User login
A Case of Malignant Transformation of Myositis Ossificans
Cloud-based network reduces repeat trauma imaging
NAPLES, FLA. – Implementing a cloud-based network for sharing radiological images reduced radiation exposure and costs at a Level 1 tertiary care trauma center.
The number of trauma patients who underwent the exact same imaging study decreased significantly from 62% to 47% in the 6 months after the network was available (P less than .01).
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
This resulted in a significant 19.2% decline in radiation exposure (mean 8.39 mSv vs. 7.23 mSv; P less than .01), Dr. Mayur Narayan reported at the annual meeting of the Eastern Association for the Surgery of Trauma.
"I think it’s a potential game changer," he said. "... The concept is important. The way we’re doing business currently is not sustainable."
Patients transferred within a regional trauma system to a tertiary care referral center frequently have prior radiology studies, but often undergo repeat imaging because of poor image quality, incompatible imaging software, or misplaced imaging discs. This can delay patient management, bring unwelcome radiation, and raise health care costs, explained Dr. Narayan, with the R Adams Cowley Shock Trauma Center at the University of Maryland Medical Center in Baltimore.
To reverse this trend, the center set up a secure, electronic (lifeIMAGE) network that uses a single platform for all medical image exchanges. Physicians can retrieve outside exams via the cloud or merge any or all picture archiving and communication system (PACS) data into their own PACS.
Ten hospitals are now on board and prospective data have been analyzed for 1,950 patients transferred to the trauma center between Jan. 1 and June 30, 2011, (pre-network) and between Jan. 1 and June 30, 2012, (post-network). About 8,500 patients are transferred to the trauma center annually from across the state. Patients in both time periods had similar demographics and Injury Severity Scores (mean 12).
In the 6 months after the network was implemented, the cost of imaging per patient dropped 18.7% ($413 vs. $333; P less than .01), while total imaging costs declined from $401,765 to $326,756 during the same period, Dr. Narayan said.
The most common repeat study in the analysis was an abdominal pelvic CT scan.
Inexplicably, hospital length of stay also declined from 4.4 days to 3.8 days (P = .07), he said. In-hospital mortality was unchanged (3.8% vs. 4.4%; P = .52).
The network cost Cowley Shock Trauma about $30,000 to set up, but other hospital groups are working to provide similar software for free, Dr. Narayan noted.
"The upfront costs should go away," he said.
During a discussion of the poster, one attendee said they set up a similar cloud-based system for their region, but no one used it. "I think it’s because small hospitals don’t see a lot of trauma. They have one or two patients maybe a month that would require transfer and with different people at the CT scanner every night, there’s no uniformity on how to do it," he said.
Others, however, noted that image sharing between hospitals has been fully integrated into their transfer algorithm, prompting calls between hospital radiology units, so that PAC files and patient record numbers have already been merged before the patient ever arrives in the trauma bay.
The Radiological Society of North America has also harnessed cloud-based computing to allow consumers to share their images and interpretations with any provider, regardless of institutional affiliation. According to 20-month follow-up data reported in late 2013, four out of five patients and 9 out of 10 physicians were satisfied with the RSNA Image Share network. A full 90% of patients were comfortable with the amount of privacy provided by the network, though some criticized the site for being "clunky" to navigate.
Dr. Narayan reported having no financial disclosures.
NAPLES, FLA. – Implementing a cloud-based network for sharing radiological images reduced radiation exposure and costs at a Level 1 tertiary care trauma center.
The number of trauma patients who underwent the exact same imaging study decreased significantly from 62% to 47% in the 6 months after the network was available (P less than .01).
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
This resulted in a significant 19.2% decline in radiation exposure (mean 8.39 mSv vs. 7.23 mSv; P less than .01), Dr. Mayur Narayan reported at the annual meeting of the Eastern Association for the Surgery of Trauma.
"I think it’s a potential game changer," he said. "... The concept is important. The way we’re doing business currently is not sustainable."
Patients transferred within a regional trauma system to a tertiary care referral center frequently have prior radiology studies, but often undergo repeat imaging because of poor image quality, incompatible imaging software, or misplaced imaging discs. This can delay patient management, bring unwelcome radiation, and raise health care costs, explained Dr. Narayan, with the R Adams Cowley Shock Trauma Center at the University of Maryland Medical Center in Baltimore.
To reverse this trend, the center set up a secure, electronic (lifeIMAGE) network that uses a single platform for all medical image exchanges. Physicians can retrieve outside exams via the cloud or merge any or all picture archiving and communication system (PACS) data into their own PACS.
Ten hospitals are now on board and prospective data have been analyzed for 1,950 patients transferred to the trauma center between Jan. 1 and June 30, 2011, (pre-network) and between Jan. 1 and June 30, 2012, (post-network). About 8,500 patients are transferred to the trauma center annually from across the state. Patients in both time periods had similar demographics and Injury Severity Scores (mean 12).
In the 6 months after the network was implemented, the cost of imaging per patient dropped 18.7% ($413 vs. $333; P less than .01), while total imaging costs declined from $401,765 to $326,756 during the same period, Dr. Narayan said.
The most common repeat study in the analysis was an abdominal pelvic CT scan.
Inexplicably, hospital length of stay also declined from 4.4 days to 3.8 days (P = .07), he said. In-hospital mortality was unchanged (3.8% vs. 4.4%; P = .52).
The network cost Cowley Shock Trauma about $30,000 to set up, but other hospital groups are working to provide similar software for free, Dr. Narayan noted.
"The upfront costs should go away," he said.
During a discussion of the poster, one attendee said they set up a similar cloud-based system for their region, but no one used it. "I think it’s because small hospitals don’t see a lot of trauma. They have one or two patients maybe a month that would require transfer and with different people at the CT scanner every night, there’s no uniformity on how to do it," he said.
Others, however, noted that image sharing between hospitals has been fully integrated into their transfer algorithm, prompting calls between hospital radiology units, so that PAC files and patient record numbers have already been merged before the patient ever arrives in the trauma bay.
The Radiological Society of North America has also harnessed cloud-based computing to allow consumers to share their images and interpretations with any provider, regardless of institutional affiliation. According to 20-month follow-up data reported in late 2013, four out of five patients and 9 out of 10 physicians were satisfied with the RSNA Image Share network. A full 90% of patients were comfortable with the amount of privacy provided by the network, though some criticized the site for being "clunky" to navigate.
Dr. Narayan reported having no financial disclosures.
NAPLES, FLA. – Implementing a cloud-based network for sharing radiological images reduced radiation exposure and costs at a Level 1 tertiary care trauma center.
The number of trauma patients who underwent the exact same imaging study decreased significantly from 62% to 47% in the 6 months after the network was available (P less than .01).
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
This resulted in a significant 19.2% decline in radiation exposure (mean 8.39 mSv vs. 7.23 mSv; P less than .01), Dr. Mayur Narayan reported at the annual meeting of the Eastern Association for the Surgery of Trauma.
"I think it’s a potential game changer," he said. "... The concept is important. The way we’re doing business currently is not sustainable."
Patients transferred within a regional trauma system to a tertiary care referral center frequently have prior radiology studies, but often undergo repeat imaging because of poor image quality, incompatible imaging software, or misplaced imaging discs. This can delay patient management, bring unwelcome radiation, and raise health care costs, explained Dr. Narayan, with the R Adams Cowley Shock Trauma Center at the University of Maryland Medical Center in Baltimore.
To reverse this trend, the center set up a secure, electronic (lifeIMAGE) network that uses a single platform for all medical image exchanges. Physicians can retrieve outside exams via the cloud or merge any or all picture archiving and communication system (PACS) data into their own PACS.
Ten hospitals are now on board and prospective data have been analyzed for 1,950 patients transferred to the trauma center between Jan. 1 and June 30, 2011, (pre-network) and between Jan. 1 and June 30, 2012, (post-network). About 8,500 patients are transferred to the trauma center annually from across the state. Patients in both time periods had similar demographics and Injury Severity Scores (mean 12).
In the 6 months after the network was implemented, the cost of imaging per patient dropped 18.7% ($413 vs. $333; P less than .01), while total imaging costs declined from $401,765 to $326,756 during the same period, Dr. Narayan said.
The most common repeat study in the analysis was an abdominal pelvic CT scan.
Inexplicably, hospital length of stay also declined from 4.4 days to 3.8 days (P = .07), he said. In-hospital mortality was unchanged (3.8% vs. 4.4%; P = .52).
The network cost Cowley Shock Trauma about $30,000 to set up, but other hospital groups are working to provide similar software for free, Dr. Narayan noted.
"The upfront costs should go away," he said.
During a discussion of the poster, one attendee said they set up a similar cloud-based system for their region, but no one used it. "I think it’s because small hospitals don’t see a lot of trauma. They have one or two patients maybe a month that would require transfer and with different people at the CT scanner every night, there’s no uniformity on how to do it," he said.
Others, however, noted that image sharing between hospitals has been fully integrated into their transfer algorithm, prompting calls between hospital radiology units, so that PAC files and patient record numbers have already been merged before the patient ever arrives in the trauma bay.
