User login
Analysis characterizes common wound microbes in epidermolysis bullosa
– in a retrospective analysis of over 700 wound cultures from 158 patients across the United States and Canada.
The findings from the EB Clinical Characterization and Outcomes Database speak to the value of surveillance cultures with routine testing for microbial resistance – including mupirocin resistance – and to the importance of antibiotic stewardship not only for oral antibiotics but for topicals as well, according to Laura E. Levin, MD, and Kimberly D. Morel, MD, of the departments of dermatology and pediatrics, Columbia University Irving Medical Center, New York, the lead and senior authors, respectively, of the paper recently published in Pediatric Dermatology.
Almost all of the 158 patients with at least one wound culture recorded in the database from the period of 2001-2018 had one or more positive culture results. Of 152 patients with positive cultures, 131 (86%) were positive for SA and 56 (37%) and 34 (22%) were positive for PA and GAS, respectively. Other bacteria isolated included Corynebacterium spp and Proteus spp. Nearly half (47%) of patients with SA-positive cultures had methicillin-resistant SA, and 68% had methicillin-susceptible SA. (Some patients grew both MSSA and MRSA at different points in time.)
Mupirocin-susceptibility testing was performed at only some of the 13 participating centers. Of 15 patients whose cultures had recorded SA mupirocin-susceptibility testing, 11 had cultures positive for mupirocin-susceptible SA and 6 (40%) had mupirocin-resistant SA isolates (2 patients grew both). Of these six patients, half had isolates that were also methicillin-resistant.
Mupirocin, a topical antibiotic, has been a cornerstone of decolonization regimens for MSSA and MRSA, but resistance has been demonstrated in other research as well and is not specific to EB, wrote Dr. Levin, Dr. Morel, and coauthors.
“Pediatric dermatologists often rely on topical antimicrobials in the treatment of patients’ open wounds to both prevent and treat infection, depending on the clinical scenario,” and surveillance cultures with routine testing for mupirocin resistance can help guide antibiotic choice and management strategies, Dr. Levin said in an interview.
More broadly, she added, “it’s helpful to know what bacteria are routinely colonizing wounds, not causing infection, versus those that are more likely to be associated with infection, chronic wounds, or the risk of developing skin cancer ... [to know] which wounds need to be treated more aggressively.”
A subset of patients with EB have been known to be at risk for squamous cell carcinoma, and research is implicating certain bacteria “as contributing to wound inflammation,” Dr. Morel said in an interview.
SCC was reported in 23 out of 717 patients in the database – but fewer than half of the patients with SCC had recorded wound cultures. The small numbers precluded the identification of microbes that may confer significant risk.
Correlating particular microbes with clinical features also will take more research. About half (57%) of the patients with recorded wound cultures had wounds with purulent exudate or other features of clinical infection. However, the presence or absence of clinical signs of infection was not temporally correlated with culture results in the database.
The 158 patients with recorded wound cultures had a mean age of 12.8 years and represented a range of EB subtypes.
PA was present in the wounds of patients as young as 1 month old, the authors noted. Investigators are “looking to further study PA and characterize clinical features ... to understand more about this microbe and its impact on patients with EB,” Dr. Morel said.
In the meantime, the analysis reaffirms the importance of antibiotic stewardship. Mupirocin is labeled to be used three times a day for a short period of time, but “tends to be prescribed and used less judiciously than intended,” Dr. Morel said. “It’s important [not to overuse it]. We have seen that patients’ culture results become sensitive to mupirocin again in the future when they avoid it for a period of time.”
The work was supported by the EB Research Partnership and EB Medical Research Foundation, as well as an NIH/NCATS grant. No investigator disclosures were listed.
SOURCE: Pediatr Dermatol. 2020 Nov 28. doi: 10.1111/pde.14444.
– in a retrospective analysis of over 700 wound cultures from 158 patients across the United States and Canada.
The findings from the EB Clinical Characterization and Outcomes Database speak to the value of surveillance cultures with routine testing for microbial resistance – including mupirocin resistance – and to the importance of antibiotic stewardship not only for oral antibiotics but for topicals as well, according to Laura E. Levin, MD, and Kimberly D. Morel, MD, of the departments of dermatology and pediatrics, Columbia University Irving Medical Center, New York, the lead and senior authors, respectively, of the paper recently published in Pediatric Dermatology.
Almost all of the 158 patients with at least one wound culture recorded in the database from the period of 2001-2018 had one or more positive culture results. Of 152 patients with positive cultures, 131 (86%) were positive for SA and 56 (37%) and 34 (22%) were positive for PA and GAS, respectively. Other bacteria isolated included Corynebacterium spp and Proteus spp. Nearly half (47%) of patients with SA-positive cultures had methicillin-resistant SA, and 68% had methicillin-susceptible SA. (Some patients grew both MSSA and MRSA at different points in time.)
Mupirocin-susceptibility testing was performed at only some of the 13 participating centers. Of 15 patients whose cultures had recorded SA mupirocin-susceptibility testing, 11 had cultures positive for mupirocin-susceptible SA and 6 (40%) had mupirocin-resistant SA isolates (2 patients grew both). Of these six patients, half had isolates that were also methicillin-resistant.
Mupirocin, a topical antibiotic, has been a cornerstone of decolonization regimens for MSSA and MRSA, but resistance has been demonstrated in other research as well and is not specific to EB, wrote Dr. Levin, Dr. Morel, and coauthors.
“Pediatric dermatologists often rely on topical antimicrobials in the treatment of patients’ open wounds to both prevent and treat infection, depending on the clinical scenario,” and surveillance cultures with routine testing for mupirocin resistance can help guide antibiotic choice and management strategies, Dr. Levin said in an interview.
More broadly, she added, “it’s helpful to know what bacteria are routinely colonizing wounds, not causing infection, versus those that are more likely to be associated with infection, chronic wounds, or the risk of developing skin cancer ... [to know] which wounds need to be treated more aggressively.”
A subset of patients with EB have been known to be at risk for squamous cell carcinoma, and research is implicating certain bacteria “as contributing to wound inflammation,” Dr. Morel said in an interview.
SCC was reported in 23 out of 717 patients in the database – but fewer than half of the patients with SCC had recorded wound cultures. The small numbers precluded the identification of microbes that may confer significant risk.
Correlating particular microbes with clinical features also will take more research. About half (57%) of the patients with recorded wound cultures had wounds with purulent exudate or other features of clinical infection. However, the presence or absence of clinical signs of infection was not temporally correlated with culture results in the database.
The 158 patients with recorded wound cultures had a mean age of 12.8 years and represented a range of EB subtypes.
PA was present in the wounds of patients as young as 1 month old, the authors noted. Investigators are “looking to further study PA and characterize clinical features ... to understand more about this microbe and its impact on patients with EB,” Dr. Morel said.
In the meantime, the analysis reaffirms the importance of antibiotic stewardship. Mupirocin is labeled to be used three times a day for a short period of time, but “tends to be prescribed and used less judiciously than intended,” Dr. Morel said. “It’s important [not to overuse it]. We have seen that patients’ culture results become sensitive to mupirocin again in the future when they avoid it for a period of time.”
The work was supported by the EB Research Partnership and EB Medical Research Foundation, as well as an NIH/NCATS grant. No investigator disclosures were listed.
SOURCE: Pediatr Dermatol. 2020 Nov 28. doi: 10.1111/pde.14444.
– in a retrospective analysis of over 700 wound cultures from 158 patients across the United States and Canada.
The findings from the EB Clinical Characterization and Outcomes Database speak to the value of surveillance cultures with routine testing for microbial resistance – including mupirocin resistance – and to the importance of antibiotic stewardship not only for oral antibiotics but for topicals as well, according to Laura E. Levin, MD, and Kimberly D. Morel, MD, of the departments of dermatology and pediatrics, Columbia University Irving Medical Center, New York, the lead and senior authors, respectively, of the paper recently published in Pediatric Dermatology.
Almost all of the 158 patients with at least one wound culture recorded in the database from the period of 2001-2018 had one or more positive culture results. Of 152 patients with positive cultures, 131 (86%) were positive for SA and 56 (37%) and 34 (22%) were positive for PA and GAS, respectively. Other bacteria isolated included Corynebacterium spp and Proteus spp. Nearly half (47%) of patients with SA-positive cultures had methicillin-resistant SA, and 68% had methicillin-susceptible SA. (Some patients grew both MSSA and MRSA at different points in time.)
Mupirocin-susceptibility testing was performed at only some of the 13 participating centers. Of 15 patients whose cultures had recorded SA mupirocin-susceptibility testing, 11 had cultures positive for mupirocin-susceptible SA and 6 (40%) had mupirocin-resistant SA isolates (2 patients grew both). Of these six patients, half had isolates that were also methicillin-resistant.
Mupirocin, a topical antibiotic, has been a cornerstone of decolonization regimens for MSSA and MRSA, but resistance has been demonstrated in other research as well and is not specific to EB, wrote Dr. Levin, Dr. Morel, and coauthors.
“Pediatric dermatologists often rely on topical antimicrobials in the treatment of patients’ open wounds to both prevent and treat infection, depending on the clinical scenario,” and surveillance cultures with routine testing for mupirocin resistance can help guide antibiotic choice and management strategies, Dr. Levin said in an interview.
More broadly, she added, “it’s helpful to know what bacteria are routinely colonizing wounds, not causing infection, versus those that are more likely to be associated with infection, chronic wounds, or the risk of developing skin cancer ... [to know] which wounds need to be treated more aggressively.”
A subset of patients with EB have been known to be at risk for squamous cell carcinoma, and research is implicating certain bacteria “as contributing to wound inflammation,” Dr. Morel said in an interview.
SCC was reported in 23 out of 717 patients in the database – but fewer than half of the patients with SCC had recorded wound cultures. The small numbers precluded the identification of microbes that may confer significant risk.
Correlating particular microbes with clinical features also will take more research. About half (57%) of the patients with recorded wound cultures had wounds with purulent exudate or other features of clinical infection. However, the presence or absence of clinical signs of infection was not temporally correlated with culture results in the database.
The 158 patients with recorded wound cultures had a mean age of 12.8 years and represented a range of EB subtypes.
PA was present in the wounds of patients as young as 1 month old, the authors noted. Investigators are “looking to further study PA and characterize clinical features ... to understand more about this microbe and its impact on patients with EB,” Dr. Morel said.
In the meantime, the analysis reaffirms the importance of antibiotic stewardship. Mupirocin is labeled to be used three times a day for a short period of time, but “tends to be prescribed and used less judiciously than intended,” Dr. Morel said. “It’s important [not to overuse it]. We have seen that patients’ culture results become sensitive to mupirocin again in the future when they avoid it for a period of time.”
The work was supported by the EB Research Partnership and EB Medical Research Foundation, as well as an NIH/NCATS grant. No investigator disclosures were listed.
SOURCE: Pediatr Dermatol. 2020 Nov 28. doi: 10.1111/pde.14444.
FROM PEDIATRIC DERMATOLOGY
Seeking new vaccines against whooping cough: The PERISCOPE project
Although there is an effective vaccine against Bordetella pertussis, whooping cough remains a leading cause of death. Cases are increasing, and scientists face challenges in developing new vaccines.
In a key research session at the start of the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year, Dimitri Diavatopoulos, PhD, associate professor at the Radboud University Medical Centre Nijmegen, the Netherlands, summarized the pertussis vaccination problem and what the Pertussis Correlates of Protection Europe (PERISCOPE) project seeks to achieve. Dr. Diavatopoulos has a longstanding interest in pertussis and immunity and will soon take over as the scientific coordinator of PERISCOPE.
Pertussis is a highly contagious infectious disease that causes uncontrollable coughing. The disease begins with an atypical cough and rhinorrhea before entering a paroxysmal stage characterized by cyanosis, lymphocytosis, vomiting, and whoops. Generally, fever is absent and coughing increases at night. Finally, after weeks to months, the patient enters a convalescent stage. The World Health Organization estimates that there are 16 million pertussis cases annually and approximately 195,000 deaths in children. Most cases are caused by Bordetella pertussis and are preventable by vaccination.
In the United States, following the introduction of a national immunization program using a whole-cell vaccine in the 1950s, cases fell significantly. After a lag phase, the adoption of an acellular vaccine in the United States in 1997 and the Netherlands in 2005 – usually in combination with diphtheria and tetanus via DTaP – saw an increase in case numbers. Dr. Diavatopoulos stated that control is no longer as good, compared with other infectious diseases prevented by the MMR vaccine, such as mumps, measles, and rubella.
In the face of increasing numbers, how do we move to the next generation of vaccines to improve control? There are several barriers to licensure, including the following:
• Universal recommendation for pertussis prevention means that more than 90% of the population will have received DTaP (usually in combination with polio and Haemophilus influenzae B) and be protected for several years after vaccination.
• Because DTaP vaccines are only efficacious for a limited time, the problem is not immediately apparent.
• Pertussis epidemics are cyclical, occurring every 3-5 years. These peaks and troughs complicate the development of epidemiological studies.
What this means is that large-scale Phase III efficacy studies, in which disease is used as the endpoint, are not feasible. Also, formal correlates of protection have not been identified.
The PERISCOPE Project started in March 2016 and is designed to respond to some of these issues. Funding is made available by a public private consortium involving the Bill & Melinda Gates foundation, the European Union, and European Federation of Pharmaceutical Industries and Associations (EFPIA) partners, and in this case, GlaxoSmithKline and Sanofi Pasteur. In total, there are 22 partners in this project.
The strategic objectives of this partnership include the following:
• Foster expertise and increase capacity in Europe to evaluate new pertussis vaccines both in clinical and preclinical models.
• Identify early biomarkers of long-lasting protective immunity to pertussis in humans. (This step will accelerate and de-risk clinical development of next generation pertussis vaccines.)
• Investigate the impact of maternal vaccination on infant response to pertussis vaccination.
The problem is that there is no one single study design that addresses all questions about the pertussis vaccine. For example, in PERISCOPE, the results of preclinical studies using the baboon or mouse models and addressing disease and colonization endpoints or immunogenicity do not perfectly model human infection and disease.
By comparison, controlled human infection studies provide information on colonization but not disease endpoints. Such studies, however, do provide information on immunogenicity endpoints. Also available are booster vaccination studies and infant vaccination studies providing data on immunogenicity, as well as safety information.
Finally, there are patient studies, such as household contact studies where immunogenicity can be correlated to disease endpoints. From these studies, it will be seen that what is needed is integration of evidence from clinical and preclinical studies to support a new vaccine registration.
PERISCOPE addresses these issues by developing novel, functional antibody and cellular assays and employing cutting-edge methods to characterize innate immune responses and cell-mediated systemic and mucosal immunity. PERISCOPE combines two major industrial partners with public researchers from academic and public health institutes and small and medium-sized enterprises with expertise in clinical trials, vaccinology, immunology, molecular microbiology, challenge models, and bioinformatics.
Andrew Gorringe, PhD, from Public Health England and the Research and Development Institute at Porton Down, Wiltshire, England, said, “Vaccines have greatly reduced the incidence of pertussis, but it remains the most prevalent ‘vaccine preventable’ disease. This is an exciting period for pertussis vaccine research as we find new ways to understand the immunity that protects from both infection and disease. The PERISCOPE project provides a collaborative environment that combines expertise across Europe to provide a route to the development of new, more effective vaccines.”
GSK and Sanofi Pasteur have cofunded the PERISCOPE Project. Dr. Diavatopoulos made no other financial disclosures.
Although there is an effective vaccine against Bordetella pertussis, whooping cough remains a leading cause of death. Cases are increasing, and scientists face challenges in developing new vaccines.
In a key research session at the start of the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year, Dimitri Diavatopoulos, PhD, associate professor at the Radboud University Medical Centre Nijmegen, the Netherlands, summarized the pertussis vaccination problem and what the Pertussis Correlates of Protection Europe (PERISCOPE) project seeks to achieve. Dr. Diavatopoulos has a longstanding interest in pertussis and immunity and will soon take over as the scientific coordinator of PERISCOPE.
Pertussis is a highly contagious infectious disease that causes uncontrollable coughing. The disease begins with an atypical cough and rhinorrhea before entering a paroxysmal stage characterized by cyanosis, lymphocytosis, vomiting, and whoops. Generally, fever is absent and coughing increases at night. Finally, after weeks to months, the patient enters a convalescent stage. The World Health Organization estimates that there are 16 million pertussis cases annually and approximately 195,000 deaths in children. Most cases are caused by Bordetella pertussis and are preventable by vaccination.
In the United States, following the introduction of a national immunization program using a whole-cell vaccine in the 1950s, cases fell significantly. After a lag phase, the adoption of an acellular vaccine in the United States in 1997 and the Netherlands in 2005 – usually in combination with diphtheria and tetanus via DTaP – saw an increase in case numbers. Dr. Diavatopoulos stated that control is no longer as good, compared with other infectious diseases prevented by the MMR vaccine, such as mumps, measles, and rubella.
In the face of increasing numbers, how do we move to the next generation of vaccines to improve control? There are several barriers to licensure, including the following:
• Universal recommendation for pertussis prevention means that more than 90% of the population will have received DTaP (usually in combination with polio and Haemophilus influenzae B) and be protected for several years after vaccination.
• Because DTaP vaccines are only efficacious for a limited time, the problem is not immediately apparent.
• Pertussis epidemics are cyclical, occurring every 3-5 years. These peaks and troughs complicate the development of epidemiological studies.
What this means is that large-scale Phase III efficacy studies, in which disease is used as the endpoint, are not feasible. Also, formal correlates of protection have not been identified.
The PERISCOPE Project started in March 2016 and is designed to respond to some of these issues. Funding is made available by a public private consortium involving the Bill & Melinda Gates foundation, the European Union, and European Federation of Pharmaceutical Industries and Associations (EFPIA) partners, and in this case, GlaxoSmithKline and Sanofi Pasteur. In total, there are 22 partners in this project.
The strategic objectives of this partnership include the following:
• Foster expertise and increase capacity in Europe to evaluate new pertussis vaccines both in clinical and preclinical models.
• Identify early biomarkers of long-lasting protective immunity to pertussis in humans. (This step will accelerate and de-risk clinical development of next generation pertussis vaccines.)
• Investigate the impact of maternal vaccination on infant response to pertussis vaccination.
The problem is that there is no one single study design that addresses all questions about the pertussis vaccine. For example, in PERISCOPE, the results of preclinical studies using the baboon or mouse models and addressing disease and colonization endpoints or immunogenicity do not perfectly model human infection and disease.
By comparison, controlled human infection studies provide information on colonization but not disease endpoints. Such studies, however, do provide information on immunogenicity endpoints. Also available are booster vaccination studies and infant vaccination studies providing data on immunogenicity, as well as safety information.
Finally, there are patient studies, such as household contact studies where immunogenicity can be correlated to disease endpoints. From these studies, it will be seen that what is needed is integration of evidence from clinical and preclinical studies to support a new vaccine registration.
PERISCOPE addresses these issues by developing novel, functional antibody and cellular assays and employing cutting-edge methods to characterize innate immune responses and cell-mediated systemic and mucosal immunity. PERISCOPE combines two major industrial partners with public researchers from academic and public health institutes and small and medium-sized enterprises with expertise in clinical trials, vaccinology, immunology, molecular microbiology, challenge models, and bioinformatics.
Andrew Gorringe, PhD, from Public Health England and the Research and Development Institute at Porton Down, Wiltshire, England, said, “Vaccines have greatly reduced the incidence of pertussis, but it remains the most prevalent ‘vaccine preventable’ disease. This is an exciting period for pertussis vaccine research as we find new ways to understand the immunity that protects from both infection and disease. The PERISCOPE project provides a collaborative environment that combines expertise across Europe to provide a route to the development of new, more effective vaccines.”
GSK and Sanofi Pasteur have cofunded the PERISCOPE Project. Dr. Diavatopoulos made no other financial disclosures.
Although there is an effective vaccine against Bordetella pertussis, whooping cough remains a leading cause of death. Cases are increasing, and scientists face challenges in developing new vaccines.
