User login
Purpura Fulminans in an Asplenic Intravenous Drug User
To the Editor:
A 56-year-old man with a history of opioid abuse and splenectomy decades prior due to a motor vehicle accident was brought to an outside emergency department with confusion, slurred speech, and difficulty breathing. Over the next few days, he became febrile and hypotensive, requiring vasopressors. Clinical laboratory testing revealed a urine drug screen positive for opioids and a low platelet count in the setting of a rapidly evolving retiform purpuric rash.
The patient was transferred to our institution 6 days after initial presentation with primary diagnoses of septic shock with multiorgan failure and disseminated intravascular coagulation (DIC). Blood cultures were positive for gram-negative rods. After several days of broad-spectrum antibiotics and supportive care, cultures were reported as positive for Capnocytophaga canimorsus. Upon further questioning, the patient’s wife reported that the couple had a new puppy and that the patient often allowed the dog to bite him playfully and lick abrasions on his hands and legs. He had not received medical treatment for any of the dog’s bites.
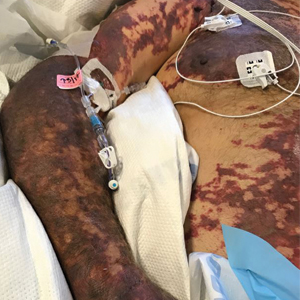
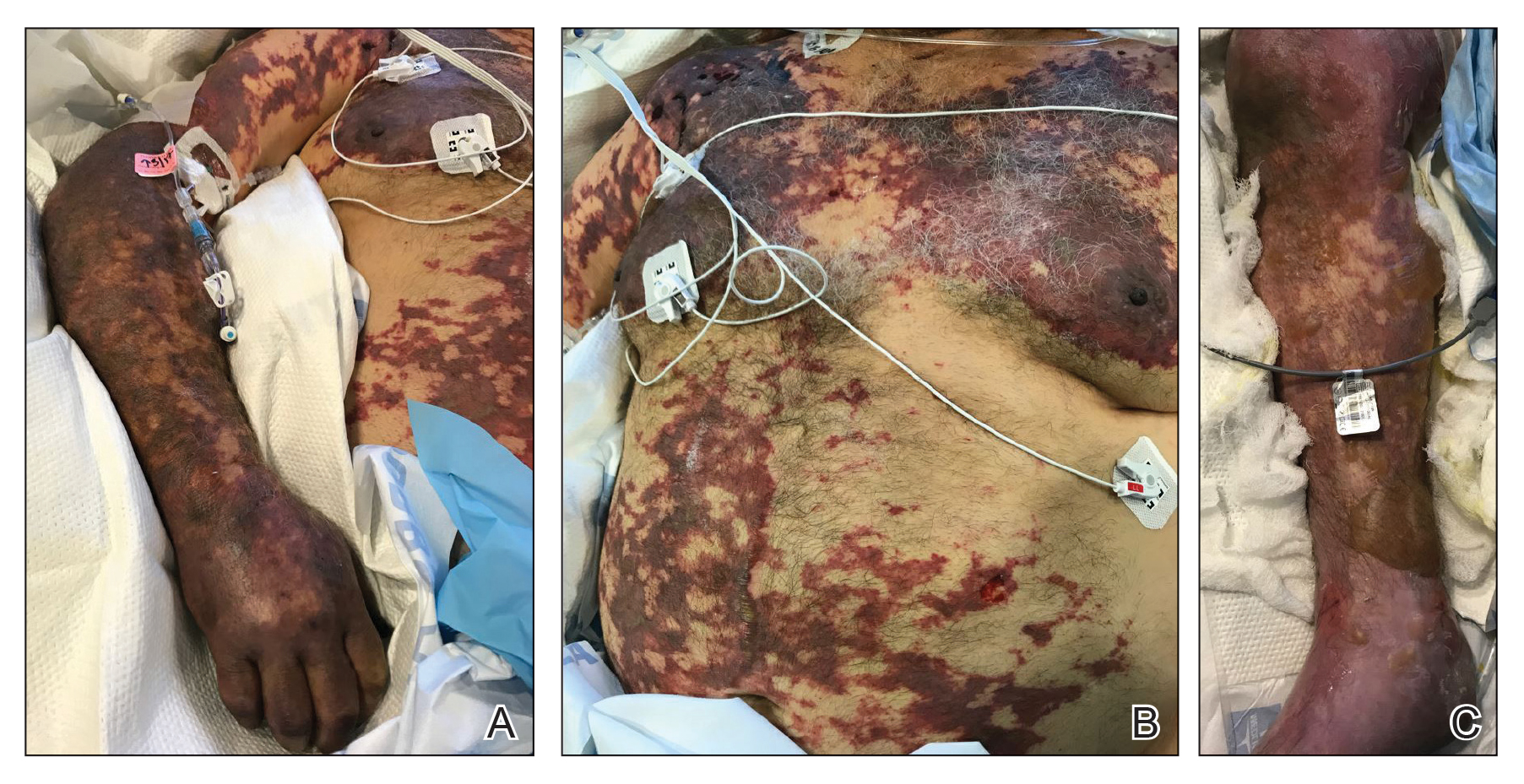
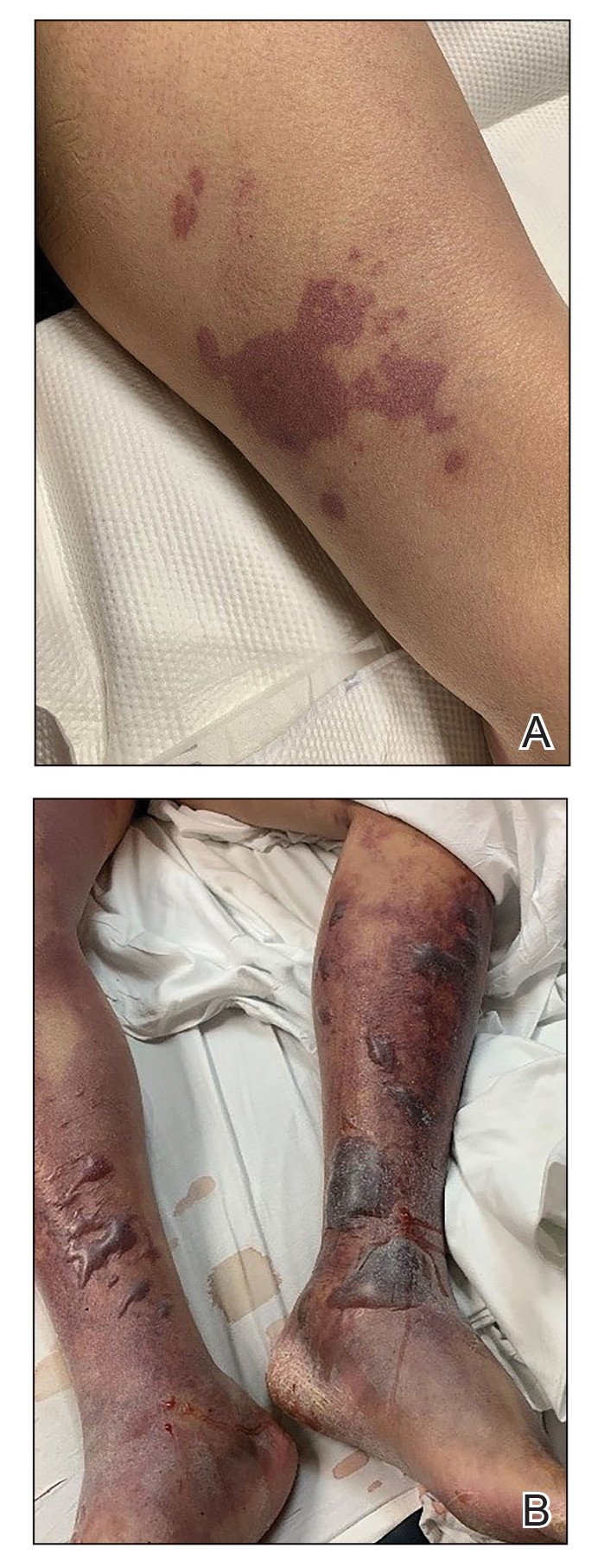
On initial examination at the time of transfer, the patient’s skin was remarkable for diffuse areas of stellate and retiform purpura with dusky centers and necrosis of the nasal tip and earlobes. Both hands were purpuric, with necrosis of the fingertips (Figure 1A). The flank was marked by large areas of full-thickness sloughing of the skin (Figure 1B). The lower extremities were edematous, with some areas of stellate purpura and numerous large bullae that drained straw-colored fluid (Figure 1C). Lower extremity pulses were found with Doppler ultrasonography.
Given the presence of rapidly developing retiform purpura in the clinical context of severe sepsis, purpura fulminans (PF) was the primary consideration in the differential diagnosis. Levamisole-induced necrosis syndrome also was considered because of necrosis of the ears and nose as well as the history of substance use; however, the patient was not known to have a history of cocaine abuse, and a test of antineutrophil cytoplasmic antibody was negative.
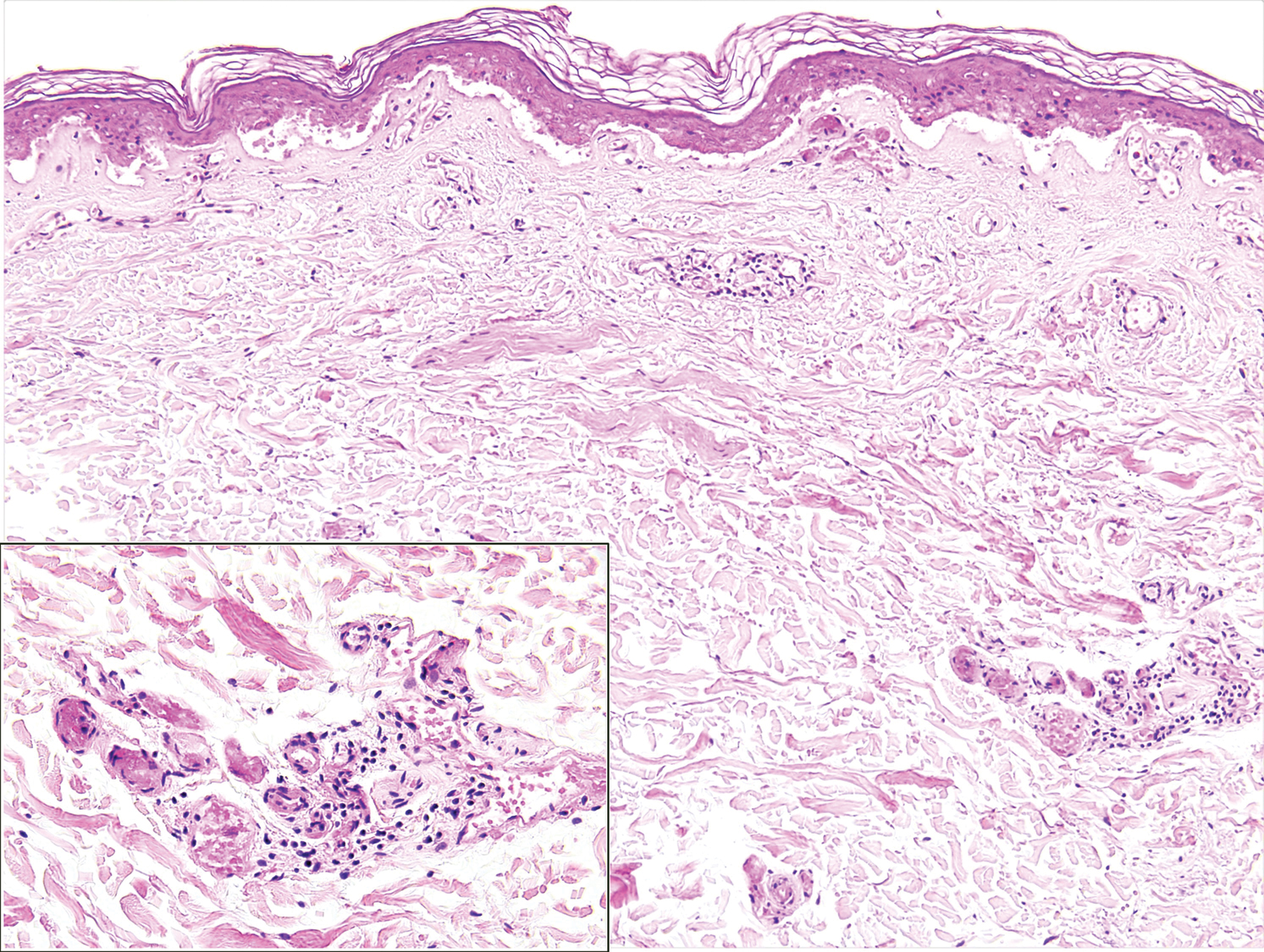
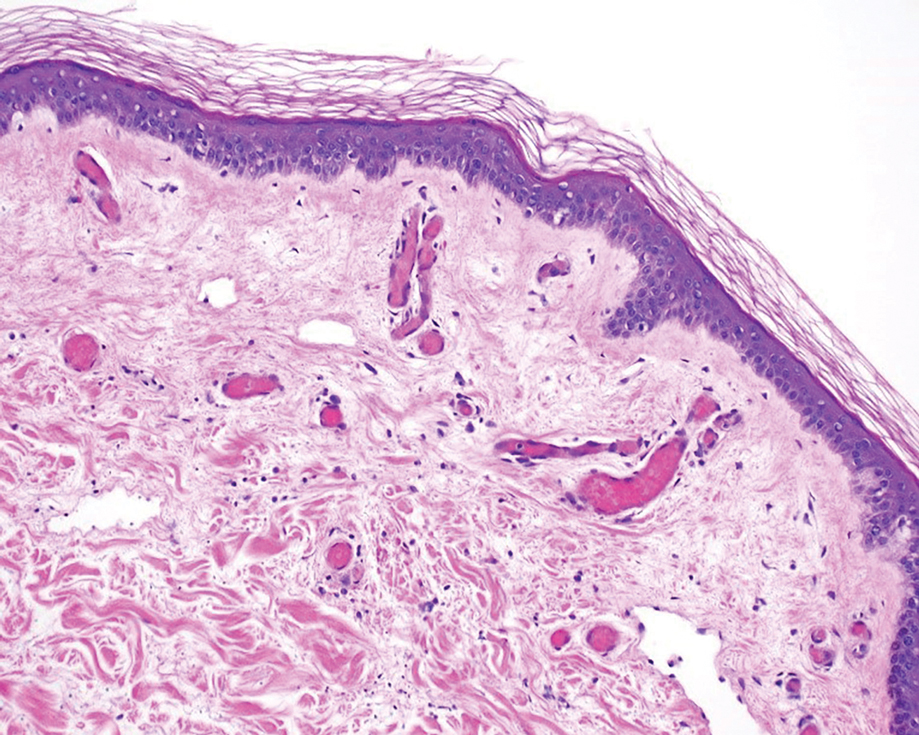
A punch biopsy of the abdomen revealed intravascular thrombi with epidermal and sweat gland necrosis, consistent with PF (Figure 2). Gram, Giemsa, and Gomori methenamine-silver stains were negative for organisms. Tissue culture remained negative. Repeat blood cultures demonstrated Candida parapsilosis fungemia. Respiratory culture was positive for budding yeast.
The patient was treated with antimicrobials, intravenous argatroban, and subcutaneous heparin. Purpura and bullae on the trunk slowly resolved with systemic therapy and wound care with petrolatum and nonadherent dressings. However, lesions on the nasal tip, all fingers of both hands, and several toes evolved into dry gangrene. The hospital course was complicated by renal failure requiring continuous renal replacement therapy; respiratory failure requiring ventilator support; and elevated levels of liver enzymes, consistent with involvement of the hepatic microvasculature.
The patient was in the medical intensive care unit at our institution for 2 weeks and was transferred to a burn center for specialized wound care. At transfer, he was still on a ventilator and receiving continuous renal replacement therapy. Subsequently, the patient required a left above-the-knee amputation, right below-the-knee amputation, and amputation of several digits of the upper extremities. In the months after the amputations, he required multiple stump revisions and experienced surgical site infections that complicated healing.
Purpura fulminans is an uncommon syndrome characterized by intravascular thrombosis and hemorrhagic infarction of the skin. The condition commonly is associated with septic shock, causing vascular collapse and DIC. It often develops rapidly.
Because of associated high mortality, it is important to differentiate PF from other causes of cutaneous retiform purpura, including other causes of thrombosis and large vessel vasculitis. Leading causes of PF include infection and hereditary or acquired deficiency of protein C, protein S, or antithrombin III. Regardless of cause, biopsy results demonstrate vascular thrombosis out of proportion to vasculitis. The mortality rate is 42% to 50%. The incidence of postinfectious sepsis sequelae in PF is higher than in survivors of sepsis only, especially amputation.1-3 Most patients do not die from complications of sepsis but from sequelae of the hypercoagulable and prothrombotic state associated with PF.4 Hemorrhagic infarction can affect the kidneys, brain, lungs, heart, eyes, and adrenal glands (ie, necrosis, namely Waterhouse-Friderichsen syndrome).5
The most common infectious cause of PF is sepsis secondary to Neisseria meningitidis, with as many as 25% of infected patients developing PF.6Streptococcus pneumoniae is another common cause. Other important causative organisms include Streptococcus pyogenes; Staphylococcus aureus (in the setting of intravenous substance use); Klebsiella oxytoca; Klebsiella aerogenes; rickettsial organisms; and viruses, including cytomegalovirus and varicella-zoster virus.2,7-13 Two earlier cases associated with Capnocytophaga were characterized by concomitant renal failure, metabolic acidosis, hemolytic anemia, and DIC.14
It is estimated that Capnocytophaga causes 11% to 46% of all cases of sepsis15; sepsis resulting from Capnocytophaga has extremely poor outcomes, with mortality reaching as high as 60%. The organism is part of the normal oral flora of cats and dogs, and a bite (less often, a scratch) is the cause of most Capnocytophaga infections. The clinical spectrum of C canimorsus infection associated with dog saliva exposure more commonly includes cellulitis at or around the site of inoculation, meningitis, and endocarditis.16
Although patients affected by PF can be young and healthy, several risk factors for PF have been identified2,6,16: asplenia, an immunocompromised state, systemic corticosteroid use, cirrhosis, and alcoholism. Asplenic patients have been shown to be particularly susceptible to systemic Capnocytophaga infection; when bitten by a dog, they should be treated with prophylactic antibiotics to cover Capnocytophaga.17 Immunocompetent patients rarely develop severe infection with Capnocytophaga.16,18,19 The complement system in particular is critically important in defending against C canimorsus.20
The underlying pathophysiology of acute infectious PF is multifactorial, encompassing increased expression of procoagulant tissue factor by monocytes and endothelial cells in the presence of bacterial pathogens. Dysfunction of protein C, an anticoagulant component of the coagulation cascade, often is cited as a crucial derangement leading to the development of a prothrombotic state in acute infectious PF.21 Serum protein S and antithrombin deficiency also can play a role.22 Specific in vitro examination of C canimorsus has revealed a protease that catalyzes N-terminal cleavage of procoagulant factor X, resulting in loss of function.15
Retiform purpura is a hallmark feature of PF, often beginning as nonblanching erythema with localized edema and petechiae before evolving into the characteristic stellate lesions with hemorrhagic bullae and subsequent necrosis.23 Pathologic examination reveals microthrombi involving arterioles and smaller vessels.24 There typically is laboratory evidence of DIC in PF, including elevated prothrombin time and partial thromboplastin time, thrombocytopenia, elevated D-dimer, and a decreased fibrinogen level.6,23
Capnocytophaga bacteria are challenging to grow on standard culture media. Optimal media for growth include 5% sheep’s blood and chocolate agar.16 Polymerase chain reaction can identify Capnocytophaga; in cases in which blood culture does not produce growth, 16S ribosomal RNA gene sequencing of tissue from skin biopsy has identified the pathogen.25
Some Capnocytophaga isolates have been shown to produce beta-lactamase; individual strains can be resistant to penicillins, cephalosporins, and imipenem.26 Factors associated with an increased risk for death include decreased leukocyte and platelet counts and an increased level of arterial lactate.27
Empiric antibiotic therapy for Capnocytophaga sepsis should include a beta-lactam and beta-lactamase inhibitor, such as piperacillin-tazobactam. Management of DIC can include therapeutic heparin or low-molecular-weight heparin and prophylactic platelet transfusion to maintain a pre-established value.28-30 Debridement should be conservative; it is important to wait for definite delineation between viable and necrotic tissue,31 which might take several months.32 Human skin allografts, in addition to artificial skin, are utilized as supplemental therapy for more rapid wound closure after removal of necrotic tissue.33,34 Hyperoxygenated fatty acids have been noted to aid in more rapid wound healing in infants with PF.35
Fresh frozen plasma is one method to replace missing factors, but it contains little protein C.36 Outcomes with recombinant human activated protein C (drotrecogin alfa) are mixed, and studies have shown no benefit in reducing the risk for death.37,38 Protein C concentrate has shown therapeutic benefit in some case reports and small retrospective studies.4 In one case report, protein C concentrate and heparin were utilized in combination with antithrombin III.21
Hyperbaric O2 might be of benefit when initiated within 5 days after onset of PF. However, hyperbaric O2 does carry risk; O2 toxicity, barotrauma, and barriers to timely resuscitation when the patient is inside the pressurized chamber can occur.2
There is a single report of successful use of the vasodilator iloprost for meningococcal PF without need for surgical intervention; the team also utilized topical nitroglycerin patches on the fingers to avoid digital amputation.39 Epoprostenol, tissue plasminogen activator, and antithrombin have been utilized in cases of extensive PF. Fibrinolytic therapy might have some utility, but only in a setting of malignancy-associated DIC.40
Treatment of acute infectious PF lacks a high level of evidence. Options include replacement of anticoagulant factors, anticoagulant therapy, hyperbaric O2, topical and systemic vasodilators, and, in the setting of underlying cancer, fibrinolytics. Even with therapy, prognosis is guarded.
