User login
Advancing Order Set Design
In the current health care environment, hospitals are constantly challenged to improve quality metrics and deliver better health care outcomes. One means to achieving quality improvement is through the use of order sets, groups of related orders that a health care provider (HCP) can place with either a few keystrokes or mouse clicks.1
Historically, design of order sets has largely focused on clicking checkboxes containing evidence-based practices. According to Bates and colleagues and the Institute for Safe Medication Practices, incorporating evidence-based medicine (EBM) into order sets is not by itself sufficient.2,3Execution of proper design coupled with simplicity and provider efficiency is paramount to HCP buy-in, increased likelihood of order set adherence, and to potentially better outcomes.
In this article, we outline advancements in order set design. These improvements increase provider efficiency and ease of use; incorporate human factors engineering (HFE); apply failure mode and effects analysis; and include EBM.
Methods
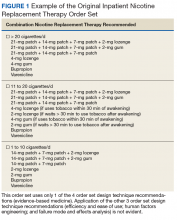
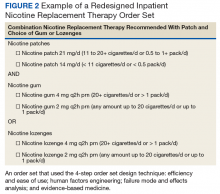
An inpatient nicotine replacement therapy (NRT) order was developed as part of a multifaceted solution to improve tobacco cessation care at the James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, a complexity level 1a facility. This NRT order set used the 4-step order set design framework the authors’ developed (for additional information about the NRT order set, contact the authors). We distinguish order set design technique between 2 different inpatient NRT order sets. The first order set in the comparison (Figure 1) is an inpatient NRT order set of unknown origin—it is common for US Department of Veterans Affairs (VA) medical facilities to share order sets and other resources. The second order set (Figure 2) is an inpatient NRT order set we designed using our 4-step process for comparison in this article. No institutional review board approval was required as this work met criteria for operational improvement activities exempt from ethics review.
Justin Iannello, DO, MBA, was the team leader and developer of the 4-step order set design technique. The intervention team consisted of 4 internal medicine physicians with expertise in quality improvement and patient safety: 1 certified professional in patient safety and certified as a Lean Six Sigma Black Belt; 2 physicians certified as Lean Six Sigma Black Belts; and 1 physician certified as a Lean Six Sigma Green Belt. Two inpatient clinical pharmacists and 1 quality management specialist also were involved in its development.
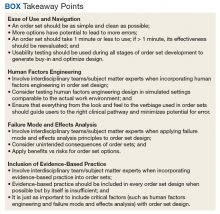
Development of a new NRT order set was felt to be an integral part of the tobacco cessation care delivery process. An NRT order set perceived by users as value-added required a solution that merged EBM with standardization and applied quality improvement principles. The result was an approach to order set design that focused on 4 key questions: Is the order set efficient and easy to use/navigate? Is human factors engineering incorporated? Is failure mode and effects analysis applied? Are evidence-based practices included?
Ease of Use and Navigation
Implementing an order set that is efficient and easy to use or navigate seems straightforward but can be difficult to execute. Figure 1 shows many detailed options consisting of different combinations of nicotine patches, lozenges, and gum. Also included are oral tobacco cessation options (bupropion and varenicline). Although more options may seem better, confusion about appropriate medication selection can occur.
According to Heath and Heath, too many options can result in lack of action.4 For example, Heath and Heath discuss a food store that offered 6 free samples of different jams on one day and 24 jams the following day. The customers who sampled 6 different types of jam were 10 times more likely to buy jam. The authors concluded that the more options available, the more difficulty a potential buyer has in deciding on a course of action.4
In clinical situations where a HCP is using an order set, the number of options can mean the difference between use vs avoidance if the choices are overwhelming. HCPs process layers of detail every day when creating differential diagnoses and treatment plans. While that level of detail is necessary clinically, that same level of detail included in orders sets can create challenges for HCPs.
Figure 2 advances the order set in Figure 1 by providing a simpler and cleaner design, so HCPs can more easily review and process the information. This order set design minimizes the number of options available to help users make the right decision, focusing on value for the appropriate setting and audience. In other words, order sets should not be a “one size fits all” approach.
Order sets should be tailored to the appropriate clinical setting (eg, inpatient acute care, outpatient clinic setting, etc) and HCP (eg, hospitalist, tobacco cessation specialist, etc). We are comparing NRT order sets designed for HCPs who do not routinely prescribe oral tobacco cessation products in the inpatient setting. When possible, autogenerated bundle orders should also be used according to evidence-based recommendations (such as nicotine patch tapers) for ease of use and further simplification of order sets.
Finally, usability testing known as “evaluating a product or service by testing it with representative users” helps further refine an order set.5Usability testing should be applied during all phases of order set development with end user(s) as it helps identify problems with order set design prior to implementation. By applying usability testing, the order set becomes more meaningful and valued by the user.
Human Factors Engineering
HFE is “the study of all the factors that make it easier to do the work in the right way.”6 HFE seeks to identify, align, and apply processes for people and the world within which they live and work to promote safe and efficient practices, especially in relation to the technology and physical design features in their work environment.6
The average American adult makes about 35,000 decisions per day.7 Thus, there is potential for error at any moment. Design that does not take HFE into account can be dangerous. For example, when tube feed and IV line connectors look similar and are compatible, patients may inadvertently receive food administered directly into their bloodstream.8
HFE can and should be applied to order sets. Everything from the look, feel, and verbiage of an order set affects potential outcomes. For example, consider the impact even seemingly minor modifications can have on outcomes simply by guiding users in a different way: Figure 1 provides NRT options based on cigarette use per day, whereas Figure 2 conveys pack use per day in relation to the equivalent number of cigarettes used daily. These differences may seem small; however, it helps guide users to the right choice when considering that health care providers have been historically trained on social history gathering that emphasizes packs per day and pack-years.
Failure Mode and Effects Analysis
Failure mode and effects analysis (FMEA) is “a structured way to identify and address potential problems, or failures and their resulting effects on the system or process before an adverse event occurs.”9 The benefit of an order set must be weighed against the risk during development. FMEA should be applied during order set design to assess and limit risk just as with any other clinical care process.
FMEA examines both level of risk and frequency of risk occurrence associated with a new proposed process. For example, let’s evaluate an order set designed for pain control after surgery that consists of multiple high-risk opioids along with antihistamine medications for as-needed itch relief (a non-life-threatening adverse event (AE) of opioids well known by the medical community). An interdisciplinary FMEA team consisting of subject matter experts may examine how the process should flow in step-by-step detail and then discuss the benefit of a process and risk for potential error. A FMEA team would then analyze what could go wrong with each part of the process and assign a level of risk and risk frequency for various steps in the process, and then decide that certain steps should be modified or eliminated. Perhaps after FMEA, a facility might conclude that the risk of serious complications is high when you combine opioid use with antihistamine medications. The facility could decide to remove antihistamine medications from an order set if it is determined that risks outweigh benefits. While a root cause analysis might identify the cause of an AE after order set use, these situations can be prevented with FMEA.
When applying FMEA to Figure 1, while bupropion is known as an evidence-based oral tobacco cessation option, there is the possibility that bupropion could be inadvertently prescribed from the order set in a hospitalized patient with alcohol withdrawal and withdrawal seizure history. These potentially dangerous situations can be avoided with FMEA. Thus, although bupropion may be evidence-based for NRT, decisions regarding order set design using EBM alone are insufficient.
The practitioner must consider possible unintended consequences within order sets and target treatment options to the appropriate setting and audience. Although Figure 1 may appear to be more inclusive, the interdisciplinary committee designing the inpatient NRT order set felt there was heightened risk with introducing bupropion in Figure 1 and decided the risk would be lowered by removing bupropion from the redesigned NRT order set (Figure 2). In addition to the goal of balancing availability of NRT options with acceptable risk, Figure 2 also focused on building an NRT order set most applicable to the inpatient setting.
Including Evidence-Based Practices
EBM has become a routine part of clinical decision making. Therefore, including EBM in order set design is vital. EBM for NRT has demonstrated that combination therapy is more effective than is monotherapy to help tobacco users quit. Incremental doses of NRT are recommended for patients who use tobacco more frequently.10
As shown in Figures 1 and 2, both order set designs incorporate EBM for NRT. Although the importance of implementing EBM is evident, critical factors, such as HFE and FMEA make a difference with well-designed order sets.
Results
The 4-step order set design technique was used during development of an inpatient NRT order set at the JAHVH. Results for the inpatient Joint Commission Tobacco Treatment Measures were obtained from the Veterans Health Administration quality metric reporting system known as Strategic Analytics for Improvement and Learning (SAIL). SAIL performance measure outcomes, which include the inpatient Joint Commission Tobacco Treatment Measures, are derived from chart reviews conducted by the External Peer Review Program. Outcomes demonstrated that TOB-2 and TOB-3 (2 inpatient Joint Commission Tobacco Treatment Measures) known as tob20 and tob40, respectively, within SAIL improved by more than 300% after development of an NRT order set using the 4-step order set design framework along with implementation of a multifaceted tobacco cessation care delivery system at JAHVH.
Discussion
While the overall tobacco cessation care delivery system contributed to improved outcomes with the inpatient Joint Commission Tobacco Treatment Measures at JAHVH, the NRT order set was a cornerstone of the design. Although using our order set design technique does not necessarily guarantee successful outcomes, we believe using the 4-step order set design process increases the value of order sets and has potential to improve quality outcomes.
Limitations
Although improved outcomes following implementation of our NRT order set suggest correlation, causation cannot be proven. Also while the NRT order set is believed to have helped tremendously with outcomes, the entire tobacco cessation care delivery system at JAHVH contributed to the results. In addition, the inpatient Joint Commission Tobacco Treatment Measures help improve processes for tobacco cessation care. However, we are uncertain whether the results of our improvement efforts helped patients stop tobacco use. Further studies are needed to determine impact on population health. Finally, our results were based on improvement work done at a single center. Further studies are necessary to see whether results are reproducible.
Conclusion
There was significant improvement with the inpatient Joint Commission Tobacco Treatment Measures outcomes following development of a tobacco cessation care delivery system that included design of an inpatient NRT order set using a 4-step process we developed. This 4-step structure includes emphasis on efficiency and ease of use; human factors engineering; failure mode and effects analysis; and incorporation of evidence-based medicine (Box.) Postimplementation results showed improvement of the inpatient Joint Commission Tobacco Treatment Measures by greater than 3-fold at a single hospital.
The next steps for this initiative include testing the 4-step order set design process in multiple clinical settings to determine the effectiveness of this approach in other areas of clinical care.
1. Order set. http://clinfowiki.org/wiki/index.php/Order_set. Updated October 15, 2015. Accessed August 30, 2019.
2. Bates DW, Kuperman GJ, Wang S, et al. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc. 2003;10(6):523-530.
3. Institute for Safe Medication Practices. Guidelines for standard order sets. https://www.ismp.org/tools/guidelines/standardordersets.pdf. Published January 12, 2010. Accessed August 30, 2019.
4. Heath C, Heath D. Switch: How to Change Things When Change Is Hard. New York, NY: Crown Business; 2010:50-51.
5. US Department of Health and Human Services. Usability testing. https://www.usability.gov/how-to-and-tools/methods/usability-testing.html. Accessed August 30, 2019.
6. World Health Organization. What is human factors and why is it important to patient safety? www.who.int/patientsafety/education/curriculum/who_mc_topic-2.pdf. Accessed August 30, 2019.
7. Sollisch J. The cure for decision fatigue. Wall Street Journal. June 10, 2016. https://www.wsj.com/articles/the-cure-for-decision-fatigue-1465596928. Accessed August 30, 2019.
8. ECRI Institute. Implementing the ENFit initiative for preventing enteral tubing misconnections. https://www.ecri.org/components/HDJournal/Pages/ENFit-for-Preventing-Enteral-Tubing-Misconnections.aspx. Published March 29, 2017. Accessed August 30, 2019.
9. Guidance for performing failure mode and effects analysis with performance improvement projects. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/QAPI/downloads/GuidanceForFMEA.pdf. Accessed August 30, 2019.
10. Diefanbach LJ, Smith PO, Nashelsky JM, Lindbloom E. What is the most effective nicotine replacement therapy? J Fam Pract. 2003;52(6):492-497.
In the current health care environment, hospitals are constantly challenged to improve quality metrics and deliver better health care outcomes. One means to achieving quality improvement is through the use of order sets, groups of related orders that a health care provider (HCP) can place with either a few keystrokes or mouse clicks.1
Historically, design of order sets has largely focused on clicking checkboxes containing evidence-based practices. According to Bates and colleagues and the Institute for Safe Medication Practices, incorporating evidence-based medicine (EBM) into order sets is not by itself sufficient.2,3Execution of proper design coupled with simplicity and provider efficiency is paramount to HCP buy-in, increased likelihood of order set adherence, and to potentially better outcomes.
In this article, we outline advancements in order set design. These improvements increase provider efficiency and ease of use; incorporate human factors engineering (HFE); apply failure mode and effects analysis; and include EBM.
Methods
An inpatient nicotine replacement therapy (NRT) order was developed as part of a multifaceted solution to improve tobacco cessation care at the James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, a complexity level 1a facility. This NRT order set used the 4-step order set design framework the authors’ developed (for additional information about the NRT order set, contact the authors). We distinguish order set design technique between 2 different inpatient NRT order sets. The first order set in the comparison (Figure 1) is an inpatient NRT order set of unknown origin—it is common for US Department of Veterans Affairs (VA) medical facilities to share order sets and other resources. The second order set (Figure 2) is an inpatient NRT order set we designed using our 4-step process for comparison in this article. No institutional review board approval was required as this work met criteria for operational improvement activities exempt from ethics review.
Justin Iannello, DO, MBA, was the team leader and developer of the 4-step order set design technique. The intervention team consisted of 4 internal medicine physicians with expertise in quality improvement and patient safety: 1 certified professional in patient safety and certified as a Lean Six Sigma Black Belt; 2 physicians certified as Lean Six Sigma Black Belts; and 1 physician certified as a Lean Six Sigma Green Belt. Two inpatient clinical pharmacists and 1 quality management specialist also were involved in its development.
Development of a new NRT order set was felt to be an integral part of the tobacco cessation care delivery process. An NRT order set perceived by users as value-added required a solution that merged EBM with standardization and applied quality improvement principles. The result was an approach to order set design that focused on 4 key questions: Is the order set efficient and easy to use/navigate? Is human factors engineering incorporated? Is failure mode and effects analysis applied? Are evidence-based practices included?
Ease of Use and Navigation
Implementing an order set that is efficient and easy to use or navigate seems straightforward but can be difficult to execute. Figure 1 shows many detailed options consisting of different combinations of nicotine patches, lozenges, and gum. Also included are oral tobacco cessation options (bupropion and varenicline). Although more options may seem better, confusion about appropriate medication selection can occur.
According to Heath and Heath, too many options can result in lack of action.4 For example, Heath and Heath discuss a food store that offered 6 free samples of different jams on one day and 24 jams the following day. The customers who sampled 6 different types of jam were 10 times more likely to buy jam. The authors concluded that the more options available, the more difficulty a potential buyer has in deciding on a course of action.4
In clinical situations where a HCP is using an order set, the number of options can mean the difference between use vs avoidance if the choices are overwhelming. HCPs process layers of detail every day when creating differential diagnoses and treatment plans. While that level of detail is necessary clinically, that same level of detail included in orders sets can create challenges for HCPs.
Figure 2 advances the order set in Figure 1 by providing a simpler and cleaner design, so HCPs can more easily review and process the information. This order set design minimizes the number of options available to help users make the right decision, focusing on value for the appropriate setting and audience. In other words, order sets should not be a “one size fits all” approach.
Order sets should be tailored to the appropriate clinical setting (eg, inpatient acute care, outpatient clinic setting, etc) and HCP (eg, hospitalist, tobacco cessation specialist, etc). We are comparing NRT order sets designed for HCPs who do not routinely prescribe oral tobacco cessation products in the inpatient setting. When possible, autogenerated bundle orders should also be used according to evidence-based recommendations (such as nicotine patch tapers) for ease of use and further simplification of order sets.
Finally, usability testing known as “evaluating a product or service by testing it with representative users” helps further refine an order set.5Usability testing should be applied during all phases of order set development with end user(s) as it helps identify problems with order set design prior to implementation. By applying usability testing, the order set becomes more meaningful and valued by the user.
Human Factors Engineering
HFE is “the study of all the factors that make it easier to do the work in the right way.”6 HFE seeks to identify, align, and apply processes for people and the world within which they live and work to promote safe and efficient practices, especially in relation to the technology and physical design features in their work environment.6
The average American adult makes about 35,000 decisions per day.7 Thus, there is potential for error at any moment. Design that does not take HFE into account can be dangerous. For example, when tube feed and IV line connectors look similar and are compatible, patients may inadvertently receive food administered directly into their bloodstream.8
HFE can and should be applied to order sets. Everything from the look, feel, and verbiage of an order set affects potential outcomes. For example, consider the impact even seemingly minor modifications can have on outcomes simply by guiding users in a different way: Figure 1 provides NRT options based on cigarette use per day, whereas Figure 2 conveys pack use per day in relation to the equivalent number of cigarettes used daily. These differences may seem small; however, it helps guide users to the right choice when considering that health care providers have been historically trained on social history gathering that emphasizes packs per day and pack-years.
Failure Mode and Effects Analysis
Failure mode and effects analysis (FMEA) is “a structured way to identify and address potential problems, or failures and their resulting effects on the system or process before an adverse event occurs.”9 The benefit of an order set must be weighed against the risk during development. FMEA should be applied during order set design to assess and limit risk just as with any other clinical care process.
FMEA examines both level of risk and frequency of risk occurrence associated with a new proposed process. For example, let’s evaluate an order set designed for pain control after surgery that consists of multiple high-risk opioids along with antihistamine medications for as-needed itch relief (a non-life-threatening adverse event (AE) of opioids well known by the medical community). An interdisciplinary FMEA team consisting of subject matter experts may examine how the process should flow in step-by-step detail and then discuss the benefit of a process and risk for potential error. A FMEA team would then analyze what could go wrong with each part of the process and assign a level of risk and risk frequency for various steps in the process, and then decide that certain steps should be modified or eliminated. Perhaps after FMEA, a facility might conclude that the risk of serious complications is high when you combine opioid use with antihistamine medications. The facility could decide to remove antihistamine medications from an order set if it is determined that risks outweigh benefits. While a root cause analysis might identify the cause of an AE after order set use, these situations can be prevented with FMEA.
When applying FMEA to Figure 1, while bupropion is known as an evidence-based oral tobacco cessation option, there is the possibility that bupropion could be inadvertently prescribed from the order set in a hospitalized patient with alcohol withdrawal and withdrawal seizure history. These potentially dangerous situations can be avoided with FMEA. Thus, although bupropion may be evidence-based for NRT, decisions regarding order set design using EBM alone are insufficient.
The practitioner must consider possible unintended consequences within order sets and target treatment options to the appropriate setting and audience. Although Figure 1 may appear to be more inclusive, the interdisciplinary committee designing the inpatient NRT order set felt there was heightened risk with introducing bupropion in Figure 1 and decided the risk would be lowered by removing bupropion from the redesigned NRT order set (Figure 2). In addition to the goal of balancing availability of NRT options with acceptable risk, Figure 2 also focused on building an NRT order set most applicable to the inpatient setting.
Including Evidence-Based Practices
EBM has become a routine part of clinical decision making. Therefore, including EBM in order set design is vital. EBM for NRT has demonstrated that combination therapy is more effective than is monotherapy to help tobacco users quit. Incremental doses of NRT are recommended for patients who use tobacco more frequently.10
As shown in Figures 1 and 2, both order set designs incorporate EBM for NRT. Although the importance of implementing EBM is evident, critical factors, such as HFE and FMEA make a difference with well-designed order sets.
Results
The 4-step order set design technique was used during development of an inpatient NRT order set at the JAHVH. Results for the inpatient Joint Commission Tobacco Treatment Measures were obtained from the Veterans Health Administration quality metric reporting system known as Strategic Analytics for Improvement and Learning (SAIL). SAIL performance measure outcomes, which include the inpatient Joint Commission Tobacco Treatment Measures, are derived from chart reviews conducted by the External Peer Review Program. Outcomes demonstrated that TOB-2 and TOB-3 (2 inpatient Joint Commission Tobacco Treatment Measures) known as tob20 and tob40, respectively, within SAIL improved by more than 300% after development of an NRT order set using the 4-step order set design framework along with implementation of a multifaceted tobacco cessation care delivery system at JAHVH.
Discussion
While the overall tobacco cessation care delivery system contributed to improved outcomes with the inpatient Joint Commission Tobacco Treatment Measures at JAHVH, the NRT order set was a cornerstone of the design. Although using our order set design technique does not necessarily guarantee successful outcomes, we believe using the 4-step order set design process increases the value of order sets and has potential to improve quality outcomes.
Limitations
Although improved outcomes following implementation of our NRT order set suggest correlation, causation cannot be proven. Also while the NRT order set is believed to have helped tremendously with outcomes, the entire tobacco cessation care delivery system at JAHVH contributed to the results. In addition, the inpatient Joint Commission Tobacco Treatment Measures help improve processes for tobacco cessation care. However, we are uncertain whether the results of our improvement efforts helped patients stop tobacco use. Further studies are needed to determine impact on population health. Finally, our results were based on improvement work done at a single center. Further studies are necessary to see whether results are reproducible.
Conclusion
There was significant improvement with the inpatient Joint Commission Tobacco Treatment Measures outcomes following development of a tobacco cessation care delivery system that included design of an inpatient NRT order set using a 4-step process we developed. This 4-step structure includes emphasis on efficiency and ease of use; human factors engineering; failure mode and effects analysis; and incorporation of evidence-based medicine (Box.) Postimplementation results showed improvement of the inpatient Joint Commission Tobacco Treatment Measures by greater than 3-fold at a single hospital.
The next steps for this initiative include testing the 4-step order set design process in multiple clinical settings to determine the effectiveness of this approach in other areas of clinical care.
In the current health care environment, hospitals are constantly challenged to improve quality metrics and deliver better health care outcomes. One means to achieving quality improvement is through the use of order sets, groups of related orders that a health care provider (HCP) can place with either a few keystrokes or mouse clicks.1
Historically, design of order sets has largely focused on clicking checkboxes containing evidence-based practices. According to Bates and colleagues and the Institute for Safe Medication Practices, incorporating evidence-based medicine (EBM) into order sets is not by itself sufficient.2,3Execution of proper design coupled with simplicity and provider efficiency is paramount to HCP buy-in, increased likelihood of order set adherence, and to potentially better outcomes.
In this article, we outline advancements in order set design. These improvements increase provider efficiency and ease of use; incorporate human factors engineering (HFE); apply failure mode and effects analysis; and include EBM.
Methods
An inpatient nicotine replacement therapy (NRT) order was developed as part of a multifaceted solution to improve tobacco cessation care at the James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, a complexity level 1a facility. This NRT order set used the 4-step order set design framework the authors’ developed (for additional information about the NRT order set, contact the authors). We distinguish order set design technique between 2 different inpatient NRT order sets. The first order set in the comparison (Figure 1) is an inpatient NRT order set of unknown origin—it is common for US Department of Veterans Affairs (VA) medical facilities to share order sets and other resources. The second order set (Figure 2) is an inpatient NRT order set we designed using our 4-step process for comparison in this article. No institutional review board approval was required as this work met criteria for operational improvement activities exempt from ethics review.
Justin Iannello, DO, MBA, was the team leader and developer of the 4-step order set design technique. The intervention team consisted of 4 internal medicine physicians with expertise in quality improvement and patient safety: 1 certified professional in patient safety and certified as a Lean Six Sigma Black Belt; 2 physicians certified as Lean Six Sigma Black Belts; and 1 physician certified as a Lean Six Sigma Green Belt. Two inpatient clinical pharmacists and 1 quality management specialist also were involved in its development.
Development of a new NRT order set was felt to be an integral part of the tobacco cessation care delivery process. An NRT order set perceived by users as value-added required a solution that merged EBM with standardization and applied quality improvement principles. The result was an approach to order set design that focused on 4 key questions: Is the order set efficient and easy to use/navigate? Is human factors engineering incorporated? Is failure mode and effects analysis applied? Are evidence-based practices included?
Ease of Use and Navigation
Implementing an order set that is efficient and easy to use or navigate seems straightforward but can be difficult to execute. Figure 1 shows many detailed options consisting of different combinations of nicotine patches, lozenges, and gum. Also included are oral tobacco cessation options (bupropion and varenicline). Although more options may seem better, confusion about appropriate medication selection can occur.
According to Heath and Heath, too many options can result in lack of action.4 For example, Heath and Heath discuss a food store that offered 6 free samples of different jams on one day and 24 jams the following day. The customers who sampled 6 different types of jam were 10 times more likely to buy jam. The authors concluded that the more options available, the more difficulty a potential buyer has in deciding on a course of action.4
In clinical situations where a HCP is using an order set, the number of options can mean the difference between use vs avoidance if the choices are overwhelming. HCPs process layers of detail every day when creating differential diagnoses and treatment plans. While that level of detail is necessary clinically, that same level of detail included in orders sets can create challenges for HCPs.
Figure 2 advances the order set in Figure 1 by providing a simpler and cleaner design, so HCPs can more easily review and process the information. This order set design minimizes the number of options available to help users make the right decision, focusing on value for the appropriate setting and audience. In other words, order sets should not be a “one size fits all” approach.
Order sets should be tailored to the appropriate clinical setting (eg, inpatient acute care, outpatient clinic setting, etc) and HCP (eg, hospitalist, tobacco cessation specialist, etc). We are comparing NRT order sets designed for HCPs who do not routinely prescribe oral tobacco cessation products in the inpatient setting. When possible, autogenerated bundle orders should also be used according to evidence-based recommendations (such as nicotine patch tapers) for ease of use and further simplification of order sets.
Finally, usability testing known as “evaluating a product or service by testing it with representative users” helps further refine an order set.5Usability testing should be applied during all phases of order set development with end user(s) as it helps identify problems with order set design prior to implementation. By applying usability testing, the order set becomes more meaningful and valued by the user.
Human Factors Engineering
HFE is “the study of all the factors that make it easier to do the work in the right way.”6 HFE seeks to identify, align, and apply processes for people and the world within which they live and work to promote safe and efficient practices, especially in relation to the technology and physical design features in their work environment.6
The average American adult makes about 35,000 decisions per day.7 Thus, there is potential for error at any moment. Design that does not take HFE into account can be dangerous. For example, when tube feed and IV line connectors look similar and are compatible, patients may inadvertently receive food administered directly into their bloodstream.8
HFE can and should be applied to order sets. Everything from the look, feel, and verbiage of an order set affects potential outcomes. For example, consider the impact even seemingly minor modifications can have on outcomes simply by guiding users in a different way: Figure 1 provides NRT options based on cigarette use per day, whereas Figure 2 conveys pack use per day in relation to the equivalent number of cigarettes used daily. These differences may seem small; however, it helps guide users to the right choice when considering that health care providers have been historically trained on social history gathering that emphasizes packs per day and pack-years.
Failure Mode and Effects Analysis
Failure mode and effects analysis (FMEA) is “a structured way to identify and address potential problems, or failures and their resulting effects on the system or process before an adverse event occurs.”9 The benefit of an order set must be weighed against the risk during development. FMEA should be applied during order set design to assess and limit risk just as with any other clinical care process.
FMEA examines both level of risk and frequency of risk occurrence associated with a new proposed process. For example, let’s evaluate an order set designed for pain control after surgery that consists of multiple high-risk opioids along with antihistamine medications for as-needed itch relief (a non-life-threatening adverse event (AE) of opioids well known by the medical community). An interdisciplinary FMEA team consisting of subject matter experts may examine how the process should flow in step-by-step detail and then discuss the benefit of a process and risk for potential error. A FMEA team would then analyze what could go wrong with each part of the process and assign a level of risk and risk frequency for various steps in the process, and then decide that certain steps should be modified or eliminated. Perhaps after FMEA, a facility might conclude that the risk of serious complications is high when you combine opioid use with antihistamine medications. The facility could decide to remove antihistamine medications from an order set if it is determined that risks outweigh benefits. While a root cause analysis might identify the cause of an AE after order set use, these situations can be prevented with FMEA.
When applying FMEA to Figure 1, while bupropion is known as an evidence-based oral tobacco cessation option, there is the possibility that bupropion could be inadvertently prescribed from the order set in a hospitalized patient with alcohol withdrawal and withdrawal seizure history. These potentially dangerous situations can be avoided with FMEA. Thus, although bupropion may be evidence-based for NRT, decisions regarding order set design using EBM alone are insufficient.
The practitioner must consider possible unintended consequences within order sets and target treatment options to the appropriate setting and audience. Although Figure 1 may appear to be more inclusive, the interdisciplinary committee designing the inpatient NRT order set felt there was heightened risk with introducing bupropion in Figure 1 and decided the risk would be lowered by removing bupropion from the redesigned NRT order set (Figure 2). In addition to the goal of balancing availability of NRT options with acceptable risk, Figure 2 also focused on building an NRT order set most applicable to the inpatient setting.
Including Evidence-Based Practices
EBM has become a routine part of clinical decision making. Therefore, including EBM in order set design is vital. EBM for NRT has demonstrated that combination therapy is more effective than is monotherapy to help tobacco users quit. Incremental doses of NRT are recommended for patients who use tobacco more frequently.10
As shown in Figures 1 and 2, both order set designs incorporate EBM for NRT. Although the importance of implementing EBM is evident, critical factors, such as HFE and FMEA make a difference with well-designed order sets.
Results
The 4-step order set design technique was used during development of an inpatient NRT order set at the JAHVH. Results for the inpatient Joint Commission Tobacco Treatment Measures were obtained from the Veterans Health Administration quality metric reporting system known as Strategic Analytics for Improvement and Learning (SAIL). SAIL performance measure outcomes, which include the inpatient Joint Commission Tobacco Treatment Measures, are derived from chart reviews conducted by the External Peer Review Program. Outcomes demonstrated that TOB-2 and TOB-3 (2 inpatient Joint Commission Tobacco Treatment Measures) known as tob20 and tob40, respectively, within SAIL improved by more than 300% after development of an NRT order set using the 4-step order set design framework along with implementation of a multifaceted tobacco cessation care delivery system at JAHVH.
Discussion
While the overall tobacco cessation care delivery system contributed to improved outcomes with the inpatient Joint Commission Tobacco Treatment Measures at JAHVH, the NRT order set was a cornerstone of the design. Although using our order set design technique does not necessarily guarantee successful outcomes, we believe using the 4-step order set design process increases the value of order sets and has potential to improve quality outcomes.
Limitations
Although improved outcomes following implementation of our NRT order set suggest correlation, causation cannot be proven. Also while the NRT order set is believed to have helped tremendously with outcomes, the entire tobacco cessation care delivery system at JAHVH contributed to the results. In addition, the inpatient Joint Commission Tobacco Treatment Measures help improve processes for tobacco cessation care. However, we are uncertain whether the results of our improvement efforts helped patients stop tobacco use. Further studies are needed to determine impact on population health. Finally, our results were based on improvement work done at a single center. Further studies are necessary to see whether results are reproducible.
Conclusion
There was significant improvement with the inpatient Joint Commission Tobacco Treatment Measures outcomes following development of a tobacco cessation care delivery system that included design of an inpatient NRT order set using a 4-step process we developed. This 4-step structure includes emphasis on efficiency and ease of use; human factors engineering; failure mode and effects analysis; and incorporation of evidence-based medicine (Box.) Postimplementation results showed improvement of the inpatient Joint Commission Tobacco Treatment Measures by greater than 3-fold at a single hospital.
The next steps for this initiative include testing the 4-step order set design process in multiple clinical settings to determine the effectiveness of this approach in other areas of clinical care.
1. Order set. http://clinfowiki.org/wiki/index.php/Order_set. Updated October 15, 2015. Accessed August 30, 2019.
2. Bates DW, Kuperman GJ, Wang S, et al. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc. 2003;10(6):523-530.
3. Institute for Safe Medication Practices. Guidelines for standard order sets. https://www.ismp.org/tools/guidelines/standardordersets.pdf. Published January 12, 2010. Accessed August 30, 2019.
4. Heath C, Heath D. Switch: How to Change Things When Change Is Hard. New York, NY: Crown Business; 2010:50-51.
5. US Department of Health and Human Services. Usability testing. https://www.usability.gov/how-to-and-tools/methods/usability-testing.html. Accessed August 30, 2019.
6. World Health Organization. What is human factors and why is it important to patient safety? www.who.int/patientsafety/education/curriculum/who_mc_topic-2.pdf. Accessed August 30, 2019.
7. Sollisch J. The cure for decision fatigue. Wall Street Journal. June 10, 2016. https://www.wsj.com/articles/the-cure-for-decision-fatigue-1465596928. Accessed August 30, 2019.
8. ECRI Institute. Implementing the ENFit initiative for preventing enteral tubing misconnections. https://www.ecri.org/components/HDJournal/Pages/ENFit-for-Preventing-Enteral-Tubing-Misconnections.aspx. Published March 29, 2017. Accessed August 30, 2019.
9. Guidance for performing failure mode and effects analysis with performance improvement projects. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/QAPI/downloads/GuidanceForFMEA.pdf. Accessed August 30, 2019.
10. Diefanbach LJ, Smith PO, Nashelsky JM, Lindbloom E. What is the most effective nicotine replacement therapy? J Fam Pract. 2003;52(6):492-497.
1. Order set. http://clinfowiki.org/wiki/index.php/Order_set. Updated October 15, 2015. Accessed August 30, 2019.
2. Bates DW, Kuperman GJ, Wang S, et al. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc. 2003;10(6):523-530.
3. Institute for Safe Medication Practices. Guidelines for standard order sets. https://www.ismp.org/tools/guidelines/standardordersets.pdf. Published January 12, 2010. Accessed August 30, 2019.
4. Heath C, Heath D. Switch: How to Change Things When Change Is Hard. New York, NY: Crown Business; 2010:50-51.
5. US Department of Health and Human Services. Usability testing. https://www.usability.gov/how-to-and-tools/methods/usability-testing.html. Accessed August 30, 2019.
6. World Health Organization. What is human factors and why is it important to patient safety? www.who.int/patientsafety/education/curriculum/who_mc_topic-2.pdf. Accessed August 30, 2019.
7. Sollisch J. The cure for decision fatigue. Wall Street Journal. June 10, 2016. https://www.wsj.com/articles/the-cure-for-decision-fatigue-1465596928. Accessed August 30, 2019.
8. ECRI Institute. Implementing the ENFit initiative for preventing enteral tubing misconnections. https://www.ecri.org/components/HDJournal/Pages/ENFit-for-Preventing-Enteral-Tubing-Misconnections.aspx. Published March 29, 2017. Accessed August 30, 2019.
9. Guidance for performing failure mode and effects analysis with performance improvement projects. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/QAPI/downloads/GuidanceForFMEA.pdf. Accessed August 30, 2019.
10. Diefanbach LJ, Smith PO, Nashelsky JM, Lindbloom E. What is the most effective nicotine replacement therapy? J Fam Pract. 2003;52(6):492-497.
The VA Ketamine Controversies
"Extreme remedies are very appropriate for extreme diseases"
- Hippocrates Aphorisms
On March 5, 2019, the US Food and Drug Administration (FDA) approved a nasal spray formulation of the drug ketamine, an old anesthetic that has been put to a new use over the past 10 years as therapy for treatment-resistant severe depression. Ketamine, known on the street as Special K, has long been known to cause dissociation, hallucinations, and other hallucinogenic effects. In many randomized controlled trials, subanesthetic doses administered intravenously have demonstrated rapid and often dramatic relief of depressive symptoms.
Neuroscientists have heralded ketamine as the paradigm
When the FDA approved Spravato (esketamine), a nasal administration of ketamine, many people hoped that researchers had succeeded in overcoming these barriers. The risks of serious adverse events (AEs) as well as the potential for abuse and diversion led the FDA to limit prescriptions under a Risk Evaluation and Mitigation Strategy (REMS).3 Patients self-administer the nasal spray but only in a certified medical facility under the observation of a health care practitioner. Patients also must agree to remain on site for 2 hours after administration of the drug to ensure their safety. The FDA recommends the drug be given twice a week for 4 weeks along with a conventional monoamine-acting antidepressant.When the US Department of Veterans Affairs (VA) cleared the way for use of esketamine, less than 2 weeks after the FDA approval, it also launched a series of controversies over how to use the drug in its massive health care system, which is the subject of this editorial. On March 19, 2019, the VA announced that VA practitioners would be able to prescribe the nasal spray for patients who were determined to have treatment-resistant depression but only after appropriate clinical assessment and in accordance with their patients’ preferences.
A number of controversies have emerged surrounding the VA adoption of esketamine, including its cost/benefit/risk ratio and who should be able to access the medication. Each of these issues has onion layers of political, regulatory, and ethical concerns that can only be superficially noted here and warrant fuller unpeeling. In June The New York Times featured a story alleging that in response to the tragic tide of ever-increasing veteran suicides, the VA sanctioned esketamine prescribing despite its cost and the serious questions experts raised about the data the FDA cited to establish its safety and efficacy. Although the cost to the VA of Spravato is unclear, it is much higher than generic IV ketamine.4
The access controversy is almost the ethical inverse of the first. In June 2019, a Veterans Health Administration advisory panel voted against allowing general use of esketamine, limiting it to individual cases of patients who are preapproved and have failed 2 antidepressant trials. Esketamine will not be on the VA formulary for widespread use. Congressional and public advocacy groups have noted that the formulary decision came in the wake of ongoing attention to the role of the pharmaceutical industry in the VA’s rapid adoption of the drug.5,6 For the thousands of veterans for whom the data show conventional antidepressants even in combination with other psychotropic medications and evidence-based psychotherapies resulted in AEs or only partial remission of depression symptoms, the VA’s restriction will likely seem unfair and even uncaring.7
As a practicing VA psychiatrist, I know firsthand how desperately we need new, more effective, and better-tolerated treatments for severe unipolar and bipolar depression. Although I have not prescribed ketamine or esketamine, several of my most respected colleagues do. I have seen patients with chronic, severe, depression respond and even recover in ways that seem just a little short of miraculous when compared with other therapies. Yet as a longtime student of the history of psychiatry, I have also seen that often the treatments that initially seem so auspicious, in time, turn out to have a dark side. Families, communities, the country, VA, and the US Department of Defense and its practitioners in and out of mental health cannot in any moral universe abide by the fact that 20 plus men and women who served take their lives every day.8
As the epigraph to this column notes, we must often try radical therapies for grave cases in drastic crises. Yet we must also in making serious public health decisions fraught with unseen consequences take all due and considered diligence that we do not violate the even more fundamental dictum of the Hippocratic School, “at least do not harm.” That means trying to balance safety and availability while VA conducts its own research in a precarious way that leaves almost no stakeholder completely happy.
1. Lener MS, Kadriu B, Zarate CA Jr. Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs. 2017;77(4):381-401.
2. Thielking M. “Is the Ketamine Boon Getting out of Hand?” STAT. September 24, 2018. https://www.statnews.com/2018/09/24/ketamine-clinics-severe-depression-treatment. Accessed September 17, 2019.
3. US Food and Drug Administration. FDA approves new nasal spray medication for treatment-resistant depression: available only at a certified doctor’s office or clinic [press release]. https://www.fda.gov/news-events/press-announcements/fda-approves-new-nasal-spray-medication-treatment-resistant-depression-available-only-certified. Published March 5, 2019. Accessed September 17, 2019.
4. Carey B, Steinhauser J. Veterans agency to offer new depression drug, despite safety and efficacy concerns. The New York Times. June 21, 2019. https://www.nytimes.com/2019/06/21/health/ketamine-depression-veterans.html. Accessed September 17, 2019.
5. US House of Representatives, Committee on Veterans Affairs. Chairman Takano statement following reports that VA fast-tracked controversial drug Spravato to treat veterans [press release]. https://veterans.house.gov/news/press-releases/chairman-takano-statement-following-reports-that-va-fast-tracked-controversial-drug-spravato-to-treat-veterans. Published June 18, 2019. Accessed September 17, 2019.
6. Cary P. Trump’s praise put drug for vets on fast track, but experts are not sure it works. https://publicintegrity.org/federal-politics/trumps-raves-put-drug-for-vets-on-fast-track-but-experts-arent-sure-it-works. Published June 18, 2019. Accessed September 17, 2019.
7. Zisook S, Tal I, Weingart K, et al. Characteristics of U.S. veteran patients with major depressive disorder who require ‘next-step’ treatments: A VAST-D report. J Affect Disord. 2016;206:232-240.
8. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. VA National Suicide Data Report 2005-2016. https://www.mentalhealth.va.gov/docs/data-sheets/OMHSP_National_Suicide_Data_Report_2005-2016_508.pdf. Updated 2018. Accessed September 17, 2019.
"Extreme remedies are very appropriate for extreme diseases"
- Hippocrates Aphorisms
On March 5, 2019, the US Food and Drug Administration (FDA) approved a nasal spray formulation of the drug ketamine, an old anesthetic that has been put to a new use over the past 10 years as therapy for treatment-resistant severe depression. Ketamine, known on the street as Special K, has long been known to cause dissociation, hallucinations, and other hallucinogenic effects. In many randomized controlled trials, subanesthetic doses administered intravenously have demonstrated rapid and often dramatic relief of depressive symptoms.
Neuroscientists have heralded ketamine as the paradigm
When the FDA approved Spravato (esketamine), a nasal administration of ketamine, many people hoped that researchers had succeeded in overcoming these barriers. The risks of serious adverse events (AEs) as well as the potential for abuse and diversion led the FDA to limit prescriptions under a Risk Evaluation and Mitigation Strategy (REMS).3 Patients self-administer the nasal spray but only in a certified medical facility under the observation of a health care practitioner. Patients also must agree to remain on site for 2 hours after administration of the drug to ensure their safety. The FDA recommends the drug be given twice a week for 4 weeks along with a conventional monoamine-acting antidepressant.When the US Department of Veterans Affairs (VA) cleared the way for use of esketamine, less than 2 weeks after the FDA approval, it also launched a series of controversies over how to use the drug in its massive health care system, which is the subject of this editorial. On March 19, 2019, the VA announced that VA practitioners would be able to prescribe the nasal spray for patients who were determined to have treatment-resistant depression but only after appropriate clinical assessment and in accordance with their patients’ preferences.
