User login
Do Clinicians Understand Quality Metric Data?
Central line-associated bloodstream infections (CLABSIs) are common and serious occurrences across healthcare systems, with an attributable mortality of 12% to 25%.1,2 Given this burden,3–5 CLABSI is a focus for both high-profile public reporting and quality improvement interventions. An integral component of such interventions is audit and feedback via quality metrics. These measures are intended to allow decision makers to assess their own performance and appropriately allocate resources. Quality metrics present a substantial cost to health systems, with an estimated $15.4 billion dollars spent annually simply for reporting.6 Despite this toll, “audit and feedback” interventions have proven to be variably successful.7–9 The mechanisms that limit the effectiveness of these interventions remain
poorly understood.
One plausible explanation for limited efficacy of quality metrics is inadequate clinician numeracy—that is, “the ability to understand the quantitative aspects of clinical medicine, original research, quality improvement, and financial matters.”10 Indeed, clinicians are not consistently able to interpret probabilities and or clinical test characteristics. For example, Wegwarth et al. identified shortcomings in physician application of lead-time bias toward cancer screening.11 Additionally, studies have demonstrated systematic misinterpretations of probabilistic information in clinical settings, along with misconceptions regarding the impact of prevalence on post-test probabilities.12,13 Effective interpretation of rates may be a key—if unstated—requirement of many CLABSI quality improvement efforts.14–19 Our broader hypothesis is that clinicians who can more accurately interpret quality data, even if only from their own institution, are more likely to act on it appropriately and persistently than those who feel they must depend on a preprocessed interpretation of that same data by some other expert.
Therefore, we designed a survey to assess the numeracy of clinicians on CLABSI data presented in a prototypical feedback report. We studied 3 domains of comprehension: (1) basic numeracy: numerical tasks related to simple data; (2) risk-adjustment numeracy: numerical tasks related to risk-adjusted data; and (3) risk-adjustment interpretation: inferential tasks concerning risk-adjusted data. We hypothesized that clinician performance would vary substantially across domains, with the poorest performance in risk-
adjusted data.
METHODS
We conducted a cross-sectional survey of clinician numeracy regarding CLABSI feedback data. Respondents were also asked to provide demographic information and opinions regarding the reliability of quality metric data. Survey recruitment occurred on Twitter, a novel approach that leveraged social media to facilitate rapid recruitment of participants. The study instrument was administered using a web survey with randomized question order to preclude any possibility of order effects between questions. The study was deemed Institutional Review Board exempt by the University of Michigan: protocol HUM00106696.
Data Presentation Method
To determine the optimal mode of presenting data, we reviewed the literature on quality metric numeracy and presentation methods. Additionally, we evaluated quality metric presentation methods used by the Centers for Disease Control and Prevention (CDC), Centers for Medicare & Medicaid Services (CMS), and a tertiary academic medical center. After assessing the available literature and options, we adapted a CLABSI data presentation array from a study that had qualitatively validated the format using physician feedback (Appendix).20 We used hypothetical CLABSI data for our survey.
Survey Development
We developed a survey that included an 11-item test regarding CLABSI numeracy and data interpretation. Additional questions related to quality metric reliability and demographic information were included. No preexisting assessment tools existed for our areas of interest. Therefore, we developed a novel instrument using a broad, exploratory approach as others have employed.21
First, we defined 3 conceptual categories related to CLABSI data. Within this conceptual framework, an iterative process of development and revision was used to assemble a question bank from which the survey would be constructed. A series of think-aloud sessions were held to evaluate each prompt for precision, clarity, and accuracy in assessing the conceptual categories. Correct and incorrect answers were defined based on literature review in conjunction with input from methodological and content experts (TJI and VC) (see Appendix for answer explanations).
Within the conceptual categories related to CLABSI risk-adjustment, a key measure is the standardized infection ratio (SIR). This value is defined as the ratio of observed number of CLABSI over the expected number of CLABSIs.22 This is the primary measure to stratify hospital performance, and it was used in our assessment of risk-adjustment comprehension. In total, 54 question prompts were developed and subsequently narrowed to 11 study questions for the initial survey.
The instrument was then pretested in a cohort of 8 hospitalists and intensivists to ensure appropriate comprehension, retrieval, and judgment processes.23 Questions were revised based on feedback from this cognitive testing to constitute the final instrument. During the survey, the data table was reshown on each page directly above each question and so was always on the same screen for the respondents.
Survey Sample
We innovated by using Twitter as an online platform for recruiting participants; we used Survey Monkey to host the electronic instrument. Two authors (TJI, VC) systematically sent out solicitation tweets to their followers. These tweets clearly indicated that the recruitment was for the purpose of a research study, and participants would receive no financial reward/incentive (Appendix). A link to the survey was provided in each tweet, and the period of recruitment was 30 days. To ensure respondents were clinicians, they needed to first answer a screening question recognizing that central lines were placed in the subclavian site but not the aorta, iliac, or radial sites.
To prevent systematic or anchoring biases, the order of questions was electronically randomized for each respondent. The primary outcome was the percentage correct of attempted questions.
Statistical Analysis
Descriptive statistics were calculated for all demographic variables. The primary outcome was evaluated as a dichotomous variable for each question (correct vs. incorrect response), and as a continuous variable when assessing mean percent correct on the overall survey. Demographic and conceptual associations were assessed via t-tests, chi-square, or Fisher exact tests. Point biserial correlations were calculated to assess for associations between response to a single question and overall performance on the survey.
To evaluate the association between various respondent characteristics and responses, logistic regression analyses were performed. An ANOVA was performed to assess the association between self-reported reliability of quality metric data and the overall performance on attempted items. Analyses were conducted using STATA MP 14.0 (College Station, TX); P <0.05 was considered statistically significant.
RESULTS
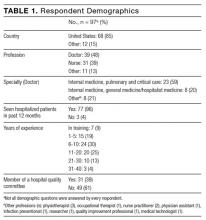
A total of 97 respondents attempted at least 1 question on the survey, and 72 respondents attempted all 11 questions, yielding 939 unique responses for analysis. Seventy respondents (87%) identified as doctors or nurses, and 44 (55%) reported having 6 to 20 years of experience; the survey cohort also came from 6 nations (Table 1). All respondents answered the CLABSI knowledge filter question correctly.
Primary Outcome
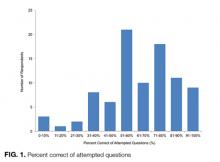
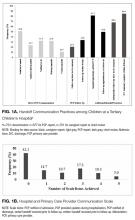
The mean percent correct of attempted questions was 61% (standard deviation 21%, interquartile range 50%-75%) (Figure 1). Of those who answered all 11 CLABSI questions, the mean percent correct was 63% (95% CI, 59%-67%). Some questions were answered correctly more often than others—ranging from 17% to 95% (Table 2). Doctors answered 68% of questions correctly (95% CI, 63%-73%), while nurses and other respondents answered 57% of questions correctly (95% CI, 52%-62%) (P = 0.003). Other demographic variables—including self-reported involvement in a quality improvement committee and being from the United States versus elsewhere—were not associated with survey performance. The point biserial correlations for each individual question with overall performance were all more than 0.2 (range 0.24–0.62) and all statistically significant at P < 0.05.
Concept-Specific Performance
Average percent correct declined across categories as numeracy requirements increased (P < 0.05 for all pairwise comparisons). In the area of basic numeracy, respondents’ mean percent correct was 82% (95% CI, 77%-87%) of attempted. This category had 4 questions, with a performance range of 77% to 90%. For example, on the question, “Which hospital has the lowest CLABSI rate?”, 80% of respondents answered correctly. For risk-adjustment numeracy, the mean percent correct was 70% (95% CI, 64%-76%); 2 items assessed this category. For “Which is better: a higher or lower SIR?”, 95% of the cohort answered correctly. However, on “If hospital B had its number of projected infection halved, what is its SIR?”, only 46% of those who attempted the question answered correctly.
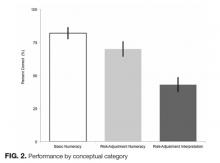
Questions featuring risk-adjustment interpretation had an average percent correct of 43% (95% CI, 37%-49%). Five questions made up this category, with a percent correct range of 17% to 75%. For example, on the question, “Which hospital’s patients are the most predisposed to developing CLABSI?”, only 32% of respondents answered this correctly. In contrast, for the question “Which hospital is most effective at preventing CLABSI?”, 51% answered correctly. Figure 2 illustrates the cohort’s performance on each conceptual category while Table 2 displays question-by-question results.
Opinions Regarding CLABSI Data Reliability
Respondents were also asked about their opinion regarding the reliability of CLABSI quality metric data. Forty-three percent of respondents stated that such data were reliable at best 50% of the time. Notably, 10% of respondents indicated that CLABSI quality metric data were rarely or never reliable. There was no association between perceived reliability of quality metric data and survey performance (P = 0.87).
DISCUSSION
This Twitter-based study found wide variation in clinician interpretation of CLABSI quality data, with low overall performance. In particular, comprehension and interpretation of risk-adjusted data were substantially worse than unadjusted data. Although doctors performed somewhat better than nurses and other respondents, those involved in quality improvement initiatives performed no better than respondents who were not. Collectively, these findings suggest clinicians may not reliably comprehend quality metric data, potentially affecting their ability to utilize audit and feedback data. These results may have important implications for policy efforts that seek to leverage quality metric data to improve patient safety.
An integral component of many contemporary quality improvement initiatives is audit and feedback through metrics.6 Unfortunately, formal audit and feedback, along with other similar methods that benchmark data, have not consistently improved outcomes.24–27 A recent meta-analysis noted that audit and feedback interventions are not becoming more efficacious over time; the study further asserted that “new trials have provided little new knowledge regarding key effect modifiers.”9 Our findings suggest that numeracy and comprehension of quality metrics may be important candidate effect modifiers not previously considered. Simply put: we hypothesize that without intrinsic comprehension of data, impetus or insight to change practice might be diminished. In other words, clinicians may be more apt to act on insights they themselves derive from the data than when they are simply told what the data “mean.”
The present study further demonstrates that clinicians do not understand risk-adjusted data as well as raw data. Risk-adjustment has long been recognized as necessary to compare outcomes among hospitals.28,29 However, risk-adjustment is complex and, by its nature, difficult to understand. Although efforts have focused on improving the statistical reliability of quality metrics, this may represent but one half of the equation. Numeracy and interpretation of the data by decision makers are potentially equally important to effecting change. Because clinicians seem to have difficulty understanding risk-adjusted data, this deficit may be of growing importance as our risk-adjustment techniques become more sophisticated.
We note that clinicians expressed concerns regarding the reliability of quality metric feedback. These findings corroborate recent research that has reported reservations from hospital leaders concerning quality data.30,31 However, as shown in the context of patients and healthcare decisions, the aversion associated with quality metrics may be related to incomplete understanding of the data.32 Whether perceptions of unreliability drive lack of understanding or, conversely, whether lack of understanding fuels perceived unreliability is an important question that requires further study.
This study has several strengths. First, we used rigorous survey development techniques to evaluate the understudied issue of quality metric numeracy. Second, our sample size was sufficient to show statistically significant differences in numeracy and comprehension of CLABSI quality metric data. Third, we leveraged social media to rapidly acquire this sample. Finally, our results provided new insights that may have important implications in the area of quality metrics.
There were also limitations to our study. First, the Twitter-derived sample precludes the calculation of a response rate and may not be representative of individuals engaged in CLABSI prevention. However, respondents were solicited from the Twitter-followers of 2 health services researchers (TJI, VC) who are actively engaged in scholarly activities pertaining to critically ill patients and hospital-acquired complications. Thus, our sample likely represents a highly motivated subset that engages in these topics on a regular basis—potentially making them more numerate than average clinicians. Second, we did not ask whether the respondents had previously seen CLABSI data specifically, so we cannot stratify by exposure to such data. Third, this study assessed only CLABSI quality metric data; generalizations regarding numeracy with other metrics should be made with caution. However, as many such data are presented in similar formats, we suspect our findings are applicable to similar audit-and-feedback initiatives.
The findings of this study serve as a stimulus for further inquiry. Research of this nature needs to be carried out in samples drawn from specific, policy-relevant populations (eg, infection control practitioners, bedside nurses, intensive care unit directors). Such studies should include longitudinal assessments of numeracy that attempt to mechanistically examine its impact on CLABSI prevention efforts and outcomes. The latter is an important issue as the link between numeracy and behavioral response, while plausible, cannot be assumed, particularly given the complexity of issues related to behavioral modification.33 Additionally, whether alternate presentations of quality data affect numeracy, interpretation, and performance is worthy of further testing; indeed, this has been shown to be the case in other forms of communication.34–37 Until data from larger samples are available, it may be prudent for quality improvement leaders to assess the comprehension of local clinicians regarding feedback and whether lack of adequate comprehension is a barrier to deploying quality improvement interventions.
Quality measurement is a cornerstone of patient safety as it seeks to assess and improve the care delivered at the bedside. Rigorous metric development is important; however, ensuring that decision makers understand complex quality metrics may be equally fundamental. Given the cost of examining quality, elucidating the mechanisms of numeracy and interpretation as decision makers engage with quality metric data is necessary, along with whether improved comprehension leads to behavior change. Such inquiry may provide an evidence-base to shape alterations in quality metric deployment that will ensure maximal efficacy in driving practice change.
Disclosures
This work was supported by VA HSR&D IIR-13-079 (TJI). Dr. Chopra is supported by a career development award from the Agency of Healthcare Research and Quality (1-K08-HS022835-01). The views expressed here are the authors’ own and do not necessarily represent the view of the US Government or the Department of Veterans’ Affairs. The authors report no conflicts of interest.
1. Scott RD II. The direct medical costs of healthcare-associated infections in us hospitals and the benefits of prevention. Centers for Disease Control and Prevention. Available at: http://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf. Published March 2009. Accessed November 8, 2016.
2. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39(4 suppl 1)::S1-S34. PubMed
3. Blot K, Bergs J, Vogelaers D, Blot S, Vandijck D. Prevention of central line-associated bloodstream infections through quality improvement interventions: a systematic review and meta-analysis. Clin Infect Dis. 2014;59(1):96-105. PubMed
4. Mermel LA. Prevention of intravascular catheter-related infections. Ann Intern Med. 2000;132(5):391-402. PubMed
5. Siempos II, Kopterides P, Tsangaris I, Dimopoulou I, Armaganidis AE. Impact of catheter-related bloodstream infections on the mortality of critically ill patients: a meta-analysis. Crit Care Med. 2009;37(7):2283-2289. PubMed
6. Casalino LP, Gans D, Weber R, et al. US physician practices spend more than $15.4 billion annually to report quality measures. Health Aff (Millwood). 2016;35(3):401-406. PubMed
7. Hysong SJ. Meta-analysis: audit and feedback features impact effectiveness on care quality. Med Care. 2009;47(3):356-363. PubMed
8. Ilgen DR, Fisher CD, Taylor MS. Consequences of individual feedback on behavior in organizations. J Appl Psychol. 1979;64:349-371.
9. Ivers NM, Grimshaw JM, Jamtvedt G, et al. Growing literature, stagnant science? Systematic review, meta-regression and cumulative analysis of audit and feedback interventions in health care. J Gen Intern Med. 2014;29(11):1534-1541. PubMed
10. Rao G. Physician numeracy: essential skills for practicing evidence-based medicine. Fam Med. 2008;40(5):354-358. PubMed
11. Wegwarth O, Schwartz LM, Woloshin S, Gaissmaier W, Gigerenzer G. Do physicians understand cancer screening statistics? A national survey of primary care physicians in the United States. Ann Intern Med. 2012;156(5):340-349. PubMed
12. Bramwell R, West H, Salmon P. Health professionals’ and service users’ interpretation of screening test results: experimental study. BMJ. 2006;333(7562):284. PubMed
13. Agoritsas T, Courvoisier DS, Combescure C, Deom M, Perneger TV. Does prevalence matter to physicians in estimating post-test probability of disease? A randomized trial. J Gen Intern Med. 2011;26(4):373-378. PubMed
14. Warren DK, Zack JE, Mayfield JL, et al. The effect of an education program on the incidence of central venous catheter-associated bloodstream infection in a medical ICU. Chest. 2004;126(5):1612-1618. PubMed
15. Rinke ML, Bundy DG, Chen AR, et al. Central line maintenance bundles and CLABSIs in ambulatory oncology patients. Pediatrics. 2013;132(5):e1403-e1412. PubMed
16. Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):
2725-2732. PubMed
17. Rinke ML, Chen AR, Bundy DG, et al. Implementation of a central line maintenance care bundle in hospitalized pediatric oncology patients. Pediatrics. 2012;130(4):e996-e1004. PubMed
18. Sacks GD, Diggs BS, Hadjizacharia P, Green D, Salim A, Malinoski DJ. Reducing the rate of catheter-associated bloodstream infections in a surgical intensive care unit using the Institute for Healthcare Improvement Central Line Bundle. Am J Surg. 2014;207(6):817-823. PubMed
19. Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014-2020. PubMed
20. Rajwan YG, Barclay PW, Lee T, Sun IF, Passaretti C, Lehmann H. Visualizing central line-associated blood stream infection (CLABSI) outcome data for decision making by health care consumers and practitioners—an evaluation study. Online J Public Health Inform. 2013;5(2):218. PubMed
21. Fagerlin A, Zikmund-Fisher BJ, Ubel PA, Jankovic A, Derry HA, Smith DM. Measuring numeracy without a math test: development of the Subjective Numeracy Scale. Med Decis Making 2007;27(5):672-680. PubMed
22. HAI progress report FAQ. 2016. Available at: http://www.cdc.gov/hai/surveillance/progress-report/faq.html. Last updated March 2, 2016. Accessed November 8, 2016.
23. Collins D. Pretesting survey instruments: an overview of cognitive methods. Qual Life Res. 2003;12(3):229-238. PubMed
24. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259. PubMed
25. Chatterjee P, Joynt KE. Do cardiology quality measures actually improve patient outcomes? J Am Heart Assoc. 2014;3(1):e000404. PubMed
26. Joynt KE, Blumenthal DM, Orav EJ, Resnic FS, Jha AK. Association of public reporting for percutaneous coronary intervention with utilization and outcomes among Medicare beneficiaries with acute myocardial infarction. JAMA. 2012;308(14):1460-1468. PubMed
27. Ryan AM, Nallamothu BK, Dimick JB. Medicare’s public reporting initiative on hospital quality had modest or no impact on mortality from three key conditions. Health Aff (Millwood). 2012;31(3):585-592. PubMed
28. Thomas JW. Risk adjustment for measuring health care outcomes, 3rd edition. Int J Qual Health Care. 2004;16(2):181-182.
29. Iezzoni LI. Risk Adjustment for Measuring Health Care Outcomes. Ann Arbor, Michigan: Health Administration Press; 1994.
30. Goff SL, Lagu T, Pekow PS, et al. A qualitative analysis of hospital leaders’ opinions about publicly reported measures of health care quality. Jt Comm J Qual Patient Saf. 2015;41(4):169-176. PubMed
31. Lindenauer PK, Lagu T, Ross JS, et al. Attitudes of hospital leaders toward publicly reported measures of health care quality. JAMA Intern Med. 2014;174(12):
1904-1911. PubMed
32. Peters E, Hibbard J, Slovic P, Dieckmann N. Numeracy skill and the communication, comprehension, and use of risk-benefit information. Health Aff (Millwood). 2007;26(3):741-748. PubMed
33. Montano DE, Kasprzyk D. Theory of reasoned action, theory of planned behavior, and the integrated behavioral model. In: Glanz K, Rimer BK, Viswanath K, eds. Health Behavior and Health Education: Theory, Research and Practice. 5th ed. San Francisco, CA: Jossey-Bass; 2015:95–124.
34. Hamstra DA, Johnson SB, Daignault S, et al. The impact of numeracy on verbatim knowledge of the longitudinal risk for prostate cancer recurrence following radiation therapy. Med Decis Making. 2015;35(1):27-36. PubMed
35. Hawley ST, Zikmund-Fisher B, Ubel P, Jancovic A, Lucas T, Fagerlin A. The impact of the format of graphical presentation on health-related knowledge and treatment choices. Patient Educ Couns. 2008;73(3):448-455. PubMed
36. Zikmund-Fisher BJ, Witteman HO, Dickson M, et al. Blocks, ovals, or people? Icon type affects risk perceptions and recall of pictographs. Med Decis Making. 2014;34(4):443-453. PubMed
37. Korfage IJ, Fuhrel-Forbis A, Ubel PA, et al. Informed choice about breast cancer prevention: randomized controlled trial of an online decision aid intervention. Breast Cancer Res. 2013;15(5):R74. PubMed
Central line-associated bloodstream infections (CLABSIs) are common and serious occurrences across healthcare systems, with an attributable mortality of 12% to 25%.1,2 Given this burden,3–5 CLABSI is a focus for both high-profile public reporting and quality improvement interventions. An integral component of such interventions is audit and feedback via quality metrics. These measures are intended to allow decision makers to assess their own performance and appropriately allocate resources. Quality metrics present a substantial cost to health systems, with an estimated $15.4 billion dollars spent annually simply for reporting.6 Despite this toll, “audit and feedback” interventions have proven to be variably successful.7–9 The mechanisms that limit the effectiveness of these interventions remain
poorly understood.
One plausible explanation for limited efficacy of quality metrics is inadequate clinician numeracy—that is, “the ability to understand the quantitative aspects of clinical medicine, original research, quality improvement, and financial matters.”10 Indeed, clinicians are not consistently able to interpret probabilities and or clinical test characteristics. For example, Wegwarth et al. identified shortcomings in physician application of lead-time bias toward cancer screening.11 Additionally, studies have demonstrated systematic misinterpretations of probabilistic information in clinical settings, along with misconceptions regarding the impact of prevalence on post-test probabilities.12,13 Effective interpretation of rates may be a key—if unstated—requirement of many CLABSI quality improvement efforts.14–19 Our broader hypothesis is that clinicians who can more accurately interpret quality data, even if only from their own institution, are more likely to act on it appropriately and persistently than those who feel they must depend on a preprocessed interpretation of that same data by some other expert.
Therefore, we designed a survey to assess the numeracy of clinicians on CLABSI data presented in a prototypical feedback report. We studied 3 domains of comprehension: (1) basic numeracy: numerical tasks related to simple data; (2) risk-adjustment numeracy: numerical tasks related to risk-adjusted data; and (3) risk-adjustment interpretation: inferential tasks concerning risk-adjusted data. We hypothesized that clinician performance would vary substantially across domains, with the poorest performance in risk-
adjusted data.
METHODS
We conducted a cross-sectional survey of clinician numeracy regarding CLABSI feedback data. Respondents were also asked to provide demographic information and opinions regarding the reliability of quality metric data. Survey recruitment occurred on Twitter, a novel approach that leveraged social media to facilitate rapid recruitment of participants. The study instrument was administered using a web survey with randomized question order to preclude any possibility of order effects between questions. The study was deemed Institutional Review Board exempt by the University of Michigan: protocol HUM00106696.
Data Presentation Method
To determine the optimal mode of presenting data, we reviewed the literature on quality metric numeracy and presentation methods. Additionally, we evaluated quality metric presentation methods used by the Centers for Disease Control and Prevention (CDC), Centers for Medicare & Medicaid Services (CMS), and a tertiary academic medical center. After assessing the available literature and options, we adapted a CLABSI data presentation array from a study that had qualitatively validated the format using physician feedback (Appendix).20 We used hypothetical CLABSI data for our survey.
Survey Development
We developed a survey that included an 11-item test regarding CLABSI numeracy and data interpretation. Additional questions related to quality metric reliability and demographic information were included. No preexisting assessment tools existed for our areas of interest. Therefore, we developed a novel instrument using a broad, exploratory approach as others have employed.21
First, we defined 3 conceptual categories related to CLABSI data. Within this conceptual framework, an iterative process of development and revision was used to assemble a question bank from which the survey would be constructed. A series of think-aloud sessions were held to evaluate each prompt for precision, clarity, and accuracy in assessing the conceptual categories. Correct and incorrect answers were defined based on literature review in conjunction with input from methodological and content experts (TJI and VC) (see Appendix for answer explanations).
Within the conceptual categories related to CLABSI risk-adjustment, a key measure is the standardized infection ratio (SIR). This value is defined as the ratio of observed number of CLABSI over the expected number of CLABSIs.22 This is the primary measure to stratify hospital performance, and it was used in our assessment of risk-adjustment comprehension. In total, 54 question prompts were developed and subsequently narrowed to 11 study questions for the initial survey.
The instrument was then pretested in a cohort of 8 hospitalists and intensivists to ensure appropriate comprehension, retrieval, and judgment processes.23 Questions were revised based on feedback from this cognitive testing to constitute the final instrument. During the survey, the data table was reshown on each page directly above each question and so was always on the same screen for the respondents.
Survey Sample
We innovated by using Twitter as an online platform for recruiting participants; we used Survey Monkey to host the electronic instrument. Two authors (TJI, VC) systematically sent out solicitation tweets to their followers. These tweets clearly indicated that the recruitment was for the purpose of a research study, and participants would receive no financial reward/incentive (Appendix). A link to the survey was provided in each tweet, and the period of recruitment was 30 days. To ensure respondents were clinicians, they needed to first answer a screening question recognizing that central lines were placed in the subclavian site but not the aorta, iliac, or radial sites.
To prevent systematic or anchoring biases, the order of questions was electronically randomized for each respondent. The primary outcome was the percentage correct of attempted questions.
Statistical Analysis
Descriptive statistics were calculated for all demographic variables. The primary outcome was evaluated as a dichotomous variable for each question (correct vs. incorrect response), and as a continuous variable when assessing mean percent correct on the overall survey. Demographic and conceptual associations were assessed via t-tests, chi-square, or Fisher exact tests. Point biserial correlations were calculated to assess for associations between response to a single question and overall performance on the survey.
To evaluate the association between various respondent characteristics and responses, logistic regression analyses were performed. An ANOVA was performed to assess the association between self-reported reliability of quality metric data and the overall performance on attempted items. Analyses were conducted using STATA MP 14.0 (College Station, TX); P <0.05 was considered statistically significant.
RESULTS
A total of 97 respondents attempted at least 1 question on the survey, and 72 respondents attempted all 11 questions, yielding 939 unique responses for analysis. Seventy respondents (87%) identified as doctors or nurses, and 44 (55%) reported having 6 to 20 years of experience; the survey cohort also came from 6 nations (Table 1). All respondents answered the CLABSI knowledge filter question correctly.
Primary Outcome
The mean percent correct of attempted questions was 61% (standard deviation 21%, interquartile range 50%-75%) (Figure 1). Of those who answered all 11 CLABSI questions, the mean percent correct was 63% (95% CI, 59%-67%). Some questions were answered correctly more often than others—ranging from 17% to 95% (Table 2). Doctors answered 68% of questions correctly (95% CI, 63%-73%), while nurses and other respondents answered 57% of questions correctly (95% CI, 52%-62%) (P = 0.003). Other demographic variables—including self-reported involvement in a quality improvement committee and being from the United States versus elsewhere—were not associated with survey performance. The point biserial correlations for each individual question with overall performance were all more than 0.2 (range 0.24–0.62) and all statistically significant at P < 0.05.
Concept-Specific Performance
Average percent correct declined across categories as numeracy requirements increased (P < 0.05 for all pairwise comparisons). In the area of basic numeracy, respondents’ mean percent correct was 82% (95% CI, 77%-87%) of attempted. This category had 4 questions, with a performance range of 77% to 90%. For example, on the question, “Which hospital has the lowest CLABSI rate?”, 80% of respondents answered correctly. For risk-adjustment numeracy, the mean percent correct was 70% (95% CI, 64%-76%); 2 items assessed this category. For “Which is better: a higher or lower SIR?”, 95% of the cohort answered correctly. However, on “If hospital B had its number of projected infection halved, what is its SIR?”, only 46% of those who attempted the question answered correctly.
Questions featuring risk-adjustment interpretation had an average percent correct of 43% (95% CI, 37%-49%). Five questions made up this category, with a percent correct range of 17% to 75%. For example, on the question, “Which hospital’s patients are the most predisposed to developing CLABSI?”, only 32% of respondents answered this correctly. In contrast, for the question “Which hospital is most effective at preventing CLABSI?”, 51% answered correctly. Figure 2 illustrates the cohort’s performance on each conceptual category while Table 2 displays question-by-question results.
Opinions Regarding CLABSI Data Reliability
Respondents were also asked about their opinion regarding the reliability of CLABSI quality metric data. Forty-three percent of respondents stated that such data were reliable at best 50% of the time. Notably, 10% of respondents indicated that CLABSI quality metric data were rarely or never reliable. There was no association between perceived reliability of quality metric data and survey performance (P = 0.87).
DISCUSSION
This Twitter-based study found wide variation in clinician interpretation of CLABSI quality data, with low overall performance. In particular, comprehension and interpretation of risk-adjusted data were substantially worse than unadjusted data. Although doctors performed somewhat better than nurses and other respondents, those involved in quality improvement initiatives performed no better than respondents who were not. Collectively, these findings suggest clinicians may not reliably comprehend quality metric data, potentially affecting their ability to utilize audit and feedback data. These results may have important implications for policy efforts that seek to leverage quality metric data to improve patient safety.
An integral component of many contemporary quality improvement initiatives is audit and feedback through metrics.6 Unfortunately, formal audit and feedback, along with other similar methods that benchmark data, have not consistently improved outcomes.24–27 A recent meta-analysis noted that audit and feedback interventions are not becoming more efficacious over time; the study further asserted that “new trials have provided little new knowledge regarding key effect modifiers.”9 Our findings suggest that numeracy and comprehension of quality metrics may be important candidate effect modifiers not previously considered. Simply put: we hypothesize that without intrinsic comprehension of data, impetus or insight to change practice might be diminished. In other words, clinicians may be more apt to act on insights they themselves derive from the data than when they are simply told what the data “mean.”
The present study further demonstrates that clinicians do not understand risk-adjusted data as well as raw data. Risk-adjustment has long been recognized as necessary to compare outcomes among hospitals.28,29 However, risk-adjustment is complex and, by its nature, difficult to understand. Although efforts have focused on improving the statistical reliability of quality metrics, this may represent but one half of the equation. Numeracy and interpretation of the data by decision makers are potentially equally important to effecting change. Because clinicians seem to have difficulty understanding risk-adjusted data, this deficit may be of growing importance as our risk-adjustment techniques become more sophisticated.
We note that clinicians expressed concerns regarding the reliability of quality metric feedback. These findings corroborate recent research that has reported reservations from hospital leaders concerning quality data.30,31 However, as shown in the context of patients and healthcare decisions, the aversion associated with quality metrics may be related to incomplete understanding of the data.32 Whether perceptions of unreliability drive lack of understanding or, conversely, whether lack of understanding fuels perceived unreliability is an important question that requires further study.
This study has several strengths. First, we used rigorous survey development techniques to evaluate the understudied issue of quality metric numeracy. Second, our sample size was sufficient to show statistically significant differences in numeracy and comprehension of CLABSI quality metric data. Third, we leveraged social media to rapidly acquire this sample. Finally, our results provided new insights that may have important implications in the area of quality metrics.
There were also limitations to our study. First, the Twitter-derived sample precludes the calculation of a response rate and may not be representative of individuals engaged in CLABSI prevention. However, respondents were solicited from the Twitter-followers of 2 health services researchers (TJI, VC) who are actively engaged in scholarly activities pertaining to critically ill patients and hospital-acquired complications. Thus, our sample likely represents a highly motivated subset that engages in these topics on a regular basis—potentially making them more numerate than average clinicians. Second, we did not ask whether the respondents had previously seen CLABSI data specifically, so we cannot stratify by exposure to such data. Third, this study assessed only CLABSI quality metric data; generalizations regarding numeracy with other metrics should be made with caution. However, as many such data are presented in similar formats, we suspect our findings are applicable to similar audit-and-feedback initiatives.
The findings of this study serve as a stimulus for further inquiry. Research of this nature needs to be carried out in samples drawn from specific, policy-relevant populations (eg, infection control practitioners, bedside nurses, intensive care unit directors). Such studies should include longitudinal assessments of numeracy that attempt to mechanistically examine its impact on CLABSI prevention efforts and outcomes. The latter is an important issue as the link between numeracy and behavioral response, while plausible, cannot be assumed, particularly given the complexity of issues related to behavioral modification.33 Additionally, whether alternate presentations of quality data affect numeracy, interpretation, and performance is worthy of further testing; indeed, this has been shown to be the case in other forms of communication.34–37 Until data from larger samples are available, it may be prudent for quality improvement leaders to assess the comprehension of local clinicians regarding feedback and whether lack of adequate comprehension is a barrier to deploying quality improvement interventions.
Quality measurement is a cornerstone of patient safety as it seeks to assess and improve the care delivered at the bedside. Rigorous metric development is important; however, ensuring that decision makers understand complex quality metrics may be equally fundamental. Given the cost of examining quality, elucidating the mechanisms of numeracy and interpretation as decision makers engage with quality metric data is necessary, along with whether improved comprehension leads to behavior change. Such inquiry may provide an evidence-base to shape alterations in quality metric deployment that will ensure maximal efficacy in driving practice change.
Disclosures
This work was supported by VA HSR&D IIR-13-079 (TJI). Dr. Chopra is supported by a career development award from the Agency of Healthcare Research and Quality (1-K08-HS022835-01). The views expressed here are the authors’ own and do not necessarily represent the view of the US Government or the Department of Veterans’ Affairs. The authors report no conflicts of interest.
Central line-associated bloodstream infections (CLABSIs) are common and serious occurrences across healthcare systems, with an attributable mortality of 12% to 25%.1,2 Given this burden,3–5 CLABSI is a focus for both high-profile public reporting and quality improvement interventions. An integral component of such interventions is audit and feedback via quality metrics. These measures are intended to allow decision makers to assess their own performance and appropriately allocate resources. Quality metrics present a substantial cost to health systems, with an estimated $15.4 billion dollars spent annually simply for reporting.6 Despite this toll, “audit and feedback” interventions have proven to be variably successful.7–9 The mechanisms that limit the effectiveness of these interventions remain
poorly understood.
One plausible explanation for limited efficacy of quality metrics is inadequate clinician numeracy—that is, “the ability to understand the quantitative aspects of clinical medicine, original research, quality improvement, and financial matters.”10 Indeed, clinicians are not consistently able to interpret probabilities and or clinical test characteristics. For example, Wegwarth et al. identified shortcomings in physician application of lead-time bias toward cancer screening.11 Additionally, studies have demonstrated systematic misinterpretations of probabilistic information in clinical settings, along with misconceptions regarding the impact of prevalence on post-test probabilities.12,13 Effective interpretation of rates may be a key—if unstated—requirement of many CLABSI quality improvement efforts.14–19 Our broader hypothesis is that clinicians who can more accurately interpret quality data, even if only from their own institution, are more likely to act on it appropriately and persistently than those who feel they must depend on a preprocessed interpretation of that same data by some other expert.
Therefore, we designed a survey to assess the numeracy of clinicians on CLABSI data presented in a prototypical feedback report. We studied 3 domains of comprehension: (1) basic numeracy: numerical tasks related to simple data; (2) risk-adjustment numeracy: numerical tasks related to risk-adjusted data; and (3) risk-adjustment interpretation: inferential tasks concerning risk-adjusted data. We hypothesized that clinician performance would vary substantially across domains, with the poorest performance in risk-
adjusted data.
METHODS
We conducted a cross-sectional survey of clinician numeracy regarding CLABSI feedback data. Respondents were also asked to provide demographic information and opinions regarding the reliability of quality metric data. Survey recruitment occurred on Twitter, a novel approach that leveraged social media to facilitate rapid recruitment of participants. The study instrument was administered using a web survey with randomized question order to preclude any possibility of order effects between questions. The study was deemed Institutional Review Board exempt by the University of Michigan: protocol HUM00106696.
Data Presentation Method
To determine the optimal mode of presenting data, we reviewed the literature on quality metric numeracy and presentation methods. Additionally, we evaluated quality metric presentation methods used by the Centers for Disease Control and Prevention (CDC), Centers for Medicare & Medicaid Services (CMS), and a tertiary academic medical center. After assessing the available literature and options, we adapted a CLABSI data presentation array from a study that had qualitatively validated the format using physician feedback (Appendix).20 We used hypothetical CLABSI data for our survey.
Survey Development
We developed a survey that included an 11-item test regarding CLABSI numeracy and data interpretation. Additional questions related to quality metric reliability and demographic information were included. No preexisting assessment tools existed for our areas of interest. Therefore, we developed a novel instrument using a broad, exploratory approach as others have employed.21
First, we defined 3 conceptual categories related to CLABSI data. Within this conceptual framework, an iterative process of development and revision was used to assemble a question bank from which the survey would be constructed. A series of think-aloud sessions were held to evaluate each prompt for precision, clarity, and accuracy in assessing the conceptual categories. Correct and incorrect answers were defined based on literature review in conjunction with input from methodological and content experts (TJI and VC) (see Appendix for answer explanations).
Within the conceptual categories related to CLABSI risk-adjustment, a key measure is the standardized infection ratio (SIR). This value is defined as the ratio of observed number of CLABSI over the expected number of CLABSIs.22 This is the primary measure to stratify hospital performance, and it was used in our assessment of risk-adjustment comprehension. In total, 54 question prompts were developed and subsequently narrowed to 11 study questions for the initial survey.
The instrument was then pretested in a cohort of 8 hospitalists and intensivists to ensure appropriate comprehension, retrieval, and judgment processes.23 Questions were revised based on feedback from this cognitive testing to constitute the final instrument. During the survey, the data table was reshown on each page directly above each question and so was always on the same screen for the respondents.
Survey Sample
We innovated by using Twitter as an online platform for recruiting participants; we used Survey Monkey to host the electronic instrument. Two authors (TJI, VC) systematically sent out solicitation tweets to their followers. These tweets clearly indicated that the recruitment was for the purpose of a research study, and participants would receive no financial reward/incentive (Appendix). A link to the survey was provided in each tweet, and the period of recruitment was 30 days. To ensure respondents were clinicians, they needed to first answer a screening question recognizing that central lines were placed in the subclavian site but not the aorta, iliac, or radial sites.
To prevent systematic or anchoring biases, the order of questions was electronically randomized for each respondent. The primary outcome was the percentage correct of attempted questions.
Statistical Analysis
Descriptive statistics were calculated for all demographic variables. The primary outcome was evaluated as a dichotomous variable for each question (correct vs. incorrect response), and as a continuous variable when assessing mean percent correct on the overall survey. Demographic and conceptual associations were assessed via t-tests, chi-square, or Fisher exact tests. Point biserial correlations were calculated to assess for associations between response to a single question and overall performance on the survey.
To evaluate the association between various respondent characteristics and responses, logistic regression analyses were performed. An ANOVA was performed to assess the association between self-reported reliability of quality metric data and the overall performance on attempted items. Analyses were conducted using STATA MP 14.0 (College Station, TX); P <0.05 was considered statistically significant.
RESULTS
A total of 97 respondents attempted at least 1 question on the survey, and 72 respondents attempted all 11 questions, yielding 939 unique responses for analysis. Seventy respondents (87%) identified as doctors or nurses, and 44 (55%) reported having 6 to 20 years of experience; the survey cohort also came from 6 nations (Table 1). All respondents answered the CLABSI knowledge filter question correctly.
Primary Outcome
The mean percent correct of attempted questions was 61% (standard deviation 21%, interquartile range 50%-75%) (Figure 1). Of those who answered all 11 CLABSI questions, the mean percent correct was 63% (95% CI, 59%-67%). Some questions were answered correctly more often than others—ranging from 17% to 95% (Table 2). Doctors answered 68% of questions correctly (95% CI, 63%-73%), while nurses and other respondents answered 57% of questions correctly (95% CI, 52%-62%) (P = 0.003). Other demographic variables—including self-reported involvement in a quality improvement committee and being from the United States versus elsewhere—were not associated with survey performance. The point biserial correlations for each individual question with overall performance were all more than 0.2 (range 0.24–0.62) and all statistically significant at P < 0.05.
Concept-Specific Performance
Average percent correct declined across categories as numeracy requirements increased (P < 0.05 for all pairwise comparisons). In the area of basic numeracy, respondents’ mean percent correct was 82% (95% CI, 77%-87%) of attempted. This category had 4 questions, with a performance range of 77% to 90%. For example, on the question, “Which hospital has the lowest CLABSI rate?”, 80% of respondents answered correctly. For risk-adjustment numeracy, the mean percent correct was 70% (95% CI, 64%-76%); 2 items assessed this category. For “Which is better: a higher or lower SIR?”, 95% of the cohort answered correctly. However, on “If hospital B had its number of projected infection halved, what is its SIR?”, only 46% of those who attempted the question answered correctly.
Questions featuring risk-adjustment interpretation had an average percent correct of 43% (95% CI, 37%-49%). Five questions made up this category, with a percent correct range of 17% to 75%. For example, on the question, “Which hospital’s patients are the most predisposed to developing CLABSI?”, only 32% of respondents answered this correctly. In contrast, for the question “Which hospital is most effective at preventing CLABSI?”, 51% answered correctly. Figure 2 illustrates the cohort’s performance on each conceptual category while Table 2 displays question-by-question results.
Opinions Regarding CLABSI Data Reliability
Respondents were also asked about their opinion regarding the reliability of CLABSI quality metric data. Forty-three percent of respondents stated that such data were reliable at best 50% of the time. Notably, 10% of respondents indicated that CLABSI quality metric data were rarely or never reliable. There was no association between perceived reliability of quality metric data and survey performance (P = 0.87).
DISCUSSION
This Twitter-based study found wide variation in clinician interpretation of CLABSI quality data, with low overall performance. In particular, comprehension and interpretation of risk-adjusted data were substantially worse than unadjusted data. Although doctors performed somewhat better than nurses and other respondents, those involved in quality improvement initiatives performed no better than respondents who were not. Collectively, these findings suggest clinicians may not reliably comprehend quality metric data, potentially affecting their ability to utilize audit and feedback data. These results may have important implications for policy efforts that seek to leverage quality metric data to improve patient safety.
An integral component of many contemporary quality improvement initiatives is audit and feedback through metrics.6 Unfortunately, formal audit and feedback, along with other similar methods that benchmark data, have not consistently improved outcomes.24–27 A recent meta-analysis noted that audit and feedback interventions are not becoming more efficacious over time; the study further asserted that “new trials have provided little new knowledge regarding key effect modifiers.”9 Our findings suggest that numeracy and comprehension of quality metrics may be important candidate effect modifiers not previously considered. Simply put: we hypothesize that without intrinsic comprehension of data, impetus or insight to change practice might be diminished. In other words, clinicians may be more apt to act on insights they themselves derive from the data than when they are simply told what the data “mean.”
The present study further demonstrates that clinicians do not understand risk-adjusted data as well as raw data. Risk-adjustment has long been recognized as necessary to compare outcomes among hospitals.28,29 However, risk-adjustment is complex and, by its nature, difficult to understand. Although efforts have focused on improving the statistical reliability of quality metrics, this may represent but one half of the equation. Numeracy and interpretation of the data by decision makers are potentially equally important to effecting change. Because clinicians seem to have difficulty understanding risk-adjusted data, this deficit may be of growing importance as our risk-adjustment techniques become more sophisticated.
We note that clinicians expressed concerns regarding the reliability of quality metric feedback. These findings corroborate recent research that has reported reservations from hospital leaders concerning quality data.30,31 However, as shown in the context of patients and healthcare decisions, the aversion associated with quality metrics may be related to incomplete understanding of the data.32 Whether perceptions of unreliability drive lack of understanding or, conversely, whether lack of understanding fuels perceived unreliability is an important question that requires further study.
This study has several strengths. First, we used rigorous survey development techniques to evaluate the understudied issue of quality metric numeracy. Second, our sample size was sufficient to show statistically significant differences in numeracy and comprehension of CLABSI quality metric data. Third, we leveraged social media to rapidly acquire this sample. Finally, our results provided new insights that may have important implications in the area of quality metrics.
There were also limitations to our study. First, the Twitter-derived sample precludes the calculation of a response rate and may not be representative of individuals engaged in CLABSI prevention. However, respondents were solicited from the Twitter-followers of 2 health services researchers (TJI, VC) who are actively engaged in scholarly activities pertaining to critically ill patients and hospital-acquired complications. Thus, our sample likely represents a highly motivated subset that engages in these topics on a regular basis—potentially making them more numerate than average clinicians. Second, we did not ask whether the respondents had previously seen CLABSI data specifically, so we cannot stratify by exposure to such data. Third, this study assessed only CLABSI quality metric data; generalizations regarding numeracy with other metrics should be made with caution. However, as many such data are presented in similar formats, we suspect our findings are applicable to similar audit-and-feedback initiatives.
The findings of this study serve as a stimulus for further inquiry. Research of this nature needs to be carried out in samples drawn from specific, policy-relevant populations (eg, infection control practitioners, bedside nurses, intensive care unit directors). Such studies should include longitudinal assessments of numeracy that attempt to mechanistically examine its impact on CLABSI prevention efforts and outcomes. The latter is an important issue as the link between numeracy and behavioral response, while plausible, cannot be assumed, particularly given the complexity of issues related to behavioral modification.33 Additionally, whether alternate presentations of quality data affect numeracy, interpretation, and performance is worthy of further testing; indeed, this has been shown to be the case in other forms of communication.34–37 Until data from larger samples are available, it may be prudent for quality improvement leaders to assess the comprehension of local clinicians regarding feedback and whether lack of adequate comprehension is a barrier to deploying quality improvement interventions.
Quality measurement is a cornerstone of patient safety as it seeks to assess and improve the care delivered at the bedside. Rigorous metric development is important; however, ensuring that decision makers understand complex quality metrics may be equally fundamental. Given the cost of examining quality, elucidating the mechanisms of numeracy and interpretation as decision makers engage with quality metric data is necessary, along with whether improved comprehension leads to behavior change. Such inquiry may provide an evidence-base to shape alterations in quality metric deployment that will ensure maximal efficacy in driving practice change.
Disclosures
This work was supported by VA HSR&D IIR-13-079 (TJI). Dr. Chopra is supported by a career development award from the Agency of Healthcare Research and Quality (1-K08-HS022835-01). The views expressed here are the authors’ own and do not necessarily represent the view of the US Government or the Department of Veterans’ Affairs. The authors report no conflicts of interest.
1. Scott RD II. The direct medical costs of healthcare-associated infections in us hospitals and the benefits of prevention. Centers for Disease Control and Prevention. Available at: http://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf. Published March 2009. Accessed November 8, 2016.
2. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39(4 suppl 1)::S1-S34. PubMed
3. Blot K, Bergs J, Vogelaers D, Blot S, Vandijck D. Prevention of central line-associated bloodstream infections through quality improvement interventions: a systematic review and meta-analysis. Clin Infect Dis. 2014;59(1):96-105. PubMed
4. Mermel LA. Prevention of intravascular catheter-related infections. Ann Intern Med. 2000;132(5):391-402. PubMed
5. Siempos II, Kopterides P, Tsangaris I, Dimopoulou I, Armaganidis AE. Impact of catheter-related bloodstream infections on the mortality of critically ill patients: a meta-analysis. Crit Care Med. 2009;37(7):2283-2289. PubMed
6. Casalino LP, Gans D, Weber R, et al. US physician practices spend more than $15.4 billion annually to report quality measures. Health Aff (Millwood). 2016;35(3):401-406. PubMed
7. Hysong SJ. Meta-analysis: audit and feedback features impact effectiveness on care quality. Med Care. 2009;47(3):356-363. PubMed
8. Ilgen DR, Fisher CD, Taylor MS. Consequences of individual feedback on behavior in organizations. J Appl Psychol. 1979;64:349-371.
