User login
Oral contrast rarely needed before CT scans of adults with acute abdomen
SEATTLE – Oral contrast is almost always unnecessary when performing a CT scan to work up adults with acute abdomens. Further, intravenous contrast is needed only when vascular causes of pain are suspected, according to Dr. Phillips Perera, a clinical associate professor of emergency medicine at Stanford (Calif.) University Medical Center.
As with everything in medicine, there are rare exceptions to this advice, he said. Oral contrast can be helpful to confirm the presence of a fistula in a postcolectomy patient, for example.
With the growth of endovascular repair of abdominal aortic aneurysms, patients with procedural complications including leaks and postop pain are increasingly presenting in emergency departments. Intravenous contrast is needed in those cases, and "more and more of these patients will be coming into the emergency department in the next few years," he said.
Forgoing oral contrast "allows us to get our patients through the emergency department much faster, and we don’t lose [diagnostic] accuracy." It also reduces radiation exposure, because noncontrast CT studies take less time, he said. The sensitivity of noncontrast CT is 93% for detecting acute appendicitis, with a specificity of 96% (Ann. Emerg. Med. 2010;55:51-59).
Recent studies indicate noncontrast CTs work well to diagnose most causes of acute abdominal pain in adults, including appendicitis, diverticulitis, kidney stones, and large ovarian cysts at risk for ovarian torsion (J. Endourol. 2008;22:2441-5).
"You only [lose] about two percentage points" on diagnostic accuracy by forgoing contrast, and the difference in one large study (World J. Surg. 2010;34:699-703) "was not statistically significant, which I think is the most important thing," Dr. Perera said in a literature review discussion at the annual meeting of the American College of Emergency Physicians.
Radiologists still require emergency physicians in some places "to make patients drink those big bottles" of contrast. "It takes about 6 hours to drink that contrast and let it pass through; that bed is pretty much done for your shift," he observed.
"We would like not to have to do IV contrast [too], but we need to move with radiologists" on that decision, and the literature has not reached that conclusion, he said. Meanwhile, "if you’re thinking about mesenteric ischemia, thrombosis, abdominal aortic aneurysm" or some other vascular cause of abdominal pain, "you want to consider giving IV contrast."
Pancreatic and intestinal fluid alone adequately opacifies the lumen of the bowel, enabling visualization of bowel loops and abrupt, diagnostic changes in lumen caliber, he said.
Alternatively, IV contrast is needed to detect bowel ischemia. The wall of ischemic intestines will not take up contrast, and the twisting of mesenteric vessels will often be apparent.
Dr. Perera reported having no relevant conflicts of interest.
SEATTLE – Oral contrast is almost always unnecessary when performing a CT scan to work up adults with acute abdomens. Further, intravenous contrast is needed only when vascular causes of pain are suspected, according to Dr. Phillips Perera, a clinical associate professor of emergency medicine at Stanford (Calif.) University Medical Center.
As with everything in medicine, there are rare exceptions to this advice, he said. Oral contrast can be helpful to confirm the presence of a fistula in a postcolectomy patient, for example.
With the growth of endovascular repair of abdominal aortic aneurysms, patients with procedural complications including leaks and postop pain are increasingly presenting in emergency departments. Intravenous contrast is needed in those cases, and "more and more of these patients will be coming into the emergency department in the next few years," he said.
Forgoing oral contrast "allows us to get our patients through the emergency department much faster, and we don’t lose [diagnostic] accuracy." It also reduces radiation exposure, because noncontrast CT studies take less time, he said. The sensitivity of noncontrast CT is 93% for detecting acute appendicitis, with a specificity of 96% (Ann. Emerg. Med. 2010;55:51-59).
Recent studies indicate noncontrast CTs work well to diagnose most causes of acute abdominal pain in adults, including appendicitis, diverticulitis, kidney stones, and large ovarian cysts at risk for ovarian torsion (J. Endourol. 2008;22:2441-5).
"You only [lose] about two percentage points" on diagnostic accuracy by forgoing contrast, and the difference in one large study (World J. Surg. 2010;34:699-703) "was not statistically significant, which I think is the most important thing," Dr. Perera said in a literature review discussion at the annual meeting of the American College of Emergency Physicians.
Radiologists still require emergency physicians in some places "to make patients drink those big bottles" of contrast. "It takes about 6 hours to drink that contrast and let it pass through; that bed is pretty much done for your shift," he observed.
"We would like not to have to do IV contrast [too], but we need to move with radiologists" on that decision, and the literature has not reached that conclusion, he said. Meanwhile, "if you’re thinking about mesenteric ischemia, thrombosis, abdominal aortic aneurysm" or some other vascular cause of abdominal pain, "you want to consider giving IV contrast."
Pancreatic and intestinal fluid alone adequately opacifies the lumen of the bowel, enabling visualization of bowel loops and abrupt, diagnostic changes in lumen caliber, he said.
Alternatively, IV contrast is needed to detect bowel ischemia. The wall of ischemic intestines will not take up contrast, and the twisting of mesenteric vessels will often be apparent.
Dr. Perera reported having no relevant conflicts of interest.
SEATTLE – Oral contrast is almost always unnecessary when performing a CT scan to work up adults with acute abdomens. Further, intravenous contrast is needed only when vascular causes of pain are suspected, according to Dr. Phillips Perera, a clinical associate professor of emergency medicine at Stanford (Calif.) University Medical Center.
As with everything in medicine, there are rare exceptions to this advice, he said. Oral contrast can be helpful to confirm the presence of a fistula in a postcolectomy patient, for example.
With the growth of endovascular repair of abdominal aortic aneurysms, patients with procedural complications including leaks and postop pain are increasingly presenting in emergency departments. Intravenous contrast is needed in those cases, and "more and more of these patients will be coming into the emergency department in the next few years," he said.
Forgoing oral contrast "allows us to get our patients through the emergency department much faster, and we don’t lose [diagnostic] accuracy." It also reduces radiation exposure, because noncontrast CT studies take less time, he said. The sensitivity of noncontrast CT is 93% for detecting acute appendicitis, with a specificity of 96% (Ann. Emerg. Med. 2010;55:51-59).
Recent studies indicate noncontrast CTs work well to diagnose most causes of acute abdominal pain in adults, including appendicitis, diverticulitis, kidney stones, and large ovarian cysts at risk for ovarian torsion (J. Endourol. 2008;22:2441-5).
"You only [lose] about two percentage points" on diagnostic accuracy by forgoing contrast, and the difference in one large study (World J. Surg. 2010;34:699-703) "was not statistically significant, which I think is the most important thing," Dr. Perera said in a literature review discussion at the annual meeting of the American College of Emergency Physicians.
Radiologists still require emergency physicians in some places "to make patients drink those big bottles" of contrast. "It takes about 6 hours to drink that contrast and let it pass through; that bed is pretty much done for your shift," he observed.
"We would like not to have to do IV contrast [too], but we need to move with radiologists" on that decision, and the literature has not reached that conclusion, he said. Meanwhile, "if you’re thinking about mesenteric ischemia, thrombosis, abdominal aortic aneurysm" or some other vascular cause of abdominal pain, "you want to consider giving IV contrast."
Pancreatic and intestinal fluid alone adequately opacifies the lumen of the bowel, enabling visualization of bowel loops and abrupt, diagnostic changes in lumen caliber, he said.
Alternatively, IV contrast is needed to detect bowel ischemia. The wall of ischemic intestines will not take up contrast, and the twisting of mesenteric vessels will often be apparent.
Dr. Perera reported having no relevant conflicts of interest.
EXPERT ANALYSIS FROM THE ACEP SCIENTIFIC ASSEMBLY 2013
Mixed results with angiography for splenic injuries
SAN FRANCISCO – Trauma centers that nonselectively performed angiography on patients with high-grade blunt splenic injury did not significantly reduce the likelihood of delayed splenectomy in a retrospective analysis of data on 6,870 patients treated at 267 hospitals.
On an individual patient level, however, use of angiography was associated with a reduced risk of delayed splenectomy (more than 6 hours after admission) after researchers controlled for the influence of multiple other factors, Dr. Ben L. Zarzaur and his associates reported at the annual meeting of the American Association for the Surgery of Trauma.
These somewhat conflicting findings suggest that "nonselective protocol-driven use of angiography at the hospital in the setting of high-grade blunt splenic injury does not benefit in terms of splenic salvage. Angiography use should be tailored to the individual patient," said Dr. Zarzaur of the University of Tennessee, Memphis.
"Attention should be paid to overall injury severity and splenic injury severity" because more severe injuries were associated with delayed splenectomy in the study, he said, adding, "Particular attention should be considered for screening for splenic vascular abnormalities."
The investigators used data from the National Trauma Data Bank (NTDB) on adults treated for high-grade blunt splenic injury at Level I or II trauma centers that admitted at least 10 such patients in 2007-2010, with high-grade injury defined as Abbreviated Injury Scale grade 3 or higher. They stratified hospital angiography use as none, low (in less than 20% of patients with high-grade blunt splenic injury), or high (in 20% or more of these patients).
Approximately 30% of patients at high-angiography centers underwent urgent splenectomy, compared with 33%-36% at hospitals with no or low-angiography use, a difference that was statistically significant. While the likelihood of a delayed splenectomy was 33% higher at low-angiography hospitals and 49% higher at hospitals without angiography, compared with high-angiography hospitals, these differences were not significant, Dr. Zarzaur reported.
The investigators used the classification of hospitals – no-, low-, or high-angiography use – to represent the three schools of thought that have developed over the past few decades regarding angiography for patients with blunt splenic injury who do not undergo immediate urgent splenectomy. The minimalist school of thought recommends using observation, not angiography for blunt splenic injury. The maximalist school of thought favors protocol-driven use of angiography for patients with certain grades of spleen injury. In between, physicians who favor a selective strategy use CT or clinical criteria or both to try and identify patients at high risk for delayed splenectomy, and reserve the risks of angiography for those patients, he said.
They chose a cutoff of 20% angiography use in patients with high-grade blunt splenic injury to discriminate between low- and high-angiography use because that represented the 90th percentile for all trauma centers in the study.
Nine percent of patients were treated at hospitals that did not use angiography for blunt splenic injury, 66% at low-angiography hospitals, and 25% at high-angiography hospitals.
Patients with grade 5 blunt splenic injury were more than twice as likely to need delayed splenectomy, compared with patients with grade 3 or 4 injury. Higher overall Injury Severity Scores (10 or higher) also doubled the risk for delayed splenectomy.
Patients with grades 4 or 5 blunt splenic injury were significantly more likely to undergo angiography at high-angiography centers than at low-angiography centers. High-angiography centers were more likely than were low-angiography centers to remove spleens with grade 5 injury after angiography, though this difference did not reach statistical significance.
Continuing controversy around the use of angiography for blunt splenic injury is illustrated by a 2011 survey of members of the American Association for the Surgery of Trauma. Members favored observation, not angiography, for grades 1 and 2 spleen injuries but showed no consensus on higher-grade injuries (J. Trauma 2011;70:1026-31).
A recent study of 1,275 patients treated for blunt splenic injury at four trauma centers that showed a significantly better chance of saving the spleen at hospitals with higher use of splenic artery embolization, especially in patients with higher-grade splenic injury (J. Trauma Acute Care Surg. 2013;75:69-74).
The current study excluded patients who died on arrival at the hospital, patients who were admitted more than 24 hours after injury, and patients who underwent splenectomy within 6 hours of admission (early splenectomy).
Dr. Zarzaur reported having no financial disclosures.
On Twitter @sherryboschert
Almost 20 years after the initial description and 30 years after we began using splenic angiography in the management of blunt splenic injury, why is it that we simply can’t settle this question? When is splenic angiography and/or catheter therapy useful in high-grade injuries?
In this study, the authors have reviewed the National Trauma Data Bank (NTDB) and have demonstrated that, at hospitals that use angiography more frequently than other hospitals, the rate of delayed splenectomy in high-grade splenic injury (defined as grades 3-5) is not different. They suggest that angiography should be selective, with particular attention to screening for vascular abnormalities.
 |
|
The authors collected information on both angiography and angiography with embolization. However, in the manuscript and the talk, they only refer to high-angiography centers. This seems to me to be a fundamental problem. This is particularly true because recent data from Jacksonville suggest that embolization of truly high-grade injury such as grade 4 injuries (even in the absence of blush) improves the salvage rate of nonoperative management.
In addition, the authors selected 20% as their cutoff for high- and low-angiography centers because 20% represented the 90th percentile of centers with regard to angiography use. I think that’s French for "it made the data analyzable." However, 20% is relatively low. If the authors wish to look at nonselective angiography they should look at us. We’re maximalists. We do angiography on 100% of patients with grades 3, 4 and 5 injuries. That’s nonselective use of angiography.
The intelligent use of this technique requires interpretation of CT and then the details of the patient presentation. For instance, a grade 4 splenic injury with or without blush but no hemoperitoneum and a totally stable patient is, in my mind, amenable to catheter therapy. Another patient with a grade 4 injury and reactive extravasation outside of the spleen and a huge peritoneum is probably best served by operative exploration. Both are grade 4 injuries, but the patients are fundamentally different.
The authors’ conclusions suggest that there is a relationship between splenic vascular injury identified on CT and success or failure of nonoperative management. This is clearly the authors’ prejudice, as they have published these findings a number of times. We’ve known for years, thanks to work from the authors’ institution, that expectant management of a patient with blush on CT fails 70% of the time and a significant number of the blushes are seen on day 3 but not day 1. Since the authors have absolutely no information on the presence or absence of blush in this data set, I fail to see how that can be one of their conclusions.
How, then, can we make sense of this? I believe the answer is in the manuscript’s last paragraph, which begins, "Another limitation of this study stems from the limitations of the NTDB." There’s little doubt that the NTDB can record an accurate snapshot of practice in the United States, but in my mind, it lacks the specificity to really answer the question, when is splenic angiography useful in high-grade injuries?
The authors have no information on presence of absence of blush, hemodynamic status other than at admission, blood transfusion rate, or technique of embolization, and they recognize that some of the data may not be accurate. I just don’t believe that the NTDB can actually answer this question.
In the end, rules are rarely helpful in the care of patients. Intelligent application of innovative techniques cannot solely be governed by rules. Perhaps the take-home message here is that the use of angiography and embolization to treat higher-grade splenic injuries is perhaps not something that everybody should be using. It may be that this technique is best preserved for high-volume centers with a real interest and a real expertise in this subject.
Dr. Thomas M. Scalea is a professor of surgery at the University of Maryland, Baltimore. These are excerpts of his remarks as a discussant of the study at the meeting. He reported having no financial disclosures.
Almost 20 years after the initial description and 30 years after we began using splenic angiography in the management of blunt splenic injury, why is it that we simply can’t settle this question? When is splenic angiography and/or catheter therapy useful in high-grade injuries?
In this study, the authors have reviewed the National Trauma Data Bank (NTDB) and have demonstrated that, at hospitals that use angiography more frequently than other hospitals, the rate of delayed splenectomy in high-grade splenic injury (defined as grades 3-5) is not different. They suggest that angiography should be selective, with particular attention to screening for vascular abnormalities.
 |
|
The authors collected information on both angiography and angiography with embolization. However, in the manuscript and the talk, they only refer to high-angiography centers. This seems to me to be a fundamental problem. This is particularly true because recent data from Jacksonville suggest that embolization of truly high-grade injury such as grade 4 injuries (even in the absence of blush) improves the salvage rate of nonoperative management.
In addition, the authors selected 20% as their cutoff for high- and low-angiography centers because 20% represented the 90th percentile of centers with regard to angiography use. I think that’s French for "it made the data analyzable." However, 20% is relatively low. If the authors wish to look at nonselective angiography they should look at us. We’re maximalists. We do angiography on 100% of patients with grades 3, 4 and 5 injuries. That’s nonselective use of angiography.
The intelligent use of this technique requires interpretation of CT and then the details of the patient presentation. For instance, a grade 4 splenic injury with or without blush but no hemoperitoneum and a totally stable patient is, in my mind, amenable to catheter therapy. Another patient with a grade 4 injury and reactive extravasation outside of the spleen and a huge peritoneum is probably best served by operative exploration. Both are grade 4 injuries, but the patients are fundamentally different.
The authors’ conclusions suggest that there is a relationship between splenic vascular injury identified on CT and success or failure of nonoperative management. This is clearly the authors’ prejudice, as they have published these findings a number of times. We’ve known for years, thanks to work from the authors’ institution, that expectant management of a patient with blush on CT fails 70% of the time and a significant number of the blushes are seen on day 3 but not day 1. Since the authors have absolutely no information on the presence or absence of blush in this data set, I fail to see how that can be one of their conclusions.
How, then, can we make sense of this? I believe the answer is in the manuscript’s last paragraph, which begins, "Another limitation of this study stems from the limitations of the NTDB." There’s little doubt that the NTDB can record an accurate snapshot of practice in the United States, but in my mind, it lacks the specificity to really answer the question, when is splenic angiography useful in high-grade injuries?
The authors have no information on presence of absence of blush, hemodynamic status other than at admission, blood transfusion rate, or technique of embolization, and they recognize that some of the data may not be accurate. I just don’t believe that the NTDB can actually answer this question.
In the end, rules are rarely helpful in the care of patients. Intelligent application of innovative techniques cannot solely be governed by rules. Perhaps the take-home message here is that the use of angiography and embolization to treat higher-grade splenic injuries is perhaps not something that everybody should be using. It may be that this technique is best preserved for high-volume centers with a real interest and a real expertise in this subject.
Dr. Thomas M. Scalea is a professor of surgery at the University of Maryland, Baltimore. These are excerpts of his remarks as a discussant of the study at the meeting. He reported having no financial disclosures.
Almost 20 years after the initial description and 30 years after we began using splenic angiography in the management of blunt splenic injury, why is it that we simply can’t settle this question? When is splenic angiography and/or catheter therapy useful in high-grade injuries?
In this study, the authors have reviewed the National Trauma Data Bank (NTDB) and have demonstrated that, at hospitals that use angiography more frequently than other hospitals, the rate of delayed splenectomy in high-grade splenic injury (defined as grades 3-5) is not different. They suggest that angiography should be selective, with particular attention to screening for vascular abnormalities.
 |
|
The authors collected information on both angiography and angiography with embolization. However, in the manuscript and the talk, they only refer to high-angiography centers. This seems to me to be a fundamental problem. This is particularly true because recent data from Jacksonville suggest that embolization of truly high-grade injury such as grade 4 injuries (even in the absence of blush) improves the salvage rate of nonoperative management.
In addition, the authors selected 20% as their cutoff for high- and low-angiography centers because 20% represented the 90th percentile of centers with regard to angiography use. I think that’s French for "it made the data analyzable." However, 20% is relatively low. If the authors wish to look at nonselective angiography they should look at us. We’re maximalists. We do angiography on 100% of patients with grades 3, 4 and 5 injuries. That’s nonselective use of angiography.
The intelligent use of this technique requires interpretation of CT and then the details of the patient presentation. For instance, a grade 4 splenic injury with or without blush but no hemoperitoneum and a totally stable patient is, in my mind, amenable to catheter therapy. Another patient with a grade 4 injury and reactive extravasation outside of the spleen and a huge peritoneum is probably best served by operative exploration. Both are grade 4 injuries, but the patients are fundamentally different.
The authors’ conclusions suggest that there is a relationship between splenic vascular injury identified on CT and success or failure of nonoperative management. This is clearly the authors’ prejudice, as they have published these findings a number of times. We’ve known for years, thanks to work from the authors’ institution, that expectant management of a patient with blush on CT fails 70% of the time and a significant number of the blushes are seen on day 3 but not day 1. Since the authors have absolutely no information on the presence or absence of blush in this data set, I fail to see how that can be one of their conclusions.
How, then, can we make sense of this? I believe the answer is in the manuscript’s last paragraph, which begins, "Another limitation of this study stems from the limitations of the NTDB." There’s little doubt that the NTDB can record an accurate snapshot of practice in the United States, but in my mind, it lacks the specificity to really answer the question, when is splenic angiography useful in high-grade injuries?
The authors have no information on presence of absence of blush, hemodynamic status other than at admission, blood transfusion rate, or technique of embolization, and they recognize that some of the data may not be accurate. I just don’t believe that the NTDB can actually answer this question.
In the end, rules are rarely helpful in the care of patients. Intelligent application of innovative techniques cannot solely be governed by rules. Perhaps the take-home message here is that the use of angiography and embolization to treat higher-grade splenic injuries is perhaps not something that everybody should be using. It may be that this technique is best preserved for high-volume centers with a real interest and a real expertise in this subject.
Dr. Thomas M. Scalea is a professor of surgery at the University of Maryland, Baltimore. These are excerpts of his remarks as a discussant of the study at the meeting. He reported having no financial disclosures.
SAN FRANCISCO – Trauma centers that nonselectively performed angiography on patients with high-grade blunt splenic injury did not significantly reduce the likelihood of delayed splenectomy in a retrospective analysis of data on 6,870 patients treated at 267 hospitals.
On an individual patient level, however, use of angiography was associated with a reduced risk of delayed splenectomy (more than 6 hours after admission) after researchers controlled for the influence of multiple other factors, Dr. Ben L. Zarzaur and his associates reported at the annual meeting of the American Association for the Surgery of Trauma.
These somewhat conflicting findings suggest that "nonselective protocol-driven use of angiography at the hospital in the setting of high-grade blunt splenic injury does not benefit in terms of splenic salvage. Angiography use should be tailored to the individual patient," said Dr. Zarzaur of the University of Tennessee, Memphis.
"Attention should be paid to overall injury severity and splenic injury severity" because more severe injuries were associated with delayed splenectomy in the study, he said, adding, "Particular attention should be considered for screening for splenic vascular abnormalities."
The investigators used data from the National Trauma Data Bank (NTDB) on adults treated for high-grade blunt splenic injury at Level I or II trauma centers that admitted at least 10 such patients in 2007-2010, with high-grade injury defined as Abbreviated Injury Scale grade 3 or higher. They stratified hospital angiography use as none, low (in less than 20% of patients with high-grade blunt splenic injury), or high (in 20% or more of these patients).
Approximately 30% of patients at high-angiography centers underwent urgent splenectomy, compared with 33%-36% at hospitals with no or low-angiography use, a difference that was statistically significant. While the likelihood of a delayed splenectomy was 33% higher at low-angiography hospitals and 49% higher at hospitals without angiography, compared with high-angiography hospitals, these differences were not significant, Dr. Zarzaur reported.
The investigators used the classification of hospitals – no-, low-, or high-angiography use – to represent the three schools of thought that have developed over the past few decades regarding angiography for patients with blunt splenic injury who do not undergo immediate urgent splenectomy. The minimalist school of thought recommends using observation, not angiography for blunt splenic injury. The maximalist school of thought favors protocol-driven use of angiography for patients with certain grades of spleen injury. In between, physicians who favor a selective strategy use CT or clinical criteria or both to try and identify patients at high risk for delayed splenectomy, and reserve the risks of angiography for those patients, he said.
They chose a cutoff of 20% angiography use in patients with high-grade blunt splenic injury to discriminate between low- and high-angiography use because that represented the 90th percentile for all trauma centers in the study.
Nine percent of patients were treated at hospitals that did not use angiography for blunt splenic injury, 66% at low-angiography hospitals, and 25% at high-angiography hospitals.
Patients with grade 5 blunt splenic injury were more than twice as likely to need delayed splenectomy, compared with patients with grade 3 or 4 injury. Higher overall Injury Severity Scores (10 or higher) also doubled the risk for delayed splenectomy.
Patients with grades 4 or 5 blunt splenic injury were significantly more likely to undergo angiography at high-angiography centers than at low-angiography centers. High-angiography centers were more likely than were low-angiography centers to remove spleens with grade 5 injury after angiography, though this difference did not reach statistical significance.
Continuing controversy around the use of angiography for blunt splenic injury is illustrated by a 2011 survey of members of the American Association for the Surgery of Trauma. Members favored observation, not angiography, for grades 1 and 2 spleen injuries but showed no consensus on higher-grade injuries (J. Trauma 2011;70:1026-31).
A recent study of 1,275 patients treated for blunt splenic injury at four trauma centers that showed a significantly better chance of saving the spleen at hospitals with higher use of splenic artery embolization, especially in patients with higher-grade splenic injury (J. Trauma Acute Care Surg. 2013;75:69-74).
The current study excluded patients who died on arrival at the hospital, patients who were admitted more than 24 hours after injury, and patients who underwent splenectomy within 6 hours of admission (early splenectomy).
Dr. Zarzaur reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Trauma centers that nonselectively performed angiography on patients with high-grade blunt splenic injury did not significantly reduce the likelihood of delayed splenectomy in a retrospective analysis of data on 6,870 patients treated at 267 hospitals.
On an individual patient level, however, use of angiography was associated with a reduced risk of delayed splenectomy (more than 6 hours after admission) after researchers controlled for the influence of multiple other factors, Dr. Ben L. Zarzaur and his associates reported at the annual meeting of the American Association for the Surgery of Trauma.
These somewhat conflicting findings suggest that "nonselective protocol-driven use of angiography at the hospital in the setting of high-grade blunt splenic injury does not benefit in terms of splenic salvage. Angiography use should be tailored to the individual patient," said Dr. Zarzaur of the University of Tennessee, Memphis.
"Attention should be paid to overall injury severity and splenic injury severity" because more severe injuries were associated with delayed splenectomy in the study, he said, adding, "Particular attention should be considered for screening for splenic vascular abnormalities."
The investigators used data from the National Trauma Data Bank (NTDB) on adults treated for high-grade blunt splenic injury at Level I or II trauma centers that admitted at least 10 such patients in 2007-2010, with high-grade injury defined as Abbreviated Injury Scale grade 3 or higher. They stratified hospital angiography use as none, low (in less than 20% of patients with high-grade blunt splenic injury), or high (in 20% or more of these patients).
Approximately 30% of patients at high-angiography centers underwent urgent splenectomy, compared with 33%-36% at hospitals with no or low-angiography use, a difference that was statistically significant. While the likelihood of a delayed splenectomy was 33% higher at low-angiography hospitals and 49% higher at hospitals without angiography, compared with high-angiography hospitals, these differences were not significant, Dr. Zarzaur reported.
The investigators used the classification of hospitals – no-, low-, or high-angiography use – to represent the three schools of thought that have developed over the past few decades regarding angiography for patients with blunt splenic injury who do not undergo immediate urgent splenectomy. The minimalist school of thought recommends using observation, not angiography for blunt splenic injury. The maximalist school of thought favors protocol-driven use of angiography for patients with certain grades of spleen injury. In between, physicians who favor a selective strategy use CT or clinical criteria or both to try and identify patients at high risk for delayed splenectomy, and reserve the risks of angiography for those patients, he said.
They chose a cutoff of 20% angiography use in patients with high-grade blunt splenic injury to discriminate between low- and high-angiography use because that represented the 90th percentile for all trauma centers in the study.
Nine percent of patients were treated at hospitals that did not use angiography for blunt splenic injury, 66% at low-angiography hospitals, and 25% at high-angiography hospitals.
Patients with grade 5 blunt splenic injury were more than twice as likely to need delayed splenectomy, compared with patients with grade 3 or 4 injury. Higher overall Injury Severity Scores (10 or higher) also doubled the risk for delayed splenectomy.
Patients with grades 4 or 5 blunt splenic injury were significantly more likely to undergo angiography at high-angiography centers than at low-angiography centers. High-angiography centers were more likely than were low-angiography centers to remove spleens with grade 5 injury after angiography, though this difference did not reach statistical significance.
Continuing controversy around the use of angiography for blunt splenic injury is illustrated by a 2011 survey of members of the American Association for the Surgery of Trauma. Members favored observation, not angiography, for grades 1 and 2 spleen injuries but showed no consensus on higher-grade injuries (J. Trauma 2011;70:1026-31).
A recent study of 1,275 patients treated for blunt splenic injury at four trauma centers that showed a significantly better chance of saving the spleen at hospitals with higher use of splenic artery embolization, especially in patients with higher-grade splenic injury (J. Trauma Acute Care Surg. 2013;75:69-74).
The current study excluded patients who died on arrival at the hospital, patients who were admitted more than 24 hours after injury, and patients who underwent splenectomy within 6 hours of admission (early splenectomy).
Dr. Zarzaur reported having no financial disclosures.
On Twitter @sherryboschert
AT THE AAST ANNUAL MEETING
Major finding: The likelihood of delayed splenectomy was 33% higher at centers without angiography and 49% higher at low-angiography centers, compared with high-angiography centers, but the differences were not statistically significant.
Data source: Retrospective analysis of data from the National Trauma Data Bank on 6,870 patients treated for blunt splenic injury at 267 hospitals.
Disclosures: Dr. Zarzaur reported having no financial disclosures.
CT says it all: Quitting smoking cuts cardiac risk
AMSTERDAM – A prospective analysis of CT angiography of more than 13,000 patients bears some good news and some bad news for patients who have quit smoking, and yet another warning for those who continue to smoke.
Current smokers had nearly a twofold increase in risk of major adverse cardiac events (MACE), compared with those who had quit and those who had never smoked. However, they – along with past smokers – still had a significantly higher prevalence, extent, and severity of coronary artery disease (CAD), compared with individuals who never smoked.
The unpublished study, which is from the CONFIRM Registry, was presented by Dr. James K. Min of Weill Cornell Medical College, New York, and New York-Presbyterian Hospital, at the annual congress of the European Society of Cardiology.
Researchers evaluated the extent and severity of CAD, as well as the risk of MACE, for active smokers, past smokers, and nonsmokers undergoing coronary CT angiography.
Of the 13,372 patients without known CAD who underwent CT, 21% were current smokers, 24% were past smokers who had quit more than 3 months prior to the CT, and 55% were nonsmokers.
The average age of the patients was 56 years, and half were men. Patients were followed up for 2 years, and MACE occurred in 279 cases (2.1%).
Analysis showed that current and past smokers had a 50% or higher risk of obstructive CAD than did nonsmokers. One-vessel disease had a frequency of 11.1% among nonsmokers, compared with 16.6% and 16.2% in current and past smokers, respectively; the frequency of two-vessel disease was 4.8% among nonsmokers vs. 7.3% and 7.8%; and the frequency of three-vessel disease was 2.3% vs. 5.1% and 5%.
In addition, current smokers had a higher risk of MACE than did nonsmokers (P less than .001), but past smokers did not (P = .29).
Even after matched-cohort analysis, the relationship remained the same, and current smoking was still significantly associated with MACE risk, but past smoking was not.
"You’re never too old to quit smoking," said Dr. Freek Verheugt, who moderated the session.
Dr. Min and Dr. Verheugt had no disclosures.
On Twitter @naseemsmiller
AMSTERDAM – A prospective analysis of CT angiography of more than 13,000 patients bears some good news and some bad news for patients who have quit smoking, and yet another warning for those who continue to smoke.
Current smokers had nearly a twofold increase in risk of major adverse cardiac events (MACE), compared with those who had quit and those who had never smoked. However, they – along with past smokers – still had a significantly higher prevalence, extent, and severity of coronary artery disease (CAD), compared with individuals who never smoked.
The unpublished study, which is from the CONFIRM Registry, was presented by Dr. James K. Min of Weill Cornell Medical College, New York, and New York-Presbyterian Hospital, at the annual congress of the European Society of Cardiology.
Researchers evaluated the extent and severity of CAD, as well as the risk of MACE, for active smokers, past smokers, and nonsmokers undergoing coronary CT angiography.
Of the 13,372 patients without known CAD who underwent CT, 21% were current smokers, 24% were past smokers who had quit more than 3 months prior to the CT, and 55% were nonsmokers.
The average age of the patients was 56 years, and half were men. Patients were followed up for 2 years, and MACE occurred in 279 cases (2.1%).
Analysis showed that current and past smokers had a 50% or higher risk of obstructive CAD than did nonsmokers. One-vessel disease had a frequency of 11.1% among nonsmokers, compared with 16.6% and 16.2% in current and past smokers, respectively; the frequency of two-vessel disease was 4.8% among nonsmokers vs. 7.3% and 7.8%; and the frequency of three-vessel disease was 2.3% vs. 5.1% and 5%.
In addition, current smokers had a higher risk of MACE than did nonsmokers (P less than .001), but past smokers did not (P = .29).
Even after matched-cohort analysis, the relationship remained the same, and current smoking was still significantly associated with MACE risk, but past smoking was not.
"You’re never too old to quit smoking," said Dr. Freek Verheugt, who moderated the session.
Dr. Min and Dr. Verheugt had no disclosures.
On Twitter @naseemsmiller
AMSTERDAM – A prospective analysis of CT angiography of more than 13,000 patients bears some good news and some bad news for patients who have quit smoking, and yet another warning for those who continue to smoke.
Current smokers had nearly a twofold increase in risk of major adverse cardiac events (MACE), compared with those who had quit and those who had never smoked. However, they – along with past smokers – still had a significantly higher prevalence, extent, and severity of coronary artery disease (CAD), compared with individuals who never smoked.
The unpublished study, which is from the CONFIRM Registry, was presented by Dr. James K. Min of Weill Cornell Medical College, New York, and New York-Presbyterian Hospital, at the annual congress of the European Society of Cardiology.
Researchers evaluated the extent and severity of CAD, as well as the risk of MACE, for active smokers, past smokers, and nonsmokers undergoing coronary CT angiography.
Of the 13,372 patients without known CAD who underwent CT, 21% were current smokers, 24% were past smokers who had quit more than 3 months prior to the CT, and 55% were nonsmokers.
The average age of the patients was 56 years, and half were men. Patients were followed up for 2 years, and MACE occurred in 279 cases (2.1%).
Analysis showed that current and past smokers had a 50% or higher risk of obstructive CAD than did nonsmokers. One-vessel disease had a frequency of 11.1% among nonsmokers, compared with 16.6% and 16.2% in current and past smokers, respectively; the frequency of two-vessel disease was 4.8% among nonsmokers vs. 7.3% and 7.8%; and the frequency of three-vessel disease was 2.3% vs. 5.1% and 5%.
In addition, current smokers had a higher risk of MACE than did nonsmokers (P less than .001), but past smokers did not (P = .29).
Even after matched-cohort analysis, the relationship remained the same, and current smoking was still significantly associated with MACE risk, but past smoking was not.
"You’re never too old to quit smoking," said Dr. Freek Verheugt, who moderated the session.
Dr. Min and Dr. Verheugt had no disclosures.
On Twitter @naseemsmiller
AT THE ESC CONGRESS 2013
Major finding: Current smokers had a higher risk of MACE than did nonsmokers (P less than .001), but past smokers did not (P = .29).
Data source: Prospective analysis of CT angiography of 13,000 patients from the CONFIRM registry.
Disclosures: Dr. Min and Dr. Verheugt had no disclosures.
Protocol boosts antimicrobial dosing practices during CRRT
DENVER – Before a new protocol was implemented, antimicrobial dosing in patients receiving continuous renal replacement therapy varied and was adherent to evidence-based recommendations in about one-quarter of antimicrobial orders, results from a single-center study showed.
