User login
Pro basketball players’ hearts: LV keeps growing, aortic root doesn’t
For the first time, cardiologists have characterized the adaptive cardiac remodeling in a large cohort of National Basketball Association players, which establishes a normative database and allows physicians to distinguish it from occult pathologic changes that may precipitate sudden cardiac death, according to an imaging study.
“We hope that the present data will help to focus decision making and improve clinical acumen for the purpose of primary prevention of cardiac emergencies in U.S. basketball players and in the athletic community at large,” said Dr. David J. Engel and his associates of Columbia University, New York.
Until now, most of the literature concerning the structural features of the athletic heart has been based on European studies, where comprehensive cardiac screening of all elite athletes is mandatory. The typical sports activities and the demographics of athletes in the U.S. are different, and their cardiologic profiles have not been well studied because detailed cardiac examinations are not compulsory. But the NBA recently mandated that all athletes undergo annual preseason medical evaluations including stress echocardiograms, and allowed the division of cardiology at Columbia to assess the results each year.
“A detailed understanding of normal and expected cardiac remodeling in U.S. basketball players has significant clinical importance given that the incidence of sports-related sudden cardiac death in the U.S. is highest among basketball players and that the most common cause ... in this population is hypertrophic cardiomyopathy,” the investigators noted.
Their analysis of all 526 ECGs performed on NBA players during a 1-year period “will provide an invaluable frame of reference to enhance player safety for the large group of U.S. basketball players in training at all skill levels, and in the athletic community at large,” they said.
The study participants were aged 18-39 years (mean age, 25.7 years). Roughly 77% were African American, 20% were white, 2% were Hispanic, and 1% were Asian or other ethnicities. The mean height was 200.2 cm (6’7”).
Left ventricular cavity size was larger than that in the general population, but LV size was proportional to the athletes’ large body size. “Scaling LV size to body size is vitally important in the cardiac evaluation of basketball players, whose heights extend to 218 cm and body surface areas to 2.8 m2,” Dr. Engel and his associates said (JAMA Cardiol. 2016 Feb 24. doi: 10.1001/jamacardio.2015.0252).
Left ventricular hypertrophy (LVH) was identified in only 27% of the athletes. African Americans had increased indices of LVH, compared with whites, and had a higher incidence of nondilated concentric hypertrophy, while whites showed predominantly eccentric dilated hypertrophy. These findings should help clinicians recognize genuine hypertrophic cardiomyopathy, which is a contraindication to participating in all but the most low-intensity competitive sports.
Most of the participants had a normal left ventricular ejection fraction, and all showed normal augmentation of LV systolic function with exercise.
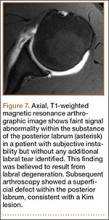
Aortic root diameter was larger than that in the general population but similar to that in other elite athletes. Surprisingly, aortic root diameter increased with increasing body size only to a certain point, reaching a plateau at 31-35 mm. Fewer than 5% of the participants had an aortic root diameter of 40 mm or more, and the maximal diameter was 42 mm. “These data have important implications in the evaluation of exceptionally large athletes and question the applicability in individuals with significantly increased biometrics of the traditional formula to estimate aortic root diameter that assumes a linear association between [it] and body surface area,” they noted.
“We hope that the results of this study will assist recognition of cardiac pathologic change and provide a framework to help avoid unnecessary exclusions of athletes from competition. We believe that these data have additional applicability to other sports that preselect for athletes with height, such as volleyball, rowing, and track and field,” Dr. Engel and his associates added.
This study was supported by the National Basketball Association as part of a medical services agreement with Columbia University. Dr. Engel and his associates reported having no relevant financial disclosures.
The most interesting finding of this study was that despite the immense body size of the athletes, aortic root diameter exceeded 40 mm in less than 5%, and when dilation did occur it was of a very small magnitude, with a maximal diameter of 42 mm.
This important finding confirms that only mild aortic dilation should be considered physiologic among athletes, and that even athletes at the extreme end of the height spectrum should not be expected to show proportionally extreme aortic dilation.
Unlike ventricular size, which increases proportionally with body size, aortic dilation has an upper limit. Athletes with aortic dimensions that exceed this limit should be considered at risk for aortopathy and either prohibited from competitive sports or closely monitored if they do participate.
Dr. Aaron L. Baggish of the Cardiovascular Performance Program at Massachusetts General Hospital, Boston, made these remarks in an accompanying editorial (JAMA Cardiol. 2016 Feb 24. doi: 10.1001/jamacardio.2015.0289). He reported having no relevant financial conflicts of interest.
The most interesting finding of this study was that despite the immense body size of the athletes, aortic root diameter exceeded 40 mm in less than 5%, and when dilation did occur it was of a very small magnitude, with a maximal diameter of 42 mm.
This important finding confirms that only mild aortic dilation should be considered physiologic among athletes, and that even athletes at the extreme end of the height spectrum should not be expected to show proportionally extreme aortic dilation.
Unlike ventricular size, which increases proportionally with body size, aortic dilation has an upper limit. Athletes with aortic dimensions that exceed this limit should be considered at risk for aortopathy and either prohibited from competitive sports or closely monitored if they do participate.
Dr. Aaron L. Baggish of the Cardiovascular Performance Program at Massachusetts General Hospital, Boston, made these remarks in an accompanying editorial (JAMA Cardiol. 2016 Feb 24. doi: 10.1001/jamacardio.2015.0289). He reported having no relevant financial conflicts of interest.
The most interesting finding of this study was that despite the immense body size of the athletes, aortic root diameter exceeded 40 mm in less than 5%, and when dilation did occur it was of a very small magnitude, with a maximal diameter of 42 mm.
This important finding confirms that only mild aortic dilation should be considered physiologic among athletes, and that even athletes at the extreme end of the height spectrum should not be expected to show proportionally extreme aortic dilation.
Unlike ventricular size, which increases proportionally with body size, aortic dilation has an upper limit. Athletes with aortic dimensions that exceed this limit should be considered at risk for aortopathy and either prohibited from competitive sports or closely monitored if they do participate.
Dr. Aaron L. Baggish of the Cardiovascular Performance Program at Massachusetts General Hospital, Boston, made these remarks in an accompanying editorial (JAMA Cardiol. 2016 Feb 24. doi: 10.1001/jamacardio.2015.0289). He reported having no relevant financial conflicts of interest.
For the first time, cardiologists have characterized the adaptive cardiac remodeling in a large cohort of National Basketball Association players, which establishes a normative database and allows physicians to distinguish it from occult pathologic changes that may precipitate sudden cardiac death, according to an imaging study.
“We hope that the present data will help to focus decision making and improve clinical acumen for the purpose of primary prevention of cardiac emergencies in U.S. basketball players and in the athletic community at large,” said Dr. David J. Engel and his associates of Columbia University, New York.
Until now, most of the literature concerning the structural features of the athletic heart has been based on European studies, where comprehensive cardiac screening of all elite athletes is mandatory. The typical sports activities and the demographics of athletes in the U.S. are different, and their cardiologic profiles have not been well studied because detailed cardiac examinations are not compulsory. But the NBA recently mandated that all athletes undergo annual preseason medical evaluations including stress echocardiograms, and allowed the division of cardiology at Columbia to assess the results each year.
“A detailed understanding of normal and expected cardiac remodeling in U.S. basketball players has significant clinical importance given that the incidence of sports-related sudden cardiac death in the U.S. is highest among basketball players and that the most common cause ... in this population is hypertrophic cardiomyopathy,” the investigators noted.
Their analysis of all 526 ECGs performed on NBA players during a 1-year period “will provide an invaluable frame of reference to enhance player safety for the large group of U.S. basketball players in training at all skill levels, and in the athletic community at large,” they said.
The study participants were aged 18-39 years (mean age, 25.7 years). Roughly 77% were African American, 20% were white, 2% were Hispanic, and 1% were Asian or other ethnicities. The mean height was 200.2 cm (6’7”).
Left ventricular cavity size was larger than that in the general population, but LV size was proportional to the athletes’ large body size. “Scaling LV size to body size is vitally important in the cardiac evaluation of basketball players, whose heights extend to 218 cm and body surface areas to 2.8 m2,” Dr. Engel and his associates said (JAMA Cardiol. 2016 Feb 24. doi: 10.1001/jamacardio.2015.0252).
Left ventricular hypertrophy (LVH) was identified in only 27% of the athletes. African Americans had increased indices of LVH, compared with whites, and had a higher incidence of nondilated concentric hypertrophy, while whites showed predominantly eccentric dilated hypertrophy. These findings should help clinicians recognize genuine hypertrophic cardiomyopathy, which is a contraindication to participating in all but the most low-intensity competitive sports.
Most of the participants had a normal left ventricular ejection fraction, and all showed normal augmentation of LV systolic function with exercise.
Aortic root diameter was larger than that in the general population but similar to that in other elite athletes. Surprisingly, aortic root diameter increased with increasing body size only to a certain point, reaching a plateau at 31-35 mm. Fewer than 5% of the participants had an aortic root diameter of 40 mm or more, and the maximal diameter was 42 mm. “These data have important implications in the evaluation of exceptionally large athletes and question the applicability in individuals with significantly increased biometrics of the traditional formula to estimate aortic root diameter that assumes a linear association between [it] and body surface area,” they noted.
“We hope that the results of this study will assist recognition of cardiac pathologic change and provide a framework to help avoid unnecessary exclusions of athletes from competition. We believe that these data have additional applicability to other sports that preselect for athletes with height, such as volleyball, rowing, and track and field,” Dr. Engel and his associates added.
This study was supported by the National Basketball Association as part of a medical services agreement with Columbia University. Dr. Engel and his associates reported having no relevant financial disclosures.
For the first time, cardiologists have characterized the adaptive cardiac remodeling in a large cohort of National Basketball Association players, which establishes a normative database and allows physicians to distinguish it from occult pathologic changes that may precipitate sudden cardiac death, according to an imaging study.
“We hope that the present data will help to focus decision making and improve clinical acumen for the purpose of primary prevention of cardiac emergencies in U.S. basketball players and in the athletic community at large,” said Dr. David J. Engel and his associates of Columbia University, New York.
Until now, most of the literature concerning the structural features of the athletic heart has been based on European studies, where comprehensive cardiac screening of all elite athletes is mandatory. The typical sports activities and the demographics of athletes in the U.S. are different, and their cardiologic profiles have not been well studied because detailed cardiac examinations are not compulsory. But the NBA recently mandated that all athletes undergo annual preseason medical evaluations including stress echocardiograms, and allowed the division of cardiology at Columbia to assess the results each year.
“A detailed understanding of normal and expected cardiac remodeling in U.S. basketball players has significant clinical importance given that the incidence of sports-related sudden cardiac death in the U.S. is highest among basketball players and that the most common cause ... in this population is hypertrophic cardiomyopathy,” the investigators noted.
Their analysis of all 526 ECGs performed on NBA players during a 1-year period “will provide an invaluable frame of reference to enhance player safety for the large group of U.S. basketball players in training at all skill levels, and in the athletic community at large,” they said.
The study participants were aged 18-39 years (mean age, 25.7 years). Roughly 77% were African American, 20% were white, 2% were Hispanic, and 1% were Asian or other ethnicities. The mean height was 200.2 cm (6’7”).
Left ventricular cavity size was larger than that in the general population, but LV size was proportional to the athletes’ large body size. “Scaling LV size to body size is vitally important in the cardiac evaluation of basketball players, whose heights extend to 218 cm and body surface areas to 2.8 m2,” Dr. Engel and his associates said (JAMA Cardiol. 2016 Feb 24. doi: 10.1001/jamacardio.2015.0252).
Left ventricular hypertrophy (LVH) was identified in only 27% of the athletes. African Americans had increased indices of LVH, compared with whites, and had a higher incidence of nondilated concentric hypertrophy, while whites showed predominantly eccentric dilated hypertrophy. These findings should help clinicians recognize genuine hypertrophic cardiomyopathy, which is a contraindication to participating in all but the most low-intensity competitive sports.
Most of the participants had a normal left ventricular ejection fraction, and all showed normal augmentation of LV systolic function with exercise.
Aortic root diameter was larger than that in the general population but similar to that in other elite athletes. Surprisingly, aortic root diameter increased with increasing body size only to a certain point, reaching a plateau at 31-35 mm. Fewer than 5% of the participants had an aortic root diameter of 40 mm or more, and the maximal diameter was 42 mm. “These data have important implications in the evaluation of exceptionally large athletes and question the applicability in individuals with significantly increased biometrics of the traditional formula to estimate aortic root diameter that assumes a linear association between [it] and body surface area,” they noted.
“We hope that the results of this study will assist recognition of cardiac pathologic change and provide a framework to help avoid unnecessary exclusions of athletes from competition. We believe that these data have additional applicability to other sports that preselect for athletes with height, such as volleyball, rowing, and track and field,” Dr. Engel and his associates added.
This study was supported by the National Basketball Association as part of a medical services agreement with Columbia University. Dr. Engel and his associates reported having no relevant financial disclosures.
FROM JAMA CARDIOLOGY
Key clinical point: Cardiologists characterized normal, adaptive cardiac remodeling in NBA players, allowing physicians to distinguish it from occult pathologic changes that may precipitate sudden cardiac death.
Major finding: Aortic root diameter increased with increasing body size only to a certain point, reaching a plateau at 31-35 mm.
Data source: An observational cohort study in which echocardiograms of 526 professional athletes were analyzed.
Disclosures: This study was supported by the National Basketball Association as part of a medical services agreement with Columbia University. Dr. Engel and his associates reported having no relevant financial disclosures.
Emergency Ultrasound: Musculoskeletal Shoulder Dislocation
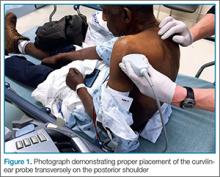
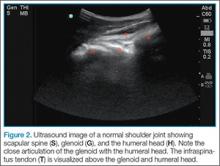
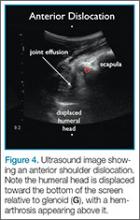
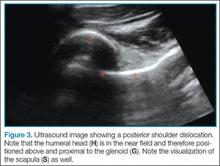
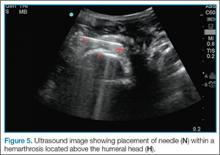
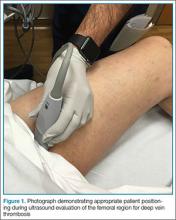
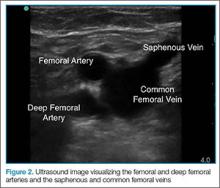
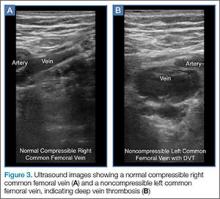
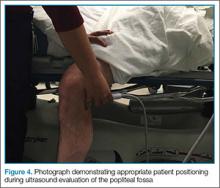
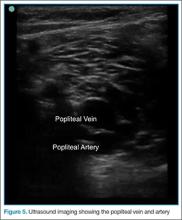
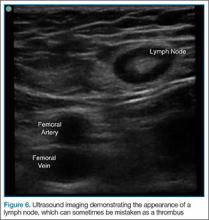
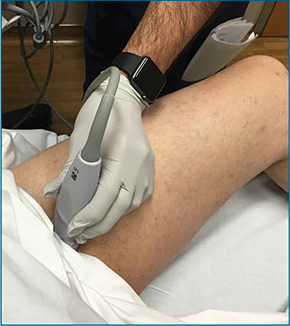
Point-of-care (POC) ultrasound is a great adjunct to the evaluation and treatment of shoulder dislocations. This modality can assist with identification of the dislocation—especially posterior dislocations, which can be notoriously difficult to diagnose on plain radiography.1,2 Moreover, it can aid with reduction by guiding intra-articular anesthetic injection, regional anesthesia with an interscalene brachial plexus nerve block, or suprascapular nerve block. Following treatment, POC ultrasound also can immediately confirm successful reduction.
Imaging Technique
Facilitation of Reduction
Summary
Bedside ultrasound is an excellent adjunct to traditional radiographs in the evaluation of patients presenting with shoulder injuries. In addition to its high sensitivity in detecting dislocation, this modality can be used to guide intra-articular treatment and to confirm successful reduction.
Dr Meer is an assistant professor and director of emergency ultrasound, department of emergency medicine, Emory University School of Medicine, Atlanta. Dr Beck is an assistant professor, department of emergency medicine, Emory University School of Medicine, Atlanta. Dr Taylor is an assistant professor and director of postgraduate medical education, department of emergency medicine, Emory University School of Medicine, Atlanta.
- Abbasi S, Molaie H, Hafezimoghadam P, et al. Diagnostic accuracy of ultrasonographic examination in the management of shoulder dislocation in the emergency department. Ann Emerg Med. 2013;62(2):170-175. doi:10.1016/j.annemergmed.2013.01.022.
- Beck S, Chilstrom M. Point-of-care ultrasound diagnosis and treatment of posterior shoulder dislocation. Am J Emerg Med. 2013;31(2):449.e3-449.e5. doi:10.1016/j.ajem.2012.06.017.
- Breslin K, Boniface K, Cohen J. Ultrasound-guided intra-articular lidocaine block for reduction of anterior shoulder dislocation in the pediatric emergency department. Pediatr Emerg Care. 2014;30(3):217-220. doi:10.1097/PEC.0000000000000095.
Point-of-care (POC) ultrasound is a great adjunct to the evaluation and treatment of shoulder dislocations. This modality can assist with identification of the dislocation—especially posterior dislocations, which can be notoriously difficult to diagnose on plain radiography.1,2 Moreover, it can aid with reduction by guiding intra-articular anesthetic injection, regional anesthesia with an interscalene brachial plexus nerve block, or suprascapular nerve block. Following treatment, POC ultrasound also can immediately confirm successful reduction.
Imaging Technique
Facilitation of Reduction
Summary
Bedside ultrasound is an excellent adjunct to traditional radiographs in the evaluation of patients presenting with shoulder injuries. In addition to its high sensitivity in detecting dislocation, this modality can be used to guide intra-articular treatment and to confirm successful reduction.
Dr Meer is an assistant professor and director of emergency ultrasound, department of emergency medicine, Emory University School of Medicine, Atlanta. Dr Beck is an assistant professor, department of emergency medicine, Emory University School of Medicine, Atlanta. Dr Taylor is an assistant professor and director of postgraduate medical education, department of emergency medicine, Emory University School of Medicine, Atlanta.
Point-of-care (POC) ultrasound is a great adjunct to the evaluation and treatment of shoulder dislocations. This modality can assist with identification of the dislocation—especially posterior dislocations, which can be notoriously difficult to diagnose on plain radiography.1,2 Moreover, it can aid with reduction by guiding intra-articular anesthetic injection, regional anesthesia with an interscalene brachial plexus nerve block, or suprascapular nerve block. Following treatment, POC ultrasound also can immediately confirm successful reduction.
Imaging Technique
Facilitation of Reduction
Summary
Bedside ultrasound is an excellent adjunct to traditional radiographs in the evaluation of patients presenting with shoulder injuries. In addition to its high sensitivity in detecting dislocation, this modality can be used to guide intra-articular treatment and to confirm successful reduction.
Dr Meer is an assistant professor and director of emergency ultrasound, department of emergency medicine, Emory University School of Medicine, Atlanta. Dr Beck is an assistant professor, department of emergency medicine, Emory University School of Medicine, Atlanta. Dr Taylor is an assistant professor and director of postgraduate medical education, department of emergency medicine, Emory University School of Medicine, Atlanta.
- Abbasi S, Molaie H, Hafezimoghadam P, et al. Diagnostic accuracy of ultrasonographic examination in the management of shoulder dislocation in the emergency department. Ann Emerg Med. 2013;62(2):170-175. doi:10.1016/j.annemergmed.2013.01.022.
- Beck S, Chilstrom M. Point-of-care ultrasound diagnosis and treatment of posterior shoulder dislocation. Am J Emerg Med. 2013;31(2):449.e3-449.e5. doi:10.1016/j.ajem.2012.06.017.
- Breslin K, Boniface K, Cohen J. Ultrasound-guided intra-articular lidocaine block for reduction of anterior shoulder dislocation in the pediatric emergency department. Pediatr Emerg Care. 2014;30(3):217-220. doi:10.1097/PEC.0000000000000095.
- Abbasi S, Molaie H, Hafezimoghadam P, et al. Diagnostic accuracy of ultrasonographic examination in the management of shoulder dislocation in the emergency department. Ann Emerg Med. 2013;62(2):170-175. doi:10.1016/j.annemergmed.2013.01.022.
- Beck S, Chilstrom M. Point-of-care ultrasound diagnosis and treatment of posterior shoulder dislocation. Am J Emerg Med. 2013;31(2):449.e3-449.e5. doi:10.1016/j.ajem.2012.06.017.
- Breslin K, Boniface K, Cohen J. Ultrasound-guided intra-articular lidocaine block for reduction of anterior shoulder dislocation in the pediatric emergency department. Pediatr Emerg Care. 2014;30(3):217-220. doi:10.1097/PEC.0000000000000095.
A Guide to Ultrasound of the Shoulder, Part 1: Coding and Reimbursement
Although ultrasound has been around for many years, the technology is underutilized. It has been used primarily by the radiologists and obstetricians-gynecologists. However, orthopedic surgeons and sports medicine doctors are beginning to realize the utility of this imaging modality for their specialties. Ultrasound has classically been used as a diagnostic tool. This usage is beneficial to sports medicine specialists for on-field coverage at sports competitions to efficiently evaluate injuries without the need for taking the athletes back to the locker room for an x-ray or magnetic resonance imaging (MRI). Ultrasound can quickly assess for damage to soft tissue, joints, and superficial bones. Another of ultrasound’s benefits is its use as an adjunct to treatment. Ultrasound has been shown to vastly increase the accuracy of injections and can be used in surgery to accurately guide percutaneous techniques or to identify structures that previously required radiation-exposing fluoroscopy or large incisions to find by feel or eye.
Ultrasound is a technician-dependent modality. The surgeon and physician must become facile with the use of the probe and how ultrasound works. The use of the probe is similar to an arthroscope, requiring small movements of the hand to reveal the best imaging of the tissues. Rather than relying on just the patient’s position with an immobile machine, the user must use the probe position and the placement of the patient’s limb or body to optimize the use of ultrasound. Doing so saves time, money, and exposure to dangerous radiation. In a retrospective study of 1012 patients treated over a 10-month period, Sivan and colleagues1 concluded that the use of clinic-based musculoskeletal (MSK) ultrasound enables a one-stop approach, reduces repeat hospital appointments, and improves quality of care.With the increased use of ultrasound comes the need to accurately code and bill for the use of ultrasound. According to the College of Radiology, Medicare reimbursements for MSK ultrasound studies has increased by 316% from 2000-2009.2 Paradoxically, ultrasound has still been relatively underutilized when compared to the use of MSK MRI.
Diagnostic Ultrasound
Ultrasound is based off sound waves, emitted from a transducer, which are then bounced back off the underlying structures based on the density of that structure. The computer interprets the returning sound waves and produces an image reflecting the quality and strength of those returning waves. When the sound waves are bounced back strongly and quickly, like when hitting bone, we see an image that is intensely white (“hyperechoic”). When the sound waves encounter a substance that transmits those waves easily and do not return, like air or fluid, the image is dark (“hypoechoic”).
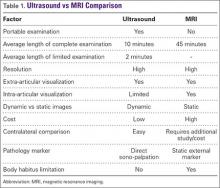
Ultrasound’s fundamental advantages start with every patient being able to have an ultrasound: no interference from metal, pacemakers, claustrophobia, or obesity. Contralateral comparisons, sono-palpation at the site of pathology, and real-time dynamic studies allow for a more comprehensive diagnostic evaluation. Doppler capabilities can further expand the usefulness of the evaluation and guide safer interventions. With the advent of high-resolution portable ultrasound machines, these studies can essentially be performed anywhere, and are typically done in a timely and cost-effective manner.
Ultrasound has many diagnostic uses for soft tissue, joint, and bone disorders. For soft tissues, ultrasound can image tears of muscles, tendons, and ligaments; show inflammation like tenosynovitis; demonstrate masses like hematomas, cysts, solid tumors, or calcific tendonitis; display nerve disorders like Morton’s neuroma; or confirm foreign bodies or infections.3-5 For joint disorders, ultrasound can show erosions on bones, loose bodies, pannus, inflammation, or effusions. For bone disorders, ultrasound can diagnose fractures and, sometimes, even stress fractures. Tomer and colleagues6 compared 51 patients with bone contusions and fractures; they determined that ultrasound was most reliable in the diagnosis of long bone diaphyseal fractures. The one disadvantage, especially when compared to MRI, is ultrasound’s inability to fully evaluate intra-articular or deep structures such as articular cartilage, the glenohumeral labrum, the biceps’ anchor, etc.
Magnetic Resonance Imaging
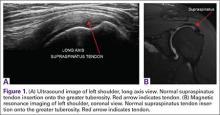
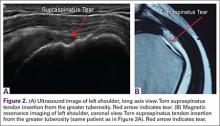
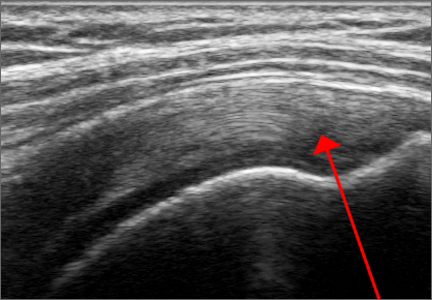
Ultrasound is similar to MRI as it images tissues and gives us ideas whether that tissue is normal, damaged, or diseased (Figures 1A, 1B). MRI is based on magnetics and large machines that cannot be moved. MRI yields planar images that can only be changed by changing the position of the limb or body in the MRI tube. This can create an issue with obese patients or with postoperative patients who cannot maintain the operated body part in one position for the length of the MRI scan. Ultrasound is better tolerated by patients without the need for claustrophobic large machines (Table 1). In 2004, Middleton and colleagues7 surveyed 118 patients who obtained an ultrasound and MRI of the shoulder for suspected rotator cuff pathology; ultrasound had higher satisfaction levels, and 93% of patients preferred ultrasound to MRI.
For rotator cuff tears, ultrasound is also comparable diagnostically with MRI (Figures 2A, 2B). In a prospective study of 124 patients, MRI and ultrasound had comparable accuracy for identifying and measuring the size of full-thickness and partial-thickness rotator cuff tears, with arthroscopic findings used as the standard.8 A 2015 meta-analysis published in the British Journal of Sports Medicine showed that the diagnostic accuracy of ultrasound, MRI, and MR arthrography in the characterization of full thickness rotator cuff tears had >90% sensitivity and specificity. As for partial rotator cuff tears and tendinopathy, overall estimates of specificity were also high (>90%), while sensitivity was as high as 83%. Diagnostic accuracy of ultrasound was similar whether it was performed by a trained radiologist, sonographer, or orthopedist.9
Medicare reimbursements for MSK ultrasound studies has increased by 316% in the past decade.2 Private practice MSK ultrasound procedures increased from 19,372 in 2000 to 158,351 in 2009.2 In 2010, non-radiologists accounted for more ultrasound-guided procedures than radiologists for the first time.10 MSK ultrasound is still underutilized compared to MRI. This underutilization is also unfortunate economically. The cost of MRIs is significantly higher. According to Parker and colleagues10, the projected Medicare cost for MSK imaging in 2020 is $3.6 billion, with MRI accounting for $2 billion. They also concluded that replacing MSK MRI with MSK ultrasound when clinically indicated could save over $6.9 billion between 2006 and 2020.11
Ultrasound-Guided Procedures
MSK ultrasound has gained significant ground on other imaging modalities when it comes to procedures, both in office and in the operating room. The ability to have a small, mobile, inexpensive machine that can be used in real time has dramatically changed how interventions are done. Most imaging modalities used to perform injections or percutaneous surgery use fluoroscopy machines. This exposes the patients to significant radiation, costs significantly more, and usually requires a secondary consultation with radiologists in a different facility. This wastes time and money, and results in potentially unnecessary exposure to radiation.
Accuracy is the most common reason for referral for guided injections. The guidance can help avoid nerves, vessels, and other sensitive tissues. However, accuracy is also important to make sure the injection is placed in the correct location. When injections are placed into a muscle, tendon, or ligament, it causes significant pain; however, injections placed into a bursal space or joint do not cause pain. Numerous studies have shown that even in the hands of experts, “simple” injections can still miss their mark over 30% of the time.12-19 Therefore, if a patient experiences pain during a bursal space or joint injection, the injection was not placed properly.
The American Medical Society for Sports Medicine Position Paper on MSK ultrasound is based on a systematic review of the literature, including 124 studies. It states that ultrasound-guided joint injections (USGI) are more accurate and efficacious than landmark guided injections (LMGI), with a strength of recommendation taxonomy (SORT) evidence rating of A and B, respectively.19 In terms of patient satisfaction, in a randomized controlled trial of 148 patients undergoing knee injections, Sibbitt and colleagues20 showed that USGI had a 48% reduction (P < .001) in procedural pain, a 58.5% reduction (P < .001) in absolute pain scores at the 2-week outcome mark, and a 75% reduction (P < .001) in significant pain and 62% reduction in nonresponder rate.20 From a financial point of view, Sibbitt and colleagues20 also demonstrated a 13% reduction in cost per patient per year, and a 58% reduction in cost per responder per year for a hospital outpatient center (P < .001).
Coding
Coding for diagnostic MSK ultrasound requires an understanding of a few current procedural terminology (CPT) codes (Table 2). Ultrasound usage should follow the usual requirements of medical necessity and the CPT code selected should be based on the elements of the study performed. A complete examination, described by CPT code 76881, includes the examination and documentation of the muscles, tendons, joint, and other soft tissue structures and any identifiable abnormality of the joint being evaluated. If anything less is done, then the CPT code 76882 should be used.
New CPT codes for joint injections became effective January 2015 (Table 3). The new changes affect only the joint injection series (20600-20610). Previously, injections could be billed with CPT code 76942, which was “Ultrasonic guidance for needle placement (eg, biopsy, aspiration, injection, localization device), imaging supervision and interpretation.” This code can still be used, but with only specific injections, when the verbiage “with ultrasound/image guidance” is not included in the injection CPT code descriptor (Table 4).
Under the National Correct Coding Initiative (NCCI), which sets Centers for Medicare & Medicaid Services (CMS) payment policy as well as that of many private payers, one unit of service is allowed for CPT code 76942 in a single patient encounter regardless of the number of needle placements performed. Per NCCI, “The unit of service for these codes is the patient encounter, not number of lesions, number of aspirations, number of biopsies, number of injections, or number of localizations.”
Per the Radiology section of the NCCI, “Ultrasound guidance and diagnostic ultrasound (echography) procedures may be reported separately only if each service is distinct and separate. If a diagnostic ultrasound study identifies a previously unknown abnormality that requires a therapeutic procedure with ultrasound guidance at the same patient encounter, both the diagnostic ultrasound and ultrasound guidance procedure codes may be reported separately. However, a previously unknown abnormality identified during ultrasound guidance for a procedure should not be reported separately as a diagnostic ultrasound procedure.”
Under the Medicare program, the International Classification of Diseases 10th Revision (ICD-10) code selected should be based on the test results, with 2 exceptions. If the test does not yield a diagnosis or was normal, the physician should use the pre-service signs, symptoms, and conditions that prompted the study. If the test is a screening examination ordered in the absence of any signs or symptoms of illness or injury, the physician should select “screening” as the primary reason for the service and record the test results, if any, as additional diagnoses.
Modifiers must be used in specific settings. In the office, physicians who own the equipment and perform the service themselves (or the service is performed by an employed or contracted sonographer) may bill the global fee without any modifiers. However, if billing for a procedure on the same day as an office visit, the -25 modifier must be used. This indicates “[a] significant, separately identifiable evaluation and management service.” This modifier should not be used routinely. If the service is performed in a hospital, the -26 modifier must be used to indicate that the professional service only was provided when the physician does not own the machine (Tables 2, 3, 4). The payers will not reimburse physicians for the technical component in the hospital setting.
Reimbursement
In general, medical insurance plans will cover ultrasound studies when they are medically indicated. However, we recommend checking with each individual private payer directly, including Medicare. Medicare Part B will generally reimburse physicians for medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors require that the physician who performs and/or interprets some types of ultrasound examinations be capable of demonstrating relevant, documented training through recent residency training or post-graduate continuing medical education (CME) and experience. Medicare does not differentiate by medical specialty with respect to billing medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors have coverage policies regarding either the diagnostic study or ultrasound guidance of certain injections, or both.
Payment policies for beneficiaries enrolled in Medicare Part C, known as the Medicare Advantage plans, will reflect those of the private insurance administrator. The Medicare Advantage plan may be either a health maintenance organization (HMO) or a preferred provider organization (PPO). Private insurance payment rules vary by payer and plan with respect to which specialties may perform and receive reimbursement for ultrasound services. Some payers will reimburse providers of any specialty for ultrasound services, while others may restrict imaging procedures to specific specialties or providers possessing specific certifications or accreditations. Some insurers require physicians to submit applications requesting ultrasound be added to their list of services performed in their practice. Physicians should contact private payers before submitting claims to determine their requirements and request that they add ultrasound to the list of services.
When contacting the private payers, ask the following questions:
- What do I need to do to have ultrasound added to my practice’s contract or list of services?
- Are there any specific training requirements that I must meet or credentials that I must obtain in order to be privileged to perform ultrasound in my office?
- Do I need to send a letter or can I submit the request verbally?
- Is there an application that must be completed?
- If there is a privileging program, how long will it take after submission of the application before we are accepted?
- What is the fee schedule associated with these codes?
- Are there any bundling edits in place covering any of the services I am considering performing? (Be prepared to provide the codes for any non-ultrasound services you will be performing in conjunction with the ultrasound services.)
- Are there any preauthorization requirements for specific ultrasound studies?
- Are there any preauthorization requirements for specific ultrasound studies?
Documentation Requirements
All diagnostic ultrasound examinations, including those when ultrasound is used to guide a procedure, require that permanently recorded images be maintained in the patient record. The images can be kept in the patient record or some other archive—they do not need to be submitted with the claim. Images can be stored as printed images, on a tape or electronic medium. Documentation of the study must be available to the insurer upon request.
A written report of all ultrasound studies should be maintained in the patient’s record. In the case of ultrasound guidance, the written report may be filed as a separate item in the patient’s record or it may be included within the report of the procedure for which the guidance is utilized.
As examples of our documentation in the office, copies of 3 of our standard forms are available: “Ultrasound report of the shoulder” (Appendix 1), “Procedure note for an ultrasound-guided injection of cortisone” (Appendix 2), and “Procedure note for an ultrasound-guided injection of platelet-rich plasma” (Appendix 3).
Appropriate Use Criteria (AUC)
The Protecting Access to Medicare Act of 2014 was an effort to help reduce unnecessary imaging services and reduce costs; the Secretary of Health and Human Services was to establish a program to promote the use of “appropriate use criteria” (AUC) for advanced imaging services such as MRI, computed tomography, positron emission tomography, and nuclear cardiology. AUC are criteria that are developed or endorsed by national professional medical specialty societies or other provider-led entities to assist ordering professionals and furnishing professionals in making the most appropriate treatment decision for a specific clinical condition for an individual. The law also noted that the criteria should be evidence-based, meaning they should have stakeholder consensus, be scientifically valid, and be based on studies that are published and reviewable by stakeholders.
By April 2016, the Secretary will identify and publish the list of qualified clinical decision support mechanisms, which are tools that could be used by ordering professionals to ensure that AUC is met for applicable imaging services. These may include certified health electronic record technology, private sector clinical decision support mechanisms, and others. Actual use of the AUC will begin in January 2017. This legislation applies only to Medicare services, but other payers have cited concerns and may follow in the future.
Conclusion
Ultrasound is being increasingly used in varying specialties, especially orthopedic surgery. It provides a time- and cost-efficient modality with diagnostic power comparable to MRI. Portability and a high safety profile allows for ease of implementation as an in-office or sideline tool. Needle guidance and other intraoperative applications highlight its versatility as an adjunct to orthopedic treatments. This article provides a comprehensive guide to billing and coding for both diagnostic and therapeutic MSK ultrasound of the shoulder. Providers should stay up to date with upcoming appropriate use criteria and adjustments to current billing procedures.
1. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
2. Sharpe R, Nazarian L, Parker L, Rao V, Levin D. Dramatically increased musculoskeletal ultrasound utilization from 2000 to 2009, especially by podiatrists in private offices. Department of Radiology Faculty Papers. Paper 16. http://jdc.jefferson.edu/radiologyfp/16. Accessed January 7, 2016.
3. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: The orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
4. Sinha TP, Bhoi S, Kumar S, et al. Diagnostic accuracy of bedside emergency ultrasound screening for fractures in pediatric trauma patients. J Emerg Trauma Shock. 2011;4(4);443-445.
5. Bica D, Armen J, Kulas AS, Young K, Womack Z. Reliability and precision of stress sonography of the ulnar collateral ligament. J Ultrasound Med. 2015;34(3):371-376.
6. Tomer K, Kleinbaum Y, Heyman Z, Dudkiewicz I, Blankstein A. Ultrasound diagnosis of fractures in adults. Akt Traumatol. 2006;36(4):171-174.
7. Middleton W, Payne WT, Teefey SA, Hidebolt CF, Rubin DA, Yamaguchi K. Sonography and MRI of the shoulder: comparison of patient satisfaction. AJR Am J Roentgenol. 2004;183(5):1449-1452.
8. Teefey SA, Rubin DA, Middleton WD, Hildebolt CF, Leibold RA, Yamaguchi K. Detection and quantification of rotator cuff tears. Comparison of ultrasonographic, magnetic resonance and arthroscopic finding in seventy-one consecutive cases. J Bone Joint Surg Am. 2004;86-A(4):708-716.
9. Roy-JS, Braën C, Leblond J, et al. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterization of rotator cuff disorders: a meta-analysis. Br J Sports Med. 2015;49(20):1316-1328.
10. Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal Imaging: Medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5(3):182-188.
11. Eustace J, Brophy D, Gibney R, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
12. Partington P, Broome G. Diagnostic injection around the shoulder: Hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
13. Rutten M, Maresch B, Jager G, de Waal Malefijt M. Injection of the subacromial-subdeltoid bursa: Blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
14. Kang M, Rizio L, Prybicien M, Middlemas D, Blacksin M. The accuracy of subacromial corticosteroid injections: A comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 Suppl):61S-66S.
15. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: An arthrographic evaluation. Arthroscopy. 2002;19(8):887-891.
16. Henkus HE, Cobben M, Coerkamp E, Nelissen R, van Arkel E. The accuracy of subacromial injections: A prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
17. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
18. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
19. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
20. Sibbitt WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intra-articular injections? J Rheumatol. 2009;36(9):1892-1902.
Although ultrasound has been around for many years, the technology is underutilized. It has been used primarily by the radiologists and obstetricians-gynecologists. However, orthopedic surgeons and sports medicine doctors are beginning to realize the utility of this imaging modality for their specialties. Ultrasound has classically been used as a diagnostic tool. This usage is beneficial to sports medicine specialists for on-field coverage at sports competitions to efficiently evaluate injuries without the need for taking the athletes back to the locker room for an x-ray or magnetic resonance imaging (MRI). Ultrasound can quickly assess for damage to soft tissue, joints, and superficial bones. Another of ultrasound’s benefits is its use as an adjunct to treatment. Ultrasound has been shown to vastly increase the accuracy of injections and can be used in surgery to accurately guide percutaneous techniques or to identify structures that previously required radiation-exposing fluoroscopy or large incisions to find by feel or eye.
Ultrasound is a technician-dependent modality. The surgeon and physician must become facile with the use of the probe and how ultrasound works. The use of the probe is similar to an arthroscope, requiring small movements of the hand to reveal the best imaging of the tissues. Rather than relying on just the patient’s position with an immobile machine, the user must use the probe position and the placement of the patient’s limb or body to optimize the use of ultrasound. Doing so saves time, money, and exposure to dangerous radiation. In a retrospective study of 1012 patients treated over a 10-month period, Sivan and colleagues1 concluded that the use of clinic-based musculoskeletal (MSK) ultrasound enables a one-stop approach, reduces repeat hospital appointments, and improves quality of care.With the increased use of ultrasound comes the need to accurately code and bill for the use of ultrasound. According to the College of Radiology, Medicare reimbursements for MSK ultrasound studies has increased by 316% from 2000-2009.2 Paradoxically, ultrasound has still been relatively underutilized when compared to the use of MSK MRI.
Diagnostic Ultrasound
Ultrasound is based off sound waves, emitted from a transducer, which are then bounced back off the underlying structures based on the density of that structure. The computer interprets the returning sound waves and produces an image reflecting the quality and strength of those returning waves. When the sound waves are bounced back strongly and quickly, like when hitting bone, we see an image that is intensely white (“hyperechoic”). When the sound waves encounter a substance that transmits those waves easily and do not return, like air or fluid, the image is dark (“hypoechoic”).
Ultrasound’s fundamental advantages start with every patient being able to have an ultrasound: no interference from metal, pacemakers, claustrophobia, or obesity. Contralateral comparisons, sono-palpation at the site of pathology, and real-time dynamic studies allow for a more comprehensive diagnostic evaluation. Doppler capabilities can further expand the usefulness of the evaluation and guide safer interventions. With the advent of high-resolution portable ultrasound machines, these studies can essentially be performed anywhere, and are typically done in a timely and cost-effective manner.
Ultrasound has many diagnostic uses for soft tissue, joint, and bone disorders. For soft tissues, ultrasound can image tears of muscles, tendons, and ligaments; show inflammation like tenosynovitis; demonstrate masses like hematomas, cysts, solid tumors, or calcific tendonitis; display nerve disorders like Morton’s neuroma; or confirm foreign bodies or infections.3-5 For joint disorders, ultrasound can show erosions on bones, loose bodies, pannus, inflammation, or effusions. For bone disorders, ultrasound can diagnose fractures and, sometimes, even stress fractures. Tomer and colleagues6 compared 51 patients with bone contusions and fractures; they determined that ultrasound was most reliable in the diagnosis of long bone diaphyseal fractures. The one disadvantage, especially when compared to MRI, is ultrasound’s inability to fully evaluate intra-articular or deep structures such as articular cartilage, the glenohumeral labrum, the biceps’ anchor, etc.
