User login
Treating Tibia Fractures With Far Cortical Locking Implants
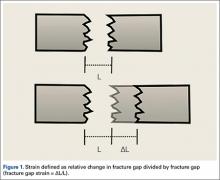
Fracture healing can be categorized as primary or secondary. Primary healing requires precise reapproximation of bone fragments and compression of cortices. Osteons are formed across the fracture line, allowing blood supply and endothelial cells to gain access, leading to osteoblast infiltration and subsequent bone formation.1 This type of bone healing can be accomplished only with absolute stability—specifically, only with less than 2% strain at the fracture site, necessitating operative intervention with compression plating (Figure 1).2 This type of construct generates friction between the bone fragments against a metal plate, created by tightening screws that purchase both far and near cortices of bone.3 Although this type of fixation works well with many fractures, there are several instances in which compression plating is not ideal.4 Osteoporotic bone, for example, limits the amount of compression that can be developed, as screws strip the bone more readily, leading to weakened constructs prone to failure. Metaphyseal fractures in which there is minimal cortex for screw thread purchase are a similar challenge.5 Highly comminuted fractures do not allow for sufficient fragment compression and stability. In addition, compression plating requires periosteal stripping at the fracture, and often substantial soft-tissue disruption, which is especially a problem in areas of tenuous blood supply (eg, the tibia).
Locked plating therefore has become a valuable technique in managing osteoporotic fractures.2 Locking plates may be used to achieve secondary bone healing through a small amount of interfragmentary motion, 0.2 to 10 mm, as seen with bridge plating for example, whereby the locking plates act as internal fixators. Much as with external fixators, as the distance from the fixator bar (or plate) to bone decreases, construct stiffness increases. Thus, locking plates function as extremely stiff fixators when the plate is very near bone. It has therefore been speculated that such stiffness is insufficient to provide optimal secondary healing conditions.6,7 Titanium (vs stainless steel) plates have been used, and screws have been omitted just adjacent to either side of the fracture site, in attempts to increase plate flexibility and thus interfragmentary motion.8,9 In addition, biomechanical and animal model studies have demonstrated that, with use of locking plates, motion at the fracture site is asymmetric and leads to unequal callus formation at the near and far cortices, thus weakening the fracture site.10,11
The locking plate design was recently modified to address these concerns. Far cortical locking (FCL) uses locking screws threaded only distally (Figure 2), which allows for purchase into the far cortex but not the near cortex, which increases pin length from plate to bone. The near cortex is no longer anchored to the plate and thus increases construct flexibility. Pilot holes in the near cortex allow for movement of the nonthreaded screw shaft in a controlled, biphasic manner.12 This design decreases stiffness while sacrificing very little construct strength.10 In addition, motion at the far and near cortices is nearly parallel. It has been shown in an ovine tibial osteotomy model that, compared with the traditional locking plate design, FCL generates symmetric callus formation and improved fracture healing.11 Although these results are promising, there are only limited clinical data on use of the FCL technique in fracture repair. Our null hypothesis was that, despite the theoretical advantages of FCL constructs over conventional locking plates, there would be no clinically observed differences between the constructs.
Patients and Methods
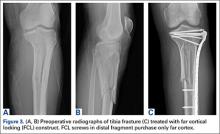
After obtaining Institutional Review Board approval from the 2 level I trauma centers and 1 level II trauma center involved in this study, we retrospectively reviewed the cases of all adults who presented with a tibia fracture and were treated with FCL technology (MotionLoc, Zimmer) by a fellowship-trained trauma surgeon at these hospitals (Figures 3A–3C). Any primary tibia fracture treated with FCL was considered. Only patients with follow-up of at least 20 weeks were included in the analysis. Exclusion criteria were tibial malunions or nonunions treated with FCL and fractures treated with a combination of intramedullary fixation and plating.
We reviewed the patient charts for demographic data, mechanism of injury, fracture type, and comorbidities. Risk factors for poor healing—such as diabetes and tobacco use, either current or prior—were recorded. We also reviewed the radiographs of the initial injuries for analysis of the tibia fracture types (Table 1) as well as the follow-up radiographs for evaluation of fracture healing. Using the Orthopaedic Trauma Association classification system, we identified a variety of fracture patterns. Fracture healing rates were recorded and used to calculate the overall healing rates for each group. Union was defined as either radiographic evidence of a completely healed fracture (≥3 cortices) or radiographic evidence of osseous bridging at the fracture site in addition to full weight-bearing without pain. Infection was defined as positive intraoperative cultures or grossly infected wounds with purulence and erythema.
