User login
Pneumatosis cystoides intestinalis: Is surgery always indicated?
A 57-year-old man with long-standing systemic sclerosis presented with worsening diffuse abdominal pain associated with several episodes of nonbloody emesis for 5 days. He had been hospitalized numerous times over the past 2 years for similar symptoms. In those instances, abdominal radiography and computed tomography (CT) had revealed nonspecific intestinal pseudo-obstruction that had resolved within a few days with bowel rest, antibiotics for small-intestinal bacterial overgrowth, and supportive care.
At the time of this presentation, physical examination showed stable vital signs, a tympanic, distended abdomen with diffuse tenderness, and diminished bowel sounds with no sign of peritonitis. Complete blood cell counts, renal function testing, and serum lactate levels were unremarkable.
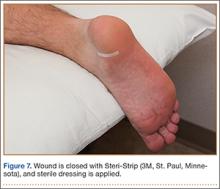
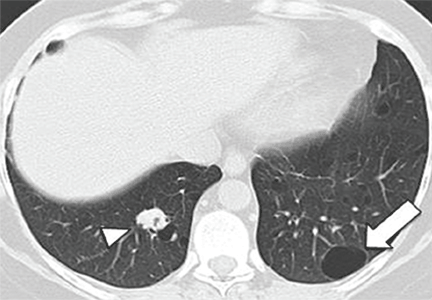
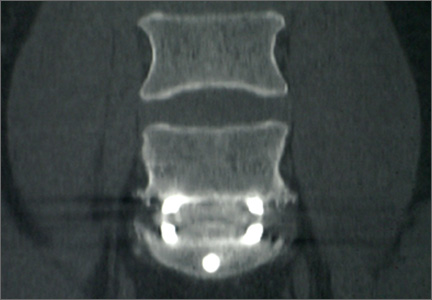
Abdominal radiography showed mildly dilated loops of small bowel with multiple fluid levels, raising concern for intestinal obstruction. Interestingly, abdominal CT revealed extensive pneumatosis cystoides intestinalis of the entire small bowel with sparing of the colon, which raised concern for acute bowel ischemia (Figure 1). However, given the patient’s underlying systemic sclerosis and current stable condition, the general surgeon recommended conservative management with bowel rest, rifaximin to treat the small-intestinal bacterial overgrowth, and intravenous fluids, which resulted in significant clinical improvement. A liquid diet was initiated and advanced as tolerated to a soft diet before he was discharged home after 8 days of hospitalization.
A RARE, USUALLY BENIGN COMPLICATION OF SYSTEMIC SCLEROSIS
Pneumatosis cystoides intestinalis is a rare gastrointestinal complication of systemic sclerosis characterized by intramural accumulation of gas within thin-walled cysts. It is postulated to result either from excess hydrogen gas produced by intraluminal bacterial fermentation and altered partial pressure of nitrogen within the intestinal wall (the bacterial theory),1 or from the transgression of gas cysts through the layers of bowel wall as a result of high luminal pressure from intestinal obstruction (the mechanical theory).2
The more widespread use of diagnostic CT in recent years has led to increased recognition of this condition, a finding that also often raises concern for intestinal necrosis or perforation.3 Meticulous correlation of the clinical presentation with corroborative laboratory testing should determine whether a conservative medical approach or emergency surgical exploration is appropriate.4
Pneumatosis cystoides intestinalis in patients with systemic sclerosis is a benign condition that generally resolves with bowel rest, antibiotics, inhalational oxygen therapy, and supportive care.5 An elevated venous oxygen concentration from high-flow oxygen therapy is believed to attenuate the gaseous cysts by decreasing the partial pressure of the nitrogenous gases and by being toxic to the anaerobic gut bacteria.
About 3% of patients with pneumatosis cystoides intestinalis develop complications such as pneumoperitoneum, intestinal volvulus, obstruction, or hemorrhage. Evidence of pneumoperitoneum or bowel infarction—such as the presence of portomesenteric venous gas, a decreased arterial pH, or an elevated lactic acid or amylase level—warrants immediate surgical intervention. Overall, early recognition and watchful monitoring for bowel necrosis or perforation are preferred over reflexive surgical exploration.
- Levitt MD, Olsson S. Pneumatosis cystoides intestinalis and high breath H2 excretion: insights into the role of H2 in this condition. Gastroenterology 1995; 108:1560–1565.
- Pieterse AS, Leong AS, Rowland R. The mucosal changes and pathogenesis of pneumatosis cystoides intestinalis. Hum Pathol 1985; 16:683–688.
- Ho LM, Paulson EK, Thompson WM. Pneumatosis intestinalis in the adult: benign to life-threatening causes. AJR Am J Roentgenol 2007; 188:1604–1613.
- Khalil PN, Huber-Wagner S, Ladurner R, et al. Natural history, clinical pattern, and surgical considerations of pneumatosis intestinalis. Eur J Med Res 2009; 14:231–239.
- Vischio J, Matlyuk-Urman Z, Lakshminarayanan S. Benign spontaneous pneumoperitoneum in systemic sclerosis. J Clin Rheumatol 2010; 16:379–381.
A 57-year-old man with long-standing systemic sclerosis presented with worsening diffuse abdominal pain associated with several episodes of nonbloody emesis for 5 days. He had been hospitalized numerous times over the past 2 years for similar symptoms. In those instances, abdominal radiography and computed tomography (CT) had revealed nonspecific intestinal pseudo-obstruction that had resolved within a few days with bowel rest, antibiotics for small-intestinal bacterial overgrowth, and supportive care.
At the time of this presentation, physical examination showed stable vital signs, a tympanic, distended abdomen with diffuse tenderness, and diminished bowel sounds with no sign of peritonitis. Complete blood cell counts, renal function testing, and serum lactate levels were unremarkable.
Abdominal radiography showed mildly dilated loops of small bowel with multiple fluid levels, raising concern for intestinal obstruction. Interestingly, abdominal CT revealed extensive pneumatosis cystoides intestinalis of the entire small bowel with sparing of the colon, which raised concern for acute bowel ischemia (Figure 1). However, given the patient’s underlying systemic sclerosis and current stable condition, the general surgeon recommended conservative management with bowel rest, rifaximin to treat the small-intestinal bacterial overgrowth, and intravenous fluids, which resulted in significant clinical improvement. A liquid diet was initiated and advanced as tolerated to a soft diet before he was discharged home after 8 days of hospitalization.
A RARE, USUALLY BENIGN COMPLICATION OF SYSTEMIC SCLEROSIS
Pneumatosis cystoides intestinalis is a rare gastrointestinal complication of systemic sclerosis characterized by intramural accumulation of gas within thin-walled cysts. It is postulated to result either from excess hydrogen gas produced by intraluminal bacterial fermentation and altered partial pressure of nitrogen within the intestinal wall (the bacterial theory),1 or from the transgression of gas cysts through the layers of bowel wall as a result of high luminal pressure from intestinal obstruction (the mechanical theory).2
The more widespread use of diagnostic CT in recent years has led to increased recognition of this condition, a finding that also often raises concern for intestinal necrosis or perforation.3 Meticulous correlation of the clinical presentation with corroborative laboratory testing should determine whether a conservative medical approach or emergency surgical exploration is appropriate.4
Pneumatosis cystoides intestinalis in patients with systemic sclerosis is a benign condition that generally resolves with bowel rest, antibiotics, inhalational oxygen therapy, and supportive care.5 An elevated venous oxygen concentration from high-flow oxygen therapy is believed to attenuate the gaseous cysts by decreasing the partial pressure of the nitrogenous gases and by being toxic to the anaerobic gut bacteria.
About 3% of patients with pneumatosis cystoides intestinalis develop complications such as pneumoperitoneum, intestinal volvulus, obstruction, or hemorrhage. Evidence of pneumoperitoneum or bowel infarction—such as the presence of portomesenteric venous gas, a decreased arterial pH, or an elevated lactic acid or amylase level—warrants immediate surgical intervention. Overall, early recognition and watchful monitoring for bowel necrosis or perforation are preferred over reflexive surgical exploration.
A 57-year-old man with long-standing systemic sclerosis presented with worsening diffuse abdominal pain associated with several episodes of nonbloody emesis for 5 days. He had been hospitalized numerous times over the past 2 years for similar symptoms. In those instances, abdominal radiography and computed tomography (CT) had revealed nonspecific intestinal pseudo-obstruction that had resolved within a few days with bowel rest, antibiotics for small-intestinal bacterial overgrowth, and supportive care.
At the time of this presentation, physical examination showed stable vital signs, a tympanic, distended abdomen with diffuse tenderness, and diminished bowel sounds with no sign of peritonitis. Complete blood cell counts, renal function testing, and serum lactate levels were unremarkable.
Abdominal radiography showed mildly dilated loops of small bowel with multiple fluid levels, raising concern for intestinal obstruction. Interestingly, abdominal CT revealed extensive pneumatosis cystoides intestinalis of the entire small bowel with sparing of the colon, which raised concern for acute bowel ischemia (Figure 1). However, given the patient’s underlying systemic sclerosis and current stable condition, the general surgeon recommended conservative management with bowel rest, rifaximin to treat the small-intestinal bacterial overgrowth, and intravenous fluids, which resulted in significant clinical improvement. A liquid diet was initiated and advanced as tolerated to a soft diet before he was discharged home after 8 days of hospitalization.
A RARE, USUALLY BENIGN COMPLICATION OF SYSTEMIC SCLEROSIS
Pneumatosis cystoides intestinalis is a rare gastrointestinal complication of systemic sclerosis characterized by intramural accumulation of gas within thin-walled cysts. It is postulated to result either from excess hydrogen gas produced by intraluminal bacterial fermentation and altered partial pressure of nitrogen within the intestinal wall (the bacterial theory),1 or from the transgression of gas cysts through the layers of bowel wall as a result of high luminal pressure from intestinal obstruction (the mechanical theory).2
The more widespread use of diagnostic CT in recent years has led to increased recognition of this condition, a finding that also often raises concern for intestinal necrosis or perforation.3 Meticulous correlation of the clinical presentation with corroborative laboratory testing should determine whether a conservative medical approach or emergency surgical exploration is appropriate.4
Pneumatosis cystoides intestinalis in patients with systemic sclerosis is a benign condition that generally resolves with bowel rest, antibiotics, inhalational oxygen therapy, and supportive care.5 An elevated venous oxygen concentration from high-flow oxygen therapy is believed to attenuate the gaseous cysts by decreasing the partial pressure of the nitrogenous gases and by being toxic to the anaerobic gut bacteria.
About 3% of patients with pneumatosis cystoides intestinalis develop complications such as pneumoperitoneum, intestinal volvulus, obstruction, or hemorrhage. Evidence of pneumoperitoneum or bowel infarction—such as the presence of portomesenteric venous gas, a decreased arterial pH, or an elevated lactic acid or amylase level—warrants immediate surgical intervention. Overall, early recognition and watchful monitoring for bowel necrosis or perforation are preferred over reflexive surgical exploration.
- Levitt MD, Olsson S. Pneumatosis cystoides intestinalis and high breath H2 excretion: insights into the role of H2 in this condition. Gastroenterology 1995; 108:1560–1565.
- Pieterse AS, Leong AS, Rowland R. The mucosal changes and pathogenesis of pneumatosis cystoides intestinalis. Hum Pathol 1985; 16:683–688.
- Ho LM, Paulson EK, Thompson WM. Pneumatosis intestinalis in the adult: benign to life-threatening causes. AJR Am J Roentgenol 2007; 188:1604–1613.
- Khalil PN, Huber-Wagner S, Ladurner R, et al. Natural history, clinical pattern, and surgical considerations of pneumatosis intestinalis. Eur J Med Res 2009; 14:231–239.
- Vischio J, Matlyuk-Urman Z, Lakshminarayanan S. Benign spontaneous pneumoperitoneum in systemic sclerosis. J Clin Rheumatol 2010; 16:379–381.
- Levitt MD, Olsson S. Pneumatosis cystoides intestinalis and high breath H2 excretion: insights into the role of H2 in this condition. Gastroenterology 1995; 108:1560–1565.
- Pieterse AS, Leong AS, Rowland R. The mucosal changes and pathogenesis of pneumatosis cystoides intestinalis. Hum Pathol 1985; 16:683–688.
- Ho LM, Paulson EK, Thompson WM. Pneumatosis intestinalis in the adult: benign to life-threatening causes. AJR Am J Roentgenol 2007; 188:1604–1613.
- Khalil PN, Huber-Wagner S, Ladurner R, et al. Natural history, clinical pattern, and surgical considerations of pneumatosis intestinalis. Eur J Med Res 2009; 14:231–239.
- Vischio J, Matlyuk-Urman Z, Lakshminarayanan S. Benign spontaneous pneumoperitoneum in systemic sclerosis. J Clin Rheumatol 2010; 16:379–381.
Poor response to statins predicts growth in plaque
For about one in five patients with known atherosclerotic coronary artery disease, standard-dose therapy with statins did not result in significant lowering of LDL cholesterol.
Furthermore, the results of this large pooled data sample showed that for statin hyporesponders, statin therapy did not prevent progression of intravascular plaque volume as measured by grayscale intravascular ultrasound.
Patients exhibit a wide range of response to standard statin dosing, and the effect of minimal LDL-C lowering on atherosclerotic disease progression had not previously been determined, according to Dr. Yu Kataoka of the University of Adelaide, Australia, and his colleagues (Arterioscler. Thromb. Vasc. Biol. 2015 [doi:10.1161/ATVBAHA.114.304477]).
Investigators pooled data from seven clinical trials that examined 647 total patients with angiographically confirmed CAD who were initiated on statins and followed by serial intravascular ultrasound. The present study analyzed baseline characteristics, serial lipid profile, and atheroma burden for the group.
In all, 130 patients of the 647 (20%) had minimal LDL-C lowering with statin therapy, showing nonsignificant lowering or even an increase in LDL-C levels during the study period. This group of hyporesponders differed in being slightly younger, more obese, less likely to have hypertension and dyslipidemia, and less likely to be receiving beta-blockers than were the statin responders. Other patient characteristics were similar between the two groups. A variety of agents were used, including atorvastatin, rosuvastatin, simvastatin, and pravastatin. Concurrent administration of other antiatherosclerotic agents was permitted and was similar between the groups. Atheroma burden at baseline was also similar between the two groups.
Measuring serial changes in atheroma burden showed a significant difference between statin responders and hyporesponders. The adjusted change in atheroma volume was –0.21% for the responders, compared with +0.83% for the hyporesponders (P = .006). Lumen volume decreased 11.64 mm3 for the responders, while the reduction was 16.54 mm3 for the hyporesponders (P = .006). Of those who responded to lipid therapy with LDL-C lowering, 29.8% had substantial atheroma regression, while 25.9% had substantial plaque progression; among hyporesponders, however, just 13.8% experienced significant plaque regression, while 37.7% had significant atheroma progression, both significant differences.
Dr. Kataoka and his colleagues emphasized that the factors contributing to poor statin response are not well understood. They noted that for this study, the pooled trials all showed adherence rates over 90%, eliminating patient compliance as a variable. Rigorous statistical techniques were used to control for comorbidities and coadministered medications. There are known genetic polymorphisms and phenotypic variations in statin metabolism, though these were not reported here. Although the results were not statistically significant, C-reactive protein levels were higher for the hyporesponse group, suggesting that another factor may be individual response to the anti-inflammatory effect that is among the known pleiotropic effects of this drug class.
In an interview, lead author Stephen Nicholls noted that many clinicians are still reluctant to treat to full effect. Citing the concept of “clinical inertia,” Dr. Nicholls pointed out that “Even when statins are prescribed, they are often at lower doses than ideal. That translated to more plaque growth, which leads directly to more heart attacks and more revascularization procedures.”
Study limitations included the potential residual confounding effects of pooling data from seven discrete clinical trials, though mixed modeling techniques attempted to correct for this effect. The present study also reported atheroma burden, but not actual clinical events. The study authors noted, however, that they had previously reported a direct relationship between atheroma progression and the occurrence of cardiovascular events.
Dr. Nicholls has received speaking honoraria and research support from many pharmaceutical companies, and from Infraredx. Dr. Steven E. Nissen of the Cleveland Clinic was a coinvestigator and has received research support from and is a consultant/adviser to numerous pharmaceutical companies; all honoraria or consulting fees go directly to charity so that he receives neither income nor a tax deduction. The other authors report no conflicts.
For about one in five patients with known atherosclerotic coronary artery disease, standard-dose therapy with statins did not result in significant lowering of LDL cholesterol.
Furthermore, the results of this large pooled data sample showed that for statin hyporesponders, statin therapy did not prevent progression of intravascular plaque volume as measured by grayscale intravascular ultrasound.
Patients exhibit a wide range of response to standard statin dosing, and the effect of minimal LDL-C lowering on atherosclerotic disease progression had not previously been determined, according to Dr. Yu Kataoka of the University of Adelaide, Australia, and his colleagues (Arterioscler. Thromb. Vasc. Biol. 2015 [doi:10.1161/ATVBAHA.114.304477]).
Investigators pooled data from seven clinical trials that examined 647 total patients with angiographically confirmed CAD who were initiated on statins and followed by serial intravascular ultrasound. The present study analyzed baseline characteristics, serial lipid profile, and atheroma burden for the group.
In all, 130 patients of the 647 (20%) had minimal LDL-C lowering with statin therapy, showing nonsignificant lowering or even an increase in LDL-C levels during the study period. This group of hyporesponders differed in being slightly younger, more obese, less likely to have hypertension and dyslipidemia, and less likely to be receiving beta-blockers than were the statin responders. Other patient characteristics were similar between the two groups. A variety of agents were used, including atorvastatin, rosuvastatin, simvastatin, and pravastatin. Concurrent administration of other antiatherosclerotic agents was permitted and was similar between the groups. Atheroma burden at baseline was also similar between the two groups.
Measuring serial changes in atheroma burden showed a significant difference between statin responders and hyporesponders. The adjusted change in atheroma volume was –0.21% for the responders, compared with +0.83% for the hyporesponders (P = .006). Lumen volume decreased 11.64 mm3 for the responders, while the reduction was 16.54 mm3 for the hyporesponders (P = .006). Of those who responded to lipid therapy with LDL-C lowering, 29.8% had substantial atheroma regression, while 25.9% had substantial plaque progression; among hyporesponders, however, just 13.8% experienced significant plaque regression, while 37.7% had significant atheroma progression, both significant differences.
Dr. Kataoka and his colleagues emphasized that the factors contributing to poor statin response are not well understood. They noted that for this study, the pooled trials all showed adherence rates over 90%, eliminating patient compliance as a variable. Rigorous statistical techniques were used to control for comorbidities and coadministered medications. There are known genetic polymorphisms and phenotypic variations in statin metabolism, though these were not reported here. Although the results were not statistically significant, C-reactive protein levels were higher for the hyporesponse group, suggesting that another factor may be individual response to the anti-inflammatory effect that is among the known pleiotropic effects of this drug class.
In an interview, lead author Stephen Nicholls noted that many clinicians are still reluctant to treat to full effect. Citing the concept of “clinical inertia,” Dr. Nicholls pointed out that “Even when statins are prescribed, they are often at lower doses than ideal. That translated to more plaque growth, which leads directly to more heart attacks and more revascularization procedures.”
Study limitations included the potential residual confounding effects of pooling data from seven discrete clinical trials, though mixed modeling techniques attempted to correct for this effect. The present study also reported atheroma burden, but not actual clinical events. The study authors noted, however, that they had previously reported a direct relationship between atheroma progression and the occurrence of cardiovascular events.
Dr. Nicholls has received speaking honoraria and research support from many pharmaceutical companies, and from Infraredx. Dr. Steven E. Nissen of the Cleveland Clinic was a coinvestigator and has received research support from and is a consultant/adviser to numerous pharmaceutical companies; all honoraria or consulting fees go directly to charity so that he receives neither income nor a tax deduction. The other authors report no conflicts.
For about one in five patients with known atherosclerotic coronary artery disease, standard-dose therapy with statins did not result in significant lowering of LDL cholesterol.
Furthermore, the results of this large pooled data sample showed that for statin hyporesponders, statin therapy did not prevent progression of intravascular plaque volume as measured by grayscale intravascular ultrasound.
Patients exhibit a wide range of response to standard statin dosing, and the effect of minimal LDL-C lowering on atherosclerotic disease progression had not previously been determined, according to Dr. Yu Kataoka of the University of Adelaide, Australia, and his colleagues (Arterioscler. Thromb. Vasc. Biol. 2015 [doi:10.1161/ATVBAHA.114.304477]).
Investigators pooled data from seven clinical trials that examined 647 total patients with angiographically confirmed CAD who were initiated on statins and followed by serial intravascular ultrasound. The present study analyzed baseline characteristics, serial lipid profile, and atheroma burden for the group.
In all, 130 patients of the 647 (20%) had minimal LDL-C lowering with statin therapy, showing nonsignificant lowering or even an increase in LDL-C levels during the study period. This group of hyporesponders differed in being slightly younger, more obese, less likely to have hypertension and dyslipidemia, and less likely to be receiving beta-blockers than were the statin responders. Other patient characteristics were similar between the two groups. A variety of agents were used, including atorvastatin, rosuvastatin, simvastatin, and pravastatin. Concurrent administration of other antiatherosclerotic agents was permitted and was similar between the groups. Atheroma burden at baseline was also similar between the two groups.
Measuring serial changes in atheroma burden showed a significant difference between statin responders and hyporesponders. The adjusted change in atheroma volume was –0.21% for the responders, compared with +0.83% for the hyporesponders (P = .006). Lumen volume decreased 11.64 mm3 for the responders, while the reduction was 16.54 mm3 for the hyporesponders (P = .006). Of those who responded to lipid therapy with LDL-C lowering, 29.8% had substantial atheroma regression, while 25.9% had substantial plaque progression; among hyporesponders, however, just 13.8% experienced significant plaque regression, while 37.7% had significant atheroma progression, both significant differences.
Dr. Kataoka and his colleagues emphasized that the factors contributing to poor statin response are not well understood. They noted that for this study, the pooled trials all showed adherence rates over 90%, eliminating patient compliance as a variable. Rigorous statistical techniques were used to control for comorbidities and coadministered medications. There are known genetic polymorphisms and phenotypic variations in statin metabolism, though these were not reported here. Although the results were not statistically significant, C-reactive protein levels were higher for the hyporesponse group, suggesting that another factor may be individual response to the anti-inflammatory effect that is among the known pleiotropic effects of this drug class.
In an interview, lead author Stephen Nicholls noted that many clinicians are still reluctant to treat to full effect. Citing the concept of “clinical inertia,” Dr. Nicholls pointed out that “Even when statins are prescribed, they are often at lower doses than ideal. That translated to more plaque growth, which leads directly to more heart attacks and more revascularization procedures.”
Study limitations included the potential residual confounding effects of pooling data from seven discrete clinical trials, though mixed modeling techniques attempted to correct for this effect. The present study also reported atheroma burden, but not actual clinical events. The study authors noted, however, that they had previously reported a direct relationship between atheroma progression and the occurrence of cardiovascular events.
Dr. Nicholls has received speaking honoraria and research support from many pharmaceutical companies, and from Infraredx. Dr. Steven E. Nissen of the Cleveland Clinic was a coinvestigator and has received research support from and is a consultant/adviser to numerous pharmaceutical companies; all honoraria or consulting fees go directly to charity so that he receives neither income nor a tax deduction. The other authors report no conflicts.
FROM ARTERIOSCLEROSIS, THROMBOSIS, AND VASCULAR BIOLOGY
Key clinical point: Patients on statins who had minimal LDL-C lowering also showed increased atheroma progression.
Major finding: Of 647 patients with CAD, 20% were hyporesponders to statin therapy and experienced greater progression of atheroma volume than statin responders (adjusted +0.83% vs. –0.21%, P = .006).
Data source: Pooled data from seven clinical trials, yielding 647 patients with angiographically confirmed CAD who were initiated on standard lipid dosing and followed by baseline and serial grayscale intravascular ultrasounds.
Disclosures: Dr. Nicholls has received speaking honoraria and research support from many pharmaceutical companies, and from Infraredx. Dr. Steven E. Nissen of the Cleveland Clinic was a coinvestigator and has received research support from and is a consultant/adviser to numerous pharmaceutical companies; all honoraria or consulting fees go directly to charity so that he receives neither income nor a tax deduction. The other authors report no conflicts.
Wrisberg-Variant Discoid Lateral Meniscus: Current Concepts, Treatment Options, and Imaging Features With Emphasis on Dynamic Ultrasonography
First described by Young1 in 1889, discoid lateral meniscus covers a spectrum of meniscal disorders of varying morphology and stability. Determining the true incidence of discoid lateral menisci is difficult because of the large number of asymptomatic cases, though published estimates range from 1% to 17%2-4 of the population, with bilaterality occurring in up to 20%.5 The most commonly used classification system for discoid lateral menisci—reported by Watanabe and colleagues6—describes 3 types of meniscal pathology based on stability to probing and arthroscopic appearance. Type I is stable to probing, has normal tibial attachments, and is “block-shaped,” with increased thickness spanning the entire lateral tibial plateau. Type II is stable to probing and has normal tibial attachments as well, but covers less than 80% of the lateral tibial plateau. Type III (the Wrisberg variant) is unstable because it lacks a posterior meniscotibial (coronary) ligament and has only 1 posterior attachment, the posterior meniscofemoral ligament, or Wrisberg ligament. Wrisberg-variant discoid lateral menisci are rare; estimated incidence is 0.2%.7
Pathophysiology
The normal lateral meniscus, with its flat tibial and concave femoral surfaces, is crucial to load transmission across the knee joint.8 Embryologically differentiating from mesenchymal tissue within the limb bud during fetal development, a normal lateral meniscus never has a discoid shape.8-10 The implication, that discoid lateral menisci represent a congenital anomaly, is further supported by ultrastructural studies involving transmission electron microscopy. These studies have demonstrated that discoid menisci have fewer collagen fibers with a more disorganized course compared with normal menisci.11
With considerable variability, the average normal lateral meniscus is 12 mm wide and 4 mm thick.2 The blood supply to the lateral meniscus recedes during growth, with only the peripheral third remaining in adulthood8 and the inner two-thirds receiving nutrients by diffusion from the intra-articular fluid.5 In comparison, discoid lateral menisci often have poorer vascularity than normal menisci and therefore are more susceptible to tears.8,12,13
Ligamentous attachments to the lateral meniscus include the lateral meniscocapsular ligament, which attaches to the lateral joint capsule. In addition, 70% to 100% of people have accessory meniscofemoral ligaments, which insert anterior (ligament of Humphrey) or posterior (ligament of Wrisberg) to the posterior cruciate ligament.14 There are no ligamentous attachments at the popliteus hiatus or lateral collateral ligament, allowing for 9- to 11-mm excursion of the lateral meniscus during knee flexion and extension.3 Morphologically, the lack of a meniscotibial (coronary) ligament in the setting of a discoid lateral meniscus (Wrisberg variant) results in meniscal hypermobility. During knee range of motion, compressive forces between the femoral condyle and the tibial plateau spread through the peripheral portion of the meniscus and, without ligamentous attachments, allow it to displace anteriorly into the femoral intercondylar notch. This displacement results in impingement between the femur and the tibia15-18 and leads to the characteristic symptoms of “snapping knee syndrome.”10
Clinical Features
Snapping knee syndrome was first described by Kroiss19 in 1910.5 Multiple authors have described patients’ primary complaints as pain, swelling, locking, and a palpable or visible snap at terminal extension. Sudden movement of a soft-tissue structure across a bony prominence during a provocative maneuver is the source of the snapping. The syndrome has many etiologies. Extra-articular causes of lateral snapping knee syndrome include iliotibial band friction syndrome, soft-tissue tumors, hypermobile popliteus tendons, and abnormal anterior insertions of the biceps femoris tendons.20,21 Common intra-articular etiologies include ganglion, synovial, and parameniscal cysts; intra-articular loose bodies; lateral meniscal tears; and discoid lateral menisci.22 Patients with discoid lateral menisci often present with knee pain, popping, range-of-motion limitations, and snapping.23,24 However, the symptoms are quite variable and depend on type of discoid meniscus, presence of a tear, and stability of the rim.2,7,18
Obtaining a thorough history is essential in evaluating patients with suspected discoid lateral menisci. Physical examination should include evaluation of the lateral joint line for bulges, effusion, and tenderness. Patients may experience knee pain with flexion to 30° to 40° when varus or valgus stress (modified McMurray maneuver) is applied.10 In addition, a clunk may be appreciated with McMurray testing as a result of subluxation of the unstable lateral meniscus.10 The contralateral knee should be carefully evaluated, given the frequency of bilateral discoid menisci.10
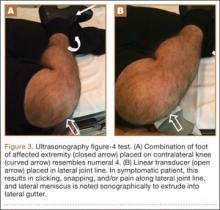
The figure-4 test, a maneuver developed by LaPrade and Konowalchuk25 to detect peripheral meniscal tears or tears of the popliteomeniscal fascicles, is performed with the patient in the supine position, with the foot of the affected extremity placed on the contralateral knee. Normally, the popliteus tendon pulls the meniscus out of the joint when the knee is brought into the figure-4 position. However, without popliteomeniscal fascicles, the meniscus subluxes into the joint and becomes impinged. With the patient in the figure-4 position, reproduction of symptoms over the lateral joint line is a positive test and suggests peripheral meniscal tears and/or tears or absence of the popliteomeniscal fascicles.25
In the series reported by LaPrade and Konowalchuk,25 all of the patients who experienced symptoms during figure-4 testing were found, on arthroscopic examination, to have lateral meniscal hypermobility caused by tears of the popliteomeniscal fascicles. Despite the success of those authors in using the figure-4 technique for diagnosis, others have reported that the accuracy of the clinical examination (vs arthroscopy) in diagnosing Wrisberg-variant discoid lateral menisci ranges from 29% to 93%.5,26,27 This emphasizes the importance of diagnostic imaging in the work-up of patients with suspected Wrisberg-variant discoid lateral menisci.
Imaging Features
Radiography
In 1964, Picard and Constantin28 recommended that patients with suspected discoid lateral menisci undergo standard anteroposterior, lateral, tunnel, and skyline radiographs as part of the diagnostic work-up. In patients with discoid lateral menisci, plain film radiographs are often normal10 but may demonstrate lateral femoral condyle squaring, widening of the lateral joint line, lateral tibial plateau cupping, tibial eminence hypoplasia, and fibular head elevation.5,29 Plain radiography is unreliable, however, and patients often require advanced imaging, such as knee magnetic resonance imaging (MRI).10
Magnetic Resonance Imaging
Because it clearly depicts soft-tissue structures, MRI is widely used to diagnose musculoskeletal pathology in and around the knee. Criteria for the diagnosis of discoid menisci include meniscal width of 15 mm or more, ratio of minimum meniscal width to maximum tibial width on coronal slice of more than 20%, ratio of sum of width of both lateral horns to meniscal diameter (on sagittal slice showing maximum meniscal diameter) of more than 75%, and continuity of anterior and posterior horns on at least 3 consecutive sagittal slices (bow tie sign).5,30,31 Even in the presence of a tear, the described ratios have sensitivity and specificity of 95% and 97% in detecting discoid lateral menisci.30
However, the Wrisberg variant, which may consist of only a thickened portion of the posterior horn, is often more difficult to diagnose using these criteria and can even appear normal on MRI.26,32 In a series by Neuschwander and colleagues,7 none of the Wrisberg-variant menisci had a true discoid shape, suggesting that the size of the lateral meniscus may appear normal in affected patients. Appropriate positioning during MRI evaluation of patients suspected of having the Wrisberg variant was emphasized by Moser and colleagues,33 who described a case of discoid lateral meniscus not observable on initial MRI but visible on MRI performed with the affected knee extended in the locked position.
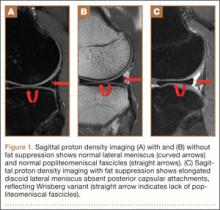
The unstable lateral meniscus may be seen subluxed anteriorly or laterally because of lack of posterior attachments. A deficiency of normal popliteomeniscal fascicles and coronary ligaments is represented by a high T2 signal interposed between the discoid lateral meniscus and the posterior joint capsule, simulating a vertical peripheral tear and suggesting presence of the Wrisberg variant (Figures 1A–1C). In addition, the posterior horn of the enlarged discoid lateral meniscus may connect to a prominent and thickened meniscofemoral ligament of Wrisberg. Despite these characteristic imaging features, some studies have found low sensitivity of MRI in the diagnosis of Wrisberg-variant discoid lateral menisci.26
Ultrasonography
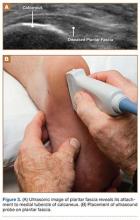
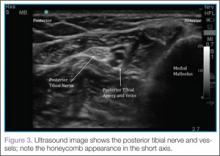
There is a growing interest in using ultrasonography in the diagnosis of Wrisberg-variant discoid lateral menisci because of its availability, multiplanar capability, and lower cost compared with MRI. Ultrasonographic criteria for the diagnosis of discoid menisci include absence of normal triangular shape, presence of abnormally elongated and thickened meniscal tissue, and demonstration of a heterogeneous central pattern.5 Through use of a high-resolution probe, which better fits the anatomical concavity of the popliteal fossa, a positive predictive value of 95% and a negative predictive value of 100% have been reported for ultrasonography in the diagnosis of meniscal tears.34
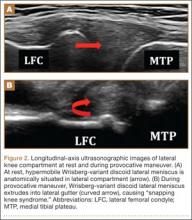
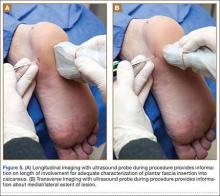
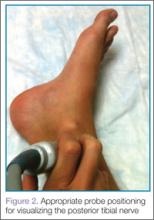
Perhaps the main advantage of ultrasonography is the possibility of performing a dynamic study to evaluate the extrusion of the meniscus into the lateral gutter and to correlate this with knee snapping (Figures 2A, 2B).35 One technique for sonographic evaluation of a hypermobile lateral meniscus involves placing the patient supine with the high-resolution (9 or 12 MHz) linear transducer along the lateral knee joint line. The patient is then asked to place the foot of the affected extremity on the contralateral knee; the combination resembles the numeral 4 (figure-4 test) (Figures 3A, 3B). In a symptomatic patient, this results in clicking, snapping, and/or pain along the lateral joint line, and the lateral meniscus is noted sonographically to extrude into the lateral gutter (Figure 2B), either the result of torn popliteomeniscal fascicles or the increased meniscal mobility of Wrisberg variants.
The main drawback of ultrasonography is operator dependence. As clinicians become more familiar with ultrasonography, dynamic ultrasonography should be used for what is often a difficult diagnosis both clinically and with nondynamic imaging.
Management
The historical treatment for symptomatic discoid lateral menisci, open total meniscectomy,5,7,15,36 is no longer performed, as studies have shown it increases contact stresses proportional to the amount of meniscus removed, with up to a 235% increase after total meniscectomy,37 predisposing patients to early degenerative changes and osteoarthritis.38-41
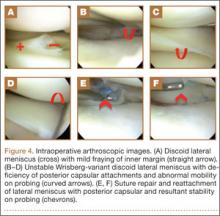
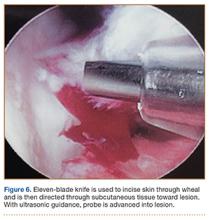
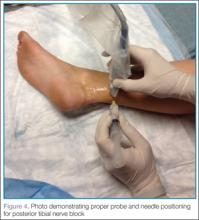
With an appreciation of the role of menisci as load distributors and joint stabilizers in cartilage nutrition, current treatments aim to preserve as much stable meniscal tissue as possible.5 Surgical management of Wrisberg-variant discoid lateral menisci involves posterior stabilization with or without saucerization.7,33,42 The goal of arthroscopic saucerization is to preserve healthy tissue and create a stable remaining meniscus (6-8 mm in width)2,7,43,44 that provides adequate shock absorption without retearing.10 Wrisberg-variant discoid menisci can be stabilized with use of all-inside sutures from the meniscus to the joint capsule (Figures 4A–4F) when there is sufficient residual meniscus to allow for suture fixation to the posterior capsule after débridement. In contrast, some prefer an inside-out technique, as described by Neuschwander and colleagues,7 with inclusion of a mini-open approach. Any meniscal tears are addressed at time of surgery, either by partial meniscectomy or repair. Relative indications for meniscal repair include longitudinal, vertical, nondegenerative tears that are within 3 mm of the periphery (vascular zone) and are less than 3 cm in length.45 However, the majority of tears in adults are degenerative cleavage tears outside the vascular zone and therefore not amenable to repair.45,46 Before surgery, patients treated with stabilization with or without saucerization are prescribed partial weight-bearing in a hinged knee brace with gradual range of motion to 90° by 6 weeks and return to sports in 3 to 4 months.
Clinical Results
As has been consistently demonstrated, the long-term outcomes of total meniscectomy are poor function39,40,47 and radiographic evidence of lateral compartment arthritis.48 Patients who previously underwent total meniscectomy should be offered meniscal allograft transplantation, as it may offset the increased peak local contact pressures in the lateral compartment10 and improve function.49
With an appreciation for the importance of meniscus preservation, more recent studies have found encouraging results for arthroscopic saucerization and stabilization of Wrisberg-variant discoid lateral menisci. For example, Woods and Whelan44 reported excellent results in 75% of patients at 37.5-month follow-up after open repair of discoid lateral menisci lacking posterior attachments. In another study, by Neuschwander and colleagues,7 4 of 6 patients who underwent arthroscopic repair of unstable discoid lateral menisci without posterior coronary ligaments had excellent outcomes. Although these studies demonstrated symptom resolution and lack of radiographic evidence of degenerative changes at midterm follow-up,50 additional long-term studies should be performed to determine whether saucerization and stabilization prevent the onset of lateral compartment osteoarthritis.
Conclusion
Abnormally mobile discoid lateral menisci can result in painful lateral snapping knee syndromes but are often challenging to diagnose clinically and with traditional static imaging. Dynamic ultrasonography with provocative maneuvers can reveal lateral meniscal subluxation, which often cannot be appreciated on MRI, allowing for timely stabilization and symptom resolution.
1. Young RB. The external semilunar cartilage as a complete disc. In: Cleland J, Mackey JY, Young RB, eds. Memoirs and Memoranda in Anatomy. London, England: Williams & Norgate; 1889:179.
2. Jordan MR. Lateral meniscal variants: evaluation and treatment. J Am Acad Orthop Surg. 1996;4(4):191-200.
3. Greis PE, Bardana DD, Holmstrom MC, Burks RT. Meniscal injury: I. Basic science and evaluation. J Am Acad Orthop Surg. 2002;10(3):168-176.
4. Ikeuchi H. Arthroscopic treatment of the discoid lateral meniscus. Technique and long-term results. Clin Orthop. 1982;(167):19-28.
5. Yaniv M, Blumberg N. The discoid meniscus. J Child Orthop. 2007;1(2):89-96.
6. Watanabe M, Takeda S, Ikeuchi H. Atlas of Arthroscopy. Tokyo, Japan: Igaku-Shoin; 1978.
7. Neuschwander DC, Drez D Jr, Finney TP. Lateral meniscal variant with absence of the posterior coronary ligament. J Bone Joint Surg Am. 1992;74(8):1186-1190.
8. Clark CR, Ogden JA. Development of the menisci of the human knee joint. Morphological changes and their potential role in childhood meniscal injury. J Bone Joint Surg Am. 1983;65(4):538-547.
9. Kaplan EB. Discoid lateral meniscus of the knee joint; nature, mechanism, and operative treatment. J Bone Joint Surg Am. 1957;39(1):77-87.
10. Kramer DE, Micheli LJ. Meniscal tears and discoid meniscus in children: diagnosis and treatment. J Am Acad Orthop Surg. 2009;17(11):698-707.
11. Atay OA, Pekmezci M, Doral MN, Sargon MF, Ayvaz M, Johnson DL. Discoid meniscus: an ultrastructural study with transmission electron microscopy. Am J Sports Med. 2007;35(3):475-478.
12. Nathan PA, Cole SC. Discoid meniscus. A clinical and pathologic study. Clin Orthop. 1969;(64):107-113.
13. Good CR, Green DW, Griffith MH, Valen AW, Widmann RF, Rodeo SA. Arthroscopic treatment of symptomatic discoid meniscus in children: classification, technique, and results. Arthroscopy. 2007;23(2):157-163.
14. Harner CD, Xerogeanes JW, Livesay GA, et al. The human posterior cruciate ligament complex: an interdisciplinary study. Ligament morphology and biomechanical evaluation. Am J Sports Med. 1995;23(6):736-745.
15. Smillie IS. The congenital discoid meniscus. J Bone Joint Surg Br. 1948;30(4):671-682.
16. Yoo WJ, Choi IH, Chung CY, et al. Discoid lateral meniscus in children: limited knee extension and meniscal instability in the posterior segment. J Pediatr Orthop. 2008;28(5):544-548.
17. Simonian PT, Sussmann PS, Wickiewicz TL, et al. Popliteomeniscal fasciculi and the unstable lateral meniscus: clinical correlation and magnetic resonance diagnosis. Arthroscopy. 1997;13(5):590-596.
18. Dickhaut SC, DeLee JC. The discoid lateral-meniscus syndrome. J Bone Joint Surg Am. 1982;64(7):1068-1073.
19. Kroiss F. Die Verletzungen der Kniegelenkoszwischenknorpel und ihrer Verbindungen. Beitr Klin Chir. 1910;66:598-801.
20. Lokiec F, Velkes S, Schindler A, Pritsch M. The snapping biceps femoris syndrome. Clin Orthop. 1992;(283):205-206.
21. Cooper DE. Snapping popliteus tendon syndrome. A cause of mechanical knee popping in athletes. Am J Sports Med. 1999;27(5):671-674.
22. Liu PC, Chen CH, Huang HT, et al. Snapping knee symptoms caused by an intra-articular ganglion cyst. Knee. 2007;14(2):167-168.
23. Bellier G, Dupont JY, Larrain M, Caudron C, Carlioz H. Lateral discoid menisci in children. Arthroscopy. 1989;5(1):52-56.
24. Washington ER 3rd, Root L, Liener UC. Discoid lateral meniscus in children. Long-term follow-up after excision. J Bone Joint Surg Am. 1995;77(9):1357-1361.
25. LaPrade RF, Konowalchuk BK. Popliteomeniscal fascicle tears causing symptomatic lateral compartment knee pain: diagnosis by the figure-4 test and treatment by open repair. Am J Sports Med. 2005;33(8):1231-1236.
26. Kocher MS, DiCanzio J, Zurakowski D, Micheli LJ. Diagnostic performance of clinical examination and selective magnetic resonance imaging in the evaluation of intraarticular knee disorders in children and adolescents. Am J Sports Med. 2001;29(3):292-296.
27. Stanitski CL. Correlation of arthroscopic and clinical examinations with magnetic resonance imaging findings of injured knees in children and adolescents. Am J Sports Med. 1998;26(1):2-6.
28. Picard JJ, Constantin L. Radiological aspects of the discoid meniscus [in French]. J Radiol Electrol Med Nucl. 1964;45:839-841.
29. Kerr R. Radiologic case study. Discoid lateral meniscus. Orthopedics. 1986;9(8):1142, 1145-1147.
30. Samoto N, Kozuma M, Tokuhisa T, Kobayashi K. Diagnosis of discoid lateral meniscus of the knee on MR imaging. Magn Reson Imaging. 2002;20(1):59-64.
31. Silverman JM, Mink JH, Deutsch AL. Discoid menisci of the knee: MR imaging appearance. Radiology. 1989;173(2):351-354.
32. Singh K, Helms CA, Jacobs MT, Higgins LD. MRI appearance of Wrisberg variant of discoid lateral meniscus. AJR Am J Roentgenol. 2006;187(2):384-387.
33. Moser MW, Dugas J, Hartzell J, Thornton DD. A hypermobile Wrisberg variant lateral discoid meniscus seen on MRI. Clin Orthop. 2007;(456):264-267.
34. Najafi J, Bagheri S, Lahiji FA. The value of sonography with micro convex probes in diagnosing meniscal tears compared with arthroscopy. J Ultrasound Med. 2006;25(5):593-597.
35. Marchand AJ, Proisy M, Ropars M, Cohen M, Duvauferrier R, Guillin R. Snapping knee: imaging findings with an emphasis on dynamic sonography. AJR Am J Roentgenol. 2012;199(1):142-150.
36. Nathan PA, Cole SC. Discoid meniscus. A clinical and pathologic study. Clin Orthop. 1969;(64):107-113.
37. Baratz ME, Fu FH, Mengato R. Meniscal tears: the effect of meniscectomy and of repair on intraarticular contact areas and stress in the human knee. A preliminary report. Am J Sports Med. 1986;14(4):270-275.
38. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30(4):664-670.
39. Manzione M, Pizzutillo PD, Peoples AB, Schweizer PA. Meniscectomy in children: a long-term follow-up study. Am J Sports Med. 1983;11(3):111-115.
40. Wroble RR, Henderson RC, Campion ER, el-Khoury GY, Albright JP. Meniscectomy in children and adolescents. A long-term follow-up study. Clin Orthop. 1992;(279):180-189.
41. Abdon P, Turner MS, Pettersson H, Lindstrand A, Stenstrom A, Swanson AJ. A long-term follow-up study of total meniscectomy in children. Clin Orthop. 1990;(257):166-170.
42. Rosenberg TD, Paulos LE, Parker RD, Harner CD, Gurley WD. Discoid lateral meniscus: case report of arthroscopic attachment of a symptomatic Wrisberg-ligament type. Arthroscopy. 1987;3(4):277-282.
43. Fleissner PR, Eilert RE. Discoid lateral meniscus. Am J Knee Surg. 1999;12(2):125-131.
44. Woods GW, Whelan JM. Discoid meniscus. Clin Sports Med. 1990;9(3):695-706.
45. Yue BW, Gupta AK, Moorman CT 3rd, Garrett WE, Helms CA. Wrisberg variant of the discoid lateral meniscus with flipped meniscal fragments simulating bucket-handle tear: MRI and arthroscopic correlation. Skeletal Radiol. 2011;40(8):1089-1094.
46. Weiss CB, Lundberg M, Hamberg P, DeHaven KE, Gillquist J. Non-operative treatment of meniscal tears. J Bone Joint Surg Am. 1989;71(6):811-822.
47. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769.
48. Kim SJ, Chun YM, Jeong JH, Ryu SW, Oh KS, Lubis AM. Effects of arthroscopic meniscectomy on the long-term prognosis for the discoid lateral meniscus. Knee Surg Sports Traumatol Arthrosc. 2007;15(11):1315-1320.
49. Kim JM, Bin SI. Meniscal allograft transplantation after total meniscectomy of torn discoid lateral meniscus. Arthroscopy. 2006;22(12):1344-1350.e1.
50. Ogut T, Kesmezacar H, Akgun I, Cansu E. Arthroscopic meniscectomy for discoid lateral meniscus in children and adolescents: 4.5 year follow-up. J Pediatr Orthop B. 2003;12(6):390-397.