In a key research session at the start of the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year, Dimitri Diavatopoulos, PhD, associate professor at the Radboud University Medical Centre Nijmegen, the Netherlands, summarized the pertussis vaccination problem and what the Pertussis Correlates of Protection Europe (PERISCOPE) project seeks to achieve. Dr. Diavatopoulos has a longstanding interest in pertussis and immunity and will soon take over as the scientific coordinator of PERISCOPE.
Pertussis is a highly contagious infectious disease that causes uncontrollable coughing. The disease begins with an atypical cough and rhinorrhea before entering a paroxysmal stage characterized by cyanosis, lymphocytosis, vomiting, and whoops. Generally, fever is absent and coughing increases at night. Finally, after weeks to months, the patient enters a convalescent stage. The World Health Organization estimates that there are 16 million pertussis cases annually and approximately 195,000 deaths in children. Most cases are caused by Bordetella pertussis and are preventable by vaccination.
In the United States, following the introduction of a national immunization program using a whole-cell vaccine in the 1950s, cases fell significantly. After a lag phase, the adoption of an acellular vaccine in the United States in 1997 and the Netherlands in 2005 – usually in combination with diphtheria and tetanus via DTaP – saw an increase in case numbers. Dr. Diavatopoulos stated that control is no longer as good, compared with other infectious diseases prevented by the MMR vaccine, such as mumps, measles, and rubella.
In the face of increasing numbers, how do we move to the next generation of vaccines to improve control? There are several barriers to licensure, including the following:
• Universal recommendation for pertussis prevention means that more than 90% of the population will have received DTaP (usually in combination with polio and Haemophilus influenzae B) and be protected for several years after vaccination.
• Because DTaP vaccines are only efficacious for a limited time, the problem is not immediately apparent.
• Pertussis epidemics are cyclical, occurring every 3-5 years. These peaks and troughs complicate the development of epidemiological studies.
What this means is that large-scale Phase III efficacy studies, in which disease is used as the endpoint, are not feasible. Also, formal correlates of protection have not been identified.
The PERISCOPE Project started in March 2016 and is designed to respond to some of these issues. Funding is made available by a public private consortium involving the Bill & Melinda Gates foundation, the European Union, and European Federation of Pharmaceutical Industries and Associations (EFPIA) partners, and in this case, GlaxoSmithKline and Sanofi Pasteur. In total, there are 22 partners in this project.
The strategic objectives of this partnership include the following:
• Foster expertise and increase capacity in Europe to evaluate new pertussis vaccines both in clinical and preclinical models.
• Identify early biomarkers of long-lasting protective immunity to pertussis in humans. (This step will accelerate and de-risk clinical development of next generation pertussis vaccines.)
• Investigate the impact of maternal vaccination on infant response to pertussis vaccination.
The problem is that there is no one single study design that addresses all questions about the pertussis vaccine. For example, in PERISCOPE, the results of preclinical studies using the baboon or mouse models and addressing disease and colonization endpoints or immunogenicity do not perfectly model human infection and disease.
By comparison, controlled human infection studies provide information on colonization but not disease endpoints. Such studies, however, do provide information on immunogenicity endpoints. Also available are booster vaccination studies and infant vaccination studies providing data on immunogenicity, as well as safety information.
Finally, there are patient studies, such as household contact studies where immunogenicity can be correlated to disease endpoints. From these studies, it will be seen that what is needed is integration of evidence from clinical and preclinical studies to support a new vaccine registration.
PERISCOPE addresses these issues by developing novel, functional antibody and cellular assays and employing cutting-edge methods to characterize innate immune responses and cell-mediated systemic and mucosal immunity. PERISCOPE combines two major industrial partners with public researchers from academic and public health institutes and small and medium-sized enterprises with expertise in clinical trials, vaccinology, immunology, molecular microbiology, challenge models, and bioinformatics.
Andrew Gorringe, PhD, from Public Health England and the Research and Development Institute at Porton Down, Wiltshire, England, said, “Vaccines have greatly reduced the incidence of pertussis, but it remains the most prevalent ‘vaccine preventable’ disease. This is an exciting period for pertussis vaccine research as we find new ways to understand the immunity that protects from both infection and disease. The PERISCOPE project provides a collaborative environment that combines expertise across Europe to provide a route to the development of new, more effective vaccines.”
GSK and Sanofi Pasteur have cofunded the PERISCOPE Project. Dr. Diavatopoulos made no other financial disclosures.
FROM ESPID 2020
Current PERISCOPE vaccine studies: Toward better pertussis prevention?
With increasing whooping cough numbers, developing an effective new vaccine against Bordetella pertussis is a priority. Results from the multifactorial PERISCOPE Project will help scientists and clinicians move forward.
Dominic Kelly, PhD, talked about vaccine-induced immunity and provided an overview of ongoing clinical trials in the PERISCOPE (Pertussis Correlates of Protection Europe) project in a key research session at the start of the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year. Dr. Kelly, a pediatrician at the Children’s Hospital in Oxford and a member of the Oxford Vaccines Group, leads one of the studies in the project looking at infant vaccination.
Dr. Kelly began his presentation by showing a figure depicting where vaccine-induced immunity fits into the larger suite of clinical studies. These studies involve mouse models, human challenge models, and infection patients. A key theme is the use of a core group of immunoassays across all studies, with the hope that they will allow effective cross comparisons.
Dr. Kelly stated, “If we find a correlate of protection in the challenge model, we can then interpret the vaccine studies in the light of that because we are using standardized constant immunoassays.”
The assays being used depend in part on the specific study and the volume of blood available. They will generally include Bordetella-specific antibody and functional antibody assays, as well as interesting studies collecting mucosal samples from infants and adults to look at serological responses. Also under examination are a range of enzyme-linked immune absorbent spot, flow cytometry, and culture techniques looking at Memory B cells, T cells, and gene expression.
Complementing these assay studies, PERISCOPE includes a series of clinical investigations designed to throw light on three areas of interest, described below:
First, researchers hope to gain a better understanding regarding the effects of the original whole cell vaccine versus the current acellular variety. The former uses an inactivated version of the whole organism. Epidemiological studies, animal data, and experience in the field demonstrate that whole-cell vaccination results in a broad, long-lasting, and effective immune response.
By comparison, the acellular pertussis vaccine consists of between three and five protein components, which are purified from cultured Bordetella pertussis. While it is an effective vaccine, its effects are less durable; routine use in some countries is associated with cyclical outbreaks of increasing severity.
A second issue for researchers involved in the PERISCOPE project concerns the effects of maternal immunization. In the United Kingdom in 2012, for example, an increasing number of cases were noted 6-7 years after adoption of an acellular vaccine for routine vaccination in the 2nd-3rd trimester of pregnancy. Vaccination appears to effectively control neonatal disease, but whether this influences infant immune responses and long-term control of pertussis for a population is unknown.
Finally, the group is interested in the effects of an acellular booster across all age groups. While the effects may be short-lived, the booster is a potential strategy for controlling a population by repeated boosting of immunity. This is another area where using novel immunoassays may aid better understanding.
To find answers, the consortium has established four studies: the Gambia Pertussis study (GaPs) in Gambia and AWARE, the sister study to GaPs in the United Kingdom, addressing the acellular pertussis versus cellular pertussis question; the Pertussis Maternal Immunization Study in Finland (MIFI) addressing maternal immunization; and the Booster against Pertussis (BERT) study across three countries (U.K., the Netherlands, and Finland) looking at acellular booster across age groups.
Gambia pertussis study
GaPs is the largest single study in the project and is being run at the Medical Research Council–funded London School of Tropical Medicine center in Gambia. Beate Kampmann, MD, PhD, of Imperial College London, England, is the project lead. It is due to complete in 2022. GaPs seeks to enroll 600 mother/infant pairs and randomize the mothers to either an acellular pertussis booster in pregnancy or a tetanus toxoid control vaccine. Infants are subsequently randomized to an acellular or whole-cell pertussis schedule of primary immunization. The vaccine doses are being given at 2, 3, and 4 months. The primary endpoint is a serological finding being measured at 9 months of age, when the infant would usually receive yellow fever, measles, and rubella vaccination.
GaPs has a number of pathways. Within each of the four arms generated by the two randomizations, the maternal randomization and the infant randomization, there are five subgroups. They are designed to study time points in subgroups A and B after the first dose in more detail, looking at the innate immune responses using gene expression. It will enable researchers to study adaptive immune responses to T cells and B cells after the second dose of vaccine. By employing a range of subgroups, the team can explore the immune profile using the assays referred to above. Such information should provide new insights into the differences between acellular and whole-cell vaccines.
The AWARE study
AWARE is the sister study to GaPs and looks at the acellular/whole pertussis issue. Because many developed countries, such as the United Kingdom, have established maternal immunization programs, it is not possible to randomize mothers. Consequently, researchers have opted to recruit infants of mothers who have received an acellular vaccine in pregnancy and randomize them to either an acellular schedule of primary immunization or a whole-cell schedule.
The selected vaccine is ComVac5 from Bharat Biotech. This whole-cell vaccine differs from that used in Gambia. An early obstacle for AWARE has been seeking permission to import a non-conventional vaccine into Europe. It has delayed the anticipated end date to 2023. Participating infants will receive a two-dose schedule at 2 and 4 months of age per their randomization; then, both groups will go on to receive an acellular pertussis booster at 12 months. At all time points, the team will sample blood for cells and serum, as well as mucosal fluid from the nose. Because the mucosal surface is where the action is, this approach will likely generate new data around antibody responses.
The MIFI
The Pertussis Maternal Immunization Study in Finland is being run by Jussi Mertsola, of the University of Turku, Finland, and Qiushui He, of the National Public Health Institute, Turku. It is due to complete in late 2021. Where, in the United Kingdom, researchers are unable to randomize mothers because of the current guidelines, researchers in Finland do not have a maternal immunization program to consider. MIFI will randomize 80 mothers, 40 to immunization with acellular pertussis and 40 to a control group. Dr. Kelly stated that whole cell vaccines are not available for use in Finland. Participants will receive a two-dose schedule at 3 and 5 months. Blood samples will then be taken to compare the serological and cellular responses, which will help researchers understand the effects of maternal immunization. In addition, there will be sampling of mucosal fluid using a device that collects a standardized aliquot of fluid.
The BERT study
The final clinical element of PERISCOPE presented by Dr. Kelly was the Booster against Pertussis study. This study is near completion. It seeks to examine the use of an acellular booster across different age groups and three countries: the United Kingdom, the Netherlands, and Finland. The study is being coordinated by Guy Berbers, PhD, at the National Institute for Public Health and the Environment in the Netherlands.
BERT comprises four cohorts (A, B, C, D) of different ages: 7-10 years (36 participants), 11-15 years (36 participants), mid-adult (25 participants), and older age (25 participants). After receiving an acellular booster, participants will undergo intense sampling. Sampling will take place immediately after immunization at day 7 and look at adaptive effects, then again at day 28 and day 365.
Because some participants will have already received whole cell or acellular vaccination, this approach will allow researchers to look at the effects of priming (i.e., how long the B cell/T cell antibody responses last).
Involving different countries across Europe ensures wide applicability of results, but also allows researchers to compare the effects of very different immunization histories.
At the end of this ESPID session, Dimitri Diavatopoulos, PhD, assistant professor at the Radboud University Medical Centre Nijmegen, the Netherlands, commented that a future problem in studying pertussis vaccines and their potential clinical application is that most vaccination schedules now involve combination products. Obtaining a stand-alone vaccination may prove difficult, and there may be resistance if it complicates current vaccination programs.
Dr. Kelly acknowledged funding for the PERISCOPE project from GlaxoSmithKline and Pasteur Sanofi.
With increasing whooping cough numbers, developing an effective new vaccine against Bordetella pertussis is a priority. Results from the multifactorial PERISCOPE Project will help scientists and clinicians move forward.
Dominic Kelly, PhD, talked about vaccine-induced immunity and provided an overview of ongoing clinical trials in the PERISCOPE (Pertussis Correlates of Protection Europe) project in a key research session at the start of the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year. Dr. Kelly, a pediatrician at the Children’s Hospital in Oxford and a member of the Oxford Vaccines Group, leads one of the studies in the project looking at infant vaccination.
Dr. Kelly began his presentation by showing a figure depicting where vaccine-induced immunity fits into the larger suite of clinical studies. These studies involve mouse models, human challenge models, and infection patients. A key theme is the use of a core group of immunoassays across all studies, with the hope that they will allow effective cross comparisons.
Dr. Kelly stated, “If we find a correlate of protection in the challenge model, we can then interpret the vaccine studies in the light of that because we are using standardized constant immunoassays.”
The assays being used depend in part on the specific study and the volume of blood available. They will generally include Bordetella-specific antibody and functional antibody assays, as well as interesting studies collecting mucosal samples from infants and adults to look at serological responses. Also under examination are a range of enzyme-linked immune absorbent spot, flow cytometry, and culture techniques looking at Memory B cells, T cells, and gene expression.
Complementing these assay studies, PERISCOPE includes a series of clinical investigations designed to throw light on three areas of interest, described below:
First, researchers hope to gain a better understanding regarding the effects of the original whole cell vaccine versus the current acellular variety. The former uses an inactivated version of the whole organism. Epidemiological studies, animal data, and experience in the field demonstrate that whole-cell vaccination results in a broad, long-lasting, and effective immune response.
By comparison, the acellular pertussis vaccine consists of between three and five protein components, which are purified from cultured Bordetella pertussis. While it is an effective vaccine, its effects are less durable; routine use in some countries is associated with cyclical outbreaks of increasing severity.
A second issue for researchers involved in the PERISCOPE project concerns the effects of maternal immunization. In the United Kingdom in 2012, for example, an increasing number of cases were noted 6-7 years after adoption of an acellular vaccine for routine vaccination in the 2nd-3rd trimester of pregnancy. Vaccination appears to effectively control neonatal disease, but whether this influences infant immune responses and long-term control of pertussis for a population is unknown.
Finally, the group is interested in the effects of an acellular booster across all age groups. While the effects may be short-lived, the booster is a potential strategy for controlling a population by repeated boosting of immunity. This is another area where using novel immunoassays may aid better understanding.
To find answers, the consortium has established four studies: the Gambia Pertussis study (GaPs) in Gambia and AWARE, the sister study to GaPs in the United Kingdom, addressing the acellular pertussis versus cellular pertussis question; the Pertussis Maternal Immunization Study in Finland (MIFI) addressing maternal immunization; and the Booster against Pertussis (BERT) study across three countries (U.K., the Netherlands, and Finland) looking at acellular booster across age groups.
Gambia pertussis study
GaPs is the largest single study in the project and is being run at the Medical Research Council–funded London School of Tropical Medicine center in Gambia. Beate Kampmann, MD, PhD, of Imperial College London, England, is the project lead. It is due to complete in 2022. GaPs seeks to enroll 600 mother/infant pairs and randomize the mothers to either an acellular pertussis booster in pregnancy or a tetanus toxoid control vaccine. Infants are subsequently randomized to an acellular or whole-cell pertussis schedule of primary immunization. The vaccine doses are being given at 2, 3, and 4 months. The primary endpoint is a serological finding being measured at 9 months of age, when the infant would usually receive yellow fever, measles, and rubella vaccination.
GaPs has a number of pathways. Within each of the four arms generated by the two randomizations, the maternal randomization and the infant randomization, there are five subgroups. They are designed to study time points in subgroups A and B after the first dose in more detail, looking at the innate immune responses using gene expression. It will enable researchers to study adaptive immune responses to T cells and B cells after the second dose of vaccine. By employing a range of subgroups, the team can explore the immune profile using the assays referred to above. Such information should provide new insights into the differences between acellular and whole-cell vaccines.
The AWARE study
AWARE is the sister study to GaPs and looks at the acellular/whole pertussis issue. Because many developed countries, such as the United Kingdom, have established maternal immunization programs, it is not possible to randomize mothers. Consequently, researchers have opted to recruit infants of mothers who have received an acellular vaccine in pregnancy and randomize them to either an acellular schedule of primary immunization or a whole-cell schedule.
The selected vaccine is ComVac5 from Bharat Biotech. This whole-cell vaccine differs from that used in Gambia. An early obstacle for AWARE has been seeking permission to import a non-conventional vaccine into Europe. It has delayed the anticipated end date to 2023. Participating infants will receive a two-dose schedule at 2 and 4 months of age per their randomization; then, both groups will go on to receive an acellular pertussis booster at 12 months. At all time points, the team will sample blood for cells and serum, as well as mucosal fluid from the nose. Because the mucosal surface is where the action is, this approach will likely generate new data around antibody responses.
The MIFI
The Pertussis Maternal Immunization Study in Finland is being run by Jussi Mertsola, of the University of Turku, Finland, and Qiushui He, of the National Public Health Institute, Turku. It is due to complete in late 2021. Where, in the United Kingdom, researchers are unable to randomize mothers because of the current guidelines, researchers in Finland do not have a maternal immunization program to consider. MIFI will randomize 80 mothers, 40 to immunization with acellular pertussis and 40 to a control group. Dr. Kelly stated that whole cell vaccines are not available for use in Finland. Participants will receive a two-dose schedule at 3 and 5 months. Blood samples will then be taken to compare the serological and cellular responses, which will help researchers understand the effects of maternal immunization. In addition, there will be sampling of mucosal fluid using a device that collects a standardized aliquot of fluid.
The BERT study
The final clinical element of PERISCOPE presented by Dr. Kelly was the Booster against Pertussis study. This study is near completion. It seeks to examine the use of an acellular booster across different age groups and three countries: the United Kingdom, the Netherlands, and Finland. The study is being coordinated by Guy Berbers, PhD, at the National Institute for Public Health and the Environment in the Netherlands.
BERT comprises four cohorts (A, B, C, D) of different ages: 7-10 years (36 participants), 11-15 years (36 participants), mid-adult (25 participants), and older age (25 participants). After receiving an acellular booster, participants will undergo intense sampling. Sampling will take place immediately after immunization at day 7 and look at adaptive effects, then again at day 28 and day 365.
Because some participants will have already received whole cell or acellular vaccination, this approach will allow researchers to look at the effects of priming (i.e., how long the B cell/T cell antibody responses last).
Involving different countries across Europe ensures wide applicability of results, but also allows researchers to compare the effects of very different immunization histories.
At the end of this ESPID session, Dimitri Diavatopoulos, PhD, assistant professor at the Radboud University Medical Centre Nijmegen, the Netherlands, commented that a future problem in studying pertussis vaccines and their potential clinical application is that most vaccination schedules now involve combination products. Obtaining a stand-alone vaccination may prove difficult, and there may be resistance if it complicates current vaccination programs.
Dr. Kelly acknowledged funding for the PERISCOPE project from GlaxoSmithKline and Pasteur Sanofi.
With increasing whooping cough numbers, developing an effective new vaccine against Bordetella pertussis is a priority. Results from the multifactorial PERISCOPE Project will help scientists and clinicians move forward.
Dominic Kelly, PhD, talked about vaccine-induced immunity and provided an overview of ongoing clinical trials in the PERISCOPE (Pertussis Correlates of Protection Europe) project in a key research session at the start of the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year. Dr. Kelly, a pediatrician at the Children’s Hospital in Oxford and a member of the Oxford Vaccines Group, leads one of the studies in the project looking at infant vaccination.
Dr. Kelly began his presentation by showing a figure depicting where vaccine-induced immunity fits into the larger suite of clinical studies. These studies involve mouse models, human challenge models, and infection patients. A key theme is the use of a core group of immunoassays across all studies, with the hope that they will allow effective cross comparisons.
Dr. Kelly stated, “If we find a correlate of protection in the challenge model, we can then interpret the vaccine studies in the light of that because we are using standardized constant immunoassays.”
The assays being used depend in part on the specific study and the volume of blood available. They will generally include Bordetella-specific antibody and functional antibody assays, as well as interesting studies collecting mucosal samples from infants and adults to look at serological responses. Also under examination are a range of enzyme-linked immune absorbent spot, flow cytometry, and culture techniques looking at Memory B cells, T cells, and gene expression.