For statistical analysis, we used Welch 2-sample t test to compare categorical data, including rates of fracture union, infection, and revision surgery. We chose this test because it was unclear whether variance in the groups would be similar. FCL and control data were compared for significant differences by calculating P values. Similarly, for continuous data, Fisher exact test was used to calculate P values for mean time to union and mean time to full weight-bearing in order to compare FCL and control outcomes.
Results
Twelve patients treated at 2 level I and 1 level II trauma centers between November 2010 and May 2012 met the inclusion and exclusion criteria for this study. Another 10 patients were treated with standard plating techniques (control group). Mean age was 52 years (range, 25-72 years) for the FCL group and 46 years (range, 28-67 years) for the control group. The FCL group included 2 open fractures (control, 0) and 2 patients with diabetes (control, 1) (Table 1).
Eleven of the 12 FCL patients and all 10 control patients achieved fracture union by most recent follow-up (Table 2). The difference was not statistically significant (P = .363). The FCL-treated fracture that did not heal received an interfragmentary screw in addition to the standard FCL technology construct. The interfragmentary screw inhibited motion at the fracture site and could potentially have led to nonunion. For this patient, revision surgery to an intramedullary nail was required. Removal of the interfragmentary screw was uneventful. Each of the 2 open fractures in the FCL group required bone grafting because of large segmental bone loss. One of these fractures, a type 3B, became infected after bone grafting, and complete healing required plate removal. The patient was eventually treated with a brace. An infection that occurred after union in a closed tibia fracture in the FCL group required hardware removal. No patient in either group experienced loss or failure of fixation.
Discussion
Far cortical locking is a relatively new technology designed to increase fracture fixation flexibility by functionally lengthening the distance between the locking plate and the screw cortical purchase, which occurs at the far cortex rather than the near cortex. This construct thereby functions as an internal fixator and is functionally similar to an external fixator. Rather than there being bars external to the skin, a plate is placed internally, adjacent to but without compressing fracture fragments or the plate to the bone. This theoretically leads to a desirable amount of interfragmentary motion, promoting callus formation and secondary healing. However, too much motion at the fracture site disrupts healing by shearing proliferating cells attempting to bridge the fracture gap. Therefore, there is a narrow target zone of desirable motion between fracture fragments required to promote secondary bone healing—defined as 2% to 10% gap strain.2 FCL constructs are thought to fall in this range of gap strain and thus better promote secondary healing over standard locked plates. Although biomechanical studies have been used as proof of concept, there are no published clinical data on the effectiveness of FCL implants. The present article describes early data on clinical outcomes of this new type of implant.
The main limitation of this study is its small cohort size, which is largely a result of the short time these implants have been available and our attempt to compare only similar fractures in this analysis. In addition, follow-up was on average less than 1 year. We consider such follow-up acceptable, though, as all fractures essentially reached final healing status within that period. Another limitation is that we combined compression plating and locked plating in the control group. Considering the mechanism of the theoretical advantage of FCL implants, with larger cohorts it would be useful to perform a subanalysis in which compression and standard locking plates are separately compared with FCL implants.
This study found no statistically significant difference between FCL and standard plating, suggesting FCL likely is not inferior to standard plating. Although the FCL group included a nonunion, it is important to note that, in this case, there was a technical discrepancy in the ideal technique whereby another interfragmentary screw was placed, eliminating the interfragmentary motion that establishes the premise of FCL technology. This case thereby demonstrated that a breach in the FCL technique, as with standard locking techniques, may lead to fracture-healing complications. In the FCL group, 2 open fractures with significant segmental bone loss requiring bone graft subsequently healed. In addition, compared with the control group, the FCL group included more patients with diabetes and more tobacco users (both diabetes and tobacco use are associated with poor bone and wound healing). The FCL group was also, on average, 6 years older than the control group. None of these group differences, however, reached statistical significance. Indeed, part of the impetus to use FCL implants in this population was that these patients likely were at higher risk for poor healing and nonunion. This factor therefore represents a selection bias—the FCL group was more predisposed to nonunion—and a study limitation.