First described by Young1 in 1889, discoid lateral meniscus covers a spectrum of meniscal disorders of varying morphology and stability. Determining the true incidence of discoid lateral menisci is difficult because of the large number of asymptomatic cases, though published estimates range from 1% to 17%2-4 of the population, with bilaterality occurring in up to 20%.5 The most commonly used classification system for discoid lateral menisci—reported by Watanabe and colleagues6—describes 3 types of meniscal pathology based on stability to probing and arthroscopic appearance. Type I is stable to probing, has normal tibial attachments, and is “block-shaped,” with increased thickness spanning the entire lateral tibial plateau. Type II is stable to probing and has normal tibial attachments as well, but covers less than 80% of the lateral tibial plateau. Type III (the Wrisberg variant) is unstable because it lacks a posterior meniscotibial (coronary) ligament and has only 1 posterior attachment, the posterior meniscofemoral ligament, or Wrisberg ligament. Wrisberg-variant discoid lateral menisci are rare; estimated incidence is 0.2%.7
Pathophysiology
The normal lateral meniscus, with its flat tibial and concave femoral surfaces, is crucial to load transmission across the knee joint.8 Embryologically differentiating from mesenchymal tissue within the limb bud during fetal development, a normal lateral meniscus never has a discoid shape.8-10 The implication, that discoid lateral menisci represent a congenital anomaly, is further supported by ultrastructural studies involving transmission electron microscopy. These studies have demonstrated that discoid menisci have fewer collagen fibers with a more disorganized course compared with normal menisci.11
With considerable variability, the average normal lateral meniscus is 12 mm wide and 4 mm thick.2 The blood supply to the lateral meniscus recedes during growth, with only the peripheral third remaining in adulthood8 and the inner two-thirds receiving nutrients by diffusion from the intra-articular fluid.5 In comparison, discoid lateral menisci often have poorer vascularity than normal menisci and therefore are more susceptible to tears.8,12,13
Ligamentous attachments to the lateral meniscus include the lateral meniscocapsular ligament, which attaches to the lateral joint capsule. In addition, 70% to 100% of people have accessory meniscofemoral ligaments, which insert anterior (ligament of Humphrey) or posterior (ligament of Wrisberg) to the posterior cruciate ligament.14 There are no ligamentous attachments at the popliteus hiatus or lateral collateral ligament, allowing for 9- to 11-mm excursion of the lateral meniscus during knee flexion and extension.3 Morphologically, the lack of a meniscotibial (coronary) ligament in the setting of a discoid lateral meniscus (Wrisberg variant) results in meniscal hypermobility. During knee range of motion, compressive forces between the femoral condyle and the tibial plateau spread through the peripheral portion of the meniscus and, without ligamentous attachments, allow it to displace anteriorly into the femoral intercondylar notch. This displacement results in impingement between the femur and the tibia15-18 and leads to the characteristic symptoms of “snapping knee syndrome.”10
Clinical Features
Snapping knee syndrome was first described by Kroiss19 in 1910.5 Multiple authors have described patients’ primary complaints as pain, swelling, locking, and a palpable or visible snap at terminal extension. Sudden movement of a soft-tissue structure across a bony prominence during a provocative maneuver is the source of the snapping. The syndrome has many etiologies. Extra-articular causes of lateral snapping knee syndrome include iliotibial band friction syndrome, soft-tissue tumors, hypermobile popliteus tendons, and abnormal anterior insertions of the biceps femoris tendons.20,21 Common intra-articular etiologies include ganglion, synovial, and parameniscal cysts; intra-articular loose bodies; lateral meniscal tears; and discoid lateral menisci.22 Patients with discoid lateral menisci often present with knee pain, popping, range-of-motion limitations, and snapping.23,24 However, the symptoms are quite variable and depend on type of discoid meniscus, presence of a tear, and stability of the rim.2,7,18
Obtaining a thorough history is essential in evaluating patients with suspected discoid lateral menisci. Physical examination should include evaluation of the lateral joint line for bulges, effusion, and tenderness. Patients may experience knee pain with flexion to 30° to 40° when varus or valgus stress (modified McMurray maneuver) is applied.10 In addition, a clunk may be appreciated with McMurray testing as a result of subluxation of the unstable lateral meniscus.10 The contralateral knee should be carefully evaluated, given the frequency of bilateral discoid menisci.10
The figure-4 test, a maneuver developed by LaPrade and Konowalchuk25 to detect peripheral meniscal tears or tears of the popliteomeniscal fascicles, is performed with the patient in the supine position, with the foot of the affected extremity placed on the contralateral knee. Normally, the popliteus tendon pulls the meniscus out of the joint when the knee is brought into the figure-4 position. However, without popliteomeniscal fascicles, the meniscus subluxes into the joint and becomes impinged. With the patient in the figure-4 position, reproduction of symptoms over the lateral joint line is a positive test and suggests peripheral meniscal tears and/or tears or absence of the popliteomeniscal fascicles.25
In the series reported by LaPrade and Konowalchuk,25 all of the patients who experienced symptoms during figure-4 testing were found, on arthroscopic examination, to have lateral meniscal hypermobility caused by tears of the popliteomeniscal fascicles. Despite the success of those authors in using the figure-4 technique for diagnosis, others have reported that the accuracy of the clinical examination (vs arthroscopy) in diagnosing Wrisberg-variant discoid lateral menisci ranges from 29% to 93%.5,26,27 This emphasizes the importance of diagnostic imaging in the work-up of patients with suspected Wrisberg-variant discoid lateral menisci.
Imaging Features
Radiography
In 1964, Picard and Constantin28 recommended that patients with suspected discoid lateral menisci undergo standard anteroposterior, lateral, tunnel, and skyline radiographs as part of the diagnostic work-up. In patients with discoid lateral menisci, plain film radiographs are often normal10 but may demonstrate lateral femoral condyle squaring, widening of the lateral joint line, lateral tibial plateau cupping, tibial eminence hypoplasia, and fibular head elevation.5,29 Plain radiography is unreliable, however, and patients often require advanced imaging, such as knee magnetic resonance imaging (MRI).10
Magnetic Resonance Imaging
Because it clearly depicts soft-tissue structures, MRI is widely used to diagnose musculoskeletal pathology in and around the knee. Criteria for the diagnosis of discoid menisci include meniscal width of 15 mm or more, ratio of minimum meniscal width to maximum tibial width on coronal slice of more than 20%, ratio of sum of width of both lateral horns to meniscal diameter (on sagittal slice showing maximum meniscal diameter) of more than 75%, and continuity of anterior and posterior horns on at least 3 consecutive sagittal slices (bow tie sign).5,30,31 Even in the presence of a tear, the described ratios have sensitivity and specificity of 95% and 97% in detecting discoid lateral menisci.30
However, the Wrisberg variant, which may consist of only a thickened portion of the posterior horn, is often more difficult to diagnose using these criteria and can even appear normal on MRI.26,32 In a series by Neuschwander and colleagues,7 none of the Wrisberg-variant menisci had a true discoid shape, suggesting that the size of the lateral meniscus may appear normal in affected patients. Appropriate positioning during MRI evaluation of patients suspected of having the Wrisberg variant was emphasized by Moser and colleagues,33 who described a case of discoid lateral meniscus not observable on initial MRI but visible on MRI performed with the affected knee extended in the locked position.
The unstable lateral meniscus may be seen subluxed anteriorly or laterally because of lack of posterior attachments. A deficiency of normal popliteomeniscal fascicles and coronary ligaments is represented by a high T2 signal interposed between the discoid lateral meniscus and the posterior joint capsule, simulating a vertical peripheral tear and suggesting presence of the Wrisberg variant (Figures 1A–1C). In addition, the posterior horn of the enlarged discoid lateral meniscus may connect to a prominent and thickened meniscofemoral ligament of Wrisberg. Despite these characteristic imaging features, some studies have found low sensitivity of MRI in the diagnosis of Wrisberg-variant discoid lateral menisci.26
Ultrasonography
There is a growing interest in using ultrasonography in the diagnosis of Wrisberg-variant discoid lateral menisci because of its availability, multiplanar capability, and lower cost compared with MRI. Ultrasonographic criteria for the diagnosis of discoid menisci include absence of normal triangular shape, presence of abnormally elongated and thickened meniscal tissue, and demonstration of a heterogeneous central pattern.5 Through use of a high-resolution probe, which better fits the anatomical concavity of the popliteal fossa, a positive predictive value of 95% and a negative predictive value of 100% have been reported for ultrasonography in the diagnosis of meniscal tears.34
Perhaps the main advantage of ultrasonography is the possibility of performing a dynamic study to evaluate the extrusion of the meniscus into the lateral gutter and to correlate this with knee snapping (Figures 2A, 2B).35 One technique for sonographic evaluation of a hypermobile lateral meniscus involves placing the patient supine with the high-resolution (9 or 12 MHz) linear transducer along the lateral knee joint line. The patient is then asked to place the foot of the affected extremity on the contralateral knee; the combination resembles the numeral 4 (figure-4 test) (Figures 3A, 3B). In a symptomatic patient, this results in clicking, snapping, and/or pain along the lateral joint line, and the lateral meniscus is noted sonographically to extrude into the lateral gutter (Figure 2B), either the result of torn popliteomeniscal fascicles or the increased meniscal mobility of Wrisberg variants.
The main drawback of ultrasonography is operator dependence. As clinicians become more familiar with ultrasonography, dynamic ultrasonography should be used for what is often a difficult diagnosis both clinically and with nondynamic imaging.
Management
The historical treatment for symptomatic discoid lateral menisci, open total meniscectomy,5,7,15,36 is no longer performed, as studies have shown it increases contact stresses proportional to the amount of meniscus removed, with up to a 235% increase after total meniscectomy,37 predisposing patients to early degenerative changes and osteoarthritis.38-41
With an appreciation of the role of menisci as load distributors and joint stabilizers in cartilage nutrition, current treatments aim to preserve as much stable meniscal tissue as possible.5 Surgical management of Wrisberg-variant discoid lateral menisci involves posterior stabilization with or without saucerization.7,33,42 The goal of arthroscopic saucerization is to preserve healthy tissue and create a stable remaining meniscus (6-8 mm in width)2,7,43,44 that provides adequate shock absorption without retearing.10 Wrisberg-variant discoid menisci can be stabilized with use of all-inside sutures from the meniscus to the joint capsule (Figures 4A–4F) when there is sufficient residual meniscus to allow for suture fixation to the posterior capsule after débridement. In contrast, some prefer an inside-out technique, as described by Neuschwander and colleagues,7 with inclusion of a mini-open approach. Any meniscal tears are addressed at time of surgery, either by partial meniscectomy or repair. Relative indications for meniscal repair include longitudinal, vertical, nondegenerative tears that are within 3 mm of the periphery (vascular zone) and are less than 3 cm in length.45 However, the majority of tears in adults are degenerative cleavage tears outside the vascular zone and therefore not amenable to repair.45,46 Before surgery, patients treated with stabilization with or without saucerization are prescribed partial weight-bearing in a hinged knee brace with gradual range of motion to 90° by 6 weeks and return to sports in 3 to 4 months.
Clinical Results
As has been consistently demonstrated, the long-term outcomes of total meniscectomy are poor function39,40,47 and radiographic evidence of lateral compartment arthritis.48 Patients who previously underwent total meniscectomy should be offered meniscal allograft transplantation, as it may offset the increased peak local contact pressures in the lateral compartment10 and improve function.49
With an appreciation for the importance of meniscus preservation, more recent studies have found encouraging results for arthroscopic saucerization and stabilization of Wrisberg-variant discoid lateral menisci. For example, Woods and Whelan44 reported excellent results in 75% of patients at 37.5-month follow-up after open repair of discoid lateral menisci lacking posterior attachments. In another study, by Neuschwander and colleagues,7 4 of 6 patients who underwent arthroscopic repair of unstable discoid lateral menisci without posterior coronary ligaments had excellent outcomes. Although these studies demonstrated symptom resolution and lack of radiographic evidence of degenerative changes at midterm follow-up,50 additional long-term studies should be performed to determine whether saucerization and stabilization prevent the onset of lateral compartment osteoarthritis.
Conclusion
Abnormally mobile discoid lateral menisci can result in painful lateral snapping knee syndromes but are often challenging to diagnose clinically and with traditional static imaging. Dynamic ultrasonography with provocative maneuvers can reveal lateral meniscal subluxation, which often cannot be appreciated on MRI, allowing for timely stabilization and symptom resolution.
First described by Young1 in 1889, discoid lateral meniscus covers a spectrum of meniscal disorders of varying morphology and stability. Determining the true incidence of discoid lateral menisci is difficult because of the large number of asymptomatic cases, though published estimates range from 1% to 17%2-4 of the population, with bilaterality occurring in up to 20%.5 The most commonly used classification system for discoid lateral menisci—reported by Watanabe and colleagues6—describes 3 types of meniscal pathology based on stability to probing and arthroscopic appearance. Type I is stable to probing, has normal tibial attachments, and is “block-shaped,” with increased thickness spanning the entire lateral tibial plateau. Type II is stable to probing and has normal tibial attachments as well, but covers less than 80% of the lateral tibial plateau. Type III (the Wrisberg variant) is unstable because it lacks a posterior meniscotibial (coronary) ligament and has only 1 posterior attachment, the posterior meniscofemoral ligament, or Wrisberg ligament. Wrisberg-variant discoid lateral menisci are rare; estimated incidence is 0.2%.7
Pathophysiology
The normal lateral meniscus, with its flat tibial and concave femoral surfaces, is crucial to load transmission across the knee joint.8 Embryologically differentiating from mesenchymal tissue within the limb bud during fetal development, a normal lateral meniscus never has a discoid shape.8-10 The implication, that discoid lateral menisci represent a congenital anomaly, is further supported by ultrastructural studies involving transmission electron microscopy. These studies have demonstrated that discoid menisci have fewer collagen fibers with a more disorganized course compared with normal menisci.11
With considerable variability, the average normal lateral meniscus is 12 mm wide and 4 mm thick.2 The blood supply to the lateral meniscus recedes during growth, with only the peripheral third remaining in adulthood8 and the inner two-thirds receiving nutrients by diffusion from the intra-articular fluid.5 In comparison, discoid lateral menisci often have poorer vascularity than normal menisci and therefore are more susceptible to tears.8,12,13
Ligamentous attachments to the lateral meniscus include the lateral meniscocapsular ligament, which attaches to the lateral joint capsule. In addition, 70% to 100% of people have accessory meniscofemoral ligaments, which insert anterior (ligament of Humphrey) or posterior (ligament of Wrisberg) to the posterior cruciate ligament.14 There are no ligamentous attachments at the popliteus hiatus or lateral collateral ligament, allowing for 9- to 11-mm excursion of the lateral meniscus during knee flexion and extension.3 Morphologically, the lack of a meniscotibial (coronary) ligament in the setting of a discoid lateral meniscus (Wrisberg variant) results in meniscal hypermobility. During knee range of motion, compressive forces between the femoral condyle and the tibial plateau spread through the peripheral portion of the meniscus and, without ligamentous attachments, allow it to displace anteriorly into the femoral intercondylar notch. This displacement results in impingement between the femur and the tibia15-18 and leads to the characteristic symptoms of “snapping knee syndrome.”10
Clinical Features
Snapping knee syndrome was first described by Kroiss19 in 1910.5 Multiple authors have described patients’ primary complaints as pain, swelling, locking, and a palpable or visible snap at terminal extension. Sudden movement of a soft-tissue structure across a bony prominence during a provocative maneuver is the source of the snapping. The syndrome has many etiologies. Extra-articular causes of lateral snapping knee syndrome include iliotibial band friction syndrome, soft-tissue tumors, hypermobile popliteus tendons, and abnormal anterior insertions of the biceps femoris tendons.20,21 Common intra-articular etiologies include ganglion, synovial, and parameniscal cysts; intra-articular loose bodies; lateral meniscal tears; and discoid lateral menisci.22 Patients with discoid lateral menisci often present with knee pain, popping, range-of-motion limitations, and snapping.23,24 However, the symptoms are quite variable and depend on type of discoid meniscus, presence of a tear, and stability of the rim.2,7,18
Obtaining a thorough history is essential in evaluating patients with suspected discoid lateral menisci. Physical examination should include evaluation of the lateral joint line for bulges, effusion, and tenderness. Patients may experience knee pain with flexion to 30° to 40° when varus or valgus stress (modified McMurray maneuver) is applied.10 In addition, a clunk may be appreciated with McMurray testing as a result of subluxation of the unstable lateral meniscus.10 The contralateral knee should be carefully evaluated, given the frequency of bilateral discoid menisci.10
The figure-4 test, a maneuver developed by LaPrade and Konowalchuk25 to detect peripheral meniscal tears or tears of the popliteomeniscal fascicles, is performed with the patient in the supine position, with the foot of the affected extremity placed on the contralateral knee. Normally, the popliteus tendon pulls the meniscus out of the joint when the knee is brought into the figure-4 position. However, without popliteomeniscal fascicles, the meniscus subluxes into the joint and becomes impinged. With the patient in the figure-4 position, reproduction of symptoms over the lateral joint line is a positive test and suggests peripheral meniscal tears and/or tears or absence of the popliteomeniscal fascicles.25
In the series reported by LaPrade and Konowalchuk,25 all of the patients who experienced symptoms during figure-4 testing were found, on arthroscopic examination, to have lateral meniscal hypermobility caused by tears of the popliteomeniscal fascicles. Despite the success of those authors in using the figure-4 technique for diagnosis, others have reported that the accuracy of the clinical examination (vs arthroscopy) in diagnosing Wrisberg-variant discoid lateral menisci ranges from 29% to 93%.5,26,27 This emphasizes the importance of diagnostic imaging in the work-up of patients with suspected Wrisberg-variant discoid lateral menisci.
Imaging Features
Radiography
In 1964, Picard and Constantin28 recommended that patients with suspected discoid lateral menisci undergo standard anteroposterior, lateral, tunnel, and skyline radiographs as part of the diagnostic work-up. In patients with discoid lateral menisci, plain film radiographs are often normal10 but may demonstrate lateral femoral condyle squaring, widening of the lateral joint line, lateral tibial plateau cupping, tibial eminence hypoplasia, and fibular head elevation.5,29 Plain radiography is unreliable, however, and patients often require advanced imaging, such as knee magnetic resonance imaging (MRI).10
Magnetic Resonance Imaging
Because it clearly depicts soft-tissue structures, MRI is widely used to diagnose musculoskeletal pathology in and around the knee. Criteria for the diagnosis of discoid menisci include meniscal width of 15 mm or more, ratio of minimum meniscal width to maximum tibial width on coronal slice of more than 20%, ratio of sum of width of both lateral horns to meniscal diameter (on sagittal slice showing maximum meniscal diameter) of more than 75%, and continuity of anterior and posterior horns on at least 3 consecutive sagittal slices (bow tie sign).5,30,31 Even in the presence of a tear, the described ratios have sensitivity and specificity of 95% and 97% in detecting discoid lateral menisci.30
However, the Wrisberg variant, which may consist of only a thickened portion of the posterior horn, is often more difficult to diagnose using these criteria and can even appear normal on MRI.26,32 In a series by Neuschwander and colleagues,7 none of the Wrisberg-variant menisci had a true discoid shape, suggesting that the size of the lateral meniscus may appear normal in affected patients. Appropriate positioning during MRI evaluation of patients suspected of having the Wrisberg variant was emphasized by Moser and colleagues,33 who described a case of discoid lateral meniscus not observable on initial MRI but visible on MRI performed with the affected knee extended in the locked position.
The unstable lateral meniscus may be seen subluxed anteriorly or laterally because of lack of posterior attachments. A deficiency of normal popliteomeniscal fascicles and coronary ligaments is represented by a high T2 signal interposed between the discoid lateral meniscus and the posterior joint capsule, simulating a vertical peripheral tear and suggesting presence of the Wrisberg variant (Figures 1A–1C). In addition, the posterior horn of the enlarged discoid lateral meniscus may connect to a prominent and thickened meniscofemoral ligament of Wrisberg. Despite these characteristic imaging features, some studies have found low sensitivity of MRI in the diagnosis of Wrisberg-variant discoid lateral menisci.26
Ultrasonography
There is a growing interest in using ultrasonography in the diagnosis of Wrisberg-variant discoid lateral menisci because of its availability, multiplanar capability, and lower cost compared with MRI. Ultrasonographic criteria for the diagnosis of discoid menisci include absence of normal triangular shape, presence of abnormally elongated and thickened meniscal tissue, and demonstration of a heterogeneous central pattern.5 Through use of a high-resolution probe, which better fits the anatomical concavity of the popliteal fossa, a positive predictive value of 95% and a negative predictive value of 100% have been reported for ultrasonography in the diagnosis of meniscal tears.34
Perhaps the main advantage of ultrasonography is the possibility of performing a dynamic study to evaluate the extrusion of the meniscus into the lateral gutter and to correlate this with knee snapping (Figures 2A, 2B).35 One technique for sonographic evaluation of a hypermobile lateral meniscus involves placing the patient supine with the high-resolution (9 or 12 MHz) linear transducer along the lateral knee joint line. The patient is then asked to place the foot of the affected extremity on the contralateral knee; the combination resembles the numeral 4 (figure-4 test) (Figures 3A, 3B). In a symptomatic patient, this results in clicking, snapping, and/or pain along the lateral joint line, and the lateral meniscus is noted sonographically to extrude into the lateral gutter (Figure 2B), either the result of torn popliteomeniscal fascicles or the increased meniscal mobility of Wrisberg variants.
The main drawback of ultrasonography is operator dependence. As clinicians become more familiar with ultrasonography, dynamic ultrasonography should be used for what is often a difficult diagnosis both clinically and with nondynamic imaging.
Management
The historical treatment for symptomatic discoid lateral menisci, open total meniscectomy,5,7,15,36 is no longer performed, as studies have shown it increases contact stresses proportional to the amount of meniscus removed, with up to a 235% increase after total meniscectomy,37 predisposing patients to early degenerative changes and osteoarthritis.38-41
With an appreciation of the role of menisci as load distributors and joint stabilizers in cartilage nutrition, current treatments aim to preserve as much stable meniscal tissue as possible.5 Surgical management of Wrisberg-variant discoid lateral menisci involves posterior stabilization with or without saucerization.7,33,42 The goal of arthroscopic saucerization is to preserve healthy tissue and create a stable remaining meniscus (6-8 mm in width)2,7,43,44 that provides adequate shock absorption without retearing.10 Wrisberg-variant discoid menisci can be stabilized with use of all-inside sutures from the meniscus to the joint capsule (Figures 4A–4F) when there is sufficient residual meniscus to allow for suture fixation to the posterior capsule after débridement. In contrast, some prefer an inside-out technique, as described by Neuschwander and colleagues,7 with inclusion of a mini-open approach. Any meniscal tears are addressed at time of surgery, either by partial meniscectomy or repair. Relative indications for meniscal repair include longitudinal, vertical, nondegenerative tears that are within 3 mm of the periphery (vascular zone) and are less than 3 cm in length.45 However, the majority of tears in adults are degenerative cleavage tears outside the vascular zone and therefore not amenable to repair.45,46 Before surgery, patients treated with stabilization with or without saucerization are prescribed partial weight-bearing in a hinged knee brace with gradual range of motion to 90° by 6 weeks and return to sports in 3 to 4 months.
Clinical Results
As has been consistently demonstrated, the long-term outcomes of total meniscectomy are poor function39,40,47 and radiographic evidence of lateral compartment arthritis.48 Patients who previously underwent total meniscectomy should be offered meniscal allograft transplantation, as it may offset the increased peak local contact pressures in the lateral compartment10 and improve function.49
With an appreciation for the importance of meniscus preservation, more recent studies have found encouraging results for arthroscopic saucerization and stabilization of Wrisberg-variant discoid lateral menisci. For example, Woods and Whelan44 reported excellent results in 75% of patients at 37.5-month follow-up after open repair of discoid lateral menisci lacking posterior attachments. In another study, by Neuschwander and colleagues,7 4 of 6 patients who underwent arthroscopic repair of unstable discoid lateral menisci without posterior coronary ligaments had excellent outcomes. Although these studies demonstrated symptom resolution and lack of radiographic evidence of degenerative changes at midterm follow-up,50 additional long-term studies should be performed to determine whether saucerization and stabilization prevent the onset of lateral compartment osteoarthritis.
Conclusion
Abnormally mobile discoid lateral menisci can result in painful lateral snapping knee syndromes but are often challenging to diagnose clinically and with traditional static imaging. Dynamic ultrasonography with provocative maneuvers can reveal lateral meniscal subluxation, which often cannot be appreciated on MRI, allowing for timely stabilization and symptom resolution.
1. Young RB. The external semilunar cartilage as a complete disc. In: Cleland J, Mackey JY, Young RB, eds. Memoirs and Memoranda in Anatomy. London, England: Williams & Norgate; 1889:179.
2. Jordan MR. Lateral meniscal variants: evaluation and treatment. J Am Acad Orthop Surg. 1996;4(4):191-200.
3. Greis PE, Bardana DD, Holmstrom MC, Burks RT. Meniscal injury: I. Basic science and evaluation. J Am Acad Orthop Surg. 2002;10(3):168-176.
4. Ikeuchi H. Arthroscopic treatment of the discoid lateral meniscus. Technique and long-term results. Clin Orthop. 1982;(167):19-28.
5. Yaniv M, Blumberg N. The discoid meniscus. J Child Orthop. 2007;1(2):89-96.
6. Watanabe M, Takeda S, Ikeuchi H. Atlas of Arthroscopy. Tokyo, Japan: Igaku-Shoin; 1978.
7. Neuschwander DC, Drez D Jr, Finney TP. Lateral meniscal variant with absence of the posterior coronary ligament. J Bone Joint Surg Am. 1992;74(8):1186-1190.
8. Clark CR, Ogden JA. Development of the menisci of the human knee joint. Morphological changes and their potential role in childhood meniscal injury. J Bone Joint Surg Am. 1983;65(4):538-547.
9. Kaplan EB. Discoid lateral meniscus of the knee joint; nature, mechanism, and operative treatment. J Bone Joint Surg Am. 1957;39(1):77-87.
10. Kramer DE, Micheli LJ. Meniscal tears and discoid meniscus in children: diagnosis and treatment. J Am Acad Orthop Surg. 2009;17(11):698-707.
11. Atay OA, Pekmezci M, Doral MN, Sargon MF, Ayvaz M, Johnson DL. Discoid meniscus: an ultrastructural study with transmission electron microscopy. Am J Sports Med. 2007;35(3):475-478.
12. Nathan PA, Cole SC. Discoid meniscus. A clinical and pathologic study. Clin Orthop. 1969;(64):107-113.
13. Good CR, Green DW, Griffith MH, Valen AW, Widmann RF, Rodeo SA. Arthroscopic treatment of symptomatic discoid meniscus in children: classification, technique, and results. Arthroscopy. 2007;23(2):157-163.
14. Harner CD, Xerogeanes JW, Livesay GA, et al. The human posterior cruciate ligament complex: an interdisciplinary study. Ligament morphology and biomechanical evaluation. Am J Sports Med. 1995;23(6):736-745.
15. Smillie IS. The congenital discoid meniscus. J Bone Joint Surg Br. 1948;30(4):671-682.
16. Yoo WJ, Choi IH, Chung CY, et al. Discoid lateral meniscus in children: limited knee extension and meniscal instability in the posterior segment. J Pediatr Orthop. 2008;28(5):544-548.
17. Simonian PT, Sussmann PS, Wickiewicz TL, et al. Popliteomeniscal fasciculi and the unstable lateral meniscus: clinical correlation and magnetic resonance diagnosis. Arthroscopy. 1997;13(5):590-596.
18. Dickhaut SC, DeLee JC. The discoid lateral-meniscus syndrome. J Bone Joint Surg Am. 1982;64(7):1068-1073.
19. Kroiss F. Die Verletzungen der Kniegelenkoszwischenknorpel und ihrer Verbindungen. Beitr Klin Chir. 1910;66:598-801.
20. Lokiec F, Velkes S, Schindler A, Pritsch M. The snapping biceps femoris syndrome. Clin Orthop. 1992;(283):205-206.
21. Cooper DE. Snapping popliteus tendon syndrome. A cause of mechanical knee popping in athletes. Am J Sports Med. 1999;27(5):671-674.
22. Liu PC, Chen CH, Huang HT, et al. Snapping knee symptoms caused by an intra-articular ganglion cyst. Knee. 2007;14(2):167-168.
23. Bellier G, Dupont JY, Larrain M, Caudron C, Carlioz H. Lateral discoid menisci in children. Arthroscopy. 1989;5(1):52-56.
24. Washington ER 3rd, Root L, Liener UC. Discoid lateral meniscus in children. Long-term follow-up after excision. J Bone Joint Surg Am. 1995;77(9):1357-1361.
25. LaPrade RF, Konowalchuk BK. Popliteomeniscal fascicle tears causing symptomatic lateral compartment knee pain: diagnosis by the figure-4 test and treatment by open repair. Am J Sports Med. 2005;33(8):1231-1236.
26. Kocher MS, DiCanzio J, Zurakowski D, Micheli LJ. Diagnostic performance of clinical examination and selective magnetic resonance imaging in the evaluation of intraarticular knee disorders in children and adolescents. Am J Sports Med. 2001;29(3):292-296.
27. Stanitski CL. Correlation of arthroscopic and clinical examinations with magnetic resonance imaging findings of injured knees in children and adolescents. Am J Sports Med. 1998;26(1):2-6.
28. Picard JJ, Constantin L. Radiological aspects of the discoid meniscus [in French]. J Radiol Electrol Med Nucl. 1964;45:839-841.
29. Kerr R. Radiologic case study. Discoid lateral meniscus. Orthopedics. 1986;9(8):1142, 1145-1147.
30. Samoto N, Kozuma M, Tokuhisa T, Kobayashi K. Diagnosis of discoid lateral meniscus of the knee on MR imaging. Magn Reson Imaging. 2002;20(1):59-64.
31. Silverman JM, Mink JH, Deutsch AL. Discoid menisci of the knee: MR imaging appearance. Radiology. 1989;173(2):351-354.
32. Singh K, Helms CA, Jacobs MT, Higgins LD. MRI appearance of Wrisberg variant of discoid lateral meniscus. AJR Am J Roentgenol. 2006;187(2):384-387.
33. Moser MW, Dugas J, Hartzell J, Thornton DD. A hypermobile Wrisberg variant lateral discoid meniscus seen on MRI. Clin Orthop. 2007;(456):264-267.
34. Najafi J, Bagheri S, Lahiji FA. The value of sonography with micro convex probes in diagnosing meniscal tears compared with arthroscopy. J Ultrasound Med. 2006;25(5):593-597.
35. Marchand AJ, Proisy M, Ropars M, Cohen M, Duvauferrier R, Guillin R. Snapping knee: imaging findings with an emphasis on dynamic sonography. AJR Am J Roentgenol. 2012;199(1):142-150.
36. Nathan PA, Cole SC. Discoid meniscus. A clinical and pathologic study. Clin Orthop. 1969;(64):107-113.
37. Baratz ME, Fu FH, Mengato R. Meniscal tears: the effect of meniscectomy and of repair on intraarticular contact areas and stress in the human knee. A preliminary report. Am J Sports Med. 1986;14(4):270-275.
38. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30(4):664-670.
39. Manzione M, Pizzutillo PD, Peoples AB, Schweizer PA. Meniscectomy in children: a long-term follow-up study. Am J Sports Med. 1983;11(3):111-115.
40. Wroble RR, Henderson RC, Campion ER, el-Khoury GY, Albright JP. Meniscectomy in children and adolescents. A long-term follow-up study. Clin Orthop. 1992;(279):180-189.
41. Abdon P, Turner MS, Pettersson H, Lindstrand A, Stenstrom A, Swanson AJ. A long-term follow-up study of total meniscectomy in children. Clin Orthop. 1990;(257):166-170.
42. Rosenberg TD, Paulos LE, Parker RD, Harner CD, Gurley WD. Discoid lateral meniscus: case report of arthroscopic attachment of a symptomatic Wrisberg-ligament type. Arthroscopy. 1987;3(4):277-282.
43. Fleissner PR, Eilert RE. Discoid lateral meniscus. Am J Knee Surg. 1999;12(2):125-131.
44. Woods GW, Whelan JM. Discoid meniscus. Clin Sports Med. 1990;9(3):695-706.
45. Yue BW, Gupta AK, Moorman CT 3rd, Garrett WE, Helms CA. Wrisberg variant of the discoid lateral meniscus with flipped meniscal fragments simulating bucket-handle tear: MRI and arthroscopic correlation. Skeletal Radiol. 2011;40(8):1089-1094.
46. Weiss CB, Lundberg M, Hamberg P, DeHaven KE, Gillquist J. Non-operative treatment of meniscal tears. J Bone Joint Surg Am. 1989;71(6):811-822.
47. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769.
48. Kim SJ, Chun YM, Jeong JH, Ryu SW, Oh KS, Lubis AM. Effects of arthroscopic meniscectomy on the long-term prognosis for the discoid lateral meniscus. Knee Surg Sports Traumatol Arthrosc. 2007;15(11):1315-1320.
49. Kim JM, Bin SI. Meniscal allograft transplantation after total meniscectomy of torn discoid lateral meniscus. Arthroscopy. 2006;22(12):1344-1350.e1.
50. Ogut T, Kesmezacar H, Akgun I, Cansu E. Arthroscopic meniscectomy for discoid lateral meniscus in children and adolescents: 4.5 year follow-up. J Pediatr Orthop B. 2003;12(6):390-397.
1. Young RB. The external semilunar cartilage as a complete disc. In: Cleland J, Mackey JY, Young RB, eds. Memoirs and Memoranda in Anatomy. London, England: Williams & Norgate; 1889:179.
2. Jordan MR. Lateral meniscal variants: evaluation and treatment. J Am Acad Orthop Surg. 1996;4(4):191-200.
3. Greis PE, Bardana DD, Holmstrom MC, Burks RT. Meniscal injury: I. Basic science and evaluation. J Am Acad Orthop Surg. 2002;10(3):168-176.
4. Ikeuchi H. Arthroscopic treatment of the discoid lateral meniscus. Technique and long-term results. Clin Orthop. 1982;(167):19-28.
5. Yaniv M, Blumberg N. The discoid meniscus. J Child Orthop. 2007;1(2):89-96.
6. Watanabe M, Takeda S, Ikeuchi H. Atlas of Arthroscopy. Tokyo, Japan: Igaku-Shoin; 1978.
7. Neuschwander DC, Drez D Jr, Finney TP. Lateral meniscal variant with absence of the posterior coronary ligament. J Bone Joint Surg Am. 1992;74(8):1186-1190.
8. Clark CR, Ogden JA. Development of the menisci of the human knee joint. Morphological changes and their potential role in childhood meniscal injury. J Bone Joint Surg Am. 1983;65(4):538-547.
9. Kaplan EB. Discoid lateral meniscus of the knee joint; nature, mechanism, and operative treatment. J Bone Joint Surg Am. 1957;39(1):77-87.
10. Kramer DE, Micheli LJ. Meniscal tears and discoid meniscus in children: diagnosis and treatment. J Am Acad Orthop Surg. 2009;17(11):698-707.
11. Atay OA, Pekmezci M, Doral MN, Sargon MF, Ayvaz M, Johnson DL. Discoid meniscus: an ultrastructural study with transmission electron microscopy. Am J Sports Med. 2007;35(3):475-478.
12. Nathan PA, Cole SC. Discoid meniscus. A clinical and pathologic study. Clin Orthop. 1969;(64):107-113.
13. Good CR, Green DW, Griffith MH, Valen AW, Widmann RF, Rodeo SA. Arthroscopic treatment of symptomatic discoid meniscus in children: classification, technique, and results. Arthroscopy. 2007;23(2):157-163.
14. Harner CD, Xerogeanes JW, Livesay GA, et al. The human posterior cruciate ligament complex: an interdisciplinary study. Ligament morphology and biomechanical evaluation. Am J Sports Med. 1995;23(6):736-745.
15. Smillie IS. The congenital discoid meniscus. J Bone Joint Surg Br. 1948;30(4):671-682.
16. Yoo WJ, Choi IH, Chung CY, et al. Discoid lateral meniscus in children: limited knee extension and meniscal instability in the posterior segment. J Pediatr Orthop. 2008;28(5):544-548.
17. Simonian PT, Sussmann PS, Wickiewicz TL, et al. Popliteomeniscal fasciculi and the unstable lateral meniscus: clinical correlation and magnetic resonance diagnosis. Arthroscopy. 1997;13(5):590-596.
18. Dickhaut SC, DeLee JC. The discoid lateral-meniscus syndrome. J Bone Joint Surg Am. 1982;64(7):1068-1073.
19. Kroiss F. Die Verletzungen der Kniegelenkoszwischenknorpel und ihrer Verbindungen. Beitr Klin Chir. 1910;66:598-801.
20. Lokiec F, Velkes S, Schindler A, Pritsch M. The snapping biceps femoris syndrome. Clin Orthop. 1992;(283):205-206.
21. Cooper DE. Snapping popliteus tendon syndrome. A cause of mechanical knee popping in athletes. Am J Sports Med. 1999;27(5):671-674.
22. Liu PC, Chen CH, Huang HT, et al. Snapping knee symptoms caused by an intra-articular ganglion cyst. Knee. 2007;14(2):167-168.
23. Bellier G, Dupont JY, Larrain M, Caudron C, Carlioz H. Lateral discoid menisci in children. Arthroscopy. 1989;5(1):52-56.
24. Washington ER 3rd, Root L, Liener UC. Discoid lateral meniscus in children. Long-term follow-up after excision. J Bone Joint Surg Am. 1995;77(9):1357-1361.
25. LaPrade RF, Konowalchuk BK. Popliteomeniscal fascicle tears causing symptomatic lateral compartment knee pain: diagnosis by the figure-4 test and treatment by open repair. Am J Sports Med. 2005;33(8):1231-1236.
26. Kocher MS, DiCanzio J, Zurakowski D, Micheli LJ. Diagnostic performance of clinical examination and selective magnetic resonance imaging in the evaluation of intraarticular knee disorders in children and adolescents. Am J Sports Med. 2001;29(3):292-296.
27. Stanitski CL. Correlation of arthroscopic and clinical examinations with magnetic resonance imaging findings of injured knees in children and adolescents. Am J Sports Med. 1998;26(1):2-6.
28. Picard JJ, Constantin L. Radiological aspects of the discoid meniscus [in French]. J Radiol Electrol Med Nucl. 1964;45:839-841.
29. Kerr R. Radiologic case study. Discoid lateral meniscus. Orthopedics. 1986;9(8):1142, 1145-1147.
30. Samoto N, Kozuma M, Tokuhisa T, Kobayashi K. Diagnosis of discoid lateral meniscus of the knee on MR imaging. Magn Reson Imaging. 2002;20(1):59-64.
31. Silverman JM, Mink JH, Deutsch AL. Discoid menisci of the knee: MR imaging appearance. Radiology. 1989;173(2):351-354.
32. Singh K, Helms CA, Jacobs MT, Higgins LD. MRI appearance of Wrisberg variant of discoid lateral meniscus. AJR Am J Roentgenol. 2006;187(2):384-387.
33. Moser MW, Dugas J, Hartzell J, Thornton DD. A hypermobile Wrisberg variant lateral discoid meniscus seen on MRI. Clin Orthop. 2007;(456):264-267.
34. Najafi J, Bagheri S, Lahiji FA. The value of sonography with micro convex probes in diagnosing meniscal tears compared with arthroscopy. J Ultrasound Med. 2006;25(5):593-597.
35. Marchand AJ, Proisy M, Ropars M, Cohen M, Duvauferrier R, Guillin R. Snapping knee: imaging findings with an emphasis on dynamic sonography. AJR Am J Roentgenol. 2012;199(1):142-150.
36. Nathan PA, Cole SC. Discoid meniscus. A clinical and pathologic study. Clin Orthop. 1969;(64):107-113.
37. Baratz ME, Fu FH, Mengato R. Meniscal tears: the effect of meniscectomy and of repair on intraarticular contact areas and stress in the human knee. A preliminary report. Am J Sports Med. 1986;14(4):270-275.
38. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30(4):664-670.
39. Manzione M, Pizzutillo PD, Peoples AB, Schweizer PA. Meniscectomy in children: a long-term follow-up study. Am J Sports Med. 1983;11(3):111-115.
40. Wroble RR, Henderson RC, Campion ER, el-Khoury GY, Albright JP. Meniscectomy in children and adolescents. A long-term follow-up study. Clin Orthop. 1992;(279):180-189.
41. Abdon P, Turner MS, Pettersson H, Lindstrand A, Stenstrom A, Swanson AJ. A long-term follow-up study of total meniscectomy in children. Clin Orthop. 1990;(257):166-170.
42. Rosenberg TD, Paulos LE, Parker RD, Harner CD, Gurley WD. Discoid lateral meniscus: case report of arthroscopic attachment of a symptomatic Wrisberg-ligament type. Arthroscopy. 1987;3(4):277-282.
43. Fleissner PR, Eilert RE. Discoid lateral meniscus. Am J Knee Surg. 1999;12(2):125-131.
44. Woods GW, Whelan JM. Discoid meniscus. Clin Sports Med. 1990;9(3):695-706.
45. Yue BW, Gupta AK, Moorman CT 3rd, Garrett WE, Helms CA. Wrisberg variant of the discoid lateral meniscus with flipped meniscal fragments simulating bucket-handle tear: MRI and arthroscopic correlation. Skeletal Radiol. 2011;40(8):1089-1094.
46. Weiss CB, Lundberg M, Hamberg P, DeHaven KE, Gillquist J. Non-operative treatment of meniscal tears. J Bone Joint Surg Am. 1989;71(6):811-822.
47. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769.
48. Kim SJ, Chun YM, Jeong JH, Ryu SW, Oh KS, Lubis AM. Effects of arthroscopic meniscectomy on the long-term prognosis for the discoid lateral meniscus. Knee Surg Sports Traumatol Arthrosc. 2007;15(11):1315-1320.
49. Kim JM, Bin SI. Meniscal allograft transplantation after total meniscectomy of torn discoid lateral meniscus. Arthroscopy. 2006;22(12):1344-1350.e1.
50. Ogut T, Kesmezacar H, Akgun I, Cansu E. Arthroscopic meniscectomy for discoid lateral meniscus in children and adolescents: 4.5 year follow-up. J Pediatr Orthop B. 2003;12(6):390-397.
Intraoperative Radiofrequency Ablation for Osteoid Osteoma
Osteoid osteoma (OO) is one of the most common benign tumors of bone, representing roughly 10% of all benign bone-forming tumors and 5% of all primary bone tumors.1 The majority of cases occur in individuals under age 20 years and more frequently in males (2:1).2 These lesions tend to be cortically based and most often located about the hip and in the diaphysis of long bones. They typically are characterized radiographically by a nidus less than 2 cm in diameter surrounded by dense, reactive bone of variable thickness.
The classic presentation of OO is localized, dull, aching pain that is worse at night and that is relieved with use of salicylates or other nonsteroidal anti-inflammatory drugs (NSAIDs).3 The diagnosis is made by patient history and plain radiographs, often supported by computed tomography (CT) or magnetic resonance imaging for appropriate identification of the tumor nidus. Despite effective pain relief with NSAIDs as well as evidence suggesting that the natural history of these tumors is self-limited, most patients forgo medical management in favor of elective surgical treatment.4,5
Initially, treatment for OO focused on either symptom management or en bloc surgical resection of the tumor nidus. Several different minimally invasive therapies have since been developed, and good results reported.6-8 More recently, use of percutaneous radiofrequency ablation (RFA) has increased, as this method has demonstrated high efficacy and minimal morbidity.9-11 RFA for OO traditionally has been performed by radiologists under CT guidance in the radiology suite, but advances in intraoperative imaging techniques now allow orthopedic oncologists to perform image-guided RFA in the operating room.
To our knowledge, there have been no reports documenting use of intraoperative CT for localization of OO and use of RFA in the treatment of this lesion. In this article, we report the results of a series of 28 patients with OO treated with intraoperative CT-guided RFA by a single surgeon. We also provide a brief description of this novel technique.
Materials and Methods
The protocol used was approved by our institutional review board. All patients and/or their legal guardians provided informed consent to participate in the study and were informed at the time consent was obtained that case-related data would be submitted for publication.
Patients
Between September 2004 and December 2008, 28 patients (19 males, 9 females) with OO underwent intraoperative percutaneous image-guided RFA at a university hospital. Mean age was 19.5 years, median age was 16 years (range, 7-54 years). Patients were referred for RFA if they had clinical and radiographic features of OO (Figures 1, 2) and wanted to forgo continued medical management. As we selected only patients with lesions that we thought were amenable to percutaneous RFA—lesions involving the long and short bones of the upper or lower extremity and selected flat bones—en bloc surgical resection was not offered to these patients. Lesions were located in the upper extremity (n = 1), lower extremity (n = 24), and pelvis (n = 3) (Figure 3). Twenty-seven procedures were performed for initial tumor treatment and 1 for recurrence after previous open excision. Two additional procedures were later performed on separate patients with recurrent symptoms after the index procedure. All procedures were performed by the senior author (DML).
Procedure
With each patient, all options were discussed, including continued medical management versus surgical treatment, and informed consent was obtained. All procedures were performed with the patient under general anesthesia in the operating room. RFA for an upper extremity lesion was performed with the patient in the supine position with the ipsilateral extremity draped over a hand table. The 2 procedures for lesions in the talus or calcaneus were performed with the patient in the supine position using a standard table with the bottom of the table flexed down 90° to allow the nonaffected leg to hang over the end of the table. The affected extremity in each case was then positioned in a well-padded leg holder to allow the foot and ankle to be draped free for 360° imaging.
All other procedures for lower extremity diaphyseal or pelvic lesions were performed with a fracture table. After successful induction of general anesthesia, the patient was positioned supine on the table with the contralateral lower extremity abducted and externally rotated in a well-leg holder. The ipsilateral leg was held in the traction apparatus without traction applied and was prepared and draped accordingly (Figure 4). With use of the Siemens Siremobil ISO-C3D fluoroscopic C-arm (Siemens Medical Solutions, Malvern, Pennsylvania), a radiograph was taken of the affected area to identify the lesion. Local anesthetic was infiltrated into the surgical site down to the periosteum. A stab incision was made, and, with fluoroscopic guidance, a 0.062-mm Kirschner wire (K-wire) was placed into the lesion. Location within the tumor nidus was confirmed with biplanar fluoroscopic imaging. A Bonopty cannula (AprioMed, Uppsala, Sweden) was then passed over the K-wire. After the wire was removed, a 5-mm radiofrequency probe (Radionics, Burlington, Massachusetts) was placed through the cannula, and positioning within the nidus was confirmed with 3-dimensional (3-D) CT reconstructions in the sagittal, coronal, and axial planes (Figure 5). A radiofrequency generator (Radionics) was used to heat the lesion at 93°C for 7 minutes. The probe and trocar were then removed. Steri-strips and a sterile dressing were used to cover the wound, and the patient was taken to the recovery area after extubation. All patients were discharged home the day of the procedure.
Follow-Up
We phoned all the patients to ask about symptom recurrence, outside treatment, and satisfaction with RFA and to obtain informed consent to participate in our study. Only 1 of the 28 patients could not be reached and was lost to follow-up. Mean follow-up at time of study completion was 31.1 months (range, 5.2-55.8 months).
The 27 patients were asked a series of questions about their treatment: Have you had any recurrence of symptoms following treatment for your OO? Have you received treatment elsewhere? Were you satisfied with your treatment? Would you have the procedure again if you had a recurrence of symptoms?
Primary success was defined as complete pain relief after initial RFA with no evidence of recurrence at time of final follow-up, and secondary success was defined as presence of recurrent symptoms after initial RFA with complete pain relief after a second procedure with no evidence of recurrence.
Results
All RFAs were technically successful with adequate localization of the tumor nidus and subsequent probe placement within the lesion. There were no intraoperative or postoperative complications. All 28 patients were discharged home the day of procedure. Twenty-six patients (92.8%) experienced complete pain relief after primary RFA, had no evidence of recurrence at final follow-up, and denied symptom recurrence at time of study completion.
The other 2 patients reported symptom recurrence after the index treatment (1 proximal femur lesion, 1 distal femur lesion). One of these patients did well initially but had a recurrence about 2 months after the primary RFA; a second RFA provided complete resolution of pain with no evidence of recurrence at time of study completion. In the other patient’s case, intermittent pain persisted for 2 weeks after the primary RFA, and evidence of recurrence was documented 3 months after surgery; a second RFA was performed shortly thereafter, but the patient was subsequently lost to follow-up.
At time of study completion, all 27 patients who had been contacted by phone denied seeking additional treatment elsewhere and stated they would have the procedure again if their symptoms ever recurred.
Discussion
Osteoid osteoma is one of the most common benign tumors of bone. Over the past 2 decades, percutaneous RFA, in comparison with open excision, has emerged as a safe and effective treatment option with minimal patient morbidity.9-11 RFA traditionally has been performed by radiologists under CT guidance in the radiology suite. However, now orthopedic surgeons can obtain advanced intraoperative imaging beyond standard fluoroscopy. The Siemens Siremobil ISO-C3D fluoroscopic C-arm is an innovative intraoperative imaging device that functions as a standard fluoroscope but also generates 3-D reconstructions of surgical anatomy. The isocentric design and integrated motor unit allow the C-arm to move through a 190º arc while centering its beam directly on the area of interest. This data set is transferred to a computer workstation, where it is reformatted so that CT-quality images are generated in axial, sagittal, and coronal planes. This acquisition process takes only minutes, and the multiplanar images produced may be simultaneously displayed and manipulated on the screen in real time.
One concern about this technology is the amount of radiation exposure for patients, surgeons, and operating room staff. The device measures only radiation time, and the amount of exposure during that time depends on the volume and density of the radiated body. We did not calculate the amount of exposure for this study. Mean exposure time was between 20 and 40 seconds, reflecting the number of attempts required to localize the lesion and the surgeon’s experience with the technique. Although the potential for increased exposure is a valid concern, previous studies using this technology have demonstrated that a similar average exposure time is equivalent to that of standard CT, and that use of the device, over conventional techniques, potentially can lead to decreased overall radiation exposure.12,13
This series demonstrated that OO can be safely and effectively treated with intraoperative percutaneous RFA by an orthopedic oncologist. Our success rate is very similar to rates reported in the radiology literature. Studies are needed to confirm the efficacy of this novel technique in comparison with what has been reported in that literature. Given these promising preliminary results, and the relative ease of use and minimal learning curve associated with this technology, all orthopedic oncologists should be able to offer this treatment for OO. Furthermore, this technique allows orthopedic oncologists to provide appropriate definitive treatment and care directly, rather than by referring patients to radiologists.