A number of controversies have emerged surrounding the VA adoption of esketamine, including its cost/benefit/risk ratio and who should be able to access the medication. Each of these issues has onion layers of political, regulatory, and ethical concerns that can only be superficially noted here and warrant fuller unpeeling. In June The New York Times featured a story alleging that in response to the tragic tide of ever-increasing veteran suicides, the VA sanctioned esketamine prescribing despite its cost and the serious questions experts raised about the data the FDA cited to establish its safety and efficacy. Although the cost to the VA of Spravato is unclear, it is much higher than generic IV ketamine.4
The access controversy is almost the ethical inverse of the first. In June 2019, a Veterans Health Administration advisory panel voted against allowing general use of esketamine, limiting it to individual cases of patients who are preapproved and have failed 2 antidepressant trials. Esketamine will not be on the VA formulary for widespread use. Congressional and public advocacy groups have noted that the formulary decision came in the wake of ongoing attention to the role of the pharmaceutical industry in the VA’s rapid adoption of the drug.5,6 For the thousands of veterans for whom the data show conventional antidepressants even in combination with other psychotropic medications and evidence-based psychotherapies resulted in AEs or only partial remission of depression symptoms, the VA’s restriction will likely seem unfair and even uncaring.7
As a practicing VA psychiatrist, I know firsthand how desperately we need new, more effective, and better-tolerated treatments for severe unipolar and bipolar depression. Although I have not prescribed ketamine or esketamine, several of my most respected colleagues do. I have seen patients with chronic, severe, depression respond and even recover in ways that seem just a little short of miraculous when compared with other therapies. Yet as a longtime student of the history of psychiatry, I have also seen that often the treatments that initially seem so auspicious, in time, turn out to have a dark side. Families, communities, the country, VA, and the US Department of Defense and its practitioners in and out of mental health cannot in any moral universe abide by the fact that 20 plus men and women who served take their lives every day.8
As the epigraph to this column notes, we must often try radical therapies for grave cases in drastic crises. Yet we must also in making serious public health decisions fraught with unseen consequences take all due and considered diligence that we do not violate the even more fundamental dictum of the Hippocratic School, “at least do not harm.” That means trying to balance safety and availability while VA conducts its own research in a precarious way that leaves almost no stakeholder completely happy.
"Extreme remedies are very appropriate for extreme diseases"
- Hippocrates Aphorisms
On March 5, 2019, the US Food and Drug Administration (FDA) approved a nasal spray formulation of the drug ketamine, an old anesthetic that has been put to a new use over the past 10 years as therapy for treatment-resistant severe depression. Ketamine, known on the street as Special K, has long been known to cause dissociation, hallucinations, and other hallucinogenic effects. In many randomized controlled trials, subanesthetic doses administered intravenously have demonstrated rapid and often dramatic relief of depressive symptoms.
Neuroscientists have heralded ketamine as the paradigm
When the FDA approved Spravato (esketamine), a nasal administration of ketamine, many people hoped that researchers had succeeded in overcoming these barriers. The risks of serious adverse events (AEs) as well as the potential for abuse and diversion led the FDA to limit prescriptions under a Risk Evaluation and Mitigation Strategy (REMS).3 Patients self-administer the nasal spray but only in a certified medical facility under the observation of a health care practitioner. Patients also must agree to remain on site for 2 hours after administration of the drug to ensure their safety. The FDA recommends the drug be given twice a week for 4 weeks along with a conventional monoamine-acting antidepressant.When the US Department of Veterans Affairs (VA) cleared the way for use of esketamine, less than 2 weeks after the FDA approval, it also launched a series of controversies over how to use the drug in its massive health care system, which is the subject of this editorial. On March 19, 2019, the VA announced that VA practitioners would be able to prescribe the nasal spray for patients who were determined to have treatment-resistant depression but only after appropriate clinical assessment and in accordance with their patients’ preferences.
A number of controversies have emerged surrounding the VA adoption of esketamine, including its cost/benefit/risk ratio and who should be able to access the medication. Each of these issues has onion layers of political, regulatory, and ethical concerns that can only be superficially noted here and warrant fuller unpeeling. In June The New York Times featured a story alleging that in response to the tragic tide of ever-increasing veteran suicides, the VA sanctioned esketamine prescribing despite its cost and the serious questions experts raised about the data the FDA cited to establish its safety and efficacy. Although the cost to the VA of Spravato is unclear, it is much higher than generic IV ketamine.4
The access controversy is almost the ethical inverse of the first. In June 2019, a Veterans Health Administration advisory panel voted against allowing general use of esketamine, limiting it to individual cases of patients who are preapproved and have failed 2 antidepressant trials. Esketamine will not be on the VA formulary for widespread use. Congressional and public advocacy groups have noted that the formulary decision came in the wake of ongoing attention to the role of the pharmaceutical industry in the VA’s rapid adoption of the drug.5,6 For the thousands of veterans for whom the data show conventional antidepressants even in combination with other psychotropic medications and evidence-based psychotherapies resulted in AEs or only partial remission of depression symptoms, the VA’s restriction will likely seem unfair and even uncaring.7
As a practicing VA psychiatrist, I know firsthand how desperately we need new, more effective, and better-tolerated treatments for severe unipolar and bipolar depression. Although I have not prescribed ketamine or esketamine, several of my most respected colleagues do. I have seen patients with chronic, severe, depression respond and even recover in ways that seem just a little short of miraculous when compared with other therapies. Yet as a longtime student of the history of psychiatry, I have also seen that often the treatments that initially seem so auspicious, in time, turn out to have a dark side. Families, communities, the country, VA, and the US Department of Defense and its practitioners in and out of mental health cannot in any moral universe abide by the fact that 20 plus men and women who served take their lives every day.8
As the epigraph to this column notes, we must often try radical therapies for grave cases in drastic crises. Yet we must also in making serious public health decisions fraught with unseen consequences take all due and considered diligence that we do not violate the even more fundamental dictum of the Hippocratic School, “at least do not harm.” That means trying to balance safety and availability while VA conducts its own research in a precarious way that leaves almost no stakeholder completely happy.
1. Lener MS, Kadriu B, Zarate CA Jr. Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs. 2017;77(4):381-401.
2. Thielking M. “Is the Ketamine Boon Getting out of Hand?” STAT. September 24, 2018. https://www.statnews.com/2018/09/24/ketamine-clinics-severe-depression-treatment. Accessed September 17, 2019.
3. US Food and Drug Administration. FDA approves new nasal spray medication for treatment-resistant depression: available only at a certified doctor’s office or clinic [press release]. https://www.fda.gov/news-events/press-announcements/fda-approves-new-nasal-spray-medication-treatment-resistant-depression-available-only-certified. Published March 5, 2019. Accessed September 17, 2019.
4. Carey B, Steinhauser J. Veterans agency to offer new depression drug, despite safety and efficacy concerns. The New York Times. June 21, 2019. https://www.nytimes.com/2019/06/21/health/ketamine-depression-veterans.html. Accessed September 17, 2019.
5. US House of Representatives, Committee on Veterans Affairs. Chairman Takano statement following reports that VA fast-tracked controversial drug Spravato to treat veterans [press release]. https://veterans.house.gov/news/press-releases/chairman-takano-statement-following-reports-that-va-fast-tracked-controversial-drug-spravato-to-treat-veterans. Published June 18, 2019. Accessed September 17, 2019.
6. Cary P. Trump’s praise put drug for vets on fast track, but experts are not sure it works. https://publicintegrity.org/federal-politics/trumps-raves-put-drug-for-vets-on-fast-track-but-experts-arent-sure-it-works. Published June 18, 2019. Accessed September 17, 2019.
7. Zisook S, Tal I, Weingart K, et al. Characteristics of U.S. veteran patients with major depressive disorder who require ‘next-step’ treatments: A VAST-D report. J Affect Disord. 2016;206:232-240.
8. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. VA National Suicide Data Report 2005-2016. https://www.mentalhealth.va.gov/docs/data-sheets/OMHSP_National_Suicide_Data_Report_2005-2016_508.pdf. Updated 2018. Accessed September 17, 2019.
1. Lener MS, Kadriu B, Zarate CA Jr. Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs. 2017;77(4):381-401.
2. Thielking M. “Is the Ketamine Boon Getting out of Hand?” STAT. September 24, 2018. https://www.statnews.com/2018/09/24/ketamine-clinics-severe-depression-treatment. Accessed September 17, 2019.
3. US Food and Drug Administration. FDA approves new nasal spray medication for treatment-resistant depression: available only at a certified doctor’s office or clinic [press release]. https://www.fda.gov/news-events/press-announcements/fda-approves-new-nasal-spray-medication-treatment-resistant-depression-available-only-certified. Published March 5, 2019. Accessed September 17, 2019.
4. Carey B, Steinhauser J. Veterans agency to offer new depression drug, despite safety and efficacy concerns. The New York Times. June 21, 2019. https://www.nytimes.com/2019/06/21/health/ketamine-depression-veterans.html. Accessed September 17, 2019.
5. US House of Representatives, Committee on Veterans Affairs. Chairman Takano statement following reports that VA fast-tracked controversial drug Spravato to treat veterans [press release]. https://veterans.house.gov/news/press-releases/chairman-takano-statement-following-reports-that-va-fast-tracked-controversial-drug-spravato-to-treat-veterans. Published June 18, 2019. Accessed September 17, 2019.
6. Cary P. Trump’s praise put drug for vets on fast track, but experts are not sure it works. https://publicintegrity.org/federal-politics/trumps-raves-put-drug-for-vets-on-fast-track-but-experts-arent-sure-it-works. Published June 18, 2019. Accessed September 17, 2019.
7. Zisook S, Tal I, Weingart K, et al. Characteristics of U.S. veteran patients with major depressive disorder who require ‘next-step’ treatments: A VAST-D report. J Affect Disord. 2016;206:232-240.
8. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. VA National Suicide Data Report 2005-2016. https://www.mentalhealth.va.gov/docs/data-sheets/OMHSP_National_Suicide_Data_Report_2005-2016_508.pdf. Updated 2018. Accessed September 17, 2019.
Heparin Drug Shortage Conservation Strategies
Heparin is the anticoagulant of choice when a rapid anticoagulant is indicated: Onset of action is immediate when administered IV as a bolus.1 The major anticoagulant effect of heparin is mediated by heparin/antithrombin (AT) interaction. Heparin/AT inactivates factor IIa (thrombin) and factors Xa, IXa, XIa, and XIIa. Heparin is approved for multiple indications, such as venous thromboembolism (VTE) treatment and prophylaxis of medical and surgical patients; stroke prevention in atrial fibrillation (AF); acute coronary syndrome (ACS); vascular and cardiac surgeries; and various interventional procedures (eg, diagnostic angiography and percutaneous coronary intervention [PCI]). It also is used as an anticoagulant in blood transfusions, extracorporeal circulation, and for maintaining patency of central vascular access devices (CVADs).
About 60% of the crude heparin used to manufacture heparin in the US originates in China, derived from porcine mucosa. African swine fever, a contagious virus with no cure, has eliminated about 25% to 35% of China’s pig population, or about 150 million pigs. In July 2019, members of the US House of Representatives Committee on Energy and Commerce sent a letter to the US Food and Drug Administration asking for details on the potential impact of African swine fever on the supply of heparin.2
The US Department of Veterans Affairs (VA) heath care system is currently experiencing a shortage of heparin vials and syringes. It is unclear when resolution of this shortage will occur as it could resolve within several weeks or as late as January 2020.3 Although vials and syringes are the current products that are affected, it is possible the shortage may eventually include IV heparin bags as well.
Since the foremost objective of VA health care providers is to provide timely access to medications for veterans, strategies to conserve unfractionated heparin (UfH) must be used since it is a first-line therapy where few evidence-based alternatives exist. Conservation strategies may include drug rationing, therapeutic substitution, and compounding of needed products using the limited stock available in the pharmacy.4 It is important that all staff are educated on facility strategies in order to be familiar with alternatives and limit the potential for near misses, adverse events, and provider frustration.
In shortage situations, the VA-Pharmacy Benefits Management (PBM) defers decisions regarding drug preservation, processes to shift to viable alternatives, and the best practice for safe transitions to local facilities and their subject matter experts.5 At the VA Tennessee Valley Healthcare System, a 1A, tertiary, dual campus health care system, a pharmacy task force has formed to track drug shortages impacting the facility’s efficiencies and budgets. This group communicates with the Pharmacy and Therapeutics committee about potential risks to patient care and develops shortage briefs (following an SBAR [situation, background, assessment, recommendation] design) generally authored and championed by at least 1 clinical pharmacy specialist and supervising physicians who are field experts. Prior to dissemination, the SBAR undergoes a rapid peer-review process.
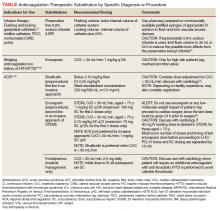
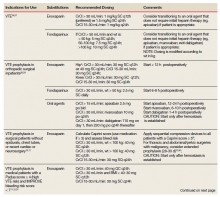
To date, VA PBM has not issued specific guidance on how pharmacists should proceed in case of a shortage. However, we recommend strategies that may be considered for implementation during a potential UfH shortage. For example, pharmacists can use therapeutic alternatives for which best available evidence suggests no disadvantage.4 The Table lists alternative agents according to indication and patient-specific considerations that may preclude use. Existing UfH products may also be used for drug compounding (eg, use current stock to provide an indicated aliquot) to meet the need of prioritized patients.4 In addition, we suggest prioritizing current UfH/heparinized saline for use for the following groups of patients4:
- Emergent/urgent cardiac surgery1,6;
- Hemodialysis patients1,7-9 for which the low-molecular-weight heparin (LMWH) dalteparin is deemed inappropriate or the patient is not monitored in the intensive care unit for regional citrate administration;
- VTE prophylaxis for patients with epidurals or chest tubes for which urgent invasive management may occur, recent cardiac or neurosurgery, or for patients with a creatine clearance < 15 mL/min or receiving hemodialysis10-12;
- Vascular surgery (eg, limb ischemia) and interventions (eg, carotid stenting, endarterectomy)13,14;
- Mesenteric ischemia (venous thrombosis) with a potential to proceed to laparotomy15;
- Critically ill patients with arterial lines for which normal saline is deemed inappropriate for line flushing16;
- Electrophysiology procedures (eg, AF ablation)17; and
- Contraindication to use of a long-acting alternative listed in the table or a medical necessity exists for using a rapidly reversible agent. Examples for this category include but are not limited to recent gastrointestinal bleeding, central nervous system lesion, and select neurologic diagnoses (eg, cerebral venous sinus thrombosis with hemorrhage, thrombus in vertebral basilar system or anterior circulation, intraparenchymal hemorrhage plus mechanical valve, medium to large cardioembolic stroke with intracardiac thrombus).
Conclusion
The UfH drug shortage represents a significant threat to public health and is a major challenge for US health care systems, including the Veterans Health Administration. Overreliance on a predominant source of crude heparin has affected multiple UfH manufacturers and products. Current alternatives to UfH include low-molecular-weight heparins, IV direct thrombin inhibitors, and SC fondaparinux, with selection supported by guidelines or evolving literature. However, the shortage has the potential to expand to other injectables, such as dalteparin and enoxaparin, and severely limit care for veterans. It is vital that clinicians rapidly address the current shortage by creating a plan to develop efficient and equitable access to UfH, continue to assess supply and update stakeholders, and select evidence-based alternatives while maintaining focus on efficacy and safety.
Acknowledgments
The authors thank Ashley Yost, PharmD, for her coordination of the multidisciplinary task force assigned to efficiently manage the heparin drug shortage. This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville, Tennessee.
1. Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(1):64S-94S.
2. Bipartisan E&C leaders request FDA briefing on threat to U.S. heparin supply [press release]. Washington, DC: House Committee on Energy and Commerce; July 30, 2019. https://energycommerce.house.gov/newsroom/press-releases/bipartisan-ec-leaders-request-fda-briefing-on-threat-to-us-heparin-supply. Accessed September 19, 2019.
3. American Society of Health-System Pharmacists. Drug Shortages. Heparin injection. https://www.ashp.org/Drug-Shortages/Current-Shortages/Drug-Shortages-List?page=CurrentShortages. Accessed September 19, 2019.
4. Reed BN, Fox ER, Konig M, et al. The impact of drug shortages on patients with cardiovascular disease: causes, consequences, and a call to action. Am Heart J. 2016;175:130-141.
5. US Department of Veterans Affairs. Pharmacy Benefits Management Services, Medical Advisory Panel, VISN Pharmacist Executives, The Center For Medication Safety. Heparin supply status: frequently asked questions. PBM-2018-02. https://www.pbm.va.gov/PBM/vacenterformedicationsafety/HeparinandSalineSyringeRecallDuetoContamination_NationalPBMPati.pdf. Published May 3, 2018. Accessed September 11, 2019.
6. Shore-Lesserson I, Baker RA, Ferraris VA, et al. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and the American Society of ExtraCorporeal Technology: Clinical Practice Guidelines-anticoagulation during cardiopulmonary bypass. Ann Thorac Surg. 2018;105(2):650-662.
7. Soroka S, Agharazii M, Donnelly S, et al. An adjustable dalteparin sodium dose regimen for the prevention of clotting in the extracorporeal circuit in hemodialysis: a clinical trial of safety and efficacy (the PARROT Study). Can J Kidney Health Dis. 2018;5:1-12.
8. Shantha GPS, Kumar AA, Sethi M, Khanna RC, Pancholy SB. Efficacy and safety of low molecular weight heparin compared to unfractionated heparin for chronic outpatient hemodialysis in end stage renal disease: systematic review and meta-analysis. Peer J. 2015;3:e835.
9. Kessler M, Moureau F, and Nguyen P. Anticoagulation in chronic hemodialysis: progress toward an optimal approach. Semin Dial. 2015;28(5):474-489.
10. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e227s-e277S.
11. Kaye AD, Brunk AJ, Kaye AJ, et al. Regional anesthesia in patients on anticoagulation therapies—evidence-based recommendations. Curr Pain Headache Rep. 2019;23(9):67.
12. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e195S-e226S.
13. Naylor AR, Ricco JB, de Borst GJ, et al. Management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg. 2018;55:3-81.
14. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. JACC. 2017;69(11): e71-e126.
15. Bjorck M, Koelemaya M, Acosta S, et al. Management of diseases of mesenteric arteries and veins. Eur J Vasc Endovasc Surg. 2017;53(4):460-510.
16. Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Infusion therapy standards of practice. J Infusion Nurs. 2016;39:S1-S156.
17. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444.
18. Spyropoulos AC, Al-Badri A, Sherwood MW, Douketis JD. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost. 2016;14(5):875-885.
, . Periprocedural bridging management of anticoagulation. Circulation. 2012;126(4):486-490.
20. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e326S-e350S.
21. Sousa-Uva M, Neumann F-J, Ahlsson A, et al; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. The Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with a special contribution of the European Association for Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2019;55(1):4-90.
22. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes. JACC. 2014;64(24):e139-e228.
23. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of patients with ST-elevation myocardial infarction. JACC. 2013;61(4):e78-e140.
24. Angiomax [package insert]. Parsippany, NJ: The Medicines Company; March 2016.
25. Sousa-Uva, Head SJ, Milojevic M, et al. 2017 EACTS guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg. 2018;53(1):5-33.
26. Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for the management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018: 2(22):3257-3291
27. Kearon C, Akl EA, Blaivas A, et al. Antithrombotic therapy for VTE disease: Chest guideline and expert panel report. Chest. 2016;149(2):315-352.
28. US Department of Veterans Affairs, Pharmacy Benefits Manager Service. Direct oral anticoagulants criteria for use and algorithm for venous thromboembolism treatment. https://www.pbm.va.gov/PBM/clinicalguidance/criteriaforuse.asp. Updated December 2016. [Source not verified]
29. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e278S-e325S.
30. Raja S, Idrees JJ, Blackstone EH, et al. Routine venous thromboembolism screening after pneumonectomy: the more you look, the more you see. J Thorac Cardiovasc Surg. 2016;152(2):524-532.e2.
31. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized patients. Blood Adv. 2018;2(22):3198-3225.
32. Naidu SS, Aronow HD, Box LC, et al. SCAI expert consensus statement: 2016 best practices in the cardiac catheterization laboratory:(endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista; affirmation of value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention). Catheter Cardiovasc Interv. 2016;88(3):407-423.
33. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. JACC. 2011;58(24):e44-e122.
34. Mason PJ, Shah B, Tamis-Holland JE, et al; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Peripheral Vascular Disease; and Council on Genomic and Precision Medicine. AHA scientific statement: an update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome. Circ Cardiovasc Interv. 2018;11(9):e000035.
35. Rao SV, Tremmel JA, Gilchrist IC, et al; Society for Cardiovascular Angiography and Intervention’s Transradial Working Group. Best practices for transradial angiography and intervention: a consensus statement from the society for cardiovascular angiography and interventions’ transradial working group. Catheter Cardiovasc Interv. 2014;83(2):228-236. 36. Moran JE, Ash SR. Locking solutions for hemodialysis catheters; heparin and citrate: a position paper by ASDIN. Semin Dial. 2008;21(5):490-492.
Heparin is the anticoagulant of choice when a rapid anticoagulant is indicated: Onset of action is immediate when administered IV as a bolus.1 The major anticoagulant effect of heparin is mediated by heparin/antithrombin (AT) interaction. Heparin/AT inactivates factor IIa (thrombin) and factors Xa, IXa, XIa, and XIIa. Heparin is approved for multiple indications, such as venous thromboembolism (VTE) treatment and prophylaxis of medical and surgical patients; stroke prevention in atrial fibrillation (AF); acute coronary syndrome (ACS); vascular and cardiac surgeries; and various interventional procedures (eg, diagnostic angiography and percutaneous coronary intervention [PCI]). It also is used as an anticoagulant in blood transfusions, extracorporeal circulation, and for maintaining patency of central vascular access devices (CVADs).
About 60% of the crude heparin used to manufacture heparin in the US originates in China, derived from porcine mucosa. African swine fever, a contagious virus with no cure, has eliminated about 25% to 35% of China’s pig population, or about 150 million pigs. In July 2019, members of the US House of Representatives Committee on Energy and Commerce sent a letter to the US Food and Drug Administration asking for details on the potential impact of African swine fever on the supply of heparin.2
The US Department of Veterans Affairs (VA) heath care system is currently experiencing a shortage of heparin vials and syringes. It is unclear when resolution of this shortage will occur as it could resolve within several weeks or as late as January 2020.3 Although vials and syringes are the current products that are affected, it is possible the shortage may eventually include IV heparin bags as well.
Since the foremost objective of VA health care providers is to provide timely access to medications for veterans, strategies to conserve unfractionated heparin (UfH) must be used since it is a first-line therapy where few evidence-based alternatives exist. Conservation strategies may include drug rationing, therapeutic substitution, and compounding of needed products using the limited stock available in the pharmacy.4 It is important that all staff are educated on facility strategies in order to be familiar with alternatives and limit the potential for near misses, adverse events, and provider frustration.
In shortage situations, the VA-Pharmacy Benefits Management (PBM) defers decisions regarding drug preservation, processes to shift to viable alternatives, and the best practice for safe transitions to local facilities and their subject matter experts.5 At the VA Tennessee Valley Healthcare System, a 1A, tertiary, dual campus health care system, a pharmacy task force has formed to track drug shortages impacting the facility’s efficiencies and budgets. This group communicates with the Pharmacy and Therapeutics committee about potential risks to patient care and develops shortage briefs (following an SBAR [situation, background, assessment, recommendation] design) generally authored and championed by at least 1 clinical pharmacy specialist and supervising physicians who are field experts. Prior to dissemination, the SBAR undergoes a rapid peer-review process.
To date, VA PBM has not issued specific guidance on how pharmacists should proceed in case of a shortage. However, we recommend strategies that may be considered for implementation during a potential UfH shortage. For example, pharmacists can use therapeutic alternatives for which best available evidence suggests no disadvantage.4 The Table lists alternative agents according to indication and patient-specific considerations that may preclude use. Existing UfH products may also be used for drug compounding (eg, use current stock to provide an indicated aliquot) to meet the need of prioritized patients.4 In addition, we suggest prioritizing current UfH/heparinized saline for use for the following groups of patients4:
- Emergent/urgent cardiac surgery1,6;
- Hemodialysis patients1,7-9 for which the low-molecular-weight heparin (LMWH) dalteparin is deemed inappropriate or the patient is not monitored in the intensive care unit for regional citrate administration;
- VTE prophylaxis for patients with epidurals or chest tubes for which urgent invasive management may occur, recent cardiac or neurosurgery, or for patients with a creatine clearance < 15 mL/min or receiving hemodialysis10-12;
- Vascular surgery (eg, limb ischemia) and interventions (eg, carotid stenting, endarterectomy)13,14;
- Mesenteric ischemia (venous thrombosis) with a potential to proceed to laparotomy15;
- Critically ill patients with arterial lines for which normal saline is deemed inappropriate for line flushing16;
- Electrophysiology procedures (eg, AF ablation)17; and
- Contraindication to use of a long-acting alternative listed in the table or a medical necessity exists for using a rapidly reversible agent. Examples for this category include but are not limited to recent gastrointestinal bleeding, central nervous system lesion, and select neurologic diagnoses (eg, cerebral venous sinus thrombosis with hemorrhage, thrombus in vertebral basilar system or anterior circulation, intraparenchymal hemorrhage plus mechanical valve, medium to large cardioembolic stroke with intracardiac thrombus).
Conclusion
The UfH drug shortage represents a significant threat to public health and is a major challenge for US health care systems, including the Veterans Health Administration. Overreliance on a predominant source of crude heparin has affected multiple UfH manufacturers and products. Current alternatives to UfH include low-molecular-weight heparins, IV direct thrombin inhibitors, and SC fondaparinux, with selection supported by guidelines or evolving literature. However, the shortage has the potential to expand to other injectables, such as dalteparin and enoxaparin, and severely limit care for veterans. It is vital that clinicians rapidly address the current shortage by creating a plan to develop efficient and equitable access to UfH, continue to assess supply and update stakeholders, and select evidence-based alternatives while maintaining focus on efficacy and safety.
Acknowledgments
The authors thank Ashley Yost, PharmD, for her coordination of the multidisciplinary task force assigned to efficiently manage the heparin drug shortage. This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville, Tennessee.
Heparin is the anticoagulant of choice when a rapid anticoagulant is indicated: Onset of action is immediate when administered IV as a bolus.1 The major anticoagulant effect of heparin is mediated by heparin/antithrombin (AT) interaction. Heparin/AT inactivates factor IIa (thrombin) and factors Xa, IXa, XIa, and XIIa. Heparin is approved for multiple indications, such as venous thromboembolism (VTE) treatment and prophylaxis of medical and surgical patients; stroke prevention in atrial fibrillation (AF); acute coronary syndrome (ACS); vascular and cardiac surgeries; and various interventional procedures (eg, diagnostic angiography and percutaneous coronary intervention [PCI]). It also is used as an anticoagulant in blood transfusions, extracorporeal circulation, and for maintaining patency of central vascular access devices (CVADs).
About 60% of the crude heparin used to manufacture heparin in the US originates in China, derived from porcine mucosa. African swine fever, a contagious virus with no cure, has eliminated about 25% to 35% of China’s pig population, or about 150 million pigs. In July 2019, members of the US House of Representatives Committee on Energy and Commerce sent a letter to the US Food and Drug Administration asking for details on the potential impact of African swine fever on the supply of heparin.2
The US Department of Veterans Affairs (VA) heath care system is currently experiencing a shortage of heparin vials and syringes. It is unclear when resolution of this shortage will occur as it could resolve within several weeks or as late as January 2020.3 Although vials and syringes are the current products that are affected, it is possible the shortage may eventually include IV heparin bags as well.
Since the foremost objective of VA health care providers is to provide timely access to medications for veterans, strategies to conserve unfractionated heparin (UfH) must be used since it is a first-line therapy where few evidence-based alternatives exist. Conservation strategies may include drug rationing, therapeutic substitution, and compounding of needed products using the limited stock available in the pharmacy.4 It is important that all staff are educated on facility strategies in order to be familiar with alternatives and limit the potential for near misses, adverse events, and provider frustration.
In shortage situations, the VA-Pharmacy Benefits Management (PBM) defers decisions regarding drug preservation, processes to shift to viable alternatives, and the best practice for safe transitions to local facilities and their subject matter experts.5 At the VA Tennessee Valley Healthcare System, a 1A, tertiary, dual campus health care system, a pharmacy task force has formed to track drug shortages impacting the facility’s efficiencies and budgets. This group communicates with the Pharmacy and Therapeutics committee about potential risks to patient care and develops shortage briefs (following an SBAR [situation, background, assessment, recommendation] design) generally authored and championed by at least 1 clinical pharmacy specialist and supervising physicians who are field experts. Prior to dissemination, the SBAR undergoes a rapid peer-review process.
To date, VA PBM has not issued specific guidance on how pharmacists should proceed in case of a shortage. However, we recommend strategies that may be considered for implementation during a potential UfH shortage. For example, pharmacists can use therapeutic alternatives for which best available evidence suggests no disadvantage.4 The Table lists alternative agents according to indication and patient-specific considerations that may preclude use. Existing UfH products may also be used for drug compounding (eg, use current stock to provide an indicated aliquot) to meet the need of prioritized patients.4 In addition, we suggest prioritizing current UfH/heparinized saline for use for the following groups of patients4:
- Emergent/urgent cardiac surgery1,6;
- Hemodialysis patients1,7-9 for which the low-molecular-weight heparin (LMWH) dalteparin is deemed inappropriate or the patient is not monitored in the intensive care unit for regional citrate administration;
- VTE prophylaxis for patients with epidurals or chest tubes for which urgent invasive management may occur, recent cardiac or neurosurgery, or for patients with a creatine clearance < 15 mL/min or receiving hemodialysis10-12;
- Vascular surgery (eg, limb ischemia) and interventions (eg, carotid stenting, endarterectomy)13,14;
- Mesenteric ischemia (venous thrombosis) with a potential to proceed to laparotomy15;
- Critically ill patients with arterial lines for which normal saline is deemed inappropriate for line flushing16;
- Electrophysiology procedures (eg, AF ablation)17; and
- Contraindication to use of a long-acting alternative listed in the table or a medical necessity exists for using a rapidly reversible agent. Examples for this category include but are not limited to recent gastrointestinal bleeding, central nervous system lesion, and select neurologic diagnoses (eg, cerebral venous sinus thrombosis with hemorrhage, thrombus in vertebral basilar system or anterior circulation, intraparenchymal hemorrhage plus mechanical valve, medium to large cardioembolic stroke with intracardiac thrombus).
Conclusion
The UfH drug shortage represents a significant threat to public health and is a major challenge for US health care systems, including the Veterans Health Administration. Overreliance on a predominant source of crude heparin has affected multiple UfH manufacturers and products. Current alternatives to UfH include low-molecular-weight heparins, IV direct thrombin inhibitors, and SC fondaparinux, with selection supported by guidelines or evolving literature. However, the shortage has the potential to expand to other injectables, such as dalteparin and enoxaparin, and severely limit care for veterans. It is vital that clinicians rapidly address the current shortage by creating a plan to develop efficient and equitable access to UfH, continue to assess supply and update stakeholders, and select evidence-based alternatives while maintaining focus on efficacy and safety.
Acknowledgments
The authors thank Ashley Yost, PharmD, for her coordination of the multidisciplinary task force assigned to efficiently manage the heparin drug shortage. This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville, Tennessee.
1. Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(1):64S-94S.
2. Bipartisan E&C leaders request FDA briefing on threat to U.S. heparin supply [press release]. Washington, DC: House Committee on Energy and Commerce; July 30, 2019. https://energycommerce.house.gov/newsroom/press-releases/bipartisan-ec-leaders-request-fda-briefing-on-threat-to-us-heparin-supply. Accessed September 19, 2019.
3. American Society of Health-System Pharmacists. Drug Shortages. Heparin injection. https://www.ashp.org/Drug-Shortages/Current-Shortages/Drug-Shortages-List?page=CurrentShortages. Accessed September 19, 2019.
4. Reed BN, Fox ER, Konig M, et al. The impact of drug shortages on patients with cardiovascular disease: causes, consequences, and a call to action. Am Heart J. 2016;175:130-141.
5. US Department of Veterans Affairs. Pharmacy Benefits Management Services, Medical Advisory Panel, VISN Pharmacist Executives, The Center For Medication Safety. Heparin supply status: frequently asked questions. PBM-2018-02. https://www.pbm.va.gov/PBM/vacenterformedicationsafety/HeparinandSalineSyringeRecallDuetoContamination_NationalPBMPati.pdf. Published May 3, 2018. Accessed September 11, 2019.
6. Shore-Lesserson I, Baker RA, Ferraris VA, et al. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and the American Society of ExtraCorporeal Technology: Clinical Practice Guidelines-anticoagulation during cardiopulmonary bypass. Ann Thorac Surg. 2018;105(2):650-662.
7. Soroka S, Agharazii M, Donnelly S, et al. An adjustable dalteparin sodium dose regimen for the prevention of clotting in the extracorporeal circuit in hemodialysis: a clinical trial of safety and efficacy (the PARROT Study). Can J Kidney Health Dis. 2018;5:1-12.
8. Shantha GPS, Kumar AA, Sethi M, Khanna RC, Pancholy SB. Efficacy and safety of low molecular weight heparin compared to unfractionated heparin for chronic outpatient hemodialysis in end stage renal disease: systematic review and meta-analysis. Peer J. 2015;3:e835.
9. Kessler M, Moureau F, and Nguyen P. Anticoagulation in chronic hemodialysis: progress toward an optimal approach. Semin Dial. 2015;28(5):474-489.
10. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e227s-e277S.
11. Kaye AD, Brunk AJ, Kaye AJ, et al. Regional anesthesia in patients on anticoagulation therapies—evidence-based recommendations. Curr Pain Headache Rep. 2019;23(9):67.
12. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e195S-e226S.
13. Naylor AR, Ricco JB, de Borst GJ, et al. Management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg. 2018;55:3-81.
14. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. JACC. 2017;69(11): e71-e126.
15. Bjorck M, Koelemaya M, Acosta S, et al. Management of diseases of mesenteric arteries and veins. Eur J Vasc Endovasc Surg. 2017;53(4):460-510.
16. Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Infusion therapy standards of practice. J Infusion Nurs. 2016;39:S1-S156.
17. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444.
18. Spyropoulos AC, Al-Badri A, Sherwood MW, Douketis JD. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost. 2016;14(5):875-885.
, . Periprocedural bridging management of anticoagulation. Circulation. 2012;126(4):486-490.
20. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e326S-e350S.
21. Sousa-Uva M, Neumann F-J, Ahlsson A, et al; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. The Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with a special contribution of the European Association for Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2019;55(1):4-90.
22. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes. JACC. 2014;64(24):e139-e228.
23. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of patients with ST-elevation myocardial infarction. JACC. 2013;61(4):e78-e140.
24. Angiomax [package insert]. Parsippany, NJ: The Medicines Company; March 2016.
25. Sousa-Uva, Head SJ, Milojevic M, et al. 2017 EACTS guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg. 2018;53(1):5-33.
26. Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for the management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018: 2(22):3257-3291
27. Kearon C, Akl EA, Blaivas A, et al. Antithrombotic therapy for VTE disease: Chest guideline and expert panel report. Chest. 2016;149(2):315-352.
28. US Department of Veterans Affairs, Pharmacy Benefits Manager Service. Direct oral anticoagulants criteria for use and algorithm for venous thromboembolism treatment. https://www.pbm.va.gov/PBM/clinicalguidance/criteriaforuse.asp. Updated December 2016. [Source not verified]
29. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e278S-e325S.
30. Raja S, Idrees JJ, Blackstone EH, et al. Routine venous thromboembolism screening after pneumonectomy: the more you look, the more you see. J Thorac Cardiovasc Surg. 2016;152(2):524-532.e2.
31. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized patients. Blood Adv. 2018;2(22):3198-3225.
32. Naidu SS, Aronow HD, Box LC, et al. SCAI expert consensus statement: 2016 best practices in the cardiac catheterization laboratory:(endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista; affirmation of value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention). Catheter Cardiovasc Interv. 2016;88(3):407-423.
33. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. JACC. 2011;58(24):e44-e122.
34. Mason PJ, Shah B, Tamis-Holland JE, et al; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Peripheral Vascular Disease; and Council on Genomic and Precision Medicine. AHA scientific statement: an update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome. Circ Cardiovasc Interv. 2018;11(9):e000035.
35. Rao SV, Tremmel JA, Gilchrist IC, et al; Society for Cardiovascular Angiography and Intervention’s Transradial Working Group. Best practices for transradial angiography and intervention: a consensus statement from the society for cardiovascular angiography and interventions’ transradial working group. Catheter Cardiovasc Interv. 2014;83(2):228-236. 36. Moran JE, Ash SR. Locking solutions for hemodialysis catheters; heparin and citrate: a position paper by ASDIN. Semin Dial. 2008;21(5):490-492.
1. Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(1):64S-94S.
2. Bipartisan E&C leaders request FDA briefing on threat to U.S. heparin supply [press release]. Washington, DC: House Committee on Energy and Commerce; July 30, 2019. https://energycommerce.house.gov/newsroom/press-releases/bipartisan-ec-leaders-request-fda-briefing-on-threat-to-us-heparin-supply. Accessed September 19, 2019.
3. American Society of Health-System Pharmacists. Drug Shortages. Heparin injection. https://www.ashp.org/Drug-Shortages/Current-Shortages/Drug-Shortages-List?page=CurrentShortages. Accessed September 19, 2019.
4. Reed BN, Fox ER, Konig M, et al. The impact of drug shortages on patients with cardiovascular disease: causes, consequences, and a call to action. Am Heart J. 2016;175:130-141.
5. US Department of Veterans Affairs. Pharmacy Benefits Management Services, Medical Advisory Panel, VISN Pharmacist Executives, The Center For Medication Safety. Heparin supply status: frequently asked questions. PBM-2018-02. https://www.pbm.va.gov/PBM/vacenterformedicationsafety/HeparinandSalineSyringeRecallDuetoContamination_NationalPBMPati.pdf. Published May 3, 2018. Accessed September 11, 2019.
6. Shore-Lesserson I, Baker RA, Ferraris VA, et al. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and the American Society of ExtraCorporeal Technology: Clinical Practice Guidelines-anticoagulation during cardiopulmonary bypass. Ann Thorac Surg. 2018;105(2):650-662.
7. Soroka S, Agharazii M, Donnelly S, et al. An adjustable dalteparin sodium dose regimen for the prevention of clotting in the extracorporeal circuit in hemodialysis: a clinical trial of safety and efficacy (the PARROT Study). Can J Kidney Health Dis. 2018;5:1-12.
8. Shantha GPS, Kumar AA, Sethi M, Khanna RC, Pancholy SB. Efficacy and safety of low molecular weight heparin compared to unfractionated heparin for chronic outpatient hemodialysis in end stage renal disease: systematic review and meta-analysis. Peer J. 2015;3:e835.
9. Kessler M, Moureau F, and Nguyen P. Anticoagulation in chronic hemodialysis: progress toward an optimal approach. Semin Dial. 2015;28(5):474-489.
10. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e227s-e277S.
11. Kaye AD, Brunk AJ, Kaye AJ, et al. Regional anesthesia in patients on anticoagulation therapies—evidence-based recommendations. Curr Pain Headache Rep. 2019;23(9):67.
12. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e195S-e226S.
13. Naylor AR, Ricco JB, de Borst GJ, et al. Management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg. 2018;55:3-81.
14. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. JACC. 2017;69(11): e71-e126.
15. Bjorck M, Koelemaya M, Acosta S, et al. Management of diseases of mesenteric arteries and veins. Eur J Vasc Endovasc Surg. 2017;53(4):460-510.
16. Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Infusion therapy standards of practice. J Infusion Nurs. 2016;39:S1-S156.
17. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444.
18. Spyropoulos AC, Al-Badri A, Sherwood MW, Douketis JD. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost. 2016;14(5):875-885.
, . Periprocedural bridging management of anticoagulation. Circulation. 2012;126(4):486-490.
20. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e326S-e350S.
21. Sousa-Uva M, Neumann F-J, Ahlsson A, et al; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. The Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with a special contribution of the European Association for Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2019;55(1):4-90.
22. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes. JACC. 2014;64(24):e139-e228.
23. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of patients with ST-elevation myocardial infarction. JACC. 2013;61(4):e78-e140.
24. Angiomax [package insert]. Parsippany, NJ: The Medicines Company; March 2016.
25. Sousa-Uva, Head SJ, Milojevic M, et al. 2017 EACTS guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg. 2018;53(1):5-33.
26. Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for the management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018: 2(22):3257-3291
27. Kearon C, Akl EA, Blaivas A, et al. Antithrombotic therapy for VTE disease: Chest guideline and expert panel report. Chest. 2016;149(2):315-352.
28. US Department of Veterans Affairs, Pharmacy Benefits Manager Service. Direct oral anticoagulants criteria for use and algorithm for venous thromboembolism treatment. https://www.pbm.va.gov/PBM/clinicalguidance/criteriaforuse.asp. Updated December 2016. [Source not verified]
29. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e278S-e325S.
30. Raja S, Idrees JJ, Blackstone EH, et al. Routine venous thromboembolism screening after pneumonectomy: the more you look, the more you see. J Thorac Cardiovasc Surg. 2016;152(2):524-532.e2.
31. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized patients. Blood Adv. 2018;2(22):3198-3225.
32. Naidu SS, Aronow HD, Box LC, et al. SCAI expert consensus statement: 2016 best practices in the cardiac catheterization laboratory:(endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista; affirmation of value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention). Catheter Cardiovasc Interv. 2016;88(3):407-423.
33. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. JACC. 2011;58(24):e44-e122.
34. Mason PJ, Shah B, Tamis-Holland JE, et al; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Peripheral Vascular Disease; and Council on Genomic and Precision Medicine. AHA scientific statement: an update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome. Circ Cardiovasc Interv. 2018;11(9):e000035.
35. Rao SV, Tremmel JA, Gilchrist IC, et al; Society for Cardiovascular Angiography and Intervention’s Transradial Working Group. Best practices for transradial angiography and intervention: a consensus statement from the society for cardiovascular angiography and interventions’ transradial working group. Catheter Cardiovasc Interv. 2014;83(2):228-236. 36. Moran JE, Ash SR. Locking solutions for hemodialysis catheters; heparin and citrate: a position paper by ASDIN. Semin Dial. 2008;21(5):490-492.