Together, our data show neither superiority nor inferiority of the FCL technique. This study is an important step in furthering investigations into FCL constructs. The finding of similar efficacy with FCL and conventional plating may assuage safety concerns and pave the way for more definitive studies of FCL technology and fuller evaluations of its effectiveness. These studies will be essential in determining whether the theoretical advantage of FCL translates into better clinical outcomes. Larger, prospective randomized studies with longer follow-ups will be needed to better compare FCL technology with current implants and techniques. At this early stage, however, FCL technology appears to be a viable option for complex fractures of the tibia.
1. Bernstein J, ed. Musculoskeletal Medicine. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2003.
2. Egol KA, Kubiak EN, Fulkerson E, Kummer FJ, Koval KJ. Biomechanics of locked plates and screws. J Orthop Trauma. 2004;18(8):488-493.
3. Bagby GW. Compression bone-plating: historical considerations. J Bone Joint Surg Am. 1977;59(5):625-631.
4. Kubiak EN, Fulkerson E, Strauss E, Egol KA. The evolution of locked plates. J Bone Joint Surg Am. 2006;88(suppl 4):189-200.
5. Fitzpatrick DC, Doornink J, Madey SM, Bottlang M. Relative stability of conventional and locked plating fixation in a model of the osteoporotic femoral diaphysis. Clin Biomech. 2009;24(2):203-209.
6. Henderson CE, Bottlang M, Marsh JL, Fitzpatrick DC, Madey SM. Does locked plating of periprosthetic supracondylar femur fractures promote bone healing by callus formation? Two cases with opposite outcomes. Iowa Orthop J. 2008;28:73-76.
7. Lujan TJ, Henderson CE, Madey SM, Fitzpatrick DC, Marsh JL, Bottlang M. Locked plating of distal femur fractures leads to inconsistent and asymmetric callus formation. J Orthop Trauma. 2010;24(3):156-162.
8. Stoffel K, Dieter U, Stachowiak G, Gächter A, Kuster MS. Biomechanical testing of the LCP—how can stability in locked internal fixators be controlled? Injury. 2003;34(suppl 2):B11-B19.
9. Schmal H, Strohm PC, Jaeger M, Südkamp NP. Flexible fixation and fracture healing: do locked plating ‘internal fixators’ resemble external fixators? J Orthop Trauma. 2011;25(suppl 1):S15-S20.
10. Bottlang M, Doornink J, Fitzpatrick DC, Madey SM. Far cortical locking can reduce stiffness of locked plating constructs while retaining construct strength. J Bone Joint Surg Am. 2009;91(8):1985-1994.
11. Bottlang M, Lesser M, Koerber J, et al. Far cortical locking can improve healing of fractures stabilized with locking plates. J Bone Joint Surg Am. 2010;92(7):1652-1660.
12. Bottlang M, Feist F. Biomechanics of far cortical locking. J Orthop Trauma. 2011;25(suppl 1):S21-S28.
Fracture healing can be categorized as primary or secondary. Primary healing requires precise reapproximation of bone fragments and compression of cortices. Osteons are formed across the fracture line, allowing blood supply and endothelial cells to gain access, leading to osteoblast infiltration and subsequent bone formation.1 This type of bone healing can be accomplished only with absolute stability—specifically, only with less than 2% strain at the fracture site, necessitating operative intervention with compression plating (Figure 1).2 This type of construct generates friction between the bone fragments against a metal plate, created by tightening screws that purchase both far and near cortices of bone.3 Although this type of fixation works well with many fractures, there are several instances in which compression plating is not ideal.4 Osteoporotic bone, for example, limits the amount of compression that can be developed, as screws strip the bone more readily, leading to weakened constructs prone to failure. Metaphyseal fractures in which there is minimal cortex for screw thread purchase are a similar challenge.5 Highly comminuted fractures do not allow for sufficient fragment compression and stability. In addition, compression plating requires periosteal stripping at the fracture, and often substantial soft-tissue disruption, which is especially a problem in areas of tenuous blood supply (eg, the tibia).