"For any kind of renal replacement therapy, there is always an uncertainty as to how much residual antibiotic is being removed, how much residual renal function the patient has, and how much of the antibiotic is actually staying within the patient for them to achieve therapeutic levels of the drug to combat their infection," Jamie Wagner, Pharm.D., said in an interview during a poster session at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
"With renal replacement therapy, everything is dependent on the filter, the flow rate, and how much residual renal function the patient has. We set out to try to determine how well the interdisciplinary teams were adhering with dosing recommendations, defined as use of evidence-based dose for each CRRT [continuous renal replacement therapy] modality employed for the entire duration of antibiotics used during CRRT."
Dr. Wagner, an infectious diseases pharmacy fellow at the 802-bed Henry Ford Hospital, Detroit, and her associates evaluated 246 antimicrobial orders placed for 43 patients from November 2008 to May 2012. Patients were included in the analysis if they had an order placed for a beta-lactam, vancomycin, tobramycin, gentamicin, or daptomycin; if they received the drug in the ICU; and if they were on CRRT at the time the drug was administered. Patients receiving intermittent hemodialysis or peritoneal dialysis were excluded from the study.
Using medical records, the researchers evaluated demographics, CRRT modality, dates of changes in CRRT, and antibiotic dosing information. Each antibiotic order was evaluated for adherence to evidence-based dosing recommendation, which was the primary outcome of interest.
In August 2011, the Henry Ford Health System implemented an institutional guideline for antibiotic dosing in CRRT, which contained a summary of evidence-based dosing recommendations for the most common antimicrobial agents used in the ICU.
Of the 43 patients, 14 met study inclusion criteria before implementation of the guideline (group A), while the remaining 29 met inclusion criteria after implementation of the guideline (group B). The mean ages of patients in both groups were similar (55 years in group A vs. 59 years in group B), as were other variables.
Dr. Wagner reported that no differences were observed in antibiotic use between pre- and postguideline antibiotic orders. The three most commonly prescribed agents were vancomycin (32%), cefepime (21%), and aminoglycosides (15%). Following implementation of the guideline, overall adherence with evidence-based dosing recommendations improved from 24% to 49% between groups A and B, a difference which reached significance (P less than .001).
Four CRRT modalities changed significantly between groups A and B: continuous venovenous hemofiltration (CVVH) for 8-12 hours (24% vs. 0%, respectively); sustained, low-efficiency, daily diafiltration (SLEDD) for 8-12 hours with an F8 filter (29% vs. 6%); SLEDD for 8-12 hours with an F250 filter (14% vs. 1%); and SLEDD for 24 hours (11% vs. 75%).
Changes between modalities occurred in 13% of all orders assessed. Variables found to be associated with nonadherent orders were change of CRRT mode that resulted in a new recommended dose (7%), SLEDD for 8-12 hours (15%), and the use of any aminoglycoside (15%).
"Communication is key between all patient care providers on a daily basis," Dr. Wagner concluded. "At Henry Ford Hospital, providers must submit a new order for CRRT every single day for patients requiring antibiotic dosing. There needs to be communication about this between all providers involved in that patient’s care."
She acknowledged certain limitations of the study, including increased use of 24-hour SLEDD during the postguideline period, strict definition for adherence to the guideline, and the inability to systematically evaluate clinical response or residual function.
"Understanding factors associated with nonadherent orders can provide a starting point for clinicians to improve the antimicrobial use process in CRRT," she said.
Dr. Wagner said that she had no relevant conflicts of interest to disclose.
DENVER – Before a new protocol was implemented, antimicrobial dosing in patients receiving continuous renal replacement therapy varied and was adherent to evidence-based recommendations in about one-quarter of antimicrobial orders, results from a single-center study showed.
"For any kind of renal replacement therapy, there is always an uncertainty as to how much residual antibiotic is being removed, how much residual renal function the patient has, and how much of the antibiotic is actually staying within the patient for them to achieve therapeutic levels of the drug to combat their infection," Jamie Wagner, Pharm.D., said in an interview during a poster session at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
"With renal replacement therapy, everything is dependent on the filter, the flow rate, and how much residual renal function the patient has. We set out to try to determine how well the interdisciplinary teams were adhering with dosing recommendations, defined as use of evidence-based dose for each CRRT [continuous renal replacement therapy] modality employed for the entire duration of antibiotics used during CRRT."
Dr. Wagner, an infectious diseases pharmacy fellow at the 802-bed Henry Ford Hospital, Detroit, and her associates evaluated 246 antimicrobial orders placed for 43 patients from November 2008 to May 2012. Patients were included in the analysis if they had an order placed for a beta-lactam, vancomycin, tobramycin, gentamicin, or daptomycin; if they received the drug in the ICU; and if they were on CRRT at the time the drug was administered. Patients receiving intermittent hemodialysis or peritoneal dialysis were excluded from the study.
Using medical records, the researchers evaluated demographics, CRRT modality, dates of changes in CRRT, and antibiotic dosing information. Each antibiotic order was evaluated for adherence to evidence-based dosing recommendation, which was the primary outcome of interest.
In August 2011, the Henry Ford Health System implemented an institutional guideline for antibiotic dosing in CRRT, which contained a summary of evidence-based dosing recommendations for the most common antimicrobial agents used in the ICU.
Of the 43 patients, 14 met study inclusion criteria before implementation of the guideline (group A), while the remaining 29 met inclusion criteria after implementation of the guideline (group B). The mean ages of patients in both groups were similar (55 years in group A vs. 59 years in group B), as were other variables.
Dr. Wagner reported that no differences were observed in antibiotic use between pre- and postguideline antibiotic orders. The three most commonly prescribed agents were vancomycin (32%), cefepime (21%), and aminoglycosides (15%). Following implementation of the guideline, overall adherence with evidence-based dosing recommendations improved from 24% to 49% between groups A and B, a difference which reached significance (P less than .001).
Four CRRT modalities changed significantly between groups A and B: continuous venovenous hemofiltration (CVVH) for 8-12 hours (24% vs. 0%, respectively); sustained, low-efficiency, daily diafiltration (SLEDD) for 8-12 hours with an F8 filter (29% vs. 6%); SLEDD for 8-12 hours with an F250 filter (14% vs. 1%); and SLEDD for 24 hours (11% vs. 75%).
Changes between modalities occurred in 13% of all orders assessed. Variables found to be associated with nonadherent orders were change of CRRT mode that resulted in a new recommended dose (7%), SLEDD for 8-12 hours (15%), and the use of any aminoglycoside (15%).
"Communication is key between all patient care providers on a daily basis," Dr. Wagner concluded. "At Henry Ford Hospital, providers must submit a new order for CRRT every single day for patients requiring antibiotic dosing. There needs to be communication about this between all providers involved in that patient’s care."
She acknowledged certain limitations of the study, including increased use of 24-hour SLEDD during the postguideline period, strict definition for adherence to the guideline, and the inability to systematically evaluate clinical response or residual function.
"Understanding factors associated with nonadherent orders can provide a starting point for clinicians to improve the antimicrobial use process in CRRT," she said.
Dr. Wagner said that she had no relevant conflicts of interest to disclose.
DENVER – Before a new protocol was implemented, antimicrobial dosing in patients receiving continuous renal replacement therapy varied and was adherent to evidence-based recommendations in about one-quarter of antimicrobial orders, results from a single-center study showed.
"For any kind of renal replacement therapy, there is always an uncertainty as to how much residual antibiotic is being removed, how much residual renal function the patient has, and how much of the antibiotic is actually staying within the patient for them to achieve therapeutic levels of the drug to combat their infection," Jamie Wagner, Pharm.D., said in an interview during a poster session at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
"With renal replacement therapy, everything is dependent on the filter, the flow rate, and how much residual renal function the patient has. We set out to try to determine how well the interdisciplinary teams were adhering with dosing recommendations, defined as use of evidence-based dose for each CRRT [continuous renal replacement therapy] modality employed for the entire duration of antibiotics used during CRRT."
Dr. Wagner, an infectious diseases pharmacy fellow at the 802-bed Henry Ford Hospital, Detroit, and her associates evaluated 246 antimicrobial orders placed for 43 patients from November 2008 to May 2012. Patients were included in the analysis if they had an order placed for a beta-lactam, vancomycin, tobramycin, gentamicin, or daptomycin; if they received the drug in the ICU; and if they were on CRRT at the time the drug was administered. Patients receiving intermittent hemodialysis or peritoneal dialysis were excluded from the study.
Using medical records, the researchers evaluated demographics, CRRT modality, dates of changes in CRRT, and antibiotic dosing information. Each antibiotic order was evaluated for adherence to evidence-based dosing recommendation, which was the primary outcome of interest.
In August 2011, the Henry Ford Health System implemented an institutional guideline for antibiotic dosing in CRRT, which contained a summary of evidence-based dosing recommendations for the most common antimicrobial agents used in the ICU.
Of the 43 patients, 14 met study inclusion criteria before implementation of the guideline (group A), while the remaining 29 met inclusion criteria after implementation of the guideline (group B). The mean ages of patients in both groups were similar (55 years in group A vs. 59 years in group B), as were other variables.
Dr. Wagner reported that no differences were observed in antibiotic use between pre- and postguideline antibiotic orders. The three most commonly prescribed agents were vancomycin (32%), cefepime (21%), and aminoglycosides (15%). Following implementation of the guideline, overall adherence with evidence-based dosing recommendations improved from 24% to 49% between groups A and B, a difference which reached significance (P less than .001).
Four CRRT modalities changed significantly between groups A and B: continuous venovenous hemofiltration (CVVH) for 8-12 hours (24% vs. 0%, respectively); sustained, low-efficiency, daily diafiltration (SLEDD) for 8-12 hours with an F8 filter (29% vs. 6%); SLEDD for 8-12 hours with an F250 filter (14% vs. 1%); and SLEDD for 24 hours (11% vs. 75%).
Changes between modalities occurred in 13% of all orders assessed. Variables found to be associated with nonadherent orders were change of CRRT mode that resulted in a new recommended dose (7%), SLEDD for 8-12 hours (15%), and the use of any aminoglycoside (15%).
"Communication is key between all patient care providers on a daily basis," Dr. Wagner concluded. "At Henry Ford Hospital, providers must submit a new order for CRRT every single day for patients requiring antibiotic dosing. There needs to be communication about this between all providers involved in that patient’s care."
She acknowledged certain limitations of the study, including increased use of 24-hour SLEDD during the postguideline period, strict definition for adherence to the guideline, and the inability to systematically evaluate clinical response or residual function.
"Understanding factors associated with nonadherent orders can provide a starting point for clinicians to improve the antimicrobial use process in CRRT," she said.
Dr. Wagner said that she had no relevant conflicts of interest to disclose.
AT ICAAC 2013
Emergency Radiology: Current and advanced imaging techniques in the ED
As EDs have evolved to handle the increasing volume and complexity of patients requiring immediate care, so too, has the field of emergency radiology. Many EDs across the country now have multiple advanced imaging modalities available 24 hours a day, including Xray, computed tomography (CT), ultrasound, and magnetic resonance imaging (MRI). While many emergency medicine physicians are now trained in performing and evaluating ultrasound images—similar to X-rays in the past—most are less comfortable with CT and MRI.
Emergency radiology, now a recognized subspecialty of diagnostic imaging, has proliferated to meet the demands for immediate interpretation of these images. This combination of around-the-clock access to equipment and expertise has brought cutting-edge, advanced imaging to the front lines of emergency care. In this special feature, we invited a group of emergency radiologists and an expert in cardiovascular imaging to discuss the applicability and utility of several of these techniques in the ED setting.
As illustrated by this panel, advanced imaging has become increasingly valuable and available in providing care to ED patients. In this presentation, however, you will notice the conspicuous absence of specific techniques that are utilized in diagnosis and evaluation of stroke, head/spine trauma, and other neurologic conditions that are commonplace in the ED. Advanced imaging has become critical in these instances and will be discussed in a future article.
Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and the executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center. He is a member of the EMER GENCY MEDICINE editorial board.
Faster than FAST: Single Pass Whole-Body Computed Tomography for Rapid Evaluation of Trauma Patients
Ashwin Asrani, MD
Dr Asrani is an assistant professor of radiology at Weill Cornell Medical College in New York City and an
assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center in New York City.
As part of initial assessment and resuscitation in the ED, severely injured trauma patients frequently undergo a bedside focused abdominal sonography for trauma (FAST) scan to assess for intraperitoneal hemorrhage from an underlying major abdominal visceral injury, as well as the presence of pleural and/or pericardial effusion. However, the use and role of a FAST scan in hemodynamically stable patients has recently been questioned since, based on its relatively low sensitivity for visceral injury, many of these cases eventually require computed tomography (CT) (See Case). In addition, patients in this subset with a positive FAST scan frequently have subsequent CT to further assist clinicians in understanding the nature of injury and to guide management recommendations (eg, operative versus nonoperative options).1
Advances in the speed of CT have made it possible to obtain images of the entire body with only a single dose of contrast injection. The elimination of segmental acquisition of torso images (eg, head, neck, chest, abdomen) eliminates the need to reposition the patient, repeat scout images, and postpone the reconstruction of reformats and other special views until the end of the examination. Depending on the original protocol, this in turn minimizes patient time in the CT-scan room by approximately 31% to 42%,2,3 and significantly reduces radiation exposure by 17% compared to conventional segmental acquisition of different body parts.2
Evidence suggests that direct single-pass whole-body CT improves outcomes in both hemodynamically stable and unstable patients and can reveal significant findings not apparent on initial clinical examination.4,5 Whole-body CT increases injury severity by detecting lesions that would not otherwise have been detected by conventional methods. While this information does not affect treatment options, it does artificially lower the ratio of observed-to-expected deaths.6 The Randomized Study of Early Assessment by CT Scanning in Trauma Patients (REACT-2), a recent international multicenter randomized clinical trial, is expected to provide evidence supporting the value of immediate total-body CT scanning during the primary evaluation of severely injured trauma patients; the results of this trial should become available in 2014.7
As EDs install and upgrade to newer multidetector CT scanners, single-pass whole-body scanning has the potential to save time in situations when it really matters.
References
1. Natarajan B, Gupta PK, Cemaj S, Sorensen M, Hatzoudis GI, Forse RA. FAST scan: Is it worth doing in hemodynamically stable blunt trauma patients? Surgery. 2010;148(4):695-700; discussion 700-701.
2. Fanucci E, Fiaschetti V, Rotili A, Floris R, Simonetti G. Whole body 16-row multislice CT in emergency room: Effects of different protocols on scanning time, image quality and radiation exposure. Emerg Radiol. 2007;13(5):251-257.
3. Nguyen D, Platon A, Shanmuganathan K, Mirvis SE, Becker CD, Poletti PA. Evaluation of a single-pass continuous whole-body 16-MDCT protocol for patients with polytrauma. AJR Am J Roentgenol. 2009;192(1):3-10.
4. Huber-Wagner S, Biberthaler P, Haberle S, et al. Whole-body CT in haemodynamically unstable severely injured patients – A retrospective, multicentre study. PLoS One. 2013;8(7):e68880.
5. Salim A, Sangthong B, Martin M, Brown C, Plurad D, Demetriades D. Whole body imaging in blunt multisystem trauma patients without obvious signs of injury: Results of a prospective study. Arch Surg. 2006;141(5):468-473; discussion 473-475.
6. Stengel D, Frank M, Matthes G, et al. Primary pan-computed tomography for blunt multiple trauma: Can the whole be better than its parts? Injury. 2009;40 Suppl 4:S36-S46.
7. Sierink JC, Saltzherr TP, Beenen LF, et al. A multicenter, randomized controlled trial of immediate total-body CT scanning in trauma patients (REACT-2). BMC Emerg Med. 2012;12:4-227X-12-4.
Obstructed Views: Imaging in Urinary Tract Obstruction
Lily M. Belfi, MD
Dr Belfi is an assistant professor of radiology at Weill Cornell Medical College in New York City and an assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center.
Urinary tract (UT) obstruction is one of the most common indications for genitourinary (GU) imaging in the ED. Until recently, diagnosis was made using intravenous (IV) pyelography. Today, improved imaging modalities, such as noncontrast computed tomography (CT), computed tomography urography (CTU), magnetic resonance urography (MRU), and magnetic resonance imaging (MRI), are available to better visualize and evaluate the underlying causes of obstruction.
Noncontrast Computed Tomography
|
|
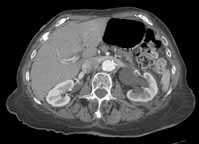
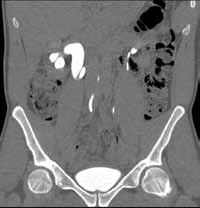
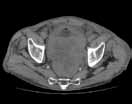
Noncontrast CT of the abdomen and pelvis is the imaging study of choice in evaluating acute GU obstruction, particularly when there is clinical suspicion of stone disease. CT is extremely sensitive and specific in detecting renal calculi and provides information about stone burden, size, location, and composition. In addition to renal calculi, noncontrast CT is also useful in identifying other, less common causes of UT obstruction such as ureteral herniation, which can occur in the inguinal, femoral, or sciatic region and result in acute obstruction. Patients with neuromuscular disorders that cause piriformis muscle atrophy (eg, multiple sclerosis) may be predisposed to ureteral sciatic herniation. CT reconstructions in the coronal plane are useful in detecting this condition (Figure 1).
|
|
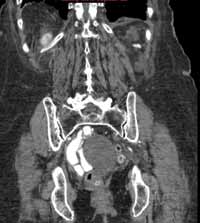
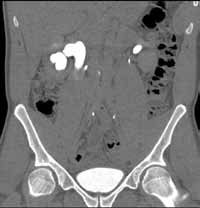
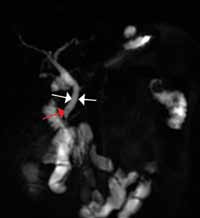
Reconstructed CT images can also reveal underlying congenital abnormalities that make a patient susceptible to obstruction. For example, CT can reveal the presence of a duplicated collecting system, which is often accompanied by an obstructing ureterocele associated with the upper pole moiety (Figure 2).
Computed Tomography Urography
|
| ||
| Figure 3. Computed tomography urography images (A and B) reveal "fish-hook" ureter (white arrows) classically seen in patients with with retrocaval ureter. | |||
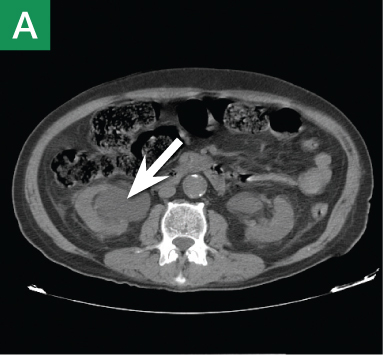
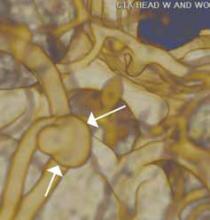
Other types of congenital anomalies may require additional imaging with CTU, in which IV iodinated contrast media is administered for increased sensitivity and visualization. For instance, patients with a retrocaval ureter often present with GU obstruction and hematuria; the classic “fish-hook” or “sickle-shaped” deformity of the ureter characterizing this disease is best visualized on CTU as the ureter is well-opacified by excreted contrast (Figure 3).
Magnetic Resonance Urography and Magnetic Resonance Imaging
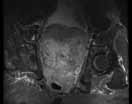
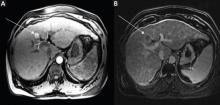
MRU and MRI are alternative modalities to CTU for patients in whom iodinated CT contrast is contraindicated or in cases where the avoidance of radiation exposure is indicated (eg, pregnant and pediatric patients). MRI and MRU are especially useful in further characterizing obstructive lesions in the GU tract. While initial noncontrast CT may suggest soft-tissue lesions within the ureter or urinary bladder, follow-up MRI clearly delineates the condition, helping clinicians quantify the extent of tumor involvement and determine disease stage (Figure 4). Pelvic MRI is also an essential tool in identifying causes of bladder outlet obstruction, especially in those involving the prostate gland (Figure 5).
|
|
|
|
|
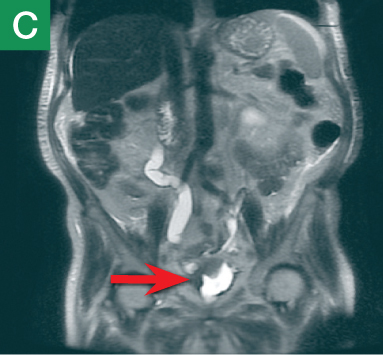
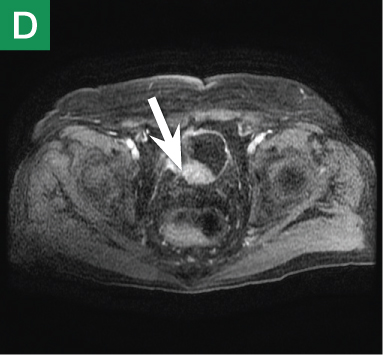
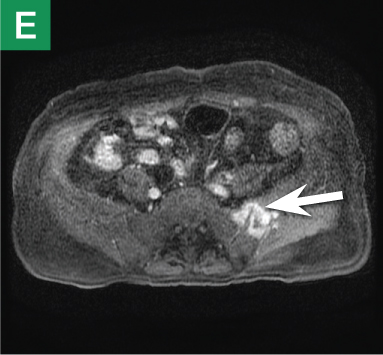
Figure 4. Noncontrast pelvic computed tomography images (A and B) of a 72-year-old man with flank pain and difficulty voiding reveal hydronephrosis (white arrow) and bladder mass (red arrow). Magnetic resonance urography (C) further visualizes the bladder mass (red arrow), and postcontrast magnetic resonance images (D and E) show the extent of the tumor (red arrow) as well as a metastatic lesion (white arrow).
| ||||
Conclusion
|
|
| |||
| Figure 5. Noncontrast pelvic computed tomography images (A and B) of a 73-yearold man with pelvic pain demonstrate bilateral hydronephrosis. (white arrows) and a large mass in the expected location of the prostate (red arrow). Further evaluation of the large heterogenous mass with pelvic magnetic resonance imaging (C) reveals prostate carcinoma (red arrows). | |||||
Based on its sensitivity in detecting both common and uncommon causes of obstruction, noncontrast abdominal and pelvic CT is an excellent first-line imaging choice for evaluating patients presenting to the ED with UT obstruction. Moreover, noncontrast CT also helps guide clinicians in determining which, if any, additional studies with CTU, MRU, and/or MRI are warranted.
Sound Advice: Ultrasound in the Emergency Department
Kemi Babagbemi, MD
Dr Babagbemi is an assistant professor of radiology and assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical College, New York.
In today’s evolving health-care environment, emphasis must be placed on providing accurate, safe, cost-effective, and timely diagnosis to the wide range of patients that present to the ED. To meet this need, emergency medicine physicians and radiologists have universally embraced the use of ultrasound in this setting.
Ultrasound is less expensive than computed tomography (CT) and magnetic resonance imaging (MRI), and its portability allows for bedside application, enabling its use in the most critical patients. It does not require extended preparation (eg, oral contrast) or carry the risk for adverse reactions associated with intravenously administered contrast. Also, since ultrasound does not use ionizing radiation, it is a particularly appropriate imaging option in susceptible populations such as pregnant and pediatric patients.1 Although a noninvasive modality, it can also be used to guide interventional procedures, including vascular access, thoracentesis/paracentesis, and specialized anesthesia.
Emergency physicians routinely perform ultrasound at bedside and are familiar with its utility in evaluating abdominal trauma, right upper quadrant pain, and acute biliary abnormalities; assessing vascular thrombosis or injury; and determining the etiology of pelvic pain and bleeding in both pregnant and nonpregnant patients. However, ultrasound is also increasingly being utilized to
diagnose other conditions for which CT and MRI were once thought the superior diagnostic tool.
Trauma (The Extended FAST Scan)
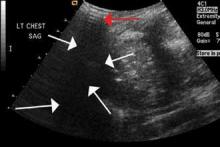
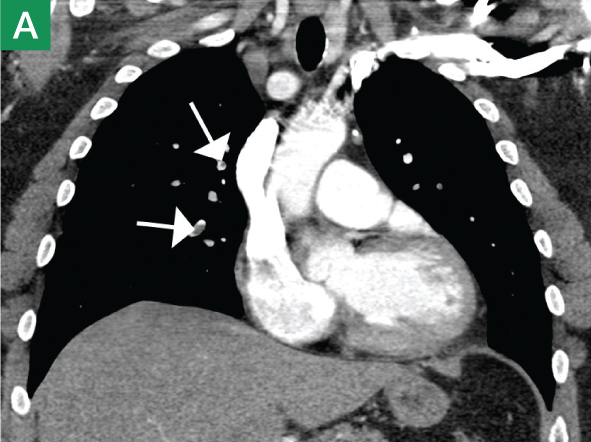
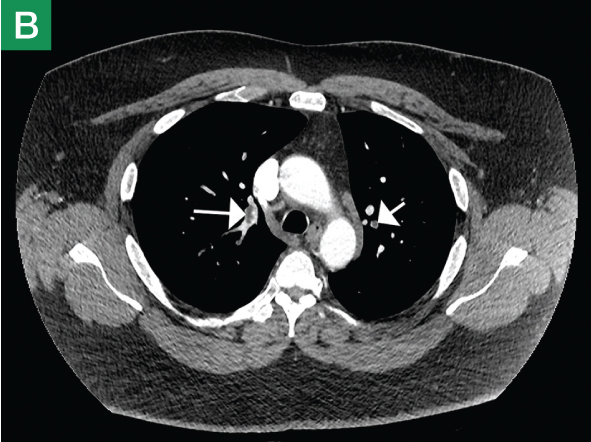
The focused abdominal sonography for trauma (FAST) scan has been used for rapid and immediate assessment of unstable trauma patients with suspected abdominal injury. In a recent study of 4,029 patients with blunt abdominal trauma, FAST scans had a sensitivity, specificity, and accuracy for detection of hemorrhage in patients with hypotension of 85%, 60%, and 77%, respectively.2 Other studies suggest that sonography of the chest may be useful in the rapid detection of additional life-threatening pathology such as pneumothorax (Figure 1), with data suggesting improved sensitivity over radiography.3 The inclusion of the pleural space in evaluation is increasingly more common and is referred to as the extended FAST (EFAST).
Appendicitis
The most common cause of abdominal pain requiring surgical intervention is appendicitis.4 Although certain clinically based prediction scores (eg, Alvarado scores) may be used, imaging is considered far superior in accurately diagnosing the condition.5 In a meta-analysis of data from 26 ultrasound and CT studies (15 prospective, 11 retrospective), there was a pooled 88% sensitivity and 94% specificity for ultrasound compared with CT, which exhibited a pooled sensitivity of 94% and specificity of 95%.6 As previously noted, because there is no ionizing radiation in ultrasound, it should be the preferred modality in both children and first-trimester pregnant patients.
Musculoskeletal Trauma
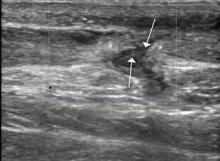
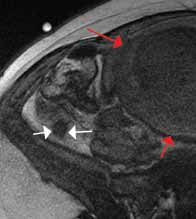
Ultrasound is an ideal imaging modality to evaluate the musculoskeletal system. Despite its widespread use in Europe for many years, musculoskeletal sonography is only now beginning to be adopted in the United States. Its ability to visualize soft-tissue structures makes it effective in evaluating for muscle, tendon, or ligament injury, and can even do so dynamically with stress maneuvers (Figure 2). With respect to fractures, sonography has also proved effective in evaluating for cortical disruption. For example, a recent study demonstrated overall 92% sensitivity and 100% sensitivity for fractures with high potential for complication in radiographically occult scaphoid fractures.7
Conclusion
As a true point-of-care imaging modality, ultrasound has an established place in the practice of emergency medicine. Future improvements in technology, including the ability to obtain true three-dimensional volumetric data sets, will expand its role even further.
References
1. Image Gently and Ultrasound. Image Gently Campaign. The Alliance for Radiation Safety in Pediatric Imaging Web site. http://www.pedrad.org/associations/5364/ig/?page=787. Accessed September 19, 2013.
2. Lee BC, Ormsby EL, McGahan JP, Melendres GM, Richards JR. The utility of sonography for the triage of blunt abdominal trauma patients to exploratory laparotomy. AJR Am J Roentgenol. 2007;188(2):415-421.
3. Nandipati KC, Allamaneni S, Kakarla, et al. Extended focused assessment with sonography for trauma (EFAST) in the diagnosis of pneumothorax: experience at a community based level I trauma center. Injury. 2011;42(5):511-514.
4. Addiss DG, Shaffer N, Fowler BS, Tauxe RV. The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol. 1990;132(5):910-925.
5. Sun JS, Noh HW, Min YG, et al. Receiver operating characteristic analysis of the diagnostic performance of a computed tomographic examination and the Alvarado score for diagnosing acute appendicitis: emphasis on age and sex of the patients.
J Comput Assist Tomogr. 2008;32(3):386-391.
6. Doria AS, Moineddin R, Kellenberger CJ, et al. US or CT for Diagnosis of Appendicitis in Children and Adults? A MetaAnalysis. Radiology. 2006;241(1):83-94.
7. Platon A, Poletti PA, Van Aaken J, Fusetti C, Della Santa D, Beaulieu JY, Becker CD. Occult fractures of the scaphoid: the role of Ultrasonography in the emergency department. Skeletal Radiol. 2011;40(7):869-875.
Stuck or Not? Noninvasive Vascular Imaging in the Emergency Setting
Michael L. Loftus, MD
Dr Loftus is assistant professor of radiology at New York-Presbyterian Hospital/Weill Cornell Medical College, New York.
Conventional catheter-directed angiography has played an important role in the history of ED imaging, providing timely information about vessel integrity throughout the body and guiding potentially life-saving interventions. However, this imaging modality carries significant potential risks, including puncture-site hematoma or pseudoaneurysm, catheter-induced vasospasm, vascular occlusion or dissection, anesthesia-associated risks, and neurological deterioration or stroke.1 Moreover, emergent angiography is not universally available in all EDs.
Computed tomography angiography (CTA) and magnetic resonance angiography (MRA) have opened new windows of opportunity for noninvasive vascular imaging to play a role in clinical decision-making in the ED. Cross-sectional angiography has virtually eliminated the need for acute catheter-directed angiography in several clinical settings, including pulmonary angiography, and the clinical applicability of CTA and MRA continues to expand as imaging techniques improve and achieve widespread acceptance and implementation. Multidetector CT is now widely available in most EDs, and the
accessibility of magnetic resonance imaging and MRA is expanding rapidly.
CTA allows evaluation of the vasculature on contrast-enhanced axial source images and also utilizes computer-generated maximum intensity projection reformations to create diagnostic images of the vascular region of interest. Similarly, MRA can be performed either with or without the administration of an intravenous gadolinium contrast agent, and provides additional information about directionality of flow, as well as detailed images of the surrounding soft tissues.
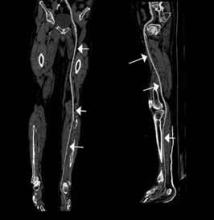
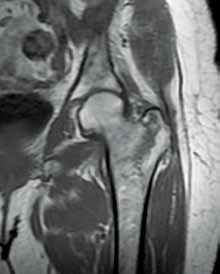
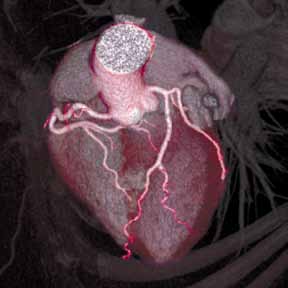
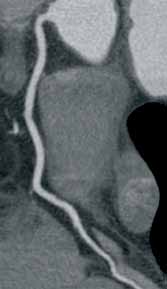
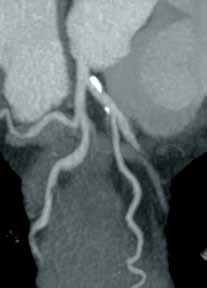
An emerging clinical situation in which contrast-enhanced cross-sectional imaging may supplant the need for arterial puncture and digital subtraction angiography is multiligament trauma to the knee. When significant kinetic force is applied to the knee, the joint is at risk for translocation and/or dislocation, with resultant injury to the surrounding soft-tissue envelope and potential trauma to the neurovascular structures around the knee. In this type of injury, cross-sectional imaging is routinely ordered to further evaluate and classify trauma, revealing potentially treatment-altering information concerning the integrity of the vascular structures with minimal risk to the patient (Figure 1).
The largest series of MRAs performed specifically in patients with knee dislocation reviewed 17 cases and found two cases of vascular pathology: one case of an intimal flap and one case of acute vasospasm. Digital subtraction angiography was performed on 6 of 17 cases and had 100% concordance with MRA findings.2 MRI is often performed in the setting of suspected multiligament knee injury to aid in preoperative planning, and the addition of MRA should be considered if trauma to the periarticular vasculature is suspected.
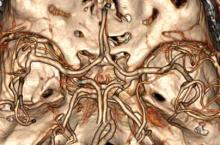
There is ample evidence that cross-sectional imaging performs well relative to conventional catheter angiography in the setting of peripheral vascular occlusion from atherosclerotic etiologies.3 Furthermore, there is precedence for utilizing contrast-enhanced CTA, as well as contrast-enhanced or three-dimensional time-of-flight MRA in other areas of the body—particularly the brain, head, and neck4 (Figure 2). Several MRA techniques are becoming available that will allow a high resolution angiographic without the use of contrast. Upcoming advances in CTA include dual-energy CT, which has the potential to allow angiography using very small amounts of contrast.
In general, when ordering cross-sectional imaging in the setting of trauma, consideration should be given to the potential for vascular compromise; thus, the addition of contrast-enhanced CTA or MRA can add valuable clinical information, with relatively little excess risk or time, sparing patients the risks of catheter-directed angiography.
References
1. Eisenberg, RL, Bank, WO, Hedgcock, MW. Neurologic complications of angiography for cerebrovascular disease. Neurology. 1980;30(8):895-897.
2. Potter HG, Weinstein M, Allen AA, Wickiewicz TL, Helfet DL. Magnetic resonance imaging of the multiple-ligament injured knee. J Orthop Trauma. 2002;16(5);330-339.
3. Chin AS, Rubin GD. CT angiography of peripheral arterial occlusive disease. Tech Vasc Interv Radiol. 2006;9(4):143-149.
4. Riles TS, Eidelman EM, Litt AW, Pinto RS, Oldford F, Schwartzenberg GW. Comparison of magnetic resonance angiography, conventional angiography, and duplex scanning. Stroke. 1992;23(3):341-346.
What’s Hip in 2013?
Roger J. Bartolotta, MD
Dr Bartolotta is an assistant professor of radiology at Weill Cornell Medical College in New York City and assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center.