2019 Update in perioperative cardiovascular medicine
Perioperative medicine is an evolving field with a rapidly growing body of literature, particularly in cardiology.
In this update, we review 6 articles to answer questions related to preoperative cardiac risk assessment, perioperative medication management, and postoperative cardiac complications. We surveyed perioperative literature from February 2018 through January 2019 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
These summaries are derived from “Updates in Perioperative Medicine” presented at the 14th Annual Perioperative Medicine Summit (Orlando, FL, February 13–16, 2019) and the 2019 Society of Hospital Medicine Annual Meeting (National Harbor, MD, March 24–27, 2019).
PREOPERATIVE CARDIAC EVALUATION
How well do measures of functional capacity predict perioperative complications and mortality in noncardiac surgical patients?
Functional capacity is commonly assessed in preoperative evaluations to estimate patients’ risks of perioperative complications and death. The American College of Cardiology/American Heart Association1 and the European Society of Cardiology2 guidelines both include estimation of cardiopulmonary fitness as a step in preoperative assessment before major noncardiac surgery.
“Subjective assessment” is one way to estimate functional capacity. Simply put, clinicians try to form a rough idea about the fitness of patients by asking questions about routine activities such as walking or climbing stairs. Although commonly used, subjective assessment of functional capacity lacks strong evidence that it predicts adverse perioperative events.
Cardiopulmonary exercise testing is a third option. It measures peak oxygen consumption and anaerobic threshold during exercise. It is probably the best objective measurement of functional capacity, but not necessarily for predicting postoperative cardiac complications, and it is performed relatively infrequently.
[Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0]
In a multicenter, prospective cohort study, Wijeysundera et al4 compared subjective functional capacity assessment, the Duke Activity Status Index, cardiopulmonary exercise testing, and the preoperative N-terminal pro-B-type natriuretic peptide (NT-proBNP) level in their ability to predict complications and death in 1,401 noncardiac surgery patients older than 40 with at least 1 cardiovascular risk factor. After surgery, patients had daily electrocardiograms and troponin measurements until postoperative day 3 or discharge.
The primary outcome was the 30-day incidence of death or myocardial infarction (MI). Additional outcomes included the 30-day incidence of death or myocardial injury after noncardiac surgery (MINS), the 1-year mortality rate, and moderate to severe in-hospital perioperative complications.
Findings. Two percent of patients died or had an MI within 30 days of surgery.4
Subjective assessment had only a 19.2% sensitivity (95% confidence interval [CI] 14.2–25) but a 94.7% specificity (95% CI 93.2–95.9) for predicting inability to attain 4 metabolic equivalents during exercise.4
A lower Duke Activity Status Index predicted the primary outcome of death or MI within 30 days (adjusted odds ratio [OR] 0.96, 95% CI 0.83–0.99, P = .03), and it was the only measure that did so. Additionally, the Duke index and NT-proBNP level predicted the risk of death or MINS within 30 days.4
Only elevated NT-proBNP was associated with death at 1 year.4
On exercise testing, low peak oxygen consumption was significantly associated with perioperative complications.
Limitations. The number of primary outcome events (death and MI) was low, potentially affecting the statistical power of the study.
Conclusions. Subjective assessment of functional capacity misclassifies too many patients as being at low risk of perioperative complications and should not be used for preoperative risk stratification. Other tools, such as the Duke Activity Status Index and NT-proBNP levels, are better predictors of adverse perioperative cardiovascular outcomes and should be considered for use in preoperative cardiac risk assessment.
Although the Duke Activity Status Index is a better predictor of adverse outcomes than subjective functional capacity assessment, a specific perioperative threshold for risk classification has not been established. Its correlate for metabolic equivalents should be considered for use in clinical practice at this point.
PERIOPERATIVE MEDICATION MANAGEMENT
Is perioperative aspirin beneficial in patients undergoing vascular surgery?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial,5 a 2-by-2 factorial randomized controlled trial in which patients received perioperative aspirin, clonidine, both, or neither, demonstrated that perioperative aspirin did not reduce cardiovascular events and increased major bleeding. Patients with recently placed coronary stents and those undergoing carotid endarterectomy were excluded because aspirin is known to have a beneficial effect in these patients.
A subsequent substudy6 found perioperative aspirin to be beneficial in patients with coronary stents placed more than a year before noncardiac surgery. Whether perioperative aspirin is beneficial in other subgroups was unknown.
[Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925]
Biccard et al7 investigated the effect of perioperative aspirin in the subgroup of patients from the POISE-2 trial who underwent vascular surgery. The primary outcome was death or MI within 30 days. Secondary outcomes in this substudy included vascular occlusive complications (amputation and peripheral arterial thrombosis) and major or life-threatening bleeding.
Limitations. There were few adverse events, and this substudy was underpowered for the primary and secondary outcomes.
Conclusion. Starting or continuing aspirin did not improve outcomes, and withdrawing it did not increase cardiovascular or occlusive complications.
Do ACE inhibitors affect risk in noncardiac nonvascular surgery?
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are some of the most commonly used medications for treating hypertension. But whether patients should continue receiving them on the day of surgery or whether they should be held remains unclear.
Although current recommendations are inconsistent, the most recent American College of Cardiology/American Heart Association1 perioperative practice guidelines say that continuing ACE inhibitors or ARBs is reasonable perioperatively. This recommendation, however, acknowledges that published evidence is limited. There is general agreement that preoperative exposure to ACE inhibitors and ARBs is associated with intraoperative hypotension, but whether this increases the risk of adverse clinical outcomes remains unclear. Needed was a study to determine the effect on perioperative morbidity and mortality of continuing vs withholding ACE inhibitors and ARBs before surgery.
[Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036]
Shiffermiller et al8 performed a randomized controlled trial comparing the effect of 2 preoperative ACE inhibitor management protocols in patients undergoing noncardiac nonvascular surgery. Patients were randomized to either receive or not receive their final preoperative ACE inhibitor dose, whether scheduled on the morning of surgery or the night before.
Exclusion criteria included hypotension or hypertension at their preoperative clinic appointment (defined as systolic blood pressure < 90 or ≥ 160 mm Hg, and diastolic blood pressure < 60 or ≥ 95 mm Hg), moderate to severe heart failure, and end-stage renal disease requiring dialysis. Excluded surgery types were cardiac, vascular, organ transplant, oncologic, and all outpatient procedures. Patients taking ARBs were also excluded.
The primary outcome was intraoperative hypotension defined as any systolic blood pressure less than 80 mm Hg from the time of anesthesia induction until transfer to the postanesthesia care unit. Secondary outcomes were measured until hospital discharge and included postoperative acute kidney injury, postoperative hypotension (systolic pressure < 90 mm Hg) and hypertension (systolic pressure > 180 mm Hg), major cardiac events (composite of acute coronary syndrome, acute heart failure, or new-onset arrhythmia), and death.
Findings. A total of 453 patients were screened for eligibility, and of these, 291 were included for randomization. Their average age was 64, 48% were men, and 87% were white. About 50% underwent general anesthesia, 25% spinal, and 25% regional. Over half of the surgeries were orthopedic, and 20% were spine surgeries.
The primary outcome of intraoperative hypotension occurred significantly less often in patients randomized to ACE inhibitor omission than in the continuation group (55% vs 69%, relative risk [RR] 0.81, 95% CI 0.67–0.97, P = .03). This translates to 1 case of intraoperative hypotension for every 7.5 patients continuing an ACE inhibitor perioperatively (number needed to harm 7.5). Intraoperative hypotension associated with vasopressor administration also occurred significantly less frequently in the ACE inhibitor omission group.
Patients in the ACE inhibitor omission group were also less likely to experience postoperative hypotension, but on the other hand, they were more likely to experience severe postoperative hypertension (defined as any systolic blood pressure > 180 mm Hg). The two groups fared the same in terms of rates of acute kidney injury and major adverse cardiac events (MACE) and hospital length of stay, and no patients died in either group.
Limitations. Several factors limit the generalizability of this single-center study, including the many exclusion criteria, the predominance of orthopedic and spine surgeries, and the low-risk patient population (the average Revised Cardiac Risk Index score was 0, range 0–3). Other limitations include not controlling for the specific ACE inhibitor used and not including the precise timing of the final dose in relation to surgery. Lastly, this study lacked power to measure postoperative outcomes.
Conclusions. Continuing ACE inhibitor treatment before noncardiac nonvascular surgery is associated with a greater frequency and duration of intraoperative hypotension, but it did not increase the incidences of acute kidney injury, MACE, or death nor the hospital length of stay.
[Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837]
Hollmann et al9 performed a meta-analysis to determine whether it is better to continue or withhold ACE inhibitors and ARBs before surgery. The patients were adults undergoing noncardiac surgery and receiving an ACE inhibitor or ARB, which was either withheld or continued on the morning of surgery.
Primary outcomes were all-cause mortality and MACE, while secondary outcomes included the incidence of acute kidney injury, heart failure, stroke, intraoperative and postoperative hypotension, and length of hospital stay. Randomized controlled trials and observational studies were included, while case reports and case-control studies were excluded.
Findings. This meta-analysis included 5 randomized controlled trials and 4 cohort studies, with a total of 6,022 patients; 1,816 had their ACE inhibitor or ARB withheld before surgery, while 4,206 continued therapy. It found no difference between the 2 groups in the incidence of death or MACE, and there were not enough data to determine a difference in heart failure, stroke, acute kidney injury, or hospital length of stay.
Seven studies, with 5,414 patients, examined intraoperative hypotension. The overall incidence was 30%, but was significantly lower if the ACE inhibitor or ARB was withheld (OR 0.63, 95% CI 0.47–0.85, P = .002). Findings were similar in an analysis of only the randomized controlled trials. No difference was observed in postoperative hypotension.
Limitations. There was no standard definition of the morbidity outcomes, including hypotension and MACE. The assessment of MACE included data only for MI and not MINS. The specific duration of hypotension was not reported, and this meta-analysis did not take into account different anesthetic techniques. The duration of follow-up varied widely among studies, ranging from the day of hospital discharge to 30 days after surgery. And the randomized controlled trial performed by Shiffermiller et al8 was not included.
Conclusions. While continuing ACE inhibitors or ARBs before noncardiac surgery was associated with intraoperative hypotension, it did not seem to affect other outcomes, including death and MACE. The authors propose that a large randomized controlled trial is needed to determine whether continuing or withholding ACE inhibitor or ARB therapy before surgery is safer.
POSTOPERATIVE CARDIAC COMPLICATIONS
How should we treat MINS?
MINS is associated with an increased risk of cardiovascular events and death in both the short term and long term. MINS is defined as an elevated postoperative troponin level related to an ischemic etiology. However, whether to routinely measure troponin after surgery is unclear, as most patients do not present with ischemic symptoms, and there is no standard of care for treatment of this entity. Limited observational data suggest that starting or intensifying cardiac medications, particularly aspirin and statins, may be beneficial in terms of reducing 30-day mortality rates in patients with MI or cardiac events at 1 year in vascular surgery patients with MINS.
The Management of Myocardial Injury After Noncardiac Surgery (MANAGE) trial was designed to evaluate the potential of the anticoagulant dabigatran to prevent major vascular complications in patients with MINS.
[Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8]
Devereaux et al10 randomized patients who were at least 45 years old and had developed MINS within the previous 35 days to receive dabigatran 110 mg orally twice daily or placebo for up to 2 years. Patients not already taking a proton pump inhibitor were also randomized to take either omeprazole 20 mg once daily or placebo.
The primary efficacy outcome initially was major vascular complications, which included vascular mortality, nonfatal MI, nonhemorrhagic stroke, and peripheral arterial thrombosis. However, amputation and symptomatic venous thromboembolism were subsequently added during the study.
The primary safety outcome was a composite of life-threatening, major, and critical organ bleeding. Major bleeding required a decrease in hemoglobin of at least 4 g/dL, transfusion of at least 3 units of red blood cells within a 24-hour period, or a procedure to stop the bleeding.
Findings. The original goal was to recruit 3,200 patients, but due to slow enrollment and loss of funding, the sample was reduced to 1,754 patients (877 in each group). Approximately 45% of each group stopped taking the study drug prematurely.
The primary efficacy outcome occurred in significantly fewer patients receiving dabigatran (97, 11%) than placebo (133, 15%, HR 0.72, 95% CI 0.55–0.93, P = .0115). The incidence of the primary safety outcome was similar in both groups: 3% with dabigatran and 4% with placebo (HR 0.92, 95% CI 0.55–1.53, P = .76). The only individual efficacy outcome meeting statistical significance was a lower rate of nonhemorrhagic stroke in the dabigatran group. Subgroup analyses showed a trend benefiting patients randomized within 5 days of MINS or with a diagnosis of MI, although it was not statistically significant.
Limitations. The efficacy outcomes were expanded to include venous thromboembolism and others not directly related to MINS, raising questions about the conclusions. Further, as defined by the protocol, bleeding had to be fairly severe to be deemed major. The high number of patients who discontinued the study drug is another limitation of this study.
Conclusion. Dabigatran lowered the risk of major vascular complications with no significant increase in major bleeding in patients with MINS.
What is the risk of thromboembolism in postoperative atrial fibrillation, and what are the benefits of anticoagulation?
Although nonvalvular atrial fibrillation is associated with increased risks of ischemic stroke and systemic embolic events in nonsurgical patients, the association of new-onset postoperative atrial fibrillation with long-term thromboembolic events in the noncardiac surgical population is not well established.
[Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088]
In this retrospective cohort study using a nationwide registry in Denmark, Butt et al11 assessed the long-term risk of thromboembolic events in noncardiac surgical patients with new postoperative atrial fibrillation. Patients were identified who had no previous history of atrial fibrillation and developed it after noncardiac, nonobstetric surgeries, and were matched in a 1:4 ratio with patients who developed nonvalvular atrial fibrillation during nonsurgical hospitalizations. Matching was based on age, sex, heart failure, hypertension, diabetes, known history of thromboembolic events, ischemic heart disease, and the year patients presented with new atrial fibrillation.
Patients were excluded if they received antiarrhythmic drugs or oral anticoagulants before hospitalization or surgery, had cancer in the year prior, or died in the hospital.
The primary outcome of the study was thromboembolic events—a composite of ischemic stroke, transient cerebral ischemia, and peripheral arterial thrombosis or embolism. Secondary outcomes included rehospitalization for atrial fibrillation and all-cause mortality.
Findings. Overall, 0.4% of patients developed new postoperative atrial fibrillation, of whom 3,380 were matched with 15,320 patients with nonvalvular atrial fibrillation. Over a median follow-up of 3.2 years, the risk of thromboembolic events was similar in both groups (31.7 and 29.9 per 1,000 person-years, HR 0.95, 95% CI 0.85–1.07). The groups did not differ in their CHA2DS2-VASc risk scores, HAS-BLED risk scores, or year in which patients were diagnosed.
Anticoagulation lowered the risk of thromboembolic events to a similar extent in both groups compared with no anticoagulation:
- In postoperative atrial fibrillation—HR 0.57, 95% CI 0.40–0.67
- In nonvalvular atrial fibrillation—HR 0.56, 95% CI 0.51–0.62.
Despite the similar reduction in thromboembolic events, only 24.4% of the postoperative atrial fibrillation patients were started on anticoagulation therapy within 30 days of discharge, compared with 41.5% of those with nonvalvular atrial fibrillation.
Limitations. Although this was a large study with excellent follow-up data, it was observational. It may have underestimated the number of patients who developed postoperative atrial fibrillation because episodes that were judged not to be clinically significant may not have been charted. Many patients are not monitored with continuous telemetry postoperatively, which also may have led to underestimation of the number of atrial fibrillation events.
The study also did not examine the number of atrial fibrillation episodes per patient, the heart rhythm at discharge or long-term, or indication for and duration of anticoagulation. There were no data regarding international normalized ratio levels.
Conclusions. Postoperative atrial fibrillation is associated with outcomes similar to those of nonsurgical nonvalvular atrial fibrillation. Anticoagulation decreases the risks of stroke and death. However, substantially fewer patients with postoperative atrial fibrillation receive anticoagulation. Anticoagulation should be considered in these patients, while noting bleeding risk.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64(22):e77–137. doi:10.1016/j.jacc.2014.07.944
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35(35):2383–2431. doi:10.1093/eurheartj/ehu282
- Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (The Duke Activity Status Index). Am J Cardiol 1989; 64(10):651–654. doi:10.1016/0002-9149(89)90496-7
- Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018;168(4):237–244. pmid:29132159
- Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925
- Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036
- Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088
Perioperative medicine is an evolving field with a rapidly growing body of literature, particularly in cardiology.
In this update, we review 6 articles to answer questions related to preoperative cardiac risk assessment, perioperative medication management, and postoperative cardiac complications. We surveyed perioperative literature from February 2018 through January 2019 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
These summaries are derived from “Updates in Perioperative Medicine” presented at the 14th Annual Perioperative Medicine Summit (Orlando, FL, February 13–16, 2019) and the 2019 Society of Hospital Medicine Annual Meeting (National Harbor, MD, March 24–27, 2019).
PREOPERATIVE CARDIAC EVALUATION
How well do measures of functional capacity predict perioperative complications and mortality in noncardiac surgical patients?
Functional capacity is commonly assessed in preoperative evaluations to estimate patients’ risks of perioperative complications and death. The American College of Cardiology/American Heart Association1 and the European Society of Cardiology2 guidelines both include estimation of cardiopulmonary fitness as a step in preoperative assessment before major noncardiac surgery.
“Subjective assessment” is one way to estimate functional capacity. Simply put, clinicians try to form a rough idea about the fitness of patients by asking questions about routine activities such as walking or climbing stairs. Although commonly used, subjective assessment of functional capacity lacks strong evidence that it predicts adverse perioperative events.
Cardiopulmonary exercise testing is a third option. It measures peak oxygen consumption and anaerobic threshold during exercise. It is probably the best objective measurement of functional capacity, but not necessarily for predicting postoperative cardiac complications, and it is performed relatively infrequently.
[Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0]
In a multicenter, prospective cohort study, Wijeysundera et al4 compared subjective functional capacity assessment, the Duke Activity Status Index, cardiopulmonary exercise testing, and the preoperative N-terminal pro-B-type natriuretic peptide (NT-proBNP) level in their ability to predict complications and death in 1,401 noncardiac surgery patients older than 40 with at least 1 cardiovascular risk factor. After surgery, patients had daily electrocardiograms and troponin measurements until postoperative day 3 or discharge.
The primary outcome was the 30-day incidence of death or myocardial infarction (MI). Additional outcomes included the 30-day incidence of death or myocardial injury after noncardiac surgery (MINS), the 1-year mortality rate, and moderate to severe in-hospital perioperative complications.
Findings. Two percent of patients died or had an MI within 30 days of surgery.4
Subjective assessment had only a 19.2% sensitivity (95% confidence interval [CI] 14.2–25) but a 94.7% specificity (95% CI 93.2–95.9) for predicting inability to attain 4 metabolic equivalents during exercise.4
A lower Duke Activity Status Index predicted the primary outcome of death or MI within 30 days (adjusted odds ratio [OR] 0.96, 95% CI 0.83–0.99, P = .03), and it was the only measure that did so. Additionally, the Duke index and NT-proBNP level predicted the risk of death or MINS within 30 days.4
Only elevated NT-proBNP was associated with death at 1 year.4
On exercise testing, low peak oxygen consumption was significantly associated with perioperative complications.
Limitations. The number of primary outcome events (death and MI) was low, potentially affecting the statistical power of the study.
Conclusions. Subjective assessment of functional capacity misclassifies too many patients as being at low risk of perioperative complications and should not be used for preoperative risk stratification. Other tools, such as the Duke Activity Status Index and NT-proBNP levels, are better predictors of adverse perioperative cardiovascular outcomes and should be considered for use in preoperative cardiac risk assessment.
Although the Duke Activity Status Index is a better predictor of adverse outcomes than subjective functional capacity assessment, a specific perioperative threshold for risk classification has not been established. Its correlate for metabolic equivalents should be considered for use in clinical practice at this point.
PERIOPERATIVE MEDICATION MANAGEMENT
Is perioperative aspirin beneficial in patients undergoing vascular surgery?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial,5 a 2-by-2 factorial randomized controlled trial in which patients received perioperative aspirin, clonidine, both, or neither, demonstrated that perioperative aspirin did not reduce cardiovascular events and increased major bleeding. Patients with recently placed coronary stents and those undergoing carotid endarterectomy were excluded because aspirin is known to have a beneficial effect in these patients.
A subsequent substudy6 found perioperative aspirin to be beneficial in patients with coronary stents placed more than a year before noncardiac surgery. Whether perioperative aspirin is beneficial in other subgroups was unknown.
[Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925]
Biccard et al7 investigated the effect of perioperative aspirin in the subgroup of patients from the POISE-2 trial who underwent vascular surgery. The primary outcome was death or MI within 30 days. Secondary outcomes in this substudy included vascular occlusive complications (amputation and peripheral arterial thrombosis) and major or life-threatening bleeding.
Limitations. There were few adverse events, and this substudy was underpowered for the primary and secondary outcomes.
Conclusion. Starting or continuing aspirin did not improve outcomes, and withdrawing it did not increase cardiovascular or occlusive complications.
Do ACE inhibitors affect risk in noncardiac nonvascular surgery?
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are some of the most commonly used medications for treating hypertension. But whether patients should continue receiving them on the day of surgery or whether they should be held remains unclear.
Although current recommendations are inconsistent, the most recent American College of Cardiology/American Heart Association1 perioperative practice guidelines say that continuing ACE inhibitors or ARBs is reasonable perioperatively. This recommendation, however, acknowledges that published evidence is limited. There is general agreement that preoperative exposure to ACE inhibitors and ARBs is associated with intraoperative hypotension, but whether this increases the risk of adverse clinical outcomes remains unclear. Needed was a study to determine the effect on perioperative morbidity and mortality of continuing vs withholding ACE inhibitors and ARBs before surgery.
[Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036]
Shiffermiller et al8 performed a randomized controlled trial comparing the effect of 2 preoperative ACE inhibitor management protocols in patients undergoing noncardiac nonvascular surgery. Patients were randomized to either receive or not receive their final preoperative ACE inhibitor dose, whether scheduled on the morning of surgery or the night before.
Exclusion criteria included hypotension or hypertension at their preoperative clinic appointment (defined as systolic blood pressure < 90 or ≥ 160 mm Hg, and diastolic blood pressure < 60 or ≥ 95 mm Hg), moderate to severe heart failure, and end-stage renal disease requiring dialysis. Excluded surgery types were cardiac, vascular, organ transplant, oncologic, and all outpatient procedures. Patients taking ARBs were also excluded.
The primary outcome was intraoperative hypotension defined as any systolic blood pressure less than 80 mm Hg from the time of anesthesia induction until transfer to the postanesthesia care unit. Secondary outcomes were measured until hospital discharge and included postoperative acute kidney injury, postoperative hypotension (systolic pressure < 90 mm Hg) and hypertension (systolic pressure > 180 mm Hg), major cardiac events (composite of acute coronary syndrome, acute heart failure, or new-onset arrhythmia), and death.
Findings. A total of 453 patients were screened for eligibility, and of these, 291 were included for randomization. Their average age was 64, 48% were men, and 87% were white. About 50% underwent general anesthesia, 25% spinal, and 25% regional. Over half of the surgeries were orthopedic, and 20% were spine surgeries.
The primary outcome of intraoperative hypotension occurred significantly less often in patients randomized to ACE inhibitor omission than in the continuation group (55% vs 69%, relative risk [RR] 0.81, 95% CI 0.67–0.97, P = .03). This translates to 1 case of intraoperative hypotension for every 7.5 patients continuing an ACE inhibitor perioperatively (number needed to harm 7.5). Intraoperative hypotension associated with vasopressor administration also occurred significantly less frequently in the ACE inhibitor omission group.
Patients in the ACE inhibitor omission group were also less likely to experience postoperative hypotension, but on the other hand, they were more likely to experience severe postoperative hypertension (defined as any systolic blood pressure > 180 mm Hg). The two groups fared the same in terms of rates of acute kidney injury and major adverse cardiac events (MACE) and hospital length of stay, and no patients died in either group.
Limitations. Several factors limit the generalizability of this single-center study, including the many exclusion criteria, the predominance of orthopedic and spine surgeries, and the low-risk patient population (the average Revised Cardiac Risk Index score was 0, range 0–3). Other limitations include not controlling for the specific ACE inhibitor used and not including the precise timing of the final dose in relation to surgery. Lastly, this study lacked power to measure postoperative outcomes.
Conclusions. Continuing ACE inhibitor treatment before noncardiac nonvascular surgery is associated with a greater frequency and duration of intraoperative hypotension, but it did not increase the incidences of acute kidney injury, MACE, or death nor the hospital length of stay.
[Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837]
Hollmann et al9 performed a meta-analysis to determine whether it is better to continue or withhold ACE inhibitors and ARBs before surgery. The patients were adults undergoing noncardiac surgery and receiving an ACE inhibitor or ARB, which was either withheld or continued on the morning of surgery.
Primary outcomes were all-cause mortality and MACE, while secondary outcomes included the incidence of acute kidney injury, heart failure, stroke, intraoperative and postoperative hypotension, and length of hospital stay. Randomized controlled trials and observational studies were included, while case reports and case-control studies were excluded.
Findings. This meta-analysis included 5 randomized controlled trials and 4 cohort studies, with a total of 6,022 patients; 1,816 had their ACE inhibitor or ARB withheld before surgery, while 4,206 continued therapy. It found no difference between the 2 groups in the incidence of death or MACE, and there were not enough data to determine a difference in heart failure, stroke, acute kidney injury, or hospital length of stay.
Seven studies, with 5,414 patients, examined intraoperative hypotension. The overall incidence was 30%, but was significantly lower if the ACE inhibitor or ARB was withheld (OR 0.63, 95% CI 0.47–0.85, P = .002). Findings were similar in an analysis of only the randomized controlled trials. No difference was observed in postoperative hypotension.
Limitations. There was no standard definition of the morbidity outcomes, including hypotension and MACE. The assessment of MACE included data only for MI and not MINS. The specific duration of hypotension was not reported, and this meta-analysis did not take into account different anesthetic techniques. The duration of follow-up varied widely among studies, ranging from the day of hospital discharge to 30 days after surgery. And the randomized controlled trial performed by Shiffermiller et al8 was not included.
Conclusions. While continuing ACE inhibitors or ARBs before noncardiac surgery was associated with intraoperative hypotension, it did not seem to affect other outcomes, including death and MACE. The authors propose that a large randomized controlled trial is needed to determine whether continuing or withholding ACE inhibitor or ARB therapy before surgery is safer.
POSTOPERATIVE CARDIAC COMPLICATIONS
How should we treat MINS?
MINS is associated with an increased risk of cardiovascular events and death in both the short term and long term. MINS is defined as an elevated postoperative troponin level related to an ischemic etiology. However, whether to routinely measure troponin after surgery is unclear, as most patients do not present with ischemic symptoms, and there is no standard of care for treatment of this entity. Limited observational data suggest that starting or intensifying cardiac medications, particularly aspirin and statins, may be beneficial in terms of reducing 30-day mortality rates in patients with MI or cardiac events at 1 year in vascular surgery patients with MINS.
The Management of Myocardial Injury After Noncardiac Surgery (MANAGE) trial was designed to evaluate the potential of the anticoagulant dabigatran to prevent major vascular complications in patients with MINS.
[Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8]
Devereaux et al10 randomized patients who were at least 45 years old and had developed MINS within the previous 35 days to receive dabigatran 110 mg orally twice daily or placebo for up to 2 years. Patients not already taking a proton pump inhibitor were also randomized to take either omeprazole 20 mg once daily or placebo.
The primary efficacy outcome initially was major vascular complications, which included vascular mortality, nonfatal MI, nonhemorrhagic stroke, and peripheral arterial thrombosis. However, amputation and symptomatic venous thromboembolism were subsequently added during the study.
The primary safety outcome was a composite of life-threatening, major, and critical organ bleeding. Major bleeding required a decrease in hemoglobin of at least 4 g/dL, transfusion of at least 3 units of red blood cells within a 24-hour period, or a procedure to stop the bleeding.
Findings. The original goal was to recruit 3,200 patients, but due to slow enrollment and loss of funding, the sample was reduced to 1,754 patients (877 in each group). Approximately 45% of each group stopped taking the study drug prematurely.
The primary efficacy outcome occurred in significantly fewer patients receiving dabigatran (97, 11%) than placebo (133, 15%, HR 0.72, 95% CI 0.55–0.93, P = .0115). The incidence of the primary safety outcome was similar in both groups: 3% with dabigatran and 4% with placebo (HR 0.92, 95% CI 0.55–1.53, P = .76). The only individual efficacy outcome meeting statistical significance was a lower rate of nonhemorrhagic stroke in the dabigatran group. Subgroup analyses showed a trend benefiting patients randomized within 5 days of MINS or with a diagnosis of MI, although it was not statistically significant.
Limitations. The efficacy outcomes were expanded to include venous thromboembolism and others not directly related to MINS, raising questions about the conclusions. Further, as defined by the protocol, bleeding had to be fairly severe to be deemed major. The high number of patients who discontinued the study drug is another limitation of this study.
Conclusion. Dabigatran lowered the risk of major vascular complications with no significant increase in major bleeding in patients with MINS.
What is the risk of thromboembolism in postoperative atrial fibrillation, and what are the benefits of anticoagulation?
Although nonvalvular atrial fibrillation is associated with increased risks of ischemic stroke and systemic embolic events in nonsurgical patients, the association of new-onset postoperative atrial fibrillation with long-term thromboembolic events in the noncardiac surgical population is not well established.
[Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088]
In this retrospective cohort study using a nationwide registry in Denmark, Butt et al11 assessed the long-term risk of thromboembolic events in noncardiac surgical patients with new postoperative atrial fibrillation. Patients were identified who had no previous history of atrial fibrillation and developed it after noncardiac, nonobstetric surgeries, and were matched in a 1:4 ratio with patients who developed nonvalvular atrial fibrillation during nonsurgical hospitalizations. Matching was based on age, sex, heart failure, hypertension, diabetes, known history of thromboembolic events, ischemic heart disease, and the year patients presented with new atrial fibrillation.
Patients were excluded if they received antiarrhythmic drugs or oral anticoagulants before hospitalization or surgery, had cancer in the year prior, or died in the hospital.
The primary outcome of the study was thromboembolic events—a composite of ischemic stroke, transient cerebral ischemia, and peripheral arterial thrombosis or embolism. Secondary outcomes included rehospitalization for atrial fibrillation and all-cause mortality.
Findings. Overall, 0.4% of patients developed new postoperative atrial fibrillation, of whom 3,380 were matched with 15,320 patients with nonvalvular atrial fibrillation. Over a median follow-up of 3.2 years, the risk of thromboembolic events was similar in both groups (31.7 and 29.9 per 1,000 person-years, HR 0.95, 95% CI 0.85–1.07). The groups did not differ in their CHA2DS2-VASc risk scores, HAS-BLED risk scores, or year in which patients were diagnosed.
Anticoagulation lowered the risk of thromboembolic events to a similar extent in both groups compared with no anticoagulation:
- In postoperative atrial fibrillation—HR 0.57, 95% CI 0.40–0.67
- In nonvalvular atrial fibrillation—HR 0.56, 95% CI 0.51–0.62.
Despite the similar reduction in thromboembolic events, only 24.4% of the postoperative atrial fibrillation patients were started on anticoagulation therapy within 30 days of discharge, compared with 41.5% of those with nonvalvular atrial fibrillation.
Limitations. Although this was a large study with excellent follow-up data, it was observational. It may have underestimated the number of patients who developed postoperative atrial fibrillation because episodes that were judged not to be clinically significant may not have been charted. Many patients are not monitored with continuous telemetry postoperatively, which also may have led to underestimation of the number of atrial fibrillation events.
The study also did not examine the number of atrial fibrillation episodes per patient, the heart rhythm at discharge or long-term, or indication for and duration of anticoagulation. There were no data regarding international normalized ratio levels.
Conclusions. Postoperative atrial fibrillation is associated with outcomes similar to those of nonsurgical nonvalvular atrial fibrillation. Anticoagulation decreases the risks of stroke and death. However, substantially fewer patients with postoperative atrial fibrillation receive anticoagulation. Anticoagulation should be considered in these patients, while noting bleeding risk.
Perioperative medicine is an evolving field with a rapidly growing body of literature, particularly in cardiology.
In this update, we review 6 articles to answer questions related to preoperative cardiac risk assessment, perioperative medication management, and postoperative cardiac complications. We surveyed perioperative literature from February 2018 through January 2019 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
These summaries are derived from “Updates in Perioperative Medicine” presented at the 14th Annual Perioperative Medicine Summit (Orlando, FL, February 13–16, 2019) and the 2019 Society of Hospital Medicine Annual Meeting (National Harbor, MD, March 24–27, 2019).
PREOPERATIVE CARDIAC EVALUATION
How well do measures of functional capacity predict perioperative complications and mortality in noncardiac surgical patients?
Functional capacity is commonly assessed in preoperative evaluations to estimate patients’ risks of perioperative complications and death. The American College of Cardiology/American Heart Association1 and the European Society of Cardiology2 guidelines both include estimation of cardiopulmonary fitness as a step in preoperative assessment before major noncardiac surgery.
“Subjective assessment” is one way to estimate functional capacity. Simply put, clinicians try to form a rough idea about the fitness of patients by asking questions about routine activities such as walking or climbing stairs. Although commonly used, subjective assessment of functional capacity lacks strong evidence that it predicts adverse perioperative events.
Cardiopulmonary exercise testing is a third option. It measures peak oxygen consumption and anaerobic threshold during exercise. It is probably the best objective measurement of functional capacity, but not necessarily for predicting postoperative cardiac complications, and it is performed relatively infrequently.
[Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0]
In a multicenter, prospective cohort study, Wijeysundera et al4 compared subjective functional capacity assessment, the Duke Activity Status Index, cardiopulmonary exercise testing, and the preoperative N-terminal pro-B-type natriuretic peptide (NT-proBNP) level in their ability to predict complications and death in 1,401 noncardiac surgery patients older than 40 with at least 1 cardiovascular risk factor. After surgery, patients had daily electrocardiograms and troponin measurements until postoperative day 3 or discharge.
The primary outcome was the 30-day incidence of death or myocardial infarction (MI). Additional outcomes included the 30-day incidence of death or myocardial injury after noncardiac surgery (MINS), the 1-year mortality rate, and moderate to severe in-hospital perioperative complications.
Findings. Two percent of patients died or had an MI within 30 days of surgery.4
Subjective assessment had only a 19.2% sensitivity (95% confidence interval [CI] 14.2–25) but a 94.7% specificity (95% CI 93.2–95.9) for predicting inability to attain 4 metabolic equivalents during exercise.4
A lower Duke Activity Status Index predicted the primary outcome of death or MI within 30 days (adjusted odds ratio [OR] 0.96, 95% CI 0.83–0.99, P = .03), and it was the only measure that did so. Additionally, the Duke index and NT-proBNP level predicted the risk of death or MINS within 30 days.4
Only elevated NT-proBNP was associated with death at 1 year.4
On exercise testing, low peak oxygen consumption was significantly associated with perioperative complications.
Limitations. The number of primary outcome events (death and MI) was low, potentially affecting the statistical power of the study.
Conclusions. Subjective assessment of functional capacity misclassifies too many patients as being at low risk of perioperative complications and should not be used for preoperative risk stratification. Other tools, such as the Duke Activity Status Index and NT-proBNP levels, are better predictors of adverse perioperative cardiovascular outcomes and should be considered for use in preoperative cardiac risk assessment.
Although the Duke Activity Status Index is a better predictor of adverse outcomes than subjective functional capacity assessment, a specific perioperative threshold for risk classification has not been established. Its correlate for metabolic equivalents should be considered for use in clinical practice at this point.
PERIOPERATIVE MEDICATION MANAGEMENT
Is perioperative aspirin beneficial in patients undergoing vascular surgery?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial,5 a 2-by-2 factorial randomized controlled trial in which patients received perioperative aspirin, clonidine, both, or neither, demonstrated that perioperative aspirin did not reduce cardiovascular events and increased major bleeding. Patients with recently placed coronary stents and those undergoing carotid endarterectomy were excluded because aspirin is known to have a beneficial effect in these patients.
A subsequent substudy6 found perioperative aspirin to be beneficial in patients with coronary stents placed more than a year before noncardiac surgery. Whether perioperative aspirin is beneficial in other subgroups was unknown.
[Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925]
Biccard et al7 investigated the effect of perioperative aspirin in the subgroup of patients from the POISE-2 trial who underwent vascular surgery. The primary outcome was death or MI within 30 days. Secondary outcomes in this substudy included vascular occlusive complications (amputation and peripheral arterial thrombosis) and major or life-threatening bleeding.
Limitations. There were few adverse events, and this substudy was underpowered for the primary and secondary outcomes.
Conclusion. Starting or continuing aspirin did not improve outcomes, and withdrawing it did not increase cardiovascular or occlusive complications.
Do ACE inhibitors affect risk in noncardiac nonvascular surgery?
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are some of the most commonly used medications for treating hypertension. But whether patients should continue receiving them on the day of surgery or whether they should be held remains unclear.
Although current recommendations are inconsistent, the most recent American College of Cardiology/American Heart Association1 perioperative practice guidelines say that continuing ACE inhibitors or ARBs is reasonable perioperatively. This recommendation, however, acknowledges that published evidence is limited. There is general agreement that preoperative exposure to ACE inhibitors and ARBs is associated with intraoperative hypotension, but whether this increases the risk of adverse clinical outcomes remains unclear. Needed was a study to determine the effect on perioperative morbidity and mortality of continuing vs withholding ACE inhibitors and ARBs before surgery.
[Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036]
Shiffermiller et al8 performed a randomized controlled trial comparing the effect of 2 preoperative ACE inhibitor management protocols in patients undergoing noncardiac nonvascular surgery. Patients were randomized to either receive or not receive their final preoperative ACE inhibitor dose, whether scheduled on the morning of surgery or the night before.
Exclusion criteria included hypotension or hypertension at their preoperative clinic appointment (defined as systolic blood pressure < 90 or ≥ 160 mm Hg, and diastolic blood pressure < 60 or ≥ 95 mm Hg), moderate to severe heart failure, and end-stage renal disease requiring dialysis. Excluded surgery types were cardiac, vascular, organ transplant, oncologic, and all outpatient procedures. Patients taking ARBs were also excluded.
The primary outcome was intraoperative hypotension defined as any systolic blood pressure less than 80 mm Hg from the time of anesthesia induction until transfer to the postanesthesia care unit. Secondary outcomes were measured until hospital discharge and included postoperative acute kidney injury, postoperative hypotension (systolic pressure < 90 mm Hg) and hypertension (systolic pressure > 180 mm Hg), major cardiac events (composite of acute coronary syndrome, acute heart failure, or new-onset arrhythmia), and death.
Findings. A total of 453 patients were screened for eligibility, and of these, 291 were included for randomization. Their average age was 64, 48% were men, and 87% were white. About 50% underwent general anesthesia, 25% spinal, and 25% regional. Over half of the surgeries were orthopedic, and 20% were spine surgeries.
The primary outcome of intraoperative hypotension occurred significantly less often in patients randomized to ACE inhibitor omission than in the continuation group (55% vs 69%, relative risk [RR] 0.81, 95% CI 0.67–0.97, P = .03). This translates to 1 case of intraoperative hypotension for every 7.5 patients continuing an ACE inhibitor perioperatively (number needed to harm 7.5). Intraoperative hypotension associated with vasopressor administration also occurred significantly less frequently in the ACE inhibitor omission group.
Patients in the ACE inhibitor omission group were also less likely to experience postoperative hypotension, but on the other hand, they were more likely to experience severe postoperative hypertension (defined as any systolic blood pressure > 180 mm Hg). The two groups fared the same in terms of rates of acute kidney injury and major adverse cardiac events (MACE) and hospital length of stay, and no patients died in either group.
Limitations. Several factors limit the generalizability of this single-center study, including the many exclusion criteria, the predominance of orthopedic and spine surgeries, and the low-risk patient population (the average Revised Cardiac Risk Index score was 0, range 0–3). Other limitations include not controlling for the specific ACE inhibitor used and not including the precise timing of the final dose in relation to surgery. Lastly, this study lacked power to measure postoperative outcomes.
Conclusions. Continuing ACE inhibitor treatment before noncardiac nonvascular surgery is associated with a greater frequency and duration of intraoperative hypotension, but it did not increase the incidences of acute kidney injury, MACE, or death nor the hospital length of stay.
[Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837]
Hollmann et al9 performed a meta-analysis to determine whether it is better to continue or withhold ACE inhibitors and ARBs before surgery. The patients were adults undergoing noncardiac surgery and receiving an ACE inhibitor or ARB, which was either withheld or continued on the morning of surgery.
Primary outcomes were all-cause mortality and MACE, while secondary outcomes included the incidence of acute kidney injury, heart failure, stroke, intraoperative and postoperative hypotension, and length of hospital stay. Randomized controlled trials and observational studies were included, while case reports and case-control studies were excluded.
Findings. This meta-analysis included 5 randomized controlled trials and 4 cohort studies, with a total of 6,022 patients; 1,816 had their ACE inhibitor or ARB withheld before surgery, while 4,206 continued therapy. It found no difference between the 2 groups in the incidence of death or MACE, and there were not enough data to determine a difference in heart failure, stroke, acute kidney injury, or hospital length of stay.
Seven studies, with 5,414 patients, examined intraoperative hypotension. The overall incidence was 30%, but was significantly lower if the ACE inhibitor or ARB was withheld (OR 0.63, 95% CI 0.47–0.85, P = .002). Findings were similar in an analysis of only the randomized controlled trials. No difference was observed in postoperative hypotension.
Limitations. There was no standard definition of the morbidity outcomes, including hypotension and MACE. The assessment of MACE included data only for MI and not MINS. The specific duration of hypotension was not reported, and this meta-analysis did not take into account different anesthetic techniques. The duration of follow-up varied widely among studies, ranging from the day of hospital discharge to 30 days after surgery. And the randomized controlled trial performed by Shiffermiller et al8 was not included.
Conclusions. While continuing ACE inhibitors or ARBs before noncardiac surgery was associated with intraoperative hypotension, it did not seem to affect other outcomes, including death and MACE. The authors propose that a large randomized controlled trial is needed to determine whether continuing or withholding ACE inhibitor or ARB therapy before surgery is safer.
