User login
Treating Tibia Fractures With Far Cortical Locking Implants
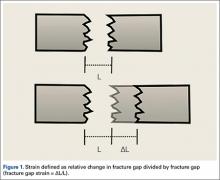
Fracture healing can be categorized as primary or secondary. Primary healing requires precise reapproximation of bone fragments and compression of cortices. Osteons are formed across the fracture line, allowing blood supply and endothelial cells to gain access, leading to osteoblast infiltration and subsequent bone formation.1 This type of bone healing can be accomplished only with absolute stability—specifically, only with less than 2% strain at the fracture site, necessitating operative intervention with compression plating (Figure 1).2 This type of construct generates friction between the bone fragments against a metal plate, created by tightening screws that purchase both far and near cortices of bone.3 Although this type of fixation works well with many fractures, there are several instances in which compression plating is not ideal.4 Osteoporotic bone, for example, limits the amount of compression that can be developed, as screws strip the bone more readily, leading to weakened constructs prone to failure. Metaphyseal fractures in which there is minimal cortex for screw thread purchase are a similar challenge.5 Highly comminuted fractures do not allow for sufficient fragment compression and stability. In addition, compression plating requires periosteal stripping at the fracture, and often substantial soft-tissue disruption, which is especially a problem in areas of tenuous blood supply (eg, the tibia).
Locked plating therefore has become a valuable technique in managing osteoporotic fractures.2 Locking plates may be used to achieve secondary bone healing through a small amount of interfragmentary motion, 0.2 to 10 mm, as seen with bridge plating for example, whereby the locking plates act as internal fixators. Much as with external fixators, as the distance from the fixator bar (or plate) to bone decreases, construct stiffness increases. Thus, locking plates function as extremely stiff fixators when the plate is very near bone. It has therefore been speculated that such stiffness is insufficient to provide optimal secondary healing conditions.6,7 Titanium (vs stainless steel) plates have been used, and screws have been omitted just adjacent to either side of the fracture site, in attempts to increase plate flexibility and thus interfragmentary motion.8,9 In addition, biomechanical and animal model studies have demonstrated that, with use of locking plates, motion at the fracture site is asymmetric and leads to unequal callus formation at the near and far cortices, thus weakening the fracture site.10,11
The locking plate design was recently modified to address these concerns. Far cortical locking (FCL) uses locking screws threaded only distally (Figure 2), which allows for purchase into the far cortex but not the near cortex, which increases pin length from plate to bone. The near cortex is no longer anchored to the plate and thus increases construct flexibility. Pilot holes in the near cortex allow for movement of the nonthreaded screw shaft in a controlled, biphasic manner.12 This design decreases stiffness while sacrificing very little construct strength.10 In addition, motion at the far and near cortices is nearly parallel. It has been shown in an ovine tibial osteotomy model that, compared with the traditional locking plate design, FCL generates symmetric callus formation and improved fracture healing.11 Although these results are promising, there are only limited clinical data on use of the FCL technique in fracture repair. Our null hypothesis was that, despite the theoretical advantages of FCL constructs over conventional locking plates, there would be no clinically observed differences between the constructs.
Patients and Methods
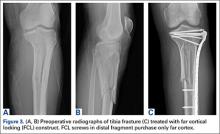
After obtaining Institutional Review Board approval from the 2 level I trauma centers and 1 level II trauma center involved in this study, we retrospectively reviewed the cases of all adults who presented with a tibia fracture and were treated with FCL technology (MotionLoc, Zimmer) by a fellowship-trained trauma surgeon at these hospitals (Figures 3A–3C). Any primary tibia fracture treated with FCL was considered. Only patients with follow-up of at least 20 weeks were included in the analysis. Exclusion criteria were tibial malunions or nonunions treated with FCL and fractures treated with a combination of intramedullary fixation and plating.
We reviewed the patient charts for demographic data, mechanism of injury, fracture type, and comorbidities. Risk factors for poor healing—such as diabetes and tobacco use, either current or prior—were recorded. We also reviewed the radiographs of the initial injuries for analysis of the tibia fracture types (Table 1) as well as the follow-up radiographs for evaluation of fracture healing. Using the Orthopaedic Trauma Association classification system, we identified a variety of fracture patterns. Fracture healing rates were recorded and used to calculate the overall healing rates for each group. Union was defined as either radiographic evidence of a completely healed fracture (≥3 cortices) or radiographic evidence of osseous bridging at the fracture site in addition to full weight-bearing without pain. Infection was defined as positive intraoperative cultures or grossly infected wounds with purulence and erythema.
For statistical analysis, we used Welch 2-sample t test to compare categorical data, including rates of fracture union, infection, and revision surgery. We chose this test because it was unclear whether variance in the groups would be similar. FCL and control data were compared for significant differences by calculating P values. Similarly, for continuous data, Fisher exact test was used to calculate P values for mean time to union and mean time to full weight-bearing in order to compare FCL and control outcomes.
Results
Twelve patients treated at 2 level I and 1 level II trauma centers between November 2010 and May 2012 met the inclusion and exclusion criteria for this study. Another 10 patients were treated with standard plating techniques (control group). Mean age was 52 years (range, 25-72 years) for the FCL group and 46 years (range, 28-67 years) for the control group. The FCL group included 2 open fractures (control, 0) and 2 patients with diabetes (control, 1) (Table 1).
Eleven of the 12 FCL patients and all 10 control patients achieved fracture union by most recent follow-up (Table 2). The difference was not statistically significant (P = .363). The FCL-treated fracture that did not heal received an interfragmentary screw in addition to the standard FCL technology construct. The interfragmentary screw inhibited motion at the fracture site and could potentially have led to nonunion. For this patient, revision surgery to an intramedullary nail was required. Removal of the interfragmentary screw was uneventful. Each of the 2 open fractures in the FCL group required bone grafting because of large segmental bone loss. One of these fractures, a type 3B, became infected after bone grafting, and complete healing required plate removal. The patient was eventually treated with a brace. An infection that occurred after union in a closed tibia fracture in the FCL group required hardware removal. No patient in either group experienced loss or failure of fixation.
Discussion
Far cortical locking is a relatively new technology designed to increase fracture fixation flexibility by functionally lengthening the distance between the locking plate and the screw cortical purchase, which occurs at the far cortex rather than the near cortex. This construct thereby functions as an internal fixator and is functionally similar to an external fixator. Rather than there being bars external to the skin, a plate is placed internally, adjacent to but without compressing fracture fragments or the plate to the bone. This theoretically leads to a desirable amount of interfragmentary motion, promoting callus formation and secondary healing. However, too much motion at the fracture site disrupts healing by shearing proliferating cells attempting to bridge the fracture gap. Therefore, there is a narrow target zone of desirable motion between fracture fragments required to promote secondary bone healing—defined as 2% to 10% gap strain.2 FCL constructs are thought to fall in this range of gap strain and thus better promote secondary healing over standard locked plates. Although biomechanical studies have been used as proof of concept, there are no published clinical data on the effectiveness of FCL implants. The present article describes early data on clinical outcomes of this new type of implant.
The main limitation of this study is its small cohort size, which is largely a result of the short time these implants have been available and our attempt to compare only similar fractures in this analysis. In addition, follow-up was on average less than 1 year. We consider such follow-up acceptable, though, as all fractures essentially reached final healing status within that period. Another limitation is that we combined compression plating and locked plating in the control group. Considering the mechanism of the theoretical advantage of FCL implants, with larger cohorts it would be useful to perform a subanalysis in which compression and standard locking plates are separately compared with FCL implants.
This study found no statistically significant difference between FCL and standard plating, suggesting FCL likely is not inferior to standard plating. Although the FCL group included a nonunion, it is important to note that, in this case, there was a technical discrepancy in the ideal technique whereby another interfragmentary screw was placed, eliminating the interfragmentary motion that establishes the premise of FCL technology. This case thereby demonstrated that a breach in the FCL technique, as with standard locking techniques, may lead to fracture-healing complications. In the FCL group, 2 open fractures with significant segmental bone loss requiring bone graft subsequently healed. In addition, compared with the control group, the FCL group included more patients with diabetes and more tobacco users (both diabetes and tobacco use are associated with poor bone and wound healing). The FCL group was also, on average, 6 years older than the control group. None of these group differences, however, reached statistical significance. Indeed, part of the impetus to use FCL implants in this population was that these patients likely were at higher risk for poor healing and nonunion. This factor therefore represents a selection bias—the FCL group was more predisposed to nonunion—and a study limitation.
Together, our data show neither superiority nor inferiority of the FCL technique. This study is an important step in furthering investigations into FCL constructs. The finding of similar efficacy with FCL and conventional plating may assuage safety concerns and pave the way for more definitive studies of FCL technology and fuller evaluations of its effectiveness. These studies will be essential in determining whether the theoretical advantage of FCL translates into better clinical outcomes. Larger, prospective randomized studies with longer follow-ups will be needed to better compare FCL technology with current implants and techniques. At this early stage, however, FCL technology appears to be a viable option for complex fractures of the tibia.
1. Bernstein J, ed. Musculoskeletal Medicine. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2003.
2. Egol KA, Kubiak EN, Fulkerson E, Kummer FJ, Koval KJ. Biomechanics of locked plates and screws. J Orthop Trauma. 2004;18(8):488-493.
3. Bagby GW. Compression bone-plating: historical considerations. J Bone Joint Surg Am. 1977;59(5):625-631.
4. Kubiak EN, Fulkerson E, Strauss E, Egol KA. The evolution of locked plates. J Bone Joint Surg Am. 2006;88(suppl 4):189-200.
5. Fitzpatrick DC, Doornink J, Madey SM, Bottlang M. Relative stability of conventional and locked plating fixation in a model of the osteoporotic femoral diaphysis. Clin Biomech. 2009;24(2):203-209.
6. Henderson CE, Bottlang M, Marsh JL, Fitzpatrick DC, Madey SM. Does locked plating of periprosthetic supracondylar femur fractures promote bone healing by callus formation? Two cases with opposite outcomes. Iowa Orthop J. 2008;28:73-76.
7. Lujan TJ, Henderson CE, Madey SM, Fitzpatrick DC, Marsh JL, Bottlang M. Locked plating of distal femur fractures leads to inconsistent and asymmetric callus formation. J Orthop Trauma. 2010;24(3):156-162.
8. Stoffel K, Dieter U, Stachowiak G, Gächter A, Kuster MS. Biomechanical testing of the LCP—how can stability in locked internal fixators be controlled? Injury. 2003;34(suppl 2):B11-B19.
9. Schmal H, Strohm PC, Jaeger M, Südkamp NP. Flexible fixation and fracture healing: do locked plating ‘internal fixators’ resemble external fixators? J Orthop Trauma. 2011;25(suppl 1):S15-S20.
10. Bottlang M, Doornink J, Fitzpatrick DC, Madey SM. Far cortical locking can reduce stiffness of locked plating constructs while retaining construct strength. J Bone Joint Surg Am. 2009;91(8):1985-1994.