Post-traumatic hip pain is a common chief concern among ED patients. While routine radiography, the standard imaging modality for the initial evaluation of suspected hip fracture, detects most fractures, its sensitivity is decreased in the setting of osteoporosis—particularly with nondisplaced fractures. Thus, though elderly/osteoporotic patients are more likely to fracture, these fractures are harder to detect on radiography. In a study of 70 patients with negative radiographs but high clinical concern for fracture, magnetic resonance imaging (MRI) detected occult femoral fractures in 37% and occult pelvic fractures in 23%.1
Hip fractures in elderly patients are associated with substantial mortality and morbidity, the risks for which increase with delayed diagnosis.2 When there is high clinical suspicion for a radiographically occult hip fracture in this population, cross-sectional imaging should be considered for further evaluation (Figure). The decision of whether to use computed tomography (CT) or MRI for the cross-sectional examination must be made on both an institutional and patient-specific basis. CT is faster, less expensive, and more widely and temporally available. Although CT has increased sensitivity for fracture detection compared to radiography, studies have demonstrated false-negative CT examinations in the setting of nondisplaced proximal femoral fractures, especially in osteoporotic patients. Hakkarinen et al3 reported that among 235 hip fractures, 10% were occult radiographically; approximately 17% of these fractures (4 out of 24) were also occult on CT but visible on MRI. Moreover, while radiography and CT may demonstrate a seemingly isolated fracture at the femoral greater trochanter, a subset of these fractures exhibit intertrochanteric extension that is only evident on MRI (Figure). Isolated greater trochanteric fractures are typically treated conservatively, while some incomplete intertrochanteric fractures warrant internal fixation, especially fractures that cross the intertrochanteric midline on coronal MRI.4,5
|
|
| ||
Figure. Anteroposterior left hip radiograph (A) in a patient with hip pain (A) shows no definite evidence of fracture. Coronal reformatted image from left hip computed tomography (B) demonstrates nondisplaced, seemingly isolated fracture of the left femoral greater trochanter (white arrows). Coronal T1-weighted image from left hip magnetic resonance imaging (C) reveals intertrochanteric extension of the fracture spanning greater than 50% of the intertrochanteric diameter (red arrows). | ||||
In addition to improved fracture detection, MRI also provides superior evaluation of the underlying bone marrow for coexisting conditions, such as osteomyelitis, osteonecrosis, and primary or metastatic neoplasm in the setting of pathologic fracture. Additional benefits of MRI over radiography and CT include its lack of ionizing radiation and improved evaluation of adjacent soft tissue injuries, such as labral and/or musculotendinous tears.
MRI, however, does require a longer examination time in which the patient must remain still. This may be difficult for acutely post-traumatic patients, notably those with baseline dementia and/or claustrophobia. For patients in whom MRI is indicated (eg, patients who do not have an implantable device such as a cardiac pacemaker) and where it is institutionally available, the decision to utilize it over CT is largely rooted in health-care economics. MRI is more expensive than radiography and CT, and even in the largest medical centers, the examination requires substantially more time than CT, which inherently decreases patient throughput in the ED. Cannon et al6 present an evidence-based algorithm for patient stratification, in which patients at high-risk for osteoporosis and low-energy trauma should be considered for immediate MRI rather than CT. These risk factors optimize MRI utilization by selecting those patients with the greatest likelihood of nondisplaced, radiographically occult fracture.
References
1. Bogost GA, Lizerbram EK, Crues JV 3rd. MR imaging in evaluation of suspected hip fracture: frequency of unsuspected bone and soft-tissue injury. Radiology. 1995;197(1):263-267.
2. Zuckerman JD, Skovron ML, Koval KJ, Aharonoff G, Frankel VH. Postoperative complications and mortality associated with operative delay in older patients who have a fracture of the hip. J Bone Joint Surg Am. 1995;77(10):1551-1556.
3. Hakkarinen DK, Banh KV, Hendey GW. Magnetic resonance imaging identifies occult hip fractures missed by 64-slice computed tomography. J Emerg Med. 2012;43(2):303-307.
4. Feldman F, Staron RB. MRI of seemingly isolated greater trochanteric fractures. AJR Am J Roentgenol. 2004;183(2):323-329.
5. Schultz E, Miller TT, Boruchov SD, Schmell EB, Toledano B. Incomplete intertrochanteric fractures: imaging features and clinical management. Radiology. 1999;211(1):237-240.
6. Cannon J, Silvestri S, Munro M. Imaging choices in occult hip fracture. J Emerg Med. 2009;37(2):144-152.
PE or Not PE: That Is the Question
Jessica Fisher, MD
Dr Fisher is an instructor of radiology at Weill Cornell Medical College in New York City.
Pulmonary embolism (PE) represents the third most common cause of death from cardiovascular disease after myocardial infarction and stroke.1 Given the potential for fatal outcome, prompt diagnosis and management are essential. To avoid overdiagnosis of PE and unnecessary treatment with anticoagulation therapy, diagnostic tests with both a high sensitivity and high specificity are essential.
Clinical stratification of patient risk for PE, in combination with D-dimer assays, is typically used to determine the need for imaging. Preliminary studies with chest X-ray are utilized to evaluate alternate causes of clinical symptoms but do not provide a definitive diagnosis. In the ED setting, advanced imaging options to detect PE include computed tomography angiography (CTA), ventilation perfusion scintigraphy (V/Q lung scan), and magnetic resonance angiography (MRA).
Computed Tomography Angiography
|
| ||
| Figure 1. Coronal (A) and axial (B) contrast-enhanced computed tomography angiography scans reveal pulmonary embolism (white arrows).. | |||
CTA has long been the standard technique for evaluating PE. In addition to reported sensitivities of 96% to 100% and specificities of 97% to 98% with multislice detectors,2 CTA also provides information on disease severity, such as clot burden, evidence of right heart strain, and the presence of pulmonary infarct (Figure 1). It also can reveal alternative etiology and diagnosis in negative cases (Figure 2).
CTA is readily available in today’s ED and can be performed quickly and efficiently. However, it does require intravenous (IV) contrast and emits a high-dose of radiation, which may be contraindicated in some patient populations (eg, patients with renal failure, allergy to contrast, and pregnant and pediatric patients). Also, to avoid degradation of images and accurately visualize peripheral branches, patient cooperation (ie, suspension of respiratory motion) during the examination is essential.
Ventilation Perfusion Scintigraphy
Before the advent of CTA, V/Q lung scan was the first-line imaging choice to assess for PE.3 This modality continues to have a role in modern practice, especially given its estimated 6-fold lower whole-body effective radiation dose compared to CTA.4 Current applications of this modality include patients with contraindications to IV contrast as well as pregnant and pediatric patients in whom perfusion-only studies should be used to significantly lower the radiation profile.
In patients with a clear chest X-ray, the negative predictive value of V/Q is not significantly different from CT.5 This suggests a growing role for V/Q scans in the younger, healthier patient population where limited/reduced exposure to radiation is particularly desired—eg, pediatric patients and women younger than age 20 years in whom there is an increased risk of malignancy associated with radiation exposure to the breast/chest area.6
The limitations of V/Q lung scan include a higher rate of indeterminate results compared to CTA (particularly in patients with an abnormal chest X-ray). Moreover, V/Q does not provide alternative diagnoses or qualify disease severity. Also, the longer imaging times compared with CTA make this modality impractical in critically ill patients.
Magnetic Resonance Angiography
MRA has emerged in recent years as a potential alternative to CTA. Since this modality does not use ionizing radiation or iodine-based contrast, it is a reasonable and appropriate option for pediatric patients and those with a contrast-dye allergy. Initial meta-analysis of MRA studies demonstrated sensitivity ranging from 77% to 100% and a specificity of 95% to 98%.7-9 However, subsequent studies have found unacceptably high rates of technically inadequate examinations resulting in nondiagnostic results.10 As progress to reduce respiratory and cardiac motion artifacts and improve spatial resolution continues, along with the growing availability of MRA in the ED, this modality may soon emerge as a useful diagnostic option.
Conclusion
New advances in the diagnosis of PE continue to emerge. Evidence suggests improved sensitivity of CTA with the use of dual-energy CT scanners.11 The recent advance of V/Q single photon emission CT has also shown promise in improving detection accuracy.12 Ongoing research will help continue to expand the emergency physician’s range of imaging choices in the diagnosis of PE.
References
1. Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet. 2012;379(9828):1835-1846.
2. Burns SK, Haramati LB. Diagnostic imaging and risk stratification of patients with acute pulmonary embolism. Cardiol Rev. 2012;20(1):15-24.
3. Mos IC, Klok FA, Kroft LJ, de Roos A, Huisman MV. Imaging tests in the diagnosis of pulmonary embolism. Semin Respir Crit Care Med. 2012;33(2):138-143.
4. Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248(1):254-263.
5. Stein EG, Haramati LB, Chamarthy M, Sprayregen S, Davitt MM, Freeman LM. Success of a safe and simple algorithm to reduce use of CT pulmonary angiography in the emergency department. Am J Roentgenol. 2010;194(2):392-397.
6. Hill DA, Preston-Martin S, Ross RK, Bernstein L. Medical radiation, family history of cancer, and benign breast disease in relation to breast cancer risk in young women, USA. Cancer Causes Control. 2002;13(8):711-718.
7. Oudkerk M, van Beek EJ, Wielopolski P, et al. Comparison of contrast-enhanced magnetic resonance angiography and conventional pulmonary angiography for the diagnosis of pulmonary embolism: a prospective study. Lancet. 2002;359(9318):1643-1647.
8. Meaney JF, Weg JG, Chenevert TL, Stafford-Johnson D, Hamilton BH, Prince MR. Diagnosis of pulmonary embolism with magnetic resonance angiography. N Engl J Med 1997;336(20):1422-1427.
9. Gupta A, Frazer CK, Ferguson JM, et al. Acute pulmonary embolism: diagnosis with MR angiography. Radiology. 1999;210(2):353-359.
10. Stein PD, Chenevert TL, Fowler SE. Gadolinium-enhanced magnetic resonance angiography for pulmonary embolism: a multicenter prospective study (PIOPED III). Ann Intern Med. 2010;152(7):434-443, W142, W143.
11. Pontana F, Faivre JB, Remy-Jardin M. Lung perfusion with dual-energy multidetector-row CT (MDCT): feasibility for the evaluation of acute pulmonary embolism in 117 consecutive patients. Acad Radiol. 2008;15(12):1494-1504.
12. Gutte H, Mortensen J, Jensen CV, et al. Comparison of V/Q SPECT and planar V/Q lung scintigraphy in diagnosing acute pulmonary embolism. Nucl Med Commun. 2010;31(1):82-86.
Just MoRe Imaging?
MRI Evaluation of Acute Abdominal Pain
David A. Boyajian, MD
Dr Boyajian is clinical director of radiology at New York-Presbyterian/Lower Manhattan Hospital, and vice chairman and assistant professor of radiology, Department of Radiology, Weill Cornell Medical College, New York.
For the past few decades, the established cross-sectional imaging modalities used to evaluate acute abdominal pain in the ED have been computed tomography (CT) and ultrasound. Advantages of these modalities include the widespread availability of equipment and qualified technologists and radiologists, the ability to obtain images quickly, and relative cost-effectiveness. There are, however, several subsets of indications for which traditional cross-sectional imaging approaches are either undesirable or have a low sensitivity for accuracy (eg, suspected nonobstetric pathology in the pregnant patient, biliary ductal pathology).
Magnetic resonance imaging (MRI) has become more widely available to ED practices across the country and an increasingly important tool in the arsenal of the emergency radiologist. Advantages of MRI include better soft-tissue characterization, ability to directly image in any plane, and the lack of ionizing radiation.
There are, however, barriers to using MRI in the emergency setting, including availability and location of the MRI scanners, long examination time, difficulty in monitoring unstable patients, and higher cost.1 In addition, relative to CT and ultrasound, there is poorer spatial resolution and greater potential for artifacts. Advances in MRI technology and workflow, including rapidly acquired imaging sequences and active radiologist management of such cases, can significantly reduce examination acquisition time and improve image quality. Due to the significant advantages in visualizing the brain and spine, MRI has become the gold standard in many emergent conditions such as suspected stroke and spinal cord compression. Increasingly, MRI is being used to diagnosis causes of abdominal pain, examples of such are discussed below.
Appendicitis in Pregnancy
Although acute appendicitis is the most common nonobstetric surgical condition during pregnancy, several factors such as physiological leukocytosis and appendiceal displacement (which reduces the sensitivity of ultrasound) confound the diagnosis. Due to the radiation conveyed by CT, MRI is considered a more appropriate imaging modality in this patient population.2 In a retrospective study of 23,290 pregnant patients, Pedrosa et al3 reported a sensitivity of 100%, specificity of 93.6%, and accuracy of 94% in detecting acute appendicitis in patients for whom ultrasound was inconclusive.
Biliary Pathology
Biliary pathology, particularly choledocholithiasis, may not be demonstrated to advantage with cross-sectional imaging. For example, CT sensitivity for detection is decreased in the absence of ductal dilatation and/or in poorly mineralized stones. Technical factors, such as obesity, patient positioning, and bowel gas, limit evaluation with ultrasound. MRI is able to depict both the intrahepatic and extrahepatic biliary ducts and identify any intraductal lesions (eg, masses, stones). Special MRI contrast agents that are excreted through the bile ducts have come to market in recent years and can assist in evaluation for biliary pathology. The sequences have replaced many of the diagnostic endoscopic retrograde cholangiopancreatograms (ERCP) that used to be performed. An advantage of magnetic resonance cholangiopancreatography (MRCP) is that structures outside of the biliary system may also be evaluated (eg, liver, kidneys).
|
|
Conclusion
The use of MRI for evaluation of abdominal pain continues to increase. While currently limited to specific vulnerable populations (eg, pregnant patients) or for specific conditions (eg, biliary disease), improving technology and availability will allow this technique to expand to more patients and conditions, including evaluation for inflammatory bowel conditions.
References
1. Saini S, Seltzer SE, Bramson RT, et al. Technical cost of radiologic examinations: Analysis across imaging modalities. Radiology. 2000; 216(1):269-272.
2. Appropriateness criteria. American College of Radiology Web site. http://www.acr.org/Quality-Safety/Appropriateness-Criteria. Accessed September 17, 2013.
3. Pedrosa I, Levine D, Eyvazzadeh AD, Siewert B, Ngo L, Rofsky NM. MR imaging evaluation of acute appendicitis in pregnancy. Radiology. 2006;238(3):891-899.
When a Picture Is Worth a Thousand Images:
3D Reconstruction in Emergency and Trauma Imaging
Jamlik-Omari Johnson, MD; Waqas Shuaib, MD
Dr Johnson is assistant professor of radiology and division director of radiology in the department of radiology and imaging services, division of emergency medicine at Emory University School of Medicine, Atlanta, Georgia. Dr Shuaib is a research associate in the department of radiology and imaging services, division of emergency medicine at Emory University School of Medicine, Atlanta, Georgia.
Three-dimensional (3D) reconstruction creates a digital image of a real-life object, capturing both its original shape and appearance. These techniques simulate reality through the use of lighting, color, and motion.1 Advantages of 3D reconstruction in the emergency and trauma setting include enhanced views of anatomy, making pathology that may be difficult to see in the axial plane easily visible (eg, rib fractures). 3D images may also increase interpretation efficiency, allowing rapid review of the large data sets associated with multidetector computed tomography (MDCT) of trauma patients. Since these images can be attached to the radiology report, this modality also enhances service to referring physicians. In addition, as 3D images succinctly illustrate a diagnosis, the treating physician may share them with patients to demonstrate a condition and explain treatment recommendations.
MDCT technology evolved rapidly over the past 25 years. Between 1992 and 2004, MDCT made quantum leaps from dual detectors to 64-slice scanners, and over the last decade, dual-energy source technology and 128-slice scanners have become integrated into clinical practice. These newer devices, along with technical enhancements, provide high temporal and superior spatial resolution, considerably improved image quality, and expanded clinical applications. With MDCT, scans are performed quickly, resulting in improved temporal resolution and reduced motion artifacts. Leverage of this technology in the emergency and trauma setting has expanded the spectrum of indications for use and increased the utility of CT in urgent care. Newer algorithms allow rapid and detailed examinations of the musculoskeletal and the vascular systems in submillimeter slices, without limitation of scan volume. The high-resolution source images are the basis for high quality 3D reconstructions.
The 3D reconstruction toolbox may benefit the surveillance of anatomy, detection of pathology, and planning of therapy. Examples of three such commonly used techniques are shaded surface display (SSD), maximum intensity projection (MIP), and volume rendering (VR).2
SSD provides a realistic 3D view of the surface of a structure within the acquired volume data set and is good at depicting aneurysms and other focal disease (Figure 1). This is particularly useful for evaluating long and tortuous structures, such as the arterial and venous structures. MIP is often used to detect lung nodules by making them stand-out from bronchi and vasculature (Figure 1). Adapted from the computer graphics field, VR creates a 2D image from a 3D model. VR is employed in the exploration of hollow structures, such as the airways or the colon (Figure 2).
Emergency and trauma environments are often fast-paced and high-stakes, and timely and accurate diagnosis is paramount. Therefore, to be useful, advanced 3D-processing tools should be embedded into the diagnostic viewing application (eg, PACS) to allow efficient reconstruction of images, simultaneous comparison of 2D and 3D images, and referencing of historical data in real time.
References
1. Blank M, Kalender WA. Medical volume exploration: gaining insights virtually. Eur J Radiol. 2000;33(3):161-169.
2. Philipp MO, Kubin K, Mang T, Hörmann M, Metz VM. Three-dimensional volume rendering of multidetector-row CT data: applicable for emergency radiology. Eur J Radiol. 2003;48(1):33-38.
After Imaging: Interventional Radiology in the ED
Joshua L. Weintraub, MD, and Thomas J. Ward, MD
Dr Weintraub is an interventional radiologist and executive vice chairman of the department of radiology
at New York Presbyterian/Columbia University Medical Center, New York. Dr Ward is a postgraduate fifth-year resident in interventional radiologist in the department of radiology at Mount Sinai Medical Center, New York, NY.
Interventional radiologists play an increasing role in treating patients in the ED setting. Simplistically, these roles are usually divided into one of three broad categories: to drain or aspirate infected fluid, to halt active bleeding, or to restore blood flow through an occluded vessel. While all of these procedures are critical, the newest advance in interventional radiology (IR) set to benefit the ED patient is not technical or procedural at all, but rather a philosophical one.
Fluid Aspiration and Drainage
In the ED, the need to drain or aspirate infected fluid is probably the most common indication for consult with an interventional radiologist. Perforated appendicitis or diverticulitis with an intra-abdominal abscess is generally managed with percutaneous drainage and antibiotics. If the clinical situation necessitates, and the location of the collection allows, this procedure may be performed at bedside with local anesthesia under ultrasound guidance. In the septic patient, the minimally invasive nature of this procedure confers significant benefits.
Nonoperative patients presenting with acute cholecystitis rapidly improve after the placement of a percutaneous cholecystostomy tube, and this procedure can be performed under ultrasound guidance. IR may also be indicated in cases of failed endoscopic retrograde cholangiopancreatography or cystoscopy in patients with cholangitis or urosepsis for benign or malignant obstruction. Percutaneous access is facilitated by a dilated system, and temporary decompression can be obtained until more definitive therapy is planned.
Active Bleeding
Active bleeding is another common presentation warranting an IR evaluation. Gastrointestinal, intracranial, posttraumatic, postsurgical, and postpartum bleeding, as well as massive hemoptysis, can all be managed endovascularly. Advances in microcatheter technology, covered vascular stents, and embolic agents have increased the efficacy of these interventions, and improved computed tomography angiography protocols facilitate accurate and timely diagnosis of active bleeding. With the availability of these techniques, waiting several hours for a tagged red blood cell nuclear scan is a thing of the past at many institutions. Embolization has become the mainstay of treating bleeding related to trauma in most major trauma centers.
Ischemia
An IR consult may be ordered for patients presenting with the sequelae of ischemia, which can range from a diabetic foot to an acute stroke. Percutaneous balloon angioplasty, endovascular stents, and catheter-directed thrombolysis were all monumental advances in the treatment of ischemia—conceptualized or introduced into clinical practice by interventional radiologists. This spirit of innovation continues. A variety of technical advances, atherectomy, and re-entry devices have been introduced to help recanalize chronically occluded vessels. New devices allow the interventional radiologist to quickly restore blood flow and function to patients suffering from cerebral embolus. There is increased interest in the use of catheter-assisted-embolectomy for submassive pulmonary embolism when intravenous fibrinolysis is unsuccessful or contraindicated.
Conclusion
The five decades of pioneering technical innovation highlighted in this article allow for minimally invasive treatment of the ED patient. The newest advance in IR is not technical, but rather philosophical, and a change in the role that the interventional radiologist plays. The American Board of Radiology has recently approved a new IR training pathway that more than doubles the amount of clinical training that graduating IR fellows now receive. This signals a renewed commitment to running a truly clinical service. Patients in the ED can be evaluated and treated by an interventional radiologist in the hospital and then discharged under the care of an interventional radiologist at an IR clinic. Several IR sections across the country currently practice in such a manner. Although this model is currently the exception and not the rule, the goal of the new training pathway hopes to change this, with increased advances and benefits to patients in the ED setting.
Suggested Reading
1. Gasior AC, Marty Knott E, Ostlie DJ, St Peter SD. To drain or not to drain: an analysis of abscess drains in the treatment of appendicitis with abscess. Pediatr Surg Int. 2013;29(5):455-458.
2. Sato KT. Percutaneous management of biliary emergencies. Semin Intervent Radiol. 2006;23(3):
249-257.
3. Funaki B. On-call treatment of acute gastrointestinal hemorrhage. Intervent Radiol. 2006;23(3):
215-222.
4. Yoon W, Kim JK, Kim YH, Chung TW, Kang HK. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics. 2002;22(6):1395-1409.
5. Stead LG, Gilmore RM, Bellolio MF, Rabinstein AA, Decker WW. Percutaneous clot removal devices in acute ischemic stroke: a systematic review and meta-analysis. Arch Neurol. 2008;65(8):
1024-1030.
6. Kucher N. Catheter embolectomy for acute pulmonary embolism. Chest. 2007;132(2):657-663.
7. Initial certification: vascular/interventional. American Board of Radiology Web site. http://www.theabr.org/ic-vir-landing. Accessed September 24, 2013.
Figure 2. Surface shaded display image of a virtual colonoscopy.
Evaluation of Chest Pain in the Emergency Department by Coronary CT Angiography
James K. Min, MD
Dr Min is director of the Institute of Cardiovascular Imaging at New York-Presbyterian Hospital/Weill-Cornell Medical College, New York.
In the United States each year, approximately 6 million patients present with complaints of chest pain suspicious for acute coronary syndromes (ACS), including unstable angina and myocardial infarction.1 Most of these patients are diagnosed with noncardiac conditions, and nearly half are due to noncardiac etiology. There are an array of diagnostic tests to identify and exclude patients with suspected ACS, such as medical history, cardiac enzyme measurements, electrocardiographic changes, and clinical risk scores. For those with nonnegligible risk for ACS for which this condition cannot be definitively diagnosed or excluded, many are often admitted to the hospital for further testing and observation. Among these patients, less than 30% are found to have ACS. And yet, despite these very careful clinical pathways, between 2% and 8% of patients with ACS are unknowingly discharged to home.2
In recent years, coronary computed tomography angiography (CCTA) has emerged as a noninvasive method that permits direct anatomic visualization of coronary atherosclerosis and luminal stenosis.3 Since the introduction of 64-multidetector row CT scanners in 2005, there have been significant advances in CT technology that now allow for reliable performance of CCTA with very low-dose radiation. Pertaining to the former, improvements in spatial resolution, temporal resolution, and volume coverage enable evaluation of coronary arteries at the submillimeter level, and can be performed in approximately 1 to 5 seconds. Concomitant to the progress in CT technology has been the parallel developments in radiation-dose reduction. As compared to the background radiation exposure of an individual living at sea level for 1 year (~3 millisieverts of radiation), current generation CCTA can be performed at doses <1 millisieverts, with doses approximating a screening mammogram now achievable.
|
|
| ||
Figure. Volume-rendered coronary computed tomographic angiography showing the coronary vascular bed and myocardium (A); normal right coronary artery without evidence of atherosclerosis (B); and (C) left anterior descending artery with mild nonobstructive calcified plaque (white area). | ||||
The diagnostic accuracy of CCTA against invasive coronary angiography has been tested in several prospective multicenter trials.3 For patients without known but suspected coronary artery disease (CAD), the sensitivity and negative predictive value has ranged between 95% to 99%—that is, CCTA can exclude anatomically obstructive CAD with near 100% certainty (Figure). It is these diagnostic performance characteristics that have encouraged several investigators to evaluate the use of CCTA in the diagnostic algorithm of patients presenting to the ED with acute chest pain—the primary intent being to identify sufficiently low-risk patients without significant CAD who can be safely discharged home.
Since 2011, three prospective multicenter randomized controlled trials have evaluated the incorporation of CCTA into a diagnostic chest pain pathway, as compared to standard-of-care algorithms.4-6 Now comprising more than 3,000 patients, these trials have demonstrated remarkably consistent results, with a reduced time-to-diagnosis of 40% to 50%, reduced lengths of stay in the ED of 25%, and reduced ED costs of 20% to 40%. Importantly, these salient effects on resource utilization and economics were underscored by exceptionally safe outcomes, which never exceeded that of the standard of care. Several studies to date have subsequently assessed the duration of safety conferred by a normal CCTA, or its “warranty period.” These studies have shown the warranty period to last at least 7 years for major adverse cardiac events and mortality.7 Moreover, they also engender hope that the chest pain pathways used for millions of Americans annually can indeed be improved, with CCTA playing an essential role.
As its technology continues to iterate, the diagnostic and prognostic performance of CCTA will invariably continue to improve. However, even at present, CCTA is robust and more accurate for exclusion of CAD when compared to other traditional methods of evaluation, and its use for patients presenting to the ED with chest pain improves throughput, reduces costs, and maximizes patient safety.
References
1. Pitts SR, Niska RW, Xu J, Burt CW. National Hospital Ambulatory Medical Care Surviva: 2006 emergency department summary. Natl Health Stat Report. 2008;7:1-38.
2. Pope JH, Aufderheidi TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342(16):1163-1170.
3. Min JK, Shaw LJ, Berman DS. The present state of coronary computed tomography angiography: a process in evolution. J Am Coll Cardiol. 2010;55(:957-965.
4. Litt HI, Gatsonis C, Snyder B, et al. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med. 2012;366(15):1393-403.
5. Hoffmann U, Truong QA, Schoenfeld DA, et al. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med. 2012;367(4):299-308.
6. Goldstein JA, Chinnaiyan KM, Abidov A, et al. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol. 2011;58(14):1414-1422.
7. Andreini D, Pontone G, Mushtaq S, et al. A long-term prognostic value of coronary CT angiography in suspected coronary artery disease. JACC Cardiovasc Imaging. 201
As EDs have evolved to handle the increasing volume and complexity of patients requiring immediate care, so too, has the field of emergency radiology. Many EDs across the country now have multiple advanced imaging modalities available 24 hours a day, including Xray, computed tomography (CT), ultrasound, and magnetic resonance imaging (MRI). While many emergency medicine physicians are now trained in performing and evaluating ultrasound images—similar to X-rays in the past—most are less comfortable with CT and MRI.
Emergency radiology, now a recognized subspecialty of diagnostic imaging, has proliferated to meet the demands for immediate interpretation of these images. This combination of around-the-clock access to equipment and expertise has brought cutting-edge, advanced imaging to the front lines of emergency care. In this special feature, we invited a group of emergency radiologists and an expert in cardiovascular imaging to discuss the applicability and utility of several of these techniques in the ED setting.
As illustrated by this panel, advanced imaging has become increasingly valuable and available in providing care to ED patients. In this presentation, however, you will notice the conspicuous absence of specific techniques that are utilized in diagnosis and evaluation of stroke, head/spine trauma, and other neurologic conditions that are commonplace in the ED. Advanced imaging has become critical in these instances and will be discussed in a future article.
Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and the executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center. He is a member of the EMER GENCY MEDICINE editorial board.
Faster than FAST: Single Pass Whole-Body Computed Tomography for Rapid Evaluation of Trauma Patients
Ashwin Asrani, MD
Dr Asrani is an assistant professor of radiology at Weill Cornell Medical College in New York City and an
assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center in New York City.
As part of initial assessment and resuscitation in the ED, severely injured trauma patients frequently undergo a bedside focused abdominal sonography for trauma (FAST) scan to assess for intraperitoneal hemorrhage from an underlying major abdominal visceral injury, as well as the presence of pleural and/or pericardial effusion. However, the use and role of a FAST scan in hemodynamically stable patients has recently been questioned since, based on its relatively low sensitivity for visceral injury, many of these cases eventually require computed tomography (CT) (See Case). In addition, patients in this subset with a positive FAST scan frequently have subsequent CT to further assist clinicians in understanding the nature of injury and to guide management recommendations (eg, operative versus nonoperative options).1
Advances in the speed of CT have made it possible to obtain images of the entire body with only a single dose of contrast injection. The elimination of segmental acquisition of torso images (eg, head, neck, chest, abdomen) eliminates the need to reposition the patient, repeat scout images, and postpone the reconstruction of reformats and other special views until the end of the examination. Depending on the original protocol, this in turn minimizes patient time in the CT-scan room by approximately 31% to 42%,2,3 and significantly reduces radiation exposure by 17% compared to conventional segmental acquisition of different body parts.2
Evidence suggests that direct single-pass whole-body CT improves outcomes in both hemodynamically stable and unstable patients and can reveal significant findings not apparent on initial clinical examination.4,5 Whole-body CT increases injury severity by detecting lesions that would not otherwise have been detected by conventional methods. While this information does not affect treatment options, it does artificially lower the ratio of observed-to-expected deaths.6 The Randomized Study of Early Assessment by CT Scanning in Trauma Patients (REACT-2), a recent international multicenter randomized clinical trial, is expected to provide evidence supporting the value of immediate total-body CT scanning during the primary evaluation of severely injured trauma patients; the results of this trial should become available in 2014.7
As EDs install and upgrade to newer multidetector CT scanners, single-pass whole-body scanning has the potential to save time in situations when it really matters.
References
1. Natarajan B, Gupta PK, Cemaj S, Sorensen M, Hatzoudis GI, Forse RA. FAST scan: Is it worth doing in hemodynamically stable blunt trauma patients? Surgery. 2010;148(4):695-700; discussion 700-701.
2. Fanucci E, Fiaschetti V, Rotili A, Floris R, Simonetti G. Whole body 16-row multislice CT in emergency room: Effects of different protocols on scanning time, image quality and radiation exposure. Emerg Radiol. 2007;13(5):251-257.
3. Nguyen D, Platon A, Shanmuganathan K, Mirvis SE, Becker CD, Poletti PA. Evaluation of a single-pass continuous whole-body 16-MDCT protocol for patients with polytrauma. AJR Am J Roentgenol. 2009;192(1):3-10.
4. Huber-Wagner S, Biberthaler P, Haberle S, et al. Whole-body CT in haemodynamically unstable severely injured patients – A retrospective, multicentre study. PLoS One. 2013;8(7):e68880.
5. Salim A, Sangthong B, Martin M, Brown C, Plurad D, Demetriades D. Whole body imaging in blunt multisystem trauma patients without obvious signs of injury: Results of a prospective study. Arch Surg. 2006;141(5):468-473; discussion 473-475.
6. Stengel D, Frank M, Matthes G, et al. Primary pan-computed tomography for blunt multiple trauma: Can the whole be better than its parts? Injury. 2009;40 Suppl 4:S36-S46.
7. Sierink JC, Saltzherr TP, Beenen LF, et al. A multicenter, randomized controlled trial of immediate total-body CT scanning in trauma patients (REACT-2). BMC Emerg Med. 2012;12:4-227X-12-4.
Obstructed Views: Imaging in Urinary Tract Obstruction
Lily M. Belfi, MD
Dr Belfi is an assistant professor of radiology at Weill Cornell Medical College in New York City and an assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center.
Urinary tract (UT) obstruction is one of the most common indications for genitourinary (GU) imaging in the ED. Until recently, diagnosis was made using intravenous (IV) pyelography. Today, improved imaging modalities, such as noncontrast computed tomography (CT), computed tomography urography (CTU), magnetic resonance urography (MRU), and magnetic resonance imaging (MRI), are available to better visualize and evaluate the underlying causes of obstruction.
Noncontrast Computed Tomography
|
|
Noncontrast CT of the abdomen and pelvis is the imaging study of choice in evaluating acute GU obstruction, particularly when there is clinical suspicion of stone disease. CT is extremely sensitive and specific in detecting renal calculi and provides information about stone burden, size, location, and composition. In addition to renal calculi, noncontrast CT is also useful in identifying other, less common causes of UT obstruction such as ureteral herniation, which can occur in the inguinal, femoral, or sciatic region and result in acute obstruction. Patients with neuromuscular disorders that cause piriformis muscle atrophy (eg, multiple sclerosis) may be predisposed to ureteral sciatic herniation. CT reconstructions in the coronal plane are useful in detecting this condition (Figure 1).
|
|
Reconstructed CT images can also reveal underlying congenital abnormalities that make a patient susceptible to obstruction. For example, CT can reveal the presence of a duplicated collecting system, which is often accompanied by an obstructing ureterocele associated with the upper pole moiety (Figure 2).
Computed Tomography Urography
|
| ||
| Figure 3. Computed tomography urography images (A and B) reveal "fish-hook" ureter (white arrows) classically seen in patients with with retrocaval ureter. | |||
Other types of congenital anomalies may require additional imaging with CTU, in which IV iodinated contrast media is administered for increased sensitivity and visualization. For instance, patients with a retrocaval ureter often present with GU obstruction and hematuria; the classic “fish-hook” or “sickle-shaped” deformity of the ureter characterizing this disease is best visualized on CTU as the ureter is well-opacified by excreted contrast (Figure 3).
Magnetic Resonance Urography and Magnetic Resonance Imaging
MRU and MRI are alternative modalities to CTU for patients in whom iodinated CT contrast is contraindicated or in cases where the avoidance of radiation exposure is indicated (eg, pregnant and pediatric patients). MRI and MRU are especially useful in further characterizing obstructive lesions in the GU tract. While initial noncontrast CT may suggest soft-tissue lesions within the ureter or urinary bladder, follow-up MRI clearly delineates the condition, helping clinicians quantify the extent of tumor involvement and determine disease stage (Figure 4). Pelvic MRI is also an essential tool in identifying causes of bladder outlet obstruction, especially in those involving the prostate gland (Figure 5).
|
|
|
|
|
Figure 4. Noncontrast pelvic computed tomography images (A and B) of a 72-year-old man with flank pain and difficulty voiding reveal hydronephrosis (white arrow) and bladder mass (red arrow). Magnetic resonance urography (C) further visualizes the bladder mass (red arrow), and postcontrast magnetic resonance images (D and E) show the extent of the tumor (red arrow) as well as a metastatic lesion (white arrow).
| ||||
Conclusion
|
|
| |||
| Figure 5. Noncontrast pelvic computed tomography images (A and B) of a 73-yearold man with pelvic pain demonstrate bilateral hydronephrosis. (white arrows) and a large mass in the expected location of the prostate (red arrow). Further evaluation of the large heterogenous mass with pelvic magnetic resonance imaging (C) reveals prostate carcinoma (red arrows). | |||||
Based on its sensitivity in detecting both common and uncommon causes of obstruction, noncontrast abdominal and pelvic CT is an excellent first-line imaging choice for evaluating patients presenting to the ED with UT obstruction. Moreover, noncontrast CT also helps guide clinicians in determining which, if any, additional studies with CTU, MRU, and/or MRI are warranted.
