User login
HIV testing dips during pandemic raise transmission concerns
raising concerns of a subsequent increase in transmission by people unaware of their HIV-positive status.
“Testing strategies need to be ramped up to cover this decrease in testing while adapting to the continuing COVID-19 environment,” reported Deesha Patel, MPH, and colleagues with the Centers for Disease Control and Prevention’s division of HIV prevention, Atlanta, in research presented at the annual meeting of the United States Conference on HIV/AIDS.
According to their data from the National HIV Prevention Program Monitoring and Evaluation system, the number of CDC-funded HIV tests declined by more than 1 million in 2020 amid the COVID-19 restrictions, with 1,228,142 tests reported that year, compared with 2,301,669 tests in 2019, a reduction of 46.6%.
The number of persons who were newly diagnosed with HIV, based on the tests, declined by 29.7%, from 7,692 newly diagnosed in 2019 to 5,409 persons in 2020, the authors reported.
The reasons for the reduction in new HIV diagnoses in 2020 could be multifactorial, possibly reflecting not just the reduced rates of testing but also possibly lower rates of transmission because of the lockdowns and social distancing, Mr. Patel said in an interview.
“Both [of those] interpretations are plausible, and the reductions are likely due to a combination of reasons,” she said.
Of note, the percentage of tests that were positive did not show a decline and was in fact slightly higher in 2020 (0.4%), compared with 2019 (0.3%; rate ratio, 1.32). But the increase may reflect that those seeking testing during the pandemic were more likely to be symptomatic.
“It is plausible that the smaller pool of people getting tested represented those with a higher likelihood of receiving a positive HIV test, [for instance] having a recent exposure, exhibiting symptoms,” Mr. Patel explained. “Furthermore, it is possible that some health departments specifically focused outreach efforts to serve persons with increased potential for HIV acquisition, thus identifying a higher proportion of persons with HIV.”
The declines in testing are nevertheless of particular concern in light of recent pre-COVID data indicating that as many as 13% of people who were infected with HIV were unaware of their positive status, placing them at high risk of transmitting the virus.
And on a broader level, the declines could negatively affect the goal to eradicate HIV through the federal Ending the HIV Epidemic in the U.S. (EHE) initiative, which aims to reduce new HIV infections in the United States by 90% by 2030 through the scaling up of key HIV prevention and treatment strategies, Mr. Patel noted.
“The first pillar of EHE is to diagnose all people with HIV as early as possible, and to accomplish that, there needs to be sufficient HIV testing,” Mr. Patel explained. “With fewer HIV tests being conducted, there are missed opportunities to identify persons with newly diagnosed HIV, which affects the entire continuum of care, [including] linkage to medical care, receiving antiretroviral treatment, getting and keeping viral suppression, and reducing transmission.”
At the local level: Adaptations allowed for continued testing
In a separate report presented at the meeting detailing the experiences at a more local level, Joseph Olsen, MPH, and colleagues with CrescentCare, New Orleans, described a similar reduction of HIV testing in 2020 of 49% in their system, compared with the previous year, down from 7,952 rapid HIV tests in 2019 to 4,034 in 2020.
However, through efforts to continue to provide services during the pandemic, the program was able to link 182 patients to HIV care in 2020, which was up from 172 in 2019.
In addition to offering the rapid HIV testing in conjunction with COVID-19 testing at their urgent care centers, the center adapted to the pandemic’s challenges with strategies including a new at-home testing program; providing testing at a hotel shelter for the homeless; and testing as part of walk-in testing with a syringe access component.
Mr. Olsen credited the swift program adaptations with maintaining testing during the time of crisis.
“Without [those] measures, it would have been a near-zero number of tests provided,” he said in an interview. “It would have been easy to blame the pandemic and not try to find innovations to deliver services, but I credit our incredibly motivated team for wanting to make sure every possible resource was available.”
But now there are signs of possible fallout from the testing reductions that did occur, Mr. Olsen said.
“We are already seeing the increase with other sexually transmitted infections [STIs], and I expect that we will see this with HIV as well,” he said.
In response, clinicians should use diligence in providing HIV testing, Mr. Olsen asserted.
“The take-home message for clinicians is that anyone having sex should get tested for HIV. It’s as easy as that!” he said.
“If they are getting tested for any other STI, make sure an HIV panel is added and discussed. If someone is pregnant, make sure an HIV panel is added and discussed. If someone has never had an HIV test before in their life – and I would add if they haven’t had an HIV test since March of 2020 – make sure an HIV panel is added/discussed,” he said. “Doing this for everyone also reduces stigma around testing. It’s not because any one person or group or risk behavior is being targeted, it is just good public health practice.”
The authors disclosed no relevant financial relationships. Mr. Patel noted that the findings and conclusions of her poster are those of the authors and do not necessarily represent the official position of the CDC.
A version of this article first appeared on Medscape.com.
raising concerns of a subsequent increase in transmission by people unaware of their HIV-positive status.
“Testing strategies need to be ramped up to cover this decrease in testing while adapting to the continuing COVID-19 environment,” reported Deesha Patel, MPH, and colleagues with the Centers for Disease Control and Prevention’s division of HIV prevention, Atlanta, in research presented at the annual meeting of the United States Conference on HIV/AIDS.
According to their data from the National HIV Prevention Program Monitoring and Evaluation system, the number of CDC-funded HIV tests declined by more than 1 million in 2020 amid the COVID-19 restrictions, with 1,228,142 tests reported that year, compared with 2,301,669 tests in 2019, a reduction of 46.6%.
The number of persons who were newly diagnosed with HIV, based on the tests, declined by 29.7%, from 7,692 newly diagnosed in 2019 to 5,409 persons in 2020, the authors reported.
The reasons for the reduction in new HIV diagnoses in 2020 could be multifactorial, possibly reflecting not just the reduced rates of testing but also possibly lower rates of transmission because of the lockdowns and social distancing, Mr. Patel said in an interview.
“Both [of those] interpretations are plausible, and the reductions are likely due to a combination of reasons,” she said.
Of note, the percentage of tests that were positive did not show a decline and was in fact slightly higher in 2020 (0.4%), compared with 2019 (0.3%; rate ratio, 1.32). But the increase may reflect that those seeking testing during the pandemic were more likely to be symptomatic.
“It is plausible that the smaller pool of people getting tested represented those with a higher likelihood of receiving a positive HIV test, [for instance] having a recent exposure, exhibiting symptoms,” Mr. Patel explained. “Furthermore, it is possible that some health departments specifically focused outreach efforts to serve persons with increased potential for HIV acquisition, thus identifying a higher proportion of persons with HIV.”
The declines in testing are nevertheless of particular concern in light of recent pre-COVID data indicating that as many as 13% of people who were infected with HIV were unaware of their positive status, placing them at high risk of transmitting the virus.
And on a broader level, the declines could negatively affect the goal to eradicate HIV through the federal Ending the HIV Epidemic in the U.S. (EHE) initiative, which aims to reduce new HIV infections in the United States by 90% by 2030 through the scaling up of key HIV prevention and treatment strategies, Mr. Patel noted.
“The first pillar of EHE is to diagnose all people with HIV as early as possible, and to accomplish that, there needs to be sufficient HIV testing,” Mr. Patel explained. “With fewer HIV tests being conducted, there are missed opportunities to identify persons with newly diagnosed HIV, which affects the entire continuum of care, [including] linkage to medical care, receiving antiretroviral treatment, getting and keeping viral suppression, and reducing transmission.”
At the local level: Adaptations allowed for continued testing
In a separate report presented at the meeting detailing the experiences at a more local level, Joseph Olsen, MPH, and colleagues with CrescentCare, New Orleans, described a similar reduction of HIV testing in 2020 of 49% in their system, compared with the previous year, down from 7,952 rapid HIV tests in 2019 to 4,034 in 2020.
However, through efforts to continue to provide services during the pandemic, the program was able to link 182 patients to HIV care in 2020, which was up from 172 in 2019.
In addition to offering the rapid HIV testing in conjunction with COVID-19 testing at their urgent care centers, the center adapted to the pandemic’s challenges with strategies including a new at-home testing program; providing testing at a hotel shelter for the homeless; and testing as part of walk-in testing with a syringe access component.
Mr. Olsen credited the swift program adaptations with maintaining testing during the time of crisis.
“Without [those] measures, it would have been a near-zero number of tests provided,” he said in an interview. “It would have been easy to blame the pandemic and not try to find innovations to deliver services, but I credit our incredibly motivated team for wanting to make sure every possible resource was available.”
But now there are signs of possible fallout from the testing reductions that did occur, Mr. Olsen said.
“We are already seeing the increase with other sexually transmitted infections [STIs], and I expect that we will see this with HIV as well,” he said.
In response, clinicians should use diligence in providing HIV testing, Mr. Olsen asserted.
“The take-home message for clinicians is that anyone having sex should get tested for HIV. It’s as easy as that!” he said.
“If they are getting tested for any other STI, make sure an HIV panel is added and discussed. If someone is pregnant, make sure an HIV panel is added and discussed. If someone has never had an HIV test before in their life – and I would add if they haven’t had an HIV test since March of 2020 – make sure an HIV panel is added/discussed,” he said. “Doing this for everyone also reduces stigma around testing. It’s not because any one person or group or risk behavior is being targeted, it is just good public health practice.”
The authors disclosed no relevant financial relationships. Mr. Patel noted that the findings and conclusions of her poster are those of the authors and do not necessarily represent the official position of the CDC.
A version of this article first appeared on Medscape.com.
raising concerns of a subsequent increase in transmission by people unaware of their HIV-positive status.
“Testing strategies need to be ramped up to cover this decrease in testing while adapting to the continuing COVID-19 environment,” reported Deesha Patel, MPH, and colleagues with the Centers for Disease Control and Prevention’s division of HIV prevention, Atlanta, in research presented at the annual meeting of the United States Conference on HIV/AIDS.
According to their data from the National HIV Prevention Program Monitoring and Evaluation system, the number of CDC-funded HIV tests declined by more than 1 million in 2020 amid the COVID-19 restrictions, with 1,228,142 tests reported that year, compared with 2,301,669 tests in 2019, a reduction of 46.6%.
The number of persons who were newly diagnosed with HIV, based on the tests, declined by 29.7%, from 7,692 newly diagnosed in 2019 to 5,409 persons in 2020, the authors reported.
The reasons for the reduction in new HIV diagnoses in 2020 could be multifactorial, possibly reflecting not just the reduced rates of testing but also possibly lower rates of transmission because of the lockdowns and social distancing, Mr. Patel said in an interview.
“Both [of those] interpretations are plausible, and the reductions are likely due to a combination of reasons,” she said.
Of note, the percentage of tests that were positive did not show a decline and was in fact slightly higher in 2020 (0.4%), compared with 2019 (0.3%; rate ratio, 1.32). But the increase may reflect that those seeking testing during the pandemic were more likely to be symptomatic.
“It is plausible that the smaller pool of people getting tested represented those with a higher likelihood of receiving a positive HIV test, [for instance] having a recent exposure, exhibiting symptoms,” Mr. Patel explained. “Furthermore, it is possible that some health departments specifically focused outreach efforts to serve persons with increased potential for HIV acquisition, thus identifying a higher proportion of persons with HIV.”
The declines in testing are nevertheless of particular concern in light of recent pre-COVID data indicating that as many as 13% of people who were infected with HIV were unaware of their positive status, placing them at high risk of transmitting the virus.
And on a broader level, the declines could negatively affect the goal to eradicate HIV through the federal Ending the HIV Epidemic in the U.S. (EHE) initiative, which aims to reduce new HIV infections in the United States by 90% by 2030 through the scaling up of key HIV prevention and treatment strategies, Mr. Patel noted.
“The first pillar of EHE is to diagnose all people with HIV as early as possible, and to accomplish that, there needs to be sufficient HIV testing,” Mr. Patel explained. “With fewer HIV tests being conducted, there are missed opportunities to identify persons with newly diagnosed HIV, which affects the entire continuum of care, [including] linkage to medical care, receiving antiretroviral treatment, getting and keeping viral suppression, and reducing transmission.”
At the local level: Adaptations allowed for continued testing
In a separate report presented at the meeting detailing the experiences at a more local level, Joseph Olsen, MPH, and colleagues with CrescentCare, New Orleans, described a similar reduction of HIV testing in 2020 of 49% in their system, compared with the previous year, down from 7,952 rapid HIV tests in 2019 to 4,034 in 2020.
However, through efforts to continue to provide services during the pandemic, the program was able to link 182 patients to HIV care in 2020, which was up from 172 in 2019.
In addition to offering the rapid HIV testing in conjunction with COVID-19 testing at their urgent care centers, the center adapted to the pandemic’s challenges with strategies including a new at-home testing program; providing testing at a hotel shelter for the homeless; and testing as part of walk-in testing with a syringe access component.
Mr. Olsen credited the swift program adaptations with maintaining testing during the time of crisis.
“Without [those] measures, it would have been a near-zero number of tests provided,” he said in an interview. “It would have been easy to blame the pandemic and not try to find innovations to deliver services, but I credit our incredibly motivated team for wanting to make sure every possible resource was available.”
But now there are signs of possible fallout from the testing reductions that did occur, Mr. Olsen said.
“We are already seeing the increase with other sexually transmitted infections [STIs], and I expect that we will see this with HIV as well,” he said.
In response, clinicians should use diligence in providing HIV testing, Mr. Olsen asserted.
“The take-home message for clinicians is that anyone having sex should get tested for HIV. It’s as easy as that!” he said.
“If they are getting tested for any other STI, make sure an HIV panel is added and discussed. If someone is pregnant, make sure an HIV panel is added and discussed. If someone has never had an HIV test before in their life – and I would add if they haven’t had an HIV test since March of 2020 – make sure an HIV panel is added/discussed,” he said. “Doing this for everyone also reduces stigma around testing. It’s not because any one person or group or risk behavior is being targeted, it is just good public health practice.”
The authors disclosed no relevant financial relationships. Mr. Patel noted that the findings and conclusions of her poster are those of the authors and do not necessarily represent the official position of the CDC.
A version of this article first appeared on Medscape.com.
Inadequate routine diabetes screening common in HIV
, research shows.
“Despite known risk in this patient population, most patients were not up to date with routine preventative screenings,” report Maya Hardman, PharmD, and colleagues with Southwest CARE Center, in Santa Fe, New Mexico, in research presented at the United States Conference on HIV/AIDS (USCHA) 2021 Annual Meeting.
“Routine preventative screenings can help identify chronic complications of diabetes early, if performed at the recommended intervals,” they write.
People with HIV are known to be at an increased risk of diabetes and the long-term complications of the disease, making the need for routine screening to prevent such complications all the more pressing due to their higher-risk health status.
Among the key routine diabetes care quality measures recommended by the Healthcare Effectiveness Data and Information Set (HEDIS) for people with HIV are testing for A1c once every 3 months, foot and eye exams every 12 months, urine albumin creatinine ratio (UACR) screenings every 12 months, and two controlled blood pressure readings every 12 months.
To investigate the rates of adherence to the HEDIS screening recommendations and identify predictors of poor compliance among people with HIV, Dr. Hardman and her colleagues evaluated data on 121 adult patients at the Southwest CARE Center who had been diagnosed with diabetes and HIV and were treated between 2019 and 2020.
The patients had a mean age of 57.5, and 9% were female. Their mean duration of being HIV positive was 19.8 years, and they had an intermediate Atherosclerotic Cardiovascular Disease (ASCVD) risk score of 17.08%.
Despite their known diagnoses of having diabetes, as many as 93.4% were found not to be up to date on their routine preventive screenings.
Of the 121 patients, only 30 had received the recommended A1c screenings, 37 had the recommended UACR screenings, and just 18 had received the recommended foot exam screenings.
Only blood pressure screenings, reported in 90 of the 121 patients, were up to date in the majority of patients in the group.
In looking at factors associated with compliance with A1c screening, only age (OR, 0.95; P = .04) was a significant predictor.
The authors pointed out that routine screenings for diabetes complications are relatively easy to implement.
“Screening for these chronic complications is minimally invasive and can be provided by individuals trained in diabetes management during routine clinic appointments.”
The team’s ongoing research is evaluating the potential benefits of clinical pharmacy services in assisting with the screenings for patients with HIV.
Research underscoring the increased risk and poorer treatment outcomes of diabetes in people with HIV include a study comparing 337 people with HIV in 2005 with a cohort of 338 participants in 2015.
The study showed the prevalence of type 2 diabetes had increased to 15.1% in 2015 from 6.8% 10 years earlier, for a relative risk of 2.4 compared with the general population.
“The alarmingly high prevalence of type 2 diabetes in HIV requires improved screening, targeted to older patients and those with a longer duration of exposure to antiretrovirals,” the authors wrote.
“Effective diabetes prevention and management strategies are needed urgently to reduce this risk; such interventions should target both conventional risk factors, such as abdominal obesity and HIV-specific risk factors such as weight gain following initiation of antiretrovirals.”
Of note, the 2015 cohort was significantly older and had higher BMI and higher hypertension than the 2005 cohort.
First author Alastair Duncan, PhD, principal dietitian at Guy’s & St. Thomas’ Hospital and lecturer, King’s College London, noted that since that 2015 study was published, concerns particularly with weight gain in the HIV population have only increased.
“Weight gain appears to be more of an issue [now],” he told this news organization in an interview.
“As in the general population, people living with HIV experienced significant weight gain during COVID-related lockdowns. Added to the high number of people living with HIV being treated with integrase inhibitors, weight gain remains a challenge.”
Meanwhile, “there are not enough studies comparing people living with HIV with the general population,” Dr. Duncan added. “We need to conduct studies where participants are matched.”
Sudipa Sarkar, MD, who co-authored a report on the issue of diabetes and HIV this year but was not involved in the study presented at USCHA, noted that the setting of care could play an important role in the quality of screening for diabetes that people with HIV receive.
“It may depend on factors such as whether a patient is being followed regularly by an HIV care provider and the larger health care system that the patient is in,” Dr. Sarkar, an assistant professor of medicine at Johns Hopkins University School of Medicine, Division of Endocrinology, Diabetes, and Metabolism, told this news organization.
“For example, one might find differences between a patient being seen in a managed care group versus not.”
The issue of how the strikingly high rates of inadequate screening in the current study compare with routine screening in the general diabetes population “is a good question and warrants more research,” she said.
The authors and Dr. Sarkar have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, research shows.
“Despite known risk in this patient population, most patients were not up to date with routine preventative screenings,” report Maya Hardman, PharmD, and colleagues with Southwest CARE Center, in Santa Fe, New Mexico, in research presented at the United States Conference on HIV/AIDS (USCHA) 2021 Annual Meeting.
“Routine preventative screenings can help identify chronic complications of diabetes early, if performed at the recommended intervals,” they write.
People with HIV are known to be at an increased risk of diabetes and the long-term complications of the disease, making the need for routine screening to prevent such complications all the more pressing due to their higher-risk health status.
Among the key routine diabetes care quality measures recommended by the Healthcare Effectiveness Data and Information Set (HEDIS) for people with HIV are testing for A1c once every 3 months, foot and eye exams every 12 months, urine albumin creatinine ratio (UACR) screenings every 12 months, and two controlled blood pressure readings every 12 months.
To investigate the rates of adherence to the HEDIS screening recommendations and identify predictors of poor compliance among people with HIV, Dr. Hardman and her colleagues evaluated data on 121 adult patients at the Southwest CARE Center who had been diagnosed with diabetes and HIV and were treated between 2019 and 2020.
The patients had a mean age of 57.5, and 9% were female. Their mean duration of being HIV positive was 19.8 years, and they had an intermediate Atherosclerotic Cardiovascular Disease (ASCVD) risk score of 17.08%.
Despite their known diagnoses of having diabetes, as many as 93.4% were found not to be up to date on their routine preventive screenings.
Of the 121 patients, only 30 had received the recommended A1c screenings, 37 had the recommended UACR screenings, and just 18 had received the recommended foot exam screenings.