In the treatment of OO, we reserve RFA for lesions involving the long and short bones of the upper and lower extremities, as well as selected flat bones, such as those in the pelvis. Although percutaneous RFA of spinal lesions has been reported in the literature, we think these represent a relative contraindication for this technique; image resolution, in our opinion, is not high enough to justify risking injury to the nerves in the spinal canal, lateral recesses, and neural foramina. In addition, given the radiation exposure, we recommend caution when using this technique for a pelvic or proximal femoral lesion in a woman of childbearing age.
1. Gitelis S, Wilkins R, Conrad EU 2nd. Benign bone tumors. Instr Course Lect. 1996;45:425-424.
2. Schajowicz F. Bone forming tumors. In: Tumors and Tumorlike Lesions of Bone. 2nd ed. New York, NY: Springer-Verlag; 1994:36-62.
3. Frassica FJ, Waltrip RL, Sponseller PD, Ma LD, McCarthy EF Jr. Clinicopathologic features and treatment of osteoid osteoma and osteoblastoma in children and adolescents. Orthop Clin North Am. 1996;27(3):559-574.
4. Golding JS. The natural history of osteoid osteoma; with a report of twenty cases. J Bone Joint Surg Br. 1954;36(2):218-229.
5. Simm RJ. The natural history of osteoid osteoma. Aust N Z J Surg. 1975;45(4):412-415.
6. Sans N, Galy-Fourcade D, Assoun J, et al. Osteoid osteoma: CT-guided percutaneous resection and follow-up in 38 patients. Radiology. 1999;212(3):687-692.
7. Skjeldal S, Lilleås F, Follerås G, et al. Real time MRI-guided excision and cryo-treatment of osteoid osteoma in os ischii—a case report. Acta Orthop Scand. 2000;71(6):637-638.
8. Sanhaji L, Gharbaoui IS, Hassani RE, Chakir N, Jiddane M, Boukhrissi N. A new treatment of osteoid osteoma: percutaneous sclerosis with ethanol under scanner guidance [in French]. J Radiol. 1996;77(1):37-40.
9. Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229(1):171-175.
10. Cantwell CP, Obyrne J, Eustace S. Current trends in treatment of osteoid osteoma with an emphasis on radiofrequency ablation. Eur Radiol. 2004;14(4):607-617.
11. Ruiz Santiago F, Castellano García Mdel M, Guzmán Álvarez L, Martínez Montes JL, Ruiz García M, Tristán Fernández JM. Percutaneous treatment of bone tumors by radiofrequency thermal ablation. Eur J Radiol. 2011;77(1):156-163.
12. Richter M, Geerling J, Zech S, Goesling T, Krettek C. Intraoperative three-dimensional imaging with a motorized mobile C-Arm (SIREMOBIL ISO-C-3D) in foot and ankle trauma care: a preliminary report. J Orthop Trauma. 2005;19(4):259-266.
13. Gebhard F, Kraus M, Schneider E, et al. Radiation dosage in orthopedics—a comparison of computer-assisted procedures [in German]. Unfallchirurg. 2003;106(6):492-497.
Osteoid osteoma (OO) is one of the most common benign tumors of bone, representing roughly 10% of all benign bone-forming tumors and 5% of all primary bone tumors.1 The majority of cases occur in individuals under age 20 years and more frequently in males (2:1).2 These lesions tend to be cortically based and most often located about the hip and in the diaphysis of long bones. They typically are characterized radiographically by a nidus less than 2 cm in diameter surrounded by dense, reactive bone of variable thickness.
The classic presentation of OO is localized, dull, aching pain that is worse at night and that is relieved with use of salicylates or other nonsteroidal anti-inflammatory drugs (NSAIDs).3 The diagnosis is made by patient history and plain radiographs, often supported by computed tomography (CT) or magnetic resonance imaging for appropriate identification of the tumor nidus. Despite effective pain relief with NSAIDs as well as evidence suggesting that the natural history of these tumors is self-limited, most patients forgo medical management in favor of elective surgical treatment.4,5
Initially, treatment for OO focused on either symptom management or en bloc surgical resection of the tumor nidus. Several different minimally invasive therapies have since been developed, and good results reported.6-8 More recently, use of percutaneous radiofrequency ablation (RFA) has increased, as this method has demonstrated high efficacy and minimal morbidity.9-11 RFA for OO traditionally has been performed by radiologists under CT guidance in the radiology suite, but advances in intraoperative imaging techniques now allow orthopedic oncologists to perform image-guided RFA in the operating room.
To our knowledge, there have been no reports documenting use of intraoperative CT for localization of OO and use of RFA in the treatment of this lesion. In this article, we report the results of a series of 28 patients with OO treated with intraoperative CT-guided RFA by a single surgeon. We also provide a brief description of this novel technique.
Materials and Methods
The protocol used was approved by our institutional review board. All patients and/or their legal guardians provided informed consent to participate in the study and were informed at the time consent was obtained that case-related data would be submitted for publication.
Patients
Between September 2004 and December 2008, 28 patients (19 males, 9 females) with OO underwent intraoperative percutaneous image-guided RFA at a university hospital. Mean age was 19.5 years, median age was 16 years (range, 7-54 years). Patients were referred for RFA if they had clinical and radiographic features of OO (Figures 1, 2) and wanted to forgo continued medical management. As we selected only patients with lesions that we thought were amenable to percutaneous RFA—lesions involving the long and short bones of the upper or lower extremity and selected flat bones—en bloc surgical resection was not offered to these patients. Lesions were located in the upper extremity (n = 1), lower extremity (n = 24), and pelvis (n = 3) (Figure 3). Twenty-seven procedures were performed for initial tumor treatment and 1 for recurrence after previous open excision. Two additional procedures were later performed on separate patients with recurrent symptoms after the index procedure. All procedures were performed by the senior author (DML).
Procedure
With each patient, all options were discussed, including continued medical management versus surgical treatment, and informed consent was obtained. All procedures were performed with the patient under general anesthesia in the operating room. RFA for an upper extremity lesion was performed with the patient in the supine position with the ipsilateral extremity draped over a hand table. The 2 procedures for lesions in the talus or calcaneus were performed with the patient in the supine position using a standard table with the bottom of the table flexed down 90° to allow the nonaffected leg to hang over the end of the table. The affected extremity in each case was then positioned in a well-padded leg holder to allow the foot and ankle to be draped free for 360° imaging.
All other procedures for lower extremity diaphyseal or pelvic lesions were performed with a fracture table. After successful induction of general anesthesia, the patient was positioned supine on the table with the contralateral lower extremity abducted and externally rotated in a well-leg holder. The ipsilateral leg was held in the traction apparatus without traction applied and was prepared and draped accordingly (Figure 4). With use of the Siemens Siremobil ISO-C3D fluoroscopic C-arm (Siemens Medical Solutions, Malvern, Pennsylvania), a radiograph was taken of the affected area to identify the lesion. Local anesthetic was infiltrated into the surgical site down to the periosteum. A stab incision was made, and, with fluoroscopic guidance, a 0.062-mm Kirschner wire (K-wire) was placed into the lesion. Location within the tumor nidus was confirmed with biplanar fluoroscopic imaging. A Bonopty cannula (AprioMed, Uppsala, Sweden) was then passed over the K-wire. After the wire was removed, a 5-mm radiofrequency probe (Radionics, Burlington, Massachusetts) was placed through the cannula, and positioning within the nidus was confirmed with 3-dimensional (3-D) CT reconstructions in the sagittal, coronal, and axial planes (Figure 5). A radiofrequency generator (Radionics) was used to heat the lesion at 93°C for 7 minutes. The probe and trocar were then removed. Steri-strips and a sterile dressing were used to cover the wound, and the patient was taken to the recovery area after extubation. All patients were discharged home the day of the procedure.
Follow-Up
We phoned all the patients to ask about symptom recurrence, outside treatment, and satisfaction with RFA and to obtain informed consent to participate in our study. Only 1 of the 28 patients could not be reached and was lost to follow-up. Mean follow-up at time of study completion was 31.1 months (range, 5.2-55.8 months).
The 27 patients were asked a series of questions about their treatment: Have you had any recurrence of symptoms following treatment for your OO? Have you received treatment elsewhere? Were you satisfied with your treatment? Would you have the procedure again if you had a recurrence of symptoms?
Primary success was defined as complete pain relief after initial RFA with no evidence of recurrence at time of final follow-up, and secondary success was defined as presence of recurrent symptoms after initial RFA with complete pain relief after a second procedure with no evidence of recurrence.
Results
All RFAs were technically successful with adequate localization of the tumor nidus and subsequent probe placement within the lesion. There were no intraoperative or postoperative complications. All 28 patients were discharged home the day of procedure. Twenty-six patients (92.8%) experienced complete pain relief after primary RFA, had no evidence of recurrence at final follow-up, and denied symptom recurrence at time of study completion.
The other 2 patients reported symptom recurrence after the index treatment (1 proximal femur lesion, 1 distal femur lesion). One of these patients did well initially but had a recurrence about 2 months after the primary RFA; a second RFA provided complete resolution of pain with no evidence of recurrence at time of study completion. In the other patient’s case, intermittent pain persisted for 2 weeks after the primary RFA, and evidence of recurrence was documented 3 months after surgery; a second RFA was performed shortly thereafter, but the patient was subsequently lost to follow-up.
At time of study completion, all 27 patients who had been contacted by phone denied seeking additional treatment elsewhere and stated they would have the procedure again if their symptoms ever recurred.
Discussion
Osteoid osteoma is one of the most common benign tumors of bone. Over the past 2 decades, percutaneous RFA, in comparison with open excision, has emerged as a safe and effective treatment option with minimal patient morbidity.9-11 RFA traditionally has been performed by radiologists under CT guidance in the radiology suite. However, now orthopedic surgeons can obtain advanced intraoperative imaging beyond standard fluoroscopy. The Siemens Siremobil ISO-C3D fluoroscopic C-arm is an innovative intraoperative imaging device that functions as a standard fluoroscope but also generates 3-D reconstructions of surgical anatomy. The isocentric design and integrated motor unit allow the C-arm to move through a 190º arc while centering its beam directly on the area of interest. This data set is transferred to a computer workstation, where it is reformatted so that CT-quality images are generated in axial, sagittal, and coronal planes. This acquisition process takes only minutes, and the multiplanar images produced may be simultaneously displayed and manipulated on the screen in real time.
One concern about this technology is the amount of radiation exposure for patients, surgeons, and operating room staff. The device measures only radiation time, and the amount of exposure during that time depends on the volume and density of the radiated body. We did not calculate the amount of exposure for this study. Mean exposure time was between 20 and 40 seconds, reflecting the number of attempts required to localize the lesion and the surgeon’s experience with the technique. Although the potential for increased exposure is a valid concern, previous studies using this technology have demonstrated that a similar average exposure time is equivalent to that of standard CT, and that use of the device, over conventional techniques, potentially can lead to decreased overall radiation exposure.12,13
This series demonstrated that OO can be safely and effectively treated with intraoperative percutaneous RFA by an orthopedic oncologist. Our success rate is very similar to rates reported in the radiology literature. Studies are needed to confirm the efficacy of this novel technique in comparison with what has been reported in that literature. Given these promising preliminary results, and the relative ease of use and minimal learning curve associated with this technology, all orthopedic oncologists should be able to offer this treatment for OO. Furthermore, this technique allows orthopedic oncologists to provide appropriate definitive treatment and care directly, rather than by referring patients to radiologists.
In the treatment of OO, we reserve RFA for lesions involving the long and short bones of the upper and lower extremities, as well as selected flat bones, such as those in the pelvis. Although percutaneous RFA of spinal lesions has been reported in the literature, we think these represent a relative contraindication for this technique; image resolution, in our opinion, is not high enough to justify risking injury to the nerves in the spinal canal, lateral recesses, and neural foramina. In addition, given the radiation exposure, we recommend caution when using this technique for a pelvic or proximal femoral lesion in a woman of childbearing age.
Osteoid osteoma (OO) is one of the most common benign tumors of bone, representing roughly 10% of all benign bone-forming tumors and 5% of all primary bone tumors.1 The majority of cases occur in individuals under age 20 years and more frequently in males (2:1).2 These lesions tend to be cortically based and most often located about the hip and in the diaphysis of long bones. They typically are characterized radiographically by a nidus less than 2 cm in diameter surrounded by dense, reactive bone of variable thickness.
The classic presentation of OO is localized, dull, aching pain that is worse at night and that is relieved with use of salicylates or other nonsteroidal anti-inflammatory drugs (NSAIDs).3 The diagnosis is made by patient history and plain radiographs, often supported by computed tomography (CT) or magnetic resonance imaging for appropriate identification of the tumor nidus. Despite effective pain relief with NSAIDs as well as evidence suggesting that the natural history of these tumors is self-limited, most patients forgo medical management in favor of elective surgical treatment.4,5
Initially, treatment for OO focused on either symptom management or en bloc surgical resection of the tumor nidus. Several different minimally invasive therapies have since been developed, and good results reported.6-8 More recently, use of percutaneous radiofrequency ablation (RFA) has increased, as this method has demonstrated high efficacy and minimal morbidity.9-11 RFA for OO traditionally has been performed by radiologists under CT guidance in the radiology suite, but advances in intraoperative imaging techniques now allow orthopedic oncologists to perform image-guided RFA in the operating room.
To our knowledge, there have been no reports documenting use of intraoperative CT for localization of OO and use of RFA in the treatment of this lesion. In this article, we report the results of a series of 28 patients with OO treated with intraoperative CT-guided RFA by a single surgeon. We also provide a brief description of this novel technique.
Materials and Methods
The protocol used was approved by our institutional review board. All patients and/or their legal guardians provided informed consent to participate in the study and were informed at the time consent was obtained that case-related data would be submitted for publication.
Patients
Between September 2004 and December 2008, 28 patients (19 males, 9 females) with OO underwent intraoperative percutaneous image-guided RFA at a university hospital. Mean age was 19.5 years, median age was 16 years (range, 7-54 years). Patients were referred for RFA if they had clinical and radiographic features of OO (Figures 1, 2) and wanted to forgo continued medical management. As we selected only patients with lesions that we thought were amenable to percutaneous RFA—lesions involving the long and short bones of the upper or lower extremity and selected flat bones—en bloc surgical resection was not offered to these patients. Lesions were located in the upper extremity (n = 1), lower extremity (n = 24), and pelvis (n = 3) (Figure 3). Twenty-seven procedures were performed for initial tumor treatment and 1 for recurrence after previous open excision. Two additional procedures were later performed on separate patients with recurrent symptoms after the index procedure. All procedures were performed by the senior author (DML).
Procedure
With each patient, all options were discussed, including continued medical management versus surgical treatment, and informed consent was obtained. All procedures were performed with the patient under general anesthesia in the operating room. RFA for an upper extremity lesion was performed with the patient in the supine position with the ipsilateral extremity draped over a hand table. The 2 procedures for lesions in the talus or calcaneus were performed with the patient in the supine position using a standard table with the bottom of the table flexed down 90° to allow the nonaffected leg to hang over the end of the table. The affected extremity in each case was then positioned in a well-padded leg holder to allow the foot and ankle to be draped free for 360° imaging.
All other procedures for lower extremity diaphyseal or pelvic lesions were performed with a fracture table. After successful induction of general anesthesia, the patient was positioned supine on the table with the contralateral lower extremity abducted and externally rotated in a well-leg holder. The ipsilateral leg was held in the traction apparatus without traction applied and was prepared and draped accordingly (Figure 4). With use of the Siemens Siremobil ISO-C3D fluoroscopic C-arm (Siemens Medical Solutions, Malvern, Pennsylvania), a radiograph was taken of the affected area to identify the lesion. Local anesthetic was infiltrated into the surgical site down to the periosteum. A stab incision was made, and, with fluoroscopic guidance, a 0.062-mm Kirschner wire (K-wire) was placed into the lesion. Location within the tumor nidus was confirmed with biplanar fluoroscopic imaging. A Bonopty cannula (AprioMed, Uppsala, Sweden) was then passed over the K-wire. After the wire was removed, a 5-mm radiofrequency probe (Radionics, Burlington, Massachusetts) was placed through the cannula, and positioning within the nidus was confirmed with 3-dimensional (3-D) CT reconstructions in the sagittal, coronal, and axial planes (Figure 5). A radiofrequency generator (Radionics) was used to heat the lesion at 93°C for 7 minutes. The probe and trocar were then removed. Steri-strips and a sterile dressing were used to cover the wound, and the patient was taken to the recovery area after extubation. All patients were discharged home the day of the procedure.
Follow-Up
We phoned all the patients to ask about symptom recurrence, outside treatment, and satisfaction with RFA and to obtain informed consent to participate in our study. Only 1 of the 28 patients could not be reached and was lost to follow-up. Mean follow-up at time of study completion was 31.1 months (range, 5.2-55.8 months).
The 27 patients were asked a series of questions about their treatment: Have you had any recurrence of symptoms following treatment for your OO? Have you received treatment elsewhere? Were you satisfied with your treatment? Would you have the procedure again if you had a recurrence of symptoms?
Primary success was defined as complete pain relief after initial RFA with no evidence of recurrence at time of final follow-up, and secondary success was defined as presence of recurrent symptoms after initial RFA with complete pain relief after a second procedure with no evidence of recurrence.
Results
All RFAs were technically successful with adequate localization of the tumor nidus and subsequent probe placement within the lesion. There were no intraoperative or postoperative complications. All 28 patients were discharged home the day of procedure. Twenty-six patients (92.8%) experienced complete pain relief after primary RFA, had no evidence of recurrence at final follow-up, and denied symptom recurrence at time of study completion.
The other 2 patients reported symptom recurrence after the index treatment (1 proximal femur lesion, 1 distal femur lesion). One of these patients did well initially but had a recurrence about 2 months after the primary RFA; a second RFA provided complete resolution of pain with no evidence of recurrence at time of study completion. In the other patient’s case, intermittent pain persisted for 2 weeks after the primary RFA, and evidence of recurrence was documented 3 months after surgery; a second RFA was performed shortly thereafter, but the patient was subsequently lost to follow-up.
At time of study completion, all 27 patients who had been contacted by phone denied seeking additional treatment elsewhere and stated they would have the procedure again if their symptoms ever recurred.
Discussion
Osteoid osteoma is one of the most common benign tumors of bone. Over the past 2 decades, percutaneous RFA, in comparison with open excision, has emerged as a safe and effective treatment option with minimal patient morbidity.9-11 RFA traditionally has been performed by radiologists under CT guidance in the radiology suite. However, now orthopedic surgeons can obtain advanced intraoperative imaging beyond standard fluoroscopy. The Siemens Siremobil ISO-C3D fluoroscopic C-arm is an innovative intraoperative imaging device that functions as a standard fluoroscope but also generates 3-D reconstructions of surgical anatomy. The isocentric design and integrated motor unit allow the C-arm to move through a 190º arc while centering its beam directly on the area of interest. This data set is transferred to a computer workstation, where it is reformatted so that CT-quality images are generated in axial, sagittal, and coronal planes. This acquisition process takes only minutes, and the multiplanar images produced may be simultaneously displayed and manipulated on the screen in real time.
One concern about this technology is the amount of radiation exposure for patients, surgeons, and operating room staff. The device measures only radiation time, and the amount of exposure during that time depends on the volume and density of the radiated body. We did not calculate the amount of exposure for this study. Mean exposure time was between 20 and 40 seconds, reflecting the number of attempts required to localize the lesion and the surgeon’s experience with the technique. Although the potential for increased exposure is a valid concern, previous studies using this technology have demonstrated that a similar average exposure time is equivalent to that of standard CT, and that use of the device, over conventional techniques, potentially can lead to decreased overall radiation exposure.12,13
This series demonstrated that OO can be safely and effectively treated with intraoperative percutaneous RFA by an orthopedic oncologist. Our success rate is very similar to rates reported in the radiology literature. Studies are needed to confirm the efficacy of this novel technique in comparison with what has been reported in that literature. Given these promising preliminary results, and the relative ease of use and minimal learning curve associated with this technology, all orthopedic oncologists should be able to offer this treatment for OO. Furthermore, this technique allows orthopedic oncologists to provide appropriate definitive treatment and care directly, rather than by referring patients to radiologists.
In the treatment of OO, we reserve RFA for lesions involving the long and short bones of the upper and lower extremities, as well as selected flat bones, such as those in the pelvis. Although percutaneous RFA of spinal lesions has been reported in the literature, we think these represent a relative contraindication for this technique; image resolution, in our opinion, is not high enough to justify risking injury to the nerves in the spinal canal, lateral recesses, and neural foramina. In addition, given the radiation exposure, we recommend caution when using this technique for a pelvic or proximal femoral lesion in a woman of childbearing age.
1. Gitelis S, Wilkins R, Conrad EU 2nd. Benign bone tumors. Instr Course Lect. 1996;45:425-424.
2. Schajowicz F. Bone forming tumors. In: Tumors and Tumorlike Lesions of Bone. 2nd ed. New York, NY: Springer-Verlag; 1994:36-62.
3. Frassica FJ, Waltrip RL, Sponseller PD, Ma LD, McCarthy EF Jr. Clinicopathologic features and treatment of osteoid osteoma and osteoblastoma in children and adolescents. Orthop Clin North Am. 1996;27(3):559-574.
4. Golding JS. The natural history of osteoid osteoma; with a report of twenty cases. J Bone Joint Surg Br. 1954;36(2):218-229.
5. Simm RJ. The natural history of osteoid osteoma. Aust N Z J Surg. 1975;45(4):412-415.
6. Sans N, Galy-Fourcade D, Assoun J, et al. Osteoid osteoma: CT-guided percutaneous resection and follow-up in 38 patients. Radiology. 1999;212(3):687-692.
7. Skjeldal S, Lilleås F, Follerås G, et al. Real time MRI-guided excision and cryo-treatment of osteoid osteoma in os ischii—a case report. Acta Orthop Scand. 2000;71(6):637-638.
8. Sanhaji L, Gharbaoui IS, Hassani RE, Chakir N, Jiddane M, Boukhrissi N. A new treatment of osteoid osteoma: percutaneous sclerosis with ethanol under scanner guidance [in French]. J Radiol. 1996;77(1):37-40.
9. Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229(1):171-175.
10. Cantwell CP, Obyrne J, Eustace S. Current trends in treatment of osteoid osteoma with an emphasis on radiofrequency ablation. Eur Radiol. 2004;14(4):607-617.
11. Ruiz Santiago F, Castellano García Mdel M, Guzmán Álvarez L, Martínez Montes JL, Ruiz García M, Tristán Fernández JM. Percutaneous treatment of bone tumors by radiofrequency thermal ablation. Eur J Radiol. 2011;77(1):156-163.
12. Richter M, Geerling J, Zech S, Goesling T, Krettek C. Intraoperative three-dimensional imaging with a motorized mobile C-Arm (SIREMOBIL ISO-C-3D) in foot and ankle trauma care: a preliminary report. J Orthop Trauma. 2005;19(4):259-266.
13. Gebhard F, Kraus M, Schneider E, et al. Radiation dosage in orthopedics—a comparison of computer-assisted procedures [in German]. Unfallchirurg. 2003;106(6):492-497.
1. Gitelis S, Wilkins R, Conrad EU 2nd. Benign bone tumors. Instr Course Lect. 1996;45:425-424.
2. Schajowicz F. Bone forming tumors. In: Tumors and Tumorlike Lesions of Bone. 2nd ed. New York, NY: Springer-Verlag; 1994:36-62.
3. Frassica FJ, Waltrip RL, Sponseller PD, Ma LD, McCarthy EF Jr. Clinicopathologic features and treatment of osteoid osteoma and osteoblastoma in children and adolescents. Orthop Clin North Am. 1996;27(3):559-574.
4. Golding JS. The natural history of osteoid osteoma; with a report of twenty cases. J Bone Joint Surg Br. 1954;36(2):218-229.
5. Simm RJ. The natural history of osteoid osteoma. Aust N Z J Surg. 1975;45(4):412-415.
6. Sans N, Galy-Fourcade D, Assoun J, et al. Osteoid osteoma: CT-guided percutaneous resection and follow-up in 38 patients. Radiology. 1999;212(3):687-692.
7. Skjeldal S, Lilleås F, Follerås G, et al. Real time MRI-guided excision and cryo-treatment of osteoid osteoma in os ischii—a case report. Acta Orthop Scand. 2000;71(6):637-638.
8. Sanhaji L, Gharbaoui IS, Hassani RE, Chakir N, Jiddane M, Boukhrissi N. A new treatment of osteoid osteoma: percutaneous sclerosis with ethanol under scanner guidance [in French]. J Radiol. 1996;77(1):37-40.
9. Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229(1):171-175.
10. Cantwell CP, Obyrne J, Eustace S. Current trends in treatment of osteoid osteoma with an emphasis on radiofrequency ablation. Eur Radiol. 2004;14(4):607-617.
11. Ruiz Santiago F, Castellano García Mdel M, Guzmán Álvarez L, Martínez Montes JL, Ruiz García M, Tristán Fernández JM. Percutaneous treatment of bone tumors by radiofrequency thermal ablation. Eur J Radiol. 2011;77(1):156-163.
12. Richter M, Geerling J, Zech S, Goesling T, Krettek C. Intraoperative three-dimensional imaging with a motorized mobile C-Arm (SIREMOBIL ISO-C-3D) in foot and ankle trauma care: a preliminary report. J Orthop Trauma. 2005;19(4):259-266.
13. Gebhard F, Kraus M, Schneider E, et al. Radiation dosage in orthopedics—a comparison of computer-assisted procedures [in German]. Unfallchirurg. 2003;106(6):492-497.
A Novel Treatment for Refractory Plantar Fasciitis
Chronic plantar fasciitis is a major health care problem worldwide and affects nearly 10% of the US population. Plantar fasciitis presents as heel pain in the mornings and usually gets better and then gets worse. Inflammation at the plantar fascia attachment causes acute and sometimes disabling pain. Chronic pain at the site can develop as time goes on because of long-standing inflammatory changes. Fibrotic tissues may develop at the site. On a continuum, symptoms may begin in an insidious phase and progress to chronic pain. Although most cases resolve with conservative care, the numerous treatments for refractory plantar fasciitis attest to the lack of consensus regarding these cases. The condition frustrates patient and physician alike.
Treatments for refractory plantar fasciitis include conservative measures, including rest, analgesics, walking orthosis, heel cup, night splint, walking boot, and then, in a standard and logical progression, cortisone or platelet-rich plasma injections. Improved magnetic resonance imaging and ultrasonographic imaging allow accurate localization of the pathologic process,1-3 and this localization in turn provides an opportunity to deliver a more reliable and focused intervention, as in needle-guided therapy.4 Surgical procedures for plantar fasciitis have included open or endoscopically assisted plantar fasciectomies with or without gastrocnemius recession; these procedures have had varying results. The emerging goals for this condition are a minimally invasive percutaneous intervention that is safe, effective, and well-tolerated and has minimal morbidity and a low complication rate.
We conducted a prospective study in which patients were allowed either to continue with noninvasive treatment or to undergo focal aspiration and partial fasciotomy with an ultrasonic probe. Study inclusion criteria were plantar fasciitis symptoms lasting 12 months or longer. Exclusion criteria were unwillingness to participate in the study. Prior treatments, even surgeries, were not exclusionary.
Twelve patients with refractory plantar fasciitis lasting a mean of 19 months (minimum, 12 months; range, 12-24 months) chose the procedure. They all had failed conservative care, including physical therapy, casting, shockwave therapy, and invasive procedures such as injections and endoscopic partial releases. Four of the 12 had undergone an open or endoscopic partial release at a different institution but had experienced no improvement in symptoms.
Based on the study protocol, patients continued noninvasive care (night splint, stretching exercises) for 2 to 6 weeks after the initial visit. When this conservative care failed, they were offered focal partial fasciectomy with a percutaneous ultrasonic probe. American Orthopaedic Foot and Ankle Society (AOFAS) scores were obtained before and after surgery. Follow-up consisted of clinic visits 2 weeks after surgery and monthly thereafter. I saw all 12 patients 3 months after surgery (range, 11-14 weeks), and all 12 underwent postoperative physical therapy.
Technique
The TX1 Tissue Removal System (Tenex Health, Lake Forest, California) (Figure 1) consists of an energy module, a pump/suction cassette that provides irrigation and suction through a probe, and the probe itself, the TX1, which is the size of an 18-gauge needle and delivers ultrasonic energy. The cassette is inserted into the energy module, and the ultrasonic energy probe is primed so it will deliver the irrigation fluid, normal saline. The safety features of the energy module are such that no energy is expended unless the system is properly irrigating and aspirating the diseased tissue. Ultrasonic treatment may be performed in a clinical or ambulatory surgical center. The patient is placed supine on an operating table, on a clinical examining table, or, if in a cast room, on a cart. A pillow is placed under the distal tibia so the knees can flex slightly, and the patient is positioned so the feet are free of the edge of the bed or gurney (Figure 2).
The pathology is first confirmed by ultrasonography (Figures 3–5). The first step is to identify the calcaneus with the sensor along the long axis of the foot. Then the plantar fascia is visualized and followed along its long axis to the site of attachment at the medial tubercle. As the pathologic process involves the medial site of attachment, a transverse image may also be obtained to better understand the medial/lateral extent of the disease process. The ultrasonographic image of plantar fasciitis has been well characterized.2,5 The pathology is visualized as an area of edema or of disruption of the linear appearance of the fascia as it attaches to the calcaneus. While the diagnosis is being confirmed, the optimal site for probe insertion should be considered based on the location of the pain and the localization of the pathology by the 2 orthogonal images.
The area is prepared as if for an injection and is squared off with sterile towels. Then the sensor is placed in the sterile sleeve. The area of maximum tenderness is again confirmed. Determining the location of the probe insertion site is a crucial step. We use the ultrasonic sensor in the longitudinal and transverse planes to direct the injection of a fast-acting local anesthetic to the medial aspect of the calcaneus. A skin wheal is created, and the fast-acting local anesthetic (3-4 mL) is injected into the region of the fascia pathology.
An 11-blade knife is used to create a site for the probe through the skin wheal at the medial aspect of the heel, in line with the pathology (Figure 6). The probe is then introduced through the puncture site and is identified, along with the pathology, with the sensor, which may be oriented transverse or longitudinal to the long axis of the foot.
Once the pathologic area is identified, the ultrasonic energy is delivered to the region by the probe, which is activated with a foot pedal, effectively releasing the pathologic tissue from its insertion at the medial tubercle of the calcaneus. The probe is moved in a linear fashion medially and laterally within the lesion across the site of attachment. Treatment continues until the entire soft-tissue lesion is addressed.
Postoperative Care
The wound or wounds are closed with a nylon stitch and Steri-Strip (3M, St. Paul, Minnesota) and covered with Tegaderm (3M) or similar dressing (Figure 7). A compressive dressing is applied. The dressing is removed in 2 to 3 days; the Steri-Strip and stitch are removed in 10 to 14 days. A walking boot is put on immediately after the procedure (most patients in this study already have a boot) and is worn for a few days, or until the symptoms have resolved. How long the boot is used is very much based on patient preference. Patients may continue stretching exercises at home, but there should be no high-impact activity. As-needed ice and analgesics are recommended for the first few days.
The 12 patients had a mean preoperative AOFAS score of 30 (range, 17-46) and a mean postoperative score of 88 (range, 25-92). By the 3-month postoperative visit, symptoms were resolved in 11 patients (no activity restricted by plantar fascia pain). On physical examination, 11 patients had no palpable tenderness at the site of preoperative pain. Pain relief was documented as having occurred between 5 and 13 weeks after treatment. One patient had bilateral procedures. One foot was treated, pain resolved by the 3-month postoperative visit, and the patient asked for the other foot to be treated. Three months after the second procedure, he had minimal non-activity-restricting pain. There were no postoperative infections or wound complications.
I phoned my patients during postoperative month 24. All 12 patients (13 feet) indicated they were essentially pain-free. None admitted to activity restriction or required over-the-counter pain medication. All indicated they were satisfied with the procedure and would have it again.
The refractory nature of plantar fasciitis, and the resistance to and unpredictability of current treatment options, is well known. Considerable efforts have been made to develop treatment guidelines and algorithms.6 A standard and logical treatment plan involves initial attempts with rest, analgesics, and a walking orthosis and then, if those fail, cortisone or platelet-rich plasma injections. Reluctance to perform surgery is well justified because of the unpredictability of the intervention. As might be expected, the utility of ultrasonography has been on the rise. The diagnostic value of ultrasonography, first recognized in the early 1970s, is of increasing importance.7,8 Subsequent use of ultrasonographic imaging as guidance for various treatments, including percutaneous release, has also been recognized and documented.4,9-12 The present article is the first to describe and document the outcome of using ultrasonic energy for percutaneous release of the diseased attachment of the plantar fascia.
This report is preliminary and was designed to alert the orthopedic community to a safe and promising treatment for a chronic, refractory condition. The safety and efficacy of this treatment are reflected in our experience and have been documented for tennis elbow as well.13
This study was limited by its single-surgeon and relatively small clinical experience. Nevertheless, the benefits of this novel technique—effectiveness, safety, tolerability, and rapid recovery—are encouraging enough to share at this time. Prospective randomized controlled studies are needed.
Conclusion
This is the first report of a plantar fascia partial release guided by ultrasonic energy delivered by a percutaneously inserted probe under local anesthesia. The procedure appears to be a safe, effective, well-tolerated treatment for a condition that is refractory to other options. More studies are needed to further validate the safety and efficacy of this innovative treatment modality.
1. Wall JR, Harkness MA, Crawford A. Ultrasound diagnosis of plantar fasciitis. Foot Ankle. 1993;14(8):465-470.
2. Maffulli N, Regine R, Angelillo M, Capasso G, Filice S. Ultrasound diagnosis of Achilles tendon pathology in runners. Br J Sports Med. 1987;21(4):158-162.
3. Patil P, Dasgupta B. Role of diagnostic ultrasound in the assessment of musculoskeletal diseases. Ther Adv Musculoskelet Dis. 2012;4(5):341-355.
4. Royall NA, Farrin E, Bahner DP, Stawicki SP. Ultrasound-assisted musculoskeletal procedures: a practical overview of current literature. World J Orthop. 2011;2(7):57-66.
5. Tsai WC, Chiu MF, Wang CL, Tang FT, Wong MK. Ultrasound evaluation of plantar fasciitis. Scand J Rheumatol. 2000;29(4):255-259.
6. Thomas JL, Christensen JC, Kravitz SR, et al; American College of Foot and Ankle Surgeons Heel Pain Committee. The diagnosis and treatment of heel pain: a clinical practice guideline—revision 2010. J Foot Ankle Surg. 2010;49(3 suppl):S1-S19.
7. McDonald DG, Leopold GR. Ultrasound B–scanning in the differentiation of Baker’s cyst and thrombophlebitis. Br J Radiol. 1972;45(538):729-732.
8. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: the orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
9. Rubens DJ, Fultz PJ, Gottlieb RH, Rubin SJ. Effective ultrasonographically guided intervention for diagnosis of musculoskeletal lesions. J Ultrasound Med. 1997;16(12):831-842.
10. Testa V, Capasso G, Benazzo F, Maffulli N. Management of Achilles tendinopathy by ultrasound-guided percutaneous tenotomy. Med Sci Sports Exerc. 2002;34(4):573-580.
11. Debrule MB. Ultrasound-guided Weil percutaneous plantar fasciotomy. J Am Podiatr Med Assoc. 2010;100(2):146-148.
12. Vohra PK, Japour CJ. Ultrasound-guided plantar fascia release technique: a retrospective study of 46 feet. J Am Podiatr Med Assoc. 2009;99(3):183-190.
13. Koh JS, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
Chronic plantar fasciitis is a major health care problem worldwide and affects nearly 10% of the US population. Plantar fasciitis presents as heel pain in the mornings and usually gets better and then gets worse. Inflammation at the plantar fascia attachment causes acute and sometimes disabling pain. Chronic pain at the site can develop as time goes on because of long-standing inflammatory changes. Fibrotic tissues may develop at the site. On a continuum, symptoms may begin in an insidious phase and progress to chronic pain. Although most cases resolve with conservative care, the numerous treatments for refractory plantar fasciitis attest to the lack of consensus regarding these cases. The condition frustrates patient and physician alike.
Treatments for refractory plantar fasciitis include conservative measures, including rest, analgesics, walking orthosis, heel cup, night splint, walking boot, and then, in a standard and logical progression, cortisone or platelet-rich plasma injections. Improved magnetic resonance imaging and ultrasonographic imaging allow accurate localization of the pathologic process,1-3 and this localization in turn provides an opportunity to deliver a more reliable and focused intervention, as in needle-guided therapy.4 Surgical procedures for plantar fasciitis have included open or endoscopically assisted plantar fasciectomies with or without gastrocnemius recession; these procedures have had varying results. The emerging goals for this condition are a minimally invasive percutaneous intervention that is safe, effective, and well-tolerated and has minimal morbidity and a low complication rate.
We conducted a prospective study in which patients were allowed either to continue with noninvasive treatment or to undergo focal aspiration and partial fasciotomy with an ultrasonic probe. Study inclusion criteria were plantar fasciitis symptoms lasting 12 months or longer. Exclusion criteria were unwillingness to participate in the study. Prior treatments, even surgeries, were not exclusionary.
Twelve patients with refractory plantar fasciitis lasting a mean of 19 months (minimum, 12 months; range, 12-24 months) chose the procedure. They all had failed conservative care, including physical therapy, casting, shockwave therapy, and invasive procedures such as injections and endoscopic partial releases. Four of the 12 had undergone an open or endoscopic partial release at a different institution but had experienced no improvement in symptoms.
Based on the study protocol, patients continued noninvasive care (night splint, stretching exercises) for 2 to 6 weeks after the initial visit. When this conservative care failed, they were offered focal partial fasciectomy with a percutaneous ultrasonic probe. American Orthopaedic Foot and Ankle Society (AOFAS) scores were obtained before and after surgery. Follow-up consisted of clinic visits 2 weeks after surgery and monthly thereafter. I saw all 12 patients 3 months after surgery (range, 11-14 weeks), and all 12 underwent postoperative physical therapy.
Technique
The TX1 Tissue Removal System (Tenex Health, Lake Forest, California) (Figure 1) consists of an energy module, a pump/suction cassette that provides irrigation and suction through a probe, and the probe itself, the TX1, which is the size of an 18-gauge needle and delivers ultrasonic energy. The cassette is inserted into the energy module, and the ultrasonic energy probe is primed so it will deliver the irrigation fluid, normal saline. The safety features of the energy module are such that no energy is expended unless the system is properly irrigating and aspirating the diseased tissue. Ultrasonic treatment may be performed in a clinical or ambulatory surgical center. The patient is placed supine on an operating table, on a clinical examining table, or, if in a cast room, on a cart. A pillow is placed under the distal tibia so the knees can flex slightly, and the patient is positioned so the feet are free of the edge of the bed or gurney (Figure 2).
The pathology is first confirmed by ultrasonography (Figures 3–5). The first step is to identify the calcaneus with the sensor along the long axis of the foot. Then the plantar fascia is visualized and followed along its long axis to the site of attachment at the medial tubercle. As the pathologic process involves the medial site of attachment, a transverse image may also be obtained to better understand the medial/lateral extent of the disease process. The ultrasonographic image of plantar fasciitis has been well characterized.2,5 The pathology is visualized as an area of edema or of disruption of the linear appearance of the fascia as it attaches to the calcaneus. While the diagnosis is being confirmed, the optimal site for probe insertion should be considered based on the location of the pain and the localization of the pathology by the 2 orthogonal images.
The area is prepared as if for an injection and is squared off with sterile towels. Then the sensor is placed in the sterile sleeve. The area of maximum tenderness is again confirmed. Determining the location of the probe insertion site is a crucial step. We use the ultrasonic sensor in the longitudinal and transverse planes to direct the injection of a fast-acting local anesthetic to the medial aspect of the calcaneus. A skin wheal is created, and the fast-acting local anesthetic (3-4 mL) is injected into the region of the fascia pathology.
An 11-blade knife is used to create a site for the probe through the skin wheal at the medial aspect of the heel, in line with the pathology (Figure 6). The probe is then introduced through the puncture site and is identified, along with the pathology, with the sensor, which may be oriented transverse or longitudinal to the long axis of the foot.
Once the pathologic area is identified, the ultrasonic energy is delivered to the region by the probe, which is activated with a foot pedal, effectively releasing the pathologic tissue from its insertion at the medial tubercle of the calcaneus. The probe is moved in a linear fashion medially and laterally within the lesion across the site of attachment. Treatment continues until the entire soft-tissue lesion is addressed.
Postoperative Care
The wound or wounds are closed with a nylon stitch and Steri-Strip (3M, St. Paul, Minnesota) and covered with Tegaderm (3M) or similar dressing (Figure 7). A compressive dressing is applied. The dressing is removed in 2 to 3 days; the Steri-Strip and stitch are removed in 10 to 14 days. A walking boot is put on immediately after the procedure (most patients in this study already have a boot) and is worn for a few days, or until the symptoms have resolved. How long the boot is used is very much based on patient preference. Patients may continue stretching exercises at home, but there should be no high-impact activity. As-needed ice and analgesics are recommended for the first few days.
The 12 patients had a mean preoperative AOFAS score of 30 (range, 17-46) and a mean postoperative score of 88 (range, 25-92). By the 3-month postoperative visit, symptoms were resolved in 11 patients (no activity restricted by plantar fascia pain). On physical examination, 11 patients had no palpable tenderness at the site of preoperative pain. Pain relief was documented as having occurred between 5 and 13 weeks after treatment. One patient had bilateral procedures. One foot was treated, pain resolved by the 3-month postoperative visit, and the patient asked for the other foot to be treated. Three months after the second procedure, he had minimal non-activity-restricting pain. There were no postoperative infections or wound complications.
I phoned my patients during postoperative month 24. All 12 patients (13 feet) indicated they were essentially pain-free. None admitted to activity restriction or required over-the-counter pain medication. All indicated they were satisfied with the procedure and would have it again.
The refractory nature of plantar fasciitis, and the resistance to and unpredictability of current treatment options, is well known. Considerable efforts have been made to develop treatment guidelines and algorithms.6 A standard and logical treatment plan involves initial attempts with rest, analgesics, and a walking orthosis and then, if those fail, cortisone or platelet-rich plasma injections. Reluctance to perform surgery is well justified because of the unpredictability of the intervention. As might be expected, the utility of ultrasonography has been on the rise. The diagnostic value of ultrasonography, first recognized in the early 1970s, is of increasing importance.7,8 Subsequent use of ultrasonographic imaging as guidance for various treatments, including percutaneous release, has also been recognized and documented.4,9-12 The present article is the first to describe and document the outcome of using ultrasonic energy for percutaneous release of the diseased attachment of the plantar fascia.
This report is preliminary and was designed to alert the orthopedic community to a safe and promising treatment for a chronic, refractory condition. The safety and efficacy of this treatment are reflected in our experience and have been documented for tennis elbow as well.13
This study was limited by its single-surgeon and relatively small clinical experience. Nevertheless, the benefits of this novel technique—effectiveness, safety, tolerability, and rapid recovery—are encouraging enough to share at this time. Prospective randomized controlled studies are needed.
Conclusion
This is the first report of a plantar fascia partial release guided by ultrasonic energy delivered by a percutaneously inserted probe under local anesthesia. The procedure appears to be a safe, effective, well-tolerated treatment for a condition that is refractory to other options. More studies are needed to further validate the safety and efficacy of this innovative treatment modality.
Chronic plantar fasciitis is a major health care problem worldwide and affects nearly 10% of the US population. Plantar fasciitis presents as heel pain in the mornings and usually gets better and then gets worse. Inflammation at the plantar fascia attachment causes acute and sometimes disabling pain. Chronic pain at the site can develop as time goes on because of long-standing inflammatory changes. Fibrotic tissues may develop at the site. On a continuum, symptoms may begin in an insidious phase and progress to chronic pain. Although most cases resolve with conservative care, the numerous treatments for refractory plantar fasciitis attest to the lack of consensus regarding these cases. The condition frustrates patient and physician alike.
Treatments for refractory plantar fasciitis include conservative measures, including rest, analgesics, walking orthosis, heel cup, night splint, walking boot, and then, in a standard and logical progression, cortisone or platelet-rich plasma injections. Improved magnetic resonance imaging and ultrasonographic imaging allow accurate localization of the pathologic process,1-3 and this localization in turn provides an opportunity to deliver a more reliable and focused intervention, as in needle-guided therapy.4 Surgical procedures for plantar fasciitis have included open or endoscopically assisted plantar fasciectomies with or without gastrocnemius recession; these procedures have had varying results. The emerging goals for this condition are a minimally invasive percutaneous intervention that is safe, effective, and well-tolerated and has minimal morbidity and a low complication rate.
We conducted a prospective study in which patients were allowed either to continue with noninvasive treatment or to undergo focal aspiration and partial fasciotomy with an ultrasonic probe. Study inclusion criteria were plantar fasciitis symptoms lasting 12 months or longer. Exclusion criteria were unwillingness to participate in the study. Prior treatments, even surgeries, were not exclusionary.
Twelve patients with refractory plantar fasciitis lasting a mean of 19 months (minimum, 12 months; range, 12-24 months) chose the procedure. They all had failed conservative care, including physical therapy, casting, shockwave therapy, and invasive procedures such as injections and endoscopic partial releases. Four of the 12 had undergone an open or endoscopic partial release at a different institution but had experienced no improvement in symptoms.
Based on the study protocol, patients continued noninvasive care (night splint, stretching exercises) for 2 to 6 weeks after the initial visit. When this conservative care failed, they were offered focal partial fasciectomy with a percutaneous ultrasonic probe. American Orthopaedic Foot and Ankle Society (AOFAS) scores were obtained before and after surgery. Follow-up consisted of clinic visits 2 weeks after surgery and monthly thereafter. I saw all 12 patients 3 months after surgery (range, 11-14 weeks), and all 12 underwent postoperative physical therapy.
Technique
The TX1 Tissue Removal System (Tenex Health, Lake Forest, California) (Figure 1) consists of an energy module, a pump/suction cassette that provides irrigation and suction through a probe, and the probe itself, the TX1, which is the size of an 18-gauge needle and delivers ultrasonic energy. The cassette is inserted into the energy module, and the ultrasonic energy probe is primed so it will deliver the irrigation fluid, normal saline. The safety features of the energy module are such that no energy is expended unless the system is properly irrigating and aspirating the diseased tissue. Ultrasonic treatment may be performed in a clinical or ambulatory surgical center. The patient is placed supine on an operating table, on a clinical examining table, or, if in a cast room, on a cart. A pillow is placed under the distal tibia so the knees can flex slightly, and the patient is positioned so the feet are free of the edge of the bed or gurney (Figure 2).
The pathology is first confirmed by ultrasonography (Figures 3–5). The first step is to identify the calcaneus with the sensor along the long axis of the foot. Then the plantar fascia is visualized and followed along its long axis to the site of attachment at the medial tubercle. As the pathologic process involves the medial site of attachment, a transverse image may also be obtained to better understand the medial/lateral extent of the disease process. The ultrasonographic image of plantar fasciitis has been well characterized.2,5 The pathology is visualized as an area of edema or of disruption of the linear appearance of the fascia as it attaches to the calcaneus. While the diagnosis is being confirmed, the optimal site for probe insertion should be considered based on the location of the pain and the localization of the pathology by the 2 orthogonal images.
The area is prepared as if for an injection and is squared off with sterile towels. Then the sensor is placed in the sterile sleeve. The area of maximum tenderness is again confirmed. Determining the location of the probe insertion site is a crucial step. We use the ultrasonic sensor in the longitudinal and transverse planes to direct the injection of a fast-acting local anesthetic to the medial aspect of the calcaneus. A skin wheal is created, and the fast-acting local anesthetic (3-4 mL) is injected into the region of the fascia pathology.
An 11-blade knife is used to create a site for the probe through the skin wheal at the medial aspect of the heel, in line with the pathology (Figure 6). The probe is then introduced through the puncture site and is identified, along with the pathology, with the sensor, which may be oriented transverse or longitudinal to the long axis of the foot.
Once the pathologic area is identified, the ultrasonic energy is delivered to the region by the probe, which is activated with a foot pedal, effectively releasing the pathologic tissue from its insertion at the medial tubercle of the calcaneus. The probe is moved in a linear fashion medially and laterally within the lesion across the site of attachment. Treatment continues until the entire soft-tissue lesion is addressed.