Locked plating therefore has become a valuable technique in managing osteoporotic fractures.2 Locking plates may be used to achieve secondary bone healing through a small amount of interfragmentary motion, 0.2 to 10 mm, as seen with bridge plating for example, whereby the locking plates act as internal fixators. Much as with external fixators, as the distance from the fixator bar (or plate) to bone decreases, construct stiffness increases. Thus, locking plates function as extremely stiff fixators when the plate is very near bone. It has therefore been speculated that such stiffness is insufficient to provide optimal secondary healing conditions.6,7 Titanium (vs stainless steel) plates have been used, and screws have been omitted just adjacent to either side of the fracture site, in attempts to increase plate flexibility and thus interfragmentary motion.8,9 In addition, biomechanical and animal model studies have demonstrated that, with use of locking plates, motion at the fracture site is asymmetric and leads to unequal callus formation at the near and far cortices, thus weakening the fracture site.10,11
The locking plate design was recently modified to address these concerns. Far cortical locking (FCL) uses locking screws threaded only distally (Figure 2), which allows for purchase into the far cortex but not the near cortex, which increases pin length from plate to bone. The near cortex is no longer anchored to the plate and thus increases construct flexibility. Pilot holes in the near cortex allow for movement of the nonthreaded screw shaft in a controlled, biphasic manner.12 This design decreases stiffness while sacrificing very little construct strength.10 In addition, motion at the far and near cortices is nearly parallel. It has been shown in an ovine tibial osteotomy model that, compared with the traditional locking plate design, FCL generates symmetric callus formation and improved fracture healing.11 Although these results are promising, there are only limited clinical data on use of the FCL technique in fracture repair. Our null hypothesis was that, despite the theoretical advantages of FCL constructs over conventional locking plates, there would be no clinically observed differences between the constructs.
Patients and Methods
After obtaining Institutional Review Board approval from the 2 level I trauma centers and 1 level II trauma center involved in this study, we retrospectively reviewed the cases of all adults who presented with a tibia fracture and were treated with FCL technology (MotionLoc, Zimmer) by a fellowship-trained trauma surgeon at these hospitals (Figures 3A–3C). Any primary tibia fracture treated with FCL was considered. Only patients with follow-up of at least 20 weeks were included in the analysis. Exclusion criteria were tibial malunions or nonunions treated with FCL and fractures treated with a combination of intramedullary fixation and plating.
We reviewed the patient charts for demographic data, mechanism of injury, fracture type, and comorbidities. Risk factors for poor healing—such as diabetes and tobacco use, either current or prior—were recorded. We also reviewed the radiographs of the initial injuries for analysis of the tibia fracture types (Table 1) as well as the follow-up radiographs for evaluation of fracture healing. Using the Orthopaedic Trauma Association classification system, we identified a variety of fracture patterns. Fracture healing rates were recorded and used to calculate the overall healing rates for each group. Union was defined as either radiographic evidence of a completely healed fracture (≥3 cortices) or radiographic evidence of osseous bridging at the fracture site in addition to full weight-bearing without pain. Infection was defined as positive intraoperative cultures or grossly infected wounds with purulence and erythema.
For statistical analysis, we used Welch 2-sample t test to compare categorical data, including rates of fracture union, infection, and revision surgery. We chose this test because it was unclear whether variance in the groups would be similar. FCL and control data were compared for significant differences by calculating P values. Similarly, for continuous data, Fisher exact test was used to calculate P values for mean time to union and mean time to full weight-bearing in order to compare FCL and control outcomes.
Results
Twelve patients treated at 2 level I and 1 level II trauma centers between November 2010 and May 2012 met the inclusion and exclusion criteria for this study. Another 10 patients were treated with standard plating techniques (control group). Mean age was 52 years (range, 25-72 years) for the FCL group and 46 years (range, 28-67 years) for the control group. The FCL group included 2 open fractures (control, 0) and 2 patients with diabetes (control, 1) (Table 1).
Eleven of the 12 FCL patients and all 10 control patients achieved fracture union by most recent follow-up (Table 2). The difference was not statistically significant (P = .363). The FCL-treated fracture that did not heal received an interfragmentary screw in addition to the standard FCL technology construct. The interfragmentary screw inhibited motion at the fracture site and could potentially have led to nonunion. For this patient, revision surgery to an intramedullary nail was required. Removal of the interfragmentary screw was uneventful. Each of the 2 open fractures in the FCL group required bone grafting because of large segmental bone loss. One of these fractures, a type 3B, became infected after bone grafting, and complete healing required plate removal. The patient was eventually treated with a brace. An infection that occurred after union in a closed tibia fracture in the FCL group required hardware removal. No patient in either group experienced loss or failure of fixation.
