User login
Commentary to "Patient-Specific Imaging and Missed Tumors: A Catastrophic Outcome"
The idiom “penny wise, pound foolish” certainly applies in this report of 2 cases of missed bone tumors that were present but not recognized on preoperative imaging prior to placement of patient-specific knee arthroplasties. The case report appeared in the December 2013 issue of The American Journal of Orthopedics. The term “non-diagnostic imaging,” itself a paradox, used in the context of preoperative imaging performed solely for the purpose of component templating for patient-specific instrumentation (PSI) and not intended to be diagnostic in purpose, would be anathematic to most radiologists and should be discarded as a concept.
Bearing in mind the costs incurred by the patient undergoing a total knee arthroplasty (TKA), such as professional consultation, preoperative magnetic resonance imaging, customized manufacture of the components, surgery and associated costs, and postoperative rehabilitation, the fee for a formal report by a musculoskeletal radiologist is comparatively minuscule. As correctly pointed out by the authors, the price associated with bypassing any assessment and missing malignant disease is far greater.
It is well recognized that unreported radiologic examinations can lead to misdiagnosis, compromised patient care, and liability concerns. As PSI is relatively new and has good potential to increase the accuracy, precision and efficiency of TKA, it is even more vital that this promising technology not be marred by disrepute due to possible devastating outcomes resulting from lack of a radiologic report. From the professional point of view of a radiologist, the issuance of a formal report is part and parcel of any radiological examination. I would argue that obtaining radiologic images without an accompanying report constitutes an incomplete study, and will not be in the best interest of patients.
Let the lessons learned from these 2 cases be a springboard to establish protocols for proper utilization of technologies involved in PSI for TKA and other orthopedic procedures. It is imperative to put into place mandatory reporting of all diagnostic images obtained for preoperative evaluation, particularly those that are meant to be sent directly to implant manufacturers for component design.
Menge TJ, Hartley KG, Holt GE. Patient-Specific Imaging and Missed Tumors: A Catastrophic Outcome. Am J Orthop. 2013;42(12):553-556.
The idiom “penny wise, pound foolish” certainly applies in this report of 2 cases of missed bone tumors that were present but not recognized on preoperative imaging prior to placement of patient-specific knee arthroplasties. The case report appeared in the December 2013 issue of The American Journal of Orthopedics. The term “non-diagnostic imaging,” itself a paradox, used in the context of preoperative imaging performed solely for the purpose of component templating for patient-specific instrumentation (PSI) and not intended to be diagnostic in purpose, would be anathematic to most radiologists and should be discarded as a concept.
Bearing in mind the costs incurred by the patient undergoing a total knee arthroplasty (TKA), such as professional consultation, preoperative magnetic resonance imaging, customized manufacture of the components, surgery and associated costs, and postoperative rehabilitation, the fee for a formal report by a musculoskeletal radiologist is comparatively minuscule. As correctly pointed out by the authors, the price associated with bypassing any assessment and missing malignant disease is far greater.
It is well recognized that unreported radiologic examinations can lead to misdiagnosis, compromised patient care, and liability concerns. As PSI is relatively new and has good potential to increase the accuracy, precision and efficiency of TKA, it is even more vital that this promising technology not be marred by disrepute due to possible devastating outcomes resulting from lack of a radiologic report. From the professional point of view of a radiologist, the issuance of a formal report is part and parcel of any radiological examination. I would argue that obtaining radiologic images without an accompanying report constitutes an incomplete study, and will not be in the best interest of patients.
Let the lessons learned from these 2 cases be a springboard to establish protocols for proper utilization of technologies involved in PSI for TKA and other orthopedic procedures. It is imperative to put into place mandatory reporting of all diagnostic images obtained for preoperative evaluation, particularly those that are meant to be sent directly to implant manufacturers for component design.
Menge TJ, Hartley KG, Holt GE. Patient-Specific Imaging and Missed Tumors: A Catastrophic Outcome. Am J Orthop. 2013;42(12):553-556.
The idiom “penny wise, pound foolish” certainly applies in this report of 2 cases of missed bone tumors that were present but not recognized on preoperative imaging prior to placement of patient-specific knee arthroplasties. The case report appeared in the December 2013 issue of The American Journal of Orthopedics. The term “non-diagnostic imaging,” itself a paradox, used in the context of preoperative imaging performed solely for the purpose of component templating for patient-specific instrumentation (PSI) and not intended to be diagnostic in purpose, would be anathematic to most radiologists and should be discarded as a concept.
Bearing in mind the costs incurred by the patient undergoing a total knee arthroplasty (TKA), such as professional consultation, preoperative magnetic resonance imaging, customized manufacture of the components, surgery and associated costs, and postoperative rehabilitation, the fee for a formal report by a musculoskeletal radiologist is comparatively minuscule. As correctly pointed out by the authors, the price associated with bypassing any assessment and missing malignant disease is far greater.
It is well recognized that unreported radiologic examinations can lead to misdiagnosis, compromised patient care, and liability concerns. As PSI is relatively new and has good potential to increase the accuracy, precision and efficiency of TKA, it is even more vital that this promising technology not be marred by disrepute due to possible devastating outcomes resulting from lack of a radiologic report. From the professional point of view of a radiologist, the issuance of a formal report is part and parcel of any radiological examination. I would argue that obtaining radiologic images without an accompanying report constitutes an incomplete study, and will not be in the best interest of patients.
Let the lessons learned from these 2 cases be a springboard to establish protocols for proper utilization of technologies involved in PSI for TKA and other orthopedic procedures. It is imperative to put into place mandatory reporting of all diagnostic images obtained for preoperative evaluation, particularly those that are meant to be sent directly to implant manufacturers for component design.
Menge TJ, Hartley KG, Holt GE. Patient-Specific Imaging and Missed Tumors: A Catastrophic Outcome. Am J Orthop. 2013;42(12):553-556.
Featured Article: A Pain in the Neck--Nontraumatic Causes of Neck Pain
The English expression, “a pain in the neck” is said to have originated in the early 1900s as a euphemism for the less polite phrase, “a pain in the ass.”1 While one might wonder how the expressions of such disparate discomforts came to be idiomatically equivalent, the focus of this article is on etiology of the former. All wryness aside, since most ED presentations of neck pain are musculoskeletal in origin, one may easily fail to consider the myriad of less common, but possibly serious, causes.
Pain can originate from any part of the neck and occur as a result of inflammation (eg, infections and arthritides), vascular pathology (eg, cervical artery dissection [CAD]), spaceoccupying lesions (eg, hematomas, cysts, tumors), or even as referred pain from noncervical sources (eg, heart, diaphragm, lung apex). Any lesion encroaching on the limited space of the neck can quickly compromise the airway, compress nerves, or inhibit blood flow to the brain; therefore, knowledge of the causes of such conditions is critical. This article reviews some of the less common and generally atraumatic etiologies of nontraumatic neck pain of which the emergency physician should be familiar.
Vascular Disorders
Vascular-associated neck pain can originate from vessels within the neck or represent referred pain from a more distant structure. In both cases, however, the potential for morbidity is high and the need for consideration and timely recognition crucial.
Cervical Artery Dissection
The typical initial presenting symptom of CAD—ie, internal CAD (ICAD) or vertebral artery dissection (VAD)—is severe pain in the ipsilateral neck and/or head. Onset of pain may be sudden or gradual.2 CAD occurs in an estimated 2 or 3 of every 100,000 people per year, mostly in patients between ages 20 and 40 years, and it is considered the most common cause of stroke in patients younger than age 45 years.2 The pain associated with CAD generally follows trauma. While the precipitating trauma can be a major blunt or penetrating one, it is often caused by something seemingly trivial, such as “trauma” associated with coughing, painting a ceiling, yoga, or (classically and notoriously) chiropractic manipulation.3 There is frequently some rotational component to CAD-associated trauma,4 though dissection may occur spontaneously.5
The typical triad of symptoms is ipsilateral neck and/or head pain, partial Horner’s syndrome (ptosis and miosis without anhidrosis), and signs of cerebral ischemia. However, patients do not always present with all three of these symptoms, which can complicate the diagnosis. For example, in some patients, neck pain is the sole presenting symptom and can mimic the musculoskeletal pain expected from the mechanical strain that precipitated the dissection.6 In addition, partial Horner’s syndrome occurs in only 50% of cases, and ischemic symptoms might not present for hours to weeks after the onset of neck pain.6
In almost all cases of CAD, initial symptoms are otherwise unexplained pain described as a constant, steady aching. 7 Since cervical arteries are heavily invested with pain fibers,8 an intimal tear with dissecting intramural hematoma provokes pain. Pain associated with VAD is usually severe, unilateral, posterior neck, and/or occipital, while ICAD-associated pain is ipsilateral, anterolateral neck, head, and/or face. It is important to note that head or neck pain caused by a dissection normally precedes the ischemic manifestation as opposed to the more common stroke, in which the ischemia precedes or is simultaneous with the accompanying headache.9
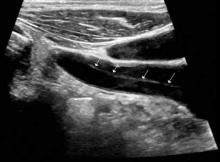
Ischemic neurological symptoms can arise from stenosis of the arterial lumen, secondary to an expanding intramural hematoma; a luminal thrombus developing at the intimal defect; or an embolization accompanied by ipsilateral Horner’s syndrome, any cranial nerve abnormality, or followed by cerebral or ocular ischemic symptoms (even if transient). A diagnosis is usually made through vascular ultrasound (Figure 1) and confirmed with computed tomography angiography (CTA) or magnetic resonance angiography. When requesting a CTA of the neck, the emergency physician should specifically make note of suspected CAD in the order. Immediate treatment includes a cervical collar and neurosurgical consultation even though treatment is essentially medical and surgery is rarely required. Anticoagulation therapy is routinely initiated to prevent thrombus propagation or embolization (unless there is brain hemorrhage). Antiplatelet therapy may be equally efficacious, 10 and can be initiated upon suspicions of CAD and while confirmatory studies are in progress. The prognosis for extracranial dissections is generally good.
Cervical Epidural Hematoma Cervical (spinal) epidural hematoma is an uncommon but potentially catastrophic event that can lead to permanent neurological deficits and death from respiratory failure. It presents as sudden and severe local neck pain with rapid development of radicular pain at the corresponding dermatomes. Motor and sensory deficits follow within minutes to days.12,13 Bleeding can occur spontaneously or secondary to trauma, surgery, or coagulopathy (which itself may be pathological—eg, hemophilia or iatrogenic in origin).14,15 Untreated, progressive cord compression can lead to permanent neurological deficits and death from respiratory failure. In the patient with acute neurological deficits, immediate correction of coagulation issues is required before decompressive surgery.
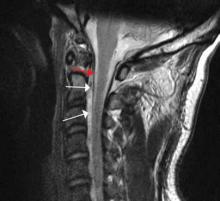
Diagnosis of cervical epidural hematoma is complicated by the rarity of the event and the lack of specific symptoms. When trauma is involved, cervical disc or nerve root injury is a more likely cause of sudden onset of neck pain, with rapid development of a radicular component. However, when symptoms occur following minor exertion (eg, sneezing, coitus, coughing) and in the presence of risk factors such as hematologic disorders, pregnancy, rheumatologic disorders, or liver dysfunction, epidural hematoma must be considered.16 Emergent magnetic resonance imaging (MRI) is the modality of choice for detecting this condition (Figure 2).
Coronary Ischemia Angina pectoris secondary to coronary ischemia is described as retrosternal “heaviness” or pressure, which may spread to either or both arms, the neck, or jaw. Pathology originating in the neck can be experienced as chest pain and may confound the diagnosis. Because cervical nerve roots C4-C8 contribute to the innervation of the anterior chest wall, irritation of any one of these nerves secondary to neck pathology can mimic true angina.17,18 Conversely, the likelihood that the only pain caused by coronary ischemia might be felt in the neck is low, but possible— especially in women.19,20 Coronary ischemia should be considered in patients with cardiac risk factors but no other obvious etiology for neck pain.21
Secondary Infection
Since emergency physicians are accustomed to dealing with infection, it is hard to imagine that we could fail to recognize infection as the etiology in a patient with a chief complaint of neck pain. Diagnosis in such cases is complicated by the anatomical location of deep neck-space infections, which limits the usefulness of standard physical examination. These sites are difficult to palpate and often impossible to visualize because they are covered with noninfected tissue. Unless specifically considered in the differential, more obscure causes of neck pain associated with infection may be missed, including retropharyngeal abscess, epiglottitis, Ludwig’s angina, vertebral osteomyelitis and discitis, cervical epidural abscess, and Lemierre’s syndrome.
Epiglottitis
Epiglottitis is inflammation of the epiglottis and adjacent supraglottic structures including the pharynx, uvula, and base of the tongue. The first recorded case is thought to have been that of George Washington, who is believed to have died from this disease.22 The high mortality rate (7% to 20% in the adult population) is a direct result of airway obstruction from inflammatory edema of the epiglottis and adjacent tissues.
Epiglottitis was originally considered a childhood disease; however, the widespread use of Haemophilus influenza vaccination has resulted in a decline in pediatric incidence. Most cases are now seen in adults (mean age of 46 years).23,24
Bacterial infection, especially from the genera Hemophilus, Streptococcus, Staphylococcus, and Klebsiella, is by far the most frequent cause of acute epiglottitis; viral and fungal-associated infections are rare. Thermal injury from swallowing hot foods or liquids, and even from inhaling crack cocaine,25 also has been implicated.
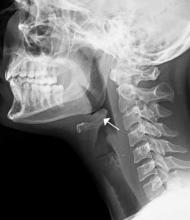
Clinical presentations of epiglottitis differ between children and adults. While children are typically dyspneic, drooling, stridorous and febrile, adults tend to present with a milder form of the disease and have painful swallowing, sore throat, and a muffled voice. In both children and adults, the larynx and upper trachea are tender to light palpation at the anterior neck.26 Although sore throat and odynophagia are more often symptoms of pharyngitis, suspicion should be aroused when pain is severe and/or there is dyspnea, severe pain with an unremarkable oropharynx examination, or anterior neck tenderness. When present, muffled voice and stridor indicate greater potential for airway compromise.27 In cases of significant airway obstruction, patients may assume the “tripod position,” leaning forward with neck extended and mouth open—panting. Since soft-tissue lateral neck radiographs are about 90% sensitive and specific for epiglottitis, a normalappearing film cannot reliably exclude the diagnosis.28 Evaluation for the classic “thumb sign” of epiglottic swelling27 (Figure 3) should be combined with the newly described “vallecula sign” for greatest accuracy.29 The vallecula sign is described as the partial or complete obliteration of a well-defined linear air pocket between the base of the tongue and the epiglottis seen on a closed mouth lateral neck X-ray.
Although CT is a useful modality for detecting epiglottic, peritonsillar, or deep neck space abscess, there are risks to patients with airway compromise; moreover, placing patients in a supine position for the study increases the likelihood of respiratory distress. Despite these risks, when indicated, CT is useful in differentiating these abscesses from similarly presenting entities such as lingual tonsillitis and upper airway foreign body.
Direct visualization via flexible oral or nasolaryngoscopy is the diagnostic gold standard but may be deferred in a stable patient. When absolutely indicated, it must be performed with caution, ideally by an anesthesiologist/otolaryngologist in a controlled setting, lest it precipitate further obstruction. Through the use of fiber optics, the need for emergent intubation can be more directly assessed and, if necessary, performed by “tube-over-scope” technique. In the ED, standby equipment for intubation and cricothyrotomy/needle cricothyrotomy should be immediately available and ready in the event of rapid deterioration, at the same time as intravenous (IV) infusion of third-generation cephalosporin or ampicillin/sulbactam, and methicillin-resistant Staphylococcus aureus (MRSA) coverage. Though the rationale for empirical use of antibiotics is evident, the role of corticosteroids and of nebulized racemic epinephrine is controversial.
Death, airway obstruction, epiglottic abscess, necrotizing epiglottitis, and secondary infections (eg, pneumonia, cervical adenitis, septic arthritis, meningitis) are the potential complications that make this source of neck pain one not to be missed. If epiglottitis is suspected, the patient must be admitted to an intensive care setting.
Retropharyngeal Abscess
The retropharyngeal space, immediately behind the posterior pharynx and esophagus, extends from the base of the skull to the mediastinum. It lies anterior to the deep cervical fascia and is bound laterally by the carotid sheaths.30 Because it is fused down the midline, abscesses in this area tend to be unilateral. The space cannot be directly assessed by physical examination, and infections in this area are rare. Timely diagnosis demands consideration of retropharyngeal abscess in patients presenting with fever, neck stiffness, and sore throat. The potential for serious morbidity and mortality is related to the host of vital structures immediately adjacent to the retropharyngeal space. Complications include mediastinitis, carotid artery erosion, jugular vein thrombosis, pericarditis, epidural abscess, sepsis, and airway compromise.
Most cases are typically observed in children younger than age 6 years. In this pediatric population, the retropharyngeal space has two parallel chains of lymph nodes draining the nose, sinuses, and pharynx; retropharyngeal abscesses usually occur as a suppurative extension from infections of these upper airway structures structures. Penetrating trauma, eg, from objects held in the mouth, is another possible cause. These nodes atrophy around 6 years of age; thereafter, the main cause of retropharyngeal abscess is purulent extension of an adjacent (frequently odontogenic) infection or posterior pharyngeal trauma (eg, from a fish bone or instrumentation).31 As befits its origin with oral flora, cultures are almost always polymicrobial (eg, Streptococci viridans and pyogenes, Staphylococcus, H influenza, Klebsiella, anaerobes).
Although retropharyngeal abscess is considered a disease of childhood, like epiglottitis, its incidence in adults is increasing. 32 Presenting symptoms are signs of respiratory distress, such as wheezing, stridor, and drooling with impending airway obstruction from the expanding posterior pharyngeal mass. Late signs of the illness are respiratory failure due to airway obstruction and septic shock, but an astute clinician should recognize the entity long before these symptoms present. Early symptoms include fever, sore throat, odynophagia, and neck pain and stiffness (typically manifesting as a reluctance to turn the neck).33 Patients may also complain of feeling a lump in the throat or pain in the posterior neck or shoulder with swallowing.34 Ninety-seven percent of pediatric patients present with neck pain,32 which could manifest dramatically as torticollis. Most likely, a child will have a subtle reluctance to move his or her neck during the course of the physical examination. In addition, there may be posterior pharyngeal edema and/or a visible unilateral posterior pharyngeal bulge, cervical adenopathy, and a “croupy” cry or cough resembling a duck’s quack—the “cri du canard.”35 Definitive diagnosis is made using X-ray and/or CT. A lateral soft-tissue neck X-ray will demonstrate widening of the prevertebral soft tissues. CT with contrast provides a more definitive diagnosis, and is also useful to differentiate abscess (ie, a hypodense lesion with ring enhancement) from cellulitis.
Regarding treatment, empiric IV antibiotics must be started immediately and may alone prevent progression if the diagnosis is made before cellulitis has progressed to abscess. Intravenous clindamycin is a reasonable first-line antibiotic; other suggested drugs include a penicillin/ beta lactamase inhibitor, penicillin G plus metronidazole, and cefoxitin.36 Airway protection is mandatory, and an otolaryngologist should be consulted early. Because of the potential for sudden airway deterioration, the emergency physician must be prepared to establish a surgical airway.
Ludwig’s Angina
Ludwig’s angina derives its name from the German physician Wilhelm Friedrich von Ludwig, who first described this deadly, rapidly progressive, fascial space/ connective tissue gangrenous cellulitis of the floor of the mouth and adjoining neck in 1836. In a curious twist of fate, it is believed that Dr Ludwig died from this very disease that bears his name.37
Ninety percent of cases of Ludwig’s angina are odontogenic, often due to periapical abscesses. This condition may result secondary to any oral or parapharyngeal infection that spreads by continuity from the submandibular space into the contiguous sublingual and submental spaces. The potential for airway obstruction comes from elevation and displacement of the tongue, resulting in a mortality rate greater than 50% if untreated. Causative organisms mirror normal, polymicrobial oral flora and include Staphylococcus, Streptococcus, Fusobacterium, and Bacteroides.38,23
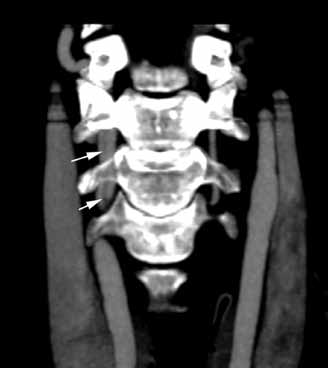
Diagnosis of Ludwig’s angina is primarily clinical. Neck pain and swelling, dental pain, dysphagia, malaise, and fever, along with a protruding or elevated tongue, are typical. Submandibular swelling, which is seen in 95% of patients, develops in advanced cases into an intense “woody” induration above the hyoid bone that portends the impending airway crisis.39 If the patient is sufficiently clinically stable and able to lie flat, definitive diagnosis can be made with a contrastenhanced, soft-tissue neck CT (Figure 4), which can also evaluate for a drainable abscess, soft-tissue gas, and mediastinal extension; this modality can also define the extent of soft-tissue swelling and airway patency.
Airway management is the primary consideration because of its potential for rapid deterioration. Traditional management has been aggressive and surgical, with the standard being early tracheostomy. More recent reports have encouraged more conservative management when possible.40 Impending or actual airway compromise, as manifest by significant trismus, inability to flex the neck without compromising the airway, inability to protrude the tongue, or actual resting dyspnea demand that a surgical airway be readied at bedside until fiber optic nasotracheal intubation is secured.
Antibiotics must be given early and include coverage for gram-positive, gramnegative, and anaerobic organisms. Intravenous metronidazole and penicillin (cefazolin or clindamycin if patient has an allergy to penicillin) are commonly prescribed.38,23 Although controversial, administration of IV dexamethasone (8 mg to 12 mg) and nebulized epinephrine (1:1000, 1 mL diluted to 5 mL with normal saline) to reduce edema has been advocated. 41
Lemierre’s Syndrome Lemierre’s syndrome, septic thrombophlebitis of the internal jugular vein, was first described in 1936 by André Lemierre, who published a series of cases of previously healthy young adults in whom oropharyngeal infections were followed by “anaerobic postanginal septicaemias.”42 Most of these patients presented with sore throat (referred to as “angina” in “old skool” speak) and worsening pain and tenderness at the anterolateral neck, with pulmonary symptoms manifesting several days to 2 weeks later. The causative organism, Fusobacterium necrophorum, is a gram-negative anaerobe that is part of the normal commensal oropharyngeal flora. It invades the internal jugular (IJ) vein via the lateral pharyngeal space and releases a hemagglutinin that promotes thrombus formation in the IJ and, ultimately, metastatic septic emboli. These emboli typically invade the lungs and cause multiple nodular infiltrates and small pleural effusions. Unfortunately, as each case is unique, diagnosis is often delayed. Septic emboli can migrate to other sites and cause arthritis (hip, knee, shoulder, sacroiliac, and other joints), osteomyelitis, young adult with a history of recent sore throat and fever who subsequently developed neck pain and tenderness (with or without swelling) over the IJ, rigors, pulmonary infiltrates, and possibly other signs of septic emboli.