Only blood pressure screenings, reported in 90 of the 121 patients, were up to date in the majority of patients in the group.
In looking at factors associated with compliance with A1c screening, only age (OR, 0.95; P = .04) was a significant predictor.
The authors pointed out that routine screenings for diabetes complications are relatively easy to implement.
“Screening for these chronic complications is minimally invasive and can be provided by individuals trained in diabetes management during routine clinic appointments.”
The team’s ongoing research is evaluating the potential benefits of clinical pharmacy services in assisting with the screenings for patients with HIV.
Research underscoring the increased risk and poorer treatment outcomes of diabetes in people with HIV include a study comparing 337 people with HIV in 2005 with a cohort of 338 participants in 2015.
The study showed the prevalence of type 2 diabetes had increased to 15.1% in 2015 from 6.8% 10 years earlier, for a relative risk of 2.4 compared with the general population.
“The alarmingly high prevalence of type 2 diabetes in HIV requires improved screening, targeted to older patients and those with a longer duration of exposure to antiretrovirals,” the authors wrote.
“Effective diabetes prevention and management strategies are needed urgently to reduce this risk; such interventions should target both conventional risk factors, such as abdominal obesity and HIV-specific risk factors such as weight gain following initiation of antiretrovirals.”
Of note, the 2015 cohort was significantly older and had higher BMI and higher hypertension than the 2005 cohort.
First author Alastair Duncan, PhD, principal dietitian at Guy’s & St. Thomas’ Hospital and lecturer, King’s College London, noted that since that 2015 study was published, concerns particularly with weight gain in the HIV population have only increased.
“Weight gain appears to be more of an issue [now],” he told this news organization in an interview.
“As in the general population, people living with HIV experienced significant weight gain during COVID-related lockdowns. Added to the high number of people living with HIV being treated with integrase inhibitors, weight gain remains a challenge.”
Meanwhile, “there are not enough studies comparing people living with HIV with the general population,” Dr. Duncan added. “We need to conduct studies where participants are matched.”
Sudipa Sarkar, MD, who co-authored a report on the issue of diabetes and HIV this year but was not involved in the study presented at USCHA, noted that the setting of care could play an important role in the quality of screening for diabetes that people with HIV receive.
“It may depend on factors such as whether a patient is being followed regularly by an HIV care provider and the larger health care system that the patient is in,” Dr. Sarkar, an assistant professor of medicine at Johns Hopkins University School of Medicine, Division of Endocrinology, Diabetes, and Metabolism, told this news organization.
“For example, one might find differences between a patient being seen in a managed care group versus not.”
The issue of how the strikingly high rates of inadequate screening in the current study compare with routine screening in the general diabetes population “is a good question and warrants more research,” she said.
The authors and Dr. Sarkar have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, research shows.
“Despite known risk in this patient population, most patients were not up to date with routine preventative screenings,” report Maya Hardman, PharmD, and colleagues with Southwest CARE Center, in Santa Fe, New Mexico, in research presented at the United States Conference on HIV/AIDS (USCHA) 2021 Annual Meeting.
“Routine preventative screenings can help identify chronic complications of diabetes early, if performed at the recommended intervals,” they write.
People with HIV are known to be at an increased risk of diabetes and the long-term complications of the disease, making the need for routine screening to prevent such complications all the more pressing due to their higher-risk health status.
Among the key routine diabetes care quality measures recommended by the Healthcare Effectiveness Data and Information Set (HEDIS) for people with HIV are testing for A1c once every 3 months, foot and eye exams every 12 months, urine albumin creatinine ratio (UACR) screenings every 12 months, and two controlled blood pressure readings every 12 months.
To investigate the rates of adherence to the HEDIS screening recommendations and identify predictors of poor compliance among people with HIV, Dr. Hardman and her colleagues evaluated data on 121 adult patients at the Southwest CARE Center who had been diagnosed with diabetes and HIV and were treated between 2019 and 2020.
The patients had a mean age of 57.5, and 9% were female. Their mean duration of being HIV positive was 19.8 years, and they had an intermediate Atherosclerotic Cardiovascular Disease (ASCVD) risk score of 17.08%.
Despite their known diagnoses of having diabetes, as many as 93.4% were found not to be up to date on their routine preventive screenings.
Of the 121 patients, only 30 had received the recommended A1c screenings, 37 had the recommended UACR screenings, and just 18 had received the recommended foot exam screenings.
Only blood pressure screenings, reported in 90 of the 121 patients, were up to date in the majority of patients in the group.
In looking at factors associated with compliance with A1c screening, only age (OR, 0.95; P = .04) was a significant predictor.
The authors pointed out that routine screenings for diabetes complications are relatively easy to implement.
“Screening for these chronic complications is minimally invasive and can be provided by individuals trained in diabetes management during routine clinic appointments.”
The team’s ongoing research is evaluating the potential benefits of clinical pharmacy services in assisting with the screenings for patients with HIV.
Research underscoring the increased risk and poorer treatment outcomes of diabetes in people with HIV include a study comparing 337 people with HIV in 2005 with a cohort of 338 participants in 2015.
The study showed the prevalence of type 2 diabetes had increased to 15.1% in 2015 from 6.8% 10 years earlier, for a relative risk of 2.4 compared with the general population.
“The alarmingly high prevalence of type 2 diabetes in HIV requires improved screening, targeted to older patients and those with a longer duration of exposure to antiretrovirals,” the authors wrote.
“Effective diabetes prevention and management strategies are needed urgently to reduce this risk; such interventions should target both conventional risk factors, such as abdominal obesity and HIV-specific risk factors such as weight gain following initiation of antiretrovirals.”
Of note, the 2015 cohort was significantly older and had higher BMI and higher hypertension than the 2005 cohort.
First author Alastair Duncan, PhD, principal dietitian at Guy’s & St. Thomas’ Hospital and lecturer, King’s College London, noted that since that 2015 study was published, concerns particularly with weight gain in the HIV population have only increased.
“Weight gain appears to be more of an issue [now],” he told this news organization in an interview.
“As in the general population, people living with HIV experienced significant weight gain during COVID-related lockdowns. Added to the high number of people living with HIV being treated with integrase inhibitors, weight gain remains a challenge.”
Meanwhile, “there are not enough studies comparing people living with HIV with the general population,” Dr. Duncan added. “We need to conduct studies where participants are matched.”
Sudipa Sarkar, MD, who co-authored a report on the issue of diabetes and HIV this year but was not involved in the study presented at USCHA, noted that the setting of care could play an important role in the quality of screening for diabetes that people with HIV receive.
“It may depend on factors such as whether a patient is being followed regularly by an HIV care provider and the larger health care system that the patient is in,” Dr. Sarkar, an assistant professor of medicine at Johns Hopkins University School of Medicine, Division of Endocrinology, Diabetes, and Metabolism, told this news organization.
“For example, one might find differences between a patient being seen in a managed care group versus not.”
The issue of how the strikingly high rates of inadequate screening in the current study compare with routine screening in the general diabetes population “is a good question and warrants more research,” she said.
The authors and Dr. Sarkar have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 asymptomatic infection rate remains high
Based on data from a meta-analysis of 95 studies that included nearly 30,000,000 individuals, the pooled percentage of asymptomatic COVID-19 infections was 0.25% in the tested population and 40.5% among confirmed cases.
, wrote Qiuyue Ma, PhD, and colleagues of Peking University, Beijing.
In a study published in JAMA Network Open the researchers identified 44 cross-sectional studies, 41 cohort studies, seven case series, and three case series on transmission studies. A total of 74 studies were conducted in developed countries, including those in Europe, North America, and Asia. Approximately one-third (37) of the studies were conducted among health care workers or in-hospital patients, 17 among nursing home staff or residents, and 14 among community residents. In addition, 13 studies involved pregnant women, eight involved air or cruise ship travelers, and six involved close contacts of individuals with confirmed infections.
The meta-analysis included 29,776,306 tested individuals; 11,516 of them had asymptomatic infections.
Overall, the pooled percentage of asymptomatic infections among the tested population was 0.25%. In an analysis of different study populations, the percentage was higher in nursing home residents or staff (4.52%), air or cruise ship travelers (2.02%), and pregnant women (2.34%), compared against the pooled percentage.
The pooled percentage of asymptomatic infections among the confirmed population was 40.50%, and this percentage was higher in pregnant women (54.11%), air or cruise ship travelers (52.91%), and nursing home residents or staff (47.53%).
The pooled percentage in the tested population was higher than the overall percentage when the mean age of the study population was 60 years or older (3.69%). By contrast, in the confirmed population, the pooled percentage was higher than the overall percentage when the study population was younger than 20 years (60.2%) or aged 20 to 39 years (49.5%).
The researchers noted in their discussion that the varying percentage of asymptomatic individuals according to community prevalence might impact the heterogeneity of the included studies. They also noted the high number of studies conducted in nursing home populations, groups in which asymptomatic individuals were more likely to be tested.
The study findings were limited by several factors, including the potential for missed studies that were not published at the time of the meta-analysis, as well as the exclusion of studies written in Chinese, the researchers noted. Other limitations included lack of follow-up on presymptomatic and covert infections, and the focus on specific populations, factors that may limit the degree to which the results can be generalized.
However, the results highlight the need to screen for asymptomatic infections, especially in countries where COVID-19 has been better controlled, the researchers said. Management strategies for asymptomatic infections, when identified, should include isolation and contact tracing similar to strategies used with confirmed cases, they added.
More testing needed to catch cases early
“During the initial phase of [the] COVID-19 pandemic, testing was not widely available in the United States or the rest of the world,” Setu Patolia, MD, of Saint Louis University School of Medicine, Missouri, said in an interview. Much of the world still lacks access to COVID-19 testing, and early in the pandemic only severely symptomatic patients were tested, he said. “With new variants, particularly the Omicron variant, which may have mild or minimally symptomatic disease, asymptomatic carriers play an important role in propagation of the pandemic,” he explained. “It is important to know the asymptomatic carrier rate among the general population for the future control of [the] pandemic,” he added.
Dr. Patolia said he was surprised by the study finding that one in 400 people in the general population could be asymptomatic carriers of COVID-19.
“Also, nursing home patients are more at risk of complications of COVID, and I expected that they would have a higher rate of symptomatic disease as compared to [the] general population,” said Dr. Patolia. He was also surprised by the high rate of asymptomatic infections in travelers.
“Physicians should be more aware about the asymptomatic carrier rate, particularly in travelers and nursing home patients,” he noted. “Travelers carry high risk of transferring infection from one region to another region of the world, and physicians should advise them to get tested despite the absence of symptoms,” Dr. Patolia emphasized. “Similarly, once any nursing home patient has been diagnosed with COVID-19, physicians should be more careful with the rest of the nursing home patients and test them despite the absence of the symptoms,” he added.
Dr. Patolia also recommended that pregnant women wear masks to help prevent disease transmission when visiting a doctor’s office or labor unit.
Looking ahead, there is a need for cheaper at-home testing kits so that all vulnerable populations can be tested fast and frequently, Dr. Patolia said.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose. Dr. Patolia has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Based on data from a meta-analysis of 95 studies that included nearly 30,000,000 individuals, the pooled percentage of asymptomatic COVID-19 infections was 0.25% in the tested population and 40.5% among confirmed cases.
, wrote Qiuyue Ma, PhD, and colleagues of Peking University, Beijing.
In a study published in JAMA Network Open the researchers identified 44 cross-sectional studies, 41 cohort studies, seven case series, and three case series on transmission studies. A total of 74 studies were conducted in developed countries, including those in Europe, North America, and Asia. Approximately one-third (37) of the studies were conducted among health care workers or in-hospital patients, 17 among nursing home staff or residents, and 14 among community residents. In addition, 13 studies involved pregnant women, eight involved air or cruise ship travelers, and six involved close contacts of individuals with confirmed infections.
The meta-analysis included 29,776,306 tested individuals; 11,516 of them had asymptomatic infections.
Overall, the pooled percentage of asymptomatic infections among the tested population was 0.25%. In an analysis of different study populations, the percentage was higher in nursing home residents or staff (4.52%), air or cruise ship travelers (2.02%), and pregnant women (2.34%), compared against the pooled percentage.
The pooled percentage of asymptomatic infections among the confirmed population was 40.50%, and this percentage was higher in pregnant women (54.11%), air or cruise ship travelers (52.91%), and nursing home residents or staff (47.53%).
The pooled percentage in the tested population was higher than the overall percentage when the mean age of the study population was 60 years or older (3.69%). By contrast, in the confirmed population, the pooled percentage was higher than the overall percentage when the study population was younger than 20 years (60.2%) or aged 20 to 39 years (49.5%).
The researchers noted in their discussion that the varying percentage of asymptomatic individuals according to community prevalence might impact the heterogeneity of the included studies. They also noted the high number of studies conducted in nursing home populations, groups in which asymptomatic individuals were more likely to be tested.
The study findings were limited by several factors, including the potential for missed studies that were not published at the time of the meta-analysis, as well as the exclusion of studies written in Chinese, the researchers noted. Other limitations included lack of follow-up on presymptomatic and covert infections, and the focus on specific populations, factors that may limit the degree to which the results can be generalized.
However, the results highlight the need to screen for asymptomatic infections, especially in countries where COVID-19 has been better controlled, the researchers said. Management strategies for asymptomatic infections, when identified, should include isolation and contact tracing similar to strategies used with confirmed cases, they added.
More testing needed to catch cases early
“During the initial phase of [the] COVID-19 pandemic, testing was not widely available in the United States or the rest of the world,” Setu Patolia, MD, of Saint Louis University School of Medicine, Missouri, said in an interview. Much of the world still lacks access to COVID-19 testing, and early in the pandemic only severely symptomatic patients were tested, he said. “With new variants, particularly the Omicron variant, which may have mild or minimally symptomatic disease, asymptomatic carriers play an important role in propagation of the pandemic,” he explained. “It is important to know the asymptomatic carrier rate among the general population for the future control of [the] pandemic,” he added.
Dr. Patolia said he was surprised by the study finding that one in 400 people in the general population could be asymptomatic carriers of COVID-19.
“Also, nursing home patients are more at risk of complications of COVID, and I expected that they would have a higher rate of symptomatic disease as compared to [the] general population,” said Dr. Patolia. He was also surprised by the high rate of asymptomatic infections in travelers.
“Physicians should be more aware about the asymptomatic carrier rate, particularly in travelers and nursing home patients,” he noted. “Travelers carry high risk of transferring infection from one region to another region of the world, and physicians should advise them to get tested despite the absence of symptoms,” Dr. Patolia emphasized. “Similarly, once any nursing home patient has been diagnosed with COVID-19, physicians should be more careful with the rest of the nursing home patients and test them despite the absence of the symptoms,” he added.
Dr. Patolia also recommended that pregnant women wear masks to help prevent disease transmission when visiting a doctor’s office or labor unit.
Looking ahead, there is a need for cheaper at-home testing kits so that all vulnerable populations can be tested fast and frequently, Dr. Patolia said.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose. Dr. Patolia has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Based on data from a meta-analysis of 95 studies that included nearly 30,000,000 individuals, the pooled percentage of asymptomatic COVID-19 infections was 0.25% in the tested population and 40.5% among confirmed cases.
, wrote Qiuyue Ma, PhD, and colleagues of Peking University, Beijing.
In a study published in JAMA Network Open the researchers identified 44 cross-sectional studies, 41 cohort studies, seven case series, and three case series on transmission studies. A total of 74 studies were conducted in developed countries, including those in Europe, North America, and Asia. Approximately one-third (37) of the studies were conducted among health care workers or in-hospital patients, 17 among nursing home staff or residents, and 14 among community residents. In addition, 13 studies involved pregnant women, eight involved air or cruise ship travelers, and six involved close contacts of individuals with confirmed infections.
The meta-analysis included 29,776,306 tested individuals; 11,516 of them had asymptomatic infections.
Overall, the pooled percentage of asymptomatic infections among the tested population was 0.25%. In an analysis of different study populations, the percentage was higher in nursing home residents or staff (4.52%), air or cruise ship travelers (2.02%), and pregnant women (2.34%), compared against the pooled percentage.
The pooled percentage of asymptomatic infections among the confirmed population was 40.50%, and this percentage was higher in pregnant women (54.11%), air or cruise ship travelers (52.91%), and nursing home residents or staff (47.53%).
The pooled percentage in the tested population was higher than the overall percentage when the mean age of the study population was 60 years or older (3.69%). By contrast, in the confirmed population, the pooled percentage was higher than the overall percentage when the study population was younger than 20 years (60.2%) or aged 20 to 39 years (49.5%).
The researchers noted in their discussion that the varying percentage of asymptomatic individuals according to community prevalence might impact the heterogeneity of the included studies. They also noted the high number of studies conducted in nursing home populations, groups in which asymptomatic individuals were more likely to be tested.
The study findings were limited by several factors, including the potential for missed studies that were not published at the time of the meta-analysis, as well as the exclusion of studies written in Chinese, the researchers noted. Other limitations included lack of follow-up on presymptomatic and covert infections, and the focus on specific populations, factors that may limit the degree to which the results can be generalized.
However, the results highlight the need to screen for asymptomatic infections, especially in countries where COVID-19 has been better controlled, the researchers said. Management strategies for asymptomatic infections, when identified, should include isolation and contact tracing similar to strategies used with confirmed cases, they added.
More testing needed to catch cases early
“During the initial phase of [the] COVID-19 pandemic, testing was not widely available in the United States or the rest of the world,” Setu Patolia, MD, of Saint Louis University School of Medicine, Missouri, said in an interview. Much of the world still lacks access to COVID-19 testing, and early in the pandemic only severely symptomatic patients were tested, he said. “With new variants, particularly the Omicron variant, which may have mild or minimally symptomatic disease, asymptomatic carriers play an important role in propagation of the pandemic,” he explained. “It is important to know the asymptomatic carrier rate among the general population for the future control of [the] pandemic,” he added.
Dr. Patolia said he was surprised by the study finding that one in 400 people in the general population could be asymptomatic carriers of COVID-19.
“Also, nursing home patients are more at risk of complications of COVID, and I expected that they would have a higher rate of symptomatic disease as compared to [the] general population,” said Dr. Patolia. He was also surprised by the high rate of asymptomatic infections in travelers.
“Physicians should be more aware about the asymptomatic carrier rate, particularly in travelers and nursing home patients,” he noted. “Travelers carry high risk of transferring infection from one region to another region of the world, and physicians should advise them to get tested despite the absence of symptoms,” Dr. Patolia emphasized. “Similarly, once any nursing home patient has been diagnosed with COVID-19, physicians should be more careful with the rest of the nursing home patients and test them despite the absence of the symptoms,” he added.
Dr. Patolia also recommended that pregnant women wear masks to help prevent disease transmission when visiting a doctor’s office or labor unit.
Looking ahead, there is a need for cheaper at-home testing kits so that all vulnerable populations can be tested fast and frequently, Dr. Patolia said.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose. Dr. Patolia has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Appendicitis: Up-front antibiotics OK in select patients
a comprehensive review of the literature suggests.
“I think this is a wonderful thing that we have for our patients now, because think about the patient who had a heart attack yesterday and has appendicitis today – you don’t want to operate on that patient – so this gives us a wonderful option in an environment where sometimes surgery is just bad timing,” Theodore Pappas, MD, professor of surgery, Duke University, Durham, N.C., told this news organization.
“It’s not that every 25-year-old who comes in should get antibiotics instead of surgery. It’s really better to say that this gives us flexibility for patients who we may not want to operate on immediately, and now we have a great option,” he stressed.
The study was published Dec. 14, 2021, in JAMA.
Acute appendicitis is the most common abdominal surgical emergency in the world, as the authors pointed out.
“We think it’s going to be 60%-70% of patients who are good candidates for consideration of antibiotics,” they speculated.