The Radiological Society of North America has also harnessed cloud-based computing to allow consumers to share their images and interpretations with any provider, regardless of institutional affiliation. According to 20-month follow-up data reported in late 2013, four out of five patients and 9 out of 10 physicians were satisfied with the RSNA Image Share network. A full 90% of patients were comfortable with the amount of privacy provided by the network, though some criticized the site for being "clunky" to navigate.
Dr. Narayan reported having no financial disclosures.
AT EAST 2014
Major finding: The number of patients who underwent repeat imaging declined from 62% to 47% in the 6 months after the network was implemented (P less than .01).
Data source: Prospective data review of 1,950 trauma patients.
Disclosures: Dr. Narayan reported having no financial disclosures.
Injury cause alone insufficient to justify CT scanning in children
Using computed tomographic imaging (CT scans) based only on a child’s method of injury in blunt trauma cases incurs more risks than benefits, according to a recent study.
The ionizing radiation from CT scanning has been linked to long-term risk of cancer, with an estimated risk of one cancer case per 10,000 CT scans, and the U.S. Environmental Protection Agency attributes 25% of all radiation in the United States to CT scanning.
"The benefit of identifying or excluding life-threatening injuries with a high sensitivity is an invaluable tool," wrote Dr. Hunter B. Moore and his colleagues at the University of Colorado at Denver, Aurora, in the Journal of Trauma and Acute Care Surgery. "However, application in the more radiosensitive pediatric population requires critical analysis."
Dr. Moore’s team found that the only clinically significant factor in determining the value of using CT scans was an abnormal Glasgow Coma Score (GCS). The GCS neurological scale rates patients from 3 to 14 on their level of consciousness; the highest score (14 on the original scale, 15 on the revised scale) refers to normal verbal, motor, and eye functioning. This study used the original scale, according to corresponding author Denis Bensard.
"Most concerning was that injured children imaged based on the mechanism of injury alone yielded no significant findings on CT imaging," the researchers wrote (J. Trauma Acute Care Surg. 2013;75:995-1001). "When anatomic or physiologic abnormalities were present, a serious CT finding was observed in more than 20% of the children imaged."
The researchers classified 174 patients, all meeting trauma team activation criteria at a Level 2 pediatric trauma center, into four groups to study the clinical value of CT scanning based on the children’s mechanism of injury. The patients, with a mean age of 7 years and a mean Injury Severity Score of 10, were admitted from January 2006 through December 2011.
The first group had normal GCS scores and normal vital signs and physical examinations. CT scanning for this group was considered to be done based on mechanism of injury alone. The second group had abnormal GCS scores but normal vital signs and physical exams. The third group had normal GCS scores but abnormal vital signs or exam findings. The fourth group had both abnormal GCS scores and abnormal findings in vital signs and/or exams.
Across all groups, motor vehicle collisions accounted for the most common injury causes, followed by being struck by autos as pedestrians, and falls. Positive CT scan findings included extra axial blood or parenchymal injury in the head; bony, vascular injury in the neck; great vessel injury in the chest; or solid organ or hollow visceral injury in the abdomen.
Of the 54 patients (82% of 66 children) in the group with normal exams, vital signs, and GCS scores who received CT scans, the patients were exposed to an average 17 mSv through an average 1.7 scans per child. The annual environmental dose limit for radiation is established at 1 mSv per year. "Remarkably, no patient imaged, based on [injury] mechanism alone, had a serious or life-threatening finding on CT scan," the researchers wrote.
All 25 patients in the group with abnormal GCS scores but normal exams and vital signs were scanned, with an average of 3.1 scans and 29 mSv of radiation per child. While 22% of the scans revealed a serious injury, the only surgeries required were one craniotomy and one nephrectomy.
Among the 57 children with normal GCS scores but abnormal exams or vital signs, 49 of them (86%) were scanned, with an average of two scans and 20 mSv per child. One splenectomy resulted from among the 23% of scans revealing significant findings.
All but 1 of the 26 children with abnormal GCS scores and abnormal vital signs or exams were scanned, with an average of 2.8 scans and 27 mSv per child. A quarter of the scans revealed significant findings, and two children required emergency craniotomies.
"We found that only one in four CT scans found a serious finding, but emergent operative interventions were required in less than 3% of injured children imaged," the researchers wrote. "Focused assessment with sonography for trauma [FAST] examination for the cohort was found to have a high specificity of 98%, but low sensitivity of 30%."
They determined the low sensitivity to result from the scans’ inability to identify injuries in solid organs without "detectable blood or retroperitoneal injury," though CT scans did appear valuable for identifying intra-abdominal hemorrhage. Abdominal CT scans were most likely to identify serious injuries when initial exams revealed anatomic or physiologic abnormalities, but chest scans had little to no utility.
The authors noted that current cancer risk estimates from CT scan radiation may be low because of the time it can take for cancers to manifest (up to 40 years) and the short time span (10 years) of the retrospective study that validated the 1 in 10,000 per CT scan risk. "Commentary on this article cautions that these preliminary data are similar to atomic bomb survivors, and the true incidence of cancer from CT scanning may be 10 times more after more time elapses following CT scans," they wrote.
The researchers did not use external funding. They reported no disclosures
Using computed tomographic imaging (CT scans) based only on a child’s method of injury in blunt trauma cases incurs more risks than benefits, according to a recent study.
The ionizing radiation from CT scanning has been linked to long-term risk of cancer, with an estimated risk of one cancer case per 10,000 CT scans, and the U.S. Environmental Protection Agency attributes 25% of all radiation in the United States to CT scanning.
"The benefit of identifying or excluding life-threatening injuries with a high sensitivity is an invaluable tool," wrote Dr. Hunter B. Moore and his colleagues at the University of Colorado at Denver, Aurora, in the Journal of Trauma and Acute Care Surgery. "However, application in the more radiosensitive pediatric population requires critical analysis."
Dr. Moore’s team found that the only clinically significant factor in determining the value of using CT scans was an abnormal Glasgow Coma Score (GCS). The GCS neurological scale rates patients from 3 to 14 on their level of consciousness; the highest score (14 on the original scale, 15 on the revised scale) refers to normal verbal, motor, and eye functioning. This study used the original scale, according to corresponding author Denis Bensard.
"Most concerning was that injured children imaged based on the mechanism of injury alone yielded no significant findings on CT imaging," the researchers wrote (J. Trauma Acute Care Surg. 2013;75:995-1001). "When anatomic or physiologic abnormalities were present, a serious CT finding was observed in more than 20% of the children imaged."
The researchers classified 174 patients, all meeting trauma team activation criteria at a Level 2 pediatric trauma center, into four groups to study the clinical value of CT scanning based on the children’s mechanism of injury. The patients, with a mean age of 7 years and a mean Injury Severity Score of 10, were admitted from January 2006 through December 2011.
The first group had normal GCS scores and normal vital signs and physical examinations. CT scanning for this group was considered to be done based on mechanism of injury alone. The second group had abnormal GCS scores but normal vital signs and physical exams. The third group had normal GCS scores but abnormal vital signs or exam findings. The fourth group had both abnormal GCS scores and abnormal findings in vital signs and/or exams.
Across all groups, motor vehicle collisions accounted for the most common injury causes, followed by being struck by autos as pedestrians, and falls. Positive CT scan findings included extra axial blood or parenchymal injury in the head; bony, vascular injury in the neck; great vessel injury in the chest; or solid organ or hollow visceral injury in the abdomen.
Of the 54 patients (82% of 66 children) in the group with normal exams, vital signs, and GCS scores who received CT scans, the patients were exposed to an average 17 mSv through an average 1.7 scans per child. The annual environmental dose limit for radiation is established at 1 mSv per year. "Remarkably, no patient imaged, based on [injury] mechanism alone, had a serious or life-threatening finding on CT scan," the researchers wrote.
All 25 patients in the group with abnormal GCS scores but normal exams and vital signs were scanned, with an average of 3.1 scans and 29 mSv of radiation per child. While 22% of the scans revealed a serious injury, the only surgeries required were one craniotomy and one nephrectomy.
Among the 57 children with normal GCS scores but abnormal exams or vital signs, 49 of them (86%) were scanned, with an average of two scans and 20 mSv per child. One splenectomy resulted from among the 23% of scans revealing significant findings.
All but 1 of the 26 children with abnormal GCS scores and abnormal vital signs or exams were scanned, with an average of 2.8 scans and 27 mSv per child. A quarter of the scans revealed significant findings, and two children required emergency craniotomies.
"We found that only one in four CT scans found a serious finding, but emergent operative interventions were required in less than 3% of injured children imaged," the researchers wrote. "Focused assessment with sonography for trauma [FAST] examination for the cohort was found to have a high specificity of 98%, but low sensitivity of 30%."
They determined the low sensitivity to result from the scans’ inability to identify injuries in solid organs without "detectable blood or retroperitoneal injury," though CT scans did appear valuable for identifying intra-abdominal hemorrhage. Abdominal CT scans were most likely to identify serious injuries when initial exams revealed anatomic or physiologic abnormalities, but chest scans had little to no utility.
The authors noted that current cancer risk estimates from CT scan radiation may be low because of the time it can take for cancers to manifest (up to 40 years) and the short time span (10 years) of the retrospective study that validated the 1 in 10,000 per CT scan risk. "Commentary on this article cautions that these preliminary data are similar to atomic bomb survivors, and the true incidence of cancer from CT scanning may be 10 times more after more time elapses following CT scans," they wrote.