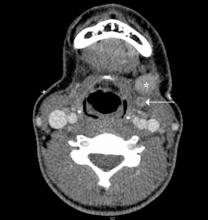
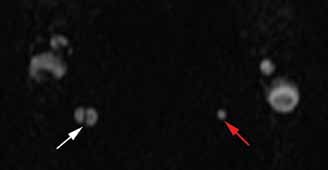
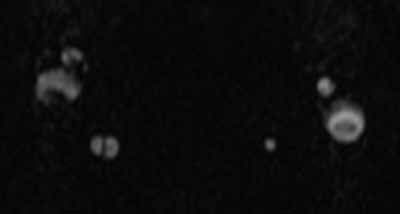
Doppler ultrasound or CT will show IJ thrombosis43 (Figure 5). Purulent discharge, if obtained, has a characteristic foul smell that has been likened to “limburger or overripe Camembert cheese.”44 Treatment is with high-dose IV penicillin and metronidazole or with clindamycin as single coverage. Heparin could potentially aid in dissemination of emboli, but it is used only when there is retrograde propagation of clot to the cavernous sinus.
With the routine antibiotic treatment of pharyngitis in the 1960s and 1970s, cases of Lemierre’s syndrome became so rare that it was referred to as the “forgotten disease.”45 Unfortunately, its incidence has increased over the past few years.43 It is unclear whether this rise is due to increasing antibiotic resistance or to an increasing resistance of clinicians to use antibiotics for “sore throats.”
Cervical Spinal Infections
Vertebral osteomyelitis, discitis, and spinal epidural abscess are rare in developed countries. Most cases stem from hematogenous seeding, skin abscesses, and urinary tract infections but can also originate from a host of other sites, including penetrating trauma and invasive spinal procedures (eg, lumbar punctures, epidural injections). 46,47 Cervical spine infections are associated with immune-compromising situations or conditions (eg, IV drug use, diabetes mellitus, malignancy, acquired immunodeficiency syndrome, renal insufficiency, long-term use of systemic corticosteroids).
All three of these conditions present similarly, often as localized neck pain that grows more intense over a period of days to weeks and worsens with neck movement. Neurological signs ordinarily appear late in the course of the illness. Fever is a classic symptom but is not always present.48 There is usually tenderness over the involved spinous process. The development of motor or sensory loss suggests formation of an abscess,49 which can rapidly lead to further compressive symptoms and sepsis.
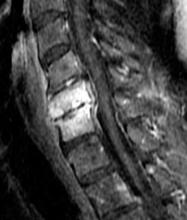
Leukocytosis may be absent but erythrocyte sedimentation rate and C-reactive protein are often elevated. A CT scan with contrast is frequently required for diagnosis, though when available, MRI with IV gadolinium is the test of choice (Figure 6). Most cases are caused by S aureus, but antibiotic coverage for gram-positive organisms (including MRSA), gram-negative organisms, and anaerobes should be started as soon as blood cultures are drawn. Neurosurgery should be consulted emergently since, with cervical epidural abscess, neurological deterioration—even to the point of total paralysis—can develop in a matter of hours.50
Conclusion
Although most patients presenting to the ED with neck pain are musculoskeletal and associated with a traumatic event, other infrequent but potentially serious atraumatic causes may be present. Based on a patient’s symptoms, emergency physicians should also consider these conditions in the differential diagnosis to ensure rapid treatment to prevent further complications.
- Ammer C. The American heritage dictionary of idioms. Boston, MA: Houghton Mifflin Company; 1997:489.
- Fusco MR, Harrigan MR. Cerebrovascular dissections—a review part I: spontaneous dissections. Neurosurgery. 2011;68(1):242-257.
- Rubinstein SM, Peerdeman SM, van Tulder MW, Riphagen I, Haldeman S. A systematic review of the risk factors for cervical artery dissection. Stroke.2005;36(7):1575-1580.
- Bergin M, Bird P, Wright A. Internal carotid artery dissection following canalith repositioning procedure. J Laryngol Otol. 2010;124(5):575, 576.
- Brandt T, Grond-Ginsbach C. Spontaneous cervical artery dissection: from risk factors toward pathogenesis. Stroke. 2002;33(3):657,658.
- Arnold M, Cumurciuc R, Stapf C, Favrole P, Berthet K, Bousser MG. Pain as the only symptom of cervical artery dissection. J Neurol Neurosurg Psychiatry. 2006;77(9):1021-1024.
- Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906.
- Caplan LR. Dissections of brain-supplying arteries. Nat Clin Pract Neurol. 2008;4(1):34-42.
- Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45(8):1517-1522.
- Engelter ST, Brandt T, Debette S; Cervical Artery Dissection in Ischemic Stroke Patients (CADISP) Study Group. Antiplatelets versus anticoagulation in cervical artery dissection. Stroke. 2007;38(9):2605-2611.
- Arnold M, Bousser M, Fahrni G, et al. Vertebral artery dissection: presenting findings and predictors of outcome. Stroke. 2006;37(10):2499-2503.
- Hsieh CT, Chang CF, Lin EY, Tsai TH, Chiang YH, Ju DT. Spontaneous spinal epidural hematomas of cervical spine: report of 4 cases and literature review. Am J Emerg Med. 2006;24(6):736-740.
- Sei A, Nakamura T, Hashimoto N, Mizuta H, Sasaki A, Takagi K. Cervical spinal epidural hematoma with spontaneous remission. J Spinal Disord. 1991;4(2):234-237.
- Williams JM, Allegra JR. Spontaneous cervical epidural hematoma. Ann Emerg Med. 1994;23(6):1368-1370.
- Demierre B, Unger PF, Bongioanni F. Sudden cervical pain: spontaneous cervical epidural hematoma. Am J Emerg Med. 1991;9(1):54-56.
- Broder J, L’Italien A. Evaluation and management of the patient with neck pain. In: Mattu A, Goyal DG eds. Emergency Medicine: Avoiding the Pitfalls and Improving the Outcomes. Malden, MA: Blackwell Publishing, Inc; 2007:46-54. http://onlinelibrary. wiley.com/book/10.1002/9780470755938. Accessed November 15, 2013.
- Brodsky AE. Cervical angina. A correlative study with emphasis on the use of coronary arteriography. Spine. 1985;10(8):699-709.
- Hanflig SS. Pain in the shoulder girdle, arm and precordium due to cervical arthritis. JAMA. 1936;106(7):523-526.
- Goldberg R, Goff D, Cooper L, et al. Age and sex differences in presentation of symptoms among patients with acute coronary disease: the REACT Trial. Rapid Early Action for Coronary Treatment. Coron Artery Dis. 2000;11(5):399-407.
- Coventry LL, Finn J, Bremner AP. Sex differences in symptom presentation in acute myocardial infarction: A systematic review and meta-analysis. Heart Lung. 2011;40(6):477-491.
- Lipetz JS, Ledon J, Silber J. Severe coronary artery disease presenting with a chief complaint of cervical pain. Am J Phys Med Rehabil. 2003;82(9):716-720.
- Morens DM. Death of a president. N Engl J Med. 1999;341(24):1845-1849.
- Winters M. Evidence-based diagnosis and management of ENT emergencies. Medscape. 2007. http://www.medscape.com/viewarticle/551650_1. Accessed November 15, 2013.
- Mayo-Smith MF, Spinale JW, Donskey CJ, Yukawa M, Li RH, Schiffman FJ. Acute epiglottitis: An 18-year experience in Rhode Island. Chest. 1995;108(6):1640-1670.
- Mayo-Smith MF, Spinale J. Thermal epiglottitis in adults: a new complication of illicit drug use. J Emerg Med. 1997;15(4):483-485.
- Bansal A, Miskoff J, Lis RJ. Otolaryngologic critical care. Crit Care Clin. 2003;19(1):55-72.
- Katori H, Tsukuda M. Acute epiglottitis: analysis of factors associated with airway intervention. J Laryngol Otol. 2005;119(12):967-972.
- Rothrock SG, Pignatiello GA, Howard RM. Radiologic diagnosis of epiglottitis: objective criteria for all ages. Ann Emerg Med. 1990;19(9):978-982.
- Ducic Y, Hébert PC, MacLachlan L, Neufeld K, Lamothe A. Description and evaluation of the vallecula sign: a new radiologic sign in the diagnosis of adult epiglottitis. Ann Emerg Med. 1997;30(1):1-6.
- Vieira F, Allen SM, Stocks RM, Thompson JW. Deep neck infection. Otolaryngol Clin North Am. 2008;41(3):459-483.
- Shores CG. Infections and disorders of the neck and upper airway. In: Tintinalli JE, Stapczynski JS, Kelen GD, eds. In: Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 6th ed. New York, NY: McGraw-Hill; 2004:1494-1499.
- Kahn JH. Retropharyngeal Abscess in Emergency Medicine. Medscape Review. 2008.
- Gibson CG. Do not rely on the presence of respiratory compromise to make the diagnosis of retropharyngeal abscess. In: Mattu A, Chanmugam AS, Swadron SP, Tibbles CD, Woolridge DP, eds. Avoiding Common Errors in the Emergency Department. New York, NY: Lippincott Williams & Wilkins; 2010:212.
- Greene JS, Asher IM. Retropharyngeal abscess: A previously unreported symptom. Ann Emerg Med. 1984;13(8):615-619.
- Melio FR. Upper respiratory tract infections. In: Marx J, Hockberger R, Walls R, eds. Rosen’s Emergency Medicine-Concepts and Clinical Practice 7th ed. Philadelphia, PA: Mosby Elsevier; 2009:921-923.
- Sanford JP, Gilbert DN, Moellering RC, Sande MA, Eliopoulos GM, eds. The Sanford guide to Antimicrobial Therapy 2006-2007. 37th ed. Sperryville, VA: Antimicrobial Therapy, Inc; 2007:30.
- Murphy SC. The person behind the eponym: Wilhelm Frederick von Ludwig (1790-1865). J Oral Pathol Med. 1996;25(9):513-515.
- Hasan W, Leonard D, Russell J. Ludwig’s Angina—A controversial surgical emergency: How we do it. Int J Otolaryngol. 2011;2011:231816.
- Saifeldeen K, Evans R. Ludwig’s angina. Emerg Med J. 2004;21(2):242,243.
- Marple BF. Ludwig angina: a review of current airway management. Arch Otolaryngol Head Neck Surg. 1999;125(5):596-599.
- Buckley MF, O’Connor K. Ludwig’s angina in a 76-year-old man. Emerg Med J. 2009;26(9):679-680.
- Lemierre A. On certain septicaemias due to anaerobic organisms. Lancet. 1936;227(5874):701-703.
- Karkos PD, Asrani S, Karkos CD, et al. Lemierre’s syndrome: a systematic review. Laryngoscope. 2009;119(8):1552-1559.
- Alston JM. Necrobacillosis in Great Britain. Brit Med J. 1955;2(4955):1524-1528.
- Vargiami EG, Zafeiriou D. Eponym: The Lemierre syndrome. Eur J Pediatr. 2010;169(4):411-414.
- Martínez Hernández PL, Amer López M, Zamora Vargas F, et al. Spontaneous infectious spondylodiscitis in an internal medicine department: epidemiological and clinical study in 41 cases. Rev Clin Esp. 2008;208(7):347-352.
- Urrutia J, Bono CM, Mery P, Rojas C, Gana N, Campos M. Chronic liver failure and concomitant distant infections are associated with high rates of neurological involvement in pyogenic spinal infections. Spine. 2009;34(7):E240-E244.
- Buranapanitkit B, Lim A, Kiriratnikom T. Clinical manifestation of tuberculous and pyogenic spine infection. J Med Assoc Thai. 2001;84(11):1522-1526.
- Schimmer RC, Jeanneret C, Nunley PD, Jeanneret B. Osteomyelitis of the cervical spine: a potentially dramatic disease. J Spinal Disord Tech. 2002;15(2):110-117.
- Darouiche RO. Spinal epidural abscess. N Engl J Med. 2006;355(19):2012-2020.
The English expression, “a pain in the neck” is said to have originated in the early 1900s as a euphemism for the less polite phrase, “a pain in the ass.”1 While one might wonder how the expressions of such disparate discomforts came to be idiomatically equivalent, the focus of this article is on etiology of the former. All wryness aside, since most ED presentations of neck pain are musculoskeletal in origin, one may easily fail to consider the myriad of less common, but possibly serious, causes.
Pain can originate from any part of the neck and occur as a result of inflammation (eg, infections and arthritides), vascular pathology (eg, cervical artery dissection [CAD]), spaceoccupying lesions (eg, hematomas, cysts, tumors), or even as referred pain from noncervical sources (eg, heart, diaphragm, lung apex). Any lesion encroaching on the limited space of the neck can quickly compromise the airway, compress nerves, or inhibit blood flow to the brain; therefore, knowledge of the causes of such conditions is critical. This article reviews some of the less common and generally atraumatic etiologies of nontraumatic neck pain of which the emergency physician should be familiar.
Vascular Disorders
Vascular-associated neck pain can originate from vessels within the neck or represent referred pain from a more distant structure. In both cases, however, the potential for morbidity is high and the need for consideration and timely recognition crucial.
Cervical Artery Dissection
The typical initial presenting symptom of CAD—ie, internal CAD (ICAD) or vertebral artery dissection (VAD)—is severe pain in the ipsilateral neck and/or head. Onset of pain may be sudden or gradual.2 CAD occurs in an estimated 2 or 3 of every 100,000 people per year, mostly in patients between ages 20 and 40 years, and it is considered the most common cause of stroke in patients younger than age 45 years.2 The pain associated with CAD generally follows trauma. While the precipitating trauma can be a major blunt or penetrating one, it is often caused by something seemingly trivial, such as “trauma” associated with coughing, painting a ceiling, yoga, or (classically and notoriously) chiropractic manipulation.3 There is frequently some rotational component to CAD-associated trauma,4 though dissection may occur spontaneously.5
The typical triad of symptoms is ipsilateral neck and/or head pain, partial Horner’s syndrome (ptosis and miosis without anhidrosis), and signs of cerebral ischemia. However, patients do not always present with all three of these symptoms, which can complicate the diagnosis. For example, in some patients, neck pain is the sole presenting symptom and can mimic the musculoskeletal pain expected from the mechanical strain that precipitated the dissection.6 In addition, partial Horner’s syndrome occurs in only 50% of cases, and ischemic symptoms might not present for hours to weeks after the onset of neck pain.6
In almost all cases of CAD, initial symptoms are otherwise unexplained pain described as a constant, steady aching. 7 Since cervical arteries are heavily invested with pain fibers,8 an intimal tear with dissecting intramural hematoma provokes pain. Pain associated with VAD is usually severe, unilateral, posterior neck, and/or occipital, while ICAD-associated pain is ipsilateral, anterolateral neck, head, and/or face. It is important to note that head or neck pain caused by a dissection normally precedes the ischemic manifestation as opposed to the more common stroke, in which the ischemia precedes or is simultaneous with the accompanying headache.9
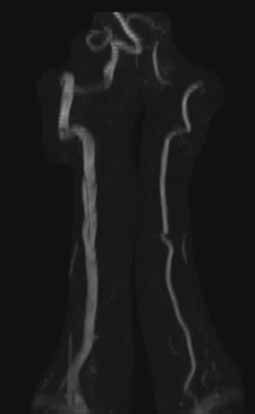
Ischemic neurological symptoms can arise from stenosis of the arterial lumen, secondary to an expanding intramural hematoma; a luminal thrombus developing at the intimal defect; or an embolization accompanied by ipsilateral Horner’s syndrome, any cranial nerve abnormality, or followed by cerebral or ocular ischemic symptoms (even if transient). A diagnosis is usually made through vascular ultrasound (Figure 1) and confirmed with computed tomography angiography (CTA) or magnetic resonance angiography. When requesting a CTA of the neck, the emergency physician should specifically make note of suspected CAD in the order. Immediate treatment includes a cervical collar and neurosurgical consultation even though treatment is essentially medical and surgery is rarely required. Anticoagulation therapy is routinely initiated to prevent thrombus propagation or embolization (unless there is brain hemorrhage). Antiplatelet therapy may be equally efficacious, 10 and can be initiated upon suspicions of CAD and while confirmatory studies are in progress. The prognosis for extracranial dissections is generally good.
Cervical Epidural Hematoma Cervical (spinal) epidural hematoma is an uncommon but potentially catastrophic event that can lead to permanent neurological deficits and death from respiratory failure. It presents as sudden and severe local neck pain with rapid development of radicular pain at the corresponding dermatomes. Motor and sensory deficits follow within minutes to days.12,13 Bleeding can occur spontaneously or secondary to trauma, surgery, or coagulopathy (which itself may be pathological—eg, hemophilia or iatrogenic in origin).14,15 Untreated, progressive cord compression can lead to permanent neurological deficits and death from respiratory failure. In the patient with acute neurological deficits, immediate correction of coagulation issues is required before decompressive surgery.
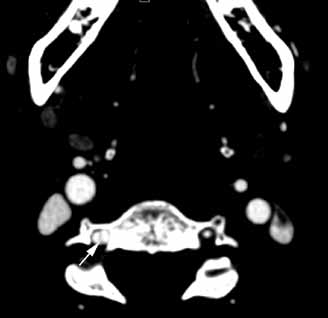
Diagnosis of cervical epidural hematoma is complicated by the rarity of the event and the lack of specific symptoms. When trauma is involved, cervical disc or nerve root injury is a more likely cause of sudden onset of neck pain, with rapid development of a radicular component. However, when symptoms occur following minor exertion (eg, sneezing, coitus, coughing) and in the presence of risk factors such as hematologic disorders, pregnancy, rheumatologic disorders, or liver dysfunction, epidural hematoma must be considered.16 Emergent magnetic resonance imaging (MRI) is the modality of choice for detecting this condition (Figure 2).
Coronary Ischemia Angina pectoris secondary to coronary ischemia is described as retrosternal “heaviness” or pressure, which may spread to either or both arms, the neck, or jaw. Pathology originating in the neck can be experienced as chest pain and may confound the diagnosis. Because cervical nerve roots C4-C8 contribute to the innervation of the anterior chest wall, irritation of any one of these nerves secondary to neck pathology can mimic true angina.17,18 Conversely, the likelihood that the only pain caused by coronary ischemia might be felt in the neck is low, but possible— especially in women.19,20 Coronary ischemia should be considered in patients with cardiac risk factors but no other obvious etiology for neck pain.21
Secondary Infection
Since emergency physicians are accustomed to dealing with infection, it is hard to imagine that we could fail to recognize infection as the etiology in a patient with a chief complaint of neck pain. Diagnosis in such cases is complicated by the anatomical location of deep neck-space infections, which limits the usefulness of standard physical examination. These sites are difficult to palpate and often impossible to visualize because they are covered with noninfected tissue. Unless specifically considered in the differential, more obscure causes of neck pain associated with infection may be missed, including retropharyngeal abscess, epiglottitis, Ludwig’s angina, vertebral osteomyelitis and discitis, cervical epidural abscess, and Lemierre’s syndrome.
Epiglottitis
Epiglottitis is inflammation of the epiglottis and adjacent supraglottic structures including the pharynx, uvula, and base of the tongue. The first recorded case is thought to have been that of George Washington, who is believed to have died from this disease.22 The high mortality rate (7% to 20% in the adult population) is a direct result of airway obstruction from inflammatory edema of the epiglottis and adjacent tissues.
Epiglottitis was originally considered a childhood disease; however, the widespread use of Haemophilus influenza vaccination has resulted in a decline in pediatric incidence. Most cases are now seen in adults (mean age of 46 years).23,24
Bacterial infection, especially from the genera Hemophilus, Streptococcus, Staphylococcus, and Klebsiella, is by far the most frequent cause of acute epiglottitis; viral and fungal-associated infections are rare. Thermal injury from swallowing hot foods or liquids, and even from inhaling crack cocaine,25 also has been implicated.
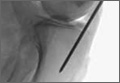
Clinical presentations of epiglottitis differ between children and adults. While children are typically dyspneic, drooling, stridorous and febrile, adults tend to present with a milder form of the disease and have painful swallowing, sore throat, and a muffled voice. In both children and adults, the larynx and upper trachea are tender to light palpation at the anterior neck.26 Although sore throat and odynophagia are more often symptoms of pharyngitis, suspicion should be aroused when pain is severe and/or there is dyspnea, severe pain with an unremarkable oropharynx examination, or anterior neck tenderness. When present, muffled voice and stridor indicate greater potential for airway compromise.27 In cases of significant airway obstruction, patients may assume the “tripod position,” leaning forward with neck extended and mouth open—panting. Since soft-tissue lateral neck radiographs are about 90% sensitive and specific for epiglottitis, a normalappearing film cannot reliably exclude the diagnosis.28 Evaluation for the classic “thumb sign” of epiglottic swelling27 (Figure 3) should be combined with the newly described “vallecula sign” for greatest accuracy.29 The vallecula sign is described as the partial or complete obliteration of a well-defined linear air pocket between the base of the tongue and the epiglottis seen on a closed mouth lateral neck X-ray.
Although CT is a useful modality for detecting epiglottic, peritonsillar, or deep neck space abscess, there are risks to patients with airway compromise; moreover, placing patients in a supine position for the study increases the likelihood of respiratory distress. Despite these risks, when indicated, CT is useful in differentiating these abscesses from similarly presenting entities such as lingual tonsillitis and upper airway foreign body.
Direct visualization via flexible oral or nasolaryngoscopy is the diagnostic gold standard but may be deferred in a stable patient. When absolutely indicated, it must be performed with caution, ideally by an anesthesiologist/otolaryngologist in a controlled setting, lest it precipitate further obstruction. Through the use of fiber optics, the need for emergent intubation can be more directly assessed and, if necessary, performed by “tube-over-scope” technique. In the ED, standby equipment for intubation and cricothyrotomy/needle cricothyrotomy should be immediately available and ready in the event of rapid deterioration, at the same time as intravenous (IV) infusion of third-generation cephalosporin or ampicillin/sulbactam, and methicillin-resistant Staphylococcus aureus (MRSA) coverage. Though the rationale for empirical use of antibiotics is evident, the role of corticosteroids and of nebulized racemic epinephrine is controversial.
Death, airway obstruction, epiglottic abscess, necrotizing epiglottitis, and secondary infections (eg, pneumonia, cervical adenitis, septic arthritis, meningitis) are the potential complications that make this source of neck pain one not to be missed. If epiglottitis is suspected, the patient must be admitted to an intensive care setting.
Retropharyngeal Abscess
The retropharyngeal space, immediately behind the posterior pharynx and esophagus, extends from the base of the skull to the mediastinum. It lies anterior to the deep cervical fascia and is bound laterally by the carotid sheaths.30 Because it is fused down the midline, abscesses in this area tend to be unilateral. The space cannot be directly assessed by physical examination, and infections in this area are rare. Timely diagnosis demands consideration of retropharyngeal abscess in patients presenting with fever, neck stiffness, and sore throat. The potential for serious morbidity and mortality is related to the host of vital structures immediately adjacent to the retropharyngeal space. Complications include mediastinitis, carotid artery erosion, jugular vein thrombosis, pericarditis, epidural abscess, sepsis, and airway compromise.