Current evidence
The review summarizes current evidence regarding the diagnosis and management of acute appendicitis based on a total of 71 articles including 10 systematic reviews, 9 meta-analyses, and 11 practice guidelines. “Appendicitis is classified as uncomplicated or complicated,” the authors explained. Uncomplicated appendicitis is acute appendicitis in the absence of clinical or radiographic signs of perforation.
In contrast, complicated appendicitis is when there is appendiceal rupture with subsequent abscess of phlegmon formation, the definitive diagnosis of which can be confirmed by CT scan. “In cases of diagnostic uncertainty imaging should be performed,” investigators cautioned – usually with ultrasound and CT scans.
If uncomplicated appendicitis is confirmed, three different guidelines now support the role of an antibiotics-first approach, including guidelines from the American Association for Surgery of Trauma. For this group of patients, empirical broad-spectrum antibiotic coverage that can be transitioned to outpatient treatment is commonly used. For example, patients may be initially treated with intravenous ertapenem monotherapy or intravenous cephalosporin plus metronidazole, then on discharge put on oral fluoroquinolones plus metronidazole.
Antibiotics that cover streptococci, nonresistant Enterobacteriaceae, and the anaerobes are usually adequate, they added. “The recommended duration of antibiotics is 10 days,” they noted. In most of the clinical trials comparing antibiotics first to surgery, the primary endpoint was treatment failure at 1 year, in other words, recurrence of symptoms during that year-long period. Across a number of clinical trials, that recurrence rate ranged from a low of 15% to a high of 41%.
In contrast, recurrence rarely occurs after surgical appendectomy. Early treatment failure, defined as clinical deterioration or lack of clinical improvement within 24-72 hours following initiation of antibiotics, is much less likely to occur, with a reported rate of between 8% and 12% of patients. The only long-term follow-up of an antibiotics-first approach in uncomplicated appendicitis was done in the Appendicitis Acuta (APPAC) trial, where at 5 years, the recurrence rate of acute appendicitis was 39% (95% confidence interval, 33.1%-45.3%) in patients initially treated with antibiotics alone.
Typically, there have been no differences in the length of hospital stay in most of the clinical trials reviewed. As Dr. Pappas explained, following a standard appendectomy, patients are typically sent home within 24 hours of undergoing surgery. On the other hand, if treated with intravenous antibiotics first, patients are usually admitted overnight then switched to oral antibiotics on discharge – suggesting that there is little difference in the time spent in hospital between the two groups.
However, there are groups of patients who predictably will not do well on antibiotics first, he cautioned. For example, patients who present with a high fever, shaking and chills, and severe abdominal pain do not have a mild case of appendicitis. Neither do patients who may not look sick but on CT scan, they have a hard piece of stool jammed into the end of the appendix that’s causing the blockage: These patients are also more likely to fail antibiotics, Dr. Pappas added.
“There is also a group of patients who have a much more dilated appendix with some fluid around it,” he noted, “and these patients are less likely to be managed with antibiotics successfully as well.” Lastly, though not part of this review and for whom an antibiotics-first protocol has long been in place, there is a subset of patients who have a perforated appendix, and that perforation has been walled off in a pocket of pus.
“These patients are treated with an antibiotic first because if you operate on them, it’s a mess, whereas if patients are reasonably stable, you can drain the abscess and then put them on antibiotics, and then you can decide 6-8 weeks later if you are going to take the appendix out,” Dr. Pappas said, adding: “Most of the time, what should be happening is the surgeon should consult with the patient and then they can weigh in – here are the options and here’s what I recommend.
“But patients will pick what they pick, and surgery is a very compelling argument: It’s laparoscopic surgery, patients are home in 24 hours, and the complication rate [and the recurrence rate] are incredibly low, so you have to think through all sorts of issues and when you come to a certain conclusion, it has to make a lot of sense to the patient,” Dr. Pappas emphasized.
Asked to comment on the findings, Ram Nirula, MD, D. Rees and Eleanor T. Jensen Presidential Chair in Surgery, University of Utah, Salt Lake City, noted that, as with all things in medicine, nothing is 100%.
“There are times where antibiotics for uncomplicated appendicitis may be appropriate, and times where appendectomy is most appropriate,” he said in an interview. Most of the evidence now shows that the risk of treatment failure following nonoperative management for uncomplicated appendicitis is significant, ranging from 15% to 40%, as Dr. Nirula reaffirmed.
A more recent randomized controlled trial from the CODA collaborative found that quality of life was similar for patients who got up-front antibiotics as for those who got surgery at 30 days, but the failure rate was high, particularly for those with appendicolith (what review authors would have classified as complicated appendicitis).
Moreover, when looking at this subset of patients, quality of life and patient satisfaction in the antibiotic treatment group were lower than it was for surgical controls, as Dr. Nirula also pointed out. While length of hospital stay was similar, overall health care resource utilization was higher in the antibiotic group. “So, if it were me, I would want my appendix removed at this stage in my life, however, for those who are poor surgical candidates, I would favor antibiotics,” Dr. Nirula stressed. He added that the presence of an appendicolith makes the argument for surgery more compelling, although he would still try antibiotics in patients with an appendicolith who are poor surgical candidates.
Dr. Pappas reported serving as a paid consultant for Transenterix. Dr. Nirula disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a comprehensive review of the literature suggests.
“I think this is a wonderful thing that we have for our patients now, because think about the patient who had a heart attack yesterday and has appendicitis today – you don’t want to operate on that patient – so this gives us a wonderful option in an environment where sometimes surgery is just bad timing,” Theodore Pappas, MD, professor of surgery, Duke University, Durham, N.C., told this news organization.
“It’s not that every 25-year-old who comes in should get antibiotics instead of surgery. It’s really better to say that this gives us flexibility for patients who we may not want to operate on immediately, and now we have a great option,” he stressed.
The study was published Dec. 14, 2021, in JAMA.
Acute appendicitis is the most common abdominal surgical emergency in the world, as the authors pointed out.
“We think it’s going to be 60%-70% of patients who are good candidates for consideration of antibiotics,” they speculated.
Current evidence
The review summarizes current evidence regarding the diagnosis and management of acute appendicitis based on a total of 71 articles including 10 systematic reviews, 9 meta-analyses, and 11 practice guidelines. “Appendicitis is classified as uncomplicated or complicated,” the authors explained. Uncomplicated appendicitis is acute appendicitis in the absence of clinical or radiographic signs of perforation.
In contrast, complicated appendicitis is when there is appendiceal rupture with subsequent abscess of phlegmon formation, the definitive diagnosis of which can be confirmed by CT scan. “In cases of diagnostic uncertainty imaging should be performed,” investigators cautioned – usually with ultrasound and CT scans.
If uncomplicated appendicitis is confirmed, three different guidelines now support the role of an antibiotics-first approach, including guidelines from the American Association for Surgery of Trauma. For this group of patients, empirical broad-spectrum antibiotic coverage that can be transitioned to outpatient treatment is commonly used. For example, patients may be initially treated with intravenous ertapenem monotherapy or intravenous cephalosporin plus metronidazole, then on discharge put on oral fluoroquinolones plus metronidazole.
Antibiotics that cover streptococci, nonresistant Enterobacteriaceae, and the anaerobes are usually adequate, they added. “The recommended duration of antibiotics is 10 days,” they noted. In most of the clinical trials comparing antibiotics first to surgery, the primary endpoint was treatment failure at 1 year, in other words, recurrence of symptoms during that year-long period. Across a number of clinical trials, that recurrence rate ranged from a low of 15% to a high of 41%.
In contrast, recurrence rarely occurs after surgical appendectomy. Early treatment failure, defined as clinical deterioration or lack of clinical improvement within 24-72 hours following initiation of antibiotics, is much less likely to occur, with a reported rate of between 8% and 12% of patients. The only long-term follow-up of an antibiotics-first approach in uncomplicated appendicitis was done in the Appendicitis Acuta (APPAC) trial, where at 5 years, the recurrence rate of acute appendicitis was 39% (95% confidence interval, 33.1%-45.3%) in patients initially treated with antibiotics alone.
Typically, there have been no differences in the length of hospital stay in most of the clinical trials reviewed. As Dr. Pappas explained, following a standard appendectomy, patients are typically sent home within 24 hours of undergoing surgery. On the other hand, if treated with intravenous antibiotics first, patients are usually admitted overnight then switched to oral antibiotics on discharge – suggesting that there is little difference in the time spent in hospital between the two groups.
However, there are groups of patients who predictably will not do well on antibiotics first, he cautioned. For example, patients who present with a high fever, shaking and chills, and severe abdominal pain do not have a mild case of appendicitis. Neither do patients who may not look sick but on CT scan, they have a hard piece of stool jammed into the end of the appendix that’s causing the blockage: These patients are also more likely to fail antibiotics, Dr. Pappas added.
“There is also a group of patients who have a much more dilated appendix with some fluid around it,” he noted, “and these patients are less likely to be managed with antibiotics successfully as well.” Lastly, though not part of this review and for whom an antibiotics-first protocol has long been in place, there is a subset of patients who have a perforated appendix, and that perforation has been walled off in a pocket of pus.
“These patients are treated with an antibiotic first because if you operate on them, it’s a mess, whereas if patients are reasonably stable, you can drain the abscess and then put them on antibiotics, and then you can decide 6-8 weeks later if you are going to take the appendix out,” Dr. Pappas said, adding: “Most of the time, what should be happening is the surgeon should consult with the patient and then they can weigh in – here are the options and here’s what I recommend.
“But patients will pick what they pick, and surgery is a very compelling argument: It’s laparoscopic surgery, patients are home in 24 hours, and the complication rate [and the recurrence rate] are incredibly low, so you have to think through all sorts of issues and when you come to a certain conclusion, it has to make a lot of sense to the patient,” Dr. Pappas emphasized.
Asked to comment on the findings, Ram Nirula, MD, D. Rees and Eleanor T. Jensen Presidential Chair in Surgery, University of Utah, Salt Lake City, noted that, as with all things in medicine, nothing is 100%.
“There are times where antibiotics for uncomplicated appendicitis may be appropriate, and times where appendectomy is most appropriate,” he said in an interview. Most of the evidence now shows that the risk of treatment failure following nonoperative management for uncomplicated appendicitis is significant, ranging from 15% to 40%, as Dr. Nirula reaffirmed.
A more recent randomized controlled trial from the CODA collaborative found that quality of life was similar for patients who got up-front antibiotics as for those who got surgery at 30 days, but the failure rate was high, particularly for those with appendicolith (what review authors would have classified as complicated appendicitis).
Moreover, when looking at this subset of patients, quality of life and patient satisfaction in the antibiotic treatment group were lower than it was for surgical controls, as Dr. Nirula also pointed out. While length of hospital stay was similar, overall health care resource utilization was higher in the antibiotic group. “So, if it were me, I would want my appendix removed at this stage in my life, however, for those who are poor surgical candidates, I would favor antibiotics,” Dr. Nirula stressed. He added that the presence of an appendicolith makes the argument for surgery more compelling, although he would still try antibiotics in patients with an appendicolith who are poor surgical candidates.
Dr. Pappas reported serving as a paid consultant for Transenterix. Dr. Nirula disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a comprehensive review of the literature suggests.
“I think this is a wonderful thing that we have for our patients now, because think about the patient who had a heart attack yesterday and has appendicitis today – you don’t want to operate on that patient – so this gives us a wonderful option in an environment where sometimes surgery is just bad timing,” Theodore Pappas, MD, professor of surgery, Duke University, Durham, N.C., told this news organization.
“It’s not that every 25-year-old who comes in should get antibiotics instead of surgery. It’s really better to say that this gives us flexibility for patients who we may not want to operate on immediately, and now we have a great option,” he stressed.
The study was published Dec. 14, 2021, in JAMA.
Acute appendicitis is the most common abdominal surgical emergency in the world, as the authors pointed out.
“We think it’s going to be 60%-70% of patients who are good candidates for consideration of antibiotics,” they speculated.
Current evidence
The review summarizes current evidence regarding the diagnosis and management of acute appendicitis based on a total of 71 articles including 10 systematic reviews, 9 meta-analyses, and 11 practice guidelines. “Appendicitis is classified as uncomplicated or complicated,” the authors explained. Uncomplicated appendicitis is acute appendicitis in the absence of clinical or radiographic signs of perforation.
In contrast, complicated appendicitis is when there is appendiceal rupture with subsequent abscess of phlegmon formation, the definitive diagnosis of which can be confirmed by CT scan. “In cases of diagnostic uncertainty imaging should be performed,” investigators cautioned – usually with ultrasound and CT scans.
If uncomplicated appendicitis is confirmed, three different guidelines now support the role of an antibiotics-first approach, including guidelines from the American Association for Surgery of Trauma. For this group of patients, empirical broad-spectrum antibiotic coverage that can be transitioned to outpatient treatment is commonly used. For example, patients may be initially treated with intravenous ertapenem monotherapy or intravenous cephalosporin plus metronidazole, then on discharge put on oral fluoroquinolones plus metronidazole.
Antibiotics that cover streptococci, nonresistant Enterobacteriaceae, and the anaerobes are usually adequate, they added. “The recommended duration of antibiotics is 10 days,” they noted. In most of the clinical trials comparing antibiotics first to surgery, the primary endpoint was treatment failure at 1 year, in other words, recurrence of symptoms during that year-long period. Across a number of clinical trials, that recurrence rate ranged from a low of 15% to a high of 41%.
In contrast, recurrence rarely occurs after surgical appendectomy. Early treatment failure, defined as clinical deterioration or lack of clinical improvement within 24-72 hours following initiation of antibiotics, is much less likely to occur, with a reported rate of between 8% and 12% of patients. The only long-term follow-up of an antibiotics-first approach in uncomplicated appendicitis was done in the Appendicitis Acuta (APPAC) trial, where at 5 years, the recurrence rate of acute appendicitis was 39% (95% confidence interval, 33.1%-45.3%) in patients initially treated with antibiotics alone.
Typically, there have been no differences in the length of hospital stay in most of the clinical trials reviewed. As Dr. Pappas explained, following a standard appendectomy, patients are typically sent home within 24 hours of undergoing surgery. On the other hand, if treated with intravenous antibiotics first, patients are usually admitted overnight then switched to oral antibiotics on discharge – suggesting that there is little difference in the time spent in hospital between the two groups.
However, there are groups of patients who predictably will not do well on antibiotics first, he cautioned. For example, patients who present with a high fever, shaking and chills, and severe abdominal pain do not have a mild case of appendicitis. Neither do patients who may not look sick but on CT scan, they have a hard piece of stool jammed into the end of the appendix that’s causing the blockage: These patients are also more likely to fail antibiotics, Dr. Pappas added.
“There is also a group of patients who have a much more dilated appendix with some fluid around it,” he noted, “and these patients are less likely to be managed with antibiotics successfully as well.” Lastly, though not part of this review and for whom an antibiotics-first protocol has long been in place, there is a subset of patients who have a perforated appendix, and that perforation has been walled off in a pocket of pus.
“These patients are treated with an antibiotic first because if you operate on them, it’s a mess, whereas if patients are reasonably stable, you can drain the abscess and then put them on antibiotics, and then you can decide 6-8 weeks later if you are going to take the appendix out,” Dr. Pappas said, adding: “Most of the time, what should be happening is the surgeon should consult with the patient and then they can weigh in – here are the options and here’s what I recommend.
“But patients will pick what they pick, and surgery is a very compelling argument: It’s laparoscopic surgery, patients are home in 24 hours, and the complication rate [and the recurrence rate] are incredibly low, so you have to think through all sorts of issues and when you come to a certain conclusion, it has to make a lot of sense to the patient,” Dr. Pappas emphasized.
Asked to comment on the findings, Ram Nirula, MD, D. Rees and Eleanor T. Jensen Presidential Chair in Surgery, University of Utah, Salt Lake City, noted that, as with all things in medicine, nothing is 100%.
“There are times where antibiotics for uncomplicated appendicitis may be appropriate, and times where appendectomy is most appropriate,” he said in an interview. Most of the evidence now shows that the risk of treatment failure following nonoperative management for uncomplicated appendicitis is significant, ranging from 15% to 40%, as Dr. Nirula reaffirmed.
A more recent randomized controlled trial from the CODA collaborative found that quality of life was similar for patients who got up-front antibiotics as for those who got surgery at 30 days, but the failure rate was high, particularly for those with appendicolith (what review authors would have classified as complicated appendicitis).
Moreover, when looking at this subset of patients, quality of life and patient satisfaction in the antibiotic treatment group were lower than it was for surgical controls, as Dr. Nirula also pointed out. While length of hospital stay was similar, overall health care resource utilization was higher in the antibiotic group. “So, if it were me, I would want my appendix removed at this stage in my life, however, for those who are poor surgical candidates, I would favor antibiotics,” Dr. Nirula stressed. He added that the presence of an appendicolith makes the argument for surgery more compelling, although he would still try antibiotics in patients with an appendicolith who are poor surgical candidates.
Dr. Pappas reported serving as a paid consultant for Transenterix. Dr. Nirula disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
Children and COVID: Weekly cases resume their climb
After a brief lull in activity, weekly COVID-19 cases in children returned to the upward trend that began in early November, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
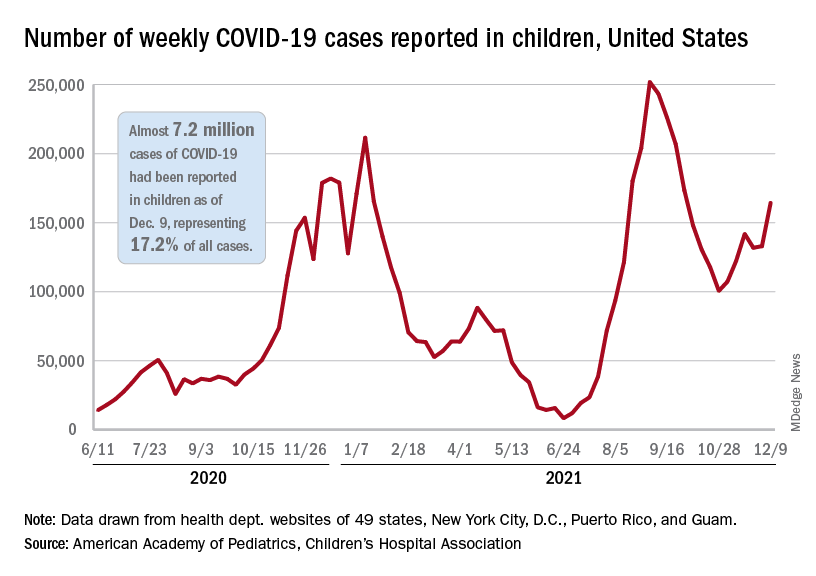
according to the Centers for Disease Control and Prevention.
New COVID-19 cases were up by 23.5% for the week of Dec. 3-9, after a 2-week period that saw a drop and then just a slight increase, the AAP and CHA said in their latest weekly COVID report. There were 164,000 new cases from Dec. 3 to Dec. 9 in 46 states (Alabama, Nebraska, and Texas stopped reporting over the summer of 2021 and New York has never reported by age), the District of Columbia, New York City, Puerto Rico, and Guam.
The increase occurred across all four regions of the country, but the largest share came in the Midwest, with over 65,000 new cases, followed by the West (just over 35,000), the Northeast (just under 35,000), and the South (close to 28,000), the AAP/CHA data show.
The 7.2 million cumulative cases in children as of Dec. 9 represent 17.2% of all cases reported in the United States since the start of the pandemic, with available state reports showing that proportion ranges from 12.3% in Florida to 26.1% in Vermont. Alaska has the highest incidence of COVID at 19,000 cases per 100,000 children, and Hawaii has the lowest (5,300 per 100,000) among the states currently reporting, the AAP and CHA said.
State reporting on vaccinations shows that 37% of children aged 5-11 years in Massachusetts have received at least one dose, the highest of any state, while West Virginia is lowest at just 4%. The highest vaccination rate for children aged 12-17 goes to Massachusetts at 84%, with Wyoming lowest at 37%, the AAP said in a separate report.