Sound Advice: Ultrasound in the Emergency Department
Kemi Babagbemi, MD
Dr Babagbemi is an assistant professor of radiology and assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical College, New York.
In today’s evolving health-care environment, emphasis must be placed on providing accurate, safe, cost-effective, and timely diagnosis to the wide range of patients that present to the ED. To meet this need, emergency medicine physicians and radiologists have universally embraced the use of ultrasound in this setting.
Ultrasound is less expensive than computed tomography (CT) and magnetic resonance imaging (MRI), and its portability allows for bedside application, enabling its use in the most critical patients. It does not require extended preparation (eg, oral contrast) or carry the risk for adverse reactions associated with intravenously administered contrast. Also, since ultrasound does not use ionizing radiation, it is a particularly appropriate imaging option in susceptible populations such as pregnant and pediatric patients.1 Although a noninvasive modality, it can also be used to guide interventional procedures, including vascular access, thoracentesis/paracentesis, and specialized anesthesia.
Emergency physicians routinely perform ultrasound at bedside and are familiar with its utility in evaluating abdominal trauma, right upper quadrant pain, and acute biliary abnormalities; assessing vascular thrombosis or injury; and determining the etiology of pelvic pain and bleeding in both pregnant and nonpregnant patients. However, ultrasound is also increasingly being utilized to
diagnose other conditions for which CT and MRI were once thought the superior diagnostic tool.
Trauma (The Extended FAST Scan)
The focused abdominal sonography for trauma (FAST) scan has been used for rapid and immediate assessment of unstable trauma patients with suspected abdominal injury. In a recent study of 4,029 patients with blunt abdominal trauma, FAST scans had a sensitivity, specificity, and accuracy for detection of hemorrhage in patients with hypotension of 85%, 60%, and 77%, respectively.2 Other studies suggest that sonography of the chest may be useful in the rapid detection of additional life-threatening pathology such as pneumothorax (Figure 1), with data suggesting improved sensitivity over radiography.3 The inclusion of the pleural space in evaluation is increasingly more common and is referred to as the extended FAST (EFAST).
Appendicitis
The most common cause of abdominal pain requiring surgical intervention is appendicitis.4 Although certain clinically based prediction scores (eg, Alvarado scores) may be used, imaging is considered far superior in accurately diagnosing the condition.5 In a meta-analysis of data from 26 ultrasound and CT studies (15 prospective, 11 retrospective), there was a pooled 88% sensitivity and 94% specificity for ultrasound compared with CT, which exhibited a pooled sensitivity of 94% and specificity of 95%.6 As previously noted, because there is no ionizing radiation in ultrasound, it should be the preferred modality in both children and first-trimester pregnant patients.
Musculoskeletal Trauma
Ultrasound is an ideal imaging modality to evaluate the musculoskeletal system. Despite its widespread use in Europe for many years, musculoskeletal sonography is only now beginning to be adopted in the United States. Its ability to visualize soft-tissue structures makes it effective in evaluating for muscle, tendon, or ligament injury, and can even do so dynamically with stress maneuvers (Figure 2). With respect to fractures, sonography has also proved effective in evaluating for cortical disruption. For example, a recent study demonstrated overall 92% sensitivity and 100% sensitivity for fractures with high potential for complication in radiographically occult scaphoid fractures.7
Conclusion
As a true point-of-care imaging modality, ultrasound has an established place in the practice of emergency medicine. Future improvements in technology, including the ability to obtain true three-dimensional volumetric data sets, will expand its role even further.
References
1. Image Gently and Ultrasound. Image Gently Campaign. The Alliance for Radiation Safety in Pediatric Imaging Web site. http://www.pedrad.org/associations/5364/ig/?page=787. Accessed September 19, 2013.
2. Lee BC, Ormsby EL, McGahan JP, Melendres GM, Richards JR. The utility of sonography for the triage of blunt abdominal trauma patients to exploratory laparotomy. AJR Am J Roentgenol. 2007;188(2):415-421.
3. Nandipati KC, Allamaneni S, Kakarla, et al. Extended focused assessment with sonography for trauma (EFAST) in the diagnosis of pneumothorax: experience at a community based level I trauma center. Injury. 2011;42(5):511-514.
4. Addiss DG, Shaffer N, Fowler BS, Tauxe RV. The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol. 1990;132(5):910-925.
5. Sun JS, Noh HW, Min YG, et al. Receiver operating characteristic analysis of the diagnostic performance of a computed tomographic examination and the Alvarado score for diagnosing acute appendicitis: emphasis on age and sex of the patients.
J Comput Assist Tomogr. 2008;32(3):386-391.
6. Doria AS, Moineddin R, Kellenberger CJ, et al. US or CT for Diagnosis of Appendicitis in Children and Adults? A MetaAnalysis. Radiology. 2006;241(1):83-94.
7. Platon A, Poletti PA, Van Aaken J, Fusetti C, Della Santa D, Beaulieu JY, Becker CD. Occult fractures of the scaphoid: the role of Ultrasonography in the emergency department. Skeletal Radiol. 2011;40(7):869-875.
Stuck or Not? Noninvasive Vascular Imaging in the Emergency Setting
Michael L. Loftus, MD
Dr Loftus is assistant professor of radiology at New York-Presbyterian Hospital/Weill Cornell Medical College, New York.
Conventional catheter-directed angiography has played an important role in the history of ED imaging, providing timely information about vessel integrity throughout the body and guiding potentially life-saving interventions. However, this imaging modality carries significant potential risks, including puncture-site hematoma or pseudoaneurysm, catheter-induced vasospasm, vascular occlusion or dissection, anesthesia-associated risks, and neurological deterioration or stroke.1 Moreover, emergent angiography is not universally available in all EDs.
Computed tomography angiography (CTA) and magnetic resonance angiography (MRA) have opened new windows of opportunity for noninvasive vascular imaging to play a role in clinical decision-making in the ED. Cross-sectional angiography has virtually eliminated the need for acute catheter-directed angiography in several clinical settings, including pulmonary angiography, and the clinical applicability of CTA and MRA continues to expand as imaging techniques improve and achieve widespread acceptance and implementation. Multidetector CT is now widely available in most EDs, and the
accessibility of magnetic resonance imaging and MRA is expanding rapidly.
CTA allows evaluation of the vasculature on contrast-enhanced axial source images and also utilizes computer-generated maximum intensity projection reformations to create diagnostic images of the vascular region of interest. Similarly, MRA can be performed either with or without the administration of an intravenous gadolinium contrast agent, and provides additional information about directionality of flow, as well as detailed images of the surrounding soft tissues.
An emerging clinical situation in which contrast-enhanced cross-sectional imaging may supplant the need for arterial puncture and digital subtraction angiography is multiligament trauma to the knee. When significant kinetic force is applied to the knee, the joint is at risk for translocation and/or dislocation, with resultant injury to the surrounding soft-tissue envelope and potential trauma to the neurovascular structures around the knee. In this type of injury, cross-sectional imaging is routinely ordered to further evaluate and classify trauma, revealing potentially treatment-altering information concerning the integrity of the vascular structures with minimal risk to the patient (Figure 1).
The largest series of MRAs performed specifically in patients with knee dislocation reviewed 17 cases and found two cases of vascular pathology: one case of an intimal flap and one case of acute vasospasm. Digital subtraction angiography was performed on 6 of 17 cases and had 100% concordance with MRA findings.2 MRI is often performed in the setting of suspected multiligament knee injury to aid in preoperative planning, and the addition of MRA should be considered if trauma to the periarticular vasculature is suspected.
There is ample evidence that cross-sectional imaging performs well relative to conventional catheter angiography in the setting of peripheral vascular occlusion from atherosclerotic etiologies.3 Furthermore, there is precedence for utilizing contrast-enhanced CTA, as well as contrast-enhanced or three-dimensional time-of-flight MRA in other areas of the body—particularly the brain, head, and neck4 (Figure 2). Several MRA techniques are becoming available that will allow a high resolution angiographic without the use of contrast. Upcoming advances in CTA include dual-energy CT, which has the potential to allow angiography using very small amounts of contrast.
In general, when ordering cross-sectional imaging in the setting of trauma, consideration should be given to the potential for vascular compromise; thus, the addition of contrast-enhanced CTA or MRA can add valuable clinical information, with relatively little excess risk or time, sparing patients the risks of catheter-directed angiography.
References
1. Eisenberg, RL, Bank, WO, Hedgcock, MW. Neurologic complications of angiography for cerebrovascular disease. Neurology. 1980;30(8):895-897.
2. Potter HG, Weinstein M, Allen AA, Wickiewicz TL, Helfet DL. Magnetic resonance imaging of the multiple-ligament injured knee. J Orthop Trauma. 2002;16(5);330-339.
3. Chin AS, Rubin GD. CT angiography of peripheral arterial occlusive disease. Tech Vasc Interv Radiol. 2006;9(4):143-149.
4. Riles TS, Eidelman EM, Litt AW, Pinto RS, Oldford F, Schwartzenberg GW. Comparison of magnetic resonance angiography, conventional angiography, and duplex scanning. Stroke. 1992;23(3):341-346.
What’s Hip in 2013?
Roger J. Bartolotta, MD
Dr Bartolotta is an assistant professor of radiology at Weill Cornell Medical College in New York City and assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center.
Post-traumatic hip pain is a common chief concern among ED patients. While routine radiography, the standard imaging modality for the initial evaluation of suspected hip fracture, detects most fractures, its sensitivity is decreased in the setting of osteoporosis—particularly with nondisplaced fractures. Thus, though elderly/osteoporotic patients are more likely to fracture, these fractures are harder to detect on radiography. In a study of 70 patients with negative radiographs but high clinical concern for fracture, magnetic resonance imaging (MRI) detected occult femoral fractures in 37% and occult pelvic fractures in 23%.1
Hip fractures in elderly patients are associated with substantial mortality and morbidity, the risks for which increase with delayed diagnosis.2 When there is high clinical suspicion for a radiographically occult hip fracture in this population, cross-sectional imaging should be considered for further evaluation (Figure). The decision of whether to use computed tomography (CT) or MRI for the cross-sectional examination must be made on both an institutional and patient-specific basis. CT is faster, less expensive, and more widely and temporally available. Although CT has increased sensitivity for fracture detection compared to radiography, studies have demonstrated false-negative CT examinations in the setting of nondisplaced proximal femoral fractures, especially in osteoporotic patients. Hakkarinen et al3 reported that among 235 hip fractures, 10% were occult radiographically; approximately 17% of these fractures (4 out of 24) were also occult on CT but visible on MRI. Moreover, while radiography and CT may demonstrate a seemingly isolated fracture at the femoral greater trochanter, a subset of these fractures exhibit intertrochanteric extension that is only evident on MRI (Figure). Isolated greater trochanteric fractures are typically treated conservatively, while some incomplete intertrochanteric fractures warrant internal fixation, especially fractures that cross the intertrochanteric midline on coronal MRI.4,5
|
|
| ||
Figure. Anteroposterior left hip radiograph (A) in a patient with hip pain (A) shows no definite evidence of fracture. Coronal reformatted image from left hip computed tomography (B) demonstrates nondisplaced, seemingly isolated fracture of the left femoral greater trochanter (white arrows). Coronal T1-weighted image from left hip magnetic resonance imaging (C) reveals intertrochanteric extension of the fracture spanning greater than 50% of the intertrochanteric diameter (red arrows). | ||||
In addition to improved fracture detection, MRI also provides superior evaluation of the underlying bone marrow for coexisting conditions, such as osteomyelitis, osteonecrosis, and primary or metastatic neoplasm in the setting of pathologic fracture. Additional benefits of MRI over radiography and CT include its lack of ionizing radiation and improved evaluation of adjacent soft tissue injuries, such as labral and/or musculotendinous tears.
MRI, however, does require a longer examination time in which the patient must remain still. This may be difficult for acutely post-traumatic patients, notably those with baseline dementia and/or claustrophobia. For patients in whom MRI is indicated (eg, patients who do not have an implantable device such as a cardiac pacemaker) and where it is institutionally available, the decision to utilize it over CT is largely rooted in health-care economics. MRI is more expensive than radiography and CT, and even in the largest medical centers, the examination requires substantially more time than CT, which inherently decreases patient throughput in the ED. Cannon et al6 present an evidence-based algorithm for patient stratification, in which patients at high-risk for osteoporosis and low-energy trauma should be considered for immediate MRI rather than CT. These risk factors optimize MRI utilization by selecting those patients with the greatest likelihood of nondisplaced, radiographically occult fracture.
References
1. Bogost GA, Lizerbram EK, Crues JV 3rd. MR imaging in evaluation of suspected hip fracture: frequency of unsuspected bone and soft-tissue injury. Radiology. 1995;197(1):263-267.
2. Zuckerman JD, Skovron ML, Koval KJ, Aharonoff G, Frankel VH. Postoperative complications and mortality associated with operative delay in older patients who have a fracture of the hip. J Bone Joint Surg Am. 1995;77(10):1551-1556.
3. Hakkarinen DK, Banh KV, Hendey GW. Magnetic resonance imaging identifies occult hip fractures missed by 64-slice computed tomography. J Emerg Med. 2012;43(2):303-307.
4. Feldman F, Staron RB. MRI of seemingly isolated greater trochanteric fractures. AJR Am J Roentgenol. 2004;183(2):323-329.
5. Schultz E, Miller TT, Boruchov SD, Schmell EB, Toledano B. Incomplete intertrochanteric fractures: imaging features and clinical management. Radiology. 1999;211(1):237-240.
6. Cannon J, Silvestri S, Munro M. Imaging choices in occult hip fracture. J Emerg Med. 2009;37(2):144-152.
PE or Not PE: That Is the Question
Jessica Fisher, MD
Dr Fisher is an instructor of radiology at Weill Cornell Medical College in New York City.
Pulmonary embolism (PE) represents the third most common cause of death from cardiovascular disease after myocardial infarction and stroke.1 Given the potential for fatal outcome, prompt diagnosis and management are essential. To avoid overdiagnosis of PE and unnecessary treatment with anticoagulation therapy, diagnostic tests with both a high sensitivity and high specificity are essential.
Clinical stratification of patient risk for PE, in combination with D-dimer assays, is typically used to determine the need for imaging. Preliminary studies with chest X-ray are utilized to evaluate alternate causes of clinical symptoms but do not provide a definitive diagnosis. In the ED setting, advanced imaging options to detect PE include computed tomography angiography (CTA), ventilation perfusion scintigraphy (V/Q lung scan), and magnetic resonance angiography (MRA).
Computed Tomography Angiography
|
| ||
| Figure 1. Coronal (A) and axial (B) contrast-enhanced computed tomography angiography scans reveal pulmonary embolism (white arrows).. | |||
CTA has long been the standard technique for evaluating PE. In addition to reported sensitivities of 96% to 100% and specificities of 97% to 98% with multislice detectors,2 CTA also provides information on disease severity, such as clot burden, evidence of right heart strain, and the presence of pulmonary infarct (Figure 1). It also can reveal alternative etiology and diagnosis in negative cases (Figure 2).
CTA is readily available in today’s ED and can be performed quickly and efficiently. However, it does require intravenous (IV) contrast and emits a high-dose of radiation, which may be contraindicated in some patient populations (eg, patients with renal failure, allergy to contrast, and pregnant and pediatric patients). Also, to avoid degradation of images and accurately visualize peripheral branches, patient cooperation (ie, suspension of respiratory motion) during the examination is essential.
Ventilation Perfusion Scintigraphy
Before the advent of CTA, V/Q lung scan was the first-line imaging choice to assess for PE.3 This modality continues to have a role in modern practice, especially given its estimated 6-fold lower whole-body effective radiation dose compared to CTA.4 Current applications of this modality include patients with contraindications to IV contrast as well as pregnant and pediatric patients in whom perfusion-only studies should be used to significantly lower the radiation profile.
In patients with a clear chest X-ray, the negative predictive value of V/Q is not significantly different from CT.5 This suggests a growing role for V/Q scans in the younger, healthier patient population where limited/reduced exposure to radiation is particularly desired—eg, pediatric patients and women younger than age 20 years in whom there is an increased risk of malignancy associated with radiation exposure to the breast/chest area.6
The limitations of V/Q lung scan include a higher rate of indeterminate results compared to CTA (particularly in patients with an abnormal chest X-ray). Moreover, V/Q does not provide alternative diagnoses or qualify disease severity. Also, the longer imaging times compared with CTA make this modality impractical in critically ill patients.
Magnetic Resonance Angiography
MRA has emerged in recent years as a potential alternative to CTA. Since this modality does not use ionizing radiation or iodine-based contrast, it is a reasonable and appropriate option for pediatric patients and those with a contrast-dye allergy. Initial meta-analysis of MRA studies demonstrated sensitivity ranging from 77% to 100% and a specificity of 95% to 98%.7-9 However, subsequent studies have found unacceptably high rates of technically inadequate examinations resulting in nondiagnostic results.10 As progress to reduce respiratory and cardiac motion artifacts and improve spatial resolution continues, along with the growing availability of MRA in the ED, this modality may soon emerge as a useful diagnostic option.
Conclusion
New advances in the diagnosis of PE continue to emerge. Evidence suggests improved sensitivity of CTA with the use of dual-energy CT scanners.11 The recent advance of V/Q single photon emission CT has also shown promise in improving detection accuracy.12 Ongoing research will help continue to expand the emergency physician’s range of imaging choices in the diagnosis of PE.
References
1. Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet. 2012;379(9828):1835-1846.
2. Burns SK, Haramati LB. Diagnostic imaging and risk stratification of patients with acute pulmonary embolism. Cardiol Rev. 2012;20(1):15-24.
3. Mos IC, Klok FA, Kroft LJ, de Roos A, Huisman MV. Imaging tests in the diagnosis of pulmonary embolism. Semin Respir Crit Care Med. 2012;33(2):138-143.
4. Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248(1):254-263.
5. Stein EG, Haramati LB, Chamarthy M, Sprayregen S, Davitt MM, Freeman LM. Success of a safe and simple algorithm to reduce use of CT pulmonary angiography in the emergency department. Am J Roentgenol. 2010;194(2):392-397.
6. Hill DA, Preston-Martin S, Ross RK, Bernstein L. Medical radiation, family history of cancer, and benign breast disease in relation to breast cancer risk in young women, USA. Cancer Causes Control. 2002;13(8):711-718.
7. Oudkerk M, van Beek EJ, Wielopolski P, et al. Comparison of contrast-enhanced magnetic resonance angiography and conventional pulmonary angiography for the diagnosis of pulmonary embolism: a prospective study. Lancet. 2002;359(9318):1643-1647.
8. Meaney JF, Weg JG, Chenevert TL, Stafford-Johnson D, Hamilton BH, Prince MR. Diagnosis of pulmonary embolism with magnetic resonance angiography. N Engl J Med 1997;336(20):1422-1427.
9. Gupta A, Frazer CK, Ferguson JM, et al. Acute pulmonary embolism: diagnosis with MR angiography. Radiology. 1999;210(2):353-359.
10. Stein PD, Chenevert TL, Fowler SE. Gadolinium-enhanced magnetic resonance angiography for pulmonary embolism: a multicenter prospective study (PIOPED III). Ann Intern Med. 2010;152(7):434-443, W142, W143.
11. Pontana F, Faivre JB, Remy-Jardin M. Lung perfusion with dual-energy multidetector-row CT (MDCT): feasibility for the evaluation of acute pulmonary embolism in 117 consecutive patients. Acad Radiol. 2008;15(12):1494-1504.
12. Gutte H, Mortensen J, Jensen CV, et al. Comparison of V/Q SPECT and planar V/Q lung scintigraphy in diagnosing acute pulmonary embolism. Nucl Med Commun. 2010;31(1):82-86.
Just MoRe Imaging?
MRI Evaluation of Acute Abdominal Pain
David A. Boyajian, MD
Dr Boyajian is clinical director of radiology at New York-Presbyterian/Lower Manhattan Hospital, and vice chairman and assistant professor of radiology, Department of Radiology, Weill Cornell Medical College, New York.
For the past few decades, the established cross-sectional imaging modalities used to evaluate acute abdominal pain in the ED have been computed tomography (CT) and ultrasound. Advantages of these modalities include the widespread availability of equipment and qualified technologists and radiologists, the ability to obtain images quickly, and relative cost-effectiveness. There are, however, several subsets of indications for which traditional cross-sectional imaging approaches are either undesirable or have a low sensitivity for accuracy (eg, suspected nonobstetric pathology in the pregnant patient, biliary ductal pathology).
Magnetic resonance imaging (MRI) has become more widely available to ED practices across the country and an increasingly important tool in the arsenal of the emergency radiologist. Advantages of MRI include better soft-tissue characterization, ability to directly image in any plane, and the lack of ionizing radiation.
There are, however, barriers to using MRI in the emergency setting, including availability and location of the MRI scanners, long examination time, difficulty in monitoring unstable patients, and higher cost.1 In addition, relative to CT and ultrasound, there is poorer spatial resolution and greater potential for artifacts. Advances in MRI technology and workflow, including rapidly acquired imaging sequences and active radiologist management of such cases, can significantly reduce examination acquisition time and improve image quality. Due to the significant advantages in visualizing the brain and spine, MRI has become the gold standard in many emergent conditions such as suspected stroke and spinal cord compression. Increasingly, MRI is being used to diagnosis causes of abdominal pain, examples of such are discussed below.
Appendicitis in Pregnancy
Although acute appendicitis is the most common nonobstetric surgical condition during pregnancy, several factors such as physiological leukocytosis and appendiceal displacement (which reduces the sensitivity of ultrasound) confound the diagnosis. Due to the radiation conveyed by CT, MRI is considered a more appropriate imaging modality in this patient population.2 In a retrospective study of 23,290 pregnant patients, Pedrosa et al3 reported a sensitivity of 100%, specificity of 93.6%, and accuracy of 94% in detecting acute appendicitis in patients for whom ultrasound was inconclusive.
Biliary Pathology
Biliary pathology, particularly choledocholithiasis, may not be demonstrated to advantage with cross-sectional imaging. For example, CT sensitivity for detection is decreased in the absence of ductal dilatation and/or in poorly mineralized stones. Technical factors, such as obesity, patient positioning, and bowel gas, limit evaluation with ultrasound. MRI is able to depict both the intrahepatic and extrahepatic biliary ducts and identify any intraductal lesions (eg, masses, stones). Special MRI contrast agents that are excreted through the bile ducts have come to market in recent years and can assist in evaluation for biliary pathology. The sequences have replaced many of the diagnostic endoscopic retrograde cholangiopancreatograms (ERCP) that used to be performed. An advantage of magnetic resonance cholangiopancreatography (MRCP) is that structures outside of the biliary system may also be evaluated (eg, liver, kidneys).
|
|
Conclusion
The use of MRI for evaluation of abdominal pain continues to increase. While currently limited to specific vulnerable populations (eg, pregnant patients) or for specific conditions (eg, biliary disease), improving technology and availability will allow this technique to expand to more patients and conditions, including evaluation for inflammatory bowel conditions.
References
1. Saini S, Seltzer SE, Bramson RT, et al. Technical cost of radiologic examinations: Analysis across imaging modalities. Radiology. 2000; 216(1):269-272.
2. Appropriateness criteria. American College of Radiology Web site. http://www.acr.org/Quality-Safety/Appropriateness-Criteria. Accessed September 17, 2013.
3. Pedrosa I, Levine D, Eyvazzadeh AD, Siewert B, Ngo L, Rofsky NM. MR imaging evaluation of acute appendicitis in pregnancy. Radiology. 2006;238(3):891-899.
When a Picture Is Worth a Thousand Images:
3D Reconstruction in Emergency and Trauma Imaging
Jamlik-Omari Johnson, MD; Waqas Shuaib, MD
Dr Johnson is assistant professor of radiology and division director of radiology in the department of radiology and imaging services, division of emergency medicine at Emory University School of Medicine, Atlanta, Georgia. Dr Shuaib is a research associate in the department of radiology and imaging services, division of emergency medicine at Emory University School of Medicine, Atlanta, Georgia.
Three-dimensional (3D) reconstruction creates a digital image of a real-life object, capturing both its original shape and appearance. These techniques simulate reality through the use of lighting, color, and motion.1 Advantages of 3D reconstruction in the emergency and trauma setting include enhanced views of anatomy, making pathology that may be difficult to see in the axial plane easily visible (eg, rib fractures). 3D images may also increase interpretation efficiency, allowing rapid review of the large data sets associated with multidetector computed tomography (MDCT) of trauma patients. Since these images can be attached to the radiology report, this modality also enhances service to referring physicians. In addition, as 3D images succinctly illustrate a diagnosis, the treating physician may share them with patients to demonstrate a condition and explain treatment recommendations.
MDCT technology evolved rapidly over the past 25 years. Between 1992 and 2004, MDCT made quantum leaps from dual detectors to 64-slice scanners, and over the last decade, dual-energy source technology and 128-slice scanners have become integrated into clinical practice. These newer devices, along with technical enhancements, provide high temporal and superior spatial resolution, considerably improved image quality, and expanded clinical applications. With MDCT, scans are performed quickly, resulting in improved temporal resolution and reduced motion artifacts. Leverage of this technology in the emergency and trauma setting has expanded the spectrum of indications for use and increased the utility of CT in urgent care. Newer algorithms allow rapid and detailed examinations of the musculoskeletal and the vascular systems in submillimeter slices, without limitation of scan volume. The high-resolution source images are the basis for high quality 3D reconstructions.
The 3D reconstruction toolbox may benefit the surveillance of anatomy, detection of pathology, and planning of therapy. Examples of three such commonly used techniques are shaded surface display (SSD), maximum intensity projection (MIP), and volume rendering (VR).2
SSD provides a realistic 3D view of the surface of a structure within the acquired volume data set and is good at depicting aneurysms and other focal disease (Figure 1). This is particularly useful for evaluating long and tortuous structures, such as the arterial and venous structures. MIP is often used to detect lung nodules by making them stand-out from bronchi and vasculature (Figure 1). Adapted from the computer graphics field, VR creates a 2D image from a 3D model. VR is employed in the exploration of hollow structures, such as the airways or the colon (Figure 2).
Emergency and trauma environments are often fast-paced and high-stakes, and timely and accurate diagnosis is paramount. Therefore, to be useful, advanced 3D-processing tools should be embedded into the diagnostic viewing application (eg, PACS) to allow efficient reconstruction of images, simultaneous comparison of 2D and 3D images, and referencing of historical data in real time.
References
1. Blank M, Kalender WA. Medical volume exploration: gaining insights virtually. Eur J Radiol. 2000;33(3):161-169.
2. Philipp MO, Kubin K, Mang T, Hörmann M, Metz VM. Three-dimensional volume rendering of multidetector-row CT data: applicable for emergency radiology. Eur J Radiol. 2003;48(1):33-38.
After Imaging: Interventional Radiology in the ED
Joshua L. Weintraub, MD, and Thomas J. Ward, MD
Dr Weintraub is an interventional radiologist and executive vice chairman of the department of radiology
at New York Presbyterian/Columbia University Medical Center, New York. Dr Ward is a postgraduate fifth-year resident in interventional radiologist in the department of radiology at Mount Sinai Medical Center, New York, NY.
Interventional radiologists play an increasing role in treating patients in the ED setting. Simplistically, these roles are usually divided into one of three broad categories: to drain or aspirate infected fluid, to halt active bleeding, or to restore blood flow through an occluded vessel. While all of these procedures are critical, the newest advance in interventional radiology (IR) set to benefit the ED patient is not technical or procedural at all, but rather a philosophical one.
Fluid Aspiration and Drainage
In the ED, the need to drain or aspirate infected fluid is probably the most common indication for consult with an interventional radiologist. Perforated appendicitis or diverticulitis with an intra-abdominal abscess is generally managed with percutaneous drainage and antibiotics. If the clinical situation necessitates, and the location of the collection allows, this procedure may be performed at bedside with local anesthesia under ultrasound guidance. In the septic patient, the minimally invasive nature of this procedure confers significant benefits.
Nonoperative patients presenting with acute cholecystitis rapidly improve after the placement of a percutaneous cholecystostomy tube, and this procedure can be performed under ultrasound guidance. IR may also be indicated in cases of failed endoscopic retrograde cholangiopancreatography or cystoscopy in patients with cholangitis or urosepsis for benign or malignant obstruction. Percutaneous access is facilitated by a dilated system, and temporary decompression can be obtained until more definitive therapy is planned.
Active Bleeding
Active bleeding is another common presentation warranting an IR evaluation. Gastrointestinal, intracranial, posttraumatic, postsurgical, and postpartum bleeding, as well as massive hemoptysis, can all be managed endovascularly. Advances in microcatheter technology, covered vascular stents, and embolic agents have increased the efficacy of these interventions, and improved computed tomography angiography protocols facilitate accurate and timely diagnosis of active bleeding. With the availability of these techniques, waiting several hours for a tagged red blood cell nuclear scan is a thing of the past at many institutions. Embolization has become the mainstay of treating bleeding related to trauma in most major trauma centers.
Ischemia
An IR consult may be ordered for patients presenting with the sequelae of ischemia, which can range from a diabetic foot to an acute stroke. Percutaneous balloon angioplasty, endovascular stents, and catheter-directed thrombolysis were all monumental advances in the treatment of ischemia—conceptualized or introduced into clinical practice by interventional radiologists. This spirit of innovation continues. A variety of technical advances, atherectomy, and re-entry devices have been introduced to help recanalize chronically occluded vessels. New devices allow the interventional radiologist to quickly restore blood flow and function to patients suffering from cerebral embolus. There is increased interest in the use of catheter-assisted-embolectomy for submassive pulmonary embolism when intravenous fibrinolysis is unsuccessful or contraindicated.
Conclusion
The five decades of pioneering technical innovation highlighted in this article allow for minimally invasive treatment of the ED patient. The newest advance in IR is not technical, but rather philosophical, and a change in the role that the interventional radiologist plays. The American Board of Radiology has recently approved a new IR training pathway that more than doubles the amount of clinical training that graduating IR fellows now receive. This signals a renewed commitment to running a truly clinical service. Patients in the ED can be evaluated and treated by an interventional radiologist in the hospital and then discharged under the care of an interventional radiologist at an IR clinic. Several IR sections across the country currently practice in such a manner. Although this model is currently the exception and not the rule, the goal of the new training pathway hopes to change this, with increased advances and benefits to patients in the ED setting.
Suggested Reading
1. Gasior AC, Marty Knott E, Ostlie DJ, St Peter SD. To drain or not to drain: an analysis of abscess drains in the treatment of appendicitis with abscess. Pediatr Surg Int. 2013;29(5):455-458.
2. Sato KT. Percutaneous management of biliary emergencies. Semin Intervent Radiol. 2006;23(3):
249-257.
3. Funaki B. On-call treatment of acute gastrointestinal hemorrhage. Intervent Radiol. 2006;23(3):
215-222.
4. Yoon W, Kim JK, Kim YH, Chung TW, Kang HK. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics. 2002;22(6):1395-1409.
5. Stead LG, Gilmore RM, Bellolio MF, Rabinstein AA, Decker WW. Percutaneous clot removal devices in acute ischemic stroke: a systematic review and meta-analysis. Arch Neurol. 2008;65(8):
1024-1030.
6. Kucher N. Catheter embolectomy for acute pulmonary embolism. Chest. 2007;132(2):657-663.
7. Initial certification: vascular/interventional. American Board of Radiology Web site. http://www.theabr.org/ic-vir-landing. Accessed September 24, 2013.
Figure 2. Surface shaded display image of a virtual colonoscopy.
Evaluation of Chest Pain in the Emergency Department by Coronary CT Angiography
James K. Min, MD
Dr Min is director of the Institute of Cardiovascular Imaging at New York-Presbyterian Hospital/Weill-Cornell Medical College, New York.
In the United States each year, approximately 6 million patients present with complaints of chest pain suspicious for acute coronary syndromes (ACS), including unstable angina and myocardial infarction.1 Most of these patients are diagnosed with noncardiac conditions, and nearly half are due to noncardiac etiology. There are an array of diagnostic tests to identify and exclude patients with suspected ACS, such as medical history, cardiac enzyme measurements, electrocardiographic changes, and clinical risk scores. For those with nonnegligible risk for ACS for which this condition cannot be definitively diagnosed or excluded, many are often admitted to the hospital for further testing and observation. Among these patients, less than 30% are found to have ACS. And yet, despite these very careful clinical pathways, between 2% and 8% of patients with ACS are unknowingly discharged to home.2
In recent years, coronary computed tomography angiography (CCTA) has emerged as a noninvasive method that permits direct anatomic visualization of coronary atherosclerosis and luminal stenosis.3 Since the introduction of 64-multidetector row CT scanners in 2005, there have been significant advances in CT technology that now allow for reliable performance of CCTA with very low-dose radiation. Pertaining to the former, improvements in spatial resolution, temporal resolution, and volume coverage enable evaluation of coronary arteries at the submillimeter level, and can be performed in approximately 1 to 5 seconds. Concomitant to the progress in CT technology has been the parallel developments in radiation-dose reduction. As compared to the background radiation exposure of an individual living at sea level for 1 year (~3 millisieverts of radiation), current generation CCTA can be performed at doses <1 millisieverts, with doses approximating a screening mammogram now achievable.
|
|
| ||
Figure. Volume-rendered coronary computed tomographic angiography showing the coronary vascular bed and myocardium (A); normal right coronary artery without evidence of atherosclerosis (B); and (C) left anterior descending artery with mild nonobstructive calcified plaque (white area). | ||||
The diagnostic accuracy of CCTA against invasive coronary angiography has been tested in several prospective multicenter trials.3 For patients without known but suspected coronary artery disease (CAD), the sensitivity and negative predictive value has ranged between 95% to 99%—that is, CCTA can exclude anatomically obstructive CAD with near 100% certainty (Figure). It is these diagnostic performance characteristics that have encouraged several investigators to evaluate the use of CCTA in the diagnostic algorithm of patients presenting to the ED with acute chest pain—the primary intent being to identify sufficiently low-risk patients without significant CAD who can be safely discharged home.
Since 2011, three prospective multicenter randomized controlled trials have evaluated the incorporation of CCTA into a diagnostic chest pain pathway, as compared to standard-of-care algorithms.4-6 Now comprising more than 3,000 patients, these trials have demonstrated remarkably consistent results, with a reduced time-to-diagnosis of 40% to 50%, reduced lengths of stay in the ED of 25%, and reduced ED costs of 20% to 40%. Importantly, these salient effects on resource utilization and economics were underscored by exceptionally safe outcomes, which never exceeded that of the standard of care. Several studies to date have subsequently assessed the duration of safety conferred by a normal CCTA, or its “warranty period.” These studies have shown the warranty period to last at least 7 years for major adverse cardiac events and mortality.7 Moreover, they also engender hope that the chest pain pathways used for millions of Americans annually can indeed be improved, with CCTA playing an essential role.