9. Ivers NM, Grimshaw JM, Jamtvedt G, et al. Growing literature, stagnant science? Systematic review, meta-regression and cumulative analysis of audit and feedback interventions in health care. J Gen Intern Med. 2014;29(11):1534-1541. PubMed
10. Rao G. Physician numeracy: essential skills for practicing evidence-based medicine. Fam Med. 2008;40(5):354-358. PubMed
11. Wegwarth O, Schwartz LM, Woloshin S, Gaissmaier W, Gigerenzer G. Do physicians understand cancer screening statistics? A national survey of primary care physicians in the United States. Ann Intern Med. 2012;156(5):340-349. PubMed
12. Bramwell R, West H, Salmon P. Health professionals’ and service users’ interpretation of screening test results: experimental study. BMJ. 2006;333(7562):284. PubMed
13. Agoritsas T, Courvoisier DS, Combescure C, Deom M, Perneger TV. Does prevalence matter to physicians in estimating post-test probability of disease? A randomized trial. J Gen Intern Med. 2011;26(4):373-378. PubMed
14. Warren DK, Zack JE, Mayfield JL, et al. The effect of an education program on the incidence of central venous catheter-associated bloodstream infection in a medical ICU. Chest. 2004;126(5):1612-1618. PubMed
15. Rinke ML, Bundy DG, Chen AR, et al. Central line maintenance bundles and CLABSIs in ambulatory oncology patients. Pediatrics. 2013;132(5):e1403-e1412. PubMed
16. Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):
2725-2732. PubMed
17. Rinke ML, Chen AR, Bundy DG, et al. Implementation of a central line maintenance care bundle in hospitalized pediatric oncology patients. Pediatrics. 2012;130(4):e996-e1004. PubMed
18. Sacks GD, Diggs BS, Hadjizacharia P, Green D, Salim A, Malinoski DJ. Reducing the rate of catheter-associated bloodstream infections in a surgical intensive care unit using the Institute for Healthcare Improvement Central Line Bundle. Am J Surg. 2014;207(6):817-823. PubMed
19. Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014-2020. PubMed
20. Rajwan YG, Barclay PW, Lee T, Sun IF, Passaretti C, Lehmann H. Visualizing central line-associated blood stream infection (CLABSI) outcome data for decision making by health care consumers and practitioners—an evaluation study. Online J Public Health Inform. 2013;5(2):218. PubMed
21. Fagerlin A, Zikmund-Fisher BJ, Ubel PA, Jankovic A, Derry HA, Smith DM. Measuring numeracy without a math test: development of the Subjective Numeracy Scale. Med Decis Making 2007;27(5):672-680. PubMed
22. HAI progress report FAQ. 2016. Available at: http://www.cdc.gov/hai/surveillance/progress-report/faq.html. Last updated March 2, 2016. Accessed November 8, 2016.
23. Collins D. Pretesting survey instruments: an overview of cognitive methods. Qual Life Res. 2003;12(3):229-238. PubMed
24. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259. PubMed
25. Chatterjee P, Joynt KE. Do cardiology quality measures actually improve patient outcomes? J Am Heart Assoc. 2014;3(1):e000404. PubMed
26. Joynt KE, Blumenthal DM, Orav EJ, Resnic FS, Jha AK. Association of public reporting for percutaneous coronary intervention with utilization and outcomes among Medicare beneficiaries with acute myocardial infarction. JAMA. 2012;308(14):1460-1468. PubMed
27. Ryan AM, Nallamothu BK, Dimick JB. Medicare’s public reporting initiative on hospital quality had modest or no impact on mortality from three key conditions. Health Aff (Millwood). 2012;31(3):585-592. PubMed
28. Thomas JW. Risk adjustment for measuring health care outcomes, 3rd edition. Int J Qual Health Care. 2004;16(2):181-182.
29. Iezzoni LI. Risk Adjustment for Measuring Health Care Outcomes. Ann Arbor, Michigan: Health Administration Press; 1994.
30. Goff SL, Lagu T, Pekow PS, et al. A qualitative analysis of hospital leaders’ opinions about publicly reported measures of health care quality. Jt Comm J Qual Patient Saf. 2015;41(4):169-176. PubMed
31. Lindenauer PK, Lagu T, Ross JS, et al. Attitudes of hospital leaders toward publicly reported measures of health care quality. JAMA Intern Med. 2014;174(12):
1904-1911. PubMed
32. Peters E, Hibbard J, Slovic P, Dieckmann N. Numeracy skill and the communication, comprehension, and use of risk-benefit information. Health Aff (Millwood). 2007;26(3):741-748. PubMed
33. Montano DE, Kasprzyk D. Theory of reasoned action, theory of planned behavior, and the integrated behavioral model. In: Glanz K, Rimer BK, Viswanath K, eds. Health Behavior and Health Education: Theory, Research and Practice. 5th ed. San Francisco, CA: Jossey-Bass; 2015:95–124.
34. Hamstra DA, Johnson SB, Daignault S, et al. The impact of numeracy on verbatim knowledge of the longitudinal risk for prostate cancer recurrence following radiation therapy. Med Decis Making. 2015;35(1):27-36. PubMed
35. Hawley ST, Zikmund-Fisher B, Ubel P, Jancovic A, Lucas T, Fagerlin A. The impact of the format of graphical presentation on health-related knowledge and treatment choices. Patient Educ Couns. 2008;73(3):448-455. PubMed
36. Zikmund-Fisher BJ, Witteman HO, Dickson M, et al. Blocks, ovals, or people? Icon type affects risk perceptions and recall of pictographs. Med Decis Making. 2014;34(4):443-453. PubMed
37. Korfage IJ, Fuhrel-Forbis A, Ubel PA, et al. Informed choice about breast cancer prevention: randomized controlled trial of an online decision aid intervention. Breast Cancer Res. 2013;15(5):R74. PubMed
1. Scott RD II. The direct medical costs of healthcare-associated infections in us hospitals and the benefits of prevention. Centers for Disease Control and Prevention. Available at: http://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf. Published March 2009. Accessed November 8, 2016.
2. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39(4 suppl 1)::S1-S34. PubMed
3. Blot K, Bergs J, Vogelaers D, Blot S, Vandijck D. Prevention of central line-associated bloodstream infections through quality improvement interventions: a systematic review and meta-analysis. Clin Infect Dis. 2014;59(1):96-105. PubMed
4. Mermel LA. Prevention of intravascular catheter-related infections. Ann Intern Med. 2000;132(5):391-402. PubMed
5. Siempos II, Kopterides P, Tsangaris I, Dimopoulou I, Armaganidis AE. Impact of catheter-related bloodstream infections on the mortality of critically ill patients: a meta-analysis. Crit Care Med. 2009;37(7):2283-2289. PubMed
6. Casalino LP, Gans D, Weber R, et al. US physician practices spend more than $15.4 billion annually to report quality measures. Health Aff (Millwood). 2016;35(3):401-406. PubMed
7. Hysong SJ. Meta-analysis: audit and feedback features impact effectiveness on care quality. Med Care. 2009;47(3):356-363. PubMed
8. Ilgen DR, Fisher CD, Taylor MS. Consequences of individual feedback on behavior in organizations. J Appl Psychol. 1979;64:349-371.
9. Ivers NM, Grimshaw JM, Jamtvedt G, et al. Growing literature, stagnant science? Systematic review, meta-regression and cumulative analysis of audit and feedback interventions in health care. J Gen Intern Med. 2014;29(11):1534-1541. PubMed
10. Rao G. Physician numeracy: essential skills for practicing evidence-based medicine. Fam Med. 2008;40(5):354-358. PubMed
11. Wegwarth O, Schwartz LM, Woloshin S, Gaissmaier W, Gigerenzer G. Do physicians understand cancer screening statistics? A national survey of primary care physicians in the United States. Ann Intern Med. 2012;156(5):340-349. PubMed
12. Bramwell R, West H, Salmon P. Health professionals’ and service users’ interpretation of screening test results: experimental study. BMJ. 2006;333(7562):284. PubMed
13. Agoritsas T, Courvoisier DS, Combescure C, Deom M, Perneger TV. Does prevalence matter to physicians in estimating post-test probability of disease? A randomized trial. J Gen Intern Med. 2011;26(4):373-378. PubMed
14. Warren DK, Zack JE, Mayfield JL, et al. The effect of an education program on the incidence of central venous catheter-associated bloodstream infection in a medical ICU. Chest. 2004;126(5):1612-1618. PubMed
15. Rinke ML, Bundy DG, Chen AR, et al. Central line maintenance bundles and CLABSIs in ambulatory oncology patients. Pediatrics. 2013;132(5):e1403-e1412. PubMed
16. Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):
2725-2732. PubMed
17. Rinke ML, Chen AR, Bundy DG, et al. Implementation of a central line maintenance care bundle in hospitalized pediatric oncology patients. Pediatrics. 2012;130(4):e996-e1004. PubMed
18. Sacks GD, Diggs BS, Hadjizacharia P, Green D, Salim A, Malinoski DJ. Reducing the rate of catheter-associated bloodstream infections in a surgical intensive care unit using the Institute for Healthcare Improvement Central Line Bundle. Am J Surg. 2014;207(6):817-823. PubMed
19. Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014-2020. PubMed
20. Rajwan YG, Barclay PW, Lee T, Sun IF, Passaretti C, Lehmann H. Visualizing central line-associated blood stream infection (CLABSI) outcome data for decision making by health care consumers and practitioners—an evaluation study. Online J Public Health Inform. 2013;5(2):218. PubMed
21. Fagerlin A, Zikmund-Fisher BJ, Ubel PA, Jankovic A, Derry HA, Smith DM. Measuring numeracy without a math test: development of the Subjective Numeracy Scale. Med Decis Making 2007;27(5):672-680. PubMed
22. HAI progress report FAQ. 2016. Available at: http://www.cdc.gov/hai/surveillance/progress-report/faq.html. Last updated March 2, 2016. Accessed November 8, 2016.
23. Collins D. Pretesting survey instruments: an overview of cognitive methods. Qual Life Res. 2003;12(3):229-238. PubMed
24. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259. PubMed
25. Chatterjee P, Joynt KE. Do cardiology quality measures actually improve patient outcomes? J Am Heart Assoc. 2014;3(1):e000404. PubMed
26. Joynt KE, Blumenthal DM, Orav EJ, Resnic FS, Jha AK. Association of public reporting for percutaneous coronary intervention with utilization and outcomes among Medicare beneficiaries with acute myocardial infarction. JAMA. 2012;308(14):1460-1468. PubMed
27. Ryan AM, Nallamothu BK, Dimick JB. Medicare’s public reporting initiative on hospital quality had modest or no impact on mortality from three key conditions. Health Aff (Millwood). 2012;31(3):585-592. PubMed
28. Thomas JW. Risk adjustment for measuring health care outcomes, 3rd edition. Int J Qual Health Care. 2004;16(2):181-182.
29. Iezzoni LI. Risk Adjustment for Measuring Health Care Outcomes. Ann Arbor, Michigan: Health Administration Press; 1994.
30. Goff SL, Lagu T, Pekow PS, et al. A qualitative analysis of hospital leaders’ opinions about publicly reported measures of health care quality. Jt Comm J Qual Patient Saf. 2015;41(4):169-176. PubMed
31. Lindenauer PK, Lagu T, Ross JS, et al. Attitudes of hospital leaders toward publicly reported measures of health care quality. JAMA Intern Med. 2014;174(12):
1904-1911. PubMed
32. Peters E, Hibbard J, Slovic P, Dieckmann N. Numeracy skill and the communication, comprehension, and use of risk-benefit information. Health Aff (Millwood). 2007;26(3):741-748. PubMed
33. Montano DE, Kasprzyk D. Theory of reasoned action, theory of planned behavior, and the integrated behavioral model. In: Glanz K, Rimer BK, Viswanath K, eds. Health Behavior and Health Education: Theory, Research and Practice. 5th ed. San Francisco, CA: Jossey-Bass; 2015:95–124.
34. Hamstra DA, Johnson SB, Daignault S, et al. The impact of numeracy on verbatim knowledge of the longitudinal risk for prostate cancer recurrence following radiation therapy. Med Decis Making. 2015;35(1):27-36. PubMed
35. Hawley ST, Zikmund-Fisher B, Ubel P, Jancovic A, Lucas T, Fagerlin A. The impact of the format of graphical presentation on health-related knowledge and treatment choices. Patient Educ Couns. 2008;73(3):448-455. PubMed
36. Zikmund-Fisher BJ, Witteman HO, Dickson M, et al. Blocks, ovals, or people? Icon type affects risk perceptions and recall of pictographs. Med Decis Making. 2014;34(4):443-453. PubMed
37. Korfage IJ, Fuhrel-Forbis A, Ubel PA, et al. Informed choice about breast cancer prevention: randomized controlled trial of an online decision aid intervention. Breast Cancer Res. 2013;15(5):R74. PubMed
© 2017 Society of Hospital Medicine
Interhospital Transfer Handover Tool
The transfer of inpatients between hospitals for specialized services is common, affecting nearly 10% of all Medicare admissions1 and 4.5% of all critical care hospitalizations.2 At tertiary referral centers, 49% of medical intensive care unit (ICU) admissions are transferred from another hospital.3
Transfer patients have longer length of stay (LOS) than patients admitted directly from the emergency department or clinic. Among patients initially admitted to an ICU, transfer patients spend 1 day to 2.2 more days in the ICU and an additional 2 days to 4 more days total at the receiving hospital.4,5 Furthermore, transfer patients have higher mortality than nontransferred patients by 4% to 8%.3-5 Even after adjustment for case mix and comorbid disease, interhospital transfer is an independent predictor of both ICU death and LOS.6,7 As a result, interhospital transfer has been associated with a $9600 increase (on average) in hospital costs.4
Despite evidence detailing patient handovers as a key time when poor communication can lead to delays in care and significant patient risk, 8-10 most studies have focused on hospital discharge or change of shift, and scant effort has been dedicated to improving the interhospital handover. The process of interhospital transfer is often prolonged and discontinuous,11 commonly including delays of more than 24 hours between initiation and completion. This frequently precludes direct physician-to-physician contact at the time of transfer, and physicians rely on the discharge/transfer summary.12 Yet discharge summaries are frequently absent or incomplete,13 and often lack information for high-risk treatments such as systemic anticoagulation.14 The traditional reliance on discharge summaries for handover communication requires interpretation of unstandardized documentation and increases the risk for miscommunication, delays, and error.
To improve communication, we developed a 1-page handover tool for all inbound adult interhospital transfers to our academic medical center. We sought to determine whether implementation of this standardized handover tool improved the timeliness of initial care, LOS, and mortality among interhospital transfer patients.
METHODS
Study Design, Setting, Population
We conducted a retrospective cohort study of patients transferred into Vanderbilt University Hospital (VUH), an adult 626-bed quaternary care academic medical center in Nashville, Tennessee. The Vanderbilt University Institutional Review Board approved this study.
Population
We selected for inclusion all patients age 18 or older who were transferred into VUH between July 1, 2009 and December 31, 2010. We excluded patients whose transfer was routed outside the main VUH Patient Flow Center as well as patients who did not arrive alive at VUH. We also excluded patients transferred to the emergency department and patients admitted to obstetrics, burn, or trauma services, because these admitting services did not initially use the handover tool. Patients were followed for the duration of their hospitalization at VUH.
Intervention
The 1-page handover tool was developed with multidisciplinary physician input from house staff; medical directors from intensive care, neurology, and surgery; and the chief of staff. The tool was structured on the SBAR model (Situation, Background, Assessment, and Recommendation).15 Fields on the handover tool were limited to those deemed critical for immediate patient care and designed for 1 tool to be used for both ICU and non-ICU transfers. Fields included primary diagnosis; allergies; use and last dose of anticoagulants, vasopressors, sedative/paralytics, and antibiotics; isolation needs; indwelling devices; recent operations/procedures; code status; emergency contact information; problem list; active medication list; vital signs; pertinent exam; imaging; lab findings; and overall reason for transfer.
The handover tool was completed by the physician at the transferring hospital, faxed to VUH, and immediately scanned into the electronic record, allowing the receiving physicians to review information before patient arrival. Use of the tool was piloted first with 2 referring hospitals in April 2010 and universally recommended but not compulsory for all adult patients routed through the main VUH Patient Flow Center beginning July 1, 2010. Immediately before full implementation, the chief of staff sent letters to leadership of the 40 highest volume referral hospitals, highlighting the institutional goal of improving hand-off communication, framing completion of the tool as a step in the transfer acceptance process, and providing contact information for questions, feedback, or concerns. To ensure the tool was a standard part of the transfer process, the VUH Patient Flow Center maintained the responsibility of faxing the form to the outside facility and monitoring its receipt. The tool was processed in the same manner as other faxed patient records and treated as a part of the formal medical record to meet all standards for the Health Insurance Portability and Accountability Act (HIPAA) and medicolegal compliance. The medical center also has a separate cardiac transfer center where the handover tool was not implemented owing to its specialized workflow.
Data Source
The VUH Patient Flow Center maintains a database of all patients for whom transfer to VUH is requested, including information on the requesting hospital and the duration of transfer process. Outcome data and patient characteristics were extracted from the Enterprise Data Warehouse. Data related to comorbid illness were extracted from the Perioperative Data Warehouse, an IRB-approved data registry.
Measures
We evaluated 3 outcomes. First, we defined 2 measures of the timeliness of initial care, the time from arrival at VUH until entry of an admission order, and the time from arrival until entry of the first antibiotic order. Only antibiotics ordered within the first 36 hours of admission were included. Second, we evaluated the total LOS after transfer to VUH and the ICU LOS for patients transferred into an ICU setting. Finally, we examined in-hospital mortality at VUH. These metrics were chosen for their broad applicability across patient groups and feasibility of data capture. Length of stay and mortality also represent final common pathways for avoidance of complications. Specific patient safety indicators and complications were not abstracted due to their low frequency and burden of data collection. Due to system changes in our cost accounting systems, we were not able to obtain cost data pre- and postimplementation that provided meaningful comparisons.
Patient covariates included age, gender, payer, and Elixhauser comorbidity index as modified by van Walraven,16 calculated based on the admission of interest and the previous 365 days. We also examined admission characteristics including location (ICU vs. non-ICU), admitting service (medicine, surgery, neurology, or gynecology), and shift of arrival (day, 7:00 am to 6:00 pm; or night, 6:00 pm to 7:00 pm). Finally, we examined duration of the transfer process (ie, time between transfer request and arrival at VUH) and the volume of the transferring hospital (high was defined as 3 or more transfers to VUH per year).
Statistical analysis
Patient characteristics before and after implementation of the handover tool were compared using Pearson’s chi-square test and Fisher exact test for categorical variables and using Student t test and the Wilcoxon rank sum test for continuous variables. The outcome variables of time to admission order entry, time to antibiotic order entry, LOS, ICU LOS, and in-hospital mortality were compared between the before- and after-intervention time periods, using the Wilcoxon rank sum test for continuous outcomes and Pearson’s chi-square test for in-hospital mortality.
To account for temporal trends, the effect of the handover tool on time-to-admission order entry, hospital LOS, and mortality was measured using an interrupted time-series design with segmented linear regression analysis.17 The study period was divided into 2-week intervals, with 26 time periods in the pre-intervention period and 13 time periods in the postintervention period. Expected rates for the postintervention time periods were projected from the pre-intervention data using a linear regression model. To assess the observed effect of the intervention, rates from the postintervention periods were compared with these projected rates, assuming continuation of the trend. Restricted cubic spline models were also fit for time-to-admission order and hospital LOS; however, the F-statistics for these models were not significant, suggesting the linear regression provided a more appropriate model.
To further account for potential confounding of outcomes by comorbid disease and other patient factors, multivariate linear regression models assessed change in timeliness and LOS with implementation of the intervention. A multivariate logistic regression model was used to assess change in mortality with intervention implementation. All models adjusted for age, gender, payer, comorbid illness, admitting team, shift of arrival (day vs. night), transfer duration, volume of transferring hospital, and ICU status. Outcomes were further adjusted for calendar month to account for temporal trends in house staff efficiency. Because the cardiac transfer center did not adopt the use of the transfer tool, we evaluated adjusted in-hospital mortality for these patients as a concurrent control group.
All statistical testing was 2-sided at a significance level of 0.05. All analyses were conducted using STATA 12.1 statistical software (StataCorp LP, College Station, Texas).
RESULTS
Of 10,325 patients for whom transfer to VUH was requested during the study period, 1715 met inclusion criteria, including 798 patients (46.5%) initially admitted to an ICU setting. Specific patient exclusions are detailed in the Supplemental Figure; the majority of exclusions were due to patients being transferred directly to the emergency department setting. Table 1 summarizes patient characteristics before and after implementation of the handover tool. The median age was 57 years, with 48.6% male patients. Accepting services included medicine (56%), surgery (34%), neurology (9%), and gynecology (1%). The median duration of transfer was 8 hours, and the majority (93%) of patients came from higher volume transferring hospitals. Most (65%) of patients were admitted during night shift. The median modified Elixhauser comorbidity index was 11 (range of possible scores, -19 to 89). A slightly higher proportion of patients admitted postimplementation of the handover tool came from higher volume transferring hospitals; otherwise, there were no significant differences between the pre- and postintervention groups.
Vanderbilt University Hospital received transfers from more than 350 unique facilities in more than 25 U.S. states during the overall study period. During the postintervention period, adherence to the handover process was excellent, with more than 85% of patients having a completed handover tool available in their medical record at the time of transfer. The remaining 15% had either incomplete forms or no form.
Timeliness of Initial Care
There was no change in either the median time-to-admission order entry after implementation (47 vs. 45 minutes, unadjusted P = 0.36) or time to antibiotic order entry (199 vs. 202 minutes; unadjusted P = 0.81; Table 2).
In the time-series analysis, the pre-intervention period did not have a significant temporal trend in median time-to-admission order entry (ß-coefficient = -0.27; 95% confidence interval [CI] -0.85 to 0.31; R2 = 0.04; P = 0.34; Figure 1A). The postintervention period showed a trend toward a reduction in median time-to-admission order entry (ß-coefficient = -1.39; 95% CI -2.92 to 0.15; R2 = 0.27; P = 0.07). There was no significant difference between the actual time-to-admission order entry in the postintervention period when compared to the projected rates from the pre-intervention period (P = 0.18).
After multivariate adjustment, the postintervention time period was not associated with any significant change in the median time-to-admission order entry (P = 0.94, R2 = 0.09) nor time-to-antibiotic order entry (P = 0.91; R2 = 0.08; Table 2).
Length of Stay
Hospital LOS demonstrated a nonstatistically significant decline after implementation of the handover tool from 6.47 days to 5.81 days (unadjusted P = 0.18; Table 2). There was no significant change in ICU LOS postintervention (4.34 days to 4.55 days; P = 0.38).
In time series analysis, hospital LOS did not have a significant temporal trend in either the pre-intervention period (ß-coefficient = 0.00094; 95% CI, -0.07 to 0.07; R2 = 0.00; P = 0.98) or the postintervention period (ß-coefficient = 0.09; 95% CI, -0.07 to 0.25; R2 = 0.13; P = 0.23; Figure 1B). Similarly, there was no significant difference between the actual and projected hospital LOS after implementation of the handover tool (P = 0.31).
After multivariate adjustment, the postintervention time period was associated with a trend toward reduction in overall LOS (P = 0.06; R2 = 0.07) but no significant change in ICU LOS (P = 0.99; R2 = 0.09).
Mortality
In-hospital mortality declined significantly from 12.0% in the pre-intervention period to 8.9% in the postintervention period (P = 0.04; Table 2). In time-series analysis, mortality did not have a significant trend in the pre-intervention period (ß-coefficient = 0.00017, 95% CI, -0.0020 to 0.0024; P = 0.878) and had a trend toward reduction in the postintervention period (ß-coefficient = -0.0032; 95% CI, -0.0091 to 0.0027; P = 0.255; Figure 1C) but did not reach statistical significance due to relatively small numbers of deaths in each individual time period.
After multivariate adjustment, the postintervention period was associated with overall lower odds of mortality among transfer patients when compared with the pre-intervention period (adjusted OR 0.68; 95% CI, 0.47 – 0.99; R2 = 0.21; P = 0.04; Figure 2). Among the concurrent control group of patients routed through the cardiac transfer center, there was no significant change in mortality between the pre- and postintervention periods (adjusted OR 1.31; 95% CI, 0.88 – 1.93; R2 = 0.28; P = 0.18).
DISCUSSION
We developed a simple 1-page handover tool for interhospital transfer patients and aimed to improve timeliness, efficiency, and outcomes of care at the receiving hospital. Implementation of the handover tool was feasible and well accepted by transferring physicians despite a geographically large and diverse transfer network. Although implementation did not substantially improve measures of the timeliness of initial care among transfer patients, we noted a nonsignificant trend toward reduced LOS postintervention.
We observed a substantial and statistically significant reduction in mortality among transfer patients after implementation of the handover tool that persisted after controlling for time trends, comorbid illness, and several other patient factors. This effect was not seen in a concurrent control group of cardiac transfer patients for whom the handover tool was not implemented. Standardizing communication regarding high-risk clinical care processes may be responsible for the observed mortality reduction, similar to improvements seen in other small pilot studies.18 We acknowledge that the magnitude of the improvement in mortality is more than might be expected from the handover tool alone and could be due to chance.
In this initial evaluation, it was not feasible to determine whether information provided in the handover tool helped avert specific complications that could affect mortality, such as complications related to the use of ventilators, high-risk medications, or indwelling devices. Assessment of additional patient safety indices such as code events, unplanned ICU transfers, and medication errors could also help clarify the effect of the handover tool on patient-safety outcomes, and future work should include these metrics as well. Alternately, the improvement in mortality may result from other unmeasured processes that occurred concurrently and verification of this finding should be completed in other settings.
CONCLUSION
More work is needed to determine suitable process and outcome measures for interhospital transfers. Most literature has focused on cost and LOS at the exclusion of more proximal measures of initial care.3-7 The Institute of Medicine has identified timeliness as 1 of the 6 aims for care delivery redesign,19 yet standardized timeliness outcomes do not exist across broad inpatient populations. We chose to monitor the time-to-admission order entry and time-to-antibiotic order entry as 2 indicators of timeliness that would be applicable to a variety of patients. The lack of change in these selected measures should prompt examination for other measures of efficiency, including those that affect nontransferred patients. It is possible that nontransferred patients cared for by the same physician also benefit from fewer delays or disruptions and experience increased efficiency of care if transfer patient communication is improved. Further work is necessary to understand whether other measures of timely initial patient care may be more suitable.
The use of a time-series design to account for temporal trends adds substantial rigor to this study, since the majority of these patients were cared for by house staff whose experience and efficiency vary throughout the academic year. Furthermore, subsequent multivariate analysis demonstrated consistent findings after adjustment for comorbid illness and several other hospital and patient-level confounders.
This study has several limitations. The primary limitation is its nonrandomized design. Patient characteristics were stable across multiple variables before and after implementation, but it is possible that another confounding factor was responsible for observed improvements. Likewise, we collected data for only 6 rather than 12 months during the postintervention time period, which limited our sample size and statistical power. This was chosen because a significant restructuring of resident duty hours occurred in spring 2011 that had the potential to affect all measures studied.20,21 Finally, we did not collect data on accuracy of the information provided in the handover tool or end-user utilization and were unable to account for effects of these.
Since implementation in 2010, this process for interhospital transfers at VUH remains the same, although the volume of incoming transfers has significantly increased. Electronic handover tools with similar structure and content have since been adopted for patients being transferred to the emergency department or directly admitted from clinic. As VUH moves in the coming years from a locally developed electronic medical record to a national vendor, there will be an opportunity to transform this tool into an electronic template that will easily share data between institutions and further enhance communication.
Interhospital transfer patients represent a high-risk population whose unique handover needs have not been adequately measured or addressed. Our investigation demonstrated that a standardized handover aid can be implemented across a broad transfer network and may contribute to reductions in LOS and mortality. Further study is warranted to confirm these findings and assess the effect on other clinical outcomes.
Disclosures
This material is based upon work supported by the Office of Academic Affiliations, Department of Veterans Affairs, VA National Quality Scholars Program, and was made possible by the use of the facilities at VA Tennessee Valley Healthcare System, Nashville, Tennessee. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government. Additionally, this publication was supported in part by CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
1. Coleman EA, Min SJ, Chomiak A, Kramer AM. Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39:1449-1465. PubMed
2. Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47:787-793. PubMed
3. Durairaj L, Will JG, Torner JC, Doebbeling BN. Prognostic factors for mortality following interhospital transfers to the medical intensive care unit of a tertiary referral center. Crit Care Med. 2003;31:1981-1986. PubMed
4. Golestanian E, Scruggs JE, Gangnon RE, Mak RP, Wood KE. Effect of interhospital transfer on resource utilization and outcomes at a tertiary care referral center. Crit Care Med. 2007;35:1470-1476. PubMed
5. Flabouris A, Hart GK, George C. Outcomes of patients admitted to tertiary intensive care units after interhospital transfer: comparison with patients admitted from emergency departments. Crit Care Resusc. 2008;10:97-105. PubMed
6. Combes A, Luyt CE, Trouillet JL, Chastre J, Gibert C. Adverse effect on a referral intensive care unit’s performance of accepting patients transferred from another intensive care unit. Crit Care Med. 2005;33:705-710. PubMed
7. Rosenberg AL, Hofer TP, Strachan C, Watts CM, Hayward RA. Accepting critically ill transfer patients: adverse effect on a referral center’s outcome and benchmark measures. A Intern Med. 2003;138:882-890. PubMed
8. Horwitz LI, Moin T, Krumholz HM, Wang L, Bradley EH. Consequences of inadequate sign-out for patient care. Arch Intern Med. 2008;168:1755-1760. PubMed
9. Starmer AJ, Sectish TC, Simon DW, et al. Rates of medical errors and preventable adverse events among hospitalized children following implementation of a resident handoff bundle. JAMA. 2013;310:2262-2270. PubMed
10. Arora VM, Manjarrez E, Dressler DD, Basaviah P, Halasyamani L, Kripalani S. Hospitalist handoffs: a systematic review and task force recommendations. J Hosp Med. 2009;4:433-440. PubMed
11. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49:592-598. PubMed
12. Herrigel DJ, Carroll M, Fanning C, Steinberg MB, Parikh A, Usher M. Interhospital transfer handoff practices among US tertiary care centers: a descriptive survey. J Hosp Med. 2016;11:413-417. PubMed
13. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians. JAMA. 2007;297:831-841. PubMed
14. Gandara E, Moniz TT, Ungar J, et al. Deficits in discharge documentation in patients transferred to rehabilitation facilities on anticoagulation: results of a systemwide evaluation. Jt Comm J Qual Patient Saf. 2008;34:460-463. PubMed
15. Haig KM, Sutton S, Whittington J. SBAR: a shared mental model for improving communication between clinicians. Jt Comm J Qual Patient Saf. 2006;32:167-175. PubMed
16. van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626-633. PubMed
17. Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299-309. PubMed
18. Malpass HC, Enfield KB, Keim-Malpass J, Verghese GM. The interhospital medical intensive care unit transfer instrument facilitates early implementation of critical therapies and is associated with fewer emergent procedures upon arrival. J Intensive Care Med. 2015;30:351-357. PubMed
19. National Academy of Sciences. Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. March 2005:1–360. Washington, DC. PubMed
20. Theobald CN, Stover DG, Choma NN, et al. The effect of reducing maximum shift lengths to 16 hours on internal medicine interns’ educational opportunities. Acad Med. 2013;88:512-518. PubMed
21. Choma NN, Vasilevskis EE, Sponsler KC, Hathaway J, Kripalani S. Effect of the ACGME 16-hour rule on efficiency and quality of care: duty hours 2.0. JAMA Intern Med. 2013;173:819-821. PubMed
The transfer of inpatients between hospitals for specialized services is common, affecting nearly 10% of all Medicare admissions1 and 4.5% of all critical care hospitalizations.2 At tertiary referral centers, 49% of medical intensive care unit (ICU) admissions are transferred from another hospital.3
Transfer patients have longer length of stay (LOS) than patients admitted directly from the emergency department or clinic. Among patients initially admitted to an ICU, transfer patients spend 1 day to 2.2 more days in the ICU and an additional 2 days to 4 more days total at the receiving hospital.4,5 Furthermore, transfer patients have higher mortality than nontransferred patients by 4% to 8%.3-5 Even after adjustment for case mix and comorbid disease, interhospital transfer is an independent predictor of both ICU death and LOS.6,7 As a result, interhospital transfer has been associated with a $9600 increase (on average) in hospital costs.4
Despite evidence detailing patient handovers as a key time when poor communication can lead to delays in care and significant patient risk, 8-10 most studies have focused on hospital discharge or change of shift, and scant effort has been dedicated to improving the interhospital handover. The process of interhospital transfer is often prolonged and discontinuous,11 commonly including delays of more than 24 hours between initiation and completion. This frequently precludes direct physician-to-physician contact at the time of transfer, and physicians rely on the discharge/transfer summary.12 Yet discharge summaries are frequently absent or incomplete,13 and often lack information for high-risk treatments such as systemic anticoagulation.14 The traditional reliance on discharge summaries for handover communication requires interpretation of unstandardized documentation and increases the risk for miscommunication, delays, and error.
To improve communication, we developed a 1-page handover tool for all inbound adult interhospital transfers to our academic medical center. We sought to determine whether implementation of this standardized handover tool improved the timeliness of initial care, LOS, and mortality among interhospital transfer patients.
METHODS
Study Design, Setting, Population
We conducted a retrospective cohort study of patients transferred into Vanderbilt University Hospital (VUH), an adult 626-bed quaternary care academic medical center in Nashville, Tennessee. The Vanderbilt University Institutional Review Board approved this study.
Population
We selected for inclusion all patients age 18 or older who were transferred into VUH between July 1, 2009 and December 31, 2010. We excluded patients whose transfer was routed outside the main VUH Patient Flow Center as well as patients who did not arrive alive at VUH. We also excluded patients transferred to the emergency department and patients admitted to obstetrics, burn, or trauma services, because these admitting services did not initially use the handover tool. Patients were followed for the duration of their hospitalization at VUH.
Intervention
The 1-page handover tool was developed with multidisciplinary physician input from house staff; medical directors from intensive care, neurology, and surgery; and the chief of staff. The tool was structured on the SBAR model (Situation, Background, Assessment, and Recommendation).15 Fields on the handover tool were limited to those deemed critical for immediate patient care and designed for 1 tool to be used for both ICU and non-ICU transfers. Fields included primary diagnosis; allergies; use and last dose of anticoagulants, vasopressors, sedative/paralytics, and antibiotics; isolation needs; indwelling devices; recent operations/procedures; code status; emergency contact information; problem list; active medication list; vital signs; pertinent exam; imaging; lab findings; and overall reason for transfer.
The handover tool was completed by the physician at the transferring hospital, faxed to VUH, and immediately scanned into the electronic record, allowing the receiving physicians to review information before patient arrival. Use of the tool was piloted first with 2 referring hospitals in April 2010 and universally recommended but not compulsory for all adult patients routed through the main VUH Patient Flow Center beginning July 1, 2010. Immediately before full implementation, the chief of staff sent letters to leadership of the 40 highest volume referral hospitals, highlighting the institutional goal of improving hand-off communication, framing completion of the tool as a step in the transfer acceptance process, and providing contact information for questions, feedback, or concerns. To ensure the tool was a standard part of the transfer process, the VUH Patient Flow Center maintained the responsibility of faxing the form to the outside facility and monitoring its receipt. The tool was processed in the same manner as other faxed patient records and treated as a part of the formal medical record to meet all standards for the Health Insurance Portability and Accountability Act (HIPAA) and medicolegal compliance. The medical center also has a separate cardiac transfer center where the handover tool was not implemented owing to its specialized workflow.
Data Source
The VUH Patient Flow Center maintains a database of all patients for whom transfer to VUH is requested, including information on the requesting hospital and the duration of transfer process. Outcome data and patient characteristics were extracted from the Enterprise Data Warehouse. Data related to comorbid illness were extracted from the Perioperative Data Warehouse, an IRB-approved data registry.
Measures
We evaluated 3 outcomes. First, we defined 2 measures of the timeliness of initial care, the time from arrival at VUH until entry of an admission order, and the time from arrival until entry of the first antibiotic order. Only antibiotics ordered within the first 36 hours of admission were included. Second, we evaluated the total LOS after transfer to VUH and the ICU LOS for patients transferred into an ICU setting. Finally, we examined in-hospital mortality at VUH. These metrics were chosen for their broad applicability across patient groups and feasibility of data capture. Length of stay and mortality also represent final common pathways for avoidance of complications. Specific patient safety indicators and complications were not abstracted due to their low frequency and burden of data collection. Due to system changes in our cost accounting systems, we were not able to obtain cost data pre- and postimplementation that provided meaningful comparisons.
Patient covariates included age, gender, payer, and Elixhauser comorbidity index as modified by van Walraven,16 calculated based on the admission of interest and the previous 365 days. We also examined admission characteristics including location (ICU vs. non-ICU), admitting service (medicine, surgery, neurology, or gynecology), and shift of arrival (day, 7:00 am to 6:00 pm; or night, 6:00 pm to 7:00 pm). Finally, we examined duration of the transfer process (ie, time between transfer request and arrival at VUH) and the volume of the transferring hospital (high was defined as 3 or more transfers to VUH per year).
Statistical analysis
Patient characteristics before and after implementation of the handover tool were compared using Pearson’s chi-square test and Fisher exact test for categorical variables and using Student t test and the Wilcoxon rank sum test for continuous variables. The outcome variables of time to admission order entry, time to antibiotic order entry, LOS, ICU LOS, and in-hospital mortality were compared between the before- and after-intervention time periods, using the Wilcoxon rank sum test for continuous outcomes and Pearson’s chi-square test for in-hospital mortality.
To account for temporal trends, the effect of the handover tool on time-to-admission order entry, hospital LOS, and mortality was measured using an interrupted time-series design with segmented linear regression analysis.17 The study period was divided into 2-week intervals, with 26 time periods in the pre-intervention period and 13 time periods in the postintervention period. Expected rates for the postintervention time periods were projected from the pre-intervention data using a linear regression model. To assess the observed effect of the intervention, rates from the postintervention periods were compared with these projected rates, assuming continuation of the trend. Restricted cubic spline models were also fit for time-to-admission order and hospital LOS; however, the F-statistics for these models were not significant, suggesting the linear regression provided a more appropriate model.
To further account for potential confounding of outcomes by comorbid disease and other patient factors, multivariate linear regression models assessed change in timeliness and LOS with implementation of the intervention. A multivariate logistic regression model was used to assess change in mortality with intervention implementation. All models adjusted for age, gender, payer, comorbid illness, admitting team, shift of arrival (day vs. night), transfer duration, volume of transferring hospital, and ICU status. Outcomes were further adjusted for calendar month to account for temporal trends in house staff efficiency. Because the cardiac transfer center did not adopt the use of the transfer tool, we evaluated adjusted in-hospital mortality for these patients as a concurrent control group.
All statistical testing was 2-sided at a significance level of 0.05. All analyses were conducted using STATA 12.1 statistical software (StataCorp LP, College Station, Texas).
RESULTS
Of 10,325 patients for whom transfer to VUH was requested during the study period, 1715 met inclusion criteria, including 798 patients (46.5%) initially admitted to an ICU setting. Specific patient exclusions are detailed in the Supplemental Figure; the majority of exclusions were due to patients being transferred directly to the emergency department setting. Table 1 summarizes patient characteristics before and after implementation of the handover tool. The median age was 57 years, with 48.6% male patients. Accepting services included medicine (56%), surgery (34%), neurology (9%), and gynecology (1%). The median duration of transfer was 8 hours, and the majority (93%) of patients came from higher volume transferring hospitals. Most (65%) of patients were admitted during night shift. The median modified Elixhauser comorbidity index was 11 (range of possible scores, -19 to 89). A slightly higher proportion of patients admitted postimplementation of the handover tool came from higher volume transferring hospitals; otherwise, there were no significant differences between the pre- and postintervention groups.
Vanderbilt University Hospital received transfers from more than 350 unique facilities in more than 25 U.S. states during the overall study period. During the postintervention period, adherence to the handover process was excellent, with more than 85% of patients having a completed handover tool available in their medical record at the time of transfer. The remaining 15% had either incomplete forms or no form.
Timeliness of Initial Care
There was no change in either the median time-to-admission order entry after implementation (47 vs. 45 minutes, unadjusted P = 0.36) or time to antibiotic order entry (199 vs. 202 minutes; unadjusted P = 0.81; Table 2).
In the time-series analysis, the pre-intervention period did not have a significant temporal trend in median time-to-admission order entry (ß-coefficient = -0.27; 95% confidence interval [CI] -0.85 to 0.31; R2 = 0.04; P = 0.34; Figure 1A). The postintervention period showed a trend toward a reduction in median time-to-admission order entry (ß-coefficient = -1.39; 95% CI -2.92 to 0.15; R2 = 0.27; P = 0.07). There was no significant difference between the actual time-to-admission order entry in the postintervention period when compared to the projected rates from the pre-intervention period (P = 0.18).
After multivariate adjustment, the postintervention time period was not associated with any significant change in the median time-to-admission order entry (P = 0.94, R2 = 0.09) nor time-to-antibiotic order entry (P = 0.91; R2 = 0.08; Table 2).
Length of Stay
Hospital LOS demonstrated a nonstatistically significant decline after implementation of the handover tool from 6.47 days to 5.81 days (unadjusted P = 0.18; Table 2). There was no significant change in ICU LOS postintervention (4.34 days to 4.55 days; P = 0.38).
In time series analysis, hospital LOS did not have a significant temporal trend in either the pre-intervention period (ß-coefficient = 0.00094; 95% CI, -0.07 to 0.07; R2 = 0.00; P = 0.98) or the postintervention period (ß-coefficient = 0.09; 95% CI, -0.07 to 0.25; R2 = 0.13; P = 0.23; Figure 1B). Similarly, there was no significant difference between the actual and projected hospital LOS after implementation of the handover tool (P = 0.31).
After multivariate adjustment, the postintervention time period was associated with a trend toward reduction in overall LOS (P = 0.06; R2 = 0.07) but no significant change in ICU LOS (P = 0.99; R2 = 0.09).
Mortality
In-hospital mortality declined significantly from 12.0% in the pre-intervention period to 8.9% in the postintervention period (P = 0.04; Table 2). In time-series analysis, mortality did not have a significant trend in the pre-intervention period (ß-coefficient = 0.00017, 95% CI, -0.0020 to 0.0024; P = 0.878) and had a trend toward reduction in the postintervention period (ß-coefficient = -0.0032; 95% CI, -0.0091 to 0.0027; P = 0.255; Figure 1C) but did not reach statistical significance due to relatively small numbers of deaths in each individual time period.
After multivariate adjustment, the postintervention period was associated with overall lower odds of mortality among transfer patients when compared with the pre-intervention period (adjusted OR 0.68; 95% CI, 0.47 – 0.99; R2 = 0.21; P = 0.04; Figure 2). Among the concurrent control group of patients routed through the cardiac transfer center, there was no significant change in mortality between the pre- and postintervention periods (adjusted OR 1.31; 95% CI, 0.88 – 1.93; R2 = 0.28; P = 0.18).
DISCUSSION
We developed a simple 1-page handover tool for interhospital transfer patients and aimed to improve timeliness, efficiency, and outcomes of care at the receiving hospital. Implementation of the handover tool was feasible and well accepted by transferring physicians despite a geographically large and diverse transfer network. Although implementation did not substantially improve measures of the timeliness of initial care among transfer patients, we noted a nonsignificant trend toward reduced LOS postintervention.
We observed a substantial and statistically significant reduction in mortality among transfer patients after implementation of the handover tool that persisted after controlling for time trends, comorbid illness, and several other patient factors. This effect was not seen in a concurrent control group of cardiac transfer patients for whom the handover tool was not implemented. Standardizing communication regarding high-risk clinical care processes may be responsible for the observed mortality reduction, similar to improvements seen in other small pilot studies.18 We acknowledge that the magnitude of the improvement in mortality is more than might be expected from the handover tool alone and could be due to chance.
In this initial evaluation, it was not feasible to determine whether information provided in the handover tool helped avert specific complications that could affect mortality, such as complications related to the use of ventilators, high-risk medications, or indwelling devices. Assessment of additional patient safety indices such as code events, unplanned ICU transfers, and medication errors could also help clarify the effect of the handover tool on patient-safety outcomes, and future work should include these metrics as well. Alternately, the improvement in mortality may result from other unmeasured processes that occurred concurrently and verification of this finding should be completed in other settings.
CONCLUSION
More work is needed to determine suitable process and outcome measures for interhospital transfers. Most literature has focused on cost and LOS at the exclusion of more proximal measures of initial care.3-7 The Institute of Medicine has identified timeliness as 1 of the 6 aims for care delivery redesign,19 yet standardized timeliness outcomes do not exist across broad inpatient populations. We chose to monitor the time-to-admission order entry and time-to-antibiotic order entry as 2 indicators of timeliness that would be applicable to a variety of patients. The lack of change in these selected measures should prompt examination for other measures of efficiency, including those that affect nontransferred patients. It is possible that nontransferred patients cared for by the same physician also benefit from fewer delays or disruptions and experience increased efficiency of care if transfer patient communication is improved. Further work is necessary to understand whether other measures of timely initial patient care may be more suitable.
The use of a time-series design to account for temporal trends adds substantial rigor to this study, since the majority of these patients were cared for by house staff whose experience and efficiency vary throughout the academic year. Furthermore, subsequent multivariate analysis demonstrated consistent findings after adjustment for comorbid illness and several other hospital and patient-level confounders.
This study has several limitations. The primary limitation is its nonrandomized design. Patient characteristics were stable across multiple variables before and after implementation, but it is possible that another confounding factor was responsible for observed improvements. Likewise, we collected data for only 6 rather than 12 months during the postintervention time period, which limited our sample size and statistical power. This was chosen because a significant restructuring of resident duty hours occurred in spring 2011 that had the potential to affect all measures studied.20,21 Finally, we did not collect data on accuracy of the information provided in the handover tool or end-user utilization and were unable to account for effects of these.
Since implementation in 2010, this process for interhospital transfers at VUH remains the same, although the volume of incoming transfers has significantly increased. Electronic handover tools with similar structure and content have since been adopted for patients being transferred to the emergency department or directly admitted from clinic. As VUH moves in the coming years from a locally developed electronic medical record to a national vendor, there will be an opportunity to transform this tool into an electronic template that will easily share data between institutions and further enhance communication.
Interhospital transfer patients represent a high-risk population whose unique handover needs have not been adequately measured or addressed. Our investigation demonstrated that a standardized handover aid can be implemented across a broad transfer network and may contribute to reductions in LOS and mortality. Further study is warranted to confirm these findings and assess the effect on other clinical outcomes.
Disclosures
This material is based upon work supported by the Office of Academic Affiliations, Department of Veterans Affairs, VA National Quality Scholars Program, and was made possible by the use of the facilities at VA Tennessee Valley Healthcare System, Nashville, Tennessee. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government. Additionally, this publication was supported in part by CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
The transfer of inpatients between hospitals for specialized services is common, affecting nearly 10% of all Medicare admissions1 and 4.5% of all critical care hospitalizations.2 At tertiary referral centers, 49% of medical intensive care unit (ICU) admissions are transferred from another hospital.3
Transfer patients have longer length of stay (LOS) than patients admitted directly from the emergency department or clinic. Among patients initially admitted to an ICU, transfer patients spend 1 day to 2.2 more days in the ICU and an additional 2 days to 4 more days total at the receiving hospital.4,5 Furthermore, transfer patients have higher mortality than nontransferred patients by 4% to 8%.3-5 Even after adjustment for case mix and comorbid disease, interhospital transfer is an independent predictor of both ICU death and LOS.6,7 As a result, interhospital transfer has been associated with a $9600 increase (on average) in hospital costs.4
Despite evidence detailing patient handovers as a key time when poor communication can lead to delays in care and significant patient risk, 8-10 most studies have focused on hospital discharge or change of shift, and scant effort has been dedicated to improving the interhospital handover. The process of interhospital transfer is often prolonged and discontinuous,11 commonly including delays of more than 24 hours between initiation and completion. This frequently precludes direct physician-to-physician contact at the time of transfer, and physicians rely on the discharge/transfer summary.12 Yet discharge summaries are frequently absent or incomplete,13 and often lack information for high-risk treatments such as systemic anticoagulation.14 The traditional reliance on discharge summaries for handover communication requires interpretation of unstandardized documentation and increases the risk for miscommunication, delays, and error.
To improve communication, we developed a 1-page handover tool for all inbound adult interhospital transfers to our academic medical center. We sought to determine whether implementation of this standardized handover tool improved the timeliness of initial care, LOS, and mortality among interhospital transfer patients.
METHODS
Study Design, Setting, Population
We conducted a retrospective cohort study of patients transferred into Vanderbilt University Hospital (VUH), an adult 626-bed quaternary care academic medical center in Nashville, Tennessee. The Vanderbilt University Institutional Review Board approved this study.
Population
We selected for inclusion all patients age 18 or older who were transferred into VUH between July 1, 2009 and December 31, 2010. We excluded patients whose transfer was routed outside the main VUH Patient Flow Center as well as patients who did not arrive alive at VUH. We also excluded patients transferred to the emergency department and patients admitted to obstetrics, burn, or trauma services, because these admitting services did not initially use the handover tool. Patients were followed for the duration of their hospitalization at VUH.