Discussion
Far cortical locking is a relatively new technology designed to increase fracture fixation flexibility by functionally lengthening the distance between the locking plate and the screw cortical purchase, which occurs at the far cortex rather than the near cortex. This construct thereby functions as an internal fixator and is functionally similar to an external fixator. Rather than there being bars external to the skin, a plate is placed internally, adjacent to but without compressing fracture fragments or the plate to the bone. This theoretically leads to a desirable amount of interfragmentary motion, promoting callus formation and secondary healing. However, too much motion at the fracture site disrupts healing by shearing proliferating cells attempting to bridge the fracture gap. Therefore, there is a narrow target zone of desirable motion between fracture fragments required to promote secondary bone healing—defined as 2% to 10% gap strain.2 FCL constructs are thought to fall in this range of gap strain and thus better promote secondary healing over standard locked plates. Although biomechanical studies have been used as proof of concept, there are no published clinical data on the effectiveness of FCL implants. The present article describes early data on clinical outcomes of this new type of implant.
The main limitation of this study is its small cohort size, which is largely a result of the short time these implants have been available and our attempt to compare only similar fractures in this analysis. In addition, follow-up was on average less than 1 year. We consider such follow-up acceptable, though, as all fractures essentially reached final healing status within that period. Another limitation is that we combined compression plating and locked plating in the control group. Considering the mechanism of the theoretical advantage of FCL implants, with larger cohorts it would be useful to perform a subanalysis in which compression and standard locking plates are separately compared with FCL implants.
This study found no statistically significant difference between FCL and standard plating, suggesting FCL likely is not inferior to standard plating. Although the FCL group included a nonunion, it is important to note that, in this case, there was a technical discrepancy in the ideal technique whereby another interfragmentary screw was placed, eliminating the interfragmentary motion that establishes the premise of FCL technology. This case thereby demonstrated that a breach in the FCL technique, as with standard locking techniques, may lead to fracture-healing complications. In the FCL group, 2 open fractures with significant segmental bone loss requiring bone graft subsequently healed. In addition, compared with the control group, the FCL group included more patients with diabetes and more tobacco users (both diabetes and tobacco use are associated with poor bone and wound healing). The FCL group was also, on average, 6 years older than the control group. None of these group differences, however, reached statistical significance. Indeed, part of the impetus to use FCL implants in this population was that these patients likely were at higher risk for poor healing and nonunion. This factor therefore represents a selection bias—the FCL group was more predisposed to nonunion—and a study limitation.
Together, our data show neither superiority nor inferiority of the FCL technique. This study is an important step in furthering investigations into FCL constructs. The finding of similar efficacy with FCL and conventional plating may assuage safety concerns and pave the way for more definitive studies of FCL technology and fuller evaluations of its effectiveness. These studies will be essential in determining whether the theoretical advantage of FCL translates into better clinical outcomes. Larger, prospective randomized studies with longer follow-ups will be needed to better compare FCL technology with current implants and techniques. At this early stage, however, FCL technology appears to be a viable option for complex fractures of the tibia.
Fracture healing can be categorized as primary or secondary. Primary healing requires precise reapproximation of bone fragments and compression of cortices. Osteons are formed across the fracture line, allowing blood supply and endothelial cells to gain access, leading to osteoblast infiltration and subsequent bone formation.1 This type of bone healing can be accomplished only with absolute stability—specifically, only with less than 2% strain at the fracture site, necessitating operative intervention with compression plating (Figure 1).2 This type of construct generates friction between the bone fragments against a metal plate, created by tightening screws that purchase both far and near cortices of bone.3 Although this type of fixation works well with many fractures, there are several instances in which compression plating is not ideal.4 Osteoporotic bone, for example, limits the amount of compression that can be developed, as screws strip the bone more readily, leading to weakened constructs prone to failure. Metaphyseal fractures in which there is minimal cortex for screw thread purchase are a similar challenge.5 Highly comminuted fractures do not allow for sufficient fragment compression and stability. In addition, compression plating requires periosteal stripping at the fracture, and often substantial soft-tissue disruption, which is especially a problem in areas of tenuous blood supply (eg, the tibia).