Magnetic Resonance Imaging
Ultrasound is similar to MRI as it images tissues and gives us ideas whether that tissue is normal, damaged, or diseased (Figures 1A, 1B). MRI is based on magnetics and large machines that cannot be moved. MRI yields planar images that can only be changed by changing the position of the limb or body in the MRI tube. This can create an issue with obese patients or with postoperative patients who cannot maintain the operated body part in one position for the length of the MRI scan. Ultrasound is better tolerated by patients without the need for claustrophobic large machines (Table 1). In 2004, Middleton and colleagues7 surveyed 118 patients who obtained an ultrasound and MRI of the shoulder for suspected rotator cuff pathology; ultrasound had higher satisfaction levels, and 93% of patients preferred ultrasound to MRI.
For rotator cuff tears, ultrasound is also comparable diagnostically with MRI (Figures 2A, 2B). In a prospective study of 124 patients, MRI and ultrasound had comparable accuracy for identifying and measuring the size of full-thickness and partial-thickness rotator cuff tears, with arthroscopic findings used as the standard.8 A 2015 meta-analysis published in the British Journal of Sports Medicine showed that the diagnostic accuracy of ultrasound, MRI, and MR arthrography in the characterization of full thickness rotator cuff tears had >90% sensitivity and specificity. As for partial rotator cuff tears and tendinopathy, overall estimates of specificity were also high (>90%), while sensitivity was as high as 83%. Diagnostic accuracy of ultrasound was similar whether it was performed by a trained radiologist, sonographer, or orthopedist.9
Medicare reimbursements for MSK ultrasound studies has increased by 316% in the past decade.2 Private practice MSK ultrasound procedures increased from 19,372 in 2000 to 158,351 in 2009.2 In 2010, non-radiologists accounted for more ultrasound-guided procedures than radiologists for the first time.10 MSK ultrasound is still underutilized compared to MRI. This underutilization is also unfortunate economically. The cost of MRIs is significantly higher. According to Parker and colleagues10, the projected Medicare cost for MSK imaging in 2020 is $3.6 billion, with MRI accounting for $2 billion. They also concluded that replacing MSK MRI with MSK ultrasound when clinically indicated could save over $6.9 billion between 2006 and 2020.11
Ultrasound-Guided Procedures
MSK ultrasound has gained significant ground on other imaging modalities when it comes to procedures, both in office and in the operating room. The ability to have a small, mobile, inexpensive machine that can be used in real time has dramatically changed how interventions are done. Most imaging modalities used to perform injections or percutaneous surgery use fluoroscopy machines. This exposes the patients to significant radiation, costs significantly more, and usually requires a secondary consultation with radiologists in a different facility. This wastes time and money, and results in potentially unnecessary exposure to radiation.
Accuracy is the most common reason for referral for guided injections. The guidance can help avoid nerves, vessels, and other sensitive tissues. However, accuracy is also important to make sure the injection is placed in the correct location. When injections are placed into a muscle, tendon, or ligament, it causes significant pain; however, injections placed into a bursal space or joint do not cause pain. Numerous studies have shown that even in the hands of experts, “simple” injections can still miss their mark over 30% of the time.12-19 Therefore, if a patient experiences pain during a bursal space or joint injection, the injection was not placed properly.
The American Medical Society for Sports Medicine Position Paper on MSK ultrasound is based on a systematic review of the literature, including 124 studies. It states that ultrasound-guided joint injections (USGI) are more accurate and efficacious than landmark guided injections (LMGI), with a strength of recommendation taxonomy (SORT) evidence rating of A and B, respectively.19 In terms of patient satisfaction, in a randomized controlled trial of 148 patients undergoing knee injections, Sibbitt and colleagues20 showed that USGI had a 48% reduction (P < .001) in procedural pain, a 58.5% reduction (P < .001) in absolute pain scores at the 2-week outcome mark, and a 75% reduction (P < .001) in significant pain and 62% reduction in nonresponder rate.20 From a financial point of view, Sibbitt and colleagues20 also demonstrated a 13% reduction in cost per patient per year, and a 58% reduction in cost per responder per year for a hospital outpatient center (P < .001).
Coding
Coding for diagnostic MSK ultrasound requires an understanding of a few current procedural terminology (CPT) codes (Table 2). Ultrasound usage should follow the usual requirements of medical necessity and the CPT code selected should be based on the elements of the study performed. A complete examination, described by CPT code 76881, includes the examination and documentation of the muscles, tendons, joint, and other soft tissue structures and any identifiable abnormality of the joint being evaluated. If anything less is done, then the CPT code 76882 should be used.
New CPT codes for joint injections became effective January 2015 (Table 3). The new changes affect only the joint injection series (20600-20610). Previously, injections could be billed with CPT code 76942, which was “Ultrasonic guidance for needle placement (eg, biopsy, aspiration, injection, localization device), imaging supervision and interpretation.” This code can still be used, but with only specific injections, when the verbiage “with ultrasound/image guidance” is not included in the injection CPT code descriptor (Table 4).
Under the National Correct Coding Initiative (NCCI), which sets Centers for Medicare & Medicaid Services (CMS) payment policy as well as that of many private payers, one unit of service is allowed for CPT code 76942 in a single patient encounter regardless of the number of needle placements performed. Per NCCI, “The unit of service for these codes is the patient encounter, not number of lesions, number of aspirations, number of biopsies, number of injections, or number of localizations.”
Per the Radiology section of the NCCI, “Ultrasound guidance and diagnostic ultrasound (echography) procedures may be reported separately only if each service is distinct and separate. If a diagnostic ultrasound study identifies a previously unknown abnormality that requires a therapeutic procedure with ultrasound guidance at the same patient encounter, both the diagnostic ultrasound and ultrasound guidance procedure codes may be reported separately. However, a previously unknown abnormality identified during ultrasound guidance for a procedure should not be reported separately as a diagnostic ultrasound procedure.”
Under the Medicare program, the International Classification of Diseases 10th Revision (ICD-10) code selected should be based on the test results, with 2 exceptions. If the test does not yield a diagnosis or was normal, the physician should use the pre-service signs, symptoms, and conditions that prompted the study. If the test is a screening examination ordered in the absence of any signs or symptoms of illness or injury, the physician should select “screening” as the primary reason for the service and record the test results, if any, as additional diagnoses.
Modifiers must be used in specific settings. In the office, physicians who own the equipment and perform the service themselves (or the service is performed by an employed or contracted sonographer) may bill the global fee without any modifiers. However, if billing for a procedure on the same day as an office visit, the -25 modifier must be used. This indicates “[a] significant, separately identifiable evaluation and management service.” This modifier should not be used routinely. If the service is performed in a hospital, the -26 modifier must be used to indicate that the professional service only was provided when the physician does not own the machine (Tables 2, 3, 4). The payers will not reimburse physicians for the technical component in the hospital setting.
Reimbursement
In general, medical insurance plans will cover ultrasound studies when they are medically indicated. However, we recommend checking with each individual private payer directly, including Medicare. Medicare Part B will generally reimburse physicians for medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors require that the physician who performs and/or interprets some types of ultrasound examinations be capable of demonstrating relevant, documented training through recent residency training or post-graduate continuing medical education (CME) and experience. Medicare does not differentiate by medical specialty with respect to billing medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors have coverage policies regarding either the diagnostic study or ultrasound guidance of certain injections, or both.
Payment policies for beneficiaries enrolled in Medicare Part C, known as the Medicare Advantage plans, will reflect those of the private insurance administrator. The Medicare Advantage plan may be either a health maintenance organization (HMO) or a preferred provider organization (PPO). Private insurance payment rules vary by payer and plan with respect to which specialties may perform and receive reimbursement for ultrasound services. Some payers will reimburse providers of any specialty for ultrasound services, while others may restrict imaging procedures to specific specialties or providers possessing specific certifications or accreditations. Some insurers require physicians to submit applications requesting ultrasound be added to their list of services performed in their practice. Physicians should contact private payers before submitting claims to determine their requirements and request that they add ultrasound to the list of services.
When contacting the private payers, ask the following questions:
- What do I need to do to have ultrasound added to my practice’s contract or list of services?
- Are there any specific training requirements that I must meet or credentials that I must obtain in order to be privileged to perform ultrasound in my office?
- Do I need to send a letter or can I submit the request verbally?
- Is there an application that must be completed?
- If there is a privileging program, how long will it take after submission of the application before we are accepted?
- What is the fee schedule associated with these codes?
- Are there any bundling edits in place covering any of the services I am considering performing? (Be prepared to provide the codes for any non-ultrasound services you will be performing in conjunction with the ultrasound services.)
- Are there any preauthorization requirements for specific ultrasound studies?
- Are there any preauthorization requirements for specific ultrasound studies?
Documentation Requirements
All diagnostic ultrasound examinations, including those when ultrasound is used to guide a procedure, require that permanently recorded images be maintained in the patient record. The images can be kept in the patient record or some other archive—they do not need to be submitted with the claim. Images can be stored as printed images, on a tape or electronic medium. Documentation of the study must be available to the insurer upon request.
A written report of all ultrasound studies should be maintained in the patient’s record. In the case of ultrasound guidance, the written report may be filed as a separate item in the patient’s record or it may be included within the report of the procedure for which the guidance is utilized.
As examples of our documentation in the office, copies of 3 of our standard forms are available: “Ultrasound report of the shoulder” (Appendix 1), “Procedure note for an ultrasound-guided injection of cortisone” (Appendix 2), and “Procedure note for an ultrasound-guided injection of platelet-rich plasma” (Appendix 3).
Appropriate Use Criteria (AUC)
The Protecting Access to Medicare Act of 2014 was an effort to help reduce unnecessary imaging services and reduce costs; the Secretary of Health and Human Services was to establish a program to promote the use of “appropriate use criteria” (AUC) for advanced imaging services such as MRI, computed tomography, positron emission tomography, and nuclear cardiology. AUC are criteria that are developed or endorsed by national professional medical specialty societies or other provider-led entities to assist ordering professionals and furnishing professionals in making the most appropriate treatment decision for a specific clinical condition for an individual. The law also noted that the criteria should be evidence-based, meaning they should have stakeholder consensus, be scientifically valid, and be based on studies that are published and reviewable by stakeholders.
By April 2016, the Secretary will identify and publish the list of qualified clinical decision support mechanisms, which are tools that could be used by ordering professionals to ensure that AUC is met for applicable imaging services. These may include certified health electronic record technology, private sector clinical decision support mechanisms, and others. Actual use of the AUC will begin in January 2017. This legislation applies only to Medicare services, but other payers have cited concerns and may follow in the future.
Conclusion
Ultrasound is being increasingly used in varying specialties, especially orthopedic surgery. It provides a time- and cost-efficient modality with diagnostic power comparable to MRI. Portability and a high safety profile allows for ease of implementation as an in-office or sideline tool. Needle guidance and other intraoperative applications highlight its versatility as an adjunct to orthopedic treatments. This article provides a comprehensive guide to billing and coding for both diagnostic and therapeutic MSK ultrasound of the shoulder. Providers should stay up to date with upcoming appropriate use criteria and adjustments to current billing procedures.
Although ultrasound has been around for many years, the technology is underutilized. It has been used primarily by the radiologists and obstetricians-gynecologists. However, orthopedic surgeons and sports medicine doctors are beginning to realize the utility of this imaging modality for their specialties. Ultrasound has classically been used as a diagnostic tool. This usage is beneficial to sports medicine specialists for on-field coverage at sports competitions to efficiently evaluate injuries without the need for taking the athletes back to the locker room for an x-ray or magnetic resonance imaging (MRI). Ultrasound can quickly assess for damage to soft tissue, joints, and superficial bones. Another of ultrasound’s benefits is its use as an adjunct to treatment. Ultrasound has been shown to vastly increase the accuracy of injections and can be used in surgery to accurately guide percutaneous techniques or to identify structures that previously required radiation-exposing fluoroscopy or large incisions to find by feel or eye.
Ultrasound is a technician-dependent modality. The surgeon and physician must become facile with the use of the probe and how ultrasound works. The use of the probe is similar to an arthroscope, requiring small movements of the hand to reveal the best imaging of the tissues. Rather than relying on just the patient’s position with an immobile machine, the user must use the probe position and the placement of the patient’s limb or body to optimize the use of ultrasound. Doing so saves time, money, and exposure to dangerous radiation. In a retrospective study of 1012 patients treated over a 10-month period, Sivan and colleagues1 concluded that the use of clinic-based musculoskeletal (MSK) ultrasound enables a one-stop approach, reduces repeat hospital appointments, and improves quality of care.With the increased use of ultrasound comes the need to accurately code and bill for the use of ultrasound. According to the College of Radiology, Medicare reimbursements for MSK ultrasound studies has increased by 316% from 2000-2009.2 Paradoxically, ultrasound has still been relatively underutilized when compared to the use of MSK MRI.
Diagnostic Ultrasound
Ultrasound is based off sound waves, emitted from a transducer, which are then bounced back off the underlying structures based on the density of that structure. The computer interprets the returning sound waves and produces an image reflecting the quality and strength of those returning waves. When the sound waves are bounced back strongly and quickly, like when hitting bone, we see an image that is intensely white (“hyperechoic”). When the sound waves encounter a substance that transmits those waves easily and do not return, like air or fluid, the image is dark (“hypoechoic”).
Ultrasound’s fundamental advantages start with every patient being able to have an ultrasound: no interference from metal, pacemakers, claustrophobia, or obesity. Contralateral comparisons, sono-palpation at the site of pathology, and real-time dynamic studies allow for a more comprehensive diagnostic evaluation. Doppler capabilities can further expand the usefulness of the evaluation and guide safer interventions. With the advent of high-resolution portable ultrasound machines, these studies can essentially be performed anywhere, and are typically done in a timely and cost-effective manner.
Ultrasound has many diagnostic uses for soft tissue, joint, and bone disorders. For soft tissues, ultrasound can image tears of muscles, tendons, and ligaments; show inflammation like tenosynovitis; demonstrate masses like hematomas, cysts, solid tumors, or calcific tendonitis; display nerve disorders like Morton’s neuroma; or confirm foreign bodies or infections.3-5 For joint disorders, ultrasound can show erosions on bones, loose bodies, pannus, inflammation, or effusions. For bone disorders, ultrasound can diagnose fractures and, sometimes, even stress fractures. Tomer and colleagues6 compared 51 patients with bone contusions and fractures; they determined that ultrasound was most reliable in the diagnosis of long bone diaphyseal fractures. The one disadvantage, especially when compared to MRI, is ultrasound’s inability to fully evaluate intra-articular or deep structures such as articular cartilage, the glenohumeral labrum, the biceps’ anchor, etc.
Magnetic Resonance Imaging
Ultrasound is similar to MRI as it images tissues and gives us ideas whether that tissue is normal, damaged, or diseased (Figures 1A, 1B). MRI is based on magnetics and large machines that cannot be moved. MRI yields planar images that can only be changed by changing the position of the limb or body in the MRI tube. This can create an issue with obese patients or with postoperative patients who cannot maintain the operated body part in one position for the length of the MRI scan. Ultrasound is better tolerated by patients without the need for claustrophobic large machines (Table 1). In 2004, Middleton and colleagues7 surveyed 118 patients who obtained an ultrasound and MRI of the shoulder for suspected rotator cuff pathology; ultrasound had higher satisfaction levels, and 93% of patients preferred ultrasound to MRI.
For rotator cuff tears, ultrasound is also comparable diagnostically with MRI (Figures 2A, 2B). In a prospective study of 124 patients, MRI and ultrasound had comparable accuracy for identifying and measuring the size of full-thickness and partial-thickness rotator cuff tears, with arthroscopic findings used as the standard.8 A 2015 meta-analysis published in the British Journal of Sports Medicine showed that the diagnostic accuracy of ultrasound, MRI, and MR arthrography in the characterization of full thickness rotator cuff tears had >90% sensitivity and specificity. As for partial rotator cuff tears and tendinopathy, overall estimates of specificity were also high (>90%), while sensitivity was as high as 83%. Diagnostic accuracy of ultrasound was similar whether it was performed by a trained radiologist, sonographer, or orthopedist.9
Medicare reimbursements for MSK ultrasound studies has increased by 316% in the past decade.2 Private practice MSK ultrasound procedures increased from 19,372 in 2000 to 158,351 in 2009.2 In 2010, non-radiologists accounted for more ultrasound-guided procedures than radiologists for the first time.10 MSK ultrasound is still underutilized compared to MRI. This underutilization is also unfortunate economically. The cost of MRIs is significantly higher. According to Parker and colleagues10, the projected Medicare cost for MSK imaging in 2020 is $3.6 billion, with MRI accounting for $2 billion. They also concluded that replacing MSK MRI with MSK ultrasound when clinically indicated could save over $6.9 billion between 2006 and 2020.11
Ultrasound-Guided Procedures
MSK ultrasound has gained significant ground on other imaging modalities when it comes to procedures, both in office and in the operating room. The ability to have a small, mobile, inexpensive machine that can be used in real time has dramatically changed how interventions are done. Most imaging modalities used to perform injections or percutaneous surgery use fluoroscopy machines. This exposes the patients to significant radiation, costs significantly more, and usually requires a secondary consultation with radiologists in a different facility. This wastes time and money, and results in potentially unnecessary exposure to radiation.
Accuracy is the most common reason for referral for guided injections. The guidance can help avoid nerves, vessels, and other sensitive tissues. However, accuracy is also important to make sure the injection is placed in the correct location. When injections are placed into a muscle, tendon, or ligament, it causes significant pain; however, injections placed into a bursal space or joint do not cause pain. Numerous studies have shown that even in the hands of experts, “simple” injections can still miss their mark over 30% of the time.12-19 Therefore, if a patient experiences pain during a bursal space or joint injection, the injection was not placed properly.
The American Medical Society for Sports Medicine Position Paper on MSK ultrasound is based on a systematic review of the literature, including 124 studies. It states that ultrasound-guided joint injections (USGI) are more accurate and efficacious than landmark guided injections (LMGI), with a strength of recommendation taxonomy (SORT) evidence rating of A and B, respectively.19 In terms of patient satisfaction, in a randomized controlled trial of 148 patients undergoing knee injections, Sibbitt and colleagues20 showed that USGI had a 48% reduction (P < .001) in procedural pain, a 58.5% reduction (P < .001) in absolute pain scores at the 2-week outcome mark, and a 75% reduction (P < .001) in significant pain and 62% reduction in nonresponder rate.20 From a financial point of view, Sibbitt and colleagues20 also demonstrated a 13% reduction in cost per patient per year, and a 58% reduction in cost per responder per year for a hospital outpatient center (P < .001).
Coding
Coding for diagnostic MSK ultrasound requires an understanding of a few current procedural terminology (CPT) codes (Table 2). Ultrasound usage should follow the usual requirements of medical necessity and the CPT code selected should be based on the elements of the study performed. A complete examination, described by CPT code 76881, includes the examination and documentation of the muscles, tendons, joint, and other soft tissue structures and any identifiable abnormality of the joint being evaluated. If anything less is done, then the CPT code 76882 should be used.
New CPT codes for joint injections became effective January 2015 (Table 3). The new changes affect only the joint injection series (20600-20610). Previously, injections could be billed with CPT code 76942, which was “Ultrasonic guidance for needle placement (eg, biopsy, aspiration, injection, localization device), imaging supervision and interpretation.” This code can still be used, but with only specific injections, when the verbiage “with ultrasound/image guidance” is not included in the injection CPT code descriptor (Table 4).
Under the National Correct Coding Initiative (NCCI), which sets Centers for Medicare & Medicaid Services (CMS) payment policy as well as that of many private payers, one unit of service is allowed for CPT code 76942 in a single patient encounter regardless of the number of needle placements performed. Per NCCI, “The unit of service for these codes is the patient encounter, not number of lesions, number of aspirations, number of biopsies, number of injections, or number of localizations.”
Per the Radiology section of the NCCI, “Ultrasound guidance and diagnostic ultrasound (echography) procedures may be reported separately only if each service is distinct and separate. If a diagnostic ultrasound study identifies a previously unknown abnormality that requires a therapeutic procedure with ultrasound guidance at the same patient encounter, both the diagnostic ultrasound and ultrasound guidance procedure codes may be reported separately. However, a previously unknown abnormality identified during ultrasound guidance for a procedure should not be reported separately as a diagnostic ultrasound procedure.”
Under the Medicare program, the International Classification of Diseases 10th Revision (ICD-10) code selected should be based on the test results, with 2 exceptions. If the test does not yield a diagnosis or was normal, the physician should use the pre-service signs, symptoms, and conditions that prompted the study. If the test is a screening examination ordered in the absence of any signs or symptoms of illness or injury, the physician should select “screening” as the primary reason for the service and record the test results, if any, as additional diagnoses.
Modifiers must be used in specific settings. In the office, physicians who own the equipment and perform the service themselves (or the service is performed by an employed or contracted sonographer) may bill the global fee without any modifiers. However, if billing for a procedure on the same day as an office visit, the -25 modifier must be used. This indicates “[a] significant, separately identifiable evaluation and management service.” This modifier should not be used routinely. If the service is performed in a hospital, the -26 modifier must be used to indicate that the professional service only was provided when the physician does not own the machine (Tables 2, 3, 4). The payers will not reimburse physicians for the technical component in the hospital setting.
Reimbursement
In general, medical insurance plans will cover ultrasound studies when they are medically indicated. However, we recommend checking with each individual private payer directly, including Medicare. Medicare Part B will generally reimburse physicians for medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors require that the physician who performs and/or interprets some types of ultrasound examinations be capable of demonstrating relevant, documented training through recent residency training or post-graduate continuing medical education (CME) and experience. Medicare does not differentiate by medical specialty with respect to billing medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors have coverage policies regarding either the diagnostic study or ultrasound guidance of certain injections, or both.
Payment policies for beneficiaries enrolled in Medicare Part C, known as the Medicare Advantage plans, will reflect those of the private insurance administrator. The Medicare Advantage plan may be either a health maintenance organization (HMO) or a preferred provider organization (PPO). Private insurance payment rules vary by payer and plan with respect to which specialties may perform and receive reimbursement for ultrasound services. Some payers will reimburse providers of any specialty for ultrasound services, while others may restrict imaging procedures to specific specialties or providers possessing specific certifications or accreditations. Some insurers require physicians to submit applications requesting ultrasound be added to their list of services performed in their practice. Physicians should contact private payers before submitting claims to determine their requirements and request that they add ultrasound to the list of services.
When contacting the private payers, ask the following questions:
- What do I need to do to have ultrasound added to my practice’s contract or list of services?
- Are there any specific training requirements that I must meet or credentials that I must obtain in order to be privileged to perform ultrasound in my office?
- Do I need to send a letter or can I submit the request verbally?
- Is there an application that must be completed?
- If there is a privileging program, how long will it take after submission of the application before we are accepted?
- What is the fee schedule associated with these codes?
- Are there any bundling edits in place covering any of the services I am considering performing? (Be prepared to provide the codes for any non-ultrasound services you will be performing in conjunction with the ultrasound services.)
- Are there any preauthorization requirements for specific ultrasound studies?
- Are there any preauthorization requirements for specific ultrasound studies?
Documentation Requirements
All diagnostic ultrasound examinations, including those when ultrasound is used to guide a procedure, require that permanently recorded images be maintained in the patient record. The images can be kept in the patient record or some other archive—they do not need to be submitted with the claim. Images can be stored as printed images, on a tape or electronic medium. Documentation of the study must be available to the insurer upon request.
A written report of all ultrasound studies should be maintained in the patient’s record. In the case of ultrasound guidance, the written report may be filed as a separate item in the patient’s record or it may be included within the report of the procedure for which the guidance is utilized.
As examples of our documentation in the office, copies of 3 of our standard forms are available: “Ultrasound report of the shoulder” (Appendix 1), “Procedure note for an ultrasound-guided injection of cortisone” (Appendix 2), and “Procedure note for an ultrasound-guided injection of platelet-rich plasma” (Appendix 3).
Appropriate Use Criteria (AUC)
The Protecting Access to Medicare Act of 2014 was an effort to help reduce unnecessary imaging services and reduce costs; the Secretary of Health and Human Services was to establish a program to promote the use of “appropriate use criteria” (AUC) for advanced imaging services such as MRI, computed tomography, positron emission tomography, and nuclear cardiology. AUC are criteria that are developed or endorsed by national professional medical specialty societies or other provider-led entities to assist ordering professionals and furnishing professionals in making the most appropriate treatment decision for a specific clinical condition for an individual. The law also noted that the criteria should be evidence-based, meaning they should have stakeholder consensus, be scientifically valid, and be based on studies that are published and reviewable by stakeholders.
By April 2016, the Secretary will identify and publish the list of qualified clinical decision support mechanisms, which are tools that could be used by ordering professionals to ensure that AUC is met for applicable imaging services. These may include certified health electronic record technology, private sector clinical decision support mechanisms, and others. Actual use of the AUC will begin in January 2017. This legislation applies only to Medicare services, but other payers have cited concerns and may follow in the future.
Conclusion
Ultrasound is being increasingly used in varying specialties, especially orthopedic surgery. It provides a time- and cost-efficient modality with diagnostic power comparable to MRI. Portability and a high safety profile allows for ease of implementation as an in-office or sideline tool. Needle guidance and other intraoperative applications highlight its versatility as an adjunct to orthopedic treatments. This article provides a comprehensive guide to billing and coding for both diagnostic and therapeutic MSK ultrasound of the shoulder. Providers should stay up to date with upcoming appropriate use criteria and adjustments to current billing procedures.
1. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
2. Sharpe R, Nazarian L, Parker L, Rao V, Levin D. Dramatically increased musculoskeletal ultrasound utilization from 2000 to 2009, especially by podiatrists in private offices. Department of Radiology Faculty Papers. Paper 16. http://jdc.jefferson.edu/radiologyfp/16. Accessed January 7, 2016.
3. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: The orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
4. Sinha TP, Bhoi S, Kumar S, et al. Diagnostic accuracy of bedside emergency ultrasound screening for fractures in pediatric trauma patients. J Emerg Trauma Shock. 2011;4(4);443-445.
5. Bica D, Armen J, Kulas AS, Young K, Womack Z. Reliability and precision of stress sonography of the ulnar collateral ligament. J Ultrasound Med. 2015;34(3):371-376.
6. Tomer K, Kleinbaum Y, Heyman Z, Dudkiewicz I, Blankstein A. Ultrasound diagnosis of fractures in adults. Akt Traumatol. 2006;36(4):171-174.
7. Middleton W, Payne WT, Teefey SA, Hidebolt CF, Rubin DA, Yamaguchi K. Sonography and MRI of the shoulder: comparison of patient satisfaction. AJR Am J Roentgenol. 2004;183(5):1449-1452.
8. Teefey SA, Rubin DA, Middleton WD, Hildebolt CF, Leibold RA, Yamaguchi K. Detection and quantification of rotator cuff tears. Comparison of ultrasonographic, magnetic resonance and arthroscopic finding in seventy-one consecutive cases. J Bone Joint Surg Am. 2004;86-A(4):708-716.
9. Roy-JS, Braën C, Leblond J, et al. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterization of rotator cuff disorders: a meta-analysis. Br J Sports Med. 2015;49(20):1316-1328.
10. Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal Imaging: Medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5(3):182-188.
11. Eustace J, Brophy D, Gibney R, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
12. Partington P, Broome G. Diagnostic injection around the shoulder: Hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
13. Rutten M, Maresch B, Jager G, de Waal Malefijt M. Injection of the subacromial-subdeltoid bursa: Blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
14. Kang M, Rizio L, Prybicien M, Middlemas D, Blacksin M. The accuracy of subacromial corticosteroid injections: A comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 Suppl):61S-66S.
15. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: An arthrographic evaluation. Arthroscopy. 2002;19(8):887-891.
16. Henkus HE, Cobben M, Coerkamp E, Nelissen R, van Arkel E. The accuracy of subacromial injections: A prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
17. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
18. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
19. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
20. Sibbitt WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intra-articular injections? J Rheumatol. 2009;36(9):1892-1902.
1. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
2. Sharpe R, Nazarian L, Parker L, Rao V, Levin D. Dramatically increased musculoskeletal ultrasound utilization from 2000 to 2009, especially by podiatrists in private offices. Department of Radiology Faculty Papers. Paper 16. http://jdc.jefferson.edu/radiologyfp/16. Accessed January 7, 2016.
3. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: The orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
4. Sinha TP, Bhoi S, Kumar S, et al. Diagnostic accuracy of bedside emergency ultrasound screening for fractures in pediatric trauma patients. J Emerg Trauma Shock. 2011;4(4);443-445.
5. Bica D, Armen J, Kulas AS, Young K, Womack Z. Reliability and precision of stress sonography of the ulnar collateral ligament. J Ultrasound Med. 2015;34(3):371-376.
6. Tomer K, Kleinbaum Y, Heyman Z, Dudkiewicz I, Blankstein A. Ultrasound diagnosis of fractures in adults. Akt Traumatol. 2006;36(4):171-174.
7. Middleton W, Payne WT, Teefey SA, Hidebolt CF, Rubin DA, Yamaguchi K. Sonography and MRI of the shoulder: comparison of patient satisfaction. AJR Am J Roentgenol. 2004;183(5):1449-1452.
8. Teefey SA, Rubin DA, Middleton WD, Hildebolt CF, Leibold RA, Yamaguchi K. Detection and quantification of rotator cuff tears. Comparison of ultrasonographic, magnetic resonance and arthroscopic finding in seventy-one consecutive cases. J Bone Joint Surg Am. 2004;86-A(4):708-716.
9. Roy-JS, Braën C, Leblond J, et al. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterization of rotator cuff disorders: a meta-analysis. Br J Sports Med. 2015;49(20):1316-1328.
10. Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal Imaging: Medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5(3):182-188.
11. Eustace J, Brophy D, Gibney R, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
12. Partington P, Broome G. Diagnostic injection around the shoulder: Hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
13. Rutten M, Maresch B, Jager G, de Waal Malefijt M. Injection of the subacromial-subdeltoid bursa: Blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
14. Kang M, Rizio L, Prybicien M, Middlemas D, Blacksin M. The accuracy of subacromial corticosteroid injections: A comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 Suppl):61S-66S.
15. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: An arthrographic evaluation. Arthroscopy. 2002;19(8):887-891.
16. Henkus HE, Cobben M, Coerkamp E, Nelissen R, van Arkel E. The accuracy of subacromial injections: A prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
17. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
18. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
19. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
20. Sibbitt WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intra-articular injections? J Rheumatol. 2009;36(9):1892-1902.
Emergency Imaging
Case
A 63-year-old woman with multiple medical conditions presented to the ED with abdominal distention, pain, and 4-day history of fever. Physical examination revealed a distended but nontender abdomen. Her vital signs included a fever of 103.1°F, tachycardia, tachypnea, and a blood pressure of 101/69. To further evaluate the abdominal distention, supine abdominal radiographs were obtained (Figure 1a and 1b).
What is the differential diagnosis?
What would be the most appropriate next imaging test?
Answer
The abdominal radiographs demonstrated a nonobstructed bowel gas pattern—ie, there were no dilated loops of small or large bowel (>3 cm or >7 cm, respectively). Although limited by supine position, there was no evidence of perforation as no signs of free air were visualized. There was a large amount of stool within the large bowel, appearing on radiographic images as the mottled air and soft-tissue density (white asterisks, Figure 2a and 2b).
Although bowel obstruction had been ruled out in this patient, the differential for abdominal pain with fever remained wide, predominately for infectious and inflammatory conditions (eg, appendicitis, diverticulitis, inflammatory bowel disease, other forms of colitis/enteritis, pancreatitis, abscess, mesenteric).In cases such as the one presented, the most appropriate next imaging examination would be a computed tomography (CT) scan with both oral and intravenous (IV) contrast. Oral contrast is useful since several of the conditions in the differential diagnosis require evaluation of the bowel wall and, in cases of suspected abscess, it assists in differentiating a collection from adjacent loops of bowel. Intravenous contrast is useful to evaluate for inflammation and assessing the bowel.
In this case, an abdominal CT was performed, using both oral and IV contrast. Axial and sagittal images demonstrated a large amount of stool in a distended rectum (white asterisks, Figures 3a and 3b), thickening of the rectal wall (white arrows, Figures 3a and 3b), and stranding of the perirectal fat indicative of perirectal inflammation (red arrows, Figures 3a and 3b). Based on these findings, the patient was diagnosed with stercoral colitis.
Stercoral colitis is inflammation of the colonic or rectal wall secondary to increased intraluminal pressure caused by impacted fecal material. This condition is most common in elderly patients and bedridden patients with chronic constipation. As this case illustrates, the clinical presentation of stercoral colitis is nonspecific, with wide range of symptoms including constipation, abdominal distention, vomiting, abdominal tenderness, peritonitis, fever, and sepsis.1
Computed tomography of the abdomen and pelvis is the most reliable test for detecting stercoral colitis and its associated complications. Characteristic findings, as seen in the presented patient, include a large dense mass of fecal material, focal or diffuse thickening of the colonic wall, and pericolonic fat-stranding that affects the impacted region. When ulceration or perforation occurs, CT will reveal and extra luminal gas and/or abscess.1,3
Since stercoral colitis is associated with a reported mortality rate of 32% to 57%, with death often occurring within the first 24 hours from presentation, rapid diagnosis is essential.1 Once diagnosed, stercoral colitis is treated with aggressive fecal disimpaction, hyperosmolar enema, and, when indicated, surgery. The patient in this case was admitted for treatment. Unfortunately, despite all appropriate therapy efforts, she succumbed due to medical complications of her underlying illnesses.
Dr Belfi is an assistant professor of radiology at Weill Cornell Medical College in New York City, and an assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Hildick-Smith is a medical student at Weill Cornell Medical College in New York City. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center; and associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
- Saksonov M, Bachar GN, Morgenstern S. Stercoral colitis: a lethal disease-computed tomographic findings and clinical characteristic. J Comput Assist Tomogr. 2014;38(5):721-726.
- Gans SL, Stoker J, Boermeester MA. Plain abdominal radiography in acute abdominal pain; past, present, and future. Int J Gen Med. 2012;5:525-533.
- Heffernan C, Pachter HL, Megibow AJ, Macari M. Stercoral colitis leading to fatal peritonitis: CT findings. AJR Am J Roentgenol. 2005;184(4):1189-1193.
Case
A 63-year-old woman with multiple medical conditions presented to the ED with abdominal distention, pain, and 4-day history of fever. Physical examination revealed a distended but nontender abdomen. Her vital signs included a fever of 103.1°F, tachycardia, tachypnea, and a blood pressure of 101/69. To further evaluate the abdominal distention, supine abdominal radiographs were obtained (Figure 1a and 1b).
What is the differential diagnosis?
What would be the most appropriate next imaging test?
Answer
The abdominal radiographs demonstrated a nonobstructed bowel gas pattern—ie, there were no dilated loops of small or large bowel (>3 cm or >7 cm, respectively). Although limited by supine position, there was no evidence of perforation as no signs of free air were visualized. There was a large amount of stool within the large bowel, appearing on radiographic images as the mottled air and soft-tissue density (white asterisks, Figure 2a and 2b).
Although bowel obstruction had been ruled out in this patient, the differential for abdominal pain with fever remained wide, predominately for infectious and inflammatory conditions (eg, appendicitis, diverticulitis, inflammatory bowel disease, other forms of colitis/enteritis, pancreatitis, abscess, mesenteric).In cases such as the one presented, the most appropriate next imaging examination would be a computed tomography (CT) scan with both oral and intravenous (IV) contrast. Oral contrast is useful since several of the conditions in the differential diagnosis require evaluation of the bowel wall and, in cases of suspected abscess, it assists in differentiating a collection from adjacent loops of bowel. Intravenous contrast is useful to evaluate for inflammation and assessing the bowel.
In this case, an abdominal CT was performed, using both oral and IV contrast. Axial and sagittal images demonstrated a large amount of stool in a distended rectum (white asterisks, Figures 3a and 3b), thickening of the rectal wall (white arrows, Figures 3a and 3b), and stranding of the perirectal fat indicative of perirectal inflammation (red arrows, Figures 3a and 3b). Based on these findings, the patient was diagnosed with stercoral colitis.
Stercoral colitis is inflammation of the colonic or rectal wall secondary to increased intraluminal pressure caused by impacted fecal material. This condition is most common in elderly patients and bedridden patients with chronic constipation. As this case illustrates, the clinical presentation of stercoral colitis is nonspecific, with wide range of symptoms including constipation, abdominal distention, vomiting, abdominal tenderness, peritonitis, fever, and sepsis.1
Computed tomography of the abdomen and pelvis is the most reliable test for detecting stercoral colitis and its associated complications. Characteristic findings, as seen in the presented patient, include a large dense mass of fecal material, focal or diffuse thickening of the colonic wall, and pericolonic fat-stranding that affects the impacted region. When ulceration or perforation occurs, CT will reveal and extra luminal gas and/or abscess.1,3
Since stercoral colitis is associated with a reported mortality rate of 32% to 57%, with death often occurring within the first 24 hours from presentation, rapid diagnosis is essential.1 Once diagnosed, stercoral colitis is treated with aggressive fecal disimpaction, hyperosmolar enema, and, when indicated, surgery. The patient in this case was admitted for treatment. Unfortunately, despite all appropriate therapy efforts, she succumbed due to medical complications of her underlying illnesses.
Dr Belfi is an assistant professor of radiology at Weill Cornell Medical College in New York City, and an assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Hildick-Smith is a medical student at Weill Cornell Medical College in New York City. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center; and associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
Case
A 63-year-old woman with multiple medical conditions presented to the ED with abdominal distention, pain, and 4-day history of fever. Physical examination revealed a distended but nontender abdomen. Her vital signs included a fever of 103.1°F, tachycardia, tachypnea, and a blood pressure of 101/69. To further evaluate the abdominal distention, supine abdominal radiographs were obtained (Figure 1a and 1b).
What is the differential diagnosis?
What would be the most appropriate next imaging test?
Answer
The abdominal radiographs demonstrated a nonobstructed bowel gas pattern—ie, there were no dilated loops of small or large bowel (>3 cm or >7 cm, respectively). Although limited by supine position, there was no evidence of perforation as no signs of free air were visualized. There was a large amount of stool within the large bowel, appearing on radiographic images as the mottled air and soft-tissue density (white asterisks, Figure 2a and 2b).
Although bowel obstruction had been ruled out in this patient, the differential for abdominal pain with fever remained wide, predominately for infectious and inflammatory conditions (eg, appendicitis, diverticulitis, inflammatory bowel disease, other forms of colitis/enteritis, pancreatitis, abscess, mesenteric).In cases such as the one presented, the most appropriate next imaging examination would be a computed tomography (CT) scan with both oral and intravenous (IV) contrast. Oral contrast is useful since several of the conditions in the differential diagnosis require evaluation of the bowel wall and, in cases of suspected abscess, it assists in differentiating a collection from adjacent loops of bowel. Intravenous contrast is useful to evaluate for inflammation and assessing the bowel.
In this case, an abdominal CT was performed, using both oral and IV contrast. Axial and sagittal images demonstrated a large amount of stool in a distended rectum (white asterisks, Figures 3a and 3b), thickening of the rectal wall (white arrows, Figures 3a and 3b), and stranding of the perirectal fat indicative of perirectal inflammation (red arrows, Figures 3a and 3b). Based on these findings, the patient was diagnosed with stercoral colitis.