As its technology continues to iterate, the diagnostic and prognostic performance of CCTA will invariably continue to improve. However, even at present, CCTA is robust and more accurate for exclusion of CAD when compared to other traditional methods of evaluation, and its use for patients presenting to the ED with chest pain improves throughput, reduces costs, and maximizes patient safety.
References
1. Pitts SR, Niska RW, Xu J, Burt CW. National Hospital Ambulatory Medical Care Surviva: 2006 emergency department summary. Natl Health Stat Report. 2008;7:1-38.
2. Pope JH, Aufderheidi TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342(16):1163-1170.
3. Min JK, Shaw LJ, Berman DS. The present state of coronary computed tomography angiography: a process in evolution. J Am Coll Cardiol. 2010;55(:957-965.
4. Litt HI, Gatsonis C, Snyder B, et al. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med. 2012;366(15):1393-403.
5. Hoffmann U, Truong QA, Schoenfeld DA, et al. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med. 2012;367(4):299-308.
6. Goldstein JA, Chinnaiyan KM, Abidov A, et al. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol. 2011;58(14):1414-1422.
7. Andreini D, Pontone G, Mushtaq S, et al. A long-term prognostic value of coronary CT angiography in suspected coronary artery disease. JACC Cardiovasc Imaging. 201
As EDs have evolved to handle the increasing volume and complexity of patients requiring immediate care, so too, has the field of emergency radiology. Many EDs across the country now have multiple advanced imaging modalities available 24 hours a day, including Xray, computed tomography (CT), ultrasound, and magnetic resonance imaging (MRI). While many emergency medicine physicians are now trained in performing and evaluating ultrasound images—similar to X-rays in the past—most are less comfortable with CT and MRI.
Emergency radiology, now a recognized subspecialty of diagnostic imaging, has proliferated to meet the demands for immediate interpretation of these images. This combination of around-the-clock access to equipment and expertise has brought cutting-edge, advanced imaging to the front lines of emergency care. In this special feature, we invited a group of emergency radiologists and an expert in cardiovascular imaging to discuss the applicability and utility of several of these techniques in the ED setting.
As illustrated by this panel, advanced imaging has become increasingly valuable and available in providing care to ED patients. In this presentation, however, you will notice the conspicuous absence of specific techniques that are utilized in diagnosis and evaluation of stroke, head/spine trauma, and other neurologic conditions that are commonplace in the ED. Advanced imaging has become critical in these instances and will be discussed in a future article.
Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and the executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center. He is a member of the EMER GENCY MEDICINE editorial board.
Faster than FAST: Single Pass Whole-Body Computed Tomography for Rapid Evaluation of Trauma Patients
Ashwin Asrani, MD
Dr Asrani is an assistant professor of radiology at Weill Cornell Medical College in New York City and an
assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center in New York City.
As part of initial assessment and resuscitation in the ED, severely injured trauma patients frequently undergo a bedside focused abdominal sonography for trauma (FAST) scan to assess for intraperitoneal hemorrhage from an underlying major abdominal visceral injury, as well as the presence of pleural and/or pericardial effusion. However, the use and role of a FAST scan in hemodynamically stable patients has recently been questioned since, based on its relatively low sensitivity for visceral injury, many of these cases eventually require computed tomography (CT) (See Case). In addition, patients in this subset with a positive FAST scan frequently have subsequent CT to further assist clinicians in understanding the nature of injury and to guide management recommendations (eg, operative versus nonoperative options).1
Advances in the speed of CT have made it possible to obtain images of the entire body with only a single dose of contrast injection. The elimination of segmental acquisition of torso images (eg, head, neck, chest, abdomen) eliminates the need to reposition the patient, repeat scout images, and postpone the reconstruction of reformats and other special views until the end of the examination. Depending on the original protocol, this in turn minimizes patient time in the CT-scan room by approximately 31% to 42%,2,3 and significantly reduces radiation exposure by 17% compared to conventional segmental acquisition of different body parts.2
Evidence suggests that direct single-pass whole-body CT improves outcomes in both hemodynamically stable and unstable patients and can reveal significant findings not apparent on initial clinical examination.4,5 Whole-body CT increases injury severity by detecting lesions that would not otherwise have been detected by conventional methods. While this information does not affect treatment options, it does artificially lower the ratio of observed-to-expected deaths.6 The Randomized Study of Early Assessment by CT Scanning in Trauma Patients (REACT-2), a recent international multicenter randomized clinical trial, is expected to provide evidence supporting the value of immediate total-body CT scanning during the primary evaluation of severely injured trauma patients; the results of this trial should become available in 2014.7
As EDs install and upgrade to newer multidetector CT scanners, single-pass whole-body scanning has the potential to save time in situations when it really matters.
References
1. Natarajan B, Gupta PK, Cemaj S, Sorensen M, Hatzoudis GI, Forse RA. FAST scan: Is it worth doing in hemodynamically stable blunt trauma patients? Surgery. 2010;148(4):695-700; discussion 700-701.
2. Fanucci E, Fiaschetti V, Rotili A, Floris R, Simonetti G. Whole body 16-row multislice CT in emergency room: Effects of different protocols on scanning time, image quality and radiation exposure. Emerg Radiol. 2007;13(5):251-257.
3. Nguyen D, Platon A, Shanmuganathan K, Mirvis SE, Becker CD, Poletti PA. Evaluation of a single-pass continuous whole-body 16-MDCT protocol for patients with polytrauma. AJR Am J Roentgenol. 2009;192(1):3-10.
4. Huber-Wagner S, Biberthaler P, Haberle S, et al. Whole-body CT in haemodynamically unstable severely injured patients – A retrospective, multicentre study. PLoS One. 2013;8(7):e68880.
5. Salim A, Sangthong B, Martin M, Brown C, Plurad D, Demetriades D. Whole body imaging in blunt multisystem trauma patients without obvious signs of injury: Results of a prospective study. Arch Surg. 2006;141(5):468-473; discussion 473-475.
6. Stengel D, Frank M, Matthes G, et al. Primary pan-computed tomography for blunt multiple trauma: Can the whole be better than its parts? Injury. 2009;40 Suppl 4:S36-S46.
7. Sierink JC, Saltzherr TP, Beenen LF, et al. A multicenter, randomized controlled trial of immediate total-body CT scanning in trauma patients (REACT-2). BMC Emerg Med. 2012;12:4-227X-12-4.
Obstructed Views: Imaging in Urinary Tract Obstruction
Lily M. Belfi, MD
Dr Belfi is an assistant professor of radiology at Weill Cornell Medical College in New York City and an assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center.
Urinary tract (UT) obstruction is one of the most common indications for genitourinary (GU) imaging in the ED. Until recently, diagnosis was made using intravenous (IV) pyelography. Today, improved imaging modalities, such as noncontrast computed tomography (CT), computed tomography urography (CTU), magnetic resonance urography (MRU), and magnetic resonance imaging (MRI), are available to better visualize and evaluate the underlying causes of obstruction.
Noncontrast Computed Tomography
|
|
Noncontrast CT of the abdomen and pelvis is the imaging study of choice in evaluating acute GU obstruction, particularly when there is clinical suspicion of stone disease. CT is extremely sensitive and specific in detecting renal calculi and provides information about stone burden, size, location, and composition. In addition to renal calculi, noncontrast CT is also useful in identifying other, less common causes of UT obstruction such as ureteral herniation, which can occur in the inguinal, femoral, or sciatic region and result in acute obstruction. Patients with neuromuscular disorders that cause piriformis muscle atrophy (eg, multiple sclerosis) may be predisposed to ureteral sciatic herniation. CT reconstructions in the coronal plane are useful in detecting this condition (Figure 1).
|
|
Reconstructed CT images can also reveal underlying congenital abnormalities that make a patient susceptible to obstruction. For example, CT can reveal the presence of a duplicated collecting system, which is often accompanied by an obstructing ureterocele associated with the upper pole moiety (Figure 2).
Computed Tomography Urography
|
| ||
| Figure 3. Computed tomography urography images (A and B) reveal "fish-hook" ureter (white arrows) classically seen in patients with with retrocaval ureter. | |||
Other types of congenital anomalies may require additional imaging with CTU, in which IV iodinated contrast media is administered for increased sensitivity and visualization. For instance, patients with a retrocaval ureter often present with GU obstruction and hematuria; the classic “fish-hook” or “sickle-shaped” deformity of the ureter characterizing this disease is best visualized on CTU as the ureter is well-opacified by excreted contrast (Figure 3).
Magnetic Resonance Urography and Magnetic Resonance Imaging
MRU and MRI are alternative modalities to CTU for patients in whom iodinated CT contrast is contraindicated or in cases where the avoidance of radiation exposure is indicated (eg, pregnant and pediatric patients). MRI and MRU are especially useful in further characterizing obstructive lesions in the GU tract. While initial noncontrast CT may suggest soft-tissue lesions within the ureter or urinary bladder, follow-up MRI clearly delineates the condition, helping clinicians quantify the extent of tumor involvement and determine disease stage (Figure 4). Pelvic MRI is also an essential tool in identifying causes of bladder outlet obstruction, especially in those involving the prostate gland (Figure 5).
|
|
|
|
|
Figure 4. Noncontrast pelvic computed tomography images (A and B) of a 72-year-old man with flank pain and difficulty voiding reveal hydronephrosis (white arrow) and bladder mass (red arrow). Magnetic resonance urography (C) further visualizes the bladder mass (red arrow), and postcontrast magnetic resonance images (D and E) show the extent of the tumor (red arrow) as well as a metastatic lesion (white arrow).
| ||||
Conclusion
|
|
| |||
| Figure 5. Noncontrast pelvic computed tomography images (A and B) of a 73-yearold man with pelvic pain demonstrate bilateral hydronephrosis. (white arrows) and a large mass in the expected location of the prostate (red arrow). Further evaluation of the large heterogenous mass with pelvic magnetic resonance imaging (C) reveals prostate carcinoma (red arrows). | |||||
Based on its sensitivity in detecting both common and uncommon causes of obstruction, noncontrast abdominal and pelvic CT is an excellent first-line imaging choice for evaluating patients presenting to the ED with UT obstruction. Moreover, noncontrast CT also helps guide clinicians in determining which, if any, additional studies with CTU, MRU, and/or MRI are warranted.
Sound Advice: Ultrasound in the Emergency Department
Kemi Babagbemi, MD
Dr Babagbemi is an assistant professor of radiology and assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical College, New York.
In today’s evolving health-care environment, emphasis must be placed on providing accurate, safe, cost-effective, and timely diagnosis to the wide range of patients that present to the ED. To meet this need, emergency medicine physicians and radiologists have universally embraced the use of ultrasound in this setting.
Ultrasound is less expensive than computed tomography (CT) and magnetic resonance imaging (MRI), and its portability allows for bedside application, enabling its use in the most critical patients. It does not require extended preparation (eg, oral contrast) or carry the risk for adverse reactions associated with intravenously administered contrast. Also, since ultrasound does not use ionizing radiation, it is a particularly appropriate imaging option in susceptible populations such as pregnant and pediatric patients.1 Although a noninvasive modality, it can also be used to guide interventional procedures, including vascular access, thoracentesis/paracentesis, and specialized anesthesia.
Emergency physicians routinely perform ultrasound at bedside and are familiar with its utility in evaluating abdominal trauma, right upper quadrant pain, and acute biliary abnormalities; assessing vascular thrombosis or injury; and determining the etiology of pelvic pain and bleeding in both pregnant and nonpregnant patients. However, ultrasound is also increasingly being utilized to
diagnose other conditions for which CT and MRI were once thought the superior diagnostic tool.
Trauma (The Extended FAST Scan)
The focused abdominal sonography for trauma (FAST) scan has been used for rapid and immediate assessment of unstable trauma patients with suspected abdominal injury. In a recent study of 4,029 patients with blunt abdominal trauma, FAST scans had a sensitivity, specificity, and accuracy for detection of hemorrhage in patients with hypotension of 85%, 60%, and 77%, respectively.2 Other studies suggest that sonography of the chest may be useful in the rapid detection of additional life-threatening pathology such as pneumothorax (Figure 1), with data suggesting improved sensitivity over radiography.3 The inclusion of the pleural space in evaluation is increasingly more common and is referred to as the extended FAST (EFAST).
Appendicitis
The most common cause of abdominal pain requiring surgical intervention is appendicitis.4 Although certain clinically based prediction scores (eg, Alvarado scores) may be used, imaging is considered far superior in accurately diagnosing the condition.5 In a meta-analysis of data from 26 ultrasound and CT studies (15 prospective, 11 retrospective), there was a pooled 88% sensitivity and 94% specificity for ultrasound compared with CT, which exhibited a pooled sensitivity of 94% and specificity of 95%.6 As previously noted, because there is no ionizing radiation in ultrasound, it should be the preferred modality in both children and first-trimester pregnant patients.
Musculoskeletal Trauma
Ultrasound is an ideal imaging modality to evaluate the musculoskeletal system. Despite its widespread use in Europe for many years, musculoskeletal sonography is only now beginning to be adopted in the United States. Its ability to visualize soft-tissue structures makes it effective in evaluating for muscle, tendon, or ligament injury, and can even do so dynamically with stress maneuvers (Figure 2). With respect to fractures, sonography has also proved effective in evaluating for cortical disruption. For example, a recent study demonstrated overall 92% sensitivity and 100% sensitivity for fractures with high potential for complication in radiographically occult scaphoid fractures.7
Conclusion
As a true point-of-care imaging modality, ultrasound has an established place in the practice of emergency medicine. Future improvements in technology, including the ability to obtain true three-dimensional volumetric data sets, will expand its role even further.
References
1. Image Gently and Ultrasound. Image Gently Campaign. The Alliance for Radiation Safety in Pediatric Imaging Web site. http://www.pedrad.org/associations/5364/ig/?page=787. Accessed September 19, 2013.
2. Lee BC, Ormsby EL, McGahan JP, Melendres GM, Richards JR. The utility of sonography for the triage of blunt abdominal trauma patients to exploratory laparotomy. AJR Am J Roentgenol. 2007;188(2):415-421.
3. Nandipati KC, Allamaneni S, Kakarla, et al. Extended focused assessment with sonography for trauma (EFAST) in the diagnosis of pneumothorax: experience at a community based level I trauma center. Injury. 2011;42(5):511-514.
4. Addiss DG, Shaffer N, Fowler BS, Tauxe RV. The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol. 1990;132(5):910-925.
5. Sun JS, Noh HW, Min YG, et al. Receiver operating characteristic analysis of the diagnostic performance of a computed tomographic examination and the Alvarado score for diagnosing acute appendicitis: emphasis on age and sex of the patients.
J Comput Assist Tomogr. 2008;32(3):386-391.
6. Doria AS, Moineddin R, Kellenberger CJ, et al. US or CT for Diagnosis of Appendicitis in Children and Adults? A MetaAnalysis. Radiology. 2006;241(1):83-94.
7. Platon A, Poletti PA, Van Aaken J, Fusetti C, Della Santa D, Beaulieu JY, Becker CD. Occult fractures of the scaphoid: the role of Ultrasonography in the emergency department. Skeletal Radiol. 2011;40(7):869-875.
Stuck or Not? Noninvasive Vascular Imaging in the Emergency Setting
Michael L. Loftus, MD
Dr Loftus is assistant professor of radiology at New York-Presbyterian Hospital/Weill Cornell Medical College, New York.
Conventional catheter-directed angiography has played an important role in the history of ED imaging, providing timely information about vessel integrity throughout the body and guiding potentially life-saving interventions. However, this imaging modality carries significant potential risks, including puncture-site hematoma or pseudoaneurysm, catheter-induced vasospasm, vascular occlusion or dissection, anesthesia-associated risks, and neurological deterioration or stroke.1 Moreover, emergent angiography is not universally available in all EDs.
Computed tomography angiography (CTA) and magnetic resonance angiography (MRA) have opened new windows of opportunity for noninvasive vascular imaging to play a role in clinical decision-making in the ED. Cross-sectional angiography has virtually eliminated the need for acute catheter-directed angiography in several clinical settings, including pulmonary angiography, and the clinical applicability of CTA and MRA continues to expand as imaging techniques improve and achieve widespread acceptance and implementation. Multidetector CT is now widely available in most EDs, and the
accessibility of magnetic resonance imaging and MRA is expanding rapidly.
CTA allows evaluation of the vasculature on contrast-enhanced axial source images and also utilizes computer-generated maximum intensity projection reformations to create diagnostic images of the vascular region of interest. Similarly, MRA can be performed either with or without the administration of an intravenous gadolinium contrast agent, and provides additional information about directionality of flow, as well as detailed images of the surrounding soft tissues.
An emerging clinical situation in which contrast-enhanced cross-sectional imaging may supplant the need for arterial puncture and digital subtraction angiography is multiligament trauma to the knee. When significant kinetic force is applied to the knee, the joint is at risk for translocation and/or dislocation, with resultant injury to the surrounding soft-tissue envelope and potential trauma to the neurovascular structures around the knee. In this type of injury, cross-sectional imaging is routinely ordered to further evaluate and classify trauma, revealing potentially treatment-altering information concerning the integrity of the vascular structures with minimal risk to the patient (Figure 1).
The largest series of MRAs performed specifically in patients with knee dislocation reviewed 17 cases and found two cases of vascular pathology: one case of an intimal flap and one case of acute vasospasm. Digital subtraction angiography was performed on 6 of 17 cases and had 100% concordance with MRA findings.2 MRI is often performed in the setting of suspected multiligament knee injury to aid in preoperative planning, and the addition of MRA should be considered if trauma to the periarticular vasculature is suspected.
There is ample evidence that cross-sectional imaging performs well relative to conventional catheter angiography in the setting of peripheral vascular occlusion from atherosclerotic etiologies.3 Furthermore, there is precedence for utilizing contrast-enhanced CTA, as well as contrast-enhanced or three-dimensional time-of-flight MRA in other areas of the body—particularly the brain, head, and neck4 (Figure 2). Several MRA techniques are becoming available that will allow a high resolution angiographic without the use of contrast. Upcoming advances in CTA include dual-energy CT, which has the potential to allow angiography using very small amounts of contrast.
In general, when ordering cross-sectional imaging in the setting of trauma, consideration should be given to the potential for vascular compromise; thus, the addition of contrast-enhanced CTA or MRA can add valuable clinical information, with relatively little excess risk or time, sparing patients the risks of catheter-directed angiography.
References
1. Eisenberg, RL, Bank, WO, Hedgcock, MW. Neurologic complications of angiography for cerebrovascular disease. Neurology. 1980;30(8):895-897.
2. Potter HG, Weinstein M, Allen AA, Wickiewicz TL, Helfet DL. Magnetic resonance imaging of the multiple-ligament injured knee. J Orthop Trauma. 2002;16(5);330-339.
3. Chin AS, Rubin GD. CT angiography of peripheral arterial occlusive disease. Tech Vasc Interv Radiol. 2006;9(4):143-149.
4. Riles TS, Eidelman EM, Litt AW, Pinto RS, Oldford F, Schwartzenberg GW. Comparison of magnetic resonance angiography, conventional angiography, and duplex scanning. Stroke. 1992;23(3):341-346.
What’s Hip in 2013?
Roger J. Bartolotta, MD
Dr Bartolotta is an assistant professor of radiology at Weill Cornell Medical College in New York City and assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center.
Post-traumatic hip pain is a common chief concern among ED patients. While routine radiography, the standard imaging modality for the initial evaluation of suspected hip fracture, detects most fractures, its sensitivity is decreased in the setting of osteoporosis—particularly with nondisplaced fractures. Thus, though elderly/osteoporotic patients are more likely to fracture, these fractures are harder to detect on radiography. In a study of 70 patients with negative radiographs but high clinical concern for fracture, magnetic resonance imaging (MRI) detected occult femoral fractures in 37% and occult pelvic fractures in 23%.1
Hip fractures in elderly patients are associated with substantial mortality and morbidity, the risks for which increase with delayed diagnosis.2 When there is high clinical suspicion for a radiographically occult hip fracture in this population, cross-sectional imaging should be considered for further evaluation (Figure). The decision of whether to use computed tomography (CT) or MRI for the cross-sectional examination must be made on both an institutional and patient-specific basis. CT is faster, less expensive, and more widely and temporally available. Although CT has increased sensitivity for fracture detection compared to radiography, studies have demonstrated false-negative CT examinations in the setting of nondisplaced proximal femoral fractures, especially in osteoporotic patients. Hakkarinen et al3 reported that among 235 hip fractures, 10% were occult radiographically; approximately 17% of these fractures (4 out of 24) were also occult on CT but visible on MRI. Moreover, while radiography and CT may demonstrate a seemingly isolated fracture at the femoral greater trochanter, a subset of these fractures exhibit intertrochanteric extension that is only evident on MRI (Figure). Isolated greater trochanteric fractures are typically treated conservatively, while some incomplete intertrochanteric fractures warrant internal fixation, especially fractures that cross the intertrochanteric midline on coronal MRI.4,5
|
|
| ||
Figure. Anteroposterior left hip radiograph (A) in a patient with hip pain (A) shows no definite evidence of fracture. Coronal reformatted image from left hip computed tomography (B) demonstrates nondisplaced, seemingly isolated fracture of the left femoral greater trochanter (white arrows). Coronal T1-weighted image from left hip magnetic resonance imaging (C) reveals intertrochanteric extension of the fracture spanning greater than 50% of the intertrochanteric diameter (red arrows). | ||||
In addition to improved fracture detection, MRI also provides superior evaluation of the underlying bone marrow for coexisting conditions, such as osteomyelitis, osteonecrosis, and primary or metastatic neoplasm in the setting of pathologic fracture. Additional benefits of MRI over radiography and CT include its lack of ionizing radiation and improved evaluation of adjacent soft tissue injuries, such as labral and/or musculotendinous tears.
MRI, however, does require a longer examination time in which the patient must remain still. This may be difficult for acutely post-traumatic patients, notably those with baseline dementia and/or claustrophobia. For patients in whom MRI is indicated (eg, patients who do not have an implantable device such as a cardiac pacemaker) and where it is institutionally available, the decision to utilize it over CT is largely rooted in health-care economics. MRI is more expensive than radiography and CT, and even in the largest medical centers, the examination requires substantially more time than CT, which inherently decreases patient throughput in the ED. Cannon et al6 present an evidence-based algorithm for patient stratification, in which patients at high-risk for osteoporosis and low-energy trauma should be considered for immediate MRI rather than CT. These risk factors optimize MRI utilization by selecting those patients with the greatest likelihood of nondisplaced, radiographically occult fracture.
References
1. Bogost GA, Lizerbram EK, Crues JV 3rd. MR imaging in evaluation of suspected hip fracture: frequency of unsuspected bone and soft-tissue injury. Radiology. 1995;197(1):263-267.
2. Zuckerman JD, Skovron ML, Koval KJ, Aharonoff G, Frankel VH. Postoperative complications and mortality associated with operative delay in older patients who have a fracture of the hip. J Bone Joint Surg Am. 1995;77(10):1551-1556.
3. Hakkarinen DK, Banh KV, Hendey GW. Magnetic resonance imaging identifies occult hip fractures missed by 64-slice computed tomography. J Emerg Med. 2012;43(2):303-307.
4. Feldman F, Staron RB. MRI of seemingly isolated greater trochanteric fractures. AJR Am J Roentgenol. 2004;183(2):323-329.
5. Schultz E, Miller TT, Boruchov SD, Schmell EB, Toledano B. Incomplete intertrochanteric fractures: imaging features and clinical management. Radiology. 1999;211(1):237-240.
6. Cannon J, Silvestri S, Munro M. Imaging choices in occult hip fracture. J Emerg Med. 2009;37(2):144-152.
PE or Not PE: That Is the Question
Jessica Fisher, MD
Dr Fisher is an instructor of radiology at Weill Cornell Medical College in New York City.
Pulmonary embolism (PE) represents the third most common cause of death from cardiovascular disease after myocardial infarction and stroke.1 Given the potential for fatal outcome, prompt diagnosis and management are essential. To avoid overdiagnosis of PE and unnecessary treatment with anticoagulation therapy, diagnostic tests with both a high sensitivity and high specificity are essential.
Clinical stratification of patient risk for PE, in combination with D-dimer assays, is typically used to determine the need for imaging. Preliminary studies with chest X-ray are utilized to evaluate alternate causes of clinical symptoms but do not provide a definitive diagnosis. In the ED setting, advanced imaging options to detect PE include computed tomography angiography (CTA), ventilation perfusion scintigraphy (V/Q lung scan), and magnetic resonance angiography (MRA).
Computed Tomography Angiography
|
| ||
| Figure 1. Coronal (A) and axial (B) contrast-enhanced computed tomography angiography scans reveal pulmonary embolism (white arrows).. | |||
CTA has long been the standard technique for evaluating PE. In addition to reported sensitivities of 96% to 100% and specificities of 97% to 98% with multislice detectors,2 CTA also provides information on disease severity, such as clot burden, evidence of right heart strain, and the presence of pulmonary infarct (Figure 1). It also can reveal alternative etiology and diagnosis in negative cases (Figure 2).
CTA is readily available in today’s ED and can be performed quickly and efficiently. However, it does require intravenous (IV) contrast and emits a high-dose of radiation, which may be contraindicated in some patient populations (eg, patients with renal failure, allergy to contrast, and pregnant and pediatric patients). Also, to avoid degradation of images and accurately visualize peripheral branches, patient cooperation (ie, suspension of respiratory motion) during the examination is essential.
Ventilation Perfusion Scintigraphy
Before the advent of CTA, V/Q lung scan was the first-line imaging choice to assess for PE.3 This modality continues to have a role in modern practice, especially given its estimated 6-fold lower whole-body effective radiation dose compared to CTA.4 Current applications of this modality include patients with contraindications to IV contrast as well as pregnant and pediatric patients in whom perfusion-only studies should be used to significantly lower the radiation profile.
In patients with a clear chest X-ray, the negative predictive value of V/Q is not significantly different from CT.5 This suggests a growing role for V/Q scans in the younger, healthier patient population where limited/reduced exposure to radiation is particularly desired—eg, pediatric patients and women younger than age 20 years in whom there is an increased risk of malignancy associated with radiation exposure to the breast/chest area.6
The limitations of V/Q lung scan include a higher rate of indeterminate results compared to CTA (particularly in patients with an abnormal chest X-ray). Moreover, V/Q does not provide alternative diagnoses or qualify disease severity. Also, the longer imaging times compared with CTA make this modality impractical in critically ill patients.
Magnetic Resonance Angiography
MRA has emerged in recent years as a potential alternative to CTA. Since this modality does not use ionizing radiation or iodine-based contrast, it is a reasonable and appropriate option for pediatric patients and those with a contrast-dye allergy. Initial meta-analysis of MRA studies demonstrated sensitivity ranging from 77% to 100% and a specificity of 95% to 98%.7-9 However, subsequent studies have found unacceptably high rates of technically inadequate examinations resulting in nondiagnostic results.10 As progress to reduce respiratory and cardiac motion artifacts and improve spatial resolution continues, along with the growing availability of MRA in the ED, this modality may soon emerge as a useful diagnostic option.
Conclusion
New advances in the diagnosis of PE continue to emerge. Evidence suggests improved sensitivity of CTA with the use of dual-energy CT scanners.11 The recent advance of V/Q single photon emission CT has also shown promise in improving detection accuracy.12 Ongoing research will help continue to expand the emergency physician’s range of imaging choices in the diagnosis of PE.
References
1. Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet. 2012;379(9828):1835-1846.
2. Burns SK, Haramati LB. Diagnostic imaging and risk stratification of patients with acute pulmonary embolism. Cardiol Rev. 2012;20(1):15-24.
3. Mos IC, Klok FA, Kroft LJ, de Roos A, Huisman MV. Imaging tests in the diagnosis of pulmonary embolism. Semin Respir Crit Care Med. 2012;33(2):138-143.
4. Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248(1):254-263.
5. Stein EG, Haramati LB, Chamarthy M, Sprayregen S, Davitt MM, Freeman LM. Success of a safe and simple algorithm to reduce use of CT pulmonary angiography in the emergency department. Am J Roentgenol. 2010;194(2):392-397.
6. Hill DA, Preston-Martin S, Ross RK, Bernstein L. Medical radiation, family history of cancer, and benign breast disease in relation to breast cancer risk in young women, USA. Cancer Causes Control. 2002;13(8):711-718.
7. Oudkerk M, van Beek EJ, Wielopolski P, et al. Comparison of contrast-enhanced magnetic resonance angiography and conventional pulmonary angiography for the diagnosis of pulmonary embolism: a prospective study. Lancet. 2002;359(9318):1643-1647.
8. Meaney JF, Weg JG, Chenevert TL, Stafford-Johnson D, Hamilton BH, Prince MR. Diagnosis of pulmonary embolism with magnetic resonance angiography. N Engl J Med 1997;336(20):1422-1427.
9. Gupta A, Frazer CK, Ferguson JM, et al. Acute pulmonary embolism: diagnosis with MR angiography. Radiology. 1999;210(2):353-359.
10. Stein PD, Chenevert TL, Fowler SE. Gadolinium-enhanced magnetic resonance angiography for pulmonary embolism: a multicenter prospective study (PIOPED III). Ann Intern Med. 2010;152(7):434-443, W142, W143.
11. Pontana F, Faivre JB, Remy-Jardin M. Lung perfusion with dual-energy multidetector-row CT (MDCT): feasibility for the evaluation of acute pulmonary embolism in 117 consecutive patients. Acad Radiol. 2008;15(12):1494-1504.
12. Gutte H, Mortensen J, Jensen CV, et al. Comparison of V/Q SPECT and planar V/Q lung scintigraphy in diagnosing acute pulmonary embolism. Nucl Med Commun. 2010;31(1):82-86.
Just MoRe Imaging?
MRI Evaluation of Acute Abdominal Pain
David A. Boyajian, MD
Dr Boyajian is clinical director of radiology at New York-Presbyterian/Lower Manhattan Hospital, and vice chairman and assistant professor of radiology, Department of Radiology, Weill Cornell Medical College, New York.
For the past few decades, the established cross-sectional imaging modalities used to evaluate acute abdominal pain in the ED have been computed tomography (CT) and ultrasound. Advantages of these modalities include the widespread availability of equipment and qualified technologists and radiologists, the ability to obtain images quickly, and relative cost-effectiveness. There are, however, several subsets of indications for which traditional cross-sectional imaging approaches are either undesirable or have a low sensitivity for accuracy (eg, suspected nonobstetric pathology in the pregnant patient, biliary ductal pathology).
Magnetic resonance imaging (MRI) has become more widely available to ED practices across the country and an increasingly important tool in the arsenal of the emergency radiologist. Advantages of MRI include better soft-tissue characterization, ability to directly image in any plane, and the lack of ionizing radiation.
There are, however, barriers to using MRI in the emergency setting, including availability and location of the MRI scanners, long examination time, difficulty in monitoring unstable patients, and higher cost.1 In addition, relative to CT and ultrasound, there is poorer spatial resolution and greater potential for artifacts. Advances in MRI technology and workflow, including rapidly acquired imaging sequences and active radiologist management of such cases, can significantly reduce examination acquisition time and improve image quality. Due to the significant advantages in visualizing the brain and spine, MRI has become the gold standard in many emergent conditions such as suspected stroke and spinal cord compression. Increasingly, MRI is being used to diagnosis causes of abdominal pain, examples of such are discussed below.
Appendicitis in Pregnancy
Although acute appendicitis is the most common nonobstetric surgical condition during pregnancy, several factors such as physiological leukocytosis and appendiceal displacement (which reduces the sensitivity of ultrasound) confound the diagnosis. Due to the radiation conveyed by CT, MRI is considered a more appropriate imaging modality in this patient population.2 In a retrospective study of 23,290 pregnant patients, Pedrosa et al3 reported a sensitivity of 100%, specificity of 93.6%, and accuracy of 94% in detecting acute appendicitis in patients for whom ultrasound was inconclusive.
Biliary Pathology
Biliary pathology, particularly choledocholithiasis, may not be demonstrated to advantage with cross-sectional imaging. For example, CT sensitivity for detection is decreased in the absence of ductal dilatation and/or in poorly mineralized stones. Technical factors, such as obesity, patient positioning, and bowel gas, limit evaluation with ultrasound. MRI is able to depict both the intrahepatic and extrahepatic biliary ducts and identify any intraductal lesions (eg, masses, stones). Special MRI contrast agents that are excreted through the bile ducts have come to market in recent years and can assist in evaluation for biliary pathology. The sequences have replaced many of the diagnostic endoscopic retrograde cholangiopancreatograms (ERCP) that used to be performed. An advantage of magnetic resonance cholangiopancreatography (MRCP) is that structures outside of the biliary system may also be evaluated (eg, liver, kidneys).
|
|
Conclusion
The use of MRI for evaluation of abdominal pain continues to increase. While currently limited to specific vulnerable populations (eg, pregnant patients) or for specific conditions (eg, biliary disease), improving technology and availability will allow this technique to expand to more patients and conditions, including evaluation for inflammatory bowel conditions.
References
1. Saini S, Seltzer SE, Bramson RT, et al. Technical cost of radiologic examinations: Analysis across imaging modalities. Radiology. 2000; 216(1):269-272.
2. Appropriateness criteria. American College of Radiology Web site. http://www.acr.org/Quality-Safety/Appropriateness-Criteria. Accessed September 17, 2013.
3. Pedrosa I, Levine D, Eyvazzadeh AD, Siewert B, Ngo L, Rofsky NM. MR imaging evaluation of acute appendicitis in pregnancy. Radiology. 2006;238(3):891-899.
When a Picture Is Worth a Thousand Images:
3D Reconstruction in Emergency and Trauma Imaging
Jamlik-Omari Johnson, MD; Waqas Shuaib, MD
Dr Johnson is assistant professor of radiology and division director of radiology in the department of radiology and imaging services, division of emergency medicine at Emory University School of Medicine, Atlanta, Georgia. Dr Shuaib is a research associate in the department of radiology and imaging services, division of emergency medicine at Emory University School of Medicine, Atlanta, Georgia.
Three-dimensional (3D) reconstruction creates a digital image of a real-life object, capturing both its original shape and appearance. These techniques simulate reality through the use of lighting, color, and motion.1 Advantages of 3D reconstruction in the emergency and trauma setting include enhanced views of anatomy, making pathology that may be difficult to see in the axial plane easily visible (eg, rib fractures). 3D images may also increase interpretation efficiency, allowing rapid review of the large data sets associated with multidetector computed tomography (MDCT) of trauma patients. Since these images can be attached to the radiology report, this modality also enhances service to referring physicians. In addition, as 3D images succinctly illustrate a diagnosis, the treating physician may share them with patients to demonstrate a condition and explain treatment recommendations.