The researchers did not use external funding. They reported no disclosures
Using computed tomographic imaging (CT scans) based only on a child’s method of injury in blunt trauma cases incurs more risks than benefits, according to a recent study.
The ionizing radiation from CT scanning has been linked to long-term risk of cancer, with an estimated risk of one cancer case per 10,000 CT scans, and the U.S. Environmental Protection Agency attributes 25% of all radiation in the United States to CT scanning.
"The benefit of identifying or excluding life-threatening injuries with a high sensitivity is an invaluable tool," wrote Dr. Hunter B. Moore and his colleagues at the University of Colorado at Denver, Aurora, in the Journal of Trauma and Acute Care Surgery. "However, application in the more radiosensitive pediatric population requires critical analysis."
Dr. Moore’s team found that the only clinically significant factor in determining the value of using CT scans was an abnormal Glasgow Coma Score (GCS). The GCS neurological scale rates patients from 3 to 14 on their level of consciousness; the highest score (14 on the original scale, 15 on the revised scale) refers to normal verbal, motor, and eye functioning. This study used the original scale, according to corresponding author Denis Bensard.
"Most concerning was that injured children imaged based on the mechanism of injury alone yielded no significant findings on CT imaging," the researchers wrote (J. Trauma Acute Care Surg. 2013;75:995-1001). "When anatomic or physiologic abnormalities were present, a serious CT finding was observed in more than 20% of the children imaged."
The researchers classified 174 patients, all meeting trauma team activation criteria at a Level 2 pediatric trauma center, into four groups to study the clinical value of CT scanning based on the children’s mechanism of injury. The patients, with a mean age of 7 years and a mean Injury Severity Score of 10, were admitted from January 2006 through December 2011.
The first group had normal GCS scores and normal vital signs and physical examinations. CT scanning for this group was considered to be done based on mechanism of injury alone. The second group had abnormal GCS scores but normal vital signs and physical exams. The third group had normal GCS scores but abnormal vital signs or exam findings. The fourth group had both abnormal GCS scores and abnormal findings in vital signs and/or exams.
Across all groups, motor vehicle collisions accounted for the most common injury causes, followed by being struck by autos as pedestrians, and falls. Positive CT scan findings included extra axial blood or parenchymal injury in the head; bony, vascular injury in the neck; great vessel injury in the chest; or solid organ or hollow visceral injury in the abdomen.
Of the 54 patients (82% of 66 children) in the group with normal exams, vital signs, and GCS scores who received CT scans, the patients were exposed to an average 17 mSv through an average 1.7 scans per child. The annual environmental dose limit for radiation is established at 1 mSv per year. "Remarkably, no patient imaged, based on [injury] mechanism alone, had a serious or life-threatening finding on CT scan," the researchers wrote.
All 25 patients in the group with abnormal GCS scores but normal exams and vital signs were scanned, with an average of 3.1 scans and 29 mSv of radiation per child. While 22% of the scans revealed a serious injury, the only surgeries required were one craniotomy and one nephrectomy.
Among the 57 children with normal GCS scores but abnormal exams or vital signs, 49 of them (86%) were scanned, with an average of two scans and 20 mSv per child. One splenectomy resulted from among the 23% of scans revealing significant findings.
All but 1 of the 26 children with abnormal GCS scores and abnormal vital signs or exams were scanned, with an average of 2.8 scans and 27 mSv per child. A quarter of the scans revealed significant findings, and two children required emergency craniotomies.
"We found that only one in four CT scans found a serious finding, but emergent operative interventions were required in less than 3% of injured children imaged," the researchers wrote. "Focused assessment with sonography for trauma [FAST] examination for the cohort was found to have a high specificity of 98%, but low sensitivity of 30%."
They determined the low sensitivity to result from the scans’ inability to identify injuries in solid organs without "detectable blood or retroperitoneal injury," though CT scans did appear valuable for identifying intra-abdominal hemorrhage. Abdominal CT scans were most likely to identify serious injuries when initial exams revealed anatomic or physiologic abnormalities, but chest scans had little to no utility.
The authors noted that current cancer risk estimates from CT scan radiation may be low because of the time it can take for cancers to manifest (up to 40 years) and the short time span (10 years) of the retrospective study that validated the 1 in 10,000 per CT scan risk. "Commentary on this article cautions that these preliminary data are similar to atomic bomb survivors, and the true incidence of cancer from CT scanning may be 10 times more after more time elapses following CT scans," they wrote.
The researchers did not use external funding. They reported no disclosures
FROM THE JOURNAL OF TRAUMA AND ACUTE CARE SURGERY
Major finding: A Glasgow Coma Score (GCS) of less than 14 (original scale; less than 15 on revised scale) was the only clinically significant variable for identifying positive findings with CT scans in pediatric blunt trauma patients.
Data source: The findings are based on an analysis of the cases (CT scans received, significant findings, surgeries, and radiation exposure) of 174 children who met trauma team activation criteria at a Level 2 pediatric trauma center between January 2006 and December 2011.
Disclosures: The researchers did not use external funding. They reported no disclosures.
Epigastric abdominal pain
What is the diagnosis?
Is additional imaging necessary, and if so, why?
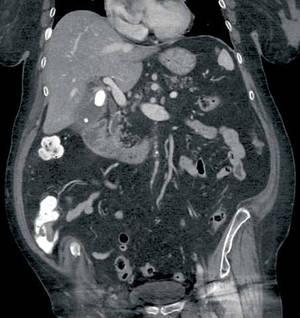
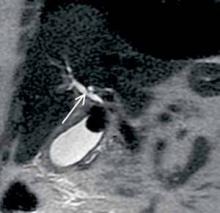
An 83-year-old woman with history of hypertension, hyperlipidemia, coronary artery disease, and gastroesophageal reflux disease presents to the ED with epigastric abdominal pain. Contrast-enhanced computed tomography (CT) was obtained; a coronal reformatted image is shown above (Figure 1).
Answer
Mirizzi syndrome, partial obstruction of the common hepatic duct by a large gallstone at the gallbladder neck (as in this patient) or within the cystic duct, was first described by Argentinian surgeon Pablo Mirizzi in the mid-20th century. Although Mirizzi syndrome occurs in less than 3% of all patients with cholelithiasis, the inflammation and fibrosis associated with this condition can lead to cholecystobiliary or choledochobiliary fistula formation. Preoperatively unrecognized cases are associated with increased morbidity and mortality compared with uncomplicated cholelithiasis. Cholecystectomy in patients with Mirizzi syndrome is often performed via an open rather than laparoscopic approach, and biliary fistula may require surgical repair with choledochoduodenostomy. 1-3
The recommended initial imaging modality for patients with clinical signs of biliary obstruction (eg, jaundice and/or intermittent cholangitis) is right upper quadrant ultrasound based on its greater sensitivity for gallstone detection compared to CT and wider availability than magnetic resonance imaging (MRI). If ultrasound demonstrates central biliary ductal prominence with calculi at the gallbladder neck and/or cystic duct, further noninvasive imaging with MRI/magnetic resonance cholangiopancreatography (MRCP) should be considered to evaluate for Mirizzi syndrome, as well as to exclude more distal common bile duct obstruction/stricture that may not be visible sonographically.
Based on this patient’s nonspecific epigastric pain, CT imaging was evaluated before ultrasound. While the CT findings and elevated liver function tests suggested Mirizzi syndrome, MRCP was subsequently performed to better characterize the partial biliary obstruction, evaluate for biliary fistula, and exclude noncalcified stones within the biliary tree.
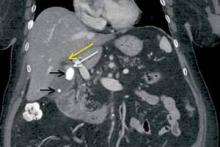
The coronal CT image demonstrates two calcified stones within the gallbladder—a large stone more superiorly at the gallbladder neck and a smaller stone more inferiorly at the gallbladder fundus (black arrows, Figure 2)—with diffuse gallbladder wall thickening/ edema, suspicious for acute cholecystitis. Focal narrowing of the common hepatic duct (white arrow, Figure 2) was caused by the larger stone as it crosses the gallbladder neck with postobstructive dilatation of the more proximal/superior aspect of the common hepatic duct (yellow arrow, Figure 2).
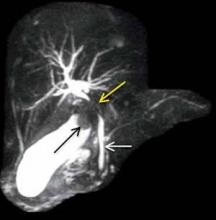
Maximum intensity projection from MRCP reveals the large gallstone at the gallbladder neck as a filling defect (black arrow, Figure 3), with focal compression of the adjacent common hepatic duct (yellow arrow, Figure 3). The more downstream common bile duct (white arrow, Figure 3) is of normal caliber without filling defect. Coronal single-shot fast spin echo image from the same examination again demonstrates narrowing of the common hepatic duct with associated upstream prominence of the central hepatic bile ducts (white arrow, Figure 4).
Given her advanced age, comorbidities, and lowimaging suspicion for biliary fistula, the patient in this case underwent successful laparoscopic, rather than open, cholecystectomy without complication.
- Ahlawat SK, Singhania R, Al-Kawas FH. Mirizzi syndrome. Curr Treat Options Gastroenterol. 2007;10(2):102-110.
- Waisberg J, Corona A, de Abreu IW, Farah JF, Lupinacci RA, Goffi FS. Benign obstruction of the common hepatic duct (Mirizzi syndrome): diagnosis and operative management. Arq Gastroenterol. 2005;42(1):13-18.
