User login
Children and COVID: Weekly cases at lowest level since August
New cases of COVID-19 in children continued their descent toward normalcy, falling below 100,000 in a week for the first time since early August 2021, according to the American Academy of Pediatrics and the Children’s Hospital Association.
and 94% since the Omicron-fueled peak of 1.15 million during the week of Jan. 14-20, the AAP and CHA said in their weekly COVID report. The total number of child cases is 12.7 million since the pandemic began, with children representing 19% of all cases.
New admissions also stayed on a downward path, as the rate dropped to 0.24 per 100,000 children aged 0-17 years on March 5, a decline of nearly 81% since hitting 1.25 per 100,000 on Jan. 15. The latest 7-day average for daily admissions, 178 per day from Feb. 27 to March 5, was 29% lower than the previous week and almost 81% lower than the peak of 914 per day for Jan. 10-16, the Centers for Disease Control and Prevention reported.
The story is the same for emergency department visits with diagnosed COVID-19, which are reported as a percentage of all ED visits. On March 4, the 7-day average for children aged 0-11 years was 0.8%, compared with a high of 13.9% in mid-January, while 12- to 15-year-olds had dropped from 12.4% to 0.5% and 16- to 17-year-olds went from 12.6% down to 0.5%, the CDC said on its COVID Data Tracker.
Florida’s surgeon general says no to the vaccine
Vaccination, in the meantime, is struggling to maintain a foothold against the current of declining cases. Florida Surgeon General Joseph Ladapo said that “the Florida Department of Health is going to be the first state to officially recommend against the COVID-19 vaccines for healthy children,” NBC News reported March 7. With such a move, “Florida would become the first state to break from the CDC on vaccines for children,” CNN said in its report.
Vaccinations among children aged 5-11 years, which hit 1.6 million in 1 week shortly after emergency use was authorized in early November, declined quickly shorty thereafter and only rose slightly during the Omicron surge. Since mid-January, the number of children receiving an initial dose has declined for seven consecutive weeks and is now lower than ever, based on CDC data compiled by the AAP.
Just over one-third of children aged 5-11 have gotten at least one dose of COVID-19 vaccine, while 26.4% are fully vaccinated. Among children aged 12-17, just over two-thirds (67.8%) have received at least one dose, 57.8% have completed the vaccine regimen, and 21.9% have gotten a booster, the CDC reported.
As of March 2, “about 8.4 million children 12-17 have yet to receive their initial COVID-19 vaccine dose,” the AAP said. About 64,000 children aged 12-17 had received their first dose in the previous week, the group noted, which was the second-lowest weekly total since the vaccine was approved for children aged 12-15 in May of 2021.
New cases of COVID-19 in children continued their descent toward normalcy, falling below 100,000 in a week for the first time since early August 2021, according to the American Academy of Pediatrics and the Children’s Hospital Association.
and 94% since the Omicron-fueled peak of 1.15 million during the week of Jan. 14-20, the AAP and CHA said in their weekly COVID report. The total number of child cases is 12.7 million since the pandemic began, with children representing 19% of all cases.
New admissions also stayed on a downward path, as the rate dropped to 0.24 per 100,000 children aged 0-17 years on March 5, a decline of nearly 81% since hitting 1.25 per 100,000 on Jan. 15. The latest 7-day average for daily admissions, 178 per day from Feb. 27 to March 5, was 29% lower than the previous week and almost 81% lower than the peak of 914 per day for Jan. 10-16, the Centers for Disease Control and Prevention reported.
The story is the same for emergency department visits with diagnosed COVID-19, which are reported as a percentage of all ED visits. On March 4, the 7-day average for children aged 0-11 years was 0.8%, compared with a high of 13.9% in mid-January, while 12- to 15-year-olds had dropped from 12.4% to 0.5% and 16- to 17-year-olds went from 12.6% down to 0.5%, the CDC said on its COVID Data Tracker.
Florida’s surgeon general says no to the vaccine
Vaccination, in the meantime, is struggling to maintain a foothold against the current of declining cases. Florida Surgeon General Joseph Ladapo said that “the Florida Department of Health is going to be the first state to officially recommend against the COVID-19 vaccines for healthy children,” NBC News reported March 7. With such a move, “Florida would become the first state to break from the CDC on vaccines for children,” CNN said in its report.
Vaccinations among children aged 5-11 years, which hit 1.6 million in 1 week shortly after emergency use was authorized in early November, declined quickly shorty thereafter and only rose slightly during the Omicron surge. Since mid-January, the number of children receiving an initial dose has declined for seven consecutive weeks and is now lower than ever, based on CDC data compiled by the AAP.
Just over one-third of children aged 5-11 have gotten at least one dose of COVID-19 vaccine, while 26.4% are fully vaccinated. Among children aged 12-17, just over two-thirds (67.8%) have received at least one dose, 57.8% have completed the vaccine regimen, and 21.9% have gotten a booster, the CDC reported.
As of March 2, “about 8.4 million children 12-17 have yet to receive their initial COVID-19 vaccine dose,” the AAP said. About 64,000 children aged 12-17 had received their first dose in the previous week, the group noted, which was the second-lowest weekly total since the vaccine was approved for children aged 12-15 in May of 2021.
New cases of COVID-19 in children continued their descent toward normalcy, falling below 100,000 in a week for the first time since early August 2021, according to the American Academy of Pediatrics and the Children’s Hospital Association.
and 94% since the Omicron-fueled peak of 1.15 million during the week of Jan. 14-20, the AAP and CHA said in their weekly COVID report. The total number of child cases is 12.7 million since the pandemic began, with children representing 19% of all cases.
New admissions also stayed on a downward path, as the rate dropped to 0.24 per 100,000 children aged 0-17 years on March 5, a decline of nearly 81% since hitting 1.25 per 100,000 on Jan. 15. The latest 7-day average for daily admissions, 178 per day from Feb. 27 to March 5, was 29% lower than the previous week and almost 81% lower than the peak of 914 per day for Jan. 10-16, the Centers for Disease Control and Prevention reported.
The story is the same for emergency department visits with diagnosed COVID-19, which are reported as a percentage of all ED visits. On March 4, the 7-day average for children aged 0-11 years was 0.8%, compared with a high of 13.9% in mid-January, while 12- to 15-year-olds had dropped from 12.4% to 0.5% and 16- to 17-year-olds went from 12.6% down to 0.5%, the CDC said on its COVID Data Tracker.
Florida’s surgeon general says no to the vaccine
Vaccination, in the meantime, is struggling to maintain a foothold against the current of declining cases. Florida Surgeon General Joseph Ladapo said that “the Florida Department of Health is going to be the first state to officially recommend against the COVID-19 vaccines for healthy children,” NBC News reported March 7. With such a move, “Florida would become the first state to break from the CDC on vaccines for children,” CNN said in its report.
Vaccinations among children aged 5-11 years, which hit 1.6 million in 1 week shortly after emergency use was authorized in early November, declined quickly shorty thereafter and only rose slightly during the Omicron surge. Since mid-January, the number of children receiving an initial dose has declined for seven consecutive weeks and is now lower than ever, based on CDC data compiled by the AAP.
Just over one-third of children aged 5-11 have gotten at least one dose of COVID-19 vaccine, while 26.4% are fully vaccinated. Among children aged 12-17, just over two-thirds (67.8%) have received at least one dose, 57.8% have completed the vaccine regimen, and 21.9% have gotten a booster, the CDC reported.
As of March 2, “about 8.4 million children 12-17 have yet to receive their initial COVID-19 vaccine dose,” the AAP said. About 64,000 children aged 12-17 had received their first dose in the previous week, the group noted, which was the second-lowest weekly total since the vaccine was approved for children aged 12-15 in May of 2021.
FDA committee recommends 2022-2023 influenza vaccine strains
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee has chosen the influenza vaccine strains for the 2022-2023 season in the northern hemisphere, which begins in the fall of 2022.
On March 3, the committee unanimously voted to endorse the World Health Organization’s recommendations as to which influenza strains to include for coverage by vaccines for the upcoming flu season. Two of the four recommended strains are different from last season.
The committee also heard updates on flu activity this season. So far, data from the U.S. Flu Vaccine Effectiveness (VE) network, which consists of seven study sites, have not shown that the vaccine is protective against influenza A. “We can say that it is not highly effective,” Brendan Flannery, PhD, who leads the U.S. Flu VE network for the Centers for Disease Control and Prevention, said in an interview. He was not involved with the advisory committee meeting. Flu activity this season has been low, he explained, so there are fewer cases his team can use to estimate vaccine efficacy. “If there’s some benefit, it’s hard for us to show that now,” he said.
Vaccine strains
The panel voted to include a A/Darwin/9/2021-like strain for the H3N2 component of the vaccine; this is changed from A/Cambodia/e0826360/2020. For the influenza B Victoria lineage component, the committee voted to include a B/Austria/1359417/2021-like virus, a swap from this year’s B/Washington/02/2019-like virus. These changes apply to the egg-based, cell-culture, and recombinant vaccines. Both new strains were included in WHO’s 2022 influenza vaccine strain recommendations for the southern hemisphere.
For the influenza A H1N1 component, the group also agreed to include a A/Victoria/2570/2019 (H1N1) pdm09-like virus for the egg-based vaccine and the A/Wisconsin/588/2019 (H1N1) pdm09-like virus for cell culture or recombinant vaccines. These strains were included for the 2021-2022 season. The panel also voted for the inclusion of a B/Phuket/3073/2013-like virus (B/Yamagata lineage) as the second influenza B strain for the quadrivalent egg-based, cell culture, or recombinant vaccines, which is unchanged from this flu season.
‘Sporadic’ flu activity
While there was an uptick in influenza activity this year compared to the 2020-2021 season, hospitalization rates are lower than in the four seasons preceding the pandemic (from 2016-2017 to 2019-2020). As of Feb. 26, the cumulative hospitalization rate for this flu season was 5.2 hospitalizations per 100,000 individuals. There have been eight pediatric deaths due to influenza so far this season, compared to one pediatric death reported to the CDC during the 2020-2021 flu season.
About 4.1% of specimens tested at clinical laboratories were positive for flu. Since Oct. 30, 2.7% of specimens have been positive for influenza this season. Nearly all viruses detected (97.7%) have been influenza A.
Lisa Grohskopf, MD, MPH, a medical officer in the influenza division at the CDC who presented the data at the meeting, described flu activity this season as “sporadic” and noted that activity is increasing in some areas of the country. According to CDC’s weekly influenza surveillance report, most states had minimal influenza-like illness (ILI) activity, although Arkansas, Idaho, Iowa, Kansas, Minnesota, and Utah had slightly higher ILI activity as of Feb. 26. Champaign-Urbana, Illinois; St. Cloud, Minnesota; and Brownwood, Texas, had the highest levels of flu activity in the country.
Low vaccine effectiveness
As of Jan. 22, results from the U.S. Flu VE network do not show statistically significant evidence that the flu vaccine is effective. Currently, the vaccine is estimated to be 8% effective against preventing influenza A infection (95% confidence interval, –31% to 36%) and 14% effective against preventing A/H3N2 infection (95% CI, –28% to 43%) for people aged 6 months and older.
The network did not have enough data to provide age-specific VE estimates or estimates of effectiveness against influenza B. This could be due to low flu activity relative to prepandemic years, Dr. Flannery said. Of the 2,758 individuals enrolled in the VE flu network this season, just 147 (5%) tested positive for the flu this season. This is the lowest positivity rate observed in the Flu VE network participants with respiratory illness over the past 10 flu seasons, Dr. Grohskopf noted. In comparison, estimates from the 2019 to 2020 season included 4,112 individuals, and 1,060 tested positive for flu.
“We are really at the bare minimum of what we can use for a flu vaccine effectiveness estimate,” Dr. Flannery said about the more recent data. The network was not able to produce any estimates about flu vaccine effectiveness for the 2020-2021 season because of historically low flu activity.
The Department of Defense also presented vaccine efficacy estimates for the 2021–2022 season. The vaccine has been 36% effective (95% CI, 28%-44%) against all strains of the virus, 33% effective against influenza A (95% CI, 24%-41%), 32% effective against A/H3N2 (95% CI, 3%-53%), and 59% effective against influenza B (95% CI, 42%-71%). These results are from a young, healthy adult population, Lieutenant Commander Courtney Gustin, DrPH, MSN, told the panel, and they may not be reflective of efficacy rates across all age groups.
Though these findings suggest there is low to no measurable benefit against influenza A, Dr. Flannery said the CDC still recommends getting the flu vaccine, as it can be protective against other circulating flu strains. “We have been able to demonstrate protection against other H3 [viruses], B viruses, and H1 viruses in the past,” he said. And as these results only show protection against mild disease, “there is still possibility that there’s benefit against more severe disease,” he added. Studies measuring effectiveness against more severe outcomes are not yet available.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee has chosen the influenza vaccine strains for the 2022-2023 season in the northern hemisphere, which begins in the fall of 2022.
On March 3, the committee unanimously voted to endorse the World Health Organization’s recommendations as to which influenza strains to include for coverage by vaccines for the upcoming flu season. Two of the four recommended strains are different from last season.
The committee also heard updates on flu activity this season. So far, data from the U.S. Flu Vaccine Effectiveness (VE) network, which consists of seven study sites, have not shown that the vaccine is protective against influenza A. “We can say that it is not highly effective,” Brendan Flannery, PhD, who leads the U.S. Flu VE network for the Centers for Disease Control and Prevention, said in an interview. He was not involved with the advisory committee meeting. Flu activity this season has been low, he explained, so there are fewer cases his team can use to estimate vaccine efficacy. “If there’s some benefit, it’s hard for us to show that now,” he said.
Vaccine strains
The panel voted to include a A/Darwin/9/2021-like strain for the H3N2 component of the vaccine; this is changed from A/Cambodia/e0826360/2020. For the influenza B Victoria lineage component, the committee voted to include a B/Austria/1359417/2021-like virus, a swap from this year’s B/Washington/02/2019-like virus. These changes apply to the egg-based, cell-culture, and recombinant vaccines. Both new strains were included in WHO’s 2022 influenza vaccine strain recommendations for the southern hemisphere.
For the influenza A H1N1 component, the group also agreed to include a A/Victoria/2570/2019 (H1N1) pdm09-like virus for the egg-based vaccine and the A/Wisconsin/588/2019 (H1N1) pdm09-like virus for cell culture or recombinant vaccines. These strains were included for the 2021-2022 season. The panel also voted for the inclusion of a B/Phuket/3073/2013-like virus (B/Yamagata lineage) as the second influenza B strain for the quadrivalent egg-based, cell culture, or recombinant vaccines, which is unchanged from this flu season.
‘Sporadic’ flu activity
While there was an uptick in influenza activity this year compared to the 2020-2021 season, hospitalization rates are lower than in the four seasons preceding the pandemic (from 2016-2017 to 2019-2020). As of Feb. 26, the cumulative hospitalization rate for this flu season was 5.2 hospitalizations per 100,000 individuals. There have been eight pediatric deaths due to influenza so far this season, compared to one pediatric death reported to the CDC during the 2020-2021 flu season.
About 4.1% of specimens tested at clinical laboratories were positive for flu. Since Oct. 30, 2.7% of specimens have been positive for influenza this season. Nearly all viruses detected (97.7%) have been influenza A.
Lisa Grohskopf, MD, MPH, a medical officer in the influenza division at the CDC who presented the data at the meeting, described flu activity this season as “sporadic” and noted that activity is increasing in some areas of the country. According to CDC’s weekly influenza surveillance report, most states had minimal influenza-like illness (ILI) activity, although Arkansas, Idaho, Iowa, Kansas, Minnesota, and Utah had slightly higher ILI activity as of Feb. 26. Champaign-Urbana, Illinois; St. Cloud, Minnesota; and Brownwood, Texas, had the highest levels of flu activity in the country.
Low vaccine effectiveness
As of Jan. 22, results from the U.S. Flu VE network do not show statistically significant evidence that the flu vaccine is effective. Currently, the vaccine is estimated to be 8% effective against preventing influenza A infection (95% confidence interval, –31% to 36%) and 14% effective against preventing A/H3N2 infection (95% CI, –28% to 43%) for people aged 6 months and older.
The network did not have enough data to provide age-specific VE estimates or estimates of effectiveness against influenza B. This could be due to low flu activity relative to prepandemic years, Dr. Flannery said. Of the 2,758 individuals enrolled in the VE flu network this season, just 147 (5%) tested positive for the flu this season. This is the lowest positivity rate observed in the Flu VE network participants with respiratory illness over the past 10 flu seasons, Dr. Grohskopf noted. In comparison, estimates from the 2019 to 2020 season included 4,112 individuals, and 1,060 tested positive for flu.
“We are really at the bare minimum of what we can use for a flu vaccine effectiveness estimate,” Dr. Flannery said about the more recent data. The network was not able to produce any estimates about flu vaccine effectiveness for the 2020-2021 season because of historically low flu activity.
The Department of Defense also presented vaccine efficacy estimates for the 2021–2022 season. The vaccine has been 36% effective (95% CI, 28%-44%) against all strains of the virus, 33% effective against influenza A (95% CI, 24%-41%), 32% effective against A/H3N2 (95% CI, 3%-53%), and 59% effective against influenza B (95% CI, 42%-71%). These results are from a young, healthy adult population, Lieutenant Commander Courtney Gustin, DrPH, MSN, told the panel, and they may not be reflective of efficacy rates across all age groups.
Though these findings suggest there is low to no measurable benefit against influenza A, Dr. Flannery said the CDC still recommends getting the flu vaccine, as it can be protective against other circulating flu strains. “We have been able to demonstrate protection against other H3 [viruses], B viruses, and H1 viruses in the past,” he said. And as these results only show protection against mild disease, “there is still possibility that there’s benefit against more severe disease,” he added. Studies measuring effectiveness against more severe outcomes are not yet available.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee has chosen the influenza vaccine strains for the 2022-2023 season in the northern hemisphere, which begins in the fall of 2022.