Most cases are typically observed in children younger than age 6 years. In this pediatric population, the retropharyngeal space has two parallel chains of lymph nodes draining the nose, sinuses, and pharynx; retropharyngeal abscesses usually occur as a suppurative extension from infections of these upper airway structures structures. Penetrating trauma, eg, from objects held in the mouth, is another possible cause. These nodes atrophy around 6 years of age; thereafter, the main cause of retropharyngeal abscess is purulent extension of an adjacent (frequently odontogenic) infection or posterior pharyngeal trauma (eg, from a fish bone or instrumentation).31 As befits its origin with oral flora, cultures are almost always polymicrobial (eg, Streptococci viridans and pyogenes, Staphylococcus, H influenza, Klebsiella, anaerobes).
Although retropharyngeal abscess is considered a disease of childhood, like epiglottitis, its incidence in adults is increasing. 32 Presenting symptoms are signs of respiratory distress, such as wheezing, stridor, and drooling with impending airway obstruction from the expanding posterior pharyngeal mass. Late signs of the illness are respiratory failure due to airway obstruction and septic shock, but an astute clinician should recognize the entity long before these symptoms present. Early symptoms include fever, sore throat, odynophagia, and neck pain and stiffness (typically manifesting as a reluctance to turn the neck).33 Patients may also complain of feeling a lump in the throat or pain in the posterior neck or shoulder with swallowing.34 Ninety-seven percent of pediatric patients present with neck pain,32 which could manifest dramatically as torticollis. Most likely, a child will have a subtle reluctance to move his or her neck during the course of the physical examination. In addition, there may be posterior pharyngeal edema and/or a visible unilateral posterior pharyngeal bulge, cervical adenopathy, and a “croupy” cry or cough resembling a duck’s quack—the “cri du canard.”35 Definitive diagnosis is made using X-ray and/or CT. A lateral soft-tissue neck X-ray will demonstrate widening of the prevertebral soft tissues. CT with contrast provides a more definitive diagnosis, and is also useful to differentiate abscess (ie, a hypodense lesion with ring enhancement) from cellulitis.
Regarding treatment, empiric IV antibiotics must be started immediately and may alone prevent progression if the diagnosis is made before cellulitis has progressed to abscess. Intravenous clindamycin is a reasonable first-line antibiotic; other suggested drugs include a penicillin/ beta lactamase inhibitor, penicillin G plus metronidazole, and cefoxitin.36 Airway protection is mandatory, and an otolaryngologist should be consulted early. Because of the potential for sudden airway deterioration, the emergency physician must be prepared to establish a surgical airway.
Ludwig’s Angina
Ludwig’s angina derives its name from the German physician Wilhelm Friedrich von Ludwig, who first described this deadly, rapidly progressive, fascial space/ connective tissue gangrenous cellulitis of the floor of the mouth and adjoining neck in 1836. In a curious twist of fate, it is believed that Dr Ludwig died from this very disease that bears his name.37
Ninety percent of cases of Ludwig’s angina are odontogenic, often due to periapical abscesses. This condition may result secondary to any oral or parapharyngeal infection that spreads by continuity from the submandibular space into the contiguous sublingual and submental spaces. The potential for airway obstruction comes from elevation and displacement of the tongue, resulting in a mortality rate greater than 50% if untreated. Causative organisms mirror normal, polymicrobial oral flora and include Staphylococcus, Streptococcus, Fusobacterium, and Bacteroides.38,23
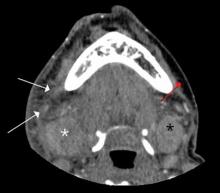
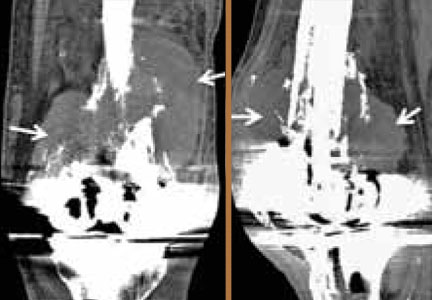
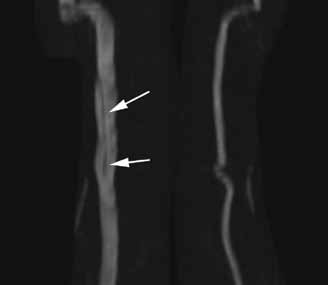
Diagnosis of Ludwig’s angina is primarily clinical. Neck pain and swelling, dental pain, dysphagia, malaise, and fever, along with a protruding or elevated tongue, are typical. Submandibular swelling, which is seen in 95% of patients, develops in advanced cases into an intense “woody” induration above the hyoid bone that portends the impending airway crisis.39 If the patient is sufficiently clinically stable and able to lie flat, definitive diagnosis can be made with a contrastenhanced, soft-tissue neck CT (Figure 4), which can also evaluate for a drainable abscess, soft-tissue gas, and mediastinal extension; this modality can also define the extent of soft-tissue swelling and airway patency.
Airway management is the primary consideration because of its potential for rapid deterioration. Traditional management has been aggressive and surgical, with the standard being early tracheostomy. More recent reports have encouraged more conservative management when possible.40 Impending or actual airway compromise, as manifest by significant trismus, inability to flex the neck without compromising the airway, inability to protrude the tongue, or actual resting dyspnea demand that a surgical airway be readied at bedside until fiber optic nasotracheal intubation is secured.
Antibiotics must be given early and include coverage for gram-positive, gramnegative, and anaerobic organisms. Intravenous metronidazole and penicillin (cefazolin or clindamycin if patient has an allergy to penicillin) are commonly prescribed.38,23 Although controversial, administration of IV dexamethasone (8 mg to 12 mg) and nebulized epinephrine (1:1000, 1 mL diluted to 5 mL with normal saline) to reduce edema has been advocated. 41
Lemierre’s Syndrome Lemierre’s syndrome, septic thrombophlebitis of the internal jugular vein, was first described in 1936 by André Lemierre, who published a series of cases of previously healthy young adults in whom oropharyngeal infections were followed by “anaerobic postanginal septicaemias.”42 Most of these patients presented with sore throat (referred to as “angina” in “old skool” speak) and worsening pain and tenderness at the anterolateral neck, with pulmonary symptoms manifesting several days to 2 weeks later. The causative organism, Fusobacterium necrophorum, is a gram-negative anaerobe that is part of the normal commensal oropharyngeal flora. It invades the internal jugular (IJ) vein via the lateral pharyngeal space and releases a hemagglutinin that promotes thrombus formation in the IJ and, ultimately, metastatic septic emboli. These emboli typically invade the lungs and cause multiple nodular infiltrates and small pleural effusions. Unfortunately, as each case is unique, diagnosis is often delayed. Septic emboli can migrate to other sites and cause arthritis (hip, knee, shoulder, sacroiliac, and other joints), osteomyelitis, young adult with a history of recent sore throat and fever who subsequently developed neck pain and tenderness (with or without swelling) over the IJ, rigors, pulmonary infiltrates, and possibly other signs of septic emboli.
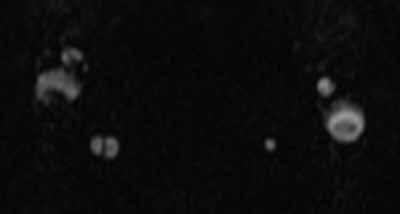
Doppler ultrasound or CT will show IJ thrombosis43 (Figure 5). Purulent discharge, if obtained, has a characteristic foul smell that has been likened to “limburger or overripe Camembert cheese.”44 Treatment is with high-dose IV penicillin and metronidazole or with clindamycin as single coverage. Heparin could potentially aid in dissemination of emboli, but it is used only when there is retrograde propagation of clot to the cavernous sinus.
With the routine antibiotic treatment of pharyngitis in the 1960s and 1970s, cases of Lemierre’s syndrome became so rare that it was referred to as the “forgotten disease.”45 Unfortunately, its incidence has increased over the past few years.43 It is unclear whether this rise is due to increasing antibiotic resistance or to an increasing resistance of clinicians to use antibiotics for “sore throats.”
Cervical Spinal Infections
Vertebral osteomyelitis, discitis, and spinal epidural abscess are rare in developed countries. Most cases stem from hematogenous seeding, skin abscesses, and urinary tract infections but can also originate from a host of other sites, including penetrating trauma and invasive spinal procedures (eg, lumbar punctures, epidural injections). 46,47 Cervical spine infections are associated with immune-compromising situations or conditions (eg, IV drug use, diabetes mellitus, malignancy, acquired immunodeficiency syndrome, renal insufficiency, long-term use of systemic corticosteroids).
All three of these conditions present similarly, often as localized neck pain that grows more intense over a period of days to weeks and worsens with neck movement. Neurological signs ordinarily appear late in the course of the illness. Fever is a classic symptom but is not always present.48 There is usually tenderness over the involved spinous process. The development of motor or sensory loss suggests formation of an abscess,49 which can rapidly lead to further compressive symptoms and sepsis.
Leukocytosis may be absent but erythrocyte sedimentation rate and C-reactive protein are often elevated. A CT scan with contrast is frequently required for diagnosis, though when available, MRI with IV gadolinium is the test of choice (Figure 6). Most cases are caused by S aureus, but antibiotic coverage for gram-positive organisms (including MRSA), gram-negative organisms, and anaerobes should be started as soon as blood cultures are drawn. Neurosurgery should be consulted emergently since, with cervical epidural abscess, neurological deterioration—even to the point of total paralysis—can develop in a matter of hours.50
Conclusion
Although most patients presenting to the ED with neck pain are musculoskeletal and associated with a traumatic event, other infrequent but potentially serious atraumatic causes may be present. Based on a patient’s symptoms, emergency physicians should also consider these conditions in the differential diagnosis to ensure rapid treatment to prevent further complications.
The English expression, “a pain in the neck” is said to have originated in the early 1900s as a euphemism for the less polite phrase, “a pain in the ass.”1 While one might wonder how the expressions of such disparate discomforts came to be idiomatically equivalent, the focus of this article is on etiology of the former. All wryness aside, since most ED presentations of neck pain are musculoskeletal in origin, one may easily fail to consider the myriad of less common, but possibly serious, causes.
Pain can originate from any part of the neck and occur as a result of inflammation (eg, infections and arthritides), vascular pathology (eg, cervical artery dissection [CAD]), spaceoccupying lesions (eg, hematomas, cysts, tumors), or even as referred pain from noncervical sources (eg, heart, diaphragm, lung apex). Any lesion encroaching on the limited space of the neck can quickly compromise the airway, compress nerves, or inhibit blood flow to the brain; therefore, knowledge of the causes of such conditions is critical. This article reviews some of the less common and generally atraumatic etiologies of nontraumatic neck pain of which the emergency physician should be familiar.
Vascular Disorders
Vascular-associated neck pain can originate from vessels within the neck or represent referred pain from a more distant structure. In both cases, however, the potential for morbidity is high and the need for consideration and timely recognition crucial.
Cervical Artery Dissection
The typical initial presenting symptom of CAD—ie, internal CAD (ICAD) or vertebral artery dissection (VAD)—is severe pain in the ipsilateral neck and/or head. Onset of pain may be sudden or gradual.2 CAD occurs in an estimated 2 or 3 of every 100,000 people per year, mostly in patients between ages 20 and 40 years, and it is considered the most common cause of stroke in patients younger than age 45 years.2 The pain associated with CAD generally follows trauma. While the precipitating trauma can be a major blunt or penetrating one, it is often caused by something seemingly trivial, such as “trauma” associated with coughing, painting a ceiling, yoga, or (classically and notoriously) chiropractic manipulation.3 There is frequently some rotational component to CAD-associated trauma,4 though dissection may occur spontaneously.5
The typical triad of symptoms is ipsilateral neck and/or head pain, partial Horner’s syndrome (ptosis and miosis without anhidrosis), and signs of cerebral ischemia. However, patients do not always present with all three of these symptoms, which can complicate the diagnosis. For example, in some patients, neck pain is the sole presenting symptom and can mimic the musculoskeletal pain expected from the mechanical strain that precipitated the dissection.6 In addition, partial Horner’s syndrome occurs in only 50% of cases, and ischemic symptoms might not present for hours to weeks after the onset of neck pain.6
In almost all cases of CAD, initial symptoms are otherwise unexplained pain described as a constant, steady aching. 7 Since cervical arteries are heavily invested with pain fibers,8 an intimal tear with dissecting intramural hematoma provokes pain. Pain associated with VAD is usually severe, unilateral, posterior neck, and/or occipital, while ICAD-associated pain is ipsilateral, anterolateral neck, head, and/or face. It is important to note that head or neck pain caused by a dissection normally precedes the ischemic manifestation as opposed to the more common stroke, in which the ischemia precedes or is simultaneous with the accompanying headache.9
Ischemic neurological symptoms can arise from stenosis of the arterial lumen, secondary to an expanding intramural hematoma; a luminal thrombus developing at the intimal defect; or an embolization accompanied by ipsilateral Horner’s syndrome, any cranial nerve abnormality, or followed by cerebral or ocular ischemic symptoms (even if transient). A diagnosis is usually made through vascular ultrasound (Figure 1) and confirmed with computed tomography angiography (CTA) or magnetic resonance angiography. When requesting a CTA of the neck, the emergency physician should specifically make note of suspected CAD in the order. Immediate treatment includes a cervical collar and neurosurgical consultation even though treatment is essentially medical and surgery is rarely required. Anticoagulation therapy is routinely initiated to prevent thrombus propagation or embolization (unless there is brain hemorrhage). Antiplatelet therapy may be equally efficacious, 10 and can be initiated upon suspicions of CAD and while confirmatory studies are in progress. The prognosis for extracranial dissections is generally good.
Cervical Epidural Hematoma Cervical (spinal) epidural hematoma is an uncommon but potentially catastrophic event that can lead to permanent neurological deficits and death from respiratory failure. It presents as sudden and severe local neck pain with rapid development of radicular pain at the corresponding dermatomes. Motor and sensory deficits follow within minutes to days.12,13 Bleeding can occur spontaneously or secondary to trauma, surgery, or coagulopathy (which itself may be pathological—eg, hemophilia or iatrogenic in origin).14,15 Untreated, progressive cord compression can lead to permanent neurological deficits and death from respiratory failure. In the patient with acute neurological deficits, immediate correction of coagulation issues is required before decompressive surgery.
Diagnosis of cervical epidural hematoma is complicated by the rarity of the event and the lack of specific symptoms. When trauma is involved, cervical disc or nerve root injury is a more likely cause of sudden onset of neck pain, with rapid development of a radicular component. However, when symptoms occur following minor exertion (eg, sneezing, coitus, coughing) and in the presence of risk factors such as hematologic disorders, pregnancy, rheumatologic disorders, or liver dysfunction, epidural hematoma must be considered.16 Emergent magnetic resonance imaging (MRI) is the modality of choice for detecting this condition (Figure 2).
Coronary Ischemia Angina pectoris secondary to coronary ischemia is described as retrosternal “heaviness” or pressure, which may spread to either or both arms, the neck, or jaw. Pathology originating in the neck can be experienced as chest pain and may confound the diagnosis. Because cervical nerve roots C4-C8 contribute to the innervation of the anterior chest wall, irritation of any one of these nerves secondary to neck pathology can mimic true angina.17,18 Conversely, the likelihood that the only pain caused by coronary ischemia might be felt in the neck is low, but possible— especially in women.19,20 Coronary ischemia should be considered in patients with cardiac risk factors but no other obvious etiology for neck pain.21
Secondary Infection
Since emergency physicians are accustomed to dealing with infection, it is hard to imagine that we could fail to recognize infection as the etiology in a patient with a chief complaint of neck pain. Diagnosis in such cases is complicated by the anatomical location of deep neck-space infections, which limits the usefulness of standard physical examination. These sites are difficult to palpate and often impossible to visualize because they are covered with noninfected tissue. Unless specifically considered in the differential, more obscure causes of neck pain associated with infection may be missed, including retropharyngeal abscess, epiglottitis, Ludwig’s angina, vertebral osteomyelitis and discitis, cervical epidural abscess, and Lemierre’s syndrome.
Epiglottitis
Epiglottitis is inflammation of the epiglottis and adjacent supraglottic structures including the pharynx, uvula, and base of the tongue. The first recorded case is thought to have been that of George Washington, who is believed to have died from this disease.22 The high mortality rate (7% to 20% in the adult population) is a direct result of airway obstruction from inflammatory edema of the epiglottis and adjacent tissues.
Epiglottitis was originally considered a childhood disease; however, the widespread use of Haemophilus influenza vaccination has resulted in a decline in pediatric incidence. Most cases are now seen in adults (mean age of 46 years).23,24
Bacterial infection, especially from the genera Hemophilus, Streptococcus, Staphylococcus, and Klebsiella, is by far the most frequent cause of acute epiglottitis; viral and fungal-associated infections are rare. Thermal injury from swallowing hot foods or liquids, and even from inhaling crack cocaine,25 also has been implicated.
Clinical presentations of epiglottitis differ between children and adults. While children are typically dyspneic, drooling, stridorous and febrile, adults tend to present with a milder form of the disease and have painful swallowing, sore throat, and a muffled voice. In both children and adults, the larynx and upper trachea are tender to light palpation at the anterior neck.26 Although sore throat and odynophagia are more often symptoms of pharyngitis, suspicion should be aroused when pain is severe and/or there is dyspnea, severe pain with an unremarkable oropharynx examination, or anterior neck tenderness. When present, muffled voice and stridor indicate greater potential for airway compromise.27 In cases of significant airway obstruction, patients may assume the “tripod position,” leaning forward with neck extended and mouth open—panting. Since soft-tissue lateral neck radiographs are about 90% sensitive and specific for epiglottitis, a normalappearing film cannot reliably exclude the diagnosis.28 Evaluation for the classic “thumb sign” of epiglottic swelling27 (Figure 3) should be combined with the newly described “vallecula sign” for greatest accuracy.29 The vallecula sign is described as the partial or complete obliteration of a well-defined linear air pocket between the base of the tongue and the epiglottis seen on a closed mouth lateral neck X-ray.
Although CT is a useful modality for detecting epiglottic, peritonsillar, or deep neck space abscess, there are risks to patients with airway compromise; moreover, placing patients in a supine position for the study increases the likelihood of respiratory distress. Despite these risks, when indicated, CT is useful in differentiating these abscesses from similarly presenting entities such as lingual tonsillitis and upper airway foreign body.
Direct visualization via flexible oral or nasolaryngoscopy is the diagnostic gold standard but may be deferred in a stable patient. When absolutely indicated, it must be performed with caution, ideally by an anesthesiologist/otolaryngologist in a controlled setting, lest it precipitate further obstruction. Through the use of fiber optics, the need for emergent intubation can be more directly assessed and, if necessary, performed by “tube-over-scope” technique. In the ED, standby equipment for intubation and cricothyrotomy/needle cricothyrotomy should be immediately available and ready in the event of rapid deterioration, at the same time as intravenous (IV) infusion of third-generation cephalosporin or ampicillin/sulbactam, and methicillin-resistant Staphylococcus aureus (MRSA) coverage. Though the rationale for empirical use of antibiotics is evident, the role of corticosteroids and of nebulized racemic epinephrine is controversial.
Death, airway obstruction, epiglottic abscess, necrotizing epiglottitis, and secondary infections (eg, pneumonia, cervical adenitis, septic arthritis, meningitis) are the potential complications that make this source of neck pain one not to be missed. If epiglottitis is suspected, the patient must be admitted to an intensive care setting.
Retropharyngeal Abscess
The retropharyngeal space, immediately behind the posterior pharynx and esophagus, extends from the base of the skull to the mediastinum. It lies anterior to the deep cervical fascia and is bound laterally by the carotid sheaths.30 Because it is fused down the midline, abscesses in this area tend to be unilateral. The space cannot be directly assessed by physical examination, and infections in this area are rare. Timely diagnosis demands consideration of retropharyngeal abscess in patients presenting with fever, neck stiffness, and sore throat. The potential for serious morbidity and mortality is related to the host of vital structures immediately adjacent to the retropharyngeal space. Complications include mediastinitis, carotid artery erosion, jugular vein thrombosis, pericarditis, epidural abscess, sepsis, and airway compromise.
Most cases are typically observed in children younger than age 6 years. In this pediatric population, the retropharyngeal space has two parallel chains of lymph nodes draining the nose, sinuses, and pharynx; retropharyngeal abscesses usually occur as a suppurative extension from infections of these upper airway structures structures. Penetrating trauma, eg, from objects held in the mouth, is another possible cause. These nodes atrophy around 6 years of age; thereafter, the main cause of retropharyngeal abscess is purulent extension of an adjacent (frequently odontogenic) infection or posterior pharyngeal trauma (eg, from a fish bone or instrumentation).31 As befits its origin with oral flora, cultures are almost always polymicrobial (eg, Streptococci viridans and pyogenes, Staphylococcus, H influenza, Klebsiella, anaerobes).
Although retropharyngeal abscess is considered a disease of childhood, like epiglottitis, its incidence in adults is increasing. 32 Presenting symptoms are signs of respiratory distress, such as wheezing, stridor, and drooling with impending airway obstruction from the expanding posterior pharyngeal mass. Late signs of the illness are respiratory failure due to airway obstruction and septic shock, but an astute clinician should recognize the entity long before these symptoms present. Early symptoms include fever, sore throat, odynophagia, and neck pain and stiffness (typically manifesting as a reluctance to turn the neck).33 Patients may also complain of feeling a lump in the throat or pain in the posterior neck or shoulder with swallowing.34 Ninety-seven percent of pediatric patients present with neck pain,32 which could manifest dramatically as torticollis. Most likely, a child will have a subtle reluctance to move his or her neck during the course of the physical examination. In addition, there may be posterior pharyngeal edema and/or a visible unilateral posterior pharyngeal bulge, cervical adenopathy, and a “croupy” cry or cough resembling a duck’s quack—the “cri du canard.”35 Definitive diagnosis is made using X-ray and/or CT. A lateral soft-tissue neck X-ray will demonstrate widening of the prevertebral soft tissues. CT with contrast provides a more definitive diagnosis, and is also useful to differentiate abscess (ie, a hypodense lesion with ring enhancement) from cellulitis.
Regarding treatment, empiric IV antibiotics must be started immediately and may alone prevent progression if the diagnosis is made before cellulitis has progressed to abscess. Intravenous clindamycin is a reasonable first-line antibiotic; other suggested drugs include a penicillin/ beta lactamase inhibitor, penicillin G plus metronidazole, and cefoxitin.36 Airway protection is mandatory, and an otolaryngologist should be consulted early. Because of the potential for sudden airway deterioration, the emergency physician must be prepared to establish a surgical airway.
Ludwig’s Angina
Ludwig’s angina derives its name from the German physician Wilhelm Friedrich von Ludwig, who first described this deadly, rapidly progressive, fascial space/ connective tissue gangrenous cellulitis of the floor of the mouth and adjoining neck in 1836. In a curious twist of fate, it is believed that Dr Ludwig died from this very disease that bears his name.37
Ninety percent of cases of Ludwig’s angina are odontogenic, often due to periapical abscesses. This condition may result secondary to any oral or parapharyngeal infection that spreads by continuity from the submandibular space into the contiguous sublingual and submental spaces. The potential for airway obstruction comes from elevation and displacement of the tongue, resulting in a mortality rate greater than 50% if untreated. Causative organisms mirror normal, polymicrobial oral flora and include Staphylococcus, Streptococcus, Fusobacterium, and Bacteroides.38,23
Diagnosis of Ludwig’s angina is primarily clinical. Neck pain and swelling, dental pain, dysphagia, malaise, and fever, along with a protruding or elevated tongue, are typical. Submandibular swelling, which is seen in 95% of patients, develops in advanced cases into an intense “woody” induration above the hyoid bone that portends the impending airway crisis.39 If the patient is sufficiently clinically stable and able to lie flat, definitive diagnosis can be made with a contrastenhanced, soft-tissue neck CT (Figure 4), which can also evaluate for a drainable abscess, soft-tissue gas, and mediastinal extension; this modality can also define the extent of soft-tissue swelling and airway patency.