- Sanal HT, Kocaoglu M, Bulakbasi N. Mirizzi syndrome in an icteric patient: MRI and MRCP findings. JBR-BTR. 2007;90(6):545.
What is the diagnosis?
Is additional imaging necessary, and if so, why?
An 83-year-old woman with history of hypertension, hyperlipidemia, coronary artery disease, and gastroesophageal reflux disease presents to the ED with epigastric abdominal pain. Contrast-enhanced computed tomography (CT) was obtained; a coronal reformatted image is shown above (Figure 1).
Answer
Mirizzi syndrome, partial obstruction of the common hepatic duct by a large gallstone at the gallbladder neck (as in this patient) or within the cystic duct, was first described by Argentinian surgeon Pablo Mirizzi in the mid-20th century. Although Mirizzi syndrome occurs in less than 3% of all patients with cholelithiasis, the inflammation and fibrosis associated with this condition can lead to cholecystobiliary or choledochobiliary fistula formation. Preoperatively unrecognized cases are associated with increased morbidity and mortality compared with uncomplicated cholelithiasis. Cholecystectomy in patients with Mirizzi syndrome is often performed via an open rather than laparoscopic approach, and biliary fistula may require surgical repair with choledochoduodenostomy. 1-3
The recommended initial imaging modality for patients with clinical signs of biliary obstruction (eg, jaundice and/or intermittent cholangitis) is right upper quadrant ultrasound based on its greater sensitivity for gallstone detection compared to CT and wider availability than magnetic resonance imaging (MRI). If ultrasound demonstrates central biliary ductal prominence with calculi at the gallbladder neck and/or cystic duct, further noninvasive imaging with MRI/magnetic resonance cholangiopancreatography (MRCP) should be considered to evaluate for Mirizzi syndrome, as well as to exclude more distal common bile duct obstruction/stricture that may not be visible sonographically.
Based on this patient’s nonspecific epigastric pain, CT imaging was evaluated before ultrasound. While the CT findings and elevated liver function tests suggested Mirizzi syndrome, MRCP was subsequently performed to better characterize the partial biliary obstruction, evaluate for biliary fistula, and exclude noncalcified stones within the biliary tree.
The coronal CT image demonstrates two calcified stones within the gallbladder—a large stone more superiorly at the gallbladder neck and a smaller stone more inferiorly at the gallbladder fundus (black arrows, Figure 2)—with diffuse gallbladder wall thickening/ edema, suspicious for acute cholecystitis. Focal narrowing of the common hepatic duct (white arrow, Figure 2) was caused by the larger stone as it crosses the gallbladder neck with postobstructive dilatation of the more proximal/superior aspect of the common hepatic duct (yellow arrow, Figure 2).
Maximum intensity projection from MRCP reveals the large gallstone at the gallbladder neck as a filling defect (black arrow, Figure 3), with focal compression of the adjacent common hepatic duct (yellow arrow, Figure 3). The more downstream common bile duct (white arrow, Figure 3) is of normal caliber without filling defect. Coronal single-shot fast spin echo image from the same examination again demonstrates narrowing of the common hepatic duct with associated upstream prominence of the central hepatic bile ducts (white arrow, Figure 4).
Given her advanced age, comorbidities, and lowimaging suspicion for biliary fistula, the patient in this case underwent successful laparoscopic, rather than open, cholecystectomy without complication.
What is the diagnosis?
Is additional imaging necessary, and if so, why?
An 83-year-old woman with history of hypertension, hyperlipidemia, coronary artery disease, and gastroesophageal reflux disease presents to the ED with epigastric abdominal pain. Contrast-enhanced computed tomography (CT) was obtained; a coronal reformatted image is shown above (Figure 1).
Answer
Mirizzi syndrome, partial obstruction of the common hepatic duct by a large gallstone at the gallbladder neck (as in this patient) or within the cystic duct, was first described by Argentinian surgeon Pablo Mirizzi in the mid-20th century. Although Mirizzi syndrome occurs in less than 3% of all patients with cholelithiasis, the inflammation and fibrosis associated with this condition can lead to cholecystobiliary or choledochobiliary fistula formation. Preoperatively unrecognized cases are associated with increased morbidity and mortality compared with uncomplicated cholelithiasis. Cholecystectomy in patients with Mirizzi syndrome is often performed via an open rather than laparoscopic approach, and biliary fistula may require surgical repair with choledochoduodenostomy. 1-3
The recommended initial imaging modality for patients with clinical signs of biliary obstruction (eg, jaundice and/or intermittent cholangitis) is right upper quadrant ultrasound based on its greater sensitivity for gallstone detection compared to CT and wider availability than magnetic resonance imaging (MRI). If ultrasound demonstrates central biliary ductal prominence with calculi at the gallbladder neck and/or cystic duct, further noninvasive imaging with MRI/magnetic resonance cholangiopancreatography (MRCP) should be considered to evaluate for Mirizzi syndrome, as well as to exclude more distal common bile duct obstruction/stricture that may not be visible sonographically.
Based on this patient’s nonspecific epigastric pain, CT imaging was evaluated before ultrasound. While the CT findings and elevated liver function tests suggested Mirizzi syndrome, MRCP was subsequently performed to better characterize the partial biliary obstruction, evaluate for biliary fistula, and exclude noncalcified stones within the biliary tree.
The coronal CT image demonstrates two calcified stones within the gallbladder—a large stone more superiorly at the gallbladder neck and a smaller stone more inferiorly at the gallbladder fundus (black arrows, Figure 2)—with diffuse gallbladder wall thickening/ edema, suspicious for acute cholecystitis. Focal narrowing of the common hepatic duct (white arrow, Figure 2) was caused by the larger stone as it crosses the gallbladder neck with postobstructive dilatation of the more proximal/superior aspect of the common hepatic duct (yellow arrow, Figure 2).
Maximum intensity projection from MRCP reveals the large gallstone at the gallbladder neck as a filling defect (black arrow, Figure 3), with focal compression of the adjacent common hepatic duct (yellow arrow, Figure 3). The more downstream common bile duct (white arrow, Figure 3) is of normal caliber without filling defect. Coronal single-shot fast spin echo image from the same examination again demonstrates narrowing of the common hepatic duct with associated upstream prominence of the central hepatic bile ducts (white arrow, Figure 4).
Given her advanced age, comorbidities, and lowimaging suspicion for biliary fistula, the patient in this case underwent successful laparoscopic, rather than open, cholecystectomy without complication.
- Ahlawat SK, Singhania R, Al-Kawas FH. Mirizzi syndrome. Curr Treat Options Gastroenterol. 2007;10(2):102-110.
- Waisberg J, Corona A, de Abreu IW, Farah JF, Lupinacci RA, Goffi FS. Benign obstruction of the common hepatic duct (Mirizzi syndrome): diagnosis and operative management. Arq Gastroenterol. 2005;42(1):13-18.
- Sanal HT, Kocaoglu M, Bulakbasi N. Mirizzi syndrome in an icteric patient: MRI and MRCP findings. JBR-BTR. 2007;90(6):545.
- Ahlawat SK, Singhania R, Al-Kawas FH. Mirizzi syndrome. Curr Treat Options Gastroenterol. 2007;10(2):102-110.
- Waisberg J, Corona A, de Abreu IW, Farah JF, Lupinacci RA, Goffi FS. Benign obstruction of the common hepatic duct (Mirizzi syndrome): diagnosis and operative management. Arq Gastroenterol. 2005;42(1):13-18.
- Sanal HT, Kocaoglu M, Bulakbasi N. Mirizzi syndrome in an icteric patient: MRI and MRCP findings. JBR-BTR. 2007;90(6):545.
CT, CEA alone or combined better than nothing to detect colon cancer recurrence
Either intensive CT imaging or frequent testing of blood levels of carcinoembryonic antigen is more likely to detect a colon cancer recurrence than is minimal follow-up, according to a report published online Jan. 14 in JAMA.
However, combining the two monitoring strategies doesn’t add to the effectiveness of either one at detecting recurrences, said Dr. John N. Primrose of the University of Southampton (England) and his associates in the FACS (Follow-Up After Colorectal Surgery) randomized clinical trial.
Several previous trials have compared different follow-up strategies for patients who have undergone potentially curative surgery – monitoring that is done in the hope that survival will be increased if metastatic disease is identified and treated before it becomes symptomatic. But these studies were of "modest" quality and failed to show any significant treatment effect on disease-specific survival, prompting calls for higher-quality trials.
The FACS, commissioned by the U.K. National Institute for Health Research, was undertaken to assess the two follow-up strategies that are available and affordable and have the greatest potential to detect isolated metastatic recurrence at an early, surgically treatable stage: serial CEA measurement and serial CT imaging of the chest, abdomen, and pelvis.
The FACS involved 1,202 patients already treated for primary colon cancer who had no residual disease and microscopically clear margins. They were scheduled to be followed for 5 years at 39 medical centers in the United Kingdom.
These participants were randomly assigned to any one of four study groups:
• CEA follow-up, with measurement of blood CEA every 3 months for 2 years, then every 6 months for the remaining 3 years, and with a single CT scan at 18 months of the chest, abdomen, and pelvis (302 patients).
