User login
Is HIV criminalization the No. 1 barrier to ending the epidemic?
For many people, being told that they are HIV positive is no longer a death sentence. But for Robert Suttle, a Black gay man and social justice educator, it is a life sentence.
Unexpectedly caught up in the HIV criminalization web at the age of 30, Mr. Suttle spent 6 months in a Louisiana state prison for a consensual sexual relationship with an adult partner. The crime? Not disclosing his HIV-positive status, a charge that Mr. Suttle says is untrue.
“I did disclose my status to my partner; however, I can’t really answer how they might have received it,” he said.
Today, at the age of 42, Mr. Suttle still carries the indelible stain of a conviction and of being a registered sex offender. “After their diagnosis, criminal charge, and/or conviction, many people think they’re done – either ‘I’ve gotten out of prison’ or ‘I’m still on probation’ – whatever the case may be,” he explained. “But we’re still living out these collateral consequences, be it with housing, moving to another state, or finding a job.”
The same is true for HIV-positive people who are charged and tried but manage to dodge prison for one reason or another. Monique Howell, a straight, 40-year-old former army soldier and single mother of five children, said that she was afraid to disclose her HIV status to a sexual partner but did advise him to wear a condom.* She points to her DD14 discharge papers (i.e., forms that verify that someone served in the military) that were issued when her military duty was rescinded following the dismissal of her court case.
“I was going to reenlist, but I got in trouble,” she said. She explained that although a DD14 separation helps to ensure that she can receive benefits and care, the papers were issued with a caveat stating “serious offense,” an indelible stain that, like Mr. Suttle’s, will follow her for the rest of her life.
Laws criminalize myths and misconceptions
HIV criminalization laws subject persons whose behaviors may expose others to HIV to felony or misdemeanor charges. Depending on the state, they can carry prison terms ranging from less than 10 years to life, according to the Centers for Disease Control and Prevention.
Originally enacted at the height of the AIDS epidemic in 1986, when fear was rampant and hundreds were dying, the laws were intended to reduce HIV transmission. But they’ve had unintended consequences: Amplifying stigmatization and discrimination and perpetuating HIV myths and misconceptions, including how HIV is transmitted.
Decades of scientific advances challenge the most basic reasoning behind laws (for example, that transmission is possible via biting or spitting or through a single sexual act, which studies have shown poses a risk as low as 0%-1.4%). In addition, few laws reflect one of the most important HIV research findings of the past decade: undetectable equals untransmittable, meaning that the virus cannot be sexually transmitted by people who are taking antiretroviral therapy and whose viral loads are undetectable.
In most of these cases, individuals who are positive for HIV are charged and punished for unintentional exposure, not deliberate intent to harm. Moreover, for the charge to stick, sexual partners don’t need to have acquired the virus or prove the transmission source if they do become HIV positive.
Ms. Howell noted that it was the Army that brought the charges against her, not her sexual partner at that time (who, incidentally, tested negative). He even testified on her behalf at the trial. “I’ll never forget it,” she said. “He said, ‘I don’t want anything to happen to Monique; even if you put her behind bars, she’s still HIV-positive and she’s still got those children. She told me to get a condom, and I chose not to.’ ”
Criminal vs. clinical fallout
In 2018, 20 scientists across the world issued a consensus statement underscoring the fact that HIV criminalization laws are based on fallacies and faulty science. The statement (which remains one of the most accessed in the Journal of the International AIDS Society) also points out that 33 countries (including the United States) use general criminal statutes such as attempted murder or reckless endangerment to lengthen sentences when people with HIV commit crimes.
When the laws were created, “many were the equivalent [to general criminal laws], because HIV was seen as a death sentence,” explained Chris Beyrer MD, MPH, professor of public health and human rights at Johns Hopkins Bloomberg School of Public Health, Baltimore. “So, failure to disclose your status, to wear a condom was seen as risking someone else’s life, which is no longer the case,” he added.
In fact, “from the perspective of the kinds of impact that these laws have had on transmission, or risk, or behavior, what you find is that they really have no public health benefit and they have real public harms,” said Dr. Beyrer.
Claire Farel, MD, assistant professor and medical director of the UNC Infectious Diseases Clinic at the University of North Carolina at Chapel Hill, concurs. “Because of the criminalization undercurrent, there are people who don’t get tested, meaning that they are at risk for worse health outcomes, such as cancer, vascular disease, and of course HIV-related poor outcomes, including progression to AIDS.”
Farel also points to the residual stigma associated with HIV. “Much of this is inextricable from that surrounding homophobia, especially among young men of color who have sex with men. It opens up a larger conversation that a lot of people don’t want to engage in,” she said.
Laws broaden existing disparities even further
The CDC released a study June 4 showing substantial declines in the overall incidence of HIV in the United States, with an important caveat: There’s been a worsening disparity in cases. Access to care and engagement with care remain poor among certain populations. For example, Black individuals accounted for 41% of new HIV infections in 2019, but they represent only 12% of the U.S. population; Hispanic/Latinx persons accounted for 29% of new infections, although they represent only 17% of the entire population.
The same is true for HIV criminalization: In 2020, more than 50% of defendants were people of color, according to U.S. case data collated by the HIV Justice Network.
Still, the momentum to change these antiquated laws is gaining speed. In May, the Illinois State Senate passed a bill repealing HIV criminalization, and this past March, Virginia’s Governor Ralph Northam signed a bill lowering HIV-related criminalization charges from a felony to a misdemeanor and changing the wording of its law to include both intent and transmission.** California, Colorado, Iowa, Michigan, Nevada, and North Carolina have also modernized or repealed their laws.
Ending the U.S. HIV epidemic: Patients first
Without true HIV criminalization reform, efforts to change the public and clinical mindset regarding HIV from its being a highly stigmatized disease to a preventable, treatable infection are likely to fall short. Dr. Beyrer emphasized that the onus lies with the scientific and activist communities working together. “I don’t know how you can end the epidemic if you are still stigmatizing the people who are actually acquiring these infections,” he said.
There are steps that patients can take while these forces push for change.
“As people first process their diagnosis, they need to learn as much about HIV and the science behind it as possible,” advised Mr. Suttle. He said that to protect oneself, it’s essential to learn about HIV criminalization and the laws in one’s state.
“Find someone you can trust, starting with your medical provider if possible, and if you have a significant other, bring that person to your appointments so they can see that you are in care and doing all that you can do to lower viral loads and protect others,” he added.
Ms. Howell said that although people should be in treatment and care, attitudes also need to change on the clinician side. “We’re just given these meds, told to take them, and are sent on our merry ways, but they don’t tell us how to live our lives properly; nobody grabs us and says, hey, these are the laws and you need to know this or that.”
When a person who is HIV positive does get caught up in the system, if possible, that person should consult an attorney who understands these laws. Mr. Suttle suggested reaching out to organizations in the movement to end HIV criminalization (e.g., the Sero Project, the Center for HIV Law and Policy, or the Positive Women’s Network) for further support, help with cases (including providing experts to testify), social services, and other resources. Mr. Suttle also encourages people who need help and direction to reach out to him directly at [email protected].
Forty years ago, the CDC published its first report of an illness in five healthy gay men living in Los Angeles. The first cases in women were reported shortly thereafter. Over the years, there have been many scientific advances in prevention and treatment. But as Dr. Beyrer aptly noted in an editorial published January 2021 in The Lancet HIV, “time has not lessened the sting of the early decades of AIDS.”
“We should not have to be afraid of who we are because we are HIV positive,” said Ms. Howell.
Dr. Farel, Mr. Suttle, and Ms. Howell report no relevant financial relationships. Dr. Beyrer has a consulting agreement with Merck.
A version of this article first appeared on Medscape.com.
*Correction, 6/14/2021: An earlier version of this story misstated Ms. Howell's age. She is 40.
**Correction, 6/14/2021: An earlier version of this story misspelled Gov. Northam's name.
For many people, being told that they are HIV positive is no longer a death sentence. But for Robert Suttle, a Black gay man and social justice educator, it is a life sentence.
Unexpectedly caught up in the HIV criminalization web at the age of 30, Mr. Suttle spent 6 months in a Louisiana state prison for a consensual sexual relationship with an adult partner. The crime? Not disclosing his HIV-positive status, a charge that Mr. Suttle says is untrue.
“I did disclose my status to my partner; however, I can’t really answer how they might have received it,” he said.
Today, at the age of 42, Mr. Suttle still carries the indelible stain of a conviction and of being a registered sex offender. “After their diagnosis, criminal charge, and/or conviction, many people think they’re done – either ‘I’ve gotten out of prison’ or ‘I’m still on probation’ – whatever the case may be,” he explained. “But we’re still living out these collateral consequences, be it with housing, moving to another state, or finding a job.”
The same is true for HIV-positive people who are charged and tried but manage to dodge prison for one reason or another. Monique Howell, a straight, 40-year-old former army soldier and single mother of five children, said that she was afraid to disclose her HIV status to a sexual partner but did advise him to wear a condom.* She points to her DD14 discharge papers (i.e., forms that verify that someone served in the military) that were issued when her military duty was rescinded following the dismissal of her court case.
“I was going to reenlist, but I got in trouble,” she said. She explained that although a DD14 separation helps to ensure that she can receive benefits and care, the papers were issued with a caveat stating “serious offense,” an indelible stain that, like Mr. Suttle’s, will follow her for the rest of her life.
Laws criminalize myths and misconceptions
HIV criminalization laws subject persons whose behaviors may expose others to HIV to felony or misdemeanor charges. Depending on the state, they can carry prison terms ranging from less than 10 years to life, according to the Centers for Disease Control and Prevention.
Originally enacted at the height of the AIDS epidemic in 1986, when fear was rampant and hundreds were dying, the laws were intended to reduce HIV transmission. But they’ve had unintended consequences: Amplifying stigmatization and discrimination and perpetuating HIV myths and misconceptions, including how HIV is transmitted.
Decades of scientific advances challenge the most basic reasoning behind laws (for example, that transmission is possible via biting or spitting or through a single sexual act, which studies have shown poses a risk as low as 0%-1.4%). In addition, few laws reflect one of the most important HIV research findings of the past decade: undetectable equals untransmittable, meaning that the virus cannot be sexually transmitted by people who are taking antiretroviral therapy and whose viral loads are undetectable.
In most of these cases, individuals who are positive for HIV are charged and punished for unintentional exposure, not deliberate intent to harm. Moreover, for the charge to stick, sexual partners don’t need to have acquired the virus or prove the transmission source if they do become HIV positive.
Ms. Howell noted that it was the Army that brought the charges against her, not her sexual partner at that time (who, incidentally, tested negative). He even testified on her behalf at the trial. “I’ll never forget it,” she said. “He said, ‘I don’t want anything to happen to Monique; even if you put her behind bars, she’s still HIV-positive and she’s still got those children. She told me to get a condom, and I chose not to.’ ”
Criminal vs. clinical fallout
In 2018, 20 scientists across the world issued a consensus statement underscoring the fact that HIV criminalization laws are based on fallacies and faulty science. The statement (which remains one of the most accessed in the Journal of the International AIDS Society) also points out that 33 countries (including the United States) use general criminal statutes such as attempted murder or reckless endangerment to lengthen sentences when people with HIV commit crimes.
When the laws were created, “many were the equivalent [to general criminal laws], because HIV was seen as a death sentence,” explained Chris Beyrer MD, MPH, professor of public health and human rights at Johns Hopkins Bloomberg School of Public Health, Baltimore. “So, failure to disclose your status, to wear a condom was seen as risking someone else’s life, which is no longer the case,” he added.
In fact, “from the perspective of the kinds of impact that these laws have had on transmission, or risk, or behavior, what you find is that they really have no public health benefit and they have real public harms,” said Dr. Beyrer.
Claire Farel, MD, assistant professor and medical director of the UNC Infectious Diseases Clinic at the University of North Carolina at Chapel Hill, concurs. “Because of the criminalization undercurrent, there are people who don’t get tested, meaning that they are at risk for worse health outcomes, such as cancer, vascular disease, and of course HIV-related poor outcomes, including progression to AIDS.”
Farel also points to the residual stigma associated with HIV. “Much of this is inextricable from that surrounding homophobia, especially among young men of color who have sex with men. It opens up a larger conversation that a lot of people don’t want to engage in,” she said.
Laws broaden existing disparities even further
The CDC released a study June 4 showing substantial declines in the overall incidence of HIV in the United States, with an important caveat: There’s been a worsening disparity in cases. Access to care and engagement with care remain poor among certain populations. For example, Black individuals accounted for 41% of new HIV infections in 2019, but they represent only 12% of the U.S. population; Hispanic/Latinx persons accounted for 29% of new infections, although they represent only 17% of the entire population.
The same is true for HIV criminalization: In 2020, more than 50% of defendants were people of color, according to U.S. case data collated by the HIV Justice Network.
Still, the momentum to change these antiquated laws is gaining speed. In May, the Illinois State Senate passed a bill repealing HIV criminalization, and this past March, Virginia’s Governor Ralph Northam signed a bill lowering HIV-related criminalization charges from a felony to a misdemeanor and changing the wording of its law to include both intent and transmission.** California, Colorado, Iowa, Michigan, Nevada, and North Carolina have also modernized or repealed their laws.
Ending the U.S. HIV epidemic: Patients first
Without true HIV criminalization reform, efforts to change the public and clinical mindset regarding HIV from its being a highly stigmatized disease to a preventable, treatable infection are likely to fall short. Dr. Beyrer emphasized that the onus lies with the scientific and activist communities working together. “I don’t know how you can end the epidemic if you are still stigmatizing the people who are actually acquiring these infections,” he said.
There are steps that patients can take while these forces push for change.
“As people first process their diagnosis, they need to learn as much about HIV and the science behind it as possible,” advised Mr. Suttle. He said that to protect oneself, it’s essential to learn about HIV criminalization and the laws in one’s state.
“Find someone you can trust, starting with your medical provider if possible, and if you have a significant other, bring that person to your appointments so they can see that you are in care and doing all that you can do to lower viral loads and protect others,” he added.
Ms. Howell said that although people should be in treatment and care, attitudes also need to change on the clinician side. “We’re just given these meds, told to take them, and are sent on our merry ways, but they don’t tell us how to live our lives properly; nobody grabs us and says, hey, these are the laws and you need to know this or that.”
When a person who is HIV positive does get caught up in the system, if possible, that person should consult an attorney who understands these laws. Mr. Suttle suggested reaching out to organizations in the movement to end HIV criminalization (e.g., the Sero Project, the Center for HIV Law and Policy, or the Positive Women’s Network) for further support, help with cases (including providing experts to testify), social services, and other resources. Mr. Suttle also encourages people who need help and direction to reach out to him directly at [email protected].
Forty years ago, the CDC published its first report of an illness in five healthy gay men living in Los Angeles. The first cases in women were reported shortly thereafter. Over the years, there have been many scientific advances in prevention and treatment. But as Dr. Beyrer aptly noted in an editorial published January 2021 in The Lancet HIV, “time has not lessened the sting of the early decades of AIDS.”
“We should not have to be afraid of who we are because we are HIV positive,” said Ms. Howell.
Dr. Farel, Mr. Suttle, and Ms. Howell report no relevant financial relationships. Dr. Beyrer has a consulting agreement with Merck.
A version of this article first appeared on Medscape.com.
*Correction, 6/14/2021: An earlier version of this story misstated Ms. Howell's age. She is 40.
**Correction, 6/14/2021: An earlier version of this story misspelled Gov. Northam's name.
For many people, being told that they are HIV positive is no longer a death sentence. But for Robert Suttle, a Black gay man and social justice educator, it is a life sentence.
Unexpectedly caught up in the HIV criminalization web at the age of 30, Mr. Suttle spent 6 months in a Louisiana state prison for a consensual sexual relationship with an adult partner. The crime? Not disclosing his HIV-positive status, a charge that Mr. Suttle says is untrue.
“I did disclose my status to my partner; however, I can’t really answer how they might have received it,” he said.
Today, at the age of 42, Mr. Suttle still carries the indelible stain of a conviction and of being a registered sex offender. “After their diagnosis, criminal charge, and/or conviction, many people think they’re done – either ‘I’ve gotten out of prison’ or ‘I’m still on probation’ – whatever the case may be,” he explained. “But we’re still living out these collateral consequences, be it with housing, moving to another state, or finding a job.”
The same is true for HIV-positive people who are charged and tried but manage to dodge prison for one reason or another. Monique Howell, a straight, 40-year-old former army soldier and single mother of five children, said that she was afraid to disclose her HIV status to a sexual partner but did advise him to wear a condom.* She points to her DD14 discharge papers (i.e., forms that verify that someone served in the military) that were issued when her military duty was rescinded following the dismissal of her court case.
“I was going to reenlist, but I got in trouble,” she said. She explained that although a DD14 separation helps to ensure that she can receive benefits and care, the papers were issued with a caveat stating “serious offense,” an indelible stain that, like Mr. Suttle’s, will follow her for the rest of her life.
Laws criminalize myths and misconceptions
HIV criminalization laws subject persons whose behaviors may expose others to HIV to felony or misdemeanor charges. Depending on the state, they can carry prison terms ranging from less than 10 years to life, according to the Centers for Disease Control and Prevention.
Originally enacted at the height of the AIDS epidemic in 1986, when fear was rampant and hundreds were dying, the laws were intended to reduce HIV transmission. But they’ve had unintended consequences: Amplifying stigmatization and discrimination and perpetuating HIV myths and misconceptions, including how HIV is transmitted.
Decades of scientific advances challenge the most basic reasoning behind laws (for example, that transmission is possible via biting or spitting or through a single sexual act, which studies have shown poses a risk as low as 0%-1.4%). In addition, few laws reflect one of the most important HIV research findings of the past decade: undetectable equals untransmittable, meaning that the virus cannot be sexually transmitted by people who are taking antiretroviral therapy and whose viral loads are undetectable.
In most of these cases, individuals who are positive for HIV are charged and punished for unintentional exposure, not deliberate intent to harm. Moreover, for the charge to stick, sexual partners don’t need to have acquired the virus or prove the transmission source if they do become HIV positive.
Ms. Howell noted that it was the Army that brought the charges against her, not her sexual partner at that time (who, incidentally, tested negative). He even testified on her behalf at the trial. “I’ll never forget it,” she said. “He said, ‘I don’t want anything to happen to Monique; even if you put her behind bars, she’s still HIV-positive and she’s still got those children. She told me to get a condom, and I chose not to.’ ”
Criminal vs. clinical fallout
In 2018, 20 scientists across the world issued a consensus statement underscoring the fact that HIV criminalization laws are based on fallacies and faulty science. The statement (which remains one of the most accessed in the Journal of the International AIDS Society) also points out that 33 countries (including the United States) use general criminal statutes such as attempted murder or reckless endangerment to lengthen sentences when people with HIV commit crimes.
When the laws were created, “many were the equivalent [to general criminal laws], because HIV was seen as a death sentence,” explained Chris Beyrer MD, MPH, professor of public health and human rights at Johns Hopkins Bloomberg School of Public Health, Baltimore. “So, failure to disclose your status, to wear a condom was seen as risking someone else’s life, which is no longer the case,” he added.
In fact, “from the perspective of the kinds of impact that these laws have had on transmission, or risk, or behavior, what you find is that they really have no public health benefit and they have real public harms,” said Dr. Beyrer.
Claire Farel, MD, assistant professor and medical director of the UNC Infectious Diseases Clinic at the University of North Carolina at Chapel Hill, concurs. “Because of the criminalization undercurrent, there are people who don’t get tested, meaning that they are at risk for worse health outcomes, such as cancer, vascular disease, and of course HIV-related poor outcomes, including progression to AIDS.”
Farel also points to the residual stigma associated with HIV. “Much of this is inextricable from that surrounding homophobia, especially among young men of color who have sex with men. It opens up a larger conversation that a lot of people don’t want to engage in,” she said.
Laws broaden existing disparities even further
The CDC released a study June 4 showing substantial declines in the overall incidence of HIV in the United States, with an important caveat: There’s been a worsening disparity in cases. Access to care and engagement with care remain poor among certain populations. For example, Black individuals accounted for 41% of new HIV infections in 2019, but they represent only 12% of the U.S. population; Hispanic/Latinx persons accounted for 29% of new infections, although they represent only 17% of the entire population.
The same is true for HIV criminalization: In 2020, more than 50% of defendants were people of color, according to U.S. case data collated by the HIV Justice Network.
Still, the momentum to change these antiquated laws is gaining speed. In May, the Illinois State Senate passed a bill repealing HIV criminalization, and this past March, Virginia’s Governor Ralph Northam signed a bill lowering HIV-related criminalization charges from a felony to a misdemeanor and changing the wording of its law to include both intent and transmission.** California, Colorado, Iowa, Michigan, Nevada, and North Carolina have also modernized or repealed their laws.
Ending the U.S. HIV epidemic: Patients first
Without true HIV criminalization reform, efforts to change the public and clinical mindset regarding HIV from its being a highly stigmatized disease to a preventable, treatable infection are likely to fall short. Dr. Beyrer emphasized that the onus lies with the scientific and activist communities working together. “I don’t know how you can end the epidemic if you are still stigmatizing the people who are actually acquiring these infections,” he said.
There are steps that patients can take while these forces push for change.
“As people first process their diagnosis, they need to learn as much about HIV and the science behind it as possible,” advised Mr. Suttle. He said that to protect oneself, it’s essential to learn about HIV criminalization and the laws in one’s state.
“Find someone you can trust, starting with your medical provider if possible, and if you have a significant other, bring that person to your appointments so they can see that you are in care and doing all that you can do to lower viral loads and protect others,” he added.
Ms. Howell said that although people should be in treatment and care, attitudes also need to change on the clinician side. “We’re just given these meds, told to take them, and are sent on our merry ways, but they don’t tell us how to live our lives properly; nobody grabs us and says, hey, these are the laws and you need to know this or that.”
When a person who is HIV positive does get caught up in the system, if possible, that person should consult an attorney who understands these laws. Mr. Suttle suggested reaching out to organizations in the movement to end HIV criminalization (e.g., the Sero Project, the Center for HIV Law and Policy, or the Positive Women’s Network) for further support, help with cases (including providing experts to testify), social services, and other resources. Mr. Suttle also encourages people who need help and direction to reach out to him directly at [email protected].
Forty years ago, the CDC published its first report of an illness in five healthy gay men living in Los Angeles. The first cases in women were reported shortly thereafter. Over the years, there have been many scientific advances in prevention and treatment. But as Dr. Beyrer aptly noted in an editorial published January 2021 in The Lancet HIV, “time has not lessened the sting of the early decades of AIDS.”
“We should not have to be afraid of who we are because we are HIV positive,” said Ms. Howell.
Dr. Farel, Mr. Suttle, and Ms. Howell report no relevant financial relationships. Dr. Beyrer has a consulting agreement with Merck.
A version of this article first appeared on Medscape.com.
*Correction, 6/14/2021: An earlier version of this story misstated Ms. Howell's age. She is 40.
**Correction, 6/14/2021: An earlier version of this story misspelled Gov. Northam's name.
Updates in clinical practice guidelines for Lyme disease
According to the Centers for Disease Control and Prevention, Lyme disease is the fastest growing vector-borne disease, affecting approximately 300,000 Americans every year. It is caused by the spirochete, Borrelia burgdorferi which is transmitted to humans by the deer tick. Lyme disease is often an overlooked diagnosis for myriad reasons, including inaccurate test results.
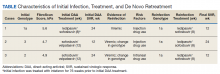
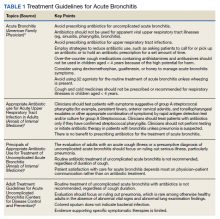
Recent guidelines for the prevention, diagnosis, and treatment of Lyme disease have been developed by a panel from the Infectious Disease Society of America (IDSA), the American Academy of Neurology (AAN), and the American College of Rheumatology (ACR) using evidence-based recommendations.
Infection prevention
We all know that the best way to treat any disease is by preventing it. The following measures are recommended as tools to prevent infection: personal protective wear, repellents, and removal of the attached tick. Recommended repellents include DEET, picaridin, IR3535, oil of lemon, eucalyptus, para-Menthane-3,8-diol (PMD), 2-undecanone, and permethrin. If a tick is found, it should be removed promptly by mechanical measures, such as with tweezers. The tweezers should be inserted between the tick body and skin to ensure removal of the entire tick. Burning an attached tick or applying a noxious chemical to the tick is not recommended.
Diagnosis
Diagnosing Lyme disease is often difficult given that tests can be negative for some time after a tick bite, even when the infection is present. There is good evidence to show that submitting the removed tick for identification is good practice. However, there is no evidence supporting testing the removed tick for the presence of Borrelia burgdorferi as it does not reliably predict infection in humans. It also is recommended to avoid testing asymptomatic people following a tick bite.
Following a high-risk tick bite, adults and children can be given prophylactic antibiotics within 72 hours. It is not helpful for low-risk bites. If the risk level is uncertain, it is better to observe before giving antibiotics. For adults, a single 200-mg dose of doxycycline can be given. In children, 4.4 mg per kg of body weight, up to 200 mg max, can be used for those under 45 kg.
For patients with a tick exposure and erythema migrans, a clinical diagnosis of Lyme disease can be made without further testing. If the clinical presentation is not typical, it is recommended to do an antibody test on an acute phase serum sample followed by a convalescent serum sample in 2-3 weeks if the initial test is negative. Recommended antibiotics for treatment include doxycycline for 10 days or amoxicillin or cefuroxime for 14 days. If a patient is unable to take these, azithromycin may be used for 7 days.
The guidelines also make recommendations regarding testing for Lyme neuroborreliosis, for which neurologic presentations, for adults with psychiatric illnesses, and for children with developmental/behavioral/psychiatric disorders. They further make recommendations for treatment of Lyme disease involving the brain or spinal column, facial nerve palsy, carditis, cardiomyopathy, and arthritis, which are beyond the scope of this discussion.
As family doctors, we are often the first ones patients call upon after a tick bite. We are the ones who diagnosis and treat Lyme disease, so it is imperative that we stay up to date with current clinical guidelines and practice evidence-based medicine. These most recent guidelines from several specialty societies can provide the answers to many of our patients’ questions. They also serve as a great tool to help with our clinical decision-making regarding tick bites. Lyme disease can be a scary infection for patients but, if we offer them the recommended measures, it doesn’t have to be.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].
According to the Centers for Disease Control and Prevention, Lyme disease is the fastest growing vector-borne disease, affecting approximately 300,000 Americans every year. It is caused by the spirochete, Borrelia burgdorferi which is transmitted to humans by the deer tick. Lyme disease is often an overlooked diagnosis for myriad reasons, including inaccurate test results.
