User login
Things We Do for No Reason™: Lumbar Punctures in Low-Risk Febrile Infants with Bronchiolitis

CLINICAL SCENARIO
A 22-day-old full-term previously healthy male infant was evaluated in the emergency department (ED). The patient’s mother reported a three-day history of nasal congestion, cough and labored breathing, decreased oral intake, and subjective fever.
In the ED, the patient was found to have a rectal temperature of 101.3 °F (38.3 °C), heart rate of 112 beats per minute, and a respiratory rate of 54 breaths per minute, with subcostal retractions and diffuse expiratory wheezing. His appearance was otherwise unremarkable. His evaluation in the ED included a normal complete blood count (CBC) with differential, a normal urinalysis, and a chest radiograph with diffuse peribronchial thickening. Blood and catheterized urine cultures were also collected. The patient’s provider informs the parents that a lumbar puncture (LP) would be performed to rule out bacterial meningitis. Is it necessary for this patient to receive an LP?
INTRODUCTION
Fever in an infant <90 days old is a common clinical presentation.1 Because a newborn’s immune system is still developing, there is a heightened concern for bacterial infections in this age group. These include bloodstream infections, meningitis, pneumonia, urinary tract infections (UTIs), skin/soft tissue infections, and osteoarticular infections. Bacterial infections collectively account for approximately 10% of illness in young febrile infants <90 days.2 Of these, UTIs are the most common. The most recent literature has narrowed the focus on infants <60 days old as the risk of serious infection is inversely correlated with age. Meningitis accounts for 1% of infections or less in children <60 days of age who present with a fever.3
Frequently, the evaluation of fever in young infants leads to cerebrospinal fluid (CSF) collection and hospitalization.4 Among febrile infants, current practice patterns regarding LPs vary across institutions.5 Some clinical practice guidelines recommend universal CSF testing for all febrile infants ≤56 days old.6
Bronchiolitis is also a common presentation. Up to 90% of children are infected with respiratory syncytial virus, the most common viral cause of bronchiolitis, within the first two years of life.7 Fever may be a presenting symptom in infants with bronchiolitis and one study found approximately 11% of febrile infants less than 90 days old met clinical criteria for bronchiolitis.8
WHY YOU MIGHT THINK LUMBAR PUNCTURE IN FEBRILE INFANTS WITH BRONCHIOLITIS IS HELPFUL
While clinical guidelines for bronchiolitis are well established,7 the evaluation and management of fever in an infant <90 days old remains a challenge because of concern for missing a bloodstream infection or meningitis. Meningitis can devastate an infant neurologically.9 Signs and symptoms of bacterial meningitis in infants are not specific, including the physical exam.10 Blood cultures are only concomitantly positive in 62% of cases of culture-confirmed bacterial meningitis.11
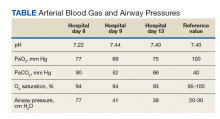
Several risk stratification algorithms exist to evaluate the likelihood of bacterial infections in febrile infants (Table). Two of the most common criteria—the Boston and Philadelphia—were validated using CSF cell count data. Other algorithms do not require an LP.12-15 All of the fever criteria algorithms have several limitations including lack of robust validation studies, under-powered methodologies (particularly for meningitis), and different inclusion criteria.2 Even with these risk stratification algorithms, some providers may continue to feel more comfortable obtaining CSF due to fear of missing meningitis in well-appearing, low-risk infants.
WHY LUMBAR PUNCTURE IN LOW-RISK FEBRILE INFANTS WITH BRONCHIOLITIS IS NOT NECESSARY
Bacterial meningitis, even in young infants, is rare. A recent meta-analysis estimated the general prevalence of meningitis in febrile neonates (regardless of risk stratification or bronchiolitis symptoms) in their first and second months of life were 1.2% (95% CI, 0.8%-1.9%) and 0.4% (95% CI, 0.2%-1.0%), respectively.3
Febrile infant risk stratification algorithms have high negative predictive values (NPVs) in ruling out meningitis. The Rochester criteria, which does not utilize CSF, has an NPV of greater than 98%.12 A recent Pediatric Emergency Care Applied Research Network Clinical Prediction Rule has an NPV of 99.9% among febrile infants <60 days, using only absolute neutrophil count, urinalysis, and procalcitonin.15
Among the patients that are already a low risk, concomitant viral infections further decrease the pretest probability. Febrile infants with lab-confirmed respiratory viral infections are at lower risk for serious bacterial infections.16,17 Multiple retrospective and prospective observational studies have demonstrated that low-risk patients with bronchiolitis symptoms are extremely unlikely to have bacterial meningitis.8,18-22 A systematic review of 1749 febrile patients under 90 days of age with clinical bronchiolitis demonstrated no cases of meningitis.23 Many of these studies included infants aged <28 days. Though the total number of neonates (<28 days) in all studies is somewhat unclear, it suggests that the cut-off to avoid an LP may be even lower.
Recent literature has advocated outpatient observation without an LP for low-risk infants as a cost-effective management tool,24 and this is particularly true in patients with concomitant viral bronchiolitis.
Based on the latest data confirming the low prevalence of meningitis among all infants,3 the ability to identify low-risk infants based on risk stratification algorithms (Table), and the decreased prevalence of meningitis in patients with clinical bronchiolitis,23 low-risk infants with bronchiolitis seem to have minimal, if any, risk of meningitis. Therefore, low-risk infants with bronchiolitis do not warrant an LP.
Importantly, LPs are not risk neutral. Their benefit versus harm should be weighed every time they are considered. Approximately 19% of LP attempts in infants under 90 days old are either traumatic or unsuccessful.25 Infants aged 28 to 60 days with traumatic or unsuccessful LPs are more frequently hospitalized.25 Increased hospitalizations are associated with higher costs.4 The majority of positive CSF cultures are deemed to be “contaminants” (87% in one study26), but the positive result still leads to unnecessary further evaluation, hospitalization, repeated invasive procedures, and family distress.27 These data further support refraining from pursuing an LP in low-risk infants with bronchiolitis.
WHY LUMBAR PUNCTURE MIGHT BE HELPFUL IN CERTAIN CIRCUMSTANCES
If the patient is not low risk based on criteria or does not have clinical bronchiolitis, consider performing an LP. A recent study demonstrated a 0.4% incidence of bacterial meningitis in febrile infants with viral co-infection,29 though it is not determined if the patients presented with symptoms of bronchiolitis or were risk-stratified using the algorithms discussed.
In the studies looking at viral infections in febrile infants, each has important exclusion criteria including prematurity, comorbidities, and recent antibiotic administration.23 For these patients, an LP may be warranted (though the evidence is lacking). In addition, in very young infants (less than seven-14 days old), viral infections may be less common than in older infants, resulting in a desire to rule out bacterial infections more thoroughly in this population.
WHAT YOU SHOULD DO INSTEAD: AVOID AN LP IN LOW-RISK FEBRILE INFANTS WITH BRONCHIOLITIS
For low-risk febrile infants with signs of bronchiolitis, evaluation for bacterial meningitis is not necessary. The low prevalence of meningitis in this age range along with the even lower likelihood of meningitis when bronchiolitis is identified suggests that the procedure is unnecessary. Moreover, the risks associated with LP—including trauma, hospitalization, costs, and family stress—likely outweigh the benefits of CSF analysis.
RECOMMENDATIONS
- In febrile infants, determine the risk of serious bacterial infections using published algorithms (Table) before considering lumbar puncture.
- In low-risk febrile infants with typical bronchiolitis, evaluation for bacterial meningitis with an LP is not necessary.
CONCLUSION
Infants under 90 days of age often present to care with fever. While there is a concern for missing bacterial meningitis, the prevalence of such an infection in infants is very low. Moreover, in low-risk patients that present with typical bronchiolitis symptoms, the prevalence is effectively zero. LP practices vary by institution and can be associated with risks. In low-risk infants with typical bronchiolitis symptoms, an LP is one of the Things We Do for No Reason™.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
1. Cioffredi L-A, Jhaveri R. Evaluation and management of febrile children. JAMA Pediatr. 2016;170(8):794. https://doi.org/10.1001/jamapediatrics.2016.0596.
2. Huppler AR, Eickhoff JC, Wald ER. Performance of low-risk criteria in the evaluation of young infants with fever: review of the literature. Pediatrics. 2010;125(2):228-233. https://doi.org/10.1542/peds.2009-1070.
3. Biondi EA, Lee B, Ralston SL, et al. Prevalence of bacteremia and bacterial meningitis in febrile neonates and infants in the second month of life a systematic review and meta-analysis + supplemental content. JAMA Netw Open. 2019;2(3):190874. https://doi.org/10.1001/jamanetworkopen.2019.0874.
4. Aronson PL, Thurm C, Williams DJ, et al. Association of clinical practice guidelines with emergency department management of febrile infants ≤56 days of age. J Hosp Med. 2015;10(6):358-365. https://doi.org/10.1002/jhm.2329.
5. Aronson PL, Thurm C, Alpern ER, et al. Variation in care of the febrile young infant <90 days in us pediatric emergency departments. Pediatrics. 2014;134(4):667-677. https://doi.org/10.1542/peds.2014-1382.
6. Aronson PL, Thurm C, Williams DJ, et al. Association of clinical practice guidelines with emergency department management of febrile infants ≤56 days of age. J Hosp Med. 2015;10(6):358-365. https://doi.org/10.1002/jhm.2329.
7. Mendonca EA, Meissner HC, Gadomski AM, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742.
8. Melendez E, Harper MB. Utility of sepsis evaluation in infants 90 days of age or younger with fever and clinical bronchiolitis. Pediatr Infect Dis J. 2003;22(12):1053-1056. https://doi.org/10.1097/01.inf.0000101296.68993.4d.
9. Pruitt CM, Neuman MI, Shah SS, et al. Factors associated with adverse outcomes among febrile young infants with invasive bacterial infections. J. Pediatr. 2018;204:177-182. https://doi.org/10.1016/j.jpeds.2018.08.066.
10. Casper TC, Mahajan PV., Tzimenatos L, et al. The Yale Observation Scale Score and the risk of serious bacterial infections in febrile infants. Pediatrics. 2017;140(1):e20170695. https://doi.org/10.1542/peds.2017-0695.
11. Garges HP. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics. 2006;117(4):1094-1100. https://doi.org/10.1542/peds.2005-1132.
12. Jaskiewicz JA, McCarthy CA, Richardson AC, et al. Febrile infants at low risk for serious bacterial infection-an appraisal of the Rochester criteria and implications for management. Pediatrics. 1994;94(3):390-396. http://www.ncbi.nlm.nih.gov/pubmed/8065869. Accessed March 23, 2019.
13. Aronson P, Wang M, Shapiro E, et al. Risk stratification of febrile infants ≤60 days old without routine lumbar puncture. Pediatrics. 2018;142(6):e20181879. https://doi.org/10.1542/peds.2018-1879.
14. Galetto-Lacour A, Zamora SA, Andreola B, et al. Validation of a laboratory risk index score for the identification of severe bacterial infection in children with fever without source. Arch Dis Child. 2010;95(12):968-973. https://doi.org/10.1136/adc.2009.176800.
15. Kuppermann N, Dayan PS, Levine DA, et al. A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342. https://doi.org/10.1001/jamapediatrics.2018.5501.
16. Byington CL, Enriquez FR, Hoff C, et al. Serious bacterial infections in febrile infants 1 to 90 days old with and without viral infections. Pediatrics. 2004;113(6):1662-1666. https://doi.org/10.1542/peds.113.6.1662.
17. Cioffredi LA, Jhaveri R. Evaluation and management of febrile children: a review. JAMA Pediatr. 2016;170(8):794-800. https://doi.org/10.1001/jamapediatrics.2016.0596.
18. Dayan PS, Roskind CG, Levine DA, Kuppermann N. Controversies in the management of children with bronchiolitis. Clin Pediatr Emerg Med. 2004;5(1):41-53. https://doi.org/10.1016/j.cpem.2003.11.001.
19. Oray-Schrom P, Phoenix C, St. Martin D, Amoateng-Adjepong Y. Sepsis workup in febrile infants 0-90 days of age with respiratory syncytial virus infection. Pediatr Emerg Care. 2003;19(5):314-319. https://doi.org/10.1097/01.pec.0000092576.40174.28.
20. Purcell K, Fergie J. Concurrent serious bacterial infections in 2396 infants and children hospitalized with respiratory syncytial virus lower respiratory tract infections. Arch Pediatr Adolesc Med. 2002;156(4):322-324. https://doi.org/10.1001/archpedi.156.4.322.
21. Purcell K, Fergie J. Concurrent serious bacterial infections in 912 infants and children hospitalized for treatment of respiratory syncytial virus lower respiratory tract infection. Pediatr Infect Dis J. 2004;23(3):267-269. https://doi.org/10.1097/01.inf.0000116759.21252.29.
22. Yarden-Bilavsky H, Ashkenazi-Hoffnung L, Livni G, Amir J, Bilavsky E. Month-by-month age analysis of the risk for serious bacterial infections in febrile infants with bronchiolitis. Clin Pediatr (Phila). 2011;50(11):1052-1056. https://doi.org/10.1177/0009922811412949.
23. Ralston S, Hill V, Waters A. Occult serious bacterial infection in infants younger than 60 to 90 days with bronchiolitis: a systematic review. Arch Pediatr Adolesc Med. 2011;165(10):951-956. https://doi.org/10.1001/archpediatrics.2011.155.
24. Lee TJ, Aronson PL. To spinal tap or not to spinal tap, that is the question. Hosp Pediatr. 2018;8(4):236-238. https://doi.org/10.1542/hpeds.2017-0207.
25. Pingree EW, Kimia AA, Nigrovic LE. The effect of traumatic lumbar puncture on hospitalization rate for febrile infants 28 to 60 days of age. Acad Emerg Med. 2015;22(2):240-243. https://doi.org/10.1111/acem.12582.
26. Leazer R, Erickson N, Paulson J, et al. epidemiology of cerebrospinal fluid cultures and time to detection in term infants. Pediatrics. 2017;139(5):e20163268. https://doi.org/10.1542/peds.2016-3268.
27. Paxton RD, Byington CL. An examination of the unintended consequences of the rule-out sepsis evaluation: a parental perspective. Clin Pediatr (Phila). 2001;40(2):71-77. https://doi.org/10.1177/000992280104000202.
28. Mahajan P, Br owne LR, Levine DA, et al. Risk of bacterial coinfections in febrile infants 60 days old and younger with documented viral infections. J Pediatr. 2018;203:86-91.e2. https://doi.org/10.1016/j.jpeds.2018.07.073.

CLINICAL SCENARIO
A 22-day-old full-term previously healthy male infant was evaluated in the emergency department (ED). The patient’s mother reported a three-day history of nasal congestion, cough and labored breathing, decreased oral intake, and subjective fever.
In the ED, the patient was found to have a rectal temperature of 101.3 °F (38.3 °C), heart rate of 112 beats per minute, and a respiratory rate of 54 breaths per minute, with subcostal retractions and diffuse expiratory wheezing. His appearance was otherwise unremarkable. His evaluation in the ED included a normal complete blood count (CBC) with differential, a normal urinalysis, and a chest radiograph with diffuse peribronchial thickening. Blood and catheterized urine cultures were also collected. The patient’s provider informs the parents that a lumbar puncture (LP) would be performed to rule out bacterial meningitis. Is it necessary for this patient to receive an LP?
INTRODUCTION
Fever in an infant <90 days old is a common clinical presentation.1 Because a newborn’s immune system is still developing, there is a heightened concern for bacterial infections in this age group. These include bloodstream infections, meningitis, pneumonia, urinary tract infections (UTIs), skin/soft tissue infections, and osteoarticular infections. Bacterial infections collectively account for approximately 10% of illness in young febrile infants <90 days.2 Of these, UTIs are the most common. The most recent literature has narrowed the focus on infants <60 days old as the risk of serious infection is inversely correlated with age. Meningitis accounts for 1% of infections or less in children <60 days of age who present with a fever.3
Frequently, the evaluation of fever in young infants leads to cerebrospinal fluid (CSF) collection and hospitalization.4 Among febrile infants, current practice patterns regarding LPs vary across institutions.5 Some clinical practice guidelines recommend universal CSF testing for all febrile infants ≤56 days old.6
Bronchiolitis is also a common presentation. Up to 90% of children are infected with respiratory syncytial virus, the most common viral cause of bronchiolitis, within the first two years of life.7 Fever may be a presenting symptom in infants with bronchiolitis and one study found approximately 11% of febrile infants less than 90 days old met clinical criteria for bronchiolitis.8
WHY YOU MIGHT THINK LUMBAR PUNCTURE IN FEBRILE INFANTS WITH BRONCHIOLITIS IS HELPFUL
While clinical guidelines for bronchiolitis are well established,7 the evaluation and management of fever in an infant <90 days old remains a challenge because of concern for missing a bloodstream infection or meningitis. Meningitis can devastate an infant neurologically.9 Signs and symptoms of bacterial meningitis in infants are not specific, including the physical exam.10 Blood cultures are only concomitantly positive in 62% of cases of culture-confirmed bacterial meningitis.11
Several risk stratification algorithms exist to evaluate the likelihood of bacterial infections in febrile infants (Table). Two of the most common criteria—the Boston and Philadelphia—were validated using CSF cell count data. Other algorithms do not require an LP.12-15 All of the fever criteria algorithms have several limitations including lack of robust validation studies, under-powered methodologies (particularly for meningitis), and different inclusion criteria.2 Even with these risk stratification algorithms, some providers may continue to feel more comfortable obtaining CSF due to fear of missing meningitis in well-appearing, low-risk infants.
WHY LUMBAR PUNCTURE IN LOW-RISK FEBRILE INFANTS WITH BRONCHIOLITIS IS NOT NECESSARY
Bacterial meningitis, even in young infants, is rare. A recent meta-analysis estimated the general prevalence of meningitis in febrile neonates (regardless of risk stratification or bronchiolitis symptoms) in their first and second months of life were 1.2% (95% CI, 0.8%-1.9%) and 0.4% (95% CI, 0.2%-1.0%), respectively.3
Febrile infant risk stratification algorithms have high negative predictive values (NPVs) in ruling out meningitis. The Rochester criteria, which does not utilize CSF, has an NPV of greater than 98%.12 A recent Pediatric Emergency Care Applied Research Network Clinical Prediction Rule has an NPV of 99.9% among febrile infants <60 days, using only absolute neutrophil count, urinalysis, and procalcitonin.15
Among the patients that are already a low risk, concomitant viral infections further decrease the pretest probability. Febrile infants with lab-confirmed respiratory viral infections are at lower risk for serious bacterial infections.16,17 Multiple retrospective and prospective observational studies have demonstrated that low-risk patients with bronchiolitis symptoms are extremely unlikely to have bacterial meningitis.8,18-22 A systematic review of 1749 febrile patients under 90 days of age with clinical bronchiolitis demonstrated no cases of meningitis.23 Many of these studies included infants aged <28 days. Though the total number of neonates (<28 days) in all studies is somewhat unclear, it suggests that the cut-off to avoid an LP may be even lower.
Recent literature has advocated outpatient observation without an LP for low-risk infants as a cost-effective management tool,24 and this is particularly true in patients with concomitant viral bronchiolitis.
Based on the latest data confirming the low prevalence of meningitis among all infants,3 the ability to identify low-risk infants based on risk stratification algorithms (Table), and the decreased prevalence of meningitis in patients with clinical bronchiolitis,23 low-risk infants with bronchiolitis seem to have minimal, if any, risk of meningitis. Therefore, low-risk infants with bronchiolitis do not warrant an LP.
Importantly, LPs are not risk neutral. Their benefit versus harm should be weighed every time they are considered. Approximately 19% of LP attempts in infants under 90 days old are either traumatic or unsuccessful.25 Infants aged 28 to 60 days with traumatic or unsuccessful LPs are more frequently hospitalized.25 Increased hospitalizations are associated with higher costs.4 The majority of positive CSF cultures are deemed to be “contaminants” (87% in one study26), but the positive result still leads to unnecessary further evaluation, hospitalization, repeated invasive procedures, and family distress.27 These data further support refraining from pursuing an LP in low-risk infants with bronchiolitis.
WHY LUMBAR PUNCTURE MIGHT BE HELPFUL IN CERTAIN CIRCUMSTANCES
If the patient is not low risk based on criteria or does not have clinical bronchiolitis, consider performing an LP. A recent study demonstrated a 0.4% incidence of bacterial meningitis in febrile infants with viral co-infection,29 though it is not determined if the patients presented with symptoms of bronchiolitis or were risk-stratified using the algorithms discussed.
In the studies looking at viral infections in febrile infants, each has important exclusion criteria including prematurity, comorbidities, and recent antibiotic administration.23 For these patients, an LP may be warranted (though the evidence is lacking). In addition, in very young infants (less than seven-14 days old), viral infections may be less common than in older infants, resulting in a desire to rule out bacterial infections more thoroughly in this population.
WHAT YOU SHOULD DO INSTEAD: AVOID AN LP IN LOW-RISK FEBRILE INFANTS WITH BRONCHIOLITIS
For low-risk febrile infants with signs of bronchiolitis, evaluation for bacterial meningitis is not necessary. The low prevalence of meningitis in this age range along with the even lower likelihood of meningitis when bronchiolitis is identified suggests that the procedure is unnecessary. Moreover, the risks associated with LP—including trauma, hospitalization, costs, and family stress—likely outweigh the benefits of CSF analysis.
RECOMMENDATIONS
- In febrile infants, determine the risk of serious bacterial infections using published algorithms (Table) before considering lumbar puncture.
- In low-risk febrile infants with typical bronchiolitis, evaluation for bacterial meningitis with an LP is not necessary.
CONCLUSION
Infants under 90 days of age often present to care with fever. While there is a concern for missing bacterial meningitis, the prevalence of such an infection in infants is very low. Moreover, in low-risk patients that present with typical bronchiolitis symptoms, the prevalence is effectively zero. LP practices vary by institution and can be associated with risks. In low-risk infants with typical bronchiolitis symptoms, an LP is one of the Things We Do for No Reason™.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].

CLINICAL SCENARIO
A 22-day-old full-term previously healthy male infant was evaluated in the emergency department (ED). The patient’s mother reported a three-day history of nasal congestion, cough and labored breathing, decreased oral intake, and subjective fever.
In the ED, the patient was found to have a rectal temperature of 101.3 °F (38.3 °C), heart rate of 112 beats per minute, and a respiratory rate of 54 breaths per minute, with subcostal retractions and diffuse expiratory wheezing. His appearance was otherwise unremarkable. His evaluation in the ED included a normal complete blood count (CBC) with differential, a normal urinalysis, and a chest radiograph with diffuse peribronchial thickening. Blood and catheterized urine cultures were also collected. The patient’s provider informs the parents that a lumbar puncture (LP) would be performed to rule out bacterial meningitis. Is it necessary for this patient to receive an LP?
INTRODUCTION
Fever in an infant <90 days old is a common clinical presentation.1 Because a newborn’s immune system is still developing, there is a heightened concern for bacterial infections in this age group. These include bloodstream infections, meningitis, pneumonia, urinary tract infections (UTIs), skin/soft tissue infections, and osteoarticular infections. Bacterial infections collectively account for approximately 10% of illness in young febrile infants <90 days.2 Of these, UTIs are the most common. The most recent literature has narrowed the focus on infants <60 days old as the risk of serious infection is inversely correlated with age. Meningitis accounts for 1% of infections or less in children <60 days of age who present with a fever.3
Frequently, the evaluation of fever in young infants leads to cerebrospinal fluid (CSF) collection and hospitalization.4 Among febrile infants, current practice patterns regarding LPs vary across institutions.5 Some clinical practice guidelines recommend universal CSF testing for all febrile infants ≤56 days old.6
Bronchiolitis is also a common presentation. Up to 90% of children are infected with respiratory syncytial virus, the most common viral cause of bronchiolitis, within the first two years of life.7 Fever may be a presenting symptom in infants with bronchiolitis and one study found approximately 11% of febrile infants less than 90 days old met clinical criteria for bronchiolitis.8
WHY YOU MIGHT THINK LUMBAR PUNCTURE IN FEBRILE INFANTS WITH BRONCHIOLITIS IS HELPFUL
While clinical guidelines for bronchiolitis are well established,7 the evaluation and management of fever in an infant <90 days old remains a challenge because of concern for missing a bloodstream infection or meningitis. Meningitis can devastate an infant neurologically.9 Signs and symptoms of bacterial meningitis in infants are not specific, including the physical exam.10 Blood cultures are only concomitantly positive in 62% of cases of culture-confirmed bacterial meningitis.11
Several risk stratification algorithms exist to evaluate the likelihood of bacterial infections in febrile infants (Table). Two of the most common criteria—the Boston and Philadelphia—were validated using CSF cell count data. Other algorithms do not require an LP.12-15 All of the fever criteria algorithms have several limitations including lack of robust validation studies, under-powered methodologies (particularly for meningitis), and different inclusion criteria.2 Even with these risk stratification algorithms, some providers may continue to feel more comfortable obtaining CSF due to fear of missing meningitis in well-appearing, low-risk infants.
WHY LUMBAR PUNCTURE IN LOW-RISK FEBRILE INFANTS WITH BRONCHIOLITIS IS NOT NECESSARY
Bacterial meningitis, even in young infants, is rare. A recent meta-analysis estimated the general prevalence of meningitis in febrile neonates (regardless of risk stratification or bronchiolitis symptoms) in their first and second months of life were 1.2% (95% CI, 0.8%-1.9%) and 0.4% (95% CI, 0.2%-1.0%), respectively.3
Febrile infant risk stratification algorithms have high negative predictive values (NPVs) in ruling out meningitis. The Rochester criteria, which does not utilize CSF, has an NPV of greater than 98%.12 A recent Pediatric Emergency Care Applied Research Network Clinical Prediction Rule has an NPV of 99.9% among febrile infants <60 days, using only absolute neutrophil count, urinalysis, and procalcitonin.15
Among the patients that are already a low risk, concomitant viral infections further decrease the pretest probability. Febrile infants with lab-confirmed respiratory viral infections are at lower risk for serious bacterial infections.16,17 Multiple retrospective and prospective observational studies have demonstrated that low-risk patients with bronchiolitis symptoms are extremely unlikely to have bacterial meningitis.8,18-22 A systematic review of 1749 febrile patients under 90 days of age with clinical bronchiolitis demonstrated no cases of meningitis.23 Many of these studies included infants aged <28 days. Though the total number of neonates (<28 days) in all studies is somewhat unclear, it suggests that the cut-off to avoid an LP may be even lower.
Recent literature has advocated outpatient observation without an LP for low-risk infants as a cost-effective management tool,24 and this is particularly true in patients with concomitant viral bronchiolitis.
Based on the latest data confirming the low prevalence of meningitis among all infants,3 the ability to identify low-risk infants based on risk stratification algorithms (Table), and the decreased prevalence of meningitis in patients with clinical bronchiolitis,23 low-risk infants with bronchiolitis seem to have minimal, if any, risk of meningitis. Therefore, low-risk infants with bronchiolitis do not warrant an LP.
Importantly, LPs are not risk neutral. Their benefit versus harm should be weighed every time they are considered. Approximately 19% of LP attempts in infants under 90 days old are either traumatic or unsuccessful.25 Infants aged 28 to 60 days with traumatic or unsuccessful LPs are more frequently hospitalized.25 Increased hospitalizations are associated with higher costs.4 The majority of positive CSF cultures are deemed to be “contaminants” (87% in one study26), but the positive result still leads to unnecessary further evaluation, hospitalization, repeated invasive procedures, and family distress.27 These data further support refraining from pursuing an LP in low-risk infants with bronchiolitis.
WHY LUMBAR PUNCTURE MIGHT BE HELPFUL IN CERTAIN CIRCUMSTANCES
If the patient is not low risk based on criteria or does not have clinical bronchiolitis, consider performing an LP. A recent study demonstrated a 0.4% incidence of bacterial meningitis in febrile infants with viral co-infection,29 though it is not determined if the patients presented with symptoms of bronchiolitis or were risk-stratified using the algorithms discussed.
In the studies looking at viral infections in febrile infants, each has important exclusion criteria including prematurity, comorbidities, and recent antibiotic administration.23 For these patients, an LP may be warranted (though the evidence is lacking). In addition, in very young infants (less than seven-14 days old), viral infections may be less common than in older infants, resulting in a desire to rule out bacterial infections more thoroughly in this population.
WHAT YOU SHOULD DO INSTEAD: AVOID AN LP IN LOW-RISK FEBRILE INFANTS WITH BRONCHIOLITIS
For low-risk febrile infants with signs of bronchiolitis, evaluation for bacterial meningitis is not necessary. The low prevalence of meningitis in this age range along with the even lower likelihood of meningitis when bronchiolitis is identified suggests that the procedure is unnecessary. Moreover, the risks associated with LP—including trauma, hospitalization, costs, and family stress—likely outweigh the benefits of CSF analysis.
RECOMMENDATIONS
- In febrile infants, determine the risk of serious bacterial infections using published algorithms (Table) before considering lumbar puncture.
- In low-risk febrile infants with typical bronchiolitis, evaluation for bacterial meningitis with an LP is not necessary.
CONCLUSION
Infants under 90 days of age often present to care with fever. While there is a concern for missing bacterial meningitis, the prevalence of such an infection in infants is very low. Moreover, in low-risk patients that present with typical bronchiolitis symptoms, the prevalence is effectively zero. LP practices vary by institution and can be associated with risks. In low-risk infants with typical bronchiolitis symptoms, an LP is one of the Things We Do for No Reason™.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
1. Cioffredi L-A, Jhaveri R. Evaluation and management of febrile children. JAMA Pediatr. 2016;170(8):794. https://doi.org/10.1001/jamapediatrics.2016.0596.
2. Huppler AR, Eickhoff JC, Wald ER. Performance of low-risk criteria in the evaluation of young infants with fever: review of the literature. Pediatrics. 2010;125(2):228-233. https://doi.org/10.1542/peds.2009-1070.
3. Biondi EA, Lee B, Ralston SL, et al. Prevalence of bacteremia and bacterial meningitis in febrile neonates and infants in the second month of life a systematic review and meta-analysis + supplemental content. JAMA Netw Open. 2019;2(3):190874. https://doi.org/10.1001/jamanetworkopen.2019.0874.
4. Aronson PL, Thurm C, Williams DJ, et al. Association of clinical practice guidelines with emergency department management of febrile infants ≤56 days of age. J Hosp Med. 2015;10(6):358-365. https://doi.org/10.1002/jhm.2329.
5. Aronson PL, Thurm C, Alpern ER, et al. Variation in care of the febrile young infant <90 days in us pediatric emergency departments. Pediatrics. 2014;134(4):667-677. https://doi.org/10.1542/peds.2014-1382.
6. Aronson PL, Thurm C, Williams DJ, et al. Association of clinical practice guidelines with emergency department management of febrile infants ≤56 days of age. J Hosp Med. 2015;10(6):358-365. https://doi.org/10.1002/jhm.2329.
7. Mendonca EA, Meissner HC, Gadomski AM, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742.
8. Melendez E, Harper MB. Utility of sepsis evaluation in infants 90 days of age or younger with fever and clinical bronchiolitis. Pediatr Infect Dis J. 2003;22(12):1053-1056. https://doi.org/10.1097/01.inf.0000101296.68993.4d.
9. Pruitt CM, Neuman MI, Shah SS, et al. Factors associated with adverse outcomes among febrile young infants with invasive bacterial infections. J. Pediatr. 2018;204:177-182. https://doi.org/10.1016/j.jpeds.2018.08.066.
10. Casper TC, Mahajan PV., Tzimenatos L, et al. The Yale Observation Scale Score and the risk of serious bacterial infections in febrile infants. Pediatrics. 2017;140(1):e20170695. https://doi.org/10.1542/peds.2017-0695.
11. Garges HP. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics. 2006;117(4):1094-1100. https://doi.org/10.1542/peds.2005-1132.
12. Jaskiewicz JA, McCarthy CA, Richardson AC, et al. Febrile infants at low risk for serious bacterial infection-an appraisal of the Rochester criteria and implications for management. Pediatrics. 1994;94(3):390-396. http://www.ncbi.nlm.nih.gov/pubmed/8065869. Accessed March 23, 2019.
13. Aronson P, Wang M, Shapiro E, et al. Risk stratification of febrile infants ≤60 days old without routine lumbar puncture. Pediatrics. 2018;142(6):e20181879. https://doi.org/10.1542/peds.2018-1879.
14. Galetto-Lacour A, Zamora SA, Andreola B, et al. Validation of a laboratory risk index score for the identification of severe bacterial infection in children with fever without source. Arch Dis Child. 2010;95(12):968-973. https://doi.org/10.1136/adc.2009.176800.
15. Kuppermann N, Dayan PS, Levine DA, et al. A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342. https://doi.org/10.1001/jamapediatrics.2018.5501.
16. Byington CL, Enriquez FR, Hoff C, et al. Serious bacterial infections in febrile infants 1 to 90 days old with and without viral infections. Pediatrics. 2004;113(6):1662-1666. https://doi.org/10.1542/peds.113.6.1662.
17. Cioffredi LA, Jhaveri R. Evaluation and management of febrile children: a review. JAMA Pediatr. 2016;170(8):794-800. https://doi.org/10.1001/jamapediatrics.2016.0596.
18. Dayan PS, Roskind CG, Levine DA, Kuppermann N. Controversies in the management of children with bronchiolitis. Clin Pediatr Emerg Med. 2004;5(1):41-53. https://doi.org/10.1016/j.cpem.2003.11.001.
19. Oray-Schrom P, Phoenix C, St. Martin D, Amoateng-Adjepong Y. Sepsis workup in febrile infants 0-90 days of age with respiratory syncytial virus infection. Pediatr Emerg Care. 2003;19(5):314-319. https://doi.org/10.1097/01.pec.0000092576.40174.28.
20. Purcell K, Fergie J. Concurrent serious bacterial infections in 2396 infants and children hospitalized with respiratory syncytial virus lower respiratory tract infections. Arch Pediatr Adolesc Med. 2002;156(4):322-324. https://doi.org/10.1001/archpedi.156.4.322.
21. Purcell K, Fergie J. Concurrent serious bacterial infections in 912 infants and children hospitalized for treatment of respiratory syncytial virus lower respiratory tract infection. Pediatr Infect Dis J. 2004;23(3):267-269. https://doi.org/10.1097/01.inf.0000116759.21252.29.
22. Yarden-Bilavsky H, Ashkenazi-Hoffnung L, Livni G, Amir J, Bilavsky E. Month-by-month age analysis of the risk for serious bacterial infections in febrile infants with bronchiolitis. Clin Pediatr (Phila). 2011;50(11):1052-1056. https://doi.org/10.1177/0009922811412949.
23. Ralston S, Hill V, Waters A. Occult serious bacterial infection in infants younger than 60 to 90 days with bronchiolitis: a systematic review. Arch Pediatr Adolesc Med. 2011;165(10):951-956. https://doi.org/10.1001/archpediatrics.2011.155.
24. Lee TJ, Aronson PL. To spinal tap or not to spinal tap, that is the question. Hosp Pediatr. 2018;8(4):236-238. https://doi.org/10.1542/hpeds.2017-0207.
25. Pingree EW, Kimia AA, Nigrovic LE. The effect of traumatic lumbar puncture on hospitalization rate for febrile infants 28 to 60 days of age. Acad Emerg Med. 2015;22(2):240-243. https://doi.org/10.1111/acem.12582.
26. Leazer R, Erickson N, Paulson J, et al. epidemiology of cerebrospinal fluid cultures and time to detection in term infants. Pediatrics. 2017;139(5):e20163268. https://doi.org/10.1542/peds.2016-3268.
27. Paxton RD, Byington CL. An examination of the unintended consequences of the rule-out sepsis evaluation: a parental perspective. Clin Pediatr (Phila). 2001;40(2):71-77. https://doi.org/10.1177/000992280104000202.
28. Mahajan P, Br owne LR, Levine DA, et al. Risk of bacterial coinfections in febrile infants 60 days old and younger with documented viral infections. J Pediatr. 2018;203:86-91.e2. https://doi.org/10.1016/j.jpeds.2018.07.073.
1. Cioffredi L-A, Jhaveri R. Evaluation and management of febrile children. JAMA Pediatr. 2016;170(8):794. https://doi.org/10.1001/jamapediatrics.2016.0596.
2. Huppler AR, Eickhoff JC, Wald ER. Performance of low-risk criteria in the evaluation of young infants with fever: review of the literature. Pediatrics. 2010;125(2):228-233. https://doi.org/10.1542/peds.2009-1070.
3. Biondi EA, Lee B, Ralston SL, et al. Prevalence of bacteremia and bacterial meningitis in febrile neonates and infants in the second month of life a systematic review and meta-analysis + supplemental content. JAMA Netw Open. 2019;2(3):190874. https://doi.org/10.1001/jamanetworkopen.2019.0874.
4. Aronson PL, Thurm C, Williams DJ, et al. Association of clinical practice guidelines with emergency department management of febrile infants ≤56 days of age. J Hosp Med. 2015;10(6):358-365. https://doi.org/10.1002/jhm.2329.
5. Aronson PL, Thurm C, Alpern ER, et al. Variation in care of the febrile young infant <90 days in us pediatric emergency departments. Pediatrics. 2014;134(4):667-677. https://doi.org/10.1542/peds.2014-1382.
6. Aronson PL, Thurm C, Williams DJ, et al. Association of clinical practice guidelines with emergency department management of febrile infants ≤56 days of age. J Hosp Med. 2015;10(6):358-365. https://doi.org/10.1002/jhm.2329.
7. Mendonca EA, Meissner HC, Gadomski AM, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742.
8. Melendez E, Harper MB. Utility of sepsis evaluation in infants 90 days of age or younger with fever and clinical bronchiolitis. Pediatr Infect Dis J. 2003;22(12):1053-1056. https://doi.org/10.1097/01.inf.0000101296.68993.4d.
9. Pruitt CM, Neuman MI, Shah SS, et al. Factors associated with adverse outcomes among febrile young infants with invasive bacterial infections. J. Pediatr. 2018;204:177-182. https://doi.org/10.1016/j.jpeds.2018.08.066.
10. Casper TC, Mahajan PV., Tzimenatos L, et al. The Yale Observation Scale Score and the risk of serious bacterial infections in febrile infants. Pediatrics. 2017;140(1):e20170695. https://doi.org/10.1542/peds.2017-0695.
11. Garges HP. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics. 2006;117(4):1094-1100. https://doi.org/10.1542/peds.2005-1132.
12. Jaskiewicz JA, McCarthy CA, Richardson AC, et al. Febrile infants at low risk for serious bacterial infection-an appraisal of the Rochester criteria and implications for management. Pediatrics. 1994;94(3):390-396. http://www.ncbi.nlm.nih.gov/pubmed/8065869. Accessed March 23, 2019.
13. Aronson P, Wang M, Shapiro E, et al. Risk stratification of febrile infants ≤60 days old without routine lumbar puncture. Pediatrics. 2018;142(6):e20181879. https://doi.org/10.1542/peds.2018-1879.
14. Galetto-Lacour A, Zamora SA, Andreola B, et al. Validation of a laboratory risk index score for the identification of severe bacterial infection in children with fever without source. Arch Dis Child. 2010;95(12):968-973. https://doi.org/10.1136/adc.2009.176800.
15. Kuppermann N, Dayan PS, Levine DA, et al. A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342. https://doi.org/10.1001/jamapediatrics.2018.5501.
16. Byington CL, Enriquez FR, Hoff C, et al. Serious bacterial infections in febrile infants 1 to 90 days old with and without viral infections. Pediatrics. 2004;113(6):1662-1666. https://doi.org/10.1542/peds.113.6.1662.
17. Cioffredi LA, Jhaveri R. Evaluation and management of febrile children: a review. JAMA Pediatr. 2016;170(8):794-800. https://doi.org/10.1001/jamapediatrics.2016.0596.
18. Dayan PS, Roskind CG, Levine DA, Kuppermann N. Controversies in the management of children with bronchiolitis. Clin Pediatr Emerg Med. 2004;5(1):41-53. https://doi.org/10.1016/j.cpem.2003.11.001.
19. Oray-Schrom P, Phoenix C, St. Martin D, Amoateng-Adjepong Y. Sepsis workup in febrile infants 0-90 days of age with respiratory syncytial virus infection. Pediatr Emerg Care. 2003;19(5):314-319. https://doi.org/10.1097/01.pec.0000092576.40174.28.
20. Purcell K, Fergie J. Concurrent serious bacterial infections in 2396 infants and children hospitalized with respiratory syncytial virus lower respiratory tract infections. Arch Pediatr Adolesc Med. 2002;156(4):322-324. https://doi.org/10.1001/archpedi.156.4.322.
21. Purcell K, Fergie J. Concurrent serious bacterial infections in 912 infants and children hospitalized for treatment of respiratory syncytial virus lower respiratory tract infection. Pediatr Infect Dis J. 2004;23(3):267-269. https://doi.org/10.1097/01.inf.0000116759.21252.29.
22. Yarden-Bilavsky H, Ashkenazi-Hoffnung L, Livni G, Amir J, Bilavsky E. Month-by-month age analysis of the risk for serious bacterial infections in febrile infants with bronchiolitis. Clin Pediatr (Phila). 2011;50(11):1052-1056. https://doi.org/10.1177/0009922811412949.
23. Ralston S, Hill V, Waters A. Occult serious bacterial infection in infants younger than 60 to 90 days with bronchiolitis: a systematic review. Arch Pediatr Adolesc Med. 2011;165(10):951-956. https://doi.org/10.1001/archpediatrics.2011.155.
24. Lee TJ, Aronson PL. To spinal tap or not to spinal tap, that is the question. Hosp Pediatr. 2018;8(4):236-238. https://doi.org/10.1542/hpeds.2017-0207.
25. Pingree EW, Kimia AA, Nigrovic LE. The effect of traumatic lumbar puncture on hospitalization rate for febrile infants 28 to 60 days of age. Acad Emerg Med. 2015;22(2):240-243. https://doi.org/10.1111/acem.12582.
26. Leazer R, Erickson N, Paulson J, et al. epidemiology of cerebrospinal fluid cultures and time to detection in term infants. Pediatrics. 2017;139(5):e20163268. https://doi.org/10.1542/peds.2016-3268.
27. Paxton RD, Byington CL. An examination of the unintended consequences of the rule-out sepsis evaluation: a parental perspective. Clin Pediatr (Phila). 2001;40(2):71-77. https://doi.org/10.1177/000992280104000202.
28. Mahajan P, Br owne LR, Levine DA, et al. Risk of bacterial coinfections in febrile infants 60 days old and younger with documented viral infections. J Pediatr. 2018;203:86-91.e2. https://doi.org/10.1016/j.jpeds.2018.07.073.
© 2019 Society of Hospital Medicine
A Branching Algorithm
A 21-year-old man with a history of hypertension presented to the emergency department with four days of generalized abdominal pain, nausea, and vomiting as well as one month of loose stools. He also had a headache (not further specified) for one day. Due to his nausea, he had been unable to take his medications for two days. Home blood pressure measurements over the preceding two days revealed systolic pressures exceeding 200 mm Hg. He did not experience fever, dyspnea, chest pain, vision changes, numbness, weakness, diaphoresis, or palpitations.
Abdominal pain with vomiting and diarrhea is often caused by a self-limited gastroenteritis. However, the priority initially is to exclude serious intraabdominal processes including arterial insufficiency, bowel obstruction, organ perforation, or organ-based infection or inflammation (eg, appendicitis, cholecystitis, pancreatitis). Essential hypertension accounts for 95% of cases of hypertension in the United States, but given this patient’s young age, secondary causes should be evaluated. These include primary aldosteronism (the most common endocrine cause for hypertension in young patients), chronic kidney disease, fibromuscular dysplasia, illicit drug use, hypercortisolism, pheochromocytoma, and coarctation of the aorta. Thyrotoxicosis can elevate blood pressure (although usually not to this extent) and cause hyperdefecation. While the etiology of the chronic hypertension is uncertain, the proximate cause of the acute rise in blood pressure is likely the stress of his acute illness and the inability to take his prescribed antihypertensive medications. In the setting of severe hypertension, his headache may reflect an intracranial hemorrhage and his abdominal pain could signal an aortic dissection.
His medical history included hypertension diagnosed at age 16 as well as anxiety diagnosed following a panic attack at age 19. Over the past year, he had also developed persistent nausea, which was attributed to gastroesophageal reflux disease. His medications included metoprolol 50 mg daily, amlodipine 5 mg daily, hydrochlorothiazide 12.5 mg daily, escitalopram 20 mg daily, and omeprazole 20 mg daily. His father and 15-year-old brother also had hypertension. He was a part-time student while working at a car dealership. He did not smoke or use drugs and he rarely drank alcohol.
The need for three antihypertensive medications (albeit at submaximal doses) reflects the severity of his hypertension (provided challenges with medication adherence have been excluded). His family history, especially that of his brother who was diagnosed with hypertension at an early age, and the patient’s own early onset hypertension point toward an inherited form of hypertension. Autosomal dominant polycystic kidney disease often results in hypertension before chronic kidney disease develops. Rare inherited forms of hypertension include familial hyperaldosteronism, apparent mineralocorticoid excess, Liddle syndrome, or a hereditary endocrine tumor syndrome predisposing to pheochromocytoma. Even among patients who report classic pheochromocytoma symptoms, such as headache and anxiety, the diagnosis remains unlikely as these symptoms are nonspecific and highly prevalent in the general population. However, once secondary hypertension is plausible or suspected, testing for hyperadrenergic states, which can also cause nausea and vomiting during times of catecholamine excess, should be pursued.
His temperature was 97.5°F, heart rate 95 beats per minute and regular, respiratory rate 18 breaths per minute, blood pressure 181/118 mm Hg (systolic and diastolic pressures in each arm were within 10 mm Hg), and oxygen saturation 100% on room air. Systolic and diastolic pressures did not decrease by more than 20 mm Hg and 10 mm Hg, respectively, after he stood for two minutes. His body mass index was 24 kg/m2. He was alert and appeared slightly anxious. There was a bounding point of maximal impulse in the fifth intercostal space at the midclavicular line and a 3/6 systolic murmur at the left upper sternal border with radiation to the carotid arteries. His abdomen was soft with generalized tenderness to palpation and without rebound tenderness, masses, organomegaly, or bruits. There was no costovertebral angle tenderness. No lymphadenopathy was present. His fundoscopic, pulmonary, skin and neurologic examinations were normal.
Laboratory studies revealed a white blood cell count of 13.3 × 103/uL with a normal differential, hemoglobin 13.9 g/dL, platelet count 373 × 103/uL, sodium 142 mmol/L, potassium 3.8 mmol/L, chloride 103 mmol/L,bicarbonate 25 mmol/L, blood urea nitrogen 12 mg/dL, creatinine 1.3 mg/dL (a baseline creatinine level was not available), glucose 88 mg/dL, calcium 10.6 mg/dL, albumin 4.9 g/dL, aspartate aminotransferase 27 IU/L, alanine aminotransferase 37 IU/L, and lipase 40 IU/L. Urinalysis revealed 5-10 white blood cells per high power field without casts and 10 mg/dL protein. Urine toxicology was not performed. Electrocardiogram (ECG) showed left ventricular hypertrophy (LVH). Chest radiography was normal.
The abdominal examination does not suggest peritonitis. The laboratory tests do not suggest inflammation of the liver, pancreas, or biliary tree as the cause of his abdominal pain or diarrhea. The murmur may indicate hypertrophic cardiomyopathy or a congenital anomaly such as bicuspid aortic valve; but neither would explain hypertension unless they were associated with another developmental abnormality, such as coarctation of the aorta. Tricuspid regurgitation is conceivable and if confirmed, might raise concern for carcinoid syndrome, which can cause diarrhea. The normal neurologic examination, including the absence of papilledema, lowers suspicion of intracranial hemorrhage as a cause of his headache.
The albumin of 4.9 g/dL likely reflects hypovolemia resulting from vomiting and diarrhea. Vasoconstriction associated with pheochromocytoma can cause pressure diuresis and resultant hypovolemia. Hyperaldosteronism arising from bilateral adrenal hyperplasia or adrenal adenoma commonly causes hypokalemia, although this is not a universal feature.
The duration of his mildly decreased glomerular filtration rate is uncertain. He may have chronic kidney disease from sustained hypertension, or acute kidney injury from hypovolemia. The mild pyuria could indicate infection or renal calculi, either of which could account for generalized abdominal pain or could reflect an acute renal injury from acute interstitial nephritis from his proton pump inhibitor or hydrochlorothiazide.
LVH on the ECG indicates longstanding hypertension. The chest radiograph does not reveal clues to the etiology of or sequelae from hypertension. In particular, there is no widened aorta to suggest aortic dissection, no pulmonary edema to indicate heart failure, and no rib notching that points toward aortic coarctation. A transthoracic echocardiogram to assess for valvular and other structural abnormalities is warranted.
Tests for secondary hypertension should be sent, including serum aldosterone and renin levels to assess for primary aldosteronism and plasma or 24-hour urine normetanephrine and metanephrine levels to assess for pheochromocytoma. Biochemical evaluation is the mainstay for endocrine hypertension evaluation and should be followed by imaging if abnormal results are found.
Intact parathyroid hormone (PTH) was 78 pg/mL (normal, 10-65 pg/mL), thyroid stimulating hormone 3.6 mIU/L (normal, 0.30-5.50 mIU/L), and morning cortisol 4.1 ug/dL (normal, >7.0 ug/dL). Plasma aldosterone was 14.6 ng/dL (normal, 1-16 ng/dL), plasma renin activity 3.6 ng/mL/hr (normal, 0.5-3.5 ng/mL/hr), and aldosterone-renin ratio 4.1 (normal, <20). Transthoracic echocardiogram showed LVH with normal valves, wall motion, and proximal aorta; the left ventricular ejection fraction was 70%. Magnetic resonance angiography of the renal vessels demonstrated no abnormalities.
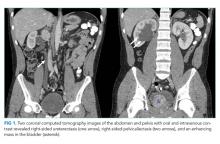
Computed tomography (CT) of the abdomen and pelvis with oral and intravenous contrast revealed a 5 cm heterogeneous enhancing mass associated with the prostate gland extending into the base of the bladder. The mass obstructed the right renal collecting system and ureter causing severe right-sided ureterectasis and hydronephrosis. There was also 2.8 cm right-sided paracaval lymph node enlargement and 2.1 cm right-sided and 1.5 cm left-sided external iliac lymph node enlargement (Figure 1). There were no adrenal masses.
He is young for prostate, bladder, or colorectal cancer, but early onset variations of these tumors, along with metastatic testicular cancer, must be considered for the pelvic mass and associated lymphadenopathy. Prostatic masses can be infectious (eg, abscess) or malignant (eg, adenocarcinoma, small cell carcinoma). Additional considerations for abdominopelvic cancer are sarcomas, germ cell tumors, or lymphoma. A low aldosterone-renin ratio coupled with a normal potassium level makes primary aldosteronism unlikely. The normal angiography excludes renovascular hypertension.
His abdominal pain and gastrointestinal symptoms could arise from irritation of the bowel, distension of the right-sided urinary collecting system, or products secreted from the mass (eg, catecholamines). The hyperdynamic precordium, elevated ejection fraction, and murmur may reflect augmented blood flow from a hyperadrenergic state. A unifying diagnosis would be a pheochromocytoma. However, given the normal appearance of the adrenal glands on CT imaging, catecholamines arising from a paraganglioma, a tumor of the autonomic nervous system, is more likely. These tumors often secrete catecholamines and can be metastatic (suggested here by the lymphadenopathy). Functional imaging or biopsy of either the mass or an adjacent lymph node is indicated. However, because of the possibility of a catecholamine-secreting tumor, he should be treated with an alpha-adrenergic receptor antagonist before undergoing a biopsy to prevent unopposed vasoconstriction from catecholamine leakage.
