User login
HPV vaccines reduce cervical cancer rates in young females
Two different studies have found that, provided young females are immunized with the human papilloma virus (HPV) vaccine at a young enough age, both the incidence of and mortality from cervical cancer can be dramatically curtailed, data from the United Kingdom and to a lesser extent, the United States indicate.
In the U.K. study, published online in The Lancet, researchers showed that the national vaccination program against HPV, initiated in England in 2008, has all but eradicated cervical cancer and cervical intraepithelial neoplasia (CIN3) in young girls who received the vaccine at the age of 12 and 13 years (school year 8) prior to their sexual debut.
In this age group, cervical cancer rates were 87% lower than rates among previously nonvaccinated generations, while CIN3 rates were reduced by 97%, as researchers report. “It’s been incredible to see the impact of HPV vaccination, and now we can prove it prevented hundreds of women from developing cancer in England,” senior author Peter Sasieni, MD, King’s College London, said in a statement. “To see the real-life impact of the vaccine has been truly rewarding,” he added.
“This study provides the first direct evidence of the impact of the UK HPV vaccination campaign on cervical cancer incidence, showing a large reduction in cervical cancer rates in vaccinated cohorts,” Kate Soldan, MD, UK Health Security Agency, London, said in the same statement.
“This represents an important step forward in cervical cancer prevention, and we hope that these new results encourage uptake as the success of the vaccination programme relies not only on the efficacy of the vaccine but also the proportion of the population vaccinated,” she added.
Vanessa Saliba, MD, a consultant epidemiologist for the UK Health Security Agency, agreed, adding that “these remarkable findings confirm that the HPV vaccine saves lives by dramatically reducing cervical cancer rates among women.”
“This reminds us that vaccines are one of the most important tools we have to help us live longer, healthier lives,” she reemphasized.
British HPV program
When initiated in 2008, the national HPV vaccination program used the bivalent, Cervarix vaccine against HPV 16 and 18. As researchers noted, these two HPV types are responsible for 70%-80% of all cervical cancers in England.
However, in 2012, the program switched to the quadrivalent HPV vaccine (Gardasil) which is also effective against two additional HPV types, 6 and 11, both of which cause genital warts. The program also originally recommended the three-dose regimen for both HPV vaccines.
Now, only two doses of the vaccine are given to girls under the age of 15 even though it has been shown that a single dose of the HPV vaccine provides good protection against persistent infection, with efficacy rates that are similar to that of three doses, as the authors point out.
Among the cohort eligible for vaccination at 12 or 13 years of age, 89% received at least one dose of the HPV vaccine while 85% of the same age group received all three shots.
Cancer registry
Data from a population-based cancer registry was used to estimate the early effect of the bivalent HPV program on the incidence of cervical cancer and CIN3 in England between January 2006 and June 2019. During the study interval, there were 27,946 diagnoses of cervical cancer and 318,058 diagnoses of CIN3, lead author Milena Falcaro, MD, King’s College London, and colleagues report. Participants were then analyzed separately according to their age at the time of vaccination and the incidence rates calculated for both cervical cancer and CIN3 in the three separate groups.
For slightly older girls who received the vaccine between 14 and 16 years of age (school year 10-11), cervical cancer was reduced by 62% while CIN3 rates were reduced by 75%. For those who received the vaccine between 16 and 18 years of age (school year 12-13), cervical cancer rates were reduced by 34% while CIN3 rates were reduced by 39%, study authors add.
Indeed, the authors estimate that by June 2019 there were approximately 450 fewer cases of cervical cancer and 17,200 fewer cases of CIN3 than would otherwise have been expected in the vaccinated population in England.
The authors acknowledge that cervical cancer is rare in young women and vaccinated populations are still young. For example, the youngest recipients would have been immunized at the age of 12 in 2008 and would still be only 23 years old in 2019 when the study ended.
Thus, the authors emphasize that, because the vaccinated populations are still young, it’s too early to assess the full effect of HPV vaccination on cervical cancer rates.
Asked to comment on the study, Maurice Markman, MD, president, Medicine and Science Cancer Treatment Centers of America, pointed out that results from the British study are very similar to those from a Swedish study assessing the effect of the quadrivalent vaccine alone.
“You can put any superlatives you want in here, but these are stunningly positive results,” Dr. Markman said in an interview. As an oncologist who has been treating cervical cancer for 40 years – particularly advanced cervical cancer – “I can tell you this is one of the most devastating diseases to women, and the ability to eliminate this cancer with something as simple as a vaccine is the goal of cancer therapy, and it’s been remarkably successful,” he stressed.
Editorial commentary
Commenting on the findings, editorialists Maggie Cruickshank, MD, University of Aberdeen (Scotland), and Mihaela Grigore, MD, University of Medicine and Pharmacy, Lasi, Romania, point out that published reports evaluating the effect of HPV vaccination on cervical cancer rates have been scarce until now.
“The most important issue, besides the availability of the vaccine ... is the education of the population to accept vaccination because a high rate of immunization is a key element of success,” they emphasize. “Even in a wealthy country such as England with free access to HPV immunization, uptake has not reached the 90% vaccination target of girls aged 15 years set by the WHO [World Health Organization],” the editorialists add.
Dr. Cruickshank and Dr. Grigore also suggest that the effect HPV vaccination is having on cervical cancer rates as shown in this study should also stimulate vaccination programs in low- and middle-income countries where cervical cancer is a far greater public health issue than it is in countries with established systems of vaccination and screening.
HPV vaccination in the United States
The HPV vaccination program is similarly reducing the incidence of and mortality from cervical cancer among younger women in the United States who are most likely to have received the vaccine. As reported by lead author, Justin Barnes, MD, Washington University, St. Louis, the incidence of cervical cancer dropped by 37.7% from 2001 through 2005 to 2010 through 2017 in girls and young women between 15 and 24 years of age.
The U.S. study was published online in JAMA Pediatrics.
“HPV vaccine coverage in the U.S. has improved over the last few years although it was quite poor for many years,” senior author of the U.K. study, Peter Sasieni, MD, King’s College London, said in an interview. “Thus, one would anticipate a lower impact on the population in the U.S., because vaccine uptake, particularly in those aged 11-14 years was so much lower than it was in the U.K.,” he noted.
SEER databases
National age-adjusted cervical cancer incidence and mortality data from January 2001 through December 2017 for women and girls between 15 and 39 years of age were obtained from the combined Surveillance, Epidemiology, and End Results as well as the National Program of Cancer Registries databases. Mortality data was obtained from the National Center for Health Statistics.
Investigators then compared percentage changes in the incidence of and mortality from cervical cancer from January 2001 through December 2005 during the prevaccination years to that observed between January 2010 through December 2017 during the postvaccination years. They also compared incidence and mortality rates in three different cohorts: females between 15 and 24 years of age, those between 25 and 29 years of age, and those between 30 and 39 years of age.
“The older two groups were included as comparison, given their low vaccination rates,” the authors explained. Results showed that, during the same study interval from 2001 through 2005 to 2010 through 2017, the incidence of cervical cancer dropped by only 16.1% in women between 25 and 29 years of age and by only 8% for women between 30 and 39 years of age, the investigators report.
Reductions in mortality from cervical cancer were only strikingly so in the youngest age group of females between 15 and 24 years of age, among whom there was a 43.3% reduction in mortality from 2001-2005 to 2010-2017, as Dr. Barnes and colleagues note.
This pattern changed substantially in women between the ages of 25 and 29, among whom there was a 4.3% increase in mortality from cervical cancer during the same study interval and a small, 4.7% reduction among women between 30 and 39 years of age, investigators add. In actual numbers, mortality rates from cervical cancer were very low at only 0.6 per 100,000 in females between 15 and 24 years of age.
This compared to a mortality rate of 0.57 per 100,000 in women between 25 and 29 years of age and 1.89 per 100,000 in the oldest age group. “These nationwide data showed decreased cervical cancer incidence and mortality among women and girls aged 15-24 years after HPV vaccine introduction,” Dr. Barnes notes.
“Thus, the current study adds to knowledge by quantitatively comparing changes in cervical cancer incidence by age-based vaccine eligibility and providing suggestive evidence for vaccine-associated decreases in cervical cancer mortality,” investigators add.
However, as the authors also point out, while the reduction in mortality from cervical cancer associated with HPV vaccination may translate to older age groups as HPV-vaccinated cohorts age, “the number of deaths and hence the number of potentially averted deaths in young women and girls was small,” they caution, “and efforts to further improve vaccination uptake remain important.”
None of the authors or the editorialists had any conflicts of interest to declare.
Two different studies have found that, provided young females are immunized with the human papilloma virus (HPV) vaccine at a young enough age, both the incidence of and mortality from cervical cancer can be dramatically curtailed, data from the United Kingdom and to a lesser extent, the United States indicate.
In the U.K. study, published online in The Lancet, researchers showed that the national vaccination program against HPV, initiated in England in 2008, has all but eradicated cervical cancer and cervical intraepithelial neoplasia (CIN3) in young girls who received the vaccine at the age of 12 and 13 years (school year 8) prior to their sexual debut.
In this age group, cervical cancer rates were 87% lower than rates among previously nonvaccinated generations, while CIN3 rates were reduced by 97%, as researchers report. “It’s been incredible to see the impact of HPV vaccination, and now we can prove it prevented hundreds of women from developing cancer in England,” senior author Peter Sasieni, MD, King’s College London, said in a statement. “To see the real-life impact of the vaccine has been truly rewarding,” he added.
“This study provides the first direct evidence of the impact of the UK HPV vaccination campaign on cervical cancer incidence, showing a large reduction in cervical cancer rates in vaccinated cohorts,” Kate Soldan, MD, UK Health Security Agency, London, said in the same statement.
“This represents an important step forward in cervical cancer prevention, and we hope that these new results encourage uptake as the success of the vaccination programme relies not only on the efficacy of the vaccine but also the proportion of the population vaccinated,” she added.
Vanessa Saliba, MD, a consultant epidemiologist for the UK Health Security Agency, agreed, adding that “these remarkable findings confirm that the HPV vaccine saves lives by dramatically reducing cervical cancer rates among women.”
“This reminds us that vaccines are one of the most important tools we have to help us live longer, healthier lives,” she reemphasized.
British HPV program
When initiated in 2008, the national HPV vaccination program used the bivalent, Cervarix vaccine against HPV 16 and 18. As researchers noted, these two HPV types are responsible for 70%-80% of all cervical cancers in England.
However, in 2012, the program switched to the quadrivalent HPV vaccine (Gardasil) which is also effective against two additional HPV types, 6 and 11, both of which cause genital warts. The program also originally recommended the three-dose regimen for both HPV vaccines.
Now, only two doses of the vaccine are given to girls under the age of 15 even though it has been shown that a single dose of the HPV vaccine provides good protection against persistent infection, with efficacy rates that are similar to that of three doses, as the authors point out.
Among the cohort eligible for vaccination at 12 or 13 years of age, 89% received at least one dose of the HPV vaccine while 85% of the same age group received all three shots.
Cancer registry
Data from a population-based cancer registry was used to estimate the early effect of the bivalent HPV program on the incidence of cervical cancer and CIN3 in England between January 2006 and June 2019. During the study interval, there were 27,946 diagnoses of cervical cancer and 318,058 diagnoses of CIN3, lead author Milena Falcaro, MD, King’s College London, and colleagues report. Participants were then analyzed separately according to their age at the time of vaccination and the incidence rates calculated for both cervical cancer and CIN3 in the three separate groups.
For slightly older girls who received the vaccine between 14 and 16 years of age (school year 10-11), cervical cancer was reduced by 62% while CIN3 rates were reduced by 75%. For those who received the vaccine between 16 and 18 years of age (school year 12-13), cervical cancer rates were reduced by 34% while CIN3 rates were reduced by 39%, study authors add.
Indeed, the authors estimate that by June 2019 there were approximately 450 fewer cases of cervical cancer and 17,200 fewer cases of CIN3 than would otherwise have been expected in the vaccinated population in England.
The authors acknowledge that cervical cancer is rare in young women and vaccinated populations are still young. For example, the youngest recipients would have been immunized at the age of 12 in 2008 and would still be only 23 years old in 2019 when the study ended.
Thus, the authors emphasize that, because the vaccinated populations are still young, it’s too early to assess the full effect of HPV vaccination on cervical cancer rates.
Asked to comment on the study, Maurice Markman, MD, president, Medicine and Science Cancer Treatment Centers of America, pointed out that results from the British study are very similar to those from a Swedish study assessing the effect of the quadrivalent vaccine alone.
“You can put any superlatives you want in here, but these are stunningly positive results,” Dr. Markman said in an interview. As an oncologist who has been treating cervical cancer for 40 years – particularly advanced cervical cancer – “I can tell you this is one of the most devastating diseases to women, and the ability to eliminate this cancer with something as simple as a vaccine is the goal of cancer therapy, and it’s been remarkably successful,” he stressed.
Editorial commentary
Commenting on the findings, editorialists Maggie Cruickshank, MD, University of Aberdeen (Scotland), and Mihaela Grigore, MD, University of Medicine and Pharmacy, Lasi, Romania, point out that published reports evaluating the effect of HPV vaccination on cervical cancer rates have been scarce until now.
“The most important issue, besides the availability of the vaccine ... is the education of the population to accept vaccination because a high rate of immunization is a key element of success,” they emphasize. “Even in a wealthy country such as England with free access to HPV immunization, uptake has not reached the 90% vaccination target of girls aged 15 years set by the WHO [World Health Organization],” the editorialists add.
Dr. Cruickshank and Dr. Grigore also suggest that the effect HPV vaccination is having on cervical cancer rates as shown in this study should also stimulate vaccination programs in low- and middle-income countries where cervical cancer is a far greater public health issue than it is in countries with established systems of vaccination and screening.
HPV vaccination in the United States
The HPV vaccination program is similarly reducing the incidence of and mortality from cervical cancer among younger women in the United States who are most likely to have received the vaccine. As reported by lead author, Justin Barnes, MD, Washington University, St. Louis, the incidence of cervical cancer dropped by 37.7% from 2001 through 2005 to 2010 through 2017 in girls and young women between 15 and 24 years of age.
The U.S. study was published online in JAMA Pediatrics.
“HPV vaccine coverage in the U.S. has improved over the last few years although it was quite poor for many years,” senior author of the U.K. study, Peter Sasieni, MD, King’s College London, said in an interview. “Thus, one would anticipate a lower impact on the population in the U.S., because vaccine uptake, particularly in those aged 11-14 years was so much lower than it was in the U.K.,” he noted.
SEER databases
National age-adjusted cervical cancer incidence and mortality data from January 2001 through December 2017 for women and girls between 15 and 39 years of age were obtained from the combined Surveillance, Epidemiology, and End Results as well as the National Program of Cancer Registries databases. Mortality data was obtained from the National Center for Health Statistics.
Investigators then compared percentage changes in the incidence of and mortality from cervical cancer from January 2001 through December 2005 during the prevaccination years to that observed between January 2010 through December 2017 during the postvaccination years. They also compared incidence and mortality rates in three different cohorts: females between 15 and 24 years of age, those between 25 and 29 years of age, and those between 30 and 39 years of age.
“The older two groups were included as comparison, given their low vaccination rates,” the authors explained. Results showed that, during the same study interval from 2001 through 2005 to 2010 through 2017, the incidence of cervical cancer dropped by only 16.1% in women between 25 and 29 years of age and by only 8% for women between 30 and 39 years of age, the investigators report.
Reductions in mortality from cervical cancer were only strikingly so in the youngest age group of females between 15 and 24 years of age, among whom there was a 43.3% reduction in mortality from 2001-2005 to 2010-2017, as Dr. Barnes and colleagues note.
This pattern changed substantially in women between the ages of 25 and 29, among whom there was a 4.3% increase in mortality from cervical cancer during the same study interval and a small, 4.7% reduction among women between 30 and 39 years of age, investigators add. In actual numbers, mortality rates from cervical cancer were very low at only 0.6 per 100,000 in females between 15 and 24 years of age.
This compared to a mortality rate of 0.57 per 100,000 in women between 25 and 29 years of age and 1.89 per 100,000 in the oldest age group. “These nationwide data showed decreased cervical cancer incidence and mortality among women and girls aged 15-24 years after HPV vaccine introduction,” Dr. Barnes notes.
“Thus, the current study adds to knowledge by quantitatively comparing changes in cervical cancer incidence by age-based vaccine eligibility and providing suggestive evidence for vaccine-associated decreases in cervical cancer mortality,” investigators add.
However, as the authors also point out, while the reduction in mortality from cervical cancer associated with HPV vaccination may translate to older age groups as HPV-vaccinated cohorts age, “the number of deaths and hence the number of potentially averted deaths in young women and girls was small,” they caution, “and efforts to further improve vaccination uptake remain important.”
None of the authors or the editorialists had any conflicts of interest to declare.
Two different studies have found that, provided young females are immunized with the human papilloma virus (HPV) vaccine at a young enough age, both the incidence of and mortality from cervical cancer can be dramatically curtailed, data from the United Kingdom and to a lesser extent, the United States indicate.
In the U.K. study, published online in The Lancet, researchers showed that the national vaccination program against HPV, initiated in England in 2008, has all but eradicated cervical cancer and cervical intraepithelial neoplasia (CIN3) in young girls who received the vaccine at the age of 12 and 13 years (school year 8) prior to their sexual debut.
In this age group, cervical cancer rates were 87% lower than rates among previously nonvaccinated generations, while CIN3 rates were reduced by 97%, as researchers report. “It’s been incredible to see the impact of HPV vaccination, and now we can prove it prevented hundreds of women from developing cancer in England,” senior author Peter Sasieni, MD, King’s College London, said in a statement. “To see the real-life impact of the vaccine has been truly rewarding,” he added.
“This study provides the first direct evidence of the impact of the UK HPV vaccination campaign on cervical cancer incidence, showing a large reduction in cervical cancer rates in vaccinated cohorts,” Kate Soldan, MD, UK Health Security Agency, London, said in the same statement.
“This represents an important step forward in cervical cancer prevention, and we hope that these new results encourage uptake as the success of the vaccination programme relies not only on the efficacy of the vaccine but also the proportion of the population vaccinated,” she added.
Vanessa Saliba, MD, a consultant epidemiologist for the UK Health Security Agency, agreed, adding that “these remarkable findings confirm that the HPV vaccine saves lives by dramatically reducing cervical cancer rates among women.”
“This reminds us that vaccines are one of the most important tools we have to help us live longer, healthier lives,” she reemphasized.
British HPV program
When initiated in 2008, the national HPV vaccination program used the bivalent, Cervarix vaccine against HPV 16 and 18. As researchers noted, these two HPV types are responsible for 70%-80% of all cervical cancers in England.
However, in 2012, the program switched to the quadrivalent HPV vaccine (Gardasil) which is also effective against two additional HPV types, 6 and 11, both of which cause genital warts. The program also originally recommended the three-dose regimen for both HPV vaccines.
Now, only two doses of the vaccine are given to girls under the age of 15 even though it has been shown that a single dose of the HPV vaccine provides good protection against persistent infection, with efficacy rates that are similar to that of three doses, as the authors point out.
Among the cohort eligible for vaccination at 12 or 13 years of age, 89% received at least one dose of the HPV vaccine while 85% of the same age group received all three shots.
Cancer registry
Data from a population-based cancer registry was used to estimate the early effect of the bivalent HPV program on the incidence of cervical cancer and CIN3 in England between January 2006 and June 2019. During the study interval, there were 27,946 diagnoses of cervical cancer and 318,058 diagnoses of CIN3, lead author Milena Falcaro, MD, King’s College London, and colleagues report. Participants were then analyzed separately according to their age at the time of vaccination and the incidence rates calculated for both cervical cancer and CIN3 in the three separate groups.