- Ghosh SK, Bandyopadhyay D, Dutta A. Purpura fulminans: a cutaneous marker of disseminated intravascular coagulation. West J Emerg Med. 2009;10:41.
- Ursin Rein P, Jacobsen D, Ormaasen V, et al. Pneumococcal sepsis requiring mechanical ventilation: cohort study in 38 patients with rapid progression to septic shock. Acta Anaesthesiol Scand. 2018;62:1428-1435. doi:10.1111/aas
- Contou D, Canoui-Poitrine F, Coudroy R, et al; Hopeful Study Group. Long-term quality of life in adult patients surviving purpura fulminans: an exposed-unexposed multicenter cohort study. Clin Infect Dis. 2019;69:332-340. doi:10.1093/cid/ciy901
- Chalmers E, Cooper P, Forman K, et al. Purpura fulminans: recognition, diagnosis and management. Arch Dis Child. 2011;96:1066-1071. doi:10.1136/adc.2010.199919
- Karimi K, Odhav A, Kollipara R, et al. Acute cutaneous necrosis: a guide to early diagnosis and treatment. J Cutan Med Surg. 2017;21:425-437. doi:10.1177/1203475417708164
- Colling ME, Bendapudi PK. Purpura fulminans: mechanism and management of dysregulated hemostasis. Transfus Med Rev. 2018;32:69-76. doi:10.1016/j.tmrv.2017.10.001
- Kankeu Fonkoua L, Zhang S, Canty E, et al. Purpura fulminans from reduced protein S following cytomegalovirus and varicella infection. Am J Hematol. 2019;94:491-495. doi:10.1002/ajh.25386
- Okuzono S, Ishimura M, Kanno S, et al. Streptococcus pyogenes-purpura fulminans as an invasive form of group A streptococcal infection. Ann Clin Microbiol Antimicrob. 2018;17:31. doi:10.1186/s12941-018-0282-9
- Gupta D, Chandrashekar L, Srinivas BH, et al. Acute infectious purpura fulminans caused by group A β-hemolytic Streptococcus: an uncommon organism. Indian Dermatol Online J. 2016;7:132-133. doi:10.4103/2229-5178.178093
- Saini S, Duncan RA. Sloughing skin in intravenous drug user. IDCases. 2018;12:74-75. doi:10.1016/j.idcr.2018.03.007
- Tsubouchi N, Tsurukiri J, Numata J, et al. Acute infectious purpura fulminans caused by Klebsiella oxytoca. Intern Med. 2019;58:1801-1802. doi:10.2169/internalmedicine.2350-18
- Yamamoto S, Ito R. Acute infectious purpura fulminans with Enterobacter aerogenes post-neurosurgery. IDCases. 2019;15:e00514. doi:10.1016/j.idcr.2019.e00514
- Dalugama C, Gawarammana IB. Rare presentation of rickettsial infection as purpura fulminans: a case report. J Med Case Rep. 2018;12:145. doi:10.1186/s13256-018-1672-5
- Kazandjieva J, Antonov D, Kamarashev J, et al. Acrally distributed dermatoses: vascular dermatoses (purpura and vasculitis). Clin Dermatol. 2017;35:68-80. doi:10.1016/j.clindermatol.2016.09.013
- Hack K, Renzi F, Hess E, et al. Inactivation of human coagulation factor X by a protease of the pathogen Capnocytophaga canimorsus. J Thromb Haemost. 2017;15:487-499. doi:10.1111/jth.13605
- Zajkowska J, M, Falkowski D, et al. Capnocytophaga canimorsus—an underestimated danger after dog or cat bite - review of literature. Przegl Epidemiol. 2016;70:289-295.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet. 2011;378:86-97. doi:10.1016/S0140-6736(10)61493-6
- Behrend Christiansen C, Berg RMG, Plovsing RR, et al. Two cases of infectious purpura fulminans and septic shock caused by Capnocytophaga canimorsus transmitted from dogs. Scand J Infect Dis. 2012;44:635-639. doi:10.3109/00365548.2012.672765
- Ruddock TL, Rindler JM, Bergfeld WF. Capnocytophaga canimorsus septicemia in an asplenic patient. Cutis. 1997;60:95-97.
- Mantovani E, Busani S, Biagioni E, et al. Purpura fulminans and septic shock due to Capnocytophaga canimorsus after dog bite: a case report and review of the literature. Case Rep Crit Care. 2018;2018:7090268. doi:10.1155/2018/7090268
- Bendapudi PK, Robbins A, LeBoeuf N, et al. Persistence of endothelial thrombomodulin in a patient with infectious purpura fulminans treated with protein C concentrate. Blood Adv. 2018;2:2917-2921. doi:10.1182/bloodadvances.2018024430
- Lerolle N, Carlotti A, Melican K, et al. Assessment of the interplay between blood and skin vascular abnormalities in adult purpura fulminans. Am J Respir Crit Care Med. 2013;188:684-692. doi:10.1164/rccm.201302-0228OC.
- Thornsberry LA, LoSicco KI, English JC III. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462. doi:10.1016/j.jaad.2013.01.043
- Adcock DM, Hicks MJ. Dermatopathology of skin necrosis associated with purpura fulminans. Semin Thromb Hemost. 1990;16:283-292. doi:10.1055/s-2007-1002681
- Dautzenberg KHW, Polderman FN, van Suylen RJ, et al. Purpura fulminans mimicking toxic epidermal necrolysis—additional value of 16S rRNA sequencing and skin biopsy. Neth J Med. 2017;75:165-168.
- Zangenah S, Andersson AF, V, et al. Genomic analysis reveals the presence of a class D beta-lactamase with broad substrate specificity in animal bite associated Capnocytophaga species. Eur J Clin Microbiol Infect Dis. 2017;36:657-662. doi:10.1007/s10096-016-2842-2
- Contou D, Sonneville R, Canoui-Poitrine F, et al; Hopeful Study Group. Clinical spectrum and short-term outcome of adult patients with purpura fulminans: a French multicenter retrospective cohort study. Intensive Care Med. 2018;44:1502-1511. doi:10.1007/s00134-018-5341-3
- Zenz W, Zoehrer B, Levin M, et al; . Use of recombinant tissue plasminogen activator in children with meningococcal purpura fulminans: a retrospective study. Crit Care Med. 2004;32:1777-1780. doi:10.1097/01.ccm.0000133667.86429.5d
- Wallace JS, Hall JC. Use of drug therapy to manage acute cutaneous necrosis of the skin. J Drugs Dermatol. 2010;9:341-349.
- Squizzato A, Hunt BJ, Kinasewitz GT, et al. Supportive management strategies for disseminated intravascular coagulation. an international consensus. Thromb Haemost. 2016;115:896-904. doi:10.1160/TH15-09-0740
- Herrera R, Hobar PC, Ginsburg CM. Surgical intervention for the complications of meningococcal-induced purpura fulminans. Pediatr Infect Dis J. 1994;13:734-737. doi:10.1097/00006454-199408000-00011
- Pino PA, JA, F. Delayed surgical debridement and use of semiocclusive dressings for salvage of fingers after purpura fulminans. Hand (N Y). 2016;11:NP34-NP37. doi:10.1177/1558944716661996
- Gaucher S, J, Jarraya M. Human skin allografts as a useful adjunct in the treatment of purpura fulminans. J Wound Care. 2010;19:355-358. doi:10.12968/jowc.2010.19.8.77714
- Mazzone L, Schiestl C. Management of septic skin necroses. Eur J Pediatr Surg. 2013;23:349-358. doi:10.1055/s-0033-1352530
- G, Torra-Bou JE, Manzano-Canillas ML, et al. Management of purpura fulminans skin lesions in a premature neonate with sepsis: a case study. J Wound Care. 2019;28:198-203. doi:10.12968/jowc.2019.28.4.198
- Kizilocak H, Ozdemir N, Dikme G, et al. Homozygous protein C deficiency presenting as neonatal purpura fulminans: management with fresh frozen plasma, low molecular weight heparin and protein C concentrate. J Thromb Thrombolysis. 2018;45:315-318. doi:10.1007/s11239-017-1606-x
- Ranieri VM, Thompson BT, Barie PS, et al; . Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055-2064. doi:10.1056/NEJMoa1202290
- Bernard GR, Vincent J-L, Laterre P-F, et al; . Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699-709. doi:10.1056/NEJM200103083441001
- Hage-Sleiman M, Derre N, Verdet C, et al. Meningococcal purpura fulminans and severe myocarditis with clinical meningitis but no meningeal inflammation: a case report. BMC Infect Dis. 2019;19:252. doi:10.1186/s12879-019-3866-x
- Levi M, Toh CH, Thachil J, et al. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24-33. doi:10.1111/j.1365-2141.2009.07600.x
To the Editor:
A 56-year-old man with a history of opioid abuse and splenectomy decades prior due to a motor vehicle accident was brought to an outside emergency department with confusion, slurred speech, and difficulty breathing. Over the next few days, he became febrile and hypotensive, requiring vasopressors. Clinical laboratory testing revealed a urine drug screen positive for opioids and a low platelet count in the setting of a rapidly evolving retiform purpuric rash.
The patient was transferred to our institution 6 days after initial presentation with primary diagnoses of septic shock with multiorgan failure and disseminated intravascular coagulation (DIC). Blood cultures were positive for gram-negative rods. After several days of broad-spectrum antibiotics and supportive care, cultures were reported as positive for Capnocytophaga canimorsus. Upon further questioning, the patient’s wife reported that the couple had a new puppy and that the patient often allowed the dog to bite him playfully and lick abrasions on his hands and legs. He had not received medical treatment for any of the dog’s bites.
On initial examination at the time of transfer, the patient’s skin was remarkable for diffuse areas of stellate and retiform purpura with dusky centers and necrosis of the nasal tip and earlobes. Both hands were purpuric, with necrosis of the fingertips (Figure 1A). The flank was marked by large areas of full-thickness sloughing of the skin (Figure 1B). The lower extremities were edematous, with some areas of stellate purpura and numerous large bullae that drained straw-colored fluid (Figure 1C). Lower extremity pulses were found with Doppler ultrasonography.

Given the presence of rapidly developing retiform purpura in the clinical context of severe sepsis, purpura fulminans (PF) was the primary consideration in the differential diagnosis. Levamisole-induced necrosis syndrome also was considered because of necrosis of the ears and nose as well as the history of substance use; however, the patient was not known to have a history of cocaine abuse, and a test of antineutrophil cytoplasmic antibody was negative.
A punch biopsy of the abdomen revealed intravascular thrombi with epidermal and sweat gland necrosis, consistent with PF (Figure 2). Gram, Giemsa, and Gomori methenamine-silver stains were negative for organisms. Tissue culture remained negative. Repeat blood cultures demonstrated Candida parapsilosis fungemia. Respiratory culture was positive for budding yeast.

The patient was treated with antimicrobials, intravenous argatroban, and subcutaneous heparin. Purpura and bullae on the trunk slowly resolved with systemic therapy and wound care with petrolatum and nonadherent dressings. However, lesions on the nasal tip, all fingers of both hands, and several toes evolved into dry gangrene. The hospital course was complicated by renal failure requiring continuous renal replacement therapy; respiratory failure requiring ventilator support; and elevated levels of liver enzymes, consistent with involvement of the hepatic microvasculature.
The patient was in the medical intensive care unit at our institution for 2 weeks and was transferred to a burn center for specialized wound care. At transfer, he was still on a ventilator and receiving continuous renal replacement therapy. Subsequently, the patient required a left above-the-knee amputation, right below-the-knee amputation, and amputation of several digits of the upper extremities. In the months after the amputations, he required multiple stump revisions and experienced surgical site infections that complicated healing.
Purpura fulminans is an uncommon syndrome characterized by intravascular thrombosis and hemorrhagic infarction of the skin. The condition commonly is associated with septic shock, causing vascular collapse and DIC. It often develops rapidly.
Because of associated high mortality, it is important to differentiate PF from other causes of cutaneous retiform purpura, including other causes of thrombosis and large vessel vasculitis. Leading causes of PF include infection and hereditary or acquired deficiency of protein C, protein S, or antithrombin III. Regardless of cause, biopsy results demonstrate vascular thrombosis out of proportion to vasculitis. The mortality rate is 42% to 50%. The incidence of postinfectious sepsis sequelae in PF is higher than in survivors of sepsis only, especially amputation.1-3 Most patients do not die from complications of sepsis but from sequelae of the hypercoagulable and prothrombotic state associated with PF.4 Hemorrhagic infarction can affect the kidneys, brain, lungs, heart, eyes, and adrenal glands (ie, necrosis, namely Waterhouse-Friderichsen syndrome).5
The most common infectious cause of PF is sepsis secondary to Neisseria meningitidis, with as many as 25% of infected patients developing PF.6Streptococcus pneumoniae is another common cause. Other important causative organisms include Streptococcus pyogenes; Staphylococcus aureus (in the setting of intravenous substance use); Klebsiella oxytoca; Klebsiella aerogenes; rickettsial organisms; and viruses, including cytomegalovirus and varicella-zoster virus.2,7-13 Two earlier cases associated with Capnocytophaga were characterized by concomitant renal failure, metabolic acidosis, hemolytic anemia, and DIC.14
It is estimated that Capnocytophaga causes 11% to 46% of all cases of sepsis15; sepsis resulting from Capnocytophaga has extremely poor outcomes, with mortality reaching as high as 60%. The organism is part of the normal oral flora of cats and dogs, and a bite (less often, a scratch) is the cause of most Capnocytophaga infections. The clinical spectrum of C canimorsus infection associated with dog saliva exposure more commonly includes cellulitis at or around the site of inoculation, meningitis, and endocarditis.16
Although patients affected by PF can be young and healthy, several risk factors for PF have been identified2,6,16: asplenia, an immunocompromised state, systemic corticosteroid use, cirrhosis, and alcoholism. Asplenic patients have been shown to be particularly susceptible to systemic Capnocytophaga infection; when bitten by a dog, they should be treated with prophylactic antibiotics to cover Capnocytophaga.17 Immunocompetent patients rarely develop severe infection with Capnocytophaga.16,18,19 The complement system in particular is critically important in defending against C canimorsus.20
The underlying pathophysiology of acute infectious PF is multifactorial, encompassing increased expression of procoagulant tissue factor by monocytes and endothelial cells in the presence of bacterial pathogens. Dysfunction of protein C, an anticoagulant component of the coagulation cascade, often is cited as a crucial derangement leading to the development of a prothrombotic state in acute infectious PF.21 Serum protein S and antithrombin deficiency also can play a role.22 Specific in vitro examination of C canimorsus has revealed a protease that catalyzes N-terminal cleavage of procoagulant factor X, resulting in loss of function.15
Retiform purpura is a hallmark feature of PF, often beginning as nonblanching erythema with localized edema and petechiae before evolving into the characteristic stellate lesions with hemorrhagic bullae and subsequent necrosis.23 Pathologic examination reveals microthrombi involving arterioles and smaller vessels.24 There typically is laboratory evidence of DIC in PF, including elevated prothrombin time and partial thromboplastin time, thrombocytopenia, elevated D-dimer, and a decreased fibrinogen level.6,23
Capnocytophaga bacteria are challenging to grow on standard culture media. Optimal media for growth include 5% sheep’s blood and chocolate agar.16 Polymerase chain reaction can identify Capnocytophaga; in cases in which blood culture does not produce growth, 16S ribosomal RNA gene sequencing of tissue from skin biopsy has identified the pathogen.25
Some Capnocytophaga isolates have been shown to produce beta-lactamase; individual strains can be resistant to penicillins, cephalosporins, and imipenem.26 Factors associated with an increased risk for death include decreased leukocyte and platelet counts and an increased level of arterial lactate.27
Empiric antibiotic therapy for Capnocytophaga sepsis should include a beta-lactam and beta-lactamase inhibitor, such as piperacillin-tazobactam. Management of DIC can include therapeutic heparin or low-molecular-weight heparin and prophylactic platelet transfusion to maintain a pre-established value.28-30 Debridement should be conservative; it is important to wait for definite delineation between viable and necrotic tissue,31 which might take several months.32 Human skin allografts, in addition to artificial skin, are utilized as supplemental therapy for more rapid wound closure after removal of necrotic tissue.33,34 Hyperoxygenated fatty acids have been noted to aid in more rapid wound healing in infants with PF.35
Fresh frozen plasma is one method to replace missing factors, but it contains little protein C.36 Outcomes with recombinant human activated protein C (drotrecogin alfa) are mixed, and studies have shown no benefit in reducing the risk for death.37,38 Protein C concentrate has shown therapeutic benefit in some case reports and small retrospective studies.4 In one case report, protein C concentrate and heparin were utilized in combination with antithrombin III.21
Hyperbaric O2 might be of benefit when initiated within 5 days after onset of PF. However, hyperbaric O2 does carry risk; O2 toxicity, barotrauma, and barriers to timely resuscitation when the patient is inside the pressurized chamber can occur.2
There is a single report of successful use of the vasodilator iloprost for meningococcal PF without need for surgical intervention; the team also utilized topical nitroglycerin patches on the fingers to avoid digital amputation.39 Epoprostenol, tissue plasminogen activator, and antithrombin have been utilized in cases of extensive PF. Fibrinolytic therapy might have some utility, but only in a setting of malignancy-associated DIC.40
Treatment of acute infectious PF lacks a high level of evidence. Options include replacement of anticoagulant factors, anticoagulant therapy, hyperbaric O2, topical and systemic vasodilators, and, in the setting of underlying cancer, fibrinolytics. Even with therapy, prognosis is guarded.
To the Editor:
A 56-year-old man with a history of opioid abuse and splenectomy decades prior due to a motor vehicle accident was brought to an outside emergency department with confusion, slurred speech, and difficulty breathing. Over the next few days, he became febrile and hypotensive, requiring vasopressors. Clinical laboratory testing revealed a urine drug screen positive for opioids and a low platelet count in the setting of a rapidly evolving retiform purpuric rash.
The patient was transferred to our institution 6 days after initial presentation with primary diagnoses of septic shock with multiorgan failure and disseminated intravascular coagulation (DIC). Blood cultures were positive for gram-negative rods. After several days of broad-spectrum antibiotics and supportive care, cultures were reported as positive for Capnocytophaga canimorsus. Upon further questioning, the patient’s wife reported that the couple had a new puppy and that the patient often allowed the dog to bite him playfully and lick abrasions on his hands and legs. He had not received medical treatment for any of the dog’s bites.
On initial examination at the time of transfer, the patient’s skin was remarkable for diffuse areas of stellate and retiform purpura with dusky centers and necrosis of the nasal tip and earlobes. Both hands were purpuric, with necrosis of the fingertips (Figure 1A). The flank was marked by large areas of full-thickness sloughing of the skin (Figure 1B). The lower extremities were edematous, with some areas of stellate purpura and numerous large bullae that drained straw-colored fluid (Figure 1C). Lower extremity pulses were found with Doppler ultrasonography.

Given the presence of rapidly developing retiform purpura in the clinical context of severe sepsis, purpura fulminans (PF) was the primary consideration in the differential diagnosis. Levamisole-induced necrosis syndrome also was considered because of necrosis of the ears and nose as well as the history of substance use; however, the patient was not known to have a history of cocaine abuse, and a test of antineutrophil cytoplasmic antibody was negative.
A punch biopsy of the abdomen revealed intravascular thrombi with epidermal and sweat gland necrosis, consistent with PF (Figure 2). Gram, Giemsa, and Gomori methenamine-silver stains were negative for organisms. Tissue culture remained negative. Repeat blood cultures demonstrated Candida parapsilosis fungemia. Respiratory culture was positive for budding yeast.