On March 3, the committee unanimously voted to endorse the World Health Organization’s recommendations as to which influenza strains to include for coverage by vaccines for the upcoming flu season. Two of the four recommended strains are different from last season.
The committee also heard updates on flu activity this season. So far, data from the U.S. Flu Vaccine Effectiveness (VE) network, which consists of seven study sites, have not shown that the vaccine is protective against influenza A. “We can say that it is not highly effective,” Brendan Flannery, PhD, who leads the U.S. Flu VE network for the Centers for Disease Control and Prevention, said in an interview. He was not involved with the advisory committee meeting. Flu activity this season has been low, he explained, so there are fewer cases his team can use to estimate vaccine efficacy. “If there’s some benefit, it’s hard for us to show that now,” he said.
Vaccine strains
The panel voted to include a A/Darwin/9/2021-like strain for the H3N2 component of the vaccine; this is changed from A/Cambodia/e0826360/2020. For the influenza B Victoria lineage component, the committee voted to include a B/Austria/1359417/2021-like virus, a swap from this year’s B/Washington/02/2019-like virus. These changes apply to the egg-based, cell-culture, and recombinant vaccines. Both new strains were included in WHO’s 2022 influenza vaccine strain recommendations for the southern hemisphere.
For the influenza A H1N1 component, the group also agreed to include a A/Victoria/2570/2019 (H1N1) pdm09-like virus for the egg-based vaccine and the A/Wisconsin/588/2019 (H1N1) pdm09-like virus for cell culture or recombinant vaccines. These strains were included for the 2021-2022 season. The panel also voted for the inclusion of a B/Phuket/3073/2013-like virus (B/Yamagata lineage) as the second influenza B strain for the quadrivalent egg-based, cell culture, or recombinant vaccines, which is unchanged from this flu season.
‘Sporadic’ flu activity
While there was an uptick in influenza activity this year compared to the 2020-2021 season, hospitalization rates are lower than in the four seasons preceding the pandemic (from 2016-2017 to 2019-2020). As of Feb. 26, the cumulative hospitalization rate for this flu season was 5.2 hospitalizations per 100,000 individuals. There have been eight pediatric deaths due to influenza so far this season, compared to one pediatric death reported to the CDC during the 2020-2021 flu season.
About 4.1% of specimens tested at clinical laboratories were positive for flu. Since Oct. 30, 2.7% of specimens have been positive for influenza this season. Nearly all viruses detected (97.7%) have been influenza A.
Lisa Grohskopf, MD, MPH, a medical officer in the influenza division at the CDC who presented the data at the meeting, described flu activity this season as “sporadic” and noted that activity is increasing in some areas of the country. According to CDC’s weekly influenza surveillance report, most states had minimal influenza-like illness (ILI) activity, although Arkansas, Idaho, Iowa, Kansas, Minnesota, and Utah had slightly higher ILI activity as of Feb. 26. Champaign-Urbana, Illinois; St. Cloud, Minnesota; and Brownwood, Texas, had the highest levels of flu activity in the country.
Low vaccine effectiveness
As of Jan. 22, results from the U.S. Flu VE network do not show statistically significant evidence that the flu vaccine is effective. Currently, the vaccine is estimated to be 8% effective against preventing influenza A infection (95% confidence interval, –31% to 36%) and 14% effective against preventing A/H3N2 infection (95% CI, –28% to 43%) for people aged 6 months and older.
The network did not have enough data to provide age-specific VE estimates or estimates of effectiveness against influenza B. This could be due to low flu activity relative to prepandemic years, Dr. Flannery said. Of the 2,758 individuals enrolled in the VE flu network this season, just 147 (5%) tested positive for the flu this season. This is the lowest positivity rate observed in the Flu VE network participants with respiratory illness over the past 10 flu seasons, Dr. Grohskopf noted. In comparison, estimates from the 2019 to 2020 season included 4,112 individuals, and 1,060 tested positive for flu.
“We are really at the bare minimum of what we can use for a flu vaccine effectiveness estimate,” Dr. Flannery said about the more recent data. The network was not able to produce any estimates about flu vaccine effectiveness for the 2020-2021 season because of historically low flu activity.
The Department of Defense also presented vaccine efficacy estimates for the 2021–2022 season. The vaccine has been 36% effective (95% CI, 28%-44%) against all strains of the virus, 33% effective against influenza A (95% CI, 24%-41%), 32% effective against A/H3N2 (95% CI, 3%-53%), and 59% effective against influenza B (95% CI, 42%-71%). These results are from a young, healthy adult population, Lieutenant Commander Courtney Gustin, DrPH, MSN, told the panel, and they may not be reflective of efficacy rates across all age groups.
Though these findings suggest there is low to no measurable benefit against influenza A, Dr. Flannery said the CDC still recommends getting the flu vaccine, as it can be protective against other circulating flu strains. “We have been able to demonstrate protection against other H3 [viruses], B viruses, and H1 viruses in the past,” he said. And as these results only show protection against mild disease, “there is still possibility that there’s benefit against more severe disease,” he added. Studies measuring effectiveness against more severe outcomes are not yet available.
A version of this article first appeared on Medscape.com.
Vaccine update: The latest recommendations from ACIP
In a typical year, the Advisory Committee on Immunization Practices (ACIP) has three 1.5- to 2-day meetings to make recommendations for the use of new and existing vaccines in the US population. However, 2021 was not a typical year. Last year, ACIP held 17 meetings for a total of 127 hours. Most of these were related to vaccines to prevent COVID-19. There are now 3 COVID-19 vaccines authorized for use in the United States: the 2-dose mRNA-based Pfizer-BioNTech/Comirnaty and Moderna COVID-19 vaccines and the single-dose adenovirus, vector-based Janssen (Johnson & Johnson) COVID-19 vaccine.
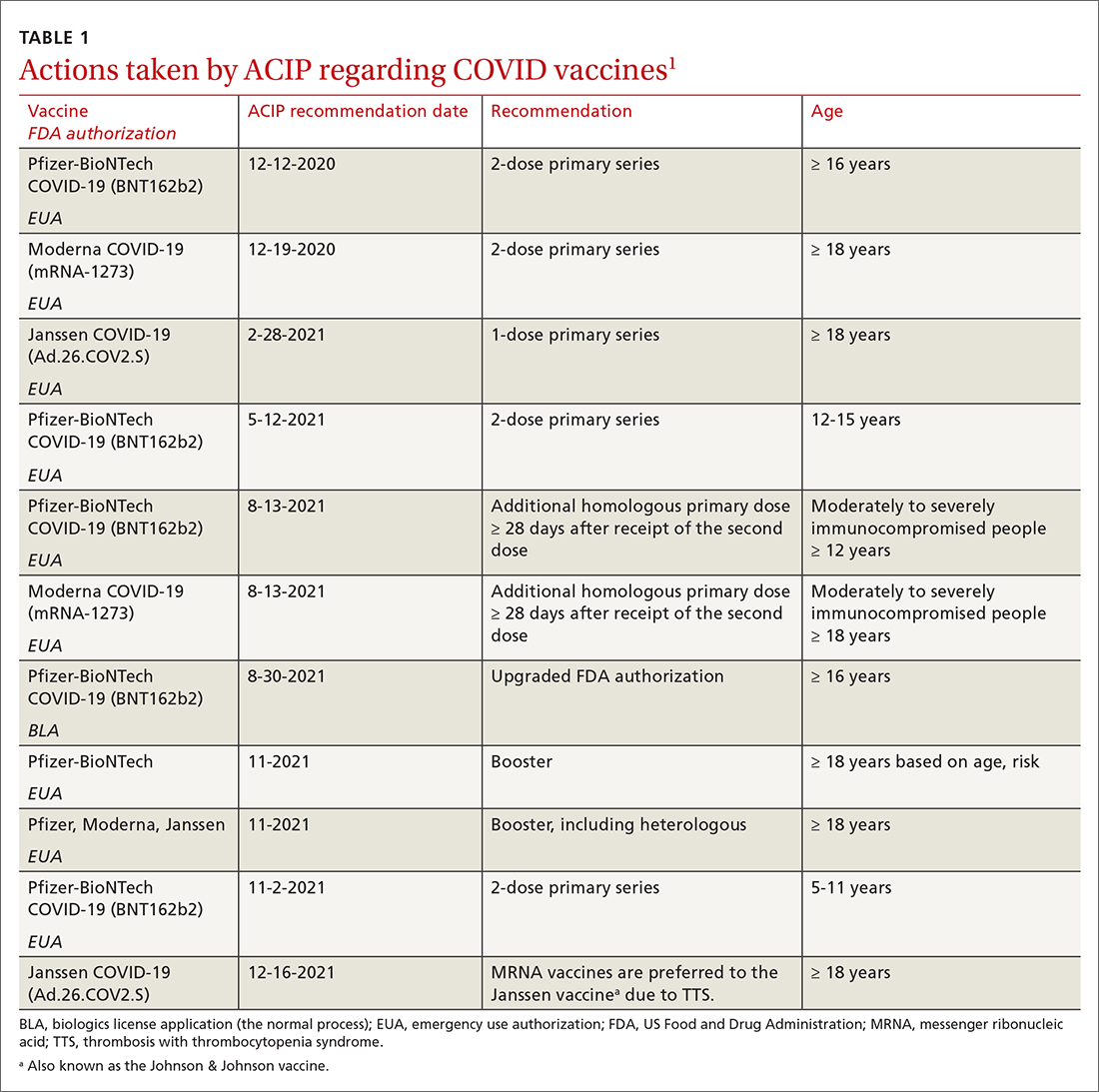
TABLE 11 includes the actions taken by the ACIP from late 2020 through 2021 related to COVID-19 vaccines. All of these recommendations except 1 occurred after the US Food and Drug Administration (FDA) approved the product using an emergency use authorization (EUA). The exception is the recommendation for use of the Pfizer-BioNTech COVID-19 vaccine (BNT162b2) for those ages 16 years and older, which was approved under the normal process 8 months after widespread use under an EUA.
Hepatitis B vaccine now for all nonimmune adults up through 59 years
Since the introduction of hepatitis B (HepB) vaccines in 1980, the incidence of hepatitis B virus (HBV) infections in the United States has been reduced dramatically; there were an estimated 287,000 cases in 19852 and 19,200 in 2014.3 However, the incidence among adults has not declined in recent years and among someage groups has actually increased. Among those ages 40 to 49 years, the rate went from 1.9 per 100,000 in 20114 to 2.7 per 100,000 population in 2019.5 In those ages 50 to 59, there was an increase from 1.1 to 1.6 per 100,000 population over the same period of time.4,5
Recommendations for using HepB vaccine in adults have been based on risk that involves individual behavior, occupation, and medical conditions (TABLE 26). The presence of these risk factors is often unknown to medical professionals, who rarely ask about or document them. And patients can be reluctant to disclose them for fear of being stigmatized. The consequence has been a low rate of vaccination in at-risk adults.

At its November 2021 meeting, ACIP accepted the advice of the Hepatitis Work Group to move to a universal adult recommendation through age 59.7 ACIP believed that the incidence of acute infection in those ages 60 and older was too low to merit a universal recommendation. The new recommendation states that
Multiple HepB vaccine products are available for adults. Two are recombinant-based and require 3 doses: Engerix-B (GlaxoSmithKline) and Recombivax HB (Merck). One is recombinant based and requires only 2 doses: Heplisav-B (Dynavax Technologies). A new product recently approved by the FDA, PREHEVBRIO (VBI Vaccines), is another recombinant 3-dose option that the ACIP will consider early in 2022. HepB and HepA vaccines can also be co-administered with Twinrix (GlaxoSmithKline).
Pneumococcal vaccines: New PCV vaccines alter prescribing choices
The ACIP recommendations for pneumococcal vaccines in adults have been very confusing, involving 2 vaccines: PCV13 (Prevnar13, Pfizer) and PPSV23 (Pneumovax23, Merck). Both PCV13 and PPSV23 given in series were recommended for immunocompromised patients, but only PPSV23 was recommended for those with chronic medical conditions. For those 65 and older, PPSV23 was recommended for all individuals (including those with no chronic or immunocompromising condition), and PCV13 was recommended for those with immunocompromising conditions. Other adults in this older age group could receive PCV13 based on individual risk and shared clinical decision making.8
Continue to: This past year...
This past year, 2 new PCV vaccines were approved by the FDA: PCV15 (Vaxneuvance, Merck) and PCV20 (Prevnar20, Pfizer). While considering these new vaccines, the ACIP re-assessed its entire approval of pneumococcal vaccines. First, they retained the cutoff for universal pneumococcal vaccination at 65 years. For those younger than 65, they combined chronic medical conditions and immunocompromising conditions into a single at-risk group (TABLE 39). They then issued the same recommendation for older adults and those younger than 65 with risks: to receive a PCV vaccine, either PCV15 or PCV20. If they receive PCV15, it should be followed by PPSV23. PPSV23 is not recommended for those who receive PCV20. Therefore,

Recombinant zoster vaccine (RZV) has been licensed and recommended in the United States since 2017 in a 2-dose schedule for adults ages 50 years and older. In the summer of 2021, the FDA expanded the indication for use of RZV to include individuals 18 to 49 years of age who are or will be immunodeficient or immunosuppressed due to known disease or therapy. In October, the ACIP agreed and recommended 2 RZV doses for those 19 years and older in these risk groups (TABLE 410).

This recommendation was based on the elevated risk of herpes zoster documented in those with immune-suppressing conditions and therapies. In the conditions studied, the incidence in these younger adults exceeded that for older adults, for whom the vaccine is recommended.10 There are many immune conditions and immune-suppressing medications. The ACIP Zoster Work Group did not have efficacy and safety information on the use of RZV in each one of them, even though their recommendation includes them all. Many of these patients are under the care of specialists whose specialty societies had been recommending zoster vaccine for their patients, off label, prior to the FDA authorization.
Rabies vaccine is now available in 2-dose schedule
People who should receive rabies pre-exposure prophylaxis (PrEP) with rabies vaccine include laboratory personnel who work with rabies virus, biologists who work with bats, animal care professionals, wildlife biologists, veterinarians, and travelers who may be at risk of encountering rabid dogs. The recommendation has been for 3 doses of rabies vaccine at 0, 7, and 21-28 days. The ACIP voted at its June 2021 meeting to adopt a 2-dose PrEP schedule of 0 and 7 days.11 This will be especially helpful to travelers who want to complete the recommended doses prior to departure. Those who have sustained risk over time can elect to have a third dose after 21 days and before 3 years, or elect to have titers checked. More detailed clinical advice will be published in the CDC’s Morbidity and Mortality Weekly Report in 2022.
Dengue vaccine: New rec for those 9-16 years
In 2019, the FDA approved the first dengue vaccine for use in the United States for children 9 to 16 years old who had laboratory-confirmed previous dengue virus infection and who were living in an area where dengue is endemic. The CYD-TDV dengue vaccine (Dengvaxia) is a live-attenuated tetravalent vaccine built on a yellow fever vaccine backbone. Its effectiveness is 82% for prevention of symptomatic dengue, 79% for prevention of dengue-associated hospitalizations, and 84% against severe dengue.12
Continue to: Dengue viruses...
Dengue viruses (DENV) are transmitted by Aedes mosquitoes. There are 4 serotypes of dengue, and all 4 appear to be circulating in most endemic countries. Clinical disease varies from a mild febrile illness to severe disease. The most common clinical presentation includes sudden onset of fever, headache, retro-orbital pain, myalgia and arthralgia, abdominal pain, and nausea.
Severe disease includes plasma leakage, shock, respiratory distress, severe bleeding, and organ failure. While severe dengue can occur with a primary infection, a second infection with a different DENV increases the risk of severe dengue. A small increased risk of severe dengue occurs when dengue infection occurs after vaccination in those with no evidence of previous dengue infection. It is felt that the vaccine serves as a primary infection that increases the risk of severe dengue with subsequent infections. This is the reason that the vaccine is recommended only for those with a documented previous dengue infection.
At its June 2021 meeting, the ACIP recommended 3-doses of Dengvaxia, administered at 0, 6, and 12 months, for individuals 9 to 16 years of age who have laboratory confirmation of previous dengue infection and live in endemic areas.12 These areas include the territories and affiliated states of Puerto Rico, American Samoa, US Virgin Islands, Federated States of Micronesia, Republic of Marshall Islands, and the Republic of Palau. Puerto Rico accounts for 85% of the population of these areas and 95% of reported dengue cases.12The reason for the delay between FDA approval and the ACIP recommendation was the need to wait for a readily available, accurate laboratory test to confirm previous dengue infection, which is now available. There are other dengue vaccines in development including 2 live-attenuated, tetravalent vaccine candidates in Phase 3 trials.
1. ACIP. COVID-19 vaccine recommendations. Accessed February 8, 2022. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
2. CDC. Division of viral hepatitis. Disease burden from viral hepatitis A, B, and C in the United States. Accessed February 8 2022. www.cdc.gov/hepatitis/PDFs/disease_burden.pdf
3. CDC. Surveillance for viral hepatitis – United States, 2014. Hepatitis B. Accessed February 8, 2022. https://www.cdc.gov/hepatitis/statistics/2014surveillance/commentary.htm#:~:text=HEPATITIS%20B-,Acute%20Hepatitis%20B,B%20cases%20occurred%20in%202014
4. CDC. Viral hepatitis surveillance: United States, 2011. Hepatitis B. Accessed February 8, 2022. www.cdc.gov/hepatitis/statistics/2011surveillance/pdfs/2011HepSurveillanceRpt.pdf
5. CDC. Viral hepatitis surveillance report, 2019. Hepatitis B. Accessed February 8, 2022. www.cdc.gov/hepatitis/statistics/2019surveillance/HepB.htm
6. Schillie S, Harris A, Link-Gelles R, et al. Recommendations of the Advisory Committee on Immunization Practices for use of a hepatitis B vaccine with a novel adjuvant. MMWR Morb Mortal Wkly Rep. 2018;67:455-458.