Postoperative Care
The wound or wounds are closed with a nylon stitch and Steri-Strip (3M, St. Paul, Minnesota) and covered with Tegaderm (3M) or similar dressing (Figure 7). A compressive dressing is applied. The dressing is removed in 2 to 3 days; the Steri-Strip and stitch are removed in 10 to 14 days. A walking boot is put on immediately after the procedure (most patients in this study already have a boot) and is worn for a few days, or until the symptoms have resolved. How long the boot is used is very much based on patient preference. Patients may continue stretching exercises at home, but there should be no high-impact activity. As-needed ice and analgesics are recommended for the first few days.
The 12 patients had a mean preoperative AOFAS score of 30 (range, 17-46) and a mean postoperative score of 88 (range, 25-92). By the 3-month postoperative visit, symptoms were resolved in 11 patients (no activity restricted by plantar fascia pain). On physical examination, 11 patients had no palpable tenderness at the site of preoperative pain. Pain relief was documented as having occurred between 5 and 13 weeks after treatment. One patient had bilateral procedures. One foot was treated, pain resolved by the 3-month postoperative visit, and the patient asked for the other foot to be treated. Three months after the second procedure, he had minimal non-activity-restricting pain. There were no postoperative infections or wound complications.
I phoned my patients during postoperative month 24. All 12 patients (13 feet) indicated they were essentially pain-free. None admitted to activity restriction or required over-the-counter pain medication. All indicated they were satisfied with the procedure and would have it again.
The refractory nature of plantar fasciitis, and the resistance to and unpredictability of current treatment options, is well known. Considerable efforts have been made to develop treatment guidelines and algorithms.6 A standard and logical treatment plan involves initial attempts with rest, analgesics, and a walking orthosis and then, if those fail, cortisone or platelet-rich plasma injections. Reluctance to perform surgery is well justified because of the unpredictability of the intervention. As might be expected, the utility of ultrasonography has been on the rise. The diagnostic value of ultrasonography, first recognized in the early 1970s, is of increasing importance.7,8 Subsequent use of ultrasonographic imaging as guidance for various treatments, including percutaneous release, has also been recognized and documented.4,9-12 The present article is the first to describe and document the outcome of using ultrasonic energy for percutaneous release of the diseased attachment of the plantar fascia.
This report is preliminary and was designed to alert the orthopedic community to a safe and promising treatment for a chronic, refractory condition. The safety and efficacy of this treatment are reflected in our experience and have been documented for tennis elbow as well.13
This study was limited by its single-surgeon and relatively small clinical experience. Nevertheless, the benefits of this novel technique—effectiveness, safety, tolerability, and rapid recovery—are encouraging enough to share at this time. Prospective randomized controlled studies are needed.
Conclusion
This is the first report of a plantar fascia partial release guided by ultrasonic energy delivered by a percutaneously inserted probe under local anesthesia. The procedure appears to be a safe, effective, well-tolerated treatment for a condition that is refractory to other options. More studies are needed to further validate the safety and efficacy of this innovative treatment modality.
1. Wall JR, Harkness MA, Crawford A. Ultrasound diagnosis of plantar fasciitis. Foot Ankle. 1993;14(8):465-470.
2. Maffulli N, Regine R, Angelillo M, Capasso G, Filice S. Ultrasound diagnosis of Achilles tendon pathology in runners. Br J Sports Med. 1987;21(4):158-162.
3. Patil P, Dasgupta B. Role of diagnostic ultrasound in the assessment of musculoskeletal diseases. Ther Adv Musculoskelet Dis. 2012;4(5):341-355.
4. Royall NA, Farrin E, Bahner DP, Stawicki SP. Ultrasound-assisted musculoskeletal procedures: a practical overview of current literature. World J Orthop. 2011;2(7):57-66.
5. Tsai WC, Chiu MF, Wang CL, Tang FT, Wong MK. Ultrasound evaluation of plantar fasciitis. Scand J Rheumatol. 2000;29(4):255-259.
6. Thomas JL, Christensen JC, Kravitz SR, et al; American College of Foot and Ankle Surgeons Heel Pain Committee. The diagnosis and treatment of heel pain: a clinical practice guideline—revision 2010. J Foot Ankle Surg. 2010;49(3 suppl):S1-S19.
7. McDonald DG, Leopold GR. Ultrasound B–scanning in the differentiation of Baker’s cyst and thrombophlebitis. Br J Radiol. 1972;45(538):729-732.
8. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: the orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
9. Rubens DJ, Fultz PJ, Gottlieb RH, Rubin SJ. Effective ultrasonographically guided intervention for diagnosis of musculoskeletal lesions. J Ultrasound Med. 1997;16(12):831-842.
10. Testa V, Capasso G, Benazzo F, Maffulli N. Management of Achilles tendinopathy by ultrasound-guided percutaneous tenotomy. Med Sci Sports Exerc. 2002;34(4):573-580.
11. Debrule MB. Ultrasound-guided Weil percutaneous plantar fasciotomy. J Am Podiatr Med Assoc. 2010;100(2):146-148.
12. Vohra PK, Japour CJ. Ultrasound-guided plantar fascia release technique: a retrospective study of 46 feet. J Am Podiatr Med Assoc. 2009;99(3):183-190.
13. Koh JS, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
1. Wall JR, Harkness MA, Crawford A. Ultrasound diagnosis of plantar fasciitis. Foot Ankle. 1993;14(8):465-470.
2. Maffulli N, Regine R, Angelillo M, Capasso G, Filice S. Ultrasound diagnosis of Achilles tendon pathology in runners. Br J Sports Med. 1987;21(4):158-162.
3. Patil P, Dasgupta B. Role of diagnostic ultrasound in the assessment of musculoskeletal diseases. Ther Adv Musculoskelet Dis. 2012;4(5):341-355.
4. Royall NA, Farrin E, Bahner DP, Stawicki SP. Ultrasound-assisted musculoskeletal procedures: a practical overview of current literature. World J Orthop. 2011;2(7):57-66.
5. Tsai WC, Chiu MF, Wang CL, Tang FT, Wong MK. Ultrasound evaluation of plantar fasciitis. Scand J Rheumatol. 2000;29(4):255-259.
6. Thomas JL, Christensen JC, Kravitz SR, et al; American College of Foot and Ankle Surgeons Heel Pain Committee. The diagnosis and treatment of heel pain: a clinical practice guideline—revision 2010. J Foot Ankle Surg. 2010;49(3 suppl):S1-S19.
7. McDonald DG, Leopold GR. Ultrasound B–scanning in the differentiation of Baker’s cyst and thrombophlebitis. Br J Radiol. 1972;45(538):729-732.
8. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: the orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
9. Rubens DJ, Fultz PJ, Gottlieb RH, Rubin SJ. Effective ultrasonographically guided intervention for diagnosis of musculoskeletal lesions. J Ultrasound Med. 1997;16(12):831-842.
10. Testa V, Capasso G, Benazzo F, Maffulli N. Management of Achilles tendinopathy by ultrasound-guided percutaneous tenotomy. Med Sci Sports Exerc. 2002;34(4):573-580.
11. Debrule MB. Ultrasound-guided Weil percutaneous plantar fasciotomy. J Am Podiatr Med Assoc. 2010;100(2):146-148.
12. Vohra PK, Japour CJ. Ultrasound-guided plantar fascia release technique: a retrospective study of 46 feet. J Am Podiatr Med Assoc. 2009;99(3):183-190.
13. Koh JS, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
Blood Product Selection and Administration
Overview
In 2011, nearly 14 million units of whole blood and RBCs were transfused in US hospitals according to the 2011 National Blood Collection and Utilization Survey Report. In the United States, United Kingdom, Western Europe, and Canada, approximately 40% of critically ill patients received a mean of 5 U of PRBC per hospitalization.1,2
In the ED, hemodynamic instability due to acute hemorrhage is the most common indication for transfusion of PRBCs. Common emergent sources include gastrointestinal (GI) bleeding, dysfunctional uterine bleeding, and bleeding secondary to trauma. For every unit of PRBCs transfused, the typical result in the average adult is an increase in hemoglobin (Hgb) by 1 g/dL and hematocrit by 3%. In the pediatric population, a 3 mL/kg intravenous (IV) dose achieves equivalent results.3
Blood Components and Type Compatibility
After donated blood is collected, blood banks divide the blood into type and components, including red cell concentrate, FFP, cryoprecipitates, and platelets.
Packed Red Blood Cells
After RBCs are separated from whole blood, they can be further processed through leukoreduction, which removes most white blood cells at the expense of a 10% to 15% loss of RBCs. Leukoreduced RBCs (LRBCs) are used in patients with a history of two or more febrile nonhemolytic transfusion reactions (FNHTR). In addition to preventing FNHTR, LRBCs may also be effective in preventing cytomegalovirus (CMV) transmission or human leukocyte antigen (HLA) alloimmunization.4
Cytomegalovirus negative PRBCs and blood components are indicated for the following patients: premature and all infants younger than age 4 weeks; intrauterine transfusions; bone marrow or organ transplant recipients (including transplant candidates); immunocompromised and asplenic patients; and pregnant women.
Irradiated PRBCs and blood products are exposed to 2,500 rad of gamma radiation to destroy lymphoproliferative processes. This irradiation prevents transfusion-associated graft-versus-host disease (TA-GVHD) in susceptible patients. Absolute indications for irradiated blood products include bone marrow transplant recipients and donors, stem-cell donors, T-cell immunodeficiency, intrauterine transfusion, and HLA-matched platelet transfusions. Relative indications include patients with leukemia, Hodgkin disease, non-Hodgkin lymphoma, neonatal exchange transfusion, premature infants, neuroblastoma, and glioblastoma.3
Divided RBC units or “pedi-packs” are derived from dividing single units of PRBCs into 4 units. Pedi-packs are type O irradiated, leukoreduced, and Hgb S negative PRBCs; however, they are not necessarily CMV negative. Pedi-packs minimize blood wasting and donor exposure when a small volume transfusion is indicated.5
Type O
Often, cross-matched blood is not immediately available. If PRBCs are needed within the first 15 minutes of resuscitation and the patient’s condition cannot be stabilized with 2 L of crystalloid fluids, type O blood is warranted. In general, women of childbearing age should be transfused with type O Rh-negative blood.6
Of 4,241 trauma patients who received uncrossmatched PRBCs (URBCs) or type O transfusions in a retrospective study at a level 1 trauma center, those receiving URBCs had a 39.6% mortality compared to 11.9% of those with crossmatched PRBCs (P<.001). In general, the use of URBCs is an independent predictor of mortality after adjusting for gender, mechanism, age, hypotension, intubation, initial Hgb, abbreviated injury scale, Glasgow coma scale, injury severity score, and the amount of blood products received. Crossmatched blood should be used whenever available, and a request for uncrossmatched blood products should trigger the blood bank to release crossmatched blood in anticipation of massive transfusion.7
Platelets
Platelets are separated and concentrated through serial centrifugation, then re-suspended in residual plasma. A therapeutic adult dose is comprised of four to six platelet concentrates of the same blood type. This raises platelet counts by 5,000 mL/U. Even though hemostasis may be maintained at platelet counts of 5,000/mL, it is acceptable to transfuse for platelet counts below 10,000/mL. Patients who are bleeding due to platelet dysfunction, and/or thrombocytopenia require platelets. Platelet transfusion is generally ineffective in the case of immune-mediated platelet consumption such as thrombotic thrombocytopenic purpura (TTP).8
ABO Compatible Platelets
Infants and small children require ABO compatible or volume-reduced platelets.9 Type ABO compatibility is less clinically significant in adults; however, Rh sensitization may occur. Conditions refractory to platelet therapy include fever, sepsis disseminated intravascular coagulation (DIC), splenomegaly, idiopathic thrombocytopenic purpura, and platelet alloimmunization. Patients frequently transfused with platelets or those with platelet alloimmunization require leukoreduced and HLA-matched products to minimize HLA antibody-induced immune destruction.10
Fresh Frozen Plasma
Plasma is removed from whole blood and frozen below 55˚F to make FFP. It contains all of the coagulation factors but is not a concentrate. Fresh frozen plasma contains both stable and labile components of the fibrinolytic, coagulation, and complement systems, as well as proteins that maintain oncotic pressure. Unlike PRBC, where type O is the universal donor, in FFP, AB type is the universal donor for transfusion. In the ED, FFP is used for the reversal of coagulopathy in bleeding patients and for replacement of coagulation factors when specific factors are unavailable. It is also given to patients requiring large volumes of blood components (ie, massive blood transfusion protocol).10
A typical FFP unit is approximately 250 mL and is administered within 6 hours of thawing. Every 1 mL/kg of body weight of FFP raises clotting factors by 1%. For warfarin reversal, 5 to 8 mL/kg of FFP should be administered IV. One milliliter of FFP has 1 U of activity of all coagulation factors; 15 mL/kg of FFP achieves approximately 30% of plasma factor concentration.10,11
Patients with active bleeding and documented liver disease, congenital factor deficiency, or mass transfusion recipients are candidates for FFP in the ED. Patients with TTP should also receive FFP with plasma exchange. When FFP is administered for emergent reversal in life-threatening bleeding or intracranial hemorrhage, it is given in conjunction with IV vitamin K and either Factor VIIa or prothrombin.12
Cryoprecipitate
Cryoprecipitate contains factor VIII, von Willebrand factor (vWF), and fibrinogen with some amounts of factor XIII and fibronectin. Actively bleeding patients with hypofibrinogenemia (<100 mg/dL fibrin) are candidates for cryoprecipitate. Cryoprecipitate is used in the therapeutic management of hemophilia A (factor VIII deficiency) when factor VIII concentrates are not available. Cryoprecipitate is given as type ABO compatible when possible and, like FFP, type AB is the universal donor. Each unit of cryoprecipitate raises fibrinogen 75 mg/dL, with a typical dose being 10 U or 1 U per 5 kg of patient body weight.13,14
Factor VIII, Von Willebrand Factor, and Factor IX
Patients with hemophilia typically present to the ED with bleeding episodes ranging from benign abrasion to life-threatening epidural hematomas. Factor VIII concentrates are purified from plasma to treat bleeding patients with hemophilia A or von Willebrand disease (VWD). For emergent use, the amount of factor VIII should be calculated as follows: estimated dose = weight (kg) x 0.5 x desired factor (%) increase. The targeted factor VIII increase is typically 80% to 100% for severe bleeding in patients with hemophilia A.
Another component of factor VIII is vWF activity (factor VIII/vWF). Von Willebrand disease is characterized by the lack of factor VIII/vWF, resulting in normal platelet counts and morphologies, but with impaired adhesion ability. Humate-P and Alphanate SD/HT, are factor VIII replacement therapies with significant amounts of vWF, and are approved for use in patients with hemophilia A and vWD. The initial dose of Humate-P for severe bleeding episodes is 40 to 60 U/kg IV. An administered dose of 50 IU/kg of Alphanate is expected to increase circulating FVIII levels to 100% of normal.
Factor IX (FIX) concentrates are used to treat patients with hemophilia B, a condition in which patients lack factor IX, a vitamin K-dependent glycoprotein. The FIX concentrates may also benefit patients with factor X or prothrombin deficiency. In the United States, since 1992, commercially available FIX is produced from genetically engineered recombinant factor replacement (rFIX). Second-generation rFIX and monoclonal antibody solvents do not contain human plasma and are free of viral contaminants, including parvovirus B19.15
Etiology and Treatment
Gastrointestinal Bleeding
Sources of GI bleeding vary from hemorrhoids to Mallory-Weiss tears. The heterogeneous population of patients with GI bleeds complicates the identification of high-risk patients needing transfusion. Bleeding is traditionally characterized as either upper GI bleeding (UGIB) or lower GI bleeding (LGIB)—the former requiring endoscopy, the latter colonoscopy or other expensive strategies to differentiate one form from the other.
In patients with LGIB, the differential diagnosis is broad, ranging from hemorrhoidal bleeding, cancer, or life-threatening diverticular hemorrhage. Prompt volume replacement with isotonic crystalloid IV fluids must be initiated. In nonvariceal UGIB, blood transfusions should be initiated for Hgb levels <70g/L.16
Clinical prediction rules for acute GI bleeding can help identify those patients who require transfusions. One study collected data on seven established independent predictors of severe LGIB, including heart rate, systolic blood pressure (SBP), syncope, nontender abdomen, rectal bleeding in the first 4 hours of evaluation, aspirin use, and more than two comorbid conditions. A nontender abdomen was the best predictor of severe bleeding, likely due to the fact that vascular disorders, such as diverticulitis, result in brisk bleeding without tenderness; whereas inflammatory processes, such as ischemic colitis, are associated with less severe bleeding and abdominal tenderness. Patients with one or more of the seven risk factors were stratified into low (0-7 risk factors), moderate (1-3 risk factors), and high-risk groups (>3 risk factors). Low-risk patients had a ≤ 9% risk of a severe LGIB, moderate-risk patients had a 43% risk, and high-risk patients had >79% likelihood of bleeding. The high-risk patients were more likely to require early transfusion of PRBCs. Tachycardia, hypotension, syncope, nontender abdomen, and rectal bleeding were identified as the most significant predictors of patients requiring 4 or more units of PRBC in the first 24 hours. Such clinical prediction rules may aid in the initial triage of patients with acute LGIB and identify those most likely to require transfusion in the ED.17
Similar to LGIB transfusion prediction rules, the BLEED (ongoing bleeding, low systolic blood pressure, elevated prothrombin time [PT], erratic mental status, unstable comorbid disease) classification identified patients with UGIB most likely to require transfusion. High-risk patients had one or more of the following: ongoing bleeding, SBP <100 mm Hg, PT more than 1.2 times the control value, altered mental status, the presence of an unstable comorbid disease, or a disease process requiring management in the intensive care unit.18
Trauma
Within the first 48 hours of presentation, blood loss accounts for more than 50% of all trauma deaths.19,20 Posttraumatic bleeding is attributed to several factors, including vascular injury and coagulopathy. Hemodilution from large amounts of crystalloid infusion, hypothermia, and acidosis in early resuscitation adversely affect coagulation, platelet function, protein C consumption, and increases levels of tissue plasminogen activator inhibitor.21 A recent study comparing coagulation tests at the trauma scene and for 1 hour after injury, demonstrated significant activation and consumption of Factors V and XIII, fibrinogen, and proteins C and S.22 Patients with acute coagulopathy of trauma-shock (ACTS) were 4 times more likely to die than those without ACTS.22
In patients with evidence of hemorrhagic shock, hemodynamic instability, and inadequate oxygen (O2) delivery, a restrictive approach to transfusion is favored to maintain a goal hemoglobin of 7 to 9 g/dL. Generally, transfusion is considered when Hgb drops to <7 g/dL, especially in mechanically ventilated and other critically ill patients. Red blood cell transfusion should not be considered the singular or absolute method to improve tissue O2 consumption.23
Obstetric Hemorrhage
Postpartum hemorrhage (PPH) is a catastrophic maternal complication of delivery and a leading cause of maternal morbidity and mortality. Delayed hemorrhage may be seen in the ED days to weeks postpartum. Initial measures to control bleeding include uterine massage, uterotonic medications (ie, oxytocin), and blood-product components. Coagulopathy may be rapidly identified and FFP considered if a clot does not form within 7 minutes in a collection tube containing no anticoagulant (ie, red-top tube).12 During an ED delivery, uterine atony should be anticipated if the uterus is enlarged or the fundus is “doughy.” Atony is the most common cause of PPH within 24 hours and is managed with oxytocin 20 to 30 U/L at 200 mL/h. Alternatively, methylergonovine maleate 0.2 mg may be administered intramuscularly.24
Transfusion Complications
Several immediate complications may arise from transfusion, including intravascular hemolytic transfusion reactions, fever, urticaria, and transfusion-related lung injury (TRALI). Delayed complications include extravascular hemolytic reactions, and TA-GVHD. Other complications include acute bacteremia from contamination, viral infection, electrolyte derangements, cardiogenic pulmonary edema, and transfusion-associated circulatory overload (TACO).
Intravascular Hemolytic Reactions
Intravascular hemolytic reactions resulting from ABO incompatibility are the most severe transfusion complication. Immediate onset symptoms include fever, chills, headache, nausea, vomiting, chest discomfort, and severe back pain. Treatment involves immediate cessation of the transfusion, replacement of all tubing components, and aggressive IV crystalloid fluid therapy with diuretics to maintain a urine output of 1 to 2 mL/kg/h. All remaining blood, along with the patient’s blood and urine samples, should be sent to the laboratory to detect free Hgb. A positive Coombs test on the posttransfusion blood confirms the diagnosis.
The most common reaction is a 1°C temperature elevation with no other cause. Treatment for fever and urticaria consists of antihistamines and antipyretics. However, febrile patients receiving blood for the first time should be managed as an intravascular hemolytic transfusion reaction until proved otherwise by a negative Coombs test. Mild reactions may be due to an allergic response to donor plasma proteins, but in patients with genetic immunoglobulin A (IgA) deficiency can represent an afebrile life-threatening reaction characterized by hypotension and respiratory symptoms. An IgA deficiency should be considered in patients of European descent as a cause of transfusion-related anaphylactic reactions.25
The most common cause of mortality from transfusions is due to transfusion-related acute lung injury (TRALI), which presents within the first 6 hours of transfusion. Signs and symptoms of TRALI include noncardiogenic pulmonary edema, dyspnea, hypoxemia, fever, and hypotension. A portable chest X-ray may reveal bilateral infiltrates, and a complete blood count may demonstrate transient leukopenia. While the underlying mechanism is likely multifactorial, TRALI may be precipitated by a “leaky” pulmonary endothelium as a direct or indirect result of antibodies against the recipient. One strategy to reduce the incidence of TRALI is to use male donors for plasma to reduce the incidence of allotypic leukocyte antibodies that can occur in women who have had prior pregnancies. Management of TRALI includes immediately stopping the transfusion, notifying the blood bank, and providing respiratory support. Blood products may be transfused from a different donor. Unlike TACO or cardiogenic pulmonary edema, TRALI demonstrates no evidence of circulatory overload, and it does not respond to diuretic therapy.26 Circulatory overload may be avoided by infusing a single unit of PRBCs over 4 hours.
Extravascular Hemolytic Reactions
Delayed extravascular hemolytic reactions are most likely due to previous sensitization to red cell antigens from prior transfusion, pregnancy, or transplant. Extravascular hemolysis can occur days to weeks after repeat exposure. Patients present with fever, anemia, and jaundice without hemoglobinemia or hemoglobinuria. Symptoms are usually benign, though oliguria and DIC have been reported.27,28
Transfusion-associated graft-versus-host disease is a delayed extravascular hemolytic reaction that occurs in immunosuppressed recipients of transfused blood. Most deaths are due to coagulopathy or infection. Transfused lymphocytes proliferate and attack the blood recipient. Symptoms (eg, fever, rash, diarrhea, elevated liver transaminases, pancytopenia) begin 3 to 30 days posttransfusion, and a bone marrow transplant is indicated. Irradiated and leukoreduced blood components prevent TA-GVHD.29,30
Bacteremia and Viral Infection
Among significant bacterial contaminants from donor blood, Yersinia enterocolitica is the most common and has a mortality rate of greater than 50%.31 Typical symptoms include rigors, vomiting, abdominal cramps, fever, shock, renal failure, or DIC during transfusion. Immediate cessation of blood products and broad spectrum antibiotics are warranted. Risks among viral contaminants include human immunodeficiency disease (HIV), CMV, and hepatitis. Hepatitis B infection occurs in one in 1 million transfusion recipients, while the risk of hepatitis C is one in 1.2 million and HIV infection one in 1.5 million.32,33
Electrolyte Derangement
Electrolyte derangements after multiple-unit transfusions include hypocalcemia, hyperkalemia, and acid-base disorders. Massive blood transfusions with blood anticoagulated with sodium citrate and citric acid may contribute to metabolic alkalosis and hypocalcemia. Potassium may move into cells in exchange for hydrogen ions moving out of cells to minimize extracellular alkalosis, contributing to hypokalemia.34-36 To avoid hypocalcemia and alkalosis, the maximum citrate infusion rate should be 0.02 mmol/kg/minute, with the citrate concentration in whole blood measured as 15 mmol/L. If liver function is impaired in the setting of hypocalcemia-related blood transfusion, calcium chloride is preferred over calcium gluconate because it decreases citrate metabolism resulting in a slower release of ionized calcium.37 Calcium replacement should be considered in patients with liver dysfunction or patients with normal liver function who have received greater than 10 U pRBCs per hour. Calcium chloride (10%) is preferred over calcium gluconate to correct ionized hypocalcemia with 2 to 5 mL given for every 500mL of blood.37 Hyperkalemia risks are minimized by avoiding prolonged blood storage or irradiation.38
Conclusion
Timely administration of blood products is crucial in resuscitation and can be life-saving in a variety of bleeding disorders. Emergent reversal of warfarin therapy, correction of thrombocytopenia, bleeding due to hemophilia, GI bleeding, trauma, and obstetric hemorrhage are among the most common disorders managed in the ED. To select the most appropriate treatment, one must know the merits of the various blood products including PRBCs, platelets, FFP, and cryoprecipitate. The clinician must also be prepared to manage the immediate complications that may arise from transfusion including intravascular hemolytic reactions, fever, urticaria, and TRALI, as well as the delayed complications of extravascular hemolytic reactions, TA-GVHD, acute bacteremia, viral infection, electrolyte derangements, cardiogenic pulmonary edema, and TACO.
Dr Stewart is an emergency physician in the department of emergency medicine, Eastern Virginia Medical School, Norfolk, Virginia, and Riverside Medical Group, Newport News, Virginia. Dr Devine is an emergency physician in the department of emergency medicine, Eastern Virginia Medical School, Norfolk, and Emergency Physicians of Tidewater, Norfolk, Virginia. The authors report no conflicts of interest.
- Napolitano LM, Kurek S, Luchette FA, et al; EAST Practice Management Workgroup; American College of Critical Care Medicine (ACCM) Taskforce of the Society of Critical Care Medicine (SCCM). Clinical Practice Guideline: Red Blood Cell Transfusion and Critical Care. J Trauma. 2009;67(6):1439-1442.
- Leal-Noval SR, Munoz-Gomez M, Jimenez-Sanchez M et al. Red blood cell transfusion in nonbleeding critically ill patients with moderate anemia: is there a benefit? Intensive Care Med. 2013;39(3):445-453.
- Carson JL, Grossman BJ, Kleinman S, et al; Clinical Transfusion Medicine Committee of the AABB. Red blood cell transfusion: a clinical practice guideline from the AAAB. Ann Intern Med. 2012;157(1):49-58.
- Gilliss BM, Looney MR, Gropper MA. Reducing noninfectious risks of blood transfusion. Anesthesiology. 2011;115(3):635-649.
- van Straaten HL, de Wildt-Eggen J, Huisveld IA. Evaluation of a strategy to limit blood donor exposure in high risk premature newborns based on clinical estimation of transfusion need. J Perniat Med. 2000;28(2):122-128.
- Anstee DJ. Red cell genotyping and the future of pretransfusion testing. Blood. 2009;114(2):248-256.
- Inaba K, Teixeira PG, Shulman I. The impact of uncross-matched blood transfusion on the need for massive transfusion and mortality: analysis of 5,166 uncross-matched units. J Trauma. 2008;65(6):1222-1226.
- Slichter SJ. Platelet transfusion therapy. Hematol Oncol Clin North Am. 2007;21(4):697-729.
- Uppal P, Lodha R, Kabra SK. Transfusion of blood and components in critically ill children. Indian J Pediatr. 2010;77(12):1424-1428.
- Shah A, Stanworth SJ, McKechnie. Evidence and triggers for the transfusion of blood and blood products. Anesthesia. 2015;70(Suppl 1):10-19.
- Emery, M. Blood and Blood Components. In: Marx JA, Hockberger RS, Walls RM ed. Rosen’s emergency medicine: concepts and clinical practice. 7th ed. Philadelphia, PA: Mosby/Elsevier; 2009:42-46.
- Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G; American College of Chest Physicians. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):160S-198S.
- Santen S A Transfusion Therapy. In: Tintinalli, J. E., Kelen, G. D., & Stapczynski, J. S. ed. Emergency medicine: a comprehensive study guide. 6th ed. New York, NY: McGraw-Hill Medical; 20041349-1351.
- Osterman JL, Arora S. Blood product transfusions and reactions. Emerg Med Clin North Am. 2014;32(3): 727-738.
- Azzi A, De Santis R, Morfini M, et al. TT virus contaminates first-generation recombinant factor VIII concentrates. Blood. 2001;98(8):2571-2573.
- Barkun AN, Bardou M, Kuipers EJ, et al; International Consensus Upper Gastrointestinal Bleeding Conference Group International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152(2):101-113.
- Strate L, Saltzman J, and Ookubo R. Validation of a clinical prediction rule for severe acute lower intestinal bleeding. Am J Gastroenterol. 2005;100(8):1821-1827.
- Kollef MH, O’Brien JD, Zuckerman GR, Shannon W. BLEED: a classification tool to predict outcomes in patients with acute upper and lower gastrointestinal hemorrhage. Crit Care Med. 1997;25(7):1125-1132.
- Sauaia A, Moore FA, Moore EE, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38(2):185-193.
- Simmons JW, Pittet JF, Pierce B. Trauma-induced coagulopathy. Curr Anesthisiol Rep. 2014;4(3):189-199.
- Zehtabchi S, Nishijima DK. Impact of transfusion of fresh-frozen plasma and packed red blood cells in a 1:1 ratio on survival of emergency department patients with severe trauma. Acad Emerg Med. 2009;16(5):371-378.
- Theusinger OM, Baulig W, Seifert B, Müller SM, Mariotti S, Spahn DR.. Changes in coagulation in standard laboratory tests and ROTEM in trauma patients between on-scene and arrival in the emergency department. Anesth Analg. 2014. [Epub ahead of print]
- Bouillon B, Brohi K, Hess JR, Holcomb JB, Parr MJ, Hoyt DB. Educational initiative on critical bleeding in trauma: Chicago, July 11-13, 2008. J Trauma. 2010;68(1):225-230.
- Phillips LE, McLintock C, Pollock W, et al; Australian and New Zealand Haemostasis Registry. Recombinant activated Factor VII in obstetric hemorrhage: experiences from the Australian and New Zealand Haemostasis Registry. Anesth Analg. 2009;109(6):1908-1915.
- Hirayama F. Current understanding of allergic transfusion reactions: incidence, pathogenesis, laboratory tests, prevention and treatment. Br J Haematol. 2013;160(4);434-444.
- Lieberman L, Maskens C, Cserti-Gazdewich C, et al. A retrospective review of patient factors, transfusion practices, and outcomes in patients with transfusion-associated circulatory overload. Transfus Med Rev. 2013;27(4)206-212.
- Welling KL, Taaning E, Lund BV, Rosenkvist J, Heslet L. Post-transfusion purpura (PTP) and disseminated intravascular coagulation (DIC). Eur J Haematol. 2003;71(1):68-71
- Kawai M, Takeda M, Tsugawa Y. Hemolytic anemia and acute renal failure caused by blood transfusions. Rinsho Ketusueki. 1990;31(10):1706-1710.
- Przepiorka D, LeParc GF, Stovall MA, Werch J, Lichtiger B. Use of irradiated blood components: practice parameter. Am J Clin Pathol. 1996;106(1):6-11.
- Rühl H, Bein G, Sachs UJ. Transfusion-associated graft-versus-host disease. Transfus Med Rev. 2009;23(1):62-71.
- Guinet F, Carniel E, Leclercq A. Transfusion-transmitted Yersinia enterolitica sepsis. Clin Infect Dis. 2011;53(6):583-591.
- Stramer SL, Notari EP, Krysztof DE, Dodd RY. Hepatitis B virus testing by minipool nuclear acid testing: does it improve blood safety? Transfusion. 2013; 53(10):2449-2458.
- Zou S, Stramer SL, Dodd RY. Donor testing and risk: current prevalence, incidence, and residual risk of transfusion-transmissible agents in US allogenic donations. Transfus Med Rev. 2012;26(2):119-128.
- Dzik WH, Kirkley SA. Citrate toxicity during massive blood transfusion. Transfus Med Rev. 1988;2(2):76-94.
- Lier H, Krep H, Schroeder S, Stuber F. Preconditions of hemostasis in trauma: a review. The influence of acidosis, hypocalcemia, anemia, and hypothermia on functional hemostasis in trauma. J Trauma. 2008;65(4):951-960.
- Bruining HA, Boelhouwer RU, Ong GK. Unexpected hypopotassemia after multiple blood transfusions during an operation. Neth J Surg. 1986;38(2):48-51.
- British Committee for Standards in Haematology; Stainsby D, MacLennan S, Thomas D, Isaac J, Hamilton PJ. Guidelines on the management of massive blood loss. Br J Haematol. 2006;135(5):634-641.
- Smith HM, Farrow SJ, Ackerman JD, Stubbs JR, Sprung J. Cardiac arrests associated with hyperkalemia during red blood cell transfusion: a case series. Anesth Analg. 2008;106(4):1062-1069.
Overview
In 2011, nearly 14 million units of whole blood and RBCs were transfused in US hospitals according to the 2011 National Blood Collection and Utilization Survey Report. In the United States, United Kingdom, Western Europe, and Canada, approximately 40% of critically ill patients received a mean of 5 U of PRBC per hospitalization.1,2
In the ED, hemodynamic instability due to acute hemorrhage is the most common indication for transfusion of PRBCs. Common emergent sources include gastrointestinal (GI) bleeding, dysfunctional uterine bleeding, and bleeding secondary to trauma. For every unit of PRBCs transfused, the typical result in the average adult is an increase in hemoglobin (Hgb) by 1 g/dL and hematocrit by 3%. In the pediatric population, a 3 mL/kg intravenous (IV) dose achieves equivalent results.3
Blood Components and Type Compatibility
After donated blood is collected, blood banks divide the blood into type and components, including red cell concentrate, FFP, cryoprecipitates, and platelets.
Packed Red Blood Cells
After RBCs are separated from whole blood, they can be further processed through leukoreduction, which removes most white blood cells at the expense of a 10% to 15% loss of RBCs. Leukoreduced RBCs (LRBCs) are used in patients with a history of two or more febrile nonhemolytic transfusion reactions (FNHTR). In addition to preventing FNHTR, LRBCs may also be effective in preventing cytomegalovirus (CMV) transmission or human leukocyte antigen (HLA) alloimmunization.4
Cytomegalovirus negative PRBCs and blood components are indicated for the following patients: premature and all infants younger than age 4 weeks; intrauterine transfusions; bone marrow or organ transplant recipients (including transplant candidates); immunocompromised and asplenic patients; and pregnant women.
Irradiated PRBCs and blood products are exposed to 2,500 rad of gamma radiation to destroy lymphoproliferative processes. This irradiation prevents transfusion-associated graft-versus-host disease (TA-GVHD) in susceptible patients. Absolute indications for irradiated blood products include bone marrow transplant recipients and donors, stem-cell donors, T-cell immunodeficiency, intrauterine transfusion, and HLA-matched platelet transfusions. Relative indications include patients with leukemia, Hodgkin disease, non-Hodgkin lymphoma, neonatal exchange transfusion, premature infants, neuroblastoma, and glioblastoma.3
Divided RBC units or “pedi-packs” are derived from dividing single units of PRBCs into 4 units. Pedi-packs are type O irradiated, leukoreduced, and Hgb S negative PRBCs; however, they are not necessarily CMV negative. Pedi-packs minimize blood wasting and donor exposure when a small volume transfusion is indicated.5
Type O
Often, cross-matched blood is not immediately available. If PRBCs are needed within the first 15 minutes of resuscitation and the patient’s condition cannot be stabilized with 2 L of crystalloid fluids, type O blood is warranted. In general, women of childbearing age should be transfused with type O Rh-negative blood.6
Of 4,241 trauma patients who received uncrossmatched PRBCs (URBCs) or type O transfusions in a retrospective study at a level 1 trauma center, those receiving URBCs had a 39.6% mortality compared to 11.9% of those with crossmatched PRBCs (P<.001). In general, the use of URBCs is an independent predictor of mortality after adjusting for gender, mechanism, age, hypotension, intubation, initial Hgb, abbreviated injury scale, Glasgow coma scale, injury severity score, and the amount of blood products received. Crossmatched blood should be used whenever available, and a request for uncrossmatched blood products should trigger the blood bank to release crossmatched blood in anticipation of massive transfusion.7
Platelets
Platelets are separated and concentrated through serial centrifugation, then re-suspended in residual plasma. A therapeutic adult dose is comprised of four to six platelet concentrates of the same blood type. This raises platelet counts by 5,000 mL/U. Even though hemostasis may be maintained at platelet counts of 5,000/mL, it is acceptable to transfuse for platelet counts below 10,000/mL. Patients who are bleeding due to platelet dysfunction, and/or thrombocytopenia require platelets. Platelet transfusion is generally ineffective in the case of immune-mediated platelet consumption such as thrombotic thrombocytopenic purpura (TTP).8
ABO Compatible Platelets
Infants and small children require ABO compatible or volume-reduced platelets.9 Type ABO compatibility is less clinically significant in adults; however, Rh sensitization may occur. Conditions refractory to platelet therapy include fever, sepsis disseminated intravascular coagulation (DIC), splenomegaly, idiopathic thrombocytopenic purpura, and platelet alloimmunization. Patients frequently transfused with platelets or those with platelet alloimmunization require leukoreduced and HLA-matched products to minimize HLA antibody-induced immune destruction.10
Fresh Frozen Plasma
Plasma is removed from whole blood and frozen below 55˚F to make FFP. It contains all of the coagulation factors but is not a concentrate. Fresh frozen plasma contains both stable and labile components of the fibrinolytic, coagulation, and complement systems, as well as proteins that maintain oncotic pressure. Unlike PRBC, where type O is the universal donor, in FFP, AB type is the universal donor for transfusion. In the ED, FFP is used for the reversal of coagulopathy in bleeding patients and for replacement of coagulation factors when specific factors are unavailable. It is also given to patients requiring large volumes of blood components (ie, massive blood transfusion protocol).10
A typical FFP unit is approximately 250 mL and is administered within 6 hours of thawing. Every 1 mL/kg of body weight of FFP raises clotting factors by 1%. For warfarin reversal, 5 to 8 mL/kg of FFP should be administered IV. One milliliter of FFP has 1 U of activity of all coagulation factors; 15 mL/kg of FFP achieves approximately 30% of plasma factor concentration.10,11
Patients with active bleeding and documented liver disease, congenital factor deficiency, or mass transfusion recipients are candidates for FFP in the ED. Patients with TTP should also receive FFP with plasma exchange. When FFP is administered for emergent reversal in life-threatening bleeding or intracranial hemorrhage, it is given in conjunction with IV vitamin K and either Factor VIIa or prothrombin.12
Cryoprecipitate
Cryoprecipitate contains factor VIII, von Willebrand factor (vWF), and fibrinogen with some amounts of factor XIII and fibronectin. Actively bleeding patients with hypofibrinogenemia (<100 mg/dL fibrin) are candidates for cryoprecipitate. Cryoprecipitate is used in the therapeutic management of hemophilia A (factor VIII deficiency) when factor VIII concentrates are not available. Cryoprecipitate is given as type ABO compatible when possible and, like FFP, type AB is the universal donor. Each unit of cryoprecipitate raises fibrinogen 75 mg/dL, with a typical dose being 10 U or 1 U per 5 kg of patient body weight.13,14
Factor VIII, Von Willebrand Factor, and Factor IX
Patients with hemophilia typically present to the ED with bleeding episodes ranging from benign abrasion to life-threatening epidural hematomas. Factor VIII concentrates are purified from plasma to treat bleeding patients with hemophilia A or von Willebrand disease (VWD). For emergent use, the amount of factor VIII should be calculated as follows: estimated dose = weight (kg) x 0.5 x desired factor (%) increase. The targeted factor VIII increase is typically 80% to 100% for severe bleeding in patients with hemophilia A.
Another component of factor VIII is vWF activity (factor VIII/vWF). Von Willebrand disease is characterized by the lack of factor VIII/vWF, resulting in normal platelet counts and morphologies, but with impaired adhesion ability. Humate-P and Alphanate SD/HT, are factor VIII replacement therapies with significant amounts of vWF, and are approved for use in patients with hemophilia A and vWD. The initial dose of Humate-P for severe bleeding episodes is 40 to 60 U/kg IV. An administered dose of 50 IU/kg of Alphanate is expected to increase circulating FVIII levels to 100% of normal.
Factor IX (FIX) concentrates are used to treat patients with hemophilia B, a condition in which patients lack factor IX, a vitamin K-dependent glycoprotein. The FIX concentrates may also benefit patients with factor X or prothrombin deficiency. In the United States, since 1992, commercially available FIX is produced from genetically engineered recombinant factor replacement (rFIX). Second-generation rFIX and monoclonal antibody solvents do not contain human plasma and are free of viral contaminants, including parvovirus B19.15
Etiology and Treatment
Gastrointestinal Bleeding
Sources of GI bleeding vary from hemorrhoids to Mallory-Weiss tears. The heterogeneous population of patients with GI bleeds complicates the identification of high-risk patients needing transfusion. Bleeding is traditionally characterized as either upper GI bleeding (UGIB) or lower GI bleeding (LGIB)—the former requiring endoscopy, the latter colonoscopy or other expensive strategies to differentiate one form from the other.
In patients with LGIB, the differential diagnosis is broad, ranging from hemorrhoidal bleeding, cancer, or life-threatening diverticular hemorrhage. Prompt volume replacement with isotonic crystalloid IV fluids must be initiated. In nonvariceal UGIB, blood transfusions should be initiated for Hgb levels <70g/L.16
Clinical prediction rules for acute GI bleeding can help identify those patients who require transfusions. One study collected data on seven established independent predictors of severe LGIB, including heart rate, systolic blood pressure (SBP), syncope, nontender abdomen, rectal bleeding in the first 4 hours of evaluation, aspirin use, and more than two comorbid conditions. A nontender abdomen was the best predictor of severe bleeding, likely due to the fact that vascular disorders, such as diverticulitis, result in brisk bleeding without tenderness; whereas inflammatory processes, such as ischemic colitis, are associated with less severe bleeding and abdominal tenderness. Patients with one or more of the seven risk factors were stratified into low (0-7 risk factors), moderate (1-3 risk factors), and high-risk groups (>3 risk factors). Low-risk patients had a ≤ 9% risk of a severe LGIB, moderate-risk patients had a 43% risk, and high-risk patients had >79% likelihood of bleeding. The high-risk patients were more likely to require early transfusion of PRBCs. Tachycardia, hypotension, syncope, nontender abdomen, and rectal bleeding were identified as the most significant predictors of patients requiring 4 or more units of PRBC in the first 24 hours. Such clinical prediction rules may aid in the initial triage of patients with acute LGIB and identify those most likely to require transfusion in the ED.17
Similar to LGIB transfusion prediction rules, the BLEED (ongoing bleeding, low systolic blood pressure, elevated prothrombin time [PT], erratic mental status, unstable comorbid disease) classification identified patients with UGIB most likely to require transfusion. High-risk patients had one or more of the following: ongoing bleeding, SBP <100 mm Hg, PT more than 1.2 times the control value, altered mental status, the presence of an unstable comorbid disease, or a disease process requiring management in the intensive care unit.18
Trauma
Within the first 48 hours of presentation, blood loss accounts for more than 50% of all trauma deaths.19,20 Posttraumatic bleeding is attributed to several factors, including vascular injury and coagulopathy. Hemodilution from large amounts of crystalloid infusion, hypothermia, and acidosis in early resuscitation adversely affect coagulation, platelet function, protein C consumption, and increases levels of tissue plasminogen activator inhibitor.21 A recent study comparing coagulation tests at the trauma scene and for 1 hour after injury, demonstrated significant activation and consumption of Factors V and XIII, fibrinogen, and proteins C and S.22 Patients with acute coagulopathy of trauma-shock (ACTS) were 4 times more likely to die than those without ACTS.22
In patients with evidence of hemorrhagic shock, hemodynamic instability, and inadequate oxygen (O2) delivery, a restrictive approach to transfusion is favored to maintain a goal hemoglobin of 7 to 9 g/dL. Generally, transfusion is considered when Hgb drops to <7 g/dL, especially in mechanically ventilated and other critically ill patients. Red blood cell transfusion should not be considered the singular or absolute method to improve tissue O2 consumption.23
Obstetric Hemorrhage
Postpartum hemorrhage (PPH) is a catastrophic maternal complication of delivery and a leading cause of maternal morbidity and mortality. Delayed hemorrhage may be seen in the ED days to weeks postpartum. Initial measures to control bleeding include uterine massage, uterotonic medications (ie, oxytocin), and blood-product components. Coagulopathy may be rapidly identified and FFP considered if a clot does not form within 7 minutes in a collection tube containing no anticoagulant (ie, red-top tube).12 During an ED delivery, uterine atony should be anticipated if the uterus is enlarged or the fundus is “doughy.” Atony is the most common cause of PPH within 24 hours and is managed with oxytocin 20 to 30 U/L at 200 mL/h. Alternatively, methylergonovine maleate 0.2 mg may be administered intramuscularly.24
Transfusion Complications
Several immediate complications may arise from transfusion, including intravascular hemolytic transfusion reactions, fever, urticaria, and transfusion-related lung injury (TRALI). Delayed complications include extravascular hemolytic reactions, and TA-GVHD. Other complications include acute bacteremia from contamination, viral infection, electrolyte derangements, cardiogenic pulmonary edema, and transfusion-associated circulatory overload (TACO).
Intravascular Hemolytic Reactions
Intravascular hemolytic reactions resulting from ABO incompatibility are the most severe transfusion complication. Immediate onset symptoms include fever, chills, headache, nausea, vomiting, chest discomfort, and severe back pain. Treatment involves immediate cessation of the transfusion, replacement of all tubing components, and aggressive IV crystalloid fluid therapy with diuretics to maintain a urine output of 1 to 2 mL/kg/h. All remaining blood, along with the patient’s blood and urine samples, should be sent to the laboratory to detect free Hgb. A positive Coombs test on the posttransfusion blood confirms the diagnosis.
The most common reaction is a 1°C temperature elevation with no other cause. Treatment for fever and urticaria consists of antihistamines and antipyretics. However, febrile patients receiving blood for the first time should be managed as an intravascular hemolytic transfusion reaction until proved otherwise by a negative Coombs test. Mild reactions may be due to an allergic response to donor plasma proteins, but in patients with genetic immunoglobulin A (IgA) deficiency can represent an afebrile life-threatening reaction characterized by hypotension and respiratory symptoms. An IgA deficiency should be considered in patients of European descent as a cause of transfusion-related anaphylactic reactions.25
The most common cause of mortality from transfusions is due to transfusion-related acute lung injury (TRALI), which presents within the first 6 hours of transfusion. Signs and symptoms of TRALI include noncardiogenic pulmonary edema, dyspnea, hypoxemia, fever, and hypotension. A portable chest X-ray may reveal bilateral infiltrates, and a complete blood count may demonstrate transient leukopenia. While the underlying mechanism is likely multifactorial, TRALI may be precipitated by a “leaky” pulmonary endothelium as a direct or indirect result of antibodies against the recipient. One strategy to reduce the incidence of TRALI is to use male donors for plasma to reduce the incidence of allotypic leukocyte antibodies that can occur in women who have had prior pregnancies. Management of TRALI includes immediately stopping the transfusion, notifying the blood bank, and providing respiratory support. Blood products may be transfused from a different donor. Unlike TACO or cardiogenic pulmonary edema, TRALI demonstrates no evidence of circulatory overload, and it does not respond to diuretic therapy.26 Circulatory overload may be avoided by infusing a single unit of PRBCs over 4 hours.