Scrotal ultrasound revealed no evidence of a testicular tumor. Lactate dehydrogenase (LDH) was 179 IU/L (normal, 120-240 IU/L) and prostate specific antigen (PSA) was 0.7 ng/mL (normal, <2.5 ng/mL). The patient was given amlodipine and labetalol with improvement of blood pressures to 160s/100s. His creatinine decreased to 1.1 mg/dL. He underwent CT-guided biopsy of a pelvic lymph node. CT of the head without intravenous contrast demonstrated no intracranial abnormalities. His headache resolved with improvement in blood pressure, and he had minimal gastrointestinal symptoms during his hospitalization. No stool studies were sent. A right-sided percutaneous nephrostomy was placed which yielded >15 L of urine from the tube over the next four days.
Upon the first episode of micturition through the urethra four days after percutaneous nephrostomy placement, he experienced severe lightheadedness, diaphoresis, and palpitations. These symptoms prompted him to recall similar episodes following micturition for several months prior to his hospitalization.
It is likely that contraction of the bladder during episodes of urination caused irritation of the pelvic mass, leading to catecholamine secretion. Another explanation for his recurrent lightheadedness would be a neurocardiogenic reflex with micturition (which when it culminates with loss of consciousness is called micturition syncope), but this would not explain his hypertension or bladder mass.
Biochemical tests that were ordered on admission but sent to a reference lab then returned. Plasma metanephrine was 0.2 nmol/L (normal, <0.5 nmol/L) and plasma normetanephrine 34.6 nmol/L (normal, <0.9 nmol/L). His 24-hour urine metanephrine was 72 ug/24 hr (normal, 0-300 ug/24 hr) and normetanephrine 8,511 ug/24 hr (normal, 50-800 ug/24 hr).
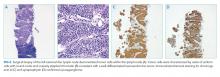
The markedly elevated plasma and urine normetanephrine levels confirm a diagnosis of a catecholamine-secreting tumor (paraganglioma). The tissue obtained from the CT-guided lymph node biopsy should be sent for markers of neuroendocrine tumors including chromogranin.
Lymph node biopsy revealed metastatic paraganglioma that was chromogranin A and synaptophysin positive (Figure 2). A fluorodeoxyglucose positron emission tomography (FDG-PET) scan disclosed skull metastases. He was treated with phenoxybenzamine, amlodipine, and labetalol. Surgical resection of the pelvic mass was discussed, but the patient elected to defer surgery as the location of the primary tumor made it challenging to resect and would have required an ileal conduit.
After the diagnosis was made, the patient’s family recalled that a maternal uncle had been diagnosed with a paraganglioma of the carotid body. Genetic testing of the patient identified a succinate dehydrogenase complex subunit B (SDHB) pathogenic variant and confirmed hereditary paraganglioma syndrome (HPGL). One year after the diagnosis, liver and lung metastases developed. He was treated with lanreotide (somatostatin analogue), capecitabine, and temozolomide, as well as a craniotomy and radiotherapy for palliation of bony metastases. The patient died less than two years after diagnosis.
DISCUSSION
Most patients with hypertension (defined as blood pressure >130/80 mm Hg1) do not have an identifiable etiology (primary hypertension). Many components of this patient’s history, however, including his young age of onset, a teenage sibling with hypertension, lack of obesity, hypertension refractory to multiple medications, and LVH suggested secondary hypertension. Hypertension onset at an age less than 30 years, resistance to three or more medications,1,2 and/or acute onset hypertension at any age should prompt an evaluation for secondary causes.1 The prevalence of secondary hypertension is approximately 30% in hypertensive patients ages 18 to 40 years compared with 5%-10% in the overall adult population with hypertension.3 Among children and adolescents ages 0 to 19 years with hypertension, the prevalence of secondary hypertension may be as high as 57%.4
The most common etiology of secondary hypertension is primary aldosteronism.5,6 However, in young adults (ages 19 to 39 years), common etiologies also include renovascular disease and renal parenchymal disease.7 Other causes include obstructive sleep apnea, medications, stimulants (cocaine and amphetamines),8 and endocrinopathies such as thyrotoxicosis, Cushing syndrome, and catecholamine-secreting tumors.7 Less than 1% of secondary hypertension in all adults is due to catecholamine-secreting tumors, and the minority of those catecholamine-secreting tumors are paragangliomas.9
Paragangliomas are tumors of the peripheral autonomic nervous system. These neoplasms arise in the sympathetic and parasympathetic chains along the paravertebral and paraaortic axes. They are closely related to pheochromocytomas, which arise in the adrenal medulla.9 Most head and neck paragangliomas are biochemically silent and are generally discovered due to mass effect.10 The subset of paragangliomas that secrete catecholamines most often arise in the abdomen and pelvis, and their clinical presentation mimics that of pheochromocytomas, including episodic hypertension, palpitations, pallor, and diaphoresis.
This patient had persistent, nonepisodic hypertension, while palpitations and diaphoresis only manifested following micturition. Other cases of urinary bladder paragangliomas have described micturition-associated symptoms and hypertensive crises. Three-fold increases of catecholamine secretion after micturition have been observed in these patients, likely due to muscle contraction and pressure changes in the bladder leading to the systemic release of catecholamines.11
Epinephrine and norepinephrine are monoamine neurotransmitters that activate alpha-adrenergic and beta-adrenergic receptors. Adrenergic receptors are present in all tissues of the body but have prominent effects on the smooth muscle in the vasculature, gastrointestinal tract, urinary tract, and airways.12 Alpha-adrenergic vasoconstriction causes hypertension, which is commonly observed in patients with catecholamine-secreting tumors.10 Catecholamine excess due to secretion from these tumors causes headache in 60%-80% of patients, tachycardia/palpitations in 50%-70%, anxiety in 20%-40%, and nausea in 20%-25%.10 Other symptoms include sweating, pallor, dyspnea, and vertigo.9,10 This patient’s chronic nausea, which was attributed to gastroesophageal reflux, and his anxiety, attributed to generalized anxiety disorder, were likely symptoms of catecholamine excess.13
The best test for the diagnosis of paragangliomas and pheochromocytomas is the measurement of plasma free or 24-hour urinary fractionated metanephrines (test sensitivity of >90% and >90%, respectively).14 Screening for pheochromocytoma should be considered in hypertensive patients who have symptoms of catecholamine excess, refractory or paroxysmal hypertension, and/or familial pheochromocytoma/paraganglioma syndromes.15 Screening for pheochromocytoma should also be performed in children and adolescents with systolic or diastolic blood pressure that is greater than the 95th percentile for their age plus 5 mm Hg.16
While a typical tumor location and elevated metanephrine levels are sufficient to make the diagnosis of a pheochromocytoma or catecholamine-secreting paraganglioma, functional imaging with FDG-PET, Ga-DOTATATE-PET, or 123I-meta-iodobenzylguanidine (123I-MIBG) can further confirm the diagnosis and detect distant metastases. However, imaging has low sensitivity for these tumors and thus should only be considered for patients in whom metastatic disease is suspected.14 Biopsy is rarely needed and should be reserved for unusual metastatic locations. Treatment with an alpha-adrenergic receptor antagonist often reduces symptoms and lowers blood pressure. Definitive management typically involves surgical resection for benign disease. Surgery, radionuclide therapy, or chemotherapy is used for malignant disease.
While most pheochromocytomas are sporadic, up to 40% of paragangliomas are due to germline pathogenic variants.17 Mutations in the succinate dehydrogenase (SDH) group of genes are the most common germline pathogenic variants in the autosomal dominant hereditary paraganglioma syndrome (HPGL). Most paragangliomas and pheochromocytomas are localized and benign, but 10%-15% are metastatic.18 SDHB mutations are associated with a high risk of metastasis.19 Thus, genetic testing for patients and subsequent cascade testing to identify at-risk family members is advised in all patients with pheochromocytomas or paragangliomas.20 This patient’s younger brother and mother were both found to carry the same pathogenic SDHB variant, but neither was found to have paragangliomas. Annual metanephrine levels (urine or plasma) and every other year whole-body magnetic resonance imaging (MRI) scans were recommended for tumor surveillance.
The clinician team followed a logical branching algorithm for the diagnosis of severe hypertension with biochemical testing, advanced imaging, histology, and genetic testing to arrive at the final diagnosis of hereditary paraganglioma syndrome. Although this patient presented for urgent care because of the acute effects of catecholamine excess, he suffered from chronic effects (nausea, anxiety, and hypertension) for years. Each symptom had been diagnosed and treated in isolation, but the combination and severity in a young patient suggested a unifying diagnosis. The family history of hypertension (brother and father) suggested an inherited diagnosis from the father’s family, but the final answer rested on the other branch (maternal uncle) of the family tree.
KEY TEACHING POINTS
- Hypertension in a young adult is due to a secondary cause in up to 30% of patients.
- Pathologic catecholamine excess leads to hypertension, tachycardia, pallor, sweating, anxiety, and nausea. A sustained and unexplained combination of these symptoms should prompt a biochemical evaluation for pheochromocytoma or paraganglioma.
- Paragangliomas are tumors of the autonomic nervous system. The frequency of catecholamine secretion depends on their location in the body, and they are commonly caused by germline pathogenic variants.
Acknowledgments
This conundrum was presented during a live Grand Rounds with the expert clinician’s responses recorded and edited for space and clarity.
Disclosures
Dr. Dhaliwal reports speaking honoraria from ISMIE Mutual Insurance Company and GE Healthcare. All other authors have nothing to disclose.
Funding
No sources of funding.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13-e115. https://doi.org/10.1161/HYP.0000000000000065.
2. Acelajado MC, Calhoun DA. Resistant hypertension, secondary hypertension, and hypertensive crises: diagnostic evaluation and treatment. Cardiol Clin. 2010;28(4):639-654. https://doi.org/10.1016/j.ccl.2010.07.002.
3. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206-1252. https://doi.org/10.1161/01.HYP.0000107251.49515.c2.
4. Gupta-Malhotra M, Banker A, Shete S, et al. Essential hypertension vs. secondary hypertension among children. Am J Hypertens. 2015;28(1):73-80. https://doi.org/10.1093/ajh/hpu083.
5. Mosso L, Carvajal C, Gonzalez A, et al. Primary aldosteronism and hypertensive disease. Hypertension. 2003;42(2):161-165. https://doi.org/10.1161/01.HYP.0000079505.25750.11.
6. Kayser SC, Dekkers T, Groenewoud HJ, et al. Study heterogeneity and estimation of prevalence of primary aldosteronism: a systematic review and meta-regression analysis. J Clin Endocrinol Metab. 2016;101(7):2826-2835. https://doi.org/10.1210/jc.2016-1472.
7. Charles L, Triscott J, Dobbs B. Secondary hypertension: discovering the underlying cause. Am Fam Physician. 2017;96(7):453-461.
8. Aronow WS. Drug-induced causes of secondary hypertension. Ann Transl Med. 2017;5(17):349. https://doi.org/10.21037/atm.2017.06.16.
9. Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366(9486):665-675. https://doi.org/10.1016/S0140-6736(05)67139-5.
10. Mannelli M, Lenders JW, Pacak K, Parenti G, Eisenhofer G. Subclinical phaeochromocytoma. Best Pract Res Clin Endocrinol Metab. 2012;26(4):507-515. https://doi.org/10.1016/j.beem.2011.10.008.
11. Kappers MH, van den Meiracker AH, Alwani RA, Kats E, Baggen MG. Paraganglioma of the urinary bladder. Neth J Med. 2008;66(4):163-165.
12. Paravati S, Warrington SJ. Physiology, Catecholamines. In: StatPearls. Treasure Island, FL: StatPearls Publishing LLC; 2019.
13. King KS, Darmani NA, Hughes MS, Adams KT, Pacak K. Exercise-induced nausea and vomiting: another sign and symptom of pheochromocytoma and paraganglioma. Endocrine. 2010;37(3):403-407. https://doi.org/10.1007/s12020-010-9319-3.
14. Lenders JW, Duh QY, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915-1942. https://doi.org/10.1210/jc.2014-1498.
15. Lenders JWM, Eisenhofer G. Update on modern management of pheochromocytoma and paraganglioma. Endocrinol Metab (Seoul). 2017;32(2):152-161. https://doi.org/10.3803/EnM.2017.32.2.152.
16. National High Blood Pressure Education Program Working Group. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2):555-576.
17. Else T, Greenberg S, Fishbein L. Hereditary Paraganglioma-Pheochromocytoma Syndromes. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. Gene Reviews. Seattle, WA: University of Washington; 1993.
18. Goldstein RE, O’Neill JA, Jr., Holcomb GW, 3rd, et al. Clinical experience over 48 years with pheochromocytoma. Ann Surg. 1999;229(6):755-764; discussion 764-756. https://doi.org/10.1097/00000658-199906000-00001.
19. Amar L, Baudin E, Burnichon N, et al. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrinol Metab. 2007;92(10):3822-3828. https://doi.org/10.1210/jc.2007-0709.
20. Favier J, Amar L, Gimenez-Roqueplo AP. Paraganglioma and phaeochromocytoma: from genetics to personalized medicine. Nat Rev Endocrinol. 2015;11(2):101-111. https://doi.org/10.1038/nrendo.2014.188.
A 21-year-old man with a history of hypertension presented to the emergency department with four days of generalized abdominal pain, nausea, and vomiting as well as one month of loose stools. He also had a headache (not further specified) for one day. Due to his nausea, he had been unable to take his medications for two days. Home blood pressure measurements over the preceding two days revealed systolic pressures exceeding 200 mm Hg. He did not experience fever, dyspnea, chest pain, vision changes, numbness, weakness, diaphoresis, or palpitations.
Abdominal pain with vomiting and diarrhea is often caused by a self-limited gastroenteritis. However, the priority initially is to exclude serious intraabdominal processes including arterial insufficiency, bowel obstruction, organ perforation, or organ-based infection or inflammation (eg, appendicitis, cholecystitis, pancreatitis). Essential hypertension accounts for 95% of cases of hypertension in the United States, but given this patient’s young age, secondary causes should be evaluated. These include primary aldosteronism (the most common endocrine cause for hypertension in young patients), chronic kidney disease, fibromuscular dysplasia, illicit drug use, hypercortisolism, pheochromocytoma, and coarctation of the aorta. Thyrotoxicosis can elevate blood pressure (although usually not to this extent) and cause hyperdefecation. While the etiology of the chronic hypertension is uncertain, the proximate cause of the acute rise in blood pressure is likely the stress of his acute illness and the inability to take his prescribed antihypertensive medications. In the setting of severe hypertension, his headache may reflect an intracranial hemorrhage and his abdominal pain could signal an aortic dissection.
His medical history included hypertension diagnosed at age 16 as well as anxiety diagnosed following a panic attack at age 19. Over the past year, he had also developed persistent nausea, which was attributed to gastroesophageal reflux disease. His medications included metoprolol 50 mg daily, amlodipine 5 mg daily, hydrochlorothiazide 12.5 mg daily, escitalopram 20 mg daily, and omeprazole 20 mg daily. His father and 15-year-old brother also had hypertension. He was a part-time student while working at a car dealership. He did not smoke or use drugs and he rarely drank alcohol.
The need for three antihypertensive medications (albeit at submaximal doses) reflects the severity of his hypertension (provided challenges with medication adherence have been excluded). His family history, especially that of his brother who was diagnosed with hypertension at an early age, and the patient’s own early onset hypertension point toward an inherited form of hypertension. Autosomal dominant polycystic kidney disease often results in hypertension before chronic kidney disease develops. Rare inherited forms of hypertension include familial hyperaldosteronism, apparent mineralocorticoid excess, Liddle syndrome, or a hereditary endocrine tumor syndrome predisposing to pheochromocytoma. Even among patients who report classic pheochromocytoma symptoms, such as headache and anxiety, the diagnosis remains unlikely as these symptoms are nonspecific and highly prevalent in the general population. However, once secondary hypertension is plausible or suspected, testing for hyperadrenergic states, which can also cause nausea and vomiting during times of catecholamine excess, should be pursued.
His temperature was 97.5°F, heart rate 95 beats per minute and regular, respiratory rate 18 breaths per minute, blood pressure 181/118 mm Hg (systolic and diastolic pressures in each arm were within 10 mm Hg), and oxygen saturation 100% on room air. Systolic and diastolic pressures did not decrease by more than 20 mm Hg and 10 mm Hg, respectively, after he stood for two minutes. His body mass index was 24 kg/m2. He was alert and appeared slightly anxious. There was a bounding point of maximal impulse in the fifth intercostal space at the midclavicular line and a 3/6 systolic murmur at the left upper sternal border with radiation to the carotid arteries. His abdomen was soft with generalized tenderness to palpation and without rebound tenderness, masses, organomegaly, or bruits. There was no costovertebral angle tenderness. No lymphadenopathy was present. His fundoscopic, pulmonary, skin and neurologic examinations were normal.
Laboratory studies revealed a white blood cell count of 13.3 × 103/uL with a normal differential, hemoglobin 13.9 g/dL, platelet count 373 × 103/uL, sodium 142 mmol/L, potassium 3.8 mmol/L, chloride 103 mmol/L,bicarbonate 25 mmol/L, blood urea nitrogen 12 mg/dL, creatinine 1.3 mg/dL (a baseline creatinine level was not available), glucose 88 mg/dL, calcium 10.6 mg/dL, albumin 4.9 g/dL, aspartate aminotransferase 27 IU/L, alanine aminotransferase 37 IU/L, and lipase 40 IU/L. Urinalysis revealed 5-10 white blood cells per high power field without casts and 10 mg/dL protein. Urine toxicology was not performed. Electrocardiogram (ECG) showed left ventricular hypertrophy (LVH). Chest radiography was normal.
The abdominal examination does not suggest peritonitis. The laboratory tests do not suggest inflammation of the liver, pancreas, or biliary tree as the cause of his abdominal pain or diarrhea. The murmur may indicate hypertrophic cardiomyopathy or a congenital anomaly such as bicuspid aortic valve; but neither would explain hypertension unless they were associated with another developmental abnormality, such as coarctation of the aorta. Tricuspid regurgitation is conceivable and if confirmed, might raise concern for carcinoid syndrome, which can cause diarrhea. The normal neurologic examination, including the absence of papilledema, lowers suspicion of intracranial hemorrhage as a cause of his headache.
The albumin of 4.9 g/dL likely reflects hypovolemia resulting from vomiting and diarrhea. Vasoconstriction associated with pheochromocytoma can cause pressure diuresis and resultant hypovolemia. Hyperaldosteronism arising from bilateral adrenal hyperplasia or adrenal adenoma commonly causes hypokalemia, although this is not a universal feature.
The duration of his mildly decreased glomerular filtration rate is uncertain. He may have chronic kidney disease from sustained hypertension, or acute kidney injury from hypovolemia. The mild pyuria could indicate infection or renal calculi, either of which could account for generalized abdominal pain or could reflect an acute renal injury from acute interstitial nephritis from his proton pump inhibitor or hydrochlorothiazide.
LVH on the ECG indicates longstanding hypertension. The chest radiograph does not reveal clues to the etiology of or sequelae from hypertension. In particular, there is no widened aorta to suggest aortic dissection, no pulmonary edema to indicate heart failure, and no rib notching that points toward aortic coarctation. A transthoracic echocardiogram to assess for valvular and other structural abnormalities is warranted.
Tests for secondary hypertension should be sent, including serum aldosterone and renin levels to assess for primary aldosteronism and plasma or 24-hour urine normetanephrine and metanephrine levels to assess for pheochromocytoma. Biochemical evaluation is the mainstay for endocrine hypertension evaluation and should be followed by imaging if abnormal results are found.
Intact parathyroid hormone (PTH) was 78 pg/mL (normal, 10-65 pg/mL), thyroid stimulating hormone 3.6 mIU/L (normal, 0.30-5.50 mIU/L), and morning cortisol 4.1 ug/dL (normal, >7.0 ug/dL). Plasma aldosterone was 14.6 ng/dL (normal, 1-16 ng/dL), plasma renin activity 3.6 ng/mL/hr (normal, 0.5-3.5 ng/mL/hr), and aldosterone-renin ratio 4.1 (normal, <20). Transthoracic echocardiogram showed LVH with normal valves, wall motion, and proximal aorta; the left ventricular ejection fraction was 70%. Magnetic resonance angiography of the renal vessels demonstrated no abnormalities.
Computed tomography (CT) of the abdomen and pelvis with oral and intravenous contrast revealed a 5 cm heterogeneous enhancing mass associated with the prostate gland extending into the base of the bladder. The mass obstructed the right renal collecting system and ureter causing severe right-sided ureterectasis and hydronephrosis. There was also 2.8 cm right-sided paracaval lymph node enlargement and 2.1 cm right-sided and 1.5 cm left-sided external iliac lymph node enlargement (Figure 1). There were no adrenal masses.
He is young for prostate, bladder, or colorectal cancer, but early onset variations of these tumors, along with metastatic testicular cancer, must be considered for the pelvic mass and associated lymphadenopathy. Prostatic masses can be infectious (eg, abscess) or malignant (eg, adenocarcinoma, small cell carcinoma). Additional considerations for abdominopelvic cancer are sarcomas, germ cell tumors, or lymphoma. A low aldosterone-renin ratio coupled with a normal potassium level makes primary aldosteronism unlikely. The normal angiography excludes renovascular hypertension.
His abdominal pain and gastrointestinal symptoms could arise from irritation of the bowel, distension of the right-sided urinary collecting system, or products secreted from the mass (eg, catecholamines). The hyperdynamic precordium, elevated ejection fraction, and murmur may reflect augmented blood flow from a hyperadrenergic state. A unifying diagnosis would be a pheochromocytoma. However, given the normal appearance of the adrenal glands on CT imaging, catecholamines arising from a paraganglioma, a tumor of the autonomic nervous system, is more likely. These tumors often secrete catecholamines and can be metastatic (suggested here by the lymphadenopathy). Functional imaging or biopsy of either the mass or an adjacent lymph node is indicated. However, because of the possibility of a catecholamine-secreting tumor, he should be treated with an alpha-adrenergic receptor antagonist before undergoing a biopsy to prevent unopposed vasoconstriction from catecholamine leakage.
Scrotal ultrasound revealed no evidence of a testicular tumor. Lactate dehydrogenase (LDH) was 179 IU/L (normal, 120-240 IU/L) and prostate specific antigen (PSA) was 0.7 ng/mL (normal, <2.5 ng/mL). The patient was given amlodipine and labetalol with improvement of blood pressures to 160s/100s. His creatinine decreased to 1.1 mg/dL. He underwent CT-guided biopsy of a pelvic lymph node. CT of the head without intravenous contrast demonstrated no intracranial abnormalities. His headache resolved with improvement in blood pressure, and he had minimal gastrointestinal symptoms during his hospitalization. No stool studies were sent. A right-sided percutaneous nephrostomy was placed which yielded >15 L of urine from the tube over the next four days.
Upon the first episode of micturition through the urethra four days after percutaneous nephrostomy placement, he experienced severe lightheadedness, diaphoresis, and palpitations. These symptoms prompted him to recall similar episodes following micturition for several months prior to his hospitalization.
It is likely that contraction of the bladder during episodes of urination caused irritation of the pelvic mass, leading to catecholamine secretion. Another explanation for his recurrent lightheadedness would be a neurocardiogenic reflex with micturition (which when it culminates with loss of consciousness is called micturition syncope), but this would not explain his hypertension or bladder mass.
Biochemical tests that were ordered on admission but sent to a reference lab then returned. Plasma metanephrine was 0.2 nmol/L (normal, <0.5 nmol/L) and plasma normetanephrine 34.6 nmol/L (normal, <0.9 nmol/L). His 24-hour urine metanephrine was 72 ug/24 hr (normal, 0-300 ug/24 hr) and normetanephrine 8,511 ug/24 hr (normal, 50-800 ug/24 hr).
The markedly elevated plasma and urine normetanephrine levels confirm a diagnosis of a catecholamine-secreting tumor (paraganglioma). The tissue obtained from the CT-guided lymph node biopsy should be sent for markers of neuroendocrine tumors including chromogranin.
Lymph node biopsy revealed metastatic paraganglioma that was chromogranin A and synaptophysin positive (Figure 2). A fluorodeoxyglucose positron emission tomography (FDG-PET) scan disclosed skull metastases. He was treated with phenoxybenzamine, amlodipine, and labetalol. Surgical resection of the pelvic mass was discussed, but the patient elected to defer surgery as the location of the primary tumor made it challenging to resect and would have required an ileal conduit.
After the diagnosis was made, the patient’s family recalled that a maternal uncle had been diagnosed with a paraganglioma of the carotid body. Genetic testing of the patient identified a succinate dehydrogenase complex subunit B (SDHB) pathogenic variant and confirmed hereditary paraganglioma syndrome (HPGL). One year after the diagnosis, liver and lung metastases developed. He was treated with lanreotide (somatostatin analogue), capecitabine, and temozolomide, as well as a craniotomy and radiotherapy for palliation of bony metastases. The patient died less than two years after diagnosis.
DISCUSSION
Most patients with hypertension (defined as blood pressure >130/80 mm Hg1) do not have an identifiable etiology (primary hypertension). Many components of this patient’s history, however, including his young age of onset, a teenage sibling with hypertension, lack of obesity, hypertension refractory to multiple medications, and LVH suggested secondary hypertension. Hypertension onset at an age less than 30 years, resistance to three or more medications,1,2 and/or acute onset hypertension at any age should prompt an evaluation for secondary causes.1 The prevalence of secondary hypertension is approximately 30% in hypertensive patients ages 18 to 40 years compared with 5%-10% in the overall adult population with hypertension.3 Among children and adolescents ages 0 to 19 years with hypertension, the prevalence of secondary hypertension may be as high as 57%.4
The most common etiology of secondary hypertension is primary aldosteronism.5,6 However, in young adults (ages 19 to 39 years), common etiologies also include renovascular disease and renal parenchymal disease.7 Other causes include obstructive sleep apnea, medications, stimulants (cocaine and amphetamines),8 and endocrinopathies such as thyrotoxicosis, Cushing syndrome, and catecholamine-secreting tumors.7 Less than 1% of secondary hypertension in all adults is due to catecholamine-secreting tumors, and the minority of those catecholamine-secreting tumors are paragangliomas.9
Paragangliomas are tumors of the peripheral autonomic nervous system. These neoplasms arise in the sympathetic and parasympathetic chains along the paravertebral and paraaortic axes. They are closely related to pheochromocytomas, which arise in the adrenal medulla.9 Most head and neck paragangliomas are biochemically silent and are generally discovered due to mass effect.10 The subset of paragangliomas that secrete catecholamines most often arise in the abdomen and pelvis, and their clinical presentation mimics that of pheochromocytomas, including episodic hypertension, palpitations, pallor, and diaphoresis.
This patient had persistent, nonepisodic hypertension, while palpitations and diaphoresis only manifested following micturition. Other cases of urinary bladder paragangliomas have described micturition-associated symptoms and hypertensive crises. Three-fold increases of catecholamine secretion after micturition have been observed in these patients, likely due to muscle contraction and pressure changes in the bladder leading to the systemic release of catecholamines.11
Epinephrine and norepinephrine are monoamine neurotransmitters that activate alpha-adrenergic and beta-adrenergic receptors. Adrenergic receptors are present in all tissues of the body but have prominent effects on the smooth muscle in the vasculature, gastrointestinal tract, urinary tract, and airways.12 Alpha-adrenergic vasoconstriction causes hypertension, which is commonly observed in patients with catecholamine-secreting tumors.10 Catecholamine excess due to secretion from these tumors causes headache in 60%-80% of patients, tachycardia/palpitations in 50%-70%, anxiety in 20%-40%, and nausea in 20%-25%.10 Other symptoms include sweating, pallor, dyspnea, and vertigo.9,10 This patient’s chronic nausea, which was attributed to gastroesophageal reflux, and his anxiety, attributed to generalized anxiety disorder, were likely symptoms of catecholamine excess.13
The best test for the diagnosis of paragangliomas and pheochromocytomas is the measurement of plasma free or 24-hour urinary fractionated metanephrines (test sensitivity of >90% and >90%, respectively).14 Screening for pheochromocytoma should be considered in hypertensive patients who have symptoms of catecholamine excess, refractory or paroxysmal hypertension, and/or familial pheochromocytoma/paraganglioma syndromes.15 Screening for pheochromocytoma should also be performed in children and adolescents with systolic or diastolic blood pressure that is greater than the 95th percentile for their age plus 5 mm Hg.16
While a typical tumor location and elevated metanephrine levels are sufficient to make the diagnosis of a pheochromocytoma or catecholamine-secreting paraganglioma, functional imaging with FDG-PET, Ga-DOTATATE-PET, or 123I-meta-iodobenzylguanidine (123I-MIBG) can further confirm the diagnosis and detect distant metastases. However, imaging has low sensitivity for these tumors and thus should only be considered for patients in whom metastatic disease is suspected.14 Biopsy is rarely needed and should be reserved for unusual metastatic locations. Treatment with an alpha-adrenergic receptor antagonist often reduces symptoms and lowers blood pressure. Definitive management typically involves surgical resection for benign disease. Surgery, radionuclide therapy, or chemotherapy is used for malignant disease.
While most pheochromocytomas are sporadic, up to 40% of paragangliomas are due to germline pathogenic variants.17 Mutations in the succinate dehydrogenase (SDH) group of genes are the most common germline pathogenic variants in the autosomal dominant hereditary paraganglioma syndrome (HPGL). Most paragangliomas and pheochromocytomas are localized and benign, but 10%-15% are metastatic.18 SDHB mutations are associated with a high risk of metastasis.19 Thus, genetic testing for patients and subsequent cascade testing to identify at-risk family members is advised in all patients with pheochromocytomas or paragangliomas.20 This patient’s younger brother and mother were both found to carry the same pathogenic SDHB variant, but neither was found to have paragangliomas. Annual metanephrine levels (urine or plasma) and every other year whole-body magnetic resonance imaging (MRI) scans were recommended for tumor surveillance.
The clinician team followed a logical branching algorithm for the diagnosis of severe hypertension with biochemical testing, advanced imaging, histology, and genetic testing to arrive at the final diagnosis of hereditary paraganglioma syndrome. Although this patient presented for urgent care because of the acute effects of catecholamine excess, he suffered from chronic effects (nausea, anxiety, and hypertension) for years. Each symptom had been diagnosed and treated in isolation, but the combination and severity in a young patient suggested a unifying diagnosis. The family history of hypertension (brother and father) suggested an inherited diagnosis from the father’s family, but the final answer rested on the other branch (maternal uncle) of the family tree.
KEY TEACHING POINTS
- Hypertension in a young adult is due to a secondary cause in up to 30% of patients.
- Pathologic catecholamine excess leads to hypertension, tachycardia, pallor, sweating, anxiety, and nausea. A sustained and unexplained combination of these symptoms should prompt a biochemical evaluation for pheochromocytoma or paraganglioma.
- Paragangliomas are tumors of the autonomic nervous system. The frequency of catecholamine secretion depends on their location in the body, and they are commonly caused by germline pathogenic variants.
Acknowledgments
This conundrum was presented during a live Grand Rounds with the expert clinician’s responses recorded and edited for space and clarity.
Disclosures
Dr. Dhaliwal reports speaking honoraria from ISMIE Mutual Insurance Company and GE Healthcare. All other authors have nothing to disclose.
Funding
No sources of funding.
A 21-year-old man with a history of hypertension presented to the emergency department with four days of generalized abdominal pain, nausea, and vomiting as well as one month of loose stools. He also had a headache (not further specified) for one day. Due to his nausea, he had been unable to take his medications for two days. Home blood pressure measurements over the preceding two days revealed systolic pressures exceeding 200 mm Hg. He did not experience fever, dyspnea, chest pain, vision changes, numbness, weakness, diaphoresis, or palpitations.
Abdominal pain with vomiting and diarrhea is often caused by a self-limited gastroenteritis. However, the priority initially is to exclude serious intraabdominal processes including arterial insufficiency, bowel obstruction, organ perforation, or organ-based infection or inflammation (eg, appendicitis, cholecystitis, pancreatitis). Essential hypertension accounts for 95% of cases of hypertension in the United States, but given this patient’s young age, secondary causes should be evaluated. These include primary aldosteronism (the most common endocrine cause for hypertension in young patients), chronic kidney disease, fibromuscular dysplasia, illicit drug use, hypercortisolism, pheochromocytoma, and coarctation of the aorta. Thyrotoxicosis can elevate blood pressure (although usually not to this extent) and cause hyperdefecation. While the etiology of the chronic hypertension is uncertain, the proximate cause of the acute rise in blood pressure is likely the stress of his acute illness and the inability to take his prescribed antihypertensive medications. In the setting of severe hypertension, his headache may reflect an intracranial hemorrhage and his abdominal pain could signal an aortic dissection.
His medical history included hypertension diagnosed at age 16 as well as anxiety diagnosed following a panic attack at age 19. Over the past year, he had also developed persistent nausea, which was attributed to gastroesophageal reflux disease. His medications included metoprolol 50 mg daily, amlodipine 5 mg daily, hydrochlorothiazide 12.5 mg daily, escitalopram 20 mg daily, and omeprazole 20 mg daily. His father and 15-year-old brother also had hypertension. He was a part-time student while working at a car dealership. He did not smoke or use drugs and he rarely drank alcohol.
The need for three antihypertensive medications (albeit at submaximal doses) reflects the severity of his hypertension (provided challenges with medication adherence have been excluded). His family history, especially that of his brother who was diagnosed with hypertension at an early age, and the patient’s own early onset hypertension point toward an inherited form of hypertension. Autosomal dominant polycystic kidney disease often results in hypertension before chronic kidney disease develops. Rare inherited forms of hypertension include familial hyperaldosteronism, apparent mineralocorticoid excess, Liddle syndrome, or a hereditary endocrine tumor syndrome predisposing to pheochromocytoma. Even among patients who report classic pheochromocytoma symptoms, such as headache and anxiety, the diagnosis remains unlikely as these symptoms are nonspecific and highly prevalent in the general population. However, once secondary hypertension is plausible or suspected, testing for hyperadrenergic states, which can also cause nausea and vomiting during times of catecholamine excess, should be pursued.
His temperature was 97.5°F, heart rate 95 beats per minute and regular, respiratory rate 18 breaths per minute, blood pressure 181/118 mm Hg (systolic and diastolic pressures in each arm were within 10 mm Hg), and oxygen saturation 100% on room air. Systolic and diastolic pressures did not decrease by more than 20 mm Hg and 10 mm Hg, respectively, after he stood for two minutes. His body mass index was 24 kg/m2. He was alert and appeared slightly anxious. There was a bounding point of maximal impulse in the fifth intercostal space at the midclavicular line and a 3/6 systolic murmur at the left upper sternal border with radiation to the carotid arteries. His abdomen was soft with generalized tenderness to palpation and without rebound tenderness, masses, organomegaly, or bruits. There was no costovertebral angle tenderness. No lymphadenopathy was present. His fundoscopic, pulmonary, skin and neurologic examinations were normal.
Laboratory studies revealed a white blood cell count of 13.3 × 103/uL with a normal differential, hemoglobin 13.9 g/dL, platelet count 373 × 103/uL, sodium 142 mmol/L, potassium 3.8 mmol/L, chloride 103 mmol/L,bicarbonate 25 mmol/L, blood urea nitrogen 12 mg/dL, creatinine 1.3 mg/dL (a baseline creatinine level was not available), glucose 88 mg/dL, calcium 10.6 mg/dL, albumin 4.9 g/dL, aspartate aminotransferase 27 IU/L, alanine aminotransferase 37 IU/L, and lipase 40 IU/L. Urinalysis revealed 5-10 white blood cells per high power field without casts and 10 mg/dL protein. Urine toxicology was not performed. Electrocardiogram (ECG) showed left ventricular hypertrophy (LVH). Chest radiography was normal.
The abdominal examination does not suggest peritonitis. The laboratory tests do not suggest inflammation of the liver, pancreas, or biliary tree as the cause of his abdominal pain or diarrhea. The murmur may indicate hypertrophic cardiomyopathy or a congenital anomaly such as bicuspid aortic valve; but neither would explain hypertension unless they were associated with another developmental abnormality, such as coarctation of the aorta. Tricuspid regurgitation is conceivable and if confirmed, might raise concern for carcinoid syndrome, which can cause diarrhea. The normal neurologic examination, including the absence of papilledema, lowers suspicion of intracranial hemorrhage as a cause of his headache.
The albumin of 4.9 g/dL likely reflects hypovolemia resulting from vomiting and diarrhea. Vasoconstriction associated with pheochromocytoma can cause pressure diuresis and resultant hypovolemia. Hyperaldosteronism arising from bilateral adrenal hyperplasia or adrenal adenoma commonly causes hypokalemia, although this is not a universal feature.
The duration of his mildly decreased glomerular filtration rate is uncertain. He may have chronic kidney disease from sustained hypertension, or acute kidney injury from hypovolemia. The mild pyuria could indicate infection or renal calculi, either of which could account for generalized abdominal pain or could reflect an acute renal injury from acute interstitial nephritis from his proton pump inhibitor or hydrochlorothiazide.
LVH on the ECG indicates longstanding hypertension. The chest radiograph does not reveal clues to the etiology of or sequelae from hypertension. In particular, there is no widened aorta to suggest aortic dissection, no pulmonary edema to indicate heart failure, and no rib notching that points toward aortic coarctation. A transthoracic echocardiogram to assess for valvular and other structural abnormalities is warranted.
Tests for secondary hypertension should be sent, including serum aldosterone and renin levels to assess for primary aldosteronism and plasma or 24-hour urine normetanephrine and metanephrine levels to assess for pheochromocytoma. Biochemical evaluation is the mainstay for endocrine hypertension evaluation and should be followed by imaging if abnormal results are found.
Intact parathyroid hormone (PTH) was 78 pg/mL (normal, 10-65 pg/mL), thyroid stimulating hormone 3.6 mIU/L (normal, 0.30-5.50 mIU/L), and morning cortisol 4.1 ug/dL (normal, >7.0 ug/dL). Plasma aldosterone was 14.6 ng/dL (normal, 1-16 ng/dL), plasma renin activity 3.6 ng/mL/hr (normal, 0.5-3.5 ng/mL/hr), and aldosterone-renin ratio 4.1 (normal, <20). Transthoracic echocardiogram showed LVH with normal valves, wall motion, and proximal aorta; the left ventricular ejection fraction was 70%. Magnetic resonance angiography of the renal vessels demonstrated no abnormalities.
Computed tomography (CT) of the abdomen and pelvis with oral and intravenous contrast revealed a 5 cm heterogeneous enhancing mass associated with the prostate gland extending into the base of the bladder. The mass obstructed the right renal collecting system and ureter causing severe right-sided ureterectasis and hydronephrosis. There was also 2.8 cm right-sided paracaval lymph node enlargement and 2.1 cm right-sided and 1.5 cm left-sided external iliac lymph node enlargement (Figure 1). There were no adrenal masses.
He is young for prostate, bladder, or colorectal cancer, but early onset variations of these tumors, along with metastatic testicular cancer, must be considered for the pelvic mass and associated lymphadenopathy. Prostatic masses can be infectious (eg, abscess) or malignant (eg, adenocarcinoma, small cell carcinoma). Additional considerations for abdominopelvic cancer are sarcomas, germ cell tumors, or lymphoma. A low aldosterone-renin ratio coupled with a normal potassium level makes primary aldosteronism unlikely. The normal angiography excludes renovascular hypertension.
His abdominal pain and gastrointestinal symptoms could arise from irritation of the bowel, distension of the right-sided urinary collecting system, or products secreted from the mass (eg, catecholamines). The hyperdynamic precordium, elevated ejection fraction, and murmur may reflect augmented blood flow from a hyperadrenergic state. A unifying diagnosis would be a pheochromocytoma. However, given the normal appearance of the adrenal glands on CT imaging, catecholamines arising from a paraganglioma, a tumor of the autonomic nervous system, is more likely. These tumors often secrete catecholamines and can be metastatic (suggested here by the lymphadenopathy). Functional imaging or biopsy of either the mass or an adjacent lymph node is indicated. However, because of the possibility of a catecholamine-secreting tumor, he should be treated with an alpha-adrenergic receptor antagonist before undergoing a biopsy to prevent unopposed vasoconstriction from catecholamine leakage.
Scrotal ultrasound revealed no evidence of a testicular tumor. Lactate dehydrogenase (LDH) was 179 IU/L (normal, 120-240 IU/L) and prostate specific antigen (PSA) was 0.7 ng/mL (normal, <2.5 ng/mL). The patient was given amlodipine and labetalol with improvement of blood pressures to 160s/100s. His creatinine decreased to 1.1 mg/dL. He underwent CT-guided biopsy of a pelvic lymph node. CT of the head without intravenous contrast demonstrated no intracranial abnormalities. His headache resolved with improvement in blood pressure, and he had minimal gastrointestinal symptoms during his hospitalization. No stool studies were sent. A right-sided percutaneous nephrostomy was placed which yielded >15 L of urine from the tube over the next four days.
Upon the first episode of micturition through the urethra four days after percutaneous nephrostomy placement, he experienced severe lightheadedness, diaphoresis, and palpitations. These symptoms prompted him to recall similar episodes following micturition for several months prior to his hospitalization.
It is likely that contraction of the bladder during episodes of urination caused irritation of the pelvic mass, leading to catecholamine secretion. Another explanation for his recurrent lightheadedness would be a neurocardiogenic reflex with micturition (which when it culminates with loss of consciousness is called micturition syncope), but this would not explain his hypertension or bladder mass.
Biochemical tests that were ordered on admission but sent to a reference lab then returned. Plasma metanephrine was 0.2 nmol/L (normal, <0.5 nmol/L) and plasma normetanephrine 34.6 nmol/L (normal, <0.9 nmol/L). His 24-hour urine metanephrine was 72 ug/24 hr (normal, 0-300 ug/24 hr) and normetanephrine 8,511 ug/24 hr (normal, 50-800 ug/24 hr).
The markedly elevated plasma and urine normetanephrine levels confirm a diagnosis of a catecholamine-secreting tumor (paraganglioma). The tissue obtained from the CT-guided lymph node biopsy should be sent for markers of neuroendocrine tumors including chromogranin.
Lymph node biopsy revealed metastatic paraganglioma that was chromogranin A and synaptophysin positive (Figure 2). A fluorodeoxyglucose positron emission tomography (FDG-PET) scan disclosed skull metastases. He was treated with phenoxybenzamine, amlodipine, and labetalol. Surgical resection of the pelvic mass was discussed, but the patient elected to defer surgery as the location of the primary tumor made it challenging to resect and would have required an ileal conduit.
After the diagnosis was made, the patient’s family recalled that a maternal uncle had been diagnosed with a paraganglioma of the carotid body. Genetic testing of the patient identified a succinate dehydrogenase complex subunit B (SDHB) pathogenic variant and confirmed hereditary paraganglioma syndrome (HPGL). One year after the diagnosis, liver and lung metastases developed. He was treated with lanreotide (somatostatin analogue), capecitabine, and temozolomide, as well as a craniotomy and radiotherapy for palliation of bony metastases. The patient died less than two years after diagnosis.
DISCUSSION
Most patients with hypertension (defined as blood pressure >130/80 mm Hg1) do not have an identifiable etiology (primary hypertension). Many components of this patient’s history, however, including his young age of onset, a teenage sibling with hypertension, lack of obesity, hypertension refractory to multiple medications, and LVH suggested secondary hypertension. Hypertension onset at an age less than 30 years, resistance to three or more medications,1,2 and/or acute onset hypertension at any age should prompt an evaluation for secondary causes.1 The prevalence of secondary hypertension is approximately 30% in hypertensive patients ages 18 to 40 years compared with 5%-10% in the overall adult population with hypertension.3 Among children and adolescents ages 0 to 19 years with hypertension, the prevalence of secondary hypertension may be as high as 57%.4
The most common etiology of secondary hypertension is primary aldosteronism.5,6 However, in young adults (ages 19 to 39 years), common etiologies also include renovascular disease and renal parenchymal disease.7 Other causes include obstructive sleep apnea, medications, stimulants (cocaine and amphetamines),8 and endocrinopathies such as thyrotoxicosis, Cushing syndrome, and catecholamine-secreting tumors.7 Less than 1% of secondary hypertension in all adults is due to catecholamine-secreting tumors, and the minority of those catecholamine-secreting tumors are paragangliomas.9
Paragangliomas are tumors of the peripheral autonomic nervous system. These neoplasms arise in the sympathetic and parasympathetic chains along the paravertebral and paraaortic axes. They are closely related to pheochromocytomas, which arise in the adrenal medulla.9 Most head and neck paragangliomas are biochemically silent and are generally discovered due to mass effect.10 The subset of paragangliomas that secrete catecholamines most often arise in the abdomen and pelvis, and their clinical presentation mimics that of pheochromocytomas, including episodic hypertension, palpitations, pallor, and diaphoresis.
This patient had persistent, nonepisodic hypertension, while palpitations and diaphoresis only manifested following micturition. Other cases of urinary bladder paragangliomas have described micturition-associated symptoms and hypertensive crises. Three-fold increases of catecholamine secretion after micturition have been observed in these patients, likely due to muscle contraction and pressure changes in the bladder leading to the systemic release of catecholamines.11
Epinephrine and norepinephrine are monoamine neurotransmitters that activate alpha-adrenergic and beta-adrenergic receptors. Adrenergic receptors are present in all tissues of the body but have prominent effects on the smooth muscle in the vasculature, gastrointestinal tract, urinary tract, and airways.12 Alpha-adrenergic vasoconstriction causes hypertension, which is commonly observed in patients with catecholamine-secreting tumors.10 Catecholamine excess due to secretion from these tumors causes headache in 60%-80% of patients, tachycardia/palpitations in 50%-70%, anxiety in 20%-40%, and nausea in 20%-25%.10 Other symptoms include sweating, pallor, dyspnea, and vertigo.9,10 This patient’s chronic nausea, which was attributed to gastroesophageal reflux, and his anxiety, attributed to generalized anxiety disorder, were likely symptoms of catecholamine excess.13
The best test for the diagnosis of paragangliomas and pheochromocytomas is the measurement of plasma free or 24-hour urinary fractionated metanephrines (test sensitivity of >90% and >90%, respectively).14 Screening for pheochromocytoma should be considered in hypertensive patients who have symptoms of catecholamine excess, refractory or paroxysmal hypertension, and/or familial pheochromocytoma/paraganglioma syndromes.15 Screening for pheochromocytoma should also be performed in children and adolescents with systolic or diastolic blood pressure that is greater than the 95th percentile for their age plus 5 mm Hg.16
While a typical tumor location and elevated metanephrine levels are sufficient to make the diagnosis of a pheochromocytoma or catecholamine-secreting paraganglioma, functional imaging with FDG-PET, Ga-DOTATATE-PET, or 123I-meta-iodobenzylguanidine (123I-MIBG) can further confirm the diagnosis and detect distant metastases. However, imaging has low sensitivity for these tumors and thus should only be considered for patients in whom metastatic disease is suspected.14 Biopsy is rarely needed and should be reserved for unusual metastatic locations. Treatment with an alpha-adrenergic receptor antagonist often reduces symptoms and lowers blood pressure. Definitive management typically involves surgical resection for benign disease. Surgery, radionuclide therapy, or chemotherapy is used for malignant disease.
While most pheochromocytomas are sporadic, up to 40% of paragangliomas are due to germline pathogenic variants.17 Mutations in the succinate dehydrogenase (SDH) group of genes are the most common germline pathogenic variants in the autosomal dominant hereditary paraganglioma syndrome (HPGL). Most paragangliomas and pheochromocytomas are localized and benign, but 10%-15% are metastatic.18 SDHB mutations are associated with a high risk of metastasis.19 Thus, genetic testing for patients and subsequent cascade testing to identify at-risk family members is advised in all patients with pheochromocytomas or paragangliomas.20 This patient’s younger brother and mother were both found to carry the same pathogenic SDHB variant, but neither was found to have paragangliomas. Annual metanephrine levels (urine or plasma) and every other year whole-body magnetic resonance imaging (MRI) scans were recommended for tumor surveillance.
The clinician team followed a logical branching algorithm for the diagnosis of severe hypertension with biochemical testing, advanced imaging, histology, and genetic testing to arrive at the final diagnosis of hereditary paraganglioma syndrome. Although this patient presented for urgent care because of the acute effects of catecholamine excess, he suffered from chronic effects (nausea, anxiety, and hypertension) for years. Each symptom had been diagnosed and treated in isolation, but the combination and severity in a young patient suggested a unifying diagnosis. The family history of hypertension (brother and father) suggested an inherited diagnosis from the father’s family, but the final answer rested on the other branch (maternal uncle) of the family tree.
KEY TEACHING POINTS
- Hypertension in a young adult is due to a secondary cause in up to 30% of patients.
- Pathologic catecholamine excess leads to hypertension, tachycardia, pallor, sweating, anxiety, and nausea. A sustained and unexplained combination of these symptoms should prompt a biochemical evaluation for pheochromocytoma or paraganglioma.
- Paragangliomas are tumors of the autonomic nervous system. The frequency of catecholamine secretion depends on their location in the body, and they are commonly caused by germline pathogenic variants.
Acknowledgments
This conundrum was presented during a live Grand Rounds with the expert clinician’s responses recorded and edited for space and clarity.
Disclosures
Dr. Dhaliwal reports speaking honoraria from ISMIE Mutual Insurance Company and GE Healthcare. All other authors have nothing to disclose.
Funding
No sources of funding.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13-e115. https://doi.org/10.1161/HYP.0000000000000065.
2. Acelajado MC, Calhoun DA. Resistant hypertension, secondary hypertension, and hypertensive crises: diagnostic evaluation and treatment. Cardiol Clin. 2010;28(4):639-654. https://doi.org/10.1016/j.ccl.2010.07.002.
3. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206-1252. https://doi.org/10.1161/01.HYP.0000107251.49515.c2.
4. Gupta-Malhotra M, Banker A, Shete S, et al. Essential hypertension vs. secondary hypertension among children. Am J Hypertens. 2015;28(1):73-80. https://doi.org/10.1093/ajh/hpu083.
5. Mosso L, Carvajal C, Gonzalez A, et al. Primary aldosteronism and hypertensive disease. Hypertension. 2003;42(2):161-165. https://doi.org/10.1161/01.HYP.0000079505.25750.11.
6. Kayser SC, Dekkers T, Groenewoud HJ, et al. Study heterogeneity and estimation of prevalence of primary aldosteronism: a systematic review and meta-regression analysis. J Clin Endocrinol Metab. 2016;101(7):2826-2835. https://doi.org/10.1210/jc.2016-1472.
7. Charles L, Triscott J, Dobbs B. Secondary hypertension: discovering the underlying cause. Am Fam Physician. 2017;96(7):453-461.
8. Aronow WS. Drug-induced causes of secondary hypertension. Ann Transl Med. 2017;5(17):349. https://doi.org/10.21037/atm.2017.06.16.
9. Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366(9486):665-675. https://doi.org/10.1016/S0140-6736(05)67139-5.
10. Mannelli M, Lenders JW, Pacak K, Parenti G, Eisenhofer G. Subclinical phaeochromocytoma. Best Pract Res Clin Endocrinol Metab. 2012;26(4):507-515. https://doi.org/10.1016/j.beem.2011.10.008.
11. Kappers MH, van den Meiracker AH, Alwani RA, Kats E, Baggen MG. Paraganglioma of the urinary bladder. Neth J Med. 2008;66(4):163-165.
12. Paravati S, Warrington SJ. Physiology, Catecholamines. In: StatPearls. Treasure Island, FL: StatPearls Publishing LLC; 2019.
13. King KS, Darmani NA, Hughes MS, Adams KT, Pacak K. Exercise-induced nausea and vomiting: another sign and symptom of pheochromocytoma and paraganglioma. Endocrine. 2010;37(3):403-407. https://doi.org/10.1007/s12020-010-9319-3.