• CT follow-up, with CT scans of the same regions every 6 months for 2 years, then annually for the remaining 3 years (302 patients).
• Combined CEA and CT follow-up (303 patients).
• Minimal follow-up (the control group), with only a single CT scan at 12-18 months if the hospital clinician requested one at study entry (304 patients).
Follow-up colonoscopy was provided to all the patients because it is standard treatment in such cases.
Colon cancer recurred in 199 patients.
The primary outcome measure was surgery for colon cancer recurrence after a minimum of 3 years, to help distinguish true recurrence from residual disease that was missed at the initial surgery. (Neither overall nor cancer-specific mortality could be used as a primary outcome measure because the sample size was too small and the follow-up period too short for sufficient power to estimate survival accurately.)
This rate was higher with all of the interventions, compared with minimal follow-up. Surgery for recurrence was performed in 6.7% of the CEA-only group, 8% of the CT-only group, and 6.6% of the combined CEA plus CT group, compared with only 2.3% of the control group, according to the investigators (JAMA 2014;311:263-70 [doi:10.1001/jama.2013.285718]).
These rates for the three intensive interventions were not significantly different from each other, indicating that CEA and CT follow-up yielded comparable early detection of recurrences. No additive effect was seen for combining the two strategies.
The results of a per-protocol analysis, which excluded the 308 patients who did not adhere strictly to the study protocol, were consistent with those of the main analysis.
The study findings indicate that only 12-20 patients need to be followed up using either CEA or CT to identify one potentially curable recurrence, Dr. Primrose and his associates said.
They added that they are continuing follow-up so they can more accurately assess disease-specific mortality. "If there is a survival advantage to any [follow-up] strategy, it is likely to be small," they noted.
The FACS trial was funded by the U.K. National Institute for Health Research Health Technology Assessment program. No financial conflicts of interest were reported.
Either intensive CT imaging or frequent testing of blood levels of carcinoembryonic antigen is more likely to detect a colon cancer recurrence than is minimal follow-up, according to a report published online Jan. 14 in JAMA.
However, combining the two monitoring strategies doesn’t add to the effectiveness of either one at detecting recurrences, said Dr. John N. Primrose of the University of Southampton (England) and his associates in the FACS (Follow-Up After Colorectal Surgery) randomized clinical trial.
Several previous trials have compared different follow-up strategies for patients who have undergone potentially curative surgery – monitoring that is done in the hope that survival will be increased if metastatic disease is identified and treated before it becomes symptomatic. But these studies were of "modest" quality and failed to show any significant treatment effect on disease-specific survival, prompting calls for higher-quality trials.
The FACS, commissioned by the U.K. National Institute for Health Research, was undertaken to assess the two follow-up strategies that are available and affordable and have the greatest potential to detect isolated metastatic recurrence at an early, surgically treatable stage: serial CEA measurement and serial CT imaging of the chest, abdomen, and pelvis.
The FACS involved 1,202 patients already treated for primary colon cancer who had no residual disease and microscopically clear margins. They were scheduled to be followed for 5 years at 39 medical centers in the United Kingdom.
These participants were randomly assigned to any one of four study groups:
• CEA follow-up, with measurement of blood CEA every 3 months for 2 years, then every 6 months for the remaining 3 years, and with a single CT scan at 18 months of the chest, abdomen, and pelvis (302 patients).
• CT follow-up, with CT scans of the same regions every 6 months for 2 years, then annually for the remaining 3 years (302 patients).
• Combined CEA and CT follow-up (303 patients).
• Minimal follow-up (the control group), with only a single CT scan at 12-18 months if the hospital clinician requested one at study entry (304 patients).
Follow-up colonoscopy was provided to all the patients because it is standard treatment in such cases.
Colon cancer recurred in 199 patients.
The primary outcome measure was surgery for colon cancer recurrence after a minimum of 3 years, to help distinguish true recurrence from residual disease that was missed at the initial surgery. (Neither overall nor cancer-specific mortality could be used as a primary outcome measure because the sample size was too small and the follow-up period too short for sufficient power to estimate survival accurately.)
This rate was higher with all of the interventions, compared with minimal follow-up. Surgery for recurrence was performed in 6.7% of the CEA-only group, 8% of the CT-only group, and 6.6% of the combined CEA plus CT group, compared with only 2.3% of the control group, according to the investigators (JAMA 2014;311:263-70 [doi:10.1001/jama.2013.285718]).
These rates for the three intensive interventions were not significantly different from each other, indicating that CEA and CT follow-up yielded comparable early detection of recurrences. No additive effect was seen for combining the two strategies.
The results of a per-protocol analysis, which excluded the 308 patients who did not adhere strictly to the study protocol, were consistent with those of the main analysis.
The study findings indicate that only 12-20 patients need to be followed up using either CEA or CT to identify one potentially curable recurrence, Dr. Primrose and his associates said.
They added that they are continuing follow-up so they can more accurately assess disease-specific mortality. "If there is a survival advantage to any [follow-up] strategy, it is likely to be small," they noted.
The FACS trial was funded by the U.K. National Institute for Health Research Health Technology Assessment program. No financial conflicts of interest were reported.
Either intensive CT imaging or frequent testing of blood levels of carcinoembryonic antigen is more likely to detect a colon cancer recurrence than is minimal follow-up, according to a report published online Jan. 14 in JAMA.
However, combining the two monitoring strategies doesn’t add to the effectiveness of either one at detecting recurrences, said Dr. John N. Primrose of the University of Southampton (England) and his associates in the FACS (Follow-Up After Colorectal Surgery) randomized clinical trial.
Several previous trials have compared different follow-up strategies for patients who have undergone potentially curative surgery – monitoring that is done in the hope that survival will be increased if metastatic disease is identified and treated before it becomes symptomatic. But these studies were of "modest" quality and failed to show any significant treatment effect on disease-specific survival, prompting calls for higher-quality trials.
The FACS, commissioned by the U.K. National Institute for Health Research, was undertaken to assess the two follow-up strategies that are available and affordable and have the greatest potential to detect isolated metastatic recurrence at an early, surgically treatable stage: serial CEA measurement and serial CT imaging of the chest, abdomen, and pelvis.
The FACS involved 1,202 patients already treated for primary colon cancer who had no residual disease and microscopically clear margins. They were scheduled to be followed for 5 years at 39 medical centers in the United Kingdom.
These participants were randomly assigned to any one of four study groups:
• CEA follow-up, with measurement of blood CEA every 3 months for 2 years, then every 6 months for the remaining 3 years, and with a single CT scan at 18 months of the chest, abdomen, and pelvis (302 patients).
• CT follow-up, with CT scans of the same regions every 6 months for 2 years, then annually for the remaining 3 years (302 patients).
• Combined CEA and CT follow-up (303 patients).
• Minimal follow-up (the control group), with only a single CT scan at 12-18 months if the hospital clinician requested one at study entry (304 patients).
Follow-up colonoscopy was provided to all the patients because it is standard treatment in such cases.
Colon cancer recurred in 199 patients.
The primary outcome measure was surgery for colon cancer recurrence after a minimum of 3 years, to help distinguish true recurrence from residual disease that was missed at the initial surgery. (Neither overall nor cancer-specific mortality could be used as a primary outcome measure because the sample size was too small and the follow-up period too short for sufficient power to estimate survival accurately.)
This rate was higher with all of the interventions, compared with minimal follow-up. Surgery for recurrence was performed in 6.7% of the CEA-only group, 8% of the CT-only group, and 6.6% of the combined CEA plus CT group, compared with only 2.3% of the control group, according to the investigators (JAMA 2014;311:263-70 [doi:10.1001/jama.2013.285718]).
These rates for the three intensive interventions were not significantly different from each other, indicating that CEA and CT follow-up yielded comparable early detection of recurrences. No additive effect was seen for combining the two strategies.
The results of a per-protocol analysis, which excluded the 308 patients who did not adhere strictly to the study protocol, were consistent with those of the main analysis.
The study findings indicate that only 12-20 patients need to be followed up using either CEA or CT to identify one potentially curable recurrence, Dr. Primrose and his associates said.
They added that they are continuing follow-up so they can more accurately assess disease-specific mortality. "If there is a survival advantage to any [follow-up] strategy, it is likely to be small," they noted.
The FACS trial was funded by the U.K. National Institute for Health Research Health Technology Assessment program. No financial conflicts of interest were reported.
FROM JAMA
Major finding: Colon cancer recurrences were detected (and operated upon) in 6.7% of the CEA-only group, 8% of the CT-only group, and 6.6% of the combined CEA plus CT group, compared with only 2.3% of the control group.
Data source: A multicenter prospective randomized clinical trial comparing the rates of surgery for colon cancer recurrences among 1,202 patients followed for 5 years using serial CEA testing, serial CT exams, both of these strategies together, or minimal follow-up.
Disclosures: The FACS trial was funded by the U.K. National Institute for Health Research Health Technology Assessment program. No financial conflicts of interest were reported.
Cardiac CT angiography feasible at ultralow radiation doses
CHICAGO – Coronary computed tomography angiography with diagnostic image quality is feasible at an ultralow radiation dose of 0.2 millisievert using model-based iterative reconstruction.