Intervention
The 1-page handover tool was developed with multidisciplinary physician input from house staff; medical directors from intensive care, neurology, and surgery; and the chief of staff. The tool was structured on the SBAR model (Situation, Background, Assessment, and Recommendation).15 Fields on the handover tool were limited to those deemed critical for immediate patient care and designed for 1 tool to be used for both ICU and non-ICU transfers. Fields included primary diagnosis; allergies; use and last dose of anticoagulants, vasopressors, sedative/paralytics, and antibiotics; isolation needs; indwelling devices; recent operations/procedures; code status; emergency contact information; problem list; active medication list; vital signs; pertinent exam; imaging; lab findings; and overall reason for transfer.
The handover tool was completed by the physician at the transferring hospital, faxed to VUH, and immediately scanned into the electronic record, allowing the receiving physicians to review information before patient arrival. Use of the tool was piloted first with 2 referring hospitals in April 2010 and universally recommended but not compulsory for all adult patients routed through the main VUH Patient Flow Center beginning July 1, 2010. Immediately before full implementation, the chief of staff sent letters to leadership of the 40 highest volume referral hospitals, highlighting the institutional goal of improving hand-off communication, framing completion of the tool as a step in the transfer acceptance process, and providing contact information for questions, feedback, or concerns. To ensure the tool was a standard part of the transfer process, the VUH Patient Flow Center maintained the responsibility of faxing the form to the outside facility and monitoring its receipt. The tool was processed in the same manner as other faxed patient records and treated as a part of the formal medical record to meet all standards for the Health Insurance Portability and Accountability Act (HIPAA) and medicolegal compliance. The medical center also has a separate cardiac transfer center where the handover tool was not implemented owing to its specialized workflow.
Data Source
The VUH Patient Flow Center maintains a database of all patients for whom transfer to VUH is requested, including information on the requesting hospital and the duration of transfer process. Outcome data and patient characteristics were extracted from the Enterprise Data Warehouse. Data related to comorbid illness were extracted from the Perioperative Data Warehouse, an IRB-approved data registry.
Measures
We evaluated 3 outcomes. First, we defined 2 measures of the timeliness of initial care, the time from arrival at VUH until entry of an admission order, and the time from arrival until entry of the first antibiotic order. Only antibiotics ordered within the first 36 hours of admission were included. Second, we evaluated the total LOS after transfer to VUH and the ICU LOS for patients transferred into an ICU setting. Finally, we examined in-hospital mortality at VUH. These metrics were chosen for their broad applicability across patient groups and feasibility of data capture. Length of stay and mortality also represent final common pathways for avoidance of complications. Specific patient safety indicators and complications were not abstracted due to their low frequency and burden of data collection. Due to system changes in our cost accounting systems, we were not able to obtain cost data pre- and postimplementation that provided meaningful comparisons.
Patient covariates included age, gender, payer, and Elixhauser comorbidity index as modified by van Walraven,16 calculated based on the admission of interest and the previous 365 days. We also examined admission characteristics including location (ICU vs. non-ICU), admitting service (medicine, surgery, neurology, or gynecology), and shift of arrival (day, 7:00 am to 6:00 pm; or night, 6:00 pm to 7:00 pm). Finally, we examined duration of the transfer process (ie, time between transfer request and arrival at VUH) and the volume of the transferring hospital (high was defined as 3 or more transfers to VUH per year).
Statistical analysis
Patient characteristics before and after implementation of the handover tool were compared using Pearson’s chi-square test and Fisher exact test for categorical variables and using Student t test and the Wilcoxon rank sum test for continuous variables. The outcome variables of time to admission order entry, time to antibiotic order entry, LOS, ICU LOS, and in-hospital mortality were compared between the before- and after-intervention time periods, using the Wilcoxon rank sum test for continuous outcomes and Pearson’s chi-square test for in-hospital mortality.
To account for temporal trends, the effect of the handover tool on time-to-admission order entry, hospital LOS, and mortality was measured using an interrupted time-series design with segmented linear regression analysis.17 The study period was divided into 2-week intervals, with 26 time periods in the pre-intervention period and 13 time periods in the postintervention period. Expected rates for the postintervention time periods were projected from the pre-intervention data using a linear regression model. To assess the observed effect of the intervention, rates from the postintervention periods were compared with these projected rates, assuming continuation of the trend. Restricted cubic spline models were also fit for time-to-admission order and hospital LOS; however, the F-statistics for these models were not significant, suggesting the linear regression provided a more appropriate model.
To further account for potential confounding of outcomes by comorbid disease and other patient factors, multivariate linear regression models assessed change in timeliness and LOS with implementation of the intervention. A multivariate logistic regression model was used to assess change in mortality with intervention implementation. All models adjusted for age, gender, payer, comorbid illness, admitting team, shift of arrival (day vs. night), transfer duration, volume of transferring hospital, and ICU status. Outcomes were further adjusted for calendar month to account for temporal trends in house staff efficiency. Because the cardiac transfer center did not adopt the use of the transfer tool, we evaluated adjusted in-hospital mortality for these patients as a concurrent control group.
All statistical testing was 2-sided at a significance level of 0.05. All analyses were conducted using STATA 12.1 statistical software (StataCorp LP, College Station, Texas).
RESULTS
Of 10,325 patients for whom transfer to VUH was requested during the study period, 1715 met inclusion criteria, including 798 patients (46.5%) initially admitted to an ICU setting. Specific patient exclusions are detailed in the Supplemental Figure; the majority of exclusions were due to patients being transferred directly to the emergency department setting. Table 1 summarizes patient characteristics before and after implementation of the handover tool. The median age was 57 years, with 48.6% male patients. Accepting services included medicine (56%), surgery (34%), neurology (9%), and gynecology (1%). The median duration of transfer was 8 hours, and the majority (93%) of patients came from higher volume transferring hospitals. Most (65%) of patients were admitted during night shift. The median modified Elixhauser comorbidity index was 11 (range of possible scores, -19 to 89). A slightly higher proportion of patients admitted postimplementation of the handover tool came from higher volume transferring hospitals; otherwise, there were no significant differences between the pre- and postintervention groups.
Vanderbilt University Hospital received transfers from more than 350 unique facilities in more than 25 U.S. states during the overall study period. During the postintervention period, adherence to the handover process was excellent, with more than 85% of patients having a completed handover tool available in their medical record at the time of transfer. The remaining 15% had either incomplete forms or no form.
Timeliness of Initial Care
There was no change in either the median time-to-admission order entry after implementation (47 vs. 45 minutes, unadjusted P = 0.36) or time to antibiotic order entry (199 vs. 202 minutes; unadjusted P = 0.81; Table 2).
In the time-series analysis, the pre-intervention period did not have a significant temporal trend in median time-to-admission order entry (ß-coefficient = -0.27; 95% confidence interval [CI] -0.85 to 0.31; R2 = 0.04; P = 0.34; Figure 1A). The postintervention period showed a trend toward a reduction in median time-to-admission order entry (ß-coefficient = -1.39; 95% CI -2.92 to 0.15; R2 = 0.27; P = 0.07). There was no significant difference between the actual time-to-admission order entry in the postintervention period when compared to the projected rates from the pre-intervention period (P = 0.18).
After multivariate adjustment, the postintervention time period was not associated with any significant change in the median time-to-admission order entry (P = 0.94, R2 = 0.09) nor time-to-antibiotic order entry (P = 0.91; R2 = 0.08; Table 2).
Length of Stay
Hospital LOS demonstrated a nonstatistically significant decline after implementation of the handover tool from 6.47 days to 5.81 days (unadjusted P = 0.18; Table 2). There was no significant change in ICU LOS postintervention (4.34 days to 4.55 days; P = 0.38).
In time series analysis, hospital LOS did not have a significant temporal trend in either the pre-intervention period (ß-coefficient = 0.00094; 95% CI, -0.07 to 0.07; R2 = 0.00; P = 0.98) or the postintervention period (ß-coefficient = 0.09; 95% CI, -0.07 to 0.25; R2 = 0.13; P = 0.23; Figure 1B). Similarly, there was no significant difference between the actual and projected hospital LOS after implementation of the handover tool (P = 0.31).
After multivariate adjustment, the postintervention time period was associated with a trend toward reduction in overall LOS (P = 0.06; R2 = 0.07) but no significant change in ICU LOS (P = 0.99; R2 = 0.09).
Mortality
In-hospital mortality declined significantly from 12.0% in the pre-intervention period to 8.9% in the postintervention period (P = 0.04; Table 2). In time-series analysis, mortality did not have a significant trend in the pre-intervention period (ß-coefficient = 0.00017, 95% CI, -0.0020 to 0.0024; P = 0.878) and had a trend toward reduction in the postintervention period (ß-coefficient = -0.0032; 95% CI, -0.0091 to 0.0027; P = 0.255; Figure 1C) but did not reach statistical significance due to relatively small numbers of deaths in each individual time period.
After multivariate adjustment, the postintervention period was associated with overall lower odds of mortality among transfer patients when compared with the pre-intervention period (adjusted OR 0.68; 95% CI, 0.47 – 0.99; R2 = 0.21; P = 0.04; Figure 2). Among the concurrent control group of patients routed through the cardiac transfer center, there was no significant change in mortality between the pre- and postintervention periods (adjusted OR 1.31; 95% CI, 0.88 – 1.93; R2 = 0.28; P = 0.18).
DISCUSSION
We developed a simple 1-page handover tool for interhospital transfer patients and aimed to improve timeliness, efficiency, and outcomes of care at the receiving hospital. Implementation of the handover tool was feasible and well accepted by transferring physicians despite a geographically large and diverse transfer network. Although implementation did not substantially improve measures of the timeliness of initial care among transfer patients, we noted a nonsignificant trend toward reduced LOS postintervention.
We observed a substantial and statistically significant reduction in mortality among transfer patients after implementation of the handover tool that persisted after controlling for time trends, comorbid illness, and several other patient factors. This effect was not seen in a concurrent control group of cardiac transfer patients for whom the handover tool was not implemented. Standardizing communication regarding high-risk clinical care processes may be responsible for the observed mortality reduction, similar to improvements seen in other small pilot studies.18 We acknowledge that the magnitude of the improvement in mortality is more than might be expected from the handover tool alone and could be due to chance.
In this initial evaluation, it was not feasible to determine whether information provided in the handover tool helped avert specific complications that could affect mortality, such as complications related to the use of ventilators, high-risk medications, or indwelling devices. Assessment of additional patient safety indices such as code events, unplanned ICU transfers, and medication errors could also help clarify the effect of the handover tool on patient-safety outcomes, and future work should include these metrics as well. Alternately, the improvement in mortality may result from other unmeasured processes that occurred concurrently and verification of this finding should be completed in other settings.
CONCLUSION
More work is needed to determine suitable process and outcome measures for interhospital transfers. Most literature has focused on cost and LOS at the exclusion of more proximal measures of initial care.3-7 The Institute of Medicine has identified timeliness as 1 of the 6 aims for care delivery redesign,19 yet standardized timeliness outcomes do not exist across broad inpatient populations. We chose to monitor the time-to-admission order entry and time-to-antibiotic order entry as 2 indicators of timeliness that would be applicable to a variety of patients. The lack of change in these selected measures should prompt examination for other measures of efficiency, including those that affect nontransferred patients. It is possible that nontransferred patients cared for by the same physician also benefit from fewer delays or disruptions and experience increased efficiency of care if transfer patient communication is improved. Further work is necessary to understand whether other measures of timely initial patient care may be more suitable.
The use of a time-series design to account for temporal trends adds substantial rigor to this study, since the majority of these patients were cared for by house staff whose experience and efficiency vary throughout the academic year. Furthermore, subsequent multivariate analysis demonstrated consistent findings after adjustment for comorbid illness and several other hospital and patient-level confounders.
This study has several limitations. The primary limitation is its nonrandomized design. Patient characteristics were stable across multiple variables before and after implementation, but it is possible that another confounding factor was responsible for observed improvements. Likewise, we collected data for only 6 rather than 12 months during the postintervention time period, which limited our sample size and statistical power. This was chosen because a significant restructuring of resident duty hours occurred in spring 2011 that had the potential to affect all measures studied.20,21 Finally, we did not collect data on accuracy of the information provided in the handover tool or end-user utilization and were unable to account for effects of these.
Since implementation in 2010, this process for interhospital transfers at VUH remains the same, although the volume of incoming transfers has significantly increased. Electronic handover tools with similar structure and content have since been adopted for patients being transferred to the emergency department or directly admitted from clinic. As VUH moves in the coming years from a locally developed electronic medical record to a national vendor, there will be an opportunity to transform this tool into an electronic template that will easily share data between institutions and further enhance communication.
Interhospital transfer patients represent a high-risk population whose unique handover needs have not been adequately measured or addressed. Our investigation demonstrated that a standardized handover aid can be implemented across a broad transfer network and may contribute to reductions in LOS and mortality. Further study is warranted to confirm these findings and assess the effect on other clinical outcomes.
Disclosures
This material is based upon work supported by the Office of Academic Affiliations, Department of Veterans Affairs, VA National Quality Scholars Program, and was made possible by the use of the facilities at VA Tennessee Valley Healthcare System, Nashville, Tennessee. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government. Additionally, this publication was supported in part by CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
1. Coleman EA, Min SJ, Chomiak A, Kramer AM. Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39:1449-1465. PubMed
2. Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47:787-793. PubMed
3. Durairaj L, Will JG, Torner JC, Doebbeling BN. Prognostic factors for mortality following interhospital transfers to the medical intensive care unit of a tertiary referral center. Crit Care Med. 2003;31:1981-1986. PubMed
4. Golestanian E, Scruggs JE, Gangnon RE, Mak RP, Wood KE. Effect of interhospital transfer on resource utilization and outcomes at a tertiary care referral center. Crit Care Med. 2007;35:1470-1476. PubMed
5. Flabouris A, Hart GK, George C. Outcomes of patients admitted to tertiary intensive care units after interhospital transfer: comparison with patients admitted from emergency departments. Crit Care Resusc. 2008;10:97-105. PubMed
6. Combes A, Luyt CE, Trouillet JL, Chastre J, Gibert C. Adverse effect on a referral intensive care unit’s performance of accepting patients transferred from another intensive care unit. Crit Care Med. 2005;33:705-710. PubMed
7. Rosenberg AL, Hofer TP, Strachan C, Watts CM, Hayward RA. Accepting critically ill transfer patients: adverse effect on a referral center’s outcome and benchmark measures. A Intern Med. 2003;138:882-890. PubMed
8. Horwitz LI, Moin T, Krumholz HM, Wang L, Bradley EH. Consequences of inadequate sign-out for patient care. Arch Intern Med. 2008;168:1755-1760. PubMed
9. Starmer AJ, Sectish TC, Simon DW, et al. Rates of medical errors and preventable adverse events among hospitalized children following implementation of a resident handoff bundle. JAMA. 2013;310:2262-2270. PubMed
10. Arora VM, Manjarrez E, Dressler DD, Basaviah P, Halasyamani L, Kripalani S. Hospitalist handoffs: a systematic review and task force recommendations. J Hosp Med. 2009;4:433-440. PubMed
11. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49:592-598. PubMed
12. Herrigel DJ, Carroll M, Fanning C, Steinberg MB, Parikh A, Usher M. Interhospital transfer handoff practices among US tertiary care centers: a descriptive survey. J Hosp Med. 2016;11:413-417. PubMed
13. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians. JAMA. 2007;297:831-841. PubMed
14. Gandara E, Moniz TT, Ungar J, et al. Deficits in discharge documentation in patients transferred to rehabilitation facilities on anticoagulation: results of a systemwide evaluation. Jt Comm J Qual Patient Saf. 2008;34:460-463. PubMed
15. Haig KM, Sutton S, Whittington J. SBAR: a shared mental model for improving communication between clinicians. Jt Comm J Qual Patient Saf. 2006;32:167-175. PubMed
16. van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626-633. PubMed
17. Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299-309. PubMed
18. Malpass HC, Enfield KB, Keim-Malpass J, Verghese GM. The interhospital medical intensive care unit transfer instrument facilitates early implementation of critical therapies and is associated with fewer emergent procedures upon arrival. J Intensive Care Med. 2015;30:351-357. PubMed
19. National Academy of Sciences. Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. March 2005:1–360. Washington, DC. PubMed
20. Theobald CN, Stover DG, Choma NN, et al. The effect of reducing maximum shift lengths to 16 hours on internal medicine interns’ educational opportunities. Acad Med. 2013;88:512-518. PubMed
21. Choma NN, Vasilevskis EE, Sponsler KC, Hathaway J, Kripalani S. Effect of the ACGME 16-hour rule on efficiency and quality of care: duty hours 2.0. JAMA Intern Med. 2013;173:819-821. PubMed
1. Coleman EA, Min SJ, Chomiak A, Kramer AM. Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39:1449-1465. PubMed
2. Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47:787-793. PubMed
3. Durairaj L, Will JG, Torner JC, Doebbeling BN. Prognostic factors for mortality following interhospital transfers to the medical intensive care unit of a tertiary referral center. Crit Care Med. 2003;31:1981-1986. PubMed
4. Golestanian E, Scruggs JE, Gangnon RE, Mak RP, Wood KE. Effect of interhospital transfer on resource utilization and outcomes at a tertiary care referral center. Crit Care Med. 2007;35:1470-1476. PubMed
5. Flabouris A, Hart GK, George C. Outcomes of patients admitted to tertiary intensive care units after interhospital transfer: comparison with patients admitted from emergency departments. Crit Care Resusc. 2008;10:97-105. PubMed
6. Combes A, Luyt CE, Trouillet JL, Chastre J, Gibert C. Adverse effect on a referral intensive care unit’s performance of accepting patients transferred from another intensive care unit. Crit Care Med. 2005;33:705-710. PubMed
7. Rosenberg AL, Hofer TP, Strachan C, Watts CM, Hayward RA. Accepting critically ill transfer patients: adverse effect on a referral center’s outcome and benchmark measures. A Intern Med. 2003;138:882-890. PubMed
8. Horwitz LI, Moin T, Krumholz HM, Wang L, Bradley EH. Consequences of inadequate sign-out for patient care. Arch Intern Med. 2008;168:1755-1760. PubMed
9. Starmer AJ, Sectish TC, Simon DW, et al. Rates of medical errors and preventable adverse events among hospitalized children following implementation of a resident handoff bundle. JAMA. 2013;310:2262-2270. PubMed
10. Arora VM, Manjarrez E, Dressler DD, Basaviah P, Halasyamani L, Kripalani S. Hospitalist handoffs: a systematic review and task force recommendations. J Hosp Med. 2009;4:433-440. PubMed
11. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49:592-598. PubMed
12. Herrigel DJ, Carroll M, Fanning C, Steinberg MB, Parikh A, Usher M. Interhospital transfer handoff practices among US tertiary care centers: a descriptive survey. J Hosp Med. 2016;11:413-417. PubMed
13. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians. JAMA. 2007;297:831-841. PubMed
14. Gandara E, Moniz TT, Ungar J, et al. Deficits in discharge documentation in patients transferred to rehabilitation facilities on anticoagulation: results of a systemwide evaluation. Jt Comm J Qual Patient Saf. 2008;34:460-463. PubMed
15. Haig KM, Sutton S, Whittington J. SBAR: a shared mental model for improving communication between clinicians. Jt Comm J Qual Patient Saf. 2006;32:167-175. PubMed
16. van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626-633. PubMed
17. Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299-309. PubMed
18. Malpass HC, Enfield KB, Keim-Malpass J, Verghese GM. The interhospital medical intensive care unit transfer instrument facilitates early implementation of critical therapies and is associated with fewer emergent procedures upon arrival. J Intensive Care Med. 2015;30:351-357. PubMed
19. National Academy of Sciences. Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. March 2005:1–360. Washington, DC. PubMed
20. Theobald CN, Stover DG, Choma NN, et al. The effect of reducing maximum shift lengths to 16 hours on internal medicine interns’ educational opportunities. Acad Med. 2013;88:512-518. PubMed
21. Choma NN, Vasilevskis EE, Sponsler KC, Hathaway J, Kripalani S. Effect of the ACGME 16-hour rule on efficiency and quality of care: duty hours 2.0. JAMA Intern Med. 2013;173:819-821. PubMed
© 2017 Society of Hospital Medicine
Hospital Handoffs and Readmissions in Children
Although much has been written about pediatric discharge and readmissions1-5 over the past several years, surprisingly little is known about which care practices are most effective at preventing postdischarge utilization.5 Major collaborations across the U.S. are currently focused on improving pediatric discharge processes,6-8 although the impact that these efforts will have on readmissions remains to be seen.
Research on handoffs between hospitals and primary care has mixed associations with postdischarge utilization. Although some studies observe positive relationships between specific activities and reduced postdischarge utilization,1 others suggest no relationship9-12 or, paradoxically, more utilization.13,14 Brittan et al15 found that outpatient visits were associated with more readmissions when occurring less than 4 days after discharge, but fewer readmissions when occurring 4 days to 29 days after discharge. Most studies, however, investigate single or limited sets of care activities, such as having an outpatient visit,15 timeliness of that visit,16 or receipt of a discharge summary.11 Inclusion of a more comprehensive set of hospital- to primary-care communication practices may better unravel this complex relationship between discharge care and postdischarge outcomes for children.
The purpose of this study was to characterize a set of traditional discharge handoff practices between hospital and primary care providers (PCPs) and to explore their relationships to readmissions. We hypothesized that handoff practices would be associated with fewer unplanned readmissions.
METHODS
Study Design, Setting, Participants
This project was part of a prospective cohort study with 2 aims: to investigate relationships between medical home experience and postdischarge utilization,17 and to identify relationships between common discharge communication practices and postdischarge utilization. This manuscript is focused on the second aim. Randomly selected pediatric patients and their caregivers were enrolled from any medical or surgical service during an acute hospitalization lasting more than 24 hours from October 1, 2012 to January 1, 2014, at a 100-bed tertiary children’s hospital. Patients who transferred to another facility, died, were older than 18 years or in neonatal care (ie, newborn nursery or neonatal intensive care unit) were excluded since their discharge experiences would be significantly distinct from the population of interest. Patients were enrolled once in the study.
Outcome
The study’s primary outcome was 30-day unplanned readmissions, defined as a hospitalization occurring within 30 days of the index (ie, study enrollment) hospitalization, identified through caregiver report or administrative sources.17 Although the study site is a single hospital system, readmissions could have occurred to any hospital reported by caregivers, (ie, readmissions could have occurred within or outside our health system). Readmissions for chemotherapy, radiation, dialysis, rehabilitation, or labor and delivery were excluded. If caregivers reported an admission as planned or chart review of the index discharge summary noted that a rehospitalization was scheduled in the subsequent 30 days, the readmission was labeled “planned” and excluded.
Discharge Handoff Communication
Transitional care is a set of actions designed to ensure continuity and coordination of healthcare during transfer from 1 location or level of care to another.18,19 The study team, comprised of a division chief of general pediatrics, a division chief of hospital medicine, 2 departmental vice-chairs, and the medical director for quality at the study site, identified 11 common handoff activities and reporting sources. These consensus-based activities were expected by the study team to improve continuity and coordination during hospital-to-home transfer, and included:
- verifying PCP identity during the hospitalization (caregiver report);
- notifying the PCP of admission, discharge, and providing updates during the hospitalization (PCP report);
- PCP follow-up appointment set prior to discharge (caregiver report);
- documenting planned PCP and subspecialty follow-up in the discharge summary (chart review);
- completing the discharge summary within 48 hours (chart review);
- providing a verbal or written handoff to the PCP prior to follow-up (PCP report); and
- having a PCP follow-up visit within 30 days of discharge (caregiver report).
We also asked PCPs whether they thought the follow-up interval was appropriate and whether phone follow-up with the patient would have been as appropriate as a face-to-face visit.
Covariates
Patient demographics that might confound the relationship between handoff practices and readmissions based on pediatric research20,21 were included. Medical complexity was accounted for by length-of-index stay, the number of hospitalizations and emergency department (ED) visits in past 12 months, complex chronic conditions,22,23 and seeing 3 or more subspecialists.24,25 Variables from related work included PCP scope (general pediatrics or subspecialist) and presence of a usual source for well and sick care.17
The Care Transitions Measure-3 (CTM-3), originally developed to assess the patient-centeredness of hospital transition,26,27 can discriminate adult patients at risk for readmission.26 We adapted the original CTM-3 to be answered by caregiver respondents after pilot testing with 5 caregivers not enrolled in the study: 1) “The hospital staff took my preferences and those of my family into account in deciding what my child’s health care needs would be when I left the hospital;” 2) “When I left the hospital, I had a good understanding of the things I was responsible for in managing my child’s health;” and 3) “When I left the hospital, I clearly understood the purpose for giving each of my child’s medications.” We analyzed the adapted CTM-3 on a transformed 0-100 scale as designed,26 initially hypothesizing that the CTM-3 would mediate the relationship between handoff practices and readmissions.
We assessed caregiver confidence to avoid a readmission, based on a strong independent association with readmissions described in Coller et al.17 Using questions developed for this study, caregivers were asked to rate “How confident are you that [child’s name] will stay out of the hospital for the next 30 days?” with instructions to refer to unplanned hospital visits only. Responses were reported on a 4-point Likert scale (1 = very confident, 4 = not very confident). Responses were dichotomized into very confident (ie, “1”) or not very confident (ie, “2-4”).
Enrollment and Data Collection
Computer-generated random numbers were assigned to patients admitted the previous day, and families were enrolled sequentially until the daily enrollment target was reached. Data were obtained from 3 sources: medical record, caregiver report, and PCP report. Trained research assistants systematically extracted chart review data documenting the transitions practices above, while a hospital information technology analyst extracted claims and demographic data to complement what was reported by parents and PCPs. After study conclusion, these medical record data were merged with caregiver and PCP-reported data.
Trained bilingual research assistants collected caregiver- and PCP-reported data using structured questionnaires in English or Spanish, according to preference. Timing of data collection differed by data source; caregiver-reported data were collected immediately after discharge and at 30 days postdischarge; PCP-reported data were collected at 30 days postdischarge.
Caregiver-reported data were collected through 2 separate phone calls following index discharge: immediately after discharge (caregiver confidence and CTM-3 measures) and at 30 days (readmission measures). Caregiver confidence questions were asked after (rather than immediately before) discharge to avoid biasing clinical care and revisit risk, consistent with previous work.28
PCP-reported data were collected using structured questionnaires with the PCP who was identified by the family during study enrollment. PCP-reported data were collected by telephone or fax 30 days after discharge, with up to 5 telephone attempts and 3 fax attempts. At the beginning of the questionnaire, PCPs were asked if they agreed with the designation, although they were asked to complete the questionnaire regardless.
Analyses
Descriptive statistics compared differences in handoff practices and 30-day unplanned readmissions. Exploratory factor analysis assessed whether certain handoff practices were sufficiently correlated to allow grouping of items and construction of scales. Relationships between handoff practices and readmissions were examined using bivariate, followed by multivariate, logistic regression adjusting for the covariates described. Collinearity was tested before constructing final models. Because no relationship was observed between CTM-3 and readmissions, additional mediation analyses were not pursued. All analyses were completed using STATA (SE version 14.0, StataCorp LP, College Station, Texas). This study was approved by the Institutional Review Boards at UCLA (study site) and University of Wisconsin (lead author site).
RESULTS
This study enrolled 701 of 816 eligible participants (85.9%) between October 2012 and January 2014. More than 99% of administrative data and 97% of caregiver questionnaires were complete. Of 685 patients with a reported PCP, we obtained responses from 577 PCPs (84.2%). Patient characteristics and outcomes were not significantly different for patients with and without a responding PCP; however, patients of nonresponding PCPs were more often publicly insured (64.5% vs. 48.2% for responding PCPs, P = 0.004) or seen by a subspecialist as opposed to a generalist (28.1% vs. 13.8% for responding PCPs, P = 0.001).
The overall population characteristics are summarized in Table 1: 27.4% of the cohort was younger 2 years, 49.2% were Hispanic, and the majority (51.1%) had public insurance. The average length of the index hospitalization for the overall population was 4.8 days (standard deviation = 9.6), and 53.5% had at least 1 complex chronic condition. Eighty-four percent of the cohort reported using a generalist (vs. subspecialist) for primary care.
Discharge Handoff Communication
Practices varied widely (Figure 1a). Verbal handoffs between hospital-based and PCPs were least common (10.7%), whereas discharge summary completion within 48 hours was most common (84.9%). Of variables measuring direct communication with PCPs, only notification of admission occurred at least half the time (50.8%).
Exploratory factor analysis identified 5 well-correlated items (Cronbach α = 0.77), which were combined and labeled the Hospital and Primary Care Provider Communication scale (Figure 1b). Items included PCP notification of admission, discharge, and receipt of updates during hospitalization, as well as receipt of verbal and written handoffs prior to follow-up. While these 5 items were analyzed only in this scale, other practices were analyzed as independent variables. In this assessment, 42.1% of patients had a scale score of 0 (no items performed), while 5% had all 5 items completed
Readmissions
The 30-day unplanned readmission rate to any hospital was 12.4%. Demographic characteristics were similar in patients with and without an unplanned readmission (Table 1); however, patients with a readmission were more often younger (P = 0.03) and used a subspecialist for primary care (P = 0.03). Fewer than 60% of those with an unplanned readmission had a usual source of sick and well care compared with 77.5% of those without a readmission (P < 0.001). The length of index stay was nearly 4 days longer for those with an unplanned readmission (9.3 days vs. 4.4 days, P < 0.001). These patients also had more hospitalizations or ED visits in the past year (P = 0.002 and P = 0.04, respectively) and saw more subspecialists (P < 0.001).
Frequencies of communication practices between those with and without an unplanned readmission are illustrated in Table 2. Nearly three-quarters of caregivers whose children were readmitted reported having follow-up appointments scheduled before discharge, compared to 48.9% without a readmission (P < 0.001). In 71% of discharges followed by a readmission, caregivers were not very confident about avoiding readmission, vs. 44.8% of discharges with no readmission (P < 0.001).
Readmissions were largely unrelated to handoff practices in multivariate analyses (Table 3). Having a follow-up visit scheduled prior to discharge was the only activity with a statistically significant association; however, it was actually associated with more than double the odds of readmission (adjusted odds ratio 2.20, 95% confidence interval 1.08-4.46).
DISCUSSION
The complex nature of hospital discharge care has led to general optimism that improved handoff processes might reduce readmissions for pediatric patients. Although the current literature linking transition practices to readmissions in pediatrics has mixed results,1,4,5 most studies are fragmented—investigating a single or small number of transitional care activities, such as outpatient follow-up visits, postdischarge caregiver phone calls, or PCP receipt of discharge summaries. Despite finding limited relationships with readmissions, a strength of our study was its inclusion of a more comprehensive set of traditional communication practices that the study team anticipates many primary care and hospital medicine providers would expect to be carried out for most, if not all, patients during the hospital-to-home transition.
Although our study was developed earlier, the variables in our analyses align with each domain of the conceptual model for readmission risk proposed by the Seamless Transitions and Re(admissions) Network (STARNet).6 This model identifies 7 elements believed to directly impact readmission risk in children: hospital and ED utilization, underlying diseases, ability to care for diseases, access to outpatient care, discharge processes, and discharge readiness. For example, our study included ED and hospital visits in the past year, complex chronic conditions, number of subspecialists, caregiver confidence, having a usual source of care, insurance status, and the 11 consensus-based handoff practices identified by our study team. Therefore, although the included handoff practices we included were a limited set, our models provide a relatively comprehensive analysis of readmission risk, confirming caregiver confidence, usual source of care, and hospitalizations to be associated with unplanned readmissions.
With the exception of having scheduled follow-up appointments before discharge – which was associated with more rather than fewer readmissions—the included care practices were not associated with readmissions. We suspect that these findings likely represent selection bias, with hospital providers taking additional steps in communicating with outpatient providers when they are most concerned about a patient’s vulnerability at discharge, eg, due to severity of illness, sociodemographics, health literacy, access to care, or other factors. Such selection bias could have 2 potential effects: (1) creating associations between the performance of certain handoff practices and higher readmission risk (eg, hospital providers are more likely to set follow-up appointments with the sickest patients who are also most likely to be readmitted, or (2) negating weakly effective communication practices that have small effect sizes. The currently mixed literature suggests that if associations between these handoff practices and postdischarge outcomes exist, they are often opposite to our expectation and likely driven by selection bias. If there are real effects that are hidden by this selection bias, they may be weak or inconsistent.
Recent qualitative research highlights the needs and preferences of caregivers of children with chronic or complex conditions to promote their sense of self-efficacy at discharge.29 Such needs include support from within and beyond the health system, comprehensive discharge education, and written instructions, ultimately leading to confidence and comfort in executing the home-management plan. Consistent with our work,17 a strong independent relationship between caregiver confidence and postdischarge outcomes remained even after accounting for these conventional handoff activities.
Transitions research in pediatrics has started only recently to move beyond traditional handoff communication between hospital and outpatient providers. Over the last several years, more ambitious conceptualizations of hospital discharge care have evolved2 and include constructs such as family-centeredness,4,28,29 discharge readiness,30 and social determinants of health.31 Interventions targeting these constructs are largely missing from the literature and are greatly needed. If transitions are to have an effect on downstream utilization, their focus likely needs to evolve to address such areas.
Finally, our study underscores the need to identify relevant outcomes of improved transitional care. Although the preventability of postdischarge utilization continues to be debated, most would agree that this should not detract from the importance of high-quality transitional care. The STARNet collaborative provides some examples of outcomes potentially impacted through improved transitional care,6 although the authors note that reliability, validity, and feasibility of the measures are not well understood. High-quality transitional care presumably would lead to improvements in patient and family experience and perhaps safer care. Although caregiver experience measured by an adapted CTM-3 was neither a mediator nor a predictor of postdischarge utilization for children in our study, use of more rigorously developed tools for pediatric patients32 may provide a better assessment of caregiver experience. Finally, given the well-described risks of poor communication between hospital and outpatient providers,33-35 safety events may be a better outcome of high-quality transitional care than readmissions. Investment in transitional care initiatives would be well justified if the positive patient, provider, and health system impacts can be better demonstrated through improved outcomes.
Future readmissions research should aim to accomplish several goals. Because observational studies will continue to be challenged by the selection biases described above, more rigorously designed and controlled experimental pediatric studies are needed. Family, social, and primary care characteristics should continue to be incorporated into pediatric readmission analyses given their increasingly recognized critical role. These variables, some of which could be modifiable, might represent potential targets for innovative readmission reduction interventions. Recently published conceptual models6,29,36 provide a useful starting framework.
Limitations
Because of the observational study design, we cannot draw conclusions about causal relationships between handoff practices and the measured outcomes. The tertiary care single-center nature of the study limits generalizability. Response biases are possible given that we often could not verify accuracy of PCP and caregiver responses. As noted above, we suspect that handoff practices were driven by important selection bias, not all of which could be controlled by the measured patient and clinical characteristics. The handoff practices included in this study were a limited set primarily focused on communication between hospital providers and PCPs. Therefore, the study does not rule out the possibility that other aspects of transitional care may reduce readmissions. Subsequent work investigating innovative interventions may find reductions in readmissions and other important outcomes. Additionally, not all practices have standardized definitions, eg, what 1 PCP considers a verbal handoff may be different from that of another provider. Although we assessed whether communication occurred, we were not able to assess the content or quality of communication, which may have important implications for its effectiveness.37,38
CONCLUSION
Improvements in handoffs between hospital and PCPs may have an important impact on postdischarge outcomes, but it is not clear that unplanned 30-day readmissions is among them. Efforts to reduce postdischarge utilization, if possible, likely need to focus on broader constructs such as caregiver self-efficacy, discharge readiness, and social determinants of health.
Disclosures
This study was supported by a grant from the Lucile Packard Foundation for Children’s Health, Palo Alto, California, as well as grant R40MC25677 Maternal and Child Health Research Program, Maternal and Child Health Bureau (Title V, Social Security Act), Health Resources and Services Administration, Department of Health and Human Services. The authors report no financial conflicts of interest.
1. Auger KA, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9:251-260. PubMed
2. Berry JG, Blaine K, Rogers J, et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168:955-962; quiz 965-956. PubMed
3. Snow V, Beck D, Budnitz T, et al, American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, Society of Academic Emergency Medicine. Transitions of Care Consensus Policy Statement. American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24:971-976. PubMed
4. Desai AD, Popalisky J, Simon TD, Mangione-Smith RM. The effectiveness of family-centered transition processes from hospital settings to home: a review of the literature. Hosp Pediatr. 2015;5:219-231. PubMed
5. Berry JG, Gay JC. Preventing readmissions in children: how do we do that? Hosp Pediatr. 2015;5:602-604. PubMed
6. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: Seamless Transitions and (Re)admissions Network. Pediatrics. 2015;135:164-175. PubMed
7. Value in inpatient pediatrics network projects. American Academy of Pediatrics. Available at: https://www.aap.org/en-us/professional-resources/quality-improvement/Quality-Improvement-Innovation-Networks/Value-in-Inpatient-Pediatrics-Network/Pages/Value-in-Inpatient-Pediatrics-Network.aspx. Accessed May 18, 2015.
8. Ohio Children’s Hospitals. Solutions for patient safety. Available at: http://www.solutionsforpatientsafety.org/about-us/our-goals/. Accessed May 18, 2015.
9. Bell CM, Schnipper JL, Auerbach AD, et al. Association of communication between hospital-based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24:381-386. PubMed
10. Oduyebo I, Lehmann CU, Pollack CE, et al. Association of self-reported hospital discharge handoffs with 30-day readmissions. JAMA Intern Med. 2013;173:624-629. PubMed
11. van Walraven C, Seth R, Austin PC, Laupacis A. Effect of discharge summary availability during post-discharge visits on hospital readmission. J Gen Intern Med. 2002;17:186-192. PubMed
12. Kashiwagi DT, Burton MC, Kirkland LL, Cha S, Varkey P. Do timely outpatient follow-up visits decrease hospital readmission rates? Am J Med Qual. 2012;27:11-15. PubMed
13. Coller RJ, Klitzner TS, Lerner CF, Chung PJ. Predictors of 30-day readmission and association with primary care follow-up plans. J Pediatr. 2013;163:1027-1033. PubMed
14. Feudtner C, Pati S, Goodman DM, et al. State-level child health system performance and the likelihood of readmission to children’s hospitals. J Pediatr. 2010;157:98-102. PubMed
15. Brittan MS, Sills MR, Fox D, et al. Outpatient follow-up visits and readmission in medically complex children enrolled in Medicaid. J Pediatr. 2015;166:998-1005. PubMed
16. Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: Examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5:392-397. PubMed
17. Coller RJ, Klitzner TS, Saenz AA, Lerner CF, Nelson BB, Chung PJ. The medical home and hospital readmissions. Pediatrics. 2015;136:e1550-e1560. PubMed
18. Coleman EA, Berenson RA. Lost in transition: challenges and opportunities for improving the quality of transitional care. Ann Intern Med. 2004;141:533-536. PubMed
19. Coleman EA, Boult C; American Geriatrics Society Health Care Systems Committee. Improving the quality of transitional care for persons with complex care needs. J Am Geriatr Soc. 2003;51:556-557. PubMed
20. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305:682-690. PubMed
21. Feudtner C, Levin JE, Srivastava R, et al. How well can hospital readmission be predicted in a cohort of hospitalized children? A retrospective, multicenter study. Pediatrics. 2009;123:286-293. PubMed
22. Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980-1997. Pediatrics. 2000;106:205-209. PubMed
23. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. PubMed
24. Berry JG, Agrawal R, Kuo DZ, et al. Characteristics of hospitalizations for patients who use a structured clinical care program for children with medical complexity. J Pediatr. 2011;159:284-290. PubMed
25. Kuo DZ, Cohen E, Agrawal R, Berry JG, Casey PH. A national profile of caregiver challenges among more medically complex children with special health care needs. Arch Pediatr Adolesc Med. 2011;165:1020-1026. PubMed
26. Parry C, Mahoney E, Chalmers SA, Coleman EA. Assessing the quality of transitional care: further applications of the care transitions measure. Med Care. 2008;46:317-322. PubMed
27. Coleman EA, Mahoney E, Parry C. Assessing the quality of preparation for posthospital care from the patient’s perspective: the care transitions measure. Med Care. 2005;43:246-255. PubMed
28. Berry JG, Ziniel SI, Freeman L, et al. Hospital readmission and parent perceptions of their child’s hospital discharge. Int J Qual Health Care. 2013;25:573-581. PubMed
29. Desai AD, Durkin LK, Jacob-Files EA, Mangione-Smith R. Caregiver perceptions of hospital to home transitions according to medical complexity: a qualitative study. Acad Pediatr. 2016;16:136-144. PubMed
30. Weiss ME, Bobay KL, Bahr SJ, Costa L, Hughes RG, Holland DE. A model for hospital discharge preparation: from case management to care transition. J Nurs Adm. 2015;45:606-614. PubMed
31. Sills MR, Hall M, Colvin JD, et al. Association of social determinants with children’s hospitals’ preventable readmissions performance. JAMA Pediatr. 2016;170:350-358. PubMed
32. Toomey SL, Zaslavsky AM, Elliott MN, et al. The development of a pediatric inpatient experience of care measure: child HCAHPS. Pediatrics. 2015;136:360-369. PubMed
33. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297:831-841. PubMed
34. Harlan G, Srivastava R, Harrison L, McBride G, Maloney C. Pediatric hospitalists and primary care providers: a communication needs assessment. J Hosp Med. 2009;4:187-193. PubMed
35. Forster AJ, Clark HD, Menard A, et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170:345-349. PubMed
36. Nakamura MM, Toomey SL, Zaslavsky AM, et al. Measuring pediatric hospital readmission rates to drive quality improvement. Acad Pediatr. 2014;14:S39-S46. PubMed
37. Smith K. Effective communication with primary care providers. Pediatr Clin North Am. 2014;61671-679. PubMed
38. Leyenaar JK, Bergert L, Mallory LA, et al. Pediatric primary care providers’ perspectives regarding hospital discharge communication: a mixed methods analysis. Acad Pediatr. 2015;15:61-68. PubMed
Although much has been written about pediatric discharge and readmissions1-5 over the past several years, surprisingly little is known about which care practices are most effective at preventing postdischarge utilization.5 Major collaborations across the U.S. are currently focused on improving pediatric discharge processes,6-8 although the impact that these efforts will have on readmissions remains to be seen.
Research on handoffs between hospitals and primary care has mixed associations with postdischarge utilization. Although some studies observe positive relationships between specific activities and reduced postdischarge utilization,1 others suggest no relationship9-12 or, paradoxically, more utilization.13,14 Brittan et al15 found that outpatient visits were associated with more readmissions when occurring less than 4 days after discharge, but fewer readmissions when occurring 4 days to 29 days after discharge. Most studies, however, investigate single or limited sets of care activities, such as having an outpatient visit,15 timeliness of that visit,16 or receipt of a discharge summary.11 Inclusion of a more comprehensive set of hospital- to primary-care communication practices may better unravel this complex relationship between discharge care and postdischarge outcomes for children.
The purpose of this study was to characterize a set of traditional discharge handoff practices between hospital and primary care providers (PCPs) and to explore their relationships to readmissions. We hypothesized that handoff practices would be associated with fewer unplanned readmissions.
METHODS
Study Design, Setting, Participants
This project was part of a prospective cohort study with 2 aims: to investigate relationships between medical home experience and postdischarge utilization,17 and to identify relationships between common discharge communication practices and postdischarge utilization. This manuscript is focused on the second aim. Randomly selected pediatric patients and their caregivers were enrolled from any medical or surgical service during an acute hospitalization lasting more than 24 hours from October 1, 2012 to January 1, 2014, at a 100-bed tertiary children’s hospital. Patients who transferred to another facility, died, were older than 18 years or in neonatal care (ie, newborn nursery or neonatal intensive care unit) were excluded since their discharge experiences would be significantly distinct from the population of interest. Patients were enrolled once in the study.
Outcome
The study’s primary outcome was 30-day unplanned readmissions, defined as a hospitalization occurring within 30 days of the index (ie, study enrollment) hospitalization, identified through caregiver report or administrative sources.17 Although the study site is a single hospital system, readmissions could have occurred to any hospital reported by caregivers, (ie, readmissions could have occurred within or outside our health system). Readmissions for chemotherapy, radiation, dialysis, rehabilitation, or labor and delivery were excluded. If caregivers reported an admission as planned or chart review of the index discharge summary noted that a rehospitalization was scheduled in the subsequent 30 days, the readmission was labeled “planned” and excluded.
Discharge Handoff Communication
Transitional care is a set of actions designed to ensure continuity and coordination of healthcare during transfer from 1 location or level of care to another.18,19 The study team, comprised of a division chief of general pediatrics, a division chief of hospital medicine, 2 departmental vice-chairs, and the medical director for quality at the study site, identified 11 common handoff activities and reporting sources. These consensus-based activities were expected by the study team to improve continuity and coordination during hospital-to-home transfer, and included:
- verifying PCP identity during the hospitalization (caregiver report);
- notifying the PCP of admission, discharge, and providing updates during the hospitalization (PCP report);
- PCP follow-up appointment set prior to discharge (caregiver report);
- documenting planned PCP and subspecialty follow-up in the discharge summary (chart review);
- completing the discharge summary within 48 hours (chart review);
- providing a verbal or written handoff to the PCP prior to follow-up (PCP report); and
- having a PCP follow-up visit within 30 days of discharge (caregiver report).
We also asked PCPs whether they thought the follow-up interval was appropriate and whether phone follow-up with the patient would have been as appropriate as a face-to-face visit.
Covariates
Patient demographics that might confound the relationship between handoff practices and readmissions based on pediatric research20,21 were included. Medical complexity was accounted for by length-of-index stay, the number of hospitalizations and emergency department (ED) visits in past 12 months, complex chronic conditions,22,23 and seeing 3 or more subspecialists.24,25 Variables from related work included PCP scope (general pediatrics or subspecialist) and presence of a usual source for well and sick care.17
The Care Transitions Measure-3 (CTM-3), originally developed to assess the patient-centeredness of hospital transition,26,27 can discriminate adult patients at risk for readmission.26 We adapted the original CTM-3 to be answered by caregiver respondents after pilot testing with 5 caregivers not enrolled in the study: 1) “The hospital staff took my preferences and those of my family into account in deciding what my child’s health care needs would be when I left the hospital;” 2) “When I left the hospital, I had a good understanding of the things I was responsible for in managing my child’s health;” and 3) “When I left the hospital, I clearly understood the purpose for giving each of my child’s medications.” We analyzed the adapted CTM-3 on a transformed 0-100 scale as designed,26 initially hypothesizing that the CTM-3 would mediate the relationship between handoff practices and readmissions.
We assessed caregiver confidence to avoid a readmission, based on a strong independent association with readmissions described in Coller et al.17 Using questions developed for this study, caregivers were asked to rate “How confident are you that [child’s name] will stay out of the hospital for the next 30 days?” with instructions to refer to unplanned hospital visits only. Responses were reported on a 4-point Likert scale (1 = very confident, 4 = not very confident). Responses were dichotomized into very confident (ie, “1”) or not very confident (ie, “2-4”).
Enrollment and Data Collection
Computer-generated random numbers were assigned to patients admitted the previous day, and families were enrolled sequentially until the daily enrollment target was reached. Data were obtained from 3 sources: medical record, caregiver report, and PCP report. Trained research assistants systematically extracted chart review data documenting the transitions practices above, while a hospital information technology analyst extracted claims and demographic data to complement what was reported by parents and PCPs. After study conclusion, these medical record data were merged with caregiver and PCP-reported data.
Trained bilingual research assistants collected caregiver- and PCP-reported data using structured questionnaires in English or Spanish, according to preference. Timing of data collection differed by data source; caregiver-reported data were collected immediately after discharge and at 30 days postdischarge; PCP-reported data were collected at 30 days postdischarge.
Caregiver-reported data were collected through 2 separate phone calls following index discharge: immediately after discharge (caregiver confidence and CTM-3 measures) and at 30 days (readmission measures). Caregiver confidence questions were asked after (rather than immediately before) discharge to avoid biasing clinical care and revisit risk, consistent with previous work.28
PCP-reported data were collected using structured questionnaires with the PCP who was identified by the family during study enrollment. PCP-reported data were collected by telephone or fax 30 days after discharge, with up to 5 telephone attempts and 3 fax attempts. At the beginning of the questionnaire, PCPs were asked if they agreed with the designation, although they were asked to complete the questionnaire regardless.