The patient was treated with antimicrobials, intravenous argatroban, and subcutaneous heparin. Purpura and bullae on the trunk slowly resolved with systemic therapy and wound care with petrolatum and nonadherent dressings. However, lesions on the nasal tip, all fingers of both hands, and several toes evolved into dry gangrene. The hospital course was complicated by renal failure requiring continuous renal replacement therapy; respiratory failure requiring ventilator support; and elevated levels of liver enzymes, consistent with involvement of the hepatic microvasculature.
The patient was in the medical intensive care unit at our institution for 2 weeks and was transferred to a burn center for specialized wound care. At transfer, he was still on a ventilator and receiving continuous renal replacement therapy. Subsequently, the patient required a left above-the-knee amputation, right below-the-knee amputation, and amputation of several digits of the upper extremities. In the months after the amputations, he required multiple stump revisions and experienced surgical site infections that complicated healing.
Purpura fulminans is an uncommon syndrome characterized by intravascular thrombosis and hemorrhagic infarction of the skin. The condition commonly is associated with septic shock, causing vascular collapse and DIC. It often develops rapidly.
Because of associated high mortality, it is important to differentiate PF from other causes of cutaneous retiform purpura, including other causes of thrombosis and large vessel vasculitis. Leading causes of PF include infection and hereditary or acquired deficiency of protein C, protein S, or antithrombin III. Regardless of cause, biopsy results demonstrate vascular thrombosis out of proportion to vasculitis. The mortality rate is 42% to 50%. The incidence of postinfectious sepsis sequelae in PF is higher than in survivors of sepsis only, especially amputation.1-3 Most patients do not die from complications of sepsis but from sequelae of the hypercoagulable and prothrombotic state associated with PF.4 Hemorrhagic infarction can affect the kidneys, brain, lungs, heart, eyes, and adrenal glands (ie, necrosis, namely Waterhouse-Friderichsen syndrome).5
The most common infectious cause of PF is sepsis secondary to Neisseria meningitidis, with as many as 25% of infected patients developing PF.6Streptococcus pneumoniae is another common cause. Other important causative organisms include Streptococcus pyogenes; Staphylococcus aureus (in the setting of intravenous substance use); Klebsiella oxytoca; Klebsiella aerogenes; rickettsial organisms; and viruses, including cytomegalovirus and varicella-zoster virus.2,7-13 Two earlier cases associated with Capnocytophaga were characterized by concomitant renal failure, metabolic acidosis, hemolytic anemia, and DIC.14
It is estimated that Capnocytophaga causes 11% to 46% of all cases of sepsis15; sepsis resulting from Capnocytophaga has extremely poor outcomes, with mortality reaching as high as 60%. The organism is part of the normal oral flora of cats and dogs, and a bite (less often, a scratch) is the cause of most Capnocytophaga infections. The clinical spectrum of C canimorsus infection associated with dog saliva exposure more commonly includes cellulitis at or around the site of inoculation, meningitis, and endocarditis.16
Although patients affected by PF can be young and healthy, several risk factors for PF have been identified2,6,16: asplenia, an immunocompromised state, systemic corticosteroid use, cirrhosis, and alcoholism. Asplenic patients have been shown to be particularly susceptible to systemic Capnocytophaga infection; when bitten by a dog, they should be treated with prophylactic antibiotics to cover Capnocytophaga.17 Immunocompetent patients rarely develop severe infection with Capnocytophaga.16,18,19 The complement system in particular is critically important in defending against C canimorsus.20
The underlying pathophysiology of acute infectious PF is multifactorial, encompassing increased expression of procoagulant tissue factor by monocytes and endothelial cells in the presence of bacterial pathogens. Dysfunction of protein C, an anticoagulant component of the coagulation cascade, often is cited as a crucial derangement leading to the development of a prothrombotic state in acute infectious PF.21 Serum protein S and antithrombin deficiency also can play a role.22 Specific in vitro examination of C canimorsus has revealed a protease that catalyzes N-terminal cleavage of procoagulant factor X, resulting in loss of function.15
Retiform purpura is a hallmark feature of PF, often beginning as nonblanching erythema with localized edema and petechiae before evolving into the characteristic stellate lesions with hemorrhagic bullae and subsequent necrosis.23 Pathologic examination reveals microthrombi involving arterioles and smaller vessels.24 There typically is laboratory evidence of DIC in PF, including elevated prothrombin time and partial thromboplastin time, thrombocytopenia, elevated D-dimer, and a decreased fibrinogen level.6,23
Capnocytophaga bacteria are challenging to grow on standard culture media. Optimal media for growth include 5% sheep’s blood and chocolate agar.16 Polymerase chain reaction can identify Capnocytophaga; in cases in which blood culture does not produce growth, 16S ribosomal RNA gene sequencing of tissue from skin biopsy has identified the pathogen.25
Some Capnocytophaga isolates have been shown to produce beta-lactamase; individual strains can be resistant to penicillins, cephalosporins, and imipenem.26 Factors associated with an increased risk for death include decreased leukocyte and platelet counts and an increased level of arterial lactate.27
Empiric antibiotic therapy for Capnocytophaga sepsis should include a beta-lactam and beta-lactamase inhibitor, such as piperacillin-tazobactam. Management of DIC can include therapeutic heparin or low-molecular-weight heparin and prophylactic platelet transfusion to maintain a pre-established value.28-30 Debridement should be conservative; it is important to wait for definite delineation between viable and necrotic tissue,31 which might take several months.32 Human skin allografts, in addition to artificial skin, are utilized as supplemental therapy for more rapid wound closure after removal of necrotic tissue.33,34 Hyperoxygenated fatty acids have been noted to aid in more rapid wound healing in infants with PF.35
Fresh frozen plasma is one method to replace missing factors, but it contains little protein C.36 Outcomes with recombinant human activated protein C (drotrecogin alfa) are mixed, and studies have shown no benefit in reducing the risk for death.37,38 Protein C concentrate has shown therapeutic benefit in some case reports and small retrospective studies.4 In one case report, protein C concentrate and heparin were utilized in combination with antithrombin III.21
Hyperbaric O2 might be of benefit when initiated within 5 days after onset of PF. However, hyperbaric O2 does carry risk; O2 toxicity, barotrauma, and barriers to timely resuscitation when the patient is inside the pressurized chamber can occur.2
There is a single report of successful use of the vasodilator iloprost for meningococcal PF without need for surgical intervention; the team also utilized topical nitroglycerin patches on the fingers to avoid digital amputation.39 Epoprostenol, tissue plasminogen activator, and antithrombin have been utilized in cases of extensive PF. Fibrinolytic therapy might have some utility, but only in a setting of malignancy-associated DIC.40
Treatment of acute infectious PF lacks a high level of evidence. Options include replacement of anticoagulant factors, anticoagulant therapy, hyperbaric O2, topical and systemic vasodilators, and, in the setting of underlying cancer, fibrinolytics. Even with therapy, prognosis is guarded.
- Ghosh SK, Bandyopadhyay D, Dutta A. Purpura fulminans: a cutaneous marker of disseminated intravascular coagulation. West J Emerg Med. 2009;10:41.
- Ursin Rein P, Jacobsen D, Ormaasen V, et al. Pneumococcal sepsis requiring mechanical ventilation: cohort study in 38 patients with rapid progression to septic shock. Acta Anaesthesiol Scand. 2018;62:1428-1435. doi:10.1111/aas
- Contou D, Canoui-Poitrine F, Coudroy R, et al; Hopeful Study Group. Long-term quality of life in adult patients surviving purpura fulminans: an exposed-unexposed multicenter cohort study. Clin Infect Dis. 2019;69:332-340. doi:10.1093/cid/ciy901
- Chalmers E, Cooper P, Forman K, et al. Purpura fulminans: recognition, diagnosis and management. Arch Dis Child. 2011;96:1066-1071. doi:10.1136/adc.2010.199919
- Karimi K, Odhav A, Kollipara R, et al. Acute cutaneous necrosis: a guide to early diagnosis and treatment. J Cutan Med Surg. 2017;21:425-437. doi:10.1177/1203475417708164
- Colling ME, Bendapudi PK. Purpura fulminans: mechanism and management of dysregulated hemostasis. Transfus Med Rev. 2018;32:69-76. doi:10.1016/j.tmrv.2017.10.001
- Kankeu Fonkoua L, Zhang S, Canty E, et al. Purpura fulminans from reduced protein S following cytomegalovirus and varicella infection. Am J Hematol. 2019;94:491-495. doi:10.1002/ajh.25386
- Okuzono S, Ishimura M, Kanno S, et al. Streptococcus pyogenes-purpura fulminans as an invasive form of group A streptococcal infection. Ann Clin Microbiol Antimicrob. 2018;17:31. doi:10.1186/s12941-018-0282-9
- Gupta D, Chandrashekar L, Srinivas BH, et al. Acute infectious purpura fulminans caused by group A β-hemolytic Streptococcus: an uncommon organism. Indian Dermatol Online J. 2016;7:132-133. doi:10.4103/2229-5178.178093
- Saini S, Duncan RA. Sloughing skin in intravenous drug user. IDCases. 2018;12:74-75. doi:10.1016/j.idcr.2018.03.007
- Tsubouchi N, Tsurukiri J, Numata J, et al. Acute infectious purpura fulminans caused by Klebsiella oxytoca. Intern Med. 2019;58:1801-1802. doi:10.2169/internalmedicine.2350-18
- Yamamoto S, Ito R. Acute infectious purpura fulminans with Enterobacter aerogenes post-neurosurgery. IDCases. 2019;15:e00514. doi:10.1016/j.idcr.2019.e00514
- Dalugama C, Gawarammana IB. Rare presentation of rickettsial infection as purpura fulminans: a case report. J Med Case Rep. 2018;12:145. doi:10.1186/s13256-018-1672-5
- Kazandjieva J, Antonov D, Kamarashev J, et al. Acrally distributed dermatoses: vascular dermatoses (purpura and vasculitis). Clin Dermatol. 2017;35:68-80. doi:10.1016/j.clindermatol.2016.09.013
- Hack K, Renzi F, Hess E, et al. Inactivation of human coagulation factor X by a protease of the pathogen Capnocytophaga canimorsus. J Thromb Haemost. 2017;15:487-499. doi:10.1111/jth.13605
- Zajkowska J, M, Falkowski D, et al. Capnocytophaga canimorsus—an underestimated danger after dog or cat bite - review of literature. Przegl Epidemiol. 2016;70:289-295.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet. 2011;378:86-97. doi:10.1016/S0140-6736(10)61493-6
- Behrend Christiansen C, Berg RMG, Plovsing RR, et al. Two cases of infectious purpura fulminans and septic shock caused by Capnocytophaga canimorsus transmitted from dogs. Scand J Infect Dis. 2012;44:635-639. doi:10.3109/00365548.2012.672765
- Ruddock TL, Rindler JM, Bergfeld WF. Capnocytophaga canimorsus septicemia in an asplenic patient. Cutis. 1997;60:95-97.
- Mantovani E, Busani S, Biagioni E, et al. Purpura fulminans and septic shock due to Capnocytophaga canimorsus after dog bite: a case report and review of the literature. Case Rep Crit Care. 2018;2018:7090268. doi:10.1155/2018/7090268
- Bendapudi PK, Robbins A, LeBoeuf N, et al. Persistence of endothelial thrombomodulin in a patient with infectious purpura fulminans treated with protein C concentrate. Blood Adv. 2018;2:2917-2921. doi:10.1182/bloodadvances.2018024430
- Lerolle N, Carlotti A, Melican K, et al. Assessment of the interplay between blood and skin vascular abnormalities in adult purpura fulminans. Am J Respir Crit Care Med. 2013;188:684-692. doi:10.1164/rccm.201302-0228OC.
- Thornsberry LA, LoSicco KI, English JC III. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462. doi:10.1016/j.jaad.2013.01.043
- Adcock DM, Hicks MJ. Dermatopathology of skin necrosis associated with purpura fulminans. Semin Thromb Hemost. 1990;16:283-292. doi:10.1055/s-2007-1002681
- Dautzenberg KHW, Polderman FN, van Suylen RJ, et al. Purpura fulminans mimicking toxic epidermal necrolysis—additional value of 16S rRNA sequencing and skin biopsy. Neth J Med. 2017;75:165-168.
- Zangenah S, Andersson AF, V, et al. Genomic analysis reveals the presence of a class D beta-lactamase with broad substrate specificity in animal bite associated Capnocytophaga species. Eur J Clin Microbiol Infect Dis. 2017;36:657-662. doi:10.1007/s10096-016-2842-2
- Contou D, Sonneville R, Canoui-Poitrine F, et al; Hopeful Study Group. Clinical spectrum and short-term outcome of adult patients with purpura fulminans: a French multicenter retrospective cohort study. Intensive Care Med. 2018;44:1502-1511. doi:10.1007/s00134-018-5341-3
- Zenz W, Zoehrer B, Levin M, et al; . Use of recombinant tissue plasminogen activator in children with meningococcal purpura fulminans: a retrospective study. Crit Care Med. 2004;32:1777-1780. doi:10.1097/01.ccm.0000133667.86429.5d
- Wallace JS, Hall JC. Use of drug therapy to manage acute cutaneous necrosis of the skin. J Drugs Dermatol. 2010;9:341-349.
- Squizzato A, Hunt BJ, Kinasewitz GT, et al. Supportive management strategies for disseminated intravascular coagulation. an international consensus. Thromb Haemost. 2016;115:896-904. doi:10.1160/TH15-09-0740
- Herrera R, Hobar PC, Ginsburg CM. Surgical intervention for the complications of meningococcal-induced purpura fulminans. Pediatr Infect Dis J. 1994;13:734-737. doi:10.1097/00006454-199408000-00011
- Pino PA, JA, F. Delayed surgical debridement and use of semiocclusive dressings for salvage of fingers after purpura fulminans. Hand (N Y). 2016;11:NP34-NP37. doi:10.1177/1558944716661996
- Gaucher S, J, Jarraya M. Human skin allografts as a useful adjunct in the treatment of purpura fulminans. J Wound Care. 2010;19:355-358. doi:10.12968/jowc.2010.19.8.77714
- Mazzone L, Schiestl C. Management of septic skin necroses. Eur J Pediatr Surg. 2013;23:349-358. doi:10.1055/s-0033-1352530
- G, Torra-Bou JE, Manzano-Canillas ML, et al. Management of purpura fulminans skin lesions in a premature neonate with sepsis: a case study. J Wound Care. 2019;28:198-203. doi:10.12968/jowc.2019.28.4.198
- Kizilocak H, Ozdemir N, Dikme G, et al. Homozygous protein C deficiency presenting as neonatal purpura fulminans: management with fresh frozen plasma, low molecular weight heparin and protein C concentrate. J Thromb Thrombolysis. 2018;45:315-318. doi:10.1007/s11239-017-1606-x
- Ranieri VM, Thompson BT, Barie PS, et al; . Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055-2064. doi:10.1056/NEJMoa1202290
- Bernard GR, Vincent J-L, Laterre P-F, et al; . Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699-709. doi:10.1056/NEJM200103083441001
- Hage-Sleiman M, Derre N, Verdet C, et al. Meningococcal purpura fulminans and severe myocarditis with clinical meningitis but no meningeal inflammation: a case report. BMC Infect Dis. 2019;19:252. doi:10.1186/s12879-019-3866-x
- Levi M, Toh CH, Thachil J, et al. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24-33. doi:10.1111/j.1365-2141.2009.07600.x
- Ghosh SK, Bandyopadhyay D, Dutta A. Purpura fulminans: a cutaneous marker of disseminated intravascular coagulation. West J Emerg Med. 2009;10:41.
- Ursin Rein P, Jacobsen D, Ormaasen V, et al. Pneumococcal sepsis requiring mechanical ventilation: cohort study in 38 patients with rapid progression to septic shock. Acta Anaesthesiol Scand. 2018;62:1428-1435. doi:10.1111/aas
- Contou D, Canoui-Poitrine F, Coudroy R, et al; Hopeful Study Group. Long-term quality of life in adult patients surviving purpura fulminans: an exposed-unexposed multicenter cohort study. Clin Infect Dis. 2019;69:332-340. doi:10.1093/cid/ciy901
- Chalmers E, Cooper P, Forman K, et al. Purpura fulminans: recognition, diagnosis and management. Arch Dis Child. 2011;96:1066-1071. doi:10.1136/adc.2010.199919
- Karimi K, Odhav A, Kollipara R, et al. Acute cutaneous necrosis: a guide to early diagnosis and treatment. J Cutan Med Surg. 2017;21:425-437. doi:10.1177/1203475417708164
- Colling ME, Bendapudi PK. Purpura fulminans: mechanism and management of dysregulated hemostasis. Transfus Med Rev. 2018;32:69-76. doi:10.1016/j.tmrv.2017.10.001
- Kankeu Fonkoua L, Zhang S, Canty E, et al. Purpura fulminans from reduced protein S following cytomegalovirus and varicella infection. Am J Hematol. 2019;94:491-495. doi:10.1002/ajh.25386
- Okuzono S, Ishimura M, Kanno S, et al. Streptococcus pyogenes-purpura fulminans as an invasive form of group A streptococcal infection. Ann Clin Microbiol Antimicrob. 2018;17:31. doi:10.1186/s12941-018-0282-9
- Gupta D, Chandrashekar L, Srinivas BH, et al. Acute infectious purpura fulminans caused by group A β-hemolytic Streptococcus: an uncommon organism. Indian Dermatol Online J. 2016;7:132-133. doi:10.4103/2229-5178.178093
- Saini S, Duncan RA. Sloughing skin in intravenous drug user. IDCases. 2018;12:74-75. doi:10.1016/j.idcr.2018.03.007
- Tsubouchi N, Tsurukiri J, Numata J, et al. Acute infectious purpura fulminans caused by Klebsiella oxytoca. Intern Med. 2019;58:1801-1802. doi:10.2169/internalmedicine.2350-18
- Yamamoto S, Ito R. Acute infectious purpura fulminans with Enterobacter aerogenes post-neurosurgery. IDCases. 2019;15:e00514. doi:10.1016/j.idcr.2019.e00514
- Dalugama C, Gawarammana IB. Rare presentation of rickettsial infection as purpura fulminans: a case report. J Med Case Rep. 2018;12:145. doi:10.1186/s13256-018-1672-5
- Kazandjieva J, Antonov D, Kamarashev J, et al. Acrally distributed dermatoses: vascular dermatoses (purpura and vasculitis). Clin Dermatol. 2017;35:68-80. doi:10.1016/j.clindermatol.2016.09.013
- Hack K, Renzi F, Hess E, et al. Inactivation of human coagulation factor X by a protease of the pathogen Capnocytophaga canimorsus. J Thromb Haemost. 2017;15:487-499. doi:10.1111/jth.13605
- Zajkowska J, M, Falkowski D, et al. Capnocytophaga canimorsus—an underestimated danger after dog or cat bite - review of literature. Przegl Epidemiol. 2016;70:289-295.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet. 2011;378:86-97. doi:10.1016/S0140-6736(10)61493-6
- Behrend Christiansen C, Berg RMG, Plovsing RR, et al. Two cases of infectious purpura fulminans and septic shock caused by Capnocytophaga canimorsus transmitted from dogs. Scand J Infect Dis. 2012;44:635-639. doi:10.3109/00365548.2012.672765
- Ruddock TL, Rindler JM, Bergfeld WF. Capnocytophaga canimorsus septicemia in an asplenic patient. Cutis. 1997;60:95-97.
- Mantovani E, Busani S, Biagioni E, et al. Purpura fulminans and septic shock due to Capnocytophaga canimorsus after dog bite: a case report and review of the literature. Case Rep Crit Care. 2018;2018:7090268. doi:10.1155/2018/7090268
- Bendapudi PK, Robbins A, LeBoeuf N, et al. Persistence of endothelial thrombomodulin in a patient with infectious purpura fulminans treated with protein C concentrate. Blood Adv. 2018;2:2917-2921. doi:10.1182/bloodadvances.2018024430
- Lerolle N, Carlotti A, Melican K, et al. Assessment of the interplay between blood and skin vascular abnormalities in adult purpura fulminans. Am J Respir Crit Care Med. 2013;188:684-692. doi:10.1164/rccm.201302-0228OC.
- Thornsberry LA, LoSicco KI, English JC III. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462. doi:10.1016/j.jaad.2013.01.043
- Adcock DM, Hicks MJ. Dermatopathology of skin necrosis associated with purpura fulminans. Semin Thromb Hemost. 1990;16:283-292. doi:10.1055/s-2007-1002681
- Dautzenberg KHW, Polderman FN, van Suylen RJ, et al. Purpura fulminans mimicking toxic epidermal necrolysis—additional value of 16S rRNA sequencing and skin biopsy. Neth J Med. 2017;75:165-168.