Stercoral colitis is inflammation of the colonic or rectal wall secondary to increased intraluminal pressure caused by impacted fecal material. This condition is most common in elderly patients and bedridden patients with chronic constipation. As this case illustrates, the clinical presentation of stercoral colitis is nonspecific, with wide range of symptoms including constipation, abdominal distention, vomiting, abdominal tenderness, peritonitis, fever, and sepsis.1
Computed tomography of the abdomen and pelvis is the most reliable test for detecting stercoral colitis and its associated complications. Characteristic findings, as seen in the presented patient, include a large dense mass of fecal material, focal or diffuse thickening of the colonic wall, and pericolonic fat-stranding that affects the impacted region. When ulceration or perforation occurs, CT will reveal and extra luminal gas and/or abscess.1,3
Since stercoral colitis is associated with a reported mortality rate of 32% to 57%, with death often occurring within the first 24 hours from presentation, rapid diagnosis is essential.1 Once diagnosed, stercoral colitis is treated with aggressive fecal disimpaction, hyperosmolar enema, and, when indicated, surgery. The patient in this case was admitted for treatment. Unfortunately, despite all appropriate therapy efforts, she succumbed due to medical complications of her underlying illnesses.
Dr Belfi is an assistant professor of radiology at Weill Cornell Medical College in New York City, and an assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Hildick-Smith is a medical student at Weill Cornell Medical College in New York City. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center; and associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
- Saksonov M, Bachar GN, Morgenstern S. Stercoral colitis: a lethal disease-computed tomographic findings and clinical characteristic. J Comput Assist Tomogr. 2014;38(5):721-726.
- Gans SL, Stoker J, Boermeester MA. Plain abdominal radiography in acute abdominal pain; past, present, and future. Int J Gen Med. 2012;5:525-533.
- Heffernan C, Pachter HL, Megibow AJ, Macari M. Stercoral colitis leading to fatal peritonitis: CT findings. AJR Am J Roentgenol. 2005;184(4):1189-1193.
- Saksonov M, Bachar GN, Morgenstern S. Stercoral colitis: a lethal disease-computed tomographic findings and clinical characteristic. J Comput Assist Tomogr. 2014;38(5):721-726.
- Gans SL, Stoker J, Boermeester MA. Plain abdominal radiography in acute abdominal pain; past, present, and future. Int J Gen Med. 2012;5:525-533.
- Heffernan C, Pachter HL, Megibow AJ, Macari M. Stercoral colitis leading to fatal peritonitis: CT findings. AJR Am J Roentgenol. 2005;184(4):1189-1193.
Novel Intraoperative Technique to Visualize the Lower Cervical Spine: A Case Series
Two adequate views of the lower cervical vertebrae are necessary to confirm the 3-dimensional location of any hardware placed during cervical spine fusion. Visualizing the lower cervical vertebrae in 2 planes intraoperatively is often a challenge because the shoulders obstruct the lateral view.1 Techniques have been described to improve lateral visualization, including gentle traction of the arms via wrist restraints or taping the shoulders down inferiorly.2,3 These techniques have their inadequacies, including an association with peripheral nerve injury and brachial plexopathy.4 In patients with stout necks, these methods may still be insufficient to achieve adequate visualization of the lower cervical vertebrae.
Invasive techniques to improve visualization have also been described. In 1 study, exposure had to be extended cephalad to allow for manual counting of cervical vertebrae when the mid- to lower cervical vertebrae had to be identified in a morbidly obese patient.5 More invasive spine procedures are associated with higher rates of complications, increased blood loss, more soft-tissue trauma, and longer hospital stays.6 We present a view 30º oblique from horizontal and 30º cephalad from neutral as a variation of the lateral radiograph that improves visualization of the mid- to lower cervical vertebrae. The authors have obtained the patients’ informed written consent for print and electronic publication of these case reports.
Technique
We used either the Smith-Robinson or Cloward approach to the anterior spine. Both techniques use the avascular plane between the medially located esophagus and trachea and the lateral sternocleidomastoid and carotid sheath to approach the anterior cervical spine. Once adequate exposure was achieved, standard anteroposterior and lateral radiographs were obtained to confirm the correct vertebral level. Gentle caudal traction was applied to the patient’s wrist straps, and when visualization continued to be compromised, a view 30º oblique from horizontal and 30º cephalad from neutral was obtained (Figure 1).
Case Series
Case 1
A 54-year-old man with a body mass index (BMI) of 50 presented with neck and bilateral arm pain, with left greater than right radicular symptoms in the C6 and C7 distribution. Magnetic resonance imaging (MRI) showed disc herniations at C5-C6 and C6-C7 with spinal cord signal changes, and he underwent a C5-C6 and C6-C7 anterior cervical discectomy and fusion. Initial localization was determined using a lateral radiograph and vertebral needle. During hardware placement, anteroposterior and lateral fluoroscopic radiographs confirmed adequate placement of the superior screw, but visualization of the inferior portion of the plate and inferior screw was challenging (Figure 2). Our oblique 30º–30º view provided better visualization of the plate and screws in the lower cervical vertebrae than lateral imaging, and allowed confirmation that the hardware was positioned correctly (Figure 3). It took 1 attempt to achieve adequate visualization with the 30º–30º view.
Postoperatively, the patient’s radiculopathy and motor weakness improved. Radiographs confirmed adequate hardware placement, and he was discharged on postoperative day 1 (Figure 4). Imaging at the patient’s 6-week follow-up confirmed adequate fusion from C5-C7, anatomically aligned facet joints, and no hardware failure. The patient’s Neck Disability Index was 31/50 preoperatively and 26/50 at this visit.
Case 2
A 51-year-old man with a BMI of 29 presented with a long-standing history of neck pain and bilateral arm pain left greater than right in the C6 and C7 dermomyotome. MRI showed a broad-based disc herniation with foraminal narrowing at C5-C6 and C6-C7, and the patient underwent a 2-level anterior cervical discectomy and fusion. This patient had pronounced neck musculature, and a deeper than normal incision was required.
Intraoperative lateral fluoroscopy was obtained to confirm the C5-C6 and C6-C7 level prior to discectomy. The musculature of the patient’s neck and shoulder made visualization of the C6-C7 disc space difficult on the lateral radiograph (Figure 5). One attempt was required to obtain the 30º–30º oblique view, which was used to ensure correct placement of the screws and plate (Figure 6).
Postoperatively, the patient’s pain had improved, and radiographs confirmed adequate hardware placement. He was discharged 1 day after surgery (Figure 7). Imaging at the patient’s 6-week follow-up confirmed adequate fusion from C5-C7, stable disc spaces, and anatomically aligned facet joints. His Neck Disability Index was 34/50 preoperatively and 32/50 at 2-week follow-up.
Discussion
The aim of this study was to describe an alternative to the lateral radiograph for imaging the cervical spine in patients with challenging anatomy or in procedures involving hardware placement at the lower cervical vertebrae. Techniques have been developed to assist with improved lateral visualization, including gentle traction of the arms via wrist restraints or taping the shoulders down inferiorly.2,3 However, visualization in 2 planes continues to be a challenge in a subset of patients. It is particularly difficult to obtain adequate lateral radiographs of the cervical spine in patients with stout necks.3 In patients with stout necks, there is more obstruction of the radiography path through the cervical spine. This leads to imaging that is unclear or may fail to show the mid- to lower cervical spine. The extent to which one should rely on the 30º–30º oblique technique for adequate visualization of the cervical spine depends on the anatomy of a particular patient. Historically, it is more challenging to obtain satisfactory lateral radiographs in patients with stout necks,3 and these patients have benefited the most from using the 30º–30º degree oblique view.
Lack of visualization can lead to aborted surgeries or, potentially, surgery at the wrong level.3 A 2008 American Academy of Neurological Surgeons survey indicated that 50% of spine surgeons had performed a wrong-level surgery at least once in their career, and the cervical spine accounted for 21% of all incorrect-level spine surgeries.7 Intraoperative factors reported during cases of wrong-level spinal surgeries included misinterpretation of intraoperative imaging, no intraoperative imaging, and unusual anatomy or physical characteristics.8 Such complications can lead to revision surgery and other significant morbidities for the patient.
In most patients, fluoroscopy allows confirmation of the correct level before disc incision.3 However, operating at a lower cervical level in a patient with a short neck or prominent shoulders poses a significant problem.3 A case report from Singh and colleagues9 described a modified intraoperative fluoroscopic view for spinal level localization at cervicothoracic levels. Their method focuses on identifying the bony lamina and using them as landmarks to count spinal levels, whereas our 30º–30º oblique image is useful for confirmation of adequate hardware placement during anterior cervical spinal fusions. Often, the initial localization of cervical vertebral levels can be achieved with a standard lateral radiograph. We recognized the utility of the 30º–30º oblique view when we were attempting to visualize the inferior aspect of the plate and inferior screw placement.
In patients with stout necks, a lateral radiograph may show only visualization down to C4 or C5.3 Even with applying traction to the arms or taping the shoulders down, it can be impossible to visualize C6, C7, or T1 because the shoulder bones and muscles obstruct the image.3 Using a 30º–30º oblique view, we were able to obtain adequate visualization and assess the accurate placement of hardware.
Conclusion
A 30º oblique view from horizontal and 30º cephalad from neutral radiograph can be used intraoperatively in patients with challenging anatomy to identify placement of hardware at the correct vertebral level in the lower cervical spine. It is a noninvasive technique that can help reduce the risk of wrong-site surgeries without prolonging operation time. This technique describes an alternative to the lateral radiograph and provides a solution to the difficult problem of intraoperative imaging of the mid- to lower cervical spine in 2 adequate planes.
1. Bebawy JF, Koht A, Mirkovic S. Anterior cervical spine surgery. In: Khot A, Sloan TB, Toleikis JR, eds. Monitoring the Nervous System for Anesthesiologists and Other Health Care Professionals. New York, NY: Springer; 2012:539-554.
2. Abumi K, Shono Y, Ito M, Taneichi H, Kotani Y, Kaneda K. Complications of pedicle screw fixation in reconstructive surgery of the cervical spine. Spine. 2000;25(8):962-969.
3. Irace C. Intraoperative imaging for verification of the correct level during spinal surgery. In: Fountas KN, ed. Novel Frontiers of Advanced Neuroimaging. Rijeka, Croatia: Intech; 2013:175-188.
4. Schwartz DM, Sestokas AK, Hilibrand AS, et al. Neurophysiological identification of position-induced neurologic injury during anterior cervical spine surgery. J Clin Monit Comput. 2006;20(6):437-444.
5. Telfeian AE, Reiter GT, Durham SR, Marcotte P. Spine surgery in morbidly obese patients. J Neurosurg Spine. 2002;97(1):20-24.
6. Oppenheimer JH, DeCastro I, McDonnell DE. Minimally invasive spine technology and minimally invasive spine surgery: a historical review. Neurosurg Focus. 2009;27(3):E9.
7. Mody MG, Nourbakhsh A, Stahl DL, Gibbs M, Alfawareh M, Garges KJ. The prevalence of wrong level surgery among spine surgeons. Spine. 2008;33(2):194.
8. Jhawar BS, Mitsis D, Duggal N. Wrong-sided and wrong-level neurosurgery: A national survey. J Neurosurg Spine. 2007;7(5):467-472.
9. Singh H, Meyer SA, Hecht AC, Jenkins AL 3rd. Novel fluoroscopic technique for localization at cervicothoracic levels. J Spinal Disord Tech. 2009;22(8):615-618.
Two adequate views of the lower cervical vertebrae are necessary to confirm the 3-dimensional location of any hardware placed during cervical spine fusion. Visualizing the lower cervical vertebrae in 2 planes intraoperatively is often a challenge because the shoulders obstruct the lateral view.1 Techniques have been described to improve lateral visualization, including gentle traction of the arms via wrist restraints or taping the shoulders down inferiorly.2,3 These techniques have their inadequacies, including an association with peripheral nerve injury and brachial plexopathy.4 In patients with stout necks, these methods may still be insufficient to achieve adequate visualization of the lower cervical vertebrae.
Invasive techniques to improve visualization have also been described. In 1 study, exposure had to be extended cephalad to allow for manual counting of cervical vertebrae when the mid- to lower cervical vertebrae had to be identified in a morbidly obese patient.5 More invasive spine procedures are associated with higher rates of complications, increased blood loss, more soft-tissue trauma, and longer hospital stays.6 We present a view 30º oblique from horizontal and 30º cephalad from neutral as a variation of the lateral radiograph that improves visualization of the mid- to lower cervical vertebrae. The authors have obtained the patients’ informed written consent for print and electronic publication of these case reports.
Technique
We used either the Smith-Robinson or Cloward approach to the anterior spine. Both techniques use the avascular plane between the medially located esophagus and trachea and the lateral sternocleidomastoid and carotid sheath to approach the anterior cervical spine. Once adequate exposure was achieved, standard anteroposterior and lateral radiographs were obtained to confirm the correct vertebral level. Gentle caudal traction was applied to the patient’s wrist straps, and when visualization continued to be compromised, a view 30º oblique from horizontal and 30º cephalad from neutral was obtained (Figure 1).
Case Series
Case 1
A 54-year-old man with a body mass index (BMI) of 50 presented with neck and bilateral arm pain, with left greater than right radicular symptoms in the C6 and C7 distribution. Magnetic resonance imaging (MRI) showed disc herniations at C5-C6 and C6-C7 with spinal cord signal changes, and he underwent a C5-C6 and C6-C7 anterior cervical discectomy and fusion. Initial localization was determined using a lateral radiograph and vertebral needle. During hardware placement, anteroposterior and lateral fluoroscopic radiographs confirmed adequate placement of the superior screw, but visualization of the inferior portion of the plate and inferior screw was challenging (Figure 2). Our oblique 30º–30º view provided better visualization of the plate and screws in the lower cervical vertebrae than lateral imaging, and allowed confirmation that the hardware was positioned correctly (Figure 3). It took 1 attempt to achieve adequate visualization with the 30º–30º view.
Postoperatively, the patient’s radiculopathy and motor weakness improved. Radiographs confirmed adequate hardware placement, and he was discharged on postoperative day 1 (Figure 4). Imaging at the patient’s 6-week follow-up confirmed adequate fusion from C5-C7, anatomically aligned facet joints, and no hardware failure. The patient’s Neck Disability Index was 31/50 preoperatively and 26/50 at this visit.
Case 2
A 51-year-old man with a BMI of 29 presented with a long-standing history of neck pain and bilateral arm pain left greater than right in the C6 and C7 dermomyotome. MRI showed a broad-based disc herniation with foraminal narrowing at C5-C6 and C6-C7, and the patient underwent a 2-level anterior cervical discectomy and fusion. This patient had pronounced neck musculature, and a deeper than normal incision was required.
Intraoperative lateral fluoroscopy was obtained to confirm the C5-C6 and C6-C7 level prior to discectomy. The musculature of the patient’s neck and shoulder made visualization of the C6-C7 disc space difficult on the lateral radiograph (Figure 5). One attempt was required to obtain the 30º–30º oblique view, which was used to ensure correct placement of the screws and plate (Figure 6).
Postoperatively, the patient’s pain had improved, and radiographs confirmed adequate hardware placement. He was discharged 1 day after surgery (Figure 7). Imaging at the patient’s 6-week follow-up confirmed adequate fusion from C5-C7, stable disc spaces, and anatomically aligned facet joints. His Neck Disability Index was 34/50 preoperatively and 32/50 at 2-week follow-up.
Discussion
The aim of this study was to describe an alternative to the lateral radiograph for imaging the cervical spine in patients with challenging anatomy or in procedures involving hardware placement at the lower cervical vertebrae. Techniques have been developed to assist with improved lateral visualization, including gentle traction of the arms via wrist restraints or taping the shoulders down inferiorly.2,3 However, visualization in 2 planes continues to be a challenge in a subset of patients. It is particularly difficult to obtain adequate lateral radiographs of the cervical spine in patients with stout necks.3 In patients with stout necks, there is more obstruction of the radiography path through the cervical spine. This leads to imaging that is unclear or may fail to show the mid- to lower cervical spine. The extent to which one should rely on the 30º–30º oblique technique for adequate visualization of the cervical spine depends on the anatomy of a particular patient. Historically, it is more challenging to obtain satisfactory lateral radiographs in patients with stout necks,3 and these patients have benefited the most from using the 30º–30º degree oblique view.
Lack of visualization can lead to aborted surgeries or, potentially, surgery at the wrong level.3 A 2008 American Academy of Neurological Surgeons survey indicated that 50% of spine surgeons had performed a wrong-level surgery at least once in their career, and the cervical spine accounted for 21% of all incorrect-level spine surgeries.7 Intraoperative factors reported during cases of wrong-level spinal surgeries included misinterpretation of intraoperative imaging, no intraoperative imaging, and unusual anatomy or physical characteristics.8 Such complications can lead to revision surgery and other significant morbidities for the patient.
In most patients, fluoroscopy allows confirmation of the correct level before disc incision.3 However, operating at a lower cervical level in a patient with a short neck or prominent shoulders poses a significant problem.3 A case report from Singh and colleagues9 described a modified intraoperative fluoroscopic view for spinal level localization at cervicothoracic levels. Their method focuses on identifying the bony lamina and using them as landmarks to count spinal levels, whereas our 30º–30º oblique image is useful for confirmation of adequate hardware placement during anterior cervical spinal fusions. Often, the initial localization of cervical vertebral levels can be achieved with a standard lateral radiograph. We recognized the utility of the 30º–30º oblique view when we were attempting to visualize the inferior aspect of the plate and inferior screw placement.
In patients with stout necks, a lateral radiograph may show only visualization down to C4 or C5.3 Even with applying traction to the arms or taping the shoulders down, it can be impossible to visualize C6, C7, or T1 because the shoulder bones and muscles obstruct the image.3 Using a 30º–30º oblique view, we were able to obtain adequate visualization and assess the accurate placement of hardware.
Conclusion
A 30º oblique view from horizontal and 30º cephalad from neutral radiograph can be used intraoperatively in patients with challenging anatomy to identify placement of hardware at the correct vertebral level in the lower cervical spine. It is a noninvasive technique that can help reduce the risk of wrong-site surgeries without prolonging operation time. This technique describes an alternative to the lateral radiograph and provides a solution to the difficult problem of intraoperative imaging of the mid- to lower cervical spine in 2 adequate planes.
Two adequate views of the lower cervical vertebrae are necessary to confirm the 3-dimensional location of any hardware placed during cervical spine fusion. Visualizing the lower cervical vertebrae in 2 planes intraoperatively is often a challenge because the shoulders obstruct the lateral view.1 Techniques have been described to improve lateral visualization, including gentle traction of the arms via wrist restraints or taping the shoulders down inferiorly.2,3 These techniques have their inadequacies, including an association with peripheral nerve injury and brachial plexopathy.4 In patients with stout necks, these methods may still be insufficient to achieve adequate visualization of the lower cervical vertebrae.
Invasive techniques to improve visualization have also been described. In 1 study, exposure had to be extended cephalad to allow for manual counting of cervical vertebrae when the mid- to lower cervical vertebrae had to be identified in a morbidly obese patient.5 More invasive spine procedures are associated with higher rates of complications, increased blood loss, more soft-tissue trauma, and longer hospital stays.6 We present a view 30º oblique from horizontal and 30º cephalad from neutral as a variation of the lateral radiograph that improves visualization of the mid- to lower cervical vertebrae. The authors have obtained the patients’ informed written consent for print and electronic publication of these case reports.
Technique
We used either the Smith-Robinson or Cloward approach to the anterior spine. Both techniques use the avascular plane between the medially located esophagus and trachea and the lateral sternocleidomastoid and carotid sheath to approach the anterior cervical spine. Once adequate exposure was achieved, standard anteroposterior and lateral radiographs were obtained to confirm the correct vertebral level. Gentle caudal traction was applied to the patient’s wrist straps, and when visualization continued to be compromised, a view 30º oblique from horizontal and 30º cephalad from neutral was obtained (Figure 1).
Case Series
Case 1
A 54-year-old man with a body mass index (BMI) of 50 presented with neck and bilateral arm pain, with left greater than right radicular symptoms in the C6 and C7 distribution. Magnetic resonance imaging (MRI) showed disc herniations at C5-C6 and C6-C7 with spinal cord signal changes, and he underwent a C5-C6 and C6-C7 anterior cervical discectomy and fusion. Initial localization was determined using a lateral radiograph and vertebral needle. During hardware placement, anteroposterior and lateral fluoroscopic radiographs confirmed adequate placement of the superior screw, but visualization of the inferior portion of the plate and inferior screw was challenging (Figure 2). Our oblique 30º–30º view provided better visualization of the plate and screws in the lower cervical vertebrae than lateral imaging, and allowed confirmation that the hardware was positioned correctly (Figure 3). It took 1 attempt to achieve adequate visualization with the 30º–30º view.
Postoperatively, the patient’s radiculopathy and motor weakness improved. Radiographs confirmed adequate hardware placement, and he was discharged on postoperative day 1 (Figure 4). Imaging at the patient’s 6-week follow-up confirmed adequate fusion from C5-C7, anatomically aligned facet joints, and no hardware failure. The patient’s Neck Disability Index was 31/50 preoperatively and 26/50 at this visit.
Case 2
A 51-year-old man with a BMI of 29 presented with a long-standing history of neck pain and bilateral arm pain left greater than right in the C6 and C7 dermomyotome. MRI showed a broad-based disc herniation with foraminal narrowing at C5-C6 and C6-C7, and the patient underwent a 2-level anterior cervical discectomy and fusion. This patient had pronounced neck musculature, and a deeper than normal incision was required.
Intraoperative lateral fluoroscopy was obtained to confirm the C5-C6 and C6-C7 level prior to discectomy. The musculature of the patient’s neck and shoulder made visualization of the C6-C7 disc space difficult on the lateral radiograph (Figure 5). One attempt was required to obtain the 30º–30º oblique view, which was used to ensure correct placement of the screws and plate (Figure 6).
Postoperatively, the patient’s pain had improved, and radiographs confirmed adequate hardware placement. He was discharged 1 day after surgery (Figure 7). Imaging at the patient’s 6-week follow-up confirmed adequate fusion from C5-C7, stable disc spaces, and anatomically aligned facet joints. His Neck Disability Index was 34/50 preoperatively and 32/50 at 2-week follow-up.
Discussion
The aim of this study was to describe an alternative to the lateral radiograph for imaging the cervical spine in patients with challenging anatomy or in procedures involving hardware placement at the lower cervical vertebrae. Techniques have been developed to assist with improved lateral visualization, including gentle traction of the arms via wrist restraints or taping the shoulders down inferiorly.2,3 However, visualization in 2 planes continues to be a challenge in a subset of patients. It is particularly difficult to obtain adequate lateral radiographs of the cervical spine in patients with stout necks.3 In patients with stout necks, there is more obstruction of the radiography path through the cervical spine. This leads to imaging that is unclear or may fail to show the mid- to lower cervical spine. The extent to which one should rely on the 30º–30º oblique technique for adequate visualization of the cervical spine depends on the anatomy of a particular patient. Historically, it is more challenging to obtain satisfactory lateral radiographs in patients with stout necks,3 and these patients have benefited the most from using the 30º–30º degree oblique view.
Lack of visualization can lead to aborted surgeries or, potentially, surgery at the wrong level.3 A 2008 American Academy of Neurological Surgeons survey indicated that 50% of spine surgeons had performed a wrong-level surgery at least once in their career, and the cervical spine accounted for 21% of all incorrect-level spine surgeries.7 Intraoperative factors reported during cases of wrong-level spinal surgeries included misinterpretation of intraoperative imaging, no intraoperative imaging, and unusual anatomy or physical characteristics.8 Such complications can lead to revision surgery and other significant morbidities for the patient.
In most patients, fluoroscopy allows confirmation of the correct level before disc incision.3 However, operating at a lower cervical level in a patient with a short neck or prominent shoulders poses a significant problem.3 A case report from Singh and colleagues9 described a modified intraoperative fluoroscopic view for spinal level localization at cervicothoracic levels. Their method focuses on identifying the bony lamina and using them as landmarks to count spinal levels, whereas our 30º–30º oblique image is useful for confirmation of adequate hardware placement during anterior cervical spinal fusions. Often, the initial localization of cervical vertebral levels can be achieved with a standard lateral radiograph. We recognized the utility of the 30º–30º oblique view when we were attempting to visualize the inferior aspect of the plate and inferior screw placement.
In patients with stout necks, a lateral radiograph may show only visualization down to C4 or C5.3 Even with applying traction to the arms or taping the shoulders down, it can be impossible to visualize C6, C7, or T1 because the shoulder bones and muscles obstruct the image.3 Using a 30º–30º oblique view, we were able to obtain adequate visualization and assess the accurate placement of hardware.
Conclusion
A 30º oblique view from horizontal and 30º cephalad from neutral radiograph can be used intraoperatively in patients with challenging anatomy to identify placement of hardware at the correct vertebral level in the lower cervical spine. It is a noninvasive technique that can help reduce the risk of wrong-site surgeries without prolonging operation time. This technique describes an alternative to the lateral radiograph and provides a solution to the difficult problem of intraoperative imaging of the mid- to lower cervical spine in 2 adequate planes.
1. Bebawy JF, Koht A, Mirkovic S. Anterior cervical spine surgery. In: Khot A, Sloan TB, Toleikis JR, eds. Monitoring the Nervous System for Anesthesiologists and Other Health Care Professionals. New York, NY: Springer; 2012:539-554.
2. Abumi K, Shono Y, Ito M, Taneichi H, Kotani Y, Kaneda K. Complications of pedicle screw fixation in reconstructive surgery of the cervical spine. Spine. 2000;25(8):962-969.
3. Irace C. Intraoperative imaging for verification of the correct level during spinal surgery. In: Fountas KN, ed. Novel Frontiers of Advanced Neuroimaging. Rijeka, Croatia: Intech; 2013:175-188.
4. Schwartz DM, Sestokas AK, Hilibrand AS, et al. Neurophysiological identification of position-induced neurologic injury during anterior cervical spine surgery. J Clin Monit Comput. 2006;20(6):437-444.
5. Telfeian AE, Reiter GT, Durham SR, Marcotte P. Spine surgery in morbidly obese patients. J Neurosurg Spine. 2002;97(1):20-24.
6. Oppenheimer JH, DeCastro I, McDonnell DE. Minimally invasive spine technology and minimally invasive spine surgery: a historical review. Neurosurg Focus. 2009;27(3):E9.
7. Mody MG, Nourbakhsh A, Stahl DL, Gibbs M, Alfawareh M, Garges KJ. The prevalence of wrong level surgery among spine surgeons. Spine. 2008;33(2):194.
8. Jhawar BS, Mitsis D, Duggal N. Wrong-sided and wrong-level neurosurgery: A national survey. J Neurosurg Spine. 2007;7(5):467-472.
9. Singh H, Meyer SA, Hecht AC, Jenkins AL 3rd. Novel fluoroscopic technique for localization at cervicothoracic levels. J Spinal Disord Tech. 2009;22(8):615-618.
1. Bebawy JF, Koht A, Mirkovic S. Anterior cervical spine surgery. In: Khot A, Sloan TB, Toleikis JR, eds. Monitoring the Nervous System for Anesthesiologists and Other Health Care Professionals. New York, NY: Springer; 2012:539-554.
2. Abumi K, Shono Y, Ito M, Taneichi H, Kotani Y, Kaneda K. Complications of pedicle screw fixation in reconstructive surgery of the cervical spine. Spine. 2000;25(8):962-969.
3. Irace C. Intraoperative imaging for verification of the correct level during spinal surgery. In: Fountas KN, ed. Novel Frontiers of Advanced Neuroimaging. Rijeka, Croatia: Intech; 2013:175-188.
4. Schwartz DM, Sestokas AK, Hilibrand AS, et al. Neurophysiological identification of position-induced neurologic injury during anterior cervical spine surgery. J Clin Monit Comput. 2006;20(6):437-444.
5. Telfeian AE, Reiter GT, Durham SR, Marcotte P. Spine surgery in morbidly obese patients. J Neurosurg Spine. 2002;97(1):20-24.
6. Oppenheimer JH, DeCastro I, McDonnell DE. Minimally invasive spine technology and minimally invasive spine surgery: a historical review. Neurosurg Focus. 2009;27(3):E9.
7. Mody MG, Nourbakhsh A, Stahl DL, Gibbs M, Alfawareh M, Garges KJ. The prevalence of wrong level surgery among spine surgeons. Spine. 2008;33(2):194.
8. Jhawar BS, Mitsis D, Duggal N. Wrong-sided and wrong-level neurosurgery: A national survey. J Neurosurg Spine. 2007;7(5):467-472.
9. Singh H, Meyer SA, Hecht AC, Jenkins AL 3rd. Novel fluoroscopic technique for localization at cervicothoracic levels. J Spinal Disord Tech. 2009;22(8):615-618.
Navigating the Alphabet Soup of Labroligamentous Pathology of the Shoulder
The widespread use of eponyms and acronyms to describe labroligamentous findings in the shoulder has made interpretation of shoulder magnetic resonance imaging (MRI) reports challenging. We review and discuss the appearance of these lesions on shoulder MRI to help the orthopedic surgeon understand these entities as imaging findings.
Glenolabral articular disruption (GLAD) occurs secondary to impaction of the humeral head on the glenoid articular cartilage. There is a resultant defect in the glenoid articular cartilage, which extends to the glenoid labrum. A GLAD lesion is diagnosed only if the glenohumeral ligament and scapular periosteum remain intact1 (Figure 1).
Complete detachment of the anteroinferior labrum with tearing of the anterior glenoid periosteum represents a Bankart lesion. Cartilaginous Bankart lesions are caused by an anterior glenohumeral dislocation with resultant avulsion of the anteroinferior labrum and disruption of the scapular periosteum because of acute traction on the anterior band of the inferior glenohumeral ligament (Figure 2). Anterior instability, caused by disruption of the anterior labroligamentous complex, results. Osseous Bankart lesions occur when the anterior displaced humeral head impacts the anterior inferior glenoid rim, causing a fracture (Figure 3). This loss of the glenoid articular surface area can result in glenohumeral instability. Posterior shoulder dislocations can result in corresponding findings in the posterior inferior glenoid labrum (reverse Bankart lesion) and anterior medial humeral head (reverse Hill-Sachs lesion) (Figure 2).
A variant of the Bankart lesion is the anterior labroligamentous periosteal sleeve avulsion (ALPSA). This refers to a medially displaced tear of the anterior labrum with intact periosteal stripping along the medial glenoid2with medial rotation and inferior displacement of the anterior inferior labrum along the scapular neck. An ALPSA lesion can heal via the intact periosteal blood supply. If not repaired, anterior instability will result because of malposition of the labrum, causing a patulous anterior capsule.3 When a corresponding lesion occurs in the posterior labrum because of a posterior dislocation, it is called a posterior labrocapsular periosteal sleeve avulsion (POLPSA) (Figure 4).
Another variant of the Bankart lesion is the Perthes lesion, which is a nondisplaced tear of the anteroinferior labrum with periosteal stripping. This differs from the ALPSA because the detached labrum and periosteum are held in anatomic position, possibly making the lesion difficult to detect on magnetic resonance arthrography (MRA).3 Obtaining images in the abduction external rotation (ABER) position exerts traction on the anterior inferior joint capsule and may make the Perthes lesion more conspicuous.4 When this occurs in the posterior labrum, it is called a reverse Perthes lesion (Figure 5).
In a patient with anterior glenohumeral instability without a Bankart lesion, pathology of the anterior band of the inferior glenohumeral ligament (IGHL) at its humeral attachment must be suspected. Humeral avulsion of the IGHL (HAGL) or its variants can be overlooked on arthroscopy. HAGL is diagnosed on MRA when the normally U-shaped IGHL takes on a J-shape, and joint fluid extravasates across the torn humeral attachment (Figure 6). If there is an avulsed bony fragment from the medial humeral neck, the lesion is termed a bony HAGL (BHAGL). In addition to the findings of a HAGL, a BHAGL shows the osseous fragment and donor site on MRI. Since a BHAGL is a bony avulsion, it can even be suggested on radiography if a bony fragment is seen adjacent to the medial humeral neck.5 These lesions are highly associated with other shoulder injuries, particularly Hill-Sachs deformities and subscapularis tendon tears, and it is imperative, therefore, to search for additional injuries if a HAGL-type injury is seen.6
A more uncommon type of HAGL can occur in the setting of posterior capsulolabral injury. A posterior-band IGHL avulsion from the humerus (PHAGL) has similar imaging findings to a HAGL, except that it involves the posterior band of the IGHL. PHAGLs are usually not associated with an acute injury and are thought to be related to repetitive microtrauma, perhaps since the posterior band of the IGHL is the thinnest portion of the IGHL complex.7
A Kim lesion is an arthroscopic finding described in patients with posterior instability as a superficial defect at the undersurface of the posterior labrum and adjacent glenoid cartilage without detachment or extension to the chondrolabral junction.8 It is, by its nature, a concealed finding on routine MRI but can be more conspicuous in FADIR (flexed, adducted, internally rotated) positioning on MRA, which exerts traction on the posterior joint capsule, allowing intra-articular contrast to fill the tear (Figure 7).
This list describes several of the most commonly encountered acronyms in shoulder MRI. A review of SLAP (superior labrum anterior to posterior) lesions was described in a previous article in the journal’s Imaging Series.9 A thorough understanding of these lesions is helpful in interpreting reports and determining the appropriate treatment for patients with shoulder injuries.
1. Sanders TG, Tirman PF, Linares R, Feller JF, Richardson R. The glenolabral articular disruption lesion: MR arthrography with arthroscopic correlation. AJR Am J Roentgenol. 1999;172(1):171-175.
2. Beltran J, Jbara M, Maimon R. Shoulder: labrum and bicipital tendon. Top Magn Reson Imaging. 2003;14(1):35-50.
3. Waldt S, Burkart A, Imhoff AB, Bruegel M, Rummeny EJ, Woertler K. Anterior shoulder instability: accuracy of MR arthrography in the classification of anteroinferior labroligamentous injuries. Radiology. 2005;237(2):578-583.
4. Schreinemachers SA, van der Hulst VP, Willems J, Bipat S, van der Woude H. Is a single direct MR arthrography series in ABER position as accurate in detecting anteroinferior labroligamentous lesions as conventional MR arthrography? Skeletal Radiol. 2009;38(7):675-683.
5. Bui-Mansfield LT, Taylor DC, Uhorchak JM, Tenuta JT. Humeral avulsions of the glenohumeral ligament: imaging features and a review of the literature. AJR Am J Roentgenol. 2002;179(3):649-655.
6. Magee T. Prevalence of HAGL lesions and associated abnormalities on shoulder MR examination. Skeletal Radiol. 2014;43(3):307-313.
7. Chung CB, Sorenson S, Dwek JR, Resnick D. Humeral avulsion of the posterior band of the inferior glenohumeral ligament: MR arthrography and clinical correlation in 17 patients. AJR Am J Roentgenol. 2004;183(2):355-359.
8. Kim SH, Ha KI, Yoo JC, Noh KC. Kim’s lesion: an incomplete and concealed avulsion of the posteroinferior labrum in posterior or multidirectional posteroinferior instability of the shoulder. Arthroscopy. 2004;20(7):712-720.
9. Grubin J, Maderazo A, Fitzpatrick D. Imaging evaluation of superior labral anteroposterior (SLAP) tears. Am J Orthop. 2015;44(10):476-477.
The widespread use of eponyms and acronyms to describe labroligamentous findings in the shoulder has made interpretation of shoulder magnetic resonance imaging (MRI) reports challenging. We review and discuss the appearance of these lesions on shoulder MRI to help the orthopedic surgeon understand these entities as imaging findings.
Glenolabral articular disruption (GLAD) occurs secondary to impaction of the humeral head on the glenoid articular cartilage. There is a resultant defect in the glenoid articular cartilage, which extends to the glenoid labrum. A GLAD lesion is diagnosed only if the glenohumeral ligament and scapular periosteum remain intact1 (Figure 1).
Complete detachment of the anteroinferior labrum with tearing of the anterior glenoid periosteum represents a Bankart lesion. Cartilaginous Bankart lesions are caused by an anterior glenohumeral dislocation with resultant avulsion of the anteroinferior labrum and disruption of the scapular periosteum because of acute traction on the anterior band of the inferior glenohumeral ligament (Figure 2). Anterior instability, caused by disruption of the anterior labroligamentous complex, results. Osseous Bankart lesions occur when the anterior displaced humeral head impacts the anterior inferior glenoid rim, causing a fracture (Figure 3). This loss of the glenoid articular surface area can result in glenohumeral instability. Posterior shoulder dislocations can result in corresponding findings in the posterior inferior glenoid labrum (reverse Bankart lesion) and anterior medial humeral head (reverse Hill-Sachs lesion) (Figure 2).
A variant of the Bankart lesion is the anterior labroligamentous periosteal sleeve avulsion (ALPSA). This refers to a medially displaced tear of the anterior labrum with intact periosteal stripping along the medial glenoid2with medial rotation and inferior displacement of the anterior inferior labrum along the scapular neck. An ALPSA lesion can heal via the intact periosteal blood supply. If not repaired, anterior instability will result because of malposition of the labrum, causing a patulous anterior capsule.3 When a corresponding lesion occurs in the posterior labrum because of a posterior dislocation, it is called a posterior labrocapsular periosteal sleeve avulsion (POLPSA) (Figure 4).
Another variant of the Bankart lesion is the Perthes lesion, which is a nondisplaced tear of the anteroinferior labrum with periosteal stripping. This differs from the ALPSA because the detached labrum and periosteum are held in anatomic position, possibly making the lesion difficult to detect on magnetic resonance arthrography (MRA).3 Obtaining images in the abduction external rotation (ABER) position exerts traction on the anterior inferior joint capsule and may make the Perthes lesion more conspicuous.4 When this occurs in the posterior labrum, it is called a reverse Perthes lesion (Figure 5).
In a patient with anterior glenohumeral instability without a Bankart lesion, pathology of the anterior band of the inferior glenohumeral ligament (IGHL) at its humeral attachment must be suspected. Humeral avulsion of the IGHL (HAGL) or its variants can be overlooked on arthroscopy. HAGL is diagnosed on MRA when the normally U-shaped IGHL takes on a J-shape, and joint fluid extravasates across the torn humeral attachment (Figure 6). If there is an avulsed bony fragment from the medial humeral neck, the lesion is termed a bony HAGL (BHAGL). In addition to the findings of a HAGL, a BHAGL shows the osseous fragment and donor site on MRI. Since a BHAGL is a bony avulsion, it can even be suggested on radiography if a bony fragment is seen adjacent to the medial humeral neck.5 These lesions are highly associated with other shoulder injuries, particularly Hill-Sachs deformities and subscapularis tendon tears, and it is imperative, therefore, to search for additional injuries if a HAGL-type injury is seen.6
A more uncommon type of HAGL can occur in the setting of posterior capsulolabral injury. A posterior-band IGHL avulsion from the humerus (PHAGL) has similar imaging findings to a HAGL, except that it involves the posterior band of the IGHL. PHAGLs are usually not associated with an acute injury and are thought to be related to repetitive microtrauma, perhaps since the posterior band of the IGHL is the thinnest portion of the IGHL complex.7
A Kim lesion is an arthroscopic finding described in patients with posterior instability as a superficial defect at the undersurface of the posterior labrum and adjacent glenoid cartilage without detachment or extension to the chondrolabral junction.8 It is, by its nature, a concealed finding on routine MRI but can be more conspicuous in FADIR (flexed, adducted, internally rotated) positioning on MRA, which exerts traction on the posterior joint capsule, allowing intra-articular contrast to fill the tear (Figure 7).
This list describes several of the most commonly encountered acronyms in shoulder MRI. A review of SLAP (superior labrum anterior to posterior) lesions was described in a previous article in the journal’s Imaging Series.9 A thorough understanding of these lesions is helpful in interpreting reports and determining the appropriate treatment for patients with shoulder injuries.
The widespread use of eponyms and acronyms to describe labroligamentous findings in the shoulder has made interpretation of shoulder magnetic resonance imaging (MRI) reports challenging. We review and discuss the appearance of these lesions on shoulder MRI to help the orthopedic surgeon understand these entities as imaging findings.
Glenolabral articular disruption (GLAD) occurs secondary to impaction of the humeral head on the glenoid articular cartilage. There is a resultant defect in the glenoid articular cartilage, which extends to the glenoid labrum. A GLAD lesion is diagnosed only if the glenohumeral ligament and scapular periosteum remain intact1 (Figure 1).
Complete detachment of the anteroinferior labrum with tearing of the anterior glenoid periosteum represents a Bankart lesion. Cartilaginous Bankart lesions are caused by an anterior glenohumeral dislocation with resultant avulsion of the anteroinferior labrum and disruption of the scapular periosteum because of acute traction on the anterior band of the inferior glenohumeral ligament (Figure 2). Anterior instability, caused by disruption of the anterior labroligamentous complex, results. Osseous Bankart lesions occur when the anterior displaced humeral head impacts the anterior inferior glenoid rim, causing a fracture (Figure 3). This loss of the glenoid articular surface area can result in glenohumeral instability. Posterior shoulder dislocations can result in corresponding findings in the posterior inferior glenoid labrum (reverse Bankart lesion) and anterior medial humeral head (reverse Hill-Sachs lesion) (Figure 2).
A variant of the Bankart lesion is the anterior labroligamentous periosteal sleeve avulsion (ALPSA). This refers to a medially displaced tear of the anterior labrum with intact periosteal stripping along the medial glenoid2with medial rotation and inferior displacement of the anterior inferior labrum along the scapular neck. An ALPSA lesion can heal via the intact periosteal blood supply. If not repaired, anterior instability will result because of malposition of the labrum, causing a patulous anterior capsule.3 When a corresponding lesion occurs in the posterior labrum because of a posterior dislocation, it is called a posterior labrocapsular periosteal sleeve avulsion (POLPSA) (Figure 4).
Another variant of the Bankart lesion is the Perthes lesion, which is a nondisplaced tear of the anteroinferior labrum with periosteal stripping. This differs from the ALPSA because the detached labrum and periosteum are held in anatomic position, possibly making the lesion difficult to detect on magnetic resonance arthrography (MRA).3 Obtaining images in the abduction external rotation (ABER) position exerts traction on the anterior inferior joint capsule and may make the Perthes lesion more conspicuous.4 When this occurs in the posterior labrum, it is called a reverse Perthes lesion (Figure 5).
In a patient with anterior glenohumeral instability without a Bankart lesion, pathology of the anterior band of the inferior glenohumeral ligament (IGHL) at its humeral attachment must be suspected. Humeral avulsion of the IGHL (HAGL) or its variants can be overlooked on arthroscopy. HAGL is diagnosed on MRA when the normally U-shaped IGHL takes on a J-shape, and joint fluid extravasates across the torn humeral attachment (Figure 6). If there is an avulsed bony fragment from the medial humeral neck, the lesion is termed a bony HAGL (BHAGL). In addition to the findings of a HAGL, a BHAGL shows the osseous fragment and donor site on MRI. Since a BHAGL is a bony avulsion, it can even be suggested on radiography if a bony fragment is seen adjacent to the medial humeral neck.5 These lesions are highly associated with other shoulder injuries, particularly Hill-Sachs deformities and subscapularis tendon tears, and it is imperative, therefore, to search for additional injuries if a HAGL-type injury is seen.6
A more uncommon type of HAGL can occur in the setting of posterior capsulolabral injury. A posterior-band IGHL avulsion from the humerus (PHAGL) has similar imaging findings to a HAGL, except that it involves the posterior band of the IGHL. PHAGLs are usually not associated with an acute injury and are thought to be related to repetitive microtrauma, perhaps since the posterior band of the IGHL is the thinnest portion of the IGHL complex.7