Recent guidelines for the prevention, diagnosis, and treatment of Lyme disease have been developed by a panel from the Infectious Disease Society of America (IDSA), the American Academy of Neurology (AAN), and the American College of Rheumatology (ACR) using evidence-based recommendations.
Infection prevention
We all know that the best way to treat any disease is by preventing it. The following measures are recommended as tools to prevent infection: personal protective wear, repellents, and removal of the attached tick. Recommended repellents include DEET, picaridin, IR3535, oil of lemon, eucalyptus, para-Menthane-3,8-diol (PMD), 2-undecanone, and permethrin. If a tick is found, it should be removed promptly by mechanical measures, such as with tweezers. The tweezers should be inserted between the tick body and skin to ensure removal of the entire tick. Burning an attached tick or applying a noxious chemical to the tick is not recommended.
Diagnosis
Diagnosing Lyme disease is often difficult given that tests can be negative for some time after a tick bite, even when the infection is present. There is good evidence to show that submitting the removed tick for identification is good practice. However, there is no evidence supporting testing the removed tick for the presence of Borrelia burgdorferi as it does not reliably predict infection in humans. It also is recommended to avoid testing asymptomatic people following a tick bite.
Following a high-risk tick bite, adults and children can be given prophylactic antibiotics within 72 hours. It is not helpful for low-risk bites. If the risk level is uncertain, it is better to observe before giving antibiotics. For adults, a single 200-mg dose of doxycycline can be given. In children, 4.4 mg per kg of body weight, up to 200 mg max, can be used for those under 45 kg.
For patients with a tick exposure and erythema migrans, a clinical diagnosis of Lyme disease can be made without further testing. If the clinical presentation is not typical, it is recommended to do an antibody test on an acute phase serum sample followed by a convalescent serum sample in 2-3 weeks if the initial test is negative. Recommended antibiotics for treatment include doxycycline for 10 days or amoxicillin or cefuroxime for 14 days. If a patient is unable to take these, azithromycin may be used for 7 days.
The guidelines also make recommendations regarding testing for Lyme neuroborreliosis, for which neurologic presentations, for adults with psychiatric illnesses, and for children with developmental/behavioral/psychiatric disorders. They further make recommendations for treatment of Lyme disease involving the brain or spinal column, facial nerve palsy, carditis, cardiomyopathy, and arthritis, which are beyond the scope of this discussion.
As family doctors, we are often the first ones patients call upon after a tick bite. We are the ones who diagnosis and treat Lyme disease, so it is imperative that we stay up to date with current clinical guidelines and practice evidence-based medicine. These most recent guidelines from several specialty societies can provide the answers to many of our patients’ questions. They also serve as a great tool to help with our clinical decision-making regarding tick bites. Lyme disease can be a scary infection for patients but, if we offer them the recommended measures, it doesn’t have to be.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].
According to the Centers for Disease Control and Prevention, Lyme disease is the fastest growing vector-borne disease, affecting approximately 300,000 Americans every year. It is caused by the spirochete, Borrelia burgdorferi which is transmitted to humans by the deer tick. Lyme disease is often an overlooked diagnosis for myriad reasons, including inaccurate test results.
Recent guidelines for the prevention, diagnosis, and treatment of Lyme disease have been developed by a panel from the Infectious Disease Society of America (IDSA), the American Academy of Neurology (AAN), and the American College of Rheumatology (ACR) using evidence-based recommendations.
Infection prevention
We all know that the best way to treat any disease is by preventing it. The following measures are recommended as tools to prevent infection: personal protective wear, repellents, and removal of the attached tick. Recommended repellents include DEET, picaridin, IR3535, oil of lemon, eucalyptus, para-Menthane-3,8-diol (PMD), 2-undecanone, and permethrin. If a tick is found, it should be removed promptly by mechanical measures, such as with tweezers. The tweezers should be inserted between the tick body and skin to ensure removal of the entire tick. Burning an attached tick or applying a noxious chemical to the tick is not recommended.
Diagnosis
Diagnosing Lyme disease is often difficult given that tests can be negative for some time after a tick bite, even when the infection is present. There is good evidence to show that submitting the removed tick for identification is good practice. However, there is no evidence supporting testing the removed tick for the presence of Borrelia burgdorferi as it does not reliably predict infection in humans. It also is recommended to avoid testing asymptomatic people following a tick bite.
Following a high-risk tick bite, adults and children can be given prophylactic antibiotics within 72 hours. It is not helpful for low-risk bites. If the risk level is uncertain, it is better to observe before giving antibiotics. For adults, a single 200-mg dose of doxycycline can be given. In children, 4.4 mg per kg of body weight, up to 200 mg max, can be used for those under 45 kg.
For patients with a tick exposure and erythema migrans, a clinical diagnosis of Lyme disease can be made without further testing. If the clinical presentation is not typical, it is recommended to do an antibody test on an acute phase serum sample followed by a convalescent serum sample in 2-3 weeks if the initial test is negative. Recommended antibiotics for treatment include doxycycline for 10 days or amoxicillin or cefuroxime for 14 days. If a patient is unable to take these, azithromycin may be used for 7 days.
The guidelines also make recommendations regarding testing for Lyme neuroborreliosis, for which neurologic presentations, for adults with psychiatric illnesses, and for children with developmental/behavioral/psychiatric disorders. They further make recommendations for treatment of Lyme disease involving the brain or spinal column, facial nerve palsy, carditis, cardiomyopathy, and arthritis, which are beyond the scope of this discussion.
As family doctors, we are often the first ones patients call upon after a tick bite. We are the ones who diagnosis and treat Lyme disease, so it is imperative that we stay up to date with current clinical guidelines and practice evidence-based medicine. These most recent guidelines from several specialty societies can provide the answers to many of our patients’ questions. They also serve as a great tool to help with our clinical decision-making regarding tick bites. Lyme disease can be a scary infection for patients but, if we offer them the recommended measures, it doesn’t have to be.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].
The pandemic changed smokers, but farming didn’t change humans
Pandemic smoking: More or less?
The COVID-19 pandemic has changed a lot of habits in people, for better or worse. Some people may have turned to food and alcohol for comfort, while others started on health kicks to emerge from the ordeal as new people. Well, the same can be said about smokers.
New evidence comes from a survey conducted from May to July 2020 of 694 current and former smokers with an average age of 53 years. All had been hospitalized prior to the pandemic and had previously participated in clinical trials to for smoking cessation in Boston, Nashville, and Pittsburgh hospitals.
Researchers found that 32% of participants smoked more, 37% smoked less, and 31% made no change in their smoking habits. By the time of the survey, 28% of former smokers had relapsed. Although 68% of the participants believed smoking increased the risk of getting COVID-19, that still didn’t stop some people from smoking more. Why?
Respondents “might have increased their smoking due to stress and boredom. On the other hand, the fear of catching COVID might have led them to cut down or quit smoking,” said lead author Nancy A. Rigotti, MD. “Even before the pandemic, tobacco smoking was the leading preventable cause of death in the United States. COVID-19 has given smokers yet another good reason to stop smoking.”
This creates an opportunity for physicians to preach the gospel to smokers about their vulnerability to respiratory disease in hopes of getting them to quit for good. We just wish the same could be said for all of our excessive pandemic online shopping.
3,000 years and just one pair of genomes to wear
Men and women are different. We’ll give you a moment to pick your jaw off the ground.
It makes sense though, the sexes being different, especially when you look at the broader animal kingdom. The males and females of many species are slightly different when it comes to size and shape, but there’s a big question that literally only anthropologists have asked: Were human males and females more different in the past than they are today?
To be more specific, some scientists believe that males and females grew more similar when humans shifted from a hunter-gatherer lifestyle to a farming-based lifestyle, as agriculture encouraged a more equitable division of labor. Others believe that the differences come down to random chance.
Researchers from Penn State University analyzed genomic data from over 350,000 males and females stored in the UK Biobank and looked at the recent (within the last ~3,000 years; post-agriculture adoption in Britain) evolutionary histories of these loci. Height, body mass, hip circumference, body fat percentage, and waist circumference were analyzed, and while there were thousands of differences in the genomes, only one trait occurred more frequently during that time period: Females gained a significantly higher body fat content than males.
It’s a sad day then for the millions of people who were big fans of the “farming caused men and women to become more similar” theory. Count the LOTME crew among them. Be honest: Wouldn’t life be so much simpler if men and women were exactly the same? Just think about it, no more arguments about leaving the toilet seat up. It’d be worth it just for that.
Proteins don’t lie
Research published in Open Biology shows that the human brain contains 14,315 different proteins. The team conducting that study wanted to find out which organ was the most similar to the old brain box, so they did protein counts for the 32 other major tissue types, including heart, salivary gland, lung, spleen, and endometrium.
The tissue with the most proteins in common with the center of human intelligence? You’re thinking it has to be colon at this point, right? We were sure it was going to be colon, but it’s not.
The winner, with 13,442 shared proteins, is the testes. The testes have 15,687 proteins, of which 85.7% are shared with the brain. The researchers, sadly, did not provide protein counts for the other tissue types, but we bet colon was a close second.
Dreaming about COVID?
We thought we were the only ones who have been having crazy dreams lately. Each one seems crazier and more vivid than the one before. Have you been having weird dreams lately?
This is likely your brain’s coping mechanism to handle your pandemic stress, according to Dr. Erik Hoel of Tufts University. Dreams that are crazy and scary might make real life seem lighter and simpler. He calls it the “overfitted brain hypothesis.”
“It is their very strangeness that gives them their biological function,” Dr. Hoel said. It literally makes you feel like COVID-19 and lockdowns aren’t as scary as they seem.
We always knew our minds were powerful things. Apparently, your brain gets tired of everyday familiarity just like you do, and it creates crazy dreams to keep things interesting.
Just remember: That recurring dream that you’re back in college and missing 10 assignments is there to help you, not scare you! Even though it is pretty scary.
Pandemic smoking: More or less?
The COVID-19 pandemic has changed a lot of habits in people, for better or worse. Some people may have turned to food and alcohol for comfort, while others started on health kicks to emerge from the ordeal as new people. Well, the same can be said about smokers.
New evidence comes from a survey conducted from May to July 2020 of 694 current and former smokers with an average age of 53 years. All had been hospitalized prior to the pandemic and had previously participated in clinical trials to for smoking cessation in Boston, Nashville, and Pittsburgh hospitals.
Researchers found that 32% of participants smoked more, 37% smoked less, and 31% made no change in their smoking habits. By the time of the survey, 28% of former smokers had relapsed. Although 68% of the participants believed smoking increased the risk of getting COVID-19, that still didn’t stop some people from smoking more. Why?
Respondents “might have increased their smoking due to stress and boredom. On the other hand, the fear of catching COVID might have led them to cut down or quit smoking,” said lead author Nancy A. Rigotti, MD. “Even before the pandemic, tobacco smoking was the leading preventable cause of death in the United States. COVID-19 has given smokers yet another good reason to stop smoking.”
This creates an opportunity for physicians to preach the gospel to smokers about their vulnerability to respiratory disease in hopes of getting them to quit for good. We just wish the same could be said for all of our excessive pandemic online shopping.
3,000 years and just one pair of genomes to wear
Men and women are different. We’ll give you a moment to pick your jaw off the ground.
It makes sense though, the sexes being different, especially when you look at the broader animal kingdom. The males and females of many species are slightly different when it comes to size and shape, but there’s a big question that literally only anthropologists have asked: Were human males and females more different in the past than they are today?
To be more specific, some scientists believe that males and females grew more similar when humans shifted from a hunter-gatherer lifestyle to a farming-based lifestyle, as agriculture encouraged a more equitable division of labor. Others believe that the differences come down to random chance.
Researchers from Penn State University analyzed genomic data from over 350,000 males and females stored in the UK Biobank and looked at the recent (within the last ~3,000 years; post-agriculture adoption in Britain) evolutionary histories of these loci. Height, body mass, hip circumference, body fat percentage, and waist circumference were analyzed, and while there were thousands of differences in the genomes, only one trait occurred more frequently during that time period: Females gained a significantly higher body fat content than males.
It’s a sad day then for the millions of people who were big fans of the “farming caused men and women to become more similar” theory. Count the LOTME crew among them. Be honest: Wouldn’t life be so much simpler if men and women were exactly the same? Just think about it, no more arguments about leaving the toilet seat up. It’d be worth it just for that.
Proteins don’t lie
Research published in Open Biology shows that the human brain contains 14,315 different proteins. The team conducting that study wanted to find out which organ was the most similar to the old brain box, so they did protein counts for the 32 other major tissue types, including heart, salivary gland, lung, spleen, and endometrium.
The tissue with the most proteins in common with the center of human intelligence? You’re thinking it has to be colon at this point, right? We were sure it was going to be colon, but it’s not.
The winner, with 13,442 shared proteins, is the testes. The testes have 15,687 proteins, of which 85.7% are shared with the brain. The researchers, sadly, did not provide protein counts for the other tissue types, but we bet colon was a close second.
Dreaming about COVID?
We thought we were the only ones who have been having crazy dreams lately. Each one seems crazier and more vivid than the one before. Have you been having weird dreams lately?
This is likely your brain’s coping mechanism to handle your pandemic stress, according to Dr. Erik Hoel of Tufts University. Dreams that are crazy and scary might make real life seem lighter and simpler. He calls it the “overfitted brain hypothesis.”
“It is their very strangeness that gives them their biological function,” Dr. Hoel said. It literally makes you feel like COVID-19 and lockdowns aren’t as scary as they seem.
We always knew our minds were powerful things. Apparently, your brain gets tired of everyday familiarity just like you do, and it creates crazy dreams to keep things interesting.
Just remember: That recurring dream that you’re back in college and missing 10 assignments is there to help you, not scare you! Even though it is pretty scary.
Pandemic smoking: More or less?
The COVID-19 pandemic has changed a lot of habits in people, for better or worse. Some people may have turned to food and alcohol for comfort, while others started on health kicks to emerge from the ordeal as new people. Well, the same can be said about smokers.
New evidence comes from a survey conducted from May to July 2020 of 694 current and former smokers with an average age of 53 years. All had been hospitalized prior to the pandemic and had previously participated in clinical trials to for smoking cessation in Boston, Nashville, and Pittsburgh hospitals.
Researchers found that 32% of participants smoked more, 37% smoked less, and 31% made no change in their smoking habits. By the time of the survey, 28% of former smokers had relapsed. Although 68% of the participants believed smoking increased the risk of getting COVID-19, that still didn’t stop some people from smoking more. Why?
Respondents “might have increased their smoking due to stress and boredom. On the other hand, the fear of catching COVID might have led them to cut down or quit smoking,” said lead author Nancy A. Rigotti, MD. “Even before the pandemic, tobacco smoking was the leading preventable cause of death in the United States. COVID-19 has given smokers yet another good reason to stop smoking.”
This creates an opportunity for physicians to preach the gospel to smokers about their vulnerability to respiratory disease in hopes of getting them to quit for good. We just wish the same could be said for all of our excessive pandemic online shopping.
3,000 years and just one pair of genomes to wear
Men and women are different. We’ll give you a moment to pick your jaw off the ground.
It makes sense though, the sexes being different, especially when you look at the broader animal kingdom. The males and females of many species are slightly different when it comes to size and shape, but there’s a big question that literally only anthropologists have asked: Were human males and females more different in the past than they are today?
To be more specific, some scientists believe that males and females grew more similar when humans shifted from a hunter-gatherer lifestyle to a farming-based lifestyle, as agriculture encouraged a more equitable division of labor. Others believe that the differences come down to random chance.
Researchers from Penn State University analyzed genomic data from over 350,000 males and females stored in the UK Biobank and looked at the recent (within the last ~3,000 years; post-agriculture adoption in Britain) evolutionary histories of these loci. Height, body mass, hip circumference, body fat percentage, and waist circumference were analyzed, and while there were thousands of differences in the genomes, only one trait occurred more frequently during that time period: Females gained a significantly higher body fat content than males.
It’s a sad day then for the millions of people who were big fans of the “farming caused men and women to become more similar” theory. Count the LOTME crew among them. Be honest: Wouldn’t life be so much simpler if men and women were exactly the same? Just think about it, no more arguments about leaving the toilet seat up. It’d be worth it just for that.
Proteins don’t lie
Research published in Open Biology shows that the human brain contains 14,315 different proteins. The team conducting that study wanted to find out which organ was the most similar to the old brain box, so they did protein counts for the 32 other major tissue types, including heart, salivary gland, lung, spleen, and endometrium.
The tissue with the most proteins in common with the center of human intelligence? You’re thinking it has to be colon at this point, right? We were sure it was going to be colon, but it’s not.
The winner, with 13,442 shared proteins, is the testes. The testes have 15,687 proteins, of which 85.7% are shared with the brain. The researchers, sadly, did not provide protein counts for the other tissue types, but we bet colon was a close second.
Dreaming about COVID?
We thought we were the only ones who have been having crazy dreams lately. Each one seems crazier and more vivid than the one before. Have you been having weird dreams lately?
This is likely your brain’s coping mechanism to handle your pandemic stress, according to Dr. Erik Hoel of Tufts University. Dreams that are crazy and scary might make real life seem lighter and simpler. He calls it the “overfitted brain hypothesis.”
“It is their very strangeness that gives them their biological function,” Dr. Hoel said. It literally makes you feel like COVID-19 and lockdowns aren’t as scary as they seem.
We always knew our minds were powerful things. Apparently, your brain gets tired of everyday familiarity just like you do, and it creates crazy dreams to keep things interesting.
Just remember: That recurring dream that you’re back in college and missing 10 assignments is there to help you, not scare you! Even though it is pretty scary.
Treating Hepatitis C Virus Reinfection With 8 Weeks of Ledipasvir/Sofosbuvir Achieves Sustained Virologic Response
Three patients reinfected with hepatitis C virus after a sustained virologic response were considered treatment naïve and treated with a short-course direct acting antiviral regimen.
To decrease the incidence and prevalence of hepatitis C virus (HCV) in the United States, hepatology experts, public health officials, and patient advocates agree that linkage to care is essential for treatment of people who inject drugs (PWID). The most recent surveillance report from the Centers for Disease Control and Prevention (CDC) estimates that injection drug use accounts for the transmission of approximately 72% of new HCV infections.1,2
Although recent studies of direct-acting antiviral (DAA) agents have not been designed to investigate the long-term rates of reinfection in this population, various population-based studies in multiple countries have attempted to describe the rate of reinfection for this cohort.3-7 This rate varies widely based on the defined population of PWID, definition of reinfection, and the prevalence of HCV in a given PWID population. However, studies have consistently shown a relatively low historic rate of reinfection, which varies from 1 to 5 per 100 person-years in patients who have ever injected drugs, to 3 to 33 per 100 person-years in patients who continue injection drug use (IDU). Higher rates are found in those who engage in high-risk behaviors such as needle sharing.3-7 Yet, the US opioid crisis is attributable to a recent rise in both overall incidence and reinfections, highlighting the importance of determining the best treatment strategy for those who become reinfected.1
Current HCV guidelines from the American Association for the Study of Liver Diseases AASLD) and Infectious Diseases Society of America (IDSA) encourage access to retreatment for PWID who become reinfected, stating that new reinfections should follow treatment-naïve therapy recommendations.8 However, to date this recommendation has not been validated by published clinical trials or patient case reports. This is likely due in part both to the small number of reinfections among PWID requiring retreatment and barriers to payment for treatment, particularly for individuals with substance use disorders.9 While this recommendation can be found under the key population section for the “Identification and Management of HCV in People Who Inject Drugs,” health care providers (HCPs) may easily miss this statement if they alternatively refer to the “Treatment-Experienced” section that recommends escalation to either sofosbuvir/velpatasvir/voxilaprevir or glecaprevir/pibrentasvir in patients who are NS5A inhibitor DAA-experienced.8 Anecdotally, the first instinct for many HCPs when considering a treatment regimen for a reinfected patient is to refer to treatment-experienced regimen recommendations rather than appreciating the reinfected virus to be treatment naïve.
A treatment-escalation approach could have the consequence of limiting the number of times a patient could undergo treatment on successive reinfections. Additionally, these retreatment regimens often are more expensive, resulting in further cost barriers for payors approving retreatment for individuals with HCV reinfection. In contrast, demonstrating efficacy of a less costly short-course regimen would support increased access to initial and retreatment courses for PWID. The implications of enabling improved access to care is essential in the setting of the ongoing opioid epidemic in the United States.
Given the perspective that the virus should be considered treatment naïve for patients who become reinfected, we describe here 3 cases of patients previously achieving sustained virologic response (SVR) being retreated with the cost-effective 8-week regimen of ledipasvir/sofosbuvir following reinfection.
Case Reports
Case 1
A 59-year-old male presented for his third treatment course for HCV genotype 1a. The patient initially underwent 76 weeks of interferon-based HCV treatment in 2007 and 2008, from which he was determined to have achieved SVR in 24 weeks (SVR24) in April 2009. His viral load remained undetected through February 2010 but subsequently had detectable virus again in 2011 following relapsed use of alcohol, cocaine, and injection drugs. The patient elected to await approval of DAAs and eventually completed an 8-week regimen of ledipasvir/sofosbuvir from May to July 2016, achieving SVR24 in December 2016. The patient’s viral load was rechecked in October 2018 and he was again viremic following recent IDU, suggesting a second reinfection.
In preparation for his third HCV treatment, the patient was included in shared decision making to consider retreating his de novo infection as treatment naïve to provide a briefer (ie, 8 weeks) and more cost-effective treatment given his low likelihood of advanced fibrotic liver disease—his FibroScan score was 6.5 kPa, whereas scores ≥ 12.5 kPa in patients with chronic HCV suggest a higher likelihood of cirrhosis.10 At week 4, the patient’s viral load was undetected, he completed his 8-week regimen of ledipasvir/sofosbuvir as planned and achieved SVR12 (Table). He had reported excellent adherence throughout treatment with assistance of a pill box and validated by a reported pill count.
Case 2
A 32-year-old male presented with HCV genotype 1a. Like case 1, this patient had a low FibroScan score of 4.7 kPa. He was previously infected with genotype 3 and completed a 12-week course of sofosbuvir/velpatasvir in November 2016. He achieved SVR12 as evidenced by an undetected viral load in February 2017 despite questionable adherence throughout and relapsed use of heroin by the end of his regimen. He continued intermittent IDU and presented in October 2018 with a detectable viral load, now with genotype 1a. The patient similarly agreed to undergo an 8-week regimen of ledipasvir/sofosbuvir, considering his de novo infection to be treatment naïve. His viral load at treatment week 3 was quantitatively negative while qualitatively detectable at < 15 U/mL. He completed his treatment course in March 2019 and was determined to have achieved SVR24 in September 2019.
Case 3
A 51-year-old male presented with a history of HCV genotype 1a and a low FibroScan score (4.9 kPa ). The patient was previously infected with genotype 2 and had achieved SVR24 following a 12-week regimen of sofosbuvir/velpatasvir in 2017. The patient subsequently was reinfected with genotype 1a and completed an 8-week course of ledipasvir/sofosbuvir in May 2019. The patient had his SVR12 lab drawn 9 days early and was undetectable at that time. He reported 0 missed doses during treatment and achieved an undetected viral load by treatment week 4.
Discussion
We demonstrate that HCV reinfection after treatment with previous interferon and/or DAA-based regimens can be treated with less costly 8-week treatment regimens. Current guidelines include a statement allowing for reinfected patients to follow initial treatment guidelines, but this statement has previously lacked published evidence and may be overlooked by HCPs who refer to recommendations for treatment-experienced patients. Given the increasing likelihood of HCPs encountering patients who have become reinfected with HCV after achieving SVR from a DAA regimen, further delineation may be needed in the recommendations for treatment-experienced patients to highlight the important nuance of recognizing that reinfections should follow initial treatment guidance.
While all 3 of these cases met criteria for the least costly and simplest 1 pill once daily 8-week regimen of ledipasvir/sofosbuvir, patients requiring retreatment with alternative genotypes or evidence of advanced fibrotic liver disease could benefit from a similar approach of using the least expensive and/or shortest duration regimen for which they meet eligibility. With this approach, coverage could be further expanded to the PWID population to help limit HCV transmission amid the opioid crisis.1
Studies have established that PWID are able to achieve similar SVR efficacy rates similar to that of the general population when treated in the setting of an interdisciplinary treatment team that offers collaborative management of complex psychosocial comorbidities and harm reduction strategies.11,12 These integrative patient-centric strategies may include personalized behavioral health pretreatment evaluations, access to substance use treatment, harm reduction counseling, needle exchange programs, and close follow-up by a case manager.2,13 Current DAA regimens combined with 1 or more of these strategies have demonstrated SVR12 rates of 90 to 95% for initial treatment regimens.11 These high SVR12 rates were even achieved in a recent study in which 74% (76/103) of participants had self-reported IDU within 30 days of HCV treatment start and similar IDU rates throughout treatment.12 A meta-analysis, including real-world studies of DAA treatment outcomes yielded a pooled SVR of 88% (95% CI, 83‐92%) for recent PWID and 91% (95% CI, 88‐95%) for individuals using opiate substitution therapy (OST).14 Additionally, linking PWID with OST also reduces risk for reinfection.14,15
For any patient with detectable HCV after completing the initial DAA regimen, it is important to distinguish between relapse and reinfection. SVR12 is generally synonymous with a clinical cure. Patients with ongoing risk factors posttreatment should continue to have their HCV viral load monitored for evidence of reinfection. Patients without known risk factors may benefit from repeat viral load only if there is clinical concern for reinfection, for example, a rise in liver enzymes.
We have shown that patients with ongoing risk factors who are reinfected can be treated successfully with cost-effective 8-week regimens. For comparison this 8-week regimen of ledipasvir/sofosbuvir has an average wholesale price (AWP) of $28,800, while alternative regimens approved for treatment-naïve patients vary in AWP from $31,680 to $43,200, and regimens approved for retreatment of DAA failures have an AWP as high as $89,712.
An 8-week treatment regimen for both initial and reinfection regimens affords many advantages in medication adherence and both medication and provider resource cost-effectiveness. First, new HCV reinfections are disproportionally younger individuals often with complex psychosocial issues that impact retention in treatment. An 8-week course of treatment can be initiated concurrently with substance abuse treatment programs, including intensive outpatient programs and residential treatment programs that are usually at least 28 days. Many of these programs provide aftercare options that would extend the entire course of treatment. These opportunities afford individuals to receive HCV treatment in a setting that supports medication adherence, sobriety efforts, and education on harm reduction to reduce risk for reinfection.