Locked plating therefore has become a valuable technique in managing osteoporotic fractures.2 Locking plates may be used to achieve secondary bone healing through a small amount of interfragmentary motion, 0.2 to 10 mm, as seen with bridge plating for example, whereby the locking plates act as internal fixators. Much as with external fixators, as the distance from the fixator bar (or plate) to bone decreases, construct stiffness increases. Thus, locking plates function as extremely stiff fixators when the plate is very near bone. It has therefore been speculated that such stiffness is insufficient to provide optimal secondary healing conditions.6,7 Titanium (vs stainless steel) plates have been used, and screws have been omitted just adjacent to either side of the fracture site, in attempts to increase plate flexibility and thus interfragmentary motion.8,9 In addition, biomechanical and animal model studies have demonstrated that, with use of locking plates, motion at the fracture site is asymmetric and leads to unequal callus formation at the near and far cortices, thus weakening the fracture site.10,11
The locking plate design was recently modified to address these concerns. Far cortical locking (FCL) uses locking screws threaded only distally (Figure 2), which allows for purchase into the far cortex but not the near cortex, which increases pin length from plate to bone. The near cortex is no longer anchored to the plate and thus increases construct flexibility. Pilot holes in the near cortex allow for movement of the nonthreaded screw shaft in a controlled, biphasic manner.12 This design decreases stiffness while sacrificing very little construct strength.10 In addition, motion at the far and near cortices is nearly parallel. It has been shown in an ovine tibial osteotomy model that, compared with the traditional locking plate design, FCL generates symmetric callus formation and improved fracture healing.11 Although these results are promising, there are only limited clinical data on use of the FCL technique in fracture repair. Our null hypothesis was that, despite the theoretical advantages of FCL constructs over conventional locking plates, there would be no clinically observed differences between the constructs.
Patients and Methods
After obtaining Institutional Review Board approval from the 2 level I trauma centers and 1 level II trauma center involved in this study, we retrospectively reviewed the cases of all adults who presented with a tibia fracture and were treated with FCL technology (MotionLoc, Zimmer) by a fellowship-trained trauma surgeon at these hospitals (Figures 3A–3C). Any primary tibia fracture treated with FCL was considered. Only patients with follow-up of at least 20 weeks were included in the analysis. Exclusion criteria were tibial malunions or nonunions treated with FCL and fractures treated with a combination of intramedullary fixation and plating.
We reviewed the patient charts for demographic data, mechanism of injury, fracture type, and comorbidities. Risk factors for poor healing—such as diabetes and tobacco use, either current or prior—were recorded. We also reviewed the radiographs of the initial injuries for analysis of the tibia fracture types (Table 1) as well as the follow-up radiographs for evaluation of fracture healing. Using the Orthopaedic Trauma Association classification system, we identified a variety of fracture patterns. Fracture healing rates were recorded and used to calculate the overall healing rates for each group. Union was defined as either radiographic evidence of a completely healed fracture (≥3 cortices) or radiographic evidence of osseous bridging at the fracture site in addition to full weight-bearing without pain. Infection was defined as positive intraoperative cultures or grossly infected wounds with purulence and erythema.
For statistical analysis, we used Welch 2-sample t test to compare categorical data, including rates of fracture union, infection, and revision surgery. We chose this test because it was unclear whether variance in the groups would be similar. FCL and control data were compared for significant differences by calculating P values. Similarly, for continuous data, Fisher exact test was used to calculate P values for mean time to union and mean time to full weight-bearing in order to compare FCL and control outcomes.
Results
Twelve patients treated at 2 level I and 1 level II trauma centers between November 2010 and May 2012 met the inclusion and exclusion criteria for this study. Another 10 patients were treated with standard plating techniques (control group). Mean age was 52 years (range, 25-72 years) for the FCL group and 46 years (range, 28-67 years) for the control group. The FCL group included 2 open fractures (control, 0) and 2 patients with diabetes (control, 1) (Table 1).