A Kim lesion is an arthroscopic finding described in patients with posterior instability as a superficial defect at the undersurface of the posterior labrum and adjacent glenoid cartilage without detachment or extension to the chondrolabral junction.8 It is, by its nature, a concealed finding on routine MRI but can be more conspicuous in FADIR (flexed, adducted, internally rotated) positioning on MRA, which exerts traction on the posterior joint capsule, allowing intra-articular contrast to fill the tear (Figure 7).
This list describes several of the most commonly encountered acronyms in shoulder MRI. A review of SLAP (superior labrum anterior to posterior) lesions was described in a previous article in the journal’s Imaging Series.9 A thorough understanding of these lesions is helpful in interpreting reports and determining the appropriate treatment for patients with shoulder injuries.
1. Sanders TG, Tirman PF, Linares R, Feller JF, Richardson R. The glenolabral articular disruption lesion: MR arthrography with arthroscopic correlation. AJR Am J Roentgenol. 1999;172(1):171-175.
2. Beltran J, Jbara M, Maimon R. Shoulder: labrum and bicipital tendon. Top Magn Reson Imaging. 2003;14(1):35-50.
3. Waldt S, Burkart A, Imhoff AB, Bruegel M, Rummeny EJ, Woertler K. Anterior shoulder instability: accuracy of MR arthrography in the classification of anteroinferior labroligamentous injuries. Radiology. 2005;237(2):578-583.
4. Schreinemachers SA, van der Hulst VP, Willems J, Bipat S, van der Woude H. Is a single direct MR arthrography series in ABER position as accurate in detecting anteroinferior labroligamentous lesions as conventional MR arthrography? Skeletal Radiol. 2009;38(7):675-683.
5. Bui-Mansfield LT, Taylor DC, Uhorchak JM, Tenuta JT. Humeral avulsions of the glenohumeral ligament: imaging features and a review of the literature. AJR Am J Roentgenol. 2002;179(3):649-655.
6. Magee T. Prevalence of HAGL lesions and associated abnormalities on shoulder MR examination. Skeletal Radiol. 2014;43(3):307-313.
7. Chung CB, Sorenson S, Dwek JR, Resnick D. Humeral avulsion of the posterior band of the inferior glenohumeral ligament: MR arthrography and clinical correlation in 17 patients. AJR Am J Roentgenol. 2004;183(2):355-359.
8. Kim SH, Ha KI, Yoo JC, Noh KC. Kim’s lesion: an incomplete and concealed avulsion of the posteroinferior labrum in posterior or multidirectional posteroinferior instability of the shoulder. Arthroscopy. 2004;20(7):712-720.
9. Grubin J, Maderazo A, Fitzpatrick D. Imaging evaluation of superior labral anteroposterior (SLAP) tears. Am J Orthop. 2015;44(10):476-477.
1. Sanders TG, Tirman PF, Linares R, Feller JF, Richardson R. The glenolabral articular disruption lesion: MR arthrography with arthroscopic correlation. AJR Am J Roentgenol. 1999;172(1):171-175.
2. Beltran J, Jbara M, Maimon R. Shoulder: labrum and bicipital tendon. Top Magn Reson Imaging. 2003;14(1):35-50.
3. Waldt S, Burkart A, Imhoff AB, Bruegel M, Rummeny EJ, Woertler K. Anterior shoulder instability: accuracy of MR arthrography in the classification of anteroinferior labroligamentous injuries. Radiology. 2005;237(2):578-583.
4. Schreinemachers SA, van der Hulst VP, Willems J, Bipat S, van der Woude H. Is a single direct MR arthrography series in ABER position as accurate in detecting anteroinferior labroligamentous lesions as conventional MR arthrography? Skeletal Radiol. 2009;38(7):675-683.
5. Bui-Mansfield LT, Taylor DC, Uhorchak JM, Tenuta JT. Humeral avulsions of the glenohumeral ligament: imaging features and a review of the literature. AJR Am J Roentgenol. 2002;179(3):649-655.
6. Magee T. Prevalence of HAGL lesions and associated abnormalities on shoulder MR examination. Skeletal Radiol. 2014;43(3):307-313.
7. Chung CB, Sorenson S, Dwek JR, Resnick D. Humeral avulsion of the posterior band of the inferior glenohumeral ligament: MR arthrography and clinical correlation in 17 patients. AJR Am J Roentgenol. 2004;183(2):355-359.
8. Kim SH, Ha KI, Yoo JC, Noh KC. Kim’s lesion: an incomplete and concealed avulsion of the posteroinferior labrum in posterior or multidirectional posteroinferior instability of the shoulder. Arthroscopy. 2004;20(7):712-720.
9. Grubin J, Maderazo A, Fitzpatrick D. Imaging evaluation of superior labral anteroposterior (SLAP) tears. Am J Orthop. 2015;44(10):476-477.
3D imaging tracks causes of post-TAVR aortic regurgitation
Three-dimensional transesophageal echocardiography identified several significant predictors of aortic regurgitation after transcatheter aortic valve replacement, according to a study published online Jan. 5 in JACC Cardiovascular Imaging.
“This is the first study to demonstrate that large prosthetic expansion, elliptical prosthetic shape, and anti-anatomical position are 3D features associated with transvalvular AR,” said Dr. Kentaro Shibayama of Cedars-Sinai Heart Institute in Los Angeles, and his associates. The study also showed that paravalvular AR was inversely related to effective area oversizing, the investigators said (JACC Cardiovasc Imag. 2016 Jan. 6).
Post-TAVR AR continues to affect substantial numbers of patients, despite progress in prosthesis design. Past research has linked paravalvular AR to prosthetic undersizing, long-axis malpositioning, and aortic annular calcification, but the causes of transvalvular AR have not been adequately studied, the researchers said. Using intraprocedural 3D transesophageal echocardiography, they imaged the native annuluses and postoperative prosthetic valves of 201 patients with severe aortic stenosis who received the Edwards SAPIEN device. The investigators also used transthoracic echocardiography to separately grade post-TAVR transvalvular and paravalvular AR as none or trivial, mild, moderate, or severe according to the 2012 Valve Academic Research Consortium criteria (J Am Coll Cardiol. 2012;60:1438-54).Fully 44% of patients developed mild or moderate aortic regurgitation after TAVR, while the rest had no or trivial AR, the investigators said. About three-quarters of AR cases were mild, nearly 25% were moderate, and none were severe. Only 3% of patients had transvalvular AR only, 34% had paravalvular AR only, and 7% had both types of AR. Patients with post-TAVR transvalvular AR had significantly more prosthetic expansion (P less than .05), a more elliptical prosthetic shape at the level of the prosthetic commissure (P less than .01), and malpositioning of the prosthetic commissures in relation to the native commissures (P less than .001), compared with patients without transvalvular AR.
Patients were more likely to have paravalvular AR if they had a lower percentage of effective area oversizing, defined as the prosthetic frame area divided by the area of the native aortic annulus (odds ratio, 0.97; 95% CI: 0.93-0.99, P less than .05). “A mismatch between a larger native aortic valve annulus area and a smaller deployed prosthesis found by intra-procedural 3D TEE may increase the risk of developing mild or greater paravalvular AR,” the researchers explained. Older age also was slightly but significantly linked with mild or moderate paravalvular AR(OR, 1.05; 95% CI, 1.01-1.09, P less than .05).
“Abnormalities related to transvalvular AR after TAVR found in this study may contribute to further deterioration of the prosthesis, warranting careful prospective studies to assess the long-term prognosis of these patients,” the investigators concluded. They cautioned that the number of patients with post-TAVR transvalvular AR was too small to carry out detailed analyses.
The researchers reported no funding sources. Senior author Dr. Takahiro Shiota reported being a speaker for Philips Ultrasound, and three of the other seven coinvestigators reported financial relationships with Edwards, Medtronic, Abbott, Capricor, St. Jude Medical, Philips Ultrasound, and Venus Medtech.
This study is important because it reinforces the important role that 3D TEE can play in procedural planning for TAVR and in predicting which patients are more likely to suffer from post-TAVR AR. It is the first study that has highlighted the practical utilization of 3D TEE in this way.
Multislice computed tomography is the preferred imaging modality for TAVR planning in many centers. However, since the imaging resolution of both techniques is similar, and they both have software capable of generating multiplane reconstructions from 3D datasets, I believe that the skill and experience of the imaging expert analyzing the datasets are more important than the modality itself, and the results from this study could probably translate to MSCT.
Although the manufacturers of TAVR valves would have us believe that the issue of postimplant AR has largely been solved by newer valve design, it still remains an important issue and will continue to be so as the technique competes with surgical alternatives. Imaging will continue to play a pivotal role in procedure planning and guidance and, as has been demonstrated by Shibayama et al., 3D TEE can be extremely useful for anticipating and potentially avoiding post-TAVR AR.
Mark Monaghan, Ph.D., is the FESC director of noninvasive cardiology at King’s College Hospital Denmark Hill in London. These comments were taken from his editorial (JACC Cardiovasc Imaging 2016 Jan. 6).
This study is important because it reinforces the important role that 3D TEE can play in procedural planning for TAVR and in predicting which patients are more likely to suffer from post-TAVR AR. It is the first study that has highlighted the practical utilization of 3D TEE in this way.
Multislice computed tomography is the preferred imaging modality for TAVR planning in many centers. However, since the imaging resolution of both techniques is similar, and they both have software capable of generating multiplane reconstructions from 3D datasets, I believe that the skill and experience of the imaging expert analyzing the datasets are more important than the modality itself, and the results from this study could probably translate to MSCT.
Although the manufacturers of TAVR valves would have us believe that the issue of postimplant AR has largely been solved by newer valve design, it still remains an important issue and will continue to be so as the technique competes with surgical alternatives. Imaging will continue to play a pivotal role in procedure planning and guidance and, as has been demonstrated by Shibayama et al., 3D TEE can be extremely useful for anticipating and potentially avoiding post-TAVR AR.
Mark Monaghan, Ph.D., is the FESC director of noninvasive cardiology at King’s College Hospital Denmark Hill in London. These comments were taken from his editorial (JACC Cardiovasc Imaging 2016 Jan. 6).
This study is important because it reinforces the important role that 3D TEE can play in procedural planning for TAVR and in predicting which patients are more likely to suffer from post-TAVR AR. It is the first study that has highlighted the practical utilization of 3D TEE in this way.
Multislice computed tomography is the preferred imaging modality for TAVR planning in many centers. However, since the imaging resolution of both techniques is similar, and they both have software capable of generating multiplane reconstructions from 3D datasets, I believe that the skill and experience of the imaging expert analyzing the datasets are more important than the modality itself, and the results from this study could probably translate to MSCT.
Although the manufacturers of TAVR valves would have us believe that the issue of postimplant AR has largely been solved by newer valve design, it still remains an important issue and will continue to be so as the technique competes with surgical alternatives. Imaging will continue to play a pivotal role in procedure planning and guidance and, as has been demonstrated by Shibayama et al., 3D TEE can be extremely useful for anticipating and potentially avoiding post-TAVR AR.
Mark Monaghan, Ph.D., is the FESC director of noninvasive cardiology at King’s College Hospital Denmark Hill in London. These comments were taken from his editorial (JACC Cardiovasc Imaging 2016 Jan. 6).
Three-dimensional transesophageal echocardiography identified several significant predictors of aortic regurgitation after transcatheter aortic valve replacement, according to a study published online Jan. 5 in JACC Cardiovascular Imaging.
“This is the first study to demonstrate that large prosthetic expansion, elliptical prosthetic shape, and anti-anatomical position are 3D features associated with transvalvular AR,” said Dr. Kentaro Shibayama of Cedars-Sinai Heart Institute in Los Angeles, and his associates. The study also showed that paravalvular AR was inversely related to effective area oversizing, the investigators said (JACC Cardiovasc Imag. 2016 Jan. 6).
Post-TAVR AR continues to affect substantial numbers of patients, despite progress in prosthesis design. Past research has linked paravalvular AR to prosthetic undersizing, long-axis malpositioning, and aortic annular calcification, but the causes of transvalvular AR have not been adequately studied, the researchers said. Using intraprocedural 3D transesophageal echocardiography, they imaged the native annuluses and postoperative prosthetic valves of 201 patients with severe aortic stenosis who received the Edwards SAPIEN device. The investigators also used transthoracic echocardiography to separately grade post-TAVR transvalvular and paravalvular AR as none or trivial, mild, moderate, or severe according to the 2012 Valve Academic Research Consortium criteria (J Am Coll Cardiol. 2012;60:1438-54).Fully 44% of patients developed mild or moderate aortic regurgitation after TAVR, while the rest had no or trivial AR, the investigators said. About three-quarters of AR cases were mild, nearly 25% were moderate, and none were severe. Only 3% of patients had transvalvular AR only, 34% had paravalvular AR only, and 7% had both types of AR. Patients with post-TAVR transvalvular AR had significantly more prosthetic expansion (P less than .05), a more elliptical prosthetic shape at the level of the prosthetic commissure (P less than .01), and malpositioning of the prosthetic commissures in relation to the native commissures (P less than .001), compared with patients without transvalvular AR.
Patients were more likely to have paravalvular AR if they had a lower percentage of effective area oversizing, defined as the prosthetic frame area divided by the area of the native aortic annulus (odds ratio, 0.97; 95% CI: 0.93-0.99, P less than .05). “A mismatch between a larger native aortic valve annulus area and a smaller deployed prosthesis found by intra-procedural 3D TEE may increase the risk of developing mild or greater paravalvular AR,” the researchers explained. Older age also was slightly but significantly linked with mild or moderate paravalvular AR(OR, 1.05; 95% CI, 1.01-1.09, P less than .05).
“Abnormalities related to transvalvular AR after TAVR found in this study may contribute to further deterioration of the prosthesis, warranting careful prospective studies to assess the long-term prognosis of these patients,” the investigators concluded. They cautioned that the number of patients with post-TAVR transvalvular AR was too small to carry out detailed analyses.
The researchers reported no funding sources. Senior author Dr. Takahiro Shiota reported being a speaker for Philips Ultrasound, and three of the other seven coinvestigators reported financial relationships with Edwards, Medtronic, Abbott, Capricor, St. Jude Medical, Philips Ultrasound, and Venus Medtech.
Three-dimensional transesophageal echocardiography identified several significant predictors of aortic regurgitation after transcatheter aortic valve replacement, according to a study published online Jan. 5 in JACC Cardiovascular Imaging.
“This is the first study to demonstrate that large prosthetic expansion, elliptical prosthetic shape, and anti-anatomical position are 3D features associated with transvalvular AR,” said Dr. Kentaro Shibayama of Cedars-Sinai Heart Institute in Los Angeles, and his associates. The study also showed that paravalvular AR was inversely related to effective area oversizing, the investigators said (JACC Cardiovasc Imag. 2016 Jan. 6).
Post-TAVR AR continues to affect substantial numbers of patients, despite progress in prosthesis design. Past research has linked paravalvular AR to prosthetic undersizing, long-axis malpositioning, and aortic annular calcification, but the causes of transvalvular AR have not been adequately studied, the researchers said. Using intraprocedural 3D transesophageal echocardiography, they imaged the native annuluses and postoperative prosthetic valves of 201 patients with severe aortic stenosis who received the Edwards SAPIEN device. The investigators also used transthoracic echocardiography to separately grade post-TAVR transvalvular and paravalvular AR as none or trivial, mild, moderate, or severe according to the 2012 Valve Academic Research Consortium criteria (J Am Coll Cardiol. 2012;60:1438-54).Fully 44% of patients developed mild or moderate aortic regurgitation after TAVR, while the rest had no or trivial AR, the investigators said. About three-quarters of AR cases were mild, nearly 25% were moderate, and none were severe. Only 3% of patients had transvalvular AR only, 34% had paravalvular AR only, and 7% had both types of AR. Patients with post-TAVR transvalvular AR had significantly more prosthetic expansion (P less than .05), a more elliptical prosthetic shape at the level of the prosthetic commissure (P less than .01), and malpositioning of the prosthetic commissures in relation to the native commissures (P less than .001), compared with patients without transvalvular AR.
Patients were more likely to have paravalvular AR if they had a lower percentage of effective area oversizing, defined as the prosthetic frame area divided by the area of the native aortic annulus (odds ratio, 0.97; 95% CI: 0.93-0.99, P less than .05). “A mismatch between a larger native aortic valve annulus area and a smaller deployed prosthesis found by intra-procedural 3D TEE may increase the risk of developing mild or greater paravalvular AR,” the researchers explained. Older age also was slightly but significantly linked with mild or moderate paravalvular AR(OR, 1.05; 95% CI, 1.01-1.09, P less than .05).
“Abnormalities related to transvalvular AR after TAVR found in this study may contribute to further deterioration of the prosthesis, warranting careful prospective studies to assess the long-term prognosis of these patients,” the investigators concluded. They cautioned that the number of patients with post-TAVR transvalvular AR was too small to carry out detailed analyses.
The researchers reported no funding sources. Senior author Dr. Takahiro Shiota reported being a speaker for Philips Ultrasound, and three of the other seven coinvestigators reported financial relationships with Edwards, Medtronic, Abbott, Capricor, St. Jude Medical, Philips Ultrasound, and Venus Medtech.
FROM JACC CARDIOVASCULAR IMAGING
Key clinical point: Three-dimensional transesophageal echocardiography identified significant predictors of aortic regurgitation after transcatheter aortic valve replacement.
Major finding: Patients with post-TAVR transvalvular AR had significantly more prosthetic expansion (P less than .05), a more elliptical prosthetic shape (P less than .01), and malpositioning of the prosthetic commissures (P less than .001) compared with patients without transvalvular AR.
Data source: A 3D TEE study of 201 patients with severe aortic stenosis who underwent TAVR with the Edwards SAPIEN device.
Disclosures: The investigators reported no funding sources. Senior author Dr. Takahiro Shiota reported being a speaker for Philips Ultrasound, and three of the other seven coinvestigators reported financial relationships with Edwards, Medtronic, Abbott, Capricor, St. Jude Medical, Philips Ultrasound, and Venus Medtech.
Managing interstitial lung disease detected on CT during lung cancer screening
Primary care physicians are playing a bigger role in evaluating the incidental finding of interstitial lung diseases since the recent publication of guidelines recommending computed tomography (CT) to screen for lung cancer.
In August 2011, the National Cancer Institute published its findings from the National Lung Screening Trial, which demonstrated a 20% reduction in mortality from lung cancer in patients at high risk screened with low-dose CT.1 Based on these results, the American Cancer Society, the American College of Chest Physicians, the American Society of Clinical Oncology, and the National Comprehensive Cancer Network recommended annual screening for lung cancer with low-dose CT in adults ages 55 to 74 who have a 30-pack-year smoking history and who currently smoke or have quit within the past 15 years.2 In December 2013, the US Preventive Services Task Force published similar guidelines but increased the age range to include high-risk patients ages 55 to 80.3
Bach et al4 estimated that, in 2010 in the United States, 8.6 million people met the criteria used in the National Lung Screening Trial for low-dose CT screening. These are the same criteria as in the multisociety recommendations cited above.2 With such large numbers of patients eligible for CT screening, internists and other primary care physicians are undoubtedly encountering the incidental discovery of nonmalignant pulmonary diseases such as interstitial lung disease.
This article reviews the radiographic characteristics of the most common interstitial lung diseases the internist may encounter on screening CT in long-term smokers.
Referral to a specialist has been associated with lower rates of morbidity and death,5 and a diagnosis of interstitial lung disease should be confirmed by a pulmonologist and a radiologist specializing in differentiating the subtypes. But the primary care physician now plays a critical role in recognizing the need for further evaluation.
HOW COMMON IS INTERSTITIAL LUNG DISEASE IN SMOKERS?
Several studies have published data on the prevalence of interstitial lung disease in patients undergoing low-dose CT for lung cancer screening.
A trial at Mayo Clinic in current and former smokers identified “diffuse lung disease” in 9 (0.9%) of 1,049 participants.6
A trial in Ireland identified idiopathic pulmonary fibrosis in 6 (1.3%) of 449 current smokers who underwent low-dose CT screening for lung cancer.7
Sverzellati et al8 evaluated 692 participants in the Multicentric Italian Lung Detection CT screening study and reported a respiratory bronchiolitis pattern in 109 (15.7%), a usual interstitial pneumonia pattern in 2 (0.3%), and other patterns of chronic interstitial pneumonia in 26 (3.8%).
The National Lung Screening Trial reported that the frequency of “clinically significant” incidental findings (including pulmonary fibrosis) in all participants was 7.5%.1 A retrospective analysis of 884 participants at a single site in this trial identified interstitial lung abnormalities in 86 participants (9.7%).9 These abnormalities were further categorized as nonfibrotic in 52 (5.9%) of 884, fibrotic in 19 (2.1%) of 884, and mixed fibrotic and nonfibrotic in 15 (1.7%) of 884.
Follow-up CT at 2 years in this trial demonstrated improvement in 50% and progression in 11% of patients who had nonfibrotic abnormalities, while fibrotic abnormalities improved in no cases and progressed in 37%. Interstitial lung abnormalities were more common in those who currently smoked and in those with more pack-years of cigarette smoking.9
In sum, these trials suggest that low-dose CT screening for lung cancer can detect the most common forms of interstitial lung disease in this at-risk population and can characterize them as fibrotic or nonfibrotic, a distinction important for prognosis and subsequent management.
NONFIBROTIC VS FIBROTIC DISEASE
It is important to distinguish between nonfibrotic and fibrotic interstitial lung disease, as fibrotic disease carries a worse prognosis and is treated differently.
Features of nonfibrotic interstitial lung disease:
- Ground-glass opacities
- Nodules
- Mosaic attenuation or consolidation.
Features of fibrotic interstitial lung disease:
- Combination of ground-glass opacities and reticulation
- Reticulation by itself
- Traction bronchiectasis
- Honeycombing
- Loss of lung volume.
NONFIBROTIC INTERSTITIAL LUNG DISEASES
Given the strong likelihood that a patient undergoing screening CT is either a current or former smoker, physicians may encounter, in addition to emphysema and lung cancer, the following smoking-related interstitial lung diseases, which are primarily nonfibrotic and which frequently coexist (Table 1):
- Respiratory bronchiolitis
- Respiratory bronchiolitis-interstitial lung disease
- Desquamative interstitial pneumonia
- Pulmonary Langerhans cell histiocytosis.
Respiratory bronchiolitis
Respiratory bronchiolitis occurs mostly in smokers and does not necessarily lead to respiratory symptoms in all patients.10 It cannot always be identified radiographically but occasionally appears as predominantly upper-lobe, patchy ground-glass opacities or ill-defined centrilobular nodules without evidence of fibrosis (Figure 1).
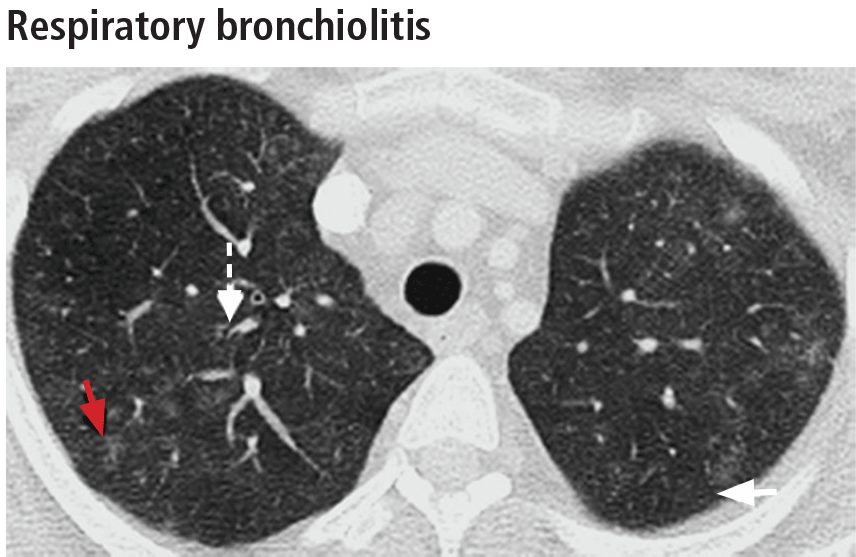
Respiratory bronchiolitis-interstitial lung disease
In rare cases, respiratory bronchiolitis leads to peribronchial fibrosis invading the alveolar walls, which is then classified as respiratory bronchiolitis-interstitial lung disease.11 The CT findings in respiratory bronchiolitis-interstitial lung disease are upper-lobe-predominant centrilobular ground-glass nodules, patchy ground-glass opacities, and bronchial wall thickening (Figure 2).10 Occasionally, mild reticulation is noted without honeycombing. Mild air trapping can be seen in the lower lobes, with centrilobular emphysema in the upper lobes.12
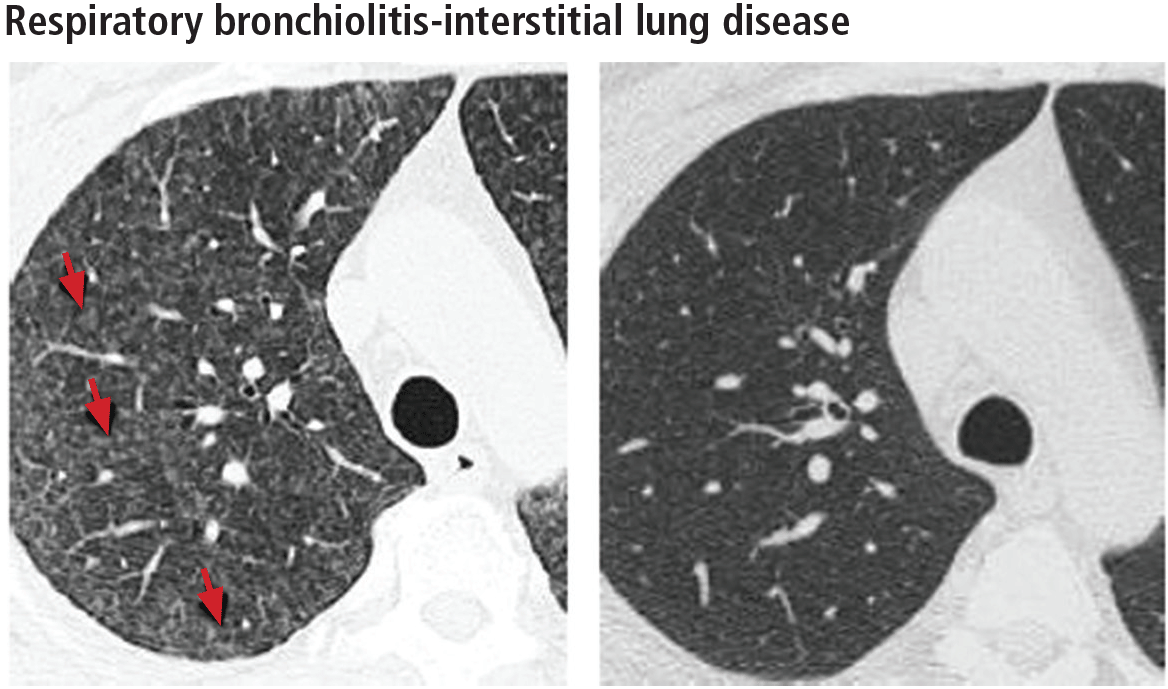
The only successful therapy for respiratory bronchiolitis and respiratory bronchiolitis-interstitial lung disease is smoking cessation. Finding either of these diseases should prompt aggressive counseling by the internist and consideration of referral to a specialist in interstitial lung disease.
Desquamative interstitial pneumonia
Although pathologically different from respiratory bronchiolitis-interstitial lung disease, desquamative interstitial pneumonia has a similar clinical and radiographic presentation. Because their features significantly overlap, they are considered a pathomorphologic continuum, representing degrees of severity of the same disease process caused by prolonged tobacco inhalation.10,13
Widespread ground-glass opacities are the predominant CT finding. These are bilateral and symmetric in distribution in 86%, basal and peripheral in 60%, patchy in 20%, and diffuse in 20% (Figure 3).14 Other frequent findings are mild reticulation with traction bronchiectasis and coexistent emphysema (Figure 4).15 The small peripheral cystic spaces noted in this disease most likely represent dilated bronchioles and alveolar ducts rather than honeycombing.16
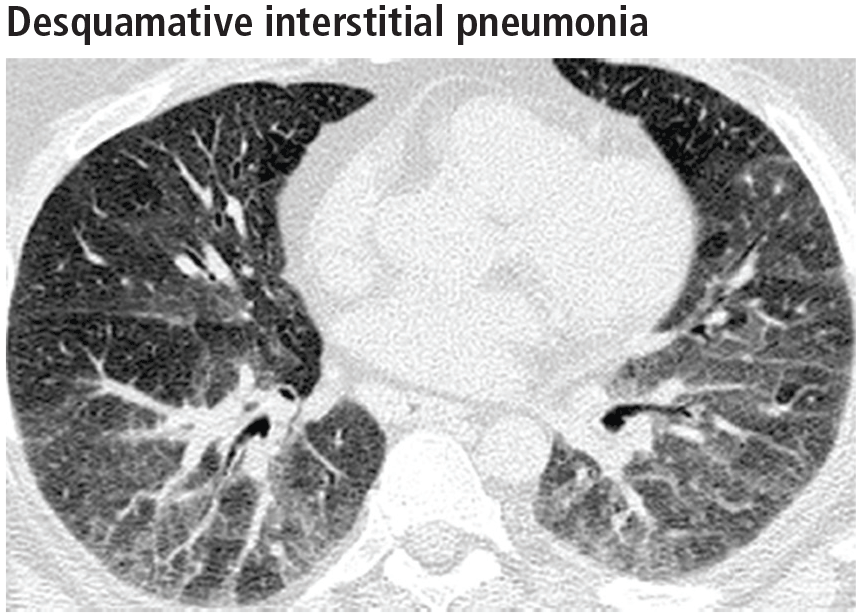
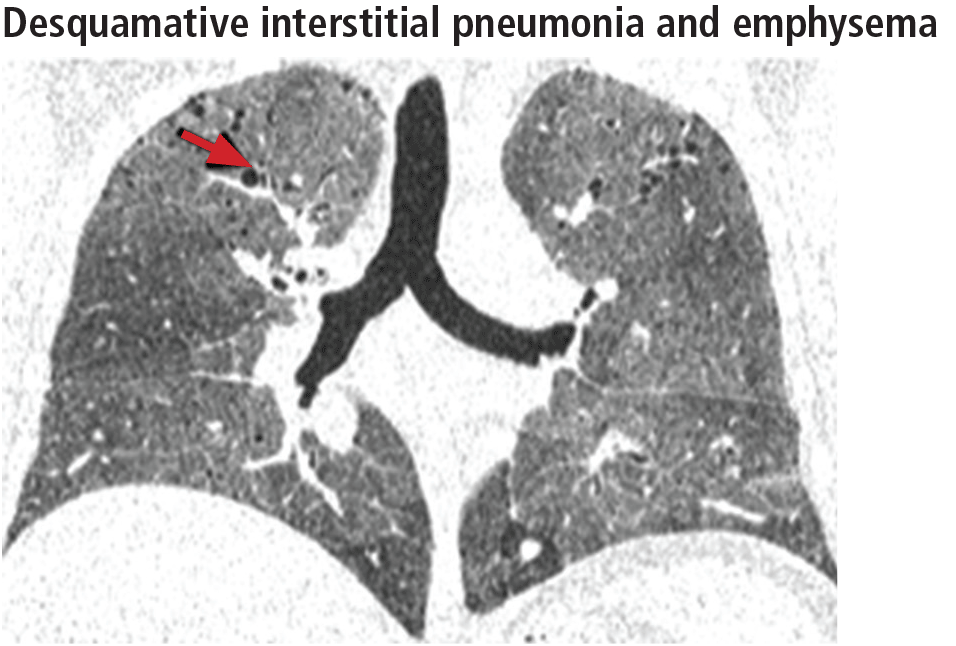
No additional treatment beyond elimination of smoking has been proven effective for desquamative interstitial pneumonia, and patients who manage to quit smoking generally have a favorable prognosis.17,18
Pulmonary Langerhans cell histiocytosis
The combination of upper-lobe-predominant cysts and nodules in a young heavy smoker are diagnostic of pulmonary Langerhans cell histiocytosis. The cysts are bizarrely shaped, thin- or thick-walled, and nonuniform in size (Figure 5). The irregular cavitary nodules are centrilobular. The disease characteristically spares the costophrenic angles.
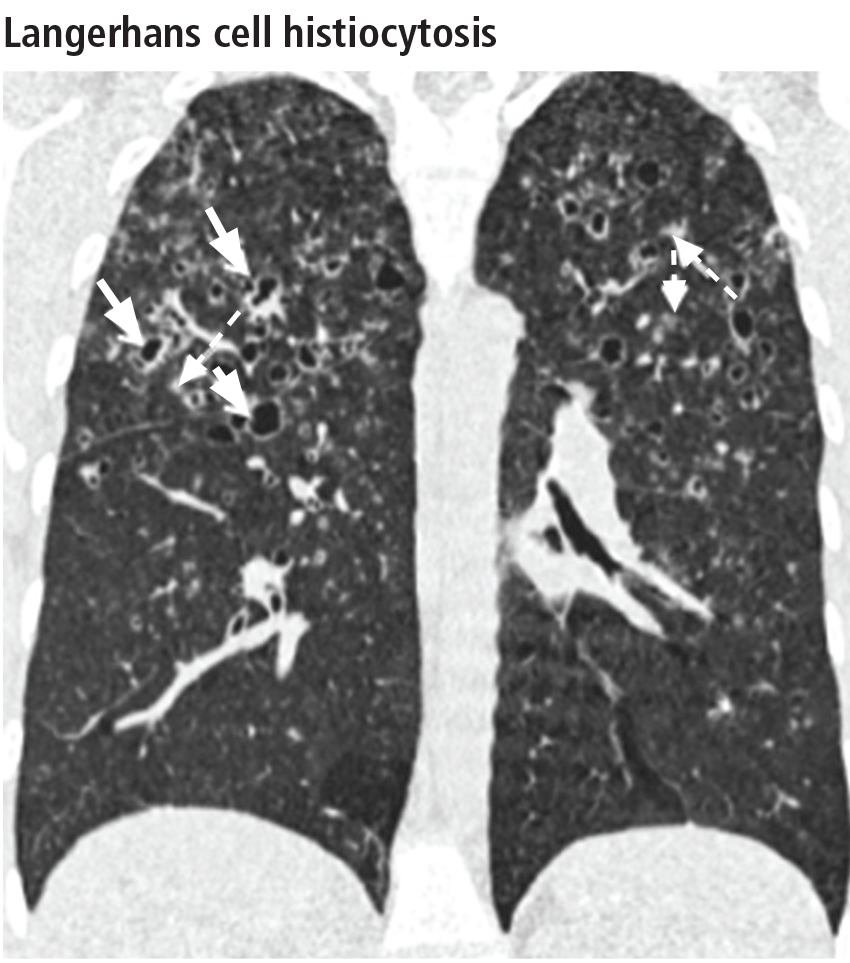
Spontaneous pneumothorax is the initial clinical presentation in 15% of patients.16 In the early stages of the disease (nodule-predominant disease without cysts), infection and metastatic disease need to be excluded (Figure 6). In the later stages, the cysts become coalescent, making the distinction between this disease and “burned-out” lymphangioleiomyomatosis or severe emphysema extremely difficult (Figure 7).17 Smoking cessation and corticosteroids are the mainstay of medical therapy for pulmonary Langerhans cell histiocytosis, and about 50% of patients who quit smoking and receive corticosteroids demonstrate partial or complete clearing of the radiographic abnormalities and symptoms (Figure 8).
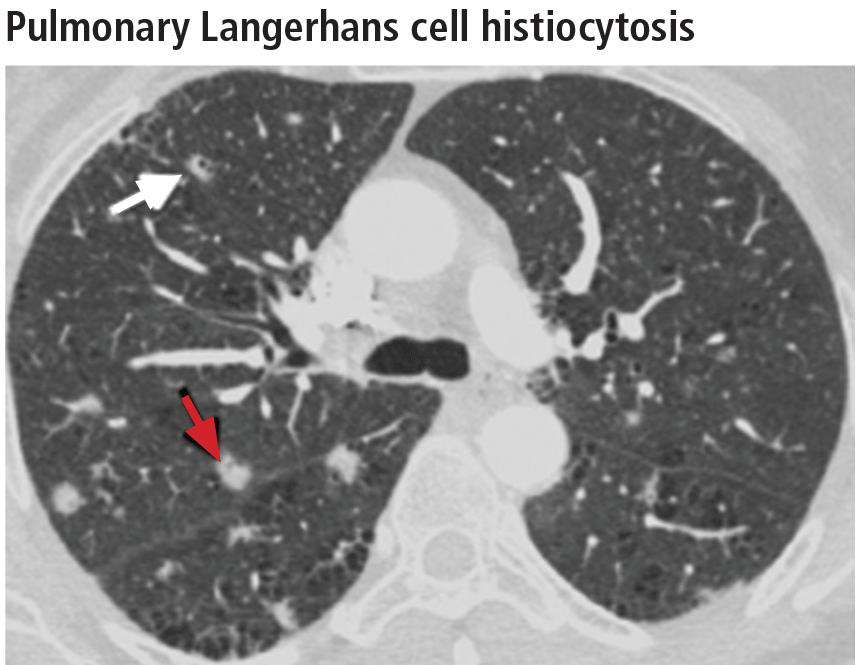
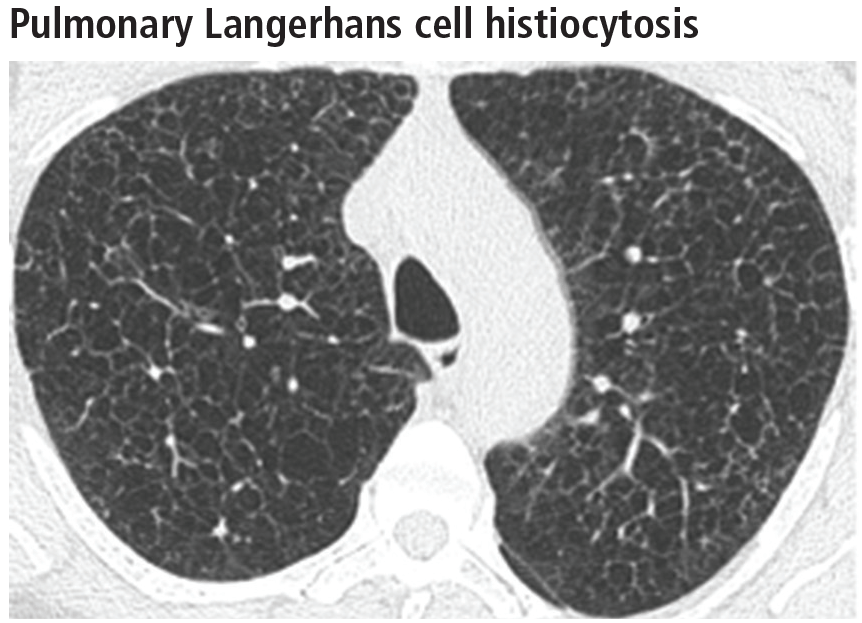
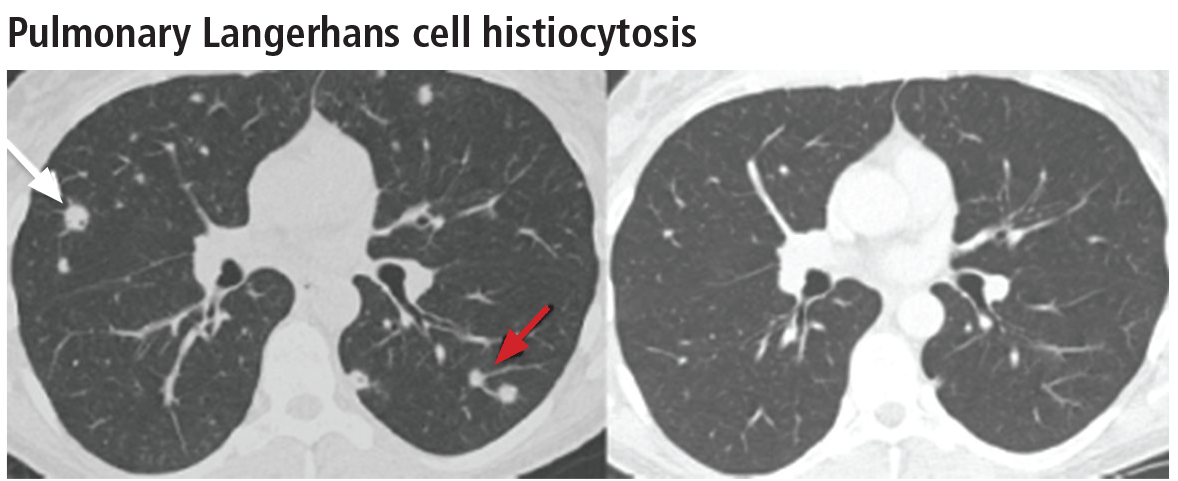
FIBROTIC INTERSTITIAL LUNG DISEASES
If CT identifies a diffuse fibrotic pattern, the two most common possibilities (Table 2) are:
- Nonspecific interstitial pneumonia
- Usual interstitial pneumonia.
As noted above, these carry a worse prognosis than the nonfibrotic interstitial lung diseases.
Nonspecific interstitial pneumonia
While most frequently idiopathic, the nonspecific interstitial pneumonia pattern can often be seen in connective tissue diseases. It has also been associated with chronic hypersensitivity pneumonitis, drug toxicity, and slowly resolving diffuse alveolar damage.19 Although it is not the only pathologic pattern in interstitial lung disease associated with connective tissue disease, it is the most common pattern in systemic sclerosis, systemic lupus erythematosus, dermatomyositis-polymyositis, and mixed connective tissue disease.20
The parenchymal changes are typically subpleural and symmetric in distribution (Figure 9). In about one-third of cases, there is a peribronchovascular distribution of the abnormalities (Figure 10).
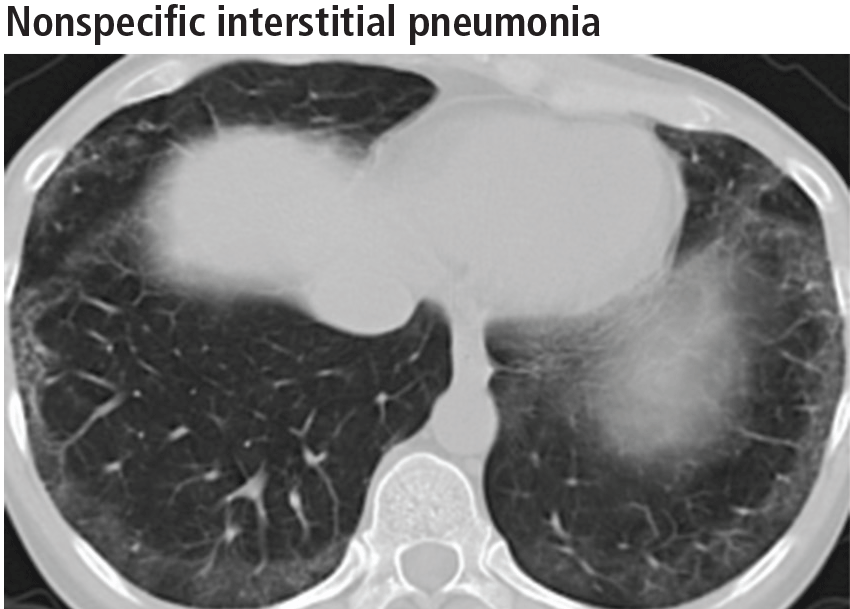
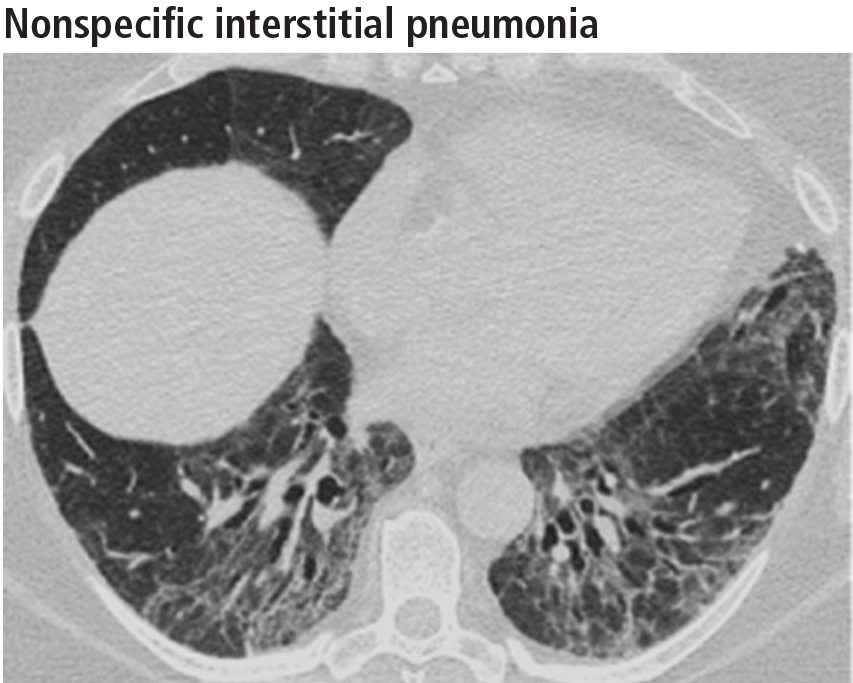
Ground-glass opacities are the dominant imaging findings, seen in 80% of cases.18 In advanced disease (also referred to as fibrotic nonspecific interstitial pneumonia), patients have accompanying fine or coarse reticular opacities, traction bronchiectasis, and consolidation (Figure 11). Honeycombing is seen in 1% to 5% of patients.21
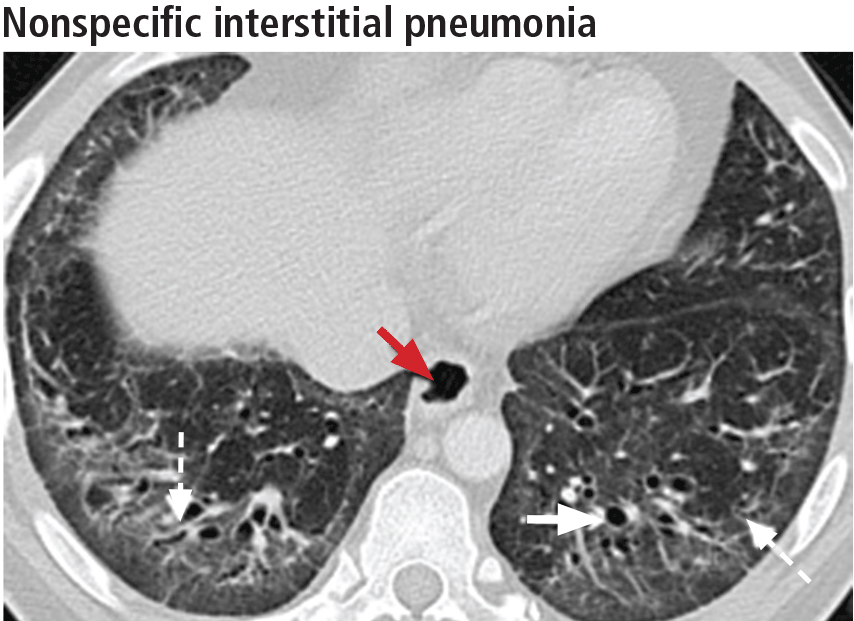
The most specific sign of nonspecific interstitial pneumonia is sparing of the immediate subpleural lung, apparent in 30% to 50% of patients (Figure 12).22 Subpleural sparing with a peribronchovascular distribution of abnormalities, absence of lobular areas with decreased attenuation, and lack of honeycombing are imaging features that increase the diagnostic confidence of nonspecific interstitial pneumonia (Table 3).23 Clinically, compared with those who have usual interstitial pneumonia (see below), patients are younger and more of them are female. These patients also present with extrapulmonary manifestations such as joint involvement, rash, and Raynaud phenomenon. Therefore, these associated symptoms on presentation can help distinguish nonspecific interstitial pneumonia or usual interstitial pneumonia related to connective tissue disease from the idiopathic forms.
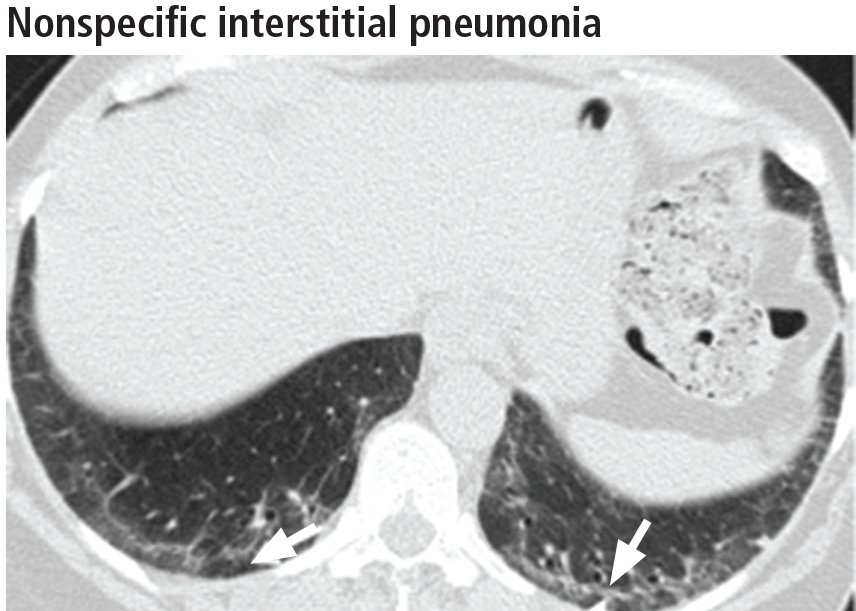
The first step in managing nonspecific interstitial pneumonia is to remove all potential exposure to inhaled substances or to drugs. Although immunosuppressive therapy has never been studied in a randomized controlled trial in this disease, numerous reports suggest that patients may respond to prednisone and to steroid-sparing immunosuppressants.24
In several studies, survival rates in nonspecific interstitial pneumonia were significantly greater than in usual interstitial pneumonia independent of the treatment strategy. In long-term follow-up of patients with idiopathic nonspecific interstitial pneumonia treated with immunosuppressive therapy, two-thirds remained stable or improved.25–27
Although most connective tissue diseases cause a lung pattern of nonspecific interstitial pneumonia, some (eg, rheumatoid arthritis) may present with a pattern of usual interstitial pneumonia. In these cases and in those of advanced fibrotic nonspecific interstitial pneumonia, the prognosis is worse, as the disease is less responsive to immunosuppressive therapy.20
Usual interstitial pneumonia
Usual interstitial pneumonia is the most severe form of lung fibrosis. Most cases are idiopathic and are termed idiopathic pulmonary fibrosis. Other causes of the usual interstitial pneumonia pattern include domestic and occupational environmental exposures, connective tissue disease, and drug toxicity.28 An epidemiologic association between smoking and usual interstitial pneumonia is well documented.28
Idiopathic pulmonary fibrosis typically affects men ages 50 to 70. Because its risk factors coincide with those of lung cancer, there is a high likelihood of detecting idiopathic pulmonary fibrosis early in this screening population. It has an especially poor prognosis, with a mean survival of 2 to 5 years from the time of diagnosis.18
The distribution of disease in usual interstitial pneumonia is characteristically subpleural and basal. CT features include coarse subpleural reticulation and honeycombing combined with traction bronchiectasis or bronchiolectasis and architectural distortion (Figure 13).18 Honeycombing is the most specific and key diagnostic CT finding for establishing a definitive diagnosis of usual interstitial pneumonia.29 However, ground-glass opacities are present in most patients, typically in the region of interstitial fibrosis, and are always less extensive than the reticulation.30 The findings demonstrate morphologic heterogeneity, with areas of fibrosis adjacent to areas of normal lung (Figure 14).
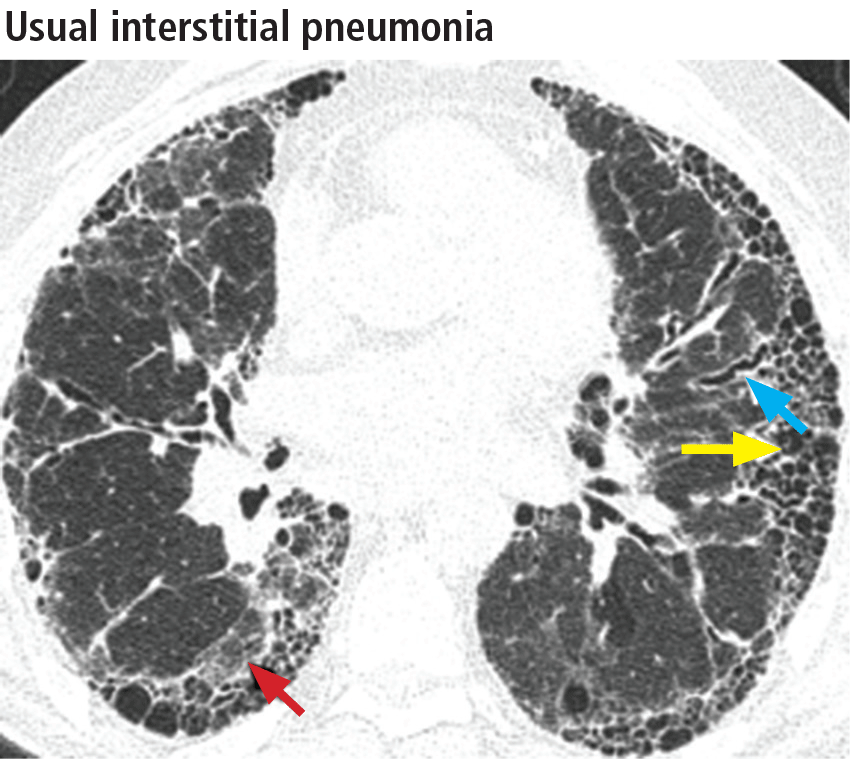
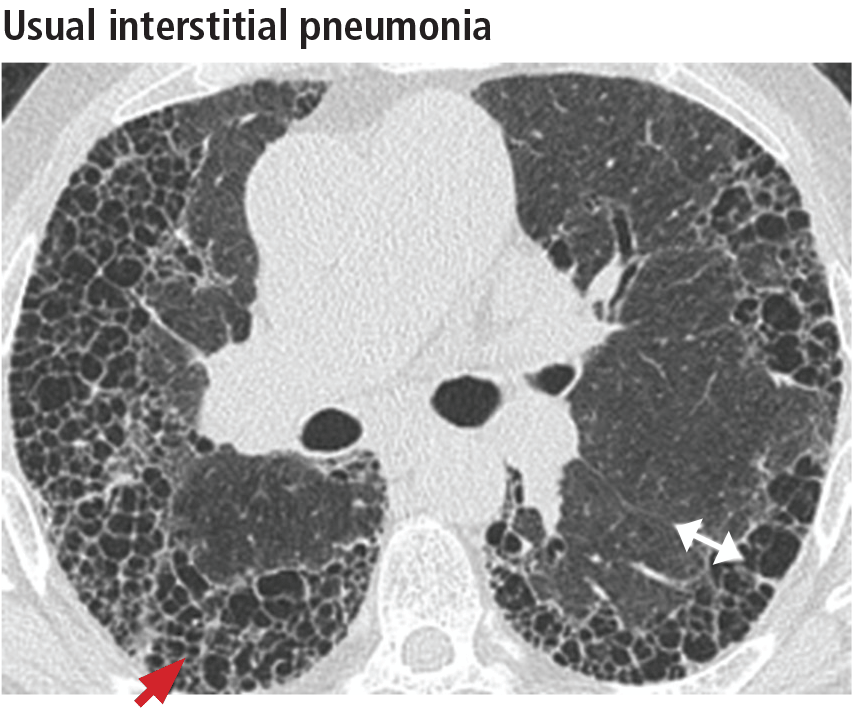
In addition to the aforementioned imaging features, the 2011 American Thoracic Society and European Respiratory Society joint guidelines for the CT diagnosis of usual interstitial pneumonia patterns require the absence of atypical features that suggest an alternative diagnosis, including those seen in nonspecific interstitial pneumonia, such as an upper, midlung, or peribronchovascular distribution and extensive ground-glass attenuation.28 Mild mediastinal lymphadenopathy (usually < 1.5 cm in the short axis) is common in usual interstitial pneumonia.31
Because other chronic interstitial pneumonias that may resemble usual interstitial pneumonia have a more favorable course and may respond to immunosuppressive therapy, establishing an early and accurate diagnosis is of the utmost importance.5 Additionally, the emergence of possible new therapies for idiopathic pulmonary fibrosis makes early referral to a specialist paramount in these cases. Recent studies have demonstrated significant slowing of the progression of disease in idiopathic pulmonary fibrosis with both pirfenidone and nintedanib.32,33
DIAGNOSIS AND MANAGEMENT
The diagnosis of these nonfibrotic and fibrotic lung diseases is complex. In all cases in which interstitial lung disease is detected on screening CT for lung cancer, the internist should strongly consider further evaluation with dedicated high-resolution CT and early referral to a specialist (Figure 15).
Because smoking cessation is the only recommended treatment for nonfibrotic smoking-related interstitial lung diseases, particular emphasis on smoking cessation counseling is essential.
Referral for bronchoscopy with transbronchial lung biopsy is generally not helpful in the diagnosis of the interstitial lung diseases discussed in this article unless there is a need to rule out infection or neoplasm.34 Referral for surgical lung biopsy may be indicated in some cases of suspected pulmonary Langerhans cell histiocytosis, desquamative interstitial pneumonia, nonspecific interstitial pneumonia, or usual interstitial pneumonia if the diagnosis is uncertain (Tables 1 and 2).35
The American Thoracic Society/European Respiratory Society guidelines suggest a multidisciplinary team approach that includes a pathologist, radiologist, and clinician.35 This approach more readily determines the correct diagnosis and relies less on invasive methods such as surgical biopsy and more on noninvasive methods such as radiology and clinical history. Overall, this will promote earlier access to appropriate therapies, clinical trial enrollment, and in more severe cases, lung transplant.
Currently, 23% of all lung transplants worldwide are performed in patients with idiopathic pulmonary fibrosis. Other forms of pulmonary fibrosis account for 3% to 4% of lung transplants performed.36
Evidence suggests that early referral reduces rates of morbidity and death in these patients. The results of a single-center study37 of patients with idiopathic pulmonary fibrosis demonstrated that a longer delay from the onset of symptoms to evaluation by a specialist at a tertiary care referral center was associated with a higher rate of death from this disease independent of disease severity. Those with the longest delay in referral had a multivariable-adjusted death rate 3.4 times higher than those with the shortest delay.5,37
In summary, with implementation of the new lung cancer screening guidelines, primary care physicians are more often encountering the incidental finding of interstitial lung disease in their patients. Prompt diagnosis of interstitial lung disease helps ensure that patients receive appropriate care and early consideration for clinical trials and lung transplant.
Primary care physicians play a critical role in the initial identification of key characteristics of the interstitial abnormality—namely, whether the pattern is nonfibrotic or fibrotic—and in the correlation of the history and physical findings to expedite the diagnosis. Subsequently, ordering high-resolution CT for more detailed characterization and prompt referral to a specialist in interstitial lung disease allow for a more rapid and accurate diagnosis, specialized therapy, and supportive care.
- National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365:395–409.
- Detterbeck FC, Lewis SZ, Diekemper R, Addrizzo-Harris D, Alberts WM. Executive summary: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143(suppl 5):7S–37S.
- Moyer VA; US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 160:330–338.
- Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA 2012; 307:2418–2429.
- Lamas DJ, Kawut SM, Bagiella E, Philip N, Arcasoy SM, Lederer DJ. Delayed access and survival in idiopathic pulmonary fibrosis: a cohort study. Am J Respir Crit Care Med 2011; 184:842–847.
- Swensen SJ, Jett JR, Hartman TE, et al. Lung cancer screening with CT: Mayo Clinic experience. Radiology 2003; 226:756–761.
- MacRedmond R, Logan PM, Lee M, Kenny D, Foley C, Costello RW. Screening for lung cancer using low dose CT scanning. Thorax 2004; 59:237–241.
- Sverzellati N, Guerci L, Randi G, et al. Interstitial lung diseases in a lung cancer screening trial. Eur Respir J 2011; 38:392–400.
- Jin GY, Lynch D, Chawla A, et al. Interstitial lung abnormalities in a CT lung cancer screening population: prevalence and progression rate. Radiology 2013; 268:563–571.
- Heyneman LE, Ward S, Lynch DA, Remy-Jardin M, Johkoh T, Müller NL. Respiratory bronchiolitis, respiratory bronchiolitis-associated interstitial lung disease, and desquamative interstitial pneumonia: different entities or part of the spectrum of the same disease process? AJR Am J Roentgenol 1999; 173:1617–1622.
- Moon J, du Bois RM, Colby TV, Hansell DM, Nicholson AG. Clinical significance of respiratory bronchiolitis on open lung biopsy and its relationship to smoking related interstitial lung disease. Thorax 1999; 54:1009–1014.
- Holt RM, Schmidt RA, Godwin JD, Raghu G. High resolution CT in respiratory bronchiolitis-associated interstitial lung disease. J Comput Assist Tomogr 1993; 17:46–50.
- Ryu JH, Myers JL, Capizzi SA, Douglas WW, Vassallo R, Decker PA. Desquamative interstitial pneumonia and respiratory bronchiolitis-associated interstitial lung disease. Chest 2005; 127:178–184.
- Hartman TE, Primack SL, Swensen SJ, Hansell D, McGuinness G, Müller NL. Desquamative interstitial pneumonia: thin-section CT findings in 22 patients. Radiology 1993; 187:787–790.
- Akira M, Yamamoto S, Hara H, Sakatani M, Ueda E. Serial computed tomographic evaluation in desquamative interstitial pneumonia. Thorax 1997; 52:333–337.
- Lacronique J, Roth C, Battesti JP, Basset F, Chretien J. Chest radiological features of pulmonary histiocytosis X: a report based on 50 adult cases. Thorax 1982; 37:104–109.
- Remy-Jardin M, Edme JL, Boulenguez C, Remy J, Mastora I, Sobaszek A. Longitudinal follow-up study of smoker’s lung with thin-section CT in correlation with pulmonary function tests. Radiology 2002; 222:261–270.
- Mueller-Mang C, Grosse C, Schmid K, Stiebellehner L, Bankier AA. What every radiologist should know about idiopathic interstitial pneumonias. Radiographics 2007; 27:595–615.
- Katzenstein AL, Fiorelli RF. Nonspecific interstitial pneumonia/fibrosis. Histologic features and clinical significance. Am J Surg Pathol 1994; 18:136–147.
- Bryson T, Sundaram B, Khanna D, Kazerooni EA. Connective tissue disease-associated interstitial pneumonia and idiopathic interstitial pneumonia: similarity and difference. Semin Ultrasound CT MR 2014; 35:29–38.
- Desai SR, Veeraraghavan S, Hansell DM, et al. CT features of lung disease in patients with systemic sclerosis: comparison with idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia. Radiology 2004; 232:560–567.
- Tsubamoto M, Müller NL, Johkoh T, et al. Pathologic subgroups of nonspecific interstitial pneumonia: differential diagnosis from other idiopathic interstitial pneumonias on high-resolution computed tomography. J Comput Assist Tomogr 2005; 29:793–800.
- Silva CI, Müller NL, Lynch DA, et al. Chronic hypersensitivity pneumonitis: differentiation from idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia by using thin-section CT. Radiology 2008; 246:288–297.
- Antin-Ozerkis D, Rubinowitz A. An update on nonspecific interstitial pneumonia. Clin Pulm Med 2010; 17:122–128.
- Daniil ZD, Gilchrist FC, Nicholson AG, et al. A histologic pattern of nonspecific interstitial pneumonia is associated with a better prognosis than usual interstitial pneumonia in patients with cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med 1999; 160:899–905.
- Travis WD, Matsui K, Moss J, Ferrans VJ. Idiopathic nonspecific interstitial pneumonia: prognostic significance of cellular and fibrosing patterns: survival comparison with usual interstitial pneumonia and desquamative interstitial pneumonia. Am J Surg Pathol 2000; 24:19–33.
- Riha RL, Duhig EE, Clarke BE, Steele RH, Slaughter RE, Zimmerman PV. Survival of patients with biopsy-proven usual interstitial pneumonia and nonspecific interstitial pneumonia. Eur Respir J 2002; 19:1114–1118.
- Raghu G, Collard HR, Egan JJ, et al; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183:788–824.
- du Bois RM. An earlier and more confident diagnosis of idiopathic pulmonary fibrosis. Eur Respir Rev 2012; 21:141–146.
- Nishimura K, Kitaichi M, Izumi T, Nagai S, Kanaoka M, Itoh H. Usual interstitial pneumonia: histologic correlation with high-resolution CT. Radiology 1992; 182:337–342.
- Souza CA, Müller NL, Lee KS, Johkoh T, Mitsuhiro H, Chong S. Idiopathic interstitial pneumonias: prevalence of mediastinal lymph node enlargement in 206 patients. AJR Am J Roentgenol 2006; 186:995–999.
- King TE Jr, Bradford WZ, Castro-Bernardini S, et al; ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014; 370:2083–2092.
- Richeldi L, du Bois RM, Raghu G, et al; INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014; 370:2071–2082.
- Bradley B, Branley HM, Egan JJ, et al; British Thoracic Society Interstitial Lung Disease Guideline Group, British Thoracic Society Standards of Care Committee; Thoracic Society of Australia; New Zealand Thoracic Society; Irish Thoracic Society. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax 2008; 63(suppl 5):v1–v58.
- Travis WD, Costabel U, Hansell DM, et al; ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013; 188:733–748.
- Stehlik J, Edwards LB, Kucheryavaya AY, et al; International Society of Heart and Lung Transplantation. The Registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report—2012. J Heart Lung Transplant 2012; 31:1052–1064.
- Oldham JM, Noth I. Idiopathic pulmonary fibrosis: early detection and referral. Respir Med 2014; 108:819–829.
Primary care physicians are playing a bigger role in evaluating the incidental finding of interstitial lung diseases since the recent publication of guidelines recommending computed tomography (CT) to screen for lung cancer.
In August 2011, the National Cancer Institute published its findings from the National Lung Screening Trial, which demonstrated a 20% reduction in mortality from lung cancer in patients at high risk screened with low-dose CT.1 Based on these results, the American Cancer Society, the American College of Chest Physicians, the American Society of Clinical Oncology, and the National Comprehensive Cancer Network recommended annual screening for lung cancer with low-dose CT in adults ages 55 to 74 who have a 30-pack-year smoking history and who currently smoke or have quit within the past 15 years.2 In December 2013, the US Preventive Services Task Force published similar guidelines but increased the age range to include high-risk patients ages 55 to 80.3
Bach et al4 estimated that, in 2010 in the United States, 8.6 million people met the criteria used in the National Lung Screening Trial for low-dose CT screening. These are the same criteria as in the multisociety recommendations cited above.2 With such large numbers of patients eligible for CT screening, internists and other primary care physicians are undoubtedly encountering the incidental discovery of nonmalignant pulmonary diseases such as interstitial lung disease.
This article reviews the radiographic characteristics of the most common interstitial lung diseases the internist may encounter on screening CT in long-term smokers.
Referral to a specialist has been associated with lower rates of morbidity and death,5 and a diagnosis of interstitial lung disease should be confirmed by a pulmonologist and a radiologist specializing in differentiating the subtypes. But the primary care physician now plays a critical role in recognizing the need for further evaluation.
HOW COMMON IS INTERSTITIAL LUNG DISEASE IN SMOKERS?
Several studies have published data on the prevalence of interstitial lung disease in patients undergoing low-dose CT for lung cancer screening.
A trial at Mayo Clinic in current and former smokers identified “diffuse lung disease” in 9 (0.9%) of 1,049 participants.6
A trial in Ireland identified idiopathic pulmonary fibrosis in 6 (1.3%) of 449 current smokers who underwent low-dose CT screening for lung cancer.7
Sverzellati et al8 evaluated 692 participants in the Multicentric Italian Lung Detection CT screening study and reported a respiratory bronchiolitis pattern in 109 (15.7%), a usual interstitial pneumonia pattern in 2 (0.3%), and other patterns of chronic interstitial pneumonia in 26 (3.8%).
The National Lung Screening Trial reported that the frequency of “clinically significant” incidental findings (including pulmonary fibrosis) in all participants was 7.5%.1 A retrospective analysis of 884 participants at a single site in this trial identified interstitial lung abnormalities in 86 participants (9.7%).9 These abnormalities were further categorized as nonfibrotic in 52 (5.9%) of 884, fibrotic in 19 (2.1%) of 884, and mixed fibrotic and nonfibrotic in 15 (1.7%) of 884.
Follow-up CT at 2 years in this trial demonstrated improvement in 50% and progression in 11% of patients who had nonfibrotic abnormalities, while fibrotic abnormalities improved in no cases and progressed in 37%. Interstitial lung abnormalities were more common in those who currently smoked and in those with more pack-years of cigarette smoking.9
In sum, these trials suggest that low-dose CT screening for lung cancer can detect the most common forms of interstitial lung disease in this at-risk population and can characterize them as fibrotic or nonfibrotic, a distinction important for prognosis and subsequent management.
NONFIBROTIC VS FIBROTIC DISEASE
It is important to distinguish between nonfibrotic and fibrotic interstitial lung disease, as fibrotic disease carries a worse prognosis and is treated differently.
Features of nonfibrotic interstitial lung disease:
- Ground-glass opacities
- Nodules
- Mosaic attenuation or consolidation.
Features of fibrotic interstitial lung disease:
- Combination of ground-glass opacities and reticulation
- Reticulation by itself
- Traction bronchiectasis
- Honeycombing
- Loss of lung volume.
NONFIBROTIC INTERSTITIAL LUNG DISEASES
Given the strong likelihood that a patient undergoing screening CT is either a current or former smoker, physicians may encounter, in addition to emphysema and lung cancer, the following smoking-related interstitial lung diseases, which are primarily nonfibrotic and which frequently coexist (Table 1):
- Respiratory bronchiolitis
- Respiratory bronchiolitis-interstitial lung disease
- Desquamative interstitial pneumonia
- Pulmonary Langerhans cell histiocytosis.
Respiratory bronchiolitis
Respiratory bronchiolitis occurs mostly in smokers and does not necessarily lead to respiratory symptoms in all patients.10 It cannot always be identified radiographically but occasionally appears as predominantly upper-lobe, patchy ground-glass opacities or ill-defined centrilobular nodules without evidence of fibrosis (Figure 1).