Nationally, new vaccinations fell by a third during the week of Dec. 7-13, compared with the previous week, with the largest decline (34.7%) coming from the 5- to 11-year-olds, who still represented the majority (almost 84%) of the 430,000 new child vaccinations received, according to the CDC’s COVID Data Tracker. Corresponding declines for the last week were 27.5% for 12- to 15-year-olds and 22.7% for those aged 16-17.
Altogether, 21.2 million children aged 5-17 had received at least one dose and 16.0 million were fully vaccinated as of Dec. 13. By age group, 19.2% of children aged 5-11 years have gotten at least one dose and 9.6% are fully vaccinated, compared with 62.1% and 52.3%, respectively, among children aged 12-17, the CDC said.
After a brief lull in activity, weekly COVID-19 cases in children returned to the upward trend that began in early November, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.

according to the Centers for Disease Control and Prevention.
New COVID-19 cases were up by 23.5% for the week of Dec. 3-9, after a 2-week period that saw a drop and then just a slight increase, the AAP and CHA said in their latest weekly COVID report. There were 164,000 new cases from Dec. 3 to Dec. 9 in 46 states (Alabama, Nebraska, and Texas stopped reporting over the summer of 2021 and New York has never reported by age), the District of Columbia, New York City, Puerto Rico, and Guam.
The increase occurred across all four regions of the country, but the largest share came in the Midwest, with over 65,000 new cases, followed by the West (just over 35,000), the Northeast (just under 35,000), and the South (close to 28,000), the AAP/CHA data show.
The 7.2 million cumulative cases in children as of Dec. 9 represent 17.2% of all cases reported in the United States since the start of the pandemic, with available state reports showing that proportion ranges from 12.3% in Florida to 26.1% in Vermont. Alaska has the highest incidence of COVID at 19,000 cases per 100,000 children, and Hawaii has the lowest (5,300 per 100,000) among the states currently reporting, the AAP and CHA said.
State reporting on vaccinations shows that 37% of children aged 5-11 years in Massachusetts have received at least one dose, the highest of any state, while West Virginia is lowest at just 4%. The highest vaccination rate for children aged 12-17 goes to Massachusetts at 84%, with Wyoming lowest at 37%, the AAP said in a separate report.
Nationally, new vaccinations fell by a third during the week of Dec. 7-13, compared with the previous week, with the largest decline (34.7%) coming from the 5- to 11-year-olds, who still represented the majority (almost 84%) of the 430,000 new child vaccinations received, according to the CDC’s COVID Data Tracker. Corresponding declines for the last week were 27.5% for 12- to 15-year-olds and 22.7% for those aged 16-17.
Altogether, 21.2 million children aged 5-17 had received at least one dose and 16.0 million were fully vaccinated as of Dec. 13. By age group, 19.2% of children aged 5-11 years have gotten at least one dose and 9.6% are fully vaccinated, compared with 62.1% and 52.3%, respectively, among children aged 12-17, the CDC said.
After a brief lull in activity, weekly COVID-19 cases in children returned to the upward trend that began in early November, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.

according to the Centers for Disease Control and Prevention.
New COVID-19 cases were up by 23.5% for the week of Dec. 3-9, after a 2-week period that saw a drop and then just a slight increase, the AAP and CHA said in their latest weekly COVID report. There were 164,000 new cases from Dec. 3 to Dec. 9 in 46 states (Alabama, Nebraska, and Texas stopped reporting over the summer of 2021 and New York has never reported by age), the District of Columbia, New York City, Puerto Rico, and Guam.
The increase occurred across all four regions of the country, but the largest share came in the Midwest, with over 65,000 new cases, followed by the West (just over 35,000), the Northeast (just under 35,000), and the South (close to 28,000), the AAP/CHA data show.
The 7.2 million cumulative cases in children as of Dec. 9 represent 17.2% of all cases reported in the United States since the start of the pandemic, with available state reports showing that proportion ranges from 12.3% in Florida to 26.1% in Vermont. Alaska has the highest incidence of COVID at 19,000 cases per 100,000 children, and Hawaii has the lowest (5,300 per 100,000) among the states currently reporting, the AAP and CHA said.
State reporting on vaccinations shows that 37% of children aged 5-11 years in Massachusetts have received at least one dose, the highest of any state, while West Virginia is lowest at just 4%. The highest vaccination rate for children aged 12-17 goes to Massachusetts at 84%, with Wyoming lowest at 37%, the AAP said in a separate report.
Nationally, new vaccinations fell by a third during the week of Dec. 7-13, compared with the previous week, with the largest decline (34.7%) coming from the 5- to 11-year-olds, who still represented the majority (almost 84%) of the 430,000 new child vaccinations received, according to the CDC’s COVID Data Tracker. Corresponding declines for the last week were 27.5% for 12- to 15-year-olds and 22.7% for those aged 16-17.
Altogether, 21.2 million children aged 5-17 had received at least one dose and 16.0 million were fully vaccinated as of Dec. 13. By age group, 19.2% of children aged 5-11 years have gotten at least one dose and 9.6% are fully vaccinated, compared with 62.1% and 52.3%, respectively, among children aged 12-17, the CDC said.
Widespread Necrotizing Purpura and Lucio Phenomenon as the First Diagnostic Presentation of Diffuse Nonnodular Lepromatous Leprosy
Case Report
A 70-year-old man living in Esna, Luxor, Egypt presented to the Department of Rheumatology and Rehabilitation with widespread gangrenous skin lesions associated with ulcers of 2 weeks’ duration. One year prior, the patient had an insidious onset of nocturnal fever, bilateral leg edema, and numbness and a tingling sensation in both hands. He presented some laboratory and radiologic investigations that were performed at another hospital prior to the current presentation, which revealed thrombocytopenia, mild splenomegaly, and generalized lymphadenopathy. An excisional left axillary lymph node biopsy was performed at another hospital prior to the current presentation, and the pathology report provided by the patient described a reactive, foamy, histiocyte-rich lesion, suggesting a diagnosis of hemophagocytic lymphohistiocytosis. The patient had no diabetes or hypertension and no history of deep vein thrombosis, stroke, or unintentional weight loss. No medications were taken prior to the onset of the skin lesions, and his family history was irrelevant.
General examination at the current presentation revealed a fever (temperature, 101.3 °F [38.5 °C]), a normal heart rate (90 beats per minute), normal blood pressure (120/80 mmHg), normal respiratory rate (14 breaths per minute), accentuated heart sounds, and normal vesicular breathing without adventitious sounds. He had saddle nose, loss of the outer third of the eyebrows, and marked reduction in the density of the eyelashes (madarosis). Bilateral pitting edema of the legs also was present. Neurologic examination revealed hypoesthesia in a glove-and-stocking pattern, thickened peripheral nerves, and trophic changes over both hands; however, he had normal muscle power and deep reflexes. Joint examination revealed no abnormalities. Skin examination revealed widespread, reticulated, necrotizing, purpuric lesions on the arms, legs, abdomen, and ears, some associated with gangrenous ulcerations and hemorrhagic blisters. Scattered vasculitic ulcers and gangrenous patches were seen on the fingers. A gangrenous ulcer mimicking Fournier gangrene was seen involving the scrotal skin in addition to a gangrenous lesion on the glans penis (Figure 1–3). Unaffected skin appeared smooth, shiny, and edematous and showed no nodular lesions. Peripheral pulsations were intact.
Positive findings from a wide panel of laboratory investigations included an elevated erythrocyte sedimentation rate (103 mm for the first hour [reference range, 0–22 mm]), high C-reactive protein (50.7 mg/L [reference range, up to 6 mg/L]), anemia (hemoglobin count, 7.3 g/dL [reference range, 13.5–17.5 g/dL]), thrombocytopenia (45×103/mm3 [reference range, 150×103/mm3), low serum albumin (2.3 g/dL [reference range, 3.4–5.4 g/dL]), elevated IgG and IgM anticardiolipin antibodies (IgG, 21.4 IgG phospholipid [GPL] units [reference range, <10 IgG phospholipid (GPL) units]; IgM, 59.4 IgM phospholipid (MPL) units [reference range, <7 IgM phospholipid (MPL) units]), positive lupus anticoagulant panel test, elevated anti-β2 glycoprotein antibodies (IgG, 17.5
Nerve conduction velocity showed axonal sensory polyneuropathy. Motor nerve conduction studies for median and ulnar nerves were within normal range. Lower-limb nerves assessment was limited by the ulcerated areas and marked edema. Echocardiography was unremarkable. Arterial Doppler studies were only available for the upper limbs and were unremarkable.
A punch biopsy was taken from one of the necrotizing purpuric lesions on the legs, and histopathologic examination revealed foci of epidermal necrosis and subepidermal separation and superficial and deep perivascular and periadnexal infiltrates extending into the fat lobules. The infiltrates were mainly made up of foamy macrophages, and some contained globi (lepra cells), in addition to lymphocytes and many neutrophils with nuclear dust. Blood vessels in the superficial and deep dermis and in the subcutaneous fat showed fibrinoid necrosis in their walls with neutrophils infiltrating the walls and thrombi in the lumens (Figure 4). Modified Ziehl-Neelsen staining revealed clumps of acid-fast lepra bacilli inside vascular lumina and endothelial cell lining and within the foamy macrophages (Figure 5). Slit-skin smear examination was performed twice and yielded negative results. The slide and paraffin block of the already performed lymph node biopsy were retrieved. Examination revealed aggregates of foamy histiocytes surrounded by lymphocytes and plasma cells replacing normal lymphoid follicles. Modified Ziehl-Neelsen stain was performed, and clusters of acid-fast bacilli were detected within the foamy histiocytic infiltrate (Figure 6).
According to the results of the skin biopsy, the revised result of the lymph node biopsy, and the pattern of neurologic deficit together with clinical and laboratory correlation, the patient was diagnosed with diffuse nonnodular lepromatous leprosy presenting with Lucio phenomenon (Lucio leprosy) and associated with lepromatous lymphadenitis.
The patient received the following treatment: methylprednisolone 500 mg (intravenous pulse therapy) followed by daily oral administration of prednisolone 10 mg, rifampicin 300 mg, dapsone 100 mg, clofazimine 100 mg, acetylsalicylic acid 150 mg, and enoxaparin sodium 80 mg. In addition, the scrotal Fournier gangrene–like lesion was treated by surgical debridement followed by vacuum therapy. By the second week after treatment, the gangrenous lesions of the fingers developed a line of demarcation, and the skin infarctions started to recede.
Comment
Despite a decrease in its prevalence through a World Health Organization (WHO)–empowered eradication program, leprosy still represents a health problem in endemic areas.1,2 It is characterized by a wide range of immune responses to Mycobacterium leprae, displaying a spectrum of clinical and histopathologic manifestations that vary from the tuberculoid or paucibacillary pole with a strong cell-mediated immune response and fewer organisms to the lepromatous or multibacillary pole with weaker cell-mediated immune response and higher loads of organisms.3 In addition to its well-known cutaneous and neurologic manifestations, leprosy can present with a variety of manifestations, including constitutional symptoms, musculoskeletal manifestations, and serologic abnormalities; thus, leprosy can mimic rheumatoid arthritis, spondyloarthritis, and vasculitis—a pitfall that may result in misdiagnosis as a rheumatologic disorder.3-7
The chronic course of leprosy can be disrupted by acute, immunologically mediated reactions known as lepra reactions, of which there are 3 types.8 Type I lepra reactions are cell mediated and occur mainly in patients with borderline disease, often representing an upgrade toward the tuberculoid pole; less often they represent a downgrade reaction. Nerves become painful and swollen with possible loss of function, and skin lesions become edematous and tender; sometimes arthritis develops.9 Type II lepra reactions, also known as erythema nodosum leprosum (ENL), occur in borderline lepromatous and lepromatous patients with a high bacillary load. They are characterized by fever, body aches, tender cutaneous/subcutaneous nodules that may ulcerate, possible bullous lesions, painful nerve swellings, swollen joints, iritis, lymphadenitis, glomerulonephritis, epididymo-orchitis, and hepatic affection. Both immune-complex and delayed hypersensitivity reactions play a role in ENL.8,10 The third reaction is a rare aggressive type known as Lucio phenomenon or Lucio leprosy, which presents with irregular-shaped, angulated, or stellar necrotizing purpuric lesions (hemorrhagic infacrtions) developing mainly on the extremities. The lesions evolve into ulcers that heal with atrophic scarring.2,11 Lucio phenomenon develops as a result of thrombotic vascular occlusion secondary to massive invasion of vascular endothelial cells by lepra bacilli.2,11-14 Involvement of the scrotal skin, such as in our patient, is rare.
Lucio phenomenon mainly is seen in Mexico and Central America, and few cases have been documented in Cuba, South America, the United States, India, Polynesia, South Africa, and Southeast Asia.15-17 It specifically occurs in patients with untreated, diffuse, nonnodular lepromatous leprosy (pure and primitive diffuse lepromatous leprosy (DLL)/diffuse leprosy of Lucio and Latapí). This type of leprosy was first described by Lucio and Alvarado18 in 1852 as a distinct form of lepromatous leprosy characterized by widespread and dense infiltration of the whole skin by lepra bacilli without the typical nodular lesions of leprosy, rendering its diagnosis challenging, especially in sporadic cases. Other manifestations of DLL include complete alopecia of the eyebrows and eyelashes, destructive rhinitis, and areas of anhidrosis and dyesthesia.2
Latapí and Chévez-Zomora19 defined Lucio phenomenon in 1948 as a form of histopathologic vasculitis restricted to patients with DLL. Histopathologically, in addition to the infiltration of the skin with acid-fast bacilli–laden foamy histiocytes, lesions of Lucio phenomenon show features of necrotizing (leukocytoclastic) vasculitis with fibrinoid necrosis20 or vascular thrombi with minimal perivascular lymphocytic infiltrate and no evidence of vasculitis.11 Medium to large vessels in the deep dermis and subcutaneous tissue show infiltration of their walls with a large number of macrophages laden with acid-fast bacilli.11 Cases with histopathologic features mimicking antiphospholipid syndrome with endothelial cell proliferation, thrombosis, and mild mononuclear cell infiltrate also may be seen.20 In all cases, ischemic epidermal necrosis is seen, as well as acid-fast bacilli, both singly and in clusters (globi) within endothelial cells and inside blood vessel lumina.
Although Lucio phenomenon initially was thought to be immune-complex mediated like ENL, it has been suggested that the main trigger is thrombotic vascular occlusion secondary to massive invasion of the vascular endothelial cells by the lepra bacilli, resulting in necrosis.14 Bacterial lipopolysaccharides promote the release of IL-1 and tumor necrosis factor α, which in turn stimulate the production of prostaglandins, IL-6, and coagulation factor III, leading to vascular thrombosis and tissue necrosis.21,22 Moreover, antiphospholipid antibodies, which have been found to be induced in response to certain infectious agents in genetically predisposed individuals,23 have been reported in patients with leprosy, mainly in association with lepromatous leprosy. The reported prevalence of anticardiolipin antibodies ranged from 37% to 98%, whereas anti-β2-glycoprotein I antibodies ranged from 3% to 19%, and antiprothrombin antibodies ranged from 6% to 45%.24,25 Antiphospholipid antibodies have been reported to play a role in the pathogenesis of Lucio phenomenon.11,13,15,26 Our case supports this hypothesis with positive anticardiolipin antibodies, anti-β2 glycoprotein antibodies, and positive lupus anticoagulant.
In accordance with Curi et al,2 who reported 5 cases of DLL with Lucio phenomenon, our patient showed a similar presentation with positive inflammatory markers in association with a negative autoimmune profile (ANA, ANCA-C&P) and negative venereal disease research laboratory test. It is important to mention that a positive autoimmune profile (ANA, ANCA-C&P) can be present in leprotic patients, causing possible diagnostic confusion with collagen diseases.27,28
An interesting finding in our case was the negative slit-skin smear results. Although the specificity of slit-skin smear is 100%, as it directly demonstrates the presence of acid-fast bacilli,29 its sensitivity is low and varies from 10% to 50%.30 The detection of acid-fast bacilli in tissue sections is reported to be a better method for confirming the diagnosis of leprosy.31
The provisional impression of hemophagocytic lymphohistiocytosis in the lymph node biopsy in our patient was excluded upon detection of acid-fast bacilli in the foamy histiocytes infiltrating the lymph node; moreover, the normal serum lipids and serum ferritin argued against this diagnosis.32 Leprosy tends to involve the lymph nodes, particularly in borderline, borderline lepromatous, and lepromatous forms.33 The incidence of lymph node involvement accompanied by skin lesions with the presence of acid-fast bacilli in the lymph nodes is 92.2%.34
Our patient showed an excellent response to antileprotic treatment, which was administered according to the WHO multidrug therapy guidelines for multibacillary leprosy,35 combined with low-dose prednisolone, acetylsalicylic acid, and anticoagulant treatment. Thalidomide and high-dose prednisolone (60 mg/d) combined with antileprotic treatment also have been reported to be successful in managing recurrent infarctions in leprosy.36 The Fournier-like gangrenous ulcer of the scrotum was managed by surgical debridement and vacuum therapy.
It is noteworthy that the WHO elimination goal for leprosy was to reduce the prevalence to less than 1 case per 10,000 population. Egypt is among the first countries in North Africa and the Middle East regions to achieve this target supervised by the National Leprosy Control Program as early as 1994; this was further reduced to 0.33 cases per 10,000 population in 2004, and reduced again in 2009; however, certain foci showed a prevalence rate more than the elimination target, particularly in the cities of Qena (1.12) and Sohag (2.47).37 Esna, where our patient is from, is an endemic area in Egypt.38
Conclusion
1. World Health Organization. World Health Statistics: 2011. World Health Organization; 2011. https://www.who.int/gho/publications/world_health_statistics/EN_WHS2011_Full.pdf
2. Curi PF, Villaroel JS, Migliore N, et al. Lucio’s phenomenon: report of five cases. Clin Rheumatol. 2016;35:1397-1401.
3. Shrestha B, Li YQ, Fu P. Leprosy mimics adult onset Still’s disease in a Chinese patient. Egypt Rheumatol. 2018;40:217-220.
4. Prasad S, Misra R, Aggarwal A, et al. Leprosy revealed in a rheumatology clinic: a case series. Int J Rheum Dis. 2013;16:129-133.
5. Chao G, Fang L, Lu C. Leprosy with ANA positive mistaken for connective tissue disease. Clin Rheumatol. 2013;32:645-648.
6. Chauhan S, Wakhlu A, Agarwal V. Arthritis in leprosy. Rheumatology. 2010;49:2237-2242.
7. Rath D, Bhargava S, Kundu BK. Leprosy mimicking common rheumatologic entities: a trial for the clinician in the era of biologics. Case Rep Rheumatol. 2014;2014:429698.
8. Cuevas J, Rodríguez-Peralto JL, Carrillo R, et al. Erythema nodosum leprosum: reactional leprosy. Semin Cutan Med Surg. 2007;26:126-130.
9. Henriques CC, Lopéz B, Mestre T, et al. Leprosy and rheumatoid arthritis: consequence or association? BMJ Case Rep. 2012;13:1-4.
10. Vázquez-Botet M, Sánchez JL. Erythema nodosum leprosum. Int J Dermatol. 1987;26:436-437.
11. Nunzie E, Ortega Cabrera LV, Macanchi Moncayo FM, et al. Lucio leprosy with Lucio’s phenomenon, digital gangrene and anticardiolipin antibodies. Lepr Rev. 2014;85:194-200.
12. Salvi S, Chopra A. Leprosy in a rheumatology setting: a challenging mimic to expose. Clin Rheumatol. 2013;32:1557-1563.
13. Azulay-Abulafia L, Pereira SL, Hardmann D, et al. Lucio phenomenon. vasculitis or occlusive vasculopathy? Hautarzt. 2006;57:1101-1105.
14. Benard G, Sakai-Valente NY, Bianconcini Trindade MA. Concomittant Lucio phenomenon and erythema nodosum in a leprosy patient: clues for their distinct pathogenesis. Am J Dermatopathol. 2009;31:288-292.
