at risk through sexual practices, should also be vaccinated.14
ACIP recommends routine HPV vaccination at age 11 or 12 years, but it can be started as early as 9 years.15 Catch-up vaccination is recommended for males and females through age 26 years.15 The vaccine is approved for use in individuals ages 27 through 45 years, but ACIP has not recommended it for routine use in this age group, and has instead recommended shared clinical decision-making to evaluate whether there is potential individual benefit from the vaccine.15
Public health implications
All STIs are reportable to local or state health departments. This is important for tracking community infection trends and, if resources are available, for contact notification and testing. In most jurisdictions, local health department resources are limited and contact tracing may be restricted to syphilis and HIV infections. When this is the case, it is especially important to instruct patients in whom STIs have been detected to notify their recent sex partners and advise them to be tested or preventively treated.
Expedited partner therapy (EPT)—providing treatment for exposed sexual contacts without a clinical encounter—is allowed in some states and is a tool that can prevent re-infection in the treated patient and suppress spread in the community. This is most useful for partners of those with gonorrhea, chlamydia, or trichomonas. The CDC has published guidance on how to implement EPT in a clinical setting if state law allows it.16
In August 2020, the US Preventive Services Task Force published an update of its recommendation on preventing sexually transmitted infections (STIs) with behavioral counseling interventions.1
Whom to counsel. The USPSTF continues to recommend behavioral counseling for all sexually active adolescents and for adults at increased risk for STIs. Adults at increased risk include those who have been diagnosed with an STI in the past year, those with multiple sex partners or a sex partner at high risk for an STI, those not using condoms consistently, and those belonging to populations with high prevalence rates of STIs. These populations with high prevalence rates include1
- individuals seeking care at STI clinics,
- sexual and gender minorities, and
- those who are positive for human immunodeficiency virus (HIV), use injection drugs, exchange sex for drugs or money, or have recently been in a correctional facility.
Features of effective counseling. The Task Force recommends that primary care clinicians provide behavioral counseling or refer to counseling services or suggest media-based interventions. The most effective counseling interventions are those that span more than 120 minutes over several sessions. But the Task Force also states that counseling lasting about 30 minutes in a single session can also be effective. Counseling should include information about common STIs and their modes of transmission; encouragement in the use of safer sex practices; and training in proper condom use, how to communicate with partners about safer sex practices, and problem-solving. Various approaches to this counseling can be found at https://uspreventiveservicestaskforce.org/uspstf/recommendation/sexually-transmitted-infections-behavioral-counseling.
This updated recommendation is timely because most STIs in the United States have been increasing in incidence for the past decade or longer.2 Per 100,000 population, the total number of chlamydia cases since 2000 has risen from 251.4 to 539.9 (115%);gonorrhea cases since 2009 have risen from 98.1 to 179.1 (83%).3 And since 2000, the total number of reported syphilis cases per 100,000 has risen from 2.1 to 10.8 (414%).3
Chlamydia affects primarily those ages 15 to 24 years, with highest rates occurring in females (FIGURE 1).2 Gonorrhea affects women and men fairly evenly with slightly higher rates in men; the highest rates are seen in those ages 20 to 29 (FIGURE 2).2 Syphilis predominantly affects men who have sex with men, and the highest rates are in those ages 20 to 34 (FIGURE 3).2 In contrast to these upward trends, the number of HIV cases diagnosed has been relatively steady, with a slight downward trend over the past decade.4Other STIs that can be prevented through behavioral counseling include herpes simplex, human papillomavirus (HPV), hepatitis B virus (HBV) and trichomonas vaginalis.



Continue to: How to integrate STI preventioninto the primary care encounter
How to integrate STI preventioninto the primary care encounter
A key resource for learning to recognize the signs and symptoms of STIs, to correctly diagnose them, and to treat them according to CDC guidelines can be found at www.cdc.gov/std/tg2015/default.htm.5 Equally important is to integrate the prevention of STIs into the clinical routine by using a 4-step approach: risk assessment, risk reduction (counseling and chemoprevention), screening, and vaccination.