Complementing these assay studies, PERISCOPE includes a series of clinical investigations designed to throw light on three areas of interest, described below:
First, researchers hope to gain a better understanding regarding the effects of the original whole cell vaccine versus the current acellular variety. The former uses an inactivated version of the whole organism. Epidemiological studies, animal data, and experience in the field demonstrate that whole-cell vaccination results in a broad, long-lasting, and effective immune response.
By comparison, the acellular pertussis vaccine consists of between three and five protein components, which are purified from cultured Bordetella pertussis. While it is an effective vaccine, its effects are less durable; routine use in some countries is associated with cyclical outbreaks of increasing severity.
A second issue for researchers involved in the PERISCOPE project concerns the effects of maternal immunization. In the United Kingdom in 2012, for example, an increasing number of cases were noted 6-7 years after adoption of an acellular vaccine for routine vaccination in the 2nd-3rd trimester of pregnancy. Vaccination appears to effectively control neonatal disease, but whether this influences infant immune responses and long-term control of pertussis for a population is unknown.
Finally, the group is interested in the effects of an acellular booster across all age groups. While the effects may be short-lived, the booster is a potential strategy for controlling a population by repeated boosting of immunity. This is another area where using novel immunoassays may aid better understanding.
To find answers, the consortium has established four studies: the Gambia Pertussis study (GaPs) in Gambia and AWARE, the sister study to GaPs in the United Kingdom, addressing the acellular pertussis versus cellular pertussis question; the Pertussis Maternal Immunization Study in Finland (MIFI) addressing maternal immunization; and the Booster against Pertussis (BERT) study across three countries (U.K., the Netherlands, and Finland) looking at acellular booster across age groups.
Gambia pertussis study
GaPs is the largest single study in the project and is being run at the Medical Research Council–funded London School of Tropical Medicine center in Gambia. Beate Kampmann, MD, PhD, of Imperial College London, England, is the project lead. It is due to complete in 2022. GaPs seeks to enroll 600 mother/infant pairs and randomize the mothers to either an acellular pertussis booster in pregnancy or a tetanus toxoid control vaccine. Infants are subsequently randomized to an acellular or whole-cell pertussis schedule of primary immunization. The vaccine doses are being given at 2, 3, and 4 months. The primary endpoint is a serological finding being measured at 9 months of age, when the infant would usually receive yellow fever, measles, and rubella vaccination.
GaPs has a number of pathways. Within each of the four arms generated by the two randomizations, the maternal randomization and the infant randomization, there are five subgroups. They are designed to study time points in subgroups A and B after the first dose in more detail, looking at the innate immune responses using gene expression. It will enable researchers to study adaptive immune responses to T cells and B cells after the second dose of vaccine. By employing a range of subgroups, the team can explore the immune profile using the assays referred to above. Such information should provide new insights into the differences between acellular and whole-cell vaccines.
The AWARE study
AWARE is the sister study to GaPs and looks at the acellular/whole pertussis issue. Because many developed countries, such as the United Kingdom, have established maternal immunization programs, it is not possible to randomize mothers. Consequently, researchers have opted to recruit infants of mothers who have received an acellular vaccine in pregnancy and randomize them to either an acellular schedule of primary immunization or a whole-cell schedule.
The selected vaccine is ComVac5 from Bharat Biotech. This whole-cell vaccine differs from that used in Gambia. An early obstacle for AWARE has been seeking permission to import a non-conventional vaccine into Europe. It has delayed the anticipated end date to 2023. Participating infants will receive a two-dose schedule at 2 and 4 months of age per their randomization; then, both groups will go on to receive an acellular pertussis booster at 12 months. At all time points, the team will sample blood for cells and serum, as well as mucosal fluid from the nose. Because the mucosal surface is where the action is, this approach will likely generate new data around antibody responses.
The MIFI
The Pertussis Maternal Immunization Study in Finland is being run by Jussi Mertsola, of the University of Turku, Finland, and Qiushui He, of the National Public Health Institute, Turku. It is due to complete in late 2021. Where, in the United Kingdom, researchers are unable to randomize mothers because of the current guidelines, researchers in Finland do not have a maternal immunization program to consider. MIFI will randomize 80 mothers, 40 to immunization with acellular pertussis and 40 to a control group. Dr. Kelly stated that whole cell vaccines are not available for use in Finland. Participants will receive a two-dose schedule at 3 and 5 months. Blood samples will then be taken to compare the serological and cellular responses, which will help researchers understand the effects of maternal immunization. In addition, there will be sampling of mucosal fluid using a device that collects a standardized aliquot of fluid.
The BERT study
The final clinical element of PERISCOPE presented by Dr. Kelly was the Booster against Pertussis study. This study is near completion. It seeks to examine the use of an acellular booster across different age groups and three countries: the United Kingdom, the Netherlands, and Finland. The study is being coordinated by Guy Berbers, PhD, at the National Institute for Public Health and the Environment in the Netherlands.
BERT comprises four cohorts (A, B, C, D) of different ages: 7-10 years (36 participants), 11-15 years (36 participants), mid-adult (25 participants), and older age (25 participants). After receiving an acellular booster, participants will undergo intense sampling. Sampling will take place immediately after immunization at day 7 and look at adaptive effects, then again at day 28 and day 365.
Because some participants will have already received whole cell or acellular vaccination, this approach will allow researchers to look at the effects of priming (i.e., how long the B cell/T cell antibody responses last).
Involving different countries across Europe ensures wide applicability of results, but also allows researchers to compare the effects of very different immunization histories.
At the end of this ESPID session, Dimitri Diavatopoulos, PhD, assistant professor at the Radboud University Medical Centre Nijmegen, the Netherlands, commented that a future problem in studying pertussis vaccines and their potential clinical application is that most vaccination schedules now involve combination products. Obtaining a stand-alone vaccination may prove difficult, and there may be resistance if it complicates current vaccination programs.
Dr. Kelly acknowledged funding for the PERISCOPE project from GlaxoSmithKline and Pasteur Sanofi.
FROM ESPID 2020
COVID-19–induced drop in first measles vaccinations sparks resurgence concerns
Widespread use of the MMR vaccine is not only crucial for protecting the community against infectious outbreaks, but also serves as the overall pacesetter for preventive services, said Sara M. Bode, MD and colleagues at Nationwide Children’s Hospital in Columbus.
As part of a bivariate logistic regression analysis, Dr. Bode and colleagues sought to evaluate changes in measles vaccination rates across 12 clinic sites of the Nationwide Children’s Hospital pediatric primary care network in Columbus among 23,534 children aged 16 months. The study period targeted the time between April and May 2020, when clinic access and appointment attendance declined following the start of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, until the June-to-August 2020 time period, when clinical care was allowed to return.
The need for the study was prompted by Centers for Disease Control and Prevention reporting on a state-specific precipitous decline in MMR vaccination rates shortly after the onset of COVID-19 in May 2020. Citing the results of one study, such reductions in vaccination have raised concerns over the possibility of a measles resurgence, noted Dr. Bode and associates.
MMR vaccination rates begin to drop with onset of COVID-19 pandemic.
From March 2017 to March 2020, the average rate of MMR vaccination in 16-month-olds was 72%. It subsequently decreased to 67% from April to May 2020, and then dropped further to 62% during the period June to August, 2020 (P = .001). Those without insurance were less likely to be vaccinated than were those carrying private insurance or Medicaid.
Among patients who had not attended a preventive care visit after 12 months of age, the proportion who received vaccines declined during the same time periods, from 10% before the pandemic to 6% at the start of the pandemic and 3% during the summer months of 2020.
“Given the baseline low vaccination rates even before the pandemic and the subsequent decline, we face a critical need to improve timely vaccination and provide catch-up opportunities” in areas with the highest incidence of COVID-19, observed Dr. Bode and colleagues.
Innovative approaches are needed to encourage families to seek preventive care.
In response, the researchers announced the implementation of new community-based vaccination approaches in Ohio, including pop-up vaccine clinics, mobile clinics, and school-based clinics to provide families, who are reluctant to visit health care facilities over COVID-19 related concerns, with safe alternatives. “We believe that it is critical to develop innovative approaches to have families return for preventive care,” they added.
In a separate interview, Herschel Lessin, MD, a private practice pediatrician in Poughkeepsie, N.Y., noted: “This study confirms the anecdotal experience of pediatricians around the country, and our greatest fear that the pandemic will interfere with herd immunity of children for vaccine-preventable illness. Although the study was of urban offices with a primarily Medicaid population, I believe the results to be very worrisome should they prove to be generalizable to the country, as a whole. The significant reduction of well-child visits due to COVID-19 (and fear of COVID-19) seriously impaired the vaccination status of a standard required vaccine in a large population. What is even more worrisome is that the rates continued to fall even after the initial closure of many offices and well into their reopening, despite concerted efforts to try to catch up these missed visits and immunizations.”
Measles is an intensely contagious illness that has not been eradicated, as evidenced by the enormous measles outbreak stemming from Disneyland in 2014-2015, and again with the possible exposure of hundreds to an infected Disneyland visitor last fall, where coverage rates were even higher than in this study, added Dr. Lessin. “This phenomenon, unless forcefully remedied, could easily result in large outbreaks of other vaccine-preventable illness besides COVID-19,” he cautioned.
Dr. Bode and colleagues as well as Dr. Lessin had no conflicts of interest and no relevant financial disclosures.
SOURCE: Bode SM et al. Pediatrics. 2021. doi: 10.1542/peds.2020-035576.
Widespread use of the MMR vaccine is not only crucial for protecting the community against infectious outbreaks, but also serves as the overall pacesetter for preventive services, said Sara M. Bode, MD and colleagues at Nationwide Children’s Hospital in Columbus.
As part of a bivariate logistic regression analysis, Dr. Bode and colleagues sought to evaluate changes in measles vaccination rates across 12 clinic sites of the Nationwide Children’s Hospital pediatric primary care network in Columbus among 23,534 children aged 16 months. The study period targeted the time between April and May 2020, when clinic access and appointment attendance declined following the start of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, until the June-to-August 2020 time period, when clinical care was allowed to return.
The need for the study was prompted by Centers for Disease Control and Prevention reporting on a state-specific precipitous decline in MMR vaccination rates shortly after the onset of COVID-19 in May 2020. Citing the results of one study, such reductions in vaccination have raised concerns over the possibility of a measles resurgence, noted Dr. Bode and associates.
MMR vaccination rates begin to drop with onset of COVID-19 pandemic.
From March 2017 to March 2020, the average rate of MMR vaccination in 16-month-olds was 72%. It subsequently decreased to 67% from April to May 2020, and then dropped further to 62% during the period June to August, 2020 (P = .001). Those without insurance were less likely to be vaccinated than were those carrying private insurance or Medicaid.
Among patients who had not attended a preventive care visit after 12 months of age, the proportion who received vaccines declined during the same time periods, from 10% before the pandemic to 6% at the start of the pandemic and 3% during the summer months of 2020.
“Given the baseline low vaccination rates even before the pandemic and the subsequent decline, we face a critical need to improve timely vaccination and provide catch-up opportunities” in areas with the highest incidence of COVID-19, observed Dr. Bode and colleagues.
Innovative approaches are needed to encourage families to seek preventive care.
In response, the researchers announced the implementation of new community-based vaccination approaches in Ohio, including pop-up vaccine clinics, mobile clinics, and school-based clinics to provide families, who are reluctant to visit health care facilities over COVID-19 related concerns, with safe alternatives. “We believe that it is critical to develop innovative approaches to have families return for preventive care,” they added.
In a separate interview, Herschel Lessin, MD, a private practice pediatrician in Poughkeepsie, N.Y., noted: “This study confirms the anecdotal experience of pediatricians around the country, and our greatest fear that the pandemic will interfere with herd immunity of children for vaccine-preventable illness. Although the study was of urban offices with a primarily Medicaid population, I believe the results to be very worrisome should they prove to be generalizable to the country, as a whole. The significant reduction of well-child visits due to COVID-19 (and fear of COVID-19) seriously impaired the vaccination status of a standard required vaccine in a large population. What is even more worrisome is that the rates continued to fall even after the initial closure of many offices and well into their reopening, despite concerted efforts to try to catch up these missed visits and immunizations.”
Measles is an intensely contagious illness that has not been eradicated, as evidenced by the enormous measles outbreak stemming from Disneyland in 2014-2015, and again with the possible exposure of hundreds to an infected Disneyland visitor last fall, where coverage rates were even higher than in this study, added Dr. Lessin. “This phenomenon, unless forcefully remedied, could easily result in large outbreaks of other vaccine-preventable illness besides COVID-19,” he cautioned.
Dr. Bode and colleagues as well as Dr. Lessin had no conflicts of interest and no relevant financial disclosures.
SOURCE: Bode SM et al. Pediatrics. 2021. doi: 10.1542/peds.2020-035576.
Widespread use of the MMR vaccine is not only crucial for protecting the community against infectious outbreaks, but also serves as the overall pacesetter for preventive services, said Sara M. Bode, MD and colleagues at Nationwide Children’s Hospital in Columbus.
As part of a bivariate logistic regression analysis, Dr. Bode and colleagues sought to evaluate changes in measles vaccination rates across 12 clinic sites of the Nationwide Children’s Hospital pediatric primary care network in Columbus among 23,534 children aged 16 months. The study period targeted the time between April and May 2020, when clinic access and appointment attendance declined following the start of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, until the June-to-August 2020 time period, when clinical care was allowed to return.
The need for the study was prompted by Centers for Disease Control and Prevention reporting on a state-specific precipitous decline in MMR vaccination rates shortly after the onset of COVID-19 in May 2020. Citing the results of one study, such reductions in vaccination have raised concerns over the possibility of a measles resurgence, noted Dr. Bode and associates.
MMR vaccination rates begin to drop with onset of COVID-19 pandemic.
From March 2017 to March 2020, the average rate of MMR vaccination in 16-month-olds was 72%. It subsequently decreased to 67% from April to May 2020, and then dropped further to 62% during the period June to August, 2020 (P = .001). Those without insurance were less likely to be vaccinated than were those carrying private insurance or Medicaid.
Among patients who had not attended a preventive care visit after 12 months of age, the proportion who received vaccines declined during the same time periods, from 10% before the pandemic to 6% at the start of the pandemic and 3% during the summer months of 2020.
“Given the baseline low vaccination rates even before the pandemic and the subsequent decline, we face a critical need to improve timely vaccination and provide catch-up opportunities” in areas with the highest incidence of COVID-19, observed Dr. Bode and colleagues.
Innovative approaches are needed to encourage families to seek preventive care.
In response, the researchers announced the implementation of new community-based vaccination approaches in Ohio, including pop-up vaccine clinics, mobile clinics, and school-based clinics to provide families, who are reluctant to visit health care facilities over COVID-19 related concerns, with safe alternatives. “We believe that it is critical to develop innovative approaches to have families return for preventive care,” they added.
In a separate interview, Herschel Lessin, MD, a private practice pediatrician in Poughkeepsie, N.Y., noted: “This study confirms the anecdotal experience of pediatricians around the country, and our greatest fear that the pandemic will interfere with herd immunity of children for vaccine-preventable illness. Although the study was of urban offices with a primarily Medicaid population, I believe the results to be very worrisome should they prove to be generalizable to the country, as a whole. The significant reduction of well-child visits due to COVID-19 (and fear of COVID-19) seriously impaired the vaccination status of a standard required vaccine in a large population. What is even more worrisome is that the rates continued to fall even after the initial closure of many offices and well into their reopening, despite concerted efforts to try to catch up these missed visits and immunizations.”
Measles is an intensely contagious illness that has not been eradicated, as evidenced by the enormous measles outbreak stemming from Disneyland in 2014-2015, and again with the possible exposure of hundreds to an infected Disneyland visitor last fall, where coverage rates were even higher than in this study, added Dr. Lessin. “This phenomenon, unless forcefully remedied, could easily result in large outbreaks of other vaccine-preventable illness besides COVID-19,” he cautioned.
Dr. Bode and colleagues as well as Dr. Lessin had no conflicts of interest and no relevant financial disclosures.
SOURCE: Bode SM et al. Pediatrics. 2021. doi: 10.1542/peds.2020-035576.
FROM PEDIATRICS
Latest rise in child COVID-19 cases is relatively small
For the seventh week out of the last eight, more new cases of COVID-19 in children were reported in the United States than any week before, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
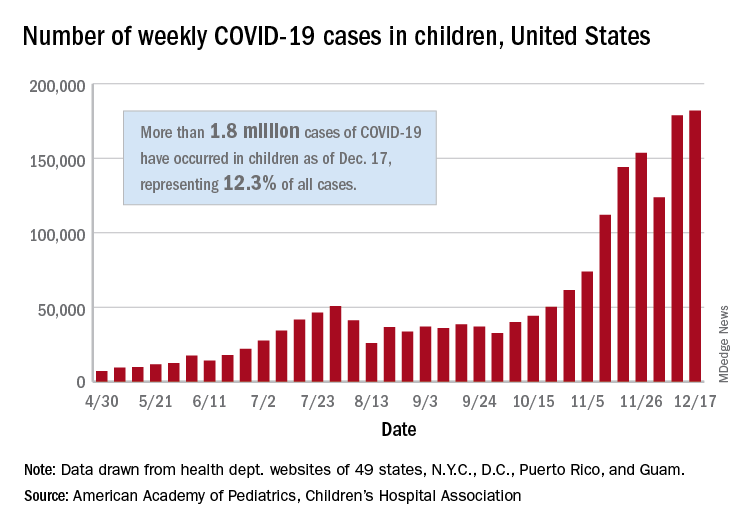
There were just over 182,000 new cases of COVID-19 in children during the week ending Dec. 17, topping the previous high of almost 179,000 set the previous week. – a stretch of 11 weeks that has produced only one decline, based on data from the latest AAP/CHA weekly report.
As of Dec. 17, there had been over 1.8 million cases of COVID-19 in children, which represents 12.3% of all U.S. cases. For the week, 14% of all cases occurred in children, which was up slightly from 13.8% the week before (Dec. 10). The overall rate of coronavirus infection is now 2,420 cases per 100,000 children in the population, the AAP and CHA said.
A total of 30 states are above that national rate, with North Dakota the highest at 7,515 cases per 100,000 children, followed by South Dakota (5,618), Wyoming (5,157), Wisconsin (5,106), and Tennessee (4,994). Wyoming has the highest proportion of cases occurring in children at 20.8%, but that is down from 23.4% in mid-November, based on data collected by the AAP and CHA from the health department websites of 49 states (New York does not provide age distributions), the District of Columbia, New York City, Puerto Rico, and Guam.
In the last 2 weeks, however, the largest percent increases in new cases came in states with low-to-average rates of cumulative child infection. California, Connecticut, Delaware, Maine, Maryland, New Hampshire, and Vermont all saw increases of over 35% from Dec. 3 to Dec. 17, while the smallest increases occurred in Hawaii, North Dakota, and Wyoming, the AAP and CHA reported.
For the seventh week out of the last eight, more new cases of COVID-19 in children were reported in the United States than any week before, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

There were just over 182,000 new cases of COVID-19 in children during the week ending Dec. 17, topping the previous high of almost 179,000 set the previous week. – a stretch of 11 weeks that has produced only one decline, based on data from the latest AAP/CHA weekly report.
As of Dec. 17, there had been over 1.8 million cases of COVID-19 in children, which represents 12.3% of all U.S. cases. For the week, 14% of all cases occurred in children, which was up slightly from 13.8% the week before (Dec. 10). The overall rate of coronavirus infection is now 2,420 cases per 100,000 children in the population, the AAP and CHA said.
A total of 30 states are above that national rate, with North Dakota the highest at 7,515 cases per 100,000 children, followed by South Dakota (5,618), Wyoming (5,157), Wisconsin (5,106), and Tennessee (4,994). Wyoming has the highest proportion of cases occurring in children at 20.8%, but that is down from 23.4% in mid-November, based on data collected by the AAP and CHA from the health department websites of 49 states (New York does not provide age distributions), the District of Columbia, New York City, Puerto Rico, and Guam.
