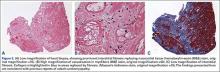
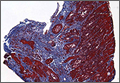
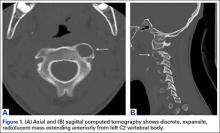
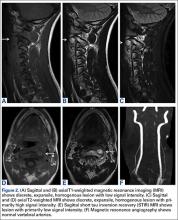
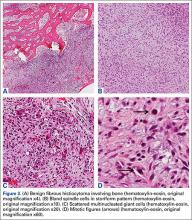
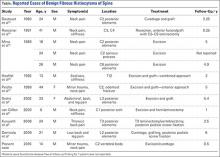
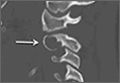
User login
Novel drug fails to prevent contrast-induced nephropathy
CHICAGO – CMX-2043, a novel agent intended for prevention of contrast-induced nephropathy, failed in the phase II, double-blind, placebo-controlled CARIN clinical trial presented at the annual meeting of the American College of Cardiology.
The drug had also shown promise in small preliminary studies for the prevention of periprocedural myocardial infarction in patients undergoing coronary stenting. There again, however, CMX-2043 – a derivative of alpha lipoic acid with antioxidant and cell membrane–stabilizing properties – proved ineffective in the 361-patient, 31-center phase II trial, reported Dr. Deepak L. Bhatt, professor of medicine at Harvard Medical School and executive director of interventional cardiovascular programs at Brigham and Women’s Hospital, both in Boston.
All participants in CARIN had baseline severe impairment of kidney function or mild to moderate renal impairment plus another risk factor, such as diabetes or age greater than 75 years. One hour prior to coronary angiography, they received various doses of CMX-2043 or placebo.
Unfortunately, no difference between the four treatment arms was present in terms of the primary study endpoint: the incidence of acute kidney injury as defined by at least a 0.3 mg/dL rise in serum creatinine from baseline on day 4. No dose response to CMX-2043 was evident, nor did the investigational agent have any impact on the risk of major adverse cardiovascular events.
Immediately prior to Dr. Bhatt’s presentation, Dr. Michelle L. O’Donoghue of Brigham and Women’s Hospital presented the equally negative results of the LATITUDE-TIMI 60 trial, a phase III trial of the investigational mitogen-activated protein kinase inhibitor losmapimod, a drug developed to improve outcomes in patients with an acute coronary syndrome.
“It’s a bit distressing” to witness back to back presentations of clinical trials that proved resoundingly negative despite very strong-looking preliminary data, commented discussant Dr. Anthony N. DeMaria, professor of medicine at the University of California, San Diego. What’s going on here? he asked.
“I think it’s a fundamental truth that a lot of things that look good in preclinical work, even when backed up by a lot of solid science, don’t pan out in human studies,” Dr. Bhatt replied. “That’s a challenge, and probably in no other arena more so than in tackling inflammation and antioxidant therapy.
“There’s a graveyard of compounds that have not worked, and now we’ve perhaps added another one,” Dr. Bhatt continued. “But it doesn’t mean that scientific inquiry isn’t important, because I think eventually we’ll have drugs for these problems, whether it’s reperfusion injury or contrast-induced nephropathy. It’ll probably just take a lot more time and effort.”
The one solace regarding the CARIN trial, in Dr. Bhatt’s view, is that it highlighted the advantages of what is known as an adaptive trial design. Instead of jumping from positive early-phase results straight to a definitive 10,000-patient phase III clinical trial, investigators were able to obtain answers regarding the drug’s ability to prevent two major problems in patients undergoing coronary angiography – contrast-induced nephropathy and major adverse cardiac events – by means of a single 361-patient trial that was comparatively inexpensive.
Acute kidney injury secondary to exposure to contrast agents remains a significant problem, with an incidence of 20%-25% in high-risk patients. Numerous proposed prophylactic agents have ultimately proved not useful, including sodium bicarbonate, N-acetylcysteine, and intravenous fenoldopam.
Indeed, the only preventive measures of proven effectiveness are hydration with saline for 12 hours preangioplasty, and limiting the volume of contrast agent used. In real-world clinical practice, however, it’s often impractical to administer the optimal 12 hours of saline because of hospital pressure to get patients out quickly, Dr. Bhatt observed.
“There remains an important unmet clinical need to find agents that reduce the occurrence of contrast nephropathy,” he stressed.
Ischemix funded the CARIN trial. Dr. Bhatt reported receiving a research grant from the company that was directed to Brigham and Women’s Hospital.
CHICAGO – CMX-2043, a novel agent intended for prevention of contrast-induced nephropathy, failed in the phase II, double-blind, placebo-controlled CARIN clinical trial presented at the annual meeting of the American College of Cardiology.
The drug had also shown promise in small preliminary studies for the prevention of periprocedural myocardial infarction in patients undergoing coronary stenting. There again, however, CMX-2043 – a derivative of alpha lipoic acid with antioxidant and cell membrane–stabilizing properties – proved ineffective in the 361-patient, 31-center phase II trial, reported Dr. Deepak L. Bhatt, professor of medicine at Harvard Medical School and executive director of interventional cardiovascular programs at Brigham and Women’s Hospital, both in Boston.
All participants in CARIN had baseline severe impairment of kidney function or mild to moderate renal impairment plus another risk factor, such as diabetes or age greater than 75 years. One hour prior to coronary angiography, they received various doses of CMX-2043 or placebo.
Unfortunately, no difference between the four treatment arms was present in terms of the primary study endpoint: the incidence of acute kidney injury as defined by at least a 0.3 mg/dL rise in serum creatinine from baseline on day 4. No dose response to CMX-2043 was evident, nor did the investigational agent have any impact on the risk of major adverse cardiovascular events.
Immediately prior to Dr. Bhatt’s presentation, Dr. Michelle L. O’Donoghue of Brigham and Women’s Hospital presented the equally negative results of the LATITUDE-TIMI 60 trial, a phase III trial of the investigational mitogen-activated protein kinase inhibitor losmapimod, a drug developed to improve outcomes in patients with an acute coronary syndrome.
“It’s a bit distressing” to witness back to back presentations of clinical trials that proved resoundingly negative despite very strong-looking preliminary data, commented discussant Dr. Anthony N. DeMaria, professor of medicine at the University of California, San Diego. What’s going on here? he asked.
“I think it’s a fundamental truth that a lot of things that look good in preclinical work, even when backed up by a lot of solid science, don’t pan out in human studies,” Dr. Bhatt replied. “That’s a challenge, and probably in no other arena more so than in tackling inflammation and antioxidant therapy.
“There’s a graveyard of compounds that have not worked, and now we’ve perhaps added another one,” Dr. Bhatt continued. “But it doesn’t mean that scientific inquiry isn’t important, because I think eventually we’ll have drugs for these problems, whether it’s reperfusion injury or contrast-induced nephropathy. It’ll probably just take a lot more time and effort.”
The one solace regarding the CARIN trial, in Dr. Bhatt’s view, is that it highlighted the advantages of what is known as an adaptive trial design. Instead of jumping from positive early-phase results straight to a definitive 10,000-patient phase III clinical trial, investigators were able to obtain answers regarding the drug’s ability to prevent two major problems in patients undergoing coronary angiography – contrast-induced nephropathy and major adverse cardiac events – by means of a single 361-patient trial that was comparatively inexpensive.
Acute kidney injury secondary to exposure to contrast agents remains a significant problem, with an incidence of 20%-25% in high-risk patients. Numerous proposed prophylactic agents have ultimately proved not useful, including sodium bicarbonate, N-acetylcysteine, and intravenous fenoldopam.
Indeed, the only preventive measures of proven effectiveness are hydration with saline for 12 hours preangioplasty, and limiting the volume of contrast agent used. In real-world clinical practice, however, it’s often impractical to administer the optimal 12 hours of saline because of hospital pressure to get patients out quickly, Dr. Bhatt observed.
“There remains an important unmet clinical need to find agents that reduce the occurrence of contrast nephropathy,” he stressed.
Ischemix funded the CARIN trial. Dr. Bhatt reported receiving a research grant from the company that was directed to Brigham and Women’s Hospital.
CHICAGO – CMX-2043, a novel agent intended for prevention of contrast-induced nephropathy, failed in the phase II, double-blind, placebo-controlled CARIN clinical trial presented at the annual meeting of the American College of Cardiology.
The drug had also shown promise in small preliminary studies for the prevention of periprocedural myocardial infarction in patients undergoing coronary stenting. There again, however, CMX-2043 – a derivative of alpha lipoic acid with antioxidant and cell membrane–stabilizing properties – proved ineffective in the 361-patient, 31-center phase II trial, reported Dr. Deepak L. Bhatt, professor of medicine at Harvard Medical School and executive director of interventional cardiovascular programs at Brigham and Women’s Hospital, both in Boston.
All participants in CARIN had baseline severe impairment of kidney function or mild to moderate renal impairment plus another risk factor, such as diabetes or age greater than 75 years. One hour prior to coronary angiography, they received various doses of CMX-2043 or placebo.
Unfortunately, no difference between the four treatment arms was present in terms of the primary study endpoint: the incidence of acute kidney injury as defined by at least a 0.3 mg/dL rise in serum creatinine from baseline on day 4. No dose response to CMX-2043 was evident, nor did the investigational agent have any impact on the risk of major adverse cardiovascular events.
Immediately prior to Dr. Bhatt’s presentation, Dr. Michelle L. O’Donoghue of Brigham and Women’s Hospital presented the equally negative results of the LATITUDE-TIMI 60 trial, a phase III trial of the investigational mitogen-activated protein kinase inhibitor losmapimod, a drug developed to improve outcomes in patients with an acute coronary syndrome.
“It’s a bit distressing” to witness back to back presentations of clinical trials that proved resoundingly negative despite very strong-looking preliminary data, commented discussant Dr. Anthony N. DeMaria, professor of medicine at the University of California, San Diego. What’s going on here? he asked.
“I think it’s a fundamental truth that a lot of things that look good in preclinical work, even when backed up by a lot of solid science, don’t pan out in human studies,” Dr. Bhatt replied. “That’s a challenge, and probably in no other arena more so than in tackling inflammation and antioxidant therapy.
“There’s a graveyard of compounds that have not worked, and now we’ve perhaps added another one,” Dr. Bhatt continued. “But it doesn’t mean that scientific inquiry isn’t important, because I think eventually we’ll have drugs for these problems, whether it’s reperfusion injury or contrast-induced nephropathy. It’ll probably just take a lot more time and effort.”
The one solace regarding the CARIN trial, in Dr. Bhatt’s view, is that it highlighted the advantages of what is known as an adaptive trial design. Instead of jumping from positive early-phase results straight to a definitive 10,000-patient phase III clinical trial, investigators were able to obtain answers regarding the drug’s ability to prevent two major problems in patients undergoing coronary angiography – contrast-induced nephropathy and major adverse cardiac events – by means of a single 361-patient trial that was comparatively inexpensive.
Acute kidney injury secondary to exposure to contrast agents remains a significant problem, with an incidence of 20%-25% in high-risk patients. Numerous proposed prophylactic agents have ultimately proved not useful, including sodium bicarbonate, N-acetylcysteine, and intravenous fenoldopam.
Indeed, the only preventive measures of proven effectiveness are hydration with saline for 12 hours preangioplasty, and limiting the volume of contrast agent used. In real-world clinical practice, however, it’s often impractical to administer the optimal 12 hours of saline because of hospital pressure to get patients out quickly, Dr. Bhatt observed.
“There remains an important unmet clinical need to find agents that reduce the occurrence of contrast nephropathy,” he stressed.
Ischemix funded the CARIN trial. Dr. Bhatt reported receiving a research grant from the company that was directed to Brigham and Women’s Hospital.
AT ACC 16
Key clinical point: There continues to be a major unmet need for agents that reduce the risk of contrast-induced nephropathy.
Major finding: The once-promising investigational antioxidant and cell membrane stabilizer CMX-2043 proved ineffective for prevention of renal or cardiac injuries in patients undergoing coronary angiography.
Data source: This randomized, double-blind, placebo-controlled, 31-center, phase II study involved 361 patients with baseline renal impairment, all of whom were scheduled for coronary angiography.
Disclosures: Ischemix funded the study. Dr. Bhatt reported receiving a research grant from the company that was directed to Brigham and Women’s Hospital.
Three things hospitalists ‘do for no reason’... and should stop
SAN DIEGO – Head CTs for patients with in-hospital delirium. Ammonia tests to check for hepatic encephalopathy in chronic liver disease. Renal ultrasounds for acute kidney injury.
Those are three low value tests highlighted in hospitalist Dr. Leonard Feldman’s latest iteration of his lecture series “Things We Do for No Reason.”
Dr. Feldman, associate professor of internal medicine and pediatrics at Johns Hopkins University, Baltimore, has presented his list of usually unnecessary hospitalist practices for five years at the Society of Hospital Medicine’s annual meetings. With three new ones explained during the 2016 meeting, there are now 19 on the list and more to come, he said.
“So far, I’ve picked things that are relatively low-hanging fruit, things for which there’s good evidence we shouldn’t be doing and if you saw the evidence, you’d say ‘that’s right, we shouldn’t,’” he said.
Dr. Feldman’s intent is to help clinicians stop certain “learned behaviors,” tests and procedures which research and experience now show “are not helping people, sometimes harm people, and often result in a cascade” of further unnecessary tests and care.
The conference presentations have been so popular, the Journal of Hospital Medicine in October 2015 started a “Things We Do for No Reason” series.
Here are the three most recent tests hospitalists should avoid:
Ammonia levels for chronic liver disease
Dr. Feldman said doctors were taught in medical school that ammonia levels rise in patients with cirrhosis and when they rise too high, the patient may develop hepatic encephalopathy. They also learned that if levels are normal, the patient should not have hepatic encephalopathy.
But a number of studies have found “neither of those is true,” he said. What’s possibly worse is that “you close your mind to other possible diagnoses way too early.” Nevertheless, the practice at many hospitals is to perform multiple tests to trend those levels.”
“I had a patient who had an ammonia test sent the other day while in the emergency room, and it was elevated,” Dr. Feldman recalled in a recent phone interview. “The patient got admitted, but when we re-tested, it wasn’t.”
Part of the problem is that blood samples are often incorrectly processed. “When you draw the blood, you have to put it on ice and it needs to get to the lab very quickly. And I think we do neither of those things on a regular basis,” he said. Also, if the patient has a tourniquet or is clenching a fist, use of muscle creates ammonia.
Dr. Feldman said that at a hospital like Johns Hopkins in Baltimore, where there are high rates of hepatitis C, there might be 50 patients with chronic liver disease, or 20% of patients on medicine service. It’s not the cost of the blood test that he’s worried about because that’s probably minimal. Rather, it’s the test’s downstream provocation of more unnecessary care “and missed opportunities to intervene with a treatable diagnosis.”
In general, he said, “for patients with chronic liver disease, we shouldn’t be checking ammonia.”
Head CTs for inpatients with new onset delirium
Performing a costly head CT scan on a patient who presents in the emergency department with delirium is appropriate. But for low-risk patients who develop delirium inside the hospital without a clear reason, such as a fall or focal neurologic symptoms suggesting a stroke, a head CT is probably not necessary, Dr. Feldman said.
“But we have this knee-jerk reaction, this reflex, that when a patient becomes delirious, we probably should run a head CT on them,” he added.
Dr. Feldman acknowledged that the frequency of head CTs on inpatients with delirium has been hard to tease out.
“But all the studies indicate that patients who develop delirium while in the hospital, without any sort of risk factor, are very unlikely to have pathology found on a head CT,” he said, noting that the cause of their delirium is likely something else, like dehydration, an infection, disruption of sleep, urinary retention, or medication effect.
Of course, if patients aren’t getting better without the CT, order the CT, he said. “Even if the patient has no risk factor, there’s still a 3% chance of having an abnormality like a tumor or stroke.”
Renal ultrasound for patients with new acute kidney injury
To determine if an acute kidney injury is caused by a treatable obstruction, such as a large prostate causing urinary retention, doctors often first order a renal ultrasound, a test that can cost $300, and must be read by a radiologist.
But a much less expensive simple bladder scan, which can be performed by a nurse, is a much better substitute for the first pass, Dr. Feldman said. He said it’s logical that “a bladder scan is a much higher value test” in the early diagnostic process.
“The studies have been pretty clear. If you don’t have risk factors for having an obstruction, a history of kidney stones, it hasn’t happened before, or other reasons kidneys aren’t working, it’s extraordinarily unlikely you’re going to find anything on that renal ultrasound that could be intervened to fix that acute kidney injury,” Dr. Feldman said. He pointed to a study that found 223 renal ultrasounds were necessary to find one patient who needed an intervention.
“You can probably get a good sense from the history and physical” and start to treat them, he said, and if they’re not getting better, then order the ultrasound.
Each of the items on Feldman’s list don’t necessarily save a lot of money, but they add up. “The more we ask ‘Why are we doing this? Can we stop it if it’s not helping people, and particularly if it’s harming people?’ the more we can prevent the cascade that happens because you did one unnecessary diagnostic test,” he concluded.
SAN DIEGO – Head CTs for patients with in-hospital delirium. Ammonia tests to check for hepatic encephalopathy in chronic liver disease. Renal ultrasounds for acute kidney injury.
Those are three low value tests highlighted in hospitalist Dr. Leonard Feldman’s latest iteration of his lecture series “Things We Do for No Reason.”
Dr. Feldman, associate professor of internal medicine and pediatrics at Johns Hopkins University, Baltimore, has presented his list of usually unnecessary hospitalist practices for five years at the Society of Hospital Medicine’s annual meetings. With three new ones explained during the 2016 meeting, there are now 19 on the list and more to come, he said.
“So far, I’ve picked things that are relatively low-hanging fruit, things for which there’s good evidence we shouldn’t be doing and if you saw the evidence, you’d say ‘that’s right, we shouldn’t,’” he said.
Dr. Feldman’s intent is to help clinicians stop certain “learned behaviors,” tests and procedures which research and experience now show “are not helping people, sometimes harm people, and often result in a cascade” of further unnecessary tests and care.
The conference presentations have been so popular, the Journal of Hospital Medicine in October 2015 started a “Things We Do for No Reason” series.
Here are the three most recent tests hospitalists should avoid:
Ammonia levels for chronic liver disease
Dr. Feldman said doctors were taught in medical school that ammonia levels rise in patients with cirrhosis and when they rise too high, the patient may develop hepatic encephalopathy. They also learned that if levels are normal, the patient should not have hepatic encephalopathy.
But a number of studies have found “neither of those is true,” he said. What’s possibly worse is that “you close your mind to other possible diagnoses way too early.” Nevertheless, the practice at many hospitals is to perform multiple tests to trend those levels.”
“I had a patient who had an ammonia test sent the other day while in the emergency room, and it was elevated,” Dr. Feldman recalled in a recent phone interview. “The patient got admitted, but when we re-tested, it wasn’t.”
Part of the problem is that blood samples are often incorrectly processed. “When you draw the blood, you have to put it on ice and it needs to get to the lab very quickly. And I think we do neither of those things on a regular basis,” he said. Also, if the patient has a tourniquet or is clenching a fist, use of muscle creates ammonia.
Dr. Feldman said that at a hospital like Johns Hopkins in Baltimore, where there are high rates of hepatitis C, there might be 50 patients with chronic liver disease, or 20% of patients on medicine service. It’s not the cost of the blood test that he’s worried about because that’s probably minimal. Rather, it’s the test’s downstream provocation of more unnecessary care “and missed opportunities to intervene with a treatable diagnosis.”
In general, he said, “for patients with chronic liver disease, we shouldn’t be checking ammonia.”
Head CTs for inpatients with new onset delirium
Performing a costly head CT scan on a patient who presents in the emergency department with delirium is appropriate. But for low-risk patients who develop delirium inside the hospital without a clear reason, such as a fall or focal neurologic symptoms suggesting a stroke, a head CT is probably not necessary, Dr. Feldman said.
“But we have this knee-jerk reaction, this reflex, that when a patient becomes delirious, we probably should run a head CT on them,” he added.
Dr. Feldman acknowledged that the frequency of head CTs on inpatients with delirium has been hard to tease out.
“But all the studies indicate that patients who develop delirium while in the hospital, without any sort of risk factor, are very unlikely to have pathology found on a head CT,” he said, noting that the cause of their delirium is likely something else, like dehydration, an infection, disruption of sleep, urinary retention, or medication effect.
Of course, if patients aren’t getting better without the CT, order the CT, he said. “Even if the patient has no risk factor, there’s still a 3% chance of having an abnormality like a tumor or stroke.”
Renal ultrasound for patients with new acute kidney injury
To determine if an acute kidney injury is caused by a treatable obstruction, such as a large prostate causing urinary retention, doctors often first order a renal ultrasound, a test that can cost $300, and must be read by a radiologist.
But a much less expensive simple bladder scan, which can be performed by a nurse, is a much better substitute for the first pass, Dr. Feldman said. He said it’s logical that “a bladder scan is a much higher value test” in the early diagnostic process.
“The studies have been pretty clear. If you don’t have risk factors for having an obstruction, a history of kidney stones, it hasn’t happened before, or other reasons kidneys aren’t working, it’s extraordinarily unlikely you’re going to find anything on that renal ultrasound that could be intervened to fix that acute kidney injury,” Dr. Feldman said. He pointed to a study that found 223 renal ultrasounds were necessary to find one patient who needed an intervention.
“You can probably get a good sense from the history and physical” and start to treat them, he said, and if they’re not getting better, then order the ultrasound.
Each of the items on Feldman’s list don’t necessarily save a lot of money, but they add up. “The more we ask ‘Why are we doing this? Can we stop it if it’s not helping people, and particularly if it’s harming people?’ the more we can prevent the cascade that happens because you did one unnecessary diagnostic test,” he concluded.
SAN DIEGO – Head CTs for patients with in-hospital delirium. Ammonia tests to check for hepatic encephalopathy in chronic liver disease. Renal ultrasounds for acute kidney injury.
Those are three low value tests highlighted in hospitalist Dr. Leonard Feldman’s latest iteration of his lecture series “Things We Do for No Reason.”
Dr. Feldman, associate professor of internal medicine and pediatrics at Johns Hopkins University, Baltimore, has presented his list of usually unnecessary hospitalist practices for five years at the Society of Hospital Medicine’s annual meetings. With three new ones explained during the 2016 meeting, there are now 19 on the list and more to come, he said.
“So far, I’ve picked things that are relatively low-hanging fruit, things for which there’s good evidence we shouldn’t be doing and if you saw the evidence, you’d say ‘that’s right, we shouldn’t,’” he said.
Dr. Feldman’s intent is to help clinicians stop certain “learned behaviors,” tests and procedures which research and experience now show “are not helping people, sometimes harm people, and often result in a cascade” of further unnecessary tests and care.
The conference presentations have been so popular, the Journal of Hospital Medicine in October 2015 started a “Things We Do for No Reason” series.
Here are the three most recent tests hospitalists should avoid:
Ammonia levels for chronic liver disease
Dr. Feldman said doctors were taught in medical school that ammonia levels rise in patients with cirrhosis and when they rise too high, the patient may develop hepatic encephalopathy. They also learned that if levels are normal, the patient should not have hepatic encephalopathy.
But a number of studies have found “neither of those is true,” he said. What’s possibly worse is that “you close your mind to other possible diagnoses way too early.” Nevertheless, the practice at many hospitals is to perform multiple tests to trend those levels.”
“I had a patient who had an ammonia test sent the other day while in the emergency room, and it was elevated,” Dr. Feldman recalled in a recent phone interview. “The patient got admitted, but when we re-tested, it wasn’t.”
Part of the problem is that blood samples are often incorrectly processed. “When you draw the blood, you have to put it on ice and it needs to get to the lab very quickly. And I think we do neither of those things on a regular basis,” he said. Also, if the patient has a tourniquet or is clenching a fist, use of muscle creates ammonia.
Dr. Feldman said that at a hospital like Johns Hopkins in Baltimore, where there are high rates of hepatitis C, there might be 50 patients with chronic liver disease, or 20% of patients on medicine service. It’s not the cost of the blood test that he’s worried about because that’s probably minimal. Rather, it’s the test’s downstream provocation of more unnecessary care “and missed opportunities to intervene with a treatable diagnosis.”
In general, he said, “for patients with chronic liver disease, we shouldn’t be checking ammonia.”
Head CTs for inpatients with new onset delirium
Performing a costly head CT scan on a patient who presents in the emergency department with delirium is appropriate. But for low-risk patients who develop delirium inside the hospital without a clear reason, such as a fall or focal neurologic symptoms suggesting a stroke, a head CT is probably not necessary, Dr. Feldman said.
“But we have this knee-jerk reaction, this reflex, that when a patient becomes delirious, we probably should run a head CT on them,” he added.
Dr. Feldman acknowledged that the frequency of head CTs on inpatients with delirium has been hard to tease out.
“But all the studies indicate that patients who develop delirium while in the hospital, without any sort of risk factor, are very unlikely to have pathology found on a head CT,” he said, noting that the cause of their delirium is likely something else, like dehydration, an infection, disruption of sleep, urinary retention, or medication effect.
Of course, if patients aren’t getting better without the CT, order the CT, he said. “Even if the patient has no risk factor, there’s still a 3% chance of having an abnormality like a tumor or stroke.”
Renal ultrasound for patients with new acute kidney injury
To determine if an acute kidney injury is caused by a treatable obstruction, such as a large prostate causing urinary retention, doctors often first order a renal ultrasound, a test that can cost $300, and must be read by a radiologist.
But a much less expensive simple bladder scan, which can be performed by a nurse, is a much better substitute for the first pass, Dr. Feldman said. He said it’s logical that “a bladder scan is a much higher value test” in the early diagnostic process.
“The studies have been pretty clear. If you don’t have risk factors for having an obstruction, a history of kidney stones, it hasn’t happened before, or other reasons kidneys aren’t working, it’s extraordinarily unlikely you’re going to find anything on that renal ultrasound that could be intervened to fix that acute kidney injury,” Dr. Feldman said. He pointed to a study that found 223 renal ultrasounds were necessary to find one patient who needed an intervention.
“You can probably get a good sense from the history and physical” and start to treat them, he said, and if they’re not getting better, then order the ultrasound.
Each of the items on Feldman’s list don’t necessarily save a lot of money, but they add up. “The more we ask ‘Why are we doing this? Can we stop it if it’s not helping people, and particularly if it’s harming people?’ the more we can prevent the cascade that happens because you did one unnecessary diagnostic test,” he concluded.
FROM HOSPITAL MEDICINE 2016
FDA: CT scans safe for patients with electronic medical devices
There’s no need to let fear of electronic interference between computed tomography and electronic medical devices preclude the ordering of such scans for patients with insulin pumps, cardiac implantable electronic devices, or neurostimulators, the Food and Drug Administration said in a written notification.
“The probability of an adverse event being caused by exposing these devices to CT irradiation is extremely low, and it is greatly outweighed by the clinical benefit of a medically indicated CT examination,” according to the new notification, which updates and replaces a preliminary health notification released on July 14, 2008.
The preliminary notification said there was a “possibility that the x-rays used during CT examinations may cause some implanted and external electronic medical devices to malfunction.” It also included recommendations to reduce the potential risk of such events from occurring and cited adverse events experienced by a few patients with medical devices who had undergone CT scanning, including unintended shocks from neurostimulators, malfunctions of insulin infusion pumps, and transient changes in pacemaker output pulse rate.
The new notification says there is an extremely low probability that a CT scanner directly irradiating the circuitry of certain implantable or wearable electronic medical devices can cause sufficient electronic interference to affect the function and operation of the medical device, and this probability is even lower when the radiation dose and the radiation dose rate are reduced. The FDA also notes that the interference is completely avoided when the medical device is outside of the primary x-ray beam of the CT scanner.
The update, which provides additional reports of adverse events by patients with electronic medical devices who had CT scans, states that the number of such events was small, compared with the number of patients with insulin pumps, cardiac implantable electronic devices, and neurostimulators who were scanned without adverse effects.
The FDA encourages health care providers and patients who suspect a problem with a medical imaging device to file a voluntary report through MedWatch, the FDA Safety Information and Adverse Event Reporting Program.
There’s no need to let fear of electronic interference between computed tomography and electronic medical devices preclude the ordering of such scans for patients with insulin pumps, cardiac implantable electronic devices, or neurostimulators, the Food and Drug Administration said in a written notification.
“The probability of an adverse event being caused by exposing these devices to CT irradiation is extremely low, and it is greatly outweighed by the clinical benefit of a medically indicated CT examination,” according to the new notification, which updates and replaces a preliminary health notification released on July 14, 2008.
The preliminary notification said there was a “possibility that the x-rays used during CT examinations may cause some implanted and external electronic medical devices to malfunction.” It also included recommendations to reduce the potential risk of such events from occurring and cited adverse events experienced by a few patients with medical devices who had undergone CT scanning, including unintended shocks from neurostimulators, malfunctions of insulin infusion pumps, and transient changes in pacemaker output pulse rate.
The new notification says there is an extremely low probability that a CT scanner directly irradiating the circuitry of certain implantable or wearable electronic medical devices can cause sufficient electronic interference to affect the function and operation of the medical device, and this probability is even lower when the radiation dose and the radiation dose rate are reduced. The FDA also notes that the interference is completely avoided when the medical device is outside of the primary x-ray beam of the CT scanner.
The update, which provides additional reports of adverse events by patients with electronic medical devices who had CT scans, states that the number of such events was small, compared with the number of patients with insulin pumps, cardiac implantable electronic devices, and neurostimulators who were scanned without adverse effects.
The FDA encourages health care providers and patients who suspect a problem with a medical imaging device to file a voluntary report through MedWatch, the FDA Safety Information and Adverse Event Reporting Program.
There’s no need to let fear of electronic interference between computed tomography and electronic medical devices preclude the ordering of such scans for patients with insulin pumps, cardiac implantable electronic devices, or neurostimulators, the Food and Drug Administration said in a written notification.
“The probability of an adverse event being caused by exposing these devices to CT irradiation is extremely low, and it is greatly outweighed by the clinical benefit of a medically indicated CT examination,” according to the new notification, which updates and replaces a preliminary health notification released on July 14, 2008.