Finally, statistical models indicate eradication of HCV will require scaling up the treatment of PWID in conjunction with harm reduction strategies such as OST and needle exchange programs.16 In contrast, there are low risks associated with retreatment given these medications are well-tolerated, treatment of PWID lowers the risk of further HCV transmission, and the understanding of these reinfections being treatment naïve disavows concerns of these patients having resistance to regimens that cleared their prior infections. The opportunity to provide retreatment without escalating regimen complexity or cost increases access to care for a vulnerable population while aiding in the eradication of HCV.
1. Centers for Disease Control and Prevention. Viral Hepatitis Surveillance - United States, 2018. Updated August 28, 2020. Accessed May 18, 2021. https://www.cdc.gov/hepatitis/statistics/2018surveillance/HepC.htm 2. Grebely J, Robaeys G, Bruggmann P, et al; International Network for Hepatitis in Substance Users. Recommendations for the management of hepatitis C virus infection among people who inject drugs. Int J Drug Policy. 2015;26(10):1028-1038. doi:10.1016/j.drugpo.2015.07.005
3. Marco A, Esteban JI, Solé C, et al. Hepatitis C virus reinfection among prisoners with sustained virological response after treatment for chronic hepatitis C. J Hepatol. 2013;59(1):45-51. doi:10.1016/j.jhep.2013.03.008
4. Midgard H, Bjøro B, Mæland A, et al. Hepatitis C reinfection after sustained virological response. J Hepatol. 2016;64(5):1020-1026. doi:10.1016/j.jhep.2016.01.001
5. Currie SL, Ryan JC, Tracy D, et al. A prospective study to examine persistent HCV reinfection in injection drug users who have previously cleared the virus [published correction appears in Drug Alcohol Depend. 2008 Jul;96(1-2):192]. Drug Alcohol Depend. 2008;93(1-2):148-154. doi:10.1016/j.drugalcdep.2007.09.011
6. Grady BP, Vanhommerig JW, Schinkel J, et al. Low incidence of reinfection with the hepatitis C virus following treatment in active drug users in Amsterdam. Eur J Gastroenterol Hepatol. 2012;24(11):1302-1307. doi:10.1097/MEG.0b013e32835702a8
7. Grebely J, Pham ST, Matthews GV, et al; ATAHC Study Group. Hepatitis C virus reinfection and superinfection among treated and untreated participants with recent infection. Hepatology. 2012;55(4):1058-1069. doi:10.1002/hep.24754
8. American Association for the Study of Liver Diseases and the Infectious Diseases Society of America. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. Accessed May 26, 2021. https://www.hcvguidelines.org
9. National Viral Hepatitis Roundtable, Center for Health Law and Policy Innovation, Harvard Law School. Hepatitis C: The State of Medicaid Access. 2017 National Summary Report. Updated October 23, 2017. Accessed May 26, 2021. https://hepcstage.wpengine.com/wp-content/uploads/2017/10/State-of-HepC_2017_FINAL.pdf
10. Singh S, Muir AJ, Dieterich DT, Falck-Ytter YT. American Gastroenterological Association Institute technical review on the role of elastography in chronic liver diseases. Gastroenterology. 2017;152(6):1544-1577. doi:10.1053/j.gastro.2017.03.016
11. Dore GJ, Altice F, Litwin AH, et al; C-EDGE CO-STAR Study Group. Elbasvir-grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med. 2016;165(9):625-634. doi:10.7326/M16-0816
12. Grebely J, Dalgard O, Conway B, et al; SIMPLIFY Study Group. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol. 2018;3(3):153-161. doi:10.1016/S2468-1253(17)30404-1
13. Cos TA, Bartholomew TS, Huynh, KJ. Role of behavioral health providers in treating hepatitis C. Professional Psychol Res Pract. 2019;50(4):246–254. doi:10.1037/pro0000243
14. Latham NH, Doyle JS, Palmer AY, et al. Staying hepatitis C negative: a systematic review and meta-analysis of cure and reinfection in people who inject drugs. Liver Int. 2019;39(12):2244-2260. doi:10.1111/liv.14152
15. Platt L, Minozzi S, Reed J, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev. 2017;9(9):CD012021. Published 2017 Sep 18. doi:10.1002/14651858.CD012021.pub2
16. Fraser H, Martin NK, Brummer-Korvenkontio H, et al. Model projections on the impact of HCV treatment in the prevention of HCV transmission among people who inject drugs in Europe. J Hepatol. 2018;68(3):402-411. doi:10.1016/j.jhep.2017.10.010
Three patients reinfected with hepatitis C virus after a sustained virologic response were considered treatment naïve and treated with a short-course direct acting antiviral regimen.
Three patients reinfected with hepatitis C virus after a sustained virologic response were considered treatment naïve and treated with a short-course direct acting antiviral regimen.
To decrease the incidence and prevalence of hepatitis C virus (HCV) in the United States, hepatology experts, public health officials, and patient advocates agree that linkage to care is essential for treatment of people who inject drugs (PWID). The most recent surveillance report from the Centers for Disease Control and Prevention (CDC) estimates that injection drug use accounts for the transmission of approximately 72% of new HCV infections.1,2
Although recent studies of direct-acting antiviral (DAA) agents have not been designed to investigate the long-term rates of reinfection in this population, various population-based studies in multiple countries have attempted to describe the rate of reinfection for this cohort.3-7 This rate varies widely based on the defined population of PWID, definition of reinfection, and the prevalence of HCV in a given PWID population. However, studies have consistently shown a relatively low historic rate of reinfection, which varies from 1 to 5 per 100 person-years in patients who have ever injected drugs, to 3 to 33 per 100 person-years in patients who continue injection drug use (IDU). Higher rates are found in those who engage in high-risk behaviors such as needle sharing.3-7 Yet, the US opioid crisis is attributable to a recent rise in both overall incidence and reinfections, highlighting the importance of determining the best treatment strategy for those who become reinfected.1
Current HCV guidelines from the American Association for the Study of Liver Diseases AASLD) and Infectious Diseases Society of America (IDSA) encourage access to retreatment for PWID who become reinfected, stating that new reinfections should follow treatment-naïve therapy recommendations.8 However, to date this recommendation has not been validated by published clinical trials or patient case reports. This is likely due in part both to the small number of reinfections among PWID requiring retreatment and barriers to payment for treatment, particularly for individuals with substance use disorders.9 While this recommendation can be found under the key population section for the “Identification and Management of HCV in People Who Inject Drugs,” health care providers (HCPs) may easily miss this statement if they alternatively refer to the “Treatment-Experienced” section that recommends escalation to either sofosbuvir/velpatasvir/voxilaprevir or glecaprevir/pibrentasvir in patients who are NS5A inhibitor DAA-experienced.8 Anecdotally, the first instinct for many HCPs when considering a treatment regimen for a reinfected patient is to refer to treatment-experienced regimen recommendations rather than appreciating the reinfected virus to be treatment naïve.
A treatment-escalation approach could have the consequence of limiting the number of times a patient could undergo treatment on successive reinfections. Additionally, these retreatment regimens often are more expensive, resulting in further cost barriers for payors approving retreatment for individuals with HCV reinfection. In contrast, demonstrating efficacy of a less costly short-course regimen would support increased access to initial and retreatment courses for PWID. The implications of enabling improved access to care is essential in the setting of the ongoing opioid epidemic in the United States.
Given the perspective that the virus should be considered treatment naïve for patients who become reinfected, we describe here 3 cases of patients previously achieving sustained virologic response (SVR) being retreated with the cost-effective 8-week regimen of ledipasvir/sofosbuvir following reinfection.
Case Reports
Case 1
A 59-year-old male presented for his third treatment course for HCV genotype 1a. The patient initially underwent 76 weeks of interferon-based HCV treatment in 2007 and 2008, from which he was determined to have achieved SVR in 24 weeks (SVR24) in April 2009. His viral load remained undetected through February 2010 but subsequently had detectable virus again in 2011 following relapsed use of alcohol, cocaine, and injection drugs. The patient elected to await approval of DAAs and eventually completed an 8-week regimen of ledipasvir/sofosbuvir from May to July 2016, achieving SVR24 in December 2016. The patient’s viral load was rechecked in October 2018 and he was again viremic following recent IDU, suggesting a second reinfection.
In preparation for his third HCV treatment, the patient was included in shared decision making to consider retreating his de novo infection as treatment naïve to provide a briefer (ie, 8 weeks) and more cost-effective treatment given his low likelihood of advanced fibrotic liver disease—his FibroScan score was 6.5 kPa, whereas scores ≥ 12.5 kPa in patients with chronic HCV suggest a higher likelihood of cirrhosis.10 At week 4, the patient’s viral load was undetected, he completed his 8-week regimen of ledipasvir/sofosbuvir as planned and achieved SVR12 (Table). He had reported excellent adherence throughout treatment with assistance of a pill box and validated by a reported pill count.
Case 2
A 32-year-old male presented with HCV genotype 1a. Like case 1, this patient had a low FibroScan score of 4.7 kPa. He was previously infected with genotype 3 and completed a 12-week course of sofosbuvir/velpatasvir in November 2016. He achieved SVR12 as evidenced by an undetected viral load in February 2017 despite questionable adherence throughout and relapsed use of heroin by the end of his regimen. He continued intermittent IDU and presented in October 2018 with a detectable viral load, now with genotype 1a. The patient similarly agreed to undergo an 8-week regimen of ledipasvir/sofosbuvir, considering his de novo infection to be treatment naïve. His viral load at treatment week 3 was quantitatively negative while qualitatively detectable at < 15 U/mL. He completed his treatment course in March 2019 and was determined to have achieved SVR24 in September 2019.
Case 3
A 51-year-old male presented with a history of HCV genotype 1a and a low FibroScan score (4.9 kPa ). The patient was previously infected with genotype 2 and had achieved SVR24 following a 12-week regimen of sofosbuvir/velpatasvir in 2017. The patient subsequently was reinfected with genotype 1a and completed an 8-week course of ledipasvir/sofosbuvir in May 2019. The patient had his SVR12 lab drawn 9 days early and was undetectable at that time. He reported 0 missed doses during treatment and achieved an undetected viral load by treatment week 4.
Discussion
We demonstrate that HCV reinfection after treatment with previous interferon and/or DAA-based regimens can be treated with less costly 8-week treatment regimens. Current guidelines include a statement allowing for reinfected patients to follow initial treatment guidelines, but this statement has previously lacked published evidence and may be overlooked by HCPs who refer to recommendations for treatment-experienced patients. Given the increasing likelihood of HCPs encountering patients who have become reinfected with HCV after achieving SVR from a DAA regimen, further delineation may be needed in the recommendations for treatment-experienced patients to highlight the important nuance of recognizing that reinfections should follow initial treatment guidance.
While all 3 of these cases met criteria for the least costly and simplest 1 pill once daily 8-week regimen of ledipasvir/sofosbuvir, patients requiring retreatment with alternative genotypes or evidence of advanced fibrotic liver disease could benefit from a similar approach of using the least expensive and/or shortest duration regimen for which they meet eligibility. With this approach, coverage could be further expanded to the PWID population to help limit HCV transmission amid the opioid crisis.1
Studies have established that PWID are able to achieve similar SVR efficacy rates similar to that of the general population when treated in the setting of an interdisciplinary treatment team that offers collaborative management of complex psychosocial comorbidities and harm reduction strategies.11,12 These integrative patient-centric strategies may include personalized behavioral health pretreatment evaluations, access to substance use treatment, harm reduction counseling, needle exchange programs, and close follow-up by a case manager.2,13 Current DAA regimens combined with 1 or more of these strategies have demonstrated SVR12 rates of 90 to 95% for initial treatment regimens.11 These high SVR12 rates were even achieved in a recent study in which 74% (76/103) of participants had self-reported IDU within 30 days of HCV treatment start and similar IDU rates throughout treatment.12 A meta-analysis, including real-world studies of DAA treatment outcomes yielded a pooled SVR of 88% (95% CI, 83‐92%) for recent PWID and 91% (95% CI, 88‐95%) for individuals using opiate substitution therapy (OST).14 Additionally, linking PWID with OST also reduces risk for reinfection.14,15
For any patient with detectable HCV after completing the initial DAA regimen, it is important to distinguish between relapse and reinfection. SVR12 is generally synonymous with a clinical cure. Patients with ongoing risk factors posttreatment should continue to have their HCV viral load monitored for evidence of reinfection. Patients without known risk factors may benefit from repeat viral load only if there is clinical concern for reinfection, for example, a rise in liver enzymes.
We have shown that patients with ongoing risk factors who are reinfected can be treated successfully with cost-effective 8-week regimens. For comparison this 8-week regimen of ledipasvir/sofosbuvir has an average wholesale price (AWP) of $28,800, while alternative regimens approved for treatment-naïve patients vary in AWP from $31,680 to $43,200, and regimens approved for retreatment of DAA failures have an AWP as high as $89,712.
An 8-week treatment regimen for both initial and reinfection regimens affords many advantages in medication adherence and both medication and provider resource cost-effectiveness. First, new HCV reinfections are disproportionally younger individuals often with complex psychosocial issues that impact retention in treatment. An 8-week course of treatment can be initiated concurrently with substance abuse treatment programs, including intensive outpatient programs and residential treatment programs that are usually at least 28 days. Many of these programs provide aftercare options that would extend the entire course of treatment. These opportunities afford individuals to receive HCV treatment in a setting that supports medication adherence, sobriety efforts, and education on harm reduction to reduce risk for reinfection.
Finally, statistical models indicate eradication of HCV will require scaling up the treatment of PWID in conjunction with harm reduction strategies such as OST and needle exchange programs.16 In contrast, there are low risks associated with retreatment given these medications are well-tolerated, treatment of PWID lowers the risk of further HCV transmission, and the understanding of these reinfections being treatment naïve disavows concerns of these patients having resistance to regimens that cleared their prior infections. The opportunity to provide retreatment without escalating regimen complexity or cost increases access to care for a vulnerable population while aiding in the eradication of HCV.
To decrease the incidence and prevalence of hepatitis C virus (HCV) in the United States, hepatology experts, public health officials, and patient advocates agree that linkage to care is essential for treatment of people who inject drugs (PWID). The most recent surveillance report from the Centers for Disease Control and Prevention (CDC) estimates that injection drug use accounts for the transmission of approximately 72% of new HCV infections.1,2
Although recent studies of direct-acting antiviral (DAA) agents have not been designed to investigate the long-term rates of reinfection in this population, various population-based studies in multiple countries have attempted to describe the rate of reinfection for this cohort.3-7 This rate varies widely based on the defined population of PWID, definition of reinfection, and the prevalence of HCV in a given PWID population. However, studies have consistently shown a relatively low historic rate of reinfection, which varies from 1 to 5 per 100 person-years in patients who have ever injected drugs, to 3 to 33 per 100 person-years in patients who continue injection drug use (IDU). Higher rates are found in those who engage in high-risk behaviors such as needle sharing.3-7 Yet, the US opioid crisis is attributable to a recent rise in both overall incidence and reinfections, highlighting the importance of determining the best treatment strategy for those who become reinfected.1
Current HCV guidelines from the American Association for the Study of Liver Diseases AASLD) and Infectious Diseases Society of America (IDSA) encourage access to retreatment for PWID who become reinfected, stating that new reinfections should follow treatment-naïve therapy recommendations.8 However, to date this recommendation has not been validated by published clinical trials or patient case reports. This is likely due in part both to the small number of reinfections among PWID requiring retreatment and barriers to payment for treatment, particularly for individuals with substance use disorders.9 While this recommendation can be found under the key population section for the “Identification and Management of HCV in People Who Inject Drugs,” health care providers (HCPs) may easily miss this statement if they alternatively refer to the “Treatment-Experienced” section that recommends escalation to either sofosbuvir/velpatasvir/voxilaprevir or glecaprevir/pibrentasvir in patients who are NS5A inhibitor DAA-experienced.8 Anecdotally, the first instinct for many HCPs when considering a treatment regimen for a reinfected patient is to refer to treatment-experienced regimen recommendations rather than appreciating the reinfected virus to be treatment naïve.
A treatment-escalation approach could have the consequence of limiting the number of times a patient could undergo treatment on successive reinfections. Additionally, these retreatment regimens often are more expensive, resulting in further cost barriers for payors approving retreatment for individuals with HCV reinfection. In contrast, demonstrating efficacy of a less costly short-course regimen would support increased access to initial and retreatment courses for PWID. The implications of enabling improved access to care is essential in the setting of the ongoing opioid epidemic in the United States.
Given the perspective that the virus should be considered treatment naïve for patients who become reinfected, we describe here 3 cases of patients previously achieving sustained virologic response (SVR) being retreated with the cost-effective 8-week regimen of ledipasvir/sofosbuvir following reinfection.
Case Reports
Case 1
A 59-year-old male presented for his third treatment course for HCV genotype 1a. The patient initially underwent 76 weeks of interferon-based HCV treatment in 2007 and 2008, from which he was determined to have achieved SVR in 24 weeks (SVR24) in April 2009. His viral load remained undetected through February 2010 but subsequently had detectable virus again in 2011 following relapsed use of alcohol, cocaine, and injection drugs. The patient elected to await approval of DAAs and eventually completed an 8-week regimen of ledipasvir/sofosbuvir from May to July 2016, achieving SVR24 in December 2016. The patient’s viral load was rechecked in October 2018 and he was again viremic following recent IDU, suggesting a second reinfection.
In preparation for his third HCV treatment, the patient was included in shared decision making to consider retreating his de novo infection as treatment naïve to provide a briefer (ie, 8 weeks) and more cost-effective treatment given his low likelihood of advanced fibrotic liver disease—his FibroScan score was 6.5 kPa, whereas scores ≥ 12.5 kPa in patients with chronic HCV suggest a higher likelihood of cirrhosis.10 At week 4, the patient’s viral load was undetected, he completed his 8-week regimen of ledipasvir/sofosbuvir as planned and achieved SVR12 (Table). He had reported excellent adherence throughout treatment with assistance of a pill box and validated by a reported pill count.
Case 2
A 32-year-old male presented with HCV genotype 1a. Like case 1, this patient had a low FibroScan score of 4.7 kPa. He was previously infected with genotype 3 and completed a 12-week course of sofosbuvir/velpatasvir in November 2016. He achieved SVR12 as evidenced by an undetected viral load in February 2017 despite questionable adherence throughout and relapsed use of heroin by the end of his regimen. He continued intermittent IDU and presented in October 2018 with a detectable viral load, now with genotype 1a. The patient similarly agreed to undergo an 8-week regimen of ledipasvir/sofosbuvir, considering his de novo infection to be treatment naïve. His viral load at treatment week 3 was quantitatively negative while qualitatively detectable at < 15 U/mL. He completed his treatment course in March 2019 and was determined to have achieved SVR24 in September 2019.
Case 3
A 51-year-old male presented with a history of HCV genotype 1a and a low FibroScan score (4.9 kPa ). The patient was previously infected with genotype 2 and had achieved SVR24 following a 12-week regimen of sofosbuvir/velpatasvir in 2017. The patient subsequently was reinfected with genotype 1a and completed an 8-week course of ledipasvir/sofosbuvir in May 2019. The patient had his SVR12 lab drawn 9 days early and was undetectable at that time. He reported 0 missed doses during treatment and achieved an undetected viral load by treatment week 4.
Discussion
We demonstrate that HCV reinfection after treatment with previous interferon and/or DAA-based regimens can be treated with less costly 8-week treatment regimens. Current guidelines include a statement allowing for reinfected patients to follow initial treatment guidelines, but this statement has previously lacked published evidence and may be overlooked by HCPs who refer to recommendations for treatment-experienced patients. Given the increasing likelihood of HCPs encountering patients who have become reinfected with HCV after achieving SVR from a DAA regimen, further delineation may be needed in the recommendations for treatment-experienced patients to highlight the important nuance of recognizing that reinfections should follow initial treatment guidance.
While all 3 of these cases met criteria for the least costly and simplest 1 pill once daily 8-week regimen of ledipasvir/sofosbuvir, patients requiring retreatment with alternative genotypes or evidence of advanced fibrotic liver disease could benefit from a similar approach of using the least expensive and/or shortest duration regimen for which they meet eligibility. With this approach, coverage could be further expanded to the PWID population to help limit HCV transmission amid the opioid crisis.1
Studies have established that PWID are able to achieve similar SVR efficacy rates similar to that of the general population when treated in the setting of an interdisciplinary treatment team that offers collaborative management of complex psychosocial comorbidities and harm reduction strategies.11,12 These integrative patient-centric strategies may include personalized behavioral health pretreatment evaluations, access to substance use treatment, harm reduction counseling, needle exchange programs, and close follow-up by a case manager.2,13 Current DAA regimens combined with 1 or more of these strategies have demonstrated SVR12 rates of 90 to 95% for initial treatment regimens.11 These high SVR12 rates were even achieved in a recent study in which 74% (76/103) of participants had self-reported IDU within 30 days of HCV treatment start and similar IDU rates throughout treatment.12 A meta-analysis, including real-world studies of DAA treatment outcomes yielded a pooled SVR of 88% (95% CI, 83‐92%) for recent PWID and 91% (95% CI, 88‐95%) for individuals using opiate substitution therapy (OST).14 Additionally, linking PWID with OST also reduces risk for reinfection.14,15
For any patient with detectable HCV after completing the initial DAA regimen, it is important to distinguish between relapse and reinfection. SVR12 is generally synonymous with a clinical cure. Patients with ongoing risk factors posttreatment should continue to have their HCV viral load monitored for evidence of reinfection. Patients without known risk factors may benefit from repeat viral load only if there is clinical concern for reinfection, for example, a rise in liver enzymes.
We have shown that patients with ongoing risk factors who are reinfected can be treated successfully with cost-effective 8-week regimens. For comparison this 8-week regimen of ledipasvir/sofosbuvir has an average wholesale price (AWP) of $28,800, while alternative regimens approved for treatment-naïve patients vary in AWP from $31,680 to $43,200, and regimens approved for retreatment of DAA failures have an AWP as high as $89,712.
An 8-week treatment regimen for both initial and reinfection regimens affords many advantages in medication adherence and both medication and provider resource cost-effectiveness. First, new HCV reinfections are disproportionally younger individuals often with complex psychosocial issues that impact retention in treatment. An 8-week course of treatment can be initiated concurrently with substance abuse treatment programs, including intensive outpatient programs and residential treatment programs that are usually at least 28 days. Many of these programs provide aftercare options that would extend the entire course of treatment. These opportunities afford individuals to receive HCV treatment in a setting that supports medication adherence, sobriety efforts, and education on harm reduction to reduce risk for reinfection.
Finally, statistical models indicate eradication of HCV will require scaling up the treatment of PWID in conjunction with harm reduction strategies such as OST and needle exchange programs.16 In contrast, there are low risks associated with retreatment given these medications are well-tolerated, treatment of PWID lowers the risk of further HCV transmission, and the understanding of these reinfections being treatment naïve disavows concerns of these patients having resistance to regimens that cleared their prior infections. The opportunity to provide retreatment without escalating regimen complexity or cost increases access to care for a vulnerable population while aiding in the eradication of HCV.