POSTOPERATIVE CARDIAC COMPLICATIONS
How should we treat MINS?
MINS is associated with an increased risk of cardiovascular events and death in both the short term and long term. MINS is defined as an elevated postoperative troponin level related to an ischemic etiology. However, whether to routinely measure troponin after surgery is unclear, as most patients do not present with ischemic symptoms, and there is no standard of care for treatment of this entity. Limited observational data suggest that starting or intensifying cardiac medications, particularly aspirin and statins, may be beneficial in terms of reducing 30-day mortality rates in patients with MI or cardiac events at 1 year in vascular surgery patients with MINS.
The Management of Myocardial Injury After Noncardiac Surgery (MANAGE) trial was designed to evaluate the potential of the anticoagulant dabigatran to prevent major vascular complications in patients with MINS.
[Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8]
Devereaux et al10 randomized patients who were at least 45 years old and had developed MINS within the previous 35 days to receive dabigatran 110 mg orally twice daily or placebo for up to 2 years. Patients not already taking a proton pump inhibitor were also randomized to take either omeprazole 20 mg once daily or placebo.
The primary efficacy outcome initially was major vascular complications, which included vascular mortality, nonfatal MI, nonhemorrhagic stroke, and peripheral arterial thrombosis. However, amputation and symptomatic venous thromboembolism were subsequently added during the study.
The primary safety outcome was a composite of life-threatening, major, and critical organ bleeding. Major bleeding required a decrease in hemoglobin of at least 4 g/dL, transfusion of at least 3 units of red blood cells within a 24-hour period, or a procedure to stop the bleeding.
Findings. The original goal was to recruit 3,200 patients, but due to slow enrollment and loss of funding, the sample was reduced to 1,754 patients (877 in each group). Approximately 45% of each group stopped taking the study drug prematurely.
The primary efficacy outcome occurred in significantly fewer patients receiving dabigatran (97, 11%) than placebo (133, 15%, HR 0.72, 95% CI 0.55–0.93, P = .0115). The incidence of the primary safety outcome was similar in both groups: 3% with dabigatran and 4% with placebo (HR 0.92, 95% CI 0.55–1.53, P = .76). The only individual efficacy outcome meeting statistical significance was a lower rate of nonhemorrhagic stroke in the dabigatran group. Subgroup analyses showed a trend benefiting patients randomized within 5 days of MINS or with a diagnosis of MI, although it was not statistically significant.
Limitations. The efficacy outcomes were expanded to include venous thromboembolism and others not directly related to MINS, raising questions about the conclusions. Further, as defined by the protocol, bleeding had to be fairly severe to be deemed major. The high number of patients who discontinued the study drug is another limitation of this study.
Conclusion. Dabigatran lowered the risk of major vascular complications with no significant increase in major bleeding in patients with MINS.
What is the risk of thromboembolism in postoperative atrial fibrillation, and what are the benefits of anticoagulation?
Although nonvalvular atrial fibrillation is associated with increased risks of ischemic stroke and systemic embolic events in nonsurgical patients, the association of new-onset postoperative atrial fibrillation with long-term thromboembolic events in the noncardiac surgical population is not well established.
[Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088]
In this retrospective cohort study using a nationwide registry in Denmark, Butt et al11 assessed the long-term risk of thromboembolic events in noncardiac surgical patients with new postoperative atrial fibrillation. Patients were identified who had no previous history of atrial fibrillation and developed it after noncardiac, nonobstetric surgeries, and were matched in a 1:4 ratio with patients who developed nonvalvular atrial fibrillation during nonsurgical hospitalizations. Matching was based on age, sex, heart failure, hypertension, diabetes, known history of thromboembolic events, ischemic heart disease, and the year patients presented with new atrial fibrillation.
Patients were excluded if they received antiarrhythmic drugs or oral anticoagulants before hospitalization or surgery, had cancer in the year prior, or died in the hospital.
The primary outcome of the study was thromboembolic events—a composite of ischemic stroke, transient cerebral ischemia, and peripheral arterial thrombosis or embolism. Secondary outcomes included rehospitalization for atrial fibrillation and all-cause mortality.
Findings. Overall, 0.4% of patients developed new postoperative atrial fibrillation, of whom 3,380 were matched with 15,320 patients with nonvalvular atrial fibrillation. Over a median follow-up of 3.2 years, the risk of thromboembolic events was similar in both groups (31.7 and 29.9 per 1,000 person-years, HR 0.95, 95% CI 0.85–1.07). The groups did not differ in their CHA2DS2-VASc risk scores, HAS-BLED risk scores, or year in which patients were diagnosed.
Anticoagulation lowered the risk of thromboembolic events to a similar extent in both groups compared with no anticoagulation:
- In postoperative atrial fibrillation—HR 0.57, 95% CI 0.40–0.67
- In nonvalvular atrial fibrillation—HR 0.56, 95% CI 0.51–0.62.
Despite the similar reduction in thromboembolic events, only 24.4% of the postoperative atrial fibrillation patients were started on anticoagulation therapy within 30 days of discharge, compared with 41.5% of those with nonvalvular atrial fibrillation.
Limitations. Although this was a large study with excellent follow-up data, it was observational. It may have underestimated the number of patients who developed postoperative atrial fibrillation because episodes that were judged not to be clinically significant may not have been charted. Many patients are not monitored with continuous telemetry postoperatively, which also may have led to underestimation of the number of atrial fibrillation events.
The study also did not examine the number of atrial fibrillation episodes per patient, the heart rhythm at discharge or long-term, or indication for and duration of anticoagulation. There were no data regarding international normalized ratio levels.
Conclusions. Postoperative atrial fibrillation is associated with outcomes similar to those of nonsurgical nonvalvular atrial fibrillation. Anticoagulation decreases the risks of stroke and death. However, substantially fewer patients with postoperative atrial fibrillation receive anticoagulation. Anticoagulation should be considered in these patients, while noting bleeding risk.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64(22):e77–137. doi:10.1016/j.jacc.2014.07.944
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35(35):2383–2431. doi:10.1093/eurheartj/ehu282
- Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (The Duke Activity Status Index). Am J Cardiol 1989; 64(10):651–654. doi:10.1016/0002-9149(89)90496-7
- Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018;168(4):237–244. pmid:29132159
- Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925
- Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036
- Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64(22):e77–137. doi:10.1016/j.jacc.2014.07.944
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35(35):2383–2431. doi:10.1093/eurheartj/ehu282
- Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (The Duke Activity Status Index). Am J Cardiol 1989; 64(10):651–654. doi:10.1016/0002-9149(89)90496-7
- Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018;168(4):237–244. pmid:29132159
- Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925
- Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036
- Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088
KEY POINTS
- The Duke Activity Status Index is a better tool for assessing cardiopulmonary fitness than subjective assessment, and it should be considered for use in guideline algorithms.
- Aspirin should not be given perioperatively in patients undergoing vascular surgery other than carotid endarterectomy.
- Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are associated with intraoperative hypotension if given before surgery. Further study is needed to determined how best to manage ACE inhibitors and ARBs perioperatively.
- In a study, dabigatran given to patients with myocardial injury after noncardiac surgery lowered the risk of major vascular complications, with no significant increase in major bleeding. But the study had major limitations.
- Postoperative atrial fibrillation is associated with outcomes similar to those of nonsurgical nonvalvular atrial fibrillation. Anticoagulation decreases its stroke and mortality risk.
A complication of enoxaparin injection
A 78-year-old woman presented to the emergency department with shortness of breath and palpitations and was found to have atrial fibrillation with rapid ventricular response. Medical therapy with drug therapy and cardioversion proved ineffective. She then underwent atrioventricular node ablation and placement of a pacemaker.
At the time of admission, anticoagulation was started with full-dose enoxaparin, injected subcutaneously on the left side of the abdominal wall, as her CHA2DS2-VASc score (http://chadvasc.org) was 5, due to age, female sex, and history of heart failure and hypertension.
Four days after admission, she reported lower abdominal pain, and her urine output was minimal. A bladder scan showed more than 500 mL of residual urine. She was hemodynamically stable, but physical examination revealed mild abdominal distention and tenderness in the suprapubic region. Laboratory testing showed a sharp rise in serum creatinine and a drop in hematocrit.
The patient was initially managed conservatively with serial physical examinations, monitoring of the hematocrit, serial imaging studies, and discontinuation of anticoagulation, but the pain and anuria persisted. Repeat computed tomography 15 days after admission showed that the hematoma had expanded, and she now had hydronephrosis on the right side as well, requiring urologic intervention with bilateral nephrostomy tube placement.
The size of the hematoma was evaluated with serial abdominal and pelvic examinations. After several days, her urine output had improved, the nephrostomy tubes were removed, and she was discharged.
RECTUS SHEATH HEMATOMA
Our patient had a giant pelvic hematoma, probably arising from the rectus sheath. This uncommon problem can arise from trauma, anticoagulation, or increased intra-abdominal pressure, but it can also occur spontaneously.1
In rectus sheath hematoma, a branch of the inferior epigastric artery is injured at its insertion into the rectus abdominis muscle. Symptoms arise if bleeding does not stop spontaneously from a tamponade effect.2
We speculate that in our patient, deep injection of enoxaparin into the abdominal wall injured the inferior epigastric artery, which started the hematoma, and the bleeding was exacerbated by the anticoagulation effect of the enoxaparin.
Another form of pelvic hematoma is retroperitoneal. It is most commonly caused by trauma but can occur due to rupture of the aorta, compression from tumors, or, infrequently, anticoagulation therapy.3
The role of anticoagulation
Spontaneous pelvic hematoma is usually missed as a cause of abdominal pain in patients on anticoagulation therapy and is mistaken for common acute conditions such as ulcer, diverticulitis, appendicitis, ovarian cyst torsion, and tumor.4 It usually develops within 5 days of starting anticoagulation therapy. Symptoms vary depending on the location of the hematoma and are best diagnosed with abdominal computed tomography, with sensitivity as high as 100%.
MANAGEMENT
Conservative management, reserved for patients in stable condition, includes temporarily stopping and reevaluating the risks and benefits of anticoagulation and antiplatelet agents, giving blood transfusions, and controlling pain. If conservative measures fail, options are arterial embolization, stent grafting, and blood vessel ligation.5 If these measures fail, patients should undergo surgical evacuation of the hematoma and ligation of bleeding vessels.6
TAKE-HOME MESSAGE
Subcutaneous injections, especially of anticoagulants, into the abdominal wall can increase the risk of hematoma. Other risk factors are older age, female sex, and thin body habitus with less abdominal fat.7 Healthcare professionals should avoid deep injections into the abdomen and should counsel patients and their caregivers about this, as well. The deltoid region could be a safer alternative.
- Cherry WB, Mueller PS. Rectus sheath hematoma: review of 126 cases at a single institution. Medicine (Baltimore) 2006; 85(2):105–110. doi:10.1097/01.md.0000216818.13067.5a
- Hatjipetrou A, Anyfantakis D, Kastanakis M. Rectus sheath hematoma: a review of the literature. Int J Surg 2015; 13:267–271. doi:10.1016/j.ijsu.2014.12.015
- Haq MM, Taimur SDM, Khan SR, Rahman MA. Retroperitoneal hematoma following enoxaparin treatment in an elderly woman—a case report. Cardiovasc J 2010; 3(1):94–97. doi:10.3329/cardio.v3i1.6434
- Luhmann A, Williams EV. Rectus sheath hematoma: a series of unfortunate events. World J Surg 2006; 30(11):2050–2055. doi:10.1007/s00268-005-0702-9
- Pace F, Colombo GM, Del Vecchio LR, et al. Low molecular weight heparin and fatal spontaneous extraperitoneal hematoma in the elderly. Geriatr Gerontol Int 2012; 12(1):172–174. doi:10.1111/j.1447-0594.2011.00742.x
- Velicki L, Cemerlic-Adic N, Bogdanovic D, Mrdanin T. Rectus sheath haematoma: enoxaparin-related complication. Acta Clin Belg 2013; 68(2):147–149. doi:10.2143/ACB.68.2.3213
- Sheth HS, Kumar R, DiNella J, Janov C, Kaldas H, Smith RE. Evaluation of risk factors for rectus sheath hematoma. Clin Appl Thromb Hemost 2016; 22(3):292–296. doi:10.1177/1076029614553024
A 78-year-old woman presented to the emergency department with shortness of breath and palpitations and was found to have atrial fibrillation with rapid ventricular response. Medical therapy with drug therapy and cardioversion proved ineffective. She then underwent atrioventricular node ablation and placement of a pacemaker.
At the time of admission, anticoagulation was started with full-dose enoxaparin, injected subcutaneously on the left side of the abdominal wall, as her CHA2DS2-VASc score (http://chadvasc.org) was 5, due to age, female sex, and history of heart failure and hypertension.
Four days after admission, she reported lower abdominal pain, and her urine output was minimal. A bladder scan showed more than 500 mL of residual urine. She was hemodynamically stable, but physical examination revealed mild abdominal distention and tenderness in the suprapubic region. Laboratory testing showed a sharp rise in serum creatinine and a drop in hematocrit.
The patient was initially managed conservatively with serial physical examinations, monitoring of the hematocrit, serial imaging studies, and discontinuation of anticoagulation, but the pain and anuria persisted. Repeat computed tomography 15 days after admission showed that the hematoma had expanded, and she now had hydronephrosis on the right side as well, requiring urologic intervention with bilateral nephrostomy tube placement.
The size of the hematoma was evaluated with serial abdominal and pelvic examinations. After several days, her urine output had improved, the nephrostomy tubes were removed, and she was discharged.
RECTUS SHEATH HEMATOMA
Our patient had a giant pelvic hematoma, probably arising from the rectus sheath. This uncommon problem can arise from trauma, anticoagulation, or increased intra-abdominal pressure, but it can also occur spontaneously.1
In rectus sheath hematoma, a branch of the inferior epigastric artery is injured at its insertion into the rectus abdominis muscle. Symptoms arise if bleeding does not stop spontaneously from a tamponade effect.2
We speculate that in our patient, deep injection of enoxaparin into the abdominal wall injured the inferior epigastric artery, which started the hematoma, and the bleeding was exacerbated by the anticoagulation effect of the enoxaparin.
Another form of pelvic hematoma is retroperitoneal. It is most commonly caused by trauma but can occur due to rupture of the aorta, compression from tumors, or, infrequently, anticoagulation therapy.3
The role of anticoagulation
Spontaneous pelvic hematoma is usually missed as a cause of abdominal pain in patients on anticoagulation therapy and is mistaken for common acute conditions such as ulcer, diverticulitis, appendicitis, ovarian cyst torsion, and tumor.4 It usually develops within 5 days of starting anticoagulation therapy. Symptoms vary depending on the location of the hematoma and are best diagnosed with abdominal computed tomography, with sensitivity as high as 100%.
MANAGEMENT
Conservative management, reserved for patients in stable condition, includes temporarily stopping and reevaluating the risks and benefits of anticoagulation and antiplatelet agents, giving blood transfusions, and controlling pain. If conservative measures fail, options are arterial embolization, stent grafting, and blood vessel ligation.5 If these measures fail, patients should undergo surgical evacuation of the hematoma and ligation of bleeding vessels.6
TAKE-HOME MESSAGE
Subcutaneous injections, especially of anticoagulants, into the abdominal wall can increase the risk of hematoma. Other risk factors are older age, female sex, and thin body habitus with less abdominal fat.7 Healthcare professionals should avoid deep injections into the abdomen and should counsel patients and their caregivers about this, as well. The deltoid region could be a safer alternative.
A 78-year-old woman presented to the emergency department with shortness of breath and palpitations and was found to have atrial fibrillation with rapid ventricular response. Medical therapy with drug therapy and cardioversion proved ineffective. She then underwent atrioventricular node ablation and placement of a pacemaker.
At the time of admission, anticoagulation was started with full-dose enoxaparin, injected subcutaneously on the left side of the abdominal wall, as her CHA2DS2-VASc score (http://chadvasc.org) was 5, due to age, female sex, and history of heart failure and hypertension.
Four days after admission, she reported lower abdominal pain, and her urine output was minimal. A bladder scan showed more than 500 mL of residual urine. She was hemodynamically stable, but physical examination revealed mild abdominal distention and tenderness in the suprapubic region. Laboratory testing showed a sharp rise in serum creatinine and a drop in hematocrit.
The patient was initially managed conservatively with serial physical examinations, monitoring of the hematocrit, serial imaging studies, and discontinuation of anticoagulation, but the pain and anuria persisted. Repeat computed tomography 15 days after admission showed that the hematoma had expanded, and she now had hydronephrosis on the right side as well, requiring urologic intervention with bilateral nephrostomy tube placement.
The size of the hematoma was evaluated with serial abdominal and pelvic examinations. After several days, her urine output had improved, the nephrostomy tubes were removed, and she was discharged.
RECTUS SHEATH HEMATOMA
Our patient had a giant pelvic hematoma, probably arising from the rectus sheath. This uncommon problem can arise from trauma, anticoagulation, or increased intra-abdominal pressure, but it can also occur spontaneously.1
In rectus sheath hematoma, a branch of the inferior epigastric artery is injured at its insertion into the rectus abdominis muscle. Symptoms arise if bleeding does not stop spontaneously from a tamponade effect.2
We speculate that in our patient, deep injection of enoxaparin into the abdominal wall injured the inferior epigastric artery, which started the hematoma, and the bleeding was exacerbated by the anticoagulation effect of the enoxaparin.
Another form of pelvic hematoma is retroperitoneal. It is most commonly caused by trauma but can occur due to rupture of the aorta, compression from tumors, or, infrequently, anticoagulation therapy.3
The role of anticoagulation
Spontaneous pelvic hematoma is usually missed as a cause of abdominal pain in patients on anticoagulation therapy and is mistaken for common acute conditions such as ulcer, diverticulitis, appendicitis, ovarian cyst torsion, and tumor.4 It usually develops within 5 days of starting anticoagulation therapy. Symptoms vary depending on the location of the hematoma and are best diagnosed with abdominal computed tomography, with sensitivity as high as 100%.
MANAGEMENT
Conservative management, reserved for patients in stable condition, includes temporarily stopping and reevaluating the risks and benefits of anticoagulation and antiplatelet agents, giving blood transfusions, and controlling pain. If conservative measures fail, options are arterial embolization, stent grafting, and blood vessel ligation.5 If these measures fail, patients should undergo surgical evacuation of the hematoma and ligation of bleeding vessels.6
TAKE-HOME MESSAGE
Subcutaneous injections, especially of anticoagulants, into the abdominal wall can increase the risk of hematoma. Other risk factors are older age, female sex, and thin body habitus with less abdominal fat.7 Healthcare professionals should avoid deep injections into the abdomen and should counsel patients and their caregivers about this, as well. The deltoid region could be a safer alternative.
- Cherry WB, Mueller PS. Rectus sheath hematoma: review of 126 cases at a single institution. Medicine (Baltimore) 2006; 85(2):105–110. doi:10.1097/01.md.0000216818.13067.5a
- Hatjipetrou A, Anyfantakis D, Kastanakis M. Rectus sheath hematoma: a review of the literature. Int J Surg 2015; 13:267–271. doi:10.1016/j.ijsu.2014.12.015
- Haq MM, Taimur SDM, Khan SR, Rahman MA. Retroperitoneal hematoma following enoxaparin treatment in an elderly woman—a case report. Cardiovasc J 2010; 3(1):94–97. doi:10.3329/cardio.v3i1.6434
- Luhmann A, Williams EV. Rectus sheath hematoma: a series of unfortunate events. World J Surg 2006; 30(11):2050–2055. doi:10.1007/s00268-005-0702-9
- Pace F, Colombo GM, Del Vecchio LR, et al. Low molecular weight heparin and fatal spontaneous extraperitoneal hematoma in the elderly. Geriatr Gerontol Int 2012; 12(1):172–174. doi:10.1111/j.1447-0594.2011.00742.x
- Velicki L, Cemerlic-Adic N, Bogdanovic D, Mrdanin T. Rectus sheath haematoma: enoxaparin-related complication. Acta Clin Belg 2013; 68(2):147–149. doi:10.2143/ACB.68.2.3213
- Sheth HS, Kumar R, DiNella J, Janov C, Kaldas H, Smith RE. Evaluation of risk factors for rectus sheath hematoma. Clin Appl Thromb Hemost 2016; 22(3):292–296. doi:10.1177/1076029614553024
- Cherry WB, Mueller PS. Rectus sheath hematoma: review of 126 cases at a single institution. Medicine (Baltimore) 2006; 85(2):105–110. doi:10.1097/01.md.0000216818.13067.5a
- Hatjipetrou A, Anyfantakis D, Kastanakis M. Rectus sheath hematoma: a review of the literature. Int J Surg 2015; 13:267–271. doi:10.1016/j.ijsu.2014.12.015
- Haq MM, Taimur SDM, Khan SR, Rahman MA. Retroperitoneal hematoma following enoxaparin treatment in an elderly woman—a case report. Cardiovasc J 2010; 3(1):94–97. doi:10.3329/cardio.v3i1.6434
- Luhmann A, Williams EV. Rectus sheath hematoma: a series of unfortunate events. World J Surg 2006; 30(11):2050–2055. doi:10.1007/s00268-005-0702-9
- Pace F, Colombo GM, Del Vecchio LR, et al. Low molecular weight heparin and fatal spontaneous extraperitoneal hematoma in the elderly. Geriatr Gerontol Int 2012; 12(1):172–174. doi:10.1111/j.1447-0594.2011.00742.x
- Velicki L, Cemerlic-Adic N, Bogdanovic D, Mrdanin T. Rectus sheath haematoma: enoxaparin-related complication. Acta Clin Belg 2013; 68(2):147–149. doi:10.2143/ACB.68.2.3213
- Sheth HS, Kumar R, DiNella J, Janov C, Kaldas H, Smith RE. Evaluation of risk factors for rectus sheath hematoma. Clin Appl Thromb Hemost 2016; 22(3):292–296. doi:10.1177/1076029614553024
What are the risks to inpatients during hospital construction or renovation?
Hospital-acquired infections related to construction and renovation activities account for more than 5,000 deaths per year across the United States.1
Hospital construction, renovation, and demolition projects ultimately serve the interests of patients, but they also can put inpatients at risk of mold infection, Legionnaires disease, sleep deprivation, exacerbation of lung disease, and in rare cases, physical injury.
Hospitals are in a continuous state of transformation to meet the needs of medical and technologic advances and an increasing patient population,1 and in the last 10 years, more than $200 billion has been spent on construction projects at US healthcare facilities. Therefore, constant attention is needed to reduce the risks to the health of hospitalized patients during these projects.
HOSPITAL-ACQUIRED INFECTIONS
Mold infections
Construction can cause substantial dust contamination and scatter large amounts of fungal spores. An analysis conducted during a period of excavation at a hospital campus showed a significant association between excavation activities and hospital-acquired mold infections (hazard ratio [HR] 2.8, P = .01) but not yeast infections (HR 0.75, P = .78).2
Aspergillus species have been the organisms most commonly involved in hospital-acquired mold infection. In a review of 53 studies including 458 patients,3A fumigatus was identified in 154 patients, and A flavus was identified in 101 patients. A niger, A terreus, A nidulans, Zygomycetes, and other fungi were also identified, but to a much lesser extent. Hematologic malignancies were the predominant underlying morbidity in 299 patients. Half of the sources of healthcare-associated Aspergillus outbreaks were estimated to result from construction and renovation activities within or surrounding the hospital.3
Heavy demolition and transportation of wreckage have been found to cause the greatest concentrations of Aspergillus species,1 but even small concentrations may be sufficient to cause infection in high-risk hospitalized patients.3 Invasive pulmonary aspergillosis is the mold infection most commonly associated with these activities, particularly in immunocompromised and critically ill patients. It is characterized by invasion of lung tissue by Aspergillus hyphae. Hematogenous dissemination occurs in about 25% of patients, and the death rate often exceeds 50%.4
A review of cases of fungal infection during hospital construction, renovation, and demolition projects from 1976 to 2014 identified 372 infected patients, of whom 180 died.5 The majority of infections were due to Aspergillus. Other fungi included Rhizopus, Candida, and Fusarium. Infections occurred mainly in patients with hematologic malignancies and patients who had undergone stem cell transplant (76%), followed by patients with other malignancies or transplant (19%). Rarely affected were patients in the intensive care unit or patients with rheumatologic diseases or on hemodialysis.5
Legionnaires disease
Legionnaires disease is a form of atypical pneumonia caused by the bacterium Legionella, often associated with differing degrees of gastrointestinal symptoms. Legionella species are the bacteria most often associated with construction in hospitals, as construction and demolition often result in collections of stagnant water.
The primary mode of transmission is inhalation of contaminated mist or aerosols. Legionella species can also colonize newly constructed hospital buildings within weeks of installation of water fixtures.
In a large university-affiliated hospital, 2 cases of nosocomial legionellosis were identified during a period of major construction.6 An epidemiologic investigation traced the source to a widespread contamination of potable water within the hospital. One patient’s isolate was similar to that of a water sample from the faucet in his room, and an association between Legionnaires disease and construction was postulated.
Another institution’s newly constructed hematology-oncology unit identified 10 cases of Legionnaires disease over a 12-week period in patients and visitors with exposure to the unit during and within the incubation period.7 A clinical and environmental assessment found 3 clinical isolates of Legionella identical to environmental isolates found from the unit, strongly implicating the potable water system as the likely source.7
In Ohio, 11 cases of hospital-acquired Legionnaires disease were identified in patients moved to a newly constructed 12-story addition to a hospital, and 1 of those died.8
Legionella infections appear to be less common than mold infections when reviewing the available literature on patients exposed to hospital construction, renovation, or demolition activities. Yet unlike mold infections, which occur mostly in immunocompromised patients, Legionella also affects people with normal immunity.1
NONCOMMUNICABLE ILLNESSES
Sleep deprivation
Noise in hospitals has been linked to sleep disturbances in inpatients. A study using noise dosimeters in a university hospital found a mean continuous noise level of 63.5 dBA (A-weighting of decibels indicates risk of hearing loss) over a 24-hour period, a level more than 2 times higher than the recommended 30 dBA.9 The same study also found a significant correlation between sleep disturbance in inpatients and increasing noise levels, in a dose-response manner.
Common sources of noise during construction may include power generators, welding and cutting equipment, and transport of materials. While construction activities themselves have yet to be directly linked to sleep deprivation in patients, construction is inevitably accompanied by noise.
Noise is the most common factor interfering with sleep reported by hospitalized patients. Other effects of noise on patients include a rise in heart rate and blood pressure, increased cholesterol and triglyceride levels, increased use of sedatives, and longer length of stay.9,10 Although construction is rarely done at night, patients generally take naps during the day, so the noise is disruptive.
Physical injuries
Hospitalized patients rarely suffer injuries related to hospital construction. However, these incidents may be underreported. Few cases of physical injury in patients exposed to construction or renovation in healthcare facilities can be found through a Web search.11,12
Exacerbation of lung disease
Inhalation of indoor air pollutants exposed during renovation can directly trigger an inflammatory response and cause exacerbation in patients with chronic lung diseases such as asthma and chronic obstructive pulmonary disease. No study has specifically examined the effect of hospital construction or renovation on exacerbation of chronic lung diseases in hospitalized patients. Nevertheless, dust and indoor air pollutants from building renovation have often been reported as agents associated with work-related asthma.13
THE MESSAGE
Although the risks to inpatients during hospital construction projects appear minimal, their effect can at times be detrimental, especially to the immunocompromised. Hospitals should adhere to infection control risk assessment protocols during construction events. The small number of outbreaks of construction-related infections can make the diagnosis of nosocomial origin of these infections challenging; a high index of suspicion is needed.
Currently in the United States, there is no standard regarding acceptable levels of airborne mold concentrations, and data to support routine hospital air sampling or validation of available air samplers are inadequate. This remains an area for future research.14,15
Certain measures have been shown to significantly decrease the risk of mold infections and other nosocomial infections during construction projects, including16:
- Effective dust control through containment units and barriers
- Consistent use of high-efficiency particulate air filters in hospital units that care for immunocompromised and critically ill patients
- Routine surveillance.
Noise and vibration can be reduced by temporary walls and careful tool selection and scheduling. Similarly, temporary walls and other barriers help protect healthcare employees and patients from the risk of direct physical injury.
Preconstruction risk assessments that address infection control, safety, noise, and air quality are crucial, and the Joint Commission generally requires such assessments. Further, education of hospital staff and members of the construction team about the potential detrimental effects of hospital construction and renovation is essential to secure a safe environment.
- Clair JD, Colatrella S. Opening Pandora’s (tool) box: health care construction and associated risk for nosocomial infection. Infect Disord Drug Targets 2013; 13(3):177–183. pmid:23961740
- Pokala HR, Leonard D, Cox J, et al. Association of hospital construction with the development of healthcare associated environmental mold infections (HAEMI) in pediatric patients with leukemia. Pediatr Blood Cancer 2014; 61(2):276–280. doi:10.1002/pbc.24685
- Vonberg RP, Gastmeier P. Nosocomial aspergillosis in outbreak settings. J Hosp Infect 2006; 63(3):246–254. doi:10.1016/j.jhin.2006.02.014
- Kanj A, Abdallah N, Soubani AO. The spectrum of pulmonary aspergillosis. Respir Med 2018; 141:121–131. doi:10.1016/j.rmed.2018.06.029
- Kanamori H, Rutala WA, Sickbert-Bennett EE, Weber DJ. Review of fungal outbreaks and infection prevention in healthcare settings during construction and renovation. Clin Infect Dis 2015; 61(3):433–444. doi:10.1093/cid/civ297
- Perola O, Kauppinen J, Kusnetsov J, Heikkinen J, Jokinen C, Katila ML. Nosocomial Legionella pneumophila serogroup 5 outbreak associated with persistent colonization of a hospital water system. APMIS 2002; 110(12):863–868. pmid:12645664
- Francois Watkins LK, Toews KE, Harris AM, et al. Lessons from an outbreak of Legionnaires disease on a hematology-oncology unit. Infect Control Hosp Epidemiol 2017; 38(3):306–313. doi:10.1017/ice.2016.281
- Lin YE, Stout JE, Yu VL. Prevention of hospital-acquired legionellosis. Curr Opin Infect Dis 2011; 24(4):350–356. doi:10.1097/QCO.0b013e3283486c6e
- Park MJ, Yoo JH, Cho BW, Kim KT, Jeong WC, Ha M. Noise in hospital rooms and sleep disturbance in hospitalized medical patients. Environ Health Toxicol 2014; 29:e2014006. doi:10.5620/eht.2014.29.e2014006
- Buxton OM, Ellenbogen JM, Wang W, et al. Sleep disruption due to hospital noises: a prospective evaluation. Ann Intern Med 2012; 157(3):170–179. doi:10.7326/0003-4819-157-3-201208070-00472
- Heldt D; The Gazette. Accident will delay University of Iowa Hospitals construction work for several days. www.thegazette.com/2013/03/08/university-of-iowa-hospitals-patient-injured-by-falling-construction-debris. Accessed July 22, 2019.
- Darrah N; Fox News. Texas hospital explosion kills 1, leaves 12 injured. www.foxnews.com/us/texas-hospital-explosion-kills-1-leaves-12-injured. Accessed July 22, 2019.
- Centers for Disease Control and Prevention (CDC). Work-related asthma: most frequently reported agents associated with work-related asthma cases by state, 2009–2012. wwwn.cdc.gov/eworld/Data/926. Accessed July 22, 2019.
- Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of Aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 63(4):e1–e60. doi:10.1093/cid/ciw326
- Chang CC, Athan E, Morrissey CO, Slavin MA. Preventing invasive fungal infection during hospital building works. Intern Med J 2008; 38(6b):538–541. doi:10.1111/j.1445-5994.2008.01727.x
- Oren I, Haddad N, Finkelstein R, Rowe JM. Invasive pulmonary aspergillosis in neutropenic patients during hospital construction: before and after chemoprophylaxis and institution of HEPA filters. Am J Hematol 2001; 66(4):257–262. doi:10.1002/ajh.1054
Hospital-acquired infections related to construction and renovation activities account for more than 5,000 deaths per year across the United States.1
Hospital construction, renovation, and demolition projects ultimately serve the interests of patients, but they also can put inpatients at risk of mold infection, Legionnaires disease, sleep deprivation, exacerbation of lung disease, and in rare cases, physical injury.
Hospitals are in a continuous state of transformation to meet the needs of medical and technologic advances and an increasing patient population,1 and in the last 10 years, more than $200 billion has been spent on construction projects at US healthcare facilities. Therefore, constant attention is needed to reduce the risks to the health of hospitalized patients during these projects.
HOSPITAL-ACQUIRED INFECTIONS
Mold infections
Construction can cause substantial dust contamination and scatter large amounts of fungal spores. An analysis conducted during a period of excavation at a hospital campus showed a significant association between excavation activities and hospital-acquired mold infections (hazard ratio [HR] 2.8, P = .01) but not yeast infections (HR 0.75, P = .78).2
Aspergillus species have been the organisms most commonly involved in hospital-acquired mold infection. In a review of 53 studies including 458 patients,3A fumigatus was identified in 154 patients, and A flavus was identified in 101 patients. A niger, A terreus, A nidulans, Zygomycetes, and other fungi were also identified, but to a much lesser extent. Hematologic malignancies were the predominant underlying morbidity in 299 patients. Half of the sources of healthcare-associated Aspergillus outbreaks were estimated to result from construction and renovation activities within or surrounding the hospital.3
Heavy demolition and transportation of wreckage have been found to cause the greatest concentrations of Aspergillus species,1 but even small concentrations may be sufficient to cause infection in high-risk hospitalized patients.3 Invasive pulmonary aspergillosis is the mold infection most commonly associated with these activities, particularly in immunocompromised and critically ill patients. It is characterized by invasion of lung tissue by Aspergillus hyphae. Hematogenous dissemination occurs in about 25% of patients, and the death rate often exceeds 50%.4
A review of cases of fungal infection during hospital construction, renovation, and demolition projects from 1976 to 2014 identified 372 infected patients, of whom 180 died.5 The majority of infections were due to Aspergillus. Other fungi included Rhizopus, Candida, and Fusarium. Infections occurred mainly in patients with hematologic malignancies and patients who had undergone stem cell transplant (76%), followed by patients with other malignancies or transplant (19%). Rarely affected were patients in the intensive care unit or patients with rheumatologic diseases or on hemodialysis.5
Legionnaires disease
Legionnaires disease is a form of atypical pneumonia caused by the bacterium Legionella, often associated with differing degrees of gastrointestinal symptoms. Legionella species are the bacteria most often associated with construction in hospitals, as construction and demolition often result in collections of stagnant water.
The primary mode of transmission is inhalation of contaminated mist or aerosols. Legionella species can also colonize newly constructed hospital buildings within weeks of installation of water fixtures.
In a large university-affiliated hospital, 2 cases of nosocomial legionellosis were identified during a period of major construction.6 An epidemiologic investigation traced the source to a widespread contamination of potable water within the hospital. One patient’s isolate was similar to that of a water sample from the faucet in his room, and an association between Legionnaires disease and construction was postulated.
Another institution’s newly constructed hematology-oncology unit identified 10 cases of Legionnaires disease over a 12-week period in patients and visitors with exposure to the unit during and within the incubation period.7 A clinical and environmental assessment found 3 clinical isolates of Legionella identical to environmental isolates found from the unit, strongly implicating the potable water system as the likely source.7
In Ohio, 11 cases of hospital-acquired Legionnaires disease were identified in patients moved to a newly constructed 12-story addition to a hospital, and 1 of those died.8
Legionella infections appear to be less common than mold infections when reviewing the available literature on patients exposed to hospital construction, renovation, or demolition activities. Yet unlike mold infections, which occur mostly in immunocompromised patients, Legionella also affects people with normal immunity.1
NONCOMMUNICABLE ILLNESSES
Sleep deprivation
Noise in hospitals has been linked to sleep disturbances in inpatients. A study using noise dosimeters in a university hospital found a mean continuous noise level of 63.5 dBA (A-weighting of decibels indicates risk of hearing loss) over a 24-hour period, a level more than 2 times higher than the recommended 30 dBA.9 The same study also found a significant correlation between sleep disturbance in inpatients and increasing noise levels, in a dose-response manner.
Common sources of noise during construction may include power generators, welding and cutting equipment, and transport of materials. While construction activities themselves have yet to be directly linked to sleep deprivation in patients, construction is inevitably accompanied by noise.
Noise is the most common factor interfering with sleep reported by hospitalized patients. Other effects of noise on patients include a rise in heart rate and blood pressure, increased cholesterol and triglyceride levels, increased use of sedatives, and longer length of stay.9,10 Although construction is rarely done at night, patients generally take naps during the day, so the noise is disruptive.
Physical injuries
Hospitalized patients rarely suffer injuries related to hospital construction. However, these incidents may be underreported. Few cases of physical injury in patients exposed to construction or renovation in healthcare facilities can be found through a Web search.11,12
Exacerbation of lung disease
Inhalation of indoor air pollutants exposed during renovation can directly trigger an inflammatory response and cause exacerbation in patients with chronic lung diseases such as asthma and chronic obstructive pulmonary disease. No study has specifically examined the effect of hospital construction or renovation on exacerbation of chronic lung diseases in hospitalized patients. Nevertheless, dust and indoor air pollutants from building renovation have often been reported as agents associated with work-related asthma.13
THE MESSAGE
Although the risks to inpatients during hospital construction projects appear minimal, their effect can at times be detrimental, especially to the immunocompromised. Hospitals should adhere to infection control risk assessment protocols during construction events. The small number of outbreaks of construction-related infections can make the diagnosis of nosocomial origin of these infections challenging; a high index of suspicion is needed.
Currently in the United States, there is no standard regarding acceptable levels of airborne mold concentrations, and data to support routine hospital air sampling or validation of available air samplers are inadequate. This remains an area for future research.14,15
Certain measures have been shown to significantly decrease the risk of mold infections and other nosocomial infections during construction projects, including16:
- Effective dust control through containment units and barriers
- Consistent use of high-efficiency particulate air filters in hospital units that care for immunocompromised and critically ill patients
- Routine surveillance.
Noise and vibration can be reduced by temporary walls and careful tool selection and scheduling. Similarly, temporary walls and other barriers help protect healthcare employees and patients from the risk of direct physical injury.
Preconstruction risk assessments that address infection control, safety, noise, and air quality are crucial, and the Joint Commission generally requires such assessments. Further, education of hospital staff and members of the construction team about the potential detrimental effects of hospital construction and renovation is essential to secure a safe environment.
Hospital-acquired infections related to construction and renovation activities account for more than 5,000 deaths per year across the United States.1
Hospital construction, renovation, and demolition projects ultimately serve the interests of patients, but they also can put inpatients at risk of mold infection, Legionnaires disease, sleep deprivation, exacerbation of lung disease, and in rare cases, physical injury.
Hospitals are in a continuous state of transformation to meet the needs of medical and technologic advances and an increasing patient population,1 and in the last 10 years, more than $200 billion has been spent on construction projects at US healthcare facilities. Therefore, constant attention is needed to reduce the risks to the health of hospitalized patients during these projects.
HOSPITAL-ACQUIRED INFECTIONS
Mold infections
Construction can cause substantial dust contamination and scatter large amounts of fungal spores. An analysis conducted during a period of excavation at a hospital campus showed a significant association between excavation activities and hospital-acquired mold infections (hazard ratio [HR] 2.8, P = .01) but not yeast infections (HR 0.75, P = .78).2
Aspergillus species have been the organisms most commonly involved in hospital-acquired mold infection. In a review of 53 studies including 458 patients,3A fumigatus was identified in 154 patients, and A flavus was identified in 101 patients. A niger, A terreus, A nidulans, Zygomycetes, and other fungi were also identified, but to a much lesser extent. Hematologic malignancies were the predominant underlying morbidity in 299 patients. Half of the sources of healthcare-associated Aspergillus outbreaks were estimated to result from construction and renovation activities within or surrounding the hospital.3
Heavy demolition and transportation of wreckage have been found to cause the greatest concentrations of Aspergillus species,1 but even small concentrations may be sufficient to cause infection in high-risk hospitalized patients.3 Invasive pulmonary aspergillosis is the mold infection most commonly associated with these activities, particularly in immunocompromised and critically ill patients. It is characterized by invasion of lung tissue by Aspergillus hyphae. Hematogenous dissemination occurs in about 25% of patients, and the death rate often exceeds 50%.4
A review of cases of fungal infection during hospital construction, renovation, and demolition projects from 1976 to 2014 identified 372 infected patients, of whom 180 died.5 The majority of infections were due to Aspergillus. Other fungi included Rhizopus, Candida, and Fusarium. Infections occurred mainly in patients with hematologic malignancies and patients who had undergone stem cell transplant (76%), followed by patients with other malignancies or transplant (19%). Rarely affected were patients in the intensive care unit or patients with rheumatologic diseases or on hemodialysis.5
Legionnaires disease
Legionnaires disease is a form of atypical pneumonia caused by the bacterium Legionella, often associated with differing degrees of gastrointestinal symptoms. Legionella species are the bacteria most often associated with construction in hospitals, as construction and demolition often result in collections of stagnant water.
The primary mode of transmission is inhalation of contaminated mist or aerosols. Legionella species can also colonize newly constructed hospital buildings within weeks of installation of water fixtures.
In a large university-affiliated hospital, 2 cases of nosocomial legionellosis were identified during a period of major construction.6 An epidemiologic investigation traced the source to a widespread contamination of potable water within the hospital. One patient’s isolate was similar to that of a water sample from the faucet in his room, and an association between Legionnaires disease and construction was postulated.
Another institution’s newly constructed hematology-oncology unit identified 10 cases of Legionnaires disease over a 12-week period in patients and visitors with exposure to the unit during and within the incubation period.7 A clinical and environmental assessment found 3 clinical isolates of Legionella identical to environmental isolates found from the unit, strongly implicating the potable water system as the likely source.7
In Ohio, 11 cases of hospital-acquired Legionnaires disease were identified in patients moved to a newly constructed 12-story addition to a hospital, and 1 of those died.8
Legionella infections appear to be less common than mold infections when reviewing the available literature on patients exposed to hospital construction, renovation, or demolition activities. Yet unlike mold infections, which occur mostly in immunocompromised patients, Legionella also affects people with normal immunity.1
NONCOMMUNICABLE ILLNESSES
Sleep deprivation
Noise in hospitals has been linked to sleep disturbances in inpatients. A study using noise dosimeters in a university hospital found a mean continuous noise level of 63.5 dBA (A-weighting of decibels indicates risk of hearing loss) over a 24-hour period, a level more than 2 times higher than the recommended 30 dBA.9 The same study also found a significant correlation between sleep disturbance in inpatients and increasing noise levels, in a dose-response manner.