11. Bottlang M, Lesser M, Koerber J, et al. Far cortical locking can improve healing of fractures stabilized with locking plates. J Bone Joint Surg Am. 2010;92(7):1652-1660.
12. Bottlang M, Feist F. Biomechanics of far cortical locking. J Orthop Trauma. 2011;25(suppl 1):S21-S28.
Fracture healing can be categorized as primary or secondary. Primary healing requires precise reapproximation of bone fragments and compression of cortices. Osteons are formed across the fracture line, allowing blood supply and endothelial cells to gain access, leading to osteoblast infiltration and subsequent bone formation.1 This type of bone healing can be accomplished only with absolute stability—specifically, only with less than 2% strain at the fracture site, necessitating operative intervention with compression plating (Figure 1).2 This type of construct generates friction between the bone fragments against a metal plate, created by tightening screws that purchase both far and near cortices of bone.3 Although this type of fixation works well with many fractures, there are several instances in which compression plating is not ideal.4 Osteoporotic bone, for example, limits the amount of compression that can be developed, as screws strip the bone more readily, leading to weakened constructs prone to failure. Metaphyseal fractures in which there is minimal cortex for screw thread purchase are a similar challenge.5 Highly comminuted fractures do not allow for sufficient fragment compression and stability. In addition, compression plating requires periosteal stripping at the fracture, and often substantial soft-tissue disruption, which is especially a problem in areas of tenuous blood supply (eg, the tibia).
Locked plating therefore has become a valuable technique in managing osteoporotic fractures.2 Locking plates may be used to achieve secondary bone healing through a small amount of interfragmentary motion, 0.2 to 10 mm, as seen with bridge plating for example, whereby the locking plates act as internal fixators. Much as with external fixators, as the distance from the fixator bar (or plate) to bone decreases, construct stiffness increases. Thus, locking plates function as extremely stiff fixators when the plate is very near bone. It has therefore been speculated that such stiffness is insufficient to provide optimal secondary healing conditions.6,7 Titanium (vs stainless steel) plates have been used, and screws have been omitted just adjacent to either side of the fracture site, in attempts to increase plate flexibility and thus interfragmentary motion.8,9 In addition, biomechanical and animal model studies have demonstrated that, with use of locking plates, motion at the fracture site is asymmetric and leads to unequal callus formation at the near and far cortices, thus weakening the fracture site.10,11
The locking plate design was recently modified to address these concerns. Far cortical locking (FCL) uses locking screws threaded only distally (Figure 2), which allows for purchase into the far cortex but not the near cortex, which increases pin length from plate to bone. The near cortex is no longer anchored to the plate and thus increases construct flexibility. Pilot holes in the near cortex allow for movement of the nonthreaded screw shaft in a controlled, biphasic manner.12 This design decreases stiffness while sacrificing very little construct strength.10 In addition, motion at the far and near cortices is nearly parallel. It has been shown in an ovine tibial osteotomy model that, compared with the traditional locking plate design, FCL generates symmetric callus formation and improved fracture healing.11 Although these results are promising, there are only limited clinical data on use of the FCL technique in fracture repair. Our null hypothesis was that, despite the theoretical advantages of FCL constructs over conventional locking plates, there would be no clinically observed differences between the constructs.
Patients and Methods
After obtaining Institutional Review Board approval from the 2 level I trauma centers and 1 level II trauma center involved in this study, we retrospectively reviewed the cases of all adults who presented with a tibia fracture and were treated with FCL technology (MotionLoc, Zimmer) by a fellowship-trained trauma surgeon at these hospitals (Figures 3A–3C). Any primary tibia fracture treated with FCL was considered. Only patients with follow-up of at least 20 weeks were included in the analysis. Exclusion criteria were tibial malunions or nonunions treated with FCL and fractures treated with a combination of intramedullary fixation and plating.
We reviewed the patient charts for demographic data, mechanism of injury, fracture type, and comorbidities. Risk factors for poor healing—such as diabetes and tobacco use, either current or prior—were recorded. We also reviewed the radiographs of the initial injuries for analysis of the tibia fracture types (Table 1) as well as the follow-up radiographs for evaluation of fracture healing. Using the Orthopaedic Trauma Association classification system, we identified a variety of fracture patterns. Fracture healing rates were recorded and used to calculate the overall healing rates for each group. Union was defined as either radiographic evidence of a completely healed fracture (≥3 cortices) or radiographic evidence of osseous bridging at the fracture site in addition to full weight-bearing without pain. Infection was defined as positive intraoperative cultures or grossly infected wounds with purulence and erythema.
For statistical analysis, we used Welch 2-sample t test to compare categorical data, including rates of fracture union, infection, and revision surgery. We chose this test because it was unclear whether variance in the groups would be similar. FCL and control data were compared for significant differences by calculating P values. Similarly, for continuous data, Fisher exact test was used to calculate P values for mean time to union and mean time to full weight-bearing in order to compare FCL and control outcomes.
Results
Twelve patients treated at 2 level I and 1 level II trauma centers between November 2010 and May 2012 met the inclusion and exclusion criteria for this study. Another 10 patients were treated with standard plating techniques (control group). Mean age was 52 years (range, 25-72 years) for the FCL group and 46 years (range, 28-67 years) for the control group. The FCL group included 2 open fractures (control, 0) and 2 patients with diabetes (control, 1) (Table 1).
Eleven of the 12 FCL patients and all 10 control patients achieved fracture union by most recent follow-up (Table 2). The difference was not statistically significant (P = .363). The FCL-treated fracture that did not heal received an interfragmentary screw in addition to the standard FCL technology construct. The interfragmentary screw inhibited motion at the fracture site and could potentially have led to nonunion. For this patient, revision surgery to an intramedullary nail was required. Removal of the interfragmentary screw was uneventful. Each of the 2 open fractures in the FCL group required bone grafting because of large segmental bone loss. One of these fractures, a type 3B, became infected after bone grafting, and complete healing required plate removal. The patient was eventually treated with a brace. An infection that occurred after union in a closed tibia fracture in the FCL group required hardware removal. No patient in either group experienced loss or failure of fixation.
Discussion
Far cortical locking is a relatively new technology designed to increase fracture fixation flexibility by functionally lengthening the distance between the locking plate and the screw cortical purchase, which occurs at the far cortex rather than the near cortex. This construct thereby functions as an internal fixator and is functionally similar to an external fixator. Rather than there being bars external to the skin, a plate is placed internally, adjacent to but without compressing fracture fragments or the plate to the bone. This theoretically leads to a desirable amount of interfragmentary motion, promoting callus formation and secondary healing. However, too much motion at the fracture site disrupts healing by shearing proliferating cells attempting to bridge the fracture gap. Therefore, there is a narrow target zone of desirable motion between fracture fragments required to promote secondary bone healing—defined as 2% to 10% gap strain.2 FCL constructs are thought to fall in this range of gap strain and thus better promote secondary healing over standard locked plates. Although biomechanical studies have been used as proof of concept, there are no published clinical data on the effectiveness of FCL implants. The present article describes early data on clinical outcomes of this new type of implant.
The main limitation of this study is its small cohort size, which is largely a result of the short time these implants have been available and our attempt to compare only similar fractures in this analysis. In addition, follow-up was on average less than 1 year. We consider such follow-up acceptable, though, as all fractures essentially reached final healing status within that period. Another limitation is that we combined compression plating and locked plating in the control group. Considering the mechanism of the theoretical advantage of FCL implants, with larger cohorts it would be useful to perform a subanalysis in which compression and standard locking plates are separately compared with FCL implants.
This study found no statistically significant difference between FCL and standard plating, suggesting FCL likely is not inferior to standard plating. Although the FCL group included a nonunion, it is important to note that, in this case, there was a technical discrepancy in the ideal technique whereby another interfragmentary screw was placed, eliminating the interfragmentary motion that establishes the premise of FCL technology. This case thereby demonstrated that a breach in the FCL technique, as with standard locking techniques, may lead to fracture-healing complications. In the FCL group, 2 open fractures with significant segmental bone loss requiring bone graft subsequently healed. In addition, compared with the control group, the FCL group included more patients with diabetes and more tobacco users (both diabetes and tobacco use are associated with poor bone and wound healing). The FCL group was also, on average, 6 years older than the control group. None of these group differences, however, reached statistical significance. Indeed, part of the impetus to use FCL implants in this population was that these patients likely were at higher risk for poor healing and nonunion. This factor therefore represents a selection bias—the FCL group was more predisposed to nonunion—and a study limitation.
Together, our data show neither superiority nor inferiority of the FCL technique. This study is an important step in furthering investigations into FCL constructs. The finding of similar efficacy with FCL and conventional plating may assuage safety concerns and pave the way for more definitive studies of FCL technology and fuller evaluations of its effectiveness. These studies will be essential in determining whether the theoretical advantage of FCL translates into better clinical outcomes. Larger, prospective randomized studies with longer follow-ups will be needed to better compare FCL technology with current implants and techniques. At this early stage, however, FCL technology appears to be a viable option for complex fractures of the tibia.
Fracture healing can be categorized as primary or secondary. Primary healing requires precise reapproximation of bone fragments and compression of cortices. Osteons are formed across the fracture line, allowing blood supply and endothelial cells to gain access, leading to osteoblast infiltration and subsequent bone formation.1 This type of bone healing can be accomplished only with absolute stability—specifically, only with less than 2% strain at the fracture site, necessitating operative intervention with compression plating (Figure 1).2 This type of construct generates friction between the bone fragments against a metal plate, created by tightening screws that purchase both far and near cortices of bone.3 Although this type of fixation works well with many fractures, there are several instances in which compression plating is not ideal.4 Osteoporotic bone, for example, limits the amount of compression that can be developed, as screws strip the bone more readily, leading to weakened constructs prone to failure. Metaphyseal fractures in which there is minimal cortex for screw thread purchase are a similar challenge.5 Highly comminuted fractures do not allow for sufficient fragment compression and stability. In addition, compression plating requires periosteal stripping at the fracture, and often substantial soft-tissue disruption, which is especially a problem in areas of tenuous blood supply (eg, the tibia).
Locked plating therefore has become a valuable technique in managing osteoporotic fractures.2 Locking plates may be used to achieve secondary bone healing through a small amount of interfragmentary motion, 0.2 to 10 mm, as seen with bridge plating for example, whereby the locking plates act as internal fixators. Much as with external fixators, as the distance from the fixator bar (or plate) to bone decreases, construct stiffness increases. Thus, locking plates function as extremely stiff fixators when the plate is very near bone. It has therefore been speculated that such stiffness is insufficient to provide optimal secondary healing conditions.6,7 Titanium (vs stainless steel) plates have been used, and screws have been omitted just adjacent to either side of the fracture site, in attempts to increase plate flexibility and thus interfragmentary motion.8,9 In addition, biomechanical and animal model studies have demonstrated that, with use of locking plates, motion at the fracture site is asymmetric and leads to unequal callus formation at the near and far cortices, thus weakening the fracture site.10,11
The locking plate design was recently modified to address these concerns. Far cortical locking (FCL) uses locking screws threaded only distally (Figure 2), which allows for purchase into the far cortex but not the near cortex, which increases pin length from plate to bone. The near cortex is no longer anchored to the plate and thus increases construct flexibility. Pilot holes in the near cortex allow for movement of the nonthreaded screw shaft in a controlled, biphasic manner.12 This design decreases stiffness while sacrificing very little construct strength.10 In addition, motion at the far and near cortices is nearly parallel. It has been shown in an ovine tibial osteotomy model that, compared with the traditional locking plate design, FCL generates symmetric callus formation and improved fracture healing.11 Although these results are promising, there are only limited clinical data on use of the FCL technique in fracture repair. Our null hypothesis was that, despite the theoretical advantages of FCL constructs over conventional locking plates, there would be no clinically observed differences between the constructs.