7. CDC. Advisory Committee on Immunization Practices. Meeting recommendations, November 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/index.html
8. Matanock A, Lee G, Gierke R, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:1069-1075.
9. Kobayashi M. Considerations for use of PCV15 and PCV20 in U.S. adults. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-02/24-25/05-Pneumococcal-Kobayashi.pdf
10. Anderson TC, Masters NB, Guo A, et al. Use of recombinant zoster vaccine in immunocompromised adults aged ≥19 years: recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:80-84.
11. CDC. ACIP recommendations. June 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/recommendations.html
12. Paz-Bailey G. Dengue vaccine. Evidence to recommendation framework. Presented to the ACIP June 24, 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-Dengue-Paz-Bailey-508.pdf
In a typical year, the Advisory Committee on Immunization Practices (ACIP) has three 1.5- to 2-day meetings to make recommendations for the use of new and existing vaccines in the US population. However, 2021 was not a typical year. Last year, ACIP held 17 meetings for a total of 127 hours. Most of these were related to vaccines to prevent COVID-19. There are now 3 COVID-19 vaccines authorized for use in the United States: the 2-dose mRNA-based Pfizer-BioNTech/Comirnaty and Moderna COVID-19 vaccines and the single-dose adenovirus, vector-based Janssen (Johnson & Johnson) COVID-19 vaccine.
TABLE 11 includes the actions taken by the ACIP from late 2020 through 2021 related to COVID-19 vaccines. All of these recommendations except 1 occurred after the US Food and Drug Administration (FDA) approved the product using an emergency use authorization (EUA). The exception is the recommendation for use of the Pfizer-BioNTech COVID-19 vaccine (BNT162b2) for those ages 16 years and older, which was approved under the normal process 8 months after widespread use under an EUA.

Hepatitis B vaccine now for all nonimmune adults up through 59 years
Since the introduction of hepatitis B (HepB) vaccines in 1980, the incidence of hepatitis B virus (HBV) infections in the United States has been reduced dramatically; there were an estimated 287,000 cases in 19852 and 19,200 in 2014.3 However, the incidence among adults has not declined in recent years and among someage groups has actually increased. Among those ages 40 to 49 years, the rate went from 1.9 per 100,000 in 20114 to 2.7 per 100,000 population in 2019.5 In those ages 50 to 59, there was an increase from 1.1 to 1.6 per 100,000 population over the same period of time.4,5
Recommendations for using HepB vaccine in adults have been based on risk that involves individual behavior, occupation, and medical conditions (TABLE 26). The presence of these risk factors is often unknown to medical professionals, who rarely ask about or document them. And patients can be reluctant to disclose them for fear of being stigmatized. The consequence has been a low rate of vaccination in at-risk adults.

At its November 2021 meeting, ACIP accepted the advice of the Hepatitis Work Group to move to a universal adult recommendation through age 59.7 ACIP believed that the incidence of acute infection in those ages 60 and older was too low to merit a universal recommendation. The new recommendation states that
Multiple HepB vaccine products are available for adults. Two are recombinant-based and require 3 doses: Engerix-B (GlaxoSmithKline) and Recombivax HB (Merck). One is recombinant based and requires only 2 doses: Heplisav-B (Dynavax Technologies). A new product recently approved by the FDA, PREHEVBRIO (VBI Vaccines), is another recombinant 3-dose option that the ACIP will consider early in 2022. HepB and HepA vaccines can also be co-administered with Twinrix (GlaxoSmithKline).
Pneumococcal vaccines: New PCV vaccines alter prescribing choices
The ACIP recommendations for pneumococcal vaccines in adults have been very confusing, involving 2 vaccines: PCV13 (Prevnar13, Pfizer) and PPSV23 (Pneumovax23, Merck). Both PCV13 and PPSV23 given in series were recommended for immunocompromised patients, but only PPSV23 was recommended for those with chronic medical conditions. For those 65 and older, PPSV23 was recommended for all individuals (including those with no chronic or immunocompromising condition), and PCV13 was recommended for those with immunocompromising conditions. Other adults in this older age group could receive PCV13 based on individual risk and shared clinical decision making.8
Continue to: This past year...
This past year, 2 new PCV vaccines were approved by the FDA: PCV15 (Vaxneuvance, Merck) and PCV20 (Prevnar20, Pfizer). While considering these new vaccines, the ACIP re-assessed its entire approval of pneumococcal vaccines. First, they retained the cutoff for universal pneumococcal vaccination at 65 years. For those younger than 65, they combined chronic medical conditions and immunocompromising conditions into a single at-risk group (TABLE 39). They then issued the same recommendation for older adults and those younger than 65 with risks: to receive a PCV vaccine, either PCV15 or PCV20. If they receive PCV15, it should be followed by PPSV23. PPSV23 is not recommended for those who receive PCV20. Therefore,

Recombinant zoster vaccine (RZV) has been licensed and recommended in the United States since 2017 in a 2-dose schedule for adults ages 50 years and older. In the summer of 2021, the FDA expanded the indication for use of RZV to include individuals 18 to 49 years of age who are or will be immunodeficient or immunosuppressed due to known disease or therapy. In October, the ACIP agreed and recommended 2 RZV doses for those 19 years and older in these risk groups (TABLE 410).

This recommendation was based on the elevated risk of herpes zoster documented in those with immune-suppressing conditions and therapies. In the conditions studied, the incidence in these younger adults exceeded that for older adults, for whom the vaccine is recommended.10 There are many immune conditions and immune-suppressing medications. The ACIP Zoster Work Group did not have efficacy and safety information on the use of RZV in each one of them, even though their recommendation includes them all. Many of these patients are under the care of specialists whose specialty societies had been recommending zoster vaccine for their patients, off label, prior to the FDA authorization.
Rabies vaccine is now available in 2-dose schedule
People who should receive rabies pre-exposure prophylaxis (PrEP) with rabies vaccine include laboratory personnel who work with rabies virus, biologists who work with bats, animal care professionals, wildlife biologists, veterinarians, and travelers who may be at risk of encountering rabid dogs. The recommendation has been for 3 doses of rabies vaccine at 0, 7, and 21-28 days. The ACIP voted at its June 2021 meeting to adopt a 2-dose PrEP schedule of 0 and 7 days.11 This will be especially helpful to travelers who want to complete the recommended doses prior to departure. Those who have sustained risk over time can elect to have a third dose after 21 days and before 3 years, or elect to have titers checked. More detailed clinical advice will be published in the CDC’s Morbidity and Mortality Weekly Report in 2022.
Dengue vaccine: New rec for those 9-16 years
In 2019, the FDA approved the first dengue vaccine for use in the United States for children 9 to 16 years old who had laboratory-confirmed previous dengue virus infection and who were living in an area where dengue is endemic. The CYD-TDV dengue vaccine (Dengvaxia) is a live-attenuated tetravalent vaccine built on a yellow fever vaccine backbone. Its effectiveness is 82% for prevention of symptomatic dengue, 79% for prevention of dengue-associated hospitalizations, and 84% against severe dengue.12
Continue to: Dengue viruses...
Dengue viruses (DENV) are transmitted by Aedes mosquitoes. There are 4 serotypes of dengue, and all 4 appear to be circulating in most endemic countries. Clinical disease varies from a mild febrile illness to severe disease. The most common clinical presentation includes sudden onset of fever, headache, retro-orbital pain, myalgia and arthralgia, abdominal pain, and nausea.
Severe disease includes plasma leakage, shock, respiratory distress, severe bleeding, and organ failure. While severe dengue can occur with a primary infection, a second infection with a different DENV increases the risk of severe dengue. A small increased risk of severe dengue occurs when dengue infection occurs after vaccination in those with no evidence of previous dengue infection. It is felt that the vaccine serves as a primary infection that increases the risk of severe dengue with subsequent infections. This is the reason that the vaccine is recommended only for those with a documented previous dengue infection.
At its June 2021 meeting, the ACIP recommended 3-doses of Dengvaxia, administered at 0, 6, and 12 months, for individuals 9 to 16 years of age who have laboratory confirmation of previous dengue infection and live in endemic areas.12 These areas include the territories and affiliated states of Puerto Rico, American Samoa, US Virgin Islands, Federated States of Micronesia, Republic of Marshall Islands, and the Republic of Palau. Puerto Rico accounts for 85% of the population of these areas and 95% of reported dengue cases.12The reason for the delay between FDA approval and the ACIP recommendation was the need to wait for a readily available, accurate laboratory test to confirm previous dengue infection, which is now available. There are other dengue vaccines in development including 2 live-attenuated, tetravalent vaccine candidates in Phase 3 trials.
In a typical year, the Advisory Committee on Immunization Practices (ACIP) has three 1.5- to 2-day meetings to make recommendations for the use of new and existing vaccines in the US population. However, 2021 was not a typical year. Last year, ACIP held 17 meetings for a total of 127 hours. Most of these were related to vaccines to prevent COVID-19. There are now 3 COVID-19 vaccines authorized for use in the United States: the 2-dose mRNA-based Pfizer-BioNTech/Comirnaty and Moderna COVID-19 vaccines and the single-dose adenovirus, vector-based Janssen (Johnson & Johnson) COVID-19 vaccine.
TABLE 11 includes the actions taken by the ACIP from late 2020 through 2021 related to COVID-19 vaccines. All of these recommendations except 1 occurred after the US Food and Drug Administration (FDA) approved the product using an emergency use authorization (EUA). The exception is the recommendation for use of the Pfizer-BioNTech COVID-19 vaccine (BNT162b2) for those ages 16 years and older, which was approved under the normal process 8 months after widespread use under an EUA.

Hepatitis B vaccine now for all nonimmune adults up through 59 years
Since the introduction of hepatitis B (HepB) vaccines in 1980, the incidence of hepatitis B virus (HBV) infections in the United States has been reduced dramatically; there were an estimated 287,000 cases in 19852 and 19,200 in 2014.3 However, the incidence among adults has not declined in recent years and among someage groups has actually increased. Among those ages 40 to 49 years, the rate went from 1.9 per 100,000 in 20114 to 2.7 per 100,000 population in 2019.5 In those ages 50 to 59, there was an increase from 1.1 to 1.6 per 100,000 population over the same period of time.4,5
Recommendations for using HepB vaccine in adults have been based on risk that involves individual behavior, occupation, and medical conditions (TABLE 26). The presence of these risk factors is often unknown to medical professionals, who rarely ask about or document them. And patients can be reluctant to disclose them for fear of being stigmatized. The consequence has been a low rate of vaccination in at-risk adults.

At its November 2021 meeting, ACIP accepted the advice of the Hepatitis Work Group to move to a universal adult recommendation through age 59.7 ACIP believed that the incidence of acute infection in those ages 60 and older was too low to merit a universal recommendation. The new recommendation states that
Multiple HepB vaccine products are available for adults. Two are recombinant-based and require 3 doses: Engerix-B (GlaxoSmithKline) and Recombivax HB (Merck). One is recombinant based and requires only 2 doses: Heplisav-B (Dynavax Technologies). A new product recently approved by the FDA, PREHEVBRIO (VBI Vaccines), is another recombinant 3-dose option that the ACIP will consider early in 2022. HepB and HepA vaccines can also be co-administered with Twinrix (GlaxoSmithKline).
Pneumococcal vaccines: New PCV vaccines alter prescribing choices
The ACIP recommendations for pneumococcal vaccines in adults have been very confusing, involving 2 vaccines: PCV13 (Prevnar13, Pfizer) and PPSV23 (Pneumovax23, Merck). Both PCV13 and PPSV23 given in series were recommended for immunocompromised patients, but only PPSV23 was recommended for those with chronic medical conditions. For those 65 and older, PPSV23 was recommended for all individuals (including those with no chronic or immunocompromising condition), and PCV13 was recommended for those with immunocompromising conditions. Other adults in this older age group could receive PCV13 based on individual risk and shared clinical decision making.8
Continue to: This past year...
This past year, 2 new PCV vaccines were approved by the FDA: PCV15 (Vaxneuvance, Merck) and PCV20 (Prevnar20, Pfizer). While considering these new vaccines, the ACIP re-assessed its entire approval of pneumococcal vaccines. First, they retained the cutoff for universal pneumococcal vaccination at 65 years. For those younger than 65, they combined chronic medical conditions and immunocompromising conditions into a single at-risk group (TABLE 39). They then issued the same recommendation for older adults and those younger than 65 with risks: to receive a PCV vaccine, either PCV15 or PCV20. If they receive PCV15, it should be followed by PPSV23. PPSV23 is not recommended for those who receive PCV20. Therefore,

Recombinant zoster vaccine (RZV) has been licensed and recommended in the United States since 2017 in a 2-dose schedule for adults ages 50 years and older. In the summer of 2021, the FDA expanded the indication for use of RZV to include individuals 18 to 49 years of age who are or will be immunodeficient or immunosuppressed due to known disease or therapy. In October, the ACIP agreed and recommended 2 RZV doses for those 19 years and older in these risk groups (TABLE 410).

This recommendation was based on the elevated risk of herpes zoster documented in those with immune-suppressing conditions and therapies. In the conditions studied, the incidence in these younger adults exceeded that for older adults, for whom the vaccine is recommended.10 There are many immune conditions and immune-suppressing medications. The ACIP Zoster Work Group did not have efficacy and safety information on the use of RZV in each one of them, even though their recommendation includes them all. Many of these patients are under the care of specialists whose specialty societies had been recommending zoster vaccine for their patients, off label, prior to the FDA authorization.
Rabies vaccine is now available in 2-dose schedule
People who should receive rabies pre-exposure prophylaxis (PrEP) with rabies vaccine include laboratory personnel who work with rabies virus, biologists who work with bats, animal care professionals, wildlife biologists, veterinarians, and travelers who may be at risk of encountering rabid dogs. The recommendation has been for 3 doses of rabies vaccine at 0, 7, and 21-28 days. The ACIP voted at its June 2021 meeting to adopt a 2-dose PrEP schedule of 0 and 7 days.11 This will be especially helpful to travelers who want to complete the recommended doses prior to departure. Those who have sustained risk over time can elect to have a third dose after 21 days and before 3 years, or elect to have titers checked. More detailed clinical advice will be published in the CDC’s Morbidity and Mortality Weekly Report in 2022.
Dengue vaccine: New rec for those 9-16 years
In 2019, the FDA approved the first dengue vaccine for use in the United States for children 9 to 16 years old who had laboratory-confirmed previous dengue virus infection and who were living in an area where dengue is endemic. The CYD-TDV dengue vaccine (Dengvaxia) is a live-attenuated tetravalent vaccine built on a yellow fever vaccine backbone. Its effectiveness is 82% for prevention of symptomatic dengue, 79% for prevention of dengue-associated hospitalizations, and 84% against severe dengue.12
Continue to: Dengue viruses...
Dengue viruses (DENV) are transmitted by Aedes mosquitoes. There are 4 serotypes of dengue, and all 4 appear to be circulating in most endemic countries. Clinical disease varies from a mild febrile illness to severe disease. The most common clinical presentation includes sudden onset of fever, headache, retro-orbital pain, myalgia and arthralgia, abdominal pain, and nausea.
Severe disease includes plasma leakage, shock, respiratory distress, severe bleeding, and organ failure. While severe dengue can occur with a primary infection, a second infection with a different DENV increases the risk of severe dengue. A small increased risk of severe dengue occurs when dengue infection occurs after vaccination in those with no evidence of previous dengue infection. It is felt that the vaccine serves as a primary infection that increases the risk of severe dengue with subsequent infections. This is the reason that the vaccine is recommended only for those with a documented previous dengue infection.
At its June 2021 meeting, the ACIP recommended 3-doses of Dengvaxia, administered at 0, 6, and 12 months, for individuals 9 to 16 years of age who have laboratory confirmation of previous dengue infection and live in endemic areas.12 These areas include the territories and affiliated states of Puerto Rico, American Samoa, US Virgin Islands, Federated States of Micronesia, Republic of Marshall Islands, and the Republic of Palau. Puerto Rico accounts for 85% of the population of these areas and 95% of reported dengue cases.12The reason for the delay between FDA approval and the ACIP recommendation was the need to wait for a readily available, accurate laboratory test to confirm previous dengue infection, which is now available. There are other dengue vaccines in development including 2 live-attenuated, tetravalent vaccine candidates in Phase 3 trials.
1. ACIP. COVID-19 vaccine recommendations. Accessed February 8, 2022. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
2. CDC. Division of viral hepatitis. Disease burden from viral hepatitis A, B, and C in the United States. Accessed February 8 2022. www.cdc.gov/hepatitis/PDFs/disease_burden.pdf
3. CDC. Surveillance for viral hepatitis – United States, 2014. Hepatitis B. Accessed February 8, 2022. https://www.cdc.gov/hepatitis/statistics/2014surveillance/commentary.htm#:~:text=HEPATITIS%20B-,Acute%20Hepatitis%20B,B%20cases%20occurred%20in%202014
4. CDC. Viral hepatitis surveillance: United States, 2011. Hepatitis B. Accessed February 8, 2022. www.cdc.gov/hepatitis/statistics/2011surveillance/pdfs/2011HepSurveillanceRpt.pdf
5. CDC. Viral hepatitis surveillance report, 2019. Hepatitis B. Accessed February 8, 2022. www.cdc.gov/hepatitis/statistics/2019surveillance/HepB.htm
6. Schillie S, Harris A, Link-Gelles R, et al. Recommendations of the Advisory Committee on Immunization Practices for use of a hepatitis B vaccine with a novel adjuvant. MMWR Morb Mortal Wkly Rep. 2018;67:455-458.
7. CDC. Advisory Committee on Immunization Practices. Meeting recommendations, November 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/index.html
8. Matanock A, Lee G, Gierke R, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:1069-1075.
9. Kobayashi M. Considerations for use of PCV15 and PCV20 in U.S. adults. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-02/24-25/05-Pneumococcal-Kobayashi.pdf
10. Anderson TC, Masters NB, Guo A, et al. Use of recombinant zoster vaccine in immunocompromised adults aged ≥19 years: recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:80-84.