Common sources of noise during construction may include power generators, welding and cutting equipment, and transport of materials. While construction activities themselves have yet to be directly linked to sleep deprivation in patients, construction is inevitably accompanied by noise.
Noise is the most common factor interfering with sleep reported by hospitalized patients. Other effects of noise on patients include a rise in heart rate and blood pressure, increased cholesterol and triglyceride levels, increased use of sedatives, and longer length of stay.9,10 Although construction is rarely done at night, patients generally take naps during the day, so the noise is disruptive.
Physical injuries
Hospitalized patients rarely suffer injuries related to hospital construction. However, these incidents may be underreported. Few cases of physical injury in patients exposed to construction or renovation in healthcare facilities can be found through a Web search.11,12
Exacerbation of lung disease
Inhalation of indoor air pollutants exposed during renovation can directly trigger an inflammatory response and cause exacerbation in patients with chronic lung diseases such as asthma and chronic obstructive pulmonary disease. No study has specifically examined the effect of hospital construction or renovation on exacerbation of chronic lung diseases in hospitalized patients. Nevertheless, dust and indoor air pollutants from building renovation have often been reported as agents associated with work-related asthma.13
THE MESSAGE
Although the risks to inpatients during hospital construction projects appear minimal, their effect can at times be detrimental, especially to the immunocompromised. Hospitals should adhere to infection control risk assessment protocols during construction events. The small number of outbreaks of construction-related infections can make the diagnosis of nosocomial origin of these infections challenging; a high index of suspicion is needed.
Currently in the United States, there is no standard regarding acceptable levels of airborne mold concentrations, and data to support routine hospital air sampling or validation of available air samplers are inadequate. This remains an area for future research.14,15
Certain measures have been shown to significantly decrease the risk of mold infections and other nosocomial infections during construction projects, including16:
- Effective dust control through containment units and barriers
- Consistent use of high-efficiency particulate air filters in hospital units that care for immunocompromised and critically ill patients
- Routine surveillance.
Noise and vibration can be reduced by temporary walls and careful tool selection and scheduling. Similarly, temporary walls and other barriers help protect healthcare employees and patients from the risk of direct physical injury.
Preconstruction risk assessments that address infection control, safety, noise, and air quality are crucial, and the Joint Commission generally requires such assessments. Further, education of hospital staff and members of the construction team about the potential detrimental effects of hospital construction and renovation is essential to secure a safe environment.
- Clair JD, Colatrella S. Opening Pandora’s (tool) box: health care construction and associated risk for nosocomial infection. Infect Disord Drug Targets 2013; 13(3):177–183. pmid:23961740
- Pokala HR, Leonard D, Cox J, et al. Association of hospital construction with the development of healthcare associated environmental mold infections (HAEMI) in pediatric patients with leukemia. Pediatr Blood Cancer 2014; 61(2):276–280. doi:10.1002/pbc.24685
- Vonberg RP, Gastmeier P. Nosocomial aspergillosis in outbreak settings. J Hosp Infect 2006; 63(3):246–254. doi:10.1016/j.jhin.2006.02.014
- Kanj A, Abdallah N, Soubani AO. The spectrum of pulmonary aspergillosis. Respir Med 2018; 141:121–131. doi:10.1016/j.rmed.2018.06.029
- Kanamori H, Rutala WA, Sickbert-Bennett EE, Weber DJ. Review of fungal outbreaks and infection prevention in healthcare settings during construction and renovation. Clin Infect Dis 2015; 61(3):433–444. doi:10.1093/cid/civ297
- Perola O, Kauppinen J, Kusnetsov J, Heikkinen J, Jokinen C, Katila ML. Nosocomial Legionella pneumophila serogroup 5 outbreak associated with persistent colonization of a hospital water system. APMIS 2002; 110(12):863–868. pmid:12645664
- Francois Watkins LK, Toews KE, Harris AM, et al. Lessons from an outbreak of Legionnaires disease on a hematology-oncology unit. Infect Control Hosp Epidemiol 2017; 38(3):306–313. doi:10.1017/ice.2016.281
- Lin YE, Stout JE, Yu VL. Prevention of hospital-acquired legionellosis. Curr Opin Infect Dis 2011; 24(4):350–356. doi:10.1097/QCO.0b013e3283486c6e
- Park MJ, Yoo JH, Cho BW, Kim KT, Jeong WC, Ha M. Noise in hospital rooms and sleep disturbance in hospitalized medical patients. Environ Health Toxicol 2014; 29:e2014006. doi:10.5620/eht.2014.29.e2014006
- Buxton OM, Ellenbogen JM, Wang W, et al. Sleep disruption due to hospital noises: a prospective evaluation. Ann Intern Med 2012; 157(3):170–179. doi:10.7326/0003-4819-157-3-201208070-00472
- Heldt D; The Gazette. Accident will delay University of Iowa Hospitals construction work for several days. www.thegazette.com/2013/03/08/university-of-iowa-hospitals-patient-injured-by-falling-construction-debris. Accessed July 22, 2019.
- Darrah N; Fox News. Texas hospital explosion kills 1, leaves 12 injured. www.foxnews.com/us/texas-hospital-explosion-kills-1-leaves-12-injured. Accessed July 22, 2019.
- Centers for Disease Control and Prevention (CDC). Work-related asthma: most frequently reported agents associated with work-related asthma cases by state, 2009–2012. wwwn.cdc.gov/eworld/Data/926. Accessed July 22, 2019.
- Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of Aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 63(4):e1–e60. doi:10.1093/cid/ciw326
- Chang CC, Athan E, Morrissey CO, Slavin MA. Preventing invasive fungal infection during hospital building works. Intern Med J 2008; 38(6b):538–541. doi:10.1111/j.1445-5994.2008.01727.x
- Oren I, Haddad N, Finkelstein R, Rowe JM. Invasive pulmonary aspergillosis in neutropenic patients during hospital construction: before and after chemoprophylaxis and institution of HEPA filters. Am J Hematol 2001; 66(4):257–262. doi:10.1002/ajh.1054
- Clair JD, Colatrella S. Opening Pandora’s (tool) box: health care construction and associated risk for nosocomial infection. Infect Disord Drug Targets 2013; 13(3):177–183. pmid:23961740
- Pokala HR, Leonard D, Cox J, et al. Association of hospital construction with the development of healthcare associated environmental mold infections (HAEMI) in pediatric patients with leukemia. Pediatr Blood Cancer 2014; 61(2):276–280. doi:10.1002/pbc.24685
- Vonberg RP, Gastmeier P. Nosocomial aspergillosis in outbreak settings. J Hosp Infect 2006; 63(3):246–254. doi:10.1016/j.jhin.2006.02.014
- Kanj A, Abdallah N, Soubani AO. The spectrum of pulmonary aspergillosis. Respir Med 2018; 141:121–131. doi:10.1016/j.rmed.2018.06.029
- Kanamori H, Rutala WA, Sickbert-Bennett EE, Weber DJ. Review of fungal outbreaks and infection prevention in healthcare settings during construction and renovation. Clin Infect Dis 2015; 61(3):433–444. doi:10.1093/cid/civ297
- Perola O, Kauppinen J, Kusnetsov J, Heikkinen J, Jokinen C, Katila ML. Nosocomial Legionella pneumophila serogroup 5 outbreak associated with persistent colonization of a hospital water system. APMIS 2002; 110(12):863–868. pmid:12645664
- Francois Watkins LK, Toews KE, Harris AM, et al. Lessons from an outbreak of Legionnaires disease on a hematology-oncology unit. Infect Control Hosp Epidemiol 2017; 38(3):306–313. doi:10.1017/ice.2016.281
- Lin YE, Stout JE, Yu VL. Prevention of hospital-acquired legionellosis. Curr Opin Infect Dis 2011; 24(4):350–356. doi:10.1097/QCO.0b013e3283486c6e
- Park MJ, Yoo JH, Cho BW, Kim KT, Jeong WC, Ha M. Noise in hospital rooms and sleep disturbance in hospitalized medical patients. Environ Health Toxicol 2014; 29:e2014006. doi:10.5620/eht.2014.29.e2014006
- Buxton OM, Ellenbogen JM, Wang W, et al. Sleep disruption due to hospital noises: a prospective evaluation. Ann Intern Med 2012; 157(3):170–179. doi:10.7326/0003-4819-157-3-201208070-00472
- Heldt D; The Gazette. Accident will delay University of Iowa Hospitals construction work for several days. www.thegazette.com/2013/03/08/university-of-iowa-hospitals-patient-injured-by-falling-construction-debris. Accessed July 22, 2019.
- Darrah N; Fox News. Texas hospital explosion kills 1, leaves 12 injured. www.foxnews.com/us/texas-hospital-explosion-kills-1-leaves-12-injured. Accessed July 22, 2019.
- Centers for Disease Control and Prevention (CDC). Work-related asthma: most frequently reported agents associated with work-related asthma cases by state, 2009–2012. wwwn.cdc.gov/eworld/Data/926. Accessed July 22, 2019.
- Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of Aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 63(4):e1–e60. doi:10.1093/cid/ciw326
- Chang CC, Athan E, Morrissey CO, Slavin MA. Preventing invasive fungal infection during hospital building works. Intern Med J 2008; 38(6b):538–541. doi:10.1111/j.1445-5994.2008.01727.x
- Oren I, Haddad N, Finkelstein R, Rowe JM. Invasive pulmonary aspergillosis in neutropenic patients during hospital construction: before and after chemoprophylaxis and institution of HEPA filters. Am J Hematol 2001; 66(4):257–262. doi:10.1002/ajh.1054
Does my patient need maintenance fluids?
My adult nonacutely ill patient, weighing 70 kg with a glomerular filtration rate (GFR) greater than 60 mL/min/1.73 m2, is admitted to the general medical service. She is to receive nothing by mouth for at least the next 24 hours for testing. Do I need to provide maintenance fluids intravenously?
The question seems like it should have an easy answer. However, there is no consensus either on the type of fluids or the need for them at all.
Mortiz and Ayus1 have described the role of maintenance intravenous (IV) fluids in acutely ill patients and made the case for isotonic saline (0.9% NaCl) to minimize the risk of hyponatremia, while acknowledging that it provides 7 to 10 g of sodium per day.
Recommendations for IV fluids for nonacutely ill hospitalized patients range from isotonic solutions such as 0.9% NaCl and lactated Ringer’s, to hypotonic fluids such as 5% dextrose in water (D5W) in 0.45% NaCl and D5W in 0.2% NaCl.2–5
The 2013 guidelines of the UK National Institute for Health and Care Excellence (NICE) recommend hypotonic fluids to provide 25 to 30 mL/kg/day of water with 1 mmol/kg/day of sodium. For a 70-kg patient (body surface area 1.7 m2), this would be 1,750 to 2,000 mL of water, with a maximum of 70 mEq/L of sodium (35 mEq/L).5 An option would be D5W in 0.2% NaCl, which has 34 mEq/L of sodium.
When choosing maintenance IV fluids, we need to consider the following questions:
- What is my patient’s volume status?
- What is the baseline serum sodium and renal function?
- Are there comorbid conditions that may affect antidiuretic hormone (ADH) status such as physiologic stimulation from volume depletion, drugs, pathologic medical conditions, or syndrome of inappropriate ADH stimulation?
- Will my patient be receiving strictly nothing by mouth?
- Are there unusual fluid losses?
SCENARIO 1: ‘USUAL’ MAINTENANCE
If the patient is euvolemic, with a normal serum osmolality, a GFR more than 60 mL/min/1.73 m2, no stimuli for ADH secretion, and no unusual fluid losses, “usual” maintenance would be expected. The usual volume for this patient can be estimated by the following formulas:
- Maintenance volume: 2,550 mL (1,500 mL × 1.7 m2 body surface area)
- Holliday-Segar method6: 2,500 mL (1,500 mL plus 20 mL/kg for every kilogram over 20 kg).
The usual sodium can be also estimated by the following formulas:
- 2 g Na/day = 2,000 mg/day = 87 mEq/day
- Holliday-Segar6: 3 mEq Na/100 mL and 2 mEq K/100 mL of maintenance fluid.
Maintenance IV fluids for our nonacutely ill adult patient could be:
- NICE guideline5: D5W in 0.2% NaCl with 20 mEq KCl, to run at 75 mL/hour
- Holliday-Segar method6: D5W in 0.2% NaCl with 20 mEq KCl, to run at 100 mL/hour.
Twenty-four hours later, assuming no unusual fluid losses or stimulation of ADH secretion, our patient would weigh the same and would have no significant change in serum osmolality.
OTHER OPTIONS
What if I provide 0.9% NaCl instead?
Each 1 L of normal saline provides 154 mEq of sodium, equivalent to 3.5 g of sodium. Thus, for the 24 hours, with administration of 2 to 2.5 L, the patient would receive a sodium load of 7 to 8.75 g. The consequences of this can be debated, but for 24 hours, more than likely, nothing will happen or be noticeable. The kidneys have a wonderful ability to “dump” excess sodium ingested in the diet, as evidenced by the average Western diet with a sodium load in the range of 4 g per day.7,8
What if I provide 0.45% NaCl instead?
Each liter provides 50% of the sodium load of 0.9% NaCl. With the 24-hour administration of 2 to 2.5 L of D5W in 0.45% NaCl, the sodium load would be 3.5 to 4.8 g, and the kidneys would dump the excess sodium.
What if I provide ‘catch-up’ fluids after 24 hours, not maintenance fluids?
Assuming only usual losses and no unusual ADH stimulation except for the physiologic stimuli from volume depletion for 24 hours, our patient would lose 2 kg (1 L fluid loss = 1 kg weight loss) and 87 mEq of sodium. This is approximately 4.5% dehydration; thus, other than increased thirst, no physical findings of volume depletion would be clinically evident.
However, serum osmolality and sodium would increase. After 24 hours of nothing by mouth with usual fluid losses, there would be a rise in serum osmolality of 13.5 mOsm/L (a rise in sodium of 6 to 7 mEq/L), which would stimulate ADH in an attempt to minimize further urinary losses. There would be an intracellular volume loss of 1.3 L (Table 1). Clinically, just as with the administration of 0.9% sodium, these changes would not likely be of any clinical consequence in the first 24 hours.
SCENARIO 2: IMPAIRED WATER EXCRETION, AND FLUIDS GIVEN
If the patient is euvolemic but has or is at risk for ADH stimulation,1,9 providing maintenance IV fluids according to the NICE or Holliday-Segar recommendations (a total of 2 L of 0.2% NaCl = 34 mEq Na/L = 68 mOsm/L) would result in an excess of free water, as an increase in ADH secretion impairs free water clearance. A potential scenario with impaired water excretion is shown in Table 2.
After 24 hours, the patient’s serum osmolality would drop by about 7 mOsm/L, and the serum sodium would decrease by 3 or 4 mEq. The consequence of the intracellular fluid shift would be seen by the expansion of the intracellular volume from 28 to 28.7 L.
If this patient were to have received 2 L of 0.9% NaCl (308 mOsm/L × 2 L = 616 Osm) as suggested by Moritz and Ayus,1 the result would be a serum osmolality of 284 mOsm/L, thus avoiding hyponatremia and intracellular fluid shifts.
THE BOTTOM LINE
Know your patient, answer the clinical questions noted above, and decide.
For a euvolemic patient with normal serum sodium, GFR greater than 60 mL/1.73 m2, and no ADH stimulation, for 24 hours it probably doesn’t matter that much, but a daily reassessment of the continued need for and type of intravenous fluids is critical.
For patients not meeting the criteria noted above such as a patient with systolic or diastolic heart failure, advanced or end-stage renal disease puts the patient at risk for early potential complications of either hyponatremia or sodium overload. For these patients, maintenance intravenous fluids need to be chosen wisely. Daily weights, examinations, and laboratory testing will let you know if something is not right and will allow for early detection and treatment.
- Mortiz ML, Ayus JC. Maintenance intravenous fluids in acutely ill patients. N Engl J Med 2015; 373(14):1350–1360. doi:10.1056/NEJMra1412877
- Feld LG, Neuspiel DR, Foster BA, et al; Subcommittee on Fluid and Electrolyte Therapy. Clinical practice guideline: maintenance intravenous fluids in children. Pediatrics 2018;142(6). doi:10.1542/peds.2018-3083
- Sterns RH. Maintenance and replacement fluid therapy in adults. www.uptodate.com/contents/maintenance-and-replacement-fluid-therapy-in-adults. Accessed August 21, 2019.
- Shafiee MA, Bohn D, Hoorn EJ, Halperin ML. How to select optimal maintenance intravenous fluid therapy. QJM 2003; 96(8):601–610. doi:10.1093/qjmed/hcg101
- National Institute for Health and Care Excellence (NICE). Intravenous fluid therapy in adults in hospital. www.nice.org.uk/guidance/cg174. Accessed August 21, 2019.
- Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics 1957; 19(5):823–832. pmid:13431307
- Appel LJ, Foti K. Sources of dietary sodium: implications for patients, physicians, and policy. Circulation 2017; 135(19):1784–1787. doi:10.1161/CIRCULATIONAHA.117.027933
- Harnack LJ, Cogswell ME, Shikany JM, et al. Sources of sodium in US adults from 3 geographic regions. Circulation 2017; 135(19):1775–1783. doi:10.1161/CIRCULATIONAHA.116.024446
- Sterns RH. Pathophysiology and etiology of the syndrome of inappropriate antidiuretic hormone secretion (SIADH). www.uptodate.com/contents/pathophysiology-and-etiology-of-the-syndrome-of-inappropriate-antidiuretic-hormone-secretion-siadh. Accessed August 21, 2019.
My adult nonacutely ill patient, weighing 70 kg with a glomerular filtration rate (GFR) greater than 60 mL/min/1.73 m2, is admitted to the general medical service. She is to receive nothing by mouth for at least the next 24 hours for testing. Do I need to provide maintenance fluids intravenously?
The question seems like it should have an easy answer. However, there is no consensus either on the type of fluids or the need for them at all.
Mortiz and Ayus1 have described the role of maintenance intravenous (IV) fluids in acutely ill patients and made the case for isotonic saline (0.9% NaCl) to minimize the risk of hyponatremia, while acknowledging that it provides 7 to 10 g of sodium per day.
Recommendations for IV fluids for nonacutely ill hospitalized patients range from isotonic solutions such as 0.9% NaCl and lactated Ringer’s, to hypotonic fluids such as 5% dextrose in water (D5W) in 0.45% NaCl and D5W in 0.2% NaCl.2–5
The 2013 guidelines of the UK National Institute for Health and Care Excellence (NICE) recommend hypotonic fluids to provide 25 to 30 mL/kg/day of water with 1 mmol/kg/day of sodium. For a 70-kg patient (body surface area 1.7 m2), this would be 1,750 to 2,000 mL of water, with a maximum of 70 mEq/L of sodium (35 mEq/L).5 An option would be D5W in 0.2% NaCl, which has 34 mEq/L of sodium.
When choosing maintenance IV fluids, we need to consider the following questions:
- What is my patient’s volume status?
- What is the baseline serum sodium and renal function?
- Are there comorbid conditions that may affect antidiuretic hormone (ADH) status such as physiologic stimulation from volume depletion, drugs, pathologic medical conditions, or syndrome of inappropriate ADH stimulation?
- Will my patient be receiving strictly nothing by mouth?
- Are there unusual fluid losses?
SCENARIO 1: ‘USUAL’ MAINTENANCE
If the patient is euvolemic, with a normal serum osmolality, a GFR more than 60 mL/min/1.73 m2, no stimuli for ADH secretion, and no unusual fluid losses, “usual” maintenance would be expected. The usual volume for this patient can be estimated by the following formulas:
- Maintenance volume: 2,550 mL (1,500 mL × 1.7 m2 body surface area)
- Holliday-Segar method6: 2,500 mL (1,500 mL plus 20 mL/kg for every kilogram over 20 kg).
The usual sodium can be also estimated by the following formulas:
- 2 g Na/day = 2,000 mg/day = 87 mEq/day
- Holliday-Segar6: 3 mEq Na/100 mL and 2 mEq K/100 mL of maintenance fluid.
Maintenance IV fluids for our nonacutely ill adult patient could be:
- NICE guideline5: D5W in 0.2% NaCl with 20 mEq KCl, to run at 75 mL/hour
- Holliday-Segar method6: D5W in 0.2% NaCl with 20 mEq KCl, to run at 100 mL/hour.
Twenty-four hours later, assuming no unusual fluid losses or stimulation of ADH secretion, our patient would weigh the same and would have no significant change in serum osmolality.
OTHER OPTIONS
What if I provide 0.9% NaCl instead?
Each 1 L of normal saline provides 154 mEq of sodium, equivalent to 3.5 g of sodium. Thus, for the 24 hours, with administration of 2 to 2.5 L, the patient would receive a sodium load of 7 to 8.75 g. The consequences of this can be debated, but for 24 hours, more than likely, nothing will happen or be noticeable. The kidneys have a wonderful ability to “dump” excess sodium ingested in the diet, as evidenced by the average Western diet with a sodium load in the range of 4 g per day.7,8
What if I provide 0.45% NaCl instead?
Each liter provides 50% of the sodium load of 0.9% NaCl. With the 24-hour administration of 2 to 2.5 L of D5W in 0.45% NaCl, the sodium load would be 3.5 to 4.8 g, and the kidneys would dump the excess sodium.
What if I provide ‘catch-up’ fluids after 24 hours, not maintenance fluids?
Assuming only usual losses and no unusual ADH stimulation except for the physiologic stimuli from volume depletion for 24 hours, our patient would lose 2 kg (1 L fluid loss = 1 kg weight loss) and 87 mEq of sodium. This is approximately 4.5% dehydration; thus, other than increased thirst, no physical findings of volume depletion would be clinically evident.
However, serum osmolality and sodium would increase. After 24 hours of nothing by mouth with usual fluid losses, there would be a rise in serum osmolality of 13.5 mOsm/L (a rise in sodium of 6 to 7 mEq/L), which would stimulate ADH in an attempt to minimize further urinary losses. There would be an intracellular volume loss of 1.3 L (Table 1). Clinically, just as with the administration of 0.9% sodium, these changes would not likely be of any clinical consequence in the first 24 hours.
SCENARIO 2: IMPAIRED WATER EXCRETION, AND FLUIDS GIVEN
If the patient is euvolemic but has or is at risk for ADH stimulation,1,9 providing maintenance IV fluids according to the NICE or Holliday-Segar recommendations (a total of 2 L of 0.2% NaCl = 34 mEq Na/L = 68 mOsm/L) would result in an excess of free water, as an increase in ADH secretion impairs free water clearance. A potential scenario with impaired water excretion is shown in Table 2.
After 24 hours, the patient’s serum osmolality would drop by about 7 mOsm/L, and the serum sodium would decrease by 3 or 4 mEq. The consequence of the intracellular fluid shift would be seen by the expansion of the intracellular volume from 28 to 28.7 L.
If this patient were to have received 2 L of 0.9% NaCl (308 mOsm/L × 2 L = 616 Osm) as suggested by Moritz and Ayus,1 the result would be a serum osmolality of 284 mOsm/L, thus avoiding hyponatremia and intracellular fluid shifts.
THE BOTTOM LINE
Know your patient, answer the clinical questions noted above, and decide.
For a euvolemic patient with normal serum sodium, GFR greater than 60 mL/1.73 m2, and no ADH stimulation, for 24 hours it probably doesn’t matter that much, but a daily reassessment of the continued need for and type of intravenous fluids is critical.
For patients not meeting the criteria noted above such as a patient with systolic or diastolic heart failure, advanced or end-stage renal disease puts the patient at risk for early potential complications of either hyponatremia or sodium overload. For these patients, maintenance intravenous fluids need to be chosen wisely. Daily weights, examinations, and laboratory testing will let you know if something is not right and will allow for early detection and treatment.
My adult nonacutely ill patient, weighing 70 kg with a glomerular filtration rate (GFR) greater than 60 mL/min/1.73 m2, is admitted to the general medical service. She is to receive nothing by mouth for at least the next 24 hours for testing. Do I need to provide maintenance fluids intravenously?
The question seems like it should have an easy answer. However, there is no consensus either on the type of fluids or the need for them at all.
Mortiz and Ayus1 have described the role of maintenance intravenous (IV) fluids in acutely ill patients and made the case for isotonic saline (0.9% NaCl) to minimize the risk of hyponatremia, while acknowledging that it provides 7 to 10 g of sodium per day.
Recommendations for IV fluids for nonacutely ill hospitalized patients range from isotonic solutions such as 0.9% NaCl and lactated Ringer’s, to hypotonic fluids such as 5% dextrose in water (D5W) in 0.45% NaCl and D5W in 0.2% NaCl.2–5
The 2013 guidelines of the UK National Institute for Health and Care Excellence (NICE) recommend hypotonic fluids to provide 25 to 30 mL/kg/day of water with 1 mmol/kg/day of sodium. For a 70-kg patient (body surface area 1.7 m2), this would be 1,750 to 2,000 mL of water, with a maximum of 70 mEq/L of sodium (35 mEq/L).5 An option would be D5W in 0.2% NaCl, which has 34 mEq/L of sodium.
When choosing maintenance IV fluids, we need to consider the following questions:
- What is my patient’s volume status?
- What is the baseline serum sodium and renal function?
- Are there comorbid conditions that may affect antidiuretic hormone (ADH) status such as physiologic stimulation from volume depletion, drugs, pathologic medical conditions, or syndrome of inappropriate ADH stimulation?
- Will my patient be receiving strictly nothing by mouth?
- Are there unusual fluid losses?
SCENARIO 1: ‘USUAL’ MAINTENANCE
If the patient is euvolemic, with a normal serum osmolality, a GFR more than 60 mL/min/1.73 m2, no stimuli for ADH secretion, and no unusual fluid losses, “usual” maintenance would be expected. The usual volume for this patient can be estimated by the following formulas:
- Maintenance volume: 2,550 mL (1,500 mL × 1.7 m2 body surface area)
- Holliday-Segar method6: 2,500 mL (1,500 mL plus 20 mL/kg for every kilogram over 20 kg).
The usual sodium can be also estimated by the following formulas:
- 2 g Na/day = 2,000 mg/day = 87 mEq/day
- Holliday-Segar6: 3 mEq Na/100 mL and 2 mEq K/100 mL of maintenance fluid.
Maintenance IV fluids for our nonacutely ill adult patient could be:
- NICE guideline5: D5W in 0.2% NaCl with 20 mEq KCl, to run at 75 mL/hour
- Holliday-Segar method6: D5W in 0.2% NaCl with 20 mEq KCl, to run at 100 mL/hour.
Twenty-four hours later, assuming no unusual fluid losses or stimulation of ADH secretion, our patient would weigh the same and would have no significant change in serum osmolality.
OTHER OPTIONS
What if I provide 0.9% NaCl instead?
Each 1 L of normal saline provides 154 mEq of sodium, equivalent to 3.5 g of sodium. Thus, for the 24 hours, with administration of 2 to 2.5 L, the patient would receive a sodium load of 7 to 8.75 g. The consequences of this can be debated, but for 24 hours, more than likely, nothing will happen or be noticeable. The kidneys have a wonderful ability to “dump” excess sodium ingested in the diet, as evidenced by the average Western diet with a sodium load in the range of 4 g per day.7,8
What if I provide 0.45% NaCl instead?
Each liter provides 50% of the sodium load of 0.9% NaCl. With the 24-hour administration of 2 to 2.5 L of D5W in 0.45% NaCl, the sodium load would be 3.5 to 4.8 g, and the kidneys would dump the excess sodium.
What if I provide ‘catch-up’ fluids after 24 hours, not maintenance fluids?
Assuming only usual losses and no unusual ADH stimulation except for the physiologic stimuli from volume depletion for 24 hours, our patient would lose 2 kg (1 L fluid loss = 1 kg weight loss) and 87 mEq of sodium. This is approximately 4.5% dehydration; thus, other than increased thirst, no physical findings of volume depletion would be clinically evident.
However, serum osmolality and sodium would increase. After 24 hours of nothing by mouth with usual fluid losses, there would be a rise in serum osmolality of 13.5 mOsm/L (a rise in sodium of 6 to 7 mEq/L), which would stimulate ADH in an attempt to minimize further urinary losses. There would be an intracellular volume loss of 1.3 L (Table 1). Clinically, just as with the administration of 0.9% sodium, these changes would not likely be of any clinical consequence in the first 24 hours.
SCENARIO 2: IMPAIRED WATER EXCRETION, AND FLUIDS GIVEN
If the patient is euvolemic but has or is at risk for ADH stimulation,1,9 providing maintenance IV fluids according to the NICE or Holliday-Segar recommendations (a total of 2 L of 0.2% NaCl = 34 mEq Na/L = 68 mOsm/L) would result in an excess of free water, as an increase in ADH secretion impairs free water clearance. A potential scenario with impaired water excretion is shown in Table 2.
After 24 hours, the patient’s serum osmolality would drop by about 7 mOsm/L, and the serum sodium would decrease by 3 or 4 mEq. The consequence of the intracellular fluid shift would be seen by the expansion of the intracellular volume from 28 to 28.7 L.
If this patient were to have received 2 L of 0.9% NaCl (308 mOsm/L × 2 L = 616 Osm) as suggested by Moritz and Ayus,1 the result would be a serum osmolality of 284 mOsm/L, thus avoiding hyponatremia and intracellular fluid shifts.
THE BOTTOM LINE
Know your patient, answer the clinical questions noted above, and decide.
For a euvolemic patient with normal serum sodium, GFR greater than 60 mL/1.73 m2, and no ADH stimulation, for 24 hours it probably doesn’t matter that much, but a daily reassessment of the continued need for and type of intravenous fluids is critical.
For patients not meeting the criteria noted above such as a patient with systolic or diastolic heart failure, advanced or end-stage renal disease puts the patient at risk for early potential complications of either hyponatremia or sodium overload. For these patients, maintenance intravenous fluids need to be chosen wisely. Daily weights, examinations, and laboratory testing will let you know if something is not right and will allow for early detection and treatment.
- Mortiz ML, Ayus JC. Maintenance intravenous fluids in acutely ill patients. N Engl J Med 2015; 373(14):1350–1360. doi:10.1056/NEJMra1412877
- Feld LG, Neuspiel DR, Foster BA, et al; Subcommittee on Fluid and Electrolyte Therapy. Clinical practice guideline: maintenance intravenous fluids in children. Pediatrics 2018;142(6). doi:10.1542/peds.2018-3083
- Sterns RH. Maintenance and replacement fluid therapy in adults. www.uptodate.com/contents/maintenance-and-replacement-fluid-therapy-in-adults. Accessed August 21, 2019.
- Shafiee MA, Bohn D, Hoorn EJ, Halperin ML. How to select optimal maintenance intravenous fluid therapy. QJM 2003; 96(8):601–610. doi:10.1093/qjmed/hcg101
- National Institute for Health and Care Excellence (NICE). Intravenous fluid therapy in adults in hospital. www.nice.org.uk/guidance/cg174. Accessed August 21, 2019.
- Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics 1957; 19(5):823–832. pmid:13431307
- Appel LJ, Foti K. Sources of dietary sodium: implications for patients, physicians, and policy. Circulation 2017; 135(19):1784–1787. doi:10.1161/CIRCULATIONAHA.117.027933
- Harnack LJ, Cogswell ME, Shikany JM, et al. Sources of sodium in US adults from 3 geographic regions. Circulation 2017; 135(19):1775–1783. doi:10.1161/CIRCULATIONAHA.116.024446
- Sterns RH. Pathophysiology and etiology of the syndrome of inappropriate antidiuretic hormone secretion (SIADH). www.uptodate.com/contents/pathophysiology-and-etiology-of-the-syndrome-of-inappropriate-antidiuretic-hormone-secretion-siadh. Accessed August 21, 2019.
- Mortiz ML, Ayus JC. Maintenance intravenous fluids in acutely ill patients. N Engl J Med 2015; 373(14):1350–1360. doi:10.1056/NEJMra1412877
- Feld LG, Neuspiel DR, Foster BA, et al; Subcommittee on Fluid and Electrolyte Therapy. Clinical practice guideline: maintenance intravenous fluids in children. Pediatrics 2018;142(6). doi:10.1542/peds.2018-3083
- Sterns RH. Maintenance and replacement fluid therapy in adults. www.uptodate.com/contents/maintenance-and-replacement-fluid-therapy-in-adults. Accessed August 21, 2019.
- Shafiee MA, Bohn D, Hoorn EJ, Halperin ML. How to select optimal maintenance intravenous fluid therapy. QJM 2003; 96(8):601–610. doi:10.1093/qjmed/hcg101
- National Institute for Health and Care Excellence (NICE). Intravenous fluid therapy in adults in hospital. www.nice.org.uk/guidance/cg174. Accessed August 21, 2019.
- Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics 1957; 19(5):823–832. pmid:13431307
- Appel LJ, Foti K. Sources of dietary sodium: implications for patients, physicians, and policy. Circulation 2017; 135(19):1784–1787. doi:10.1161/CIRCULATIONAHA.117.027933
- Harnack LJ, Cogswell ME, Shikany JM, et al. Sources of sodium in US adults from 3 geographic regions. Circulation 2017; 135(19):1775–1783. doi:10.1161/CIRCULATIONAHA.116.024446
- Sterns RH. Pathophysiology and etiology of the syndrome of inappropriate antidiuretic hormone secretion (SIADH). www.uptodate.com/contents/pathophysiology-and-etiology-of-the-syndrome-of-inappropriate-antidiuretic-hormone-secretion-siadh. Accessed August 21, 2019.
A 66-year-old man with abnormal thyroid function tests
A 66-year-old man presented to the emergency department with increasing shortness of breath and productive cough, which had begun 5 days earlier. Three years previously, he had been diagnosed with chronic obstructive pulmonary disease (COPD).
One week before the current presentation, he developed a sore throat, rhinorrhea, and nasal congestion, and the shortness of breath had started 2 days after that. Although he could speak in sentences, he was breathless even at rest. His dyspnea was associated with noisy breathing and cough productive of yellowish sputum; there was no hemoptysis. He reported fever, but he had no chills, night sweats, chest pain, or paroxysmal nocturnal dyspnea. The review of other systems was unremarkable.
His COPD was known to be mild, in Global Initiative for Chronic Obstructive Lung Disease (GOLD) grade 1, group A. His postbronchodilator ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) was less than 0.70, and his FEV1 was 84% of predicted. Apart from mild intermittent cough with white sputum, his COPD had been under good control with inhaled ipratropium 4 times daily and inhaled albuterol as needed. He said he did not have shortness of breath except when hurrying on level ground or walking up a slight hill (Modified Medical Research Council dyspnea scale grade 1; COPD Assessment Test score < 10). In the last 3 years, he had 2 exacerbations of COPD, 1 year apart, both requiring oral prednisone and antibiotic therapy.
Other relevant history included hypertension and dyslipidemia of 15-year duration, for which he was taking candesartan 16 mg twice daily and atorvastatin 20 mg daily. He was compliant with his medications.
Though he usually received an influenza vaccine every year, he did not get it the previous year. Also, 3 years previously, he received the 23-valent pneumococcal polysaccharide vaccine (PPSV23), and the year before that he received the pneumococcal conjugate vaccine (PCV13). In addition, he was immunized against herpes zoster and tetanus.
The patient had smoked 1 pack per day for the past 38 years. His primary care physician had advised him many times to quit smoking. He had enrolled in a smoking cessation program 2 years previously, in which he received varenicline in addition to behavioral counseling in the form of motivational interviewing and a telephone quit-line. Nevertheless, he continued to smoke.
He was a retired engineer. He did not drink alcohol or use illicit drugs.
PHYSICAL EXAMINATION
On physical examination, the patient was sitting up in bed, leaning forward. He was alert and oriented but was breathing rapidly and looked sick. He had no cyanosis, clubbing, pallor, or jaundice. His blood pressure was 145/90 mm Hg, heart rate 110 beats per minute and regular, respiratory rate 29 breaths per minute, and oral temperature 38.1°C (100.6°F). His oxygen saturation was 88% while breathing room air. His body mass index was 27.1 kg/m2.
His throat was mildly congested. His neck veins were flat, and there were no carotid bruits. His thyroid examination was normal, without goiter, nodules, or tenderness.
Intercostal retractions were noted around the anterolateral costal margins. He had no chest wall deformities. Chest expansion was reduced bilaterally. There was hyperresonance bilaterally. Expiratory wheezes were heard over both lungs, without crackles.
His heart had no murmurs or added sounds. There was no lower-limb edema or swelling. The rest of his physical examination was unremarkable.
Results of initial laboratory testing are shown in Table 1.
Assessment: A 66-year-old man with GOLD grade 1, group A COPD, presenting with a severe exacerbation, most likely due to viral bronchitis.
INITIAL MANAGEMENT
The patient was given oxygen 28% by Venturi mask, and his oxygen saturation went up to 90%. He was started on nebulized albuterol 2.5 mg with ipratropium bromide 500 µg every 4 hours, prednisone 40 mg orally daily for 5 days, and ceftriaxone 1 g intravenously every 24 hours. The first dose of each medication was given in the emergency department.
The patient was then admitted to a progressive care unit, where he was placed on noninvasive positive pressure ventilation, continuous cardiac monitoring, and pulse oximetry. He was started on enoxaparin 40 mg subcutaneously daily to prevent venous thromboembolism, and the oral medications he had been taking at home were continued. Because he was receiving a glucocorticoid, his blood glucose was monitored in the fasting state, 2 hours after each meal, and as needed.
Two hours after he started noninvasive positive pressure ventilation, his arterial blood gases were remeasured and showed the following results:
- pH 7.35
- Partial pressure of carbon dioxide (Paco2) 52 mm Hg
- Bicarbonate 28 mmol/L
- Partial pressure of oxygen (Pao2) 60 mm Hg
- Oxygen saturation 90%.
HOSPITAL COURSE
On hospital day 3, his dyspnea had slightly improved. His respiratory rate was 26 to 28 breaths per minute. His oxygen saturation remained between 90% and 92%.
At 10:21 pm, his cardiac monitor showed an episode of focal atrial tachycardia at a rate of 129 beats per minute that lasted for 3 minutes and 21 seconds, terminating spontaneously. He denied any change in his clinical condition during the episode, with no chest pain, palpitation, or change in dyspnea. There was no change in his vital signs. He had another similar asymptomatic episode lasting 4 minutes and 9 seconds at 6:30 am of hospital day 4.
Because of these episodes, the attending physician ordered thyroid function tests.
THYROID FUNCTION TESTING
1. Which thyroid function test is most likely to be helpful in the assessment of this patient’s thyroid status?
- Serum thyroid-stimulating hormone (TSH) alone
- Serum TSH and total thyroxine (T4)
- Serum TSH and total triiodothyronine (T3)
- Serum TSH and free T4
- Serum TSH and free T3
There are several tests to assess thyroid function: the serum TSH, total T4, free T4, total T3, and free T3 concentrations.1
In normal physiology, TSH from the pituitary stimulates the thyroid gland to produce and secrete T4 and T3, which in turn inhibit TSH secretion through negative feedback. A negative log-linear relation exists between serum free T4 and TSH levels.2 Thus, the serum free T4 level can remain within the normal reference range even if the TSH level is high or low.
TSH assays can have different detection limits. A third-generation TSH assay with a detection limit of 0.01 mU/L is recommended for use in clinical practice.3
TSH testing alone. Given its superior sensitivity and specificity, serum TSH measurement is considered the best single test for assessing thyroid function in most cases.4 Nevertheless, measurement of the serum TSH level alone could be misleading in several situations, eg, hypothalamic or pituitary disorders, recent treatment of thyrotoxicosis, impaired sensitivity to thyroid hormone, and acute nonthyroidal illness.4
Free vs total T4 and T3 levels
Serum total T4 includes a fraction that is bound, mainly to thyroxin-binding globulin, and a very small unbound (free) fraction. The same applies to T3. Only free thyroid hormones represent the “active” fraction available for interaction with their protein receptors in the nucleus.8 Patients with conditions that can affect the thyroid-binding protein concentrations usually have altered serum total T4 and T3 levels, whereas their free hormone concentrations remain normal. Accordingly, measurement of free hormone levels, especially free T4, is usually recommended.
Although equilibrium dialysis is the method most likely to provide an accurate serum free T4 measurement, it is not commonly used because of its limited availability and high cost. Thus, most commercial laboratories use “direct” free T4 measurement or, to a lesser degree, the free T4 index.9 However, none of the currently available free T4 tests actually measure free T4 directly; rather, they estimate it.10
Commercial laboratories can provide a direct free T3 estimate, but it may be less reliable than total T3. If serum T3 measurement is indicated, serum total T3 is usually measured. However, total T3 measurement is rarely indicated for patients with hypothyroidism because it usually remains within the normal reference range.11 Nevertheless, serum total T3 measurement could be useful in patients with T3 toxicosis and in those who are acutely ill.
Accordingly, in acutely ill hospitalized patients like ours, measuring serum TSH using a third-generation assay and free T4 is essential to assess thyroid function. Many clinicians also measure serum total T3.
CASE CONTINUED: LOW TSH, LOW-NORMAL FREE T4, LOW TOTAL T3
The attending physician ordered serum TSH, free T4, and total T3 measurements, which yielded the following:
- TSH 0.1 mU/L (0.5–5.0)
- Total T3 55 ng/dL (80–180)
- Free T4 0.9 ng/dL (0.9–2.4).
2. Which best explains this patient’s abnormal thyroid test results?
- His acute illness
- Central hypothyroidism due to pituitary infarction
- His albuterol therapy
- Subclinical thyrotoxicosis
- Hashimoto thyroiditis
Since euthyroid patients with an acute illness may have abnormal thyroid test results (Table 2),5–7 thyroid function testing is not recommended unless there is a strong indication for it, such as new-onset atrial fibrillation, atrial flutter, or focal atrial tachycardia.1 In such patients, it is important to know whether the test abnormalities represent true thyroid disorder or are the result of a nonthyroidal illness.