Airway management is the primary consideration because of its potential for rapid deterioration. Traditional management has been aggressive and surgical, with the standard being early tracheostomy. More recent reports have encouraged more conservative management when possible.40 Impending or actual airway compromise, as manifest by significant trismus, inability to flex the neck without compromising the airway, inability to protrude the tongue, or actual resting dyspnea demand that a surgical airway be readied at bedside until fiber optic nasotracheal intubation is secured.
Antibiotics must be given early and include coverage for gram-positive, gramnegative, and anaerobic organisms. Intravenous metronidazole and penicillin (cefazolin or clindamycin if patient has an allergy to penicillin) are commonly prescribed.38,23 Although controversial, administration of IV dexamethasone (8 mg to 12 mg) and nebulized epinephrine (1:1000, 1 mL diluted to 5 mL with normal saline) to reduce edema has been advocated. 41
Lemierre’s Syndrome Lemierre’s syndrome, septic thrombophlebitis of the internal jugular vein, was first described in 1936 by André Lemierre, who published a series of cases of previously healthy young adults in whom oropharyngeal infections were followed by “anaerobic postanginal septicaemias.”42 Most of these patients presented with sore throat (referred to as “angina” in “old skool” speak) and worsening pain and tenderness at the anterolateral neck, with pulmonary symptoms manifesting several days to 2 weeks later. The causative organism, Fusobacterium necrophorum, is a gram-negative anaerobe that is part of the normal commensal oropharyngeal flora. It invades the internal jugular (IJ) vein via the lateral pharyngeal space and releases a hemagglutinin that promotes thrombus formation in the IJ and, ultimately, metastatic septic emboli. These emboli typically invade the lungs and cause multiple nodular infiltrates and small pleural effusions. Unfortunately, as each case is unique, diagnosis is often delayed. Septic emboli can migrate to other sites and cause arthritis (hip, knee, shoulder, sacroiliac, and other joints), osteomyelitis, young adult with a history of recent sore throat and fever who subsequently developed neck pain and tenderness (with or without swelling) over the IJ, rigors, pulmonary infiltrates, and possibly other signs of septic emboli.
Doppler ultrasound or CT will show IJ thrombosis43 (Figure 5). Purulent discharge, if obtained, has a characteristic foul smell that has been likened to “limburger or overripe Camembert cheese.”44 Treatment is with high-dose IV penicillin and metronidazole or with clindamycin as single coverage. Heparin could potentially aid in dissemination of emboli, but it is used only when there is retrograde propagation of clot to the cavernous sinus.
With the routine antibiotic treatment of pharyngitis in the 1960s and 1970s, cases of Lemierre’s syndrome became so rare that it was referred to as the “forgotten disease.”45 Unfortunately, its incidence has increased over the past few years.43 It is unclear whether this rise is due to increasing antibiotic resistance or to an increasing resistance of clinicians to use antibiotics for “sore throats.”
Cervical Spinal Infections
Vertebral osteomyelitis, discitis, and spinal epidural abscess are rare in developed countries. Most cases stem from hematogenous seeding, skin abscesses, and urinary tract infections but can also originate from a host of other sites, including penetrating trauma and invasive spinal procedures (eg, lumbar punctures, epidural injections). 46,47 Cervical spine infections are associated with immune-compromising situations or conditions (eg, IV drug use, diabetes mellitus, malignancy, acquired immunodeficiency syndrome, renal insufficiency, long-term use of systemic corticosteroids).
All three of these conditions present similarly, often as localized neck pain that grows more intense over a period of days to weeks and worsens with neck movement. Neurological signs ordinarily appear late in the course of the illness. Fever is a classic symptom but is not always present.48 There is usually tenderness over the involved spinous process. The development of motor or sensory loss suggests formation of an abscess,49 which can rapidly lead to further compressive symptoms and sepsis.
Leukocytosis may be absent but erythrocyte sedimentation rate and C-reactive protein are often elevated. A CT scan with contrast is frequently required for diagnosis, though when available, MRI with IV gadolinium is the test of choice (Figure 6). Most cases are caused by S aureus, but antibiotic coverage for gram-positive organisms (including MRSA), gram-negative organisms, and anaerobes should be started as soon as blood cultures are drawn. Neurosurgery should be consulted emergently since, with cervical epidural abscess, neurological deterioration—even to the point of total paralysis—can develop in a matter of hours.50
Conclusion
Although most patients presenting to the ED with neck pain are musculoskeletal and associated with a traumatic event, other infrequent but potentially serious atraumatic causes may be present. Based on a patient’s symptoms, emergency physicians should also consider these conditions in the differential diagnosis to ensure rapid treatment to prevent further complications.
- Ammer C. The American heritage dictionary of idioms. Boston, MA: Houghton Mifflin Company; 1997:489.
- Fusco MR, Harrigan MR. Cerebrovascular dissections—a review part I: spontaneous dissections. Neurosurgery. 2011;68(1):242-257.
- Rubinstein SM, Peerdeman SM, van Tulder MW, Riphagen I, Haldeman S. A systematic review of the risk factors for cervical artery dissection. Stroke.2005;36(7):1575-1580.
- Bergin M, Bird P, Wright A. Internal carotid artery dissection following canalith repositioning procedure. J Laryngol Otol. 2010;124(5):575, 576.
- Brandt T, Grond-Ginsbach C. Spontaneous cervical artery dissection: from risk factors toward pathogenesis. Stroke. 2002;33(3):657,658.
- Arnold M, Cumurciuc R, Stapf C, Favrole P, Berthet K, Bousser MG. Pain as the only symptom of cervical artery dissection. J Neurol Neurosurg Psychiatry. 2006;77(9):1021-1024.
- Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906.
- Caplan LR. Dissections of brain-supplying arteries. Nat Clin Pract Neurol. 2008;4(1):34-42.
- Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45(8):1517-1522.
- Engelter ST, Brandt T, Debette S; Cervical Artery Dissection in Ischemic Stroke Patients (CADISP) Study Group. Antiplatelets versus anticoagulation in cervical artery dissection. Stroke. 2007;38(9):2605-2611.
- Arnold M, Bousser M, Fahrni G, et al. Vertebral artery dissection: presenting findings and predictors of outcome. Stroke. 2006;37(10):2499-2503.
- Hsieh CT, Chang CF, Lin EY, Tsai TH, Chiang YH, Ju DT. Spontaneous spinal epidural hematomas of cervical spine: report of 4 cases and literature review. Am J Emerg Med. 2006;24(6):736-740.
- Sei A, Nakamura T, Hashimoto N, Mizuta H, Sasaki A, Takagi K. Cervical spinal epidural hematoma with spontaneous remission. J Spinal Disord. 1991;4(2):234-237.
- Williams JM, Allegra JR. Spontaneous cervical epidural hematoma. Ann Emerg Med. 1994;23(6):1368-1370.
- Demierre B, Unger PF, Bongioanni F. Sudden cervical pain: spontaneous cervical epidural hematoma. Am J Emerg Med. 1991;9(1):54-56.
- Broder J, L’Italien A. Evaluation and management of the patient with neck pain. In: Mattu A, Goyal DG eds. Emergency Medicine: Avoiding the Pitfalls and Improving the Outcomes. Malden, MA: Blackwell Publishing, Inc; 2007:46-54. http://onlinelibrary. wiley.com/book/10.1002/9780470755938. Accessed November 15, 2013.
- Brodsky AE. Cervical angina. A correlative study with emphasis on the use of coronary arteriography. Spine. 1985;10(8):699-709.
- Hanflig SS. Pain in the shoulder girdle, arm and precordium due to cervical arthritis. JAMA. 1936;106(7):523-526.
- Goldberg R, Goff D, Cooper L, et al. Age and sex differences in presentation of symptoms among patients with acute coronary disease: the REACT Trial. Rapid Early Action for Coronary Treatment. Coron Artery Dis. 2000;11(5):399-407.
- Coventry LL, Finn J, Bremner AP. Sex differences in symptom presentation in acute myocardial infarction: A systematic review and meta-analysis. Heart Lung. 2011;40(6):477-491.
- Lipetz JS, Ledon J, Silber J. Severe coronary artery disease presenting with a chief complaint of cervical pain. Am J Phys Med Rehabil. 2003;82(9):716-720.
- Morens DM. Death of a president. N Engl J Med. 1999;341(24):1845-1849.
- Winters M. Evidence-based diagnosis and management of ENT emergencies. Medscape. 2007. http://www.medscape.com/viewarticle/551650_1. Accessed November 15, 2013.
- Mayo-Smith MF, Spinale JW, Donskey CJ, Yukawa M, Li RH, Schiffman FJ. Acute epiglottitis: An 18-year experience in Rhode Island. Chest. 1995;108(6):1640-1670.
- Mayo-Smith MF, Spinale J. Thermal epiglottitis in adults: a new complication of illicit drug use. J Emerg Med. 1997;15(4):483-485.
- Bansal A, Miskoff J, Lis RJ. Otolaryngologic critical care. Crit Care Clin. 2003;19(1):55-72.
- Katori H, Tsukuda M. Acute epiglottitis: analysis of factors associated with airway intervention. J Laryngol Otol. 2005;119(12):967-972.
- Rothrock SG, Pignatiello GA, Howard RM. Radiologic diagnosis of epiglottitis: objective criteria for all ages. Ann Emerg Med. 1990;19(9):978-982.
- Ducic Y, Hébert PC, MacLachlan L, Neufeld K, Lamothe A. Description and evaluation of the vallecula sign: a new radiologic sign in the diagnosis of adult epiglottitis. Ann Emerg Med. 1997;30(1):1-6.
- Vieira F, Allen SM, Stocks RM, Thompson JW. Deep neck infection. Otolaryngol Clin North Am. 2008;41(3):459-483.
- Shores CG. Infections and disorders of the neck and upper airway. In: Tintinalli JE, Stapczynski JS, Kelen GD, eds. In: Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 6th ed. New York, NY: McGraw-Hill; 2004:1494-1499.
- Kahn JH. Retropharyngeal Abscess in Emergency Medicine. Medscape Review. 2008.
- Gibson CG. Do not rely on the presence of respiratory compromise to make the diagnosis of retropharyngeal abscess. In: Mattu A, Chanmugam AS, Swadron SP, Tibbles CD, Woolridge DP, eds. Avoiding Common Errors in the Emergency Department. New York, NY: Lippincott Williams & Wilkins; 2010:212.
- Greene JS, Asher IM. Retropharyngeal abscess: A previously unreported symptom. Ann Emerg Med. 1984;13(8):615-619.
- Melio FR. Upper respiratory tract infections. In: Marx J, Hockberger R, Walls R, eds. Rosen’s Emergency Medicine-Concepts and Clinical Practice 7th ed. Philadelphia, PA: Mosby Elsevier; 2009:921-923.
- Sanford JP, Gilbert DN, Moellering RC, Sande MA, Eliopoulos GM, eds. The Sanford guide to Antimicrobial Therapy 2006-2007. 37th ed. Sperryville, VA: Antimicrobial Therapy, Inc; 2007:30.
- Murphy SC. The person behind the eponym: Wilhelm Frederick von Ludwig (1790-1865). J Oral Pathol Med. 1996;25(9):513-515.
- Hasan W, Leonard D, Russell J. Ludwig’s Angina—A controversial surgical emergency: How we do it. Int J Otolaryngol. 2011;2011:231816.
- Saifeldeen K, Evans R. Ludwig’s angina. Emerg Med J. 2004;21(2):242,243.
- Marple BF. Ludwig angina: a review of current airway management. Arch Otolaryngol Head Neck Surg. 1999;125(5):596-599.
- Buckley MF, O’Connor K. Ludwig’s angina in a 76-year-old man. Emerg Med J. 2009;26(9):679-680.
- Lemierre A. On certain septicaemias due to anaerobic organisms. Lancet. 1936;227(5874):701-703.
- Karkos PD, Asrani S, Karkos CD, et al. Lemierre’s syndrome: a systematic review. Laryngoscope. 2009;119(8):1552-1559.
- Alston JM. Necrobacillosis in Great Britain. Brit Med J. 1955;2(4955):1524-1528.
- Vargiami EG, Zafeiriou D. Eponym: The Lemierre syndrome. Eur J Pediatr. 2010;169(4):411-414.
- Martínez Hernández PL, Amer López M, Zamora Vargas F, et al. Spontaneous infectious spondylodiscitis in an internal medicine department: epidemiological and clinical study in 41 cases. Rev Clin Esp. 2008;208(7):347-352.
- Urrutia J, Bono CM, Mery P, Rojas C, Gana N, Campos M. Chronic liver failure and concomitant distant infections are associated with high rates of neurological involvement in pyogenic spinal infections. Spine. 2009;34(7):E240-E244.
- Buranapanitkit B, Lim A, Kiriratnikom T. Clinical manifestation of tuberculous and pyogenic spine infection. J Med Assoc Thai. 2001;84(11):1522-1526.
- Schimmer RC, Jeanneret C, Nunley PD, Jeanneret B. Osteomyelitis of the cervical spine: a potentially dramatic disease. J Spinal Disord Tech. 2002;15(2):110-117.
- Darouiche RO. Spinal epidural abscess. N Engl J Med. 2006;355(19):2012-2020.
- Ammer C. The American heritage dictionary of idioms. Boston, MA: Houghton Mifflin Company; 1997:489.
- Fusco MR, Harrigan MR. Cerebrovascular dissections—a review part I: spontaneous dissections. Neurosurgery. 2011;68(1):242-257.
- Rubinstein SM, Peerdeman SM, van Tulder MW, Riphagen I, Haldeman S. A systematic review of the risk factors for cervical artery dissection. Stroke.2005;36(7):1575-1580.
- Bergin M, Bird P, Wright A. Internal carotid artery dissection following canalith repositioning procedure. J Laryngol Otol. 2010;124(5):575, 576.
- Brandt T, Grond-Ginsbach C. Spontaneous cervical artery dissection: from risk factors toward pathogenesis. Stroke. 2002;33(3):657,658.
- Arnold M, Cumurciuc R, Stapf C, Favrole P, Berthet K, Bousser MG. Pain as the only symptom of cervical artery dissection. J Neurol Neurosurg Psychiatry. 2006;77(9):1021-1024.
- Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906.
- Caplan LR. Dissections of brain-supplying arteries. Nat Clin Pract Neurol. 2008;4(1):34-42.
- Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45(8):1517-1522.
- Engelter ST, Brandt T, Debette S; Cervical Artery Dissection in Ischemic Stroke Patients (CADISP) Study Group. Antiplatelets versus anticoagulation in cervical artery dissection. Stroke. 2007;38(9):2605-2611.
- Arnold M, Bousser M, Fahrni G, et al. Vertebral artery dissection: presenting findings and predictors of outcome. Stroke. 2006;37(10):2499-2503.
- Hsieh CT, Chang CF, Lin EY, Tsai TH, Chiang YH, Ju DT. Spontaneous spinal epidural hematomas of cervical spine: report of 4 cases and literature review. Am J Emerg Med. 2006;24(6):736-740.
- Sei A, Nakamura T, Hashimoto N, Mizuta H, Sasaki A, Takagi K. Cervical spinal epidural hematoma with spontaneous remission. J Spinal Disord. 1991;4(2):234-237.
- Williams JM, Allegra JR. Spontaneous cervical epidural hematoma. Ann Emerg Med. 1994;23(6):1368-1370.
- Demierre B, Unger PF, Bongioanni F. Sudden cervical pain: spontaneous cervical epidural hematoma. Am J Emerg Med. 1991;9(1):54-56.
- Broder J, L’Italien A. Evaluation and management of the patient with neck pain. In: Mattu A, Goyal DG eds. Emergency Medicine: Avoiding the Pitfalls and Improving the Outcomes. Malden, MA: Blackwell Publishing, Inc; 2007:46-54. http://onlinelibrary. wiley.com/book/10.1002/9780470755938. Accessed November 15, 2013.
- Brodsky AE. Cervical angina. A correlative study with emphasis on the use of coronary arteriography. Spine. 1985;10(8):699-709.
- Hanflig SS. Pain in the shoulder girdle, arm and precordium due to cervical arthritis. JAMA. 1936;106(7):523-526.
- Goldberg R, Goff D, Cooper L, et al. Age and sex differences in presentation of symptoms among patients with acute coronary disease: the REACT Trial. Rapid Early Action for Coronary Treatment. Coron Artery Dis. 2000;11(5):399-407.
- Coventry LL, Finn J, Bremner AP. Sex differences in symptom presentation in acute myocardial infarction: A systematic review and meta-analysis. Heart Lung. 2011;40(6):477-491.
- Lipetz JS, Ledon J, Silber J. Severe coronary artery disease presenting with a chief complaint of cervical pain. Am J Phys Med Rehabil. 2003;82(9):716-720.
- Morens DM. Death of a president. N Engl J Med. 1999;341(24):1845-1849.
- Winters M. Evidence-based diagnosis and management of ENT emergencies. Medscape. 2007. http://www.medscape.com/viewarticle/551650_1. Accessed November 15, 2013.
- Mayo-Smith MF, Spinale JW, Donskey CJ, Yukawa M, Li RH, Schiffman FJ. Acute epiglottitis: An 18-year experience in Rhode Island. Chest. 1995;108(6):1640-1670.
- Mayo-Smith MF, Spinale J. Thermal epiglottitis in adults: a new complication of illicit drug use. J Emerg Med. 1997;15(4):483-485.
- Bansal A, Miskoff J, Lis RJ. Otolaryngologic critical care. Crit Care Clin. 2003;19(1):55-72.
- Katori H, Tsukuda M. Acute epiglottitis: analysis of factors associated with airway intervention. J Laryngol Otol. 2005;119(12):967-972.
- Rothrock SG, Pignatiello GA, Howard RM. Radiologic diagnosis of epiglottitis: objective criteria for all ages. Ann Emerg Med. 1990;19(9):978-982.
- Ducic Y, Hébert PC, MacLachlan L, Neufeld K, Lamothe A. Description and evaluation of the vallecula sign: a new radiologic sign in the diagnosis of adult epiglottitis. Ann Emerg Med. 1997;30(1):1-6.
- Vieira F, Allen SM, Stocks RM, Thompson JW. Deep neck infection. Otolaryngol Clin North Am. 2008;41(3):459-483.
- Shores CG. Infections and disorders of the neck and upper airway. In: Tintinalli JE, Stapczynski JS, Kelen GD, eds. In: Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 6th ed. New York, NY: McGraw-Hill; 2004:1494-1499.
- Kahn JH. Retropharyngeal Abscess in Emergency Medicine. Medscape Review. 2008.
- Gibson CG. Do not rely on the presence of respiratory compromise to make the diagnosis of retropharyngeal abscess. In: Mattu A, Chanmugam AS, Swadron SP, Tibbles CD, Woolridge DP, eds. Avoiding Common Errors in the Emergency Department. New York, NY: Lippincott Williams & Wilkins; 2010:212.
- Greene JS, Asher IM. Retropharyngeal abscess: A previously unreported symptom. Ann Emerg Med. 1984;13(8):615-619.
- Melio FR. Upper respiratory tract infections. In: Marx J, Hockberger R, Walls R, eds. Rosen’s Emergency Medicine-Concepts and Clinical Practice 7th ed. Philadelphia, PA: Mosby Elsevier; 2009:921-923.
- Sanford JP, Gilbert DN, Moellering RC, Sande MA, Eliopoulos GM, eds. The Sanford guide to Antimicrobial Therapy 2006-2007. 37th ed. Sperryville, VA: Antimicrobial Therapy, Inc; 2007:30.
- Murphy SC. The person behind the eponym: Wilhelm Frederick von Ludwig (1790-1865). J Oral Pathol Med. 1996;25(9):513-515.
- Hasan W, Leonard D, Russell J. Ludwig’s Angina—A controversial surgical emergency: How we do it. Int J Otolaryngol. 2011;2011:231816.
- Saifeldeen K, Evans R. Ludwig’s angina. Emerg Med J. 2004;21(2):242,243.
- Marple BF. Ludwig angina: a review of current airway management. Arch Otolaryngol Head Neck Surg. 1999;125(5):596-599.
- Buckley MF, O’Connor K. Ludwig’s angina in a 76-year-old man. Emerg Med J. 2009;26(9):679-680.
- Lemierre A. On certain septicaemias due to anaerobic organisms. Lancet. 1936;227(5874):701-703.
- Karkos PD, Asrani S, Karkos CD, et al. Lemierre’s syndrome: a systematic review. Laryngoscope. 2009;119(8):1552-1559.
- Alston JM. Necrobacillosis in Great Britain. Brit Med J. 1955;2(4955):1524-1528.
- Vargiami EG, Zafeiriou D. Eponym: The Lemierre syndrome. Eur J Pediatr. 2010;169(4):411-414.
- Martínez Hernández PL, Amer López M, Zamora Vargas F, et al. Spontaneous infectious spondylodiscitis in an internal medicine department: epidemiological and clinical study in 41 cases. Rev Clin Esp. 2008;208(7):347-352.
- Urrutia J, Bono CM, Mery P, Rojas C, Gana N, Campos M. Chronic liver failure and concomitant distant infections are associated with high rates of neurological involvement in pyogenic spinal infections. Spine. 2009;34(7):E240-E244.
- Buranapanitkit B, Lim A, Kiriratnikom T. Clinical manifestation of tuberculous and pyogenic spine infection. J Med Assoc Thai. 2001;84(11):1522-1526.
- Schimmer RC, Jeanneret C, Nunley PD, Jeanneret B. Osteomyelitis of the cervical spine: a potentially dramatic disease. J Spinal Disord Tech. 2002;15(2):110-117.
- Darouiche RO. Spinal epidural abscess. N Engl J Med. 2006;355(19):2012-2020.
The conundrum of explaining breast density to patients
Density: The quality or state of being dense; the quantity per unit volume, unit area, or unit length; the degree of opacity of a translucent medium, or the common logarithm of the opacity.
—Merriam-Webster’s dictionary1
For more than a decade, federal law in the United States has compelled breast imaging centers to give every mammography patient a letter explaining her result.2
Often, however, the first person a woman speaks to about her findings is her primary care clinician, particularly if she has had a screening mammogram at a center where films are “batch-read” and are not viewed by the radiologist at the time of the appointment. Internal medicine physicians are often called on to help women understand their findings and to order follow-up tests recommended by the radiologist—a not uncommon occurrence. Also, internists often need to address patients’ anxieties about the possibility of breast cancer and provide them with enough information to make an informed decision about an appropriate action plan.
Meanwhile, discussing mammography has become more complicated. In 2009, the United States Preventive Services Task Force stopped recommending that women under age 50 be routinely screened for breast cancer, and instead stated that the decision to begin screening these women should consider “patient context” and the patient’s personal “values”3—with the implication that women’s primary clinicians would play an important role in helping them weigh the test’s potential benefits and harms.