- Zangenah S, Andersson AF, V, et al. Genomic analysis reveals the presence of a class D beta-lactamase with broad substrate specificity in animal bite associated Capnocytophaga species. Eur J Clin Microbiol Infect Dis. 2017;36:657-662. doi:10.1007/s10096-016-2842-2
- Contou D, Sonneville R, Canoui-Poitrine F, et al; Hopeful Study Group. Clinical spectrum and short-term outcome of adult patients with purpura fulminans: a French multicenter retrospective cohort study. Intensive Care Med. 2018;44:1502-1511. doi:10.1007/s00134-018-5341-3
- Zenz W, Zoehrer B, Levin M, et al; . Use of recombinant tissue plasminogen activator in children with meningococcal purpura fulminans: a retrospective study. Crit Care Med. 2004;32:1777-1780. doi:10.1097/01.ccm.0000133667.86429.5d
- Wallace JS, Hall JC. Use of drug therapy to manage acute cutaneous necrosis of the skin. J Drugs Dermatol. 2010;9:341-349.
- Squizzato A, Hunt BJ, Kinasewitz GT, et al. Supportive management strategies for disseminated intravascular coagulation. an international consensus. Thromb Haemost. 2016;115:896-904. doi:10.1160/TH15-09-0740
- Herrera R, Hobar PC, Ginsburg CM. Surgical intervention for the complications of meningococcal-induced purpura fulminans. Pediatr Infect Dis J. 1994;13:734-737. doi:10.1097/00006454-199408000-00011
- Pino PA, JA, F. Delayed surgical debridement and use of semiocclusive dressings for salvage of fingers after purpura fulminans. Hand (N Y). 2016;11:NP34-NP37. doi:10.1177/1558944716661996
- Gaucher S, J, Jarraya M. Human skin allografts as a useful adjunct in the treatment of purpura fulminans. J Wound Care. 2010;19:355-358. doi:10.12968/jowc.2010.19.8.77714
- Mazzone L, Schiestl C. Management of septic skin necroses. Eur J Pediatr Surg. 2013;23:349-358. doi:10.1055/s-0033-1352530
- G, Torra-Bou JE, Manzano-Canillas ML, et al. Management of purpura fulminans skin lesions in a premature neonate with sepsis: a case study. J Wound Care. 2019;28:198-203. doi:10.12968/jowc.2019.28.4.198
- Kizilocak H, Ozdemir N, Dikme G, et al. Homozygous protein C deficiency presenting as neonatal purpura fulminans: management with fresh frozen plasma, low molecular weight heparin and protein C concentrate. J Thromb Thrombolysis. 2018;45:315-318. doi:10.1007/s11239-017-1606-x
- Ranieri VM, Thompson BT, Barie PS, et al; . Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055-2064. doi:10.1056/NEJMoa1202290
- Bernard GR, Vincent J-L, Laterre P-F, et al; . Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699-709. doi:10.1056/NEJM200103083441001
- Hage-Sleiman M, Derre N, Verdet C, et al. Meningococcal purpura fulminans and severe myocarditis with clinical meningitis but no meningeal inflammation: a case report. BMC Infect Dis. 2019;19:252. doi:10.1186/s12879-019-3866-x
- Levi M, Toh CH, Thachil J, et al. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24-33. doi:10.1111/j.1365-2141.2009.07600.x
Practice Points
- Capnocytophaga species are fastidious, slow-growing microorganisms. It is important, therefore, to maintain a high degree of suspicion and alertthe microbiology laboratory to increase the likelihood of isolation.
- Patients should be cautioned regarding the need for prophylactic antibiotics in the event of an animal bite; asplenic patients are at particular risk for infection.
- In patients with severe purpura fulminans and a gangrenous limb, it is important to allow adequate time for demarcation of gangrene and not rush to amputation.
Purpura Fulminans Induced by Vibrio vulnificus
To the Editor:
Purpura fulminans (PF) is an acute, life-threatening condition characterized by intravascular thrombosis and hemorrhagic necrosis of the skin. It classically presents as retiform purpura with branched or angular purpuric lesions. Purpura fulminans often occurs in the setting of disseminated intravascular coagulation, secondary to sepsis, trauma, malignancy, autoimmune disease, and congenital or acquired protein C or S deficiency, among other abnormalities.1 Rapid identification and treatment of the underlying cause are mainstays of management. We report a case of PF secondary to Vibrio vulnificus infection and highlight the importance of timely consideration of this etiologic agent due to the high mortality rate and specific treatment required.
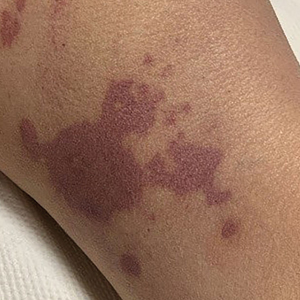
A 58-year-old man with liver cirrhosis and hepatitis B virus presented with pain, swelling, and localized erythema affecting both legs as well as a fever. He reported vomiting blood and an episode of bloody diarrhea over the preceding 24 hours. He denied exposure to sick contacts or a history of autoimmune disease. At initial presentation to the emergency department, physical examination revealed few scattered, sharply demarcated, erythematous to violaceous patches that rapidly progressed overnight to hemorrhagic bullae and widespread retiform purpuric patches on both legs (Figure 1). As the patient’s skin condition worsened, he had a blood pressure of 80/50 mm Hg and a pulse rate of 110/min. Serum analysis was notable for mild leukocytosis (10.74×109/L [reference range, 4.8–10.8×109/L), thrombocytopenia (39×109/L [reference range, 150–450×109/L]), and decreased C3 (25 mg/dL [reference range, 81–157 mg/dL]) and C4 (8 mg/dL [reference range, 13–39 mg/dL]). Laboratory findings also were remarkable for prothrombin time (23.3 seconds [reference range, 8.8–12.3 seconds]), partial thromboplastin time (52.5 seconds [reference range, 23.6–35.8 seconds]), and international normalized ratio (2.01 [reference range, 0.8–1.13]). Aspartate transaminase (237 U/L [reference range, 11–39 U/L]) and alanine transaminase (80 U/L [reference range, 11–35 U/L]) were elevated, while antineutrophil cytoplasmic antibodies, serum immunoglobulin, and cryoglobulins were unremarkable. Punch biopsies of the left thigh were performed, and histopathology revealed small vessel thrombosis and ischemic changes consistent with PF (Figure 2). Vancomycin, clindamycin, cefepime injection, and piperacillin-tazobactam were administered intravenously for empiric broad-spectrum sepsis coverage. Within hours, the patient experienced refractory septic shock with disseminated intravascular coagulation and died from pulmonary embolism and subsequent cardiac arrest. Tissue and blood cultures grew V vulnificus.
Vibrio vulnificus is a gram-negative bacillus and a rare cause of primary septicemia following consumption of shellfish, especially oysters. Wounds exposed to saltwater or brackish water contaminated with the microorganism can produce soft-tissue infections. Individuals with chronic liver disease are at greater risk for V vulnificus infection.2 The clinical presentation of V vulnificus includes early cellulitislike patches, late purpura with hemorrhagic bullae, and rapidly progressing shock.3
Mortality rates from V vulnificus infection are high.4 Therefore, it is recommended to presumptively diagnose V vulnificus septicemia in any individual at risk for infection who presents with the characteristic history in the setting of hypotension, fever, or septic shock. It is crucial for providers to be aware that broad-spectrum antibiotics commonly used for sepsis are inadequate for the treatment of V vulnificus. Immediate treatment with tetracycline (minocycline or doxycycline) and a third-generation cephalosporin (cefotaxime or ceftriaxone injection) or in combination with ciprofloxacin has been proven effective.4,5
Vibrio vulnificus rarely is described in the literature as inducing PF. In one previously reported case, the patient was otherwise healthy and managed to recover following antibiotic therapy and wound debridement,6 whereas in another case the patient had undiagnosed liver cirrhosis and died from the infection.6,7 In the latter case, the patient presented to the emergency department in a coma. Our patient did not have the clinical signs of sepsis upon initial presentation to the emergency department. It is possible the infection rapidly progressed because of his underlying liver disease. Genotyping analysis of V vulnificus has shown that strains with low pathogenicity can cause primary septicemia in humans.7
Our case reinforces the need to quickly recognize V vulnificus as a rare underlying cause of PF and administer the appropriate treatment.
- Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999;341:586-592.
- Tacket CO, Brenner F, Blake PA. Clinical features and an epidemiological study of Vibrio vulnificus infections. J Infect Dis. 1984;149:558-561.
- Blake PA, Merson MH, Weaver RE et al. Disease caused by a marine Vibrio: clinical characteristics and epidemiology. N Engl J Med. 1979;300:1-5.
- Liu JW, Lee IK, Tang HJ, et al. Prognostic factors and antibiotics in Vibrio vulnificus septicemia. Arch Intern Med. 2006;166:2117-2123.
- Chen SC, Lee YT, Tsai SJ, et al. Antibiotic therapy for necrotizing fasciitis caused by Vibrio vulnificus: retrospective analysis of an 8 year period.J Antimicrob Chemother. 2012;67:488-493.
- Choi HJ, Lee DK, Lee MW et al. Vibrio vulnificus septicemia presenting as purpura fulminans. J Dermatol. 2005;32:48-51.
- Hori M, Nakayama A, Kitagawa D et al. A case of Vibrio vulnificus infection complicated with fulminant purpura: gene and biotype analysis of the pathogen. JMM Case Rep. 2017;4:e005096.
To the Editor:
Purpura fulminans (PF) is an acute, life-threatening condition characterized by intravascular thrombosis and hemorrhagic necrosis of the skin. It classically presents as retiform purpura with branched or angular purpuric lesions. Purpura fulminans often occurs in the setting of disseminated intravascular coagulation, secondary to sepsis, trauma, malignancy, autoimmune disease, and congenital or acquired protein C or S deficiency, among other abnormalities.1 Rapid identification and treatment of the underlying cause are mainstays of management. We report a case of PF secondary to Vibrio vulnificus infection and highlight the importance of timely consideration of this etiologic agent due to the high mortality rate and specific treatment required.
A 58-year-old man with liver cirrhosis and hepatitis B virus presented with pain, swelling, and localized erythema affecting both legs as well as a fever. He reported vomiting blood and an episode of bloody diarrhea over the preceding 24 hours. He denied exposure to sick contacts or a history of autoimmune disease. At initial presentation to the emergency department, physical examination revealed few scattered, sharply demarcated, erythematous to violaceous patches that rapidly progressed overnight to hemorrhagic bullae and widespread retiform purpuric patches on both legs (Figure 1). As the patient’s skin condition worsened, he had a blood pressure of 80/50 mm Hg and a pulse rate of 110/min. Serum analysis was notable for mild leukocytosis (10.74×109/L [reference range, 4.8–10.8×109/L), thrombocytopenia (39×109/L [reference range, 150–450×109/L]), and decreased C3 (25 mg/dL [reference range, 81–157 mg/dL]) and C4 (8 mg/dL [reference range, 13–39 mg/dL]). Laboratory findings also were remarkable for prothrombin time (23.3 seconds [reference range, 8.8–12.3 seconds]), partial thromboplastin time (52.5 seconds [reference range, 23.6–35.8 seconds]), and international normalized ratio (2.01 [reference range, 0.8–1.13]). Aspartate transaminase (237 U/L [reference range, 11–39 U/L]) and alanine transaminase (80 U/L [reference range, 11–35 U/L]) were elevated, while antineutrophil cytoplasmic antibodies, serum immunoglobulin, and cryoglobulins were unremarkable. Punch biopsies of the left thigh were performed, and histopathology revealed small vessel thrombosis and ischemic changes consistent with PF (Figure 2). Vancomycin, clindamycin, cefepime injection, and piperacillin-tazobactam were administered intravenously for empiric broad-spectrum sepsis coverage. Within hours, the patient experienced refractory septic shock with disseminated intravascular coagulation and died from pulmonary embolism and subsequent cardiac arrest. Tissue and blood cultures grew V vulnificus.

Vibrio vulnificus is a gram-negative bacillus and a rare cause of primary septicemia following consumption of shellfish, especially oysters. Wounds exposed to saltwater or brackish water contaminated with the microorganism can produce soft-tissue infections. Individuals with chronic liver disease are at greater risk for V vulnificus infection.2 The clinical presentation of V vulnificus includes early cellulitislike patches, late purpura with hemorrhagic bullae, and rapidly progressing shock.3

Mortality rates from V vulnificus infection are high.4 Therefore, it is recommended to presumptively diagnose V vulnificus septicemia in any individual at risk for infection who presents with the characteristic history in the setting of hypotension, fever, or septic shock. It is crucial for providers to be aware that broad-spectrum antibiotics commonly used for sepsis are inadequate for the treatment of V vulnificus. Immediate treatment with tetracycline (minocycline or doxycycline) and a third-generation cephalosporin (cefotaxime or ceftriaxone injection) or in combination with ciprofloxacin has been proven effective.4,5
Vibrio vulnificus rarely is described in the literature as inducing PF. In one previously reported case, the patient was otherwise healthy and managed to recover following antibiotic therapy and wound debridement,6 whereas in another case the patient had undiagnosed liver cirrhosis and died from the infection.6,7 In the latter case, the patient presented to the emergency department in a coma. Our patient did not have the clinical signs of sepsis upon initial presentation to the emergency department. It is possible the infection rapidly progressed because of his underlying liver disease. Genotyping analysis of V vulnificus has shown that strains with low pathogenicity can cause primary septicemia in humans.7
Our case reinforces the need to quickly recognize V vulnificus as a rare underlying cause of PF and administer the appropriate treatment.
To the Editor:
Purpura fulminans (PF) is an acute, life-threatening condition characterized by intravascular thrombosis and hemorrhagic necrosis of the skin. It classically presents as retiform purpura with branched or angular purpuric lesions. Purpura fulminans often occurs in the setting of disseminated intravascular coagulation, secondary to sepsis, trauma, malignancy, autoimmune disease, and congenital or acquired protein C or S deficiency, among other abnormalities.1 Rapid identification and treatment of the underlying cause are mainstays of management. We report a case of PF secondary to Vibrio vulnificus infection and highlight the importance of timely consideration of this etiologic agent due to the high mortality rate and specific treatment required.
A 58-year-old man with liver cirrhosis and hepatitis B virus presented with pain, swelling, and localized erythema affecting both legs as well as a fever. He reported vomiting blood and an episode of bloody diarrhea over the preceding 24 hours. He denied exposure to sick contacts or a history of autoimmune disease. At initial presentation to the emergency department, physical examination revealed few scattered, sharply demarcated, erythematous to violaceous patches that rapidly progressed overnight to hemorrhagic bullae and widespread retiform purpuric patches on both legs (Figure 1). As the patient’s skin condition worsened, he had a blood pressure of 80/50 mm Hg and a pulse rate of 110/min. Serum analysis was notable for mild leukocytosis (10.74×109/L [reference range, 4.8–10.8×109/L), thrombocytopenia (39×109/L [reference range, 150–450×109/L]), and decreased C3 (25 mg/dL [reference range, 81–157 mg/dL]) and C4 (8 mg/dL [reference range, 13–39 mg/dL]). Laboratory findings also were remarkable for prothrombin time (23.3 seconds [reference range, 8.8–12.3 seconds]), partial thromboplastin time (52.5 seconds [reference range, 23.6–35.8 seconds]), and international normalized ratio (2.01 [reference range, 0.8–1.13]). Aspartate transaminase (237 U/L [reference range, 11–39 U/L]) and alanine transaminase (80 U/L [reference range, 11–35 U/L]) were elevated, while antineutrophil cytoplasmic antibodies, serum immunoglobulin, and cryoglobulins were unremarkable. Punch biopsies of the left thigh were performed, and histopathology revealed small vessel thrombosis and ischemic changes consistent with PF (Figure 2). Vancomycin, clindamycin, cefepime injection, and piperacillin-tazobactam were administered intravenously for empiric broad-spectrum sepsis coverage. Within hours, the patient experienced refractory septic shock with disseminated intravascular coagulation and died from pulmonary embolism and subsequent cardiac arrest. Tissue and blood cultures grew V vulnificus.

Vibrio vulnificus is a gram-negative bacillus and a rare cause of primary septicemia following consumption of shellfish, especially oysters. Wounds exposed to saltwater or brackish water contaminated with the microorganism can produce soft-tissue infections. Individuals with chronic liver disease are at greater risk for V vulnificus infection.2 The clinical presentation of V vulnificus includes early cellulitislike patches, late purpura with hemorrhagic bullae, and rapidly progressing shock.3

Mortality rates from V vulnificus infection are high.4 Therefore, it is recommended to presumptively diagnose V vulnificus septicemia in any individual at risk for infection who presents with the characteristic history in the setting of hypotension, fever, or septic shock. It is crucial for providers to be aware that broad-spectrum antibiotics commonly used for sepsis are inadequate for the treatment of V vulnificus. Immediate treatment with tetracycline (minocycline or doxycycline) and a third-generation cephalosporin (cefotaxime or ceftriaxone injection) or in combination with ciprofloxacin has been proven effective.4,5
Vibrio vulnificus rarely is described in the literature as inducing PF. In one previously reported case, the patient was otherwise healthy and managed to recover following antibiotic therapy and wound debridement,6 whereas in another case the patient had undiagnosed liver cirrhosis and died from the infection.6,7 In the latter case, the patient presented to the emergency department in a coma. Our patient did not have the clinical signs of sepsis upon initial presentation to the emergency department. It is possible the infection rapidly progressed because of his underlying liver disease. Genotyping analysis of V vulnificus has shown that strains with low pathogenicity can cause primary septicemia in humans.7
Our case reinforces the need to quickly recognize V vulnificus as a rare underlying cause of PF and administer the appropriate treatment.
- Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999;341:586-592.
- Tacket CO, Brenner F, Blake PA. Clinical features and an epidemiological study of Vibrio vulnificus infections. J Infect Dis. 1984;149:558-561.
- Blake PA, Merson MH, Weaver RE et al. Disease caused by a marine Vibrio: clinical characteristics and epidemiology. N Engl J Med. 1979;300:1-5.
- Liu JW, Lee IK, Tang HJ, et al. Prognostic factors and antibiotics in Vibrio vulnificus septicemia. Arch Intern Med. 2006;166:2117-2123.
- Chen SC, Lee YT, Tsai SJ, et al. Antibiotic therapy for necrotizing fasciitis caused by Vibrio vulnificus: retrospective analysis of an 8 year period.J Antimicrob Chemother. 2012;67:488-493.
- Choi HJ, Lee DK, Lee MW et al. Vibrio vulnificus septicemia presenting as purpura fulminans. J Dermatol. 2005;32:48-51.
- Hori M, Nakayama A, Kitagawa D et al. A case of Vibrio vulnificus infection complicated with fulminant purpura: gene and biotype analysis of the pathogen. JMM Case Rep. 2017;4:e005096.
- Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999;341:586-592.
- Tacket CO, Brenner F, Blake PA. Clinical features and an epidemiological study of Vibrio vulnificus infections. J Infect Dis. 1984;149:558-561.
- Blake PA, Merson MH, Weaver RE et al. Disease caused by a marine Vibrio: clinical characteristics and epidemiology. N Engl J Med. 1979;300:1-5.
- Liu JW, Lee IK, Tang HJ, et al. Prognostic factors and antibiotics in Vibrio vulnificus septicemia. Arch Intern Med. 2006;166:2117-2123.
- Chen SC, Lee YT, Tsai SJ, et al. Antibiotic therapy for necrotizing fasciitis caused by Vibrio vulnificus: retrospective analysis of an 8 year period.J Antimicrob Chemother. 2012;67:488-493.
- Choi HJ, Lee DK, Lee MW et al. Vibrio vulnificus septicemia presenting as purpura fulminans. J Dermatol. 2005;32:48-51.
- Hori M, Nakayama A, Kitagawa D et al. A case of Vibrio vulnificus infection complicated with fulminant purpura: gene and biotype analysis of the pathogen. JMM Case Rep. 2017;4:e005096.
Practice Points
- Purpura fulminans (PF) is a life-threatening condition characterized by intravascular coagulation and skin necrosis.
- Patients with underlying liver disease are at greater risk for PF secondary to Vibrio vulnificus infection.
- Given the high mortality rate, rapid identification of the etiologic agent and timely antibiotic treatment are necessary.
Second woman spontaneously clears HIV: ‘We think more are out there’
It sounds like a fairy tale steeped in HIV stigma: A woman wakes up one morning and, poof, the HIV she’s been living with for 8 years is gone. But for a 30-year-old Argentinian woman from the aptly named village of Esperanza, that’s close to the truth, according to an article published in Annals of Internal Medicine.
The woman, the so-called Esperanza Patient, appears to be the second person whose immune system cleared the virus without the use of stem cell transplantation. The first was Loreen Willenberg, a California woman who, after living with HIV for 27 years, no longer had replicating HIV in her system. That case was reported last year.
“That’s the beauty of this name, right? Esperanza,” said Xu Yu, MD, principal investigator of the Ragon Institute of Massachusetts General Hospital, the Massachusetts Institute of Technology, and Harvard University, Boston, referring to the Spanish word for “hope.” “This makes us hopeful that a natural cure of HIV is actually possible.”