Thyroid function testing in patients with nonthyroidal illness usually shows low serum total T3, normal or low serum TSH, and normal, low, or high serum free T4. However, transient mild serum TSH elevation can be seen in some patients during the recovery period.16 These abnormalities with their mechanisms are shown in Table 2.5–7 In several commercial kits, serum direct free T4 can be falsely decreased or increased.8
THE DIFFERENTIAL DIAGNOSIS
Our patient had low serum TSH, low-normal serum direct free T4, and low serum total T3. This profile could be caused by a nonthyroidal illness, “true” central hypothyroidism, or his glucocorticoid treatment. The reason we use the term “true” in this setting is that some experts suggest that the thyroid function test abnormalities in patients with acute nonthyroidal illness represent a transient central hypothyroidism.17 The clinical presentation is key in differentiating true central hypothyroidism from nonthyroidal illness.
In addition, measuring serum cortisol may help to differentiate between the 2 states, as it would be elevated in patients with nonthyroidal illness as part of a stress response but low in patients with true central hypothyroidism, since it is usually part of combined pituitary hormone deficiency.18 Of note, some critically ill patients have low serum cortisol because of transient central adrenal insufficiency.19,20
The serum concentration of reverse T3 has been suggested as a way to differentiate between hypothyroidism (low) and nonthyroidal illness (high); however, further studies showed that it does not reliably differentiate between the conditions.21
GLUCOCORTICOIDS AND THYROID FUNCTION TESTS
By inhibiting D1, glucocorticoids can decrease peripheral conversion of T4 to T3 and thus decrease serum total T3. This effect depends on the type and dose of the glucocorticoid and the duration of therapy.
In one study,22 there was a significant reduction in serum total T3 concentration 24 hours after a single oral dose of dexamethasone 12 mg in normal participants. This effect lasted 48 hours, after which serum total T3 returned to its pretreatment level.
In another study,23 a daily oral dose of betamethasone 1.5 mg for 5 days did not significantly reduce the serum total T3 in healthy volunteers, but a daily dose of 3 mg did. This effect was more pronounced at a daily dose of 4.5 mg, whereas a dose of 6.0 mg had no further effect.
Long-term glucocorticoid therapy also decreases serum total T4 and total T3 by lowering serum thyroid-binding globulin.24
Finally, glucocorticoids can decrease TSH secretion by directly inhibiting thyrotropin-releasing hormone.25,26 However, chronic hypercortisolism, whether endogenous or exogenous, does not cause clinically central hypothyroidism, possibly because of the negative feedback mechanism of low thyroid hormones on the pituitary and the hypothalamus.27
Other drugs including dopamine, dopamine agonists, dobutamine, and somatostatin analogues can suppress serum TSH. As with glucocorticoids, these drugs do not cause clinically evident central hypothyroidism.28 Bexarotene, a retinoid X receptor ligand used in the treatment of cutaneous T-cell lymphoma, has been reported to cause clinically evident central hypothyroidism by suppressing TSH and increasing T4 clearance.29
BETA-BLOCKERS, BETA-AGONISTS AND THYROID FUNCTION
While there is general agreement that beta-adrenergic antagonists (beta-blockers) do not affect the serum TSH concentration, conflicting data have been reported concerning their effect on other thyroid function tests. This may be due to several factors, including dose, duration of therapy, the patient’s thyroid status, and differences in laboratory methodology.30
In studies of propranolol, serum total T4 concentrations did not change or were increased with daily doses of 160 mg or more in both euthyroid participants and hyperthyroid patients31–33; serum total T3 concentrations did not change or were decreased with 40 mg or more daily34; and serum reverse T3 concentrations were increased with daily doses of 80 mg or more.31 It is most likely that propranolol exerts these changes by inhibiting D1 activity in peripheral tissues.
Furthermore, a significant decrease in serum total T3 concentrations was observed in hyperthyroid patients treated with atenolol 100 mg daily, metoprolol 100 mg daily, and alprenolol 100 mg daily, but not with sotalol 80 mg daily or nadolol (up to 240 mg daily).35,36
On the other hand, beta-adrenergic agonists have not been reported to cause significant changes in thyroid function tests.37
SUBCLINICAL THYROTOXICOSIS OR HASHIMOTO THYROIDITIS?
Our patient’s thyroid function test results are more likely due to his nonthyroidal illness and glucocorticoid therapy, as there is no clinical evidence to point to a hypothalamic-pituitary disorder accounting for true central hypothyroidism.
The other options mentioned in question 2 are unlikely to explain our patient’s thyroid function test results.
Subclinical thyrotoxicosis is characterized by suppressed serum TSH, but both serum free T4 and total T3 remain within the normal reference ranges. In addition, the serum TSH level may help to differentiate between thyrotoxicosis and nonthyroidal illness. In the former, serum TSH is usually suppressed (< 0.01 mU/L), whereas in the latter it is usually low but detectable (0.05– 0.3 mU/L).38,39
Hashimoto thyroiditis is a chronic autoimmune thyroid disease characterized by diffuse lymphocytic infiltration of the thyroid gland. Almost all patients with Hashimoto thyroiditis have elevated levels of antibodies to thyroid peroxidase or thyroglobulin.40 Clinically, patients with Hashimoto thyroiditis can either be hypothyroid or have normal thyroid function, which is not the case in our patient.
CASE CONTINUED
An endocrinologist, consulted for a second opinion, agreed that the patient’s thyroid function test results were most likely due to his nonthyroidal illness and glucocorticoid therapy.
3. In view of the endocrinologist’s opinion, which should be the next step in the management of the patient’s thyroid condition?
- Start levothyroxine (T4) therapy
- Start liothyronine (T3) therapy
- Start N-acetylcysteine therapy
- Start thyrotropin-releasing hormone therapy
- Remeasure thyroid hormones after full recovery from his acute illness
It is not clear whether the changes in thyroid hormone levels during an acute illness are a pathologic alteration for which thyroid hormone therapy may be beneficial, or a physiologic adaptation for which such therapy would not be indicated.41
However, current data argue against thyroid hormone therapy using T4 or T3 for patients with nonthyroidal illness syndrome (also called euthyroid sick syndrome).42 Indeed, several randomized controlled trials showed that thyroid hormone therapy is not beneficial in such patients and may be detrimental.41,43
Therapies other than thyroid hormone have been investigated to ameliorate thyroid hormone abnormalities in patients with nonthyroidal illness. These include N-acetylcysteine, thyrotropin-releasing hormone therapy, and nutritional support.
Some studies showed that giving N-acetylcysteine, an antioxidant, increased serum T3 and decreased serum reverse T3 concentrations in patients with acute myocardial infarction.44 Nevertheless, the mortality rate and length of hospitalization were not affected. Further studies are needed to know whether N-acetylcysteine therapy is beneficial for such patients.
Similarly, a study using a thyrotropin-releasing hormone analogue along with growth hormone-releasing peptide 2 showed an increase in serum TSH, T4, and T3 levels in critically ill patients.45 The benefit of this therapy has yet to be determined. On the other hand, early nutritional support was reported to prevent thyroid hormonal changes in patients postoperatively.46
Measuring thyroid hormone levels after full recovery is the most appropriate next step in our patient, as the changes in thyroid hormone concentrations subside as the acute illness resolves.47
CASE CONTINUED
The patient continued to improve. On hospital day 6, he was feeling better but still had mild respiratory distress. There had been no further episodes of arrhythmia since day 4. His blood pressure was 136/86 mm Hg, heart rate 88 beats per minute and regular, respiratory rate 18 breaths per minute, and oral temperature 37.1°C. His oxygen saturation was 92% on room air.
Before discharge, he was encouraged to quit smoking. He was offered behavioral counseling and medication therapy, but he only said that he would think about it. He was discharged on oral cefixime for 4 more days and was instructed to switch to a long-acting bronchodilator along with his other home medications and to return in 1 week to have his thyroid hormones checked.
One week later, his laboratory results were:
- TSH 11.2 mU/L (reference range 0.5–5.0)
- Free T4 1.2 ng/dL (0.9–2.4)
- Total T3 92 ng/dL (80–180).
Clinically, the patient was euthyroid, and examination of his thyroid was unremarkable.
4. Based on these last test results, which statement is correct?
- Levothyroxine therapy should be started
- His serum TSH elevation is most likely transient
- Thyroid ultrasonography is strongly indicated
- A radioactive iodine uptake study should be performed
- Measurement of thyroid-stimulating immunoglobulins is indicated
During recovery from nonthyroidal illness, some patients may have elevated serum TSH levels that are usually transient and modest (< 20 mU/L).48 Normalization of the thyroid function tests including serum TSH may take several weeks49 or months.50 However, a systematic review found that the likelihood of permanent primary hypothyroidism is high in patients with serum TSH levels higher than 20 mU/L during the recovery phase of their nonthyroidal illness.51
Ultrasonography is useful for evaluating patients with thyroid nodules or goiter but is of little benefit for patients like ours, in whom the thyroid is normal on examination.
Similarly, a radioactive iodine uptake study is not indicated, as it is principally used to help differentiate between types of thyrotoxicosis. (Radioactive iodine is also used to treat differentiated thyroid cancer.)
Thyroid-stimulating immunoglobins are TSH receptor-stimulating antibodies that cause Graves disease. Nevertheless, measuring them is not routinely indicated for its diagnosis. However, their measurement is of significant help in the diagnosis of Graves disease if a radioactive iodine uptake study cannot be performed (as in pregnancy) and in atypical presentations such as euthyroid Graves ophthalmopathy.52 Other indications for thyroid-stimulating immunoglobin measurement are beyond the scope of the article. Our patient’s test results are not consistent with hyperthyroidism, so measuring thyroid-stimulating immunoglobins is not indicated.
CASE CONCLUSION: BETTER, BUT STILL SMOKING
The patient missed his 1-month clinic follow-up, but he visited the clinic for follow-up 3 months later. He was feeling well with no complaints. Test results including serum TSH, free T4, and total T3 were within normal ranges. His COPD was under control, with an FEV1 88% of predicted.
He was again encouraged to quit smoking and was offered drug therapy and behavioral counseling, but he declined. In addition, he was instructed to adhere to his annual influenza vaccination.
KEY POINTS
- In patients with acute illness, it is recommended that thyroid function not be assessed unless there is a strong indication.
- If thyroid function assessment is indicated for critically ill patients, serum TSH and free T4 concentrations should be measured. Some clinicians also measure serum total T3 level.
- Thyroid function testing in critically ill patients usually shows low serum total T3, normal or low serum TSH, and normal or low serum free T4.
- Many drugs can alter thyroid hormone levels.
- Thyroid hormone therapy is not recommended for critically ill patients with low T3, low T4, or both.
- During recovery from nonthyroidal illness, some patients may have mild elevation in serum TSH levels (< 20 mU/L).
- Thyroid hormone levels may take several weeks or months to return to normal after the acute illness.
- Patients with serum TSH levels higher than 20 mU/L during the recovery phase of their nonthyroidal illness are more likely to have permanent primary hypothyroidism.
- Lamb EJ, Martin J. Thyroid function tests: often justified in the acutely ill. Ann Clin Biochem 2000; 37(pt 2):158–164. doi:10.1258/0004563001899159
- Spencer CA, LoPresti JS, Patel A, et al. Applications of a new chemiluminometric thyrotropin assay to subnormal measurement. J Clin Endocrinol Metab 1990; 70(2):453–460. doi:10.1210/jcem-70-2-453
- Ross DS, Ardisson LJ, Meskell MJ. Measurement of thyrotropin in clinical and subclinical hyperthyroidism using a new chemiluminescent assay. J Clin Endocrinol Metab 1989; 69(3):684–688. doi:10.1210/jcem-69-3-684
- Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab 2013; 27(6):745–762. doi:10.1016/j.beem.2013.10.003
- Lechan RM, Fekete C. Role of thyroid hormone deiodination in the hypothalamus. Thyroid 2005; 15(8):883–897. doi:10.1089/thy.2005.15.883
- Chopra IJ, Hershman JM, Pardridge WM, Nicoloff JT. Thyroid function in nonthyroidal ilnesses. Ann Intern Med 1983; 98(6):946–957. doi:10.7326/0003-4819-98-6-946
- Chopra IJ, Solomon DH, Hepner HW, Mortenstein AA. Misleadingly low free thyroxine index and usefulness of reverse triiodothyronine measurement in nonthyroidal illnesses. Ann Intern Med 1979; 90(6):905–912. doi:10.7326/0003-4819-90-6-905
- Pontecorvi A, Robbins J. The plasma membrane and thyroid hormone entry into cells. Trends Endocrinol Metab 1989; 1(2):90–94. pmid:18411097
- Hennemann G, Krenning EP. Pitfalls in the interpretation of thyroid function tests in old age and non-thyroidal illness. Horm Res 1987; 26(1–4):100–104. doi:10.1159/000180688
- Baloch Z, Carayon P, Conte-Devolx B, et al; Guidelines Committee, National Academy of Clinical Biochemistry. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 2003; 13(1):3–126. doi:10.1089/105072503321086962
- Lum S, Nicoloff JT, Spencer CA, Kaptein EM. Peripheral tissue mechanism for maintenance of serum triiodothyronine values in a thyroxine-deficient state in man. J Clin Invest 1984; 73(2):570–575. doi:10.1172/JCI111245
- Ortiga-Carvalho TM, Chiamolera MI, Pazos-Moura CC, Wondisford FE. Hypothalamus-pituitary-thyroid axis. Compr Physiol 2016; 6(3):1387–1428. doi:10.1002/cphy.c150027
- de Vries EM, Fliers E, Boelen A. The molecular basis of the non-thyroidal illness syndrome. J Endocrinol 2015; 225(3):R67–R81. doi:10.1530/JOE-15-0133
- Chopra IJ, Huang TS, Beredo A, Solomon DH, Teco GN, Mean JF. Evidence for an inhibitor of extrathyroidal conversion of thyroxine to 3, 5, 3'-triiodothyronine in sera of patients with nonthyroidal illnesses. J Clin Endocrinol Metab 1985; 60(4):666–672. doi:10.1210/jcem-60-4-666
- Peeters RP, Debaveye Y, Fliers E, Visser TJ. Changes within the thyroid axis during critical illness. Crit Care Clin 2006; 22(1):41–55. doi:10.1016/j.ccc.2005.08.006
- Spencer C, Eigen A, Shen D, et al. Specificity of sensitive assays of thyrotropin (TSH) used to screen for thyroid disease in hospitalized patients. Clin Chem 1987; 33(8):1391–1396. pmid:3301067
- Adler SM, Wartofsky L. The nonthyroidal illness syndrome. Endocrinol Metab Clin North Am 2007; 36(3):657–672. doi:10.1016/j.ecl.2007.04.007
- Persani L. Central hypothyroidism: pathogenic, diagnostic, and therapeutic challenges. J Clin Endocrinol Metab 2012; 97(9):3068–3078. doi:10.1210/jc.2012-1616
- Kidess AI, Caplan RH, Reynertson RH, Wickus GG, Goodnough DE. Transient corticotropin deficiency in critical illness. Mayo Clin Proc 1993; 68(5):435–441. doi:10.1016/s0025-6196(12)60188-8
- Lamberts SW, Bruining HA, De Jong FH. Corticosteroid therapy in severe illness. N Engl J Med 1997; 337(18):1285–1292. doi:10.1056/NEJM199710303371807
- Burmeister LA. Reverse T3 does not reliably differentiate hypothyroid sick syndrome from euthyroid sick syndrome. Thyroid 1995; 5(6):435–441. doi:10.1089/thy.1995.5.435
- Duick DS, Warren DW, Nicoloff JT, Otis CL, Croxson MS. Effect of single dose dexamethasone on the concentration of serum triiodothyronine in man. J Clin Endocrinol Metab 1974; 39(6):1151–1154. doi:10.1210/jcem-39-6-1151
- Gamstedt A, Järnerot G, Kågedal B. Dose related effects of betamethasone on iodothyronines and thyroid hormone-binding proteins in serum. Acta Endocrinol (Copenh) 1981; 96(4):484–490. doi:10.1530/acta.0.0960484
- Wartofsky L, Burman KD. Alterations in thyroid function in patients with systemic illness: the “euthyroid sick syndrome.” Endocr Rev 1982; 3(2):164–217. doi:10.1210/edrv-3-2-164
- Wilber JF, Utiger RD. The effect of glucocorticoids on thyrotropin secretion. J Clin Invest 1969; 48(11):2096–2103. doi:10.1172/JCI106176
- Nicoloff JT, Fisher DA, Appleman MD Jr. The role of glucocorticoids in the regulation of thyroid function in man. J Clin Invest 1970; 49(10):1922–1929. doi:10.1172/JCI106411
- Surks MI, Sievert R. Drugs and thyroid function. N Engl J Med 1995; 333(25):1688–1694. doi:10.1056/NEJM199512213332507
- Haugen BR. Drugs that suppress TSH or cause central hypothyroidism. Best Pract Res Clin Endocrinol Metab 2009; 23(6):793–800. doi:10.1016/j.beem.2009.08.003
- Sherman SI, Gopal J, Haugen BR, et al. Central hypothyroidism associated with retinoid X receptor–selective ligands. N Engl J Med 1999; 340(14):1075–1079. doi:10.1056/NEJM199904083401404
- Murchison LE, How J, Bewsher PD. Comparison of propranolol and metoprolol in the management of hyperthyroidism. Br J Clin Pharmacol 1979; 8(6):581–587. doi:10.1111/j.1365-2125.1979.tb01048.x
- Faber J, Friis T, Kirkegaard C, et al. Serum T4, T3 and reverse T3 during treatment with propranolol in hyperthyroidism, L-T4 treated myxedema and in normal man. Horm Metab Res 1979; 11(1):34–36. doi:10.1055/s-0028-1092678
- Kristensen BO, Weeke J. Propranolol-induced increments in total and free serum thyroxine in patients with essential hypertension. Clin Pharmacol Ther 1977; 22(6):864–867. doi:10.1002/cpt1977226864
- Murchison LE, Bewsher PD, Chesters MI, Ferrier WR. Comparison of propranolol and practolol in the management of hyperthyroidism. Br J Clin Pharmacol 1976; 3(2):273–277. doi:10.1111/j.1365-2125.1976.tb00603.x
- Lotti G, Delitala G, Devilla L, Alagna S, Masala A. Reduction of plasma triiodothyronine (T3) induced by propranolol. Clin Endocrinol 1977; 6(6):405–410. doi:10.1111/j.1365-2265.1977.tb03322.x
- Perrild H, Hansen JM, Skovsted L, Christensen LK. Different effects of propranolol, alprenolol, sotalol, atenolol and metoprolol on serum T3 and serum rT3 in hyperthyroidism. Clin Endocrinol (Oxf) 1983; 18(2):139–142. pmid:6133659
- Reeves RA, From GL, Paul W, Leenen FH. Nadolol, propranolol, and thyroid hormones: evidence for a membrane-stabilizing action of propranolol. Clin Pharmacol Ther 1985; 37(2):157–161. doi:10.1038/clpt.1985.28
- Walker N, Jung RT, Jennings G, James WP. The effect of a beta-receptor agonist (salbutamol) on peripheral thyroid metabolism in euthyroid subjects. Horm Metab Res 1981; 13(10):590–591. doi:10.1055/s-2007-1019346
- Melmed S, Geola FL, Reed AW, Pekary AE, Park J, Hershman JM. A comparison of methods for assessing thyroid function in nonthyroidal illness. J Clin Endocrinol Metab 1982; 54(2):300–306. doi:10.1210/jcem-54-2-300
- Docter R, Krenning E, De Jong M, Hennemann G. The sick euthyroid syndrome: changes in thyroid hormone serum parameters and hormone metabolism. Clin Endocrinol (Oxf) 1993; 39(5):499–518. pmid:8252737
- Mariotti S, Caturegli P, Piccolo P, Barbesino G, Pinchera A. Antithyroid peroxidase autoantibodies in thyroid diseases. J Clin Endocrinol Metab 1990; 71(3):661–669. doi:10.1210/jcem-71-3-661
- De Groot LJ. Non-thyroidal illness syndrome is a manifestation of hypothalamic-pituitary dysfunction, and in view of current evidence, should be treated with appropriate replacement therapies. Crit Care Clin 2006; 22(1):57–86. doi:10.1016/j.ccc.2005.10.001
- Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 2014; 24(12):1670–1751. doi:10.1089/thy.2014.0028
- Kaptein EM, Beale E, Chan LS. Thyroid hormone therapy for obesity and nonthyroidal illnesses: a systematic review. J Clin Endocrinol Metab 2009; 94(10):3663–3675. doi:10.1210/jc.2009-0899
- Vidart J, Wajner SM, Leite RS, et al. N-acetylcysteine administration prevents nonthyroidal illness syndrome in patients with acute myocardial infarction: a randomized clinical trial. J Clin Endocrinol Metab 2014; 99(12):4537–4545. doi:10.1210/jc.2014-2192
- Van den Berghe G, Wouters P, Weekers F, et al. Reactivation of pituitary hormone release and metabolic improvement by infusion of growth hormone-releasing peptide and thyrotropin-releasing hormone in patients with protracted critical illness. J Clin Endocrinol Metab 1999; 84(4):1311–1323. doi:10.1210/jcem.84.4.5636
- Langouche L, Vander Perre S, Marques M, et al. Impact of early nutrient restriction during critical illness on the nonthyroidal illness syndrome and its relation with outcome: a randomized, controlled clinical study. J Clin Endocrinol Metab 2013; 98(3):1006–1013. doi:10.1210/jc.2012-2809
- Economidou F, Douka E, Tzanela M, Nanas S, Kotanidou A. Thyroid function during critical illness. Hormones (Athens) 2011; 10(2):117–124. doi:10.14310/horm.2002.1301
- Hamblin PS, Dyer SA, Mohr VS, et al. Relationship between thyrotropin and thyroxine changes during recovery from severe hypothyroxinemia of critical illness. J Clin Endocrinol Metab 1986; 62(4):717–722. doi:10.1210/jcem-62-4-717
- Iglesias P, Diez JJ. Thyroid dysfunction and kidney disease. Eur J Endocrinol 2009; 160(4):503–515. doi:10.1530/EJE-08-0837
- Spencer CA. Clinical utility and cost-effectiveness of sensitive thyrotropin assays in ambulatory and hospitalized patients. Mayo Clin Proc 1988; 63(12):1214–1222. doi:10.1016/s0025-6196(12)65408-1
- Attia J, Margetts P, Guyatt G. Diagnosis of thyroid disease in hospitalized patients: a systematic review. Arch Intern Med 1999; 159(7):658–665. pmid:10218744
- Barbesino G, Tomer Y. Clinical review: clinical utility of TSH receptor antibodies. J Clin Endocrinol Metab 2013; 98(6):2247–2255. doi:10.1210/jc.2012-4309
A 66-year-old man presented to the emergency department with increasing shortness of breath and productive cough, which had begun 5 days earlier. Three years previously, he had been diagnosed with chronic obstructive pulmonary disease (COPD).
One week before the current presentation, he developed a sore throat, rhinorrhea, and nasal congestion, and the shortness of breath had started 2 days after that. Although he could speak in sentences, he was breathless even at rest. His dyspnea was associated with noisy breathing and cough productive of yellowish sputum; there was no hemoptysis. He reported fever, but he had no chills, night sweats, chest pain, or paroxysmal nocturnal dyspnea. The review of other systems was unremarkable.
His COPD was known to be mild, in Global Initiative for Chronic Obstructive Lung Disease (GOLD) grade 1, group A. His postbronchodilator ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) was less than 0.70, and his FEV1 was 84% of predicted. Apart from mild intermittent cough with white sputum, his COPD had been under good control with inhaled ipratropium 4 times daily and inhaled albuterol as needed. He said he did not have shortness of breath except when hurrying on level ground or walking up a slight hill (Modified Medical Research Council dyspnea scale grade 1; COPD Assessment Test score < 10). In the last 3 years, he had 2 exacerbations of COPD, 1 year apart, both requiring oral prednisone and antibiotic therapy.
Other relevant history included hypertension and dyslipidemia of 15-year duration, for which he was taking candesartan 16 mg twice daily and atorvastatin 20 mg daily. He was compliant with his medications.
Though he usually received an influenza vaccine every year, he did not get it the previous year. Also, 3 years previously, he received the 23-valent pneumococcal polysaccharide vaccine (PPSV23), and the year before that he received the pneumococcal conjugate vaccine (PCV13). In addition, he was immunized against herpes zoster and tetanus.
The patient had smoked 1 pack per day for the past 38 years. His primary care physician had advised him many times to quit smoking. He had enrolled in a smoking cessation program 2 years previously, in which he received varenicline in addition to behavioral counseling in the form of motivational interviewing and a telephone quit-line. Nevertheless, he continued to smoke.
He was a retired engineer. He did not drink alcohol or use illicit drugs.
PHYSICAL EXAMINATION
On physical examination, the patient was sitting up in bed, leaning forward. He was alert and oriented but was breathing rapidly and looked sick. He had no cyanosis, clubbing, pallor, or jaundice. His blood pressure was 145/90 mm Hg, heart rate 110 beats per minute and regular, respiratory rate 29 breaths per minute, and oral temperature 38.1°C (100.6°F). His oxygen saturation was 88% while breathing room air. His body mass index was 27.1 kg/m2.
His throat was mildly congested. His neck veins were flat, and there were no carotid bruits. His thyroid examination was normal, without goiter, nodules, or tenderness.
Intercostal retractions were noted around the anterolateral costal margins. He had no chest wall deformities. Chest expansion was reduced bilaterally. There was hyperresonance bilaterally. Expiratory wheezes were heard over both lungs, without crackles.
His heart had no murmurs or added sounds. There was no lower-limb edema or swelling. The rest of his physical examination was unremarkable.
Results of initial laboratory testing are shown in Table 1.
Assessment: A 66-year-old man with GOLD grade 1, group A COPD, presenting with a severe exacerbation, most likely due to viral bronchitis.
INITIAL MANAGEMENT
The patient was given oxygen 28% by Venturi mask, and his oxygen saturation went up to 90%. He was started on nebulized albuterol 2.5 mg with ipratropium bromide 500 µg every 4 hours, prednisone 40 mg orally daily for 5 days, and ceftriaxone 1 g intravenously every 24 hours. The first dose of each medication was given in the emergency department.
The patient was then admitted to a progressive care unit, where he was placed on noninvasive positive pressure ventilation, continuous cardiac monitoring, and pulse oximetry. He was started on enoxaparin 40 mg subcutaneously daily to prevent venous thromboembolism, and the oral medications he had been taking at home were continued. Because he was receiving a glucocorticoid, his blood glucose was monitored in the fasting state, 2 hours after each meal, and as needed.
Two hours after he started noninvasive positive pressure ventilation, his arterial blood gases were remeasured and showed the following results:
- pH 7.35
- Partial pressure of carbon dioxide (Paco2) 52 mm Hg
- Bicarbonate 28 mmol/L
- Partial pressure of oxygen (Pao2) 60 mm Hg
- Oxygen saturation 90%.
HOSPITAL COURSE
On hospital day 3, his dyspnea had slightly improved. His respiratory rate was 26 to 28 breaths per minute. His oxygen saturation remained between 90% and 92%.
At 10:21 pm, his cardiac monitor showed an episode of focal atrial tachycardia at a rate of 129 beats per minute that lasted for 3 minutes and 21 seconds, terminating spontaneously. He denied any change in his clinical condition during the episode, with no chest pain, palpitation, or change in dyspnea. There was no change in his vital signs. He had another similar asymptomatic episode lasting 4 minutes and 9 seconds at 6:30 am of hospital day 4.
Because of these episodes, the attending physician ordered thyroid function tests.
THYROID FUNCTION TESTING
1. Which thyroid function test is most likely to be helpful in the assessment of this patient’s thyroid status?
- Serum thyroid-stimulating hormone (TSH) alone
- Serum TSH and total thyroxine (T4)
- Serum TSH and total triiodothyronine (T3)
- Serum TSH and free T4
- Serum TSH and free T3
There are several tests to assess thyroid function: the serum TSH, total T4, free T4, total T3, and free T3 concentrations.1
In normal physiology, TSH from the pituitary stimulates the thyroid gland to produce and secrete T4 and T3, which in turn inhibit TSH secretion through negative feedback. A negative log-linear relation exists between serum free T4 and TSH levels.2 Thus, the serum free T4 level can remain within the normal reference range even if the TSH level is high or low.
TSH assays can have different detection limits. A third-generation TSH assay with a detection limit of 0.01 mU/L is recommended for use in clinical practice.3
TSH testing alone. Given its superior sensitivity and specificity, serum TSH measurement is considered the best single test for assessing thyroid function in most cases.4 Nevertheless, measurement of the serum TSH level alone could be misleading in several situations, eg, hypothalamic or pituitary disorders, recent treatment of thyrotoxicosis, impaired sensitivity to thyroid hormone, and acute nonthyroidal illness.4
Free vs total T4 and T3 levels
Serum total T4 includes a fraction that is bound, mainly to thyroxin-binding globulin, and a very small unbound (free) fraction. The same applies to T3. Only free thyroid hormones represent the “active” fraction available for interaction with their protein receptors in the nucleus.8 Patients with conditions that can affect the thyroid-binding protein concentrations usually have altered serum total T4 and T3 levels, whereas their free hormone concentrations remain normal. Accordingly, measurement of free hormone levels, especially free T4, is usually recommended.
Although equilibrium dialysis is the method most likely to provide an accurate serum free T4 measurement, it is not commonly used because of its limited availability and high cost. Thus, most commercial laboratories use “direct” free T4 measurement or, to a lesser degree, the free T4 index.9 However, none of the currently available free T4 tests actually measure free T4 directly; rather, they estimate it.10
Commercial laboratories can provide a direct free T3 estimate, but it may be less reliable than total T3. If serum T3 measurement is indicated, serum total T3 is usually measured. However, total T3 measurement is rarely indicated for patients with hypothyroidism because it usually remains within the normal reference range.11 Nevertheless, serum total T3 measurement could be useful in patients with T3 toxicosis and in those who are acutely ill.
Accordingly, in acutely ill hospitalized patients like ours, measuring serum TSH using a third-generation assay and free T4 is essential to assess thyroid function. Many clinicians also measure serum total T3.
CASE CONTINUED: LOW TSH, LOW-NORMAL FREE T4, LOW TOTAL T3
The attending physician ordered serum TSH, free T4, and total T3 measurements, which yielded the following:
- TSH 0.1 mU/L (0.5–5.0)
- Total T3 55 ng/dL (80–180)
- Free T4 0.9 ng/dL (0.9–2.4).
2. Which best explains this patient’s abnormal thyroid test results?
- His acute illness
- Central hypothyroidism due to pituitary infarction
- His albuterol therapy
- Subclinical thyrotoxicosis
- Hashimoto thyroiditis
Since euthyroid patients with an acute illness may have abnormal thyroid test results (Table 2),5–7 thyroid function testing is not recommended unless there is a strong indication for it, such as new-onset atrial fibrillation, atrial flutter, or focal atrial tachycardia.1 In such patients, it is important to know whether the test abnormalities represent true thyroid disorder or are the result of a nonthyroidal illness.
Thyroid function testing in patients with nonthyroidal illness usually shows low serum total T3, normal or low serum TSH, and normal, low, or high serum free T4. However, transient mild serum TSH elevation can be seen in some patients during the recovery period.16 These abnormalities with their mechanisms are shown in Table 2.5–7 In several commercial kits, serum direct free T4 can be falsely decreased or increased.8
THE DIFFERENTIAL DIAGNOSIS
Our patient had low serum TSH, low-normal serum direct free T4, and low serum total T3. This profile could be caused by a nonthyroidal illness, “true” central hypothyroidism, or his glucocorticoid treatment. The reason we use the term “true” in this setting is that some experts suggest that the thyroid function test abnormalities in patients with acute nonthyroidal illness represent a transient central hypothyroidism.17 The clinical presentation is key in differentiating true central hypothyroidism from nonthyroidal illness.
In addition, measuring serum cortisol may help to differentiate between the 2 states, as it would be elevated in patients with nonthyroidal illness as part of a stress response but low in patients with true central hypothyroidism, since it is usually part of combined pituitary hormone deficiency.18 Of note, some critically ill patients have low serum cortisol because of transient central adrenal insufficiency.19,20
The serum concentration of reverse T3 has been suggested as a way to differentiate between hypothyroidism (low) and nonthyroidal illness (high); however, further studies showed that it does not reliably differentiate between the conditions.21
GLUCOCORTICOIDS AND THYROID FUNCTION TESTS
By inhibiting D1, glucocorticoids can decrease peripheral conversion of T4 to T3 and thus decrease serum total T3. This effect depends on the type and dose of the glucocorticoid and the duration of therapy.
In one study,22 there was a significant reduction in serum total T3 concentration 24 hours after a single oral dose of dexamethasone 12 mg in normal participants. This effect lasted 48 hours, after which serum total T3 returned to its pretreatment level.
In another study,23 a daily oral dose of betamethasone 1.5 mg for 5 days did not significantly reduce the serum total T3 in healthy volunteers, but a daily dose of 3 mg did. This effect was more pronounced at a daily dose of 4.5 mg, whereas a dose of 6.0 mg had no further effect.
Long-term glucocorticoid therapy also decreases serum total T4 and total T3 by lowering serum thyroid-binding globulin.24
Finally, glucocorticoids can decrease TSH secretion by directly inhibiting thyrotropin-releasing hormone.25,26 However, chronic hypercortisolism, whether endogenous or exogenous, does not cause clinically central hypothyroidism, possibly because of the negative feedback mechanism of low thyroid hormones on the pituitary and the hypothalamus.27
Other drugs including dopamine, dopamine agonists, dobutamine, and somatostatin analogues can suppress serum TSH. As with glucocorticoids, these drugs do not cause clinically evident central hypothyroidism.28 Bexarotene, a retinoid X receptor ligand used in the treatment of cutaneous T-cell lymphoma, has been reported to cause clinically evident central hypothyroidism by suppressing TSH and increasing T4 clearance.29
BETA-BLOCKERS, BETA-AGONISTS AND THYROID FUNCTION
While there is general agreement that beta-adrenergic antagonists (beta-blockers) do not affect the serum TSH concentration, conflicting data have been reported concerning their effect on other thyroid function tests. This may be due to several factors, including dose, duration of therapy, the patient’s thyroid status, and differences in laboratory methodology.30
In studies of propranolol, serum total T4 concentrations did not change or were increased with daily doses of 160 mg or more in both euthyroid participants and hyperthyroid patients31–33; serum total T3 concentrations did not change or were decreased with 40 mg or more daily34; and serum reverse T3 concentrations were increased with daily doses of 80 mg or more.31 It is most likely that propranolol exerts these changes by inhibiting D1 activity in peripheral tissues.
Furthermore, a significant decrease in serum total T3 concentrations was observed in hyperthyroid patients treated with atenolol 100 mg daily, metoprolol 100 mg daily, and alprenolol 100 mg daily, but not with sotalol 80 mg daily or nadolol (up to 240 mg daily).35,36
On the other hand, beta-adrenergic agonists have not been reported to cause significant changes in thyroid function tests.37
SUBCLINICAL THYROTOXICOSIS OR HASHIMOTO THYROIDITIS?
Our patient’s thyroid function test results are more likely due to his nonthyroidal illness and glucocorticoid therapy, as there is no clinical evidence to point to a hypothalamic-pituitary disorder accounting for true central hypothyroidism.
The other options mentioned in question 2 are unlikely to explain our patient’s thyroid function test results.
Subclinical thyrotoxicosis is characterized by suppressed serum TSH, but both serum free T4 and total T3 remain within the normal reference ranges. In addition, the serum TSH level may help to differentiate between thyrotoxicosis and nonthyroidal illness. In the former, serum TSH is usually suppressed (< 0.01 mU/L), whereas in the latter it is usually low but detectable (0.05– 0.3 mU/L).38,39
Hashimoto thyroiditis is a chronic autoimmune thyroid disease characterized by diffuse lymphocytic infiltration of the thyroid gland. Almost all patients with Hashimoto thyroiditis have elevated levels of antibodies to thyroid peroxidase or thyroglobulin.40 Clinically, patients with Hashimoto thyroiditis can either be hypothyroid or have normal thyroid function, which is not the case in our patient.
CASE CONTINUED
An endocrinologist, consulted for a second opinion, agreed that the patient’s thyroid function test results were most likely due to his nonthyroidal illness and glucocorticoid therapy.
3. In view of the endocrinologist’s opinion, which should be the next step in the management of the patient’s thyroid condition?
- Start levothyroxine (T4) therapy
- Start liothyronine (T3) therapy
- Start N-acetylcysteine therapy
- Start thyrotropin-releasing hormone therapy
- Remeasure thyroid hormones after full recovery from his acute illness
It is not clear whether the changes in thyroid hormone levels during an acute illness are a pathologic alteration for which thyroid hormone therapy may be beneficial, or a physiologic adaptation for which such therapy would not be indicated.41
However, current data argue against thyroid hormone therapy using T4 or T3 for patients with nonthyroidal illness syndrome (also called euthyroid sick syndrome).42 Indeed, several randomized controlled trials showed that thyroid hormone therapy is not beneficial in such patients and may be detrimental.41,43
Therapies other than thyroid hormone have been investigated to ameliorate thyroid hormone abnormalities in patients with nonthyroidal illness. These include N-acetylcysteine, thyrotropin-releasing hormone therapy, and nutritional support.
Some studies showed that giving N-acetylcysteine, an antioxidant, increased serum T3 and decreased serum reverse T3 concentrations in patients with acute myocardial infarction.44 Nevertheless, the mortality rate and length of hospitalization were not affected. Further studies are needed to know whether N-acetylcysteine therapy is beneficial for such patients.
Similarly, a study using a thyrotropin-releasing hormone analogue along with growth hormone-releasing peptide 2 showed an increase in serum TSH, T4, and T3 levels in critically ill patients.45 The benefit of this therapy has yet to be determined. On the other hand, early nutritional support was reported to prevent thyroid hormonal changes in patients postoperatively.46
Measuring thyroid hormone levels after full recovery is the most appropriate next step in our patient, as the changes in thyroid hormone concentrations subside as the acute illness resolves.47
CASE CONTINUED
The patient continued to improve. On hospital day 6, he was feeling better but still had mild respiratory distress. There had been no further episodes of arrhythmia since day 4. His blood pressure was 136/86 mm Hg, heart rate 88 beats per minute and regular, respiratory rate 18 breaths per minute, and oral temperature 37.1°C. His oxygen saturation was 92% on room air.
Before discharge, he was encouraged to quit smoking. He was offered behavioral counseling and medication therapy, but he only said that he would think about it. He was discharged on oral cefixime for 4 more days and was instructed to switch to a long-acting bronchodilator along with his other home medications and to return in 1 week to have his thyroid hormones checked.
One week later, his laboratory results were:
- TSH 11.2 mU/L (reference range 0.5–5.0)
- Free T4 1.2 ng/dL (0.9–2.4)
- Total T3 92 ng/dL (80–180).
Clinically, the patient was euthyroid, and examination of his thyroid was unremarkable.
4. Based on these last test results, which statement is correct?
- Levothyroxine therapy should be started
- His serum TSH elevation is most likely transient
- Thyroid ultrasonography is strongly indicated
- A radioactive iodine uptake study should be performed
- Measurement of thyroid-stimulating immunoglobulins is indicated
During recovery from nonthyroidal illness, some patients may have elevated serum TSH levels that are usually transient and modest (< 20 mU/L).48 Normalization of the thyroid function tests including serum TSH may take several weeks49 or months.50 However, a systematic review found that the likelihood of permanent primary hypothyroidism is high in patients with serum TSH levels higher than 20 mU/L during the recovery phase of their nonthyroidal illness.51
Ultrasonography is useful for evaluating patients with thyroid nodules or goiter but is of little benefit for patients like ours, in whom the thyroid is normal on examination.
Similarly, a radioactive iodine uptake study is not indicated, as it is principally used to help differentiate between types of thyrotoxicosis. (Radioactive iodine is also used to treat differentiated thyroid cancer.)
Thyroid-stimulating immunoglobins are TSH receptor-stimulating antibodies that cause Graves disease. Nevertheless, measuring them is not routinely indicated for its diagnosis. However, their measurement is of significant help in the diagnosis of Graves disease if a radioactive iodine uptake study cannot be performed (as in pregnancy) and in atypical presentations such as euthyroid Graves ophthalmopathy.52 Other indications for thyroid-stimulating immunoglobin measurement are beyond the scope of the article. Our patient’s test results are not consistent with hyperthyroidism, so measuring thyroid-stimulating immunoglobins is not indicated.
CASE CONCLUSION: BETTER, BUT STILL SMOKING
The patient missed his 1-month clinic follow-up, but he visited the clinic for follow-up 3 months later. He was feeling well with no complaints. Test results including serum TSH, free T4, and total T3 were within normal ranges. His COPD was under control, with an FEV1 88% of predicted.
He was again encouraged to quit smoking and was offered drug therapy and behavioral counseling, but he declined. In addition, he was instructed to adhere to his annual influenza vaccination.
KEY POINTS
- In patients with acute illness, it is recommended that thyroid function not be assessed unless there is a strong indication.
- If thyroid function assessment is indicated for critically ill patients, serum TSH and free T4 concentrations should be measured. Some clinicians also measure serum total T3 level.
- Thyroid function testing in critically ill patients usually shows low serum total T3, normal or low serum TSH, and normal or low serum free T4.
- Many drugs can alter thyroid hormone levels.
- Thyroid hormone therapy is not recommended for critically ill patients with low T3, low T4, or both.
- During recovery from nonthyroidal illness, some patients may have mild elevation in serum TSH levels (< 20 mU/L).
- Thyroid hormone levels may take several weeks or months to return to normal after the acute illness.
- Patients with serum TSH levels higher than 20 mU/L during the recovery phase of their nonthyroidal illness are more likely to have permanent primary hypothyroidism.
A 66-year-old man presented to the emergency department with increasing shortness of breath and productive cough, which had begun 5 days earlier. Three years previously, he had been diagnosed with chronic obstructive pulmonary disease (COPD).
One week before the current presentation, he developed a sore throat, rhinorrhea, and nasal congestion, and the shortness of breath had started 2 days after that. Although he could speak in sentences, he was breathless even at rest. His dyspnea was associated with noisy breathing and cough productive of yellowish sputum; there was no hemoptysis. He reported fever, but he had no chills, night sweats, chest pain, or paroxysmal nocturnal dyspnea. The review of other systems was unremarkable.