1. Centers for Disease Control and Prevention. Viral Hepatitis Surveillance - United States, 2018. Updated August 28, 2020. Accessed May 18, 2021. https://www.cdc.gov/hepatitis/statistics/2018surveillance/HepC.htm 2. Grebely J, Robaeys G, Bruggmann P, et al; International Network for Hepatitis in Substance Users. Recommendations for the management of hepatitis C virus infection among people who inject drugs. Int J Drug Policy. 2015;26(10):1028-1038. doi:10.1016/j.drugpo.2015.07.005
3. Marco A, Esteban JI, Solé C, et al. Hepatitis C virus reinfection among prisoners with sustained virological response after treatment for chronic hepatitis C. J Hepatol. 2013;59(1):45-51. doi:10.1016/j.jhep.2013.03.008
4. Midgard H, Bjøro B, Mæland A, et al. Hepatitis C reinfection after sustained virological response. J Hepatol. 2016;64(5):1020-1026. doi:10.1016/j.jhep.2016.01.001
5. Currie SL, Ryan JC, Tracy D, et al. A prospective study to examine persistent HCV reinfection in injection drug users who have previously cleared the virus [published correction appears in Drug Alcohol Depend. 2008 Jul;96(1-2):192]. Drug Alcohol Depend. 2008;93(1-2):148-154. doi:10.1016/j.drugalcdep.2007.09.011
6. Grady BP, Vanhommerig JW, Schinkel J, et al. Low incidence of reinfection with the hepatitis C virus following treatment in active drug users in Amsterdam. Eur J Gastroenterol Hepatol. 2012;24(11):1302-1307. doi:10.1097/MEG.0b013e32835702a8
7. Grebely J, Pham ST, Matthews GV, et al; ATAHC Study Group. Hepatitis C virus reinfection and superinfection among treated and untreated participants with recent infection. Hepatology. 2012;55(4):1058-1069. doi:10.1002/hep.24754
8. American Association for the Study of Liver Diseases and the Infectious Diseases Society of America. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. Accessed May 26, 2021. https://www.hcvguidelines.org
9. National Viral Hepatitis Roundtable, Center for Health Law and Policy Innovation, Harvard Law School. Hepatitis C: The State of Medicaid Access. 2017 National Summary Report. Updated October 23, 2017. Accessed May 26, 2021. https://hepcstage.wpengine.com/wp-content/uploads/2017/10/State-of-HepC_2017_FINAL.pdf
10. Singh S, Muir AJ, Dieterich DT, Falck-Ytter YT. American Gastroenterological Association Institute technical review on the role of elastography in chronic liver diseases. Gastroenterology. 2017;152(6):1544-1577. doi:10.1053/j.gastro.2017.03.016
11. Dore GJ, Altice F, Litwin AH, et al; C-EDGE CO-STAR Study Group. Elbasvir-grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med. 2016;165(9):625-634. doi:10.7326/M16-0816
12. Grebely J, Dalgard O, Conway B, et al; SIMPLIFY Study Group. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol. 2018;3(3):153-161. doi:10.1016/S2468-1253(17)30404-1
13. Cos TA, Bartholomew TS, Huynh, KJ. Role of behavioral health providers in treating hepatitis C. Professional Psychol Res Pract. 2019;50(4):246–254. doi:10.1037/pro0000243
14. Latham NH, Doyle JS, Palmer AY, et al. Staying hepatitis C negative: a systematic review and meta-analysis of cure and reinfection in people who inject drugs. Liver Int. 2019;39(12):2244-2260. doi:10.1111/liv.14152
15. Platt L, Minozzi S, Reed J, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev. 2017;9(9):CD012021. Published 2017 Sep 18. doi:10.1002/14651858.CD012021.pub2
16. Fraser H, Martin NK, Brummer-Korvenkontio H, et al. Model projections on the impact of HCV treatment in the prevention of HCV transmission among people who inject drugs in Europe. J Hepatol. 2018;68(3):402-411. doi:10.1016/j.jhep.2017.10.010
1. Centers for Disease Control and Prevention. Viral Hepatitis Surveillance - United States, 2018. Updated August 28, 2020. Accessed May 18, 2021. https://www.cdc.gov/hepatitis/statistics/2018surveillance/HepC.htm 2. Grebely J, Robaeys G, Bruggmann P, et al; International Network for Hepatitis in Substance Users. Recommendations for the management of hepatitis C virus infection among people who inject drugs. Int J Drug Policy. 2015;26(10):1028-1038. doi:10.1016/j.drugpo.2015.07.005
3. Marco A, Esteban JI, Solé C, et al. Hepatitis C virus reinfection among prisoners with sustained virological response after treatment for chronic hepatitis C. J Hepatol. 2013;59(1):45-51. doi:10.1016/j.jhep.2013.03.008
4. Midgard H, Bjøro B, Mæland A, et al. Hepatitis C reinfection after sustained virological response. J Hepatol. 2016;64(5):1020-1026. doi:10.1016/j.jhep.2016.01.001
5. Currie SL, Ryan JC, Tracy D, et al. A prospective study to examine persistent HCV reinfection in injection drug users who have previously cleared the virus [published correction appears in Drug Alcohol Depend. 2008 Jul;96(1-2):192]. Drug Alcohol Depend. 2008;93(1-2):148-154. doi:10.1016/j.drugalcdep.2007.09.011
6. Grady BP, Vanhommerig JW, Schinkel J, et al. Low incidence of reinfection with the hepatitis C virus following treatment in active drug users in Amsterdam. Eur J Gastroenterol Hepatol. 2012;24(11):1302-1307. doi:10.1097/MEG.0b013e32835702a8
7. Grebely J, Pham ST, Matthews GV, et al; ATAHC Study Group. Hepatitis C virus reinfection and superinfection among treated and untreated participants with recent infection. Hepatology. 2012;55(4):1058-1069. doi:10.1002/hep.24754
8. American Association for the Study of Liver Diseases and the Infectious Diseases Society of America. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. Accessed May 26, 2021. https://www.hcvguidelines.org
9. National Viral Hepatitis Roundtable, Center for Health Law and Policy Innovation, Harvard Law School. Hepatitis C: The State of Medicaid Access. 2017 National Summary Report. Updated October 23, 2017. Accessed May 26, 2021. https://hepcstage.wpengine.com/wp-content/uploads/2017/10/State-of-HepC_2017_FINAL.pdf
10. Singh S, Muir AJ, Dieterich DT, Falck-Ytter YT. American Gastroenterological Association Institute technical review on the role of elastography in chronic liver diseases. Gastroenterology. 2017;152(6):1544-1577. doi:10.1053/j.gastro.2017.03.016
11. Dore GJ, Altice F, Litwin AH, et al; C-EDGE CO-STAR Study Group. Elbasvir-grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med. 2016;165(9):625-634. doi:10.7326/M16-0816
12. Grebely J, Dalgard O, Conway B, et al; SIMPLIFY Study Group. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol. 2018;3(3):153-161. doi:10.1016/S2468-1253(17)30404-1
13. Cos TA, Bartholomew TS, Huynh, KJ. Role of behavioral health providers in treating hepatitis C. Professional Psychol Res Pract. 2019;50(4):246–254. doi:10.1037/pro0000243
14. Latham NH, Doyle JS, Palmer AY, et al. Staying hepatitis C negative: a systematic review and meta-analysis of cure and reinfection in people who inject drugs. Liver Int. 2019;39(12):2244-2260. doi:10.1111/liv.14152
15. Platt L, Minozzi S, Reed J, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev. 2017;9(9):CD012021. Published 2017 Sep 18. doi:10.1002/14651858.CD012021.pub2
16. Fraser H, Martin NK, Brummer-Korvenkontio H, et al. Model projections on the impact of HCV treatment in the prevention of HCV transmission among people who inject drugs in Europe. J Hepatol. 2018;68(3):402-411. doi:10.1016/j.jhep.2017.10.010
Audit and Feedback: A Quality Improvement Study to Improve Antimicrobial Stewardship
Antibiotics are commonly overused for several viral respiratory conditions where antibiotic treatment is not clinically indicated. For example, a 2016 study by Fleming-Dutra and colleagues showed that at least 30% of all antibiotics prescribed in an outpatient setting were inappropriate and for acute bronchitis, antibiotic prescriptions were inappropriate in 50% of cases.1 Acute bronchitis is predominantly a viral illness where antibiotics should be rarely used.2-8 The Healthcare Effectiveness Data and Information Set has measured the avoidance of antibiotic treatment in adults with acute bronchitis since 2006. The National Committee for Quality Assurance reported in 2018 that about 75% of adults received antibiotics for acute bronchitis.9 Inappropriate antibiotic use contributes to antimicrobial resistance, resulting in the increase of morbidity and mortality of treatable infections.10 Reducing inappropriate antibiotic use in outpatient settings is a high-priority public health issue and is a Healthy People 2030 objective.11
Antimicrobial Stewardship
Antimicrobial stewardship programs measure and track how antibiotics are prescribed by health care providers (HCPs) and used by patients. The Centers for Disease Control and Prevention (CDC) created a framework for outpatient antimicrobial stewardship programs by outlining 4 core elements: (1) commitment from every person involved in patient care to act as an antibiotic steward; (2) policies and interventions to promote appropriate antibiotic prescribing practices; (3) antibiotic prescription tracking and reporting; and (4) appropriate antibiotic use education.12
Audit and feedback (A&F) is a form of antibiotic prescription tracking and reporting that involves measuring and comparing a HCP’s performance (ie, antibiotic prescribing) with a standard, and the results of this audit are shared with the HCP. This strategy is based on the belief that a HCP is motivated to modify practice habits when given feedback showing that his or her performance is inconsistent with targeted expectations. A&F is most effective when feedback is provided by a supervisor or respected peer, presented more than once, individualized, delivered in both verbal and written formats, and includes explicit targets and an action plan.13,14
This study focuses on an antimicrobial stewardship program implemented in an outpatient Indian Health Service ambulatory care clinic in the Pacific Northwest. The clinic was staffed by 9 HCPs serving about 12,000 American Indian and Alaskan Native patients. The clinic includes a full-service pharmacy where nearly all prescriptions issued by in-house HCPs are filled. The clinic’s antibiotic prescribing rate for adult patients with acute bronchitis was similar to the national mean in 2018 (75%).9 The study objective was to reduce the rate of potentially inappropriate (not guideline-concordant) antibiotic prescribing in patients with acute bronchitis without underlying chronic lung disease or evidence of bacterial infection through A&F.
Methods
The antimicrobial stewardship program was implemented by 3 pharmacists, including a pharmacy resident. HCPs received education by pharmacy staff on evidence-based prescribing for adult acute bronchitis and quarterly feedback on antibiotic prescribing rates. All prescribing and dispensing records necessary for the program were available in the clinic electronic health record. The rate of potentially inappropriate antibiotic prescribing was calculated as the proportion of eligible bronchitis cases who received antibiotics.
In October 2018, a 60-minute educational session was provided by 2 pharmacists to HCPs. The material covered an overview of acute bronchitis presentation, diagnosis, treatment (Table 1), and a comparison of national and local prescribing data (baseline audit).2-4 The educational session concluded with prescription strategies to reduce inappropriate antibiotic prescribing, including but not limited to: delayed prescriptions, patient and caregiver education, use of nonantibiotic medications to control symptoms, and use of A&F reports.5-8 At the conclusion of the session, HCPs committed to engage in the antimicrobial stewardship program.
Audit
To determine the total number of eligible bronchitis cases (denominator), a visit report was generated by a pharmacist for a primary diagnosis of acute bronchitis using International Statistical Classification of Diseases, Tenth Revision (ICD 10) codes (J20.3 - J20.9) for the review period. Only adults aged ≥ 18 years were included. Patients with a chronic lung disease (eg, chronic obstructive pulmonary disease, asthma) and those who had a concomitant bacterial infection (eg, urinary tract infection, cellulitis) were excluded. A visit for acute bronchitis that included additional ICD 10 codes indicating the patient had a chronic lung disease or concomitant bacterial infection were used to determine exclusion. The remaining patients who received a potentially inappropriate antibiotic prescription (numerator) were those who were prescribed or dispensed antibiotics on the date of service.
Feedback
Baseline data were presented to HCPs during the educational session in October 2018. Prospective audits were performed quarterly thereafter (January, April, and July) by the pharmacy resident using the criteria described above. Audit data were compiled into personalized reports and provided to HCPs by the pharmacy resident with written and verbal individual feedback. Written feedback was sent by email to each HCP containing the HCP’s rate, the clinic rate in aggregate, rates from the prior year and quarter(s) for comparison, and clinical pearls from the guidelines (Figure). Verbal feedback included a review of the written feedback and answering any questions concerning the report.
Implementation
Study periods were chosen to coincide with the pharmacy residency training year, which starts in July and ends in June. The start date of October 2018 differed from the start of the residency year (July 2018) owing to delays in obtaining permissions. A&F and analysis of prescribing rates continued through the end of the residency year, for total duration of 9 months (October 1, 2018 to June 30, 2019). For ease of reporting, quarterly reports followed the federal government’s fiscal year (FY) which runs from October 1 of the prior calendar year through September 30 of the year being described. HCPs received 4 feedback reports: baseline (October 1, 2018 - June 30, 2018) in October 2018, quarter 1 (October 1, 2018 - December 31, 2018) in January 2019, quarter 2 (January 1, 2019 - March 31, 2019) in April 2019, and quarter 3 (April 1, 2019 - June 30, 2019) in July 2019.
Statistical Analysis
Prescribing rates were compared between identical 9 -month periods. A 2-sample binomial test for proportions was used to derive an approximate CI of prescribing rates at the patient level. However, to account for clustering of patients within HCP panels and dependence of observations over study periods stemming from examining the same HCPs within each of the periods, the Wilcoxon signed rank test for paired data was used to evaluate prescribing rates at the HCP level. Statistical analysis was performed using R statistical software version 4.0.3. Differences were considered significant at P < .05 set a priori.
This study was approved by the Portland Area Indian Health Service Institutional Review Board (Study ID: 1316730).
Results
All 9 HCPs who see adult patients at the clinic agreed to participate and were all fully present in each study period. Among HCPs, there were 5 physicians and 4 physician assistants or nurse practitioners. There was a total of 213 visits that met study criteria during the baseline period (October 1, 2017 to June 30, 2018) and 177 visits in the posteducation period (October 1, 2018 to June 30, 2019). The total number of acute bronchitis encounters varied by HCP (Ranges, 5-63 [baseline] and 2-57 [posteducation]); however, the relative number of encounters each HCP contributed was similar in each study period (Table 2). The pharmacy resident spent about 2 hours each quarter to generate 9 feedback reports, 1 for each HCP.
Antibiotic Prescribing
Antibiotic prescribing rates decreased from 75% at baseline to 60% at posteducation month 9 (absolute difference, -15% [95% CI, 5 - 24%]; P ≤ .01) (Table 3). The clinic rate was lower for each quarter in FY 2019 (posteducation) compared with the same quarter of FY 2018 (baseline), with the lowest rate observed in the final quarter of the study. Comparing pre- and post- A&F, the rates for HCPs prescribing antibiotics were lower for 7 HCPs, unchanged for 1 HCP, and slightly increased for 1 HCP(P = .02).
Discussion
Acute bronchitis remains a common diagnosis where antibiotics are prescribed despite being a predominately viral illness. Guidelines and evidence-based practices advise against antibiotics for this diagnosis. According to the American Academy of Family Physicians, antibiotics are reserved for cases where chronic lung disease is present as these patients are at a high risk of developing pneumonia.3 The decision to prescribe antibiotics is complex and driven by several interdependent factors, such as patient expectations, health system limitations, clinician training, and specialty.15 HCPs may more aggressively treat acute bronchitis among American Indian/Alaskan Native (AI/AN) people due to a high risk of developing serious complications from respiratory illnesses.16 A clinician’s background, usual patient cohort (ie, mostly pediatric or geriatric), and time spent in urgent care or in activities outside of patient care (administration) may account for the difference in patient encounters by HCP for acute bronchitis.
Following the CDC framework, this antimicrobial stewardship program helped empower people involved in patient care (eg, pharmacists, HCPs), educate staff on proper use of antibiotics for acute bronchitis, and track and report antibiotic prescribing through the A&F process. Educational interventions coupled with ongoing A&F are reproducible by other health care facilities and are not usually time consuming. This study showcases a successful example of implementing A&F in an antimicrobial stewardship quality improvement project that could be translated toward other conditions (eg, sinusitis, urinary tract infection, community-acquired pneumonia).
In a similar study, Meeker and colleagues used a variation of an A&F intervention using a monthly email showing peer comparisons to notify clinicians who were prescribing too many unnecessary antibiotics for common respiratory illnesses that did not require antibiotics, such as the common cold.17 The peer comparison intervention arm emailed a rank order that listed prescribers by the number of prescriptions for common respiratory illnesses. This intervention demonstrated a reduction of 5.2% in inappropriate antibiotic prescribing.
Limitations
This quality improvement study had several limitations. The study did not account for the duration of symptoms as a factor to judge appropriateness. Although this was identified early in the study, it was unavoidable since there was no report that could extract the duration of symptoms in the electronic health record. Future studies should consider a manual review of each encounter to overcome this limitation. Another limitation was that only three-quarters of the year and not the entire year were reviewed. Future studies should include longer time frames to measure the durability of changes to antibiotic prescriptions. Lastly, the study did not assess diagnosis shifting (the practice of changing the proportion of antibiotic-appropriate acute respiratory tract infection diagnosis over time), effects of patient demographics (patient age and sex were not recorded), or any sustained effect on prescribing rates after the study ended.
Conclusions
Clinician education coupled with A&F are components of the CDC’s framework for an effective antimicrobial stewardship program. The intervention seem to be an effective means toward reducing inappropriate antibiotic prescribing for acute bronchitis and has the potential for application to other antimicrobial stewardship initiatives. The present study adds to the growing body of evidence on the importance and impact an antimicrobial stewardship program has on a clinic or health system.
Acknowledgment
The results of this study have been reported at the 2019 IHS Southwest Regional Pharmacy Continuing Education Seminar, April 12-14, 2019.
1. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315(17):1864-1873. doi:10.1001/jama.2016.4151
2. Barnett ML, Linder JA. Antibiotic prescribing for adults with acute bronchitis in the United States, 1996-2010. JAMA. 2014;311(19):2020-2022. doi:10.1001/jama.2013.286141
3. Kinkade S, Long NA. Acute bronchitis. Am Fam Physician. 2016;94(7):560-565.
4. Harris AM, Hicks LA, Qaseem A; High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164(6):425-434. doi:10.7326/M15-1840
5. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med. 2001;134(6):521-529. doi:10.7326/0003-4819-134-6-200103200-00021
6. Centers for Disease Control and Prevention. Adult outpatient treatment recommendations. Updated October 3, 2017. Accessed May 19, 2021. www.cdc.gov/antibiotic-use/community/for-hcp/outpatient-hcp/adult-treatment-rec.html
7. Braman SS. Chronic cough due to chronic bronchitis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 suppl):104S-115S. doi:10.1378/chest.129.1_suppl.104S
8. Petersen I, Johnson AM, Islam A, Duckworth G, Livermore DM, Hayward AC. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ. 2007;335(7627):982. doi:10.1136/bmj.39345.405243.BE
9. National Committee for Quality Assurance. Avoidance of antibiotic treatment in adults with acute bronchitis (AAB). Accessed May 19, 2021. https://www.ncqa.org/hedis/measures/avoidance-of-antibiotic-treatment-in-adults-with-acute-bronchitis
10. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Published April 23, 2013. Accessed May 19, 2021. https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf
11. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2030: reduce inappropriate antibiotic use in outpatient settings — HAI‑D01. Accessed May 19, 2021. https://health.gov/healthypeople/objectives-and-data/browse-objectives/healthcare-associated-infections/reduce-inappropriate-antibiotic-use-outpatient-settings-hai-d01
12. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1-12. Published 2016 Nov 11. doi:10.15585/mmwr.rr6506a1
13. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259. Published 2012 Jun 13. doi:10.1002/14651858.CD000259.pub3
14. Ivers NM, Grimshaw JM, Jamtvedt G, et al. Growing literature, stagnant science? Systematic review, meta-regression and cumulative analysis of audit and feedback interventions in health care. J Gen Intern Med. 2014;29(11):1534-1541. doi:10.1007/s11606-014-2913-y
15. Ranji SR, Steinman MA, Shojania KG, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Vol. 4: Antibiotic Prescribing Behavior. Agency for Healthcare Research and Quality (US); 2006. Accessed May 20, 2021. https://www.ncbi.nlm.nih.gov/books/NBK43956/
16. Groom AV, Hennessy TW, Singleton RJ, Butler JC, Holve S, Cheek JE. Pneumonia and influenza mortality among American Indian and Alaska Native people, 1990-2009. Am J Public Health. 2014;104 Suppl 3(suppl 3):S460-S469. doi:10.2105/AJPH.2013.301740
17. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315(6):562-570. doi:10.1001/jama.2016.0275
Antibiotics are commonly overused for several viral respiratory conditions where antibiotic treatment is not clinically indicated. For example, a 2016 study by Fleming-Dutra and colleagues showed that at least 30% of all antibiotics prescribed in an outpatient setting were inappropriate and for acute bronchitis, antibiotic prescriptions were inappropriate in 50% of cases.1 Acute bronchitis is predominantly a viral illness where antibiotics should be rarely used.2-8 The Healthcare Effectiveness Data and Information Set has measured the avoidance of antibiotic treatment in adults with acute bronchitis since 2006. The National Committee for Quality Assurance reported in 2018 that about 75% of adults received antibiotics for acute bronchitis.9 Inappropriate antibiotic use contributes to antimicrobial resistance, resulting in the increase of morbidity and mortality of treatable infections.10 Reducing inappropriate antibiotic use in outpatient settings is a high-priority public health issue and is a Healthy People 2030 objective.11
Antimicrobial Stewardship
Antimicrobial stewardship programs measure and track how antibiotics are prescribed by health care providers (HCPs) and used by patients. The Centers for Disease Control and Prevention (CDC) created a framework for outpatient antimicrobial stewardship programs by outlining 4 core elements: (1) commitment from every person involved in patient care to act as an antibiotic steward; (2) policies and interventions to promote appropriate antibiotic prescribing practices; (3) antibiotic prescription tracking and reporting; and (4) appropriate antibiotic use education.12
Audit and feedback (A&F) is a form of antibiotic prescription tracking and reporting that involves measuring and comparing a HCP’s performance (ie, antibiotic prescribing) with a standard, and the results of this audit are shared with the HCP. This strategy is based on the belief that a HCP is motivated to modify practice habits when given feedback showing that his or her performance is inconsistent with targeted expectations. A&F is most effective when feedback is provided by a supervisor or respected peer, presented more than once, individualized, delivered in both verbal and written formats, and includes explicit targets and an action plan.13,14
This study focuses on an antimicrobial stewardship program implemented in an outpatient Indian Health Service ambulatory care clinic in the Pacific Northwest. The clinic was staffed by 9 HCPs serving about 12,000 American Indian and Alaskan Native patients. The clinic includes a full-service pharmacy where nearly all prescriptions issued by in-house HCPs are filled. The clinic’s antibiotic prescribing rate for adult patients with acute bronchitis was similar to the national mean in 2018 (75%).9 The study objective was to reduce the rate of potentially inappropriate (not guideline-concordant) antibiotic prescribing in patients with acute bronchitis without underlying chronic lung disease or evidence of bacterial infection through A&F.
Methods
The antimicrobial stewardship program was implemented by 3 pharmacists, including a pharmacy resident. HCPs received education by pharmacy staff on evidence-based prescribing for adult acute bronchitis and quarterly feedback on antibiotic prescribing rates. All prescribing and dispensing records necessary for the program were available in the clinic electronic health record. The rate of potentially inappropriate antibiotic prescribing was calculated as the proportion of eligible bronchitis cases who received antibiotics.
In October 2018, a 60-minute educational session was provided by 2 pharmacists to HCPs. The material covered an overview of acute bronchitis presentation, diagnosis, treatment (Table 1), and a comparison of national and local prescribing data (baseline audit).2-4 The educational session concluded with prescription strategies to reduce inappropriate antibiotic prescribing, including but not limited to: delayed prescriptions, patient and caregiver education, use of nonantibiotic medications to control symptoms, and use of A&F reports.5-8 At the conclusion of the session, HCPs committed to engage in the antimicrobial stewardship program.
Audit
To determine the total number of eligible bronchitis cases (denominator), a visit report was generated by a pharmacist for a primary diagnosis of acute bronchitis using International Statistical Classification of Diseases, Tenth Revision (ICD 10) codes (J20.3 - J20.9) for the review period. Only adults aged ≥ 18 years were included. Patients with a chronic lung disease (eg, chronic obstructive pulmonary disease, asthma) and those who had a concomitant bacterial infection (eg, urinary tract infection, cellulitis) were excluded. A visit for acute bronchitis that included additional ICD 10 codes indicating the patient had a chronic lung disease or concomitant bacterial infection were used to determine exclusion. The remaining patients who received a potentially inappropriate antibiotic prescription (numerator) were those who were prescribed or dispensed antibiotics on the date of service.
Feedback
Baseline data were presented to HCPs during the educational session in October 2018. Prospective audits were performed quarterly thereafter (January, April, and July) by the pharmacy resident using the criteria described above. Audit data were compiled into personalized reports and provided to HCPs by the pharmacy resident with written and verbal individual feedback. Written feedback was sent by email to each HCP containing the HCP’s rate, the clinic rate in aggregate, rates from the prior year and quarter(s) for comparison, and clinical pearls from the guidelines (Figure). Verbal feedback included a review of the written feedback and answering any questions concerning the report.
Implementation
Study periods were chosen to coincide with the pharmacy residency training year, which starts in July and ends in June. The start date of October 2018 differed from the start of the residency year (July 2018) owing to delays in obtaining permissions. A&F and analysis of prescribing rates continued through the end of the residency year, for total duration of 9 months (October 1, 2018 to June 30, 2019). For ease of reporting, quarterly reports followed the federal government’s fiscal year (FY) which runs from October 1 of the prior calendar year through September 30 of the year being described. HCPs received 4 feedback reports: baseline (October 1, 2018 - June 30, 2018) in October 2018, quarter 1 (October 1, 2018 - December 31, 2018) in January 2019, quarter 2 (January 1, 2019 - March 31, 2019) in April 2019, and quarter 3 (April 1, 2019 - June 30, 2019) in July 2019.
Statistical Analysis
Prescribing rates were compared between identical 9 -month periods. A 2-sample binomial test for proportions was used to derive an approximate CI of prescribing rates at the patient level. However, to account for clustering of patients within HCP panels and dependence of observations over study periods stemming from examining the same HCPs within each of the periods, the Wilcoxon signed rank test for paired data was used to evaluate prescribing rates at the HCP level. Statistical analysis was performed using R statistical software version 4.0.3. Differences were considered significant at P < .05 set a priori.
This study was approved by the Portland Area Indian Health Service Institutional Review Board (Study ID: 1316730).
Results
All 9 HCPs who see adult patients at the clinic agreed to participate and were all fully present in each study period. Among HCPs, there were 5 physicians and 4 physician assistants or nurse practitioners. There was a total of 213 visits that met study criteria during the baseline period (October 1, 2017 to June 30, 2018) and 177 visits in the posteducation period (October 1, 2018 to June 30, 2019). The total number of acute bronchitis encounters varied by HCP (Ranges, 5-63 [baseline] and 2-57 [posteducation]); however, the relative number of encounters each HCP contributed was similar in each study period (Table 2). The pharmacy resident spent about 2 hours each quarter to generate 9 feedback reports, 1 for each HCP.
Antibiotic Prescribing
Antibiotic prescribing rates decreased from 75% at baseline to 60% at posteducation month 9 (absolute difference, -15% [95% CI, 5 - 24%]; P ≤ .01) (Table 3). The clinic rate was lower for each quarter in FY 2019 (posteducation) compared with the same quarter of FY 2018 (baseline), with the lowest rate observed in the final quarter of the study. Comparing pre- and post- A&F, the rates for HCPs prescribing antibiotics were lower for 7 HCPs, unchanged for 1 HCP, and slightly increased for 1 HCP(P = .02).
Discussion
Acute bronchitis remains a common diagnosis where antibiotics are prescribed despite being a predominately viral illness. Guidelines and evidence-based practices advise against antibiotics for this diagnosis. According to the American Academy of Family Physicians, antibiotics are reserved for cases where chronic lung disease is present as these patients are at a high risk of developing pneumonia.3 The decision to prescribe antibiotics is complex and driven by several interdependent factors, such as patient expectations, health system limitations, clinician training, and specialty.15 HCPs may more aggressively treat acute bronchitis among American Indian/Alaskan Native (AI/AN) people due to a high risk of developing serious complications from respiratory illnesses.16 A clinician’s background, usual patient cohort (ie, mostly pediatric or geriatric), and time spent in urgent care or in activities outside of patient care (administration) may account for the difference in patient encounters by HCP for acute bronchitis.
Following the CDC framework, this antimicrobial stewardship program helped empower people involved in patient care (eg, pharmacists, HCPs), educate staff on proper use of antibiotics for acute bronchitis, and track and report antibiotic prescribing through the A&F process. Educational interventions coupled with ongoing A&F are reproducible by other health care facilities and are not usually time consuming. This study showcases a successful example of implementing A&F in an antimicrobial stewardship quality improvement project that could be translated toward other conditions (eg, sinusitis, urinary tract infection, community-acquired pneumonia).
In a similar study, Meeker and colleagues used a variation of an A&F intervention using a monthly email showing peer comparisons to notify clinicians who were prescribing too many unnecessary antibiotics for common respiratory illnesses that did not require antibiotics, such as the common cold.17 The peer comparison intervention arm emailed a rank order that listed prescribers by the number of prescriptions for common respiratory illnesses. This intervention demonstrated a reduction of 5.2% in inappropriate antibiotic prescribing.