Two other people appear to have cleared HIV, but only after full replacement of the immune system via stem cell transplantation – the Berlin Patient, Timothy Ray Brown, and the London Patient. Another man, from Brazil, appeared to have an undetectable viral load after receiving intensified antiretroviral treatment plus supplemental vitamin B3.
The rarest of the rare
The Esperanza Patient is among a rare group of people living with HIV called elite controllers. These people’s immune systems can control HIV without antiretrovirals. Most elite controllers’ immune systems, however, can’t mount the immune attack necessary to eliminate all replicating HIV from their systems. Instead, their immune systems control the virus without affecting the reservoirs where HIV continues to make copies of itself and can spread.
The Esperanza Patient and Ms. Willenberg, however, appear to be the rarest of the rare. Their own immune systems seem not only to have stopped HIV replication outside of reservoirs but also to have stormed those reservoirs and killed all virus that might have continued to replicate.
The two women are connected in another way: At an HIV conference in 2019, Dr. Yu was presenting data on Ms. Willenberg’s case. At that conference, she met Natalia Laufer, MD, PhD, associate researcher at the Instituto de Investigaciones Biomédicas en Retrovirs y SIDA at the University of Buenos Aires. Dr. Laufer had been studying the Esperanza Patient at the time and asked Dr. Yu whether she and her team at the Ragon Institute could help her sequence the patient’s HIV genome to see whether, indeed, the virus had been spontaneously cleared from the patient’s system.
So that’s what the pair did, in collaboration with several other researchers into cures for HIV. The Esperanza Patient first acquired HIV in 2013, but in the 8 years that followed, results of 10 conventional viral load tests indicated the virus was undetectable (that is, below the level of quantification for standard technology). During that time, the woman’s boyfriend, from whom she had acquired HIV, died of AIDS-defining illnesses. She subsequently married and had a baby. Both her partner and baby are HIV negative. She only received HIV treatment for 6 months while she was pregnant.
A fossil record of HIV
Yet, there was still HIV in the woman’s system. Dr. Laufer and Dr. Yu wanted to know whether that HIV was transmissible or whether it was a relic from when HIV was still replicating and was now defective and incapable of replicating. They performed extensive genome sequencing on nearly 1.2 billion cells that Dr. Laufer had taken from the patient’s blood in 2017, 2018, 2019, and 2020, an additional 503 million cells that were from the placenta of the baby she gave birth to in 2020, and 150 million resting CD4 T cells. Proviral sequencing was undertaken of the full DNA of the HIV to detect whether the virus was still intact. The DNA was then analyzed by use of an algorithm and was tested for mutations. The investigators tested the patient’s CD4 cells to determine whether the cells still harbored any latent HIV.
In this way, they conducted a full viral workup using tests that are far more sensitive than the viral load tests the woman had undergone in the clinic. The investigators then assessed the patient’s immune system to see what the various cells of the immune system could tell them about how well her natural immune system could identify and kill HIV. They isolated the Esperanza Patient’s immune cells and subjected those cells to HIV in the lab to see whether the cells could detect and eliminate the virus.
And just to be safe, they checked to make sure there were no antiretroviral drugs in the patient’s system.
What they found was that without treatment, her CD4 count hovered around 1,000 cells – a sign of a functioning immune system. DNA sequences revealed large chunks of missing DNA, and one sequence had an immune-induced hypermutation. In total, seven proviruses were found, but none were capable of replicating. The CD4 cells they evaluated showed no evidence of latent HIV.
In other words, they had uncovered a fossil record.
“These HIV-1 DNA products clearly indicate that this person was infected with HIV-1 in the past and that active cycles of viral replication had occurred at one point,” Dr. Yu and colleagues write in their recent article.
What may be more useful to researchers looking to turn this spontaneous cure into treatment for millions of people living with active HIV was the evidence that the woman’s immune system had trained itself to attack HIV through a number of genetic mutations. What they found, the researchers write, was evidence of “an incomplete seroconversion” – that is, when the patient was acquiring HIV, the infection was stopped in its tracks.
Yet, Dr. Yu and colleagues say that they can’t prove that the woman is fully cured of HIV.
“Although this might sound unsatisfying, it reflects an intrinsic limitation of scientific research,” they write. “Scientific concepts can never be proved through empirical data collection; they can only be disproved.”
There are more out there
Are these women the only ones to have spontaneously cleared HIV? That’s the question, said Carl Dieffenbach, PhD, director of the Division of AIDS at the National Institute of Allergy and Infectious Diseases at the National Institutes of Health. Just like they can’t disprove that the women cured themselves, they can’t prove that she and Ms. Willenberg are the only two people to have experienced this cure.
“We’re all struggling with this,” Dr. Dieffenbach told this news organization. “The goal is to get enough of these people so maybe there’s a road map to how to induce, trigger, change immunity. But this could well be a unique event at the time of initiation of infection. We just don’t know.”
What is needed, Dr. Yu said, is for clinicians to reach out to them regarding cases that could mimic the cases of Ms. Willenberg and the Esperanza Patient. Elaborate testing could then be conducted to see whether these cases are similar to those of Ms. Willenberg and the Esperanza Patient.
“We do think there are more out there,” Dr. Yu said in an interview.
Asked whether we’re still far away from applying these one-off cures to the millions of people taking HIV treatment daily, Dr. Yu responded, “We might be close. That’s the beauty of scientific discovery. We don’t know, but that’s why we need more engagement of the community and care providers to help us.”
The research was funded by the Bill and Melinda Gates Foundation and the National Institutes of Health. Dr. Yu and Dr. Dieffenbach have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It sounds like a fairy tale steeped in HIV stigma: A woman wakes up one morning and, poof, the HIV she’s been living with for 8 years is gone. But for a 30-year-old Argentinian woman from the aptly named village of Esperanza, that’s close to the truth, according to an article published in Annals of Internal Medicine.
The woman, the so-called Esperanza Patient, appears to be the second person whose immune system cleared the virus without the use of stem cell transplantation. The first was Loreen Willenberg, a California woman who, after living with HIV for 27 years, no longer had replicating HIV in her system. That case was reported last year.
“That’s the beauty of this name, right? Esperanza,” said Xu Yu, MD, principal investigator of the Ragon Institute of Massachusetts General Hospital, the Massachusetts Institute of Technology, and Harvard University, Boston, referring to the Spanish word for “hope.” “This makes us hopeful that a natural cure of HIV is actually possible.”
Two other people appear to have cleared HIV, but only after full replacement of the immune system via stem cell transplantation – the Berlin Patient, Timothy Ray Brown, and the London Patient. Another man, from Brazil, appeared to have an undetectable viral load after receiving intensified antiretroviral treatment plus supplemental vitamin B3.
The rarest of the rare
The Esperanza Patient is among a rare group of people living with HIV called elite controllers. These people’s immune systems can control HIV without antiretrovirals. Most elite controllers’ immune systems, however, can’t mount the immune attack necessary to eliminate all replicating HIV from their systems. Instead, their immune systems control the virus without affecting the reservoirs where HIV continues to make copies of itself and can spread.
The Esperanza Patient and Ms. Willenberg, however, appear to be the rarest of the rare. Their own immune systems seem not only to have stopped HIV replication outside of reservoirs but also to have stormed those reservoirs and killed all virus that might have continued to replicate.
The two women are connected in another way: At an HIV conference in 2019, Dr. Yu was presenting data on Ms. Willenberg’s case. At that conference, she met Natalia Laufer, MD, PhD, associate researcher at the Instituto de Investigaciones Biomédicas en Retrovirs y SIDA at the University of Buenos Aires. Dr. Laufer had been studying the Esperanza Patient at the time and asked Dr. Yu whether she and her team at the Ragon Institute could help her sequence the patient’s HIV genome to see whether, indeed, the virus had been spontaneously cleared from the patient’s system.
So that’s what the pair did, in collaboration with several other researchers into cures for HIV. The Esperanza Patient first acquired HIV in 2013, but in the 8 years that followed, results of 10 conventional viral load tests indicated the virus was undetectable (that is, below the level of quantification for standard technology). During that time, the woman’s boyfriend, from whom she had acquired HIV, died of AIDS-defining illnesses. She subsequently married and had a baby. Both her partner and baby are HIV negative. She only received HIV treatment for 6 months while she was pregnant.
A fossil record of HIV
Yet, there was still HIV in the woman’s system. Dr. Laufer and Dr. Yu wanted to know whether that HIV was transmissible or whether it was a relic from when HIV was still replicating and was now defective and incapable of replicating. They performed extensive genome sequencing on nearly 1.2 billion cells that Dr. Laufer had taken from the patient’s blood in 2017, 2018, 2019, and 2020, an additional 503 million cells that were from the placenta of the baby she gave birth to in 2020, and 150 million resting CD4 T cells. Proviral sequencing was undertaken of the full DNA of the HIV to detect whether the virus was still intact. The DNA was then analyzed by use of an algorithm and was tested for mutations. The investigators tested the patient’s CD4 cells to determine whether the cells still harbored any latent HIV.
In this way, they conducted a full viral workup using tests that are far more sensitive than the viral load tests the woman had undergone in the clinic. The investigators then assessed the patient’s immune system to see what the various cells of the immune system could tell them about how well her natural immune system could identify and kill HIV. They isolated the Esperanza Patient’s immune cells and subjected those cells to HIV in the lab to see whether the cells could detect and eliminate the virus.
And just to be safe, they checked to make sure there were no antiretroviral drugs in the patient’s system.
What they found was that without treatment, her CD4 count hovered around 1,000 cells – a sign of a functioning immune system. DNA sequences revealed large chunks of missing DNA, and one sequence had an immune-induced hypermutation. In total, seven proviruses were found, but none were capable of replicating. The CD4 cells they evaluated showed no evidence of latent HIV.
In other words, they had uncovered a fossil record.
“These HIV-1 DNA products clearly indicate that this person was infected with HIV-1 in the past and that active cycles of viral replication had occurred at one point,” Dr. Yu and colleagues write in their recent article.
What may be more useful to researchers looking to turn this spontaneous cure into treatment for millions of people living with active HIV was the evidence that the woman’s immune system had trained itself to attack HIV through a number of genetic mutations. What they found, the researchers write, was evidence of “an incomplete seroconversion” – that is, when the patient was acquiring HIV, the infection was stopped in its tracks.
Yet, Dr. Yu and colleagues say that they can’t prove that the woman is fully cured of HIV.
“Although this might sound unsatisfying, it reflects an intrinsic limitation of scientific research,” they write. “Scientific concepts can never be proved through empirical data collection; they can only be disproved.”
There are more out there
Are these women the only ones to have spontaneously cleared HIV? That’s the question, said Carl Dieffenbach, PhD, director of the Division of AIDS at the National Institute of Allergy and Infectious Diseases at the National Institutes of Health. Just like they can’t disprove that the women cured themselves, they can’t prove that she and Ms. Willenberg are the only two people to have experienced this cure.
“We’re all struggling with this,” Dr. Dieffenbach told this news organization. “The goal is to get enough of these people so maybe there’s a road map to how to induce, trigger, change immunity. But this could well be a unique event at the time of initiation of infection. We just don’t know.”
What is needed, Dr. Yu said, is for clinicians to reach out to them regarding cases that could mimic the cases of Ms. Willenberg and the Esperanza Patient. Elaborate testing could then be conducted to see whether these cases are similar to those of Ms. Willenberg and the Esperanza Patient.
“We do think there are more out there,” Dr. Yu said in an interview.
Asked whether we’re still far away from applying these one-off cures to the millions of people taking HIV treatment daily, Dr. Yu responded, “We might be close. That’s the beauty of scientific discovery. We don’t know, but that’s why we need more engagement of the community and care providers to help us.”
The research was funded by the Bill and Melinda Gates Foundation and the National Institutes of Health. Dr. Yu and Dr. Dieffenbach have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It sounds like a fairy tale steeped in HIV stigma: A woman wakes up one morning and, poof, the HIV she’s been living with for 8 years is gone. But for a 30-year-old Argentinian woman from the aptly named village of Esperanza, that’s close to the truth, according to an article published in Annals of Internal Medicine.
The woman, the so-called Esperanza Patient, appears to be the second person whose immune system cleared the virus without the use of stem cell transplantation. The first was Loreen Willenberg, a California woman who, after living with HIV for 27 years, no longer had replicating HIV in her system. That case was reported last year.
“That’s the beauty of this name, right? Esperanza,” said Xu Yu, MD, principal investigator of the Ragon Institute of Massachusetts General Hospital, the Massachusetts Institute of Technology, and Harvard University, Boston, referring to the Spanish word for “hope.” “This makes us hopeful that a natural cure of HIV is actually possible.”
Two other people appear to have cleared HIV, but only after full replacement of the immune system via stem cell transplantation – the Berlin Patient, Timothy Ray Brown, and the London Patient. Another man, from Brazil, appeared to have an undetectable viral load after receiving intensified antiretroviral treatment plus supplemental vitamin B3.
The rarest of the rare
The Esperanza Patient is among a rare group of people living with HIV called elite controllers. These people’s immune systems can control HIV without antiretrovirals. Most elite controllers’ immune systems, however, can’t mount the immune attack necessary to eliminate all replicating HIV from their systems. Instead, their immune systems control the virus without affecting the reservoirs where HIV continues to make copies of itself and can spread.
The Esperanza Patient and Ms. Willenberg, however, appear to be the rarest of the rare. Their own immune systems seem not only to have stopped HIV replication outside of reservoirs but also to have stormed those reservoirs and killed all virus that might have continued to replicate.
The two women are connected in another way: At an HIV conference in 2019, Dr. Yu was presenting data on Ms. Willenberg’s case. At that conference, she met Natalia Laufer, MD, PhD, associate researcher at the Instituto de Investigaciones Biomédicas en Retrovirs y SIDA at the University of Buenos Aires. Dr. Laufer had been studying the Esperanza Patient at the time and asked Dr. Yu whether she and her team at the Ragon Institute could help her sequence the patient’s HIV genome to see whether, indeed, the virus had been spontaneously cleared from the patient’s system.
So that’s what the pair did, in collaboration with several other researchers into cures for HIV. The Esperanza Patient first acquired HIV in 2013, but in the 8 years that followed, results of 10 conventional viral load tests indicated the virus was undetectable (that is, below the level of quantification for standard technology). During that time, the woman’s boyfriend, from whom she had acquired HIV, died of AIDS-defining illnesses. She subsequently married and had a baby. Both her partner and baby are HIV negative. She only received HIV treatment for 6 months while she was pregnant.
A fossil record of HIV
Yet, there was still HIV in the woman’s system. Dr. Laufer and Dr. Yu wanted to know whether that HIV was transmissible or whether it was a relic from when HIV was still replicating and was now defective and incapable of replicating. They performed extensive genome sequencing on nearly 1.2 billion cells that Dr. Laufer had taken from the patient’s blood in 2017, 2018, 2019, and 2020, an additional 503 million cells that were from the placenta of the baby she gave birth to in 2020, and 150 million resting CD4 T cells. Proviral sequencing was undertaken of the full DNA of the HIV to detect whether the virus was still intact. The DNA was then analyzed by use of an algorithm and was tested for mutations. The investigators tested the patient’s CD4 cells to determine whether the cells still harbored any latent HIV.
In this way, they conducted a full viral workup using tests that are far more sensitive than the viral load tests the woman had undergone in the clinic. The investigators then assessed the patient’s immune system to see what the various cells of the immune system could tell them about how well her natural immune system could identify and kill HIV. They isolated the Esperanza Patient’s immune cells and subjected those cells to HIV in the lab to see whether the cells could detect and eliminate the virus.
And just to be safe, they checked to make sure there were no antiretroviral drugs in the patient’s system.
What they found was that without treatment, her CD4 count hovered around 1,000 cells – a sign of a functioning immune system. DNA sequences revealed large chunks of missing DNA, and one sequence had an immune-induced hypermutation. In total, seven proviruses were found, but none were capable of replicating. The CD4 cells they evaluated showed no evidence of latent HIV.
In other words, they had uncovered a fossil record.
“These HIV-1 DNA products clearly indicate that this person was infected with HIV-1 in the past and that active cycles of viral replication had occurred at one point,” Dr. Yu and colleagues write in their recent article.
What may be more useful to researchers looking to turn this spontaneous cure into treatment for millions of people living with active HIV was the evidence that the woman’s immune system had trained itself to attack HIV through a number of genetic mutations. What they found, the researchers write, was evidence of “an incomplete seroconversion” – that is, when the patient was acquiring HIV, the infection was stopped in its tracks.
Yet, Dr. Yu and colleagues say that they can’t prove that the woman is fully cured of HIV.
“Although this might sound unsatisfying, it reflects an intrinsic limitation of scientific research,” they write. “Scientific concepts can never be proved through empirical data collection; they can only be disproved.”
There are more out there
Are these women the only ones to have spontaneously cleared HIV? That’s the question, said Carl Dieffenbach, PhD, director of the Division of AIDS at the National Institute of Allergy and Infectious Diseases at the National Institutes of Health. Just like they can’t disprove that the women cured themselves, they can’t prove that she and Ms. Willenberg are the only two people to have experienced this cure.
“We’re all struggling with this,” Dr. Dieffenbach told this news organization. “The goal is to get enough of these people so maybe there’s a road map to how to induce, trigger, change immunity. But this could well be a unique event at the time of initiation of infection. We just don’t know.”
What is needed, Dr. Yu said, is for clinicians to reach out to them regarding cases that could mimic the cases of Ms. Willenberg and the Esperanza Patient. Elaborate testing could then be conducted to see whether these cases are similar to those of Ms. Willenberg and the Esperanza Patient.
“We do think there are more out there,” Dr. Yu said in an interview.
Asked whether we’re still far away from applying these one-off cures to the millions of people taking HIV treatment daily, Dr. Yu responded, “We might be close. That’s the beauty of scientific discovery. We don’t know, but that’s why we need more engagement of the community and care providers to help us.”
The research was funded by the Bill and Melinda Gates Foundation and the National Institutes of Health. Dr. Yu and Dr. Dieffenbach have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Text-based COVID monitoring system could reduce deaths, relieve ED in winter surge
COVID Watch, a text message–based remote monitoring program developed by the University of Pennsylvania Health System, was associated with a 68% reduction in the risk of death, compared with those who received usual care. This was the main finding of a paper published in the Annals of Internal Medicine.
The investigators also determined that patients who enrolled in the program were more likely to seek care in the ED and when they did, they came in on average 2 days sooner than those who received usual care.
“When our clinical team designed COVID Watch the goal was to facilitate hospital care for patients who require it, while supporting access to care for patients who can safely remain at home,” study author M. Kit Delgado, MD, MS, an assistant professor of emergency medicine and epidemiology at Penn Presbyterian Medical Center in Philadelphia, said in an interview.
Researchers had initially hoped COVID Watch would relieve pressure on EDs, Dr. Delgado said.
Significantly lower mortality seen among COVID Watch group
For the study, Dr. Delgado and colleagues enrolled 3,488 patients in COVID Watch and 4,377 in the usual care group to compare outcomes at 30 and 60 days.
“We didn’t include patients who were diagnosed with COVID in the ER or hospital, so this is a lower-risk cohort of patients who test positive in outpatient settings,” Dr. Delgado noted. “Outpatients who received usual care and COVID Watch both had relatively low mortality, but it was significantly lower in those who were in COVID Watch.”
The researchers found that 3 patients in the COVID Watch group died within 30 days of their enrollment, compared with 12 in the control group. At 60 days after enrollment, 5 people within COVID Watch died, compared with 16 not using the system. More than one-third of the deaths in the usual care group occurred outside the hospital, compared with zero deaths among those in COVID Watch.
More than half of program participants were Black or Latino
The messaging system also reduced mortality rates among “all major racial and ethnic subgroups,” the researchers said, with more than 50% of the patients enrolled in COVID Watch having been Black or Latino.
“This is important because Black and Hispanic communities have experienced higher exposure and infection rates, decreased access to care, and have had higher mortality rates,” Dr. Delgado said. “Therefore, the results imply that this type of program could play a role in decreasing disparities in COVID outcomes if scaled more broadly.”
Outside expert: COVID Watch bring new approach to digital health monitoring
The study not only highlights the efficacy and sustainment of the COVID Watch program, but it sheds light on the possibility of using text message monitoring systems on other chronic disease conditions, said Jamie Faro, PhD, who was not involved in the study.
“It brings a new approach to health monitoring using digital means, which may lessen the burden on health care providers and be more cost effective than usual care approaches,” said Dr. Faro, who is assistant professor at the department of population and quantitative health sciences at the University of Massachusetts, Worcester. “Text messaging, which is used by over 80% of Americans, can allow us to reach a large percentage of the population for remote health care monitoring.”
Researchers of the current study said the findings “reveal a model for outpatient health system management of patients with COVID-19 and possibly other conditions where the early detection of clinical declines is critical.” Dr. Faro said that COVID Watch can have a measurable impact on an outcome that is truly life or death. However, it would be critical to understand how to reach those who either “were not offered or refused to take part in the program.”
The authors of the paper and Dr. Faro had no disclosures.