For slightly older girls who received the vaccine between 14 and 16 years of age (school year 10-11), cervical cancer was reduced by 62% while CIN3 rates were reduced by 75%. For those who received the vaccine between 16 and 18 years of age (school year 12-13), cervical cancer rates were reduced by 34% while CIN3 rates were reduced by 39%, study authors add.
Indeed, the authors estimate that by June 2019 there were approximately 450 fewer cases of cervical cancer and 17,200 fewer cases of CIN3 than would otherwise have been expected in the vaccinated population in England.
The authors acknowledge that cervical cancer is rare in young women and vaccinated populations are still young. For example, the youngest recipients would have been immunized at the age of 12 in 2008 and would still be only 23 years old in 2019 when the study ended.
Thus, the authors emphasize that, because the vaccinated populations are still young, it’s too early to assess the full effect of HPV vaccination on cervical cancer rates.
Asked to comment on the study, Maurice Markman, MD, president, Medicine and Science Cancer Treatment Centers of America, pointed out that results from the British study are very similar to those from a Swedish study assessing the effect of the quadrivalent vaccine alone.
“You can put any superlatives you want in here, but these are stunningly positive results,” Dr. Markman said in an interview. As an oncologist who has been treating cervical cancer for 40 years – particularly advanced cervical cancer – “I can tell you this is one of the most devastating diseases to women, and the ability to eliminate this cancer with something as simple as a vaccine is the goal of cancer therapy, and it’s been remarkably successful,” he stressed.
Editorial commentary
Commenting on the findings, editorialists Maggie Cruickshank, MD, University of Aberdeen (Scotland), and Mihaela Grigore, MD, University of Medicine and Pharmacy, Lasi, Romania, point out that published reports evaluating the effect of HPV vaccination on cervical cancer rates have been scarce until now.
“The most important issue, besides the availability of the vaccine ... is the education of the population to accept vaccination because a high rate of immunization is a key element of success,” they emphasize. “Even in a wealthy country such as England with free access to HPV immunization, uptake has not reached the 90% vaccination target of girls aged 15 years set by the WHO [World Health Organization],” the editorialists add.
Dr. Cruickshank and Dr. Grigore also suggest that the effect HPV vaccination is having on cervical cancer rates as shown in this study should also stimulate vaccination programs in low- and middle-income countries where cervical cancer is a far greater public health issue than it is in countries with established systems of vaccination and screening.
HPV vaccination in the United States
The HPV vaccination program is similarly reducing the incidence of and mortality from cervical cancer among younger women in the United States who are most likely to have received the vaccine. As reported by lead author, Justin Barnes, MD, Washington University, St. Louis, the incidence of cervical cancer dropped by 37.7% from 2001 through 2005 to 2010 through 2017 in girls and young women between 15 and 24 years of age.
The U.S. study was published online in JAMA Pediatrics.
“HPV vaccine coverage in the U.S. has improved over the last few years although it was quite poor for many years,” senior author of the U.K. study, Peter Sasieni, MD, King’s College London, said in an interview. “Thus, one would anticipate a lower impact on the population in the U.S., because vaccine uptake, particularly in those aged 11-14 years was so much lower than it was in the U.K.,” he noted.
SEER databases
National age-adjusted cervical cancer incidence and mortality data from January 2001 through December 2017 for women and girls between 15 and 39 years of age were obtained from the combined Surveillance, Epidemiology, and End Results as well as the National Program of Cancer Registries databases. Mortality data was obtained from the National Center for Health Statistics.
Investigators then compared percentage changes in the incidence of and mortality from cervical cancer from January 2001 through December 2005 during the prevaccination years to that observed between January 2010 through December 2017 during the postvaccination years. They also compared incidence and mortality rates in three different cohorts: females between 15 and 24 years of age, those between 25 and 29 years of age, and those between 30 and 39 years of age.
“The older two groups were included as comparison, given their low vaccination rates,” the authors explained. Results showed that, during the same study interval from 2001 through 2005 to 2010 through 2017, the incidence of cervical cancer dropped by only 16.1% in women between 25 and 29 years of age and by only 8% for women between 30 and 39 years of age, the investigators report.
Reductions in mortality from cervical cancer were only strikingly so in the youngest age group of females between 15 and 24 years of age, among whom there was a 43.3% reduction in mortality from 2001-2005 to 2010-2017, as Dr. Barnes and colleagues note.
This pattern changed substantially in women between the ages of 25 and 29, among whom there was a 4.3% increase in mortality from cervical cancer during the same study interval and a small, 4.7% reduction among women between 30 and 39 years of age, investigators add. In actual numbers, mortality rates from cervical cancer were very low at only 0.6 per 100,000 in females between 15 and 24 years of age.
This compared to a mortality rate of 0.57 per 100,000 in women between 25 and 29 years of age and 1.89 per 100,000 in the oldest age group. “These nationwide data showed decreased cervical cancer incidence and mortality among women and girls aged 15-24 years after HPV vaccine introduction,” Dr. Barnes notes.
“Thus, the current study adds to knowledge by quantitatively comparing changes in cervical cancer incidence by age-based vaccine eligibility and providing suggestive evidence for vaccine-associated decreases in cervical cancer mortality,” investigators add.
However, as the authors also point out, while the reduction in mortality from cervical cancer associated with HPV vaccination may translate to older age groups as HPV-vaccinated cohorts age, “the number of deaths and hence the number of potentially averted deaths in young women and girls was small,” they caution, “and efforts to further improve vaccination uptake remain important.”
None of the authors or the editorialists had any conflicts of interest to declare.
For older adults, smelling the roses may be more difficult
Young and old alike are affected – more than 80%-90% of those diagnosed with the virus, according to some estimates. While most people recover in a few months, 16% take half a year or longer to do so, research has found. According to new estimates, up to 1.6 million Americans have chronic olfactory dysfunction due to COVID-19.
Seniors are especially vulnerable, experts suggest. “We know that many older adults have a compromised sense of smell to begin with. Add to that the insult of COVID, and it made these problems worse,” said Dr. Jayant Pinto, professor of surgery and a specialist in sinus and nasal diseases at the University of Chicago Medical Center.
Recent data highlight the interaction between COVID-19, advanced age, and loss of smell. When Italian researchers evaluated 101 patients who’d been hospitalized for mild to moderate COVID-19, 50 showed objective signs of smell impairment 6 months later. Those 65 or older were nearly twice as likely to be impaired; those 75 or older were more than 2½ times as likely.
Most people aren’t aware of the extent to which smell can be diminished in later life. More than half of 65- to 80-year-olds have some degree of smell loss, or olfactory dysfunction, as it’s known in the scientific literature. That rises to as high as 80% for those even older. People affected often report concerns about safety, less enjoyment eating, and an impaired quality of life.
But because the ability to detect, identify, and discriminate among odors declines gradually, most older adults – up to 75% of those with some degree of olfactory dysfunction – don’t realize they’re affected.
A host of factors are believed to contribute to age-related smell loss, including a reduction in the number of olfactory sensory neurons in the nose, which are essential for detecting odors; changes in stem cells that replenish these neurons every few months; atrophy of the processing center for smell in the brain, called the olfactory bulb; and the shrinkage of brain centers closely connected with the olfactory bulb, such as the hippocampus, a region central to learning and memory.
Also, environmental toxic substances such as air pollution play a part, research shows. “Olfactory neurons in your nose are basically little pieces of your brain hanging out in the outside world,” and exposure to them over time damages those neurons and the tissues that support them, explained Pamela Dalton, PhD, a principal investigator at the Monell Chemical Senses Center, a smell and taste research institute in Philadelphia.
Still, the complex workings of the olfactory system have not been mapped in detail yet, and much remains unknown, said Dr. Sandeep Robert Datta, professor of neurobiology at Harvard Medical School, Boston.
“We tend to think of our sense of smell as primarily aesthetic,” he said. “What’s very clear is that it’s far more important. The olfactory system plays a key role in maintaining our emotional well-being and connecting us with the world.”
Dr. Datta experienced this after having a bone marrow transplant followed by chemotherapy years ago. Unable to smell or taste food, he said, he felt “very disoriented” in his environment.
Common consequences of smell loss include a loss of appetite (without smell, taste is deeply compromised), difficulty monitoring personal hygiene, depression, and an inability to detect noxious fumes. In older adults, this can lead to weight loss, malnutrition, frailty, inadequate personal care, and accidents caused by gas leaks or fires.
Jerome Pisano, 75, of Bloomington, Ill., has been living with smell loss for 5 years. Repeated tests and consultations with physicians haven’t pinpointed a reason for this ailment, and sometimes he feels “hopeless,” he admitted.
Before he became smell-impaired, Mr. Pisano was certified as a wine specialist. He has an 800-bottle wine cellar. “I can’t appreciate that as much as I’d like. I miss the smell of cut grass. Flowers. My wife’s cooking,” he said. “It certainly does decrease my quality of life.”
Smell loss is also associated in various research studies with a higher risk of death for older adults. One study, authored by Dr. Pinto and colleagues, found that older adults with olfactory dysfunction were nearly three times as likely to die over a period of 5 years as were seniors whose sense of smell remained intact.
“Our sense of smell signals how our nervous system is doing and how well our brain is doing overall,” Dr. Pinto said. According to a review published earlier this year, 90% of people with early-stage Parkinson’s disease and more than 80% of people with Alzheimer’s disease have olfactory dysfunction – a symptom that can precede other symptoms by many years.
There is no treatment for smell loss associated with neurological illness or head trauma, but if someone has persistent sinus problems or allergies that cause congestion, an over-the-counter antihistamine or nasal steroid spray can help. Usually, smell returns in a few weeks.
For smell loss following a viral infection, the picture is less clear. It’s not known, yet, which viruses are associated with olfactory dysfunction, why they damage smell, and what trajectory recovery takes. COVID-19 may help shine a light on this since it has inspired a wave of research on olfaction loss around the world.
“What characteristics make people more vulnerable to a persistent loss of smell after a virus? We don’t know that, but I think we will because that research is underway and we’ve never had a cohort [of people with smell loss] this large to study,” said Dr. Dalton, of the Monell center.
Some experts recommend smell training, noting evidence of efficacy and no indication of harm. This involves sniffing four distinct scents (often eucalyptus, lemon, rose, and cloves) twice a day for 30 seconds each, usually for 4 weeks. Sometimes the practice is combined with pictures of the items being smelled, a form of visual reinforcement.
The theory is that “practice, practice, practice” will stimulate the olfactory system, said Charles Greer, PhD, professor of neurosurgery and neuroscience at Yale University, New Haven, Conn. Although scientific support isn’t well established, he said, he often recommends that people who think their smell is declining “get a shelf full of spices and smell them on a regular basis.”
Richard Doty, PhD, director of the University of Pennsylvania’s Smell and Taste Center, remains skeptical. He’s writing a review of smell training and notes that 20%-30% of people with viral infections and smell loss recover in a relatively short time, whether or not they pursue this therapy.
“The main thing we recommend is avoid polluted environments and get your full complement of vitamins,” since several vitamins play an important role in maintaining the olfactory system, he said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Young and old alike are affected – more than 80%-90% of those diagnosed with the virus, according to some estimates. While most people recover in a few months, 16% take half a year or longer to do so, research has found. According to new estimates, up to 1.6 million Americans have chronic olfactory dysfunction due to COVID-19.
Seniors are especially vulnerable, experts suggest. “We know that many older adults have a compromised sense of smell to begin with. Add to that the insult of COVID, and it made these problems worse,” said Dr. Jayant Pinto, professor of surgery and a specialist in sinus and nasal diseases at the University of Chicago Medical Center.
Recent data highlight the interaction between COVID-19, advanced age, and loss of smell. When Italian researchers evaluated 101 patients who’d been hospitalized for mild to moderate COVID-19, 50 showed objective signs of smell impairment 6 months later. Those 65 or older were nearly twice as likely to be impaired; those 75 or older were more than 2½ times as likely.
Most people aren’t aware of the extent to which smell can be diminished in later life. More than half of 65- to 80-year-olds have some degree of smell loss, or olfactory dysfunction, as it’s known in the scientific literature. That rises to as high as 80% for those even older. People affected often report concerns about safety, less enjoyment eating, and an impaired quality of life.
But because the ability to detect, identify, and discriminate among odors declines gradually, most older adults – up to 75% of those with some degree of olfactory dysfunction – don’t realize they’re affected.
A host of factors are believed to contribute to age-related smell loss, including a reduction in the number of olfactory sensory neurons in the nose, which are essential for detecting odors; changes in stem cells that replenish these neurons every few months; atrophy of the processing center for smell in the brain, called the olfactory bulb; and the shrinkage of brain centers closely connected with the olfactory bulb, such as the hippocampus, a region central to learning and memory.
Also, environmental toxic substances such as air pollution play a part, research shows. “Olfactory neurons in your nose are basically little pieces of your brain hanging out in the outside world,” and exposure to them over time damages those neurons and the tissues that support them, explained Pamela Dalton, PhD, a principal investigator at the Monell Chemical Senses Center, a smell and taste research institute in Philadelphia.
Still, the complex workings of the olfactory system have not been mapped in detail yet, and much remains unknown, said Dr. Sandeep Robert Datta, professor of neurobiology at Harvard Medical School, Boston.
“We tend to think of our sense of smell as primarily aesthetic,” he said. “What’s very clear is that it’s far more important. The olfactory system plays a key role in maintaining our emotional well-being and connecting us with the world.”
Dr. Datta experienced this after having a bone marrow transplant followed by chemotherapy years ago. Unable to smell or taste food, he said, he felt “very disoriented” in his environment.
Common consequences of smell loss include a loss of appetite (without smell, taste is deeply compromised), difficulty monitoring personal hygiene, depression, and an inability to detect noxious fumes. In older adults, this can lead to weight loss, malnutrition, frailty, inadequate personal care, and accidents caused by gas leaks or fires.
Jerome Pisano, 75, of Bloomington, Ill., has been living with smell loss for 5 years. Repeated tests and consultations with physicians haven’t pinpointed a reason for this ailment, and sometimes he feels “hopeless,” he admitted.
Before he became smell-impaired, Mr. Pisano was certified as a wine specialist. He has an 800-bottle wine cellar. “I can’t appreciate that as much as I’d like. I miss the smell of cut grass. Flowers. My wife’s cooking,” he said. “It certainly does decrease my quality of life.”
Smell loss is also associated in various research studies with a higher risk of death for older adults. One study, authored by Dr. Pinto and colleagues, found that older adults with olfactory dysfunction were nearly three times as likely to die over a period of 5 years as were seniors whose sense of smell remained intact.
“Our sense of smell signals how our nervous system is doing and how well our brain is doing overall,” Dr. Pinto said. According to a review published earlier this year, 90% of people with early-stage Parkinson’s disease and more than 80% of people with Alzheimer’s disease have olfactory dysfunction – a symptom that can precede other symptoms by many years.
There is no treatment for smell loss associated with neurological illness or head trauma, but if someone has persistent sinus problems or allergies that cause congestion, an over-the-counter antihistamine or nasal steroid spray can help. Usually, smell returns in a few weeks.
For smell loss following a viral infection, the picture is less clear. It’s not known, yet, which viruses are associated with olfactory dysfunction, why they damage smell, and what trajectory recovery takes. COVID-19 may help shine a light on this since it has inspired a wave of research on olfaction loss around the world.
“What characteristics make people more vulnerable to a persistent loss of smell after a virus? We don’t know that, but I think we will because that research is underway and we’ve never had a cohort [of people with smell loss] this large to study,” said Dr. Dalton, of the Monell center.
Some experts recommend smell training, noting evidence of efficacy and no indication of harm. This involves sniffing four distinct scents (often eucalyptus, lemon, rose, and cloves) twice a day for 30 seconds each, usually for 4 weeks. Sometimes the practice is combined with pictures of the items being smelled, a form of visual reinforcement.
The theory is that “practice, practice, practice” will stimulate the olfactory system, said Charles Greer, PhD, professor of neurosurgery and neuroscience at Yale University, New Haven, Conn. Although scientific support isn’t well established, he said, he often recommends that people who think their smell is declining “get a shelf full of spices and smell them on a regular basis.”
Richard Doty, PhD, director of the University of Pennsylvania’s Smell and Taste Center, remains skeptical. He’s writing a review of smell training and notes that 20%-30% of people with viral infections and smell loss recover in a relatively short time, whether or not they pursue this therapy.
“The main thing we recommend is avoid polluted environments and get your full complement of vitamins,” since several vitamins play an important role in maintaining the olfactory system, he said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Young and old alike are affected – more than 80%-90% of those diagnosed with the virus, according to some estimates. While most people recover in a few months, 16% take half a year or longer to do so, research has found. According to new estimates, up to 1.6 million Americans have chronic olfactory dysfunction due to COVID-19.
Seniors are especially vulnerable, experts suggest. “We know that many older adults have a compromised sense of smell to begin with. Add to that the insult of COVID, and it made these problems worse,” said Dr. Jayant Pinto, professor of surgery and a specialist in sinus and nasal diseases at the University of Chicago Medical Center.
Recent data highlight the interaction between COVID-19, advanced age, and loss of smell. When Italian researchers evaluated 101 patients who’d been hospitalized for mild to moderate COVID-19, 50 showed objective signs of smell impairment 6 months later. Those 65 or older were nearly twice as likely to be impaired; those 75 or older were more than 2½ times as likely.
Most people aren’t aware of the extent to which smell can be diminished in later life. More than half of 65- to 80-year-olds have some degree of smell loss, or olfactory dysfunction, as it’s known in the scientific literature. That rises to as high as 80% for those even older. People affected often report concerns about safety, less enjoyment eating, and an impaired quality of life.
But because the ability to detect, identify, and discriminate among odors declines gradually, most older adults – up to 75% of those with some degree of olfactory dysfunction – don’t realize they’re affected.
A host of factors are believed to contribute to age-related smell loss, including a reduction in the number of olfactory sensory neurons in the nose, which are essential for detecting odors; changes in stem cells that replenish these neurons every few months; atrophy of the processing center for smell in the brain, called the olfactory bulb; and the shrinkage of brain centers closely connected with the olfactory bulb, such as the hippocampus, a region central to learning and memory.
Also, environmental toxic substances such as air pollution play a part, research shows. “Olfactory neurons in your nose are basically little pieces of your brain hanging out in the outside world,” and exposure to them over time damages those neurons and the tissues that support them, explained Pamela Dalton, PhD, a principal investigator at the Monell Chemical Senses Center, a smell and taste research institute in Philadelphia.
Still, the complex workings of the olfactory system have not been mapped in detail yet, and much remains unknown, said Dr. Sandeep Robert Datta, professor of neurobiology at Harvard Medical School, Boston.
“We tend to think of our sense of smell as primarily aesthetic,” he said. “What’s very clear is that it’s far more important. The olfactory system plays a key role in maintaining our emotional well-being and connecting us with the world.”
Dr. Datta experienced this after having a bone marrow transplant followed by chemotherapy years ago. Unable to smell or taste food, he said, he felt “very disoriented” in his environment.
Common consequences of smell loss include a loss of appetite (without smell, taste is deeply compromised), difficulty monitoring personal hygiene, depression, and an inability to detect noxious fumes. In older adults, this can lead to weight loss, malnutrition, frailty, inadequate personal care, and accidents caused by gas leaks or fires.