Eleven of the 12 FCL patients and all 10 control patients achieved fracture union by most recent follow-up (Table 2). The difference was not statistically significant (P = .363). The FCL-treated fracture that did not heal received an interfragmentary screw in addition to the standard FCL technology construct. The interfragmentary screw inhibited motion at the fracture site and could potentially have led to nonunion. For this patient, revision surgery to an intramedullary nail was required. Removal of the interfragmentary screw was uneventful. Each of the 2 open fractures in the FCL group required bone grafting because of large segmental bone loss. One of these fractures, a type 3B, became infected after bone grafting, and complete healing required plate removal. The patient was eventually treated with a brace. An infection that occurred after union in a closed tibia fracture in the FCL group required hardware removal. No patient in either group experienced loss or failure of fixation.
Discussion
Far cortical locking is a relatively new technology designed to increase fracture fixation flexibility by functionally lengthening the distance between the locking plate and the screw cortical purchase, which occurs at the far cortex rather than the near cortex. This construct thereby functions as an internal fixator and is functionally similar to an external fixator. Rather than there being bars external to the skin, a plate is placed internally, adjacent to but without compressing fracture fragments or the plate to the bone. This theoretically leads to a desirable amount of interfragmentary motion, promoting callus formation and secondary healing. However, too much motion at the fracture site disrupts healing by shearing proliferating cells attempting to bridge the fracture gap. Therefore, there is a narrow target zone of desirable motion between fracture fragments required to promote secondary bone healing—defined as 2% to 10% gap strain.2 FCL constructs are thought to fall in this range of gap strain and thus better promote secondary healing over standard locked plates. Although biomechanical studies have been used as proof of concept, there are no published clinical data on the effectiveness of FCL implants. The present article describes early data on clinical outcomes of this new type of implant.
The main limitation of this study is its small cohort size, which is largely a result of the short time these implants have been available and our attempt to compare only similar fractures in this analysis. In addition, follow-up was on average less than 1 year. We consider such follow-up acceptable, though, as all fractures essentially reached final healing status within that period. Another limitation is that we combined compression plating and locked plating in the control group. Considering the mechanism of the theoretical advantage of FCL implants, with larger cohorts it would be useful to perform a subanalysis in which compression and standard locking plates are separately compared with FCL implants.
This study found no statistically significant difference between FCL and standard plating, suggesting FCL likely is not inferior to standard plating. Although the FCL group included a nonunion, it is important to note that, in this case, there was a technical discrepancy in the ideal technique whereby another interfragmentary screw was placed, eliminating the interfragmentary motion that establishes the premise of FCL technology. This case thereby demonstrated that a breach in the FCL technique, as with standard locking techniques, may lead to fracture-healing complications. In the FCL group, 2 open fractures with significant segmental bone loss requiring bone graft subsequently healed. In addition, compared with the control group, the FCL group included more patients with diabetes and more tobacco users (both diabetes and tobacco use are associated with poor bone and wound healing). The FCL group was also, on average, 6 years older than the control group. None of these group differences, however, reached statistical significance. Indeed, part of the impetus to use FCL implants in this population was that these patients likely were at higher risk for poor healing and nonunion. This factor therefore represents a selection bias—the FCL group was more predisposed to nonunion—and a study limitation.
Together, our data show neither superiority nor inferiority of the FCL technique. This study is an important step in furthering investigations into FCL constructs. The finding of similar efficacy with FCL and conventional plating may assuage safety concerns and pave the way for more definitive studies of FCL technology and fuller evaluations of its effectiveness. These studies will be essential in determining whether the theoretical advantage of FCL translates into better clinical outcomes. Larger, prospective randomized studies with longer follow-ups will be needed to better compare FCL technology with current implants and techniques. At this early stage, however, FCL technology appears to be a viable option for complex fractures of the tibia.
1. Bernstein J, ed. Musculoskeletal Medicine. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2003.
2. Egol KA, Kubiak EN, Fulkerson E, Kummer FJ, Koval KJ. Biomechanics of locked plates and screws. J Orthop Trauma. 2004;18(8):488-493.
3. Bagby GW. Compression bone-plating: historical considerations. J Bone Joint Surg Am. 1977;59(5):625-631.
4. Kubiak EN, Fulkerson E, Strauss E, Egol KA. The evolution of locked plates. J Bone Joint Surg Am. 2006;88(suppl 4):189-200.