Patients and Methods
After obtaining Institutional Review Board approval from the 2 level I trauma centers and 1 level II trauma center involved in this study, we retrospectively reviewed the cases of all adults who presented with a tibia fracture and were treated with FCL technology (MotionLoc, Zimmer) by a fellowship-trained trauma surgeon at these hospitals (Figures 3A–3C). Any primary tibia fracture treated with FCL was considered. Only patients with follow-up of at least 20 weeks were included in the analysis. Exclusion criteria were tibial malunions or nonunions treated with FCL and fractures treated with a combination of intramedullary fixation and plating.
We reviewed the patient charts for demographic data, mechanism of injury, fracture type, and comorbidities. Risk factors for poor healing—such as diabetes and tobacco use, either current or prior—were recorded. We also reviewed the radiographs of the initial injuries for analysis of the tibia fracture types (Table 1) as well as the follow-up radiographs for evaluation of fracture healing. Using the Orthopaedic Trauma Association classification system, we identified a variety of fracture patterns. Fracture healing rates were recorded and used to calculate the overall healing rates for each group. Union was defined as either radiographic evidence of a completely healed fracture (≥3 cortices) or radiographic evidence of osseous bridging at the fracture site in addition to full weight-bearing without pain. Infection was defined as positive intraoperative cultures or grossly infected wounds with purulence and erythema.
For statistical analysis, we used Welch 2-sample t test to compare categorical data, including rates of fracture union, infection, and revision surgery. We chose this test because it was unclear whether variance in the groups would be similar. FCL and control data were compared for significant differences by calculating P values. Similarly, for continuous data, Fisher exact test was used to calculate P values for mean time to union and mean time to full weight-bearing in order to compare FCL and control outcomes.
Results
Twelve patients treated at 2 level I and 1 level II trauma centers between November 2010 and May 2012 met the inclusion and exclusion criteria for this study. Another 10 patients were treated with standard plating techniques (control group). Mean age was 52 years (range, 25-72 years) for the FCL group and 46 years (range, 28-67 years) for the control group. The FCL group included 2 open fractures (control, 0) and 2 patients with diabetes (control, 1) (Table 1).
Eleven of the 12 FCL patients and all 10 control patients achieved fracture union by most recent follow-up (Table 2). The difference was not statistically significant (P = .363). The FCL-treated fracture that did not heal received an interfragmentary screw in addition to the standard FCL technology construct. The interfragmentary screw inhibited motion at the fracture site and could potentially have led to nonunion. For this patient, revision surgery to an intramedullary nail was required. Removal of the interfragmentary screw was uneventful. Each of the 2 open fractures in the FCL group required bone grafting because of large segmental bone loss. One of these fractures, a type 3B, became infected after bone grafting, and complete healing required plate removal. The patient was eventually treated with a brace. An infection that occurred after union in a closed tibia fracture in the FCL group required hardware removal. No patient in either group experienced loss or failure of fixation.
Discussion
Far cortical locking is a relatively new technology designed to increase fracture fixation flexibility by functionally lengthening the distance between the locking plate and the screw cortical purchase, which occurs at the far cortex rather than the near cortex. This construct thereby functions as an internal fixator and is functionally similar to an external fixator. Rather than there being bars external to the skin, a plate is placed internally, adjacent to but without compressing fracture fragments or the plate to the bone. This theoretically leads to a desirable amount of interfragmentary motion, promoting callus formation and secondary healing. However, too much motion at the fracture site disrupts healing by shearing proliferating cells attempting to bridge the fracture gap. Therefore, there is a narrow target zone of desirable motion between fracture fragments required to promote secondary bone healing—defined as 2% to 10% gap strain.2 FCL constructs are thought to fall in this range of gap strain and thus better promote secondary healing over standard locked plates. Although biomechanical studies have been used as proof of concept, there are no published clinical data on the effectiveness of FCL implants. The present article describes early data on clinical outcomes of this new type of implant.
The main limitation of this study is its small cohort size, which is largely a result of the short time these implants have been available and our attempt to compare only similar fractures in this analysis. In addition, follow-up was on average less than 1 year. We consider such follow-up acceptable, though, as all fractures essentially reached final healing status within that period. Another limitation is that we combined compression plating and locked plating in the control group. Considering the mechanism of the theoretical advantage of FCL implants, with larger cohorts it would be useful to perform a subanalysis in which compression and standard locking plates are separately compared with FCL implants.
This study found no statistically significant difference between FCL and standard plating, suggesting FCL likely is not inferior to standard plating. Although the FCL group included a nonunion, it is important to note that, in this case, there was a technical discrepancy in the ideal technique whereby another interfragmentary screw was placed, eliminating the interfragmentary motion that establishes the premise of FCL technology. This case thereby demonstrated that a breach in the FCL technique, as with standard locking techniques, may lead to fracture-healing complications. In the FCL group, 2 open fractures with significant segmental bone loss requiring bone graft subsequently healed. In addition, compared with the control group, the FCL group included more patients with diabetes and more tobacco users (both diabetes and tobacco use are associated with poor bone and wound healing). The FCL group was also, on average, 6 years older than the control group. None of these group differences, however, reached statistical significance. Indeed, part of the impetus to use FCL implants in this population was that these patients likely were at higher risk for poor healing and nonunion. This factor therefore represents a selection bias—the FCL group was more predisposed to nonunion—and a study limitation.
Together, our data show neither superiority nor inferiority of the FCL technique. This study is an important step in furthering investigations into FCL constructs. The finding of similar efficacy with FCL and conventional plating may assuage safety concerns and pave the way for more definitive studies of FCL technology and fuller evaluations of its effectiveness. These studies will be essential in determining whether the theoretical advantage of FCL translates into better clinical outcomes. Larger, prospective randomized studies with longer follow-ups will be needed to better compare FCL technology with current implants and techniques. At this early stage, however, FCL technology appears to be a viable option for complex fractures of the tibia.
1. Bernstein J, ed. Musculoskeletal Medicine. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2003.
2. Egol KA, Kubiak EN, Fulkerson E, Kummer FJ, Koval KJ. Biomechanics of locked plates and screws. J Orthop Trauma. 2004;18(8):488-493.
3. Bagby GW. Compression bone-plating: historical considerations. J Bone Joint Surg Am. 1977;59(5):625-631.
4. Kubiak EN, Fulkerson E, Strauss E, Egol KA. The evolution of locked plates. J Bone Joint Surg Am. 2006;88(suppl 4):189-200.
5. Fitzpatrick DC, Doornink J, Madey SM, Bottlang M. Relative stability of conventional and locked plating fixation in a model of the osteoporotic femoral diaphysis. Clin Biomech. 2009;24(2):203-209.
6. Henderson CE, Bottlang M, Marsh JL, Fitzpatrick DC, Madey SM. Does locked plating of periprosthetic supracondylar femur fractures promote bone healing by callus formation? Two cases with opposite outcomes. Iowa Orthop J. 2008;28:73-76.
7. Lujan TJ, Henderson CE, Madey SM, Fitzpatrick DC, Marsh JL, Bottlang M. Locked plating of distal femur fractures leads to inconsistent and asymmetric callus formation. J Orthop Trauma. 2010;24(3):156-162.
8. Stoffel K, Dieter U, Stachowiak G, Gächter A, Kuster MS. Biomechanical testing of the LCP—how can stability in locked internal fixators be controlled? Injury. 2003;34(suppl 2):B11-B19.
9. Schmal H, Strohm PC, Jaeger M, Südkamp NP. Flexible fixation and fracture healing: do locked plating ‘internal fixators’ resemble external fixators? J Orthop Trauma. 2011;25(suppl 1):S15-S20.
10. Bottlang M, Doornink J, Fitzpatrick DC, Madey SM. Far cortical locking can reduce stiffness of locked plating constructs while retaining construct strength. J Bone Joint Surg Am. 2009;91(8):1985-1994.
11. Bottlang M, Lesser M, Koerber J, et al. Far cortical locking can improve healing of fractures stabilized with locking plates. J Bone Joint Surg Am. 2010;92(7):1652-1660.
12. Bottlang M, Feist F. Biomechanics of far cortical locking. J Orthop Trauma. 2011;25(suppl 1):S21-S28.
1. Bernstein J, ed. Musculoskeletal Medicine. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2003.
2. Egol KA, Kubiak EN, Fulkerson E, Kummer FJ, Koval KJ. Biomechanics of locked plates and screws. J Orthop Trauma. 2004;18(8):488-493.
3. Bagby GW. Compression bone-plating: historical considerations. J Bone Joint Surg Am. 1977;59(5):625-631.
4. Kubiak EN, Fulkerson E, Strauss E, Egol KA. The evolution of locked plates. J Bone Joint Surg Am. 2006;88(suppl 4):189-200.
5. Fitzpatrick DC, Doornink J, Madey SM, Bottlang M. Relative stability of conventional and locked plating fixation in a model of the osteoporotic femoral diaphysis. Clin Biomech. 2009;24(2):203-209.
6. Henderson CE, Bottlang M, Marsh JL, Fitzpatrick DC, Madey SM. Does locked plating of periprosthetic supracondylar femur fractures promote bone healing by callus formation? Two cases with opposite outcomes. Iowa Orthop J. 2008;28:73-76.
7. Lujan TJ, Henderson CE, Madey SM, Fitzpatrick DC, Marsh JL, Bottlang M. Locked plating of distal femur fractures leads to inconsistent and asymmetric callus formation. J Orthop Trauma. 2010;24(3):156-162.
8. Stoffel K, Dieter U, Stachowiak G, Gächter A, Kuster MS. Biomechanical testing of the LCP—how can stability in locked internal fixators be controlled? Injury. 2003;34(suppl 2):B11-B19.
9. Schmal H, Strohm PC, Jaeger M, Südkamp NP. Flexible fixation and fracture healing: do locked plating ‘internal fixators’ resemble external fixators? J Orthop Trauma. 2011;25(suppl 1):S15-S20.
10. Bottlang M, Doornink J, Fitzpatrick DC, Madey SM. Far cortical locking can reduce stiffness of locked plating constructs while retaining construct strength. J Bone Joint Surg Am. 2009;91(8):1985-1994.
11. Bottlang M, Lesser M, Koerber J, et al. Far cortical locking can improve healing of fractures stabilized with locking plates. J Bone Joint Surg Am. 2010;92(7):1652-1660.