COVID Watch, a text message–based remote monitoring program developed by the University of Pennsylvania Health System, was associated with a 68% reduction in the risk of death, compared with those who received usual care. This was the main finding of a paper published in the Annals of Internal Medicine.
The investigators also determined that patients who enrolled in the program were more likely to seek care in the ED and when they did, they came in on average 2 days sooner than those who received usual care.
“When our clinical team designed COVID Watch the goal was to facilitate hospital care for patients who require it, while supporting access to care for patients who can safely remain at home,” study author M. Kit Delgado, MD, MS, an assistant professor of emergency medicine and epidemiology at Penn Presbyterian Medical Center in Philadelphia, said in an interview.
Researchers had initially hoped COVID Watch would relieve pressure on EDs, Dr. Delgado said.
Significantly lower mortality seen among COVID Watch group
For the study, Dr. Delgado and colleagues enrolled 3,488 patients in COVID Watch and 4,377 in the usual care group to compare outcomes at 30 and 60 days.
“We didn’t include patients who were diagnosed with COVID in the ER or hospital, so this is a lower-risk cohort of patients who test positive in outpatient settings,” Dr. Delgado noted. “Outpatients who received usual care and COVID Watch both had relatively low mortality, but it was significantly lower in those who were in COVID Watch.”
The researchers found that 3 patients in the COVID Watch group died within 30 days of their enrollment, compared with 12 in the control group. At 60 days after enrollment, 5 people within COVID Watch died, compared with 16 not using the system. More than one-third of the deaths in the usual care group occurred outside the hospital, compared with zero deaths among those in COVID Watch.
More than half of program participants were Black or Latino
The messaging system also reduced mortality rates among “all major racial and ethnic subgroups,” the researchers said, with more than 50% of the patients enrolled in COVID Watch having been Black or Latino.
“This is important because Black and Hispanic communities have experienced higher exposure and infection rates, decreased access to care, and have had higher mortality rates,” Dr. Delgado said. “Therefore, the results imply that this type of program could play a role in decreasing disparities in COVID outcomes if scaled more broadly.”
Outside expert: COVID Watch bring new approach to digital health monitoring
The study not only highlights the efficacy and sustainment of the COVID Watch program, but it sheds light on the possibility of using text message monitoring systems on other chronic disease conditions, said Jamie Faro, PhD, who was not involved in the study.
“It brings a new approach to health monitoring using digital means, which may lessen the burden on health care providers and be more cost effective than usual care approaches,” said Dr. Faro, who is assistant professor at the department of population and quantitative health sciences at the University of Massachusetts, Worcester. “Text messaging, which is used by over 80% of Americans, can allow us to reach a large percentage of the population for remote health care monitoring.”
Researchers of the current study said the findings “reveal a model for outpatient health system management of patients with COVID-19 and possibly other conditions where the early detection of clinical declines is critical.” Dr. Faro said that COVID Watch can have a measurable impact on an outcome that is truly life or death. However, it would be critical to understand how to reach those who either “were not offered or refused to take part in the program.”
The authors of the paper and Dr. Faro had no disclosures.
COVID Watch, a text message–based remote monitoring program developed by the University of Pennsylvania Health System, was associated with a 68% reduction in the risk of death, compared with those who received usual care. This was the main finding of a paper published in the Annals of Internal Medicine.
The investigators also determined that patients who enrolled in the program were more likely to seek care in the ED and when they did, they came in on average 2 days sooner than those who received usual care.
“When our clinical team designed COVID Watch the goal was to facilitate hospital care for patients who require it, while supporting access to care for patients who can safely remain at home,” study author M. Kit Delgado, MD, MS, an assistant professor of emergency medicine and epidemiology at Penn Presbyterian Medical Center in Philadelphia, said in an interview.
Researchers had initially hoped COVID Watch would relieve pressure on EDs, Dr. Delgado said.
Significantly lower mortality seen among COVID Watch group
For the study, Dr. Delgado and colleagues enrolled 3,488 patients in COVID Watch and 4,377 in the usual care group to compare outcomes at 30 and 60 days.
“We didn’t include patients who were diagnosed with COVID in the ER or hospital, so this is a lower-risk cohort of patients who test positive in outpatient settings,” Dr. Delgado noted. “Outpatients who received usual care and COVID Watch both had relatively low mortality, but it was significantly lower in those who were in COVID Watch.”
The researchers found that 3 patients in the COVID Watch group died within 30 days of their enrollment, compared with 12 in the control group. At 60 days after enrollment, 5 people within COVID Watch died, compared with 16 not using the system. More than one-third of the deaths in the usual care group occurred outside the hospital, compared with zero deaths among those in COVID Watch.
More than half of program participants were Black or Latino
The messaging system also reduced mortality rates among “all major racial and ethnic subgroups,” the researchers said, with more than 50% of the patients enrolled in COVID Watch having been Black or Latino.
“This is important because Black and Hispanic communities have experienced higher exposure and infection rates, decreased access to care, and have had higher mortality rates,” Dr. Delgado said. “Therefore, the results imply that this type of program could play a role in decreasing disparities in COVID outcomes if scaled more broadly.”
Outside expert: COVID Watch bring new approach to digital health monitoring
The study not only highlights the efficacy and sustainment of the COVID Watch program, but it sheds light on the possibility of using text message monitoring systems on other chronic disease conditions, said Jamie Faro, PhD, who was not involved in the study.
“It brings a new approach to health monitoring using digital means, which may lessen the burden on health care providers and be more cost effective than usual care approaches,” said Dr. Faro, who is assistant professor at the department of population and quantitative health sciences at the University of Massachusetts, Worcester. “Text messaging, which is used by over 80% of Americans, can allow us to reach a large percentage of the population for remote health care monitoring.”
Researchers of the current study said the findings “reveal a model for outpatient health system management of patients with COVID-19 and possibly other conditions where the early detection of clinical declines is critical.” Dr. Faro said that COVID Watch can have a measurable impact on an outcome that is truly life or death. However, it would be critical to understand how to reach those who either “were not offered or refused to take part in the program.”
The authors of the paper and Dr. Faro had no disclosures.
FROM ANNALS OF INTERNAL MEDICINE
Children and COVID: Youngest vaccinees off to a slower start

Specific figures for children aged 5-11 years are not yet available, but CDC data show that 1.55 million children under the age of 12 years had received at least one dose of COVID-19 vaccine as of Nov. 15, of whom almost 204,000 already had been vaccinated before Nov. 2. For children aged 12-15, the first 2 weeks after approval on May 12 produced almost 2.1 million vaccine initiations, according to the CDC’s COVID Data Tracker.
That dataset reveals several other noteworthy differences between the two age groups in the 10 days after approval:
- There were over 7,000 vaccine initiations on the first day in the 12-15 group; the younger group had 32.
- The older children reached 100,000 per day in 3 days; the younger children took 8 days.
- The older group topped 200,000 vaccinations per day on six different days; the younger group didn’t get above 175,000.
Children under 12 made up 27.5% of vaccine initiations in all age groups during the 2 weeks from Nov. 2 to Nov. 15, versus 3.4% for 12- to 15-year-olds and 1.2% for 16- and 17-year-olds, the CDC said, while also reporting that 3.6% of children under age 12 had received at least one dose of the COVID vaccine, compared with 57.8% of those aged 12-15 and 64.4% of 16- to 17-year-olds.
Meanwhile, the first full week of November marked the second consecutive increase in the number of weekly child COVID cases, with 122,000 reported for Nov. 5-11. The number of new cases has now surpassed 100,000 for 14 consecutive weeks, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report. That report, which covers state health departments, has not included current information from Alabama, Nebraska, and Texas since the summer.
Regionally, the increases over the past 2 weeks were spread out among the East, the Midwest, and the West, while the decline that had been going on for several weeks in the South has largely come to a halt. The states with the highest percent increases over those 2 weeks are all in New England: Maine, New Hampshire, and Vermont, the AAP and CHA noted. In a separate report, the AAP said that Vermont has the second-highest child vaccination rate (81%) in the country, just behind Massachusetts (82%).

Specific figures for children aged 5-11 years are not yet available, but CDC data show that 1.55 million children under the age of 12 years had received at least one dose of COVID-19 vaccine as of Nov. 15, of whom almost 204,000 already had been vaccinated before Nov. 2. For children aged 12-15, the first 2 weeks after approval on May 12 produced almost 2.1 million vaccine initiations, according to the CDC’s COVID Data Tracker.
That dataset reveals several other noteworthy differences between the two age groups in the 10 days after approval:
- There were over 7,000 vaccine initiations on the first day in the 12-15 group; the younger group had 32.
- The older children reached 100,000 per day in 3 days; the younger children took 8 days.
- The older group topped 200,000 vaccinations per day on six different days; the younger group didn’t get above 175,000.
Children under 12 made up 27.5% of vaccine initiations in all age groups during the 2 weeks from Nov. 2 to Nov. 15, versus 3.4% for 12- to 15-year-olds and 1.2% for 16- and 17-year-olds, the CDC said, while also reporting that 3.6% of children under age 12 had received at least one dose of the COVID vaccine, compared with 57.8% of those aged 12-15 and 64.4% of 16- to 17-year-olds.
Meanwhile, the first full week of November marked the second consecutive increase in the number of weekly child COVID cases, with 122,000 reported for Nov. 5-11. The number of new cases has now surpassed 100,000 for 14 consecutive weeks, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report. That report, which covers state health departments, has not included current information from Alabama, Nebraska, and Texas since the summer.
Regionally, the increases over the past 2 weeks were spread out among the East, the Midwest, and the West, while the decline that had been going on for several weeks in the South has largely come to a halt. The states with the highest percent increases over those 2 weeks are all in New England: Maine, New Hampshire, and Vermont, the AAP and CHA noted. In a separate report, the AAP said that Vermont has the second-highest child vaccination rate (81%) in the country, just behind Massachusetts (82%).

Specific figures for children aged 5-11 years are not yet available, but CDC data show that 1.55 million children under the age of 12 years had received at least one dose of COVID-19 vaccine as of Nov. 15, of whom almost 204,000 already had been vaccinated before Nov. 2. For children aged 12-15, the first 2 weeks after approval on May 12 produced almost 2.1 million vaccine initiations, according to the CDC’s COVID Data Tracker.
That dataset reveals several other noteworthy differences between the two age groups in the 10 days after approval:
- There were over 7,000 vaccine initiations on the first day in the 12-15 group; the younger group had 32.
- The older children reached 100,000 per day in 3 days; the younger children took 8 days.
- The older group topped 200,000 vaccinations per day on six different days; the younger group didn’t get above 175,000.
Children under 12 made up 27.5% of vaccine initiations in all age groups during the 2 weeks from Nov. 2 to Nov. 15, versus 3.4% for 12- to 15-year-olds and 1.2% for 16- and 17-year-olds, the CDC said, while also reporting that 3.6% of children under age 12 had received at least one dose of the COVID vaccine, compared with 57.8% of those aged 12-15 and 64.4% of 16- to 17-year-olds.
Meanwhile, the first full week of November marked the second consecutive increase in the number of weekly child COVID cases, with 122,000 reported for Nov. 5-11. The number of new cases has now surpassed 100,000 for 14 consecutive weeks, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report. That report, which covers state health departments, has not included current information from Alabama, Nebraska, and Texas since the summer.
Regionally, the increases over the past 2 weeks were spread out among the East, the Midwest, and the West, while the decline that had been going on for several weeks in the South has largely come to a halt. The states with the highest percent increases over those 2 weeks are all in New England: Maine, New Hampshire, and Vermont, the AAP and CHA noted. In a separate report, the AAP said that Vermont has the second-highest child vaccination rate (81%) in the country, just behind Massachusetts (82%).
ACIP simplifies adult vaccinations for HepB and pneumonia
REFERENCES
- Weng MK. Universal adult hepatitis B vaccinations: work group considerations. Presented to the Advisory Committee on Immunization Practices on November 3, 2021. Accessed November 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-11-2-3/02-HepWG-weng-508.pdf
- Kovayashi M. Considerations for age-based and risk-based use of PCV15 and PCV20 among US adults and proposed policy options. Presented to the Advisory Committee on Immunization Practices on October 20, 2021. Accessed November 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-10-20-21/02-Pneumococcal-Kobayashi-508.pdf
- Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1-31.
- Matanock A, Lee G, Gierke R, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morbid Mortal Wkly Rep. 2019;68:1069-1075.
REFERENCES
- Weng MK. Universal adult hepatitis B vaccinations: work group considerations. Presented to the Advisory Committee on Immunization Practices on November 3, 2021. Accessed November 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-11-2-3/02-HepWG-weng-508.pdf
- Kovayashi M. Considerations for age-based and risk-based use of PCV15 and PCV20 among US adults and proposed policy options. Presented to the Advisory Committee on Immunization Practices on October 20, 2021. Accessed November 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-10-20-21/02-Pneumococcal-Kobayashi-508.pdf
- Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1-31.
- Matanock A, Lee G, Gierke R, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morbid Mortal Wkly Rep. 2019;68:1069-1075.
REFERENCES
- Weng MK. Universal adult hepatitis B vaccinations: work group considerations. Presented to the Advisory Committee on Immunization Practices on November 3, 2021. Accessed November 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-11-2-3/02-HepWG-weng-508.pdf
- Kovayashi M. Considerations for age-based and risk-based use of PCV15 and PCV20 among US adults and proposed policy options. Presented to the Advisory Committee on Immunization Practices on October 20, 2021. Accessed November 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-10-20-21/02-Pneumococcal-Kobayashi-508.pdf
- Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1-31.
- Matanock A, Lee G, Gierke R, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morbid Mortal Wkly Rep. 2019;68:1069-1075.
To boost HIV screening, ED nurses need institutional support
, according to a national survey of ED nurses. Nearly 43% of respondents said they had received “little” or “very little” HIV education as part of their professional development and practice.
This lack of continuing HIV education “often translated into attitudes that did not support the policy” of routine HIV screening in EDs, lead author Candace Elam, DNP, a family nurse practitioner at the Institute of Family Health in the Bronx, New York City, told this news organization. “But more than individual attitudes, what came out most clearly in the research was that organizational support for HIV screening in EDs was the one factor that could make or break whether an emergency nurse performs HIV screening,” she said. This includes working routine HIV screening into ED workflows and providing resources to streamline screening and testing efforts.
In 2006, the Centers for Disease Control and Prevention released guidance recommending routine HIV screening in all healthcare settings, including urgent care and EDs. Elam, who conducted the research as a student at Rutgers University School of Nursing in New Brunswick, N.J., noticed during her time as an ED nurse that, while her department had a policy supporting routine HIV screening, the practice was not consistent across all nursing staff. To find out how HIV screening varied nationally, Elam ran a national survey from Oct. through Dec. 2020, recruiting participants both by email outreach and Facebook.
In the 30- to 45-minute survey, respondents reported:
- Demographic information
- Knowledge of the CDC HIV screening recommendations
- Workplace HIV screening policy
- Self-reported performance of HIV screening
- Beliefs and attitudes pertaining to HIV screening
Overall, 371 individuals from 43 states filled out at least some part of the survey, and 171 individuals completed it. Of the 251 individuals who answered whether their EDs routinely conducted HIV screening, 76.9% responded affirmatively. Overall, 28.5% of respondents thought HIV screening was “not important” or “not at all important.” Nearly half – 47.6% – reported never offering HIV testing to all eligible patients regardless of risk factors, and only 14.3% reported offering testing all of the time. Only 25% of participants said they received “adequate” or “a lot” of HIV-related nursing education, and 42.9% reported “little” or “very little” education.
“For the most part, those of us working in hospitals, all the education that we get about HIV took place in school,” Elam said. “So, if you went to school in the early 2000s or in the 1990s, you don’t know much else.” Elam noted that she keeps informed on HIV research issues because it is an area of interest, but the hospital she had worked at did not contribute much to her knowledge.
Elam also found that in practice there were several barriers to performing screening, such as lack of availability of a dedicated HIV educator, tester, or counselors; not knowing where to refer patients who had a positive HIV test result; and insufficient time to address positive HIV test results in ED practice.
“A lot of these things are outside an individual nurse’s control,” said Elam, and can result in missing patients who would benefit from care. Lisa Leimer, RN, a nurse at Primary Health Care in Des Moines, works with patients after they have been diagnosed with HIV, but noted that many of her patients could have been identified earlier. “Once we get someone, you look back at medical records and you see that they have been in and out of the hospital,” she said. “There’s been multiple encounters,” she said.
Prioritizing HIV screening in all healthcare settings and including HIV education for all medical professionals – not just nurses – could help in the continuing battle against HIV. “So much has changed in the world of HIV,” she said. “We’re trying to end the epidemic, and it could happen if we identified, diagnosed, and treated the people that are living with it.”
Elam and Leimer have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a national survey of ED nurses. Nearly 43% of respondents said they had received “little” or “very little” HIV education as part of their professional development and practice.
This lack of continuing HIV education “often translated into attitudes that did not support the policy” of routine HIV screening in EDs, lead author Candace Elam, DNP, a family nurse practitioner at the Institute of Family Health in the Bronx, New York City, told this news organization. “But more than individual attitudes, what came out most clearly in the research was that organizational support for HIV screening in EDs was the one factor that could make or break whether an emergency nurse performs HIV screening,” she said. This includes working routine HIV screening into ED workflows and providing resources to streamline screening and testing efforts.
In 2006, the Centers for Disease Control and Prevention released guidance recommending routine HIV screening in all healthcare settings, including urgent care and EDs. Elam, who conducted the research as a student at Rutgers University School of Nursing in New Brunswick, N.J., noticed during her time as an ED nurse that, while her department had a policy supporting routine HIV screening, the practice was not consistent across all nursing staff. To find out how HIV screening varied nationally, Elam ran a national survey from Oct. through Dec. 2020, recruiting participants both by email outreach and Facebook.
In the 30- to 45-minute survey, respondents reported:
- Demographic information
- Knowledge of the CDC HIV screening recommendations
- Workplace HIV screening policy
- Self-reported performance of HIV screening
- Beliefs and attitudes pertaining to HIV screening
Overall, 371 individuals from 43 states filled out at least some part of the survey, and 171 individuals completed it. Of the 251 individuals who answered whether their EDs routinely conducted HIV screening, 76.9% responded affirmatively. Overall, 28.5% of respondents thought HIV screening was “not important” or “not at all important.” Nearly half – 47.6% – reported never offering HIV testing to all eligible patients regardless of risk factors, and only 14.3% reported offering testing all of the time. Only 25% of participants said they received “adequate” or “a lot” of HIV-related nursing education, and 42.9% reported “little” or “very little” education.
“For the most part, those of us working in hospitals, all the education that we get about HIV took place in school,” Elam said. “So, if you went to school in the early 2000s or in the 1990s, you don’t know much else.” Elam noted that she keeps informed on HIV research issues because it is an area of interest, but the hospital she had worked at did not contribute much to her knowledge.
Elam also found that in practice there were several barriers to performing screening, such as lack of availability of a dedicated HIV educator, tester, or counselors; not knowing where to refer patients who had a positive HIV test result; and insufficient time to address positive HIV test results in ED practice.
“A lot of these things are outside an individual nurse’s control,” said Elam, and can result in missing patients who would benefit from care. Lisa Leimer, RN, a nurse at Primary Health Care in Des Moines, works with patients after they have been diagnosed with HIV, but noted that many of her patients could have been identified earlier. “Once we get someone, you look back at medical records and you see that they have been in and out of the hospital,” she said. “There’s been multiple encounters,” she said.
Prioritizing HIV screening in all healthcare settings and including HIV education for all medical professionals – not just nurses – could help in the continuing battle against HIV. “So much has changed in the world of HIV,” she said. “We’re trying to end the epidemic, and it could happen if we identified, diagnosed, and treated the people that are living with it.”
Elam and Leimer have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a national survey of ED nurses. Nearly 43% of respondents said they had received “little” or “very little” HIV education as part of their professional development and practice.
This lack of continuing HIV education “often translated into attitudes that did not support the policy” of routine HIV screening in EDs, lead author Candace Elam, DNP, a family nurse practitioner at the Institute of Family Health in the Bronx, New York City, told this news organization. “But more than individual attitudes, what came out most clearly in the research was that organizational support for HIV screening in EDs was the one factor that could make or break whether an emergency nurse performs HIV screening,” she said. This includes working routine HIV screening into ED workflows and providing resources to streamline screening and testing efforts.
In 2006, the Centers for Disease Control and Prevention released guidance recommending routine HIV screening in all healthcare settings, including urgent care and EDs. Elam, who conducted the research as a student at Rutgers University School of Nursing in New Brunswick, N.J., noticed during her time as an ED nurse that, while her department had a policy supporting routine HIV screening, the practice was not consistent across all nursing staff. To find out how HIV screening varied nationally, Elam ran a national survey from Oct. through Dec. 2020, recruiting participants both by email outreach and Facebook.