The preliminary notification said there was a “possibility that the x-rays used during CT examinations may cause some implanted and external electronic medical devices to malfunction.” It also included recommendations to reduce the potential risk of such events from occurring and cited adverse events experienced by a few patients with medical devices who had undergone CT scanning, including unintended shocks from neurostimulators, malfunctions of insulin infusion pumps, and transient changes in pacemaker output pulse rate.
The new notification says there is an extremely low probability that a CT scanner directly irradiating the circuitry of certain implantable or wearable electronic medical devices can cause sufficient electronic interference to affect the function and operation of the medical device, and this probability is even lower when the radiation dose and the radiation dose rate are reduced. The FDA also notes that the interference is completely avoided when the medical device is outside of the primary x-ray beam of the CT scanner.
The update, which provides additional reports of adverse events by patients with electronic medical devices who had CT scans, states that the number of such events was small, compared with the number of patients with insulin pumps, cardiac implantable electronic devices, and neurostimulators who were scanned without adverse effects.
The FDA encourages health care providers and patients who suspect a problem with a medical imaging device to file a voluntary report through MedWatch, the FDA Safety Information and Adverse Event Reporting Program.
What is the best approach to a high systolic pulmonary artery pressure on echocardiography?
The incidental finding of high systolic pulmonary artery pressure on echocardiography is common. What we should do about it varies according to clinical presentation, comorbidities, and results of other tests, including assessment of the right ventricle. Thus, the optimal approach ranges from no further investigation to right heart catheterization and, in some cases, referral to a pulmonary hypertension center.
THE TWO MEASUREMENTS COMPARED
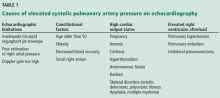
Although it raises concern, the finding of high systolic pulmonary artery pressure is not enough to diagnose pulmonary hypertension. In fact, several other conditions are associated with high systolic pulmonary artery pressure on echocardiography (Table 1). The diagnosis must be confirmed with right heart catheterization.1
Echocardiography provides an estimate of the systolic pulmonary artery pressure that is calculated from other values, whereas right heart catheterization gives a direct measurement of the mean pulmonary artery pressure, which is necessary for diagnosing pulmonary hypertension. The two values are correlated, but the differences are noteworthy.
WHAT IS PULMONARY HYPERTENSION?
Pulmonary hypertension is defined by a resting mean pulmonary artery pressure 25 mm Hg or greater during right heart catheterization.1 The large number of conditions associated with pulmonary hypertension can be divided into five groups2:
- Group 1, pulmonary artery hypertension
- Group 2, pulmonary hypertension associated with left heart disease
- Group 3, pulmonary hypertension due to chronic lung disease or hypoxia
- Group 4, chronic thromboembolic pulmonary hypertension
- Group 5, pulmonary hypertension due to unclear multifactorial mechanisms.2
Pulmonary artery hypertension (group 1) is a syndrome characterized by a restricted flow of small pulmonary arteries that can be idiopathic, heritable, or induced by anorexigens, connective tissue disease, congenital heart disease, portal hypertension, human immunodeficiency virus (HIV), or schistosomiasis.2,3 In spite of significant advances in therapy in the last 3 decades, pulmonary artery hypertension continues to lead to right heart failure and death,4 and the diagnosis has adverse prognostic implications. Therefore, it is essential to be attentive when reviewing the echocardiogram, since an elevated systolic pulmonary artery pressure may be an important clue to pulmonary hypertension.
ESTIMATED PRESSURE: HOW HIGH IS TOO HIGH?
There is no consensus on the optimal cutoff of echocardiographic systolic pulmonary artery pressure to trigger a further evaluation for pulmonary hypertension.
A retrospective evaluation of nearly 16,000 normal echocardiograms found that the 95% upper limit for systolic pulmonary artery pressure was 37 mm Hg.5
European guidelines6 propose that pulmonary hypertension is unlikely if the estimated systolic pulmonary artery pressure is 36 mm Hg or lower, possible if it is 37 to 50 mm Hg, and likely if it is higher than 50 mm Hg.6
The 2009 consensus document of the American College of Cardiology Foundation and American Heart Association3 recommends a systolic pulmonary artery pressure greater than 40 mm Hg as the threshold to suggest further evaluation in a patient with unexplained dyspnea.
Converting the systolic pulmonary artery pressure to the mean pressure
Although not validated to use with echocardiography, the most accurate estimate of mean pulmonary artery pressure was shown in one study7 to be obtained with the equation:
0.61 × systolic pulmonary artery pressure
+ 2 mm Hg
Using this formula, a systolic pulmonary artery pressure of 37 mm Hg would correspond to a mean pulmonary artery pressure of 24.6 mm Hg. A systolic pulmonary artery pressure of 40 mm Hg would correspond to a mean pulmonary artery pressure of 26.4 mm Hg.
Estimated systolic pulmonary artery pressure depends on several variables
Systolic pulmonary artery pressure is estimated using the simplified Bernoulli equation8:
4 × tricuspid regurgitation jet velocity2 (m/s)
+ right atrial pressure (mm Hg)
Tricuspid regurgitation is present in over 75% of the normal population. The regurgitation velocity across the tricuspid valve must be measured to estimate the pressure gradient between the right ventricle and the right atrium. The right atrial pressure is estimated from the diameter of the inferior vena cava and the degree of inspiratory collapse with the sniff test. As the right atrial pressure increases, the inferior vena cava dilates and inspiratory collapse decreases.8 If there is no gradient across the right ventricular outflow tract or pulmonary valve, the right ventricular systolic pressure is equal to the systolic pulmonary artery pressure.
Since tricuspid regurgitation velocity is squared and then multiplied by 4, small deviations of this measurement lead to markedly different systolic pulmonary artery pressure values. To avoid this problem, the tricuspid regurgitation velocity needs to be looked at in multiple echocardiographic views to find the best alignment with the flow and an adequate envelope.
Many causes of high estimated systolic pulmonary artery pressure
Table 1 shows conditions associated with a high estimated systolic pulmonary artery pressure. Echocardiographic limitations, constitutional factors, and high cardiac output states can lead to an apparent elevation in systolic pulmonary artery pressure, which is not confirmed later during right heart catheterization.
Systolic pulmonary artery pressure increases with age and body mass index as a result of worsening left ventricular diastolic dysfunction.8 In fact, an estimated pressure greater than 40 mm Hg is found5 in 6% of people over age 50 and in 5% of people with a body mass index greater than 30 kg/m2. It can also be high in conditions in which there is an increase in cardiac output, such as pregnancy, anemia (sickle cell disease, thalassemia), cirrhosis, and arteriovenous fistula.
The estimated systolic value often differs from the measured value
Studies have compared the systolic pulmonary artery pressure measured during right heart catheterization with the estimated value on echocardiography.9,10 These studies noted a reasonable degree of agreement between the tests but a substantial variability.
Both underestimation and overestimation of the systolic pulmonary artery pressure by echocardiography were common, with 95% limits of agreement ranging from minus 40 mm Hg to plus 40 mm Hg.9,10 A difference of plus or minus 10 mm Hg in systolic pulmonary artery pressure between echocardiography and catheterization was observed in 48% to 51% of patients with pulmonary hypertension, particularly in those with higher systolic pulmonary artery pressure.9,10
An important reason for overestimation of systolic pulmonary artery pressure is the inaccurate estimation of the right atrial pressure by echocardiography.9,10 Indeed, this factor may account for half of the cases in which the systolic pulmonary artery pressure is overestimated.10 Although the traditional methods to estimate the right atrial pressure have been revisited,8,11 this estimation is less reliable for intermediate pressure values, for patients on mechanical ventilation, and for young athletes.8
Other explanations for the variability between measured and estimated systolic pulmonary artery pressure include suboptimal alignment between the Doppler beam and the regurgitant jet, severe tricuspid regurgitation, arrhythmias, and limitations inherent to the simplified Bernoulli equation.12 The estimated value is particularly inaccurate in patients with advanced lung disease, possibly owing to lung hyperinflation and alteration in the thoracic cavity and position of the heart—all factors that limit visualization and measurement of the tricuspid regurgitant jet.13
OTHER SIGNS OF PULMONARY HYPERTENSION ON ECHOCARDIOGRAPHY
Echocardiography provides information that is useful in assessing the accuracy of the estimated systolic pulmonary artery pressure, particularly right ventricular size and function.
As pulmonary hypertension progresses, the right ventricle dilates, and its function is compromised. Therefore, it is important to determine the right ventricular size and function by using objective echocardiographic findings such as right ventricular diameters (basal, mid, apical) and area, right ventricular fractional area change, tricuspid annular plane systolic excursion, myocardial performance index, and the pulsed tissue Doppler tricuspid annular peak systolic excursion velocity.8
Other echocardiographic features that suggest pulmonary hypertension include a dilated right atrial area, flattening of the interventricular septum, notching of the right ventricular outflow tract flow, and dilation of the main pulmonary artery. Interestingly, left ventricular diastolic dysfunction of the impaired relaxation type (grade I) is commonly observed in pulmonary hypertension14; however, more advanced degrees of diastolic dysfunction, ie, pseudonormalization (grade II) or restrictive left ventricular filling (grade III),15 particularly when associated with a left atrial enlargement, suggest pulmonary hypertension associated with left heart disease and not pulmonary artery hypertension.
WHAT TO DO IF ECHOCARDIOGRAPHY INDICATES PULMONARY HYPERTENSION
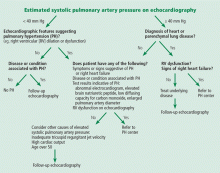
An algorithm showing the approach to an elevated systolic pulmonary artery pressure on echocardiography is presented in Figure 1.
In the appropriate clinical setting, if the systolic pulmonary artery pressure is 40 mm Hg or greater or if other echocardiographic variables suggest pulmonary hypertension, our practice is to proceed with right heart catheterization.
Clinical variables that suggest pulmonary hypertension include progressive dyspnea, chest pain, presyncope-syncope, lower extremity edema, hepatomegaly, jugular vein distention, hepatojugular reflux, sternal heave, loud second heart sound (P2), murmur of tricuspid or pulmonary regurgitation, and right ventricular third heart sound.16 These are of particular interest when associated with conditions known to cause pulmonary hypertension,2such as connective tissue disease, portal hypertension, congenital heart disease, HIV infection, and certain drugs and toxins.
Other tests that raise suspicion of pulmonary hypertension are an electrocardiogram suggesting a dilated right atrium or ventricle, an elevated brain natriuretic peptide level, a low carbon monoxide diffusing capacity on pulmonary function testing, and an enlarged pulmonary artery diameter on imaging.
Given the high prevalence of pulmonary hypertension, the Fifth World Symposium on Pulmonary Hypertension recommended first considering heart or parenchymal lung disease when an echocardiogram suggests pulmonary hypertension.6 If there are signs of severe pulmonary hypertension or right ventricular dysfunction, referral to a center specializing in pulmonary hypertension is recommended. Referral is also appropriate when there is no major heart or lung disease and the echocardiogram shows an elevated systolic pulmonary artery pressure, particularly when the clinical presentation or results of other testing suggest pulmonary hypertension.
TAKE-HOME POINTS
In the appropriate context, a high systolic pulmonary artery pressure on echocardiography suggests pulmonary hypertension, but right heart catheterization is needed to confirm the diagnosis. Estimating the systolic pulmonary artery pressure with echocardiography has limitations, including false-positive results, predominantly when the pretest probability of pulmonary hypertension is low.
- Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013; 62(suppl D):D42–D50.
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62(suppl D):D34–D41.
- McLaughlin VV, Archer SL, Badesch DB, et al; American College of Cardiology Foundation Task Force on Expert Consensus Documents; American Heart Association; American College of Chest Physicians; American Thoracic Society, Inc; Pulmonary Hypertension Association. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009; 53:1573–1619.
- Tonelli AR, Arelli V, Minai OA, et al. Causes and circumstances of death in pulmonary arterial hypertension. Am J Respir Crit Care Med 2013; 188:365–369.
- McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation 2001; 104:2797–2802.
- Galiè N, Hoeper MM, Humbert M, et al; ESC Committee for Practice Guidelines (CPG). Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30:2493–2537.
- Chemla D, Castelain V, Provencher S, Humbert M, Simonneau G, Herve P. Evaluation of various empirical formulas for estimating mean pulmonary artery pressure by using systolic pulmonary artery pressure in adults. Chest 2009; 135:760–768.
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23:685–713.
- Rich JD, Shah SJ, Swamy RS, Kamp A, Rich S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest 2011; 139:988–993.
- Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med 2009; 179:615–621.
- Brennan JM, Blair JE, Goonewardena S, et al. Reappraisal of the use of inferior vena cava for estimating right atrial pressure. J Am Soc Echocardiogr 2007; 20:857–861.
- Giardini A, Tacy TA. Non-invasive estimation of pressure gradients in regurgitant jets: an overdue consideration. Eur J Echocardiogr 2008; 9:578–584.
- Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med 2003; 167:735–740.
- Tonelli AR, Plana JC, Heresi GA, Dweik RA. Prevalence and prognostic value of left ventricular diastolic dysfunction in idiopathic and heritable pulmonary arterial hypertension. Chest 2012; 141:1457–1465.
- Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009; 22:107–133.
- Barst RJ, McGoon M, Torbicki A, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2004; 43(suppl S):40S–47S.
The incidental finding of high systolic pulmonary artery pressure on echocardiography is common. What we should do about it varies according to clinical presentation, comorbidities, and results of other tests, including assessment of the right ventricle. Thus, the optimal approach ranges from no further investigation to right heart catheterization and, in some cases, referral to a pulmonary hypertension center.
THE TWO MEASUREMENTS COMPARED
Although it raises concern, the finding of high systolic pulmonary artery pressure is not enough to diagnose pulmonary hypertension. In fact, several other conditions are associated with high systolic pulmonary artery pressure on echocardiography (Table 1). The diagnosis must be confirmed with right heart catheterization.1
Echocardiography provides an estimate of the systolic pulmonary artery pressure that is calculated from other values, whereas right heart catheterization gives a direct measurement of the mean pulmonary artery pressure, which is necessary for diagnosing pulmonary hypertension. The two values are correlated, but the differences are noteworthy.
WHAT IS PULMONARY HYPERTENSION?
Pulmonary hypertension is defined by a resting mean pulmonary artery pressure 25 mm Hg or greater during right heart catheterization.1 The large number of conditions associated with pulmonary hypertension can be divided into five groups2:
- Group 1, pulmonary artery hypertension
- Group 2, pulmonary hypertension associated with left heart disease
- Group 3, pulmonary hypertension due to chronic lung disease or hypoxia
- Group 4, chronic thromboembolic pulmonary hypertension
- Group 5, pulmonary hypertension due to unclear multifactorial mechanisms.2
Pulmonary artery hypertension (group 1) is a syndrome characterized by a restricted flow of small pulmonary arteries that can be idiopathic, heritable, or induced by anorexigens, connective tissue disease, congenital heart disease, portal hypertension, human immunodeficiency virus (HIV), or schistosomiasis.2,3 In spite of significant advances in therapy in the last 3 decades, pulmonary artery hypertension continues to lead to right heart failure and death,4 and the diagnosis has adverse prognostic implications. Therefore, it is essential to be attentive when reviewing the echocardiogram, since an elevated systolic pulmonary artery pressure may be an important clue to pulmonary hypertension.
ESTIMATED PRESSURE: HOW HIGH IS TOO HIGH?
There is no consensus on the optimal cutoff of echocardiographic systolic pulmonary artery pressure to trigger a further evaluation for pulmonary hypertension.
A retrospective evaluation of nearly 16,000 normal echocardiograms found that the 95% upper limit for systolic pulmonary artery pressure was 37 mm Hg.5
European guidelines6 propose that pulmonary hypertension is unlikely if the estimated systolic pulmonary artery pressure is 36 mm Hg or lower, possible if it is 37 to 50 mm Hg, and likely if it is higher than 50 mm Hg.6
The 2009 consensus document of the American College of Cardiology Foundation and American Heart Association3 recommends a systolic pulmonary artery pressure greater than 40 mm Hg as the threshold to suggest further evaluation in a patient with unexplained dyspnea.
Converting the systolic pulmonary artery pressure to the mean pressure
Although not validated to use with echocardiography, the most accurate estimate of mean pulmonary artery pressure was shown in one study7 to be obtained with the equation:
0.61 × systolic pulmonary artery pressure
+ 2 mm Hg
Using this formula, a systolic pulmonary artery pressure of 37 mm Hg would correspond to a mean pulmonary artery pressure of 24.6 mm Hg. A systolic pulmonary artery pressure of 40 mm Hg would correspond to a mean pulmonary artery pressure of 26.4 mm Hg.
Estimated systolic pulmonary artery pressure depends on several variables
Systolic pulmonary artery pressure is estimated using the simplified Bernoulli equation8:
4 × tricuspid regurgitation jet velocity2 (m/s)
+ right atrial pressure (mm Hg)
Tricuspid regurgitation is present in over 75% of the normal population. The regurgitation velocity across the tricuspid valve must be measured to estimate the pressure gradient between the right ventricle and the right atrium. The right atrial pressure is estimated from the diameter of the inferior vena cava and the degree of inspiratory collapse with the sniff test. As the right atrial pressure increases, the inferior vena cava dilates and inspiratory collapse decreases.8 If there is no gradient across the right ventricular outflow tract or pulmonary valve, the right ventricular systolic pressure is equal to the systolic pulmonary artery pressure.
Since tricuspid regurgitation velocity is squared and then multiplied by 4, small deviations of this measurement lead to markedly different systolic pulmonary artery pressure values. To avoid this problem, the tricuspid regurgitation velocity needs to be looked at in multiple echocardiographic views to find the best alignment with the flow and an adequate envelope.
Many causes of high estimated systolic pulmonary artery pressure
Table 1 shows conditions associated with a high estimated systolic pulmonary artery pressure. Echocardiographic limitations, constitutional factors, and high cardiac output states can lead to an apparent elevation in systolic pulmonary artery pressure, which is not confirmed later during right heart catheterization.
Systolic pulmonary artery pressure increases with age and body mass index as a result of worsening left ventricular diastolic dysfunction.8 In fact, an estimated pressure greater than 40 mm Hg is found5 in 6% of people over age 50 and in 5% of people with a body mass index greater than 30 kg/m2. It can also be high in conditions in which there is an increase in cardiac output, such as pregnancy, anemia (sickle cell disease, thalassemia), cirrhosis, and arteriovenous fistula.
The estimated systolic value often differs from the measured value
Studies have compared the systolic pulmonary artery pressure measured during right heart catheterization with the estimated value on echocardiography.9,10 These studies noted a reasonable degree of agreement between the tests but a substantial variability.
Both underestimation and overestimation of the systolic pulmonary artery pressure by echocardiography were common, with 95% limits of agreement ranging from minus 40 mm Hg to plus 40 mm Hg.9,10 A difference of plus or minus 10 mm Hg in systolic pulmonary artery pressure between echocardiography and catheterization was observed in 48% to 51% of patients with pulmonary hypertension, particularly in those with higher systolic pulmonary artery pressure.9,10
An important reason for overestimation of systolic pulmonary artery pressure is the inaccurate estimation of the right atrial pressure by echocardiography.9,10 Indeed, this factor may account for half of the cases in which the systolic pulmonary artery pressure is overestimated.10 Although the traditional methods to estimate the right atrial pressure have been revisited,8,11 this estimation is less reliable for intermediate pressure values, for patients on mechanical ventilation, and for young athletes.8
Other explanations for the variability between measured and estimated systolic pulmonary artery pressure include suboptimal alignment between the Doppler beam and the regurgitant jet, severe tricuspid regurgitation, arrhythmias, and limitations inherent to the simplified Bernoulli equation.12 The estimated value is particularly inaccurate in patients with advanced lung disease, possibly owing to lung hyperinflation and alteration in the thoracic cavity and position of the heart—all factors that limit visualization and measurement of the tricuspid regurgitant jet.13
OTHER SIGNS OF PULMONARY HYPERTENSION ON ECHOCARDIOGRAPHY
Echocardiography provides information that is useful in assessing the accuracy of the estimated systolic pulmonary artery pressure, particularly right ventricular size and function.
As pulmonary hypertension progresses, the right ventricle dilates, and its function is compromised. Therefore, it is important to determine the right ventricular size and function by using objective echocardiographic findings such as right ventricular diameters (basal, mid, apical) and area, right ventricular fractional area change, tricuspid annular plane systolic excursion, myocardial performance index, and the pulsed tissue Doppler tricuspid annular peak systolic excursion velocity.8
Other echocardiographic features that suggest pulmonary hypertension include a dilated right atrial area, flattening of the interventricular septum, notching of the right ventricular outflow tract flow, and dilation of the main pulmonary artery. Interestingly, left ventricular diastolic dysfunction of the impaired relaxation type (grade I) is commonly observed in pulmonary hypertension14; however, more advanced degrees of diastolic dysfunction, ie, pseudonormalization (grade II) or restrictive left ventricular filling (grade III),15 particularly when associated with a left atrial enlargement, suggest pulmonary hypertension associated with left heart disease and not pulmonary artery hypertension.
WHAT TO DO IF ECHOCARDIOGRAPHY INDICATES PULMONARY HYPERTENSION
An algorithm showing the approach to an elevated systolic pulmonary artery pressure on echocardiography is presented in Figure 1.
In the appropriate clinical setting, if the systolic pulmonary artery pressure is 40 mm Hg or greater or if other echocardiographic variables suggest pulmonary hypertension, our practice is to proceed with right heart catheterization.
Clinical variables that suggest pulmonary hypertension include progressive dyspnea, chest pain, presyncope-syncope, lower extremity edema, hepatomegaly, jugular vein distention, hepatojugular reflux, sternal heave, loud second heart sound (P2), murmur of tricuspid or pulmonary regurgitation, and right ventricular third heart sound.16 These are of particular interest when associated with conditions known to cause pulmonary hypertension,2such as connective tissue disease, portal hypertension, congenital heart disease, HIV infection, and certain drugs and toxins.
Other tests that raise suspicion of pulmonary hypertension are an electrocardiogram suggesting a dilated right atrium or ventricle, an elevated brain natriuretic peptide level, a low carbon monoxide diffusing capacity on pulmonary function testing, and an enlarged pulmonary artery diameter on imaging.
Given the high prevalence of pulmonary hypertension, the Fifth World Symposium on Pulmonary Hypertension recommended first considering heart or parenchymal lung disease when an echocardiogram suggests pulmonary hypertension.6 If there are signs of severe pulmonary hypertension or right ventricular dysfunction, referral to a center specializing in pulmonary hypertension is recommended. Referral is also appropriate when there is no major heart or lung disease and the echocardiogram shows an elevated systolic pulmonary artery pressure, particularly when the clinical presentation or results of other testing suggest pulmonary hypertension.
TAKE-HOME POINTS
In the appropriate context, a high systolic pulmonary artery pressure on echocardiography suggests pulmonary hypertension, but right heart catheterization is needed to confirm the diagnosis. Estimating the systolic pulmonary artery pressure with echocardiography has limitations, including false-positive results, predominantly when the pretest probability of pulmonary hypertension is low.
The incidental finding of high systolic pulmonary artery pressure on echocardiography is common. What we should do about it varies according to clinical presentation, comorbidities, and results of other tests, including assessment of the right ventricle. Thus, the optimal approach ranges from no further investigation to right heart catheterization and, in some cases, referral to a pulmonary hypertension center.
THE TWO MEASUREMENTS COMPARED
Although it raises concern, the finding of high systolic pulmonary artery pressure is not enough to diagnose pulmonary hypertension. In fact, several other conditions are associated with high systolic pulmonary artery pressure on echocardiography (Table 1). The diagnosis must be confirmed with right heart catheterization.1
Echocardiography provides an estimate of the systolic pulmonary artery pressure that is calculated from other values, whereas right heart catheterization gives a direct measurement of the mean pulmonary artery pressure, which is necessary for diagnosing pulmonary hypertension. The two values are correlated, but the differences are noteworthy.
WHAT IS PULMONARY HYPERTENSION?
Pulmonary hypertension is defined by a resting mean pulmonary artery pressure 25 mm Hg or greater during right heart catheterization.1 The large number of conditions associated with pulmonary hypertension can be divided into five groups2:
- Group 1, pulmonary artery hypertension
- Group 2, pulmonary hypertension associated with left heart disease
- Group 3, pulmonary hypertension due to chronic lung disease or hypoxia
- Group 4, chronic thromboembolic pulmonary hypertension
- Group 5, pulmonary hypertension due to unclear multifactorial mechanisms.2
Pulmonary artery hypertension (group 1) is a syndrome characterized by a restricted flow of small pulmonary arteries that can be idiopathic, heritable, or induced by anorexigens, connective tissue disease, congenital heart disease, portal hypertension, human immunodeficiency virus (HIV), or schistosomiasis.2,3 In spite of significant advances in therapy in the last 3 decades, pulmonary artery hypertension continues to lead to right heart failure and death,4 and the diagnosis has adverse prognostic implications. Therefore, it is essential to be attentive when reviewing the echocardiogram, since an elevated systolic pulmonary artery pressure may be an important clue to pulmonary hypertension.
ESTIMATED PRESSURE: HOW HIGH IS TOO HIGH?
There is no consensus on the optimal cutoff of echocardiographic systolic pulmonary artery pressure to trigger a further evaluation for pulmonary hypertension.
A retrospective evaluation of nearly 16,000 normal echocardiograms found that the 95% upper limit for systolic pulmonary artery pressure was 37 mm Hg.5
European guidelines6 propose that pulmonary hypertension is unlikely if the estimated systolic pulmonary artery pressure is 36 mm Hg or lower, possible if it is 37 to 50 mm Hg, and likely if it is higher than 50 mm Hg.6
The 2009 consensus document of the American College of Cardiology Foundation and American Heart Association3 recommends a systolic pulmonary artery pressure greater than 40 mm Hg as the threshold to suggest further evaluation in a patient with unexplained dyspnea.
Converting the systolic pulmonary artery pressure to the mean pressure
Although not validated to use with echocardiography, the most accurate estimate of mean pulmonary artery pressure was shown in one study7 to be obtained with the equation:
0.61 × systolic pulmonary artery pressure
+ 2 mm Hg
Using this formula, a systolic pulmonary artery pressure of 37 mm Hg would correspond to a mean pulmonary artery pressure of 24.6 mm Hg. A systolic pulmonary artery pressure of 40 mm Hg would correspond to a mean pulmonary artery pressure of 26.4 mm Hg.
Estimated systolic pulmonary artery pressure depends on several variables
Systolic pulmonary artery pressure is estimated using the simplified Bernoulli equation8:
4 × tricuspid regurgitation jet velocity2 (m/s)
+ right atrial pressure (mm Hg)
Tricuspid regurgitation is present in over 75% of the normal population. The regurgitation velocity across the tricuspid valve must be measured to estimate the pressure gradient between the right ventricle and the right atrium. The right atrial pressure is estimated from the diameter of the inferior vena cava and the degree of inspiratory collapse with the sniff test. As the right atrial pressure increases, the inferior vena cava dilates and inspiratory collapse decreases.8 If there is no gradient across the right ventricular outflow tract or pulmonary valve, the right ventricular systolic pressure is equal to the systolic pulmonary artery pressure.
Since tricuspid regurgitation velocity is squared and then multiplied by 4, small deviations of this measurement lead to markedly different systolic pulmonary artery pressure values. To avoid this problem, the tricuspid regurgitation velocity needs to be looked at in multiple echocardiographic views to find the best alignment with the flow and an adequate envelope.
Many causes of high estimated systolic pulmonary artery pressure
Table 1 shows conditions associated with a high estimated systolic pulmonary artery pressure. Echocardiographic limitations, constitutional factors, and high cardiac output states can lead to an apparent elevation in systolic pulmonary artery pressure, which is not confirmed later during right heart catheterization.
Systolic pulmonary artery pressure increases with age and body mass index as a result of worsening left ventricular diastolic dysfunction.8 In fact, an estimated pressure greater than 40 mm Hg is found5 in 6% of people over age 50 and in 5% of people with a body mass index greater than 30 kg/m2. It can also be high in conditions in which there is an increase in cardiac output, such as pregnancy, anemia (sickle cell disease, thalassemia), cirrhosis, and arteriovenous fistula.
The estimated systolic value often differs from the measured value
Studies have compared the systolic pulmonary artery pressure measured during right heart catheterization with the estimated value on echocardiography.9,10 These studies noted a reasonable degree of agreement between the tests but a substantial variability.
Both underestimation and overestimation of the systolic pulmonary artery pressure by echocardiography were common, with 95% limits of agreement ranging from minus 40 mm Hg to plus 40 mm Hg.9,10 A difference of plus or minus 10 mm Hg in systolic pulmonary artery pressure between echocardiography and catheterization was observed in 48% to 51% of patients with pulmonary hypertension, particularly in those with higher systolic pulmonary artery pressure.9,10
An important reason for overestimation of systolic pulmonary artery pressure is the inaccurate estimation of the right atrial pressure by echocardiography.9,10 Indeed, this factor may account for half of the cases in which the systolic pulmonary artery pressure is overestimated.10 Although the traditional methods to estimate the right atrial pressure have been revisited,8,11 this estimation is less reliable for intermediate pressure values, for patients on mechanical ventilation, and for young athletes.8
Other explanations for the variability between measured and estimated systolic pulmonary artery pressure include suboptimal alignment between the Doppler beam and the regurgitant jet, severe tricuspid regurgitation, arrhythmias, and limitations inherent to the simplified Bernoulli equation.12 The estimated value is particularly inaccurate in patients with advanced lung disease, possibly owing to lung hyperinflation and alteration in the thoracic cavity and position of the heart—all factors that limit visualization and measurement of the tricuspid regurgitant jet.13
OTHER SIGNS OF PULMONARY HYPERTENSION ON ECHOCARDIOGRAPHY
Echocardiography provides information that is useful in assessing the accuracy of the estimated systolic pulmonary artery pressure, particularly right ventricular size and function.