In the last 2 weeks, however, the largest percent increases in new cases came in states with low-to-average rates of cumulative child infection. California, Connecticut, Delaware, Maine, Maryland, New Hampshire, and Vermont all saw increases of over 35% from Dec. 3 to Dec. 17, while the smallest increases occurred in Hawaii, North Dakota, and Wyoming, the AAP and CHA reported.
For the seventh week out of the last eight, more new cases of COVID-19 in children were reported in the United States than any week before, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

There were just over 182,000 new cases of COVID-19 in children during the week ending Dec. 17, topping the previous high of almost 179,000 set the previous week. – a stretch of 11 weeks that has produced only one decline, based on data from the latest AAP/CHA weekly report.
As of Dec. 17, there had been over 1.8 million cases of COVID-19 in children, which represents 12.3% of all U.S. cases. For the week, 14% of all cases occurred in children, which was up slightly from 13.8% the week before (Dec. 10). The overall rate of coronavirus infection is now 2,420 cases per 100,000 children in the population, the AAP and CHA said.
A total of 30 states are above that national rate, with North Dakota the highest at 7,515 cases per 100,000 children, followed by South Dakota (5,618), Wyoming (5,157), Wisconsin (5,106), and Tennessee (4,994). Wyoming has the highest proportion of cases occurring in children at 20.8%, but that is down from 23.4% in mid-November, based on data collected by the AAP and CHA from the health department websites of 49 states (New York does not provide age distributions), the District of Columbia, New York City, Puerto Rico, and Guam.
In the last 2 weeks, however, the largest percent increases in new cases came in states with low-to-average rates of cumulative child infection. California, Connecticut, Delaware, Maine, Maryland, New Hampshire, and Vermont all saw increases of over 35% from Dec. 3 to Dec. 17, while the smallest increases occurred in Hawaii, North Dakota, and Wyoming, the AAP and CHA reported.
Strategies for tracking SARS-CoV-2 could help detect next pandemic
Two recently published studies indicate that COVID-19 infections were already circulating in the United States in December 2019. The question is whether these methodologies that could be applied to track the next pandemic.
One study evaluating blood donations found antibodies on the West coast as early as Dec. 13, 2019, and in blood donated on the East Coast by early January 2020 (Clin Infect Dis. 2020; Nov 30. doi: 10.1093/cid/ciaa1785). Both preceded the first documented COVID-19 infection in the United States, which has been widely reported as occurring on Jan. 19, 2020, in a traveler returning from China.
The other study, utilizing electronic medical record (EMR) analytics, demonstrated a spike in visits or hospitalizations for cough, a trend that persisted from Dec. 22, 2019, onward, exceeding norms for seasonal flu ( J Med Internet Res. 2020;22:e21562). This spike was interpreted as evidence that the SARS-CoV-2 pandemic was already underway before the first case was established.
While the ongoing serologic testing of blood donations for viral antibodies “will advance understanding of the epidemiology” for SARS-CoV-2 and “inform allocation of resources and public health prevention interventions to mitigate morbidity and mortality,” it might also be a strategy for disease surveillance in the next pandemic, according to a team led by investigators at the Centers for Disease Control and Prevention.
Blood donation surveillance is not now used routinely to monitor for population-based health threats, but it is not a new idea, according to the lead author of the study, Sridhar V. Basavaraju, MD, of Emory University and director of the CDC’s Office of Blood, Organ, and Other Tissue Safety, Atlanta, and his coinvestigators. Most recently, blood donation surveillance was used in the United States to track the penetration of the Zika virus.
For early detection of respiratory infections, blood donations might have unique advantages over alternatives, such as surveillance of respiratory specimens from symptomatic patients. Not least, blood donation surveillance captures individuals who are not seeking medical care, according to the investigators.
EMR surveillance might also have unique advantages for population-based monitoring of health threats. For one, aggregate data from large EMR systems have the potential to reveal symptom patterns before they become apparent at level of clinical care, according to a team of collaborating investigators from the University of California, Los Angeles, and the University of Washington, Seattle.
Emphasizing an urgent need for “agile healthcare analytics” to enable “disease surveillance in real time,” the first author of the EMR study, Joann G. Elmore, MD, professor in the department of health policy and management at the University of California, Los Angeles, expressed the hope that the approach will “lead to better preparation and the ability to quickly provide warnings and track the next pandemic.”
In the blood donation surveillance study, the goal was simply to determine whether SARS-CoV-2 reactive antibodies could be found in blood donations before the first case was identified. Of the 7,389 archived blood samples tested between Dec. 13, 2019, and Jan. 17, 2020, 106 (1.4%) were reactive.
These were not true positives, acknowledged the investigators. True positives would require reactive antibodies in the context of a positive molecular diagnostic test or paired acute convalescent sera with rising titers. The investigators also cautioned that false positives could not be completely ruled out, particularly in light of cross-reactivity that has been reported with other human coronaviruses.
Nevertheless, the monitoring of blood donations offers substantial promise for “understanding the dynamics of SARS-CoV-2 pandemic from early introduction,” and the CDC is now collaborating on ongoing surveillance with the goal of contributing information that could be applied “to mitigate morbidity and mortality.”
Lessons learned from this pandemic are potentially relevant to the next.
The EMR study simply looked at whether the word “cough” was included more often in the notes from visits or hospitalizations between December 2019 and February 2020 relative to the preceding 5 years. The investigators drew on data from three hospitals and more than 180 clinics.
From Dec. 22, 2019, onward, cough was noted above the 95% prediction interval for all 10 weeks of the study. The excess was seen in the outpatient setting and among hospitalized patients. There was also significant excess in the number of patients hospitalized with acute respiratory failure during the study period.
“Our approach to analyzing electronic records could be helpful in the future as we included consideration of data from the outpatient clinics in addition to the emergency departments and inpatient settings,” Dr. Elmore reported.
Surveillance of influenza and influenza-like infections has been undertaken in the United States for more than 20 years, but Dr. Elmore contends that EMR data, particularly data from outpatient clinics are “usually a harbinger of what is to come” for emergency department visits and, ultimately, hospitalizations. She thinks that this is a resource not yet fully exploited.
“There are always opportunities to better harness EMR data,” Dr. Elmore said.
These are intriguing studies and “useful” for reconsidering when SARS-CoV-2 was introduced in the United States, according to Janet G. Basemen, PhD, a professor of epidemiology and the associate dean of the University of Washington School of Public Health, Seattle. However, she noted that the task of translating data like these into actionable public health strategies has proven difficult in the past.
Symptom-based surveillance systems “have mostly served as situational awareness rather than early detection tools,” Dr. Baseman said. The problem is timely interpretation of a given signal.
Not that she doubts such tools “would be an incredible resource for humanity” if the current limitations can be resolved or that technological advances will lead to better methods of detecting and monitoring pandemics “at some point.” Rather, “we’re just not there yet,” she said.
SOURCE: Basavaraju SV et al. Clin Infect Dis. 2020 Nov 30. doi: 10.1093/cid/ciaa1785); Elmore JG et al. J Med Internet Res. 2020;22:e21562).
Two recently published studies indicate that COVID-19 infections were already circulating in the United States in December 2019. The question is whether these methodologies that could be applied to track the next pandemic.
One study evaluating blood donations found antibodies on the West coast as early as Dec. 13, 2019, and in blood donated on the East Coast by early January 2020 (Clin Infect Dis. 2020; Nov 30. doi: 10.1093/cid/ciaa1785). Both preceded the first documented COVID-19 infection in the United States, which has been widely reported as occurring on Jan. 19, 2020, in a traveler returning from China.
The other study, utilizing electronic medical record (EMR) analytics, demonstrated a spike in visits or hospitalizations for cough, a trend that persisted from Dec. 22, 2019, onward, exceeding norms for seasonal flu ( J Med Internet Res. 2020;22:e21562). This spike was interpreted as evidence that the SARS-CoV-2 pandemic was already underway before the first case was established.
While the ongoing serologic testing of blood donations for viral antibodies “will advance understanding of the epidemiology” for SARS-CoV-2 and “inform allocation of resources and public health prevention interventions to mitigate morbidity and mortality,” it might also be a strategy for disease surveillance in the next pandemic, according to a team led by investigators at the Centers for Disease Control and Prevention.
Blood donation surveillance is not now used routinely to monitor for population-based health threats, but it is not a new idea, according to the lead author of the study, Sridhar V. Basavaraju, MD, of Emory University and director of the CDC’s Office of Blood, Organ, and Other Tissue Safety, Atlanta, and his coinvestigators. Most recently, blood donation surveillance was used in the United States to track the penetration of the Zika virus.
For early detection of respiratory infections, blood donations might have unique advantages over alternatives, such as surveillance of respiratory specimens from symptomatic patients. Not least, blood donation surveillance captures individuals who are not seeking medical care, according to the investigators.
EMR surveillance might also have unique advantages for population-based monitoring of health threats. For one, aggregate data from large EMR systems have the potential to reveal symptom patterns before they become apparent at level of clinical care, according to a team of collaborating investigators from the University of California, Los Angeles, and the University of Washington, Seattle.
Emphasizing an urgent need for “agile healthcare analytics” to enable “disease surveillance in real time,” the first author of the EMR study, Joann G. Elmore, MD, professor in the department of health policy and management at the University of California, Los Angeles, expressed the hope that the approach will “lead to better preparation and the ability to quickly provide warnings and track the next pandemic.”
In the blood donation surveillance study, the goal was simply to determine whether SARS-CoV-2 reactive antibodies could be found in blood donations before the first case was identified. Of the 7,389 archived blood samples tested between Dec. 13, 2019, and Jan. 17, 2020, 106 (1.4%) were reactive.
These were not true positives, acknowledged the investigators. True positives would require reactive antibodies in the context of a positive molecular diagnostic test or paired acute convalescent sera with rising titers. The investigators also cautioned that false positives could not be completely ruled out, particularly in light of cross-reactivity that has been reported with other human coronaviruses.
Nevertheless, the monitoring of blood donations offers substantial promise for “understanding the dynamics of SARS-CoV-2 pandemic from early introduction,” and the CDC is now collaborating on ongoing surveillance with the goal of contributing information that could be applied “to mitigate morbidity and mortality.”
Lessons learned from this pandemic are potentially relevant to the next.
The EMR study simply looked at whether the word “cough” was included more often in the notes from visits or hospitalizations between December 2019 and February 2020 relative to the preceding 5 years. The investigators drew on data from three hospitals and more than 180 clinics.
From Dec. 22, 2019, onward, cough was noted above the 95% prediction interval for all 10 weeks of the study. The excess was seen in the outpatient setting and among hospitalized patients. There was also significant excess in the number of patients hospitalized with acute respiratory failure during the study period.
“Our approach to analyzing electronic records could be helpful in the future as we included consideration of data from the outpatient clinics in addition to the emergency departments and inpatient settings,” Dr. Elmore reported.
Surveillance of influenza and influenza-like infections has been undertaken in the United States for more than 20 years, but Dr. Elmore contends that EMR data, particularly data from outpatient clinics are “usually a harbinger of what is to come” for emergency department visits and, ultimately, hospitalizations. She thinks that this is a resource not yet fully exploited.
“There are always opportunities to better harness EMR data,” Dr. Elmore said.
These are intriguing studies and “useful” for reconsidering when SARS-CoV-2 was introduced in the United States, according to Janet G. Basemen, PhD, a professor of epidemiology and the associate dean of the University of Washington School of Public Health, Seattle. However, she noted that the task of translating data like these into actionable public health strategies has proven difficult in the past.
Symptom-based surveillance systems “have mostly served as situational awareness rather than early detection tools,” Dr. Baseman said. The problem is timely interpretation of a given signal.
Not that she doubts such tools “would be an incredible resource for humanity” if the current limitations can be resolved or that technological advances will lead to better methods of detecting and monitoring pandemics “at some point.” Rather, “we’re just not there yet,” she said.
SOURCE: Basavaraju SV et al. Clin Infect Dis. 2020 Nov 30. doi: 10.1093/cid/ciaa1785); Elmore JG et al. J Med Internet Res. 2020;22:e21562).
Two recently published studies indicate that COVID-19 infections were already circulating in the United States in December 2019. The question is whether these methodologies that could be applied to track the next pandemic.
One study evaluating blood donations found antibodies on the West coast as early as Dec. 13, 2019, and in blood donated on the East Coast by early January 2020 (Clin Infect Dis. 2020; Nov 30. doi: 10.1093/cid/ciaa1785). Both preceded the first documented COVID-19 infection in the United States, which has been widely reported as occurring on Jan. 19, 2020, in a traveler returning from China.
The other study, utilizing electronic medical record (EMR) analytics, demonstrated a spike in visits or hospitalizations for cough, a trend that persisted from Dec. 22, 2019, onward, exceeding norms for seasonal flu ( J Med Internet Res. 2020;22:e21562). This spike was interpreted as evidence that the SARS-CoV-2 pandemic was already underway before the first case was established.
While the ongoing serologic testing of blood donations for viral antibodies “will advance understanding of the epidemiology” for SARS-CoV-2 and “inform allocation of resources and public health prevention interventions to mitigate morbidity and mortality,” it might also be a strategy for disease surveillance in the next pandemic, according to a team led by investigators at the Centers for Disease Control and Prevention.
Blood donation surveillance is not now used routinely to monitor for population-based health threats, but it is not a new idea, according to the lead author of the study, Sridhar V. Basavaraju, MD, of Emory University and director of the CDC’s Office of Blood, Organ, and Other Tissue Safety, Atlanta, and his coinvestigators. Most recently, blood donation surveillance was used in the United States to track the penetration of the Zika virus.
For early detection of respiratory infections, blood donations might have unique advantages over alternatives, such as surveillance of respiratory specimens from symptomatic patients. Not least, blood donation surveillance captures individuals who are not seeking medical care, according to the investigators.
EMR surveillance might also have unique advantages for population-based monitoring of health threats. For one, aggregate data from large EMR systems have the potential to reveal symptom patterns before they become apparent at level of clinical care, according to a team of collaborating investigators from the University of California, Los Angeles, and the University of Washington, Seattle.
Emphasizing an urgent need for “agile healthcare analytics” to enable “disease surveillance in real time,” the first author of the EMR study, Joann G. Elmore, MD, professor in the department of health policy and management at the University of California, Los Angeles, expressed the hope that the approach will “lead to better preparation and the ability to quickly provide warnings and track the next pandemic.”
In the blood donation surveillance study, the goal was simply to determine whether SARS-CoV-2 reactive antibodies could be found in blood donations before the first case was identified. Of the 7,389 archived blood samples tested between Dec. 13, 2019, and Jan. 17, 2020, 106 (1.4%) were reactive.
These were not true positives, acknowledged the investigators. True positives would require reactive antibodies in the context of a positive molecular diagnostic test or paired acute convalescent sera with rising titers. The investigators also cautioned that false positives could not be completely ruled out, particularly in light of cross-reactivity that has been reported with other human coronaviruses.
Nevertheless, the monitoring of blood donations offers substantial promise for “understanding the dynamics of SARS-CoV-2 pandemic from early introduction,” and the CDC is now collaborating on ongoing surveillance with the goal of contributing information that could be applied “to mitigate morbidity and mortality.”
Lessons learned from this pandemic are potentially relevant to the next.
The EMR study simply looked at whether the word “cough” was included more often in the notes from visits or hospitalizations between December 2019 and February 2020 relative to the preceding 5 years. The investigators drew on data from three hospitals and more than 180 clinics.
From Dec. 22, 2019, onward, cough was noted above the 95% prediction interval for all 10 weeks of the study. The excess was seen in the outpatient setting and among hospitalized patients. There was also significant excess in the number of patients hospitalized with acute respiratory failure during the study period.
“Our approach to analyzing electronic records could be helpful in the future as we included consideration of data from the outpatient clinics in addition to the emergency departments and inpatient settings,” Dr. Elmore reported.
Surveillance of influenza and influenza-like infections has been undertaken in the United States for more than 20 years, but Dr. Elmore contends that EMR data, particularly data from outpatient clinics are “usually a harbinger of what is to come” for emergency department visits and, ultimately, hospitalizations. She thinks that this is a resource not yet fully exploited.
“There are always opportunities to better harness EMR data,” Dr. Elmore said.
These are intriguing studies and “useful” for reconsidering when SARS-CoV-2 was introduced in the United States, according to Janet G. Basemen, PhD, a professor of epidemiology and the associate dean of the University of Washington School of Public Health, Seattle. However, she noted that the task of translating data like these into actionable public health strategies has proven difficult in the past.
Symptom-based surveillance systems “have mostly served as situational awareness rather than early detection tools,” Dr. Baseman said. The problem is timely interpretation of a given signal.
Not that she doubts such tools “would be an incredible resource for humanity” if the current limitations can be resolved or that technological advances will lead to better methods of detecting and monitoring pandemics “at some point.” Rather, “we’re just not there yet,” she said.
SOURCE: Basavaraju SV et al. Clin Infect Dis. 2020 Nov 30. doi: 10.1093/cid/ciaa1785); Elmore JG et al. J Med Internet Res. 2020;22:e21562).
The top pediatric articles of 2019
Updates in pediatric hospital medicine
The expansion of the field of pediatric hospital medicine in the past 30 years has resulted in improved health care outcomes for hospitalized children1,2 and has been accompanied by a robust increase in the amount of scholarly work related to the field.3 We performed a review of the literature published in 2019 to identify the 10 articles that had the most impact on pediatric hospital medicine, and presented the findings at HM20 Virtual, the 2020 annual conference of the Society of Hospital Medicine. Five of the selected articles are highlighted here.
STUDY 1
Wechsler ME et al. Step-up therapy in black children and adults with poorly controlled asthma. N Engl J Med. 2019 Sep 26;381(13):1227-39.
Background
Current pediatric asthma guidelines suggest adding a long-acting beta-agonist (LABA) to inhaled corticosteroid (ICS) therapy, rather than increasing the ICS dose, for children with poorly controlled asthma. However, these data are based on trials with disproportionately few Black subjects. This study aimed to determine the best step-up therapy for Black patients whose asthma was poorly controlled on ICS monotherapy.
Study overview and results
The authors reported two parallel double-blind, randomized, controlled trials, one in children and one in adolescents and adults. The study of children included 280 subjects ranging in age from 5 to 11, with at least one Black grandparent, and with poorly controlled asthma on low-dose ICS therapy. It used a four-way crossover design in which each subject was treated with four different 14-week treatment regimens: either double (medium-dose) or quintuple (high-dose) their baseline ICS dose, with or without the addition of a LABA. A superior response was defined by the composite outcome of at least one fewer asthma exacerbation, more asthma-control days, or a 5–percentage point difference in predicted FEV1. Forty-six percent of children had improved asthma outcomes when the ICS dose was increased rather than with the addition of a LABA. In contrast, Black adolescents and Black adults had superior responses to the addition of a LABA. There was no significant interaction between the percentage of African ancestry as determined by DNA genotyping and the primary composite outcome. High-dose ICS was associated with a decrease in the ratio of urinary cortisol to creatinine in children younger than 8 years.
Limitations
Approximately 25% of children dropped out of the study, with disproportionately more children dropping out while on a high-dose ICS regimen. Additionally, the difference in the composite outcome was primarily driven by differences in FEV1, with few subjects demonstrating a difference in asthma exacerbations or asthma-control days. Although a decrease in urinary cortisol to creatinine ratio was noted in children under 8 on high-dose ICS, the study period was not long enough to determine the clinical implications of this finding.
Important findings and implications
While studies with a majority of white children have suggested a superior response from adding a LABA compared to increasing the dose of an ICS, almost half of Black children showed a superior response when the dose of an ICS was increased rather than adding a LABA. It is important to note that current guidelines are based on studies with a disproportionate majority of white subjects and may not accurately reflect optimal care for patients in other racial groups. This study underscores the need to include a diverse patient population in research studies.