Risk assessment. The first step in prevention is taking a sexual history to accurately assess a patient’s risk for STIs. The CDC provides a tool (www.cdc.gov/std/products/provider-pocket-guides.htm) that can assist in gathering information in a nonjudgmental fashion about 5 Ps: partners, practices, protection from STIs, past history of STIs, and prevention of pregnancy.
Risk reduction. Following STI risk assessment, recommend risk-reduction interventions, as appropriate. Notable in the new Task Force recommendation are behavioral counseling methods that work. Additionally, when needed, pre-exposure prophylaxis with effective antiretroviral agents can be offered to those at high risk of HIV.6
Screening. Task Force recommendations for STI screening are described in the TABLE.7-12 Screening for HIV, chlamydia, gonorrhea, syphilis, and HBV are also recommended for pregnant women. And, although pregnant women are not specifically mentioned in the recommendation on chlamydia screening, it is reasonable to include it in prenatal care testing for STIs.

The Task Force has made an “I” statement regarding screening for gonorrhea and chlamydia in males. This does not mean that screening should be avoided, but only that there is insufficient evidence to support a firm statement regarding the harms and benefits in males. Keep in mind that this applies to asymptomatic males, and that testing and preventive treatment are warranted after documented exposure to either infection.
The Task Force recommends against screening for genital herpes, including in pregnant women, because of a lack of evidence of benefit from such screening, the high rate of false-positive tests, and the potential to cause anxiety and harm to personal relationships.
Continue to: Although hepatitis C virus...
Although hepatitis C virus (HCV) is transmitted mainly through intravenous drug use, it can also be transmitted sexually. The Task Force recommends screening for HCV in all adults ages 18 to 79 years.13
Vaccination. Two STIs can be prevented by immunizations: HPV and HBV. The current recommendations by the Advisory Committee on Immunization Practices (ACIP) are to vaccinate all infants with HBV vaccine and all unvaccinated children and adolescents through age 18.14 Unvaccinated adults who are at risk for HBV infection, including those at risk through sexual practices, should also be vaccinated.14
ACIP recommends routine HPV vaccination at age 11 or 12 years, but it can be started as early as 9 years.15 Catch-up vaccination is recommended for males and females through age 26 years.15 The vaccine is approved for use in individuals ages 27 through 45 years, but ACIP has not recommended it for routine use in this age group, and has instead recommended shared clinical decision-making to evaluate whether there is potential individual benefit from the vaccine.15
Public health implications
All STIs are reportable to local or state health departments. This is important for tracking community infection trends and, if resources are available, for contact notification and testing. In most jurisdictions, local health department resources are limited and contact tracing may be restricted to syphilis and HIV infections. When this is the case, it is especially important to instruct patients in whom STIs have been detected to notify their recent sex partners and advise them to be tested or preventively treated.
Expedited partner therapy (EPT)—providing treatment for exposed sexual contacts without a clinical encounter—is allowed in some states and is a tool that can prevent re-infection in the treated patient and suppress spread in the community. This is most useful for partners of those with gonorrhea, chlamydia, or trichomonas. The CDC has published guidance on how to implement EPT in a clinical setting if state law allows it.16
1. Henderson JT, Senger CA, Henninger M, et al. Behavioral counseling interventions to prevent sexually transmitted infections. JAMA. 2020;324:682-699.
2. CDC. Sexually transmitted disease surveillance, 2018. www.cdc.gov/std/stats18/slides.htm. Accessed November 25, 2020.
3. CDC. Sexually transmitted disease surveillance 2018. www.cdc.gov/std/stats18/tables/1.htm. Accessed November 25, 2020.
4. CDC. Estimated HIV incidence and prevalence in the United States (2010-2018). www.cdc.gov/hiv/pdf/library/slidesets/cdc-hiv-surveillance-epidemiology-2018.pdf. Accessed November 25, 2020.