As pulmonary hypertension progresses, the right ventricle dilates, and its function is compromised. Therefore, it is important to determine the right ventricular size and function by using objective echocardiographic findings such as right ventricular diameters (basal, mid, apical) and area, right ventricular fractional area change, tricuspid annular plane systolic excursion, myocardial performance index, and the pulsed tissue Doppler tricuspid annular peak systolic excursion velocity.8
Other echocardiographic features that suggest pulmonary hypertension include a dilated right atrial area, flattening of the interventricular septum, notching of the right ventricular outflow tract flow, and dilation of the main pulmonary artery. Interestingly, left ventricular diastolic dysfunction of the impaired relaxation type (grade I) is commonly observed in pulmonary hypertension14; however, more advanced degrees of diastolic dysfunction, ie, pseudonormalization (grade II) or restrictive left ventricular filling (grade III),15 particularly when associated with a left atrial enlargement, suggest pulmonary hypertension associated with left heart disease and not pulmonary artery hypertension.
WHAT TO DO IF ECHOCARDIOGRAPHY INDICATES PULMONARY HYPERTENSION
An algorithm showing the approach to an elevated systolic pulmonary artery pressure on echocardiography is presented in Figure 1.
In the appropriate clinical setting, if the systolic pulmonary artery pressure is 40 mm Hg or greater or if other echocardiographic variables suggest pulmonary hypertension, our practice is to proceed with right heart catheterization.
Clinical variables that suggest pulmonary hypertension include progressive dyspnea, chest pain, presyncope-syncope, lower extremity edema, hepatomegaly, jugular vein distention, hepatojugular reflux, sternal heave, loud second heart sound (P2), murmur of tricuspid or pulmonary regurgitation, and right ventricular third heart sound.16 These are of particular interest when associated with conditions known to cause pulmonary hypertension,2such as connective tissue disease, portal hypertension, congenital heart disease, HIV infection, and certain drugs and toxins.
Other tests that raise suspicion of pulmonary hypertension are an electrocardiogram suggesting a dilated right atrium or ventricle, an elevated brain natriuretic peptide level, a low carbon monoxide diffusing capacity on pulmonary function testing, and an enlarged pulmonary artery diameter on imaging.
Given the high prevalence of pulmonary hypertension, the Fifth World Symposium on Pulmonary Hypertension recommended first considering heart or parenchymal lung disease when an echocardiogram suggests pulmonary hypertension.6 If there are signs of severe pulmonary hypertension or right ventricular dysfunction, referral to a center specializing in pulmonary hypertension is recommended. Referral is also appropriate when there is no major heart or lung disease and the echocardiogram shows an elevated systolic pulmonary artery pressure, particularly when the clinical presentation or results of other testing suggest pulmonary hypertension.
TAKE-HOME POINTS
In the appropriate context, a high systolic pulmonary artery pressure on echocardiography suggests pulmonary hypertension, but right heart catheterization is needed to confirm the diagnosis. Estimating the systolic pulmonary artery pressure with echocardiography has limitations, including false-positive results, predominantly when the pretest probability of pulmonary hypertension is low.
- Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013; 62(suppl D):D42–D50.
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62(suppl D):D34–D41.
- McLaughlin VV, Archer SL, Badesch DB, et al; American College of Cardiology Foundation Task Force on Expert Consensus Documents; American Heart Association; American College of Chest Physicians; American Thoracic Society, Inc; Pulmonary Hypertension Association. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009; 53:1573–1619.
- Tonelli AR, Arelli V, Minai OA, et al. Causes and circumstances of death in pulmonary arterial hypertension. Am J Respir Crit Care Med 2013; 188:365–369.
- McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation 2001; 104:2797–2802.
- Galiè N, Hoeper MM, Humbert M, et al; ESC Committee for Practice Guidelines (CPG). Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30:2493–2537.
- Chemla D, Castelain V, Provencher S, Humbert M, Simonneau G, Herve P. Evaluation of various empirical formulas for estimating mean pulmonary artery pressure by using systolic pulmonary artery pressure in adults. Chest 2009; 135:760–768.
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23:685–713.
- Rich JD, Shah SJ, Swamy RS, Kamp A, Rich S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest 2011; 139:988–993.
- Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med 2009; 179:615–621.
- Brennan JM, Blair JE, Goonewardena S, et al. Reappraisal of the use of inferior vena cava for estimating right atrial pressure. J Am Soc Echocardiogr 2007; 20:857–861.
- Giardini A, Tacy TA. Non-invasive estimation of pressure gradients in regurgitant jets: an overdue consideration. Eur J Echocardiogr 2008; 9:578–584.
- Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med 2003; 167:735–740.
- Tonelli AR, Plana JC, Heresi GA, Dweik RA. Prevalence and prognostic value of left ventricular diastolic dysfunction in idiopathic and heritable pulmonary arterial hypertension. Chest 2012; 141:1457–1465.
- Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009; 22:107–133.
- Barst RJ, McGoon M, Torbicki A, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2004; 43(suppl S):40S–47S.
- Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013; 62(suppl D):D42–D50.
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62(suppl D):D34–D41.
- McLaughlin VV, Archer SL, Badesch DB, et al; American College of Cardiology Foundation Task Force on Expert Consensus Documents; American Heart Association; American College of Chest Physicians; American Thoracic Society, Inc; Pulmonary Hypertension Association. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009; 53:1573–1619.
- Tonelli AR, Arelli V, Minai OA, et al. Causes and circumstances of death in pulmonary arterial hypertension. Am J Respir Crit Care Med 2013; 188:365–369.
- McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation 2001; 104:2797–2802.
- Galiè N, Hoeper MM, Humbert M, et al; ESC Committee for Practice Guidelines (CPG). Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30:2493–2537.
- Chemla D, Castelain V, Provencher S, Humbert M, Simonneau G, Herve P. Evaluation of various empirical formulas for estimating mean pulmonary artery pressure by using systolic pulmonary artery pressure in adults. Chest 2009; 135:760–768.
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23:685–713.
- Rich JD, Shah SJ, Swamy RS, Kamp A, Rich S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest 2011; 139:988–993.
- Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med 2009; 179:615–621.
- Brennan JM, Blair JE, Goonewardena S, et al. Reappraisal of the use of inferior vena cava for estimating right atrial pressure. J Am Soc Echocardiogr 2007; 20:857–861.
- Giardini A, Tacy TA. Non-invasive estimation of pressure gradients in regurgitant jets: an overdue consideration. Eur J Echocardiogr 2008; 9:578–584.
- Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med 2003; 167:735–740.
- Tonelli AR, Plana JC, Heresi GA, Dweik RA. Prevalence and prognostic value of left ventricular diastolic dysfunction in idiopathic and heritable pulmonary arterial hypertension. Chest 2012; 141:1457–1465.
- Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009; 22:107–133.
- Barst RJ, McGoon M, Torbicki A, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2004; 43(suppl S):40S–47S.
When does asymptomatic aortic stenosis warrant surgery? Assessment techniques
Aortic stenosis is the most common valvular heart condition in the developed world, affecting 3% of people between ages 75 and 851 and 4% of people over age 85.2 Aortic valve replacement remains the only treatment proven to reduce the rates of mortality and morbidity in this condition.3 Under current guidelines,4,5 the onset of symptoms of exertional angina, syncope, or dyspnea in a patient who has severe aortic stenosis is a class I indication for surgery—ie, surgery should be performed.
However, high-gradient, severe aortic stenosis that is asymptomatic often poses a dilemma. The annual rate of sudden death in patients with this condition is estimated at 1% to 3%,6–9 but the surgical mortality rate in aortic valve replacement has been as high as 6% in Medicare patients (varying by center and comorbidities).10 Therefore, the traditional teaching was to not surgically replace the valve in asymptomatic patients, based on an adverse risk-benefit ratio. But with improvements in surgical techniques and prostheses, these rates have been reduced to 2.41% at high-volume centers11 (and to less than 1% at some hospitals),12 arguing in favor of earlier intervention.
Complicating the issue, transcatheter aortic valve replacement has become widely available, but further investigation into its use in this patient cohort is warranted.
Furthermore, many patients with severe but apparently asymptomatic aortic stenosis and normal left ventricular ejection fraction may actually have impaired exercise capacity, or they may have structural left ventricular changes such as severe hypertrophy or reduction in global strain, which may worsen the long-term survival rate.13,14
A prospective trial in patients with severe aortic stenosis found that mortality rates were significantly lower in those who underwent surgery early than in those who received conventional treatment, ie, watchful waiting (no specific medical treatment for aortic stenosis is available).15
Patients with asymptomatic severe aortic stenosis are a diverse group; some have a far worse prognosis than others, with or without surgery.
This paper reviews the guidelines for valve replacement in this patient group and the factors useful in establishing who should be considered for early intervention even if they have no classic symptoms (Figure 1).
SIGNS AND SYMPTOMS OF STENOSIS
Aortic stenosis is often first suspected when a patient presents with angina, dyspnea, and syncope, or when an ejection systolic murmur is heard incidentally on physical examination—typically a high-pitched, crescendo-decrescendo, midsystolic ejection murmur that is best heard at the right upper sternal border and that radiates to the carotid arteries.
Several physical findings may help in assessing the severity of aortic stenosis. In mild stenosis, the murmur peaks in early systole, but as the disease progresses the peak moves later into systole. The corollary of this phenomenon is a weak and delayed carotid upstroke known as “pulsus parvus et tardus.” This can be assessed by palpating the carotid artery while auscultating the heart.
The second heart sound becomes progressively softer as the stenosis advances until it is no longer audible. If a fourth heart sound is present, it may be due to concentric left ventricular hypertrophy with reduced left ventricular compliance, and a third heart sound indicates severe left ventricular dysfunction. Both of these findings suggest severe aortic stenosis.
ECHOCARDIOGRAPHIC MEASURES OF SEVERITY
Echocardiography is the best established and most important initial investigation in the assessment of a patient with suspected aortic stenosis. It usually provides accurate information on the severity and the mechanism of stenosis. The following findings indicate severe aortic stenosis:
- Mean pressure gradient > 40 mm Hg
- Peak aortic jet velocity > 4.0 m/s
- Aortic valve area < 1 cm2.
RECOMMENDATIONS FOR SURGERY BASED ON SEVERITY AND SYMPTOMS
The American College of Cardiology and American Heart Association (ACC/AHA)4 have issued the following recommendations for aortic valve replacement, based on the severity of stenosis and on whether the patient has symptoms (Figure 2):
Severe stenosis, with symptoms: class I recommendation (surgery should be done). Without surgery, these patients have a very poor prognosis, with an overall mortality rate of 75% at 3 years.3
Severe stenosis, no symptoms, in patients undergoing cardiac surgery for another indication (eg, coronary artery bypass grafting, ascending aortic surgery, or surgery on other valves): class I recommendation for concomitant aortic valve replacement.
Moderate stenosis, no symptoms, in patients undergoing cardiac surgery for another indication: class IIa recommendation (ie, aortic valve replacement “is reasonable”).
Very severe stenosis (aortic peak velocity > 5.0 m/s or mean pressure gradient ≥ 60 mm Hg), no symptoms, and low risk of death during surgery: class IIa recommendation.
Severe stenosis, no symptoms, and an increase in transaortic velocity of 0.3 m/s or more per year on serial testing or in patients considered to be at high risk for rapid disease progression, such as elderly patients with severe calcification: class IIb recommendation (surgery “can be considered”). The threshold to replace the valve is lower for patients who cannot make serial follow-up appointments because they live far away or lack transportation, or because they have problems with compliance.
Surgery for those with left ventricular dysfunction
Echocardiography also provides information on left ventricular function, and patients with left ventricular dysfunction have significantly worse outcomes. Studies have shown substantial differences in survival in patients who had an ejection fraction of less than 50% before valve replacement compared with those with a normal ejection fraction.3
Thus, the ACC/AHA guidelines recommend immediate referral for aortic valve replacement in asymptomatic patients whose left ventricular ejection fraction is less than 50% (class I recommendation, level of evidence B) in the hope of preventing irreversible ventricular dysfunction.4
TREADMILL EXERCISE TESTING UNMASKS SYMPTOMS
In the past, severe aortic stenosis was considered a contraindication to stress testing because of concerns of precipitating severe, life-threatening complications. However, studies over the past 10 years have shown that a supervised modified Bruce protocol is safe in patients with severe asymptomatic aortic stenosis.16,17
However, treadmill exercise testing clearly is absolutely contraindicated in patients with severe symptomatic aortic stenosis because of the risk of syncope or of precipitating a malignant arrhythmia. Nevertheless, it may play an essential role in the workup of a physically active patient with no symptoms.
Symptoms can develop insidiously in patients with chronic valve disease and may often go unrecognized by patients and their physicians. Many patients who state they have no symptoms may actually be subconsciously limiting their exercise to avoid symptoms.
Amato et al13 examined the exercise capacity of 66 patients reported to have severe asymptomatic aortic stenosis. Treadmill exercise testing was considered positive in this study if the patient developed symptoms or complex ventricular arrhythmias, had blood pressure that failed to rise by 20 mm Hg, or developed horizontal or down-sloping ST depression (≥ 1 mm in men, ≥ 2 mm in women). Twenty (30.3%) of the 66 patients developed symptoms during exercise testing, and they had a significantly worse prognosis: the 2-year event-free survival rate was only 19% in those with a positive test compared with 85% in those with a negative test.13 This study highlights the problem of patients subconsciously reducing their level of activity, thereby masking their true symptoms.
A meta-analysis by Rafique et al18 found that asymptomatic patients with abnormal results on exercise testing had a risk of cardiac events during follow-up that was eight times higher than normal, and a risk of sudden death 5.5 times higher.
With trials demonstrating that exercise testing is safe and prognostically useful in patients with aortic stenosis, the ACC/AHA guidelines emphasize its role, giving a class I recommendation for aortic valve replacement in patients who develop symptoms on exercise testing, and a class IIa recommendation in asymptomatic patients with decreased exercise tolerance or an exercise-related fall in blood pressure (Figure 2).4
STRESS ECHOCARDIOGRAPHY
Stress echocardiography has been used since the 1980s to assess the hemodynamic consequences of valvular heart disease, and many studies highlight its prognostic usefulness in patients with asymptomatic aortic stenosis.
In a 2005 study by Lancellotti et al,19 69 patients with severe asymptomatic aortic stenosis underwent a symptom-limited bicycle exercise stress test using quantitative Doppler echocardiography both at rest and at peak exercise, and a number of independent predictors of poor outcome (ie, symptoms, aortic valve replacement, death) were identified. These predictors included an abnormal test result, defined as any of the following: angina, dyspnea, ST-segment depression of 2 mm Hg or more, a fall or a small (< 20 mm Hg) rise in systolic blood pressure during the test, an aortic valve area of 0.75 cm2 or less, or a mean increase in valve gradient of 18 mm Hg or more.
Subsequently, a multicenter prospective trial assessed the value of exercise stress echocardiography in 186 patients with asymptomatic moderate or severe aortic stenosis.20 A mean increase in the aortic valve gradient of 20 mm Hg or more after exercise was associated with a rate of cardiovascular events (death, aortic valve replacement) 3.8 times higher, independent of other risk factors and whether moderate or severe stenosis was present (Table 1).20
Exercise-induced changes in systolic pulmonary artery pressure, which can be assessed using stress echocardiography, also have prognostic utility. Elevated systolic pulmonary artery pressure (> 50 mm Hg) seems to portend a poorer prognosis21,22 and a higher mortality rate after valve replacement,23 making it an independent predictor of hospital mortality and postoperative major adverse cardiovascular and cerebrovascular events (Table 1).
Exercise echocardiography also can be used to assess the patient’s contractile reserve. Left ventricular contractile reserve can be defined as an exercise-induced increase in left ventricular ejection fraction. In a study by Maréchaux et al24 in 50 patients with asymptomatic aortic stenosis and a normal resting left ventricular ejection fraction (> 50%), 40% of patients did not have left ventricular contractile reserve. In fact, their left ventricular ejection fraction decreased with exercise (from 64 ± 10% to 53 ± 12%). The subgroup of patients without contractile reserve developed symptoms more frequently during exercise and had lower event-free survival (Table 1).
Stress echocardiography has recently been introduced into the European Society of Cardiology guidelines, which give a class IIb indication for aortic valve replacement in asymptomatic patients who have severe aortic stenosis, a normal ejection fraction, and a greater than 20-mm Hg increase in mean gradient on exercise.5 But it has yet to be introduced into the ACC/AHA guidelines as a consideration for surgery.
LEFT VENTRICULAR FUNCTION: BEYOND EJECTION FRACTION
Left ventricular dysfunction is a bad sign for patients with aortic stenosis. Struggling to empty its contents through the narrowed aortic valve, the left ventricle is subjected to increased wall stress and eventually develops hypertrophy. The hypertrophied heart muscle requires more oxygen but receives less perfusion. Eventually, myocardial fibrosis develops, leading to systolic dysfunction and a reduction in the ejection fraction. As described above, patients with asymptomatic aortic stenosis and a left ventricular ejection fraction less than 50% have a poor prognosis,14 and therefore the ACC/AHA guidelines give this condition a class I recommendation for surgery.4
However, the ejection fraction has limitations as a marker of left ventricular function. It reflects changes in left ventricular cavity volume but not in the complex structure of the left ventricle. Several studies show that up to one-third of patients with severe aortic stenosis have considerable impairment of intrinsic myocardial systolic function despite a preserved ejection fraction.8,25,26
Thus, other variables such as left atrial size, left ventricular hypertrophy, myocardial deformation (assessed using strain imaging), and B-type natriuretic peptide (BNP) level may also be considered in assessing the effect of severe aortic stenosis on left ventricular function in the context of a normal ejection fraction (Table 2).
Left ventricular hypertrophy
The development of left ventricular hypertrophy is one of the earliest compensatory responses of the ventricle to the increase in afterload. This leads to impaired myocardial relaxation and reduced myocardial compliance, with resultant diastolic dysfunction with increased filling pressures.
Cioffi et al,27 in a study in 209 patients with severe but asymptomatic aortic stenosis, found that inappropriately high left ventricular mass (> 110% of that expected for body size, sex, and wall stress) portended a 4.5-times higher risk of death, independent of other risk factors.
Severe left ventricular hypertrophy may have a long-term effect on prognosis irrespective of valve replacement. An observational study14 of 3,049 patients who underwent aortic valve replacement for severe aortic stenosis showed that the 10-year survival rate was 45% in those whose left ventricular mass was greater than 185 g/m2, compared with 65% in patients whose left ventricular mass was less than 100 g/m2.
Thus, as surgical mortality and morbidity rates decrease, the impact of these structural changes in left ventricular wall thickness may affect the decision to intervene earlier in order to improve longer-term outcomes in select asymptomatic patients with high-risk features.
Left atrial size
Diastolic dysfunction is caused by increased afterload and results in elevated left ventricular end-diastolic pressure and elevated left atrial pressure. The left atrium responds by dilating, which increases the risk of atrial fibrillation.
Lancellotti et al8 investigated the negative prognostic implications of a large indexed left atrial area in asymptomatic patients with severe aortic stenosis. They found that patients with an indexed left atrial area greater than 12.2 cm2/m2 had a 77% 2-year probability of aortic valve replacement or death.
Beach et al28 examined cardiac remodeling after surgery and found that the left atrial diameter did not decrease after aortic valve replacement, even after left ventricular hypertrophy reversed. This observation has major prognostic implications. Patients with a severely enlarged left atrium (> 5.0 cm in diameter) had considerably lower survival rates than patients with a diameter less than 3.55 cm at 5 years (61% vs 85%) and at 10 years (28% vs 62%) after aortic valve replacement.
Therefore, left atrial size appears to have an important long-term impact on prognosis in patients with aortic stenosis even after aortic valve replacement and adds valuable information when assessing the effect of aortic stenosis on myocardial function.
B-type natriuretic peptide
Natriuretic peptides are cardiac hormones released in response to myocyte stretch. In aortic stenosis, increased afterload induces significant expression of BNP, N-terminal proBNP,29 and atrial natriuretic peptide,30 with numerous studies showing a good correlation between plasma natriuretic peptide levels and severity of aortic stenosis.31–34
Bergler-Klein et al33 showed that patients with asymptomatic aortic stenosis who developed symptoms during follow-up had higher levels of these biomarkers than patients who remained asymptomatic. Of note, patients with BNP levels lower than 130 pg/mL had significantly better symptom-free survival than those with higher levels, 66% vs 34% at 12 months.
However, these biomarkers are not specific to aortic stenosis and can be elevated in any condition that increases left ventricular stress. Nevertheless, they offer an easy and low-cost way to assess left ventricular function and may give an indication of the total burden of disease on the left ventricle.
Global left ventricular longitudinal strain
In view of the limitations of the left ventricular ejection fraction in identifying changes in the structure of the heart and in early detection of myocardial dysfunction, assessment of myocardial deformation using strain imaging is proving an attractive alternative.
Strain is the normalized, dimensionless measure of deformation of a solid object (such as a segment of myocardium) in response to an applied force or stress.35 A novel echocardiographic technique allows assessment of segmental myocardial deformation and thereby overcomes the limitation of tethering, which limits other echocardiographic techniques in the assessment of systolic function. Strain can be circumferential, longitudinal, or radial and is generally assessed using either tissue Doppler velocities or 2D echocardiographic speckle-tracking techniques. Longitudinal strain has proven to be a more sensitive method than left ventricular ejection fraction in detecting subclinical myocardial dysfunction and is a superior prognosticator in a variety of clinical conditions.36,37
Abnormal strain develops very early in the disease process and can even be seen in patients with mild aortic stenosis.
A study by Kearney et al38 in 146 patients with various degrees of aortic stenosis (26% mild, 21% moderate, and 53% severe) and preserved left ventricular ejection fraction demonstrated that global longitudinal strain worsened with increasing severity of aortic stenosis. Furthermore, global longitudinal strain was a strong independent predictor of all-cause mortality (hazard ratio 1.38, P < .001).
Similarly, in a study by Lancellotti et al8 in 163 patients with at least moderate to severe asymptomatic aortic stenosis, impaired longitudinal myocardial strain was an independent predictor of survival. Patients with longitudinal strain greater than 15.9% had significantly better outcomes than patients with strain of 15.9% or less (4-year survival 63% vs 22%, P < .001).
Hence, left ventricular global longitudinal strain offers an alternative—perhaps a superior alternative—to left ventricular ejection fraction in detecting and quantifying left ventricular dysfunction in asymptomatic aortic stenosis. It is an exciting new marker for the future in aortic stenosis, with a threshold of strain below 15.9% as a possible cutoff for those at higher risk of poorer outcomes.
WHERE ARE WE NOW? WHERE ARE WE GOING?
Aortic valve replacement in patients with severe but asymptomatic aortic stenosis remains a topic of debate, but support is growing for earlier intervention.
Now that concerns over the safety of exercise stress testing in patients with severe asymptomatic aortic stenosis have subsided following multiple studies,16,17 exercise testing should be performed in patients with asymptomatic severe aortic stenosis suspected of having reduced exercise capacity, with stress echocardiography providing added prognostic information through its assessment of exercise-induced changes in mean pressure gradient19 and systolic pulmonary artery pressure.21–23
Assessing left ventricular function provides important information about prognosis, with left ventricular ejection fraction, left ventricular diameter, left atrial size, BNP, and global longitudinal strain all helping identify asymptomatic patients at higher risk of death. Surgical intervention in asymptomatic patients with severe aortic stenosis may be considered when there is evidence of higher longer-term mortality risk based on reduced functional capacity, excess left ventricular hypertrophy, and abnormal left ventricular function as detected by ancillary methods such as global longitudinal strain and BNP elevation despite a normal left ventricular ejection fraction.
Figure 3 shows a possible algorithm to define which patients would benefit from earlier intervention. However, left ventricular hypertrophy, left atrial diameter, BNP, left ventricular longitudinal strain, and changes in systolic pulmonary artery pressure are not included in the current ACC/AHA guidelines for the management of asymptomatic patients with severe aortic stenosis. Further study is needed to determine whether earlier intervention in those with adverse risk profiles based on the newer evaluation techniques described above leads to better long-term outcomes.
Intervention should especially be considered in those in whom the measured surgical risk is low and in surgical centers at which the mortality rate is low.
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006; 368:1005–1011.
- Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 1997; 29:630–634.
- Schwarz F, Baumann P, Manthey J, et al. The effect of aortic valve replacement on survival. Circulation 1982; 66:1105–1110.
- Nishimura RA, Otto CM, Bonow RO, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:e57–e185.
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS); Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012; 33:2451–2496.
- Rosenhek R, Binder T, Porenta G, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000; 343:611–617.
- Rosenhek R, Zilberszac R, Schemper M, at al. Natural history of very severe aortic stenosis. Circulation 2010; 121:151–156.
- Lancellotti P, Donal E, Magne J, et al. Risk stratification in asymptomatic moderate to severe aortic stenosis: the importance of the valvular, arterial and ventricular interplay. Heart 2010; 96:1364–1371.
- Pai R, Kapoor N, Bansal RC, Varadarajan P. Natural malignant history of asymptomatic severe aortic stenosis: benefit of aortic valve replacement. Ann Thorac Surg 2006; 82:2116–2122.
- American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease); Society of Cardiovascular Anesthesiologists; Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 2006; 48:e1–e148.
- Patel HJ, Herbert MA, Drake DH, et al. Aortic valve replacement: using a statewide cardiac surgical database identifies a procedural volume hinge point. Ann Thorac Surg 2013; 96:1560–1566.
- Johnston DR, Roselli EE. Minimally invasive aortic valve surgery: Cleveland Clinic experience. Ann Cardiothorac Surg 2015;4:140–147.
- Amato MC, Moffa PJ, Werner KE, Ramires JA. Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart 2001; 86:381–386.
- Mihaljevic T, Nowicki ER, Rajeswaran J, et al. Survival after valve replacement for aortic stenosis: implications for decision making. J Thorac Cardiovasc Surg 2008; 135:1270–1279.
- Kang DH, Park SJ, Rim JH, et al. Early surgery versus conventional treatment in asymptomatic very severe aortic stenosis. Circulation 2010; 121:1502–1509.
- Alborino D, Hoffmann JL, Fournet PC, Bloch A. Value of exercise testing to evaluate the indication for surgery in asymptomatic patients with valvular aortic stenosis. J Heart Valve Dis 2002; 11:204–209.
- Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J 2005; 26:1309–1313.
- Rafique AM, Biner S, Ray I, Forrester JS, Tolstrup K, Siegel RJ. Meta-analysis of prognostic value of stress testing in patients with asymptomatic severe aortic stenosis. Am J Cardiol 2009; 104:972–977.
- Lancellotti P, Lebois F, Simon M, Tombeux C, Chauvel C, Pierard LA. Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation 2005; 112(suppl I):I377–I382.
- Marechaux S, Hachicha Z, Bellouin A, et al. Usefulness of exercise-stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur Heart J 2010; 31:1390–1397.
- Cooper R, Ghali J, Simmons BE, Castaner A. Elevated pulmonary artery pressure. An independent predictor of mortality. Chest 1991; 99:112–120.
- McHenry MM, Rice J, Matlof HJ, Flamm MD Jr. Pulmonary hypertension and sudden death in aortic stenosis. Br Heart J 1979; 41:463–467.
- Copeland JG, Griepp RB, Stinson EB, Shumway NE. Long-term follow-up after isolated aortic valve replacement. J Thorac Cardiovasc Surg 1977; 74: 875–889.
- Maréchaux S, Ennezat PV, LeJemtel TH, et al. Left ventricular response to exercise in aortic stenosis: an exercise echocardiographic study. Echocardiography 2007; 24:955–959.
- Cramariuc D, Cioffi G, Rieck AE, et al. Low-flow aortic stenosis in asymptomatic patients: valvular arterial impedance and systolic function from the SEAS substudy. JACC Cardiovasc Imaging 2009; 2:390–399.
- Dumesnil JG, Shoucri RM, Laurenceau JL, Turcot J. A mathematical model of the dynamic geometry of the intact left ventricle and its application to clinical data. Circulation 1979; 59:1024–1034.
- Cioffi G, Faggiano P, Vizzardi E, et al. Prognostic effect of inappropriately high left ventricular mass in asymptomatic severe aortic stenosis. Heart 2011; 97:301–307.
- Beach JM, Mihaljevic T, Rajeswaran J, et al. Ventricular hypertrophy and left atrial dilatation persist and are associated with reduced survival after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg 2014; 147:362–369.e8.
- Vanderheyden M, Goethals M, Verstreken S, et al. Wall stress modulates brain natriuretic peptide production in pressure overload cardiomyopathy. J Am Coll Cardiol 2004; 44:2349–2354.
- Ikeda T, Matsuda K, Itoh H, et al. Plasma levels of brain and atrial natriuretic peptides elevate in proportion to left ventricular end-systolic wall stress in patients with aortic stenosis. Am Heart J 1997; 133:307–314.
- Qi W, Mathisen P, Kjekshus J, et al. Natriuretic peptides in patients with aortic stenosis. Am Heart J 2001; 142:725–732.
- Weber M, Arnold R, Rau M, et al. Relation of N-terminal pro-B-type natriuretic peptide to severity of valvular aortic stenosis. Am J Cardiol 2004; 94:740–745.
- Bergler-Klein J, Klaar U, Heger M, et al. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation 2004; 109:2302–2308.
- Lim P, Monin JL, Monchi M, et al. Predictors of outcome in patients with severe aortic stenosis and normal left ventricular function: role of B-type natriuretic peptide. Eur Heart J 2004; 25:2048–2053.
- Holt B. Strain and strain rate echocardiography and coronary artery disease. Circ Cardiovasc Imaging 2011; 4:179–190.
- Ng AC, Delgado V, Bertini M, et al. Alterations in multidirectional myocardial functions in patients with aortic stenosis and preserved ejection fraction: a two-dimensional speckle tracking analysis. Eur Heart J 2011; 32:1542–1550.
- Ng AC, Delgado V, Bertini M, et al. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol 2009; 104:1398–1401
- Kearney LG, Lu K, Ord M, et al. Global longitudinal strain is a strong independent predictor of all-cause mortality in patients with aortic stenosis. Eur Heart J Cardiovasc Imag 2012; 13:827–833.
Aortic stenosis is the most common valvular heart condition in the developed world, affecting 3% of people between ages 75 and 851 and 4% of people over age 85.2 Aortic valve replacement remains the only treatment proven to reduce the rates of mortality and morbidity in this condition.3 Under current guidelines,4,5 the onset of symptoms of exertional angina, syncope, or dyspnea in a patient who has severe aortic stenosis is a class I indication for surgery—ie, surgery should be performed.