Respiratory bronchiolitis-interstitial lung disease
In rare cases, respiratory bronchiolitis leads to peribronchial fibrosis invading the alveolar walls, which is then classified as respiratory bronchiolitis-interstitial lung disease.11 The CT findings in respiratory bronchiolitis-interstitial lung disease are upper-lobe-predominant centrilobular ground-glass nodules, patchy ground-glass opacities, and bronchial wall thickening (Figure 2).10 Occasionally, mild reticulation is noted without honeycombing. Mild air trapping can be seen in the lower lobes, with centrilobular emphysema in the upper lobes.12

The only successful therapy for respiratory bronchiolitis and respiratory bronchiolitis-interstitial lung disease is smoking cessation. Finding either of these diseases should prompt aggressive counseling by the internist and consideration of referral to a specialist in interstitial lung disease.
Desquamative interstitial pneumonia
Although pathologically different from respiratory bronchiolitis-interstitial lung disease, desquamative interstitial pneumonia has a similar clinical and radiographic presentation. Because their features significantly overlap, they are considered a pathomorphologic continuum, representing degrees of severity of the same disease process caused by prolonged tobacco inhalation.10,13
Widespread ground-glass opacities are the predominant CT finding. These are bilateral and symmetric in distribution in 86%, basal and peripheral in 60%, patchy in 20%, and diffuse in 20% (Figure 3).14 Other frequent findings are mild reticulation with traction bronchiectasis and coexistent emphysema (Figure 4).15 The small peripheral cystic spaces noted in this disease most likely represent dilated bronchioles and alveolar ducts rather than honeycombing.16