This represents roughly an 80% reduction in radiation dose compared with standard coronary CT angiography, Dr. Julia Stehli said at the annual meeting of the Radiological Society of North America.
Increasing concerns about radiation exposure have prompted the use of prospective ECG triggering to reduce radiation doses from 20 mSv to 2 mSv or less. Several vendors also have developed new raw-data–based iterative reconstruction algorithms to further reduce radiation doses, but the trade-off can be increased image noise.
The model-based iterative reconstruction (MIBR) algorithm (GE Healthcare), however, has shown promising results for noise reduction, said Dr. Stehli of University Hospital Zurich. The technology, known as Veo, is already in use in the United States, Europe, and Asia for abdominal CT scans but is not yet commercially available for cardiac scans because of the added complexity of ECG triggering.
Dr. Stehli reported on the hospital’s first clinical experience with MIBR in 25 consecutive prospectively enrolled patients with suspected coronary artery disease who underwent standard low-dose coronary CT angiography (CCTA) and same-day ultralow-dose CCTA on a 64-slice CT scanner with prospective ECG triggering. Tube voltage and current were adapted to body mass index, which covered a wide range from 18.4 kg/m2 to 40.2 kg/m2. Contrast media volume and flow rate were adapted to body surface area. Intravenous beta-blockers were used prior to CCTA in 20 patients.
Standard CCTA was reconstructed using 30% of adaptive statistical iterative reconstruction (ASIR) according to usual hospital practice, while the ultralow-dose images were sent to the vendor for reconstruction with MIBR.
The effective radiation dose was 1.3 mSv with standard CCTA and 0.2 mSv in the ultralow-dose CCTA group (P less than .001), which is in the range reported for a postero-anterior and lateral chest X-ray, Dr. Stehli said.
A total of 100 vessels and 330 coronary artery segments were semiquantitatively assessed by two blinded, independent readers using a 4-point Likert scale, with 1 being nondiagnostic, 2 good, 3 adequate, and 4 excellent. The Kappa value for interobserver agreement of image quality was 0.8.
The average image quality score per segment was 3.3 with standard CCTA vs. 3.4 with ultralow-dose MBIR (P less than .05), she said.
Diagnostic image quality (score 2-4) was found in 319 segments (97%) and 317 segments (96%), respectively.
"These numbers are quite revolutionary," session comoderator Dr. Konstantin Nikolaou, professor of radiology at the University of Munich, said in an interview. "We’ve heard about 1.0 [mSv], so 0.2 [mSv] is great."
Still, more details are needed on exactly how the protocol works and the need to send images to the vendor for MBIR reconstruction, he said.
During a discussion of the results, Dr. Stehli said that reconstruction by the vendor typically took about 15 minutes, but Dr. Nikolau said that "it’s hard to say if that is feasible in routine clinical practice."
The ultimate test for the ultralow-dose protocol will be the clinical outcomes data, expected to be reported in 2014.
"If that proves to be robust and works in many patients and rates a good diagnostic accuracy, it would be great," Dr. Nikolau said.
The investigators would not release details on the clinical outcomes but said sensitivity and specificity for the new protocol are good.
"We believe this will have clinical applications in the near future," Dr. Stehli said in an interview.
Most patients in the study presented with chest pain (72%), and 56% were smokers, 44% had arterial hypertension, and 36% had a family history of cardiovascular disease. Their mean age was 58 years.
Dr. Stehli and her associates reported having no financial disclosures.
CHICAGO – Coronary computed tomography angiography with diagnostic image quality is feasible at an ultralow radiation dose of 0.2 millisievert using model-based iterative reconstruction.
This represents roughly an 80% reduction in radiation dose compared with standard coronary CT angiography, Dr. Julia Stehli said at the annual meeting of the Radiological Society of North America.
Increasing concerns about radiation exposure have prompted the use of prospective ECG triggering to reduce radiation doses from 20 mSv to 2 mSv or less. Several vendors also have developed new raw-data–based iterative reconstruction algorithms to further reduce radiation doses, but the trade-off can be increased image noise.
The model-based iterative reconstruction (MIBR) algorithm (GE Healthcare), however, has shown promising results for noise reduction, said Dr. Stehli of University Hospital Zurich. The technology, known as Veo, is already in use in the United States, Europe, and Asia for abdominal CT scans but is not yet commercially available for cardiac scans because of the added complexity of ECG triggering.
Dr. Stehli reported on the hospital’s first clinical experience with MIBR in 25 consecutive prospectively enrolled patients with suspected coronary artery disease who underwent standard low-dose coronary CT angiography (CCTA) and same-day ultralow-dose CCTA on a 64-slice CT scanner with prospective ECG triggering. Tube voltage and current were adapted to body mass index, which covered a wide range from 18.4 kg/m2 to 40.2 kg/m2. Contrast media volume and flow rate were adapted to body surface area. Intravenous beta-blockers were used prior to CCTA in 20 patients.
Standard CCTA was reconstructed using 30% of adaptive statistical iterative reconstruction (ASIR) according to usual hospital practice, while the ultralow-dose images were sent to the vendor for reconstruction with MIBR.
The effective radiation dose was 1.3 mSv with standard CCTA and 0.2 mSv in the ultralow-dose CCTA group (P less than .001), which is in the range reported for a postero-anterior and lateral chest X-ray, Dr. Stehli said.
A total of 100 vessels and 330 coronary artery segments were semiquantitatively assessed by two blinded, independent readers using a 4-point Likert scale, with 1 being nondiagnostic, 2 good, 3 adequate, and 4 excellent. The Kappa value for interobserver agreement of image quality was 0.8.
The average image quality score per segment was 3.3 with standard CCTA vs. 3.4 with ultralow-dose MBIR (P less than .05), she said.
Diagnostic image quality (score 2-4) was found in 319 segments (97%) and 317 segments (96%), respectively.
"These numbers are quite revolutionary," session comoderator Dr. Konstantin Nikolaou, professor of radiology at the University of Munich, said in an interview. "We’ve heard about 1.0 [mSv], so 0.2 [mSv] is great."
Still, more details are needed on exactly how the protocol works and the need to send images to the vendor for MBIR reconstruction, he said.
During a discussion of the results, Dr. Stehli said that reconstruction by the vendor typically took about 15 minutes, but Dr. Nikolau said that "it’s hard to say if that is feasible in routine clinical practice."
The ultimate test for the ultralow-dose protocol will be the clinical outcomes data, expected to be reported in 2014.
"If that proves to be robust and works in many patients and rates a good diagnostic accuracy, it would be great," Dr. Nikolau said.
The investigators would not release details on the clinical outcomes but said sensitivity and specificity for the new protocol are good.
"We believe this will have clinical applications in the near future," Dr. Stehli said in an interview.
Most patients in the study presented with chest pain (72%), and 56% were smokers, 44% had arterial hypertension, and 36% had a family history of cardiovascular disease. Their mean age was 58 years.
Dr. Stehli and her associates reported having no financial disclosures.
CHICAGO – Coronary computed tomography angiography with diagnostic image quality is feasible at an ultralow radiation dose of 0.2 millisievert using model-based iterative reconstruction.
This represents roughly an 80% reduction in radiation dose compared with standard coronary CT angiography, Dr. Julia Stehli said at the annual meeting of the Radiological Society of North America.
Increasing concerns about radiation exposure have prompted the use of prospective ECG triggering to reduce radiation doses from 20 mSv to 2 mSv or less. Several vendors also have developed new raw-data–based iterative reconstruction algorithms to further reduce radiation doses, but the trade-off can be increased image noise.
The model-based iterative reconstruction (MIBR) algorithm (GE Healthcare), however, has shown promising results for noise reduction, said Dr. Stehli of University Hospital Zurich. The technology, known as Veo, is already in use in the United States, Europe, and Asia for abdominal CT scans but is not yet commercially available for cardiac scans because of the added complexity of ECG triggering.
Dr. Stehli reported on the hospital’s first clinical experience with MIBR in 25 consecutive prospectively enrolled patients with suspected coronary artery disease who underwent standard low-dose coronary CT angiography (CCTA) and same-day ultralow-dose CCTA on a 64-slice CT scanner with prospective ECG triggering. Tube voltage and current were adapted to body mass index, which covered a wide range from 18.4 kg/m2 to 40.2 kg/m2. Contrast media volume and flow rate were adapted to body surface area. Intravenous beta-blockers were used prior to CCTA in 20 patients.
Standard CCTA was reconstructed using 30% of adaptive statistical iterative reconstruction (ASIR) according to usual hospital practice, while the ultralow-dose images were sent to the vendor for reconstruction with MIBR.
The effective radiation dose was 1.3 mSv with standard CCTA and 0.2 mSv in the ultralow-dose CCTA group (P less than .001), which is in the range reported for a postero-anterior and lateral chest X-ray, Dr. Stehli said.
A total of 100 vessels and 330 coronary artery segments were semiquantitatively assessed by two blinded, independent readers using a 4-point Likert scale, with 1 being nondiagnostic, 2 good, 3 adequate, and 4 excellent. The Kappa value for interobserver agreement of image quality was 0.8.
The average image quality score per segment was 3.3 with standard CCTA vs. 3.4 with ultralow-dose MBIR (P less than .05), she said.
Diagnostic image quality (score 2-4) was found in 319 segments (97%) and 317 segments (96%), respectively.