In the 30- to 45-minute survey, respondents reported:
- Demographic information
- Knowledge of the CDC HIV screening recommendations
- Workplace HIV screening policy
- Self-reported performance of HIV screening
- Beliefs and attitudes pertaining to HIV screening
Overall, 371 individuals from 43 states filled out at least some part of the survey, and 171 individuals completed it. Of the 251 individuals who answered whether their EDs routinely conducted HIV screening, 76.9% responded affirmatively. Overall, 28.5% of respondents thought HIV screening was “not important” or “not at all important.” Nearly half – 47.6% – reported never offering HIV testing to all eligible patients regardless of risk factors, and only 14.3% reported offering testing all of the time. Only 25% of participants said they received “adequate” or “a lot” of HIV-related nursing education, and 42.9% reported “little” or “very little” education.
“For the most part, those of us working in hospitals, all the education that we get about HIV took place in school,” Elam said. “So, if you went to school in the early 2000s or in the 1990s, you don’t know much else.” Elam noted that she keeps informed on HIV research issues because it is an area of interest, but the hospital she had worked at did not contribute much to her knowledge.
Elam also found that in practice there were several barriers to performing screening, such as lack of availability of a dedicated HIV educator, tester, or counselors; not knowing where to refer patients who had a positive HIV test result; and insufficient time to address positive HIV test results in ED practice.
“A lot of these things are outside an individual nurse’s control,” said Elam, and can result in missing patients who would benefit from care. Lisa Leimer, RN, a nurse at Primary Health Care in Des Moines, works with patients after they have been diagnosed with HIV, but noted that many of her patients could have been identified earlier. “Once we get someone, you look back at medical records and you see that they have been in and out of the hospital,” she said. “There’s been multiple encounters,” she said.
Prioritizing HIV screening in all healthcare settings and including HIV education for all medical professionals – not just nurses – could help in the continuing battle against HIV. “So much has changed in the world of HIV,” she said. “We’re trying to end the epidemic, and it could happen if we identified, diagnosed, and treated the people that are living with it.”
Elam and Leimer have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children and COVID: New cases up again after dropping for 8 weeks
As children aged 5-11 years began to receive the first officially approved doses of COVID-19 vaccine, new pediatric cases increased after 8 consecutive weeks of declines, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Weekly cases peaked at almost 252,000 in early September and then dropped for 8 straight weeks before this latest rise, the AAP and the CHA said in their weekly COVID report, which is based on data reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The end of that 8-week drop, unfortunately, allowed another streak to continue: New cases have been above 100,000 for 13 consecutive weeks, the AAP and CHA noted.
The cumulative COVID count in children as of Nov. 4 was 6.5 million, the AAP/CHA said, although that figure does not fully cover Alabama, Nebraska, and Texas, which stopped public reporting over the summer. The Centers for Disease Control and Prevention, with input from all states and territories, puts the total through Nov. 8 at almost 5.7 million cases in children under 18 years of age, while most states define a child as someone aged 0-19 years.
As for the newest group of vaccinees, the CDC said that “updated vaccination data for 5-11 year-olds will be added to COVID Data Tracker later this week,” meaning the week of Nov. 7-13. Currently available data, however, show that almost 157,000 children under age 12 initiated vaccination in the 14 days ending Nov. 8, which was more than those aged 12-15 and 16-17 years combined (127,000).
Among those older groups, the CDC reports that 57.1% of 12- to 15-year-olds have received at least one dose and 47.9% are fully vaccinated, while 64.0% of those aged 16-17 have gotten at least one dose and 55.2% are fully vaccinated. Altogether, about 13.9 million children under age 18 have gotten at least one dose and almost 11.6 million are fully vaccinated, according to the CDC.
As children aged 5-11 years began to receive the first officially approved doses of COVID-19 vaccine, new pediatric cases increased after 8 consecutive weeks of declines, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Weekly cases peaked at almost 252,000 in early September and then dropped for 8 straight weeks before this latest rise, the AAP and the CHA said in their weekly COVID report, which is based on data reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The end of that 8-week drop, unfortunately, allowed another streak to continue: New cases have been above 100,000 for 13 consecutive weeks, the AAP and CHA noted.
The cumulative COVID count in children as of Nov. 4 was 6.5 million, the AAP/CHA said, although that figure does not fully cover Alabama, Nebraska, and Texas, which stopped public reporting over the summer. The Centers for Disease Control and Prevention, with input from all states and territories, puts the total through Nov. 8 at almost 5.7 million cases in children under 18 years of age, while most states define a child as someone aged 0-19 years.
As for the newest group of vaccinees, the CDC said that “updated vaccination data for 5-11 year-olds will be added to COVID Data Tracker later this week,” meaning the week of Nov. 7-13. Currently available data, however, show that almost 157,000 children under age 12 initiated vaccination in the 14 days ending Nov. 8, which was more than those aged 12-15 and 16-17 years combined (127,000).
Among those older groups, the CDC reports that 57.1% of 12- to 15-year-olds have received at least one dose and 47.9% are fully vaccinated, while 64.0% of those aged 16-17 have gotten at least one dose and 55.2% are fully vaccinated. Altogether, about 13.9 million children under age 18 have gotten at least one dose and almost 11.6 million are fully vaccinated, according to the CDC.
As children aged 5-11 years began to receive the first officially approved doses of COVID-19 vaccine, new pediatric cases increased after 8 consecutive weeks of declines, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Weekly cases peaked at almost 252,000 in early September and then dropped for 8 straight weeks before this latest rise, the AAP and the CHA said in their weekly COVID report, which is based on data reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The end of that 8-week drop, unfortunately, allowed another streak to continue: New cases have been above 100,000 for 13 consecutive weeks, the AAP and CHA noted.
The cumulative COVID count in children as of Nov. 4 was 6.5 million, the AAP/CHA said, although that figure does not fully cover Alabama, Nebraska, and Texas, which stopped public reporting over the summer. The Centers for Disease Control and Prevention, with input from all states and territories, puts the total through Nov. 8 at almost 5.7 million cases in children under 18 years of age, while most states define a child as someone aged 0-19 years.
As for the newest group of vaccinees, the CDC said that “updated vaccination data for 5-11 year-olds will be added to COVID Data Tracker later this week,” meaning the week of Nov. 7-13. Currently available data, however, show that almost 157,000 children under age 12 initiated vaccination in the 14 days ending Nov. 8, which was more than those aged 12-15 and 16-17 years combined (127,000).
Among those older groups, the CDC reports that 57.1% of 12- to 15-year-olds have received at least one dose and 47.9% are fully vaccinated, while 64.0% of those aged 16-17 have gotten at least one dose and 55.2% are fully vaccinated. Altogether, about 13.9 million children under age 18 have gotten at least one dose and almost 11.6 million are fully vaccinated, according to the CDC.
72-year-old man • fever • new-onset urinary frequency • altered mental state • Dx?
THE CASE
A 72-year-old man was admitted to our Dallas hospital with a 4-day history of fevers and new-onset urinary frequency. He did not report any joint pain, sick contacts, or recent travel or recall any skin findings (rashes, insect bites). Past medical history was significant for hypertension, hyperlipidemia, diabetes, benign prostatic hyperplasia, recurrent urinary tract infections, and lumbar radiculopathy.
Initial signs and symptoms were suggestive of sepsis: a temperature of 102.7 °F, tachycardia, and a suspected genitourinary infection. This was supported by initial labs concerning for end-organ damage: elevated creatinine of 1.58 mg/dL (reference range, 0.67-1.17 mg/dL), elevated international normalized ratio (INR) of 1.6 (reference range, 0.9-1.1), hemoglobin of 12.8 g/dL (reference range, 13.5 - 17.5 g/dL), and platelet count of 99 ×109/L (reference range, 160-383 ×109/L).
Over the next several days, the patient’s condition worsened, and he experienced a decline in mental status, despite initiation of broad-spectrum antibiotics and fluid resuscitation. Although lumbar puncture was warranted, neither Neurology nor Interventional Radiology were willing to risk the procedure given the patient’s worsening hemoglobin (8.3 g/dL) and platelet count (51 ×109/L).
Preliminary work-up included a urinalysis negative for leukocytes, nitrites, and bacteria—despite a urine culture that showed gram-positive cocci. His chest x-ray was unremarkable, and computed tomography of his brain showed generalized atrophy without acute changes. The work-up was expanded to fungal cultures and immunochemical assays. Empiric treatment with micafungin and acyclovir was started without improvement.
Further conversation with family revealed that the patient liked to spend time outdoors and he’d had a similar episode in which he’d been diagnosed with an unknown disease from an insect bite. Pertinent negative tests included: HIV, syphilis, rapid heterophile antibody, influenza, respiratory virus panel, blood culture, fungal culture, antineutrophil cytoplasmic antibodies, histoplasmosis, brucellosis, malaria, Epstein-Barr virus, cytomegalovirus, and parvovirus. Coxiella burnetii and West Nile virus immunoglobulin (Ig) G were positive, suggesting a prior exposure.
THE DIAGNOSIS
Given these new findings and reported outdoor activities, Infectious Diseases recommended we start our patient on doxycycline for possible rickettsia infection. On Day 8, doxycycline 200 mg IV once daily was started. (The IV form was initiated due to the patient’s altered mentation.) The patient started to show improvement, and on Day 14, an immunofluorescence antibody (IFA) assay revealed Rickettsia typhi IgM titers 1:512 (< 1:64) and IgG titers 1:256 (< 1:64), consistent with a diagnosis of murine (endemic) typhus.
DISCUSSION
Murine typhus is an acute febrile disease caused by R typhi, an obligate, intracellular gram-negative organism.1 Worldwide, transmission to humans occurs mainly from infected rat fleas harbored by rodents. In the United States, it’s been suggested that opossums serve as an important reservoir in peri-domestic settings, with cat fleas as vectors.2-4 The disease is endemic to southern California and south Texas.4
Continue to: Incidence of murine typhus
Incidence of murine typhus has declined in the United States since 1945 with the use of the insecticide dichlorodiphenyltrichloroethane (DDT). However, a recent rise in murine typhus cases—likely due to ecological changes—makes timely diagnosis and treatment essential.5 An epidemiologic study of 1762 confirmed cases in Texas from 2003 to 2013 found an increase in the number of cases and an expansion of the geographic areas impacted.3 Thus, in the work-up of acute fever of unknown origin, it is not unreasonable to include murine typhus in the differential.
Murine typhus can be difficult to diagnose due to nonspecific clinical manifestation.3,4 A 2016 systematic review of 2074 patients reported common symptoms of fever, headache, malaise, chills, and myalgia.6 The most common laboratory abnormalities in adults were elevated aminotransferases, lactate dehydrogenase, hypoalbuminemia, and thrombocytopenia.6 A 4-fold increase in typhus group IgM or IgG-specific antibody titer by IFA is supportive of diagnosis.4
The differential diagnosis included urosepsis, prostatitis, syphilis, HIV, and meningitis. However, lack of response to broad-spectrum antibiotics and antifungals made a diagnosis of urologic infection unlikely. A negative sexually transmitted infection screen ruled out syphilis and HIV.
Treatment may begin without a definitive diagnosis
Serologic testing with IFA is the preferred diagnostic method; however, a definitive diagnosis is not needed before treatment can be initiated. Doxycycline is the first-line therapy for all rickettsioses. Adults are advised to take doxycycline 200 mg orally once, followed by 100 mg twice daily until the patient improves, has been afebrile for 48 hours, and has received treatment for at least 7 days.7 Oral chloramphenicol is considered a second-line treatment; however it is not available in the United States and is associated with adverse hematologic effects.7
Our patient responded remarkably well to the doxycycline. After a 14-day course was completed, he was discharged to a skilled nursing facility for physical rehabilitation.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Rickettsia diseases, such as murine typhus, should be considered in the differential if a patient presents with a worsening clinical picture of unresolved delirium; fever despite use of broad-spectrum antibiotics, antifungals, and antivirals; and a history of potential outdoor exposure. Sources include opossums or cats when flea contact is likely. Rickettsia diseases belong in the differential when there is a history of travel to tropical areas, as well. All suspected cases should be reported to the local health department.
CORRESPONDENCE
Tenzin Tsewang MD, 5200 Harry Hines Boulevard, Dallas, TX 75235; [email protected]
1. Afzal Z, Kallumadanda S, Wang F, et al. Acute febrile illness and complications due to murine typhus, Texas, USA. Emerg Infect Dis. 2017;23:1268-1273. doi: 10.3201/eid2308.161861
2. Stern RM, Luskin MR, Clark RP, et al. A headache of a diagnosis. N Engl J Med. 2018;379:475-479. doi: 10.1056/NEJMcps1803584
3. Murray KO, Evert N, Mayes B, et al. Typhus group rickettsiosis, Texas, USA, 2003–2013. Emerg Iinfect Dis. 2017;23:645-648. doi: 10.3201/eid2304.160958
4. Blanton LS, Idowu BM, Tatsch TN, et al. Opossums and cat fleas: new insights in the ecology of murine typhus in Galveston, Texas. Am J Trop Med Hyg. 2016;95:457-461. doi: 10.4269/ajtmh.16-0197
5. Civen R, Ngo V. Murine typhus: an unrecognized suburban vectorborne disease. Clin Infect Dis. 2008;46:913-918. doi: 10.1086/527443
6. Tsioutis C, Zafeiri M, Avramopoulos A, et al. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16-24. doi: 10.1016/j.actatropica.2016.10.018
7. Petri WA Jr. Murine (Endemic) Typhus. Merck Manual Professional Version. Modified July 2020. Accessed October 25, 2021. www.merckmanuals.com/professional/infectious-diseases/rickettsiae-and-related-organisms/murine-endemic-typhus
THE CASE
A 72-year-old man was admitted to our Dallas hospital with a 4-day history of fevers and new-onset urinary frequency. He did not report any joint pain, sick contacts, or recent travel or recall any skin findings (rashes, insect bites). Past medical history was significant for hypertension, hyperlipidemia, diabetes, benign prostatic hyperplasia, recurrent urinary tract infections, and lumbar radiculopathy.
Initial signs and symptoms were suggestive of sepsis: a temperature of 102.7 °F, tachycardia, and a suspected genitourinary infection. This was supported by initial labs concerning for end-organ damage: elevated creatinine of 1.58 mg/dL (reference range, 0.67-1.17 mg/dL), elevated international normalized ratio (INR) of 1.6 (reference range, 0.9-1.1), hemoglobin of 12.8 g/dL (reference range, 13.5 - 17.5 g/dL), and platelet count of 99 ×109/L (reference range, 160-383 ×109/L).
Over the next several days, the patient’s condition worsened, and he experienced a decline in mental status, despite initiation of broad-spectrum antibiotics and fluid resuscitation. Although lumbar puncture was warranted, neither Neurology nor Interventional Radiology were willing to risk the procedure given the patient’s worsening hemoglobin (8.3 g/dL) and platelet count (51 ×109/L).
Preliminary work-up included a urinalysis negative for leukocytes, nitrites, and bacteria—despite a urine culture that showed gram-positive cocci. His chest x-ray was unremarkable, and computed tomography of his brain showed generalized atrophy without acute changes. The work-up was expanded to fungal cultures and immunochemical assays. Empiric treatment with micafungin and acyclovir was started without improvement.
Further conversation with family revealed that the patient liked to spend time outdoors and he’d had a similar episode in which he’d been diagnosed with an unknown disease from an insect bite. Pertinent negative tests included: HIV, syphilis, rapid heterophile antibody, influenza, respiratory virus panel, blood culture, fungal culture, antineutrophil cytoplasmic antibodies, histoplasmosis, brucellosis, malaria, Epstein-Barr virus, cytomegalovirus, and parvovirus. Coxiella burnetii and West Nile virus immunoglobulin (Ig) G were positive, suggesting a prior exposure.
THE DIAGNOSIS
Given these new findings and reported outdoor activities, Infectious Diseases recommended we start our patient on doxycycline for possible rickettsia infection. On Day 8, doxycycline 200 mg IV once daily was started. (The IV form was initiated due to the patient’s altered mentation.) The patient started to show improvement, and on Day 14, an immunofluorescence antibody (IFA) assay revealed Rickettsia typhi IgM titers 1:512 (< 1:64) and IgG titers 1:256 (< 1:64), consistent with a diagnosis of murine (endemic) typhus.
DISCUSSION
Murine typhus is an acute febrile disease caused by R typhi, an obligate, intracellular gram-negative organism.1 Worldwide, transmission to humans occurs mainly from infected rat fleas harbored by rodents. In the United States, it’s been suggested that opossums serve as an important reservoir in peri-domestic settings, with cat fleas as vectors.2-4 The disease is endemic to southern California and south Texas.4
Continue to: Incidence of murine typhus
Incidence of murine typhus has declined in the United States since 1945 with the use of the insecticide dichlorodiphenyltrichloroethane (DDT). However, a recent rise in murine typhus cases—likely due to ecological changes—makes timely diagnosis and treatment essential.5 An epidemiologic study of 1762 confirmed cases in Texas from 2003 to 2013 found an increase in the number of cases and an expansion of the geographic areas impacted.3 Thus, in the work-up of acute fever of unknown origin, it is not unreasonable to include murine typhus in the differential.
Murine typhus can be difficult to diagnose due to nonspecific clinical manifestation.3,4 A 2016 systematic review of 2074 patients reported common symptoms of fever, headache, malaise, chills, and myalgia.6 The most common laboratory abnormalities in adults were elevated aminotransferases, lactate dehydrogenase, hypoalbuminemia, and thrombocytopenia.6 A 4-fold increase in typhus group IgM or IgG-specific antibody titer by IFA is supportive of diagnosis.4
The differential diagnosis included urosepsis, prostatitis, syphilis, HIV, and meningitis. However, lack of response to broad-spectrum antibiotics and antifungals made a diagnosis of urologic infection unlikely. A negative sexually transmitted infection screen ruled out syphilis and HIV.
Treatment may begin without a definitive diagnosis
Serologic testing with IFA is the preferred diagnostic method; however, a definitive diagnosis is not needed before treatment can be initiated. Doxycycline is the first-line therapy for all rickettsioses. Adults are advised to take doxycycline 200 mg orally once, followed by 100 mg twice daily until the patient improves, has been afebrile for 48 hours, and has received treatment for at least 7 days.7 Oral chloramphenicol is considered a second-line treatment; however it is not available in the United States and is associated with adverse hematologic effects.7
Our patient responded remarkably well to the doxycycline. After a 14-day course was completed, he was discharged to a skilled nursing facility for physical rehabilitation.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Rickettsia diseases, such as murine typhus, should be considered in the differential if a patient presents with a worsening clinical picture of unresolved delirium; fever despite use of broad-spectrum antibiotics, antifungals, and antivirals; and a history of potential outdoor exposure. Sources include opossums or cats when flea contact is likely. Rickettsia diseases belong in the differential when there is a history of travel to tropical areas, as well. All suspected cases should be reported to the local health department.
CORRESPONDENCE
Tenzin Tsewang MD, 5200 Harry Hines Boulevard, Dallas, TX 75235; [email protected]
THE CASE
A 72-year-old man was admitted to our Dallas hospital with a 4-day history of fevers and new-onset urinary frequency. He did not report any joint pain, sick contacts, or recent travel or recall any skin findings (rashes, insect bites). Past medical history was significant for hypertension, hyperlipidemia, diabetes, benign prostatic hyperplasia, recurrent urinary tract infections, and lumbar radiculopathy.
Initial signs and symptoms were suggestive of sepsis: a temperature of 102.7 °F, tachycardia, and a suspected genitourinary infection. This was supported by initial labs concerning for end-organ damage: elevated creatinine of 1.58 mg/dL (reference range, 0.67-1.17 mg/dL), elevated international normalized ratio (INR) of 1.6 (reference range, 0.9-1.1), hemoglobin of 12.8 g/dL (reference range, 13.5 - 17.5 g/dL), and platelet count of 99 ×109/L (reference range, 160-383 ×109/L).
Over the next several days, the patient’s condition worsened, and he experienced a decline in mental status, despite initiation of broad-spectrum antibiotics and fluid resuscitation. Although lumbar puncture was warranted, neither Neurology nor Interventional Radiology were willing to risk the procedure given the patient’s worsening hemoglobin (8.3 g/dL) and platelet count (51 ×109/L).
Preliminary work-up included a urinalysis negative for leukocytes, nitrites, and bacteria—despite a urine culture that showed gram-positive cocci. His chest x-ray was unremarkable, and computed tomography of his brain showed generalized atrophy without acute changes. The work-up was expanded to fungal cultures and immunochemical assays. Empiric treatment with micafungin and acyclovir was started without improvement.
Further conversation with family revealed that the patient liked to spend time outdoors and he’d had a similar episode in which he’d been diagnosed with an unknown disease from an insect bite. Pertinent negative tests included: HIV, syphilis, rapid heterophile antibody, influenza, respiratory virus panel, blood culture, fungal culture, antineutrophil cytoplasmic antibodies, histoplasmosis, brucellosis, malaria, Epstein-Barr virus, cytomegalovirus, and parvovirus. Coxiella burnetii and West Nile virus immunoglobulin (Ig) G were positive, suggesting a prior exposure.