15. Rocha RH, Emerich PS, Diniz LM, et al. Lucio’s phenomenon: exuberant case report and review of Brazilian cases. An Bras Dermatol. 2016;91(suppl 5):S60-S63.
16. Costa IM, Kawano LB, Pereira CP, et al. Lucio’s phenomenon: a case report and review of the literature. Int J Dermatol. 2005;44:566-571.
17. Kumari R, Thappa DM, Basu D. A fatal case of Lucio phenomenon from India. Dermatol Online J. 2008;14:10.
18. Lucio R, Alvarado I. Opúsculo Sobre el Mal de San Lázaro o Elefantiasis de los Griegos. M. Murguía; 1852.
19. Latapí F, Chévez-Zamora A. The “spotted” leprosy of Lucio: an introduction to its clinical and histological study. Int J Lepr. 1948;16:421-437.
20. Vargas OF. Diffuse leprosy of Lucio and Latapí: a histologic study. Lepr Rev. 2007;78:248-260.
21. Latapí FR, Chevez-Zamora A. La lepra manchada de Lucio. Rev Dermatol Mex. 1978;22:102-107.
22. Monteiro R, Abreu MA, Tiezzi MG, et al. Fenômeno de Lúcio: mais um caso relatado no Brasil. An Bras Dermatol. 2012;87:296-300.
23. Gharavi EE, Chaimovich H, Cucucrull E, et al. Induction of antiphospholipid antibodies by immunization with synthetic bacterial & viral peptides. Lupus. 1999;8:449-455.
24. de Larrañaga GF, Forastiero RR, Martinuzzo ME, et al. High prevalence of antiphospholipid antibodies in leprosy: evaluation of antigen reactivity. Lupus. 2000;9:594-600.
25. Loizou S, Singh S, Wypkema E, et al. Anticardiolipin, anti-beta(2)-glycoprotein I and antiprothrombin antibodies in black South African patients with infectious disease. Ann Rheum Dis. 2003;62:1106-1111.
26. Akerkar SM, Bichile LS. Leprosy & gangrene: a rare association; role of antiphospholipid antibodies. BMC Infect Dis. 2005,5:74.
27. Horta-Baas G, Hernández-Cabrera MF, Barile-Fabris LA, et al. Multibacillary leprosy mimicking systemic lupus erythematosus: case report and literature review. Lupus. 2015;24:1095-1102.
28. Pradhan V, Badakere SS, Shankar KU. Increased incidence of cytoplasmic ANCA (cANCA) and other auto antibodies in leprosy patients from western India. Lepr Rev. 2004;75:50-56.
29. Oskam L. Diagnosis and classification of leprosy. Lepr Rev. 2002;73:17-26.
30. Rao PN. Recent advances in the control programs and therapy of leprosy. Indian J Dermatol Venereol Leprol. 2004;70:269-276.
31. Rao PN, Pratap D, Ramana Reddy AV, et al. Evaluation of leprosy patients with 1 to 5 skin lesions with relevance to their grouping into paucibacillary or multibacillary disease. Indian J Dermatol Venereol Leprol. 2006;72:207-210.
32. Rosado FGN, Kim AS. Hemophagocytic lymphohistiocytosis. an update on diagnosis and pathogenesis. Am J Clin Pathol. 2013;139:713-727.
33. Kar HK, Mohanty HC, Mohanty GN, et al. Clinicopathological study of lymph node involvement in leprosy. Lepr India. 1983;55:725-738.
34. Gupta JC, Panda PK, Shrivastava KK, et al. A histopathologic study of lymph nodes in 43 cases of leprosy. Lepr India. 1978;50:196-203.
35. WHO Expert Committee on Leprosy. Seventh Report. World Health Organization; 1998. https://apps.who.int/iris/bitstream/handle/10665/42060/WHO_TRS_874.pdf?sequence=1&isAllowed=y
36. Misra DP, Parida JR, Chowdhury AC, et al. Lepra reaction with Lucio phenomenon mimicking cutaneous vasculitis. Case Rep Immunol. 2014;2014:641989.
37. Amer A, Mansour A. Epidemiological study of leprosy in Egypt: 2005-2009. Egypt J Dermatol Venereol. 2014;34:70-73.
38. World Health Organization. Screening campaign aims to eliminate leprosy in Egypt. Published May 9, 2018. Accessed September 8, 2021. http://www.emro.who.int/egy/egypt-events/last-miless-activities-on-eliminating-leprosy-from-egypt.html
Case Report
A 70-year-old man living in Esna, Luxor, Egypt presented to the Department of Rheumatology and Rehabilitation with widespread gangrenous skin lesions associated with ulcers of 2 weeks’ duration. One year prior, the patient had an insidious onset of nocturnal fever, bilateral leg edema, and numbness and a tingling sensation in both hands. He presented some laboratory and radiologic investigations that were performed at another hospital prior to the current presentation, which revealed thrombocytopenia, mild splenomegaly, and generalized lymphadenopathy. An excisional left axillary lymph node biopsy was performed at another hospital prior to the current presentation, and the pathology report provided by the patient described a reactive, foamy, histiocyte-rich lesion, suggesting a diagnosis of hemophagocytic lymphohistiocytosis. The patient had no diabetes or hypertension and no history of deep vein thrombosis, stroke, or unintentional weight loss. No medications were taken prior to the onset of the skin lesions, and his family history was irrelevant.
General examination at the current presentation revealed a fever (temperature, 101.3 °F [38.5 °C]), a normal heart rate (90 beats per minute), normal blood pressure (120/80 mmHg), normal respiratory rate (14 breaths per minute), accentuated heart sounds, and normal vesicular breathing without adventitious sounds. He had saddle nose, loss of the outer third of the eyebrows, and marked reduction in the density of the eyelashes (madarosis). Bilateral pitting edema of the legs also was present. Neurologic examination revealed hypoesthesia in a glove-and-stocking pattern, thickened peripheral nerves, and trophic changes over both hands; however, he had normal muscle power and deep reflexes. Joint examination revealed no abnormalities. Skin examination revealed widespread, reticulated, necrotizing, purpuric lesions on the arms, legs, abdomen, and ears, some associated with gangrenous ulcerations and hemorrhagic blisters. Scattered vasculitic ulcers and gangrenous patches were seen on the fingers. A gangrenous ulcer mimicking Fournier gangrene was seen involving the scrotal skin in addition to a gangrenous lesion on the glans penis (Figure 1–3). Unaffected skin appeared smooth, shiny, and edematous and showed no nodular lesions. Peripheral pulsations were intact.



Positive findings from a wide panel of laboratory investigations included an elevated erythrocyte sedimentation rate (103 mm for the first hour [reference range, 0–22 mm]), high C-reactive protein (50.7 mg/L [reference range, up to 6 mg/L]), anemia (hemoglobin count, 7.3 g/dL [reference range, 13.5–17.5 g/dL]), thrombocytopenia (45×103/mm3 [reference range, 150×103/mm3), low serum albumin (2.3 g/dL [reference range, 3.4–5.4 g/dL]), elevated IgG and IgM anticardiolipin antibodies (IgG, 21.4 IgG phospholipid [GPL] units [reference range, <10 IgG phospholipid (GPL) units]; IgM, 59.4 IgM phospholipid (MPL) units [reference range, <7 IgM phospholipid (MPL) units]), positive lupus anticoagulant panel test, elevated anti-β2 glycoprotein antibodies (IgG, 17.5
Nerve conduction velocity showed axonal sensory polyneuropathy. Motor nerve conduction studies for median and ulnar nerves were within normal range. Lower-limb nerves assessment was limited by the ulcerated areas and marked edema. Echocardiography was unremarkable. Arterial Doppler studies were only available for the upper limbs and were unremarkable.
A punch biopsy was taken from one of the necrotizing purpuric lesions on the legs, and histopathologic examination revealed foci of epidermal necrosis and subepidermal separation and superficial and deep perivascular and periadnexal infiltrates extending into the fat lobules. The infiltrates were mainly made up of foamy macrophages, and some contained globi (lepra cells), in addition to lymphocytes and many neutrophils with nuclear dust. Blood vessels in the superficial and deep dermis and in the subcutaneous fat showed fibrinoid necrosis in their walls with neutrophils infiltrating the walls and thrombi in the lumens (Figure 4). Modified Ziehl-Neelsen staining revealed clumps of acid-fast lepra bacilli inside vascular lumina and endothelial cell lining and within the foamy macrophages (Figure 5). Slit-skin smear examination was performed twice and yielded negative results. The slide and paraffin block of the already performed lymph node biopsy were retrieved. Examination revealed aggregates of foamy histiocytes surrounded by lymphocytes and plasma cells replacing normal lymphoid follicles. Modified Ziehl-Neelsen stain was performed, and clusters of acid-fast bacilli were detected within the foamy histiocytic infiltrate (Figure 6).



According to the results of the skin biopsy, the revised result of the lymph node biopsy, and the pattern of neurologic deficit together with clinical and laboratory correlation, the patient was diagnosed with diffuse nonnodular lepromatous leprosy presenting with Lucio phenomenon (Lucio leprosy) and associated with lepromatous lymphadenitis.
The patient received the following treatment: methylprednisolone 500 mg (intravenous pulse therapy) followed by daily oral administration of prednisolone 10 mg, rifampicin 300 mg, dapsone 100 mg, clofazimine 100 mg, acetylsalicylic acid 150 mg, and enoxaparin sodium 80 mg. In addition, the scrotal Fournier gangrene–like lesion was treated by surgical debridement followed by vacuum therapy. By the second week after treatment, the gangrenous lesions of the fingers developed a line of demarcation, and the skin infarctions started to recede.
Comment
Despite a decrease in its prevalence through a World Health Organization (WHO)–empowered eradication program, leprosy still represents a health problem in endemic areas.1,2 It is characterized by a wide range of immune responses to Mycobacterium leprae, displaying a spectrum of clinical and histopathologic manifestations that vary from the tuberculoid or paucibacillary pole with a strong cell-mediated immune response and fewer organisms to the lepromatous or multibacillary pole with weaker cell-mediated immune response and higher loads of organisms.3 In addition to its well-known cutaneous and neurologic manifestations, leprosy can present with a variety of manifestations, including constitutional symptoms, musculoskeletal manifestations, and serologic abnormalities; thus, leprosy can mimic rheumatoid arthritis, spondyloarthritis, and vasculitis—a pitfall that may result in misdiagnosis as a rheumatologic disorder.3-7
The chronic course of leprosy can be disrupted by acute, immunologically mediated reactions known as lepra reactions, of which there are 3 types.8 Type I lepra reactions are cell mediated and occur mainly in patients with borderline disease, often representing an upgrade toward the tuberculoid pole; less often they represent a downgrade reaction. Nerves become painful and swollen with possible loss of function, and skin lesions become edematous and tender; sometimes arthritis develops.9 Type II lepra reactions, also known as erythema nodosum leprosum (ENL), occur in borderline lepromatous and lepromatous patients with a high bacillary load. They are characterized by fever, body aches, tender cutaneous/subcutaneous nodules that may ulcerate, possible bullous lesions, painful nerve swellings, swollen joints, iritis, lymphadenitis, glomerulonephritis, epididymo-orchitis, and hepatic affection. Both immune-complex and delayed hypersensitivity reactions play a role in ENL.8,10 The third reaction is a rare aggressive type known as Lucio phenomenon or Lucio leprosy, which presents with irregular-shaped, angulated, or stellar necrotizing purpuric lesions (hemorrhagic infacrtions) developing mainly on the extremities. The lesions evolve into ulcers that heal with atrophic scarring.2,11 Lucio phenomenon develops as a result of thrombotic vascular occlusion secondary to massive invasion of vascular endothelial cells by lepra bacilli.2,11-14 Involvement of the scrotal skin, such as in our patient, is rare.
Lucio phenomenon mainly is seen in Mexico and Central America, and few cases have been documented in Cuba, South America, the United States, India, Polynesia, South Africa, and Southeast Asia.15-17 It specifically occurs in patients with untreated, diffuse, nonnodular lepromatous leprosy (pure and primitive diffuse lepromatous leprosy (DLL)/diffuse leprosy of Lucio and Latapí). This type of leprosy was first described by Lucio and Alvarado18 in 1852 as a distinct form of lepromatous leprosy characterized by widespread and dense infiltration of the whole skin by lepra bacilli without the typical nodular lesions of leprosy, rendering its diagnosis challenging, especially in sporadic cases. Other manifestations of DLL include complete alopecia of the eyebrows and eyelashes, destructive rhinitis, and areas of anhidrosis and dyesthesia.2
Latapí and Chévez-Zomora19 defined Lucio phenomenon in 1948 as a form of histopathologic vasculitis restricted to patients with DLL. Histopathologically, in addition to the infiltration of the skin with acid-fast bacilli–laden foamy histiocytes, lesions of Lucio phenomenon show features of necrotizing (leukocytoclastic) vasculitis with fibrinoid necrosis20 or vascular thrombi with minimal perivascular lymphocytic infiltrate and no evidence of vasculitis.11 Medium to large vessels in the deep dermis and subcutaneous tissue show infiltration of their walls with a large number of macrophages laden with acid-fast bacilli.11 Cases with histopathologic features mimicking antiphospholipid syndrome with endothelial cell proliferation, thrombosis, and mild mononuclear cell infiltrate also may be seen.20 In all cases, ischemic epidermal necrosis is seen, as well as acid-fast bacilli, both singly and in clusters (globi) within endothelial cells and inside blood vessel lumina.
Although Lucio phenomenon initially was thought to be immune-complex mediated like ENL, it has been suggested that the main trigger is thrombotic vascular occlusion secondary to massive invasion of the vascular endothelial cells by the lepra bacilli, resulting in necrosis.14 Bacterial lipopolysaccharides promote the release of IL-1 and tumor necrosis factor α, which in turn stimulate the production of prostaglandins, IL-6, and coagulation factor III, leading to vascular thrombosis and tissue necrosis.21,22 Moreover, antiphospholipid antibodies, which have been found to be induced in response to certain infectious agents in genetically predisposed individuals,23 have been reported in patients with leprosy, mainly in association with lepromatous leprosy. The reported prevalence of anticardiolipin antibodies ranged from 37% to 98%, whereas anti-β2-glycoprotein I antibodies ranged from 3% to 19%, and antiprothrombin antibodies ranged from 6% to 45%.24,25 Antiphospholipid antibodies have been reported to play a role in the pathogenesis of Lucio phenomenon.11,13,15,26 Our case supports this hypothesis with positive anticardiolipin antibodies, anti-β2 glycoprotein antibodies, and positive lupus anticoagulant.
In accordance with Curi et al,2 who reported 5 cases of DLL with Lucio phenomenon, our patient showed a similar presentation with positive inflammatory markers in association with a negative autoimmune profile (ANA, ANCA-C&P) and negative venereal disease research laboratory test. It is important to mention that a positive autoimmune profile (ANA, ANCA-C&P) can be present in leprotic patients, causing possible diagnostic confusion with collagen diseases.27,28
An interesting finding in our case was the negative slit-skin smear results. Although the specificity of slit-skin smear is 100%, as it directly demonstrates the presence of acid-fast bacilli,29 its sensitivity is low and varies from 10% to 50%.30 The detection of acid-fast bacilli in tissue sections is reported to be a better method for confirming the diagnosis of leprosy.31
The provisional impression of hemophagocytic lymphohistiocytosis in the lymph node biopsy in our patient was excluded upon detection of acid-fast bacilli in the foamy histiocytes infiltrating the lymph node; moreover, the normal serum lipids and serum ferritin argued against this diagnosis.32 Leprosy tends to involve the lymph nodes, particularly in borderline, borderline lepromatous, and lepromatous forms.33 The incidence of lymph node involvement accompanied by skin lesions with the presence of acid-fast bacilli in the lymph nodes is 92.2%.34
Our patient showed an excellent response to antileprotic treatment, which was administered according to the WHO multidrug therapy guidelines for multibacillary leprosy,35 combined with low-dose prednisolone, acetylsalicylic acid, and anticoagulant treatment. Thalidomide and high-dose prednisolone (60 mg/d) combined with antileprotic treatment also have been reported to be successful in managing recurrent infarctions in leprosy.36 The Fournier-like gangrenous ulcer of the scrotum was managed by surgical debridement and vacuum therapy.
It is noteworthy that the WHO elimination goal for leprosy was to reduce the prevalence to less than 1 case per 10,000 population. Egypt is among the first countries in North Africa and the Middle East regions to achieve this target supervised by the National Leprosy Control Program as early as 1994; this was further reduced to 0.33 cases per 10,000 population in 2004, and reduced again in 2009; however, certain foci showed a prevalence rate more than the elimination target, particularly in the cities of Qena (1.12) and Sohag (2.47).37 Esna, where our patient is from, is an endemic area in Egypt.38
Conclusion
Case Report
A 70-year-old man living in Esna, Luxor, Egypt presented to the Department of Rheumatology and Rehabilitation with widespread gangrenous skin lesions associated with ulcers of 2 weeks’ duration. One year prior, the patient had an insidious onset of nocturnal fever, bilateral leg edema, and numbness and a tingling sensation in both hands. He presented some laboratory and radiologic investigations that were performed at another hospital prior to the current presentation, which revealed thrombocytopenia, mild splenomegaly, and generalized lymphadenopathy. An excisional left axillary lymph node biopsy was performed at another hospital prior to the current presentation, and the pathology report provided by the patient described a reactive, foamy, histiocyte-rich lesion, suggesting a diagnosis of hemophagocytic lymphohistiocytosis. The patient had no diabetes or hypertension and no history of deep vein thrombosis, stroke, or unintentional weight loss. No medications were taken prior to the onset of the skin lesions, and his family history was irrelevant.
General examination at the current presentation revealed a fever (temperature, 101.3 °F [38.5 °C]), a normal heart rate (90 beats per minute), normal blood pressure (120/80 mmHg), normal respiratory rate (14 breaths per minute), accentuated heart sounds, and normal vesicular breathing without adventitious sounds. He had saddle nose, loss of the outer third of the eyebrows, and marked reduction in the density of the eyelashes (madarosis). Bilateral pitting edema of the legs also was present. Neurologic examination revealed hypoesthesia in a glove-and-stocking pattern, thickened peripheral nerves, and trophic changes over both hands; however, he had normal muscle power and deep reflexes. Joint examination revealed no abnormalities. Skin examination revealed widespread, reticulated, necrotizing, purpuric lesions on the arms, legs, abdomen, and ears, some associated with gangrenous ulcerations and hemorrhagic blisters. Scattered vasculitic ulcers and gangrenous patches were seen on the fingers. A gangrenous ulcer mimicking Fournier gangrene was seen involving the scrotal skin in addition to a gangrenous lesion on the glans penis (Figure 1–3). Unaffected skin appeared smooth, shiny, and edematous and showed no nodular lesions. Peripheral pulsations were intact.



Positive findings from a wide panel of laboratory investigations included an elevated erythrocyte sedimentation rate (103 mm for the first hour [reference range, 0–22 mm]), high C-reactive protein (50.7 mg/L [reference range, up to 6 mg/L]), anemia (hemoglobin count, 7.3 g/dL [reference range, 13.5–17.5 g/dL]), thrombocytopenia (45×103/mm3 [reference range, 150×103/mm3), low serum albumin (2.3 g/dL [reference range, 3.4–5.4 g/dL]), elevated IgG and IgM anticardiolipin antibodies (IgG, 21.4 IgG phospholipid [GPL] units [reference range, <10 IgG phospholipid (GPL) units]; IgM, 59.4 IgM phospholipid (MPL) units [reference range, <7 IgM phospholipid (MPL) units]), positive lupus anticoagulant panel test, elevated anti-β2 glycoprotein antibodies (IgG, 17.5
Nerve conduction velocity showed axonal sensory polyneuropathy. Motor nerve conduction studies for median and ulnar nerves were within normal range. Lower-limb nerves assessment was limited by the ulcerated areas and marked edema. Echocardiography was unremarkable. Arterial Doppler studies were only available for the upper limbs and were unremarkable.