Extravascular Hemolytic Reactions
Delayed extravascular hemolytic reactions are most likely due to previous sensitization to red cell antigens from prior transfusion, pregnancy, or transplant. Extravascular hemolysis can occur days to weeks after repeat exposure. Patients present with fever, anemia, and jaundice without hemoglobinemia or hemoglobinuria. Symptoms are usually benign, though oliguria and DIC have been reported.27,28
Transfusion-associated graft-versus-host disease is a delayed extravascular hemolytic reaction that occurs in immunosuppressed recipients of transfused blood. Most deaths are due to coagulopathy or infection. Transfused lymphocytes proliferate and attack the blood recipient. Symptoms (eg, fever, rash, diarrhea, elevated liver transaminases, pancytopenia) begin 3 to 30 days posttransfusion, and a bone marrow transplant is indicated. Irradiated and leukoreduced blood components prevent TA-GVHD.29,30
Bacteremia and Viral Infection
Among significant bacterial contaminants from donor blood, Yersinia enterocolitica is the most common and has a mortality rate of greater than 50%.31 Typical symptoms include rigors, vomiting, abdominal cramps, fever, shock, renal failure, or DIC during transfusion. Immediate cessation of blood products and broad spectrum antibiotics are warranted. Risks among viral contaminants include human immunodeficiency disease (HIV), CMV, and hepatitis. Hepatitis B infection occurs in one in 1 million transfusion recipients, while the risk of hepatitis C is one in 1.2 million and HIV infection one in 1.5 million.32,33
Electrolyte Derangement
Electrolyte derangements after multiple-unit transfusions include hypocalcemia, hyperkalemia, and acid-base disorders. Massive blood transfusions with blood anticoagulated with sodium citrate and citric acid may contribute to metabolic alkalosis and hypocalcemia. Potassium may move into cells in exchange for hydrogen ions moving out of cells to minimize extracellular alkalosis, contributing to hypokalemia.34-36 To avoid hypocalcemia and alkalosis, the maximum citrate infusion rate should be 0.02 mmol/kg/minute, with the citrate concentration in whole blood measured as 15 mmol/L. If liver function is impaired in the setting of hypocalcemia-related blood transfusion, calcium chloride is preferred over calcium gluconate because it decreases citrate metabolism resulting in a slower release of ionized calcium.37 Calcium replacement should be considered in patients with liver dysfunction or patients with normal liver function who have received greater than 10 U pRBCs per hour. Calcium chloride (10%) is preferred over calcium gluconate to correct ionized hypocalcemia with 2 to 5 mL given for every 500mL of blood.37 Hyperkalemia risks are minimized by avoiding prolonged blood storage or irradiation.38
Conclusion
Timely administration of blood products is crucial in resuscitation and can be life-saving in a variety of bleeding disorders. Emergent reversal of warfarin therapy, correction of thrombocytopenia, bleeding due to hemophilia, GI bleeding, trauma, and obstetric hemorrhage are among the most common disorders managed in the ED. To select the most appropriate treatment, one must know the merits of the various blood products including PRBCs, platelets, FFP, and cryoprecipitate. The clinician must also be prepared to manage the immediate complications that may arise from transfusion including intravascular hemolytic reactions, fever, urticaria, and TRALI, as well as the delayed complications of extravascular hemolytic reactions, TA-GVHD, acute bacteremia, viral infection, electrolyte derangements, cardiogenic pulmonary edema, and TACO.
Dr Stewart is an emergency physician in the department of emergency medicine, Eastern Virginia Medical School, Norfolk, Virginia, and Riverside Medical Group, Newport News, Virginia. Dr Devine is an emergency physician in the department of emergency medicine, Eastern Virginia Medical School, Norfolk, and Emergency Physicians of Tidewater, Norfolk, Virginia. The authors report no conflicts of interest.
Overview
In 2011, nearly 14 million units of whole blood and RBCs were transfused in US hospitals according to the 2011 National Blood Collection and Utilization Survey Report. In the United States, United Kingdom, Western Europe, and Canada, approximately 40% of critically ill patients received a mean of 5 U of PRBC per hospitalization.1,2
In the ED, hemodynamic instability due to acute hemorrhage is the most common indication for transfusion of PRBCs. Common emergent sources include gastrointestinal (GI) bleeding, dysfunctional uterine bleeding, and bleeding secondary to trauma. For every unit of PRBCs transfused, the typical result in the average adult is an increase in hemoglobin (Hgb) by 1 g/dL and hematocrit by 3%. In the pediatric population, a 3 mL/kg intravenous (IV) dose achieves equivalent results.3
Blood Components and Type Compatibility
After donated blood is collected, blood banks divide the blood into type and components, including red cell concentrate, FFP, cryoprecipitates, and platelets.
Packed Red Blood Cells
After RBCs are separated from whole blood, they can be further processed through leukoreduction, which removes most white blood cells at the expense of a 10% to 15% loss of RBCs. Leukoreduced RBCs (LRBCs) are used in patients with a history of two or more febrile nonhemolytic transfusion reactions (FNHTR). In addition to preventing FNHTR, LRBCs may also be effective in preventing cytomegalovirus (CMV) transmission or human leukocyte antigen (HLA) alloimmunization.4
Cytomegalovirus negative PRBCs and blood components are indicated for the following patients: premature and all infants younger than age 4 weeks; intrauterine transfusions; bone marrow or organ transplant recipients (including transplant candidates); immunocompromised and asplenic patients; and pregnant women.
Irradiated PRBCs and blood products are exposed to 2,500 rad of gamma radiation to destroy lymphoproliferative processes. This irradiation prevents transfusion-associated graft-versus-host disease (TA-GVHD) in susceptible patients. Absolute indications for irradiated blood products include bone marrow transplant recipients and donors, stem-cell donors, T-cell immunodeficiency, intrauterine transfusion, and HLA-matched platelet transfusions. Relative indications include patients with leukemia, Hodgkin disease, non-Hodgkin lymphoma, neonatal exchange transfusion, premature infants, neuroblastoma, and glioblastoma.3
Divided RBC units or “pedi-packs” are derived from dividing single units of PRBCs into 4 units. Pedi-packs are type O irradiated, leukoreduced, and Hgb S negative PRBCs; however, they are not necessarily CMV negative. Pedi-packs minimize blood wasting and donor exposure when a small volume transfusion is indicated.5
Type O
Often, cross-matched blood is not immediately available. If PRBCs are needed within the first 15 minutes of resuscitation and the patient’s condition cannot be stabilized with 2 L of crystalloid fluids, type O blood is warranted. In general, women of childbearing age should be transfused with type O Rh-negative blood.6
Of 4,241 trauma patients who received uncrossmatched PRBCs (URBCs) or type O transfusions in a retrospective study at a level 1 trauma center, those receiving URBCs had a 39.6% mortality compared to 11.9% of those with crossmatched PRBCs (P<.001). In general, the use of URBCs is an independent predictor of mortality after adjusting for gender, mechanism, age, hypotension, intubation, initial Hgb, abbreviated injury scale, Glasgow coma scale, injury severity score, and the amount of blood products received. Crossmatched blood should be used whenever available, and a request for uncrossmatched blood products should trigger the blood bank to release crossmatched blood in anticipation of massive transfusion.7
Platelets
Platelets are separated and concentrated through serial centrifugation, then re-suspended in residual plasma. A therapeutic adult dose is comprised of four to six platelet concentrates of the same blood type. This raises platelet counts by 5,000 mL/U. Even though hemostasis may be maintained at platelet counts of 5,000/mL, it is acceptable to transfuse for platelet counts below 10,000/mL. Patients who are bleeding due to platelet dysfunction, and/or thrombocytopenia require platelets. Platelet transfusion is generally ineffective in the case of immune-mediated platelet consumption such as thrombotic thrombocytopenic purpura (TTP).8
ABO Compatible Platelets
Infants and small children require ABO compatible or volume-reduced platelets.9 Type ABO compatibility is less clinically significant in adults; however, Rh sensitization may occur. Conditions refractory to platelet therapy include fever, sepsis disseminated intravascular coagulation (DIC), splenomegaly, idiopathic thrombocytopenic purpura, and platelet alloimmunization. Patients frequently transfused with platelets or those with platelet alloimmunization require leukoreduced and HLA-matched products to minimize HLA antibody-induced immune destruction.10
Fresh Frozen Plasma
Plasma is removed from whole blood and frozen below 55˚F to make FFP. It contains all of the coagulation factors but is not a concentrate. Fresh frozen plasma contains both stable and labile components of the fibrinolytic, coagulation, and complement systems, as well as proteins that maintain oncotic pressure. Unlike PRBC, where type O is the universal donor, in FFP, AB type is the universal donor for transfusion. In the ED, FFP is used for the reversal of coagulopathy in bleeding patients and for replacement of coagulation factors when specific factors are unavailable. It is also given to patients requiring large volumes of blood components (ie, massive blood transfusion protocol).10
A typical FFP unit is approximately 250 mL and is administered within 6 hours of thawing. Every 1 mL/kg of body weight of FFP raises clotting factors by 1%. For warfarin reversal, 5 to 8 mL/kg of FFP should be administered IV. One milliliter of FFP has 1 U of activity of all coagulation factors; 15 mL/kg of FFP achieves approximately 30% of plasma factor concentration.10,11
Patients with active bleeding and documented liver disease, congenital factor deficiency, or mass transfusion recipients are candidates for FFP in the ED. Patients with TTP should also receive FFP with plasma exchange. When FFP is administered for emergent reversal in life-threatening bleeding or intracranial hemorrhage, it is given in conjunction with IV vitamin K and either Factor VIIa or prothrombin.12
Cryoprecipitate
Cryoprecipitate contains factor VIII, von Willebrand factor (vWF), and fibrinogen with some amounts of factor XIII and fibronectin. Actively bleeding patients with hypofibrinogenemia (<100 mg/dL fibrin) are candidates for cryoprecipitate. Cryoprecipitate is used in the therapeutic management of hemophilia A (factor VIII deficiency) when factor VIII concentrates are not available. Cryoprecipitate is given as type ABO compatible when possible and, like FFP, type AB is the universal donor. Each unit of cryoprecipitate raises fibrinogen 75 mg/dL, with a typical dose being 10 U or 1 U per 5 kg of patient body weight.13,14
Factor VIII, Von Willebrand Factor, and Factor IX
Patients with hemophilia typically present to the ED with bleeding episodes ranging from benign abrasion to life-threatening epidural hematomas. Factor VIII concentrates are purified from plasma to treat bleeding patients with hemophilia A or von Willebrand disease (VWD). For emergent use, the amount of factor VIII should be calculated as follows: estimated dose = weight (kg) x 0.5 x desired factor (%) increase. The targeted factor VIII increase is typically 80% to 100% for severe bleeding in patients with hemophilia A.
Another component of factor VIII is vWF activity (factor VIII/vWF). Von Willebrand disease is characterized by the lack of factor VIII/vWF, resulting in normal platelet counts and morphologies, but with impaired adhesion ability. Humate-P and Alphanate SD/HT, are factor VIII replacement therapies with significant amounts of vWF, and are approved for use in patients with hemophilia A and vWD. The initial dose of Humate-P for severe bleeding episodes is 40 to 60 U/kg IV. An administered dose of 50 IU/kg of Alphanate is expected to increase circulating FVIII levels to 100% of normal.
Factor IX (FIX) concentrates are used to treat patients with hemophilia B, a condition in which patients lack factor IX, a vitamin K-dependent glycoprotein. The FIX concentrates may also benefit patients with factor X or prothrombin deficiency. In the United States, since 1992, commercially available FIX is produced from genetically engineered recombinant factor replacement (rFIX). Second-generation rFIX and monoclonal antibody solvents do not contain human plasma and are free of viral contaminants, including parvovirus B19.15
Etiology and Treatment
Gastrointestinal Bleeding
Sources of GI bleeding vary from hemorrhoids to Mallory-Weiss tears. The heterogeneous population of patients with GI bleeds complicates the identification of high-risk patients needing transfusion. Bleeding is traditionally characterized as either upper GI bleeding (UGIB) or lower GI bleeding (LGIB)—the former requiring endoscopy, the latter colonoscopy or other expensive strategies to differentiate one form from the other.
In patients with LGIB, the differential diagnosis is broad, ranging from hemorrhoidal bleeding, cancer, or life-threatening diverticular hemorrhage. Prompt volume replacement with isotonic crystalloid IV fluids must be initiated. In nonvariceal UGIB, blood transfusions should be initiated for Hgb levels <70g/L.16
Clinical prediction rules for acute GI bleeding can help identify those patients who require transfusions. One study collected data on seven established independent predictors of severe LGIB, including heart rate, systolic blood pressure (SBP), syncope, nontender abdomen, rectal bleeding in the first 4 hours of evaluation, aspirin use, and more than two comorbid conditions. A nontender abdomen was the best predictor of severe bleeding, likely due to the fact that vascular disorders, such as diverticulitis, result in brisk bleeding without tenderness; whereas inflammatory processes, such as ischemic colitis, are associated with less severe bleeding and abdominal tenderness. Patients with one or more of the seven risk factors were stratified into low (0-7 risk factors), moderate (1-3 risk factors), and high-risk groups (>3 risk factors). Low-risk patients had a ≤ 9% risk of a severe LGIB, moderate-risk patients had a 43% risk, and high-risk patients had >79% likelihood of bleeding. The high-risk patients were more likely to require early transfusion of PRBCs. Tachycardia, hypotension, syncope, nontender abdomen, and rectal bleeding were identified as the most significant predictors of patients requiring 4 or more units of PRBC in the first 24 hours. Such clinical prediction rules may aid in the initial triage of patients with acute LGIB and identify those most likely to require transfusion in the ED.17
Similar to LGIB transfusion prediction rules, the BLEED (ongoing bleeding, low systolic blood pressure, elevated prothrombin time [PT], erratic mental status, unstable comorbid disease) classification identified patients with UGIB most likely to require transfusion. High-risk patients had one or more of the following: ongoing bleeding, SBP <100 mm Hg, PT more than 1.2 times the control value, altered mental status, the presence of an unstable comorbid disease, or a disease process requiring management in the intensive care unit.18
Trauma
Within the first 48 hours of presentation, blood loss accounts for more than 50% of all trauma deaths.19,20 Posttraumatic bleeding is attributed to several factors, including vascular injury and coagulopathy. Hemodilution from large amounts of crystalloid infusion, hypothermia, and acidosis in early resuscitation adversely affect coagulation, platelet function, protein C consumption, and increases levels of tissue plasminogen activator inhibitor.21 A recent study comparing coagulation tests at the trauma scene and for 1 hour after injury, demonstrated significant activation and consumption of Factors V and XIII, fibrinogen, and proteins C and S.22 Patients with acute coagulopathy of trauma-shock (ACTS) were 4 times more likely to die than those without ACTS.22
In patients with evidence of hemorrhagic shock, hemodynamic instability, and inadequate oxygen (O2) delivery, a restrictive approach to transfusion is favored to maintain a goal hemoglobin of 7 to 9 g/dL. Generally, transfusion is considered when Hgb drops to <7 g/dL, especially in mechanically ventilated and other critically ill patients. Red blood cell transfusion should not be considered the singular or absolute method to improve tissue O2 consumption.23
Obstetric Hemorrhage
Postpartum hemorrhage (PPH) is a catastrophic maternal complication of delivery and a leading cause of maternal morbidity and mortality. Delayed hemorrhage may be seen in the ED days to weeks postpartum. Initial measures to control bleeding include uterine massage, uterotonic medications (ie, oxytocin), and blood-product components. Coagulopathy may be rapidly identified and FFP considered if a clot does not form within 7 minutes in a collection tube containing no anticoagulant (ie, red-top tube).12 During an ED delivery, uterine atony should be anticipated if the uterus is enlarged or the fundus is “doughy.” Atony is the most common cause of PPH within 24 hours and is managed with oxytocin 20 to 30 U/L at 200 mL/h. Alternatively, methylergonovine maleate 0.2 mg may be administered intramuscularly.24
Transfusion Complications
Several immediate complications may arise from transfusion, including intravascular hemolytic transfusion reactions, fever, urticaria, and transfusion-related lung injury (TRALI). Delayed complications include extravascular hemolytic reactions, and TA-GVHD. Other complications include acute bacteremia from contamination, viral infection, electrolyte derangements, cardiogenic pulmonary edema, and transfusion-associated circulatory overload (TACO).
Intravascular Hemolytic Reactions
Intravascular hemolytic reactions resulting from ABO incompatibility are the most severe transfusion complication. Immediate onset symptoms include fever, chills, headache, nausea, vomiting, chest discomfort, and severe back pain. Treatment involves immediate cessation of the transfusion, replacement of all tubing components, and aggressive IV crystalloid fluid therapy with diuretics to maintain a urine output of 1 to 2 mL/kg/h. All remaining blood, along with the patient’s blood and urine samples, should be sent to the laboratory to detect free Hgb. A positive Coombs test on the posttransfusion blood confirms the diagnosis.
The most common reaction is a 1°C temperature elevation with no other cause. Treatment for fever and urticaria consists of antihistamines and antipyretics. However, febrile patients receiving blood for the first time should be managed as an intravascular hemolytic transfusion reaction until proved otherwise by a negative Coombs test. Mild reactions may be due to an allergic response to donor plasma proteins, but in patients with genetic immunoglobulin A (IgA) deficiency can represent an afebrile life-threatening reaction characterized by hypotension and respiratory symptoms. An IgA deficiency should be considered in patients of European descent as a cause of transfusion-related anaphylactic reactions.25
The most common cause of mortality from transfusions is due to transfusion-related acute lung injury (TRALI), which presents within the first 6 hours of transfusion. Signs and symptoms of TRALI include noncardiogenic pulmonary edema, dyspnea, hypoxemia, fever, and hypotension. A portable chest X-ray may reveal bilateral infiltrates, and a complete blood count may demonstrate transient leukopenia. While the underlying mechanism is likely multifactorial, TRALI may be precipitated by a “leaky” pulmonary endothelium as a direct or indirect result of antibodies against the recipient. One strategy to reduce the incidence of TRALI is to use male donors for plasma to reduce the incidence of allotypic leukocyte antibodies that can occur in women who have had prior pregnancies. Management of TRALI includes immediately stopping the transfusion, notifying the blood bank, and providing respiratory support. Blood products may be transfused from a different donor. Unlike TACO or cardiogenic pulmonary edema, TRALI demonstrates no evidence of circulatory overload, and it does not respond to diuretic therapy.26 Circulatory overload may be avoided by infusing a single unit of PRBCs over 4 hours.
Extravascular Hemolytic Reactions
Delayed extravascular hemolytic reactions are most likely due to previous sensitization to red cell antigens from prior transfusion, pregnancy, or transplant. Extravascular hemolysis can occur days to weeks after repeat exposure. Patients present with fever, anemia, and jaundice without hemoglobinemia or hemoglobinuria. Symptoms are usually benign, though oliguria and DIC have been reported.27,28
Transfusion-associated graft-versus-host disease is a delayed extravascular hemolytic reaction that occurs in immunosuppressed recipients of transfused blood. Most deaths are due to coagulopathy or infection. Transfused lymphocytes proliferate and attack the blood recipient. Symptoms (eg, fever, rash, diarrhea, elevated liver transaminases, pancytopenia) begin 3 to 30 days posttransfusion, and a bone marrow transplant is indicated. Irradiated and leukoreduced blood components prevent TA-GVHD.29,30
Bacteremia and Viral Infection
Among significant bacterial contaminants from donor blood, Yersinia enterocolitica is the most common and has a mortality rate of greater than 50%.31 Typical symptoms include rigors, vomiting, abdominal cramps, fever, shock, renal failure, or DIC during transfusion. Immediate cessation of blood products and broad spectrum antibiotics are warranted. Risks among viral contaminants include human immunodeficiency disease (HIV), CMV, and hepatitis. Hepatitis B infection occurs in one in 1 million transfusion recipients, while the risk of hepatitis C is one in 1.2 million and HIV infection one in 1.5 million.32,33
Electrolyte Derangement
Electrolyte derangements after multiple-unit transfusions include hypocalcemia, hyperkalemia, and acid-base disorders. Massive blood transfusions with blood anticoagulated with sodium citrate and citric acid may contribute to metabolic alkalosis and hypocalcemia. Potassium may move into cells in exchange for hydrogen ions moving out of cells to minimize extracellular alkalosis, contributing to hypokalemia.34-36 To avoid hypocalcemia and alkalosis, the maximum citrate infusion rate should be 0.02 mmol/kg/minute, with the citrate concentration in whole blood measured as 15 mmol/L. If liver function is impaired in the setting of hypocalcemia-related blood transfusion, calcium chloride is preferred over calcium gluconate because it decreases citrate metabolism resulting in a slower release of ionized calcium.37 Calcium replacement should be considered in patients with liver dysfunction or patients with normal liver function who have received greater than 10 U pRBCs per hour. Calcium chloride (10%) is preferred over calcium gluconate to correct ionized hypocalcemia with 2 to 5 mL given for every 500mL of blood.37 Hyperkalemia risks are minimized by avoiding prolonged blood storage or irradiation.38
Conclusion
Timely administration of blood products is crucial in resuscitation and can be life-saving in a variety of bleeding disorders. Emergent reversal of warfarin therapy, correction of thrombocytopenia, bleeding due to hemophilia, GI bleeding, trauma, and obstetric hemorrhage are among the most common disorders managed in the ED. To select the most appropriate treatment, one must know the merits of the various blood products including PRBCs, platelets, FFP, and cryoprecipitate. The clinician must also be prepared to manage the immediate complications that may arise from transfusion including intravascular hemolytic reactions, fever, urticaria, and TRALI, as well as the delayed complications of extravascular hemolytic reactions, TA-GVHD, acute bacteremia, viral infection, electrolyte derangements, cardiogenic pulmonary edema, and TACO.
Dr Stewart is an emergency physician in the department of emergency medicine, Eastern Virginia Medical School, Norfolk, Virginia, and Riverside Medical Group, Newport News, Virginia. Dr Devine is an emergency physician in the department of emergency medicine, Eastern Virginia Medical School, Norfolk, and Emergency Physicians of Tidewater, Norfolk, Virginia. The authors report no conflicts of interest.
- Napolitano LM, Kurek S, Luchette FA, et al; EAST Practice Management Workgroup; American College of Critical Care Medicine (ACCM) Taskforce of the Society of Critical Care Medicine (SCCM). Clinical Practice Guideline: Red Blood Cell Transfusion and Critical Care. J Trauma. 2009;67(6):1439-1442.
- Leal-Noval SR, Munoz-Gomez M, Jimenez-Sanchez M et al. Red blood cell transfusion in nonbleeding critically ill patients with moderate anemia: is there a benefit? Intensive Care Med. 2013;39(3):445-453.
- Carson JL, Grossman BJ, Kleinman S, et al; Clinical Transfusion Medicine Committee of the AABB. Red blood cell transfusion: a clinical practice guideline from the AAAB. Ann Intern Med. 2012;157(1):49-58.
- Gilliss BM, Looney MR, Gropper MA. Reducing noninfectious risks of blood transfusion. Anesthesiology. 2011;115(3):635-649.
- van Straaten HL, de Wildt-Eggen J, Huisveld IA. Evaluation of a strategy to limit blood donor exposure in high risk premature newborns based on clinical estimation of transfusion need. J Perniat Med. 2000;28(2):122-128.
- Anstee DJ. Red cell genotyping and the future of pretransfusion testing. Blood. 2009;114(2):248-256.
- Inaba K, Teixeira PG, Shulman I. The impact of uncross-matched blood transfusion on the need for massive transfusion and mortality: analysis of 5,166 uncross-matched units. J Trauma. 2008;65(6):1222-1226.
- Slichter SJ. Platelet transfusion therapy. Hematol Oncol Clin North Am. 2007;21(4):697-729.
- Uppal P, Lodha R, Kabra SK. Transfusion of blood and components in critically ill children. Indian J Pediatr. 2010;77(12):1424-1428.
- Shah A, Stanworth SJ, McKechnie. Evidence and triggers for the transfusion of blood and blood products. Anesthesia. 2015;70(Suppl 1):10-19.
- Emery, M. Blood and Blood Components. In: Marx JA, Hockberger RS, Walls RM ed. Rosen’s emergency medicine: concepts and clinical practice. 7th ed. Philadelphia, PA: Mosby/Elsevier; 2009:42-46.
- Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G; American College of Chest Physicians. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):160S-198S.
- Santen S A Transfusion Therapy. In: Tintinalli, J. E., Kelen, G. D., & Stapczynski, J. S. ed. Emergency medicine: a comprehensive study guide. 6th ed. New York, NY: McGraw-Hill Medical; 20041349-1351.
- Osterman JL, Arora S. Blood product transfusions and reactions. Emerg Med Clin North Am. 2014;32(3): 727-738.
- Azzi A, De Santis R, Morfini M, et al. TT virus contaminates first-generation recombinant factor VIII concentrates. Blood. 2001;98(8):2571-2573.
- Barkun AN, Bardou M, Kuipers EJ, et al; International Consensus Upper Gastrointestinal Bleeding Conference Group International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152(2):101-113.
- Strate L, Saltzman J, and Ookubo R. Validation of a clinical prediction rule for severe acute lower intestinal bleeding. Am J Gastroenterol. 2005;100(8):1821-1827.
- Kollef MH, O’Brien JD, Zuckerman GR, Shannon W. BLEED: a classification tool to predict outcomes in patients with acute upper and lower gastrointestinal hemorrhage. Crit Care Med. 1997;25(7):1125-1132.
- Sauaia A, Moore FA, Moore EE, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38(2):185-193.
- Simmons JW, Pittet JF, Pierce B. Trauma-induced coagulopathy. Curr Anesthisiol Rep. 2014;4(3):189-199.
- Zehtabchi S, Nishijima DK. Impact of transfusion of fresh-frozen plasma and packed red blood cells in a 1:1 ratio on survival of emergency department patients with severe trauma. Acad Emerg Med. 2009;16(5):371-378.
- Theusinger OM, Baulig W, Seifert B, Müller SM, Mariotti S, Spahn DR.. Changes in coagulation in standard laboratory tests and ROTEM in trauma patients between on-scene and arrival in the emergency department. Anesth Analg. 2014. [Epub ahead of print]
- Bouillon B, Brohi K, Hess JR, Holcomb JB, Parr MJ, Hoyt DB. Educational initiative on critical bleeding in trauma: Chicago, July 11-13, 2008. J Trauma. 2010;68(1):225-230.
- Phillips LE, McLintock C, Pollock W, et al; Australian and New Zealand Haemostasis Registry. Recombinant activated Factor VII in obstetric hemorrhage: experiences from the Australian and New Zealand Haemostasis Registry. Anesth Analg. 2009;109(6):1908-1915.
- Hirayama F. Current understanding of allergic transfusion reactions: incidence, pathogenesis, laboratory tests, prevention and treatment. Br J Haematol. 2013;160(4);434-444.
- Lieberman L, Maskens C, Cserti-Gazdewich C, et al. A retrospective review of patient factors, transfusion practices, and outcomes in patients with transfusion-associated circulatory overload. Transfus Med Rev. 2013;27(4)206-212.
- Welling KL, Taaning E, Lund BV, Rosenkvist J, Heslet L. Post-transfusion purpura (PTP) and disseminated intravascular coagulation (DIC). Eur J Haematol. 2003;71(1):68-71
- Kawai M, Takeda M, Tsugawa Y. Hemolytic anemia and acute renal failure caused by blood transfusions. Rinsho Ketusueki. 1990;31(10):1706-1710.
- Przepiorka D, LeParc GF, Stovall MA, Werch J, Lichtiger B. Use of irradiated blood components: practice parameter. Am J Clin Pathol. 1996;106(1):6-11.
- Rühl H, Bein G, Sachs UJ. Transfusion-associated graft-versus-host disease. Transfus Med Rev. 2009;23(1):62-71.
- Guinet F, Carniel E, Leclercq A. Transfusion-transmitted Yersinia enterolitica sepsis. Clin Infect Dis. 2011;53(6):583-591.
- Stramer SL, Notari EP, Krysztof DE, Dodd RY. Hepatitis B virus testing by minipool nuclear acid testing: does it improve blood safety? Transfusion. 2013; 53(10):2449-2458.
- Zou S, Stramer SL, Dodd RY. Donor testing and risk: current prevalence, incidence, and residual risk of transfusion-transmissible agents in US allogenic donations. Transfus Med Rev. 2012;26(2):119-128.
- Dzik WH, Kirkley SA. Citrate toxicity during massive blood transfusion. Transfus Med Rev. 1988;2(2):76-94.
- Lier H, Krep H, Schroeder S, Stuber F. Preconditions of hemostasis in trauma: a review. The influence of acidosis, hypocalcemia, anemia, and hypothermia on functional hemostasis in trauma. J Trauma. 2008;65(4):951-960.
- Bruining HA, Boelhouwer RU, Ong GK. Unexpected hypopotassemia after multiple blood transfusions during an operation. Neth J Surg. 1986;38(2):48-51.
- British Committee for Standards in Haematology; Stainsby D, MacLennan S, Thomas D, Isaac J, Hamilton PJ. Guidelines on the management of massive blood loss. Br J Haematol. 2006;135(5):634-641.
- Smith HM, Farrow SJ, Ackerman JD, Stubbs JR, Sprung J. Cardiac arrests associated with hyperkalemia during red blood cell transfusion: a case series. Anesth Analg. 2008;106(4):1062-1069.
- Napolitano LM, Kurek S, Luchette FA, et al; EAST Practice Management Workgroup; American College of Critical Care Medicine (ACCM) Taskforce of the Society of Critical Care Medicine (SCCM). Clinical Practice Guideline: Red Blood Cell Transfusion and Critical Care. J Trauma. 2009;67(6):1439-1442.
- Leal-Noval SR, Munoz-Gomez M, Jimenez-Sanchez M et al. Red blood cell transfusion in nonbleeding critically ill patients with moderate anemia: is there a benefit? Intensive Care Med. 2013;39(3):445-453.
- Carson JL, Grossman BJ, Kleinman S, et al; Clinical Transfusion Medicine Committee of the AABB. Red blood cell transfusion: a clinical practice guideline from the AAAB. Ann Intern Med. 2012;157(1):49-58.
- Gilliss BM, Looney MR, Gropper MA. Reducing noninfectious risks of blood transfusion. Anesthesiology. 2011;115(3):635-649.
- van Straaten HL, de Wildt-Eggen J, Huisveld IA. Evaluation of a strategy to limit blood donor exposure in high risk premature newborns based on clinical estimation of transfusion need. J Perniat Med. 2000;28(2):122-128.
- Anstee DJ. Red cell genotyping and the future of pretransfusion testing. Blood. 2009;114(2):248-256.
- Inaba K, Teixeira PG, Shulman I. The impact of uncross-matched blood transfusion on the need for massive transfusion and mortality: analysis of 5,166 uncross-matched units. J Trauma. 2008;65(6):1222-1226.
- Slichter SJ. Platelet transfusion therapy. Hematol Oncol Clin North Am. 2007;21(4):697-729.
- Uppal P, Lodha R, Kabra SK. Transfusion of blood and components in critically ill children. Indian J Pediatr. 2010;77(12):1424-1428.
- Shah A, Stanworth SJ, McKechnie. Evidence and triggers for the transfusion of blood and blood products. Anesthesia. 2015;70(Suppl 1):10-19.
- Emery, M. Blood and Blood Components. In: Marx JA, Hockberger RS, Walls RM ed. Rosen’s emergency medicine: concepts and clinical practice. 7th ed. Philadelphia, PA: Mosby/Elsevier; 2009:42-46.
- Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G; American College of Chest Physicians. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):160S-198S.
- Santen S A Transfusion Therapy. In: Tintinalli, J. E., Kelen, G. D., & Stapczynski, J. S. ed. Emergency medicine: a comprehensive study guide. 6th ed. New York, NY: McGraw-Hill Medical; 20041349-1351.
- Osterman JL, Arora S. Blood product transfusions and reactions. Emerg Med Clin North Am. 2014;32(3): 727-738.
- Azzi A, De Santis R, Morfini M, et al. TT virus contaminates first-generation recombinant factor VIII concentrates. Blood. 2001;98(8):2571-2573.
- Barkun AN, Bardou M, Kuipers EJ, et al; International Consensus Upper Gastrointestinal Bleeding Conference Group International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152(2):101-113.
- Strate L, Saltzman J, and Ookubo R. Validation of a clinical prediction rule for severe acute lower intestinal bleeding. Am J Gastroenterol. 2005;100(8):1821-1827.
- Kollef MH, O’Brien JD, Zuckerman GR, Shannon W. BLEED: a classification tool to predict outcomes in patients with acute upper and lower gastrointestinal hemorrhage. Crit Care Med. 1997;25(7):1125-1132.
- Sauaia A, Moore FA, Moore EE, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38(2):185-193.
- Simmons JW, Pittet JF, Pierce B. Trauma-induced coagulopathy. Curr Anesthisiol Rep. 2014;4(3):189-199.
- Zehtabchi S, Nishijima DK. Impact of transfusion of fresh-frozen plasma and packed red blood cells in a 1:1 ratio on survival of emergency department patients with severe trauma. Acad Emerg Med. 2009;16(5):371-378.
- Theusinger OM, Baulig W, Seifert B, Müller SM, Mariotti S, Spahn DR.. Changes in coagulation in standard laboratory tests and ROTEM in trauma patients between on-scene and arrival in the emergency department. Anesth Analg. 2014. [Epub ahead of print]
- Bouillon B, Brohi K, Hess JR, Holcomb JB, Parr MJ, Hoyt DB. Educational initiative on critical bleeding in trauma: Chicago, July 11-13, 2008. J Trauma. 2010;68(1):225-230.
- Phillips LE, McLintock C, Pollock W, et al; Australian and New Zealand Haemostasis Registry. Recombinant activated Factor VII in obstetric hemorrhage: experiences from the Australian and New Zealand Haemostasis Registry. Anesth Analg. 2009;109(6):1908-1915.
- Hirayama F. Current understanding of allergic transfusion reactions: incidence, pathogenesis, laboratory tests, prevention and treatment. Br J Haematol. 2013;160(4);434-444.
- Lieberman L, Maskens C, Cserti-Gazdewich C, et al. A retrospective review of patient factors, transfusion practices, and outcomes in patients with transfusion-associated circulatory overload. Transfus Med Rev. 2013;27(4)206-212.
- Welling KL, Taaning E, Lund BV, Rosenkvist J, Heslet L. Post-transfusion purpura (PTP) and disseminated intravascular coagulation (DIC). Eur J Haematol. 2003;71(1):68-71
- Kawai M, Takeda M, Tsugawa Y. Hemolytic anemia and acute renal failure caused by blood transfusions. Rinsho Ketusueki. 1990;31(10):1706-1710.
- Przepiorka D, LeParc GF, Stovall MA, Werch J, Lichtiger B. Use of irradiated blood components: practice parameter. Am J Clin Pathol. 1996;106(1):6-11.
- Rühl H, Bein G, Sachs UJ. Transfusion-associated graft-versus-host disease. Transfus Med Rev. 2009;23(1):62-71.
- Guinet F, Carniel E, Leclercq A. Transfusion-transmitted Yersinia enterolitica sepsis. Clin Infect Dis. 2011;53(6):583-591.
- Stramer SL, Notari EP, Krysztof DE, Dodd RY. Hepatitis B virus testing by minipool nuclear acid testing: does it improve blood safety? Transfusion. 2013; 53(10):2449-2458.
- Zou S, Stramer SL, Dodd RY. Donor testing and risk: current prevalence, incidence, and residual risk of transfusion-transmissible agents in US allogenic donations. Transfus Med Rev. 2012;26(2):119-128.
- Dzik WH, Kirkley SA. Citrate toxicity during massive blood transfusion. Transfus Med Rev. 1988;2(2):76-94.
- Lier H, Krep H, Schroeder S, Stuber F. Preconditions of hemostasis in trauma: a review. The influence of acidosis, hypocalcemia, anemia, and hypothermia on functional hemostasis in trauma. J Trauma. 2008;65(4):951-960.
- Bruining HA, Boelhouwer RU, Ong GK. Unexpected hypopotassemia after multiple blood transfusions during an operation. Neth J Surg. 1986;38(2):48-51.
- British Committee for Standards in Haematology; Stainsby D, MacLennan S, Thomas D, Isaac J, Hamilton PJ. Guidelines on the management of massive blood loss. Br J Haematol. 2006;135(5):634-641.
- Smith HM, Farrow SJ, Ackerman JD, Stubbs JR, Sprung J. Cardiac arrests associated with hyperkalemia during red blood cell transfusion: a case series. Anesth Analg. 2008;106(4):1062-1069.
Emergency Ultrasound: Ultrasound-Guided Posterior Tibial Nerve Block
Posterior Tibial Nerve Block
Identifying the Posterior Tibial Nerve
Performing the Block
It is important to remember that the medial and lateral aspects of the sole of the foot receive variable degrees of innervation from the saphenous and sural nerves respectively; therefore, the EP should always ensure the area of interest is completely anesthetized before beginning the procedure.
Conclusion
Penetrating injuries to the sole of the foot are a common presentation in the ED. Achieving adequate anesthesia to the area with local anesthetic alone can be a difficult and painful process. However, with practice, ultrasound guidance can improve procedural success and decrease the risk of nerve injury as compared to blind nerve blocks.
Dr Beck is an assistant professor, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Taylor is an assistant professor and director of postgraduate medical education, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Meer is an assistant professor and director of emergency ultrasound, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia.
Reference
- Redborg KE, Antonakakis JG, Beach ML, Chinn CD, Sites BD. Ultrasound improves the success rate of a tibial nerve block at the ankle regional anesthesia and pain medicine. Reg Anesth Pain Med. 2009;34(3):256-260.
Posterior Tibial Nerve Block
Identifying the Posterior Tibial Nerve
Performing the Block
It is important to remember that the medial and lateral aspects of the sole of the foot receive variable degrees of innervation from the saphenous and sural nerves respectively; therefore, the EP should always ensure the area of interest is completely anesthetized before beginning the procedure.
Conclusion
Penetrating injuries to the sole of the foot are a common presentation in the ED. Achieving adequate anesthesia to the area with local anesthetic alone can be a difficult and painful process. However, with practice, ultrasound guidance can improve procedural success and decrease the risk of nerve injury as compared to blind nerve blocks.
Dr Beck is an assistant professor, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Taylor is an assistant professor and director of postgraduate medical education, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Meer is an assistant professor and director of emergency ultrasound, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia.
Posterior Tibial Nerve Block
Identifying the Posterior Tibial Nerve
Performing the Block
It is important to remember that the medial and lateral aspects of the sole of the foot receive variable degrees of innervation from the saphenous and sural nerves respectively; therefore, the EP should always ensure the area of interest is completely anesthetized before beginning the procedure.
Conclusion
Penetrating injuries to the sole of the foot are a common presentation in the ED. Achieving adequate anesthesia to the area with local anesthetic alone can be a difficult and painful process. However, with practice, ultrasound guidance can improve procedural success and decrease the risk of nerve injury as compared to blind nerve blocks.
Dr Beck is an assistant professor, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Taylor is an assistant professor and director of postgraduate medical education, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Meer is an assistant professor and director of emergency ultrasound, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia.
Reference
- Redborg KE, Antonakakis JG, Beach ML, Chinn CD, Sites BD. Ultrasound improves the success rate of a tibial nerve block at the ankle regional anesthesia and pain medicine. Reg Anesth Pain Med. 2009;34(3):256-260.
Reference
- Redborg KE, Antonakakis JG, Beach ML, Chinn CD, Sites BD. Ultrasound improves the success rate of a tibial nerve block at the ankle regional anesthesia and pain medicine. Reg Anesth Pain Med. 2009;34(3):256-260.
Cystic lung disease: Systematic, stepwise diagnosis
Air-filled pulmonary lesions commonly detected on chest computed tomography. Cystic lung lesions should be distinguished from other air-filled lesions to facilitate diagnosis. Primary care physicians play an integral role in the recognition of cystic lung disease.
The differential diagnosis of cystic lung disease is broad and includes isolated pulmonary, systemic, infectious, and congenital etiologies.
Here, we aim to provide a systematic, stepwise approach to help differentiate among the various cystic lung diseases and devise an algorithm for diagnosis. In doing so, we will discuss the clinical and radiographic features of many of these diseases:
- Lymphangioleiomyomatosis
- Birt-Hogg-Dubé syndrome
- Pulmonary Langerhans cell histiocytosis
- Interstitial pneumonia (desquamative interstitial pneumonia, lymphocytic interstitial pneumonia)
- Congenital cystic lung disease (congenital pulmonary airway malformation, pulmonary sequestration, bronchogenic cyst) Pulmonary infection
- Systemic disease (amyloidosis, light chain deposition disease, neurofibromatosis type 1).
STEP 1: RULE OUT CYST-MIMICS
A pulmonary cyst is a round, circumscribed space surrounded by an epithelial or fibrous wall of variable thickness.1 On chest radiography and computed tomography, a cyst appears as a round parenchymal lucency or low-attenuating area with a well-defined interface with normal lung.1 Cysts vary in wall thickness but usually have a thin wall (< 2 mm) and occur without associated pulmonary emphysema.1 They typically contain air but occasionally contain fluid or solid material.
A pulmonary cyst can be categorized as a bulla, bleb, or pneumatocele.
Bullae are larger than 1 cm in diameter, sharply demarcated by a thin wall, and usually accompanied by emphysematous changes in the adjacent lung.1
Blebs are no larger than 1 cm in diameter, are located within the visceral pleura or the subpleural space, and appear on computed tomography as thin-walled air spaces that are contiguous with the pleura.1 The distinction between a bleb and a bulla is of little clinical importance, and is often unnecessary.
Pneumatoceles are cysts that are frequently caused by acute pneumonia, trauma, or aspiration of hydrocarbon fluid, and are usually transient.1
Mimics of pulmonary cysts include pulmonary cavities, emphysema, loculated pneumothoraces, honeycomb lung, and bronchiectasis (Figure 1).2
Pulmonary cavities differ from cysts in that their walls are typically thicker (usually > 4 mm).3
Emphysema differs from cystic lung disease as it typically leads to focal areas or regions of decreased lung attenuation that do not have defined walls.1
Honeycombing refers to a cluster or row of cysts, 1 to 3 mm in wall thickness and typically 3 to 10 mm in diameter, that are associated with end-stage lung fibrosis.1 They are typically subpleural in distribution and are accompanied by fibrotic features such as reticulation and traction bronchiectasis.1
Bronchiectasis is dilation and distortion of bronchi and bronchioles and can be mistaken for cysts when viewed en face.1
Loculated pneumothoraces can also mimic pulmonary cysts, but they typically fail to adhere to a defined anatomic unit and are subpleural in distribution.
STEP 2: CHARACTERIZE THE CLINICAL PRESENTATION
Clinical signs and symptoms of cystic lung disease play a key role in diagnosis (Table 1). For instance, spontaneous pneumothorax is commonly associated with diffuse cystic lung disease (lymphangioleiomyomatosis and Birt-Hogg-Dubé syndrome), while insidious dyspnea, with or without associated pneumothorax, is usually associated with the interstitial pneumonias (lymphocytic interstitial pneumonia and desquamative interstitial pneumonia).
In addition, congenital abnormalities of the lung can lead to cyst formation. These abnormalities, especially when associated with other congenital abnormalities, are often diagnosed in the prenatal and perinatal periods. However, some remain undetected until incidentally found later in adulthood or if superimposing infection develops.
Primary pulmonary infections can also cause parenchymal necrosis, which in turn cavitates or forms cysts.4
Lastly, cystic lung diseases can occur as part of a multiorgan or systemic illness in which the lung is one of the organs involved. Although usually diagnosed before the discovery of cysts or manifestations of pulmonary symptoms, they can present as a diagnostic challenge, especially when lung cysts are the initial presentation.bsence of amyloid fibrils.
In view of the features of the different types of cystic lung disease, adults with cystic lung disease can be grouped according to their typical clinical presentations (Table 2):
- Insidious dyspnea or spontaneous pneumothorax
- Incidentally found cysts or recurrent pneumonia
- Signs and symptoms of primary pulmonary infection
- Signs and symptoms that are primarily nonpulmonary.
Insidious dyspnea or spontaneous pneumothorax
Insidious dyspnea or spontaneous pneumothorax can be manifestations of lymphangioleiomyomatosis, Birt-Hogg-Dubé syndrome, pulmonary Langerhans cell histiocytosis, desquamative interstitial pneumonia, or lymphocytic interstitial pneumonia.
Lymphangioleiomyomatosis is characterized by abnormal cellular proliferation within the lung, kidney, lymphatic system, or any combination.5 The peak prevalence is in the third to fourth decades of life, and most patients are women of childbearing age.6 In addition to progressive dyspnea on exertion and pneumothorax, other signs and symptoms include hemoptysis, nonproductive cough, chylous pleural effusion, and ascites.7,8
Birt-Hogg-Dubé syndrome is caused by germline mutations in the folliculin (FLCN) gene.9 It is characterized by skin fibrofolliculomas, pulmonary cysts, spontaneous pneumothorax, and renal cancer.10
Pulmonary Langerhans cell histiocytosis is part of the spectrum of Langerhans cell histiocytosis that, in addition to the lungs, can also involve the bone, pituitary gland, thyroid, skin, lymph nodes, and liver.11 It occurs almost exclusively in smokers, affecting individuals in their 20s and 30s, with no gender predilection.12,13 In addition to nonproductive cough and dyspnea, patients can also present with fever, anorexia, and weight loss,13 but approximately 25% of patients are asymptomatic.14
Desquamative interstitial pneumonia is an idiopathic interstitial pneumonia that, like pulmonary Langerhans cell histiocytosis, is seen almost exclusively in current or former smokers, who account for about 90% of patients with this disease. It affects almost twice as many men as women.15,16 The mean age at onset is 42 to 46.15,16 In addition to insidious cough and dyspnea, digital clubbing develops in 26% to 40% of patients.16,17
Lymphocytic interstitial pneumonia is another rare idiopathic pneumonia, usually associated with connective tissue disease, Sjögren syndrome, immunodeficiencies, and viral infections.18–21 It is more common in women, presenting between the 4th and 7th decades of life, with a mean age at diagnosis of 50 to 56.18,22 In addition to progressive dyspnea and cough, other symptoms include weight loss, pleuritic pain, arthralgias, fatigue, night sweats, and fever.23
In summary, in this clinical group, lymphangioleiomyomatosis and Birt-Hogg-Dubé syndrome should be considered when patients present with spontaneous pneumothorax; those with Birt-Hogg-Dubé syndrome also present with skin lesions or renal cancer. In patients with progressive dyspnea and cough, lymphocytic interstitial pneumonia should be considered in those with a known history of connective tissue disease or immunodeficiency. Pulmonary Langerhans cell histiocytosis typically presents at a younger age (20 to 30 years old) than desquamative interstitial pneumonia (smokers in their 40s). Making the distinction, however, will likely require imaging with computed tomography.
Incidentally found cysts or recurrent pneumonia
Incidentally found cysts or recurrent pneumonia can be manifestations of congenital pulmonary airway malformation, pulmonary sequestration, or bronchogenic cyst.
Congenital pulmonary airway malformation, of which there are five types, is the most common pulmonary congenital abnormality. It accounts for up to 95% of cases of congenital cystic lung disease.24,25 About 85% of cases are detected in the prenatal or perinatal periods.26 Late-onset congenital pulmonary airway malformation (arising in childhood to adulthood) presents with recurrent pneumonia in about 75% of cases and can be misdiagnosed as lung abscess, pulmonary tuberculosis, or bronchiectasis.27
Pulmonary sequestration, the second most common pulmonary congenital abnormality, is characterized by a portion of lung that does not connect to the tracheobronchial tree and has its own systemic arterial supply.24 Intralobar sequestration, which shares the pleural investment with normal lung, accounts for about 80% of cases of pulmonary sequestration.28–30 In addition to signs or symptoms of pulmonary infection, patients with pulmonary sequestration can remain asymp-
tomatic (about 25% of cases), or can present with hemoptysis or hemothorax.28–30 In adults, the typical age at presentation is between 20 and 25.29,30
Bronchogenic cyst is usually life-threatening in children. In adults, it commonly causes cough and chest pain.31 Hemoptysis, dysphagia, hoarseness, and diaphragmatic paralysis can also occur.32,33 The mean age at diagnosis in adults is 35 to 40.31,32
In summary, most cases of recurrent pneumonia with cysts are due to congenital pulmonary airway malformation. Pulmonary sequestration is the second most common cause of cystic lung disease in this group. Bronchogenic cyst is usually fatal in fetal development; smaller cysts can go unnoticed during the earlier years and are later found incidentally as imaging abnormalities in adults.
Signs and symptoms of primary pulmonary infections
Signs and symptoms of primary pulmonary infections can be due to Pneumocystis jirovecii pneumonia or echinococcal infections.
P jirovecii pneumonia commonly develops in patients with human immunodeficiency virus infection and low CD4 counts, recipients of hematologic or solid-organ transplants, and those receiving immunosuppressive therapy (eg, glucocorticoids or chemotherapy).
Echinococcal infections (with Echinococcus granulosus or multilocularis species) are more common in less-developed countries such as those in South America or the Middle East, in China, or in patients who have traveled to endemic areas.34
In summary, cystic lung disease in patients with primary pulmonary infections can be diagnosed by the patient’s clinical history and risk factors for infections. Those with human immunodeficiency virus infection and other causes of immunodeficiency are predisposed to P jirovecii pneumonia. Echinococcal infections occur in those with a history of travel to an endemic area.
Primarily nonpulmonary signs and symptoms
If the patient has primarily nonpulmonary signs and symptoms, think about pulmonary amyloidosis, light chain deposition disease, and neurofibromatosis type 1.
Pulmonary amyloidosis has a variety of manifestations, including tracheobronchial disease, nodular parenchymal disease, diffuse or alveolar septal pattern, pleural disease, lymphadenopathy, and pulmonary cysts.4
Light chain deposition disease shares some clinical features with amyloidosis. However, the light chain fragments in this disease do not form amyloid fibrils and therefore do not stain positively with Congo red. The kidney is the most commonly involved organ.4
Neurofibromatosis type 1 is characterized by collections of neurofibromas, café-au-lait spots, and pigmented hamartomas in the iris (Lisch nodules).35
In summary, patients in this group typically present with complications related to systemic involvement. Those with neurofibromatosis type 1 present with ophthalmologic, dermatologic, and neurologic manifestations. Amyloidosis and light chain deposition disease most commonly involve the renal system; their distinction will likely require tissue biopsy and Congo-red staining.