12. Bottlang M, Feist F. Biomechanics of far cortical locking. J Orthop Trauma. 2011;25(suppl 1):S21-S28.
Navigating the Alphabet Soup of Labroligamentous Pathology of the Shoulder
The widespread use of eponyms and acronyms to describe labroligamentous findings in the shoulder has made interpretation of shoulder magnetic resonance imaging (MRI) reports challenging. We review and discuss the appearance of these lesions on shoulder MRI to help the orthopedic surgeon understand these entities as imaging findings.
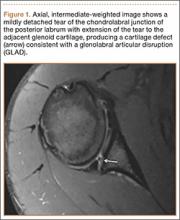
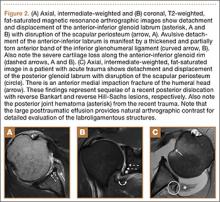
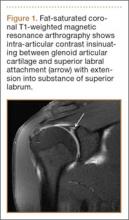
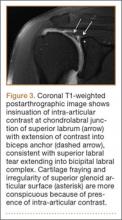
Glenolabral articular disruption (GLAD) occurs secondary to impaction of the humeral head on the glenoid articular cartilage. There is a resultant defect in the glenoid articular cartilage, which extends to the glenoid labrum. A GLAD lesion is diagnosed only if the glenohumeral ligament and scapular periosteum remain intact1 (Figure 1).
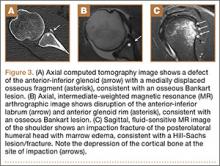
Complete detachment of the anteroinferior labrum with tearing of the anterior glenoid periosteum represents a Bankart lesion. Cartilaginous Bankart lesions are caused by an anterior glenohumeral dislocation with resultant avulsion of the anteroinferior labrum and disruption of the scapular periosteum because of acute traction on the anterior band of the inferior glenohumeral ligament (Figure 2). Anterior instability, caused by disruption of the anterior labroligamentous complex, results. Osseous Bankart lesions occur when the anterior displaced humeral head impacts the anterior inferior glenoid rim, causing a fracture (Figure 3). This loss of the glenoid articular surface area can result in glenohumeral instability. Posterior shoulder dislocations can result in corresponding findings in the posterior inferior glenoid labrum (reverse Bankart lesion) and anterior medial humeral head (reverse Hill-Sachs lesion) (Figure 2).
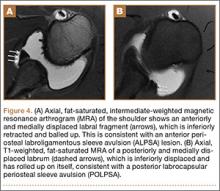
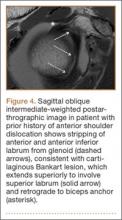
A variant of the Bankart lesion is the anterior labroligamentous periosteal sleeve avulsion (ALPSA). This refers to a medially displaced tear of the anterior labrum with intact periosteal stripping along the medial glenoid2with medial rotation and inferior displacement of the anterior inferior labrum along the scapular neck. An ALPSA lesion can heal via the intact periosteal blood supply. If not repaired, anterior instability will result because of malposition of the labrum, causing a patulous anterior capsule.3 When a corresponding lesion occurs in the posterior labrum because of a posterior dislocation, it is called a posterior labrocapsular periosteal sleeve avulsion (POLPSA) (Figure 4).
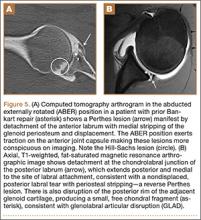
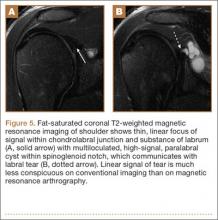
Another variant of the Bankart lesion is the Perthes lesion, which is a nondisplaced tear of the anteroinferior labrum with periosteal stripping. This differs from the ALPSA because the detached labrum and periosteum are held in anatomic position, possibly making the lesion difficult to detect on magnetic resonance arthrography (MRA).3 Obtaining images in the abduction external rotation (ABER) position exerts traction on the anterior inferior joint capsule and may make the Perthes lesion more conspicuous.4 When this occurs in the posterior labrum, it is called a reverse Perthes lesion (Figure 5).
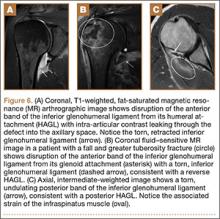
In a patient with anterior glenohumeral instability without a Bankart lesion, pathology of the anterior band of the inferior glenohumeral ligament (IGHL) at its humeral attachment must be suspected. Humeral avulsion of the IGHL (HAGL) or its variants can be overlooked on arthroscopy. HAGL is diagnosed on MRA when the normally U-shaped IGHL takes on a J-shape, and joint fluid extravasates across the torn humeral attachment (Figure 6). If there is an avulsed bony fragment from the medial humeral neck, the lesion is termed a bony HAGL (BHAGL). In addition to the findings of a HAGL, a BHAGL shows the osseous fragment and donor site on MRI. Since a BHAGL is a bony avulsion, it can even be suggested on radiography if a bony fragment is seen adjacent to the medial humeral neck.5 These lesions are highly associated with other shoulder injuries, particularly Hill-Sachs deformities and subscapularis tendon tears, and it is imperative, therefore, to search for additional injuries if a HAGL-type injury is seen.6
A more uncommon type of HAGL can occur in the setting of posterior capsulolabral injury. A posterior-band IGHL avulsion from the humerus (PHAGL) has similar imaging findings to a HAGL, except that it involves the posterior band of the IGHL. PHAGLs are usually not associated with an acute injury and are thought to be related to repetitive microtrauma, perhaps since the posterior band of the IGHL is the thinnest portion of the IGHL complex.7
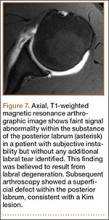
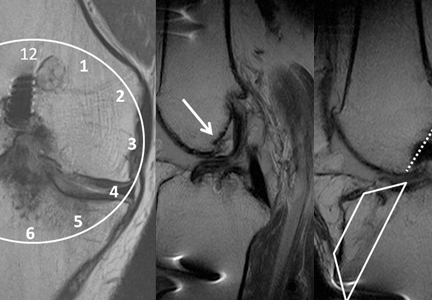
A Kim lesion is an arthroscopic finding described in patients with posterior instability as a superficial defect at the undersurface of the posterior labrum and adjacent glenoid cartilage without detachment or extension to the chondrolabral junction.8 It is, by its nature, a concealed finding on routine MRI but can be more conspicuous in FADIR (flexed, adducted, internally rotated) positioning on MRA, which exerts traction on the posterior joint capsule, allowing intra-articular contrast to fill the tear (Figure 7).
This list describes several of the most commonly encountered acronyms in shoulder MRI. A review of SLAP (superior labrum anterior to posterior) lesions was described in a previous article in the journal’s Imaging Series.9 A thorough understanding of these lesions is helpful in interpreting reports and determining the appropriate treatment for patients with shoulder injuries.
1. Sanders TG, Tirman PF, Linares R, Feller JF, Richardson R. The glenolabral articular disruption lesion: MR arthrography with arthroscopic correlation. AJR Am J Roentgenol. 1999;172(1):171-175.
2. Beltran J, Jbara M, Maimon R. Shoulder: labrum and bicipital tendon. Top Magn Reson Imaging. 2003;14(1):35-50.
3. Waldt S, Burkart A, Imhoff AB, Bruegel M, Rummeny EJ, Woertler K. Anterior shoulder instability: accuracy of MR arthrography in the classification of anteroinferior labroligamentous injuries. Radiology. 2005;237(2):578-583.
4. Schreinemachers SA, van der Hulst VP, Willems J, Bipat S, van der Woude H. Is a single direct MR arthrography series in ABER position as accurate in detecting anteroinferior labroligamentous lesions as conventional MR arthrography? Skeletal Radiol. 2009;38(7):675-683.
5. Bui-Mansfield LT, Taylor DC, Uhorchak JM, Tenuta JT. Humeral avulsions of the glenohumeral ligament: imaging features and a review of the literature. AJR Am J Roentgenol. 2002;179(3):649-655.
6. Magee T. Prevalence of HAGL lesions and associated abnormalities on shoulder MR examination. Skeletal Radiol. 2014;43(3):307-313.
7. Chung CB, Sorenson S, Dwek JR, Resnick D. Humeral avulsion of the posterior band of the inferior glenohumeral ligament: MR arthrography and clinical correlation in 17 patients. AJR Am J Roentgenol. 2004;183(2):355-359.
8. Kim SH, Ha KI, Yoo JC, Noh KC. Kim’s lesion: an incomplete and concealed avulsion of the posteroinferior labrum in posterior or multidirectional posteroinferior instability of the shoulder. Arthroscopy. 2004;20(7):712-720.
9. Grubin J, Maderazo A, Fitzpatrick D. Imaging evaluation of superior labral anteroposterior (SLAP) tears. Am J Orthop. 2015;44(10):476-477.
The widespread use of eponyms and acronyms to describe labroligamentous findings in the shoulder has made interpretation of shoulder magnetic resonance imaging (MRI) reports challenging. We review and discuss the appearance of these lesions on shoulder MRI to help the orthopedic surgeon understand these entities as imaging findings.
Glenolabral articular disruption (GLAD) occurs secondary to impaction of the humeral head on the glenoid articular cartilage. There is a resultant defect in the glenoid articular cartilage, which extends to the glenoid labrum. A GLAD lesion is diagnosed only if the glenohumeral ligament and scapular periosteum remain intact1 (Figure 1).
Complete detachment of the anteroinferior labrum with tearing of the anterior glenoid periosteum represents a Bankart lesion. Cartilaginous Bankart lesions are caused by an anterior glenohumeral dislocation with resultant avulsion of the anteroinferior labrum and disruption of the scapular periosteum because of acute traction on the anterior band of the inferior glenohumeral ligament (Figure 2). Anterior instability, caused by disruption of the anterior labroligamentous complex, results. Osseous Bankart lesions occur when the anterior displaced humeral head impacts the anterior inferior glenoid rim, causing a fracture (Figure 3). This loss of the glenoid articular surface area can result in glenohumeral instability. Posterior shoulder dislocations can result in corresponding findings in the posterior inferior glenoid labrum (reverse Bankart lesion) and anterior medial humeral head (reverse Hill-Sachs lesion) (Figure 2).
A variant of the Bankart lesion is the anterior labroligamentous periosteal sleeve avulsion (ALPSA). This refers to a medially displaced tear of the anterior labrum with intact periosteal stripping along the medial glenoid2with medial rotation and inferior displacement of the anterior inferior labrum along the scapular neck. An ALPSA lesion can heal via the intact periosteal blood supply. If not repaired, anterior instability will result because of malposition of the labrum, causing a patulous anterior capsule.3 When a corresponding lesion occurs in the posterior labrum because of a posterior dislocation, it is called a posterior labrocapsular periosteal sleeve avulsion (POLPSA) (Figure 4).