THE DIAGNOSIS
Given these new findings and reported outdoor activities, Infectious Diseases recommended we start our patient on doxycycline for possible rickettsia infection. On Day 8, doxycycline 200 mg IV once daily was started. (The IV form was initiated due to the patient’s altered mentation.) The patient started to show improvement, and on Day 14, an immunofluorescence antibody (IFA) assay revealed Rickettsia typhi IgM titers 1:512 (< 1:64) and IgG titers 1:256 (< 1:64), consistent with a diagnosis of murine (endemic) typhus.
DISCUSSION
Murine typhus is an acute febrile disease caused by R typhi, an obligate, intracellular gram-negative organism.1 Worldwide, transmission to humans occurs mainly from infected rat fleas harbored by rodents. In the United States, it’s been suggested that opossums serve as an important reservoir in peri-domestic settings, with cat fleas as vectors.2-4 The disease is endemic to southern California and south Texas.4
Continue to: Incidence of murine typhus
Incidence of murine typhus has declined in the United States since 1945 with the use of the insecticide dichlorodiphenyltrichloroethane (DDT). However, a recent rise in murine typhus cases—likely due to ecological changes—makes timely diagnosis and treatment essential.5 An epidemiologic study of 1762 confirmed cases in Texas from 2003 to 2013 found an increase in the number of cases and an expansion of the geographic areas impacted.3 Thus, in the work-up of acute fever of unknown origin, it is not unreasonable to include murine typhus in the differential.
Murine typhus can be difficult to diagnose due to nonspecific clinical manifestation.3,4 A 2016 systematic review of 2074 patients reported common symptoms of fever, headache, malaise, chills, and myalgia.6 The most common laboratory abnormalities in adults were elevated aminotransferases, lactate dehydrogenase, hypoalbuminemia, and thrombocytopenia.6 A 4-fold increase in typhus group IgM or IgG-specific antibody titer by IFA is supportive of diagnosis.4
The differential diagnosis included urosepsis, prostatitis, syphilis, HIV, and meningitis. However, lack of response to broad-spectrum antibiotics and antifungals made a diagnosis of urologic infection unlikely. A negative sexually transmitted infection screen ruled out syphilis and HIV.
Treatment may begin without a definitive diagnosis
Serologic testing with IFA is the preferred diagnostic method; however, a definitive diagnosis is not needed before treatment can be initiated. Doxycycline is the first-line therapy for all rickettsioses. Adults are advised to take doxycycline 200 mg orally once, followed by 100 mg twice daily until the patient improves, has been afebrile for 48 hours, and has received treatment for at least 7 days.7 Oral chloramphenicol is considered a second-line treatment; however it is not available in the United States and is associated with adverse hematologic effects.7
Our patient responded remarkably well to the doxycycline. After a 14-day course was completed, he was discharged to a skilled nursing facility for physical rehabilitation.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Rickettsia diseases, such as murine typhus, should be considered in the differential if a patient presents with a worsening clinical picture of unresolved delirium; fever despite use of broad-spectrum antibiotics, antifungals, and antivirals; and a history of potential outdoor exposure. Sources include opossums or cats when flea contact is likely. Rickettsia diseases belong in the differential when there is a history of travel to tropical areas, as well. All suspected cases should be reported to the local health department.
CORRESPONDENCE
Tenzin Tsewang MD, 5200 Harry Hines Boulevard, Dallas, TX 75235; [email protected]
1. Afzal Z, Kallumadanda S, Wang F, et al. Acute febrile illness and complications due to murine typhus, Texas, USA. Emerg Infect Dis. 2017;23:1268-1273. doi: 10.3201/eid2308.161861
2. Stern RM, Luskin MR, Clark RP, et al. A headache of a diagnosis. N Engl J Med. 2018;379:475-479. doi: 10.1056/NEJMcps1803584
3. Murray KO, Evert N, Mayes B, et al. Typhus group rickettsiosis, Texas, USA, 2003–2013. Emerg Iinfect Dis. 2017;23:645-648. doi: 10.3201/eid2304.160958
4. Blanton LS, Idowu BM, Tatsch TN, et al. Opossums and cat fleas: new insights in the ecology of murine typhus in Galveston, Texas. Am J Trop Med Hyg. 2016;95:457-461. doi: 10.4269/ajtmh.16-0197
5. Civen R, Ngo V. Murine typhus: an unrecognized suburban vectorborne disease. Clin Infect Dis. 2008;46:913-918. doi: 10.1086/527443
6. Tsioutis C, Zafeiri M, Avramopoulos A, et al. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16-24. doi: 10.1016/j.actatropica.2016.10.018
7. Petri WA Jr. Murine (Endemic) Typhus. Merck Manual Professional Version. Modified July 2020. Accessed October 25, 2021. www.merckmanuals.com/professional/infectious-diseases/rickettsiae-and-related-organisms/murine-endemic-typhus
1. Afzal Z, Kallumadanda S, Wang F, et al. Acute febrile illness and complications due to murine typhus, Texas, USA. Emerg Infect Dis. 2017;23:1268-1273. doi: 10.3201/eid2308.161861
2. Stern RM, Luskin MR, Clark RP, et al. A headache of a diagnosis. N Engl J Med. 2018;379:475-479. doi: 10.1056/NEJMcps1803584
3. Murray KO, Evert N, Mayes B, et al. Typhus group rickettsiosis, Texas, USA, 2003–2013. Emerg Iinfect Dis. 2017;23:645-648. doi: 10.3201/eid2304.160958
4. Blanton LS, Idowu BM, Tatsch TN, et al. Opossums and cat fleas: new insights in the ecology of murine typhus in Galveston, Texas. Am J Trop Med Hyg. 2016;95:457-461. doi: 10.4269/ajtmh.16-0197
5. Civen R, Ngo V. Murine typhus: an unrecognized suburban vectorborne disease. Clin Infect Dis. 2008;46:913-918. doi: 10.1086/527443
6. Tsioutis C, Zafeiri M, Avramopoulos A, et al. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16-24. doi: 10.1016/j.actatropica.2016.10.018
7. Petri WA Jr. Murine (Endemic) Typhus. Merck Manual Professional Version. Modified July 2020. Accessed October 25, 2021. www.merckmanuals.com/professional/infectious-diseases/rickettsiae-and-related-organisms/murine-endemic-typhus
More than half of people living with HIV have coronary plaque
More than half of people living with HIV and suppressed viral loads nonetheless had imaging-confirmed coronary artery disease – and despite longtime use of HIV drugs that have been associated with cardiovascular trouble, none of those drugs were implicated in disease risk in this study.
“Traditional risk factors and duration of HIV infection were associated with severe coronary artery disease,” said Andreas Knudsen, MD, PhD, an infectious disease provider at Copenhagen University Hospital, Hvidovre, Denmark, during his presentation at the 18th European AIDS Conference. “When we adjusted for time since diagnosis of HIV, none of the drugs remained associated with the severity of coronary artery disease.”
Notably, that included abacavir, which was found in another EACS presentation and in past research to be associated with increased rates of heart attacks. Abacavir is sold individually as a generic as well as a component of Epzicom (abacavir/lamivudine) and the single-drug regimen Triumeq (dolutegravir/abacavir/lamivudine).
The Copenhagen Comorbidity in HIV Infection (COCOMO) study enrolled 1,099 people living with HIV in the Danish capital beginning in 2015, and 705 of them had angiographies via CT available to include in the results. The participants were almost all male (89%), at a healthy weight (BMI of 25), and 96% had undetectable viral loads.
Large minorities of participants also had traditional risk factors for coronary artery disease. More than one in four smoked, one in five had high cholesterol, and 42% had high blood pressure. In addition, many had used drugs that have been associated with cardiovascular trouble, including abacavir, which 26% of participants had used; indinavir, used by 17% of participants; zidovudine/AZT, used by 47%; and didanosine, which 14% used. (While abacavir is still in use, the other three drugs are considered legacy drugs and are not in current use.)
In addition, nearly one in three (29%) were currently using a protease inhibitor, which has been associated with heart failure.
When the investigators looked at participants’ CTs, they found that, by the Coronary Artery Disease-Reporting and Data Systems (CAD-RAMS) scoring system, close to half (46%) had clear arteries with no signs of coronary artery disease. But that also meant that 54% had some blockage or stiffening of the arteries. The good news is that 27% of those people had minimal or mild coronary artery disease.
But a full 17% had confirmed obstructive coronary artery disease, and another 1 in 10 participants had the highest level of blockages. When they broke the data down by traditional and HIV medication–related risk factors for coronary artery disease, they found something interesting. Although obesity was associated with the presence of atherosclerosis, it wasn’t associated with severe disease. But diabetes was the reverse of that: It wasn’t associated with the presence of the disease, but it was associated with more severe disease.
And when they looked at abacavir, they found no relationship between the drug and atherosclerosis. “Abacavir was not associated with the presence of atherosclerosis and was also not associated with severity of disease,” said Dr. Knudsen.
Although past use of AZT, indinavir, and didanosine were associated with severity of atherosclerosis, that association went away when Dr. Knudsen and team adjusted the findings for time since diagnosis. What was associated atherosclerosis was length of time living with HIV itself. For every 5 years a person lived with HIV, the study found the risk of having any atherosclerosis increased 20% and severity increased 23%. In addition, being a man was associated with a nearly 2.5-times increased risk of having any atherosclerosis and a 96% increased chance of having more severe atherosclerosis. Having diabetes was associated with a nearly threefold increased risk of atherosclerosis, as was every additional decade of life for a person who was living with HIV.
The findings confirm the baseline data of the REPRIEVE trial, which recently released data showing similarly high rates of atherosclerotic plaque in people living with HIV who didn’t register as “at risk” for cardiovascular disease using traditional scoring methods.
“It’s important in that it’s a huge study that’s confirmatory [of] what we know, which is that there are high levels of subclinical coronary artery disease in people living with HIV,” said Steven Grinspoon, MD, professor at Harvard Medical School in Boston, Massachusetts, and principal investigator of REPRIEVE.
As for the lack of association between abacavir and cardiovascular risk, he said he’s taking the findings with a grain of salt.
“It’s hard to make a lot out of that,” he said. “It’s hard to know in a cross-sectional study. People put people on different things.”
In Spain, where Jose Ignacio Bernardino, MD, treats people living with HIV at La Paz University Hospital in Madrid, abacavir is mostly a moot point, as clinicians have long since moved away from maintaining people living with HIV on any abacavir-containing regimens. What’s more important in the study, he told this news organization, is that “worrisome” high level of risk. REPRIEVE will test whether statins can reduce heart disease events in people living with HIV. But in the meantime, he said the take-away for clinicians from the study is the primary importance of traditional cardiovascular risk factors.
“We have to acknowledge that the major cardiovascular risk factor is age,” he said. “When patients are approaching their 50s, I usually try to stress a lot about cardiovascular risk factors in general. I stress healthy lifestyle – get physical exercise, hypertension, glucose, lipids – in every single patient.”
Dr. Knudsen and Dr. Bernardino have disclosed no relevant financial relationships. Dr. Grinspoon reports receiving personal and consulting fees from Theratechnologies and ViiV Healthcare.
A version of this article first appeared on Medscape.com.
More than half of people living with HIV and suppressed viral loads nonetheless had imaging-confirmed coronary artery disease – and despite longtime use of HIV drugs that have been associated with cardiovascular trouble, none of those drugs were implicated in disease risk in this study.
“Traditional risk factors and duration of HIV infection were associated with severe coronary artery disease,” said Andreas Knudsen, MD, PhD, an infectious disease provider at Copenhagen University Hospital, Hvidovre, Denmark, during his presentation at the 18th European AIDS Conference. “When we adjusted for time since diagnosis of HIV, none of the drugs remained associated with the severity of coronary artery disease.”
Notably, that included abacavir, which was found in another EACS presentation and in past research to be associated with increased rates of heart attacks. Abacavir is sold individually as a generic as well as a component of Epzicom (abacavir/lamivudine) and the single-drug regimen Triumeq (dolutegravir/abacavir/lamivudine).
The Copenhagen Comorbidity in HIV Infection (COCOMO) study enrolled 1,099 people living with HIV in the Danish capital beginning in 2015, and 705 of them had angiographies via CT available to include in the results. The participants were almost all male (89%), at a healthy weight (BMI of 25), and 96% had undetectable viral loads.
Large minorities of participants also had traditional risk factors for coronary artery disease. More than one in four smoked, one in five had high cholesterol, and 42% had high blood pressure. In addition, many had used drugs that have been associated with cardiovascular trouble, including abacavir, which 26% of participants had used; indinavir, used by 17% of participants; zidovudine/AZT, used by 47%; and didanosine, which 14% used. (While abacavir is still in use, the other three drugs are considered legacy drugs and are not in current use.)
In addition, nearly one in three (29%) were currently using a protease inhibitor, which has been associated with heart failure.
When the investigators looked at participants’ CTs, they found that, by the Coronary Artery Disease-Reporting and Data Systems (CAD-RAMS) scoring system, close to half (46%) had clear arteries with no signs of coronary artery disease. But that also meant that 54% had some blockage or stiffening of the arteries. The good news is that 27% of those people had minimal or mild coronary artery disease.
But a full 17% had confirmed obstructive coronary artery disease, and another 1 in 10 participants had the highest level of blockages. When they broke the data down by traditional and HIV medication–related risk factors for coronary artery disease, they found something interesting. Although obesity was associated with the presence of atherosclerosis, it wasn’t associated with severe disease. But diabetes was the reverse of that: It wasn’t associated with the presence of the disease, but it was associated with more severe disease.
And when they looked at abacavir, they found no relationship between the drug and atherosclerosis. “Abacavir was not associated with the presence of atherosclerosis and was also not associated with severity of disease,” said Dr. Knudsen.
Although past use of AZT, indinavir, and didanosine were associated with severity of atherosclerosis, that association went away when Dr. Knudsen and team adjusted the findings for time since diagnosis. What was associated atherosclerosis was length of time living with HIV itself. For every 5 years a person lived with HIV, the study found the risk of having any atherosclerosis increased 20% and severity increased 23%. In addition, being a man was associated with a nearly 2.5-times increased risk of having any atherosclerosis and a 96% increased chance of having more severe atherosclerosis. Having diabetes was associated with a nearly threefold increased risk of atherosclerosis, as was every additional decade of life for a person who was living with HIV.
The findings confirm the baseline data of the REPRIEVE trial, which recently released data showing similarly high rates of atherosclerotic plaque in people living with HIV who didn’t register as “at risk” for cardiovascular disease using traditional scoring methods.
“It’s important in that it’s a huge study that’s confirmatory [of] what we know, which is that there are high levels of subclinical coronary artery disease in people living with HIV,” said Steven Grinspoon, MD, professor at Harvard Medical School in Boston, Massachusetts, and principal investigator of REPRIEVE.
As for the lack of association between abacavir and cardiovascular risk, he said he’s taking the findings with a grain of salt.
“It’s hard to make a lot out of that,” he said. “It’s hard to know in a cross-sectional study. People put people on different things.”
In Spain, where Jose Ignacio Bernardino, MD, treats people living with HIV at La Paz University Hospital in Madrid, abacavir is mostly a moot point, as clinicians have long since moved away from maintaining people living with HIV on any abacavir-containing regimens. What’s more important in the study, he told this news organization, is that “worrisome” high level of risk. REPRIEVE will test whether statins can reduce heart disease events in people living with HIV. But in the meantime, he said the take-away for clinicians from the study is the primary importance of traditional cardiovascular risk factors.
“We have to acknowledge that the major cardiovascular risk factor is age,” he said. “When patients are approaching their 50s, I usually try to stress a lot about cardiovascular risk factors in general. I stress healthy lifestyle – get physical exercise, hypertension, glucose, lipids – in every single patient.”
Dr. Knudsen and Dr. Bernardino have disclosed no relevant financial relationships. Dr. Grinspoon reports receiving personal and consulting fees from Theratechnologies and ViiV Healthcare.
A version of this article first appeared on Medscape.com.
More than half of people living with HIV and suppressed viral loads nonetheless had imaging-confirmed coronary artery disease – and despite longtime use of HIV drugs that have been associated with cardiovascular trouble, none of those drugs were implicated in disease risk in this study.
“Traditional risk factors and duration of HIV infection were associated with severe coronary artery disease,” said Andreas Knudsen, MD, PhD, an infectious disease provider at Copenhagen University Hospital, Hvidovre, Denmark, during his presentation at the 18th European AIDS Conference. “When we adjusted for time since diagnosis of HIV, none of the drugs remained associated with the severity of coronary artery disease.”
Notably, that included abacavir, which was found in another EACS presentation and in past research to be associated with increased rates of heart attacks. Abacavir is sold individually as a generic as well as a component of Epzicom (abacavir/lamivudine) and the single-drug regimen Triumeq (dolutegravir/abacavir/lamivudine).
The Copenhagen Comorbidity in HIV Infection (COCOMO) study enrolled 1,099 people living with HIV in the Danish capital beginning in 2015, and 705 of them had angiographies via CT available to include in the results. The participants were almost all male (89%), at a healthy weight (BMI of 25), and 96% had undetectable viral loads.
Large minorities of participants also had traditional risk factors for coronary artery disease. More than one in four smoked, one in five had high cholesterol, and 42% had high blood pressure. In addition, many had used drugs that have been associated with cardiovascular trouble, including abacavir, which 26% of participants had used; indinavir, used by 17% of participants; zidovudine/AZT, used by 47%; and didanosine, which 14% used. (While abacavir is still in use, the other three drugs are considered legacy drugs and are not in current use.)
In addition, nearly one in three (29%) were currently using a protease inhibitor, which has been associated with heart failure.
When the investigators looked at participants’ CTs, they found that, by the Coronary Artery Disease-Reporting and Data Systems (CAD-RAMS) scoring system, close to half (46%) had clear arteries with no signs of coronary artery disease. But that also meant that 54% had some blockage or stiffening of the arteries. The good news is that 27% of those people had minimal or mild coronary artery disease.
But a full 17% had confirmed obstructive coronary artery disease, and another 1 in 10 participants had the highest level of blockages. When they broke the data down by traditional and HIV medication–related risk factors for coronary artery disease, they found something interesting. Although obesity was associated with the presence of atherosclerosis, it wasn’t associated with severe disease. But diabetes was the reverse of that: It wasn’t associated with the presence of the disease, but it was associated with more severe disease.
And when they looked at abacavir, they found no relationship between the drug and atherosclerosis. “Abacavir was not associated with the presence of atherosclerosis and was also not associated with severity of disease,” said Dr. Knudsen.
Although past use of AZT, indinavir, and didanosine were associated with severity of atherosclerosis, that association went away when Dr. Knudsen and team adjusted the findings for time since diagnosis. What was associated atherosclerosis was length of time living with HIV itself. For every 5 years a person lived with HIV, the study found the risk of having any atherosclerosis increased 20% and severity increased 23%. In addition, being a man was associated with a nearly 2.5-times increased risk of having any atherosclerosis and a 96% increased chance of having more severe atherosclerosis. Having diabetes was associated with a nearly threefold increased risk of atherosclerosis, as was every additional decade of life for a person who was living with HIV.
The findings confirm the baseline data of the REPRIEVE trial, which recently released data showing similarly high rates of atherosclerotic plaque in people living with HIV who didn’t register as “at risk” for cardiovascular disease using traditional scoring methods.
“It’s important in that it’s a huge study that’s confirmatory [of] what we know, which is that there are high levels of subclinical coronary artery disease in people living with HIV,” said Steven Grinspoon, MD, professor at Harvard Medical School in Boston, Massachusetts, and principal investigator of REPRIEVE.
As for the lack of association between abacavir and cardiovascular risk, he said he’s taking the findings with a grain of salt.
“It’s hard to make a lot out of that,” he said. “It’s hard to know in a cross-sectional study. People put people on different things.”
In Spain, where Jose Ignacio Bernardino, MD, treats people living with HIV at La Paz University Hospital in Madrid, abacavir is mostly a moot point, as clinicians have long since moved away from maintaining people living with HIV on any abacavir-containing regimens. What’s more important in the study, he told this news organization, is that “worrisome” high level of risk. REPRIEVE will test whether statins can reduce heart disease events in people living with HIV. But in the meantime, he said the take-away for clinicians from the study is the primary importance of traditional cardiovascular risk factors.
“We have to acknowledge that the major cardiovascular risk factor is age,” he said. “When patients are approaching their 50s, I usually try to stress a lot about cardiovascular risk factors in general. I stress healthy lifestyle – get physical exercise, hypertension, glucose, lipids – in every single patient.”
Dr. Knudsen and Dr. Bernardino have disclosed no relevant financial relationships. Dr. Grinspoon reports receiving personal and consulting fees from Theratechnologies and ViiV Healthcare.
A version of this article first appeared on Medscape.com.