STEP 3: CHARACTERIZE THE RADIOGRAPHIC FEATURES
Characterization of pulmonary cysts and their distribution plays a key role in the diagnosis. Radiographically, cystic lung diseases can be subclassified into two major categories according to their cystic distribution:
- Discrete (focal or multifocal)
- Diffuse (unilobular or panlobular).2,3
Discrete cystic lung diseases include congenital abnormalities, infectious diseases, and interstitial pneumonias.2,3
Diffuse, panlobular cystic lung diseases include lymphangioleiomyomatosis, pulmonary Langerhans cell histiocytosis, Birt-Hogg-Dubé syndrome, amyloidosis, light chain deposition disease, and neurofibromatosis type 1.7,13,36–39
In addition, other associated radiographic findings play a major role in diagnosis.
Cysts in patients presenting with insidious dyspnea or spontaneous pneumothorax
Lymphangioleiomyomatosis. Cysts are seen in nearly all cases of advanced lymphangioleiomyomatosis, typically in a diffuse pattern, varying from 2 mm to 40 mm in diameter, and uniform in shape (Figure 2A).7,8,40–42
Other radiographic features include vessels located at the periphery of the cysts (in contrast to the centrilobular pattern seen with emphysema), and chylous pleural effusions (in about 22% of patients).40 Nodules are typically not seen with lymphangioleiomyomatosis, and if found represent type 2 pneumocyte hyperplasia.
Pulmonary Langerhans cell histiocytosis. Nodules measuring 1 to 10 mm in diameter and favoring a centrilobular location are often seen on computed tomography. Pulmonary cysts occur in about 61% of patients.13,43 Cysts are variable in size and shape (Figure 2B), in contrast to their uniform appearance in lymphangioleiomyomatosis. Most cysts are less than 10 mm in diameter; however, they can be up to 80 mm.13,43 Early in its course, nodules may predominate in the upper and middle lobes. Over time, diffuse cysts become more common and can be difficult to differentiate from advanced smoking-induced emphysema.44
Birt-Hogg-Dubé syndrome. Approximately 70% to 100% of patients with Birt-Hogg-Dubé syndrome will have multiple pulmonary cysts detected on computed tomography. These cysts are characteristically basal and subpleural in location, with varying sizes and irregular shapes in otherwise normal lung parenchyma (Figure 2C).36,45,46
Desquamative interstitial pneumonia. Pulmonary cysts are present on computed tomography in about 32% of patients.47 They are usually round and less than 20 mm in diameter.48 Ground-glass opacity is present in almost all cases of desquamative interstitial pneumonia, with a diffuse pattern in 25% to 44% of patients.16,17,47
Pulmonary cysts occur in up to two-thirds of those with lymphocytic interstitial pneumonia. Cysts are usually multifocal and perivascular in distribution and have varying sizes and shapes (Figure 2D).22 Ground-glass opacity and poorly defined centrilobular nodules are also frequently seen. Other computed tomographic findings include thickening of the bronchovascular bundles, focal consolidation, interseptal lobular thickening, pleural thickening, and lymph node enlargement.22
In summary, in this group of patients, diffuse panlobular cysts are due to lymphangioleiomyomatosis, pulmonary Langerhans cell histiocytosis, or Birt-Hogg-Dubé syndrome. Cysts due to lymphangioleiomyomatosis have a diffuse distribution, while those due to pulmonary Langerhans cell histiocytosis tend to be upper-lobe-predominant and in the early stages are associated with stellate centrilobular nodules. Cysts in Birt-Hogg-Dubé syndrome tend to be subpleural and those due to lymphocytic interstitial pneumonia are perivascular in distribution.
Cysts that are incidentally found or occur in patients with recurrent pneumonia
Congenital pulmonary airway malformation types 1, 2, and 4 (Figure 3A, 3B). Cysts are typically discrete and focal or multifocal in distribution, but cases of multilobar and bilateral distribution have also been reported.27,49 The lower lobes are more often involved.49 Cysts vary in size and shape and can contain air, fluid, or both.27,49 Up to 50% of cases can occur in conjunction with pulmonary sequestration.50
Pulmonary sequestration displays an anomalous arterial supply on computed tomography (Figure 3C). Other imaging findings include mass lesions (49%), cystic lesions (29%), cavitary lesions (12%), and bronchiectasis.30 Air trapping can be seen in the adjacent lung. Lower lobe involvement accounts for more than 95% of total cases of sequestration.30 The cysts are usually discrete or focal in distribution. Misdiagnosis of pulmonary sequestration is common, and can include pulmonary abscess, pneumonia, bronchiectasis, and lung cancer.30
Bronchogenic cyst. Cyst contents generally demonstrate water attenuation, or higher attenuation if filled with proteinaceous/mucoid material or calcium deposits; air-fluid levels are seen in infected cysts.32 Intrapulmonary cysts have a predilection for the lower lobes and are usually discrete or focal in distribution.31,32 Mediastinal cysts are usually homogeneous, solitary, and located in the middle mediastinum.32 Cysts vary in size from 20 to 90 mm, with a mean diameter of 40 mm.31
In summary, in this group of cystic lung diseases, characteristic computed tomographic findings will suggest the diagnosis—air-filled cysts of varying sizes for congenital pulmonary airway malformation and anomalous vascular supply for pulmonary sequestration. Bronchogenic cysts will tend to have water or higher-than-water attenuation due to proteinaceous-mucoid material or calcium deposits.
Cysts in patients with signs and symptoms of primary pulmonary infections
P jirovecii pneumonia. Between 10% and 15% of patients have cysts, and about 18% present with spontaneous pneumothorax.51 Cysts in P jirovecii pneumonia vary in size from 15 to 85 mm in diameter and tend to occur in the upper lobes (Figure 4A).51,52
Echinococcal infection. Echinococcal pulmonary cysts typically are single and located more often in the lower lobes (Figure 4B).53,54 Cysts can be complicated by air-fluid levels, hydropneumothorax, or pneumothorax, or they can turn into cavitary lesions.
The diagnoses of these pulmonary infections are usually made by clinical and computed tomographic findings and depend less on detecting and characterizing lung cysts. Patients with P jirovecii pneumonia tend to have bilateral perihilar ground-glass opacities, while air-fluid levels suggest echinococcal infections. Cysts in this group of patients tend to be discrete or focal or multifocal in distribution, and vary in size.
Cysts in patients with primarily nonpulmonary signs and symptoms
Amyloidosis. Cyst formation is rare in amyloidosis.4 When present, cysts can be diffuse and scattered in distribution, in varying sizes (usually < 30 mm in diameter) and irregular shapes (Figure 5).55,56
Pulmonary light chain deposition disease usually presents as linear opacities and small nodules on chest computed tomography. Numerous cysts that are diffuse in distribution and have no topographic predominance can also be present. They can progress in number and size and coalesce to form irregular shapes.57
Neurofibromatosis type 1. In neurofibromatosis type 1, the most common radiographic presentations are bibasilar reticular opacities (50%), bullae (50%), and ground glass opacities (37%).58 Well-formed cysts occur in up to 25% of patients and tend to be diffuse and smaller (2 to 18 mm in diameter), with upper lobe predominance.58,59
In summary, in this group of patients, bibasilar reticular and ground-glass opacities suggest neurofibromatosis type 1, while nodules and linear opacities suggest amyloidosis or light chain deposition disease. Cysts tend to be diffuse with varying sizes.
STEP 4: PUT IT ALL TOGETHER
Diagnosis in insidious dyspnea or spontaneous pneumothorax
For patients who present with insidious dyspnea or spontaneous pneumothorax, the diagnosis of cystic lung disease can be made by characterizing the distribution, size, and shape of the cysts (Table 3).
Diffuse, panlobular distribution. Cystic lung diseases with this pattern include lymphangioleiomyomatosis, pulmonary Langerhans cell histiocytosis, and Birt-Hogg-Dubé syndrome. In this group, cysts that are uniform in size and regular in shape are invariably due to lymphangioleiomyomatosis. Those with variable size and irregular shapes can be due to pulmonary Langerhans cell histiocytosis or Birt-Hogg-Dubé syndrome. Patients with pulmonary Langerhans cell histiocytosis tend to be smokers and their cysts tend to be upper- lobe-predominant. Those with Birt-Hogg-Dubé syndrome will likely have renal cancer or skin lesions; their cysts tend to be basilar and subpleural in distribution.
Cysts that are focal or multifocal and unilobular are due to lymphocytic interstitial pneumonia or desquamative interstitial pneumonia. Patients with lymphocytic interstitial pneumonia tend to have underlying connective tissue disease; those with desquamative interstitial pneumonia are almost always smokers. The definitive diagnosis for lymphocytic interstitial pneumonia or desquamative interstitial pneumonia can require a tissue biopsy.
Diagnosis in patients with incidentally found cysts or recurrent pneumonia
In those who present with incidentally found cysts or recurrent pneumonia, suspicion for a congenital lung malformation should be raised. Patients with a type 1, 2, or 4 congenital pulmonary airway malformation typically have air-filled cysts in varying sizes; those with pulmonary sequestration have an anomalous arterial supply in addition to cysts that are usually located in the lower lobes. Bronchogenic cysts tend to be larger, with attenuation equal to or greater than that of water, and distinguishing them from congenital pulmonary airway malformation will likely require surgical examination.
Diagnosis in patients with signs and symptoms of pulmonary infections
Patients with signs and symptoms of pulmonary infections should be investigated according to clinical risk factors for P jirovecii pneumonia or echinococcal infections.
Diagnosis in patients with primarily nonpulmonary presentations
The distinction between amyloidosis and neurofibromatosis type 1 can be made by the history and the clinical examination. However, a definitive diagnosis of amyloidosis or light chain deposition disease requires tissue examination for the presence or absence of amyloid fibrils.
- Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008; 246:697–722.
- Cosgrove GP, Frankel SK, Brown KK. Challenges in pulmonary fibrosis. 3: cystic lung disease. Thorax 2007; 62:820–829.
- Ryu JH, Swensen SJ. Cystic and cavitary lung diseases: focal and diffuse. Mayo Clin Proc 2003; 78:744–752.
- Ryu JH, Tian X, Baqir M, Xu K. Diffuse cystic lung diseases. Front Med 2013; 7:316–327.
- McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest 2008; 133:507–516.
- Johnson SR, Cordier JF, Lazor R, et al; Review Panel of the ERS LAM Task Force. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J 2010; 35:14–26.
- Taylor JR, Ryu J, Colby TV, Raffin TA. Lymphangioleiomyomatosis. Clinical course in 32 patients. N Engl J Med 1990; 323:1254–1260.
- Chu SC, Horiba K, Usuki J. Comprehensive evaluation of 35 patients with lymphangioleiomyomatosis. Chest 1999; 115:1041–1052.
- Graham RB, Nolasco M, Peterlin B, Garcia CK. Nonsense mutations in folliculin presenting as isolated familial spontaneous pneumothorax in adults. Am J Respir Crit Care Med 2005; 172:39–44.
- Birt AR, Hogg GR, Dubé WJ. Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch Dermatol 1977; 113:1674–1677.
- Sundar KM, Gosselin MV, Chung HL, Cahill BC. Pulmonary Langerhans cell histiocytosis: emerging concepts in pathobiology, radiology, and clinical evolution of disease. Chest 2003; 123:1673–1683.
- Vassallo R, Ryu JH, Colby TV, Hartman T, Limper AH. Pulmonary Langerhans’-cell histiocytosis. N Engl J Med 2000; 342:1969–1978.
- Vassallo R, Ryu JH, Schroeder DR, Decker PA, Limper AH. Clinical outcomes of pulmonary Langerhans’-cell histiocytosis in adults. N Engl J Med 2002; 346:484–490.
- Mendez JL, Nadrous HF, Vassallo R, Decker PA, Ryu JH. Pneumothorax in pulmonary Langerhans cell histiocytosis. Chest 2004; 125:1028–1032.
- Carrington CB, Gaensler EA, Coutu RE, FitzGerald MX, Gupta RG. Natural history and treated course of usual and desquamative interstitial pneumonia. N Engl J Med 1978; 298:801–809.
- Ryu JH, Myers JL, Capizzi SA, Douglas WW, Vassallo R, Decker PA. Desquamative interstitial pneumonia and respiratory bronchiolitis-associated interstitial lung disease. Chest 2005; 127:178–184.
- Lynch DA, Travis WD, Müller NL, et al. Idiopathic interstitial pneumonias: CT features. Radiology 2005; 236:10–21.
- Strimlan CV, Rosenow EC 3rd, Weiland LH, Brown LR. Lymphocytic interstitial pneumonitis. Review of 13 cases. Ann Intern Med 1978; 88:616–621.
- Arish N, Eldor R, Fellig Y, et al. Lymphocytic interstitial pneumonia associated with common variable immunodeficiency resolved with intravenous immunoglobulins. Thorax 2006; 61:1096–1097.
- Schooley RT, Carey RW, Miller G, et al. Chronic Epstein-Barr virus infection associated with fever and interstitial pneumonitis. Clinical and serologic features and response to antiviral chemotherapy. Ann Intern Med 1986; 104:636–643.
- Kramer MR, Saldana MJ, Ramos M, Pitchenik AE. High titers of Epstein-Barr virus antibodies in adult patients with lymphocytic interstitial pneumonitis associated with AIDS. Respir Med 1992; 86:49–52.
- Johkoh T, Müller NL, Pickford HA, et al. Lymphocytic interstitial pneumonia: thin-section CT findings in 22 patients. Radiology 1999; 212:567–572.
- Swigris JJ, Berry GJ, Raffin TA, Kuschner WG. Lymphoid interstitial pneumonia: a narrative review. Chest 2002; 122:2150–2164.
- Biyyam DR, Chapman T, Ferguson MR, Deutsch G, Dighe MK. Congenital lung abnormalities: embryologic features, prenatal diagnosis, and postnatal radiologic-pathologic correlation. Radiographics 2010; 30:1721–1738.
- Cloutier MM, Schaeffer DA, Hight D. Congenital cystic adenomatoid malformation. Chest 1993; 103:761–764.
- Luján M, Bosque M, Mirapeix RM, Marco MT, Asensio O, Domingo C. Late-onset congenital cystic adenomatoid malformation of the lung. Embryology, clinical symptomatology, diagnostic procedures, therapeutic approach and clinical follow-up. Respiration 2002; 69:148–154.
- Oh BJ, Lee JS, Kim JS, Lim CM, Koh Y. Congenital cystic adenomatoid malformation of the lung in adults: clinical and CT evaluation of seven patients. Respirology 2006; 11:496–501.
- Tsolakis CC, Kollias VD, Panayotopoulos PP. Pulmonary sequestration. Experience with eight consecutive cases. Scand Cardiovasc J 1997; 31:229–232.
- Sauvanet A, Regnard JF, Calanducci F, Rojas-Miranda A, Dartevelle P, Levasseur P. Pulmonary sequestration. Surgical aspects based on 61 cases. Rev Pneumol Clin 1991; 47:126–132. Article in French.
- Wei Y, Li F. Pulmonary sequestration: a retrospective analysis of 2,625 cases in China. Eur J Cardiothorac Surg 2011; 40:e39–e42.
- Patel SR, Meeker DP, Biscotti CV, Kirby TJ, Rice TW. Presentation and management of bronchogenic cysts in the adult. Chest 1994; 106:79–85.
- Limaïem F, Ayadi-Kaddour A, Djilani H, Kilani T, El Mezni F. Pulmonary and mediastinal bronchogenic cysts: a clinicopathologic study of 33 cases. Lung 2008; 186:55–61.
- Liu HS, Li SQ, Cao ZL, Zhang ZY, Ren H. Clinical features and treatment of bronchogenic cyst in adults. Chin Med Sci J 2009; 24:60–63.
- Jenkins DJ, Romig T, Thompson RC. Emergence/re-emergence of Echinococcus spp.—a global update. Int J Parasitol 2005; 35:1205–1219.
- Riccardi VM. Von Recklinghausen neurofibromatosis. N Engl J Med 1981; 305:1617–1627.
- Toro JR, Pautler SE, Stewart L, et al. Lung cysts, spontaneous pneumothorax, and genetic associations in 89 families with Birt-Hogg-Dubé syndrome. Am J Respir Crit Care Med 2007; 175:1044–1053.
- Biko DM, Schwartz M, Anupindi SA, Altes TA. Subpleural lung cysts in Down syndrome: prevalence and association with coexisting diagnoses. Pediatr Radiol 2008; 38:280–284.
- Colombat M, Stern M, Groussard O, et al. Pulmonary cystic disorder related to light chain deposition disease. Am J Respir Crit Care Med 2006; 173:777–780.
- Ohdama S, Akagawa S, Matsubara O, Yoshizawa Y. Primary diffuse alveolar septal amyloidosis with multiple cysts and calcification. Eur Respir J 1996; 9:1569–1571.
- Johnson SR, Tattersfield AE. Clinical experience of lymphangioleiomyomatosis in the UK. Thorax 2000; 55:1052–1057.
- Kitaichi M, Nishimura K, Itoh H, Izumi T. Pulmonary lymphangioleiomyomatosis: a report of 46 patients including a clinicopathologic study of prognostic factors. Am J Respir Crit Care Med 1995; 151:527–533.
- Urban T, Lazor R, Lacronique J, et al. Pulmonary lymphangioleiomyomatosis. A study of 69 patients. Groupe d’Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM”O”P). Medicine (Baltimore) 1999; 78:321–337.
- Schönfeld N, Frank W, Wenig S, et al. Clinical and radiologic features, lung function and therapeutic results in pulmonary histiocytosis X. Respiration 1993; 60:38–44.
- Lacronique J, Roth C, Battesti JP, Basset F, Chretien J. Chest radiological features of pulmonary histiocytosis X: a report based on 50 adult cases. Thorax 1982; 37:104–109.
- Kluger N, Giraud S, Coupier I, et al. Birt-Hogg-Dubé syndrome: clinical and genetic studies of 10 French families. Br J Dermatol 2010; 162:527–537.
- Tobino K, Gunji Y, Kurihara M, et al. Characteristics of pulmonary cysts in Birt-Hogg-Dubé syndrome: thin-section CT findings of the chest in 12 patients. Eur J Radiol 2011; 77:403–409.
- Hartman TE, Primack SL, Swensen SJ, Hansell D, McGuinness G, Müller NL. Desquamative interstitial pneumonia: thin-section CT findings in 22 patients. Radiology 1993; 187:787–790.
- Koyama M, Johkoh T, Honda O, et al. Chronic cystic lung disease: diagnostic accuracy of high-resolution CT in 92 patients. AJR Am J Roentgenol 2003; 180:827–835.
- Patz EF Jr, Müller NL, Swensen SJ, Dodd LG. Congenital cystic adenomatoid malformation in adults: CT findings. J Comput Assist Tomogr 1995; 19:361–364.
- Conran RM, Stocker JT. Extralobar sequestration with frequently associated congenital cystic adenomatoid malformation, type 2: report of 50 cases. Pediatr Dev Pathol 1999; 2:454–463.
- Kennedy CA, Goetz MB. Atypical roentgenographic manifestations of Pneumocystis carinii pneumonia. Arch Intern Med 1992; 152:1390–1398.
- Sandhu JS, Goodman PC. Pulmonary cysts associated with Pneumocystis carinii pneumonia in patients with AIDS. Radiology 1989; 173:33–35.
- Doğan R, Yüksel M, Cetin G, et al. Surgical treatment of hydatid cysts of the lung: report on 1,055 patients. Thorax 1989; 44:192–199.
- Salih OK, Topcuoğlu MS, Celik SK, Ulus T, Tokcan A. Surgical treatment of hydatid cysts of the lung: analysis of 405 patients. Can J Surg 1998; 41:131–135.
- Ohdama S, Akagawa S, Matsubara O, Yoshizawa Y. Primary diffuse alveolar septal amyloidosis with multiple cysts and calcification. Eur Respir J 1996; 9:1569–1571.
- Sakai M, Yamaoka M, Kawaguchi M, Hizawa N, Sato Y. Multiple cystic pulmonary amyloidosis. Ann Thorac Surg 2011; 92:e109.
- Colombat M, Caudroy S, Lagonotte E, et al. Pathomechanisms of cyst formation in pulmonary light chain deposition disease. Eur Respir J 2008; 32:1399–1403.
- Zamora AC, Collard HR, Wolters PJ, Webb WR, King TE. Neurofibromatosis-associated lung disease: a case series and literature review. Eur Respir J 2007; 29:210–214.
- Oikonomou A, Vadikolias K, Birbilis T, Bouros D, Prassopoulos P. HRCT findings in the lungs of non-smokers with neurofibromatosis. Eur J Radiol 2011; 80:e520–e523.
Air-filled pulmonary lesions commonly detected on chest computed tomography. Cystic lung lesions should be distinguished from other air-filled lesions to facilitate diagnosis. Primary care physicians play an integral role in the recognition of cystic lung disease.
The differential diagnosis of cystic lung disease is broad and includes isolated pulmonary, systemic, infectious, and congenital etiologies.
Here, we aim to provide a systematic, stepwise approach to help differentiate among the various cystic lung diseases and devise an algorithm for diagnosis. In doing so, we will discuss the clinical and radiographic features of many of these diseases:
- Lymphangioleiomyomatosis
- Birt-Hogg-Dubé syndrome
- Pulmonary Langerhans cell histiocytosis
- Interstitial pneumonia (desquamative interstitial pneumonia, lymphocytic interstitial pneumonia)
- Congenital cystic lung disease (congenital pulmonary airway malformation, pulmonary sequestration, bronchogenic cyst) Pulmonary infection
- Systemic disease (amyloidosis, light chain deposition disease, neurofibromatosis type 1).
STEP 1: RULE OUT CYST-MIMICS
A pulmonary cyst is a round, circumscribed space surrounded by an epithelial or fibrous wall of variable thickness.1 On chest radiography and computed tomography, a cyst appears as a round parenchymal lucency or low-attenuating area with a well-defined interface with normal lung.1 Cysts vary in wall thickness but usually have a thin wall (< 2 mm) and occur without associated pulmonary emphysema.1 They typically contain air but occasionally contain fluid or solid material.
A pulmonary cyst can be categorized as a bulla, bleb, or pneumatocele.
Bullae are larger than 1 cm in diameter, sharply demarcated by a thin wall, and usually accompanied by emphysematous changes in the adjacent lung.1
Blebs are no larger than 1 cm in diameter, are located within the visceral pleura or the subpleural space, and appear on computed tomography as thin-walled air spaces that are contiguous with the pleura.1 The distinction between a bleb and a bulla is of little clinical importance, and is often unnecessary.
Pneumatoceles are cysts that are frequently caused by acute pneumonia, trauma, or aspiration of hydrocarbon fluid, and are usually transient.1
Mimics of pulmonary cysts include pulmonary cavities, emphysema, loculated pneumothoraces, honeycomb lung, and bronchiectasis (Figure 1).2
Pulmonary cavities differ from cysts in that their walls are typically thicker (usually > 4 mm).3
Emphysema differs from cystic lung disease as it typically leads to focal areas or regions of decreased lung attenuation that do not have defined walls.1
Honeycombing refers to a cluster or row of cysts, 1 to 3 mm in wall thickness and typically 3 to 10 mm in diameter, that are associated with end-stage lung fibrosis.1 They are typically subpleural in distribution and are accompanied by fibrotic features such as reticulation and traction bronchiectasis.1
Bronchiectasis is dilation and distortion of bronchi and bronchioles and can be mistaken for cysts when viewed en face.1
Loculated pneumothoraces can also mimic pulmonary cysts, but they typically fail to adhere to a defined anatomic unit and are subpleural in distribution.
STEP 2: CHARACTERIZE THE CLINICAL PRESENTATION
Clinical signs and symptoms of cystic lung disease play a key role in diagnosis (Table 1). For instance, spontaneous pneumothorax is commonly associated with diffuse cystic lung disease (lymphangioleiomyomatosis and Birt-Hogg-Dubé syndrome), while insidious dyspnea, with or without associated pneumothorax, is usually associated with the interstitial pneumonias (lymphocytic interstitial pneumonia and desquamative interstitial pneumonia).
In addition, congenital abnormalities of the lung can lead to cyst formation. These abnormalities, especially when associated with other congenital abnormalities, are often diagnosed in the prenatal and perinatal periods. However, some remain undetected until incidentally found later in adulthood or if superimposing infection develops.
Primary pulmonary infections can also cause parenchymal necrosis, which in turn cavitates or forms cysts.4
Lastly, cystic lung diseases can occur as part of a multiorgan or systemic illness in which the lung is one of the organs involved. Although usually diagnosed before the discovery of cysts or manifestations of pulmonary symptoms, they can present as a diagnostic challenge, especially when lung cysts are the initial presentation.bsence of amyloid fibrils.
In view of the features of the different types of cystic lung disease, adults with cystic lung disease can be grouped according to their typical clinical presentations (Table 2):
- Insidious dyspnea or spontaneous pneumothorax
- Incidentally found cysts or recurrent pneumonia
- Signs and symptoms of primary pulmonary infection
- Signs and symptoms that are primarily nonpulmonary.
Insidious dyspnea or spontaneous pneumothorax
Insidious dyspnea or spontaneous pneumothorax can be manifestations of lymphangioleiomyomatosis, Birt-Hogg-Dubé syndrome, pulmonary Langerhans cell histiocytosis, desquamative interstitial pneumonia, or lymphocytic interstitial pneumonia.
Lymphangioleiomyomatosis is characterized by abnormal cellular proliferation within the lung, kidney, lymphatic system, or any combination.5 The peak prevalence is in the third to fourth decades of life, and most patients are women of childbearing age.6 In addition to progressive dyspnea on exertion and pneumothorax, other signs and symptoms include hemoptysis, nonproductive cough, chylous pleural effusion, and ascites.7,8
Birt-Hogg-Dubé syndrome is caused by germline mutations in the folliculin (FLCN) gene.9 It is characterized by skin fibrofolliculomas, pulmonary cysts, spontaneous pneumothorax, and renal cancer.10
Pulmonary Langerhans cell histiocytosis is part of the spectrum of Langerhans cell histiocytosis that, in addition to the lungs, can also involve the bone, pituitary gland, thyroid, skin, lymph nodes, and liver.11 It occurs almost exclusively in smokers, affecting individuals in their 20s and 30s, with no gender predilection.12,13 In addition to nonproductive cough and dyspnea, patients can also present with fever, anorexia, and weight loss,13 but approximately 25% of patients are asymptomatic.14
Desquamative interstitial pneumonia is an idiopathic interstitial pneumonia that, like pulmonary Langerhans cell histiocytosis, is seen almost exclusively in current or former smokers, who account for about 90% of patients with this disease. It affects almost twice as many men as women.15,16 The mean age at onset is 42 to 46.15,16 In addition to insidious cough and dyspnea, digital clubbing develops in 26% to 40% of patients.16,17
Lymphocytic interstitial pneumonia is another rare idiopathic pneumonia, usually associated with connective tissue disease, Sjögren syndrome, immunodeficiencies, and viral infections.18–21 It is more common in women, presenting between the 4th and 7th decades of life, with a mean age at diagnosis of 50 to 56.18,22 In addition to progressive dyspnea and cough, other symptoms include weight loss, pleuritic pain, arthralgias, fatigue, night sweats, and fever.23
In summary, in this clinical group, lymphangioleiomyomatosis and Birt-Hogg-Dubé syndrome should be considered when patients present with spontaneous pneumothorax; those with Birt-Hogg-Dubé syndrome also present with skin lesions or renal cancer. In patients with progressive dyspnea and cough, lymphocytic interstitial pneumonia should be considered in those with a known history of connective tissue disease or immunodeficiency. Pulmonary Langerhans cell histiocytosis typically presents at a younger age (20 to 30 years old) than desquamative interstitial pneumonia (smokers in their 40s). Making the distinction, however, will likely require imaging with computed tomography.
Incidentally found cysts or recurrent pneumonia
Incidentally found cysts or recurrent pneumonia can be manifestations of congenital pulmonary airway malformation, pulmonary sequestration, or bronchogenic cyst.
Congenital pulmonary airway malformation, of which there are five types, is the most common pulmonary congenital abnormality. It accounts for up to 95% of cases of congenital cystic lung disease.24,25 About 85% of cases are detected in the prenatal or perinatal periods.26 Late-onset congenital pulmonary airway malformation (arising in childhood to adulthood) presents with recurrent pneumonia in about 75% of cases and can be misdiagnosed as lung abscess, pulmonary tuberculosis, or bronchiectasis.27
Pulmonary sequestration, the second most common pulmonary congenital abnormality, is characterized by a portion of lung that does not connect to the tracheobronchial tree and has its own systemic arterial supply.24 Intralobar sequestration, which shares the pleural investment with normal lung, accounts for about 80% of cases of pulmonary sequestration.28–30 In addition to signs or symptoms of pulmonary infection, patients with pulmonary sequestration can remain asymp-
tomatic (about 25% of cases), or can present with hemoptysis or hemothorax.28–30 In adults, the typical age at presentation is between 20 and 25.29,30
Bronchogenic cyst is usually life-threatening in children. In adults, it commonly causes cough and chest pain.31 Hemoptysis, dysphagia, hoarseness, and diaphragmatic paralysis can also occur.32,33 The mean age at diagnosis in adults is 35 to 40.31,32
In summary, most cases of recurrent pneumonia with cysts are due to congenital pulmonary airway malformation. Pulmonary sequestration is the second most common cause of cystic lung disease in this group. Bronchogenic cyst is usually fatal in fetal development; smaller cysts can go unnoticed during the earlier years and are later found incidentally as imaging abnormalities in adults.
Signs and symptoms of primary pulmonary infections
Signs and symptoms of primary pulmonary infections can be due to Pneumocystis jirovecii pneumonia or echinococcal infections.
P jirovecii pneumonia commonly develops in patients with human immunodeficiency virus infection and low CD4 counts, recipients of hematologic or solid-organ transplants, and those receiving immunosuppressive therapy (eg, glucocorticoids or chemotherapy).
Echinococcal infections (with Echinococcus granulosus or multilocularis species) are more common in less-developed countries such as those in South America or the Middle East, in China, or in patients who have traveled to endemic areas.34
In summary, cystic lung disease in patients with primary pulmonary infections can be diagnosed by the patient’s clinical history and risk factors for infections. Those with human immunodeficiency virus infection and other causes of immunodeficiency are predisposed to P jirovecii pneumonia. Echinococcal infections occur in those with a history of travel to an endemic area.
Primarily nonpulmonary signs and symptoms
If the patient has primarily nonpulmonary signs and symptoms, think about pulmonary amyloidosis, light chain deposition disease, and neurofibromatosis type 1.
Pulmonary amyloidosis has a variety of manifestations, including tracheobronchial disease, nodular parenchymal disease, diffuse or alveolar septal pattern, pleural disease, lymphadenopathy, and pulmonary cysts.4
Light chain deposition disease shares some clinical features with amyloidosis. However, the light chain fragments in this disease do not form amyloid fibrils and therefore do not stain positively with Congo red. The kidney is the most commonly involved organ.4
Neurofibromatosis type 1 is characterized by collections of neurofibromas, café-au-lait spots, and pigmented hamartomas in the iris (Lisch nodules).35
In summary, patients in this group typically present with complications related to systemic involvement. Those with neurofibromatosis type 1 present with ophthalmologic, dermatologic, and neurologic manifestations. Amyloidosis and light chain deposition disease most commonly involve the renal system; their distinction will likely require tissue biopsy and Congo-red staining.
STEP 3: CHARACTERIZE THE RADIOGRAPHIC FEATURES
Characterization of pulmonary cysts and their distribution plays a key role in the diagnosis. Radiographically, cystic lung diseases can be subclassified into two major categories according to their cystic distribution:
- Discrete (focal or multifocal)
- Diffuse (unilobular or panlobular).2,3
Discrete cystic lung diseases include congenital abnormalities, infectious diseases, and interstitial pneumonias.2,3
Diffuse, panlobular cystic lung diseases include lymphangioleiomyomatosis, pulmonary Langerhans cell histiocytosis, Birt-Hogg-Dubé syndrome, amyloidosis, light chain deposition disease, and neurofibromatosis type 1.7,13,36–39
In addition, other associated radiographic findings play a major role in diagnosis.
Cysts in patients presenting with insidious dyspnea or spontaneous pneumothorax
Lymphangioleiomyomatosis. Cysts are seen in nearly all cases of advanced lymphangioleiomyomatosis, typically in a diffuse pattern, varying from 2 mm to 40 mm in diameter, and uniform in shape (Figure 2A).7,8,40–42
Other radiographic features include vessels located at the periphery of the cysts (in contrast to the centrilobular pattern seen with emphysema), and chylous pleural effusions (in about 22% of patients).40 Nodules are typically not seen with lymphangioleiomyomatosis, and if found represent type 2 pneumocyte hyperplasia.
Pulmonary Langerhans cell histiocytosis. Nodules measuring 1 to 10 mm in diameter and favoring a centrilobular location are often seen on computed tomography. Pulmonary cysts occur in about 61% of patients.13,43 Cysts are variable in size and shape (Figure 2B), in contrast to their uniform appearance in lymphangioleiomyomatosis. Most cysts are less than 10 mm in diameter; however, they can be up to 80 mm.13,43 Early in its course, nodules may predominate in the upper and middle lobes. Over time, diffuse cysts become more common and can be difficult to differentiate from advanced smoking-induced emphysema.44
Birt-Hogg-Dubé syndrome. Approximately 70% to 100% of patients with Birt-Hogg-Dubé syndrome will have multiple pulmonary cysts detected on computed tomography. These cysts are characteristically basal and subpleural in location, with varying sizes and irregular shapes in otherwise normal lung parenchyma (Figure 2C).36,45,46
Desquamative interstitial pneumonia. Pulmonary cysts are present on computed tomography in about 32% of patients.47 They are usually round and less than 20 mm in diameter.48 Ground-glass opacity is present in almost all cases of desquamative interstitial pneumonia, with a diffuse pattern in 25% to 44% of patients.16,17,47
Pulmonary cysts occur in up to two-thirds of those with lymphocytic interstitial pneumonia. Cysts are usually multifocal and perivascular in distribution and have varying sizes and shapes (Figure 2D).22 Ground-glass opacity and poorly defined centrilobular nodules are also frequently seen. Other computed tomographic findings include thickening of the bronchovascular bundles, focal consolidation, interseptal lobular thickening, pleural thickening, and lymph node enlargement.22
In summary, in this group of patients, diffuse panlobular cysts are due to lymphangioleiomyomatosis, pulmonary Langerhans cell histiocytosis, or Birt-Hogg-Dubé syndrome. Cysts due to lymphangioleiomyomatosis have a diffuse distribution, while those due to pulmonary Langerhans cell histiocytosis tend to be upper-lobe-predominant and in the early stages are associated with stellate centrilobular nodules. Cysts in Birt-Hogg-Dubé syndrome tend to be subpleural and those due to lymphocytic interstitial pneumonia are perivascular in distribution.
Cysts that are incidentally found or occur in patients with recurrent pneumonia
Congenital pulmonary airway malformation types 1, 2, and 4 (Figure 3A, 3B). Cysts are typically discrete and focal or multifocal in distribution, but cases of multilobar and bilateral distribution have also been reported.27,49 The lower lobes are more often involved.49 Cysts vary in size and shape and can contain air, fluid, or both.27,49 Up to 50% of cases can occur in conjunction with pulmonary sequestration.50
Pulmonary sequestration displays an anomalous arterial supply on computed tomography (Figure 3C). Other imaging findings include mass lesions (49%), cystic lesions (29%), cavitary lesions (12%), and bronchiectasis.30 Air trapping can be seen in the adjacent lung. Lower lobe involvement accounts for more than 95% of total cases of sequestration.30 The cysts are usually discrete or focal in distribution. Misdiagnosis of pulmonary sequestration is common, and can include pulmonary abscess, pneumonia, bronchiectasis, and lung cancer.30
Bronchogenic cyst. Cyst contents generally demonstrate water attenuation, or higher attenuation if filled with proteinaceous/mucoid material or calcium deposits; air-fluid levels are seen in infected cysts.32 Intrapulmonary cysts have a predilection for the lower lobes and are usually discrete or focal in distribution.31,32 Mediastinal cysts are usually homogeneous, solitary, and located in the middle mediastinum.32 Cysts vary in size from 20 to 90 mm, with a mean diameter of 40 mm.31
In summary, in this group of cystic lung diseases, characteristic computed tomographic findings will suggest the diagnosis—air-filled cysts of varying sizes for congenital pulmonary airway malformation and anomalous vascular supply for pulmonary sequestration. Bronchogenic cysts will tend to have water or higher-than-water attenuation due to proteinaceous-mucoid material or calcium deposits.
Cysts in patients with signs and symptoms of primary pulmonary infections
P jirovecii pneumonia. Between 10% and 15% of patients have cysts, and about 18% present with spontaneous pneumothorax.51 Cysts in P jirovecii pneumonia vary in size from 15 to 85 mm in diameter and tend to occur in the upper lobes (Figure 4A).51,52
Echinococcal infection. Echinococcal pulmonary cysts typically are single and located more often in the lower lobes (Figure 4B).53,54 Cysts can be complicated by air-fluid levels, hydropneumothorax, or pneumothorax, or they can turn into cavitary lesions.
The diagnoses of these pulmonary infections are usually made by clinical and computed tomographic findings and depend less on detecting and characterizing lung cysts. Patients with P jirovecii pneumonia tend to have bilateral perihilar ground-glass opacities, while air-fluid levels suggest echinococcal infections. Cysts in this group of patients tend to be discrete or focal or multifocal in distribution, and vary in size.
Cysts in patients with primarily nonpulmonary signs and symptoms
Amyloidosis. Cyst formation is rare in amyloidosis.4 When present, cysts can be diffuse and scattered in distribution, in varying sizes (usually < 30 mm in diameter) and irregular shapes (Figure 5).55,56
Pulmonary light chain deposition disease usually presents as linear opacities and small nodules on chest computed tomography. Numerous cysts that are diffuse in distribution and have no topographic predominance can also be present. They can progress in number and size and coalesce to form irregular shapes.57
Neurofibromatosis type 1. In neurofibromatosis type 1, the most common radiographic presentations are bibasilar reticular opacities (50%), bullae (50%), and ground glass opacities (37%).58 Well-formed cysts occur in up to 25% of patients and tend to be diffuse and smaller (2 to 18 mm in diameter), with upper lobe predominance.58,59
In summary, in this group of patients, bibasilar reticular and ground-glass opacities suggest neurofibromatosis type 1, while nodules and linear opacities suggest amyloidosis or light chain deposition disease. Cysts tend to be diffuse with varying sizes.
STEP 4: PUT IT ALL TOGETHER
Diagnosis in insidious dyspnea or spontaneous pneumothorax
For patients who present with insidious dyspnea or spontaneous pneumothorax, the diagnosis of cystic lung disease can be made by characterizing the distribution, size, and shape of the cysts (Table 3).
Diffuse, panlobular distribution. Cystic lung diseases with this pattern include lymphangioleiomyomatosis, pulmonary Langerhans cell histiocytosis, and Birt-Hogg-Dubé syndrome. In this group, cysts that are uniform in size and regular in shape are invariably due to lymphangioleiomyomatosis. Those with variable size and irregular shapes can be due to pulmonary Langerhans cell histiocytosis or Birt-Hogg-Dubé syndrome. Patients with pulmonary Langerhans cell histiocytosis tend to be smokers and their cysts tend to be upper- lobe-predominant. Those with Birt-Hogg-Dubé syndrome will likely have renal cancer or skin lesions; their cysts tend to be basilar and subpleural in distribution.
Cysts that are focal or multifocal and unilobular are due to lymphocytic interstitial pneumonia or desquamative interstitial pneumonia. Patients with lymphocytic interstitial pneumonia tend to have underlying connective tissue disease; those with desquamative interstitial pneumonia are almost always smokers. The definitive diagnosis for lymphocytic interstitial pneumonia or desquamative interstitial pneumonia can require a tissue biopsy.
Diagnosis in patients with incidentally found cysts or recurrent pneumonia
In those who present with incidentally found cysts or recurrent pneumonia, suspicion for a congenital lung malformation should be raised. Patients with a type 1, 2, or 4 congenital pulmonary airway malformation typically have air-filled cysts in varying sizes; those with pulmonary sequestration have an anomalous arterial supply in addition to cysts that are usually located in the lower lobes. Bronchogenic cysts tend to be larger, with attenuation equal to or greater than that of water, and distinguishing them from congenital pulmonary airway malformation will likely require surgical examination.
Diagnosis in patients with signs and symptoms of pulmonary infections
Patients with signs and symptoms of pulmonary infections should be investigated according to clinical risk factors for P jirovecii pneumonia or echinococcal infections.
Diagnosis in patients with primarily nonpulmonary presentations
The distinction between amyloidosis and neurofibromatosis type 1 can be made by the history and the clinical examination. However, a definitive diagnosis of amyloidosis or light chain deposition disease requires tissue examination for the presence or absence of amyloid fibrils.
Air-filled pulmonary lesions commonly detected on chest computed tomography. Cystic lung lesions should be distinguished from other air-filled lesions to facilitate diagnosis. Primary care physicians play an integral role in the recognition of cystic lung disease.
The differential diagnosis of cystic lung disease is broad and includes isolated pulmonary, systemic, infectious, and congenital etiologies.
Here, we aim to provide a systematic, stepwise approach to help differentiate among the various cystic lung diseases and devise an algorithm for diagnosis. In doing so, we will discuss the clinical and radiographic features of many of these diseases:
- Lymphangioleiomyomatosis
- Birt-Hogg-Dubé syndrome
- Pulmonary Langerhans cell histiocytosis
- Interstitial pneumonia (desquamative interstitial pneumonia, lymphocytic interstitial pneumonia)
- Congenital cystic lung disease (congenital pulmonary airway malformation, pulmonary sequestration, bronchogenic cyst) Pulmonary infection
- Systemic disease (amyloidosis, light chain deposition disease, neurofibromatosis type 1).
STEP 1: RULE OUT CYST-MIMICS
A pulmonary cyst is a round, circumscribed space surrounded by an epithelial or fibrous wall of variable thickness.1 On chest radiography and computed tomography, a cyst appears as a round parenchymal lucency or low-attenuating area with a well-defined interface with normal lung.1 Cysts vary in wall thickness but usually have a thin wall (< 2 mm) and occur without associated pulmonary emphysema.1 They typically contain air but occasionally contain fluid or solid material.
A pulmonary cyst can be categorized as a bulla, bleb, or pneumatocele.
Bullae are larger than 1 cm in diameter, sharply demarcated by a thin wall, and usually accompanied by emphysematous changes in the adjacent lung.1
Blebs are no larger than 1 cm in diameter, are located within the visceral pleura or the subpleural space, and appear on computed tomography as thin-walled air spaces that are contiguous with the pleura.1 The distinction between a bleb and a bulla is of little clinical importance, and is often unnecessary.
Pneumatoceles are cysts that are frequently caused by acute pneumonia, trauma, or aspiration of hydrocarbon fluid, and are usually transient.1
Mimics of pulmonary cysts include pulmonary cavities, emphysema, loculated pneumothoraces, honeycomb lung, and bronchiectasis (Figure 1).2
Pulmonary cavities differ from cysts in that their walls are typically thicker (usually > 4 mm).3
Emphysema differs from cystic lung disease as it typically leads to focal areas or regions of decreased lung attenuation that do not have defined walls.1
Honeycombing refers to a cluster or row of cysts, 1 to 3 mm in wall thickness and typically 3 to 10 mm in diameter, that are associated with end-stage lung fibrosis.1 They are typically subpleural in distribution and are accompanied by fibrotic features such as reticulation and traction bronchiectasis.1
Bronchiectasis is dilation and distortion of bronchi and bronchioles and can be mistaken for cysts when viewed en face.1
Loculated pneumothoraces can also mimic pulmonary cysts, but they typically fail to adhere to a defined anatomic unit and are subpleural in distribution.
STEP 2: CHARACTERIZE THE CLINICAL PRESENTATION
Clinical signs and symptoms of cystic lung disease play a key role in diagnosis (Table 1). For instance, spontaneous pneumothorax is commonly associated with diffuse cystic lung disease (lymphangioleiomyomatosis and Birt-Hogg-Dubé syndrome), while insidious dyspnea, with or without associated pneumothorax, is usually associated with the interstitial pneumonias (lymphocytic interstitial pneumonia and desquamative interstitial pneumonia).
In addition, congenital abnormalities of the lung can lead to cyst formation. These abnormalities, especially when associated with other congenital abnormalities, are often diagnosed in the prenatal and perinatal periods. However, some remain undetected until incidentally found later in adulthood or if superimposing infection develops.
Primary pulmonary infections can also cause parenchymal necrosis, which in turn cavitates or forms cysts.4
Lastly, cystic lung diseases can occur as part of a multiorgan or systemic illness in which the lung is one of the organs involved. Although usually diagnosed before the discovery of cysts or manifestations of pulmonary symptoms, they can present as a diagnostic challenge, especially when lung cysts are the initial presentation.bsence of amyloid fibrils.
In view of the features of the different types of cystic lung disease, adults with cystic lung disease can be grouped according to their typical clinical presentations (Table 2):
- Insidious dyspnea or spontaneous pneumothorax
- Incidentally found cysts or recurrent pneumonia
- Signs and symptoms of primary pulmonary infection
- Signs and symptoms that are primarily nonpulmonary.
Insidious dyspnea or spontaneous pneumothorax
Insidious dyspnea or spontaneous pneumothorax can be manifestations of lymphangioleiomyomatosis, Birt-Hogg-Dubé syndrome, pulmonary Langerhans cell histiocytosis, desquamative interstitial pneumonia, or lymphocytic interstitial pneumonia.
Lymphangioleiomyomatosis is characterized by abnormal cellular proliferation within the lung, kidney, lymphatic system, or any combination.5 The peak prevalence is in the third to fourth decades of life, and most patients are women of childbearing age.6 In addition to progressive dyspnea on exertion and pneumothorax, other signs and symptoms include hemoptysis, nonproductive cough, chylous pleural effusion, and ascites.7,8
Birt-Hogg-Dubé syndrome is caused by germline mutations in the folliculin (FLCN) gene.9 It is characterized by skin fibrofolliculomas, pulmonary cysts, spontaneous pneumothorax, and renal cancer.10
Pulmonary Langerhans cell histiocytosis is part of the spectrum of Langerhans cell histiocytosis that, in addition to the lungs, can also involve the bone, pituitary gland, thyroid, skin, lymph nodes, and liver.11 It occurs almost exclusively in smokers, affecting individuals in their 20s and 30s, with no gender predilection.12,13 In addition to nonproductive cough and dyspnea, patients can also present with fever, anorexia, and weight loss,13 but approximately 25% of patients are asymptomatic.14
Desquamative interstitial pneumonia is an idiopathic interstitial pneumonia that, like pulmonary Langerhans cell histiocytosis, is seen almost exclusively in current or former smokers, who account for about 90% of patients with this disease. It affects almost twice as many men as women.15,16 The mean age at onset is 42 to 46.15,16 In addition to insidious cough and dyspnea, digital clubbing develops in 26% to 40% of patients.16,17
Lymphocytic interstitial pneumonia is another rare idiopathic pneumonia, usually associated with connective tissue disease, Sjögren syndrome, immunodeficiencies, and viral infections.18–21 It is more common in women, presenting between the 4th and 7th decades of life, with a mean age at diagnosis of 50 to 56.18,22 In addition to progressive dyspnea and cough, other symptoms include weight loss, pleuritic pain, arthralgias, fatigue, night sweats, and fever.23
In summary, in this clinical group, lymphangioleiomyomatosis and Birt-Hogg-Dubé syndrome should be considered when patients present with spontaneous pneumothorax; those with Birt-Hogg-Dubé syndrome also present with skin lesions or renal cancer. In patients with progressive dyspnea and cough, lymphocytic interstitial pneumonia should be considered in those with a known history of connective tissue disease or immunodeficiency. Pulmonary Langerhans cell histiocytosis typically presents at a younger age (20 to 30 years old) than desquamative interstitial pneumonia (smokers in their 40s). Making the distinction, however, will likely require imaging with computed tomography.
Incidentally found cysts or recurrent pneumonia
Incidentally found cysts or recurrent pneumonia can be manifestations of congenital pulmonary airway malformation, pulmonary sequestration, or bronchogenic cyst.