11. CDC. ACIP recommendations. June 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/recommendations.html
12. Paz-Bailey G. Dengue vaccine. Evidence to recommendation framework. Presented to the ACIP June 24, 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-Dengue-Paz-Bailey-508.pdf
1. ACIP. COVID-19 vaccine recommendations. Accessed February 8, 2022. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
2. CDC. Division of viral hepatitis. Disease burden from viral hepatitis A, B, and C in the United States. Accessed February 8 2022. www.cdc.gov/hepatitis/PDFs/disease_burden.pdf
3. CDC. Surveillance for viral hepatitis – United States, 2014. Hepatitis B. Accessed February 8, 2022. https://www.cdc.gov/hepatitis/statistics/2014surveillance/commentary.htm#:~:text=HEPATITIS%20B-,Acute%20Hepatitis%20B,B%20cases%20occurred%20in%202014
4. CDC. Viral hepatitis surveillance: United States, 2011. Hepatitis B. Accessed February 8, 2022. www.cdc.gov/hepatitis/statistics/2011surveillance/pdfs/2011HepSurveillanceRpt.pdf
5. CDC. Viral hepatitis surveillance report, 2019. Hepatitis B. Accessed February 8, 2022. www.cdc.gov/hepatitis/statistics/2019surveillance/HepB.htm
6. Schillie S, Harris A, Link-Gelles R, et al. Recommendations of the Advisory Committee on Immunization Practices for use of a hepatitis B vaccine with a novel adjuvant. MMWR Morb Mortal Wkly Rep. 2018;67:455-458.
7. CDC. Advisory Committee on Immunization Practices. Meeting recommendations, November 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/index.html
8. Matanock A, Lee G, Gierke R, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:1069-1075.
9. Kobayashi M. Considerations for use of PCV15 and PCV20 in U.S. adults. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-02/24-25/05-Pneumococcal-Kobayashi.pdf
10. Anderson TC, Masters NB, Guo A, et al. Use of recombinant zoster vaccine in immunocompromised adults aged ≥19 years: recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:80-84.
11. CDC. ACIP recommendations. June 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/recommendations.html
12. Paz-Bailey G. Dengue vaccine. Evidence to recommendation framework. Presented to the ACIP June 24, 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-Dengue-Paz-Bailey-508.pdf
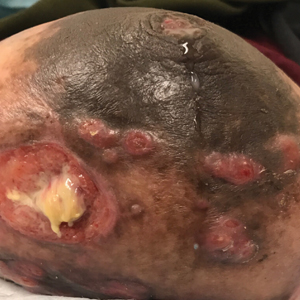
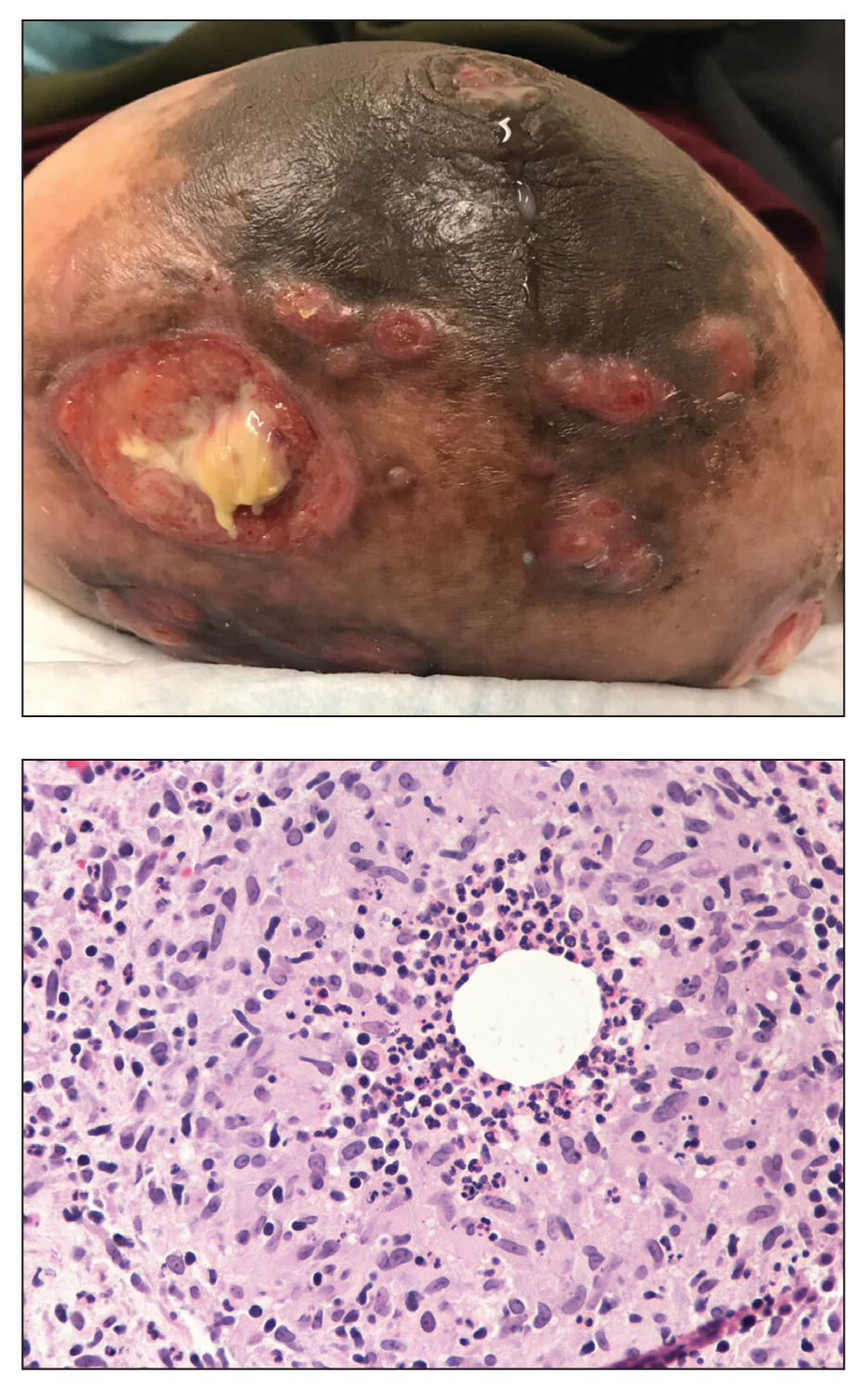
Painful Ulcerating Lesions on the Breast
The Diagnosis: Cystic Neutrophilic Granulomatous Mastitis
The histopathologic findings in our patient were characteristic of cystic neutrophilic granulomatous mastitis (CNGM), a rare granulomatous mastitis associated with Corynebacterium and suppurative lipogranulomas. Although not seen in our patient, the lipid vacuoles may contain gram-positive bacilli.1 The surrounding mixed inflammatory infiltrate contains Langerhans giant cells, lymphocytes, and neutrophils. Cystic neutrophilic granulomatous mastitis is seen in parous women of reproductive age. Physical examination demonstrates a palpable painful mass on the breast. Wound cultures frequently are negative, likely due to difficulty culturing Corynebacterium and prophylactic antibiotic treatment. Given the association with Corynebacterium species, early diagnosis of CNGM is essential in offering patients the most appropriate treatment. Prolonged antibiotic therapy specifically directed to corynebacteria is required, sometimes even beyond resolution of clinical symptoms. The diagnosis of CNGM often is missed or delayed due to its rarity and many potential mimickers. Clinically, CNGM may be virtually impossible to discern from invasive carcinoma.1
Our patient was treated with vancomycin and cefepime with incision and drainage as an inpatient. Upon discharge, she was started on prednisone 1 mg/kg daily tapered by 10 mg every 5 days over 1 month and doxycycline 100 mg twice daily. She was then transitioned to topical hydrocortisone and bacitracin; she reported decreased swelling and pain. No new lesions formed after the initiation of therapy; however, most lesions remained open. Cystic neutrophilic granulomatous mastitis remains a challenging entity to treat, with a variable response rate reported in the literature for antibiotics such as doxycycline and systemic and topical steroids as well as immunosuppressants including methotrexate.2,3
Cystic neutrophilic granulomatous mastitis can be distinguished from hidradenitis suppurativa clinically because ulcerating lesions can involve the superior portions of the breast in CNGM, whereas hidradenitis suppurativa typically is restricted to the lower intertriginous parts of the breast. Other mimics of CNGM can be distinguished with biopsy. Histology of pyoderma gangrenosum lacks prominent granuloma formation. Although sarcoidosis and mycobacterial infection show prominent granulomas, neither show the characteristic lipogranulomas seen in CNGM. Additionally, the granulomas of sarcoidosis are much larger and deeper than CNGM. Mycobacterial granulomas also typically reveal bacilli with acid-fast bacilli staining or via wound culture.
- Wu JM, Turashvili G. Cystic neutrophilic granulomatous mastitis: an update. J Clin Pathol. 2020;73:445-453. doi:10.1136/jclinpath-2019-206180
- Steuer AB, Stern MJ, Cobos G, et al. Clinical characteristics and medical management of idiopathic granulomatous mastitis. JAMA Dermatol. 2020;156:460-464. doi:10.1001/jamadermatol.2019.4516
- Dobinson HC, Anderson TP, Chambers ST, et al. Antimicrobial treatment options for granulomatous mastitis caused by Corynebacterium species [published online July 1, 2015]. J Clin Microbiol. 2015;53:2895-2899. doi:10.1128/JCM.00760-15
The Diagnosis: Cystic Neutrophilic Granulomatous Mastitis
The histopathologic findings in our patient were characteristic of cystic neutrophilic granulomatous mastitis (CNGM), a rare granulomatous mastitis associated with Corynebacterium and suppurative lipogranulomas. Although not seen in our patient, the lipid vacuoles may contain gram-positive bacilli.1 The surrounding mixed inflammatory infiltrate contains Langerhans giant cells, lymphocytes, and neutrophils. Cystic neutrophilic granulomatous mastitis is seen in parous women of reproductive age. Physical examination demonstrates a palpable painful mass on the breast. Wound cultures frequently are negative, likely due to difficulty culturing Corynebacterium and prophylactic antibiotic treatment. Given the association with Corynebacterium species, early diagnosis of CNGM is essential in offering patients the most appropriate treatment. Prolonged antibiotic therapy specifically directed to corynebacteria is required, sometimes even beyond resolution of clinical symptoms. The diagnosis of CNGM often is missed or delayed due to its rarity and many potential mimickers. Clinically, CNGM may be virtually impossible to discern from invasive carcinoma.1
Our patient was treated with vancomycin and cefepime with incision and drainage as an inpatient. Upon discharge, she was started on prednisone 1 mg/kg daily tapered by 10 mg every 5 days over 1 month and doxycycline 100 mg twice daily. She was then transitioned to topical hydrocortisone and bacitracin; she reported decreased swelling and pain. No new lesions formed after the initiation of therapy; however, most lesions remained open. Cystic neutrophilic granulomatous mastitis remains a challenging entity to treat, with a variable response rate reported in the literature for antibiotics such as doxycycline and systemic and topical steroids as well as immunosuppressants including methotrexate.2,3
Cystic neutrophilic granulomatous mastitis can be distinguished from hidradenitis suppurativa clinically because ulcerating lesions can involve the superior portions of the breast in CNGM, whereas hidradenitis suppurativa typically is restricted to the lower intertriginous parts of the breast. Other mimics of CNGM can be distinguished with biopsy. Histology of pyoderma gangrenosum lacks prominent granuloma formation. Although sarcoidosis and mycobacterial infection show prominent granulomas, neither show the characteristic lipogranulomas seen in CNGM. Additionally, the granulomas of sarcoidosis are much larger and deeper than CNGM. Mycobacterial granulomas also typically reveal bacilli with acid-fast bacilli staining or via wound culture.
The Diagnosis: Cystic Neutrophilic Granulomatous Mastitis
The histopathologic findings in our patient were characteristic of cystic neutrophilic granulomatous mastitis (CNGM), a rare granulomatous mastitis associated with Corynebacterium and suppurative lipogranulomas. Although not seen in our patient, the lipid vacuoles may contain gram-positive bacilli.1 The surrounding mixed inflammatory infiltrate contains Langerhans giant cells, lymphocytes, and neutrophils. Cystic neutrophilic granulomatous mastitis is seen in parous women of reproductive age. Physical examination demonstrates a palpable painful mass on the breast. Wound cultures frequently are negative, likely due to difficulty culturing Corynebacterium and prophylactic antibiotic treatment. Given the association with Corynebacterium species, early diagnosis of CNGM is essential in offering patients the most appropriate treatment. Prolonged antibiotic therapy specifically directed to corynebacteria is required, sometimes even beyond resolution of clinical symptoms. The diagnosis of CNGM often is missed or delayed due to its rarity and many potential mimickers. Clinically, CNGM may be virtually impossible to discern from invasive carcinoma.1
Our patient was treated with vancomycin and cefepime with incision and drainage as an inpatient. Upon discharge, she was started on prednisone 1 mg/kg daily tapered by 10 mg every 5 days over 1 month and doxycycline 100 mg twice daily. She was then transitioned to topical hydrocortisone and bacitracin; she reported decreased swelling and pain. No new lesions formed after the initiation of therapy; however, most lesions remained open. Cystic neutrophilic granulomatous mastitis remains a challenging entity to treat, with a variable response rate reported in the literature for antibiotics such as doxycycline and systemic and topical steroids as well as immunosuppressants including methotrexate.2,3
Cystic neutrophilic granulomatous mastitis can be distinguished from hidradenitis suppurativa clinically because ulcerating lesions can involve the superior portions of the breast in CNGM, whereas hidradenitis suppurativa typically is restricted to the lower intertriginous parts of the breast. Other mimics of CNGM can be distinguished with biopsy. Histology of pyoderma gangrenosum lacks prominent granuloma formation. Although sarcoidosis and mycobacterial infection show prominent granulomas, neither show the characteristic lipogranulomas seen in CNGM. Additionally, the granulomas of sarcoidosis are much larger and deeper than CNGM. Mycobacterial granulomas also typically reveal bacilli with acid-fast bacilli staining or via wound culture.
- Wu JM, Turashvili G. Cystic neutrophilic granulomatous mastitis: an update. J Clin Pathol. 2020;73:445-453. doi:10.1136/jclinpath-2019-206180
- Steuer AB, Stern MJ, Cobos G, et al. Clinical characteristics and medical management of idiopathic granulomatous mastitis. JAMA Dermatol. 2020;156:460-464. doi:10.1001/jamadermatol.2019.4516
- Dobinson HC, Anderson TP, Chambers ST, et al. Antimicrobial treatment options for granulomatous mastitis caused by Corynebacterium species [published online July 1, 2015]. J Clin Microbiol. 2015;53:2895-2899. doi:10.1128/JCM.00760-15
- Wu JM, Turashvili G. Cystic neutrophilic granulomatous mastitis: an update. J Clin Pathol. 2020;73:445-453. doi:10.1136/jclinpath-2019-206180
- Steuer AB, Stern MJ, Cobos G, et al. Clinical characteristics and medical management of idiopathic granulomatous mastitis. JAMA Dermatol. 2020;156:460-464. doi:10.1001/jamadermatol.2019.4516
- Dobinson HC, Anderson TP, Chambers ST, et al. Antimicrobial treatment options for granulomatous mastitis caused by Corynebacterium species [published online July 1, 2015]. J Clin Microbiol. 2015;53:2895-2899. doi:10.1128/JCM.00760-15
A 36-year-old puerperal woman presented with painful, unilateral, ulcerating breast lesions (top) of 3 months’ duration that developed during pregnancy and drained pus with blood. No improvement was seen with antibiotics or incision and drainage. Biopsy of a lesion showed stellate granulomas with cystic spaces and suppurative lipogranulomas where central lipid vacuoles were rimmed by neutrophils and an outer cuff of epithelioid histiocytes (bottom). Acid-fast bacilli, Grocott-Gomori methenamine-silver, Gram, and Steiner staining did not reveal any microorganisms. Additionally, wound cultures were negative.
Veterans Potentially Exposed to HIV, HCV at Georgia Hospital
Testing is ongoing after more than 4,600 veterans who had received care at the Carl Vinson Veterans Affairs Medical Center in Dublin, Georgia, were alerted that they may have been exposed to HIV, hepatitis B, and hepatitis C. The exposure was due to improperly sterilized equipment. At least some of the patients have tested positive, but the facility has not indicated the number, the diseases, or whether the infections were the result of the exposure.
A mid-January internal review at the hospital found that not all steps were being followed in the procedures for sterilizing equipment between patients. Patients who had dentistry, endoscopy, urology, podiatry, optometry, or surgical procedures in 2021 may have been exposed to blood-borne pathogens.
In response, the VA sent teams from other hospitals to help, including a team from the Augusta Veterans Affairs Medical Center to reprocess all equipment and staff from VA facilities in Atlanta, South Carolina, and Alabama to provide personnel training. All staff at Carl Vinson Veterans Affairs Medical Center have since been retrained on all current guidelines.
The hospital says it’s still testing exposed veterans. Hospital spokesperson James Huckfeldt told a Macon-based newspaper, The Telegraph, that veterans with positive test results will undergo additional testing to determine whether the transmission is new or preexisting. “The findings from the additional testing will be used to accurately diagnose any impacted veterans and ensure that they receive appropriate medical treatment,” he said.
Manuel M. Davila, director of the hospital, sent letters to the patients at risk, alerting them to the exposure. “We sincerely apologize and accept responsibility for this mistake and are taking steps to prevent it from happening in the future,” Davilla wrote. “This event is unacceptable to us as well, and we want to work with you to correct the situation and ensure your safety and well-being. Because your safety is important to us and because we want to honor your trust in us, we want you to know that when concerns are raised over our processes or procedures, we take immediate steps to stop everything and make sure things are.”
Davilla reassured the veterans that “we are confident that the risk of infectious disease is very low.”