Limitations
This quality improvement study had several limitations. The study did not account for the duration of symptoms as a factor to judge appropriateness. Although this was identified early in the study, it was unavoidable since there was no report that could extract the duration of symptoms in the electronic health record. Future studies should consider a manual review of each encounter to overcome this limitation. Another limitation was that only three-quarters of the year and not the entire year were reviewed. Future studies should include longer time frames to measure the durability of changes to antibiotic prescriptions. Lastly, the study did not assess diagnosis shifting (the practice of changing the proportion of antibiotic-appropriate acute respiratory tract infection diagnosis over time), effects of patient demographics (patient age and sex were not recorded), or any sustained effect on prescribing rates after the study ended.
Conclusions
Clinician education coupled with A&F are components of the CDC’s framework for an effective antimicrobial stewardship program. The intervention seem to be an effective means toward reducing inappropriate antibiotic prescribing for acute bronchitis and has the potential for application to other antimicrobial stewardship initiatives. The present study adds to the growing body of evidence on the importance and impact an antimicrobial stewardship program has on a clinic or health system.
Acknowledgment
The results of this study have been reported at the 2019 IHS Southwest Regional Pharmacy Continuing Education Seminar, April 12-14, 2019.
Antibiotics are commonly overused for several viral respiratory conditions where antibiotic treatment is not clinically indicated. For example, a 2016 study by Fleming-Dutra and colleagues showed that at least 30% of all antibiotics prescribed in an outpatient setting were inappropriate and for acute bronchitis, antibiotic prescriptions were inappropriate in 50% of cases.1 Acute bronchitis is predominantly a viral illness where antibiotics should be rarely used.2-8 The Healthcare Effectiveness Data and Information Set has measured the avoidance of antibiotic treatment in adults with acute bronchitis since 2006. The National Committee for Quality Assurance reported in 2018 that about 75% of adults received antibiotics for acute bronchitis.9 Inappropriate antibiotic use contributes to antimicrobial resistance, resulting in the increase of morbidity and mortality of treatable infections.10 Reducing inappropriate antibiotic use in outpatient settings is a high-priority public health issue and is a Healthy People 2030 objective.11
Antimicrobial Stewardship
Antimicrobial stewardship programs measure and track how antibiotics are prescribed by health care providers (HCPs) and used by patients. The Centers for Disease Control and Prevention (CDC) created a framework for outpatient antimicrobial stewardship programs by outlining 4 core elements: (1) commitment from every person involved in patient care to act as an antibiotic steward; (2) policies and interventions to promote appropriate antibiotic prescribing practices; (3) antibiotic prescription tracking and reporting; and (4) appropriate antibiotic use education.12
Audit and feedback (A&F) is a form of antibiotic prescription tracking and reporting that involves measuring and comparing a HCP’s performance (ie, antibiotic prescribing) with a standard, and the results of this audit are shared with the HCP. This strategy is based on the belief that a HCP is motivated to modify practice habits when given feedback showing that his or her performance is inconsistent with targeted expectations. A&F is most effective when feedback is provided by a supervisor or respected peer, presented more than once, individualized, delivered in both verbal and written formats, and includes explicit targets and an action plan.13,14
This study focuses on an antimicrobial stewardship program implemented in an outpatient Indian Health Service ambulatory care clinic in the Pacific Northwest. The clinic was staffed by 9 HCPs serving about 12,000 American Indian and Alaskan Native patients. The clinic includes a full-service pharmacy where nearly all prescriptions issued by in-house HCPs are filled. The clinic’s antibiotic prescribing rate for adult patients with acute bronchitis was similar to the national mean in 2018 (75%).9 The study objective was to reduce the rate of potentially inappropriate (not guideline-concordant) antibiotic prescribing in patients with acute bronchitis without underlying chronic lung disease or evidence of bacterial infection through A&F.
Methods
The antimicrobial stewardship program was implemented by 3 pharmacists, including a pharmacy resident. HCPs received education by pharmacy staff on evidence-based prescribing for adult acute bronchitis and quarterly feedback on antibiotic prescribing rates. All prescribing and dispensing records necessary for the program were available in the clinic electronic health record. The rate of potentially inappropriate antibiotic prescribing was calculated as the proportion of eligible bronchitis cases who received antibiotics.
In October 2018, a 60-minute educational session was provided by 2 pharmacists to HCPs. The material covered an overview of acute bronchitis presentation, diagnosis, treatment (Table 1), and a comparison of national and local prescribing data (baseline audit).2-4 The educational session concluded with prescription strategies to reduce inappropriate antibiotic prescribing, including but not limited to: delayed prescriptions, patient and caregiver education, use of nonantibiotic medications to control symptoms, and use of A&F reports.5-8 At the conclusion of the session, HCPs committed to engage in the antimicrobial stewardship program.
Audit
To determine the total number of eligible bronchitis cases (denominator), a visit report was generated by a pharmacist for a primary diagnosis of acute bronchitis using International Statistical Classification of Diseases, Tenth Revision (ICD 10) codes (J20.3 - J20.9) for the review period. Only adults aged ≥ 18 years were included. Patients with a chronic lung disease (eg, chronic obstructive pulmonary disease, asthma) and those who had a concomitant bacterial infection (eg, urinary tract infection, cellulitis) were excluded. A visit for acute bronchitis that included additional ICD 10 codes indicating the patient had a chronic lung disease or concomitant bacterial infection were used to determine exclusion. The remaining patients who received a potentially inappropriate antibiotic prescription (numerator) were those who were prescribed or dispensed antibiotics on the date of service.
Feedback
Baseline data were presented to HCPs during the educational session in October 2018. Prospective audits were performed quarterly thereafter (January, April, and July) by the pharmacy resident using the criteria described above. Audit data were compiled into personalized reports and provided to HCPs by the pharmacy resident with written and verbal individual feedback. Written feedback was sent by email to each HCP containing the HCP’s rate, the clinic rate in aggregate, rates from the prior year and quarter(s) for comparison, and clinical pearls from the guidelines (Figure). Verbal feedback included a review of the written feedback and answering any questions concerning the report.
Implementation
Study periods were chosen to coincide with the pharmacy residency training year, which starts in July and ends in June. The start date of October 2018 differed from the start of the residency year (July 2018) owing to delays in obtaining permissions. A&F and analysis of prescribing rates continued through the end of the residency year, for total duration of 9 months (October 1, 2018 to June 30, 2019). For ease of reporting, quarterly reports followed the federal government’s fiscal year (FY) which runs from October 1 of the prior calendar year through September 30 of the year being described. HCPs received 4 feedback reports: baseline (October 1, 2018 - June 30, 2018) in October 2018, quarter 1 (October 1, 2018 - December 31, 2018) in January 2019, quarter 2 (January 1, 2019 - March 31, 2019) in April 2019, and quarter 3 (April 1, 2019 - June 30, 2019) in July 2019.
Statistical Analysis
Prescribing rates were compared between identical 9 -month periods. A 2-sample binomial test for proportions was used to derive an approximate CI of prescribing rates at the patient level. However, to account for clustering of patients within HCP panels and dependence of observations over study periods stemming from examining the same HCPs within each of the periods, the Wilcoxon signed rank test for paired data was used to evaluate prescribing rates at the HCP level. Statistical analysis was performed using R statistical software version 4.0.3. Differences were considered significant at P < .05 set a priori.
This study was approved by the Portland Area Indian Health Service Institutional Review Board (Study ID: 1316730).
Results
All 9 HCPs who see adult patients at the clinic agreed to participate and were all fully present in each study period. Among HCPs, there were 5 physicians and 4 physician assistants or nurse practitioners. There was a total of 213 visits that met study criteria during the baseline period (October 1, 2017 to June 30, 2018) and 177 visits in the posteducation period (October 1, 2018 to June 30, 2019). The total number of acute bronchitis encounters varied by HCP (Ranges, 5-63 [baseline] and 2-57 [posteducation]); however, the relative number of encounters each HCP contributed was similar in each study period (Table 2). The pharmacy resident spent about 2 hours each quarter to generate 9 feedback reports, 1 for each HCP.
Antibiotic Prescribing
Antibiotic prescribing rates decreased from 75% at baseline to 60% at posteducation month 9 (absolute difference, -15% [95% CI, 5 - 24%]; P ≤ .01) (Table 3). The clinic rate was lower for each quarter in FY 2019 (posteducation) compared with the same quarter of FY 2018 (baseline), with the lowest rate observed in the final quarter of the study. Comparing pre- and post- A&F, the rates for HCPs prescribing antibiotics were lower for 7 HCPs, unchanged for 1 HCP, and slightly increased for 1 HCP(P = .02).
Discussion
Acute bronchitis remains a common diagnosis where antibiotics are prescribed despite being a predominately viral illness. Guidelines and evidence-based practices advise against antibiotics for this diagnosis. According to the American Academy of Family Physicians, antibiotics are reserved for cases where chronic lung disease is present as these patients are at a high risk of developing pneumonia.3 The decision to prescribe antibiotics is complex and driven by several interdependent factors, such as patient expectations, health system limitations, clinician training, and specialty.15 HCPs may more aggressively treat acute bronchitis among American Indian/Alaskan Native (AI/AN) people due to a high risk of developing serious complications from respiratory illnesses.16 A clinician’s background, usual patient cohort (ie, mostly pediatric or geriatric), and time spent in urgent care or in activities outside of patient care (administration) may account for the difference in patient encounters by HCP for acute bronchitis.
Following the CDC framework, this antimicrobial stewardship program helped empower people involved in patient care (eg, pharmacists, HCPs), educate staff on proper use of antibiotics for acute bronchitis, and track and report antibiotic prescribing through the A&F process. Educational interventions coupled with ongoing A&F are reproducible by other health care facilities and are not usually time consuming. This study showcases a successful example of implementing A&F in an antimicrobial stewardship quality improvement project that could be translated toward other conditions (eg, sinusitis, urinary tract infection, community-acquired pneumonia).
In a similar study, Meeker and colleagues used a variation of an A&F intervention using a monthly email showing peer comparisons to notify clinicians who were prescribing too many unnecessary antibiotics for common respiratory illnesses that did not require antibiotics, such as the common cold.17 The peer comparison intervention arm emailed a rank order that listed prescribers by the number of prescriptions for common respiratory illnesses. This intervention demonstrated a reduction of 5.2% in inappropriate antibiotic prescribing.
Limitations
This quality improvement study had several limitations. The study did not account for the duration of symptoms as a factor to judge appropriateness. Although this was identified early in the study, it was unavoidable since there was no report that could extract the duration of symptoms in the electronic health record. Future studies should consider a manual review of each encounter to overcome this limitation. Another limitation was that only three-quarters of the year and not the entire year were reviewed. Future studies should include longer time frames to measure the durability of changes to antibiotic prescriptions. Lastly, the study did not assess diagnosis shifting (the practice of changing the proportion of antibiotic-appropriate acute respiratory tract infection diagnosis over time), effects of patient demographics (patient age and sex were not recorded), or any sustained effect on prescribing rates after the study ended.
Conclusions
Clinician education coupled with A&F are components of the CDC’s framework for an effective antimicrobial stewardship program. The intervention seem to be an effective means toward reducing inappropriate antibiotic prescribing for acute bronchitis and has the potential for application to other antimicrobial stewardship initiatives. The present study adds to the growing body of evidence on the importance and impact an antimicrobial stewardship program has on a clinic or health system.
Acknowledgment
The results of this study have been reported at the 2019 IHS Southwest Regional Pharmacy Continuing Education Seminar, April 12-14, 2019.
1. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315(17):1864-1873. doi:10.1001/jama.2016.4151
2. Barnett ML, Linder JA. Antibiotic prescribing for adults with acute bronchitis in the United States, 1996-2010. JAMA. 2014;311(19):2020-2022. doi:10.1001/jama.2013.286141
3. Kinkade S, Long NA. Acute bronchitis. Am Fam Physician. 2016;94(7):560-565.
4. Harris AM, Hicks LA, Qaseem A; High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164(6):425-434. doi:10.7326/M15-1840
5. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med. 2001;134(6):521-529. doi:10.7326/0003-4819-134-6-200103200-00021
6. Centers for Disease Control and Prevention. Adult outpatient treatment recommendations. Updated October 3, 2017. Accessed May 19, 2021. www.cdc.gov/antibiotic-use/community/for-hcp/outpatient-hcp/adult-treatment-rec.html
7. Braman SS. Chronic cough due to chronic bronchitis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 suppl):104S-115S. doi:10.1378/chest.129.1_suppl.104S
8. Petersen I, Johnson AM, Islam A, Duckworth G, Livermore DM, Hayward AC. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ. 2007;335(7627):982. doi:10.1136/bmj.39345.405243.BE
9. National Committee for Quality Assurance. Avoidance of antibiotic treatment in adults with acute bronchitis (AAB). Accessed May 19, 2021. https://www.ncqa.org/hedis/measures/avoidance-of-antibiotic-treatment-in-adults-with-acute-bronchitis
10. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Published April 23, 2013. Accessed May 19, 2021. https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf
11. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2030: reduce inappropriate antibiotic use in outpatient settings — HAI‑D01. Accessed May 19, 2021. https://health.gov/healthypeople/objectives-and-data/browse-objectives/healthcare-associated-infections/reduce-inappropriate-antibiotic-use-outpatient-settings-hai-d01
12. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1-12. Published 2016 Nov 11. doi:10.15585/mmwr.rr6506a1
13. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259. Published 2012 Jun 13. doi:10.1002/14651858.CD000259.pub3
14. Ivers NM, Grimshaw JM, Jamtvedt G, et al. Growing literature, stagnant science? Systematic review, meta-regression and cumulative analysis of audit and feedback interventions in health care. J Gen Intern Med. 2014;29(11):1534-1541. doi:10.1007/s11606-014-2913-y
15. Ranji SR, Steinman MA, Shojania KG, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Vol. 4: Antibiotic Prescribing Behavior. Agency for Healthcare Research and Quality (US); 2006. Accessed May 20, 2021. https://www.ncbi.nlm.nih.gov/books/NBK43956/
16. Groom AV, Hennessy TW, Singleton RJ, Butler JC, Holve S, Cheek JE. Pneumonia and influenza mortality among American Indian and Alaska Native people, 1990-2009. Am J Public Health. 2014;104 Suppl 3(suppl 3):S460-S469. doi:10.2105/AJPH.2013.301740
17. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315(6):562-570. doi:10.1001/jama.2016.0275
1. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315(17):1864-1873. doi:10.1001/jama.2016.4151
2. Barnett ML, Linder JA. Antibiotic prescribing for adults with acute bronchitis in the United States, 1996-2010. JAMA. 2014;311(19):2020-2022. doi:10.1001/jama.2013.286141
3. Kinkade S, Long NA. Acute bronchitis. Am Fam Physician. 2016;94(7):560-565.
4. Harris AM, Hicks LA, Qaseem A; High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164(6):425-434. doi:10.7326/M15-1840
5. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med. 2001;134(6):521-529. doi:10.7326/0003-4819-134-6-200103200-00021
6. Centers for Disease Control and Prevention. Adult outpatient treatment recommendations. Updated October 3, 2017. Accessed May 19, 2021. www.cdc.gov/antibiotic-use/community/for-hcp/outpatient-hcp/adult-treatment-rec.html
7. Braman SS. Chronic cough due to chronic bronchitis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 suppl):104S-115S. doi:10.1378/chest.129.1_suppl.104S
8. Petersen I, Johnson AM, Islam A, Duckworth G, Livermore DM, Hayward AC. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ. 2007;335(7627):982. doi:10.1136/bmj.39345.405243.BE
9. National Committee for Quality Assurance. Avoidance of antibiotic treatment in adults with acute bronchitis (AAB). Accessed May 19, 2021. https://www.ncqa.org/hedis/measures/avoidance-of-antibiotic-treatment-in-adults-with-acute-bronchitis
10. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Published April 23, 2013. Accessed May 19, 2021. https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf
11. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2030: reduce inappropriate antibiotic use in outpatient settings — HAI‑D01. Accessed May 19, 2021. https://health.gov/healthypeople/objectives-and-data/browse-objectives/healthcare-associated-infections/reduce-inappropriate-antibiotic-use-outpatient-settings-hai-d01
12. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1-12. Published 2016 Nov 11. doi:10.15585/mmwr.rr6506a1
13. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259. Published 2012 Jun 13. doi:10.1002/14651858.CD000259.pub3
14. Ivers NM, Grimshaw JM, Jamtvedt G, et al. Growing literature, stagnant science? Systematic review, meta-regression and cumulative analysis of audit and feedback interventions in health care. J Gen Intern Med. 2014;29(11):1534-1541. doi:10.1007/s11606-014-2913-y
15. Ranji SR, Steinman MA, Shojania KG, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Vol. 4: Antibiotic Prescribing Behavior. Agency for Healthcare Research and Quality (US); 2006. Accessed May 20, 2021. https://www.ncbi.nlm.nih.gov/books/NBK43956/
16. Groom AV, Hennessy TW, Singleton RJ, Butler JC, Holve S, Cheek JE. Pneumonia and influenza mortality among American Indian and Alaska Native people, 1990-2009. Am J Public Health. 2014;104 Suppl 3(suppl 3):S460-S469. doi:10.2105/AJPH.2013.301740
17. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315(6):562-570. doi:10.1001/jama.2016.0275
By the numbers: Children and COVID-19 prevention
Over 6.3 million doses of COVID-19 vaccine have been administered to children aged 12-17 years as of June 7, according to data from the Centers for Disease Control and Prevention.
The latest results from the CDC’s COVID Data Tracker show that , with the corresponding figures for vaccine completion coming in at 4.1% and 26.4%. Compared with a week earlier, those numbers are up by 15.4% (one dose) and 486% (completion) for the younger group and by 4.7% and 8.6%, respectively, for the older children.
Children aged 12-15 represented 17.9% of all persons who initiated vaccination in the last 14 days up to June 7, while children aged 16-17 made up 4.8% of vaccine initiation over that period. The 25- to 39-year-olds, at 23.7% of all vaccine initiators, were the only group ahead of those aged 12-15, and the 50- to 64-year-olds were just behind at 17.7%, the CDC data show.
Both groups of children were on the low side, however, when it came to vaccine completion in the last 14 days, with those aged 12-15 at 6.7% of the total and those aged 16-17 years at 4.3%. The only age groups lower than that were ≥75 at 3.5% and <12 at 0.2%, and the highest share of vaccine completion was 26.0% for those aged 25-39, which also happens to be the group with the largest share of the U.S. population (20.5%), the CDC said.
People considered fully vaccinated are those who have received the second dose of a two-dose series or one dose of a single-shot vaccine, but children under age 18 years are eligible only for the Pfizer-BioNTech version, the CDC noted.
Meanwhile, back on the incidence side of the COVID-19 pandemic, the number of new cases in U.S. children for the week ending June 3 was at its lowest point (16,281) since mid-June of 2020, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
Cases among children now total 3.99 million, which represents 14.1% of cases among all ages, a proportion that hasn’t increased since mid-May, which hasn’t happened since the two groups started keeping track in mid-April of 2020 in the 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam that report such data by age.
Less encouraging was the CDC’s report that “COVID-19-associated hospitalization rates among adolescents ages 12-17 years increased during March and April, following declines in January and February 2021.”
Children have been experiencing much lower rates of severe disease than those of adults throughout the pandemic, the CDC pointed out, but “recent increases in COVID-19-associated hospitalization rates and the potential for severe disease in adolescents reinforce the importance of continued prevention strategies, including vaccination and the correct and consistent use of masks in those who are not yet fully vaccinated.”
Over 6.3 million doses of COVID-19 vaccine have been administered to children aged 12-17 years as of June 7, according to data from the Centers for Disease Control and Prevention.

The latest results from the CDC’s COVID Data Tracker show that , with the corresponding figures for vaccine completion coming in at 4.1% and 26.4%. Compared with a week earlier, those numbers are up by 15.4% (one dose) and 486% (completion) for the younger group and by 4.7% and 8.6%, respectively, for the older children.
Children aged 12-15 represented 17.9% of all persons who initiated vaccination in the last 14 days up to June 7, while children aged 16-17 made up 4.8% of vaccine initiation over that period. The 25- to 39-year-olds, at 23.7% of all vaccine initiators, were the only group ahead of those aged 12-15, and the 50- to 64-year-olds were just behind at 17.7%, the CDC data show.
Both groups of children were on the low side, however, when it came to vaccine completion in the last 14 days, with those aged 12-15 at 6.7% of the total and those aged 16-17 years at 4.3%. The only age groups lower than that were ≥75 at 3.5% and <12 at 0.2%, and the highest share of vaccine completion was 26.0% for those aged 25-39, which also happens to be the group with the largest share of the U.S. population (20.5%), the CDC said.
People considered fully vaccinated are those who have received the second dose of a two-dose series or one dose of a single-shot vaccine, but children under age 18 years are eligible only for the Pfizer-BioNTech version, the CDC noted.
Meanwhile, back on the incidence side of the COVID-19 pandemic, the number of new cases in U.S. children for the week ending June 3 was at its lowest point (16,281) since mid-June of 2020, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
Cases among children now total 3.99 million, which represents 14.1% of cases among all ages, a proportion that hasn’t increased since mid-May, which hasn’t happened since the two groups started keeping track in mid-April of 2020 in the 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam that report such data by age.
Less encouraging was the CDC’s report that “COVID-19-associated hospitalization rates among adolescents ages 12-17 years increased during March and April, following declines in January and February 2021.”
Children have been experiencing much lower rates of severe disease than those of adults throughout the pandemic, the CDC pointed out, but “recent increases in COVID-19-associated hospitalization rates and the potential for severe disease in adolescents reinforce the importance of continued prevention strategies, including vaccination and the correct and consistent use of masks in those who are not yet fully vaccinated.”
Over 6.3 million doses of COVID-19 vaccine have been administered to children aged 12-17 years as of June 7, according to data from the Centers for Disease Control and Prevention.

The latest results from the CDC’s COVID Data Tracker show that , with the corresponding figures for vaccine completion coming in at 4.1% and 26.4%. Compared with a week earlier, those numbers are up by 15.4% (one dose) and 486% (completion) for the younger group and by 4.7% and 8.6%, respectively, for the older children.
Children aged 12-15 represented 17.9% of all persons who initiated vaccination in the last 14 days up to June 7, while children aged 16-17 made up 4.8% of vaccine initiation over that period. The 25- to 39-year-olds, at 23.7% of all vaccine initiators, were the only group ahead of those aged 12-15, and the 50- to 64-year-olds were just behind at 17.7%, the CDC data show.
Both groups of children were on the low side, however, when it came to vaccine completion in the last 14 days, with those aged 12-15 at 6.7% of the total and those aged 16-17 years at 4.3%. The only age groups lower than that were ≥75 at 3.5% and <12 at 0.2%, and the highest share of vaccine completion was 26.0% for those aged 25-39, which also happens to be the group with the largest share of the U.S. population (20.5%), the CDC said.
People considered fully vaccinated are those who have received the second dose of a two-dose series or one dose of a single-shot vaccine, but children under age 18 years are eligible only for the Pfizer-BioNTech version, the CDC noted.
Meanwhile, back on the incidence side of the COVID-19 pandemic, the number of new cases in U.S. children for the week ending June 3 was at its lowest point (16,281) since mid-June of 2020, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
Cases among children now total 3.99 million, which represents 14.1% of cases among all ages, a proportion that hasn’t increased since mid-May, which hasn’t happened since the two groups started keeping track in mid-April of 2020 in the 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam that report such data by age.
Less encouraging was the CDC’s report that “COVID-19-associated hospitalization rates among adolescents ages 12-17 years increased during March and April, following declines in January and February 2021.”
Children have been experiencing much lower rates of severe disease than those of adults throughout the pandemic, the CDC pointed out, but “recent increases in COVID-19-associated hospitalization rates and the potential for severe disease in adolescents reinforce the importance of continued prevention strategies, including vaccination and the correct and consistent use of masks in those who are not yet fully vaccinated.”
NIAID advances universal flu vaccine candidate into phase 1 trial
Last month, U.S. government researchers began a test of an experimental influenza vaccine that they hope will provide long-lasting immunity against multiple strains of the virus. Their project adds to the many approaches that have been tried in the decades-long quest for a universal flu shot.
For the first time, the National Institute of Allergy and Infectious Diseases (NIAID) is testing an investigational flu vaccine, known as FluMos-v1, on people. Researchers in recent years have targeted the stalk or stem of an influenza surface protein called hemagglutinin (HA) in trying to develop better flu vaccines. NIAID said FluMos-v1 is designed to spark production of antibodies against the HA protein from different virus strains, which could make it superior to vaccines now available, NIAID said.
“It could be longer lasting than the traditional flu vaccine and give us what we call super seasonal protection that might go beyond just one flu season to next year’s or the year after, or offer additional protection in a pandemic setting,” Alicia T. Widge, MD, of NIAID’s Vaccine Research Center, who is the principal investigator of the trial, said in an interview.
The phase 1 study (NCT04896086) aims to enroll 35 participants, 15 of whom will receive a single intramuscular injection of a comparator treatment, Flucelvax, which has already been approved by the U.S. Food and Drug Administration. The FluMos-v1 group will start with five participants who will receive one 20-μg dose. If no safety problems emerge at that dosage, another 15 volunteers will receive one 60-μg dose of the investigational vaccine.
The incorporation of a comparator group in the phase 1 study may help investigators get an early idea of how well FluMos-v1 compares to a marketed product, Dr. Widge said. The test will be carried out through the National Institutes of Health Clinical Center.
‘Renaissance’ of flu-vaccine research?
Currently, flu vaccines are reformulated each year in an attempt to match the dominant strain for the upcoming season, an effort that often falls notably short. The estimated vaccine effectiveness rate in the United States has ranged from a low of 19% to a high of 60% in recent years, according to the Centers for Disease Control and Prevention.
Scientists have been working for decades on a universal flu vaccine that would offer better results but haven’t yet identified the right strategy to outwit mutations in the virus. Recent setbacks include BiondVax Pharmaceuticals’ October 2020 announcement of a failed phase 3 trial of its experimental M-001 universal flu vaccine candidate.
But advances in understanding the immune system may set the stage for a “renaissance” in efforts to develop a universal flu vaccine, Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, said in an interview.
The COVID-19 pandemic has spurred greater interest in the need to develop a universal flu vaccine, he said. Dr. Osterholm said he is “more optimistic now than ever” about the chances for developing vaccines that can fend off multiple strains over longer periods, although the goal of a shot that can ward off influenza in all cases may remain elusive.