However, high-gradient, severe aortic stenosis that is asymptomatic often poses a dilemma. The annual rate of sudden death in patients with this condition is estimated at 1% to 3%,6–9 but the surgical mortality rate in aortic valve replacement has been as high as 6% in Medicare patients (varying by center and comorbidities).10 Therefore, the traditional teaching was to not surgically replace the valve in asymptomatic patients, based on an adverse risk-benefit ratio. But with improvements in surgical techniques and prostheses, these rates have been reduced to 2.41% at high-volume centers11 (and to less than 1% at some hospitals),12 arguing in favor of earlier intervention.
Complicating the issue, transcatheter aortic valve replacement has become widely available, but further investigation into its use in this patient cohort is warranted.
Furthermore, many patients with severe but apparently asymptomatic aortic stenosis and normal left ventricular ejection fraction may actually have impaired exercise capacity, or they may have structural left ventricular changes such as severe hypertrophy or reduction in global strain, which may worsen the long-term survival rate.13,14
A prospective trial in patients with severe aortic stenosis found that mortality rates were significantly lower in those who underwent surgery early than in those who received conventional treatment, ie, watchful waiting (no specific medical treatment for aortic stenosis is available).15
Patients with asymptomatic severe aortic stenosis are a diverse group; some have a far worse prognosis than others, with or without surgery.
This paper reviews the guidelines for valve replacement in this patient group and the factors useful in establishing who should be considered for early intervention even if they have no classic symptoms (Figure 1).
SIGNS AND SYMPTOMS OF STENOSIS
Aortic stenosis is often first suspected when a patient presents with angina, dyspnea, and syncope, or when an ejection systolic murmur is heard incidentally on physical examination—typically a high-pitched, crescendo-decrescendo, midsystolic ejection murmur that is best heard at the right upper sternal border and that radiates to the carotid arteries.
Several physical findings may help in assessing the severity of aortic stenosis. In mild stenosis, the murmur peaks in early systole, but as the disease progresses the peak moves later into systole. The corollary of this phenomenon is a weak and delayed carotid upstroke known as “pulsus parvus et tardus.” This can be assessed by palpating the carotid artery while auscultating the heart.
The second heart sound becomes progressively softer as the stenosis advances until it is no longer audible. If a fourth heart sound is present, it may be due to concentric left ventricular hypertrophy with reduced left ventricular compliance, and a third heart sound indicates severe left ventricular dysfunction. Both of these findings suggest severe aortic stenosis.
ECHOCARDIOGRAPHIC MEASURES OF SEVERITY
Echocardiography is the best established and most important initial investigation in the assessment of a patient with suspected aortic stenosis. It usually provides accurate information on the severity and the mechanism of stenosis. The following findings indicate severe aortic stenosis:
- Mean pressure gradient > 40 mm Hg
- Peak aortic jet velocity > 4.0 m/s
- Aortic valve area < 1 cm2.
RECOMMENDATIONS FOR SURGERY BASED ON SEVERITY AND SYMPTOMS
The American College of Cardiology and American Heart Association (ACC/AHA)4 have issued the following recommendations for aortic valve replacement, based on the severity of stenosis and on whether the patient has symptoms (Figure 2):
Severe stenosis, with symptoms: class I recommendation (surgery should be done). Without surgery, these patients have a very poor prognosis, with an overall mortality rate of 75% at 3 years.3
Severe stenosis, no symptoms, in patients undergoing cardiac surgery for another indication (eg, coronary artery bypass grafting, ascending aortic surgery, or surgery on other valves): class I recommendation for concomitant aortic valve replacement.
Moderate stenosis, no symptoms, in patients undergoing cardiac surgery for another indication: class IIa recommendation (ie, aortic valve replacement “is reasonable”).
Very severe stenosis (aortic peak velocity > 5.0 m/s or mean pressure gradient ≥ 60 mm Hg), no symptoms, and low risk of death during surgery: class IIa recommendation.
Severe stenosis, no symptoms, and an increase in transaortic velocity of 0.3 m/s or more per year on serial testing or in patients considered to be at high risk for rapid disease progression, such as elderly patients with severe calcification: class IIb recommendation (surgery “can be considered”). The threshold to replace the valve is lower for patients who cannot make serial follow-up appointments because they live far away or lack transportation, or because they have problems with compliance.
Surgery for those with left ventricular dysfunction
Echocardiography also provides information on left ventricular function, and patients with left ventricular dysfunction have significantly worse outcomes. Studies have shown substantial differences in survival in patients who had an ejection fraction of less than 50% before valve replacement compared with those with a normal ejection fraction.3
Thus, the ACC/AHA guidelines recommend immediate referral for aortic valve replacement in asymptomatic patients whose left ventricular ejection fraction is less than 50% (class I recommendation, level of evidence B) in the hope of preventing irreversible ventricular dysfunction.4
TREADMILL EXERCISE TESTING UNMASKS SYMPTOMS
In the past, severe aortic stenosis was considered a contraindication to stress testing because of concerns of precipitating severe, life-threatening complications. However, studies over the past 10 years have shown that a supervised modified Bruce protocol is safe in patients with severe asymptomatic aortic stenosis.16,17
However, treadmill exercise testing clearly is absolutely contraindicated in patients with severe symptomatic aortic stenosis because of the risk of syncope or of precipitating a malignant arrhythmia. Nevertheless, it may play an essential role in the workup of a physically active patient with no symptoms.
Symptoms can develop insidiously in patients with chronic valve disease and may often go unrecognized by patients and their physicians. Many patients who state they have no symptoms may actually be subconsciously limiting their exercise to avoid symptoms.
Amato et al13 examined the exercise capacity of 66 patients reported to have severe asymptomatic aortic stenosis. Treadmill exercise testing was considered positive in this study if the patient developed symptoms or complex ventricular arrhythmias, had blood pressure that failed to rise by 20 mm Hg, or developed horizontal or down-sloping ST depression (≥ 1 mm in men, ≥ 2 mm in women). Twenty (30.3%) of the 66 patients developed symptoms during exercise testing, and they had a significantly worse prognosis: the 2-year event-free survival rate was only 19% in those with a positive test compared with 85% in those with a negative test.13 This study highlights the problem of patients subconsciously reducing their level of activity, thereby masking their true symptoms.
A meta-analysis by Rafique et al18 found that asymptomatic patients with abnormal results on exercise testing had a risk of cardiac events during follow-up that was eight times higher than normal, and a risk of sudden death 5.5 times higher.
With trials demonstrating that exercise testing is safe and prognostically useful in patients with aortic stenosis, the ACC/AHA guidelines emphasize its role, giving a class I recommendation for aortic valve replacement in patients who develop symptoms on exercise testing, and a class IIa recommendation in asymptomatic patients with decreased exercise tolerance or an exercise-related fall in blood pressure (Figure 2).4
STRESS ECHOCARDIOGRAPHY
Stress echocardiography has been used since the 1980s to assess the hemodynamic consequences of valvular heart disease, and many studies highlight its prognostic usefulness in patients with asymptomatic aortic stenosis.
In a 2005 study by Lancellotti et al,19 69 patients with severe asymptomatic aortic stenosis underwent a symptom-limited bicycle exercise stress test using quantitative Doppler echocardiography both at rest and at peak exercise, and a number of independent predictors of poor outcome (ie, symptoms, aortic valve replacement, death) were identified. These predictors included an abnormal test result, defined as any of the following: angina, dyspnea, ST-segment depression of 2 mm Hg or more, a fall or a small (< 20 mm Hg) rise in systolic blood pressure during the test, an aortic valve area of 0.75 cm2 or less, or a mean increase in valve gradient of 18 mm Hg or more.
Subsequently, a multicenter prospective trial assessed the value of exercise stress echocardiography in 186 patients with asymptomatic moderate or severe aortic stenosis.20 A mean increase in the aortic valve gradient of 20 mm Hg or more after exercise was associated with a rate of cardiovascular events (death, aortic valve replacement) 3.8 times higher, independent of other risk factors and whether moderate or severe stenosis was present (Table 1).20
Exercise-induced changes in systolic pulmonary artery pressure, which can be assessed using stress echocardiography, also have prognostic utility. Elevated systolic pulmonary artery pressure (> 50 mm Hg) seems to portend a poorer prognosis21,22 and a higher mortality rate after valve replacement,23 making it an independent predictor of hospital mortality and postoperative major adverse cardiovascular and cerebrovascular events (Table 1).
Exercise echocardiography also can be used to assess the patient’s contractile reserve. Left ventricular contractile reserve can be defined as an exercise-induced increase in left ventricular ejection fraction. In a study by Maréchaux et al24 in 50 patients with asymptomatic aortic stenosis and a normal resting left ventricular ejection fraction (> 50%), 40% of patients did not have left ventricular contractile reserve. In fact, their left ventricular ejection fraction decreased with exercise (from 64 ± 10% to 53 ± 12%). The subgroup of patients without contractile reserve developed symptoms more frequently during exercise and had lower event-free survival (Table 1).
Stress echocardiography has recently been introduced into the European Society of Cardiology guidelines, which give a class IIb indication for aortic valve replacement in asymptomatic patients who have severe aortic stenosis, a normal ejection fraction, and a greater than 20-mm Hg increase in mean gradient on exercise.5 But it has yet to be introduced into the ACC/AHA guidelines as a consideration for surgery.
LEFT VENTRICULAR FUNCTION: BEYOND EJECTION FRACTION
Left ventricular dysfunction is a bad sign for patients with aortic stenosis. Struggling to empty its contents through the narrowed aortic valve, the left ventricle is subjected to increased wall stress and eventually develops hypertrophy. The hypertrophied heart muscle requires more oxygen but receives less perfusion. Eventually, myocardial fibrosis develops, leading to systolic dysfunction and a reduction in the ejection fraction. As described above, patients with asymptomatic aortic stenosis and a left ventricular ejection fraction less than 50% have a poor prognosis,14 and therefore the ACC/AHA guidelines give this condition a class I recommendation for surgery.4
However, the ejection fraction has limitations as a marker of left ventricular function. It reflects changes in left ventricular cavity volume but not in the complex structure of the left ventricle. Several studies show that up to one-third of patients with severe aortic stenosis have considerable impairment of intrinsic myocardial systolic function despite a preserved ejection fraction.8,25,26
Thus, other variables such as left atrial size, left ventricular hypertrophy, myocardial deformation (assessed using strain imaging), and B-type natriuretic peptide (BNP) level may also be considered in assessing the effect of severe aortic stenosis on left ventricular function in the context of a normal ejection fraction (Table 2).
Left ventricular hypertrophy
The development of left ventricular hypertrophy is one of the earliest compensatory responses of the ventricle to the increase in afterload. This leads to impaired myocardial relaxation and reduced myocardial compliance, with resultant diastolic dysfunction with increased filling pressures.
Cioffi et al,27 in a study in 209 patients with severe but asymptomatic aortic stenosis, found that inappropriately high left ventricular mass (> 110% of that expected for body size, sex, and wall stress) portended a 4.5-times higher risk of death, independent of other risk factors.
Severe left ventricular hypertrophy may have a long-term effect on prognosis irrespective of valve replacement. An observational study14 of 3,049 patients who underwent aortic valve replacement for severe aortic stenosis showed that the 10-year survival rate was 45% in those whose left ventricular mass was greater than 185 g/m2, compared with 65% in patients whose left ventricular mass was less than 100 g/m2.
Thus, as surgical mortality and morbidity rates decrease, the impact of these structural changes in left ventricular wall thickness may affect the decision to intervene earlier in order to improve longer-term outcomes in select asymptomatic patients with high-risk features.
Left atrial size
Diastolic dysfunction is caused by increased afterload and results in elevated left ventricular end-diastolic pressure and elevated left atrial pressure. The left atrium responds by dilating, which increases the risk of atrial fibrillation.
Lancellotti et al8 investigated the negative prognostic implications of a large indexed left atrial area in asymptomatic patients with severe aortic stenosis. They found that patients with an indexed left atrial area greater than 12.2 cm2/m2 had a 77% 2-year probability of aortic valve replacement or death.
Beach et al28 examined cardiac remodeling after surgery and found that the left atrial diameter did not decrease after aortic valve replacement, even after left ventricular hypertrophy reversed. This observation has major prognostic implications. Patients with a severely enlarged left atrium (> 5.0 cm in diameter) had considerably lower survival rates than patients with a diameter less than 3.55 cm at 5 years (61% vs 85%) and at 10 years (28% vs 62%) after aortic valve replacement.
Therefore, left atrial size appears to have an important long-term impact on prognosis in patients with aortic stenosis even after aortic valve replacement and adds valuable information when assessing the effect of aortic stenosis on myocardial function.
B-type natriuretic peptide
Natriuretic peptides are cardiac hormones released in response to myocyte stretch. In aortic stenosis, increased afterload induces significant expression of BNP, N-terminal proBNP,29 and atrial natriuretic peptide,30 with numerous studies showing a good correlation between plasma natriuretic peptide levels and severity of aortic stenosis.31–34
Bergler-Klein et al33 showed that patients with asymptomatic aortic stenosis who developed symptoms during follow-up had higher levels of these biomarkers than patients who remained asymptomatic. Of note, patients with BNP levels lower than 130 pg/mL had significantly better symptom-free survival than those with higher levels, 66% vs 34% at 12 months.
However, these biomarkers are not specific to aortic stenosis and can be elevated in any condition that increases left ventricular stress. Nevertheless, they offer an easy and low-cost way to assess left ventricular function and may give an indication of the total burden of disease on the left ventricle.
Global left ventricular longitudinal strain
In view of the limitations of the left ventricular ejection fraction in identifying changes in the structure of the heart and in early detection of myocardial dysfunction, assessment of myocardial deformation using strain imaging is proving an attractive alternative.
Strain is the normalized, dimensionless measure of deformation of a solid object (such as a segment of myocardium) in response to an applied force or stress.35 A novel echocardiographic technique allows assessment of segmental myocardial deformation and thereby overcomes the limitation of tethering, which limits other echocardiographic techniques in the assessment of systolic function. Strain can be circumferential, longitudinal, or radial and is generally assessed using either tissue Doppler velocities or 2D echocardiographic speckle-tracking techniques. Longitudinal strain has proven to be a more sensitive method than left ventricular ejection fraction in detecting subclinical myocardial dysfunction and is a superior prognosticator in a variety of clinical conditions.36,37
Abnormal strain develops very early in the disease process and can even be seen in patients with mild aortic stenosis.
A study by Kearney et al38 in 146 patients with various degrees of aortic stenosis (26% mild, 21% moderate, and 53% severe) and preserved left ventricular ejection fraction demonstrated that global longitudinal strain worsened with increasing severity of aortic stenosis. Furthermore, global longitudinal strain was a strong independent predictor of all-cause mortality (hazard ratio 1.38, P < .001).
Similarly, in a study by Lancellotti et al8 in 163 patients with at least moderate to severe asymptomatic aortic stenosis, impaired longitudinal myocardial strain was an independent predictor of survival. Patients with longitudinal strain greater than 15.9% had significantly better outcomes than patients with strain of 15.9% or less (4-year survival 63% vs 22%, P < .001).
Hence, left ventricular global longitudinal strain offers an alternative—perhaps a superior alternative—to left ventricular ejection fraction in detecting and quantifying left ventricular dysfunction in asymptomatic aortic stenosis. It is an exciting new marker for the future in aortic stenosis, with a threshold of strain below 15.9% as a possible cutoff for those at higher risk of poorer outcomes.
WHERE ARE WE NOW? WHERE ARE WE GOING?
Aortic valve replacement in patients with severe but asymptomatic aortic stenosis remains a topic of debate, but support is growing for earlier intervention.
Now that concerns over the safety of exercise stress testing in patients with severe asymptomatic aortic stenosis have subsided following multiple studies,16,17 exercise testing should be performed in patients with asymptomatic severe aortic stenosis suspected of having reduced exercise capacity, with stress echocardiography providing added prognostic information through its assessment of exercise-induced changes in mean pressure gradient19 and systolic pulmonary artery pressure.21–23
Assessing left ventricular function provides important information about prognosis, with left ventricular ejection fraction, left ventricular diameter, left atrial size, BNP, and global longitudinal strain all helping identify asymptomatic patients at higher risk of death. Surgical intervention in asymptomatic patients with severe aortic stenosis may be considered when there is evidence of higher longer-term mortality risk based on reduced functional capacity, excess left ventricular hypertrophy, and abnormal left ventricular function as detected by ancillary methods such as global longitudinal strain and BNP elevation despite a normal left ventricular ejection fraction.
Figure 3 shows a possible algorithm to define which patients would benefit from earlier intervention. However, left ventricular hypertrophy, left atrial diameter, BNP, left ventricular longitudinal strain, and changes in systolic pulmonary artery pressure are not included in the current ACC/AHA guidelines for the management of asymptomatic patients with severe aortic stenosis. Further study is needed to determine whether earlier intervention in those with adverse risk profiles based on the newer evaluation techniques described above leads to better long-term outcomes.
Intervention should especially be considered in those in whom the measured surgical risk is low and in surgical centers at which the mortality rate is low.
Aortic stenosis is the most common valvular heart condition in the developed world, affecting 3% of people between ages 75 and 851 and 4% of people over age 85.2 Aortic valve replacement remains the only treatment proven to reduce the rates of mortality and morbidity in this condition.3 Under current guidelines,4,5 the onset of symptoms of exertional angina, syncope, or dyspnea in a patient who has severe aortic stenosis is a class I indication for surgery—ie, surgery should be performed.
However, high-gradient, severe aortic stenosis that is asymptomatic often poses a dilemma. The annual rate of sudden death in patients with this condition is estimated at 1% to 3%,6–9 but the surgical mortality rate in aortic valve replacement has been as high as 6% in Medicare patients (varying by center and comorbidities).10 Therefore, the traditional teaching was to not surgically replace the valve in asymptomatic patients, based on an adverse risk-benefit ratio. But with improvements in surgical techniques and prostheses, these rates have been reduced to 2.41% at high-volume centers11 (and to less than 1% at some hospitals),12 arguing in favor of earlier intervention.
Complicating the issue, transcatheter aortic valve replacement has become widely available, but further investigation into its use in this patient cohort is warranted.
Furthermore, many patients with severe but apparently asymptomatic aortic stenosis and normal left ventricular ejection fraction may actually have impaired exercise capacity, or they may have structural left ventricular changes such as severe hypertrophy or reduction in global strain, which may worsen the long-term survival rate.13,14
A prospective trial in patients with severe aortic stenosis found that mortality rates were significantly lower in those who underwent surgery early than in those who received conventional treatment, ie, watchful waiting (no specific medical treatment for aortic stenosis is available).15
Patients with asymptomatic severe aortic stenosis are a diverse group; some have a far worse prognosis than others, with or without surgery.
This paper reviews the guidelines for valve replacement in this patient group and the factors useful in establishing who should be considered for early intervention even if they have no classic symptoms (Figure 1).
SIGNS AND SYMPTOMS OF STENOSIS
Aortic stenosis is often first suspected when a patient presents with angina, dyspnea, and syncope, or when an ejection systolic murmur is heard incidentally on physical examination—typically a high-pitched, crescendo-decrescendo, midsystolic ejection murmur that is best heard at the right upper sternal border and that radiates to the carotid arteries.
Several physical findings may help in assessing the severity of aortic stenosis. In mild stenosis, the murmur peaks in early systole, but as the disease progresses the peak moves later into systole. The corollary of this phenomenon is a weak and delayed carotid upstroke known as “pulsus parvus et tardus.” This can be assessed by palpating the carotid artery while auscultating the heart.
The second heart sound becomes progressively softer as the stenosis advances until it is no longer audible. If a fourth heart sound is present, it may be due to concentric left ventricular hypertrophy with reduced left ventricular compliance, and a third heart sound indicates severe left ventricular dysfunction. Both of these findings suggest severe aortic stenosis.
ECHOCARDIOGRAPHIC MEASURES OF SEVERITY
Echocardiography is the best established and most important initial investigation in the assessment of a patient with suspected aortic stenosis. It usually provides accurate information on the severity and the mechanism of stenosis. The following findings indicate severe aortic stenosis:
- Mean pressure gradient > 40 mm Hg
- Peak aortic jet velocity > 4.0 m/s
- Aortic valve area < 1 cm2.
RECOMMENDATIONS FOR SURGERY BASED ON SEVERITY AND SYMPTOMS
The American College of Cardiology and American Heart Association (ACC/AHA)4 have issued the following recommendations for aortic valve replacement, based on the severity of stenosis and on whether the patient has symptoms (Figure 2):
Severe stenosis, with symptoms: class I recommendation (surgery should be done). Without surgery, these patients have a very poor prognosis, with an overall mortality rate of 75% at 3 years.3
Severe stenosis, no symptoms, in patients undergoing cardiac surgery for another indication (eg, coronary artery bypass grafting, ascending aortic surgery, or surgery on other valves): class I recommendation for concomitant aortic valve replacement.
Moderate stenosis, no symptoms, in patients undergoing cardiac surgery for another indication: class IIa recommendation (ie, aortic valve replacement “is reasonable”).
Very severe stenosis (aortic peak velocity > 5.0 m/s or mean pressure gradient ≥ 60 mm Hg), no symptoms, and low risk of death during surgery: class IIa recommendation.
Severe stenosis, no symptoms, and an increase in transaortic velocity of 0.3 m/s or more per year on serial testing or in patients considered to be at high risk for rapid disease progression, such as elderly patients with severe calcification: class IIb recommendation (surgery “can be considered”). The threshold to replace the valve is lower for patients who cannot make serial follow-up appointments because they live far away or lack transportation, or because they have problems with compliance.
Surgery for those with left ventricular dysfunction
Echocardiography also provides information on left ventricular function, and patients with left ventricular dysfunction have significantly worse outcomes. Studies have shown substantial differences in survival in patients who had an ejection fraction of less than 50% before valve replacement compared with those with a normal ejection fraction.3
Thus, the ACC/AHA guidelines recommend immediate referral for aortic valve replacement in asymptomatic patients whose left ventricular ejection fraction is less than 50% (class I recommendation, level of evidence B) in the hope of preventing irreversible ventricular dysfunction.4
TREADMILL EXERCISE TESTING UNMASKS SYMPTOMS
In the past, severe aortic stenosis was considered a contraindication to stress testing because of concerns of precipitating severe, life-threatening complications. However, studies over the past 10 years have shown that a supervised modified Bruce protocol is safe in patients with severe asymptomatic aortic stenosis.16,17
However, treadmill exercise testing clearly is absolutely contraindicated in patients with severe symptomatic aortic stenosis because of the risk of syncope or of precipitating a malignant arrhythmia. Nevertheless, it may play an essential role in the workup of a physically active patient with no symptoms.
Symptoms can develop insidiously in patients with chronic valve disease and may often go unrecognized by patients and their physicians. Many patients who state they have no symptoms may actually be subconsciously limiting their exercise to avoid symptoms.
Amato et al13 examined the exercise capacity of 66 patients reported to have severe asymptomatic aortic stenosis. Treadmill exercise testing was considered positive in this study if the patient developed symptoms or complex ventricular arrhythmias, had blood pressure that failed to rise by 20 mm Hg, or developed horizontal or down-sloping ST depression (≥ 1 mm in men, ≥ 2 mm in women). Twenty (30.3%) of the 66 patients developed symptoms during exercise testing, and they had a significantly worse prognosis: the 2-year event-free survival rate was only 19% in those with a positive test compared with 85% in those with a negative test.13 This study highlights the problem of patients subconsciously reducing their level of activity, thereby masking their true symptoms.
A meta-analysis by Rafique et al18 found that asymptomatic patients with abnormal results on exercise testing had a risk of cardiac events during follow-up that was eight times higher than normal, and a risk of sudden death 5.5 times higher.
With trials demonstrating that exercise testing is safe and prognostically useful in patients with aortic stenosis, the ACC/AHA guidelines emphasize its role, giving a class I recommendation for aortic valve replacement in patients who develop symptoms on exercise testing, and a class IIa recommendation in asymptomatic patients with decreased exercise tolerance or an exercise-related fall in blood pressure (Figure 2).4
STRESS ECHOCARDIOGRAPHY
Stress echocardiography has been used since the 1980s to assess the hemodynamic consequences of valvular heart disease, and many studies highlight its prognostic usefulness in patients with asymptomatic aortic stenosis.
In a 2005 study by Lancellotti et al,19 69 patients with severe asymptomatic aortic stenosis underwent a symptom-limited bicycle exercise stress test using quantitative Doppler echocardiography both at rest and at peak exercise, and a number of independent predictors of poor outcome (ie, symptoms, aortic valve replacement, death) were identified. These predictors included an abnormal test result, defined as any of the following: angina, dyspnea, ST-segment depression of 2 mm Hg or more, a fall or a small (< 20 mm Hg) rise in systolic blood pressure during the test, an aortic valve area of 0.75 cm2 or less, or a mean increase in valve gradient of 18 mm Hg or more.
Subsequently, a multicenter prospective trial assessed the value of exercise stress echocardiography in 186 patients with asymptomatic moderate or severe aortic stenosis.20 A mean increase in the aortic valve gradient of 20 mm Hg or more after exercise was associated with a rate of cardiovascular events (death, aortic valve replacement) 3.8 times higher, independent of other risk factors and whether moderate or severe stenosis was present (Table 1).20
Exercise-induced changes in systolic pulmonary artery pressure, which can be assessed using stress echocardiography, also have prognostic utility. Elevated systolic pulmonary artery pressure (> 50 mm Hg) seems to portend a poorer prognosis21,22 and a higher mortality rate after valve replacement,23 making it an independent predictor of hospital mortality and postoperative major adverse cardiovascular and cerebrovascular events (Table 1).
Exercise echocardiography also can be used to assess the patient’s contractile reserve. Left ventricular contractile reserve can be defined as an exercise-induced increase in left ventricular ejection fraction. In a study by Maréchaux et al24 in 50 patients with asymptomatic aortic stenosis and a normal resting left ventricular ejection fraction (> 50%), 40% of patients did not have left ventricular contractile reserve. In fact, their left ventricular ejection fraction decreased with exercise (from 64 ± 10% to 53 ± 12%). The subgroup of patients without contractile reserve developed symptoms more frequently during exercise and had lower event-free survival (Table 1).
Stress echocardiography has recently been introduced into the European Society of Cardiology guidelines, which give a class IIb indication for aortic valve replacement in asymptomatic patients who have severe aortic stenosis, a normal ejection fraction, and a greater than 20-mm Hg increase in mean gradient on exercise.5 But it has yet to be introduced into the ACC/AHA guidelines as a consideration for surgery.
LEFT VENTRICULAR FUNCTION: BEYOND EJECTION FRACTION
Left ventricular dysfunction is a bad sign for patients with aortic stenosis. Struggling to empty its contents through the narrowed aortic valve, the left ventricle is subjected to increased wall stress and eventually develops hypertrophy. The hypertrophied heart muscle requires more oxygen but receives less perfusion. Eventually, myocardial fibrosis develops, leading to systolic dysfunction and a reduction in the ejection fraction. As described above, patients with asymptomatic aortic stenosis and a left ventricular ejection fraction less than 50% have a poor prognosis,14 and therefore the ACC/AHA guidelines give this condition a class I recommendation for surgery.4
However, the ejection fraction has limitations as a marker of left ventricular function. It reflects changes in left ventricular cavity volume but not in the complex structure of the left ventricle. Several studies show that up to one-third of patients with severe aortic stenosis have considerable impairment of intrinsic myocardial systolic function despite a preserved ejection fraction.8,25,26
Thus, other variables such as left atrial size, left ventricular hypertrophy, myocardial deformation (assessed using strain imaging), and B-type natriuretic peptide (BNP) level may also be considered in assessing the effect of severe aortic stenosis on left ventricular function in the context of a normal ejection fraction (Table 2).
Left ventricular hypertrophy
The development of left ventricular hypertrophy is one of the earliest compensatory responses of the ventricle to the increase in afterload. This leads to impaired myocardial relaxation and reduced myocardial compliance, with resultant diastolic dysfunction with increased filling pressures.
Cioffi et al,27 in a study in 209 patients with severe but asymptomatic aortic stenosis, found that inappropriately high left ventricular mass (> 110% of that expected for body size, sex, and wall stress) portended a 4.5-times higher risk of death, independent of other risk factors.
Severe left ventricular hypertrophy may have a long-term effect on prognosis irrespective of valve replacement. An observational study14 of 3,049 patients who underwent aortic valve replacement for severe aortic stenosis showed that the 10-year survival rate was 45% in those whose left ventricular mass was greater than 185 g/m2, compared with 65% in patients whose left ventricular mass was less than 100 g/m2.
Thus, as surgical mortality and morbidity rates decrease, the impact of these structural changes in left ventricular wall thickness may affect the decision to intervene earlier in order to improve longer-term outcomes in select asymptomatic patients with high-risk features.
Left atrial size
Diastolic dysfunction is caused by increased afterload and results in elevated left ventricular end-diastolic pressure and elevated left atrial pressure. The left atrium responds by dilating, which increases the risk of atrial fibrillation.
Lancellotti et al8 investigated the negative prognostic implications of a large indexed left atrial area in asymptomatic patients with severe aortic stenosis. They found that patients with an indexed left atrial area greater than 12.2 cm2/m2 had a 77% 2-year probability of aortic valve replacement or death.
Beach et al28 examined cardiac remodeling after surgery and found that the left atrial diameter did not decrease after aortic valve replacement, even after left ventricular hypertrophy reversed. This observation has major prognostic implications. Patients with a severely enlarged left atrium (> 5.0 cm in diameter) had considerably lower survival rates than patients with a diameter less than 3.55 cm at 5 years (61% vs 85%) and at 10 years (28% vs 62%) after aortic valve replacement.
Therefore, left atrial size appears to have an important long-term impact on prognosis in patients with aortic stenosis even after aortic valve replacement and adds valuable information when assessing the effect of aortic stenosis on myocardial function.
B-type natriuretic peptide
Natriuretic peptides are cardiac hormones released in response to myocyte stretch. In aortic stenosis, increased afterload induces significant expression of BNP, N-terminal proBNP,29 and atrial natriuretic peptide,30 with numerous studies showing a good correlation between plasma natriuretic peptide levels and severity of aortic stenosis.31–34
Bergler-Klein et al33 showed that patients with asymptomatic aortic stenosis who developed symptoms during follow-up had higher levels of these biomarkers than patients who remained asymptomatic. Of note, patients with BNP levels lower than 130 pg/mL had significantly better symptom-free survival than those with higher levels, 66% vs 34% at 12 months.