Congenital pulmonary airway malformation, of which there are five types, is the most common pulmonary congenital abnormality. It accounts for up to 95% of cases of congenital cystic lung disease.24,25 About 85% of cases are detected in the prenatal or perinatal periods.26 Late-onset congenital pulmonary airway malformation (arising in childhood to adulthood) presents with recurrent pneumonia in about 75% of cases and can be misdiagnosed as lung abscess, pulmonary tuberculosis, or bronchiectasis.27
Pulmonary sequestration, the second most common pulmonary congenital abnormality, is characterized by a portion of lung that does not connect to the tracheobronchial tree and has its own systemic arterial supply.24 Intralobar sequestration, which shares the pleural investment with normal lung, accounts for about 80% of cases of pulmonary sequestration.28–30 In addition to signs or symptoms of pulmonary infection, patients with pulmonary sequestration can remain asymp-
tomatic (about 25% of cases), or can present with hemoptysis or hemothorax.28–30 In adults, the typical age at presentation is between 20 and 25.29,30
Bronchogenic cyst is usually life-threatening in children. In adults, it commonly causes cough and chest pain.31 Hemoptysis, dysphagia, hoarseness, and diaphragmatic paralysis can also occur.32,33 The mean age at diagnosis in adults is 35 to 40.31,32
In summary, most cases of recurrent pneumonia with cysts are due to congenital pulmonary airway malformation. Pulmonary sequestration is the second most common cause of cystic lung disease in this group. Bronchogenic cyst is usually fatal in fetal development; smaller cysts can go unnoticed during the earlier years and are later found incidentally as imaging abnormalities in adults.
Signs and symptoms of primary pulmonary infections
Signs and symptoms of primary pulmonary infections can be due to Pneumocystis jirovecii pneumonia or echinococcal infections.
P jirovecii pneumonia commonly develops in patients with human immunodeficiency virus infection and low CD4 counts, recipients of hematologic or solid-organ transplants, and those receiving immunosuppressive therapy (eg, glucocorticoids or chemotherapy).
Echinococcal infections (with Echinococcus granulosus or multilocularis species) are more common in less-developed countries such as those in South America or the Middle East, in China, or in patients who have traveled to endemic areas.34
In summary, cystic lung disease in patients with primary pulmonary infections can be diagnosed by the patient’s clinical history and risk factors for infections. Those with human immunodeficiency virus infection and other causes of immunodeficiency are predisposed to P jirovecii pneumonia. Echinococcal infections occur in those with a history of travel to an endemic area.
Primarily nonpulmonary signs and symptoms
If the patient has primarily nonpulmonary signs and symptoms, think about pulmonary amyloidosis, light chain deposition disease, and neurofibromatosis type 1.
Pulmonary amyloidosis has a variety of manifestations, including tracheobronchial disease, nodular parenchymal disease, diffuse or alveolar septal pattern, pleural disease, lymphadenopathy, and pulmonary cysts.4
Light chain deposition disease shares some clinical features with amyloidosis. However, the light chain fragments in this disease do not form amyloid fibrils and therefore do not stain positively with Congo red. The kidney is the most commonly involved organ.4
Neurofibromatosis type 1 is characterized by collections of neurofibromas, café-au-lait spots, and pigmented hamartomas in the iris (Lisch nodules).35
In summary, patients in this group typically present with complications related to systemic involvement. Those with neurofibromatosis type 1 present with ophthalmologic, dermatologic, and neurologic manifestations. Amyloidosis and light chain deposition disease most commonly involve the renal system; their distinction will likely require tissue biopsy and Congo-red staining.
STEP 3: CHARACTERIZE THE RADIOGRAPHIC FEATURES
Characterization of pulmonary cysts and their distribution plays a key role in the diagnosis. Radiographically, cystic lung diseases can be subclassified into two major categories according to their cystic distribution:
- Discrete (focal or multifocal)
- Diffuse (unilobular or panlobular).2,3
Discrete cystic lung diseases include congenital abnormalities, infectious diseases, and interstitial pneumonias.2,3
Diffuse, panlobular cystic lung diseases include lymphangioleiomyomatosis, pulmonary Langerhans cell histiocytosis, Birt-Hogg-Dubé syndrome, amyloidosis, light chain deposition disease, and neurofibromatosis type 1.7,13,36–39
In addition, other associated radiographic findings play a major role in diagnosis.
Cysts in patients presenting with insidious dyspnea or spontaneous pneumothorax
Lymphangioleiomyomatosis. Cysts are seen in nearly all cases of advanced lymphangioleiomyomatosis, typically in a diffuse pattern, varying from 2 mm to 40 mm in diameter, and uniform in shape (Figure 2A).7,8,40–42
Other radiographic features include vessels located at the periphery of the cysts (in contrast to the centrilobular pattern seen with emphysema), and chylous pleural effusions (in about 22% of patients).40 Nodules are typically not seen with lymphangioleiomyomatosis, and if found represent type 2 pneumocyte hyperplasia.
Pulmonary Langerhans cell histiocytosis. Nodules measuring 1 to 10 mm in diameter and favoring a centrilobular location are often seen on computed tomography. Pulmonary cysts occur in about 61% of patients.13,43 Cysts are variable in size and shape (Figure 2B), in contrast to their uniform appearance in lymphangioleiomyomatosis. Most cysts are less than 10 mm in diameter; however, they can be up to 80 mm.13,43 Early in its course, nodules may predominate in the upper and middle lobes. Over time, diffuse cysts become more common and can be difficult to differentiate from advanced smoking-induced emphysema.44
Birt-Hogg-Dubé syndrome. Approximately 70% to 100% of patients with Birt-Hogg-Dubé syndrome will have multiple pulmonary cysts detected on computed tomography. These cysts are characteristically basal and subpleural in location, with varying sizes and irregular shapes in otherwise normal lung parenchyma (Figure 2C).36,45,46
Desquamative interstitial pneumonia. Pulmonary cysts are present on computed tomography in about 32% of patients.47 They are usually round and less than 20 mm in diameter.48 Ground-glass opacity is present in almost all cases of desquamative interstitial pneumonia, with a diffuse pattern in 25% to 44% of patients.16,17,47
Pulmonary cysts occur in up to two-thirds of those with lymphocytic interstitial pneumonia. Cysts are usually multifocal and perivascular in distribution and have varying sizes and shapes (Figure 2D).22 Ground-glass opacity and poorly defined centrilobular nodules are also frequently seen. Other computed tomographic findings include thickening of the bronchovascular bundles, focal consolidation, interseptal lobular thickening, pleural thickening, and lymph node enlargement.22
In summary, in this group of patients, diffuse panlobular cysts are due to lymphangioleiomyomatosis, pulmonary Langerhans cell histiocytosis, or Birt-Hogg-Dubé syndrome. Cysts due to lymphangioleiomyomatosis have a diffuse distribution, while those due to pulmonary Langerhans cell histiocytosis tend to be upper-lobe-predominant and in the early stages are associated with stellate centrilobular nodules. Cysts in Birt-Hogg-Dubé syndrome tend to be subpleural and those due to lymphocytic interstitial pneumonia are perivascular in distribution.
Cysts that are incidentally found or occur in patients with recurrent pneumonia
Congenital pulmonary airway malformation types 1, 2, and 4 (Figure 3A, 3B). Cysts are typically discrete and focal or multifocal in distribution, but cases of multilobar and bilateral distribution have also been reported.27,49 The lower lobes are more often involved.49 Cysts vary in size and shape and can contain air, fluid, or both.27,49 Up to 50% of cases can occur in conjunction with pulmonary sequestration.50
Pulmonary sequestration displays an anomalous arterial supply on computed tomography (Figure 3C). Other imaging findings include mass lesions (49%), cystic lesions (29%), cavitary lesions (12%), and bronchiectasis.30 Air trapping can be seen in the adjacent lung. Lower lobe involvement accounts for more than 95% of total cases of sequestration.30 The cysts are usually discrete or focal in distribution. Misdiagnosis of pulmonary sequestration is common, and can include pulmonary abscess, pneumonia, bronchiectasis, and lung cancer.30
Bronchogenic cyst. Cyst contents generally demonstrate water attenuation, or higher attenuation if filled with proteinaceous/mucoid material or calcium deposits; air-fluid levels are seen in infected cysts.32 Intrapulmonary cysts have a predilection for the lower lobes and are usually discrete or focal in distribution.31,32 Mediastinal cysts are usually homogeneous, solitary, and located in the middle mediastinum.32 Cysts vary in size from 20 to 90 mm, with a mean diameter of 40 mm.31
In summary, in this group of cystic lung diseases, characteristic computed tomographic findings will suggest the diagnosis—air-filled cysts of varying sizes for congenital pulmonary airway malformation and anomalous vascular supply for pulmonary sequestration. Bronchogenic cysts will tend to have water or higher-than-water attenuation due to proteinaceous-mucoid material or calcium deposits.
Cysts in patients with signs and symptoms of primary pulmonary infections
P jirovecii pneumonia. Between 10% and 15% of patients have cysts, and about 18% present with spontaneous pneumothorax.51 Cysts in P jirovecii pneumonia vary in size from 15 to 85 mm in diameter and tend to occur in the upper lobes (Figure 4A).51,52
Echinococcal infection. Echinococcal pulmonary cysts typically are single and located more often in the lower lobes (Figure 4B).53,54 Cysts can be complicated by air-fluid levels, hydropneumothorax, or pneumothorax, or they can turn into cavitary lesions.
The diagnoses of these pulmonary infections are usually made by clinical and computed tomographic findings and depend less on detecting and characterizing lung cysts. Patients with P jirovecii pneumonia tend to have bilateral perihilar ground-glass opacities, while air-fluid levels suggest echinococcal infections. Cysts in this group of patients tend to be discrete or focal or multifocal in distribution, and vary in size.
Cysts in patients with primarily nonpulmonary signs and symptoms
Amyloidosis. Cyst formation is rare in amyloidosis.4 When present, cysts can be diffuse and scattered in distribution, in varying sizes (usually < 30 mm in diameter) and irregular shapes (Figure 5).55,56
Pulmonary light chain deposition disease usually presents as linear opacities and small nodules on chest computed tomography. Numerous cysts that are diffuse in distribution and have no topographic predominance can also be present. They can progress in number and size and coalesce to form irregular shapes.57
Neurofibromatosis type 1. In neurofibromatosis type 1, the most common radiographic presentations are bibasilar reticular opacities (50%), bullae (50%), and ground glass opacities (37%).58 Well-formed cysts occur in up to 25% of patients and tend to be diffuse and smaller (2 to 18 mm in diameter), with upper lobe predominance.58,59
In summary, in this group of patients, bibasilar reticular and ground-glass opacities suggest neurofibromatosis type 1, while nodules and linear opacities suggest amyloidosis or light chain deposition disease. Cysts tend to be diffuse with varying sizes.
STEP 4: PUT IT ALL TOGETHER
Diagnosis in insidious dyspnea or spontaneous pneumothorax
For patients who present with insidious dyspnea or spontaneous pneumothorax, the diagnosis of cystic lung disease can be made by characterizing the distribution, size, and shape of the cysts (Table 3).
Diffuse, panlobular distribution. Cystic lung diseases with this pattern include lymphangioleiomyomatosis, pulmonary Langerhans cell histiocytosis, and Birt-Hogg-Dubé syndrome. In this group, cysts that are uniform in size and regular in shape are invariably due to lymphangioleiomyomatosis. Those with variable size and irregular shapes can be due to pulmonary Langerhans cell histiocytosis or Birt-Hogg-Dubé syndrome. Patients with pulmonary Langerhans cell histiocytosis tend to be smokers and their cysts tend to be upper- lobe-predominant. Those with Birt-Hogg-Dubé syndrome will likely have renal cancer or skin lesions; their cysts tend to be basilar and subpleural in distribution.
Cysts that are focal or multifocal and unilobular are due to lymphocytic interstitial pneumonia or desquamative interstitial pneumonia. Patients with lymphocytic interstitial pneumonia tend to have underlying connective tissue disease; those with desquamative interstitial pneumonia are almost always smokers. The definitive diagnosis for lymphocytic interstitial pneumonia or desquamative interstitial pneumonia can require a tissue biopsy.
Diagnosis in patients with incidentally found cysts or recurrent pneumonia
In those who present with incidentally found cysts or recurrent pneumonia, suspicion for a congenital lung malformation should be raised. Patients with a type 1, 2, or 4 congenital pulmonary airway malformation typically have air-filled cysts in varying sizes; those with pulmonary sequestration have an anomalous arterial supply in addition to cysts that are usually located in the lower lobes. Bronchogenic cysts tend to be larger, with attenuation equal to or greater than that of water, and distinguishing them from congenital pulmonary airway malformation will likely require surgical examination.
Diagnosis in patients with signs and symptoms of pulmonary infections
Patients with signs and symptoms of pulmonary infections should be investigated according to clinical risk factors for P jirovecii pneumonia or echinococcal infections.
Diagnosis in patients with primarily nonpulmonary presentations
The distinction between amyloidosis and neurofibromatosis type 1 can be made by the history and the clinical examination. However, a definitive diagnosis of amyloidosis or light chain deposition disease requires tissue examination for the presence or absence of amyloid fibrils.
- Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008; 246:697–722.
- Cosgrove GP, Frankel SK, Brown KK. Challenges in pulmonary fibrosis. 3: cystic lung disease. Thorax 2007; 62:820–829.
- Ryu JH, Swensen SJ. Cystic and cavitary lung diseases: focal and diffuse. Mayo Clin Proc 2003; 78:744–752.
- Ryu JH, Tian X, Baqir M, Xu K. Diffuse cystic lung diseases. Front Med 2013; 7:316–327.
- McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest 2008; 133:507–516.
- Johnson SR, Cordier JF, Lazor R, et al; Review Panel of the ERS LAM Task Force. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J 2010; 35:14–26.
- Taylor JR, Ryu J, Colby TV, Raffin TA. Lymphangioleiomyomatosis. Clinical course in 32 patients. N Engl J Med 1990; 323:1254–1260.
- Chu SC, Horiba K, Usuki J. Comprehensive evaluation of 35 patients with lymphangioleiomyomatosis. Chest 1999; 115:1041–1052.
- Graham RB, Nolasco M, Peterlin B, Garcia CK. Nonsense mutations in folliculin presenting as isolated familial spontaneous pneumothorax in adults. Am J Respir Crit Care Med 2005; 172:39–44.
- Birt AR, Hogg GR, Dubé WJ. Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch Dermatol 1977; 113:1674–1677.
- Sundar KM, Gosselin MV, Chung HL, Cahill BC. Pulmonary Langerhans cell histiocytosis: emerging concepts in pathobiology, radiology, and clinical evolution of disease. Chest 2003; 123:1673–1683.
- Vassallo R, Ryu JH, Colby TV, Hartman T, Limper AH. Pulmonary Langerhans’-cell histiocytosis. N Engl J Med 2000; 342:1969–1978.
- Vassallo R, Ryu JH, Schroeder DR, Decker PA, Limper AH. Clinical outcomes of pulmonary Langerhans’-cell histiocytosis in adults. N Engl J Med 2002; 346:484–490.
- Mendez JL, Nadrous HF, Vassallo R, Decker PA, Ryu JH. Pneumothorax in pulmonary Langerhans cell histiocytosis. Chest 2004; 125:1028–1032.
- Carrington CB, Gaensler EA, Coutu RE, FitzGerald MX, Gupta RG. Natural history and treated course of usual and desquamative interstitial pneumonia. N Engl J Med 1978; 298:801–809.
- Ryu JH, Myers JL, Capizzi SA, Douglas WW, Vassallo R, Decker PA. Desquamative interstitial pneumonia and respiratory bronchiolitis-associated interstitial lung disease. Chest 2005; 127:178–184.
- Lynch DA, Travis WD, Müller NL, et al. Idiopathic interstitial pneumonias: CT features. Radiology 2005; 236:10–21.
- Strimlan CV, Rosenow EC 3rd, Weiland LH, Brown LR. Lymphocytic interstitial pneumonitis. Review of 13 cases. Ann Intern Med 1978; 88:616–621.
- Arish N, Eldor R, Fellig Y, et al. Lymphocytic interstitial pneumonia associated with common variable immunodeficiency resolved with intravenous immunoglobulins. Thorax 2006; 61:1096–1097.
- Schooley RT, Carey RW, Miller G, et al. Chronic Epstein-Barr virus infection associated with fever and interstitial pneumonitis. Clinical and serologic features and response to antiviral chemotherapy. Ann Intern Med 1986; 104:636–643.
- Kramer MR, Saldana MJ, Ramos M, Pitchenik AE. High titers of Epstein-Barr virus antibodies in adult patients with lymphocytic interstitial pneumonitis associated with AIDS. Respir Med 1992; 86:49–52.
- Johkoh T, Müller NL, Pickford HA, et al. Lymphocytic interstitial pneumonia: thin-section CT findings in 22 patients. Radiology 1999; 212:567–572.
- Swigris JJ, Berry GJ, Raffin TA, Kuschner WG. Lymphoid interstitial pneumonia: a narrative review. Chest 2002; 122:2150–2164.
- Biyyam DR, Chapman T, Ferguson MR, Deutsch G, Dighe MK. Congenital lung abnormalities: embryologic features, prenatal diagnosis, and postnatal radiologic-pathologic correlation. Radiographics 2010; 30:1721–1738.
- Cloutier MM, Schaeffer DA, Hight D. Congenital cystic adenomatoid malformation. Chest 1993; 103:761–764.
- Luján M, Bosque M, Mirapeix RM, Marco MT, Asensio O, Domingo C. Late-onset congenital cystic adenomatoid malformation of the lung. Embryology, clinical symptomatology, diagnostic procedures, therapeutic approach and clinical follow-up. Respiration 2002; 69:148–154.
- Oh BJ, Lee JS, Kim JS, Lim CM, Koh Y. Congenital cystic adenomatoid malformation of the lung in adults: clinical and CT evaluation of seven patients. Respirology 2006; 11:496–501.
- Tsolakis CC, Kollias VD, Panayotopoulos PP. Pulmonary sequestration. Experience with eight consecutive cases. Scand Cardiovasc J 1997; 31:229–232.
- Sauvanet A, Regnard JF, Calanducci F, Rojas-Miranda A, Dartevelle P, Levasseur P. Pulmonary sequestration. Surgical aspects based on 61 cases. Rev Pneumol Clin 1991; 47:126–132. Article in French.
- Wei Y, Li F. Pulmonary sequestration: a retrospective analysis of 2,625 cases in China. Eur J Cardiothorac Surg 2011; 40:e39–e42.
- Patel SR, Meeker DP, Biscotti CV, Kirby TJ, Rice TW. Presentation and management of bronchogenic cysts in the adult. Chest 1994; 106:79–85.
- Limaïem F, Ayadi-Kaddour A, Djilani H, Kilani T, El Mezni F. Pulmonary and mediastinal bronchogenic cysts: a clinicopathologic study of 33 cases. Lung 2008; 186:55–61.
- Liu HS, Li SQ, Cao ZL, Zhang ZY, Ren H. Clinical features and treatment of bronchogenic cyst in adults. Chin Med Sci J 2009; 24:60–63.
- Jenkins DJ, Romig T, Thompson RC. Emergence/re-emergence of Echinococcus spp.—a global update. Int J Parasitol 2005; 35:1205–1219.
- Riccardi VM. Von Recklinghausen neurofibromatosis. N Engl J Med 1981; 305:1617–1627.
- Toro JR, Pautler SE, Stewart L, et al. Lung cysts, spontaneous pneumothorax, and genetic associations in 89 families with Birt-Hogg-Dubé syndrome. Am J Respir Crit Care Med 2007; 175:1044–1053.
- Biko DM, Schwartz M, Anupindi SA, Altes TA. Subpleural lung cysts in Down syndrome: prevalence and association with coexisting diagnoses. Pediatr Radiol 2008; 38:280–284.
- Colombat M, Stern M, Groussard O, et al. Pulmonary cystic disorder related to light chain deposition disease. Am J Respir Crit Care Med 2006; 173:777–780.
- Ohdama S, Akagawa S, Matsubara O, Yoshizawa Y. Primary diffuse alveolar septal amyloidosis with multiple cysts and calcification. Eur Respir J 1996; 9:1569–1571.
- Johnson SR, Tattersfield AE. Clinical experience of lymphangioleiomyomatosis in the UK. Thorax 2000; 55:1052–1057.
- Kitaichi M, Nishimura K, Itoh H, Izumi T. Pulmonary lymphangioleiomyomatosis: a report of 46 patients including a clinicopathologic study of prognostic factors. Am J Respir Crit Care Med 1995; 151:527–533.
- Urban T, Lazor R, Lacronique J, et al. Pulmonary lymphangioleiomyomatosis. A study of 69 patients. Groupe d’Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM”O”P). Medicine (Baltimore) 1999; 78:321–337.
- Schönfeld N, Frank W, Wenig S, et al. Clinical and radiologic features, lung function and therapeutic results in pulmonary histiocytosis X. Respiration 1993; 60:38–44.
- Lacronique J, Roth C, Battesti JP, Basset F, Chretien J. Chest radiological features of pulmonary histiocytosis X: a report based on 50 adult cases. Thorax 1982; 37:104–109.
- Kluger N, Giraud S, Coupier I, et al. Birt-Hogg-Dubé syndrome: clinical and genetic studies of 10 French families. Br J Dermatol 2010; 162:527–537.
- Tobino K, Gunji Y, Kurihara M, et al. Characteristics of pulmonary cysts in Birt-Hogg-Dubé syndrome: thin-section CT findings of the chest in 12 patients. Eur J Radiol 2011; 77:403–409.
- Hartman TE, Primack SL, Swensen SJ, Hansell D, McGuinness G, Müller NL. Desquamative interstitial pneumonia: thin-section CT findings in 22 patients. Radiology 1993; 187:787–790.
- Koyama M, Johkoh T, Honda O, et al. Chronic cystic lung disease: diagnostic accuracy of high-resolution CT in 92 patients. AJR Am J Roentgenol 2003; 180:827–835.
- Patz EF Jr, Müller NL, Swensen SJ, Dodd LG. Congenital cystic adenomatoid malformation in adults: CT findings. J Comput Assist Tomogr 1995; 19:361–364.
- Conran RM, Stocker JT. Extralobar sequestration with frequently associated congenital cystic adenomatoid malformation, type 2: report of 50 cases. Pediatr Dev Pathol 1999; 2:454–463.
- Kennedy CA, Goetz MB. Atypical roentgenographic manifestations of Pneumocystis carinii pneumonia. Arch Intern Med 1992; 152:1390–1398.
- Sandhu JS, Goodman PC. Pulmonary cysts associated with Pneumocystis carinii pneumonia in patients with AIDS. Radiology 1989; 173:33–35.
- Doğan R, Yüksel M, Cetin G, et al. Surgical treatment of hydatid cysts of the lung: report on 1,055 patients. Thorax 1989; 44:192–199.
- Salih OK, Topcuoğlu MS, Celik SK, Ulus T, Tokcan A. Surgical treatment of hydatid cysts of the lung: analysis of 405 patients. Can J Surg 1998; 41:131–135.
- Ohdama S, Akagawa S, Matsubara O, Yoshizawa Y. Primary diffuse alveolar septal amyloidosis with multiple cysts and calcification. Eur Respir J 1996; 9:1569–1571.
- Sakai M, Yamaoka M, Kawaguchi M, Hizawa N, Sato Y. Multiple cystic pulmonary amyloidosis. Ann Thorac Surg 2011; 92:e109.
- Colombat M, Caudroy S, Lagonotte E, et al. Pathomechanisms of cyst formation in pulmonary light chain deposition disease. Eur Respir J 2008; 32:1399–1403.
- Zamora AC, Collard HR, Wolters PJ, Webb WR, King TE. Neurofibromatosis-associated lung disease: a case series and literature review. Eur Respir J 2007; 29:210–214.
- Oikonomou A, Vadikolias K, Birbilis T, Bouros D, Prassopoulos P. HRCT findings in the lungs of non-smokers with neurofibromatosis. Eur J Radiol 2011; 80:e520–e523.
- Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008; 246:697–722.
- Cosgrove GP, Frankel SK, Brown KK. Challenges in pulmonary fibrosis. 3: cystic lung disease. Thorax 2007; 62:820–829.
- Ryu JH, Swensen SJ. Cystic and cavitary lung diseases: focal and diffuse. Mayo Clin Proc 2003; 78:744–752.
- Ryu JH, Tian X, Baqir M, Xu K. Diffuse cystic lung diseases. Front Med 2013; 7:316–327.
- McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest 2008; 133:507–516.
- Johnson SR, Cordier JF, Lazor R, et al; Review Panel of the ERS LAM Task Force. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J 2010; 35:14–26.
- Taylor JR, Ryu J, Colby TV, Raffin TA. Lymphangioleiomyomatosis. Clinical course in 32 patients. N Engl J Med 1990; 323:1254–1260.
- Chu SC, Horiba K, Usuki J. Comprehensive evaluation of 35 patients with lymphangioleiomyomatosis. Chest 1999; 115:1041–1052.
- Graham RB, Nolasco M, Peterlin B, Garcia CK. Nonsense mutations in folliculin presenting as isolated familial spontaneous pneumothorax in adults. Am J Respir Crit Care Med 2005; 172:39–44.
- Birt AR, Hogg GR, Dubé WJ. Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch Dermatol 1977; 113:1674–1677.
- Sundar KM, Gosselin MV, Chung HL, Cahill BC. Pulmonary Langerhans cell histiocytosis: emerging concepts in pathobiology, radiology, and clinical evolution of disease. Chest 2003; 123:1673–1683.
- Vassallo R, Ryu JH, Colby TV, Hartman T, Limper AH. Pulmonary Langerhans’-cell histiocytosis. N Engl J Med 2000; 342:1969–1978.
- Vassallo R, Ryu JH, Schroeder DR, Decker PA, Limper AH. Clinical outcomes of pulmonary Langerhans’-cell histiocytosis in adults. N Engl J Med 2002; 346:484–490.
- Mendez JL, Nadrous HF, Vassallo R, Decker PA, Ryu JH. Pneumothorax in pulmonary Langerhans cell histiocytosis. Chest 2004; 125:1028–1032.
- Carrington CB, Gaensler EA, Coutu RE, FitzGerald MX, Gupta RG. Natural history and treated course of usual and desquamative interstitial pneumonia. N Engl J Med 1978; 298:801–809.
- Ryu JH, Myers JL, Capizzi SA, Douglas WW, Vassallo R, Decker PA. Desquamative interstitial pneumonia and respiratory bronchiolitis-associated interstitial lung disease. Chest 2005; 127:178–184.
- Lynch DA, Travis WD, Müller NL, et al. Idiopathic interstitial pneumonias: CT features. Radiology 2005; 236:10–21.
- Strimlan CV, Rosenow EC 3rd, Weiland LH, Brown LR. Lymphocytic interstitial pneumonitis. Review of 13 cases. Ann Intern Med 1978; 88:616–621.
- Arish N, Eldor R, Fellig Y, et al. Lymphocytic interstitial pneumonia associated with common variable immunodeficiency resolved with intravenous immunoglobulins. Thorax 2006; 61:1096–1097.
- Schooley RT, Carey RW, Miller G, et al. Chronic Epstein-Barr virus infection associated with fever and interstitial pneumonitis. Clinical and serologic features and response to antiviral chemotherapy. Ann Intern Med 1986; 104:636–643.
- Kramer MR, Saldana MJ, Ramos M, Pitchenik AE. High titers of Epstein-Barr virus antibodies in adult patients with lymphocytic interstitial pneumonitis associated with AIDS. Respir Med 1992; 86:49–52.
- Johkoh T, Müller NL, Pickford HA, et al. Lymphocytic interstitial pneumonia: thin-section CT findings in 22 patients. Radiology 1999; 212:567–572.
- Swigris JJ, Berry GJ, Raffin TA, Kuschner WG. Lymphoid interstitial pneumonia: a narrative review. Chest 2002; 122:2150–2164.
- Biyyam DR, Chapman T, Ferguson MR, Deutsch G, Dighe MK. Congenital lung abnormalities: embryologic features, prenatal diagnosis, and postnatal radiologic-pathologic correlation. Radiographics 2010; 30:1721–1738.
- Cloutier MM, Schaeffer DA, Hight D. Congenital cystic adenomatoid malformation. Chest 1993; 103:761–764.
- Luján M, Bosque M, Mirapeix RM, Marco MT, Asensio O, Domingo C. Late-onset congenital cystic adenomatoid malformation of the lung. Embryology, clinical symptomatology, diagnostic procedures, therapeutic approach and clinical follow-up. Respiration 2002; 69:148–154.
- Oh BJ, Lee JS, Kim JS, Lim CM, Koh Y. Congenital cystic adenomatoid malformation of the lung in adults: clinical and CT evaluation of seven patients. Respirology 2006; 11:496–501.
- Tsolakis CC, Kollias VD, Panayotopoulos PP. Pulmonary sequestration. Experience with eight consecutive cases. Scand Cardiovasc J 1997; 31:229–232.
- Sauvanet A, Regnard JF, Calanducci F, Rojas-Miranda A, Dartevelle P, Levasseur P. Pulmonary sequestration. Surgical aspects based on 61 cases. Rev Pneumol Clin 1991; 47:126–132. Article in French.
- Wei Y, Li F. Pulmonary sequestration: a retrospective analysis of 2,625 cases in China. Eur J Cardiothorac Surg 2011; 40:e39–e42.
- Patel SR, Meeker DP, Biscotti CV, Kirby TJ, Rice TW. Presentation and management of bronchogenic cysts in the adult. Chest 1994; 106:79–85.
- Limaïem F, Ayadi-Kaddour A, Djilani H, Kilani T, El Mezni F. Pulmonary and mediastinal bronchogenic cysts: a clinicopathologic study of 33 cases. Lung 2008; 186:55–61.
- Liu HS, Li SQ, Cao ZL, Zhang ZY, Ren H. Clinical features and treatment of bronchogenic cyst in adults. Chin Med Sci J 2009; 24:60–63.
- Jenkins DJ, Romig T, Thompson RC. Emergence/re-emergence of Echinococcus spp.—a global update. Int J Parasitol 2005; 35:1205–1219.
- Riccardi VM. Von Recklinghausen neurofibromatosis. N Engl J Med 1981; 305:1617–1627.
- Toro JR, Pautler SE, Stewart L, et al. Lung cysts, spontaneous pneumothorax, and genetic associations in 89 families with Birt-Hogg-Dubé syndrome. Am J Respir Crit Care Med 2007; 175:1044–1053.
- Biko DM, Schwartz M, Anupindi SA, Altes TA. Subpleural lung cysts in Down syndrome: prevalence and association with coexisting diagnoses. Pediatr Radiol 2008; 38:280–284.
- Colombat M, Stern M, Groussard O, et al. Pulmonary cystic disorder related to light chain deposition disease. Am J Respir Crit Care Med 2006; 173:777–780.
- Ohdama S, Akagawa S, Matsubara O, Yoshizawa Y. Primary diffuse alveolar septal amyloidosis with multiple cysts and calcification. Eur Respir J 1996; 9:1569–1571.
- Johnson SR, Tattersfield AE. Clinical experience of lymphangioleiomyomatosis in the UK. Thorax 2000; 55:1052–1057.
- Kitaichi M, Nishimura K, Itoh H, Izumi T. Pulmonary lymphangioleiomyomatosis: a report of 46 patients including a clinicopathologic study of prognostic factors. Am J Respir Crit Care Med 1995; 151:527–533.
- Urban T, Lazor R, Lacronique J, et al. Pulmonary lymphangioleiomyomatosis. A study of 69 patients. Groupe d’Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM”O”P). Medicine (Baltimore) 1999; 78:321–337.
- Schönfeld N, Frank W, Wenig S, et al. Clinical and radiologic features, lung function and therapeutic results in pulmonary histiocytosis X. Respiration 1993; 60:38–44.
- Lacronique J, Roth C, Battesti JP, Basset F, Chretien J. Chest radiological features of pulmonary histiocytosis X: a report based on 50 adult cases. Thorax 1982; 37:104–109.
- Kluger N, Giraud S, Coupier I, et al. Birt-Hogg-Dubé syndrome: clinical and genetic studies of 10 French families. Br J Dermatol 2010; 162:527–537.
- Tobino K, Gunji Y, Kurihara M, et al. Characteristics of pulmonary cysts in Birt-Hogg-Dubé syndrome: thin-section CT findings of the chest in 12 patients. Eur J Radiol 2011; 77:403–409.
- Hartman TE, Primack SL, Swensen SJ, Hansell D, McGuinness G, Müller NL. Desquamative interstitial pneumonia: thin-section CT findings in 22 patients. Radiology 1993; 187:787–790.
- Koyama M, Johkoh T, Honda O, et al. Chronic cystic lung disease: diagnostic accuracy of high-resolution CT in 92 patients. AJR Am J Roentgenol 2003; 180:827–835.
- Patz EF Jr, Müller NL, Swensen SJ, Dodd LG. Congenital cystic adenomatoid malformation in adults: CT findings. J Comput Assist Tomogr 1995; 19:361–364.
- Conran RM, Stocker JT. Extralobar sequestration with frequently associated congenital cystic adenomatoid malformation, type 2: report of 50 cases. Pediatr Dev Pathol 1999; 2:454–463.
- Kennedy CA, Goetz MB. Atypical roentgenographic manifestations of Pneumocystis carinii pneumonia. Arch Intern Med 1992; 152:1390–1398.
- Sandhu JS, Goodman PC. Pulmonary cysts associated with Pneumocystis carinii pneumonia in patients with AIDS. Radiology 1989; 173:33–35.
- Doğan R, Yüksel M, Cetin G, et al. Surgical treatment of hydatid cysts of the lung: report on 1,055 patients. Thorax 1989; 44:192–199.
- Salih OK, Topcuoğlu MS, Celik SK, Ulus T, Tokcan A. Surgical treatment of hydatid cysts of the lung: analysis of 405 patients. Can J Surg 1998; 41:131–135.
- Ohdama S, Akagawa S, Matsubara O, Yoshizawa Y. Primary diffuse alveolar septal amyloidosis with multiple cysts and calcification. Eur Respir J 1996; 9:1569–1571.
- Sakai M, Yamaoka M, Kawaguchi M, Hizawa N, Sato Y. Multiple cystic pulmonary amyloidosis. Ann Thorac Surg 2011; 92:e109.
- Colombat M, Caudroy S, Lagonotte E, et al. Pathomechanisms of cyst formation in pulmonary light chain deposition disease. Eur Respir J 2008; 32:1399–1403.
- Zamora AC, Collard HR, Wolters PJ, Webb WR, King TE. Neurofibromatosis-associated lung disease: a case series and literature review. Eur Respir J 2007; 29:210–214.
- Oikonomou A, Vadikolias K, Birbilis T, Bouros D, Prassopoulos P. HRCT findings in the lungs of non-smokers with neurofibromatosis. Eur J Radiol 2011; 80:e520–e523.
KEY POINTS
- Pulmonary cysts should be differentiated from cyst-mimics.
- Adults with cystic lung disease can be grouped by the clinical presentation: ie, insidious dyspnea or spontaneous pneumothorax; incidentally found cysts or recurrent pneumonia; signs and symptoms of primary pulmonary infection; or signs and symptoms that are primarily nonpulmonary.
- Characterization of pulmonary cysts and their distribution plays a key role in diagnosis. Radiographically, cystic lung disease can be subclassified into two major categories according to the distribution of cysts: discrete (focal or multifocal) and diffuse (unilobular or panlobular).
Cutaneous Burn Caused by Radiofrequency Ablation Probe During Shoulder Arthroscopy
Cautery and radiofrequency ablation (RFA) devices are commonly used in shoulder arthroscopic surgery for hemostasis and ablation of soft tissue. Although these devices are easily used and applied, complications (eg, extensive release of deltoid muscle,1 nerve damage,2 tendon damage,3 cartilage damage from heat transfer4) can occur during arthroscopic surgery. Radiofrequency devices can elevate fluid temperatures to unsafe levels and directly or indirectly injure surrounding tissue.5,6 Skin complications from using these devices include direct burns to the subcutaneous tissues from the joint to the skin surface7 and skin burns related to overheated arthroscopic fluid.8
In our English-language literature review, however, we found no report of a skin burn secondary to contact between a RFA device and a spinal needle used in identifying structures during an arthroscopic acromioplasty. We report such a case here. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman injured her left, nondominant shoulder when a descending garage door hit her directly on the superior aspect of the shoulder. She had immediate onset of pain on the top and lateral side of the shoulder and was evaluated by a primary care physician. Radiographs and magnetic resonance imaging (MRI) were normal. The patient was referred to an orthopedic surgeon for further evaluation.
The orthopedic surgeon found her to be in good health, with no history of diabetes, vascular conditions, or skin disorders. The initial diagnosis after history taking and physical examination was impingement syndrome with subacromial bursitis. The surgeon recommended nonoperative treatment: ice, nonsteroidal anti-inflammatory drugs, and physical therapy. After 3 months, the patient’s examination was unchanged, and there was no improvement in pain. Cortisone injected into the subacromial space helped for a few weeks, but the pain returned. After 2 more cortisone injections over 9 months failed, repeat MRI showed no tears of the rotator cuff or any other salient abnormalities. The treatment options were discussed with the patient, and, because the physical examination findings were consistent with impingement syndrome and nonoperative measures had failed, she consented to arthroscopic evaluation of the shoulder and arthroscopic partial anterior-lateral acromioplasty.
The procedure was performed 8 months after initial injury. With the patient under general anesthesia and in a lateral decubitus position, her arm was placed in an arm holder. Before the partial acromioplasty, two 18-gauge spinal needles were inserted from the skin surface into the subacromial space to help localize the anterolateral acromion and the acromioclavicular joint. The procedure was performed with a pump using saline bags kept at room temperature. A bipolar radiofrequency device (Stryker Energy Radiofrequency Ablation System; Stryker, Mahwah, New Jersey) was used to débride the subacromial bursa and the periosteum of the undersurface of the acromion. While the bursa was being débrided, the radiofrequency device inadvertently touched the anterior lateral needle probe, and a small skin burn formed around the needle on the surface of the shoulder (Figure). The radiofrequency device did not directly contact the skin, and the deltoid fascia was intact. The spinal needle was removed, and the skin around the burn was excised; the muscle beneath the skin was intact and showed no signs of thermal damage. The skin was mobilized and closed with interrupted simple sutures using a 4-0 nylon suture. The procedure was then completed with no other complications.
After surgery, the patient recovered without complications, and the skin lesion healed with no signs of infection and no skin or muscle defects. Some stiffness was treated with medication and physical therapy. Nine months after surgery, the patient reported mild shoulder stiffness and remained dissatisfied with the appearance of the skin in the area of the burn.
Discussion
Our patient’s case is a reminder that contact between a radiofrequency device and metal needles can transfer heat to tissues and cause skin burns. When using a radiofrequency device around metal needles or cannulas, surgeons should be sure to avoid prolonged contact with the metal. Our patient’s case is the first reported case of a thermal skin injury occurring when a spinal needle was heated by an arthroscopic ablater.
Other authors have reported indirect thermal skin injuries caused by radiofrequency devices during arthroscopic surgery, but the causes were postulated to be direct contact between device and skin7 and overheating of the arthroscopy fluid.5,6,8 Huang and colleagues8 reported that full-thickness skin burns occurred when normal saline used during routine knee arthroscopy overheated from use of a radiofrequency device. Burn lesions, noted on their patient’s leg within 1 day after surgery, required subsequent débridement, a muscle flap, and split-skin grafting. Skin burns caused by overheated fluid have occurred irrespective of type of fluid used (eg, 1.5% glycine or lactated Ringer solution).6 There was no evidence that our patient’s burn resulted from extravasated overheated fluid, as the lesion was localized to the area immediately around the needle and was not geographic, as was described by Huang and colleagues.8
Other possible causes of skin burns during arthroscopic surgery have been described, but none applies in our patient’s case. Segami and colleagues7 described a burn resulting from direct transfer of heat from the radiofrequency device to the skin because of their proximity. This mechanism was not the cause in our patient’s case; there was no evidence of a defect or burned deltoid muscle at time of surgery. Lau and Dao9 reported 2 small full-thickness skin burns caused by a fiberoptic-light cable tip placed on a patient’s leg; in addition, the hot (>170°C) cables caused the paper drapes to combust.9 Skin burns secondary to use of skin antiseptics have been reported,10 but such lesions typically are located beneath tourniquets or in areas of friction from surgical drapes. In some cases, lesions described as skin burns may actually have been pressure lesions secondary to moist skin and friction.11
Whether type of radiofrequency device contributes to the occurrence of heat-related lesions during arthroscopic surgery is unknown. Some investigators have suggested there is more potential for harm with bipolar RFA devices than with monopolar devices.12,13 Monopolar devices pass energy between a probe and a grounding plate, whereas bipolar devices pass energy through 2 points on the probe.14 Because the heat for the monopolar probe derives from the frictional resistance of tissues to each other rather than from the probe itself, the bipolar probe theoretically allows for better temperature control. In addition, bipolar probes require less current to achieve the same heating effect. However, recent studies have suggested that, compared with monopolar radiofrequency devices, bipolar radiofrequency devices are associated with larger increases in temperature at equal depths after an equal number of applications.12,13
To our knowledge, no one has specifically investigated the type of bipolar device used in the present case. This case report, the first to describe a thermal skin injury caused by direct contact between a radiofrequency device and a metal needle inserted in the skin, is a reminder that contact between radiofrequency devices and spinal needles or other metal cannulas used in arthroscopic surgery should be avoided.
1. Bonsell S. Detached deltoid during arthroscopic subacromial decompression. Arthroscopy. 2000;16(7):745-748.
2. Mohammed KD, Hayes MG, Saies AD. Unusual complications of shoulder arthroscopy. J Shoulder Elbow Surg. 2000;9(4):350-353.
3. Pell RF 4th, Uhl RL. Complications of thermal ablation in wrist arthroscopy. Arthroscopy. 2004;20(suppl 2):84-86.
4. Lu Y, Hayashi K, Hecht P, et al. The effect of monopolar radiofrequency energy on partial-thickness defects of articular cartilage. Arthroscopy. 2000;16(5):527-536.
5. Kouk SN, Zoric B, Stetson WB. Complication of the use of a radiofrequency device in arthroscopic shoulder surgery: second-degree burn of the shoulder girdle. Arthroscopy. 2011;27(1):136-141.
6. Lord MJ, Maltry JA, Shall LM. Thermal injury resulting from arthroscopic lateral retinacular release by electrocautery: report of three cases and a review of the literature. Arthroscopy. 1991;7(1):33-37.
7. Segami N, Yamada T, Nishimura M. Thermal injury during temporomandibular joint arthroscopy: a case report. J Oral Maxillofac Surg. 2004;62(4):508-510.
8. Huang S, Gateley D, Moss ALH. Accidental burn injury during knee arthroscopy. Arthroscopy. 2007;23(12):1363.e1-e3.
9. Lau YJ, Dao Q. Cutaneous burns from a fiberoptic cable tip during arthroscopy of the knee. Knee. 2008;15(4):333-335.
10. Sanders TH, Hawken SM. Chlorhexidine burns after shoulder arthroscopy. Am J Orthop. 2012;41(4):172-174.
11. Keyurapan E, Hu SJ, Redett R, McCarthy EF, McFarland EG. Pressure ulcers of the thorax after shoulder surgery. Knee Surg Sports Traumatol Arthrosc. 2007;15(12):1489-1493.
12. Edwards RB 3rd, Lu Y, Rodriguez E, Markel MD. Thermometric determination of cartilage matrix temperatures during thermal chondroplasty: comparison of bipolar and monopolar radiofrequency devices. Arthroscopy. 2002;18(4):339-346.
13. Figueroa D, Calvo R, Vaisman A, et al. Bipolar radiofrequency in the human meniscus. Comparative study between patients younger and older than 40 years of age. Knee. 2007;14(5):357-360.
14. Sahasrabudhe A, McMahon PJ. Thermal probes: what’s available in 2004. Oper Tech Sports Med. 2004;12:206-209.
Cautery and radiofrequency ablation (RFA) devices are commonly used in shoulder arthroscopic surgery for hemostasis and ablation of soft tissue. Although these devices are easily used and applied, complications (eg, extensive release of deltoid muscle,1 nerve damage,2 tendon damage,3 cartilage damage from heat transfer4) can occur during arthroscopic surgery. Radiofrequency devices can elevate fluid temperatures to unsafe levels and directly or indirectly injure surrounding tissue.5,6 Skin complications from using these devices include direct burns to the subcutaneous tissues from the joint to the skin surface7 and skin burns related to overheated arthroscopic fluid.8
In our English-language literature review, however, we found no report of a skin burn secondary to contact between a RFA device and a spinal needle used in identifying structures during an arthroscopic acromioplasty. We report such a case here. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman injured her left, nondominant shoulder when a descending garage door hit her directly on the superior aspect of the shoulder. She had immediate onset of pain on the top and lateral side of the shoulder and was evaluated by a primary care physician. Radiographs and magnetic resonance imaging (MRI) were normal. The patient was referred to an orthopedic surgeon for further evaluation.
The orthopedic surgeon found her to be in good health, with no history of diabetes, vascular conditions, or skin disorders. The initial diagnosis after history taking and physical examination was impingement syndrome with subacromial bursitis. The surgeon recommended nonoperative treatment: ice, nonsteroidal anti-inflammatory drugs, and physical therapy. After 3 months, the patient’s examination was unchanged, and there was no improvement in pain. Cortisone injected into the subacromial space helped for a few weeks, but the pain returned. After 2 more cortisone injections over 9 months failed, repeat MRI showed no tears of the rotator cuff or any other salient abnormalities. The treatment options were discussed with the patient, and, because the physical examination findings were consistent with impingement syndrome and nonoperative measures had failed, she consented to arthroscopic evaluation of the shoulder and arthroscopic partial anterior-lateral acromioplasty.
The procedure was performed 8 months after initial injury. With the patient under general anesthesia and in a lateral decubitus position, her arm was placed in an arm holder. Before the partial acromioplasty, two 18-gauge spinal needles were inserted from the skin surface into the subacromial space to help localize the anterolateral acromion and the acromioclavicular joint. The procedure was performed with a pump using saline bags kept at room temperature. A bipolar radiofrequency device (Stryker Energy Radiofrequency Ablation System; Stryker, Mahwah, New Jersey) was used to débride the subacromial bursa and the periosteum of the undersurface of the acromion. While the bursa was being débrided, the radiofrequency device inadvertently touched the anterior lateral needle probe, and a small skin burn formed around the needle on the surface of the shoulder (Figure). The radiofrequency device did not directly contact the skin, and the deltoid fascia was intact. The spinal needle was removed, and the skin around the burn was excised; the muscle beneath the skin was intact and showed no signs of thermal damage. The skin was mobilized and closed with interrupted simple sutures using a 4-0 nylon suture. The procedure was then completed with no other complications.
After surgery, the patient recovered without complications, and the skin lesion healed with no signs of infection and no skin or muscle defects. Some stiffness was treated with medication and physical therapy. Nine months after surgery, the patient reported mild shoulder stiffness and remained dissatisfied with the appearance of the skin in the area of the burn.
Discussion
Our patient’s case is a reminder that contact between a radiofrequency device and metal needles can transfer heat to tissues and cause skin burns. When using a radiofrequency device around metal needles or cannulas, surgeons should be sure to avoid prolonged contact with the metal. Our patient’s case is the first reported case of a thermal skin injury occurring when a spinal needle was heated by an arthroscopic ablater.
Other authors have reported indirect thermal skin injuries caused by radiofrequency devices during arthroscopic surgery, but the causes were postulated to be direct contact between device and skin7 and overheating of the arthroscopy fluid.5,6,8 Huang and colleagues8 reported that full-thickness skin burns occurred when normal saline used during routine knee arthroscopy overheated from use of a radiofrequency device. Burn lesions, noted on their patient’s leg within 1 day after surgery, required subsequent débridement, a muscle flap, and split-skin grafting. Skin burns caused by overheated fluid have occurred irrespective of type of fluid used (eg, 1.5% glycine or lactated Ringer solution).6 There was no evidence that our patient’s burn resulted from extravasated overheated fluid, as the lesion was localized to the area immediately around the needle and was not geographic, as was described by Huang and colleagues.8
Other possible causes of skin burns during arthroscopic surgery have been described, but none applies in our patient’s case. Segami and colleagues7 described a burn resulting from direct transfer of heat from the radiofrequency device to the skin because of their proximity. This mechanism was not the cause in our patient’s case; there was no evidence of a defect or burned deltoid muscle at time of surgery. Lau and Dao9 reported 2 small full-thickness skin burns caused by a fiberoptic-light cable tip placed on a patient’s leg; in addition, the hot (>170°C) cables caused the paper drapes to combust.9 Skin burns secondary to use of skin antiseptics have been reported,10 but such lesions typically are located beneath tourniquets or in areas of friction from surgical drapes. In some cases, lesions described as skin burns may actually have been pressure lesions secondary to moist skin and friction.11
Whether type of radiofrequency device contributes to the occurrence of heat-related lesions during arthroscopic surgery is unknown. Some investigators have suggested there is more potential for harm with bipolar RFA devices than with monopolar devices.12,13 Monopolar devices pass energy between a probe and a grounding plate, whereas bipolar devices pass energy through 2 points on the probe.14 Because the heat for the monopolar probe derives from the frictional resistance of tissues to each other rather than from the probe itself, the bipolar probe theoretically allows for better temperature control. In addition, bipolar probes require less current to achieve the same heating effect. However, recent studies have suggested that, compared with monopolar radiofrequency devices, bipolar radiofrequency devices are associated with larger increases in temperature at equal depths after an equal number of applications.12,13
To our knowledge, no one has specifically investigated the type of bipolar device used in the present case. This case report, the first to describe a thermal skin injury caused by direct contact between a radiofrequency device and a metal needle inserted in the skin, is a reminder that contact between radiofrequency devices and spinal needles or other metal cannulas used in arthroscopic surgery should be avoided.