Jerome Pisano, 75, of Bloomington, Ill., has been living with smell loss for 5 years. Repeated tests and consultations with physicians haven’t pinpointed a reason for this ailment, and sometimes he feels “hopeless,” he admitted.
Before he became smell-impaired, Mr. Pisano was certified as a wine specialist. He has an 800-bottle wine cellar. “I can’t appreciate that as much as I’d like. I miss the smell of cut grass. Flowers. My wife’s cooking,” he said. “It certainly does decrease my quality of life.”
Smell loss is also associated in various research studies with a higher risk of death for older adults. One study, authored by Dr. Pinto and colleagues, found that older adults with olfactory dysfunction were nearly three times as likely to die over a period of 5 years as were seniors whose sense of smell remained intact.
“Our sense of smell signals how our nervous system is doing and how well our brain is doing overall,” Dr. Pinto said. According to a review published earlier this year, 90% of people with early-stage Parkinson’s disease and more than 80% of people with Alzheimer’s disease have olfactory dysfunction – a symptom that can precede other symptoms by many years.
There is no treatment for smell loss associated with neurological illness or head trauma, but if someone has persistent sinus problems or allergies that cause congestion, an over-the-counter antihistamine or nasal steroid spray can help. Usually, smell returns in a few weeks.
For smell loss following a viral infection, the picture is less clear. It’s not known, yet, which viruses are associated with olfactory dysfunction, why they damage smell, and what trajectory recovery takes. COVID-19 may help shine a light on this since it has inspired a wave of research on olfaction loss around the world.
“What characteristics make people more vulnerable to a persistent loss of smell after a virus? We don’t know that, but I think we will because that research is underway and we’ve never had a cohort [of people with smell loss] this large to study,” said Dr. Dalton, of the Monell center.
Some experts recommend smell training, noting evidence of efficacy and no indication of harm. This involves sniffing four distinct scents (often eucalyptus, lemon, rose, and cloves) twice a day for 30 seconds each, usually for 4 weeks. Sometimes the practice is combined with pictures of the items being smelled, a form of visual reinforcement.
The theory is that “practice, practice, practice” will stimulate the olfactory system, said Charles Greer, PhD, professor of neurosurgery and neuroscience at Yale University, New Haven, Conn. Although scientific support isn’t well established, he said, he often recommends that people who think their smell is declining “get a shelf full of spices and smell them on a regular basis.”
Richard Doty, PhD, director of the University of Pennsylvania’s Smell and Taste Center, remains skeptical. He’s writing a review of smell training and notes that 20%-30% of people with viral infections and smell loss recover in a relatively short time, whether or not they pursue this therapy.
“The main thing we recommend is avoid polluted environments and get your full complement of vitamins,” since several vitamins play an important role in maintaining the olfactory system, he said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Children and COVID: New cases, vaccinations both decline
States reported 131,828 new pediatric cases for the week of Nov. 19-25, a decline of 7.1% over the previous week but still enough to surpass 100,000 for the 16th consecutive week. The weekly count had risen for 3 straight weeks since the last decrease in late October, the American Academy of Pediatrics and the Children’s Hospital Association said Nov. 30 in their weekly COVID report.
The AAP/CHA analysis, based on data from state and territorial health departments, puts the total number of cases in children at 6.9 million since the pandemic began, representing 17.0% of cases in Americans of all ages. The Centers for Disease Control and Prevention, which uses an age limit of 18 years to define a child, unlike some states, reports numbers of 6.1 million and 15.5%.
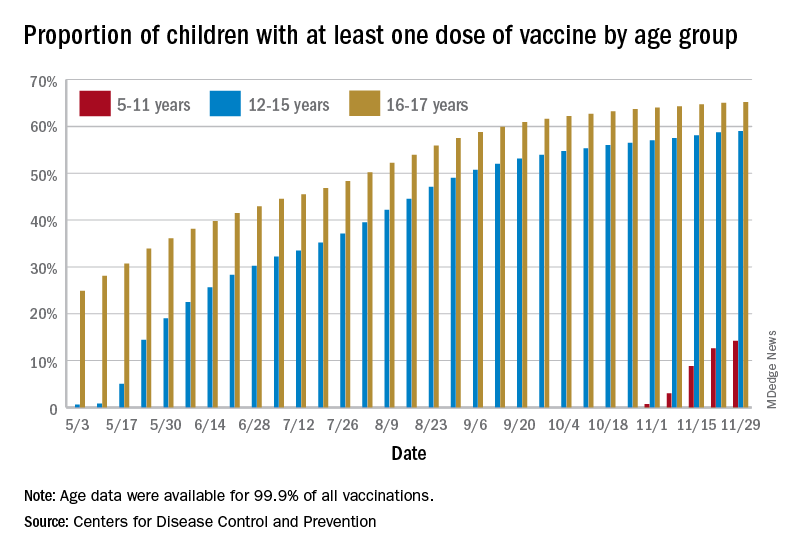
New vaccinations among the youngest eligible children, those aged 5-11 years, were down for the second week in a row after reaching almost 1.7 million during the first full week after approval on Nov. 2. Since then, the vaccination counts have been 1.2 million (Nov. 16-22) and 333,000 (Nov. 23-29), the CDC said on its COVID Data Tracker. A similar drop in the last week – from 127,000 to just 50,000 – also was seen for those aged 12-17 years.
Altogether, 14.2% of children aged 5-11, almost 4.1 million individuals, have received at least one dose of the vaccine, compared with 59.0% (10 million) of the 12- to 15-year-olds and 65.2% (5.5 million) of those aged 16-17. Just under 1% of the youngest group has been fully vaccinated, versus 49.0% and 55.8% for the older children, the CDC said.
It has been reported that Pfizer and BioNTech, which produce the only COVID vaccine approved for children, are planning to apply to the Food and Drug Administration during the first week of December for authorization for a booster dose for 16- and 17-year-olds.

States reported 131,828 new pediatric cases for the week of Nov. 19-25, a decline of 7.1% over the previous week but still enough to surpass 100,000 for the 16th consecutive week. The weekly count had risen for 3 straight weeks since the last decrease in late October, the American Academy of Pediatrics and the Children’s Hospital Association said Nov. 30 in their weekly COVID report.
The AAP/CHA analysis, based on data from state and territorial health departments, puts the total number of cases in children at 6.9 million since the pandemic began, representing 17.0% of cases in Americans of all ages. The Centers for Disease Control and Prevention, which uses an age limit of 18 years to define a child, unlike some states, reports numbers of 6.1 million and 15.5%.
New vaccinations among the youngest eligible children, those aged 5-11 years, were down for the second week in a row after reaching almost 1.7 million during the first full week after approval on Nov. 2. Since then, the vaccination counts have been 1.2 million (Nov. 16-22) and 333,000 (Nov. 23-29), the CDC said on its COVID Data Tracker. A similar drop in the last week – from 127,000 to just 50,000 – also was seen for those aged 12-17 years.
Altogether, 14.2% of children aged 5-11, almost 4.1 million individuals, have received at least one dose of the vaccine, compared with 59.0% (10 million) of the 12- to 15-year-olds and 65.2% (5.5 million) of those aged 16-17. Just under 1% of the youngest group has been fully vaccinated, versus 49.0% and 55.8% for the older children, the CDC said.
It has been reported that Pfizer and BioNTech, which produce the only COVID vaccine approved for children, are planning to apply to the Food and Drug Administration during the first week of December for authorization for a booster dose for 16- and 17-year-olds.

States reported 131,828 new pediatric cases for the week of Nov. 19-25, a decline of 7.1% over the previous week but still enough to surpass 100,000 for the 16th consecutive week. The weekly count had risen for 3 straight weeks since the last decrease in late October, the American Academy of Pediatrics and the Children’s Hospital Association said Nov. 30 in their weekly COVID report.
The AAP/CHA analysis, based on data from state and territorial health departments, puts the total number of cases in children at 6.9 million since the pandemic began, representing 17.0% of cases in Americans of all ages. The Centers for Disease Control and Prevention, which uses an age limit of 18 years to define a child, unlike some states, reports numbers of 6.1 million and 15.5%.
New vaccinations among the youngest eligible children, those aged 5-11 years, were down for the second week in a row after reaching almost 1.7 million during the first full week after approval on Nov. 2. Since then, the vaccination counts have been 1.2 million (Nov. 16-22) and 333,000 (Nov. 23-29), the CDC said on its COVID Data Tracker. A similar drop in the last week – from 127,000 to just 50,000 – also was seen for those aged 12-17 years.
Altogether, 14.2% of children aged 5-11, almost 4.1 million individuals, have received at least one dose of the vaccine, compared with 59.0% (10 million) of the 12- to 15-year-olds and 65.2% (5.5 million) of those aged 16-17. Just under 1% of the youngest group has been fully vaccinated, versus 49.0% and 55.8% for the older children, the CDC said.
It has been reported that Pfizer and BioNTech, which produce the only COVID vaccine approved for children, are planning to apply to the Food and Drug Administration during the first week of December for authorization for a booster dose for 16- and 17-year-olds.
FDA approves first drug for treatment of resistant cytomegalovirus infection
The Food and Drug Administration has approved the first treatment for posttransplant cytomegalovirus (CMV) that is resistant to other drugs.
There are an estimated 200,000 adult transplants every year globally. CMV, a type of herpes virus, is one of the most common infections in transplant patients, occurring in 16%-56% of solid organ transplant recipients and 30%-70% of hematopoietic stem cell transplant recipients, according to Takeda Pharmaceutical Company Limited, the company that manufactures Livtencity. For immunosuppressed transplant patients, CMV infection can lead to complications that include loss of the transplanted or organ or even death.
“Cytomegalovirus infections that are resistant or do not respond to available drugs are of even greater concern,” John Farley, MD, MPH, the director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research, said in a statement. “Today’s approval helps meet a significant unmet medical need by providing a treatment option for this patient population.”
Livtencity, which is taken orally, works by preventing the activity of the enzyme responsible for virus replication. The approval, announced Nov. 23, was based on a phase 3 clinical trial that compared Livtencity with conventional antiviral treatments in the achievement of CMV DNA concentration levels below what is measurable in transplant patients with CMV infection that is refractory or treatment-resistant. After 8 weeks, of the 235 patients who received Livtencity, 56% achieved this primary endpoint, compared with 24% of the 117 patients who received conventional antiviral treatments, the press release says.
The most reported adverse reactions of Livtencity were taste disturbance, nausea, diarrhea, vomiting, and fatigue.
“We are grateful for the contributions of the patients and clinicians who participated in our clinical trials, as well as the dedication of our scientists and researchers,” Ramona Sequeira, president of the Takeda’s U.S. Business Unit and Global Portfolio Commercialization, said in a statement. “People undergoing transplants have a lengthy and complex health care journey; with the approval of this treatment, we’re proud to offer these individuals a new oral antiviral to fight CMV infection and disease.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the first treatment for posttransplant cytomegalovirus (CMV) that is resistant to other drugs.
There are an estimated 200,000 adult transplants every year globally. CMV, a type of herpes virus, is one of the most common infections in transplant patients, occurring in 16%-56% of solid organ transplant recipients and 30%-70% of hematopoietic stem cell transplant recipients, according to Takeda Pharmaceutical Company Limited, the company that manufactures Livtencity. For immunosuppressed transplant patients, CMV infection can lead to complications that include loss of the transplanted or organ or even death.
“Cytomegalovirus infections that are resistant or do not respond to available drugs are of even greater concern,” John Farley, MD, MPH, the director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research, said in a statement. “Today’s approval helps meet a significant unmet medical need by providing a treatment option for this patient population.”
Livtencity, which is taken orally, works by preventing the activity of the enzyme responsible for virus replication. The approval, announced Nov. 23, was based on a phase 3 clinical trial that compared Livtencity with conventional antiviral treatments in the achievement of CMV DNA concentration levels below what is measurable in transplant patients with CMV infection that is refractory or treatment-resistant. After 8 weeks, of the 235 patients who received Livtencity, 56% achieved this primary endpoint, compared with 24% of the 117 patients who received conventional antiviral treatments, the press release says.
The most reported adverse reactions of Livtencity were taste disturbance, nausea, diarrhea, vomiting, and fatigue.
“We are grateful for the contributions of the patients and clinicians who participated in our clinical trials, as well as the dedication of our scientists and researchers,” Ramona Sequeira, president of the Takeda’s U.S. Business Unit and Global Portfolio Commercialization, said in a statement. “People undergoing transplants have a lengthy and complex health care journey; with the approval of this treatment, we’re proud to offer these individuals a new oral antiviral to fight CMV infection and disease.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the first treatment for posttransplant cytomegalovirus (CMV) that is resistant to other drugs.
There are an estimated 200,000 adult transplants every year globally. CMV, a type of herpes virus, is one of the most common infections in transplant patients, occurring in 16%-56% of solid organ transplant recipients and 30%-70% of hematopoietic stem cell transplant recipients, according to Takeda Pharmaceutical Company Limited, the company that manufactures Livtencity. For immunosuppressed transplant patients, CMV infection can lead to complications that include loss of the transplanted or organ or even death.
“Cytomegalovirus infections that are resistant or do not respond to available drugs are of even greater concern,” John Farley, MD, MPH, the director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research, said in a statement. “Today’s approval helps meet a significant unmet medical need by providing a treatment option for this patient population.”
Livtencity, which is taken orally, works by preventing the activity of the enzyme responsible for virus replication. The approval, announced Nov. 23, was based on a phase 3 clinical trial that compared Livtencity with conventional antiviral treatments in the achievement of CMV DNA concentration levels below what is measurable in transplant patients with CMV infection that is refractory or treatment-resistant. After 8 weeks, of the 235 patients who received Livtencity, 56% achieved this primary endpoint, compared with 24% of the 117 patients who received conventional antiviral treatments, the press release says.
The most reported adverse reactions of Livtencity were taste disturbance, nausea, diarrhea, vomiting, and fatigue.
“We are grateful for the contributions of the patients and clinicians who participated in our clinical trials, as well as the dedication of our scientists and researchers,” Ramona Sequeira, president of the Takeda’s U.S. Business Unit and Global Portfolio Commercialization, said in a statement. “People undergoing transplants have a lengthy and complex health care journey; with the approval of this treatment, we’re proud to offer these individuals a new oral antiviral to fight CMV infection and disease.”
A version of this article first appeared on Medscape.com.
Big drop in U.S. cervical cancer rates, mortality in younger women
The analysis adds to a growing body of evidence demonstrating vaccine-associated changes in cervical cancer incidence and mortality.
Previous data from the United Kingdom, published earlier in November, showed that cervical cancer rates were 87% lower among girls who received the HPV vaccine compared to previously unvaccinated generations. Based on the analysis, the authors concluded that the UK’s HPV immunization program “almost eliminated cervical cancer” in women born since September 1995.
The latest study, published Nov. 29 in JAMA Pediatrics , reports a 38% drop in cervical cancer incidence and a 43% decline in mortality among young women and girls after HPV vaccination was introduced in the United States.
“These results are encouraging,” Peter Sasieni, MD, of King’s College London, and senior author on the U.K. study, told this news organization in an email.
The difference in incidence rates between the U.K. and U.S. studies, Dr. Sasieni explained, is likely due to HPV vaccine coverage not expanding as significantly in the United States as it has in the United Kingdom, and “thus one would anticipate a lower impact on the population in the U.S.”
In the U.S. analysis, Justin Barnes, MD, a radiation oncology resident at Washington University, St. Louis, and colleagues examined cervical cancer incidence between January 2001 and December 2017 using Surveillance, Epidemiology, and End Results and National Program of Cancer Registries data as well as mortality data from the National Center for Health Statistics.
Dr. Barnes and colleagues then compared changes in cervical cancer incidence and mortality between prevaccination years (January 2001 to December 2005) and postvaccination years (January 2010 to December 2017) among three age cohorts – 15-24 years, 25-29 years, and 30-39 years.
“The older 2 groups were included as comparison, given their low vaccination rates,” Dr. Barnes and colleagues explained.
Results show that between the prevaccination and postvaccination periods, the incidence of cervical cancer dropped by 38% in the youngest cohort and by only 16% in the middle-aged group and 8% in the oldest cohort.
Women and girls in the youngest group saw a striking drop in mortality: a 43% decline, which translated to a mortality rate of 0.6 per 100,000.
On the other hand, the authors report a 4.7% decline in mortality in the oldest group and a 4.3% increase in mortality in the middle-aged group – translating to a mortality rate of 1.89 per 100,000 and 0.57 per 100,000, respectively.
Overall, “these nationwide data showed decreased cervical cancer incidence and mortality among women and girls aged 15-24 years after HPV vaccine introduction,” Dr. Barnes and colleagues wrote. The changes in cervical cancer incidence and mortality observed in the youngest age group “were greater than changes in those aged 25 to 29 years and 30 to 39 years, suggesting possible associations with HPV vaccination.”
This analysis lines up with previous evidence from U.S. epidemiologic data, which “have shown decreased cervical cancer incidence after vaccine implementation in women and girls aged 15 to 24 years but not older women.”
Although “the number of deaths and hence the number of potentially averted deaths in young women and girls was small,” the study adds to the current literature by “providing suggestive evidence for vaccine-associated decreases in cervical cancer mortality,” investigators concluded.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The analysis adds to a growing body of evidence demonstrating vaccine-associated changes in cervical cancer incidence and mortality.
Previous data from the United Kingdom, published earlier in November, showed that cervical cancer rates were 87% lower among girls who received the HPV vaccine compared to previously unvaccinated generations. Based on the analysis, the authors concluded that the UK’s HPV immunization program “almost eliminated cervical cancer” in women born since September 1995.
The latest study, published Nov. 29 in JAMA Pediatrics , reports a 38% drop in cervical cancer incidence and a 43% decline in mortality among young women and girls after HPV vaccination was introduced in the United States.
“These results are encouraging,” Peter Sasieni, MD, of King’s College London, and senior author on the U.K. study, told this news organization in an email.
The difference in incidence rates between the U.K. and U.S. studies, Dr. Sasieni explained, is likely due to HPV vaccine coverage not expanding as significantly in the United States as it has in the United Kingdom, and “thus one would anticipate a lower impact on the population in the U.S.”
In the U.S. analysis, Justin Barnes, MD, a radiation oncology resident at Washington University, St. Louis, and colleagues examined cervical cancer incidence between January 2001 and December 2017 using Surveillance, Epidemiology, and End Results and National Program of Cancer Registries data as well as mortality data from the National Center for Health Statistics.
Dr. Barnes and colleagues then compared changes in cervical cancer incidence and mortality between prevaccination years (January 2001 to December 2005) and postvaccination years (January 2010 to December 2017) among three age cohorts – 15-24 years, 25-29 years, and 30-39 years.
“The older 2 groups were included as comparison, given their low vaccination rates,” Dr. Barnes and colleagues explained.
Results show that between the prevaccination and postvaccination periods, the incidence of cervical cancer dropped by 38% in the youngest cohort and by only 16% in the middle-aged group and 8% in the oldest cohort.
Women and girls in the youngest group saw a striking drop in mortality: a 43% decline, which translated to a mortality rate of 0.6 per 100,000.
On the other hand, the authors report a 4.7% decline in mortality in the oldest group and a 4.3% increase in mortality in the middle-aged group – translating to a mortality rate of 1.89 per 100,000 and 0.57 per 100,000, respectively.
Overall, “these nationwide data showed decreased cervical cancer incidence and mortality among women and girls aged 15-24 years after HPV vaccine introduction,” Dr. Barnes and colleagues wrote. The changes in cervical cancer incidence and mortality observed in the youngest age group “were greater than changes in those aged 25 to 29 years and 30 to 39 years, suggesting possible associations with HPV vaccination.”