The Carl Vinson Medical Center has set up a communication center to answer questions for veterans: (478) 274-5400.
Testing is ongoing after more than 4,600 veterans who had received care at the Carl Vinson Veterans Affairs Medical Center in Dublin, Georgia, were alerted that they may have been exposed to HIV, hepatitis B, and hepatitis C. The exposure was due to improperly sterilized equipment. At least some of the patients have tested positive, but the facility has not indicated the number, the diseases, or whether the infections were the result of the exposure.
A mid-January internal review at the hospital found that not all steps were being followed in the procedures for sterilizing equipment between patients. Patients who had dentistry, endoscopy, urology, podiatry, optometry, or surgical procedures in 2021 may have been exposed to blood-borne pathogens.
In response, the VA sent teams from other hospitals to help, including a team from the Augusta Veterans Affairs Medical Center to reprocess all equipment and staff from VA facilities in Atlanta, South Carolina, and Alabama to provide personnel training. All staff at Carl Vinson Veterans Affairs Medical Center have since been retrained on all current guidelines.
The hospital says it’s still testing exposed veterans. Hospital spokesperson James Huckfeldt told a Macon-based newspaper, The Telegraph, that veterans with positive test results will undergo additional testing to determine whether the transmission is new or preexisting. “The findings from the additional testing will be used to accurately diagnose any impacted veterans and ensure that they receive appropriate medical treatment,” he said.
Manuel M. Davila, director of the hospital, sent letters to the patients at risk, alerting them to the exposure. “We sincerely apologize and accept responsibility for this mistake and are taking steps to prevent it from happening in the future,” Davilla wrote. “This event is unacceptable to us as well, and we want to work with you to correct the situation and ensure your safety and well-being. Because your safety is important to us and because we want to honor your trust in us, we want you to know that when concerns are raised over our processes or procedures, we take immediate steps to stop everything and make sure things are.”
Davilla reassured the veterans that “we are confident that the risk of infectious disease is very low.”
The Carl Vinson Medical Center has set up a communication center to answer questions for veterans: (478) 274-5400.
Testing is ongoing after more than 4,600 veterans who had received care at the Carl Vinson Veterans Affairs Medical Center in Dublin, Georgia, were alerted that they may have been exposed to HIV, hepatitis B, and hepatitis C. The exposure was due to improperly sterilized equipment. At least some of the patients have tested positive, but the facility has not indicated the number, the diseases, or whether the infections were the result of the exposure.
A mid-January internal review at the hospital found that not all steps were being followed in the procedures for sterilizing equipment between patients. Patients who had dentistry, endoscopy, urology, podiatry, optometry, or surgical procedures in 2021 may have been exposed to blood-borne pathogens.
In response, the VA sent teams from other hospitals to help, including a team from the Augusta Veterans Affairs Medical Center to reprocess all equipment and staff from VA facilities in Atlanta, South Carolina, and Alabama to provide personnel training. All staff at Carl Vinson Veterans Affairs Medical Center have since been retrained on all current guidelines.
The hospital says it’s still testing exposed veterans. Hospital spokesperson James Huckfeldt told a Macon-based newspaper, The Telegraph, that veterans with positive test results will undergo additional testing to determine whether the transmission is new or preexisting. “The findings from the additional testing will be used to accurately diagnose any impacted veterans and ensure that they receive appropriate medical treatment,” he said.
Manuel M. Davila, director of the hospital, sent letters to the patients at risk, alerting them to the exposure. “We sincerely apologize and accept responsibility for this mistake and are taking steps to prevent it from happening in the future,” Davilla wrote. “This event is unacceptable to us as well, and we want to work with you to correct the situation and ensure your safety and well-being. Because your safety is important to us and because we want to honor your trust in us, we want you to know that when concerns are raised over our processes or procedures, we take immediate steps to stop everything and make sure things are.”
Davilla reassured the veterans that “we are confident that the risk of infectious disease is very low.”
The Carl Vinson Medical Center has set up a communication center to answer questions for veterans: (478) 274-5400.
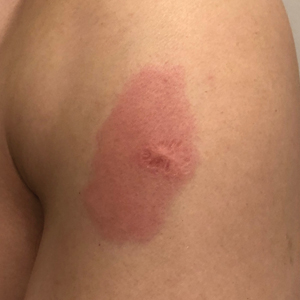
Reactivation of a BCG Vaccination Scar Following the First Dose of the Moderna COVID-19 Vaccine
The COVID-19 pandemic has resulted in notable morbidity and mortality worldwide. In December 2020, the US Food and Drug Administration issued an Emergency Use Authorization for 2 messenger RNA (mRNA) vaccines—produced by Pfizer-BioNTech and Moderna—for the prevention of COVID-19. Phase 3 trials of the vaccine developed by Moderna showed 94.1% efficacy at preventing COVID-19 after 2 doses.1
Common cutaneous adverse effects of the Moderna COVID-19 Vaccine include injection-site reactions, such as pain, induration, and erythema. Less frequently reported dermatologic adverse effects include diffuse bullous rash and hypersensitivity reactions.1 We report a case of reactivation of a BCG vaccination scar after the first dose of the Moderna COVID-19 Vaccine.
Case Report
A 48-year-old Asian man who was otherwise healthy presented with erythema, induration, and mild pruritus on the deltoid muscle of the left arm, near the scar from an earlier BCG vaccine, which he received at approximately 5 years of age when living in Taiwan. The patient received the first dose of the Moderna COVID-19 Vaccine approximately 5 to 7 cm distant from the BCG vaccination scar. One to 2 days after inoculation, the patient endorsed tenderness at the site of COVID-19 vaccination but denied systemic symptoms. He had never been given a diagnosis of COVID-19. His SARS-CoV-2 antibody status was unknown.
Eight days later, the patient noticed a well-defined, erythematous, indurated plaque with mild itchiness overlying and around the BCG vaccination scar that did not involve the COVID-19 vaccination site. The following day, the redness and induration became worse (Figure).
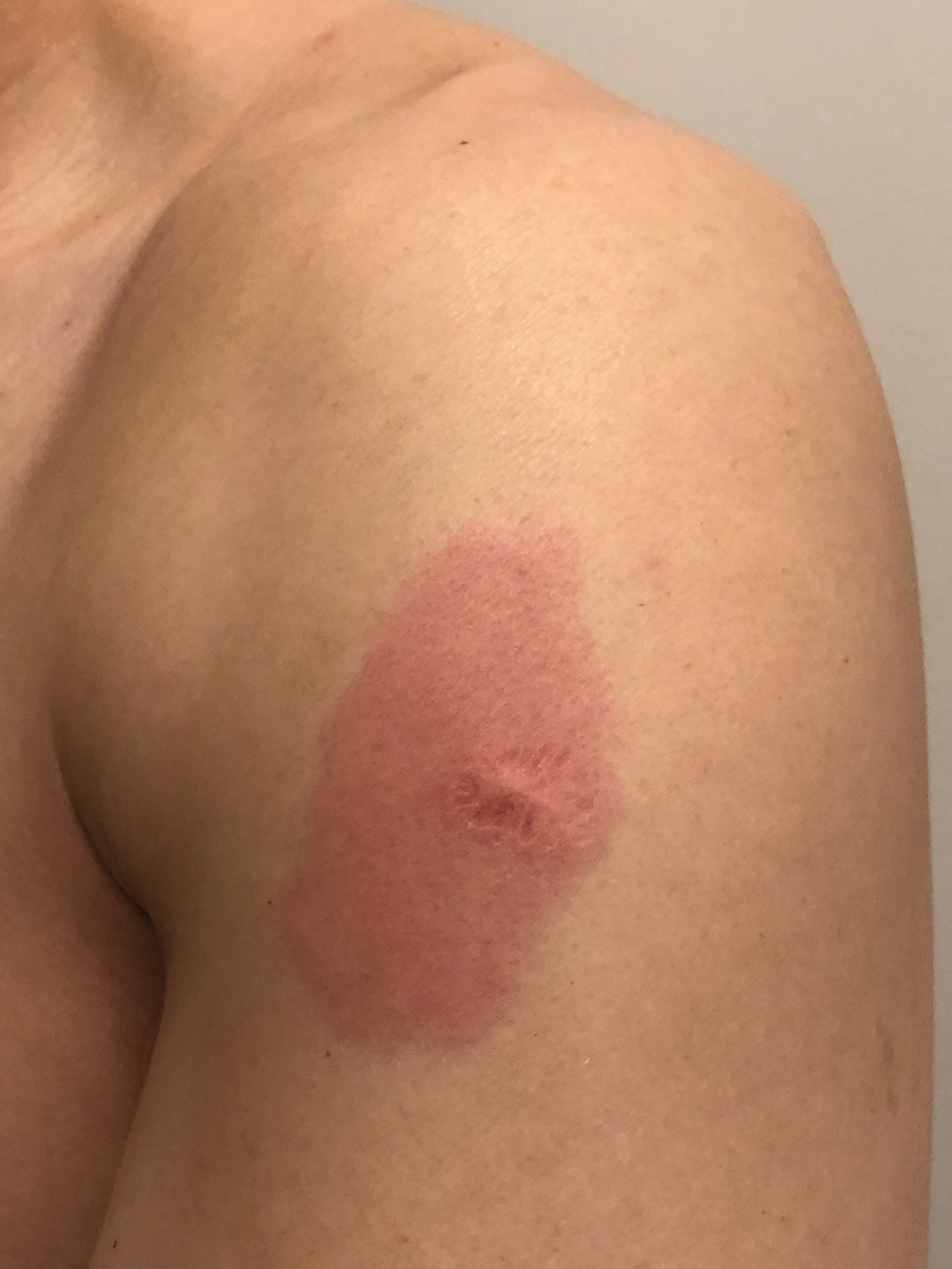
The patient was otherwise well. Vital signs were normal; there was no lymphadenopathy. The rash resolved without treatment over the next 4 days.
Comment
The BCG vaccine is an intradermal live attenuated virus vaccine used to prevent certain forms of tuberculosis and potentially other Mycobacterium infections. Although the vaccine is not routinely administered in the United States, it is part of the vaccination schedule in most countries, administered most often to newborns and infants. Administration of the BCG vaccine commonly results in mild localized erythema, swelling, and pain at the injection site. Most inoculated patients also develop an ulcer that heals with the characteristic BCG vaccination scar.2,3
There is evidence that the BCG vaccine can enhance the innate immune system response and might decrease the rate of infection by unrelated pathogens, including viruses.4 Several epidemiologic studies have suggested that the BCG vaccine might offer some protection against COVID-19, possibly due to a resemblance of the amino acid sequences of BCG and SARS-CoV-2, which might provoke cross-reactive T cells.5,6 Further studies are underway to determine whether the BCG vaccine is truly protective against COVID-19.
BCG vaccination scar reactivation presents as redness, swelling, or ulceration at the BCG injection site months to years after inoculation. Although erythema and induration of the BCG scar are not included in the diagnostic criteria of Kawasaki disease, likely due to variable vaccine requirements in different countries, these findings are largely recognized as specific for Kawasaki disease and present in approximately half of affected patients who received the BCG vaccine.2
Heat Shock Proteins—Heat shock proteins (HSPs) are produced by cells in response to stressors. The proposed mechanism of BCG vaccination scar reactivation is a cross-reaction between human homologue HSP 63 and Mycobacterium HSP 65, leading to hyperactivity of the immune system against BCG.7 There also are reports of reactivation of a BCG vaccination scar from measles infection and influenza vaccination.2,8,9 Most prior reports of BCG vaccination scar reactivation have been in pediatric patients; our patient is an adult who received the BCG vaccine more than 40 years ago.
Mechanism of Reactivation—The mechanism of BCG vaccination scar reactivation in our patient, who received the Moderna COVID-19 Vaccine, is unclear. Possible mechanisms include (1) release of HSP mediated by the COVID-19 vaccine, leading to an immune response at the BCG vaccine scar, or (2) another immune-mediated cross-reaction between BCG and the Moderna COVID-19 Vaccine mRNA nanoparticle or encoded spike protein antigen. It has been hypothesized that the BCG vaccine might offer some protection against COVID-19; this remains uncertain and is under further investigation.10 A recent retrospective cohort study showed that a BCG vaccination booster may decrease COVID-19 infection rates in higher-risk populations.11
Conclusion
We present a case of BCG vaccine scar reactivation occurring after a dose of the Moderna COVID-19 Vaccine, a likely underreported, self-limiting, cutaneous adverse effect of this mRNA vaccine.
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384:403-416. doi:10.1056/NEJMoa2035389
- Muthuvelu S, Lim KS, Huang L-Y, et al. Measles infection causing bacillus Calmette-Guérin reactivation: a case report. BMC Pediatr. 2019;19:251. doi:10.1186/s12887-019-1635-z
- Fatima S, Kumari A, Das G, et al. Tuberculosis vaccine: a journey from BCG to present. Life Sci. 2020;252:117594. doi:10.1016/j.lfs.2020.117594
- O’Neill LAJ, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20:335-337. doi:10.1038/s41577-020-0337-y
- Brooks NA, Puri A, Garg S, et al. The association of coronavirus disease-19 mortality and prior bacille Calmette-Guérin vaccination: a robust ecological analysis using unsupervised machine learning. Sci Rep. 2021;11:774. doi:10.1038/s41598-020-80787-z
- Tomita Y, Sato R, Ikeda T, et al. BCG vaccine may generate cross-reactive T-cells against SARS-CoV-2: in silico analyses and a hypothesis. Vaccine. 2020;38:6352-6356. doi:10.1016/j.vaccine.2020.08.045
- Lim KYY, Chua MC, Tan NWH, et al. Reactivation of BCG inoculation site in a child with febrile exanthema of 3 days duration: an early indicator of incomplete Kawasaki disease. BMJ Case Rep. 2020;13:E239648. doi:10.1136/bcr-2020-239648
- Kondo M, Goto H, Yamamoto S. First case of redness and erosion at bacillus Calmette-Guérin inoculation site after vaccination against influenza. J Dermatol. 2016;43:1229-1231. doi:10.1111/1346-8138.13365
- Chavarri-Guerra Y, Soto-Pérez-de-Celis E. Erythema at the bacillus Calmette-Guerin scar after influenza vaccination. Rev Soc Bras Med Trop. 2019;53:E20190390. doi:10.1590/0037-8682-0390-2019
- Fu W, Ho P-C, Liu C-L, et al. Reconcile the debate over protective effects of BCG vaccine against COVID-19. Sci Rep. 2021;11:8356. doi:10.1038/s41598-021-87731-9
- Amirlak L, Haddad R, Hardy JD, et al. Effectiveness of booster BCG vaccination in preventing COVID-19 infection. Hum Vaccin Immunother. 2021;17:3913-3915. doi:10.1080/21645515.2021.1956228
The COVID-19 pandemic has resulted in notable morbidity and mortality worldwide. In December 2020, the US Food and Drug Administration issued an Emergency Use Authorization for 2 messenger RNA (mRNA) vaccines—produced by Pfizer-BioNTech and Moderna—for the prevention of COVID-19. Phase 3 trials of the vaccine developed by Moderna showed 94.1% efficacy at preventing COVID-19 after 2 doses.1
Common cutaneous adverse effects of the Moderna COVID-19 Vaccine include injection-site reactions, such as pain, induration, and erythema. Less frequently reported dermatologic adverse effects include diffuse bullous rash and hypersensitivity reactions.1 We report a case of reactivation of a BCG vaccination scar after the first dose of the Moderna COVID-19 Vaccine.
Case Report
A 48-year-old Asian man who was otherwise healthy presented with erythema, induration, and mild pruritus on the deltoid muscle of the left arm, near the scar from an earlier BCG vaccine, which he received at approximately 5 years of age when living in Taiwan. The patient received the first dose of the Moderna COVID-19 Vaccine approximately 5 to 7 cm distant from the BCG vaccination scar. One to 2 days after inoculation, the patient endorsed tenderness at the site of COVID-19 vaccination but denied systemic symptoms. He had never been given a diagnosis of COVID-19. His SARS-CoV-2 antibody status was unknown.
Eight days later, the patient noticed a well-defined, erythematous, indurated plaque with mild itchiness overlying and around the BCG vaccination scar that did not involve the COVID-19 vaccination site. The following day, the redness and induration became worse (Figure).

The patient was otherwise well. Vital signs were normal; there was no lymphadenopathy. The rash resolved without treatment over the next 4 days.
Comment
The BCG vaccine is an intradermal live attenuated virus vaccine used to prevent certain forms of tuberculosis and potentially other Mycobacterium infections. Although the vaccine is not routinely administered in the United States, it is part of the vaccination schedule in most countries, administered most often to newborns and infants. Administration of the BCG vaccine commonly results in mild localized erythema, swelling, and pain at the injection site. Most inoculated patients also develop an ulcer that heals with the characteristic BCG vaccination scar.2,3
There is evidence that the BCG vaccine can enhance the innate immune system response and might decrease the rate of infection by unrelated pathogens, including viruses.4 Several epidemiologic studies have suggested that the BCG vaccine might offer some protection against COVID-19, possibly due to a resemblance of the amino acid sequences of BCG and SARS-CoV-2, which might provoke cross-reactive T cells.5,6 Further studies are underway to determine whether the BCG vaccine is truly protective against COVID-19.
BCG vaccination scar reactivation presents as redness, swelling, or ulceration at the BCG injection site months to years after inoculation. Although erythema and induration of the BCG scar are not included in the diagnostic criteria of Kawasaki disease, likely due to variable vaccine requirements in different countries, these findings are largely recognized as specific for Kawasaki disease and present in approximately half of affected patients who received the BCG vaccine.2
Heat Shock Proteins—Heat shock proteins (HSPs) are produced by cells in response to stressors. The proposed mechanism of BCG vaccination scar reactivation is a cross-reaction between human homologue HSP 63 and Mycobacterium HSP 65, leading to hyperactivity of the immune system against BCG.7 There also are reports of reactivation of a BCG vaccination scar from measles infection and influenza vaccination.2,8,9 Most prior reports of BCG vaccination scar reactivation have been in pediatric patients; our patient is an adult who received the BCG vaccine more than 40 years ago.