Another variant of the Bankart lesion is the Perthes lesion, which is a nondisplaced tear of the anteroinferior labrum with periosteal stripping. This differs from the ALPSA because the detached labrum and periosteum are held in anatomic position, possibly making the lesion difficult to detect on magnetic resonance arthrography (MRA).3 Obtaining images in the abduction external rotation (ABER) position exerts traction on the anterior inferior joint capsule and may make the Perthes lesion more conspicuous.4 When this occurs in the posterior labrum, it is called a reverse Perthes lesion (Figure 5).
In a patient with anterior glenohumeral instability without a Bankart lesion, pathology of the anterior band of the inferior glenohumeral ligament (IGHL) at its humeral attachment must be suspected. Humeral avulsion of the IGHL (HAGL) or its variants can be overlooked on arthroscopy. HAGL is diagnosed on MRA when the normally U-shaped IGHL takes on a J-shape, and joint fluid extravasates across the torn humeral attachment (Figure 6). If there is an avulsed bony fragment from the medial humeral neck, the lesion is termed a bony HAGL (BHAGL). In addition to the findings of a HAGL, a BHAGL shows the osseous fragment and donor site on MRI. Since a BHAGL is a bony avulsion, it can even be suggested on radiography if a bony fragment is seen adjacent to the medial humeral neck.5 These lesions are highly associated with other shoulder injuries, particularly Hill-Sachs deformities and subscapularis tendon tears, and it is imperative, therefore, to search for additional injuries if a HAGL-type injury is seen.6
A more uncommon type of HAGL can occur in the setting of posterior capsulolabral injury. A posterior-band IGHL avulsion from the humerus (PHAGL) has similar imaging findings to a HAGL, except that it involves the posterior band of the IGHL. PHAGLs are usually not associated with an acute injury and are thought to be related to repetitive microtrauma, perhaps since the posterior band of the IGHL is the thinnest portion of the IGHL complex.7
A Kim lesion is an arthroscopic finding described in patients with posterior instability as a superficial defect at the undersurface of the posterior labrum and adjacent glenoid cartilage without detachment or extension to the chondrolabral junction.8 It is, by its nature, a concealed finding on routine MRI but can be more conspicuous in FADIR (flexed, adducted, internally rotated) positioning on MRA, which exerts traction on the posterior joint capsule, allowing intra-articular contrast to fill the tear (Figure 7).
This list describes several of the most commonly encountered acronyms in shoulder MRI. A review of SLAP (superior labrum anterior to posterior) lesions was described in a previous article in the journal’s Imaging Series.9 A thorough understanding of these lesions is helpful in interpreting reports and determining the appropriate treatment for patients with shoulder injuries.
The widespread use of eponyms and acronyms to describe labroligamentous findings in the shoulder has made interpretation of shoulder magnetic resonance imaging (MRI) reports challenging. We review and discuss the appearance of these lesions on shoulder MRI to help the orthopedic surgeon understand these entities as imaging findings.
Glenolabral articular disruption (GLAD) occurs secondary to impaction of the humeral head on the glenoid articular cartilage. There is a resultant defect in the glenoid articular cartilage, which extends to the glenoid labrum. A GLAD lesion is diagnosed only if the glenohumeral ligament and scapular periosteum remain intact1 (Figure 1).
Complete detachment of the anteroinferior labrum with tearing of the anterior glenoid periosteum represents a Bankart lesion. Cartilaginous Bankart lesions are caused by an anterior glenohumeral dislocation with resultant avulsion of the anteroinferior labrum and disruption of the scapular periosteum because of acute traction on the anterior band of the inferior glenohumeral ligament (Figure 2). Anterior instability, caused by disruption of the anterior labroligamentous complex, results. Osseous Bankart lesions occur when the anterior displaced humeral head impacts the anterior inferior glenoid rim, causing a fracture (Figure 3). This loss of the glenoid articular surface area can result in glenohumeral instability. Posterior shoulder dislocations can result in corresponding findings in the posterior inferior glenoid labrum (reverse Bankart lesion) and anterior medial humeral head (reverse Hill-Sachs lesion) (Figure 2).
A variant of the Bankart lesion is the anterior labroligamentous periosteal sleeve avulsion (ALPSA). This refers to a medially displaced tear of the anterior labrum with intact periosteal stripping along the medial glenoid2with medial rotation and inferior displacement of the anterior inferior labrum along the scapular neck. An ALPSA lesion can heal via the intact periosteal blood supply. If not repaired, anterior instability will result because of malposition of the labrum, causing a patulous anterior capsule.3 When a corresponding lesion occurs in the posterior labrum because of a posterior dislocation, it is called a posterior labrocapsular periosteal sleeve avulsion (POLPSA) (Figure 4).
Another variant of the Bankart lesion is the Perthes lesion, which is a nondisplaced tear of the anteroinferior labrum with periosteal stripping. This differs from the ALPSA because the detached labrum and periosteum are held in anatomic position, possibly making the lesion difficult to detect on magnetic resonance arthrography (MRA).3 Obtaining images in the abduction external rotation (ABER) position exerts traction on the anterior inferior joint capsule and may make the Perthes lesion more conspicuous.4 When this occurs in the posterior labrum, it is called a reverse Perthes lesion (Figure 5).
In a patient with anterior glenohumeral instability without a Bankart lesion, pathology of the anterior band of the inferior glenohumeral ligament (IGHL) at its humeral attachment must be suspected. Humeral avulsion of the IGHL (HAGL) or its variants can be overlooked on arthroscopy. HAGL is diagnosed on MRA when the normally U-shaped IGHL takes on a J-shape, and joint fluid extravasates across the torn humeral attachment (Figure 6). If there is an avulsed bony fragment from the medial humeral neck, the lesion is termed a bony HAGL (BHAGL). In addition to the findings of a HAGL, a BHAGL shows the osseous fragment and donor site on MRI. Since a BHAGL is a bony avulsion, it can even be suggested on radiography if a bony fragment is seen adjacent to the medial humeral neck.5 These lesions are highly associated with other shoulder injuries, particularly Hill-Sachs deformities and subscapularis tendon tears, and it is imperative, therefore, to search for additional injuries if a HAGL-type injury is seen.6
A more uncommon type of HAGL can occur in the setting of posterior capsulolabral injury. A posterior-band IGHL avulsion from the humerus (PHAGL) has similar imaging findings to a HAGL, except that it involves the posterior band of the IGHL. PHAGLs are usually not associated with an acute injury and are thought to be related to repetitive microtrauma, perhaps since the posterior band of the IGHL is the thinnest portion of the IGHL complex.7
A Kim lesion is an arthroscopic finding described in patients with posterior instability as a superficial defect at the undersurface of the posterior labrum and adjacent glenoid cartilage without detachment or extension to the chondrolabral junction.8 It is, by its nature, a concealed finding on routine MRI but can be more conspicuous in FADIR (flexed, adducted, internally rotated) positioning on MRA, which exerts traction on the posterior joint capsule, allowing intra-articular contrast to fill the tear (Figure 7).
This list describes several of the most commonly encountered acronyms in shoulder MRI. A review of SLAP (superior labrum anterior to posterior) lesions was described in a previous article in the journal’s Imaging Series.9 A thorough understanding of these lesions is helpful in interpreting reports and determining the appropriate treatment for patients with shoulder injuries.
1. Sanders TG, Tirman PF, Linares R, Feller JF, Richardson R. The glenolabral articular disruption lesion: MR arthrography with arthroscopic correlation. AJR Am J Roentgenol. 1999;172(1):171-175.
2. Beltran J, Jbara M, Maimon R. Shoulder: labrum and bicipital tendon. Top Magn Reson Imaging. 2003;14(1):35-50.
3. Waldt S, Burkart A, Imhoff AB, Bruegel M, Rummeny EJ, Woertler K. Anterior shoulder instability: accuracy of MR arthrography in the classification of anteroinferior labroligamentous injuries. Radiology. 2005;237(2):578-583.
4. Schreinemachers SA, van der Hulst VP, Willems J, Bipat S, van der Woude H. Is a single direct MR arthrography series in ABER position as accurate in detecting anteroinferior labroligamentous lesions as conventional MR arthrography? Skeletal Radiol. 2009;38(7):675-683.
5. Bui-Mansfield LT, Taylor DC, Uhorchak JM, Tenuta JT. Humeral avulsions of the glenohumeral ligament: imaging features and a review of the literature. AJR Am J Roentgenol. 2002;179(3):649-655.
6. Magee T. Prevalence of HAGL lesions and associated abnormalities on shoulder MR examination. Skeletal Radiol. 2014;43(3):307-313.
7. Chung CB, Sorenson S, Dwek JR, Resnick D. Humeral avulsion of the posterior band of the inferior glenohumeral ligament: MR arthrography and clinical correlation in 17 patients. AJR Am J Roentgenol. 2004;183(2):355-359.
8. Kim SH, Ha KI, Yoo JC, Noh KC. Kim’s lesion: an incomplete and concealed avulsion of the posteroinferior labrum in posterior or multidirectional posteroinferior instability of the shoulder. Arthroscopy. 2004;20(7):712-720.
9. Grubin J, Maderazo A, Fitzpatrick D. Imaging evaluation of superior labral anteroposterior (SLAP) tears. Am J Orthop. 2015;44(10):476-477.
1. Sanders TG, Tirman PF, Linares R, Feller JF, Richardson R. The glenolabral articular disruption lesion: MR arthrography with arthroscopic correlation. AJR Am J Roentgenol. 1999;172(1):171-175.
2. Beltran J, Jbara M, Maimon R. Shoulder: labrum and bicipital tendon. Top Magn Reson Imaging. 2003;14(1):35-50.
3. Waldt S, Burkart A, Imhoff AB, Bruegel M, Rummeny EJ, Woertler K. Anterior shoulder instability: accuracy of MR arthrography in the classification of anteroinferior labroligamentous injuries. Radiology. 2005;237(2):578-583.
4. Schreinemachers SA, van der Hulst VP, Willems J, Bipat S, van der Woude H. Is a single direct MR arthrography series in ABER position as accurate in detecting anteroinferior labroligamentous lesions as conventional MR arthrography? Skeletal Radiol. 2009;38(7):675-683.
5. Bui-Mansfield LT, Taylor DC, Uhorchak JM, Tenuta JT. Humeral avulsions of the glenohumeral ligament: imaging features and a review of the literature. AJR Am J Roentgenol. 2002;179(3):649-655.
6. Magee T. Prevalence of HAGL lesions and associated abnormalities on shoulder MR examination. Skeletal Radiol. 2014;43(3):307-313.
7. Chung CB, Sorenson S, Dwek JR, Resnick D. Humeral avulsion of the posterior band of the inferior glenohumeral ligament: MR arthrography and clinical correlation in 17 patients. AJR Am J Roentgenol. 2004;183(2):355-359.
8. Kim SH, Ha KI, Yoo JC, Noh KC. Kim’s lesion: an incomplete and concealed avulsion of the posteroinferior labrum in posterior or multidirectional posteroinferior instability of the shoulder. Arthroscopy. 2004;20(7):712-720.
9. Grubin J, Maderazo A, Fitzpatrick D. Imaging evaluation of superior labral anteroposterior (SLAP) tears. Am J Orthop. 2015;44(10):476-477.