His COPD was known to be mild, in Global Initiative for Chronic Obstructive Lung Disease (GOLD) grade 1, group A. His postbronchodilator ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) was less than 0.70, and his FEV1 was 84% of predicted. Apart from mild intermittent cough with white sputum, his COPD had been under good control with inhaled ipratropium 4 times daily and inhaled albuterol as needed. He said he did not have shortness of breath except when hurrying on level ground or walking up a slight hill (Modified Medical Research Council dyspnea scale grade 1; COPD Assessment Test score < 10). In the last 3 years, he had 2 exacerbations of COPD, 1 year apart, both requiring oral prednisone and antibiotic therapy.
Other relevant history included hypertension and dyslipidemia of 15-year duration, for which he was taking candesartan 16 mg twice daily and atorvastatin 20 mg daily. He was compliant with his medications.
Though he usually received an influenza vaccine every year, he did not get it the previous year. Also, 3 years previously, he received the 23-valent pneumococcal polysaccharide vaccine (PPSV23), and the year before that he received the pneumococcal conjugate vaccine (PCV13). In addition, he was immunized against herpes zoster and tetanus.
The patient had smoked 1 pack per day for the past 38 years. His primary care physician had advised him many times to quit smoking. He had enrolled in a smoking cessation program 2 years previously, in which he received varenicline in addition to behavioral counseling in the form of motivational interviewing and a telephone quit-line. Nevertheless, he continued to smoke.
He was a retired engineer. He did not drink alcohol or use illicit drugs.
PHYSICAL EXAMINATION
On physical examination, the patient was sitting up in bed, leaning forward. He was alert and oriented but was breathing rapidly and looked sick. He had no cyanosis, clubbing, pallor, or jaundice. His blood pressure was 145/90 mm Hg, heart rate 110 beats per minute and regular, respiratory rate 29 breaths per minute, and oral temperature 38.1°C (100.6°F). His oxygen saturation was 88% while breathing room air. His body mass index was 27.1 kg/m2.
His throat was mildly congested. His neck veins were flat, and there were no carotid bruits. His thyroid examination was normal, without goiter, nodules, or tenderness.
Intercostal retractions were noted around the anterolateral costal margins. He had no chest wall deformities. Chest expansion was reduced bilaterally. There was hyperresonance bilaterally. Expiratory wheezes were heard over both lungs, without crackles.
His heart had no murmurs or added sounds. There was no lower-limb edema or swelling. The rest of his physical examination was unremarkable.
Results of initial laboratory testing are shown in Table 1.
Assessment: A 66-year-old man with GOLD grade 1, group A COPD, presenting with a severe exacerbation, most likely due to viral bronchitis.
INITIAL MANAGEMENT
The patient was given oxygen 28% by Venturi mask, and his oxygen saturation went up to 90%. He was started on nebulized albuterol 2.5 mg with ipratropium bromide 500 µg every 4 hours, prednisone 40 mg orally daily for 5 days, and ceftriaxone 1 g intravenously every 24 hours. The first dose of each medication was given in the emergency department.
The patient was then admitted to a progressive care unit, where he was placed on noninvasive positive pressure ventilation, continuous cardiac monitoring, and pulse oximetry. He was started on enoxaparin 40 mg subcutaneously daily to prevent venous thromboembolism, and the oral medications he had been taking at home were continued. Because he was receiving a glucocorticoid, his blood glucose was monitored in the fasting state, 2 hours after each meal, and as needed.
Two hours after he started noninvasive positive pressure ventilation, his arterial blood gases were remeasured and showed the following results:
- pH 7.35
- Partial pressure of carbon dioxide (Paco2) 52 mm Hg
- Bicarbonate 28 mmol/L
- Partial pressure of oxygen (Pao2) 60 mm Hg
- Oxygen saturation 90%.
HOSPITAL COURSE
On hospital day 3, his dyspnea had slightly improved. His respiratory rate was 26 to 28 breaths per minute. His oxygen saturation remained between 90% and 92%.
At 10:21 pm, his cardiac monitor showed an episode of focal atrial tachycardia at a rate of 129 beats per minute that lasted for 3 minutes and 21 seconds, terminating spontaneously. He denied any change in his clinical condition during the episode, with no chest pain, palpitation, or change in dyspnea. There was no change in his vital signs. He had another similar asymptomatic episode lasting 4 minutes and 9 seconds at 6:30 am of hospital day 4.
Because of these episodes, the attending physician ordered thyroid function tests.
THYROID FUNCTION TESTING
1. Which thyroid function test is most likely to be helpful in the assessment of this patient’s thyroid status?
- Serum thyroid-stimulating hormone (TSH) alone
- Serum TSH and total thyroxine (T4)
- Serum TSH and total triiodothyronine (T3)
- Serum TSH and free T4
- Serum TSH and free T3
There are several tests to assess thyroid function: the serum TSH, total T4, free T4, total T3, and free T3 concentrations.1
In normal physiology, TSH from the pituitary stimulates the thyroid gland to produce and secrete T4 and T3, which in turn inhibit TSH secretion through negative feedback. A negative log-linear relation exists between serum free T4 and TSH levels.2 Thus, the serum free T4 level can remain within the normal reference range even if the TSH level is high or low.
TSH assays can have different detection limits. A third-generation TSH assay with a detection limit of 0.01 mU/L is recommended for use in clinical practice.3
TSH testing alone. Given its superior sensitivity and specificity, serum TSH measurement is considered the best single test for assessing thyroid function in most cases.4 Nevertheless, measurement of the serum TSH level alone could be misleading in several situations, eg, hypothalamic or pituitary disorders, recent treatment of thyrotoxicosis, impaired sensitivity to thyroid hormone, and acute nonthyroidal illness.4
Free vs total T4 and T3 levels
Serum total T4 includes a fraction that is bound, mainly to thyroxin-binding globulin, and a very small unbound (free) fraction. The same applies to T3. Only free thyroid hormones represent the “active” fraction available for interaction with their protein receptors in the nucleus.8 Patients with conditions that can affect the thyroid-binding protein concentrations usually have altered serum total T4 and T3 levels, whereas their free hormone concentrations remain normal. Accordingly, measurement of free hormone levels, especially free T4, is usually recommended.
Although equilibrium dialysis is the method most likely to provide an accurate serum free T4 measurement, it is not commonly used because of its limited availability and high cost. Thus, most commercial laboratories use “direct” free T4 measurement or, to a lesser degree, the free T4 index.9 However, none of the currently available free T4 tests actually measure free T4 directly; rather, they estimate it.10
Commercial laboratories can provide a direct free T3 estimate, but it may be less reliable than total T3. If serum T3 measurement is indicated, serum total T3 is usually measured. However, total T3 measurement is rarely indicated for patients with hypothyroidism because it usually remains within the normal reference range.11 Nevertheless, serum total T3 measurement could be useful in patients with T3 toxicosis and in those who are acutely ill.
Accordingly, in acutely ill hospitalized patients like ours, measuring serum TSH using a third-generation assay and free T4 is essential to assess thyroid function. Many clinicians also measure serum total T3.
CASE CONTINUED: LOW TSH, LOW-NORMAL FREE T4, LOW TOTAL T3
The attending physician ordered serum TSH, free T4, and total T3 measurements, which yielded the following:
- TSH 0.1 mU/L (0.5–5.0)
- Total T3 55 ng/dL (80–180)
- Free T4 0.9 ng/dL (0.9–2.4).
2. Which best explains this patient’s abnormal thyroid test results?
- His acute illness
- Central hypothyroidism due to pituitary infarction
- His albuterol therapy
- Subclinical thyrotoxicosis
- Hashimoto thyroiditis
Since euthyroid patients with an acute illness may have abnormal thyroid test results (Table 2),5–7 thyroid function testing is not recommended unless there is a strong indication for it, such as new-onset atrial fibrillation, atrial flutter, or focal atrial tachycardia.1 In such patients, it is important to know whether the test abnormalities represent true thyroid disorder or are the result of a nonthyroidal illness.
Thyroid function testing in patients with nonthyroidal illness usually shows low serum total T3, normal or low serum TSH, and normal, low, or high serum free T4. However, transient mild serum TSH elevation can be seen in some patients during the recovery period.16 These abnormalities with their mechanisms are shown in Table 2.5–7 In several commercial kits, serum direct free T4 can be falsely decreased or increased.8
THE DIFFERENTIAL DIAGNOSIS
Our patient had low serum TSH, low-normal serum direct free T4, and low serum total T3. This profile could be caused by a nonthyroidal illness, “true” central hypothyroidism, or his glucocorticoid treatment. The reason we use the term “true” in this setting is that some experts suggest that the thyroid function test abnormalities in patients with acute nonthyroidal illness represent a transient central hypothyroidism.17 The clinical presentation is key in differentiating true central hypothyroidism from nonthyroidal illness.
In addition, measuring serum cortisol may help to differentiate between the 2 states, as it would be elevated in patients with nonthyroidal illness as part of a stress response but low in patients with true central hypothyroidism, since it is usually part of combined pituitary hormone deficiency.18 Of note, some critically ill patients have low serum cortisol because of transient central adrenal insufficiency.19,20
The serum concentration of reverse T3 has been suggested as a way to differentiate between hypothyroidism (low) and nonthyroidal illness (high); however, further studies showed that it does not reliably differentiate between the conditions.21
GLUCOCORTICOIDS AND THYROID FUNCTION TESTS
By inhibiting D1, glucocorticoids can decrease peripheral conversion of T4 to T3 and thus decrease serum total T3. This effect depends on the type and dose of the glucocorticoid and the duration of therapy.
In one study,22 there was a significant reduction in serum total T3 concentration 24 hours after a single oral dose of dexamethasone 12 mg in normal participants. This effect lasted 48 hours, after which serum total T3 returned to its pretreatment level.
In another study,23 a daily oral dose of betamethasone 1.5 mg for 5 days did not significantly reduce the serum total T3 in healthy volunteers, but a daily dose of 3 mg did. This effect was more pronounced at a daily dose of 4.5 mg, whereas a dose of 6.0 mg had no further effect.
Long-term glucocorticoid therapy also decreases serum total T4 and total T3 by lowering serum thyroid-binding globulin.24
Finally, glucocorticoids can decrease TSH secretion by directly inhibiting thyrotropin-releasing hormone.25,26 However, chronic hypercortisolism, whether endogenous or exogenous, does not cause clinically central hypothyroidism, possibly because of the negative feedback mechanism of low thyroid hormones on the pituitary and the hypothalamus.27
Other drugs including dopamine, dopamine agonists, dobutamine, and somatostatin analogues can suppress serum TSH. As with glucocorticoids, these drugs do not cause clinically evident central hypothyroidism.28 Bexarotene, a retinoid X receptor ligand used in the treatment of cutaneous T-cell lymphoma, has been reported to cause clinically evident central hypothyroidism by suppressing TSH and increasing T4 clearance.29
BETA-BLOCKERS, BETA-AGONISTS AND THYROID FUNCTION
While there is general agreement that beta-adrenergic antagonists (beta-blockers) do not affect the serum TSH concentration, conflicting data have been reported concerning their effect on other thyroid function tests. This may be due to several factors, including dose, duration of therapy, the patient’s thyroid status, and differences in laboratory methodology.30
In studies of propranolol, serum total T4 concentrations did not change or were increased with daily doses of 160 mg or more in both euthyroid participants and hyperthyroid patients31–33; serum total T3 concentrations did not change or were decreased with 40 mg or more daily34; and serum reverse T3 concentrations were increased with daily doses of 80 mg or more.31 It is most likely that propranolol exerts these changes by inhibiting D1 activity in peripheral tissues.
Furthermore, a significant decrease in serum total T3 concentrations was observed in hyperthyroid patients treated with atenolol 100 mg daily, metoprolol 100 mg daily, and alprenolol 100 mg daily, but not with sotalol 80 mg daily or nadolol (up to 240 mg daily).35,36
On the other hand, beta-adrenergic agonists have not been reported to cause significant changes in thyroid function tests.37
SUBCLINICAL THYROTOXICOSIS OR HASHIMOTO THYROIDITIS?
Our patient’s thyroid function test results are more likely due to his nonthyroidal illness and glucocorticoid therapy, as there is no clinical evidence to point to a hypothalamic-pituitary disorder accounting for true central hypothyroidism.
The other options mentioned in question 2 are unlikely to explain our patient’s thyroid function test results.
Subclinical thyrotoxicosis is characterized by suppressed serum TSH, but both serum free T4 and total T3 remain within the normal reference ranges. In addition, the serum TSH level may help to differentiate between thyrotoxicosis and nonthyroidal illness. In the former, serum TSH is usually suppressed (< 0.01 mU/L), whereas in the latter it is usually low but detectable (0.05– 0.3 mU/L).38,39
Hashimoto thyroiditis is a chronic autoimmune thyroid disease characterized by diffuse lymphocytic infiltration of the thyroid gland. Almost all patients with Hashimoto thyroiditis have elevated levels of antibodies to thyroid peroxidase or thyroglobulin.40 Clinically, patients with Hashimoto thyroiditis can either be hypothyroid or have normal thyroid function, which is not the case in our patient.
CASE CONTINUED
An endocrinologist, consulted for a second opinion, agreed that the patient’s thyroid function test results were most likely due to his nonthyroidal illness and glucocorticoid therapy.
3. In view of the endocrinologist’s opinion, which should be the next step in the management of the patient’s thyroid condition?
- Start levothyroxine (T4) therapy
- Start liothyronine (T3) therapy
- Start N-acetylcysteine therapy
- Start thyrotropin-releasing hormone therapy
- Remeasure thyroid hormones after full recovery from his acute illness
It is not clear whether the changes in thyroid hormone levels during an acute illness are a pathologic alteration for which thyroid hormone therapy may be beneficial, or a physiologic adaptation for which such therapy would not be indicated.41
However, current data argue against thyroid hormone therapy using T4 or T3 for patients with nonthyroidal illness syndrome (also called euthyroid sick syndrome).42 Indeed, several randomized controlled trials showed that thyroid hormone therapy is not beneficial in such patients and may be detrimental.41,43
Therapies other than thyroid hormone have been investigated to ameliorate thyroid hormone abnormalities in patients with nonthyroidal illness. These include N-acetylcysteine, thyrotropin-releasing hormone therapy, and nutritional support.
Some studies showed that giving N-acetylcysteine, an antioxidant, increased serum T3 and decreased serum reverse T3 concentrations in patients with acute myocardial infarction.44 Nevertheless, the mortality rate and length of hospitalization were not affected. Further studies are needed to know whether N-acetylcysteine therapy is beneficial for such patients.
Similarly, a study using a thyrotropin-releasing hormone analogue along with growth hormone-releasing peptide 2 showed an increase in serum TSH, T4, and T3 levels in critically ill patients.45 The benefit of this therapy has yet to be determined. On the other hand, early nutritional support was reported to prevent thyroid hormonal changes in patients postoperatively.46
Measuring thyroid hormone levels after full recovery is the most appropriate next step in our patient, as the changes in thyroid hormone concentrations subside as the acute illness resolves.47
CASE CONTINUED
The patient continued to improve. On hospital day 6, he was feeling better but still had mild respiratory distress. There had been no further episodes of arrhythmia since day 4. His blood pressure was 136/86 mm Hg, heart rate 88 beats per minute and regular, respiratory rate 18 breaths per minute, and oral temperature 37.1°C. His oxygen saturation was 92% on room air.
Before discharge, he was encouraged to quit smoking. He was offered behavioral counseling and medication therapy, but he only said that he would think about it. He was discharged on oral cefixime for 4 more days and was instructed to switch to a long-acting bronchodilator along with his other home medications and to return in 1 week to have his thyroid hormones checked.
One week later, his laboratory results were:
- TSH 11.2 mU/L (reference range 0.5–5.0)
- Free T4 1.2 ng/dL (0.9–2.4)
- Total T3 92 ng/dL (80–180).
Clinically, the patient was euthyroid, and examination of his thyroid was unremarkable.
4. Based on these last test results, which statement is correct?
- Levothyroxine therapy should be started
- His serum TSH elevation is most likely transient
- Thyroid ultrasonography is strongly indicated
- A radioactive iodine uptake study should be performed
- Measurement of thyroid-stimulating immunoglobulins is indicated
During recovery from nonthyroidal illness, some patients may have elevated serum TSH levels that are usually transient and modest (< 20 mU/L).48 Normalization of the thyroid function tests including serum TSH may take several weeks49 or months.50 However, a systematic review found that the likelihood of permanent primary hypothyroidism is high in patients with serum TSH levels higher than 20 mU/L during the recovery phase of their nonthyroidal illness.51
Ultrasonography is useful for evaluating patients with thyroid nodules or goiter but is of little benefit for patients like ours, in whom the thyroid is normal on examination.
Similarly, a radioactive iodine uptake study is not indicated, as it is principally used to help differentiate between types of thyrotoxicosis. (Radioactive iodine is also used to treat differentiated thyroid cancer.)
Thyroid-stimulating immunoglobins are TSH receptor-stimulating antibodies that cause Graves disease. Nevertheless, measuring them is not routinely indicated for its diagnosis. However, their measurement is of significant help in the diagnosis of Graves disease if a radioactive iodine uptake study cannot be performed (as in pregnancy) and in atypical presentations such as euthyroid Graves ophthalmopathy.52 Other indications for thyroid-stimulating immunoglobin measurement are beyond the scope of the article. Our patient’s test results are not consistent with hyperthyroidism, so measuring thyroid-stimulating immunoglobins is not indicated.
CASE CONCLUSION: BETTER, BUT STILL SMOKING
The patient missed his 1-month clinic follow-up, but he visited the clinic for follow-up 3 months later. He was feeling well with no complaints. Test results including serum TSH, free T4, and total T3 were within normal ranges. His COPD was under control, with an FEV1 88% of predicted.
He was again encouraged to quit smoking and was offered drug therapy and behavioral counseling, but he declined. In addition, he was instructed to adhere to his annual influenza vaccination.
KEY POINTS
- In patients with acute illness, it is recommended that thyroid function not be assessed unless there is a strong indication.
- If thyroid function assessment is indicated for critically ill patients, serum TSH and free T4 concentrations should be measured. Some clinicians also measure serum total T3 level.
- Thyroid function testing in critically ill patients usually shows low serum total T3, normal or low serum TSH, and normal or low serum free T4.
- Many drugs can alter thyroid hormone levels.
- Thyroid hormone therapy is not recommended for critically ill patients with low T3, low T4, or both.
- During recovery from nonthyroidal illness, some patients may have mild elevation in serum TSH levels (< 20 mU/L).
- Thyroid hormone levels may take several weeks or months to return to normal after the acute illness.
- Patients with serum TSH levels higher than 20 mU/L during the recovery phase of their nonthyroidal illness are more likely to have permanent primary hypothyroidism.
- Lamb EJ, Martin J. Thyroid function tests: often justified in the acutely ill. Ann Clin Biochem 2000; 37(pt 2):158–164. doi:10.1258/0004563001899159
- Spencer CA, LoPresti JS, Patel A, et al. Applications of a new chemiluminometric thyrotropin assay to subnormal measurement. J Clin Endocrinol Metab 1990; 70(2):453–460. doi:10.1210/jcem-70-2-453
- Ross DS, Ardisson LJ, Meskell MJ. Measurement of thyrotropin in clinical and subclinical hyperthyroidism using a new chemiluminescent assay. J Clin Endocrinol Metab 1989; 69(3):684–688. doi:10.1210/jcem-69-3-684
- Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab 2013; 27(6):745–762. doi:10.1016/j.beem.2013.10.003
- Lechan RM, Fekete C. Role of thyroid hormone deiodination in the hypothalamus. Thyroid 2005; 15(8):883–897. doi:10.1089/thy.2005.15.883
- Chopra IJ, Hershman JM, Pardridge WM, Nicoloff JT. Thyroid function in nonthyroidal ilnesses. Ann Intern Med 1983; 98(6):946–957. doi:10.7326/0003-4819-98-6-946
- Chopra IJ, Solomon DH, Hepner HW, Mortenstein AA. Misleadingly low free thyroxine index and usefulness of reverse triiodothyronine measurement in nonthyroidal illnesses. Ann Intern Med 1979; 90(6):905–912. doi:10.7326/0003-4819-90-6-905
- Pontecorvi A, Robbins J. The plasma membrane and thyroid hormone entry into cells. Trends Endocrinol Metab 1989; 1(2):90–94. pmid:18411097
- Hennemann G, Krenning EP. Pitfalls in the interpretation of thyroid function tests in old age and non-thyroidal illness. Horm Res 1987; 26(1–4):100–104. doi:10.1159/000180688
- Baloch Z, Carayon P, Conte-Devolx B, et al; Guidelines Committee, National Academy of Clinical Biochemistry. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 2003; 13(1):3–126. doi:10.1089/105072503321086962
- Lum S, Nicoloff JT, Spencer CA, Kaptein EM. Peripheral tissue mechanism for maintenance of serum triiodothyronine values in a thyroxine-deficient state in man. J Clin Invest 1984; 73(2):570–575. doi:10.1172/JCI111245
- Ortiga-Carvalho TM, Chiamolera MI, Pazos-Moura CC, Wondisford FE. Hypothalamus-pituitary-thyroid axis. Compr Physiol 2016; 6(3):1387–1428. doi:10.1002/cphy.c150027
- de Vries EM, Fliers E, Boelen A. The molecular basis of the non-thyroidal illness syndrome. J Endocrinol 2015; 225(3):R67–R81. doi:10.1530/JOE-15-0133
- Chopra IJ, Huang TS, Beredo A, Solomon DH, Teco GN, Mean JF. Evidence for an inhibitor of extrathyroidal conversion of thyroxine to 3, 5, 3'-triiodothyronine in sera of patients with nonthyroidal illnesses. J Clin Endocrinol Metab 1985; 60(4):666–672. doi:10.1210/jcem-60-4-666
- Peeters RP, Debaveye Y, Fliers E, Visser TJ. Changes within the thyroid axis during critical illness. Crit Care Clin 2006; 22(1):41–55. doi:10.1016/j.ccc.2005.08.006
- Spencer C, Eigen A, Shen D, et al. Specificity of sensitive assays of thyrotropin (TSH) used to screen for thyroid disease in hospitalized patients. Clin Chem 1987; 33(8):1391–1396. pmid:3301067
- Adler SM, Wartofsky L. The nonthyroidal illness syndrome. Endocrinol Metab Clin North Am 2007; 36(3):657–672. doi:10.1016/j.ecl.2007.04.007
- Persani L. Central hypothyroidism: pathogenic, diagnostic, and therapeutic challenges. J Clin Endocrinol Metab 2012; 97(9):3068–3078. doi:10.1210/jc.2012-1616
- Kidess AI, Caplan RH, Reynertson RH, Wickus GG, Goodnough DE. Transient corticotropin deficiency in critical illness. Mayo Clin Proc 1993; 68(5):435–441. doi:10.1016/s0025-6196(12)60188-8
- Lamberts SW, Bruining HA, De Jong FH. Corticosteroid therapy in severe illness. N Engl J Med 1997; 337(18):1285–1292. doi:10.1056/NEJM199710303371807
- Burmeister LA. Reverse T3 does not reliably differentiate hypothyroid sick syndrome from euthyroid sick syndrome. Thyroid 1995; 5(6):435–441. doi:10.1089/thy.1995.5.435
- Duick DS, Warren DW, Nicoloff JT, Otis CL, Croxson MS. Effect of single dose dexamethasone on the concentration of serum triiodothyronine in man. J Clin Endocrinol Metab 1974; 39(6):1151–1154. doi:10.1210/jcem-39-6-1151
- Gamstedt A, Järnerot G, Kågedal B. Dose related effects of betamethasone on iodothyronines and thyroid hormone-binding proteins in serum. Acta Endocrinol (Copenh) 1981; 96(4):484–490. doi:10.1530/acta.0.0960484
- Wartofsky L, Burman KD. Alterations in thyroid function in patients with systemic illness: the “euthyroid sick syndrome.” Endocr Rev 1982; 3(2):164–217. doi:10.1210/edrv-3-2-164
- Wilber JF, Utiger RD. The effect of glucocorticoids on thyrotropin secretion. J Clin Invest 1969; 48(11):2096–2103. doi:10.1172/JCI106176
- Nicoloff JT, Fisher DA, Appleman MD Jr. The role of glucocorticoids in the regulation of thyroid function in man. J Clin Invest 1970; 49(10):1922–1929. doi:10.1172/JCI106411
- Surks MI, Sievert R. Drugs and thyroid function. N Engl J Med 1995; 333(25):1688–1694. doi:10.1056/NEJM199512213332507
- Haugen BR. Drugs that suppress TSH or cause central hypothyroidism. Best Pract Res Clin Endocrinol Metab 2009; 23(6):793–800. doi:10.1016/j.beem.2009.08.003
- Sherman SI, Gopal J, Haugen BR, et al. Central hypothyroidism associated with retinoid X receptor–selective ligands. N Engl J Med 1999; 340(14):1075–1079. doi:10.1056/NEJM199904083401404
- Murchison LE, How J, Bewsher PD. Comparison of propranolol and metoprolol in the management of hyperthyroidism. Br J Clin Pharmacol 1979; 8(6):581–587. doi:10.1111/j.1365-2125.1979.tb01048.x
- Faber J, Friis T, Kirkegaard C, et al. Serum T4, T3 and reverse T3 during treatment with propranolol in hyperthyroidism, L-T4 treated myxedema and in normal man. Horm Metab Res 1979; 11(1):34–36. doi:10.1055/s-0028-1092678
- Kristensen BO, Weeke J. Propranolol-induced increments in total and free serum thyroxine in patients with essential hypertension. Clin Pharmacol Ther 1977; 22(6):864–867. doi:10.1002/cpt1977226864
- Murchison LE, Bewsher PD, Chesters MI, Ferrier WR. Comparison of propranolol and practolol in the management of hyperthyroidism. Br J Clin Pharmacol 1976; 3(2):273–277. doi:10.1111/j.1365-2125.1976.tb00603.x
- Lotti G, Delitala G, Devilla L, Alagna S, Masala A. Reduction of plasma triiodothyronine (T3) induced by propranolol. Clin Endocrinol 1977; 6(6):405–410. doi:10.1111/j.1365-2265.1977.tb03322.x
- Perrild H, Hansen JM, Skovsted L, Christensen LK. Different effects of propranolol, alprenolol, sotalol, atenolol and metoprolol on serum T3 and serum rT3 in hyperthyroidism. Clin Endocrinol (Oxf) 1983; 18(2):139–142. pmid:6133659
- Reeves RA, From GL, Paul W, Leenen FH. Nadolol, propranolol, and thyroid hormones: evidence for a membrane-stabilizing action of propranolol. Clin Pharmacol Ther 1985; 37(2):157–161. doi:10.1038/clpt.1985.28
- Walker N, Jung RT, Jennings G, James WP. The effect of a beta-receptor agonist (salbutamol) on peripheral thyroid metabolism in euthyroid subjects. Horm Metab Res 1981; 13(10):590–591. doi:10.1055/s-2007-1019346
- Melmed S, Geola FL, Reed AW, Pekary AE, Park J, Hershman JM. A comparison of methods for assessing thyroid function in nonthyroidal illness. J Clin Endocrinol Metab 1982; 54(2):300–306. doi:10.1210/jcem-54-2-300
- Docter R, Krenning E, De Jong M, Hennemann G. The sick euthyroid syndrome: changes in thyroid hormone serum parameters and hormone metabolism. Clin Endocrinol (Oxf) 1993; 39(5):499–518. pmid:8252737
- Mariotti S, Caturegli P, Piccolo P, Barbesino G, Pinchera A. Antithyroid peroxidase autoantibodies in thyroid diseases. J Clin Endocrinol Metab 1990; 71(3):661–669. doi:10.1210/jcem-71-3-661
- De Groot LJ. Non-thyroidal illness syndrome is a manifestation of hypothalamic-pituitary dysfunction, and in view of current evidence, should be treated with appropriate replacement therapies. Crit Care Clin 2006; 22(1):57–86. doi:10.1016/j.ccc.2005.10.001
- Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 2014; 24(12):1670–1751. doi:10.1089/thy.2014.0028
- Kaptein EM, Beale E, Chan LS. Thyroid hormone therapy for obesity and nonthyroidal illnesses: a systematic review. J Clin Endocrinol Metab 2009; 94(10):3663–3675. doi:10.1210/jc.2009-0899
- Vidart J, Wajner SM, Leite RS, et al. N-acetylcysteine administration prevents nonthyroidal illness syndrome in patients with acute myocardial infarction: a randomized clinical trial. J Clin Endocrinol Metab 2014; 99(12):4537–4545. doi:10.1210/jc.2014-2192
- Van den Berghe G, Wouters P, Weekers F, et al. Reactivation of pituitary hormone release and metabolic improvement by infusion of growth hormone-releasing peptide and thyrotropin-releasing hormone in patients with protracted critical illness. J Clin Endocrinol Metab 1999; 84(4):1311–1323. doi:10.1210/jcem.84.4.5636
- Langouche L, Vander Perre S, Marques M, et al. Impact of early nutrient restriction during critical illness on the nonthyroidal illness syndrome and its relation with outcome: a randomized, controlled clinical study. J Clin Endocrinol Metab 2013; 98(3):1006–1013. doi:10.1210/jc.2012-2809
- Economidou F, Douka E, Tzanela M, Nanas S, Kotanidou A. Thyroid function during critical illness. Hormones (Athens) 2011; 10(2):117–124. doi:10.14310/horm.2002.1301
- Hamblin PS, Dyer SA, Mohr VS, et al. Relationship between thyrotropin and thyroxine changes during recovery from severe hypothyroxinemia of critical illness. J Clin Endocrinol Metab 1986; 62(4):717–722. doi:10.1210/jcem-62-4-717
- Iglesias P, Diez JJ. Thyroid dysfunction and kidney disease. Eur J Endocrinol 2009; 160(4):503–515. doi:10.1530/EJE-08-0837
- Spencer CA. Clinical utility and cost-effectiveness of sensitive thyrotropin assays in ambulatory and hospitalized patients. Mayo Clin Proc 1988; 63(12):1214–1222. doi:10.1016/s0025-6196(12)65408-1
- Attia J, Margetts P, Guyatt G. Diagnosis of thyroid disease in hospitalized patients: a systematic review. Arch Intern Med 1999; 159(7):658–665. pmid:10218744
- Barbesino G, Tomer Y. Clinical review: clinical utility of TSH receptor antibodies. J Clin Endocrinol Metab 2013; 98(6):2247–2255. doi:10.1210/jc.2012-4309
- Lamb EJ, Martin J. Thyroid function tests: often justified in the acutely ill. Ann Clin Biochem 2000; 37(pt 2):158–164. doi:10.1258/0004563001899159
- Spencer CA, LoPresti JS, Patel A, et al. Applications of a new chemiluminometric thyrotropin assay to subnormal measurement. J Clin Endocrinol Metab 1990; 70(2):453–460. doi:10.1210/jcem-70-2-453
- Ross DS, Ardisson LJ, Meskell MJ. Measurement of thyrotropin in clinical and subclinical hyperthyroidism using a new chemiluminescent assay. J Clin Endocrinol Metab 1989; 69(3):684–688. doi:10.1210/jcem-69-3-684
- Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab 2013; 27(6):745–762. doi:10.1016/j.beem.2013.10.003
- Lechan RM, Fekete C. Role of thyroid hormone deiodination in the hypothalamus. Thyroid 2005; 15(8):883–897. doi:10.1089/thy.2005.15.883
- Chopra IJ, Hershman JM, Pardridge WM, Nicoloff JT. Thyroid function in nonthyroidal ilnesses. Ann Intern Med 1983; 98(6):946–957. doi:10.7326/0003-4819-98-6-946
- Chopra IJ, Solomon DH, Hepner HW, Mortenstein AA. Misleadingly low free thyroxine index and usefulness of reverse triiodothyronine measurement in nonthyroidal illnesses. Ann Intern Med 1979; 90(6):905–912. doi:10.7326/0003-4819-90-6-905
- Pontecorvi A, Robbins J. The plasma membrane and thyroid hormone entry into cells. Trends Endocrinol Metab 1989; 1(2):90–94. pmid:18411097
- Hennemann G, Krenning EP. Pitfalls in the interpretation of thyroid function tests in old age and non-thyroidal illness. Horm Res 1987; 26(1–4):100–104. doi:10.1159/000180688
- Baloch Z, Carayon P, Conte-Devolx B, et al; Guidelines Committee, National Academy of Clinical Biochemistry. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 2003; 13(1):3–126. doi:10.1089/105072503321086962
- Lum S, Nicoloff JT, Spencer CA, Kaptein EM. Peripheral tissue mechanism for maintenance of serum triiodothyronine values in a thyroxine-deficient state in man. J Clin Invest 1984; 73(2):570–575. doi:10.1172/JCI111245
- Ortiga-Carvalho TM, Chiamolera MI, Pazos-Moura CC, Wondisford FE. Hypothalamus-pituitary-thyroid axis. Compr Physiol 2016; 6(3):1387–1428. doi:10.1002/cphy.c150027
- de Vries EM, Fliers E, Boelen A. The molecular basis of the non-thyroidal illness syndrome. J Endocrinol 2015; 225(3):R67–R81. doi:10.1530/JOE-15-0133
- Chopra IJ, Huang TS, Beredo A, Solomon DH, Teco GN, Mean JF. Evidence for an inhibitor of extrathyroidal conversion of thyroxine to 3, 5, 3'-triiodothyronine in sera of patients with nonthyroidal illnesses. J Clin Endocrinol Metab 1985; 60(4):666–672. doi:10.1210/jcem-60-4-666
- Peeters RP, Debaveye Y, Fliers E, Visser TJ. Changes within the thyroid axis during critical illness. Crit Care Clin 2006; 22(1):41–55. doi:10.1016/j.ccc.2005.08.006
- Spencer C, Eigen A, Shen D, et al. Specificity of sensitive assays of thyrotropin (TSH) used to screen for thyroid disease in hospitalized patients. Clin Chem 1987; 33(8):1391–1396. pmid:3301067
- Adler SM, Wartofsky L. The nonthyroidal illness syndrome. Endocrinol Metab Clin North Am 2007; 36(3):657–672. doi:10.1016/j.ecl.2007.04.007
- Persani L. Central hypothyroidism: pathogenic, diagnostic, and therapeutic challenges. J Clin Endocrinol Metab 2012; 97(9):3068–3078. doi:10.1210/jc.2012-1616
- Kidess AI, Caplan RH, Reynertson RH, Wickus GG, Goodnough DE. Transient corticotropin deficiency in critical illness. Mayo Clin Proc 1993; 68(5):435–441. doi:10.1016/s0025-6196(12)60188-8
- Lamberts SW, Bruining HA, De Jong FH. Corticosteroid therapy in severe illness. N Engl J Med 1997; 337(18):1285–1292. doi:10.1056/NEJM199710303371807
- Burmeister LA. Reverse T3 does not reliably differentiate hypothyroid sick syndrome from euthyroid sick syndrome. Thyroid 1995; 5(6):435–441. doi:10.1089/thy.1995.5.435
- Duick DS, Warren DW, Nicoloff JT, Otis CL, Croxson MS. Effect of single dose dexamethasone on the concentration of serum triiodothyronine in man. J Clin Endocrinol Metab 1974; 39(6):1151–1154. doi:10.1210/jcem-39-6-1151
- Gamstedt A, Järnerot G, Kågedal B. Dose related effects of betamethasone on iodothyronines and thyroid hormone-binding proteins in serum. Acta Endocrinol (Copenh) 1981; 96(4):484–490. doi:10.1530/acta.0.0960484
- Wartofsky L, Burman KD. Alterations in thyroid function in patients with systemic illness: the “euthyroid sick syndrome.” Endocr Rev 1982; 3(2):164–217. doi:10.1210/edrv-3-2-164
- Wilber JF, Utiger RD. The effect of glucocorticoids on thyrotropin secretion. J Clin Invest 1969; 48(11):2096–2103. doi:10.1172/JCI106176
- Nicoloff JT, Fisher DA, Appleman MD Jr. The role of glucocorticoids in the regulation of thyroid function in man. J Clin Invest 1970; 49(10):1922–1929. doi:10.1172/JCI106411
- Surks MI, Sievert R. Drugs and thyroid function. N Engl J Med 1995; 333(25):1688–1694. doi:10.1056/NEJM199512213332507
- Haugen BR. Drugs that suppress TSH or cause central hypothyroidism. Best Pract Res Clin Endocrinol Metab 2009; 23(6):793–800. doi:10.1016/j.beem.2009.08.003
- Sherman SI, Gopal J, Haugen BR, et al. Central hypothyroidism associated with retinoid X receptor–selective ligands. N Engl J Med 1999; 340(14):1075–1079. doi:10.1056/NEJM199904083401404
- Murchison LE, How J, Bewsher PD. Comparison of propranolol and metoprolol in the management of hyperthyroidism. Br J Clin Pharmacol 1979; 8(6):581–587. doi:10.1111/j.1365-2125.1979.tb01048.x
- Faber J, Friis T, Kirkegaard C, et al. Serum T4, T3 and reverse T3 during treatment with propranolol in hyperthyroidism, L-T4 treated myxedema and in normal man. Horm Metab Res 1979; 11(1):34–36. doi:10.1055/s-0028-1092678
- Kristensen BO, Weeke J. Propranolol-induced increments in total and free serum thyroxine in patients with essential hypertension. Clin Pharmacol Ther 1977; 22(6):864–867. doi:10.1002/cpt1977226864
- Murchison LE, Bewsher PD, Chesters MI, Ferrier WR. Comparison of propranolol and practolol in the management of hyperthyroidism. Br J Clin Pharmacol 1976; 3(2):273–277. doi:10.1111/j.1365-2125.1976.tb00603.x
- Lotti G, Delitala G, Devilla L, Alagna S, Masala A. Reduction of plasma triiodothyronine (T3) induced by propranolol. Clin Endocrinol 1977; 6(6):405–410. doi:10.1111/j.1365-2265.1977.tb03322.x
- Perrild H, Hansen JM, Skovsted L, Christensen LK. Different effects of propranolol, alprenolol, sotalol, atenolol and metoprolol on serum T3 and serum rT3 in hyperthyroidism. Clin Endocrinol (Oxf) 1983; 18(2):139–142. pmid:6133659
- Reeves RA, From GL, Paul W, Leenen FH. Nadolol, propranolol, and thyroid hormones: evidence for a membrane-stabilizing action of propranolol. Clin Pharmacol Ther 1985; 37(2):157–161. doi:10.1038/clpt.1985.28
- Walker N, Jung RT, Jennings G, James WP. The effect of a beta-receptor agonist (salbutamol) on peripheral thyroid metabolism in euthyroid subjects. Horm Metab Res 1981; 13(10):590–591. doi:10.1055/s-2007-1019346
- Melmed S, Geola FL, Reed AW, Pekary AE, Park J, Hershman JM. A comparison of methods for assessing thyroid function in nonthyroidal illness. J Clin Endocrinol Metab 1982; 54(2):300–306. doi:10.1210/jcem-54-2-300
- Docter R, Krenning E, De Jong M, Hennemann G. The sick euthyroid syndrome: changes in thyroid hormone serum parameters and hormone metabolism. Clin Endocrinol (Oxf) 1993; 39(5):499–518. pmid:8252737
- Mariotti S, Caturegli P, Piccolo P, Barbesino G, Pinchera A. Antithyroid peroxidase autoantibodies in thyroid diseases. J Clin Endocrinol Metab 1990; 71(3):661–669. doi:10.1210/jcem-71-3-661
- De Groot LJ. Non-thyroidal illness syndrome is a manifestation of hypothalamic-pituitary dysfunction, and in view of current evidence, should be treated with appropriate replacement therapies. Crit Care Clin 2006; 22(1):57–86. doi:10.1016/j.ccc.2005.10.001
- Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 2014; 24(12):1670–1751. doi:10.1089/thy.2014.0028
- Kaptein EM, Beale E, Chan LS. Thyroid hormone therapy for obesity and nonthyroidal illnesses: a systematic review. J Clin Endocrinol Metab 2009; 94(10):3663–3675. doi:10.1210/jc.2009-0899
- Vidart J, Wajner SM, Leite RS, et al. N-acetylcysteine administration prevents nonthyroidal illness syndrome in patients with acute myocardial infarction: a randomized clinical trial. J Clin Endocrinol Metab 2014; 99(12):4537–4545. doi:10.1210/jc.2014-2192
- Van den Berghe G, Wouters P, Weekers F, et al. Reactivation of pituitary hormone release and metabolic improvement by infusion of growth hormone-releasing peptide and thyrotropin-releasing hormone in patients with protracted critical illness. J Clin Endocrinol Metab 1999; 84(4):1311–1323. doi:10.1210/jcem.84.4.5636
- Langouche L, Vander Perre S, Marques M, et al. Impact of early nutrient restriction during critical illness on the nonthyroidal illness syndrome and its relation with outcome: a randomized, controlled clinical study. J Clin Endocrinol Metab 2013; 98(3):1006–1013. doi:10.1210/jc.2012-2809
- Economidou F, Douka E, Tzanela M, Nanas S, Kotanidou A. Thyroid function during critical illness. Hormones (Athens) 2011; 10(2):117–124. doi:10.14310/horm.2002.1301
- Hamblin PS, Dyer SA, Mohr VS, et al. Relationship between thyrotropin and thyroxine changes during recovery from severe hypothyroxinemia of critical illness. J Clin Endocrinol Metab 1986; 62(4):717–722. doi:10.1210/jcem-62-4-717
- Iglesias P, Diez JJ. Thyroid dysfunction and kidney disease. Eur J Endocrinol 2009; 160(4):503–515. doi:10.1530/EJE-08-0837
- Spencer CA. Clinical utility and cost-effectiveness of sensitive thyrotropin assays in ambulatory and hospitalized patients. Mayo Clin Proc 1988; 63(12):1214–1222. doi:10.1016/s0025-6196(12)65408-1
- Attia J, Margetts P, Guyatt G. Diagnosis of thyroid disease in hospitalized patients: a systematic review. Arch Intern Med 1999; 159(7):658–665. pmid:10218744
- Barbesino G, Tomer Y. Clinical review: clinical utility of TSH receptor antibodies. J Clin Endocrinol Metab 2013; 98(6):2247–2255. doi:10.1210/jc.2012-4309
Are Pediatric Readmission Reduction Efforts Falling Flat?