“How good can we make them? Will they ever be really universal? Will they have long periods of protection? I don’t think any of us know that yet,” Dr. Osterholm said. “But this is not the influenza vaccine world of 5 or 7 years ago.”
The mRNA technology used to develop the world’s first approved COVID-19 vaccines, for example, may be applied against influenza, Dr. Osterholm said.
In January 2021, Moderna announced plans to test three development candidates for a seasonal influenza vaccine and aims to start a phase 1 study this year. In an April interview on CNBC’s Squawk Box program, Moderna’s chief executive, Stephané Bancel, spoke about the company’s plans to eventually create a combination vaccine for SARS-Cov-2 and flu viruses.
SARS-CoV-2 “is not going away.” Like flu, this virus will persist and change forms, Ms. Bancel said. Creating a flu shot that outperforms the existing ones would boost confidence in influenza vaccines, which many people now skip, Ms. Bancel said. People might someday be able to get a combination of this more effective flu shot with a COVID-19 vaccine booster in their local pharmacies.
“You can take one dose and then have a nice winter,” Ms. Bancel said of Moderna’s goal for a combination vaccine.
A version of this article first appeared on Medscape.com.
Last month, U.S. government researchers began a test of an experimental influenza vaccine that they hope will provide long-lasting immunity against multiple strains of the virus. Their project adds to the many approaches that have been tried in the decades-long quest for a universal flu shot.
For the first time, the National Institute of Allergy and Infectious Diseases (NIAID) is testing an investigational flu vaccine, known as FluMos-v1, on people. Researchers in recent years have targeted the stalk or stem of an influenza surface protein called hemagglutinin (HA) in trying to develop better flu vaccines. NIAID said FluMos-v1 is designed to spark production of antibodies against the HA protein from different virus strains, which could make it superior to vaccines now available, NIAID said.
“It could be longer lasting than the traditional flu vaccine and give us what we call super seasonal protection that might go beyond just one flu season to next year’s or the year after, or offer additional protection in a pandemic setting,” Alicia T. Widge, MD, of NIAID’s Vaccine Research Center, who is the principal investigator of the trial, said in an interview.
The phase 1 study (NCT04896086) aims to enroll 35 participants, 15 of whom will receive a single intramuscular injection of a comparator treatment, Flucelvax, which has already been approved by the U.S. Food and Drug Administration. The FluMos-v1 group will start with five participants who will receive one 20-μg dose. If no safety problems emerge at that dosage, another 15 volunteers will receive one 60-μg dose of the investigational vaccine.
The incorporation of a comparator group in the phase 1 study may help investigators get an early idea of how well FluMos-v1 compares to a marketed product, Dr. Widge said. The test will be carried out through the National Institutes of Health Clinical Center.
‘Renaissance’ of flu-vaccine research?
Currently, flu vaccines are reformulated each year in an attempt to match the dominant strain for the upcoming season, an effort that often falls notably short. The estimated vaccine effectiveness rate in the United States has ranged from a low of 19% to a high of 60% in recent years, according to the Centers for Disease Control and Prevention.
Scientists have been working for decades on a universal flu vaccine that would offer better results but haven’t yet identified the right strategy to outwit mutations in the virus. Recent setbacks include BiondVax Pharmaceuticals’ October 2020 announcement of a failed phase 3 trial of its experimental M-001 universal flu vaccine candidate.
But advances in understanding the immune system may set the stage for a “renaissance” in efforts to develop a universal flu vaccine, Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, said in an interview.
The COVID-19 pandemic has spurred greater interest in the need to develop a universal flu vaccine, he said. Dr. Osterholm said he is “more optimistic now than ever” about the chances for developing vaccines that can fend off multiple strains over longer periods, although the goal of a shot that can ward off influenza in all cases may remain elusive.
“How good can we make them? Will they ever be really universal? Will they have long periods of protection? I don’t think any of us know that yet,” Dr. Osterholm said. “But this is not the influenza vaccine world of 5 or 7 years ago.”
The mRNA technology used to develop the world’s first approved COVID-19 vaccines, for example, may be applied against influenza, Dr. Osterholm said.
In January 2021, Moderna announced plans to test three development candidates for a seasonal influenza vaccine and aims to start a phase 1 study this year. In an April interview on CNBC’s Squawk Box program, Moderna’s chief executive, Stephané Bancel, spoke about the company’s plans to eventually create a combination vaccine for SARS-Cov-2 and flu viruses.
SARS-CoV-2 “is not going away.” Like flu, this virus will persist and change forms, Ms. Bancel said. Creating a flu shot that outperforms the existing ones would boost confidence in influenza vaccines, which many people now skip, Ms. Bancel said. People might someday be able to get a combination of this more effective flu shot with a COVID-19 vaccine booster in their local pharmacies.
“You can take one dose and then have a nice winter,” Ms. Bancel said of Moderna’s goal for a combination vaccine.
A version of this article first appeared on Medscape.com.
Last month, U.S. government researchers began a test of an experimental influenza vaccine that they hope will provide long-lasting immunity against multiple strains of the virus. Their project adds to the many approaches that have been tried in the decades-long quest for a universal flu shot.
For the first time, the National Institute of Allergy and Infectious Diseases (NIAID) is testing an investigational flu vaccine, known as FluMos-v1, on people. Researchers in recent years have targeted the stalk or stem of an influenza surface protein called hemagglutinin (HA) in trying to develop better flu vaccines. NIAID said FluMos-v1 is designed to spark production of antibodies against the HA protein from different virus strains, which could make it superior to vaccines now available, NIAID said.
“It could be longer lasting than the traditional flu vaccine and give us what we call super seasonal protection that might go beyond just one flu season to next year’s or the year after, or offer additional protection in a pandemic setting,” Alicia T. Widge, MD, of NIAID’s Vaccine Research Center, who is the principal investigator of the trial, said in an interview.
The phase 1 study (NCT04896086) aims to enroll 35 participants, 15 of whom will receive a single intramuscular injection of a comparator treatment, Flucelvax, which has already been approved by the U.S. Food and Drug Administration. The FluMos-v1 group will start with five participants who will receive one 20-μg dose. If no safety problems emerge at that dosage, another 15 volunteers will receive one 60-μg dose of the investigational vaccine.
The incorporation of a comparator group in the phase 1 study may help investigators get an early idea of how well FluMos-v1 compares to a marketed product, Dr. Widge said. The test will be carried out through the National Institutes of Health Clinical Center.
‘Renaissance’ of flu-vaccine research?
Currently, flu vaccines are reformulated each year in an attempt to match the dominant strain for the upcoming season, an effort that often falls notably short. The estimated vaccine effectiveness rate in the United States has ranged from a low of 19% to a high of 60% in recent years, according to the Centers for Disease Control and Prevention.
Scientists have been working for decades on a universal flu vaccine that would offer better results but haven’t yet identified the right strategy to outwit mutations in the virus. Recent setbacks include BiondVax Pharmaceuticals’ October 2020 announcement of a failed phase 3 trial of its experimental M-001 universal flu vaccine candidate.
But advances in understanding the immune system may set the stage for a “renaissance” in efforts to develop a universal flu vaccine, Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, said in an interview.
The COVID-19 pandemic has spurred greater interest in the need to develop a universal flu vaccine, he said. Dr. Osterholm said he is “more optimistic now than ever” about the chances for developing vaccines that can fend off multiple strains over longer periods, although the goal of a shot that can ward off influenza in all cases may remain elusive.
“How good can we make them? Will they ever be really universal? Will they have long periods of protection? I don’t think any of us know that yet,” Dr. Osterholm said. “But this is not the influenza vaccine world of 5 or 7 years ago.”
The mRNA technology used to develop the world’s first approved COVID-19 vaccines, for example, may be applied against influenza, Dr. Osterholm said.
In January 2021, Moderna announced plans to test three development candidates for a seasonal influenza vaccine and aims to start a phase 1 study this year. In an April interview on CNBC’s Squawk Box program, Moderna’s chief executive, Stephané Bancel, spoke about the company’s plans to eventually create a combination vaccine for SARS-Cov-2 and flu viruses.
SARS-CoV-2 “is not going away.” Like flu, this virus will persist and change forms, Ms. Bancel said. Creating a flu shot that outperforms the existing ones would boost confidence in influenza vaccines, which many people now skip, Ms. Bancel said. People might someday be able to get a combination of this more effective flu shot with a COVID-19 vaccine booster in their local pharmacies.
“You can take one dose and then have a nice winter,” Ms. Bancel said of Moderna’s goal for a combination vaccine.
A version of this article first appeared on Medscape.com.
COVID-19 Vaccine Reactions in Dermatology: “Filling” in the Gaps
As we marked the 1-year anniversary of the COVID-19 pandemic, nearly 100 million Americans had received their first dose of the COVID-19 vaccine, heralding some sense of relief and enabling us to envision a return to something resembling life before lockdown.1 Amid these breakthroughs and vaccination campaigns forging ahead worldwide, we saw new questions and problems arise. Vaccine hesitancy was already an issue in many segments of society where misinformation and mistrust of the medical establishment have served as barriers to the progress of public health. Once reports of adverse reactions following COVID-19 vaccination—such as those linked to use of facial fillers—made news headlines, many in the dermatology community began facing inquiries from patients questioning if they should wait to receive the vaccine or skip it entirely. As dermatologists, we must be informed and prepared to address these situations, to manage adverse reactions when they arise, and to encourage and promote vaccination during this critical time for public health in our society.
Cutaneous Vaccine Reactions and Facial Fillers
As public COVID-19 vaccinations move forward, dermatologic side effects, which were first noted during clinical trials, have received amplified attention, despite the fact that these cutaneous reactions—including localized injection-site redness and swelling, generalized urticarial and morbilliform eruptions, and even facial filler reactions—have been reported as relatively minor and self-limited.2 The excipient polyethylene glycol has been suspected as a possible etiology of vaccine-related allergic and hypersensitivity reactions, suggesting care be taken in those who are patch-test positive or have a history of allergy to polyethylene glycol–containing products (eg, penicillin, laxatives, makeup, certain dermal fillers).2,3 Although rare, facial and lip swelling reactions in those with a prior history of facial fillers in COVID-19 vaccine trials have drawn particular public concern and potential vaccine hesitancy given that more than 2.7 million Americans seek treatment with dermal fillers annually. There has been continued demand for these treatments during the pandemic, particularly due to aesthetic sensitivity surrounding video conferencing.4
Release of trial data from the Moderna COVID-19 vaccine prompted a discourse around safety and recommended protocols for filler procedures in the community of aesthetic medicine, as 3 participants in the experimental arm—all of whom had a history of treatment with facial filler injections—were reported to have facial or lip swelling shortly following vaccination. Two of these cases were considered to be serious adverse events due to extensive facial swelling, with the participants having received filler injections 6 months and 2 weeks prior to vaccination, respectively.5 A third participant experienced lip swelling only, which according to the US Food and Drug Administration briefing document was considered “medically significant” but not a serious adverse event, with unknown timing of the most recent filler injection. In all cases, symptom onset began 1 or 2 days following vaccination, and all resolved with either no or minimal intervention.6 The US Food and Drug Administration briefing document does not detail which type of fillers each participant had received, but subsequent reports indicated hyaluronic acid (HA) fillers. Of note, one patient in the placebo arm of the trial also developed progressive periorbital and facial edema in the setting of known filler injections performed 5 weeks prior, requiring treatment with corticosteroids and barring her from receiving a second injection in the trial.7
After public vaccination started, additional reports have emerged of facial edema occurring following administration of both the Pfizer and Moderna COVID-19 vaccines.2,8,9 In one series, 4 cases of facial swelling were reported in patients who had HA filler placed more than 1 year prior to vaccination.9 The first patient, who had a history of HA fillers in the temples and cheeks, developed moderate periorbital swelling 2 days following her second dose of the Pfizer vaccine. Another patient who had received a series of filler injections over the last 3 years experienced facial swelling 24 hours after her second dose of the Moderna vaccine and also reported a similar reaction in the past following an upper respiratory tract infection. The third patient developed perioral and infraorbital edema 18 hours after her first dose of the Moderna vaccine. The fourth patient developed inflammation in filler-treated areas 10 days after the first dose of the Pfizer vaccine and notably had a history of filler reaction to an unknown trigger in 2019 that was treated with hyaluronidase, intralesional steroids, and 5-fluorouracil. All cases of facial edema reportedly resolved.9
The observed adverse events have been proposed as delayed-type hypersensitivity reactions (DTRs) to facial fillers and are suspected to be triggered by the COVID-19 spike protein and subsequent immunogenic response. This reaction is not unique to the COVID-19 vaccines; in fact, many inflammatory stimuli such as sinus infections, flulike illnesses, facial injury, dental procedures, and exposure to certain medications and chemotherapeutics have triggered DTRs in filler patients, especially in those with genetic or immunologic risk factors including certain human leukocyte antigen subtypes or autoimmune disorders.3
Counseling Patients and Reducing Risks
As reports of DTRs to facial fillers after COVID-19 vaccination continue to emerge, it is not surprising that patients may become confused by potential side effects and postpone vaccination as a result. This evolving situation has called upon aesthetic physicians to adapt our practice and prepare our patients. Most importantly, we must continue to follow the data and integrate evidence-based COVID-19 vaccine–related counseling into our office visits. It is paramount to encourage vaccination and inform patients that these rare adverse events are both temporary and treatable. Given the currently available data, patients with a history of treatment with dermal fillers should not be discouraged from receiving the vaccine; however, we may provide suggestions to lessen the likelihood of adverse reactions and ease patient concerns. For example, it may be helpful to consider a time frame between vaccination and filler procedures that is longer than 2 weeks, just as would be advised for those having dental procedures or with recent infections, and potentially longer windows for those with risk factors such as prior sensitivity to dermal fillers, autoimmune disorders, or those on immunomodulatory medications. Dilution of fillers with saline or lidocaine or use of non-HA fillers also may be suggested around the time of vaccination to mitigate the risk of DTRs.3
Managing Vaccine Reactions
If facial swelling does occur despite these precautions and lasts longer than 48 hours, treatment with antihistamines, steroids, and/or hyaluronidase has been successful in vaccine trial and posttrial patients, both alone or in combination, and are likely to resolve edema promptly without altering the effectiveness of the vaccine.3,5,9 Angiotensin-converting enzyme inhibitors such as lisinopril more recently have been recommended for treatment of facial edema following COVID-19 vaccination,9 but questions remain regarding the true efficacy in this scenario given that the majority of swelling reactions resolve without this treatment. Additionally, there were no controls to indicate treatment with the angiotensin-converting enzyme inhibitor demonstrated an actual impact. Dermatologists generally are wary of adding medications of questionable utility that are associated with potential side effects and drug reactions, given that we often are tasked with managing the consequences of such mistakes. Thus, to avoid additional harm in the setting of insufficient evidence, as was seen following widespread use of hydroxychloroquine at the outset of the COVID-19 pandemic, well-structured studies are required before such interventions can be recommended.
If symptoms arise following the first vaccine injection, they can be managed if needed while patients are reassured and advised to obtain their second dose, with pretreatment considerations including antihistamines and instruction to present to the emergency department if a more severe reaction is suspected.2 In a larger sense, we also can contribute to the collective knowledge, growth, and preparedness of the medical community by reporting cases of adverse events to vaccine reporting systems and registries, such as the US Department of Health and Human Services’ Vaccine Adverse Event Reporting System, the Centers for Disease Control and Prevention’s V-Safe After Vaccination Health Checker, and the American Academy of Dermatology’s COVID-19 Dermatology Registry.
Final Thoughts
As dermatologists, we now find ourselves in the familiar role of balancing the aesthetic goals of our patients with our primary mission of public health and safety at a time when their health and well-being is particularly vulnerable. Adverse reactions will continue to occur as larger segments of the world’s population become vaccinated. Meanwhile, we must continue to manage symptoms, dispel myths, emphasize that any dermatologic risk posed by the COVID-19 vaccines is far outweighed by the benefits of immunization, and promote health and education, looking ahead to life beyond the pandemic.
- Ritchie H, Ortiz-Ospina E, Beltekian D, et al. Coronavirus (COVID-19) vaccinations. Our World in Data website. Accessed May 10, 2021. https://ourworldindata.org/covid-vaccinations
- McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases [published online April 7, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.092
- Rice SM, Ferree SD, Mesinkovska NA, et al. The art of prevention: COVID-19 vaccine preparedness for the dermatologist. Int J Womens Dermatol. 2021;7:209-212. doi:10.1016/j.ijwd.2021.01.007
- Rice SM, Siegel JA, Libby T, et al. Zooming into cosmetic procedures during the COVID-19 pandemic: the provider’s perspective. Int J Womens Dermatol. 2021;7:213-216.
- FDA Briefing Document: Moderna COVID-19 Vaccine. US Department of Health and Human Services; 2020. Accessed May 11, 2021. https://www.fda.gov/media/144434/download
- Moderna’s COVID-19 vaccine may cause swelling, inflammation in those with facial fillers. American Society of Plastic Surgeons website. Published December 27, 2020. Accessed May 11, 2021. http://www.plasticsurgery.org/for-medical-professionals/publications/psn-extra/news/modernas-covid19-vaccine-may-cause-swelling-inflammation-in-those-with-facial-fillers
- Munavalli GG, Guthridge R, Knutsen-Larson S, et al. COVID-19/SARS-CoV-2 virus spike protein-related delayed inflammatory reaction to hyaluronic acid dermal fillers: a challenging clinical conundrum in diagnosis and treatment [published online February 9, 2021]. Arch Dermatol Res. doi:10.1007/s00403-021-02190-6
- Schlessinger J. Update on COVID-19 vaccines and dermal fillers. Practical Dermatol. February 2021:46-47. Accessed May 10, 2021. https://practicaldermatology.com/articles/2021-feb/update-on-covid-19-vaccines-and-dermal-fillers/pdf
- Munavalli GG, Knutsen-Larson S, Lupo MP, et al. Oral angiotensin-converting enzyme inhibitors for treatment of delayed inflammatory reaction to dermal hyaluronic acid fillers following COVID-19 vaccination—a model for inhibition of angiotensin II-induced cutaneous inflammation. JAAD Case Rep. 2021;10:63-68. doi:10.1016/j.jdcr.2021.02.018
As we marked the 1-year anniversary of the COVID-19 pandemic, nearly 100 million Americans had received their first dose of the COVID-19 vaccine, heralding some sense of relief and enabling us to envision a return to something resembling life before lockdown.1 Amid these breakthroughs and vaccination campaigns forging ahead worldwide, we saw new questions and problems arise. Vaccine hesitancy was already an issue in many segments of society where misinformation and mistrust of the medical establishment have served as barriers to the progress of public health. Once reports of adverse reactions following COVID-19 vaccination—such as those linked to use of facial fillers—made news headlines, many in the dermatology community began facing inquiries from patients questioning if they should wait to receive the vaccine or skip it entirely. As dermatologists, we must be informed and prepared to address these situations, to manage adverse reactions when they arise, and to encourage and promote vaccination during this critical time for public health in our society.
Cutaneous Vaccine Reactions and Facial Fillers
As public COVID-19 vaccinations move forward, dermatologic side effects, which were first noted during clinical trials, have received amplified attention, despite the fact that these cutaneous reactions—including localized injection-site redness and swelling, generalized urticarial and morbilliform eruptions, and even facial filler reactions—have been reported as relatively minor and self-limited.2 The excipient polyethylene glycol has been suspected as a possible etiology of vaccine-related allergic and hypersensitivity reactions, suggesting care be taken in those who are patch-test positive or have a history of allergy to polyethylene glycol–containing products (eg, penicillin, laxatives, makeup, certain dermal fillers).2,3 Although rare, facial and lip swelling reactions in those with a prior history of facial fillers in COVID-19 vaccine trials have drawn particular public concern and potential vaccine hesitancy given that more than 2.7 million Americans seek treatment with dermal fillers annually. There has been continued demand for these treatments during the pandemic, particularly due to aesthetic sensitivity surrounding video conferencing.4
Release of trial data from the Moderna COVID-19 vaccine prompted a discourse around safety and recommended protocols for filler procedures in the community of aesthetic medicine, as 3 participants in the experimental arm—all of whom had a history of treatment with facial filler injections—were reported to have facial or lip swelling shortly following vaccination. Two of these cases were considered to be serious adverse events due to extensive facial swelling, with the participants having received filler injections 6 months and 2 weeks prior to vaccination, respectively.5 A third participant experienced lip swelling only, which according to the US Food and Drug Administration briefing document was considered “medically significant” but not a serious adverse event, with unknown timing of the most recent filler injection. In all cases, symptom onset began 1 or 2 days following vaccination, and all resolved with either no or minimal intervention.6 The US Food and Drug Administration briefing document does not detail which type of fillers each participant had received, but subsequent reports indicated hyaluronic acid (HA) fillers. Of note, one patient in the placebo arm of the trial also developed progressive periorbital and facial edema in the setting of known filler injections performed 5 weeks prior, requiring treatment with corticosteroids and barring her from receiving a second injection in the trial.7
After public vaccination started, additional reports have emerged of facial edema occurring following administration of both the Pfizer and Moderna COVID-19 vaccines.2,8,9 In one series, 4 cases of facial swelling were reported in patients who had HA filler placed more than 1 year prior to vaccination.9 The first patient, who had a history of HA fillers in the temples and cheeks, developed moderate periorbital swelling 2 days following her second dose of the Pfizer vaccine. Another patient who had received a series of filler injections over the last 3 years experienced facial swelling 24 hours after her second dose of the Moderna vaccine and also reported a similar reaction in the past following an upper respiratory tract infection. The third patient developed perioral and infraorbital edema 18 hours after her first dose of the Moderna vaccine. The fourth patient developed inflammation in filler-treated areas 10 days after the first dose of the Pfizer vaccine and notably had a history of filler reaction to an unknown trigger in 2019 that was treated with hyaluronidase, intralesional steroids, and 5-fluorouracil. All cases of facial edema reportedly resolved.9
The observed adverse events have been proposed as delayed-type hypersensitivity reactions (DTRs) to facial fillers and are suspected to be triggered by the COVID-19 spike protein and subsequent immunogenic response. This reaction is not unique to the COVID-19 vaccines; in fact, many inflammatory stimuli such as sinus infections, flulike illnesses, facial injury, dental procedures, and exposure to certain medications and chemotherapeutics have triggered DTRs in filler patients, especially in those with genetic or immunologic risk factors including certain human leukocyte antigen subtypes or autoimmune disorders.3
Counseling Patients and Reducing Risks
As reports of DTRs to facial fillers after COVID-19 vaccination continue to emerge, it is not surprising that patients may become confused by potential side effects and postpone vaccination as a result. This evolving situation has called upon aesthetic physicians to adapt our practice and prepare our patients. Most importantly, we must continue to follow the data and integrate evidence-based COVID-19 vaccine–related counseling into our office visits. It is paramount to encourage vaccination and inform patients that these rare adverse events are both temporary and treatable. Given the currently available data, patients with a history of treatment with dermal fillers should not be discouraged from receiving the vaccine; however, we may provide suggestions to lessen the likelihood of adverse reactions and ease patient concerns. For example, it may be helpful to consider a time frame between vaccination and filler procedures that is longer than 2 weeks, just as would be advised for those having dental procedures or with recent infections, and potentially longer windows for those with risk factors such as prior sensitivity to dermal fillers, autoimmune disorders, or those on immunomodulatory medications. Dilution of fillers with saline or lidocaine or use of non-HA fillers also may be suggested around the time of vaccination to mitigate the risk of DTRs.3
Managing Vaccine Reactions
If facial swelling does occur despite these precautions and lasts longer than 48 hours, treatment with antihistamines, steroids, and/or hyaluronidase has been successful in vaccine trial and posttrial patients, both alone or in combination, and are likely to resolve edema promptly without altering the effectiveness of the vaccine.3,5,9 Angiotensin-converting enzyme inhibitors such as lisinopril more recently have been recommended for treatment of facial edema following COVID-19 vaccination,9 but questions remain regarding the true efficacy in this scenario given that the majority of swelling reactions resolve without this treatment. Additionally, there were no controls to indicate treatment with the angiotensin-converting enzyme inhibitor demonstrated an actual impact. Dermatologists generally are wary of adding medications of questionable utility that are associated with potential side effects and drug reactions, given that we often are tasked with managing the consequences of such mistakes. Thus, to avoid additional harm in the setting of insufficient evidence, as was seen following widespread use of hydroxychloroquine at the outset of the COVID-19 pandemic, well-structured studies are required before such interventions can be recommended.
If symptoms arise following the first vaccine injection, they can be managed if needed while patients are reassured and advised to obtain their second dose, with pretreatment considerations including antihistamines and instruction to present to the emergency department if a more severe reaction is suspected.2 In a larger sense, we also can contribute to the collective knowledge, growth, and preparedness of the medical community by reporting cases of adverse events to vaccine reporting systems and registries, such as the US Department of Health and Human Services’ Vaccine Adverse Event Reporting System, the Centers for Disease Control and Prevention’s V-Safe After Vaccination Health Checker, and the American Academy of Dermatology’s COVID-19 Dermatology Registry.
Final Thoughts
As dermatologists, we now find ourselves in the familiar role of balancing the aesthetic goals of our patients with our primary mission of public health and safety at a time when their health and well-being is particularly vulnerable. Adverse reactions will continue to occur as larger segments of the world’s population become vaccinated. Meanwhile, we must continue to manage symptoms, dispel myths, emphasize that any dermatologic risk posed by the COVID-19 vaccines is far outweighed by the benefits of immunization, and promote health and education, looking ahead to life beyond the pandemic.
As we marked the 1-year anniversary of the COVID-19 pandemic, nearly 100 million Americans had received their first dose of the COVID-19 vaccine, heralding some sense of relief and enabling us to envision a return to something resembling life before lockdown.1 Amid these breakthroughs and vaccination campaigns forging ahead worldwide, we saw new questions and problems arise. Vaccine hesitancy was already an issue in many segments of society where misinformation and mistrust of the medical establishment have served as barriers to the progress of public health. Once reports of adverse reactions following COVID-19 vaccination—such as those linked to use of facial fillers—made news headlines, many in the dermatology community began facing inquiries from patients questioning if they should wait to receive the vaccine or skip it entirely. As dermatologists, we must be informed and prepared to address these situations, to manage adverse reactions when they arise, and to encourage and promote vaccination during this critical time for public health in our society.