This analysis lines up with previous evidence from U.S. epidemiologic data, which “have shown decreased cervical cancer incidence after vaccine implementation in women and girls aged 15 to 24 years but not older women.”
Although “the number of deaths and hence the number of potentially averted deaths in young women and girls was small,” the study adds to the current literature by “providing suggestive evidence for vaccine-associated decreases in cervical cancer mortality,” investigators concluded.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The analysis adds to a growing body of evidence demonstrating vaccine-associated changes in cervical cancer incidence and mortality.
Previous data from the United Kingdom, published earlier in November, showed that cervical cancer rates were 87% lower among girls who received the HPV vaccine compared to previously unvaccinated generations. Based on the analysis, the authors concluded that the UK’s HPV immunization program “almost eliminated cervical cancer” in women born since September 1995.
The latest study, published Nov. 29 in JAMA Pediatrics , reports a 38% drop in cervical cancer incidence and a 43% decline in mortality among young women and girls after HPV vaccination was introduced in the United States.
“These results are encouraging,” Peter Sasieni, MD, of King’s College London, and senior author on the U.K. study, told this news organization in an email.
The difference in incidence rates between the U.K. and U.S. studies, Dr. Sasieni explained, is likely due to HPV vaccine coverage not expanding as significantly in the United States as it has in the United Kingdom, and “thus one would anticipate a lower impact on the population in the U.S.”
In the U.S. analysis, Justin Barnes, MD, a radiation oncology resident at Washington University, St. Louis, and colleagues examined cervical cancer incidence between January 2001 and December 2017 using Surveillance, Epidemiology, and End Results and National Program of Cancer Registries data as well as mortality data from the National Center for Health Statistics.
Dr. Barnes and colleagues then compared changes in cervical cancer incidence and mortality between prevaccination years (January 2001 to December 2005) and postvaccination years (January 2010 to December 2017) among three age cohorts – 15-24 years, 25-29 years, and 30-39 years.
“The older 2 groups were included as comparison, given their low vaccination rates,” Dr. Barnes and colleagues explained.
Results show that between the prevaccination and postvaccination periods, the incidence of cervical cancer dropped by 38% in the youngest cohort and by only 16% in the middle-aged group and 8% in the oldest cohort.
Women and girls in the youngest group saw a striking drop in mortality: a 43% decline, which translated to a mortality rate of 0.6 per 100,000.
On the other hand, the authors report a 4.7% decline in mortality in the oldest group and a 4.3% increase in mortality in the middle-aged group – translating to a mortality rate of 1.89 per 100,000 and 0.57 per 100,000, respectively.
Overall, “these nationwide data showed decreased cervical cancer incidence and mortality among women and girls aged 15-24 years after HPV vaccine introduction,” Dr. Barnes and colleagues wrote. The changes in cervical cancer incidence and mortality observed in the youngest age group “were greater than changes in those aged 25 to 29 years and 30 to 39 years, suggesting possible associations with HPV vaccination.”
This analysis lines up with previous evidence from U.S. epidemiologic data, which “have shown decreased cervical cancer incidence after vaccine implementation in women and girls aged 15 to 24 years but not older women.”
Although “the number of deaths and hence the number of potentially averted deaths in young women and girls was small,” the study adds to the current literature by “providing suggestive evidence for vaccine-associated decreases in cervical cancer mortality,” investigators concluded.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PEDIATRICS
Pfizer COVID vaccine is 100% effective in adolescents: Study
Pfizer announced on Nov. 22 that its COVID-19 vaccine provided long-term protection against the virus in a late-stage clinical trial among adolescents ages 12-15.
A two-dose series was 100% effective against COVID-19, which was measured between 7 days and 4 months after the second dose.
“As the global health community works to increase the number of vaccinated people around the world, these additional data provide further confidence in our vaccine safety and effectiveness profile in adolescents,” Albert Bourla, PhD, chairman and CEO of Pfizer, said in a statement.
The clinical trial researchers found no serious safety concerns while following patients for 6 months. The adverse events were consistent with other clinical safety data for the vaccine, the company said.
Pfizer will incorporate the data into its submissions for full regulatory approval of the vaccine for ages 12-15 in the United States and worldwide.
The company will request clearance for a 30-mcg dose of the vaccines for ages 12 and older. The shot received FDA emergency use authorization for ages 12-15 in May and full approval for ages 16 and older in August.
The study included 2,228 clinical trial participants who were monitored between November 2020 and September 2021. There were 30 confirmed symptomatic cases of COVID-19 in the placebo group that didn’t receive the vaccine and 0 COVID-19 cases among the vaccinated group.
The efficacy was consistently high across gender, race, ethnicity, and health conditions, the company said.
“This is especially important as we see rates of COVID-19 climbing in this age group in some regions, while vaccine uptake has slowed,” Mr. Bourla said. “We look forward to sharing these data with the FDA and other regulators.”
A version of this article first appeared on WebMD.com.
Pfizer announced on Nov. 22 that its COVID-19 vaccine provided long-term protection against the virus in a late-stage clinical trial among adolescents ages 12-15.
A two-dose series was 100% effective against COVID-19, which was measured between 7 days and 4 months after the second dose.
“As the global health community works to increase the number of vaccinated people around the world, these additional data provide further confidence in our vaccine safety and effectiveness profile in adolescents,” Albert Bourla, PhD, chairman and CEO of Pfizer, said in a statement.
The clinical trial researchers found no serious safety concerns while following patients for 6 months. The adverse events were consistent with other clinical safety data for the vaccine, the company said.
Pfizer will incorporate the data into its submissions for full regulatory approval of the vaccine for ages 12-15 in the United States and worldwide.
The company will request clearance for a 30-mcg dose of the vaccines for ages 12 and older. The shot received FDA emergency use authorization for ages 12-15 in May and full approval for ages 16 and older in August.
The study included 2,228 clinical trial participants who were monitored between November 2020 and September 2021. There were 30 confirmed symptomatic cases of COVID-19 in the placebo group that didn’t receive the vaccine and 0 COVID-19 cases among the vaccinated group.
The efficacy was consistently high across gender, race, ethnicity, and health conditions, the company said.
“This is especially important as we see rates of COVID-19 climbing in this age group in some regions, while vaccine uptake has slowed,” Mr. Bourla said. “We look forward to sharing these data with the FDA and other regulators.”
A version of this article first appeared on WebMD.com.
Pfizer announced on Nov. 22 that its COVID-19 vaccine provided long-term protection against the virus in a late-stage clinical trial among adolescents ages 12-15.
A two-dose series was 100% effective against COVID-19, which was measured between 7 days and 4 months after the second dose.
“As the global health community works to increase the number of vaccinated people around the world, these additional data provide further confidence in our vaccine safety and effectiveness profile in adolescents,” Albert Bourla, PhD, chairman and CEO of Pfizer, said in a statement.
The clinical trial researchers found no serious safety concerns while following patients for 6 months. The adverse events were consistent with other clinical safety data for the vaccine, the company said.
Pfizer will incorporate the data into its submissions for full regulatory approval of the vaccine for ages 12-15 in the United States and worldwide.
The company will request clearance for a 30-mcg dose of the vaccines for ages 12 and older. The shot received FDA emergency use authorization for ages 12-15 in May and full approval for ages 16 and older in August.
The study included 2,228 clinical trial participants who were monitored between November 2020 and September 2021. There were 30 confirmed symptomatic cases of COVID-19 in the placebo group that didn’t receive the vaccine and 0 COVID-19 cases among the vaccinated group.
The efficacy was consistently high across gender, race, ethnicity, and health conditions, the company said.
“This is especially important as we see rates of COVID-19 climbing in this age group in some regions, while vaccine uptake has slowed,” Mr. Bourla said. “We look forward to sharing these data with the FDA and other regulators.”
A version of this article first appeared on WebMD.com.
Surveillance for measles is a victim of the COVID pandemic
Although the estimated annual number of measles deaths decreased 94% from 2000 to 2020, the COVID-19 pandemic took a toll on both measles vaccination and surveillance, according to a recent report in Morbidity and Mortality Weekly Report.
The number of World Health Organization member states that achieved more than 90% coverage with the first dose of the measles vaccine (MCV1) declined 37% from 2019 to 2020. In 2020, 23 million infants did not receive MCV1 through routine immunization services, and another 93 million were affected by the postponement of mass immunizations or supplementary immunization activities because of the pandemic. Also, endemic transmission was reestablished in nine countries that had previously eliminated measles.
But perhaps the most overlooked aspect of COVID-19 is its effect on surveillance.
“The entire COVID pandemic really put a lot of strain on the surveillance systems, not only for measles but for all vaccine-preventable disease, because there’s a lot of overlap in the staff who work for surveillance,” said Katrina Kretsinger, MD, a medical epidemiologist at the Centers for Disease Control and Prevention, who contributed to the MMWR report.
Because of the stress on the systems, a lot fewer specimens were tested, she said in an interview. And it’s not just measles that is at risk. This has had an impact on the Global Polio Eradication Initiative, which lost staff.
In addition, many vaccination campaigns “were postponed and curtailed throughout 2020,” Dr. Kretsinger said. The strengthening of surveillance systems – and immunization systems, more broadly – needs to be a priority.
“It’s not clear that the children who were missed during that year were subsequently caught up,” she explained. Having a “cohort of children who have missed measles vaccine creates the reservoir of susceptibility that will provide the nidus for the next big outbreak.”
Measles is the indicator disease. That could mean a resurgence of other vaccine-preventable diseases as well.
This report “was written by some of the world’s experts in measles, and it raises concerns about potential resurgence of measles,” said Walter Orenstein, MD, professor of medicine, epidemiology, global health, and pediatrics at Emory University, Atlanta. “Measles is sort of a canary in the coal mine. If you look at vaccine-preventable diseases, measles is probably the most contagious, so the herd-immunity threshold is highest. Usually on the order of 92%-94% immunity is needed to stop transmission.”
“Measles is the indicator disease,” he said in an interview. “That could mean a resurgence of other vaccine-preventable diseases as well.” Outbreaks don’t just affect the countries where infections are occurring, they “also affect our own domestic health security.”
“Some sort of periodic intensified routine immunization” would be helpful, said Dr. Kretsinger, who recommends “going through and selectively doing some sort of intensified efforts to catch children up early for the entire range of vaccines that they may have missed.”
“Some of these capture campaigns in areas that are thought to have the major problem would be very, very important,” agreed Dr. Orenstein. “A school entry check is one way of trying to look at kids, let’s say at 4-6 years of age, in schools around the world,” offering doses if they’re unvaccinated or inadequately vaccinated. “Another is to try to improve surveillance and try to understand if the cases are vaccine failure or failure to vaccinate.”
“Where the health systems are the most fragile is where those gaps will be the last to be filled, if they are at all, and where we have the basic concerns,” Dr. Kretsinger explained.
“Years ago, WHO recognized that vaccine hesitancy is a top global health threat,” said Dr. Orenstein. “People may not see these diseases so they don’t mean much to them. Since vaccines, we’re victims of our own success.” There’s also a lot of incorrect information circulating.
“We need to realize – and it’s been shown with COVID – that a decision not to vaccinate is not just a decision for your own child. It’s a community decision,” he pointed out. “It’s not my freedom to drive drunk, because not only do I put myself at risk, but others can’t control the car. We have speed limits and other examples where we restrict personal choice because it can adversely affect individuals.”
“My favorite line is vaccines don’t save lives, vaccinations save lives,” Dr. Orenstein said. “The vaccine dose that remains in the vial is 0% effective, no matter what the clinical trials show. And the issue, I think, is that we need to determine how to convince the hesitant to get confident enough to accept vaccination. For that, there is behavioral research; there’s a whole bunch of things that need to be supported. Just purchasing the vaccine doesn’t get it into the bodies.”
Dr. Kretsinger and Dr. Orenstein disclosed no relevant financial relationships .
A version of this article first appeared on Medscape.com.
Although the estimated annual number of measles deaths decreased 94% from 2000 to 2020, the COVID-19 pandemic took a toll on both measles vaccination and surveillance, according to a recent report in Morbidity and Mortality Weekly Report.
The number of World Health Organization member states that achieved more than 90% coverage with the first dose of the measles vaccine (MCV1) declined 37% from 2019 to 2020. In 2020, 23 million infants did not receive MCV1 through routine immunization services, and another 93 million were affected by the postponement of mass immunizations or supplementary immunization activities because of the pandemic. Also, endemic transmission was reestablished in nine countries that had previously eliminated measles.
But perhaps the most overlooked aspect of COVID-19 is its effect on surveillance.
“The entire COVID pandemic really put a lot of strain on the surveillance systems, not only for measles but for all vaccine-preventable disease, because there’s a lot of overlap in the staff who work for surveillance,” said Katrina Kretsinger, MD, a medical epidemiologist at the Centers for Disease Control and Prevention, who contributed to the MMWR report.
Because of the stress on the systems, a lot fewer specimens were tested, she said in an interview. And it’s not just measles that is at risk. This has had an impact on the Global Polio Eradication Initiative, which lost staff.
In addition, many vaccination campaigns “were postponed and curtailed throughout 2020,” Dr. Kretsinger said. The strengthening of surveillance systems – and immunization systems, more broadly – needs to be a priority.
“It’s not clear that the children who were missed during that year were subsequently caught up,” she explained. Having a “cohort of children who have missed measles vaccine creates the reservoir of susceptibility that will provide the nidus for the next big outbreak.”
Measles is the indicator disease. That could mean a resurgence of other vaccine-preventable diseases as well.
This report “was written by some of the world’s experts in measles, and it raises concerns about potential resurgence of measles,” said Walter Orenstein, MD, professor of medicine, epidemiology, global health, and pediatrics at Emory University, Atlanta. “Measles is sort of a canary in the coal mine. If you look at vaccine-preventable diseases, measles is probably the most contagious, so the herd-immunity threshold is highest. Usually on the order of 92%-94% immunity is needed to stop transmission.”
“Measles is the indicator disease,” he said in an interview. “That could mean a resurgence of other vaccine-preventable diseases as well.” Outbreaks don’t just affect the countries where infections are occurring, they “also affect our own domestic health security.”
“Some sort of periodic intensified routine immunization” would be helpful, said Dr. Kretsinger, who recommends “going through and selectively doing some sort of intensified efforts to catch children up early for the entire range of vaccines that they may have missed.”
“Some of these capture campaigns in areas that are thought to have the major problem would be very, very important,” agreed Dr. Orenstein. “A school entry check is one way of trying to look at kids, let’s say at 4-6 years of age, in schools around the world,” offering doses if they’re unvaccinated or inadequately vaccinated. “Another is to try to improve surveillance and try to understand if the cases are vaccine failure or failure to vaccinate.”
“Where the health systems are the most fragile is where those gaps will be the last to be filled, if they are at all, and where we have the basic concerns,” Dr. Kretsinger explained.
“Years ago, WHO recognized that vaccine hesitancy is a top global health threat,” said Dr. Orenstein. “People may not see these diseases so they don’t mean much to them. Since vaccines, we’re victims of our own success.” There’s also a lot of incorrect information circulating.
“We need to realize – and it’s been shown with COVID – that a decision not to vaccinate is not just a decision for your own child. It’s a community decision,” he pointed out. “It’s not my freedom to drive drunk, because not only do I put myself at risk, but others can’t control the car. We have speed limits and other examples where we restrict personal choice because it can adversely affect individuals.”
“My favorite line is vaccines don’t save lives, vaccinations save lives,” Dr. Orenstein said. “The vaccine dose that remains in the vial is 0% effective, no matter what the clinical trials show. And the issue, I think, is that we need to determine how to convince the hesitant to get confident enough to accept vaccination. For that, there is behavioral research; there’s a whole bunch of things that need to be supported. Just purchasing the vaccine doesn’t get it into the bodies.”
Dr. Kretsinger and Dr. Orenstein disclosed no relevant financial relationships .
A version of this article first appeared on Medscape.com.
Although the estimated annual number of measles deaths decreased 94% from 2000 to 2020, the COVID-19 pandemic took a toll on both measles vaccination and surveillance, according to a recent report in Morbidity and Mortality Weekly Report.
The number of World Health Organization member states that achieved more than 90% coverage with the first dose of the measles vaccine (MCV1) declined 37% from 2019 to 2020. In 2020, 23 million infants did not receive MCV1 through routine immunization services, and another 93 million were affected by the postponement of mass immunizations or supplementary immunization activities because of the pandemic. Also, endemic transmission was reestablished in nine countries that had previously eliminated measles.
But perhaps the most overlooked aspect of COVID-19 is its effect on surveillance.
“The entire COVID pandemic really put a lot of strain on the surveillance systems, not only for measles but for all vaccine-preventable disease, because there’s a lot of overlap in the staff who work for surveillance,” said Katrina Kretsinger, MD, a medical epidemiologist at the Centers for Disease Control and Prevention, who contributed to the MMWR report.
Because of the stress on the systems, a lot fewer specimens were tested, she said in an interview. And it’s not just measles that is at risk. This has had an impact on the Global Polio Eradication Initiative, which lost staff.
In addition, many vaccination campaigns “were postponed and curtailed throughout 2020,” Dr. Kretsinger said. The strengthening of surveillance systems – and immunization systems, more broadly – needs to be a priority.
“It’s not clear that the children who were missed during that year were subsequently caught up,” she explained. Having a “cohort of children who have missed measles vaccine creates the reservoir of susceptibility that will provide the nidus for the next big outbreak.”
Measles is the indicator disease. That could mean a resurgence of other vaccine-preventable diseases as well.
This report “was written by some of the world’s experts in measles, and it raises concerns about potential resurgence of measles,” said Walter Orenstein, MD, professor of medicine, epidemiology, global health, and pediatrics at Emory University, Atlanta. “Measles is sort of a canary in the coal mine. If you look at vaccine-preventable diseases, measles is probably the most contagious, so the herd-immunity threshold is highest. Usually on the order of 92%-94% immunity is needed to stop transmission.”
“Measles is the indicator disease,” he said in an interview. “That could mean a resurgence of other vaccine-preventable diseases as well.” Outbreaks don’t just affect the countries where infections are occurring, they “also affect our own domestic health security.”
“Some sort of periodic intensified routine immunization” would be helpful, said Dr. Kretsinger, who recommends “going through and selectively doing some sort of intensified efforts to catch children up early for the entire range of vaccines that they may have missed.”
“Some of these capture campaigns in areas that are thought to have the major problem would be very, very important,” agreed Dr. Orenstein. “A school entry check is one way of trying to look at kids, let’s say at 4-6 years of age, in schools around the world,” offering doses if they’re unvaccinated or inadequately vaccinated. “Another is to try to improve surveillance and try to understand if the cases are vaccine failure or failure to vaccinate.”
“Where the health systems are the most fragile is where those gaps will be the last to be filled, if they are at all, and where we have the basic concerns,” Dr. Kretsinger explained.
“Years ago, WHO recognized that vaccine hesitancy is a top global health threat,” said Dr. Orenstein. “People may not see these diseases so they don’t mean much to them. Since vaccines, we’re victims of our own success.” There’s also a lot of incorrect information circulating.
“We need to realize – and it’s been shown with COVID – that a decision not to vaccinate is not just a decision for your own child. It’s a community decision,” he pointed out. “It’s not my freedom to drive drunk, because not only do I put myself at risk, but others can’t control the car. We have speed limits and other examples where we restrict personal choice because it can adversely affect individuals.”