Cautery and radiofrequency ablation (RFA) devices are commonly used in shoulder arthroscopic surgery for hemostasis and ablation of soft tissue. Although these devices are easily used and applied, complications (eg, extensive release of deltoid muscle,1 nerve damage,2 tendon damage,3 cartilage damage from heat transfer4) can occur during arthroscopic surgery. Radiofrequency devices can elevate fluid temperatures to unsafe levels and directly or indirectly injure surrounding tissue.5,6 Skin complications from using these devices include direct burns to the subcutaneous tissues from the joint to the skin surface7 and skin burns related to overheated arthroscopic fluid.8
In our English-language literature review, however, we found no report of a skin burn secondary to contact between a RFA device and a spinal needle used in identifying structures during an arthroscopic acromioplasty. We report such a case here. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman injured her left, nondominant shoulder when a descending garage door hit her directly on the superior aspect of the shoulder. She had immediate onset of pain on the top and lateral side of the shoulder and was evaluated by a primary care physician. Radiographs and magnetic resonance imaging (MRI) were normal. The patient was referred to an orthopedic surgeon for further evaluation.
The orthopedic surgeon found her to be in good health, with no history of diabetes, vascular conditions, or skin disorders. The initial diagnosis after history taking and physical examination was impingement syndrome with subacromial bursitis. The surgeon recommended nonoperative treatment: ice, nonsteroidal anti-inflammatory drugs, and physical therapy. After 3 months, the patient’s examination was unchanged, and there was no improvement in pain. Cortisone injected into the subacromial space helped for a few weeks, but the pain returned. After 2 more cortisone injections over 9 months failed, repeat MRI showed no tears of the rotator cuff or any other salient abnormalities. The treatment options were discussed with the patient, and, because the physical examination findings were consistent with impingement syndrome and nonoperative measures had failed, she consented to arthroscopic evaluation of the shoulder and arthroscopic partial anterior-lateral acromioplasty.
The procedure was performed 8 months after initial injury. With the patient under general anesthesia and in a lateral decubitus position, her arm was placed in an arm holder. Before the partial acromioplasty, two 18-gauge spinal needles were inserted from the skin surface into the subacromial space to help localize the anterolateral acromion and the acromioclavicular joint. The procedure was performed with a pump using saline bags kept at room temperature. A bipolar radiofrequency device (Stryker Energy Radiofrequency Ablation System; Stryker, Mahwah, New Jersey) was used to débride the subacromial bursa and the periosteum of the undersurface of the acromion. While the bursa was being débrided, the radiofrequency device inadvertently touched the anterior lateral needle probe, and a small skin burn formed around the needle on the surface of the shoulder (Figure). The radiofrequency device did not directly contact the skin, and the deltoid fascia was intact. The spinal needle was removed, and the skin around the burn was excised; the muscle beneath the skin was intact and showed no signs of thermal damage. The skin was mobilized and closed with interrupted simple sutures using a 4-0 nylon suture. The procedure was then completed with no other complications.
After surgery, the patient recovered without complications, and the skin lesion healed with no signs of infection and no skin or muscle defects. Some stiffness was treated with medication and physical therapy. Nine months after surgery, the patient reported mild shoulder stiffness and remained dissatisfied with the appearance of the skin in the area of the burn.
Discussion
Our patient’s case is a reminder that contact between a radiofrequency device and metal needles can transfer heat to tissues and cause skin burns. When using a radiofrequency device around metal needles or cannulas, surgeons should be sure to avoid prolonged contact with the metal. Our patient’s case is the first reported case of a thermal skin injury occurring when a spinal needle was heated by an arthroscopic ablater.
Other authors have reported indirect thermal skin injuries caused by radiofrequency devices during arthroscopic surgery, but the causes were postulated to be direct contact between device and skin7 and overheating of the arthroscopy fluid.5,6,8 Huang and colleagues8 reported that full-thickness skin burns occurred when normal saline used during routine knee arthroscopy overheated from use of a radiofrequency device. Burn lesions, noted on their patient’s leg within 1 day after surgery, required subsequent débridement, a muscle flap, and split-skin grafting. Skin burns caused by overheated fluid have occurred irrespective of type of fluid used (eg, 1.5% glycine or lactated Ringer solution).6 There was no evidence that our patient’s burn resulted from extravasated overheated fluid, as the lesion was localized to the area immediately around the needle and was not geographic, as was described by Huang and colleagues.8
Other possible causes of skin burns during arthroscopic surgery have been described, but none applies in our patient’s case. Segami and colleagues7 described a burn resulting from direct transfer of heat from the radiofrequency device to the skin because of their proximity. This mechanism was not the cause in our patient’s case; there was no evidence of a defect or burned deltoid muscle at time of surgery. Lau and Dao9 reported 2 small full-thickness skin burns caused by a fiberoptic-light cable tip placed on a patient’s leg; in addition, the hot (>170°C) cables caused the paper drapes to combust.9 Skin burns secondary to use of skin antiseptics have been reported,10 but such lesions typically are located beneath tourniquets or in areas of friction from surgical drapes. In some cases, lesions described as skin burns may actually have been pressure lesions secondary to moist skin and friction.11
Whether type of radiofrequency device contributes to the occurrence of heat-related lesions during arthroscopic surgery is unknown. Some investigators have suggested there is more potential for harm with bipolar RFA devices than with monopolar devices.12,13 Monopolar devices pass energy between a probe and a grounding plate, whereas bipolar devices pass energy through 2 points on the probe.14 Because the heat for the monopolar probe derives from the frictional resistance of tissues to each other rather than from the probe itself, the bipolar probe theoretically allows for better temperature control. In addition, bipolar probes require less current to achieve the same heating effect. However, recent studies have suggested that, compared with monopolar radiofrequency devices, bipolar radiofrequency devices are associated with larger increases in temperature at equal depths after an equal number of applications.12,13
To our knowledge, no one has specifically investigated the type of bipolar device used in the present case. This case report, the first to describe a thermal skin injury caused by direct contact between a radiofrequency device and a metal needle inserted in the skin, is a reminder that contact between radiofrequency devices and spinal needles or other metal cannulas used in arthroscopic surgery should be avoided.
1. Bonsell S. Detached deltoid during arthroscopic subacromial decompression. Arthroscopy. 2000;16(7):745-748.
2. Mohammed KD, Hayes MG, Saies AD. Unusual complications of shoulder arthroscopy. J Shoulder Elbow Surg. 2000;9(4):350-353.
3. Pell RF 4th, Uhl RL. Complications of thermal ablation in wrist arthroscopy. Arthroscopy. 2004;20(suppl 2):84-86.
4. Lu Y, Hayashi K, Hecht P, et al. The effect of monopolar radiofrequency energy on partial-thickness defects of articular cartilage. Arthroscopy. 2000;16(5):527-536.
5. Kouk SN, Zoric B, Stetson WB. Complication of the use of a radiofrequency device in arthroscopic shoulder surgery: second-degree burn of the shoulder girdle. Arthroscopy. 2011;27(1):136-141.
6. Lord MJ, Maltry JA, Shall LM. Thermal injury resulting from arthroscopic lateral retinacular release by electrocautery: report of three cases and a review of the literature. Arthroscopy. 1991;7(1):33-37.
7. Segami N, Yamada T, Nishimura M. Thermal injury during temporomandibular joint arthroscopy: a case report. J Oral Maxillofac Surg. 2004;62(4):508-510.
8. Huang S, Gateley D, Moss ALH. Accidental burn injury during knee arthroscopy. Arthroscopy. 2007;23(12):1363.e1-e3.
9. Lau YJ, Dao Q. Cutaneous burns from a fiberoptic cable tip during arthroscopy of the knee. Knee. 2008;15(4):333-335.
10. Sanders TH, Hawken SM. Chlorhexidine burns after shoulder arthroscopy. Am J Orthop. 2012;41(4):172-174.
11. Keyurapan E, Hu SJ, Redett R, McCarthy EF, McFarland EG. Pressure ulcers of the thorax after shoulder surgery. Knee Surg Sports Traumatol Arthrosc. 2007;15(12):1489-1493.
12. Edwards RB 3rd, Lu Y, Rodriguez E, Markel MD. Thermometric determination of cartilage matrix temperatures during thermal chondroplasty: comparison of bipolar and monopolar radiofrequency devices. Arthroscopy. 2002;18(4):339-346.
13. Figueroa D, Calvo R, Vaisman A, et al. Bipolar radiofrequency in the human meniscus. Comparative study between patients younger and older than 40 years of age. Knee. 2007;14(5):357-360.
14. Sahasrabudhe A, McMahon PJ. Thermal probes: what’s available in 2004. Oper Tech Sports Med. 2004;12:206-209.
1. Bonsell S. Detached deltoid during arthroscopic subacromial decompression. Arthroscopy. 2000;16(7):745-748.
2. Mohammed KD, Hayes MG, Saies AD. Unusual complications of shoulder arthroscopy. J Shoulder Elbow Surg. 2000;9(4):350-353.
3. Pell RF 4th, Uhl RL. Complications of thermal ablation in wrist arthroscopy. Arthroscopy. 2004;20(suppl 2):84-86.
4. Lu Y, Hayashi K, Hecht P, et al. The effect of monopolar radiofrequency energy on partial-thickness defects of articular cartilage. Arthroscopy. 2000;16(5):527-536.
5. Kouk SN, Zoric B, Stetson WB. Complication of the use of a radiofrequency device in arthroscopic shoulder surgery: second-degree burn of the shoulder girdle. Arthroscopy. 2011;27(1):136-141.
6. Lord MJ, Maltry JA, Shall LM. Thermal injury resulting from arthroscopic lateral retinacular release by electrocautery: report of three cases and a review of the literature. Arthroscopy. 1991;7(1):33-37.
7. Segami N, Yamada T, Nishimura M. Thermal injury during temporomandibular joint arthroscopy: a case report. J Oral Maxillofac Surg. 2004;62(4):508-510.
8. Huang S, Gateley D, Moss ALH. Accidental burn injury during knee arthroscopy. Arthroscopy. 2007;23(12):1363.e1-e3.
9. Lau YJ, Dao Q. Cutaneous burns from a fiberoptic cable tip during arthroscopy of the knee. Knee. 2008;15(4):333-335.
10. Sanders TH, Hawken SM. Chlorhexidine burns after shoulder arthroscopy. Am J Orthop. 2012;41(4):172-174.
11. Keyurapan E, Hu SJ, Redett R, McCarthy EF, McFarland EG. Pressure ulcers of the thorax after shoulder surgery. Knee Surg Sports Traumatol Arthrosc. 2007;15(12):1489-1493.
12. Edwards RB 3rd, Lu Y, Rodriguez E, Markel MD. Thermometric determination of cartilage matrix temperatures during thermal chondroplasty: comparison of bipolar and monopolar radiofrequency devices. Arthroscopy. 2002;18(4):339-346.
13. Figueroa D, Calvo R, Vaisman A, et al. Bipolar radiofrequency in the human meniscus. Comparative study between patients younger and older than 40 years of age. Knee. 2007;14(5):357-360.
14. Sahasrabudhe A, McMahon PJ. Thermal probes: what’s available in 2004. Oper Tech Sports Med. 2004;12:206-209.
Interobserver Agreement Using Computed Tomography to Assess Radiographic Fusion Criteria With a Unique Titanium Interbody Device
The accuracy of using computed tomography (CT) to assess lumbar interbody fusion with titanium implants has been questioned in the past.1-4 Reports have most often focused on older technologies using paired, threaded, smooth-surface titanium devices. Some authors have reported they could not confidently assess the quality of fusions using CT because of implant artifact.1-3
When pseudarthrosis is suspected clinically, and imaging results are inconclusive, surgical explorations may be performed with mechanical stressing of the segment to assess for motion.2,5-7 However, surgical exploration not only has the morbidity of another surgery but may not be conclusive. Direct exploration of an interbody fusion is problematic. In some cases, there may be residual normal springing motion through posterior elements, even in the presence of a solid interbody fusion, which can be confusing.5 Radiologic confirmation of fusion status is therefore preferred over surgical exploration. CT is the imaging modality used most often to assess spinal fusions.8,9
A new titanium interbody fusion implant (Endoskeleton TA; Titan Spine, Mequon, Wisconsin) preserves the endplate and has an acid-etched titanium surface for osseous integration and a wide central aperture for bone graft (Figure 1). Compared with earlier titanium implants, this design may allow for more accurate CT imaging and fusion assessment. We conducted a study to determine the interobserver reliability of using CT to evaluate bone formation and other radiographic variables with this new titanium interbody device.
Materials and Methods
After receiving institutional review board approval for this study, as well as patient consent, we obtained and analyzed CT scans of patients after they had undergone anterior lumbar interbody fusion (ALIF) at L3–S1 as part of a separate clinical outcomes study.
Each patient received an Endoskeleton TA implant. The fusion cage was packed with 2 sponges (3.0 mg per fusion level) of bone morphogenetic protein, or BMP (InFuse; Medtronic, Minneapolis, Minnesota). In addition, 1 to 3 cm3 of hydroxyapatite/β-tricalcium phosphate (MasterGraft, Medtronic) collagen sponge was used as graft extender to fill any remaining gaps within the cage. Pedicle screw fixation was used in all cases.
Patients were randomly assigned to have fine-cut CT scans with reconstructed images at 6, 9, or 12 months. The scans were reviewed by 2 independent radiologists who were blinded to each other’s interpretations and the clinical results. The radiographic fusion criteria are listed in Tables 1 to 3. Interobserver agreement (κ) was calculated separately for each radiographic criterion and could range from 0.00 (no agreement) to 1.00 (perfect agreement).10,11
Results
The study involved 33 patients (17 men, 16 women) with 56 lumbar spinal fusion levels. Mean age was 46 years (range, 23-66 years). Six patients (18%) were nicotine users. Seventeen patients were scanned at 6 months, 9 at 9 months, and 7 at 12 months. There were no significant differences in results between men and women, between nicotine users and nonusers, or among patients evaluated at 6, 9, or 12 months.
The radiologists agreed on 345 of the 392 data points reviewed (κ = 0.88). Interobserver agreement results for the fusion criteria are listed in Tables 1 and 3. Interobserver agreement was 0.77 for overall fusion grade, with the radiologists noting definite fusion (grade 5) in 80% and 91% of the levels (Table 1). Other radiographic criteria are listed in Tables 2 and 3. Interobserver agreement was 0.80 for degree of artifact, 0.95 for subsidence, 0.96 for both lucency and trabecular bone, 0.77 for anterior osseous bridging, and 0.95 for cystic vertebral changes.
Discussion
Radiographic analysis of interbody fusions is an important clinical issue. Investigators have shown that CT is the radiographic method of choice for assessing fusion.8,9 Others have reported that assessing fusion with metallic interbody implants is more difficult compared with PEEK (polyether ether ketone) or allograft bone.3,4,5,12
Heithoff and colleagues1,2 reported on difficulties they encountered in assessing interbody fusion with titanium implants, and their research has often been cited. The authors concluded that they could not accurately assess fusion in these cases because of artifact from the small apertures in the cages and metallic scatter. Their study was very small (8 patients, 12 surgical levels) and used paired BAK (Bagby and Kuslich) cages (Zimmer, Warsaw, Indiana).
Recently, a unique surface technology, used to manufacture osseointegrative dental implants, has been adapted for use in the spine.13-15 Acid etching modifies the surface of titanium to create a nano-scale (micron-level) alteration. Compared with PEEK and smooth titanium, acid-etched titanium stimulates a better osteogenic environment.16,17 As this technology is now used clinically in spinal surgery, we thought it important to revisit the issue of CT analysis for fusion assessment with the newer titanium implants.
Artifact
The results of our study support the idea that the design of a titanium interbody fusion implant is important to radiographic analysis. The implant studied has a large open central aperture that appears to generate less artifact than historical controls (paired cylindrical cages) have.1-4 Other investigators have reported fewer problems with artifact in their studies of implants incorporating larger openings for bone graft.6,18 The radiologists in the present study found no significant problems with artifact. Less artifact is clinically important, as the remaining fusion variables can be more clearly visualized (Table 2, Figure 2).
Anterior Osseous Bridging, Subsidence, Lysis
In this study, the bony endplates were preserved. The disc and endplate cartilage was removed without reaming or drilling. Endplate reaming most likely contributes to subsidence and loss of original fixation between implant and bone interface.1,4,12 Some authors have advocated recessing the cages deeply and then packing bone anteriorly to create a “sentinel fusion sign.”1,2,6 Deeply seating interbody implants, instead of resting them more widely on the apophyseal ring of the vertebral endplate, may also lead to subsidence.4,12 The issue of identifying a sentinel fusion sign is relevant only if the surgeon tries to create one. In the present study, the implant used was an impacted cage positioned on the apophyseal perimeter of the disc space, just slightly recessed, so there was no attempt to create a sentinel fusion sign, as reflected in the relatively low scores on anterior osseous bridging (48%, 52%).
Subsidence and peri-implant lysis are pathologic variables associated with motion and bone loss. Sethi and colleagues19 noted a high percentage of endplate resorption and subsidence in cases reviewed using PEEK or allograft spacers paired with BMP-2. Although BMP-2 was used in the present study, we found very low rates of subsidence (0%, 5%) and no significant peri-implant lucencies (2%, 4%) (Figure 2). Interobserver agreement for these variables was high (0.95, 0.96). We hypothesize that the combination of endplate-sparing surgical technique and implant–bone integration contributed to these results.
Trabecular Bone and Fusion Grade
The primary radiographic criterion for solid interbody fusion is trabecular bone throughout the cage, bridging the vertebral bodies. In our study, the success rates for this variable were 96% and 100%, and there was very high interobserver agreement (0.96) (Figure 3). This very high fusion rate may preclude detecting subtle differences in interobserver agreement, but to what degree, if any, is unknown. Other investigators have effectively identified trabecular bone across the interspace and throughout the cages.6,18 The openings for bone formation were larger in the implants they used than in first-generation fusion cages but not as large as the implant openings in the present study. Larger openings may correlate with improved ability to visualize bridging bone on CT.
Radiologists and surgeons must ultimately arrive at a conclusion regarding the likelihood a fusion has occurred. Our radiologists integrated all the separate radiologic variables cited here, as well as their overall impressions of the scans, to arrive at a final grade regarding fusion quality (Figures 3, 4). Although this category provides the most interpretive latitude of all the variables examined, the results demonstrate high interobserver reliability. Agreement to exactly the same fusion grade was 0.77, and agreement to within 1 category grade was 0.95.
This study had several limitations. Surgical explorations were not clinically indicated and were not performed. There were no suspected nonunions or hardware complications, two of the most common indications for exploration. In addition, this study was conducted not to determine specific accuracy of CT (compared with surgery exploration) for fusion assessment but to assess interobserver reliability. The clinical success rates for this population were high, and no patient required revision surgery for suspected pseudarthrosis. To assess the true accuracy of CT for fusion assessment, one would have to subject patients to follow-up exploratory surgery to test fusions mechanically.
Another limitation is the lack of a single industry-accepted radiographic fusion grading system. Fusion criteria are not standardized across all studies. Our radiologists have extensive research experience and limit their practices to neuromuscular radiology with a concentration on the spine. The radiographic criteria cited here are the same criteria they use in clinical practice, when reviewing CT scans for clinicians. Last, there was no control group for direct comparison against other cages. Historical controls were cited. This does not adversely affect the conclusions of this investigation.
Conclusion
Clinicians have been reluctant to rely on CT with titanium devices because of concerns about the accuracy of image interpretations. The interbody device used in this study demonstrated minimal artifact and minimal subsidence, and trabecular bone was easily identified throughout the implant in the majority of cases reviewed. We found high interobserver agreement scores across all fusion criteria. Although surgical exploration remains the gold standard for fusion assessment, surgeons should have confidence in using CT with this titanium implant.
1. Gilbert TJ, Heithoff KB, Mullin WJ. Radiographic assessment of cage-assisted interbody fusions in the lumbar spine. Semin Spine Surg. 2001;13:311-315.
2. Heithoff KB, Mullin WJ, Renfrew DL, Gilbert TJ. The failure of radiographic detection of pseudarthrosis in patients with titanium lumbar interbody fusion cages. In: Proceedings of the 14th Annual Meeting of the North American Spine Society; October 20-23, 1999; Chicago, IL. Abstract 14.
3. Cizek GR, Boyd LM. Imaging pitfalls of interbody implants. Spine. 2000;25(20):2633-2636.
4. Dorchak JD, Burkus JK, Foor BD, Sanders DL. Dual paired proximity and combined BAK/proximity interbody fusion cages: radiographic results. In: Proceedings of the 15th Annual Meeting of the North American Spine Society. New Orleans, LA: North American Spine Society; 2000:83-85.
5. Santos ER, Goss DG, Morcom RK, Fraser RD. Radiologic assessment of interbody fusion using carbon fiber cages. Spine. 2003;28(10):997-1001.
6. Carreon LY, Glassman SD, Schwender JD, Subach BR, Gornet MF, Ohno S. Reliability and accuracy of fine-cut computed tomography scans to determine the status of anterior interbody fusions with metallic cages. Spine J. 2008;8(6):998-1002.
7. Fogel GR, Toohey JS, Neidre A, Brantigan JW. Fusion assessment of posterior lumbar interbody fusion using radiolucent cages: x-ray films and helical computed tomography scans compared with surgical exploration of fusion. Spine J. 2008;8(4):570-577.
8. Selby MD, Clark SR, Hall DJ, Freeman BJ. Radiologic assessment of spinal fusion. J Am Acad Orthop Surg. 2012;20(11):694-703.
9. Chafetz N, Cann CE, Morris JM, Steinbach LS, Goldberg HI, Ax L. Pseudarthrosis following lumbar fusion: detection by direct coronal CT scanning. Radiology. 1987;162(3):803-805.
10. Landis RJ, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174.
11. Viera AJ, Garrett JM. Understanding interobserver agreement; the kappa statistic. Fam Med. 2005;37(5):360-363.
12. Burkus JK, Foley K, Haid RW, Lehuec JC. Surgical Interbody Research Group—radiographic assessment of interbody fusion devices: fusion criteria for anterior lumbar interbody surgery. Neurosurg Focus. 2001;10(4):E11.
13. Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1(1):11-25.
14. De Leonardis D, Garg AK, Pecora GE. Osseointegration of rough acid-etched titanium implants: 5-year follow-up of 100 Minimatic implants. Int J Oral Maxillofac Implants. 1999;14(3):384-391.
15. Schwartz Z, Raz P, Zhao G, et al. Effect of micrometer-scale roughness on the surface of Ti6Al4V pedicle screws in vitro and in vivo. J Bone Joint Surg Am. 2008;90(11):2485-2498.
16. Olivares-Navarrete R, Gittens RA, Schneider JM, et al. Osteoblasts exhibit a more differentiated phenotype and increased bone morphogenetic protein production on titanium alloy substrates than on poly-ether-ether-ketone. Spine J. 2012;12(3):265-272.
17. Olivares-Navarrete R, Hyzy SL, Gittens RA 1st, et al. Rough titanium alloys regulate osteoblast production of angiogenic factors. Spine J. 2013;13(11):1563-1570.
18. Burkus JK, Dorchak JD, Sanders DL. Radiographic assessment of interbody fusion using recombinant human bone morphogenetic protein type 2. Spine. 2003;28(4):372-377.
19. Sethi A, Craig J, Bartol S, et al. Radiographic and CT evaluation of recombinant human bone morphogenetic protein-2–assisted spinal interbody fusion. AJR Am J Roentgenol. 2011;197(1):W128-W133.
The accuracy of using computed tomography (CT) to assess lumbar interbody fusion with titanium implants has been questioned in the past.1-4 Reports have most often focused on older technologies using paired, threaded, smooth-surface titanium devices. Some authors have reported they could not confidently assess the quality of fusions using CT because of implant artifact.1-3
When pseudarthrosis is suspected clinically, and imaging results are inconclusive, surgical explorations may be performed with mechanical stressing of the segment to assess for motion.2,5-7 However, surgical exploration not only has the morbidity of another surgery but may not be conclusive. Direct exploration of an interbody fusion is problematic. In some cases, there may be residual normal springing motion through posterior elements, even in the presence of a solid interbody fusion, which can be confusing.5 Radiologic confirmation of fusion status is therefore preferred over surgical exploration. CT is the imaging modality used most often to assess spinal fusions.8,9
A new titanium interbody fusion implant (Endoskeleton TA; Titan Spine, Mequon, Wisconsin) preserves the endplate and has an acid-etched titanium surface for osseous integration and a wide central aperture for bone graft (Figure 1). Compared with earlier titanium implants, this design may allow for more accurate CT imaging and fusion assessment. We conducted a study to determine the interobserver reliability of using CT to evaluate bone formation and other radiographic variables with this new titanium interbody device.
Materials and Methods
After receiving institutional review board approval for this study, as well as patient consent, we obtained and analyzed CT scans of patients after they had undergone anterior lumbar interbody fusion (ALIF) at L3–S1 as part of a separate clinical outcomes study.
Each patient received an Endoskeleton TA implant. The fusion cage was packed with 2 sponges (3.0 mg per fusion level) of bone morphogenetic protein, or BMP (InFuse; Medtronic, Minneapolis, Minnesota). In addition, 1 to 3 cm3 of hydroxyapatite/β-tricalcium phosphate (MasterGraft, Medtronic) collagen sponge was used as graft extender to fill any remaining gaps within the cage. Pedicle screw fixation was used in all cases.
Patients were randomly assigned to have fine-cut CT scans with reconstructed images at 6, 9, or 12 months. The scans were reviewed by 2 independent radiologists who were blinded to each other’s interpretations and the clinical results. The radiographic fusion criteria are listed in Tables 1 to 3. Interobserver agreement (κ) was calculated separately for each radiographic criterion and could range from 0.00 (no agreement) to 1.00 (perfect agreement).10,11
Results
The study involved 33 patients (17 men, 16 women) with 56 lumbar spinal fusion levels. Mean age was 46 years (range, 23-66 years). Six patients (18%) were nicotine users. Seventeen patients were scanned at 6 months, 9 at 9 months, and 7 at 12 months. There were no significant differences in results between men and women, between nicotine users and nonusers, or among patients evaluated at 6, 9, or 12 months.
The radiologists agreed on 345 of the 392 data points reviewed (κ = 0.88). Interobserver agreement results for the fusion criteria are listed in Tables 1 and 3. Interobserver agreement was 0.77 for overall fusion grade, with the radiologists noting definite fusion (grade 5) in 80% and 91% of the levels (Table 1). Other radiographic criteria are listed in Tables 2 and 3. Interobserver agreement was 0.80 for degree of artifact, 0.95 for subsidence, 0.96 for both lucency and trabecular bone, 0.77 for anterior osseous bridging, and 0.95 for cystic vertebral changes.
Discussion
Radiographic analysis of interbody fusions is an important clinical issue. Investigators have shown that CT is the radiographic method of choice for assessing fusion.8,9 Others have reported that assessing fusion with metallic interbody implants is more difficult compared with PEEK (polyether ether ketone) or allograft bone.3,4,5,12
Heithoff and colleagues1,2 reported on difficulties they encountered in assessing interbody fusion with titanium implants, and their research has often been cited. The authors concluded that they could not accurately assess fusion in these cases because of artifact from the small apertures in the cages and metallic scatter. Their study was very small (8 patients, 12 surgical levels) and used paired BAK (Bagby and Kuslich) cages (Zimmer, Warsaw, Indiana).
Recently, a unique surface technology, used to manufacture osseointegrative dental implants, has been adapted for use in the spine.13-15 Acid etching modifies the surface of titanium to create a nano-scale (micron-level) alteration. Compared with PEEK and smooth titanium, acid-etched titanium stimulates a better osteogenic environment.16,17 As this technology is now used clinically in spinal surgery, we thought it important to revisit the issue of CT analysis for fusion assessment with the newer titanium implants.
Artifact
The results of our study support the idea that the design of a titanium interbody fusion implant is important to radiographic analysis. The implant studied has a large open central aperture that appears to generate less artifact than historical controls (paired cylindrical cages) have.1-4 Other investigators have reported fewer problems with artifact in their studies of implants incorporating larger openings for bone graft.6,18 The radiologists in the present study found no significant problems with artifact. Less artifact is clinically important, as the remaining fusion variables can be more clearly visualized (Table 2, Figure 2).
Anterior Osseous Bridging, Subsidence, Lysis
In this study, the bony endplates were preserved. The disc and endplate cartilage was removed without reaming or drilling. Endplate reaming most likely contributes to subsidence and loss of original fixation between implant and bone interface.1,4,12 Some authors have advocated recessing the cages deeply and then packing bone anteriorly to create a “sentinel fusion sign.”1,2,6 Deeply seating interbody implants, instead of resting them more widely on the apophyseal ring of the vertebral endplate, may also lead to subsidence.4,12 The issue of identifying a sentinel fusion sign is relevant only if the surgeon tries to create one. In the present study, the implant used was an impacted cage positioned on the apophyseal perimeter of the disc space, just slightly recessed, so there was no attempt to create a sentinel fusion sign, as reflected in the relatively low scores on anterior osseous bridging (48%, 52%).
Subsidence and peri-implant lysis are pathologic variables associated with motion and bone loss. Sethi and colleagues19 noted a high percentage of endplate resorption and subsidence in cases reviewed using PEEK or allograft spacers paired with BMP-2. Although BMP-2 was used in the present study, we found very low rates of subsidence (0%, 5%) and no significant peri-implant lucencies (2%, 4%) (Figure 2). Interobserver agreement for these variables was high (0.95, 0.96). We hypothesize that the combination of endplate-sparing surgical technique and implant–bone integration contributed to these results.
Trabecular Bone and Fusion Grade
The primary radiographic criterion for solid interbody fusion is trabecular bone throughout the cage, bridging the vertebral bodies. In our study, the success rates for this variable were 96% and 100%, and there was very high interobserver agreement (0.96) (Figure 3). This very high fusion rate may preclude detecting subtle differences in interobserver agreement, but to what degree, if any, is unknown. Other investigators have effectively identified trabecular bone across the interspace and throughout the cages.6,18 The openings for bone formation were larger in the implants they used than in first-generation fusion cages but not as large as the implant openings in the present study. Larger openings may correlate with improved ability to visualize bridging bone on CT.
Radiologists and surgeons must ultimately arrive at a conclusion regarding the likelihood a fusion has occurred. Our radiologists integrated all the separate radiologic variables cited here, as well as their overall impressions of the scans, to arrive at a final grade regarding fusion quality (Figures 3, 4). Although this category provides the most interpretive latitude of all the variables examined, the results demonstrate high interobserver reliability. Agreement to exactly the same fusion grade was 0.77, and agreement to within 1 category grade was 0.95.
This study had several limitations. Surgical explorations were not clinically indicated and were not performed. There were no suspected nonunions or hardware complications, two of the most common indications for exploration. In addition, this study was conducted not to determine specific accuracy of CT (compared with surgery exploration) for fusion assessment but to assess interobserver reliability. The clinical success rates for this population were high, and no patient required revision surgery for suspected pseudarthrosis. To assess the true accuracy of CT for fusion assessment, one would have to subject patients to follow-up exploratory surgery to test fusions mechanically.
Another limitation is the lack of a single industry-accepted radiographic fusion grading system. Fusion criteria are not standardized across all studies. Our radiologists have extensive research experience and limit their practices to neuromuscular radiology with a concentration on the spine. The radiographic criteria cited here are the same criteria they use in clinical practice, when reviewing CT scans for clinicians. Last, there was no control group for direct comparison against other cages. Historical controls were cited. This does not adversely affect the conclusions of this investigation.
Conclusion
Clinicians have been reluctant to rely on CT with titanium devices because of concerns about the accuracy of image interpretations. The interbody device used in this study demonstrated minimal artifact and minimal subsidence, and trabecular bone was easily identified throughout the implant in the majority of cases reviewed. We found high interobserver agreement scores across all fusion criteria. Although surgical exploration remains the gold standard for fusion assessment, surgeons should have confidence in using CT with this titanium implant.
The accuracy of using computed tomography (CT) to assess lumbar interbody fusion with titanium implants has been questioned in the past.1-4 Reports have most often focused on older technologies using paired, threaded, smooth-surface titanium devices. Some authors have reported they could not confidently assess the quality of fusions using CT because of implant artifact.1-3
When pseudarthrosis is suspected clinically, and imaging results are inconclusive, surgical explorations may be performed with mechanical stressing of the segment to assess for motion.2,5-7 However, surgical exploration not only has the morbidity of another surgery but may not be conclusive. Direct exploration of an interbody fusion is problematic. In some cases, there may be residual normal springing motion through posterior elements, even in the presence of a solid interbody fusion, which can be confusing.5 Radiologic confirmation of fusion status is therefore preferred over surgical exploration. CT is the imaging modality used most often to assess spinal fusions.8,9
A new titanium interbody fusion implant (Endoskeleton TA; Titan Spine, Mequon, Wisconsin) preserves the endplate and has an acid-etched titanium surface for osseous integration and a wide central aperture for bone graft (Figure 1). Compared with earlier titanium implants, this design may allow for more accurate CT imaging and fusion assessment. We conducted a study to determine the interobserver reliability of using CT to evaluate bone formation and other radiographic variables with this new titanium interbody device.
Materials and Methods
After receiving institutional review board approval for this study, as well as patient consent, we obtained and analyzed CT scans of patients after they had undergone anterior lumbar interbody fusion (ALIF) at L3–S1 as part of a separate clinical outcomes study.
Each patient received an Endoskeleton TA implant. The fusion cage was packed with 2 sponges (3.0 mg per fusion level) of bone morphogenetic protein, or BMP (InFuse; Medtronic, Minneapolis, Minnesota). In addition, 1 to 3 cm3 of hydroxyapatite/β-tricalcium phosphate (MasterGraft, Medtronic) collagen sponge was used as graft extender to fill any remaining gaps within the cage. Pedicle screw fixation was used in all cases.
Patients were randomly assigned to have fine-cut CT scans with reconstructed images at 6, 9, or 12 months. The scans were reviewed by 2 independent radiologists who were blinded to each other’s interpretations and the clinical results. The radiographic fusion criteria are listed in Tables 1 to 3. Interobserver agreement (κ) was calculated separately for each radiographic criterion and could range from 0.00 (no agreement) to 1.00 (perfect agreement).10,11
Results
The study involved 33 patients (17 men, 16 women) with 56 lumbar spinal fusion levels. Mean age was 46 years (range, 23-66 years). Six patients (18%) were nicotine users. Seventeen patients were scanned at 6 months, 9 at 9 months, and 7 at 12 months. There were no significant differences in results between men and women, between nicotine users and nonusers, or among patients evaluated at 6, 9, or 12 months.
The radiologists agreed on 345 of the 392 data points reviewed (κ = 0.88). Interobserver agreement results for the fusion criteria are listed in Tables 1 and 3. Interobserver agreement was 0.77 for overall fusion grade, with the radiologists noting definite fusion (grade 5) in 80% and 91% of the levels (Table 1). Other radiographic criteria are listed in Tables 2 and 3. Interobserver agreement was 0.80 for degree of artifact, 0.95 for subsidence, 0.96 for both lucency and trabecular bone, 0.77 for anterior osseous bridging, and 0.95 for cystic vertebral changes.
Discussion
Radiographic analysis of interbody fusions is an important clinical issue. Investigators have shown that CT is the radiographic method of choice for assessing fusion.8,9 Others have reported that assessing fusion with metallic interbody implants is more difficult compared with PEEK (polyether ether ketone) or allograft bone.3,4,5,12
Heithoff and colleagues1,2 reported on difficulties they encountered in assessing interbody fusion with titanium implants, and their research has often been cited. The authors concluded that they could not accurately assess fusion in these cases because of artifact from the small apertures in the cages and metallic scatter. Their study was very small (8 patients, 12 surgical levels) and used paired BAK (Bagby and Kuslich) cages (Zimmer, Warsaw, Indiana).
Recently, a unique surface technology, used to manufacture osseointegrative dental implants, has been adapted for use in the spine.13-15 Acid etching modifies the surface of titanium to create a nano-scale (micron-level) alteration. Compared with PEEK and smooth titanium, acid-etched titanium stimulates a better osteogenic environment.16,17 As this technology is now used clinically in spinal surgery, we thought it important to revisit the issue of CT analysis for fusion assessment with the newer titanium implants.
Artifact
The results of our study support the idea that the design of a titanium interbody fusion implant is important to radiographic analysis. The implant studied has a large open central aperture that appears to generate less artifact than historical controls (paired cylindrical cages) have.1-4 Other investigators have reported fewer problems with artifact in their studies of implants incorporating larger openings for bone graft.6,18 The radiologists in the present study found no significant problems with artifact. Less artifact is clinically important, as the remaining fusion variables can be more clearly visualized (Table 2, Figure 2).
Anterior Osseous Bridging, Subsidence, Lysis
In this study, the bony endplates were preserved. The disc and endplate cartilage was removed without reaming or drilling. Endplate reaming most likely contributes to subsidence and loss of original fixation between implant and bone interface.1,4,12 Some authors have advocated recessing the cages deeply and then packing bone anteriorly to create a “sentinel fusion sign.”1,2,6 Deeply seating interbody implants, instead of resting them more widely on the apophyseal ring of the vertebral endplate, may also lead to subsidence.4,12 The issue of identifying a sentinel fusion sign is relevant only if the surgeon tries to create one. In the present study, the implant used was an impacted cage positioned on the apophyseal perimeter of the disc space, just slightly recessed, so there was no attempt to create a sentinel fusion sign, as reflected in the relatively low scores on anterior osseous bridging (48%, 52%).
Subsidence and peri-implant lysis are pathologic variables associated with motion and bone loss. Sethi and colleagues19 noted a high percentage of endplate resorption and subsidence in cases reviewed using PEEK or allograft spacers paired with BMP-2. Although BMP-2 was used in the present study, we found very low rates of subsidence (0%, 5%) and no significant peri-implant lucencies (2%, 4%) (Figure 2). Interobserver agreement for these variables was high (0.95, 0.96). We hypothesize that the combination of endplate-sparing surgical technique and implant–bone integration contributed to these results.
Trabecular Bone and Fusion Grade
The primary radiographic criterion for solid interbody fusion is trabecular bone throughout the cage, bridging the vertebral bodies. In our study, the success rates for this variable were 96% and 100%, and there was very high interobserver agreement (0.96) (Figure 3). This very high fusion rate may preclude detecting subtle differences in interobserver agreement, but to what degree, if any, is unknown. Other investigators have effectively identified trabecular bone across the interspace and throughout the cages.6,18 The openings for bone formation were larger in the implants they used than in first-generation fusion cages but not as large as the implant openings in the present study. Larger openings may correlate with improved ability to visualize bridging bone on CT.
Radiologists and surgeons must ultimately arrive at a conclusion regarding the likelihood a fusion has occurred. Our radiologists integrated all the separate radiologic variables cited here, as well as their overall impressions of the scans, to arrive at a final grade regarding fusion quality (Figures 3, 4). Although this category provides the most interpretive latitude of all the variables examined, the results demonstrate high interobserver reliability. Agreement to exactly the same fusion grade was 0.77, and agreement to within 1 category grade was 0.95.
This study had several limitations. Surgical explorations were not clinically indicated and were not performed. There were no suspected nonunions or hardware complications, two of the most common indications for exploration. In addition, this study was conducted not to determine specific accuracy of CT (compared with surgery exploration) for fusion assessment but to assess interobserver reliability. The clinical success rates for this population were high, and no patient required revision surgery for suspected pseudarthrosis. To assess the true accuracy of CT for fusion assessment, one would have to subject patients to follow-up exploratory surgery to test fusions mechanically.
Another limitation is the lack of a single industry-accepted radiographic fusion grading system. Fusion criteria are not standardized across all studies. Our radiologists have extensive research experience and limit their practices to neuromuscular radiology with a concentration on the spine. The radiographic criteria cited here are the same criteria they use in clinical practice, when reviewing CT scans for clinicians. Last, there was no control group for direct comparison against other cages. Historical controls were cited. This does not adversely affect the conclusions of this investigation.
Conclusion
Clinicians have been reluctant to rely on CT with titanium devices because of concerns about the accuracy of image interpretations. The interbody device used in this study demonstrated minimal artifact and minimal subsidence, and trabecular bone was easily identified throughout the implant in the majority of cases reviewed. We found high interobserver agreement scores across all fusion criteria. Although surgical exploration remains the gold standard for fusion assessment, surgeons should have confidence in using CT with this titanium implant.
1. Gilbert TJ, Heithoff KB, Mullin WJ. Radiographic assessment of cage-assisted interbody fusions in the lumbar spine. Semin Spine Surg. 2001;13:311-315.
2. Heithoff KB, Mullin WJ, Renfrew DL, Gilbert TJ. The failure of radiographic detection of pseudarthrosis in patients with titanium lumbar interbody fusion cages. In: Proceedings of the 14th Annual Meeting of the North American Spine Society; October 20-23, 1999; Chicago, IL. Abstract 14.
3. Cizek GR, Boyd LM. Imaging pitfalls of interbody implants. Spine. 2000;25(20):2633-2636.
4. Dorchak JD, Burkus JK, Foor BD, Sanders DL. Dual paired proximity and combined BAK/proximity interbody fusion cages: radiographic results. In: Proceedings of the 15th Annual Meeting of the North American Spine Society. New Orleans, LA: North American Spine Society; 2000:83-85.
5. Santos ER, Goss DG, Morcom RK, Fraser RD. Radiologic assessment of interbody fusion using carbon fiber cages. Spine. 2003;28(10):997-1001.
6. Carreon LY, Glassman SD, Schwender JD, Subach BR, Gornet MF, Ohno S. Reliability and accuracy of fine-cut computed tomography scans to determine the status of anterior interbody fusions with metallic cages. Spine J. 2008;8(6):998-1002.
7. Fogel GR, Toohey JS, Neidre A, Brantigan JW. Fusion assessment of posterior lumbar interbody fusion using radiolucent cages: x-ray films and helical computed tomography scans compared with surgical exploration of fusion. Spine J. 2008;8(4):570-577.
8. Selby MD, Clark SR, Hall DJ, Freeman BJ. Radiologic assessment of spinal fusion. J Am Acad Orthop Surg. 2012;20(11):694-703.
9. Chafetz N, Cann CE, Morris JM, Steinbach LS, Goldberg HI, Ax L. Pseudarthrosis following lumbar fusion: detection by direct coronal CT scanning. Radiology. 1987;162(3):803-805.
10. Landis RJ, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174.
11. Viera AJ, Garrett JM. Understanding interobserver agreement; the kappa statistic. Fam Med. 2005;37(5):360-363.
12. Burkus JK, Foley K, Haid RW, Lehuec JC. Surgical Interbody Research Group—radiographic assessment of interbody fusion devices: fusion criteria for anterior lumbar interbody surgery. Neurosurg Focus. 2001;10(4):E11.
13. Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1(1):11-25.
14. De Leonardis D, Garg AK, Pecora GE. Osseointegration of rough acid-etched titanium implants: 5-year follow-up of 100 Minimatic implants. Int J Oral Maxillofac Implants. 1999;14(3):384-391.
15. Schwartz Z, Raz P, Zhao G, et al. Effect of micrometer-scale roughness on the surface of Ti6Al4V pedicle screws in vitro and in vivo. J Bone Joint Surg Am. 2008;90(11):2485-2498.
16. Olivares-Navarrete R, Gittens RA, Schneider JM, et al. Osteoblasts exhibit a more differentiated phenotype and increased bone morphogenetic protein production on titanium alloy substrates than on poly-ether-ether-ketone. Spine J. 2012;12(3):265-272.
17. Olivares-Navarrete R, Hyzy SL, Gittens RA 1st, et al. Rough titanium alloys regulate osteoblast production of angiogenic factors. Spine J. 2013;13(11):1563-1570.
18. Burkus JK, Dorchak JD, Sanders DL. Radiographic assessment of interbody fusion using recombinant human bone morphogenetic protein type 2. Spine. 2003;28(4):372-377.
19. Sethi A, Craig J, Bartol S, et al. Radiographic and CT evaluation of recombinant human bone morphogenetic protein-2–assisted spinal interbody fusion. AJR Am J Roentgenol. 2011;197(1):W128-W133.
1. Gilbert TJ, Heithoff KB, Mullin WJ. Radiographic assessment of cage-assisted interbody fusions in the lumbar spine. Semin Spine Surg. 2001;13:311-315.
2. Heithoff KB, Mullin WJ, Renfrew DL, Gilbert TJ. The failure of radiographic detection of pseudarthrosis in patients with titanium lumbar interbody fusion cages. In: Proceedings of the 14th Annual Meeting of the North American Spine Society; October 20-23, 1999; Chicago, IL. Abstract 14.
3. Cizek GR, Boyd LM. Imaging pitfalls of interbody implants. Spine. 2000;25(20):2633-2636.
4. Dorchak JD, Burkus JK, Foor BD, Sanders DL. Dual paired proximity and combined BAK/proximity interbody fusion cages: radiographic results. In: Proceedings of the 15th Annual Meeting of the North American Spine Society. New Orleans, LA: North American Spine Society; 2000:83-85.
5. Santos ER, Goss DG, Morcom RK, Fraser RD. Radiologic assessment of interbody fusion using carbon fiber cages. Spine. 2003;28(10):997-1001.
6. Carreon LY, Glassman SD, Schwender JD, Subach BR, Gornet MF, Ohno S. Reliability and accuracy of fine-cut computed tomography scans to determine the status of anterior interbody fusions with metallic cages. Spine J. 2008;8(6):998-1002.
7. Fogel GR, Toohey JS, Neidre A, Brantigan JW. Fusion assessment of posterior lumbar interbody fusion using radiolucent cages: x-ray films and helical computed tomography scans compared with surgical exploration of fusion. Spine J. 2008;8(4):570-577.
8. Selby MD, Clark SR, Hall DJ, Freeman BJ. Radiologic assessment of spinal fusion. J Am Acad Orthop Surg. 2012;20(11):694-703.
9. Chafetz N, Cann CE, Morris JM, Steinbach LS, Goldberg HI, Ax L. Pseudarthrosis following lumbar fusion: detection by direct coronal CT scanning. Radiology. 1987;162(3):803-805.
10. Landis RJ, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174.
11. Viera AJ, Garrett JM. Understanding interobserver agreement; the kappa statistic. Fam Med. 2005;37(5):360-363.
12. Burkus JK, Foley K, Haid RW, Lehuec JC. Surgical Interbody Research Group—radiographic assessment of interbody fusion devices: fusion criteria for anterior lumbar interbody surgery. Neurosurg Focus. 2001;10(4):E11.
13. Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1(1):11-25.
14. De Leonardis D, Garg AK, Pecora GE. Osseointegration of rough acid-etched titanium implants: 5-year follow-up of 100 Minimatic implants. Int J Oral Maxillofac Implants. 1999;14(3):384-391.
15. Schwartz Z, Raz P, Zhao G, et al. Effect of micrometer-scale roughness on the surface of Ti6Al4V pedicle screws in vitro and in vivo. J Bone Joint Surg Am. 2008;90(11):2485-2498.
16. Olivares-Navarrete R, Gittens RA, Schneider JM, et al. Osteoblasts exhibit a more differentiated phenotype and increased bone morphogenetic protein production on titanium alloy substrates than on poly-ether-ether-ketone. Spine J. 2012;12(3):265-272.
17. Olivares-Navarrete R, Hyzy SL, Gittens RA 1st, et al. Rough titanium alloys regulate osteoblast production of angiogenic factors. Spine J. 2013;13(11):1563-1570.
18. Burkus JK, Dorchak JD, Sanders DL. Radiographic assessment of interbody fusion using recombinant human bone morphogenetic protein type 2. Spine. 2003;28(4):372-377.
19. Sethi A, Craig J, Bartol S, et al. Radiographic and CT evaluation of recombinant human bone morphogenetic protein-2–assisted spinal interbody fusion. AJR Am J Roentgenol. 2011;197(1):W128-W133.