More and more, internists must grapple with the task of how to help women decipher the concept of “breast density,” understand their personal density results, and make an informed decision about whether to undergo additional imaging studies, such as ultrasonography and magnetic resonance imaging (MRI).
LEGISLATION REQUIRING DENSITY NOTIFICATION
The impetus for this change in practice has been spurred in large part by patient advocates, who have argued that women deserve to know their density because mammography is less sensitive in women with dense breasts. So far, at least 12 states have enacted laws requiring breast imaging centers to add information about breast density in the result notification letters they mail to patients. Legislatures in several other states are considering breast density notification laws,4 and federal legislation has been proposed.
Some of the state laws, such as those in Connecticut, Texas, and Virginia, require informing all mammography patients about their density findings, whether or not they have dense breast tissue. Other states, such as California, Hawaii, and New York, require informing only those found to have dense tissue. And some states, such as California, Connecticut, Hawaii, Texas, and Virginia, require specific wording in the density notification letter (Table 1).
The details of all these notification laws may differ in how they specify which patients must be notified and in how the information should be worded, but the goal is the same: to raise women’s awareness so that they can embark on an informed decision with their physician about whether to undergo further testing.
Because of liability concerns, some breast imaging centers in states that currently lack such notification laws have begun informing women about their density results.
Unfortunately, at this point clinicians have no clear guidelines for helping patients with dense breasts decide whether to undergo additional testing. In addition, the evidence is equivocal, and the tests have risks as well as benefits. The patient needs to understand all this by discussing it with her physician. And to discuss this decision effectively, the physician must be well versed in the evolving literature on breast density. Below, we present important points to keep in mind as we foster these discussions with our patients.
BREAST TISSUE DENSITY IS STILL A SUBJECTIVE MEASUREMENT
Breast density limits the sensitivity of mammography. This is widely established. Yet the interpretation of breast density today is subjective. It is determined by the interpreting radiologist based on the Breast Imaging and Reporting Data System (BI-RADS), which defines “heterogeneously dense” breasts as those containing 50% to 75% dense tissue and “dense” breasts as those with more than 75%5 (Figure 1). This subjective measurement is based on two-dimensional imaging, which may underestimate or overestimate the percentage of breast density because of tissue summation. Ideally, density should be measured using three-dimensional imaging with automated software,6 but this technology is not yet widely available.
INCREASED DETECTION OF BENIGN LESIONS
Although adding ultrasonography to mammography in patients with dense breast tissue detects additional cancers,7,8 it also leads to a significant increase in the detection of lesions that are not malignant yet require additional workup or biopsy.
The largest study to examine this was the American College of Radiology Imaging Network Protocol 6666 (ACRIN 6666),7 a multi-institutional study evaluating the diagnostic yield, sensitivity, and specificity of adding ultrasonography in high-risk patients who presented with negative mammograms and had heterogeneously dense tissue in at least one quadrant.7 (High risk was defined as a threefold higher risk of breast cancer as determined by risk factors such as personal history of breast cancer or high-risk lesions, or elevated risk using the Gail or Claus model.) The supplemental yield was 4.2 cancers per 1,000 women (95% confidence interval 1.1 to 7.2 per 1,000) on a single prevalent screen. Of 12 cancers detected solely by ultrasonography, 11 were invasive and had a median size of 10 mm. Of those reported, 8 of 9 were node-negative. Despite this additional yield, the positive predictive value of biopsy prompted by ultrasonography was only 8.9%.7 Other investigators have reported similar findings.8
RELATIONSHIP BETWEEN DENSITY AND CANCER RISK STILL NOT CLEAR
The relationship between breast density and cancer risk is not entirely clear. Higher breast density has been associated with a higher risk of breast cancer,9,10 presumably because cancer usually develops in parenchyma, and not fatty tissue. Yet obesity and age, which are inversely associated with density, are also risk factors for the development of breast cancer. Some prominent radiologists have cast doubt on the methodology used in these density studies, which relied on density measurements calculated by two-dimensional views of the breast, and have called for a re-evaluation of the relationship between density and cancer risk.6
LIMITED HEALTH LITERACY: A CHALLENGE
The term “breast density” is unfamiliar to most lay people. As physicians, we need to keep in mind that more than a third of US adults have limited health literacy and thus have difficulty processing basic health information.11 But even the 1 in 10 US women with “proficient” health literacy skills may find the term “density” confusing.
As the definition at the opening of this article suggests, the word itself is nuanced and has different meanings. Anecdotally, both of the authors, a general internist (E.M.) and a breast imaging specialist (M.Y.), have encountered numerous quizzical and sometimes distrustful reactions when telling patients—including some with graduate degrees—that they have “dense” breast tissue and might benefit from additional ultrasonographic testing. Avoiding jargon is key; studies have found that terms such as “benign” can be confusing when used in a mammogram result notification letter.12
How can we explain the concept of breast density to our patients?
Supplemental educational materials that feature simple pictures can also be helpful in conveying complex health information,13 although their effect on the communication of breast density has not been studied. The American College of Radiology and the Society of Breast Imaging produce a freely available, downloadable patient brochure on breast density that includes photographs of mammograms with high and low breast density. The brochure is available from the American College of Radiology online at www.acr.org, under “Tools you can use.”
We recommend introducing women to the concept of breast density before they undergo mammography—at the time the test is ordered—and provide them with supplemental materials such as the above-mentioned brochure. About 1 out of every 10 women who undergo screening mammography has a result requiring additional testing that does not result in a cancer diagnosis. Yet a body of research suggests that many women don’t realize that mammograms don’t always yield a cut-and-dried “cancer” or “no cancer” result. In past studies, women have said they were unaware of how common it is to be called back after routine screening mammography, and they wanted to be prepared for this in advance.12,14 Similarly, many women are unaware of the concept of breast density and don’t know that they may be told about these findings when they get their mammogram report.
Avoid causing anxiety
When explaining results to women with dense breasts, we should emphasize that there are no abnormalities on the current mammogram, and that the only reason to consider additional imaging is the breast density. But regardless of the ultimate outcome, an abnormal mammogram can trigger long-standing anxiety, 15 and it is reasonable to assume that some women will become anxious when told they have highly dense breasts. It is important that clinicians be aware of this potential anxiety and inquire about any personal cancer-related concerns at the time they discuss their findings.16
Helping the patient choose the type of additional screening
If a patient is found to have dense breasts and chooses to undergo additional screening, the decision about which test—ultrasonography or MRI—can be based on the woman’s lifetime risk of breast cancer.
The American Cancer Society recommends that patients with a lifetime risk of 20% or greater—according to a risk model such as BRCAPRO, Tyrer-Cuzick, or BOADICEA (Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm)—should be screened annually with breast MRI regardless of breast density. Patients in this category are those who carry the BRCA gene mutations and their untested first-degree relatives, and patients with Li-Fraumeni, Cowden, or Bannayan-Riley-Ruvalcaba syndrome. Also considered are women who underwent chest radiation between the ages of 10 and 30, and patients who have more than one first-degree relative with breast cancer but who do not have an identifiable genetic mutation.17
Patients with dense breasts who have an increased lifetime risk but who do not meet these criteria and those who are at average risk may be offered breast ultrasonography. If risk factors are unclear, genetic counseling can help determine the lifetime risk and thus help the patient choose the additional screening test.18
MORE WORK TO DO
Clearly, we still do not know how to explain breast density results to our patients in a way that will help them make a fully informed decision about additional screening. Research suggests that letters alone are insufficient,13,19,20 and there is no guarantee that simply adding breast density notification language to result letters will enhance a woman’s understanding and empower her to choose a course of action that is sensitive to her personal preferences.
As more states adopt notification legislation, we must develop effective methods to improve our patients’ understanding of the meaning and implications of having dense breasts and to help them decide how to proceed. Such tools could include videos, Web sites, and pictorials, as well as specialized training for patient educators and health navigators. Otherwise, including this additional, conceptually difficult information to result notification letters could make the doctor-patient interaction even more “dense”—and could increase women’s uncertainty and anxiety about their personal risk of cancer.21
- Merriam-Webster online dictionary. Density http://www.merriam-webster.com/dictionary/density. Accessed November 12, 2013.
- US Food and Drug Administration (FDA). Radiation-emitting products: Frequently asked questions about MQSA. http://www.fda.gov/Radiation-EmittingProducts/MammographyQualityStandardsActandProgram/ConsumerInformation/ucm113968.htm. Accessed November 12, 2013.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 151:716–726, W–236.
- Are You Dense Advocacy Inc. Are you dense? http://areyoudenseadvocacy.org. Accessed November 12, 2013.
- American College of Radiology. Breast Imaging Reporting and Data System (BI-RADS). 4th ed. http://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Resources/BIRADS/MammoBIRADS.pdf. Accessed November 12, 2013.
- Kopans DB. Basic physics and doubts about relationship between mammographically determined tissue density and breast cancer risk. Radiology 2008; 246:348–353.
- Berg WA, Blume JD, Cormack JB, et al; ACRIN 6666 Investigators. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA 2008; 299:2151–2163.
- Hooley RJ, Greenberg KL, Stackhouse RM, Geisel JL, Butler RS, Philpotts LE. Screening US in patients with mammographically dense breasts: initial experience with Connecticut Public Act 09-41. Radiology 2012; 265:59–69.
- Vacek PM, Geller BM. A prospective study of breast cancer risk using routine mammographic breast density measurements. Cancer Epidemiol Biomarkers Prev 2004; 13:715–722.
- Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med 2007; 356:227–236.
- Kutner M, Greenberg E, Jin Y, Paulsen C; National Center for Education Statistics. The health literacy of America’s adults: Results from the 2003 national assessment of adult literacy. US Department of Education. http://nces.ed.gov/pubs2006/2006483.pdf. Accessed November 12, 2013.
- Marcus EN, Drummond D, Dietz N. Urban women’s p for learning of their mammogram result: a qualitative study. J Cancer Educ 2012; 27:156–164.
- Houts PS, Doak CC, Doak LG, Loscalzo MJ. The role of pictures in improving health communication: a review of research on attention, comprehension, recall, and adherence. Patient Educ Couns 2006; 61:173–190.
- Nekhlyudov L, Li R, Fletcher SW. Information and involvement p of women in their 40s before their first screening mammogram. Arch Intern Med 2005; 165:1370–1374.
- Barton MB, Moore S, Polk S, Shtatland E, Elmore JG, Fletcher SW. Increased patient concern after false-positive mammograms: clinician documentation and subsequent ambulatory visits. J Gen Intern Med 2001; 16:150–156.
- Politi MC, Street RL. The importance of communication in collaborative decision making: facilitating shared mind and the management of uncertainty. J Eval Clin Pract 2011; 17:579–584.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007; 57:75–89.
- Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol 2009; 192:390–399.
- Jones BA, Reams K, Calvocoressi L, Dailey A, Kasl SV, Liston NM. Adequacy of communicating results from screening mammograms to African American and white women. Am J Public Health 2007; 97:531–538.
- Karliner LS, Patricia Kaplan C, Juarbe T, Pasick R, Pérez-Stable EJ. Poor patient comprehension of abnormal mammography results. J Gen Intern Med 2005; 20:432–437.
- Marcus EN. Post-mammogram letters often confuse more than they help. Washington Post, February 25, 2013. http://articles.washingtonpost.com/2013-02-25/national/37287736_1_mammogram-letters-densebreasts/2. Accessed November 12, 2013.
Density: The quality or state of being dense; the quantity per unit volume, unit area, or unit length; the degree of opacity of a translucent medium, or the common logarithm of the opacity.
—Merriam-Webster’s dictionary1
For more than a decade, federal law in the United States has compelled breast imaging centers to give every mammography patient a letter explaining her result.2
Often, however, the first person a woman speaks to about her findings is her primary care clinician, particularly if she has had a screening mammogram at a center where films are “batch-read” and are not viewed by the radiologist at the time of the appointment. Internal medicine physicians are often called on to help women understand their findings and to order follow-up tests recommended by the radiologist—a not uncommon occurrence. Also, internists often need to address patients’ anxieties about the possibility of breast cancer and provide them with enough information to make an informed decision about an appropriate action plan.
Meanwhile, discussing mammography has become more complicated. In 2009, the United States Preventive Services Task Force stopped recommending that women under age 50 be routinely screened for breast cancer, and instead stated that the decision to begin screening these women should consider “patient context” and the patient’s personal “values”3—with the implication that women’s primary clinicians would play an important role in helping them weigh the test’s potential benefits and harms.
More and more, internists must grapple with the task of how to help women decipher the concept of “breast density,” understand their personal density results, and make an informed decision about whether to undergo additional imaging studies, such as ultrasonography and magnetic resonance imaging (MRI).
LEGISLATION REQUIRING DENSITY NOTIFICATION
The impetus for this change in practice has been spurred in large part by patient advocates, who have argued that women deserve to know their density because mammography is less sensitive in women with dense breasts. So far, at least 12 states have enacted laws requiring breast imaging centers to add information about breast density in the result notification letters they mail to patients. Legislatures in several other states are considering breast density notification laws,4 and federal legislation has been proposed.
Some of the state laws, such as those in Connecticut, Texas, and Virginia, require informing all mammography patients about their density findings, whether or not they have dense breast tissue. Other states, such as California, Hawaii, and New York, require informing only those found to have dense tissue. And some states, such as California, Connecticut, Hawaii, Texas, and Virginia, require specific wording in the density notification letter (Table 1).
The details of all these notification laws may differ in how they specify which patients must be notified and in how the information should be worded, but the goal is the same: to raise women’s awareness so that they can embark on an informed decision with their physician about whether to undergo further testing.
Because of liability concerns, some breast imaging centers in states that currently lack such notification laws have begun informing women about their density results.
Unfortunately, at this point clinicians have no clear guidelines for helping patients with dense breasts decide whether to undergo additional testing. In addition, the evidence is equivocal, and the tests have risks as well as benefits. The patient needs to understand all this by discussing it with her physician. And to discuss this decision effectively, the physician must be well versed in the evolving literature on breast density. Below, we present important points to keep in mind as we foster these discussions with our patients.
BREAST TISSUE DENSITY IS STILL A SUBJECTIVE MEASUREMENT
Breast density limits the sensitivity of mammography. This is widely established. Yet the interpretation of breast density today is subjective. It is determined by the interpreting radiologist based on the Breast Imaging and Reporting Data System (BI-RADS), which defines “heterogeneously dense” breasts as those containing 50% to 75% dense tissue and “dense” breasts as those with more than 75%5 (Figure 1). This subjective measurement is based on two-dimensional imaging, which may underestimate or overestimate the percentage of breast density because of tissue summation. Ideally, density should be measured using three-dimensional imaging with automated software,6 but this technology is not yet widely available.
INCREASED DETECTION OF BENIGN LESIONS
Although adding ultrasonography to mammography in patients with dense breast tissue detects additional cancers,7,8 it also leads to a significant increase in the detection of lesions that are not malignant yet require additional workup or biopsy.
The largest study to examine this was the American College of Radiology Imaging Network Protocol 6666 (ACRIN 6666),7 a multi-institutional study evaluating the diagnostic yield, sensitivity, and specificity of adding ultrasonography in high-risk patients who presented with negative mammograms and had heterogeneously dense tissue in at least one quadrant.7 (High risk was defined as a threefold higher risk of breast cancer as determined by risk factors such as personal history of breast cancer or high-risk lesions, or elevated risk using the Gail or Claus model.) The supplemental yield was 4.2 cancers per 1,000 women (95% confidence interval 1.1 to 7.2 per 1,000) on a single prevalent screen. Of 12 cancers detected solely by ultrasonography, 11 were invasive and had a median size of 10 mm. Of those reported, 8 of 9 were node-negative. Despite this additional yield, the positive predictive value of biopsy prompted by ultrasonography was only 8.9%.7 Other investigators have reported similar findings.8
RELATIONSHIP BETWEEN DENSITY AND CANCER RISK STILL NOT CLEAR
The relationship between breast density and cancer risk is not entirely clear. Higher breast density has been associated with a higher risk of breast cancer,9,10 presumably because cancer usually develops in parenchyma, and not fatty tissue. Yet obesity and age, which are inversely associated with density, are also risk factors for the development of breast cancer. Some prominent radiologists have cast doubt on the methodology used in these density studies, which relied on density measurements calculated by two-dimensional views of the breast, and have called for a re-evaluation of the relationship between density and cancer risk.6
LIMITED HEALTH LITERACY: A CHALLENGE
The term “breast density” is unfamiliar to most lay people. As physicians, we need to keep in mind that more than a third of US adults have limited health literacy and thus have difficulty processing basic health information.11 But even the 1 in 10 US women with “proficient” health literacy skills may find the term “density” confusing.
As the definition at the opening of this article suggests, the word itself is nuanced and has different meanings. Anecdotally, both of the authors, a general internist (E.M.) and a breast imaging specialist (M.Y.), have encountered numerous quizzical and sometimes distrustful reactions when telling patients—including some with graduate degrees—that they have “dense” breast tissue and might benefit from additional ultrasonographic testing. Avoiding jargon is key; studies have found that terms such as “benign” can be confusing when used in a mammogram result notification letter.12
How can we explain the concept of breast density to our patients?
Supplemental educational materials that feature simple pictures can also be helpful in conveying complex health information,13 although their effect on the communication of breast density has not been studied. The American College of Radiology and the Society of Breast Imaging produce a freely available, downloadable patient brochure on breast density that includes photographs of mammograms with high and low breast density. The brochure is available from the American College of Radiology online at www.acr.org, under “Tools you can use.”
We recommend introducing women to the concept of breast density before they undergo mammography—at the time the test is ordered—and provide them with supplemental materials such as the above-mentioned brochure. About 1 out of every 10 women who undergo screening mammography has a result requiring additional testing that does not result in a cancer diagnosis. Yet a body of research suggests that many women don’t realize that mammograms don’t always yield a cut-and-dried “cancer” or “no cancer” result. In past studies, women have said they were unaware of how common it is to be called back after routine screening mammography, and they wanted to be prepared for this in advance.12,14 Similarly, many women are unaware of the concept of breast density and don’t know that they may be told about these findings when they get their mammogram report.
Avoid causing anxiety
When explaining results to women with dense breasts, we should emphasize that there are no abnormalities on the current mammogram, and that the only reason to consider additional imaging is the breast density. But regardless of the ultimate outcome, an abnormal mammogram can trigger long-standing anxiety, 15 and it is reasonable to assume that some women will become anxious when told they have highly dense breasts. It is important that clinicians be aware of this potential anxiety and inquire about any personal cancer-related concerns at the time they discuss their findings.16
Helping the patient choose the type of additional screening
If a patient is found to have dense breasts and chooses to undergo additional screening, the decision about which test—ultrasonography or MRI—can be based on the woman’s lifetime risk of breast cancer.
The American Cancer Society recommends that patients with a lifetime risk of 20% or greater—according to a risk model such as BRCAPRO, Tyrer-Cuzick, or BOADICEA (Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm)—should be screened annually with breast MRI regardless of breast density. Patients in this category are those who carry the BRCA gene mutations and their untested first-degree relatives, and patients with Li-Fraumeni, Cowden, or Bannayan-Riley-Ruvalcaba syndrome. Also considered are women who underwent chest radiation between the ages of 10 and 30, and patients who have more than one first-degree relative with breast cancer but who do not have an identifiable genetic mutation.17
Patients with dense breasts who have an increased lifetime risk but who do not meet these criteria and those who are at average risk may be offered breast ultrasonography. If risk factors are unclear, genetic counseling can help determine the lifetime risk and thus help the patient choose the additional screening test.18
MORE WORK TO DO
Clearly, we still do not know how to explain breast density results to our patients in a way that will help them make a fully informed decision about additional screening. Research suggests that letters alone are insufficient,13,19,20 and there is no guarantee that simply adding breast density notification language to result letters will enhance a woman’s understanding and empower her to choose a course of action that is sensitive to her personal preferences.
As more states adopt notification legislation, we must develop effective methods to improve our patients’ understanding of the meaning and implications of having dense breasts and to help them decide how to proceed. Such tools could include videos, Web sites, and pictorials, as well as specialized training for patient educators and health navigators. Otherwise, including this additional, conceptually difficult information to result notification letters could make the doctor-patient interaction even more “dense”—and could increase women’s uncertainty and anxiety about their personal risk of cancer.21
Density: The quality or state of being dense; the quantity per unit volume, unit area, or unit length; the degree of opacity of a translucent medium, or the common logarithm of the opacity.
—Merriam-Webster’s dictionary1
For more than a decade, federal law in the United States has compelled breast imaging centers to give every mammography patient a letter explaining her result.2
Often, however, the first person a woman speaks to about her findings is her primary care clinician, particularly if she has had a screening mammogram at a center where films are “batch-read” and are not viewed by the radiologist at the time of the appointment. Internal medicine physicians are often called on to help women understand their findings and to order follow-up tests recommended by the radiologist—a not uncommon occurrence. Also, internists often need to address patients’ anxieties about the possibility of breast cancer and provide them with enough information to make an informed decision about an appropriate action plan.
Meanwhile, discussing mammography has become more complicated. In 2009, the United States Preventive Services Task Force stopped recommending that women under age 50 be routinely screened for breast cancer, and instead stated that the decision to begin screening these women should consider “patient context” and the patient’s personal “values”3—with the implication that women’s primary clinicians would play an important role in helping them weigh the test’s potential benefits and harms.
More and more, internists must grapple with the task of how to help women decipher the concept of “breast density,” understand their personal density results, and make an informed decision about whether to undergo additional imaging studies, such as ultrasonography and magnetic resonance imaging (MRI).
LEGISLATION REQUIRING DENSITY NOTIFICATION
The impetus for this change in practice has been spurred in large part by patient advocates, who have argued that women deserve to know their density because mammography is less sensitive in women with dense breasts. So far, at least 12 states have enacted laws requiring breast imaging centers to add information about breast density in the result notification letters they mail to patients. Legislatures in several other states are considering breast density notification laws,4 and federal legislation has been proposed.
Some of the state laws, such as those in Connecticut, Texas, and Virginia, require informing all mammography patients about their density findings, whether or not they have dense breast tissue. Other states, such as California, Hawaii, and New York, require informing only those found to have dense tissue. And some states, such as California, Connecticut, Hawaii, Texas, and Virginia, require specific wording in the density notification letter (Table 1).
The details of all these notification laws may differ in how they specify which patients must be notified and in how the information should be worded, but the goal is the same: to raise women’s awareness so that they can embark on an informed decision with their physician about whether to undergo further testing.
Because of liability concerns, some breast imaging centers in states that currently lack such notification laws have begun informing women about their density results.
Unfortunately, at this point clinicians have no clear guidelines for helping patients with dense breasts decide whether to undergo additional testing. In addition, the evidence is equivocal, and the tests have risks as well as benefits. The patient needs to understand all this by discussing it with her physician. And to discuss this decision effectively, the physician must be well versed in the evolving literature on breast density. Below, we present important points to keep in mind as we foster these discussions with our patients.