However, these biomarkers are not specific to aortic stenosis and can be elevated in any condition that increases left ventricular stress. Nevertheless, they offer an easy and low-cost way to assess left ventricular function and may give an indication of the total burden of disease on the left ventricle.
Global left ventricular longitudinal strain
In view of the limitations of the left ventricular ejection fraction in identifying changes in the structure of the heart and in early detection of myocardial dysfunction, assessment of myocardial deformation using strain imaging is proving an attractive alternative.
Strain is the normalized, dimensionless measure of deformation of a solid object (such as a segment of myocardium) in response to an applied force or stress.35 A novel echocardiographic technique allows assessment of segmental myocardial deformation and thereby overcomes the limitation of tethering, which limits other echocardiographic techniques in the assessment of systolic function. Strain can be circumferential, longitudinal, or radial and is generally assessed using either tissue Doppler velocities or 2D echocardiographic speckle-tracking techniques. Longitudinal strain has proven to be a more sensitive method than left ventricular ejection fraction in detecting subclinical myocardial dysfunction and is a superior prognosticator in a variety of clinical conditions.36,37
Abnormal strain develops very early in the disease process and can even be seen in patients with mild aortic stenosis.
A study by Kearney et al38 in 146 patients with various degrees of aortic stenosis (26% mild, 21% moderate, and 53% severe) and preserved left ventricular ejection fraction demonstrated that global longitudinal strain worsened with increasing severity of aortic stenosis. Furthermore, global longitudinal strain was a strong independent predictor of all-cause mortality (hazard ratio 1.38, P < .001).
Similarly, in a study by Lancellotti et al8 in 163 patients with at least moderate to severe asymptomatic aortic stenosis, impaired longitudinal myocardial strain was an independent predictor of survival. Patients with longitudinal strain greater than 15.9% had significantly better outcomes than patients with strain of 15.9% or less (4-year survival 63% vs 22%, P < .001).
Hence, left ventricular global longitudinal strain offers an alternative—perhaps a superior alternative—to left ventricular ejection fraction in detecting and quantifying left ventricular dysfunction in asymptomatic aortic stenosis. It is an exciting new marker for the future in aortic stenosis, with a threshold of strain below 15.9% as a possible cutoff for those at higher risk of poorer outcomes.
WHERE ARE WE NOW? WHERE ARE WE GOING?
Aortic valve replacement in patients with severe but asymptomatic aortic stenosis remains a topic of debate, but support is growing for earlier intervention.
Now that concerns over the safety of exercise stress testing in patients with severe asymptomatic aortic stenosis have subsided following multiple studies,16,17 exercise testing should be performed in patients with asymptomatic severe aortic stenosis suspected of having reduced exercise capacity, with stress echocardiography providing added prognostic information through its assessment of exercise-induced changes in mean pressure gradient19 and systolic pulmonary artery pressure.21–23
Assessing left ventricular function provides important information about prognosis, with left ventricular ejection fraction, left ventricular diameter, left atrial size, BNP, and global longitudinal strain all helping identify asymptomatic patients at higher risk of death. Surgical intervention in asymptomatic patients with severe aortic stenosis may be considered when there is evidence of higher longer-term mortality risk based on reduced functional capacity, excess left ventricular hypertrophy, and abnormal left ventricular function as detected by ancillary methods such as global longitudinal strain and BNP elevation despite a normal left ventricular ejection fraction.
Figure 3 shows a possible algorithm to define which patients would benefit from earlier intervention. However, left ventricular hypertrophy, left atrial diameter, BNP, left ventricular longitudinal strain, and changes in systolic pulmonary artery pressure are not included in the current ACC/AHA guidelines for the management of asymptomatic patients with severe aortic stenosis. Further study is needed to determine whether earlier intervention in those with adverse risk profiles based on the newer evaluation techniques described above leads to better long-term outcomes.
Intervention should especially be considered in those in whom the measured surgical risk is low and in surgical centers at which the mortality rate is low.
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006; 368:1005–1011.
- Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 1997; 29:630–634.
- Schwarz F, Baumann P, Manthey J, et al. The effect of aortic valve replacement on survival. Circulation 1982; 66:1105–1110.
- Nishimura RA, Otto CM, Bonow RO, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:e57–e185.
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS); Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012; 33:2451–2496.
- Rosenhek R, Binder T, Porenta G, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000; 343:611–617.
- Rosenhek R, Zilberszac R, Schemper M, at al. Natural history of very severe aortic stenosis. Circulation 2010; 121:151–156.
- Lancellotti P, Donal E, Magne J, et al. Risk stratification in asymptomatic moderate to severe aortic stenosis: the importance of the valvular, arterial and ventricular interplay. Heart 2010; 96:1364–1371.
- Pai R, Kapoor N, Bansal RC, Varadarajan P. Natural malignant history of asymptomatic severe aortic stenosis: benefit of aortic valve replacement. Ann Thorac Surg 2006; 82:2116–2122.
- American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease); Society of Cardiovascular Anesthesiologists; Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 2006; 48:e1–e148.
- Patel HJ, Herbert MA, Drake DH, et al. Aortic valve replacement: using a statewide cardiac surgical database identifies a procedural volume hinge point. Ann Thorac Surg 2013; 96:1560–1566.
- Johnston DR, Roselli EE. Minimally invasive aortic valve surgery: Cleveland Clinic experience. Ann Cardiothorac Surg 2015;4:140–147.
- Amato MC, Moffa PJ, Werner KE, Ramires JA. Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart 2001; 86:381–386.
- Mihaljevic T, Nowicki ER, Rajeswaran J, et al. Survival after valve replacement for aortic stenosis: implications for decision making. J Thorac Cardiovasc Surg 2008; 135:1270–1279.
- Kang DH, Park SJ, Rim JH, et al. Early surgery versus conventional treatment in asymptomatic very severe aortic stenosis. Circulation 2010; 121:1502–1509.
- Alborino D, Hoffmann JL, Fournet PC, Bloch A. Value of exercise testing to evaluate the indication for surgery in asymptomatic patients with valvular aortic stenosis. J Heart Valve Dis 2002; 11:204–209.
- Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J 2005; 26:1309–1313.
- Rafique AM, Biner S, Ray I, Forrester JS, Tolstrup K, Siegel RJ. Meta-analysis of prognostic value of stress testing in patients with asymptomatic severe aortic stenosis. Am J Cardiol 2009; 104:972–977.
- Lancellotti P, Lebois F, Simon M, Tombeux C, Chauvel C, Pierard LA. Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation 2005; 112(suppl I):I377–I382.
- Marechaux S, Hachicha Z, Bellouin A, et al. Usefulness of exercise-stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur Heart J 2010; 31:1390–1397.
- Cooper R, Ghali J, Simmons BE, Castaner A. Elevated pulmonary artery pressure. An independent predictor of mortality. Chest 1991; 99:112–120.
- McHenry MM, Rice J, Matlof HJ, Flamm MD Jr. Pulmonary hypertension and sudden death in aortic stenosis. Br Heart J 1979; 41:463–467.
- Copeland JG, Griepp RB, Stinson EB, Shumway NE. Long-term follow-up after isolated aortic valve replacement. J Thorac Cardiovasc Surg 1977; 74: 875–889.
- Maréchaux S, Ennezat PV, LeJemtel TH, et al. Left ventricular response to exercise in aortic stenosis: an exercise echocardiographic study. Echocardiography 2007; 24:955–959.
- Cramariuc D, Cioffi G, Rieck AE, et al. Low-flow aortic stenosis in asymptomatic patients: valvular arterial impedance and systolic function from the SEAS substudy. JACC Cardiovasc Imaging 2009; 2:390–399.
- Dumesnil JG, Shoucri RM, Laurenceau JL, Turcot J. A mathematical model of the dynamic geometry of the intact left ventricle and its application to clinical data. Circulation 1979; 59:1024–1034.
- Cioffi G, Faggiano P, Vizzardi E, et al. Prognostic effect of inappropriately high left ventricular mass in asymptomatic severe aortic stenosis. Heart 2011; 97:301–307.
- Beach JM, Mihaljevic T, Rajeswaran J, et al. Ventricular hypertrophy and left atrial dilatation persist and are associated with reduced survival after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg 2014; 147:362–369.e8.
- Vanderheyden M, Goethals M, Verstreken S, et al. Wall stress modulates brain natriuretic peptide production in pressure overload cardiomyopathy. J Am Coll Cardiol 2004; 44:2349–2354.
- Ikeda T, Matsuda K, Itoh H, et al. Plasma levels of brain and atrial natriuretic peptides elevate in proportion to left ventricular end-systolic wall stress in patients with aortic stenosis. Am Heart J 1997; 133:307–314.
- Qi W, Mathisen P, Kjekshus J, et al. Natriuretic peptides in patients with aortic stenosis. Am Heart J 2001; 142:725–732.
- Weber M, Arnold R, Rau M, et al. Relation of N-terminal pro-B-type natriuretic peptide to severity of valvular aortic stenosis. Am J Cardiol 2004; 94:740–745.
- Bergler-Klein J, Klaar U, Heger M, et al. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation 2004; 109:2302–2308.
- Lim P, Monin JL, Monchi M, et al. Predictors of outcome in patients with severe aortic stenosis and normal left ventricular function: role of B-type natriuretic peptide. Eur Heart J 2004; 25:2048–2053.
- Holt B. Strain and strain rate echocardiography and coronary artery disease. Circ Cardiovasc Imaging 2011; 4:179–190.
- Ng AC, Delgado V, Bertini M, et al. Alterations in multidirectional myocardial functions in patients with aortic stenosis and preserved ejection fraction: a two-dimensional speckle tracking analysis. Eur Heart J 2011; 32:1542–1550.
- Ng AC, Delgado V, Bertini M, et al. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol 2009; 104:1398–1401
- Kearney LG, Lu K, Ord M, et al. Global longitudinal strain is a strong independent predictor of all-cause mortality in patients with aortic stenosis. Eur Heart J Cardiovasc Imag 2012; 13:827–833.
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006; 368:1005–1011.
- Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 1997; 29:630–634.
- Schwarz F, Baumann P, Manthey J, et al. The effect of aortic valve replacement on survival. Circulation 1982; 66:1105–1110.
- Nishimura RA, Otto CM, Bonow RO, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:e57–e185.
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS); Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012; 33:2451–2496.
- Rosenhek R, Binder T, Porenta G, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000; 343:611–617.
- Rosenhek R, Zilberszac R, Schemper M, at al. Natural history of very severe aortic stenosis. Circulation 2010; 121:151–156.
- Lancellotti P, Donal E, Magne J, et al. Risk stratification in asymptomatic moderate to severe aortic stenosis: the importance of the valvular, arterial and ventricular interplay. Heart 2010; 96:1364–1371.
- Pai R, Kapoor N, Bansal RC, Varadarajan P. Natural malignant history of asymptomatic severe aortic stenosis: benefit of aortic valve replacement. Ann Thorac Surg 2006; 82:2116–2122.
- American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease); Society of Cardiovascular Anesthesiologists; Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 2006; 48:e1–e148.
- Patel HJ, Herbert MA, Drake DH, et al. Aortic valve replacement: using a statewide cardiac surgical database identifies a procedural volume hinge point. Ann Thorac Surg 2013; 96:1560–1566.
- Johnston DR, Roselli EE. Minimally invasive aortic valve surgery: Cleveland Clinic experience. Ann Cardiothorac Surg 2015;4:140–147.
- Amato MC, Moffa PJ, Werner KE, Ramires JA. Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart 2001; 86:381–386.
- Mihaljevic T, Nowicki ER, Rajeswaran J, et al. Survival after valve replacement for aortic stenosis: implications for decision making. J Thorac Cardiovasc Surg 2008; 135:1270–1279.
- Kang DH, Park SJ, Rim JH, et al. Early surgery versus conventional treatment in asymptomatic very severe aortic stenosis. Circulation 2010; 121:1502–1509.
- Alborino D, Hoffmann JL, Fournet PC, Bloch A. Value of exercise testing to evaluate the indication for surgery in asymptomatic patients with valvular aortic stenosis. J Heart Valve Dis 2002; 11:204–209.
- Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J 2005; 26:1309–1313.
- Rafique AM, Biner S, Ray I, Forrester JS, Tolstrup K, Siegel RJ. Meta-analysis of prognostic value of stress testing in patients with asymptomatic severe aortic stenosis. Am J Cardiol 2009; 104:972–977.
- Lancellotti P, Lebois F, Simon M, Tombeux C, Chauvel C, Pierard LA. Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation 2005; 112(suppl I):I377–I382.
- Marechaux S, Hachicha Z, Bellouin A, et al. Usefulness of exercise-stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur Heart J 2010; 31:1390–1397.
- Cooper R, Ghali J, Simmons BE, Castaner A. Elevated pulmonary artery pressure. An independent predictor of mortality. Chest 1991; 99:112–120.
- McHenry MM, Rice J, Matlof HJ, Flamm MD Jr. Pulmonary hypertension and sudden death in aortic stenosis. Br Heart J 1979; 41:463–467.
- Copeland JG, Griepp RB, Stinson EB, Shumway NE. Long-term follow-up after isolated aortic valve replacement. J Thorac Cardiovasc Surg 1977; 74: 875–889.
- Maréchaux S, Ennezat PV, LeJemtel TH, et al. Left ventricular response to exercise in aortic stenosis: an exercise echocardiographic study. Echocardiography 2007; 24:955–959.
- Cramariuc D, Cioffi G, Rieck AE, et al. Low-flow aortic stenosis in asymptomatic patients: valvular arterial impedance and systolic function from the SEAS substudy. JACC Cardiovasc Imaging 2009; 2:390–399.
- Dumesnil JG, Shoucri RM, Laurenceau JL, Turcot J. A mathematical model of the dynamic geometry of the intact left ventricle and its application to clinical data. Circulation 1979; 59:1024–1034.
- Cioffi G, Faggiano P, Vizzardi E, et al. Prognostic effect of inappropriately high left ventricular mass in asymptomatic severe aortic stenosis. Heart 2011; 97:301–307.
- Beach JM, Mihaljevic T, Rajeswaran J, et al. Ventricular hypertrophy and left atrial dilatation persist and are associated with reduced survival after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg 2014; 147:362–369.e8.
- Vanderheyden M, Goethals M, Verstreken S, et al. Wall stress modulates brain natriuretic peptide production in pressure overload cardiomyopathy. J Am Coll Cardiol 2004; 44:2349–2354.
- Ikeda T, Matsuda K, Itoh H, et al. Plasma levels of brain and atrial natriuretic peptides elevate in proportion to left ventricular end-systolic wall stress in patients with aortic stenosis. Am Heart J 1997; 133:307–314.
- Qi W, Mathisen P, Kjekshus J, et al. Natriuretic peptides in patients with aortic stenosis. Am Heart J 2001; 142:725–732.
- Weber M, Arnold R, Rau M, et al. Relation of N-terminal pro-B-type natriuretic peptide to severity of valvular aortic stenosis. Am J Cardiol 2004; 94:740–745.
- Bergler-Klein J, Klaar U, Heger M, et al. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation 2004; 109:2302–2308.
- Lim P, Monin JL, Monchi M, et al. Predictors of outcome in patients with severe aortic stenosis and normal left ventricular function: role of B-type natriuretic peptide. Eur Heart J 2004; 25:2048–2053.
- Holt B. Strain and strain rate echocardiography and coronary artery disease. Circ Cardiovasc Imaging 2011; 4:179–190.
- Ng AC, Delgado V, Bertini M, et al. Alterations in multidirectional myocardial functions in patients with aortic stenosis and preserved ejection fraction: a two-dimensional speckle tracking analysis. Eur Heart J 2011; 32:1542–1550.
- Ng AC, Delgado V, Bertini M, et al. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol 2009; 104:1398–1401
- Kearney LG, Lu K, Ord M, et al. Global longitudinal strain is a strong independent predictor of all-cause mortality in patients with aortic stenosis. Eur Heart J Cardiovasc Imag 2012; 13:827–833.
KEY POINTS
- Echocardiography is the best established and most important initial test in patients with suspected aortic stenosis.
- Traditional echocardiographic variables used in assessing aortic stenosis and the need for surgery are the pressure gradient across the valve, the velocity through the valve, the valve area, and the left ventricular ejection fraction.
- Aortic valve replacement is recommended for severe aortic stenosis if the patient has symptoms. It is also recommended if the left ventricular ejection fraction is less than 50%, if the patient is undergoing other cardiac surgery, or if symptoms arise on exercise stress testing.
- Novel assessment variables include left ventricular hypertrophy, left atrial size, B-type natriuretic peptide level, and global left ventricular longitudinal strain.
Phrenic nerve paralysis induced by brachial plexus block
A 72-year-old man underwent elective ambulatory arthroscopic repair of the right shoulder rotator cuff. To manage postoperative pain, a supraclavicular catheter was placed for brachial plexus block, and he was sent home with a ropivacaine infusion pump.
The next day, he presented to the emergency department with right-sided chest pain and mild shortness of breath. He had normal vital signs and adequate oxygen saturation on room air. On physical examination, breath sounds were decreased at the right lung base, and chest radiography (Figure 1) revealed an isolated elevated right hemidiaphragm, a clear indication of phrenic nerve paralysis from local infiltration of the infusion.
The ropivacaine infusion was stopped, and the supraclavicular catheter was removed under anesthesia. He was admitted to the hospital for observation, and over the course of 8 to 12 hours his shortness of breath resolved, and his findings on lung examination normalized. Repeat chest radiography 24 hours after his emergency room presentation showed regular positioning of his diaphragm (Figure 2).
RECOGNIZING AND MANAGING PHRENIC NERVE PARALYSIS
The scenario described here illustrates the importance of recognizing symptomatic phrenic nerve paralysis as a result of local infiltration of anesthetic from supraclavicular brachial plexus block. Regional anesthesia is commonly used for perioperative analgesia for minor shoulder surgeries. Because these blocks anesthetize the trunks formed by the C5–T1 nerve roots, infiltration of the anesthetic agent to the proximal nerve roots resulting in phrenic nerve paralysis is a common complication.
Although phrenic nerve paralysis has been reported to some degree in nearly all patients, reports of significant shortness of breath and radiographic evidence of hemidiaphragm are few.1–4 When it occurs, the analgesic regimen must be changed from regional anesthesia to oral or parenteral pain medications. Resolution of symptoms and radiographic abnormalities usually occurs spontaneously.
When available, an ultrasonographically guided approach for supraclavicular brachial plexus blocks is preferred over a blind approach and is associated with a higher success rate and a lower rate of complications.5,6
A potentially life-threatening complication of brachial plexus block is pneumothorax.
Contraindications to brachial plexus block include severe lung disease and previous surgery or interventions with the potential for phrenic nerve injury that could result in bilateral paralysis of the diaphragm. Ultimately, preprocedural chest radiography in selected patients at high risk should be considered to mitigate this risk.
- Tran QH, Clemente A, Doan J, Finlayson RJ. Brachial plexus blocks: a review of approaches and techniques. Can J Anaesth 2007; 54:662–674.
- Mian A, Chaudhry I, Huang R, Rizk E, Tubbs RS, Loukas M. Brachial plexus anesthesia: a review of the relevant anatomy, complications, and anatomical variations. Clin Anat 2014; 27:210–221.
- Knoblanche GE. The incidence and aetiology of phrenic nerve blockade associated with supraclavicular brachial plexus block. Anaesth Intensive Care 1979; 7:346–349.
- Urmey WF, Talts KH, Sharrock NE. One hundred percent incidence of hemidiaphragmatic paresis associated with interscalene brachial plexus anesthesia as diagnosed by ultrasonography. Anesth Analg 1991; 72:498–503.
- Gelfand HJ, Ouanes JP, Lesley MR, et al. Analgesic efficacy of ultrasound-guided regional anesthesia: a meta-analysis. J Clin Anesth 2011; 23:90–96.
- Sandhu NS, Capan LM. Ultrasound-guided infraclavicular brachial plexus block. Br J Anaesth 2002; 89:254–259.
A 72-year-old man underwent elective ambulatory arthroscopic repair of the right shoulder rotator cuff. To manage postoperative pain, a supraclavicular catheter was placed for brachial plexus block, and he was sent home with a ropivacaine infusion pump.
The next day, he presented to the emergency department with right-sided chest pain and mild shortness of breath. He had normal vital signs and adequate oxygen saturation on room air. On physical examination, breath sounds were decreased at the right lung base, and chest radiography (Figure 1) revealed an isolated elevated right hemidiaphragm, a clear indication of phrenic nerve paralysis from local infiltration of the infusion.
The ropivacaine infusion was stopped, and the supraclavicular catheter was removed under anesthesia. He was admitted to the hospital for observation, and over the course of 8 to 12 hours his shortness of breath resolved, and his findings on lung examination normalized. Repeat chest radiography 24 hours after his emergency room presentation showed regular positioning of his diaphragm (Figure 2).
RECOGNIZING AND MANAGING PHRENIC NERVE PARALYSIS
The scenario described here illustrates the importance of recognizing symptomatic phrenic nerve paralysis as a result of local infiltration of anesthetic from supraclavicular brachial plexus block. Regional anesthesia is commonly used for perioperative analgesia for minor shoulder surgeries. Because these blocks anesthetize the trunks formed by the C5–T1 nerve roots, infiltration of the anesthetic agent to the proximal nerve roots resulting in phrenic nerve paralysis is a common complication.
Although phrenic nerve paralysis has been reported to some degree in nearly all patients, reports of significant shortness of breath and radiographic evidence of hemidiaphragm are few.1–4 When it occurs, the analgesic regimen must be changed from regional anesthesia to oral or parenteral pain medications. Resolution of symptoms and radiographic abnormalities usually occurs spontaneously.
When available, an ultrasonographically guided approach for supraclavicular brachial plexus blocks is preferred over a blind approach and is associated with a higher success rate and a lower rate of complications.5,6
A potentially life-threatening complication of brachial plexus block is pneumothorax.
Contraindications to brachial plexus block include severe lung disease and previous surgery or interventions with the potential for phrenic nerve injury that could result in bilateral paralysis of the diaphragm. Ultimately, preprocedural chest radiography in selected patients at high risk should be considered to mitigate this risk.
A 72-year-old man underwent elective ambulatory arthroscopic repair of the right shoulder rotator cuff. To manage postoperative pain, a supraclavicular catheter was placed for brachial plexus block, and he was sent home with a ropivacaine infusion pump.
The next day, he presented to the emergency department with right-sided chest pain and mild shortness of breath. He had normal vital signs and adequate oxygen saturation on room air. On physical examination, breath sounds were decreased at the right lung base, and chest radiography (Figure 1) revealed an isolated elevated right hemidiaphragm, a clear indication of phrenic nerve paralysis from local infiltration of the infusion.
The ropivacaine infusion was stopped, and the supraclavicular catheter was removed under anesthesia. He was admitted to the hospital for observation, and over the course of 8 to 12 hours his shortness of breath resolved, and his findings on lung examination normalized. Repeat chest radiography 24 hours after his emergency room presentation showed regular positioning of his diaphragm (Figure 2).
RECOGNIZING AND MANAGING PHRENIC NERVE PARALYSIS
The scenario described here illustrates the importance of recognizing symptomatic phrenic nerve paralysis as a result of local infiltration of anesthetic from supraclavicular brachial plexus block. Regional anesthesia is commonly used for perioperative analgesia for minor shoulder surgeries. Because these blocks anesthetize the trunks formed by the C5–T1 nerve roots, infiltration of the anesthetic agent to the proximal nerve roots resulting in phrenic nerve paralysis is a common complication.
Although phrenic nerve paralysis has been reported to some degree in nearly all patients, reports of significant shortness of breath and radiographic evidence of hemidiaphragm are few.1–4 When it occurs, the analgesic regimen must be changed from regional anesthesia to oral or parenteral pain medications. Resolution of symptoms and radiographic abnormalities usually occurs spontaneously.
When available, an ultrasonographically guided approach for supraclavicular brachial plexus blocks is preferred over a blind approach and is associated with a higher success rate and a lower rate of complications.5,6
A potentially life-threatening complication of brachial plexus block is pneumothorax.
Contraindications to brachial plexus block include severe lung disease and previous surgery or interventions with the potential for phrenic nerve injury that could result in bilateral paralysis of the diaphragm. Ultimately, preprocedural chest radiography in selected patients at high risk should be considered to mitigate this risk.
- Tran QH, Clemente A, Doan J, Finlayson RJ. Brachial plexus blocks: a review of approaches and techniques. Can J Anaesth 2007; 54:662–674.
- Mian A, Chaudhry I, Huang R, Rizk E, Tubbs RS, Loukas M. Brachial plexus anesthesia: a review of the relevant anatomy, complications, and anatomical variations. Clin Anat 2014; 27:210–221.
- Knoblanche GE. The incidence and aetiology of phrenic nerve blockade associated with supraclavicular brachial plexus block. Anaesth Intensive Care 1979; 7:346–349.
- Urmey WF, Talts KH, Sharrock NE. One hundred percent incidence of hemidiaphragmatic paresis associated with interscalene brachial plexus anesthesia as diagnosed by ultrasonography. Anesth Analg 1991; 72:498–503.
- Gelfand HJ, Ouanes JP, Lesley MR, et al. Analgesic efficacy of ultrasound-guided regional anesthesia: a meta-analysis. J Clin Anesth 2011; 23:90–96.
- Sandhu NS, Capan LM. Ultrasound-guided infraclavicular brachial plexus block. Br J Anaesth 2002; 89:254–259.
- Tran QH, Clemente A, Doan J, Finlayson RJ. Brachial plexus blocks: a review of approaches and techniques. Can J Anaesth 2007; 54:662–674.
- Mian A, Chaudhry I, Huang R, Rizk E, Tubbs RS, Loukas M. Brachial plexus anesthesia: a review of the relevant anatomy, complications, and anatomical variations. Clin Anat 2014; 27:210–221.
- Knoblanche GE. The incidence and aetiology of phrenic nerve blockade associated with supraclavicular brachial plexus block. Anaesth Intensive Care 1979; 7:346–349.
- Urmey WF, Talts KH, Sharrock NE. One hundred percent incidence of hemidiaphragmatic paresis associated with interscalene brachial plexus anesthesia as diagnosed by ultrasonography. Anesth Analg 1991; 72:498–503.
- Gelfand HJ, Ouanes JP, Lesley MR, et al. Analgesic efficacy of ultrasound-guided regional anesthesia: a meta-analysis. J Clin Anesth 2011; 23:90–96.
- Sandhu NS, Capan LM. Ultrasound-guided infraclavicular brachial plexus block. Br J Anaesth 2002; 89:254–259.
Emergency Imaging: Presyncopal episode
An 85-year-old man presented to the ED with a presyncopal episode, which included lightheadedness and sharp chest pain. His medical history was significant for atrial fibrillation, for which he was taking warfarin. In addition to warfarin, the patient had recently completed a 5-day dose pack of azithromycin for pneumonia. Despite treatment for the pneumonia, he reported persistent episodes of cough and mild hemoptysis.
Radiographs and a noncontrast computed tomography (CT) scan of the chest were obtained. A representative posterior-anterior radiograph (Figure 1a) and a coronal noncontrast CT image (Figure 2a) are shown above.
What is your diagnosis?
What additional imaging, if any, should be performed?
Answer
The frontal chest radiograph demonstrated abnormal peripheral opacity at the left lung base (white arrow, Figure 1b), and the noncontrast chest CT demonstrated a peripheral, wedge/pyramid-shaped subpleural ground-glass opacity (white arrow, Figure 2b). Based on the persistent peripheral opacity despite treatment, and the patient’s clinical symptoms of acute sharp chest pain/hemoptysis, a pulmonary infarct was considered as part of the differential diagnosis, and a contrast-enhanced pulmonary embolism (PE) protocol CT was obtained for further evaluation. A coronal image from the contrast-enhanced CT demonstrated the wedge-shaped peripheral opacity (white arrow, Figure 3) as well as filling defects in the bilateral pulmonary arteries (red arrows, Figure 3), indicating the presence of PE.
Large PE, such as those seen in this case, may result in peripheral infarcts due to occlusion of the pulmonary arteries. The subpleural location of the infarcts typically causes acute pleuritic chest pain, which this patient experienced.
The radiographic appearance of pulmonary infarct was originally described in 1940 by Hampton and Castleman and is commonly referred to as Hampton’s hump.1 Chest radiographic imaging, however, is often not specific in patients with suspected PE. In the Prospective Investigation of Pulmonary Embolism Diagnosis Study, the most common chest radiographic findings in patients with angiographically documented PE were atelectasis and/or parenchymal opacities in the affected lung zone, but there was no significant difference in prevalence seen in patients without PE. Although a Hampton’s hump is a more specific finding, it is often not present, and is therefore not a reliable marker for PE.2 As this case illustrates, in patients with high clinical probability of PE, peripheral areas of consolidation may not always represent pneumonia and should be evaluated further with contrast-enhanced CT.
1. Hampton AO, Castleman B. Correlation of postmortem chest teleroentgenograms with autopsy findings with special reference to pulmonary embolism and infarction. Am J Roentgenol Radium Ther. 1940;43:305-326.
2. Worsley DF, Alavi A, Aronchick JM, Chen JT, Greenspan RH, Ravin CE. Chest radiographic findings in patients with acute pulmonary embolism: observations from the PIOPED Study. Radiology. 1993;189(1):133-136.
An 85-year-old man presented to the ED with a presyncopal episode, which included lightheadedness and sharp chest pain. His medical history was significant for atrial fibrillation, for which he was taking warfarin. In addition to warfarin, the patient had recently completed a 5-day dose pack of azithromycin for pneumonia. Despite treatment for the pneumonia, he reported persistent episodes of cough and mild hemoptysis.
Radiographs and a noncontrast computed tomography (CT) scan of the chest were obtained. A representative posterior-anterior radiograph (Figure 1a) and a coronal noncontrast CT image (Figure 2a) are shown above.
What is your diagnosis?
What additional imaging, if any, should be performed?