No additional treatment beyond elimination of smoking has been proven effective for desquamative interstitial pneumonia, and patients who manage to quit smoking generally have a favorable prognosis.17,18
Pulmonary Langerhans cell histiocytosis
The combination of upper-lobe-predominant cysts and nodules in a young heavy smoker are diagnostic of pulmonary Langerhans cell histiocytosis. The cysts are bizarrely shaped, thin- or thick-walled, and nonuniform in size (Figure 5). The irregular cavitary nodules are centrilobular. The disease characteristically spares the costophrenic angles.

Spontaneous pneumothorax is the initial clinical presentation in 15% of patients.16 In the early stages of the disease (nodule-predominant disease without cysts), infection and metastatic disease need to be excluded (Figure 6). In the later stages, the cysts become coalescent, making the distinction between this disease and “burned-out” lymphangioleiomyomatosis or severe emphysema extremely difficult (Figure 7).17 Smoking cessation and corticosteroids are the mainstay of medical therapy for pulmonary Langerhans cell histiocytosis, and about 50% of patients who quit smoking and receive corticosteroids demonstrate partial or complete clearing of the radiographic abnormalities and symptoms (Figure 8).



FIBROTIC INTERSTITIAL LUNG DISEASES
If CT identifies a diffuse fibrotic pattern, the two most common possibilities (Table 2) are:
- Nonspecific interstitial pneumonia
- Usual interstitial pneumonia.
As noted above, these carry a worse prognosis than the nonfibrotic interstitial lung diseases.
Nonspecific interstitial pneumonia
While most frequently idiopathic, the nonspecific interstitial pneumonia pattern can often be seen in connective tissue diseases. It has also been associated with chronic hypersensitivity pneumonitis, drug toxicity, and slowly resolving diffuse alveolar damage.19 Although it is not the only pathologic pattern in interstitial lung disease associated with connective tissue disease, it is the most common pattern in systemic sclerosis, systemic lupus erythematosus, dermatomyositis-polymyositis, and mixed connective tissue disease.20
The parenchymal changes are typically subpleural and symmetric in distribution (Figure 9). In about one-third of cases, there is a peribronchovascular distribution of the abnormalities (Figure 10).