STUDY 2
Chang PW, Newman TB. A simpler prediction rule for rebound hyperbilirubinemia. Pediatrics. 2019 Jul;144(1):e20183712.
Background
Hyperbilirubinemia (jaundice) is estimated to affect 50%-60% of all newborns. Rebound hyperbilirubinemia – a rise in bilirubin after cessation of phototherapy – is common and can lead to recently discharged infants being readmitted for additional therapy. Lack of clear guidelines regarding when to discharge infants with hyperbilirubinemia has likely contributed to practice variation and some trepidation regarding whether a bilirubin level is “low enough” to discontinue therapy.
Study overview and results
The authors had previously proposed a three-factor hyperbilirubinemia risk model and sought to simplify their rule further.4 They examined a retrospective cohort of 7,048 infants greater than or equal to 35 weeks’ gestation using a random split sample. The authors derived a two-factor model using the same methods and compared its performance to the three-factor model. The two-factor formula was shown to be a good fit as a logistic regression model (Hosmer-Lemeshow test 9.21; P = .33), and the AUROC (area under the receiver operating characteristic) curves for the derivation and validation cohorts were similar between the two-factor (0.877 and 0.876, respectively) and three-factor risk models (0.887 and 0.881, respectively).
Limitations
These data are limited to infants receiving their first treatment of phototherapy and have not been externally validated. An important variable, serum bilirubin at phototherapy termination, was estimated in most subjects, which may have affected the accuracy of the prediction rule. Whether infants received home phototherapy was based only on equipment orders, and some infants may have received phototherapy unbeknownst to investigators. Last, infants with rebound hyperbilirubinemia at less than 72 hours after phototherapy discontinuation may have been missed.
Important findings and implications
This prediction model provides evidence-based, concrete data that can be used in making joint decisions with families regarding discharge timing of infants with hyperbilirubinemia. It also could be beneficial when deciding appropriate follow-up time after discharge.
STUDY 3
Ramgopal S et al. Risk of serious bacterial infection in infants aged ≤60 days presenting to emergency departments with a history of fever only. J Pediatr. 2019 Jan;204:191-195. doi: 10.1016/j.jpeds.2018.08.043.
Background
Febrile infants aged 60 days and younger are at risk for serious bacterial infections (SBI) including urinary tract infections (UTI), bacteremia, and meningitis. As physical exam is a poor discriminator of SBI in this age group, providers frequently rely on laboratory values and risk factors to guide management. Infants presenting with documented fevers by caregivers but found to have no fever in the emergency department are a challenge, and there are limited data regarding SBI frequency in this population.
Study overview and results
The authors performed a secondary analysis of a prospectively gathered cohort of infants aged 60 days and younger within the Pediatric Emergency Care Applied Research Network (PECARN) who had blood, urine, and CSF data available. Notable exclusions included infants who were premature, had a focal infection, were clinically ill, had recent antibiotic use, did not have blood, urine, and CSF data available, or were lost to telephone follow-up at 7 days to ensure wellness. The study cohort included 6,014 infants, 1,233 (32%) who were febrile by history alone. Rates of overall SBI were lower in the afebrile group (8.8% vs. 12.8%). For infants 0-28 days, rates of UTI were lower for the afebrile group (9.5% vs. 14.5%), but there was no difference in the rates of bacteremia or meningitis. For infants 29-60 days, rates of UTI (6.6% vs. 9.3%) and bacteremia (.5% vs. 1.7%) were lower in the afebrile group.
Limitations
Neither the use of home antipyretics nor the method of temperature taking at home were studied. Also, as this was a secondary analysis, it is possible that not all infants who presented with history of fever only were captured, as work-up was dictated by individual treating providers who may have chosen not to work up certain afebrile infants.
Important findings and implications
Nearly one-third of infants presenting for fever evaluation are afebrile on arrival. Although overall rates of SBI were lower in the group with fever by history only, this difference is largely accounted for by differing rates of UTI. Rates of bacteremia and meningitis remained substantial between groups, particularly for infants aged 0-28 days. Because of the significant morbidity associated with these infections, it is reasonable to suggest that absence of fever on presentation alone should not alter clinical or laboratory work-up, particularly in infants 0-28 days.
STUDY 4
Humphrey-Murto S et al. The influence of prior performance information on ratings of current performance and implications for learner handover: A scoping review. Acad Med. 2019 Jul;94(7):1050-7.
Background
Learner Handover (LH) or “forward feeding” occurs when information about trainees is shared between faculty supervisors. Although this can be helpful to tailor educational experiences and build upon previous assessments, it risks stigmatizing trainees and adding bias to future feedback and assessments as the trainee never really has a “clean slate.” In this study, the authors sought to uncover the key concepts of how prior performance information (PPI) influences assessments and any implications for medical education.
Study overview and results
The authors performed a cross-disciplinary scoping review looking at over 17,000 articles published between 1980 and 2017 across the domains of psychology, sports, business, and education. Seven themes were identified with the following notable findings. Raters exposed to positive PPI scored a learner’s performance higher, and vice versa. There was a dose-response relationship with more positive and more negative PPI resulting in higher and lower assessments, respectively. General standards, such as a direction to complete all work in a timely manner, caused an assimilation effect, while specific standards, such as a direction to complete a certain task by a certain day, did not. More motivated and more experienced raters are less affected by PPI, and those who believe that people can change (incremental theorists) are less affected by PPI while those who believe personal attributes are fixed (entity theorists) are more affected.
Limitations
The heterogeneity of the studies and the fact that they were largely conducted in experimental settings may limit generalizability to medical education. Slightly less than half of the studies included a control arm. Last, most of the studies looked at the ratings of only one target performance, not multiple performances over time.
Important findings and implications
Ratings of current performance displace toward PPI direction, with negative PPI more influential than positive PPI. In a formative setting, PPI may help the assessor focus on areas of possible weakness. In contrast, for a summative assessment, PPI may be prejudicial and have an impact on the rating given to the student. Clinicians should be mindful of the information they share with future raters about learners and the potential bias on future assessments that can manifest as a result.
STUDY 5
McCann ME et al. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): An international, multicentre, randomised, controlled equivalence trial. The Lancet. 2019 Feb;393:664-77.
Background
Animal models and observational studies have suggested a link between early anesthesia exposure and adverse neurocognitive outcomes; however, findings have been mixed and studies are prone to confounding. This study is the first randomized controlled trial to compare neurocognitive outcomes for infants exposed to general anesthesia versus awake-regional anesthesia.
Study overview and results
In this international, multicenter, assessor-masked trial, 722 infants undergoing inguinal hernia repair were randomized to awake-regional anesthesia or single-agent sevoflurane-based general anesthesia. Infants born at greater than 26 weeks’ gestational age were eligible, while those with prior anesthesia exposure or risks for neurocognitive delay were excluded. The primary outcome was full-scale intelligence quotient (FSIQ) testing at 5 years of age on the Wechsler Preschool and Primary Scale of Intelligence, third edition (WPPSI-III). Seven additional neurodevelopmental assessments and parental questionnaires regarding behavior were administered as secondary outcomes. Average anesthesia exposure was 54 minutes and no infant had exposure greater than 120 minutes. There was no significant difference in mean scores on WPPSI-III FSIQ testing, and no difference in the additional neurocognitive assessments or parent-reported outcomes used as secondary outcomes.
Limitations
This study was limited to single, short periods of single-agent anesthesia exposure in children with no additional neurologic risk factors, so caution should be used in extrapolating these data to children with medical complexity and children undergoing multiple procedures, longer surgeries, or multidrug anesthetic regimens. The study population was majority male because of the surgical pathology selected and included only children in the narrow range of postmenstrual age 60 weeks or less. While this population represents a suspected a period of high cerebral vulnerability based on animal models, the implications of anesthesia exposure at other ages are unclear.
Important findings and implications
An estimated 10% of children from developed countries are exposed to general anesthesia during the first 3 years of life. While hospitalists do not typically select the route of anesthesia, they frequently care for patients undergoing procedures and must address parental concerns regarding the safety of anesthesia exposure. Given the rigorous study methods and long-term follow up in the current study, these data should provide reassurance that, for healthy infants undergoing short, single-agent anesthetic exposure, there is no evidence of future adverse neurologic outcomes.
Dr. Russo is director of pediatrics, medical director for quality and innovation, at WellSpan Health, York, Pa. Dr. Money is a pediatric hospitalist at Primary Children’s Hospital, University of Utah School of Medicine, Salt Lake City. Dr. Steed is instructor of hospital medicine, Northwestern Memorial Hospital and Ann and Robert H. Lurie Children’s Hospital of Chicago, Northwestern University School of Medicine, Chicago. The authors would like to thank Dr. Klint M. Schwenk and the Society for Hospital Medicine Pediatric Special Interest Group Executive Council.
References
1. Roberts KB, Fisher ER, and Rauch DA. The history of pediatric hospital medicine in the United States, 1996-2019. J Hosp Med. 2020 Jul;15(7):424-7.
2. Mussman GM and Conway PH. Pediatric hospitalist systems versus traditional models of care: Effect on quality and cost outcomes. J Hosp Med. 2012 Apr;7(4):350-7.
3. Wang ME, Shaughnessy EE, and Leyenaar JK. The future of pediatric hospital medicine: Challenges and opportunities. J Hosp Med. 2020 Jul;15(7):428-30.
4. Chang PW et al. A clinical prediction rule for rebound hyperbilirubinemia following inpatient phototherapy. Pediatrics. 2017;139 Mar;139(3):e20162896.
Updates in pediatric hospital medicine
Updates in pediatric hospital medicine
The expansion of the field of pediatric hospital medicine in the past 30 years has resulted in improved health care outcomes for hospitalized children1,2 and has been accompanied by a robust increase in the amount of scholarly work related to the field.3 We performed a review of the literature published in 2019 to identify the 10 articles that had the most impact on pediatric hospital medicine, and presented the findings at HM20 Virtual, the 2020 annual conference of the Society of Hospital Medicine. Five of the selected articles are highlighted here.
STUDY 1
Wechsler ME et al. Step-up therapy in black children and adults with poorly controlled asthma. N Engl J Med. 2019 Sep 26;381(13):1227-39.
Background
Current pediatric asthma guidelines suggest adding a long-acting beta-agonist (LABA) to inhaled corticosteroid (ICS) therapy, rather than increasing the ICS dose, for children with poorly controlled asthma. However, these data are based on trials with disproportionately few Black subjects. This study aimed to determine the best step-up therapy for Black patients whose asthma was poorly controlled on ICS monotherapy.
Study overview and results
The authors reported two parallel double-blind, randomized, controlled trials, one in children and one in adolescents and adults. The study of children included 280 subjects ranging in age from 5 to 11, with at least one Black grandparent, and with poorly controlled asthma on low-dose ICS therapy. It used a four-way crossover design in which each subject was treated with four different 14-week treatment regimens: either double (medium-dose) or quintuple (high-dose) their baseline ICS dose, with or without the addition of a LABA. A superior response was defined by the composite outcome of at least one fewer asthma exacerbation, more asthma-control days, or a 5–percentage point difference in predicted FEV1. Forty-six percent of children had improved asthma outcomes when the ICS dose was increased rather than with the addition of a LABA. In contrast, Black adolescents and Black adults had superior responses to the addition of a LABA. There was no significant interaction between the percentage of African ancestry as determined by DNA genotyping and the primary composite outcome. High-dose ICS was associated with a decrease in the ratio of urinary cortisol to creatinine in children younger than 8 years.
Limitations
Approximately 25% of children dropped out of the study, with disproportionately more children dropping out while on a high-dose ICS regimen. Additionally, the difference in the composite outcome was primarily driven by differences in FEV1, with few subjects demonstrating a difference in asthma exacerbations or asthma-control days. Although a decrease in urinary cortisol to creatinine ratio was noted in children under 8 on high-dose ICS, the study period was not long enough to determine the clinical implications of this finding.
Important findings and implications
While studies with a majority of white children have suggested a superior response from adding a LABA compared to increasing the dose of an ICS, almost half of Black children showed a superior response when the dose of an ICS was increased rather than adding a LABA. It is important to note that current guidelines are based on studies with a disproportionate majority of white subjects and may not accurately reflect optimal care for patients in other racial groups. This study underscores the need to include a diverse patient population in research studies.
STUDY 2
Chang PW, Newman TB. A simpler prediction rule for rebound hyperbilirubinemia. Pediatrics. 2019 Jul;144(1):e20183712.
Background
Hyperbilirubinemia (jaundice) is estimated to affect 50%-60% of all newborns. Rebound hyperbilirubinemia – a rise in bilirubin after cessation of phototherapy – is common and can lead to recently discharged infants being readmitted for additional therapy. Lack of clear guidelines regarding when to discharge infants with hyperbilirubinemia has likely contributed to practice variation and some trepidation regarding whether a bilirubin level is “low enough” to discontinue therapy.
Study overview and results
The authors had previously proposed a three-factor hyperbilirubinemia risk model and sought to simplify their rule further.4 They examined a retrospective cohort of 7,048 infants greater than or equal to 35 weeks’ gestation using a random split sample. The authors derived a two-factor model using the same methods and compared its performance to the three-factor model. The two-factor formula was shown to be a good fit as a logistic regression model (Hosmer-Lemeshow test 9.21; P = .33), and the AUROC (area under the receiver operating characteristic) curves for the derivation and validation cohorts were similar between the two-factor (0.877 and 0.876, respectively) and three-factor risk models (0.887 and 0.881, respectively).
Limitations
These data are limited to infants receiving their first treatment of phototherapy and have not been externally validated. An important variable, serum bilirubin at phototherapy termination, was estimated in most subjects, which may have affected the accuracy of the prediction rule. Whether infants received home phototherapy was based only on equipment orders, and some infants may have received phototherapy unbeknownst to investigators. Last, infants with rebound hyperbilirubinemia at less than 72 hours after phototherapy discontinuation may have been missed.
Important findings and implications
This prediction model provides evidence-based, concrete data that can be used in making joint decisions with families regarding discharge timing of infants with hyperbilirubinemia. It also could be beneficial when deciding appropriate follow-up time after discharge.
STUDY 3
Ramgopal S et al. Risk of serious bacterial infection in infants aged ≤60 days presenting to emergency departments with a history of fever only. J Pediatr. 2019 Jan;204:191-195. doi: 10.1016/j.jpeds.2018.08.043.
Background
Febrile infants aged 60 days and younger are at risk for serious bacterial infections (SBI) including urinary tract infections (UTI), bacteremia, and meningitis. As physical exam is a poor discriminator of SBI in this age group, providers frequently rely on laboratory values and risk factors to guide management. Infants presenting with documented fevers by caregivers but found to have no fever in the emergency department are a challenge, and there are limited data regarding SBI frequency in this population.
Study overview and results
The authors performed a secondary analysis of a prospectively gathered cohort of infants aged 60 days and younger within the Pediatric Emergency Care Applied Research Network (PECARN) who had blood, urine, and CSF data available. Notable exclusions included infants who were premature, had a focal infection, were clinically ill, had recent antibiotic use, did not have blood, urine, and CSF data available, or were lost to telephone follow-up at 7 days to ensure wellness. The study cohort included 6,014 infants, 1,233 (32%) who were febrile by history alone. Rates of overall SBI were lower in the afebrile group (8.8% vs. 12.8%). For infants 0-28 days, rates of UTI were lower for the afebrile group (9.5% vs. 14.5%), but there was no difference in the rates of bacteremia or meningitis. For infants 29-60 days, rates of UTI (6.6% vs. 9.3%) and bacteremia (.5% vs. 1.7%) were lower in the afebrile group.
Limitations
Neither the use of home antipyretics nor the method of temperature taking at home were studied. Also, as this was a secondary analysis, it is possible that not all infants who presented with history of fever only were captured, as work-up was dictated by individual treating providers who may have chosen not to work up certain afebrile infants.
Important findings and implications
Nearly one-third of infants presenting for fever evaluation are afebrile on arrival. Although overall rates of SBI were lower in the group with fever by history only, this difference is largely accounted for by differing rates of UTI. Rates of bacteremia and meningitis remained substantial between groups, particularly for infants aged 0-28 days. Because of the significant morbidity associated with these infections, it is reasonable to suggest that absence of fever on presentation alone should not alter clinical or laboratory work-up, particularly in infants 0-28 days.
STUDY 4
Humphrey-Murto S et al. The influence of prior performance information on ratings of current performance and implications for learner handover: A scoping review. Acad Med. 2019 Jul;94(7):1050-7.
Background
Learner Handover (LH) or “forward feeding” occurs when information about trainees is shared between faculty supervisors. Although this can be helpful to tailor educational experiences and build upon previous assessments, it risks stigmatizing trainees and adding bias to future feedback and assessments as the trainee never really has a “clean slate.” In this study, the authors sought to uncover the key concepts of how prior performance information (PPI) influences assessments and any implications for medical education.
Study overview and results
The authors performed a cross-disciplinary scoping review looking at over 17,000 articles published between 1980 and 2017 across the domains of psychology, sports, business, and education. Seven themes were identified with the following notable findings. Raters exposed to positive PPI scored a learner’s performance higher, and vice versa. There was a dose-response relationship with more positive and more negative PPI resulting in higher and lower assessments, respectively. General standards, such as a direction to complete all work in a timely manner, caused an assimilation effect, while specific standards, such as a direction to complete a certain task by a certain day, did not. More motivated and more experienced raters are less affected by PPI, and those who believe that people can change (incremental theorists) are less affected by PPI while those who believe personal attributes are fixed (entity theorists) are more affected.
Limitations
The heterogeneity of the studies and the fact that they were largely conducted in experimental settings may limit generalizability to medical education. Slightly less than half of the studies included a control arm. Last, most of the studies looked at the ratings of only one target performance, not multiple performances over time.
Important findings and implications
Ratings of current performance displace toward PPI direction, with negative PPI more influential than positive PPI. In a formative setting, PPI may help the assessor focus on areas of possible weakness. In contrast, for a summative assessment, PPI may be prejudicial and have an impact on the rating given to the student. Clinicians should be mindful of the information they share with future raters about learners and the potential bias on future assessments that can manifest as a result.
STUDY 5
McCann ME et al. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): An international, multicentre, randomised, controlled equivalence trial. The Lancet. 2019 Feb;393:664-77.
Background
Animal models and observational studies have suggested a link between early anesthesia exposure and adverse neurocognitive outcomes; however, findings have been mixed and studies are prone to confounding. This study is the first randomized controlled trial to compare neurocognitive outcomes for infants exposed to general anesthesia versus awake-regional anesthesia.
Study overview and results
In this international, multicenter, assessor-masked trial, 722 infants undergoing inguinal hernia repair were randomized to awake-regional anesthesia or single-agent sevoflurane-based general anesthesia. Infants born at greater than 26 weeks’ gestational age were eligible, while those with prior anesthesia exposure or risks for neurocognitive delay were excluded. The primary outcome was full-scale intelligence quotient (FSIQ) testing at 5 years of age on the Wechsler Preschool and Primary Scale of Intelligence, third edition (WPPSI-III). Seven additional neurodevelopmental assessments and parental questionnaires regarding behavior were administered as secondary outcomes. Average anesthesia exposure was 54 minutes and no infant had exposure greater than 120 minutes. There was no significant difference in mean scores on WPPSI-III FSIQ testing, and no difference in the additional neurocognitive assessments or parent-reported outcomes used as secondary outcomes.
Limitations
This study was limited to single, short periods of single-agent anesthesia exposure in children with no additional neurologic risk factors, so caution should be used in extrapolating these data to children with medical complexity and children undergoing multiple procedures, longer surgeries, or multidrug anesthetic regimens. The study population was majority male because of the surgical pathology selected and included only children in the narrow range of postmenstrual age 60 weeks or less. While this population represents a suspected a period of high cerebral vulnerability based on animal models, the implications of anesthesia exposure at other ages are unclear.