BREAST TISSUE DENSITY IS STILL A SUBJECTIVE MEASUREMENT
Breast density limits the sensitivity of mammography. This is widely established. Yet the interpretation of breast density today is subjective. It is determined by the interpreting radiologist based on the Breast Imaging and Reporting Data System (BI-RADS), which defines “heterogeneously dense” breasts as those containing 50% to 75% dense tissue and “dense” breasts as those with more than 75%5 (Figure 1). This subjective measurement is based on two-dimensional imaging, which may underestimate or overestimate the percentage of breast density because of tissue summation. Ideally, density should be measured using three-dimensional imaging with automated software,6 but this technology is not yet widely available.
INCREASED DETECTION OF BENIGN LESIONS
Although adding ultrasonography to mammography in patients with dense breast tissue detects additional cancers,7,8 it also leads to a significant increase in the detection of lesions that are not malignant yet require additional workup or biopsy.
The largest study to examine this was the American College of Radiology Imaging Network Protocol 6666 (ACRIN 6666),7 a multi-institutional study evaluating the diagnostic yield, sensitivity, and specificity of adding ultrasonography in high-risk patients who presented with negative mammograms and had heterogeneously dense tissue in at least one quadrant.7 (High risk was defined as a threefold higher risk of breast cancer as determined by risk factors such as personal history of breast cancer or high-risk lesions, or elevated risk using the Gail or Claus model.) The supplemental yield was 4.2 cancers per 1,000 women (95% confidence interval 1.1 to 7.2 per 1,000) on a single prevalent screen. Of 12 cancers detected solely by ultrasonography, 11 were invasive and had a median size of 10 mm. Of those reported, 8 of 9 were node-negative. Despite this additional yield, the positive predictive value of biopsy prompted by ultrasonography was only 8.9%.7 Other investigators have reported similar findings.8
RELATIONSHIP BETWEEN DENSITY AND CANCER RISK STILL NOT CLEAR
The relationship between breast density and cancer risk is not entirely clear. Higher breast density has been associated with a higher risk of breast cancer,9,10 presumably because cancer usually develops in parenchyma, and not fatty tissue. Yet obesity and age, which are inversely associated with density, are also risk factors for the development of breast cancer. Some prominent radiologists have cast doubt on the methodology used in these density studies, which relied on density measurements calculated by two-dimensional views of the breast, and have called for a re-evaluation of the relationship between density and cancer risk.6
LIMITED HEALTH LITERACY: A CHALLENGE
The term “breast density” is unfamiliar to most lay people. As physicians, we need to keep in mind that more than a third of US adults have limited health literacy and thus have difficulty processing basic health information.11 But even the 1 in 10 US women with “proficient” health literacy skills may find the term “density” confusing.
As the definition at the opening of this article suggests, the word itself is nuanced and has different meanings. Anecdotally, both of the authors, a general internist (E.M.) and a breast imaging specialist (M.Y.), have encountered numerous quizzical and sometimes distrustful reactions when telling patients—including some with graduate degrees—that they have “dense” breast tissue and might benefit from additional ultrasonographic testing. Avoiding jargon is key; studies have found that terms such as “benign” can be confusing when used in a mammogram result notification letter.12
How can we explain the concept of breast density to our patients?
Supplemental educational materials that feature simple pictures can also be helpful in conveying complex health information,13 although their effect on the communication of breast density has not been studied. The American College of Radiology and the Society of Breast Imaging produce a freely available, downloadable patient brochure on breast density that includes photographs of mammograms with high and low breast density. The brochure is available from the American College of Radiology online at www.acr.org, under “Tools you can use.”
We recommend introducing women to the concept of breast density before they undergo mammography—at the time the test is ordered—and provide them with supplemental materials such as the above-mentioned brochure. About 1 out of every 10 women who undergo screening mammography has a result requiring additional testing that does not result in a cancer diagnosis. Yet a body of research suggests that many women don’t realize that mammograms don’t always yield a cut-and-dried “cancer” or “no cancer” result. In past studies, women have said they were unaware of how common it is to be called back after routine screening mammography, and they wanted to be prepared for this in advance.12,14 Similarly, many women are unaware of the concept of breast density and don’t know that they may be told about these findings when they get their mammogram report.
Avoid causing anxiety
When explaining results to women with dense breasts, we should emphasize that there are no abnormalities on the current mammogram, and that the only reason to consider additional imaging is the breast density. But regardless of the ultimate outcome, an abnormal mammogram can trigger long-standing anxiety, 15 and it is reasonable to assume that some women will become anxious when told they have highly dense breasts. It is important that clinicians be aware of this potential anxiety and inquire about any personal cancer-related concerns at the time they discuss their findings.16
Helping the patient choose the type of additional screening
If a patient is found to have dense breasts and chooses to undergo additional screening, the decision about which test—ultrasonography or MRI—can be based on the woman’s lifetime risk of breast cancer.
The American Cancer Society recommends that patients with a lifetime risk of 20% or greater—according to a risk model such as BRCAPRO, Tyrer-Cuzick, or BOADICEA (Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm)—should be screened annually with breast MRI regardless of breast density. Patients in this category are those who carry the BRCA gene mutations and their untested first-degree relatives, and patients with Li-Fraumeni, Cowden, or Bannayan-Riley-Ruvalcaba syndrome. Also considered are women who underwent chest radiation between the ages of 10 and 30, and patients who have more than one first-degree relative with breast cancer but who do not have an identifiable genetic mutation.17
Patients with dense breasts who have an increased lifetime risk but who do not meet these criteria and those who are at average risk may be offered breast ultrasonography. If risk factors are unclear, genetic counseling can help determine the lifetime risk and thus help the patient choose the additional screening test.18
MORE WORK TO DO
Clearly, we still do not know how to explain breast density results to our patients in a way that will help them make a fully informed decision about additional screening. Research suggests that letters alone are insufficient,13,19,20 and there is no guarantee that simply adding breast density notification language to result letters will enhance a woman’s understanding and empower her to choose a course of action that is sensitive to her personal preferences.
As more states adopt notification legislation, we must develop effective methods to improve our patients’ understanding of the meaning and implications of having dense breasts and to help them decide how to proceed. Such tools could include videos, Web sites, and pictorials, as well as specialized training for patient educators and health navigators. Otherwise, including this additional, conceptually difficult information to result notification letters could make the doctor-patient interaction even more “dense”—and could increase women’s uncertainty and anxiety about their personal risk of cancer.21
- Merriam-Webster online dictionary. Density http://www.merriam-webster.com/dictionary/density. Accessed November 12, 2013.
- US Food and Drug Administration (FDA). Radiation-emitting products: Frequently asked questions about MQSA. http://www.fda.gov/Radiation-EmittingProducts/MammographyQualityStandardsActandProgram/ConsumerInformation/ucm113968.htm. Accessed November 12, 2013.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 151:716–726, W–236.
- Are You Dense Advocacy Inc. Are you dense? http://areyoudenseadvocacy.org. Accessed November 12, 2013.
- American College of Radiology. Breast Imaging Reporting and Data System (BI-RADS). 4th ed. http://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Resources/BIRADS/MammoBIRADS.pdf. Accessed November 12, 2013.
- Kopans DB. Basic physics and doubts about relationship between mammographically determined tissue density and breast cancer risk. Radiology 2008; 246:348–353.
- Berg WA, Blume JD, Cormack JB, et al; ACRIN 6666 Investigators. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA 2008; 299:2151–2163.
- Hooley RJ, Greenberg KL, Stackhouse RM, Geisel JL, Butler RS, Philpotts LE. Screening US in patients with mammographically dense breasts: initial experience with Connecticut Public Act 09-41. Radiology 2012; 265:59–69.
- Vacek PM, Geller BM. A prospective study of breast cancer risk using routine mammographic breast density measurements. Cancer Epidemiol Biomarkers Prev 2004; 13:715–722.
- Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med 2007; 356:227–236.
- Kutner M, Greenberg E, Jin Y, Paulsen C; National Center for Education Statistics. The health literacy of America’s adults: Results from the 2003 national assessment of adult literacy. US Department of Education. http://nces.ed.gov/pubs2006/2006483.pdf. Accessed November 12, 2013.
- Marcus EN, Drummond D, Dietz N. Urban women’s p for learning of their mammogram result: a qualitative study. J Cancer Educ 2012; 27:156–164.
- Houts PS, Doak CC, Doak LG, Loscalzo MJ. The role of pictures in improving health communication: a review of research on attention, comprehension, recall, and adherence. Patient Educ Couns 2006; 61:173–190.
- Nekhlyudov L, Li R, Fletcher SW. Information and involvement p of women in their 40s before their first screening mammogram. Arch Intern Med 2005; 165:1370–1374.
- Barton MB, Moore S, Polk S, Shtatland E, Elmore JG, Fletcher SW. Increased patient concern after false-positive mammograms: clinician documentation and subsequent ambulatory visits. J Gen Intern Med 2001; 16:150–156.
- Politi MC, Street RL. The importance of communication in collaborative decision making: facilitating shared mind and the management of uncertainty. J Eval Clin Pract 2011; 17:579–584.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007; 57:75–89.
- Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol 2009; 192:390–399.
- Jones BA, Reams K, Calvocoressi L, Dailey A, Kasl SV, Liston NM. Adequacy of communicating results from screening mammograms to African American and white women. Am J Public Health 2007; 97:531–538.
- Karliner LS, Patricia Kaplan C, Juarbe T, Pasick R, Pérez-Stable EJ. Poor patient comprehension of abnormal mammography results. J Gen Intern Med 2005; 20:432–437.
- Marcus EN. Post-mammogram letters often confuse more than they help. Washington Post, February 25, 2013. http://articles.washingtonpost.com/2013-02-25/national/37287736_1_mammogram-letters-densebreasts/2. Accessed November 12, 2013.
- Merriam-Webster online dictionary. Density http://www.merriam-webster.com/dictionary/density. Accessed November 12, 2013.
- US Food and Drug Administration (FDA). Radiation-emitting products: Frequently asked questions about MQSA. http://www.fda.gov/Radiation-EmittingProducts/MammographyQualityStandardsActandProgram/ConsumerInformation/ucm113968.htm. Accessed November 12, 2013.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 151:716–726, W–236.
- Are You Dense Advocacy Inc. Are you dense? http://areyoudenseadvocacy.org. Accessed November 12, 2013.
- American College of Radiology. Breast Imaging Reporting and Data System (BI-RADS). 4th ed. http://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Resources/BIRADS/MammoBIRADS.pdf. Accessed November 12, 2013.
- Kopans DB. Basic physics and doubts about relationship between mammographically determined tissue density and breast cancer risk. Radiology 2008; 246:348–353.
- Berg WA, Blume JD, Cormack JB, et al; ACRIN 6666 Investigators. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA 2008; 299:2151–2163.
- Hooley RJ, Greenberg KL, Stackhouse RM, Geisel JL, Butler RS, Philpotts LE. Screening US in patients with mammographically dense breasts: initial experience with Connecticut Public Act 09-41. Radiology 2012; 265:59–69.
- Vacek PM, Geller BM. A prospective study of breast cancer risk using routine mammographic breast density measurements. Cancer Epidemiol Biomarkers Prev 2004; 13:715–722.
- Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med 2007; 356:227–236.
- Kutner M, Greenberg E, Jin Y, Paulsen C; National Center for Education Statistics. The health literacy of America’s adults: Results from the 2003 national assessment of adult literacy. US Department of Education. http://nces.ed.gov/pubs2006/2006483.pdf. Accessed November 12, 2013.
- Marcus EN, Drummond D, Dietz N. Urban women’s p for learning of their mammogram result: a qualitative study. J Cancer Educ 2012; 27:156–164.
- Houts PS, Doak CC, Doak LG, Loscalzo MJ. The role of pictures in improving health communication: a review of research on attention, comprehension, recall, and adherence. Patient Educ Couns 2006; 61:173–190.
- Nekhlyudov L, Li R, Fletcher SW. Information and involvement p of women in their 40s before their first screening mammogram. Arch Intern Med 2005; 165:1370–1374.
- Barton MB, Moore S, Polk S, Shtatland E, Elmore JG, Fletcher SW. Increased patient concern after false-positive mammograms: clinician documentation and subsequent ambulatory visits. J Gen Intern Med 2001; 16:150–156.
- Politi MC, Street RL. The importance of communication in collaborative decision making: facilitating shared mind and the management of uncertainty. J Eval Clin Pract 2011; 17:579–584.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007; 57:75–89.
- Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol 2009; 192:390–399.
- Jones BA, Reams K, Calvocoressi L, Dailey A, Kasl SV, Liston NM. Adequacy of communicating results from screening mammograms to African American and white women. Am J Public Health 2007; 97:531–538.
- Karliner LS, Patricia Kaplan C, Juarbe T, Pasick R, Pérez-Stable EJ. Poor patient comprehension of abnormal mammography results. J Gen Intern Med 2005; 20:432–437.
- Marcus EN. Post-mammogram letters often confuse more than they help. Washington Post, February 25, 2013. http://articles.washingtonpost.com/2013-02-25/national/37287736_1_mammogram-letters-densebreasts/2. Accessed November 12, 2013.
Dense breasts and legislating medicine
Recently, Nevada,1 North Carolina, and Oregon joined a number of other US states (as of this writing, nine other states) in enacting laws that require informing women if they have dense breast tissue detected on mammography.2 Laws are pending in other states. Federal legislation has also been introduced in the US House of Representatives.
THE POWER OF ADVOCACY TO CHANGE MEDICAL PRACTICE
One such bill3 was introduced as a result of the advocacy of a single patient, Nancy Cappello, a Connecticut woman who was not informed that she had dense breasts and was later found to have node-positive breast cancer.4
While new medical practices are rarely credited to the efforts of single physician or researcher, these “dense-breast laws” show the power a single patient may play in health care. The evidence behind these laws and their implications bring to the forefront the role of advocacy and legislation in the practice of medicine.
Dense-breast laws are the latest chapter in how legislative action can change the practice of medicine. Proof that advocacy could use law to change medical practice emerged in the early 1990s in the wake of AIDS activism. Patient-advocacy activists lobbied for early access to investigational agents, arguing that traditional pathways of clinical testing would deprive terminally ill patients of potentially lifesaving treatments. These efforts led the US Food and Drug Administration (FDA) to create the Accelerated Approval Program, which allows new drugs to garner approval based on surrogate end-point data for terminal or neglected diseases. Accelerated approval was codified into law in 1997 in the FDA’s Modernization Act.5 In 2012, legislative action further broadened the ability of the FDA to approve new products based on surrogate data,6 with the FDA’s Safety and Innovation Act, which provides for first-time approval of a drug based on “pharmacologic” end points that are even more limited.6
Although proponents have declared success when legislative action lowers the bar for drug and device approval, independent analyses have been more critical. In 2009, accelerated approval underwent significant scrutiny when the Government Accountability Office issued a report summarizing 16 years of the program.7 Over the program’s life span, the FDA called for 144 postmarketing studies, but more than one-third of these remained incomplete. Moreover, in 13 years, the FDA never exercised its power to expedite the withdrawal of a drug from the market.
Many accelerated approvals have created considerable controversy. Bevacizumab for metastatic breast cancer was ultimately found to confer no survival benefit, and its approval was revoked.8 Gemtuzumab ozogamicin for acute myeloid leukemia may be effective, but not at the dose that was approved.9 And midodrine hydrochloride and many other drugs remain untested.10
DOES THIS INFORMATION HELP PATIENTS? WHAT WOULD THEY DO WITH IT?
The question with dense-breast laws is similar to that facing other legal efforts to change medicine: Does it actually help patents? Will the information doctors disclose lead to appropriate interventions that improve health outcomes, or, instead, lead to cascades of testing and biopsies that worsen overdiagnosis?
Like accelerated approval, mandating disclosure of breast density is an intervention with uncertain efficacy. While increased breast density has been shown to increase a woman’s risk of developing breast cancer, it is also neutral regarding a woman’s chances of dying of breast cancer.11 In other words, it does not identify patients who experience aggressive disease.
Next comes the larger question of what women would do with this information. Will they simply be more compliant with existing screening recommendations, or will they seek additional testing? This is where the greatest uncertainty lies. The utility of additional testing with ultrasonography or magnetic resonance imaging (MRI) remains uncertain in this population. We will certainly find more cancers if we use MRI to screen women, but it remains unclear if this translates to improved outcomes.
A recent study shows just this.12 In Connecticut, breast density notification is mandatory, as is insurance coverage for screening (or whole-breast) ultrasonography. Since the passage of these laws, the Yale Medical Center has screened 935 women with dense breasts using ultrasonography. Over this time, they performed roughly 16,000 mammograms; thus, the breast density law applied roughly to 1 out of 16 (6.25%) studies. Of the 935 women, biopsies were performed in 54 (5.8%). These were mostly needle biopsies (46), but 3 patients underwent surgical excision, and five cysts were aspirated. From these efforts, two sub-centimeter cancers were found and one case of ductal carcinoma in situ was found. Thus, only 3.7% of women undergoing biopsy and fewer than 1% of women undergoing ultrasonography were found to have cancer.
Of course, given the nature of this study, we cannot know what would have happened without referral and testing. However, empirical research suggests that detecting a breast cancer with screening does not mean a life was saved.13 In fact, only a minority of such women (13%) can credit screening with a survival gain.13
In a study14 that compared women with dense breasts who underwent annual vs biannual screening, no difference in the rate of advanced or metastatic disease was seen with more frequent screening, but the rates of false-positive results and biopsies were higher.14
Notably, dense-breast legislation comes at a time when fundamental questions have been raised about the impact of screening on breast cancer. A prominent study of trends in US breast cancer incidence and death rates over the last 30 years shows that even under the most favorable assumptions, mammography has led to a huge surplus in the diagnosis of breast cancer but little change in the breast cancer mortality rate.15 It is entirely possible that more-aggressive screening in women with dense breasts will only exacerbate this problem. Advocacy may harm rather than help these patients.
We are often told that laws such as the dense-breast bills are motivated by the public’s desire and patient advocacy. However, we are unsure if the vocal proponents of dense-breast laws represent the average women’s desires. These efforts may simply be another case of how a vocal and passionate minority can overcome a large and indifferent majority.16
LEGISLATING MEDICAL PRACTICE IS A BOLD STEP
Dense-breast laws present an additional challenge: they cannot be changed as quickly as scientific understanding. In other words, if the medical field comes to believe that notification is generally harmful because it leads to increased biopsies but not better health, can the law be changed rapidly enough to reflect this? There is a large precedent for the reversal of medical practices,17,18 particularly those based on scant evidence, including cases of recommended screening tests (most notably, recent changes to prostate-specific antigen guidelines). But in all these other cases, law did not mandate the practice or recommendation. Laws are often slow to adapt to changes in understanding.
Legislating medical practice is a bold step, and even those who feel it is occasionally warranted must hold themselves to a rational guiding principle. We have incontrovertible evidence that flexible sigmoidoscopy can reduce the number of deaths from colorectal cancer, but no state mandates that doctors inform their patients of this fact. A patient’s ejection fraction serves as a marker of benefit for several lifesaving drugs and devices, yet no state mandates that physicians disclose this information to patients after echocardiography.
All of us in health care—physicians, researchers, nurses, practitioners, and patients—are patient advocates, and we all want policies that promote human health. However, doing so means adhering to practices grounded in evidence. Dense-breast laws serve as a reminder that good intentions and good people may be necessary—but are not sufficient—for sound policy.
- Nevada Legislature. Requires the notification of patients regarding breast density. (BDR 40-172). http://www.leg.state.nv.us/Session/77th2013/Reports/history.cfm?ID=371. Accessed November 7, 2013.
- ImagingBIZ Newswire. Nevada Governor Signs Breast Density Law June 10, 2013. http://www.imagingbiz.com/articles/news/nevada-governor-signs-breast-density-law. Accessed August 1, 2013.
- Are You Dense Advocacy. H.R.3102Latest 112th Congress. Breast Density and Mammography Reporting Act of 2011 http://www.congressweb.com/areyoudenseadvocacy/Bills/Detail/id/12734. Accessed November 7, 2013.
- The New York Times. New Laws Add a Divisive Component to Breast Screening. http://www.nytimes.com/2012/10/25/health/laws-tell-mammogram-clinics-to-address-breast-density.html?pagewanted=all. Accessed November 7, 2013.
- Reichert JM. Trends in development and approval times for new therapeutics in the United States. Nat Rev Drug Discov 2003; 2:695–702.
- Kramer DB, Kesselheim AS. User fees and beyond—the FDA Safety and Innovation Act of 2012. N Engl J Med 2012; 367:1277–1279.
- US Government Accountability Office (GAO). New Drug Approval: FDA Needs to Enhance Its Oversight of Drugs Approved on the Basis of Surrogate Endpoints. GAO-09-866. http://www.gao.gov/products/GAO-09-866. Accessed November 7, 2013.
- Ocaña A, Amir E, Vera F, Eisenhauer EA, Tannock IF. Addition of bevacizumab to chemotherapy for treatment of solid tumors: similar results but different conclusions. J Clin Oncol 2011; 29:254–256.
- Rowe JM, Löwenberg B. Gemtuzumab ozogamicin in acute myeloid leukemia: a remarkable saga about an active drug. Blood 2013; 121:4838–4841.
- Dhruva SS, Redberg RF. Accelerated approval and possible withdrawal of midodrine. JAMA 2010; 304:2172–2173.
- Gierach GL, Ichikawa L, Kerlikowske K, et al. Relationship between mammographic density and breast cancer death in the Breast Cancer Surveillance Consortium. J Natl Cancer Inst 2012; 104:1218–1227.
- Hooley RJ, Greenberg KL, Stackhouse RM, Geisel JL, Butler RS, Philpotts LE. Screening US in patients with mammographically dense breasts: initial experience with Connecticut Public Act 09-41. Radiology 2012; 265:59–69.
- Welch HG, Frankel BA. Likelihood that a woman with screen-detected breast cancer has had her “life saved” by that screening. Arch Intern Med 2011; 171:2043–2046.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med 2013; 173:807–816.
- Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med 2012; 367:1998–2005.
- New York Review of Books. Facing the Real Gun Problem. http://www.nybooks.com/articles/archives/2013/jun/20/facing-real-gunproblem. Accessed November 7, 2013.
- Prasad V, Gall V, Cifu A. The frequency of medical reversal. Arch Intern Med 2011; 171:1675–1676.
- Prasad V, Cifu A, Ioannidis JP. Reversals of established medical practices: evidence to abandon ship. JAMA 2012; 307:37–38.
Recently, Nevada,1 North Carolina, and Oregon joined a number of other US states (as of this writing, nine other states) in enacting laws that require informing women if they have dense breast tissue detected on mammography.2 Laws are pending in other states. Federal legislation has also been introduced in the US House of Representatives.
THE POWER OF ADVOCACY TO CHANGE MEDICAL PRACTICE
One such bill3 was introduced as a result of the advocacy of a single patient, Nancy Cappello, a Connecticut woman who was not informed that she had dense breasts and was later found to have node-positive breast cancer.4
While new medical practices are rarely credited to the efforts of single physician or researcher, these “dense-breast laws” show the power a single patient may play in health care. The evidence behind these laws and their implications bring to the forefront the role of advocacy and legislation in the practice of medicine.