Ground-glass opacities are the dominant imaging findings, seen in 80% of cases.18 In advanced disease (also referred to as fibrotic nonspecific interstitial pneumonia), patients have accompanying fine or coarse reticular opacities, traction bronchiectasis, and consolidation (Figure 11). Honeycombing is seen in 1% to 5% of patients.21

The most specific sign of nonspecific interstitial pneumonia is sparing of the immediate subpleural lung, apparent in 30% to 50% of patients (Figure 12).22 Subpleural sparing with a peribronchovascular distribution of abnormalities, absence of lobular areas with decreased attenuation, and lack of honeycombing are imaging features that increase the diagnostic confidence of nonspecific interstitial pneumonia (Table 3).23 Clinically, compared with those who have usual interstitial pneumonia (see below), patients are younger and more of them are female. These patients also present with extrapulmonary manifestations such as joint involvement, rash, and Raynaud phenomenon. Therefore, these associated symptoms on presentation can help distinguish nonspecific interstitial pneumonia or usual interstitial pneumonia related to connective tissue disease from the idiopathic forms.

The first step in managing nonspecific interstitial pneumonia is to remove all potential exposure to inhaled substances or to drugs. Although immunosuppressive therapy has never been studied in a randomized controlled trial in this disease, numerous reports suggest that patients may respond to prednisone and to steroid-sparing immunosuppressants.24
In several studies, survival rates in nonspecific interstitial pneumonia were significantly greater than in usual interstitial pneumonia independent of the treatment strategy. In long-term follow-up of patients with idiopathic nonspecific interstitial pneumonia treated with immunosuppressive therapy, two-thirds remained stable or improved.25–27
Although most connective tissue diseases cause a lung pattern of nonspecific interstitial pneumonia, some (eg, rheumatoid arthritis) may present with a pattern of usual interstitial pneumonia. In these cases and in those of advanced fibrotic nonspecific interstitial pneumonia, the prognosis is worse, as the disease is less responsive to immunosuppressive therapy.20
Usual interstitial pneumonia
Usual interstitial pneumonia is the most severe form of lung fibrosis. Most cases are idiopathic and are termed idiopathic pulmonary fibrosis. Other causes of the usual interstitial pneumonia pattern include domestic and occupational environmental exposures, connective tissue disease, and drug toxicity.28 An epidemiologic association between smoking and usual interstitial pneumonia is well documented.28
Idiopathic pulmonary fibrosis typically affects men ages 50 to 70. Because its risk factors coincide with those of lung cancer, there is a high likelihood of detecting idiopathic pulmonary fibrosis early in this screening population. It has an especially poor prognosis, with a mean survival of 2 to 5 years from the time of diagnosis.18
The distribution of disease in usual interstitial pneumonia is characteristically subpleural and basal. CT features include coarse subpleural reticulation and honeycombing combined with traction bronchiectasis or bronchiolectasis and architectural distortion (Figure 13).18 Honeycombing is the most specific and key diagnostic CT finding for establishing a definitive diagnosis of usual interstitial pneumonia.29 However, ground-glass opacities are present in most patients, typically in the region of interstitial fibrosis, and are always less extensive than the reticulation.30 The findings demonstrate morphologic heterogeneity, with areas of fibrosis adjacent to areas of normal lung (Figure 14).


In addition to the aforementioned imaging features, the 2011 American Thoracic Society and European Respiratory Society joint guidelines for the CT diagnosis of usual interstitial pneumonia patterns require the absence of atypical features that suggest an alternative diagnosis, including those seen in nonspecific interstitial pneumonia, such as an upper, midlung, or peribronchovascular distribution and extensive ground-glass attenuation.28 Mild mediastinal lymphadenopathy (usually < 1.5 cm in the short axis) is common in usual interstitial pneumonia.31
Because other chronic interstitial pneumonias that may resemble usual interstitial pneumonia have a more favorable course and may respond to immunosuppressive therapy, establishing an early and accurate diagnosis is of the utmost importance.5 Additionally, the emergence of possible new therapies for idiopathic pulmonary fibrosis makes early referral to a specialist paramount in these cases. Recent studies have demonstrated significant slowing of the progression of disease in idiopathic pulmonary fibrosis with both pirfenidone and nintedanib.32,33
DIAGNOSIS AND MANAGEMENT
The diagnosis of these nonfibrotic and fibrotic lung diseases is complex. In all cases in which interstitial lung disease is detected on screening CT for lung cancer, the internist should strongly consider further evaluation with dedicated high-resolution CT and early referral to a specialist (Figure 15).
Because smoking cessation is the only recommended treatment for nonfibrotic smoking-related interstitial lung diseases, particular emphasis on smoking cessation counseling is essential.
Referral for bronchoscopy with transbronchial lung biopsy is generally not helpful in the diagnosis of the interstitial lung diseases discussed in this article unless there is a need to rule out infection or neoplasm.34 Referral for surgical lung biopsy may be indicated in some cases of suspected pulmonary Langerhans cell histiocytosis, desquamative interstitial pneumonia, nonspecific interstitial pneumonia, or usual interstitial pneumonia if the diagnosis is uncertain (Tables 1 and 2).35
The American Thoracic Society/European Respiratory Society guidelines suggest a multidisciplinary team approach that includes a pathologist, radiologist, and clinician.35 This approach more readily determines the correct diagnosis and relies less on invasive methods such as surgical biopsy and more on noninvasive methods such as radiology and clinical history. Overall, this will promote earlier access to appropriate therapies, clinical trial enrollment, and in more severe cases, lung transplant.
Currently, 23% of all lung transplants worldwide are performed in patients with idiopathic pulmonary fibrosis. Other forms of pulmonary fibrosis account for 3% to 4% of lung transplants performed.36
Evidence suggests that early referral reduces rates of morbidity and death in these patients. The results of a single-center study37 of patients with idiopathic pulmonary fibrosis demonstrated that a longer delay from the onset of symptoms to evaluation by a specialist at a tertiary care referral center was associated with a higher rate of death from this disease independent of disease severity. Those with the longest delay in referral had a multivariable-adjusted death rate 3.4 times higher than those with the shortest delay.5,37
In summary, with implementation of the new lung cancer screening guidelines, primary care physicians are more often encountering the incidental finding of interstitial lung disease in their patients. Prompt diagnosis of interstitial lung disease helps ensure that patients receive appropriate care and early consideration for clinical trials and lung transplant.
Primary care physicians play a critical role in the initial identification of key characteristics of the interstitial abnormality—namely, whether the pattern is nonfibrotic or fibrotic—and in the correlation of the history and physical findings to expedite the diagnosis. Subsequently, ordering high-resolution CT for more detailed characterization and prompt referral to a specialist in interstitial lung disease allow for a more rapid and accurate diagnosis, specialized therapy, and supportive care.
Primary care physicians are playing a bigger role in evaluating the incidental finding of interstitial lung diseases since the recent publication of guidelines recommending computed tomography (CT) to screen for lung cancer.
In August 2011, the National Cancer Institute published its findings from the National Lung Screening Trial, which demonstrated a 20% reduction in mortality from lung cancer in patients at high risk screened with low-dose CT.1 Based on these results, the American Cancer Society, the American College of Chest Physicians, the American Society of Clinical Oncology, and the National Comprehensive Cancer Network recommended annual screening for lung cancer with low-dose CT in adults ages 55 to 74 who have a 30-pack-year smoking history and who currently smoke or have quit within the past 15 years.2 In December 2013, the US Preventive Services Task Force published similar guidelines but increased the age range to include high-risk patients ages 55 to 80.3
Bach et al4 estimated that, in 2010 in the United States, 8.6 million people met the criteria used in the National Lung Screening Trial for low-dose CT screening. These are the same criteria as in the multisociety recommendations cited above.2 With such large numbers of patients eligible for CT screening, internists and other primary care physicians are undoubtedly encountering the incidental discovery of nonmalignant pulmonary diseases such as interstitial lung disease.
This article reviews the radiographic characteristics of the most common interstitial lung diseases the internist may encounter on screening CT in long-term smokers.
Referral to a specialist has been associated with lower rates of morbidity and death,5 and a diagnosis of interstitial lung disease should be confirmed by a pulmonologist and a radiologist specializing in differentiating the subtypes. But the primary care physician now plays a critical role in recognizing the need for further evaluation.
HOW COMMON IS INTERSTITIAL LUNG DISEASE IN SMOKERS?
Several studies have published data on the prevalence of interstitial lung disease in patients undergoing low-dose CT for lung cancer screening.
A trial at Mayo Clinic in current and former smokers identified “diffuse lung disease” in 9 (0.9%) of 1,049 participants.6
A trial in Ireland identified idiopathic pulmonary fibrosis in 6 (1.3%) of 449 current smokers who underwent low-dose CT screening for lung cancer.7
Sverzellati et al8 evaluated 692 participants in the Multicentric Italian Lung Detection CT screening study and reported a respiratory bronchiolitis pattern in 109 (15.7%), a usual interstitial pneumonia pattern in 2 (0.3%), and other patterns of chronic interstitial pneumonia in 26 (3.8%).
The National Lung Screening Trial reported that the frequency of “clinically significant” incidental findings (including pulmonary fibrosis) in all participants was 7.5%.1 A retrospective analysis of 884 participants at a single site in this trial identified interstitial lung abnormalities in 86 participants (9.7%).9 These abnormalities were further categorized as nonfibrotic in 52 (5.9%) of 884, fibrotic in 19 (2.1%) of 884, and mixed fibrotic and nonfibrotic in 15 (1.7%) of 884.
Follow-up CT at 2 years in this trial demonstrated improvement in 50% and progression in 11% of patients who had nonfibrotic abnormalities, while fibrotic abnormalities improved in no cases and progressed in 37%. Interstitial lung abnormalities were more common in those who currently smoked and in those with more pack-years of cigarette smoking.9
In sum, these trials suggest that low-dose CT screening for lung cancer can detect the most common forms of interstitial lung disease in this at-risk population and can characterize them as fibrotic or nonfibrotic, a distinction important for prognosis and subsequent management.
NONFIBROTIC VS FIBROTIC DISEASE
It is important to distinguish between nonfibrotic and fibrotic interstitial lung disease, as fibrotic disease carries a worse prognosis and is treated differently.
Features of nonfibrotic interstitial lung disease:
- Ground-glass opacities
- Nodules
- Mosaic attenuation or consolidation.
Features of fibrotic interstitial lung disease:
- Combination of ground-glass opacities and reticulation
- Reticulation by itself
- Traction bronchiectasis
- Honeycombing
- Loss of lung volume.
NONFIBROTIC INTERSTITIAL LUNG DISEASES
Given the strong likelihood that a patient undergoing screening CT is either a current or former smoker, physicians may encounter, in addition to emphysema and lung cancer, the following smoking-related interstitial lung diseases, which are primarily nonfibrotic and which frequently coexist (Table 1):
- Respiratory bronchiolitis
- Respiratory bronchiolitis-interstitial lung disease
- Desquamative interstitial pneumonia
- Pulmonary Langerhans cell histiocytosis.
Respiratory bronchiolitis
Respiratory bronchiolitis occurs mostly in smokers and does not necessarily lead to respiratory symptoms in all patients.10 It cannot always be identified radiographically but occasionally appears as predominantly upper-lobe, patchy ground-glass opacities or ill-defined centrilobular nodules without evidence of fibrosis (Figure 1).

Respiratory bronchiolitis-interstitial lung disease
In rare cases, respiratory bronchiolitis leads to peribronchial fibrosis invading the alveolar walls, which is then classified as respiratory bronchiolitis-interstitial lung disease.11 The CT findings in respiratory bronchiolitis-interstitial lung disease are upper-lobe-predominant centrilobular ground-glass nodules, patchy ground-glass opacities, and bronchial wall thickening (Figure 2).10 Occasionally, mild reticulation is noted without honeycombing. Mild air trapping can be seen in the lower lobes, with centrilobular emphysema in the upper lobes.12

The only successful therapy for respiratory bronchiolitis and respiratory bronchiolitis-interstitial lung disease is smoking cessation. Finding either of these diseases should prompt aggressive counseling by the internist and consideration of referral to a specialist in interstitial lung disease.
Desquamative interstitial pneumonia
Although pathologically different from respiratory bronchiolitis-interstitial lung disease, desquamative interstitial pneumonia has a similar clinical and radiographic presentation. Because their features significantly overlap, they are considered a pathomorphologic continuum, representing degrees of severity of the same disease process caused by prolonged tobacco inhalation.10,13
Widespread ground-glass opacities are the predominant CT finding. These are bilateral and symmetric in distribution in 86%, basal and peripheral in 60%, patchy in 20%, and diffuse in 20% (Figure 3).14 Other frequent findings are mild reticulation with traction bronchiectasis and coexistent emphysema (Figure 4).15 The small peripheral cystic spaces noted in this disease most likely represent dilated bronchioles and alveolar ducts rather than honeycombing.16


No additional treatment beyond elimination of smoking has been proven effective for desquamative interstitial pneumonia, and patients who manage to quit smoking generally have a favorable prognosis.17,18
Pulmonary Langerhans cell histiocytosis
The combination of upper-lobe-predominant cysts and nodules in a young heavy smoker are diagnostic of pulmonary Langerhans cell histiocytosis. The cysts are bizarrely shaped, thin- or thick-walled, and nonuniform in size (Figure 5). The irregular cavitary nodules are centrilobular. The disease characteristically spares the costophrenic angles.

Spontaneous pneumothorax is the initial clinical presentation in 15% of patients.16 In the early stages of the disease (nodule-predominant disease without cysts), infection and metastatic disease need to be excluded (Figure 6). In the later stages, the cysts become coalescent, making the distinction between this disease and “burned-out” lymphangioleiomyomatosis or severe emphysema extremely difficult (Figure 7).17 Smoking cessation and corticosteroids are the mainstay of medical therapy for pulmonary Langerhans cell histiocytosis, and about 50% of patients who quit smoking and receive corticosteroids demonstrate partial or complete clearing of the radiographic abnormalities and symptoms (Figure 8).



FIBROTIC INTERSTITIAL LUNG DISEASES
If CT identifies a diffuse fibrotic pattern, the two most common possibilities (Table 2) are:
- Nonspecific interstitial pneumonia
- Usual interstitial pneumonia.
As noted above, these carry a worse prognosis than the nonfibrotic interstitial lung diseases.
Nonspecific interstitial pneumonia
While most frequently idiopathic, the nonspecific interstitial pneumonia pattern can often be seen in connective tissue diseases. It has also been associated with chronic hypersensitivity pneumonitis, drug toxicity, and slowly resolving diffuse alveolar damage.19 Although it is not the only pathologic pattern in interstitial lung disease associated with connective tissue disease, it is the most common pattern in systemic sclerosis, systemic lupus erythematosus, dermatomyositis-polymyositis, and mixed connective tissue disease.20
The parenchymal changes are typically subpleural and symmetric in distribution (Figure 9). In about one-third of cases, there is a peribronchovascular distribution of the abnormalities (Figure 10).


Ground-glass opacities are the dominant imaging findings, seen in 80% of cases.18 In advanced disease (also referred to as fibrotic nonspecific interstitial pneumonia), patients have accompanying fine or coarse reticular opacities, traction bronchiectasis, and consolidation (Figure 11). Honeycombing is seen in 1% to 5% of patients.21

The most specific sign of nonspecific interstitial pneumonia is sparing of the immediate subpleural lung, apparent in 30% to 50% of patients (Figure 12).22 Subpleural sparing with a peribronchovascular distribution of abnormalities, absence of lobular areas with decreased attenuation, and lack of honeycombing are imaging features that increase the diagnostic confidence of nonspecific interstitial pneumonia (Table 3).23 Clinically, compared with those who have usual interstitial pneumonia (see below), patients are younger and more of them are female. These patients also present with extrapulmonary manifestations such as joint involvement, rash, and Raynaud phenomenon. Therefore, these associated symptoms on presentation can help distinguish nonspecific interstitial pneumonia or usual interstitial pneumonia related to connective tissue disease from the idiopathic forms.

The first step in managing nonspecific interstitial pneumonia is to remove all potential exposure to inhaled substances or to drugs. Although immunosuppressive therapy has never been studied in a randomized controlled trial in this disease, numerous reports suggest that patients may respond to prednisone and to steroid-sparing immunosuppressants.24
In several studies, survival rates in nonspecific interstitial pneumonia were significantly greater than in usual interstitial pneumonia independent of the treatment strategy. In long-term follow-up of patients with idiopathic nonspecific interstitial pneumonia treated with immunosuppressive therapy, two-thirds remained stable or improved.25–27
Although most connective tissue diseases cause a lung pattern of nonspecific interstitial pneumonia, some (eg, rheumatoid arthritis) may present with a pattern of usual interstitial pneumonia. In these cases and in those of advanced fibrotic nonspecific interstitial pneumonia, the prognosis is worse, as the disease is less responsive to immunosuppressive therapy.20
Usual interstitial pneumonia
Usual interstitial pneumonia is the most severe form of lung fibrosis. Most cases are idiopathic and are termed idiopathic pulmonary fibrosis. Other causes of the usual interstitial pneumonia pattern include domestic and occupational environmental exposures, connective tissue disease, and drug toxicity.28 An epidemiologic association between smoking and usual interstitial pneumonia is well documented.28
Idiopathic pulmonary fibrosis typically affects men ages 50 to 70. Because its risk factors coincide with those of lung cancer, there is a high likelihood of detecting idiopathic pulmonary fibrosis early in this screening population. It has an especially poor prognosis, with a mean survival of 2 to 5 years from the time of diagnosis.18
The distribution of disease in usual interstitial pneumonia is characteristically subpleural and basal. CT features include coarse subpleural reticulation and honeycombing combined with traction bronchiectasis or bronchiolectasis and architectural distortion (Figure 13).18 Honeycombing is the most specific and key diagnostic CT finding for establishing a definitive diagnosis of usual interstitial pneumonia.29 However, ground-glass opacities are present in most patients, typically in the region of interstitial fibrosis, and are always less extensive than the reticulation.30 The findings demonstrate morphologic heterogeneity, with areas of fibrosis adjacent to areas of normal lung (Figure 14).