Answer
The frontal chest radiograph demonstrated abnormal peripheral opacity at the left lung base (white arrow, Figure 1b), and the noncontrast chest CT demonstrated a peripheral, wedge/pyramid-shaped subpleural ground-glass opacity (white arrow, Figure 2b). Based on the persistent peripheral opacity despite treatment, and the patient’s clinical symptoms of acute sharp chest pain/hemoptysis, a pulmonary infarct was considered as part of the differential diagnosis, and a contrast-enhanced pulmonary embolism (PE) protocol CT was obtained for further evaluation. A coronal image from the contrast-enhanced CT demonstrated the wedge-shaped peripheral opacity (white arrow, Figure 3) as well as filling defects in the bilateral pulmonary arteries (red arrows, Figure 3), indicating the presence of PE.
Large PE, such as those seen in this case, may result in peripheral infarcts due to occlusion of the pulmonary arteries. The subpleural location of the infarcts typically causes acute pleuritic chest pain, which this patient experienced.
The radiographic appearance of pulmonary infarct was originally described in 1940 by Hampton and Castleman and is commonly referred to as Hampton’s hump.1 Chest radiographic imaging, however, is often not specific in patients with suspected PE. In the Prospective Investigation of Pulmonary Embolism Diagnosis Study, the most common chest radiographic findings in patients with angiographically documented PE were atelectasis and/or parenchymal opacities in the affected lung zone, but there was no significant difference in prevalence seen in patients without PE. Although a Hampton’s hump is a more specific finding, it is often not present, and is therefore not a reliable marker for PE.2 As this case illustrates, in patients with high clinical probability of PE, peripheral areas of consolidation may not always represent pneumonia and should be evaluated further with contrast-enhanced CT.
An 85-year-old man presented to the ED with a presyncopal episode, which included lightheadedness and sharp chest pain. His medical history was significant for atrial fibrillation, for which he was taking warfarin. In addition to warfarin, the patient had recently completed a 5-day dose pack of azithromycin for pneumonia. Despite treatment for the pneumonia, he reported persistent episodes of cough and mild hemoptysis.
Radiographs and a noncontrast computed tomography (CT) scan of the chest were obtained. A representative posterior-anterior radiograph (Figure 1a) and a coronal noncontrast CT image (Figure 2a) are shown above.
What is your diagnosis?
What additional imaging, if any, should be performed?
Answer
The frontal chest radiograph demonstrated abnormal peripheral opacity at the left lung base (white arrow, Figure 1b), and the noncontrast chest CT demonstrated a peripheral, wedge/pyramid-shaped subpleural ground-glass opacity (white arrow, Figure 2b). Based on the persistent peripheral opacity despite treatment, and the patient’s clinical symptoms of acute sharp chest pain/hemoptysis, a pulmonary infarct was considered as part of the differential diagnosis, and a contrast-enhanced pulmonary embolism (PE) protocol CT was obtained for further evaluation. A coronal image from the contrast-enhanced CT demonstrated the wedge-shaped peripheral opacity (white arrow, Figure 3) as well as filling defects in the bilateral pulmonary arteries (red arrows, Figure 3), indicating the presence of PE.
Large PE, such as those seen in this case, may result in peripheral infarcts due to occlusion of the pulmonary arteries. The subpleural location of the infarcts typically causes acute pleuritic chest pain, which this patient experienced.
The radiographic appearance of pulmonary infarct was originally described in 1940 by Hampton and Castleman and is commonly referred to as Hampton’s hump.1 Chest radiographic imaging, however, is often not specific in patients with suspected PE. In the Prospective Investigation of Pulmonary Embolism Diagnosis Study, the most common chest radiographic findings in patients with angiographically documented PE were atelectasis and/or parenchymal opacities in the affected lung zone, but there was no significant difference in prevalence seen in patients without PE. Although a Hampton’s hump is a more specific finding, it is often not present, and is therefore not a reliable marker for PE.2 As this case illustrates, in patients with high clinical probability of PE, peripheral areas of consolidation may not always represent pneumonia and should be evaluated further with contrast-enhanced CT.
1. Hampton AO, Castleman B. Correlation of postmortem chest teleroentgenograms with autopsy findings with special reference to pulmonary embolism and infarction. Am J Roentgenol Radium Ther. 1940;43:305-326.
2. Worsley DF, Alavi A, Aronchick JM, Chen JT, Greenspan RH, Ravin CE. Chest radiographic findings in patients with acute pulmonary embolism: observations from the PIOPED Study. Radiology. 1993;189(1):133-136.
1. Hampton AO, Castleman B. Correlation of postmortem chest teleroentgenograms with autopsy findings with special reference to pulmonary embolism and infarction. Am J Roentgenol Radium Ther. 1940;43:305-326.
2. Worsley DF, Alavi A, Aronchick JM, Chen JT, Greenspan RH, Ravin CE. Chest radiographic findings in patients with acute pulmonary embolism: observations from the PIOPED Study. Radiology. 1993;189(1):133-136.
Progressive Cardiomyopathy in a Patient With Elevated Cobalt Ion Levels and Bilateral Metal-on-Metal Hip Arthroplasties
Systemic cobalt toxicity has been reported in the literature after hip arthroplasty revisions for failed ceramic components secondary to third-body abrasive wear of cobalt-chrome (CoCr) components, as well as with metal-on-metal (MOM) hip arthroplasty designs. There have been several cases of systemic cobalt toxicity after revision for fractured ceramic components.1,2 Of these 7 reported cases, all patients had neurologic complaints and 4 patients developed cardiomyopathy secondary to toxic cobalt levels, with 1 case being fatal.1 MOM hip prostheses have also been associated with local and systemic problems secondary to metal debris. Adverse local tissue reactions have been reported to occur in up to 59% of patients, and, in some registries, the failure rate of MOM arthroplasty caused by these soft-tissue reactions is 2 to 3 times that of conventional metal-on-polyethylene design failures.3,4 The occurrence of systemic complications from MOM total hip arthroplasty (THA) wear debris is much less common. There have been 6 cases of systemic cobalt toxicity reported in the literature resulting from MOM total hip prosthesis design.1,2
We present a case of biopsy-confirmed cardiomyopathy secondary to cobalt toxicity from a MOM THA design with subsequent requirement for left ventricular assist device (LVAD) implantation despite prosthesis removal. To our knowledge, this is the first report in the literature of this specific implant design causing systemic cobalt toxicity. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a healthy nondiabetic man age 54 years who presented to our clinic 6 years after undergoing left THA and 5 years after undergoing right THA with the Biomet M2a-Magnum MOM prosthesis at an outside facility. The left-side components placed at the index procedure were a size 50 cup, 44 magnum head, 10 Taperloc stem (Biomet), and +9 neck. The right-side components were a size 52 cup, 46 magnum head, 10 Taperloc stem, and +3 neck. The patient emphasized that he was very happy with his hip prostheses and denied groin or thigh pain. His medical history was significant for exogenous obesity, and he denied any history of alcohol, tobacco, steroid, or recreational drug use.
The patient’s review of systems suggested that, approximately 11 months prior to presentation at our facility, he began having difficulty with his activities of daily living secondary to chest pressure with exertion, fatigue, and associated diaphoresis. He complained of decreased sensation in his feet bilaterally but denied any hearing loss, tinnitus, or vision changes. He underwent evaluation of the new-onset chest discomfort with a cardiac stress test that suggested no active cardiac ischemia. An echocardiogram revealed mitral regurgitation, stage II diastolic dysfunction with a left ventricular ejection fraction of 55%. Additionally, during this time period, the patient was being followed by his local orthopedic surgeon for an elevated cobalt level of 120 ppb and a chromium level of 109 ppb. The patient was referred to our clinic for recommendations regarding the elevated metal-ion levels. Upon initial evaluation, the patient denied any hip or groin pain. His physical examination revealed a nonantalgic gait with full range of motion and no signs of instability, tenderness, or masses. The patient was also noted to have no vibratory sensation in his feet bilaterally. The plain radiographs indicated bilateral MOM THA with acetabular inclination levels of 55º on the right and left sides. No cystic changes or other worrisome signs that would suggest implant loosening or failure were present (Figure 1). The serum metal levels were repeated and showed a cobalt level of 189 ppb and a chromium level of 71 ppb. Whole venous blood samples were drawn at our request using trace element tubes and were sent to Medtox Laboratories Inc. for analysis. Other pertinent laboratory values, including hematocrit and thyroid levels, were within normal limits. Because of concerns of systemic toxicity from significantly elevated cobalt and chromium levels, the patient elected to proceed with revision of the MOM components.
During the preoperative medical evaluation, the patient’s cardiac status was a concern, and the etiology of the cardiac dysfunction was unclear. Cardiac magnetic resonance imaging (MRI), which was performed to evaluate the extent and etiology of cardiac dysfunction, showed biventricular dysfunction. To evaluate the underlying myocardial tissue characteristics, delayed contrast imaging was performed and showed diffuse myocardial hyperenhancement of the anterior, lateral, and apical walls, with sparing of the base and midseptum. This type of extensive hyperenhancement is commonly seen with cardiac amyloidosis; however, the blood-pool kinetics during contrast administration is unusual for amyloidosis, as well as the diffuse edema noted on T2-weighted MRI. Importantly, cardiac MRI is very specific in excluding alternative diagnoses, such as postinfarct, infiltrative, acquired, viral, or alcoholic/drugs of abuse etiologies. In the absence of amyloidosis, the only other pattern that would be consistent with symptoms was diffuse, fulminant myocarditis of toxic origin lacking clinical evidence for an infectious origin. The patient’s prior exposure to cobalt was noted. Thus, the hyperenhancement and edema could be strong supportive evidence of cobalt infiltration, despite no reported cases in the literature of cobalt cardiomyopathy found on cardiac MRI.
Additional workup was initiated, and cardiac catheterization showed that the patient continued to decompensate, with worsening global left ventricular dysfunction with an ejection fraction of 30% without evidence of coronary artery disease. Also, he was noted to have mild renal impairment with a blood urea nitrogen level of 31 mg/dL and a creatinine level of 1.7 mg/dL. The etiology of the renal impairment was unknown and had not been established, according to the patient and his wife. The renal impairment was not thought to be caused by the elevated metal ions levels but likely resulted from prerenal azotemia secondary to decreased cardiac output. During catheterization, an endomyocardial biopsy was performed and the tissue sent to the Mayo Clinic pathology department for analysis. The sample showed myocyte hypertrophy and interstitial fibrosis with scattered myofibers containing large cytoplasmic vacuoles. Also present was karyomegaly consistent with myocyte hypertrophy (Figures 2A, 2B). Trichrome stain confirmed replacement of myofibers by collagen (Figure 2C). Electron microscopy performed on a paraffin block showed reduced contractile elements, vacuolar spaces, and increased lipofuscin. The findings were very consistent with, but not specific for, cardiomyopathy from cobalt toxicity. No evidence of an inflammatory infiltrate was identified. The diagnosis was cobalt cardiomyopathy based on biopsy, presentation, cobalt levels, and intraoperative findings.
The patient was admitted to the cardiac intensive care unit preoperatively and optimized with inotropic agents. A multidisciplinary consultation with the cardiology and anesthesia departments was obtained. Both recommended cardiac anesthesia with intraoperative Swan-Ganz catheter and transesophageal echo monitoring. Assuming that the patient remained hemodynamically stable with limited blood loss and the first hip was timely performed, the cardiology department recommended a single surgery, because fewer risks and complications could be expected than from a staged procedure. Subsequently, surgery was performed on the left hip via a conservative anterior approach on the fracture table. The patient remained stable with limited blood loss. During the same operating room time, revision of the right hip was performed using an anterior approach. The intraoperative findings showed evidence of pseudotumors in the adjacent soft tissues and abundant brown, creamy fluid upon entering the joint capsule, consistent with a metallic appearance. Both hips showed similar prosthetic findings. There was no significant visible wear of the large diameter metal heads or gross abnormality of the acetabular components. The trunnion area on both femoral implants was abnormal, revealing a black coating suggestive of marked corrosion. The components were all well fixed, without visible damage, and, because of his fragile cardiac status, the patient’s acetabular components were not revised. The trunnions were cleaned and the femoral heads were revised to active articulation dual-mobility metal-on-polyethylene constructs using 28-mm Biolox Option ceramic (CeramTec). The tissue specimens from the operation showed chronic inflammation with areas of fibroconnective tissue and bland fibrinoid necrosis with extensive brown pigment-laden macrophage reaction. The intraoperative cultures were negative.
The patient tolerated the surgery without complication, and his postoperative period was without incident. Nine months after surgery, the patient’s cobalt and chromium levels had declined to 16 ppb and 32 ppb, respectively (normal, <1 ppb). However, his cardiac status continued to worsen with significant shortness of breath and bilateral lower extremity edema despite diuresis. Follow-up cardiac MRI indicated progressive left and right dysfunction with ejection fractions of 23% and 25%, respectively. After progressive heart-failure symptoms, the patient was admitted to the hospital for severe congestive heart failure and underwent implantation of a HeartWare LVAD with tricuspid valve repair using an Edwards annuloplasty ring. He has since had a cardiac transplant and is doing well.
Discussion
To our knowledge, this is the first reported case of cardiomyopathy in a patient with elevated cobalt ion levels and a Biomet M2a-Magnum hip prosthesis. This is also the first reported case of cardiac MRI–defined cobalt cardiomyopathy. The cobalt levels seen in this patient were similar to those of other cases with systemic cobalt toxicity from a MOM hip construct. Mao and colleagues5 reported 2 cases of systemic cobalt toxicity in 2 patients with articular surface replacement hip prostheses.One patient presented with mild groin pain, neurologic symptoms, and a cobalt level of 410 ppb 5 years after her index procedure. The other patient presented with cardiac and neurologic symptoms but no hip complaints. The patient’s cobalt levels ranged from 185 ppb to 210 ppb. Both patients improved after their revision surgery, and their cobalt levels decreased. The 2 patients in Tower’s report6 were 49-year-old men who had articular surface replacement implants (DePuy). One patient who presented with progressive hip pain 11 months postoperatively developed neurologic symptoms and cardiomyopathy, with cobalt levels of 83 ppb before revision surgery 43 months after his index procedure. The other patient presented with hip pain and vertigo, headaches, fatigue, and dyspnea. He underwent hip revision 40 months postoperatively and required closed reduction under sedation for dislocation. Finally, and most recently, Allen and colleagues2 reported a 59-year-old woman with a cobalt level of 287 ppb whose symptoms did not resolve after implantation of an LVAD or cardiac transplantation but only after removal of her bilateral hip prosthesis. Our case is most similar to this report but significantly adds to the literature in 2 distinct manners: (1) Biomet M2a-Magnum has not been implicated in cobalt toxicity; and (2) this is the first reported use of dedicated cardiac MRI to noninvasively define underlying cardiac pathology.
The cardiac manifestations secondary to systemic cobalt toxicity in this patient represent a frightening consequence of MOM prosthetic wear. The effects of cobalt toxicity on cardiac tissues were first described in a series of alcoholic patients from Manchester in 1900;7 however, it was not until 1967, in a series of patients in Quebec, that cobalt was found to be the inciting factor. In the modern era, hip arthroplasty techniques resulting in excessive cobalt and chromium wear have demonstrated the same findings of myocyte hypertrophy, interstitial fibrosis, and scattered myofibers containing large cytoplasmic inclusions.8,9 The patient presented here has pathologic findings consistent with previous cases of cobalt cardiomyopathy; however, in the other cases of cardiomyopathy due to MOM total hip components, the patients’ cardiac conditions improved after the prostheses were revised and the cobalt levels began to diminish.5,6In our case, the patient has sustained permanent damage to his myocardium and a progressive decline in his cardiac status, which is a deviation from reported cases as of 2014.
While there is no guideline to unequivocally diagnose cobalt cardiomyopathy, the constellation of findings, including pathologic, biologic, blood levels, imaging, and surgical, all uniformly indicate a unifying diagnosis. The lack of improvement after prosthetic device removal supports a diagnosis of permanent myocardial damage, which is consistent with cardiomyopathy of advanced toxic etiology.
Conclusion
This case presents a patient with bilateral MOM THAs, acetabular cup inclinations of greater than 55º, renal impairment, and cobalt levels greater than 60 ppb, with occult cardiac failure leading to LVAD implantation as a prelude to cardiac transplantation in order to avoid certain death. These factors have been shown, in prior case reports, to be associated with cardiac damage that may be reversible.6 However; it is important for orthopedic surgeons to recognize that certain hip prostheses can be associated or lead to irreversible cardiac damage.
1. Zywiel MG, Brandt JM, Overgaard CB, Cheung AC, Turgeon TR, Syed KA. Fatal cardiomyopathy after revision total hip replacement for fracture of a ceramic liner. Bone Joint J. 2013;95(1):31-37.
2. Allen LA, Ambardekar AV, Devaraj KM, Maleszewski JJ, Wolfel EE. Clinical problem-solving. Missing elements of the history. N Engl J Med. 2014;370(6):559-566.
3. Hart AJ, Satchihananda K, Liddle AD, et al. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: a case-control study using three-dimensional tomography and magnetic resonance imaging. J Bone Joint Surg Am. 2012;94(4);317-325.
4. Kwon MK, Jacobs JJ, MacDonald SJ, Potter HG, Fehring TK, Lombardi AV. Evidence-based understanding of management perils for metal-on-metal hip arthroplasty patients. J Arthroplasty. 2012;27(8 suppl):20-25.
5. Mao X, Wong AA, Crawford RW. Cobalt toxicity- -an emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194(12):649-651.
6. Tower SS. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92(17):2847-2851.
7. Morin Y, Daniel P. Quebec beer-drinkers’ cardiomyopathy: etiological considerations. Can Med Assoc J. 1967;97(15):926-928.
8. Gilbert C, Cheung A, Butany J, et al. Hip pain and heart failure: the missing link. Can J Cardiol. 2013;29(5):639.e1-e2.
9. Seghizzi P, D’Adda F, Borleri D, Barbic F, Mosconi G. Cobalt myocardiopathy. A critical review of literature. Sci Total Environ. 1994;150(1-3):105-109.
Systemic cobalt toxicity has been reported in the literature after hip arthroplasty revisions for failed ceramic components secondary to third-body abrasive wear of cobalt-chrome (CoCr) components, as well as with metal-on-metal (MOM) hip arthroplasty designs. There have been several cases of systemic cobalt toxicity after revision for fractured ceramic components.1,2 Of these 7 reported cases, all patients had neurologic complaints and 4 patients developed cardiomyopathy secondary to toxic cobalt levels, with 1 case being fatal.1 MOM hip prostheses have also been associated with local and systemic problems secondary to metal debris. Adverse local tissue reactions have been reported to occur in up to 59% of patients, and, in some registries, the failure rate of MOM arthroplasty caused by these soft-tissue reactions is 2 to 3 times that of conventional metal-on-polyethylene design failures.3,4 The occurrence of systemic complications from MOM total hip arthroplasty (THA) wear debris is much less common. There have been 6 cases of systemic cobalt toxicity reported in the literature resulting from MOM total hip prosthesis design.1,2
We present a case of biopsy-confirmed cardiomyopathy secondary to cobalt toxicity from a MOM THA design with subsequent requirement for left ventricular assist device (LVAD) implantation despite prosthesis removal. To our knowledge, this is the first report in the literature of this specific implant design causing systemic cobalt toxicity. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a healthy nondiabetic man age 54 years who presented to our clinic 6 years after undergoing left THA and 5 years after undergoing right THA with the Biomet M2a-Magnum MOM prosthesis at an outside facility. The left-side components placed at the index procedure were a size 50 cup, 44 magnum head, 10 Taperloc stem (Biomet), and +9 neck. The right-side components were a size 52 cup, 46 magnum head, 10 Taperloc stem, and +3 neck. The patient emphasized that he was very happy with his hip prostheses and denied groin or thigh pain. His medical history was significant for exogenous obesity, and he denied any history of alcohol, tobacco, steroid, or recreational drug use.
The patient’s review of systems suggested that, approximately 11 months prior to presentation at our facility, he began having difficulty with his activities of daily living secondary to chest pressure with exertion, fatigue, and associated diaphoresis. He complained of decreased sensation in his feet bilaterally but denied any hearing loss, tinnitus, or vision changes. He underwent evaluation of the new-onset chest discomfort with a cardiac stress test that suggested no active cardiac ischemia. An echocardiogram revealed mitral regurgitation, stage II diastolic dysfunction with a left ventricular ejection fraction of 55%. Additionally, during this time period, the patient was being followed by his local orthopedic surgeon for an elevated cobalt level of 120 ppb and a chromium level of 109 ppb. The patient was referred to our clinic for recommendations regarding the elevated metal-ion levels. Upon initial evaluation, the patient denied any hip or groin pain. His physical examination revealed a nonantalgic gait with full range of motion and no signs of instability, tenderness, or masses. The patient was also noted to have no vibratory sensation in his feet bilaterally. The plain radiographs indicated bilateral MOM THA with acetabular inclination levels of 55º on the right and left sides. No cystic changes or other worrisome signs that would suggest implant loosening or failure were present (Figure 1). The serum metal levels were repeated and showed a cobalt level of 189 ppb and a chromium level of 71 ppb. Whole venous blood samples were drawn at our request using trace element tubes and were sent to Medtox Laboratories Inc. for analysis. Other pertinent laboratory values, including hematocrit and thyroid levels, were within normal limits. Because of concerns of systemic toxicity from significantly elevated cobalt and chromium levels, the patient elected to proceed with revision of the MOM components.
During the preoperative medical evaluation, the patient’s cardiac status was a concern, and the etiology of the cardiac dysfunction was unclear. Cardiac magnetic resonance imaging (MRI), which was performed to evaluate the extent and etiology of cardiac dysfunction, showed biventricular dysfunction. To evaluate the underlying myocardial tissue characteristics, delayed contrast imaging was performed and showed diffuse myocardial hyperenhancement of the anterior, lateral, and apical walls, with sparing of the base and midseptum. This type of extensive hyperenhancement is commonly seen with cardiac amyloidosis; however, the blood-pool kinetics during contrast administration is unusual for amyloidosis, as well as the diffuse edema noted on T2-weighted MRI. Importantly, cardiac MRI is very specific in excluding alternative diagnoses, such as postinfarct, infiltrative, acquired, viral, or alcoholic/drugs of abuse etiologies. In the absence of amyloidosis, the only other pattern that would be consistent with symptoms was diffuse, fulminant myocarditis of toxic origin lacking clinical evidence for an infectious origin. The patient’s prior exposure to cobalt was noted. Thus, the hyperenhancement and edema could be strong supportive evidence of cobalt infiltration, despite no reported cases in the literature of cobalt cardiomyopathy found on cardiac MRI.
Additional workup was initiated, and cardiac catheterization showed that the patient continued to decompensate, with worsening global left ventricular dysfunction with an ejection fraction of 30% without evidence of coronary artery disease. Also, he was noted to have mild renal impairment with a blood urea nitrogen level of 31 mg/dL and a creatinine level of 1.7 mg/dL. The etiology of the renal impairment was unknown and had not been established, according to the patient and his wife. The renal impairment was not thought to be caused by the elevated metal ions levels but likely resulted from prerenal azotemia secondary to decreased cardiac output. During catheterization, an endomyocardial biopsy was performed and the tissue sent to the Mayo Clinic pathology department for analysis. The sample showed myocyte hypertrophy and interstitial fibrosis with scattered myofibers containing large cytoplasmic vacuoles. Also present was karyomegaly consistent with myocyte hypertrophy (Figures 2A, 2B). Trichrome stain confirmed replacement of myofibers by collagen (Figure 2C). Electron microscopy performed on a paraffin block showed reduced contractile elements, vacuolar spaces, and increased lipofuscin. The findings were very consistent with, but not specific for, cardiomyopathy from cobalt toxicity. No evidence of an inflammatory infiltrate was identified. The diagnosis was cobalt cardiomyopathy based on biopsy, presentation, cobalt levels, and intraoperative findings.
The patient was admitted to the cardiac intensive care unit preoperatively and optimized with inotropic agents. A multidisciplinary consultation with the cardiology and anesthesia departments was obtained. Both recommended cardiac anesthesia with intraoperative Swan-Ganz catheter and transesophageal echo monitoring. Assuming that the patient remained hemodynamically stable with limited blood loss and the first hip was timely performed, the cardiology department recommended a single surgery, because fewer risks and complications could be expected than from a staged procedure. Subsequently, surgery was performed on the left hip via a conservative anterior approach on the fracture table. The patient remained stable with limited blood loss. During the same operating room time, revision of the right hip was performed using an anterior approach. The intraoperative findings showed evidence of pseudotumors in the adjacent soft tissues and abundant brown, creamy fluid upon entering the joint capsule, consistent with a metallic appearance. Both hips showed similar prosthetic findings. There was no significant visible wear of the large diameter metal heads or gross abnormality of the acetabular components. The trunnion area on both femoral implants was abnormal, revealing a black coating suggestive of marked corrosion. The components were all well fixed, without visible damage, and, because of his fragile cardiac status, the patient’s acetabular components were not revised. The trunnions were cleaned and the femoral heads were revised to active articulation dual-mobility metal-on-polyethylene constructs using 28-mm Biolox Option ceramic (CeramTec). The tissue specimens from the operation showed chronic inflammation with areas of fibroconnective tissue and bland fibrinoid necrosis with extensive brown pigment-laden macrophage reaction. The intraoperative cultures were negative.
The patient tolerated the surgery without complication, and his postoperative period was without incident. Nine months after surgery, the patient’s cobalt and chromium levels had declined to 16 ppb and 32 ppb, respectively (normal, <1 ppb). However, his cardiac status continued to worsen with significant shortness of breath and bilateral lower extremity edema despite diuresis. Follow-up cardiac MRI indicated progressive left and right dysfunction with ejection fractions of 23% and 25%, respectively. After progressive heart-failure symptoms, the patient was admitted to the hospital for severe congestive heart failure and underwent implantation of a HeartWare LVAD with tricuspid valve repair using an Edwards annuloplasty ring. He has since had a cardiac transplant and is doing well.
Discussion
To our knowledge, this is the first reported case of cardiomyopathy in a patient with elevated cobalt ion levels and a Biomet M2a-Magnum hip prosthesis. This is also the first reported case of cardiac MRI–defined cobalt cardiomyopathy. The cobalt levels seen in this patient were similar to those of other cases with systemic cobalt toxicity from a MOM hip construct. Mao and colleagues5 reported 2 cases of systemic cobalt toxicity in 2 patients with articular surface replacement hip prostheses.One patient presented with mild groin pain, neurologic symptoms, and a cobalt level of 410 ppb 5 years after her index procedure. The other patient presented with cardiac and neurologic symptoms but no hip complaints. The patient’s cobalt levels ranged from 185 ppb to 210 ppb. Both patients improved after their revision surgery, and their cobalt levels decreased. The 2 patients in Tower’s report6 were 49-year-old men who had articular surface replacement implants (DePuy). One patient who presented with progressive hip pain 11 months postoperatively developed neurologic symptoms and cardiomyopathy, with cobalt levels of 83 ppb before revision surgery 43 months after his index procedure. The other patient presented with hip pain and vertigo, headaches, fatigue, and dyspnea. He underwent hip revision 40 months postoperatively and required closed reduction under sedation for dislocation. Finally, and most recently, Allen and colleagues2 reported a 59-year-old woman with a cobalt level of 287 ppb whose symptoms did not resolve after implantation of an LVAD or cardiac transplantation but only after removal of her bilateral hip prosthesis. Our case is most similar to this report but significantly adds to the literature in 2 distinct manners: (1) Biomet M2a-Magnum has not been implicated in cobalt toxicity; and (2) this is the first reported use of dedicated cardiac MRI to noninvasively define underlying cardiac pathology.
The cardiac manifestations secondary to systemic cobalt toxicity in this patient represent a frightening consequence of MOM prosthetic wear. The effects of cobalt toxicity on cardiac tissues were first described in a series of alcoholic patients from Manchester in 1900;7 however, it was not until 1967, in a series of patients in Quebec, that cobalt was found to be the inciting factor. In the modern era, hip arthroplasty techniques resulting in excessive cobalt and chromium wear have demonstrated the same findings of myocyte hypertrophy, interstitial fibrosis, and scattered myofibers containing large cytoplasmic inclusions.8,9 The patient presented here has pathologic findings consistent with previous cases of cobalt cardiomyopathy; however, in the other cases of cardiomyopathy due to MOM total hip components, the patients’ cardiac conditions improved after the prostheses were revised and the cobalt levels began to diminish.5,6In our case, the patient has sustained permanent damage to his myocardium and a progressive decline in his cardiac status, which is a deviation from reported cases as of 2014.
While there is no guideline to unequivocally diagnose cobalt cardiomyopathy, the constellation of findings, including pathologic, biologic, blood levels, imaging, and surgical, all uniformly indicate a unifying diagnosis. The lack of improvement after prosthetic device removal supports a diagnosis of permanent myocardial damage, which is consistent with cardiomyopathy of advanced toxic etiology.
Conclusion
This case presents a patient with bilateral MOM THAs, acetabular cup inclinations of greater than 55º, renal impairment, and cobalt levels greater than 60 ppb, with occult cardiac failure leading to LVAD implantation as a prelude to cardiac transplantation in order to avoid certain death. These factors have been shown, in prior case reports, to be associated with cardiac damage that may be reversible.6 However; it is important for orthopedic surgeons to recognize that certain hip prostheses can be associated or lead to irreversible cardiac damage.
Systemic cobalt toxicity has been reported in the literature after hip arthroplasty revisions for failed ceramic components secondary to third-body abrasive wear of cobalt-chrome (CoCr) components, as well as with metal-on-metal (MOM) hip arthroplasty designs. There have been several cases of systemic cobalt toxicity after revision for fractured ceramic components.1,2 Of these 7 reported cases, all patients had neurologic complaints and 4 patients developed cardiomyopathy secondary to toxic cobalt levels, with 1 case being fatal.1 MOM hip prostheses have also been associated with local and systemic problems secondary to metal debris. Adverse local tissue reactions have been reported to occur in up to 59% of patients, and, in some registries, the failure rate of MOM arthroplasty caused by these soft-tissue reactions is 2 to 3 times that of conventional metal-on-polyethylene design failures.3,4 The occurrence of systemic complications from MOM total hip arthroplasty (THA) wear debris is much less common. There have been 6 cases of systemic cobalt toxicity reported in the literature resulting from MOM total hip prosthesis design.1,2
We present a case of biopsy-confirmed cardiomyopathy secondary to cobalt toxicity from a MOM THA design with subsequent requirement for left ventricular assist device (LVAD) implantation despite prosthesis removal. To our knowledge, this is the first report in the literature of this specific implant design causing systemic cobalt toxicity. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a healthy nondiabetic man age 54 years who presented to our clinic 6 years after undergoing left THA and 5 years after undergoing right THA with the Biomet M2a-Magnum MOM prosthesis at an outside facility. The left-side components placed at the index procedure were a size 50 cup, 44 magnum head, 10 Taperloc stem (Biomet), and +9 neck. The right-side components were a size 52 cup, 46 magnum head, 10 Taperloc stem, and +3 neck. The patient emphasized that he was very happy with his hip prostheses and denied groin or thigh pain. His medical history was significant for exogenous obesity, and he denied any history of alcohol, tobacco, steroid, or recreational drug use.