Dense-breast laws are the latest chapter in how legislative action can change the practice of medicine. Proof that advocacy could use law to change medical practice emerged in the early 1990s in the wake of AIDS activism. Patient-advocacy activists lobbied for early access to investigational agents, arguing that traditional pathways of clinical testing would deprive terminally ill patients of potentially lifesaving treatments. These efforts led the US Food and Drug Administration (FDA) to create the Accelerated Approval Program, which allows new drugs to garner approval based on surrogate end-point data for terminal or neglected diseases. Accelerated approval was codified into law in 1997 in the FDA’s Modernization Act.5 In 2012, legislative action further broadened the ability of the FDA to approve new products based on surrogate data,6 with the FDA’s Safety and Innovation Act, which provides for first-time approval of a drug based on “pharmacologic” end points that are even more limited.6
Although proponents have declared success when legislative action lowers the bar for drug and device approval, independent analyses have been more critical. In 2009, accelerated approval underwent significant scrutiny when the Government Accountability Office issued a report summarizing 16 years of the program.7 Over the program’s life span, the FDA called for 144 postmarketing studies, but more than one-third of these remained incomplete. Moreover, in 13 years, the FDA never exercised its power to expedite the withdrawal of a drug from the market.
Many accelerated approvals have created considerable controversy. Bevacizumab for metastatic breast cancer was ultimately found to confer no survival benefit, and its approval was revoked.8 Gemtuzumab ozogamicin for acute myeloid leukemia may be effective, but not at the dose that was approved.9 And midodrine hydrochloride and many other drugs remain untested.10
DOES THIS INFORMATION HELP PATIENTS? WHAT WOULD THEY DO WITH IT?
The question with dense-breast laws is similar to that facing other legal efforts to change medicine: Does it actually help patents? Will the information doctors disclose lead to appropriate interventions that improve health outcomes, or, instead, lead to cascades of testing and biopsies that worsen overdiagnosis?
Like accelerated approval, mandating disclosure of breast density is an intervention with uncertain efficacy. While increased breast density has been shown to increase a woman’s risk of developing breast cancer, it is also neutral regarding a woman’s chances of dying of breast cancer.11 In other words, it does not identify patients who experience aggressive disease.
Next comes the larger question of what women would do with this information. Will they simply be more compliant with existing screening recommendations, or will they seek additional testing? This is where the greatest uncertainty lies. The utility of additional testing with ultrasonography or magnetic resonance imaging (MRI) remains uncertain in this population. We will certainly find more cancers if we use MRI to screen women, but it remains unclear if this translates to improved outcomes.
A recent study shows just this.12 In Connecticut, breast density notification is mandatory, as is insurance coverage for screening (or whole-breast) ultrasonography. Since the passage of these laws, the Yale Medical Center has screened 935 women with dense breasts using ultrasonography. Over this time, they performed roughly 16,000 mammograms; thus, the breast density law applied roughly to 1 out of 16 (6.25%) studies. Of the 935 women, biopsies were performed in 54 (5.8%). These were mostly needle biopsies (46), but 3 patients underwent surgical excision, and five cysts were aspirated. From these efforts, two sub-centimeter cancers were found and one case of ductal carcinoma in situ was found. Thus, only 3.7% of women undergoing biopsy and fewer than 1% of women undergoing ultrasonography were found to have cancer.
Of course, given the nature of this study, we cannot know what would have happened without referral and testing. However, empirical research suggests that detecting a breast cancer with screening does not mean a life was saved.13 In fact, only a minority of such women (13%) can credit screening with a survival gain.13
In a study14 that compared women with dense breasts who underwent annual vs biannual screening, no difference in the rate of advanced or metastatic disease was seen with more frequent screening, but the rates of false-positive results and biopsies were higher.14
Notably, dense-breast legislation comes at a time when fundamental questions have been raised about the impact of screening on breast cancer. A prominent study of trends in US breast cancer incidence and death rates over the last 30 years shows that even under the most favorable assumptions, mammography has led to a huge surplus in the diagnosis of breast cancer but little change in the breast cancer mortality rate.15 It is entirely possible that more-aggressive screening in women with dense breasts will only exacerbate this problem. Advocacy may harm rather than help these patients.
We are often told that laws such as the dense-breast bills are motivated by the public’s desire and patient advocacy. However, we are unsure if the vocal proponents of dense-breast laws represent the average women’s desires. These efforts may simply be another case of how a vocal and passionate minority can overcome a large and indifferent majority.16
LEGISLATING MEDICAL PRACTICE IS A BOLD STEP
Dense-breast laws present an additional challenge: they cannot be changed as quickly as scientific understanding. In other words, if the medical field comes to believe that notification is generally harmful because it leads to increased biopsies but not better health, can the law be changed rapidly enough to reflect this? There is a large precedent for the reversal of medical practices,17,18 particularly those based on scant evidence, including cases of recommended screening tests (most notably, recent changes to prostate-specific antigen guidelines). But in all these other cases, law did not mandate the practice or recommendation. Laws are often slow to adapt to changes in understanding.
Legislating medical practice is a bold step, and even those who feel it is occasionally warranted must hold themselves to a rational guiding principle. We have incontrovertible evidence that flexible sigmoidoscopy can reduce the number of deaths from colorectal cancer, but no state mandates that doctors inform their patients of this fact. A patient’s ejection fraction serves as a marker of benefit for several lifesaving drugs and devices, yet no state mandates that physicians disclose this information to patients after echocardiography.
All of us in health care—physicians, researchers, nurses, practitioners, and patients—are patient advocates, and we all want policies that promote human health. However, doing so means adhering to practices grounded in evidence. Dense-breast laws serve as a reminder that good intentions and good people may be necessary—but are not sufficient—for sound policy.
Recently, Nevada,1 North Carolina, and Oregon joined a number of other US states (as of this writing, nine other states) in enacting laws that require informing women if they have dense breast tissue detected on mammography.2 Laws are pending in other states. Federal legislation has also been introduced in the US House of Representatives.
THE POWER OF ADVOCACY TO CHANGE MEDICAL PRACTICE
One such bill3 was introduced as a result of the advocacy of a single patient, Nancy Cappello, a Connecticut woman who was not informed that she had dense breasts and was later found to have node-positive breast cancer.4
While new medical practices are rarely credited to the efforts of single physician or researcher, these “dense-breast laws” show the power a single patient may play in health care. The evidence behind these laws and their implications bring to the forefront the role of advocacy and legislation in the practice of medicine.
Dense-breast laws are the latest chapter in how legislative action can change the practice of medicine. Proof that advocacy could use law to change medical practice emerged in the early 1990s in the wake of AIDS activism. Patient-advocacy activists lobbied for early access to investigational agents, arguing that traditional pathways of clinical testing would deprive terminally ill patients of potentially lifesaving treatments. These efforts led the US Food and Drug Administration (FDA) to create the Accelerated Approval Program, which allows new drugs to garner approval based on surrogate end-point data for terminal or neglected diseases. Accelerated approval was codified into law in 1997 in the FDA’s Modernization Act.5 In 2012, legislative action further broadened the ability of the FDA to approve new products based on surrogate data,6 with the FDA’s Safety and Innovation Act, which provides for first-time approval of a drug based on “pharmacologic” end points that are even more limited.6
Although proponents have declared success when legislative action lowers the bar for drug and device approval, independent analyses have been more critical. In 2009, accelerated approval underwent significant scrutiny when the Government Accountability Office issued a report summarizing 16 years of the program.7 Over the program’s life span, the FDA called for 144 postmarketing studies, but more than one-third of these remained incomplete. Moreover, in 13 years, the FDA never exercised its power to expedite the withdrawal of a drug from the market.
Many accelerated approvals have created considerable controversy. Bevacizumab for metastatic breast cancer was ultimately found to confer no survival benefit, and its approval was revoked.8 Gemtuzumab ozogamicin for acute myeloid leukemia may be effective, but not at the dose that was approved.9 And midodrine hydrochloride and many other drugs remain untested.10
DOES THIS INFORMATION HELP PATIENTS? WHAT WOULD THEY DO WITH IT?
The question with dense-breast laws is similar to that facing other legal efforts to change medicine: Does it actually help patents? Will the information doctors disclose lead to appropriate interventions that improve health outcomes, or, instead, lead to cascades of testing and biopsies that worsen overdiagnosis?
Like accelerated approval, mandating disclosure of breast density is an intervention with uncertain efficacy. While increased breast density has been shown to increase a woman’s risk of developing breast cancer, it is also neutral regarding a woman’s chances of dying of breast cancer.11 In other words, it does not identify patients who experience aggressive disease.
Next comes the larger question of what women would do with this information. Will they simply be more compliant with existing screening recommendations, or will they seek additional testing? This is where the greatest uncertainty lies. The utility of additional testing with ultrasonography or magnetic resonance imaging (MRI) remains uncertain in this population. We will certainly find more cancers if we use MRI to screen women, but it remains unclear if this translates to improved outcomes.
A recent study shows just this.12 In Connecticut, breast density notification is mandatory, as is insurance coverage for screening (or whole-breast) ultrasonography. Since the passage of these laws, the Yale Medical Center has screened 935 women with dense breasts using ultrasonography. Over this time, they performed roughly 16,000 mammograms; thus, the breast density law applied roughly to 1 out of 16 (6.25%) studies. Of the 935 women, biopsies were performed in 54 (5.8%). These were mostly needle biopsies (46), but 3 patients underwent surgical excision, and five cysts were aspirated. From these efforts, two sub-centimeter cancers were found and one case of ductal carcinoma in situ was found. Thus, only 3.7% of women undergoing biopsy and fewer than 1% of women undergoing ultrasonography were found to have cancer.
Of course, given the nature of this study, we cannot know what would have happened without referral and testing. However, empirical research suggests that detecting a breast cancer with screening does not mean a life was saved.13 In fact, only a minority of such women (13%) can credit screening with a survival gain.13
In a study14 that compared women with dense breasts who underwent annual vs biannual screening, no difference in the rate of advanced or metastatic disease was seen with more frequent screening, but the rates of false-positive results and biopsies were higher.14
Notably, dense-breast legislation comes at a time when fundamental questions have been raised about the impact of screening on breast cancer. A prominent study of trends in US breast cancer incidence and death rates over the last 30 years shows that even under the most favorable assumptions, mammography has led to a huge surplus in the diagnosis of breast cancer but little change in the breast cancer mortality rate.15 It is entirely possible that more-aggressive screening in women with dense breasts will only exacerbate this problem. Advocacy may harm rather than help these patients.
We are often told that laws such as the dense-breast bills are motivated by the public’s desire and patient advocacy. However, we are unsure if the vocal proponents of dense-breast laws represent the average women’s desires. These efforts may simply be another case of how a vocal and passionate minority can overcome a large and indifferent majority.16
LEGISLATING MEDICAL PRACTICE IS A BOLD STEP
Dense-breast laws present an additional challenge: they cannot be changed as quickly as scientific understanding. In other words, if the medical field comes to believe that notification is generally harmful because it leads to increased biopsies but not better health, can the law be changed rapidly enough to reflect this? There is a large precedent for the reversal of medical practices,17,18 particularly those based on scant evidence, including cases of recommended screening tests (most notably, recent changes to prostate-specific antigen guidelines). But in all these other cases, law did not mandate the practice or recommendation. Laws are often slow to adapt to changes in understanding.
Legislating medical practice is a bold step, and even those who feel it is occasionally warranted must hold themselves to a rational guiding principle. We have incontrovertible evidence that flexible sigmoidoscopy can reduce the number of deaths from colorectal cancer, but no state mandates that doctors inform their patients of this fact. A patient’s ejection fraction serves as a marker of benefit for several lifesaving drugs and devices, yet no state mandates that physicians disclose this information to patients after echocardiography.
All of us in health care—physicians, researchers, nurses, practitioners, and patients—are patient advocates, and we all want policies that promote human health. However, doing so means adhering to practices grounded in evidence. Dense-breast laws serve as a reminder that good intentions and good people may be necessary—but are not sufficient—for sound policy.
- Nevada Legislature. Requires the notification of patients regarding breast density. (BDR 40-172). http://www.leg.state.nv.us/Session/77th2013/Reports/history.cfm?ID=371. Accessed November 7, 2013.
- ImagingBIZ Newswire. Nevada Governor Signs Breast Density Law June 10, 2013. http://www.imagingbiz.com/articles/news/nevada-governor-signs-breast-density-law. Accessed August 1, 2013.
- Are You Dense Advocacy. H.R.3102Latest 112th Congress. Breast Density and Mammography Reporting Act of 2011 http://www.congressweb.com/areyoudenseadvocacy/Bills/Detail/id/12734. Accessed November 7, 2013.
- The New York Times. New Laws Add a Divisive Component to Breast Screening. http://www.nytimes.com/2012/10/25/health/laws-tell-mammogram-clinics-to-address-breast-density.html?pagewanted=all. Accessed November 7, 2013.
- Reichert JM. Trends in development and approval times for new therapeutics in the United States. Nat Rev Drug Discov 2003; 2:695–702.
- Kramer DB, Kesselheim AS. User fees and beyond—the FDA Safety and Innovation Act of 2012. N Engl J Med 2012; 367:1277–1279.
- US Government Accountability Office (GAO). New Drug Approval: FDA Needs to Enhance Its Oversight of Drugs Approved on the Basis of Surrogate Endpoints. GAO-09-866. http://www.gao.gov/products/GAO-09-866. Accessed November 7, 2013.
- Ocaña A, Amir E, Vera F, Eisenhauer EA, Tannock IF. Addition of bevacizumab to chemotherapy for treatment of solid tumors: similar results but different conclusions. J Clin Oncol 2011; 29:254–256.
- Rowe JM, Löwenberg B. Gemtuzumab ozogamicin in acute myeloid leukemia: a remarkable saga about an active drug. Blood 2013; 121:4838–4841.
- Dhruva SS, Redberg RF. Accelerated approval and possible withdrawal of midodrine. JAMA 2010; 304:2172–2173.
- Gierach GL, Ichikawa L, Kerlikowske K, et al. Relationship between mammographic density and breast cancer death in the Breast Cancer Surveillance Consortium. J Natl Cancer Inst 2012; 104:1218–1227.
- Hooley RJ, Greenberg KL, Stackhouse RM, Geisel JL, Butler RS, Philpotts LE. Screening US in patients with mammographically dense breasts: initial experience with Connecticut Public Act 09-41. Radiology 2012; 265:59–69.
- Welch HG, Frankel BA. Likelihood that a woman with screen-detected breast cancer has had her “life saved” by that screening. Arch Intern Med 2011; 171:2043–2046.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med 2013; 173:807–816.
- Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med 2012; 367:1998–2005.
- New York Review of Books. Facing the Real Gun Problem. http://www.nybooks.com/articles/archives/2013/jun/20/facing-real-gunproblem. Accessed November 7, 2013.
- Prasad V, Gall V, Cifu A. The frequency of medical reversal. Arch Intern Med 2011; 171:1675–1676.
- Prasad V, Cifu A, Ioannidis JP. Reversals of established medical practices: evidence to abandon ship. JAMA 2012; 307:37–38.
- Nevada Legislature. Requires the notification of patients regarding breast density. (BDR 40-172). http://www.leg.state.nv.us/Session/77th2013/Reports/history.cfm?ID=371. Accessed November 7, 2013.
- ImagingBIZ Newswire. Nevada Governor Signs Breast Density Law June 10, 2013. http://www.imagingbiz.com/articles/news/nevada-governor-signs-breast-density-law. Accessed August 1, 2013.
- Are You Dense Advocacy. H.R.3102Latest 112th Congress. Breast Density and Mammography Reporting Act of 2011 http://www.congressweb.com/areyoudenseadvocacy/Bills/Detail/id/12734. Accessed November 7, 2013.
- The New York Times. New Laws Add a Divisive Component to Breast Screening. http://www.nytimes.com/2012/10/25/health/laws-tell-mammogram-clinics-to-address-breast-density.html?pagewanted=all. Accessed November 7, 2013.
- Reichert JM. Trends in development and approval times for new therapeutics in the United States. Nat Rev Drug Discov 2003; 2:695–702.
- Kramer DB, Kesselheim AS. User fees and beyond—the FDA Safety and Innovation Act of 2012. N Engl J Med 2012; 367:1277–1279.
- US Government Accountability Office (GAO). New Drug Approval: FDA Needs to Enhance Its Oversight of Drugs Approved on the Basis of Surrogate Endpoints. GAO-09-866. http://www.gao.gov/products/GAO-09-866. Accessed November 7, 2013.
- Ocaña A, Amir E, Vera F, Eisenhauer EA, Tannock IF. Addition of bevacizumab to chemotherapy for treatment of solid tumors: similar results but different conclusions. J Clin Oncol 2011; 29:254–256.
- Rowe JM, Löwenberg B. Gemtuzumab ozogamicin in acute myeloid leukemia: a remarkable saga about an active drug. Blood 2013; 121:4838–4841.
- Dhruva SS, Redberg RF. Accelerated approval and possible withdrawal of midodrine. JAMA 2010; 304:2172–2173.
- Gierach GL, Ichikawa L, Kerlikowske K, et al. Relationship between mammographic density and breast cancer death in the Breast Cancer Surveillance Consortium. J Natl Cancer Inst 2012; 104:1218–1227.
- Hooley RJ, Greenberg KL, Stackhouse RM, Geisel JL, Butler RS, Philpotts LE. Screening US in patients with mammographically dense breasts: initial experience with Connecticut Public Act 09-41. Radiology 2012; 265:59–69.
- Welch HG, Frankel BA. Likelihood that a woman with screen-detected breast cancer has had her “life saved” by that screening. Arch Intern Med 2011; 171:2043–2046.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med 2013; 173:807–816.
- Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med 2012; 367:1998–2005.
- New York Review of Books. Facing the Real Gun Problem. http://www.nybooks.com/articles/archives/2013/jun/20/facing-real-gunproblem. Accessed November 7, 2013.
- Prasad V, Gall V, Cifu A. The frequency of medical reversal. Arch Intern Med 2011; 171:1675–1676.
- Prasad V, Cifu A, Ioannidis JP. Reversals of established medical practices: evidence to abandon ship. JAMA 2012; 307:37–38.
Patient-Specific Imaging and Missed Tumors: A Catastrophic Outcome
Noninvasive coronary test accurate for lesion-specific ischemia
SAN FRANCISCO – A noninvasive test that computes fractional flow reserve from coronary CT angiography images was highly accurate in detecting ischemia, compared with anatomic interpretation from CT angiography or invasive coronary angiography, in a study of 254 patients and 484 vessels.
The primary endpoint was per-patient diagnostic performance as assessed by the area under the receiver operating characteristic curve (AUC) of the test, compared with coronary CT angiography, for the diagnosis of ischemia. The AUC for the new test was 0.82, significantly better than 0.63 for coronary CT angiography, Dr. Bjarne L. Nørgaard reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
The specificity nearly doubled when the HeartFlow test was used to compute fractional flow reserve from coronary CT angiography images (FFRCT), compared with coronary CT angiography assessment. FFRCT correctly reclassified 68% of false positives from CT angiography to true negatives, said Dr. Nørgaard of Aarhus (Denmark) University.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Invasive assessment of FFR is considered the gold standard for diagnosis of lesion-specific functional ischemic disease, but it carries more risk than a noninvasive test. Coronary CT angiography detects anatomic stenosis but is not good at determining the physiologic significance of lesions, he said.
The FFRCT technology builds a quantitative model using data from conventional coronary CT images, and develops a physiological model using left ventricular and coronary anatomy and established form-function principles, Dr. Nørgaard said. A fluid model calculates flow and pressure under simulated hyperemic conditions.
In the 254 patients, FFRCT had an accuracy of 81%, compared with 64% for anatomic assessment using invasive coronary angiography and 53% with CT angiography. The specificity was 79% with FFRCT, 51% with invasive angiography, and 34% with CT angiography. Positive predictive values were 65% with FFRCT, 46% with invasive angiography, and 40% with CT angiography. In each of these categories, FFRCT performed significantly better than CT angiography.
The sensitivity in per-patient diagnosis was 86% with FFRCT, 91% with invasive angiography, and 94% with CT angiography. The negative predictive values were 92% with FFRCT, 93% with invasive angiography, and 92% with CT angiography. Differences between groups were not significant for sensitivity and negative predictive values.
Similar trends were seen in results for the 484 vessels in the study. FFRCT had an accuracy of 86%, compared with 71% for invasive angiography and 65% for CT angiography. The per-vessel specificities were 86%, 66%, and 60%, respectively, and the positive predictive value was 61% with FFRCT, 40% with invasive angiography, and 33% with CT angiography. Again, there was no significant loss in sensitivity (84%, 84%, and 83%, respectively) or in negative predictive value (95%, 94%, and 92%).
The accuracy of FFRCT and invasive assessments of FFR compares favorably with the accuracy of other tests, Dr. Nørgaard said, including stress echo, coronary CT angiography (cCTA), cCTA with transluminal attenuation gradient, single-photon emission CT, and intravenous ultrasound.
"The diagnostic performance of other tests is not impressive," he added. "I think the FFR is a major breakthrough."
The study enrolled patients at 10 centers on three continents who underwent CT and invasive coronary angiography with no more than 60 days between tests.
"I think this will be incorporated into practice," Dr. James B. Hermiller Jr. commented in a panel discussion of the study during a press briefing. A cost analysis is needed, added Dr. Hermiller of St. Vincent Heart Center of Indiana, Indianapolis.
Dr. Philippe Généreux, of Hôpital du Sacré-Coeur de Montréal, called the trial "a brilliant study" and "a breath of fresh air" in the area of noninvasive testing.
Dr. Bernard J. Gersh of the Mayo Clinic, Rochester, Minn., said, "This is a really important trial." He predicted that over the next 2-3 years, great strides will be made in noninvasive assessments of ischemia. "Stay tuned. A number of other methods for evaluating FFR" are being studied, he noted.
The meeting was cosponsored by the American College of Cardiology.
HeartFlow, which markets the FFRCT test, funded the study. Dr. Nørgaard reported having no other financial disclosures. Dr. Hermiller, Dr. Généreux, and Dr. Gersh reported financial associations with multiple companies, but not with HeartFlow.
[email protected] On Twitter @sherryboschert
SAN FRANCISCO – A noninvasive test that computes fractional flow reserve from coronary CT angiography images was highly accurate in detecting ischemia, compared with anatomic interpretation from CT angiography or invasive coronary angiography, in a study of 254 patients and 484 vessels.
The primary endpoint was per-patient diagnostic performance as assessed by the area under the receiver operating characteristic curve (AUC) of the test, compared with coronary CT angiography, for the diagnosis of ischemia. The AUC for the new test was 0.82, significantly better than 0.63 for coronary CT angiography, Dr. Bjarne L. Nørgaard reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
The specificity nearly doubled when the HeartFlow test was used to compute fractional flow reserve from coronary CT angiography images (FFRCT), compared with coronary CT angiography assessment. FFRCT correctly reclassified 68% of false positives from CT angiography to true negatives, said Dr. Nørgaard of Aarhus (Denmark) University.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Invasive assessment of FFR is considered the gold standard for diagnosis of lesion-specific functional ischemic disease, but it carries more risk than a noninvasive test. Coronary CT angiography detects anatomic stenosis but is not good at determining the physiologic significance of lesions, he said.
The FFRCT technology builds a quantitative model using data from conventional coronary CT images, and develops a physiological model using left ventricular and coronary anatomy and established form-function principles, Dr. Nørgaard said. A fluid model calculates flow and pressure under simulated hyperemic conditions.