Analyses
Descriptive statistics compared differences in handoff practices and 30-day unplanned readmissions. Exploratory factor analysis assessed whether certain handoff practices were sufficiently correlated to allow grouping of items and construction of scales. Relationships between handoff practices and readmissions were examined using bivariate, followed by multivariate, logistic regression adjusting for the covariates described. Collinearity was tested before constructing final models. Because no relationship was observed between CTM-3 and readmissions, additional mediation analyses were not pursued. All analyses were completed using STATA (SE version 14.0, StataCorp LP, College Station, Texas). This study was approved by the Institutional Review Boards at UCLA (study site) and University of Wisconsin (lead author site).
RESULTS
This study enrolled 701 of 816 eligible participants (85.9%) between October 2012 and January 2014. More than 99% of administrative data and 97% of caregiver questionnaires were complete. Of 685 patients with a reported PCP, we obtained responses from 577 PCPs (84.2%). Patient characteristics and outcomes were not significantly different for patients with and without a responding PCP; however, patients of nonresponding PCPs were more often publicly insured (64.5% vs. 48.2% for responding PCPs, P = 0.004) or seen by a subspecialist as opposed to a generalist (28.1% vs. 13.8% for responding PCPs, P = 0.001).
The overall population characteristics are summarized in Table 1: 27.4% of the cohort was younger 2 years, 49.2% were Hispanic, and the majority (51.1%) had public insurance. The average length of the index hospitalization for the overall population was 4.8 days (standard deviation = 9.6), and 53.5% had at least 1 complex chronic condition. Eighty-four percent of the cohort reported using a generalist (vs. subspecialist) for primary care.
Discharge Handoff Communication
Practices varied widely (Figure 1a). Verbal handoffs between hospital-based and PCPs were least common (10.7%), whereas discharge summary completion within 48 hours was most common (84.9%). Of variables measuring direct communication with PCPs, only notification of admission occurred at least half the time (50.8%).
Exploratory factor analysis identified 5 well-correlated items (Cronbach α = 0.77), which were combined and labeled the Hospital and Primary Care Provider Communication scale (Figure 1b). Items included PCP notification of admission, discharge, and receipt of updates during hospitalization, as well as receipt of verbal and written handoffs prior to follow-up. While these 5 items were analyzed only in this scale, other practices were analyzed as independent variables. In this assessment, 42.1% of patients had a scale score of 0 (no items performed), while 5% had all 5 items completed
Readmissions
The 30-day unplanned readmission rate to any hospital was 12.4%. Demographic characteristics were similar in patients with and without an unplanned readmission (Table 1); however, patients with a readmission were more often younger (P = 0.03) and used a subspecialist for primary care (P = 0.03). Fewer than 60% of those with an unplanned readmission had a usual source of sick and well care compared with 77.5% of those without a readmission (P < 0.001). The length of index stay was nearly 4 days longer for those with an unplanned readmission (9.3 days vs. 4.4 days, P < 0.001). These patients also had more hospitalizations or ED visits in the past year (P = 0.002 and P = 0.04, respectively) and saw more subspecialists (P < 0.001).
Frequencies of communication practices between those with and without an unplanned readmission are illustrated in Table 2. Nearly three-quarters of caregivers whose children were readmitted reported having follow-up appointments scheduled before discharge, compared to 48.9% without a readmission (P < 0.001). In 71% of discharges followed by a readmission, caregivers were not very confident about avoiding readmission, vs. 44.8% of discharges with no readmission (P < 0.001).
Readmissions were largely unrelated to handoff practices in multivariate analyses (Table 3). Having a follow-up visit scheduled prior to discharge was the only activity with a statistically significant association; however, it was actually associated with more than double the odds of readmission (adjusted odds ratio 2.20, 95% confidence interval 1.08-4.46).
DISCUSSION
The complex nature of hospital discharge care has led to general optimism that improved handoff processes might reduce readmissions for pediatric patients. Although the current literature linking transition practices to readmissions in pediatrics has mixed results,1,4,5 most studies are fragmented—investigating a single or small number of transitional care activities, such as outpatient follow-up visits, postdischarge caregiver phone calls, or PCP receipt of discharge summaries. Despite finding limited relationships with readmissions, a strength of our study was its inclusion of a more comprehensive set of traditional communication practices that the study team anticipates many primary care and hospital medicine providers would expect to be carried out for most, if not all, patients during the hospital-to-home transition.
Although our study was developed earlier, the variables in our analyses align with each domain of the conceptual model for readmission risk proposed by the Seamless Transitions and Re(admissions) Network (STARNet).6 This model identifies 7 elements believed to directly impact readmission risk in children: hospital and ED utilization, underlying diseases, ability to care for diseases, access to outpatient care, discharge processes, and discharge readiness. For example, our study included ED and hospital visits in the past year, complex chronic conditions, number of subspecialists, caregiver confidence, having a usual source of care, insurance status, and the 11 consensus-based handoff practices identified by our study team. Therefore, although the included handoff practices we included were a limited set, our models provide a relatively comprehensive analysis of readmission risk, confirming caregiver confidence, usual source of care, and hospitalizations to be associated with unplanned readmissions.
With the exception of having scheduled follow-up appointments before discharge – which was associated with more rather than fewer readmissions—the included care practices were not associated with readmissions. We suspect that these findings likely represent selection bias, with hospital providers taking additional steps in communicating with outpatient providers when they are most concerned about a patient’s vulnerability at discharge, eg, due to severity of illness, sociodemographics, health literacy, access to care, or other factors. Such selection bias could have 2 potential effects: (1) creating associations between the performance of certain handoff practices and higher readmission risk (eg, hospital providers are more likely to set follow-up appointments with the sickest patients who are also most likely to be readmitted, or (2) negating weakly effective communication practices that have small effect sizes. The currently mixed literature suggests that if associations between these handoff practices and postdischarge outcomes exist, they are often opposite to our expectation and likely driven by selection bias. If there are real effects that are hidden by this selection bias, they may be weak or inconsistent.
Recent qualitative research highlights the needs and preferences of caregivers of children with chronic or complex conditions to promote their sense of self-efficacy at discharge.29 Such needs include support from within and beyond the health system, comprehensive discharge education, and written instructions, ultimately leading to confidence and comfort in executing the home-management plan. Consistent with our work,17 a strong independent relationship between caregiver confidence and postdischarge outcomes remained even after accounting for these conventional handoff activities.
Transitions research in pediatrics has started only recently to move beyond traditional handoff communication between hospital and outpatient providers. Over the last several years, more ambitious conceptualizations of hospital discharge care have evolved2 and include constructs such as family-centeredness,4,28,29 discharge readiness,30 and social determinants of health.31 Interventions targeting these constructs are largely missing from the literature and are greatly needed. If transitions are to have an effect on downstream utilization, their focus likely needs to evolve to address such areas.
Finally, our study underscores the need to identify relevant outcomes of improved transitional care. Although the preventability of postdischarge utilization continues to be debated, most would agree that this should not detract from the importance of high-quality transitional care. The STARNet collaborative provides some examples of outcomes potentially impacted through improved transitional care,6 although the authors note that reliability, validity, and feasibility of the measures are not well understood. High-quality transitional care presumably would lead to improvements in patient and family experience and perhaps safer care. Although caregiver experience measured by an adapted CTM-3 was neither a mediator nor a predictor of postdischarge utilization for children in our study, use of more rigorously developed tools for pediatric patients32 may provide a better assessment of caregiver experience. Finally, given the well-described risks of poor communication between hospital and outpatient providers,33-35 safety events may be a better outcome of high-quality transitional care than readmissions. Investment in transitional care initiatives would be well justified if the positive patient, provider, and health system impacts can be better demonstrated through improved outcomes.
Future readmissions research should aim to accomplish several goals. Because observational studies will continue to be challenged by the selection biases described above, more rigorously designed and controlled experimental pediatric studies are needed. Family, social, and primary care characteristics should continue to be incorporated into pediatric readmission analyses given their increasingly recognized critical role. These variables, some of which could be modifiable, might represent potential targets for innovative readmission reduction interventions. Recently published conceptual models6,29,36 provide a useful starting framework.
Limitations
Because of the observational study design, we cannot draw conclusions about causal relationships between handoff practices and the measured outcomes. The tertiary care single-center nature of the study limits generalizability. Response biases are possible given that we often could not verify accuracy of PCP and caregiver responses. As noted above, we suspect that handoff practices were driven by important selection bias, not all of which could be controlled by the measured patient and clinical characteristics. The handoff practices included in this study were a limited set primarily focused on communication between hospital providers and PCPs. Therefore, the study does not rule out the possibility that other aspects of transitional care may reduce readmissions. Subsequent work investigating innovative interventions may find reductions in readmissions and other important outcomes. Additionally, not all practices have standardized definitions, eg, what 1 PCP considers a verbal handoff may be different from that of another provider. Although we assessed whether communication occurred, we were not able to assess the content or quality of communication, which may have important implications for its effectiveness.37,38
CONCLUSION
Improvements in handoffs between hospital and PCPs may have an important impact on postdischarge outcomes, but it is not clear that unplanned 30-day readmissions is among them. Efforts to reduce postdischarge utilization, if possible, likely need to focus on broader constructs such as caregiver self-efficacy, discharge readiness, and social determinants of health.
Disclosures
This study was supported by a grant from the Lucile Packard Foundation for Children’s Health, Palo Alto, California, as well as grant R40MC25677 Maternal and Child Health Research Program, Maternal and Child Health Bureau (Title V, Social Security Act), Health Resources and Services Administration, Department of Health and Human Services. The authors report no financial conflicts of interest.
Although much has been written about pediatric discharge and readmissions1-5 over the past several years, surprisingly little is known about which care practices are most effective at preventing postdischarge utilization.5 Major collaborations across the U.S. are currently focused on improving pediatric discharge processes,6-8 although the impact that these efforts will have on readmissions remains to be seen.
Research on handoffs between hospitals and primary care has mixed associations with postdischarge utilization. Although some studies observe positive relationships between specific activities and reduced postdischarge utilization,1 others suggest no relationship9-12 or, paradoxically, more utilization.13,14 Brittan et al15 found that outpatient visits were associated with more readmissions when occurring less than 4 days after discharge, but fewer readmissions when occurring 4 days to 29 days after discharge. Most studies, however, investigate single or limited sets of care activities, such as having an outpatient visit,15 timeliness of that visit,16 or receipt of a discharge summary.11 Inclusion of a more comprehensive set of hospital- to primary-care communication practices may better unravel this complex relationship between discharge care and postdischarge outcomes for children.
The purpose of this study was to characterize a set of traditional discharge handoff practices between hospital and primary care providers (PCPs) and to explore their relationships to readmissions. We hypothesized that handoff practices would be associated with fewer unplanned readmissions.
METHODS
Study Design, Setting, Participants
This project was part of a prospective cohort study with 2 aims: to investigate relationships between medical home experience and postdischarge utilization,17 and to identify relationships between common discharge communication practices and postdischarge utilization. This manuscript is focused on the second aim. Randomly selected pediatric patients and their caregivers were enrolled from any medical or surgical service during an acute hospitalization lasting more than 24 hours from October 1, 2012 to January 1, 2014, at a 100-bed tertiary children’s hospital. Patients who transferred to another facility, died, were older than 18 years or in neonatal care (ie, newborn nursery or neonatal intensive care unit) were excluded since their discharge experiences would be significantly distinct from the population of interest. Patients were enrolled once in the study.
Outcome
The study’s primary outcome was 30-day unplanned readmissions, defined as a hospitalization occurring within 30 days of the index (ie, study enrollment) hospitalization, identified through caregiver report or administrative sources.17 Although the study site is a single hospital system, readmissions could have occurred to any hospital reported by caregivers, (ie, readmissions could have occurred within or outside our health system). Readmissions for chemotherapy, radiation, dialysis, rehabilitation, or labor and delivery were excluded. If caregivers reported an admission as planned or chart review of the index discharge summary noted that a rehospitalization was scheduled in the subsequent 30 days, the readmission was labeled “planned” and excluded.
Discharge Handoff Communication
Transitional care is a set of actions designed to ensure continuity and coordination of healthcare during transfer from 1 location or level of care to another.18,19 The study team, comprised of a division chief of general pediatrics, a division chief of hospital medicine, 2 departmental vice-chairs, and the medical director for quality at the study site, identified 11 common handoff activities and reporting sources. These consensus-based activities were expected by the study team to improve continuity and coordination during hospital-to-home transfer, and included:
- verifying PCP identity during the hospitalization (caregiver report);
- notifying the PCP of admission, discharge, and providing updates during the hospitalization (PCP report);
- PCP follow-up appointment set prior to discharge (caregiver report);
- documenting planned PCP and subspecialty follow-up in the discharge summary (chart review);
- completing the discharge summary within 48 hours (chart review);
- providing a verbal or written handoff to the PCP prior to follow-up (PCP report); and
- having a PCP follow-up visit within 30 days of discharge (caregiver report).
We also asked PCPs whether they thought the follow-up interval was appropriate and whether phone follow-up with the patient would have been as appropriate as a face-to-face visit.
Covariates
Patient demographics that might confound the relationship between handoff practices and readmissions based on pediatric research20,21 were included. Medical complexity was accounted for by length-of-index stay, the number of hospitalizations and emergency department (ED) visits in past 12 months, complex chronic conditions,22,23 and seeing 3 or more subspecialists.24,25 Variables from related work included PCP scope (general pediatrics or subspecialist) and presence of a usual source for well and sick care.17
The Care Transitions Measure-3 (CTM-3), originally developed to assess the patient-centeredness of hospital transition,26,27 can discriminate adult patients at risk for readmission.26 We adapted the original CTM-3 to be answered by caregiver respondents after pilot testing with 5 caregivers not enrolled in the study: 1) “The hospital staff took my preferences and those of my family into account in deciding what my child’s health care needs would be when I left the hospital;” 2) “When I left the hospital, I had a good understanding of the things I was responsible for in managing my child’s health;” and 3) “When I left the hospital, I clearly understood the purpose for giving each of my child’s medications.” We analyzed the adapted CTM-3 on a transformed 0-100 scale as designed,26 initially hypothesizing that the CTM-3 would mediate the relationship between handoff practices and readmissions.
We assessed caregiver confidence to avoid a readmission, based on a strong independent association with readmissions described in Coller et al.17 Using questions developed for this study, caregivers were asked to rate “How confident are you that [child’s name] will stay out of the hospital for the next 30 days?” with instructions to refer to unplanned hospital visits only. Responses were reported on a 4-point Likert scale (1 = very confident, 4 = not very confident). Responses were dichotomized into very confident (ie, “1”) or not very confident (ie, “2-4”).
Enrollment and Data Collection
Computer-generated random numbers were assigned to patients admitted the previous day, and families were enrolled sequentially until the daily enrollment target was reached. Data were obtained from 3 sources: medical record, caregiver report, and PCP report. Trained research assistants systematically extracted chart review data documenting the transitions practices above, while a hospital information technology analyst extracted claims and demographic data to complement what was reported by parents and PCPs. After study conclusion, these medical record data were merged with caregiver and PCP-reported data.
Trained bilingual research assistants collected caregiver- and PCP-reported data using structured questionnaires in English or Spanish, according to preference. Timing of data collection differed by data source; caregiver-reported data were collected immediately after discharge and at 30 days postdischarge; PCP-reported data were collected at 30 days postdischarge.
Caregiver-reported data were collected through 2 separate phone calls following index discharge: immediately after discharge (caregiver confidence and CTM-3 measures) and at 30 days (readmission measures). Caregiver confidence questions were asked after (rather than immediately before) discharge to avoid biasing clinical care and revisit risk, consistent with previous work.28
PCP-reported data were collected using structured questionnaires with the PCP who was identified by the family during study enrollment. PCP-reported data were collected by telephone or fax 30 days after discharge, with up to 5 telephone attempts and 3 fax attempts. At the beginning of the questionnaire, PCPs were asked if they agreed with the designation, although they were asked to complete the questionnaire regardless.
Analyses
Descriptive statistics compared differences in handoff practices and 30-day unplanned readmissions. Exploratory factor analysis assessed whether certain handoff practices were sufficiently correlated to allow grouping of items and construction of scales. Relationships between handoff practices and readmissions were examined using bivariate, followed by multivariate, logistic regression adjusting for the covariates described. Collinearity was tested before constructing final models. Because no relationship was observed between CTM-3 and readmissions, additional mediation analyses were not pursued. All analyses were completed using STATA (SE version 14.0, StataCorp LP, College Station, Texas). This study was approved by the Institutional Review Boards at UCLA (study site) and University of Wisconsin (lead author site).
RESULTS
This study enrolled 701 of 816 eligible participants (85.9%) between October 2012 and January 2014. More than 99% of administrative data and 97% of caregiver questionnaires were complete. Of 685 patients with a reported PCP, we obtained responses from 577 PCPs (84.2%). Patient characteristics and outcomes were not significantly different for patients with and without a responding PCP; however, patients of nonresponding PCPs were more often publicly insured (64.5% vs. 48.2% for responding PCPs, P = 0.004) or seen by a subspecialist as opposed to a generalist (28.1% vs. 13.8% for responding PCPs, P = 0.001).
The overall population characteristics are summarized in Table 1: 27.4% of the cohort was younger 2 years, 49.2% were Hispanic, and the majority (51.1%) had public insurance. The average length of the index hospitalization for the overall population was 4.8 days (standard deviation = 9.6), and 53.5% had at least 1 complex chronic condition. Eighty-four percent of the cohort reported using a generalist (vs. subspecialist) for primary care.
Discharge Handoff Communication
Practices varied widely (Figure 1a). Verbal handoffs between hospital-based and PCPs were least common (10.7%), whereas discharge summary completion within 48 hours was most common (84.9%). Of variables measuring direct communication with PCPs, only notification of admission occurred at least half the time (50.8%).
Exploratory factor analysis identified 5 well-correlated items (Cronbach α = 0.77), which were combined and labeled the Hospital and Primary Care Provider Communication scale (Figure 1b). Items included PCP notification of admission, discharge, and receipt of updates during hospitalization, as well as receipt of verbal and written handoffs prior to follow-up. While these 5 items were analyzed only in this scale, other practices were analyzed as independent variables. In this assessment, 42.1% of patients had a scale score of 0 (no items performed), while 5% had all 5 items completed
Readmissions
The 30-day unplanned readmission rate to any hospital was 12.4%. Demographic characteristics were similar in patients with and without an unplanned readmission (Table 1); however, patients with a readmission were more often younger (P = 0.03) and used a subspecialist for primary care (P = 0.03). Fewer than 60% of those with an unplanned readmission had a usual source of sick and well care compared with 77.5% of those without a readmission (P < 0.001). The length of index stay was nearly 4 days longer for those with an unplanned readmission (9.3 days vs. 4.4 days, P < 0.001). These patients also had more hospitalizations or ED visits in the past year (P = 0.002 and P = 0.04, respectively) and saw more subspecialists (P < 0.001).
Frequencies of communication practices between those with and without an unplanned readmission are illustrated in Table 2. Nearly three-quarters of caregivers whose children were readmitted reported having follow-up appointments scheduled before discharge, compared to 48.9% without a readmission (P < 0.001). In 71% of discharges followed by a readmission, caregivers were not very confident about avoiding readmission, vs. 44.8% of discharges with no readmission (P < 0.001).
Readmissions were largely unrelated to handoff practices in multivariate analyses (Table 3). Having a follow-up visit scheduled prior to discharge was the only activity with a statistically significant association; however, it was actually associated with more than double the odds of readmission (adjusted odds ratio 2.20, 95% confidence interval 1.08-4.46).
DISCUSSION
The complex nature of hospital discharge care has led to general optimism that improved handoff processes might reduce readmissions for pediatric patients. Although the current literature linking transition practices to readmissions in pediatrics has mixed results,1,4,5 most studies are fragmented—investigating a single or small number of transitional care activities, such as outpatient follow-up visits, postdischarge caregiver phone calls, or PCP receipt of discharge summaries. Despite finding limited relationships with readmissions, a strength of our study was its inclusion of a more comprehensive set of traditional communication practices that the study team anticipates many primary care and hospital medicine providers would expect to be carried out for most, if not all, patients during the hospital-to-home transition.
Although our study was developed earlier, the variables in our analyses align with each domain of the conceptual model for readmission risk proposed by the Seamless Transitions and Re(admissions) Network (STARNet).6 This model identifies 7 elements believed to directly impact readmission risk in children: hospital and ED utilization, underlying diseases, ability to care for diseases, access to outpatient care, discharge processes, and discharge readiness. For example, our study included ED and hospital visits in the past year, complex chronic conditions, number of subspecialists, caregiver confidence, having a usual source of care, insurance status, and the 11 consensus-based handoff practices identified by our study team. Therefore, although the included handoff practices we included were a limited set, our models provide a relatively comprehensive analysis of readmission risk, confirming caregiver confidence, usual source of care, and hospitalizations to be associated with unplanned readmissions.
With the exception of having scheduled follow-up appointments before discharge – which was associated with more rather than fewer readmissions—the included care practices were not associated with readmissions. We suspect that these findings likely represent selection bias, with hospital providers taking additional steps in communicating with outpatient providers when they are most concerned about a patient’s vulnerability at discharge, eg, due to severity of illness, sociodemographics, health literacy, access to care, or other factors. Such selection bias could have 2 potential effects: (1) creating associations between the performance of certain handoff practices and higher readmission risk (eg, hospital providers are more likely to set follow-up appointments with the sickest patients who are also most likely to be readmitted, or (2) negating weakly effective communication practices that have small effect sizes. The currently mixed literature suggests that if associations between these handoff practices and postdischarge outcomes exist, they are often opposite to our expectation and likely driven by selection bias. If there are real effects that are hidden by this selection bias, they may be weak or inconsistent.
Recent qualitative research highlights the needs and preferences of caregivers of children with chronic or complex conditions to promote their sense of self-efficacy at discharge.29 Such needs include support from within and beyond the health system, comprehensive discharge education, and written instructions, ultimately leading to confidence and comfort in executing the home-management plan. Consistent with our work,17 a strong independent relationship between caregiver confidence and postdischarge outcomes remained even after accounting for these conventional handoff activities.
Transitions research in pediatrics has started only recently to move beyond traditional handoff communication between hospital and outpatient providers. Over the last several years, more ambitious conceptualizations of hospital discharge care have evolved2 and include constructs such as family-centeredness,4,28,29 discharge readiness,30 and social determinants of health.31 Interventions targeting these constructs are largely missing from the literature and are greatly needed. If transitions are to have an effect on downstream utilization, their focus likely needs to evolve to address such areas.
Finally, our study underscores the need to identify relevant outcomes of improved transitional care. Although the preventability of postdischarge utilization continues to be debated, most would agree that this should not detract from the importance of high-quality transitional care. The STARNet collaborative provides some examples of outcomes potentially impacted through improved transitional care,6 although the authors note that reliability, validity, and feasibility of the measures are not well understood. High-quality transitional care presumably would lead to improvements in patient and family experience and perhaps safer care. Although caregiver experience measured by an adapted CTM-3 was neither a mediator nor a predictor of postdischarge utilization for children in our study, use of more rigorously developed tools for pediatric patients32 may provide a better assessment of caregiver experience. Finally, given the well-described risks of poor communication between hospital and outpatient providers,33-35 safety events may be a better outcome of high-quality transitional care than readmissions. Investment in transitional care initiatives would be well justified if the positive patient, provider, and health system impacts can be better demonstrated through improved outcomes.
Future readmissions research should aim to accomplish several goals. Because observational studies will continue to be challenged by the selection biases described above, more rigorously designed and controlled experimental pediatric studies are needed. Family, social, and primary care characteristics should continue to be incorporated into pediatric readmission analyses given their increasingly recognized critical role. These variables, some of which could be modifiable, might represent potential targets for innovative readmission reduction interventions. Recently published conceptual models6,29,36 provide a useful starting framework.
Limitations
Because of the observational study design, we cannot draw conclusions about causal relationships between handoff practices and the measured outcomes. The tertiary care single-center nature of the study limits generalizability. Response biases are possible given that we often could not verify accuracy of PCP and caregiver responses. As noted above, we suspect that handoff practices were driven by important selection bias, not all of which could be controlled by the measured patient and clinical characteristics. The handoff practices included in this study were a limited set primarily focused on communication between hospital providers and PCPs. Therefore, the study does not rule out the possibility that other aspects of transitional care may reduce readmissions. Subsequent work investigating innovative interventions may find reductions in readmissions and other important outcomes. Additionally, not all practices have standardized definitions, eg, what 1 PCP considers a verbal handoff may be different from that of another provider. Although we assessed whether communication occurred, we were not able to assess the content or quality of communication, which may have important implications for its effectiveness.37,38
CONCLUSION
Improvements in handoffs between hospital and PCPs may have an important impact on postdischarge outcomes, but it is not clear that unplanned 30-day readmissions is among them. Efforts to reduce postdischarge utilization, if possible, likely need to focus on broader constructs such as caregiver self-efficacy, discharge readiness, and social determinants of health.
Disclosures
This study was supported by a grant from the Lucile Packard Foundation for Children’s Health, Palo Alto, California, as well as grant R40MC25677 Maternal and Child Health Research Program, Maternal and Child Health Bureau (Title V, Social Security Act), Health Resources and Services Administration, Department of Health and Human Services. The authors report no financial conflicts of interest.
1. Auger KA, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9:251-260. PubMed
2. Berry JG, Blaine K, Rogers J, et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168:955-962; quiz 965-956. PubMed
3. Snow V, Beck D, Budnitz T, et al, American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, Society of Academic Emergency Medicine. Transitions of Care Consensus Policy Statement. American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24:971-976. PubMed
4. Desai AD, Popalisky J, Simon TD, Mangione-Smith RM. The effectiveness of family-centered transition processes from hospital settings to home: a review of the literature. Hosp Pediatr. 2015;5:219-231. PubMed
5. Berry JG, Gay JC. Preventing readmissions in children: how do we do that? Hosp Pediatr. 2015;5:602-604. PubMed
6. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: Seamless Transitions and (Re)admissions Network. Pediatrics. 2015;135:164-175. PubMed
7. Value in inpatient pediatrics network projects. American Academy of Pediatrics. Available at: https://www.aap.org/en-us/professional-resources/quality-improvement/Quality-Improvement-Innovation-Networks/Value-in-Inpatient-Pediatrics-Network/Pages/Value-in-Inpatient-Pediatrics-Network.aspx. Accessed May 18, 2015.
8. Ohio Children’s Hospitals. Solutions for patient safety. Available at: http://www.solutionsforpatientsafety.org/about-us/our-goals/. Accessed May 18, 2015.
9. Bell CM, Schnipper JL, Auerbach AD, et al. Association of communication between hospital-based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24:381-386. PubMed
10. Oduyebo I, Lehmann CU, Pollack CE, et al. Association of self-reported hospital discharge handoffs with 30-day readmissions. JAMA Intern Med. 2013;173:624-629. PubMed
11. van Walraven C, Seth R, Austin PC, Laupacis A. Effect of discharge summary availability during post-discharge visits on hospital readmission. J Gen Intern Med. 2002;17:186-192. PubMed
12. Kashiwagi DT, Burton MC, Kirkland LL, Cha S, Varkey P. Do timely outpatient follow-up visits decrease hospital readmission rates? Am J Med Qual. 2012;27:11-15. PubMed
13. Coller RJ, Klitzner TS, Lerner CF, Chung PJ. Predictors of 30-day readmission and association with primary care follow-up plans. J Pediatr. 2013;163:1027-1033. PubMed
14. Feudtner C, Pati S, Goodman DM, et al. State-level child health system performance and the likelihood of readmission to children’s hospitals. J Pediatr. 2010;157:98-102. PubMed
15. Brittan MS, Sills MR, Fox D, et al. Outpatient follow-up visits and readmission in medically complex children enrolled in Medicaid. J Pediatr. 2015;166:998-1005. PubMed
16. Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: Examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5:392-397. PubMed
17. Coller RJ, Klitzner TS, Saenz AA, Lerner CF, Nelson BB, Chung PJ. The medical home and hospital readmissions. Pediatrics. 2015;136:e1550-e1560. PubMed
18. Coleman EA, Berenson RA. Lost in transition: challenges and opportunities for improving the quality of transitional care. Ann Intern Med. 2004;141:533-536. PubMed
19. Coleman EA, Boult C; American Geriatrics Society Health Care Systems Committee. Improving the quality of transitional care for persons with complex care needs. J Am Geriatr Soc. 2003;51:556-557. PubMed
20. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305:682-690. PubMed
21. Feudtner C, Levin JE, Srivastava R, et al. How well can hospital readmission be predicted in a cohort of hospitalized children? A retrospective, multicenter study. Pediatrics. 2009;123:286-293. PubMed
22. Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980-1997. Pediatrics. 2000;106:205-209. PubMed
23. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. PubMed
24. Berry JG, Agrawal R, Kuo DZ, et al. Characteristics of hospitalizations for patients who use a structured clinical care program for children with medical complexity. J Pediatr. 2011;159:284-290. PubMed
25. Kuo DZ, Cohen E, Agrawal R, Berry JG, Casey PH. A national profile of caregiver challenges among more medically complex children with special health care needs. Arch Pediatr Adolesc Med. 2011;165:1020-1026. PubMed
26. Parry C, Mahoney E, Chalmers SA, Coleman EA. Assessing the quality of transitional care: further applications of the care transitions measure. Med Care. 2008;46:317-322. PubMed
27. Coleman EA, Mahoney E, Parry C. Assessing the quality of preparation for posthospital care from the patient’s perspective: the care transitions measure. Med Care. 2005;43:246-255. PubMed
28. Berry JG, Ziniel SI, Freeman L, et al. Hospital readmission and parent perceptions of their child’s hospital discharge. Int J Qual Health Care. 2013;25:573-581. PubMed
29. Desai AD, Durkin LK, Jacob-Files EA, Mangione-Smith R. Caregiver perceptions of hospital to home transitions according to medical complexity: a qualitative study. Acad Pediatr. 2016;16:136-144. PubMed
30. Weiss ME, Bobay KL, Bahr SJ, Costa L, Hughes RG, Holland DE. A model for hospital discharge preparation: from case management to care transition. J Nurs Adm. 2015;45:606-614. PubMed
31. Sills MR, Hall M, Colvin JD, et al. Association of social determinants with children’s hospitals’ preventable readmissions performance. JAMA Pediatr. 2016;170:350-358. PubMed
32. Toomey SL, Zaslavsky AM, Elliott MN, et al. The development of a pediatric inpatient experience of care measure: child HCAHPS. Pediatrics. 2015;136:360-369. PubMed
33. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297:831-841. PubMed
34. Harlan G, Srivastava R, Harrison L, McBride G, Maloney C. Pediatric hospitalists and primary care providers: a communication needs assessment. J Hosp Med. 2009;4:187-193. PubMed
35. Forster AJ, Clark HD, Menard A, et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170:345-349. PubMed
36. Nakamura MM, Toomey SL, Zaslavsky AM, et al. Measuring pediatric hospital readmission rates to drive quality improvement. Acad Pediatr. 2014;14:S39-S46. PubMed
37. Smith K. Effective communication with primary care providers. Pediatr Clin North Am. 2014;61671-679. PubMed
38. Leyenaar JK, Bergert L, Mallory LA, et al. Pediatric primary care providers’ perspectives regarding hospital discharge communication: a mixed methods analysis. Acad Pediatr. 2015;15:61-68. PubMed
1. Auger KA, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9:251-260. PubMed
2. Berry JG, Blaine K, Rogers J, et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168:955-962; quiz 965-956. PubMed
3. Snow V, Beck D, Budnitz T, et al, American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, Society of Academic Emergency Medicine. Transitions of Care Consensus Policy Statement. American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24:971-976. PubMed
4. Desai AD, Popalisky J, Simon TD, Mangione-Smith RM. The effectiveness of family-centered transition processes from hospital settings to home: a review of the literature. Hosp Pediatr. 2015;5:219-231. PubMed
5. Berry JG, Gay JC. Preventing readmissions in children: how do we do that? Hosp Pediatr. 2015;5:602-604. PubMed
6. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: Seamless Transitions and (Re)admissions Network. Pediatrics. 2015;135:164-175. PubMed
7. Value in inpatient pediatrics network projects. American Academy of Pediatrics. Available at: https://www.aap.org/en-us/professional-resources/quality-improvement/Quality-Improvement-Innovation-Networks/Value-in-Inpatient-Pediatrics-Network/Pages/Value-in-Inpatient-Pediatrics-Network.aspx. Accessed May 18, 2015.
8. Ohio Children’s Hospitals. Solutions for patient safety. Available at: http://www.solutionsforpatientsafety.org/about-us/our-goals/. Accessed May 18, 2015.
9. Bell CM, Schnipper JL, Auerbach AD, et al. Association of communication between hospital-based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24:381-386. PubMed
10. Oduyebo I, Lehmann CU, Pollack CE, et al. Association of self-reported hospital discharge handoffs with 30-day readmissions. JAMA Intern Med. 2013;173:624-629. PubMed
11. van Walraven C, Seth R, Austin PC, Laupacis A. Effect of discharge summary availability during post-discharge visits on hospital readmission. J Gen Intern Med. 2002;17:186-192. PubMed
12. Kashiwagi DT, Burton MC, Kirkland LL, Cha S, Varkey P. Do timely outpatient follow-up visits decrease hospital readmission rates? Am J Med Qual. 2012;27:11-15. PubMed
13. Coller RJ, Klitzner TS, Lerner CF, Chung PJ. Predictors of 30-day readmission and association with primary care follow-up plans. J Pediatr. 2013;163:1027-1033. PubMed
14. Feudtner C, Pati S, Goodman DM, et al. State-level child health system performance and the likelihood of readmission to children’s hospitals. J Pediatr. 2010;157:98-102. PubMed
15. Brittan MS, Sills MR, Fox D, et al. Outpatient follow-up visits and readmission in medically complex children enrolled in Medicaid. J Pediatr. 2015;166:998-1005. PubMed
16. Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: Examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5:392-397. PubMed
17. Coller RJ, Klitzner TS, Saenz AA, Lerner CF, Nelson BB, Chung PJ. The medical home and hospital readmissions. Pediatrics. 2015;136:e1550-e1560. PubMed
18. Coleman EA, Berenson RA. Lost in transition: challenges and opportunities for improving the quality of transitional care. Ann Intern Med. 2004;141:533-536. PubMed
19. Coleman EA, Boult C; American Geriatrics Society Health Care Systems Committee. Improving the quality of transitional care for persons with complex care needs. J Am Geriatr Soc. 2003;51:556-557. PubMed
20. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305:682-690. PubMed
21. Feudtner C, Levin JE, Srivastava R, et al. How well can hospital readmission be predicted in a cohort of hospitalized children? A retrospective, multicenter study. Pediatrics. 2009;123:286-293. PubMed
22. Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980-1997. Pediatrics. 2000;106:205-209. PubMed
23. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. PubMed
24. Berry JG, Agrawal R, Kuo DZ, et al. Characteristics of hospitalizations for patients who use a structured clinical care program for children with medical complexity. J Pediatr. 2011;159:284-290. PubMed
25. Kuo DZ, Cohen E, Agrawal R, Berry JG, Casey PH. A national profile of caregiver challenges among more medically complex children with special health care needs. Arch Pediatr Adolesc Med. 2011;165:1020-1026. PubMed
26. Parry C, Mahoney E, Chalmers SA, Coleman EA. Assessing the quality of transitional care: further applications of the care transitions measure. Med Care. 2008;46:317-322. PubMed
27. Coleman EA, Mahoney E, Parry C. Assessing the quality of preparation for posthospital care from the patient’s perspective: the care transitions measure. Med Care. 2005;43:246-255. PubMed
28. Berry JG, Ziniel SI, Freeman L, et al. Hospital readmission and parent perceptions of their child’s hospital discharge. Int J Qual Health Care. 2013;25:573-581. PubMed
29. Desai AD, Durkin LK, Jacob-Files EA, Mangione-Smith R. Caregiver perceptions of hospital to home transitions according to medical complexity: a qualitative study. Acad Pediatr. 2016;16:136-144. PubMed
30. Weiss ME, Bobay KL, Bahr SJ, Costa L, Hughes RG, Holland DE. A model for hospital discharge preparation: from case management to care transition. J Nurs Adm. 2015;45:606-614. PubMed
31. Sills MR, Hall M, Colvin JD, et al. Association of social determinants with children’s hospitals’ preventable readmissions performance. JAMA Pediatr. 2016;170:350-358. PubMed
32. Toomey SL, Zaslavsky AM, Elliott MN, et al. The development of a pediatric inpatient experience of care measure: child HCAHPS. Pediatrics. 2015;136:360-369. PubMed
33. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297:831-841. PubMed
34. Harlan G, Srivastava R, Harrison L, McBride G, Maloney C. Pediatric hospitalists and primary care providers: a communication needs assessment. J Hosp Med. 2009;4:187-193. PubMed
35. Forster AJ, Clark HD, Menard A, et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170:345-349. PubMed
36. Nakamura MM, Toomey SL, Zaslavsky AM, et al. Measuring pediatric hospital readmission rates to drive quality improvement. Acad Pediatr. 2014;14:S39-S46. PubMed
37. Smith K. Effective communication with primary care providers. Pediatr Clin North Am. 2014;61671-679. PubMed
38. Leyenaar JK, Bergert L, Mallory LA, et al. Pediatric primary care providers’ perspectives regarding hospital discharge communication: a mixed methods analysis. Acad Pediatr. 2015;15:61-68. PubMed
© 2017 Society of Hospital Medicine
Routine Replacement of Peripheral Intravenous Catheters
The “Things We Do for No Reason” (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Hospitals and health systems worldwide have adopted policies for routine replacement of peripheral intravenous catheters (PIVCs) at prespecified time intervals (range, 48-96 hours). This practice accounts for a large number of PIVC reinsertions and places a significant cost burden on the healthcare infrastructure. The authors of this article examine the evidence that has been used to support this practice.
CASE PRESENTATION
A 67-year-old man with metastatic lung cancer presents to a hospital for pain control and “failure to thrive.” In the emergency department, a left antecubital peripheral intravenous catheter (PIVC) is placed. On admission, a prerenal acute kidney injury is noted. During the patient’s entire hospitalization, normal saline with parenteral hydromorphone is administered. On hospital day 4, the pain is still not adequately controlled, and the intravenous opioid is continued. On morning rounds, an intern notes that the PIVC is functioning well, and there are no signs of irritation. However, the nursing staff reminds the team that the PIVC should be changed because it has been in place for 4 days and is “due for replacement.” The patient does not want to receive another skin puncture for routine venous access. Does the PIVC need to be replaced, per routine?
WHY YOU MIGHT THINK ROUTINE PIVC REPLACEMENT IS HELPFUL
PIVC placement is easily the most common procedure performed in the United States. An estimated 200 million PIVCs are placed each year.1 Given the number of inpatient hospital stays per year in the United States alone—more than 37 million1,2—data regarding the care, maintenance, and complications of PIVCs are essential to the healthcare infrastructure.
The recommendation to routinely replace PIVCs dates to 1981, when the Centers for Disease Control and Prevention3 (CDC) issued a guideline that calls for replacing PIVCs every 24 to 48 hours. Most of the data and studies that established that recommendation originated in the 1970s, when catheters varied in length and material, and precise definitions of complications, such as phlebitis—localized vein inflammation characterized by pain, erythema, tenderness, swelling, and a palpable cord4,5—were not standardized across trials. Research at the time suggested higher rates of complications from IVCs dwelling longer than 48 to 72 hours. The latest (2011) CDC guidelines6,7 softened the recommendation but still concluded, “There is no need to replace peripheral catheters more frequently than every 72-96 hours.”
The 2011 recommendation6,7 is based on findings of a 1983 prospective observational study,8 a 1991 randomized controlled trial (RCT),9 and a 1998 prospective observational study.2 The 1983 and 1991 studies found higher rates of PIVC complications after day 2 of cannulation.8,9 The 1998 study found no increase in the rate of complications after day 3 of catheterization, and its authors, recommending a reevaluation of the need to routinely replace PIVCs, wrote, “[The] hazard for catheter-related complications, phlebitis, catheter-related infections, and mechanical complications did not increase during prolonged catheterization.”2
Results of RCTs conducted by Barker et al.10 (2004) and Nishanth et al.11 (2009) supported the claim that routine replacement of PIVCs leads to lower rates of thrombophlebitis. Nishanth et al. also included site pain and cannula dislodgement in their definition of phlebitis. Neither study compared blood stream infection rates, but both found higher rates of phlebitis between day 2.5 and day 3. However, Cochrane reviewers Webster et al.12 questioned the findings of these 2 trials, given their missing data and possibly biased results and conclusions. In the Barker study, patient numbers (screened, eligible, dropout) were unclear; each patient group was unbalanced; protocol deviations were not reported (possibly a result of incomplete data reporting or inappropriate randomization); and varied definitions of phlebitis were allowed, which may have resulted in more events being included. In the Nishanth study, the 100% phlebitis rate for the clinically indicated replacement group seemed extreme, which suggested confounding by an unknown bias or chance. Last, both samples were small: 47 patients (Barker) and 42 patients (Nishanth). Given all these concerns, the 2 trials were excluded from the Cochrane meta-analysis on the subject.12
In the 1980s and early 1990s, routine removal and exchange of PIVCs were supported by limited evidence. Current well-designed trial data cast doubt on the need for such a practice.
WHY YOU SHOULD NOT ROUTINELY REPLACE PIVCs
According to the CDC,6,7 the issue of routine PIVC replacement remains unresolved: “No recommendation is made regarding replacement of peripheral catheters in adults only when clinically indicated.”
Whereas earlier data showed a higher risk of complications with longer dwelling IVs, the majority of contemporary data has failed to support this conclusion. The recent (2015) Cochrane meta-analysis comparing routine with clinically indicated IVC replacement found “no evidence to support changing catheters every 72-96 hours.”12 Of the 7 studies that fulfilled the criteria for qualitative analysis, only 5 were included (the studies by Barker et al.10 and Nishanth et al.11 were excluded). The included studies assessed the endpoints of catheter-related blood stream infection (CRBSI), phlebitis, phlebitis per device-days, mortality, cost, and infiltration. Statistically significant differences were found only for cost (favoring clinically indicated replacement) and infiltration (occurring less with routine replacement).
The largest and most robust RCT in the meta-analysis12 was conducted by Rickard et al.13 (2012). Their nonblinded, intention-to-treat study of 3283 patients used concealed allocation to randomly assign patients to either clinically indicated or routine PIVC replacement in order to evaluate a primary endpoint, phlebitis. Secondary endpoints were CRBSI, venous port infection, IVC tip colonization, infusion failure, number of IVCs needed per patient, IV therapy duration, cost, and mortality. Need for PIVC replacement was methodically monitored (Table) with extensive nursing education and interrater validation. The study found no difference in the groups’ phlebitis rates; the rate was 7% for both routine and clinically indicated replacement (13.08% and 13.11%, respectively, adjusted for phlebitis per 1000 IVC days). In addition, there was no difference in the secondary outcome measures, except cost and number of catheters used, both of which favored clinically indicated replacement. The most serious complication, CRBSI, occurred at essentially the same rate in the 2 replacement arms: 0.11% (routine) and 0% (clinically indicated). Per-patient cost for the entire course of treatment was A$69.24 in the routine group and A$61.66 in the clinically indicated group; the difference was A$7.58 (P < 0.0001). Mean number of catheters used was 1.9 in the routine group and 1.7 in the clinically indicated group; the difference was 0.21 catheter per patient for the treatment course (P < 0.0001). Overall, the study found no important difference in significant outcomes between the 2 study arms.
The other 4 studies in the meta-analysis12 duplicated these results, with none finding a higher rate of major adverse events.14-17 All 4 showed virtually equivalent rates of phlebitis, the primary outcome; 3 also examined the secondary outcome measure of blood stream infection, and results were similar, with identical rates of complications. Only 1 trial identified any bloodstream infections (1 per group).15 The meta-analysis did find that routine catheter replacement resulted in less catheter infiltration.
Most of the data on PIVC exchange involves phlebitis and other local complications. A prospective study by Stuart et al.18 and commentary by Collignon et al.19 underscore the need for further research targeting blood stream infections (sepsis and severe sepsis in particular) as a primary outcome. Blood stream infections, especially those related to PIVC use, are rare entities overall, with most recent data yielding an estimated rate of 0.5 per 1000 catheter-days.20 Given this epidemiologic finding, researchers trying to acquire meaningful data on PIVC-related blood stream infections and subsequent complications would need to have tens of thousands of patients in routine and clinically indicated replacement arms to sufficiently power their studies.20 As they are infeasible, such trials cannot be found in the scientific literature.
Stuart et al.18 tried addressing the question. Prospectively examining more than 5 million occupied-bed days and the incidence of bloodstream infections by type of intravascular device over a 5-year period, they found that 137 (23.5%) of 583 healthcare-associated Staphylococcus aureus bacteremia (SAB) cases were attributed to PIVC use. PIVC insertions were performed equally (39.6%) in emergency departments and medical wards. About 45% of PIVCs remained in place 4 days or longer. Stuart et al. noted the “significant issue of PIVC-associated SAB” and favored routine removal of PIVCs within 96 hours (4 days). However, 55% of patients in their PIVC-related SAB group had the device in place less than 4 days. In addition, overall incidence of SAB was low: 0.3 per 10,000 occupied-bed days. Further, their study did not adjust device-specific SAB incidence for frequency of device use. For example, the rate of healthcare-acquired SAB was 19.7% for central venous catheters and 23.5% for PIVCs, despite PIVCs being used significantly more often than central lines. Device-specific adjustments would show a vastly different absolute risk of SAB in relation to individual devices. Nevertheless, the overall benefit of and need for routine PIVC replacement must be questioned. The percentage of PIVC-associated SAB in their study and the need for more research in this area should be noted. Given current information, their study and others in the literature underscore the need for selective use, appropriate maintenance, and timely removal of PIVCs.
Pure clinical outcomes are important, but procedural costs are as well. Clinically indicated replacement helps patients avoid an unpleasant procedure and saves money.21 If one third of the 37 million annual inpatient admissions require a PIVC for more than 3 days, then a strategy of “replacement when clinically indicated” could prevent almost 2.5 million unnecessary PIVC insertions each year. Equipment cost savings combined with savings of nearly 1 million staff hours could yield an estimated $400 million in savings over a 5-year period.22 Given current data suggesting no harm from clinically indicated PIVC replacement and clear evidence that routine replacement increases needle sticks and costs, it seems time to end the practice of routine PIVC replacement.
RECOMMENDATIONS
Compared with clinically indicated catheter replacement, routine replacement in the absence of a clinical indication (eg, infiltration, phlebitis, infection) provides no added benefit. Studies have consistently found that rates of phlebitis and SAB are not affected by scheduled replacement, though the largest RCT may not have been powered to show a difference in SAB. The present authors’ recommendations for PIVC care are:
- Scrutinize each patient’s need for PIVCs and remove each PIVC as soon as possible.
- Do not make routine replacement of otherwise well-functioning, well-appearing clinically necessary PIVCs the standard of care.
- Regularly examine PIVC sites for signs and symptoms of infection.
- Remove a PIVC immediately on recognition of any clinical sign of a complication (eg, infiltration, phlebitis, localized infection, blood stream infection) and replace the PIVC only if there is a clinical need.
- If replacing PIVCs on a clinical basis, establish protocols for frequency of evaluation for complications; these protocols might mirror those from prior studies (Table).10,22
- Replace as soon as possible any PIVC inserted during an urgent or emergent situation in which proper insertion technique could not be guaranteed.
- Conduct real-world observational studies to ensure that the switch to clinically driven replacement is safe and develop standardized definitions of complications.
Given the literature findings and the preceding recommendations, the authors conclude that the patient in the case example does not need routine PIVC replacement. His PIVC may remain in place as long as evaluation for local complications is routinely and methodically performed and the device is removed as soon as it is deemed unnecessary (transition to oral opioid therapy).