14. Lenders JW, Duh QY, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915-1942. https://doi.org/10.1210/jc.2014-1498.
15. Lenders JWM, Eisenhofer G. Update on modern management of pheochromocytoma and paraganglioma. Endocrinol Metab (Seoul). 2017;32(2):152-161. https://doi.org/10.3803/EnM.2017.32.2.152.
16. National High Blood Pressure Education Program Working Group. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2):555-576.
17. Else T, Greenberg S, Fishbein L. Hereditary Paraganglioma-Pheochromocytoma Syndromes. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. Gene Reviews. Seattle, WA: University of Washington; 1993.
18. Goldstein RE, O’Neill JA, Jr., Holcomb GW, 3rd, et al. Clinical experience over 48 years with pheochromocytoma. Ann Surg. 1999;229(6):755-764; discussion 764-756. https://doi.org/10.1097/00000658-199906000-00001.
19. Amar L, Baudin E, Burnichon N, et al. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrinol Metab. 2007;92(10):3822-3828. https://doi.org/10.1210/jc.2007-0709.
20. Favier J, Amar L, Gimenez-Roqueplo AP. Paraganglioma and phaeochromocytoma: from genetics to personalized medicine. Nat Rev Endocrinol. 2015;11(2):101-111. https://doi.org/10.1038/nrendo.2014.188.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13-e115. https://doi.org/10.1161/HYP.0000000000000065.
2. Acelajado MC, Calhoun DA. Resistant hypertension, secondary hypertension, and hypertensive crises: diagnostic evaluation and treatment. Cardiol Clin. 2010;28(4):639-654. https://doi.org/10.1016/j.ccl.2010.07.002.
3. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206-1252. https://doi.org/10.1161/01.HYP.0000107251.49515.c2.
4. Gupta-Malhotra M, Banker A, Shete S, et al. Essential hypertension vs. secondary hypertension among children. Am J Hypertens. 2015;28(1):73-80. https://doi.org/10.1093/ajh/hpu083.
5. Mosso L, Carvajal C, Gonzalez A, et al. Primary aldosteronism and hypertensive disease. Hypertension. 2003;42(2):161-165. https://doi.org/10.1161/01.HYP.0000079505.25750.11.
6. Kayser SC, Dekkers T, Groenewoud HJ, et al. Study heterogeneity and estimation of prevalence of primary aldosteronism: a systematic review and meta-regression analysis. J Clin Endocrinol Metab. 2016;101(7):2826-2835. https://doi.org/10.1210/jc.2016-1472.
7. Charles L, Triscott J, Dobbs B. Secondary hypertension: discovering the underlying cause. Am Fam Physician. 2017;96(7):453-461.
8. Aronow WS. Drug-induced causes of secondary hypertension. Ann Transl Med. 2017;5(17):349. https://doi.org/10.21037/atm.2017.06.16.
9. Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366(9486):665-675. https://doi.org/10.1016/S0140-6736(05)67139-5.
10. Mannelli M, Lenders JW, Pacak K, Parenti G, Eisenhofer G. Subclinical phaeochromocytoma. Best Pract Res Clin Endocrinol Metab. 2012;26(4):507-515. https://doi.org/10.1016/j.beem.2011.10.008.
11. Kappers MH, van den Meiracker AH, Alwani RA, Kats E, Baggen MG. Paraganglioma of the urinary bladder. Neth J Med. 2008;66(4):163-165.
12. Paravati S, Warrington SJ. Physiology, Catecholamines. In: StatPearls. Treasure Island, FL: StatPearls Publishing LLC; 2019.
13. King KS, Darmani NA, Hughes MS, Adams KT, Pacak K. Exercise-induced nausea and vomiting: another sign and symptom of pheochromocytoma and paraganglioma. Endocrine. 2010;37(3):403-407. https://doi.org/10.1007/s12020-010-9319-3.
14. Lenders JW, Duh QY, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915-1942. https://doi.org/10.1210/jc.2014-1498.
15. Lenders JWM, Eisenhofer G. Update on modern management of pheochromocytoma and paraganglioma. Endocrinol Metab (Seoul). 2017;32(2):152-161. https://doi.org/10.3803/EnM.2017.32.2.152.
16. National High Blood Pressure Education Program Working Group. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2):555-576.
17. Else T, Greenberg S, Fishbein L. Hereditary Paraganglioma-Pheochromocytoma Syndromes. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. Gene Reviews. Seattle, WA: University of Washington; 1993.
18. Goldstein RE, O’Neill JA, Jr., Holcomb GW, 3rd, et al. Clinical experience over 48 years with pheochromocytoma. Ann Surg. 1999;229(6):755-764; discussion 764-756. https://doi.org/10.1097/00000658-199906000-00001.
19. Amar L, Baudin E, Burnichon N, et al. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrinol Metab. 2007;92(10):3822-3828. https://doi.org/10.1210/jc.2007-0709.
20. Favier J, Amar L, Gimenez-Roqueplo AP. Paraganglioma and phaeochromocytoma: from genetics to personalized medicine. Nat Rev Endocrinol. 2015;11(2):101-111. https://doi.org/10.1038/nrendo.2014.188.
© 2019 Society of Hospital Medicine
Clinical Progress Note: Addressing Prognosis in Advanced Dementia
Advanced dementia (AD) is a serious terminal illness. Some features of AD include significant memory deficits (inability to recognize family members), inability to ambulate, very limited verbal communication, and needing assistance with all activities of daily living.1 AD carries a six-month mortality of 25% and a median survival of 1.3 years.1
Despite a limited life expectancy, patients with AD face increasingly significant symptom burden and use of burdensome interventions2 near the end of life. Among the most common interventions hospitalists routinely navigate during a hospitalization is tube feeding for enteral artificial nutrition, which has not been shown to prolong survival, improve quality of life, decrease risk of aspiration pneumonia, or decrease the risk of pressure ulcers.3,4 Recent data show that rates of hospitalizations in the last 90 days of life, especially in the last three days of life, are increasing.5 These late transitions can have significant negative impact on family perceptions of quality of care, including not being treated with respect, receiving care inconsistent with goals, receiving inadequate communication about care decisions, and not being fully informed of the medical conditions.6
Therefore, hospitalization in AD, especially a readmission, indicates a critical change in a patient’s illness, marking an opportune time to have discussions on prognosis and improve care at the end of life. While determining and sharing prognosis can be challenging in the setting of many chronic diseases, resources exist to help clinicians share prognosis in AD and understand the goals of care for each patient.7 The aim of this paper is to assist hospitalists in addressing prognosis in the setting of AD. We identify and present key knowledge and recommendations from relevant articles identified from a hand-search of articles, published in 2018, from leading palliative care journals, as well as a MEDLINE search from 2003 through December 2018 using the key words “dementia” and “prognosis.” Final presented articles and recommendations were determined based on scientific rigor and relevance to hospital-based care of patients with AD.
IMPORTANCE OF PROGNOSIS DISCUSSIONS IN ADVANCED DEMENTIA
For a myriad of reasons, most AD caregivers do not receive adequate information on the complications of dementia or prognosis.2 Conversations that provide prognostic estimates and aim to understand the goals, preferences, and values of AD patients and their surrogates can help in providing goal-concordant care. A prospective study of nursing-home patients with AD showed that having goals of care discussions was strongly associated with surrogates’ likelihood of estimating a life expectancy of less than six months in AD patients.8 Having this perception was associated with a lower likelihood of patients with AD undergoing burdensome interventions such as hospitalizations, parenteral therapy, venipuncture, feeding tube, or urinary catheterization.8 To help improve goal-concordant care, it is important that hospitalists be prepared to have prognostic conversations with patients and their caregivers.
“FORESEEING” PROGNOSIS IN ADVANCED DEMENTIA
Offering a clinical prognosis involves components of foreseeing (estimating prognosis) and foretelling (sharing prognosis).9 Foreseeing prognosis in AD can be complex due to the highly variable but slow, dwindling clinical course of AD. As a practical matter, determining if a patient has a six-month prognosis is most helpful as eligibility for hospice services may allow for a discharge to a supportive home setting instead of a transfer to an institution.10 An evidence-based clinical prediction rule, the Advanced Dementia Prognostic Tool (ADEPT, Appendix Table),11 can be used to estimate prognosis by a composite of 12 risk factors in nursing-home patients. Although the consensus-based National Hospice and Palliative Care Organization (NHPCO) guidelines for Medicare hospice eligibility12 (Table) do not perform well in predicting individual mortality, they are used as criteria for hospice enrollment. Given the variability of course of AD, evaluating the mortality risk for acute illnesses leading to hospitalization, like pneumonia or hip fracture, can further help estimate prognosis. The website, www.eprognosis.com, combines various prediction tools to help estimate prognosis. Although using these tools can often help clinicians satisfy the entry requirements to offer hospice, the ADEPT tool and the NHPCO criteria both perform poorly in discriminating those who will or will not actually die in six months. ADEPT, as a prognostic tool, has not yet been validated for community-dwelling patients. Clinicians should exercise caution in making a highly specific estimate of survival in AD, but can and should communicate the expected decline in function over time.
“FORETELLING” PROGNOSIS IN ADVANCED DEMENTIA
Having goals of care conversations and sharing prognosis has many benefits. A large multistate cohort study showed that having goals of care conversations among patients with terminal cancer was associated with less use of intensive care units, mechanical ventilation, and cardio-pulmonary resuscitation.13 Caregivers may also benefit from prognosis discussions through identifying resources to care for the patient at home and by potentially limiting their risk of major depressive order and regret, common among those witnessing patients undergoing aggressive treatment at the end of life.13 How to share prognosis can be challenging; however, tools such as the Serious Illness Conversation Guide14 can provide step-by-step guidance for providers. The key aspects of the guide are asking permission, assessing illness understanding, and exploring goals, fears, worries, and tradeoffs.
Before exploring goals, it is helpful to explain the serious illness by using “I wish,” “I worry,” and “I wonder” statements such as “I wish I had better news for you; your mom’s dementia has progressed given the recent complication of aspiration,” “I worry that she will not be able to eat on her own and will develop another serious infection very soon,” or “I wonder whether it is a good time to talk about what your mom would want if she cannot eat on her own.”
An example of an effective conversation about artificial nutrition and hydration with a surrogate of a patient with AD with recurrent aspirations may include the following elements:14,15
- Obtain the caregiver’s and/or patient’s perception of illness: “Is it OK if we have a conversation about what may lie ahead with your mother? Is there anyone else that should be present? What is your understanding of your mother’s illness?”
- Give relevant data: “Based on her current level of decline with complications and repetitive hospitalizations, I am worried that her life expectancy is likely measured in months rather than years.”
- Address emotions: “This must be very hard to hear. I cannot imagine how difficult it must be to see her in the hospital so often.”
- Elicit concerns and goals based on understanding key values: “Tell me what you are hoping for regarding your mother’s future care and what worries you have. Tell me what your mother would say if she could fully understand her current situation.”
- Present goals based on patient and caregiver values: “Based on what you have told me about your mother, she valued her interactions with family and her independence, and she would not want measures that would cause distress, especially when facing a terminal illness.”
- Be mindful of prognostic uncertainty: “While we cannot know for certain what will happen next, I am very worried that your mother will continue to aspirate even with a feeding tube.”
- Make a recommendation with permission: “From our conversation, I have an idea of what treatment might make sense to your mom. May I share my recommendation with you?” If they are willing, you might say: “As evidence shows that feeding tubes do not improve the level of family interaction or independence in patients with dementia and as your mother would not want any distressing procedures, I recommend that we do not place a feeding-tube.”
- Balance realism and hope: “Instead, we can focus on other ways to maintain dignity and quality of life for her even without a feeding tube.”
RESPONDING TO CHALLENGES
Conversations about goals of care and prognosis can be challenging and time consuming. At times, the conversations can be strained. The following tips are based on authors’ shared experiences to help in those challenging situations:
- Caregivers may show signs of emotional and/or cognitive strain: Recognize and name the emotional response and consider asking the family if they need a break to avoid overtaxing them.
“I can see that this is very difficult for you. Do you want to take a break and meet again?”
- Caregivers may have unrealistic hopes: Confirm the caregivers’ understanding of the situation, before assuming their hope is unrealistic. Try to reframe what they/we can hope for by validating their goals while avoiding unnecessary burdens or discomfort.
“I want to be sure that I have explained your mom’s situation clearly. Can you tell me in your words, what I have told you?” as this gives you an opportunity to clarify misunderstandings that may manifest as “false hope”.
“Together we can hope for the best and see if your mother can tolerate hand-feeding safely without causing any harm or distress.”
- Avoid assumptions about cultural and religious beliefs: Be curious and demonstrate cultural humility to all patients.
“Are there any cultural or spiritual beliefs that are important to you or your mother?”
- Avoid spending too much time on clinical details: Give families time to share stories about the patient in better days as this gives you an opportunity to get to know the patient.
“Tell me more about what your mother was like when she was healthy.”
- Listen first, recommend second: Refrain from making recommendations about the patient’s care before you understand his/her values and preferences.
“What would your mother say is most important to her as her health worsens?”
- Use active listening techniques: Using reflection statements can confirm your understanding of the caregiver’s view point.
“So, I hear that your mother valued being at home and being comfortable. Is that correct?”
These conversations are often an iterative process of helping the patient and family traverse the course of AD. Therefore, starting the process even during a hospitalization earlier in the course of AD can help engage in preparedness planning to provide goal-concordant care and help optimize the patient’s quality of life.
CONCLUSION
Hospitalization among patients with AD can signal a significant change in prognosis and represents an important opportunity for further dialogue. A patient- and caregiver-centered conversation, sharing prognosis and learning about values important to the patient and family, has the potential to lead to less burdensome interventions. Doing so can minimize harm, promote quality of life, and reduce unnecessary care transitions near the end of life.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Havyer was supported, in part, by the Mayo Clinic Department of Medicine Catalyst for Advancing in Academics grant. Dr. Abedini was supported by the National Clinician Scholars Program at the Institute for Healthcare Policy and Innovation, University of Michigan, Ann Arbor, MI.
1. Mitchell SL. Advanced dementia. N Engl J Med. 2015;373(13):1276-1277. https://doi.org/10.1056/NEJMcp1412652.
2. Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361(16):1529-1538. https://doi.org/10.1056/NEJMoa0902234.
3. Teno JM, Gozalo PL, Mitchell SL, et al. Does feeding tube insertion and its timing improve survival? J Am Geriatr Soc. 2012;60(10):1918-1921. https://doi.org/10.1111/j.1532-5415.2012.04148.x.
4. Teno JM, Gozalo P, Mitchell SL, Kuo S, Fulton AT, Mor V. Feeding tubes and the prevention or healing of pressure ulcers. Arch Intern Med. 2012;172(9):697-701. https://doi.org/10.1001/archinternmed.2012.1200.
5. Teno JM, Gozalo PL, Bynum JP, et al. Change in end-of-life care for medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470-477. https://doi.org/10.1001/jama.2012.207624.
6. Makaroun LK, Teno JM, Freedman VA, Kasper JD, Gozalo P, Mor V. Late transitions and bereaved family member perceptions of quality of end-of-life care. J Am Geriatr Soc. 2018;66(9):1730-1736. https://doi.org/10.1111/jgs.15455.
7. Ansari AA, Pomerantz DH, Jayes RL, Aguirre EA, Havyer RD. Promoting primary palliative care in severe chronic obstructive pulmonary disease: symptom management and preparedness planning. J Palliat Care. 2019;34(2):85-91. https://doi.org/10.1177/0825859718819437.
8. Loizeau AJ, Shaffer ML, Habtemariam DA, Hanson LC, Volandes AE, Mitchell SL. Association of prognostic estimates with burdensome interventions in nursing home residents with advanced dementia. JAMA Intern Med. 2018;178(7):922-929. https://doi.org/10.1001/jamainternmed.2018.1413.
9. Glare PA, Sinclair CT. Palliative medicine review: Prognostication. J Palliat Med. 2008;11(1):84-103. https://doi.org/10.1089/jpm.2008.9992.
10. Jayes RL, Arnold RM, Fromme EK. Does this dementia patient meet the prognosis eligibility requirements for hospice enrollment? J Pain Symptom Manage. 2012;44(5):750-756. https://doi.org/10.1016/j.jpainsymman.2012.08.004.
11. Mitchell SL, Miller SC, Teno JM, Kiely DK, Davis RB, Shaffer ML. Prediction of 6-month survival of nursing home residents with advanced dementia using ADEPT vs hospice eligibility guidelines. JAMA. 2010;304(17):1929-1935. https://doi.org/10.1001/jama.2010.1572.
12. Schonwetter RS, Han B, Small BJ, et al. Predictors of six-month survival among patients with dementia: an evaluation of hospice Medicare guidelines. Amer J Hospice & Pall Care. 2003;20(2):105-113. https://doi.org/10.1177/104990910302000208
13. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673. https://doi.org/10.1001/jama.300.14.1665.
14. Bernacki R, Hutchings M, Vick J, et al. Development of the serious illness care program: a randomised controlled trial of a palliative care communication intervention. BMJ Open. 2015;5(10):e009032-2015-009032. https://doi.org/10.1136/bmjopen-2015-009032
15. Ansari A, Pomerantz D, Smith K. Being mindful: difficult decisions in advanced dementia and end stage renal disease. SGIM Forum. 2017;40(3):4, 13.
Advanced dementia (AD) is a serious terminal illness. Some features of AD include significant memory deficits (inability to recognize family members), inability to ambulate, very limited verbal communication, and needing assistance with all activities of daily living.1 AD carries a six-month mortality of 25% and a median survival of 1.3 years.1
Despite a limited life expectancy, patients with AD face increasingly significant symptom burden and use of burdensome interventions2 near the end of life. Among the most common interventions hospitalists routinely navigate during a hospitalization is tube feeding for enteral artificial nutrition, which has not been shown to prolong survival, improve quality of life, decrease risk of aspiration pneumonia, or decrease the risk of pressure ulcers.3,4 Recent data show that rates of hospitalizations in the last 90 days of life, especially in the last three days of life, are increasing.5 These late transitions can have significant negative impact on family perceptions of quality of care, including not being treated with respect, receiving care inconsistent with goals, receiving inadequate communication about care decisions, and not being fully informed of the medical conditions.6
Therefore, hospitalization in AD, especially a readmission, indicates a critical change in a patient’s illness, marking an opportune time to have discussions on prognosis and improve care at the end of life. While determining and sharing prognosis can be challenging in the setting of many chronic diseases, resources exist to help clinicians share prognosis in AD and understand the goals of care for each patient.7 The aim of this paper is to assist hospitalists in addressing prognosis in the setting of AD. We identify and present key knowledge and recommendations from relevant articles identified from a hand-search of articles, published in 2018, from leading palliative care journals, as well as a MEDLINE search from 2003 through December 2018 using the key words “dementia” and “prognosis.” Final presented articles and recommendations were determined based on scientific rigor and relevance to hospital-based care of patients with AD.
IMPORTANCE OF PROGNOSIS DISCUSSIONS IN ADVANCED DEMENTIA
For a myriad of reasons, most AD caregivers do not receive adequate information on the complications of dementia or prognosis.2 Conversations that provide prognostic estimates and aim to understand the goals, preferences, and values of AD patients and their surrogates can help in providing goal-concordant care. A prospective study of nursing-home patients with AD showed that having goals of care discussions was strongly associated with surrogates’ likelihood of estimating a life expectancy of less than six months in AD patients.8 Having this perception was associated with a lower likelihood of patients with AD undergoing burdensome interventions such as hospitalizations, parenteral therapy, venipuncture, feeding tube, or urinary catheterization.8 To help improve goal-concordant care, it is important that hospitalists be prepared to have prognostic conversations with patients and their caregivers.
“FORESEEING” PROGNOSIS IN ADVANCED DEMENTIA
Offering a clinical prognosis involves components of foreseeing (estimating prognosis) and foretelling (sharing prognosis).9 Foreseeing prognosis in AD can be complex due to the highly variable but slow, dwindling clinical course of AD. As a practical matter, determining if a patient has a six-month prognosis is most helpful as eligibility for hospice services may allow for a discharge to a supportive home setting instead of a transfer to an institution.10 An evidence-based clinical prediction rule, the Advanced Dementia Prognostic Tool (ADEPT, Appendix Table),11 can be used to estimate prognosis by a composite of 12 risk factors in nursing-home patients. Although the consensus-based National Hospice and Palliative Care Organization (NHPCO) guidelines for Medicare hospice eligibility12 (Table) do not perform well in predicting individual mortality, they are used as criteria for hospice enrollment. Given the variability of course of AD, evaluating the mortality risk for acute illnesses leading to hospitalization, like pneumonia or hip fracture, can further help estimate prognosis. The website, www.eprognosis.com, combines various prediction tools to help estimate prognosis. Although using these tools can often help clinicians satisfy the entry requirements to offer hospice, the ADEPT tool and the NHPCO criteria both perform poorly in discriminating those who will or will not actually die in six months. ADEPT, as a prognostic tool, has not yet been validated for community-dwelling patients. Clinicians should exercise caution in making a highly specific estimate of survival in AD, but can and should communicate the expected decline in function over time.
“FORETELLING” PROGNOSIS IN ADVANCED DEMENTIA
Having goals of care conversations and sharing prognosis has many benefits. A large multistate cohort study showed that having goals of care conversations among patients with terminal cancer was associated with less use of intensive care units, mechanical ventilation, and cardio-pulmonary resuscitation.13 Caregivers may also benefit from prognosis discussions through identifying resources to care for the patient at home and by potentially limiting their risk of major depressive order and regret, common among those witnessing patients undergoing aggressive treatment at the end of life.13 How to share prognosis can be challenging; however, tools such as the Serious Illness Conversation Guide14 can provide step-by-step guidance for providers. The key aspects of the guide are asking permission, assessing illness understanding, and exploring goals, fears, worries, and tradeoffs.
Before exploring goals, it is helpful to explain the serious illness by using “I wish,” “I worry,” and “I wonder” statements such as “I wish I had better news for you; your mom’s dementia has progressed given the recent complication of aspiration,” “I worry that she will not be able to eat on her own and will develop another serious infection very soon,” or “I wonder whether it is a good time to talk about what your mom would want if she cannot eat on her own.”
An example of an effective conversation about artificial nutrition and hydration with a surrogate of a patient with AD with recurrent aspirations may include the following elements:14,15
- Obtain the caregiver’s and/or patient’s perception of illness: “Is it OK if we have a conversation about what may lie ahead with your mother? Is there anyone else that should be present? What is your understanding of your mother’s illness?”
- Give relevant data: “Based on her current level of decline with complications and repetitive hospitalizations, I am worried that her life expectancy is likely measured in months rather than years.”
- Address emotions: “This must be very hard to hear. I cannot imagine how difficult it must be to see her in the hospital so often.”
- Elicit concerns and goals based on understanding key values: “Tell me what you are hoping for regarding your mother’s future care and what worries you have. Tell me what your mother would say if she could fully understand her current situation.”
- Present goals based on patient and caregiver values: “Based on what you have told me about your mother, she valued her interactions with family and her independence, and she would not want measures that would cause distress, especially when facing a terminal illness.”
- Be mindful of prognostic uncertainty: “While we cannot know for certain what will happen next, I am very worried that your mother will continue to aspirate even with a feeding tube.”
- Make a recommendation with permission: “From our conversation, I have an idea of what treatment might make sense to your mom. May I share my recommendation with you?” If they are willing, you might say: “As evidence shows that feeding tubes do not improve the level of family interaction or independence in patients with dementia and as your mother would not want any distressing procedures, I recommend that we do not place a feeding-tube.”
- Balance realism and hope: “Instead, we can focus on other ways to maintain dignity and quality of life for her even without a feeding tube.”
RESPONDING TO CHALLENGES
Conversations about goals of care and prognosis can be challenging and time consuming. At times, the conversations can be strained. The following tips are based on authors’ shared experiences to help in those challenging situations:
- Caregivers may show signs of emotional and/or cognitive strain: Recognize and name the emotional response and consider asking the family if they need a break to avoid overtaxing them.
“I can see that this is very difficult for you. Do you want to take a break and meet again?”
- Caregivers may have unrealistic hopes: Confirm the caregivers’ understanding of the situation, before assuming their hope is unrealistic. Try to reframe what they/we can hope for by validating their goals while avoiding unnecessary burdens or discomfort.
“I want to be sure that I have explained your mom’s situation clearly. Can you tell me in your words, what I have told you?” as this gives you an opportunity to clarify misunderstandings that may manifest as “false hope”.
“Together we can hope for the best and see if your mother can tolerate hand-feeding safely without causing any harm or distress.”
- Avoid assumptions about cultural and religious beliefs: Be curious and demonstrate cultural humility to all patients.
“Are there any cultural or spiritual beliefs that are important to you or your mother?”
- Avoid spending too much time on clinical details: Give families time to share stories about the patient in better days as this gives you an opportunity to get to know the patient.
“Tell me more about what your mother was like when she was healthy.”
- Listen first, recommend second: Refrain from making recommendations about the patient’s care before you understand his/her values and preferences.
“What would your mother say is most important to her as her health worsens?”
- Use active listening techniques: Using reflection statements can confirm your understanding of the caregiver’s view point.
“So, I hear that your mother valued being at home and being comfortable. Is that correct?”
These conversations are often an iterative process of helping the patient and family traverse the course of AD. Therefore, starting the process even during a hospitalization earlier in the course of AD can help engage in preparedness planning to provide goal-concordant care and help optimize the patient’s quality of life.
CONCLUSION
Hospitalization among patients with AD can signal a significant change in prognosis and represents an important opportunity for further dialogue. A patient- and caregiver-centered conversation, sharing prognosis and learning about values important to the patient and family, has the potential to lead to less burdensome interventions. Doing so can minimize harm, promote quality of life, and reduce unnecessary care transitions near the end of life.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Havyer was supported, in part, by the Mayo Clinic Department of Medicine Catalyst for Advancing in Academics grant. Dr. Abedini was supported by the National Clinician Scholars Program at the Institute for Healthcare Policy and Innovation, University of Michigan, Ann Arbor, MI.
Advanced dementia (AD) is a serious terminal illness. Some features of AD include significant memory deficits (inability to recognize family members), inability to ambulate, very limited verbal communication, and needing assistance with all activities of daily living.1 AD carries a six-month mortality of 25% and a median survival of 1.3 years.1
Despite a limited life expectancy, patients with AD face increasingly significant symptom burden and use of burdensome interventions2 near the end of life. Among the most common interventions hospitalists routinely navigate during a hospitalization is tube feeding for enteral artificial nutrition, which has not been shown to prolong survival, improve quality of life, decrease risk of aspiration pneumonia, or decrease the risk of pressure ulcers.3,4 Recent data show that rates of hospitalizations in the last 90 days of life, especially in the last three days of life, are increasing.5 These late transitions can have significant negative impact on family perceptions of quality of care, including not being treated with respect, receiving care inconsistent with goals, receiving inadequate communication about care decisions, and not being fully informed of the medical conditions.6
Therefore, hospitalization in AD, especially a readmission, indicates a critical change in a patient’s illness, marking an opportune time to have discussions on prognosis and improve care at the end of life. While determining and sharing prognosis can be challenging in the setting of many chronic diseases, resources exist to help clinicians share prognosis in AD and understand the goals of care for each patient.7 The aim of this paper is to assist hospitalists in addressing prognosis in the setting of AD. We identify and present key knowledge and recommendations from relevant articles identified from a hand-search of articles, published in 2018, from leading palliative care journals, as well as a MEDLINE search from 2003 through December 2018 using the key words “dementia” and “prognosis.” Final presented articles and recommendations were determined based on scientific rigor and relevance to hospital-based care of patients with AD.
IMPORTANCE OF PROGNOSIS DISCUSSIONS IN ADVANCED DEMENTIA
For a myriad of reasons, most AD caregivers do not receive adequate information on the complications of dementia or prognosis.2 Conversations that provide prognostic estimates and aim to understand the goals, preferences, and values of AD patients and their surrogates can help in providing goal-concordant care. A prospective study of nursing-home patients with AD showed that having goals of care discussions was strongly associated with surrogates’ likelihood of estimating a life expectancy of less than six months in AD patients.8 Having this perception was associated with a lower likelihood of patients with AD undergoing burdensome interventions such as hospitalizations, parenteral therapy, venipuncture, feeding tube, or urinary catheterization.8 To help improve goal-concordant care, it is important that hospitalists be prepared to have prognostic conversations with patients and their caregivers.
“FORESEEING” PROGNOSIS IN ADVANCED DEMENTIA
Offering a clinical prognosis involves components of foreseeing (estimating prognosis) and foretelling (sharing prognosis).9 Foreseeing prognosis in AD can be complex due to the highly variable but slow, dwindling clinical course of AD. As a practical matter, determining if a patient has a six-month prognosis is most helpful as eligibility for hospice services may allow for a discharge to a supportive home setting instead of a transfer to an institution.10 An evidence-based clinical prediction rule, the Advanced Dementia Prognostic Tool (ADEPT, Appendix Table),11 can be used to estimate prognosis by a composite of 12 risk factors in nursing-home patients. Although the consensus-based National Hospice and Palliative Care Organization (NHPCO) guidelines for Medicare hospice eligibility12 (Table) do not perform well in predicting individual mortality, they are used as criteria for hospice enrollment. Given the variability of course of AD, evaluating the mortality risk for acute illnesses leading to hospitalization, like pneumonia or hip fracture, can further help estimate prognosis. The website, www.eprognosis.com, combines various prediction tools to help estimate prognosis. Although using these tools can often help clinicians satisfy the entry requirements to offer hospice, the ADEPT tool and the NHPCO criteria both perform poorly in discriminating those who will or will not actually die in six months. ADEPT, as a prognostic tool, has not yet been validated for community-dwelling patients. Clinicians should exercise caution in making a highly specific estimate of survival in AD, but can and should communicate the expected decline in function over time.
“FORETELLING” PROGNOSIS IN ADVANCED DEMENTIA
Having goals of care conversations and sharing prognosis has many benefits. A large multistate cohort study showed that having goals of care conversations among patients with terminal cancer was associated with less use of intensive care units, mechanical ventilation, and cardio-pulmonary resuscitation.13 Caregivers may also benefit from prognosis discussions through identifying resources to care for the patient at home and by potentially limiting their risk of major depressive order and regret, common among those witnessing patients undergoing aggressive treatment at the end of life.13 How to share prognosis can be challenging; however, tools such as the Serious Illness Conversation Guide14 can provide step-by-step guidance for providers. The key aspects of the guide are asking permission, assessing illness understanding, and exploring goals, fears, worries, and tradeoffs.
Before exploring goals, it is helpful to explain the serious illness by using “I wish,” “I worry,” and “I wonder” statements such as “I wish I had better news for you; your mom’s dementia has progressed given the recent complication of aspiration,” “I worry that she will not be able to eat on her own and will develop another serious infection very soon,” or “I wonder whether it is a good time to talk about what your mom would want if she cannot eat on her own.”
An example of an effective conversation about artificial nutrition and hydration with a surrogate of a patient with AD with recurrent aspirations may include the following elements:14,15
- Obtain the caregiver’s and/or patient’s perception of illness: “Is it OK if we have a conversation about what may lie ahead with your mother? Is there anyone else that should be present? What is your understanding of your mother’s illness?”
- Give relevant data: “Based on her current level of decline with complications and repetitive hospitalizations, I am worried that her life expectancy is likely measured in months rather than years.”
- Address emotions: “This must be very hard to hear. I cannot imagine how difficult it must be to see her in the hospital so often.”
- Elicit concerns and goals based on understanding key values: “Tell me what you are hoping for regarding your mother’s future care and what worries you have. Tell me what your mother would say if she could fully understand her current situation.”
- Present goals based on patient and caregiver values: “Based on what you have told me about your mother, she valued her interactions with family and her independence, and she would not want measures that would cause distress, especially when facing a terminal illness.”
- Be mindful of prognostic uncertainty: “While we cannot know for certain what will happen next, I am very worried that your mother will continue to aspirate even with a feeding tube.”
- Make a recommendation with permission: “From our conversation, I have an idea of what treatment might make sense to your mom. May I share my recommendation with you?” If they are willing, you might say: “As evidence shows that feeding tubes do not improve the level of family interaction or independence in patients with dementia and as your mother would not want any distressing procedures, I recommend that we do not place a feeding-tube.”
- Balance realism and hope: “Instead, we can focus on other ways to maintain dignity and quality of life for her even without a feeding tube.”
RESPONDING TO CHALLENGES
Conversations about goals of care and prognosis can be challenging and time consuming. At times, the conversations can be strained. The following tips are based on authors’ shared experiences to help in those challenging situations:
- Caregivers may show signs of emotional and/or cognitive strain: Recognize and name the emotional response and consider asking the family if they need a break to avoid overtaxing them.
“I can see that this is very difficult for you. Do you want to take a break and meet again?”
- Caregivers may have unrealistic hopes: Confirm the caregivers’ understanding of the situation, before assuming their hope is unrealistic. Try to reframe what they/we can hope for by validating their goals while avoiding unnecessary burdens or discomfort.
“I want to be sure that I have explained your mom’s situation clearly. Can you tell me in your words, what I have told you?” as this gives you an opportunity to clarify misunderstandings that may manifest as “false hope”.
“Together we can hope for the best and see if your mother can tolerate hand-feeding safely without causing any harm or distress.”
- Avoid assumptions about cultural and religious beliefs: Be curious and demonstrate cultural humility to all patients.
“Are there any cultural or spiritual beliefs that are important to you or your mother?”
- Avoid spending too much time on clinical details: Give families time to share stories about the patient in better days as this gives you an opportunity to get to know the patient.
“Tell me more about what your mother was like when she was healthy.”
- Listen first, recommend second: Refrain from making recommendations about the patient’s care before you understand his/her values and preferences.
“What would your mother say is most important to her as her health worsens?”
- Use active listening techniques: Using reflection statements can confirm your understanding of the caregiver’s view point.
“So, I hear that your mother valued being at home and being comfortable. Is that correct?”
These conversations are often an iterative process of helping the patient and family traverse the course of AD. Therefore, starting the process even during a hospitalization earlier in the course of AD can help engage in preparedness planning to provide goal-concordant care and help optimize the patient’s quality of life.
CONCLUSION
Hospitalization among patients with AD can signal a significant change in prognosis and represents an important opportunity for further dialogue. A patient- and caregiver-centered conversation, sharing prognosis and learning about values important to the patient and family, has the potential to lead to less burdensome interventions. Doing so can minimize harm, promote quality of life, and reduce unnecessary care transitions near the end of life.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Havyer was supported, in part, by the Mayo Clinic Department of Medicine Catalyst for Advancing in Academics grant. Dr. Abedini was supported by the National Clinician Scholars Program at the Institute for Healthcare Policy and Innovation, University of Michigan, Ann Arbor, MI.
1. Mitchell SL. Advanced dementia. N Engl J Med. 2015;373(13):1276-1277. https://doi.org/10.1056/NEJMcp1412652.
2. Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361(16):1529-1538. https://doi.org/10.1056/NEJMoa0902234.
3. Teno JM, Gozalo PL, Mitchell SL, et al. Does feeding tube insertion and its timing improve survival? J Am Geriatr Soc. 2012;60(10):1918-1921. https://doi.org/10.1111/j.1532-5415.2012.04148.x.
4. Teno JM, Gozalo P, Mitchell SL, Kuo S, Fulton AT, Mor V. Feeding tubes and the prevention or healing of pressure ulcers. Arch Intern Med. 2012;172(9):697-701. https://doi.org/10.1001/archinternmed.2012.1200.
5. Teno JM, Gozalo PL, Bynum JP, et al. Change in end-of-life care for medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470-477. https://doi.org/10.1001/jama.2012.207624.
6. Makaroun LK, Teno JM, Freedman VA, Kasper JD, Gozalo P, Mor V. Late transitions and bereaved family member perceptions of quality of end-of-life care. J Am Geriatr Soc. 2018;66(9):1730-1736. https://doi.org/10.1111/jgs.15455.
7. Ansari AA, Pomerantz DH, Jayes RL, Aguirre EA, Havyer RD. Promoting primary palliative care in severe chronic obstructive pulmonary disease: symptom management and preparedness planning. J Palliat Care. 2019;34(2):85-91. https://doi.org/10.1177/0825859718819437.
8. Loizeau AJ, Shaffer ML, Habtemariam DA, Hanson LC, Volandes AE, Mitchell SL. Association of prognostic estimates with burdensome interventions in nursing home residents with advanced dementia. JAMA Intern Med. 2018;178(7):922-929. https://doi.org/10.1001/jamainternmed.2018.1413.
9. Glare PA, Sinclair CT. Palliative medicine review: Prognostication. J Palliat Med. 2008;11(1):84-103. https://doi.org/10.1089/jpm.2008.9992.
10. Jayes RL, Arnold RM, Fromme EK. Does this dementia patient meet the prognosis eligibility requirements for hospice enrollment? J Pain Symptom Manage. 2012;44(5):750-756. https://doi.org/10.1016/j.jpainsymman.2012.08.004.
11. Mitchell SL, Miller SC, Teno JM, Kiely DK, Davis RB, Shaffer ML. Prediction of 6-month survival of nursing home residents with advanced dementia using ADEPT vs hospice eligibility guidelines. JAMA. 2010;304(17):1929-1935. https://doi.org/10.1001/jama.2010.1572.
12. Schonwetter RS, Han B, Small BJ, et al. Predictors of six-month survival among patients with dementia: an evaluation of hospice Medicare guidelines. Amer J Hospice & Pall Care. 2003;20(2):105-113. https://doi.org/10.1177/104990910302000208
13. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673. https://doi.org/10.1001/jama.300.14.1665.
14. Bernacki R, Hutchings M, Vick J, et al. Development of the serious illness care program: a randomised controlled trial of a palliative care communication intervention. BMJ Open. 2015;5(10):e009032-2015-009032. https://doi.org/10.1136/bmjopen-2015-009032
15. Ansari A, Pomerantz D, Smith K. Being mindful: difficult decisions in advanced dementia and end stage renal disease. SGIM Forum. 2017;40(3):4, 13.
1. Mitchell SL. Advanced dementia. N Engl J Med. 2015;373(13):1276-1277. https://doi.org/10.1056/NEJMcp1412652.
2. Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361(16):1529-1538. https://doi.org/10.1056/NEJMoa0902234.
3. Teno JM, Gozalo PL, Mitchell SL, et al. Does feeding tube insertion and its timing improve survival? J Am Geriatr Soc. 2012;60(10):1918-1921. https://doi.org/10.1111/j.1532-5415.2012.04148.x.
4. Teno JM, Gozalo P, Mitchell SL, Kuo S, Fulton AT, Mor V. Feeding tubes and the prevention or healing of pressure ulcers. Arch Intern Med. 2012;172(9):697-701. https://doi.org/10.1001/archinternmed.2012.1200.
5. Teno JM, Gozalo PL, Bynum JP, et al. Change in end-of-life care for medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470-477. https://doi.org/10.1001/jama.2012.207624.
6. Makaroun LK, Teno JM, Freedman VA, Kasper JD, Gozalo P, Mor V. Late transitions and bereaved family member perceptions of quality of end-of-life care. J Am Geriatr Soc. 2018;66(9):1730-1736. https://doi.org/10.1111/jgs.15455.
7. Ansari AA, Pomerantz DH, Jayes RL, Aguirre EA, Havyer RD. Promoting primary palliative care in severe chronic obstructive pulmonary disease: symptom management and preparedness planning. J Palliat Care. 2019;34(2):85-91. https://doi.org/10.1177/0825859718819437.
8. Loizeau AJ, Shaffer ML, Habtemariam DA, Hanson LC, Volandes AE, Mitchell SL. Association of prognostic estimates with burdensome interventions in nursing home residents with advanced dementia. JAMA Intern Med. 2018;178(7):922-929. https://doi.org/10.1001/jamainternmed.2018.1413.
9. Glare PA, Sinclair CT. Palliative medicine review: Prognostication. J Palliat Med. 2008;11(1):84-103. https://doi.org/10.1089/jpm.2008.9992.
10. Jayes RL, Arnold RM, Fromme EK. Does this dementia patient meet the prognosis eligibility requirements for hospice enrollment? J Pain Symptom Manage. 2012;44(5):750-756. https://doi.org/10.1016/j.jpainsymman.2012.08.004.
11. Mitchell SL, Miller SC, Teno JM, Kiely DK, Davis RB, Shaffer ML. Prediction of 6-month survival of nursing home residents with advanced dementia using ADEPT vs hospice eligibility guidelines. JAMA. 2010;304(17):1929-1935. https://doi.org/10.1001/jama.2010.1572.
12. Schonwetter RS, Han B, Small BJ, et al. Predictors of six-month survival among patients with dementia: an evaluation of hospice Medicare guidelines. Amer J Hospice & Pall Care. 2003;20(2):105-113. https://doi.org/10.1177/104990910302000208
13. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673. https://doi.org/10.1001/jama.300.14.1665.
14. Bernacki R, Hutchings M, Vick J, et al. Development of the serious illness care program: a randomised controlled trial of a palliative care communication intervention. BMJ Open. 2015;5(10):e009032-2015-009032. https://doi.org/10.1136/bmjopen-2015-009032
15. Ansari A, Pomerantz D, Smith K. Being mindful: difficult decisions in advanced dementia and end stage renal disease. SGIM Forum. 2017;40(3):4, 13.
© 2019 Society of Hospital Medicine
Things We Do for No Reason™: Supplemental Oxygen for Patients without Hypoxemia

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 65-year-old woman with hypertension presents to the emergency department with three days of dyspnea, malaise, and pleuritic chest pain. Her temperature is 100.1°F, heart rate 110 beats per minute, and blood pressure 110/60 mm Hg. She is breathing 24 times per minute and has an oxygen saturation (SpO2) of 94% on room air. Her exam is remarkable for dry mucous membranes and right lower lung crackles. Her nurse places her on 3 L of oxygen per minute via nasal cannula, and her SpO2 rises to 99%.
WHY YOU MIGHT THINK SUPPLEMENTAL OXYGEN FOR NORMOXEMIC PATIENTS IS HELPFUL
Shortly after the discovery of oxygen in the late 18th century, physicians began using it to treat a variety of conditions including tuberculosis, pneumonia, respiratory failure, and angina. By the 1970s, most medical texts recommended oxygen use in suspected myocardial infarction (MI) because of the theoretical appeal of increasing delivery of oxygen to the heart and other vital organs.1 Additionally, there is a tendency to believe that supplemental oxygen alleviates dyspnea regardless of etiology or oxygen saturation. Recent studies have shown widespread use of oxygen in scenarios without clear indications and without oxygen saturation goals. A 2010 survey of clinicians managing acute MI found that 98% “always or usually” used oxygen and 55% believed that oxygen “definitely or probably reduces the risk of death.”2 In a Danish prehospital study, supplemental oxygen was used in 34% of ambulance patients even though only 17% of these patients had an SpO2 less than 94%.3 A study of critically ill patients found that most of the time, SpO2 exceeded 98%. Even when the fraction of inspired oxygen (FiO2) was between 0.3 and 0.4, no one adjusted the oxygen dose.4
WHY IT IS NOT HELPFUL TO PROVIDE SUPPLEMENTAL OXYGEN TO NORMOXEMIC PATIENTS
The reflexive use of oxygen in patients with acute respiratory or cardiovascular illness is problematic for several reasons. First, when oxygen saturation is near-normal, the potential benefit from supplemental oxygen lacks physiologic plausibility. More compellingly, evidence exists that hyperoxemia may cause significant harm. Finally, the unnecessary use of supplemental oxygen incurs practical inconveniences and expenses.
To understand why the physiologic basis for reflexive oxygen use is weak, it is important to distinguish hypoxemia (low arterial oxygen tension and hemoglobin oxygen saturation), tissue hypoxia (which can occur from hypoxemia or focal abnormalities in perfusion), and dyspnea (a subjective experience of breathing discomfort). A variety of mechanisms cause dyspnea, most of which do not involve hypoxemia. A patient with acute heart failure may experience severe dyspnea caused by activation of pressure-sensitive J-receptors in the lung, even if oxygen saturation and tissue perfusion are intact. This process will be relieved by reducing pulmonary capillary pressures, but it is unaffected by supplemental oxygen. Coronary occlusion causes hypoxia of the heart muscle, but restoring perfusion is the most effective treatment. The instinct to maximize the oxygen-carrying capacity of the remaining blood flow is understandable. However, in a normoxemic patient, increasing the inspired fraction of oxygen has a marginal effect on oxygen-carrying capacity, since hemoglobin saturation and concentration rather than arterial oxygen tension (PaO2) predominantly determine oxygen-carrying capacity. On the other hand, supraphysiologic levels of dissolved oxygen may lead to toxicity.5
For over a century, we have known the potential harms of hyperoxia. Original studies in animal models showed that hyperoxia led to lung injury, altered hemodynamics, endothelial cell dysfunction, and inflammatory activation.5 Many of these detrimental effects involve the generation of reactive oxygen species and oxidative stress.5 High levels of inspired oxygen can also cause increased pulmonary shunting through inhibition of physiologic hypoxic vasoconstriction and due to absorption atelectasis.6 Oxygen negatively affects cardiovascular function by reducing coronary blood flow, increasing systemic vascular resistance, and reducing cardiac output.1
Chronic obstructive pulmonary disease (COPD) is the clinical setting in which risks of supplemental oxygen are most well-recognized historically. In patients with COPD at risk for hypercarbia, oxygen titrated to a goal SpO2 outside 88%-92% is associated with a two-fold risk of mortality.7 Worsening ventilation-perfusion matching and the Haldane effect (decreased affinity of hemoglobin for carbon dioxide as the PaO2 rises), rather than the previously theorized decrease in hypoxic drive, are now believed to contribute most to hyperoxia-induced hypercarbia. These unintended consequences may also occur in patients with other forms of acute and chronic lung disease.
The British Medical Journal published the first randomized controlled trial of oxygen use in suspected MI in 1976.1 Patients who received oxygen at 6 L per minute for 24 hours had more episodes of sinus tachycardia without any improvement in mortality, analgesic use, or infarct size.1 More recent and robust trials comparing outcomes in normoxemic patients randomized to supplemental oxygen versus room air have had similar findings: no difference in mortality, infarct size, or pain ratings.8,9 One found a significantly increased rate of MI recurrence with the use of oxygen.8 These data have led the latest guidelines for the management of ST-elevation MI from the European Society of Cardiology to discourage the use of supplemental oxygen unless SpO2 is <90%.10
Two recent trials investigated the effects of hyperoxia in critically ill patients.11,12 Girardis and colleagues randomized 480 critically ill patients in an Italian medical-surgical intensive care unit to conservative (SpO2 between 94% and 98% or PaO2 between 70 and 100 mm Hg) versus conventional oxygenation targets (SpO2 between 97% and 100% and PaO2 up to 150 mm Hg). Compared with conventional oxygen targets, conservative oxygen use was associated with an absolute risk reduction in mortality of 8.6% (11.6% vs 20.2%; P =.01).11 Another trial from 22 centers in France compared outcomes in mechanically ventilated patients with septic shock who received FiO2 at 1.0 compared with those with oxygen titration to SpO2 between 88% and 95%. The trial was stopped early for safety concerns. Those in the hyperoxemia group had a higher incidence of serious adverse events (85% vs 76%; P =.02), including pneumothorax, clinically relevant bleeding, myocardial infarction, and arrhythmias, as well as a trend toward increased mortality.12
Trials of liberal oxygen use in other settings of acute illness,13 including ischemic stroke,14 traumatic brain injury,15 and postcardiac arrest,16 have also linked liberal oxygen use with increased risk of mortality and other adverse events. “Liberal” use in these trials ranged from an FiO2 of 0.28 (equivalent to 2 L of nasal cannula) to 1.0. Significant secondary outcomes included fewer hospital-free and ventilator-free days in patients with liberal oxygen use. Furthermore, a meta-analysis of 25 trials including over 16,000 patients found dose-dependent toxicity: for every 1% increase in SpO2 above 94%-96% (the median SpO2 in the liberal oxygen groups), there was a 25% relative increase in in-hospital mortality.13
In addition to the data above, there are practical reasons to avoid unnecessary use of supplemental oxygen. Providing supplemental oxygen to a patient who is not hypoxemic may delay the recognition of cardiopulmonary decompensation by delaying detection of hypoxemia.6 Beyond the effects of oxygen itself, oxygen delivery methods carry their own potential adverse effects. These include epistaxis (with nasal cannula), claustrophobia (with face masks), decreased mobility, falls, and delirium.17 Finally, oxygen administration has direct and indirect financial costs, including those of supplies, care coordination, and monitoring.
WHEN SUPPLEMENTAL OXYGEN MIGHT BE HELPFUL
Importantly, the above discussion pertains to normoxemic patients receiving supplemental oxygen. There is no dispute that significantly hypoxemic patients should receive supplemental oxygen. There are also instances where the use of supplemental oxygen in normoxemic patients may be beneficial, such as in carbon monoxide poisoning, decompression injury, gas embolism, cluster headaches, sickle cell crisis, and pneumothorax.17
WHAT YOU SHOULD DO INSTEAD
Like any other drug, oxygen should be administered after assessment of its indications, intended benefits, and possible harms. Both significant hypoxemia and hyperoxemia should be avoided. In patients with neither hypoxemia nor the indications above, clinicians should not administer supplemental oxygen. Recent society guidelines can be applied in various clinical contexts. In patients with suspected MI, oxygen should be administered if SpO2 is <90%.10 For most other acutely ill patients, clinicians should administer supplemental oxygen if SpO2 <90%-92% and target an SpO2 of no higher than 94%-96%,18-19 as meta-analyses found evidence of harm above this level.13 Results of randomized trials currently underway should add supporting evidence for more specific oxygenation targets in different patient populations. With respect to implementation, it must be noted that factors beyond physician decision influence the use of supplemental oxygen. Appropriate institutional policies, standards of care, and educational efforts to all hospital providers must be enacted in order to reduce the unnecessary use of supplemental oxygen.
RECOMMENDATIONS
- For most acutely ill patients, do not administer supplemental oxygen when SpO2 >92%. If supplemental oxygen is used, the SpO2 should not exceed 94%-96%.
- For patients with suspected MI, only start supplemental oxygen for SpO2 <90%.
- For patients at risk for hypercapnic respiratory failure (eg, COPD patients), target SpO2 of 88%-92%.
- Provide supplemental oxygen to normoxemic patients with carbon monoxide poisoning, decompression injury, gas embolism, cluster headache, sickle cell crisis, and pneumothorax.
- Review and revise institutional practices and policies that contribute to unnecessary use of supplemental oxygen.
CONCLUSIONS
In the opening case, the patient is acutely ill and requires further workup. Her current SpO2 of 99% puts her at risk for adverse events and death, and supplemental oxygen should be titrated down or stopped to avoid SpO2 greater than 94%-96%. For years, clinicians have erred on the side of using supplemental oxygen, without recognizing its dangers. However, over a century of evidence from pathophysiologic experiments and randomized trials across multiple clinical settings have associated hyperoxemia with adverse outcomes and increased mortality. Professional societies are adopting this evidence into their guideline recommendations, and clinicians should use supplemental oxygen judiciously in their daily practice.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
1. Rawles JM, Kenmure AC. Controlled trial of oxygen in uncomplicated myocardial infarction. Br Med J. 1976;1(6018):1121-1123. https://doi.org/10.1136/bmj.1.6018.1121.
2. Burls A, Emparanza JI, Quinn T, Cabello J. Oxygen use in acute myocardial infarction: an online survey of health professionals’ practice and beliefs. Emerg Med J. 2010;27(4):283-286. https://doi.org/10.1136/emj.2009.077370.
3. Hale KE, Gavin C, O’Driscoll BR. Audit of oxygen use in emergency ambulances and in a hospital emergency department. Emerg Med J. 2008;25(11):773-776. https://doi.org/10.1136/emj.2008.059287.