“My favorite line is vaccines don’t save lives, vaccinations save lives,” Dr. Orenstein said. “The vaccine dose that remains in the vial is 0% effective, no matter what the clinical trials show. And the issue, I think, is that we need to determine how to convince the hesitant to get confident enough to accept vaccination. For that, there is behavioral research; there’s a whole bunch of things that need to be supported. Just purchasing the vaccine doesn’t get it into the bodies.”
Dr. Kretsinger and Dr. Orenstein disclosed no relevant financial relationships .
A version of this article first appeared on Medscape.com.
The Use of Nasogastric Tube Bridle Kits in COVID-19 Intensive Care Unit Patients
From Queen Elizabeth Hospital Birmingham, Mindelsohn Way, Birmingham, United Kingdom.
Objective: To ascertain the extent of nasogastric tube (NGT) dislodgment in COVID-19 intensive care unit (ICU) patients after the introduction of NGT bridle kits as a standard of practice, to see whether this would reduce the number of NGT insertions, patient irradiation, missed feeds, and overall cost.
Background: Nasogastric feeding is the mainstay of enteral feeding for ICU patients. The usual standard of practice is to secure the tube using adhesive tape. Studies show this method has a 40% to 48% dislodgment rate. The COVID-19 ICU patient population may be at even greater risk due to the need for proning, long duration of invasive ventilation, and emergence delirium.
Design: This was a 2-cycle quality improvement project. The first cycle was done retrospectively, looking at the contemporaneous standard of practice where bridle kits were not used. This gave an objective measure of the extent of NGT displacement, associated costs, and missed feeds. The second cycle was carried out prospectively, with the use of NGT bridle kits as the new standard of practice.
Setting: A large United Kingdom teaching hospital with a 100-bed, single-floor ICU.
Participants: Patients admitted to the ICU with COVID-19 who subsequently required sedation and invasive ventilation.
Measurements: Measurements included days of feeding required, hours of feeding missed due to NGT dislodgment, total number of nasogastric tubes required per ICU stay, and number of chest radiographs for NGT position confirmation. NGT-related pressure sores were also recorded.
Results: When compared to the bridled group, the unbridled group required a higher number of NGTs (2.5 vs 1.3; P < .001) and chest radiographs (3.4 vs 1.6; P < .001), had more hours of feeding missed (11.8 vs 5.0), and accumulated a slightly higher total cost (cost of NGT, chest radiographs +/- bridle kit: £211.67 vs £210, [US $284.25 vs US $282.01]).
Conclusions: The use of NGT bridle kits reduces the number of NGT insertions patients require and subsequently reduces the number of chest radiographs for each patient. These patients also miss fewer feeds, with no appreciable increase in cost.
Keywords: nasogastric, bridle, enteral, COVID-19, intensive care, quality improvement, safety.
The COVID-19 pandemic has led to a large influx of patients to critical care units in the United Kingdom (UK) and across the world. Figures from the Intensive Care National Audit & Research Centre in May 2020 show that the median length of stay for COVID-19 survivors requiring invasive ventilatory support while on the intensive care unit (ICU) was 15 days.1 For these days at the very least, patients are completely reliant on enteral feeding in order to meet their nutritional requirements.The standard method of enteral feeding when a patient is sedated and ventilated is via a nasogastric tube (NGT). Incorrect placement of an NGT can have devastating consequences, including pneumothorax, fistula formation, ulceration, sepsis, and death. Between September 2011 and March 2016, the National Patient Safety Agency in the UK recorded 95 incidents of feeding into the respiratory tract as a result of incorrect NGT placement.2 With the onset of the pandemic, the prevalence of NGT misplacement increased, with the NHS Improvement team reporting 7 cases of misplaced NGTs within just 3 months (April 1, 2020, through June 30, 2020).3 With over 3 million nasogastric or orogastric tubes inserted each year in the UK, the risk of adverse events is very real.
NGT dislodgment is common, with 1 study putting this figure at 40%.4 Recurrent dislodgment of NGTs disrupts nutrition and may lead to the patient missing a feed in a time where nutrition is vital during acute illness. Research has showed that NGT bridling reduces the rate of dislodgment significantly (from 40% to 14%).5 Moreover, a 2018 systematic review looking specifically at NGT dislodgment found 10 out of 11 studies showed a significant reduction in dislodgment following use of a bridle kit.6 Bridling an NGT has been shown to significantly reduce the need for percutaneous endoscopic gastrostomy insertion.7 NGT bridle kits have already been used successfully in ICU burn patients, where sloughed skin makes securement particularly difficult with traditional methods.8 With each repeated insertion comes the risk of incorrect placement. COVID-19 ICU patients had specific risk factors for their NGTs becoming dislodged: duration of NGT feeding (in the ICU and on the ward), requirement for proning and de-proning, and post-emergence confusion related to long duration of sedation. Repeated NGT insertion comes with potential risks to the patient and staff, as well as a financial cost. Patient-specific risks include potential for incorrect placement, missed feedings, irradiation (from the patient’s own chest radiograph and from others), and discomfort from manual handling and repeat reinsertions. Staff risk factors include radiation scatter from portable radiographs (especially when dealing with more than 1 patient per bed space), manual handling, and increased pressure on radiographers. Finally, financial costs are related to the NGTs themselves as well as the portable chest radiograph, which our Superintendent Radiographer estimates to be £55 (US $73.86).
The objective of this study was to ascertain the extent of NGT dislodgment in COVID-19 ICU patients after the introduction of NGT bridle kits as a standard of practice and to determine whether this would reduce the number of NGT insertions, patient irradiation, missed feedings, and overall costs. With the introduction of bridle kits, incidence of pressure sores related to the bridle kit were also recorded.
Methods
Data were collected over 2 cycles, the first retrospectively and the second prospectively, once NGT bridle kits were introduced as an intervention.
Cycle 1. Analyzing the current standard of practice: regular NGT insertion with no use of bridle kit
Cycle 1 was done retrospectively, looking at 30 patient notes of COVID-19 patients admitted to the critical care unit (CCU) between March 11, 2020, and April 20, 2020, at Queen Elizabeth Hospital Birmingham, Birmingham, UK. All patients admitted to the ICU with COVID-19 requiring invasive ventilation were eligible for inclusion in the study. A total of 32 patients were admitted during this time; however, 2 patients were excluded due to NGTs being inserted prior to ICU admission.
Individual patient notes were searched for:
- days of feeding required during their inpatient stay (this included NGT feeding on the ward post-ICU discharge).
- hours of feeding missed while waiting for NGT reinsertion or chest radiograph due to dislodged or displaced NGTs (during the entire period of enteral feeding, ICU, and ward).
- number of NGT insertions.
- number of chest radiographs purely for NGT position.
Each patient’s first day of feeding and NGT insertion were noted. Following that, the patient electronic note system, the Prescribing Information and Communication System, was used to look for any further chest radiograph requests, which were primarily for NGT position. Using the date and time, the “critical care observations” tab was used to look at fluids and to calculate how long NGT feeding was stopped while NGT position-check x-rays were being awaited. The notes were also checked at this date and time to work out whether a new NGT was inserted or whether an existing tube had been dislodged (if not evident from the x-ray request). Data collection was stopped once either of the following occurred:
- patient no longer required NGT feeding.
- patient was transferred to another hospital.
- death.
The cost of the NGT was averaged between the cost of size 8 and 12, which worked out to be £10 (US $13.43). As mentioned earlier, each radiograph cost was determined by the Superintendent Radiographer (£55).
Cycle 2. Implementing a change: introduction of NGT bridle kit (Applied Medical Technology Bridle) as standard of practice
The case notes of 54 patients admitted to the COVID-19 CCU at the Queen Elizabeth Hospital Birmingham, Birmingham, UK, were retrospectively reviewed between February 8, 2021, and April 17, 2021. The inclusion criteria consisted of: admitted to the CCU due to COVID-19, required NGT feeding, and was bridled on admission. Case notes were retrospectively reviewed for:
- Length of CCU stay
- Days of feeding required during the hospital stay
- Hours of feeding missed while waiting for a chest radiograph due to displaced NGTs
- Number of NGT insertions
- Number of chest radiographs to confirm NGT position
- Bridling of NGTs
- Documented pressure sores related to the bridle or NGT, or referrals for wound management advice (Tissue Viability Team) as a consequence of the NGT bridle
Results
Of the 54 patients admitted, 31 had their NGTs bridled. Data were collected as in the first cycle, with individual notes analyzed on the online system (Table). Additionally, notes were reviewed for documentation of pressure sores related to NGT bridling, and the “requests” tab as well as the “noting” function were used to identify referrals for “Wound Management Advice” (Tissue Viability Review).
The average length of stay for this ICU cohort was 17.6 days. This reiterates the reliance on NGT feeding of patients admitted to the CCU. The results from this project can be summarized as follows: The use of NGT bridle kits leads to a significant reduction in the total number of NGTs a patient requires during intensive care. As a result, there is a significant reduction in the number of chest radiographs required to confirm NGT position. Feedings missed can also be reduced by using a bridle kit. These advantages all come with no additional cost.
On average, bridled patients required 1.3 NGTs, compared to 2.5 before bridles were introduced. The fewer NGTs inserted, the less chance of an NGT-associated injury occurring.
The number of chest radiographs required to confirm NGT position after resiting also fell, from 3.4 to 1.6. This has numerous advantages. There is a financial savings of £99 (US $133.04) per patient from the reduced number of chest x-rays. Although this does not offset the price of the bridle kit itself, there are other less easily quantifiable costs that are reduced. For instance, patients are highly catabolic during severe infection, and their predominant energy source comes from their feedings. Missed feedings are associated with longer length of stay in the ICU and in the hospital in general.9 Bridle kits have the potential to reduce the number of missed feedings by ensuring the NGT remains in the correct position.
Discussion
Many of the results are aligned with what is already known in the literature. A meta-analysis from 2014 concluded that dislodgment is reduced with the use of a bridle kit.6 This change is what underpins many of the advantages seen, as an NGT that stays in place means additional radiographs are not required and feeding is not delayed.
COVID-19 critical care patients are very fragile and are dependent on ventilators for the majority of their stay. They are often on very high levels of ventilator support and moving the patient can lead to desaturation or difficulties in ventilation. Therefore, reduction in any manual handling occurring as a result of the need for portable chest radiographs minimizes the chances of further negative events. Furthermore, nursing staff, along with the radiographers, are often the ones who must move these patients in order for the x-ray film to be placed behind the patient. This task is not easy, especially with limited personnel, and has the potential to cause injuries to both patients and staff members.
The knock-on effect of reduced NGTs and x-rays is also a reduction of work for the portable radiography team, in what is a very time- and resource-consuming process of coming onto the COVID-19 CCU. Not only does the machine itself need to be wiped down thoroughly after use, but also the individual must use personal protective equipment (PPE) each time. There is a cost associated with PPE itself, as well as the time it takes to don and doff appropriately.
A reduction in chest radiographs reduces the irradiation of the patient and the potential irradiation of staff members. With bridling of the NGT, the radiation exposure is more than halved for the patient. Because the COVID ICU is often very busy, with patients in some cases being doubled up in a bed space, the scatter radiation is high. This can be reduced if fewer chest radiographs are required.
An additional benefit of a reduction in the mean number of NGT insertions per patient is also illustrated by anecdotal evidence. Over the studied period, we identified 2 traumatic pneumothoraces related to NGT insertion on the COVID-19 CCU, highlighting the potential risks of NGT insertion and the need to reduce its frequency, if possible.
One concern noted was that bridles could cause increased incidence of pressure sores. In the patients represented in this study, only 1 suffered a pressure sore (grade 2) directly related to the bridle. A subpopulation of patients not bridled was also noted. This was significantly smaller than the main group; however, we had noted 2 incidences of pressure sores from their standard NGT and securement devices. Some studies have alluded to the potential for increased skin complications with bridle kits; however, studies looking specifically at kits using umbilical tape (as in this study) show no significant increase in skin damage.10 This leaves us confident that there is no increased risk of pressure sores related to the bridling of patients when umbilical tape is used with the bridle kit.
NGT bridles require training to insert safely. With the introduction of bridling, our hospital’s nursing staff underwent training in order to be proficient with the bridle kits. This comes with a time commitment, and, like other equipment usage, it takes time to build confidence. However, in this study, there were no concerns raised from nursing staff regarding difficulty of insertion or the time taken to do so.
Our study adds an objective measure of the benefits provided by bridle kits. Not only was there a reduction in the number of NGT insertions required, but we were also able to show a significant reduction in the number of chest radiographs required as well in the amount of time feeding is missed. While apprehension regarding bridle kits may be focused on cost, this study has shown that the savings more than make up for the initial cost of the kit itself.
Although the patient demographics, systemic effects, and treatment of COVID-19 are similar between different ICUs, a single-center study does have limitations. One of these is the potential for an intervention in a single-center study to lead to a larger effect than that of multicenter studies.11 But as seen in previous studies, the dislodgment of NGTs is not just an issue in this ICU.12 COVID-19–specific risk factors for NGT dislodgment also apply to all patients requiring invasive ventilation and proning.
Identification of whether a new NGT was inserted, or whether the existing NGT was replaced following dislodging of an NGT, relied on accurate documentation by the relevant staff. The case notes did not always make this explicitly clear. Unlike other procedures commonly performed, documentation of NGT insertion is not formally done under the procedures heading, and, on occasion is not done at all. We recognize that manually searching notes only yields NGT insertions that have been formally documented. There is a potential for the number recorded to be lower than the actual number of NGTs inserted. However, when x-ray requests are cross-referenced with the notes, there is a significant degree of confidence that the vast majority of insertions are picked up.
One patient identified in the study required a Ryle’s tube as part of their critical care treatment. While similar in nature to an NGT, these are unable to fit into a bridle and are at increased risk of dislodging during the patient’s critical care stay. The intended benefit of the bridle kit does not therefore extend to patients with Ryle’s tubes.
Conclusion
The COVID-19 critical care population requires significant time on invasive ventilation and remains dependent on NGT feeding during this process. The risk of NGT dislodgment can be mitigated by using a bridle kit, as the number of NGT insertions a patient requires is significantly reduced. Not only does this reduce the risk of inadvertent misplacement but also has a cost savings, as well as increasing safety for staff and patients. From this study, the risk of pressure injuries is not significant. The benefit of NGT bridling may be extended to other non-COVID long-stay ICU patients.
Future research looking at the efficacy of bridle kits in larger patient groups will help confirm the benefits seen in this study and will also provide better information with regard to any long-term complications associated with bridles.
Corresponding author: Rajveer Atkar, MBBS, Queen Elizabeth Hospital Birmingham, Mindelsohn Way, Birmingham B15 2GW, United Kingdom; [email protected].
Financial disclosures: None.
1. Intensive Care National Audit & Research Centre. ICNARC report on COVID-19 in critical care 15 May 2020. https://www.icnarc.org/DataServices/Attachments/Download/cbcb6217-f698-ea11-9125-00505601089b
2. NHS. Nasogastric tube misplacement: continuing risk of death and severe harm. July 22, 2016. https://www.england.nhs.uk/2016/07/nasogastric-tube-misplacement-continuing-risk-of-death-severe-harm/
3. NHS. Provisional publication of never events reported as occurring between 1 April and 30 June 2020. https://www.england.nhs.uk/wp-content/uploads/2020/08/Provisional_publication_-_NE_1_April_-_30_June_2020.pdf
4. Meer JA. Inadvertent dislodgement of nasoenteral feeding tubes: incidence and prevention. JPEN J Parenter Enteral Nutr. 1987;11(2):187- 189. doi:10.1177/0148607187011002187
5. Bechtold ML, Nguyen DL, Palmer L, et al. Nasal bridles for securing nasoenteric tubes: a meta-analysis. Nutr Clin Pract. 2014;29(5):667-671. doi:10.1177/0884533614536737
6. Lynch A, Tang CS, Jeganathan LS, Rockey JG. A systematic review of the effectiveness and complications of using nasal bridles to secure nasoenteral feeding tubes. Aust J Otolaryngol. 2018;1:8. doi:10.21037/ajo.2018.01.01
7. Johnston R, O’Dell L, Patrick M, Cole OT, Cunliffe N. Outcome of patients fed via a nasogastric tube retained with a bridle loop: Do bridle loops reduce the requirement for percutaneous endoscopic gastrostomy insertion and 30-day mortality? Proc Nutr Soc. 2008;67:E116. doi:10.1017/S0029665108007489
8. Li AY, Rustad KC, Long C, et al. Reduced incidence of feeding tube dislodgement and missed feeds in burn patients with nasal bridle securement. Burns. 2018;44(5):1203-1209. doi:10.1016/j.burns.2017.05.025
9. Peev MP, Yeh DD, Quraishi SA, et al. Causes and consequences of interrupted enteral nutrition: a prospective observational study in critically ill surgical patients. JPEN J Parenter Enteral Nutr. 2015;39(1):21-27. doi:10.1177/0148607114526887
10. Seder CW, Janczyk R. The routine bridling of nasjejunal tubes is a safe and effective method of reducing dislodgement in the intensive care unit. Nutr Clin Pract. 2008;23(6):651-654. doi:10.1177/0148607114526887
11. Dechartres A, Boutron I, Trinquart L, Charles P, Ravaud P. Single-center trials show larger treatment effects than multicenter trials: evidence from a meta-epidemiologic study. Ann Intern Med. 2011;155:39-51. doi:10.7326/0003-4819-155-1-201107050-00006
12. Morton B, Hall R, Ridgway T, Al-Rawi O. Nasogastric tube dislodgement: a problem on our ICU. Crit Care. 2013;17(suppl 2):P242. doi:10.1186/cc12180
From Queen Elizabeth Hospital Birmingham, Mindelsohn Way, Birmingham, United Kingdom.
Objective: To ascertain the extent of nasogastric tube (NGT) dislodgment in COVID-19 intensive care unit (ICU) patients after the introduction of NGT bridle kits as a standard of practice, to see whether this would reduce the number of NGT insertions, patient irradiation, missed feeds, and overall cost.
Background: Nasogastric feeding is the mainstay of enteral feeding for ICU patients. The usual standard of practice is to secure the tube using adhesive tape. Studies show this method has a 40% to 48% dislodgment rate. The COVID-19 ICU patient population may be at even greater risk due to the need for proning, long duration of invasive ventilation, and emergence delirium.
Design: This was a 2-cycle quality improvement project. The first cycle was done retrospectively, looking at the contemporaneous standard of practice where bridle kits were not used. This gave an objective measure of the extent of NGT displacement, associated costs, and missed feeds. The second cycle was carried out prospectively, with the use of NGT bridle kits as the new standard of practice.
Setting: A large United Kingdom teaching hospital with a 100-bed, single-floor ICU.
Participants: Patients admitted to the ICU with COVID-19 who subsequently required sedation and invasive ventilation.
Measurements: Measurements included days of feeding required, hours of feeding missed due to NGT dislodgment, total number of nasogastric tubes required per ICU stay, and number of chest radiographs for NGT position confirmation. NGT-related pressure sores were also recorded.
Results: When compared to the bridled group, the unbridled group required a higher number of NGTs (2.5 vs 1.3; P < .001) and chest radiographs (3.4 vs 1.6; P < .001), had more hours of feeding missed (11.8 vs 5.0), and accumulated a slightly higher total cost (cost of NGT, chest radiographs +/- bridle kit: £211.67 vs £210, [US $284.25 vs US $282.01]).
Conclusions: The use of NGT bridle kits reduces the number of NGT insertions patients require and subsequently reduces the number of chest radiographs for each patient. These patients also miss fewer feeds, with no appreciable increase in cost.
Keywords: nasogastric, bridle, enteral, COVID-19, intensive care, quality improvement, safety.