Magnetic Resonance Imaging of Complications of Anterior Cruciate Ligament Reconstruction
Magnetic resonance imaging (MRI) is the preferred modality in the evaluation of complications of anterior cruciate ligament reconstruction (ACL-R).1-3 ACL-R complications may be broadly characterized as those resulting in decreased range of motion (ROM), eg, arthrofibrosis and impingement, and those resulting in increased laxity, ie, graft disruption.4 Short tau inversion recovery (STIR) sequences best minimize artifact related to field inhomogeneity in the presence of metal-containing fixation devices. Patients with contraindications to MRI may undergo high-resolution computed tomographic arthrography of the knee for evaluation of postoperative graft abnormalities.1
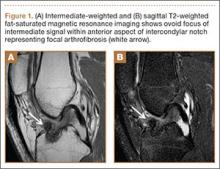
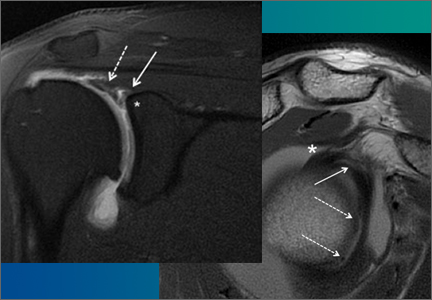
Arthrofibrosis refers to focal or diffuse synovial scar tissue, which may limit ROM. Preoperative irritation, preoperative limited ROM, and reconstruction within 4 weeks of trauma may all play a role in the development of arthrofibrosis.5,6 The focal form, cyclops lesion, named for its arthroscopic appearance, has been reported in 1% to 10% of patients with ACL-R.1 On MRI, focal arthrofibrosis may be seen as a focal or diffuse intermediate signal lesion in the anterior intercondylar notch extending linearly along the intercondylar roof1 (Figure 1).
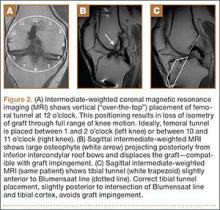
MRI can be used to accurately determine the position of the femoral and tibial tunnels. Correct femoral tunnel position results in isometry of the graft during full ROM of the knee. Graft impingement can occur when the tibial tunnel is placed too far anteriorly such that the graft contacts the roof of the intercondylar notch before the knee is able to fully extend.7 A tibial tunnel placed anterior to the intersection of the Blumensaat line and the tibia is at higher risk for impingement.1,4 Impingement may be accompanied by signal change in the graft on intermediate-weighted and fluid-sensitive sequences. The signal abnormality is usually focal and persists longer than the expected signal changes related to revascularization of immature grafts within the first year (Figure 2). If left untreated, impingement may progress to graft rupture.4
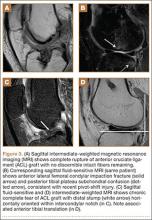
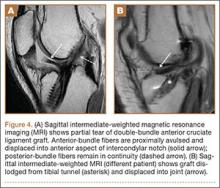
Complete graft rupture is diagnosed on the basis of discontinuity of the graft fibers. MRI findings include fluid-filled defect or absence of intact graft fibers. Other reliable signs include large joint effusion, anterior tibial translation, pivot-shift–type marrow edema pattern, and horizontal orientation, laxity, or resorption of the graft fibers.1,8,9 The diagnosis of partial graft rupture may be challenging, as there are several other causes of increased graft signal, including revascularization (within 12 months after procedure), signal heterogeneity between individual bundles of hamstring grafts, and focal signal changes related to impingment (Figures 3, 4).
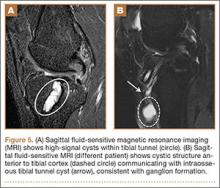
Fluid within the tunnels is a normal finding after surgery and typically resolves within the first 18 months.1 Cyst formation within the tibial tunnel is an uncommon complication of ACL-R and may be incidental to or present with clinical symptoms caused by extension into the pretibial soft tissues or expansion of the tunnel (Figure 5). Communication of cyst with joint space is important, as a noncommunicating cyst requires simple excision without need for bone grafting.7
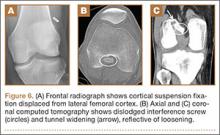
Hardware-related complications (eg, loosening of fixation devices) are uncommon but may require revision surgery (Figure 6). Septic arthritis after ACL-R has a cumulative incidence of 0.1% to 0.9% and may be difficult to diagnose clinically because of the lack of classic symptoms of a septic joint.1 Diagnosis requires joint aspiration.
MRI is reliably and accurately used to assess ACL-R complications. The clinical history helps in stratifying complications that result in decreased ROM or increased laxity.
1. Bencardino JT, Beltran J, Feldman MI, Rose DJ. MR imaging of complications of anterior cruciate ligament graft reconstruction. Radiographics. 2009;29(7):2115-2126.
2. Recht MP, Kramer J. MR imaging of the postoperative knee: a pictorial essay. Radiographics. 2002;22(4):765-774.
3. Papakonstantinou O, Chung CB, Chanchairujira K, Resnick DL. Complications of anterior cruciate ligament reconstruction: MR imaging. Eur Radiol. 2003;13(5):1106-1117.
4. Meyers AB, Haims AH, Menn K, Moukaddam H. Imaging of anterior cruciate ligament repair and its complications. AJR Am J Roentgenol. 2010;194(2):476-484.
5. Kwok CS, Harrison T, Servant C. The optimal timing for anterior cruciate ligament reconstruction with respect to the risk of postoperative stiffness. Arthroscopy. 2013;29(3):556-565.
6. Mayr HO, Weig TG, Plitz W. Arthrofibrosis following ACL reconstruction—reasons and outcome. Arch Orthop Trauma Surg. 2004;124(8):518-522.
7. Ghazikhanian V, Beltran J, Nikac V, Feldman M, Bencardino JT. Tibial tunnel and pretibial cysts following ACL graft reconstruction: MR imaging diagnosis. Skeletal Radiol. 2012;41(11):1375-1379.
8. Collins MS, Unruh KP, Bond JR, Mandrekar JN. Magnetic resonance imaging of surgically confirmed anterior cruciate ligament graft disruption. Skeletal Radiol. 2008;37(3):233-243.
9. Saupe N, White LM, Chiavaras MM, et al. Anterior cruciate ligament reconstruction grafts: MR imaging features at long-term follow-up—correlation with functional and clinical evaluation. Radiology. 2008;249(2):581-590.
Magnetic resonance imaging (MRI) is the preferred modality in the evaluation of complications of anterior cruciate ligament reconstruction (ACL-R).1-3 ACL-R complications may be broadly characterized as those resulting in decreased range of motion (ROM), eg, arthrofibrosis and impingement, and those resulting in increased laxity, ie, graft disruption.4 Short tau inversion recovery (STIR) sequences best minimize artifact related to field inhomogeneity in the presence of metal-containing fixation devices. Patients with contraindications to MRI may undergo high-resolution computed tomographic arthrography of the knee for evaluation of postoperative graft abnormalities.1
Arthrofibrosis refers to focal or diffuse synovial scar tissue, which may limit ROM. Preoperative irritation, preoperative limited ROM, and reconstruction within 4 weeks of trauma may all play a role in the development of arthrofibrosis.5,6 The focal form, cyclops lesion, named for its arthroscopic appearance, has been reported in 1% to 10% of patients with ACL-R.1 On MRI, focal arthrofibrosis may be seen as a focal or diffuse intermediate signal lesion in the anterior intercondylar notch extending linearly along the intercondylar roof1 (Figure 1).
MRI can be used to accurately determine the position of the femoral and tibial tunnels. Correct femoral tunnel position results in isometry of the graft during full ROM of the knee. Graft impingement can occur when the tibial tunnel is placed too far anteriorly such that the graft contacts the roof of the intercondylar notch before the knee is able to fully extend.7 A tibial tunnel placed anterior to the intersection of the Blumensaat line and the tibia is at higher risk for impingement.1,4 Impingement may be accompanied by signal change in the graft on intermediate-weighted and fluid-sensitive sequences. The signal abnormality is usually focal and persists longer than the expected signal changes related to revascularization of immature grafts within the first year (Figure 2). If left untreated, impingement may progress to graft rupture.4
Complete graft rupture is diagnosed on the basis of discontinuity of the graft fibers. MRI findings include fluid-filled defect or absence of intact graft fibers. Other reliable signs include large joint effusion, anterior tibial translation, pivot-shift–type marrow edema pattern, and horizontal orientation, laxity, or resorption of the graft fibers.1,8,9 The diagnosis of partial graft rupture may be challenging, as there are several other causes of increased graft signal, including revascularization (within 12 months after procedure), signal heterogeneity between individual bundles of hamstring grafts, and focal signal changes related to impingment (Figures 3, 4).
Fluid within the tunnels is a normal finding after surgery and typically resolves within the first 18 months.1 Cyst formation within the tibial tunnel is an uncommon complication of ACL-R and may be incidental to or present with clinical symptoms caused by extension into the pretibial soft tissues or expansion of the tunnel (Figure 5). Communication of cyst with joint space is important, as a noncommunicating cyst requires simple excision without need for bone grafting.7
Hardware-related complications (eg, loosening of fixation devices) are uncommon but may require revision surgery (Figure 6). Septic arthritis after ACL-R has a cumulative incidence of 0.1% to 0.9% and may be difficult to diagnose clinically because of the lack of classic symptoms of a septic joint.1 Diagnosis requires joint aspiration.
MRI is reliably and accurately used to assess ACL-R complications. The clinical history helps in stratifying complications that result in decreased ROM or increased laxity.
Magnetic resonance imaging (MRI) is the preferred modality in the evaluation of complications of anterior cruciate ligament reconstruction (ACL-R).1-3 ACL-R complications may be broadly characterized as those resulting in decreased range of motion (ROM), eg, arthrofibrosis and impingement, and those resulting in increased laxity, ie, graft disruption.4 Short tau inversion recovery (STIR) sequences best minimize artifact related to field inhomogeneity in the presence of metal-containing fixation devices. Patients with contraindications to MRI may undergo high-resolution computed tomographic arthrography of the knee for evaluation of postoperative graft abnormalities.1
Arthrofibrosis refers to focal or diffuse synovial scar tissue, which may limit ROM. Preoperative irritation, preoperative limited ROM, and reconstruction within 4 weeks of trauma may all play a role in the development of arthrofibrosis.5,6 The focal form, cyclops lesion, named for its arthroscopic appearance, has been reported in 1% to 10% of patients with ACL-R.1 On MRI, focal arthrofibrosis may be seen as a focal or diffuse intermediate signal lesion in the anterior intercondylar notch extending linearly along the intercondylar roof1 (Figure 1).