MDCT technology evolved rapidly over the past 25 years. Between 1992 and 2004, MDCT made quantum leaps from dual detectors to 64-slice scanners, and over the last decade, dual-energy source technology and 128-slice scanners have become integrated into clinical practice. These newer devices, along with technical enhancements, provide high temporal and superior spatial resolution, considerably improved image quality, and expanded clinical applications. With MDCT, scans are performed quickly, resulting in improved temporal resolution and reduced motion artifacts. Leverage of this technology in the emergency and trauma setting has expanded the spectrum of indications for use and increased the utility of CT in urgent care. Newer algorithms allow rapid and detailed examinations of the musculoskeletal and the vascular systems in submillimeter slices, without limitation of scan volume. The high-resolution source images are the basis for high quality 3D reconstructions.
The 3D reconstruction toolbox may benefit the surveillance of anatomy, detection of pathology, and planning of therapy. Examples of three such commonly used techniques are shaded surface display (SSD), maximum intensity projection (MIP), and volume rendering (VR).2
SSD provides a realistic 3D view of the surface of a structure within the acquired volume data set and is good at depicting aneurysms and other focal disease (Figure 1). This is particularly useful for evaluating long and tortuous structures, such as the arterial and venous structures. MIP is often used to detect lung nodules by making them stand-out from bronchi and vasculature (Figure 1). Adapted from the computer graphics field, VR creates a 2D image from a 3D model. VR is employed in the exploration of hollow structures, such as the airways or the colon (Figure 2).
Emergency and trauma environments are often fast-paced and high-stakes, and timely and accurate diagnosis is paramount. Therefore, to be useful, advanced 3D-processing tools should be embedded into the diagnostic viewing application (eg, PACS) to allow efficient reconstruction of images, simultaneous comparison of 2D and 3D images, and referencing of historical data in real time.
References
1. Blank M, Kalender WA. Medical volume exploration: gaining insights virtually. Eur J Radiol. 2000;33(3):161-169.
2. Philipp MO, Kubin K, Mang T, Hörmann M, Metz VM. Three-dimensional volume rendering of multidetector-row CT data: applicable for emergency radiology. Eur J Radiol. 2003;48(1):33-38.
After Imaging: Interventional Radiology in the ED
Joshua L. Weintraub, MD, and Thomas J. Ward, MD
Dr Weintraub is an interventional radiologist and executive vice chairman of the department of radiology
at New York Presbyterian/Columbia University Medical Center, New York. Dr Ward is a postgraduate fifth-year resident in interventional radiologist in the department of radiology at Mount Sinai Medical Center, New York, NY.
Interventional radiologists play an increasing role in treating patients in the ED setting. Simplistically, these roles are usually divided into one of three broad categories: to drain or aspirate infected fluid, to halt active bleeding, or to restore blood flow through an occluded vessel. While all of these procedures are critical, the newest advance in interventional radiology (IR) set to benefit the ED patient is not technical or procedural at all, but rather a philosophical one.
Fluid Aspiration and Drainage
In the ED, the need to drain or aspirate infected fluid is probably the most common indication for consult with an interventional radiologist. Perforated appendicitis or diverticulitis with an intra-abdominal abscess is generally managed with percutaneous drainage and antibiotics. If the clinical situation necessitates, and the location of the collection allows, this procedure may be performed at bedside with local anesthesia under ultrasound guidance. In the septic patient, the minimally invasive nature of this procedure confers significant benefits.
Nonoperative patients presenting with acute cholecystitis rapidly improve after the placement of a percutaneous cholecystostomy tube, and this procedure can be performed under ultrasound guidance. IR may also be indicated in cases of failed endoscopic retrograde cholangiopancreatography or cystoscopy in patients with cholangitis or urosepsis for benign or malignant obstruction. Percutaneous access is facilitated by a dilated system, and temporary decompression can be obtained until more definitive therapy is planned.
Active Bleeding
Active bleeding is another common presentation warranting an IR evaluation. Gastrointestinal, intracranial, posttraumatic, postsurgical, and postpartum bleeding, as well as massive hemoptysis, can all be managed endovascularly. Advances in microcatheter technology, covered vascular stents, and embolic agents have increased the efficacy of these interventions, and improved computed tomography angiography protocols facilitate accurate and timely diagnosis of active bleeding. With the availability of these techniques, waiting several hours for a tagged red blood cell nuclear scan is a thing of the past at many institutions. Embolization has become the mainstay of treating bleeding related to trauma in most major trauma centers.
Ischemia
An IR consult may be ordered for patients presenting with the sequelae of ischemia, which can range from a diabetic foot to an acute stroke. Percutaneous balloon angioplasty, endovascular stents, and catheter-directed thrombolysis were all monumental advances in the treatment of ischemia—conceptualized or introduced into clinical practice by interventional radiologists. This spirit of innovation continues. A variety of technical advances, atherectomy, and re-entry devices have been introduced to help recanalize chronically occluded vessels. New devices allow the interventional radiologist to quickly restore blood flow and function to patients suffering from cerebral embolus. There is increased interest in the use of catheter-assisted-embolectomy for submassive pulmonary embolism when intravenous fibrinolysis is unsuccessful or contraindicated.
Conclusion
The five decades of pioneering technical innovation highlighted in this article allow for minimally invasive treatment of the ED patient. The newest advance in IR is not technical, but rather philosophical, and a change in the role that the interventional radiologist plays. The American Board of Radiology has recently approved a new IR training pathway that more than doubles the amount of clinical training that graduating IR fellows now receive. This signals a renewed commitment to running a truly clinical service. Patients in the ED can be evaluated and treated by an interventional radiologist in the hospital and then discharged under the care of an interventional radiologist at an IR clinic. Several IR sections across the country currently practice in such a manner. Although this model is currently the exception and not the rule, the goal of the new training pathway hopes to change this, with increased advances and benefits to patients in the ED setting.
Suggested Reading
1. Gasior AC, Marty Knott E, Ostlie DJ, St Peter SD. To drain or not to drain: an analysis of abscess drains in the treatment of appendicitis with abscess. Pediatr Surg Int. 2013;29(5):455-458.
2. Sato KT. Percutaneous management of biliary emergencies. Semin Intervent Radiol. 2006;23(3):
249-257.
3. Funaki B. On-call treatment of acute gastrointestinal hemorrhage. Intervent Radiol. 2006;23(3):
215-222.
4. Yoon W, Kim JK, Kim YH, Chung TW, Kang HK. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics. 2002;22(6):1395-1409.
5. Stead LG, Gilmore RM, Bellolio MF, Rabinstein AA, Decker WW. Percutaneous clot removal devices in acute ischemic stroke: a systematic review and meta-analysis. Arch Neurol. 2008;65(8):
1024-1030.
6. Kucher N. Catheter embolectomy for acute pulmonary embolism. Chest. 2007;132(2):657-663.
7. Initial certification: vascular/interventional. American Board of Radiology Web site. http://www.theabr.org/ic-vir-landing. Accessed September 24, 2013.
Figure 2. Surface shaded display image of a virtual colonoscopy.
Evaluation of Chest Pain in the Emergency Department by Coronary CT Angiography
James K. Min, MD
Dr Min is director of the Institute of Cardiovascular Imaging at New York-Presbyterian Hospital/Weill-Cornell Medical College, New York.
In the United States each year, approximately 6 million patients present with complaints of chest pain suspicious for acute coronary syndromes (ACS), including unstable angina and myocardial infarction.1 Most of these patients are diagnosed with noncardiac conditions, and nearly half are due to noncardiac etiology. There are an array of diagnostic tests to identify and exclude patients with suspected ACS, such as medical history, cardiac enzyme measurements, electrocardiographic changes, and clinical risk scores. For those with nonnegligible risk for ACS for which this condition cannot be definitively diagnosed or excluded, many are often admitted to the hospital for further testing and observation. Among these patients, less than 30% are found to have ACS. And yet, despite these very careful clinical pathways, between 2% and 8% of patients with ACS are unknowingly discharged to home.2
In recent years, coronary computed tomography angiography (CCTA) has emerged as a noninvasive method that permits direct anatomic visualization of coronary atherosclerosis and luminal stenosis.3 Since the introduction of 64-multidetector row CT scanners in 2005, there have been significant advances in CT technology that now allow for reliable performance of CCTA with very low-dose radiation. Pertaining to the former, improvements in spatial resolution, temporal resolution, and volume coverage enable evaluation of coronary arteries at the submillimeter level, and can be performed in approximately 1 to 5 seconds. Concomitant to the progress in CT technology has been the parallel developments in radiation-dose reduction. As compared to the background radiation exposure of an individual living at sea level for 1 year (~3 millisieverts of radiation), current generation CCTA can be performed at doses <1 millisieverts, with doses approximating a screening mammogram now achievable.
|
|
| ||
Figure. Volume-rendered coronary computed tomographic angiography showing the coronary vascular bed and myocardium (A); normal right coronary artery without evidence of atherosclerosis (B); and (C) left anterior descending artery with mild nonobstructive calcified plaque (white area). | ||||
The diagnostic accuracy of CCTA against invasive coronary angiography has been tested in several prospective multicenter trials.3 For patients without known but suspected coronary artery disease (CAD), the sensitivity and negative predictive value has ranged between 95% to 99%—that is, CCTA can exclude anatomically obstructive CAD with near 100% certainty (Figure). It is these diagnostic performance characteristics that have encouraged several investigators to evaluate the use of CCTA in the diagnostic algorithm of patients presenting to the ED with acute chest pain—the primary intent being to identify sufficiently low-risk patients without significant CAD who can be safely discharged home.
Since 2011, three prospective multicenter randomized controlled trials have evaluated the incorporation of CCTA into a diagnostic chest pain pathway, as compared to standard-of-care algorithms.4-6 Now comprising more than 3,000 patients, these trials have demonstrated remarkably consistent results, with a reduced time-to-diagnosis of 40% to 50%, reduced lengths of stay in the ED of 25%, and reduced ED costs of 20% to 40%. Importantly, these salient effects on resource utilization and economics were underscored by exceptionally safe outcomes, which never exceeded that of the standard of care. Several studies to date have subsequently assessed the duration of safety conferred by a normal CCTA, or its “warranty period.” These studies have shown the warranty period to last at least 7 years for major adverse cardiac events and mortality.7 Moreover, they also engender hope that the chest pain pathways used for millions of Americans annually can indeed be improved, with CCTA playing an essential role.
As its technology continues to iterate, the diagnostic and prognostic performance of CCTA will invariably continue to improve. However, even at present, CCTA is robust and more accurate for exclusion of CAD when compared to other traditional methods of evaluation, and its use for patients presenting to the ED with chest pain improves throughput, reduces costs, and maximizes patient safety.
References
1. Pitts SR, Niska RW, Xu J, Burt CW. National Hospital Ambulatory Medical Care Surviva: 2006 emergency department summary. Natl Health Stat Report. 2008;7:1-38.
2. Pope JH, Aufderheidi TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342(16):1163-1170.
3. Min JK, Shaw LJ, Berman DS. The present state of coronary computed tomography angiography: a process in evolution. J Am Coll Cardiol. 2010;55(:957-965.
4. Litt HI, Gatsonis C, Snyder B, et al. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med. 2012;366(15):1393-403.
5. Hoffmann U, Truong QA, Schoenfeld DA, et al. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med. 2012;367(4):299-308.
6. Goldstein JA, Chinnaiyan KM, Abidov A, et al. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol. 2011;58(14):1414-1422.
7. Andreini D, Pontone G, Mushtaq S, et al. A long-term prognostic value of coronary CT angiography in suspected coronary artery disease. JACC Cardiovasc Imaging. 201
Hepatocellular carcinoma: Options for diagnosing and managing a deadly disease
Hepatocellular carcinoma (HCC) is a common cause of death worldwide. However, it can be detected early in high-risk individuals by using effective screening strategies, resulting in the ability to provide curative treatment.
Here, we review the risk factors for HCC, strategies for surveillance and diagnosis, and therapies that can be used.
EPIDEMIOLOGY
HCC is the most common primary malignancy of the liver. Overall, it is the fifth most common type of cancer in men and the seventh most common in women.1
Cirrhosis is present in 80% to 90% of patients with HCC.
Male sex. The male-to-female ratio is from 2:1 to 4:1, depending on the region.2 In the United States, the overall male-to-female ratio has been reported2 as 2.4:1. In another report,3 the incidence rate of HCC per 100,000 person-years was 3.7 for men and 2.0 for women.
Geographic areas with a high incidence of HCC include sub-Saharan Africa and eastern Asia, whereas Canada and the United States are low-incidence areas. The difference has been because of a lower prevalence of hepatitis B virus infection in North America. However, recent data show a downward trend in incidence of HCC in eastern Asia and an upward trend in North America (Figure 1).3,4
Viral hepatitis (ie, hepatitis B or hepatitis C) is the main risk factor for cirrhosis and HCC.
Diabetes mellitus can predispose to nonalcoholic steatohepatitis, which can subsequently progress to cirrhosis. Thus, it increases the risk of HCC.
Obesity increases the risk of death from liver cancer, with obese people (body mass index ≥ 30 kg/m2) having a higher HCC-related death rate than leaner individuals.5 And as obesity becomes more prevalent, the number of deaths from HCC could increase.
Other diseases that predispose to HCC include alcohol abuse, hereditary hemochromatosis, alpha-1-antitrypsin deficiency, and glycogen storage disease.
SURVEILLANCE OF PATIENTS AT RISK
Patients at high risk of developing liver cancer require frequent screening (Table 1).
Patients with cirrhosis. Sarasin et al6 calculated that surveillance is cost-effective and increases the odds of survival in patients with cirrhosis if the incidence of HCC exceeds 1.5% per year (which it does). In view of this finding, all patients with cirrhosis should be screened every 6 months, irrespective of the cause of the cirrhosis.
Hepatitis B carriers. Surveillance is also indicated in some hepatitis B carriers (Table 1), eg, those with a family history of HCC in a first-degree relative (an independent risk factor for developing the disease in this group).7 Also, Africans with hepatitis B tend to develop HCC early in life.8 Though it has been recommended that surveillance be started at a younger age in these patients,9 the age at which it should begin has not been clearly established. In addition, it is not clear if black people born outside Africa are at higher risk.
Benefit of surveillance
HCC surveillance has shown to lower the death rate. A randomized controlled trial in China compared screening (with abdominal ultrasonography and alpha-fetoprotein levels) vs no screening in patients with hepatitis B. It showed that screening led to a 37% decrease in the death rate.12 Studies have also established that patients with early-stage HCC have a better survival rate than patients with more-advanced disease.10,11 This survival benefit is largely explained by the availability of effective treatments for early-stage cancer, including liver transplantation. Therefore, early-stage asymptomatic patients diagnosed by a surveillance program should have a better survival rate than symptomatic patients.
Surveillance methods
The tests most often used in surveillance for HCC are serum alpha-fetoprotein levels and liver ultrasonography.
Serum alpha-fetoprotein levels by themselves have not been shown to be useful, whereas the combination of alpha-fetoprotein levels and ultrasonography has been shown to reduce the death rate when used for surveillance in a randomized trial.12 A 2012 study reported that the combination of alpha-fetoprotein testing and ultrasonography had a higher sensitivity (90%) than ultrasonography alone (58%), but at the expense of a lower specificity.13
Alpha-fetoprotein has a low sensitivity (ie, 54%) for HCC.14 Tumor size is one of the factors limiting the sensitivity of alpha-fetoprotein, 14 and this would imply that this test may not be helpful in detecting HCC at an early stage. Alpha-fetoprotein L3, an isoform of alpha-fetoprotein, may be helpful in patients with alpha-fetoprotein levels in the intermediate range, and it is currently being studied.
Liver ultrasonography is operator-dependent, and it may not be as accurate in overweight or obese people.
Computed tomography (CT) and magnetic resonance imaging (MRI) are not recommended for surveillance. Serial CT poses risks of radiation-induced damage, contrast-related anaphylaxis, and renal failure, and MRI is not cost-effective and can also lead to gadolinium-induced nephrogenic systemic fibrosis in patients with renal failure.
Currently, the American Association for the Study of Liver Diseases9 recommends ultrasonography only, every 6 months, for surveillance for HCC. However, it may be premature to conclude that alpha-fetoprotein measurement is no longer required for surveillance, and if new data emerge that support its role, it may be reincorporated into the guidelines.
DIAGNOSING HEPATOCELLULAR CARCINOMA
Lesions larger than 1 cm on ultrasonography
The finding of a liver lesion larger than 1 cm on ultrasonography during surveillance warrants further testing.
Noninvasive testing with four-phase multidetector CT or dynamic contrast-enhanced MRI is the next step. Typical findings on either of these imaging studies are sufficient to make a diagnosis of HCC, as they have a high specificity and positive predictive value.15 Arterial hyperenhancement with a venous-phase or delayed-phase washout of contrast medium confirms the diagnosis (Figure 2).9 If one of the two imaging studies is typical for HCC, liver biopsy is not needed.
Other imaging studies, including contrast-enhanced ultrasonography, have not been shown to be specific for this diagnosis.16
Liver biopsy is indicated in patients in whom the imaging findings are atypical for HCC.9,17 Biopsy has very good sensitivity and specificity for cancer, but false-negative findings do occur.18 Therefore, a negative biopsy does not entirely exclude HCC. In this situation, patients should be followed by serial ultrasonography, and any further growth or change in character should be reevaluated.
Lesions smaller than 1 cm
For lesions smaller than 1 cm, the incidence of HCC is low, and currently available diagnostic tests are not reliable.15,19 Lesions of this size should be followed by serial ultrasonography every 3 to 4 months until they either enlarge to greater than 1 cm or remain stable at 2 years.9 If they remain stable at the end of 2 years, regular surveillance ultrasonography once every 6 months can be continued.
CURATIVE AND PALLIATIVE THERAPIES
Therapies for HCC (Table 2) can be divided into two categories: curative and palliative.
Curative treatments include surgical resection, liver transplantation, and radiofrequency ablation. All other treatments are palliative, including transarterial chemoembolization and medical therapy with sorafenib.
The choice of treatment depends on the characteristics of the tumor, the degree of liver dysfunction, and the patient’s current level of function. The Barcelona Clinic Liver Cancer classification is widely used in making these decisions, as it incorporates both clinical features and tumor stage.9 Figure 3 shows a simplified management algorithm.
SURGICAL RESECTION
Surgical resection is the preferred treatment for patients who have a solitary HCC lesion without cirrhosis.9 It is also indicated in patients with well-compensated cirrhosis who have normal portal pressure, a normal serum bilirubin level, and a platelet count greater than 100 × 109/L.20,21 In such patients, the 5-year survival rate is about 74%, compared with 25% in patients with portal hypertension and serum bilirubin levels higher than 1 mg/dL.21
Surgical resection is not recommended for patients with decompensated cirrhosis, as it can worsen liver function postoperatively and increase the risk of death.19,20 In Western countries, where cirrhosis from hepatitis C is the commonest cause of HCC, most patients have poorly preserved hepatic function at the time of diagnosis, leaving only a minority of patients as candidates for surgical resection.
After surgical resection of HCC, the recurrence rate can be as high as 70% to 80% at 5 years.22,23 Studies have consistently found larger tumor size and vascular invasion to be factors that predict recurrence.24,25 Vascular invasion was also found to predict poor survival after recurrence.24 Studies have so far not shown any conclusive benefit from post-surgical adjuvant chemotherapy in reducing the rate of recurrence of HCC.26,27
How to treat recurrent HCC after surgical resection has not been clearly established. Radiofrequency ablation, transarterial chemoembolization, repeat resection, and liver transplantation have all improved survival when used alone or in combination.28 However, randomized controlled trials are needed to establish the effective treatment strategy and the benefit of multimodal treatment of recurrent HCC.
LIVER TRANSPLANTATION
Orthotopic liver transplantation is the preferred treatment for patients with HCC complicated by cirrhosis and portal hypertension. It has the advantage not only of being potentially curative, but also of overcoming liver cirrhosis by replacing the liver.
To qualify for liver transplantation, patients must meet the Milan criteria (ie, have a single nodule less than 5 cm in diameter or up to three nodules, with the largest being less than 3 cm in diameter, with no evidence of vascular invasion or distant metastasis). These patients have an expected 4-year survival rate of 85% and a recurrence-free survival rate of 92% after transplantation, compared with 50% and 59%, respectively, in patients whose tumors exceeded these criteria.29
Some believe that the Milan criteria are too restrictive and could be expanded. Yao et al at the University of California-San Francisco30 reported that patients with HCC meeting the criteria of having a solitary tumor smaller than 6.5 cm or having up to three nodules, with the largest smaller than 4.5 cm, and total tumor diameter less than 8 cm, had survival rates of 90% at 1 year and 75.2% at 5 years after liver transplantation, compared with 50% at 1 year for patients with tumors exceeding these limits. (These have come to be known as the UCSF criteria.) However, the United Network for Organ Sharing (UNOS) has not adopted these expanded criteria. UNOS has a point system for allocating livers for transplant called the Model for End-Stage Liver Disease (MELD). Patients who meet the Milan criteria receive extra points, putting them higher on the transplant list. This allows for early transplantation, thus reducing tumor progression and dropout from the transplant list. UNOS allocates a MELD score of 22 to all patients who meet the Milan criteria, and the score is further adjusted once every 3 months to reflect a 10% increase in the mortality rate. However, patients who have a single lesion smaller than 2 cm and are candidates for liver transplantation are not assigned additional MELD points per UNOS policy, as the risk of tumor progression beyond the Milan criteria in these patients is deemed to be low.
Therapies while awaiting transplantation
Even if they receive additional MELD points to give them priority on the waiting list, patients face a considerable wait before transplantation because of the limited availability of donor organs. In the interim, they have a risk of tumor progression beyond the Milan criteria and subsequent dropout from the transplant list.31 Patients on the waiting list may therefore undergo a locoregional therapy such as transarterial chemoembolization or radiofrequency ablation as bridging therapy.
These therapies have been shown to decrease dropout from the waiting list.31 A prospective study showed that in 48 patients who underwent transarterial chemoembolization while awaiting liver transplantation, none had tumor progression, and 41 did receive a transplant, with excellent posttransplantation survival rates.32 Similarly, radioembolization using yttrium-90-labeled microspheres or radiofrequency ablation while on the waiting list has been shown to significantly decrease the rate of dropout, with good posttransplantation outcomes.33,34
However, in spite of these benefits, these bridging therapies do not increase survival rates after transplantation. It is also unclear whether they are useful in regions with short waiting times for liver transplantation.
Adjuvant systemic chemotherapy has not been shown to improve survival in patients undergoing liver transplantation. For example, in a randomized controlled trial of doxorubicin given before, during, and after surgery, the survival rate at 5 years was 38% with doxorubicin and 40% without.35
ABLATIVE LOCOREGIONAL THERAPIES
Locoregional therapies play an important role in managing HCC. They are classified as ablative and perfusion-based.
Ablative locoregional therapies include chemical modalities such as percutaneous ethanol injection; thermal therapies such as radiofrequency ablation, microwave ablation, laser ablation, and cryotherapy; and newer methods such as irreversible electroporation and light-activated drug therapy. Of these, radiofrequency ablation is the most widely used.
Radiofrequency ablation
Radiofrequency ablation induces thermal injury, resulting in tumor necrosis. It can be used as an alternative to surgery in patients who have a single HCC lesion less than 3 to 5 cm in diameter, confined to the liver, and in a site amenable to this procedure and who have a reasonable coagulation profile. The procedure can be performed percutaneously or via laparoscopy.
Radiofrequency ablation is contraindicated in patients with decompensated cirrhosis, Child-Pugh class C cirrhosis (the most severe category), vascular or bile duct invasion, extrahepatic disease, or lesions that are not accessible or are adjacent to structures such as the gall bladder, bowel, stomach, or diaphragm.
Radiofrequency ablation has been compared with surgical resection in patients who had small tumors. Though a randomized controlled trial did not show any difference between the two treatment groups in terms of survival at 5 years and recurrence rates,36 a meta-analysis showed that overall survival rates at 3 years and 5 years were significantly higher with surgical resection than with radiofrequency ablation.37 Patients also had a higher rate of local recurrence with radiofrequency ablation than with surgical resection.37 In addition, radiofrequency ablation has been shown to be effective only in small tumors and does not perform as well in lesions larger than 2 or 3 cm.
Thus, based on current evidence, surgical resection is preferable to radiofrequency ablation as first-line treatment. The latter, however, is also used as a bridging therapy in patients awaiting liver transplantation.
Percutaneous ethanol injection
Percutaneous ethanol injection is used less frequently than radiofrequency ablation, as studies have shown the latter to be superior in regard to local recurrence-free survival rates.38 However, percutaneous ethanol injection is used instead of radiofrequency ablation in a small number of patients, when the lesion is very close to organs such as the bile duct (which could be damaged by radiofrequency ablation) or the large vessels (which may make radiofrequency ablation less effective, since heat may dissipate as a result of excessive blood flow in this region).
Microwave ablation
Microwave ablation is an emerging therapy for HCC. Its advantage over radiofrequency ablation is that its use is not limited by blood vessels in close proximity to the ablation site.
Earlier studies did not show microwave ablation to be superior to radiofrequency ablation.39,40 However, current studies involving newer techniques of microwave ablation are more promising.41
PERFUSION-BASED LOCOREGIONAL THERAPIES
Perfusion-based locoregional therapies deliver embolic particles, chemotherapeutic agents, or radioactive materials into the artery feeding the tumor. The portal blood flow allows for preservation of vital liver tissue during arterial embolization of liver tumors. Perfusionbased therapies include transarterial chemoembolization, transarterial chemoembolization with doxorubicin-eluting beads (DEB-TACE), “bland” embolization, and radioembolization.
Transarterial chemoembolization
Transarterial chemoembolization is a minimally invasive procedure in which the hepatic artery is cannulated through a percutaneous puncture, the branches of the hepatic artery supplying the tumor are identified, and then embolic particles and chemotherapeutic agents are injected. This serves a dual purpose: it embolizes the feeding vessel that supplies the tumor, causing tumor necrosis, and it focuses the chemotherapy on the tumor and thus minimizes the systemic effects of the chemotherapeutic agent.
This therapy is contraindicated in patients with portal vein thrombosis, advanced liver dysfunction, or a transjugular intrahepatic portosystemic shunt. Side effects of the procedure include a postembolization syndrome of abdominal pain and fever (occurring in about 50% of patients from ischemic injury to the liver), hepatic abscesses, injury to the hepatic artery, development of ascites, liver dysfunction, and contrast-induced renal failure.
In addition to bridging patients to liver transplantation, transarterial chemoembolization is recommended as palliative treatment to prolong survival in patients with HCC who are not candidates for liver transplantation, surgical resection, or radiofrequency ablation.9,42 Patients who have Child-Pugh grade A or B cirrhosis but do not have main portal vein thrombosis or extrahepatic spread are candidates for this therapy. Patients such as these who undergo this therapy have a better survival rate at 2 years compared with untreated patients.43,44
Transarterial chemoembolization has also been used to reduce the size of (ie, to “downstage”) tumors that are outside the Milan criteria in patients who are otherwise candidates for liver transplantation. It induces tumor necrosis and has been shown to decrease the tumor size in a selected group of patients and to bring them within the Milan criteria, thus potentially enabling them to be put on the transplant list.45 Studies have shown that patients who receive a transplant after successful down-staging may achieve a 5-year survival rate comparable with that of patients who were initially within the Milan criteria and received a transplant without the need for down-staging.45 However, factors that predict successful down-staging have not been clearly established.
Newer techniques have been developed. A randomized controlled trial found transarterial chemoembolization with doxorubicin-eluting beads to be safer and better tolerated than conventional transarterial chemembolization.46
Bland embolization is transarterial embolization without chemotherapeutic agents and is performed in patients with significant liver dysfunction who might not tolerate chemotherapy. The benefits of this approach are yet to be determined.
Radioembolization
Radioembolization with yttrium-90 microspheres has recently been introduced as an alternative to transarterial chemoembolization, especially in patients with portal vein thrombosis, a portocaval shunt, or a transjugular intrahepatic portosystemic shunt.
In observational studies, radioembolization was as effective as transarterial chemoembolization, with a similar survival benefit.47 However, significant pulmonary shunting must be ruled out before radioembolization, as it would lead to radiation-induced pulmonary disease. Randomized controlled trials are under way to compare the efficacy of the two methods.
CHEMOTHERAPY
Sorafenib
Sorafenib is an oral antiangiogenic agent. A kinase inhibitor, it interacts with multiple intracellular and cell-surface kinases, including vascular endothelial growth factor receptor, platelet-derived growth factor receptor, and Raf proto-oncogene, inhibiting tumor cell proliferation and angiogenesis.
Sorafenib has been shown to prolong survival in patients with advanced-stage HCC.48 A randomized placebo-controlled trial in patients with Child-Pugh grade A cirrhosis and advanced HCC who had not received chemotherapy showed that sorafenib increased the life expectancy by nearly 3 months compared with placebo.47 Sorafenib therapy is very expensive, but it is usually covered by insurance.
Sorafenib is recommended in patients who have advanced HCC with vascular invasion, extrahepatic dissemination, or minimal constitutional symptoms. It is not recommended for patients with severe advanced liver disease who have moderate to severe tumor-related constitutional symptoms or Child-Pugh grade C cirrhosis, or for patients with a life expectancy of less than 3 months.
The most common side effects of sorafenib are diarrhea, weight loss, and skin reactions on the hands and feet. These commonly lead to decreased tolerability and dose reductions.47 Doses should be adjusted on the basis of the bilirubin and albumin levels.49
Other chemotherapeutic agents
Several molecular targeted agents are undergoing clinical trials for the treatment of HCC. These include bevacizumab, erlotinib, brivanib, and ramucirumab. Chemotherapeutic agents such as doxorubicin and everolimus are also being studied.
PALLIATIVE TREATMENT
Patients with end-stage HCC with moderate to severe constitutional symptoms, extrahepatic disease progression, and decompensated liver disease have a survival of less than 3 months and are treated for pain and symptom control.9
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893–2917.
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007; 132:2557–2576.
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012; 142:1264–1273.e1.
- Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009; 27:1485–1491.
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med 2003; 348:1625–1638.
- Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosis. Am J Med 1996; 101:422–434.
- Yu MW, Chang HC, Liaw YF, et al. Familial risk of hepatocellular carcinoma among chronic hepatitis B carriers and their relatives. J Natl Cancer Inst 2000; 92:1159–1164.
- Kew MC, Macerollo P. Effect of age on the etiologic role of the hepatitis B virus in hepatocellular carcinoma in blacks. Gastroenterology 1988; 94:439–442.
- Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53:1020–1022.
- Bruix J, Llovet JM. Major achievements in hepatocellular carcinoma. Lancet 2009; 373:614–616.
- Gómez-Rodríguez R, Romero-Gutiérrez M, Artaza-Varasa T, et al. The value of the Barcelona Clinic Liver Cancer and alpha-fetoprotein in the prognosis of hepatocellular carcinoma. Rev Esp Enferm Dig 2012; 104:298–304.
- Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004; 130:417–422.
- Giannini EG, Erroi V, Trevisani F. Effectiveness of a-fetoprotein for hepatocellular carcinoma surveillance: the return of the living-dead? Expert Rev Gastroenterol Hepatol 2012; 6:441–444.
- Farinati F, Marino D, De Giorgio M, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol 2006; 101:524–532.
- Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008; 47:97–104.
- Vilana R, Forner A, Bianchi L, et al. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology 2010; 51:2020–2029.
- Kojiro M. Pathological diagnosis at early stage: reaching international consensus. Oncology 2010; 78(suppl 1):31–35.
- Schölmerich J, Schacherer D. Diagnostic biopsy for hepatocellular carcinoma in cirrhosis: useful, necessary, dangerous, or academic sport? Gut 2004; 53:1224–1226.
- Durand F, Regimbeau JM, Belghiti J, et al. Assessment of the benefits and risks of percutaneous biopsy before surgical resection of hepatocellular carcinoma. J Hepatol 2001; 35:254–258.
- Bruix J, Castells A, Bosch J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology 1996; 111:1018–1022.
- Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999; 30:1434–1440.
- Nagasue N, Uchida M, Makino Y, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology 1993; 105:488–494.
- Arii S, Tanaka J, Yamazoe Y, et al. Predictive factors for intrahepatic recurrence of hepatocellular carcinoma after partial hepatectomy. Cancer 1992; 69:913–919.
- Cha C, Fong Y, Jarnagin WR, Blumgart LH, DeMatteo RP. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg 2003; 197:753–758.
- Shah SA, Cleary SP, Wei AC, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery 2007; 141:330–339.
- Kohno H, Nagasue N, Hayashi T, et al. Postoperative adjuvant chemotherapy after radical hepatic resection for hepatocellular carcinoma (HCC). Hepatogastroenterology 1996; 43:1405–1409.
- Ono T, Nagasue N, Kohno H, et al. Adjuvant chemotherapy with epirubicin and carmofur after radical resection of hepatocellular carcinoma: a prospective randomized study. Semin Oncol 1997; 24(suppl 6):S6–25.
- Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: Long-term results of treatment and prognostic factors. Ann Surg 1999; 229:216–222.
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334:693–699.
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001; 33:1394–1403.
- Majno P, Lencioni R, Mornex F, Girard N, Poon RT, Cherqui D. Is the treatment of hepatocellular carcinoma on the waiting list necessary? Liver Transpl 2011; 17(suppl 2):S98–S108.
- Graziadei IW, Sandmueller H, Waldenberger P, et al. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl 2003; 9:557–563.
- Kulik LM, Atassi B, van Holsbeeck L, et al. Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol 2006; 94:572–586.
- Lu DS, Yu NC, Raman SS, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology 2005; 41:1130–1137.
- Pokorny H, Gnant M, Rasoul-Rockenschaub S, et al. Does additional doxorubicin chemotherapy improve outcome in patients with hepatocellular carcinoma treated by liver transplantation? Am J Transplant 2005; 5:788–794.
- Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 2012; 57:794–802.
- Zhou Y, Zhao Y, Li B, et al. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol 2010; 10:78.
- Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: Randomized comparison of radiofrequency thermal ablation versus percutaneous ethanol injection. Radiology 2003; 228:235–240.
- Ohmoto K, Yoshioka N, Tomiyama Y, et al. Comparison of therapeutic effects between radiofrequency ablation and percutaneous microwave coagulation therapy for small hepatocellular carcinomas. J Gastroenterol Hepatol 2009; 24:223–227.
- Shibata T, Iimuro Y, Yamamoto Y, et al. Small hepatocellular carcinoma: comparison of radiofrequency ablation and percutaneous microwave coagulation therapy. Radiology 2002; 223:331–337.
- Qian GJ, Wang N, Shen Q, et al. Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: Experimental and clinical studies. Eur Radiol 2012; 22:1983–1990.
- Burrel M, Reig M, Forner A, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using drug eluting beads. Implications for clinical practice and trial design. J Hepatol 2012; 56:1330–1335.
- Cammà C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology 2002; 224:47–54.
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 2003; 37:429–442.
- Yao FY, Kerlan RK, Hirose R, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology 2008; 48:819–827.