4. Suzuki S, Eastwood G, Peck L, Glassford N, Bellomo R. Oxygen management in mechanically ventilated patients: a prospective observational cohort study. Aust Crit Care. 2014;27(1):50-51. https://doi.org/10.1016/j.aucc.2013.10.025.
5. Helmerhorst HJ, Schultz MJ, van der Voort PH, de Jonge E, van Wasterloo DJ. Bench-to-bedside review: the effects of hyperoxia during critical illness. Crit Care. 2015;19(1):284. https://doi.org/10.1186/s13054-015-0996-4.
6. Downs JB. Has oxygen administration delayed appropriate respiratory care? Fallacies regarding oxygen therapy. Respir Care. 2003;48(6):611-620.
7. Austin MA, Willis KE, Blizzard L, Walters EH, Wood-Baker R. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010;341:c5462. https://doi.org/10.2307/20800296.
8. Stub D, Smith K, Bernard S, et al. Air versus oxygen in ST-segment-elevation myocardial infarction. Circulation. 2015;131(24):2143-2150. https://doi.org/10.1161/CIRCULATIONAHA.114.014494.
9. Hofman R. Witt N, Lagergvist B, et al. Oxygen therapy in ST-elevation myocardial infarction. Eur Heart J. 2018;39(29):2730-2739. https://doi.org/10.1093/eurheartj/ehy326.
10. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018:39(2):119-177. https://doi.org/10.1093/eurheartj/ehx393.
11. Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit. JAMA. 2016;316(15):1583-1589. https://doi.org/10.1001/jama.2016.11993.
12. Asfar P, Schortgen F, Boisramé-Helms J, et al. Hyperoxia and hypertonic saline in patients with septic shock (HYPERS2S): a two-by-two factorial, multicentre, randomised, clinical trial. Lancet Respir Med. 2017:5(3):180-190. https://doi.org/10.1016/S2213-2600(17)30046-2.
13. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693-1705. https://doi.org/10.1016/S0140-6736(18)30479-3.
14. Rincon F, Kang J, Maltenfort M, et al. Association between hyperoxia and mortality after stroke: a multicenter cohort study. Crit Care Med. 2014;42(2):387-396. https://doi.org/10.1097/CCM.0b013e3182a27732.
15. Brenner M, Stein D, Hu P, Kufera J, Woodford M, Scalea T. Association between early hyperoxia and worse outcomes after traumatic brain injury. Arch Surg. 2012;147(11):1042-1046. https://doi.org/10.1001/archsurg.2012.1560.
16. Kilgannon JH, Jones AE, Shapiro NI, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303(21):2165-2171. https://doi.org/10.1
17. Siemieniuk RA, Chu DK, Kim L, et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ. 2018;363:k4169. https://doi.org/10.1136/bmj.k4169.
18. O’Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72(1):ii1-ii90. https://doi.org/10.1136/thoraxjnl-2016-209729.
19. Beasley R, Chien J, Douglas J, et al. Thoracic Society of Australia and New Zealand oxygen guidelines for acute oxygen use in adults: ‘Swimming between the flags’. Respirology. 2015;20(8):1182-1191. https://doi.org/10.1111/resp.12620.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 65-year-old woman with hypertension presents to the emergency department with three days of dyspnea, malaise, and pleuritic chest pain. Her temperature is 100.1°F, heart rate 110 beats per minute, and blood pressure 110/60 mm Hg. She is breathing 24 times per minute and has an oxygen saturation (SpO2) of 94% on room air. Her exam is remarkable for dry mucous membranes and right lower lung crackles. Her nurse places her on 3 L of oxygen per minute via nasal cannula, and her SpO2 rises to 99%.
WHY YOU MIGHT THINK SUPPLEMENTAL OXYGEN FOR NORMOXEMIC PATIENTS IS HELPFUL
Shortly after the discovery of oxygen in the late 18th century, physicians began using it to treat a variety of conditions including tuberculosis, pneumonia, respiratory failure, and angina. By the 1970s, most medical texts recommended oxygen use in suspected myocardial infarction (MI) because of the theoretical appeal of increasing delivery of oxygen to the heart and other vital organs.1 Additionally, there is a tendency to believe that supplemental oxygen alleviates dyspnea regardless of etiology or oxygen saturation. Recent studies have shown widespread use of oxygen in scenarios without clear indications and without oxygen saturation goals. A 2010 survey of clinicians managing acute MI found that 98% “always or usually” used oxygen and 55% believed that oxygen “definitely or probably reduces the risk of death.”2 In a Danish prehospital study, supplemental oxygen was used in 34% of ambulance patients even though only 17% of these patients had an SpO2 less than 94%.3 A study of critically ill patients found that most of the time, SpO2 exceeded 98%. Even when the fraction of inspired oxygen (FiO2) was between 0.3 and 0.4, no one adjusted the oxygen dose.4
WHY IT IS NOT HELPFUL TO PROVIDE SUPPLEMENTAL OXYGEN TO NORMOXEMIC PATIENTS
The reflexive use of oxygen in patients with acute respiratory or cardiovascular illness is problematic for several reasons. First, when oxygen saturation is near-normal, the potential benefit from supplemental oxygen lacks physiologic plausibility. More compellingly, evidence exists that hyperoxemia may cause significant harm. Finally, the unnecessary use of supplemental oxygen incurs practical inconveniences and expenses.
To understand why the physiologic basis for reflexive oxygen use is weak, it is important to distinguish hypoxemia (low arterial oxygen tension and hemoglobin oxygen saturation), tissue hypoxia (which can occur from hypoxemia or focal abnormalities in perfusion), and dyspnea (a subjective experience of breathing discomfort). A variety of mechanisms cause dyspnea, most of which do not involve hypoxemia. A patient with acute heart failure may experience severe dyspnea caused by activation of pressure-sensitive J-receptors in the lung, even if oxygen saturation and tissue perfusion are intact. This process will be relieved by reducing pulmonary capillary pressures, but it is unaffected by supplemental oxygen. Coronary occlusion causes hypoxia of the heart muscle, but restoring perfusion is the most effective treatment. The instinct to maximize the oxygen-carrying capacity of the remaining blood flow is understandable. However, in a normoxemic patient, increasing the inspired fraction of oxygen has a marginal effect on oxygen-carrying capacity, since hemoglobin saturation and concentration rather than arterial oxygen tension (PaO2) predominantly determine oxygen-carrying capacity. On the other hand, supraphysiologic levels of dissolved oxygen may lead to toxicity.5
For over a century, we have known the potential harms of hyperoxia. Original studies in animal models showed that hyperoxia led to lung injury, altered hemodynamics, endothelial cell dysfunction, and inflammatory activation.5 Many of these detrimental effects involve the generation of reactive oxygen species and oxidative stress.5 High levels of inspired oxygen can also cause increased pulmonary shunting through inhibition of physiologic hypoxic vasoconstriction and due to absorption atelectasis.6 Oxygen negatively affects cardiovascular function by reducing coronary blood flow, increasing systemic vascular resistance, and reducing cardiac output.1
Chronic obstructive pulmonary disease (COPD) is the clinical setting in which risks of supplemental oxygen are most well-recognized historically. In patients with COPD at risk for hypercarbia, oxygen titrated to a goal SpO2 outside 88%-92% is associated with a two-fold risk of mortality.7 Worsening ventilation-perfusion matching and the Haldane effect (decreased affinity of hemoglobin for carbon dioxide as the PaO2 rises), rather than the previously theorized decrease in hypoxic drive, are now believed to contribute most to hyperoxia-induced hypercarbia. These unintended consequences may also occur in patients with other forms of acute and chronic lung disease.
The British Medical Journal published the first randomized controlled trial of oxygen use in suspected MI in 1976.1 Patients who received oxygen at 6 L per minute for 24 hours had more episodes of sinus tachycardia without any improvement in mortality, analgesic use, or infarct size.1 More recent and robust trials comparing outcomes in normoxemic patients randomized to supplemental oxygen versus room air have had similar findings: no difference in mortality, infarct size, or pain ratings.8,9 One found a significantly increased rate of MI recurrence with the use of oxygen.8 These data have led the latest guidelines for the management of ST-elevation MI from the European Society of Cardiology to discourage the use of supplemental oxygen unless SpO2 is <90%.10
Two recent trials investigated the effects of hyperoxia in critically ill patients.11,12 Girardis and colleagues randomized 480 critically ill patients in an Italian medical-surgical intensive care unit to conservative (SpO2 between 94% and 98% or PaO2 between 70 and 100 mm Hg) versus conventional oxygenation targets (SpO2 between 97% and 100% and PaO2 up to 150 mm Hg). Compared with conventional oxygen targets, conservative oxygen use was associated with an absolute risk reduction in mortality of 8.6% (11.6% vs 20.2%; P =.01).11 Another trial from 22 centers in France compared outcomes in mechanically ventilated patients with septic shock who received FiO2 at 1.0 compared with those with oxygen titration to SpO2 between 88% and 95%. The trial was stopped early for safety concerns. Those in the hyperoxemia group had a higher incidence of serious adverse events (85% vs 76%; P =.02), including pneumothorax, clinically relevant bleeding, myocardial infarction, and arrhythmias, as well as a trend toward increased mortality.12
Trials of liberal oxygen use in other settings of acute illness,13 including ischemic stroke,14 traumatic brain injury,15 and postcardiac arrest,16 have also linked liberal oxygen use with increased risk of mortality and other adverse events. “Liberal” use in these trials ranged from an FiO2 of 0.28 (equivalent to 2 L of nasal cannula) to 1.0. Significant secondary outcomes included fewer hospital-free and ventilator-free days in patients with liberal oxygen use. Furthermore, a meta-analysis of 25 trials including over 16,000 patients found dose-dependent toxicity: for every 1% increase in SpO2 above 94%-96% (the median SpO2 in the liberal oxygen groups), there was a 25% relative increase in in-hospital mortality.13
In addition to the data above, there are practical reasons to avoid unnecessary use of supplemental oxygen. Providing supplemental oxygen to a patient who is not hypoxemic may delay the recognition of cardiopulmonary decompensation by delaying detection of hypoxemia.6 Beyond the effects of oxygen itself, oxygen delivery methods carry their own potential adverse effects. These include epistaxis (with nasal cannula), claustrophobia (with face masks), decreased mobility, falls, and delirium.17 Finally, oxygen administration has direct and indirect financial costs, including those of supplies, care coordination, and monitoring.
WHEN SUPPLEMENTAL OXYGEN MIGHT BE HELPFUL
Importantly, the above discussion pertains to normoxemic patients receiving supplemental oxygen. There is no dispute that significantly hypoxemic patients should receive supplemental oxygen. There are also instances where the use of supplemental oxygen in normoxemic patients may be beneficial, such as in carbon monoxide poisoning, decompression injury, gas embolism, cluster headaches, sickle cell crisis, and pneumothorax.17
WHAT YOU SHOULD DO INSTEAD
Like any other drug, oxygen should be administered after assessment of its indications, intended benefits, and possible harms. Both significant hypoxemia and hyperoxemia should be avoided. In patients with neither hypoxemia nor the indications above, clinicians should not administer supplemental oxygen. Recent society guidelines can be applied in various clinical contexts. In patients with suspected MI, oxygen should be administered if SpO2 is <90%.10 For most other acutely ill patients, clinicians should administer supplemental oxygen if SpO2 <90%-92% and target an SpO2 of no higher than 94%-96%,18-19 as meta-analyses found evidence of harm above this level.13 Results of randomized trials currently underway should add supporting evidence for more specific oxygenation targets in different patient populations. With respect to implementation, it must be noted that factors beyond physician decision influence the use of supplemental oxygen. Appropriate institutional policies, standards of care, and educational efforts to all hospital providers must be enacted in order to reduce the unnecessary use of supplemental oxygen.
RECOMMENDATIONS
- For most acutely ill patients, do not administer supplemental oxygen when SpO2 >92%. If supplemental oxygen is used, the SpO2 should not exceed 94%-96%.
- For patients with suspected MI, only start supplemental oxygen for SpO2 <90%.
- For patients at risk for hypercapnic respiratory failure (eg, COPD patients), target SpO2 of 88%-92%.
- Provide supplemental oxygen to normoxemic patients with carbon monoxide poisoning, decompression injury, gas embolism, cluster headache, sickle cell crisis, and pneumothorax.
- Review and revise institutional practices and policies that contribute to unnecessary use of supplemental oxygen.
CONCLUSIONS
In the opening case, the patient is acutely ill and requires further workup. Her current SpO2 of 99% puts her at risk for adverse events and death, and supplemental oxygen should be titrated down or stopped to avoid SpO2 greater than 94%-96%. For years, clinicians have erred on the side of using supplemental oxygen, without recognizing its dangers. However, over a century of evidence from pathophysiologic experiments and randomized trials across multiple clinical settings have associated hyperoxemia with adverse outcomes and increased mortality. Professional societies are adopting this evidence into their guideline recommendations, and clinicians should use supplemental oxygen judiciously in their daily practice.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 65-year-old woman with hypertension presents to the emergency department with three days of dyspnea, malaise, and pleuritic chest pain. Her temperature is 100.1°F, heart rate 110 beats per minute, and blood pressure 110/60 mm Hg. She is breathing 24 times per minute and has an oxygen saturation (SpO2) of 94% on room air. Her exam is remarkable for dry mucous membranes and right lower lung crackles. Her nurse places her on 3 L of oxygen per minute via nasal cannula, and her SpO2 rises to 99%.
WHY YOU MIGHT THINK SUPPLEMENTAL OXYGEN FOR NORMOXEMIC PATIENTS IS HELPFUL
Shortly after the discovery of oxygen in the late 18th century, physicians began using it to treat a variety of conditions including tuberculosis, pneumonia, respiratory failure, and angina. By the 1970s, most medical texts recommended oxygen use in suspected myocardial infarction (MI) because of the theoretical appeal of increasing delivery of oxygen to the heart and other vital organs.1 Additionally, there is a tendency to believe that supplemental oxygen alleviates dyspnea regardless of etiology or oxygen saturation. Recent studies have shown widespread use of oxygen in scenarios without clear indications and without oxygen saturation goals. A 2010 survey of clinicians managing acute MI found that 98% “always or usually” used oxygen and 55% believed that oxygen “definitely or probably reduces the risk of death.”2 In a Danish prehospital study, supplemental oxygen was used in 34% of ambulance patients even though only 17% of these patients had an SpO2 less than 94%.3 A study of critically ill patients found that most of the time, SpO2 exceeded 98%. Even when the fraction of inspired oxygen (FiO2) was between 0.3 and 0.4, no one adjusted the oxygen dose.4
WHY IT IS NOT HELPFUL TO PROVIDE SUPPLEMENTAL OXYGEN TO NORMOXEMIC PATIENTS
The reflexive use of oxygen in patients with acute respiratory or cardiovascular illness is problematic for several reasons. First, when oxygen saturation is near-normal, the potential benefit from supplemental oxygen lacks physiologic plausibility. More compellingly, evidence exists that hyperoxemia may cause significant harm. Finally, the unnecessary use of supplemental oxygen incurs practical inconveniences and expenses.
To understand why the physiologic basis for reflexive oxygen use is weak, it is important to distinguish hypoxemia (low arterial oxygen tension and hemoglobin oxygen saturation), tissue hypoxia (which can occur from hypoxemia or focal abnormalities in perfusion), and dyspnea (a subjective experience of breathing discomfort). A variety of mechanisms cause dyspnea, most of which do not involve hypoxemia. A patient with acute heart failure may experience severe dyspnea caused by activation of pressure-sensitive J-receptors in the lung, even if oxygen saturation and tissue perfusion are intact. This process will be relieved by reducing pulmonary capillary pressures, but it is unaffected by supplemental oxygen. Coronary occlusion causes hypoxia of the heart muscle, but restoring perfusion is the most effective treatment. The instinct to maximize the oxygen-carrying capacity of the remaining blood flow is understandable. However, in a normoxemic patient, increasing the inspired fraction of oxygen has a marginal effect on oxygen-carrying capacity, since hemoglobin saturation and concentration rather than arterial oxygen tension (PaO2) predominantly determine oxygen-carrying capacity. On the other hand, supraphysiologic levels of dissolved oxygen may lead to toxicity.5
For over a century, we have known the potential harms of hyperoxia. Original studies in animal models showed that hyperoxia led to lung injury, altered hemodynamics, endothelial cell dysfunction, and inflammatory activation.5 Many of these detrimental effects involve the generation of reactive oxygen species and oxidative stress.5 High levels of inspired oxygen can also cause increased pulmonary shunting through inhibition of physiologic hypoxic vasoconstriction and due to absorption atelectasis.6 Oxygen negatively affects cardiovascular function by reducing coronary blood flow, increasing systemic vascular resistance, and reducing cardiac output.1
Chronic obstructive pulmonary disease (COPD) is the clinical setting in which risks of supplemental oxygen are most well-recognized historically. In patients with COPD at risk for hypercarbia, oxygen titrated to a goal SpO2 outside 88%-92% is associated with a two-fold risk of mortality.7 Worsening ventilation-perfusion matching and the Haldane effect (decreased affinity of hemoglobin for carbon dioxide as the PaO2 rises), rather than the previously theorized decrease in hypoxic drive, are now believed to contribute most to hyperoxia-induced hypercarbia. These unintended consequences may also occur in patients with other forms of acute and chronic lung disease.
The British Medical Journal published the first randomized controlled trial of oxygen use in suspected MI in 1976.1 Patients who received oxygen at 6 L per minute for 24 hours had more episodes of sinus tachycardia without any improvement in mortality, analgesic use, or infarct size.1 More recent and robust trials comparing outcomes in normoxemic patients randomized to supplemental oxygen versus room air have had similar findings: no difference in mortality, infarct size, or pain ratings.8,9 One found a significantly increased rate of MI recurrence with the use of oxygen.8 These data have led the latest guidelines for the management of ST-elevation MI from the European Society of Cardiology to discourage the use of supplemental oxygen unless SpO2 is <90%.10
Two recent trials investigated the effects of hyperoxia in critically ill patients.11,12 Girardis and colleagues randomized 480 critically ill patients in an Italian medical-surgical intensive care unit to conservative (SpO2 between 94% and 98% or PaO2 between 70 and 100 mm Hg) versus conventional oxygenation targets (SpO2 between 97% and 100% and PaO2 up to 150 mm Hg). Compared with conventional oxygen targets, conservative oxygen use was associated with an absolute risk reduction in mortality of 8.6% (11.6% vs 20.2%; P =.01).11 Another trial from 22 centers in France compared outcomes in mechanically ventilated patients with septic shock who received FiO2 at 1.0 compared with those with oxygen titration to SpO2 between 88% and 95%. The trial was stopped early for safety concerns. Those in the hyperoxemia group had a higher incidence of serious adverse events (85% vs 76%; P =.02), including pneumothorax, clinically relevant bleeding, myocardial infarction, and arrhythmias, as well as a trend toward increased mortality.12
Trials of liberal oxygen use in other settings of acute illness,13 including ischemic stroke,14 traumatic brain injury,15 and postcardiac arrest,16 have also linked liberal oxygen use with increased risk of mortality and other adverse events. “Liberal” use in these trials ranged from an FiO2 of 0.28 (equivalent to 2 L of nasal cannula) to 1.0. Significant secondary outcomes included fewer hospital-free and ventilator-free days in patients with liberal oxygen use. Furthermore, a meta-analysis of 25 trials including over 16,000 patients found dose-dependent toxicity: for every 1% increase in SpO2 above 94%-96% (the median SpO2 in the liberal oxygen groups), there was a 25% relative increase in in-hospital mortality.13
In addition to the data above, there are practical reasons to avoid unnecessary use of supplemental oxygen. Providing supplemental oxygen to a patient who is not hypoxemic may delay the recognition of cardiopulmonary decompensation by delaying detection of hypoxemia.6 Beyond the effects of oxygen itself, oxygen delivery methods carry their own potential adverse effects. These include epistaxis (with nasal cannula), claustrophobia (with face masks), decreased mobility, falls, and delirium.17 Finally, oxygen administration has direct and indirect financial costs, including those of supplies, care coordination, and monitoring.
WHEN SUPPLEMENTAL OXYGEN MIGHT BE HELPFUL
Importantly, the above discussion pertains to normoxemic patients receiving supplemental oxygen. There is no dispute that significantly hypoxemic patients should receive supplemental oxygen. There are also instances where the use of supplemental oxygen in normoxemic patients may be beneficial, such as in carbon monoxide poisoning, decompression injury, gas embolism, cluster headaches, sickle cell crisis, and pneumothorax.17
WHAT YOU SHOULD DO INSTEAD
Like any other drug, oxygen should be administered after assessment of its indications, intended benefits, and possible harms. Both significant hypoxemia and hyperoxemia should be avoided. In patients with neither hypoxemia nor the indications above, clinicians should not administer supplemental oxygen. Recent society guidelines can be applied in various clinical contexts. In patients with suspected MI, oxygen should be administered if SpO2 is <90%.10 For most other acutely ill patients, clinicians should administer supplemental oxygen if SpO2 <90%-92% and target an SpO2 of no higher than 94%-96%,18-19 as meta-analyses found evidence of harm above this level.13 Results of randomized trials currently underway should add supporting evidence for more specific oxygenation targets in different patient populations. With respect to implementation, it must be noted that factors beyond physician decision influence the use of supplemental oxygen. Appropriate institutional policies, standards of care, and educational efforts to all hospital providers must be enacted in order to reduce the unnecessary use of supplemental oxygen.
RECOMMENDATIONS
- For most acutely ill patients, do not administer supplemental oxygen when SpO2 >92%. If supplemental oxygen is used, the SpO2 should not exceed 94%-96%.
- For patients with suspected MI, only start supplemental oxygen for SpO2 <90%.
- For patients at risk for hypercapnic respiratory failure (eg, COPD patients), target SpO2 of 88%-92%.
- Provide supplemental oxygen to normoxemic patients with carbon monoxide poisoning, decompression injury, gas embolism, cluster headache, sickle cell crisis, and pneumothorax.
- Review and revise institutional practices and policies that contribute to unnecessary use of supplemental oxygen.
CONCLUSIONS
In the opening case, the patient is acutely ill and requires further workup. Her current SpO2 of 99% puts her at risk for adverse events and death, and supplemental oxygen should be titrated down or stopped to avoid SpO2 greater than 94%-96%. For years, clinicians have erred on the side of using supplemental oxygen, without recognizing its dangers. However, over a century of evidence from pathophysiologic experiments and randomized trials across multiple clinical settings have associated hyperoxemia with adverse outcomes and increased mortality. Professional societies are adopting this evidence into their guideline recommendations, and clinicians should use supplemental oxygen judiciously in their daily practice.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
1. Rawles JM, Kenmure AC. Controlled trial of oxygen in uncomplicated myocardial infarction. Br Med J. 1976;1(6018):1121-1123. https://doi.org/10.1136/bmj.1.6018.1121.
2. Burls A, Emparanza JI, Quinn T, Cabello J. Oxygen use in acute myocardial infarction: an online survey of health professionals’ practice and beliefs. Emerg Med J. 2010;27(4):283-286. https://doi.org/10.1136/emj.2009.077370.
3. Hale KE, Gavin C, O’Driscoll BR. Audit of oxygen use in emergency ambulances and in a hospital emergency department. Emerg Med J. 2008;25(11):773-776. https://doi.org/10.1136/emj.2008.059287.
4. Suzuki S, Eastwood G, Peck L, Glassford N, Bellomo R. Oxygen management in mechanically ventilated patients: a prospective observational cohort study. Aust Crit Care. 2014;27(1):50-51. https://doi.org/10.1016/j.aucc.2013.10.025.
5. Helmerhorst HJ, Schultz MJ, van der Voort PH, de Jonge E, van Wasterloo DJ. Bench-to-bedside review: the effects of hyperoxia during critical illness. Crit Care. 2015;19(1):284. https://doi.org/10.1186/s13054-015-0996-4.
6. Downs JB. Has oxygen administration delayed appropriate respiratory care? Fallacies regarding oxygen therapy. Respir Care. 2003;48(6):611-620.
7. Austin MA, Willis KE, Blizzard L, Walters EH, Wood-Baker R. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010;341:c5462. https://doi.org/10.2307/20800296.
8. Stub D, Smith K, Bernard S, et al. Air versus oxygen in ST-segment-elevation myocardial infarction. Circulation. 2015;131(24):2143-2150. https://doi.org/10.1161/CIRCULATIONAHA.114.014494.
9. Hofman R. Witt N, Lagergvist B, et al. Oxygen therapy in ST-elevation myocardial infarction. Eur Heart J. 2018;39(29):2730-2739. https://doi.org/10.1093/eurheartj/ehy326.
10. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018:39(2):119-177. https://doi.org/10.1093/eurheartj/ehx393.
11. Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit. JAMA. 2016;316(15):1583-1589. https://doi.org/10.1001/jama.2016.11993.
12. Asfar P, Schortgen F, Boisramé-Helms J, et al. Hyperoxia and hypertonic saline in patients with septic shock (HYPERS2S): a two-by-two factorial, multicentre, randomised, clinical trial. Lancet Respir Med. 2017:5(3):180-190. https://doi.org/10.1016/S2213-2600(17)30046-2.
13. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693-1705. https://doi.org/10.1016/S0140-6736(18)30479-3.
14. Rincon F, Kang J, Maltenfort M, et al. Association between hyperoxia and mortality after stroke: a multicenter cohort study. Crit Care Med. 2014;42(2):387-396. https://doi.org/10.1097/CCM.0b013e3182a27732.
15. Brenner M, Stein D, Hu P, Kufera J, Woodford M, Scalea T. Association between early hyperoxia and worse outcomes after traumatic brain injury. Arch Surg. 2012;147(11):1042-1046. https://doi.org/10.1001/archsurg.2012.1560.
16. Kilgannon JH, Jones AE, Shapiro NI, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303(21):2165-2171. https://doi.org/10.1
17. Siemieniuk RA, Chu DK, Kim L, et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ. 2018;363:k4169. https://doi.org/10.1136/bmj.k4169.
18. O’Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72(1):ii1-ii90. https://doi.org/10.1136/thoraxjnl-2016-209729.
19. Beasley R, Chien J, Douglas J, et al. Thoracic Society of Australia and New Zealand oxygen guidelines for acute oxygen use in adults: ‘Swimming between the flags’. Respirology. 2015;20(8):1182-1191. https://doi.org/10.1111/resp.12620.
1. Rawles JM, Kenmure AC. Controlled trial of oxygen in uncomplicated myocardial infarction. Br Med J. 1976;1(6018):1121-1123. https://doi.org/10.1136/bmj.1.6018.1121.
2. Burls A, Emparanza JI, Quinn T, Cabello J. Oxygen use in acute myocardial infarction: an online survey of health professionals’ practice and beliefs. Emerg Med J. 2010;27(4):283-286. https://doi.org/10.1136/emj.2009.077370.
3. Hale KE, Gavin C, O’Driscoll BR. Audit of oxygen use in emergency ambulances and in a hospital emergency department. Emerg Med J. 2008;25(11):773-776. https://doi.org/10.1136/emj.2008.059287.
4. Suzuki S, Eastwood G, Peck L, Glassford N, Bellomo R. Oxygen management in mechanically ventilated patients: a prospective observational cohort study. Aust Crit Care. 2014;27(1):50-51. https://doi.org/10.1016/j.aucc.2013.10.025.
5. Helmerhorst HJ, Schultz MJ, van der Voort PH, de Jonge E, van Wasterloo DJ. Bench-to-bedside review: the effects of hyperoxia during critical illness. Crit Care. 2015;19(1):284. https://doi.org/10.1186/s13054-015-0996-4.
6. Downs JB. Has oxygen administration delayed appropriate respiratory care? Fallacies regarding oxygen therapy. Respir Care. 2003;48(6):611-620.
7. Austin MA, Willis KE, Blizzard L, Walters EH, Wood-Baker R. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010;341:c5462. https://doi.org/10.2307/20800296.
8. Stub D, Smith K, Bernard S, et al. Air versus oxygen in ST-segment-elevation myocardial infarction. Circulation. 2015;131(24):2143-2150. https://doi.org/10.1161/CIRCULATIONAHA.114.014494.
9. Hofman R. Witt N, Lagergvist B, et al. Oxygen therapy in ST-elevation myocardial infarction. Eur Heart J. 2018;39(29):2730-2739. https://doi.org/10.1093/eurheartj/ehy326.
10. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018:39(2):119-177. https://doi.org/10.1093/eurheartj/ehx393.
11. Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit. JAMA. 2016;316(15):1583-1589. https://doi.org/10.1001/jama.2016.11993.
12. Asfar P, Schortgen F, Boisramé-Helms J, et al. Hyperoxia and hypertonic saline in patients with septic shock (HYPERS2S): a two-by-two factorial, multicentre, randomised, clinical trial. Lancet Respir Med. 2017:5(3):180-190. https://doi.org/10.1016/S2213-2600(17)30046-2.
13. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693-1705. https://doi.org/10.1016/S0140-6736(18)30479-3.
14. Rincon F, Kang J, Maltenfort M, et al. Association between hyperoxia and mortality after stroke: a multicenter cohort study. Crit Care Med. 2014;42(2):387-396. https://doi.org/10.1097/CCM.0b013e3182a27732.
15. Brenner M, Stein D, Hu P, Kufera J, Woodford M, Scalea T. Association between early hyperoxia and worse outcomes after traumatic brain injury. Arch Surg. 2012;147(11):1042-1046. https://doi.org/10.1001/archsurg.2012.1560.
16. Kilgannon JH, Jones AE, Shapiro NI, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303(21):2165-2171. https://doi.org/10.1
17. Siemieniuk RA, Chu DK, Kim L, et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ. 2018;363:k4169. https://doi.org/10.1136/bmj.k4169.
18. O’Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72(1):ii1-ii90. https://doi.org/10.1136/thoraxjnl-2016-209729.
19. Beasley R, Chien J, Douglas J, et al. Thoracic Society of Australia and New Zealand oxygen guidelines for acute oxygen use in adults: ‘Swimming between the flags’. Respirology. 2015;20(8):1182-1191. https://doi.org/10.1111/resp.12620.
© 2019 Society of Hospital Medicine
Long Peripheral Catheters: A Retrospective Review of Major Complications
Introduced in the 1950s, midline catheters have become a popular option for intravenous (IV) access.1,2 Ranging from 8 to 25 cm in length, they are inserted in the veins of the upper arm. Unlike peripherally inserted central catheters (PICCs), the tip of midline catheters terminates proximal to the axillary vein; thus, midlines are peripheral, not central venous access devices.1-3 One popular variation of a midline catheter, though nebulously defined, is the long peripheral catheter (LPC), a device ranging from 6 to 15 cm in length.4,5
Concerns regarding inappropriate use and complications such as thrombosis and central line-associated bloodstream infection (CLABSI) have spurred growth in the use of LPCs.6 However, data regarding complication rates with these devices are limited. Whether LPCs are a safe and viable option for IV access is unclear. We conducted a retrospective study to examine indications, patterns of use, and complications following LPC insertion in hospitalized patients.
METHODS
Device Selection
Our institution is a 470-bed tertiary care, safety-net hospital in Chicago, Illinois. Our vascular access team (VAT) performs a patient assessment and selects IV devices based upon published standards for device appropriateness. 7 We retrospectively collated electronic requests for LPC insertion on adult inpatients between October 2015 and June 2017. Cases where (1) duplicate orders, (2) patient refusal, (3) peripheral intravenous catheter of any length, or (4) PICCs were placed were excluded from this analysis.
VAT and Device Characteristics
We used Bard PowerGlide® (Bard Access Systems, Inc., Salt Lake City, Utah), an 18-gauge, 8-10 cm long, power-injectable, polyurethane LPC. Bundled kits (ie, device, gown, dressing, etc.) were utilized, and VAT providers underwent two weeks of training prior to the study period. All LPCs were inserted in the upper extremities under sterile technique using ultrasound guidance (accelerated Seldinger technique). Placement confirmation was verified by aspiration, flush, and ultrasound visualization of the catheter tip within the vein. An antimicrobial dressing was applied to the catheter insertion site, and daily saline flushes and weekly dressing changes by bedside nurses were used for device maintenance. LPC placement was available on all nonholiday weekdays from 8
Data Selection
For each LPC recipient, demographic and comorbidity data were collected to calculate the Charlson Comorbidity Index (Table 1). Every LPC recipient’s history of deep vein thrombosis (DVT) and catheter-related infection (CRI) was recorded. Procedural information (eg, inserter, vein, and number of attempts) was obtained from insertion notes. All data were extracted from the electronic medical record via chart review. Two reviewers verified outcomes to ensure concordance with stated definitions (ie, DVT, CRI). Device parameters, including dwell time, indication, and time to complication(s) were also collected.
Primary Outcomes
The primary outcome was the incidence of DVT and CRI (Table 2). DVT was defined as radiographically confirmed (eg, ultrasound, computed tomography) thrombosis in the presence of patient signs or symptoms. CRI was defined in accordance with Timsit et al.8 as follows: catheter-related clinical sepsis without bloodstream infection defined as (1) combination of fever (body temperature >38.5°C) or hypothermia (body temperature <36.5°C), (2) catheter-tip culture yielding ≥103 CFUs/mL, (3) pus at the insertion site or resolution of clinical sepsis after catheter removal, and (4) absence of any other infectious focus or catheter-related bloodstream infection (CRBSI). CRBSI was defined as a combination of (1) one or more positive peripheral blood cultures sampled immediately before or within 48 hours after catheter removal, (2) a quantitative catheter-tip culture testing positive for the same microorganisms (same species and susceptibility pattern) or a differential time to positivity of blood cultures ≥2 hours, and (3) no other infectious focus explaining the positive blood culture result.
Secondary Outcomes
Secondary outcomes, defined as minor complications, included infiltration, thrombophlebitis, and catheter occlusion. Infiltration was defined as localized swelling due to infusate or site leakage. Thrombophlebitis was defined as one or more of the following: localized erythema, palpable cord, tenderness, or streaking. Occlusion was defined as nonpatency of the catheter due to the inability to flush or aspirate. Definitions for secondary outcomes are consistent with those used in prior studies.9
Statistical Analysis
Patient and LPC characteristics were analyzed using descriptive statistics. Results were reported as percentages, means, medians (interquartile range [IQR]), and rates per 1,000 catheter days. All analyses were conducted in Stata v.15 (StataCorp, College Station, Texas).
RESULTS
Within the 20-month study period, a total of 539 LPCs representing 5,543 catheter days were available for analysis. The mean patient age was 53 years. A total of 90 patients (16.7%) had a history of DVT, while 6 (1.1%) had a history of CRI. We calculated a median Charlson index of 4 (interquartile range [IQR], 2-7), suggesting an estimated one-year postdischarge survival of 53% (Table 1).
The majority of LPCs (99.6% [537/539]) were single lumen catheters. No patient had more than one concurrent LPC. The cannulation success rate on the first attempt was 93.9% (507/539). The brachial or basilic veins were primarily targeted (98.7%, [532/539]). Difficult intravenous access represented 48.8% (263/539) of indications, and postdischarge parenteral antibiotics constituted 47.9% (258/539). The median catheter dwell time was eight days (IQR, 4-14 days).
Nine DVTs (1.7% [9/539]) occurred in patients with LPCs. The incidence of DVT was higher in patients with a history of DVT (5.7%, 5/90). The median time from insertion to DVT was 11 (IQR, 5-14) days. DVTs were managed with LPC removal and systemic anticoagulation in accordance with catheter-related DVT guidelines. The rate of CRI was 0.6% (3/539), or 0.54 per 1,000 catheter days. Two CRIs had positive blood cultures, while one had negative cultures. Infections occurred after a median of 12 (IQR, 8-15) days of catheter dwell. Each was treated with LPC removal and IV antibiotics, with two patients receiving two weeks and one receiving six weeks of antibiotic therapy (Table 2).
With respect to secondary outcomes, the incidence of infiltration was 0.4% (2/539), thrombophlebitis 0.7% (4/539), and catheter occlusion 0.9% (5/539). The time to event was 8.5, 3.75, and 5.4 days, respectively. Collectively, 2.0% of devices experienced a minor complication.
DISCUSSION
In our single-center study, LPCs were primarily inserted for difficult venous access or parenteral antibiotics. Despite a clinically complex population with a high number of comorbidities, rates of major and minor complications associated with LPCs were low. These data suggest that LPCs are a safe alternative to PICCs and other central access devices for short-term use.
Our incidence of CRI of 0.6% (0.54 per 1,000 catheter days) is similar to or lower than other studies.2,10,11 An incidence of 0%-1.5% was observed in two recent publications about midline catheters, with rates across individual studies and hospital sites varying widely.12,13 A systematic review of intravascular devices reported CRI rates of 0.4% (0.2 per 1,000 catheter days) for midlines and 0.1% (0.5 per 1,000 catheter days for peripheral IVs), in contrast to PICCs at 3.1% (1.1 per 1,000 catheter days).14 However, catheters of varying lengths and diameters were used in studies within the review, potentially leading to heterogeneous outcomes. In accordance with existing data, CRI incidence in our study increased with catheter dwell time.10
The 1.7% rate of DVT observed in our study is on the lower end of existing data (1.4%-5.9%).12-15 Compared with PICCs (2%-15%), the incidence of venous thrombosis appears to be lower with midlines/LPCs—justifying their use as an alternative device for IV access.7,9,12,14 There was an overall low rate of minor complications, similar to recently published results.10 As rates were greater in patients with a history of DVT (5.7%), caution is warranted when using these devices in this population.
Our experience with LPCs suggests financial and patient benefits. The cost of LPCs is lower than central access devices.4 As rates of CRI were low, costs related to CLABSIs from PICC use may be reduced by appropriate LPC use. LPCs may allow the ability to draw blood routinely, which could improve the patient experience—albeit with its own risks. Current recommendations support the use of PICCs or LPCs, somewhat interchangeably, for patients with appropriate indications needing IV therapy for more than five to six days.2,7 However, LPCs now account for 57% of vascular access procedures in our center and have led to a decrease in reliance on PICCs and attendant complications.
Our study has several limitations. First, LPCs and midlines are often used interchangeably in the literature.4,5 Therefore, reported complication rates may not reflect those of LPCs alone and may limit comparisons. Second, ours was a single-center study with experts assessing device appropriateness and performing ultrasound-guided insertions; our findings may not be generalizable to dissimilar settings. Third, we did not track LPC complications such as nonpatency and leakage. As prior studies reported high rates of complications such as these events, caution is advised when interpreting our findings.15 Finally, we retrospectively extracted data from our medical records; limitations in documentation may influence our findings.
CONCLUSION
In patients requiring short-term IV therapy, these data suggest LPCs have low complication rates and may be safely used as an alternative option for venous access.
Acknowledgments
The authors thank Drs. Laura Hernandez, Andres Mendez Hernandez, and Victor Prado for their assistance in data collection. The authors also thank Mr. Onofre Donceras and Dr. Sharon Welbel from the John H. Stroger, Jr. Hospital of Cook County Department of Infection Control & Epidemiology for their assistance in reviewing local line infection data.
Drs. Patel and Chopra developed the study design. Drs. Patel, Araujo, Parra Rodriguez, Ramirez Sanchez, and Chopra contributed to manuscript writing. Ms. Snyder provided statistical analysis. All authors have seen and approved the final manuscript for submission.
Disclosures
The authors have nothing to disclose.
1. Anderson NR. Midline catheters: the middle ground of intravenous therapy administration. J Infus Nurs. 2004;27(5):313-321.
2. Adams DZ, Little A, Vinsant C, et al. The midline catheter: a clinical review. J Emerg Med. 2016;51(3):252-258. https://doi.org/10.1016/j.jemermed.2016.05.029.
3. Scoppettuolo G, Pittiruti M, Pitoni S, et al. Ultrasound-guided “short” midline catheters for difficult venous access in the emergency department: a retrospective analysis. Int J Emerg Med. 2016;9(1):3. https://doi.org/10.1186/s12245-016-0100-0.
4. Qin KR, Nataraja RM, Pacilli M. Long peripheral catheters: is it time to address the confusion? J Vasc Access. 2018;20(5). https://doi.org/10.1177/1129729818819730.
5. Pittiruti M, Scoppettuolo G. The GAVeCeLT Manual of PICC and Midlines. Milano: EDRA; 2016.
6. Dawson RB, Moureau NL. Midline catheters: an essential tool in CLABSI reduction. Infection Control Today. https://www.infectioncontroltoday.com/clabsi/midline-catheters-essential-tool-clabsi-reduction. Accessed February 19, 2018
7. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6):S1-S40. https://doi.org/10.7326/M15-0744.
8. Timsit JF, Schwebel C, Bouadma L, et al. Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults: a randomized controlled trial. JAMA. 2009;301(12):1231-1241. https://doi.org/10.1001/jama.2009.376.
9. Bahl A, Karabon P, Chu D. Comparison of venous thrombosis complications in midlines versus peripherally inserted central catheters: are midlines the safer option? Clin Appl Thromb Hemost. 2019;25. https://doi.org/10.1177/1076029619839150.
10. Goetz AM, Miller J, Wagener MM, et al. Complications related to intravenous midline catheter usage. A 2-year study. J Intraven Nurs. 1998;21(2):76-80.
11. Xu T, Kingsley L, DiNucci S, et al. Safety and utilization of peripherally inserted central catheters versus midline catheters at a large academic medical center. Am J Infect Control. 2016;44(12):1458-1461. https://doi.org/10.1016/j.ajic.2016.09.010.
12. Chopra V, Kaatz S, Swaminathan L, et al. Variation in use and outcomes related to midline catheters: results from a multicentre pilot study. BMJ Qual Saf. 2019;28(9):714-720. https://doi.org/10.1136/bmjqs-2018-008554.
13. Badger J. Long peripheral catheters for deep arm vein venous access: A systematic review of complications. Heart Lung. 2019;48(3):222-225. https://doi.org/10.1016/j.hrtlng.2019.01.002.
14. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81(9):1159-1171. https://doi.org/10.4065/81.9.1159.
15. Zerla PA, Caravella G, De Luca G, et al. Open- vs closed-tip valved peripherally inserted central catheters and midlines: Findings from a vascular access database. J Assoc Vasc Access. 2015;20(3):169-176. https://doi.org/10.1016/j.java.2015.06.001.
Introduced in the 1950s, midline catheters have become a popular option for intravenous (IV) access.1,2 Ranging from 8 to 25 cm in length, they are inserted in the veins of the upper arm. Unlike peripherally inserted central catheters (PICCs), the tip of midline catheters terminates proximal to the axillary vein; thus, midlines are peripheral, not central venous access devices.1-3 One popular variation of a midline catheter, though nebulously defined, is the long peripheral catheter (LPC), a device ranging from 6 to 15 cm in length.4,5
Concerns regarding inappropriate use and complications such as thrombosis and central line-associated bloodstream infection (CLABSI) have spurred growth in the use of LPCs.6 However, data regarding complication rates with these devices are limited. Whether LPCs are a safe and viable option for IV access is unclear. We conducted a retrospective study to examine indications, patterns of use, and complications following LPC insertion in hospitalized patients.
METHODS
Device Selection
Our institution is a 470-bed tertiary care, safety-net hospital in Chicago, Illinois. Our vascular access team (VAT) performs a patient assessment and selects IV devices based upon published standards for device appropriateness. 7 We retrospectively collated electronic requests for LPC insertion on adult inpatients between October 2015 and June 2017. Cases where (1) duplicate orders, (2) patient refusal, (3) peripheral intravenous catheter of any length, or (4) PICCs were placed were excluded from this analysis.
VAT and Device Characteristics
We used Bard PowerGlide® (Bard Access Systems, Inc., Salt Lake City, Utah), an 18-gauge, 8-10 cm long, power-injectable, polyurethane LPC. Bundled kits (ie, device, gown, dressing, etc.) were utilized, and VAT providers underwent two weeks of training prior to the study period. All LPCs were inserted in the upper extremities under sterile technique using ultrasound guidance (accelerated Seldinger technique). Placement confirmation was verified by aspiration, flush, and ultrasound visualization of the catheter tip within the vein. An antimicrobial dressing was applied to the catheter insertion site, and daily saline flushes and weekly dressing changes by bedside nurses were used for device maintenance. LPC placement was available on all nonholiday weekdays from 8
Data Selection
For each LPC recipient, demographic and comorbidity data were collected to calculate the Charlson Comorbidity Index (Table 1). Every LPC recipient’s history of deep vein thrombosis (DVT) and catheter-related infection (CRI) was recorded. Procedural information (eg, inserter, vein, and number of attempts) was obtained from insertion notes. All data were extracted from the electronic medical record via chart review. Two reviewers verified outcomes to ensure concordance with stated definitions (ie, DVT, CRI). Device parameters, including dwell time, indication, and time to complication(s) were also collected.
Primary Outcomes
The primary outcome was the incidence of DVT and CRI (Table 2). DVT was defined as radiographically confirmed (eg, ultrasound, computed tomography) thrombosis in the presence of patient signs or symptoms. CRI was defined in accordance with Timsit et al.8 as follows: catheter-related clinical sepsis without bloodstream infection defined as (1) combination of fever (body temperature >38.5°C) or hypothermia (body temperature <36.5°C), (2) catheter-tip culture yielding ≥103 CFUs/mL, (3) pus at the insertion site or resolution of clinical sepsis after catheter removal, and (4) absence of any other infectious focus or catheter-related bloodstream infection (CRBSI). CRBSI was defined as a combination of (1) one or more positive peripheral blood cultures sampled immediately before or within 48 hours after catheter removal, (2) a quantitative catheter-tip culture testing positive for the same microorganisms (same species and susceptibility pattern) or a differential time to positivity of blood cultures ≥2 hours, and (3) no other infectious focus explaining the positive blood culture result.
Secondary Outcomes
Secondary outcomes, defined as minor complications, included infiltration, thrombophlebitis, and catheter occlusion. Infiltration was defined as localized swelling due to infusate or site leakage. Thrombophlebitis was defined as one or more of the following: localized erythema, palpable cord, tenderness, or streaking. Occlusion was defined as nonpatency of the catheter due to the inability to flush or aspirate. Definitions for secondary outcomes are consistent with those used in prior studies.9
Statistical Analysis
Patient and LPC characteristics were analyzed using descriptive statistics. Results were reported as percentages, means, medians (interquartile range [IQR]), and rates per 1,000 catheter days. All analyses were conducted in Stata v.15 (StataCorp, College Station, Texas).
RESULTS
Within the 20-month study period, a total of 539 LPCs representing 5,543 catheter days were available for analysis. The mean patient age was 53 years. A total of 90 patients (16.7%) had a history of DVT, while 6 (1.1%) had a history of CRI. We calculated a median Charlson index of 4 (interquartile range [IQR], 2-7), suggesting an estimated one-year postdischarge survival of 53% (Table 1).
The majority of LPCs (99.6% [537/539]) were single lumen catheters. No patient had more than one concurrent LPC. The cannulation success rate on the first attempt was 93.9% (507/539). The brachial or basilic veins were primarily targeted (98.7%, [532/539]). Difficult intravenous access represented 48.8% (263/539) of indications, and postdischarge parenteral antibiotics constituted 47.9% (258/539). The median catheter dwell time was eight days (IQR, 4-14 days).
Nine DVTs (1.7% [9/539]) occurred in patients with LPCs. The incidence of DVT was higher in patients with a history of DVT (5.7%, 5/90). The median time from insertion to DVT was 11 (IQR, 5-14) days. DVTs were managed with LPC removal and systemic anticoagulation in accordance with catheter-related DVT guidelines. The rate of CRI was 0.6% (3/539), or 0.54 per 1,000 catheter days. Two CRIs had positive blood cultures, while one had negative cultures. Infections occurred after a median of 12 (IQR, 8-15) days of catheter dwell. Each was treated with LPC removal and IV antibiotics, with two patients receiving two weeks and one receiving six weeks of antibiotic therapy (Table 2).
With respect to secondary outcomes, the incidence of infiltration was 0.4% (2/539), thrombophlebitis 0.7% (4/539), and catheter occlusion 0.9% (5/539). The time to event was 8.5, 3.75, and 5.4 days, respectively. Collectively, 2.0% of devices experienced a minor complication.
DISCUSSION
In our single-center study, LPCs were primarily inserted for difficult venous access or parenteral antibiotics. Despite a clinically complex population with a high number of comorbidities, rates of major and minor complications associated with LPCs were low. These data suggest that LPCs are a safe alternative to PICCs and other central access devices for short-term use.
Our incidence of CRI of 0.6% (0.54 per 1,000 catheter days) is similar to or lower than other studies.2,10,11 An incidence of 0%-1.5% was observed in two recent publications about midline catheters, with rates across individual studies and hospital sites varying widely.12,13 A systematic review of intravascular devices reported CRI rates of 0.4% (0.2 per 1,000 catheter days) for midlines and 0.1% (0.5 per 1,000 catheter days for peripheral IVs), in contrast to PICCs at 3.1% (1.1 per 1,000 catheter days).14 However, catheters of varying lengths and diameters were used in studies within the review, potentially leading to heterogeneous outcomes. In accordance with existing data, CRI incidence in our study increased with catheter dwell time.10
The 1.7% rate of DVT observed in our study is on the lower end of existing data (1.4%-5.9%).12-15 Compared with PICCs (2%-15%), the incidence of venous thrombosis appears to be lower with midlines/LPCs—justifying their use as an alternative device for IV access.7,9,12,14 There was an overall low rate of minor complications, similar to recently published results.10 As rates were greater in patients with a history of DVT (5.7%), caution is warranted when using these devices in this population.
Our experience with LPCs suggests financial and patient benefits. The cost of LPCs is lower than central access devices.4 As rates of CRI were low, costs related to CLABSIs from PICC use may be reduced by appropriate LPC use. LPCs may allow the ability to draw blood routinely, which could improve the patient experience—albeit with its own risks. Current recommendations support the use of PICCs or LPCs, somewhat interchangeably, for patients with appropriate indications needing IV therapy for more than five to six days.2,7 However, LPCs now account for 57% of vascular access procedures in our center and have led to a decrease in reliance on PICCs and attendant complications.
Our study has several limitations. First, LPCs and midlines are often used interchangeably in the literature.4,5 Therefore, reported complication rates may not reflect those of LPCs alone and may limit comparisons. Second, ours was a single-center study with experts assessing device appropriateness and performing ultrasound-guided insertions; our findings may not be generalizable to dissimilar settings. Third, we did not track LPC complications such as nonpatency and leakage. As prior studies reported high rates of complications such as these events, caution is advised when interpreting our findings.15 Finally, we retrospectively extracted data from our medical records; limitations in documentation may influence our findings.
CONCLUSION
In patients requiring short-term IV therapy, these data suggest LPCs have low complication rates and may be safely used as an alternative option for venous access.
Acknowledgments
The authors thank Drs. Laura Hernandez, Andres Mendez Hernandez, and Victor Prado for their assistance in data collection. The authors also thank Mr. Onofre Donceras and Dr. Sharon Welbel from the John H. Stroger, Jr. Hospital of Cook County Department of Infection Control & Epidemiology for their assistance in reviewing local line infection data.
Drs. Patel and Chopra developed the study design. Drs. Patel, Araujo, Parra Rodriguez, Ramirez Sanchez, and Chopra contributed to manuscript writing. Ms. Snyder provided statistical analysis. All authors have seen and approved the final manuscript for submission.
Disclosures
The authors have nothing to disclose.
Introduced in the 1950s, midline catheters have become a popular option for intravenous (IV) access.1,2 Ranging from 8 to 25 cm in length, they are inserted in the veins of the upper arm. Unlike peripherally inserted central catheters (PICCs), the tip of midline catheters terminates proximal to the axillary vein; thus, midlines are peripheral, not central venous access devices.1-3 One popular variation of a midline catheter, though nebulously defined, is the long peripheral catheter (LPC), a device ranging from 6 to 15 cm in length.4,5
Concerns regarding inappropriate use and complications such as thrombosis and central line-associated bloodstream infection (CLABSI) have spurred growth in the use of LPCs.6 However, data regarding complication rates with these devices are limited. Whether LPCs are a safe and viable option for IV access is unclear. We conducted a retrospective study to examine indications, patterns of use, and complications following LPC insertion in hospitalized patients.