5. Fitzpatrick DC, Doornink J, Madey SM, Bottlang M. Relative stability of conventional and locked plating fixation in a model of the osteoporotic femoral diaphysis. Clin Biomech. 2009;24(2):203-209.
6. Henderson CE, Bottlang M, Marsh JL, Fitzpatrick DC, Madey SM. Does locked plating of periprosthetic supracondylar femur fractures promote bone healing by callus formation? Two cases with opposite outcomes. Iowa Orthop J. 2008;28:73-76.
7. Lujan TJ, Henderson CE, Madey SM, Fitzpatrick DC, Marsh JL, Bottlang M. Locked plating of distal femur fractures leads to inconsistent and asymmetric callus formation. J Orthop Trauma. 2010;24(3):156-162.
8. Stoffel K, Dieter U, Stachowiak G, Gächter A, Kuster MS. Biomechanical testing of the LCP—how can stability in locked internal fixators be controlled? Injury. 2003;34(suppl 2):B11-B19.
9. Schmal H, Strohm PC, Jaeger M, Südkamp NP. Flexible fixation and fracture healing: do locked plating ‘internal fixators’ resemble external fixators? J Orthop Trauma. 2011;25(suppl 1):S15-S20.
10. Bottlang M, Doornink J, Fitzpatrick DC, Madey SM. Far cortical locking can reduce stiffness of locked plating constructs while retaining construct strength. J Bone Joint Surg Am. 2009;91(8):1985-1994.
11. Bottlang M, Lesser M, Koerber J, et al. Far cortical locking can improve healing of fractures stabilized with locking plates. J Bone Joint Surg Am. 2010;92(7):1652-1660.
12. Bottlang M, Feist F. Biomechanics of far cortical locking. J Orthop Trauma. 2011;25(suppl 1):S21-S28.
1. Bernstein J, ed. Musculoskeletal Medicine. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2003.
2. Egol KA, Kubiak EN, Fulkerson E, Kummer FJ, Koval KJ. Biomechanics of locked plates and screws. J Orthop Trauma. 2004;18(8):488-493.
3. Bagby GW. Compression bone-plating: historical considerations. J Bone Joint Surg Am. 1977;59(5):625-631.
4. Kubiak EN, Fulkerson E, Strauss E, Egol KA. The evolution of locked plates. J Bone Joint Surg Am. 2006;88(suppl 4):189-200.
5. Fitzpatrick DC, Doornink J, Madey SM, Bottlang M. Relative stability of conventional and locked plating fixation in a model of the osteoporotic femoral diaphysis. Clin Biomech. 2009;24(2):203-209.
6. Henderson CE, Bottlang M, Marsh JL, Fitzpatrick DC, Madey SM. Does locked plating of periprosthetic supracondylar femur fractures promote bone healing by callus formation? Two cases with opposite outcomes. Iowa Orthop J. 2008;28:73-76.
7. Lujan TJ, Henderson CE, Madey SM, Fitzpatrick DC, Marsh JL, Bottlang M. Locked plating of distal femur fractures leads to inconsistent and asymmetric callus formation. J Orthop Trauma. 2010;24(3):156-162.
8. Stoffel K, Dieter U, Stachowiak G, Gächter A, Kuster MS. Biomechanical testing of the LCP—how can stability in locked internal fixators be controlled? Injury. 2003;34(suppl 2):B11-B19.
9. Schmal H, Strohm PC, Jaeger M, Südkamp NP. Flexible fixation and fracture healing: do locked plating ‘internal fixators’ resemble external fixators? J Orthop Trauma. 2011;25(suppl 1):S15-S20.
10. Bottlang M, Doornink J, Fitzpatrick DC, Madey SM. Far cortical locking can reduce stiffness of locked plating constructs while retaining construct strength. J Bone Joint Surg Am. 2009;91(8):1985-1994.
11. Bottlang M, Lesser M, Koerber J, et al. Far cortical locking can improve healing of fractures stabilized with locking plates. J Bone Joint Surg Am. 2010;92(7):1652-1660.
12. Bottlang M, Feist F. Biomechanics of far cortical locking. J Orthop Trauma. 2011;25(suppl 1):S21-S28.