MRI can be used to accurately determine the position of the femoral and tibial tunnels. Correct femoral tunnel position results in isometry of the graft during full ROM of the knee. Graft impingement can occur when the tibial tunnel is placed too far anteriorly such that the graft contacts the roof of the intercondylar notch before the knee is able to fully extend.7 A tibial tunnel placed anterior to the intersection of the Blumensaat line and the tibia is at higher risk for impingement.1,4 Impingement may be accompanied by signal change in the graft on intermediate-weighted and fluid-sensitive sequences. The signal abnormality is usually focal and persists longer than the expected signal changes related to revascularization of immature grafts within the first year (Figure 2). If left untreated, impingement may progress to graft rupture.4
Complete graft rupture is diagnosed on the basis of discontinuity of the graft fibers. MRI findings include fluid-filled defect or absence of intact graft fibers. Other reliable signs include large joint effusion, anterior tibial translation, pivot-shift–type marrow edema pattern, and horizontal orientation, laxity, or resorption of the graft fibers.1,8,9 The diagnosis of partial graft rupture may be challenging, as there are several other causes of increased graft signal, including revascularization (within 12 months after procedure), signal heterogeneity between individual bundles of hamstring grafts, and focal signal changes related to impingment (Figures 3, 4).
Fluid within the tunnels is a normal finding after surgery and typically resolves within the first 18 months.1 Cyst formation within the tibial tunnel is an uncommon complication of ACL-R and may be incidental to or present with clinical symptoms caused by extension into the pretibial soft tissues or expansion of the tunnel (Figure 5). Communication of cyst with joint space is important, as a noncommunicating cyst requires simple excision without need for bone grafting.7
Hardware-related complications (eg, loosening of fixation devices) are uncommon but may require revision surgery (Figure 6). Septic arthritis after ACL-R has a cumulative incidence of 0.1% to 0.9% and may be difficult to diagnose clinically because of the lack of classic symptoms of a septic joint.1 Diagnosis requires joint aspiration.
MRI is reliably and accurately used to assess ACL-R complications. The clinical history helps in stratifying complications that result in decreased ROM or increased laxity.
1. Bencardino JT, Beltran J, Feldman MI, Rose DJ. MR imaging of complications of anterior cruciate ligament graft reconstruction. Radiographics. 2009;29(7):2115-2126.
2. Recht MP, Kramer J. MR imaging of the postoperative knee: a pictorial essay. Radiographics. 2002;22(4):765-774.
3. Papakonstantinou O, Chung CB, Chanchairujira K, Resnick DL. Complications of anterior cruciate ligament reconstruction: MR imaging. Eur Radiol. 2003;13(5):1106-1117.
4. Meyers AB, Haims AH, Menn K, Moukaddam H. Imaging of anterior cruciate ligament repair and its complications. AJR Am J Roentgenol. 2010;194(2):476-484.
5. Kwok CS, Harrison T, Servant C. The optimal timing for anterior cruciate ligament reconstruction with respect to the risk of postoperative stiffness. Arthroscopy. 2013;29(3):556-565.
6. Mayr HO, Weig TG, Plitz W. Arthrofibrosis following ACL reconstruction—reasons and outcome. Arch Orthop Trauma Surg. 2004;124(8):518-522.
7. Ghazikhanian V, Beltran J, Nikac V, Feldman M, Bencardino JT. Tibial tunnel and pretibial cysts following ACL graft reconstruction: MR imaging diagnosis. Skeletal Radiol. 2012;41(11):1375-1379.
8. Collins MS, Unruh KP, Bond JR, Mandrekar JN. Magnetic resonance imaging of surgically confirmed anterior cruciate ligament graft disruption. Skeletal Radiol. 2008;37(3):233-243.
9. Saupe N, White LM, Chiavaras MM, et al. Anterior cruciate ligament reconstruction grafts: MR imaging features at long-term follow-up—correlation with functional and clinical evaluation. Radiology. 2008;249(2):581-590.
1. Bencardino JT, Beltran J, Feldman MI, Rose DJ. MR imaging of complications of anterior cruciate ligament graft reconstruction. Radiographics. 2009;29(7):2115-2126.
2. Recht MP, Kramer J. MR imaging of the postoperative knee: a pictorial essay. Radiographics. 2002;22(4):765-774.
3. Papakonstantinou O, Chung CB, Chanchairujira K, Resnick DL. Complications of anterior cruciate ligament reconstruction: MR imaging. Eur Radiol. 2003;13(5):1106-1117.
4. Meyers AB, Haims AH, Menn K, Moukaddam H. Imaging of anterior cruciate ligament repair and its complications. AJR Am J Roentgenol. 2010;194(2):476-484.
5. Kwok CS, Harrison T, Servant C. The optimal timing for anterior cruciate ligament reconstruction with respect to the risk of postoperative stiffness. Arthroscopy. 2013;29(3):556-565.
6. Mayr HO, Weig TG, Plitz W. Arthrofibrosis following ACL reconstruction—reasons and outcome. Arch Orthop Trauma Surg. 2004;124(8):518-522.
7. Ghazikhanian V, Beltran J, Nikac V, Feldman M, Bencardino JT. Tibial tunnel and pretibial cysts following ACL graft reconstruction: MR imaging diagnosis. Skeletal Radiol. 2012;41(11):1375-1379.
8. Collins MS, Unruh KP, Bond JR, Mandrekar JN. Magnetic resonance imaging of surgically confirmed anterior cruciate ligament graft disruption. Skeletal Radiol. 2008;37(3):233-243.
9. Saupe N, White LM, Chiavaras MM, et al. Anterior cruciate ligament reconstruction grafts: MR imaging features at long-term follow-up—correlation with functional and clinical evaluation. Radiology. 2008;249(2):581-590.
Imaging Evaluation of Superior Labral Anteroposterior (SLAP) Tears
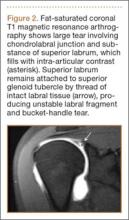
Superior labral anteroposterior (SLAP) tears are common labral injuries. They occur at the attachment of the long head of the biceps tendon on the superior glenoid and extend anterior and/or posterior to the biceps anchor. The mechanism of action for SLAP tears is traction on the superior labrum by the long head of the biceps tendon, resulting in “peeling” of the labrum off the glenoid. Such forces may result from repetitive overhead arm motion (pitching) or from direct trauma.
Clinical diagnosis is challenging with SLAP tears, as they often present with nonspecific shoulder pain and may not be associated with an acute injury. A further complication is that they are often associated with other shoulder pathology, such as rotator cuff tears.1 As physical examination is typically nonspecific, proper diagnostic imaging is essential for diagnosis.
We prefer to assess potential SLAP tears with magnetic resonance arthrography (MRA).2 Dilute (1:200) gadolinium contrast material (12-15 mL) is introduced into the glenohumeral joint under sonographic or fluoroscopic guidance. Capsular distention by dilute intra-articular contrast enables superior imaging resolution of the labroligamentous complex. We think the increase in diagnostic confidence enabled by direct arthrography outweighs the additional invasiveness and cost associated with MRA relative to noncontrast magnetic resonance imaging (MRI).
The MRA protocol differs from our routine noncontrast shoulder imaging. We perform a fat-saturated coronal oblique T1 sequence that maximizes the conspicuity of intra-articular contrast in the plane that optimally visualizes the superior labrum. Three planes of intermediate-weighted fast spin echo not only contrast the high-signal intra-articular fluid with the low-signal fibrocartilaginous labrum and the stratified intermediate signal of glenoid articular cartilage, but they also allow optimal assessment of the rotator cuff. In addition, we perform a fat-saturated coronal T2 sequence that highlights all fluid signal structures as well as edema.
SLAP tears appear on MRA as the insinuation of intra-articular contrast between the articular cartilage and the attachment of the superior labrum,3 within the substance of the labrum, or as detachment of the labrum from the glenoid rim4 (Figure 1). Findings can range from labral fraying to complete detachment with displacement. Tears can extend into other quadrants of the labrum, extend from a Bankart lesion, or involve the biceps tendon and/or the glenohumeral ligaments (Figures 2–4). Up to 10 types of SLAP tears have been described on arthroscopy. This classification scheme, however, is seldom helpful in the interpretation of SLAP tears on MRI. More important in guiding treatment is having a detailed description of the tear, including location, extent, and morphology, along with associated abnormalities.
Several findings can aid in the diagnosis of SLAP tears. Normal anatomical variants of the anterior-superior labrum do not extend posterior to the biceps anchor—an important finding for discerning normal morphologic variants from tears. Therefore, high signal within the posterior third of the superior labrum or extension of high signal laterally within the labrum and away from the glenoid suggests a SLAP tear.5 A paralabral cyst is almost always associated with a labral tear,1 so signal abnormality of the superior labrum with a paralabral cyst suggests a SLAP tear (Figure 5).
MRA is not the only method for diagnosing SLAP tears. Standard 3-Tesla MRI had 83% sensitivity and 99% specificity for diagnosing SLAP tears in a recent study, though MRA had 98% sensitivity and 99% specificity—a statistically significant sensitivity difference.6 In another study, computed tomography arthrography (CTA) had 95% sensitivity and 88% specificity for diagnosing recurrent SLAP tears after surgery.7 CTA is associated with ionizing radiation and is limited in its assessment of other structures that may show concomitant abnormalities, such as the articular cartilage and the rotator cuff. Indirect MRA, wherein magnetic resonance sequences are obtained after intravenous injection of gadolinium contrast and exercise of the affected shoulder, had a high sensitivity of detection of labral tears of all types.8
MRA is most sensitive and specific for diagnosing SLAP tears; 3-Tesla MRI, indirect MRA, and CTA are useful alternative modalities for cases in which MRA cannot be performed.
1. Chang D, Mohana-Borges A, Borso M, Chung CB. SLAP lesions: anatomy, clinical presentation, MR imaging diagnosis and characterization. Eur J Radiol. 2008;68(1):72-87.
2. Jee WH, McCauley TR, Katz LD, Matheny JM, Ruwe PA, Daigneault JP. Superior labral anterior posterior (SLAP) lesions of the glenoid labrum: reliability and accuracy of MR arthrography for diagnosis. Radiology. 2001;218(1):127-132.
3. Fitzpatrick D, Walz DM. Shoulder MR imaging normal variants and imaging artifacts. Magn Reson Imaging Clin N Am. 2010;18(4):615-632.
4. Bencardino JT, Beltran J, Rosenberg ZS, et al. Superior labrum anterior-posterior lesions: diagnosis with MR arthrography of the shoulder. Radiology. 2000;214(1):267-271.
5. Tuite MJ, Cirillo RL, De Smet AA, Orwin JF. Superior labrum anterior-posterior (SLAP) tears: evaluation of three MR signs on T2-weighted images. Radiology. 2000;215(3):841-845.
6. Magee T. 3-T MRI of the shoulder: is MR arthrography necessary? AJR Am J Roentgenol. 2009;192(1):86-92.
7. De Filippo M, Araoz PA, Pogliacomi F, et al. Recurrent superior labral anterior-to-posterior tears after surgery: detection and grading with CT arthrography. Radiology. 2009;252(3):781-788.
8. Fallahi F, Green N, Gadde S, Jeavons L, Armstrong P, Jonker L. Indirect magnetic resonance arthrography of the shoulder; a reliable diagnostic tool for investigation of suspected labral pathology. Skeletal Radiol. 2013;42(9):1225-1233.