- Ferrer Puchol MD, la Parra C, Esteban E, et al. Comparison of doxorubicin-eluting bead transarterial chemoembolization (DEBTACE) with conventional transarterial chemoembolization (TACE) for the treatment of hepatocellular carcinoma (article in Spanish). Radiologia 2011; 53:246–253.
- Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2011; 140:497–507.e2.
- Llovet JM, Ricci S, Mazzaferro V, et al; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359:378–390.
- Miller AA, Murry DJ, Owzar K, et al. Phase I and pharmacokinetic study of sorafenib in patients with hepatic or renal dysfunction: CALGB 60301. J Clin Oncol 2009; 27:1800–1805.
Hepatocellular carcinoma (HCC) is a common cause of death worldwide. However, it can be detected early in high-risk individuals by using effective screening strategies, resulting in the ability to provide curative treatment.
Here, we review the risk factors for HCC, strategies for surveillance and diagnosis, and therapies that can be used.
EPIDEMIOLOGY
HCC is the most common primary malignancy of the liver. Overall, it is the fifth most common type of cancer in men and the seventh most common in women.1
Cirrhosis is present in 80% to 90% of patients with HCC.
Male sex. The male-to-female ratio is from 2:1 to 4:1, depending on the region.2 In the United States, the overall male-to-female ratio has been reported2 as 2.4:1. In another report,3 the incidence rate of HCC per 100,000 person-years was 3.7 for men and 2.0 for women.
Geographic areas with a high incidence of HCC include sub-Saharan Africa and eastern Asia, whereas Canada and the United States are low-incidence areas. The difference has been because of a lower prevalence of hepatitis B virus infection in North America. However, recent data show a downward trend in incidence of HCC in eastern Asia and an upward trend in North America (Figure 1).3,4
Viral hepatitis (ie, hepatitis B or hepatitis C) is the main risk factor for cirrhosis and HCC.
Diabetes mellitus can predispose to nonalcoholic steatohepatitis, which can subsequently progress to cirrhosis. Thus, it increases the risk of HCC.
Obesity increases the risk of death from liver cancer, with obese people (body mass index ≥ 30 kg/m2) having a higher HCC-related death rate than leaner individuals.5 And as obesity becomes more prevalent, the number of deaths from HCC could increase.
Other diseases that predispose to HCC include alcohol abuse, hereditary hemochromatosis, alpha-1-antitrypsin deficiency, and glycogen storage disease.
SURVEILLANCE OF PATIENTS AT RISK
Patients at high risk of developing liver cancer require frequent screening (Table 1).
Patients with cirrhosis. Sarasin et al6 calculated that surveillance is cost-effective and increases the odds of survival in patients with cirrhosis if the incidence of HCC exceeds 1.5% per year (which it does). In view of this finding, all patients with cirrhosis should be screened every 6 months, irrespective of the cause of the cirrhosis.
Hepatitis B carriers. Surveillance is also indicated in some hepatitis B carriers (Table 1), eg, those with a family history of HCC in a first-degree relative (an independent risk factor for developing the disease in this group).7 Also, Africans with hepatitis B tend to develop HCC early in life.8 Though it has been recommended that surveillance be started at a younger age in these patients,9 the age at which it should begin has not been clearly established. In addition, it is not clear if black people born outside Africa are at higher risk.
Benefit of surveillance
HCC surveillance has shown to lower the death rate. A randomized controlled trial in China compared screening (with abdominal ultrasonography and alpha-fetoprotein levels) vs no screening in patients with hepatitis B. It showed that screening led to a 37% decrease in the death rate.12 Studies have also established that patients with early-stage HCC have a better survival rate than patients with more-advanced disease.10,11 This survival benefit is largely explained by the availability of effective treatments for early-stage cancer, including liver transplantation. Therefore, early-stage asymptomatic patients diagnosed by a surveillance program should have a better survival rate than symptomatic patients.
Surveillance methods
The tests most often used in surveillance for HCC are serum alpha-fetoprotein levels and liver ultrasonography.
Serum alpha-fetoprotein levels by themselves have not been shown to be useful, whereas the combination of alpha-fetoprotein levels and ultrasonography has been shown to reduce the death rate when used for surveillance in a randomized trial.12 A 2012 study reported that the combination of alpha-fetoprotein testing and ultrasonography had a higher sensitivity (90%) than ultrasonography alone (58%), but at the expense of a lower specificity.13
Alpha-fetoprotein has a low sensitivity (ie, 54%) for HCC.14 Tumor size is one of the factors limiting the sensitivity of alpha-fetoprotein, 14 and this would imply that this test may not be helpful in detecting HCC at an early stage. Alpha-fetoprotein L3, an isoform of alpha-fetoprotein, may be helpful in patients with alpha-fetoprotein levels in the intermediate range, and it is currently being studied.
Liver ultrasonography is operator-dependent, and it may not be as accurate in overweight or obese people.
Computed tomography (CT) and magnetic resonance imaging (MRI) are not recommended for surveillance. Serial CT poses risks of radiation-induced damage, contrast-related anaphylaxis, and renal failure, and MRI is not cost-effective and can also lead to gadolinium-induced nephrogenic systemic fibrosis in patients with renal failure.
Currently, the American Association for the Study of Liver Diseases9 recommends ultrasonography only, every 6 months, for surveillance for HCC. However, it may be premature to conclude that alpha-fetoprotein measurement is no longer required for surveillance, and if new data emerge that support its role, it may be reincorporated into the guidelines.
DIAGNOSING HEPATOCELLULAR CARCINOMA
Lesions larger than 1 cm on ultrasonography
The finding of a liver lesion larger than 1 cm on ultrasonography during surveillance warrants further testing.
Noninvasive testing with four-phase multidetector CT or dynamic contrast-enhanced MRI is the next step. Typical findings on either of these imaging studies are sufficient to make a diagnosis of HCC, as they have a high specificity and positive predictive value.15 Arterial hyperenhancement with a venous-phase or delayed-phase washout of contrast medium confirms the diagnosis (Figure 2).9 If one of the two imaging studies is typical for HCC, liver biopsy is not needed.
Other imaging studies, including contrast-enhanced ultrasonography, have not been shown to be specific for this diagnosis.16
Liver biopsy is indicated in patients in whom the imaging findings are atypical for HCC.9,17 Biopsy has very good sensitivity and specificity for cancer, but false-negative findings do occur.18 Therefore, a negative biopsy does not entirely exclude HCC. In this situation, patients should be followed by serial ultrasonography, and any further growth or change in character should be reevaluated.
Lesions smaller than 1 cm
For lesions smaller than 1 cm, the incidence of HCC is low, and currently available diagnostic tests are not reliable.15,19 Lesions of this size should be followed by serial ultrasonography every 3 to 4 months until they either enlarge to greater than 1 cm or remain stable at 2 years.9 If they remain stable at the end of 2 years, regular surveillance ultrasonography once every 6 months can be continued.
CURATIVE AND PALLIATIVE THERAPIES
Therapies for HCC (Table 2) can be divided into two categories: curative and palliative.
Curative treatments include surgical resection, liver transplantation, and radiofrequency ablation. All other treatments are palliative, including transarterial chemoembolization and medical therapy with sorafenib.
The choice of treatment depends on the characteristics of the tumor, the degree of liver dysfunction, and the patient’s current level of function. The Barcelona Clinic Liver Cancer classification is widely used in making these decisions, as it incorporates both clinical features and tumor stage.9 Figure 3 shows a simplified management algorithm.
SURGICAL RESECTION
Surgical resection is the preferred treatment for patients who have a solitary HCC lesion without cirrhosis.9 It is also indicated in patients with well-compensated cirrhosis who have normal portal pressure, a normal serum bilirubin level, and a platelet count greater than 100 × 109/L.20,21 In such patients, the 5-year survival rate is about 74%, compared with 25% in patients with portal hypertension and serum bilirubin levels higher than 1 mg/dL.21
Surgical resection is not recommended for patients with decompensated cirrhosis, as it can worsen liver function postoperatively and increase the risk of death.19,20 In Western countries, where cirrhosis from hepatitis C is the commonest cause of HCC, most patients have poorly preserved hepatic function at the time of diagnosis, leaving only a minority of patients as candidates for surgical resection.
After surgical resection of HCC, the recurrence rate can be as high as 70% to 80% at 5 years.22,23 Studies have consistently found larger tumor size and vascular invasion to be factors that predict recurrence.24,25 Vascular invasion was also found to predict poor survival after recurrence.24 Studies have so far not shown any conclusive benefit from post-surgical adjuvant chemotherapy in reducing the rate of recurrence of HCC.26,27
How to treat recurrent HCC after surgical resection has not been clearly established. Radiofrequency ablation, transarterial chemoembolization, repeat resection, and liver transplantation have all improved survival when used alone or in combination.28 However, randomized controlled trials are needed to establish the effective treatment strategy and the benefit of multimodal treatment of recurrent HCC.
LIVER TRANSPLANTATION
Orthotopic liver transplantation is the preferred treatment for patients with HCC complicated by cirrhosis and portal hypertension. It has the advantage not only of being potentially curative, but also of overcoming liver cirrhosis by replacing the liver.
To qualify for liver transplantation, patients must meet the Milan criteria (ie, have a single nodule less than 5 cm in diameter or up to three nodules, with the largest being less than 3 cm in diameter, with no evidence of vascular invasion or distant metastasis). These patients have an expected 4-year survival rate of 85% and a recurrence-free survival rate of 92% after transplantation, compared with 50% and 59%, respectively, in patients whose tumors exceeded these criteria.29
Some believe that the Milan criteria are too restrictive and could be expanded. Yao et al at the University of California-San Francisco30 reported that patients with HCC meeting the criteria of having a solitary tumor smaller than 6.5 cm or having up to three nodules, with the largest smaller than 4.5 cm, and total tumor diameter less than 8 cm, had survival rates of 90% at 1 year and 75.2% at 5 years after liver transplantation, compared with 50% at 1 year for patients with tumors exceeding these limits. (These have come to be known as the UCSF criteria.) However, the United Network for Organ Sharing (UNOS) has not adopted these expanded criteria. UNOS has a point system for allocating livers for transplant called the Model for End-Stage Liver Disease (MELD). Patients who meet the Milan criteria receive extra points, putting them higher on the transplant list. This allows for early transplantation, thus reducing tumor progression and dropout from the transplant list. UNOS allocates a MELD score of 22 to all patients who meet the Milan criteria, and the score is further adjusted once every 3 months to reflect a 10% increase in the mortality rate. However, patients who have a single lesion smaller than 2 cm and are candidates for liver transplantation are not assigned additional MELD points per UNOS policy, as the risk of tumor progression beyond the Milan criteria in these patients is deemed to be low.
Therapies while awaiting transplantation
Even if they receive additional MELD points to give them priority on the waiting list, patients face a considerable wait before transplantation because of the limited availability of donor organs. In the interim, they have a risk of tumor progression beyond the Milan criteria and subsequent dropout from the transplant list.31 Patients on the waiting list may therefore undergo a locoregional therapy such as transarterial chemoembolization or radiofrequency ablation as bridging therapy.
These therapies have been shown to decrease dropout from the waiting list.31 A prospective study showed that in 48 patients who underwent transarterial chemoembolization while awaiting liver transplantation, none had tumor progression, and 41 did receive a transplant, with excellent posttransplantation survival rates.32 Similarly, radioembolization using yttrium-90-labeled microspheres or radiofrequency ablation while on the waiting list has been shown to significantly decrease the rate of dropout, with good posttransplantation outcomes.33,34
However, in spite of these benefits, these bridging therapies do not increase survival rates after transplantation. It is also unclear whether they are useful in regions with short waiting times for liver transplantation.
Adjuvant systemic chemotherapy has not been shown to improve survival in patients undergoing liver transplantation. For example, in a randomized controlled trial of doxorubicin given before, during, and after surgery, the survival rate at 5 years was 38% with doxorubicin and 40% without.35
ABLATIVE LOCOREGIONAL THERAPIES
Locoregional therapies play an important role in managing HCC. They are classified as ablative and perfusion-based.
Ablative locoregional therapies include chemical modalities such as percutaneous ethanol injection; thermal therapies such as radiofrequency ablation, microwave ablation, laser ablation, and cryotherapy; and newer methods such as irreversible electroporation and light-activated drug therapy. Of these, radiofrequency ablation is the most widely used.
Radiofrequency ablation
Radiofrequency ablation induces thermal injury, resulting in tumor necrosis. It can be used as an alternative to surgery in patients who have a single HCC lesion less than 3 to 5 cm in diameter, confined to the liver, and in a site amenable to this procedure and who have a reasonable coagulation profile. The procedure can be performed percutaneously or via laparoscopy.
Radiofrequency ablation is contraindicated in patients with decompensated cirrhosis, Child-Pugh class C cirrhosis (the most severe category), vascular or bile duct invasion, extrahepatic disease, or lesions that are not accessible or are adjacent to structures such as the gall bladder, bowel, stomach, or diaphragm.
Radiofrequency ablation has been compared with surgical resection in patients who had small tumors. Though a randomized controlled trial did not show any difference between the two treatment groups in terms of survival at 5 years and recurrence rates,36 a meta-analysis showed that overall survival rates at 3 years and 5 years were significantly higher with surgical resection than with radiofrequency ablation.37 Patients also had a higher rate of local recurrence with radiofrequency ablation than with surgical resection.37 In addition, radiofrequency ablation has been shown to be effective only in small tumors and does not perform as well in lesions larger than 2 or 3 cm.
Thus, based on current evidence, surgical resection is preferable to radiofrequency ablation as first-line treatment. The latter, however, is also used as a bridging therapy in patients awaiting liver transplantation.
Percutaneous ethanol injection
Percutaneous ethanol injection is used less frequently than radiofrequency ablation, as studies have shown the latter to be superior in regard to local recurrence-free survival rates.38 However, percutaneous ethanol injection is used instead of radiofrequency ablation in a small number of patients, when the lesion is very close to organs such as the bile duct (which could be damaged by radiofrequency ablation) or the large vessels (which may make radiofrequency ablation less effective, since heat may dissipate as a result of excessive blood flow in this region).
Microwave ablation
Microwave ablation is an emerging therapy for HCC. Its advantage over radiofrequency ablation is that its use is not limited by blood vessels in close proximity to the ablation site.
Earlier studies did not show microwave ablation to be superior to radiofrequency ablation.39,40 However, current studies involving newer techniques of microwave ablation are more promising.41
PERFUSION-BASED LOCOREGIONAL THERAPIES
Perfusion-based locoregional therapies deliver embolic particles, chemotherapeutic agents, or radioactive materials into the artery feeding the tumor. The portal blood flow allows for preservation of vital liver tissue during arterial embolization of liver tumors. Perfusionbased therapies include transarterial chemoembolization, transarterial chemoembolization with doxorubicin-eluting beads (DEB-TACE), “bland” embolization, and radioembolization.
Transarterial chemoembolization
Transarterial chemoembolization is a minimally invasive procedure in which the hepatic artery is cannulated through a percutaneous puncture, the branches of the hepatic artery supplying the tumor are identified, and then embolic particles and chemotherapeutic agents are injected. This serves a dual purpose: it embolizes the feeding vessel that supplies the tumor, causing tumor necrosis, and it focuses the chemotherapy on the tumor and thus minimizes the systemic effects of the chemotherapeutic agent.
This therapy is contraindicated in patients with portal vein thrombosis, advanced liver dysfunction, or a transjugular intrahepatic portosystemic shunt. Side effects of the procedure include a postembolization syndrome of abdominal pain and fever (occurring in about 50% of patients from ischemic injury to the liver), hepatic abscesses, injury to the hepatic artery, development of ascites, liver dysfunction, and contrast-induced renal failure.
In addition to bridging patients to liver transplantation, transarterial chemoembolization is recommended as palliative treatment to prolong survival in patients with HCC who are not candidates for liver transplantation, surgical resection, or radiofrequency ablation.9,42 Patients who have Child-Pugh grade A or B cirrhosis but do not have main portal vein thrombosis or extrahepatic spread are candidates for this therapy. Patients such as these who undergo this therapy have a better survival rate at 2 years compared with untreated patients.43,44
Transarterial chemoembolization has also been used to reduce the size of (ie, to “downstage”) tumors that are outside the Milan criteria in patients who are otherwise candidates for liver transplantation. It induces tumor necrosis and has been shown to decrease the tumor size in a selected group of patients and to bring them within the Milan criteria, thus potentially enabling them to be put on the transplant list.45 Studies have shown that patients who receive a transplant after successful down-staging may achieve a 5-year survival rate comparable with that of patients who were initially within the Milan criteria and received a transplant without the need for down-staging.45 However, factors that predict successful down-staging have not been clearly established.
Newer techniques have been developed. A randomized controlled trial found transarterial chemoembolization with doxorubicin-eluting beads to be safer and better tolerated than conventional transarterial chemembolization.46
Bland embolization is transarterial embolization without chemotherapeutic agents and is performed in patients with significant liver dysfunction who might not tolerate chemotherapy. The benefits of this approach are yet to be determined.
Radioembolization
Radioembolization with yttrium-90 microspheres has recently been introduced as an alternative to transarterial chemoembolization, especially in patients with portal vein thrombosis, a portocaval shunt, or a transjugular intrahepatic portosystemic shunt.
In observational studies, radioembolization was as effective as transarterial chemoembolization, with a similar survival benefit.47 However, significant pulmonary shunting must be ruled out before radioembolization, as it would lead to radiation-induced pulmonary disease. Randomized controlled trials are under way to compare the efficacy of the two methods.
CHEMOTHERAPY
Sorafenib
Sorafenib is an oral antiangiogenic agent. A kinase inhibitor, it interacts with multiple intracellular and cell-surface kinases, including vascular endothelial growth factor receptor, platelet-derived growth factor receptor, and Raf proto-oncogene, inhibiting tumor cell proliferation and angiogenesis.
Sorafenib has been shown to prolong survival in patients with advanced-stage HCC.48 A randomized placebo-controlled trial in patients with Child-Pugh grade A cirrhosis and advanced HCC who had not received chemotherapy showed that sorafenib increased the life expectancy by nearly 3 months compared with placebo.47 Sorafenib therapy is very expensive, but it is usually covered by insurance.
Sorafenib is recommended in patients who have advanced HCC with vascular invasion, extrahepatic dissemination, or minimal constitutional symptoms. It is not recommended for patients with severe advanced liver disease who have moderate to severe tumor-related constitutional symptoms or Child-Pugh grade C cirrhosis, or for patients with a life expectancy of less than 3 months.
The most common side effects of sorafenib are diarrhea, weight loss, and skin reactions on the hands and feet. These commonly lead to decreased tolerability and dose reductions.47 Doses should be adjusted on the basis of the bilirubin and albumin levels.49
Other chemotherapeutic agents
Several molecular targeted agents are undergoing clinical trials for the treatment of HCC. These include bevacizumab, erlotinib, brivanib, and ramucirumab. Chemotherapeutic agents such as doxorubicin and everolimus are also being studied.
PALLIATIVE TREATMENT
Patients with end-stage HCC with moderate to severe constitutional symptoms, extrahepatic disease progression, and decompensated liver disease have a survival of less than 3 months and are treated for pain and symptom control.9
Hepatocellular carcinoma (HCC) is a common cause of death worldwide. However, it can be detected early in high-risk individuals by using effective screening strategies, resulting in the ability to provide curative treatment.
Here, we review the risk factors for HCC, strategies for surveillance and diagnosis, and therapies that can be used.
EPIDEMIOLOGY
HCC is the most common primary malignancy of the liver. Overall, it is the fifth most common type of cancer in men and the seventh most common in women.1
Cirrhosis is present in 80% to 90% of patients with HCC.
Male sex. The male-to-female ratio is from 2:1 to 4:1, depending on the region.2 In the United States, the overall male-to-female ratio has been reported2 as 2.4:1. In another report,3 the incidence rate of HCC per 100,000 person-years was 3.7 for men and 2.0 for women.
Geographic areas with a high incidence of HCC include sub-Saharan Africa and eastern Asia, whereas Canada and the United States are low-incidence areas. The difference has been because of a lower prevalence of hepatitis B virus infection in North America. However, recent data show a downward trend in incidence of HCC in eastern Asia and an upward trend in North America (Figure 1).3,4
Viral hepatitis (ie, hepatitis B or hepatitis C) is the main risk factor for cirrhosis and HCC.
Diabetes mellitus can predispose to nonalcoholic steatohepatitis, which can subsequently progress to cirrhosis. Thus, it increases the risk of HCC.
Obesity increases the risk of death from liver cancer, with obese people (body mass index ≥ 30 kg/m2) having a higher HCC-related death rate than leaner individuals.5 And as obesity becomes more prevalent, the number of deaths from HCC could increase.
Other diseases that predispose to HCC include alcohol abuse, hereditary hemochromatosis, alpha-1-antitrypsin deficiency, and glycogen storage disease.
SURVEILLANCE OF PATIENTS AT RISK
Patients at high risk of developing liver cancer require frequent screening (Table 1).
Patients with cirrhosis. Sarasin et al6 calculated that surveillance is cost-effective and increases the odds of survival in patients with cirrhosis if the incidence of HCC exceeds 1.5% per year (which it does). In view of this finding, all patients with cirrhosis should be screened every 6 months, irrespective of the cause of the cirrhosis.
Hepatitis B carriers. Surveillance is also indicated in some hepatitis B carriers (Table 1), eg, those with a family history of HCC in a first-degree relative (an independent risk factor for developing the disease in this group).7 Also, Africans with hepatitis B tend to develop HCC early in life.8 Though it has been recommended that surveillance be started at a younger age in these patients,9 the age at which it should begin has not been clearly established. In addition, it is not clear if black people born outside Africa are at higher risk.
Benefit of surveillance
HCC surveillance has shown to lower the death rate. A randomized controlled trial in China compared screening (with abdominal ultrasonography and alpha-fetoprotein levels) vs no screening in patients with hepatitis B. It showed that screening led to a 37% decrease in the death rate.12 Studies have also established that patients with early-stage HCC have a better survival rate than patients with more-advanced disease.10,11 This survival benefit is largely explained by the availability of effective treatments for early-stage cancer, including liver transplantation. Therefore, early-stage asymptomatic patients diagnosed by a surveillance program should have a better survival rate than symptomatic patients.
Surveillance methods
The tests most often used in surveillance for HCC are serum alpha-fetoprotein levels and liver ultrasonography.
Serum alpha-fetoprotein levels by themselves have not been shown to be useful, whereas the combination of alpha-fetoprotein levels and ultrasonography has been shown to reduce the death rate when used for surveillance in a randomized trial.12 A 2012 study reported that the combination of alpha-fetoprotein testing and ultrasonography had a higher sensitivity (90%) than ultrasonography alone (58%), but at the expense of a lower specificity.13
Alpha-fetoprotein has a low sensitivity (ie, 54%) for HCC.14 Tumor size is one of the factors limiting the sensitivity of alpha-fetoprotein, 14 and this would imply that this test may not be helpful in detecting HCC at an early stage. Alpha-fetoprotein L3, an isoform of alpha-fetoprotein, may be helpful in patients with alpha-fetoprotein levels in the intermediate range, and it is currently being studied.
Liver ultrasonography is operator-dependent, and it may not be as accurate in overweight or obese people.
Computed tomography (CT) and magnetic resonance imaging (MRI) are not recommended for surveillance. Serial CT poses risks of radiation-induced damage, contrast-related anaphylaxis, and renal failure, and MRI is not cost-effective and can also lead to gadolinium-induced nephrogenic systemic fibrosis in patients with renal failure.
Currently, the American Association for the Study of Liver Diseases9 recommends ultrasonography only, every 6 months, for surveillance for HCC. However, it may be premature to conclude that alpha-fetoprotein measurement is no longer required for surveillance, and if new data emerge that support its role, it may be reincorporated into the guidelines.
DIAGNOSING HEPATOCELLULAR CARCINOMA
Lesions larger than 1 cm on ultrasonography
The finding of a liver lesion larger than 1 cm on ultrasonography during surveillance warrants further testing.
Noninvasive testing with four-phase multidetector CT or dynamic contrast-enhanced MRI is the next step. Typical findings on either of these imaging studies are sufficient to make a diagnosis of HCC, as they have a high specificity and positive predictive value.15 Arterial hyperenhancement with a venous-phase or delayed-phase washout of contrast medium confirms the diagnosis (Figure 2).9 If one of the two imaging studies is typical for HCC, liver biopsy is not needed.
Other imaging studies, including contrast-enhanced ultrasonography, have not been shown to be specific for this diagnosis.16
Liver biopsy is indicated in patients in whom the imaging findings are atypical for HCC.9,17 Biopsy has very good sensitivity and specificity for cancer, but false-negative findings do occur.18 Therefore, a negative biopsy does not entirely exclude HCC. In this situation, patients should be followed by serial ultrasonography, and any further growth or change in character should be reevaluated.
Lesions smaller than 1 cm
For lesions smaller than 1 cm, the incidence of HCC is low, and currently available diagnostic tests are not reliable.15,19 Lesions of this size should be followed by serial ultrasonography every 3 to 4 months until they either enlarge to greater than 1 cm or remain stable at 2 years.9 If they remain stable at the end of 2 years, regular surveillance ultrasonography once every 6 months can be continued.
CURATIVE AND PALLIATIVE THERAPIES
Therapies for HCC (Table 2) can be divided into two categories: curative and palliative.
Curative treatments include surgical resection, liver transplantation, and radiofrequency ablation. All other treatments are palliative, including transarterial chemoembolization and medical therapy with sorafenib.
The choice of treatment depends on the characteristics of the tumor, the degree of liver dysfunction, and the patient’s current level of function. The Barcelona Clinic Liver Cancer classification is widely used in making these decisions, as it incorporates both clinical features and tumor stage.9 Figure 3 shows a simplified management algorithm.
SURGICAL RESECTION
Surgical resection is the preferred treatment for patients who have a solitary HCC lesion without cirrhosis.9 It is also indicated in patients with well-compensated cirrhosis who have normal portal pressure, a normal serum bilirubin level, and a platelet count greater than 100 × 109/L.20,21 In such patients, the 5-year survival rate is about 74%, compared with 25% in patients with portal hypertension and serum bilirubin levels higher than 1 mg/dL.21
Surgical resection is not recommended for patients with decompensated cirrhosis, as it can worsen liver function postoperatively and increase the risk of death.19,20 In Western countries, where cirrhosis from hepatitis C is the commonest cause of HCC, most patients have poorly preserved hepatic function at the time of diagnosis, leaving only a minority of patients as candidates for surgical resection.
After surgical resection of HCC, the recurrence rate can be as high as 70% to 80% at 5 years.22,23 Studies have consistently found larger tumor size and vascular invasion to be factors that predict recurrence.24,25 Vascular invasion was also found to predict poor survival after recurrence.24 Studies have so far not shown any conclusive benefit from post-surgical adjuvant chemotherapy in reducing the rate of recurrence of HCC.26,27
How to treat recurrent HCC after surgical resection has not been clearly established. Radiofrequency ablation, transarterial chemoembolization, repeat resection, and liver transplantation have all improved survival when used alone or in combination.28 However, randomized controlled trials are needed to establish the effective treatment strategy and the benefit of multimodal treatment of recurrent HCC.
LIVER TRANSPLANTATION
Orthotopic liver transplantation is the preferred treatment for patients with HCC complicated by cirrhosis and portal hypertension. It has the advantage not only of being potentially curative, but also of overcoming liver cirrhosis by replacing the liver.
To qualify for liver transplantation, patients must meet the Milan criteria (ie, have a single nodule less than 5 cm in diameter or up to three nodules, with the largest being less than 3 cm in diameter, with no evidence of vascular invasion or distant metastasis). These patients have an expected 4-year survival rate of 85% and a recurrence-free survival rate of 92% after transplantation, compared with 50% and 59%, respectively, in patients whose tumors exceeded these criteria.29
Some believe that the Milan criteria are too restrictive and could be expanded. Yao et al at the University of California-San Francisco30 reported that patients with HCC meeting the criteria of having a solitary tumor smaller than 6.5 cm or having up to three nodules, with the largest smaller than 4.5 cm, and total tumor diameter less than 8 cm, had survival rates of 90% at 1 year and 75.2% at 5 years after liver transplantation, compared with 50% at 1 year for patients with tumors exceeding these limits. (These have come to be known as the UCSF criteria.) However, the United Network for Organ Sharing (UNOS) has not adopted these expanded criteria. UNOS has a point system for allocating livers for transplant called the Model for End-Stage Liver Disease (MELD). Patients who meet the Milan criteria receive extra points, putting them higher on the transplant list. This allows for early transplantation, thus reducing tumor progression and dropout from the transplant list. UNOS allocates a MELD score of 22 to all patients who meet the Milan criteria, and the score is further adjusted once every 3 months to reflect a 10% increase in the mortality rate. However, patients who have a single lesion smaller than 2 cm and are candidates for liver transplantation are not assigned additional MELD points per UNOS policy, as the risk of tumor progression beyond the Milan criteria in these patients is deemed to be low.
Therapies while awaiting transplantation
Even if they receive additional MELD points to give them priority on the waiting list, patients face a considerable wait before transplantation because of the limited availability of donor organs. In the interim, they have a risk of tumor progression beyond the Milan criteria and subsequent dropout from the transplant list.31 Patients on the waiting list may therefore undergo a locoregional therapy such as transarterial chemoembolization or radiofrequency ablation as bridging therapy.
These therapies have been shown to decrease dropout from the waiting list.31 A prospective study showed that in 48 patients who underwent transarterial chemoembolization while awaiting liver transplantation, none had tumor progression, and 41 did receive a transplant, with excellent posttransplantation survival rates.32 Similarly, radioembolization using yttrium-90-labeled microspheres or radiofrequency ablation while on the waiting list has been shown to significantly decrease the rate of dropout, with good posttransplantation outcomes.33,34
However, in spite of these benefits, these bridging therapies do not increase survival rates after transplantation. It is also unclear whether they are useful in regions with short waiting times for liver transplantation.
Adjuvant systemic chemotherapy has not been shown to improve survival in patients undergoing liver transplantation. For example, in a randomized controlled trial of doxorubicin given before, during, and after surgery, the survival rate at 5 years was 38% with doxorubicin and 40% without.35
ABLATIVE LOCOREGIONAL THERAPIES
Locoregional therapies play an important role in managing HCC. They are classified as ablative and perfusion-based.
Ablative locoregional therapies include chemical modalities such as percutaneous ethanol injection; thermal therapies such as radiofrequency ablation, microwave ablation, laser ablation, and cryotherapy; and newer methods such as irreversible electroporation and light-activated drug therapy. Of these, radiofrequency ablation is the most widely used.
Radiofrequency ablation
Radiofrequency ablation induces thermal injury, resulting in tumor necrosis. It can be used as an alternative to surgery in patients who have a single HCC lesion less than 3 to 5 cm in diameter, confined to the liver, and in a site amenable to this procedure and who have a reasonable coagulation profile. The procedure can be performed percutaneously or via laparoscopy.
Radiofrequency ablation is contraindicated in patients with decompensated cirrhosis, Child-Pugh class C cirrhosis (the most severe category), vascular or bile duct invasion, extrahepatic disease, or lesions that are not accessible or are adjacent to structures such as the gall bladder, bowel, stomach, or diaphragm.
Radiofrequency ablation has been compared with surgical resection in patients who had small tumors. Though a randomized controlled trial did not show any difference between the two treatment groups in terms of survival at 5 years and recurrence rates,36 a meta-analysis showed that overall survival rates at 3 years and 5 years were significantly higher with surgical resection than with radiofrequency ablation.37 Patients also had a higher rate of local recurrence with radiofrequency ablation than with surgical resection.37 In addition, radiofrequency ablation has been shown to be effective only in small tumors and does not perform as well in lesions larger than 2 or 3 cm.
Thus, based on current evidence, surgical resection is preferable to radiofrequency ablation as first-line treatment. The latter, however, is also used as a bridging therapy in patients awaiting liver transplantation.
Percutaneous ethanol injection
Percutaneous ethanol injection is used less frequently than radiofrequency ablation, as studies have shown the latter to be superior in regard to local recurrence-free survival rates.38 However, percutaneous ethanol injection is used instead of radiofrequency ablation in a small number of patients, when the lesion is very close to organs such as the bile duct (which could be damaged by radiofrequency ablation) or the large vessels (which may make radiofrequency ablation less effective, since heat may dissipate as a result of excessive blood flow in this region).
Microwave ablation
Microwave ablation is an emerging therapy for HCC. Its advantage over radiofrequency ablation is that its use is not limited by blood vessels in close proximity to the ablation site.
Earlier studies did not show microwave ablation to be superior to radiofrequency ablation.39,40 However, current studies involving newer techniques of microwave ablation are more promising.41
PERFUSION-BASED LOCOREGIONAL THERAPIES
Perfusion-based locoregional therapies deliver embolic particles, chemotherapeutic agents, or radioactive materials into the artery feeding the tumor. The portal blood flow allows for preservation of vital liver tissue during arterial embolization of liver tumors. Perfusionbased therapies include transarterial chemoembolization, transarterial chemoembolization with doxorubicin-eluting beads (DEB-TACE), “bland” embolization, and radioembolization.
Transarterial chemoembolization
Transarterial chemoembolization is a minimally invasive procedure in which the hepatic artery is cannulated through a percutaneous puncture, the branches of the hepatic artery supplying the tumor are identified, and then embolic particles and chemotherapeutic agents are injected. This serves a dual purpose: it embolizes the feeding vessel that supplies the tumor, causing tumor necrosis, and it focuses the chemotherapy on the tumor and thus minimizes the systemic effects of the chemotherapeutic agent.
This therapy is contraindicated in patients with portal vein thrombosis, advanced liver dysfunction, or a transjugular intrahepatic portosystemic shunt. Side effects of the procedure include a postembolization syndrome of abdominal pain and fever (occurring in about 50% of patients from ischemic injury to the liver), hepatic abscesses, injury to the hepatic artery, development of ascites, liver dysfunction, and contrast-induced renal failure.
In addition to bridging patients to liver transplantation, transarterial chemoembolization is recommended as palliative treatment to prolong survival in patients with HCC who are not candidates for liver transplantation, surgical resection, or radiofrequency ablation.9,42 Patients who have Child-Pugh grade A or B cirrhosis but do not have main portal vein thrombosis or extrahepatic spread are candidates for this therapy. Patients such as these who undergo this therapy have a better survival rate at 2 years compared with untreated patients.43,44
Transarterial chemoembolization has also been used to reduce the size of (ie, to “downstage”) tumors that are outside the Milan criteria in patients who are otherwise candidates for liver transplantation. It induces tumor necrosis and has been shown to decrease the tumor size in a selected group of patients and to bring them within the Milan criteria, thus potentially enabling them to be put on the transplant list.45 Studies have shown that patients who receive a transplant after successful down-staging may achieve a 5-year survival rate comparable with that of patients who were initially within the Milan criteria and received a transplant without the need for down-staging.45 However, factors that predict successful down-staging have not been clearly established.
Newer techniques have been developed. A randomized controlled trial found transarterial chemoembolization with doxorubicin-eluting beads to be safer and better tolerated than conventional transarterial chemembolization.46
Bland embolization is transarterial embolization without chemotherapeutic agents and is performed in patients with significant liver dysfunction who might not tolerate chemotherapy. The benefits of this approach are yet to be determined.