CONCLUSION
The long-standing practice of routinely replacing PIVCs every 72 to 96 hours during a hospital stay does not affect any meaningful clinical outcome. Specifically, data do not show that routine replacement prevents phlebitis or blood stream infections. Furthermore, routine PIVC replacement increases patient discomfort, uses resources unnecessarily, and raises hospital costs. Most of the PIVC research has involved phlebitis and other local complications; more research on PIVC use and bloodstream infections is needed. Given the findings in the current literature, routine PIVC replacement should be considered a Thing We Do For No Reason.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
1. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1-45. PubMed
2. Bregenzer T, Conen D, Sakmann P, Widmer AF. Is routine replacement of peripheral intravenous catheters necessary? Arch Intern Med. 1998;158(2):151-156. PubMed
3. Centers for Disease Control Working Group. Guidelines for prevention of intravenous therapy-related infections. Infect Control. 1981;3:62-79.
4. Hershey CO, Tomford JW, McLaren CE, Porter DK, Cohen DI. The natural history of intravenous catheter-associated phlebitis. Arch Intern Med. 1984;144(7):1373-1375. PubMed
5. Widmer AF. IV-related infections. In: Wenzel RP, ed. Prevention and Control of Nosocomial Infections. 3rd ed. Baltimore, MD: Williams & Wilkins; 1997:556-579.
6. O’Grady NP, Alexander M, Burns LA, et al; Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for the Prevention of Intravascular Catheter-Related Infections, 2011. Centers for Disease Control and Prevention website. http://www.cdc.gov/hicpac/pdf/guidelines/bsi-guidelines-2011.pdf. Published April 1, 2011. Accessed November 5, 2016. PubMed
7. O’Grady NP, Alexander M, Burns LA, et al; Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162-e193. PubMed
8. Rhode Island Nosocomial Infection Consortium; Tager IB, Ginsberg MB, Ellis SE, et al. An epidemiologic study of the risks associated with peripheral intravenous catheters. Am J Epidemiol. 1983;118(6):839-851. PubMed
9. Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. A randomized controlled trial. Ann Intern Med. 1991;114(10):845-854. PubMed
10. Barker P, Anderson AD, MacFie J. Randomised clinical trial of elective re-siting of intravenous cannulae. Ann R Coll Surg Engl. 2004;86(4):281-283. PubMed
11. Nishanth S, Sivaram G, Kalayarasan R, Kate V, Ananthakrishnan N. Does elective re-siting of intravenous cannulae decrease peripheral thrombophlebitis? A randomized controlled study. Int Med J India. 2009;22(2):60-62. PubMed
12. Webster J, Osborne S, Rickard CM, New K. Clinically-indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst Rev. 2015;(8):CD007798. PubMed
13. Rickard CM, Webster J, Wallis MC, et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet. 2012;380(9847):1066-1074. PubMed
14. Webster J, Lloyd S, Hopkins T, Osborne S, Yaxley M. Developing a Research base for Intravenous Peripheral cannula re-sites (DRIP trial). A randomised controlled trial of hospital in-patients. Int J Nurs Stud. 2007;44(5):664-671. PubMed
15. Webster J, Clarke S, Paterson D, et al. Routine care of peripheral intravenous catheters versus clinically indicated replacement: randomised controlled trial. BMJ. 2008;337:a339. PubMed
16. Van Donk P, Rickard CM, McGrail MR, Doolan G. Routine replacement versus clinical monitoring of peripheral intravenous catheters in a regional hospital in the home program: a randomized controlled trial. Infect Control Hosp Epidemiol. 2009;30(9):915-917. PubMed
17. Rickard CM, McCann D, Munnings J, McGrail MR. Routine resite of peripheral intravenous devices every 3 days did not reduce complications compared with clinically indicated resite: a randomised controlled trial. BMC Med. 2010;8:53. PubMed
18. Stuart RL, Cameron DR, Scott C, et al. Peripheral intravenous catheter-associated Staphylococcus aureus bacteraemia: more than 5 years of prospective data from two tertiary health services. Med J Aust. 2013;198(10):551-553. PubMed
19. Collignon PJ, Kimber FJ, Beckingham WD, Roberts JL. Prevention of peripheral intravenous catheter-related bloodstream infections: the need for routine replacement [letter]. Med J Aust. 2013;199(11):750-751. PubMed
20. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006:81(9):1159-1171. PubMed
21. Tuffaha HW, Rickard CM, Webster J, et al. Cost-effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Health Policy. 2014;12(1):51-58. PubMed
22. Rickard CM, Webster J, Playford EG. Prevention of peripheral intravenous catheter-related bloodstream infections: the need for a new focus. Med J Aust. 2013;198(10):519-520. PubMed
The “Things We Do for No Reason” (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Hospitals and health systems worldwide have adopted policies for routine replacement of peripheral intravenous catheters (PIVCs) at prespecified time intervals (range, 48-96 hours). This practice accounts for a large number of PIVC reinsertions and places a significant cost burden on the healthcare infrastructure. The authors of this article examine the evidence that has been used to support this practice.
CASE PRESENTATION
A 67-year-old man with metastatic lung cancer presents to a hospital for pain control and “failure to thrive.” In the emergency department, a left antecubital peripheral intravenous catheter (PIVC) is placed. On admission, a prerenal acute kidney injury is noted. During the patient’s entire hospitalization, normal saline with parenteral hydromorphone is administered. On hospital day 4, the pain is still not adequately controlled, and the intravenous opioid is continued. On morning rounds, an intern notes that the PIVC is functioning well, and there are no signs of irritation. However, the nursing staff reminds the team that the PIVC should be changed because it has been in place for 4 days and is “due for replacement.” The patient does not want to receive another skin puncture for routine venous access. Does the PIVC need to be replaced, per routine?
WHY YOU MIGHT THINK ROUTINE PIVC REPLACEMENT IS HELPFUL
PIVC placement is easily the most common procedure performed in the United States. An estimated 200 million PIVCs are placed each year.1 Given the number of inpatient hospital stays per year in the United States alone—more than 37 million1,2—data regarding the care, maintenance, and complications of PIVCs are essential to the healthcare infrastructure.
The recommendation to routinely replace PIVCs dates to 1981, when the Centers for Disease Control and Prevention3 (CDC) issued a guideline that calls for replacing PIVCs every 24 to 48 hours. Most of the data and studies that established that recommendation originated in the 1970s, when catheters varied in length and material, and precise definitions of complications, such as phlebitis—localized vein inflammation characterized by pain, erythema, tenderness, swelling, and a palpable cord4,5—were not standardized across trials. Research at the time suggested higher rates of complications from IVCs dwelling longer than 48 to 72 hours. The latest (2011) CDC guidelines6,7 softened the recommendation but still concluded, “There is no need to replace peripheral catheters more frequently than every 72-96 hours.”
The 2011 recommendation6,7 is based on findings of a 1983 prospective observational study,8 a 1991 randomized controlled trial (RCT),9 and a 1998 prospective observational study.2 The 1983 and 1991 studies found higher rates of PIVC complications after day 2 of cannulation.8,9 The 1998 study found no increase in the rate of complications after day 3 of catheterization, and its authors, recommending a reevaluation of the need to routinely replace PIVCs, wrote, “[The] hazard for catheter-related complications, phlebitis, catheter-related infections, and mechanical complications did not increase during prolonged catheterization.”2
Results of RCTs conducted by Barker et al.10 (2004) and Nishanth et al.11 (2009) supported the claim that routine replacement of PIVCs leads to lower rates of thrombophlebitis. Nishanth et al. also included site pain and cannula dislodgement in their definition of phlebitis. Neither study compared blood stream infection rates, but both found higher rates of phlebitis between day 2.5 and day 3. However, Cochrane reviewers Webster et al.12 questioned the findings of these 2 trials, given their missing data and possibly biased results and conclusions. In the Barker study, patient numbers (screened, eligible, dropout) were unclear; each patient group was unbalanced; protocol deviations were not reported (possibly a result of incomplete data reporting or inappropriate randomization); and varied definitions of phlebitis were allowed, which may have resulted in more events being included. In the Nishanth study, the 100% phlebitis rate for the clinically indicated replacement group seemed extreme, which suggested confounding by an unknown bias or chance. Last, both samples were small: 47 patients (Barker) and 42 patients (Nishanth). Given all these concerns, the 2 trials were excluded from the Cochrane meta-analysis on the subject.12
In the 1980s and early 1990s, routine removal and exchange of PIVCs were supported by limited evidence. Current well-designed trial data cast doubt on the need for such a practice.
WHY YOU SHOULD NOT ROUTINELY REPLACE PIVCs
According to the CDC,6,7 the issue of routine PIVC replacement remains unresolved: “No recommendation is made regarding replacement of peripheral catheters in adults only when clinically indicated.”
Whereas earlier data showed a higher risk of complications with longer dwelling IVs, the majority of contemporary data has failed to support this conclusion. The recent (2015) Cochrane meta-analysis comparing routine with clinically indicated IVC replacement found “no evidence to support changing catheters every 72-96 hours.”12 Of the 7 studies that fulfilled the criteria for qualitative analysis, only 5 were included (the studies by Barker et al.10 and Nishanth et al.11 were excluded). The included studies assessed the endpoints of catheter-related blood stream infection (CRBSI), phlebitis, phlebitis per device-days, mortality, cost, and infiltration. Statistically significant differences were found only for cost (favoring clinically indicated replacement) and infiltration (occurring less with routine replacement).
The largest and most robust RCT in the meta-analysis12 was conducted by Rickard et al.13 (2012). Their nonblinded, intention-to-treat study of 3283 patients used concealed allocation to randomly assign patients to either clinically indicated or routine PIVC replacement in order to evaluate a primary endpoint, phlebitis. Secondary endpoints were CRBSI, venous port infection, IVC tip colonization, infusion failure, number of IVCs needed per patient, IV therapy duration, cost, and mortality. Need for PIVC replacement was methodically monitored (Table) with extensive nursing education and interrater validation. The study found no difference in the groups’ phlebitis rates; the rate was 7% for both routine and clinically indicated replacement (13.08% and 13.11%, respectively, adjusted for phlebitis per 1000 IVC days). In addition, there was no difference in the secondary outcome measures, except cost and number of catheters used, both of which favored clinically indicated replacement. The most serious complication, CRBSI, occurred at essentially the same rate in the 2 replacement arms: 0.11% (routine) and 0% (clinically indicated). Per-patient cost for the entire course of treatment was A$69.24 in the routine group and A$61.66 in the clinically indicated group; the difference was A$7.58 (P < 0.0001). Mean number of catheters used was 1.9 in the routine group and 1.7 in the clinically indicated group; the difference was 0.21 catheter per patient for the treatment course (P < 0.0001). Overall, the study found no important difference in significant outcomes between the 2 study arms.
The other 4 studies in the meta-analysis12 duplicated these results, with none finding a higher rate of major adverse events.14-17 All 4 showed virtually equivalent rates of phlebitis, the primary outcome; 3 also examined the secondary outcome measure of blood stream infection, and results were similar, with identical rates of complications. Only 1 trial identified any bloodstream infections (1 per group).15 The meta-analysis did find that routine catheter replacement resulted in less catheter infiltration.
Most of the data on PIVC exchange involves phlebitis and other local complications. A prospective study by Stuart et al.18 and commentary by Collignon et al.19 underscore the need for further research targeting blood stream infections (sepsis and severe sepsis in particular) as a primary outcome. Blood stream infections, especially those related to PIVC use, are rare entities overall, with most recent data yielding an estimated rate of 0.5 per 1000 catheter-days.20 Given this epidemiologic finding, researchers trying to acquire meaningful data on PIVC-related blood stream infections and subsequent complications would need to have tens of thousands of patients in routine and clinically indicated replacement arms to sufficiently power their studies.20 As they are infeasible, such trials cannot be found in the scientific literature.
Stuart et al.18 tried addressing the question. Prospectively examining more than 5 million occupied-bed days and the incidence of bloodstream infections by type of intravascular device over a 5-year period, they found that 137 (23.5%) of 583 healthcare-associated Staphylococcus aureus bacteremia (SAB) cases were attributed to PIVC use. PIVC insertions were performed equally (39.6%) in emergency departments and medical wards. About 45% of PIVCs remained in place 4 days or longer. Stuart et al. noted the “significant issue of PIVC-associated SAB” and favored routine removal of PIVCs within 96 hours (4 days). However, 55% of patients in their PIVC-related SAB group had the device in place less than 4 days. In addition, overall incidence of SAB was low: 0.3 per 10,000 occupied-bed days. Further, their study did not adjust device-specific SAB incidence for frequency of device use. For example, the rate of healthcare-acquired SAB was 19.7% for central venous catheters and 23.5% for PIVCs, despite PIVCs being used significantly more often than central lines. Device-specific adjustments would show a vastly different absolute risk of SAB in relation to individual devices. Nevertheless, the overall benefit of and need for routine PIVC replacement must be questioned. The percentage of PIVC-associated SAB in their study and the need for more research in this area should be noted. Given current information, their study and others in the literature underscore the need for selective use, appropriate maintenance, and timely removal of PIVCs.
Pure clinical outcomes are important, but procedural costs are as well. Clinically indicated replacement helps patients avoid an unpleasant procedure and saves money.21 If one third of the 37 million annual inpatient admissions require a PIVC for more than 3 days, then a strategy of “replacement when clinically indicated” could prevent almost 2.5 million unnecessary PIVC insertions each year. Equipment cost savings combined with savings of nearly 1 million staff hours could yield an estimated $400 million in savings over a 5-year period.22 Given current data suggesting no harm from clinically indicated PIVC replacement and clear evidence that routine replacement increases needle sticks and costs, it seems time to end the practice of routine PIVC replacement.
RECOMMENDATIONS
Compared with clinically indicated catheter replacement, routine replacement in the absence of a clinical indication (eg, infiltration, phlebitis, infection) provides no added benefit. Studies have consistently found that rates of phlebitis and SAB are not affected by scheduled replacement, though the largest RCT may not have been powered to show a difference in SAB. The present authors’ recommendations for PIVC care are:
- Scrutinize each patient’s need for PIVCs and remove each PIVC as soon as possible.
- Do not make routine replacement of otherwise well-functioning, well-appearing clinically necessary PIVCs the standard of care.
- Regularly examine PIVC sites for signs and symptoms of infection.
- Remove a PIVC immediately on recognition of any clinical sign of a complication (eg, infiltration, phlebitis, localized infection, blood stream infection) and replace the PIVC only if there is a clinical need.
- If replacing PIVCs on a clinical basis, establish protocols for frequency of evaluation for complications; these protocols might mirror those from prior studies (Table).10,22
- Replace as soon as possible any PIVC inserted during an urgent or emergent situation in which proper insertion technique could not be guaranteed.
- Conduct real-world observational studies to ensure that the switch to clinically driven replacement is safe and develop standardized definitions of complications.
Given the literature findings and the preceding recommendations, the authors conclude that the patient in the case example does not need routine PIVC replacement. His PIVC may remain in place as long as evaluation for local complications is routinely and methodically performed and the device is removed as soon as it is deemed unnecessary (transition to oral opioid therapy).
CONCLUSION
The long-standing practice of routinely replacing PIVCs every 72 to 96 hours during a hospital stay does not affect any meaningful clinical outcome. Specifically, data do not show that routine replacement prevents phlebitis or blood stream infections. Furthermore, routine PIVC replacement increases patient discomfort, uses resources unnecessarily, and raises hospital costs. Most of the PIVC research has involved phlebitis and other local complications; more research on PIVC use and bloodstream infections is needed. Given the findings in the current literature, routine PIVC replacement should be considered a Thing We Do For No Reason.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
The “Things We Do for No Reason” (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Hospitals and health systems worldwide have adopted policies for routine replacement of peripheral intravenous catheters (PIVCs) at prespecified time intervals (range, 48-96 hours). This practice accounts for a large number of PIVC reinsertions and places a significant cost burden on the healthcare infrastructure. The authors of this article examine the evidence that has been used to support this practice.
CASE PRESENTATION
A 67-year-old man with metastatic lung cancer presents to a hospital for pain control and “failure to thrive.” In the emergency department, a left antecubital peripheral intravenous catheter (PIVC) is placed. On admission, a prerenal acute kidney injury is noted. During the patient’s entire hospitalization, normal saline with parenteral hydromorphone is administered. On hospital day 4, the pain is still not adequately controlled, and the intravenous opioid is continued. On morning rounds, an intern notes that the PIVC is functioning well, and there are no signs of irritation. However, the nursing staff reminds the team that the PIVC should be changed because it has been in place for 4 days and is “due for replacement.” The patient does not want to receive another skin puncture for routine venous access. Does the PIVC need to be replaced, per routine?
WHY YOU MIGHT THINK ROUTINE PIVC REPLACEMENT IS HELPFUL
PIVC placement is easily the most common procedure performed in the United States. An estimated 200 million PIVCs are placed each year.1 Given the number of inpatient hospital stays per year in the United States alone—more than 37 million1,2—data regarding the care, maintenance, and complications of PIVCs are essential to the healthcare infrastructure.
The recommendation to routinely replace PIVCs dates to 1981, when the Centers for Disease Control and Prevention3 (CDC) issued a guideline that calls for replacing PIVCs every 24 to 48 hours. Most of the data and studies that established that recommendation originated in the 1970s, when catheters varied in length and material, and precise definitions of complications, such as phlebitis—localized vein inflammation characterized by pain, erythema, tenderness, swelling, and a palpable cord4,5—were not standardized across trials. Research at the time suggested higher rates of complications from IVCs dwelling longer than 48 to 72 hours. The latest (2011) CDC guidelines6,7 softened the recommendation but still concluded, “There is no need to replace peripheral catheters more frequently than every 72-96 hours.”
The 2011 recommendation6,7 is based on findings of a 1983 prospective observational study,8 a 1991 randomized controlled trial (RCT),9 and a 1998 prospective observational study.2 The 1983 and 1991 studies found higher rates of PIVC complications after day 2 of cannulation.8,9 The 1998 study found no increase in the rate of complications after day 3 of catheterization, and its authors, recommending a reevaluation of the need to routinely replace PIVCs, wrote, “[The] hazard for catheter-related complications, phlebitis, catheter-related infections, and mechanical complications did not increase during prolonged catheterization.”2
Results of RCTs conducted by Barker et al.10 (2004) and Nishanth et al.11 (2009) supported the claim that routine replacement of PIVCs leads to lower rates of thrombophlebitis. Nishanth et al. also included site pain and cannula dislodgement in their definition of phlebitis. Neither study compared blood stream infection rates, but both found higher rates of phlebitis between day 2.5 and day 3. However, Cochrane reviewers Webster et al.12 questioned the findings of these 2 trials, given their missing data and possibly biased results and conclusions. In the Barker study, patient numbers (screened, eligible, dropout) were unclear; each patient group was unbalanced; protocol deviations were not reported (possibly a result of incomplete data reporting or inappropriate randomization); and varied definitions of phlebitis were allowed, which may have resulted in more events being included. In the Nishanth study, the 100% phlebitis rate for the clinically indicated replacement group seemed extreme, which suggested confounding by an unknown bias or chance. Last, both samples were small: 47 patients (Barker) and 42 patients (Nishanth). Given all these concerns, the 2 trials were excluded from the Cochrane meta-analysis on the subject.12
In the 1980s and early 1990s, routine removal and exchange of PIVCs were supported by limited evidence. Current well-designed trial data cast doubt on the need for such a practice.
WHY YOU SHOULD NOT ROUTINELY REPLACE PIVCs
According to the CDC,6,7 the issue of routine PIVC replacement remains unresolved: “No recommendation is made regarding replacement of peripheral catheters in adults only when clinically indicated.”
Whereas earlier data showed a higher risk of complications with longer dwelling IVs, the majority of contemporary data has failed to support this conclusion. The recent (2015) Cochrane meta-analysis comparing routine with clinically indicated IVC replacement found “no evidence to support changing catheters every 72-96 hours.”12 Of the 7 studies that fulfilled the criteria for qualitative analysis, only 5 were included (the studies by Barker et al.10 and Nishanth et al.11 were excluded). The included studies assessed the endpoints of catheter-related blood stream infection (CRBSI), phlebitis, phlebitis per device-days, mortality, cost, and infiltration. Statistically significant differences were found only for cost (favoring clinically indicated replacement) and infiltration (occurring less with routine replacement).
The largest and most robust RCT in the meta-analysis12 was conducted by Rickard et al.13 (2012). Their nonblinded, intention-to-treat study of 3283 patients used concealed allocation to randomly assign patients to either clinically indicated or routine PIVC replacement in order to evaluate a primary endpoint, phlebitis. Secondary endpoints were CRBSI, venous port infection, IVC tip colonization, infusion failure, number of IVCs needed per patient, IV therapy duration, cost, and mortality. Need for PIVC replacement was methodically monitored (Table) with extensive nursing education and interrater validation. The study found no difference in the groups’ phlebitis rates; the rate was 7% for both routine and clinically indicated replacement (13.08% and 13.11%, respectively, adjusted for phlebitis per 1000 IVC days). In addition, there was no difference in the secondary outcome measures, except cost and number of catheters used, both of which favored clinically indicated replacement. The most serious complication, CRBSI, occurred at essentially the same rate in the 2 replacement arms: 0.11% (routine) and 0% (clinically indicated). Per-patient cost for the entire course of treatment was A$69.24 in the routine group and A$61.66 in the clinically indicated group; the difference was A$7.58 (P < 0.0001). Mean number of catheters used was 1.9 in the routine group and 1.7 in the clinically indicated group; the difference was 0.21 catheter per patient for the treatment course (P < 0.0001). Overall, the study found no important difference in significant outcomes between the 2 study arms.
The other 4 studies in the meta-analysis12 duplicated these results, with none finding a higher rate of major adverse events.14-17 All 4 showed virtually equivalent rates of phlebitis, the primary outcome; 3 also examined the secondary outcome measure of blood stream infection, and results were similar, with identical rates of complications. Only 1 trial identified any bloodstream infections (1 per group).15 The meta-analysis did find that routine catheter replacement resulted in less catheter infiltration.
Most of the data on PIVC exchange involves phlebitis and other local complications. A prospective study by Stuart et al.18 and commentary by Collignon et al.19 underscore the need for further research targeting blood stream infections (sepsis and severe sepsis in particular) as a primary outcome. Blood stream infections, especially those related to PIVC use, are rare entities overall, with most recent data yielding an estimated rate of 0.5 per 1000 catheter-days.20 Given this epidemiologic finding, researchers trying to acquire meaningful data on PIVC-related blood stream infections and subsequent complications would need to have tens of thousands of patients in routine and clinically indicated replacement arms to sufficiently power their studies.20 As they are infeasible, such trials cannot be found in the scientific literature.
Stuart et al.18 tried addressing the question. Prospectively examining more than 5 million occupied-bed days and the incidence of bloodstream infections by type of intravascular device over a 5-year period, they found that 137 (23.5%) of 583 healthcare-associated Staphylococcus aureus bacteremia (SAB) cases were attributed to PIVC use. PIVC insertions were performed equally (39.6%) in emergency departments and medical wards. About 45% of PIVCs remained in place 4 days or longer. Stuart et al. noted the “significant issue of PIVC-associated SAB” and favored routine removal of PIVCs within 96 hours (4 days). However, 55% of patients in their PIVC-related SAB group had the device in place less than 4 days. In addition, overall incidence of SAB was low: 0.3 per 10,000 occupied-bed days. Further, their study did not adjust device-specific SAB incidence for frequency of device use. For example, the rate of healthcare-acquired SAB was 19.7% for central venous catheters and 23.5% for PIVCs, despite PIVCs being used significantly more often than central lines. Device-specific adjustments would show a vastly different absolute risk of SAB in relation to individual devices. Nevertheless, the overall benefit of and need for routine PIVC replacement must be questioned. The percentage of PIVC-associated SAB in their study and the need for more research in this area should be noted. Given current information, their study and others in the literature underscore the need for selective use, appropriate maintenance, and timely removal of PIVCs.
Pure clinical outcomes are important, but procedural costs are as well. Clinically indicated replacement helps patients avoid an unpleasant procedure and saves money.21 If one third of the 37 million annual inpatient admissions require a PIVC for more than 3 days, then a strategy of “replacement when clinically indicated” could prevent almost 2.5 million unnecessary PIVC insertions each year. Equipment cost savings combined with savings of nearly 1 million staff hours could yield an estimated $400 million in savings over a 5-year period.22 Given current data suggesting no harm from clinically indicated PIVC replacement and clear evidence that routine replacement increases needle sticks and costs, it seems time to end the practice of routine PIVC replacement.
RECOMMENDATIONS
Compared with clinically indicated catheter replacement, routine replacement in the absence of a clinical indication (eg, infiltration, phlebitis, infection) provides no added benefit. Studies have consistently found that rates of phlebitis and SAB are not affected by scheduled replacement, though the largest RCT may not have been powered to show a difference in SAB. The present authors’ recommendations for PIVC care are:
- Scrutinize each patient’s need for PIVCs and remove each PIVC as soon as possible.
- Do not make routine replacement of otherwise well-functioning, well-appearing clinically necessary PIVCs the standard of care.
- Regularly examine PIVC sites for signs and symptoms of infection.
- Remove a PIVC immediately on recognition of any clinical sign of a complication (eg, infiltration, phlebitis, localized infection, blood stream infection) and replace the PIVC only if there is a clinical need.
- If replacing PIVCs on a clinical basis, establish protocols for frequency of evaluation for complications; these protocols might mirror those from prior studies (Table).10,22
- Replace as soon as possible any PIVC inserted during an urgent or emergent situation in which proper insertion technique could not be guaranteed.
- Conduct real-world observational studies to ensure that the switch to clinically driven replacement is safe and develop standardized definitions of complications.
Given the literature findings and the preceding recommendations, the authors conclude that the patient in the case example does not need routine PIVC replacement. His PIVC may remain in place as long as evaluation for local complications is routinely and methodically performed and the device is removed as soon as it is deemed unnecessary (transition to oral opioid therapy).
CONCLUSION
The long-standing practice of routinely replacing PIVCs every 72 to 96 hours during a hospital stay does not affect any meaningful clinical outcome. Specifically, data do not show that routine replacement prevents phlebitis or blood stream infections. Furthermore, routine PIVC replacement increases patient discomfort, uses resources unnecessarily, and raises hospital costs. Most of the PIVC research has involved phlebitis and other local complications; more research on PIVC use and bloodstream infections is needed. Given the findings in the current literature, routine PIVC replacement should be considered a Thing We Do For No Reason.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
1. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1-45. PubMed
2. Bregenzer T, Conen D, Sakmann P, Widmer AF. Is routine replacement of peripheral intravenous catheters necessary? Arch Intern Med. 1998;158(2):151-156. PubMed
3. Centers for Disease Control Working Group. Guidelines for prevention of intravenous therapy-related infections. Infect Control. 1981;3:62-79.
4. Hershey CO, Tomford JW, McLaren CE, Porter DK, Cohen DI. The natural history of intravenous catheter-associated phlebitis. Arch Intern Med. 1984;144(7):1373-1375. PubMed
5. Widmer AF. IV-related infections. In: Wenzel RP, ed. Prevention and Control of Nosocomial Infections. 3rd ed. Baltimore, MD: Williams & Wilkins; 1997:556-579.
6. O’Grady NP, Alexander M, Burns LA, et al; Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for the Prevention of Intravascular Catheter-Related Infections, 2011. Centers for Disease Control and Prevention website. http://www.cdc.gov/hicpac/pdf/guidelines/bsi-guidelines-2011.pdf. Published April 1, 2011. Accessed November 5, 2016. PubMed
7. O’Grady NP, Alexander M, Burns LA, et al; Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162-e193. PubMed
8. Rhode Island Nosocomial Infection Consortium; Tager IB, Ginsberg MB, Ellis SE, et al. An epidemiologic study of the risks associated with peripheral intravenous catheters. Am J Epidemiol. 1983;118(6):839-851. PubMed
9. Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. A randomized controlled trial. Ann Intern Med. 1991;114(10):845-854. PubMed
10. Barker P, Anderson AD, MacFie J. Randomised clinical trial of elective re-siting of intravenous cannulae. Ann R Coll Surg Engl. 2004;86(4):281-283. PubMed
11. Nishanth S, Sivaram G, Kalayarasan R, Kate V, Ananthakrishnan N. Does elective re-siting of intravenous cannulae decrease peripheral thrombophlebitis? A randomized controlled study. Int Med J India. 2009;22(2):60-62. PubMed
12. Webster J, Osborne S, Rickard CM, New K. Clinically-indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst Rev. 2015;(8):CD007798. PubMed
13. Rickard CM, Webster J, Wallis MC, et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet. 2012;380(9847):1066-1074. PubMed
14. Webster J, Lloyd S, Hopkins T, Osborne S, Yaxley M. Developing a Research base for Intravenous Peripheral cannula re-sites (DRIP trial). A randomised controlled trial of hospital in-patients. Int J Nurs Stud. 2007;44(5):664-671. PubMed
15. Webster J, Clarke S, Paterson D, et al. Routine care of peripheral intravenous catheters versus clinically indicated replacement: randomised controlled trial. BMJ. 2008;337:a339. PubMed
16. Van Donk P, Rickard CM, McGrail MR, Doolan G. Routine replacement versus clinical monitoring of peripheral intravenous catheters in a regional hospital in the home program: a randomized controlled trial. Infect Control Hosp Epidemiol. 2009;30(9):915-917. PubMed
17. Rickard CM, McCann D, Munnings J, McGrail MR. Routine resite of peripheral intravenous devices every 3 days did not reduce complications compared with clinically indicated resite: a randomised controlled trial. BMC Med. 2010;8:53. PubMed
18. Stuart RL, Cameron DR, Scott C, et al. Peripheral intravenous catheter-associated Staphylococcus aureus bacteraemia: more than 5 years of prospective data from two tertiary health services. Med J Aust. 2013;198(10):551-553. PubMed
19. Collignon PJ, Kimber FJ, Beckingham WD, Roberts JL. Prevention of peripheral intravenous catheter-related bloodstream infections: the need for routine replacement [letter]. Med J Aust. 2013;199(11):750-751. PubMed
20. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006:81(9):1159-1171. PubMed
21. Tuffaha HW, Rickard CM, Webster J, et al. Cost-effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Health Policy. 2014;12(1):51-58. PubMed
22. Rickard CM, Webster J, Playford EG. Prevention of peripheral intravenous catheter-related bloodstream infections: the need for a new focus. Med J Aust. 2013;198(10):519-520. PubMed
1. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1-45. PubMed
2. Bregenzer T, Conen D, Sakmann P, Widmer AF. Is routine replacement of peripheral intravenous catheters necessary? Arch Intern Med. 1998;158(2):151-156. PubMed
3. Centers for Disease Control Working Group. Guidelines for prevention of intravenous therapy-related infections. Infect Control. 1981;3:62-79.
4. Hershey CO, Tomford JW, McLaren CE, Porter DK, Cohen DI. The natural history of intravenous catheter-associated phlebitis. Arch Intern Med. 1984;144(7):1373-1375. PubMed
5. Widmer AF. IV-related infections. In: Wenzel RP, ed. Prevention and Control of Nosocomial Infections. 3rd ed. Baltimore, MD: Williams & Wilkins; 1997:556-579.
6. O’Grady NP, Alexander M, Burns LA, et al; Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for the Prevention of Intravascular Catheter-Related Infections, 2011. Centers for Disease Control and Prevention website. http://www.cdc.gov/hicpac/pdf/guidelines/bsi-guidelines-2011.pdf. Published April 1, 2011. Accessed November 5, 2016. PubMed
7. O’Grady NP, Alexander M, Burns LA, et al; Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162-e193. PubMed
8. Rhode Island Nosocomial Infection Consortium; Tager IB, Ginsberg MB, Ellis SE, et al. An epidemiologic study of the risks associated with peripheral intravenous catheters. Am J Epidemiol. 1983;118(6):839-851. PubMed
9. Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. A randomized controlled trial. Ann Intern Med. 1991;114(10):845-854. PubMed
10. Barker P, Anderson AD, MacFie J. Randomised clinical trial of elective re-siting of intravenous cannulae. Ann R Coll Surg Engl. 2004;86(4):281-283. PubMed
11. Nishanth S, Sivaram G, Kalayarasan R, Kate V, Ananthakrishnan N. Does elective re-siting of intravenous cannulae decrease peripheral thrombophlebitis? A randomized controlled study. Int Med J India. 2009;22(2):60-62. PubMed
12. Webster J, Osborne S, Rickard CM, New K. Clinically-indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst Rev. 2015;(8):CD007798. PubMed
13. Rickard CM, Webster J, Wallis MC, et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet. 2012;380(9847):1066-1074. PubMed
14. Webster J, Lloyd S, Hopkins T, Osborne S, Yaxley M. Developing a Research base for Intravenous Peripheral cannula re-sites (DRIP trial). A randomised controlled trial of hospital in-patients. Int J Nurs Stud. 2007;44(5):664-671. PubMed
15. Webster J, Clarke S, Paterson D, et al. Routine care of peripheral intravenous catheters versus clinically indicated replacement: randomised controlled trial. BMJ. 2008;337:a339. PubMed
16. Van Donk P, Rickard CM, McGrail MR, Doolan G. Routine replacement versus clinical monitoring of peripheral intravenous catheters in a regional hospital in the home program: a randomized controlled trial. Infect Control Hosp Epidemiol. 2009;30(9):915-917. PubMed
17. Rickard CM, McCann D, Munnings J, McGrail MR. Routine resite of peripheral intravenous devices every 3 days did not reduce complications compared with clinically indicated resite: a randomised controlled trial. BMC Med. 2010;8:53. PubMed
18. Stuart RL, Cameron DR, Scott C, et al. Peripheral intravenous catheter-associated Staphylococcus aureus bacteraemia: more than 5 years of prospective data from two tertiary health services. Med J Aust. 2013;198(10):551-553. PubMed
19. Collignon PJ, Kimber FJ, Beckingham WD, Roberts JL. Prevention of peripheral intravenous catheter-related bloodstream infections: the need for routine replacement [letter]. Med J Aust. 2013;199(11):750-751. PubMed
20. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006:81(9):1159-1171. PubMed
21. Tuffaha HW, Rickard CM, Webster J, et al. Cost-effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Health Policy. 2014;12(1):51-58. PubMed
22. Rickard CM, Webster J, Playford EG. Prevention of peripheral intravenous catheter-related bloodstream infections: the need for a new focus. Med J Aust. 2013;198(10):519-520. PubMed
© 2017 Society of Hospital Medicine
Getting Warmer
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 3-month-old otherwise healthy, immunized female presented to clinic with 2 days of intermittent low-grade fevers (maximum, 100º F), decreased oral intake, and sleepiness. Her pediatrician noted a faint, maculopapular rash on her trunk and extremities with mild conjunctival injection bilaterally that appeared that day, according to her mother. The infant otherwise appeared alert, well-hydrated, and without respiratory distress. She had no history of sick contacts or recent travel. She was prescribed amoxicillin for empiric treatment of a possible bacterial sinusitis or pharyngitis, despite a negative rapid strep antigen test.
At this age, multiple conditions can cause rashes. Given that this is early in the course of illness, without focal symptoms but with low-grade fevers, the initial differential diagnosis is broad and would include infectious, rheumatologic, and hematologic-oncologic etiologies, although the latter would be less likely. While the patient’s mother reports decreased oral intake, the fact that the patient is alert and appears hydrated is encouraging, suggesting time to observe and see if other symptoms present that may assist in elucidating the cause. The history of increased sleepiness warrants further investigation of meningeal signs, which would point to a central nervous system infection.
While streptococcal infection is possible, it would be uncommon at this age. The patient would have a higher fever and focal infection, and the rash does not appear consistent unless it was described as “sandpaper” in feel and appearance. A negative rapid strep test, while not sensitive, further supports this impression. A low-grade fever and rash would be consistent with a viral syndrome and, given the conjunctival injection, adenovirus, cytomegalovirus, rhinovirus, and Epstein Barr virus (EBV) are possibilities. Without ocular discharge, bacterial conjunctivitis would be unlikely. Another consideration would be Kawasaki disease, though it would be too early to diagnose this condition since at least 5 days of fever are required. Next steps include a detailed physical examination, looking for other focal signs such as swelling or desquamation of hands and feet, lymphadenopathy, strawberry tongue, and mucositis. Rather than empirically starting antibiotics, it would be more reasonable to observe her with close outpatient follow-up. The patient’s family should be instructed to monitor for additional and/or worsening symptoms, further decreased oral intake, signs of dehydration, or changes in alertness.
At home, the patient completed 5 doses of amoxicillin but continued to be febrile (maximum, 102.6º F). She was taken to a local emergency department on day 6 of her illness. She had worsening conjunctival injection and progression of the rash, involving the palms and soles. She was noted to have edema of hands and feet without desquamation (Figure 1). She had no oral mucous membrane changes and no cervical lymphadenopathy. Cerebrospinal fluid (CSF) was unremarkable, and empiric treatment with intravenous (IV) ceftriaxone was initiated. Complete blood count was notable for a white blood cell (WBC) count of 18.9 k/μL (normal range, 6.0-17.0); hemoglobin, 7.6 g/dL (normal range, 10-13); mean corpuscular volume, 84 (normal range, 74-108); and platelet count, 105 k/μL (normal range, 150-400). A peripheral blood smear revealed no abnormal cells. C-reactive protein (CRP) was elevated at 6.5 mg/dL (normal range, 0.0-0.6). She was admitted for further management.
Infection remains on the differential diagnosis given the elevated WBC count. Since the patient has completed a reasonable course of antibiotics, a bacterial infection would be less likely but not fully excluded. The cultures obtained would be helpful if they become positive, but given that the patient has been on antibiotics, a negative culture may represent partial sterilization and would not rule out infection. A viral infection continues to be high on the differential, but one would expect that symptoms and fever would have begun to abate. The normal peripheral blood smear makes a hematologic disorder less likely.
Kawasaki disease has risen on the differential with 5 days of fever surpassing 102º F. She has 3 of 5 primary clinical criteria, including conjunctival injection, rash, and edema of the hands and feet. Desquamation of the peripheral extremities would not be expected until the convalescent phase. A diagnosis of typical Kawasaki disease would require a fourth criterion, either oral mucous membrane changes or cervical lymphadenopathy. She meets the criteria for atypical or incomplete Kawasaki disease, which requires only fever for at least 5 days, elevated CRP, and 2 or 3 additional clinical criteria. She also meets supplemental laboratory criteria with an elevated WBC count greater than 15,000/μL, normocytic and normochromic anemia for age, and elevated CRP. Urinalysis positive for pyuria or serum albumin less than 3 g/dL would lend further support but is not necessary. Fever of 7 or more days in a child less than 6 months old without other explanation would also increase the likelihood of incomplete Kawasaki disease. Admission to the hospital, treatment with IV immunoglobulin (IVIg), and echocardiography to evaluate for typical cardiac involvement (eg, aneurysms, coronary arteritis, and pericardial effusion) are the appropriate next steps.
The patient was diagnosed with atypical Kawasaki disease. A transthoracic echocardiogram was normal on admission. On day 7 of her illness, she was treated with 1 dose of IVIg at 2 g/kg and high-dose aspirin at 100 mg/kg per day in divided doses. Despite this treatment, she continued to be febrile and was given a second dose of IVIg on day 9. Her fevers persisted.
In Kawasaki disease, persistent fever is concerning for long-term sequelae, including coronary artery aneurysms. Continued treatment is reasonable. After 2 doses of IVIg with a cumulative dose of 4 g/kg, it is prudent to switch therapy to IV methylprednisolone 30mg/kg with repeated doses as needed for up to 3 days should her fevers persist.
Her blood culture was negative. EBV serology, enterovirus polymerase chain reaction, and viral cultures were negative. Chest radiography on day 9 was normal. Abdominal ultrasonography on day 10 showed hydrops of the gallbladder.
The patient was started on IV corticosteroids on day 11 with resolution of her fevers and improvement in her rash. A repeat echocardiogram revealed new findings of dilated left main, left anterior descending, and right coronary arteries. On day 13, a steroid wean was attempted because she had remained afebrile for more than 48 hours, but the wean was halted due to recurrence of fevers and rash. Her high-dose aspirin was reduced to 81 mg PO daily on day 14, and she was started on enoxaparin injections.
It is unusual for Kawasaki disease not to respond to 2 doses of IVIg, followed by corticosteroids. As such, the differential diagnosis must be revisited. The findings of coronary artery dilation, prolonged fever, and rash corroborate the diagnosis of Kawasaki disease, although this could be an atypical presentation of another vasculitis. Systemic onset juvenile idiopathic arthritis usually affects children at 2 to 5 years old and is, therefore, less likely. Henoch-Schönlein purpura manifests with a rash but is often associated with diarrhea. There does not appear to be objective evidence of polyarteritis nodosa, although biopsy or angiography would be required to make this diagnosis. Hydrops of the gallbladder is an over-distention of the organ filled with watery or mucoid content. While hydrops can be noninflammatory and seen in gallstone disease, it can also occur in vasculitides. Despite the reassuring serologies, false negative results are possible. Thus, these viral infections are not eliminated, but they are less likely. Given the echocardiogram findings and continued concern for atypical Kawasaki disease, high-dose aspirin should be continued. It is reasonable to consider rheumatology consultation for assessment and recommendations as to length of steroid treatment and/or alternative interventions.
Pediatric cardiology was consulted. Repeat echocardiogram on day 16 showed an increase in the size of her coronary artery aneurysms, and her fevers persisted. Computed tomography scan of the abdomen and pelvis with contrast, obtained to further evaluate for a source of infection, was unremarkable.
The patient was transferred to a tertiary care institution on day 19, at which time she remained on aspirin, enoxaparin, and oral corticosteroids. On arrival, her temperature was 101.3º F, heart rate 225 beats per minute, and respiratory rate 57 breaths per minute. She was fussy with bilateral conjunctivitis and a maculopapular rash involving palms, soles, and right infraorbital region. Laboratory studies were significant for a WBC count of 30.3 k/μL; hemoglobin, 10.9 g/dL; platelets, 106 k/μL; and CRP, 8.3 mg/dL.
Pediatric rheumatology was consulted on day 20. The patient was treated with 3 days IV pulse-dose methylprednisolone at 30 mg/kg daily. Her fevers resolved, although her CRP level remained elevated. She was treated with 1 dose of infliximab 10 mg/kg IV on day 24, followed by 1 dose of anakinra 15 mg subcutaneously on day 27 due to persistently elevated CRP.
The symptoms and diagnostic evaluation remain most consistent with atypical Kawasaki disease. Her tachycardia and tachypnea are likely driven by her fever and fussiness, and should be followed closely. The elevated WBC is likely a consequence of the steroids and demargination of neutrophils. The elevated and increasing CRP is a marker of acute inflammation. The adage “treat the patient, not the numbers” comes to mind, because it is reassuring that the patient’s overall clinical picture seems to be improving with resolution of her fevers. However, further discussion with the pediatric rheumatology consultant is prudent, specifically regarding the significance of the persistently elevated CRP, refinement of the differential diagnosis including the potential for other vasculitides and appropriate evaluation of such, as well as recommendations for further treatment.
The patient was noted to have ongoing fevers. Based on reports of success with cyclophosphamide in refractory Kawasaki disease, she was treated with 2 doses at 60 mg IV per dose starting on day 28. Her CRP level decreased. Cardiology and rheumatology consultants recommended magnetic resonance imaging/magnetic resonance angiography of the chest, abdomen, and pelvis with and without contrast. These studies revealed dilation of the axillary and brachial arteries (Figure 2).
The response to cyclophosphamide confirms an autoimmune/inflammatory process. The imaging results and pattern are most consistent with either Kawasaki disease or polyarteritis nodosa. Therefore, rheumatology’s input will be invaluable with regard to which diagnosis is most likely, additional diagnostic testing, and appropriate medical regimen and follow-up plans.
Systemic extracoronary vascular inflammation on imaging and the refractory nature of the patient’s disease process, despite appropriate treatment for Kawasaki disease, led to the diagnosis of childhood polyarteritis nodosa (PAN). The patient was discharged home and closely followed in rheumatology clinic. Her most recent outpatient visit 1 year after the initial onset of her illness showed no further fevers or rashes, normal inflammatory markers, and stabilization of her coronary aneurysms on daily maintenance azathioprine.
DISCUSSION
Fever with an accompanying rash is a common issue in children. The extensive differential diagnosis includes infectious diseases, rheumatologic disorders, and medication reactions (Table 1). A thorough history and physical examination are essential in guiding the physician toward the proper diagnosis and management. Important information includes patient age, season, associated symptoms, exposure to sick contacts, travel history, host immune status, and immunization history. Fever duration and pattern must be elicited, as should features of the rash, including temporal relationship to the fever, distribution, progression, and morphology.1
When unexplained fever persists for 5 days or more in the pediatric patient, the diagnosis of KD must be suspected. KD is an acute, febrile, primary systemic vasculitis affecting small- and medium-sized vessels, with a predilection for coronary arteries.2 KD affects younger children, with approximately 85% of cases occurring in children under 5 years old. KD has a higher incidence in Asian populations, suggesting a possible genetic predisposition.3 The etiology of KD is not well understood, but infection and immune dysregulation have been proposed as contributing factors. KD is the leading cause of acquired heart disease in developed countries.2
The diagnosis of KD is made clinically (Table 2). Atypical KD is considered in patients with at least 5 days of fever but only 2 or 3 clinical criteria. Supportive laboratory findings include elevated inflammatory markers, anemia, neutrophilia, abnormal plasma lipids, low albumin, sterile pyuria, CSF pleocytosis, and elevated serum transaminases. Two-dimensional echocardiography should be performed in all children with definite or suspected KD at the time of diagnosis, 1 to 2 weeks later, and 6 weeks following discharge for evaluation of the coronary arteries, left ventricular function, and valve function. The American Heart Association recommends follow-up echocardiography at 1 year in children without coronary vessel involvement.4
Treatment is aimed at minimizing inflammation and coronary artery involvement, and should be initiated promptly.5 Therapy includes a single infusion of high-dose IVIg and aspirin;6,7 the latter is initially provided at high anti-inflammatory doses, followed by lower antithrombotic doses once fever and laboratory markers have resolved.2 Aspirin can be discontinued if there is no evidence of coronary involvement at the 6-week follow-up echocardiogram.5 A second dose of IVIg is given within 48 hours for refractory cases, defined as persistent fever following the first dose of IVIg.4 Fifteen percent of children have refractory illness, and refractory KD is associated with a higher risk of coronary artery lesions.5 Additional agents that suppress immune activation and cytokine secretion contributing to KD pathogenesis have been studied. Corticosteroids inhibit phospholipase A, an enzyme required for production of inflammatory markers.8 Infliximab, a tumor necrosis factor-alpha inhibitor, has been shown to reduce duration of fever and length of hospital stay.8,9 Anakinra, an interleukin-1 receptor antagonist, has been shown to decrease fever duration and prevent progression of vascular injury in cases of refractory KD.10 There is, however, a lack of sufficient evidence and consensus on best practice.8-10
If inflammation, evidenced by fever, elevated inflammatory markers (such as erythrocyte sedimentation rate, CRP), or vessel involvement on imaging, persists or worsens despite standard therapy, physicians should seek alternative diagnoses. This patient’s extracoronary vascular inflammation and favorable response only to cyclophosphamide led to the diagnosis of systemic PAN. Like KD, PAN is a multi-system vasculitis affecting small- and medium-sized vessels. Unlike KD, PAN is rarely seen in children.11 Historically, PAN was thought to represent an extreme fatal end of the KD spectrum. Today, PAN is accepted as a separate entity. Clinical features and histological findings often overlap with KD, creating a diagnostic dilemma for providers.12
At the onset of illness, clinical features of systemic PAN may include recurrent fever, weight loss, and myalgia, with gradual progression to multi-organ system involvement. Laboratory assessment reveals elevated inflammatory markers and leukocytosis. Thrombocytosis, anemia, proteinuria, and hematuria may be present. A positive antineutrophil cytoplasmic antibody is rare in PAN and should raise suspicion for a microscopic polyangiitis, which is distinguished from PAN by small vessel involvement only. When compared to KD, cardiac vessel involvement in PAN is more variable.11 Diagnostic criteria for childhood PAN are listed in Table 2.13
Treatment of PAN is aimed at inducing remission with high-dose steroids and cyclophosphamide. Maintenance of remission is achieved using low-dose steroids and azathioprine.11 Total duration of treatment averages 2 to 3 years, with a minimum of 18 months.14 Plasma exchange has been used in severe, life-threatening cases.11 Prognosis for children with PAN is more favorable compared to adults with PAN, in whom the mortality rate is as high as 20% to 30%, even with aggressive treatment. In 1 multicenter study of childhood and adolescent PAN, overall mortality was 1.1%.15
This patient initially presented with findings consistent with KD. As her inflammatory markers remained elevated and fevers persisted, her physicians appropriately reconsidered the etiology of her symptoms, thereby “getting warmer” in the search for the correct diagnosis of systemic PAN, a rare disease and a separate entity from KD. Recognizing the overlapping and distinct clinical features of each entity can promote more timely and appropriate selection of therapy, thereby minimizing clinical manifestations and complications associated with each vasculitis.