METHODS
Device Selection
Our institution is a 470-bed tertiary care, safety-net hospital in Chicago, Illinois. Our vascular access team (VAT) performs a patient assessment and selects IV devices based upon published standards for device appropriateness. 7 We retrospectively collated electronic requests for LPC insertion on adult inpatients between October 2015 and June 2017. Cases where (1) duplicate orders, (2) patient refusal, (3) peripheral intravenous catheter of any length, or (4) PICCs were placed were excluded from this analysis.
VAT and Device Characteristics
We used Bard PowerGlide® (Bard Access Systems, Inc., Salt Lake City, Utah), an 18-gauge, 8-10 cm long, power-injectable, polyurethane LPC. Bundled kits (ie, device, gown, dressing, etc.) were utilized, and VAT providers underwent two weeks of training prior to the study period. All LPCs were inserted in the upper extremities under sterile technique using ultrasound guidance (accelerated Seldinger technique). Placement confirmation was verified by aspiration, flush, and ultrasound visualization of the catheter tip within the vein. An antimicrobial dressing was applied to the catheter insertion site, and daily saline flushes and weekly dressing changes by bedside nurses were used for device maintenance. LPC placement was available on all nonholiday weekdays from 8
Data Selection
For each LPC recipient, demographic and comorbidity data were collected to calculate the Charlson Comorbidity Index (Table 1). Every LPC recipient’s history of deep vein thrombosis (DVT) and catheter-related infection (CRI) was recorded. Procedural information (eg, inserter, vein, and number of attempts) was obtained from insertion notes. All data were extracted from the electronic medical record via chart review. Two reviewers verified outcomes to ensure concordance with stated definitions (ie, DVT, CRI). Device parameters, including dwell time, indication, and time to complication(s) were also collected.
Primary Outcomes
The primary outcome was the incidence of DVT and CRI (Table 2). DVT was defined as radiographically confirmed (eg, ultrasound, computed tomography) thrombosis in the presence of patient signs or symptoms. CRI was defined in accordance with Timsit et al.8 as follows: catheter-related clinical sepsis without bloodstream infection defined as (1) combination of fever (body temperature >38.5°C) or hypothermia (body temperature <36.5°C), (2) catheter-tip culture yielding ≥103 CFUs/mL, (3) pus at the insertion site or resolution of clinical sepsis after catheter removal, and (4) absence of any other infectious focus or catheter-related bloodstream infection (CRBSI). CRBSI was defined as a combination of (1) one or more positive peripheral blood cultures sampled immediately before or within 48 hours after catheter removal, (2) a quantitative catheter-tip culture testing positive for the same microorganisms (same species and susceptibility pattern) or a differential time to positivity of blood cultures ≥2 hours, and (3) no other infectious focus explaining the positive blood culture result.
Secondary Outcomes
Secondary outcomes, defined as minor complications, included infiltration, thrombophlebitis, and catheter occlusion. Infiltration was defined as localized swelling due to infusate or site leakage. Thrombophlebitis was defined as one or more of the following: localized erythema, palpable cord, tenderness, or streaking. Occlusion was defined as nonpatency of the catheter due to the inability to flush or aspirate. Definitions for secondary outcomes are consistent with those used in prior studies.9
Statistical Analysis
Patient and LPC characteristics were analyzed using descriptive statistics. Results were reported as percentages, means, medians (interquartile range [IQR]), and rates per 1,000 catheter days. All analyses were conducted in Stata v.15 (StataCorp, College Station, Texas).
RESULTS
Within the 20-month study period, a total of 539 LPCs representing 5,543 catheter days were available for analysis. The mean patient age was 53 years. A total of 90 patients (16.7%) had a history of DVT, while 6 (1.1%) had a history of CRI. We calculated a median Charlson index of 4 (interquartile range [IQR], 2-7), suggesting an estimated one-year postdischarge survival of 53% (Table 1).
The majority of LPCs (99.6% [537/539]) were single lumen catheters. No patient had more than one concurrent LPC. The cannulation success rate on the first attempt was 93.9% (507/539). The brachial or basilic veins were primarily targeted (98.7%, [532/539]). Difficult intravenous access represented 48.8% (263/539) of indications, and postdischarge parenteral antibiotics constituted 47.9% (258/539). The median catheter dwell time was eight days (IQR, 4-14 days).
Nine DVTs (1.7% [9/539]) occurred in patients with LPCs. The incidence of DVT was higher in patients with a history of DVT (5.7%, 5/90). The median time from insertion to DVT was 11 (IQR, 5-14) days. DVTs were managed with LPC removal and systemic anticoagulation in accordance with catheter-related DVT guidelines. The rate of CRI was 0.6% (3/539), or 0.54 per 1,000 catheter days. Two CRIs had positive blood cultures, while one had negative cultures. Infections occurred after a median of 12 (IQR, 8-15) days of catheter dwell. Each was treated with LPC removal and IV antibiotics, with two patients receiving two weeks and one receiving six weeks of antibiotic therapy (Table 2).
With respect to secondary outcomes, the incidence of infiltration was 0.4% (2/539), thrombophlebitis 0.7% (4/539), and catheter occlusion 0.9% (5/539). The time to event was 8.5, 3.75, and 5.4 days, respectively. Collectively, 2.0% of devices experienced a minor complication.
DISCUSSION
In our single-center study, LPCs were primarily inserted for difficult venous access or parenteral antibiotics. Despite a clinically complex population with a high number of comorbidities, rates of major and minor complications associated with LPCs were low. These data suggest that LPCs are a safe alternative to PICCs and other central access devices for short-term use.
Our incidence of CRI of 0.6% (0.54 per 1,000 catheter days) is similar to or lower than other studies.2,10,11 An incidence of 0%-1.5% was observed in two recent publications about midline catheters, with rates across individual studies and hospital sites varying widely.12,13 A systematic review of intravascular devices reported CRI rates of 0.4% (0.2 per 1,000 catheter days) for midlines and 0.1% (0.5 per 1,000 catheter days for peripheral IVs), in contrast to PICCs at 3.1% (1.1 per 1,000 catheter days).14 However, catheters of varying lengths and diameters were used in studies within the review, potentially leading to heterogeneous outcomes. In accordance with existing data, CRI incidence in our study increased with catheter dwell time.10
The 1.7% rate of DVT observed in our study is on the lower end of existing data (1.4%-5.9%).12-15 Compared with PICCs (2%-15%), the incidence of venous thrombosis appears to be lower with midlines/LPCs—justifying their use as an alternative device for IV access.7,9,12,14 There was an overall low rate of minor complications, similar to recently published results.10 As rates were greater in patients with a history of DVT (5.7%), caution is warranted when using these devices in this population.
Our experience with LPCs suggests financial and patient benefits. The cost of LPCs is lower than central access devices.4 As rates of CRI were low, costs related to CLABSIs from PICC use may be reduced by appropriate LPC use. LPCs may allow the ability to draw blood routinely, which could improve the patient experience—albeit with its own risks. Current recommendations support the use of PICCs or LPCs, somewhat interchangeably, for patients with appropriate indications needing IV therapy for more than five to six days.2,7 However, LPCs now account for 57% of vascular access procedures in our center and have led to a decrease in reliance on PICCs and attendant complications.
Our study has several limitations. First, LPCs and midlines are often used interchangeably in the literature.4,5 Therefore, reported complication rates may not reflect those of LPCs alone and may limit comparisons. Second, ours was a single-center study with experts assessing device appropriateness and performing ultrasound-guided insertions; our findings may not be generalizable to dissimilar settings. Third, we did not track LPC complications such as nonpatency and leakage. As prior studies reported high rates of complications such as these events, caution is advised when interpreting our findings.15 Finally, we retrospectively extracted data from our medical records; limitations in documentation may influence our findings.
CONCLUSION
In patients requiring short-term IV therapy, these data suggest LPCs have low complication rates and may be safely used as an alternative option for venous access.
Acknowledgments
The authors thank Drs. Laura Hernandez, Andres Mendez Hernandez, and Victor Prado for their assistance in data collection. The authors also thank Mr. Onofre Donceras and Dr. Sharon Welbel from the John H. Stroger, Jr. Hospital of Cook County Department of Infection Control & Epidemiology for their assistance in reviewing local line infection data.
Drs. Patel and Chopra developed the study design. Drs. Patel, Araujo, Parra Rodriguez, Ramirez Sanchez, and Chopra contributed to manuscript writing. Ms. Snyder provided statistical analysis. All authors have seen and approved the final manuscript for submission.
Disclosures
The authors have nothing to disclose.
1. Anderson NR. Midline catheters: the middle ground of intravenous therapy administration. J Infus Nurs. 2004;27(5):313-321.
2. Adams DZ, Little A, Vinsant C, et al. The midline catheter: a clinical review. J Emerg Med. 2016;51(3):252-258. https://doi.org/10.1016/j.jemermed.2016.05.029.
3. Scoppettuolo G, Pittiruti M, Pitoni S, et al. Ultrasound-guided “short” midline catheters for difficult venous access in the emergency department: a retrospective analysis. Int J Emerg Med. 2016;9(1):3. https://doi.org/10.1186/s12245-016-0100-0.
4. Qin KR, Nataraja RM, Pacilli M. Long peripheral catheters: is it time to address the confusion? J Vasc Access. 2018;20(5). https://doi.org/10.1177/1129729818819730.
5. Pittiruti M, Scoppettuolo G. The GAVeCeLT Manual of PICC and Midlines. Milano: EDRA; 2016.
6. Dawson RB, Moureau NL. Midline catheters: an essential tool in CLABSI reduction. Infection Control Today. https://www.infectioncontroltoday.com/clabsi/midline-catheters-essential-tool-clabsi-reduction. Accessed February 19, 2018
7. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6):S1-S40. https://doi.org/10.7326/M15-0744.
8. Timsit JF, Schwebel C, Bouadma L, et al. Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults: a randomized controlled trial. JAMA. 2009;301(12):1231-1241. https://doi.org/10.1001/jama.2009.376.
9. Bahl A, Karabon P, Chu D. Comparison of venous thrombosis complications in midlines versus peripherally inserted central catheters: are midlines the safer option? Clin Appl Thromb Hemost. 2019;25. https://doi.org/10.1177/1076029619839150.
10. Goetz AM, Miller J, Wagener MM, et al. Complications related to intravenous midline catheter usage. A 2-year study. J Intraven Nurs. 1998;21(2):76-80.
11. Xu T, Kingsley L, DiNucci S, et al. Safety and utilization of peripherally inserted central catheters versus midline catheters at a large academic medical center. Am J Infect Control. 2016;44(12):1458-1461. https://doi.org/10.1016/j.ajic.2016.09.010.
12. Chopra V, Kaatz S, Swaminathan L, et al. Variation in use and outcomes related to midline catheters: results from a multicentre pilot study. BMJ Qual Saf. 2019;28(9):714-720. https://doi.org/10.1136/bmjqs-2018-008554.
13. Badger J. Long peripheral catheters for deep arm vein venous access: A systematic review of complications. Heart Lung. 2019;48(3):222-225. https://doi.org/10.1016/j.hrtlng.2019.01.002.
14. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81(9):1159-1171. https://doi.org/10.4065/81.9.1159.
15. Zerla PA, Caravella G, De Luca G, et al. Open- vs closed-tip valved peripherally inserted central catheters and midlines: Findings from a vascular access database. J Assoc Vasc Access. 2015;20(3):169-176. https://doi.org/10.1016/j.java.2015.06.001.
1. Anderson NR. Midline catheters: the middle ground of intravenous therapy administration. J Infus Nurs. 2004;27(5):313-321.
2. Adams DZ, Little A, Vinsant C, et al. The midline catheter: a clinical review. J Emerg Med. 2016;51(3):252-258. https://doi.org/10.1016/j.jemermed.2016.05.029.
3. Scoppettuolo G, Pittiruti M, Pitoni S, et al. Ultrasound-guided “short” midline catheters for difficult venous access in the emergency department: a retrospective analysis. Int J Emerg Med. 2016;9(1):3. https://doi.org/10.1186/s12245-016-0100-0.
4. Qin KR, Nataraja RM, Pacilli M. Long peripheral catheters: is it time to address the confusion? J Vasc Access. 2018;20(5). https://doi.org/10.1177/1129729818819730.
5. Pittiruti M, Scoppettuolo G. The GAVeCeLT Manual of PICC and Midlines. Milano: EDRA; 2016.
6. Dawson RB, Moureau NL. Midline catheters: an essential tool in CLABSI reduction. Infection Control Today. https://www.infectioncontroltoday.com/clabsi/midline-catheters-essential-tool-clabsi-reduction. Accessed February 19, 2018
7. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6):S1-S40. https://doi.org/10.7326/M15-0744.
8. Timsit JF, Schwebel C, Bouadma L, et al. Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults: a randomized controlled trial. JAMA. 2009;301(12):1231-1241. https://doi.org/10.1001/jama.2009.376.
9. Bahl A, Karabon P, Chu D. Comparison of venous thrombosis complications in midlines versus peripherally inserted central catheters: are midlines the safer option? Clin Appl Thromb Hemost. 2019;25. https://doi.org/10.1177/1076029619839150.
10. Goetz AM, Miller J, Wagener MM, et al. Complications related to intravenous midline catheter usage. A 2-year study. J Intraven Nurs. 1998;21(2):76-80.
11. Xu T, Kingsley L, DiNucci S, et al. Safety and utilization of peripherally inserted central catheters versus midline catheters at a large academic medical center. Am J Infect Control. 2016;44(12):1458-1461. https://doi.org/10.1016/j.ajic.2016.09.010.
12. Chopra V, Kaatz S, Swaminathan L, et al. Variation in use and outcomes related to midline catheters: results from a multicentre pilot study. BMJ Qual Saf. 2019;28(9):714-720. https://doi.org/10.1136/bmjqs-2018-008554.
13. Badger J. Long peripheral catheters for deep arm vein venous access: A systematic review of complications. Heart Lung. 2019;48(3):222-225. https://doi.org/10.1016/j.hrtlng.2019.01.002.
14. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81(9):1159-1171. https://doi.org/10.4065/81.9.1159.
15. Zerla PA, Caravella G, De Luca G, et al. Open- vs closed-tip valved peripherally inserted central catheters and midlines: Findings from a vascular access database. J Assoc Vasc Access. 2015;20(3):169-176. https://doi.org/10.1016/j.java.2015.06.001.
© 2019 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: Clostridium difficile Infections in Children
Clostridium difficile (name changed to Clostridioides difficile [CDI]) are a major public health problem, with 500,000 infections annually in the United States, 15,000-30,000 associated deaths, and acute care costs exceeding $4.8 billion. The recent clinical practice guideline for CDI provides recommendations about the epidemiology, diagnosis, treatment, prevention, and environmental management. A total of 52 recommendations are included, and we will review 11 with pertinence to pediatrics in this highlight.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. In infants ≤12 months of age, testing for CDI should never be routinely recommended because of the high prevalence of asymptomatic carriage of toxigenic C. difficile in infants (strong recommendation, moderate quality of evidence).
Recommendation 2. In children 1-2 years of age, testing should not be routinely performed unless other causes have been excluded (weak recommendation, low quality of evidence).Recommendation 3. In children ≥2 years of age, testing is recommended for patients with prolonged or worsening diarrhea and risk factors (eg, underlying inflammatory bowel disease) or immunocompromising conditions) or relevant exposures (eg, contact with the healthcare system or recent antibiotics) (weak recommendation, moderate quality of evidence).
The rate of C. difficile colonization among asymptomatic infants can exceed 40%. This rate declines over the first year but remains 15% at 12 months of age.1 Therefore, the guideline recommends against routinely testing infants ≤12 months of age as a positive test probably reflects colonization rather than disease. Testing in infants is recommended only when other causes have been excluded and a concern for pseudomembranous colitis, toxic megacolon, or clinically significant diarrhea exists.
The rate of asymptomatic colonization remains elevated in the second year of life. By 2-3 years, the rate is 1%-3% which is similar to that in healthy adults. However, the role of C. difficile in community-onset diarrhea in otherwise healthy children is controversial. In a study of 100 hospitalized children aged <2 years with CDI and diarrhea, all had resolution of diarrhea regardless of whether therapy was administered.2 Another study found an alternative pathogen in >50% of hospitalized children with CDI.3 Therefore, the guideline recommends against testing in children aged 1-2 years unless other causes have been excluded and in children aged >2 years only when they have prolonged or worsening diarrhea along with risk factors or exposures.
Recommendation 4. In institutions without specific required criteria for stool submissions, use a stool toxin test as part of a multistep algorithm (ie, glutamate dehydrogenase [GDH] plus toxin, GDH plus toxin arbitrated by nucleic-acid amplification tests [NAAT], or NAAT plus toxin) rather than a NAAT alone (weak recommendation, low quality of evidence).
Recommendation 5. In institutions with specific required criteria for stool submissions, use a NAAT alone or a multistep algorithm for testing (ie, GDH plus toxin, GDH plus toxin arbitrated by NAAT, or NAAT plus toxin) rather than a toxin test alone (weak recommendation, low quality of evidence).
There are a variety of testing approaches for CDI and recommendations vary based on local practice. If laboratories accept all stools, a more specific approach is recommended, including a toxin test as part of a multistep algorithm to limit false positives. If laboratories first screen for symptoms and antibiotic exposure before accepting stool samples, a more sensitive approach is recommended including NAAT alone or a multistep algorithm rather than toxin alone.
Infection Prevention and Control
Recommendation 6. There is insufficient evidence for discontinuation of PPIs (proton pump inhibitors) as a measure for preventing CDI (no recommendation).
The guideline acknowledges data suggesting an association between PPI use and CDI, but not a causal relationship. Due to the lack of high-quality evidence, it does not recommend stopping PPIs to prevent CDI.
Recommendation 7. There are insufficient data to recommend probiotics for primary prevention of CDI outside of clinical trials (no recommendation).
The guideline notes that although several meta-analyses indicate that probiotics may prevent CDI; however there were limitations, including a high incidence of CDI in placebo arms and differences in probiotic formulations and duration of use, leading to insufficient data to recommend probiotic use to prevent CDI.
Treatment
Recommendation 8. Either per os (PO) metronidazole or PO vancomycin is recommended for an initial episode or first recurrence of nonsevere pediatric CDI (weak recommendation, low quality of evidence).
Data assessing the optimal treatment for nonsevere pediatric CDI are limited. Emerging data support the use of vancomycin,4 which is now recommended for initial episodes of CDI in adults. However, there are insufficient data to recommend vancomycin over metronidazole for nonsevere pediatric CDI; therefore, either option is recommended.
Recommendation 9. For children with an initial episode of severe CDI, oral vancomycin with or without IV metronidazole is recommended over metronidazole alone (strong recommendation, moderate quality of evidence).
Recommendation 10. For children with a second or greater episode of recurrent CDI, oral vancomycin is recommended over metronidazole (weak recommendation, low quality of evidence).
There is no well-designed trial comparing metronidazole and vancomycin for severe or recurrent pediatric CDI. For children previously treated with metronidazole, vancomycin is recommended based on adult literature.4 For children previously treated with metronidazole and vancomycin, an extended course of tapered or pulse regimen vancomycin or vancomycin followed by rifaximin is recommended.
Recommendations must weigh potential harms. Metronidazole has been associated with neuropathies,5 cramping, and nausea. PO vancomycin has poor enteral absorption, minimizing systemic effects. Both vancomycin and metronidazole may promote carriage of resistant enterococci.
Recommendation 11. Fecal microbiota transplantation (FMT) should be considered for pediatric patients with multiple recurrences of CDI following standard treatments (weak recommendation, very low quality of evidence).
There are no robust data examining the effectiveness of pediatric FMT. Recommendations are guided by adult studies. Limited evidence suggests that FMT can be effective in children with multiple recurrent CDI.6 Concerns include procedure-related risks, transmission of resistant organisms and blood-borne pathogens, and induced metabolic or immunologic disorders.
CRITIQUE
Methods in Preparing a Guideline
The strength of a guideline includes representation from a diverse panel, including the Infectious Diseases Society of America (IDSA), the Society for Healthcare Epidemiology of America, the American Society of Health-Systems Pharmacists, the Society of Infectious Diseases Pharmacists, and the Pediatric Infectious Diseases Society.
The panel utilized the Grading of Recommendations Assessment, Development, and Evaluation system to weigh the strength and quality of evidence.
From a pediatric perspective, the current guideline added pediatric-specific recommendations based on a comprehensive review of the literature from 1977 to 2016. The strength of these recommendations is somewhat limited by the lack of well-designed pediatric studies. An additional limitation is that treatment recommendations are based on illness severity, although the definitions used to classify severity are not pediatric-specific and are based on unvalidated expert opinion.
Sources of Potential Conflicts or Interest or Bias
The panel complied with the IDSA policy on conflicts of interest and disclosed any interest that might be construed as a conflict, regardless of relevancy. These were evaluated by the IDSA Standards and Practice Guidelines Committee.
Generalizability
Guideline generalizability may be impacted by testing availabilities within a particular setting. Cost factors and local formularies may also limit treatment options within a given setting.
Areas in Need of Future Study
Research gaps exist regarding at what age C. difficile is pathogenic given the prevalence of asymptomatic carriage. Future studies can also focus on a newly available molecular polymerase chain reaction test platform that detects C. difficile.7
There is limited pediatric evidence to recommend metronidazole versus vancomycin in children, particularly in nonsevere cases. There is also an opportunity to further explore alternative therapies, including fidaxomicin (not currently approved for children) and bezlotoxumab, a new agent approved as adult adjunctive therapy.8
1. Donta ST, Myers MG. Clostridium difficile toxin in asymptomatic neonates. J Pediatr. 1982;100(3):431-434. https://doi.org/10.1016/s0022-3476(82)80454-x.
2. González-Del Vecchio M, Álvarez-Uria A, Marin M, et al. Clinical significance of Clostridium difficile in children less than 2 years old: a case-control study. Pediatr Infect Dis J. 2016;35(3):281-285. https://doi.org/10.1097/INF.0000000000001008.
3. Valentini D, Vittucci AC, Grandin A, et al. Coinfection in acute gastroenteritis predicts a more severe clinical course in children. Eur J Clin Microbiol Infect Dis. 2013;32(7):909-915. https://doi.org/10.1007/s10096-013-1825-9.
4. Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59(3):345-354. https://doi.org/10.1093/cid/ciu313.
5. Yamamoto T, Abe K, Anjiki H, Ishii T, Kuyama Y. Metronidazole-induced neurotoxicity developed in liver cirrhosis. J Clin Med Res. 2012;4(4):295-298. https://doi.org/10.4021/jocmr893w.
6. Russell G, Kaplan J, Ferraro M, Michelow IC. Fecal bacteriotherapy for relapsing Clostridium difficile infection in a child: a proposed treatment protocol. Pediatrics. 2010;126(1):e239-e242. https://doi.org/10.1542/peds.2009-3363.
7. Zhang H, Morrison S, Tang YW. Multiplex polymerase chain reaction tests for detection of pathogens associated with gastroenteritis. Clin Lab Med. 2015;35(2):461-486. https://doi.org/10.1016/j.cll.2015.02.006.
8. Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376(4):305-317. https://doi.org/10.1056/NEJMoa1602615.
Clostridium difficile (name changed to Clostridioides difficile [CDI]) are a major public health problem, with 500,000 infections annually in the United States, 15,000-30,000 associated deaths, and acute care costs exceeding $4.8 billion. The recent clinical practice guideline for CDI provides recommendations about the epidemiology, diagnosis, treatment, prevention, and environmental management. A total of 52 recommendations are included, and we will review 11 with pertinence to pediatrics in this highlight.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. In infants ≤12 months of age, testing for CDI should never be routinely recommended because of the high prevalence of asymptomatic carriage of toxigenic C. difficile in infants (strong recommendation, moderate quality of evidence).
Recommendation 2. In children 1-2 years of age, testing should not be routinely performed unless other causes have been excluded (weak recommendation, low quality of evidence).Recommendation 3. In children ≥2 years of age, testing is recommended for patients with prolonged or worsening diarrhea and risk factors (eg, underlying inflammatory bowel disease) or immunocompromising conditions) or relevant exposures (eg, contact with the healthcare system or recent antibiotics) (weak recommendation, moderate quality of evidence).
The rate of C. difficile colonization among asymptomatic infants can exceed 40%. This rate declines over the first year but remains 15% at 12 months of age.1 Therefore, the guideline recommends against routinely testing infants ≤12 months of age as a positive test probably reflects colonization rather than disease. Testing in infants is recommended only when other causes have been excluded and a concern for pseudomembranous colitis, toxic megacolon, or clinically significant diarrhea exists.
The rate of asymptomatic colonization remains elevated in the second year of life. By 2-3 years, the rate is 1%-3% which is similar to that in healthy adults. However, the role of C. difficile in community-onset diarrhea in otherwise healthy children is controversial. In a study of 100 hospitalized children aged <2 years with CDI and diarrhea, all had resolution of diarrhea regardless of whether therapy was administered.2 Another study found an alternative pathogen in >50% of hospitalized children with CDI.3 Therefore, the guideline recommends against testing in children aged 1-2 years unless other causes have been excluded and in children aged >2 years only when they have prolonged or worsening diarrhea along with risk factors or exposures.
Recommendation 4. In institutions without specific required criteria for stool submissions, use a stool toxin test as part of a multistep algorithm (ie, glutamate dehydrogenase [GDH] plus toxin, GDH plus toxin arbitrated by nucleic-acid amplification tests [NAAT], or NAAT plus toxin) rather than a NAAT alone (weak recommendation, low quality of evidence).
Recommendation 5. In institutions with specific required criteria for stool submissions, use a NAAT alone or a multistep algorithm for testing (ie, GDH plus toxin, GDH plus toxin arbitrated by NAAT, or NAAT plus toxin) rather than a toxin test alone (weak recommendation, low quality of evidence).
There are a variety of testing approaches for CDI and recommendations vary based on local practice. If laboratories accept all stools, a more specific approach is recommended, including a toxin test as part of a multistep algorithm to limit false positives. If laboratories first screen for symptoms and antibiotic exposure before accepting stool samples, a more sensitive approach is recommended including NAAT alone or a multistep algorithm rather than toxin alone.
Infection Prevention and Control
Recommendation 6. There is insufficient evidence for discontinuation of PPIs (proton pump inhibitors) as a measure for preventing CDI (no recommendation).
The guideline acknowledges data suggesting an association between PPI use and CDI, but not a causal relationship. Due to the lack of high-quality evidence, it does not recommend stopping PPIs to prevent CDI.
Recommendation 7. There are insufficient data to recommend probiotics for primary prevention of CDI outside of clinical trials (no recommendation).
The guideline notes that although several meta-analyses indicate that probiotics may prevent CDI; however there were limitations, including a high incidence of CDI in placebo arms and differences in probiotic formulations and duration of use, leading to insufficient data to recommend probiotic use to prevent CDI.
Treatment
Recommendation 8. Either per os (PO) metronidazole or PO vancomycin is recommended for an initial episode or first recurrence of nonsevere pediatric CDI (weak recommendation, low quality of evidence).
Data assessing the optimal treatment for nonsevere pediatric CDI are limited. Emerging data support the use of vancomycin,4 which is now recommended for initial episodes of CDI in adults. However, there are insufficient data to recommend vancomycin over metronidazole for nonsevere pediatric CDI; therefore, either option is recommended.
Recommendation 9. For children with an initial episode of severe CDI, oral vancomycin with or without IV metronidazole is recommended over metronidazole alone (strong recommendation, moderate quality of evidence).
Recommendation 10. For children with a second or greater episode of recurrent CDI, oral vancomycin is recommended over metronidazole (weak recommendation, low quality of evidence).
There is no well-designed trial comparing metronidazole and vancomycin for severe or recurrent pediatric CDI. For children previously treated with metronidazole, vancomycin is recommended based on adult literature.4 For children previously treated with metronidazole and vancomycin, an extended course of tapered or pulse regimen vancomycin or vancomycin followed by rifaximin is recommended.
Recommendations must weigh potential harms. Metronidazole has been associated with neuropathies,5 cramping, and nausea. PO vancomycin has poor enteral absorption, minimizing systemic effects. Both vancomycin and metronidazole may promote carriage of resistant enterococci.
Recommendation 11. Fecal microbiota transplantation (FMT) should be considered for pediatric patients with multiple recurrences of CDI following standard treatments (weak recommendation, very low quality of evidence).
There are no robust data examining the effectiveness of pediatric FMT. Recommendations are guided by adult studies. Limited evidence suggests that FMT can be effective in children with multiple recurrent CDI.6 Concerns include procedure-related risks, transmission of resistant organisms and blood-borne pathogens, and induced metabolic or immunologic disorders.
CRITIQUE
Methods in Preparing a Guideline
The strength of a guideline includes representation from a diverse panel, including the Infectious Diseases Society of America (IDSA), the Society for Healthcare Epidemiology of America, the American Society of Health-Systems Pharmacists, the Society of Infectious Diseases Pharmacists, and the Pediatric Infectious Diseases Society.
The panel utilized the Grading of Recommendations Assessment, Development, and Evaluation system to weigh the strength and quality of evidence.
From a pediatric perspective, the current guideline added pediatric-specific recommendations based on a comprehensive review of the literature from 1977 to 2016. The strength of these recommendations is somewhat limited by the lack of well-designed pediatric studies. An additional limitation is that treatment recommendations are based on illness severity, although the definitions used to classify severity are not pediatric-specific and are based on unvalidated expert opinion.
Sources of Potential Conflicts or Interest or Bias
The panel complied with the IDSA policy on conflicts of interest and disclosed any interest that might be construed as a conflict, regardless of relevancy. These were evaluated by the IDSA Standards and Practice Guidelines Committee.
Generalizability
Guideline generalizability may be impacted by testing availabilities within a particular setting. Cost factors and local formularies may also limit treatment options within a given setting.
Areas in Need of Future Study
Research gaps exist regarding at what age C. difficile is pathogenic given the prevalence of asymptomatic carriage. Future studies can also focus on a newly available molecular polymerase chain reaction test platform that detects C. difficile.7
There is limited pediatric evidence to recommend metronidazole versus vancomycin in children, particularly in nonsevere cases. There is also an opportunity to further explore alternative therapies, including fidaxomicin (not currently approved for children) and bezlotoxumab, a new agent approved as adult adjunctive therapy.8
Clostridium difficile (name changed to Clostridioides difficile [CDI]) are a major public health problem, with 500,000 infections annually in the United States, 15,000-30,000 associated deaths, and acute care costs exceeding $4.8 billion. The recent clinical practice guideline for CDI provides recommendations about the epidemiology, diagnosis, treatment, prevention, and environmental management. A total of 52 recommendations are included, and we will review 11 with pertinence to pediatrics in this highlight.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. In infants ≤12 months of age, testing for CDI should never be routinely recommended because of the high prevalence of asymptomatic carriage of toxigenic C. difficile in infants (strong recommendation, moderate quality of evidence).
Recommendation 2. In children 1-2 years of age, testing should not be routinely performed unless other causes have been excluded (weak recommendation, low quality of evidence).Recommendation 3. In children ≥2 years of age, testing is recommended for patients with prolonged or worsening diarrhea and risk factors (eg, underlying inflammatory bowel disease) or immunocompromising conditions) or relevant exposures (eg, contact with the healthcare system or recent antibiotics) (weak recommendation, moderate quality of evidence).
The rate of C. difficile colonization among asymptomatic infants can exceed 40%. This rate declines over the first year but remains 15% at 12 months of age.1 Therefore, the guideline recommends against routinely testing infants ≤12 months of age as a positive test probably reflects colonization rather than disease. Testing in infants is recommended only when other causes have been excluded and a concern for pseudomembranous colitis, toxic megacolon, or clinically significant diarrhea exists.
The rate of asymptomatic colonization remains elevated in the second year of life. By 2-3 years, the rate is 1%-3% which is similar to that in healthy adults. However, the role of C. difficile in community-onset diarrhea in otherwise healthy children is controversial. In a study of 100 hospitalized children aged <2 years with CDI and diarrhea, all had resolution of diarrhea regardless of whether therapy was administered.2 Another study found an alternative pathogen in >50% of hospitalized children with CDI.3 Therefore, the guideline recommends against testing in children aged 1-2 years unless other causes have been excluded and in children aged >2 years only when they have prolonged or worsening diarrhea along with risk factors or exposures.
Recommendation 4. In institutions without specific required criteria for stool submissions, use a stool toxin test as part of a multistep algorithm (ie, glutamate dehydrogenase [GDH] plus toxin, GDH plus toxin arbitrated by nucleic-acid amplification tests [NAAT], or NAAT plus toxin) rather than a NAAT alone (weak recommendation, low quality of evidence).
Recommendation 5. In institutions with specific required criteria for stool submissions, use a NAAT alone or a multistep algorithm for testing (ie, GDH plus toxin, GDH plus toxin arbitrated by NAAT, or NAAT plus toxin) rather than a toxin test alone (weak recommendation, low quality of evidence).
There are a variety of testing approaches for CDI and recommendations vary based on local practice. If laboratories accept all stools, a more specific approach is recommended, including a toxin test as part of a multistep algorithm to limit false positives. If laboratories first screen for symptoms and antibiotic exposure before accepting stool samples, a more sensitive approach is recommended including NAAT alone or a multistep algorithm rather than toxin alone.
Infection Prevention and Control
Recommendation 6. There is insufficient evidence for discontinuation of PPIs (proton pump inhibitors) as a measure for preventing CDI (no recommendation).
The guideline acknowledges data suggesting an association between PPI use and CDI, but not a causal relationship. Due to the lack of high-quality evidence, it does not recommend stopping PPIs to prevent CDI.
Recommendation 7. There are insufficient data to recommend probiotics for primary prevention of CDI outside of clinical trials (no recommendation).
The guideline notes that although several meta-analyses indicate that probiotics may prevent CDI; however there were limitations, including a high incidence of CDI in placebo arms and differences in probiotic formulations and duration of use, leading to insufficient data to recommend probiotic use to prevent CDI.
Treatment
Recommendation 8. Either per os (PO) metronidazole or PO vancomycin is recommended for an initial episode or first recurrence of nonsevere pediatric CDI (weak recommendation, low quality of evidence).
Data assessing the optimal treatment for nonsevere pediatric CDI are limited. Emerging data support the use of vancomycin,4 which is now recommended for initial episodes of CDI in adults. However, there are insufficient data to recommend vancomycin over metronidazole for nonsevere pediatric CDI; therefore, either option is recommended.
Recommendation 9. For children with an initial episode of severe CDI, oral vancomycin with or without IV metronidazole is recommended over metronidazole alone (strong recommendation, moderate quality of evidence).
Recommendation 10. For children with a second or greater episode of recurrent CDI, oral vancomycin is recommended over metronidazole (weak recommendation, low quality of evidence).
There is no well-designed trial comparing metronidazole and vancomycin for severe or recurrent pediatric CDI. For children previously treated with metronidazole, vancomycin is recommended based on adult literature.4 For children previously treated with metronidazole and vancomycin, an extended course of tapered or pulse regimen vancomycin or vancomycin followed by rifaximin is recommended.
Recommendations must weigh potential harms. Metronidazole has been associated with neuropathies,5 cramping, and nausea. PO vancomycin has poor enteral absorption, minimizing systemic effects. Both vancomycin and metronidazole may promote carriage of resistant enterococci.
Recommendation 11. Fecal microbiota transplantation (FMT) should be considered for pediatric patients with multiple recurrences of CDI following standard treatments (weak recommendation, very low quality of evidence).
There are no robust data examining the effectiveness of pediatric FMT. Recommendations are guided by adult studies. Limited evidence suggests that FMT can be effective in children with multiple recurrent CDI.6 Concerns include procedure-related risks, transmission of resistant organisms and blood-borne pathogens, and induced metabolic or immunologic disorders.
CRITIQUE
Methods in Preparing a Guideline
The strength of a guideline includes representation from a diverse panel, including the Infectious Diseases Society of America (IDSA), the Society for Healthcare Epidemiology of America, the American Society of Health-Systems Pharmacists, the Society of Infectious Diseases Pharmacists, and the Pediatric Infectious Diseases Society.
The panel utilized the Grading of Recommendations Assessment, Development, and Evaluation system to weigh the strength and quality of evidence.
From a pediatric perspective, the current guideline added pediatric-specific recommendations based on a comprehensive review of the literature from 1977 to 2016. The strength of these recommendations is somewhat limited by the lack of well-designed pediatric studies. An additional limitation is that treatment recommendations are based on illness severity, although the definitions used to classify severity are not pediatric-specific and are based on unvalidated expert opinion.
Sources of Potential Conflicts or Interest or Bias
The panel complied with the IDSA policy on conflicts of interest and disclosed any interest that might be construed as a conflict, regardless of relevancy. These were evaluated by the IDSA Standards and Practice Guidelines Committee.
Generalizability
Guideline generalizability may be impacted by testing availabilities within a particular setting. Cost factors and local formularies may also limit treatment options within a given setting.
Areas in Need of Future Study
Research gaps exist regarding at what age C. difficile is pathogenic given the prevalence of asymptomatic carriage. Future studies can also focus on a newly available molecular polymerase chain reaction test platform that detects C. difficile.7
There is limited pediatric evidence to recommend metronidazole versus vancomycin in children, particularly in nonsevere cases. There is also an opportunity to further explore alternative therapies, including fidaxomicin (not currently approved for children) and bezlotoxumab, a new agent approved as adult adjunctive therapy.8
1. Donta ST, Myers MG. Clostridium difficile toxin in asymptomatic neonates. J Pediatr. 1982;100(3):431-434. https://doi.org/10.1016/s0022-3476(82)80454-x.
2. González-Del Vecchio M, Álvarez-Uria A, Marin M, et al. Clinical significance of Clostridium difficile in children less than 2 years old: a case-control study. Pediatr Infect Dis J. 2016;35(3):281-285. https://doi.org/10.1097/INF.0000000000001008.
3. Valentini D, Vittucci AC, Grandin A, et al. Coinfection in acute gastroenteritis predicts a more severe clinical course in children. Eur J Clin Microbiol Infect Dis. 2013;32(7):909-915. https://doi.org/10.1007/s10096-013-1825-9.
4. Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59(3):345-354. https://doi.org/10.1093/cid/ciu313.
5. Yamamoto T, Abe K, Anjiki H, Ishii T, Kuyama Y. Metronidazole-induced neurotoxicity developed in liver cirrhosis. J Clin Med Res. 2012;4(4):295-298. https://doi.org/10.4021/jocmr893w.
6. Russell G, Kaplan J, Ferraro M, Michelow IC. Fecal bacteriotherapy for relapsing Clostridium difficile infection in a child: a proposed treatment protocol. Pediatrics. 2010;126(1):e239-e242. https://doi.org/10.1542/peds.2009-3363.
7. Zhang H, Morrison S, Tang YW. Multiplex polymerase chain reaction tests for detection of pathogens associated with gastroenteritis. Clin Lab Med. 2015;35(2):461-486. https://doi.org/10.1016/j.cll.2015.02.006.
8. Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376(4):305-317. https://doi.org/10.1056/NEJMoa1602615.
1. Donta ST, Myers MG. Clostridium difficile toxin in asymptomatic neonates. J Pediatr. 1982;100(3):431-434. https://doi.org/10.1016/s0022-3476(82)80454-x.
2. González-Del Vecchio M, Álvarez-Uria A, Marin M, et al. Clinical significance of Clostridium difficile in children less than 2 years old: a case-control study. Pediatr Infect Dis J. 2016;35(3):281-285. https://doi.org/10.1097/INF.0000000000001008.
3. Valentini D, Vittucci AC, Grandin A, et al. Coinfection in acute gastroenteritis predicts a more severe clinical course in children. Eur J Clin Microbiol Infect Dis. 2013;32(7):909-915. https://doi.org/10.1007/s10096-013-1825-9.
4. Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59(3):345-354. https://doi.org/10.1093/cid/ciu313.
5. Yamamoto T, Abe K, Anjiki H, Ishii T, Kuyama Y. Metronidazole-induced neurotoxicity developed in liver cirrhosis. J Clin Med Res. 2012;4(4):295-298. https://doi.org/10.4021/jocmr893w.
6. Russell G, Kaplan J, Ferraro M, Michelow IC. Fecal bacteriotherapy for relapsing Clostridium difficile infection in a child: a proposed treatment protocol. Pediatrics. 2010;126(1):e239-e242. https://doi.org/10.1542/peds.2009-3363.
7. Zhang H, Morrison S, Tang YW. Multiplex polymerase chain reaction tests for detection of pathogens associated with gastroenteritis. Clin Lab Med. 2015;35(2):461-486. https://doi.org/10.1016/j.cll.2015.02.006.
8. Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376(4):305-317. https://doi.org/10.1056/NEJMoa1602615.
© 2019 Society of Hospital Medicine
A Call to Action: Hospitalists’ Role in Addressing Substance Use Disorder
In 2017, the death toll from drug overdoses reached a record high, killing more Americans than the entire Vietnam War or the HIV/AIDS epidemic at its peak.1 Up to one-quarter of hospitalized patients have a substance use disorder (SUD) and SUD-related2,3 hospitalizations are surging. People with SUD have longer hospital stays, higher costs, and more readmissions.3,4 While the burden of SUD is staggering, it is far from hopeless. There are multiple evidence-based and highly effective interventions to treat SUD, including medications, behavioral interventions, and harm reduction strategies.
Hospitalization can be a reachable moment to initiate and coordinate addictions care.5 Hospital-based addictions care has the potential to engage sicker, highly vulnerable patients, many who are not engaged in primary care or outpatient addictions care.6 Studied effects of hospital-based addictions care include improved SUD treatment engagement, reduced alcohol and drug use, lower hospital readmissions, and improved provider experience.7-9
Most hospitals, however, do not treat SUD during hospitalization and do not connect people to treatment after discharge. Hospitals may lack staffing or financial resources to implement addiction care, may believe that SUDs are an outpatient concern, may want to avoid caring for people with SUD, or may simply not know where to begin. Whatever the reason, unaddressed SUD can lead to untreated withdrawal, disruptive patient behaviors, failure to complete recommended medical therapy, high rates of against medical advice discharge, poor patient experience, and widespread provider distress.8
Hospitalists—individually and collectively—are uniquely positioned to address this gap. By treating addiction effectively and compassionately, hospitalists can engage patients, improve care, improve patient and provider experience, and lower costs. This paper is a call to action that describes the current state of hospital-based addictions care, outlines key challenges to implementing SUD care in the hospital, debunks common misconceptions, and identifies actionable steps for hospitalists, hospital leaders, and hospitalist organizations.
MODELS TO DELIVER HOSPITAL-BASED ADDICTIONS CARE
Hospital-based addiction medicine consult services are emerging; they include a range of models, with variations in how patients are identified, team composition, service availability, and financing.10 Existing addiction medicine consult services commonly offer SUD assessments, psychological intervention, medical management of SUDs (eg, initiating methadone or buprenorphine), medical pain management, and linkage to SUD care after hospitalization. Some services also explicitly integrate harm reduction principles (eg, naloxone distribution, safe injection education, permitting patients to smoke).11 Additional consult service activities include hospital-wide SUD education, and creation and implementation of hospital guidance documents (eg, methadone policies).10 Some consult services utilize only physicians, while others include interprofessional providers, such as nurses, social workers, and peers with lived experience of addiction. Whereas addiction medicine physicians staff some consult services, hospitalists with less formal addiction credentials staff others.
Broadly, hospital-based addictions care cannot depend solely on consult services. Just as not all hospitals have cardiology consult services, not all hospitals will have addiction consult services. As such, hospitalists can play an even greater role by implementing order sets and guidelines, supporting partnerships with community SUD treatment, and independently initiating evidence-based medications.
CHALLENGES TO ADOPTION AND IMPLEMENTATION OF HOSPITAL-BASED ADDICTIONS CARE
Pervasive individual and structural stigmas12 are perhaps the most critical barriers to incorporating addiction medicine into routine hospital practice, and they are both cause and consequence of our system failures. Most medical schools and residencies lack SUD training, which means that the understanding of addiction as a moral deficiency or lack of willpower may remain unchallenged. Stigma surrounding SUDs contributes to hospitalists’ and hospital leaders’ aversion to treating patients with SUD, and to fears that providing quality SUD care will attract patients suffering from these conditions.
Recent national efforts have focused on the problem of opioid overprescribing. Without an equal emphasis on treatment, this focus can lead to undertreatment of pain and/or opioid use disorder in hospitalized patients, particularly since most hospitalists have little to no training in diagnosing SUD, prescribing life-saving medications for opioid use disorder, or managing acute pain in patients with SUD. The focus on overprescribing also diverts attention away from trends involving stimulants,2 fentanyl contamination of the drug supply,13 and alcohol, all of which have important implications for the care of hospitalized adults.
Hospital policies are often not grounded in evidence (eg, recommending clonidine for first-line treatment of opioid withdrawal and not buprenorphine/methadone), and there are widespread misconceptions about perceived legal barriers to treating opioid use disorder in the hospital, which is both safe and legal.10 People with SUD may be unjustly viewed through a criminal justice lens. Policies focused on controlling visitors and conducting room searches disproportionately burden people with SUD, which may create further harms through reinforcing negative provider cognitive biases about SUDs. Finally, hospitals may lack inpatient social work and pharmacy supports, and they rarely have pathways to connect people to SUD care after discharge.
Funding remains a widespread challenge. While some hospital administrators support addiction medicine services because of the pressing medical need and public health crisis, most services depend on billing or demonstrated savings through reduced hospital days or readmissions.
A CALL TO ACTION: HOW HOSPITALISTS CAN IMPROVE ADDICTION CARE
Individual hospitalists, hospitalist leaders, and hospitalist organizations can engage by improving individual practice, driving systems change, and through advocacy and policy change (Table).
Individual Hospitalists
Providing basic addiction medicine care should be a core competency for all hospitalists, just as every hospitalist can initiate a goals-of-care conversation or prescribe insulin. For opioid use disorder, hospitalists should treat withdrawal and offer treatment initiation with opioid agonist therapy (ie, methadone, buprenorphine), which reduces mortality by over half. Commonly, hospitalized patients are subjected to harmful, nonevidence-based treatments, such as mandated rapid methadone tapers,25 which can lead to undertreated withdrawal, increased pain, and opioid cravings. This increases patients’ risk for overdose after discharge and precludes them from receiving life-saving, evidence-based methadone maintenance, or buprenorphine treatment. Though widely misunderstood, prescribing methadone in the hospital is legal, and providers need no special waiver to prescribe buprenorphine during admission. Current laws require that hospitalists have a waiver to prescribe buprenorphine at discharge and prohibit hospitalists (or anyone outside of an opioid treatment program) from prescribing methadone for the treatment of opioid use disorder at discharge. Further, hospitalists should offer medication for alcohol use disorder (eg, naltrexone) and be good stewards of opioids during hospitalization, avoiding intravenous opioids where appropriate and curbing excessive prescribing at discharge. Given high rates of overdose and fentanyl contamination of stimulants, opioids, and benzodiazepines, hospitalists should prescribe naloxone at discharge to every patient with SUD, on chronic opioids, or who uses any nonmedical substances.
Resources exist for individual hospitalists seeking mentorship or additional training (Table). Though not necessary for in-hospital prescribing, hospitalists can obtain a waiver to prescribe buprenorphine at discharge (commonly called the X-waiver). To qualify, physicians must complete eight hours of accredited training (online and/or in-person), after which they must request a waiver from the Drug Enforcement Administration. Advanced-practice practitioners must complete 24 hours of training. Many have argued that policymakers should end this waiver requirement.26 While we support efforts to “X the X” and urgently expand treatment access, additional training can enrich providers’ knowledge and confidence to prescribe buprenorphine, and is a relatively simple way that all hospitalists could act. Finally, by treating addiction and modeling patient-centered addictions care, hospitalists can legitimize and destigmatize the disease of addiction,8 and have the potential to mentor and train students, residents, nurses, and other staff.27
Hospitalist Leaders
As leaders, hospitalists can play a key role in promoting hospital-based addictions care and tailoring solutions to meet local needs. Leaders can promote a cultural shift away from stigma, and promote evidence-based, life-saving care. Hospitalist leaders could require all hospitalists to obtain buprenorphine waivers. Leaders could initiate quality improvement projects related to SUD service delivery, develop policies that support inpatient SUD treatment, develop order sets for medication initiation, engage community substance use treatment partners, build pathways to timely addiction care after discharge, and champion development of addiction medicine consult services.
Hospitalist leaders can reference open-source guidelines, order sets, assessment and treatment tools, patient materials, pharmacy and therapeutics committee materials, and other resources for implementing services for hospitalized patients with SUD (Table).21,22 Hospitalist leaders who understand financial and quality drivers can also champion the business and quality case for hospital-based addictions care, and help pursue local and national funding opportunities.
Hospitalist Organizations
Hospitalist societies could provide training at regional and national conferences to upskill hospitalists to care for people with SUD; support addiction medicine interest groups; and partner with addiction medicine societies, harm reduction organizations, and organizations focused on trauma-informed care. They could endorse practice guidelines and position statements describing the crucial role of hospitalists in addressing the overdose crisis and offering medication for addiction (Table). Hospitalist organizations can engage national and state hospital associations, lobby medical specialties to include addiction medicine competencies in board certification requirements, and advocate with governmental leaders to reduce barriers that restrict treatment access such as the X-waiver.
MOVING FORWARD
Regardless of whether a hospitalist is serving as an individual provider, a hospitalist leader, or as part of a hospitalist organization, hospitalists can take critical steps to advance the care of people with SUD. These steps shift the culture of hospitals from one where patients are afraid to discuss their substance use, to one that creates space for connection, treatment engagement, and healing. By starting medications, utilizing widely accessible resources, and collaborating with community treatment and harm reduction organizations, each one of us can play a part in addressing the epidemic.
Acknowledgments
The authors thank Alisa Patten for help preparing this manuscript. Dr. Englander would like to thank Dr. David Bangsberg and Dr. Christina Nicolaidis for their mentorship.
1. Weiss A, Elixhauser A, Barrett M, Steiner C, Bailey M, O’Malley L. Opioid-related inpatient stays and emergency department visits by state, 2009-2014. Statistical Brief #219. Healthcare Cost and Utilization Project. 2016. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb219-Opioid-Hospital-Stays-ED-Visits-by-State.jsp. Accessed May 21, 2019.
2. Winkelman TA, Admon LK, Jennings L, Shippee ND, Richardson CR, Bart G. Evaluation of amphetamine-related hospitalizations and associated clinical outcomes and costs in the United States. JAMA Netw Open. 2018;1(6):e183758. https://doi.org/10.1001/jamanetworkopen.2018.3758.
3. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-837. https://doi.org/10.1377/hlthaff.2015.1424.
4. Walley AY, Paasche-Orlow M, Lee EC, et al. Acute care hospital utilization among medical inpatients discharged with a substance use disorder diagnosis. J Addict Med. 2012;6(1):50-56. https://doi.org/10.1097/ADM.0b013e318231de51.
5. Englander H, Weimer M, Solotaroff R, et al. Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12(5):339-342. https://doi.org/10.12788/jhm.2736.
6. Velez C, Nicolaidis C, Korthuis P, Englander H. “It’s been an experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. doi 10.1007/s11606-016-3919-4.
7. Wakeman SE, Metlay JP, Chang Y, Herman GE, Rigotti NA. Inpatient addiction consultation for hospitalized patients increases post-discharge abstinence and reduces addiction severity. J Gen Intern Med. 2017;32(8):909-916. https://doi.org/10.1007/s11606-017-4077-z.
8. Englander H, Collins D, Perry SP, Rabinowitz M, Phoutrides E, Nicolaidis C. “We’ve learned it’s a medical illness, not a moral choice”: qualitative study of the effects of a multicomponent addiction intervention on hospital providers’ attitudes and experiences. J Hosp Med. 2018;13(11):752-758. https://doi.org/10.12788/jhm.2993.