Cutaneous Vaccine Reactions and Facial Fillers
As public COVID-19 vaccinations move forward, dermatologic side effects, which were first noted during clinical trials, have received amplified attention, despite the fact that these cutaneous reactions—including localized injection-site redness and swelling, generalized urticarial and morbilliform eruptions, and even facial filler reactions—have been reported as relatively minor and self-limited.2 The excipient polyethylene glycol has been suspected as a possible etiology of vaccine-related allergic and hypersensitivity reactions, suggesting care be taken in those who are patch-test positive or have a history of allergy to polyethylene glycol–containing products (eg, penicillin, laxatives, makeup, certain dermal fillers).2,3 Although rare, facial and lip swelling reactions in those with a prior history of facial fillers in COVID-19 vaccine trials have drawn particular public concern and potential vaccine hesitancy given that more than 2.7 million Americans seek treatment with dermal fillers annually. There has been continued demand for these treatments during the pandemic, particularly due to aesthetic sensitivity surrounding video conferencing.4
Release of trial data from the Moderna COVID-19 vaccine prompted a discourse around safety and recommended protocols for filler procedures in the community of aesthetic medicine, as 3 participants in the experimental arm—all of whom had a history of treatment with facial filler injections—were reported to have facial or lip swelling shortly following vaccination. Two of these cases were considered to be serious adverse events due to extensive facial swelling, with the participants having received filler injections 6 months and 2 weeks prior to vaccination, respectively.5 A third participant experienced lip swelling only, which according to the US Food and Drug Administration briefing document was considered “medically significant” but not a serious adverse event, with unknown timing of the most recent filler injection. In all cases, symptom onset began 1 or 2 days following vaccination, and all resolved with either no or minimal intervention.6 The US Food and Drug Administration briefing document does not detail which type of fillers each participant had received, but subsequent reports indicated hyaluronic acid (HA) fillers. Of note, one patient in the placebo arm of the trial also developed progressive periorbital and facial edema in the setting of known filler injections performed 5 weeks prior, requiring treatment with corticosteroids and barring her from receiving a second injection in the trial.7
After public vaccination started, additional reports have emerged of facial edema occurring following administration of both the Pfizer and Moderna COVID-19 vaccines.2,8,9 In one series, 4 cases of facial swelling were reported in patients who had HA filler placed more than 1 year prior to vaccination.9 The first patient, who had a history of HA fillers in the temples and cheeks, developed moderate periorbital swelling 2 days following her second dose of the Pfizer vaccine. Another patient who had received a series of filler injections over the last 3 years experienced facial swelling 24 hours after her second dose of the Moderna vaccine and also reported a similar reaction in the past following an upper respiratory tract infection. The third patient developed perioral and infraorbital edema 18 hours after her first dose of the Moderna vaccine. The fourth patient developed inflammation in filler-treated areas 10 days after the first dose of the Pfizer vaccine and notably had a history of filler reaction to an unknown trigger in 2019 that was treated with hyaluronidase, intralesional steroids, and 5-fluorouracil. All cases of facial edema reportedly resolved.9
The observed adverse events have been proposed as delayed-type hypersensitivity reactions (DTRs) to facial fillers and are suspected to be triggered by the COVID-19 spike protein and subsequent immunogenic response. This reaction is not unique to the COVID-19 vaccines; in fact, many inflammatory stimuli such as sinus infections, flulike illnesses, facial injury, dental procedures, and exposure to certain medications and chemotherapeutics have triggered DTRs in filler patients, especially in those with genetic or immunologic risk factors including certain human leukocyte antigen subtypes or autoimmune disorders.3
Counseling Patients and Reducing Risks
As reports of DTRs to facial fillers after COVID-19 vaccination continue to emerge, it is not surprising that patients may become confused by potential side effects and postpone vaccination as a result. This evolving situation has called upon aesthetic physicians to adapt our practice and prepare our patients. Most importantly, we must continue to follow the data and integrate evidence-based COVID-19 vaccine–related counseling into our office visits. It is paramount to encourage vaccination and inform patients that these rare adverse events are both temporary and treatable. Given the currently available data, patients with a history of treatment with dermal fillers should not be discouraged from receiving the vaccine; however, we may provide suggestions to lessen the likelihood of adverse reactions and ease patient concerns. For example, it may be helpful to consider a time frame between vaccination and filler procedures that is longer than 2 weeks, just as would be advised for those having dental procedures or with recent infections, and potentially longer windows for those with risk factors such as prior sensitivity to dermal fillers, autoimmune disorders, or those on immunomodulatory medications. Dilution of fillers with saline or lidocaine or use of non-HA fillers also may be suggested around the time of vaccination to mitigate the risk of DTRs.3
Managing Vaccine Reactions
If facial swelling does occur despite these precautions and lasts longer than 48 hours, treatment with antihistamines, steroids, and/or hyaluronidase has been successful in vaccine trial and posttrial patients, both alone or in combination, and are likely to resolve edema promptly without altering the effectiveness of the vaccine.3,5,9 Angiotensin-converting enzyme inhibitors such as lisinopril more recently have been recommended for treatment of facial edema following COVID-19 vaccination,9 but questions remain regarding the true efficacy in this scenario given that the majority of swelling reactions resolve without this treatment. Additionally, there were no controls to indicate treatment with the angiotensin-converting enzyme inhibitor demonstrated an actual impact. Dermatologists generally are wary of adding medications of questionable utility that are associated with potential side effects and drug reactions, given that we often are tasked with managing the consequences of such mistakes. Thus, to avoid additional harm in the setting of insufficient evidence, as was seen following widespread use of hydroxychloroquine at the outset of the COVID-19 pandemic, well-structured studies are required before such interventions can be recommended.
If symptoms arise following the first vaccine injection, they can be managed if needed while patients are reassured and advised to obtain their second dose, with pretreatment considerations including antihistamines and instruction to present to the emergency department if a more severe reaction is suspected.2 In a larger sense, we also can contribute to the collective knowledge, growth, and preparedness of the medical community by reporting cases of adverse events to vaccine reporting systems and registries, such as the US Department of Health and Human Services’ Vaccine Adverse Event Reporting System, the Centers for Disease Control and Prevention’s V-Safe After Vaccination Health Checker, and the American Academy of Dermatology’s COVID-19 Dermatology Registry.
Final Thoughts
As dermatologists, we now find ourselves in the familiar role of balancing the aesthetic goals of our patients with our primary mission of public health and safety at a time when their health and well-being is particularly vulnerable. Adverse reactions will continue to occur as larger segments of the world’s population become vaccinated. Meanwhile, we must continue to manage symptoms, dispel myths, emphasize that any dermatologic risk posed by the COVID-19 vaccines is far outweighed by the benefits of immunization, and promote health and education, looking ahead to life beyond the pandemic.
- Ritchie H, Ortiz-Ospina E, Beltekian D, et al. Coronavirus (COVID-19) vaccinations. Our World in Data website. Accessed May 10, 2021. https://ourworldindata.org/covid-vaccinations
- McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases [published online April 7, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.092
- Rice SM, Ferree SD, Mesinkovska NA, et al. The art of prevention: COVID-19 vaccine preparedness for the dermatologist. Int J Womens Dermatol. 2021;7:209-212. doi:10.1016/j.ijwd.2021.01.007
- Rice SM, Siegel JA, Libby T, et al. Zooming into cosmetic procedures during the COVID-19 pandemic: the provider’s perspective. Int J Womens Dermatol. 2021;7:213-216.
- FDA Briefing Document: Moderna COVID-19 Vaccine. US Department of Health and Human Services; 2020. Accessed May 11, 2021. https://www.fda.gov/media/144434/download
- Moderna’s COVID-19 vaccine may cause swelling, inflammation in those with facial fillers. American Society of Plastic Surgeons website. Published December 27, 2020. Accessed May 11, 2021. http://www.plasticsurgery.org/for-medical-professionals/publications/psn-extra/news/modernas-covid19-vaccine-may-cause-swelling-inflammation-in-those-with-facial-fillers
- Munavalli GG, Guthridge R, Knutsen-Larson S, et al. COVID-19/SARS-CoV-2 virus spike protein-related delayed inflammatory reaction to hyaluronic acid dermal fillers: a challenging clinical conundrum in diagnosis and treatment [published online February 9, 2021]. Arch Dermatol Res. doi:10.1007/s00403-021-02190-6
- Schlessinger J. Update on COVID-19 vaccines and dermal fillers. Practical Dermatol. February 2021:46-47. Accessed May 10, 2021. https://practicaldermatology.com/articles/2021-feb/update-on-covid-19-vaccines-and-dermal-fillers/pdf
- Munavalli GG, Knutsen-Larson S, Lupo MP, et al. Oral angiotensin-converting enzyme inhibitors for treatment of delayed inflammatory reaction to dermal hyaluronic acid fillers following COVID-19 vaccination—a model for inhibition of angiotensin II-induced cutaneous inflammation. JAAD Case Rep. 2021;10:63-68. doi:10.1016/j.jdcr.2021.02.018
- Ritchie H, Ortiz-Ospina E, Beltekian D, et al. Coronavirus (COVID-19) vaccinations. Our World in Data website. Accessed May 10, 2021. https://ourworldindata.org/covid-vaccinations
- McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases [published online April 7, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.092
- Rice SM, Ferree SD, Mesinkovska NA, et al. The art of prevention: COVID-19 vaccine preparedness for the dermatologist. Int J Womens Dermatol. 2021;7:209-212. doi:10.1016/j.ijwd.2021.01.007
- Rice SM, Siegel JA, Libby T, et al. Zooming into cosmetic procedures during the COVID-19 pandemic: the provider’s perspective. Int J Womens Dermatol. 2021;7:213-216.
- FDA Briefing Document: Moderna COVID-19 Vaccine. US Department of Health and Human Services; 2020. Accessed May 11, 2021. https://www.fda.gov/media/144434/download
- Moderna’s COVID-19 vaccine may cause swelling, inflammation in those with facial fillers. American Society of Plastic Surgeons website. Published December 27, 2020. Accessed May 11, 2021. http://www.plasticsurgery.org/for-medical-professionals/publications/psn-extra/news/modernas-covid19-vaccine-may-cause-swelling-inflammation-in-those-with-facial-fillers
- Munavalli GG, Guthridge R, Knutsen-Larson S, et al. COVID-19/SARS-CoV-2 virus spike protein-related delayed inflammatory reaction to hyaluronic acid dermal fillers: a challenging clinical conundrum in diagnosis and treatment [published online February 9, 2021]. Arch Dermatol Res. doi:10.1007/s00403-021-02190-6
- Schlessinger J. Update on COVID-19 vaccines and dermal fillers. Practical Dermatol. February 2021:46-47. Accessed May 10, 2021. https://practicaldermatology.com/articles/2021-feb/update-on-covid-19-vaccines-and-dermal-fillers/pdf
- Munavalli GG, Knutsen-Larson S, Lupo MP, et al. Oral angiotensin-converting enzyme inhibitors for treatment of delayed inflammatory reaction to dermal hyaluronic acid fillers following COVID-19 vaccination—a model for inhibition of angiotensin II-induced cutaneous inflammation. JAAD Case Rep. 2021;10:63-68. doi:10.1016/j.jdcr.2021.02.018
How to Save a Limb: Identification of Pyoderma Gangrenosum
Case Report
A 67-year-old woman presented with a painful expanding ulcer on the left leg and a new nearby ulcer of 2 months’ duration. She initially was seen 2 months prior for a wound on the left knee due to a fall as well as cellulitis, which was treated with intravenous vancomycin and ceftriaxone. Wound cultures were negative for bacteria, and she was discharged without antibiotics. She presented to the emergency department 1 month later for malodorous discharge of the first ulcer with zero systemic inflammatory response syndrome criteria; no fever; and no abnormal heart rate, respiratory rate, or leukocyte count. She was discharged with wound care. After 3 weeks, she returned with a second ulcer and worsening drainage but zero systemic inflammatory response syndrome criteria. She had a medical history of Crohn disease with 9-year remission, atrial fibrillation, pacemaker, mitral valve replacement, chronic obstructive pulmonary disease, and a 51 pack-year smoking history.
Physical examination of the left leg revealed a 3×3-cm deep lesion (ulcer A) on the distal left thigh located superomedial to the knee (Figure 1) as well as a 2×1-cm deep lesion (ulcer B) on the anteromedial knee with undermining and tunneling (Figure 2). A large amount of malodorous tan bloody discharge was present on both ulcers. There were no signs of induration or crepitus.Due to concerns of skin and soft tissue infection (SSTI) or osteomyelitis, a bone scan and wound and blood cultures were ordered. The patient was started on vancomycin and piperacillin-tazobactam in the emergency department, which later was augmented with cefepime. Trauma surgery scheduled debridement for the following morning with suspicion of necrotizing fasciitis. Additional consultations were requested, including infectious disease, wound care, and dermatology. Dermatology evaluated the wound, performed a punch biopsy, and canceled debridement due to unclear diagnosis. The clinical differential at that time included pyoderma gangrenosum (PG), atypical vasculitis, or infection. Additional workup revealed positive antineutrophil cytoplasmic antibodies but negative proteinase 3 and myeloperoxidase, disfavoring vasculitis. Wound cultures grew Staphylococcus aureus and Pseudomonas aeruginosa.
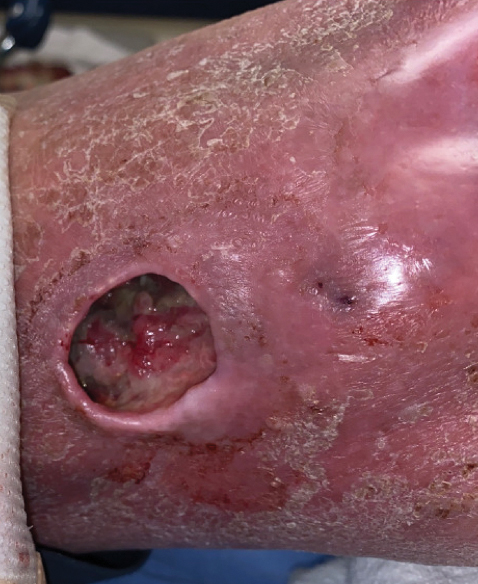
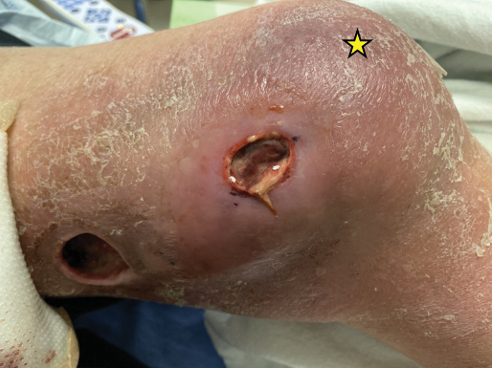
Histologic evaluation revealed deep dermal necrosis with a mixed inflammatory infiltrate (Figure 3) and no organisms or vasculitis. Antibiotics were discontinued, and she was discharged on a 14-day course of prednisone 60 mg daily for empirical treatment of PG with dermatology follow-up. Medical management included a 6-month course of dapsone that was extended to 7 months because of an intensive care unit stay for a cerebrovascular accident. Daily dosing was as follows: 100 mg for 5 months, 50 mg for 1 month, and 25 mg for 1 month, then stopped. She was followed with serial complete blood cell count every 1 to 2 months and home-health wound care. One month after dapsone initiation, the ulcers decreased in size. Ulcer B was fully healed after 4 months, and ulcer A was nearly closed at 6 months without any new flares.
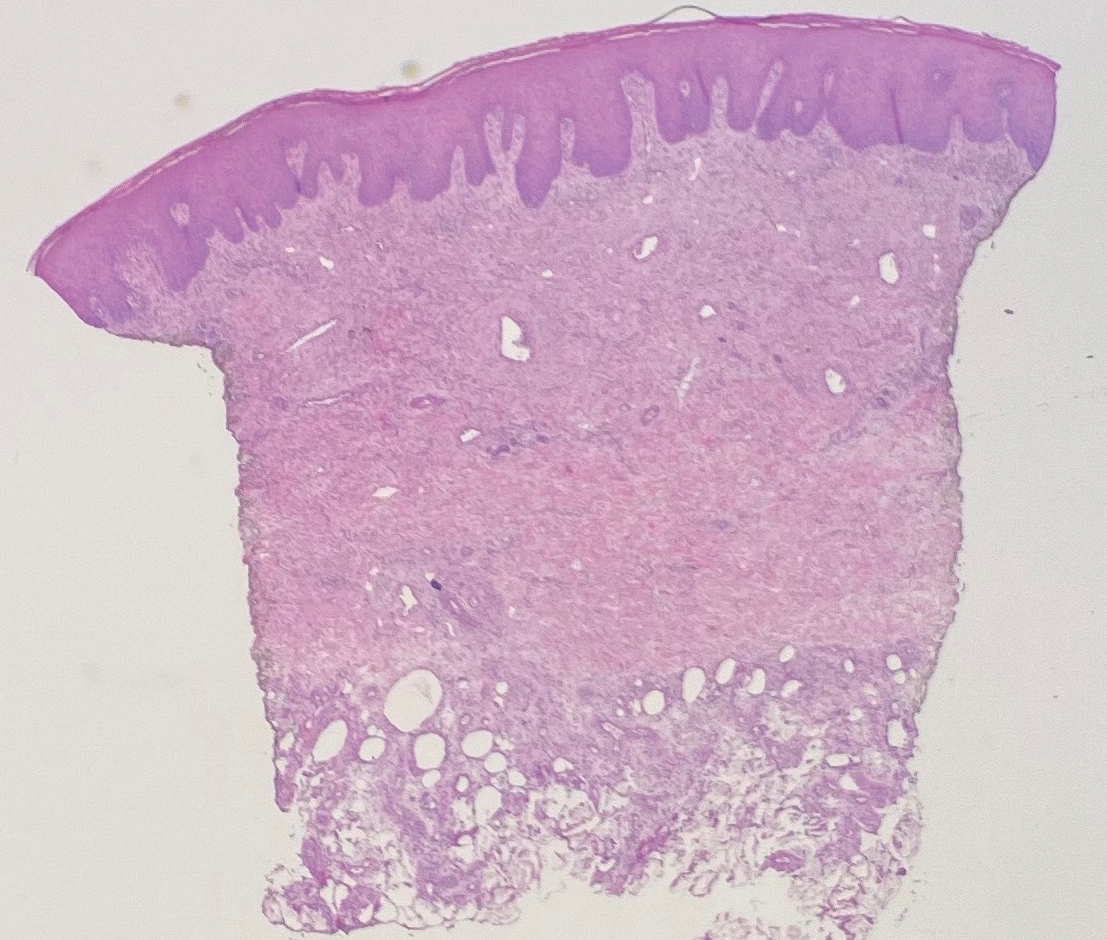
Comment
Pyoderma gangrenosum is a rare inflammatory skin condition that classically presents as tender papules or pustules evolving into painful ulcers, most commonly on the lower extremities. Pyoderma gangrenosum has a propensity to exhibit pathergy, the hyperreactivity of the skin in response to minor trauma. This phenomenon in PG manifests as the rapid evolution from pustule to ulceration with violaceous undermining borders.
Diagnosis of PG
Pyoderma gangrenosum has been described as a diagnosis of exclusion, as its findings frequently mimic SSTIs. Important findings to obtain are histology, history, ulcer morphology, and response to treatment.
In 2018, Maverakis et al1 proposed diagnostic criteria for classic ulcerative PG (Table 1). A diagnosis of PG can be made if the patient meets 1 major criterion and 4 minor criteria. Our case met 0 major criteria and 5 minor criteria: history of inflammatory bowel disease (IBD); history of pustule ulcerating within 4 days of appearing; peripheral erythema, undermining border, and tenderness at ulceration site; multiple ulcerations, with at least 1 on an anterior lower leg; and decreased ulcer size within 1 month of initiating immunosuppressive medication(s). Although our patient’s biopsy demonstrated a mixed infiltrate, PG was not excluded due to spontaneous resolution at the time of biopsy, emphasizing the need to biopsy subsequent new lesions if neutrophils are not initially seen.1 Pyoderma gangrenosum frequently is associated with IBD, most often Crohn disease, as seen in our patient.2-4 Although IBD classically is associated with smoking, studies have yet to conclude if smoking is a predictive factor of PG.5 Our patient presented with an initial ulcer that evolved into 2 ulcers, similar to a case of bilateral ulcers.6
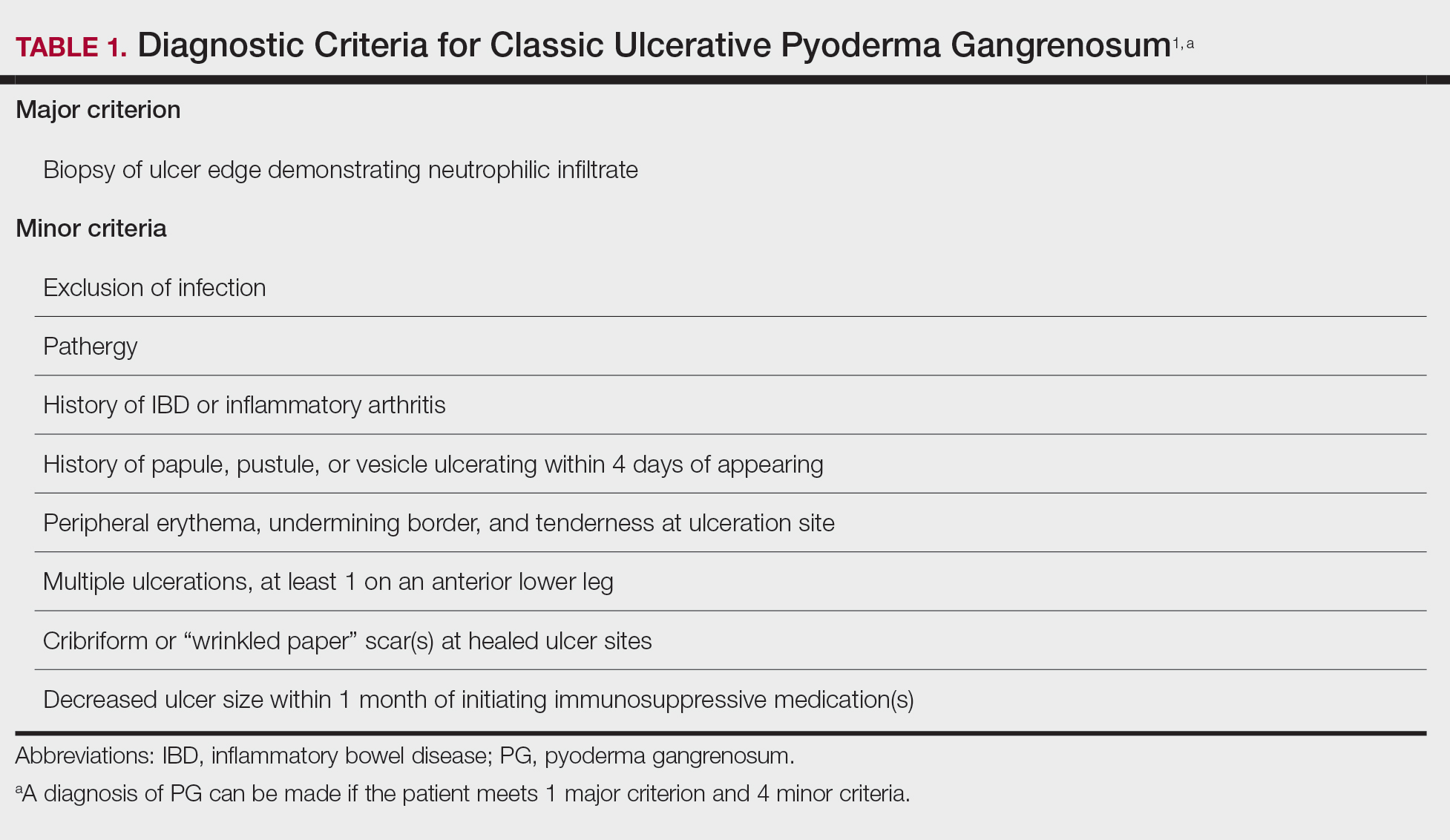
Differential Diagnosis of PG
Other possible diagnoses to consider are SSTI and vasculitis, the latter being disfavored by no evidence of vasculitis on biopsy and negative titers for proteinase 3 and myeloperoxidase antibodies. However, the presence of either, similar to a mixed infiltrate, does not exclude a diagnosis of PG, as they can occur simultaneously. Consequently, superinfection of a chronically open wound can occur due to underlying PG.7 The differences between PG and SSTI are listed in Table 2.