A punch biopsy was taken from one of the necrotizing purpuric lesions on the legs, and histopathologic examination revealed foci of epidermal necrosis and subepidermal separation and superficial and deep perivascular and periadnexal infiltrates extending into the fat lobules. The infiltrates were mainly made up of foamy macrophages, and some contained globi (lepra cells), in addition to lymphocytes and many neutrophils with nuclear dust. Blood vessels in the superficial and deep dermis and in the subcutaneous fat showed fibrinoid necrosis in their walls with neutrophils infiltrating the walls and thrombi in the lumens (Figure 4). Modified Ziehl-Neelsen staining revealed clumps of acid-fast lepra bacilli inside vascular lumina and endothelial cell lining and within the foamy macrophages (Figure 5). Slit-skin smear examination was performed twice and yielded negative results. The slide and paraffin block of the already performed lymph node biopsy were retrieved. Examination revealed aggregates of foamy histiocytes surrounded by lymphocytes and plasma cells replacing normal lymphoid follicles. Modified Ziehl-Neelsen stain was performed, and clusters of acid-fast bacilli were detected within the foamy histiocytic infiltrate (Figure 6).



According to the results of the skin biopsy, the revised result of the lymph node biopsy, and the pattern of neurologic deficit together with clinical and laboratory correlation, the patient was diagnosed with diffuse nonnodular lepromatous leprosy presenting with Lucio phenomenon (Lucio leprosy) and associated with lepromatous lymphadenitis.
The patient received the following treatment: methylprednisolone 500 mg (intravenous pulse therapy) followed by daily oral administration of prednisolone 10 mg, rifampicin 300 mg, dapsone 100 mg, clofazimine 100 mg, acetylsalicylic acid 150 mg, and enoxaparin sodium 80 mg. In addition, the scrotal Fournier gangrene–like lesion was treated by surgical debridement followed by vacuum therapy. By the second week after treatment, the gangrenous lesions of the fingers developed a line of demarcation, and the skin infarctions started to recede.
Comment
Despite a decrease in its prevalence through a World Health Organization (WHO)–empowered eradication program, leprosy still represents a health problem in endemic areas.1,2 It is characterized by a wide range of immune responses to Mycobacterium leprae, displaying a spectrum of clinical and histopathologic manifestations that vary from the tuberculoid or paucibacillary pole with a strong cell-mediated immune response and fewer organisms to the lepromatous or multibacillary pole with weaker cell-mediated immune response and higher loads of organisms.3 In addition to its well-known cutaneous and neurologic manifestations, leprosy can present with a variety of manifestations, including constitutional symptoms, musculoskeletal manifestations, and serologic abnormalities; thus, leprosy can mimic rheumatoid arthritis, spondyloarthritis, and vasculitis—a pitfall that may result in misdiagnosis as a rheumatologic disorder.3-7
The chronic course of leprosy can be disrupted by acute, immunologically mediated reactions known as lepra reactions, of which there are 3 types.8 Type I lepra reactions are cell mediated and occur mainly in patients with borderline disease, often representing an upgrade toward the tuberculoid pole; less often they represent a downgrade reaction. Nerves become painful and swollen with possible loss of function, and skin lesions become edematous and tender; sometimes arthritis develops.9 Type II lepra reactions, also known as erythema nodosum leprosum (ENL), occur in borderline lepromatous and lepromatous patients with a high bacillary load. They are characterized by fever, body aches, tender cutaneous/subcutaneous nodules that may ulcerate, possible bullous lesions, painful nerve swellings, swollen joints, iritis, lymphadenitis, glomerulonephritis, epididymo-orchitis, and hepatic affection. Both immune-complex and delayed hypersensitivity reactions play a role in ENL.8,10 The third reaction is a rare aggressive type known as Lucio phenomenon or Lucio leprosy, which presents with irregular-shaped, angulated, or stellar necrotizing purpuric lesions (hemorrhagic infacrtions) developing mainly on the extremities. The lesions evolve into ulcers that heal with atrophic scarring.2,11 Lucio phenomenon develops as a result of thrombotic vascular occlusion secondary to massive invasion of vascular endothelial cells by lepra bacilli.2,11-14 Involvement of the scrotal skin, such as in our patient, is rare.
Lucio phenomenon mainly is seen in Mexico and Central America, and few cases have been documented in Cuba, South America, the United States, India, Polynesia, South Africa, and Southeast Asia.15-17 It specifically occurs in patients with untreated, diffuse, nonnodular lepromatous leprosy (pure and primitive diffuse lepromatous leprosy (DLL)/diffuse leprosy of Lucio and Latapí). This type of leprosy was first described by Lucio and Alvarado18 in 1852 as a distinct form of lepromatous leprosy characterized by widespread and dense infiltration of the whole skin by lepra bacilli without the typical nodular lesions of leprosy, rendering its diagnosis challenging, especially in sporadic cases. Other manifestations of DLL include complete alopecia of the eyebrows and eyelashes, destructive rhinitis, and areas of anhidrosis and dyesthesia.2
Latapí and Chévez-Zomora19 defined Lucio phenomenon in 1948 as a form of histopathologic vasculitis restricted to patients with DLL. Histopathologically, in addition to the infiltration of the skin with acid-fast bacilli–laden foamy histiocytes, lesions of Lucio phenomenon show features of necrotizing (leukocytoclastic) vasculitis with fibrinoid necrosis20 or vascular thrombi with minimal perivascular lymphocytic infiltrate and no evidence of vasculitis.11 Medium to large vessels in the deep dermis and subcutaneous tissue show infiltration of their walls with a large number of macrophages laden with acid-fast bacilli.11 Cases with histopathologic features mimicking antiphospholipid syndrome with endothelial cell proliferation, thrombosis, and mild mononuclear cell infiltrate also may be seen.20 In all cases, ischemic epidermal necrosis is seen, as well as acid-fast bacilli, both singly and in clusters (globi) within endothelial cells and inside blood vessel lumina.
Although Lucio phenomenon initially was thought to be immune-complex mediated like ENL, it has been suggested that the main trigger is thrombotic vascular occlusion secondary to massive invasion of the vascular endothelial cells by the lepra bacilli, resulting in necrosis.14 Bacterial lipopolysaccharides promote the release of IL-1 and tumor necrosis factor α, which in turn stimulate the production of prostaglandins, IL-6, and coagulation factor III, leading to vascular thrombosis and tissue necrosis.21,22 Moreover, antiphospholipid antibodies, which have been found to be induced in response to certain infectious agents in genetically predisposed individuals,23 have been reported in patients with leprosy, mainly in association with lepromatous leprosy. The reported prevalence of anticardiolipin antibodies ranged from 37% to 98%, whereas anti-β2-glycoprotein I antibodies ranged from 3% to 19%, and antiprothrombin antibodies ranged from 6% to 45%.24,25 Antiphospholipid antibodies have been reported to play a role in the pathogenesis of Lucio phenomenon.11,13,15,26 Our case supports this hypothesis with positive anticardiolipin antibodies, anti-β2 glycoprotein antibodies, and positive lupus anticoagulant.
In accordance with Curi et al,2 who reported 5 cases of DLL with Lucio phenomenon, our patient showed a similar presentation with positive inflammatory markers in association with a negative autoimmune profile (ANA, ANCA-C&P) and negative venereal disease research laboratory test. It is important to mention that a positive autoimmune profile (ANA, ANCA-C&P) can be present in leprotic patients, causing possible diagnostic confusion with collagen diseases.27,28
An interesting finding in our case was the negative slit-skin smear results. Although the specificity of slit-skin smear is 100%, as it directly demonstrates the presence of acid-fast bacilli,29 its sensitivity is low and varies from 10% to 50%.30 The detection of acid-fast bacilli in tissue sections is reported to be a better method for confirming the diagnosis of leprosy.31
The provisional impression of hemophagocytic lymphohistiocytosis in the lymph node biopsy in our patient was excluded upon detection of acid-fast bacilli in the foamy histiocytes infiltrating the lymph node; moreover, the normal serum lipids and serum ferritin argued against this diagnosis.32 Leprosy tends to involve the lymph nodes, particularly in borderline, borderline lepromatous, and lepromatous forms.33 The incidence of lymph node involvement accompanied by skin lesions with the presence of acid-fast bacilli in the lymph nodes is 92.2%.34
Our patient showed an excellent response to antileprotic treatment, which was administered according to the WHO multidrug therapy guidelines for multibacillary leprosy,35 combined with low-dose prednisolone, acetylsalicylic acid, and anticoagulant treatment. Thalidomide and high-dose prednisolone (60 mg/d) combined with antileprotic treatment also have been reported to be successful in managing recurrent infarctions in leprosy.36 The Fournier-like gangrenous ulcer of the scrotum was managed by surgical debridement and vacuum therapy.
It is noteworthy that the WHO elimination goal for leprosy was to reduce the prevalence to less than 1 case per 10,000 population. Egypt is among the first countries in North Africa and the Middle East regions to achieve this target supervised by the National Leprosy Control Program as early as 1994; this was further reduced to 0.33 cases per 10,000 population in 2004, and reduced again in 2009; however, certain foci showed a prevalence rate more than the elimination target, particularly in the cities of Qena (1.12) and Sohag (2.47).37 Esna, where our patient is from, is an endemic area in Egypt.38
Conclusion
1. World Health Organization. World Health Statistics: 2011. World Health Organization; 2011. https://www.who.int/gho/publications/world_health_statistics/EN_WHS2011_Full.pdf
2. Curi PF, Villaroel JS, Migliore N, et al. Lucio’s phenomenon: report of five cases. Clin Rheumatol. 2016;35:1397-1401.
3. Shrestha B, Li YQ, Fu P. Leprosy mimics adult onset Still’s disease in a Chinese patient. Egypt Rheumatol. 2018;40:217-220.
4. Prasad S, Misra R, Aggarwal A, et al. Leprosy revealed in a rheumatology clinic: a case series. Int J Rheum Dis. 2013;16:129-133.
5. Chao G, Fang L, Lu C. Leprosy with ANA positive mistaken for connective tissue disease. Clin Rheumatol. 2013;32:645-648.
6. Chauhan S, Wakhlu A, Agarwal V. Arthritis in leprosy. Rheumatology. 2010;49:2237-2242.
7. Rath D, Bhargava S, Kundu BK. Leprosy mimicking common rheumatologic entities: a trial for the clinician in the era of biologics. Case Rep Rheumatol. 2014;2014:429698.
8. Cuevas J, Rodríguez-Peralto JL, Carrillo R, et al. Erythema nodosum leprosum: reactional leprosy. Semin Cutan Med Surg. 2007;26:126-130.
9. Henriques CC, Lopéz B, Mestre T, et al. Leprosy and rheumatoid arthritis: consequence or association? BMJ Case Rep. 2012;13:1-4.
10. Vázquez-Botet M, Sánchez JL. Erythema nodosum leprosum. Int J Dermatol. 1987;26:436-437.
11. Nunzie E, Ortega Cabrera LV, Macanchi Moncayo FM, et al. Lucio leprosy with Lucio’s phenomenon, digital gangrene and anticardiolipin antibodies. Lepr Rev. 2014;85:194-200.
12. Salvi S, Chopra A. Leprosy in a rheumatology setting: a challenging mimic to expose. Clin Rheumatol. 2013;32:1557-1563.
13. Azulay-Abulafia L, Pereira SL, Hardmann D, et al. Lucio phenomenon. vasculitis or occlusive vasculopathy? Hautarzt. 2006;57:1101-1105.
14. Benard G, Sakai-Valente NY, Bianconcini Trindade MA. Concomittant Lucio phenomenon and erythema nodosum in a leprosy patient: clues for their distinct pathogenesis. Am J Dermatopathol. 2009;31:288-292.
15. Rocha RH, Emerich PS, Diniz LM, et al. Lucio’s phenomenon: exuberant case report and review of Brazilian cases. An Bras Dermatol. 2016;91(suppl 5):S60-S63.
16. Costa IM, Kawano LB, Pereira CP, et al. Lucio’s phenomenon: a case report and review of the literature. Int J Dermatol. 2005;44:566-571.
17. Kumari R, Thappa DM, Basu D. A fatal case of Lucio phenomenon from India. Dermatol Online J. 2008;14:10.
18. Lucio R, Alvarado I. Opúsculo Sobre el Mal de San Lázaro o Elefantiasis de los Griegos. M. Murguía; 1852.
19. Latapí F, Chévez-Zamora A. The “spotted” leprosy of Lucio: an introduction to its clinical and histological study. Int J Lepr. 1948;16:421-437.
20. Vargas OF. Diffuse leprosy of Lucio and Latapí: a histologic study. Lepr Rev. 2007;78:248-260.
21. Latapí FR, Chevez-Zamora A. La lepra manchada de Lucio. Rev Dermatol Mex. 1978;22:102-107.
22. Monteiro R, Abreu MA, Tiezzi MG, et al. Fenômeno de Lúcio: mais um caso relatado no Brasil. An Bras Dermatol. 2012;87:296-300.
23. Gharavi EE, Chaimovich H, Cucucrull E, et al. Induction of antiphospholipid antibodies by immunization with synthetic bacterial & viral peptides. Lupus. 1999;8:449-455.
24. de Larrañaga GF, Forastiero RR, Martinuzzo ME, et al. High prevalence of antiphospholipid antibodies in leprosy: evaluation of antigen reactivity. Lupus. 2000;9:594-600.
25. Loizou S, Singh S, Wypkema E, et al. Anticardiolipin, anti-beta(2)-glycoprotein I and antiprothrombin antibodies in black South African patients with infectious disease. Ann Rheum Dis. 2003;62:1106-1111.
26. Akerkar SM, Bichile LS. Leprosy & gangrene: a rare association; role of antiphospholipid antibodies. BMC Infect Dis. 2005,5:74.
27. Horta-Baas G, Hernández-Cabrera MF, Barile-Fabris LA, et al. Multibacillary leprosy mimicking systemic lupus erythematosus: case report and literature review. Lupus. 2015;24:1095-1102.
28. Pradhan V, Badakere SS, Shankar KU. Increased incidence of cytoplasmic ANCA (cANCA) and other auto antibodies in leprosy patients from western India. Lepr Rev. 2004;75:50-56.
29. Oskam L. Diagnosis and classification of leprosy. Lepr Rev. 2002;73:17-26.
30. Rao PN. Recent advances in the control programs and therapy of leprosy. Indian J Dermatol Venereol Leprol. 2004;70:269-276.
31. Rao PN, Pratap D, Ramana Reddy AV, et al. Evaluation of leprosy patients with 1 to 5 skin lesions with relevance to their grouping into paucibacillary or multibacillary disease. Indian J Dermatol Venereol Leprol. 2006;72:207-210.
32. Rosado FGN, Kim AS. Hemophagocytic lymphohistiocytosis. an update on diagnosis and pathogenesis. Am J Clin Pathol. 2013;139:713-727.
33. Kar HK, Mohanty HC, Mohanty GN, et al. Clinicopathological study of lymph node involvement in leprosy. Lepr India. 1983;55:725-738.
34. Gupta JC, Panda PK, Shrivastava KK, et al. A histopathologic study of lymph nodes in 43 cases of leprosy. Lepr India. 1978;50:196-203.
35. WHO Expert Committee on Leprosy. Seventh Report. World Health Organization; 1998. https://apps.who.int/iris/bitstream/handle/10665/42060/WHO_TRS_874.pdf?sequence=1&isAllowed=y
36. Misra DP, Parida JR, Chowdhury AC, et al. Lepra reaction with Lucio phenomenon mimicking cutaneous vasculitis. Case Rep Immunol. 2014;2014:641989.
37. Amer A, Mansour A. Epidemiological study of leprosy in Egypt: 2005-2009. Egypt J Dermatol Venereol. 2014;34:70-73.
38. World Health Organization. Screening campaign aims to eliminate leprosy in Egypt. Published May 9, 2018. Accessed September 8, 2021. http://www.emro.who.int/egy/egypt-events/last-miless-activities-on-eliminating-leprosy-from-egypt.html
1. World Health Organization. World Health Statistics: 2011. World Health Organization; 2011. https://www.who.int/gho/publications/world_health_statistics/EN_WHS2011_Full.pdf
2. Curi PF, Villaroel JS, Migliore N, et al. Lucio’s phenomenon: report of five cases. Clin Rheumatol. 2016;35:1397-1401.
3. Shrestha B, Li YQ, Fu P. Leprosy mimics adult onset Still’s disease in a Chinese patient. Egypt Rheumatol. 2018;40:217-220.
4. Prasad S, Misra R, Aggarwal A, et al. Leprosy revealed in a rheumatology clinic: a case series. Int J Rheum Dis. 2013;16:129-133.
5. Chao G, Fang L, Lu C. Leprosy with ANA positive mistaken for connective tissue disease. Clin Rheumatol. 2013;32:645-648.
6. Chauhan S, Wakhlu A, Agarwal V. Arthritis in leprosy. Rheumatology. 2010;49:2237-2242.
7. Rath D, Bhargava S, Kundu BK. Leprosy mimicking common rheumatologic entities: a trial for the clinician in the era of biologics. Case Rep Rheumatol. 2014;2014:429698.
8. Cuevas J, Rodríguez-Peralto JL, Carrillo R, et al. Erythema nodosum leprosum: reactional leprosy. Semin Cutan Med Surg. 2007;26:126-130.
9. Henriques CC, Lopéz B, Mestre T, et al. Leprosy and rheumatoid arthritis: consequence or association? BMJ Case Rep. 2012;13:1-4.
10. Vázquez-Botet M, Sánchez JL. Erythema nodosum leprosum. Int J Dermatol. 1987;26:436-437.
11. Nunzie E, Ortega Cabrera LV, Macanchi Moncayo FM, et al. Lucio leprosy with Lucio’s phenomenon, digital gangrene and anticardiolipin antibodies. Lepr Rev. 2014;85:194-200.
12. Salvi S, Chopra A. Leprosy in a rheumatology setting: a challenging mimic to expose. Clin Rheumatol. 2013;32:1557-1563.
13. Azulay-Abulafia L, Pereira SL, Hardmann D, et al. Lucio phenomenon. vasculitis or occlusive vasculopathy? Hautarzt. 2006;57:1101-1105.
14. Benard G, Sakai-Valente NY, Bianconcini Trindade MA. Concomittant Lucio phenomenon and erythema nodosum in a leprosy patient: clues for their distinct pathogenesis. Am J Dermatopathol. 2009;31:288-292.
15. Rocha RH, Emerich PS, Diniz LM, et al. Lucio’s phenomenon: exuberant case report and review of Brazilian cases. An Bras Dermatol. 2016;91(suppl 5):S60-S63.
16. Costa IM, Kawano LB, Pereira CP, et al. Lucio’s phenomenon: a case report and review of the literature. Int J Dermatol. 2005;44:566-571.
17. Kumari R, Thappa DM, Basu D. A fatal case of Lucio phenomenon from India. Dermatol Online J. 2008;14:10.
18. Lucio R, Alvarado I. Opúsculo Sobre el Mal de San Lázaro o Elefantiasis de los Griegos. M. Murguía; 1852.
19. Latapí F, Chévez-Zamora A. The “spotted” leprosy of Lucio: an introduction to its clinical and histological study. Int J Lepr. 1948;16:421-437.
20. Vargas OF. Diffuse leprosy of Lucio and Latapí: a histologic study. Lepr Rev. 2007;78:248-260.
21. Latapí FR, Chevez-Zamora A. La lepra manchada de Lucio. Rev Dermatol Mex. 1978;22:102-107.
22. Monteiro R, Abreu MA, Tiezzi MG, et al. Fenômeno de Lúcio: mais um caso relatado no Brasil. An Bras Dermatol. 2012;87:296-300.