Radioembolization
Radioembolization with yttrium-90 microspheres has recently been introduced as an alternative to transarterial chemoembolization, especially in patients with portal vein thrombosis, a portocaval shunt, or a transjugular intrahepatic portosystemic shunt.
In observational studies, radioembolization was as effective as transarterial chemoembolization, with a similar survival benefit.47 However, significant pulmonary shunting must be ruled out before radioembolization, as it would lead to radiation-induced pulmonary disease. Randomized controlled trials are under way to compare the efficacy of the two methods.
CHEMOTHERAPY
Sorafenib
Sorafenib is an oral antiangiogenic agent. A kinase inhibitor, it interacts with multiple intracellular and cell-surface kinases, including vascular endothelial growth factor receptor, platelet-derived growth factor receptor, and Raf proto-oncogene, inhibiting tumor cell proliferation and angiogenesis.
Sorafenib has been shown to prolong survival in patients with advanced-stage HCC.48 A randomized placebo-controlled trial in patients with Child-Pugh grade A cirrhosis and advanced HCC who had not received chemotherapy showed that sorafenib increased the life expectancy by nearly 3 months compared with placebo.47 Sorafenib therapy is very expensive, but it is usually covered by insurance.
Sorafenib is recommended in patients who have advanced HCC with vascular invasion, extrahepatic dissemination, or minimal constitutional symptoms. It is not recommended for patients with severe advanced liver disease who have moderate to severe tumor-related constitutional symptoms or Child-Pugh grade C cirrhosis, or for patients with a life expectancy of less than 3 months.
The most common side effects of sorafenib are diarrhea, weight loss, and skin reactions on the hands and feet. These commonly lead to decreased tolerability and dose reductions.47 Doses should be adjusted on the basis of the bilirubin and albumin levels.49
Other chemotherapeutic agents
Several molecular targeted agents are undergoing clinical trials for the treatment of HCC. These include bevacizumab, erlotinib, brivanib, and ramucirumab. Chemotherapeutic agents such as doxorubicin and everolimus are also being studied.
PALLIATIVE TREATMENT
Patients with end-stage HCC with moderate to severe constitutional symptoms, extrahepatic disease progression, and decompensated liver disease have a survival of less than 3 months and are treated for pain and symptom control.9
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893–2917.
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007; 132:2557–2576.
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012; 142:1264–1273.e1.
- Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009; 27:1485–1491.
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med 2003; 348:1625–1638.
- Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosis. Am J Med 1996; 101:422–434.
- Yu MW, Chang HC, Liaw YF, et al. Familial risk of hepatocellular carcinoma among chronic hepatitis B carriers and their relatives. J Natl Cancer Inst 2000; 92:1159–1164.
- Kew MC, Macerollo P. Effect of age on the etiologic role of the hepatitis B virus in hepatocellular carcinoma in blacks. Gastroenterology 1988; 94:439–442.
- Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53:1020–1022.
- Bruix J, Llovet JM. Major achievements in hepatocellular carcinoma. Lancet 2009; 373:614–616.
- Gómez-Rodríguez R, Romero-Gutiérrez M, Artaza-Varasa T, et al. The value of the Barcelona Clinic Liver Cancer and alpha-fetoprotein in the prognosis of hepatocellular carcinoma. Rev Esp Enferm Dig 2012; 104:298–304.
- Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004; 130:417–422.
- Giannini EG, Erroi V, Trevisani F. Effectiveness of a-fetoprotein for hepatocellular carcinoma surveillance: the return of the living-dead? Expert Rev Gastroenterol Hepatol 2012; 6:441–444.
- Farinati F, Marino D, De Giorgio M, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol 2006; 101:524–532.
- Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008; 47:97–104.
- Vilana R, Forner A, Bianchi L, et al. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology 2010; 51:2020–2029.
- Kojiro M. Pathological diagnosis at early stage: reaching international consensus. Oncology 2010; 78(suppl 1):31–35.
- Schölmerich J, Schacherer D. Diagnostic biopsy for hepatocellular carcinoma in cirrhosis: useful, necessary, dangerous, or academic sport? Gut 2004; 53:1224–1226.
- Durand F, Regimbeau JM, Belghiti J, et al. Assessment of the benefits and risks of percutaneous biopsy before surgical resection of hepatocellular carcinoma. J Hepatol 2001; 35:254–258.
- Bruix J, Castells A, Bosch J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology 1996; 111:1018–1022.
- Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999; 30:1434–1440.
- Nagasue N, Uchida M, Makino Y, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology 1993; 105:488–494.
- Arii S, Tanaka J, Yamazoe Y, et al. Predictive factors for intrahepatic recurrence of hepatocellular carcinoma after partial hepatectomy. Cancer 1992; 69:913–919.
- Cha C, Fong Y, Jarnagin WR, Blumgart LH, DeMatteo RP. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg 2003; 197:753–758.
- Shah SA, Cleary SP, Wei AC, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery 2007; 141:330–339.
- Kohno H, Nagasue N, Hayashi T, et al. Postoperative adjuvant chemotherapy after radical hepatic resection for hepatocellular carcinoma (HCC). Hepatogastroenterology 1996; 43:1405–1409.
- Ono T, Nagasue N, Kohno H, et al. Adjuvant chemotherapy with epirubicin and carmofur after radical resection of hepatocellular carcinoma: a prospective randomized study. Semin Oncol 1997; 24(suppl 6):S6–25.
- Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: Long-term results of treatment and prognostic factors. Ann Surg 1999; 229:216–222.
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334:693–699.
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001; 33:1394–1403.
- Majno P, Lencioni R, Mornex F, Girard N, Poon RT, Cherqui D. Is the treatment of hepatocellular carcinoma on the waiting list necessary? Liver Transpl 2011; 17(suppl 2):S98–S108.
- Graziadei IW, Sandmueller H, Waldenberger P, et al. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl 2003; 9:557–563.
- Kulik LM, Atassi B, van Holsbeeck L, et al. Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol 2006; 94:572–586.
- Lu DS, Yu NC, Raman SS, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology 2005; 41:1130–1137.
- Pokorny H, Gnant M, Rasoul-Rockenschaub S, et al. Does additional doxorubicin chemotherapy improve outcome in patients with hepatocellular carcinoma treated by liver transplantation? Am J Transplant 2005; 5:788–794.
- Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 2012; 57:794–802.
- Zhou Y, Zhao Y, Li B, et al. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol 2010; 10:78.
- Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: Randomized comparison of radiofrequency thermal ablation versus percutaneous ethanol injection. Radiology 2003; 228:235–240.
- Ohmoto K, Yoshioka N, Tomiyama Y, et al. Comparison of therapeutic effects between radiofrequency ablation and percutaneous microwave coagulation therapy for small hepatocellular carcinomas. J Gastroenterol Hepatol 2009; 24:223–227.
- Shibata T, Iimuro Y, Yamamoto Y, et al. Small hepatocellular carcinoma: comparison of radiofrequency ablation and percutaneous microwave coagulation therapy. Radiology 2002; 223:331–337.
- Qian GJ, Wang N, Shen Q, et al. Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: Experimental and clinical studies. Eur Radiol 2012; 22:1983–1990.
- Burrel M, Reig M, Forner A, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using drug eluting beads. Implications for clinical practice and trial design. J Hepatol 2012; 56:1330–1335.
- Cammà C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology 2002; 224:47–54.
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 2003; 37:429–442.
- Yao FY, Kerlan RK, Hirose R, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology 2008; 48:819–827.
- Ferrer Puchol MD, la Parra C, Esteban E, et al. Comparison of doxorubicin-eluting bead transarterial chemoembolization (DEBTACE) with conventional transarterial chemoembolization (TACE) for the treatment of hepatocellular carcinoma (article in Spanish). Radiologia 2011; 53:246–253.
- Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2011; 140:497–507.e2.
- Llovet JM, Ricci S, Mazzaferro V, et al; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359:378–390.
- Miller AA, Murry DJ, Owzar K, et al. Phase I and pharmacokinetic study of sorafenib in patients with hepatic or renal dysfunction: CALGB 60301. J Clin Oncol 2009; 27:1800–1805.
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893–2917.
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007; 132:2557–2576.
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012; 142:1264–1273.e1.
- Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009; 27:1485–1491.
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med 2003; 348:1625–1638.
- Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosis. Am J Med 1996; 101:422–434.
- Yu MW, Chang HC, Liaw YF, et al. Familial risk of hepatocellular carcinoma among chronic hepatitis B carriers and their relatives. J Natl Cancer Inst 2000; 92:1159–1164.
- Kew MC, Macerollo P. Effect of age on the etiologic role of the hepatitis B virus in hepatocellular carcinoma in blacks. Gastroenterology 1988; 94:439–442.
- Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53:1020–1022.
- Bruix J, Llovet JM. Major achievements in hepatocellular carcinoma. Lancet 2009; 373:614–616.
- Gómez-Rodríguez R, Romero-Gutiérrez M, Artaza-Varasa T, et al. The value of the Barcelona Clinic Liver Cancer and alpha-fetoprotein in the prognosis of hepatocellular carcinoma. Rev Esp Enferm Dig 2012; 104:298–304.
- Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004; 130:417–422.
- Giannini EG, Erroi V, Trevisani F. Effectiveness of a-fetoprotein for hepatocellular carcinoma surveillance: the return of the living-dead? Expert Rev Gastroenterol Hepatol 2012; 6:441–444.
- Farinati F, Marino D, De Giorgio M, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol 2006; 101:524–532.
- Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008; 47:97–104.
- Vilana R, Forner A, Bianchi L, et al. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology 2010; 51:2020–2029.
- Kojiro M. Pathological diagnosis at early stage: reaching international consensus. Oncology 2010; 78(suppl 1):31–35.
- Schölmerich J, Schacherer D. Diagnostic biopsy for hepatocellular carcinoma in cirrhosis: useful, necessary, dangerous, or academic sport? Gut 2004; 53:1224–1226.
- Durand F, Regimbeau JM, Belghiti J, et al. Assessment of the benefits and risks of percutaneous biopsy before surgical resection of hepatocellular carcinoma. J Hepatol 2001; 35:254–258.
- Bruix J, Castells A, Bosch J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology 1996; 111:1018–1022.
- Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999; 30:1434–1440.
- Nagasue N, Uchida M, Makino Y, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology 1993; 105:488–494.
- Arii S, Tanaka J, Yamazoe Y, et al. Predictive factors for intrahepatic recurrence of hepatocellular carcinoma after partial hepatectomy. Cancer 1992; 69:913–919.
- Cha C, Fong Y, Jarnagin WR, Blumgart LH, DeMatteo RP. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg 2003; 197:753–758.
- Shah SA, Cleary SP, Wei AC, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery 2007; 141:330–339.
- Kohno H, Nagasue N, Hayashi T, et al. Postoperative adjuvant chemotherapy after radical hepatic resection for hepatocellular carcinoma (HCC). Hepatogastroenterology 1996; 43:1405–1409.
- Ono T, Nagasue N, Kohno H, et al. Adjuvant chemotherapy with epirubicin and carmofur after radical resection of hepatocellular carcinoma: a prospective randomized study. Semin Oncol 1997; 24(suppl 6):S6–25.
- Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: Long-term results of treatment and prognostic factors. Ann Surg 1999; 229:216–222.
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334:693–699.
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001; 33:1394–1403.
- Majno P, Lencioni R, Mornex F, Girard N, Poon RT, Cherqui D. Is the treatment of hepatocellular carcinoma on the waiting list necessary? Liver Transpl 2011; 17(suppl 2):S98–S108.
- Graziadei IW, Sandmueller H, Waldenberger P, et al. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl 2003; 9:557–563.
- Kulik LM, Atassi B, van Holsbeeck L, et al. Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol 2006; 94:572–586.
- Lu DS, Yu NC, Raman SS, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology 2005; 41:1130–1137.
- Pokorny H, Gnant M, Rasoul-Rockenschaub S, et al. Does additional doxorubicin chemotherapy improve outcome in patients with hepatocellular carcinoma treated by liver transplantation? Am J Transplant 2005; 5:788–794.
- Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 2012; 57:794–802.
- Zhou Y, Zhao Y, Li B, et al. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol 2010; 10:78.
- Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: Randomized comparison of radiofrequency thermal ablation versus percutaneous ethanol injection. Radiology 2003; 228:235–240.
- Ohmoto K, Yoshioka N, Tomiyama Y, et al. Comparison of therapeutic effects between radiofrequency ablation and percutaneous microwave coagulation therapy for small hepatocellular carcinomas. J Gastroenterol Hepatol 2009; 24:223–227.
- Shibata T, Iimuro Y, Yamamoto Y, et al. Small hepatocellular carcinoma: comparison of radiofrequency ablation and percutaneous microwave coagulation therapy. Radiology 2002; 223:331–337.
- Qian GJ, Wang N, Shen Q, et al. Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: Experimental and clinical studies. Eur Radiol 2012; 22:1983–1990.
- Burrel M, Reig M, Forner A, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using drug eluting beads. Implications for clinical practice and trial design. J Hepatol 2012; 56:1330–1335.
- Cammà C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology 2002; 224:47–54.
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 2003; 37:429–442.
- Yao FY, Kerlan RK, Hirose R, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology 2008; 48:819–827.
- Ferrer Puchol MD, la Parra C, Esteban E, et al. Comparison of doxorubicin-eluting bead transarterial chemoembolization (DEBTACE) with conventional transarterial chemoembolization (TACE) for the treatment of hepatocellular carcinoma (article in Spanish). Radiologia 2011; 53:246–253.
- Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2011; 140:497–507.e2.
- Llovet JM, Ricci S, Mazzaferro V, et al; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359:378–390.
- Miller AA, Murry DJ, Owzar K, et al. Phase I and pharmacokinetic study of sorafenib in patients with hepatic or renal dysfunction: CALGB 60301. J Clin Oncol 2009; 27:1800–1805.
KEY POINTS
- Surveillance for HCC is indicated in all patients with cirrhosis, regardless of the cause of the cirrhosis.
- Liver biopsy is not needed to make the diagnosis if the findings on four-phase multidetector computed tomography or dynamic contrast-enhanced magnetic resonance imaging are typical of HCC (arterial hyperenhancement with venous-phase or delayed-phase washout).
- Many treatments are available, including surgical resection, liver transplantation, ablative therapy, perfusion-based therapy, chemotherapy, and palliative therapy.
Peripheral opacity on plain chest radiography
An 82-year-old woman was admitted to the hospital with dyspnea and chest discomfort over the past 24 hours. She was known to have paroxysmal atrial fibrillation and was taking warfarin, but that had been stopped 2 weeks earlier because of an acute ischemic stroke.
At the time of admission, she had no fever, cough, orthopnea, or leg swelling. Her physical activity was restricted, with residual right-sided weakness after her stroke. Her heart rate was 125 bpm; her oxygen saturation level was 98% on 2 L of oxygen per minute via nasal cannula. She had an irregularly irregular rhythm, a jugular venous pressure of 7 cm H2O, and no cardiac murmurs. Lung sounds were reduced at the bases, with faint crepitations.
Her hemoglobin concentration and white blood cell count were normal. Her brain-natriuretic peptide level was elevated at 2,648 pg/mL (reference range < 167), but cardiac enzyme levels were normal.
Electrocardiography showed atrial fibrillation with rapid ventricular response.
Plain chest radiography showed a 3-cm wedge-shaped opacity in the right mid-thorax (Figure 1), a finding known as the Hampton hump—a sign of pulmonary infarction caused by embolism.
Contrast-enhanced computed tomography (CT) of the chest showed acute thromboembolism in the right interlobar artery and wedge-shaped consolidation in the right-middle lobe (Figure 2), indicating pulmonary infarction.
Brain CT showed a stable infarction. Anticoagulation was restarted, and the patient was discharged in stable condition.
THE HAMPTON HUMP IN PULMONARY EMBOLISM
Because the lungs have a dual blood supply, pulmonary infarction is seen in only a minority of cases of pulmonary embolism. Infarction is more common in patients with peripheral pulmonary embolism, owing to the rapid inflow of bronchial blood, and in patients with medical comorbidities such as heart failure and chronic lung disease.2
The Hampton hump, first described by Aubrey Otis Hampton in 1940, is a peripheral (pleural-based) opacity that represents alveolar hemorrhage from underlying pulmonary infarction. It is one of several radiographic features that have been associated with pulmonary embolism; another is the Westermark sign, indicating oligemia.3
Worsley et al4 examined the diagnostic value of these radiographic features and found that the Hampton hump had a sensitivity of 22% and a specificity of 82% for detecting pulmonary embolism in the right hemithorax, and 24% and 82%, respectively, in the left hemithorax. The prevalence of pleural-based opacities was not significantly different in patients with or without pulmonary embolism. The authors concluded that chest radiography has limited diagnostic value in excluding or diagnosing pulmonary embolism.
In contrast, computed tomographic pulmonary angiography is the first-line imaging test in patients with suspected pulmonary embolism, because of its high sensitivity and specificity.1
We were not specifically looking for a pulmonary embolism when we found this new opacity on our patient’s radiograph, but this prompted further imaging, which led to the diagnosis. Although a near-normal chest radiograph is the most common radiologic finding in pulmonary embolism, this case shows how careful observation can detect unusual signs.
- Mos IC, Klok FA, Kroft LJ, de Roos A, Huisman MV. Imaging tests in the diagnosis of pulmonary embolism. Semin Respir Crit Care Med 2012; 33:138–143.
- Cha SI, Shin KM, Lee J, et al. Clinical relevance of pulmonary infarction in patients with pulmonary embolism. Thromb Res 2012; 130:e1–e5.
- Algın O, GÖkalp G, Topal U. Signs in chest imaging. Diagn Interv Radiol 2011; 17:18–29.
- Worsley DF, Alavi A, Aronchick JM, Chen JT, Greenspan RH, Ravin CE. Chest radiographic findings in patients with acute pulmonary embolism: observations from the PIOPED study. Radiology 1993; 189:133–136.
An 82-year-old woman was admitted to the hospital with dyspnea and chest discomfort over the past 24 hours. She was known to have paroxysmal atrial fibrillation and was taking warfarin, but that had been stopped 2 weeks earlier because of an acute ischemic stroke.
At the time of admission, she had no fever, cough, orthopnea, or leg swelling. Her physical activity was restricted, with residual right-sided weakness after her stroke. Her heart rate was 125 bpm; her oxygen saturation level was 98% on 2 L of oxygen per minute via nasal cannula. She had an irregularly irregular rhythm, a jugular venous pressure of 7 cm H2O, and no cardiac murmurs. Lung sounds were reduced at the bases, with faint crepitations.
Her hemoglobin concentration and white blood cell count were normal. Her brain-natriuretic peptide level was elevated at 2,648 pg/mL (reference range < 167), but cardiac enzyme levels were normal.
Electrocardiography showed atrial fibrillation with rapid ventricular response.
Plain chest radiography showed a 3-cm wedge-shaped opacity in the right mid-thorax (Figure 1), a finding known as the Hampton hump—a sign of pulmonary infarction caused by embolism.
Contrast-enhanced computed tomography (CT) of the chest showed acute thromboembolism in the right interlobar artery and wedge-shaped consolidation in the right-middle lobe (Figure 2), indicating pulmonary infarction.
Brain CT showed a stable infarction. Anticoagulation was restarted, and the patient was discharged in stable condition.
THE HAMPTON HUMP IN PULMONARY EMBOLISM
Because the lungs have a dual blood supply, pulmonary infarction is seen in only a minority of cases of pulmonary embolism. Infarction is more common in patients with peripheral pulmonary embolism, owing to the rapid inflow of bronchial blood, and in patients with medical comorbidities such as heart failure and chronic lung disease.2
The Hampton hump, first described by Aubrey Otis Hampton in 1940, is a peripheral (pleural-based) opacity that represents alveolar hemorrhage from underlying pulmonary infarction. It is one of several radiographic features that have been associated with pulmonary embolism; another is the Westermark sign, indicating oligemia.3
Worsley et al4 examined the diagnostic value of these radiographic features and found that the Hampton hump had a sensitivity of 22% and a specificity of 82% for detecting pulmonary embolism in the right hemithorax, and 24% and 82%, respectively, in the left hemithorax. The prevalence of pleural-based opacities was not significantly different in patients with or without pulmonary embolism. The authors concluded that chest radiography has limited diagnostic value in excluding or diagnosing pulmonary embolism.
In contrast, computed tomographic pulmonary angiography is the first-line imaging test in patients with suspected pulmonary embolism, because of its high sensitivity and specificity.1
We were not specifically looking for a pulmonary embolism when we found this new opacity on our patient’s radiograph, but this prompted further imaging, which led to the diagnosis. Although a near-normal chest radiograph is the most common radiologic finding in pulmonary embolism, this case shows how careful observation can detect unusual signs.
An 82-year-old woman was admitted to the hospital with dyspnea and chest discomfort over the past 24 hours. She was known to have paroxysmal atrial fibrillation and was taking warfarin, but that had been stopped 2 weeks earlier because of an acute ischemic stroke.
At the time of admission, she had no fever, cough, orthopnea, or leg swelling. Her physical activity was restricted, with residual right-sided weakness after her stroke. Her heart rate was 125 bpm; her oxygen saturation level was 98% on 2 L of oxygen per minute via nasal cannula. She had an irregularly irregular rhythm, a jugular venous pressure of 7 cm H2O, and no cardiac murmurs. Lung sounds were reduced at the bases, with faint crepitations.
Her hemoglobin concentration and white blood cell count were normal. Her brain-natriuretic peptide level was elevated at 2,648 pg/mL (reference range < 167), but cardiac enzyme levels were normal.
Electrocardiography showed atrial fibrillation with rapid ventricular response.
Plain chest radiography showed a 3-cm wedge-shaped opacity in the right mid-thorax (Figure 1), a finding known as the Hampton hump—a sign of pulmonary infarction caused by embolism.
Contrast-enhanced computed tomography (CT) of the chest showed acute thromboembolism in the right interlobar artery and wedge-shaped consolidation in the right-middle lobe (Figure 2), indicating pulmonary infarction.
Brain CT showed a stable infarction. Anticoagulation was restarted, and the patient was discharged in stable condition.
THE HAMPTON HUMP IN PULMONARY EMBOLISM
Because the lungs have a dual blood supply, pulmonary infarction is seen in only a minority of cases of pulmonary embolism. Infarction is more common in patients with peripheral pulmonary embolism, owing to the rapid inflow of bronchial blood, and in patients with medical comorbidities such as heart failure and chronic lung disease.2
The Hampton hump, first described by Aubrey Otis Hampton in 1940, is a peripheral (pleural-based) opacity that represents alveolar hemorrhage from underlying pulmonary infarction. It is one of several radiographic features that have been associated with pulmonary embolism; another is the Westermark sign, indicating oligemia.3
Worsley et al4 examined the diagnostic value of these radiographic features and found that the Hampton hump had a sensitivity of 22% and a specificity of 82% for detecting pulmonary embolism in the right hemithorax, and 24% and 82%, respectively, in the left hemithorax. The prevalence of pleural-based opacities was not significantly different in patients with or without pulmonary embolism. The authors concluded that chest radiography has limited diagnostic value in excluding or diagnosing pulmonary embolism.
In contrast, computed tomographic pulmonary angiography is the first-line imaging test in patients with suspected pulmonary embolism, because of its high sensitivity and specificity.1
We were not specifically looking for a pulmonary embolism when we found this new opacity on our patient’s radiograph, but this prompted further imaging, which led to the diagnosis. Although a near-normal chest radiograph is the most common radiologic finding in pulmonary embolism, this case shows how careful observation can detect unusual signs.
- Mos IC, Klok FA, Kroft LJ, de Roos A, Huisman MV. Imaging tests in the diagnosis of pulmonary embolism. Semin Respir Crit Care Med 2012; 33:138–143.
- Cha SI, Shin KM, Lee J, et al. Clinical relevance of pulmonary infarction in patients with pulmonary embolism. Thromb Res 2012; 130:e1–e5.
- Algın O, GÖkalp G, Topal U. Signs in chest imaging. Diagn Interv Radiol 2011; 17:18–29.
- Worsley DF, Alavi A, Aronchick JM, Chen JT, Greenspan RH, Ravin CE. Chest radiographic findings in patients with acute pulmonary embolism: observations from the PIOPED study. Radiology 1993; 189:133–136.
- Mos IC, Klok FA, Kroft LJ, de Roos A, Huisman MV. Imaging tests in the diagnosis of pulmonary embolism. Semin Respir Crit Care Med 2012; 33:138–143.
- Cha SI, Shin KM, Lee J, et al. Clinical relevance of pulmonary infarction in patients with pulmonary embolism. Thromb Res 2012; 130:e1–e5.
- Algın O, GÖkalp G, Topal U. Signs in chest imaging. Diagn Interv Radiol 2011; 17:18–29.
- Worsley DF, Alavi A, Aronchick JM, Chen JT, Greenspan RH, Ravin CE. Chest radiographic findings in patients with acute pulmonary embolism: observations from the PIOPED study. Radiology 1993; 189:133–136.
Small study: MRIs okay with pacemakers and defibrillators
VANCOUVER, B.C. – When done safely, MRI imaging has an important role to play in patients with pacemakers and implanted cardioverter defibrillators, according to a study of 32 patients who underwent the procedure without complications at Allegheny General Hospital in Pittsburgh.
"It looks like this is safe in the right situation," based on the results and ongoing research elsewhere. "There are occasions – more often than you might think – when" MRI imaging of such patients has "potentially life-saving consequences. I would suggest it is probably no longer taboo," said lead investigator Dr. Robert Biederman, director of cardiovascular MRI at Allegheny and an associate professor of medicine at Drexel University in Philadelphia.
Among the 32 patients in the study, 23 underwent neurologic MRI imaging, and 9 underwent cardiac MRI imaging.
The neurologic MRI imaging picked up a case of Creutzfeldt–Jakob disease in one with unexplained altered mental status, and a syrinx in another who had unexplained arm weakness, a finding that made a "huge difference neurosurgically." Overall, neurologic imaging made or altered diagnoses in 15 patients, and confirmed them in eight, Dr. Biederman said at the 18th World Congress on Heart Disease.
Cardiac MRI imaging picked up cardiac sarcoidosis in one, and constrictive pericarditis in another. In six cases, it simply confirmed provisional diagnoses. Even so, "there is value in confirming for surgeons, patients, and family members that they are doing the right thing," he said.
Often, in the absence of a solid diagnosis, the "patients were going to die." Imaging was only done, however, after conversations that sometimes ran more than an hour with patients and family members about the risks of MRIs with pacemakers (PM) and automated implantable cardioverter-defibrillators (AICD), including tissue cauterization, fibrosis, lethal tachycardia, and device misfire. "This was absolutely done with the patient’s full knowledge of risk," he said.
Although MRIs have caused at least 12 deaths in patients with PMs and AICDs over the last 3 decades, ongoing research with newer devices is beginning to suggest it’s safe if proper device and MRI adjustments are made; well over 1,000 patients have undergone MRIs in recent years with no complications. Even so, "assuming it is safe, no one’s asked if it makes a difference. This is the first time to my knowledge that anyone has stepped beyond the question of safety to see if results from MRIs" in these patients "warrant the risk," Dr. Biederman said (e.g., Am. Heart J. 2013;165:266-72).
Four of the patients had combination PM/AICDs, 18 had dual-chamber PMs, three dependent PMs, five AICDs, and two just single pacer leads. Only one patient had a Revo MRI SureScan Pacing System, the only pacer FDA- approved for MRI use. Scans ran an average of a half hour, plus or minus 15 minutes; a few patients were scanned twice.
The MRIs were performed in a dedicated cardiovascular MRI suite with continuous ECG, blood pressure, heart rate, and oxygen saturation monitoring. "We turned all the defibrillator technology off, and tried to turn the pacemaker off. If we couldn’t, we turned it to an asynchronous mode." Sequences were done at a specific absorption rate (SAR) of less than 2 W/kg, because "there is less radiofrequency energy being sent at a slower rate, so [it’s] less likely" to heat the device. "We try to keep the specific absorption rate as low as possible," Dr. Biederman said.
Devices were reset and tested after the procedure to ensure that impedance, thresholds, amplitudes, and shock impedances were the same as before the scans.
Dr. Biederman said he has no disclosures.
VANCOUVER, B.C. – When done safely, MRI imaging has an important role to play in patients with pacemakers and implanted cardioverter defibrillators, according to a study of 32 patients who underwent the procedure without complications at Allegheny General Hospital in Pittsburgh.
"It looks like this is safe in the right situation," based on the results and ongoing research elsewhere. "There are occasions – more often than you might think – when" MRI imaging of such patients has "potentially life-saving consequences. I would suggest it is probably no longer taboo," said lead investigator Dr. Robert Biederman, director of cardiovascular MRI at Allegheny and an associate professor of medicine at Drexel University in Philadelphia.
Among the 32 patients in the study, 23 underwent neurologic MRI imaging, and 9 underwent cardiac MRI imaging.
The neurologic MRI imaging picked up a case of Creutzfeldt–Jakob disease in one with unexplained altered mental status, and a syrinx in another who had unexplained arm weakness, a finding that made a "huge difference neurosurgically." Overall, neurologic imaging made or altered diagnoses in 15 patients, and confirmed them in eight, Dr. Biederman said at the 18th World Congress on Heart Disease.
Cardiac MRI imaging picked up cardiac sarcoidosis in one, and constrictive pericarditis in another. In six cases, it simply confirmed provisional diagnoses. Even so, "there is value in confirming for surgeons, patients, and family members that they are doing the right thing," he said.
Often, in the absence of a solid diagnosis, the "patients were going to die." Imaging was only done, however, after conversations that sometimes ran more than an hour with patients and family members about the risks of MRIs with pacemakers (PM) and automated implantable cardioverter-defibrillators (AICD), including tissue cauterization, fibrosis, lethal tachycardia, and device misfire. "This was absolutely done with the patient’s full knowledge of risk," he said.
Although MRIs have caused at least 12 deaths in patients with PMs and AICDs over the last 3 decades, ongoing research with newer devices is beginning to suggest it’s safe if proper device and MRI adjustments are made; well over 1,000 patients have undergone MRIs in recent years with no complications. Even so, "assuming it is safe, no one’s asked if it makes a difference. This is the first time to my knowledge that anyone has stepped beyond the question of safety to see if results from MRIs" in these patients "warrant the risk," Dr. Biederman said (e.g., Am. Heart J. 2013;165:266-72).
Four of the patients had combination PM/AICDs, 18 had dual-chamber PMs, three dependent PMs, five AICDs, and two just single pacer leads. Only one patient had a Revo MRI SureScan Pacing System, the only pacer FDA- approved for MRI use. Scans ran an average of a half hour, plus or minus 15 minutes; a few patients were scanned twice.
The MRIs were performed in a dedicated cardiovascular MRI suite with continuous ECG, blood pressure, heart rate, and oxygen saturation monitoring. "We turned all the defibrillator technology off, and tried to turn the pacemaker off. If we couldn’t, we turned it to an asynchronous mode." Sequences were done at a specific absorption rate (SAR) of less than 2 W/kg, because "there is less radiofrequency energy being sent at a slower rate, so [it’s] less likely" to heat the device. "We try to keep the specific absorption rate as low as possible," Dr. Biederman said.
Devices were reset and tested after the procedure to ensure that impedance, thresholds, amplitudes, and shock impedances were the same as before the scans.
Dr. Biederman said he has no disclosures.
VANCOUVER, B.C. – When done safely, MRI imaging has an important role to play in patients with pacemakers and implanted cardioverter defibrillators, according to a study of 32 patients who underwent the procedure without complications at Allegheny General Hospital in Pittsburgh.
"It looks like this is safe in the right situation," based on the results and ongoing research elsewhere. "There are occasions – more often than you might think – when" MRI imaging of such patients has "potentially life-saving consequences. I would suggest it is probably no longer taboo," said lead investigator Dr. Robert Biederman, director of cardiovascular MRI at Allegheny and an associate professor of medicine at Drexel University in Philadelphia.
Among the 32 patients in the study, 23 underwent neurologic MRI imaging, and 9 underwent cardiac MRI imaging.
The neurologic MRI imaging picked up a case of Creutzfeldt–Jakob disease in one with unexplained altered mental status, and a syrinx in another who had unexplained arm weakness, a finding that made a "huge difference neurosurgically." Overall, neurologic imaging made or altered diagnoses in 15 patients, and confirmed them in eight, Dr. Biederman said at the 18th World Congress on Heart Disease.
Cardiac MRI imaging picked up cardiac sarcoidosis in one, and constrictive pericarditis in another. In six cases, it simply confirmed provisional diagnoses. Even so, "there is value in confirming for surgeons, patients, and family members that they are doing the right thing," he said.
Often, in the absence of a solid diagnosis, the "patients were going to die." Imaging was only done, however, after conversations that sometimes ran more than an hour with patients and family members about the risks of MRIs with pacemakers (PM) and automated implantable cardioverter-defibrillators (AICD), including tissue cauterization, fibrosis, lethal tachycardia, and device misfire. "This was absolutely done with the patient’s full knowledge of risk," he said.
Although MRIs have caused at least 12 deaths in patients with PMs and AICDs over the last 3 decades, ongoing research with newer devices is beginning to suggest it’s safe if proper device and MRI adjustments are made; well over 1,000 patients have undergone MRIs in recent years with no complications. Even so, "assuming it is safe, no one’s asked if it makes a difference. This is the first time to my knowledge that anyone has stepped beyond the question of safety to see if results from MRIs" in these patients "warrant the risk," Dr. Biederman said (e.g., Am. Heart J. 2013;165:266-72).
Four of the patients had combination PM/AICDs, 18 had dual-chamber PMs, three dependent PMs, five AICDs, and two just single pacer leads. Only one patient had a Revo MRI SureScan Pacing System, the only pacer FDA- approved for MRI use. Scans ran an average of a half hour, plus or minus 15 minutes; a few patients were scanned twice.
The MRIs were performed in a dedicated cardiovascular MRI suite with continuous ECG, blood pressure, heart rate, and oxygen saturation monitoring. "We turned all the defibrillator technology off, and tried to turn the pacemaker off. If we couldn’t, we turned it to an asynchronous mode." Sequences were done at a specific absorption rate (SAR) of less than 2 W/kg, because "there is less radiofrequency energy being sent at a slower rate, so [it’s] less likely" to heat the device. "We try to keep the specific absorption rate as low as possible," Dr. Biederman said.
Devices were reset and tested after the procedure to ensure that impedance, thresholds, amplitudes, and shock impedances were the same as before the scans.
Dr. Biederman said he has no disclosures.
AT THE 18TH WORLD CONGRESS ON HEART DISEASE
Major finding: MRIs made or altered neurologic diagnoses in 15 of 23 patients with pacemakers or implanted defibrillators. They confirmed diagnoses in the rest. There were no imaging complications.
Data Source: Prospective study of 32 patients with the devices who underwent MRIs
Disclosures: The lead investigator has no disclosures.