9. McQueen J, Howe TE, Allan L, Mains D, Hardy V. Brief interventions for heavy alcohol users admitted to general hospital wards. Cochrane Database Syst Rev. 2011;10(8):CD005191 https://doi.org/10.1002/14651858.CD005191.pub3.
10. Priest KC, McCarty D. Role of the hospital in the 21st century opioid overdose epidemic: the addiction medicine consult service. J Addict Med. 2019;13(2):104-112. https://doi.org/10.1097/ADM.0000000000000496.
11. Weinstein ZM, Wakeman SE, Nolan S. Inpatient addiction consult service: expertise for hospitalized patients with complex addiction problems. Med Clin North Am. 2018;102(4):587-601. https://doi.org/10.1016/j.mcna.2018.03.001.
12. McNeil R, Small W, Wood E, Kerr T. Hospitals as a “risk environment”: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66. https://doi.org/10.1016/j.socscimed.2014.01.010.
13. Ciccarone D. The triple wave epidemic: supply and demand drivers of the US opioid overdose crisis. Int J Drug Policy. 2019. pii: S0955-3959(19)30018-0. [Epub ahead of print]. https://doi.org/10.1016/j.drugpo.2019.01.010.
14. Substance Abuse and Mental Health Services Administration. TIP 63: Medications for Opioid Use Disorder-Executive Summary. February 2018. https://store.samhsa.gov/product/TIP-63-Medications-for-Opioid-Use-Disorder-Executive-Summary/sma18-5063exsumm. Accessed August 8, 2019.
15. Providers Clinical Support System. Discover the rewards of treating patients with Opioid Use Disorders. https://pcssnow.org/. Accessed August 8, 2019.
16. California Bridge Program. Treatment Starts Here: Resources for the Treatment of Substance Use Disorders from the Acute Care Setting. https://www.bridgetotreatment.org/resources. Accessed August 7, 2019.
17. Clinical Consultation Center. Substance Use Resources. 2019. https://nccc.ucsf.edu/clinical-resources/substance-use-resources/. Accessed August 8, 2019.
18. Thakarar K, Weinstein ZM, Walley AY. Optimising health and safety of people who inject drugs during transition from acute to outpatient care: narrative review with clinical checklist. Postgrad Med J. 2016;92(1088):356-363. https://doi.org/10.1136/postgradmedj-2015-133720.
19. Office of National Drug Control Policy. Changing the Language of Addiction. Washington, D.C. 2017. https://www.whitehouse.gov/sites/whitehouse.gov/files/images/Memo%20-%20Changing%20Federal%20Terminology%20Regrading%20Substance%20Use%20and%20Substance%20Use%20Disorders.pdf. Accessed August 8, 2019.
20. The University of New Mexico. Project ECHO: A Revolution in Medical Education and Care Delivery. 2019. https://echo.unm.edu/. Accessed August 8, 2019.
21. Englander H, Mahoney S, Brandt K, et al. Tools to support hospital-based addiction care: core components, values, and activities of the Improving Addiction Care Team. J Addict Med. 2019;13(2):85-89. https://doi.org/10.1097/ADM.0000000000000487.
22. Englander H, Gregg J, Gollickson J, et al. Recommendations for intergrating peer mentors in hospital-based addiction care. Subst Abus. In press. https://doi.org/10.1080/08897077.2019.1635968.
23. American College of Medical Toxicology. ACMT Position Statement: Buprenorphine Administration in the Emergency Department. https://www.acep.org/globalassets/sites/acep/media/equal-documents/policy_acmt_bupeadministration.pdf. Accessed May 21, 2019.
24. Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: a consensus statement from the society of hospital medicine. J Hosp Med. 2018;13(4):263-271. https://doi.org/10.12788/jhm.2980.
25. Winetsky D, Weinrieb RM, Perrone J. Expanding treatment opportunities for hospitalized patients with opioid use disorders. J Hosp Med. 2018;13(1):62-64. https://doi.org/10.12788/jhm.2861.
26. Frank JW, Wakeman SE, Gordon AJ. No end to the crisis without an end to the waiver. Subst Abus. 2018;39(3):263-265. https://doi.org/10.1080/08897077.2018.1543382.
27. Gorfinkel L, Klimas J, Reel B, et al. In-hospital training in addiction medicine: a mixed-methods study of health care provider benefits and differences. Subst Abus. 2019. In press. https://doi.org/10.1080/08897077.2018.1561596.
In 2017, the death toll from drug overdoses reached a record high, killing more Americans than the entire Vietnam War or the HIV/AIDS epidemic at its peak.1 Up to one-quarter of hospitalized patients have a substance use disorder (SUD) and SUD-related2,3 hospitalizations are surging. People with SUD have longer hospital stays, higher costs, and more readmissions.3,4 While the burden of SUD is staggering, it is far from hopeless. There are multiple evidence-based and highly effective interventions to treat SUD, including medications, behavioral interventions, and harm reduction strategies.
Hospitalization can be a reachable moment to initiate and coordinate addictions care.5 Hospital-based addictions care has the potential to engage sicker, highly vulnerable patients, many who are not engaged in primary care or outpatient addictions care.6 Studied effects of hospital-based addictions care include improved SUD treatment engagement, reduced alcohol and drug use, lower hospital readmissions, and improved provider experience.7-9
Most hospitals, however, do not treat SUD during hospitalization and do not connect people to treatment after discharge. Hospitals may lack staffing or financial resources to implement addiction care, may believe that SUDs are an outpatient concern, may want to avoid caring for people with SUD, or may simply not know where to begin. Whatever the reason, unaddressed SUD can lead to untreated withdrawal, disruptive patient behaviors, failure to complete recommended medical therapy, high rates of against medical advice discharge, poor patient experience, and widespread provider distress.8
Hospitalists—individually and collectively—are uniquely positioned to address this gap. By treating addiction effectively and compassionately, hospitalists can engage patients, improve care, improve patient and provider experience, and lower costs. This paper is a call to action that describes the current state of hospital-based addictions care, outlines key challenges to implementing SUD care in the hospital, debunks common misconceptions, and identifies actionable steps for hospitalists, hospital leaders, and hospitalist organizations.
MODELS TO DELIVER HOSPITAL-BASED ADDICTIONS CARE
Hospital-based addiction medicine consult services are emerging; they include a range of models, with variations in how patients are identified, team composition, service availability, and financing.10 Existing addiction medicine consult services commonly offer SUD assessments, psychological intervention, medical management of SUDs (eg, initiating methadone or buprenorphine), medical pain management, and linkage to SUD care after hospitalization. Some services also explicitly integrate harm reduction principles (eg, naloxone distribution, safe injection education, permitting patients to smoke).11 Additional consult service activities include hospital-wide SUD education, and creation and implementation of hospital guidance documents (eg, methadone policies).10 Some consult services utilize only physicians, while others include interprofessional providers, such as nurses, social workers, and peers with lived experience of addiction. Whereas addiction medicine physicians staff some consult services, hospitalists with less formal addiction credentials staff others.
Broadly, hospital-based addictions care cannot depend solely on consult services. Just as not all hospitals have cardiology consult services, not all hospitals will have addiction consult services. As such, hospitalists can play an even greater role by implementing order sets and guidelines, supporting partnerships with community SUD treatment, and independently initiating evidence-based medications.
CHALLENGES TO ADOPTION AND IMPLEMENTATION OF HOSPITAL-BASED ADDICTIONS CARE
Pervasive individual and structural stigmas12 are perhaps the most critical barriers to incorporating addiction medicine into routine hospital practice, and they are both cause and consequence of our system failures. Most medical schools and residencies lack SUD training, which means that the understanding of addiction as a moral deficiency or lack of willpower may remain unchallenged. Stigma surrounding SUDs contributes to hospitalists’ and hospital leaders’ aversion to treating patients with SUD, and to fears that providing quality SUD care will attract patients suffering from these conditions.
Recent national efforts have focused on the problem of opioid overprescribing. Without an equal emphasis on treatment, this focus can lead to undertreatment of pain and/or opioid use disorder in hospitalized patients, particularly since most hospitalists have little to no training in diagnosing SUD, prescribing life-saving medications for opioid use disorder, or managing acute pain in patients with SUD. The focus on overprescribing also diverts attention away from trends involving stimulants,2 fentanyl contamination of the drug supply,13 and alcohol, all of which have important implications for the care of hospitalized adults.
Hospital policies are often not grounded in evidence (eg, recommending clonidine for first-line treatment of opioid withdrawal and not buprenorphine/methadone), and there are widespread misconceptions about perceived legal barriers to treating opioid use disorder in the hospital, which is both safe and legal.10 People with SUD may be unjustly viewed through a criminal justice lens. Policies focused on controlling visitors and conducting room searches disproportionately burden people with SUD, which may create further harms through reinforcing negative provider cognitive biases about SUDs. Finally, hospitals may lack inpatient social work and pharmacy supports, and they rarely have pathways to connect people to SUD care after discharge.
Funding remains a widespread challenge. While some hospital administrators support addiction medicine services because of the pressing medical need and public health crisis, most services depend on billing or demonstrated savings through reduced hospital days or readmissions.
A CALL TO ACTION: HOW HOSPITALISTS CAN IMPROVE ADDICTION CARE
Individual hospitalists, hospitalist leaders, and hospitalist organizations can engage by improving individual practice, driving systems change, and through advocacy and policy change (Table).
Individual Hospitalists
Providing basic addiction medicine care should be a core competency for all hospitalists, just as every hospitalist can initiate a goals-of-care conversation or prescribe insulin. For opioid use disorder, hospitalists should treat withdrawal and offer treatment initiation with opioid agonist therapy (ie, methadone, buprenorphine), which reduces mortality by over half. Commonly, hospitalized patients are subjected to harmful, nonevidence-based treatments, such as mandated rapid methadone tapers,25 which can lead to undertreated withdrawal, increased pain, and opioid cravings. This increases patients’ risk for overdose after discharge and precludes them from receiving life-saving, evidence-based methadone maintenance, or buprenorphine treatment. Though widely misunderstood, prescribing methadone in the hospital is legal, and providers need no special waiver to prescribe buprenorphine during admission. Current laws require that hospitalists have a waiver to prescribe buprenorphine at discharge and prohibit hospitalists (or anyone outside of an opioid treatment program) from prescribing methadone for the treatment of opioid use disorder at discharge. Further, hospitalists should offer medication for alcohol use disorder (eg, naltrexone) and be good stewards of opioids during hospitalization, avoiding intravenous opioids where appropriate and curbing excessive prescribing at discharge. Given high rates of overdose and fentanyl contamination of stimulants, opioids, and benzodiazepines, hospitalists should prescribe naloxone at discharge to every patient with SUD, on chronic opioids, or who uses any nonmedical substances.
Resources exist for individual hospitalists seeking mentorship or additional training (Table). Though not necessary for in-hospital prescribing, hospitalists can obtain a waiver to prescribe buprenorphine at discharge (commonly called the X-waiver). To qualify, physicians must complete eight hours of accredited training (online and/or in-person), after which they must request a waiver from the Drug Enforcement Administration. Advanced-practice practitioners must complete 24 hours of training. Many have argued that policymakers should end this waiver requirement.26 While we support efforts to “X the X” and urgently expand treatment access, additional training can enrich providers’ knowledge and confidence to prescribe buprenorphine, and is a relatively simple way that all hospitalists could act. Finally, by treating addiction and modeling patient-centered addictions care, hospitalists can legitimize and destigmatize the disease of addiction,8 and have the potential to mentor and train students, residents, nurses, and other staff.27
Hospitalist Leaders
As leaders, hospitalists can play a key role in promoting hospital-based addictions care and tailoring solutions to meet local needs. Leaders can promote a cultural shift away from stigma, and promote evidence-based, life-saving care. Hospitalist leaders could require all hospitalists to obtain buprenorphine waivers. Leaders could initiate quality improvement projects related to SUD service delivery, develop policies that support inpatient SUD treatment, develop order sets for medication initiation, engage community substance use treatment partners, build pathways to timely addiction care after discharge, and champion development of addiction medicine consult services.
Hospitalist leaders can reference open-source guidelines, order sets, assessment and treatment tools, patient materials, pharmacy and therapeutics committee materials, and other resources for implementing services for hospitalized patients with SUD (Table).21,22 Hospitalist leaders who understand financial and quality drivers can also champion the business and quality case for hospital-based addictions care, and help pursue local and national funding opportunities.
Hospitalist Organizations
Hospitalist societies could provide training at regional and national conferences to upskill hospitalists to care for people with SUD; support addiction medicine interest groups; and partner with addiction medicine societies, harm reduction organizations, and organizations focused on trauma-informed care. They could endorse practice guidelines and position statements describing the crucial role of hospitalists in addressing the overdose crisis and offering medication for addiction (Table). Hospitalist organizations can engage national and state hospital associations, lobby medical specialties to include addiction medicine competencies in board certification requirements, and advocate with governmental leaders to reduce barriers that restrict treatment access such as the X-waiver.
MOVING FORWARD
Regardless of whether a hospitalist is serving as an individual provider, a hospitalist leader, or as part of a hospitalist organization, hospitalists can take critical steps to advance the care of people with SUD. These steps shift the culture of hospitals from one where patients are afraid to discuss their substance use, to one that creates space for connection, treatment engagement, and healing. By starting medications, utilizing widely accessible resources, and collaborating with community treatment and harm reduction organizations, each one of us can play a part in addressing the epidemic.
Acknowledgments
The authors thank Alisa Patten for help preparing this manuscript. Dr. Englander would like to thank Dr. David Bangsberg and Dr. Christina Nicolaidis for their mentorship.
In 2017, the death toll from drug overdoses reached a record high, killing more Americans than the entire Vietnam War or the HIV/AIDS epidemic at its peak.1 Up to one-quarter of hospitalized patients have a substance use disorder (SUD) and SUD-related2,3 hospitalizations are surging. People with SUD have longer hospital stays, higher costs, and more readmissions.3,4 While the burden of SUD is staggering, it is far from hopeless. There are multiple evidence-based and highly effective interventions to treat SUD, including medications, behavioral interventions, and harm reduction strategies.
Hospitalization can be a reachable moment to initiate and coordinate addictions care.5 Hospital-based addictions care has the potential to engage sicker, highly vulnerable patients, many who are not engaged in primary care or outpatient addictions care.6 Studied effects of hospital-based addictions care include improved SUD treatment engagement, reduced alcohol and drug use, lower hospital readmissions, and improved provider experience.7-9
Most hospitals, however, do not treat SUD during hospitalization and do not connect people to treatment after discharge. Hospitals may lack staffing or financial resources to implement addiction care, may believe that SUDs are an outpatient concern, may want to avoid caring for people with SUD, or may simply not know where to begin. Whatever the reason, unaddressed SUD can lead to untreated withdrawal, disruptive patient behaviors, failure to complete recommended medical therapy, high rates of against medical advice discharge, poor patient experience, and widespread provider distress.8
Hospitalists—individually and collectively—are uniquely positioned to address this gap. By treating addiction effectively and compassionately, hospitalists can engage patients, improve care, improve patient and provider experience, and lower costs. This paper is a call to action that describes the current state of hospital-based addictions care, outlines key challenges to implementing SUD care in the hospital, debunks common misconceptions, and identifies actionable steps for hospitalists, hospital leaders, and hospitalist organizations.
MODELS TO DELIVER HOSPITAL-BASED ADDICTIONS CARE
Hospital-based addiction medicine consult services are emerging; they include a range of models, with variations in how patients are identified, team composition, service availability, and financing.10 Existing addiction medicine consult services commonly offer SUD assessments, psychological intervention, medical management of SUDs (eg, initiating methadone or buprenorphine), medical pain management, and linkage to SUD care after hospitalization. Some services also explicitly integrate harm reduction principles (eg, naloxone distribution, safe injection education, permitting patients to smoke).11 Additional consult service activities include hospital-wide SUD education, and creation and implementation of hospital guidance documents (eg, methadone policies).10 Some consult services utilize only physicians, while others include interprofessional providers, such as nurses, social workers, and peers with lived experience of addiction. Whereas addiction medicine physicians staff some consult services, hospitalists with less formal addiction credentials staff others.
Broadly, hospital-based addictions care cannot depend solely on consult services. Just as not all hospitals have cardiology consult services, not all hospitals will have addiction consult services. As such, hospitalists can play an even greater role by implementing order sets and guidelines, supporting partnerships with community SUD treatment, and independently initiating evidence-based medications.
CHALLENGES TO ADOPTION AND IMPLEMENTATION OF HOSPITAL-BASED ADDICTIONS CARE
Pervasive individual and structural stigmas12 are perhaps the most critical barriers to incorporating addiction medicine into routine hospital practice, and they are both cause and consequence of our system failures. Most medical schools and residencies lack SUD training, which means that the understanding of addiction as a moral deficiency or lack of willpower may remain unchallenged. Stigma surrounding SUDs contributes to hospitalists’ and hospital leaders’ aversion to treating patients with SUD, and to fears that providing quality SUD care will attract patients suffering from these conditions.
Recent national efforts have focused on the problem of opioid overprescribing. Without an equal emphasis on treatment, this focus can lead to undertreatment of pain and/or opioid use disorder in hospitalized patients, particularly since most hospitalists have little to no training in diagnosing SUD, prescribing life-saving medications for opioid use disorder, or managing acute pain in patients with SUD. The focus on overprescribing also diverts attention away from trends involving stimulants,2 fentanyl contamination of the drug supply,13 and alcohol, all of which have important implications for the care of hospitalized adults.
Hospital policies are often not grounded in evidence (eg, recommending clonidine for first-line treatment of opioid withdrawal and not buprenorphine/methadone), and there are widespread misconceptions about perceived legal barriers to treating opioid use disorder in the hospital, which is both safe and legal.10 People with SUD may be unjustly viewed through a criminal justice lens. Policies focused on controlling visitors and conducting room searches disproportionately burden people with SUD, which may create further harms through reinforcing negative provider cognitive biases about SUDs. Finally, hospitals may lack inpatient social work and pharmacy supports, and they rarely have pathways to connect people to SUD care after discharge.
Funding remains a widespread challenge. While some hospital administrators support addiction medicine services because of the pressing medical need and public health crisis, most services depend on billing or demonstrated savings through reduced hospital days or readmissions.
A CALL TO ACTION: HOW HOSPITALISTS CAN IMPROVE ADDICTION CARE
Individual hospitalists, hospitalist leaders, and hospitalist organizations can engage by improving individual practice, driving systems change, and through advocacy and policy change (Table).
Individual Hospitalists
Providing basic addiction medicine care should be a core competency for all hospitalists, just as every hospitalist can initiate a goals-of-care conversation or prescribe insulin. For opioid use disorder, hospitalists should treat withdrawal and offer treatment initiation with opioid agonist therapy (ie, methadone, buprenorphine), which reduces mortality by over half. Commonly, hospitalized patients are subjected to harmful, nonevidence-based treatments, such as mandated rapid methadone tapers,25 which can lead to undertreated withdrawal, increased pain, and opioid cravings. This increases patients’ risk for overdose after discharge and precludes them from receiving life-saving, evidence-based methadone maintenance, or buprenorphine treatment. Though widely misunderstood, prescribing methadone in the hospital is legal, and providers need no special waiver to prescribe buprenorphine during admission. Current laws require that hospitalists have a waiver to prescribe buprenorphine at discharge and prohibit hospitalists (or anyone outside of an opioid treatment program) from prescribing methadone for the treatment of opioid use disorder at discharge. Further, hospitalists should offer medication for alcohol use disorder (eg, naltrexone) and be good stewards of opioids during hospitalization, avoiding intravenous opioids where appropriate and curbing excessive prescribing at discharge. Given high rates of overdose and fentanyl contamination of stimulants, opioids, and benzodiazepines, hospitalists should prescribe naloxone at discharge to every patient with SUD, on chronic opioids, or who uses any nonmedical substances.
Resources exist for individual hospitalists seeking mentorship or additional training (Table). Though not necessary for in-hospital prescribing, hospitalists can obtain a waiver to prescribe buprenorphine at discharge (commonly called the X-waiver). To qualify, physicians must complete eight hours of accredited training (online and/or in-person), after which they must request a waiver from the Drug Enforcement Administration. Advanced-practice practitioners must complete 24 hours of training. Many have argued that policymakers should end this waiver requirement.26 While we support efforts to “X the X” and urgently expand treatment access, additional training can enrich providers’ knowledge and confidence to prescribe buprenorphine, and is a relatively simple way that all hospitalists could act. Finally, by treating addiction and modeling patient-centered addictions care, hospitalists can legitimize and destigmatize the disease of addiction,8 and have the potential to mentor and train students, residents, nurses, and other staff.27
Hospitalist Leaders
As leaders, hospitalists can play a key role in promoting hospital-based addictions care and tailoring solutions to meet local needs. Leaders can promote a cultural shift away from stigma, and promote evidence-based, life-saving care. Hospitalist leaders could require all hospitalists to obtain buprenorphine waivers. Leaders could initiate quality improvement projects related to SUD service delivery, develop policies that support inpatient SUD treatment, develop order sets for medication initiation, engage community substance use treatment partners, build pathways to timely addiction care after discharge, and champion development of addiction medicine consult services.
Hospitalist leaders can reference open-source guidelines, order sets, assessment and treatment tools, patient materials, pharmacy and therapeutics committee materials, and other resources for implementing services for hospitalized patients with SUD (Table).21,22 Hospitalist leaders who understand financial and quality drivers can also champion the business and quality case for hospital-based addictions care, and help pursue local and national funding opportunities.
Hospitalist Organizations
Hospitalist societies could provide training at regional and national conferences to upskill hospitalists to care for people with SUD; support addiction medicine interest groups; and partner with addiction medicine societies, harm reduction organizations, and organizations focused on trauma-informed care. They could endorse practice guidelines and position statements describing the crucial role of hospitalists in addressing the overdose crisis and offering medication for addiction (Table). Hospitalist organizations can engage national and state hospital associations, lobby medical specialties to include addiction medicine competencies in board certification requirements, and advocate with governmental leaders to reduce barriers that restrict treatment access such as the X-waiver.
MOVING FORWARD
Regardless of whether a hospitalist is serving as an individual provider, a hospitalist leader, or as part of a hospitalist organization, hospitalists can take critical steps to advance the care of people with SUD. These steps shift the culture of hospitals from one where patients are afraid to discuss their substance use, to one that creates space for connection, treatment engagement, and healing. By starting medications, utilizing widely accessible resources, and collaborating with community treatment and harm reduction organizations, each one of us can play a part in addressing the epidemic.
Acknowledgments
The authors thank Alisa Patten for help preparing this manuscript. Dr. Englander would like to thank Dr. David Bangsberg and Dr. Christina Nicolaidis for their mentorship.
1. Weiss A, Elixhauser A, Barrett M, Steiner C, Bailey M, O’Malley L. Opioid-related inpatient stays and emergency department visits by state, 2009-2014. Statistical Brief #219. Healthcare Cost and Utilization Project. 2016. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb219-Opioid-Hospital-Stays-ED-Visits-by-State.jsp. Accessed May 21, 2019.
2. Winkelman TA, Admon LK, Jennings L, Shippee ND, Richardson CR, Bart G. Evaluation of amphetamine-related hospitalizations and associated clinical outcomes and costs in the United States. JAMA Netw Open. 2018;1(6):e183758. https://doi.org/10.1001/jamanetworkopen.2018.3758.
3. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-837. https://doi.org/10.1377/hlthaff.2015.1424.
4. Walley AY, Paasche-Orlow M, Lee EC, et al. Acute care hospital utilization among medical inpatients discharged with a substance use disorder diagnosis. J Addict Med. 2012;6(1):50-56. https://doi.org/10.1097/ADM.0b013e318231de51.
5. Englander H, Weimer M, Solotaroff R, et al. Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12(5):339-342. https://doi.org/10.12788/jhm.2736.
6. Velez C, Nicolaidis C, Korthuis P, Englander H. “It’s been an experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. doi 10.1007/s11606-016-3919-4.
7. Wakeman SE, Metlay JP, Chang Y, Herman GE, Rigotti NA. Inpatient addiction consultation for hospitalized patients increases post-discharge abstinence and reduces addiction severity. J Gen Intern Med. 2017;32(8):909-916. https://doi.org/10.1007/s11606-017-4077-z.
8. Englander H, Collins D, Perry SP, Rabinowitz M, Phoutrides E, Nicolaidis C. “We’ve learned it’s a medical illness, not a moral choice”: qualitative study of the effects of a multicomponent addiction intervention on hospital providers’ attitudes and experiences. J Hosp Med. 2018;13(11):752-758. https://doi.org/10.12788/jhm.2993.
9. McQueen J, Howe TE, Allan L, Mains D, Hardy V. Brief interventions for heavy alcohol users admitted to general hospital wards. Cochrane Database Syst Rev. 2011;10(8):CD005191 https://doi.org/10.1002/14651858.CD005191.pub3.
10. Priest KC, McCarty D. Role of the hospital in the 21st century opioid overdose epidemic: the addiction medicine consult service. J Addict Med. 2019;13(2):104-112. https://doi.org/10.1097/ADM.0000000000000496.
11. Weinstein ZM, Wakeman SE, Nolan S. Inpatient addiction consult service: expertise for hospitalized patients with complex addiction problems. Med Clin North Am. 2018;102(4):587-601. https://doi.org/10.1016/j.mcna.2018.03.001.
12. McNeil R, Small W, Wood E, Kerr T. Hospitals as a “risk environment”: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66. https://doi.org/10.1016/j.socscimed.2014.01.010.
13. Ciccarone D. The triple wave epidemic: supply and demand drivers of the US opioid overdose crisis. Int J Drug Policy. 2019. pii: S0955-3959(19)30018-0. [Epub ahead of print]. https://doi.org/10.1016/j.drugpo.2019.01.010.
14. Substance Abuse and Mental Health Services Administration. TIP 63: Medications for Opioid Use Disorder-Executive Summary. February 2018. https://store.samhsa.gov/product/TIP-63-Medications-for-Opioid-Use-Disorder-Executive-Summary/sma18-5063exsumm. Accessed August 8, 2019.
15. Providers Clinical Support System. Discover the rewards of treating patients with Opioid Use Disorders. https://pcssnow.org/. Accessed August 8, 2019.
16. California Bridge Program. Treatment Starts Here: Resources for the Treatment of Substance Use Disorders from the Acute Care Setting. https://www.bridgetotreatment.org/resources. Accessed August 7, 2019.
17. Clinical Consultation Center. Substance Use Resources. 2019. https://nccc.ucsf.edu/clinical-resources/substance-use-resources/. Accessed August 8, 2019.
18. Thakarar K, Weinstein ZM, Walley AY. Optimising health and safety of people who inject drugs during transition from acute to outpatient care: narrative review with clinical checklist. Postgrad Med J. 2016;92(1088):356-363. https://doi.org/10.1136/postgradmedj-2015-133720.
19. Office of National Drug Control Policy. Changing the Language of Addiction. Washington, D.C. 2017. https://www.whitehouse.gov/sites/whitehouse.gov/files/images/Memo%20-%20Changing%20Federal%20Terminology%20Regrading%20Substance%20Use%20and%20Substance%20Use%20Disorders.pdf. Accessed August 8, 2019.
20. The University of New Mexico. Project ECHO: A Revolution in Medical Education and Care Delivery. 2019. https://echo.unm.edu/. Accessed August 8, 2019.
21. Englander H, Mahoney S, Brandt K, et al. Tools to support hospital-based addiction care: core components, values, and activities of the Improving Addiction Care Team. J Addict Med. 2019;13(2):85-89. https://doi.org/10.1097/ADM.0000000000000487.
22. Englander H, Gregg J, Gollickson J, et al. Recommendations for intergrating peer mentors in hospital-based addiction care. Subst Abus. In press. https://doi.org/10.1080/08897077.2019.1635968.
23. American College of Medical Toxicology. ACMT Position Statement: Buprenorphine Administration in the Emergency Department. https://www.acep.org/globalassets/sites/acep/media/equal-documents/policy_acmt_bupeadministration.pdf. Accessed May 21, 2019.
24. Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: a consensus statement from the society of hospital medicine. J Hosp Med. 2018;13(4):263-271. https://doi.org/10.12788/jhm.2980.
25. Winetsky D, Weinrieb RM, Perrone J. Expanding treatment opportunities for hospitalized patients with opioid use disorders. J Hosp Med. 2018;13(1):62-64. https://doi.org/10.12788/jhm.2861.
26. Frank JW, Wakeman SE, Gordon AJ. No end to the crisis without an end to the waiver. Subst Abus. 2018;39(3):263-265. https://doi.org/10.1080/08897077.2018.1543382.
27. Gorfinkel L, Klimas J, Reel B, et al. In-hospital training in addiction medicine: a mixed-methods study of health care provider benefits and differences. Subst Abus. 2019. In press. https://doi.org/10.1080/08897077.2018.1561596.
1. Weiss A, Elixhauser A, Barrett M, Steiner C, Bailey M, O’Malley L. Opioid-related inpatient stays and emergency department visits by state, 2009-2014. Statistical Brief #219. Healthcare Cost and Utilization Project. 2016. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb219-Opioid-Hospital-Stays-ED-Visits-by-State.jsp. Accessed May 21, 2019.
2. Winkelman TA, Admon LK, Jennings L, Shippee ND, Richardson CR, Bart G. Evaluation of amphetamine-related hospitalizations and associated clinical outcomes and costs in the United States. JAMA Netw Open. 2018;1(6):e183758. https://doi.org/10.1001/jamanetworkopen.2018.3758.
3. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-837. https://doi.org/10.1377/hlthaff.2015.1424.
4. Walley AY, Paasche-Orlow M, Lee EC, et al. Acute care hospital utilization among medical inpatients discharged with a substance use disorder diagnosis. J Addict Med. 2012;6(1):50-56. https://doi.org/10.1097/ADM.0b013e318231de51.
5. Englander H, Weimer M, Solotaroff R, et al. Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12(5):339-342. https://doi.org/10.12788/jhm.2736.
6. Velez C, Nicolaidis C, Korthuis P, Englander H. “It’s been an experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. doi 10.1007/s11606-016-3919-4.
7. Wakeman SE, Metlay JP, Chang Y, Herman GE, Rigotti NA. Inpatient addiction consultation for hospitalized patients increases post-discharge abstinence and reduces addiction severity. J Gen Intern Med. 2017;32(8):909-916. https://doi.org/10.1007/s11606-017-4077-z.
8. Englander H, Collins D, Perry SP, Rabinowitz M, Phoutrides E, Nicolaidis C. “We’ve learned it’s a medical illness, not a moral choice”: qualitative study of the effects of a multicomponent addiction intervention on hospital providers’ attitudes and experiences. J Hosp Med. 2018;13(11):752-758. https://doi.org/10.12788/jhm.2993.
9. McQueen J, Howe TE, Allan L, Mains D, Hardy V. Brief interventions for heavy alcohol users admitted to general hospital wards. Cochrane Database Syst Rev. 2011;10(8):CD005191 https://doi.org/10.1002/14651858.CD005191.pub3.
10. Priest KC, McCarty D. Role of the hospital in the 21st century opioid overdose epidemic: the addiction medicine consult service. J Addict Med. 2019;13(2):104-112. https://doi.org/10.1097/ADM.0000000000000496.
11. Weinstein ZM, Wakeman SE, Nolan S. Inpatient addiction consult service: expertise for hospitalized patients with complex addiction problems. Med Clin North Am. 2018;102(4):587-601. https://doi.org/10.1016/j.mcna.2018.03.001.
12. McNeil R, Small W, Wood E, Kerr T. Hospitals as a “risk environment”: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66. https://doi.org/10.1016/j.socscimed.2014.01.010.
13. Ciccarone D. The triple wave epidemic: supply and demand drivers of the US opioid overdose crisis. Int J Drug Policy. 2019. pii: S0955-3959(19)30018-0. [Epub ahead of print]. https://doi.org/10.1016/j.drugpo.2019.01.010.
14. Substance Abuse and Mental Health Services Administration. TIP 63: Medications for Opioid Use Disorder-Executive Summary. February 2018. https://store.samhsa.gov/product/TIP-63-Medications-for-Opioid-Use-Disorder-Executive-Summary/sma18-5063exsumm. Accessed August 8, 2019.
15. Providers Clinical Support System. Discover the rewards of treating patients with Opioid Use Disorders. https://pcssnow.org/. Accessed August 8, 2019.
16. California Bridge Program. Treatment Starts Here: Resources for the Treatment of Substance Use Disorders from the Acute Care Setting. https://www.bridgetotreatment.org/resources. Accessed August 7, 2019.
17. Clinical Consultation Center. Substance Use Resources. 2019. https://nccc.ucsf.edu/clinical-resources/substance-use-resources/. Accessed August 8, 2019.
18. Thakarar K, Weinstein ZM, Walley AY. Optimising health and safety of people who inject drugs during transition from acute to outpatient care: narrative review with clinical checklist. Postgrad Med J. 2016;92(1088):356-363. https://doi.org/10.1136/postgradmedj-2015-133720.
19. Office of National Drug Control Policy. Changing the Language of Addiction. Washington, D.C. 2017. https://www.whitehouse.gov/sites/whitehouse.gov/files/images/Memo%20-%20Changing%20Federal%20Terminology%20Regrading%20Substance%20Use%20and%20Substance%20Use%20Disorders.pdf. Accessed August 8, 2019.
20. The University of New Mexico. Project ECHO: A Revolution in Medical Education and Care Delivery. 2019. https://echo.unm.edu/. Accessed August 8, 2019.
21. Englander H, Mahoney S, Brandt K, et al. Tools to support hospital-based addiction care: core components, values, and activities of the Improving Addiction Care Team. J Addict Med. 2019;13(2):85-89. https://doi.org/10.1097/ADM.0000000000000487.
22. Englander H, Gregg J, Gollickson J, et al. Recommendations for intergrating peer mentors in hospital-based addiction care. Subst Abus. In press. https://doi.org/10.1080/08897077.2019.1635968.
23. American College of Medical Toxicology. ACMT Position Statement: Buprenorphine Administration in the Emergency Department. https://www.acep.org/globalassets/sites/acep/media/equal-documents/policy_acmt_bupeadministration.pdf. Accessed May 21, 2019.
24. Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: a consensus statement from the society of hospital medicine. J Hosp Med. 2018;13(4):263-271. https://doi.org/10.12788/jhm.2980.
25. Winetsky D, Weinrieb RM, Perrone J. Expanding treatment opportunities for hospitalized patients with opioid use disorders. J Hosp Med. 2018;13(1):62-64. https://doi.org/10.12788/jhm.2861.
26. Frank JW, Wakeman SE, Gordon AJ. No end to the crisis without an end to the waiver. Subst Abus. 2018;39(3):263-265. https://doi.org/10.1080/08897077.2018.1543382.
27. Gorfinkel L, Klimas J, Reel B, et al. In-hospital training in addiction medicine: a mixed-methods study of health care provider benefits and differences. Subst Abus. 2019. In press. https://doi.org/10.1080/08897077.2018.1561596.
© 2020 Society of Hospital Medicine
Turning Your Passion into Action: Becoming a Physician Advocate
I stand in the hospital room of a little girl who was shot in her own home just two weeks ago. She was drawing in her sketchbook when a group of teenagers drove by her apartment and took aim. She was shot twice in the chest. Her life and her health will forever be altered. I am not part of her care team, but I am there because just hours after their arrival to the hospital her mother declared that she was going to do something, that gun violence must be stopped. She wants to speak out and she wants to give her daughter a voice. She does not want this to happen to other little girls. My colleagues know that I can help this woman by elevating her voice, by telling her daughter’s story. I have found a passion in gun violence prevention advocacy and I fight every day for little girls like this.
For almost 10 years, I studied asthma. I presented lectures. I conducted research. I published papers. It was my thing. In fact, it still is my thing. But one day shortly after the shooting at Marjory Stoneman Douglas High School in Parkland, Florida, I was dropping my oldest daughter off at Kindergarten and for the first time, I saw an armed police officer patrolling the drop-off line. It hit me like a ton of bricks. I went home and called my Senators and Representatives. As I was talking to an aide about evidence-based gun safety legislation, I lost it. I started crying. I finished the call and just sat there. I was momentarily frozen, uncertain of what to do next yet compelled to take action. I decided to attend a meeting of a local gun violence prevention group. Maybe this action of going to one meeting would quell the anxiety and fear that was building inside of me. I found my local Moms Demand Action chapter and I went. About halfway through the meeting, the chapter leader began describing their gun safety campaign, Be SMART for kids, and mentioned that they had been trying to make connections with the Children’s Hospital. That is the moment. That is when it clicked. I have a voice that this movement needs. I can help them. And I did.
Gun violence is the second leading cause of death in children.1 Gun violence is a public health epidemic. Every day in America, approximately 100 people are shot and killed.2 The rate of firearm deaths among children and teens in the United States is 36.5 times higher than that of 12 other high-income countries.1 We know that states with stricter gun laws have lower rates of child firearm mortality.3 We also know that safe gun storage practices (storing guns locked, unloaded, and separate from ammunition) reduce the risk of suicide and firearm injuries,4 yet 4.6 million American children live in a home with a loaded, unlocked firearm.5 Promoting safe gun storage practices and advocating for common sense gun safety legislation are two effective ways to address this crisis.
Gun violence prevention is my passion, but it might not be yours. Regardless of your passion, the blueprint for becoming a physician advocate is the same.
WHY DO PHYSICIANS MAKE NATURAL, EFFECTIVE ADVOCATES?
Advocacy, in its most distilled form, is speaking out for something you believe in, often for someone who cannot speak out for themselves. This is at the core of what we, as healthcare providers, do every day. We help people through some of the hardest moments of their lives, when they are sick and vulnerable. Every day, we are faced with problems that need to be solved. Our experience at the bedside helps us understand how policies affect real people. We understand evidence, data, and science. We recognize that anecdotes are powerful but if not backed up with data will be unlikely to lead to meaningful change. Perhaps most importantly, as professional members of the community, we have agency. We can use our voice and our privilege as physicians to elevate the voices of others.
As you go through medical training, you may not even realize that what you are doing on a daily basis is advocacy. But there comes a moment when you realize that the problem is bigger than the individual patient in front of you. There are systems that are broken that, if fixed, could improve the health of patients everywhere and save lives. To create change on a population level, the status quo will need to be challenged and systems may need to be disrupted.
Hospitalists are particularly well positioned to be advocates because we interact with virtually all aspects of the healthcare system either directly or indirectly. We care for patients with a myriad of disease processes and medical needs using varying levels of resources and social support systems. We often see patients in their most dire moments and, unlike outpatient physicians, we have the luxury of time. Hospitalized patients are a captive audience. We have time to educate, assess what patients need, and connect patients with community resources.
HOW TO BECOME A PHYSICIAN ADVOCATE
Find your passion. Often, your passion will find you. When it does, listen to it. Initially, most of your advocacy will be done on your own time. If you are not passionate about your cause, you will struggle and you will be less likely to be an effective advocate. Keep in mind that sometimes the deeper you dig into an issue, the bigger problems you find and, as a result, your passion can grow.
Do your research. Read the literature. Do you really understand the issue? Identify local and national experts, read their work, and follow their careers. You do not need an advanced degree. Your experience as a physician, willingness to learn, and your voice are all you need.
Start small. Do something small every day. Read an article. Make a new contact. Talk to a colleague. Be thoughtful in your approach. Is this a problem that community advocacy can solve? Will legislation be an effective way to achieve my goal? Would state or federal legislation be more appropriate? In most cases, a combination of community advocacy and legislative advocacy is necessary.
Partner with community organizations. Find local organizations that have existing infrastructure and are engaged on the issue and create partnerships. Community organizations are fighting every day and are waiting for a powerful authoritative voice like yours. They want your voice and you need their support.
Find your allies and your challengers. Identify allies in your community, your institution, your field, and in government. Anticipate potential challengers. When you encounter them, work diligently to find common ground and be respectful. If you only talk to people who agree with you, you will not make progress. Tread carefully when necessary. Develop a thick skin. Read people and try to figure out what it is that they want, what is motivating their position. Make your first ask small and as noncontroversial as possible. Stick to the facts. If you keep your patients at the heart of what you are doing, it is hard to go wrong.
Stay focused and disciplined, but do not quiet the anger and frustration that you feel. That is your fuel. Build momentum and build your team. Passion is contagious; when people see that you are making progress, they will want to join you. Together, you can create a dialog that will change minds.
Align advocacy with your other work. Ideally, this work will not be done in isolation from your other professional duties. Advocacy initiatives make excellent quality improvement projects. When you identify holes in the evidence that could potentially inform the policy debate, apply health services research methods and publish. This approach builds the evidence base to affect change and contributes to your professional development. Consider developing an advocacy curriculum for trainees. Identify trainees interested in advocacy and mentor them. Look for opportunities to speak and write on the topic. Use your unique skillset to further your cause.
Work with your employer. Find common ground. Even if they fundamentally disagree with your point of view, you can still speak out as a private citizen. Recognize the difference between speaking as a physician and speaking as an employee of a specific institution. Unless you have explicit permission, you are speaking for yourself, not your institution. Do not be afraid to push leaders at your institution. Help them see why it is important for you to speak up on a particular issue. If your professional organization has a statement on the issue, use it to support your position.
Leverage social media. Social media is a powerful method to amplify your voice. Consider the impact of the #thisisourlane movement. It will connect you with people, across the world, who share similar passions. It will help you identify local allies. It will open opportunities for speaking engagements and publications. It can be a great way to bring positive attention to your institution. It will take time to find your voice. Try to use consistent messaging. Keep it professional. Tag people who you want to see the great work you are doing. It only takes one retweet by someone with hundreds of thousands of followers to get your message in the feed of exponentially more viewers. Tag your institution when you want them to know what you are up to or when you are doing something that you think they should be proud of. Tag the professional organizations that would be interested in your work. Tag community leaders. This can be a great way to elevate their voice with your platform. Include an “opinions my own” statement in your social media profiles. Beware of disinformation. Read articles before retweeting. Ignore the trolls. I repeat, ignore the trolls.
CONCLUSION
I did not start my career with a focus on advocacy and in becoming an advocate, I have not given up my previous focus on asthma research. I did not get an advanced degree or specialized training in advocacy. I let my passion drive me. I am now an active member and leader in our Moms Demand Action chapter. The safe storage campaign in our resident clinic has had significant success. We increased the frequency of discussion of gun safety during well-child visits from 2% to 50% and shared this success at local and national scientific meetings. We have worked with our local media to spread awareness about safe gun storage. We have spent time at the state capital to discuss child access prevention laws with legislators. We have collaborated with community leaders and elected officials for gun violence awareness events. We earned support from leaders at our institution. If you walk through our hospital units, clinics, resident areas, and faculty offices, you will see evidence of our success. Physicians and nurses are still wearing their ribbons from the Wear Orange day on their name badges. “We Can End Gun Violence” signs are hanging on faculty members’ doors. Thanks to local police departments, the clinic has a constant supply of gun locks that are provided to families free of charge. Our residents proudly walk the halls with Be SMART buttons on their badges. These physical reminders of our progress are incredibly motivating as we continue this work. However, it is the quiet moments alone with children and parents who are suffering because of the epidemic of gun violence that really move me. I will not give up this fight until children in our communities are safe.
Acknowledgments
Dr. Andrews wishes to thank Dr. Kelsey Gastineau for her efforts to increase the frequency of gun safety discussions in our Pediatric Primary Care clinic and for her support in all of this work.
1. Cunningham RM, Walton MA, Carter PM. The major causes of death in children and adolescents in the United States. N Engl J Med. 2018;379(25):2468-2475. https://doi.org/10.1056/NEJMsr1804754.
2. Prevention CfDCa. National centers for injury prevention and control, web-based injury statistics query and reporting system (WISQARS) Fatal Injury Reports. 2013-2017.
3. Goyal MK, Badolato GM, Patel SJ, Iqbal SF, Parikh K, McCarter R. State gun laws and pediatric firearm-related mortality. Pediatrics. 2019;144(2). https://doi.org/10.1542/peds.2018-3283
4. Grossman DC, Mueller BA , Riedy C, Dowd MD, Villaveces A, Prodzinski J, et al. Gun storage practices and risk of youth suicide and unintentional firearm injuries. JAMA. 2005;293(6):707-714. https://doi.org/10.1001/jama.293.6.707.
5. Azrael D, Cohen J, Salhi C, Miller M. Firearm storage in gun-owning households with children: results of a 2015 national survey. J Urban Health. 2018;95(3):295-304. https://doi.org/10.1007/s11524-018-0261-7
I stand in the hospital room of a little girl who was shot in her own home just two weeks ago. She was drawing in her sketchbook when a group of teenagers drove by her apartment and took aim. She was shot twice in the chest. Her life and her health will forever be altered. I am not part of her care team, but I am there because just hours after their arrival to the hospital her mother declared that she was going to do something, that gun violence must be stopped. She wants to speak out and she wants to give her daughter a voice. She does not want this to happen to other little girls. My colleagues know that I can help this woman by elevating her voice, by telling her daughter’s story. I have found a passion in gun violence prevention advocacy and I fight every day for little girls like this.
For almost 10 years, I studied asthma. I presented lectures. I conducted research. I published papers. It was my thing. In fact, it still is my thing. But one day shortly after the shooting at Marjory Stoneman Douglas High School in Parkland, Florida, I was dropping my oldest daughter off at Kindergarten and for the first time, I saw an armed police officer patrolling the drop-off line. It hit me like a ton of bricks. I went home and called my Senators and Representatives. As I was talking to an aide about evidence-based gun safety legislation, I lost it. I started crying. I finished the call and just sat there. I was momentarily frozen, uncertain of what to do next yet compelled to take action. I decided to attend a meeting of a local gun violence prevention group. Maybe this action of going to one meeting would quell the anxiety and fear that was building inside of me. I found my local Moms Demand Action chapter and I went. About halfway through the meeting, the chapter leader began describing their gun safety campaign, Be SMART for kids, and mentioned that they had been trying to make connections with the Children’s Hospital. That is the moment. That is when it clicked. I have a voice that this movement needs. I can help them. And I did.
Gun violence is the second leading cause of death in children.1 Gun violence is a public health epidemic. Every day in America, approximately 100 people are shot and killed.2 The rate of firearm deaths among children and teens in the United States is 36.5 times higher than that of 12 other high-income countries.1 We know that states with stricter gun laws have lower rates of child firearm mortality.3 We also know that safe gun storage practices (storing guns locked, unloaded, and separate from ammunition) reduce the risk of suicide and firearm injuries,4 yet 4.6 million American children live in a home with a loaded, unlocked firearm.5 Promoting safe gun storage practices and advocating for common sense gun safety legislation are two effective ways to address this crisis.
Gun violence prevention is my passion, but it might not be yours. Regardless of your passion, the blueprint for becoming a physician advocate is the same.
WHY DO PHYSICIANS MAKE NATURAL, EFFECTIVE ADVOCATES?
Advocacy, in its most distilled form, is speaking out for something you believe in, often for someone who cannot speak out for themselves. This is at the core of what we, as healthcare providers, do every day. We help people through some of the hardest moments of their lives, when they are sick and vulnerable. Every day, we are faced with problems that need to be solved. Our experience at the bedside helps us understand how policies affect real people. We understand evidence, data, and science. We recognize that anecdotes are powerful but if not backed up with data will be unlikely to lead to meaningful change. Perhaps most importantly, as professional members of the community, we have agency. We can use our voice and our privilege as physicians to elevate the voices of others.
As you go through medical training, you may not even realize that what you are doing on a daily basis is advocacy. But there comes a moment when you realize that the problem is bigger than the individual patient in front of you. There are systems that are broken that, if fixed, could improve the health of patients everywhere and save lives. To create change on a population level, the status quo will need to be challenged and systems may need to be disrupted.
Hospitalists are particularly well positioned to be advocates because we interact with virtually all aspects of the healthcare system either directly or indirectly. We care for patients with a myriad of disease processes and medical needs using varying levels of resources and social support systems. We often see patients in their most dire moments and, unlike outpatient physicians, we have the luxury of time. Hospitalized patients are a captive audience. We have time to educate, assess what patients need, and connect patients with community resources.
HOW TO BECOME A PHYSICIAN ADVOCATE
Find your passion. Often, your passion will find you. When it does, listen to it. Initially, most of your advocacy will be done on your own time. If you are not passionate about your cause, you will struggle and you will be less likely to be an effective advocate. Keep in mind that sometimes the deeper you dig into an issue, the bigger problems you find and, as a result, your passion can grow.
Do your research. Read the literature. Do you really understand the issue? Identify local and national experts, read their work, and follow their careers. You do not need an advanced degree. Your experience as a physician, willingness to learn, and your voice are all you need.
Start small. Do something small every day. Read an article. Make a new contact. Talk to a colleague. Be thoughtful in your approach. Is this a problem that community advocacy can solve? Will legislation be an effective way to achieve my goal? Would state or federal legislation be more appropriate? In most cases, a combination of community advocacy and legislative advocacy is necessary.
Partner with community organizations. Find local organizations that have existing infrastructure and are engaged on the issue and create partnerships. Community organizations are fighting every day and are waiting for a powerful authoritative voice like yours. They want your voice and you need their support.
Find your allies and your challengers. Identify allies in your community, your institution, your field, and in government. Anticipate potential challengers. When you encounter them, work diligently to find common ground and be respectful. If you only talk to people who agree with you, you will not make progress. Tread carefully when necessary. Develop a thick skin. Read people and try to figure out what it is that they want, what is motivating their position. Make your first ask small and as noncontroversial as possible. Stick to the facts. If you keep your patients at the heart of what you are doing, it is hard to go wrong.
Stay focused and disciplined, but do not quiet the anger and frustration that you feel. That is your fuel. Build momentum and build your team. Passion is contagious; when people see that you are making progress, they will want to join you. Together, you can create a dialog that will change minds.
Align advocacy with your other work. Ideally, this work will not be done in isolation from your other professional duties. Advocacy initiatives make excellent quality improvement projects. When you identify holes in the evidence that could potentially inform the policy debate, apply health services research methods and publish. This approach builds the evidence base to affect change and contributes to your professional development. Consider developing an advocacy curriculum for trainees. Identify trainees interested in advocacy and mentor them. Look for opportunities to speak and write on the topic. Use your unique skillset to further your cause.
Work with your employer. Find common ground. Even if they fundamentally disagree with your point of view, you can still speak out as a private citizen. Recognize the difference between speaking as a physician and speaking as an employee of a specific institution. Unless you have explicit permission, you are speaking for yourself, not your institution. Do not be afraid to push leaders at your institution. Help them see why it is important for you to speak up on a particular issue. If your professional organization has a statement on the issue, use it to support your position.
Leverage social media. Social media is a powerful method to amplify your voice. Consider the impact of the #thisisourlane movement. It will connect you with people, across the world, who share similar passions. It will help you identify local allies. It will open opportunities for speaking engagements and publications. It can be a great way to bring positive attention to your institution. It will take time to find your voice. Try to use consistent messaging. Keep it professional. Tag people who you want to see the great work you are doing. It only takes one retweet by someone with hundreds of thousands of followers to get your message in the feed of exponentially more viewers. Tag your institution when you want them to know what you are up to or when you are doing something that you think they should be proud of. Tag the professional organizations that would be interested in your work. Tag community leaders. This can be a great way to elevate their voice with your platform. Include an “opinions my own” statement in your social media profiles. Beware of disinformation. Read articles before retweeting. Ignore the trolls. I repeat, ignore the trolls.