The COVID-19 pandemic has led to a large influx of patients to critical care units in the United Kingdom (UK) and across the world. Figures from the Intensive Care National Audit & Research Centre in May 2020 show that the median length of stay for COVID-19 survivors requiring invasive ventilatory support while on the intensive care unit (ICU) was 15 days.1 For these days at the very least, patients are completely reliant on enteral feeding in order to meet their nutritional requirements.The standard method of enteral feeding when a patient is sedated and ventilated is via a nasogastric tube (NGT). Incorrect placement of an NGT can have devastating consequences, including pneumothorax, fistula formation, ulceration, sepsis, and death. Between September 2011 and March 2016, the National Patient Safety Agency in the UK recorded 95 incidents of feeding into the respiratory tract as a result of incorrect NGT placement.2 With the onset of the pandemic, the prevalence of NGT misplacement increased, with the NHS Improvement team reporting 7 cases of misplaced NGTs within just 3 months (April 1, 2020, through June 30, 2020).3 With over 3 million nasogastric or orogastric tubes inserted each year in the UK, the risk of adverse events is very real.
NGT dislodgment is common, with 1 study putting this figure at 40%.4 Recurrent dislodgment of NGTs disrupts nutrition and may lead to the patient missing a feed in a time where nutrition is vital during acute illness. Research has showed that NGT bridling reduces the rate of dislodgment significantly (from 40% to 14%).5 Moreover, a 2018 systematic review looking specifically at NGT dislodgment found 10 out of 11 studies showed a significant reduction in dislodgment following use of a bridle kit.6 Bridling an NGT has been shown to significantly reduce the need for percutaneous endoscopic gastrostomy insertion.7 NGT bridle kits have already been used successfully in ICU burn patients, where sloughed skin makes securement particularly difficult with traditional methods.8 With each repeated insertion comes the risk of incorrect placement. COVID-19 ICU patients had specific risk factors for their NGTs becoming dislodged: duration of NGT feeding (in the ICU and on the ward), requirement for proning and de-proning, and post-emergence confusion related to long duration of sedation. Repeated NGT insertion comes with potential risks to the patient and staff, as well as a financial cost. Patient-specific risks include potential for incorrect placement, missed feedings, irradiation (from the patient’s own chest radiograph and from others), and discomfort from manual handling and repeat reinsertions. Staff risk factors include radiation scatter from portable radiographs (especially when dealing with more than 1 patient per bed space), manual handling, and increased pressure on radiographers. Finally, financial costs are related to the NGTs themselves as well as the portable chest radiograph, which our Superintendent Radiographer estimates to be £55 (US $73.86).
The objective of this study was to ascertain the extent of NGT dislodgment in COVID-19 ICU patients after the introduction of NGT bridle kits as a standard of practice and to determine whether this would reduce the number of NGT insertions, patient irradiation, missed feedings, and overall costs. With the introduction of bridle kits, incidence of pressure sores related to the bridle kit were also recorded.
Methods
Data were collected over 2 cycles, the first retrospectively and the second prospectively, once NGT bridle kits were introduced as an intervention.
Cycle 1. Analyzing the current standard of practice: regular NGT insertion with no use of bridle kit
Cycle 1 was done retrospectively, looking at 30 patient notes of COVID-19 patients admitted to the critical care unit (CCU) between March 11, 2020, and April 20, 2020, at Queen Elizabeth Hospital Birmingham, Birmingham, UK. All patients admitted to the ICU with COVID-19 requiring invasive ventilation were eligible for inclusion in the study. A total of 32 patients were admitted during this time; however, 2 patients were excluded due to NGTs being inserted prior to ICU admission.
Individual patient notes were searched for:
- days of feeding required during their inpatient stay (this included NGT feeding on the ward post-ICU discharge).
- hours of feeding missed while waiting for NGT reinsertion or chest radiograph due to dislodged or displaced NGTs (during the entire period of enteral feeding, ICU, and ward).
- number of NGT insertions.
- number of chest radiographs purely for NGT position.
Each patient’s first day of feeding and NGT insertion were noted. Following that, the patient electronic note system, the Prescribing Information and Communication System, was used to look for any further chest radiograph requests, which were primarily for NGT position. Using the date and time, the “critical care observations” tab was used to look at fluids and to calculate how long NGT feeding was stopped while NGT position-check x-rays were being awaited. The notes were also checked at this date and time to work out whether a new NGT was inserted or whether an existing tube had been dislodged (if not evident from the x-ray request). Data collection was stopped once either of the following occurred:
- patient no longer required NGT feeding.
- patient was transferred to another hospital.
- death.
The cost of the NGT was averaged between the cost of size 8 and 12, which worked out to be £10 (US $13.43). As mentioned earlier, each radiograph cost was determined by the Superintendent Radiographer (£55).
Cycle 2. Implementing a change: introduction of NGT bridle kit (Applied Medical Technology Bridle) as standard of practice
The case notes of 54 patients admitted to the COVID-19 CCU at the Queen Elizabeth Hospital Birmingham, Birmingham, UK, were retrospectively reviewed between February 8, 2021, and April 17, 2021. The inclusion criteria consisted of: admitted to the CCU due to COVID-19, required NGT feeding, and was bridled on admission. Case notes were retrospectively reviewed for:
- Length of CCU stay
- Days of feeding required during the hospital stay
- Hours of feeding missed while waiting for a chest radiograph due to displaced NGTs
- Number of NGT insertions
- Number of chest radiographs to confirm NGT position
- Bridling of NGTs
- Documented pressure sores related to the bridle or NGT, or referrals for wound management advice (Tissue Viability Team) as a consequence of the NGT bridle
Results
Of the 54 patients admitted, 31 had their NGTs bridled. Data were collected as in the first cycle, with individual notes analyzed on the online system (Table). Additionally, notes were reviewed for documentation of pressure sores related to NGT bridling, and the “requests” tab as well as the “noting” function were used to identify referrals for “Wound Management Advice” (Tissue Viability Review).

The average length of stay for this ICU cohort was 17.6 days. This reiterates the reliance on NGT feeding of patients admitted to the CCU. The results from this project can be summarized as follows: The use of NGT bridle kits leads to a significant reduction in the total number of NGTs a patient requires during intensive care. As a result, there is a significant reduction in the number of chest radiographs required to confirm NGT position. Feedings missed can also be reduced by using a bridle kit. These advantages all come with no additional cost.
On average, bridled patients required 1.3 NGTs, compared to 2.5 before bridles were introduced. The fewer NGTs inserted, the less chance of an NGT-associated injury occurring.
The number of chest radiographs required to confirm NGT position after resiting also fell, from 3.4 to 1.6. This has numerous advantages. There is a financial savings of £99 (US $133.04) per patient from the reduced number of chest x-rays. Although this does not offset the price of the bridle kit itself, there are other less easily quantifiable costs that are reduced. For instance, patients are highly catabolic during severe infection, and their predominant energy source comes from their feedings. Missed feedings are associated with longer length of stay in the ICU and in the hospital in general.9 Bridle kits have the potential to reduce the number of missed feedings by ensuring the NGT remains in the correct position.
Discussion
Many of the results are aligned with what is already known in the literature. A meta-analysis from 2014 concluded that dislodgment is reduced with the use of a bridle kit.6 This change is what underpins many of the advantages seen, as an NGT that stays in place means additional radiographs are not required and feeding is not delayed.
COVID-19 critical care patients are very fragile and are dependent on ventilators for the majority of their stay. They are often on very high levels of ventilator support and moving the patient can lead to desaturation or difficulties in ventilation. Therefore, reduction in any manual handling occurring as a result of the need for portable chest radiographs minimizes the chances of further negative events. Furthermore, nursing staff, along with the radiographers, are often the ones who must move these patients in order for the x-ray film to be placed behind the patient. This task is not easy, especially with limited personnel, and has the potential to cause injuries to both patients and staff members.
The knock-on effect of reduced NGTs and x-rays is also a reduction of work for the portable radiography team, in what is a very time- and resource-consuming process of coming onto the COVID-19 CCU. Not only does the machine itself need to be wiped down thoroughly after use, but also the individual must use personal protective equipment (PPE) each time. There is a cost associated with PPE itself, as well as the time it takes to don and doff appropriately.
A reduction in chest radiographs reduces the irradiation of the patient and the potential irradiation of staff members. With bridling of the NGT, the radiation exposure is more than halved for the patient. Because the COVID ICU is often very busy, with patients in some cases being doubled up in a bed space, the scatter radiation is high. This can be reduced if fewer chest radiographs are required.
An additional benefit of a reduction in the mean number of NGT insertions per patient is also illustrated by anecdotal evidence. Over the studied period, we identified 2 traumatic pneumothoraces related to NGT insertion on the COVID-19 CCU, highlighting the potential risks of NGT insertion and the need to reduce its frequency, if possible.
One concern noted was that bridles could cause increased incidence of pressure sores. In the patients represented in this study, only 1 suffered a pressure sore (grade 2) directly related to the bridle. A subpopulation of patients not bridled was also noted. This was significantly smaller than the main group; however, we had noted 2 incidences of pressure sores from their standard NGT and securement devices. Some studies have alluded to the potential for increased skin complications with bridle kits; however, studies looking specifically at kits using umbilical tape (as in this study) show no significant increase in skin damage.10 This leaves us confident that there is no increased risk of pressure sores related to the bridling of patients when umbilical tape is used with the bridle kit.
NGT bridles require training to insert safely. With the introduction of bridling, our hospital’s nursing staff underwent training in order to be proficient with the bridle kits. This comes with a time commitment, and, like other equipment usage, it takes time to build confidence. However, in this study, there were no concerns raised from nursing staff regarding difficulty of insertion or the time taken to do so.
Our study adds an objective measure of the benefits provided by bridle kits. Not only was there a reduction in the number of NGT insertions required, but we were also able to show a significant reduction in the number of chest radiographs required as well in the amount of time feeding is missed. While apprehension regarding bridle kits may be focused on cost, this study has shown that the savings more than make up for the initial cost of the kit itself.
Although the patient demographics, systemic effects, and treatment of COVID-19 are similar between different ICUs, a single-center study does have limitations. One of these is the potential for an intervention in a single-center study to lead to a larger effect than that of multicenter studies.11 But as seen in previous studies, the dislodgment of NGTs is not just an issue in this ICU.12 COVID-19–specific risk factors for NGT dislodgment also apply to all patients requiring invasive ventilation and proning.
Identification of whether a new NGT was inserted, or whether the existing NGT was replaced following dislodging of an NGT, relied on accurate documentation by the relevant staff. The case notes did not always make this explicitly clear. Unlike other procedures commonly performed, documentation of NGT insertion is not formally done under the procedures heading, and, on occasion is not done at all. We recognize that manually searching notes only yields NGT insertions that have been formally documented. There is a potential for the number recorded to be lower than the actual number of NGTs inserted. However, when x-ray requests are cross-referenced with the notes, there is a significant degree of confidence that the vast majority of insertions are picked up.
One patient identified in the study required a Ryle’s tube as part of their critical care treatment. While similar in nature to an NGT, these are unable to fit into a bridle and are at increased risk of dislodging during the patient’s critical care stay. The intended benefit of the bridle kit does not therefore extend to patients with Ryle’s tubes.
Conclusion
The COVID-19 critical care population requires significant time on invasive ventilation and remains dependent on NGT feeding during this process. The risk of NGT dislodgment can be mitigated by using a bridle kit, as the number of NGT insertions a patient requires is significantly reduced. Not only does this reduce the risk of inadvertent misplacement but also has a cost savings, as well as increasing safety for staff and patients. From this study, the risk of pressure injuries is not significant. The benefit of NGT bridling may be extended to other non-COVID long-stay ICU patients.
Future research looking at the efficacy of bridle kits in larger patient groups will help confirm the benefits seen in this study and will also provide better information with regard to any long-term complications associated with bridles.
Corresponding author: Rajveer Atkar, MBBS, Queen Elizabeth Hospital Birmingham, Mindelsohn Way, Birmingham B15 2GW, United Kingdom; [email protected].
Financial disclosures: None.
From Queen Elizabeth Hospital Birmingham, Mindelsohn Way, Birmingham, United Kingdom.
Objective: To ascertain the extent of nasogastric tube (NGT) dislodgment in COVID-19 intensive care unit (ICU) patients after the introduction of NGT bridle kits as a standard of practice, to see whether this would reduce the number of NGT insertions, patient irradiation, missed feeds, and overall cost.
Background: Nasogastric feeding is the mainstay of enteral feeding for ICU patients. The usual standard of practice is to secure the tube using adhesive tape. Studies show this method has a 40% to 48% dislodgment rate. The COVID-19 ICU patient population may be at even greater risk due to the need for proning, long duration of invasive ventilation, and emergence delirium.
Design: This was a 2-cycle quality improvement project. The first cycle was done retrospectively, looking at the contemporaneous standard of practice where bridle kits were not used. This gave an objective measure of the extent of NGT displacement, associated costs, and missed feeds. The second cycle was carried out prospectively, with the use of NGT bridle kits as the new standard of practice.
Setting: A large United Kingdom teaching hospital with a 100-bed, single-floor ICU.
Participants: Patients admitted to the ICU with COVID-19 who subsequently required sedation and invasive ventilation.
Measurements: Measurements included days of feeding required, hours of feeding missed due to NGT dislodgment, total number of nasogastric tubes required per ICU stay, and number of chest radiographs for NGT position confirmation. NGT-related pressure sores were also recorded.
Results: When compared to the bridled group, the unbridled group required a higher number of NGTs (2.5 vs 1.3; P < .001) and chest radiographs (3.4 vs 1.6; P < .001), had more hours of feeding missed (11.8 vs 5.0), and accumulated a slightly higher total cost (cost of NGT, chest radiographs +/- bridle kit: £211.67 vs £210, [US $284.25 vs US $282.01]).
Conclusions: The use of NGT bridle kits reduces the number of NGT insertions patients require and subsequently reduces the number of chest radiographs for each patient. These patients also miss fewer feeds, with no appreciable increase in cost.
Keywords: nasogastric, bridle, enteral, COVID-19, intensive care, quality improvement, safety.
The COVID-19 pandemic has led to a large influx of patients to critical care units in the United Kingdom (UK) and across the world. Figures from the Intensive Care National Audit & Research Centre in May 2020 show that the median length of stay for COVID-19 survivors requiring invasive ventilatory support while on the intensive care unit (ICU) was 15 days.1 For these days at the very least, patients are completely reliant on enteral feeding in order to meet their nutritional requirements.The standard method of enteral feeding when a patient is sedated and ventilated is via a nasogastric tube (NGT). Incorrect placement of an NGT can have devastating consequences, including pneumothorax, fistula formation, ulceration, sepsis, and death. Between September 2011 and March 2016, the National Patient Safety Agency in the UK recorded 95 incidents of feeding into the respiratory tract as a result of incorrect NGT placement.2 With the onset of the pandemic, the prevalence of NGT misplacement increased, with the NHS Improvement team reporting 7 cases of misplaced NGTs within just 3 months (April 1, 2020, through June 30, 2020).3 With over 3 million nasogastric or orogastric tubes inserted each year in the UK, the risk of adverse events is very real.
NGT dislodgment is common, with 1 study putting this figure at 40%.4 Recurrent dislodgment of NGTs disrupts nutrition and may lead to the patient missing a feed in a time where nutrition is vital during acute illness. Research has showed that NGT bridling reduces the rate of dislodgment significantly (from 40% to 14%).5 Moreover, a 2018 systematic review looking specifically at NGT dislodgment found 10 out of 11 studies showed a significant reduction in dislodgment following use of a bridle kit.6 Bridling an NGT has been shown to significantly reduce the need for percutaneous endoscopic gastrostomy insertion.7 NGT bridle kits have already been used successfully in ICU burn patients, where sloughed skin makes securement particularly difficult with traditional methods.8 With each repeated insertion comes the risk of incorrect placement. COVID-19 ICU patients had specific risk factors for their NGTs becoming dislodged: duration of NGT feeding (in the ICU and on the ward), requirement for proning and de-proning, and post-emergence confusion related to long duration of sedation. Repeated NGT insertion comes with potential risks to the patient and staff, as well as a financial cost. Patient-specific risks include potential for incorrect placement, missed feedings, irradiation (from the patient’s own chest radiograph and from others), and discomfort from manual handling and repeat reinsertions. Staff risk factors include radiation scatter from portable radiographs (especially when dealing with more than 1 patient per bed space), manual handling, and increased pressure on radiographers. Finally, financial costs are related to the NGTs themselves as well as the portable chest radiograph, which our Superintendent Radiographer estimates to be £55 (US $73.86).
The objective of this study was to ascertain the extent of NGT dislodgment in COVID-19 ICU patients after the introduction of NGT bridle kits as a standard of practice and to determine whether this would reduce the number of NGT insertions, patient irradiation, missed feedings, and overall costs. With the introduction of bridle kits, incidence of pressure sores related to the bridle kit were also recorded.
Methods
Data were collected over 2 cycles, the first retrospectively and the second prospectively, once NGT bridle kits were introduced as an intervention.
Cycle 1. Analyzing the current standard of practice: regular NGT insertion with no use of bridle kit
Cycle 1 was done retrospectively, looking at 30 patient notes of COVID-19 patients admitted to the critical care unit (CCU) between March 11, 2020, and April 20, 2020, at Queen Elizabeth Hospital Birmingham, Birmingham, UK. All patients admitted to the ICU with COVID-19 requiring invasive ventilation were eligible for inclusion in the study. A total of 32 patients were admitted during this time; however, 2 patients were excluded due to NGTs being inserted prior to ICU admission.
Individual patient notes were searched for:
- days of feeding required during their inpatient stay (this included NGT feeding on the ward post-ICU discharge).
- hours of feeding missed while waiting for NGT reinsertion or chest radiograph due to dislodged or displaced NGTs (during the entire period of enteral feeding, ICU, and ward).
- number of NGT insertions.
- number of chest radiographs purely for NGT position.
Each patient’s first day of feeding and NGT insertion were noted. Following that, the patient electronic note system, the Prescribing Information and Communication System, was used to look for any further chest radiograph requests, which were primarily for NGT position. Using the date and time, the “critical care observations” tab was used to look at fluids and to calculate how long NGT feeding was stopped while NGT position-check x-rays were being awaited. The notes were also checked at this date and time to work out whether a new NGT was inserted or whether an existing tube had been dislodged (if not evident from the x-ray request). Data collection was stopped once either of the following occurred:
- patient no longer required NGT feeding.
- patient was transferred to another hospital.
- death.
The cost of the NGT was averaged between the cost of size 8 and 12, which worked out to be £10 (US $13.43). As mentioned earlier, each radiograph cost was determined by the Superintendent Radiographer (£55).
Cycle 2. Implementing a change: introduction of NGT bridle kit (Applied Medical Technology Bridle) as standard of practice
The case notes of 54 patients admitted to the COVID-19 CCU at the Queen Elizabeth Hospital Birmingham, Birmingham, UK, were retrospectively reviewed between February 8, 2021, and April 17, 2021. The inclusion criteria consisted of: admitted to the CCU due to COVID-19, required NGT feeding, and was bridled on admission. Case notes were retrospectively reviewed for:
- Length of CCU stay
- Days of feeding required during the hospital stay
- Hours of feeding missed while waiting for a chest radiograph due to displaced NGTs
- Number of NGT insertions
- Number of chest radiographs to confirm NGT position
- Bridling of NGTs
- Documented pressure sores related to the bridle or NGT, or referrals for wound management advice (Tissue Viability Team) as a consequence of the NGT bridle
Results
Of the 54 patients admitted, 31 had their NGTs bridled. Data were collected as in the first cycle, with individual notes analyzed on the online system (Table). Additionally, notes were reviewed for documentation of pressure sores related to NGT bridling, and the “requests” tab as well as the “noting” function were used to identify referrals for “Wound Management Advice” (Tissue Viability Review).