Mechanism of Reactivation—The mechanism of BCG vaccination scar reactivation in our patient, who received the Moderna COVID-19 Vaccine, is unclear. Possible mechanisms include (1) release of HSP mediated by the COVID-19 vaccine, leading to an immune response at the BCG vaccine scar, or (2) another immune-mediated cross-reaction between BCG and the Moderna COVID-19 Vaccine mRNA nanoparticle or encoded spike protein antigen. It has been hypothesized that the BCG vaccine might offer some protection against COVID-19; this remains uncertain and is under further investigation.10 A recent retrospective cohort study showed that a BCG vaccination booster may decrease COVID-19 infection rates in higher-risk populations.11
Conclusion
We present a case of BCG vaccine scar reactivation occurring after a dose of the Moderna COVID-19 Vaccine, a likely underreported, self-limiting, cutaneous adverse effect of this mRNA vaccine.
The COVID-19 pandemic has resulted in notable morbidity and mortality worldwide. In December 2020, the US Food and Drug Administration issued an Emergency Use Authorization for 2 messenger RNA (mRNA) vaccines—produced by Pfizer-BioNTech and Moderna—for the prevention of COVID-19. Phase 3 trials of the vaccine developed by Moderna showed 94.1% efficacy at preventing COVID-19 after 2 doses.1
Common cutaneous adverse effects of the Moderna COVID-19 Vaccine include injection-site reactions, such as pain, induration, and erythema. Less frequently reported dermatologic adverse effects include diffuse bullous rash and hypersensitivity reactions.1 We report a case of reactivation of a BCG vaccination scar after the first dose of the Moderna COVID-19 Vaccine.
Case Report
A 48-year-old Asian man who was otherwise healthy presented with erythema, induration, and mild pruritus on the deltoid muscle of the left arm, near the scar from an earlier BCG vaccine, which he received at approximately 5 years of age when living in Taiwan. The patient received the first dose of the Moderna COVID-19 Vaccine approximately 5 to 7 cm distant from the BCG vaccination scar. One to 2 days after inoculation, the patient endorsed tenderness at the site of COVID-19 vaccination but denied systemic symptoms. He had never been given a diagnosis of COVID-19. His SARS-CoV-2 antibody status was unknown.
Eight days later, the patient noticed a well-defined, erythematous, indurated plaque with mild itchiness overlying and around the BCG vaccination scar that did not involve the COVID-19 vaccination site. The following day, the redness and induration became worse (Figure).

The patient was otherwise well. Vital signs were normal; there was no lymphadenopathy. The rash resolved without treatment over the next 4 days.
Comment
The BCG vaccine is an intradermal live attenuated virus vaccine used to prevent certain forms of tuberculosis and potentially other Mycobacterium infections. Although the vaccine is not routinely administered in the United States, it is part of the vaccination schedule in most countries, administered most often to newborns and infants. Administration of the BCG vaccine commonly results in mild localized erythema, swelling, and pain at the injection site. Most inoculated patients also develop an ulcer that heals with the characteristic BCG vaccination scar.2,3
There is evidence that the BCG vaccine can enhance the innate immune system response and might decrease the rate of infection by unrelated pathogens, including viruses.4 Several epidemiologic studies have suggested that the BCG vaccine might offer some protection against COVID-19, possibly due to a resemblance of the amino acid sequences of BCG and SARS-CoV-2, which might provoke cross-reactive T cells.5,6 Further studies are underway to determine whether the BCG vaccine is truly protective against COVID-19.
BCG vaccination scar reactivation presents as redness, swelling, or ulceration at the BCG injection site months to years after inoculation. Although erythema and induration of the BCG scar are not included in the diagnostic criteria of Kawasaki disease, likely due to variable vaccine requirements in different countries, these findings are largely recognized as specific for Kawasaki disease and present in approximately half of affected patients who received the BCG vaccine.2
Heat Shock Proteins—Heat shock proteins (HSPs) are produced by cells in response to stressors. The proposed mechanism of BCG vaccination scar reactivation is a cross-reaction between human homologue HSP 63 and Mycobacterium HSP 65, leading to hyperactivity of the immune system against BCG.7 There also are reports of reactivation of a BCG vaccination scar from measles infection and influenza vaccination.2,8,9 Most prior reports of BCG vaccination scar reactivation have been in pediatric patients; our patient is an adult who received the BCG vaccine more than 40 years ago.
Mechanism of Reactivation—The mechanism of BCG vaccination scar reactivation in our patient, who received the Moderna COVID-19 Vaccine, is unclear. Possible mechanisms include (1) release of HSP mediated by the COVID-19 vaccine, leading to an immune response at the BCG vaccine scar, or (2) another immune-mediated cross-reaction between BCG and the Moderna COVID-19 Vaccine mRNA nanoparticle or encoded spike protein antigen. It has been hypothesized that the BCG vaccine might offer some protection against COVID-19; this remains uncertain and is under further investigation.10 A recent retrospective cohort study showed that a BCG vaccination booster may decrease COVID-19 infection rates in higher-risk populations.11
Conclusion
We present a case of BCG vaccine scar reactivation occurring after a dose of the Moderna COVID-19 Vaccine, a likely underreported, self-limiting, cutaneous adverse effect of this mRNA vaccine.
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384:403-416. doi:10.1056/NEJMoa2035389
- Muthuvelu S, Lim KS, Huang L-Y, et al. Measles infection causing bacillus Calmette-Guérin reactivation: a case report. BMC Pediatr. 2019;19:251. doi:10.1186/s12887-019-1635-z
- Fatima S, Kumari A, Das G, et al. Tuberculosis vaccine: a journey from BCG to present. Life Sci. 2020;252:117594. doi:10.1016/j.lfs.2020.117594
- O’Neill LAJ, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20:335-337. doi:10.1038/s41577-020-0337-y
- Brooks NA, Puri A, Garg S, et al. The association of coronavirus disease-19 mortality and prior bacille Calmette-Guérin vaccination: a robust ecological analysis using unsupervised machine learning. Sci Rep. 2021;11:774. doi:10.1038/s41598-020-80787-z
- Tomita Y, Sato R, Ikeda T, et al. BCG vaccine may generate cross-reactive T-cells against SARS-CoV-2: in silico analyses and a hypothesis. Vaccine. 2020;38:6352-6356. doi:10.1016/j.vaccine.2020.08.045
- Lim KYY, Chua MC, Tan NWH, et al. Reactivation of BCG inoculation site in a child with febrile exanthema of 3 days duration: an early indicator of incomplete Kawasaki disease. BMJ Case Rep. 2020;13:E239648. doi:10.1136/bcr-2020-239648
- Kondo M, Goto H, Yamamoto S. First case of redness and erosion at bacillus Calmette-Guérin inoculation site after vaccination against influenza. J Dermatol. 2016;43:1229-1231. doi:10.1111/1346-8138.13365
- Chavarri-Guerra Y, Soto-Pérez-de-Celis E. Erythema at the bacillus Calmette-Guerin scar after influenza vaccination. Rev Soc Bras Med Trop. 2019;53:E20190390. doi:10.1590/0037-8682-0390-2019
- Fu W, Ho P-C, Liu C-L, et al. Reconcile the debate over protective effects of BCG vaccine against COVID-19. Sci Rep. 2021;11:8356. doi:10.1038/s41598-021-87731-9
- Amirlak L, Haddad R, Hardy JD, et al. Effectiveness of booster BCG vaccination in preventing COVID-19 infection. Hum Vaccin Immunother. 2021;17:3913-3915. doi:10.1080/21645515.2021.1956228
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384:403-416. doi:10.1056/NEJMoa2035389
- Muthuvelu S, Lim KS, Huang L-Y, et al. Measles infection causing bacillus Calmette-Guérin reactivation: a case report. BMC Pediatr. 2019;19:251. doi:10.1186/s12887-019-1635-z
- Fatima S, Kumari A, Das G, et al. Tuberculosis vaccine: a journey from BCG to present. Life Sci. 2020;252:117594. doi:10.1016/j.lfs.2020.117594
- O’Neill LAJ, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20:335-337. doi:10.1038/s41577-020-0337-y
- Brooks NA, Puri A, Garg S, et al. The association of coronavirus disease-19 mortality and prior bacille Calmette-Guérin vaccination: a robust ecological analysis using unsupervised machine learning. Sci Rep. 2021;11:774. doi:10.1038/s41598-020-80787-z
- Tomita Y, Sato R, Ikeda T, et al. BCG vaccine may generate cross-reactive T-cells against SARS-CoV-2: in silico analyses and a hypothesis. Vaccine. 2020;38:6352-6356. doi:10.1016/j.vaccine.2020.08.045
- Lim KYY, Chua MC, Tan NWH, et al. Reactivation of BCG inoculation site in a child with febrile exanthema of 3 days duration: an early indicator of incomplete Kawasaki disease. BMJ Case Rep. 2020;13:E239648. doi:10.1136/bcr-2020-239648
- Kondo M, Goto H, Yamamoto S. First case of redness and erosion at bacillus Calmette-Guérin inoculation site after vaccination against influenza. J Dermatol. 2016;43:1229-1231. doi:10.1111/1346-8138.13365
- Chavarri-Guerra Y, Soto-Pérez-de-Celis E. Erythema at the bacillus Calmette-Guerin scar after influenza vaccination. Rev Soc Bras Med Trop. 2019;53:E20190390. doi:10.1590/0037-8682-0390-2019
- Fu W, Ho P-C, Liu C-L, et al. Reconcile the debate over protective effects of BCG vaccine against COVID-19. Sci Rep. 2021;11:8356. doi:10.1038/s41598-021-87731-9
- Amirlak L, Haddad R, Hardy JD, et al. Effectiveness of booster BCG vaccination in preventing COVID-19 infection. Hum Vaccin Immunother. 2021;17:3913-3915. doi:10.1080/21645515.2021.1956228
Practice Points
- BCG vaccination scar reactivation is a potential benign, self-limited reaction in patients who receive the Moderna COVID-19 Vaccine.
- Symptoms of BCG vaccination scar reactivation, which is seen more commonly in children with Kawasaki disease, include redness, swelling, and ulceration.
Let’s be more careful about the data—and commentary—we publish
In a recent letter to the editor, “25-hydroxyvitamin D concentration is key to analyzing vitamin D’s effects” (J Fam Pract. 2021;70:472), Dr. Grant links vitamin D supplementation with important health outcomes. He concludes that the positivity rate of SARS-CoV-2 was only 5.9% in people with higher concentrations of 25(OH)D vs 12.5% in those with lower concentrations. This is a flawed conclusion on the face of it, because the great confabulatory factor is behavior. Is it possible that those more likely to take supplemental vitamin D do so as a result of overall healthier lifestyles and choices (eg, vaccinations)? As health care representatives, we must be very careful about the data we publish and the commentary we attach to it, lest we advertise inadvertent follies. I see so much of that in our “peer-reviewed literature.”
I came to medicine as a chemist, and the rigors of peer review impressed upon the hard (fundamental) sciences are markedly different from those we “claim” adherence to in medicine. I find that some of the medical literature and study designs fall short of what would pass muster in the fundamental science industry. That is a shame! Such statements, as discussed here, have to be served for public consumption, and even to our colleagues, with a generous helping of skepticism and qualification.
RA Segal, MD, MPH
Gainesville, FL
In a recent letter to the editor, “25-hydroxyvitamin D concentration is key to analyzing vitamin D’s effects” (J Fam Pract. 2021;70:472), Dr. Grant links vitamin D supplementation with important health outcomes. He concludes that the positivity rate of SARS-CoV-2 was only 5.9% in people with higher concentrations of 25(OH)D vs 12.5% in those with lower concentrations. This is a flawed conclusion on the face of it, because the great confabulatory factor is behavior. Is it possible that those more likely to take supplemental vitamin D do so as a result of overall healthier lifestyles and choices (eg, vaccinations)? As health care representatives, we must be very careful about the data we publish and the commentary we attach to it, lest we advertise inadvertent follies. I see so much of that in our “peer-reviewed literature.”
I came to medicine as a chemist, and the rigors of peer review impressed upon the hard (fundamental) sciences are markedly different from those we “claim” adherence to in medicine. I find that some of the medical literature and study designs fall short of what would pass muster in the fundamental science industry. That is a shame! Such statements, as discussed here, have to be served for public consumption, and even to our colleagues, with a generous helping of skepticism and qualification.
RA Segal, MD, MPH
Gainesville, FL
In a recent letter to the editor, “25-hydroxyvitamin D concentration is key to analyzing vitamin D’s effects” (J Fam Pract. 2021;70:472), Dr. Grant links vitamin D supplementation with important health outcomes. He concludes that the positivity rate of SARS-CoV-2 was only 5.9% in people with higher concentrations of 25(OH)D vs 12.5% in those with lower concentrations. This is a flawed conclusion on the face of it, because the great confabulatory factor is behavior. Is it possible that those more likely to take supplemental vitamin D do so as a result of overall healthier lifestyles and choices (eg, vaccinations)? As health care representatives, we must be very careful about the data we publish and the commentary we attach to it, lest we advertise inadvertent follies. I see so much of that in our “peer-reviewed literature.”
I came to medicine as a chemist, and the rigors of peer review impressed upon the hard (fundamental) sciences are markedly different from those we “claim” adherence to in medicine. I find that some of the medical literature and study designs fall short of what would pass muster in the fundamental science industry. That is a shame! Such statements, as discussed here, have to be served for public consumption, and even to our colleagues, with a generous helping of skepticism and qualification.
RA Segal, MD, MPH
Gainesville, FL
Nirsevimab protects healthy infants from RSV
A single injection of the experimental agent nirsevimab ahead of respiratory syncytial virus (RSV) season protects healthy infants from lower respiratory tract infections associated with the pathogen, according to the results of a phase 3 study.
A previously published trial showed that a single dose of nirsevimab was effective in preterm infants. The ability to protect all babies from RSV, which causes bronchiolitis and pneumonia and is a leading cause of hospitalization for this age group, “would be a paradigm shift in the approach to this disease,” William Muller, MD, PhD, of the Lurie Children’s Hospital of Chicago and a coauthor of the study, said in a statement.
The primary endpoint of the study was medically attended lower respiratory tract infections linked to RSV. The single injection of nirsevimab was associated with a 74.5% reduction in such infections (P < .001), according to Dr. Muller’s group, who published their findings March 2 in the New England Journal of Medicine.
Nirsevimab, a monoclonal antibody to the RSV fusion protein being developed by AstraZeneca and Sanofi, has an extended half-life, which may allow one dose to confer protection throughout a season. The only approved option to prevent RSV, palivizumab (Synagis), is used for high-risk infants, and five injections are needed to cover a viral season.
Nearly 1,500 infants in more than 20 countries studied
To assess the effectiveness of nirsevimab in late-preterm and term infants, investigators at 160 sites randomly assigned 1,490 babies born at a gestational age of at least 35 weeks to receive an intramuscular injection of nirsevimab or placebo.
During the 150 days after injection, medically attended RSV-associated lower respiratory tract infections occurred in 12 of 994 infants who received nirsevimab, compared with 25 of 496 babies who received placebo (1.2% vs. 5%).
Six of 994 infants who received nirsevimab were hospitalized for RSV-associated lower respiratory tract infections, compared with 8 of 496 infants in the placebo group (0.6% vs. 1.6%; P = .07). The proportion of children hospitalized for any respiratory illness as a result of RSV was 0.9% among those who received nirsevimab, compared with 2.2% among those who received placebo.
Serious adverse events occurred in 6.8% of the nirsevimab group and 7.3% of the placebo group. None of these events, including three deaths in the nirsevimab group, was considered related to nirsevimab or placebo, according to the researchers. One infant who received nirsevimab had a generalized macular rash without systemic features that did not require treatment and resolved in 20 days, they said.
Antidrug antibodies were detected in 6.1% of the nirsevimab group and in 1.1% of the placebo group. These antidrug antibodies tended to develop later and did not affect nirsevimab pharmacokinetics during the RSV season, the researchers reported. How they might affect subsequent doses of nirsevimab is not known, they added.
In a separate report in the journal, researcher Joseph Domachowske, MD, SUNY Upstate Medical University, Syracuse, New York, and colleagues described safety results from an ongoing study of nirsevimab that includes infants with congenital heart disease, chronic lung disease, and prematurity.
In this trial, infants received nirsevimab or palivizumab, and the treatments appeared to have similar safety profiles, the authors reported.
Other approaches to RSV protection include passive antibodies acquired from maternal vaccination in pregnancy and active vaccination of infants.
The publication follows news last month that GlaxoSmithKline is pausing a maternal RSV vaccine trial, which “had the same goal of protecting babies against severe RSV infection,” said Louis Bont, MD, PhD, with University Medical Center Utrecht, the Netherlands.
RSV infection is one of the deadliest diseases during infancy, and the nirsevimab trial, conducted in more than 20 countries, is “gamechanging,” Dr. Bont told this news organization. Still, researchers will need to monitor for RSV resistance to this treatment, he said.
Whether nirsevimab prevents the development of reactive airway disease and asthma is another open question, he said.
“Finally, we need to keep in mind that RSV mortality is almost limited to the developing world, and it is unlikely that this novel drug will become available to these countries in the coming years,” Dr. Bont said. “Nevertheless, nirsevimab has the potential to seriously decrease the annual overwhelming number of RSV infected babies.”
Nirsevimab may have advantages in low- and middle-income countries, including its potential to be incorporated into established immunization programs and to be given seasonally, said Amy Sarah Ginsburg, MD, MPH, of the University of Washington, Seattle. “However, cost remains a significant factor, as does susceptibility to pathogen escape,” she said.
MedImmune/AstraZeneca and Sanofi funded the nirsevimab studies. UMC Utrecht has received research grants and fees for advisory work from AstraZeneca for RSV-related work by Bont.