CONCLUSION
I did not start my career with a focus on advocacy and in becoming an advocate, I have not given up my previous focus on asthma research. I did not get an advanced degree or specialized training in advocacy. I let my passion drive me. I am now an active member and leader in our Moms Demand Action chapter. The safe storage campaign in our resident clinic has had significant success. We increased the frequency of discussion of gun safety during well-child visits from 2% to 50% and shared this success at local and national scientific meetings. We have worked with our local media to spread awareness about safe gun storage. We have spent time at the state capital to discuss child access prevention laws with legislators. We have collaborated with community leaders and elected officials for gun violence awareness events. We earned support from leaders at our institution. If you walk through our hospital units, clinics, resident areas, and faculty offices, you will see evidence of our success. Physicians and nurses are still wearing their ribbons from the Wear Orange day on their name badges. “We Can End Gun Violence” signs are hanging on faculty members’ doors. Thanks to local police departments, the clinic has a constant supply of gun locks that are provided to families free of charge. Our residents proudly walk the halls with Be SMART buttons on their badges. These physical reminders of our progress are incredibly motivating as we continue this work. However, it is the quiet moments alone with children and parents who are suffering because of the epidemic of gun violence that really move me. I will not give up this fight until children in our communities are safe.
Acknowledgments
Dr. Andrews wishes to thank Dr. Kelsey Gastineau for her efforts to increase the frequency of gun safety discussions in our Pediatric Primary Care clinic and for her support in all of this work.
I stand in the hospital room of a little girl who was shot in her own home just two weeks ago. She was drawing in her sketchbook when a group of teenagers drove by her apartment and took aim. She was shot twice in the chest. Her life and her health will forever be altered. I am not part of her care team, but I am there because just hours after their arrival to the hospital her mother declared that she was going to do something, that gun violence must be stopped. She wants to speak out and she wants to give her daughter a voice. She does not want this to happen to other little girls. My colleagues know that I can help this woman by elevating her voice, by telling her daughter’s story. I have found a passion in gun violence prevention advocacy and I fight every day for little girls like this.
For almost 10 years, I studied asthma. I presented lectures. I conducted research. I published papers. It was my thing. In fact, it still is my thing. But one day shortly after the shooting at Marjory Stoneman Douglas High School in Parkland, Florida, I was dropping my oldest daughter off at Kindergarten and for the first time, I saw an armed police officer patrolling the drop-off line. It hit me like a ton of bricks. I went home and called my Senators and Representatives. As I was talking to an aide about evidence-based gun safety legislation, I lost it. I started crying. I finished the call and just sat there. I was momentarily frozen, uncertain of what to do next yet compelled to take action. I decided to attend a meeting of a local gun violence prevention group. Maybe this action of going to one meeting would quell the anxiety and fear that was building inside of me. I found my local Moms Demand Action chapter and I went. About halfway through the meeting, the chapter leader began describing their gun safety campaign, Be SMART for kids, and mentioned that they had been trying to make connections with the Children’s Hospital. That is the moment. That is when it clicked. I have a voice that this movement needs. I can help them. And I did.
Gun violence is the second leading cause of death in children.1 Gun violence is a public health epidemic. Every day in America, approximately 100 people are shot and killed.2 The rate of firearm deaths among children and teens in the United States is 36.5 times higher than that of 12 other high-income countries.1 We know that states with stricter gun laws have lower rates of child firearm mortality.3 We also know that safe gun storage practices (storing guns locked, unloaded, and separate from ammunition) reduce the risk of suicide and firearm injuries,4 yet 4.6 million American children live in a home with a loaded, unlocked firearm.5 Promoting safe gun storage practices and advocating for common sense gun safety legislation are two effective ways to address this crisis.
Gun violence prevention is my passion, but it might not be yours. Regardless of your passion, the blueprint for becoming a physician advocate is the same.
WHY DO PHYSICIANS MAKE NATURAL, EFFECTIVE ADVOCATES?
Advocacy, in its most distilled form, is speaking out for something you believe in, often for someone who cannot speak out for themselves. This is at the core of what we, as healthcare providers, do every day. We help people through some of the hardest moments of their lives, when they are sick and vulnerable. Every day, we are faced with problems that need to be solved. Our experience at the bedside helps us understand how policies affect real people. We understand evidence, data, and science. We recognize that anecdotes are powerful but if not backed up with data will be unlikely to lead to meaningful change. Perhaps most importantly, as professional members of the community, we have agency. We can use our voice and our privilege as physicians to elevate the voices of others.
As you go through medical training, you may not even realize that what you are doing on a daily basis is advocacy. But there comes a moment when you realize that the problem is bigger than the individual patient in front of you. There are systems that are broken that, if fixed, could improve the health of patients everywhere and save lives. To create change on a population level, the status quo will need to be challenged and systems may need to be disrupted.
Hospitalists are particularly well positioned to be advocates because we interact with virtually all aspects of the healthcare system either directly or indirectly. We care for patients with a myriad of disease processes and medical needs using varying levels of resources and social support systems. We often see patients in their most dire moments and, unlike outpatient physicians, we have the luxury of time. Hospitalized patients are a captive audience. We have time to educate, assess what patients need, and connect patients with community resources.
HOW TO BECOME A PHYSICIAN ADVOCATE
Find your passion. Often, your passion will find you. When it does, listen to it. Initially, most of your advocacy will be done on your own time. If you are not passionate about your cause, you will struggle and you will be less likely to be an effective advocate. Keep in mind that sometimes the deeper you dig into an issue, the bigger problems you find and, as a result, your passion can grow.
Do your research. Read the literature. Do you really understand the issue? Identify local and national experts, read their work, and follow their careers. You do not need an advanced degree. Your experience as a physician, willingness to learn, and your voice are all you need.
Start small. Do something small every day. Read an article. Make a new contact. Talk to a colleague. Be thoughtful in your approach. Is this a problem that community advocacy can solve? Will legislation be an effective way to achieve my goal? Would state or federal legislation be more appropriate? In most cases, a combination of community advocacy and legislative advocacy is necessary.
Partner with community organizations. Find local organizations that have existing infrastructure and are engaged on the issue and create partnerships. Community organizations are fighting every day and are waiting for a powerful authoritative voice like yours. They want your voice and you need their support.
Find your allies and your challengers. Identify allies in your community, your institution, your field, and in government. Anticipate potential challengers. When you encounter them, work diligently to find common ground and be respectful. If you only talk to people who agree with you, you will not make progress. Tread carefully when necessary. Develop a thick skin. Read people and try to figure out what it is that they want, what is motivating their position. Make your first ask small and as noncontroversial as possible. Stick to the facts. If you keep your patients at the heart of what you are doing, it is hard to go wrong.
Stay focused and disciplined, but do not quiet the anger and frustration that you feel. That is your fuel. Build momentum and build your team. Passion is contagious; when people see that you are making progress, they will want to join you. Together, you can create a dialog that will change minds.
Align advocacy with your other work. Ideally, this work will not be done in isolation from your other professional duties. Advocacy initiatives make excellent quality improvement projects. When you identify holes in the evidence that could potentially inform the policy debate, apply health services research methods and publish. This approach builds the evidence base to affect change and contributes to your professional development. Consider developing an advocacy curriculum for trainees. Identify trainees interested in advocacy and mentor them. Look for opportunities to speak and write on the topic. Use your unique skillset to further your cause.
Work with your employer. Find common ground. Even if they fundamentally disagree with your point of view, you can still speak out as a private citizen. Recognize the difference between speaking as a physician and speaking as an employee of a specific institution. Unless you have explicit permission, you are speaking for yourself, not your institution. Do not be afraid to push leaders at your institution. Help them see why it is important for you to speak up on a particular issue. If your professional organization has a statement on the issue, use it to support your position.
Leverage social media. Social media is a powerful method to amplify your voice. Consider the impact of the #thisisourlane movement. It will connect you with people, across the world, who share similar passions. It will help you identify local allies. It will open opportunities for speaking engagements and publications. It can be a great way to bring positive attention to your institution. It will take time to find your voice. Try to use consistent messaging. Keep it professional. Tag people who you want to see the great work you are doing. It only takes one retweet by someone with hundreds of thousands of followers to get your message in the feed of exponentially more viewers. Tag your institution when you want them to know what you are up to or when you are doing something that you think they should be proud of. Tag the professional organizations that would be interested in your work. Tag community leaders. This can be a great way to elevate their voice with your platform. Include an “opinions my own” statement in your social media profiles. Beware of disinformation. Read articles before retweeting. Ignore the trolls. I repeat, ignore the trolls.
CONCLUSION
I did not start my career with a focus on advocacy and in becoming an advocate, I have not given up my previous focus on asthma research. I did not get an advanced degree or specialized training in advocacy. I let my passion drive me. I am now an active member and leader in our Moms Demand Action chapter. The safe storage campaign in our resident clinic has had significant success. We increased the frequency of discussion of gun safety during well-child visits from 2% to 50% and shared this success at local and national scientific meetings. We have worked with our local media to spread awareness about safe gun storage. We have spent time at the state capital to discuss child access prevention laws with legislators. We have collaborated with community leaders and elected officials for gun violence awareness events. We earned support from leaders at our institution. If you walk through our hospital units, clinics, resident areas, and faculty offices, you will see evidence of our success. Physicians and nurses are still wearing their ribbons from the Wear Orange day on their name badges. “We Can End Gun Violence” signs are hanging on faculty members’ doors. Thanks to local police departments, the clinic has a constant supply of gun locks that are provided to families free of charge. Our residents proudly walk the halls with Be SMART buttons on their badges. These physical reminders of our progress are incredibly motivating as we continue this work. However, it is the quiet moments alone with children and parents who are suffering because of the epidemic of gun violence that really move me. I will not give up this fight until children in our communities are safe.
Acknowledgments
Dr. Andrews wishes to thank Dr. Kelsey Gastineau for her efforts to increase the frequency of gun safety discussions in our Pediatric Primary Care clinic and for her support in all of this work.
1. Cunningham RM, Walton MA, Carter PM. The major causes of death in children and adolescents in the United States. N Engl J Med. 2018;379(25):2468-2475. https://doi.org/10.1056/NEJMsr1804754.
2. Prevention CfDCa. National centers for injury prevention and control, web-based injury statistics query and reporting system (WISQARS) Fatal Injury Reports. 2013-2017.
3. Goyal MK, Badolato GM, Patel SJ, Iqbal SF, Parikh K, McCarter R. State gun laws and pediatric firearm-related mortality. Pediatrics. 2019;144(2). https://doi.org/10.1542/peds.2018-3283
4. Grossman DC, Mueller BA , Riedy C, Dowd MD, Villaveces A, Prodzinski J, et al. Gun storage practices and risk of youth suicide and unintentional firearm injuries. JAMA. 2005;293(6):707-714. https://doi.org/10.1001/jama.293.6.707.
5. Azrael D, Cohen J, Salhi C, Miller M. Firearm storage in gun-owning households with children: results of a 2015 national survey. J Urban Health. 2018;95(3):295-304. https://doi.org/10.1007/s11524-018-0261-7
1. Cunningham RM, Walton MA, Carter PM. The major causes of death in children and adolescents in the United States. N Engl J Med. 2018;379(25):2468-2475. https://doi.org/10.1056/NEJMsr1804754.
2. Prevention CfDCa. National centers for injury prevention and control, web-based injury statistics query and reporting system (WISQARS) Fatal Injury Reports. 2013-2017.
3. Goyal MK, Badolato GM, Patel SJ, Iqbal SF, Parikh K, McCarter R. State gun laws and pediatric firearm-related mortality. Pediatrics. 2019;144(2). https://doi.org/10.1542/peds.2018-3283
4. Grossman DC, Mueller BA , Riedy C, Dowd MD, Villaveces A, Prodzinski J, et al. Gun storage practices and risk of youth suicide and unintentional firearm injuries. JAMA. 2005;293(6):707-714. https://doi.org/10.1001/jama.293.6.707.
5. Azrael D, Cohen J, Salhi C, Miller M. Firearm storage in gun-owning households with children: results of a 2015 national survey. J Urban Health. 2018;95(3):295-304. https://doi.org/10.1007/s11524-018-0261-7
© 2019 Society of Hospital Medicine
Disclosure After Adverse Medical Outcomes: A Multidimensional Challenge
From The Communication in Healthcare Group, Seattle, WA.
Abstract
- Objective: To review established approaches to disclosure and resolution following adverse medical outcomes and highlight barriers that may hinder universal implementation of effective disclosure/resolution practices.
- Methods: An overview of established approaches to disclosure and resolution of adverse medical outcomes is presented.
- Results: Clinicians must be equipped to manage situations where adverse medical outcomes occur even though the care provided was reasonable, within the standard, as well as in situations where preventable problems in the care provided were likely the cause of patient harm. Established approaches that have proven useful for investigating, disclosing, and resolving situations, captured in the acronyms AIDR, ALEE, and TEAM, can assist clinicians in the disclosure and ultimate resolution of these 2 types of situations.
- Conclusion: Health care organizations with a solid commitment and a reliable structure for ensuring adherence to full disclosure and fair resolution of adverse outcomes have demonstrated sustainable progress in ethically and effectively resolving situations where patients are harmed by medical care.
Keywords: safety; medical error; adverse outcomes; resolution; communication.
Much has been learned over the 20 years since the Institute of Medicine’s (IOM) report To Err Is Human1 was published. At the time it was published, the IOM report made it clear that only a minority of preventable patient harms were being acknowledged, investigated, and reported. In the face of adverse outcomes “dissemble, deny, and defend” was a common strategy of many clinicians, institutions, and liability carriers.2 The health care system appeared to place a priority on protecting itself from reputational and financial harm over the rights of injured patients to be given an accurate understanding of what had happened in their care and to pursue restitution, if appropriate.3-5
The emerging quality improvement movement was accompanied by calls for increased patient advocacy. This included the goal of greater transparency and more timely and equitable resolutions with patients who have been harmed by problems in care. Health care systems pressed for confidentiality protections in exchange for increased focus on quality improvement.6 Applying medical ethics of autonomy, no-maleficence, beneficence, and justice initially took a backseat, as risk management was given priority.7 Insurance carriers have no ethical obligation, and a clear disincentive, to assure that harmed patients are fully informed and offered restitution. Some self-insured health systems, however, began experimenting with more proactive and transparent approaches to disclosure and resolution. In contrast to the often-reported fear of a liability explosion, they reported reduced claims and suits, shorter time to resolution, and reduced overall financial cost,8-10 providing some evidence that perhaps greater openness could work after all.
But for providers and staff to allow transparency and candor to become the norm, institutions needed to create a more “just culture” for managing errors. Individual impairment or willful disregard of safe practice would need to be handled differently from the slips and lapses that more often contributed to preventable harm.11 For example, the nurse who was inadequately oriented to the equipment on an unfamiliar unit where she was asked to work a double shift due to a staffing shortage should not be held as accountable as an employee who knowingly violated agreed upon safe practices, even though patient harm resulted in each situation. It became clear that patient harm was usually the result of multiple factors involving individuals, communication, procedures, systems, and equipment. Blaming and disciplining individuals at the sharp end would not reliably reduce adverse outcomes.
Since the 1999 IOM report, we have developed general agreement on best practices for investigating, disclosing, and resolving situations where patients are harmed by medical care.12,13 This article reviews the perspectives and practices that appear necessary for effective disclosure and resolution after an adverse outcome and highlights barriers to reliably enacting them in practice.
Elements of Effective Disclosure
Effective disclosure to patients and families hinges on determining and providing an accurate understanding of what happened in the patient’s care. It should be the care providers’ and their institution’s responsibility to determine causation and disclose it. This should not require only the most upset patients and families initiating a legal process taking 3 years or more to complete. The most consequential question must be answered, “Was the care provided reasonable?” That is, was everything done within the standard, as would have been expected by similarly trained clinicians with the information and resources available at that time? It follows that if care was reasonable, then the adverse outcome could not normally have been prevented, no correction in care processes is called for, and no financial compensation is required. If the care review reveals deficiencies in care that were linked to patient harm, then achieving a satisfying resolution would be more complex and difficult.13 First, individuals would have to accept that they have contributed to patient harm, itself an often-contentious process and psychologically devastating realization. Then they must have this difficult conversation with patient and family, creating liability risk for themselves in the process. They must commit to correcting the problems that contributed to the harm. They must facilitate, rather than obstruct, a path to a restitution that addresses the medical, practical, and financial harms that have resulted. Given the challenges inherent to disclosure and resolution, it is no wonder that dissembling, denying, and defending was the common practice for the preceding decades.14
Disclosure and Resolution Pathways
I was the co-developer of an approach to disclosure and resolution which is now widely accepted and that has been taught across the United States and Canada to more than 50,000 health care providers and administrators over 18 years.15,16 We learned that resolving adverse medical outcomes is a 4-part process (anticipate, investigate, disclose, resolve [AIDR]). Most adverse or simply disappointing outcomes occur despite reasonable care (eg, due to biological variability, the imprecision of the science and limitations and risks of the procedures). The minority of harms are associated with deficiencies in the care (ie, unreasonable care). We need to equip ourselves to manage both situations effectively. The approach we developed can be captured in 3 acronyms: AIDR, ALEE, and TEAM,
AIDR
This acronym encapsulates the overview guidance for clinicians after an adverse event or outcome, regardless of the cause.
Anticipate the thoughts and feelings of the harmed/disappointed patient and family and reach out immediately with an expression of sympathy.
Investigate sufficiently to address questions about most likely causation and do not conjecture prior to investigation. Ask for patience—“You deserve more than a guess”—and keep in regular contact to reinforce the promise that there will be a full reporting when the review is complete.
Disclose (in a planned and coordinated manner) what has been learned in the investigation.
Resolve the situation with the patient and family consistent with our ethical principles.
If our failure caused the harm (care unreasonable/breached the standard), then working toward a fair restitution and taking corrective actions are appropriate. If the care was found to have been reasonable, then compensation would not be offered and corrective action is unwarranted. The organization would defend reasonable care if a claim was still pursued.
This process involves ethical clarity, emotional intelligence, and discipline. Clinicians must first acknowledge that a disappointing outcome or event has occurred. Clinicians involved in the care, usually led by the attending provider, then immediately reach out to the patient and family with sympathy, a plan of care to address the medical issues, and the promise to investigate and follow-up with the patient and family when the harm and its causes are more clearly determined. To disclose simply means to provide an accurate understanding (ie, the understanding determined by the investigation we conducted) of what happened, its causes, and consequences. Depending on the extent of the harm and the complexity and time needed for the investigation, a “coach” or “disclosure coordinator” who has advanced training in managing these situations is brought in to guide the process. The disclosure coach/coordinator provides a consistent and steady hand throughout the process of investigation, disclosure, and ultimately resolution with patient and family. Patients and families often move across settings during the time of the AIDR process, and it is easy to lose track of them unless someone is following the entire process until resolved.
ALEE
When the investigation of an adverse/disappointing outcome determines the care was reasonable and therefore the adverse outcome could not have been prevented, we use the ALEE pathway to guide the disclosure conversation (Step 3 in AIDR) with the patient and family:
Anticipate. What are the questions, thoughts, and feelings we would expect the patient and family will have? On this track, there is nothing to apologize for since the care was reasonable, yet expressing compassion and sympathy for the patient’s experience is essential. “I/we really sympathize with how differently this has turned out than we had hoped.”
Listen. Invite and listen for their questions and concerns, how they are seeing the situation, and where and what they are finding most upsetting and in need of explanation.
Empathize. There are 2 kinds of empathy required here. Cognitive empathy means showing that we understand their thinking from their perspective, separate from whether we fully agree. Emotional empathy involves demonstrating that their emotions are understandable given the situation, even if those emotions are painful for clinicians to experience. Listening in step 2 is how we learned their perspective and emotions. Now we can show accurate empathy: I/we can understand how upsetting it is to be facing another set of procedures to treat the unfortunate complications from your last surgery.
Explain. Even when care is reasonable, questions and perhaps suspicions are to be expected. Listening and empathizing sets us up to focus our explanations on the patient’s and family’s key questions with a level of thoughtfulness and transparency that conveys credibility. We should not assume, however, that they have accepted our explanation. Instead, solicit their reactions and unresolved questions as part of the disclosure discussion. It is normal for additional concerns to emerge in the days after the disclosure discussion, and we should be ready to address these concerns until resolved. In some instances, the patient and family will not be satisfied and it may be helpful to offer an independent review of the care. If the unresolved patient and family engages an attorney, that will be the first step taken anyway. Proactively offering an independent review signals confidence in your objectivity and sensitivity to the importance of fairness for the patient and family: Your questions and concerns are completely normal in light of the disappointing experience you have had. Let me see if I/we can address those now to your satisfaction.
TEAM
If the investigation determines that aspects of the care were unreasonable (breached the standard) and the adverse outcome/harm was related to the deficiencies in the care, then we use the TEAM pathway to disclose and resolve the situation with the patient and family
Truthful and Transparent and Teamwork. We should be offering our most accurate understanding of how the adverse outcome occurred, with sufficient depth and clarity that the patient and family can see how we reached that conclusion. In straightforward situations involving minor harm (eg, an allergic reaction to a medication that the clinician overlooked and that resulted in an urgent care center visit), a very limited investigation may clarify the situation sufficiently that the prescribing provider, accompanied by an office or staff nurse as support and witness, may be able to complete an effective disclosure in a single discussion, and simply writing off a bill or arranging to reimburse the urgent care center visit cost may satisfy the affected patient.
In more complex situations involving greater harm, a number of people must be involved to accomplish TEAM tasks: to offer an explanation, to answer questions, to make apologies, to explain changes intended to reduce the chance of harm to others in the future, and to work through any restitution that may be appropriate. Appointing a disclosure coach/coordinator/facilitator who has had extended training in the disclosure process can help guide these more complex situations. Risk management, insurance carriers, and legal counsel should be aware and advising throughout the process and participating directly in meetings with the patient and family, as appropriate. Since on the TEAM track we are admitting liability, offering a path to financial restitution may be warranted and the disclosure process may trigger reporting requirements with regulatory as well as human resource implications.
The patient and family may want to include other people on their “team” as well. Since complex disclosure meetings need to be carefully planned in advance, we should clarify who will be attending from the health care side and who the family intends to involve. We should anticipate potential requests and questions such as: Would it be OK to record this meeting? Can we ask our attorney to attend? Who are all these people and why are they in this meeting? (We should introduce all team members and clarify how their involvement is necessary to help reach the most satisfying resolution for all involved.)
Empathize. Admitting that deficient aspects in the care contributed to the harm will trigger thoughts, emotions, and expectations for the patient and family. Empathizing involves seeing the whole situation from their perspective and acknowledging their emotions as understandable. Empathizing is not the same as fully agreeing with the patient’s and family’s perspective, but we will not be able to effectively address concerns and expectations that we have not understood. Organizations should have supports in place for staff who are involved in these difficult situations. Nonetheless, we must prioritize the patient’s and family‘s feelings in a disclosure meeting.
Apologize and be Accountable. This calls for both expressions of sympathy as well as a genuine apology for having caused harm by failure in some aspect of care: We are very sorry you are going through this difficult situation. We are especially sorry to tell you that we now recognize that problems in the care we provided are the most likely cause of this harm. Would this be a good time to explain what we learned?
Having the responsible clinicians present increases the chances of achieving the most complete resolution in a single planned and well facilitated meeting. The tasks for that meeting include: offering an explanation that reveals the problems in care that contributed to the adverse outcome, making sincere apologies, and explaining changes to reduce chance of harm to others. The disclosure coach can work with individuals to help them understand how and why their involvement can be important and to help staff members become ready to participate constructively in the disclosure meeting. When individuals appear unable or unwilling to contribute constructively, a plan is needed for how their part can be replaced (eg, a charge nurse or department chair might need to step in to explain and apologize for the care of a subordinate). Managers/administrators can explain contributory factors for what may at first appear to have been simply individual negligence. Administrators can describe the actions that the organization is taking to correct problems that contributed to the patient harm: As nursing executive, it is my responsibility to see that all our staff have been adequately trained on the equipment we are asking them to use. We now recognize that the nurse’s lack of familiarity with that equipment contributed to the harm you experienced and I am very sorry for that. It is my responsibility to get that problem corrected, and we are already taking steps to assure that. Patients and families often have ideas for improving care processes and appreciate being invited to share these ideas as a service to future patients.
Manage until resolved. On the “care unreasonable” track, we must signal openness to helping with the patient’s and family’s immediate and longer-term needs, as well as their expectations about financial and other forms of restitution. Someone should be in the meeting who can describe the next steps in working towards a fair restitution and how that process will take place following the conclusion of the disclosure meeting. The close involvement of risk and claims professionals throughout the process of investigation through to the disclosure discussion itself will assure a more satisfactory handoff to questions about around financial compensation
Psychological Barriers to Implementation of Disclosure Pathways
Many organizations and researchers agree that disclosure and resolution pathways as just described are the most ethical and effective ways for all parties to resolve these painful situations. So why isn’t this approach universally practiced? In concluding this article, it may be helpful to point out some of the human dynamics that make resolution more difficult and how they might be addressed.
A key issue is the “urge to self-preservation.” Health care organizations have often been accused of disclosing only what they cannot hide. We have repeatedly observed how individuals and organizations are often initially motivated to do whatever is needed to protect themselves, even when those behaviors are frankly deceptive. This is almost to be expected. By age 4 children have learned to use deception as a defensive strategy when confronted with misbehavior. Research shows that children and adults continue the strategy to escape censure or punishment and simply get better at hiding their tracks.18 Because people want to preserve their image as ethical individuals, they have also learned to rationalize/justify this deception as necessary for self-preservation (“My dad would have killed me,” “I will lose my license,” “It is not fair that I take the blame when others have done the same thing and gotten away with it.”). Imagining the most extreme, and therefore “unfair” consequences, helps justify the individual’s use of dissembling and frank deception in order to avoid them. Clinicians and organizations may convince themselves that they are the victims entitled to protection rather than the injured patient. Patients and families often accept explanations that are less than candid, as doctors and nurses remain among the most trusted of professionals. Sufficiently understanding the complexities of the care is beyond the capability of most lay people. Successfully challenging the clinician’s or institution’s exculpatory explanation for an adverse outcome is very difficult, even though many clinicians believe that the tort system is stacked against them.
As a result, even the most sensible of best practices, toolkits, and trainings will not make full disclosure and fair resolution of adverse outcomes more likely without a counterweight of solid ethical commitment and a reliable structure for ensuring adherence. Sustainable progress has been demonstrated in those institutions8,10,17 where: (1) institutional values and ethics around disclosure were elevated above self-protection, (2) efficient processes for recognizing and objectively reviewing care involving an adverse outcome were developed and followed, (3) salaried and institutionally insured staff and providers were required to participate in and accept a fair path to resolution in the context of a just culture, and (4) the institution was able to deliver on any commitments (eg, financial, corrective actions) it has made. Conversely, disclosure and resolution programs have struggled in the following situations: where values and ethics are not clarified and made primary; where the processes for reviewing adverse outcomes are slow, inconsistent, and open to political interference; where independent providers have latitude to insist on self-protective behaviors; and where liability carriers who place highest priority on avoiding financial exposure are involved.
Conclusion
The challenge of effectively disclosing and resolving adverse medical outcomes will continue to be most formidable for health care systems with independent medical staffs with separate liability carriers. Can these systems get a firm consensus on the ethics that are paramount in disclosure situations? Can they create care review systems that are efficient and objective and reach conclusions that are binding on those involved? Are they willing to provide explanations to patients and families regardless of the consequences to themselves? Can they coordinate an efficient path to financial and other forms of restitution in those situations where problems in the care contributed to the patient being harmed? And can they enforce these practices despite the self-concerns of all the involved parties? The good news is we now know how to disclose and resolve adverse medical outcomes with patients and families in a way that is fair to providers, staff, and institutions and will not break the bank. For health care organizations, implementing effective disclosure and resolution practices starts with a commitment to both build consensus for this process and consistently enforce it.
Corresponding author: Daniel O’Connell, PhD, 2212 Queen Anne Ave. N. #810, Seattle, WA 98109; [email protected].
Financial disclosures: None.
1. Kohn L, Corrigan J, Donaldson M, eds. To Err Is Human: Building a Safer Health System. Washington, DC: Committee on Quality of Health Care in America, Institute of Medicine. National Academies Press; 1999.
2. Gibson R, Singh JP. Wall of Silence: The Untold Story of the Medical Mistakes That Kill and Injure Millions of Americans. Washington, DC: Lifeline Press; 2003.
3. Rathert C, Phillips W. Medical error disclosure training: evidence for values-based ethical environments. J Bus Ethics. 2010;97:491-503.
4. Wu AW, Cavanaugh TA, McPhee SJ, et al. To tell the truth: ethical and practical issues in disclosing medical mistakes to patients. J Gen Intern Med. 1997;12:770-775.
5. Gallagher TH, Waterman AD, Ebers AG, et al. Patients’ and doctors’ attitudes regarding the disclosure of medical errors. JAMA. 2003;289:1001-1007.
6. The Patient Safety and Quality Improvement Act of 2005 (PSQIA); Public Law 109-41, 119 Stat. 424-434, which amended the Public Health Service Act.
7. Banja J. Moral courage in medicine—disclosing medical error. Bioethics Forum. 2001;17:7-115
8. Boothman R, Imhoff SJ, Campbell DA. Nurturing a culture of patient safety and achieving lower malpractice risk through disclosure: Lessons learned and future directions. Front Health Serv Manage. 2012;28:13-27.
9. Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
10. Mello MM, Boothman RC, McDonald T, et al. Communication and resolution programs: the challenges and lessons learned from six early adopters. Health Affairs. 2014;33:20-29.
11. Marx D. Patient Safety and the Just Culture: A Primer for Health Care Executives. New York, NY: Trustees of Columbia University; 2001.
12. AHRQ Communication and Optimal Resolution (CANDOR) Toolkit. Rockville, MD: Agency for Healthcare Research and Quality; May 2016.
13. O’Connell D, White MK, Platt F. Disclosing unanticipated outcomes and medical errors. J Clin Outcomes Manag. 2003;10:25-29.
14. Berlinger N. After Harm: Medical Error and the Ethics of Forgiveness. Baltimore, MD: Johns Hopkins University Press; 2005.
15. O’Connell D, Reifsteck SW Disclosing unexpected outcomes and medical error. J Med Prac Manag. 2004;19:317-323.
16. Robson R, and Pelletier E. Giving back the pen: Disclosure, apology and early compensation discussions after harm in the healthcare setting. Healthc Q. 2008;11(3 Spec No.)85-90.
17. Kraman SS, Hamm G. Risk management: extreme honesty may be the best policy. Ann Intern Med. 1999;131:963-967.
18. Ding XP, Wellman HM, WangY, et al. Theory-of-mind training causes honest young children to lie. Psychol Sci. 2015;26:1812-1821.
From The Communication in Healthcare Group, Seattle, WA.
Abstract
- Objective: To review established approaches to disclosure and resolution following adverse medical outcomes and highlight barriers that may hinder universal implementation of effective disclosure/resolution practices.
- Methods: An overview of established approaches to disclosure and resolution of adverse medical outcomes is presented.
- Results: Clinicians must be equipped to manage situations where adverse medical outcomes occur even though the care provided was reasonable, within the standard, as well as in situations where preventable problems in the care provided were likely the cause of patient harm. Established approaches that have proven useful for investigating, disclosing, and resolving situations, captured in the acronyms AIDR, ALEE, and TEAM, can assist clinicians in the disclosure and ultimate resolution of these 2 types of situations.
- Conclusion: Health care organizations with a solid commitment and a reliable structure for ensuring adherence to full disclosure and fair resolution of adverse outcomes have demonstrated sustainable progress in ethically and effectively resolving situations where patients are harmed by medical care.
Keywords: safety; medical error; adverse outcomes; resolution; communication.
Much has been learned over the 20 years since the Institute of Medicine’s (IOM) report To Err Is Human1 was published. At the time it was published, the IOM report made it clear that only a minority of preventable patient harms were being acknowledged, investigated, and reported. In the face of adverse outcomes “dissemble, deny, and defend” was a common strategy of many clinicians, institutions, and liability carriers.2 The health care system appeared to place a priority on protecting itself from reputational and financial harm over the rights of injured patients to be given an accurate understanding of what had happened in their care and to pursue restitution, if appropriate.3-5
The emerging quality improvement movement was accompanied by calls for increased patient advocacy. This included the goal of greater transparency and more timely and equitable resolutions with patients who have been harmed by problems in care. Health care systems pressed for confidentiality protections in exchange for increased focus on quality improvement.6 Applying medical ethics of autonomy, no-maleficence, beneficence, and justice initially took a backseat, as risk management was given priority.7 Insurance carriers have no ethical obligation, and a clear disincentive, to assure that harmed patients are fully informed and offered restitution. Some self-insured health systems, however, began experimenting with more proactive and transparent approaches to disclosure and resolution. In contrast to the often-reported fear of a liability explosion, they reported reduced claims and suits, shorter time to resolution, and reduced overall financial cost,8-10 providing some evidence that perhaps greater openness could work after all.
But for providers and staff to allow transparency and candor to become the norm, institutions needed to create a more “just culture” for managing errors. Individual impairment or willful disregard of safe practice would need to be handled differently from the slips and lapses that more often contributed to preventable harm.11 For example, the nurse who was inadequately oriented to the equipment on an unfamiliar unit where she was asked to work a double shift due to a staffing shortage should not be held as accountable as an employee who knowingly violated agreed upon safe practices, even though patient harm resulted in each situation. It became clear that patient harm was usually the result of multiple factors involving individuals, communication, procedures, systems, and equipment. Blaming and disciplining individuals at the sharp end would not reliably reduce adverse outcomes.
Since the 1999 IOM report, we have developed general agreement on best practices for investigating, disclosing, and resolving situations where patients are harmed by medical care.12,13 This article reviews the perspectives and practices that appear necessary for effective disclosure and resolution after an adverse outcome and highlights barriers to reliably enacting them in practice.
Elements of Effective Disclosure
Effective disclosure to patients and families hinges on determining and providing an accurate understanding of what happened in the patient’s care. It should be the care providers’ and their institution’s responsibility to determine causation and disclose it. This should not require only the most upset patients and families initiating a legal process taking 3 years or more to complete. The most consequential question must be answered, “Was the care provided reasonable?” That is, was everything done within the standard, as would have been expected by similarly trained clinicians with the information and resources available at that time? It follows that if care was reasonable, then the adverse outcome could not normally have been prevented, no correction in care processes is called for, and no financial compensation is required. If the care review reveals deficiencies in care that were linked to patient harm, then achieving a satisfying resolution would be more complex and difficult.13 First, individuals would have to accept that they have contributed to patient harm, itself an often-contentious process and psychologically devastating realization. Then they must have this difficult conversation with patient and family, creating liability risk for themselves in the process. They must commit to correcting the problems that contributed to the harm. They must facilitate, rather than obstruct, a path to a restitution that addresses the medical, practical, and financial harms that have resulted. Given the challenges inherent to disclosure and resolution, it is no wonder that dissembling, denying, and defending was the common practice for the preceding decades.14
Disclosure and Resolution Pathways
I was the co-developer of an approach to disclosure and resolution which is now widely accepted and that has been taught across the United States and Canada to more than 50,000 health care providers and administrators over 18 years.15,16 We learned that resolving adverse medical outcomes is a 4-part process (anticipate, investigate, disclose, resolve [AIDR]). Most adverse or simply disappointing outcomes occur despite reasonable care (eg, due to biological variability, the imprecision of the science and limitations and risks of the procedures). The minority of harms are associated with deficiencies in the care (ie, unreasonable care). We need to equip ourselves to manage both situations effectively. The approach we developed can be captured in 3 acronyms: AIDR, ALEE, and TEAM,
AIDR
This acronym encapsulates the overview guidance for clinicians after an adverse event or outcome, regardless of the cause.
Anticipate the thoughts and feelings of the harmed/disappointed patient and family and reach out immediately with an expression of sympathy.
Investigate sufficiently to address questions about most likely causation and do not conjecture prior to investigation. Ask for patience—“You deserve more than a guess”—and keep in regular contact to reinforce the promise that there will be a full reporting when the review is complete.
Disclose (in a planned and coordinated manner) what has been learned in the investigation.
Resolve the situation with the patient and family consistent with our ethical principles.
If our failure caused the harm (care unreasonable/breached the standard), then working toward a fair restitution and taking corrective actions are appropriate. If the care was found to have been reasonable, then compensation would not be offered and corrective action is unwarranted. The organization would defend reasonable care if a claim was still pursued.
This process involves ethical clarity, emotional intelligence, and discipline. Clinicians must first acknowledge that a disappointing outcome or event has occurred. Clinicians involved in the care, usually led by the attending provider, then immediately reach out to the patient and family with sympathy, a plan of care to address the medical issues, and the promise to investigate and follow-up with the patient and family when the harm and its causes are more clearly determined. To disclose simply means to provide an accurate understanding (ie, the understanding determined by the investigation we conducted) of what happened, its causes, and consequences. Depending on the extent of the harm and the complexity and time needed for the investigation, a “coach” or “disclosure coordinator” who has advanced training in managing these situations is brought in to guide the process. The disclosure coach/coordinator provides a consistent and steady hand throughout the process of investigation, disclosure, and ultimately resolution with patient and family. Patients and families often move across settings during the time of the AIDR process, and it is easy to lose track of them unless someone is following the entire process until resolved.
ALEE
When the investigation of an adverse/disappointing outcome determines the care was reasonable and therefore the adverse outcome could not have been prevented, we use the ALEE pathway to guide the disclosure conversation (Step 3 in AIDR) with the patient and family:
Anticipate. What are the questions, thoughts, and feelings we would expect the patient and family will have? On this track, there is nothing to apologize for since the care was reasonable, yet expressing compassion and sympathy for the patient’s experience is essential. “I/we really sympathize with how differently this has turned out than we had hoped.”
Listen. Invite and listen for their questions and concerns, how they are seeing the situation, and where and what they are finding most upsetting and in need of explanation.
Empathize. There are 2 kinds of empathy required here. Cognitive empathy means showing that we understand their thinking from their perspective, separate from whether we fully agree. Emotional empathy involves demonstrating that their emotions are understandable given the situation, even if those emotions are painful for clinicians to experience. Listening in step 2 is how we learned their perspective and emotions. Now we can show accurate empathy: I/we can understand how upsetting it is to be facing another set of procedures to treat the unfortunate complications from your last surgery.
Explain. Even when care is reasonable, questions and perhaps suspicions are to be expected. Listening and empathizing sets us up to focus our explanations on the patient’s and family’s key questions with a level of thoughtfulness and transparency that conveys credibility. We should not assume, however, that they have accepted our explanation. Instead, solicit their reactions and unresolved questions as part of the disclosure discussion. It is normal for additional concerns to emerge in the days after the disclosure discussion, and we should be ready to address these concerns until resolved. In some instances, the patient and family will not be satisfied and it may be helpful to offer an independent review of the care. If the unresolved patient and family engages an attorney, that will be the first step taken anyway. Proactively offering an independent review signals confidence in your objectivity and sensitivity to the importance of fairness for the patient and family: Your questions and concerns are completely normal in light of the disappointing experience you have had. Let me see if I/we can address those now to your satisfaction.
TEAM
If the investigation determines that aspects of the care were unreasonable (breached the standard) and the adverse outcome/harm was related to the deficiencies in the care, then we use the TEAM pathway to disclose and resolve the situation with the patient and family
Truthful and Transparent and Teamwork. We should be offering our most accurate understanding of how the adverse outcome occurred, with sufficient depth and clarity that the patient and family can see how we reached that conclusion. In straightforward situations involving minor harm (eg, an allergic reaction to a medication that the clinician overlooked and that resulted in an urgent care center visit), a very limited investigation may clarify the situation sufficiently that the prescribing provider, accompanied by an office or staff nurse as support and witness, may be able to complete an effective disclosure in a single discussion, and simply writing off a bill or arranging to reimburse the urgent care center visit cost may satisfy the affected patient.
In more complex situations involving greater harm, a number of people must be involved to accomplish TEAM tasks: to offer an explanation, to answer questions, to make apologies, to explain changes intended to reduce the chance of harm to others in the future, and to work through any restitution that may be appropriate. Appointing a disclosure coach/coordinator/facilitator who has had extended training in the disclosure process can help guide these more complex situations. Risk management, insurance carriers, and legal counsel should be aware and advising throughout the process and participating directly in meetings with the patient and family, as appropriate. Since on the TEAM track we are admitting liability, offering a path to financial restitution may be warranted and the disclosure process may trigger reporting requirements with regulatory as well as human resource implications.
The patient and family may want to include other people on their “team” as well. Since complex disclosure meetings need to be carefully planned in advance, we should clarify who will be attending from the health care side and who the family intends to involve. We should anticipate potential requests and questions such as: Would it be OK to record this meeting? Can we ask our attorney to attend? Who are all these people and why are they in this meeting? (We should introduce all team members and clarify how their involvement is necessary to help reach the most satisfying resolution for all involved.)
Empathize. Admitting that deficient aspects in the care contributed to the harm will trigger thoughts, emotions, and expectations for the patient and family. Empathizing involves seeing the whole situation from their perspective and acknowledging their emotions as understandable. Empathizing is not the same as fully agreeing with the patient’s and family’s perspective, but we will not be able to effectively address concerns and expectations that we have not understood. Organizations should have supports in place for staff who are involved in these difficult situations. Nonetheless, we must prioritize the patient’s and family‘s feelings in a disclosure meeting.
Apologize and be Accountable. This calls for both expressions of sympathy as well as a genuine apology for having caused harm by failure in some aspect of care: We are very sorry you are going through this difficult situation. We are especially sorry to tell you that we now recognize that problems in the care we provided are the most likely cause of this harm. Would this be a good time to explain what we learned?
Having the responsible clinicians present increases the chances of achieving the most complete resolution in a single planned and well facilitated meeting. The tasks for that meeting include: offering an explanation that reveals the problems in care that contributed to the adverse outcome, making sincere apologies, and explaining changes to reduce chance of harm to others. The disclosure coach can work with individuals to help them understand how and why their involvement can be important and to help staff members become ready to participate constructively in the disclosure meeting. When individuals appear unable or unwilling to contribute constructively, a plan is needed for how their part can be replaced (eg, a charge nurse or department chair might need to step in to explain and apologize for the care of a subordinate). Managers/administrators can explain contributory factors for what may at first appear to have been simply individual negligence. Administrators can describe the actions that the organization is taking to correct problems that contributed to the patient harm: As nursing executive, it is my responsibility to see that all our staff have been adequately trained on the equipment we are asking them to use. We now recognize that the nurse’s lack of familiarity with that equipment contributed to the harm you experienced and I am very sorry for that. It is my responsibility to get that problem corrected, and we are already taking steps to assure that. Patients and families often have ideas for improving care processes and appreciate being invited to share these ideas as a service to future patients.
Manage until resolved. On the “care unreasonable” track, we must signal openness to helping with the patient’s and family’s immediate and longer-term needs, as well as their expectations about financial and other forms of restitution. Someone should be in the meeting who can describe the next steps in working towards a fair restitution and how that process will take place following the conclusion of the disclosure meeting. The close involvement of risk and claims professionals throughout the process of investigation through to the disclosure discussion itself will assure a more satisfactory handoff to questions about around financial compensation
Psychological Barriers to Implementation of Disclosure Pathways
Many organizations and researchers agree that disclosure and resolution pathways as just described are the most ethical and effective ways for all parties to resolve these painful situations. So why isn’t this approach universally practiced? In concluding this article, it may be helpful to point out some of the human dynamics that make resolution more difficult and how they might be addressed.
A key issue is the “urge to self-preservation.” Health care organizations have often been accused of disclosing only what they cannot hide. We have repeatedly observed how individuals and organizations are often initially motivated to do whatever is needed to protect themselves, even when those behaviors are frankly deceptive. This is almost to be expected. By age 4 children have learned to use deception as a defensive strategy when confronted with misbehavior. Research shows that children and adults continue the strategy to escape censure or punishment and simply get better at hiding their tracks.18 Because people want to preserve their image as ethical individuals, they have also learned to rationalize/justify this deception as necessary for self-preservation (“My dad would have killed me,” “I will lose my license,” “It is not fair that I take the blame when others have done the same thing and gotten away with it.”). Imagining the most extreme, and therefore “unfair” consequences, helps justify the individual’s use of dissembling and frank deception in order to avoid them. Clinicians and organizations may convince themselves that they are the victims entitled to protection rather than the injured patient. Patients and families often accept explanations that are less than candid, as doctors and nurses remain among the most trusted of professionals. Sufficiently understanding the complexities of the care is beyond the capability of most lay people. Successfully challenging the clinician’s or institution’s exculpatory explanation for an adverse outcome is very difficult, even though many clinicians believe that the tort system is stacked against them.
As a result, even the most sensible of best practices, toolkits, and trainings will not make full disclosure and fair resolution of adverse outcomes more likely without a counterweight of solid ethical commitment and a reliable structure for ensuring adherence. Sustainable progress has been demonstrated in those institutions8,10,17 where: (1) institutional values and ethics around disclosure were elevated above self-protection, (2) efficient processes for recognizing and objectively reviewing care involving an adverse outcome were developed and followed, (3) salaried and institutionally insured staff and providers were required to participate in and accept a fair path to resolution in the context of a just culture, and (4) the institution was able to deliver on any commitments (eg, financial, corrective actions) it has made. Conversely, disclosure and resolution programs have struggled in the following situations: where values and ethics are not clarified and made primary; where the processes for reviewing adverse outcomes are slow, inconsistent, and open to political interference; where independent providers have latitude to insist on self-protective behaviors; and where liability carriers who place highest priority on avoiding financial exposure are involved.
Conclusion
The challenge of effectively disclosing and resolving adverse medical outcomes will continue to be most formidable for health care systems with independent medical staffs with separate liability carriers. Can these systems get a firm consensus on the ethics that are paramount in disclosure situations? Can they create care review systems that are efficient and objective and reach conclusions that are binding on those involved? Are they willing to provide explanations to patients and families regardless of the consequences to themselves? Can they coordinate an efficient path to financial and other forms of restitution in those situations where problems in the care contributed to the patient being harmed? And can they enforce these practices despite the self-concerns of all the involved parties? The good news is we now know how to disclose and resolve adverse medical outcomes with patients and families in a way that is fair to providers, staff, and institutions and will not break the bank. For health care organizations, implementing effective disclosure and resolution practices starts with a commitment to both build consensus for this process and consistently enforce it.
Corresponding author: Daniel O’Connell, PhD, 2212 Queen Anne Ave. N. #810, Seattle, WA 98109; [email protected].
Financial disclosures: None.
From The Communication in Healthcare Group, Seattle, WA.
Abstract
- Objective: To review established approaches to disclosure and resolution following adverse medical outcomes and highlight barriers that may hinder universal implementation of effective disclosure/resolution practices.
- Methods: An overview of established approaches to disclosure and resolution of adverse medical outcomes is presented.
- Results: Clinicians must be equipped to manage situations where adverse medical outcomes occur even though the care provided was reasonable, within the standard, as well as in situations where preventable problems in the care provided were likely the cause of patient harm. Established approaches that have proven useful for investigating, disclosing, and resolving situations, captured in the acronyms AIDR, ALEE, and TEAM, can assist clinicians in the disclosure and ultimate resolution of these 2 types of situations.
- Conclusion: Health care organizations with a solid commitment and a reliable structure for ensuring adherence to full disclosure and fair resolution of adverse outcomes have demonstrated sustainable progress in ethically and effectively resolving situations where patients are harmed by medical care.
Keywords: safety; medical error; adverse outcomes; resolution; communication.
Much has been learned over the 20 years since the Institute of Medicine’s (IOM) report To Err Is Human1 was published. At the time it was published, the IOM report made it clear that only a minority of preventable patient harms were being acknowledged, investigated, and reported. In the face of adverse outcomes “dissemble, deny, and defend” was a common strategy of many clinicians, institutions, and liability carriers.2 The health care system appeared to place a priority on protecting itself from reputational and financial harm over the rights of injured patients to be given an accurate understanding of what had happened in their care and to pursue restitution, if appropriate.3-5
The emerging quality improvement movement was accompanied by calls for increased patient advocacy. This included the goal of greater transparency and more timely and equitable resolutions with patients who have been harmed by problems in care. Health care systems pressed for confidentiality protections in exchange for increased focus on quality improvement.6 Applying medical ethics of autonomy, no-maleficence, beneficence, and justice initially took a backseat, as risk management was given priority.7 Insurance carriers have no ethical obligation, and a clear disincentive, to assure that harmed patients are fully informed and offered restitution. Some self-insured health systems, however, began experimenting with more proactive and transparent approaches to disclosure and resolution. In contrast to the often-reported fear of a liability explosion, they reported reduced claims and suits, shorter time to resolution, and reduced overall financial cost,8-10 providing some evidence that perhaps greater openness could work after all.
But for providers and staff to allow transparency and candor to become the norm, institutions needed to create a more “just culture” for managing errors. Individual impairment or willful disregard of safe practice would need to be handled differently from the slips and lapses that more often contributed to preventable harm.11 For example, the nurse who was inadequately oriented to the equipment on an unfamiliar unit where she was asked to work a double shift due to a staffing shortage should not be held as accountable as an employee who knowingly violated agreed upon safe practices, even though patient harm resulted in each situation. It became clear that patient harm was usually the result of multiple factors involving individuals, communication, procedures, systems, and equipment. Blaming and disciplining individuals at the sharp end would not reliably reduce adverse outcomes.
Since the 1999 IOM report, we have developed general agreement on best practices for investigating, disclosing, and resolving situations where patients are harmed by medical care.12,13 This article reviews the perspectives and practices that appear necessary for effective disclosure and resolution after an adverse outcome and highlights barriers to reliably enacting them in practice.
Elements of Effective Disclosure
Effective disclosure to patients and families hinges on determining and providing an accurate understanding of what happened in the patient’s care. It should be the care providers’ and their institution’s responsibility to determine causation and disclose it. This should not require only the most upset patients and families initiating a legal process taking 3 years or more to complete. The most consequential question must be answered, “Was the care provided reasonable?” That is, was everything done within the standard, as would have been expected by similarly trained clinicians with the information and resources available at that time? It follows that if care was reasonable, then the adverse outcome could not normally have been prevented, no correction in care processes is called for, and no financial compensation is required. If the care review reveals deficiencies in care that were linked to patient harm, then achieving a satisfying resolution would be more complex and difficult.13 First, individuals would have to accept that they have contributed to patient harm, itself an often-contentious process and psychologically devastating realization. Then they must have this difficult conversation with patient and family, creating liability risk for themselves in the process. They must commit to correcting the problems that contributed to the harm. They must facilitate, rather than obstruct, a path to a restitution that addresses the medical, practical, and financial harms that have resulted. Given the challenges inherent to disclosure and resolution, it is no wonder that dissembling, denying, and defending was the common practice for the preceding decades.14
Disclosure and Resolution Pathways
I was the co-developer of an approach to disclosure and resolution which is now widely accepted and that has been taught across the United States and Canada to more than 50,000 health care providers and administrators over 18 years.15,16 We learned that resolving adverse medical outcomes is a 4-part process (anticipate, investigate, disclose, resolve [AIDR]). Most adverse or simply disappointing outcomes occur despite reasonable care (eg, due to biological variability, the imprecision of the science and limitations and risks of the procedures). The minority of harms are associated with deficiencies in the care (ie, unreasonable care). We need to equip ourselves to manage both situations effectively. The approach we developed can be captured in 3 acronyms: AIDR, ALEE, and TEAM,
AIDR
This acronym encapsulates the overview guidance for clinicians after an adverse event or outcome, regardless of the cause.
Anticipate the thoughts and feelings of the harmed/disappointed patient and family and reach out immediately with an expression of sympathy.
Investigate sufficiently to address questions about most likely causation and do not conjecture prior to investigation. Ask for patience—“You deserve more than a guess”—and keep in regular contact to reinforce the promise that there will be a full reporting when the review is complete.
Disclose (in a planned and coordinated manner) what has been learned in the investigation.
Resolve the situation with the patient and family consistent with our ethical principles.
If our failure caused the harm (care unreasonable/breached the standard), then working toward a fair restitution and taking corrective actions are appropriate. If the care was found to have been reasonable, then compensation would not be offered and corrective action is unwarranted. The organization would defend reasonable care if a claim was still pursued.