Important findings and implications
An estimated 10% of children from developed countries are exposed to general anesthesia during the first 3 years of life. While hospitalists do not typically select the route of anesthesia, they frequently care for patients undergoing procedures and must address parental concerns regarding the safety of anesthesia exposure. Given the rigorous study methods and long-term follow up in the current study, these data should provide reassurance that, for healthy infants undergoing short, single-agent anesthetic exposure, there is no evidence of future adverse neurologic outcomes.
Dr. Russo is director of pediatrics, medical director for quality and innovation, at WellSpan Health, York, Pa. Dr. Money is a pediatric hospitalist at Primary Children’s Hospital, University of Utah School of Medicine, Salt Lake City. Dr. Steed is instructor of hospital medicine, Northwestern Memorial Hospital and Ann and Robert H. Lurie Children’s Hospital of Chicago, Northwestern University School of Medicine, Chicago. The authors would like to thank Dr. Klint M. Schwenk and the Society for Hospital Medicine Pediatric Special Interest Group Executive Council.
References
1. Roberts KB, Fisher ER, and Rauch DA. The history of pediatric hospital medicine in the United States, 1996-2019. J Hosp Med. 2020 Jul;15(7):424-7.
2. Mussman GM and Conway PH. Pediatric hospitalist systems versus traditional models of care: Effect on quality and cost outcomes. J Hosp Med. 2012 Apr;7(4):350-7.
3. Wang ME, Shaughnessy EE, and Leyenaar JK. The future of pediatric hospital medicine: Challenges and opportunities. J Hosp Med. 2020 Jul;15(7):428-30.
4. Chang PW et al. A clinical prediction rule for rebound hyperbilirubinemia following inpatient phototherapy. Pediatrics. 2017;139 Mar;139(3):e20162896.
The expansion of the field of pediatric hospital medicine in the past 30 years has resulted in improved health care outcomes for hospitalized children1,2 and has been accompanied by a robust increase in the amount of scholarly work related to the field.3 We performed a review of the literature published in 2019 to identify the 10 articles that had the most impact on pediatric hospital medicine, and presented the findings at HM20 Virtual, the 2020 annual conference of the Society of Hospital Medicine. Five of the selected articles are highlighted here.
STUDY 1
Wechsler ME et al. Step-up therapy in black children and adults with poorly controlled asthma. N Engl J Med. 2019 Sep 26;381(13):1227-39.
Background
Current pediatric asthma guidelines suggest adding a long-acting beta-agonist (LABA) to inhaled corticosteroid (ICS) therapy, rather than increasing the ICS dose, for children with poorly controlled asthma. However, these data are based on trials with disproportionately few Black subjects. This study aimed to determine the best step-up therapy for Black patients whose asthma was poorly controlled on ICS monotherapy.
Study overview and results
The authors reported two parallel double-blind, randomized, controlled trials, one in children and one in adolescents and adults. The study of children included 280 subjects ranging in age from 5 to 11, with at least one Black grandparent, and with poorly controlled asthma on low-dose ICS therapy. It used a four-way crossover design in which each subject was treated with four different 14-week treatment regimens: either double (medium-dose) or quintuple (high-dose) their baseline ICS dose, with or without the addition of a LABA. A superior response was defined by the composite outcome of at least one fewer asthma exacerbation, more asthma-control days, or a 5–percentage point difference in predicted FEV1. Forty-six percent of children had improved asthma outcomes when the ICS dose was increased rather than with the addition of a LABA. In contrast, Black adolescents and Black adults had superior responses to the addition of a LABA. There was no significant interaction between the percentage of African ancestry as determined by DNA genotyping and the primary composite outcome. High-dose ICS was associated with a decrease in the ratio of urinary cortisol to creatinine in children younger than 8 years.
Limitations
Approximately 25% of children dropped out of the study, with disproportionately more children dropping out while on a high-dose ICS regimen. Additionally, the difference in the composite outcome was primarily driven by differences in FEV1, with few subjects demonstrating a difference in asthma exacerbations or asthma-control days. Although a decrease in urinary cortisol to creatinine ratio was noted in children under 8 on high-dose ICS, the study period was not long enough to determine the clinical implications of this finding.
Important findings and implications
While studies with a majority of white children have suggested a superior response from adding a LABA compared to increasing the dose of an ICS, almost half of Black children showed a superior response when the dose of an ICS was increased rather than adding a LABA. It is important to note that current guidelines are based on studies with a disproportionate majority of white subjects and may not accurately reflect optimal care for patients in other racial groups. This study underscores the need to include a diverse patient population in research studies.
STUDY 2
Chang PW, Newman TB. A simpler prediction rule for rebound hyperbilirubinemia. Pediatrics. 2019 Jul;144(1):e20183712.
Background
Hyperbilirubinemia (jaundice) is estimated to affect 50%-60% of all newborns. Rebound hyperbilirubinemia – a rise in bilirubin after cessation of phototherapy – is common and can lead to recently discharged infants being readmitted for additional therapy. Lack of clear guidelines regarding when to discharge infants with hyperbilirubinemia has likely contributed to practice variation and some trepidation regarding whether a bilirubin level is “low enough” to discontinue therapy.
Study overview and results
The authors had previously proposed a three-factor hyperbilirubinemia risk model and sought to simplify their rule further.4 They examined a retrospective cohort of 7,048 infants greater than or equal to 35 weeks’ gestation using a random split sample. The authors derived a two-factor model using the same methods and compared its performance to the three-factor model. The two-factor formula was shown to be a good fit as a logistic regression model (Hosmer-Lemeshow test 9.21; P = .33), and the AUROC (area under the receiver operating characteristic) curves for the derivation and validation cohorts were similar between the two-factor (0.877 and 0.876, respectively) and three-factor risk models (0.887 and 0.881, respectively).
Limitations
These data are limited to infants receiving their first treatment of phototherapy and have not been externally validated. An important variable, serum bilirubin at phototherapy termination, was estimated in most subjects, which may have affected the accuracy of the prediction rule. Whether infants received home phototherapy was based only on equipment orders, and some infants may have received phototherapy unbeknownst to investigators. Last, infants with rebound hyperbilirubinemia at less than 72 hours after phototherapy discontinuation may have been missed.
Important findings and implications
This prediction model provides evidence-based, concrete data that can be used in making joint decisions with families regarding discharge timing of infants with hyperbilirubinemia. It also could be beneficial when deciding appropriate follow-up time after discharge.
STUDY 3
Ramgopal S et al. Risk of serious bacterial infection in infants aged ≤60 days presenting to emergency departments with a history of fever only. J Pediatr. 2019 Jan;204:191-195. doi: 10.1016/j.jpeds.2018.08.043.
Background
Febrile infants aged 60 days and younger are at risk for serious bacterial infections (SBI) including urinary tract infections (UTI), bacteremia, and meningitis. As physical exam is a poor discriminator of SBI in this age group, providers frequently rely on laboratory values and risk factors to guide management. Infants presenting with documented fevers by caregivers but found to have no fever in the emergency department are a challenge, and there are limited data regarding SBI frequency in this population.
Study overview and results
The authors performed a secondary analysis of a prospectively gathered cohort of infants aged 60 days and younger within the Pediatric Emergency Care Applied Research Network (PECARN) who had blood, urine, and CSF data available. Notable exclusions included infants who were premature, had a focal infection, were clinically ill, had recent antibiotic use, did not have blood, urine, and CSF data available, or were lost to telephone follow-up at 7 days to ensure wellness. The study cohort included 6,014 infants, 1,233 (32%) who were febrile by history alone. Rates of overall SBI were lower in the afebrile group (8.8% vs. 12.8%). For infants 0-28 days, rates of UTI were lower for the afebrile group (9.5% vs. 14.5%), but there was no difference in the rates of bacteremia or meningitis. For infants 29-60 days, rates of UTI (6.6% vs. 9.3%) and bacteremia (.5% vs. 1.7%) were lower in the afebrile group.
Limitations
Neither the use of home antipyretics nor the method of temperature taking at home were studied. Also, as this was a secondary analysis, it is possible that not all infants who presented with history of fever only were captured, as work-up was dictated by individual treating providers who may have chosen not to work up certain afebrile infants.
Important findings and implications
Nearly one-third of infants presenting for fever evaluation are afebrile on arrival. Although overall rates of SBI were lower in the group with fever by history only, this difference is largely accounted for by differing rates of UTI. Rates of bacteremia and meningitis remained substantial between groups, particularly for infants aged 0-28 days. Because of the significant morbidity associated with these infections, it is reasonable to suggest that absence of fever on presentation alone should not alter clinical or laboratory work-up, particularly in infants 0-28 days.
STUDY 4
Humphrey-Murto S et al. The influence of prior performance information on ratings of current performance and implications for learner handover: A scoping review. Acad Med. 2019 Jul;94(7):1050-7.
Background
Learner Handover (LH) or “forward feeding” occurs when information about trainees is shared between faculty supervisors. Although this can be helpful to tailor educational experiences and build upon previous assessments, it risks stigmatizing trainees and adding bias to future feedback and assessments as the trainee never really has a “clean slate.” In this study, the authors sought to uncover the key concepts of how prior performance information (PPI) influences assessments and any implications for medical education.
Study overview and results
The authors performed a cross-disciplinary scoping review looking at over 17,000 articles published between 1980 and 2017 across the domains of psychology, sports, business, and education. Seven themes were identified with the following notable findings. Raters exposed to positive PPI scored a learner’s performance higher, and vice versa. There was a dose-response relationship with more positive and more negative PPI resulting in higher and lower assessments, respectively. General standards, such as a direction to complete all work in a timely manner, caused an assimilation effect, while specific standards, such as a direction to complete a certain task by a certain day, did not. More motivated and more experienced raters are less affected by PPI, and those who believe that people can change (incremental theorists) are less affected by PPI while those who believe personal attributes are fixed (entity theorists) are more affected.
Limitations
The heterogeneity of the studies and the fact that they were largely conducted in experimental settings may limit generalizability to medical education. Slightly less than half of the studies included a control arm. Last, most of the studies looked at the ratings of only one target performance, not multiple performances over time.
Important findings and implications
Ratings of current performance displace toward PPI direction, with negative PPI more influential than positive PPI. In a formative setting, PPI may help the assessor focus on areas of possible weakness. In contrast, for a summative assessment, PPI may be prejudicial and have an impact on the rating given to the student. Clinicians should be mindful of the information they share with future raters about learners and the potential bias on future assessments that can manifest as a result.
STUDY 5
McCann ME et al. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): An international, multicentre, randomised, controlled equivalence trial. The Lancet. 2019 Feb;393:664-77.
Background
Animal models and observational studies have suggested a link between early anesthesia exposure and adverse neurocognitive outcomes; however, findings have been mixed and studies are prone to confounding. This study is the first randomized controlled trial to compare neurocognitive outcomes for infants exposed to general anesthesia versus awake-regional anesthesia.
Study overview and results
In this international, multicenter, assessor-masked trial, 722 infants undergoing inguinal hernia repair were randomized to awake-regional anesthesia or single-agent sevoflurane-based general anesthesia. Infants born at greater than 26 weeks’ gestational age were eligible, while those with prior anesthesia exposure or risks for neurocognitive delay were excluded. The primary outcome was full-scale intelligence quotient (FSIQ) testing at 5 years of age on the Wechsler Preschool and Primary Scale of Intelligence, third edition (WPPSI-III). Seven additional neurodevelopmental assessments and parental questionnaires regarding behavior were administered as secondary outcomes. Average anesthesia exposure was 54 minutes and no infant had exposure greater than 120 minutes. There was no significant difference in mean scores on WPPSI-III FSIQ testing, and no difference in the additional neurocognitive assessments or parent-reported outcomes used as secondary outcomes.
Limitations
This study was limited to single, short periods of single-agent anesthesia exposure in children with no additional neurologic risk factors, so caution should be used in extrapolating these data to children with medical complexity and children undergoing multiple procedures, longer surgeries, or multidrug anesthetic regimens. The study population was majority male because of the surgical pathology selected and included only children in the narrow range of postmenstrual age 60 weeks or less. While this population represents a suspected a period of high cerebral vulnerability based on animal models, the implications of anesthesia exposure at other ages are unclear.
Important findings and implications
An estimated 10% of children from developed countries are exposed to general anesthesia during the first 3 years of life. While hospitalists do not typically select the route of anesthesia, they frequently care for patients undergoing procedures and must address parental concerns regarding the safety of anesthesia exposure. Given the rigorous study methods and long-term follow up in the current study, these data should provide reassurance that, for healthy infants undergoing short, single-agent anesthetic exposure, there is no evidence of future adverse neurologic outcomes.
Dr. Russo is director of pediatrics, medical director for quality and innovation, at WellSpan Health, York, Pa. Dr. Money is a pediatric hospitalist at Primary Children’s Hospital, University of Utah School of Medicine, Salt Lake City. Dr. Steed is instructor of hospital medicine, Northwestern Memorial Hospital and Ann and Robert H. Lurie Children’s Hospital of Chicago, Northwestern University School of Medicine, Chicago. The authors would like to thank Dr. Klint M. Schwenk and the Society for Hospital Medicine Pediatric Special Interest Group Executive Council.
References
1. Roberts KB, Fisher ER, and Rauch DA. The history of pediatric hospital medicine in the United States, 1996-2019. J Hosp Med. 2020 Jul;15(7):424-7.
2. Mussman GM and Conway PH. Pediatric hospitalist systems versus traditional models of care: Effect on quality and cost outcomes. J Hosp Med. 2012 Apr;7(4):350-7.
3. Wang ME, Shaughnessy EE, and Leyenaar JK. The future of pediatric hospital medicine: Challenges and opportunities. J Hosp Med. 2020 Jul;15(7):428-30.
4. Chang PW et al. A clinical prediction rule for rebound hyperbilirubinemia following inpatient phototherapy. Pediatrics. 2017;139 Mar;139(3):e20162896.
Contact tracing in hospitals falls off as COVID-19 cases rise
Like most health care workers at his hospital in Lafayette, Ind., Ramesh Adhikari, MD, FHM, occasionally gets an email noting that a patient he saw later tested positive for COVID-19. He’s reminded to self-monitor for symptoms. But 10 months into the pandemic, it has become increasingly unlikely for contact tracing investigations to result in clinicians quarantining.
The very act of working in the hospital, Dr. Adhikari said, means being likely to see COVID-19 every day, whether in a known patient or an asymptomatic person who tests positive later. If hospitalists had to quarantine after every interaction with a COVID-positive person, there wouldn’t be anyone left to do their jobs.
“It’s really hard to do [contact tracing] in health care workers thoroughly because of the way we work,” Dr. Adhikari said. “It’s impossible to do it absolutely.”
In a recently updated guidance, the Centers for Disease Control and Prevention extended more leeway in contact tracing when community rates of COVID-19 surge, even allowing that contact tracing “may not be possible” in certain situations. And by defining an exposure more narrowly – health care workers are only considered “exposed” if their contact was more than 15 minutes or lacking in some form of PPE – the guidelines suggest that hospitals can rely more on universal PPE and screening protocols, as Dr. Adhikari’s hospital does, and less on extensive contact tracing to curtail viral spread.
Accordingly, while contact tracing has gotten more lax, doctors say, universal precautions – including full PPE and screening of symptoms for patients and health care workers – have become more stringent.
It’s a shift from the beginning of the pandemic. At first, CDC recommended wearing masks only during aerosol-producing procedures. Exposures were frequently reported and health care workers sent home. With more evidence in favor of stricter PPE requirements, hospitals including the one where Shyam Odeti, MD, FHM, works in Johnson City, Tenn., have adopted a universal precaution strategy – requiring masks everywhere and a gown, face shield, gloves, and N95 to enter a COVID-positive patient’s room. Thus, most exposures fall into that low-risk category.
“If I get it and am asymptomatic, I don’t think my colleagues would be exposed by any means because of these stringent policies being enforced,” said Dr. Odeti, a hospitalist who often wears a surgical mask on top of his N95 all day. “And U.S. health care is not in a state that can afford to quarantine health care workers for 14 days.”
Can universal PPE precautions supplant contact tracing?
The extent of contact tracing varies by hospital. Larger university and community hospitals often have infection control and occupational health teams that can do their own contact tracing, while smaller institutions can’t always spare staff. And some state health departments get involved with contact tracing of health care workers while others do not.
“I would venture to say that most hospitals are doing something in terms of contact tracing,” said Pam Falk, MPH, CIC, a member of the Association for Professionals in Infection Control and Epidemiology’s COVID-19 task force and an infection control consultant. “It kind of depends on their bandwidth.”
But there’s no longer a norm. Outside of a pandemic, with ample staffing and far fewer instances that need to be investigated, standards for contact tracing are higher, Dr. Falk said: When a patient is found to have an airborne disease such as tuberculosis, measles, mumps, or chickenpox, a hospital’s infection prevention team should investigate, confirm the diagnosis and identify everyone who was exposed. The hospital’s occupational health team assists in deciding who will likely need prophylactic treatment and if employees should be furloughed. The thoroughness of such measures has always depended on a hospital’s bandwidth.
Because PPE seems to be able to contain COVID-19 better than some of the older diseases targeted by contact tracing, universal protections may be a reasonable alternative in current circumstances, doctors said – if PPE is available.
“At the end of the day, universal source control with surgical masks – and ideally eye protection for clinicians as well – should prevent most transmissions,” said Aaron Richterman, MD, from the division of infectious diseases at the Hospital of the University of Pennsylvania, Philadelphia, who coauthored a JAMA commentary on decreased transmission rates in hospitals.
Contact tracing is still useful, though, to identify weaknesses in universal protection measures, he said.
“I don’t think it’s worth abandoning. It’s like a tool in the toolbox. All are imperfect, and none work 100% of the time,” Dr. Richterman said, but using all of them can achieve a fairly high measure of safety. Of the tools, universal masking likely works the best, he contends, so it should be the top pick for hospitals without resources to use all of the tools.
A recent incident at Brigham and Women’s Hospital in Boston is a case study in how contact tracing can work together with universal protections to identify cracks in the system, said Dr. Richterman, who worked at the hospital earlier in the pandemic.
Mass General Brigham adopted a universal masking policy for staff and patients in March 2020. Then, when the system experienced an outbreak in September, the hospital did “a very detailed public evaluation that included contact tracing and universal testing,” Dr. Richterman said. Testing even included genetic analysis of the virus to confirm which cases were hospital acquired. In the end, the hospital identified weaknesses in infection control that could be rectified, such as clinicians eating too close together.
“The approach is not to point fingers, but to say: ‘What’s wrong with the system and how do we improve?’ ” Dr. Richterman said. “To ask, why did that maskless transmission happen? Is there not enough space to eat? Are people working too many hours? It’s useful for systems to understand where transmissions are happening.”
Amith Skandhan, MD, SFHM, a hospitalist in Dothan, Ala., is comfortable without much contact tracing as long as there is universal PPE use. His hospital informs clinicians of exposures, but “basically we’re trained to treat every patient as if they had COVID,” he said, so “I feel more secure in the hospital than in the community.” Masks have become so habitual they’re like part of your regular clothing, he said – you feel incomplete if you don’t have one.
While ad hoc approaches to contact tracing may be useful in the current stage of the pandemic, they are likely to be short-lived: Once a community’s positivity rate falls, the CDC’s guidance suggests how hospitals can return to full contact tracing.
A version of this article first appeared on Medscape.com.
Like most health care workers at his hospital in Lafayette, Ind., Ramesh Adhikari, MD, FHM, occasionally gets an email noting that a patient he saw later tested positive for COVID-19. He’s reminded to self-monitor for symptoms. But 10 months into the pandemic, it has become increasingly unlikely for contact tracing investigations to result in clinicians quarantining.
The very act of working in the hospital, Dr. Adhikari said, means being likely to see COVID-19 every day, whether in a known patient or an asymptomatic person who tests positive later. If hospitalists had to quarantine after every interaction with a COVID-positive person, there wouldn’t be anyone left to do their jobs.