"These numbers are quite revolutionary," session comoderator Dr. Konstantin Nikolaou, professor of radiology at the University of Munich, said in an interview. "We’ve heard about 1.0 [mSv], so 0.2 [mSv] is great."
Still, more details are needed on exactly how the protocol works and the need to send images to the vendor for MBIR reconstruction, he said.
During a discussion of the results, Dr. Stehli said that reconstruction by the vendor typically took about 15 minutes, but Dr. Nikolau said that "it’s hard to say if that is feasible in routine clinical practice."
The ultimate test for the ultralow-dose protocol will be the clinical outcomes data, expected to be reported in 2014.
"If that proves to be robust and works in many patients and rates a good diagnostic accuracy, it would be great," Dr. Nikolau said.
The investigators would not release details on the clinical outcomes but said sensitivity and specificity for the new protocol are good.
"We believe this will have clinical applications in the near future," Dr. Stehli said in an interview.
Most patients in the study presented with chest pain (72%), and 56% were smokers, 44% had arterial hypertension, and 36% had a family history of cardiovascular disease. Their mean age was 58 years.
Dr. Stehli and her associates reported having no financial disclosures.
AT RSNA 2013
Major finding: The effective radiation dose was 1.3 mSv with standard CCTA and 0.2 mSv in the ultralow-dose CCTA group (P less than .001).
Data source: A prospective study of 25 patients with suspected coronary artery disease.
Disclosures: Dr. Stehli and her coauthors reported having no financial disclosures.
Cardiac stress-imaging in stable angina patients
No one knows whether patients with stable ischemic heart disease and moderate or severe inducible ischemia benefit from revascularization when added to optimal medical therapy. A major, federally-funded trial named ISCHEMIA is underway to answer this question.
Main results from ISCHEMIA are still several years off, but the study has already produced an interesting finding about current cardiology practice and the way that cardiac stress-imaging studies are ordered.
Based on first-year enrollment data from the ISCHEMIA trial, the vast majority of both U.S. and European patients currently referred for stress-imaging assessment of ischemia have little or no inducible ischemia, Dr. Judith S. Hochman, head of the study, said during a talk at the American Heart Association’s Scientific Sessions in November.
Stable angina patients enrolled into ISCHEMIA need to have at least moderate inducible ischemia, defined as involving at least 10% of the left ventricle, in a cardiac imaging study read by a core laboratory. Since the trial began in July 2012, fewer than 700 patients had been enrolled based on imaging studies from about 15,000 patients. In the United States, about 3% of imaged patients had moderate or severe induced ischemia; the other 97% of patients referred for assessment had mild or no induced ischemia. In Europe, the rate with moderate or severe induced ischemia was slightly higher at 5%.
This observation made Dr. Hochman, a New York University cardiologist, ask why the prevalence of moderate or severe ischemia is so low in patients referred for stress imaging. She also wondered which patients with stable angina are undergoing revascularization today if so few qualify with moderate or severe inducible ischemia.
Another surprising fact she highlighted is how cardiologists manage patients found to have moderate or severe inducible ischemia. "Most of us think that all these patients with moderate to severe inducible ischemia are referred for catheterization," but that’s not what study results showed. She cited U.S. data from the mid- and late 2000s documenting that about one-third to two-thirds of these patients undergo cardiac catheterization.
This finding shows that there is "clinical equipoise" on how to manage these patients, and the ISCHEMIA trial will address that issue.
But until the results arrive in 2020, will as many stress-imaging studies continue for patients with stable angina when so many referred patients turn out to be negative for more advanced coronary disease? And, as Dr. Tracy Y. Wang, a cardiologist at Duke University, Durham, N.C., asked after hearing about the equivocal use of catheterization for patients with worse inducible ischemia: Why do physicians order these tests if they don’t intend to catheterize patients found to have moderate-to-severe inducible ischemia?
On Twitter @mitchelzoler
No one knows whether patients with stable ischemic heart disease and moderate or severe inducible ischemia benefit from revascularization when added to optimal medical therapy. A major, federally-funded trial named ISCHEMIA is underway to answer this question.
Main results from ISCHEMIA are still several years off, but the study has already produced an interesting finding about current cardiology practice and the way that cardiac stress-imaging studies are ordered.
Based on first-year enrollment data from the ISCHEMIA trial, the vast majority of both U.S. and European patients currently referred for stress-imaging assessment of ischemia have little or no inducible ischemia, Dr. Judith S. Hochman, head of the study, said during a talk at the American Heart Association’s Scientific Sessions in November.
Stable angina patients enrolled into ISCHEMIA need to have at least moderate inducible ischemia, defined as involving at least 10% of the left ventricle, in a cardiac imaging study read by a core laboratory. Since the trial began in July 2012, fewer than 700 patients had been enrolled based on imaging studies from about 15,000 patients. In the United States, about 3% of imaged patients had moderate or severe induced ischemia; the other 97% of patients referred for assessment had mild or no induced ischemia. In Europe, the rate with moderate or severe induced ischemia was slightly higher at 5%.
This observation made Dr. Hochman, a New York University cardiologist, ask why the prevalence of moderate or severe ischemia is so low in patients referred for stress imaging. She also wondered which patients with stable angina are undergoing revascularization today if so few qualify with moderate or severe inducible ischemia.
Another surprising fact she highlighted is how cardiologists manage patients found to have moderate or severe inducible ischemia. "Most of us think that all these patients with moderate to severe inducible ischemia are referred for catheterization," but that’s not what study results showed. She cited U.S. data from the mid- and late 2000s documenting that about one-third to two-thirds of these patients undergo cardiac catheterization.
This finding shows that there is "clinical equipoise" on how to manage these patients, and the ISCHEMIA trial will address that issue.
But until the results arrive in 2020, will as many stress-imaging studies continue for patients with stable angina when so many referred patients turn out to be negative for more advanced coronary disease? And, as Dr. Tracy Y. Wang, a cardiologist at Duke University, Durham, N.C., asked after hearing about the equivocal use of catheterization for patients with worse inducible ischemia: Why do physicians order these tests if they don’t intend to catheterize patients found to have moderate-to-severe inducible ischemia?
On Twitter @mitchelzoler
No one knows whether patients with stable ischemic heart disease and moderate or severe inducible ischemia benefit from revascularization when added to optimal medical therapy. A major, federally-funded trial named ISCHEMIA is underway to answer this question.
Main results from ISCHEMIA are still several years off, but the study has already produced an interesting finding about current cardiology practice and the way that cardiac stress-imaging studies are ordered.
Based on first-year enrollment data from the ISCHEMIA trial, the vast majority of both U.S. and European patients currently referred for stress-imaging assessment of ischemia have little or no inducible ischemia, Dr. Judith S. Hochman, head of the study, said during a talk at the American Heart Association’s Scientific Sessions in November.
Stable angina patients enrolled into ISCHEMIA need to have at least moderate inducible ischemia, defined as involving at least 10% of the left ventricle, in a cardiac imaging study read by a core laboratory. Since the trial began in July 2012, fewer than 700 patients had been enrolled based on imaging studies from about 15,000 patients. In the United States, about 3% of imaged patients had moderate or severe induced ischemia; the other 97% of patients referred for assessment had mild or no induced ischemia. In Europe, the rate with moderate or severe induced ischemia was slightly higher at 5%.
This observation made Dr. Hochman, a New York University cardiologist, ask why the prevalence of moderate or severe ischemia is so low in patients referred for stress imaging. She also wondered which patients with stable angina are undergoing revascularization today if so few qualify with moderate or severe inducible ischemia.
Another surprising fact she highlighted is how cardiologists manage patients found to have moderate or severe inducible ischemia. "Most of us think that all these patients with moderate to severe inducible ischemia are referred for catheterization," but that’s not what study results showed. She cited U.S. data from the mid- and late 2000s documenting that about one-third to two-thirds of these patients undergo cardiac catheterization.
This finding shows that there is "clinical equipoise" on how to manage these patients, and the ISCHEMIA trial will address that issue.
But until the results arrive in 2020, will as many stress-imaging studies continue for patients with stable angina when so many referred patients turn out to be negative for more advanced coronary disease? And, as Dr. Tracy Y. Wang, a cardiologist at Duke University, Durham, N.C., asked after hearing about the equivocal use of catheterization for patients with worse inducible ischemia: Why do physicians order these tests if they don’t intend to catheterize patients found to have moderate-to-severe inducible ischemia?
On Twitter @mitchelzoler
Locked Knee Caused by Lateral Meniscal Capsular Disruption: Verification by Magnetic Resonance Imaging and Arthroscopy
Symptomatic Carpal Coalition: Scaphotrapezial Joint
Passive leg raise may predict fluid responsiveness in sepsis
SEATTLE – Septic patients are more likely to respond to fluid therapy if their velocity time integral – a Doppler ultrasound measurement of blood flow across the left ventricular outflow tract – increases by 15% or more with a passive single-leg raise, according to a preliminary, observational study of 32 patients at New York Methodist Hospital in Brooklyn.
A passive leg raise to 45 degrees simulates a 250- to 500-cc fluid bolus. "We have found that people who don’t respond with a VTI greater than 15% have higher repeat lactate levels. Instead of giving them 2 L [of fluid] and then reassessing, maybe they’re patients you want to start on pressors right away," Dr. Andrew Balk said at the annual meeting of the American College of Emergency Physicians.