KEY TEACHING POINTS
- KD and childhood PAN are disseminated vasculitides affecting small- and medium-sized vessels. Although they are distinct entities, KD and PAN exhibit overlapping clinical and pathological features that make appropriate diagnosis and treatment challenging.
- In cases of refractory KD, alternative diagnoses should be considered.
- Recognizing the individual features of both entities is imperative because treatment differs: KD is treated with high-dose aspirin and IVIg; corticosteroids and immunosuppressive agents are used to treat PAN.
Disclosure
Nothing to report.
1. McKinnon HD Jr, Howard T. Evaluating the febrile patient with a rash. Am Fam Physician. 2000;62:804-816. PubMed
2. Dimitriades V, Brown AG, Gedalia A. Kawasaki disease: pathophysiology, clinical manifestations, and management. Curr Rheumatol Rep. 2014;16:423. PubMed
3. Callinan L, Holman RC, Vugia DJ, Schonberger LB, Belay ED. Kawasaki disease hospitalization rate among children younger than 5 years of age in California, 2003-2010. Pediatr Infect Dis J. 2014;33:781-783. PubMed
4. Newburger JW, Takahashi M, Gerber MA, Gewirtz MH, Tani LY, Burns JC, et al. Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association; American Academy of Pediatrics. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747-2771. PubMed
5. Son M, Newburger JW. Kawasaki disease. Pediatr Rev. 2013;34:151-61. PubMed
6. Newberger JW, Takahasi M, Beiser AS, et al. A single intravenous infusion of gammaglobulin as compared with four infusions in treatment of acute Kawasaki syndrome. N Engl J Med. 1991;324:1633-1639. PubMed
7. Dajani AS, Taubert KA, Gerber MA, et al. Diagnosis and therapy of Kawasaki disease in children. Circulation. 1993;87:1776-1780. PubMed
8. Saneeymehri S, Baker K, So TY. Overview of pharmacological treatment options for pediatric patients with refractory Kawasaki disease. J Pediatr Pharmacol Ther. 2015;20:163-177. PubMed
9. Brogan R, Eleftheriou D, Gnanapragasam J, Klein NJ, Brogan PA. Infliximab for the treatment of intravenous immunoglobulin resistant Kawasaki disease complicated by coronary artery aneurysms: a case report. Pediatr Rheumatol Online J. 2009;7:3. PubMed
10. Cohen S, Tacke CE, Straver B, Meijer N, Kuipers IM, Kuijpers TW. A child with severe relapsing Kawasaki disease rescued by IL-1 receptor blockade and extracorporeal membrane oxygenation. Ann Rheum Dis. 2012;71:2059-2061. PubMed
11. Kelly A, Tizard E. Vasculitis in children. Paediatrics and Child Health. 2010;20:65-72.
12. Yamazaki-Nakashimada MA, Espinosa-Lopez M, Hernandez-Bautista V, Espinosa-Padilla S, Espinosa-Rosales F. Catastrophic Kawasaki disease or juvenile polyarteritis nodosa? Semin Arthritis Rheum. 2006;35:349-354. PubMed
13. Ozen S, Pistorio A, Iusan SM, et al. EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria. Ann Rheum Dis. 2010;69:798-806. PubMed
14. Eleftheriou D, Brogan PA. Vasculitis in children. Best Pract Res Clin Rheumatol. 2009;23:309-323. PubMed
15. Ozen S, Anton J, Arisoy N, et al. Juvenile polyarteritis: results of a multicenter survey of 110 children. J Pediatr. 2004;145:517-522. PubMed
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 3-month-old otherwise healthy, immunized female presented to clinic with 2 days of intermittent low-grade fevers (maximum, 100º F), decreased oral intake, and sleepiness. Her pediatrician noted a faint, maculopapular rash on her trunk and extremities with mild conjunctival injection bilaterally that appeared that day, according to her mother. The infant otherwise appeared alert, well-hydrated, and without respiratory distress. She had no history of sick contacts or recent travel. She was prescribed amoxicillin for empiric treatment of a possible bacterial sinusitis or pharyngitis, despite a negative rapid strep antigen test.
At this age, multiple conditions can cause rashes. Given that this is early in the course of illness, without focal symptoms but with low-grade fevers, the initial differential diagnosis is broad and would include infectious, rheumatologic, and hematologic-oncologic etiologies, although the latter would be less likely. While the patient’s mother reports decreased oral intake, the fact that the patient is alert and appears hydrated is encouraging, suggesting time to observe and see if other symptoms present that may assist in elucidating the cause. The history of increased sleepiness warrants further investigation of meningeal signs, which would point to a central nervous system infection.
While streptococcal infection is possible, it would be uncommon at this age. The patient would have a higher fever and focal infection, and the rash does not appear consistent unless it was described as “sandpaper” in feel and appearance. A negative rapid strep test, while not sensitive, further supports this impression. A low-grade fever and rash would be consistent with a viral syndrome and, given the conjunctival injection, adenovirus, cytomegalovirus, rhinovirus, and Epstein Barr virus (EBV) are possibilities. Without ocular discharge, bacterial conjunctivitis would be unlikely. Another consideration would be Kawasaki disease, though it would be too early to diagnose this condition since at least 5 days of fever are required. Next steps include a detailed physical examination, looking for other focal signs such as swelling or desquamation of hands and feet, lymphadenopathy, strawberry tongue, and mucositis. Rather than empirically starting antibiotics, it would be more reasonable to observe her with close outpatient follow-up. The patient’s family should be instructed to monitor for additional and/or worsening symptoms, further decreased oral intake, signs of dehydration, or changes in alertness.
At home, the patient completed 5 doses of amoxicillin but continued to be febrile (maximum, 102.6º F). She was taken to a local emergency department on day 6 of her illness. She had worsening conjunctival injection and progression of the rash, involving the palms and soles. She was noted to have edema of hands and feet without desquamation (Figure 1). She had no oral mucous membrane changes and no cervical lymphadenopathy. Cerebrospinal fluid (CSF) was unremarkable, and empiric treatment with intravenous (IV) ceftriaxone was initiated. Complete blood count was notable for a white blood cell (WBC) count of 18.9 k/μL (normal range, 6.0-17.0); hemoglobin, 7.6 g/dL (normal range, 10-13); mean corpuscular volume, 84 (normal range, 74-108); and platelet count, 105 k/μL (normal range, 150-400). A peripheral blood smear revealed no abnormal cells. C-reactive protein (CRP) was elevated at 6.5 mg/dL (normal range, 0.0-0.6). She was admitted for further management.
Infection remains on the differential diagnosis given the elevated WBC count. Since the patient has completed a reasonable course of antibiotics, a bacterial infection would be less likely but not fully excluded. The cultures obtained would be helpful if they become positive, but given that the patient has been on antibiotics, a negative culture may represent partial sterilization and would not rule out infection. A viral infection continues to be high on the differential, but one would expect that symptoms and fever would have begun to abate. The normal peripheral blood smear makes a hematologic disorder less likely.
Kawasaki disease has risen on the differential with 5 days of fever surpassing 102º F. She has 3 of 5 primary clinical criteria, including conjunctival injection, rash, and edema of the hands and feet. Desquamation of the peripheral extremities would not be expected until the convalescent phase. A diagnosis of typical Kawasaki disease would require a fourth criterion, either oral mucous membrane changes or cervical lymphadenopathy. She meets the criteria for atypical or incomplete Kawasaki disease, which requires only fever for at least 5 days, elevated CRP, and 2 or 3 additional clinical criteria. She also meets supplemental laboratory criteria with an elevated WBC count greater than 15,000/μL, normocytic and normochromic anemia for age, and elevated CRP. Urinalysis positive for pyuria or serum albumin less than 3 g/dL would lend further support but is not necessary. Fever of 7 or more days in a child less than 6 months old without other explanation would also increase the likelihood of incomplete Kawasaki disease. Admission to the hospital, treatment with IV immunoglobulin (IVIg), and echocardiography to evaluate for typical cardiac involvement (eg, aneurysms, coronary arteritis, and pericardial effusion) are the appropriate next steps.
The patient was diagnosed with atypical Kawasaki disease. A transthoracic echocardiogram was normal on admission. On day 7 of her illness, she was treated with 1 dose of IVIg at 2 g/kg and high-dose aspirin at 100 mg/kg per day in divided doses. Despite this treatment, she continued to be febrile and was given a second dose of IVIg on day 9. Her fevers persisted.
In Kawasaki disease, persistent fever is concerning for long-term sequelae, including coronary artery aneurysms. Continued treatment is reasonable. After 2 doses of IVIg with a cumulative dose of 4 g/kg, it is prudent to switch therapy to IV methylprednisolone 30mg/kg with repeated doses as needed for up to 3 days should her fevers persist.
Her blood culture was negative. EBV serology, enterovirus polymerase chain reaction, and viral cultures were negative. Chest radiography on day 9 was normal. Abdominal ultrasonography on day 10 showed hydrops of the gallbladder.
The patient was started on IV corticosteroids on day 11 with resolution of her fevers and improvement in her rash. A repeat echocardiogram revealed new findings of dilated left main, left anterior descending, and right coronary arteries. On day 13, a steroid wean was attempted because she had remained afebrile for more than 48 hours, but the wean was halted due to recurrence of fevers and rash. Her high-dose aspirin was reduced to 81 mg PO daily on day 14, and she was started on enoxaparin injections.
It is unusual for Kawasaki disease not to respond to 2 doses of IVIg, followed by corticosteroids. As such, the differential diagnosis must be revisited. The findings of coronary artery dilation, prolonged fever, and rash corroborate the diagnosis of Kawasaki disease, although this could be an atypical presentation of another vasculitis. Systemic onset juvenile idiopathic arthritis usually affects children at 2 to 5 years old and is, therefore, less likely. Henoch-Schönlein purpura manifests with a rash but is often associated with diarrhea. There does not appear to be objective evidence of polyarteritis nodosa, although biopsy or angiography would be required to make this diagnosis. Hydrops of the gallbladder is an over-distention of the organ filled with watery or mucoid content. While hydrops can be noninflammatory and seen in gallstone disease, it can also occur in vasculitides. Despite the reassuring serologies, false negative results are possible. Thus, these viral infections are not eliminated, but they are less likely. Given the echocardiogram findings and continued concern for atypical Kawasaki disease, high-dose aspirin should be continued. It is reasonable to consider rheumatology consultation for assessment and recommendations as to length of steroid treatment and/or alternative interventions.
Pediatric cardiology was consulted. Repeat echocardiogram on day 16 showed an increase in the size of her coronary artery aneurysms, and her fevers persisted. Computed tomography scan of the abdomen and pelvis with contrast, obtained to further evaluate for a source of infection, was unremarkable.
The patient was transferred to a tertiary care institution on day 19, at which time she remained on aspirin, enoxaparin, and oral corticosteroids. On arrival, her temperature was 101.3º F, heart rate 225 beats per minute, and respiratory rate 57 breaths per minute. She was fussy with bilateral conjunctivitis and a maculopapular rash involving palms, soles, and right infraorbital region. Laboratory studies were significant for a WBC count of 30.3 k/μL; hemoglobin, 10.9 g/dL; platelets, 106 k/μL; and CRP, 8.3 mg/dL.
Pediatric rheumatology was consulted on day 20. The patient was treated with 3 days IV pulse-dose methylprednisolone at 30 mg/kg daily. Her fevers resolved, although her CRP level remained elevated. She was treated with 1 dose of infliximab 10 mg/kg IV on day 24, followed by 1 dose of anakinra 15 mg subcutaneously on day 27 due to persistently elevated CRP.
The symptoms and diagnostic evaluation remain most consistent with atypical Kawasaki disease. Her tachycardia and tachypnea are likely driven by her fever and fussiness, and should be followed closely. The elevated WBC is likely a consequence of the steroids and demargination of neutrophils. The elevated and increasing CRP is a marker of acute inflammation. The adage “treat the patient, not the numbers” comes to mind, because it is reassuring that the patient’s overall clinical picture seems to be improving with resolution of her fevers. However, further discussion with the pediatric rheumatology consultant is prudent, specifically regarding the significance of the persistently elevated CRP, refinement of the differential diagnosis including the potential for other vasculitides and appropriate evaluation of such, as well as recommendations for further treatment.
The patient was noted to have ongoing fevers. Based on reports of success with cyclophosphamide in refractory Kawasaki disease, she was treated with 2 doses at 60 mg IV per dose starting on day 28. Her CRP level decreased. Cardiology and rheumatology consultants recommended magnetic resonance imaging/magnetic resonance angiography of the chest, abdomen, and pelvis with and without contrast. These studies revealed dilation of the axillary and brachial arteries (Figure 2).
The response to cyclophosphamide confirms an autoimmune/inflammatory process. The imaging results and pattern are most consistent with either Kawasaki disease or polyarteritis nodosa. Therefore, rheumatology’s input will be invaluable with regard to which diagnosis is most likely, additional diagnostic testing, and appropriate medical regimen and follow-up plans.
Systemic extracoronary vascular inflammation on imaging and the refractory nature of the patient’s disease process, despite appropriate treatment for Kawasaki disease, led to the diagnosis of childhood polyarteritis nodosa (PAN). The patient was discharged home and closely followed in rheumatology clinic. Her most recent outpatient visit 1 year after the initial onset of her illness showed no further fevers or rashes, normal inflammatory markers, and stabilization of her coronary aneurysms on daily maintenance azathioprine.
DISCUSSION
Fever with an accompanying rash is a common issue in children. The extensive differential diagnosis includes infectious diseases, rheumatologic disorders, and medication reactions (Table 1). A thorough history and physical examination are essential in guiding the physician toward the proper diagnosis and management. Important information includes patient age, season, associated symptoms, exposure to sick contacts, travel history, host immune status, and immunization history. Fever duration and pattern must be elicited, as should features of the rash, including temporal relationship to the fever, distribution, progression, and morphology.1
When unexplained fever persists for 5 days or more in the pediatric patient, the diagnosis of KD must be suspected. KD is an acute, febrile, primary systemic vasculitis affecting small- and medium-sized vessels, with a predilection for coronary arteries.2 KD affects younger children, with approximately 85% of cases occurring in children under 5 years old. KD has a higher incidence in Asian populations, suggesting a possible genetic predisposition.3 The etiology of KD is not well understood, but infection and immune dysregulation have been proposed as contributing factors. KD is the leading cause of acquired heart disease in developed countries.2
The diagnosis of KD is made clinically (Table 2). Atypical KD is considered in patients with at least 5 days of fever but only 2 or 3 clinical criteria. Supportive laboratory findings include elevated inflammatory markers, anemia, neutrophilia, abnormal plasma lipids, low albumin, sterile pyuria, CSF pleocytosis, and elevated serum transaminases. Two-dimensional echocardiography should be performed in all children with definite or suspected KD at the time of diagnosis, 1 to 2 weeks later, and 6 weeks following discharge for evaluation of the coronary arteries, left ventricular function, and valve function. The American Heart Association recommends follow-up echocardiography at 1 year in children without coronary vessel involvement.4
Treatment is aimed at minimizing inflammation and coronary artery involvement, and should be initiated promptly.5 Therapy includes a single infusion of high-dose IVIg and aspirin;6,7 the latter is initially provided at high anti-inflammatory doses, followed by lower antithrombotic doses once fever and laboratory markers have resolved.2 Aspirin can be discontinued if there is no evidence of coronary involvement at the 6-week follow-up echocardiogram.5 A second dose of IVIg is given within 48 hours for refractory cases, defined as persistent fever following the first dose of IVIg.4 Fifteen percent of children have refractory illness, and refractory KD is associated with a higher risk of coronary artery lesions.5 Additional agents that suppress immune activation and cytokine secretion contributing to KD pathogenesis have been studied. Corticosteroids inhibit phospholipase A, an enzyme required for production of inflammatory markers.8 Infliximab, a tumor necrosis factor-alpha inhibitor, has been shown to reduce duration of fever and length of hospital stay.8,9 Anakinra, an interleukin-1 receptor antagonist, has been shown to decrease fever duration and prevent progression of vascular injury in cases of refractory KD.10 There is, however, a lack of sufficient evidence and consensus on best practice.8-10
If inflammation, evidenced by fever, elevated inflammatory markers (such as erythrocyte sedimentation rate, CRP), or vessel involvement on imaging, persists or worsens despite standard therapy, physicians should seek alternative diagnoses. This patient’s extracoronary vascular inflammation and favorable response only to cyclophosphamide led to the diagnosis of systemic PAN. Like KD, PAN is a multi-system vasculitis affecting small- and medium-sized vessels. Unlike KD, PAN is rarely seen in children.11 Historically, PAN was thought to represent an extreme fatal end of the KD spectrum. Today, PAN is accepted as a separate entity. Clinical features and histological findings often overlap with KD, creating a diagnostic dilemma for providers.12
At the onset of illness, clinical features of systemic PAN may include recurrent fever, weight loss, and myalgia, with gradual progression to multi-organ system involvement. Laboratory assessment reveals elevated inflammatory markers and leukocytosis. Thrombocytosis, anemia, proteinuria, and hematuria may be present. A positive antineutrophil cytoplasmic antibody is rare in PAN and should raise suspicion for a microscopic polyangiitis, which is distinguished from PAN by small vessel involvement only. When compared to KD, cardiac vessel involvement in PAN is more variable.11 Diagnostic criteria for childhood PAN are listed in Table 2.13
Treatment of PAN is aimed at inducing remission with high-dose steroids and cyclophosphamide. Maintenance of remission is achieved using low-dose steroids and azathioprine.11 Total duration of treatment averages 2 to 3 years, with a minimum of 18 months.14 Plasma exchange has been used in severe, life-threatening cases.11 Prognosis for children with PAN is more favorable compared to adults with PAN, in whom the mortality rate is as high as 20% to 30%, even with aggressive treatment. In 1 multicenter study of childhood and adolescent PAN, overall mortality was 1.1%.15
This patient initially presented with findings consistent with KD. As her inflammatory markers remained elevated and fevers persisted, her physicians appropriately reconsidered the etiology of her symptoms, thereby “getting warmer” in the search for the correct diagnosis of systemic PAN, a rare disease and a separate entity from KD. Recognizing the overlapping and distinct clinical features of each entity can promote more timely and appropriate selection of therapy, thereby minimizing clinical manifestations and complications associated with each vasculitis.
KEY TEACHING POINTS
- KD and childhood PAN are disseminated vasculitides affecting small- and medium-sized vessels. Although they are distinct entities, KD and PAN exhibit overlapping clinical and pathological features that make appropriate diagnosis and treatment challenging.
- In cases of refractory KD, alternative diagnoses should be considered.
- Recognizing the individual features of both entities is imperative because treatment differs: KD is treated with high-dose aspirin and IVIg; corticosteroids and immunosuppressive agents are used to treat PAN.
Disclosure
Nothing to report.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 3-month-old otherwise healthy, immunized female presented to clinic with 2 days of intermittent low-grade fevers (maximum, 100º F), decreased oral intake, and sleepiness. Her pediatrician noted a faint, maculopapular rash on her trunk and extremities with mild conjunctival injection bilaterally that appeared that day, according to her mother. The infant otherwise appeared alert, well-hydrated, and without respiratory distress. She had no history of sick contacts or recent travel. She was prescribed amoxicillin for empiric treatment of a possible bacterial sinusitis or pharyngitis, despite a negative rapid strep antigen test.
At this age, multiple conditions can cause rashes. Given that this is early in the course of illness, without focal symptoms but with low-grade fevers, the initial differential diagnosis is broad and would include infectious, rheumatologic, and hematologic-oncologic etiologies, although the latter would be less likely. While the patient’s mother reports decreased oral intake, the fact that the patient is alert and appears hydrated is encouraging, suggesting time to observe and see if other symptoms present that may assist in elucidating the cause. The history of increased sleepiness warrants further investigation of meningeal signs, which would point to a central nervous system infection.
While streptococcal infection is possible, it would be uncommon at this age. The patient would have a higher fever and focal infection, and the rash does not appear consistent unless it was described as “sandpaper” in feel and appearance. A negative rapid strep test, while not sensitive, further supports this impression. A low-grade fever and rash would be consistent with a viral syndrome and, given the conjunctival injection, adenovirus, cytomegalovirus, rhinovirus, and Epstein Barr virus (EBV) are possibilities. Without ocular discharge, bacterial conjunctivitis would be unlikely. Another consideration would be Kawasaki disease, though it would be too early to diagnose this condition since at least 5 days of fever are required. Next steps include a detailed physical examination, looking for other focal signs such as swelling or desquamation of hands and feet, lymphadenopathy, strawberry tongue, and mucositis. Rather than empirically starting antibiotics, it would be more reasonable to observe her with close outpatient follow-up. The patient’s family should be instructed to monitor for additional and/or worsening symptoms, further decreased oral intake, signs of dehydration, or changes in alertness.
At home, the patient completed 5 doses of amoxicillin but continued to be febrile (maximum, 102.6º F). She was taken to a local emergency department on day 6 of her illness. She had worsening conjunctival injection and progression of the rash, involving the palms and soles. She was noted to have edema of hands and feet without desquamation (Figure 1). She had no oral mucous membrane changes and no cervical lymphadenopathy. Cerebrospinal fluid (CSF) was unremarkable, and empiric treatment with intravenous (IV) ceftriaxone was initiated. Complete blood count was notable for a white blood cell (WBC) count of 18.9 k/μL (normal range, 6.0-17.0); hemoglobin, 7.6 g/dL (normal range, 10-13); mean corpuscular volume, 84 (normal range, 74-108); and platelet count, 105 k/μL (normal range, 150-400). A peripheral blood smear revealed no abnormal cells. C-reactive protein (CRP) was elevated at 6.5 mg/dL (normal range, 0.0-0.6). She was admitted for further management.
Infection remains on the differential diagnosis given the elevated WBC count. Since the patient has completed a reasonable course of antibiotics, a bacterial infection would be less likely but not fully excluded. The cultures obtained would be helpful if they become positive, but given that the patient has been on antibiotics, a negative culture may represent partial sterilization and would not rule out infection. A viral infection continues to be high on the differential, but one would expect that symptoms and fever would have begun to abate. The normal peripheral blood smear makes a hematologic disorder less likely.
Kawasaki disease has risen on the differential with 5 days of fever surpassing 102º F. She has 3 of 5 primary clinical criteria, including conjunctival injection, rash, and edema of the hands and feet. Desquamation of the peripheral extremities would not be expected until the convalescent phase. A diagnosis of typical Kawasaki disease would require a fourth criterion, either oral mucous membrane changes or cervical lymphadenopathy. She meets the criteria for atypical or incomplete Kawasaki disease, which requires only fever for at least 5 days, elevated CRP, and 2 or 3 additional clinical criteria. She also meets supplemental laboratory criteria with an elevated WBC count greater than 15,000/μL, normocytic and normochromic anemia for age, and elevated CRP. Urinalysis positive for pyuria or serum albumin less than 3 g/dL would lend further support but is not necessary. Fever of 7 or more days in a child less than 6 months old without other explanation would also increase the likelihood of incomplete Kawasaki disease. Admission to the hospital, treatment with IV immunoglobulin (IVIg), and echocardiography to evaluate for typical cardiac involvement (eg, aneurysms, coronary arteritis, and pericardial effusion) are the appropriate next steps.
The patient was diagnosed with atypical Kawasaki disease. A transthoracic echocardiogram was normal on admission. On day 7 of her illness, she was treated with 1 dose of IVIg at 2 g/kg and high-dose aspirin at 100 mg/kg per day in divided doses. Despite this treatment, she continued to be febrile and was given a second dose of IVIg on day 9. Her fevers persisted.
In Kawasaki disease, persistent fever is concerning for long-term sequelae, including coronary artery aneurysms. Continued treatment is reasonable. After 2 doses of IVIg with a cumulative dose of 4 g/kg, it is prudent to switch therapy to IV methylprednisolone 30mg/kg with repeated doses as needed for up to 3 days should her fevers persist.
Her blood culture was negative. EBV serology, enterovirus polymerase chain reaction, and viral cultures were negative. Chest radiography on day 9 was normal. Abdominal ultrasonography on day 10 showed hydrops of the gallbladder.
The patient was started on IV corticosteroids on day 11 with resolution of her fevers and improvement in her rash. A repeat echocardiogram revealed new findings of dilated left main, left anterior descending, and right coronary arteries. On day 13, a steroid wean was attempted because she had remained afebrile for more than 48 hours, but the wean was halted due to recurrence of fevers and rash. Her high-dose aspirin was reduced to 81 mg PO daily on day 14, and she was started on enoxaparin injections.
It is unusual for Kawasaki disease not to respond to 2 doses of IVIg, followed by corticosteroids. As such, the differential diagnosis must be revisited. The findings of coronary artery dilation, prolonged fever, and rash corroborate the diagnosis of Kawasaki disease, although this could be an atypical presentation of another vasculitis. Systemic onset juvenile idiopathic arthritis usually affects children at 2 to 5 years old and is, therefore, less likely. Henoch-Schönlein purpura manifests with a rash but is often associated with diarrhea. There does not appear to be objective evidence of polyarteritis nodosa, although biopsy or angiography would be required to make this diagnosis. Hydrops of the gallbladder is an over-distention of the organ filled with watery or mucoid content. While hydrops can be noninflammatory and seen in gallstone disease, it can also occur in vasculitides. Despite the reassuring serologies, false negative results are possible. Thus, these viral infections are not eliminated, but they are less likely. Given the echocardiogram findings and continued concern for atypical Kawasaki disease, high-dose aspirin should be continued. It is reasonable to consider rheumatology consultation for assessment and recommendations as to length of steroid treatment and/or alternative interventions.
Pediatric cardiology was consulted. Repeat echocardiogram on day 16 showed an increase in the size of her coronary artery aneurysms, and her fevers persisted. Computed tomography scan of the abdomen and pelvis with contrast, obtained to further evaluate for a source of infection, was unremarkable.
The patient was transferred to a tertiary care institution on day 19, at which time she remained on aspirin, enoxaparin, and oral corticosteroids. On arrival, her temperature was 101.3º F, heart rate 225 beats per minute, and respiratory rate 57 breaths per minute. She was fussy with bilateral conjunctivitis and a maculopapular rash involving palms, soles, and right infraorbital region. Laboratory studies were significant for a WBC count of 30.3 k/μL; hemoglobin, 10.9 g/dL; platelets, 106 k/μL; and CRP, 8.3 mg/dL.
Pediatric rheumatology was consulted on day 20. The patient was treated with 3 days IV pulse-dose methylprednisolone at 30 mg/kg daily. Her fevers resolved, although her CRP level remained elevated. She was treated with 1 dose of infliximab 10 mg/kg IV on day 24, followed by 1 dose of anakinra 15 mg subcutaneously on day 27 due to persistently elevated CRP.
The symptoms and diagnostic evaluation remain most consistent with atypical Kawasaki disease. Her tachycardia and tachypnea are likely driven by her fever and fussiness, and should be followed closely. The elevated WBC is likely a consequence of the steroids and demargination of neutrophils. The elevated and increasing CRP is a marker of acute inflammation. The adage “treat the patient, not the numbers” comes to mind, because it is reassuring that the patient’s overall clinical picture seems to be improving with resolution of her fevers. However, further discussion with the pediatric rheumatology consultant is prudent, specifically regarding the significance of the persistently elevated CRP, refinement of the differential diagnosis including the potential for other vasculitides and appropriate evaluation of such, as well as recommendations for further treatment.
The patient was noted to have ongoing fevers. Based on reports of success with cyclophosphamide in refractory Kawasaki disease, she was treated with 2 doses at 60 mg IV per dose starting on day 28. Her CRP level decreased. Cardiology and rheumatology consultants recommended magnetic resonance imaging/magnetic resonance angiography of the chest, abdomen, and pelvis with and without contrast. These studies revealed dilation of the axillary and brachial arteries (Figure 2).
The response to cyclophosphamide confirms an autoimmune/inflammatory process. The imaging results and pattern are most consistent with either Kawasaki disease or polyarteritis nodosa. Therefore, rheumatology’s input will be invaluable with regard to which diagnosis is most likely, additional diagnostic testing, and appropriate medical regimen and follow-up plans.
Systemic extracoronary vascular inflammation on imaging and the refractory nature of the patient’s disease process, despite appropriate treatment for Kawasaki disease, led to the diagnosis of childhood polyarteritis nodosa (PAN). The patient was discharged home and closely followed in rheumatology clinic. Her most recent outpatient visit 1 year after the initial onset of her illness showed no further fevers or rashes, normal inflammatory markers, and stabilization of her coronary aneurysms on daily maintenance azathioprine.
DISCUSSION
Fever with an accompanying rash is a common issue in children. The extensive differential diagnosis includes infectious diseases, rheumatologic disorders, and medication reactions (Table 1). A thorough history and physical examination are essential in guiding the physician toward the proper diagnosis and management. Important information includes patient age, season, associated symptoms, exposure to sick contacts, travel history, host immune status, and immunization history. Fever duration and pattern must be elicited, as should features of the rash, including temporal relationship to the fever, distribution, progression, and morphology.1
When unexplained fever persists for 5 days or more in the pediatric patient, the diagnosis of KD must be suspected. KD is an acute, febrile, primary systemic vasculitis affecting small- and medium-sized vessels, with a predilection for coronary arteries.2 KD affects younger children, with approximately 85% of cases occurring in children under 5 years old. KD has a higher incidence in Asian populations, suggesting a possible genetic predisposition.3 The etiology of KD is not well understood, but infection and immune dysregulation have been proposed as contributing factors. KD is the leading cause of acquired heart disease in developed countries.2
The diagnosis of KD is made clinically (Table 2). Atypical KD is considered in patients with at least 5 days of fever but only 2 or 3 clinical criteria. Supportive laboratory findings include elevated inflammatory markers, anemia, neutrophilia, abnormal plasma lipids, low albumin, sterile pyuria, CSF pleocytosis, and elevated serum transaminases. Two-dimensional echocardiography should be performed in all children with definite or suspected KD at the time of diagnosis, 1 to 2 weeks later, and 6 weeks following discharge for evaluation of the coronary arteries, left ventricular function, and valve function. The American Heart Association recommends follow-up echocardiography at 1 year in children without coronary vessel involvement.4
Treatment is aimed at minimizing inflammation and coronary artery involvement, and should be initiated promptly.5 Therapy includes a single infusion of high-dose IVIg and aspirin;6,7 the latter is initially provided at high anti-inflammatory doses, followed by lower antithrombotic doses once fever and laboratory markers have resolved.2 Aspirin can be discontinued if there is no evidence of coronary involvement at the 6-week follow-up echocardiogram.5 A second dose of IVIg is given within 48 hours for refractory cases, defined as persistent fever following the first dose of IVIg.4 Fifteen percent of children have refractory illness, and refractory KD is associated with a higher risk of coronary artery lesions.5 Additional agents that suppress immune activation and cytokine secretion contributing to KD pathogenesis have been studied. Corticosteroids inhibit phospholipase A, an enzyme required for production of inflammatory markers.8 Infliximab, a tumor necrosis factor-alpha inhibitor, has been shown to reduce duration of fever and length of hospital stay.8,9 Anakinra, an interleukin-1 receptor antagonist, has been shown to decrease fever duration and prevent progression of vascular injury in cases of refractory KD.10 There is, however, a lack of sufficient evidence and consensus on best practice.8-10
If inflammation, evidenced by fever, elevated inflammatory markers (such as erythrocyte sedimentation rate, CRP), or vessel involvement on imaging, persists or worsens despite standard therapy, physicians should seek alternative diagnoses. This patient’s extracoronary vascular inflammation and favorable response only to cyclophosphamide led to the diagnosis of systemic PAN. Like KD, PAN is a multi-system vasculitis affecting small- and medium-sized vessels. Unlike KD, PAN is rarely seen in children.11 Historically, PAN was thought to represent an extreme fatal end of the KD spectrum. Today, PAN is accepted as a separate entity. Clinical features and histological findings often overlap with KD, creating a diagnostic dilemma for providers.12
At the onset of illness, clinical features of systemic PAN may include recurrent fever, weight loss, and myalgia, with gradual progression to multi-organ system involvement. Laboratory assessment reveals elevated inflammatory markers and leukocytosis. Thrombocytosis, anemia, proteinuria, and hematuria may be present. A positive antineutrophil cytoplasmic antibody is rare in PAN and should raise suspicion for a microscopic polyangiitis, which is distinguished from PAN by small vessel involvement only. When compared to KD, cardiac vessel involvement in PAN is more variable.11 Diagnostic criteria for childhood PAN are listed in Table 2.13
Treatment of PAN is aimed at inducing remission with high-dose steroids and cyclophosphamide. Maintenance of remission is achieved using low-dose steroids and azathioprine.11 Total duration of treatment averages 2 to 3 years, with a minimum of 18 months.14 Plasma exchange has been used in severe, life-threatening cases.11 Prognosis for children with PAN is more favorable compared to adults with PAN, in whom the mortality rate is as high as 20% to 30%, even with aggressive treatment. In 1 multicenter study of childhood and adolescent PAN, overall mortality was 1.1%.15
This patient initially presented with findings consistent with KD. As her inflammatory markers remained elevated and fevers persisted, her physicians appropriately reconsidered the etiology of her symptoms, thereby “getting warmer” in the search for the correct diagnosis of systemic PAN, a rare disease and a separate entity from KD. Recognizing the overlapping and distinct clinical features of each entity can promote more timely and appropriate selection of therapy, thereby minimizing clinical manifestations and complications associated with each vasculitis.
KEY TEACHING POINTS
- KD and childhood PAN are disseminated vasculitides affecting small- and medium-sized vessels. Although they are distinct entities, KD and PAN exhibit overlapping clinical and pathological features that make appropriate diagnosis and treatment challenging.
- In cases of refractory KD, alternative diagnoses should be considered.
- Recognizing the individual features of both entities is imperative because treatment differs: KD is treated with high-dose aspirin and IVIg; corticosteroids and immunosuppressive agents are used to treat PAN.
Disclosure
Nothing to report.
1. McKinnon HD Jr, Howard T. Evaluating the febrile patient with a rash. Am Fam Physician. 2000;62:804-816. PubMed
2. Dimitriades V, Brown AG, Gedalia A. Kawasaki disease: pathophysiology, clinical manifestations, and management. Curr Rheumatol Rep. 2014;16:423. PubMed
3. Callinan L, Holman RC, Vugia DJ, Schonberger LB, Belay ED. Kawasaki disease hospitalization rate among children younger than 5 years of age in California, 2003-2010. Pediatr Infect Dis J. 2014;33:781-783. PubMed
4. Newburger JW, Takahashi M, Gerber MA, Gewirtz MH, Tani LY, Burns JC, et al. Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association; American Academy of Pediatrics. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747-2771. PubMed
5. Son M, Newburger JW. Kawasaki disease. Pediatr Rev. 2013;34:151-61. PubMed
6. Newberger JW, Takahasi M, Beiser AS, et al. A single intravenous infusion of gammaglobulin as compared with four infusions in treatment of acute Kawasaki syndrome. N Engl J Med. 1991;324:1633-1639. PubMed
7. Dajani AS, Taubert KA, Gerber MA, et al. Diagnosis and therapy of Kawasaki disease in children. Circulation. 1993;87:1776-1780. PubMed
8. Saneeymehri S, Baker K, So TY. Overview of pharmacological treatment options for pediatric patients with refractory Kawasaki disease. J Pediatr Pharmacol Ther. 2015;20:163-177. PubMed
9. Brogan R, Eleftheriou D, Gnanapragasam J, Klein NJ, Brogan PA. Infliximab for the treatment of intravenous immunoglobulin resistant Kawasaki disease complicated by coronary artery aneurysms: a case report. Pediatr Rheumatol Online J. 2009;7:3. PubMed
10. Cohen S, Tacke CE, Straver B, Meijer N, Kuipers IM, Kuijpers TW. A child with severe relapsing Kawasaki disease rescued by IL-1 receptor blockade and extracorporeal membrane oxygenation. Ann Rheum Dis. 2012;71:2059-2061. PubMed
11. Kelly A, Tizard E. Vasculitis in children. Paediatrics and Child Health. 2010;20:65-72.
12. Yamazaki-Nakashimada MA, Espinosa-Lopez M, Hernandez-Bautista V, Espinosa-Padilla S, Espinosa-Rosales F. Catastrophic Kawasaki disease or juvenile polyarteritis nodosa? Semin Arthritis Rheum. 2006;35:349-354. PubMed
13. Ozen S, Pistorio A, Iusan SM, et al. EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria. Ann Rheum Dis. 2010;69:798-806. PubMed
14. Eleftheriou D, Brogan PA. Vasculitis in children. Best Pract Res Clin Rheumatol. 2009;23:309-323. PubMed
15. Ozen S, Anton J, Arisoy N, et al. Juvenile polyarteritis: results of a multicenter survey of 110 children. J Pediatr. 2004;145:517-522. PubMed
1. McKinnon HD Jr, Howard T. Evaluating the febrile patient with a rash. Am Fam Physician. 2000;62:804-816. PubMed
2. Dimitriades V, Brown AG, Gedalia A. Kawasaki disease: pathophysiology, clinical manifestations, and management. Curr Rheumatol Rep. 2014;16:423. PubMed
3. Callinan L, Holman RC, Vugia DJ, Schonberger LB, Belay ED. Kawasaki disease hospitalization rate among children younger than 5 years of age in California, 2003-2010. Pediatr Infect Dis J. 2014;33:781-783. PubMed
4. Newburger JW, Takahashi M, Gerber MA, Gewirtz MH, Tani LY, Burns JC, et al. Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association; American Academy of Pediatrics. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747-2771. PubMed
5. Son M, Newburger JW. Kawasaki disease. Pediatr Rev. 2013;34:151-61. PubMed
6. Newberger JW, Takahasi M, Beiser AS, et al. A single intravenous infusion of gammaglobulin as compared with four infusions in treatment of acute Kawasaki syndrome. N Engl J Med. 1991;324:1633-1639. PubMed
7. Dajani AS, Taubert KA, Gerber MA, et al. Diagnosis and therapy of Kawasaki disease in children. Circulation. 1993;87:1776-1780. PubMed
8. Saneeymehri S, Baker K, So TY. Overview of pharmacological treatment options for pediatric patients with refractory Kawasaki disease. J Pediatr Pharmacol Ther. 2015;20:163-177. PubMed
9. Brogan R, Eleftheriou D, Gnanapragasam J, Klein NJ, Brogan PA. Infliximab for the treatment of intravenous immunoglobulin resistant Kawasaki disease complicated by coronary artery aneurysms: a case report. Pediatr Rheumatol Online J. 2009;7:3. PubMed
10. Cohen S, Tacke CE, Straver B, Meijer N, Kuipers IM, Kuijpers TW. A child with severe relapsing Kawasaki disease rescued by IL-1 receptor blockade and extracorporeal membrane oxygenation. Ann Rheum Dis. 2012;71:2059-2061. PubMed
11. Kelly A, Tizard E. Vasculitis in children. Paediatrics and Child Health. 2010;20:65-72.
12. Yamazaki-Nakashimada MA, Espinosa-Lopez M, Hernandez-Bautista V, Espinosa-Padilla S, Espinosa-Rosales F. Catastrophic Kawasaki disease or juvenile polyarteritis nodosa? Semin Arthritis Rheum. 2006;35:349-354. PubMed
13. Ozen S, Pistorio A, Iusan SM, et al. EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria. Ann Rheum Dis. 2010;69:798-806. PubMed
14. Eleftheriou D, Brogan PA. Vasculitis in children. Best Pract Res Clin Rheumatol. 2009;23:309-323. PubMed
15. Ozen S, Anton J, Arisoy N, et al. Juvenile polyarteritis: results of a multicenter survey of 110 children. J Pediatr. 2004;145:517-522. PubMed
© 2017 Society of Hospital Medicine
A Problem of Capacity
For a number of years, those challenged with improving discharge transitions and preventing readmissions have suggested more—more case managers, more checklists and systems, more discharge pharmacists; and better—better communication, better medication reconciliation, better discharge documentation, better follow-up. In a study by Chan Carusone et al.,1 high-need, high-complexity patients receiving treatment at Casey House, a specialized urban hospital providing inpatient and community programs, were afforded a full complement of discharge planning and posthospitalization services. Despite these services, the patients achieved little success in maintaining their health and following their discharge plans after hospitalization.
This longitudinal qualitative study detailing the lived experience of discharge extends our knowledge of challenges faced by patients during the posthospital transition,2 and further elucidates the differences between patients’ expectations and assessments of their resources and goals, and their actual abilities and priorities on discharge. Despite substantial assistance, including housing, food assistance, and case management, Chan Carusone et al. found that the exigencies of day-to-day existence exceeded the patients’ capacities to sustain themselves outside the hospital. This failure implies a question: If the interventions alluded to in this study were not enough, then how much more, and how much better, is needed?
Attention to this question of how to best serve high-need patients continues to increase,3 and success in intervening to improve care transitions for this population is limited,4 in part because providing more care and more coordination requires more resources. Observing the challenges that remain for patients treated in the highly-resourced setting that is Casey House, the authors propose a previously described theoretical construct, minimally disruptive medicine (MDM),5 as a framework to guide patients and providers in creating a discharge plan that relies on the patient’s capacity to integrate disease self-management into his or her daily circumstances. MDM hinges on the concept of balancing workload and capacity: the burden of managing disease with the resources and abilities to do so. On first consideration, this seems an attractive approach to operationalizing patient-centered care by tailoring a discharge plan to a patient’s goals and capacities. On closer examination, however, MDM, applied to a single transition episode, raises some important concerns.
As Chan Carusone et al. describe, patients may poorly judge their future resources and capacity when making decisions in the hospital setting. Likewise, physicians and other team members may lack insight, perspective, and detailed knowledge of resources and barriers in the outpatient setting. From their vantage point, they may not see the fragile contingencies of the discharge plan that is reflected in the patients’ spoken words. At any moment, a well-meant, seemingly well-crafted discharge plan could fall apart.
Within the walls of the hospital, we tend to perform what might be termed maximally disruptive medicine—the treatments provided are exactly those that can’t be delivered in a nonhospital setting. For many patients, these interventions are not curative, but rather stabilizing;6 we assuage chronic conditions that had become exacerbated by new illness, disease progression, or conditions outside the hospital. To return the patient to his or her home situation, especially one that is under-resourced, with minimized workload can feel counterproductive and demoralizing at best. What prevents one from worrying that, where capacity can’t be improved, planning for MDM is, in essence, planning for minimal care?
Viewed in the broader context of a life course health development framework,7 which integrates biological, psychological, cultural, and historical experience to explain the development of health trajectories over an individual’s lifetime, a minimally disruptive approach might be viewed as amplifying disparities. The patients contributing to the study by Chan Carusone et al. may have arrived in their respective situations through a life course marked by poverty, violence, inadequate housing, poor nutrition, discrimination, and other disadvantages that may have resulted from accident, malfeasance, or choice. Their limited personal capacity and the ongoing chaos that is reflected in many of their comments requires that discharge planning uses imagination and dialogue, with careful, compassionate listening by providers, and close partnering and decision-making by patient and providers. Approaches to building the capacity for such compassion, as well as structural interventions to provide care that is necessary and just for these most vulnerable patients by considering their experiences and beliefs,8 remain to be articulated.
In a sense, the narrative unfolded by Chan Carusone et al. appropriately emphasizes that care transitions contain both complex problems and “wicked” problems.9 While aspects of transitions are complex and can be reasonably addressed with complex solutions, these same complex solutions are inadequate to mitigate the seemingly intractable socioeconomic challenges that drive hospital dependence for many high-need patients. Addressing these likely requires a reexamination of what we expect from hospitals, what systems we are able to design and are willing to support to keep people from returning to them, and what it means that for some people returning is the best, and sometimes only, thing to do.
As we continue to seek new models for healthcare in high-need, high-risk populations, we may do well to focus further longitudinal qualitative study on building a deep understanding of when and how patients achieve success following discharge. What characterizes patients, caregivers, service networks, and communities in healthcare settings with the highest rates of effective transitions? Maintaining equilibrium outside an institutional setting is convoluted, time-consuming, nuanced, and taxing; that those who have not experienced doing so as a patient or caregiver might struggle to help others should not surprise us. The concepts of capacity and workload lend themselves to structuring discovery of the resources that patients, not providers and policy-makers, have found through their lived experience to be most crucial to their enduring well-being. Learning from these experiences may shift the balance by increasing our own capacity to understand what constitutes success.
Disclosures
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. The authors report no conflicts of interest.
References
1. Chan Carusone S, O’Leary B, McWatt S, Stewart S, Craig S, Brennan D. The lived experience of the hospital discharge “plan”: a longitudinal qualitative study of complex patients. J Hosp Med. 2017;12(1):5-10. PubMed
2. Kangovi S, Barg FK, Carter T, et al. Challenges faced by patients with low socioeconomic status during the post-hospital transition. J Gen Intern Med. 2014;29:283-289. PubMed
3. Blumenthal D, Chernof B, Fulmer T, Lumpkin J, Selberg J. Caring for high-need, high-cost patients - an urgent priority. N Engl J Med. 2016;375:909-911. PubMed
4. Powers BW, Milstein A, Jain SH. Delivery models for high-risk older patients: back to the future? JAMA. 2016;315:23-24. PubMed
5. Abu Dabrh AM, Gallacher K, Boehmer KR, Hargraves IG, Mair FS. Minimally disruptive medicine: the evidence and conceptual progress supporting a new era of healthcare. J R Coll Physicians Edinb. 2015;45:114-117. PubMed
6. Pannick S, Wachter RM, Vincent C, Sevdalis N. Rethinking medical ward quality. BMJ. 2016;355:i5417. PubMed
7. Kressin NR, Chapman SE, Magnani JW. A tale of two patients: patient-centered approaches to adherence as a gateway to reducing disparities. Circulation. 2016;133:2583-2592. PubMed
8. Thiel de Bocanegra H, Gany F. Good provider, good patient: changing behaviors to eliminate disparities in healthcare. Am J Manag Care. 2004;10:SP20-28. PubMed
9. Churchman CW. Wicked problems. Manage Sci. 1967;14(4):B141-B142.
For a number of years, those challenged with improving discharge transitions and preventing readmissions have suggested more—more case managers, more checklists and systems, more discharge pharmacists; and better—better communication, better medication reconciliation, better discharge documentation, better follow-up. In a study by Chan Carusone et al.,1 high-need, high-complexity patients receiving treatment at Casey House, a specialized urban hospital providing inpatient and community programs, were afforded a full complement of discharge planning and posthospitalization services. Despite these services, the patients achieved little success in maintaining their health and following their discharge plans after hospitalization.
This longitudinal qualitative study detailing the lived experience of discharge extends our knowledge of challenges faced by patients during the posthospital transition,2 and further elucidates the differences between patients’ expectations and assessments of their resources and goals, and their actual abilities and priorities on discharge. Despite substantial assistance, including housing, food assistance, and case management, Chan Carusone et al. found that the exigencies of day-to-day existence exceeded the patients’ capacities to sustain themselves outside the hospital. This failure implies a question: If the interventions alluded to in this study were not enough, then how much more, and how much better, is needed?
Attention to this question of how to best serve high-need patients continues to increase,3 and success in intervening to improve care transitions for this population is limited,4 in part because providing more care and more coordination requires more resources. Observing the challenges that remain for patients treated in the highly-resourced setting that is Casey House, the authors propose a previously described theoretical construct, minimally disruptive medicine (MDM),5 as a framework to guide patients and providers in creating a discharge plan that relies on the patient’s capacity to integrate disease self-management into his or her daily circumstances. MDM hinges on the concept of balancing workload and capacity: the burden of managing disease with the resources and abilities to do so. On first consideration, this seems an attractive approach to operationalizing patient-centered care by tailoring a discharge plan to a patient’s goals and capacities. On closer examination, however, MDM, applied to a single transition episode, raises some important concerns.
As Chan Carusone et al. describe, patients may poorly judge their future resources and capacity when making decisions in the hospital setting. Likewise, physicians and other team members may lack insight, perspective, and detailed knowledge of resources and barriers in the outpatient setting. From their vantage point, they may not see the fragile contingencies of the discharge plan that is reflected in the patients’ spoken words. At any moment, a well-meant, seemingly well-crafted discharge plan could fall apart.