In the 254 patients, FFRCT had an accuracy of 81%, compared with 64% for anatomic assessment using invasive coronary angiography and 53% with CT angiography. The specificity was 79% with FFRCT, 51% with invasive angiography, and 34% with CT angiography. Positive predictive values were 65% with FFRCT, 46% with invasive angiography, and 40% with CT angiography. In each of these categories, FFRCT performed significantly better than CT angiography.
The sensitivity in per-patient diagnosis was 86% with FFRCT, 91% with invasive angiography, and 94% with CT angiography. The negative predictive values were 92% with FFRCT, 93% with invasive angiography, and 92% with CT angiography. Differences between groups were not significant for sensitivity and negative predictive values.
Similar trends were seen in results for the 484 vessels in the study. FFRCT had an accuracy of 86%, compared with 71% for invasive angiography and 65% for CT angiography. The per-vessel specificities were 86%, 66%, and 60%, respectively, and the positive predictive value was 61% with FFRCT, 40% with invasive angiography, and 33% with CT angiography. Again, there was no significant loss in sensitivity (84%, 84%, and 83%, respectively) or in negative predictive value (95%, 94%, and 92%).
The accuracy of FFRCT and invasive assessments of FFR compares favorably with the accuracy of other tests, Dr. Nørgaard said, including stress echo, coronary CT angiography (cCTA), cCTA with transluminal attenuation gradient, single-photon emission CT, and intravenous ultrasound.
"The diagnostic performance of other tests is not impressive," he added. "I think the FFR is a major breakthrough."
The study enrolled patients at 10 centers on three continents who underwent CT and invasive coronary angiography with no more than 60 days between tests.
"I think this will be incorporated into practice," Dr. James B. Hermiller Jr. commented in a panel discussion of the study during a press briefing. A cost analysis is needed, added Dr. Hermiller of St. Vincent Heart Center of Indiana, Indianapolis.
Dr. Philippe Généreux, of Hôpital du Sacré-Coeur de Montréal, called the trial "a brilliant study" and "a breath of fresh air" in the area of noninvasive testing.
Dr. Bernard J. Gersh of the Mayo Clinic, Rochester, Minn., said, "This is a really important trial." He predicted that over the next 2-3 years, great strides will be made in noninvasive assessments of ischemia. "Stay tuned. A number of other methods for evaluating FFR" are being studied, he noted.
The meeting was cosponsored by the American College of Cardiology.
HeartFlow, which markets the FFRCT test, funded the study. Dr. Nørgaard reported having no other financial disclosures. Dr. Hermiller, Dr. Généreux, and Dr. Gersh reported financial associations with multiple companies, but not with HeartFlow.
[email protected] On Twitter @sherryboschert
SAN FRANCISCO – A noninvasive test that computes fractional flow reserve from coronary CT angiography images was highly accurate in detecting ischemia, compared with anatomic interpretation from CT angiography or invasive coronary angiography, in a study of 254 patients and 484 vessels.
The primary endpoint was per-patient diagnostic performance as assessed by the area under the receiver operating characteristic curve (AUC) of the test, compared with coronary CT angiography, for the diagnosis of ischemia. The AUC for the new test was 0.82, significantly better than 0.63 for coronary CT angiography, Dr. Bjarne L. Nørgaard reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
The specificity nearly doubled when the HeartFlow test was used to compute fractional flow reserve from coronary CT angiography images (FFRCT), compared with coronary CT angiography assessment. FFRCT correctly reclassified 68% of false positives from CT angiography to true negatives, said Dr. Nørgaard of Aarhus (Denmark) University.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Invasive assessment of FFR is considered the gold standard for diagnosis of lesion-specific functional ischemic disease, but it carries more risk than a noninvasive test. Coronary CT angiography detects anatomic stenosis but is not good at determining the physiologic significance of lesions, he said.
The FFRCT technology builds a quantitative model using data from conventional coronary CT images, and develops a physiological model using left ventricular and coronary anatomy and established form-function principles, Dr. Nørgaard said. A fluid model calculates flow and pressure under simulated hyperemic conditions.
In the 254 patients, FFRCT had an accuracy of 81%, compared with 64% for anatomic assessment using invasive coronary angiography and 53% with CT angiography. The specificity was 79% with FFRCT, 51% with invasive angiography, and 34% with CT angiography. Positive predictive values were 65% with FFRCT, 46% with invasive angiography, and 40% with CT angiography. In each of these categories, FFRCT performed significantly better than CT angiography.
The sensitivity in per-patient diagnosis was 86% with FFRCT, 91% with invasive angiography, and 94% with CT angiography. The negative predictive values were 92% with FFRCT, 93% with invasive angiography, and 92% with CT angiography. Differences between groups were not significant for sensitivity and negative predictive values.
Similar trends were seen in results for the 484 vessels in the study. FFRCT had an accuracy of 86%, compared with 71% for invasive angiography and 65% for CT angiography. The per-vessel specificities were 86%, 66%, and 60%, respectively, and the positive predictive value was 61% with FFRCT, 40% with invasive angiography, and 33% with CT angiography. Again, there was no significant loss in sensitivity (84%, 84%, and 83%, respectively) or in negative predictive value (95%, 94%, and 92%).
The accuracy of FFRCT and invasive assessments of FFR compares favorably with the accuracy of other tests, Dr. Nørgaard said, including stress echo, coronary CT angiography (cCTA), cCTA with transluminal attenuation gradient, single-photon emission CT, and intravenous ultrasound.
"The diagnostic performance of other tests is not impressive," he added. "I think the FFR is a major breakthrough."
The study enrolled patients at 10 centers on three continents who underwent CT and invasive coronary angiography with no more than 60 days between tests.
"I think this will be incorporated into practice," Dr. James B. Hermiller Jr. commented in a panel discussion of the study during a press briefing. A cost analysis is needed, added Dr. Hermiller of St. Vincent Heart Center of Indiana, Indianapolis.
Dr. Philippe Généreux, of Hôpital du Sacré-Coeur de Montréal, called the trial "a brilliant study" and "a breath of fresh air" in the area of noninvasive testing.
Dr. Bernard J. Gersh of the Mayo Clinic, Rochester, Minn., said, "This is a really important trial." He predicted that over the next 2-3 years, great strides will be made in noninvasive assessments of ischemia. "Stay tuned. A number of other methods for evaluating FFR" are being studied, he noted.
The meeting was cosponsored by the American College of Cardiology.
HeartFlow, which markets the FFRCT test, funded the study. Dr. Nørgaard reported having no other financial disclosures. Dr. Hermiller, Dr. Généreux, and Dr. Gersh reported financial associations with multiple companies, but not with HeartFlow.
[email protected] On Twitter @sherryboschert
AT TCT 2013
Major finding: The per-patient AUC was 0.82 with the FFRCT test, significantly better than 0.63 with CT angiography.
Data source: A prospective, international study of FFRCT in 254 patients who underwent CT and invasive angiography at 10 centers.
Disclosures: HeartFlow, which markets the FFRCT test, funded the study. Dr. Nørgaard reported having no other financial disclosures. Dr. Hermiller, Dr. Généreux, and Dr. Gersh reported financial associations with multiple companies, but not with HeartFlow.
Surgeons' Perception of Fluoroscopic Radiation Hazards to Vision
Tibia-Based Referencing for Standard Proximal Tibial Radiographs During Intramedullary Nailing
Occipital headache and unstable gait
A 41-year-old-man with a history of hypertension presented to the ED with a sudden onset of severe occipital headache and unstable gait. On physical examination, right-sided ptosis and miosis (Horner’s syndrome) were noted. An emergent head computed tomography (CT) scan was performed and showed no evidence of hemorrhage or infraction. Magnetic resonance imaging (MRI) of the brain and magnetic resonance angiography (MRA) of the head and neck were also ordered. Noncontrast MRA images of the neck are shown above (Figures 1 and 2).
|
|
Figure 1 | Figure 2 |
What is the diagnosis?
What other imaging modalities may be useful for evaluation?
Dr Wladyka is an assistant professor of radiology at Weill Cornell Medical College in New York City and an assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and the executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center. He is associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
Answer
|
|
Figure 3 | Figure 4 |
The axial MRA image demonstrates flow in two separate lumens of the right vertebral artery (white arrow, Figure 3), indicating the presence of a dissection. A normal single lumen vertebral artery can be seen on the left (red arrow, Figure 3). This dissection is confirmed on the coronal MRA image (white arrows, Figure 4).
Vertebral artery dissection (VAD) is a common cause of stroke and, less commonly, of transient ischemic attack in patients aged 18 to 45 years. Patients typically present with headache, vertigo, dizziness, and neck pain.1 Additional neurologic signs that may be related to compromise of the posterior cerebral circulation include lateral medullary syndrome (eg, dysphagia, slurred speech, ataxia, facial pain, nystagmus, diplopia, dysphonia) and Horner’s syndrome (eg, ptosis, miosis, anhidrosis). In a small percentage of patients, VAD may present as intracranial subarachnoid hemorrhage.1
VAD can occur spontaneously or following high-energy trauma or minor trauma (eg, resulting from coughing, vomiting, cervical spine manipulation, or sports injury). Approximately 15% of patients have an underlying connective tissue disorder, such as fibromuscular dysplasia.2
The diagnosis of VAD can be made with MRI/MRA or with computed tomography angiography (CTA). A recent review of the literature shows no clear advantage to using either modality; therefore, the choice CTA or MRA should be based on urgency, availability of the imaging modality, and the preferences/expertise of the radiologists.3
|
|
Figure 5 | Figure 6 |
Advantages of CTA include its widespread availability, short examination time, and ability to evaluate for concurrent injuries in a trauma patient. With respect to MRA, in addition the lack of ionizing radiation, images may be performed without intravenous contrast and completed at the same time as MRI, which is useful in detecting alternative or concurrent intracranial abnormalities such as stroke, mass, or demyelination.
In this case, axial and coronal images from follow-up CTA illustrate its ability to depict the dissection of the right vertebral artery (white arrows, Figures 5 and 6). Typical treatment for VAD includes anticoagulation and antiplatelet therapy, though there have also been reports of successful treatment with endovascular stenting and endovascular thrombolysis.4 Since timely and proper treatment decreases the risk of stroke and long-term disabilities, emergent imaging with CTA or MRA should be performed in cases of suspected VAD.
- Gottesman RF, Sharma P, Robinson KA, et al. Clinical characteristics of symptomatic vertebral artery dissection: a systematic review. Neurologist. 2012;18(5):245-254.
- Rodallec MH, Marteau V, Gerber S, Desmottes L, Zins M. Craniocervical arterial dissection: spectrum of imaging findings and differential diagnosis. Radiographics. 2008;28(6):1711-1728.
- Provenzale JM, Sarikaya B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. AJR Am J Roentgenol. 2009;193(4):1167-1174.
- Menon R, Kerry S, Norris JW, Markus HS. Treatment of cervical artery dissection: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2008;79(10):1122-1127.
A 41-year-old-man with a history of hypertension presented to the ED with a sudden onset of severe occipital headache and unstable gait. On physical examination, right-sided ptosis and miosis (Horner’s syndrome) were noted. An emergent head computed tomography (CT) scan was performed and showed no evidence of hemorrhage or infraction. Magnetic resonance imaging (MRI) of the brain and magnetic resonance angiography (MRA) of the head and neck were also ordered. Noncontrast MRA images of the neck are shown above (Figures 1 and 2).
|
|
Figure 1 | Figure 2 |
What is the diagnosis?
What other imaging modalities may be useful for evaluation?
Dr Wladyka is an assistant professor of radiology at Weill Cornell Medical College in New York City and an assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and the executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center. He is associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
Answer
|
|
Figure 3 | Figure 4 |
The axial MRA image demonstrates flow in two separate lumens of the right vertebral artery (white arrow, Figure 3), indicating the presence of a dissection. A normal single lumen vertebral artery can be seen on the left (red arrow, Figure 3). This dissection is confirmed on the coronal MRA image (white arrows, Figure 4).
Vertebral artery dissection (VAD) is a common cause of stroke and, less commonly, of transient ischemic attack in patients aged 18 to 45 years. Patients typically present with headache, vertigo, dizziness, and neck pain.1 Additional neurologic signs that may be related to compromise of the posterior cerebral circulation include lateral medullary syndrome (eg, dysphagia, slurred speech, ataxia, facial pain, nystagmus, diplopia, dysphonia) and Horner’s syndrome (eg, ptosis, miosis, anhidrosis). In a small percentage of patients, VAD may present as intracranial subarachnoid hemorrhage.1
VAD can occur spontaneously or following high-energy trauma or minor trauma (eg, resulting from coughing, vomiting, cervical spine manipulation, or sports injury). Approximately 15% of patients have an underlying connective tissue disorder, such as fibromuscular dysplasia.2
The diagnosis of VAD can be made with MRI/MRA or with computed tomography angiography (CTA). A recent review of the literature shows no clear advantage to using either modality; therefore, the choice CTA or MRA should be based on urgency, availability of the imaging modality, and the preferences/expertise of the radiologists.3
|
|
Figure 5 | Figure 6 |
Advantages of CTA include its widespread availability, short examination time, and ability to evaluate for concurrent injuries in a trauma patient. With respect to MRA, in addition the lack of ionizing radiation, images may be performed without intravenous contrast and completed at the same time as MRI, which is useful in detecting alternative or concurrent intracranial abnormalities such as stroke, mass, or demyelination.
In this case, axial and coronal images from follow-up CTA illustrate its ability to depict the dissection of the right vertebral artery (white arrows, Figures 5 and 6). Typical treatment for VAD includes anticoagulation and antiplatelet therapy, though there have also been reports of successful treatment with endovascular stenting and endovascular thrombolysis.4 Since timely and proper treatment decreases the risk of stroke and long-term disabilities, emergent imaging with CTA or MRA should be performed in cases of suspected VAD.
A 41-year-old-man with a history of hypertension presented to the ED with a sudden onset of severe occipital headache and unstable gait. On physical examination, right-sided ptosis and miosis (Horner’s syndrome) were noted. An emergent head computed tomography (CT) scan was performed and showed no evidence of hemorrhage or infraction. Magnetic resonance imaging (MRI) of the brain and magnetic resonance angiography (MRA) of the head and neck were also ordered. Noncontrast MRA images of the neck are shown above (Figures 1 and 2).
|
|
Figure 1 | Figure 2 |
What is the diagnosis?
What other imaging modalities may be useful for evaluation?
Dr Wladyka is an assistant professor of radiology at Weill Cornell Medical College in New York City and an assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and the executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center. He is associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
Answer
|
|
Figure 3 | Figure 4 |
The axial MRA image demonstrates flow in two separate lumens of the right vertebral artery (white arrow, Figure 3), indicating the presence of a dissection. A normal single lumen vertebral artery can be seen on the left (red arrow, Figure 3). This dissection is confirmed on the coronal MRA image (white arrows, Figure 4).
Vertebral artery dissection (VAD) is a common cause of stroke and, less commonly, of transient ischemic attack in patients aged 18 to 45 years. Patients typically present with headache, vertigo, dizziness, and neck pain.1 Additional neurologic signs that may be related to compromise of the posterior cerebral circulation include lateral medullary syndrome (eg, dysphagia, slurred speech, ataxia, facial pain, nystagmus, diplopia, dysphonia) and Horner’s syndrome (eg, ptosis, miosis, anhidrosis). In a small percentage of patients, VAD may present as intracranial subarachnoid hemorrhage.1
VAD can occur spontaneously or following high-energy trauma or minor trauma (eg, resulting from coughing, vomiting, cervical spine manipulation, or sports injury). Approximately 15% of patients have an underlying connective tissue disorder, such as fibromuscular dysplasia.2
The diagnosis of VAD can be made with MRI/MRA or with computed tomography angiography (CTA). A recent review of the literature shows no clear advantage to using either modality; therefore, the choice CTA or MRA should be based on urgency, availability of the imaging modality, and the preferences/expertise of the radiologists.3
|
|
Figure 5 | Figure 6 |
Advantages of CTA include its widespread availability, short examination time, and ability to evaluate for concurrent injuries in a trauma patient. With respect to MRA, in addition the lack of ionizing radiation, images may be performed without intravenous contrast and completed at the same time as MRI, which is useful in detecting alternative or concurrent intracranial abnormalities such as stroke, mass, or demyelination.
In this case, axial and coronal images from follow-up CTA illustrate its ability to depict the dissection of the right vertebral artery (white arrows, Figures 5 and 6). Typical treatment for VAD includes anticoagulation and antiplatelet therapy, though there have also been reports of successful treatment with endovascular stenting and endovascular thrombolysis.4 Since timely and proper treatment decreases the risk of stroke and long-term disabilities, emergent imaging with CTA or MRA should be performed in cases of suspected VAD.
- Gottesman RF, Sharma P, Robinson KA, et al. Clinical characteristics of symptomatic vertebral artery dissection: a systematic review. Neurologist. 2012;18(5):245-254.
- Rodallec MH, Marteau V, Gerber S, Desmottes L, Zins M. Craniocervical arterial dissection: spectrum of imaging findings and differential diagnosis. Radiographics. 2008;28(6):1711-1728.
- Provenzale JM, Sarikaya B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. AJR Am J Roentgenol. 2009;193(4):1167-1174.
- Menon R, Kerry S, Norris JW, Markus HS. Treatment of cervical artery dissection: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2008;79(10):1122-1127.
- Gottesman RF, Sharma P, Robinson KA, et al. Clinical characteristics of symptomatic vertebral artery dissection: a systematic review. Neurologist. 2012;18(5):245-254.
- Rodallec MH, Marteau V, Gerber S, Desmottes L, Zins M. Craniocervical arterial dissection: spectrum of imaging findings and differential diagnosis. Radiographics. 2008;28(6):1711-1728.
- Provenzale JM, Sarikaya B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. AJR Am J Roentgenol. 2009;193(4):1167-1174.
- Menon R, Kerry S, Norris JW, Markus HS. Treatment of cervical artery dissection: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2008;79(10):1122-1127.
Challenges in Sports Medicine and Orthopedics
After playing football, an 18-year-old man presented to the ED with severe pain in his right arm. He stated that while throwing a pass, he felt a "snap" in his right arm and has been unable to move the arm since the injury. Radiographs were completed.
What is your interpretation of the radiographic image (Figure 1)?
Dr Patterson, editor of "Challenges in Sports Medicine and Orthopedics," is a sports medicine physician at Florida Sports Injury in Clermont, Florida. Dr Patterson is board certified in family medicine and spinal cord injury medicine, and is a member of the faculty of sports and exercise medicine of the Royal College of Surgeons in Ireland.
The radiograph (Figure 2) revealed a minimally displaced pathologic fracture (red arrow) through the midshaft of the humerus, which resulted from a space-occupying lesion (green arrow) in the humeral diaphysis. Since the radial nerve is commonly affected in this type of injury due to its close proximity to the humeral midshaft, careful neurologic assessment at the wrist and hand is essential. Injury to the nerve can occur during the fracture or reduction of the fracture, causing weakness in the extensors of the hand and numbness in the first dorsal web space. The incidence of radial nerve palsy in midshaft fractures of the humerus is 16%.1
In nondisplaced or minimally displaced fractures of the humeral midshaft, conservative management with a U-shaped (sugar-tong) splint from axilla to shoulder with elasticized wrap and sling is recommended. Surgical management is indicated in comminuted, significantly displaced, nonreducible, pathologic cases or in fractures resulting in neurovascular compromise. The patient in this case was referred to an orthopedic surgeon for open treatment.
- Smith WR, Agudelo JF, Parekh AA, Shank, JR. Musculoskeletal trauma surgery. In: Skinner HB, ed. Current Diagnosis & Treatment in Orthopedics. 4th ed. McGraw Hill Companies, Inc; 2006:121.
After playing football, an 18-year-old man presented to the ED with severe pain in his right arm. He stated that while throwing a pass, he felt a "snap" in his right arm and has been unable to move the arm since the injury. Radiographs were completed.
What is your interpretation of the radiographic image (Figure 1)?
Dr Patterson, editor of "Challenges in Sports Medicine and Orthopedics," is a sports medicine physician at Florida Sports Injury in Clermont, Florida. Dr Patterson is board certified in family medicine and spinal cord injury medicine, and is a member of the faculty of sports and exercise medicine of the Royal College of Surgeons in Ireland.
The radiograph (Figure 2) revealed a minimally displaced pathologic fracture (red arrow) through the midshaft of the humerus, which resulted from a space-occupying lesion (green arrow) in the humeral diaphysis. Since the radial nerve is commonly affected in this type of injury due to its close proximity to the humeral midshaft, careful neurologic assessment at the wrist and hand is essential. Injury to the nerve can occur during the fracture or reduction of the fracture, causing weakness in the extensors of the hand and numbness in the first dorsal web space. The incidence of radial nerve palsy in midshaft fractures of the humerus is 16%.1
In nondisplaced or minimally displaced fractures of the humeral midshaft, conservative management with a U-shaped (sugar-tong) splint from axilla to shoulder with elasticized wrap and sling is recommended. Surgical management is indicated in comminuted, significantly displaced, nonreducible, pathologic cases or in fractures resulting in neurovascular compromise. The patient in this case was referred to an orthopedic surgeon for open treatment.
After playing football, an 18-year-old man presented to the ED with severe pain in his right arm. He stated that while throwing a pass, he felt a "snap" in his right arm and has been unable to move the arm since the injury. Radiographs were completed.
What is your interpretation of the radiographic image (Figure 1)?
Dr Patterson, editor of "Challenges in Sports Medicine and Orthopedics," is a sports medicine physician at Florida Sports Injury in Clermont, Florida. Dr Patterson is board certified in family medicine and spinal cord injury medicine, and is a member of the faculty of sports and exercise medicine of the Royal College of Surgeons in Ireland.
The radiograph (Figure 2) revealed a minimally displaced pathologic fracture (red arrow) through the midshaft of the humerus, which resulted from a space-occupying lesion (green arrow) in the humeral diaphysis. Since the radial nerve is commonly affected in this type of injury due to its close proximity to the humeral midshaft, careful neurologic assessment at the wrist and hand is essential. Injury to the nerve can occur during the fracture or reduction of the fracture, causing weakness in the extensors of the hand and numbness in the first dorsal web space. The incidence of radial nerve palsy in midshaft fractures of the humerus is 16%.1
In nondisplaced or minimally displaced fractures of the humeral midshaft, conservative management with a U-shaped (sugar-tong) splint from axilla to shoulder with elasticized wrap and sling is recommended. Surgical management is indicated in comminuted, significantly displaced, nonreducible, pathologic cases or in fractures resulting in neurovascular compromise. The patient in this case was referred to an orthopedic surgeon for open treatment.
- Smith WR, Agudelo JF, Parekh AA, Shank, JR. Musculoskeletal trauma surgery. In: Skinner HB, ed. Current Diagnosis & Treatment in Orthopedics. 4th ed. McGraw Hill Companies, Inc; 2006:121.
- Smith WR, Agudelo JF, Parekh AA, Shank, JR. Musculoskeletal trauma surgery. In: Skinner HB, ed. Current Diagnosis & Treatment in Orthopedics. 4th ed. McGraw Hill Companies, Inc; 2006:121.