Echocardiogram machines can automatically calculate VTI. The measurement, which Dr. Balk and his associates obtained from the apical five-chamber view, is a surrogate for, and can be used to calculate, cardiac output. Poor response to fluid challenge indicates that fluids are less likely to increase cardiac output and more likely to cause fluid overload, said Dr. Balk, associate director of the clinical ultrasound division at the hospital.
The patients’ mean age was 68 years, and those with valvular pathology and atrial fibrillation were excluded from the study.
The group’s mean baseline VTI was 22 cm (range, 15-29 cm), which leg raises raised to a mean of 26 cm (18-34 cm), an increase of about 18% (4%-36%). A subsequent 2-L normal saline challenge increased VTI to a mean of 33 cm.
The mean baseline lactate level was 3.2 mmol/L (1.2-5.2 mmol/L), and 2 mmol/L (1-3 mmol/L) after the 2-L challenge. The percent change in VTI correlated significantly with the percent change in serum lactate levels. "Below-average responsiveness to the initial small fluid bolus was associated with a higher repeat lactate value, ... which suggests an inverse relationship between a patient’s fluid responsiveness as observed by the change in VTI and the severity of sepsis," the researchers concluded.
The VTI/leg-raise approach looks promising as a possible quick bedside marker that identifies patients who need aggressive treatment, without the need for central line measurements, Dr. Balk said. "The quickest initial fluid bolus you can get is a passive leg raise. You can watch for changes" in real time, and don’t have to move the probe from the point of maximum impact.
Dr. Balk reported having no disclosures.
Dr. Steven Q. Simpson commented: The search for noninvasive measures of or predictors for volume responsiveness in septic patients continues. VTI is the integral of velocity and time, i.e., the distance a small blood bolus travels. When multiplied by cross-sectional area of the aortic outflow tract, this would result in stroke volume. Since one would not expect the cross-sectional area to change significantly after a fluid bolus, alterations in VTI should reflect alterations in stroke volume. While promising, this technique is not as easy as the authors make it sound
and is operator dependent, even though the machine does the calculating. The incident angle of the probe must remain constant during the leg raise (at least 90 seconds). The user must know whether valve pathology or LV impairment are present and, if so, the degree. Massively volume depleted patients may fail to respond adequately to a passive leg raise.
One would be remiss to rely on this small study, which does not report sensitivity or specificity, to establish a reliable percent increase for predicting lactate response or to guide fluid therapy. However, this research is certainly aimed in the right direction.
Dr. Steven Q. Simpson is professor of medicine and director of fellowship training in the pulmonary disease and critical care medicine division at the University of Kansas Medical Center, Kansas City.
SEATTLE – Septic patients are more likely to respond to fluid therapy if their velocity time integral – a Doppler ultrasound measurement of blood flow across the left ventricular outflow tract – increases by 15% or more with a passive single-leg raise, according to a preliminary, observational study of 32 patients at New York Methodist Hospital in Brooklyn.
A passive leg raise to 45 degrees simulates a 250- to 500-cc fluid bolus. "We have found that people who don’t respond with a VTI greater than 15% have higher repeat lactate levels. Instead of giving them 2 L [of fluid] and then reassessing, maybe they’re patients you want to start on pressors right away," Dr. Andrew Balk said at the annual meeting of the American College of Emergency Physicians.
Echocardiogram machines can automatically calculate VTI. The measurement, which Dr. Balk and his associates obtained from the apical five-chamber view, is a surrogate for, and can be used to calculate, cardiac output. Poor response to fluid challenge indicates that fluids are less likely to increase cardiac output and more likely to cause fluid overload, said Dr. Balk, associate director of the clinical ultrasound division at the hospital.
The patients’ mean age was 68 years, and those with valvular pathology and atrial fibrillation were excluded from the study.
The group’s mean baseline VTI was 22 cm (range, 15-29 cm), which leg raises raised to a mean of 26 cm (18-34 cm), an increase of about 18% (4%-36%). A subsequent 2-L normal saline challenge increased VTI to a mean of 33 cm.
The mean baseline lactate level was 3.2 mmol/L (1.2-5.2 mmol/L), and 2 mmol/L (1-3 mmol/L) after the 2-L challenge. The percent change in VTI correlated significantly with the percent change in serum lactate levels. "Below-average responsiveness to the initial small fluid bolus was associated with a higher repeat lactate value, ... which suggests an inverse relationship between a patient’s fluid responsiveness as observed by the change in VTI and the severity of sepsis," the researchers concluded.
The VTI/leg-raise approach looks promising as a possible quick bedside marker that identifies patients who need aggressive treatment, without the need for central line measurements, Dr. Balk said. "The quickest initial fluid bolus you can get is a passive leg raise. You can watch for changes" in real time, and don’t have to move the probe from the point of maximum impact.
Dr. Balk reported having no disclosures.
Dr. Steven Q. Simpson commented: The search for noninvasive measures of or predictors for volume responsiveness in septic patients continues. VTI is the integral of velocity and time, i.e., the distance a small blood bolus travels. When multiplied by cross-sectional area of the aortic outflow tract, this would result in stroke volume. Since one would not expect the cross-sectional area to change significantly after a fluid bolus, alterations in VTI should reflect alterations in stroke volume. While promising, this technique is not as easy as the authors make it sound
and is operator dependent, even though the machine does the calculating. The incident angle of the probe must remain constant during the leg raise (at least 90 seconds). The user must know whether valve pathology or LV impairment are present and, if so, the degree. Massively volume depleted patients may fail to respond adequately to a passive leg raise.
One would be remiss to rely on this small study, which does not report sensitivity or specificity, to establish a reliable percent increase for predicting lactate response or to guide fluid therapy. However, this research is certainly aimed in the right direction.
Dr. Steven Q. Simpson is professor of medicine and director of fellowship training in the pulmonary disease and critical care medicine division at the University of Kansas Medical Center, Kansas City.
SEATTLE – Septic patients are more likely to respond to fluid therapy if their velocity time integral – a Doppler ultrasound measurement of blood flow across the left ventricular outflow tract – increases by 15% or more with a passive single-leg raise, according to a preliminary, observational study of 32 patients at New York Methodist Hospital in Brooklyn.
A passive leg raise to 45 degrees simulates a 250- to 500-cc fluid bolus. "We have found that people who don’t respond with a VTI greater than 15% have higher repeat lactate levels. Instead of giving them 2 L [of fluid] and then reassessing, maybe they’re patients you want to start on pressors right away," Dr. Andrew Balk said at the annual meeting of the American College of Emergency Physicians.
Echocardiogram machines can automatically calculate VTI. The measurement, which Dr. Balk and his associates obtained from the apical five-chamber view, is a surrogate for, and can be used to calculate, cardiac output. Poor response to fluid challenge indicates that fluids are less likely to increase cardiac output and more likely to cause fluid overload, said Dr. Balk, associate director of the clinical ultrasound division at the hospital.
The patients’ mean age was 68 years, and those with valvular pathology and atrial fibrillation were excluded from the study.
The group’s mean baseline VTI was 22 cm (range, 15-29 cm), which leg raises raised to a mean of 26 cm (18-34 cm), an increase of about 18% (4%-36%). A subsequent 2-L normal saline challenge increased VTI to a mean of 33 cm.
The mean baseline lactate level was 3.2 mmol/L (1.2-5.2 mmol/L), and 2 mmol/L (1-3 mmol/L) after the 2-L challenge. The percent change in VTI correlated significantly with the percent change in serum lactate levels. "Below-average responsiveness to the initial small fluid bolus was associated with a higher repeat lactate value, ... which suggests an inverse relationship between a patient’s fluid responsiveness as observed by the change in VTI and the severity of sepsis," the researchers concluded.
The VTI/leg-raise approach looks promising as a possible quick bedside marker that identifies patients who need aggressive treatment, without the need for central line measurements, Dr. Balk said. "The quickest initial fluid bolus you can get is a passive leg raise. You can watch for changes" in real time, and don’t have to move the probe from the point of maximum impact.
Dr. Balk reported having no disclosures.
Dr. Steven Q. Simpson commented: The search for noninvasive measures of or predictors for volume responsiveness in septic patients continues. VTI is the integral of velocity and time, i.e., the distance a small blood bolus travels. When multiplied by cross-sectional area of the aortic outflow tract, this would result in stroke volume. Since one would not expect the cross-sectional area to change significantly after a fluid bolus, alterations in VTI should reflect alterations in stroke volume. While promising, this technique is not as easy as the authors make it sound
and is operator dependent, even though the machine does the calculating. The incident angle of the probe must remain constant during the leg raise (at least 90 seconds). The user must know whether valve pathology or LV impairment are present and, if so, the degree. Massively volume depleted patients may fail to respond adequately to a passive leg raise.
One would be remiss to rely on this small study, which does not report sensitivity or specificity, to establish a reliable percent increase for predicting lactate response or to guide fluid therapy. However, this research is certainly aimed in the right direction.
Dr. Steven Q. Simpson is professor of medicine and director of fellowship training in the pulmonary disease and critical care medicine division at the University of Kansas Medical Center, Kansas City.
AT THE ACEP SCIENTIFIC ASSEMBLY 2013