Within the walls of the hospital, we tend to perform what might be termed maximally disruptive medicine—the treatments provided are exactly those that can’t be delivered in a nonhospital setting. For many patients, these interventions are not curative, but rather stabilizing;6 we assuage chronic conditions that had become exacerbated by new illness, disease progression, or conditions outside the hospital. To return the patient to his or her home situation, especially one that is under-resourced, with minimized workload can feel counterproductive and demoralizing at best. What prevents one from worrying that, where capacity can’t be improved, planning for MDM is, in essence, planning for minimal care?
Viewed in the broader context of a life course health development framework,7 which integrates biological, psychological, cultural, and historical experience to explain the development of health trajectories over an individual’s lifetime, a minimally disruptive approach might be viewed as amplifying disparities. The patients contributing to the study by Chan Carusone et al. may have arrived in their respective situations through a life course marked by poverty, violence, inadequate housing, poor nutrition, discrimination, and other disadvantages that may have resulted from accident, malfeasance, or choice. Their limited personal capacity and the ongoing chaos that is reflected in many of their comments requires that discharge planning uses imagination and dialogue, with careful, compassionate listening by providers, and close partnering and decision-making by patient and providers. Approaches to building the capacity for such compassion, as well as structural interventions to provide care that is necessary and just for these most vulnerable patients by considering their experiences and beliefs,8 remain to be articulated.
In a sense, the narrative unfolded by Chan Carusone et al. appropriately emphasizes that care transitions contain both complex problems and “wicked” problems.9 While aspects of transitions are complex and can be reasonably addressed with complex solutions, these same complex solutions are inadequate to mitigate the seemingly intractable socioeconomic challenges that drive hospital dependence for many high-need patients. Addressing these likely requires a reexamination of what we expect from hospitals, what systems we are able to design and are willing to support to keep people from returning to them, and what it means that for some people returning is the best, and sometimes only, thing to do.
As we continue to seek new models for healthcare in high-need, high-risk populations, we may do well to focus further longitudinal qualitative study on building a deep understanding of when and how patients achieve success following discharge. What characterizes patients, caregivers, service networks, and communities in healthcare settings with the highest rates of effective transitions? Maintaining equilibrium outside an institutional setting is convoluted, time-consuming, nuanced, and taxing; that those who have not experienced doing so as a patient or caregiver might struggle to help others should not surprise us. The concepts of capacity and workload lend themselves to structuring discovery of the resources that patients, not providers and policy-makers, have found through their lived experience to be most crucial to their enduring well-being. Learning from these experiences may shift the balance by increasing our own capacity to understand what constitutes success.
Disclosures
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. The authors report no conflicts of interest.
For a number of years, those challenged with improving discharge transitions and preventing readmissions have suggested more—more case managers, more checklists and systems, more discharge pharmacists; and better—better communication, better medication reconciliation, better discharge documentation, better follow-up. In a study by Chan Carusone et al.,1 high-need, high-complexity patients receiving treatment at Casey House, a specialized urban hospital providing inpatient and community programs, were afforded a full complement of discharge planning and posthospitalization services. Despite these services, the patients achieved little success in maintaining their health and following their discharge plans after hospitalization.
This longitudinal qualitative study detailing the lived experience of discharge extends our knowledge of challenges faced by patients during the posthospital transition,2 and further elucidates the differences between patients’ expectations and assessments of their resources and goals, and their actual abilities and priorities on discharge. Despite substantial assistance, including housing, food assistance, and case management, Chan Carusone et al. found that the exigencies of day-to-day existence exceeded the patients’ capacities to sustain themselves outside the hospital. This failure implies a question: If the interventions alluded to in this study were not enough, then how much more, and how much better, is needed?
Attention to this question of how to best serve high-need patients continues to increase,3 and success in intervening to improve care transitions for this population is limited,4 in part because providing more care and more coordination requires more resources. Observing the challenges that remain for patients treated in the highly-resourced setting that is Casey House, the authors propose a previously described theoretical construct, minimally disruptive medicine (MDM),5 as a framework to guide patients and providers in creating a discharge plan that relies on the patient’s capacity to integrate disease self-management into his or her daily circumstances. MDM hinges on the concept of balancing workload and capacity: the burden of managing disease with the resources and abilities to do so. On first consideration, this seems an attractive approach to operationalizing patient-centered care by tailoring a discharge plan to a patient’s goals and capacities. On closer examination, however, MDM, applied to a single transition episode, raises some important concerns.
As Chan Carusone et al. describe, patients may poorly judge their future resources and capacity when making decisions in the hospital setting. Likewise, physicians and other team members may lack insight, perspective, and detailed knowledge of resources and barriers in the outpatient setting. From their vantage point, they may not see the fragile contingencies of the discharge plan that is reflected in the patients’ spoken words. At any moment, a well-meant, seemingly well-crafted discharge plan could fall apart.
Within the walls of the hospital, we tend to perform what might be termed maximally disruptive medicine—the treatments provided are exactly those that can’t be delivered in a nonhospital setting. For many patients, these interventions are not curative, but rather stabilizing;6 we assuage chronic conditions that had become exacerbated by new illness, disease progression, or conditions outside the hospital. To return the patient to his or her home situation, especially one that is under-resourced, with minimized workload can feel counterproductive and demoralizing at best. What prevents one from worrying that, where capacity can’t be improved, planning for MDM is, in essence, planning for minimal care?
Viewed in the broader context of a life course health development framework,7 which integrates biological, psychological, cultural, and historical experience to explain the development of health trajectories over an individual’s lifetime, a minimally disruptive approach might be viewed as amplifying disparities. The patients contributing to the study by Chan Carusone et al. may have arrived in their respective situations through a life course marked by poverty, violence, inadequate housing, poor nutrition, discrimination, and other disadvantages that may have resulted from accident, malfeasance, or choice. Their limited personal capacity and the ongoing chaos that is reflected in many of their comments requires that discharge planning uses imagination and dialogue, with careful, compassionate listening by providers, and close partnering and decision-making by patient and providers. Approaches to building the capacity for such compassion, as well as structural interventions to provide care that is necessary and just for these most vulnerable patients by considering their experiences and beliefs,8 remain to be articulated.
In a sense, the narrative unfolded by Chan Carusone et al. appropriately emphasizes that care transitions contain both complex problems and “wicked” problems.9 While aspects of transitions are complex and can be reasonably addressed with complex solutions, these same complex solutions are inadequate to mitigate the seemingly intractable socioeconomic challenges that drive hospital dependence for many high-need patients. Addressing these likely requires a reexamination of what we expect from hospitals, what systems we are able to design and are willing to support to keep people from returning to them, and what it means that for some people returning is the best, and sometimes only, thing to do.
As we continue to seek new models for healthcare in high-need, high-risk populations, we may do well to focus further longitudinal qualitative study on building a deep understanding of when and how patients achieve success following discharge. What characterizes patients, caregivers, service networks, and communities in healthcare settings with the highest rates of effective transitions? Maintaining equilibrium outside an institutional setting is convoluted, time-consuming, nuanced, and taxing; that those who have not experienced doing so as a patient or caregiver might struggle to help others should not surprise us. The concepts of capacity and workload lend themselves to structuring discovery of the resources that patients, not providers and policy-makers, have found through their lived experience to be most crucial to their enduring well-being. Learning from these experiences may shift the balance by increasing our own capacity to understand what constitutes success.
Disclosures
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. The authors report no conflicts of interest.
References
1. Chan Carusone S, O’Leary B, McWatt S, Stewart S, Craig S, Brennan D. The lived experience of the hospital discharge “plan”: a longitudinal qualitative study of complex patients. J Hosp Med. 2017;12(1):5-10. PubMed
2. Kangovi S, Barg FK, Carter T, et al. Challenges faced by patients with low socioeconomic status during the post-hospital transition. J Gen Intern Med. 2014;29:283-289. PubMed
3. Blumenthal D, Chernof B, Fulmer T, Lumpkin J, Selberg J. Caring for high-need, high-cost patients - an urgent priority. N Engl J Med. 2016;375:909-911. PubMed
4. Powers BW, Milstein A, Jain SH. Delivery models for high-risk older patients: back to the future? JAMA. 2016;315:23-24. PubMed
5. Abu Dabrh AM, Gallacher K, Boehmer KR, Hargraves IG, Mair FS. Minimally disruptive medicine: the evidence and conceptual progress supporting a new era of healthcare. J R Coll Physicians Edinb. 2015;45:114-117. PubMed
6. Pannick S, Wachter RM, Vincent C, Sevdalis N. Rethinking medical ward quality. BMJ. 2016;355:i5417. PubMed
7. Kressin NR, Chapman SE, Magnani JW. A tale of two patients: patient-centered approaches to adherence as a gateway to reducing disparities. Circulation. 2016;133:2583-2592. PubMed
8. Thiel de Bocanegra H, Gany F. Good provider, good patient: changing behaviors to eliminate disparities in healthcare. Am J Manag Care. 2004;10:SP20-28. PubMed
9. Churchman CW. Wicked problems. Manage Sci. 1967;14(4):B141-B142.
References
1. Chan Carusone S, O’Leary B, McWatt S, Stewart S, Craig S, Brennan D. The lived experience of the hospital discharge “plan”: a longitudinal qualitative study of complex patients. J Hosp Med. 2017;12(1):5-10. PubMed
2. Kangovi S, Barg FK, Carter T, et al. Challenges faced by patients with low socioeconomic status during the post-hospital transition. J Gen Intern Med. 2014;29:283-289. PubMed
3. Blumenthal D, Chernof B, Fulmer T, Lumpkin J, Selberg J. Caring for high-need, high-cost patients - an urgent priority. N Engl J Med. 2016;375:909-911. PubMed
4. Powers BW, Milstein A, Jain SH. Delivery models for high-risk older patients: back to the future? JAMA. 2016;315:23-24. PubMed
5. Abu Dabrh AM, Gallacher K, Boehmer KR, Hargraves IG, Mair FS. Minimally disruptive medicine: the evidence and conceptual progress supporting a new era of healthcare. J R Coll Physicians Edinb. 2015;45:114-117. PubMed
6. Pannick S, Wachter RM, Vincent C, Sevdalis N. Rethinking medical ward quality. BMJ. 2016;355:i5417. PubMed
7. Kressin NR, Chapman SE, Magnani JW. A tale of two patients: patient-centered approaches to adherence as a gateway to reducing disparities. Circulation. 2016;133:2583-2592. PubMed
8. Thiel de Bocanegra H, Gany F. Good provider, good patient: changing behaviors to eliminate disparities in healthcare. Am J Manag Care. 2004;10:SP20-28. PubMed
9. Churchman CW. Wicked problems. Manage Sci. 1967;14(4):B141-B142.
© 2017 Society of Hospital Medicine
Improving Quality in Against Medical Advice Discharges
Against Medical Advice (AMA) discharges, when a patient chooses to leave the hospital prior to a clinically specified and physician recommended endpoint, remain a healthcare quality problem. Patients who leave the hospital AMA challenge the healthcare professionals entrusted to care for them as well as the institutions that work to promote continuity and improved quality. AMA discharges account for up to 2% of all hospital discharges and, compared with conventional discharges, are associated with worse health and health services outcomes. Patients discharged AMA have higher rates of 30-day readmission, morbidity, and 30-day mortality.1,2 Additionally, the burden of worse health outcomes is disproportionate among disadvantaged patient populations. Patients with human immunodeficiency virus,3 substance use disorders,4 and psychiatric illness5 are more likely to be discharged AMA, as are patients with low socioeconomic status, without insurance, or with Medicaid insurance.
In this issue of the Journal of Hospital Medicine, Stearns and colleagues6 provide an important contribution to this area of medicine in need of more high quality empiric studies. The study reviewed all AMA discharges from a single year in an urban community hospital in order to assess provider perceptions and knowledge about AMA discharges. The study reconfirmed both the patient-level predictors of AMA discharges that have been demonstrated consistently (ie, male gender, younger age, Medicare or no insurance, and injection drug use) as well as the low rates of documentation of patient capacity, medication prescribed, and follow-up plans in AMA discharges.7
The authors’ investigation has also advanced the study of AMA discharges in two important directions. First, by characterizing patients with multiple AMA discharges, the authors focus on a more vulnerable population. These patients, who may have particular difficulty in consistently engaging in care, could help provide insight into the general phenomenon of AMA discharges. Second, the authors broadened their attention to include the study of nurses, a group of healthcare professionals who may play an important but not well recognized role in the AMA discharge process. In further characterizing nurses’ attitudes toward AMA discharges, medication prescriptions, and outpatient follow-up, the authors highlight nurses’ role in gathering critical patient information and promoting ethical practices in discharge planning. To better understand this dynamic and its potential role in mediating adverse health outcomes, further studies should also examine the attitudes of other central members of the treatment team (eg, pharmacists, social workers, etc.) who participate in discharge planning.
Inadequate documentation of AMA discharges remains a problem. In an attempt to address this, some institutions use AMA discharge forms to facilitate documentation of the informed consent process, the patient’s signed declination of care, medico-legal considerations, and the resulting treatment plan. Although systematic efforts to improve documentation should be encouraged, significant uncertainty about the optimal use of AMA discharge forms remains. Specifically, the use of a patient-signed AMA discharge form has not been demonstrated to advance patient care and may promote harm by stigmatizing patients8 and reducing the likelihood that they will pursue follow-up care.9 Furthermore, given that these forms may be written using institution-centered legalistic language or at an inappropriate reading level, this common hospital practice should be evaluated to assess whether patients comprehend and benefit from the forms, and how the forms influence healthcare decision making.10
Finally, the authors’ finding that 38% of nurses, 22% of physician trainees, and 6% of attendings believe patients discharged AMA lose the “right” to follow-up is noteworthy. The practice would suggest a significant lapse in understanding the professional obligation to acknowledge and communicate that the informed consent process is voluntary and patients have the right to decline recommended treatment without forfeiting future access to care. Harm reduction principles indicate that simply choosing to decline an episode of inpatient care does not make a patient ineligible for other medically indicated treatments and services. Previous studies have demonstrated that physicians may incorrectly inform patients that insurance will not pay for their care if they leave AMA, in order to persuade them to remain hospitalized.11 The current study suggests similar and potentially well-meaning but coercive attitudes about AMA discharge that can undermine a patient’s voluntary choice to accept medical care.
Stearns and colleagues6 rightly point to educational and policy interventions to improve the quality of care for patients discharged AMA. Additionally, setting patients’ expectations early in the hospitalization,12 empathically addressing their concerns,13 and sharing clinical decisions with patients by providing a medically reasonable range of clinical options rather than a single choice14 are practical bedside interventions that all clinicians can implement. System changes like developing clear policies and electronic medical records templates are particularly important, as they are more likely to lead to durable institutional change that is systematic, transparent, and fair. Moreover, research that expands the object of study beyond the physician-patient relationship could significantly improve outcomes in this vulnerable population of patients. Recent studies have begun to elucidate the deficiencies that may underlie communication failures with patients before they choose to leave AMA,15 how providers decide to designate a discharge as AMA,16 and how changing the structure and environment of care for patients who use injection drugs can reduce AMA discharges and improve health outcomes.17
AMA discharges are a persistent, complicated healthcare quality problem that defies an easy solution. Improving the quality of care for these patients will require building upon the empirical research base, providing enhanced education and guidance to healthcare professionals in the ethical and clinical management of AMA discharges, and making systems changes that promote enduring institutional change. We are moving in the right direction, but we have further to go.
Disclosures
The views expressed in this article are those of the author and do not necessarily reflect the position or policy of the US Department of Veterans Affairs or the National Center for Ethics in Health Care. The author has no conflicts of interest to disclose.
References
1. Alfandre DJ. “I’m going home”: discharges against medical advice. Mayo Clin Proc. 2009;84(3):255-260. PubMed
2. Southern WN, Nahvi S, Arnsten JH. Increased risk of mortality and readmission among patients discharged against medical advice. Am J Med. 2012;125(6):
594-602. PubMed
3. Anis AH, Sun H, Guh DP, Palepu A, Schechter MT, O’Shaughnessy MV. Leaving hospital against medical advice among HIV-positive patients. CMAJ. 2002;167(6):633-637. PubMed
4. Chan AC, Palepu A, Guh DP, et al. HIV-positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr. 2004;35(1):56-59. PubMed
5. Kuo CJ, Tsai SY, Liao YT, Lee WC, Sung XW, Chen CC. Psychiatric discharge against medical advice is a risk factor for suicide but not for other causes of death. J Clin Psychiatry. 2010;71(6):808-809. PubMed
6. Edwards J, Markert R, Bricker D. Discharge against medical advice: how often do we intervene? J Hosp Med. 2013;8(10):574-577. PubMed
7. Stearns CR, Bakamjian A, Sattar S, Ritterman Weintraub M. Discharges against medical advice at a county hospital: provider perceptions and practice. J Hosp Med. 2017;12(1):11-17. PubMed
8. Windish DM, Ratanawongsa N. Providers’ perceptions of relationships and professional roles when caring for patients who leave the hospital against medical advice. J Gen Intern Med. 2008;23(10):1698-1707. PubMed
9. Jerrard DA, Chasm RM. Patients leaving against medical advice (AMA) from the emergency department—disease prevalence and willingness to return. J Emerg Med. 2011;41(4):412-417. PubMed
10. Alfandre D. Reconsidering against medical advice discharges: embracing patient-centeredness to promote high quality care and a renewed research agenda.
J Gen Intern Med. 2013;28(12):1657-1662. PubMed
11. Schaefer GR, Matus H, Schumann JH, et al. Financial responsibility of hospitalized patients who left against medical advice: Medical urban legend? J Gen Intern Med. 2012;27(7):825-830. PubMed
12. Steinglass P, Grantham CE, Hertzman M. Predicting which patients will be discharged against medical advice: a pilot study. Am J Psychiatry. 1980;137(11):
1385-1389. PubMed
13. Clark MA, Abbott JT, Adyanthaya T. Ethics seminars: a best-practice approach to navigating the against-medical-advice discharge. Acad Emerg Med. 2014;21(9):1050-1057. PubMed
14. Alfandre D. Clinical recommendations in medical practice: a proposed framework to reduce bias and improve the quality of medical decisions. J Clin Ethics. 2016;27(1):21-27. PubMed
15. Lekas HM, Alfandre D, Gordon P, Harwood K, Yin MT. The role of patient-provider interactions: Using an accounts framework to explain hospital discharges against medical advice. Soc Sci Med. 2016;156:106-113. PubMed
16. Brenner J, Joslin J, Goulette A, Grant WD, Wojcik SM. Against medical advice: A survey of ED clinicians’ rationale for use. J Emerg Nurs. 2016;42(5):408-411. PubMed
17. McNeil R, Small W, Wood E, Kerr T. Hospitals as a ‘risk environment’: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66. PubMed
Against Medical Advice (AMA) discharges, when a patient chooses to leave the hospital prior to a clinically specified and physician recommended endpoint, remain a healthcare quality problem. Patients who leave the hospital AMA challenge the healthcare professionals entrusted to care for them as well as the institutions that work to promote continuity and improved quality. AMA discharges account for up to 2% of all hospital discharges and, compared with conventional discharges, are associated with worse health and health services outcomes. Patients discharged AMA have higher rates of 30-day readmission, morbidity, and 30-day mortality.1,2 Additionally, the burden of worse health outcomes is disproportionate among disadvantaged patient populations. Patients with human immunodeficiency virus,3 substance use disorders,4 and psychiatric illness5 are more likely to be discharged AMA, as are patients with low socioeconomic status, without insurance, or with Medicaid insurance.
In this issue of the Journal of Hospital Medicine, Stearns and colleagues6 provide an important contribution to this area of medicine in need of more high quality empiric studies. The study reviewed all AMA discharges from a single year in an urban community hospital in order to assess provider perceptions and knowledge about AMA discharges. The study reconfirmed both the patient-level predictors of AMA discharges that have been demonstrated consistently (ie, male gender, younger age, Medicare or no insurance, and injection drug use) as well as the low rates of documentation of patient capacity, medication prescribed, and follow-up plans in AMA discharges.7
The authors’ investigation has also advanced the study of AMA discharges in two important directions. First, by characterizing patients with multiple AMA discharges, the authors focus on a more vulnerable population. These patients, who may have particular difficulty in consistently engaging in care, could help provide insight into the general phenomenon of AMA discharges. Second, the authors broadened their attention to include the study of nurses, a group of healthcare professionals who may play an important but not well recognized role in the AMA discharge process. In further characterizing nurses’ attitudes toward AMA discharges, medication prescriptions, and outpatient follow-up, the authors highlight nurses’ role in gathering critical patient information and promoting ethical practices in discharge planning. To better understand this dynamic and its potential role in mediating adverse health outcomes, further studies should also examine the attitudes of other central members of the treatment team (eg, pharmacists, social workers, etc.) who participate in discharge planning.
Inadequate documentation of AMA discharges remains a problem. In an attempt to address this, some institutions use AMA discharge forms to facilitate documentation of the informed consent process, the patient’s signed declination of care, medico-legal considerations, and the resulting treatment plan. Although systematic efforts to improve documentation should be encouraged, significant uncertainty about the optimal use of AMA discharge forms remains. Specifically, the use of a patient-signed AMA discharge form has not been demonstrated to advance patient care and may promote harm by stigmatizing patients8 and reducing the likelihood that they will pursue follow-up care.9 Furthermore, given that these forms may be written using institution-centered legalistic language or at an inappropriate reading level, this common hospital practice should be evaluated to assess whether patients comprehend and benefit from the forms, and how the forms influence healthcare decision making.10
Finally, the authors’ finding that 38% of nurses, 22% of physician trainees, and 6% of attendings believe patients discharged AMA lose the “right” to follow-up is noteworthy. The practice would suggest a significant lapse in understanding the professional obligation to acknowledge and communicate that the informed consent process is voluntary and patients have the right to decline recommended treatment without forfeiting future access to care. Harm reduction principles indicate that simply choosing to decline an episode of inpatient care does not make a patient ineligible for other medically indicated treatments and services. Previous studies have demonstrated that physicians may incorrectly inform patients that insurance will not pay for their care if they leave AMA, in order to persuade them to remain hospitalized.11 The current study suggests similar and potentially well-meaning but coercive attitudes about AMA discharge that can undermine a patient’s voluntary choice to accept medical care.
Stearns and colleagues6 rightly point to educational and policy interventions to improve the quality of care for patients discharged AMA. Additionally, setting patients’ expectations early in the hospitalization,12 empathically addressing their concerns,13 and sharing clinical decisions with patients by providing a medically reasonable range of clinical options rather than a single choice14 are practical bedside interventions that all clinicians can implement. System changes like developing clear policies and electronic medical records templates are particularly important, as they are more likely to lead to durable institutional change that is systematic, transparent, and fair. Moreover, research that expands the object of study beyond the physician-patient relationship could significantly improve outcomes in this vulnerable population of patients. Recent studies have begun to elucidate the deficiencies that may underlie communication failures with patients before they choose to leave AMA,15 how providers decide to designate a discharge as AMA,16 and how changing the structure and environment of care for patients who use injection drugs can reduce AMA discharges and improve health outcomes.17
AMA discharges are a persistent, complicated healthcare quality problem that defies an easy solution. Improving the quality of care for these patients will require building upon the empirical research base, providing enhanced education and guidance to healthcare professionals in the ethical and clinical management of AMA discharges, and making systems changes that promote enduring institutional change. We are moving in the right direction, but we have further to go.
Disclosures
The views expressed in this article are those of the author and do not necessarily reflect the position or policy of the US Department of Veterans Affairs or the National Center for Ethics in Health Care. The author has no conflicts of interest to disclose.
Against Medical Advice (AMA) discharges, when a patient chooses to leave the hospital prior to a clinically specified and physician recommended endpoint, remain a healthcare quality problem. Patients who leave the hospital AMA challenge the healthcare professionals entrusted to care for them as well as the institutions that work to promote continuity and improved quality. AMA discharges account for up to 2% of all hospital discharges and, compared with conventional discharges, are associated with worse health and health services outcomes. Patients discharged AMA have higher rates of 30-day readmission, morbidity, and 30-day mortality.1,2 Additionally, the burden of worse health outcomes is disproportionate among disadvantaged patient populations. Patients with human immunodeficiency virus,3 substance use disorders,4 and psychiatric illness5 are more likely to be discharged AMA, as are patients with low socioeconomic status, without insurance, or with Medicaid insurance.
In this issue of the Journal of Hospital Medicine, Stearns and colleagues6 provide an important contribution to this area of medicine in need of more high quality empiric studies. The study reviewed all AMA discharges from a single year in an urban community hospital in order to assess provider perceptions and knowledge about AMA discharges. The study reconfirmed both the patient-level predictors of AMA discharges that have been demonstrated consistently (ie, male gender, younger age, Medicare or no insurance, and injection drug use) as well as the low rates of documentation of patient capacity, medication prescribed, and follow-up plans in AMA discharges.7
The authors’ investigation has also advanced the study of AMA discharges in two important directions. First, by characterizing patients with multiple AMA discharges, the authors focus on a more vulnerable population. These patients, who may have particular difficulty in consistently engaging in care, could help provide insight into the general phenomenon of AMA discharges. Second, the authors broadened their attention to include the study of nurses, a group of healthcare professionals who may play an important but not well recognized role in the AMA discharge process. In further characterizing nurses’ attitudes toward AMA discharges, medication prescriptions, and outpatient follow-up, the authors highlight nurses’ role in gathering critical patient information and promoting ethical practices in discharge planning. To better understand this dynamic and its potential role in mediating adverse health outcomes, further studies should also examine the attitudes of other central members of the treatment team (eg, pharmacists, social workers, etc.) who participate in discharge planning.
Inadequate documentation of AMA discharges remains a problem. In an attempt to address this, some institutions use AMA discharge forms to facilitate documentation of the informed consent process, the patient’s signed declination of care, medico-legal considerations, and the resulting treatment plan. Although systematic efforts to improve documentation should be encouraged, significant uncertainty about the optimal use of AMA discharge forms remains. Specifically, the use of a patient-signed AMA discharge form has not been demonstrated to advance patient care and may promote harm by stigmatizing patients8 and reducing the likelihood that they will pursue follow-up care.9 Furthermore, given that these forms may be written using institution-centered legalistic language or at an inappropriate reading level, this common hospital practice should be evaluated to assess whether patients comprehend and benefit from the forms, and how the forms influence healthcare decision making.10
Finally, the authors’ finding that 38% of nurses, 22% of physician trainees, and 6% of attendings believe patients discharged AMA lose the “right” to follow-up is noteworthy. The practice would suggest a significant lapse in understanding the professional obligation to acknowledge and communicate that the informed consent process is voluntary and patients have the right to decline recommended treatment without forfeiting future access to care. Harm reduction principles indicate that simply choosing to decline an episode of inpatient care does not make a patient ineligible for other medically indicated treatments and services. Previous studies have demonstrated that physicians may incorrectly inform patients that insurance will not pay for their care if they leave AMA, in order to persuade them to remain hospitalized.11 The current study suggests similar and potentially well-meaning but coercive attitudes about AMA discharge that can undermine a patient’s voluntary choice to accept medical care.
Stearns and colleagues6 rightly point to educational and policy interventions to improve the quality of care for patients discharged AMA. Additionally, setting patients’ expectations early in the hospitalization,12 empathically addressing their concerns,13 and sharing clinical decisions with patients by providing a medically reasonable range of clinical options rather than a single choice14 are practical bedside interventions that all clinicians can implement. System changes like developing clear policies and electronic medical records templates are particularly important, as they are more likely to lead to durable institutional change that is systematic, transparent, and fair. Moreover, research that expands the object of study beyond the physician-patient relationship could significantly improve outcomes in this vulnerable population of patients. Recent studies have begun to elucidate the deficiencies that may underlie communication failures with patients before they choose to leave AMA,15 how providers decide to designate a discharge as AMA,16 and how changing the structure and environment of care for patients who use injection drugs can reduce AMA discharges and improve health outcomes.17
AMA discharges are a persistent, complicated healthcare quality problem that defies an easy solution. Improving the quality of care for these patients will require building upon the empirical research base, providing enhanced education and guidance to healthcare professionals in the ethical and clinical management of AMA discharges, and making systems changes that promote enduring institutional change. We are moving in the right direction, but we have further to go.
Disclosures
The views expressed in this article are those of the author and do not necessarily reflect the position or policy of the US Department of Veterans Affairs or the National Center for Ethics in Health Care. The author has no conflicts of interest to disclose.
References
1. Alfandre DJ. “I’m going home”: discharges against medical advice. Mayo Clin Proc. 2009;84(3):255-260. PubMed
2. Southern WN, Nahvi S, Arnsten JH. Increased risk of mortality and readmission among patients discharged against medical advice. Am J Med. 2012;125(6):
594-602. PubMed
3. Anis AH, Sun H, Guh DP, Palepu A, Schechter MT, O’Shaughnessy MV. Leaving hospital against medical advice among HIV-positive patients. CMAJ. 2002;167(6):633-637. PubMed
4. Chan AC, Palepu A, Guh DP, et al. HIV-positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr. 2004;35(1):56-59. PubMed
5. Kuo CJ, Tsai SY, Liao YT, Lee WC, Sung XW, Chen CC. Psychiatric discharge against medical advice is a risk factor for suicide but not for other causes of death. J Clin Psychiatry. 2010;71(6):808-809. PubMed
6. Edwards J, Markert R, Bricker D. Discharge against medical advice: how often do we intervene? J Hosp Med. 2013;8(10):574-577. PubMed
7. Stearns CR, Bakamjian A, Sattar S, Ritterman Weintraub M. Discharges against medical advice at a county hospital: provider perceptions and practice. J Hosp Med. 2017;12(1):11-17. PubMed
8. Windish DM, Ratanawongsa N. Providers’ perceptions of relationships and professional roles when caring for patients who leave the hospital against medical advice. J Gen Intern Med. 2008;23(10):1698-1707. PubMed
9. Jerrard DA, Chasm RM. Patients leaving against medical advice (AMA) from the emergency department—disease prevalence and willingness to return. J Emerg Med. 2011;41(4):412-417. PubMed
10. Alfandre D. Reconsidering against medical advice discharges: embracing patient-centeredness to promote high quality care and a renewed research agenda.
J Gen Intern Med. 2013;28(12):1657-1662. PubMed
11. Schaefer GR, Matus H, Schumann JH, et al. Financial responsibility of hospitalized patients who left against medical advice: Medical urban legend? J Gen Intern Med. 2012;27(7):825-830. PubMed
12. Steinglass P, Grantham CE, Hertzman M. Predicting which patients will be discharged against medical advice: a pilot study. Am J Psychiatry. 1980;137(11):
1385-1389. PubMed
13. Clark MA, Abbott JT, Adyanthaya T. Ethics seminars: a best-practice approach to navigating the against-medical-advice discharge. Acad Emerg Med. 2014;21(9):1050-1057. PubMed
14. Alfandre D. Clinical recommendations in medical practice: a proposed framework to reduce bias and improve the quality of medical decisions. J Clin Ethics. 2016;27(1):21-27. PubMed
15. Lekas HM, Alfandre D, Gordon P, Harwood K, Yin MT. The role of patient-provider interactions: Using an accounts framework to explain hospital discharges against medical advice. Soc Sci Med. 2016;156:106-113. PubMed
16. Brenner J, Joslin J, Goulette A, Grant WD, Wojcik SM. Against medical advice: A survey of ED clinicians’ rationale for use. J Emerg Nurs. 2016;42(5):408-411. PubMed
17. McNeil R, Small W, Wood E, Kerr T. Hospitals as a ‘risk environment’: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66. PubMed
References
1. Alfandre DJ. “I’m going home”: discharges against medical advice. Mayo Clin Proc. 2009;84(3):255-260. PubMed
2. Southern WN, Nahvi S, Arnsten JH. Increased risk of mortality and readmission among patients discharged against medical advice. Am J Med. 2012;125(6):
594-602. PubMed
3. Anis AH, Sun H, Guh DP, Palepu A, Schechter MT, O’Shaughnessy MV. Leaving hospital against medical advice among HIV-positive patients. CMAJ. 2002;167(6):633-637. PubMed
4. Chan AC, Palepu A, Guh DP, et al. HIV-positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr. 2004;35(1):56-59. PubMed
5. Kuo CJ, Tsai SY, Liao YT, Lee WC, Sung XW, Chen CC. Psychiatric discharge against medical advice is a risk factor for suicide but not for other causes of death. J Clin Psychiatry. 2010;71(6):808-809. PubMed
6. Edwards J, Markert R, Bricker D. Discharge against medical advice: how often do we intervene? J Hosp Med. 2013;8(10):574-577. PubMed
7. Stearns CR, Bakamjian A, Sattar S, Ritterman Weintraub M. Discharges against medical advice at a county hospital: provider perceptions and practice. J Hosp Med. 2017;12(1):11-17. PubMed
8. Windish DM, Ratanawongsa N. Providers’ perceptions of relationships and professional roles when caring for patients who leave the hospital against medical advice. J Gen Intern Med. 2008;23(10):1698-1707. PubMed
9. Jerrard DA, Chasm RM. Patients leaving against medical advice (AMA) from the emergency department—disease prevalence and willingness to return. J Emerg Med. 2011;41(4):412-417. PubMed
10. Alfandre D. Reconsidering against medical advice discharges: embracing patient-centeredness to promote high quality care and a renewed research agenda.
J Gen Intern Med. 2013;28(12):1657-1662. PubMed
11. Schaefer GR, Matus H, Schumann JH, et al. Financial responsibility of hospitalized patients who left against medical advice: Medical urban legend? J Gen Intern Med. 2012;27(7):825-830. PubMed
12. Steinglass P, Grantham CE, Hertzman M. Predicting which patients will be discharged against medical advice: a pilot study. Am J Psychiatry. 1980;137(11):
1385-1389. PubMed
13. Clark MA, Abbott JT, Adyanthaya T. Ethics seminars: a best-practice approach to navigating the against-medical-advice discharge. Acad Emerg Med. 2014;21(9):1050-1057. PubMed
14. Alfandre D. Clinical recommendations in medical practice: a proposed framework to reduce bias and improve the quality of medical decisions. J Clin Ethics. 2016;27(1):21-27. PubMed
15. Lekas HM, Alfandre D, Gordon P, Harwood K, Yin MT. The role of patient-provider interactions: Using an accounts framework to explain hospital discharges against medical advice. Soc Sci Med. 2016;156:106-113. PubMed
16. Brenner J, Joslin J, Goulette A, Grant WD, Wojcik SM. Against medical advice: A survey of ED clinicians’ rationale for use. J Emerg Nurs. 2016;42(5):408-411. PubMed
17. McNeil R, Small W, Wood E, Kerr T. Hospitals as a ‘risk environment’: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66. PubMed
© 2017 Society of Hospital Medicine
In Reference to “Pilot Study Aiming to Support Sleep Quality and Duration During Hospitalizations”
We commend Gathecha et al.1 on the implementation of a well-formed, multicomponent sleep intervention to improve sleep in hospitalized patients. While they were unable to show objective improvement in sleep outcomes, they found improvements in patient-reported sleep outcomes across hospital days, implying that multiple hospital nights are needed to realize the benefits. We wish to propose an alternative strategy. To produce a more observable and immediate improvement in patient sleep outcomes, the behavioral economics principle of nudges2 could be an effective way to influence hospital staff toward sleep-promoting practices.
In focus groups at the University of Chicago Medicine, nurses, hospitalists, and residents reported unnecessary nocturnal disruptions were the “default” option hardwired in electronic medical records admission order sets. It was time-consuming to enter orders that minimized unnecessary nocturnal disruptions, such as forgo overnight vitals for stable patients. Given that changing default settings of order sets have been shown to effectively nudge physicians in other areas,3-5 altering default settings in admission orders could facilitate physicians’ adherence to sleep-promoting practices. An intervention combining these nudges with educational initiatives may be more effective in sustained reductions in nocturnal disruptions and improved inpatient sleep from the start of a hospital stay.
References
1. Gathecha E, Rios R, Buenaver LF, Landis R, Howell E, Wright S. Pilot study aiming to support sleep quality and duration during hospitalizations. J Hosp Med. 2016;11(7):467-472. doi:10.1002/jhm.2578. PubMed
2. Thaler R, Sunstein C. Nudge: Improving Decisions About Health, Wealth and Happiness. New Haven, CT: Yale University Press; 2008.
3. Bourdeaux CP, Davies KJ, Thomas MJC, Bewley JS, Gould TH. Using “nudge” principles for order set design: a before and after evaluation of an electronic prescribing template in critical care. BMJ Qual Saf. 2014;23(5):382-388. doi:10.1136/bmjqs-2013-002395. PubMed
4. Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340-1344. doi:10.1056/NEJMsb071595. PubMed
5. Ansher C, Ariely D, Nagler A, Rudd M, Schwartz J, Shah A. Better medicine by default. Med Decis Making. 2014;34(2):147-158. doi:10.1177/0272989X13507339. PubMed
We commend Gathecha et al.1 on the implementation of a well-formed, multicomponent sleep intervention to improve sleep in hospitalized patients. While they were unable to show objective improvement in sleep outcomes, they found improvements in patient-reported sleep outcomes across hospital days, implying that multiple hospital nights are needed to realize the benefits. We wish to propose an alternative strategy. To produce a more observable and immediate improvement in patient sleep outcomes, the behavioral economics principle of nudges2 could be an effective way to influence hospital staff toward sleep-promoting practices.
In focus groups at the University of Chicago Medicine, nurses, hospitalists, and residents reported unnecessary nocturnal disruptions were the “default” option hardwired in electronic medical records admission order sets. It was time-consuming to enter orders that minimized unnecessary nocturnal disruptions, such as forgo overnight vitals for stable patients. Given that changing default settings of order sets have been shown to effectively nudge physicians in other areas,3-5 altering default settings in admission orders could facilitate physicians’ adherence to sleep-promoting practices. An intervention combining these nudges with educational initiatives may be more effective in sustained reductions in nocturnal disruptions and improved inpatient sleep from the start of a hospital stay.
We commend Gathecha et al.1 on the implementation of a well-formed, multicomponent sleep intervention to improve sleep in hospitalized patients. While they were unable to show objective improvement in sleep outcomes, they found improvements in patient-reported sleep outcomes across hospital days, implying that multiple hospital nights are needed to realize the benefits. We wish to propose an alternative strategy. To produce a more observable and immediate improvement in patient sleep outcomes, the behavioral economics principle of nudges2 could be an effective way to influence hospital staff toward sleep-promoting practices.
In focus groups at the University of Chicago Medicine, nurses, hospitalists, and residents reported unnecessary nocturnal disruptions were the “default” option hardwired in electronic medical records admission order sets. It was time-consuming to enter orders that minimized unnecessary nocturnal disruptions, such as forgo overnight vitals for stable patients. Given that changing default settings of order sets have been shown to effectively nudge physicians in other areas,3-5 altering default settings in admission orders could facilitate physicians’ adherence to sleep-promoting practices. An intervention combining these nudges with educational initiatives may be more effective in sustained reductions in nocturnal disruptions and improved inpatient sleep from the start of a hospital stay.
References
1. Gathecha E, Rios R, Buenaver LF, Landis R, Howell E, Wright S. Pilot study aiming to support sleep quality and duration during hospitalizations. J Hosp Med. 2016;11(7):467-472. doi:10.1002/jhm.2578. PubMed
2. Thaler R, Sunstein C. Nudge: Improving Decisions About Health, Wealth and Happiness. New Haven, CT: Yale University Press; 2008.
3. Bourdeaux CP, Davies KJ, Thomas MJC, Bewley JS, Gould TH. Using “nudge” principles for order set design: a before and after evaluation of an electronic prescribing template in critical care. BMJ Qual Saf. 2014;23(5):382-388. doi:10.1136/bmjqs-2013-002395. PubMed
4. Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340-1344. doi:10.1056/NEJMsb071595. PubMed
5. Ansher C, Ariely D, Nagler A, Rudd M, Schwartz J, Shah A. Better medicine by default. Med Decis Making. 2014;34(2):147-158. doi:10.1177/0272989X13507339. PubMed
References
1. Gathecha E, Rios R, Buenaver LF, Landis R, Howell E, Wright S. Pilot study aiming to support sleep quality and duration during hospitalizations. J Hosp Med. 2016;11(7):467-472. doi:10.1002/jhm.2578. PubMed
2. Thaler R, Sunstein C. Nudge: Improving Decisions About Health, Wealth and Happiness. New Haven, CT: Yale University Press; 2008.
3. Bourdeaux CP, Davies KJ, Thomas MJC, Bewley JS, Gould TH. Using “nudge” principles for order set design: a before and after evaluation of an electronic prescribing template in critical care. BMJ Qual Saf. 2014;23(5):382-388. doi:10.1136/bmjqs-2013-002395. PubMed
4. Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340-1344. doi:10.1056/NEJMsb071595. PubMed
5. Ansher C, Ariely D, Nagler A, Rudd M, Schwartz J, Shah A. Better medicine by default. Med Decis Making. 2014;34(2):147-158. doi:10.1177/0272989X13507339. PubMed
© 2017 Society of Hospital Medicine
The Authors Reply, “Pilot Study Aiming to Support Sleep Quality and Duration During Hospitalizations”
We thank the authors for their comments and thoughts about our recent publication.1 Their suggestion that the incorporation of principles from the “Nudge Theory” might enhance the impact of our sleep intervention and shorten the lag time until patients appreciate the benefits is interesting.2 Our study aimed to assess the effect of a sleep-promoting intervention on sleep quality and duration among hospitalized patients within a quasi-experimental prospective study design. As is the case at the University of Chicago hospital described in Machado’s letter, nocturnal disruptions are also the “default” in order sets in our electronic medical records (EMR). Because the EMR team at our hospital is stretched thin with more requests than it can fulfill, it was not feasible or possible to incorporate any sleep supporting changes when designing the pilot.
Complementing sleep-promoting procedures for hospitalized patients with “nudge” principles, such as the use of choice architecture with appropriate EMR defaults or even incentives and mappings, seems like a wise recommendation.3 Regular nudges may be helpful for sustaining any multicomponent interventions in healthcare delivery that rely on cooperation by multiple parties. It appears as if evidence is growing that “nudge principles” can augment behavior change attributable to interventions.4,5 Sleep-promoting nudges, namely “anti-nudges” by members of the healthcare team, should help patients to sleep better during their hospitalizations, when sleep is critically important to recovery and health restitution.
1. Gathecha E, Rios R, Buenaver LF, Landis R, Howell E, Wright S. Pilot study aiming to support sleep quality and duration during hospitalizations. J Hosp Med. 2016;11(7):467-472. doi:10.1002/jhm.2578. PubMed
2. Thaler R, Sunstein C. Nudge: Improving Decisions About Health, Wealth and Happiness. New Haven, CT: Yale University Press; 2008.
3. Bourdeaux CP, Davies KJ, Thomas MJC, Bewley JS, Gould TH. Using “nudge” principles for order set design: a before and after evaluation of an electronic prescribing template in critical care. BMJ Qual Saf. 2014;23(5):382-388. doi:10.1136/bmjqs-2013-002395 PubMed
4. Hollands GJ, Shemilt I, Marteau TM, et al. Altering micro-environments to change population health behaviour: towards an evidence base for choice architecture interventions. BMC Public Health. 2013;13:1218. doi:10.1186/1471-2458-13-1218. PubMed
5. Arno A, Thomas S. The efficacy of nudge theory strategies in influencing adult dietary behavior: a systematic review and meta-analysis. BMC Public Health. 2016;16:676. doi:10.1186/s12889-016-3272-x. PubMed
We thank the authors for their comments and thoughts about our recent publication.1 Their suggestion that the incorporation of principles from the “Nudge Theory” might enhance the impact of our sleep intervention and shorten the lag time until patients appreciate the benefits is interesting.2 Our study aimed to assess the effect of a sleep-promoting intervention on sleep quality and duration among hospitalized patients within a quasi-experimental prospective study design. As is the case at the University of Chicago hospital described in Machado’s letter, nocturnal disruptions are also the “default” in order sets in our electronic medical records (EMR). Because the EMR team at our hospital is stretched thin with more requests than it can fulfill, it was not feasible or possible to incorporate any sleep supporting changes when designing the pilot.
Complementing sleep-promoting procedures for hospitalized patients with “nudge” principles, such as the use of choice architecture with appropriate EMR defaults or even incentives and mappings, seems like a wise recommendation.3 Regular nudges may be helpful for sustaining any multicomponent interventions in healthcare delivery that rely on cooperation by multiple parties. It appears as if evidence is growing that “nudge principles” can augment behavior change attributable to interventions.4,5 Sleep-promoting nudges, namely “anti-nudges” by members of the healthcare team, should help patients to sleep better during their hospitalizations, when sleep is critically important to recovery and health restitution.
We thank the authors for their comments and thoughts about our recent publication.1 Their suggestion that the incorporation of principles from the “Nudge Theory” might enhance the impact of our sleep intervention and shorten the lag time until patients appreciate the benefits is interesting.2 Our study aimed to assess the effect of a sleep-promoting intervention on sleep quality and duration among hospitalized patients within a quasi-experimental prospective study design. As is the case at the University of Chicago hospital described in Machado’s letter, nocturnal disruptions are also the “default” in order sets in our electronic medical records (EMR). Because the EMR team at our hospital is stretched thin with more requests than it can fulfill, it was not feasible or possible to incorporate any sleep supporting changes when designing the pilot.
Complementing sleep-promoting procedures for hospitalized patients with “nudge” principles, such as the use of choice architecture with appropriate EMR defaults or even incentives and mappings, seems like a wise recommendation.3 Regular nudges may be helpful for sustaining any multicomponent interventions in healthcare delivery that rely on cooperation by multiple parties. It appears as if evidence is growing that “nudge principles” can augment behavior change attributable to interventions.4,5 Sleep-promoting nudges, namely “anti-nudges” by members of the healthcare team, should help patients to sleep better during their hospitalizations, when sleep is critically important to recovery and health restitution.
1. Gathecha E, Rios R, Buenaver LF, Landis R, Howell E, Wright S. Pilot study aiming to support sleep quality and duration during hospitalizations. J Hosp Med. 2016;11(7):467-472. doi:10.1002/jhm.2578. PubMed
2. Thaler R, Sunstein C. Nudge: Improving Decisions About Health, Wealth and Happiness. New Haven, CT: Yale University Press; 2008.
3. Bourdeaux CP, Davies KJ, Thomas MJC, Bewley JS, Gould TH. Using “nudge” principles for order set design: a before and after evaluation of an electronic prescribing template in critical care. BMJ Qual Saf. 2014;23(5):382-388. doi:10.1136/bmjqs-2013-002395 PubMed
4. Hollands GJ, Shemilt I, Marteau TM, et al. Altering micro-environments to change population health behaviour: towards an evidence base for choice architecture interventions. BMC Public Health. 2013;13:1218. doi:10.1186/1471-2458-13-1218. PubMed
5. Arno A, Thomas S. The efficacy of nudge theory strategies in influencing adult dietary behavior: a systematic review and meta-analysis. BMC Public Health. 2016;16:676. doi:10.1186/s12889-016-3272-x. PubMed
1. Gathecha E, Rios R, Buenaver LF, Landis R, Howell E, Wright S. Pilot study aiming to support sleep quality and duration during hospitalizations. J Hosp Med. 2016;11(7):467-472. doi:10.1002/jhm.2578. PubMed
2. Thaler R, Sunstein C. Nudge: Improving Decisions About Health, Wealth and Happiness. New Haven, CT: Yale University Press; 2008.
3. Bourdeaux CP, Davies KJ, Thomas MJC, Bewley JS, Gould TH. Using “nudge” principles for order set design: a before and after evaluation of an electronic prescribing template in critical care. BMJ Qual Saf. 2014;23(5):382-388. doi:10.1136/bmjqs-2013-002395 PubMed
4. Hollands GJ, Shemilt I, Marteau TM, et al. Altering micro-environments to change population health behaviour: towards an evidence base for choice architecture interventions. BMC Public Health. 2013;13:1218. doi:10.1186/1471-2458-13-1218. PubMed
5. Arno A, Thomas S. The efficacy of nudge theory strategies in influencing adult dietary behavior: a systematic review and meta-analysis. BMC Public Health. 2016;16:676. doi:10.1186/s12889-016-3272-x. PubMed
© 2017 Society of Hospital Medicine
Postexposure management of infectious diseases
People who have been exposed to an infectious disease should be evaluated promptly and systematically, whether they are healthcare professionals at work,1 patients, or contacts of patients. The primary goals are to prevent acquisition and transmission of the infection, allay the exposed person’s anxiety, and avoid unnecessary interventions and loss of work days.1,2 Some may need postexposure prophylaxis.