A version of this article first appeared on Medscape.com.
A single injection of the experimental agent nirsevimab ahead of respiratory syncytial virus (RSV) season protects healthy infants from lower respiratory tract infections associated with the pathogen, according to the results of a phase 3 study.
A previously published trial showed that a single dose of nirsevimab was effective in preterm infants. The ability to protect all babies from RSV, which causes bronchiolitis and pneumonia and is a leading cause of hospitalization for this age group, “would be a paradigm shift in the approach to this disease,” William Muller, MD, PhD, of the Lurie Children’s Hospital of Chicago and a coauthor of the study, said in a statement.
The primary endpoint of the study was medically attended lower respiratory tract infections linked to RSV. The single injection of nirsevimab was associated with a 74.5% reduction in such infections (P < .001), according to Dr. Muller’s group, who published their findings March 2 in the New England Journal of Medicine.
Nirsevimab, a monoclonal antibody to the RSV fusion protein being developed by AstraZeneca and Sanofi, has an extended half-life, which may allow one dose to confer protection throughout a season. The only approved option to prevent RSV, palivizumab (Synagis), is used for high-risk infants, and five injections are needed to cover a viral season.
Nearly 1,500 infants in more than 20 countries studied
To assess the effectiveness of nirsevimab in late-preterm and term infants, investigators at 160 sites randomly assigned 1,490 babies born at a gestational age of at least 35 weeks to receive an intramuscular injection of nirsevimab or placebo.
During the 150 days after injection, medically attended RSV-associated lower respiratory tract infections occurred in 12 of 994 infants who received nirsevimab, compared with 25 of 496 babies who received placebo (1.2% vs. 5%).
Six of 994 infants who received nirsevimab were hospitalized for RSV-associated lower respiratory tract infections, compared with 8 of 496 infants in the placebo group (0.6% vs. 1.6%; P = .07). The proportion of children hospitalized for any respiratory illness as a result of RSV was 0.9% among those who received nirsevimab, compared with 2.2% among those who received placebo.
Serious adverse events occurred in 6.8% of the nirsevimab group and 7.3% of the placebo group. None of these events, including three deaths in the nirsevimab group, was considered related to nirsevimab or placebo, according to the researchers. One infant who received nirsevimab had a generalized macular rash without systemic features that did not require treatment and resolved in 20 days, they said.
Antidrug antibodies were detected in 6.1% of the nirsevimab group and in 1.1% of the placebo group. These antidrug antibodies tended to develop later and did not affect nirsevimab pharmacokinetics during the RSV season, the researchers reported. How they might affect subsequent doses of nirsevimab is not known, they added.
In a separate report in the journal, researcher Joseph Domachowske, MD, SUNY Upstate Medical University, Syracuse, New York, and colleagues described safety results from an ongoing study of nirsevimab that includes infants with congenital heart disease, chronic lung disease, and prematurity.
In this trial, infants received nirsevimab or palivizumab, and the treatments appeared to have similar safety profiles, the authors reported.
Other approaches to RSV protection include passive antibodies acquired from maternal vaccination in pregnancy and active vaccination of infants.
The publication follows news last month that GlaxoSmithKline is pausing a maternal RSV vaccine trial, which “had the same goal of protecting babies against severe RSV infection,” said Louis Bont, MD, PhD, with University Medical Center Utrecht, the Netherlands.
RSV infection is one of the deadliest diseases during infancy, and the nirsevimab trial, conducted in more than 20 countries, is “gamechanging,” Dr. Bont told this news organization. Still, researchers will need to monitor for RSV resistance to this treatment, he said.
Whether nirsevimab prevents the development of reactive airway disease and asthma is another open question, he said.
“Finally, we need to keep in mind that RSV mortality is almost limited to the developing world, and it is unlikely that this novel drug will become available to these countries in the coming years,” Dr. Bont said. “Nevertheless, nirsevimab has the potential to seriously decrease the annual overwhelming number of RSV infected babies.”
Nirsevimab may have advantages in low- and middle-income countries, including its potential to be incorporated into established immunization programs and to be given seasonally, said Amy Sarah Ginsburg, MD, MPH, of the University of Washington, Seattle. “However, cost remains a significant factor, as does susceptibility to pathogen escape,” she said.
MedImmune/AstraZeneca and Sanofi funded the nirsevimab studies. UMC Utrecht has received research grants and fees for advisory work from AstraZeneca for RSV-related work by Bont.
A version of this article first appeared on Medscape.com.
A single injection of the experimental agent nirsevimab ahead of respiratory syncytial virus (RSV) season protects healthy infants from lower respiratory tract infections associated with the pathogen, according to the results of a phase 3 study.
A previously published trial showed that a single dose of nirsevimab was effective in preterm infants. The ability to protect all babies from RSV, which causes bronchiolitis and pneumonia and is a leading cause of hospitalization for this age group, “would be a paradigm shift in the approach to this disease,” William Muller, MD, PhD, of the Lurie Children’s Hospital of Chicago and a coauthor of the study, said in a statement.
The primary endpoint of the study was medically attended lower respiratory tract infections linked to RSV. The single injection of nirsevimab was associated with a 74.5% reduction in such infections (P < .001), according to Dr. Muller’s group, who published their findings March 2 in the New England Journal of Medicine.
Nirsevimab, a monoclonal antibody to the RSV fusion protein being developed by AstraZeneca and Sanofi, has an extended half-life, which may allow one dose to confer protection throughout a season. The only approved option to prevent RSV, palivizumab (Synagis), is used for high-risk infants, and five injections are needed to cover a viral season.
Nearly 1,500 infants in more than 20 countries studied
To assess the effectiveness of nirsevimab in late-preterm and term infants, investigators at 160 sites randomly assigned 1,490 babies born at a gestational age of at least 35 weeks to receive an intramuscular injection of nirsevimab or placebo.
During the 150 days after injection, medically attended RSV-associated lower respiratory tract infections occurred in 12 of 994 infants who received nirsevimab, compared with 25 of 496 babies who received placebo (1.2% vs. 5%).
Six of 994 infants who received nirsevimab were hospitalized for RSV-associated lower respiratory tract infections, compared with 8 of 496 infants in the placebo group (0.6% vs. 1.6%; P = .07). The proportion of children hospitalized for any respiratory illness as a result of RSV was 0.9% among those who received nirsevimab, compared with 2.2% among those who received placebo.
Serious adverse events occurred in 6.8% of the nirsevimab group and 7.3% of the placebo group. None of these events, including three deaths in the nirsevimab group, was considered related to nirsevimab or placebo, according to the researchers. One infant who received nirsevimab had a generalized macular rash without systemic features that did not require treatment and resolved in 20 days, they said.
Antidrug antibodies were detected in 6.1% of the nirsevimab group and in 1.1% of the placebo group. These antidrug antibodies tended to develop later and did not affect nirsevimab pharmacokinetics during the RSV season, the researchers reported. How they might affect subsequent doses of nirsevimab is not known, they added.
In a separate report in the journal, researcher Joseph Domachowske, MD, SUNY Upstate Medical University, Syracuse, New York, and colleagues described safety results from an ongoing study of nirsevimab that includes infants with congenital heart disease, chronic lung disease, and prematurity.
In this trial, infants received nirsevimab or palivizumab, and the treatments appeared to have similar safety profiles, the authors reported.
Other approaches to RSV protection include passive antibodies acquired from maternal vaccination in pregnancy and active vaccination of infants.
The publication follows news last month that GlaxoSmithKline is pausing a maternal RSV vaccine trial, which “had the same goal of protecting babies against severe RSV infection,” said Louis Bont, MD, PhD, with University Medical Center Utrecht, the Netherlands.
RSV infection is one of the deadliest diseases during infancy, and the nirsevimab trial, conducted in more than 20 countries, is “gamechanging,” Dr. Bont told this news organization. Still, researchers will need to monitor for RSV resistance to this treatment, he said.
Whether nirsevimab prevents the development of reactive airway disease and asthma is another open question, he said.
“Finally, we need to keep in mind that RSV mortality is almost limited to the developing world, and it is unlikely that this novel drug will become available to these countries in the coming years,” Dr. Bont said. “Nevertheless, nirsevimab has the potential to seriously decrease the annual overwhelming number of RSV infected babies.”
Nirsevimab may have advantages in low- and middle-income countries, including its potential to be incorporated into established immunization programs and to be given seasonally, said Amy Sarah Ginsburg, MD, MPH, of the University of Washington, Seattle. “However, cost remains a significant factor, as does susceptibility to pathogen escape,” she said.
MedImmune/AstraZeneca and Sanofi funded the nirsevimab studies. UMC Utrecht has received research grants and fees for advisory work from AstraZeneca for RSV-related work by Bont.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Honoring Dr. Paul Farmer: Dr. Serena Koenig shares her memories of working with him
Infectious disease specialist and humanitarian, Paul Edward Farmer, MD, PhD, who cofounded Partners In Health, died suddenly on Feb. 21. To celebrate his life, this news organization interviewed Serena Koenig, MD, MPH, who met Dr. Farmer when she was an internal medicine resident at Brigham and Women’s Hospital. Dr. Koenig had worked closely with Dr. Farmer ever since they met.
Q. Can you please share one of your best memories of Dr. Farmer?
Dr. Serena Koenig: Paul and some other incredible colleagues at Partners IN Health (PIH) had started the HIV Equity Initiative, which was one of the first programs in the world to provide free, comprehensive treatment for HIV. This was at the time when millions of people in Africa were dying of HIV and many experts said it was not feasible to treat HIV in a poor country, because it was too complicated and expensive. Paul took me on some home visits with patients who had what he called the Lazarus effect, coming back from death’s door from advanced AIDS to vigorous health on antiretroviral therapy. I had just started working in Haiti with Paul and PIH, and I felt the enormous magnitude of what he was doing.
Q. What aspects of him and his work do you find most admirable?
Dr. Koenig: I most admired Paul’s humanity, his belief that every person matters and has the right to high-quality health care, and his vision of global health equity.
He said: “The idea that some lives matter less is the root of all that is wrong with the world.” Paul lived this philosophy. He has spoken extensively about harms of socialization for scarcity on behalf of those who are poor, leading policy makers to decisions regarding the feasibility of treating some diseases, but not others.
He said in an interview with the Harvard Gazette in 2018: “The most compelling thing to fight socialization for scarcity on behalf of others is health system strengthening. Health systems that integrate prevention and quality care.”
A few weeks ago, I asked him his thoughts about the high-level resources we have invested in some patients who have needed specialty care over the years, and he said: “No way that we should waste all of our emotional energy responding only to those constant, nagging critics that it’s not cost effective, not feasible, not sustainable, not even prudent. Because you know what they would have done if it was their child or family member.”
Q. When did you first meet Dr. Farmer, and what inspired you to work with him?
Dr. Koenig: When I was an internal medicine resident at the Brigham, Paul and I bonded over the care of one of my clinic patients who I followed very closely, and who was admitted to his inpatient service.
Like everyone else who has worked with Paul, I was touched by his kindness and warmth.
A couple of years later, he asked me to help him raise money to bring a young man named Wilnot from Haiti to the Brigham for an aortic valve replacement. After we raised the money, he asked me to go to Haiti to help Wilnot get his medical visa and to escort him to Boston.
That short trip to Haiti had an enormous impact on my life. I was shattered to see the poverty that the people of Haiti were enduring – and in a country a short plane flight from Miami.
Shortly after this, Paul asked me to help him find treatment for another patient, a young boy named John, who presented with neck masses that were later diagnosed as nasopharyngeal carcinoma.
It took us some time to make the diagnosis and then to arrange free care at Mass General.
When I returned to Haiti with two PIH colleagues to help John get a visa and escort him back to Boston, we found that John’s condition was much worse. We ended up medically evacuating him to Boston, because he was too sick for a commercial flight.
Tracy Kidder wrote about this heartbreaking experience in the book “Mountains Beyond Mountains.”
Throughout all of these experiences, I was deeply impressed with Paul’s commitment to do whatever it took to provide the best care for patients, as if they were members of his own family. He said “Tout Moun Se Moun” (Haitian Creole for “every person is a person”), and I could tell that he meant it.
Q. How did you collaborate with him professionally?
Dr. Koenig: I spent the first few years after residency working with Paul and Partners In Health. Initially, I served as a liaison between PIH in Haiti and the Brigham, bringing several more patients to Boston for care, and arranging specialty surgical trips to Haiti.
Later, when HIV funding became available from the Global Fund for HIV, Tuberculosis, and Malaria, I moved to rural Haiti to provide treatment for patients with HIV and/or TB at one of the first PIH expansion sites. We treated many patients with advanced stages of HIV and/or TB, and many of them recovered remarkably quickly with antiretroviral therapy.
When I returned to Boston to complete an infectious disease fellowship I switched my focus to conducting clinical research to improve HIV and TB treatment outcomes. Paul emailed his mentor and friend, Jean “Bill” Pape, the director of a Haitian NGO called GHESKIO (Haitian Group for the Study of Kaposi’s Sarcoma and Opportunistic Infections), which is an internationally celebrated center of excellence in HIV-related research and clinical care, to ask if I could collaborate with them.
Ever since that time, I have been based between the Brigham’s division of global health equity, which was led by Paul, and GHESKIO.
Paul was very supportive of our research, which aims to improve health service delivery and treatment regimens for HIV and TB.
Q. What lessons do you think other physicians can learn from him?
Dr. Koenig: As Joia Mukherjee, chief medical officer of Partners In Health, has said, Paul left us a roadmap. He wrote many books, and he was very eloquent in expressing his philosophy about equity and justice in numerous interviews. This is relevant not only for international sites, but in the United States as well, with our major disparities in health outcomes by race, geography, and socioeconomic status.
No one will be able to replace Paul, but he left us with a vision of what is achievable.
Dr. Koenig is associate physician, Brigham and Women’s Hospital, Boston, with faculty appointments in the divisions of global health equity and infectious diseases. She is also associate professor at Harvard Medical School.
Infectious disease specialist and humanitarian, Paul Edward Farmer, MD, PhD, who cofounded Partners In Health, died suddenly on Feb. 21. To celebrate his life, this news organization interviewed Serena Koenig, MD, MPH, who met Dr. Farmer when she was an internal medicine resident at Brigham and Women’s Hospital. Dr. Koenig had worked closely with Dr. Farmer ever since they met.
Q. Can you please share one of your best memories of Dr. Farmer?
Dr. Serena Koenig: Paul and some other incredible colleagues at Partners IN Health (PIH) had started the HIV Equity Initiative, which was one of the first programs in the world to provide free, comprehensive treatment for HIV. This was at the time when millions of people in Africa were dying of HIV and many experts said it was not feasible to treat HIV in a poor country, because it was too complicated and expensive. Paul took me on some home visits with patients who had what he called the Lazarus effect, coming back from death’s door from advanced AIDS to vigorous health on antiretroviral therapy. I had just started working in Haiti with Paul and PIH, and I felt the enormous magnitude of what he was doing.
Q. What aspects of him and his work do you find most admirable?
Dr. Koenig: I most admired Paul’s humanity, his belief that every person matters and has the right to high-quality health care, and his vision of global health equity.
He said: “The idea that some lives matter less is the root of all that is wrong with the world.” Paul lived this philosophy. He has spoken extensively about harms of socialization for scarcity on behalf of those who are poor, leading policy makers to decisions regarding the feasibility of treating some diseases, but not others.
He said in an interview with the Harvard Gazette in 2018: “The most compelling thing to fight socialization for scarcity on behalf of others is health system strengthening. Health systems that integrate prevention and quality care.”
A few weeks ago, I asked him his thoughts about the high-level resources we have invested in some patients who have needed specialty care over the years, and he said: “No way that we should waste all of our emotional energy responding only to those constant, nagging critics that it’s not cost effective, not feasible, not sustainable, not even prudent. Because you know what they would have done if it was their child or family member.”
Q. When did you first meet Dr. Farmer, and what inspired you to work with him?
Dr. Koenig: When I was an internal medicine resident at the Brigham, Paul and I bonded over the care of one of my clinic patients who I followed very closely, and who was admitted to his inpatient service.
Like everyone else who has worked with Paul, I was touched by his kindness and warmth.
A couple of years later, he asked me to help him raise money to bring a young man named Wilnot from Haiti to the Brigham for an aortic valve replacement. After we raised the money, he asked me to go to Haiti to help Wilnot get his medical visa and to escort him to Boston.
That short trip to Haiti had an enormous impact on my life. I was shattered to see the poverty that the people of Haiti were enduring – and in a country a short plane flight from Miami.
Shortly after this, Paul asked me to help him find treatment for another patient, a young boy named John, who presented with neck masses that were later diagnosed as nasopharyngeal carcinoma.
It took us some time to make the diagnosis and then to arrange free care at Mass General.
When I returned to Haiti with two PIH colleagues to help John get a visa and escort him back to Boston, we found that John’s condition was much worse. We ended up medically evacuating him to Boston, because he was too sick for a commercial flight.
Tracy Kidder wrote about this heartbreaking experience in the book “Mountains Beyond Mountains.”
Throughout all of these experiences, I was deeply impressed with Paul’s commitment to do whatever it took to provide the best care for patients, as if they were members of his own family. He said “Tout Moun Se Moun” (Haitian Creole for “every person is a person”), and I could tell that he meant it.
Q. How did you collaborate with him professionally?
Dr. Koenig: I spent the first few years after residency working with Paul and Partners In Health. Initially, I served as a liaison between PIH in Haiti and the Brigham, bringing several more patients to Boston for care, and arranging specialty surgical trips to Haiti.
Later, when HIV funding became available from the Global Fund for HIV, Tuberculosis, and Malaria, I moved to rural Haiti to provide treatment for patients with HIV and/or TB at one of the first PIH expansion sites. We treated many patients with advanced stages of HIV and/or TB, and many of them recovered remarkably quickly with antiretroviral therapy.