The average length of stay for this ICU cohort was 17.6 days. This reiterates the reliance on NGT feeding of patients admitted to the CCU. The results from this project can be summarized as follows: The use of NGT bridle kits leads to a significant reduction in the total number of NGTs a patient requires during intensive care. As a result, there is a significant reduction in the number of chest radiographs required to confirm NGT position. Feedings missed can also be reduced by using a bridle kit. These advantages all come with no additional cost.
On average, bridled patients required 1.3 NGTs, compared to 2.5 before bridles were introduced. The fewer NGTs inserted, the less chance of an NGT-associated injury occurring.
The number of chest radiographs required to confirm NGT position after resiting also fell, from 3.4 to 1.6. This has numerous advantages. There is a financial savings of £99 (US $133.04) per patient from the reduced number of chest x-rays. Although this does not offset the price of the bridle kit itself, there are other less easily quantifiable costs that are reduced. For instance, patients are highly catabolic during severe infection, and their predominant energy source comes from their feedings. Missed feedings are associated with longer length of stay in the ICU and in the hospital in general.9 Bridle kits have the potential to reduce the number of missed feedings by ensuring the NGT remains in the correct position.
Discussion
Many of the results are aligned with what is already known in the literature. A meta-analysis from 2014 concluded that dislodgment is reduced with the use of a bridle kit.6 This change is what underpins many of the advantages seen, as an NGT that stays in place means additional radiographs are not required and feeding is not delayed.
COVID-19 critical care patients are very fragile and are dependent on ventilators for the majority of their stay. They are often on very high levels of ventilator support and moving the patient can lead to desaturation or difficulties in ventilation. Therefore, reduction in any manual handling occurring as a result of the need for portable chest radiographs minimizes the chances of further negative events. Furthermore, nursing staff, along with the radiographers, are often the ones who must move these patients in order for the x-ray film to be placed behind the patient. This task is not easy, especially with limited personnel, and has the potential to cause injuries to both patients and staff members.
The knock-on effect of reduced NGTs and x-rays is also a reduction of work for the portable radiography team, in what is a very time- and resource-consuming process of coming onto the COVID-19 CCU. Not only does the machine itself need to be wiped down thoroughly after use, but also the individual must use personal protective equipment (PPE) each time. There is a cost associated with PPE itself, as well as the time it takes to don and doff appropriately.
A reduction in chest radiographs reduces the irradiation of the patient and the potential irradiation of staff members. With bridling of the NGT, the radiation exposure is more than halved for the patient. Because the COVID ICU is often very busy, with patients in some cases being doubled up in a bed space, the scatter radiation is high. This can be reduced if fewer chest radiographs are required.
An additional benefit of a reduction in the mean number of NGT insertions per patient is also illustrated by anecdotal evidence. Over the studied period, we identified 2 traumatic pneumothoraces related to NGT insertion on the COVID-19 CCU, highlighting the potential risks of NGT insertion and the need to reduce its frequency, if possible.
One concern noted was that bridles could cause increased incidence of pressure sores. In the patients represented in this study, only 1 suffered a pressure sore (grade 2) directly related to the bridle. A subpopulation of patients not bridled was also noted. This was significantly smaller than the main group; however, we had noted 2 incidences of pressure sores from their standard NGT and securement devices. Some studies have alluded to the potential for increased skin complications with bridle kits; however, studies looking specifically at kits using umbilical tape (as in this study) show no significant increase in skin damage.10 This leaves us confident that there is no increased risk of pressure sores related to the bridling of patients when umbilical tape is used with the bridle kit.
NGT bridles require training to insert safely. With the introduction of bridling, our hospital’s nursing staff underwent training in order to be proficient with the bridle kits. This comes with a time commitment, and, like other equipment usage, it takes time to build confidence. However, in this study, there were no concerns raised from nursing staff regarding difficulty of insertion or the time taken to do so.
Our study adds an objective measure of the benefits provided by bridle kits. Not only was there a reduction in the number of NGT insertions required, but we were also able to show a significant reduction in the number of chest radiographs required as well in the amount of time feeding is missed. While apprehension regarding bridle kits may be focused on cost, this study has shown that the savings more than make up for the initial cost of the kit itself.
Although the patient demographics, systemic effects, and treatment of COVID-19 are similar between different ICUs, a single-center study does have limitations. One of these is the potential for an intervention in a single-center study to lead to a larger effect than that of multicenter studies.11 But as seen in previous studies, the dislodgment of NGTs is not just an issue in this ICU.12 COVID-19–specific risk factors for NGT dislodgment also apply to all patients requiring invasive ventilation and proning.
Identification of whether a new NGT was inserted, or whether the existing NGT was replaced following dislodging of an NGT, relied on accurate documentation by the relevant staff. The case notes did not always make this explicitly clear. Unlike other procedures commonly performed, documentation of NGT insertion is not formally done under the procedures heading, and, on occasion is not done at all. We recognize that manually searching notes only yields NGT insertions that have been formally documented. There is a potential for the number recorded to be lower than the actual number of NGTs inserted. However, when x-ray requests are cross-referenced with the notes, there is a significant degree of confidence that the vast majority of insertions are picked up.
One patient identified in the study required a Ryle’s tube as part of their critical care treatment. While similar in nature to an NGT, these are unable to fit into a bridle and are at increased risk of dislodging during the patient’s critical care stay. The intended benefit of the bridle kit does not therefore extend to patients with Ryle’s tubes.
Conclusion
The COVID-19 critical care population requires significant time on invasive ventilation and remains dependent on NGT feeding during this process. The risk of NGT dislodgment can be mitigated by using a bridle kit, as the number of NGT insertions a patient requires is significantly reduced. Not only does this reduce the risk of inadvertent misplacement but also has a cost savings, as well as increasing safety for staff and patients. From this study, the risk of pressure injuries is not significant. The benefit of NGT bridling may be extended to other non-COVID long-stay ICU patients.
Future research looking at the efficacy of bridle kits in larger patient groups will help confirm the benefits seen in this study and will also provide better information with regard to any long-term complications associated with bridles.
Corresponding author: Rajveer Atkar, MBBS, Queen Elizabeth Hospital Birmingham, Mindelsohn Way, Birmingham B15 2GW, United Kingdom; [email protected].
Financial disclosures: None.
1. Intensive Care National Audit & Research Centre. ICNARC report on COVID-19 in critical care 15 May 2020. https://www.icnarc.org/DataServices/Attachments/Download/cbcb6217-f698-ea11-9125-00505601089b
2. NHS. Nasogastric tube misplacement: continuing risk of death and severe harm. July 22, 2016. https://www.england.nhs.uk/2016/07/nasogastric-tube-misplacement-continuing-risk-of-death-severe-harm/
3. NHS. Provisional publication of never events reported as occurring between 1 April and 30 June 2020. https://www.england.nhs.uk/wp-content/uploads/2020/08/Provisional_publication_-_NE_1_April_-_30_June_2020.pdf
4. Meer JA. Inadvertent dislodgement of nasoenteral feeding tubes: incidence and prevention. JPEN J Parenter Enteral Nutr. 1987;11(2):187- 189. doi:10.1177/0148607187011002187
5. Bechtold ML, Nguyen DL, Palmer L, et al. Nasal bridles for securing nasoenteric tubes: a meta-analysis. Nutr Clin Pract. 2014;29(5):667-671. doi:10.1177/0884533614536737
6. Lynch A, Tang CS, Jeganathan LS, Rockey JG. A systematic review of the effectiveness and complications of using nasal bridles to secure nasoenteral feeding tubes. Aust J Otolaryngol. 2018;1:8. doi:10.21037/ajo.2018.01.01
7. Johnston R, O’Dell L, Patrick M, Cole OT, Cunliffe N. Outcome of patients fed via a nasogastric tube retained with a bridle loop: Do bridle loops reduce the requirement for percutaneous endoscopic gastrostomy insertion and 30-day mortality? Proc Nutr Soc. 2008;67:E116. doi:10.1017/S0029665108007489
8. Li AY, Rustad KC, Long C, et al. Reduced incidence of feeding tube dislodgement and missed feeds in burn patients with nasal bridle securement. Burns. 2018;44(5):1203-1209. doi:10.1016/j.burns.2017.05.025
9. Peev MP, Yeh DD, Quraishi SA, et al. Causes and consequences of interrupted enteral nutrition: a prospective observational study in critically ill surgical patients. JPEN J Parenter Enteral Nutr. 2015;39(1):21-27. doi:10.1177/0148607114526887
10. Seder CW, Janczyk R. The routine bridling of nasjejunal tubes is a safe and effective method of reducing dislodgement in the intensive care unit. Nutr Clin Pract. 2008;23(6):651-654. doi:10.1177/0148607114526887
11. Dechartres A, Boutron I, Trinquart L, Charles P, Ravaud P. Single-center trials show larger treatment effects than multicenter trials: evidence from a meta-epidemiologic study. Ann Intern Med. 2011;155:39-51. doi:10.7326/0003-4819-155-1-201107050-00006
12. Morton B, Hall R, Ridgway T, Al-Rawi O. Nasogastric tube dislodgement: a problem on our ICU. Crit Care. 2013;17(suppl 2):P242. doi:10.1186/cc12180
1. Intensive Care National Audit & Research Centre. ICNARC report on COVID-19 in critical care 15 May 2020. https://www.icnarc.org/DataServices/Attachments/Download/cbcb6217-f698-ea11-9125-00505601089b
2. NHS. Nasogastric tube misplacement: continuing risk of death and severe harm. July 22, 2016. https://www.england.nhs.uk/2016/07/nasogastric-tube-misplacement-continuing-risk-of-death-severe-harm/
3. NHS. Provisional publication of never events reported as occurring between 1 April and 30 June 2020. https://www.england.nhs.uk/wp-content/uploads/2020/08/Provisional_publication_-_NE_1_April_-_30_June_2020.pdf
4. Meer JA. Inadvertent dislodgement of nasoenteral feeding tubes: incidence and prevention. JPEN J Parenter Enteral Nutr. 1987;11(2):187- 189. doi:10.1177/0148607187011002187
5. Bechtold ML, Nguyen DL, Palmer L, et al. Nasal bridles for securing nasoenteric tubes: a meta-analysis. Nutr Clin Pract. 2014;29(5):667-671. doi:10.1177/0884533614536737
6. Lynch A, Tang CS, Jeganathan LS, Rockey JG. A systematic review of the effectiveness and complications of using nasal bridles to secure nasoenteral feeding tubes. Aust J Otolaryngol. 2018;1:8. doi:10.21037/ajo.2018.01.01
7. Johnston R, O’Dell L, Patrick M, Cole OT, Cunliffe N. Outcome of patients fed via a nasogastric tube retained with a bridle loop: Do bridle loops reduce the requirement for percutaneous endoscopic gastrostomy insertion and 30-day mortality? Proc Nutr Soc. 2008;67:E116. doi:10.1017/S0029665108007489
8. Li AY, Rustad KC, Long C, et al. Reduced incidence of feeding tube dislodgement and missed feeds in burn patients with nasal bridle securement. Burns. 2018;44(5):1203-1209. doi:10.1016/j.burns.2017.05.025
9. Peev MP, Yeh DD, Quraishi SA, et al. Causes and consequences of interrupted enteral nutrition: a prospective observational study in critically ill surgical patients. JPEN J Parenter Enteral Nutr. 2015;39(1):21-27. doi:10.1177/0148607114526887
10. Seder CW, Janczyk R. The routine bridling of nasjejunal tubes is a safe and effective method of reducing dislodgement in the intensive care unit. Nutr Clin Pract. 2008;23(6):651-654. doi:10.1177/0148607114526887
11. Dechartres A, Boutron I, Trinquart L, Charles P, Ravaud P. Single-center trials show larger treatment effects than multicenter trials: evidence from a meta-epidemiologic study. Ann Intern Med. 2011;155:39-51. doi:10.7326/0003-4819-155-1-201107050-00006
12. Morton B, Hall R, Ridgway T, Al-Rawi O. Nasogastric tube dislodgement: a problem on our ICU. Crit Care. 2013;17(suppl 2):P242. doi:10.1186/cc12180
Children and COVID: New cases increase for third straight week
There were almost 142,000 new cases reported during the week of Nov. 12-18, marking an increase of 16% over the previous week and the 15th straight week with a weekly total over 100,000, the American Academy of Pediatrics and the Children’s Hospital Association said.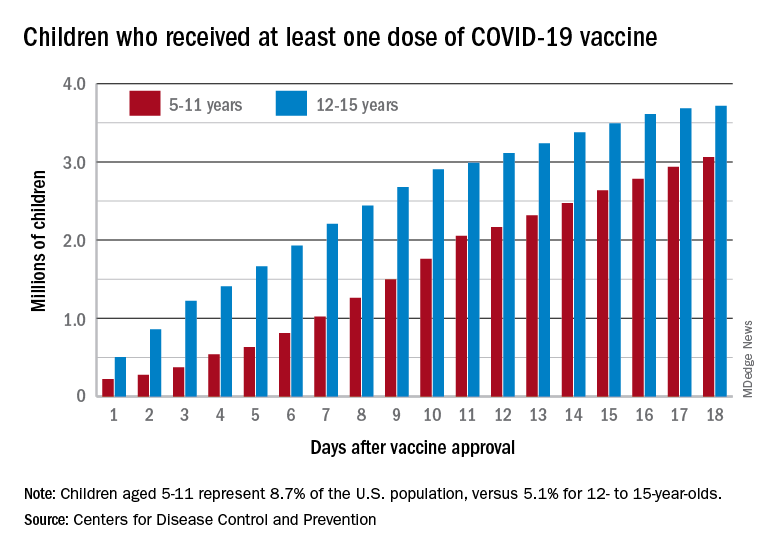
Regional data show that the Midwest has experienced the largest share of this latest surge, followed by the Northeast. Cases increased in the South during the week of Nov. 12-18 after holding steady over the previous 2 weeks, while new cases in the West dropped in the last week. At the state level, Maine, New Hampshire, and Vermont again reported the largest percent increases, with Michigan, Minnesota, and New Mexico also above average, the AAP and CHA said in their weekly COVID report.
Data from the Centers for Disease Control and Prevention show similar trends for both emergency department visits and hospital admissions, as both have risen in November after declines that began in late August and early September.
The cumulative number of pediatric cases is 6.77 million since the pandemic began, based on the AAP/CHA accounting of state cases, although Alabama, Nebraska, and Texas stopped reporting over the summer, suggesting the actual number is higher. The CDC puts the total number of COVID cases in children at 5.96 million, but there are age discrepancies between the CDC and the AAP/CHA’s state-based data.
The vaccine gap is closing
Vaccinations among the recently eligible 5- to 11-year-olds have steadily increased following a somewhat slow start. The initial pace was behind that of the 12- to 15-years-olds through the first postapproval week but has since closed the gap, based on data from the CDC’s COVID Data Tracker.
The tally of children who received at least one dose of the COVID vaccine among the 5- to 11-year-olds was behind the older group by almost 1.2 million on day 7 after the CDC’s Nov. 2 approval, but by day 18 the deficit was down to about 650,000, the CDC reported.
Altogether, just over 3 million children aged 5-11 have received at least one dose, which is 10.7% of that age group’s total population. Among children aged 12-17, the proportions are 60.7% with at least one dose and 51.1% at full vaccination. Children aged 5-11, who make up 8.7% of the total U.S. population, represented 42.8% of all vaccinations initiated over the 2 weeks ending Nov. 21, compared with 4.2% for those aged 12-17, the CDC said.
There were almost 142,000 new cases reported during the week of Nov. 12-18, marking an increase of 16% over the previous week and the 15th straight week with a weekly total over 100,000, the American Academy of Pediatrics and the Children’s Hospital Association said.
Regional data show that the Midwest has experienced the largest share of this latest surge, followed by the Northeast. Cases increased in the South during the week of Nov. 12-18 after holding steady over the previous 2 weeks, while new cases in the West dropped in the last week. At the state level, Maine, New Hampshire, and Vermont again reported the largest percent increases, with Michigan, Minnesota, and New Mexico also above average, the AAP and CHA said in their weekly COVID report.
Data from the Centers for Disease Control and Prevention show similar trends for both emergency department visits and hospital admissions, as both have risen in November after declines that began in late August and early September.
The cumulative number of pediatric cases is 6.77 million since the pandemic began, based on the AAP/CHA accounting of state cases, although Alabama, Nebraska, and Texas stopped reporting over the summer, suggesting the actual number is higher. The CDC puts the total number of COVID cases in children at 5.96 million, but there are age discrepancies between the CDC and the AAP/CHA’s state-based data.
The vaccine gap is closing
Vaccinations among the recently eligible 5- to 11-year-olds have steadily increased following a somewhat slow start. The initial pace was behind that of the 12- to 15-years-olds through the first postapproval week but has since closed the gap, based on data from the CDC’s COVID Data Tracker.
The tally of children who received at least one dose of the COVID vaccine among the 5- to 11-year-olds was behind the older group by almost 1.2 million on day 7 after the CDC’s Nov. 2 approval, but by day 18 the deficit was down to about 650,000, the CDC reported.
Altogether, just over 3 million children aged 5-11 have received at least one dose, which is 10.7% of that age group’s total population. Among children aged 12-17, the proportions are 60.7% with at least one dose and 51.1% at full vaccination. Children aged 5-11, who make up 8.7% of the total U.S. population, represented 42.8% of all vaccinations initiated over the 2 weeks ending Nov. 21, compared with 4.2% for those aged 12-17, the CDC said.
There were almost 142,000 new cases reported during the week of Nov. 12-18, marking an increase of 16% over the previous week and the 15th straight week with a weekly total over 100,000, the American Academy of Pediatrics and the Children’s Hospital Association said.
Regional data show that the Midwest has experienced the largest share of this latest surge, followed by the Northeast. Cases increased in the South during the week of Nov. 12-18 after holding steady over the previous 2 weeks, while new cases in the West dropped in the last week. At the state level, Maine, New Hampshire, and Vermont again reported the largest percent increases, with Michigan, Minnesota, and New Mexico also above average, the AAP and CHA said in their weekly COVID report.
Data from the Centers for Disease Control and Prevention show similar trends for both emergency department visits and hospital admissions, as both have risen in November after declines that began in late August and early September.
The cumulative number of pediatric cases is 6.77 million since the pandemic began, based on the AAP/CHA accounting of state cases, although Alabama, Nebraska, and Texas stopped reporting over the summer, suggesting the actual number is higher. The CDC puts the total number of COVID cases in children at 5.96 million, but there are age discrepancies between the CDC and the AAP/CHA’s state-based data.
The vaccine gap is closing
Vaccinations among the recently eligible 5- to 11-year-olds have steadily increased following a somewhat slow start. The initial pace was behind that of the 12- to 15-years-olds through the first postapproval week but has since closed the gap, based on data from the CDC’s COVID Data Tracker.
The tally of children who received at least one dose of the COVID vaccine among the 5- to 11-year-olds was behind the older group by almost 1.2 million on day 7 after the CDC’s Nov. 2 approval, but by day 18 the deficit was down to about 650,000, the CDC reported.