This process involves ethical clarity, emotional intelligence, and discipline. Clinicians must first acknowledge that a disappointing outcome or event has occurred. Clinicians involved in the care, usually led by the attending provider, then immediately reach out to the patient and family with sympathy, a plan of care to address the medical issues, and the promise to investigate and follow-up with the patient and family when the harm and its causes are more clearly determined. To disclose simply means to provide an accurate understanding (ie, the understanding determined by the investigation we conducted) of what happened, its causes, and consequences. Depending on the extent of the harm and the complexity and time needed for the investigation, a “coach” or “disclosure coordinator” who has advanced training in managing these situations is brought in to guide the process. The disclosure coach/coordinator provides a consistent and steady hand throughout the process of investigation, disclosure, and ultimately resolution with patient and family. Patients and families often move across settings during the time of the AIDR process, and it is easy to lose track of them unless someone is following the entire process until resolved.
ALEE
When the investigation of an adverse/disappointing outcome determines the care was reasonable and therefore the adverse outcome could not have been prevented, we use the ALEE pathway to guide the disclosure conversation (Step 3 in AIDR) with the patient and family:
Anticipate. What are the questions, thoughts, and feelings we would expect the patient and family will have? On this track, there is nothing to apologize for since the care was reasonable, yet expressing compassion and sympathy for the patient’s experience is essential. “I/we really sympathize with how differently this has turned out than we had hoped.”
Listen. Invite and listen for their questions and concerns, how they are seeing the situation, and where and what they are finding most upsetting and in need of explanation.
Empathize. There are 2 kinds of empathy required here. Cognitive empathy means showing that we understand their thinking from their perspective, separate from whether we fully agree. Emotional empathy involves demonstrating that their emotions are understandable given the situation, even if those emotions are painful for clinicians to experience. Listening in step 2 is how we learned their perspective and emotions. Now we can show accurate empathy: I/we can understand how upsetting it is to be facing another set of procedures to treat the unfortunate complications from your last surgery.
Explain. Even when care is reasonable, questions and perhaps suspicions are to be expected. Listening and empathizing sets us up to focus our explanations on the patient’s and family’s key questions with a level of thoughtfulness and transparency that conveys credibility. We should not assume, however, that they have accepted our explanation. Instead, solicit their reactions and unresolved questions as part of the disclosure discussion. It is normal for additional concerns to emerge in the days after the disclosure discussion, and we should be ready to address these concerns until resolved. In some instances, the patient and family will not be satisfied and it may be helpful to offer an independent review of the care. If the unresolved patient and family engages an attorney, that will be the first step taken anyway. Proactively offering an independent review signals confidence in your objectivity and sensitivity to the importance of fairness for the patient and family: Your questions and concerns are completely normal in light of the disappointing experience you have had. Let me see if I/we can address those now to your satisfaction.
TEAM
If the investigation determines that aspects of the care were unreasonable (breached the standard) and the adverse outcome/harm was related to the deficiencies in the care, then we use the TEAM pathway to disclose and resolve the situation with the patient and family
Truthful and Transparent and Teamwork. We should be offering our most accurate understanding of how the adverse outcome occurred, with sufficient depth and clarity that the patient and family can see how we reached that conclusion. In straightforward situations involving minor harm (eg, an allergic reaction to a medication that the clinician overlooked and that resulted in an urgent care center visit), a very limited investigation may clarify the situation sufficiently that the prescribing provider, accompanied by an office or staff nurse as support and witness, may be able to complete an effective disclosure in a single discussion, and simply writing off a bill or arranging to reimburse the urgent care center visit cost may satisfy the affected patient.
In more complex situations involving greater harm, a number of people must be involved to accomplish TEAM tasks: to offer an explanation, to answer questions, to make apologies, to explain changes intended to reduce the chance of harm to others in the future, and to work through any restitution that may be appropriate. Appointing a disclosure coach/coordinator/facilitator who has had extended training in the disclosure process can help guide these more complex situations. Risk management, insurance carriers, and legal counsel should be aware and advising throughout the process and participating directly in meetings with the patient and family, as appropriate. Since on the TEAM track we are admitting liability, offering a path to financial restitution may be warranted and the disclosure process may trigger reporting requirements with regulatory as well as human resource implications.
The patient and family may want to include other people on their “team” as well. Since complex disclosure meetings need to be carefully planned in advance, we should clarify who will be attending from the health care side and who the family intends to involve. We should anticipate potential requests and questions such as: Would it be OK to record this meeting? Can we ask our attorney to attend? Who are all these people and why are they in this meeting? (We should introduce all team members and clarify how their involvement is necessary to help reach the most satisfying resolution for all involved.)
Empathize. Admitting that deficient aspects in the care contributed to the harm will trigger thoughts, emotions, and expectations for the patient and family. Empathizing involves seeing the whole situation from their perspective and acknowledging their emotions as understandable. Empathizing is not the same as fully agreeing with the patient’s and family’s perspective, but we will not be able to effectively address concerns and expectations that we have not understood. Organizations should have supports in place for staff who are involved in these difficult situations. Nonetheless, we must prioritize the patient’s and family‘s feelings in a disclosure meeting.
Apologize and be Accountable. This calls for both expressions of sympathy as well as a genuine apology for having caused harm by failure in some aspect of care: We are very sorry you are going through this difficult situation. We are especially sorry to tell you that we now recognize that problems in the care we provided are the most likely cause of this harm. Would this be a good time to explain what we learned?
Having the responsible clinicians present increases the chances of achieving the most complete resolution in a single planned and well facilitated meeting. The tasks for that meeting include: offering an explanation that reveals the problems in care that contributed to the adverse outcome, making sincere apologies, and explaining changes to reduce chance of harm to others. The disclosure coach can work with individuals to help them understand how and why their involvement can be important and to help staff members become ready to participate constructively in the disclosure meeting. When individuals appear unable or unwilling to contribute constructively, a plan is needed for how their part can be replaced (eg, a charge nurse or department chair might need to step in to explain and apologize for the care of a subordinate). Managers/administrators can explain contributory factors for what may at first appear to have been simply individual negligence. Administrators can describe the actions that the organization is taking to correct problems that contributed to the patient harm: As nursing executive, it is my responsibility to see that all our staff have been adequately trained on the equipment we are asking them to use. We now recognize that the nurse’s lack of familiarity with that equipment contributed to the harm you experienced and I am very sorry for that. It is my responsibility to get that problem corrected, and we are already taking steps to assure that. Patients and families often have ideas for improving care processes and appreciate being invited to share these ideas as a service to future patients.
Manage until resolved. On the “care unreasonable” track, we must signal openness to helping with the patient’s and family’s immediate and longer-term needs, as well as their expectations about financial and other forms of restitution. Someone should be in the meeting who can describe the next steps in working towards a fair restitution and how that process will take place following the conclusion of the disclosure meeting. The close involvement of risk and claims professionals throughout the process of investigation through to the disclosure discussion itself will assure a more satisfactory handoff to questions about around financial compensation
Psychological Barriers to Implementation of Disclosure Pathways
Many organizations and researchers agree that disclosure and resolution pathways as just described are the most ethical and effective ways for all parties to resolve these painful situations. So why isn’t this approach universally practiced? In concluding this article, it may be helpful to point out some of the human dynamics that make resolution more difficult and how they might be addressed.
A key issue is the “urge to self-preservation.” Health care organizations have often been accused of disclosing only what they cannot hide. We have repeatedly observed how individuals and organizations are often initially motivated to do whatever is needed to protect themselves, even when those behaviors are frankly deceptive. This is almost to be expected. By age 4 children have learned to use deception as a defensive strategy when confronted with misbehavior. Research shows that children and adults continue the strategy to escape censure or punishment and simply get better at hiding their tracks.18 Because people want to preserve their image as ethical individuals, they have also learned to rationalize/justify this deception as necessary for self-preservation (“My dad would have killed me,” “I will lose my license,” “It is not fair that I take the blame when others have done the same thing and gotten away with it.”). Imagining the most extreme, and therefore “unfair” consequences, helps justify the individual’s use of dissembling and frank deception in order to avoid them. Clinicians and organizations may convince themselves that they are the victims entitled to protection rather than the injured patient. Patients and families often accept explanations that are less than candid, as doctors and nurses remain among the most trusted of professionals. Sufficiently understanding the complexities of the care is beyond the capability of most lay people. Successfully challenging the clinician’s or institution’s exculpatory explanation for an adverse outcome is very difficult, even though many clinicians believe that the tort system is stacked against them.
As a result, even the most sensible of best practices, toolkits, and trainings will not make full disclosure and fair resolution of adverse outcomes more likely without a counterweight of solid ethical commitment and a reliable structure for ensuring adherence. Sustainable progress has been demonstrated in those institutions8,10,17 where: (1) institutional values and ethics around disclosure were elevated above self-protection, (2) efficient processes for recognizing and objectively reviewing care involving an adverse outcome were developed and followed, (3) salaried and institutionally insured staff and providers were required to participate in and accept a fair path to resolution in the context of a just culture, and (4) the institution was able to deliver on any commitments (eg, financial, corrective actions) it has made. Conversely, disclosure and resolution programs have struggled in the following situations: where values and ethics are not clarified and made primary; where the processes for reviewing adverse outcomes are slow, inconsistent, and open to political interference; where independent providers have latitude to insist on self-protective behaviors; and where liability carriers who place highest priority on avoiding financial exposure are involved.
Conclusion
The challenge of effectively disclosing and resolving adverse medical outcomes will continue to be most formidable for health care systems with independent medical staffs with separate liability carriers. Can these systems get a firm consensus on the ethics that are paramount in disclosure situations? Can they create care review systems that are efficient and objective and reach conclusions that are binding on those involved? Are they willing to provide explanations to patients and families regardless of the consequences to themselves? Can they coordinate an efficient path to financial and other forms of restitution in those situations where problems in the care contributed to the patient being harmed? And can they enforce these practices despite the self-concerns of all the involved parties? The good news is we now know how to disclose and resolve adverse medical outcomes with patients and families in a way that is fair to providers, staff, and institutions and will not break the bank. For health care organizations, implementing effective disclosure and resolution practices starts with a commitment to both build consensus for this process and consistently enforce it.
Corresponding author: Daniel O’Connell, PhD, 2212 Queen Anne Ave. N. #810, Seattle, WA 98109; [email protected].
Financial disclosures: None.
1. Kohn L, Corrigan J, Donaldson M, eds. To Err Is Human: Building a Safer Health System. Washington, DC: Committee on Quality of Health Care in America, Institute of Medicine. National Academies Press; 1999.
2. Gibson R, Singh JP. Wall of Silence: The Untold Story of the Medical Mistakes That Kill and Injure Millions of Americans. Washington, DC: Lifeline Press; 2003.
3. Rathert C, Phillips W. Medical error disclosure training: evidence for values-based ethical environments. J Bus Ethics. 2010;97:491-503.
4. Wu AW, Cavanaugh TA, McPhee SJ, et al. To tell the truth: ethical and practical issues in disclosing medical mistakes to patients. J Gen Intern Med. 1997;12:770-775.
5. Gallagher TH, Waterman AD, Ebers AG, et al. Patients’ and doctors’ attitudes regarding the disclosure of medical errors. JAMA. 2003;289:1001-1007.
6. The Patient Safety and Quality Improvement Act of 2005 (PSQIA); Public Law 109-41, 119 Stat. 424-434, which amended the Public Health Service Act.
7. Banja J. Moral courage in medicine—disclosing medical error. Bioethics Forum. 2001;17:7-115
8. Boothman R, Imhoff SJ, Campbell DA. Nurturing a culture of patient safety and achieving lower malpractice risk through disclosure: Lessons learned and future directions. Front Health Serv Manage. 2012;28:13-27.
9. Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
10. Mello MM, Boothman RC, McDonald T, et al. Communication and resolution programs: the challenges and lessons learned from six early adopters. Health Affairs. 2014;33:20-29.
11. Marx D. Patient Safety and the Just Culture: A Primer for Health Care Executives. New York, NY: Trustees of Columbia University; 2001.
12. AHRQ Communication and Optimal Resolution (CANDOR) Toolkit. Rockville, MD: Agency for Healthcare Research and Quality; May 2016.
13. O’Connell D, White MK, Platt F. Disclosing unanticipated outcomes and medical errors. J Clin Outcomes Manag. 2003;10:25-29.
14. Berlinger N. After Harm: Medical Error and the Ethics of Forgiveness. Baltimore, MD: Johns Hopkins University Press; 2005.
15. O’Connell D, Reifsteck SW Disclosing unexpected outcomes and medical error. J Med Prac Manag. 2004;19:317-323.
16. Robson R, and Pelletier E. Giving back the pen: Disclosure, apology and early compensation discussions after harm in the healthcare setting. Healthc Q. 2008;11(3 Spec No.)85-90.
17. Kraman SS, Hamm G. Risk management: extreme honesty may be the best policy. Ann Intern Med. 1999;131:963-967.
18. Ding XP, Wellman HM, WangY, et al. Theory-of-mind training causes honest young children to lie. Psychol Sci. 2015;26:1812-1821.
1. Kohn L, Corrigan J, Donaldson M, eds. To Err Is Human: Building a Safer Health System. Washington, DC: Committee on Quality of Health Care in America, Institute of Medicine. National Academies Press; 1999.
2. Gibson R, Singh JP. Wall of Silence: The Untold Story of the Medical Mistakes That Kill and Injure Millions of Americans. Washington, DC: Lifeline Press; 2003.
3. Rathert C, Phillips W. Medical error disclosure training: evidence for values-based ethical environments. J Bus Ethics. 2010;97:491-503.
4. Wu AW, Cavanaugh TA, McPhee SJ, et al. To tell the truth: ethical and practical issues in disclosing medical mistakes to patients. J Gen Intern Med. 1997;12:770-775.
5. Gallagher TH, Waterman AD, Ebers AG, et al. Patients’ and doctors’ attitudes regarding the disclosure of medical errors. JAMA. 2003;289:1001-1007.
6. The Patient Safety and Quality Improvement Act of 2005 (PSQIA); Public Law 109-41, 119 Stat. 424-434, which amended the Public Health Service Act.
7. Banja J. Moral courage in medicine—disclosing medical error. Bioethics Forum. 2001;17:7-115
8. Boothman R, Imhoff SJ, Campbell DA. Nurturing a culture of patient safety and achieving lower malpractice risk through disclosure: Lessons learned and future directions. Front Health Serv Manage. 2012;28:13-27.
9. Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
10. Mello MM, Boothman RC, McDonald T, et al. Communication and resolution programs: the challenges and lessons learned from six early adopters. Health Affairs. 2014;33:20-29.
11. Marx D. Patient Safety and the Just Culture: A Primer for Health Care Executives. New York, NY: Trustees of Columbia University; 2001.
12. AHRQ Communication and Optimal Resolution (CANDOR) Toolkit. Rockville, MD: Agency for Healthcare Research and Quality; May 2016.
13. O’Connell D, White MK, Platt F. Disclosing unanticipated outcomes and medical errors. J Clin Outcomes Manag. 2003;10:25-29.
14. Berlinger N. After Harm: Medical Error and the Ethics of Forgiveness. Baltimore, MD: Johns Hopkins University Press; 2005.
15. O’Connell D, Reifsteck SW Disclosing unexpected outcomes and medical error. J Med Prac Manag. 2004;19:317-323.
16. Robson R, and Pelletier E. Giving back the pen: Disclosure, apology and early compensation discussions after harm in the healthcare setting. Healthc Q. 2008;11(3 Spec No.)85-90.
17. Kraman SS, Hamm G. Risk management: extreme honesty may be the best policy. Ann Intern Med. 1999;131:963-967.
18. Ding XP, Wellman HM, WangY, et al. Theory-of-mind training causes honest young children to lie. Psychol Sci. 2015;26:1812-1821.
Refractory Status Asthmaticus: Treatment With Sevoflurane
Asthma attacks account for 1.8 million emergency department (ED) visits each year in the US and for 10 deaths daily.1 Management of asthma attacks includes administration of inhaled ß2 adrenergic agonists, inhaled anticholinergic agents, IV magnesium sulfate, and corticosteroids.2 Status asthmaticus is an intense acute exacerbation of asthma that does not respond to repeated treatments of bronchodilators and corticosteroids.3 It is a medical emergency requiring immediate recognition and treatment. The decision to intubate a patient with status asthmaticus is a clinical decision based on work of breathing, respiratory acidosis, and failure to respond to medical interventions.
In refractory cases of status asthmaticus, intubation and mechanical ventilation are undertaken to provide oxygenation and ventilation until the bronchospasm resolves. However, mechanical ventilation is associated with significant risks, including high end-inspiratory pressures, barotrauma, and volutrauma.4 Rescue therapies include muscle relaxation, infusion of ketamine (central acting nonopioid analgesic with bronchodilatory properties), heliox, and general anesthesia.2,4 We report a case of a patient with life-threatening asthma and status asthmaticus treated with sevoflurane general anesthesia.
Case Presentation
A 55-year-old woman whose medical history was notable for asthma, psoriasis, hypothyroidism, tobacco, and alcohol abuse, and posttraumatic stress disorder (PTSD) presented to the ED. The patient had rarely sought medical attention and had no prior ED visits or hospitalizations in the electronic health record. Her home regimen included an albuterol inhaler used as needed. Her family reported that they had found her in distress in bed in a tripod position, unable to speak and struggling to breath.
Emergency medical services found the patient cyanotic, apneic, and pulseless. She received cardiopulmonary resuscitation for 30 seconds and 1-mg IV epinephrine, and spontaneous circulation returned. The patient arrived in the ED with an oral airway in place receiving bag valve mask ventilation. The patient expelled the oral airway. She was unable to speak due to dyspnea, exhibited persistent cyanosis, fatigue due to work of breathing, and failed to respond to nebulized albuterol/ipratropium bromide, IV methylprednisolone, and magnesium sulfate. The patient met criteria for acute severe asthma, or status asthmaticus. Thus, the patient received rapid sequence induction with rocuronium and ketamine and was intubated.
According to her family, the patient had no previous intensive care unit (ICU) admissions or prior intubations. Her only asthma medication was an albuterol inhaler as needed. The patient worked as a supervisor at a window blind manufacturing company. She lived alone, smoked 2 packs of cigarettes a day for more than 30 years, had no pets, drank unknown quantities of beer, wine, and hard liquor daily, and had smoked marijuana for several years.
The patient’s physical examination was notable for diffuse expiratory wheezes. Laboratory analysis revealed white blood cell count of 13.7 k/mcL, sodium 140 mmol/L, potassium 4.9 mmol/L, chloride 105 mmol/L, CO2 17 mmol/L, creatinine 0.98 mg/dL, troponin 0.03 ng/mL, lactate 7.2 mmol/L. Her chest X-ray showed hyperinflation but no focal opacities, pneumothorax, or pulmonary edema. Her endotracheal tube was in good position (Figure 1). A computed tomography pulmonary angiogram showed no pulmonary embolus or emphysema. There were atelectatic changes in the dependent portion of the right lower lobe, central bronchial wall thickening, and no stigmata of air trapping (Figure 2). An echocardiogram revealed a left ventricular ejection fraction of 45%, normal right ventricle and right ventricular size and function with an estimated right ventricular systolic pressure of 40 mm Hg.
The patient was admitted to the ICU and started on continuous infusion cisatracurium for paralysis and deep sedation to improve ventilatory synchrony and decrease auto positive end-expiratory pressure (PEEP). Mechanical ventilation was initiated with volume-cycled assist control ventilation, 6 mL/kg/ideal body weight (IBW) at 5-cm H2O PEEP, and 1 minute ventilation of 10 liters. The patient had severe air trapping and high airway pressures. The dynamic PEEP was 22-cm H2O (normal PEEP of 5-cm H2O), peak airway pressure (PAP) 41-cm H2O, and plateau pressure 31-cm H2O. In addition, the arterial blood gas (ABG) showed severe hypercapnic respiratory acidosis without significant hypoxemia with pH 7.15, PaCO2 90 mm Hg, and PaO2 150 mm Hg.
Pressure controlled ventilation was attempted unsuccessfully due to high airway resistance. Ultimately, the patient was set on volume control with low tidal volume, 6 mL/kg/IBW, high flow 90 L/min, PEEP 0 cm of H2O, and a low respiratory rate of 10 to achieve an inspiratory to expiratory (I:E) ratio of 1:7. Managing the ventilator to avoid dynamic hyperinflation and auto-PEEP, she remained relatively stable and improved.
By day 4 the patient’s ventilator was set to volume assist control with respiratory rate of 16, tidal volume, 6 mL/kg/IBW, PEEP 5-cm H2O with auto PEEP of 3-cm H2O, and fraction of inspired ABG O2 (FiO2) 0.35 with PAP of 46-cm H2O and plateau pressure of 17-cm H2O. The ABG was pH 7.32, PaCO2 65 mm Hg, and PaO2 74 mm Hg. However, on hospital day 5, she developed worsening PAP 60 to 77-cm H2O, plateau pressures 17-cm H2O, and a dynamic PEEP 16-cm H2O and was unresponsive to ventilator maneuvers to lower airway pressures and improve ventilation.
The patient had been receiving continuous albuterol and ipratropium nebulizer treatments. Ketamine infusion was considered fraught with potential for a dissociative reaction due to the patient’s significant PTSD. The patient’s family requested avoidance of ketamine infusion since the patient was paralyzed and psychiatric effects could not be monitored. Heliox 80/20 mixture was considered; however, it is incompatible with the ventilator that was being used since it could not account for the density of the helium gas flow in the tidal volumes. Extracorporeal membrane oxygenation (ECMO) was not available at our facility, and the patient was not a candidate for the regional ECMO center.
On hospital day 8, the patient developed worsening respiratory acidosis. The patient’s PAP increased to > 77-cm H2O, and her ABG revealed pH 7.22, PaCO2 90 mm Hg, and PaO2 77 mm Hg with FiO2 0.4. A chest X-ray demonstrated a new left lower lobe infiltrate. Fiber optic bronchoscopy was notable for scattered thick secretions throughout both lungs without obstructing mucus plug. Removal of airway secretions did not improve airway pressures or dynamic hyperinflation.
After consultation and discussion with the chief of anesthesia, the patient was placed on an anesthesia ventilator and started on sevoflurane 1.5% in the ICU. Anesthesiology was available 24 hours a day, and the anesthesiologist rounded with the intensivist frequently for this patient. The anesthesia technician worked closely with respiratory therapy regarding ventilator setting and changing the anesthesia gas scavenging charcoal canister. Within 4 hours, her gas exchange normalized (Table). The patient’s ABG was pH 7.44, PaCO2 52 mm Hg, and PaO2 69 mm Hg on FiO2 0.4. On volume cycled ventilation with a rate of 12, flow rate of 40 L/min, and tidal volume 6 mL/kg/IBW, the PAP decreased to 41-cm H2O.
Within 24 hours bronchospasm improved as evidenced by decreased airway pressures, resolution of wheezing, and decreased CO2 retention. The sevoflurane was easily weaned over the next 48 hours by decreasing the dose by 25% every 12-hour shift without rebound bronchospasm. Airway pressures and ABGs were frequently monitored during the weaning process. The patient resumed conventional mechanical ventilation, cisatracurium was discontinued, and she underwent a percutaneous tracheostomy for critical illness polymyopathy. Her respiratory muscle strength recovered more robustly than anticipated. Prior to discharge to a skilled nursing facility for continued rehabilitation, she was removed from mechanical ventilation and decannulated.
Discussion
This case illustrates the successful treatment of a patient with extreme status asthmaticus given inhalational anesthesia as supportive care while the bronchospasm and status asthmaticus abated. This is an unusual treatment in an ominous situation. Inhalational anesthetics are potent bronchodilators and have been successfully used in the management of status asthmaticus refractory to conventional therapy.4 Inhalational anesthetics have been shown to decrease airway resistance, dynamic hyperinflation, and intrinsic PEEP.5 These agents result in rapid bronchodilation by relaxing the smooth muscle and are associated with early liberation from mechanical ventilation.5,6 Although there are no guidelines regarding which inhalational agent is best, specific dosing, duration, or titration, case reports in the literature regarding the successful use of inhalational agents in life-threatening status asthmaticus exist.2,5,7
Caveats regarding the use of inhalational anesthetics in status asthmaticus include proarrhythmias, severe hepatic and renal toxicity. Although isoflurane is less likely to cause arrhythmia, both isoflurane and sevoflurane can cause dose-dependent hypotension by peripheral vasodilatation.7 Ourpatient did not manifest any adverse effects.
Additional challenges regarding the use of inhalational anesthetics for status asthmaticus include differences in ventilators and occupational hazards.8 Anesthesia or operating room ventilators differ from ICU ventilators in flow and pressure capabilities.7 The anesthesia ventilator is not capable of generating inspiratory pressures sufficient to ventilate patients with severely elevated airway resistance. Thus, the decrease inspiratory flow that occurs with increasing airway pressure limits the tidal volume delivered and consequently the minute volume. Although newer anesthesia ventilators have increased flow capabilities, they require a fully trained staff.8
Potential occupational exposure to these volatile anesthetic gases occurs as patients being treated may exhale considerable amounts of volatile anesthetics.8 An anesthesia gas scavenging device, such as a charcoal canister, must be attached to the ventilator to capture the exhaled anesthetic gases and should be changed every 12 hours.8 Finally, there is a potential for rebound bronchospasm as the anesthetic agent is tapered.6,7,9-11
Conclusion
Inhalational anesthetics are an option as rescue therapy for severe life-threatening asthma when all other therapies have failed. Use of inhalational anesthetics in status asthmaticus consists of case reports of which half are in children.2,5,7 Our patient contributes to the literature of case reports regarding using sevoflurane in refractory status asthmaticus. A decision to choose them must be a collaborative team approach with anesthesiology, pulmonary/critical care medicine, respiratory therapy, and ICU nurses, and the risks and benefits should be discussed with decision-making family members. Since there are no specific guidelines for the use of inhalational agents in status asthmaticus, close attention to inspiratory flows, gas scavenging devices, and clinical response is required. Additionally, the team must be comfortable with the plan to use an anesthesia ventilator and trained on its limitations.
1. Centers for Disease Control and Prevention. Most recent national asthma data. https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Updated May 2019. Accessed September 5, 2019.
2. Lazarus SC. Emergency treatment of asthma. N Engl J Med. 2010;363(8):755-764.
3. Shah R, Saltoun CA. Acute severe asthma (status asthmaticus). Allergy Asthma Proc. 2012;33(suppl 1):47-50.
4. Mutlu GM, Factor P, Schwartz DE, Snajder JI. Severe status asthmaticus: management with permissive hypercapnia and inhalation anesthesia Crit Care Med. 2002;30(2):477-480.
5. Maltais, F, Sovilj M, Goldber P, Gottfried SB. Respiratory mechanism in status asthmaticus. Effects of inhalational anesthesia. Chest. 1994;106(5):1401-1406.
6. Parnass SM, Feld JM, Chamberlin WH, Segil LJ. Status asthmaticus treated with isoflurane and enflurane. Anesth Analg. 1987;66(2):193-195.
7. Johnston RG, Noseworthy TW, Friesen EG, Yule HA, Shustack A. Isoflurane therapy for status asthmaticus in children and adults. Chest. 1990;97(3):698-701.
8. Meiser A, Laubenthal H. Inhalational anesthetics in the ICU: theory and practice of inhalational sedation in the ICU economics, risk-benefit. Best Pract Res Clin Anesthesiol. 2005;19(3):523-538.
9. Miller RD. Miller’s Anesthesia. 7th ed. Philadelphia, PA: Churchill Livingstone; 2010.
10. Nakao S, Hatano K, Sumi C, et al. Sevoflurane causes greater QTc interval prolongation in elderly patients than in younger patients. Anesth Analg. 2010;110(3):775-779.
11. Stachnik J. Inhaled anesthetic agents. Am J Health-Syst Pharm. 2006;63(7):623-634.
Asthma attacks account for 1.8 million emergency department (ED) visits each year in the US and for 10 deaths daily.1 Management of asthma attacks includes administration of inhaled ß2 adrenergic agonists, inhaled anticholinergic agents, IV magnesium sulfate, and corticosteroids.2 Status asthmaticus is an intense acute exacerbation of asthma that does not respond to repeated treatments of bronchodilators and corticosteroids.3 It is a medical emergency requiring immediate recognition and treatment. The decision to intubate a patient with status asthmaticus is a clinical decision based on work of breathing, respiratory acidosis, and failure to respond to medical interventions.
In refractory cases of status asthmaticus, intubation and mechanical ventilation are undertaken to provide oxygenation and ventilation until the bronchospasm resolves. However, mechanical ventilation is associated with significant risks, including high end-inspiratory pressures, barotrauma, and volutrauma.4 Rescue therapies include muscle relaxation, infusion of ketamine (central acting nonopioid analgesic with bronchodilatory properties), heliox, and general anesthesia.2,4 We report a case of a patient with life-threatening asthma and status asthmaticus treated with sevoflurane general anesthesia.
Case Presentation
A 55-year-old woman whose medical history was notable for asthma, psoriasis, hypothyroidism, tobacco, and alcohol abuse, and posttraumatic stress disorder (PTSD) presented to the ED. The patient had rarely sought medical attention and had no prior ED visits or hospitalizations in the electronic health record. Her home regimen included an albuterol inhaler used as needed. Her family reported that they had found her in distress in bed in a tripod position, unable to speak and struggling to breath.
Emergency medical services found the patient cyanotic, apneic, and pulseless. She received cardiopulmonary resuscitation for 30 seconds and 1-mg IV epinephrine, and spontaneous circulation returned. The patient arrived in the ED with an oral airway in place receiving bag valve mask ventilation. The patient expelled the oral airway. She was unable to speak due to dyspnea, exhibited persistent cyanosis, fatigue due to work of breathing, and failed to respond to nebulized albuterol/ipratropium bromide, IV methylprednisolone, and magnesium sulfate. The patient met criteria for acute severe asthma, or status asthmaticus. Thus, the patient received rapid sequence induction with rocuronium and ketamine and was intubated.
According to her family, the patient had no previous intensive care unit (ICU) admissions or prior intubations. Her only asthma medication was an albuterol inhaler as needed. The patient worked as a supervisor at a window blind manufacturing company. She lived alone, smoked 2 packs of cigarettes a day for more than 30 years, had no pets, drank unknown quantities of beer, wine, and hard liquor daily, and had smoked marijuana for several years.
The patient’s physical examination was notable for diffuse expiratory wheezes. Laboratory analysis revealed white blood cell count of 13.7 k/mcL, sodium 140 mmol/L, potassium 4.9 mmol/L, chloride 105 mmol/L, CO2 17 mmol/L, creatinine 0.98 mg/dL, troponin 0.03 ng/mL, lactate 7.2 mmol/L. Her chest X-ray showed hyperinflation but no focal opacities, pneumothorax, or pulmonary edema. Her endotracheal tube was in good position (Figure 1). A computed tomography pulmonary angiogram showed no pulmonary embolus or emphysema. There were atelectatic changes in the dependent portion of the right lower lobe, central bronchial wall thickening, and no stigmata of air trapping (Figure 2). An echocardiogram revealed a left ventricular ejection fraction of 45%, normal right ventricle and right ventricular size and function with an estimated right ventricular systolic pressure of 40 mm Hg.
The patient was admitted to the ICU and started on continuous infusion cisatracurium for paralysis and deep sedation to improve ventilatory synchrony and decrease auto positive end-expiratory pressure (PEEP). Mechanical ventilation was initiated with volume-cycled assist control ventilation, 6 mL/kg/ideal body weight (IBW) at 5-cm H2O PEEP, and 1 minute ventilation of 10 liters. The patient had severe air trapping and high airway pressures. The dynamic PEEP was 22-cm H2O (normal PEEP of 5-cm H2O), peak airway pressure (PAP) 41-cm H2O, and plateau pressure 31-cm H2O. In addition, the arterial blood gas (ABG) showed severe hypercapnic respiratory acidosis without significant hypoxemia with pH 7.15, PaCO2 90 mm Hg, and PaO2 150 mm Hg.
Pressure controlled ventilation was attempted unsuccessfully due to high airway resistance. Ultimately, the patient was set on volume control with low tidal volume, 6 mL/kg/IBW, high flow 90 L/min, PEEP 0 cm of H2O, and a low respiratory rate of 10 to achieve an inspiratory to expiratory (I:E) ratio of 1:7. Managing the ventilator to avoid dynamic hyperinflation and auto-PEEP, she remained relatively stable and improved.
By day 4 the patient’s ventilator was set to volume assist control with respiratory rate of 16, tidal volume, 6 mL/kg/IBW, PEEP 5-cm H2O with auto PEEP of 3-cm H2O, and fraction of inspired ABG O2 (FiO2) 0.35 with PAP of 46-cm H2O and plateau pressure of 17-cm H2O. The ABG was pH 7.32, PaCO2 65 mm Hg, and PaO2 74 mm Hg. However, on hospital day 5, she developed worsening PAP 60 to 77-cm H2O, plateau pressures 17-cm H2O, and a dynamic PEEP 16-cm H2O and was unresponsive to ventilator maneuvers to lower airway pressures and improve ventilation.
The patient had been receiving continuous albuterol and ipratropium nebulizer treatments. Ketamine infusion was considered fraught with potential for a dissociative reaction due to the patient’s significant PTSD. The patient’s family requested avoidance of ketamine infusion since the patient was paralyzed and psychiatric effects could not be monitored. Heliox 80/20 mixture was considered; however, it is incompatible with the ventilator that was being used since it could not account for the density of the helium gas flow in the tidal volumes. Extracorporeal membrane oxygenation (ECMO) was not available at our facility, and the patient was not a candidate for the regional ECMO center.
On hospital day 8, the patient developed worsening respiratory acidosis. The patient’s PAP increased to > 77-cm H2O, and her ABG revealed pH 7.22, PaCO2 90 mm Hg, and PaO2 77 mm Hg with FiO2 0.4. A chest X-ray demonstrated a new left lower lobe infiltrate. Fiber optic bronchoscopy was notable for scattered thick secretions throughout both lungs without obstructing mucus plug. Removal of airway secretions did not improve airway pressures or dynamic hyperinflation.
After consultation and discussion with the chief of anesthesia, the patient was placed on an anesthesia ventilator and started on sevoflurane 1.5% in the ICU. Anesthesiology was available 24 hours a day, and the anesthesiologist rounded with the intensivist frequently for this patient. The anesthesia technician worked closely with respiratory therapy regarding ventilator setting and changing the anesthesia gas scavenging charcoal canister. Within 4 hours, her gas exchange normalized (Table). The patient’s ABG was pH 7.44, PaCO2 52 mm Hg, and PaO2 69 mm Hg on FiO2 0.4. On volume cycled ventilation with a rate of 12, flow rate of 40 L/min, and tidal volume 6 mL/kg/IBW, the PAP decreased to 41-cm H2O.
Within 24 hours bronchospasm improved as evidenced by decreased airway pressures, resolution of wheezing, and decreased CO2 retention. The sevoflurane was easily weaned over the next 48 hours by decreasing the dose by 25% every 12-hour shift without rebound bronchospasm. Airway pressures and ABGs were frequently monitored during the weaning process. The patient resumed conventional mechanical ventilation, cisatracurium was discontinued, and she underwent a percutaneous tracheostomy for critical illness polymyopathy. Her respiratory muscle strength recovered more robustly than anticipated. Prior to discharge to a skilled nursing facility for continued rehabilitation, she was removed from mechanical ventilation and decannulated.
Discussion
This case illustrates the successful treatment of a patient with extreme status asthmaticus given inhalational anesthesia as supportive care while the bronchospasm and status asthmaticus abated. This is an unusual treatment in an ominous situation. Inhalational anesthetics are potent bronchodilators and have been successfully used in the management of status asthmaticus refractory to conventional therapy.4 Inhalational anesthetics have been shown to decrease airway resistance, dynamic hyperinflation, and intrinsic PEEP.5 These agents result in rapid bronchodilation by relaxing the smooth muscle and are associated with early liberation from mechanical ventilation.5,6 Although there are no guidelines regarding which inhalational agent is best, specific dosing, duration, or titration, case reports in the literature regarding the successful use of inhalational agents in life-threatening status asthmaticus exist.2,5,7
Caveats regarding the use of inhalational anesthetics in status asthmaticus include proarrhythmias, severe hepatic and renal toxicity. Although isoflurane is less likely to cause arrhythmia, both isoflurane and sevoflurane can cause dose-dependent hypotension by peripheral vasodilatation.7 Ourpatient did not manifest any adverse effects.
Additional challenges regarding the use of inhalational anesthetics for status asthmaticus include differences in ventilators and occupational hazards.8 Anesthesia or operating room ventilators differ from ICU ventilators in flow and pressure capabilities.7 The anesthesia ventilator is not capable of generating inspiratory pressures sufficient to ventilate patients with severely elevated airway resistance. Thus, the decrease inspiratory flow that occurs with increasing airway pressure limits the tidal volume delivered and consequently the minute volume. Although newer anesthesia ventilators have increased flow capabilities, they require a fully trained staff.8
Potential occupational exposure to these volatile anesthetic gases occurs as patients being treated may exhale considerable amounts of volatile anesthetics.8 An anesthesia gas scavenging device, such as a charcoal canister, must be attached to the ventilator to capture the exhaled anesthetic gases and should be changed every 12 hours.8 Finally, there is a potential for rebound bronchospasm as the anesthetic agent is tapered.6,7,9-11
Conclusion
Inhalational anesthetics are an option as rescue therapy for severe life-threatening asthma when all other therapies have failed. Use of inhalational anesthetics in status asthmaticus consists of case reports of which half are in children.2,5,7 Our patient contributes to the literature of case reports regarding using sevoflurane in refractory status asthmaticus. A decision to choose them must be a collaborative team approach with anesthesiology, pulmonary/critical care medicine, respiratory therapy, and ICU nurses, and the risks and benefits should be discussed with decision-making family members. Since there are no specific guidelines for the use of inhalational agents in status asthmaticus, close attention to inspiratory flows, gas scavenging devices, and clinical response is required. Additionally, the team must be comfortable with the plan to use an anesthesia ventilator and trained on its limitations.
Asthma attacks account for 1.8 million emergency department (ED) visits each year in the US and for 10 deaths daily.1 Management of asthma attacks includes administration of inhaled ß2 adrenergic agonists, inhaled anticholinergic agents, IV magnesium sulfate, and corticosteroids.2 Status asthmaticus is an intense acute exacerbation of asthma that does not respond to repeated treatments of bronchodilators and corticosteroids.3 It is a medical emergency requiring immediate recognition and treatment. The decision to intubate a patient with status asthmaticus is a clinical decision based on work of breathing, respiratory acidosis, and failure to respond to medical interventions.
In refractory cases of status asthmaticus, intubation and mechanical ventilation are undertaken to provide oxygenation and ventilation until the bronchospasm resolves. However, mechanical ventilation is associated with significant risks, including high end-inspiratory pressures, barotrauma, and volutrauma.4 Rescue therapies include muscle relaxation, infusion of ketamine (central acting nonopioid analgesic with bronchodilatory properties), heliox, and general anesthesia.2,4 We report a case of a patient with life-threatening asthma and status asthmaticus treated with sevoflurane general anesthesia.
Case Presentation
A 55-year-old woman whose medical history was notable for asthma, psoriasis, hypothyroidism, tobacco, and alcohol abuse, and posttraumatic stress disorder (PTSD) presented to the ED. The patient had rarely sought medical attention and had no prior ED visits or hospitalizations in the electronic health record. Her home regimen included an albuterol inhaler used as needed. Her family reported that they had found her in distress in bed in a tripod position, unable to speak and struggling to breath.
Emergency medical services found the patient cyanotic, apneic, and pulseless. She received cardiopulmonary resuscitation for 30 seconds and 1-mg IV epinephrine, and spontaneous circulation returned. The patient arrived in the ED with an oral airway in place receiving bag valve mask ventilation. The patient expelled the oral airway. She was unable to speak due to dyspnea, exhibited persistent cyanosis, fatigue due to work of breathing, and failed to respond to nebulized albuterol/ipratropium bromide, IV methylprednisolone, and magnesium sulfate. The patient met criteria for acute severe asthma, or status asthmaticus. Thus, the patient received rapid sequence induction with rocuronium and ketamine and was intubated.
According to her family, the patient had no previous intensive care unit (ICU) admissions or prior intubations. Her only asthma medication was an albuterol inhaler as needed. The patient worked as a supervisor at a window blind manufacturing company. She lived alone, smoked 2 packs of cigarettes a day for more than 30 years, had no pets, drank unknown quantities of beer, wine, and hard liquor daily, and had smoked marijuana for several years.
The patient’s physical examination was notable for diffuse expiratory wheezes. Laboratory analysis revealed white blood cell count of 13.7 k/mcL, sodium 140 mmol/L, potassium 4.9 mmol/L, chloride 105 mmol/L, CO2 17 mmol/L, creatinine 0.98 mg/dL, troponin 0.03 ng/mL, lactate 7.2 mmol/L. Her chest X-ray showed hyperinflation but no focal opacities, pneumothorax, or pulmonary edema. Her endotracheal tube was in good position (Figure 1). A computed tomography pulmonary angiogram showed no pulmonary embolus or emphysema. There were atelectatic changes in the dependent portion of the right lower lobe, central bronchial wall thickening, and no stigmata of air trapping (Figure 2). An echocardiogram revealed a left ventricular ejection fraction of 45%, normal right ventricle and right ventricular size and function with an estimated right ventricular systolic pressure of 40 mm Hg.
The patient was admitted to the ICU and started on continuous infusion cisatracurium for paralysis and deep sedation to improve ventilatory synchrony and decrease auto positive end-expiratory pressure (PEEP). Mechanical ventilation was initiated with volume-cycled assist control ventilation, 6 mL/kg/ideal body weight (IBW) at 5-cm H2O PEEP, and 1 minute ventilation of 10 liters. The patient had severe air trapping and high airway pressures. The dynamic PEEP was 22-cm H2O (normal PEEP of 5-cm H2O), peak airway pressure (PAP) 41-cm H2O, and plateau pressure 31-cm H2O. In addition, the arterial blood gas (ABG) showed severe hypercapnic respiratory acidosis without significant hypoxemia with pH 7.15, PaCO2 90 mm Hg, and PaO2 150 mm Hg.
Pressure controlled ventilation was attempted unsuccessfully due to high airway resistance. Ultimately, the patient was set on volume control with low tidal volume, 6 mL/kg/IBW, high flow 90 L/min, PEEP 0 cm of H2O, and a low respiratory rate of 10 to achieve an inspiratory to expiratory (I:E) ratio of 1:7. Managing the ventilator to avoid dynamic hyperinflation and auto-PEEP, she remained relatively stable and improved.
By day 4 the patient’s ventilator was set to volume assist control with respiratory rate of 16, tidal volume, 6 mL/kg/IBW, PEEP 5-cm H2O with auto PEEP of 3-cm H2O, and fraction of inspired ABG O2 (FiO2) 0.35 with PAP of 46-cm H2O and plateau pressure of 17-cm H2O. The ABG was pH 7.32, PaCO2 65 mm Hg, and PaO2 74 mm Hg. However, on hospital day 5, she developed worsening PAP 60 to 77-cm H2O, plateau pressures 17-cm H2O, and a dynamic PEEP 16-cm H2O and was unresponsive to ventilator maneuvers to lower airway pressures and improve ventilation.
The patient had been receiving continuous albuterol and ipratropium nebulizer treatments. Ketamine infusion was considered fraught with potential for a dissociative reaction due to the patient’s significant PTSD. The patient’s family requested avoidance of ketamine infusion since the patient was paralyzed and psychiatric effects could not be monitored. Heliox 80/20 mixture was considered; however, it is incompatible with the ventilator that was being used since it could not account for the density of the helium gas flow in the tidal volumes. Extracorporeal membrane oxygenation (ECMO) was not available at our facility, and the patient was not a candidate for the regional ECMO center.
On hospital day 8, the patient developed worsening respiratory acidosis. The patient’s PAP increased to > 77-cm H2O, and her ABG revealed pH 7.22, PaCO2 90 mm Hg, and PaO2 77 mm Hg with FiO2 0.4. A chest X-ray demonstrated a new left lower lobe infiltrate. Fiber optic bronchoscopy was notable for scattered thick secretions throughout both lungs without obstructing mucus plug. Removal of airway secretions did not improve airway pressures or dynamic hyperinflation.
After consultation and discussion with the chief of anesthesia, the patient was placed on an anesthesia ventilator and started on sevoflurane 1.5% in the ICU. Anesthesiology was available 24 hours a day, and the anesthesiologist rounded with the intensivist frequently for this patient. The anesthesia technician worked closely with respiratory therapy regarding ventilator setting and changing the anesthesia gas scavenging charcoal canister. Within 4 hours, her gas exchange normalized (Table). The patient’s ABG was pH 7.44, PaCO2 52 mm Hg, and PaO2 69 mm Hg on FiO2 0.4. On volume cycled ventilation with a rate of 12, flow rate of 40 L/min, and tidal volume 6 mL/kg/IBW, the PAP decreased to 41-cm H2O.
Within 24 hours bronchospasm improved as evidenced by decreased airway pressures, resolution of wheezing, and decreased CO2 retention. The sevoflurane was easily weaned over the next 48 hours by decreasing the dose by 25% every 12-hour shift without rebound bronchospasm. Airway pressures and ABGs were frequently monitored during the weaning process. The patient resumed conventional mechanical ventilation, cisatracurium was discontinued, and she underwent a percutaneous tracheostomy for critical illness polymyopathy. Her respiratory muscle strength recovered more robustly than anticipated. Prior to discharge to a skilled nursing facility for continued rehabilitation, she was removed from mechanical ventilation and decannulated.
Discussion
This case illustrates the successful treatment of a patient with extreme status asthmaticus given inhalational anesthesia as supportive care while the bronchospasm and status asthmaticus abated. This is an unusual treatment in an ominous situation. Inhalational anesthetics are potent bronchodilators and have been successfully used in the management of status asthmaticus refractory to conventional therapy.4 Inhalational anesthetics have been shown to decrease airway resistance, dynamic hyperinflation, and intrinsic PEEP.5 These agents result in rapid bronchodilation by relaxing the smooth muscle and are associated with early liberation from mechanical ventilation.5,6 Although there are no guidelines regarding which inhalational agent is best, specific dosing, duration, or titration, case reports in the literature regarding the successful use of inhalational agents in life-threatening status asthmaticus exist.2,5,7
Caveats regarding the use of inhalational anesthetics in status asthmaticus include proarrhythmias, severe hepatic and renal toxicity. Although isoflurane is less likely to cause arrhythmia, both isoflurane and sevoflurane can cause dose-dependent hypotension by peripheral vasodilatation.7 Ourpatient did not manifest any adverse effects.
Additional challenges regarding the use of inhalational anesthetics for status asthmaticus include differences in ventilators and occupational hazards.8 Anesthesia or operating room ventilators differ from ICU ventilators in flow and pressure capabilities.7 The anesthesia ventilator is not capable of generating inspiratory pressures sufficient to ventilate patients with severely elevated airway resistance. Thus, the decrease inspiratory flow that occurs with increasing airway pressure limits the tidal volume delivered and consequently the minute volume. Although newer anesthesia ventilators have increased flow capabilities, they require a fully trained staff.8
Potential occupational exposure to these volatile anesthetic gases occurs as patients being treated may exhale considerable amounts of volatile anesthetics.8 An anesthesia gas scavenging device, such as a charcoal canister, must be attached to the ventilator to capture the exhaled anesthetic gases and should be changed every 12 hours.8 Finally, there is a potential for rebound bronchospasm as the anesthetic agent is tapered.6,7,9-11
Conclusion
Inhalational anesthetics are an option as rescue therapy for severe life-threatening asthma when all other therapies have failed. Use of inhalational anesthetics in status asthmaticus consists of case reports of which half are in children.2,5,7 Our patient contributes to the literature of case reports regarding using sevoflurane in refractory status asthmaticus. A decision to choose them must be a collaborative team approach with anesthesiology, pulmonary/critical care medicine, respiratory therapy, and ICU nurses, and the risks and benefits should be discussed with decision-making family members. Since there are no specific guidelines for the use of inhalational agents in status asthmaticus, close attention to inspiratory flows, gas scavenging devices, and clinical response is required. Additionally, the team must be comfortable with the plan to use an anesthesia ventilator and trained on its limitations.
1. Centers for Disease Control and Prevention. Most recent national asthma data. https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Updated May 2019. Accessed September 5, 2019.
2. Lazarus SC. Emergency treatment of asthma. N Engl J Med. 2010;363(8):755-764.
3. Shah R, Saltoun CA. Acute severe asthma (status asthmaticus). Allergy Asthma Proc. 2012;33(suppl 1):47-50.
4. Mutlu GM, Factor P, Schwartz DE, Snajder JI. Severe status asthmaticus: management with permissive hypercapnia and inhalation anesthesia Crit Care Med. 2002;30(2):477-480.
5. Maltais, F, Sovilj M, Goldber P, Gottfried SB. Respiratory mechanism in status asthmaticus. Effects of inhalational anesthesia. Chest. 1994;106(5):1401-1406.
6. Parnass SM, Feld JM, Chamberlin WH, Segil LJ. Status asthmaticus treated with isoflurane and enflurane. Anesth Analg. 1987;66(2):193-195.
7. Johnston RG, Noseworthy TW, Friesen EG, Yule HA, Shustack A. Isoflurane therapy for status asthmaticus in children and adults. Chest. 1990;97(3):698-701.
8. Meiser A, Laubenthal H. Inhalational anesthetics in the ICU: theory and practice of inhalational sedation in the ICU economics, risk-benefit. Best Pract Res Clin Anesthesiol. 2005;19(3):523-538.
9. Miller RD. Miller’s Anesthesia. 7th ed. Philadelphia, PA: Churchill Livingstone; 2010.
10. Nakao S, Hatano K, Sumi C, et al. Sevoflurane causes greater QTc interval prolongation in elderly patients than in younger patients. Anesth Analg. 2010;110(3):775-779.
11. Stachnik J. Inhaled anesthetic agents. Am J Health-Syst Pharm. 2006;63(7):623-634.
1. Centers for Disease Control and Prevention. Most recent national asthma data. https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Updated May 2019. Accessed September 5, 2019.
2. Lazarus SC. Emergency treatment of asthma. N Engl J Med. 2010;363(8):755-764.
3. Shah R, Saltoun CA. Acute severe asthma (status asthmaticus). Allergy Asthma Proc. 2012;33(suppl 1):47-50.
4. Mutlu GM, Factor P, Schwartz DE, Snajder JI. Severe status asthmaticus: management with permissive hypercapnia and inhalation anesthesia Crit Care Med. 2002;30(2):477-480.
5. Maltais, F, Sovilj M, Goldber P, Gottfried SB. Respiratory mechanism in status asthmaticus. Effects of inhalational anesthesia. Chest. 1994;106(5):1401-1406.
6. Parnass SM, Feld JM, Chamberlin WH, Segil LJ. Status asthmaticus treated with isoflurane and enflurane. Anesth Analg. 1987;66(2):193-195.
7. Johnston RG, Noseworthy TW, Friesen EG, Yule HA, Shustack A. Isoflurane therapy for status asthmaticus in children and adults. Chest. 1990;97(3):698-701.
8. Meiser A, Laubenthal H. Inhalational anesthetics in the ICU: theory and practice of inhalational sedation in the ICU economics, risk-benefit. Best Pract Res Clin Anesthesiol. 2005;19(3):523-538.
9. Miller RD. Miller’s Anesthesia. 7th ed. Philadelphia, PA: Churchill Livingstone; 2010.
10. Nakao S, Hatano K, Sumi C, et al. Sevoflurane causes greater QTc interval prolongation in elderly patients than in younger patients. Anesth Analg. 2010;110(3):775-779.
11. Stachnik J. Inhaled anesthetic agents. Am J Health-Syst Pharm. 2006;63(7):623-634.