In addition to the aforementioned imaging features, the 2011 American Thoracic Society and European Respiratory Society joint guidelines for the CT diagnosis of usual interstitial pneumonia patterns require the absence of atypical features that suggest an alternative diagnosis, including those seen in nonspecific interstitial pneumonia, such as an upper, midlung, or peribronchovascular distribution and extensive ground-glass attenuation.28 Mild mediastinal lymphadenopathy (usually < 1.5 cm in the short axis) is common in usual interstitial pneumonia.31
Because other chronic interstitial pneumonias that may resemble usual interstitial pneumonia have a more favorable course and may respond to immunosuppressive therapy, establishing an early and accurate diagnosis is of the utmost importance.5 Additionally, the emergence of possible new therapies for idiopathic pulmonary fibrosis makes early referral to a specialist paramount in these cases. Recent studies have demonstrated significant slowing of the progression of disease in idiopathic pulmonary fibrosis with both pirfenidone and nintedanib.32,33
DIAGNOSIS AND MANAGEMENT
The diagnosis of these nonfibrotic and fibrotic lung diseases is complex. In all cases in which interstitial lung disease is detected on screening CT for lung cancer, the internist should strongly consider further evaluation with dedicated high-resolution CT and early referral to a specialist (Figure 15).
Because smoking cessation is the only recommended treatment for nonfibrotic smoking-related interstitial lung diseases, particular emphasis on smoking cessation counseling is essential.
Referral for bronchoscopy with transbronchial lung biopsy is generally not helpful in the diagnosis of the interstitial lung diseases discussed in this article unless there is a need to rule out infection or neoplasm.34 Referral for surgical lung biopsy may be indicated in some cases of suspected pulmonary Langerhans cell histiocytosis, desquamative interstitial pneumonia, nonspecific interstitial pneumonia, or usual interstitial pneumonia if the diagnosis is uncertain (Tables 1 and 2).35
The American Thoracic Society/European Respiratory Society guidelines suggest a multidisciplinary team approach that includes a pathologist, radiologist, and clinician.35 This approach more readily determines the correct diagnosis and relies less on invasive methods such as surgical biopsy and more on noninvasive methods such as radiology and clinical history. Overall, this will promote earlier access to appropriate therapies, clinical trial enrollment, and in more severe cases, lung transplant.
Currently, 23% of all lung transplants worldwide are performed in patients with idiopathic pulmonary fibrosis. Other forms of pulmonary fibrosis account for 3% to 4% of lung transplants performed.36
Evidence suggests that early referral reduces rates of morbidity and death in these patients. The results of a single-center study37 of patients with idiopathic pulmonary fibrosis demonstrated that a longer delay from the onset of symptoms to evaluation by a specialist at a tertiary care referral center was associated with a higher rate of death from this disease independent of disease severity. Those with the longest delay in referral had a multivariable-adjusted death rate 3.4 times higher than those with the shortest delay.5,37
In summary, with implementation of the new lung cancer screening guidelines, primary care physicians are more often encountering the incidental finding of interstitial lung disease in their patients. Prompt diagnosis of interstitial lung disease helps ensure that patients receive appropriate care and early consideration for clinical trials and lung transplant.
Primary care physicians play a critical role in the initial identification of key characteristics of the interstitial abnormality—namely, whether the pattern is nonfibrotic or fibrotic—and in the correlation of the history and physical findings to expedite the diagnosis. Subsequently, ordering high-resolution CT for more detailed characterization and prompt referral to a specialist in interstitial lung disease allow for a more rapid and accurate diagnosis, specialized therapy, and supportive care.
- National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365:395–409.
- Detterbeck FC, Lewis SZ, Diekemper R, Addrizzo-Harris D, Alberts WM. Executive summary: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143(suppl 5):7S–37S.
- Moyer VA; US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 160:330–338.
- Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA 2012; 307:2418–2429.
- Lamas DJ, Kawut SM, Bagiella E, Philip N, Arcasoy SM, Lederer DJ. Delayed access and survival in idiopathic pulmonary fibrosis: a cohort study. Am J Respir Crit Care Med 2011; 184:842–847.
- Swensen SJ, Jett JR, Hartman TE, et al. Lung cancer screening with CT: Mayo Clinic experience. Radiology 2003; 226:756–761.
- MacRedmond R, Logan PM, Lee M, Kenny D, Foley C, Costello RW. Screening for lung cancer using low dose CT scanning. Thorax 2004; 59:237–241.
- Sverzellati N, Guerci L, Randi G, et al. Interstitial lung diseases in a lung cancer screening trial. Eur Respir J 2011; 38:392–400.
- Jin GY, Lynch D, Chawla A, et al. Interstitial lung abnormalities in a CT lung cancer screening population: prevalence and progression rate. Radiology 2013; 268:563–571.
- Heyneman LE, Ward S, Lynch DA, Remy-Jardin M, Johkoh T, Müller NL. Respiratory bronchiolitis, respiratory bronchiolitis-associated interstitial lung disease, and desquamative interstitial pneumonia: different entities or part of the spectrum of the same disease process? AJR Am J Roentgenol 1999; 173:1617–1622.
- Moon J, du Bois RM, Colby TV, Hansell DM, Nicholson AG. Clinical significance of respiratory bronchiolitis on open lung biopsy and its relationship to smoking related interstitial lung disease. Thorax 1999; 54:1009–1014.
- Holt RM, Schmidt RA, Godwin JD, Raghu G. High resolution CT in respiratory bronchiolitis-associated interstitial lung disease. J Comput Assist Tomogr 1993; 17:46–50.
- Ryu JH, Myers JL, Capizzi SA, Douglas WW, Vassallo R, Decker PA. Desquamative interstitial pneumonia and respiratory bronchiolitis-associated interstitial lung disease. Chest 2005; 127:178–184.
- Hartman TE, Primack SL, Swensen SJ, Hansell D, McGuinness G, Müller NL. Desquamative interstitial pneumonia: thin-section CT findings in 22 patients. Radiology 1993; 187:787–790.
- Akira M, Yamamoto S, Hara H, Sakatani M, Ueda E. Serial computed tomographic evaluation in desquamative interstitial pneumonia. Thorax 1997; 52:333–337.
- Lacronique J, Roth C, Battesti JP, Basset F, Chretien J. Chest radiological features of pulmonary histiocytosis X: a report based on 50 adult cases. Thorax 1982; 37:104–109.
- Remy-Jardin M, Edme JL, Boulenguez C, Remy J, Mastora I, Sobaszek A. Longitudinal follow-up study of smoker’s lung with thin-section CT in correlation with pulmonary function tests. Radiology 2002; 222:261–270.
- Mueller-Mang C, Grosse C, Schmid K, Stiebellehner L, Bankier AA. What every radiologist should know about idiopathic interstitial pneumonias. Radiographics 2007; 27:595–615.
- Katzenstein AL, Fiorelli RF. Nonspecific interstitial pneumonia/fibrosis. Histologic features and clinical significance. Am J Surg Pathol 1994; 18:136–147.
- Bryson T, Sundaram B, Khanna D, Kazerooni EA. Connective tissue disease-associated interstitial pneumonia and idiopathic interstitial pneumonia: similarity and difference. Semin Ultrasound CT MR 2014; 35:29–38.
- Desai SR, Veeraraghavan S, Hansell DM, et al. CT features of lung disease in patients with systemic sclerosis: comparison with idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia. Radiology 2004; 232:560–567.
- Tsubamoto M, Müller NL, Johkoh T, et al. Pathologic subgroups of nonspecific interstitial pneumonia: differential diagnosis from other idiopathic interstitial pneumonias on high-resolution computed tomography. J Comput Assist Tomogr 2005; 29:793–800.
- Silva CI, Müller NL, Lynch DA, et al. Chronic hypersensitivity pneumonitis: differentiation from idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia by using thin-section CT. Radiology 2008; 246:288–297.
- Antin-Ozerkis D, Rubinowitz A. An update on nonspecific interstitial pneumonia. Clin Pulm Med 2010; 17:122–128.
- Daniil ZD, Gilchrist FC, Nicholson AG, et al. A histologic pattern of nonspecific interstitial pneumonia is associated with a better prognosis than usual interstitial pneumonia in patients with cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med 1999; 160:899–905.
- Travis WD, Matsui K, Moss J, Ferrans VJ. Idiopathic nonspecific interstitial pneumonia: prognostic significance of cellular and fibrosing patterns: survival comparison with usual interstitial pneumonia and desquamative interstitial pneumonia. Am J Surg Pathol 2000; 24:19–33.
- Riha RL, Duhig EE, Clarke BE, Steele RH, Slaughter RE, Zimmerman PV. Survival of patients with biopsy-proven usual interstitial pneumonia and nonspecific interstitial pneumonia. Eur Respir J 2002; 19:1114–1118.
- Raghu G, Collard HR, Egan JJ, et al; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183:788–824.
- du Bois RM. An earlier and more confident diagnosis of idiopathic pulmonary fibrosis. Eur Respir Rev 2012; 21:141–146.
- Nishimura K, Kitaichi M, Izumi T, Nagai S, Kanaoka M, Itoh H. Usual interstitial pneumonia: histologic correlation with high-resolution CT. Radiology 1992; 182:337–342.
- Souza CA, Müller NL, Lee KS, Johkoh T, Mitsuhiro H, Chong S. Idiopathic interstitial pneumonias: prevalence of mediastinal lymph node enlargement in 206 patients. AJR Am J Roentgenol 2006; 186:995–999.
- King TE Jr, Bradford WZ, Castro-Bernardini S, et al; ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014; 370:2083–2092.
- Richeldi L, du Bois RM, Raghu G, et al; INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014; 370:2071–2082.
- Bradley B, Branley HM, Egan JJ, et al; British Thoracic Society Interstitial Lung Disease Guideline Group, British Thoracic Society Standards of Care Committee; Thoracic Society of Australia; New Zealand Thoracic Society; Irish Thoracic Society. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax 2008; 63(suppl 5):v1–v58.
- Travis WD, Costabel U, Hansell DM, et al; ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013; 188:733–748.
- Stehlik J, Edwards LB, Kucheryavaya AY, et al; International Society of Heart and Lung Transplantation. The Registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report—2012. J Heart Lung Transplant 2012; 31:1052–1064.
- Oldham JM, Noth I. Idiopathic pulmonary fibrosis: early detection and referral. Respir Med 2014; 108:819–829.
- National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365:395–409.
- Detterbeck FC, Lewis SZ, Diekemper R, Addrizzo-Harris D, Alberts WM. Executive summary: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143(suppl 5):7S–37S.
- Moyer VA; US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 160:330–338.
- Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA 2012; 307:2418–2429.
- Lamas DJ, Kawut SM, Bagiella E, Philip N, Arcasoy SM, Lederer DJ. Delayed access and survival in idiopathic pulmonary fibrosis: a cohort study. Am J Respir Crit Care Med 2011; 184:842–847.
- Swensen SJ, Jett JR, Hartman TE, et al. Lung cancer screening with CT: Mayo Clinic experience. Radiology 2003; 226:756–761.
- MacRedmond R, Logan PM, Lee M, Kenny D, Foley C, Costello RW. Screening for lung cancer using low dose CT scanning. Thorax 2004; 59:237–241.
- Sverzellati N, Guerci L, Randi G, et al. Interstitial lung diseases in a lung cancer screening trial. Eur Respir J 2011; 38:392–400.
- Jin GY, Lynch D, Chawla A, et al. Interstitial lung abnormalities in a CT lung cancer screening population: prevalence and progression rate. Radiology 2013; 268:563–571.
- Heyneman LE, Ward S, Lynch DA, Remy-Jardin M, Johkoh T, Müller NL. Respiratory bronchiolitis, respiratory bronchiolitis-associated interstitial lung disease, and desquamative interstitial pneumonia: different entities or part of the spectrum of the same disease process? AJR Am J Roentgenol 1999; 173:1617–1622.
- Moon J, du Bois RM, Colby TV, Hansell DM, Nicholson AG. Clinical significance of respiratory bronchiolitis on open lung biopsy and its relationship to smoking related interstitial lung disease. Thorax 1999; 54:1009–1014.
- Holt RM, Schmidt RA, Godwin JD, Raghu G. High resolution CT in respiratory bronchiolitis-associated interstitial lung disease. J Comput Assist Tomogr 1993; 17:46–50.
- Ryu JH, Myers JL, Capizzi SA, Douglas WW, Vassallo R, Decker PA. Desquamative interstitial pneumonia and respiratory bronchiolitis-associated interstitial lung disease. Chest 2005; 127:178–184.
- Hartman TE, Primack SL, Swensen SJ, Hansell D, McGuinness G, Müller NL. Desquamative interstitial pneumonia: thin-section CT findings in 22 patients. Radiology 1993; 187:787–790.
- Akira M, Yamamoto S, Hara H, Sakatani M, Ueda E. Serial computed tomographic evaluation in desquamative interstitial pneumonia. Thorax 1997; 52:333–337.
- Lacronique J, Roth C, Battesti JP, Basset F, Chretien J. Chest radiological features of pulmonary histiocytosis X: a report based on 50 adult cases. Thorax 1982; 37:104–109.
- Remy-Jardin M, Edme JL, Boulenguez C, Remy J, Mastora I, Sobaszek A. Longitudinal follow-up study of smoker’s lung with thin-section CT in correlation with pulmonary function tests. Radiology 2002; 222:261–270.
- Mueller-Mang C, Grosse C, Schmid K, Stiebellehner L, Bankier AA. What every radiologist should know about idiopathic interstitial pneumonias. Radiographics 2007; 27:595–615.
- Katzenstein AL, Fiorelli RF. Nonspecific interstitial pneumonia/fibrosis. Histologic features and clinical significance. Am J Surg Pathol 1994; 18:136–147.
- Bryson T, Sundaram B, Khanna D, Kazerooni EA. Connective tissue disease-associated interstitial pneumonia and idiopathic interstitial pneumonia: similarity and difference. Semin Ultrasound CT MR 2014; 35:29–38.
- Desai SR, Veeraraghavan S, Hansell DM, et al. CT features of lung disease in patients with systemic sclerosis: comparison with idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia. Radiology 2004; 232:560–567.
- Tsubamoto M, Müller NL, Johkoh T, et al. Pathologic subgroups of nonspecific interstitial pneumonia: differential diagnosis from other idiopathic interstitial pneumonias on high-resolution computed tomography. J Comput Assist Tomogr 2005; 29:793–800.
- Silva CI, Müller NL, Lynch DA, et al. Chronic hypersensitivity pneumonitis: differentiation from idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia by using thin-section CT. Radiology 2008; 246:288–297.
- Antin-Ozerkis D, Rubinowitz A. An update on nonspecific interstitial pneumonia. Clin Pulm Med 2010; 17:122–128.
- Daniil ZD, Gilchrist FC, Nicholson AG, et al. A histologic pattern of nonspecific interstitial pneumonia is associated with a better prognosis than usual interstitial pneumonia in patients with cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med 1999; 160:899–905.
- Travis WD, Matsui K, Moss J, Ferrans VJ. Idiopathic nonspecific interstitial pneumonia: prognostic significance of cellular and fibrosing patterns: survival comparison with usual interstitial pneumonia and desquamative interstitial pneumonia. Am J Surg Pathol 2000; 24:19–33.
- Riha RL, Duhig EE, Clarke BE, Steele RH, Slaughter RE, Zimmerman PV. Survival of patients with biopsy-proven usual interstitial pneumonia and nonspecific interstitial pneumonia. Eur Respir J 2002; 19:1114–1118.
- Raghu G, Collard HR, Egan JJ, et al; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183:788–824.
- du Bois RM. An earlier and more confident diagnosis of idiopathic pulmonary fibrosis. Eur Respir Rev 2012; 21:141–146.
- Nishimura K, Kitaichi M, Izumi T, Nagai S, Kanaoka M, Itoh H. Usual interstitial pneumonia: histologic correlation with high-resolution CT. Radiology 1992; 182:337–342.
- Souza CA, Müller NL, Lee KS, Johkoh T, Mitsuhiro H, Chong S. Idiopathic interstitial pneumonias: prevalence of mediastinal lymph node enlargement in 206 patients. AJR Am J Roentgenol 2006; 186:995–999.
- King TE Jr, Bradford WZ, Castro-Bernardini S, et al; ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014; 370:2083–2092.
- Richeldi L, du Bois RM, Raghu G, et al; INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014; 370:2071–2082.
- Bradley B, Branley HM, Egan JJ, et al; British Thoracic Society Interstitial Lung Disease Guideline Group, British Thoracic Society Standards of Care Committee; Thoracic Society of Australia; New Zealand Thoracic Society; Irish Thoracic Society. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax 2008; 63(suppl 5):v1–v58.
- Travis WD, Costabel U, Hansell DM, et al; ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013; 188:733–748.
- Stehlik J, Edwards LB, Kucheryavaya AY, et al; International Society of Heart and Lung Transplantation. The Registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report—2012. J Heart Lung Transplant 2012; 31:1052–1064.
- Oldham JM, Noth I. Idiopathic pulmonary fibrosis: early detection and referral. Respir Med 2014; 108:819–829.
KEY POINTS
- Smoking-related interstitial lung diseases can broadly be categorized as fibrotic or nonfibrotic on the basis of their appearance on CT. Fibrotic disease generally carries a worse prognosis.
- Nonfibrotic interstitial lung diseases include respiratory bronchiolitis, respiratory bronchiolitis-interstitial lung disease, desquamative interstitial pneumonia, and pulmonary Langerhans cell histiocytosis.
- Smoking-related fibrotic interstitial lung diseases include nonspecific interstitial pneumonia and usual interstitial pneumonia. A subset of usual interstitial pneumonia, called idiopathic pulmonary fibrosis, carries the worst prognosis of all.
- If CT detects interstitial lung disease during screening for lung cancer, the clinician should strongly consider further evaluation with dedicated high-resolution CT and early referral to a specialist. Smoking cessation is extremely important.
Eventration of the diaphragm presenting as spontaneous pneumothorax
A 25-year-old man with a 2-day history of upper respiratory tract infection presents to the emergency department with the sudden onset of right-sided back and chest pain and shortness of breath after a severe coughing fit.
He is morbidly obese, is a long-time smoker, and has had recurrent exacerbations of asthma with frequent upper respiratory tract infections. He has no history of recent trauma.
A review of systems reveals no significant impairment in exercise tolerance. He has been able to continue doing manual labor at his job as a railroad worker.
Radiography shows a large right pneumothorax and an elevated right diaphragm (Figure 1). Computed tomography (CT) (Figure 2) reveals a right anterior apical pneumothorax with hypoplastic lung and significant elevation of the right diaphragm with fat, bowel, and kidney within the right thorax. He is hemodynamically stable and shows no signs of bowel obstruction.
The physical examination is normal except for diminished breath sounds on the right side. He is diagnosed with congenital diaphragmatic hernia and spontaneous pneumothorax. A 10-F locking pigtail catheter is inserted under CT guidance, leading to complete resolution of the pneumothorax. He is discharged home the next day with a plan for elective repair of the hernia.
Two months later, he returns for scheduled right thoracotomy to repair the hernia. However, while preparing the chest cavity, the surgeon finds no diaphragmatic hernia and no intra-abdominal content—but rather, a severely elevated and thinned-out diaphragm with uninterrupted continuity. The diagnosis is changed to congenital diaphragmatic eventration, and plication of the diaphragm is performed with a series of interrupted, pledgeted polypropylene sutures.
CONGENITAL EVENTRATION OF THE DIAPHRAGM
Congenital diaphragmatic eventration is a rare developmental defect of the central, muscular portion of the diaphragm. The true prevalence is not known, but early reports identified this condition in less than 0.1% of adult.1
Symptomatic patients usually experience dyspnea secondary to ventilation-perfusion mismatch resulting from chronic atelectasis and lung hypoplasia, as well as impaired ventilation resulting from the limited caudal migration of the diaphragm.2,3 Increased susceptibility to recurrent upper respiratory tract infections and pneumonia is also a common feature.
Although rare, spontaneous pneumothorax can develop in patients such as ours, whose lengthy history of smoking and asthma predisposed him to the development of emphysema-like blebs and bullae and to subsequent rupture of blebs brought on by vigorous coughing that caused an involuntary Valsalva maneuver.4
As in our patient, distinguishing congenital diaphragmatic eventration from hernia preoperatively can be difficult with plain chest radiography. Spiral CT with multiplanar reconstruction or with magnetic resonance imaging can help establish the diagnosis.3 However, a severely attenuated diaphragm can be difficult to visualize on CT, as in our patient, leading to a presumptive diagnosis of diaphragmatic hernia. In such situations, the diagnosis of eventration can only be made intraoperatively.
Surgical repair is indicated only for patients with symptoms. Other potential causes of the symptoms should first be ruled out, however, including primary pulmonary disease, cardiac dysfunction, and morbid obesity.
- Chin EF, Lynn RB. Surgery of eventration of the diaphragm. J Thorac Surg 1956; 32:6–14.
- Ridyard JB, Stewart RM. Regional lung function in unilateral diaphragmatic paralysis. Thorax 1976; 31:438–442.
- Shen C, Che G. Congenital eventration of hemidiaphragm in an adult. Ann Thorac Surg 2012; 94:e137–e139.
- Porpodis K, Zarogoulidis P, Spyratos D, et al. Pneumothorax and asthma. J Thorac Dis 2014; 6(suppl 1):S152–S161.
A 25-year-old man with a 2-day history of upper respiratory tract infection presents to the emergency department with the sudden onset of right-sided back and chest pain and shortness of breath after a severe coughing fit.
He is morbidly obese, is a long-time smoker, and has had recurrent exacerbations of asthma with frequent upper respiratory tract infections. He has no history of recent trauma.
A review of systems reveals no significant impairment in exercise tolerance. He has been able to continue doing manual labor at his job as a railroad worker.
Radiography shows a large right pneumothorax and an elevated right diaphragm (Figure 1). Computed tomography (CT) (Figure 2) reveals a right anterior apical pneumothorax with hypoplastic lung and significant elevation of the right diaphragm with fat, bowel, and kidney within the right thorax. He is hemodynamically stable and shows no signs of bowel obstruction.
The physical examination is normal except for diminished breath sounds on the right side. He is diagnosed with congenital diaphragmatic hernia and spontaneous pneumothorax. A 10-F locking pigtail catheter is inserted under CT guidance, leading to complete resolution of the pneumothorax. He is discharged home the next day with a plan for elective repair of the hernia.
Two months later, he returns for scheduled right thoracotomy to repair the hernia. However, while preparing the chest cavity, the surgeon finds no diaphragmatic hernia and no intra-abdominal content—but rather, a severely elevated and thinned-out diaphragm with uninterrupted continuity. The diagnosis is changed to congenital diaphragmatic eventration, and plication of the diaphragm is performed with a series of interrupted, pledgeted polypropylene sutures.
CONGENITAL EVENTRATION OF THE DIAPHRAGM
Congenital diaphragmatic eventration is a rare developmental defect of the central, muscular portion of the diaphragm. The true prevalence is not known, but early reports identified this condition in less than 0.1% of adult.1
Symptomatic patients usually experience dyspnea secondary to ventilation-perfusion mismatch resulting from chronic atelectasis and lung hypoplasia, as well as impaired ventilation resulting from the limited caudal migration of the diaphragm.2,3 Increased susceptibility to recurrent upper respiratory tract infections and pneumonia is also a common feature.
Although rare, spontaneous pneumothorax can develop in patients such as ours, whose lengthy history of smoking and asthma predisposed him to the development of emphysema-like blebs and bullae and to subsequent rupture of blebs brought on by vigorous coughing that caused an involuntary Valsalva maneuver.4
As in our patient, distinguishing congenital diaphragmatic eventration from hernia preoperatively can be difficult with plain chest radiography. Spiral CT with multiplanar reconstruction or with magnetic resonance imaging can help establish the diagnosis.3 However, a severely attenuated diaphragm can be difficult to visualize on CT, as in our patient, leading to a presumptive diagnosis of diaphragmatic hernia. In such situations, the diagnosis of eventration can only be made intraoperatively.
Surgical repair is indicated only for patients with symptoms. Other potential causes of the symptoms should first be ruled out, however, including primary pulmonary disease, cardiac dysfunction, and morbid obesity.
A 25-year-old man with a 2-day history of upper respiratory tract infection presents to the emergency department with the sudden onset of right-sided back and chest pain and shortness of breath after a severe coughing fit.
He is morbidly obese, is a long-time smoker, and has had recurrent exacerbations of asthma with frequent upper respiratory tract infections. He has no history of recent trauma.
A review of systems reveals no significant impairment in exercise tolerance. He has been able to continue doing manual labor at his job as a railroad worker.
Radiography shows a large right pneumothorax and an elevated right diaphragm (Figure 1). Computed tomography (CT) (Figure 2) reveals a right anterior apical pneumothorax with hypoplastic lung and significant elevation of the right diaphragm with fat, bowel, and kidney within the right thorax. He is hemodynamically stable and shows no signs of bowel obstruction.
The physical examination is normal except for diminished breath sounds on the right side. He is diagnosed with congenital diaphragmatic hernia and spontaneous pneumothorax. A 10-F locking pigtail catheter is inserted under CT guidance, leading to complete resolution of the pneumothorax. He is discharged home the next day with a plan for elective repair of the hernia.
Two months later, he returns for scheduled right thoracotomy to repair the hernia. However, while preparing the chest cavity, the surgeon finds no diaphragmatic hernia and no intra-abdominal content—but rather, a severely elevated and thinned-out diaphragm with uninterrupted continuity. The diagnosis is changed to congenital diaphragmatic eventration, and plication of the diaphragm is performed with a series of interrupted, pledgeted polypropylene sutures.
CONGENITAL EVENTRATION OF THE DIAPHRAGM
Congenital diaphragmatic eventration is a rare developmental defect of the central, muscular portion of the diaphragm. The true prevalence is not known, but early reports identified this condition in less than 0.1% of adult.1
Symptomatic patients usually experience dyspnea secondary to ventilation-perfusion mismatch resulting from chronic atelectasis and lung hypoplasia, as well as impaired ventilation resulting from the limited caudal migration of the diaphragm.2,3 Increased susceptibility to recurrent upper respiratory tract infections and pneumonia is also a common feature.
Although rare, spontaneous pneumothorax can develop in patients such as ours, whose lengthy history of smoking and asthma predisposed him to the development of emphysema-like blebs and bullae and to subsequent rupture of blebs brought on by vigorous coughing that caused an involuntary Valsalva maneuver.4
As in our patient, distinguishing congenital diaphragmatic eventration from hernia preoperatively can be difficult with plain chest radiography. Spiral CT with multiplanar reconstruction or with magnetic resonance imaging can help establish the diagnosis.3 However, a severely attenuated diaphragm can be difficult to visualize on CT, as in our patient, leading to a presumptive diagnosis of diaphragmatic hernia. In such situations, the diagnosis of eventration can only be made intraoperatively.
Surgical repair is indicated only for patients with symptoms. Other potential causes of the symptoms should first be ruled out, however, including primary pulmonary disease, cardiac dysfunction, and morbid obesity.
- Chin EF, Lynn RB. Surgery of eventration of the diaphragm. J Thorac Surg 1956; 32:6–14.
- Ridyard JB, Stewart RM. Regional lung function in unilateral diaphragmatic paralysis. Thorax 1976; 31:438–442.
- Shen C, Che G. Congenital eventration of hemidiaphragm in an adult. Ann Thorac Surg 2012; 94:e137–e139.
- Porpodis K, Zarogoulidis P, Spyratos D, et al. Pneumothorax and asthma. J Thorac Dis 2014; 6(suppl 1):S152–S161.
- Chin EF, Lynn RB. Surgery of eventration of the diaphragm. J Thorac Surg 1956; 32:6–14.
- Ridyard JB, Stewart RM. Regional lung function in unilateral diaphragmatic paralysis. Thorax 1976; 31:438–442.
- Shen C, Che G. Congenital eventration of hemidiaphragm in an adult. Ann Thorac Surg 2012; 94:e137–e139.
- Porpodis K, Zarogoulidis P, Spyratos D, et al. Pneumothorax and asthma. J Thorac Dis 2014; 6(suppl 1):S152–S161.
Emergency Ultrasound: Deep Vein Thrombosis
Femoral and Saphenous Vein
After the appropriate positioning has been achieved, the clinician should identify the common femoral vein and artery, and then slide the probe toward the head or feet until the saphenous vein, which empties into the common femoral vein, is identified
The clinician should take care not to apply too much pressure with the probe as this will compress the saphenous vein; therefore, it is important to use a light touch to ensure proper visualization. Once this key region is identified, the clinician should then use the probe to apply pressure to the vessels. The saphenous vein should compress completely upon application of sufficient pressure to the artery. In a normal vein, the anterior and posterior walls should touch and completely obliterate the lumen of the vein. When a clot is present, the walls of the vein will remain separated (Figure 3). A thrombus can be bright on ultrasound, but some DVTs may appear completely black. In some cases, a lack of compressibility may be the only tip-off to the presence of clot.
After fully evaluating the saphenous vein, the clinician should move the probe toward the feet, keeping the vessels centered in the image, while at the same time applying serial compression approximately every centimeter. As the probe is moved distally, the artery will typically bifurcate first and then the vein will bifurcate into the femoral and deep femoral branches. The femoral vein should be followed until it “dives” into the adductor canal.
Popliteal Region
The second zone of the examination is in the popliteal region. It can be helpful to prop the foot up, or hang the foot off the bed to improve access to this region (Figure 4). Similar to the femoral region, maximizing your depth and then decreasing it once the vessels are identified can help to ensure that you are not mistaking superficial vessels
Common Pitfalls
The main pitfall to performing a DVT study is failing to identify the correct vessels. It is important to identify both the artery and vein running together to ensure that the correct location. In larger patients, the relatively shallow depth of the high-frequency
Another common pitfall to keep in mind is that lymph nodes can often be mistaken for clot (Figure 6). A lymph node may be distinguished from a clot by sliding the probe up and down the patient’s leg—lymph nodes will appear as discrete structures, continuous like vasculature. It is essential to evaluate the length of the common femoral, the femoral, and popliteal veins visualized as a recent study by Adhikari et al1 showed that two-point compression studies can miss up to 6% of isolated clots.
Summary
With practice, POC compression ultrasonography of the lower extremities can be used to quickly rule in the diagnosis DVT. Proper patient and probe positioning, as well as the application of appropriate probe pressure at different stages of the examination are essential to accurately visualize and assess the femoral, saphenous, and popliteal veins for the presence of a thrombus.
For a video and more information on performing this scan:
Dr Meer is an assistant professor and director of emergency ultrasound, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Beck is an assistant professor, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Taylor is an assistant professor and director of postgraduate medical education, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia.
- Emergency Ultrasound: Deep Vein Thrombosis
- Adhikari S, Zeger W, Thom C, Fields JM. Isolated deep venous thrombosis: implications for 2-point compression ultrasonography of the lower extremity. Ann Emerg Med. 2015;66(3):262-266.
Femoral and Saphenous Vein
After the appropriate positioning has been achieved, the clinician should identify the common femoral vein and artery, and then slide the probe toward the head or feet until the saphenous vein, which empties into the common femoral vein, is identified
The clinician should take care not to apply too much pressure with the probe as this will compress the saphenous vein; therefore, it is important to use a light touch to ensure proper visualization. Once this key region is identified, the clinician should then use the probe to apply pressure to the vessels. The saphenous vein should compress completely upon application of sufficient pressure to the artery. In a normal vein, the anterior and posterior walls should touch and completely obliterate the lumen of the vein. When a clot is present, the walls of the vein will remain separated (Figure 3). A thrombus can be bright on ultrasound, but some DVTs may appear completely black. In some cases, a lack of compressibility may be the only tip-off to the presence of clot.
After fully evaluating the saphenous vein, the clinician should move the probe toward the feet, keeping the vessels centered in the image, while at the same time applying serial compression approximately every centimeter. As the probe is moved distally, the artery will typically bifurcate first and then the vein will bifurcate into the femoral and deep femoral branches. The femoral vein should be followed until it “dives” into the adductor canal.
Popliteal Region
The second zone of the examination is in the popliteal region. It can be helpful to prop the foot up, or hang the foot off the bed to improve access to this region (Figure 4). Similar to the femoral region, maximizing your depth and then decreasing it once the vessels are identified can help to ensure that you are not mistaking superficial vessels
Common Pitfalls
The main pitfall to performing a DVT study is failing to identify the correct vessels. It is important to identify both the artery and vein running together to ensure that the correct location. In larger patients, the relatively shallow depth of the high-frequency
Another common pitfall to keep in mind is that lymph nodes can often be mistaken for clot (Figure 6). A lymph node may be distinguished from a clot by sliding the probe up and down the patient’s leg—lymph nodes will appear as discrete structures, continuous like vasculature. It is essential to evaluate the length of the common femoral, the femoral, and popliteal veins visualized as a recent study by Adhikari et al1 showed that two-point compression studies can miss up to 6% of isolated clots.
Summary
With practice, POC compression ultrasonography of the lower extremities can be used to quickly rule in the diagnosis DVT. Proper patient and probe positioning, as well as the application of appropriate probe pressure at different stages of the examination are essential to accurately visualize and assess the femoral, saphenous, and popliteal veins for the presence of a thrombus.
For a video and more information on performing this scan:
Dr Meer is an assistant professor and director of emergency ultrasound, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Beck is an assistant professor, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Taylor is an assistant professor and director of postgraduate medical education, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia.
Femoral and Saphenous Vein
After the appropriate positioning has been achieved, the clinician should identify the common femoral vein and artery, and then slide the probe toward the head or feet until the saphenous vein, which empties into the common femoral vein, is identified
The clinician should take care not to apply too much pressure with the probe as this will compress the saphenous vein; therefore, it is important to use a light touch to ensure proper visualization. Once this key region is identified, the clinician should then use the probe to apply pressure to the vessels. The saphenous vein should compress completely upon application of sufficient pressure to the artery. In a normal vein, the anterior and posterior walls should touch and completely obliterate the lumen of the vein. When a clot is present, the walls of the vein will remain separated (Figure 3). A thrombus can be bright on ultrasound, but some DVTs may appear completely black. In some cases, a lack of compressibility may be the only tip-off to the presence of clot.
After fully evaluating the saphenous vein, the clinician should move the probe toward the feet, keeping the vessels centered in the image, while at the same time applying serial compression approximately every centimeter. As the probe is moved distally, the artery will typically bifurcate first and then the vein will bifurcate into the femoral and deep femoral branches. The femoral vein should be followed until it “dives” into the adductor canal.
Popliteal Region
The second zone of the examination is in the popliteal region. It can be helpful to prop the foot up, or hang the foot off the bed to improve access to this region (Figure 4). Similar to the femoral region, maximizing your depth and then decreasing it once the vessels are identified can help to ensure that you are not mistaking superficial vessels
Common Pitfalls
The main pitfall to performing a DVT study is failing to identify the correct vessels. It is important to identify both the artery and vein running together to ensure that the correct location. In larger patients, the relatively shallow depth of the high-frequency
Another common pitfall to keep in mind is that lymph nodes can often be mistaken for clot (Figure 6). A lymph node may be distinguished from a clot by sliding the probe up and down the patient’s leg—lymph nodes will appear as discrete structures, continuous like vasculature. It is essential to evaluate the length of the common femoral, the femoral, and popliteal veins visualized as a recent study by Adhikari et al1 showed that two-point compression studies can miss up to 6% of isolated clots.
Summary
With practice, POC compression ultrasonography of the lower extremities can be used to quickly rule in the diagnosis DVT. Proper patient and probe positioning, as well as the application of appropriate probe pressure at different stages of the examination are essential to accurately visualize and assess the femoral, saphenous, and popliteal veins for the presence of a thrombus.
For a video and more information on performing this scan:
Dr Meer is an assistant professor and director of emergency ultrasound, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Beck is an assistant professor, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Taylor is an assistant professor and director of postgraduate medical education, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia.
- Emergency Ultrasound: Deep Vein Thrombosis
- Adhikari S, Zeger W, Thom C, Fields JM. Isolated deep venous thrombosis: implications for 2-point compression ultrasonography of the lower extremity. Ann Emerg Med. 2015;66(3):262-266.
- Emergency Ultrasound: Deep Vein Thrombosis
- Adhikari S, Zeger W, Thom C, Fields JM. Isolated deep venous thrombosis: implications for 2-point compression ultrasonography of the lower extremity. Ann Emerg Med. 2015;66(3):262-266.