Altogether, just over 3 million children aged 5-11 have received at least one dose, which is 10.7% of that age group’s total population. Among children aged 12-17, the proportions are 60.7% with at least one dose and 51.1% at full vaccination. Children aged 5-11, who make up 8.7% of the total U.S. population, represented 42.8% of all vaccinations initiated over the 2 weeks ending Nov. 21, compared with 4.2% for those aged 12-17, the CDC said.
HCV screening in pregnancy: Reducing the risk for casualties in the quest for elimination
Because hepatitis C virus (HCV) infection is typically asymptomatic, its presence can easily be overlooked without appropriate screening efforts. For those screening efforts to be effective, they must keep pace with the changing demographic face of this increasingly prevalent but treatable disease.
Perhaps the most dramatic shift in HCV demographics in recent years has been the increase of infections among those born after 1965, a trend primarily driven by the opioid epidemic. In addition, data from the National Notifiable Diseases Surveillance System show that cases of diagnosed HCV doubled among women of childbearing age from 2006 to 2014, with new infections in younger women surpassing those in older age groups.
With such trends in mind, the Centers for Disease Control and Prevention broadened their recommendations regarding HCV in 2020 to include one-time testing in all adults aged 18 years and older and screening of all pregnant women during each pregnancy, except where the prevalence of infection is less than 0.1%, a threshold that no state has yet achieved.
The US Preventive Services Task Force (USPSTF) subsequently followed suit in their own recommendations.
The American Association for the Study of Liver Diseases/Infectious Diseases Society of America have long advocated for extensive expansion in their screening recommendations for HCV, including pregnancy.
Although the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine did not immediately adopt these recommendations, they have since endorsed them in May 2021 and June 2021, respectively.
The hepatologist perspective
As a practicing hepatologist, this seems like an uncontroversial recommendation. Obstetricians already screen for hepatitis B virus in each pregnancy. It should be easy to add HCV testing to the same lab testing.
Risk-based screening has repeatedly been demonstrated to be ineffective. It should be easier to test all women than to ask prying questions about high-risk behaviors.
Given the increase of injection drug use and resultant HCV infections in women of childbearing age, this seems like a perfect opportunity to identify chronically infected women and counsel them on transmission and cure. And pregnancy is also unique in that it is a time of near-universal health coverage.
Let’s address some of the operational issues.
The diagnostic cascade for HCV can be made very easy. HCV antibody testing is our standard screening test and, when positive, can automatically reflex to HCV polymerase chain reaction (PCR), the diagnostic test. Thus, with one blood sample, you can both screen for and diagnose infection.
Current guidelines do not recommend treating HCV during pregnancy, although therapy can be considered on an individual basis. Linkage to a knowledgeable provider who can discuss transmission and treatment, as well as assess the stage of liver injury, should decrease the burden on the ob.gyn.
The impact on pregnancy is marginal. HCV should not change either the mode of delivery or the decision to breastfeed. The AASLD/IDSA guidance outlines only four recommendations for monitoring during pregnancy:
- Obtain HCV RNA to see whether the infection is active and assess liver function at initiation of prenatal care.
- Prenatal care should be tailored to the pregnancy. There is no modification recommended to decrease mother-to-child transmission (MTCT).
- Be aware that intrahepatic is more common with HCV.
- Women with have a higher rate of adverse outcomes and should be linked to a high-risk obstetrics specialist.
But of course, what seems easy to one specialist may not be true of another. With that in mind, let’s hear the ob.gyn. perspective on these updated screening recommendations.
The ob.gyn. perspective
Recent guidelines from the CDC, ACOG, and SMFM recommend universal screening for HCV in all pregnant women. The increased availability of highly effective antiviral regimens makes universal screening a logical strategy, especially to identify candidates for this curative treatment. What is questionable, however, is the recommended timing by which this screening should take place.
HCV screening during pregnancy, as currently recommended, provides no immediate benefit for the pregnant woman or the fetus/neonate, given that antiviral treatments have not been approved during gestation, and there are no known measures that decrease MTCT or change routine perinatal care.
We also must not forget that a significant proportion of women in the United States, particularly those with limited resources, do not receive prenatal care at all. Most of them, however, will present to a hospital for delivery. Consequently, compliance with screening might be higher if performed at the time of delivery rather than antepartum.
Deferring screening until the intrapartum or immediate postpartum period, at least until antiviral treatment during pregnancy becomes a reality, was discussed. The rationale was that this approach might obviate the need to deal with the unintended consequences and burden of testing for HCV during pregnancy. Ultimately, ACOG and SMFM fell in line with the CDC recommendations.
Despite the lack of robust evidence regarding the risk for MTCT associated with commonly performed obstetric procedures (for example, genetic amniocentesis, artificial rupture of the membranes during labor, placement of an intrauterine pressure catheter), clinicians may be reluctant to perform them in HCV-infected women, resulting in potential deviations from the obstetric standard of care.
Similarly, it is likely that patients may choose to have a cesarean delivery for the sole purpose of decreasing MTCT, despite the lack of evidence for this. Such ill-advised patient-driven decisions are increasingly likely in the current environment, where social media can rapidly disseminate misinformation.
Implications for pediatric patients
One cannot isolate HCV screening in pregnancy from the consequences that may potentially occur as part of the infant’s transition to the care of a pediatrician.
Even though MTCT is estimated to occur in just 5%-15% of cases, all children born to HCV viremic mothers should be screened for HCV.
Traditionally, screening for HCV antibodies occurred after 18 months of age. In those who test positive, HCV PCR testing is recommended at 3 years. However, this algorithm is being called into question because only approximately one-third of infants are successfully screened.
HCV RNA testing in the first year after birth has been suggested. However, even proponents of this approach concur that all management decisions should be deferred until after the age of 3 years, when medications are approved for pediatric use.
In addition, HCV testing would be required again before considering therapy because children have higher rates of spontaneous clearance.
Seeking consensus beyond the controversy
Controversy remains surrounding the most recent update to the HCV screening guidelines. The current recommendation to screen during pregnancy cannot modify the risk for MTCT, has no impact on decisions regarding mode of delivery or breastfeeding, and could potentially cause harm by making obstetricians defer necessary invasive procedures even though there are no data linking them to an increase in MTCT.
Yet after extensive debate, the CDC, USPSTF, AASLD/IDSA, ACOG, and SMFM all developed their current recommendations to initiate HCV screening during pregnancy. To make this successful, screening algorithms need to be simple and consistent across all society recommendations.
HCV antibody testing should always reflex to the diagnostic test (HCV PCR) to allow confirmation in those who test positive without requiring an additional blood test. Viremic mothers (those who are HCV positive on PCR) should be linked to a provider who can discuss prognosis, transmission, and treatment. The importance of screening the infant also must be communicated to the parents and pediatrician alike.
Dr. Reau has served as a director, officer, partner, employee, adviser, consultant, or trustee for AbbVie, Gilead, Arbutus, Intercept, and Salix; received research grants from AbbVie and Gilead; and received income from AASLD. Dr. Pacheco disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Because hepatitis C virus (HCV) infection is typically asymptomatic, its presence can easily be overlooked without appropriate screening efforts. For those screening efforts to be effective, they must keep pace with the changing demographic face of this increasingly prevalent but treatable disease.
Perhaps the most dramatic shift in HCV demographics in recent years has been the increase of infections among those born after 1965, a trend primarily driven by the opioid epidemic. In addition, data from the National Notifiable Diseases Surveillance System show that cases of diagnosed HCV doubled among women of childbearing age from 2006 to 2014, with new infections in younger women surpassing those in older age groups.
With such trends in mind, the Centers for Disease Control and Prevention broadened their recommendations regarding HCV in 2020 to include one-time testing in all adults aged 18 years and older and screening of all pregnant women during each pregnancy, except where the prevalence of infection is less than 0.1%, a threshold that no state has yet achieved.
The US Preventive Services Task Force (USPSTF) subsequently followed suit in their own recommendations.
The American Association for the Study of Liver Diseases/Infectious Diseases Society of America have long advocated for extensive expansion in their screening recommendations for HCV, including pregnancy.
Although the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine did not immediately adopt these recommendations, they have since endorsed them in May 2021 and June 2021, respectively.
The hepatologist perspective
As a practicing hepatologist, this seems like an uncontroversial recommendation. Obstetricians already screen for hepatitis B virus in each pregnancy. It should be easy to add HCV testing to the same lab testing.
Risk-based screening has repeatedly been demonstrated to be ineffective. It should be easier to test all women than to ask prying questions about high-risk behaviors.
Given the increase of injection drug use and resultant HCV infections in women of childbearing age, this seems like a perfect opportunity to identify chronically infected women and counsel them on transmission and cure. And pregnancy is also unique in that it is a time of near-universal health coverage.
Let’s address some of the operational issues.
The diagnostic cascade for HCV can be made very easy. HCV antibody testing is our standard screening test and, when positive, can automatically reflex to HCV polymerase chain reaction (PCR), the diagnostic test. Thus, with one blood sample, you can both screen for and diagnose infection.
Current guidelines do not recommend treating HCV during pregnancy, although therapy can be considered on an individual basis. Linkage to a knowledgeable provider who can discuss transmission and treatment, as well as assess the stage of liver injury, should decrease the burden on the ob.gyn.
The impact on pregnancy is marginal. HCV should not change either the mode of delivery or the decision to breastfeed. The AASLD/IDSA guidance outlines only four recommendations for monitoring during pregnancy:
- Obtain HCV RNA to see whether the infection is active and assess liver function at initiation of prenatal care.
- Prenatal care should be tailored to the pregnancy. There is no modification recommended to decrease mother-to-child transmission (MTCT).
- Be aware that intrahepatic is more common with HCV.
- Women with have a higher rate of adverse outcomes and should be linked to a high-risk obstetrics specialist.
But of course, what seems easy to one specialist may not be true of another. With that in mind, let’s hear the ob.gyn. perspective on these updated screening recommendations.
The ob.gyn. perspective
Recent guidelines from the CDC, ACOG, and SMFM recommend universal screening for HCV in all pregnant women. The increased availability of highly effective antiviral regimens makes universal screening a logical strategy, especially to identify candidates for this curative treatment. What is questionable, however, is the recommended timing by which this screening should take place.
HCV screening during pregnancy, as currently recommended, provides no immediate benefit for the pregnant woman or the fetus/neonate, given that antiviral treatments have not been approved during gestation, and there are no known measures that decrease MTCT or change routine perinatal care.
We also must not forget that a significant proportion of women in the United States, particularly those with limited resources, do not receive prenatal care at all. Most of them, however, will present to a hospital for delivery. Consequently, compliance with screening might be higher if performed at the time of delivery rather than antepartum.
Deferring screening until the intrapartum or immediate postpartum period, at least until antiviral treatment during pregnancy becomes a reality, was discussed. The rationale was that this approach might obviate the need to deal with the unintended consequences and burden of testing for HCV during pregnancy. Ultimately, ACOG and SMFM fell in line with the CDC recommendations.
Despite the lack of robust evidence regarding the risk for MTCT associated with commonly performed obstetric procedures (for example, genetic amniocentesis, artificial rupture of the membranes during labor, placement of an intrauterine pressure catheter), clinicians may be reluctant to perform them in HCV-infected women, resulting in potential deviations from the obstetric standard of care.
Similarly, it is likely that patients may choose to have a cesarean delivery for the sole purpose of decreasing MTCT, despite the lack of evidence for this. Such ill-advised patient-driven decisions are increasingly likely in the current environment, where social media can rapidly disseminate misinformation.
Implications for pediatric patients
One cannot isolate HCV screening in pregnancy from the consequences that may potentially occur as part of the infant’s transition to the care of a pediatrician.
Even though MTCT is estimated to occur in just 5%-15% of cases, all children born to HCV viremic mothers should be screened for HCV.
Traditionally, screening for HCV antibodies occurred after 18 months of age. In those who test positive, HCV PCR testing is recommended at 3 years. However, this algorithm is being called into question because only approximately one-third of infants are successfully screened.
HCV RNA testing in the first year after birth has been suggested. However, even proponents of this approach concur that all management decisions should be deferred until after the age of 3 years, when medications are approved for pediatric use.
In addition, HCV testing would be required again before considering therapy because children have higher rates of spontaneous clearance.
Seeking consensus beyond the controversy
Controversy remains surrounding the most recent update to the HCV screening guidelines. The current recommendation to screen during pregnancy cannot modify the risk for MTCT, has no impact on decisions regarding mode of delivery or breastfeeding, and could potentially cause harm by making obstetricians defer necessary invasive procedures even though there are no data linking them to an increase in MTCT.
Yet after extensive debate, the CDC, USPSTF, AASLD/IDSA, ACOG, and SMFM all developed their current recommendations to initiate HCV screening during pregnancy. To make this successful, screening algorithms need to be simple and consistent across all society recommendations.
HCV antibody testing should always reflex to the diagnostic test (HCV PCR) to allow confirmation in those who test positive without requiring an additional blood test. Viremic mothers (those who are HCV positive on PCR) should be linked to a provider who can discuss prognosis, transmission, and treatment. The importance of screening the infant also must be communicated to the parents and pediatrician alike.
Dr. Reau has served as a director, officer, partner, employee, adviser, consultant, or trustee for AbbVie, Gilead, Arbutus, Intercept, and Salix; received research grants from AbbVie and Gilead; and received income from AASLD. Dr. Pacheco disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Because hepatitis C virus (HCV) infection is typically asymptomatic, its presence can easily be overlooked without appropriate screening efforts. For those screening efforts to be effective, they must keep pace with the changing demographic face of this increasingly prevalent but treatable disease.
Perhaps the most dramatic shift in HCV demographics in recent years has been the increase of infections among those born after 1965, a trend primarily driven by the opioid epidemic. In addition, data from the National Notifiable Diseases Surveillance System show that cases of diagnosed HCV doubled among women of childbearing age from 2006 to 2014, with new infections in younger women surpassing those in older age groups.
With such trends in mind, the Centers for Disease Control and Prevention broadened their recommendations regarding HCV in 2020 to include one-time testing in all adults aged 18 years and older and screening of all pregnant women during each pregnancy, except where the prevalence of infection is less than 0.1%, a threshold that no state has yet achieved.
The US Preventive Services Task Force (USPSTF) subsequently followed suit in their own recommendations.
The American Association for the Study of Liver Diseases/Infectious Diseases Society of America have long advocated for extensive expansion in their screening recommendations for HCV, including pregnancy.
Although the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine did not immediately adopt these recommendations, they have since endorsed them in May 2021 and June 2021, respectively.
The hepatologist perspective
As a practicing hepatologist, this seems like an uncontroversial recommendation. Obstetricians already screen for hepatitis B virus in each pregnancy. It should be easy to add HCV testing to the same lab testing.
Risk-based screening has repeatedly been demonstrated to be ineffective. It should be easier to test all women than to ask prying questions about high-risk behaviors.
Given the increase of injection drug use and resultant HCV infections in women of childbearing age, this seems like a perfect opportunity to identify chronically infected women and counsel them on transmission and cure. And pregnancy is also unique in that it is a time of near-universal health coverage.
Let’s address some of the operational issues.
The diagnostic cascade for HCV can be made very easy. HCV antibody testing is our standard screening test and, when positive, can automatically reflex to HCV polymerase chain reaction (PCR), the diagnostic test. Thus, with one blood sample, you can both screen for and diagnose infection.
Current guidelines do not recommend treating HCV during pregnancy, although therapy can be considered on an individual basis. Linkage to a knowledgeable provider who can discuss transmission and treatment, as well as assess the stage of liver injury, should decrease the burden on the ob.gyn.
The impact on pregnancy is marginal. HCV should not change either the mode of delivery or the decision to breastfeed. The AASLD/IDSA guidance outlines only four recommendations for monitoring during pregnancy:
- Obtain HCV RNA to see whether the infection is active and assess liver function at initiation of prenatal care.
- Prenatal care should be tailored to the pregnancy. There is no modification recommended to decrease mother-to-child transmission (MTCT).
- Be aware that intrahepatic is more common with HCV.
- Women with have a higher rate of adverse outcomes and should be linked to a high-risk obstetrics specialist.
But of course, what seems easy to one specialist may not be true of another. With that in mind, let’s hear the ob.gyn. perspective on these updated screening recommendations.
The ob.gyn. perspective
Recent guidelines from the CDC, ACOG, and SMFM recommend universal screening for HCV in all pregnant women. The increased availability of highly effective antiviral regimens makes universal screening a logical strategy, especially to identify candidates for this curative treatment. What is questionable, however, is the recommended timing by which this screening should take place.
HCV screening during pregnancy, as currently recommended, provides no immediate benefit for the pregnant woman or the fetus/neonate, given that antiviral treatments have not been approved during gestation, and there are no known measures that decrease MTCT or change routine perinatal care.
We also must not forget that a significant proportion of women in the United States, particularly those with limited resources, do not receive prenatal care at all. Most of them, however, will present to a hospital for delivery. Consequently, compliance with screening might be higher if performed at the time of delivery rather than antepartum.
Deferring screening until the intrapartum or immediate postpartum period, at least until antiviral treatment during pregnancy becomes a reality, was discussed. The rationale was that this approach might obviate the need to deal with the unintended consequences and burden of testing for HCV during pregnancy. Ultimately, ACOG and SMFM fell in line with the CDC recommendations.
Despite the lack of robust evidence regarding the risk for MTCT associated with commonly performed obstetric procedures (for example, genetic amniocentesis, artificial rupture of the membranes during labor, placement of an intrauterine pressure catheter), clinicians may be reluctant to perform them in HCV-infected women, resulting in potential deviations from the obstetric standard of care.
Similarly, it is likely that patients may choose to have a cesarean delivery for the sole purpose of decreasing MTCT, despite the lack of evidence for this. Such ill-advised patient-driven decisions are increasingly likely in the current environment, where social media can rapidly disseminate misinformation.
Implications for pediatric patients
One cannot isolate HCV screening in pregnancy from the consequences that may potentially occur as part of the infant’s transition to the care of a pediatrician.
Even though MTCT is estimated to occur in just 5%-15% of cases, all children born to HCV viremic mothers should be screened for HCV.
Traditionally, screening for HCV antibodies occurred after 18 months of age. In those who test positive, HCV PCR testing is recommended at 3 years. However, this algorithm is being called into question because only approximately one-third of infants are successfully screened.
HCV RNA testing in the first year after birth has been suggested. However, even proponents of this approach concur that all management decisions should be deferred until after the age of 3 years, when medications are approved for pediatric use.
In addition, HCV testing would be required again before considering therapy because children have higher rates of spontaneous clearance.
Seeking consensus beyond the controversy
Controversy remains surrounding the most recent update to the HCV screening guidelines. The current recommendation to screen during pregnancy cannot modify the risk for MTCT, has no impact on decisions regarding mode of delivery or breastfeeding, and could potentially cause harm by making obstetricians defer necessary invasive procedures even though there are no data linking them to an increase in MTCT.
Yet after extensive debate, the CDC, USPSTF, AASLD/IDSA, ACOG, and SMFM all developed their current recommendations to initiate HCV screening during pregnancy. To make this successful, screening algorithms need to be simple and consistent across all society recommendations.
HCV antibody testing should always reflex to the diagnostic test (HCV PCR) to allow confirmation in those who test positive without requiring an additional blood test. Viremic mothers (those who are HCV positive on PCR) should be linked to a provider who can discuss prognosis, transmission, and treatment. The importance of screening the infant also must be communicated to the parents and pediatrician alike.
Dr. Reau has served as a director, officer, partner, employee, adviser, consultant, or trustee for AbbVie, Gilead, Arbutus, Intercept, and Salix; received research grants from AbbVie and Gilead; and received income from AASLD. Dr. Pacheco disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.