When I returned to Boston to complete an infectious disease fellowship I switched my focus to conducting clinical research to improve HIV and TB treatment outcomes. Paul emailed his mentor and friend, Jean “Bill” Pape, the director of a Haitian NGO called GHESKIO (Haitian Group for the Study of Kaposi’s Sarcoma and Opportunistic Infections), which is an internationally celebrated center of excellence in HIV-related research and clinical care, to ask if I could collaborate with them.
Ever since that time, I have been based between the Brigham’s division of global health equity, which was led by Paul, and GHESKIO.
Paul was very supportive of our research, which aims to improve health service delivery and treatment regimens for HIV and TB.
Q. What lessons do you think other physicians can learn from him?
Dr. Koenig: As Joia Mukherjee, chief medical officer of Partners In Health, has said, Paul left us a roadmap. He wrote many books, and he was very eloquent in expressing his philosophy about equity and justice in numerous interviews. This is relevant not only for international sites, but in the United States as well, with our major disparities in health outcomes by race, geography, and socioeconomic status.
No one will be able to replace Paul, but he left us with a vision of what is achievable.
Dr. Koenig is associate physician, Brigham and Women’s Hospital, Boston, with faculty appointments in the divisions of global health equity and infectious diseases. She is also associate professor at Harvard Medical School.
Infectious disease specialist and humanitarian, Paul Edward Farmer, MD, PhD, who cofounded Partners In Health, died suddenly on Feb. 21. To celebrate his life, this news organization interviewed Serena Koenig, MD, MPH, who met Dr. Farmer when she was an internal medicine resident at Brigham and Women’s Hospital. Dr. Koenig had worked closely with Dr. Farmer ever since they met.
Q. Can you please share one of your best memories of Dr. Farmer?
Dr. Serena Koenig: Paul and some other incredible colleagues at Partners IN Health (PIH) had started the HIV Equity Initiative, which was one of the first programs in the world to provide free, comprehensive treatment for HIV. This was at the time when millions of people in Africa were dying of HIV and many experts said it was not feasible to treat HIV in a poor country, because it was too complicated and expensive. Paul took me on some home visits with patients who had what he called the Lazarus effect, coming back from death’s door from advanced AIDS to vigorous health on antiretroviral therapy. I had just started working in Haiti with Paul and PIH, and I felt the enormous magnitude of what he was doing.
Q. What aspects of him and his work do you find most admirable?
Dr. Koenig: I most admired Paul’s humanity, his belief that every person matters and has the right to high-quality health care, and his vision of global health equity.
He said: “The idea that some lives matter less is the root of all that is wrong with the world.” Paul lived this philosophy. He has spoken extensively about harms of socialization for scarcity on behalf of those who are poor, leading policy makers to decisions regarding the feasibility of treating some diseases, but not others.
He said in an interview with the Harvard Gazette in 2018: “The most compelling thing to fight socialization for scarcity on behalf of others is health system strengthening. Health systems that integrate prevention and quality care.”
A few weeks ago, I asked him his thoughts about the high-level resources we have invested in some patients who have needed specialty care over the years, and he said: “No way that we should waste all of our emotional energy responding only to those constant, nagging critics that it’s not cost effective, not feasible, not sustainable, not even prudent. Because you know what they would have done if it was their child or family member.”
Q. When did you first meet Dr. Farmer, and what inspired you to work with him?
Dr. Koenig: When I was an internal medicine resident at the Brigham, Paul and I bonded over the care of one of my clinic patients who I followed very closely, and who was admitted to his inpatient service.
Like everyone else who has worked with Paul, I was touched by his kindness and warmth.
A couple of years later, he asked me to help him raise money to bring a young man named Wilnot from Haiti to the Brigham for an aortic valve replacement. After we raised the money, he asked me to go to Haiti to help Wilnot get his medical visa and to escort him to Boston.
That short trip to Haiti had an enormous impact on my life. I was shattered to see the poverty that the people of Haiti were enduring – and in a country a short plane flight from Miami.
Shortly after this, Paul asked me to help him find treatment for another patient, a young boy named John, who presented with neck masses that were later diagnosed as nasopharyngeal carcinoma.
It took us some time to make the diagnosis and then to arrange free care at Mass General.
When I returned to Haiti with two PIH colleagues to help John get a visa and escort him back to Boston, we found that John’s condition was much worse. We ended up medically evacuating him to Boston, because he was too sick for a commercial flight.
Tracy Kidder wrote about this heartbreaking experience in the book “Mountains Beyond Mountains.”
Throughout all of these experiences, I was deeply impressed with Paul’s commitment to do whatever it took to provide the best care for patients, as if they were members of his own family. He said “Tout Moun Se Moun” (Haitian Creole for “every person is a person”), and I could tell that he meant it.
Q. How did you collaborate with him professionally?
Dr. Koenig: I spent the first few years after residency working with Paul and Partners In Health. Initially, I served as a liaison between PIH in Haiti and the Brigham, bringing several more patients to Boston for care, and arranging specialty surgical trips to Haiti.
Later, when HIV funding became available from the Global Fund for HIV, Tuberculosis, and Malaria, I moved to rural Haiti to provide treatment for patients with HIV and/or TB at one of the first PIH expansion sites. We treated many patients with advanced stages of HIV and/or TB, and many of them recovered remarkably quickly with antiretroviral therapy.
When I returned to Boston to complete an infectious disease fellowship I switched my focus to conducting clinical research to improve HIV and TB treatment outcomes. Paul emailed his mentor and friend, Jean “Bill” Pape, the director of a Haitian NGO called GHESKIO (Haitian Group for the Study of Kaposi’s Sarcoma and Opportunistic Infections), which is an internationally celebrated center of excellence in HIV-related research and clinical care, to ask if I could collaborate with them.
Ever since that time, I have been based between the Brigham’s division of global health equity, which was led by Paul, and GHESKIO.
Paul was very supportive of our research, which aims to improve health service delivery and treatment regimens for HIV and TB.
Q. What lessons do you think other physicians can learn from him?
Dr. Koenig: As Joia Mukherjee, chief medical officer of Partners In Health, has said, Paul left us a roadmap. He wrote many books, and he was very eloquent in expressing his philosophy about equity and justice in numerous interviews. This is relevant not only for international sites, but in the United States as well, with our major disparities in health outcomes by race, geography, and socioeconomic status.
No one will be able to replace Paul, but he left us with a vision of what is achievable.
Dr. Koenig is associate physician, Brigham and Women’s Hospital, Boston, with faculty appointments in the divisions of global health equity and infectious diseases. She is also associate professor at Harvard Medical School.
Practice guidelines highlights from past year
Based on the most recent Infectious Diseases Society of America (IDSA) guidelines, what would be the preferred therapy?
A) Metronidazole
B) Fidaxomicin + bezlotoxumab
C) Vancomycin
D) Fecal microbiota transplant
The recommendations from the 2021 guidelines would be to treat with fidaxomicin and add bezlotoxumab.1 The guidelines highlight the following changes:
- In patients with an initial Clostridioides difficile infections (CDI) fidaxomicin is preferred over vancomycin.
- In patients with a recurrent CDI episode, fidaxomicin is favored over vancomycin. For patients with multiple recurrences, vancomycin in a tapered and pulsed regimen, vancomycin followed by rifaximin, and fecal microbiota transplantation are options in addition to fidaxomicin.
- Addition of bezlotoxumab to standard of care antibiotics is recommended for recurrence of CDI within the first 6 months over standard of care antibiotics alone
The feasibility of these recommendations is up for debate. The cost of a course of fidaxomicin is $2,800, and the cost of bezlotoxumab is about $4,500. Cost effectiveness studies that helped drive the recommendations show a savings by reducing future hospitalizations for C. diff.2 Unfortunately, this enthusiasm is not shared by many insurance companies for outpatient treatment.
Knee osteoarthritis
I will save you the excitement of the new acromegaly guidelines and focus on something we see all the time: knee osteoarthritis. The American Academy of Orthopedic Surgeons has released guidelines for this condition.3 The useful points I found were as follows:
- Topical application of nonsteroidal anti-inflammatory drugs (e.g., diclofenac) should be used to improve function and quality of life in patients with knee osteoarthritis.
- Exercise routines (i.e, supervised, unsupervised, and/or aquatic) are recommended versus no exercise for improving pain and function in patients with knee osteoarthritis.
- Not recommended is the use of oral narcotics (including tramadol), as they are not effective at improving pain or function, and their use results in a significant increased risk of adverse events.
- Not recommended for routine use in symptomatic knee osteoarthritis is intra-articular injection of hyaluronic acid.
I was happy to see topical NSAIDS recommended, as they are a much safer option in older patients than oral NSAIDS (which were also recommended). The recommendation against narcotics, including tramadol, is a shift from the recommendation of tramadol in the 2013 guidelines.4 Acetaminophen was enthusiastically recommended, and is still worth a try.
Sexually transmitted infections
- The dosing for the treatment of gonorrhea has increased to 500 mg of ceftriaxone (was 250 mg in 2015 guidelines), with a dose of 1 gram for patients who weigh more than 150 kg.
- Chlamydia infections should be treated with a 7-day course of doxycycline as the preferred antibiotic, except in pregnant women (where azithromycin is recommended).
- Herpes simplex virus 2 recurrences can be treated with twice-daily dosing of 800 mg of acyclovir for 5 days, or acyclovir 800 mg three times a day for 2 days. The shortest course for recurrence is famciclovir 1 gram twice a day for 1 day.
- The Centers for Disease Control and Prevention has removed the recommendation for avoidance of alcohol when taking metronidazole.
I hope these highlights of guidelines for common issues we see are helpful!
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Johnson S et al. Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused update guidelines on management of Clostridioides difficile Infection in adults. Clin Infect Dis. 2021 Sep 7;73(5):e1029-e1044.
2. Pabhu VS et al. Cost-effectiveness of bezlotoxumab compared with placebo for the prevention of recurrent Clostridium difficile infection. Clin Infect Dis. 2018 Feb 1;66(3):355-62.
3. American Academy of Orthopaedic Surgeons: Management of osteoarthritis of the knee (non-arthroplasty) – Evidence-based clinical practice guideline (2021 Aug 31. https://www.aaos.org/oak3cpg).
4. Jevsevar DS. Treatment of osteoarthritis of the knee: Evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013: Sep;21(9):571-6.
5. Sexually transmitted infections treatment guidelines, 2021 recommendations and reports. MMWR 2021 Jul 23;70(4):1-187.
Based on the most recent Infectious Diseases Society of America (IDSA) guidelines, what would be the preferred therapy?
A) Metronidazole
B) Fidaxomicin + bezlotoxumab
C) Vancomycin
D) Fecal microbiota transplant
The recommendations from the 2021 guidelines would be to treat with fidaxomicin and add bezlotoxumab.1 The guidelines highlight the following changes:
- In patients with an initial Clostridioides difficile infections (CDI) fidaxomicin is preferred over vancomycin.
- In patients with a recurrent CDI episode, fidaxomicin is favored over vancomycin. For patients with multiple recurrences, vancomycin in a tapered and pulsed regimen, vancomycin followed by rifaximin, and fecal microbiota transplantation are options in addition to fidaxomicin.
- Addition of bezlotoxumab to standard of care antibiotics is recommended for recurrence of CDI within the first 6 months over standard of care antibiotics alone
The feasibility of these recommendations is up for debate. The cost of a course of fidaxomicin is $2,800, and the cost of bezlotoxumab is about $4,500. Cost effectiveness studies that helped drive the recommendations show a savings by reducing future hospitalizations for C. diff.2 Unfortunately, this enthusiasm is not shared by many insurance companies for outpatient treatment.
Knee osteoarthritis
I will save you the excitement of the new acromegaly guidelines and focus on something we see all the time: knee osteoarthritis. The American Academy of Orthopedic Surgeons has released guidelines for this condition.3 The useful points I found were as follows:
- Topical application of nonsteroidal anti-inflammatory drugs (e.g., diclofenac) should be used to improve function and quality of life in patients with knee osteoarthritis.
- Exercise routines (i.e, supervised, unsupervised, and/or aquatic) are recommended versus no exercise for improving pain and function in patients with knee osteoarthritis.
- Not recommended is the use of oral narcotics (including tramadol), as they are not effective at improving pain or function, and their use results in a significant increased risk of adverse events.
- Not recommended for routine use in symptomatic knee osteoarthritis is intra-articular injection of hyaluronic acid.
I was happy to see topical NSAIDS recommended, as they are a much safer option in older patients than oral NSAIDS (which were also recommended). The recommendation against narcotics, including tramadol, is a shift from the recommendation of tramadol in the 2013 guidelines.4 Acetaminophen was enthusiastically recommended, and is still worth a try.
Sexually transmitted infections
- The dosing for the treatment of gonorrhea has increased to 500 mg of ceftriaxone (was 250 mg in 2015 guidelines), with a dose of 1 gram for patients who weigh more than 150 kg.
- Chlamydia infections should be treated with a 7-day course of doxycycline as the preferred antibiotic, except in pregnant women (where azithromycin is recommended).
- Herpes simplex virus 2 recurrences can be treated with twice-daily dosing of 800 mg of acyclovir for 5 days, or acyclovir 800 mg three times a day for 2 days. The shortest course for recurrence is famciclovir 1 gram twice a day for 1 day.
- The Centers for Disease Control and Prevention has removed the recommendation for avoidance of alcohol when taking metronidazole.
I hope these highlights of guidelines for common issues we see are helpful!
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Johnson S et al. Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused update guidelines on management of Clostridioides difficile Infection in adults. Clin Infect Dis. 2021 Sep 7;73(5):e1029-e1044.
2. Pabhu VS et al. Cost-effectiveness of bezlotoxumab compared with placebo for the prevention of recurrent Clostridium difficile infection. Clin Infect Dis. 2018 Feb 1;66(3):355-62.
3. American Academy of Orthopaedic Surgeons: Management of osteoarthritis of the knee (non-arthroplasty) – Evidence-based clinical practice guideline (2021 Aug 31. https://www.aaos.org/oak3cpg).
4. Jevsevar DS. Treatment of osteoarthritis of the knee: Evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013: Sep;21(9):571-6.
5. Sexually transmitted infections treatment guidelines, 2021 recommendations and reports. MMWR 2021 Jul 23;70(4):1-187.
Based on the most recent Infectious Diseases Society of America (IDSA) guidelines, what would be the preferred therapy?
A) Metronidazole
B) Fidaxomicin + bezlotoxumab
C) Vancomycin
D) Fecal microbiota transplant
The recommendations from the 2021 guidelines would be to treat with fidaxomicin and add bezlotoxumab.1 The guidelines highlight the following changes:
- In patients with an initial Clostridioides difficile infections (CDI) fidaxomicin is preferred over vancomycin.
- In patients with a recurrent CDI episode, fidaxomicin is favored over vancomycin. For patients with multiple recurrences, vancomycin in a tapered and pulsed regimen, vancomycin followed by rifaximin, and fecal microbiota transplantation are options in addition to fidaxomicin.
- Addition of bezlotoxumab to standard of care antibiotics is recommended for recurrence of CDI within the first 6 months over standard of care antibiotics alone
The feasibility of these recommendations is up for debate. The cost of a course of fidaxomicin is $2,800, and the cost of bezlotoxumab is about $4,500. Cost effectiveness studies that helped drive the recommendations show a savings by reducing future hospitalizations for C. diff.2 Unfortunately, this enthusiasm is not shared by many insurance companies for outpatient treatment.
Knee osteoarthritis
I will save you the excitement of the new acromegaly guidelines and focus on something we see all the time: knee osteoarthritis. The American Academy of Orthopedic Surgeons has released guidelines for this condition.3 The useful points I found were as follows:
- Topical application of nonsteroidal anti-inflammatory drugs (e.g., diclofenac) should be used to improve function and quality of life in patients with knee osteoarthritis.
- Exercise routines (i.e, supervised, unsupervised, and/or aquatic) are recommended versus no exercise for improving pain and function in patients with knee osteoarthritis.
- Not recommended is the use of oral narcotics (including tramadol), as they are not effective at improving pain or function, and their use results in a significant increased risk of adverse events.
- Not recommended for routine use in symptomatic knee osteoarthritis is intra-articular injection of hyaluronic acid.
I was happy to see topical NSAIDS recommended, as they are a much safer option in older patients than oral NSAIDS (which were also recommended). The recommendation against narcotics, including tramadol, is a shift from the recommendation of tramadol in the 2013 guidelines.4 Acetaminophen was enthusiastically recommended, and is still worth a try.
Sexually transmitted infections
- The dosing for the treatment of gonorrhea has increased to 500 mg of ceftriaxone (was 250 mg in 2015 guidelines), with a dose of 1 gram for patients who weigh more than 150 kg.
- Chlamydia infections should be treated with a 7-day course of doxycycline as the preferred antibiotic, except in pregnant women (where azithromycin is recommended).
- Herpes simplex virus 2 recurrences can be treated with twice-daily dosing of 800 mg of acyclovir for 5 days, or acyclovir 800 mg three times a day for 2 days. The shortest course for recurrence is famciclovir 1 gram twice a day for 1 day.
- The Centers for Disease Control and Prevention has removed the recommendation for avoidance of alcohol when taking metronidazole.
I hope these highlights of guidelines for common issues we see are helpful!
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Johnson S et al. Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused update guidelines on management of Clostridioides difficile Infection in adults. Clin Infect Dis. 2021 Sep 7;73(5):e1029-e1044.
2. Pabhu VS et al. Cost-effectiveness of bezlotoxumab compared with placebo for the prevention of recurrent Clostridium difficile infection. Clin Infect Dis. 2018 Feb 1;66(3):355-62.
3. American Academy of Orthopaedic Surgeons: Management of osteoarthritis of the knee (non-arthroplasty) – Evidence-based clinical practice guideline (2021 Aug 31. https://www.aaos.org/oak3cpg).
4. Jevsevar DS. Treatment of osteoarthritis of the knee: Evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013: Sep;21(9):571-6.
5. Sexually transmitted infections treatment guidelines, 2021 recommendations and reports. MMWR 2021 Jul 23;70(4):1-187.