23. Gharavi EE, Chaimovich H, Cucucrull E, et al. Induction of antiphospholipid antibodies by immunization with synthetic bacterial & viral peptides. Lupus. 1999;8:449-455.
24. de Larrañaga GF, Forastiero RR, Martinuzzo ME, et al. High prevalence of antiphospholipid antibodies in leprosy: evaluation of antigen reactivity. Lupus. 2000;9:594-600.
25. Loizou S, Singh S, Wypkema E, et al. Anticardiolipin, anti-beta(2)-glycoprotein I and antiprothrombin antibodies in black South African patients with infectious disease. Ann Rheum Dis. 2003;62:1106-1111.
26. Akerkar SM, Bichile LS. Leprosy & gangrene: a rare association; role of antiphospholipid antibodies. BMC Infect Dis. 2005,5:74.
27. Horta-Baas G, Hernández-Cabrera MF, Barile-Fabris LA, et al. Multibacillary leprosy mimicking systemic lupus erythematosus: case report and literature review. Lupus. 2015;24:1095-1102.
28. Pradhan V, Badakere SS, Shankar KU. Increased incidence of cytoplasmic ANCA (cANCA) and other auto antibodies in leprosy patients from western India. Lepr Rev. 2004;75:50-56.
29. Oskam L. Diagnosis and classification of leprosy. Lepr Rev. 2002;73:17-26.
30. Rao PN. Recent advances in the control programs and therapy of leprosy. Indian J Dermatol Venereol Leprol. 2004;70:269-276.
31. Rao PN, Pratap D, Ramana Reddy AV, et al. Evaluation of leprosy patients with 1 to 5 skin lesions with relevance to their grouping into paucibacillary or multibacillary disease. Indian J Dermatol Venereol Leprol. 2006;72:207-210.
32. Rosado FGN, Kim AS. Hemophagocytic lymphohistiocytosis. an update on diagnosis and pathogenesis. Am J Clin Pathol. 2013;139:713-727.
33. Kar HK, Mohanty HC, Mohanty GN, et al. Clinicopathological study of lymph node involvement in leprosy. Lepr India. 1983;55:725-738.
34. Gupta JC, Panda PK, Shrivastava KK, et al. A histopathologic study of lymph nodes in 43 cases of leprosy. Lepr India. 1978;50:196-203.
35. WHO Expert Committee on Leprosy. Seventh Report. World Health Organization; 1998. https://apps.who.int/iris/bitstream/handle/10665/42060/WHO_TRS_874.pdf?sequence=1&isAllowed=y
36. Misra DP, Parida JR, Chowdhury AC, et al. Lepra reaction with Lucio phenomenon mimicking cutaneous vasculitis. Case Rep Immunol. 2014;2014:641989.
37. Amer A, Mansour A. Epidemiological study of leprosy in Egypt: 2005-2009. Egypt J Dermatol Venereol. 2014;34:70-73.
38. World Health Organization. Screening campaign aims to eliminate leprosy in Egypt. Published May 9, 2018. Accessed September 8, 2021. http://www.emro.who.int/egy/egypt-events/last-miless-activities-on-eliminating-leprosy-from-egypt.html
Practice Points
- Leprosy is a great mimicker of many connective tissue diseases, including vasculitis.
- Antiphospholipid antibodies are involved in Lucio phenomenon.
- Prompt treatment is important in Lucio phenomenon to avoid morbidity and mortality.
Booster recommendations for pregnant women, teens, and other groups explained
These recommendations have been widened because of the continued emergence of new variants of the virus and the wane of protection over time for both vaccinations and previous disease.
The new recommendations take away some of the questions surrounding eligibility for booster vaccinations while potentially leaving some additional questions. All in all, they provide flexibility for individuals to help protect themselves against the COVID-19 virus, as many are considering celebrating the holidays with friends and family.
The first item that has become clear is that all individuals over 18 are now not only eligible for a booster vaccination a certain time after they have completed their series, but have a recommendation for one.1
But what about a fourth dose? There is a possibility that some patients should be receiving one. For those who require a three-dose series due to a condition that makes them immunocompromised, they should receive their booster vaccination six months after completion of the three-dose series. This distinction may cause confusion for some, but is important for those immunocompromised.
Boosters in women who are pregnant
The recommendations also include specific comments about individuals who are pregnant. Although initial studies did not include pregnant individuals, there has been increasing real world data that vaccination against COVID, including booster vaccinations, is safe and recommended. As pregnancy increases the risk of severe disease if infected by COVID-19, both the CDC and the American College of Obstetricians and Gynecologists,2 along with other specialty organizations, such as the Royal College of Obstetricians and Gynaecologists, recommend vaccinations for pregnant individuals.
The CDC goes on to describe that there is no evidence of vaccination increasing the risk of infertility. The vaccine protects the pregnant individual and also provides protection to the baby once born. The same is true of breastfeeding individuals.3
I hope that this information allows physicians to feel comfortable recommending vaccinations and boosters to those who are pregnant and breast feeding.
Expanded recommendations for those aged 16-17 years
Recently, the CDC also expanded booster recommendations to include those aged 16-17 years, 6 months after completing their vaccine series.
Those under 18 are currently only able to receive the Pfizer-BioNtech vaccine. This new guidance has left some parents wondering if there will also be approval for booster vaccinations soon for those aged 12-16 who are approaching or have reached six months past the initial vaccine.1
Booster brand for those over 18 years?
Although the recommendation has been simplified for all over age 18 years, there is still a decision to be made about which vaccine to use as the booster.
The recommendations allow individuals to decide which brand of vaccine they would like to have as a booster. They may choose to be vaccinated with the same vaccine they originally received or with a different vaccine. This vaccine flexibility may cause confusion, but ultimately is a good thing as it allows individuals to receive whatever vaccine is available and most convenient. This also allows individuals who have been vaccinated outside of the United States by a different brand of vaccine to also receive a booster vaccination with one of the options available here.
Take home message
Overall, the expansion of booster recommendations will help everyone avoid severe disease from COVID-19 infections. Physicians now have more clarity on who should be receiving these vaccines. Along with testing, masking, and appropriate distancing, these recommendations should help prevent severe disease and death from COVID-19.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program, also in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. COVID-19 Vaccine Booster Shots. Centers for Disease Control and Prevention. 2021 Dec 9.
2. COVID-19 Vaccines and Pregnancy: Conversation Guide. American College of Obstetricians and Gynecologists. 2021 November.
3. COVID-19 Vaccines While Pregnant or Breastfeeding. Centers for Disease Control and Prevention. 2021 Dec 6.
These recommendations have been widened because of the continued emergence of new variants of the virus and the wane of protection over time for both vaccinations and previous disease.
The new recommendations take away some of the questions surrounding eligibility for booster vaccinations while potentially leaving some additional questions. All in all, they provide flexibility for individuals to help protect themselves against the COVID-19 virus, as many are considering celebrating the holidays with friends and family.
The first item that has become clear is that all individuals over 18 are now not only eligible for a booster vaccination a certain time after they have completed their series, but have a recommendation for one.1
But what about a fourth dose? There is a possibility that some patients should be receiving one. For those who require a three-dose series due to a condition that makes them immunocompromised, they should receive their booster vaccination six months after completion of the three-dose series. This distinction may cause confusion for some, but is important for those immunocompromised.
Boosters in women who are pregnant
The recommendations also include specific comments about individuals who are pregnant. Although initial studies did not include pregnant individuals, there has been increasing real world data that vaccination against COVID, including booster vaccinations, is safe and recommended. As pregnancy increases the risk of severe disease if infected by COVID-19, both the CDC and the American College of Obstetricians and Gynecologists,2 along with other specialty organizations, such as the Royal College of Obstetricians and Gynaecologists, recommend vaccinations for pregnant individuals.
The CDC goes on to describe that there is no evidence of vaccination increasing the risk of infertility. The vaccine protects the pregnant individual and also provides protection to the baby once born. The same is true of breastfeeding individuals.3
I hope that this information allows physicians to feel comfortable recommending vaccinations and boosters to those who are pregnant and breast feeding.
Expanded recommendations for those aged 16-17 years
Recently, the CDC also expanded booster recommendations to include those aged 16-17 years, 6 months after completing their vaccine series.
Those under 18 are currently only able to receive the Pfizer-BioNtech vaccine. This new guidance has left some parents wondering if there will also be approval for booster vaccinations soon for those aged 12-16 who are approaching or have reached six months past the initial vaccine.1
Booster brand for those over 18 years?
Although the recommendation has been simplified for all over age 18 years, there is still a decision to be made about which vaccine to use as the booster.
The recommendations allow individuals to decide which brand of vaccine they would like to have as a booster. They may choose to be vaccinated with the same vaccine they originally received or with a different vaccine. This vaccine flexibility may cause confusion, but ultimately is a good thing as it allows individuals to receive whatever vaccine is available and most convenient. This also allows individuals who have been vaccinated outside of the United States by a different brand of vaccine to also receive a booster vaccination with one of the options available here.
Take home message
Overall, the expansion of booster recommendations will help everyone avoid severe disease from COVID-19 infections. Physicians now have more clarity on who should be receiving these vaccines. Along with testing, masking, and appropriate distancing, these recommendations should help prevent severe disease and death from COVID-19.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program, also in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. COVID-19 Vaccine Booster Shots. Centers for Disease Control and Prevention. 2021 Dec 9.
2. COVID-19 Vaccines and Pregnancy: Conversation Guide. American College of Obstetricians and Gynecologists. 2021 November.
3. COVID-19 Vaccines While Pregnant or Breastfeeding. Centers for Disease Control and Prevention. 2021 Dec 6.
These recommendations have been widened because of the continued emergence of new variants of the virus and the wane of protection over time for both vaccinations and previous disease.
The new recommendations take away some of the questions surrounding eligibility for booster vaccinations while potentially leaving some additional questions. All in all, they provide flexibility for individuals to help protect themselves against the COVID-19 virus, as many are considering celebrating the holidays with friends and family.
The first item that has become clear is that all individuals over 18 are now not only eligible for a booster vaccination a certain time after they have completed their series, but have a recommendation for one.1
But what about a fourth dose? There is a possibility that some patients should be receiving one. For those who require a three-dose series due to a condition that makes them immunocompromised, they should receive their booster vaccination six months after completion of the three-dose series. This distinction may cause confusion for some, but is important for those immunocompromised.
Boosters in women who are pregnant
The recommendations also include specific comments about individuals who are pregnant. Although initial studies did not include pregnant individuals, there has been increasing real world data that vaccination against COVID, including booster vaccinations, is safe and recommended. As pregnancy increases the risk of severe disease if infected by COVID-19, both the CDC and the American College of Obstetricians and Gynecologists,2 along with other specialty organizations, such as the Royal College of Obstetricians and Gynaecologists, recommend vaccinations for pregnant individuals.
The CDC goes on to describe that there is no evidence of vaccination increasing the risk of infertility. The vaccine protects the pregnant individual and also provides protection to the baby once born. The same is true of breastfeeding individuals.3
I hope that this information allows physicians to feel comfortable recommending vaccinations and boosters to those who are pregnant and breast feeding.
Expanded recommendations for those aged 16-17 years
Recently, the CDC also expanded booster recommendations to include those aged 16-17 years, 6 months after completing their vaccine series.
Those under 18 are currently only able to receive the Pfizer-BioNtech vaccine. This new guidance has left some parents wondering if there will also be approval for booster vaccinations soon for those aged 12-16 who are approaching or have reached six months past the initial vaccine.1
Booster brand for those over 18 years?
Although the recommendation has been simplified for all over age 18 years, there is still a decision to be made about which vaccine to use as the booster.
The recommendations allow individuals to decide which brand of vaccine they would like to have as a booster. They may choose to be vaccinated with the same vaccine they originally received or with a different vaccine. This vaccine flexibility may cause confusion, but ultimately is a good thing as it allows individuals to receive whatever vaccine is available and most convenient. This also allows individuals who have been vaccinated outside of the United States by a different brand of vaccine to also receive a booster vaccination with one of the options available here.
Take home message
Overall, the expansion of booster recommendations will help everyone avoid severe disease from COVID-19 infections. Physicians now have more clarity on who should be receiving these vaccines. Along with testing, masking, and appropriate distancing, these recommendations should help prevent severe disease and death from COVID-19.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program, also in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. COVID-19 Vaccine Booster Shots. Centers for Disease Control and Prevention. 2021 Dec 9.
2. COVID-19 Vaccines and Pregnancy: Conversation Guide. American College of Obstetricians and Gynecologists. 2021 November.
3. COVID-19 Vaccines While Pregnant or Breastfeeding. Centers for Disease Control and Prevention. 2021 Dec 6.
Oral step-down therapy for infective endocarditis
Background: The standard of care for IE has been a prolonged course of IV antibiotics. Recent literature has suggested that oral antibiotics might be a safe and effective step-down therapy for IE.
Study design: Systematic review.
Setting: Literature review in October 2019, with update in February 2020, consisting of 21 observational studies and 3 randomized controlled trials.
Synopsis: Three RCTs and 21 observational studies were reviewed, with a focus on the effectiveness of antibiotics administered orally for part of the therapeutic course for IE patients. Patients included in the study had left- or right-sided IE. Pathogens included viridians streptococci, staphylococci, and enterococci, with a minority of patients infected with methicillin-resistant Staphylococcus aureus. Treatment regimens included beta-lactams, linezolid, fluoroquinolones, trimethoprim-sulfamethoxazole, or clindamycin, with or without rifampin.
In studies wherein IV antibiotics alone were compared with IV antibiotics with oral step-down therapy, there was no difference in clinical cure rate. Those given oral step-down therapy had a statistically significant lower mortality rate than patients who received only IV therapy.
Limitations include inconclusive data regarding duration of IV lead-in therapy, with the variance before conversion to oral antibiotics amongst the studies ranging from 0 to 24 days. The limited number of patients with MRSA infections makes it difficult to draw conclusions regarding this particular pathogen.
Bottom line: Highly orally bioavailable antibiotics should be considered for patients with IE who have cleared bacteremia and achieved clinical stability with IV regimens.
Citation: Spellberg B et al. Evaluation of a paradigm shift from intravenous antibiotics to oral step-down therapy for the treatment of infective endocarditis: a narrative review. JAMA Intern Med. 2020;180(5):769-77. doi: 10.1001/jamainternmed.2020.0555.
Dr. Yoo is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: The standard of care for IE has been a prolonged course of IV antibiotics. Recent literature has suggested that oral antibiotics might be a safe and effective step-down therapy for IE.
Study design: Systematic review.
Setting: Literature review in October 2019, with update in February 2020, consisting of 21 observational studies and 3 randomized controlled trials.
Synopsis: Three RCTs and 21 observational studies were reviewed, with a focus on the effectiveness of antibiotics administered orally for part of the therapeutic course for IE patients. Patients included in the study had left- or right-sided IE. Pathogens included viridians streptococci, staphylococci, and enterococci, with a minority of patients infected with methicillin-resistant Staphylococcus aureus. Treatment regimens included beta-lactams, linezolid, fluoroquinolones, trimethoprim-sulfamethoxazole, or clindamycin, with or without rifampin.
In studies wherein IV antibiotics alone were compared with IV antibiotics with oral step-down therapy, there was no difference in clinical cure rate. Those given oral step-down therapy had a statistically significant lower mortality rate than patients who received only IV therapy.
Limitations include inconclusive data regarding duration of IV lead-in therapy, with the variance before conversion to oral antibiotics amongst the studies ranging from 0 to 24 days. The limited number of patients with MRSA infections makes it difficult to draw conclusions regarding this particular pathogen.
Bottom line: Highly orally bioavailable antibiotics should be considered for patients with IE who have cleared bacteremia and achieved clinical stability with IV regimens.
Citation: Spellberg B et al. Evaluation of a paradigm shift from intravenous antibiotics to oral step-down therapy for the treatment of infective endocarditis: a narrative review. JAMA Intern Med. 2020;180(5):769-77. doi: 10.1001/jamainternmed.2020.0555.
Dr. Yoo is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: The standard of care for IE has been a prolonged course of IV antibiotics. Recent literature has suggested that oral antibiotics might be a safe and effective step-down therapy for IE.
Study design: Systematic review.
Setting: Literature review in October 2019, with update in February 2020, consisting of 21 observational studies and 3 randomized controlled trials.
Synopsis: Three RCTs and 21 observational studies were reviewed, with a focus on the effectiveness of antibiotics administered orally for part of the therapeutic course for IE patients. Patients included in the study had left- or right-sided IE. Pathogens included viridians streptococci, staphylococci, and enterococci, with a minority of patients infected with methicillin-resistant Staphylococcus aureus. Treatment regimens included beta-lactams, linezolid, fluoroquinolones, trimethoprim-sulfamethoxazole, or clindamycin, with or without rifampin.
In studies wherein IV antibiotics alone were compared with IV antibiotics with oral step-down therapy, there was no difference in clinical cure rate. Those given oral step-down therapy had a statistically significant lower mortality rate than patients who received only IV therapy.
Limitations include inconclusive data regarding duration of IV lead-in therapy, with the variance before conversion to oral antibiotics amongst the studies ranging from 0 to 24 days. The limited number of patients with MRSA infections makes it difficult to draw conclusions regarding this particular pathogen.
Bottom line: Highly orally bioavailable antibiotics should be considered for patients with IE who have cleared bacteremia and achieved clinical stability with IV regimens.
Citation: Spellberg B et al. Evaluation of a paradigm shift from intravenous antibiotics to oral step-down therapy for the treatment of infective endocarditis: a narrative review. JAMA Intern Med. 2020;180(5):769-77. doi: 10.1001/jamainternmed.2020.0555.
Dr. Yoo is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Risk for severe COVID-19 and death plummets with Pfizer booster
Both studies were completed before the advent of the Omicron variant.
In one study that included data on more than 4 million patients, led by Yinon M. Bar-On, MSc, of the Weizmann Institute of Science in Rehovot, Israel, the rate of confirmed SARS-CoV-2 infection was lower in the booster group than in the nonbooster group by a factor of about 10.
This was true across all five age groups studied (range among the groups [starting with age 16], 9.0-17.2).
The risk for severe COVID-19 in the primary analysis decreased in the booster group by a factor of 17.9 (95% confidence interval, 15.1-21.2), among those aged 60 years or older. Risk for severe illness in those ages 40-59 was lower by a factor of 21.7 (95% CI, 10.6-44.2).
Among the 60 and older age group, risk for death was also reduced by a factor of 14.7 (95% CI, 10.0-21.4).
Researchers analyzed data for the period from July 30 to Oct. 10, 2021, from the Israel Ministry of Health database on 4.69 million people at least 16 years old who had received two Pfizer doses at least 5 months earlier.
In the main analysis, the researchers compared the rates of confirmed COVID-19, severe disease, and death among those who had gotten a booster at least 12 days earlier with the rates in a nonbooster group.
The authors wrote: “Booster vaccination programs may provide a way to control transmission without costly social-distancing measures and quarantines. Our findings provide evidence for the short-term effectiveness of the booster dose against the currently dominant Delta variant in persons 16 years of age or older.”
Death risk down by 90%
A second study, led by Ronen Arbel, PhD, with the community medical services division, Clalit Health Services (CHS), Tel Aviv, which included more than 800,000 participants, also found mortality risk was greatly reduced among those who received the booster compared with those who didn’t get the booster.
Participants aged 50 years or older who received a booster at least 5 months after a second Pfizer dose had 90% lower mortality risk because of COVID-19 than participants who did not get the booster.
The adjusted hazard ratio for death as a result of COVID-19 in the booster group, as compared with the nonbooster group, was 0.10 (95% CI, 0.07-0.14; P < .001). Of the 843,208 eligible participants, 758,118 (90%) received the booster during the 54-day study period.
The study included all CHS members who were aged 50 years or older on the study start date and had received two Pfizer doses at least 5 months earlier. CHS covers about 52% of the Israeli population and is the largest of four health care organizations in Israel that provide mandatory health care.