The patient’s review of systems suggested that, approximately 11 months prior to presentation at our facility, he began having difficulty with his activities of daily living secondary to chest pressure with exertion, fatigue, and associated diaphoresis. He complained of decreased sensation in his feet bilaterally but denied any hearing loss, tinnitus, or vision changes. He underwent evaluation of the new-onset chest discomfort with a cardiac stress test that suggested no active cardiac ischemia. An echocardiogram revealed mitral regurgitation, stage II diastolic dysfunction with a left ventricular ejection fraction of 55%. Additionally, during this time period, the patient was being followed by his local orthopedic surgeon for an elevated cobalt level of 120 ppb and a chromium level of 109 ppb. The patient was referred to our clinic for recommendations regarding the elevated metal-ion levels. Upon initial evaluation, the patient denied any hip or groin pain. His physical examination revealed a nonantalgic gait with full range of motion and no signs of instability, tenderness, or masses. The patient was also noted to have no vibratory sensation in his feet bilaterally. The plain radiographs indicated bilateral MOM THA with acetabular inclination levels of 55º on the right and left sides. No cystic changes or other worrisome signs that would suggest implant loosening or failure were present (Figure 1). The serum metal levels were repeated and showed a cobalt level of 189 ppb and a chromium level of 71 ppb. Whole venous blood samples were drawn at our request using trace element tubes and were sent to Medtox Laboratories Inc. for analysis. Other pertinent laboratory values, including hematocrit and thyroid levels, were within normal limits. Because of concerns of systemic toxicity from significantly elevated cobalt and chromium levels, the patient elected to proceed with revision of the MOM components.
During the preoperative medical evaluation, the patient’s cardiac status was a concern, and the etiology of the cardiac dysfunction was unclear. Cardiac magnetic resonance imaging (MRI), which was performed to evaluate the extent and etiology of cardiac dysfunction, showed biventricular dysfunction. To evaluate the underlying myocardial tissue characteristics, delayed contrast imaging was performed and showed diffuse myocardial hyperenhancement of the anterior, lateral, and apical walls, with sparing of the base and midseptum. This type of extensive hyperenhancement is commonly seen with cardiac amyloidosis; however, the blood-pool kinetics during contrast administration is unusual for amyloidosis, as well as the diffuse edema noted on T2-weighted MRI. Importantly, cardiac MRI is very specific in excluding alternative diagnoses, such as postinfarct, infiltrative, acquired, viral, or alcoholic/drugs of abuse etiologies. In the absence of amyloidosis, the only other pattern that would be consistent with symptoms was diffuse, fulminant myocarditis of toxic origin lacking clinical evidence for an infectious origin. The patient’s prior exposure to cobalt was noted. Thus, the hyperenhancement and edema could be strong supportive evidence of cobalt infiltration, despite no reported cases in the literature of cobalt cardiomyopathy found on cardiac MRI.
Additional workup was initiated, and cardiac catheterization showed that the patient continued to decompensate, with worsening global left ventricular dysfunction with an ejection fraction of 30% without evidence of coronary artery disease. Also, he was noted to have mild renal impairment with a blood urea nitrogen level of 31 mg/dL and a creatinine level of 1.7 mg/dL. The etiology of the renal impairment was unknown and had not been established, according to the patient and his wife. The renal impairment was not thought to be caused by the elevated metal ions levels but likely resulted from prerenal azotemia secondary to decreased cardiac output. During catheterization, an endomyocardial biopsy was performed and the tissue sent to the Mayo Clinic pathology department for analysis. The sample showed myocyte hypertrophy and interstitial fibrosis with scattered myofibers containing large cytoplasmic vacuoles. Also present was karyomegaly consistent with myocyte hypertrophy (Figures 2A, 2B). Trichrome stain confirmed replacement of myofibers by collagen (Figure 2C). Electron microscopy performed on a paraffin block showed reduced contractile elements, vacuolar spaces, and increased lipofuscin. The findings were very consistent with, but not specific for, cardiomyopathy from cobalt toxicity. No evidence of an inflammatory infiltrate was identified. The diagnosis was cobalt cardiomyopathy based on biopsy, presentation, cobalt levels, and intraoperative findings.
The patient was admitted to the cardiac intensive care unit preoperatively and optimized with inotropic agents. A multidisciplinary consultation with the cardiology and anesthesia departments was obtained. Both recommended cardiac anesthesia with intraoperative Swan-Ganz catheter and transesophageal echo monitoring. Assuming that the patient remained hemodynamically stable with limited blood loss and the first hip was timely performed, the cardiology department recommended a single surgery, because fewer risks and complications could be expected than from a staged procedure. Subsequently, surgery was performed on the left hip via a conservative anterior approach on the fracture table. The patient remained stable with limited blood loss. During the same operating room time, revision of the right hip was performed using an anterior approach. The intraoperative findings showed evidence of pseudotumors in the adjacent soft tissues and abundant brown, creamy fluid upon entering the joint capsule, consistent with a metallic appearance. Both hips showed similar prosthetic findings. There was no significant visible wear of the large diameter metal heads or gross abnormality of the acetabular components. The trunnion area on both femoral implants was abnormal, revealing a black coating suggestive of marked corrosion. The components were all well fixed, without visible damage, and, because of his fragile cardiac status, the patient’s acetabular components were not revised. The trunnions were cleaned and the femoral heads were revised to active articulation dual-mobility metal-on-polyethylene constructs using 28-mm Biolox Option ceramic (CeramTec). The tissue specimens from the operation showed chronic inflammation with areas of fibroconnective tissue and bland fibrinoid necrosis with extensive brown pigment-laden macrophage reaction. The intraoperative cultures were negative.
The patient tolerated the surgery without complication, and his postoperative period was without incident. Nine months after surgery, the patient’s cobalt and chromium levels had declined to 16 ppb and 32 ppb, respectively (normal, <1 ppb). However, his cardiac status continued to worsen with significant shortness of breath and bilateral lower extremity edema despite diuresis. Follow-up cardiac MRI indicated progressive left and right dysfunction with ejection fractions of 23% and 25%, respectively. After progressive heart-failure symptoms, the patient was admitted to the hospital for severe congestive heart failure and underwent implantation of a HeartWare LVAD with tricuspid valve repair using an Edwards annuloplasty ring. He has since had a cardiac transplant and is doing well.
Discussion
To our knowledge, this is the first reported case of cardiomyopathy in a patient with elevated cobalt ion levels and a Biomet M2a-Magnum hip prosthesis. This is also the first reported case of cardiac MRI–defined cobalt cardiomyopathy. The cobalt levels seen in this patient were similar to those of other cases with systemic cobalt toxicity from a MOM hip construct. Mao and colleagues5 reported 2 cases of systemic cobalt toxicity in 2 patients with articular surface replacement hip prostheses.One patient presented with mild groin pain, neurologic symptoms, and a cobalt level of 410 ppb 5 years after her index procedure. The other patient presented with cardiac and neurologic symptoms but no hip complaints. The patient’s cobalt levels ranged from 185 ppb to 210 ppb. Both patients improved after their revision surgery, and their cobalt levels decreased. The 2 patients in Tower’s report6 were 49-year-old men who had articular surface replacement implants (DePuy). One patient who presented with progressive hip pain 11 months postoperatively developed neurologic symptoms and cardiomyopathy, with cobalt levels of 83 ppb before revision surgery 43 months after his index procedure. The other patient presented with hip pain and vertigo, headaches, fatigue, and dyspnea. He underwent hip revision 40 months postoperatively and required closed reduction under sedation for dislocation. Finally, and most recently, Allen and colleagues2 reported a 59-year-old woman with a cobalt level of 287 ppb whose symptoms did not resolve after implantation of an LVAD or cardiac transplantation but only after removal of her bilateral hip prosthesis. Our case is most similar to this report but significantly adds to the literature in 2 distinct manners: (1) Biomet M2a-Magnum has not been implicated in cobalt toxicity; and (2) this is the first reported use of dedicated cardiac MRI to noninvasively define underlying cardiac pathology.
The cardiac manifestations secondary to systemic cobalt toxicity in this patient represent a frightening consequence of MOM prosthetic wear. The effects of cobalt toxicity on cardiac tissues were first described in a series of alcoholic patients from Manchester in 1900;7 however, it was not until 1967, in a series of patients in Quebec, that cobalt was found to be the inciting factor. In the modern era, hip arthroplasty techniques resulting in excessive cobalt and chromium wear have demonstrated the same findings of myocyte hypertrophy, interstitial fibrosis, and scattered myofibers containing large cytoplasmic inclusions.8,9 The patient presented here has pathologic findings consistent with previous cases of cobalt cardiomyopathy; however, in the other cases of cardiomyopathy due to MOM total hip components, the patients’ cardiac conditions improved after the prostheses were revised and the cobalt levels began to diminish.5,6In our case, the patient has sustained permanent damage to his myocardium and a progressive decline in his cardiac status, which is a deviation from reported cases as of 2014.
While there is no guideline to unequivocally diagnose cobalt cardiomyopathy, the constellation of findings, including pathologic, biologic, blood levels, imaging, and surgical, all uniformly indicate a unifying diagnosis. The lack of improvement after prosthetic device removal supports a diagnosis of permanent myocardial damage, which is consistent with cardiomyopathy of advanced toxic etiology.
Conclusion
This case presents a patient with bilateral MOM THAs, acetabular cup inclinations of greater than 55º, renal impairment, and cobalt levels greater than 60 ppb, with occult cardiac failure leading to LVAD implantation as a prelude to cardiac transplantation in order to avoid certain death. These factors have been shown, in prior case reports, to be associated with cardiac damage that may be reversible.6 However; it is important for orthopedic surgeons to recognize that certain hip prostheses can be associated or lead to irreversible cardiac damage.
1. Zywiel MG, Brandt JM, Overgaard CB, Cheung AC, Turgeon TR, Syed KA. Fatal cardiomyopathy after revision total hip replacement for fracture of a ceramic liner. Bone Joint J. 2013;95(1):31-37.
2. Allen LA, Ambardekar AV, Devaraj KM, Maleszewski JJ, Wolfel EE. Clinical problem-solving. Missing elements of the history. N Engl J Med. 2014;370(6):559-566.
3. Hart AJ, Satchihananda K, Liddle AD, et al. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: a case-control study using three-dimensional tomography and magnetic resonance imaging. J Bone Joint Surg Am. 2012;94(4);317-325.
4. Kwon MK, Jacobs JJ, MacDonald SJ, Potter HG, Fehring TK, Lombardi AV. Evidence-based understanding of management perils for metal-on-metal hip arthroplasty patients. J Arthroplasty. 2012;27(8 suppl):20-25.
5. Mao X, Wong AA, Crawford RW. Cobalt toxicity- -an emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194(12):649-651.
6. Tower SS. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92(17):2847-2851.
7. Morin Y, Daniel P. Quebec beer-drinkers’ cardiomyopathy: etiological considerations. Can Med Assoc J. 1967;97(15):926-928.
8. Gilbert C, Cheung A, Butany J, et al. Hip pain and heart failure: the missing link. Can J Cardiol. 2013;29(5):639.e1-e2.
9. Seghizzi P, D’Adda F, Borleri D, Barbic F, Mosconi G. Cobalt myocardiopathy. A critical review of literature. Sci Total Environ. 1994;150(1-3):105-109.
1. Zywiel MG, Brandt JM, Overgaard CB, Cheung AC, Turgeon TR, Syed KA. Fatal cardiomyopathy after revision total hip replacement for fracture of a ceramic liner. Bone Joint J. 2013;95(1):31-37.
2. Allen LA, Ambardekar AV, Devaraj KM, Maleszewski JJ, Wolfel EE. Clinical problem-solving. Missing elements of the history. N Engl J Med. 2014;370(6):559-566.
3. Hart AJ, Satchihananda K, Liddle AD, et al. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: a case-control study using three-dimensional tomography and magnetic resonance imaging. J Bone Joint Surg Am. 2012;94(4);317-325.
4. Kwon MK, Jacobs JJ, MacDonald SJ, Potter HG, Fehring TK, Lombardi AV. Evidence-based understanding of management perils for metal-on-metal hip arthroplasty patients. J Arthroplasty. 2012;27(8 suppl):20-25.
5. Mao X, Wong AA, Crawford RW. Cobalt toxicity- -an emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194(12):649-651.
6. Tower SS. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92(17):2847-2851.
7. Morin Y, Daniel P. Quebec beer-drinkers’ cardiomyopathy: etiological considerations. Can Med Assoc J. 1967;97(15):926-928.
8. Gilbert C, Cheung A, Butany J, et al. Hip pain and heart failure: the missing link. Can J Cardiol. 2013;29(5):639.e1-e2.
9. Seghizzi P, D’Adda F, Borleri D, Barbic F, Mosconi G. Cobalt myocardiopathy. A critical review of literature. Sci Total Environ. 1994;150(1-3):105-109.
14-Year-Old Boy With Mild Antecedent Neck Pain in Setting of Acute Trauma: A Rare Case of Benign Fibrous Histiocytoma of the Spine
Benign fibrous histiocytoma (BFH) is a rare, well-recognized, primary skeletal tumor accounting for approximately 1% of all benign bone tumors. Spinal involvement is exceedingly rare with only 11 cases reported in the literature.1,2 We present a case of BFH located in the cervical spine of a pediatric patient that was successfully treated with curretage through an anterior surgical approach, along with a review of the literature and appropriate management concerning BFH of the spine.
Case Report
A 14-year-old boy was tackled while playing football and noticed immediate neck pain and subjective paresthesia in the upper extremities. Examination revealed a nontender spine (cervical, thoracic, lumbar) and normal strength and range of motion in all extremities. Sensation was diffusely intact, long tract signs were absent, and gait was normal. On questioning, the patient endorsed mild antecedent neck pain but denied prior history of any trauma. Neck pain did not radiate and was slightly worsened by activity but was mostly intermittent and random. As the neck pain was very mild and was not interfering with daily activities, the patient had not sought care before presenting to the emergency department. He had no pertinent past medical or surgical history.
The patient presented with a computed tomography (CT) scan of his head and cervical spine and a magnetic resonance imaging (MRI) scan of the cervical spine. A magnetic resonance angiography (MRA) scan of the neck was ordered after his arrival.
Axial and sagittal CT (Figures 1A, 1B) showed a 1×1.2-cm discrete, expansile, lytic, radiolucent mass extending anterior from the left C2 vertebral body. The mass appeared to abut the left vertebral artery foramen. The cortical bone surrounding the lesion was thin but uniform. Sagittal and axial T1-weighted MRI (Figures 2A, 2B) showed the discrete, expansile, homogenous lesion with the same intensity as normal bone marrow. Sagittal and axial T2-weighted MRI (Figures 2C, 2D) showed a discrete, expansile, homogenous lesion with primarily high signal intensity. Sagittal short tau inversion recovery (STIR) MRI (Figure 2E) again showed the lesion with primarily low intensity. Given the close proximity of the lesion to the vertebral foramen, MRA was ordered; it showed the lesion was not interfering with the vertebral artery (Figure 2F).
The tumor’s location, in the left anterior aspect of the C2 vertebral body, was not conducive to percutaneous biopsy for establishing tissue diagnosis, so the decision was made to surgically excise the lesion. A left-sided anterior incision was made 2 fingerbreadths inferior to the jaw line in a neck crease. A head and neck surgeon assisted with dissection. Dissection was carried down through the skin, subcutaneous tissue, and platysma on to the anterior part of the spine medial to the carotid sheath. Superior thyroid nerve and vessels and superior laryngeal nerve were identified and preserved. Fluoroscopy confirmed correct location at C2. The tumor was easily visualized, and the outer shell broke easily with palpation. Gentle curettage was necessary when removing the tumor off the vertebral artery. A portion of the specimen was sent during surgery for frozen section, which showed infrequent mitotic figures and no other findings concerning for malignancy. No instability was created after curettage and excision of the tumor, so no grafting or instrumentation was necessary.
Grossly, the tumor was pale tan and firm. Histologic examination with hematoxylin-eosin staining revealed a bland spindle-cell neoplasm that focally involved bone. A storiform pattern was present. The cells had scant cytoplasm and oval to elongate nuclei with tapered ends. Significant nuclear pleomorphism was not seen. The stroma was loose, with focal myxoid change. Benign multinucleated giant cells were present. Mitotic activity was infrequent (Figures 3A–3D). Two attending pathologists reviewed the case material and the frozen and formalin-fixed specimens independently and concurred with the diagnosis of BFH. In addition, the case was reviewed at the surgical pathology consensus conference; the reviewers agreed on BFH, and additional studies were deemed unnecessary.
Given the patient’s complete clinical picture, the differential diagnosis included nonossifying fibroma (NOF), eosinophilic granuloma (EG), BFH, fibrous dysplasia, giant cell tumor (GCT), aneurysmal bone cyst (ABC), and osteoblastoma (OB).
Discussion
BFH is an extremely rare bone lesion, accounting for only 1% of all surgically managed bone tumors; not counting the present case, only 11 spine cases have been reported in the literature.1,2 BFH of the spine traditionally causes nonspecific, poorly localized pain. The Table lists the reported cases of spinal BFH and their presenting symptoms, location, and treatment. BFH usually occurs in young adults, but the age range is 5 to 75 years.2-4 Mean age of the 12 patients with spinal BFH in the literature (including ours) is 25 years.1 In addition, spinal BFH appears to have no predilection for sex.
Skeletal BFH presents as a discrete, well-defined, osteolytic lesion with sharp borders and potentially a sclerotic rim.4-6 Cortical expansion and even cortical disruption with invasion into adjacent tissue have occurred in flat bones.7 Histologically, BFHs contain spindle cells, multinucleated giant cells, and foam cells in storiform pattern.6
BFH shares many of its radiologic and histologic characteristics and clinical symptoms with other benign bone lesions (the tumors listed above). Therefore, accurate diagnosis of BFH requires appropriate correlation of clinical, radiographic, and histologic data.2,3,8 Below is a comparison of BFH with related bone lesions.
Spinal BFH causes a nonspecific, poorly localized pain similar to that of EG, ABC, GCT, and OB.3,9 NOF and fibrous dysplasia generally do not cause pain, unless these lesions are discovered secondary to a pathologic fracture.8,10,11 Our patient had minor antecedent neck pain, which was brought to light by his football accident. ABC and OB are more locally aggressive than BFH and can cause neurologic symptoms by mass effect and spinal cord or nerve root compression.1,8 In this case and in the 6 other cases of BFH of the cervical spine, there were no neurologic changes.4,10
Of the tumors mentioned, NOF and EG almost always occur in children. However, NOF usually occurs in the metaphyseal region of long bones, and EG is usually accompanied by systemic symptoms, such as lymphadenopathy, hepatomegaly, and increased inflammatory markers.1,8 Fibrous dysplasia usually presents in childhood but does not become symptomatic until adulthood. GCTs and OB predominantly occur in adulthood.12,13 Our patient’s age and lack of other systemic symptoms supported the diagnosis of BFH.
Appearance on MRI is reported less with BFH than with other tumors, but heterogenous signal intensity similar to that of skeletal muscle on T1-weighted images and high signal intensity on T2-weighted images is typically reported.8,14 NOF and fibrous dysplasia do not disrupt the bony cortex unless a pathologic fracture has occurred.4 GCTs are more aggressive lytic lesions with more aggressive radiologic features. GCTs generally cause cortical expansion/attenuation, and lack a sclerotic rim. GCTs also have a heterogenous appearance on MRI and give a low to intermediate signal on both T1- and T2-weighted images.12,15 The appearance of EG is similar to that of BFH as an osteolytic lesion with a sclerotic rim, though EGs typically break through the cortex and acquire a “punched-out” look.1,8 ABC typically is described as an expansile osteolytic lesion with a “soap-bubble” appearance on radiographs; periosteal elevation and cortical attenuation can also be visualized. MRI shows the typical multilobular appearance of the lesion with fluid levels.13
OB appears as a radiolucent lesion, with or without calcifications, surrounded by a thin margin of reactive bone.14,16 A distinguishing characteristic of OB was thought to be intense radioisotope uptake on bone scintigraphy, but recently a bony BFH demonstrated intense uptake.17 OBs typically demonstrate nonspecific MRI results similar to those of BFH: low to intermediate signal on T1-weighted images and intermediate to high signal on T2-weighted images.13 In our patient’s case, the radiographic appearance and lack of specific radiographic findings consistent with the other tumors supported the diagnosis of BFH.
Histologically, BFHs contain spindle cells, multinucleated giant cells, and foam cells in a storiform pattern6 which was demonstrated in our patient’s case. In addition, significant nuclear pleomorphism, mitotic activity, and necrosis were absent—a difference between BFH and malignant fibrous histiocytoma.4,15 The microscopic characteristics of BFH readily differentiate it from OB, ABC, EG, and GCT, but not from NOF on microscopic appearance alone. Clinical and radiographic findings must be consistent, as mentioned.7,18
Complete surgical excision is the reported treatment for BFH. Prognosis after resection or curettage is usually good, and recurrences have been rare.1,2 Depending on the intraspinous location of BFH, stabilization after resection or curettage may be necessary to prevent residual instability. Three of the 11 reported cases of spinal BFH required stabilization by anterior fusion or posterior pedicle screw fixation after resection.1,2 The other 8 cases underwent excision alone or excision and grafting. All 11 patients were disease-free at a mean follow-up of 3.5 years.1 In nonspinal BFH, however, both local recurrence and lung metastasis have been reported.2,5,9,19 Clarke and colleagues9 reported local recurrences in 3 of 8 cases. These recurrences involved BFH in long bones of the leg, which had been treated with curettage and grafting. There has been no reliable report of a malignant change in BFH.2,9 The only case of lung metastasis, reported by Unni and Dahlin6 in their study of 10 cases, occurred 2 years after local recurrence in the distal femur.Our patient was doing well at most recent follow-up, 6 months after surgery. He had no pain and had returned to normal activities. Although there are no reported cases of spinal BFH recurrence, we will follow this patient with imaging on an annual basis. His case is of particular interest to orthopedic surgeons because they encounter benign bone lesions every day, and many of these lesions are in difficult anatomical locations. Knowing the characteristics, differential diagnoses, and appropriate diagnostic workups for benign bone lesions is important for optimal and timely patient care.
1. Demiralp B, Kose O, Oguz E, Sanal T, Ozcan A, Sehirlioglu A. Benign fibrous histiocytoma of the lumbar vertebrae. Skeletal Radiol. 2009;38(2):187-191.
2. Kuruvath S, O’Donovan DG, Aspoas AR, David KM. Benign fibrous histiocytoma of the thoracic spine: case report and review of the literature. J Neurosurg Spine. 2006;4(3):260-264.
3. Ceroni D, Dayer R, De Coulon G, Kaelin A. Benign fibrous histiocytoma of bone in a paediatric population: a report of 6 cases. Musculoskelet Surg. 2011;95(2):107-114.
4. Dorfman HD, Czerniak B. Bone Tumors. St. Louis, MO: Mosby; 1998.
5. Grohs JG, Nicolakis M, Kainberger F, Lang S, Kotz R. Benign fibrous histiocytoma of bone: a report of ten cases and review of literature. Wien Klin Wochenschr. 2002;114(1-2):56-63.
6. Unni KK, Dahlin DC. Dahlin’s Bone Tumors. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
7. Balasubramanian C, Rajaraman G, Singh CS, Baliga DK. Benign fibrous histiocytoma of the sacrum—diagnostic difficulties facing this rare bone tumor. Pediatr Neurosurg. 2005;41(5):253-257.
8. van Giffen NH, van Rhijn LW, van Ooij A, et al. Benign fibrous histiocytoma of the posterior arch of C1 in a 6-year old boy: a case report. Spine. 2003;28(18):E359-E363.
9. Clarke BE, Xipell JM, Thomas DP. Benign fibrous histiocytoma of bone. Am J Surg Pathol. 1985;9(11):806-815.
10. Peicha G, Siebert FJ, Bratschitsch G, Fankhauser F, Grechenig W. Pathologic odontoid fracture and benign fibrous histiocytoma of bone. Eur Spine J. 1999;8(2):161-163.
11. Unni KK, Inwards CY, Bridge JA, Kindblom LG, Wold LE. Tumors of the Bones and Joints (AFIP Atlas of Tumor Pathology Series IV). Annapolis Junction, MD: American Registry of Pathology Press; 2005.
12. Dee R. Principles of Orthopaedic Practice. 2nd ed. New York, NY: McGraw-Hill; 1997.
13. Murphey M, Andrews C, Flemming D, Temple HT, Smith WS, Smirniotopoulos JG. Primary tumors of the spine: radiologic–pathologic correlation. Radiographics. 1996;16(5):1131-1158.
14. Hamada T, Ito H, Araki Y, Fujii K, Inoue M, Ishida O. Benign fibrous histiocytoma of the femur: review of three cases. Skeletal Radiol. 1996;25(1):25-29.
15. Mirra JM, Picci P, Gold RH. Bone Tumors: Clinical, Radiologic, and Pathologic Correlations. Vol 1. Philadelphia, PA: Lea & Febiger; 1989.
16. Theodorou DJ, Theodorou SJ, Sartoris DJ. An imaging overview of primary tumors of the spine: part 1. Benign tumors. Clin Imaging. 2008;32(3):196-203.
17. Li X, Meng Z, Li D, Tan J, Song X. Benign fibrous histiocytoma of a rib. Clin Nucl Med. 2014;39(9): 837-841.
18. Roessner A, Immenkamp M, Weidner A, Hobik HP, Grundmann E. Benign fibrous histiocytoma of bone. Light- and electron-microscopic observations. J Cancer Res Clin Oncol. 1981;101(2):191-202.
19. Destouet JM, Kyriakos M, Gilula LA. Fibrous histiocytoma (fibroxanthoma) of a cervical vertebra. A report with a review of the literature. Skeletal Radiol. 1980;5(4):241-246.
20. Hoeffel JC, Bomand-Ferrand F, Tachet F, Lascombes P, Czorny A, Bernard C. So-called benign fibrous histiocytoma: report of a case. J Pediatr Surg. 1992;27(5):672-674.
Benign fibrous histiocytoma (BFH) is a rare, well-recognized, primary skeletal tumor accounting for approximately 1% of all benign bone tumors. Spinal involvement is exceedingly rare with only 11 cases reported in the literature.1,2 We present a case of BFH located in the cervical spine of a pediatric patient that was successfully treated with curretage through an anterior surgical approach, along with a review of the literature and appropriate management concerning BFH of the spine.
Case Report
A 14-year-old boy was tackled while playing football and noticed immediate neck pain and subjective paresthesia in the upper extremities. Examination revealed a nontender spine (cervical, thoracic, lumbar) and normal strength and range of motion in all extremities. Sensation was diffusely intact, long tract signs were absent, and gait was normal. On questioning, the patient endorsed mild antecedent neck pain but denied prior history of any trauma. Neck pain did not radiate and was slightly worsened by activity but was mostly intermittent and random. As the neck pain was very mild and was not interfering with daily activities, the patient had not sought care before presenting to the emergency department. He had no pertinent past medical or surgical history.
The patient presented with a computed tomography (CT) scan of his head and cervical spine and a magnetic resonance imaging (MRI) scan of the cervical spine. A magnetic resonance angiography (MRA) scan of the neck was ordered after his arrival.
Axial and sagittal CT (Figures 1A, 1B) showed a 1×1.2-cm discrete, expansile, lytic, radiolucent mass extending anterior from the left C2 vertebral body. The mass appeared to abut the left vertebral artery foramen. The cortical bone surrounding the lesion was thin but uniform. Sagittal and axial T1-weighted MRI (Figures 2A, 2B) showed the discrete, expansile, homogenous lesion with the same intensity as normal bone marrow. Sagittal and axial T2-weighted MRI (Figures 2C, 2D) showed a discrete, expansile, homogenous lesion with primarily high signal intensity. Sagittal short tau inversion recovery (STIR) MRI (Figure 2E) again showed the lesion with primarily low intensity. Given the close proximity of the lesion to the vertebral foramen, MRA was ordered; it showed the lesion was not interfering with the vertebral artery (Figure 2F).
The tumor’s location, in the left anterior aspect of the C2 vertebral body, was not conducive to percutaneous biopsy for establishing tissue diagnosis, so the decision was made to surgically excise the lesion. A left-sided anterior incision was made 2 fingerbreadths inferior to the jaw line in a neck crease. A head and neck surgeon assisted with dissection. Dissection was carried down through the skin, subcutaneous tissue, and platysma on to the anterior part of the spine medial to the carotid sheath. Superior thyroid nerve and vessels and superior laryngeal nerve were identified and preserved. Fluoroscopy confirmed correct location at C2. The tumor was easily visualized, and the outer shell broke easily with palpation. Gentle curettage was necessary when removing the tumor off the vertebral artery. A portion of the specimen was sent during surgery for frozen section, which showed infrequent mitotic figures and no other findings concerning for malignancy. No instability was created after curettage and excision of the tumor, so no grafting or instrumentation was necessary.
Grossly, the tumor was pale tan and firm. Histologic examination with hematoxylin-eosin staining revealed a bland spindle-cell neoplasm that focally involved bone. A storiform pattern was present. The cells had scant cytoplasm and oval to elongate nuclei with tapered ends. Significant nuclear pleomorphism was not seen. The stroma was loose, with focal myxoid change. Benign multinucleated giant cells were present. Mitotic activity was infrequent (Figures 3A–3D). Two attending pathologists reviewed the case material and the frozen and formalin-fixed specimens independently and concurred with the diagnosis of BFH. In addition, the case was reviewed at the surgical pathology consensus conference; the reviewers agreed on BFH, and additional studies were deemed unnecessary.