“It’s really hard to do [contact tracing] in health care workers thoroughly because of the way we work,” Dr. Adhikari said. “It’s impossible to do it absolutely.”
In a recently updated guidance, the Centers for Disease Control and Prevention extended more leeway in contact tracing when community rates of COVID-19 surge, even allowing that contact tracing “may not be possible” in certain situations. And by defining an exposure more narrowly – health care workers are only considered “exposed” if their contact was more than 15 minutes or lacking in some form of PPE – the guidelines suggest that hospitals can rely more on universal PPE and screening protocols, as Dr. Adhikari’s hospital does, and less on extensive contact tracing to curtail viral spread.
Accordingly, while contact tracing has gotten more lax, doctors say, universal precautions – including full PPE and screening of symptoms for patients and health care workers – have become more stringent.
It’s a shift from the beginning of the pandemic. At first, CDC recommended wearing masks only during aerosol-producing procedures. Exposures were frequently reported and health care workers sent home. With more evidence in favor of stricter PPE requirements, hospitals including the one where Shyam Odeti, MD, FHM, works in Johnson City, Tenn., have adopted a universal precaution strategy – requiring masks everywhere and a gown, face shield, gloves, and N95 to enter a COVID-positive patient’s room. Thus, most exposures fall into that low-risk category.
“If I get it and am asymptomatic, I don’t think my colleagues would be exposed by any means because of these stringent policies being enforced,” said Dr. Odeti, a hospitalist who often wears a surgical mask on top of his N95 all day. “And U.S. health care is not in a state that can afford to quarantine health care workers for 14 days.”
Can universal PPE precautions supplant contact tracing?
The extent of contact tracing varies by hospital. Larger university and community hospitals often have infection control and occupational health teams that can do their own contact tracing, while smaller institutions can’t always spare staff. And some state health departments get involved with contact tracing of health care workers while others do not.
“I would venture to say that most hospitals are doing something in terms of contact tracing,” said Pam Falk, MPH, CIC, a member of the Association for Professionals in Infection Control and Epidemiology’s COVID-19 task force and an infection control consultant. “It kind of depends on their bandwidth.”
But there’s no longer a norm. Outside of a pandemic, with ample staffing and far fewer instances that need to be investigated, standards for contact tracing are higher, Dr. Falk said: When a patient is found to have an airborne disease such as tuberculosis, measles, mumps, or chickenpox, a hospital’s infection prevention team should investigate, confirm the diagnosis and identify everyone who was exposed. The hospital’s occupational health team assists in deciding who will likely need prophylactic treatment and if employees should be furloughed. The thoroughness of such measures has always depended on a hospital’s bandwidth.
Because PPE seems to be able to contain COVID-19 better than some of the older diseases targeted by contact tracing, universal protections may be a reasonable alternative in current circumstances, doctors said – if PPE is available.
“At the end of the day, universal source control with surgical masks – and ideally eye protection for clinicians as well – should prevent most transmissions,” said Aaron Richterman, MD, from the division of infectious diseases at the Hospital of the University of Pennsylvania, Philadelphia, who coauthored a JAMA commentary on decreased transmission rates in hospitals.
Contact tracing is still useful, though, to identify weaknesses in universal protection measures, he said.
“I don’t think it’s worth abandoning. It’s like a tool in the toolbox. All are imperfect, and none work 100% of the time,” Dr. Richterman said, but using all of them can achieve a fairly high measure of safety. Of the tools, universal masking likely works the best, he contends, so it should be the top pick for hospitals without resources to use all of the tools.
A recent incident at Brigham and Women’s Hospital in Boston is a case study in how contact tracing can work together with universal protections to identify cracks in the system, said Dr. Richterman, who worked at the hospital earlier in the pandemic.
Mass General Brigham adopted a universal masking policy for staff and patients in March 2020. Then, when the system experienced an outbreak in September, the hospital did “a very detailed public evaluation that included contact tracing and universal testing,” Dr. Richterman said. Testing even included genetic analysis of the virus to confirm which cases were hospital acquired. In the end, the hospital identified weaknesses in infection control that could be rectified, such as clinicians eating too close together.
“The approach is not to point fingers, but to say: ‘What’s wrong with the system and how do we improve?’ ” Dr. Richterman said. “To ask, why did that maskless transmission happen? Is there not enough space to eat? Are people working too many hours? It’s useful for systems to understand where transmissions are happening.”
Amith Skandhan, MD, SFHM, a hospitalist in Dothan, Ala., is comfortable without much contact tracing as long as there is universal PPE use. His hospital informs clinicians of exposures, but “basically we’re trained to treat every patient as if they had COVID,” he said, so “I feel more secure in the hospital than in the community.” Masks have become so habitual they’re like part of your regular clothing, he said – you feel incomplete if you don’t have one.
While ad hoc approaches to contact tracing may be useful in the current stage of the pandemic, they are likely to be short-lived: Once a community’s positivity rate falls, the CDC’s guidance suggests how hospitals can return to full contact tracing.
A version of this article first appeared on Medscape.com.
Like most health care workers at his hospital in Lafayette, Ind., Ramesh Adhikari, MD, FHM, occasionally gets an email noting that a patient he saw later tested positive for COVID-19. He’s reminded to self-monitor for symptoms. But 10 months into the pandemic, it has become increasingly unlikely for contact tracing investigations to result in clinicians quarantining.
The very act of working in the hospital, Dr. Adhikari said, means being likely to see COVID-19 every day, whether in a known patient or an asymptomatic person who tests positive later. If hospitalists had to quarantine after every interaction with a COVID-positive person, there wouldn’t be anyone left to do their jobs.
“It’s really hard to do [contact tracing] in health care workers thoroughly because of the way we work,” Dr. Adhikari said. “It’s impossible to do it absolutely.”
In a recently updated guidance, the Centers for Disease Control and Prevention extended more leeway in contact tracing when community rates of COVID-19 surge, even allowing that contact tracing “may not be possible” in certain situations. And by defining an exposure more narrowly – health care workers are only considered “exposed” if their contact was more than 15 minutes or lacking in some form of PPE – the guidelines suggest that hospitals can rely more on universal PPE and screening protocols, as Dr. Adhikari’s hospital does, and less on extensive contact tracing to curtail viral spread.
Accordingly, while contact tracing has gotten more lax, doctors say, universal precautions – including full PPE and screening of symptoms for patients and health care workers – have become more stringent.
It’s a shift from the beginning of the pandemic. At first, CDC recommended wearing masks only during aerosol-producing procedures. Exposures were frequently reported and health care workers sent home. With more evidence in favor of stricter PPE requirements, hospitals including the one where Shyam Odeti, MD, FHM, works in Johnson City, Tenn., have adopted a universal precaution strategy – requiring masks everywhere and a gown, face shield, gloves, and N95 to enter a COVID-positive patient’s room. Thus, most exposures fall into that low-risk category.
“If I get it and am asymptomatic, I don’t think my colleagues would be exposed by any means because of these stringent policies being enforced,” said Dr. Odeti, a hospitalist who often wears a surgical mask on top of his N95 all day. “And U.S. health care is not in a state that can afford to quarantine health care workers for 14 days.”
Can universal PPE precautions supplant contact tracing?
The extent of contact tracing varies by hospital. Larger university and community hospitals often have infection control and occupational health teams that can do their own contact tracing, while smaller institutions can’t always spare staff. And some state health departments get involved with contact tracing of health care workers while others do not.
“I would venture to say that most hospitals are doing something in terms of contact tracing,” said Pam Falk, MPH, CIC, a member of the Association for Professionals in Infection Control and Epidemiology’s COVID-19 task force and an infection control consultant. “It kind of depends on their bandwidth.”
But there’s no longer a norm. Outside of a pandemic, with ample staffing and far fewer instances that need to be investigated, standards for contact tracing are higher, Dr. Falk said: When a patient is found to have an airborne disease such as tuberculosis, measles, mumps, or chickenpox, a hospital’s infection prevention team should investigate, confirm the diagnosis and identify everyone who was exposed. The hospital’s occupational health team assists in deciding who will likely need prophylactic treatment and if employees should be furloughed. The thoroughness of such measures has always depended on a hospital’s bandwidth.
Because PPE seems to be able to contain COVID-19 better than some of the older diseases targeted by contact tracing, universal protections may be a reasonable alternative in current circumstances, doctors said – if PPE is available.
“At the end of the day, universal source control with surgical masks – and ideally eye protection for clinicians as well – should prevent most transmissions,” said Aaron Richterman, MD, from the division of infectious diseases at the Hospital of the University of Pennsylvania, Philadelphia, who coauthored a JAMA commentary on decreased transmission rates in hospitals.
Contact tracing is still useful, though, to identify weaknesses in universal protection measures, he said.
“I don’t think it’s worth abandoning. It’s like a tool in the toolbox. All are imperfect, and none work 100% of the time,” Dr. Richterman said, but using all of them can achieve a fairly high measure of safety. Of the tools, universal masking likely works the best, he contends, so it should be the top pick for hospitals without resources to use all of the tools.
A recent incident at Brigham and Women’s Hospital in Boston is a case study in how contact tracing can work together with universal protections to identify cracks in the system, said Dr. Richterman, who worked at the hospital earlier in the pandemic.
Mass General Brigham adopted a universal masking policy for staff and patients in March 2020. Then, when the system experienced an outbreak in September, the hospital did “a very detailed public evaluation that included contact tracing and universal testing,” Dr. Richterman said. Testing even included genetic analysis of the virus to confirm which cases were hospital acquired. In the end, the hospital identified weaknesses in infection control that could be rectified, such as clinicians eating too close together.
“The approach is not to point fingers, but to say: ‘What’s wrong with the system and how do we improve?’ ” Dr. Richterman said. “To ask, why did that maskless transmission happen? Is there not enough space to eat? Are people working too many hours? It’s useful for systems to understand where transmissions are happening.”
Amith Skandhan, MD, SFHM, a hospitalist in Dothan, Ala., is comfortable without much contact tracing as long as there is universal PPE use. His hospital informs clinicians of exposures, but “basically we’re trained to treat every patient as if they had COVID,” he said, so “I feel more secure in the hospital than in the community.” Masks have become so habitual they’re like part of your regular clothing, he said – you feel incomplete if you don’t have one.
While ad hoc approaches to contact tracing may be useful in the current stage of the pandemic, they are likely to be short-lived: Once a community’s positivity rate falls, the CDC’s guidance suggests how hospitals can return to full contact tracing.
A version of this article first appeared on Medscape.com.
Antibiotic prescribing: How to manage patient pressures
Inappropriate antibiotic prescribing in the face of growing microbial resistance is a global public health problem, and a major cause is perceived patient pressure.
At the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year, Tanya Stivers, PhD, professor of sociology at the University of California, Los Angeles, presented some of her team’s work studying patterns of clinical prescription.
It is widely appreciated that inappropriate prescribing is a common problem that the medical community seems powerless to stop, particularly in primary care. Already, clinicians are running out of effective antibiotics to treat a range of serious infections. Dr. Stivers began by saying that this problem isn’t caused by a lack of understanding about disease causation and microbial resistance or patients overtly demanding antibiotics, which occurs in less than 2% of cases. Instead, the cause appears to lie in doctor-patient interactions during consultations.
In pediatric practice, physicians have previously been found to prescribe antibiotics for a clinically diagnosed respiratory viral infection in 62% of cases when they perceive that this diagnosis was expected by parents, compared with 7% in the absence of such perception. Similarly, associated ear infections were diagnosed three times more often, and sinus infections seven times more often, leading to increased prescribing.
In adult practice, Dr. Stivers reported that patients can exert subtle pressure to prescribe through:
- Priming. Patients help their physician to see the problem as relatively severe (e.g., a sore throat that “feels like a knife”).
- Nudging. Patients redirect physicians back to a bacterial problem (e.g., “I’ve tried all these medicines, and nothing worked”). Nudging was found to occur in 41% of encounters.
- Resisting. Patients contest diagnosis or treatment in 40% of consultations (e.g., “there was pus yesterday”).
Priming or nudging resulted in antibiotic prescribing in 60% of patients without signs of a bacterial infection, compared with 30% where this was not a feature (P < 0.05).
But how can these pressures be countered? Dr. Stivers offered advice based on her original data from 570 video recordings of pediatric encounters. The current findings come from an analysis of 68 adult primary care visits for upper respiratory tract infections in Southern California. Inappropriate prescribing was identified in 37%.
When researching the antibiotic prescribing problem, it is helpful to explore a typical primary care consultation. The acute medical visit structure is a stepwise process involving opening, establishing the problem, gathering information, counseling, and then closing the consultation. It is important is to recognize that patients shape prescribing decisions, and effective communication is vital in influencing the outcome. In Dr. Stivers’ experience, priming, nudging, and resisting result in antibiotic prescribing in 60% of cases in whom clinical signs of bacterial illness are absent, compared with 30% where patient pressure is not a feature.
How can we change practice? Global experience suggests that printed material aimed at physicians is only of marginal benefit. By comparison, patient education does work but needs to be repeated, and there’s always a reason why this consultation should be “special.”
Try a 3-prong communication plan
To counteract these pressures, Dr. Stivers recommends a three-prong communication plan to influence the consultation:
- Foreshadowing, where suggesting that the cause of the patient’s symptoms is likely to be viral is introduced early in the consultation. This approach was found to reduce antibiotic prescribing to 33%, compared with 59% without foreshadowing (P < .05). Resistance may also be reduced.
- Affirmative nonantibiotic treatment plans, where specific positive recommendations given early (e.g., “I’m going to put you on some medicine to try to dry that out”) are less likely to be resisted than is vague negative advice at the end of a consultation.
- Persuasion, which involves explaining the diagnosis and nature of a cough and cold, educating about viral and bacterial differences, and presenting the risks of antibiotics. When persuasion is employed, antibiotic prescribing is reduced to 33%, compared with 63% (P < .05) without persuasion. In general, effective foreshadowing and affirmation should avoid the need for persuasion.
Dr. Stivers’ research suggests that these techniques work, but to do so, they should be delivered naturally as part of routine practice. Interestingly, her data showed that physicians rarely foreshadowed, and when they encountered resistance, they adopted persuasion in 53% of cases. By comparison, affirmative recommendations were used in 89% of cases, but their effects were reduced by the physician being vague and nonspecific.
In conclusion, Dr. Stivers said that addressing inappropriate prescribing requires awareness but that is not enough. The challenge is to reconsider health policies and ways of communicating about antibiotics. There is no downside to foreshadowing a likely viral origin, delivering affirmation, or using persuasion. She added, “If we can make even a 5%-10% reduction [in prescribing], wouldn’t it be worth it?”
Questions answered
A question-and-answer session followed Dr. Stivers’ presentation, and points raised included:
- Physicians have a desire to please. Dr. Stivers countered this point by saying that satisfaction is not tied to antibiotic prescription, and that physicians often misjudge what patients want. It’s important to communicate other treatment options because patients often just want “something they can do.”
- Decision fatigue is often a factor. Evidence shows that antibiotic prescription is more frequent toward the end of a shift. Doctors should avoid negotiation because it increases consultation time. Here, foreshadowing early on may help. Setting may also be important – prescription is more frequent in the ED.
- Vaccine-resistant parents often want active treatment. Here, conversations can be challenging. Trying to persuade may be a less successful than giving positive instruction (e.g., “we’ll give you a vaccine today.”) Resistance is likely to be lower.
- Concern was expressed about manipulating patients ahead of a firm diagnosis. Could this lead to missing a serious bacterial infection? Dr. Stivers acknowledged that this was a gamble. She recommended a “neutral” early foreshadowing statement such as “we are seeing a lot of viral infections at the present.”
- Cultural differences can have an effect. In China, for example, the argument between parents and physicians no longer focuses on antibiotics versus nonantibiotics but rather on oral versus intravenous administration.
- Litigation is a factor in prescribing, especially in the United States. Dr. Stivers stated that her proposed approach to prescribing should not interfere with appropriate management. The clinical picture can change, and antibiotics should be prescribed where needed.
- Audits improve prescribing in the short term. These results were based on recorded consultations, and that factor may have influenced management. In unrecorded consultations, inappropriate antibiotic prescription would be higher.
- Increased point-of-care testing can reduce unnecessary prescribing. This has been documented in countries such as Sweden. Evidence from China suggests that many patients will still receive antibiotics even if a bacterial cause is excluded.
When patients dictate treatment, sometimes we must tell them what is best. Dr. Stivers closed her presentation by emphasizing that, “how you say things will matter.”
Louis Bont, MD, PhD, chair of this session and pediatric infectious diseases specialist at the University Medical Center Utrecht (the Netherlands), commented: “Antimicrobial resistance is a global health threat which jeopardizes sustainable health goals. The World Health Organization has declared that antimicrobial resistance is one of the top 10 global public health threats facing humanity. Resistance to ciprofloxacin varies from 8%-93% in Escherichia coli and 4%-80% in Klebsiella pneumoniae. Colistin is the only last-resort treatment for life-threatening infections caused by carbapenem-resistant enterobacteriaceae.”
Dr. Stivers stated that she has nothing to disclose.
Inappropriate antibiotic prescribing in the face of growing microbial resistance is a global public health problem, and a major cause is perceived patient pressure.
At the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year, Tanya Stivers, PhD, professor of sociology at the University of California, Los Angeles, presented some of her team’s work studying patterns of clinical prescription.
It is widely appreciated that inappropriate prescribing is a common problem that the medical community seems powerless to stop, particularly in primary care. Already, clinicians are running out of effective antibiotics to treat a range of serious infections. Dr. Stivers began by saying that this problem isn’t caused by a lack of understanding about disease causation and microbial resistance or patients overtly demanding antibiotics, which occurs in less than 2% of cases. Instead, the cause appears to lie in doctor-patient interactions during consultations.
In pediatric practice, physicians have previously been found to prescribe antibiotics for a clinically diagnosed respiratory viral infection in 62% of cases when they perceive that this diagnosis was expected by parents, compared with 7% in the absence of such perception. Similarly, associated ear infections were diagnosed three times more often, and sinus infections seven times more often, leading to increased prescribing.
In adult practice, Dr. Stivers reported that patients can exert subtle pressure to prescribe through:
- Priming. Patients help their physician to see the problem as relatively severe (e.g., a sore throat that “feels like a knife”).
- Nudging. Patients redirect physicians back to a bacterial problem (e.g., “I’ve tried all these medicines, and nothing worked”). Nudging was found to occur in 41% of encounters.
- Resisting. Patients contest diagnosis or treatment in 40% of consultations (e.g., “there was pus yesterday”).
Priming or nudging resulted in antibiotic prescribing in 60% of patients without signs of a bacterial infection, compared with 30% where this was not a feature (P < 0.05).
But how can these pressures be countered? Dr. Stivers offered advice based on her original data from 570 video recordings of pediatric encounters. The current findings come from an analysis of 68 adult primary care visits for upper respiratory tract infections in Southern California. Inappropriate prescribing was identified in 37%.
When researching the antibiotic prescribing problem, it is helpful to explore a typical primary care consultation. The acute medical visit structure is a stepwise process involving opening, establishing the problem, gathering information, counseling, and then closing the consultation. It is important is to recognize that patients shape prescribing decisions, and effective communication is vital in influencing the outcome. In Dr. Stivers’ experience, priming, nudging, and resisting result in antibiotic prescribing in 60% of cases in whom clinical signs of bacterial illness are absent, compared with 30% where patient pressure is not a feature.
How can we change practice? Global experience suggests that printed material aimed at physicians is only of marginal benefit. By comparison, patient education does work but needs to be repeated, and there’s always a reason why this consultation should be “special.”
Try a 3-prong communication plan
To counteract these pressures, Dr. Stivers recommends a three-prong communication plan to influence the consultation:
- Foreshadowing, where suggesting that the cause of the patient’s symptoms is likely to be viral is introduced early in the consultation. This approach was found to reduce antibiotic prescribing to 33%, compared with 59% without foreshadowing (P < .05). Resistance may also be reduced.