Superior labral anteroposterior (SLAP) tears are common labral injuries. They occur at the attachment of the long head of the biceps tendon on the superior glenoid and extend anterior and/or posterior to the biceps anchor. The mechanism of action for SLAP tears is traction on the superior labrum by the long head of the biceps tendon, resulting in “peeling” of the labrum off the glenoid. Such forces may result from repetitive overhead arm motion (pitching) or from direct trauma.
Clinical diagnosis is challenging with SLAP tears, as they often present with nonspecific shoulder pain and may not be associated with an acute injury. A further complication is that they are often associated with other shoulder pathology, such as rotator cuff tears.1 As physical examination is typically nonspecific, proper diagnostic imaging is essential for diagnosis.
We prefer to assess potential SLAP tears with magnetic resonance arthrography (MRA).2 Dilute (1:200) gadolinium contrast material (12-15 mL) is introduced into the glenohumeral joint under sonographic or fluoroscopic guidance. Capsular distention by dilute intra-articular contrast enables superior imaging resolution of the labroligamentous complex. We think the increase in diagnostic confidence enabled by direct arthrography outweighs the additional invasiveness and cost associated with MRA relative to noncontrast magnetic resonance imaging (MRI).
The MRA protocol differs from our routine noncontrast shoulder imaging. We perform a fat-saturated coronal oblique T1 sequence that maximizes the conspicuity of intra-articular contrast in the plane that optimally visualizes the superior labrum. Three planes of intermediate-weighted fast spin echo not only contrast the high-signal intra-articular fluid with the low-signal fibrocartilaginous labrum and the stratified intermediate signal of glenoid articular cartilage, but they also allow optimal assessment of the rotator cuff. In addition, we perform a fat-saturated coronal T2 sequence that highlights all fluid signal structures as well as edema.
SLAP tears appear on MRA as the insinuation of intra-articular contrast between the articular cartilage and the attachment of the superior labrum,3 within the substance of the labrum, or as detachment of the labrum from the glenoid rim4 (Figure 1). Findings can range from labral fraying to complete detachment with displacement. Tears can extend into other quadrants of the labrum, extend from a Bankart lesion, or involve the biceps tendon and/or the glenohumeral ligaments (Figures 2–4). Up to 10 types of SLAP tears have been described on arthroscopy. This classification scheme, however, is seldom helpful in the interpretation of SLAP tears on MRI. More important in guiding treatment is having a detailed description of the tear, including location, extent, and morphology, along with associated abnormalities.
Several findings can aid in the diagnosis of SLAP tears. Normal anatomical variants of the anterior-superior labrum do not extend posterior to the biceps anchor—an important finding for discerning normal morphologic variants from tears. Therefore, high signal within the posterior third of the superior labrum or extension of high signal laterally within the labrum and away from the glenoid suggests a SLAP tear.5 A paralabral cyst is almost always associated with a labral tear,1 so signal abnormality of the superior labrum with a paralabral cyst suggests a SLAP tear (Figure 5).
MRA is not the only method for diagnosing SLAP tears. Standard 3-Tesla MRI had 83% sensitivity and 99% specificity for diagnosing SLAP tears in a recent study, though MRA had 98% sensitivity and 99% specificity—a statistically significant sensitivity difference.6 In another study, computed tomography arthrography (CTA) had 95% sensitivity and 88% specificity for diagnosing recurrent SLAP tears after surgery.7 CTA is associated with ionizing radiation and is limited in its assessment of other structures that may show concomitant abnormalities, such as the articular cartilage and the rotator cuff. Indirect MRA, wherein magnetic resonance sequences are obtained after intravenous injection of gadolinium contrast and exercise of the affected shoulder, had a high sensitivity of detection of labral tears of all types.8
MRA is most sensitive and specific for diagnosing SLAP tears; 3-Tesla MRI, indirect MRA, and CTA are useful alternative modalities for cases in which MRA cannot be performed.
Superior labral anteroposterior (SLAP) tears are common labral injuries. They occur at the attachment of the long head of the biceps tendon on the superior glenoid and extend anterior and/or posterior to the biceps anchor. The mechanism of action for SLAP tears is traction on the superior labrum by the long head of the biceps tendon, resulting in “peeling” of the labrum off the glenoid. Such forces may result from repetitive overhead arm motion (pitching) or from direct trauma.
Clinical diagnosis is challenging with SLAP tears, as they often present with nonspecific shoulder pain and may not be associated with an acute injury. A further complication is that they are often associated with other shoulder pathology, such as rotator cuff tears.1 As physical examination is typically nonspecific, proper diagnostic imaging is essential for diagnosis.
We prefer to assess potential SLAP tears with magnetic resonance arthrography (MRA).2 Dilute (1:200) gadolinium contrast material (12-15 mL) is introduced into the glenohumeral joint under sonographic or fluoroscopic guidance. Capsular distention by dilute intra-articular contrast enables superior imaging resolution of the labroligamentous complex. We think the increase in diagnostic confidence enabled by direct arthrography outweighs the additional invasiveness and cost associated with MRA relative to noncontrast magnetic resonance imaging (MRI).
The MRA protocol differs from our routine noncontrast shoulder imaging. We perform a fat-saturated coronal oblique T1 sequence that maximizes the conspicuity of intra-articular contrast in the plane that optimally visualizes the superior labrum. Three planes of intermediate-weighted fast spin echo not only contrast the high-signal intra-articular fluid with the low-signal fibrocartilaginous labrum and the stratified intermediate signal of glenoid articular cartilage, but they also allow optimal assessment of the rotator cuff. In addition, we perform a fat-saturated coronal T2 sequence that highlights all fluid signal structures as well as edema.
SLAP tears appear on MRA as the insinuation of intra-articular contrast between the articular cartilage and the attachment of the superior labrum,3 within the substance of the labrum, or as detachment of the labrum from the glenoid rim4 (Figure 1). Findings can range from labral fraying to complete detachment with displacement. Tears can extend into other quadrants of the labrum, extend from a Bankart lesion, or involve the biceps tendon and/or the glenohumeral ligaments (Figures 2–4). Up to 10 types of SLAP tears have been described on arthroscopy. This classification scheme, however, is seldom helpful in the interpretation of SLAP tears on MRI. More important in guiding treatment is having a detailed description of the tear, including location, extent, and morphology, along with associated abnormalities.
Several findings can aid in the diagnosis of SLAP tears. Normal anatomical variants of the anterior-superior labrum do not extend posterior to the biceps anchor—an important finding for discerning normal morphologic variants from tears. Therefore, high signal within the posterior third of the superior labrum or extension of high signal laterally within the labrum and away from the glenoid suggests a SLAP tear.5 A paralabral cyst is almost always associated with a labral tear,1 so signal abnormality of the superior labrum with a paralabral cyst suggests a SLAP tear (Figure 5).
MRA is not the only method for diagnosing SLAP tears. Standard 3-Tesla MRI had 83% sensitivity and 99% specificity for diagnosing SLAP tears in a recent study, though MRA had 98% sensitivity and 99% specificity—a statistically significant sensitivity difference.6 In another study, computed tomography arthrography (CTA) had 95% sensitivity and 88% specificity for diagnosing recurrent SLAP tears after surgery.7 CTA is associated with ionizing radiation and is limited in its assessment of other structures that may show concomitant abnormalities, such as the articular cartilage and the rotator cuff. Indirect MRA, wherein magnetic resonance sequences are obtained after intravenous injection of gadolinium contrast and exercise of the affected shoulder, had a high sensitivity of detection of labral tears of all types.8
MRA is most sensitive and specific for diagnosing SLAP tears; 3-Tesla MRI, indirect MRA, and CTA are useful alternative modalities for cases in which MRA cannot be performed.
1. Chang D, Mohana-Borges A, Borso M, Chung CB. SLAP lesions: anatomy, clinical presentation, MR imaging diagnosis and characterization. Eur J Radiol. 2008;68(1):72-87.
2. Jee WH, McCauley TR, Katz LD, Matheny JM, Ruwe PA, Daigneault JP. Superior labral anterior posterior (SLAP) lesions of the glenoid labrum: reliability and accuracy of MR arthrography for diagnosis. Radiology. 2001;218(1):127-132.
3. Fitzpatrick D, Walz DM. Shoulder MR imaging normal variants and imaging artifacts. Magn Reson Imaging Clin N Am. 2010;18(4):615-632.
4. Bencardino JT, Beltran J, Rosenberg ZS, et al. Superior labrum anterior-posterior lesions: diagnosis with MR arthrography of the shoulder. Radiology. 2000;214(1):267-271.
5. Tuite MJ, Cirillo RL, De Smet AA, Orwin JF. Superior labrum anterior-posterior (SLAP) tears: evaluation of three MR signs on T2-weighted images. Radiology. 2000;215(3):841-845.
6. Magee T. 3-T MRI of the shoulder: is MR arthrography necessary? AJR Am J Roentgenol. 2009;192(1):86-92.
7. De Filippo M, Araoz PA, Pogliacomi F, et al. Recurrent superior labral anterior-to-posterior tears after surgery: detection and grading with CT arthrography. Radiology. 2009;252(3):781-788.
8. Fallahi F, Green N, Gadde S, Jeavons L, Armstrong P, Jonker L. Indirect magnetic resonance arthrography of the shoulder; a reliable diagnostic tool for investigation of suspected labral pathology. Skeletal Radiol. 2013;42(9):1225-1233.
1. Chang D, Mohana-Borges A, Borso M, Chung CB. SLAP lesions: anatomy, clinical presentation, MR imaging diagnosis and characterization. Eur J Radiol. 2008;68(1):72-87.
2. Jee WH, McCauley TR, Katz LD, Matheny JM, Ruwe PA, Daigneault JP. Superior labral anterior posterior (SLAP) lesions of the glenoid labrum: reliability and accuracy of MR arthrography for diagnosis. Radiology. 2001;218(1):127-132.
3. Fitzpatrick D, Walz DM. Shoulder MR imaging normal variants and imaging artifacts. Magn Reson Imaging Clin N Am. 2010;18(4):615-632.
4. Bencardino JT, Beltran J, Rosenberg ZS, et al. Superior labrum anterior-posterior lesions: diagnosis with MR arthrography of the shoulder. Radiology. 2000;214(1):267-271.
5. Tuite MJ, Cirillo RL, De Smet AA, Orwin JF. Superior labrum anterior-posterior (SLAP) tears: evaluation of three MR signs on T2-weighted images. Radiology. 2000;215(3):841-845.
6. Magee T. 3-T MRI of the shoulder: is MR arthrography necessary? AJR Am J Roentgenol. 2009;192(1):86-92.
7. De Filippo M, Araoz PA, Pogliacomi F, et al. Recurrent superior labral anterior-to-posterior tears after surgery: detection and grading with CT arthrography. Radiology. 2009;252(3):781-788.
8. Fallahi F, Green N, Gadde S, Jeavons L, Armstrong P, Jonker L. Indirect magnetic resonance arthrography of the shoulder; a reliable diagnostic tool for investigation of suspected labral pathology. Skeletal Radiol. 2013;42(9):1225-1233.