In an effort to improve healthcare for Americans by linking hospital payments to quality of care, Medicare’s Hospital Readmission Reduction Program (HRRP) began penalizing hospitals with “excess” readmission rates in 2012. The decision sparked widespread debate about the definition of a preventable readmission and whether a patient’s socioeconomic status should be considered for risk adjustment. Although coming back to the hospital after an admission is an undesirable outcome for any patient, the suitability of readmission as a quality measure remains a hot and debated topic. Research on the subject skyrocketed; over 12000 articles about hospital readmissions have been indexed in PubMed since 2000, and the number of publications has steadily increased since 2010 (Figure).
Although the HRRP is a Medicare initiative, there has been a substantial focus on readmissions in pediatrics as well. The National Quality Forum has endorsed three quality measures specific to readmission in children: (1) the rate of unplanned readmissions to the pediatric intensive care unit within 24 hours after discharge or transfer, (2) the pediatric lower respiratory infection readmission measure, defined as the percentage of admissions followed by one or more readmissions within 30 days of hospitalization for lower respiratory infection, and (3) the pediatric all-cause readmission measure, defined as the percentage of admissions followed by one or more readmissions within 30 days. These endorsements were preceded by studies showing that pediatric readmission rates varied substantially across hospitals and clinical conditions, and that children with chronic illnesses were at the highest risk.
Readmission is an attractive pediatric quality measure for a number of reasons. This measure is easy to apply to data at the hospital, health system, and payor levels at relatively low cost. Relatedly, the all-condition measure can be applied to all pediatric hospitalizations, overcoming the very real challenge in pediatric quality measurement of inadequate sample sizes to discern differences in healthcare quality at the hospital level for many disease-specific measures.1 In addition, this measure moves beyond process measurement to quantify an outcome relevant to families as well as healthcare systems. Finally, the measure is founded on a compelling conceptual framework (albeit one that remains challenging to prove) that efforts to improve a patient’s hospital-to-home transition and discharge readiness will reduce their likelihood of readmission.
In this issue of the Journal of Hospital Medicine, Katherine Auger and colleagues present their analysis of pediatric readmission rates from 2010 to 2016 across 66 children’s hospitals.2 They found that the median seven-day all-cause pediatric readmission rate was 5.1%, with no change in rates over the seven-year study period. Applying proprietary software to identify potentially preventable readmissions (PPR), they reported that approximately 40% of these readmissions may be preventable, a proportion that was also unchanged over time. Interestingly, 88% of the hospitals represented in their data were participating in the Solutions for Patient Safety national learning collaborative during the study period, making efforts to reduce seven-day readmission rates. Despite this, the figures presented in this paper of all-condition and potentially preventable readmission rates over time are very, very flat.
This work by Auger et al. contributes to our understanding about the preventability, or lack thereof, of pediatric all-condition readmissions. If 40% of these readmissions are indeed preventable, then why did Auger et al. not observe a declining proportion of PPR over time as a result of hospital participation in a national collaborative? Past quantitative and qualitative studies provide important context. First, the 40% rate of readmission preventability is twofold higher than that reported in past studies that relied on physician judgement to determine readmission preventability;3,4 the authors’ use of proprietary software to categorize the preventability of a readmission limits our ability to explain the differences in these rates. However, in these past studies, the rates of initial agreement between physician reviewers about readmission preventability were poor, highlighting the challenges associated with determining readmission preventability. Moreover, qualitative studies suggest that physicians and families lack a shared understanding of the preventability of readmissions.5 Finally, a systematic review of pediatric hospital discharge interventions did not identify any one intervention that was consistently effective in reducing hospital readmission rates.6 The following important questions remain: Were hospitals’ efforts to reduce PPR targeting the wrong patients? Were the interventions insufficient or ineffective? Or are readmission measures insufficiently sensitive to improved processes of care?
Recognizing that the majority of research on readmission as well as HRRP penalties focuses on adult populations, perhaps we can apply some lessons learned from the HRRP to pediatrics. Recent analyses by Medicare Payment Advisory Commission (MedPAC) suggest that raw and risk-adjusted readmission rates have declined for conditions covered by the HRRP, with readmission rates for HRRP target conditions declining more quickly than that for nontarget conditions.7 Just as the HRRP has focused on target conditions with relatively high readmission rates, analogous efforts to focus pediatric readmission reduction on children at greatest risk may enable measurement of change over time. For example, although children with complex chronic medical conditions represent a small proportion of the pediatric population, they account for 60% of all pediatric readmissions in the United States. However, similar to the above-described meta-analysis of readmission reduction efforts in children, at least one meta-analysis has demonstrated that there is no one intervention or even bundle of interventions that has consistently reduced readmissions in adults.8 Although the readmission rates for HRRP target conditions have decreased, the results of clinical trials evaluating readmission reduction efforts are difficult to translate into practice given substantial heterogeneity in study designs, interventions, and patient populations.
Does this study by Auger et al. suggest that pediatric readmission reduction efforts are misguided or futile? No. But it does provide compelling data that efforts to reduce all-cause readmissions for all children may not yield measureable changes using the current measures. A narrowed focus on children with chronic illnesses, who account for approximately half of all pediatric admissions, may be warranted. A number of studies have summarized families’ preferences regarding their hospital-to-home transitions; the results indicate that families of children with chronic illness have unique desires and needs.9,10 Perhaps it is time to take a step back from pediatric readmission reduction efforts, largely inspired by the HRRP, and redirect our resources to implement and evaluate processes and outcomes most valued by children and their families.
Disclosures
Drs. Lagu and Lindenauer have served as consultants for the Yale Center for Outcomes Research and Evaluation (under contract to the Centers for Medicare and Medicaid Services) providing clinical and methodological expertise and input on the development, reevaluation, and implementation of hospital outcome and efficiency measures.
Funding
Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award R01 HL139985-01A1 and 1R01HL146884-01. Dr. Lindenauer was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K24HL132008.
Disclaimer
The views expressed in this manuscript do not necessarily reflect those of the Yale Center for Outcomes Research and Evaluation or the Centers for Medicare and Medicaid Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
1. Berry JG, Zaslavsky AM, Toomey SL, et al. Recognizing differences in hospital quality performance for pediatric inpatient care. Pediatrics. 2015;136(2):251-262. https://doi.org/10.1542/peds.2014-3131.
2. Auger K, Harris M, Gay J, et al. Progress (?) towards reducing pediatric readmissions. J Hosp Med. 2019;14(10):618-621. https://doi.org/10.12788/jhm.3210
3. Hain PD, Gay JC, Berutti TW, Whitney GM, Wang W, Saville BR. Preventability of early readmissions at a children’s hospital. Pediatrics. 2013;131(1):e171-e181. https://doi.org/10.1542/peds.2012-0820.
4. Wallace SS, Keller SL, Falco CN, et al. An examination of physician-, caregiver-, and disease-related factors associated with readmission from a pediatric hospital medicine service. Hosp Pediatr. 2015;5(11):566-573. https://doi.org/10.1542/hpeds.2015-0015.
5. Brittan M, Albright K, Cifuentes M, Jimenez-Zambrano A, Kempe A. Parent and provider perspectives on pediatric readmissions: what can we learn about readiness for discharge?. Hosp Pediatr. 2015;5(11):559-565. https://doi.org/10.1542/hpeds.2015-0034.
6. Auger K, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9(4):251-260. https://doi.org/10.1002/jhm.2134.
7. NEJM Catalyst. Hospital Readmissions Reduction Program (HRRP). Available at: https://catalyst.nejm.org/hospital-readmissions-reduction-program-hrrp/. Accessed May 21, 2019.
8. Hansen L, Young R, Hinami K, Leung A, Williams M. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. https://doi.org/10.7326/0003-4819-155-8-201110180-00008.
9. Leyenaar J, O’Brien E, Leslie L, Lindenauer P, Mangione-Smith R. Families’ priorities regarding hospital-to-home transitions for children with medical complexity. Pediatrics. 2017;139(1): e20161581. https://doi.org/10.1542/peds.2016-1581.
10. Desai AD, Durkin LK, Jacob-Files EA, Mangione-Smith R. Caregiver perceptions of hospital to home transitions according to medical complexity: a qualitative study. Acad Pediatr. 2016;16(2):136-144. https://doi.org/10.1016/j.acap.2015.08.003.
In an effort to improve healthcare for Americans by linking hospital payments to quality of care, Medicare’s Hospital Readmission Reduction Program (HRRP) began penalizing hospitals with “excess” readmission rates in 2012. The decision sparked widespread debate about the definition of a preventable readmission and whether a patient’s socioeconomic status should be considered for risk adjustment. Although coming back to the hospital after an admission is an undesirable outcome for any patient, the suitability of readmission as a quality measure remains a hot and debated topic. Research on the subject skyrocketed; over 12000 articles about hospital readmissions have been indexed in PubMed since 2000, and the number of publications has steadily increased since 2010 (Figure).
Although the HRRP is a Medicare initiative, there has been a substantial focus on readmissions in pediatrics as well. The National Quality Forum has endorsed three quality measures specific to readmission in children: (1) the rate of unplanned readmissions to the pediatric intensive care unit within 24 hours after discharge or transfer, (2) the pediatric lower respiratory infection readmission measure, defined as the percentage of admissions followed by one or more readmissions within 30 days of hospitalization for lower respiratory infection, and (3) the pediatric all-cause readmission measure, defined as the percentage of admissions followed by one or more readmissions within 30 days. These endorsements were preceded by studies showing that pediatric readmission rates varied substantially across hospitals and clinical conditions, and that children with chronic illnesses were at the highest risk.
Readmission is an attractive pediatric quality measure for a number of reasons. This measure is easy to apply to data at the hospital, health system, and payor levels at relatively low cost. Relatedly, the all-condition measure can be applied to all pediatric hospitalizations, overcoming the very real challenge in pediatric quality measurement of inadequate sample sizes to discern differences in healthcare quality at the hospital level for many disease-specific measures.1 In addition, this measure moves beyond process measurement to quantify an outcome relevant to families as well as healthcare systems. Finally, the measure is founded on a compelling conceptual framework (albeit one that remains challenging to prove) that efforts to improve a patient’s hospital-to-home transition and discharge readiness will reduce their likelihood of readmission.
In this issue of the Journal of Hospital Medicine, Katherine Auger and colleagues present their analysis of pediatric readmission rates from 2010 to 2016 across 66 children’s hospitals.2 They found that the median seven-day all-cause pediatric readmission rate was 5.1%, with no change in rates over the seven-year study period. Applying proprietary software to identify potentially preventable readmissions (PPR), they reported that approximately 40% of these readmissions may be preventable, a proportion that was also unchanged over time. Interestingly, 88% of the hospitals represented in their data were participating in the Solutions for Patient Safety national learning collaborative during the study period, making efforts to reduce seven-day readmission rates. Despite this, the figures presented in this paper of all-condition and potentially preventable readmission rates over time are very, very flat.
This work by Auger et al. contributes to our understanding about the preventability, or lack thereof, of pediatric all-condition readmissions. If 40% of these readmissions are indeed preventable, then why did Auger et al. not observe a declining proportion of PPR over time as a result of hospital participation in a national collaborative? Past quantitative and qualitative studies provide important context. First, the 40% rate of readmission preventability is twofold higher than that reported in past studies that relied on physician judgement to determine readmission preventability;3,4 the authors’ use of proprietary software to categorize the preventability of a readmission limits our ability to explain the differences in these rates. However, in these past studies, the rates of initial agreement between physician reviewers about readmission preventability were poor, highlighting the challenges associated with determining readmission preventability. Moreover, qualitative studies suggest that physicians and families lack a shared understanding of the preventability of readmissions.5 Finally, a systematic review of pediatric hospital discharge interventions did not identify any one intervention that was consistently effective in reducing hospital readmission rates.6 The following important questions remain: Were hospitals’ efforts to reduce PPR targeting the wrong patients? Were the interventions insufficient or ineffective? Or are readmission measures insufficiently sensitive to improved processes of care?
Recognizing that the majority of research on readmission as well as HRRP penalties focuses on adult populations, perhaps we can apply some lessons learned from the HRRP to pediatrics. Recent analyses by Medicare Payment Advisory Commission (MedPAC) suggest that raw and risk-adjusted readmission rates have declined for conditions covered by the HRRP, with readmission rates for HRRP target conditions declining more quickly than that for nontarget conditions.7 Just as the HRRP has focused on target conditions with relatively high readmission rates, analogous efforts to focus pediatric readmission reduction on children at greatest risk may enable measurement of change over time. For example, although children with complex chronic medical conditions represent a small proportion of the pediatric population, they account for 60% of all pediatric readmissions in the United States. However, similar to the above-described meta-analysis of readmission reduction efforts in children, at least one meta-analysis has demonstrated that there is no one intervention or even bundle of interventions that has consistently reduced readmissions in adults.8 Although the readmission rates for HRRP target conditions have decreased, the results of clinical trials evaluating readmission reduction efforts are difficult to translate into practice given substantial heterogeneity in study designs, interventions, and patient populations.
Does this study by Auger et al. suggest that pediatric readmission reduction efforts are misguided or futile? No. But it does provide compelling data that efforts to reduce all-cause readmissions for all children may not yield measureable changes using the current measures. A narrowed focus on children with chronic illnesses, who account for approximately half of all pediatric admissions, may be warranted. A number of studies have summarized families’ preferences regarding their hospital-to-home transitions; the results indicate that families of children with chronic illness have unique desires and needs.9,10 Perhaps it is time to take a step back from pediatric readmission reduction efforts, largely inspired by the HRRP, and redirect our resources to implement and evaluate processes and outcomes most valued by children and their families.
Disclosures
Drs. Lagu and Lindenauer have served as consultants for the Yale Center for Outcomes Research and Evaluation (under contract to the Centers for Medicare and Medicaid Services) providing clinical and methodological expertise and input on the development, reevaluation, and implementation of hospital outcome and efficiency measures.
Funding
Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award R01 HL139985-01A1 and 1R01HL146884-01. Dr. Lindenauer was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K24HL132008.
Disclaimer
The views expressed in this manuscript do not necessarily reflect those of the Yale Center for Outcomes Research and Evaluation or the Centers for Medicare and Medicaid Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
In an effort to improve healthcare for Americans by linking hospital payments to quality of care, Medicare’s Hospital Readmission Reduction Program (HRRP) began penalizing hospitals with “excess” readmission rates in 2012. The decision sparked widespread debate about the definition of a preventable readmission and whether a patient’s socioeconomic status should be considered for risk adjustment. Although coming back to the hospital after an admission is an undesirable outcome for any patient, the suitability of readmission as a quality measure remains a hot and debated topic. Research on the subject skyrocketed; over 12000 articles about hospital readmissions have been indexed in PubMed since 2000, and the number of publications has steadily increased since 2010 (Figure).
Although the HRRP is a Medicare initiative, there has been a substantial focus on readmissions in pediatrics as well. The National Quality Forum has endorsed three quality measures specific to readmission in children: (1) the rate of unplanned readmissions to the pediatric intensive care unit within 24 hours after discharge or transfer, (2) the pediatric lower respiratory infection readmission measure, defined as the percentage of admissions followed by one or more readmissions within 30 days of hospitalization for lower respiratory infection, and (3) the pediatric all-cause readmission measure, defined as the percentage of admissions followed by one or more readmissions within 30 days. These endorsements were preceded by studies showing that pediatric readmission rates varied substantially across hospitals and clinical conditions, and that children with chronic illnesses were at the highest risk.
Readmission is an attractive pediatric quality measure for a number of reasons. This measure is easy to apply to data at the hospital, health system, and payor levels at relatively low cost. Relatedly, the all-condition measure can be applied to all pediatric hospitalizations, overcoming the very real challenge in pediatric quality measurement of inadequate sample sizes to discern differences in healthcare quality at the hospital level for many disease-specific measures.1 In addition, this measure moves beyond process measurement to quantify an outcome relevant to families as well as healthcare systems. Finally, the measure is founded on a compelling conceptual framework (albeit one that remains challenging to prove) that efforts to improve a patient’s hospital-to-home transition and discharge readiness will reduce their likelihood of readmission.
In this issue of the Journal of Hospital Medicine, Katherine Auger and colleagues present their analysis of pediatric readmission rates from 2010 to 2016 across 66 children’s hospitals.2 They found that the median seven-day all-cause pediatric readmission rate was 5.1%, with no change in rates over the seven-year study period. Applying proprietary software to identify potentially preventable readmissions (PPR), they reported that approximately 40% of these readmissions may be preventable, a proportion that was also unchanged over time. Interestingly, 88% of the hospitals represented in their data were participating in the Solutions for Patient Safety national learning collaborative during the study period, making efforts to reduce seven-day readmission rates. Despite this, the figures presented in this paper of all-condition and potentially preventable readmission rates over time are very, very flat.
This work by Auger et al. contributes to our understanding about the preventability, or lack thereof, of pediatric all-condition readmissions. If 40% of these readmissions are indeed preventable, then why did Auger et al. not observe a declining proportion of PPR over time as a result of hospital participation in a national collaborative? Past quantitative and qualitative studies provide important context. First, the 40% rate of readmission preventability is twofold higher than that reported in past studies that relied on physician judgement to determine readmission preventability;3,4 the authors’ use of proprietary software to categorize the preventability of a readmission limits our ability to explain the differences in these rates. However, in these past studies, the rates of initial agreement between physician reviewers about readmission preventability were poor, highlighting the challenges associated with determining readmission preventability. Moreover, qualitative studies suggest that physicians and families lack a shared understanding of the preventability of readmissions.5 Finally, a systematic review of pediatric hospital discharge interventions did not identify any one intervention that was consistently effective in reducing hospital readmission rates.6 The following important questions remain: Were hospitals’ efforts to reduce PPR targeting the wrong patients? Were the interventions insufficient or ineffective? Or are readmission measures insufficiently sensitive to improved processes of care?
Recognizing that the majority of research on readmission as well as HRRP penalties focuses on adult populations, perhaps we can apply some lessons learned from the HRRP to pediatrics. Recent analyses by Medicare Payment Advisory Commission (MedPAC) suggest that raw and risk-adjusted readmission rates have declined for conditions covered by the HRRP, with readmission rates for HRRP target conditions declining more quickly than that for nontarget conditions.7 Just as the HRRP has focused on target conditions with relatively high readmission rates, analogous efforts to focus pediatric readmission reduction on children at greatest risk may enable measurement of change over time. For example, although children with complex chronic medical conditions represent a small proportion of the pediatric population, they account for 60% of all pediatric readmissions in the United States. However, similar to the above-described meta-analysis of readmission reduction efforts in children, at least one meta-analysis has demonstrated that there is no one intervention or even bundle of interventions that has consistently reduced readmissions in adults.8 Although the readmission rates for HRRP target conditions have decreased, the results of clinical trials evaluating readmission reduction efforts are difficult to translate into practice given substantial heterogeneity in study designs, interventions, and patient populations.
Does this study by Auger et al. suggest that pediatric readmission reduction efforts are misguided or futile? No. But it does provide compelling data that efforts to reduce all-cause readmissions for all children may not yield measureable changes using the current measures. A narrowed focus on children with chronic illnesses, who account for approximately half of all pediatric admissions, may be warranted. A number of studies have summarized families’ preferences regarding their hospital-to-home transitions; the results indicate that families of children with chronic illness have unique desires and needs.9,10 Perhaps it is time to take a step back from pediatric readmission reduction efforts, largely inspired by the HRRP, and redirect our resources to implement and evaluate processes and outcomes most valued by children and their families.
Disclosures
Drs. Lagu and Lindenauer have served as consultants for the Yale Center for Outcomes Research and Evaluation (under contract to the Centers for Medicare and Medicaid Services) providing clinical and methodological expertise and input on the development, reevaluation, and implementation of hospital outcome and efficiency measures.
Funding
Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award R01 HL139985-01A1 and 1R01HL146884-01. Dr. Lindenauer was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K24HL132008.
Disclaimer
The views expressed in this manuscript do not necessarily reflect those of the Yale Center for Outcomes Research and Evaluation or the Centers for Medicare and Medicaid Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
1. Berry JG, Zaslavsky AM, Toomey SL, et al. Recognizing differences in hospital quality performance for pediatric inpatient care. Pediatrics. 2015;136(2):251-262. https://doi.org/10.1542/peds.2014-3131.
2. Auger K, Harris M, Gay J, et al. Progress (?) towards reducing pediatric readmissions. J Hosp Med. 2019;14(10):618-621. https://doi.org/10.12788/jhm.3210
3. Hain PD, Gay JC, Berutti TW, Whitney GM, Wang W, Saville BR. Preventability of early readmissions at a children’s hospital. Pediatrics. 2013;131(1):e171-e181. https://doi.org/10.1542/peds.2012-0820.
4. Wallace SS, Keller SL, Falco CN, et al. An examination of physician-, caregiver-, and disease-related factors associated with readmission from a pediatric hospital medicine service. Hosp Pediatr. 2015;5(11):566-573. https://doi.org/10.1542/hpeds.2015-0015.
5. Brittan M, Albright K, Cifuentes M, Jimenez-Zambrano A, Kempe A. Parent and provider perspectives on pediatric readmissions: what can we learn about readiness for discharge?. Hosp Pediatr. 2015;5(11):559-565. https://doi.org/10.1542/hpeds.2015-0034.
6. Auger K, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9(4):251-260. https://doi.org/10.1002/jhm.2134.
7. NEJM Catalyst. Hospital Readmissions Reduction Program (HRRP). Available at: https://catalyst.nejm.org/hospital-readmissions-reduction-program-hrrp/. Accessed May 21, 2019.
8. Hansen L, Young R, Hinami K, Leung A, Williams M. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. https://doi.org/10.7326/0003-4819-155-8-201110180-00008.
9. Leyenaar J, O’Brien E, Leslie L, Lindenauer P, Mangione-Smith R. Families’ priorities regarding hospital-to-home transitions for children with medical complexity. Pediatrics. 2017;139(1): e20161581. https://doi.org/10.1542/peds.2016-1581.
10. Desai AD, Durkin LK, Jacob-Files EA, Mangione-Smith R. Caregiver perceptions of hospital to home transitions according to medical complexity: a qualitative study. Acad Pediatr. 2016;16(2):136-144. https://doi.org/10.1016/j.acap.2015.08.003.
1. Berry JG, Zaslavsky AM, Toomey SL, et al. Recognizing differences in hospital quality performance for pediatric inpatient care. Pediatrics. 2015;136(2):251-262. https://doi.org/10.1542/peds.2014-3131.
2. Auger K, Harris M, Gay J, et al. Progress (?) towards reducing pediatric readmissions. J Hosp Med. 2019;14(10):618-621. https://doi.org/10.12788/jhm.3210
3. Hain PD, Gay JC, Berutti TW, Whitney GM, Wang W, Saville BR. Preventability of early readmissions at a children’s hospital. Pediatrics. 2013;131(1):e171-e181. https://doi.org/10.1542/peds.2012-0820.
4. Wallace SS, Keller SL, Falco CN, et al. An examination of physician-, caregiver-, and disease-related factors associated with readmission from a pediatric hospital medicine service. Hosp Pediatr. 2015;5(11):566-573. https://doi.org/10.1542/hpeds.2015-0015.
5. Brittan M, Albright K, Cifuentes M, Jimenez-Zambrano A, Kempe A. Parent and provider perspectives on pediatric readmissions: what can we learn about readiness for discharge?. Hosp Pediatr. 2015;5(11):559-565. https://doi.org/10.1542/hpeds.2015-0034.
6. Auger K, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9(4):251-260. https://doi.org/10.1002/jhm.2134.
7. NEJM Catalyst. Hospital Readmissions Reduction Program (HRRP). Available at: https://catalyst.nejm.org/hospital-readmissions-reduction-program-hrrp/. Accessed May 21, 2019.
8. Hansen L, Young R, Hinami K, Leung A, Williams M. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. https://doi.org/10.7326/0003-4819-155-8-201110180-00008.
9. Leyenaar J, O’Brien E, Leslie L, Lindenauer P, Mangione-Smith R. Families’ priorities regarding hospital-to-home transitions for children with medical complexity. Pediatrics. 2017;139(1): e20161581. https://doi.org/10.1542/peds.2016-1581.
10. Desai AD, Durkin LK, Jacob-Files EA, Mangione-Smith R. Caregiver perceptions of hospital to home transitions according to medical complexity: a qualitative study. Acad Pediatr. 2016;16(2):136-144. https://doi.org/10.1016/j.acap.2015.08.003.
© 2019 Society of Hospital Medicine
The Best Laid Plans—Medication Reconciliation Optimization in Theory and Practice
Of all the errors that occur in modern healthcare, medication errors are among the most ubiquitous and consequential. Adverse drug events (ADEs) account for approximately 700,000 emergency department visits, 100,000 hospitalizations, and 1.3 million people are injured by medication errors annually.1 Among the most frequent causes of preventable ADEs are errors on the medication lists when patients are admitted to hospitals.2 Therefore, preventing discrepancies between medications the patient is prescribed (and actually taking) inside and outside the hospital—the so-called “medication reconciliation”—is an intense, ongoing area of focus for health systems, pharmacies, and numerous quality and safety organizations seeking to reduce ADEs.
Past studies of medication reconciliation interventions have suggested benefit from restricting medication reconciliation to admission or discharge, pharmacist or pharmacy technician-led medication reconciliation, and pharmacy-led interventions (ie, telephone follow-up/home visit, patient counseling) for ensuring an accurate medication list.3-5 Recent evidence suggests that pharmacist discharge medication reconciliation is associated with decreased readmission rates, decreased medication discrepancies, and adverse events associated with drug therapy issues.4 The successful interventions were promising, but disseminating such interventions can often be very complex.6
In this issue of the Journal of Hospital Medicine, Mixon et al. report the results of a subanalysis of the MARQUIS trial,7 wherein they individually examined the on-protocol effects of the interventions that MARQUIS recommended, comparing hospitals to their own running baseline data at the implementation of each intervention to data following the implementation. The authors found that only three of the nine interventions were associated with reducing potentially harmful discrepancies in the medication list—training existing staff to perform discharge medication reconciliation, hiring additional staff for this purpose, and defining roles and responsibilities and roles clearly—and that two were actually associated with harm—training existing staff to take best possible medication histories (BPMHs) and implementing a new Electronic Medical Record (EMR). MARQUIS is unique in not just attempting but in reporting “best case” real-world implementation using available literature to design mentored, practical approaches to those same interventions at sites not involved in their initial setup and validation.
EMR implementation should in theory improve accuracy (or at least legibility), but it can also contribute to new types of inaccuracy or, as the authors propose, deprioritize quality and safety as organizational goals during the rigors of digitization. Similarly, training staff to take a BPMH might create false confidence in the results or interact with medication reconciliation in other complex ways. Opting to add more work instead of hiring additional staff may have increased the burden of medication review and thus contributed to its inaccuracy.
On the contrary, certain interventions, such as having clear accountability for the medication list, hiring additional staff to construct that list, and clearly defining the roles of those involved in the reconciliation process, were associated with improved medication reconciliation. All these strategies require resource allocation, but at least the current study provides evidence that such resource allocation can be effective in new settings as they were in their original ones.
The study has important acknowledged limitations. The on-protocol analysis limited the authors to reporting associations rather than causality. Moreover, the original trial ran from 2011 to 2014, which was a time of rapid EMR implementation and new recognition of the problems posed by the same; several organizations are in a far more mature EMR context today. Conversely, newer technologies such as patient-facing medication reconciliation applications, cross-organization medication lists available from some EMR vendors, and health platforms that collect data from multiple EMRs were not evaluated because they did not exist at the time of the original trial. Another important trend in healthcare, the rise of Accountable Care Organizations and their focus on integration and defragmentation, may have an important part to play in medication list accuracy. All the above-mentioned aspects will be important avenues for ongoing research in real-world medication reconciliation.
Mixon’s findings come at a time when medication reconciliation is again a national health informatics priority, a key component of the Medicare Access and CHIP reauthorization Act of 2015 and Merit-based Incentive Payments System8 since 2019, with hospitals reporting medication reconciliation rates for financial in addition to quality and safety reasons. Hopefully, this study and others, in combination with the abovementioned incentives, will stimulate further research into impactful strategies for medication reconciliation and ideal ways to implement them. With luck, the end result will be more generalizable interventions, with a track record of success, that would help ensure that patients are prescribed, are reporting, are taking, and are noted to be taking the medications that they and their providers intended, both on presentation to the hospital and on discharge home.
Disclosures
Vicki Jue has no conflicts of interest to report. Raman Khanna reports developing CareWeb, a communication platform that has been licensed to Voalte, Inc. This work is unrelated to the current editorial. No other conflicts of interest to report.
1. Center for Drug Evaluation and Research. Medication Errors-Medication Error Reports. https://www.fda.gov/Drugs/DrugSafety/MedicationErrors/ucm080629.htm.Accessed June 14, 2019.
2. Cornish PL, Knowles SR, Marchesano R, et al. Unintended medication discrepancies at the time of hospital admission. Arch Intern Med. 2005;165(4):424-429. https://doi.org/ 10.1001/archinte.165.4.424.
3. Mekonnen AB, McLachlan AJ, Brien JE. Pharmacy-led medication reconciliation programmes at hospital transitions: a systematic review and meta-analysis. J Clin Pharm Ther. 2016;41(2):128-144. https://doi.org/ 10.1111/jcpt.12364.
4. Kilcup M, Schultz D, Carlson J, et al. Postdischarge pharmacist medication reconciliation: impact on readmission rates and financial savings. J Am Pharm Assoc. 2013;53(1):78-84. https://doi.org/ 10.1331/JAPhA.2013.11250.
5. Cater SW, Luzum M, Serra AE, et al. A prospective cohort study of medication reconciliation using pharmacy technicians in the emergency department to reduce medication errors among patients. J Emerg Med. 2015;48(2):230-238. https://doi.org/ 10.1016/j.jemermed.2014.09.065.
6. Horton TJ, Illingworth JH, Warburton WHP. Overcoming challenges in codifying and replicating complex health care interventions. Health Aff. 2018;37(2):191-197. https://doi.org/ 10.1377/hlthaff.2017.1161.
7. Mixon A, Kripalani S, Stein J, et al. An on-treatment analysis of the MARQUIS study: interventions to improve inpatient medication reconciliation. J Hosp Med. 2019;(10):614-617. https://doi.org/ 10.12788/jhm.3258
8. Centers for Medicare & Medicaid Services. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/MACRA-MIPS-and-APMs/MACRA-MIPS-and-APMs.html.Accessed June 25, 2019.
Of all the errors that occur in modern healthcare, medication errors are among the most ubiquitous and consequential. Adverse drug events (ADEs) account for approximately 700,000 emergency department visits, 100,000 hospitalizations, and 1.3 million people are injured by medication errors annually.1 Among the most frequent causes of preventable ADEs are errors on the medication lists when patients are admitted to hospitals.2 Therefore, preventing discrepancies between medications the patient is prescribed (and actually taking) inside and outside the hospital—the so-called “medication reconciliation”—is an intense, ongoing area of focus for health systems, pharmacies, and numerous quality and safety organizations seeking to reduce ADEs.
Past studies of medication reconciliation interventions have suggested benefit from restricting medication reconciliation to admission or discharge, pharmacist or pharmacy technician-led medication reconciliation, and pharmacy-led interventions (ie, telephone follow-up/home visit, patient counseling) for ensuring an accurate medication list.3-5 Recent evidence suggests that pharmacist discharge medication reconciliation is associated with decreased readmission rates, decreased medication discrepancies, and adverse events associated with drug therapy issues.4 The successful interventions were promising, but disseminating such interventions can often be very complex.6
In this issue of the Journal of Hospital Medicine, Mixon et al. report the results of a subanalysis of the MARQUIS trial,7 wherein they individually examined the on-protocol effects of the interventions that MARQUIS recommended, comparing hospitals to their own running baseline data at the implementation of each intervention to data following the implementation. The authors found that only three of the nine interventions were associated with reducing potentially harmful discrepancies in the medication list—training existing staff to perform discharge medication reconciliation, hiring additional staff for this purpose, and defining roles and responsibilities and roles clearly—and that two were actually associated with harm—training existing staff to take best possible medication histories (BPMHs) and implementing a new Electronic Medical Record (EMR). MARQUIS is unique in not just attempting but in reporting “best case” real-world implementation using available literature to design mentored, practical approaches to those same interventions at sites not involved in their initial setup and validation.
EMR implementation should in theory improve accuracy (or at least legibility), but it can also contribute to new types of inaccuracy or, as the authors propose, deprioritize quality and safety as organizational goals during the rigors of digitization. Similarly, training staff to take a BPMH might create false confidence in the results or interact with medication reconciliation in other complex ways. Opting to add more work instead of hiring additional staff may have increased the burden of medication review and thus contributed to its inaccuracy.
On the contrary, certain interventions, such as having clear accountability for the medication list, hiring additional staff to construct that list, and clearly defining the roles of those involved in the reconciliation process, were associated with improved medication reconciliation. All these strategies require resource allocation, but at least the current study provides evidence that such resource allocation can be effective in new settings as they were in their original ones.
The study has important acknowledged limitations. The on-protocol analysis limited the authors to reporting associations rather than causality. Moreover, the original trial ran from 2011 to 2014, which was a time of rapid EMR implementation and new recognition of the problems posed by the same; several organizations are in a far more mature EMR context today. Conversely, newer technologies such as patient-facing medication reconciliation applications, cross-organization medication lists available from some EMR vendors, and health platforms that collect data from multiple EMRs were not evaluated because they did not exist at the time of the original trial. Another important trend in healthcare, the rise of Accountable Care Organizations and their focus on integration and defragmentation, may have an important part to play in medication list accuracy. All the above-mentioned aspects will be important avenues for ongoing research in real-world medication reconciliation.
Mixon’s findings come at a time when medication reconciliation is again a national health informatics priority, a key component of the Medicare Access and CHIP reauthorization Act of 2015 and Merit-based Incentive Payments System8 since 2019, with hospitals reporting medication reconciliation rates for financial in addition to quality and safety reasons. Hopefully, this study and others, in combination with the abovementioned incentives, will stimulate further research into impactful strategies for medication reconciliation and ideal ways to implement them. With luck, the end result will be more generalizable interventions, with a track record of success, that would help ensure that patients are prescribed, are reporting, are taking, and are noted to be taking the medications that they and their providers intended, both on presentation to the hospital and on discharge home.
Disclosures
Vicki Jue has no conflicts of interest to report. Raman Khanna reports developing CareWeb, a communication platform that has been licensed to Voalte, Inc. This work is unrelated to the current editorial. No other conflicts of interest to report.
Of all the errors that occur in modern healthcare, medication errors are among the most ubiquitous and consequential. Adverse drug events (ADEs) account for approximately 700,000 emergency department visits, 100,000 hospitalizations, and 1.3 million people are injured by medication errors annually.1 Among the most frequent causes of preventable ADEs are errors on the medication lists when patients are admitted to hospitals.2 Therefore, preventing discrepancies between medications the patient is prescribed (and actually taking) inside and outside the hospital—the so-called “medication reconciliation”—is an intense, ongoing area of focus for health systems, pharmacies, and numerous quality and safety organizations seeking to reduce ADEs.
Past studies of medication reconciliation interventions have suggested benefit from restricting medication reconciliation to admission or discharge, pharmacist or pharmacy technician-led medication reconciliation, and pharmacy-led interventions (ie, telephone follow-up/home visit, patient counseling) for ensuring an accurate medication list.3-5 Recent evidence suggests that pharmacist discharge medication reconciliation is associated with decreased readmission rates, decreased medication discrepancies, and adverse events associated with drug therapy issues.4 The successful interventions were promising, but disseminating such interventions can often be very complex.6
In this issue of the Journal of Hospital Medicine, Mixon et al. report the results of a subanalysis of the MARQUIS trial,7 wherein they individually examined the on-protocol effects of the interventions that MARQUIS recommended, comparing hospitals to their own running baseline data at the implementation of each intervention to data following the implementation. The authors found that only three of the nine interventions were associated with reducing potentially harmful discrepancies in the medication list—training existing staff to perform discharge medication reconciliation, hiring additional staff for this purpose, and defining roles and responsibilities and roles clearly—and that two were actually associated with harm—training existing staff to take best possible medication histories (BPMHs) and implementing a new Electronic Medical Record (EMR). MARQUIS is unique in not just attempting but in reporting “best case” real-world implementation using available literature to design mentored, practical approaches to those same interventions at sites not involved in their initial setup and validation.
EMR implementation should in theory improve accuracy (or at least legibility), but it can also contribute to new types of inaccuracy or, as the authors propose, deprioritize quality and safety as organizational goals during the rigors of digitization. Similarly, training staff to take a BPMH might create false confidence in the results or interact with medication reconciliation in other complex ways. Opting to add more work instead of hiring additional staff may have increased the burden of medication review and thus contributed to its inaccuracy.
On the contrary, certain interventions, such as having clear accountability for the medication list, hiring additional staff to construct that list, and clearly defining the roles of those involved in the reconciliation process, were associated with improved medication reconciliation. All these strategies require resource allocation, but at least the current study provides evidence that such resource allocation can be effective in new settings as they were in their original ones.
The study has important acknowledged limitations. The on-protocol analysis limited the authors to reporting associations rather than causality. Moreover, the original trial ran from 2011 to 2014, which was a time of rapid EMR implementation and new recognition of the problems posed by the same; several organizations are in a far more mature EMR context today. Conversely, newer technologies such as patient-facing medication reconciliation applications, cross-organization medication lists available from some EMR vendors, and health platforms that collect data from multiple EMRs were not evaluated because they did not exist at the time of the original trial. Another important trend in healthcare, the rise of Accountable Care Organizations and their focus on integration and defragmentation, may have an important part to play in medication list accuracy. All the above-mentioned aspects will be important avenues for ongoing research in real-world medication reconciliation.
Mixon’s findings come at a time when medication reconciliation is again a national health informatics priority, a key component of the Medicare Access and CHIP reauthorization Act of 2015 and Merit-based Incentive Payments System8 since 2019, with hospitals reporting medication reconciliation rates for financial in addition to quality and safety reasons. Hopefully, this study and others, in combination with the abovementioned incentives, will stimulate further research into impactful strategies for medication reconciliation and ideal ways to implement them. With luck, the end result will be more generalizable interventions, with a track record of success, that would help ensure that patients are prescribed, are reporting, are taking, and are noted to be taking the medications that they and their providers intended, both on presentation to the hospital and on discharge home.
Disclosures
Vicki Jue has no conflicts of interest to report. Raman Khanna reports developing CareWeb, a communication platform that has been licensed to Voalte, Inc. This work is unrelated to the current editorial. No other conflicts of interest to report.
1. Center for Drug Evaluation and Research. Medication Errors-Medication Error Reports. https://www.fda.gov/Drugs/DrugSafety/MedicationErrors/ucm080629.htm.Accessed June 14, 2019.
2. Cornish PL, Knowles SR, Marchesano R, et al. Unintended medication discrepancies at the time of hospital admission. Arch Intern Med. 2005;165(4):424-429. https://doi.org/ 10.1001/archinte.165.4.424.
3. Mekonnen AB, McLachlan AJ, Brien JE. Pharmacy-led medication reconciliation programmes at hospital transitions: a systematic review and meta-analysis. J Clin Pharm Ther. 2016;41(2):128-144. https://doi.org/ 10.1111/jcpt.12364.
4. Kilcup M, Schultz D, Carlson J, et al. Postdischarge pharmacist medication reconciliation: impact on readmission rates and financial savings. J Am Pharm Assoc. 2013;53(1):78-84. https://doi.org/ 10.1331/JAPhA.2013.11250.
5. Cater SW, Luzum M, Serra AE, et al. A prospective cohort study of medication reconciliation using pharmacy technicians in the emergency department to reduce medication errors among patients. J Emerg Med. 2015;48(2):230-238. https://doi.org/ 10.1016/j.jemermed.2014.09.065.
6. Horton TJ, Illingworth JH, Warburton WHP. Overcoming challenges in codifying and replicating complex health care interventions. Health Aff. 2018;37(2):191-197. https://doi.org/ 10.1377/hlthaff.2017.1161.
7. Mixon A, Kripalani S, Stein J, et al. An on-treatment analysis of the MARQUIS study: interventions to improve inpatient medication reconciliation. J Hosp Med. 2019;(10):614-617. https://doi.org/ 10.12788/jhm.3258
8. Centers for Medicare & Medicaid Services. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/MACRA-MIPS-and-APMs/MACRA-MIPS-and-APMs.html.Accessed June 25, 2019.
1. Center for Drug Evaluation and Research. Medication Errors-Medication Error Reports. https://www.fda.gov/Drugs/DrugSafety/MedicationErrors/ucm080629.htm.Accessed June 14, 2019.
2. Cornish PL, Knowles SR, Marchesano R, et al. Unintended medication discrepancies at the time of hospital admission. Arch Intern Med. 2005;165(4):424-429. https://doi.org/ 10.1001/archinte.165.4.424.
3. Mekonnen AB, McLachlan AJ, Brien JE. Pharmacy-led medication reconciliation programmes at hospital transitions: a systematic review and meta-analysis. J Clin Pharm Ther. 2016;41(2):128-144. https://doi.org/ 10.1111/jcpt.12364.
4. Kilcup M, Schultz D, Carlson J, et al. Postdischarge pharmacist medication reconciliation: impact on readmission rates and financial savings. J Am Pharm Assoc. 2013;53(1):78-84. https://doi.org/ 10.1331/JAPhA.2013.11250.
5. Cater SW, Luzum M, Serra AE, et al. A prospective cohort study of medication reconciliation using pharmacy technicians in the emergency department to reduce medication errors among patients. J Emerg Med. 2015;48(2):230-238. https://doi.org/ 10.1016/j.jemermed.2014.09.065.
6. Horton TJ, Illingworth JH, Warburton WHP. Overcoming challenges in codifying and replicating complex health care interventions. Health Aff. 2018;37(2):191-197. https://doi.org/ 10.1377/hlthaff.2017.1161.
7. Mixon A, Kripalani S, Stein J, et al. An on-treatment analysis of the MARQUIS study: interventions to improve inpatient medication reconciliation. J Hosp Med. 2019;(10):614-617. https://doi.org/ 10.12788/jhm.3258
8. Centers for Medicare & Medicaid Services. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/MACRA-MIPS-and-APMs/MACRA-MIPS-and-APMs.html.Accessed June 25, 2019.
© 2019 Society of Hospital Medicine