- Affirmative nonantibiotic treatment plans, where specific positive recommendations given early (e.g., “I’m going to put you on some medicine to try to dry that out”) are less likely to be resisted than is vague negative advice at the end of a consultation.
- Persuasion, which involves explaining the diagnosis and nature of a cough and cold, educating about viral and bacterial differences, and presenting the risks of antibiotics. When persuasion is employed, antibiotic prescribing is reduced to 33%, compared with 63% (P < .05) without persuasion. In general, effective foreshadowing and affirmation should avoid the need for persuasion.
Dr. Stivers’ research suggests that these techniques work, but to do so, they should be delivered naturally as part of routine practice. Interestingly, her data showed that physicians rarely foreshadowed, and when they encountered resistance, they adopted persuasion in 53% of cases. By comparison, affirmative recommendations were used in 89% of cases, but their effects were reduced by the physician being vague and nonspecific.
In conclusion, Dr. Stivers said that addressing inappropriate prescribing requires awareness but that is not enough. The challenge is to reconsider health policies and ways of communicating about antibiotics. There is no downside to foreshadowing a likely viral origin, delivering affirmation, or using persuasion. She added, “If we can make even a 5%-10% reduction [in prescribing], wouldn’t it be worth it?”
Questions answered
A question-and-answer session followed Dr. Stivers’ presentation, and points raised included:
- Physicians have a desire to please. Dr. Stivers countered this point by saying that satisfaction is not tied to antibiotic prescription, and that physicians often misjudge what patients want. It’s important to communicate other treatment options because patients often just want “something they can do.”
- Decision fatigue is often a factor. Evidence shows that antibiotic prescription is more frequent toward the end of a shift. Doctors should avoid negotiation because it increases consultation time. Here, foreshadowing early on may help. Setting may also be important – prescription is more frequent in the ED.
- Vaccine-resistant parents often want active treatment. Here, conversations can be challenging. Trying to persuade may be a less successful than giving positive instruction (e.g., “we’ll give you a vaccine today.”) Resistance is likely to be lower.
- Concern was expressed about manipulating patients ahead of a firm diagnosis. Could this lead to missing a serious bacterial infection? Dr. Stivers acknowledged that this was a gamble. She recommended a “neutral” early foreshadowing statement such as “we are seeing a lot of viral infections at the present.”
- Cultural differences can have an effect. In China, for example, the argument between parents and physicians no longer focuses on antibiotics versus nonantibiotics but rather on oral versus intravenous administration.
- Litigation is a factor in prescribing, especially in the United States. Dr. Stivers stated that her proposed approach to prescribing should not interfere with appropriate management. The clinical picture can change, and antibiotics should be prescribed where needed.
- Audits improve prescribing in the short term. These results were based on recorded consultations, and that factor may have influenced management. In unrecorded consultations, inappropriate antibiotic prescription would be higher.
- Increased point-of-care testing can reduce unnecessary prescribing. This has been documented in countries such as Sweden. Evidence from China suggests that many patients will still receive antibiotics even if a bacterial cause is excluded.
When patients dictate treatment, sometimes we must tell them what is best. Dr. Stivers closed her presentation by emphasizing that, “how you say things will matter.”
Louis Bont, MD, PhD, chair of this session and pediatric infectious diseases specialist at the University Medical Center Utrecht (the Netherlands), commented: “Antimicrobial resistance is a global health threat which jeopardizes sustainable health goals. The World Health Organization has declared that antimicrobial resistance is one of the top 10 global public health threats facing humanity. Resistance to ciprofloxacin varies from 8%-93% in Escherichia coli and 4%-80% in Klebsiella pneumoniae. Colistin is the only last-resort treatment for life-threatening infections caused by carbapenem-resistant enterobacteriaceae.”
Dr. Stivers stated that she has nothing to disclose.
Inappropriate antibiotic prescribing in the face of growing microbial resistance is a global public health problem, and a major cause is perceived patient pressure.
At the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year, Tanya Stivers, PhD, professor of sociology at the University of California, Los Angeles, presented some of her team’s work studying patterns of clinical prescription.
It is widely appreciated that inappropriate prescribing is a common problem that the medical community seems powerless to stop, particularly in primary care. Already, clinicians are running out of effective antibiotics to treat a range of serious infections. Dr. Stivers began by saying that this problem isn’t caused by a lack of understanding about disease causation and microbial resistance or patients overtly demanding antibiotics, which occurs in less than 2% of cases. Instead, the cause appears to lie in doctor-patient interactions during consultations.
In pediatric practice, physicians have previously been found to prescribe antibiotics for a clinically diagnosed respiratory viral infection in 62% of cases when they perceive that this diagnosis was expected by parents, compared with 7% in the absence of such perception. Similarly, associated ear infections were diagnosed three times more often, and sinus infections seven times more often, leading to increased prescribing.
In adult practice, Dr. Stivers reported that patients can exert subtle pressure to prescribe through:
- Priming. Patients help their physician to see the problem as relatively severe (e.g., a sore throat that “feels like a knife”).
- Nudging. Patients redirect physicians back to a bacterial problem (e.g., “I’ve tried all these medicines, and nothing worked”). Nudging was found to occur in 41% of encounters.
- Resisting. Patients contest diagnosis or treatment in 40% of consultations (e.g., “there was pus yesterday”).
Priming or nudging resulted in antibiotic prescribing in 60% of patients without signs of a bacterial infection, compared with 30% where this was not a feature (P < 0.05).
But how can these pressures be countered? Dr. Stivers offered advice based on her original data from 570 video recordings of pediatric encounters. The current findings come from an analysis of 68 adult primary care visits for upper respiratory tract infections in Southern California. Inappropriate prescribing was identified in 37%.
When researching the antibiotic prescribing problem, it is helpful to explore a typical primary care consultation. The acute medical visit structure is a stepwise process involving opening, establishing the problem, gathering information, counseling, and then closing the consultation. It is important is to recognize that patients shape prescribing decisions, and effective communication is vital in influencing the outcome. In Dr. Stivers’ experience, priming, nudging, and resisting result in antibiotic prescribing in 60% of cases in whom clinical signs of bacterial illness are absent, compared with 30% where patient pressure is not a feature.
How can we change practice? Global experience suggests that printed material aimed at physicians is only of marginal benefit. By comparison, patient education does work but needs to be repeated, and there’s always a reason why this consultation should be “special.”
Try a 3-prong communication plan
To counteract these pressures, Dr. Stivers recommends a three-prong communication plan to influence the consultation:
- Foreshadowing, where suggesting that the cause of the patient’s symptoms is likely to be viral is introduced early in the consultation. This approach was found to reduce antibiotic prescribing to 33%, compared with 59% without foreshadowing (P < .05). Resistance may also be reduced.
- Affirmative nonantibiotic treatment plans, where specific positive recommendations given early (e.g., “I’m going to put you on some medicine to try to dry that out”) are less likely to be resisted than is vague negative advice at the end of a consultation.
- Persuasion, which involves explaining the diagnosis and nature of a cough and cold, educating about viral and bacterial differences, and presenting the risks of antibiotics. When persuasion is employed, antibiotic prescribing is reduced to 33%, compared with 63% (P < .05) without persuasion. In general, effective foreshadowing and affirmation should avoid the need for persuasion.
Dr. Stivers’ research suggests that these techniques work, but to do so, they should be delivered naturally as part of routine practice. Interestingly, her data showed that physicians rarely foreshadowed, and when they encountered resistance, they adopted persuasion in 53% of cases. By comparison, affirmative recommendations were used in 89% of cases, but their effects were reduced by the physician being vague and nonspecific.
In conclusion, Dr. Stivers said that addressing inappropriate prescribing requires awareness but that is not enough. The challenge is to reconsider health policies and ways of communicating about antibiotics. There is no downside to foreshadowing a likely viral origin, delivering affirmation, or using persuasion. She added, “If we can make even a 5%-10% reduction [in prescribing], wouldn’t it be worth it?”
Questions answered
A question-and-answer session followed Dr. Stivers’ presentation, and points raised included:
- Physicians have a desire to please. Dr. Stivers countered this point by saying that satisfaction is not tied to antibiotic prescription, and that physicians often misjudge what patients want. It’s important to communicate other treatment options because patients often just want “something they can do.”
- Decision fatigue is often a factor. Evidence shows that antibiotic prescription is more frequent toward the end of a shift. Doctors should avoid negotiation because it increases consultation time. Here, foreshadowing early on may help. Setting may also be important – prescription is more frequent in the ED.
- Vaccine-resistant parents often want active treatment. Here, conversations can be challenging. Trying to persuade may be a less successful than giving positive instruction (e.g., “we’ll give you a vaccine today.”) Resistance is likely to be lower.
- Concern was expressed about manipulating patients ahead of a firm diagnosis. Could this lead to missing a serious bacterial infection? Dr. Stivers acknowledged that this was a gamble. She recommended a “neutral” early foreshadowing statement such as “we are seeing a lot of viral infections at the present.”
- Cultural differences can have an effect. In China, for example, the argument between parents and physicians no longer focuses on antibiotics versus nonantibiotics but rather on oral versus intravenous administration.
- Litigation is a factor in prescribing, especially in the United States. Dr. Stivers stated that her proposed approach to prescribing should not interfere with appropriate management. The clinical picture can change, and antibiotics should be prescribed where needed.
- Audits improve prescribing in the short term. These results were based on recorded consultations, and that factor may have influenced management. In unrecorded consultations, inappropriate antibiotic prescription would be higher.
- Increased point-of-care testing can reduce unnecessary prescribing. This has been documented in countries such as Sweden. Evidence from China suggests that many patients will still receive antibiotics even if a bacterial cause is excluded.
When patients dictate treatment, sometimes we must tell them what is best. Dr. Stivers closed her presentation by emphasizing that, “how you say things will matter.”
Louis Bont, MD, PhD, chair of this session and pediatric infectious diseases specialist at the University Medical Center Utrecht (the Netherlands), commented: “Antimicrobial resistance is a global health threat which jeopardizes sustainable health goals. The World Health Organization has declared that antimicrobial resistance is one of the top 10 global public health threats facing humanity. Resistance to ciprofloxacin varies from 8%-93% in Escherichia coli and 4%-80% in Klebsiella pneumoniae. Colistin is the only last-resort treatment for life-threatening infections caused by carbapenem-resistant enterobacteriaceae.”
Dr. Stivers stated that she has nothing to disclose.
FROM ESPID 2020
Hand hygiene in pediatric ICUs: Identifying areas for improvement
A multidisciplinary team seeking to measure compliance with hand hygiene (HH) practices in pediatric ICUs across Europe found compliance was comparable and relatively high among unit doctors and nurses, but not as high in nonunit doctors and nurses.
Ioannis Kopsidas, MD, presented these results from the RANIN-KIDS Network during the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year. RANIN-KIDS (Reducing Antimicrobial Use and Nosocomial Infections in Kids) is a European network with the aim of preventing hospital-associated infections and promoting judicial antimicrobial use in pediatric patients using a common sustainable methodology across Europe.
Infections kill. This is especially the case in pediatric ICUs, where young age and an immunocompromised status make patients particularly vulnerable to infections. Poor HH is a major cause for disease transmission. To reduce the risk, the World Health Organization recommends attention to five moments of hand hygiene and nine steps for hand washing. Various tools are available to improve adherence, but whether these measures are being followed is unclear. The researchers sought to assess the degree of compliance with HH practices in pediatric ICUs and to identify targets for improvement.
Dr. Kopsidas, of the Center of Clinical Epidemiology and Outcomes Research, the National and Kapodistrian University of Athens, and colleagues examined practices in nine pediatric ICUs across six European countries (Estonia, Germany, Greece, Italy, Spain, and Switzerland) by means of prospective observational study. All organizations were part of the RANIN-KIDS network. Over a 6-month period starting in March 2019, observations were conducted in every unit by observers using a data collection tool developed based on WHO guidelines. Training for observers was provided using a self-paced teaching kit comprising PowerPoint and video presentations, followed by the completion of a test observation form after observing staged hand hygiene exercises. Results were then compared with WHO guidance, and irregularities were explained in order to achieve interrater reliability.
Researchers observed 1,715 HH opportunities. Across all pediatric ICUs, the median HH compliance rate was 82% (interquartile range, 72%-95%). Stratified by type of professional, median compliance was comparable among unit doctors (90%) and nurses (87%), but lower for nonunit doctors and nurses (81%) and also for nondoctors and nonnurses (67%). Alcohol-based hand rub was substantially preferred to soap and water, being used in 84% of the observations (IQR, 69%-87%). Cleaning and drying technique was considered appropriate in a median of 93% of observations (IQR, 86%-96%).
Compliance to moment 5 (after touching patient surroundings) was the lowest across hospitals (median 71%), compared with a median 100% for moment 2 (before clean/aseptic procedures) and a median 93% for moment 3 (after body fluid exposure/risk). For moment 1, median compliance was 87% (before touching a patient), and for moment 4, median compliance was 82% (after touching a patient).
Dr. Kopsidas concluded that the overall level of HH compliance among doctors and nurses working in European pediatric ICUs appears to be high, with moment 5 being the most frequently missed opportunity. Nonunit doctors and nurses and other personnel show lower WHO guidelines adherence. He stated that “these results will be used to design tailor-made interventions in participating units with the aim of reducing HAIs [health care–associated infections] and spread of multidrug resistant infections.”
He also said that “unified surveillance in Europe is possible and achievable, and allows for benchmarking among countries, institutions and wards.”
For some units, improving HH is a missed opportunity. The next stop for the RANIN-KIDS network is to look at the effects of interventions on reducing spread.
Dr. Kopsidas had no relevant financial disclosures.
A multidisciplinary team seeking to measure compliance with hand hygiene (HH) practices in pediatric ICUs across Europe found compliance was comparable and relatively high among unit doctors and nurses, but not as high in nonunit doctors and nurses.
Ioannis Kopsidas, MD, presented these results from the RANIN-KIDS Network during the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year. RANIN-KIDS (Reducing Antimicrobial Use and Nosocomial Infections in Kids) is a European network with the aim of preventing hospital-associated infections and promoting judicial antimicrobial use in pediatric patients using a common sustainable methodology across Europe.
Infections kill. This is especially the case in pediatric ICUs, where young age and an immunocompromised status make patients particularly vulnerable to infections. Poor HH is a major cause for disease transmission. To reduce the risk, the World Health Organization recommends attention to five moments of hand hygiene and nine steps for hand washing. Various tools are available to improve adherence, but whether these measures are being followed is unclear. The researchers sought to assess the degree of compliance with HH practices in pediatric ICUs and to identify targets for improvement.
Dr. Kopsidas, of the Center of Clinical Epidemiology and Outcomes Research, the National and Kapodistrian University of Athens, and colleagues examined practices in nine pediatric ICUs across six European countries (Estonia, Germany, Greece, Italy, Spain, and Switzerland) by means of prospective observational study. All organizations were part of the RANIN-KIDS network. Over a 6-month period starting in March 2019, observations were conducted in every unit by observers using a data collection tool developed based on WHO guidelines. Training for observers was provided using a self-paced teaching kit comprising PowerPoint and video presentations, followed by the completion of a test observation form after observing staged hand hygiene exercises. Results were then compared with WHO guidance, and irregularities were explained in order to achieve interrater reliability.
Researchers observed 1,715 HH opportunities. Across all pediatric ICUs, the median HH compliance rate was 82% (interquartile range, 72%-95%). Stratified by type of professional, median compliance was comparable among unit doctors (90%) and nurses (87%), but lower for nonunit doctors and nurses (81%) and also for nondoctors and nonnurses (67%). Alcohol-based hand rub was substantially preferred to soap and water, being used in 84% of the observations (IQR, 69%-87%). Cleaning and drying technique was considered appropriate in a median of 93% of observations (IQR, 86%-96%).
Compliance to moment 5 (after touching patient surroundings) was the lowest across hospitals (median 71%), compared with a median 100% for moment 2 (before clean/aseptic procedures) and a median 93% for moment 3 (after body fluid exposure/risk). For moment 1, median compliance was 87% (before touching a patient), and for moment 4, median compliance was 82% (after touching a patient).
Dr. Kopsidas concluded that the overall level of HH compliance among doctors and nurses working in European pediatric ICUs appears to be high, with moment 5 being the most frequently missed opportunity. Nonunit doctors and nurses and other personnel show lower WHO guidelines adherence. He stated that “these results will be used to design tailor-made interventions in participating units with the aim of reducing HAIs [health care–associated infections] and spread of multidrug resistant infections.”
He also said that “unified surveillance in Europe is possible and achievable, and allows for benchmarking among countries, institutions and wards.”
For some units, improving HH is a missed opportunity. The next stop for the RANIN-KIDS network is to look at the effects of interventions on reducing spread.
Dr. Kopsidas had no relevant financial disclosures.
A multidisciplinary team seeking to measure compliance with hand hygiene (HH) practices in pediatric ICUs across Europe found compliance was comparable and relatively high among unit doctors and nurses, but not as high in nonunit doctors and nurses.
Ioannis Kopsidas, MD, presented these results from the RANIN-KIDS Network during the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year. RANIN-KIDS (Reducing Antimicrobial Use and Nosocomial Infections in Kids) is a European network with the aim of preventing hospital-associated infections and promoting judicial antimicrobial use in pediatric patients using a common sustainable methodology across Europe.
Infections kill. This is especially the case in pediatric ICUs, where young age and an immunocompromised status make patients particularly vulnerable to infections. Poor HH is a major cause for disease transmission. To reduce the risk, the World Health Organization recommends attention to five moments of hand hygiene and nine steps for hand washing. Various tools are available to improve adherence, but whether these measures are being followed is unclear. The researchers sought to assess the degree of compliance with HH practices in pediatric ICUs and to identify targets for improvement.
Dr. Kopsidas, of the Center of Clinical Epidemiology and Outcomes Research, the National and Kapodistrian University of Athens, and colleagues examined practices in nine pediatric ICUs across six European countries (Estonia, Germany, Greece, Italy, Spain, and Switzerland) by means of prospective observational study. All organizations were part of the RANIN-KIDS network. Over a 6-month period starting in March 2019, observations were conducted in every unit by observers using a data collection tool developed based on WHO guidelines. Training for observers was provided using a self-paced teaching kit comprising PowerPoint and video presentations, followed by the completion of a test observation form after observing staged hand hygiene exercises. Results were then compared with WHO guidance, and irregularities were explained in order to achieve interrater reliability.
Researchers observed 1,715 HH opportunities. Across all pediatric ICUs, the median HH compliance rate was 82% (interquartile range, 72%-95%). Stratified by type of professional, median compliance was comparable among unit doctors (90%) and nurses (87%), but lower for nonunit doctors and nurses (81%) and also for nondoctors and nonnurses (67%). Alcohol-based hand rub was substantially preferred to soap and water, being used in 84% of the observations (IQR, 69%-87%). Cleaning and drying technique was considered appropriate in a median of 93% of observations (IQR, 86%-96%).
Compliance to moment 5 (after touching patient surroundings) was the lowest across hospitals (median 71%), compared with a median 100% for moment 2 (before clean/aseptic procedures) and a median 93% for moment 3 (after body fluid exposure/risk). For moment 1, median compliance was 87% (before touching a patient), and for moment 4, median compliance was 82% (after touching a patient).
Dr. Kopsidas concluded that the overall level of HH compliance among doctors and nurses working in European pediatric ICUs appears to be high, with moment 5 being the most frequently missed opportunity. Nonunit doctors and nurses and other personnel show lower WHO guidelines adherence. He stated that “these results will be used to design tailor-made interventions in participating units with the aim of reducing HAIs [health care–associated infections] and spread of multidrug resistant infections.”
He also said that “unified surveillance in Europe is possible and achievable, and allows for benchmarking among countries, institutions and wards.”
For some units, improving HH is a missed opportunity. The next stop for the RANIN-KIDS network is to look at the effects of interventions on reducing spread.
Dr. Kopsidas had no relevant financial disclosures.
FROM ESPID 2020