Given the patient’s complete clinical picture, the differential diagnosis included nonossifying fibroma (NOF), eosinophilic granuloma (EG), BFH, fibrous dysplasia, giant cell tumor (GCT), aneurysmal bone cyst (ABC), and osteoblastoma (OB).
Discussion
BFH is an extremely rare bone lesion, accounting for only 1% of all surgically managed bone tumors; not counting the present case, only 11 spine cases have been reported in the literature.1,2 BFH of the spine traditionally causes nonspecific, poorly localized pain. The Table lists the reported cases of spinal BFH and their presenting symptoms, location, and treatment. BFH usually occurs in young adults, but the age range is 5 to 75 years.2-4 Mean age of the 12 patients with spinal BFH in the literature (including ours) is 25 years.1 In addition, spinal BFH appears to have no predilection for sex.
Skeletal BFH presents as a discrete, well-defined, osteolytic lesion with sharp borders and potentially a sclerotic rim.4-6 Cortical expansion and even cortical disruption with invasion into adjacent tissue have occurred in flat bones.7 Histologically, BFHs contain spindle cells, multinucleated giant cells, and foam cells in storiform pattern.6
BFH shares many of its radiologic and histologic characteristics and clinical symptoms with other benign bone lesions (the tumors listed above). Therefore, accurate diagnosis of BFH requires appropriate correlation of clinical, radiographic, and histologic data.2,3,8 Below is a comparison of BFH with related bone lesions.
Spinal BFH causes a nonspecific, poorly localized pain similar to that of EG, ABC, GCT, and OB.3,9 NOF and fibrous dysplasia generally do not cause pain, unless these lesions are discovered secondary to a pathologic fracture.8,10,11 Our patient had minor antecedent neck pain, which was brought to light by his football accident. ABC and OB are more locally aggressive than BFH and can cause neurologic symptoms by mass effect and spinal cord or nerve root compression.1,8 In this case and in the 6 other cases of BFH of the cervical spine, there were no neurologic changes.4,10
Of the tumors mentioned, NOF and EG almost always occur in children. However, NOF usually occurs in the metaphyseal region of long bones, and EG is usually accompanied by systemic symptoms, such as lymphadenopathy, hepatomegaly, and increased inflammatory markers.1,8 Fibrous dysplasia usually presents in childhood but does not become symptomatic until adulthood. GCTs and OB predominantly occur in adulthood.12,13 Our patient’s age and lack of other systemic symptoms supported the diagnosis of BFH.
Appearance on MRI is reported less with BFH than with other tumors, but heterogenous signal intensity similar to that of skeletal muscle on T1-weighted images and high signal intensity on T2-weighted images is typically reported.8,14 NOF and fibrous dysplasia do not disrupt the bony cortex unless a pathologic fracture has occurred.4 GCTs are more aggressive lytic lesions with more aggressive radiologic features. GCTs generally cause cortical expansion/attenuation, and lack a sclerotic rim. GCTs also have a heterogenous appearance on MRI and give a low to intermediate signal on both T1- and T2-weighted images.12,15 The appearance of EG is similar to that of BFH as an osteolytic lesion with a sclerotic rim, though EGs typically break through the cortex and acquire a “punched-out” look.1,8 ABC typically is described as an expansile osteolytic lesion with a “soap-bubble” appearance on radiographs; periosteal elevation and cortical attenuation can also be visualized. MRI shows the typical multilobular appearance of the lesion with fluid levels.13
OB appears as a radiolucent lesion, with or without calcifications, surrounded by a thin margin of reactive bone.14,16 A distinguishing characteristic of OB was thought to be intense radioisotope uptake on bone scintigraphy, but recently a bony BFH demonstrated intense uptake.17 OBs typically demonstrate nonspecific MRI results similar to those of BFH: low to intermediate signal on T1-weighted images and intermediate to high signal on T2-weighted images.13 In our patient’s case, the radiographic appearance and lack of specific radiographic findings consistent with the other tumors supported the diagnosis of BFH.
Histologically, BFHs contain spindle cells, multinucleated giant cells, and foam cells in a storiform pattern6 which was demonstrated in our patient’s case. In addition, significant nuclear pleomorphism, mitotic activity, and necrosis were absent—a difference between BFH and malignant fibrous histiocytoma.4,15 The microscopic characteristics of BFH readily differentiate it from OB, ABC, EG, and GCT, but not from NOF on microscopic appearance alone. Clinical and radiographic findings must be consistent, as mentioned.7,18
Complete surgical excision is the reported treatment for BFH. Prognosis after resection or curettage is usually good, and recurrences have been rare.1,2 Depending on the intraspinous location of BFH, stabilization after resection or curettage may be necessary to prevent residual instability. Three of the 11 reported cases of spinal BFH required stabilization by anterior fusion or posterior pedicle screw fixation after resection.1,2 The other 8 cases underwent excision alone or excision and grafting. All 11 patients were disease-free at a mean follow-up of 3.5 years.1 In nonspinal BFH, however, both local recurrence and lung metastasis have been reported.2,5,9,19 Clarke and colleagues9 reported local recurrences in 3 of 8 cases. These recurrences involved BFH in long bones of the leg, which had been treated with curettage and grafting. There has been no reliable report of a malignant change in BFH.2,9 The only case of lung metastasis, reported by Unni and Dahlin6 in their study of 10 cases, occurred 2 years after local recurrence in the distal femur.Our patient was doing well at most recent follow-up, 6 months after surgery. He had no pain and had returned to normal activities. Although there are no reported cases of spinal BFH recurrence, we will follow this patient with imaging on an annual basis. His case is of particular interest to orthopedic surgeons because they encounter benign bone lesions every day, and many of these lesions are in difficult anatomical locations. Knowing the characteristics, differential diagnoses, and appropriate diagnostic workups for benign bone lesions is important for optimal and timely patient care.
Benign fibrous histiocytoma (BFH) is a rare, well-recognized, primary skeletal tumor accounting for approximately 1% of all benign bone tumors. Spinal involvement is exceedingly rare with only 11 cases reported in the literature.1,2 We present a case of BFH located in the cervical spine of a pediatric patient that was successfully treated with curretage through an anterior surgical approach, along with a review of the literature and appropriate management concerning BFH of the spine.
Case Report
A 14-year-old boy was tackled while playing football and noticed immediate neck pain and subjective paresthesia in the upper extremities. Examination revealed a nontender spine (cervical, thoracic, lumbar) and normal strength and range of motion in all extremities. Sensation was diffusely intact, long tract signs were absent, and gait was normal. On questioning, the patient endorsed mild antecedent neck pain but denied prior history of any trauma. Neck pain did not radiate and was slightly worsened by activity but was mostly intermittent and random. As the neck pain was very mild and was not interfering with daily activities, the patient had not sought care before presenting to the emergency department. He had no pertinent past medical or surgical history.
The patient presented with a computed tomography (CT) scan of his head and cervical spine and a magnetic resonance imaging (MRI) scan of the cervical spine. A magnetic resonance angiography (MRA) scan of the neck was ordered after his arrival.
Axial and sagittal CT (Figures 1A, 1B) showed a 1×1.2-cm discrete, expansile, lytic, radiolucent mass extending anterior from the left C2 vertebral body. The mass appeared to abut the left vertebral artery foramen. The cortical bone surrounding the lesion was thin but uniform. Sagittal and axial T1-weighted MRI (Figures 2A, 2B) showed the discrete, expansile, homogenous lesion with the same intensity as normal bone marrow. Sagittal and axial T2-weighted MRI (Figures 2C, 2D) showed a discrete, expansile, homogenous lesion with primarily high signal intensity. Sagittal short tau inversion recovery (STIR) MRI (Figure 2E) again showed the lesion with primarily low intensity. Given the close proximity of the lesion to the vertebral foramen, MRA was ordered; it showed the lesion was not interfering with the vertebral artery (Figure 2F).
The tumor’s location, in the left anterior aspect of the C2 vertebral body, was not conducive to percutaneous biopsy for establishing tissue diagnosis, so the decision was made to surgically excise the lesion. A left-sided anterior incision was made 2 fingerbreadths inferior to the jaw line in a neck crease. A head and neck surgeon assisted with dissection. Dissection was carried down through the skin, subcutaneous tissue, and platysma on to the anterior part of the spine medial to the carotid sheath. Superior thyroid nerve and vessels and superior laryngeal nerve were identified and preserved. Fluoroscopy confirmed correct location at C2. The tumor was easily visualized, and the outer shell broke easily with palpation. Gentle curettage was necessary when removing the tumor off the vertebral artery. A portion of the specimen was sent during surgery for frozen section, which showed infrequent mitotic figures and no other findings concerning for malignancy. No instability was created after curettage and excision of the tumor, so no grafting or instrumentation was necessary.
Grossly, the tumor was pale tan and firm. Histologic examination with hematoxylin-eosin staining revealed a bland spindle-cell neoplasm that focally involved bone. A storiform pattern was present. The cells had scant cytoplasm and oval to elongate nuclei with tapered ends. Significant nuclear pleomorphism was not seen. The stroma was loose, with focal myxoid change. Benign multinucleated giant cells were present. Mitotic activity was infrequent (Figures 3A–3D). Two attending pathologists reviewed the case material and the frozen and formalin-fixed specimens independently and concurred with the diagnosis of BFH. In addition, the case was reviewed at the surgical pathology consensus conference; the reviewers agreed on BFH, and additional studies were deemed unnecessary.
Given the patient’s complete clinical picture, the differential diagnosis included nonossifying fibroma (NOF), eosinophilic granuloma (EG), BFH, fibrous dysplasia, giant cell tumor (GCT), aneurysmal bone cyst (ABC), and osteoblastoma (OB).
Discussion
BFH is an extremely rare bone lesion, accounting for only 1% of all surgically managed bone tumors; not counting the present case, only 11 spine cases have been reported in the literature.1,2 BFH of the spine traditionally causes nonspecific, poorly localized pain. The Table lists the reported cases of spinal BFH and their presenting symptoms, location, and treatment. BFH usually occurs in young adults, but the age range is 5 to 75 years.2-4 Mean age of the 12 patients with spinal BFH in the literature (including ours) is 25 years.1 In addition, spinal BFH appears to have no predilection for sex.
Skeletal BFH presents as a discrete, well-defined, osteolytic lesion with sharp borders and potentially a sclerotic rim.4-6 Cortical expansion and even cortical disruption with invasion into adjacent tissue have occurred in flat bones.7 Histologically, BFHs contain spindle cells, multinucleated giant cells, and foam cells in storiform pattern.6
BFH shares many of its radiologic and histologic characteristics and clinical symptoms with other benign bone lesions (the tumors listed above). Therefore, accurate diagnosis of BFH requires appropriate correlation of clinical, radiographic, and histologic data.2,3,8 Below is a comparison of BFH with related bone lesions.
Spinal BFH causes a nonspecific, poorly localized pain similar to that of EG, ABC, GCT, and OB.3,9 NOF and fibrous dysplasia generally do not cause pain, unless these lesions are discovered secondary to a pathologic fracture.8,10,11 Our patient had minor antecedent neck pain, which was brought to light by his football accident. ABC and OB are more locally aggressive than BFH and can cause neurologic symptoms by mass effect and spinal cord or nerve root compression.1,8 In this case and in the 6 other cases of BFH of the cervical spine, there were no neurologic changes.4,10
Of the tumors mentioned, NOF and EG almost always occur in children. However, NOF usually occurs in the metaphyseal region of long bones, and EG is usually accompanied by systemic symptoms, such as lymphadenopathy, hepatomegaly, and increased inflammatory markers.1,8 Fibrous dysplasia usually presents in childhood but does not become symptomatic until adulthood. GCTs and OB predominantly occur in adulthood.12,13 Our patient’s age and lack of other systemic symptoms supported the diagnosis of BFH.
Appearance on MRI is reported less with BFH than with other tumors, but heterogenous signal intensity similar to that of skeletal muscle on T1-weighted images and high signal intensity on T2-weighted images is typically reported.8,14 NOF and fibrous dysplasia do not disrupt the bony cortex unless a pathologic fracture has occurred.4 GCTs are more aggressive lytic lesions with more aggressive radiologic features. GCTs generally cause cortical expansion/attenuation, and lack a sclerotic rim. GCTs also have a heterogenous appearance on MRI and give a low to intermediate signal on both T1- and T2-weighted images.12,15 The appearance of EG is similar to that of BFH as an osteolytic lesion with a sclerotic rim, though EGs typically break through the cortex and acquire a “punched-out” look.1,8 ABC typically is described as an expansile osteolytic lesion with a “soap-bubble” appearance on radiographs; periosteal elevation and cortical attenuation can also be visualized. MRI shows the typical multilobular appearance of the lesion with fluid levels.13
OB appears as a radiolucent lesion, with or without calcifications, surrounded by a thin margin of reactive bone.14,16 A distinguishing characteristic of OB was thought to be intense radioisotope uptake on bone scintigraphy, but recently a bony BFH demonstrated intense uptake.17 OBs typically demonstrate nonspecific MRI results similar to those of BFH: low to intermediate signal on T1-weighted images and intermediate to high signal on T2-weighted images.13 In our patient’s case, the radiographic appearance and lack of specific radiographic findings consistent with the other tumors supported the diagnosis of BFH.
Histologically, BFHs contain spindle cells, multinucleated giant cells, and foam cells in a storiform pattern6 which was demonstrated in our patient’s case. In addition, significant nuclear pleomorphism, mitotic activity, and necrosis were absent—a difference between BFH and malignant fibrous histiocytoma.4,15 The microscopic characteristics of BFH readily differentiate it from OB, ABC, EG, and GCT, but not from NOF on microscopic appearance alone. Clinical and radiographic findings must be consistent, as mentioned.7,18
Complete surgical excision is the reported treatment for BFH. Prognosis after resection or curettage is usually good, and recurrences have been rare.1,2 Depending on the intraspinous location of BFH, stabilization after resection or curettage may be necessary to prevent residual instability. Three of the 11 reported cases of spinal BFH required stabilization by anterior fusion or posterior pedicle screw fixation after resection.1,2 The other 8 cases underwent excision alone or excision and grafting. All 11 patients were disease-free at a mean follow-up of 3.5 years.1 In nonspinal BFH, however, both local recurrence and lung metastasis have been reported.2,5,9,19 Clarke and colleagues9 reported local recurrences in 3 of 8 cases. These recurrences involved BFH in long bones of the leg, which had been treated with curettage and grafting. There has been no reliable report of a malignant change in BFH.2,9 The only case of lung metastasis, reported by Unni and Dahlin6 in their study of 10 cases, occurred 2 years after local recurrence in the distal femur.Our patient was doing well at most recent follow-up, 6 months after surgery. He had no pain and had returned to normal activities. Although there are no reported cases of spinal BFH recurrence, we will follow this patient with imaging on an annual basis. His case is of particular interest to orthopedic surgeons because they encounter benign bone lesions every day, and many of these lesions are in difficult anatomical locations. Knowing the characteristics, differential diagnoses, and appropriate diagnostic workups for benign bone lesions is important for optimal and timely patient care.
1. Demiralp B, Kose O, Oguz E, Sanal T, Ozcan A, Sehirlioglu A. Benign fibrous histiocytoma of the lumbar vertebrae. Skeletal Radiol. 2009;38(2):187-191.
2. Kuruvath S, O’Donovan DG, Aspoas AR, David KM. Benign fibrous histiocytoma of the thoracic spine: case report and review of the literature. J Neurosurg Spine. 2006;4(3):260-264.
3. Ceroni D, Dayer R, De Coulon G, Kaelin A. Benign fibrous histiocytoma of bone in a paediatric population: a report of 6 cases. Musculoskelet Surg. 2011;95(2):107-114.
4. Dorfman HD, Czerniak B. Bone Tumors. St. Louis, MO: Mosby; 1998.
5. Grohs JG, Nicolakis M, Kainberger F, Lang S, Kotz R. Benign fibrous histiocytoma of bone: a report of ten cases and review of literature. Wien Klin Wochenschr. 2002;114(1-2):56-63.
6. Unni KK, Dahlin DC. Dahlin’s Bone Tumors. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
7. Balasubramanian C, Rajaraman G, Singh CS, Baliga DK. Benign fibrous histiocytoma of the sacrum—diagnostic difficulties facing this rare bone tumor. Pediatr Neurosurg. 2005;41(5):253-257.
8. van Giffen NH, van Rhijn LW, van Ooij A, et al. Benign fibrous histiocytoma of the posterior arch of C1 in a 6-year old boy: a case report. Spine. 2003;28(18):E359-E363.
9. Clarke BE, Xipell JM, Thomas DP. Benign fibrous histiocytoma of bone. Am J Surg Pathol. 1985;9(11):806-815.
10. Peicha G, Siebert FJ, Bratschitsch G, Fankhauser F, Grechenig W. Pathologic odontoid fracture and benign fibrous histiocytoma of bone. Eur Spine J. 1999;8(2):161-163.
11. Unni KK, Inwards CY, Bridge JA, Kindblom LG, Wold LE. Tumors of the Bones and Joints (AFIP Atlas of Tumor Pathology Series IV). Annapolis Junction, MD: American Registry of Pathology Press; 2005.
12. Dee R. Principles of Orthopaedic Practice. 2nd ed. New York, NY: McGraw-Hill; 1997.
13. Murphey M, Andrews C, Flemming D, Temple HT, Smith WS, Smirniotopoulos JG. Primary tumors of the spine: radiologic–pathologic correlation. Radiographics. 1996;16(5):1131-1158.
14. Hamada T, Ito H, Araki Y, Fujii K, Inoue M, Ishida O. Benign fibrous histiocytoma of the femur: review of three cases. Skeletal Radiol. 1996;25(1):25-29.
15. Mirra JM, Picci P, Gold RH. Bone Tumors: Clinical, Radiologic, and Pathologic Correlations. Vol 1. Philadelphia, PA: Lea & Febiger; 1989.
16. Theodorou DJ, Theodorou SJ, Sartoris DJ. An imaging overview of primary tumors of the spine: part 1. Benign tumors. Clin Imaging. 2008;32(3):196-203.
17. Li X, Meng Z, Li D, Tan J, Song X. Benign fibrous histiocytoma of a rib. Clin Nucl Med. 2014;39(9): 837-841.
18. Roessner A, Immenkamp M, Weidner A, Hobik HP, Grundmann E. Benign fibrous histiocytoma of bone. Light- and electron-microscopic observations. J Cancer Res Clin Oncol. 1981;101(2):191-202.
19. Destouet JM, Kyriakos M, Gilula LA. Fibrous histiocytoma (fibroxanthoma) of a cervical vertebra. A report with a review of the literature. Skeletal Radiol. 1980;5(4):241-246.
20. Hoeffel JC, Bomand-Ferrand F, Tachet F, Lascombes P, Czorny A, Bernard C. So-called benign fibrous histiocytoma: report of a case. J Pediatr Surg. 1992;27(5):672-674.
1. Demiralp B, Kose O, Oguz E, Sanal T, Ozcan A, Sehirlioglu A. Benign fibrous histiocytoma of the lumbar vertebrae. Skeletal Radiol. 2009;38(2):187-191.
2. Kuruvath S, O’Donovan DG, Aspoas AR, David KM. Benign fibrous histiocytoma of the thoracic spine: case report and review of the literature. J Neurosurg Spine. 2006;4(3):260-264.
3. Ceroni D, Dayer R, De Coulon G, Kaelin A. Benign fibrous histiocytoma of bone in a paediatric population: a report of 6 cases. Musculoskelet Surg. 2011;95(2):107-114.
4. Dorfman HD, Czerniak B. Bone Tumors. St. Louis, MO: Mosby; 1998.
5. Grohs JG, Nicolakis M, Kainberger F, Lang S, Kotz R. Benign fibrous histiocytoma of bone: a report of ten cases and review of literature. Wien Klin Wochenschr. 2002;114(1-2):56-63.
6. Unni KK, Dahlin DC. Dahlin’s Bone Tumors. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
7. Balasubramanian C, Rajaraman G, Singh CS, Baliga DK. Benign fibrous histiocytoma of the sacrum—diagnostic difficulties facing this rare bone tumor. Pediatr Neurosurg. 2005;41(5):253-257.
8. van Giffen NH, van Rhijn LW, van Ooij A, et al. Benign fibrous histiocytoma of the posterior arch of C1 in a 6-year old boy: a case report. Spine. 2003;28(18):E359-E363.
9. Clarke BE, Xipell JM, Thomas DP. Benign fibrous histiocytoma of bone. Am J Surg Pathol. 1985;9(11):806-815.
10. Peicha G, Siebert FJ, Bratschitsch G, Fankhauser F, Grechenig W. Pathologic odontoid fracture and benign fibrous histiocytoma of bone. Eur Spine J. 1999;8(2):161-163.
11. Unni KK, Inwards CY, Bridge JA, Kindblom LG, Wold LE. Tumors of the Bones and Joints (AFIP Atlas of Tumor Pathology Series IV). Annapolis Junction, MD: American Registry of Pathology Press; 2005.
12. Dee R. Principles of Orthopaedic Practice. 2nd ed. New York, NY: McGraw-Hill; 1997.
13. Murphey M, Andrews C, Flemming D, Temple HT, Smith WS, Smirniotopoulos JG. Primary tumors of the spine: radiologic–pathologic correlation. Radiographics. 1996;16(5):1131-1158.
14. Hamada T, Ito H, Araki Y, Fujii K, Inoue M, Ishida O. Benign fibrous histiocytoma of the femur: review of three cases. Skeletal Radiol. 1996;25(1):25-29.
15. Mirra JM, Picci P, Gold RH. Bone Tumors: Clinical, Radiologic, and Pathologic Correlations. Vol 1. Philadelphia, PA: Lea & Febiger; 1989.
16. Theodorou DJ, Theodorou SJ, Sartoris DJ. An imaging overview of primary tumors of the spine: part 1. Benign tumors. Clin Imaging. 2008;32(3):196-203.
17. Li X, Meng Z, Li D, Tan J, Song X. Benign fibrous histiocytoma of a rib. Clin Nucl Med. 2014;39(9): 837-841.
18. Roessner A, Immenkamp M, Weidner A, Hobik HP, Grundmann E. Benign fibrous histiocytoma of bone. Light- and electron-microscopic observations. J Cancer Res Clin Oncol. 1981;101(2):191-202.
19. Destouet JM, Kyriakos M, Gilula LA. Fibrous histiocytoma (fibroxanthoma) of a cervical vertebra. A report with a review of the literature. Skeletal Radiol. 1980;5(4):241-246.
20. Hoeffel JC, Bomand-Ferrand F, Tachet F, Lascombes P, Czorny A, Bernard C. So-called benign fibrous histiocytoma: report of a case. J Pediatr Surg. 1992;27(5):672-674.
Subtle radiographic progression in axial SpA cannot be reliably distinguished from error
Sacroiliitis observed in patients with axial spondyloarthritis more often regressed rather than progressed on radiography over nearly 5 years of follow-up of the Assessment of SpondyloArthritis international Society (ASAS) cohort, which lead author Dr. Alexandre Sepriano and his colleagues called “strange” and “sobering.”
The findings call into question the reliability of plain pelvic radiographs for detecting subtle change in sacroiliitis and should prompt the evaluation of alternative imaging modalities such as MRI and low-dose CT, according to Dr. Sepriano of Leiden (the Netherlands) University Medical Center and his associates.
Determining the presence of radiographic sacroiliitis is prognostically relevant and can pave the way for treatment with biologics, but ambiguity in making this decision and in tracking progression has been revealed in the large inter-and intrareader variability found in previous studies. Furthermore, previous studies tracking progression of nonradiographic axial spondyloarthritis (axSpA) to radiographic axSpA have addressed only disease progression and ignored regression. While regression is likely to be rare, it cannot be ignored from a methodologic standpoint, the investigators wrote.
The researchers therefore set out in the current study to assess positive and negative changes in sacroiliitis on plain pelvic radiographs over time in 975 patients from the ASAS cohort who had chronic back pain of unknown origin or undiagnosed peripheral symptoms (Ann Rheum Dis. 2016 Feb 22. doi: 10.1136/annrheumdis-2015-208964).
Of the 357 of the patients who had paired plain pelvic radiographs available at baseline and follow-up, 17.4% (62/357) fulfilled the criteria for radiographic axSpA at baseline, as defined by modified New York criteria (mNY). At a mean follow-up of 4.4 years, this figure had risen to 22.4% (80/357), suggesting a net progression of 5%.
However, when the authors cross-tabulated their figures, more than half (36/62) of the patients considered mNY positive at baseline were assessed as mNY negative at follow-up. This would mean that radiographic sacroiliitis would have regressed in 58% of the cases; conversely, only 54 of 295 patients (18.3%) became mNY positive at follow-up.
“If only positive change (progression) is valued and negative change is ignored, one would disregard measurement error and spuriously attribute part of the observed positive change to real progression,” the research team explained. “The most likely explanation of our strange and extreme observation is that subtle radiographic progression (the signal) – if truly present – cannot be reliably distinguished from measurement error (the noise). These sobering data clearly illustrate that more research is needed in visualising progression in axSpA.”
ASAS funded the study. The authors had no competing interests to declare.
Sacroiliitis observed in patients with axial spondyloarthritis more often regressed rather than progressed on radiography over nearly 5 years of follow-up of the Assessment of SpondyloArthritis international Society (ASAS) cohort, which lead author Dr. Alexandre Sepriano and his colleagues called “strange” and “sobering.”
The findings call into question the reliability of plain pelvic radiographs for detecting subtle change in sacroiliitis and should prompt the evaluation of alternative imaging modalities such as MRI and low-dose CT, according to Dr. Sepriano of Leiden (the Netherlands) University Medical Center and his associates.
Determining the presence of radiographic sacroiliitis is prognostically relevant and can pave the way for treatment with biologics, but ambiguity in making this decision and in tracking progression has been revealed in the large inter-and intrareader variability found in previous studies. Furthermore, previous studies tracking progression of nonradiographic axial spondyloarthritis (axSpA) to radiographic axSpA have addressed only disease progression and ignored regression. While regression is likely to be rare, it cannot be ignored from a methodologic standpoint, the investigators wrote.
The researchers therefore set out in the current study to assess positive and negative changes in sacroiliitis on plain pelvic radiographs over time in 975 patients from the ASAS cohort who had chronic back pain of unknown origin or undiagnosed peripheral symptoms (Ann Rheum Dis. 2016 Feb 22. doi: 10.1136/annrheumdis-2015-208964).
Of the 357 of the patients who had paired plain pelvic radiographs available at baseline and follow-up, 17.4% (62/357) fulfilled the criteria for radiographic axSpA at baseline, as defined by modified New York criteria (mNY). At a mean follow-up of 4.4 years, this figure had risen to 22.4% (80/357), suggesting a net progression of 5%.
However, when the authors cross-tabulated their figures, more than half (36/62) of the patients considered mNY positive at baseline were assessed as mNY negative at follow-up. This would mean that radiographic sacroiliitis would have regressed in 58% of the cases; conversely, only 54 of 295 patients (18.3%) became mNY positive at follow-up.
“If only positive change (progression) is valued and negative change is ignored, one would disregard measurement error and spuriously attribute part of the observed positive change to real progression,” the research team explained. “The most likely explanation of our strange and extreme observation is that subtle radiographic progression (the signal) – if truly present – cannot be reliably distinguished from measurement error (the noise). These sobering data clearly illustrate that more research is needed in visualising progression in axSpA.”
ASAS funded the study. The authors had no competing interests to declare.
Sacroiliitis observed in patients with axial spondyloarthritis more often regressed rather than progressed on radiography over nearly 5 years of follow-up of the Assessment of SpondyloArthritis international Society (ASAS) cohort, which lead author Dr. Alexandre Sepriano and his colleagues called “strange” and “sobering.”
The findings call into question the reliability of plain pelvic radiographs for detecting subtle change in sacroiliitis and should prompt the evaluation of alternative imaging modalities such as MRI and low-dose CT, according to Dr. Sepriano of Leiden (the Netherlands) University Medical Center and his associates.
Determining the presence of radiographic sacroiliitis is prognostically relevant and can pave the way for treatment with biologics, but ambiguity in making this decision and in tracking progression has been revealed in the large inter-and intrareader variability found in previous studies. Furthermore, previous studies tracking progression of nonradiographic axial spondyloarthritis (axSpA) to radiographic axSpA have addressed only disease progression and ignored regression. While regression is likely to be rare, it cannot be ignored from a methodologic standpoint, the investigators wrote.
The researchers therefore set out in the current study to assess positive and negative changes in sacroiliitis on plain pelvic radiographs over time in 975 patients from the ASAS cohort who had chronic back pain of unknown origin or undiagnosed peripheral symptoms (Ann Rheum Dis. 2016 Feb 22. doi: 10.1136/annrheumdis-2015-208964).
Of the 357 of the patients who had paired plain pelvic radiographs available at baseline and follow-up, 17.4% (62/357) fulfilled the criteria for radiographic axSpA at baseline, as defined by modified New York criteria (mNY). At a mean follow-up of 4.4 years, this figure had risen to 22.4% (80/357), suggesting a net progression of 5%.
However, when the authors cross-tabulated their figures, more than half (36/62) of the patients considered mNY positive at baseline were assessed as mNY negative at follow-up. This would mean that radiographic sacroiliitis would have regressed in 58% of the cases; conversely, only 54 of 295 patients (18.3%) became mNY positive at follow-up.
“If only positive change (progression) is valued and negative change is ignored, one would disregard measurement error and spuriously attribute part of the observed positive change to real progression,” the research team explained. “The most likely explanation of our strange and extreme observation is that subtle radiographic progression (the signal) – if truly present – cannot be reliably distinguished from measurement error (the noise). These sobering data clearly illustrate that more research is needed in visualising progression in axSpA.”
ASAS funded the study. The authors had no competing interests to declare.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: Subtle radiographic progression in axSpA cannot be reliably distinguished from measurement error.
Major finding: Using plain radiographs, more than half of the patients identified as mNY positive for axSpA at baseline were assessed as mNY negative at a mean follow-up of 4.4 years.
Data source: 975 patients with chronic back pain of unknown origin or undiagnosed peripheral symptoms taking part in the Assessment of SpondyloArthritis international Society (ASAS) cohort.
Disclosures: ASAS funded the study. The authors had no competing interests to declare.