The authors noted that, although the study period was only 54 days (Aug. 6–Sept. 29), during that time “the incidence of COVID-19 in Israel was one of the highest in the world.”
The authors of both original articles pointed out that the studies are limited by short time periods and that longer-term studies are needed to see how the booster shots stand up to known and future variants, such as Omicron.
None of the authors involved in both studies reported relevant financial relationships.
A version of this article first appeared on Medscape.com.
Both studies were completed before the advent of the Omicron variant.
In one study that included data on more than 4 million patients, led by Yinon M. Bar-On, MSc, of the Weizmann Institute of Science in Rehovot, Israel, the rate of confirmed SARS-CoV-2 infection was lower in the booster group than in the nonbooster group by a factor of about 10.
This was true across all five age groups studied (range among the groups [starting with age 16], 9.0-17.2).
The risk for severe COVID-19 in the primary analysis decreased in the booster group by a factor of 17.9 (95% confidence interval, 15.1-21.2), among those aged 60 years or older. Risk for severe illness in those ages 40-59 was lower by a factor of 21.7 (95% CI, 10.6-44.2).
Among the 60 and older age group, risk for death was also reduced by a factor of 14.7 (95% CI, 10.0-21.4).
Researchers analyzed data for the period from July 30 to Oct. 10, 2021, from the Israel Ministry of Health database on 4.69 million people at least 16 years old who had received two Pfizer doses at least 5 months earlier.
In the main analysis, the researchers compared the rates of confirmed COVID-19, severe disease, and death among those who had gotten a booster at least 12 days earlier with the rates in a nonbooster group.
The authors wrote: “Booster vaccination programs may provide a way to control transmission without costly social-distancing measures and quarantines. Our findings provide evidence for the short-term effectiveness of the booster dose against the currently dominant Delta variant in persons 16 years of age or older.”
Death risk down by 90%
A second study, led by Ronen Arbel, PhD, with the community medical services division, Clalit Health Services (CHS), Tel Aviv, which included more than 800,000 participants, also found mortality risk was greatly reduced among those who received the booster compared with those who didn’t get the booster.
Participants aged 50 years or older who received a booster at least 5 months after a second Pfizer dose had 90% lower mortality risk because of COVID-19 than participants who did not get the booster.
The adjusted hazard ratio for death as a result of COVID-19 in the booster group, as compared with the nonbooster group, was 0.10 (95% CI, 0.07-0.14; P < .001). Of the 843,208 eligible participants, 758,118 (90%) received the booster during the 54-day study period.
The study included all CHS members who were aged 50 years or older on the study start date and had received two Pfizer doses at least 5 months earlier. CHS covers about 52% of the Israeli population and is the largest of four health care organizations in Israel that provide mandatory health care.
The authors noted that, although the study period was only 54 days (Aug. 6–Sept. 29), during that time “the incidence of COVID-19 in Israel was one of the highest in the world.”
The authors of both original articles pointed out that the studies are limited by short time periods and that longer-term studies are needed to see how the booster shots stand up to known and future variants, such as Omicron.
None of the authors involved in both studies reported relevant financial relationships.
A version of this article first appeared on Medscape.com.
Both studies were completed before the advent of the Omicron variant.
In one study that included data on more than 4 million patients, led by Yinon M. Bar-On, MSc, of the Weizmann Institute of Science in Rehovot, Israel, the rate of confirmed SARS-CoV-2 infection was lower in the booster group than in the nonbooster group by a factor of about 10.
This was true across all five age groups studied (range among the groups [starting with age 16], 9.0-17.2).
The risk for severe COVID-19 in the primary analysis decreased in the booster group by a factor of 17.9 (95% confidence interval, 15.1-21.2), among those aged 60 years or older. Risk for severe illness in those ages 40-59 was lower by a factor of 21.7 (95% CI, 10.6-44.2).
Among the 60 and older age group, risk for death was also reduced by a factor of 14.7 (95% CI, 10.0-21.4).
Researchers analyzed data for the period from July 30 to Oct. 10, 2021, from the Israel Ministry of Health database on 4.69 million people at least 16 years old who had received two Pfizer doses at least 5 months earlier.
In the main analysis, the researchers compared the rates of confirmed COVID-19, severe disease, and death among those who had gotten a booster at least 12 days earlier with the rates in a nonbooster group.
The authors wrote: “Booster vaccination programs may provide a way to control transmission without costly social-distancing measures and quarantines. Our findings provide evidence for the short-term effectiveness of the booster dose against the currently dominant Delta variant in persons 16 years of age or older.”
Death risk down by 90%
A second study, led by Ronen Arbel, PhD, with the community medical services division, Clalit Health Services (CHS), Tel Aviv, which included more than 800,000 participants, also found mortality risk was greatly reduced among those who received the booster compared with those who didn’t get the booster.
Participants aged 50 years or older who received a booster at least 5 months after a second Pfizer dose had 90% lower mortality risk because of COVID-19 than participants who did not get the booster.
The adjusted hazard ratio for death as a result of COVID-19 in the booster group, as compared with the nonbooster group, was 0.10 (95% CI, 0.07-0.14; P < .001). Of the 843,208 eligible participants, 758,118 (90%) received the booster during the 54-day study period.
The study included all CHS members who were aged 50 years or older on the study start date and had received two Pfizer doses at least 5 months earlier. CHS covers about 52% of the Israeli population and is the largest of four health care organizations in Israel that provide mandatory health care.
The authors noted that, although the study period was only 54 days (Aug. 6–Sept. 29), during that time “the incidence of COVID-19 in Israel was one of the highest in the world.”
The authors of both original articles pointed out that the studies are limited by short time periods and that longer-term studies are needed to see how the booster shots stand up to known and future variants, such as Omicron.
None of the authors involved in both studies reported relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Mumps: Sometimes forgotten but not gone
The 7-year-old boy sat at the edge of a stretcher in the emergency department, looking miserable, as his mother recounted his symptoms to a senior resident physician on duty. Low-grade fever, fatigue, and myalgias prompted rapid SARS-CoV-2 testing at his school. That test, as well as a repeat test at the pediatrician’s office, were negative. A triage protocol in the emergency department prompted a third test, which was also negative.
“Everyone has told me that it’s likely just a different virus,” the mother said. “But then his cheek started to swell. Have you ever seen anything like this?”
The boy turned his head, revealing a diffuse swelling that extended down his right cheek to the angle of his jaw.
“Only in textbooks,” the resident physician responded.
It is a credit to our national immunization program that most practicing clinicians have never actually seen a case of mumps. Before vaccination was introduced in 1967, infection in childhood was nearly universal. Unilateral or bilateral tender swelling of the parotid gland is the typical clinical finding. Low-grade fever, myalgias, decreased appetite, malaise, and headache may precede parotid swelling in some patients. Other patients infected with mumps may have only respiratory symptoms, and some may have no symptoms at all.
Two doses of measles-mumps-rubella vaccine have been recommended for children in the United States since 1989, with the first dose administered at 12-15 months of age. According to data collected through the National Immunization Survey, more than 92% of children in the United States receive at least one dose of measles-mumps-rubella vaccine by 24 months of age. The vaccine is immunogenic, with 94% of recipients developing measurable mumps antibody (range, 89%-97%). The vaccine has been a public health success: Overall, mumps cases declined more than 99% between 1967 and 2005.
But in the mid-2000s, mumps cases started to rise again, with more than 28,000 reported between 2007 and 2019. Annual cases ranged from 229 to 6,369 and while large, localized outbreaks have contributed to peak years, mumps has been reported from all 50 states and the District of Columbia. According to a recently published paper in Pediatrics, nearly a third of these cases occurred in children <18 years of age and most had been appropriately immunized for age.
Of the 9,172 cases reported in children, 5,461 or 60% occurred between 2015 and 2019. Of these, 55% were in boys. While cases occurred in children of all ages, 54% were in children 11-17 years of age, and 33% were in children 5-10 years of age. Non-Hispanic Asian and/or Pacific Islander children accounted for 38% of cases. Only 2% of cases were associated with international travel and were presumed to have been acquired outside the United States
The reason for the increase in mumps cases in recent years is not well understood. Outbreaks in fully immunized college students have prompted concern about poor B-cell memory after vaccination resulting in waning immunity over time. In the past, antibodies against mumps were boosted by exposure to wild-type mumps virus but such exposures have become fortunately rare for most of us. Cases in recently immunized children suggest there is more to the story. Notably, there is a mismatch between the genotype A mumps virus contained in the current MMR and MMRV vaccines and the genotype G virus currently circulating in the United States.
With the onset of the pandemic and implementation of mitigation measures to prevent the spread of COVID-19, circulation of some common respiratory viruses, including respiratory syncytial virus and influenza, was sharply curtailed. Mumps continued to circulate, albeit at reduced levels, with 616 cases reported in 2020. In 2021, 30 states and jurisdictions reported 139 cases through Dec. 1.
Clinicians should suspect mumps in all cases of parotitis, regardless of an individual’s age, vaccination status, or travel history. Laboratory testing is required to distinguish mumps from other infectious and noninfectious causes of parotitis. Infectious causes include gram-positive and gram-negative bacterial infection, as well as other viral infections, including Epstein-Barr virus, coxsackie viruses, parainfluenza, and rarely, influenza. Case reports also describe parotitis coincident with SARS-CoV-2 infection.
When parotitis has been present for 3 days or less, a buccal swab for RT-PCR should be obtained, massaging the parotid gland for 30 seconds before specimen collection. When parotitis has been present for >3 days, a mumps Immunoglobulin M serum antibody should be collected in addition to the buccal swab PCR. A negative IgM does not exclude the possibility of infection, especially in immunized individuals. Mumps is a nationally notifiable disease, and all confirmed and suspect cases should be reported to the state or local health department.
Back in the emergency department, the mother was counseled about the potential diagnosis of mumps and the need for her son to isolate at home for 5 days after the onset of the parotid swelling. She was also educated about potential complications of mumps, including orchitis, aseptic meningitis and encephalitis, and hearing loss. Fortunately, complications are less common in individuals who have been immunized, and orchitis rarely occurs in prepubertal boys.
The resident physician also confirmed that other members of the household had been appropriately immunized for age. While the MMR vaccine does not prevent illness in those already infected with mumps and is not indicated as postexposure prophylaxis, providing vaccine to those not already immunized can protect against future exposures. A third dose of MMR vaccine is only indicated in the setting of an outbreak and when specifically recommended by public health authorities for those deemed to be in a high-risk group. Additional information about mumps is available at www.cdc.gov/mumps/hcp.html#report.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
The 7-year-old boy sat at the edge of a stretcher in the emergency department, looking miserable, as his mother recounted his symptoms to a senior resident physician on duty. Low-grade fever, fatigue, and myalgias prompted rapid SARS-CoV-2 testing at his school. That test, as well as a repeat test at the pediatrician’s office, were negative. A triage protocol in the emergency department prompted a third test, which was also negative.
“Everyone has told me that it’s likely just a different virus,” the mother said. “But then his cheek started to swell. Have you ever seen anything like this?”
The boy turned his head, revealing a diffuse swelling that extended down his right cheek to the angle of his jaw.
“Only in textbooks,” the resident physician responded.
It is a credit to our national immunization program that most practicing clinicians have never actually seen a case of mumps. Before vaccination was introduced in 1967, infection in childhood was nearly universal. Unilateral or bilateral tender swelling of the parotid gland is the typical clinical finding. Low-grade fever, myalgias, decreased appetite, malaise, and headache may precede parotid swelling in some patients. Other patients infected with mumps may have only respiratory symptoms, and some may have no symptoms at all.
Two doses of measles-mumps-rubella vaccine have been recommended for children in the United States since 1989, with the first dose administered at 12-15 months of age. According to data collected through the National Immunization Survey, more than 92% of children in the United States receive at least one dose of measles-mumps-rubella vaccine by 24 months of age. The vaccine is immunogenic, with 94% of recipients developing measurable mumps antibody (range, 89%-97%). The vaccine has been a public health success: Overall, mumps cases declined more than 99% between 1967 and 2005.
But in the mid-2000s, mumps cases started to rise again, with more than 28,000 reported between 2007 and 2019. Annual cases ranged from 229 to 6,369 and while large, localized outbreaks have contributed to peak years, mumps has been reported from all 50 states and the District of Columbia. According to a recently published paper in Pediatrics, nearly a third of these cases occurred in children <18 years of age and most had been appropriately immunized for age.
Of the 9,172 cases reported in children, 5,461 or 60% occurred between 2015 and 2019. Of these, 55% were in boys. While cases occurred in children of all ages, 54% were in children 11-17 years of age, and 33% were in children 5-10 years of age. Non-Hispanic Asian and/or Pacific Islander children accounted for 38% of cases. Only 2% of cases were associated with international travel and were presumed to have been acquired outside the United States
The reason for the increase in mumps cases in recent years is not well understood. Outbreaks in fully immunized college students have prompted concern about poor B-cell memory after vaccination resulting in waning immunity over time. In the past, antibodies against mumps were boosted by exposure to wild-type mumps virus but such exposures have become fortunately rare for most of us. Cases in recently immunized children suggest there is more to the story. Notably, there is a mismatch between the genotype A mumps virus contained in the current MMR and MMRV vaccines and the genotype G virus currently circulating in the United States.
With the onset of the pandemic and implementation of mitigation measures to prevent the spread of COVID-19, circulation of some common respiratory viruses, including respiratory syncytial virus and influenza, was sharply curtailed. Mumps continued to circulate, albeit at reduced levels, with 616 cases reported in 2020. In 2021, 30 states and jurisdictions reported 139 cases through Dec. 1.
Clinicians should suspect mumps in all cases of parotitis, regardless of an individual’s age, vaccination status, or travel history. Laboratory testing is required to distinguish mumps from other infectious and noninfectious causes of parotitis. Infectious causes include gram-positive and gram-negative bacterial infection, as well as other viral infections, including Epstein-Barr virus, coxsackie viruses, parainfluenza, and rarely, influenza. Case reports also describe parotitis coincident with SARS-CoV-2 infection.
When parotitis has been present for 3 days or less, a buccal swab for RT-PCR should be obtained, massaging the parotid gland for 30 seconds before specimen collection. When parotitis has been present for >3 days, a mumps Immunoglobulin M serum antibody should be collected in addition to the buccal swab PCR. A negative IgM does not exclude the possibility of infection, especially in immunized individuals. Mumps is a nationally notifiable disease, and all confirmed and suspect cases should be reported to the state or local health department.
Back in the emergency department, the mother was counseled about the potential diagnosis of mumps and the need for her son to isolate at home for 5 days after the onset of the parotid swelling. She was also educated about potential complications of mumps, including orchitis, aseptic meningitis and encephalitis, and hearing loss. Fortunately, complications are less common in individuals who have been immunized, and orchitis rarely occurs in prepubertal boys.
The resident physician also confirmed that other members of the household had been appropriately immunized for age. While the MMR vaccine does not prevent illness in those already infected with mumps and is not indicated as postexposure prophylaxis, providing vaccine to those not already immunized can protect against future exposures. A third dose of MMR vaccine is only indicated in the setting of an outbreak and when specifically recommended by public health authorities for those deemed to be in a high-risk group. Additional information about mumps is available at www.cdc.gov/mumps/hcp.html#report.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
The 7-year-old boy sat at the edge of a stretcher in the emergency department, looking miserable, as his mother recounted his symptoms to a senior resident physician on duty. Low-grade fever, fatigue, and myalgias prompted rapid SARS-CoV-2 testing at his school. That test, as well as a repeat test at the pediatrician’s office, were negative. A triage protocol in the emergency department prompted a third test, which was also negative.
“Everyone has told me that it’s likely just a different virus,” the mother said. “But then his cheek started to swell. Have you ever seen anything like this?”
The boy turned his head, revealing a diffuse swelling that extended down his right cheek to the angle of his jaw.
“Only in textbooks,” the resident physician responded.
It is a credit to our national immunization program that most practicing clinicians have never actually seen a case of mumps. Before vaccination was introduced in 1967, infection in childhood was nearly universal. Unilateral or bilateral tender swelling of the parotid gland is the typical clinical finding. Low-grade fever, myalgias, decreased appetite, malaise, and headache may precede parotid swelling in some patients. Other patients infected with mumps may have only respiratory symptoms, and some may have no symptoms at all.
Two doses of measles-mumps-rubella vaccine have been recommended for children in the United States since 1989, with the first dose administered at 12-15 months of age. According to data collected through the National Immunization Survey, more than 92% of children in the United States receive at least one dose of measles-mumps-rubella vaccine by 24 months of age. The vaccine is immunogenic, with 94% of recipients developing measurable mumps antibody (range, 89%-97%). The vaccine has been a public health success: Overall, mumps cases declined more than 99% between 1967 and 2005.
But in the mid-2000s, mumps cases started to rise again, with more than 28,000 reported between 2007 and 2019. Annual cases ranged from 229 to 6,369 and while large, localized outbreaks have contributed to peak years, mumps has been reported from all 50 states and the District of Columbia. According to a recently published paper in Pediatrics, nearly a third of these cases occurred in children <18 years of age and most had been appropriately immunized for age.
Of the 9,172 cases reported in children, 5,461 or 60% occurred between 2015 and 2019. Of these, 55% were in boys. While cases occurred in children of all ages, 54% were in children 11-17 years of age, and 33% were in children 5-10 years of age. Non-Hispanic Asian and/or Pacific Islander children accounted for 38% of cases. Only 2% of cases were associated with international travel and were presumed to have been acquired outside the United States
The reason for the increase in mumps cases in recent years is not well understood. Outbreaks in fully immunized college students have prompted concern about poor B-cell memory after vaccination resulting in waning immunity over time. In the past, antibodies against mumps were boosted by exposure to wild-type mumps virus but such exposures have become fortunately rare for most of us. Cases in recently immunized children suggest there is more to the story. Notably, there is a mismatch between the genotype A mumps virus contained in the current MMR and MMRV vaccines and the genotype G virus currently circulating in the United States.
With the onset of the pandemic and implementation of mitigation measures to prevent the spread of COVID-19, circulation of some common respiratory viruses, including respiratory syncytial virus and influenza, was sharply curtailed. Mumps continued to circulate, albeit at reduced levels, with 616 cases reported in 2020. In 2021, 30 states and jurisdictions reported 139 cases through Dec. 1.
Clinicians should suspect mumps in all cases of parotitis, regardless of an individual’s age, vaccination status, or travel history. Laboratory testing is required to distinguish mumps from other infectious and noninfectious causes of parotitis. Infectious causes include gram-positive and gram-negative bacterial infection, as well as other viral infections, including Epstein-Barr virus, coxsackie viruses, parainfluenza, and rarely, influenza. Case reports also describe parotitis coincident with SARS-CoV-2 infection.
When parotitis has been present for 3 days or less, a buccal swab for RT-PCR should be obtained, massaging the parotid gland for 30 seconds before specimen collection. When parotitis has been present for >3 days, a mumps Immunoglobulin M serum antibody should be collected in addition to the buccal swab PCR. A negative IgM does not exclude the possibility of infection, especially in immunized individuals. Mumps is a nationally notifiable disease, and all confirmed and suspect cases should be reported to the state or local health department.
Back in the emergency department, the mother was counseled about the potential diagnosis of mumps and the need for her son to isolate at home for 5 days after the onset of the parotid swelling. She was also educated about potential complications of mumps, including orchitis, aseptic meningitis and encephalitis, and hearing loss. Fortunately, complications are less common in individuals who have been immunized, and orchitis rarely occurs in prepubertal boys.
The resident physician also confirmed that other members of the household had been appropriately immunized for age. While the MMR vaccine does not prevent illness in those already infected with mumps and is not indicated as postexposure prophylaxis, providing vaccine to those not already immunized can protect against future exposures. A third dose of MMR vaccine is only indicated in the setting of an outbreak and when specifically recommended by public health authorities for those deemed to be in a high-risk group. Additional information about mumps is available at www.cdc.gov/mumps/hcp.html#report.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].