5. CDC. 2015 sexually transmitted disease treatment guidelines. www.cdc.gov/std/tg2015/default.htm. Accessed November 25, 2020.
6. USPSTF. Prevention of human immunodeficiency (HIV) infection: pre-exposure prophylaxis. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis. Accessed November 25, 2020.
7. LeFevre ML, U.S. Preventive Services Task Force. Screening for chlamydia and gonorrhea: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:902-910. 8. USPSTF. Syphilis infection in nonpregnant adults and adolescents: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/syphilis-infection-in-nonpregnant-adults-and-adolescents. Accessed November 25, 2020.
9. Curry SJ, Krist AH, Owens DK, et al. Screening for syphilis in pregnant women: US Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2018;320:911-917.
10. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for HIV infection: US Preventive Services Task Force recommendation statement. JAMA. 2019;321:2326-2336.
11. USPSTF. US Preventive Services Task Force issues draft recommendation statement on screening for hepatitis B virus infection in adolescents and adults. www.uspreventiveservicestaskforce.org/uspstf/sites/default/files/file/supporting_documents/hepatitis-b-nonpregnant-adults-draft-rs-bulletin.pdf. Accessed November 25, 2020.
12. Owens DK, Davidson KW, Krist AH, et al. Screening for Hepatitis B Virus Infection in Pregnant Women: US Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2019;322:349-354.
13. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening. Accessed November 25, 2020. 14. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67;1-31.
15. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
16. CDC. Expedited partner therapy in the management of sexually transmitted diseases. www.cdc.gov/std/treatment/eptfinalreport2006.pdf. Accessed November 25, 2020.
1. Henderson JT, Senger CA, Henninger M, et al. Behavioral counseling interventions to prevent sexually transmitted infections. JAMA. 2020;324:682-699.
2. CDC. Sexually transmitted disease surveillance, 2018. www.cdc.gov/std/stats18/slides.htm. Accessed November 25, 2020.
3. CDC. Sexually transmitted disease surveillance 2018. www.cdc.gov/std/stats18/tables/1.htm. Accessed November 25, 2020.
4. CDC. Estimated HIV incidence and prevalence in the United States (2010-2018). www.cdc.gov/hiv/pdf/library/slidesets/cdc-hiv-surveillance-epidemiology-2018.pdf. Accessed November 25, 2020.
5. CDC. 2015 sexually transmitted disease treatment guidelines. www.cdc.gov/std/tg2015/default.htm. Accessed November 25, 2020.
6. USPSTF. Prevention of human immunodeficiency (HIV) infection: pre-exposure prophylaxis. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis. Accessed November 25, 2020.
7. LeFevre ML, U.S. Preventive Services Task Force. Screening for chlamydia and gonorrhea: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:902-910. 8. USPSTF. Syphilis infection in nonpregnant adults and adolescents: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/syphilis-infection-in-nonpregnant-adults-and-adolescents. Accessed November 25, 2020.
9. Curry SJ, Krist AH, Owens DK, et al. Screening for syphilis in pregnant women: US Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2018;320:911-917.
10. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for HIV infection: US Preventive Services Task Force recommendation statement. JAMA. 2019;321:2326-2336.
11. USPSTF. US Preventive Services Task Force issues draft recommendation statement on screening for hepatitis B virus infection in adolescents and adults. www.uspreventiveservicestaskforce.org/uspstf/sites/default/files/file/supporting_documents/hepatitis-b-nonpregnant-adults-draft-rs-bulletin.pdf. Accessed November 25, 2020.
12. Owens DK, Davidson KW, Krist AH, et al. Screening for Hepatitis B Virus Infection in Pregnant Women: US Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2019;322:349-354.
13. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening. Accessed November 25, 2020. 14. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67;1-31.
15. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
16. CDC. Expedited partner therapy in the management of sexually transmitted diseases. www.cdc.gov/std/treatment/eptfinalreport2006.pdf. Accessed November 25, 2020.