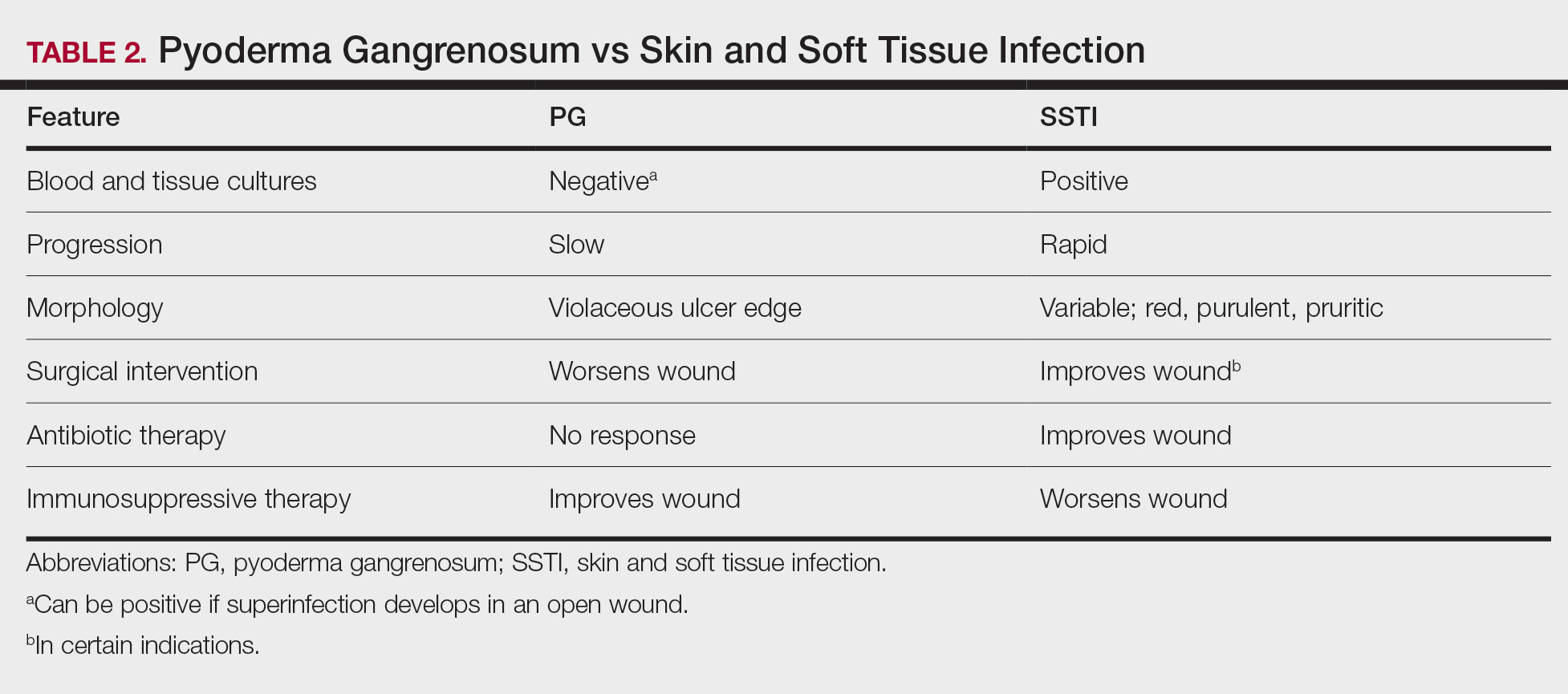
Although we know PG involves neutrophilic dysfunction, the pathophysiology remains poorly understood, contributing to the lack of clinical guidelines.8 Therefore, the diagnosis of PG often is delayed and is associated with severe consequences such as necrotizing fasciitis, osteomyelitis, cosmetic morbidity, and limb amputation.9,10 Dermatologic consultation can aid in early diagnosis and avoid amputation.7,10 Amputation has been used as a last resort to preserve optimal outcomes in patients with severe PG.11
Management of PG
A gold standard of treatment of PG does not exist, but the goal is to promote wound healing. Patients with limited disease typically can be managed with wound care and topical steroids or calcineurin inhibitors, though data on efficacy are limited. However, our patient had more extensive disease and needed to be treated with systemic therapy. First-line therapy for extensive disease includes oral prednisone or cyclosporine for patients who cannot tolerate systemic corticosteroids.12 Second-line and adjunctive therapy options include dapsone, minocycline, methotrexate, and infliximab. Our patient was prescribed a 7-month course of dapsone with outpatient dermatology and demonstrated resolution of both ulcers. Dapsone was tapered from a daily dose of 100 mg to 50 mg to 25 mg to none over the course of 2 to 3 months. Close monitoring with wound care is recommended, and petroleum jelly can be used for dry skin around the lesion for comfort.
Conclusion
The diagnosis of PG is challenging because it relies heavily on clinical signs and often mimics SSTI. Gathering a detailed medical history is critical to make the diagnosis of PG. In a patient with associated features of PG, dermatologic consultation and biopsy of skin lesions should be considered. Physicians should evaluate for suspected PG prior to proceeding with surgical intervention to avoid unnecessary amputation. The diagnostic criteria for classic ulcerative PG are gaining wider acceptance and are a useful tool for clinicians.
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466.
- Bisarya K, Azzopardi S, Lye G, et al. Necrotizing fasciitis versus pyoderma gangrenosum: securing the correct diagnosis! a case report and literature review. Eplasty. 2011;11:E24.
- Perricone G, Vangeli M. Pyoderma gangrenosum in ulcerative colitis. N Engl J Med. 2018;379:E7.
- Ashchyan HJ, Butler DC, Nelson CA, et al. The association of age with clinical presentation and comorbidities of pyoderma gangrenosum. JAMA Dermatol. 2018;154:409-413.
- Ampuero J, Rojas-Feria M, Castro-Fernández M, et al. Predictive factors for erythema nodosum and pyoderma gangrenosum in inflammatory bowel disease. J Gastroenterol Hepatol. 2014;29:291-295.
- Ebner DW, Hu M, Poterucha TH. 29-year-old woman with fever and bilateral lower extremity lesions. Mayo Clin Proc. 2018;93:1659-1663.
- Marzak H, Von Hunolstein JJ, Lipsker D, et al. Management of a superinfected pyoderma gangrenosum after pacemaker implant. HeartRhythm Case Rep. 2018;5:63-65.
- Braswell SF, Kostopoulos TC, Ortega-Loayza AG. Pathophysiology of pyoderma gangrenosum (PG): an updated review. J Am Acad Dermatol. 2015;73:691-698.
- Saffie MG, Shroff A. A case of pyoderma gangrenosum misdiagnosed as necrotizing infection: a potential diagnostic catastrophe. Case Rep Infect Dis. 2018;2018:8907542.
- Haag CK, Nutan F, Cyrus JW, et al. Pyoderma gangrenosum misdiagnosis resulting in amputation: a review. J Trauma Acute Care Surg. 2019;86:307-313.
- Sanchez IM, Lowenstein S, Johnson KA, et al. Clinical features of neutrophilic dermatosis variants resembling necrotizing fasciitis. JAMA Dermatol. 2019;155:79-84.
- Alavi A, French LE, Davis MD, et al. Pyoderma gangrenosum: an update on pathophysiology, diagnosis and treatment. Am J Clin Dermatol. 2017;18:355-372.
Case Report
A 67-year-old woman presented with a painful expanding ulcer on the left leg and a new nearby ulcer of 2 months’ duration. She initially was seen 2 months prior for a wound on the left knee due to a fall as well as cellulitis, which was treated with intravenous vancomycin and ceftriaxone. Wound cultures were negative for bacteria, and she was discharged without antibiotics. She presented to the emergency department 1 month later for malodorous discharge of the first ulcer with zero systemic inflammatory response syndrome criteria; no fever; and no abnormal heart rate, respiratory rate, or leukocyte count. She was discharged with wound care. After 3 weeks, she returned with a second ulcer and worsening drainage but zero systemic inflammatory response syndrome criteria. She had a medical history of Crohn disease with 9-year remission, atrial fibrillation, pacemaker, mitral valve replacement, chronic obstructive pulmonary disease, and a 51 pack-year smoking history.
Physical examination of the left leg revealed a 3×3-cm deep lesion (ulcer A) on the distal left thigh located superomedial to the knee (Figure 1) as well as a 2×1-cm deep lesion (ulcer B) on the anteromedial knee with undermining and tunneling (Figure 2). A large amount of malodorous tan bloody discharge was present on both ulcers. There were no signs of induration or crepitus.Due to concerns of skin and soft tissue infection (SSTI) or osteomyelitis, a bone scan and wound and blood cultures were ordered. The patient was started on vancomycin and piperacillin-tazobactam in the emergency department, which later was augmented with cefepime. Trauma surgery scheduled debridement for the following morning with suspicion of necrotizing fasciitis. Additional consultations were requested, including infectious disease, wound care, and dermatology. Dermatology evaluated the wound, performed a punch biopsy, and canceled debridement due to unclear diagnosis. The clinical differential at that time included pyoderma gangrenosum (PG), atypical vasculitis, or infection. Additional workup revealed positive antineutrophil cytoplasmic antibodies but negative proteinase 3 and myeloperoxidase, disfavoring vasculitis. Wound cultures grew Staphylococcus aureus and Pseudomonas aeruginosa.


Histologic evaluation revealed deep dermal necrosis with a mixed inflammatory infiltrate (Figure 3) and no organisms or vasculitis. Antibiotics were discontinued, and she was discharged on a 14-day course of prednisone 60 mg daily for empirical treatment of PG with dermatology follow-up. Medical management included a 6-month course of dapsone that was extended to 7 months because of an intensive care unit stay for a cerebrovascular accident. Daily dosing was as follows: 100 mg for 5 months, 50 mg for 1 month, and 25 mg for 1 month, then stopped. She was followed with serial complete blood cell count every 1 to 2 months and home-health wound care. One month after dapsone initiation, the ulcers decreased in size. Ulcer B was fully healed after 4 months, and ulcer A was nearly closed at 6 months without any new flares.

Comment
Pyoderma gangrenosum is a rare inflammatory skin condition that classically presents as tender papules or pustules evolving into painful ulcers, most commonly on the lower extremities. Pyoderma gangrenosum has a propensity to exhibit pathergy, the hyperreactivity of the skin in response to minor trauma. This phenomenon in PG manifests as the rapid evolution from pustule to ulceration with violaceous undermining borders.
Diagnosis of PG
Pyoderma gangrenosum has been described as a diagnosis of exclusion, as its findings frequently mimic SSTIs. Important findings to obtain are histology, history, ulcer morphology, and response to treatment.
In 2018, Maverakis et al1 proposed diagnostic criteria for classic ulcerative PG (Table 1). A diagnosis of PG can be made if the patient meets 1 major criterion and 4 minor criteria. Our case met 0 major criteria and 5 minor criteria: history of inflammatory bowel disease (IBD); history of pustule ulcerating within 4 days of appearing; peripheral erythema, undermining border, and tenderness at ulceration site; multiple ulcerations, with at least 1 on an anterior lower leg; and decreased ulcer size within 1 month of initiating immunosuppressive medication(s). Although our patient’s biopsy demonstrated a mixed infiltrate, PG was not excluded due to spontaneous resolution at the time of biopsy, emphasizing the need to biopsy subsequent new lesions if neutrophils are not initially seen.1 Pyoderma gangrenosum frequently is associated with IBD, most often Crohn disease, as seen in our patient.2-4 Although IBD classically is associated with smoking, studies have yet to conclude if smoking is a predictive factor of PG.5 Our patient presented with an initial ulcer that evolved into 2 ulcers, similar to a case of bilateral ulcers.6

Differential Diagnosis of PG
Other possible diagnoses to consider are SSTI and vasculitis, the latter being disfavored by no evidence of vasculitis on biopsy and negative titers for proteinase 3 and myeloperoxidase antibodies. However, the presence of either, similar to a mixed infiltrate, does not exclude a diagnosis of PG, as they can occur simultaneously. Consequently, superinfection of a chronically open wound can occur due to underlying PG.7 The differences between PG and SSTI are listed in Table 2.

Although we know PG involves neutrophilic dysfunction, the pathophysiology remains poorly understood, contributing to the lack of clinical guidelines.8 Therefore, the diagnosis of PG often is delayed and is associated with severe consequences such as necrotizing fasciitis, osteomyelitis, cosmetic morbidity, and limb amputation.9,10 Dermatologic consultation can aid in early diagnosis and avoid amputation.7,10 Amputation has been used as a last resort to preserve optimal outcomes in patients with severe PG.11
Management of PG
A gold standard of treatment of PG does not exist, but the goal is to promote wound healing. Patients with limited disease typically can be managed with wound care and topical steroids or calcineurin inhibitors, though data on efficacy are limited. However, our patient had more extensive disease and needed to be treated with systemic therapy. First-line therapy for extensive disease includes oral prednisone or cyclosporine for patients who cannot tolerate systemic corticosteroids.12 Second-line and adjunctive therapy options include dapsone, minocycline, methotrexate, and infliximab. Our patient was prescribed a 7-month course of dapsone with outpatient dermatology and demonstrated resolution of both ulcers. Dapsone was tapered from a daily dose of 100 mg to 50 mg to 25 mg to none over the course of 2 to 3 months. Close monitoring with wound care is recommended, and petroleum jelly can be used for dry skin around the lesion for comfort.
Conclusion
The diagnosis of PG is challenging because it relies heavily on clinical signs and often mimics SSTI. Gathering a detailed medical history is critical to make the diagnosis of PG. In a patient with associated features of PG, dermatologic consultation and biopsy of skin lesions should be considered. Physicians should evaluate for suspected PG prior to proceeding with surgical intervention to avoid unnecessary amputation. The diagnostic criteria for classic ulcerative PG are gaining wider acceptance and are a useful tool for clinicians.
Case Report
A 67-year-old woman presented with a painful expanding ulcer on the left leg and a new nearby ulcer of 2 months’ duration. She initially was seen 2 months prior for a wound on the left knee due to a fall as well as cellulitis, which was treated with intravenous vancomycin and ceftriaxone. Wound cultures were negative for bacteria, and she was discharged without antibiotics. She presented to the emergency department 1 month later for malodorous discharge of the first ulcer with zero systemic inflammatory response syndrome criteria; no fever; and no abnormal heart rate, respiratory rate, or leukocyte count. She was discharged with wound care. After 3 weeks, she returned with a second ulcer and worsening drainage but zero systemic inflammatory response syndrome criteria. She had a medical history of Crohn disease with 9-year remission, atrial fibrillation, pacemaker, mitral valve replacement, chronic obstructive pulmonary disease, and a 51 pack-year smoking history.
Physical examination of the left leg revealed a 3×3-cm deep lesion (ulcer A) on the distal left thigh located superomedial to the knee (Figure 1) as well as a 2×1-cm deep lesion (ulcer B) on the anteromedial knee with undermining and tunneling (Figure 2). A large amount of malodorous tan bloody discharge was present on both ulcers. There were no signs of induration or crepitus.Due to concerns of skin and soft tissue infection (SSTI) or osteomyelitis, a bone scan and wound and blood cultures were ordered. The patient was started on vancomycin and piperacillin-tazobactam in the emergency department, which later was augmented with cefepime. Trauma surgery scheduled debridement for the following morning with suspicion of necrotizing fasciitis. Additional consultations were requested, including infectious disease, wound care, and dermatology. Dermatology evaluated the wound, performed a punch biopsy, and canceled debridement due to unclear diagnosis. The clinical differential at that time included pyoderma gangrenosum (PG), atypical vasculitis, or infection. Additional workup revealed positive antineutrophil cytoplasmic antibodies but negative proteinase 3 and myeloperoxidase, disfavoring vasculitis. Wound cultures grew Staphylococcus aureus and Pseudomonas aeruginosa.


Histologic evaluation revealed deep dermal necrosis with a mixed inflammatory infiltrate (Figure 3) and no organisms or vasculitis. Antibiotics were discontinued, and she was discharged on a 14-day course of prednisone 60 mg daily for empirical treatment of PG with dermatology follow-up. Medical management included a 6-month course of dapsone that was extended to 7 months because of an intensive care unit stay for a cerebrovascular accident. Daily dosing was as follows: 100 mg for 5 months, 50 mg for 1 month, and 25 mg for 1 month, then stopped. She was followed with serial complete blood cell count every 1 to 2 months and home-health wound care. One month after dapsone initiation, the ulcers decreased in size. Ulcer B was fully healed after 4 months, and ulcer A was nearly closed at 6 months without any new flares.

Comment
Pyoderma gangrenosum is a rare inflammatory skin condition that classically presents as tender papules or pustules evolving into painful ulcers, most commonly on the lower extremities. Pyoderma gangrenosum has a propensity to exhibit pathergy, the hyperreactivity of the skin in response to minor trauma. This phenomenon in PG manifests as the rapid evolution from pustule to ulceration with violaceous undermining borders.
Diagnosis of PG
Pyoderma gangrenosum has been described as a diagnosis of exclusion, as its findings frequently mimic SSTIs. Important findings to obtain are histology, history, ulcer morphology, and response to treatment.
In 2018, Maverakis et al1 proposed diagnostic criteria for classic ulcerative PG (Table 1). A diagnosis of PG can be made if the patient meets 1 major criterion and 4 minor criteria. Our case met 0 major criteria and 5 minor criteria: history of inflammatory bowel disease (IBD); history of pustule ulcerating within 4 days of appearing; peripheral erythema, undermining border, and tenderness at ulceration site; multiple ulcerations, with at least 1 on an anterior lower leg; and decreased ulcer size within 1 month of initiating immunosuppressive medication(s). Although our patient’s biopsy demonstrated a mixed infiltrate, PG was not excluded due to spontaneous resolution at the time of biopsy, emphasizing the need to biopsy subsequent new lesions if neutrophils are not initially seen.1 Pyoderma gangrenosum frequently is associated with IBD, most often Crohn disease, as seen in our patient.2-4 Although IBD classically is associated with smoking, studies have yet to conclude if smoking is a predictive factor of PG.5 Our patient presented with an initial ulcer that evolved into 2 ulcers, similar to a case of bilateral ulcers.6

Differential Diagnosis of PG
Other possible diagnoses to consider are SSTI and vasculitis, the latter being disfavored by no evidence of vasculitis on biopsy and negative titers for proteinase 3 and myeloperoxidase antibodies. However, the presence of either, similar to a mixed infiltrate, does not exclude a diagnosis of PG, as they can occur simultaneously. Consequently, superinfection of a chronically open wound can occur due to underlying PG.7 The differences between PG and SSTI are listed in Table 2.

Although we know PG involves neutrophilic dysfunction, the pathophysiology remains poorly understood, contributing to the lack of clinical guidelines.8 Therefore, the diagnosis of PG often is delayed and is associated with severe consequences such as necrotizing fasciitis, osteomyelitis, cosmetic morbidity, and limb amputation.9,10 Dermatologic consultation can aid in early diagnosis and avoid amputation.7,10 Amputation has been used as a last resort to preserve optimal outcomes in patients with severe PG.11
Management of PG
A gold standard of treatment of PG does not exist, but the goal is to promote wound healing. Patients with limited disease typically can be managed with wound care and topical steroids or calcineurin inhibitors, though data on efficacy are limited. However, our patient had more extensive disease and needed to be treated with systemic therapy. First-line therapy for extensive disease includes oral prednisone or cyclosporine for patients who cannot tolerate systemic corticosteroids.12 Second-line and adjunctive therapy options include dapsone, minocycline, methotrexate, and infliximab. Our patient was prescribed a 7-month course of dapsone with outpatient dermatology and demonstrated resolution of both ulcers. Dapsone was tapered from a daily dose of 100 mg to 50 mg to 25 mg to none over the course of 2 to 3 months. Close monitoring with wound care is recommended, and petroleum jelly can be used for dry skin around the lesion for comfort.
Conclusion
The diagnosis of PG is challenging because it relies heavily on clinical signs and often mimics SSTI. Gathering a detailed medical history is critical to make the diagnosis of PG. In a patient with associated features of PG, dermatologic consultation and biopsy of skin lesions should be considered. Physicians should evaluate for suspected PG prior to proceeding with surgical intervention to avoid unnecessary amputation. The diagnostic criteria for classic ulcerative PG are gaining wider acceptance and are a useful tool for clinicians.
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466.
- Bisarya K, Azzopardi S, Lye G, et al. Necrotizing fasciitis versus pyoderma gangrenosum: securing the correct diagnosis! a case report and literature review. Eplasty. 2011;11:E24.
- Perricone G, Vangeli M. Pyoderma gangrenosum in ulcerative colitis. N Engl J Med. 2018;379:E7.
- Ashchyan HJ, Butler DC, Nelson CA, et al. The association of age with clinical presentation and comorbidities of pyoderma gangrenosum. JAMA Dermatol. 2018;154:409-413.
- Ampuero J, Rojas-Feria M, Castro-Fernández M, et al. Predictive factors for erythema nodosum and pyoderma gangrenosum in inflammatory bowel disease. J Gastroenterol Hepatol. 2014;29:291-295.
- Ebner DW, Hu M, Poterucha TH. 29-year-old woman with fever and bilateral lower extremity lesions. Mayo Clin Proc. 2018;93:1659-1663.
- Marzak H, Von Hunolstein JJ, Lipsker D, et al. Management of a superinfected pyoderma gangrenosum after pacemaker implant. HeartRhythm Case Rep. 2018;5:63-65.
- Braswell SF, Kostopoulos TC, Ortega-Loayza AG. Pathophysiology of pyoderma gangrenosum (PG): an updated review. J Am Acad Dermatol. 2015;73:691-698.
- Saffie MG, Shroff A. A case of pyoderma gangrenosum misdiagnosed as necrotizing infection: a potential diagnostic catastrophe. Case Rep Infect Dis. 2018;2018:8907542.
- Haag CK, Nutan F, Cyrus JW, et al. Pyoderma gangrenosum misdiagnosis resulting in amputation: a review. J Trauma Acute Care Surg. 2019;86:307-313.
- Sanchez IM, Lowenstein S, Johnson KA, et al. Clinical features of neutrophilic dermatosis variants resembling necrotizing fasciitis. JAMA Dermatol. 2019;155:79-84.
- Alavi A, French LE, Davis MD, et al. Pyoderma gangrenosum: an update on pathophysiology, diagnosis and treatment. Am J Clin Dermatol. 2017;18:355-372.
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466.
- Bisarya K, Azzopardi S, Lye G, et al. Necrotizing fasciitis versus pyoderma gangrenosum: securing the correct diagnosis! a case report and literature review. Eplasty. 2011;11:E24.
- Perricone G, Vangeli M. Pyoderma gangrenosum in ulcerative colitis. N Engl J Med. 2018;379:E7.
- Ashchyan HJ, Butler DC, Nelson CA, et al. The association of age with clinical presentation and comorbidities of pyoderma gangrenosum. JAMA Dermatol. 2018;154:409-413.
- Ampuero J, Rojas-Feria M, Castro-Fernández M, et al. Predictive factors for erythema nodosum and pyoderma gangrenosum in inflammatory bowel disease. J Gastroenterol Hepatol. 2014;29:291-295.
- Ebner DW, Hu M, Poterucha TH. 29-year-old woman with fever and bilateral lower extremity lesions. Mayo Clin Proc. 2018;93:1659-1663.
- Marzak H, Von Hunolstein JJ, Lipsker D, et al. Management of a superinfected pyoderma gangrenosum after pacemaker implant. HeartRhythm Case Rep. 2018;5:63-65.
- Braswell SF, Kostopoulos TC, Ortega-Loayza AG. Pathophysiology of pyoderma gangrenosum (PG): an updated review. J Am Acad Dermatol. 2015;73:691-698.
- Saffie MG, Shroff A. A case of pyoderma gangrenosum misdiagnosed as necrotizing infection: a potential diagnostic catastrophe. Case Rep Infect Dis. 2018;2018:8907542.
- Haag CK, Nutan F, Cyrus JW, et al. Pyoderma gangrenosum misdiagnosis resulting in amputation: a review. J Trauma Acute Care Surg. 2019;86:307-313.
- Sanchez IM, Lowenstein S, Johnson KA, et al. Clinical features of neutrophilic dermatosis variants resembling necrotizing fasciitis. JAMA Dermatol. 2019;155:79-84.
- Alavi A, French LE, Davis MD, et al. Pyoderma gangrenosum: an update on pathophysiology, diagnosis and treatment. Am J Clin Dermatol. 2017;18:355-372.
Practice Points
- Pyoderma gangrenosum (PG) frequently is misdiagnosed due to its similar presentation to other skin and soft tissue infections (SSTIs). Patients with known risk factors for PG should be evaluated with a high index of suspicion to ensure early diagnosis and avoid serious complications. Common associations include inflammatory bowel disease (IBD), hematologic malignancies, and rheumatologic disorders.
- Response to treatment may be used to guide management when the diagnosis of SSTIs vs PG cannot be distinguished with clinical and histologic findings alone. In a worsening ulcer that has failed antibiotic therapy, clinicians should consider the diagnosis of PG and the risk of pathergy prior to surgical intervention such as debridement.
- Although typically a diagnosis of exclusion, clinicians can consider the use of diagnostic criteria for PG in patients of high clinical suspicion. A trial of immunosuppressants can be considered after infection has been ruled out.
COVID-19 vaccine update: Uptake, effectiveness, and safety concerns
REFERENCES
- CDC. COVID Data Tracker. Accessed June 3, 2021. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
- WHO Coronavirus (COVID-19) Dashboard. Accessed June 3, 2021. https://covid19.who.int/
- CDC. Demographic trends of people receiving COVID-19 vaccinations in the United States. Accessed June 3, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccination-demographics-trends
- Shimabukuro T. Update: thrombosis with thrombocytopenia syndrome (TTS) following COVID-19 vaccination. Presentation to the Advisory Committee on Immunization Practices, May 12, 2021. Accessed June 3, 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/07-COVID-Shimabukuro-508.pdf
- Fleming-Dutra K. CDC COVID-19 vaccine effectiveness studies. Presentation to the Advisory Committee on Immunization Practices, May 12, 2021. Accessed June 3, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/09-COVID-Fleming-Dutra-508.pdf
- Scobie H. Update on emerging SARS-CoV-2 variants and vaccine considerations. Presentation to the Advisory Committee on Immunization Practices, May 12, 2021. Accessed June 3, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/10-COVID-Scobie-508.pdf
REFERENCES
- CDC. COVID Data Tracker. Accessed June 3, 2021. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
- WHO Coronavirus (COVID-19) Dashboard. Accessed June 3, 2021. https://covid19.who.int/
- CDC. Demographic trends of people receiving COVID-19 vaccinations in the United States. Accessed June 3, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccination-demographics-trends
- Shimabukuro T. Update: thrombosis with thrombocytopenia syndrome (TTS) following COVID-19 vaccination. Presentation to the Advisory Committee on Immunization Practices, May 12, 2021. Accessed June 3, 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/07-COVID-Shimabukuro-508.pdf
- Fleming-Dutra K. CDC COVID-19 vaccine effectiveness studies. Presentation to the Advisory Committee on Immunization Practices, May 12, 2021. Accessed June 3, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/09-COVID-Fleming-Dutra-508.pdf
- Scobie H. Update on emerging SARS-CoV-2 variants and vaccine considerations. Presentation to the Advisory Committee on Immunization Practices, May 12, 2021. Accessed June 3, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/10-COVID-Scobie-508.pdf
REFERENCES
- CDC. COVID Data Tracker. Accessed June 3, 2021. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
- WHO Coronavirus (COVID-19) Dashboard. Accessed June 3, 2021. https://covid19.who.int/
- CDC. Demographic trends of people receiving COVID-19 vaccinations in the United States. Accessed June 3, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccination-demographics-trends
- Shimabukuro T. Update: thrombosis with thrombocytopenia syndrome (TTS) following COVID-19 vaccination. Presentation to the Advisory Committee on Immunization Practices, May 12, 2021. Accessed June 3, 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/07-COVID-Shimabukuro-508.pdf
- Fleming-Dutra K. CDC COVID-19 vaccine effectiveness studies. Presentation to the Advisory Committee on Immunization Practices, May 12, 2021. Accessed June 3, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/09-COVID-Fleming-Dutra-508.pdf
- Scobie H. Update on emerging SARS-CoV-2 variants and vaccine considerations. Presentation to the Advisory Committee on Immunization Practices, May 12, 2021. Accessed June 3, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/10-COVID-Scobie-508.pdf