ESSENTIAL ELEMENTS OF POSTEXPOSURE MANAGEMENT
Because postexposure management can be challenging, an experienced clinician or expert consultant (eg, infectious disease specialist, infection control provider, or public health officer) should be involved. Institution-specific policies and procedures for postexposure prophylaxis and testing should be followed.1,2
Postexposure management should include the following elements:
- Immediate care of the wound or other site of exposure in cases of blood-borne exposures and tetanus- and rabies-prone injuries. This includes thoroughly washing with soap and water or cleansing with an antiseptic agent, flushing affected mucous membranes with water, and debridement of devitalized tissue.1–6
- Deciding whether postexposure prophylaxis is indicated and, if so, the type, dose, route, and duration.
- Initiating prophylaxis as soon as possible.
- Determining an appropriate baseline assessment and follow-up plan for the exposed individual.
- Counseling exposed women who are pregnant or breast-feeding about the risks and benefits of postexposure prophylaxis to mother, fetus, and infant.
- Identifying required infection control precautions, including work and school restriction, for exposed and source individuals.
- Counseling and psychological support for exposed individuals, who need to know about the risks of acquiring the infection and transmitting it to others, infection control precautions, benefits, and adverse effects of postexposure prophylaxis, the importance of adhering to the regimen, and the follow-up plan. They must understand that this treatment may not completely prevent the infection, and they should seek medical attention if they develop fever or any symptoms or signs of the infection of concern.1,2
IS POSTEXPOSURE PROPHYLAXIS INDICATED?
Postexposure management begins with an assessment to determine whether the exposure is likely to result in infection; whether the exposed individual is susceptible to the infection of concern or is at greater risk of complications from it than the general population; and whether postexposure prophylaxis is needed. This involves a complete focused history, physical examination, and laboratory testing of the potentially exposed individual and of the source, if possible.1,2
Postexposure prophylaxis should begin as soon as possible to maximize its effects while awaiting the results of further diagnostic tests. However, if the exposed individual seeks care after the recommended period, prophylactic therapy can still be effective for certain infections that have a long incubation period, such as tetanus and rabies.5,6 The choice of regimen should be guided by efficacy, safety, cost, toxicity, ease of adherence, drug interactions, and antimicrobial resistance.1,2
HOW GREAT IS THE RISK OF INFECTION?
Exposed individuals are not all at the same risk of acquiring a given infection. The risk depends on:
- Type and extent of exposure (see below)
- Characteristics of the infectious agent (eg, virulence, infectious dose)
- Status of the infectious source (eg, whether the disease is in its infectious period or is being treated); effective treatment can shorten the duration of microbial shedding and subsequently reduce risk of transmission of certain infections such as tuberculosis, meningococcal infection, invasive group A streptococcal infection, and pertussis7–10
- Immune status of the exposed individual (eg, prior infection or vaccination), since people who are immune to the infection of concern usually do not need postexposure prophylaxis2
- Adherence to infection prevention and control principles; postexposure prophylaxis may not be required if the potentially exposed individual was wearing appropriate personal protective equipment such as a surgical mask, gown, and gloves and was following standard precautions.1
WHO SHOULD BE RESTRICTED FROM WORK OR SCHOOL?
Most people without symptoms who were exposed to most types of infections do not need to stay home from work or school. However, susceptible people, particularly healthcare providers exposed to measles, mumps, rubella, and varicella, should be excluded from work while they are capable of transmitting these diseases, even if they have no symptoms.11,12 Moreover, people with symptoms with infections primarily transmitted via the airborne, droplet, or contact route should be restricted from work until no longer infectious.1,2,7,9–15
Most healthcare institutions have clear protocols for managing occupational exposures to infectious diseases, in particular for blood-borne pathogens such as human immunodeficiency virus (HIV). The protocol should include appropriate evaluation and laboratory testing of the source patient and exposed healthcare provider, as well as procedures for counseling the exposed provider, identifying and procuring an initial prophylactic regimen for timely administration, a mechanism for formal expert consultation (eg, with an in-house infectious diseases consultant), and a plan for outpatient follow-up.
The next section reviews postexposure management of common infections categorized by mode of transmission, including the risk of transmission, initial and follow-up evaluation, and considerations for postexposure prophylaxis.
BLOOD-BORNE INFECTIONS
Blood-borne pathogens can be transmitted by accidental needlesticks or cuts or by exposure of the eyes, mucous membranes, or nonintact skin to blood, tissue, or other potentially infectious body fluids—cerebrospinal, pericardial, pleural, peritoneal, synovial, and amniotic fluid, semen, and vaginal secretions. (Feces, nasal secretions, saliva, sputum, sweat, tears, urine, and vomitus are considered noninfectious for blood-borne pathogens unless they contain blood.16)
Healthcare professionals are commonly exposed to blood-borne pathogens as a result of needlestick injuries, and these exposures tend to be underreported.17
When someone has been exposed to blood or other infectious body fluids, the source individual and the exposed individual should be assessed for risk factors for hepatitis B virus, hepatitis C virus, HIV, and other blood-borne pathogens.3,4,16,18 If the disease status for these viruses is unknown, the source and exposed individual should be tested in accordance with institutional policies regarding consent to testing. Testing of needles or sharp instruments implicated in an exposure is not recommended.3,4,16,18
Determining the need for prophylaxis after exposure to an unknown source such as a disposed needle can be challenging. Assessment should be made on a case-by-case basis, depending on the known prevalence of the infection of concern in the local community. The risk of transmission in most source-unknown exposures is negligible.3,4,18 However, hepatitis B vaccine and hepatitis B immunoglobulin should be used liberally as postexposure prophylaxis for previously unvaccinated healthcare providers exposed to an unknown source.3,4,16,18
Hepatitis B
Hepatitis B virus (Table 1) is the most infectious of the common blood-borne viruses. The risk of transmission after percutaneous exposure to hepatitis B-infected blood ranges from 1% to 30% based on hepatitis Be antigen status and viral load (based on hepatitis B viral DNA).1,2,4,16
Hepatitis B vaccine or immunoglobulin, or both, are recommended for postexposure prophylaxis in pregnant women, based on evidence that perinatal transmission was reduced by 70% to 90% when these were given within 12 to 24 hours of exposure.4,16,19
Hepatitis C
The risk of infection after percutaneous exposure to hepatitis C virus-infected blood is estimated to be 1.8% per exposure.16 The risk is lower with exposure of a mucous membrane or nonintact skin to blood, fluids, or tissues from hepatitis C-infected patients.16,18
Since there is no effective postexposure prophylactic regimen, the goal of postexposure assessment of hepatitis C is early identification of infection (by monitoring the patient to see if he or she seroconverts) and, if infection is present, referral to an experienced clinician for further evaluation (Table 1). However, data supporting the utility of direct-acting anti-hepatitis C antiviral drugs as postexposure prophylaxis after occupational exposure to hepatitis C are lacking.
Human immunodeficiency virus
The estimated risk of HIV transmission from a known infected source after percutaneous exposure is 0.3%, and after mucosal exposures it is 0.09%.20
If postexposure prophylaxis is indicated, it should be a three-drug regimen (Table 1).3,18 The recommended antiretroviral therapies have been proven effective in clinical trials of HIV treatment, not for postexposure prophylaxis per se, but they are recommended because they are effective, safe, tolerable, and associated with high adherence rates.3,16,18,21 If the source individual is known to have HIV infection, information about his or her stage of infection, CD4+ T-cell count, results of viral load testing, current and previous antiretroviral therapy, and results of any genotypic viral resistance testing will guide the choice of postexposure prophylactic regimen.3,18
The clinician should give the exposed patient a starter pack of 5 to 7 days of medication, give the first dose then and there, and arrange follow-up with an experienced clinician within a few days of the exposure to determine whether a complete 30-day course is needed.3,16,18
SEXUALLY TRANSMITTED INFECTIONS
In the case of sexually transmitted infections, “exposure” means unprotected sexual contact with someone who has a sexually transmitted infection.22 People with sexually transmitted infections often have no symptoms but can still transmit the infection. Thus, people at risk should be identified and screened for all suspected sexually transmitted infections.23–25
Patients with sexually transmitted infections should be instructed to refer their sex partners for evaluation and treatment to prevent further transmission and reinfection. Assessment of exposed partners includes a medical history, physical examination, microbiologic testing for all potential sexually transmitted infections, and eligibility for hepatitis A virus, hepatitis B virus, and human papillomavirus vaccines.22 Ideally, exposed partners should be reassessed within 1 to 2 weeks to follow up testing results and to monitor for side effects of and adherence to postexposure prophylaxis, if applicable.
Public health departments should be notified of sexually transmitted infections such as gonorrhea, chlamydia, chancroid, and syphilis.22
Expedited partner therapy, in which index patients deliver the medication or a prescription for it directly to their partners, is an alternative for partner management where legally allowed by state and local health departments (see www.cdc.gov/std/ept/legal/).22
Recommended postexposure prophylactic regimens for sexually transmitted infections (Table 2) are based on their efficacy in the treatment of these infections.22,26–28 The regimen for HIV prophylaxis is the same as in Table 1.3,18,26
Chlamydia
Chlamydia is the most commonly reported communicable disease in the United States. The risk of transmission after sexual intercourse with a person who has an active infection is approximately 65% and increases with the number of exposures.22,29
Gonorrhea
Infection with Neisseria gonorrhoeae is the second most commonly reported communicable disease in the United States. The transmission rate of gonorrhea after sex with someone who has it ranges from 50% to 93%.22 When prescribing postexposure prophylaxis for gonorrhea, it is essential to consider the risk of antimicrobial resistance and local susceptibility data.22
Human immunodeficiency virus
Risk of HIV transmission through sexual contact varies depending on the nature of the exposure, ranging from 0.05% to 0.5%.30
Syphilis
The risk of transmission of syphilis in its early stages (primary and secondary) after sexual exposure is approximately 30%. Transmission requires open lesions such as chancres in primary syphilis and mucocutaneous lesions (mucous patches, condyloma lata) in secondary syphilis.22
After sexual assault
In cases of sexual assault, the risk of sexually transmitted infections may be increased due to trauma and bleeding. Testing for all sexually transmitted infections, including HIV, should be considered on a case-by-case basis.22
Survivors of sexual assault have been shown to be poorly compliant with follow-up visits, and thus provision of postexposure prophylaxis at the time of initial assessment is preferable to deferred treatment.22 The recommended regimen should cover chlamydia, gonorrhea, and trichomoniasis (a single dose of intramuscular ceftriaxone 250 mg, oral azithromycin 1 g, and either oral metronidazole 2 g or tinidazole 2 g), in addition to HIV if the victim presents within 72 hours of exposure (Table 2).22,26
Hepatitis B virus vaccine, not immunoglobulin, should be given if the hepatitis status of the assailant is unknown and the survivor has not been previously vaccinated. Both hepatitis B vaccine and immunoglobulin should be given to unvaccinated survivors if the assailant is known to be hepatitis B surface antigen-positive.22
Human papillomavirus vaccination is recommended for female survivors ages 9 to 26 and male survivors ages 9 to 21.
Emergency contraception should be given if there is a risk of pregnancy.22,26
In many jurisdictions, sexual assault centers provide trained examiners through Sexual Assault Nurse Examiners to perform evidence collection and to provide initial contact with the aftercare resources of the center.
Advice on medical management of sexual assault can be obtained by calling National PEPline (888–448–4911).
INFECTIONS TRANSMITTED BY THE AIRBORNE ROUTE
Airborne transmission of infections occurs by inhalation of droplet nuclei (diameter ≤ 5 μm) generated by coughing and sneezing. Certain procedures (eg, administration of nebulized medication, sputum induction, bronchoscopy) also generate droplets and aerosols, which can transmit organisms.1
Measles
Measles (Table 3) is highly contagious; up to 90% of susceptible individuals develop measles after exposure. The virus is transmitted by direct contact with infectious droplets and by the airborne route. It remains infectious in the air and on surfaces for up to 2 hours; therefore, any type of exposure, even transient, is an indication for postexposure prophylaxis in susceptible individuals.11
Both the measles, mumps, rubella (MMR) vaccine and immune globulin may prevent or modify disease severity in susceptible exposed individuals if given within 3 days of exposure (for the vaccine) or within 6 days of exposure (for immune globulin).31,32
Tuberculosis
Mycobacterium tuberculosis is transmitted from patients with pulmonary or laryngeal tuberculosis, particularly if patients cough and are sputum-positive for acid-fast bacilli. Patients with extrapulmonary tuberculosis or latent tuberculosis infection are not infectious.1,7
Postexposure management of tuberculosis occurs through contact investigation of a newly diagnosed index case of tuberculosis disease. Contacts are categorized as household contacts, close nonhousehold contacts (those having regular, extensive contact with the index case), casual contacts, and transient community contacts. The highest priority for contact investigations should be household contacts, close nonhousehold or casual contacts at high risk of progressing to tuberculosis disease (eg, those with HIV, those on dialysis, or transplant recipients), and unprotected healthcare providers exposed during aerosol-generating procedures.7,33
Postexposure management includes screening exposed individuals for tuberculosis symptoms and performing tuberculin skin testing or interferon-gamma release assay (blood testing) for those who had previously negative results (Table 3). Chest radiography is recommended for exposed immunocompromised individuals, due to high risk of tuberculosis disease and low sensitivity of skin or blood testing, and for those with a documented history of tuberculosis or previous positive skin or blood test.7,33,34
A positive tuberculin skin test for persons with recent contact with tuberculosis is defined as a wheal 5 mm or larger on baseline or follow-up screening. Prior bacillus Calmette-Guérin vaccination status should not be used in the interpretation of tuberculin skin testing in the setting of contact investigation.7,33
All exposed asymptomatic people with a positive result on testing should be treated for latent tuberculosis infection, since treatment reduces the risk of progression to tuberculosis disease by 60% to 90% .7,33,35–37
Varicella and disseminated herpes zoster
Varicella zoster virus is transmitted by direct contact with vesicular fluid of skin lesions and inhalation of aerosols from vesicular fluid or respiratory tract secretions. Varicella (chickenpox) is highly contagious, with a secondary attack rate in susceptible household contacts of 85%.12 Herpes zoster is less contagious than varicella.38
Postexposure prophylaxis against varicella is recommended for susceptible individuals who had household exposure, had face-to-face contact with an infectious patient while indoors, or shared the same hospital room with the patient.12
Postexposure prophylactic options for varicella and herpes zoster include varicella vaccine (not zoster vaccine) and varicella zoster immune globulin (Table 3).12,38–40
Varicella vaccine is approximately 90% effective if given within 3 days of exposure, and 70% effective if given within 5 days.12,39
Antiviral agents should be given if the exposed individual develops manifestations of varicella or herpes zoster.12,38
INFECTIONS TRANSMITTED BY THE DROPLET ROUTE
Droplet transmission occurs when respiratory droplets carrying infectious agents travel directly across short distances (3–6 feet) from the respiratory tract of the infected to mucosal surfaces of the susceptible exposed individual. Droplets are generated during coughing, sneezing, talking, and aerosol-generating procedures. Indirect contact with droplets can also transmit infection.1
Group A streptococcal infection
Although group A streptococcal infection (Table 4) may spread to close contacts of the index case and in closed populations (eg, military recruit camps, schools, institutions), secondary cases of invasive group A streptococcal infection rarely occur in family and institutional contacts.9,41,42
Postexposure prophylaxis for contacts of people with invasive group A streptococcal infection is debated, because it is unknown if antibiotic therapy will decrease the risk of acquiring the infection. It is generally agreed that it should not be routinely given to all contacts. The decision should be based on the clinician’s assessment of each individual’s risk and guidance from the local institution. If indicated, postexposure prophylaxis should be given to household and close contacts, particularly in high-risk groups (eg, Native Americans and those with risk factors such as old age, HIV infection, diabetes mellitus, heart disease, chickenpox, cancer, systemic corticosteroid therapy, other immunosuppressive medications, intravenous drug use, recent surgery or childbirth).9,41,42
Influenza
Influenza (Table 4) causes a significant burden in healthcare settings, given its prevalence and potential to cause outbreaks of severe respiratory illness in hospitalized patients and residents of long-term-care facilities.13,43
Neuraminidase inhibitors are effective as prophylaxis after unprotected exposure to influenza, particularly in outbreak situations. However, their use is not widely recommended, since overuse could lead to antiviral resistance. In selected cases, postexposure prophylaxis may be indicated for close contacts who are at high risk of complications of influenza (eg, age 65 or older, in third trimester of pregnancy or 2 weeks postpartum, morbid obesity, chronic comorbid conditions such as a cardiopulmonary and renal disorder, immunocompromising condition) or who are in close contact with persons at high risk of influenza-related complications.13,44,45
Meningococcal disease
N meningitidis is transmitted from individuals with meningococcal disease or from asymptomatic carriers.8
Postexposure prophylaxis is effective in eradicating N meningiditis and is recommended for all close contacts of patients with invasive meningococcal disease (Table 4).46 Close contacts include household contacts, childcare and preschool contacts, contacts exposed in dormitories or military training centers, those who had direct contact with the index case’s respiratory secretions (eg, intimate kissing, mouth-to-mouth resuscitation, unprotected contact during endotracheal intubation or endotracheal tube management), and passengers seated directly next to an index case on airplane flights of longer than 8 hours.
Postexposure prophylaxis is not indicated for those who had brief contact, those who had contact that did not involve exposure to oral or respiratory secretions, or for close contacts of patients with N meningitidis isolated in nonsterile sites only (eg, oropharynyx, trachea, conjunctiva).8,46
Pertussis
Pertussis is highly contagious, with a secondary attack rate of approximately 80% in susceptible individuals. Approximately one-third of susceptible household contacts develop pertussis after exposure.10
Postexposure prophylaxis for pertussis should be given to all household and close contacts (Table 4).10,47
Rubella
Transmission occurs through droplets or direct contact with nasopharyngeal secretions of an infectious case. Neither MMR vaccine nor immunoglobulin has been shown to prevent rubella in exposed contacts, and they are not recommended.11
INFECTIONS TRANSMITTED BY DIRECT CONTACT
Direct contact transmission includes infectious agents transmitted from an infected or colonized individual to another, whereas indirect contact transmission involves a contaminated intermediate object or person (eg, hands of healthcare providers, electronic thermometers, surgical instruments).1
There are no available postexposure prophylactic regimens for the organisms most commonly transmitted by this route (eg, methicillin-resistant Staphylococcus aureus, Clostridium difficile), but transmission can be prevented with adherence to standard precautions, including hand hygiene.1
Hepatitis A
Person-to-person transmission of hepatitis A virus occurs via the fecal-oral route. Common-source outbreaks and sporadic cases can occur from exposure to food or water contaminated with feces.1,15
Postexposure prophylaxis is indicated only for nonimmune close contacts (eg, household and sexual contacts) (Table 5). Without this treatment, secondary attack rates of 15% to 30% have been reported among households.15,48 Both hepatitis A vaccine and immune globulin are effective in preventing and ameliorating symptomatic hepatitis A infection. Advantages of vaccination include induction of longer-lasting immunity (at least 2 years), greater ease of administration, and lower cost than immune globulin.15,48
Scabies
Scabies is an infestation of the skin by the mite Sarcoptes scabiei var hominis. Person-to-person transmission typically occurs through direct, prolonged skin-to-skin contact with an infested person (eg, household and sexual contacts). However, crusted scabies can be transmitted after brief skin-to-skin contact or by exposure to bedding, clothing, or furniture used by the infested person.
All potentially infested persons should be treated concomitantly (Table 5).14,49
INFECTIONS TRANSMITTED BY MAMMAL BITES AND INJURIES
Bites and injury wounds account for approximately 1% of all visits to emergency departments.50 Human bites are associated with a risk of infection by blood-borne pathogens, herpes simplex infection, and bacterial infections (eg, skin and soft-tissue infections, bacteremia). Animal bites are associated with a risk of bacterial infections, rabies, tetanus, hepatitis B virus, and monkeypox.50
Rabies
Human rabies (Table 5) is almost always fatal. Essential factors in determining the need for postexposure prophylaxis include knowledge of the epidemiology of animal rabies in the area where the contact occurred and the species of animal involved, availability of the animal for observation or rabies testing, health status of the biting animal, and vaccination history of both the animal and exposed individual.6 Clinicians should seek assistance from public health officials for evaluating exposures and determining the need for postexposure prophylaxis in situations that are not routine.51
High-risk wild animals associated with rabies in North America include bats, raccoons, skunks, foxes, coyotes, bobcats, and woodchucks. Bats are the most common source of human rabies infections in the United States, and transmission can occur from minor, sometimes unnoticed, bites. The types of exposures that require postexposure prophylaxis include bites, abrasions, scratches, and contamination of mucous membranes or open wound with saliva or neural tissue of a suspected rabid animal.
Human-to-human transmission of rabies can rarely occur through exposure of mucous membrane or nonintact skin to an infectious material (saliva, tears, neural tissue), in addition to organ transplantation.6
Animal capture and testing is a strategy for excluding rabies risk and reducing the need for postexposure prophylaxis. A dog, cat, or ferret that bites a person should be confined and observed for 10 days without administering postexposure prophylaxis for rabies, unless the bite or exposure is on the face or neck, in which case this treatment should be given immediately.6 If the observed biting animal lives and remains healthy, postexposure prophylaxis is not recommended. However, if signs suggestive of rabies develop, postexposure prophylaxis should be given and the animal should be euthanized, with testing of brain tissue for rabies virus. Postexposure prophylaxis should be discontinued if rabies testing is negative.
The combination of rabies vaccine and human rabies immunoglobulin is nearly 100% effective in preventing rabies if administered in a timely and accurate fashion after exposure (Table 5).6
Tetanus
Tetanus transmission can occur through injuries ranging from small cuts to severe trauma and through contact with contaminated objects (eg, bites, nails, needles, splinters, neonates whose umbilical cord is cut with contaminated surgical instruments, and during circumcision or piercing with contaminated instruments).5
Tetanus is almost completely preventable with vaccination, and timely administration of postexposure prophylaxis (tetanus toxoid-containing vaccine, tetanus immune globulin) decreases disease severity (Table 5).2,5,52
- Siegel JD, Rhinehart E, Jackson M, Chiarello L; Health Care Infection Control Practices Advisory Committee. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control 2007; 35(suppl 2): S65–S164.
- Advisory Committee on Immunization Practices; Centers for Disease Control and Prevention. Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:1–45.
- Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol 2013; 34: 875–892.
- Schille S, Murphy TV, Sawyer M, et al; Centers for Disease Control and Prevention (CDC). CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR Recomm Rep 2013; 62:1–19.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:13–15.
- Manning SE, Rupprecht CE, Fishbein D, et al; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Human rabies prevention—United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2008; 57:1–28.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon R, Centers for Disease Control and Prevention (CDC). Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Morb Mortal Wkly Rep 2005; 54:1–141.
- Cohn AC, MacNeil JR, Clark TA, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2013; 62:1–28.
- Prevention of Invasive Group A Streptococcal Infections Workshop Participants. Prevention of invasive group A streptococcal disease among household contacts of case patients and among postpartum and postsurgical patients: recommendations from the Centers for Disease Control and Prevention. Clin Infect Dis 2002; 35:950–959.
- Tiwari T, Murphy TV, Moran J; National Immunization Program, Centers for Disease Control and Prevention (CDC). Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis: 2005 CDC Guidelines. MMWR Morb Mortal Wkly Rep 2005; 54:1–16.
- McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS; Centers for Disease Control and Prevention (CDC). Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2013; 62:1–34.
- Marin M, Guris D, Chaves SS, Schmid S, Seward JF; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56:1–40.
- Harper SA, Bradley JS, Englund JA, et al; Expert Panel of the Infectious Diseases Society of America. Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1003–1032.
- Centers for Disease Control and Prevention (CDC). Scabies. www.cdc.gov/parasites/scabies/. Accessed November 4, 2016.
- Advisory Committee on Immunization Practices (ACIP); Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2006; 55:1–23.
- US Public Health Service. Updated US Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep 2001; 50:1–52.
- Treakle AM, Schultz M, Giannakos GP, Joyce PC, Gordin FM. Evaluating a decade of exposures to blood and body fluids in an inner-city teaching hospital. Infect Control Hosp Epidemiol 2011; 32:903–907.
- New York State Department of Health AIDS Institute. Update: HIV prophylaxis following non-occupational exposure. www.hivguidelines.org/clinical-guidelines/post-exposure-prophylaxis/hiv-prophylaxis-following-non-occupational-exposure/. Accessed November 4, 2016.
- Beasley RP, Hwang LY, Lee GC, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet 1983; 2:1099–1102.
- Baggaley RF, Boily MC, White RG, Alary M. Risk of HIV-1 transmission for parenteral exposure and blood transfusion: a systematic review and meta-analysis. AIDS 2006; 20:805–812.
- McAllister J, Read P, McNulty A, Tong WW, Ingersoll A, Carr A. Raltegravir-emtricitabine-tenofovir as HIV nonoccupational post-exposure prophylaxis in men who have sex with men: safety, tolerability and adherence. HIV Med 2014; 15:13–22.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137.
- US Preventive Services Task Force (USPSTF). Final recommendation statement: chlamydia and gonorrhea: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/chlamydia-and-gonorrhea-screening. Accessed November 4, 2016.
- US Preventive Services Task Force (USPSTF). Human immunodeficiency virus (HIV) infection: screening. www.uspreventiveservicestaskforce.org/uspstf/uspshivi.htm. Accessed November 4, 2016.
- US Preventive Services Task Force (USPSTF). Screening for syphilis. www.uspreventiveservicestaskforce.org/uspstf/uspssyph.htm#update. Accessed November 4, 2016.
- Smith DK, Grohskopf LA, Black RJ, et al; US Department of Health and Human Services. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the US Department of Health and Human Services. MMWR Recomm Rep 2005; 54:1–20.
- Lin JS, Donegan SP, Heeren TC, et al. Transmission of Chlamydia trachomatis and Neisseria gonorrhoeae among men with urethritis and their female sex partners. J Infect Dis 1998; 178:1707–1712.
- Varghese B, Maher JE, Peterman TA, Branson BM, Steketee RW. Reducing the risk of sexual HIV transmission: quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use. Sex Transm Dis 2002; 29:38–43.
- Gülmezoglu AM, Azhar M. Interventions for trichomoniasis in pregnancy. Cochrane Database Syst Rev 2011; (5):CD000220.
- Forna F, Gülmezoglu AM. Interventions for treating trichomoniasis in women. Cochrane Database Syst Rev 2003; (2):CD000218.
- Rice P, Young Y, Cohen B, Ramsay M. MMR immunization after contact with measles virus. Lancet 2004; 363:569–570.
- Young MK, Nimmo GR, Cripps AW, Jones MA. Post-exposure passive immunization for preventing measles. Cochrane Database Syst Rev 2014; 4:CD010056.
- National Tuberculosis Controllers Association; Centers for Disease Control and Prevention (CDC). Guidelines for the investigation of contacts of persons with infectious tuberculosis. Recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Recomm Rep 2005; 54:1–47.
- Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Morb Mortal Wkly Rep 2010; 59:1–25.
- Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Morb Mortal Wkly Rep 2000; 49:1–51.
- Stagg HR, Zenner D, Harris RJ, Munoz L, Lipman MC, Abubakar I. Treatment of latent tuberculosis infection: a network meta-analysis. Ann Intern Med 2014; 161:419–428.
- Centers for Disease Control and Prevention (CDC). Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep 2011; 60:1650–1653.
- Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2008; 57:1–30.
- Macartney K, Heywood A, McIntyre P. Vaccines for post-exposure prophylaxis against varicella (chickenpox) in children and adults. Cochrane Database Syst Rev 2014; 6:CD001833.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of VariZIG—United States, 2013. MMWR Morb Mortal Wkly Rep 2013; 62: 574–576.
- Public Health Agency of Canada. Guidelines for the prevention and control of invasive group A streptococcal disease. Can Commun Dis Rep 2006; 32(suppl 2):1–26.
- Steer JA, Lamagni T, Healy B, et al. Guidelines for prevention and control of group A streptococcal infection in acute healthcare and maternity settings in the UK. J Infect 2012; 64:1–18.
- Grohskopf LA, Olsen SJ, Sokolow LZ, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2014–15 influenza season. MMWR Morb Mortal Wkly Rep 2014; 63: 691–697.
- Fiore AE, Fry A, Shay D, et al; Centers for Disease Control and Prevention (CDC). Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2011; 60:1–24.
- Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev 2014; 4:CD008965.
- Zalmanovici Trestioreanu A, Fraser A, Gafter-Gvili A, Paul M, Leibovici L. Antibiotics for preventing meningococcal infections. Cochrane Database Syst Rev 2013; 10:CD004785.
- Altunaiji S, Kukuruzovic R, Curtis N, Massie J. Antibiotics for whooping cough (pertussis). Cochrane Database Syst Rev 2007: CD004404.
- Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56:1080–1084.
- FitzGerald D, Grainger RJ, Reid A. Interventions for preventing the spread of infestation in close contacts of people with scabies. Cochrane Database Syst Rev 2014; 2:CD009943.
- Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:e10–e52.
- Rupprecht CE, Briggs D, Brown CM, et al; Centers for Disease Control and Prevention (CDC). Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies—recommendations of the Advisory Committee on Immunization Practice. MMWR Recomm Rep 2010; 59:1–9.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine in adults aged 65 years and older—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep 2012; 61:468–470.
People who have been exposed to an infectious disease should be evaluated promptly and systematically, whether they are healthcare professionals at work,1 patients, or contacts of patients. The primary goals are to prevent acquisition and transmission of the infection, allay the exposed person’s anxiety, and avoid unnecessary interventions and loss of work days.1,2 Some may need postexposure prophylaxis.
ESSENTIAL ELEMENTS OF POSTEXPOSURE MANAGEMENT
Because postexposure management can be challenging, an experienced clinician or expert consultant (eg, infectious disease specialist, infection control provider, or public health officer) should be involved. Institution-specific policies and procedures for postexposure prophylaxis and testing should be followed.1,2
Postexposure management should include the following elements:
- Immediate care of the wound or other site of exposure in cases of blood-borne exposures and tetanus- and rabies-prone injuries. This includes thoroughly washing with soap and water or cleansing with an antiseptic agent, flushing affected mucous membranes with water, and debridement of devitalized tissue.1–6
- Deciding whether postexposure prophylaxis is indicated and, if so, the type, dose, route, and duration.
- Initiating prophylaxis as soon as possible.
- Determining an appropriate baseline assessment and follow-up plan for the exposed individual.
- Counseling exposed women who are pregnant or breast-feeding about the risks and benefits of postexposure prophylaxis to mother, fetus, and infant.
- Identifying required infection control precautions, including work and school restriction, for exposed and source individuals.
- Counseling and psychological support for exposed individuals, who need to know about the risks of acquiring the infection and transmitting it to others, infection control precautions, benefits, and adverse effects of postexposure prophylaxis, the importance of adhering to the regimen, and the follow-up plan. They must understand that this treatment may not completely prevent the infection, and they should seek medical attention if they develop fever or any symptoms or signs of the infection of concern.1,2
IS POSTEXPOSURE PROPHYLAXIS INDICATED?
Postexposure management begins with an assessment to determine whether the exposure is likely to result in infection; whether the exposed individual is susceptible to the infection of concern or is at greater risk of complications from it than the general population; and whether postexposure prophylaxis is needed. This involves a complete focused history, physical examination, and laboratory testing of the potentially exposed individual and of the source, if possible.1,2
Postexposure prophylaxis should begin as soon as possible to maximize its effects while awaiting the results of further diagnostic tests. However, if the exposed individual seeks care after the recommended period, prophylactic therapy can still be effective for certain infections that have a long incubation period, such as tetanus and rabies.5,6 The choice of regimen should be guided by efficacy, safety, cost, toxicity, ease of adherence, drug interactions, and antimicrobial resistance.1,2
HOW GREAT IS THE RISK OF INFECTION?
Exposed individuals are not all at the same risk of acquiring a given infection. The risk depends on:
- Type and extent of exposure (see below)
- Characteristics of the infectious agent (eg, virulence, infectious dose)
- Status of the infectious source (eg, whether the disease is in its infectious period or is being treated); effective treatment can shorten the duration of microbial shedding and subsequently reduce risk of transmission of certain infections such as tuberculosis, meningococcal infection, invasive group A streptococcal infection, and pertussis7–10
- Immune status of the exposed individual (eg, prior infection or vaccination), since people who are immune to the infection of concern usually do not need postexposure prophylaxis2
- Adherence to infection prevention and control principles; postexposure prophylaxis may not be required if the potentially exposed individual was wearing appropriate personal protective equipment such as a surgical mask, gown, and gloves and was following standard precautions.1
WHO SHOULD BE RESTRICTED FROM WORK OR SCHOOL?
Most people without symptoms who were exposed to most types of infections do not need to stay home from work or school. However, susceptible people, particularly healthcare providers exposed to measles, mumps, rubella, and varicella, should be excluded from work while they are capable of transmitting these diseases, even if they have no symptoms.11,12 Moreover, people with symptoms with infections primarily transmitted via the airborne, droplet, or contact route should be restricted from work until no longer infectious.1,2,7,9–15
Most healthcare institutions have clear protocols for managing occupational exposures to infectious diseases, in particular for blood-borne pathogens such as human immunodeficiency virus (HIV). The protocol should include appropriate evaluation and laboratory testing of the source patient and exposed healthcare provider, as well as procedures for counseling the exposed provider, identifying and procuring an initial prophylactic regimen for timely administration, a mechanism for formal expert consultation (eg, with an in-house infectious diseases consultant), and a plan for outpatient follow-up.
The next section reviews postexposure management of common infections categorized by mode of transmission, including the risk of transmission, initial and follow-up evaluation, and considerations for postexposure prophylaxis.
BLOOD-BORNE INFECTIONS
Blood-borne pathogens can be transmitted by accidental needlesticks or cuts or by exposure of the eyes, mucous membranes, or nonintact skin to blood, tissue, or other potentially infectious body fluids—cerebrospinal, pericardial, pleural, peritoneal, synovial, and amniotic fluid, semen, and vaginal secretions. (Feces, nasal secretions, saliva, sputum, sweat, tears, urine, and vomitus are considered noninfectious for blood-borne pathogens unless they contain blood.16)
Healthcare professionals are commonly exposed to blood-borne pathogens as a result of needlestick injuries, and these exposures tend to be underreported.17
When someone has been exposed to blood or other infectious body fluids, the source individual and the exposed individual should be assessed for risk factors for hepatitis B virus, hepatitis C virus, HIV, and other blood-borne pathogens.3,4,16,18 If the disease status for these viruses is unknown, the source and exposed individual should be tested in accordance with institutional policies regarding consent to testing. Testing of needles or sharp instruments implicated in an exposure is not recommended.3,4,16,18
Determining the need for prophylaxis after exposure to an unknown source such as a disposed needle can be challenging. Assessment should be made on a case-by-case basis, depending on the known prevalence of the infection of concern in the local community. The risk of transmission in most source-unknown exposures is negligible.3,4,18 However, hepatitis B vaccine and hepatitis B immunoglobulin should be used liberally as postexposure prophylaxis for previously unvaccinated healthcare providers exposed to an unknown source.3,4,16,18
Hepatitis B
Hepatitis B virus (Table 1) is the most infectious of the common blood-borne viruses. The risk of transmission after percutaneous exposure to hepatitis B-infected blood ranges from 1% to 30% based on hepatitis Be antigen status and viral load (based on hepatitis B viral DNA).1,2,4,16
Hepatitis B vaccine or immunoglobulin, or both, are recommended for postexposure prophylaxis in pregnant women, based on evidence that perinatal transmission was reduced by 70% to 90% when these were given within 12 to 24 hours of exposure.4,16,19
Hepatitis C
The risk of infection after percutaneous exposure to hepatitis C virus-infected blood is estimated to be 1.8% per exposure.16 The risk is lower with exposure of a mucous membrane or nonintact skin to blood, fluids, or tissues from hepatitis C-infected patients.16,18
Since there is no effective postexposure prophylactic regimen, the goal of postexposure assessment of hepatitis C is early identification of infection (by monitoring the patient to see if he or she seroconverts) and, if infection is present, referral to an experienced clinician for further evaluation (Table 1). However, data supporting the utility of direct-acting anti-hepatitis C antiviral drugs as postexposure prophylaxis after occupational exposure to hepatitis C are lacking.
Human immunodeficiency virus
The estimated risk of HIV transmission from a known infected source after percutaneous exposure is 0.3%, and after mucosal exposures it is 0.09%.20
If postexposure prophylaxis is indicated, it should be a three-drug regimen (Table 1).3,18 The recommended antiretroviral therapies have been proven effective in clinical trials of HIV treatment, not for postexposure prophylaxis per se, but they are recommended because they are effective, safe, tolerable, and associated with high adherence rates.3,16,18,21 If the source individual is known to have HIV infection, information about his or her stage of infection, CD4+ T-cell count, results of viral load testing, current and previous antiretroviral therapy, and results of any genotypic viral resistance testing will guide the choice of postexposure prophylactic regimen.3,18
The clinician should give the exposed patient a starter pack of 5 to 7 days of medication, give the first dose then and there, and arrange follow-up with an experienced clinician within a few days of the exposure to determine whether a complete 30-day course is needed.3,16,18
SEXUALLY TRANSMITTED INFECTIONS
In the case of sexually transmitted infections, “exposure” means unprotected sexual contact with someone who has a sexually transmitted infection.22 People with sexually transmitted infections often have no symptoms but can still transmit the infection. Thus, people at risk should be identified and screened for all suspected sexually transmitted infections.23–25
Patients with sexually transmitted infections should be instructed to refer their sex partners for evaluation and treatment to prevent further transmission and reinfection. Assessment of exposed partners includes a medical history, physical examination, microbiologic testing for all potential sexually transmitted infections, and eligibility for hepatitis A virus, hepatitis B virus, and human papillomavirus vaccines.22 Ideally, exposed partners should be reassessed within 1 to 2 weeks to follow up testing results and to monitor for side effects of and adherence to postexposure prophylaxis, if applicable.
Public health departments should be notified of sexually transmitted infections such as gonorrhea, chlamydia, chancroid, and syphilis.22
Expedited partner therapy, in which index patients deliver the medication or a prescription for it directly to their partners, is an alternative for partner management where legally allowed by state and local health departments (see www.cdc.gov/std/ept/legal/).22
Recommended postexposure prophylactic regimens for sexually transmitted infections (Table 2) are based on their efficacy in the treatment of these infections.22,26–28 The regimen for HIV prophylaxis is the same as in Table 1.3,18,26
Chlamydia
Chlamydia is the most commonly reported communicable disease in the United States. The risk of transmission after sexual intercourse with a person who has an active infection is approximately 65% and increases with the number of exposures.22,29
Gonorrhea
Infection with Neisseria gonorrhoeae is the second most commonly reported communicable disease in the United States. The transmission rate of gonorrhea after sex with someone who has it ranges from 50% to 93%.22 When prescribing postexposure prophylaxis for gonorrhea, it is essential to consider the risk of antimicrobial resistance and local susceptibility data.22
Human immunodeficiency virus
Risk of HIV transmission through sexual contact varies depending on the nature of the exposure, ranging from 0.05% to 0.5%.30
Syphilis
The risk of transmission of syphilis in its early stages (primary and secondary) after sexual exposure is approximately 30%. Transmission requires open lesions such as chancres in primary syphilis and mucocutaneous lesions (mucous patches, condyloma lata) in secondary syphilis.22
After sexual assault
In cases of sexual assault, the risk of sexually transmitted infections may be increased due to trauma and bleeding. Testing for all sexually transmitted infections, including HIV, should be considered on a case-by-case basis.22
Survivors of sexual assault have been shown to be poorly compliant with follow-up visits, and thus provision of postexposure prophylaxis at the time of initial assessment is preferable to deferred treatment.22 The recommended regimen should cover chlamydia, gonorrhea, and trichomoniasis (a single dose of intramuscular ceftriaxone 250 mg, oral azithromycin 1 g, and either oral metronidazole 2 g or tinidazole 2 g), in addition to HIV if the victim presents within 72 hours of exposure (Table 2).22,26
Hepatitis B virus vaccine, not immunoglobulin, should be given if the hepatitis status of the assailant is unknown and the survivor has not been previously vaccinated. Both hepatitis B vaccine and immunoglobulin should be given to unvaccinated survivors if the assailant is known to be hepatitis B surface antigen-positive.22
Human papillomavirus vaccination is recommended for female survivors ages 9 to 26 and male survivors ages 9 to 21.
Emergency contraception should be given if there is a risk of pregnancy.22,26
In many jurisdictions, sexual assault centers provide trained examiners through Sexual Assault Nurse Examiners to perform evidence collection and to provide initial contact with the aftercare resources of the center.
Advice on medical management of sexual assault can be obtained by calling National PEPline (888–448–4911).
INFECTIONS TRANSMITTED BY THE AIRBORNE ROUTE
Airborne transmission of infections occurs by inhalation of droplet nuclei (diameter ≤ 5 μm) generated by coughing and sneezing. Certain procedures (eg, administration of nebulized medication, sputum induction, bronchoscopy) also generate droplets and aerosols, which can transmit organisms.1
Measles
Measles (Table 3) is highly contagious; up to 90% of susceptible individuals develop measles after exposure. The virus is transmitted by direct contact with infectious droplets and by the airborne route. It remains infectious in the air and on surfaces for up to 2 hours; therefore, any type of exposure, even transient, is an indication for postexposure prophylaxis in susceptible individuals.11
Both the measles, mumps, rubella (MMR) vaccine and immune globulin may prevent or modify disease severity in susceptible exposed individuals if given within 3 days of exposure (for the vaccine) or within 6 days of exposure (for immune globulin).31,32
Tuberculosis
Mycobacterium tuberculosis is transmitted from patients with pulmonary or laryngeal tuberculosis, particularly if patients cough and are sputum-positive for acid-fast bacilli. Patients with extrapulmonary tuberculosis or latent tuberculosis infection are not infectious.1,7
Postexposure management of tuberculosis occurs through contact investigation of a newly diagnosed index case of tuberculosis disease. Contacts are categorized as household contacts, close nonhousehold contacts (those having regular, extensive contact with the index case), casual contacts, and transient community contacts. The highest priority for contact investigations should be household contacts, close nonhousehold or casual contacts at high risk of progressing to tuberculosis disease (eg, those with HIV, those on dialysis, or transplant recipients), and unprotected healthcare providers exposed during aerosol-generating procedures.7,33
Postexposure management includes screening exposed individuals for tuberculosis symptoms and performing tuberculin skin testing or interferon-gamma release assay (blood testing) for those who had previously negative results (Table 3). Chest radiography is recommended for exposed immunocompromised individuals, due to high risk of tuberculosis disease and low sensitivity of skin or blood testing, and for those with a documented history of tuberculosis or previous positive skin or blood test.7,33,34
A positive tuberculin skin test for persons with recent contact with tuberculosis is defined as a wheal 5 mm or larger on baseline or follow-up screening. Prior bacillus Calmette-Guérin vaccination status should not be used in the interpretation of tuberculin skin testing in the setting of contact investigation.7,33
All exposed asymptomatic people with a positive result on testing should be treated for latent tuberculosis infection, since treatment reduces the risk of progression to tuberculosis disease by 60% to 90% .7,33,35–37
Varicella and disseminated herpes zoster
Varicella zoster virus is transmitted by direct contact with vesicular fluid of skin lesions and inhalation of aerosols from vesicular fluid or respiratory tract secretions. Varicella (chickenpox) is highly contagious, with a secondary attack rate in susceptible household contacts of 85%.12 Herpes zoster is less contagious than varicella.38
Postexposure prophylaxis against varicella is recommended for susceptible individuals who had household exposure, had face-to-face contact with an infectious patient while indoors, or shared the same hospital room with the patient.12
Postexposure prophylactic options for varicella and herpes zoster include varicella vaccine (not zoster vaccine) and varicella zoster immune globulin (Table 3).12,38–40
Varicella vaccine is approximately 90% effective if given within 3 days of exposure, and 70% effective if given within 5 days.12,39
Antiviral agents should be given if the exposed individual develops manifestations of varicella or herpes zoster.12,38
INFECTIONS TRANSMITTED BY THE DROPLET ROUTE
Droplet transmission occurs when respiratory droplets carrying infectious agents travel directly across short distances (3–6 feet) from the respiratory tract of the infected to mucosal surfaces of the susceptible exposed individual. Droplets are generated during coughing, sneezing, talking, and aerosol-generating procedures. Indirect contact with droplets can also transmit infection.1
Group A streptococcal infection
Although group A streptococcal infection (Table 4) may spread to close contacts of the index case and in closed populations (eg, military recruit camps, schools, institutions), secondary cases of invasive group A streptococcal infection rarely occur in family and institutional contacts.9,41,42
Postexposure prophylaxis for contacts of people with invasive group A streptococcal infection is debated, because it is unknown if antibiotic therapy will decrease the risk of acquiring the infection. It is generally agreed that it should not be routinely given to all contacts. The decision should be based on the clinician’s assessment of each individual’s risk and guidance from the local institution. If indicated, postexposure prophylaxis should be given to household and close contacts, particularly in high-risk groups (eg, Native Americans and those with risk factors such as old age, HIV infection, diabetes mellitus, heart disease, chickenpox, cancer, systemic corticosteroid therapy, other immunosuppressive medications, intravenous drug use, recent surgery or childbirth).9,41,42
Influenza
Influenza (Table 4) causes a significant burden in healthcare settings, given its prevalence and potential to cause outbreaks of severe respiratory illness in hospitalized patients and residents of long-term-care facilities.13,43
Neuraminidase inhibitors are effective as prophylaxis after unprotected exposure to influenza, particularly in outbreak situations. However, their use is not widely recommended, since overuse could lead to antiviral resistance. In selected cases, postexposure prophylaxis may be indicated for close contacts who are at high risk of complications of influenza (eg, age 65 or older, in third trimester of pregnancy or 2 weeks postpartum, morbid obesity, chronic comorbid conditions such as a cardiopulmonary and renal disorder, immunocompromising condition) or who are in close contact with persons at high risk of influenza-related complications.13,44,45
Meningococcal disease
N meningitidis is transmitted from individuals with meningococcal disease or from asymptomatic carriers.8
Postexposure prophylaxis is effective in eradicating N meningiditis and is recommended for all close contacts of patients with invasive meningococcal disease (Table 4).46 Close contacts include household contacts, childcare and preschool contacts, contacts exposed in dormitories or military training centers, those who had direct contact with the index case’s respiratory secretions (eg, intimate kissing, mouth-to-mouth resuscitation, unprotected contact during endotracheal intubation or endotracheal tube management), and passengers seated directly next to an index case on airplane flights of longer than 8 hours.
Postexposure prophylaxis is not indicated for those who had brief contact, those who had contact that did not involve exposure to oral or respiratory secretions, or for close contacts of patients with N meningitidis isolated in nonsterile sites only (eg, oropharynyx, trachea, conjunctiva).8,46
Pertussis
Pertussis is highly contagious, with a secondary attack rate of approximately 80% in susceptible individuals. Approximately one-third of susceptible household contacts develop pertussis after exposure.10
Postexposure prophylaxis for pertussis should be given to all household and close contacts (Table 4).10,47
Rubella
Transmission occurs through droplets or direct contact with nasopharyngeal secretions of an infectious case. Neither MMR vaccine nor immunoglobulin has been shown to prevent rubella in exposed contacts, and they are not recommended.11
INFECTIONS TRANSMITTED BY DIRECT CONTACT
Direct contact transmission includes infectious agents transmitted from an infected or colonized individual to another, whereas indirect contact transmission involves a contaminated intermediate object or person (eg, hands of healthcare providers, electronic thermometers, surgical instruments).1
There are no available postexposure prophylactic regimens for the organisms most commonly transmitted by this route (eg, methicillin-resistant Staphylococcus aureus, Clostridium difficile), but transmission can be prevented with adherence to standard precautions, including hand hygiene.1
Hepatitis A
Person-to-person transmission of hepatitis A virus occurs via the fecal-oral route. Common-source outbreaks and sporadic cases can occur from exposure to food or water contaminated with feces.1,15
Postexposure prophylaxis is indicated only for nonimmune close contacts (eg, household and sexual contacts) (Table 5). Without this treatment, secondary attack rates of 15% to 30% have been reported among households.15,48 Both hepatitis A vaccine and immune globulin are effective in preventing and ameliorating symptomatic hepatitis A infection. Advantages of vaccination include induction of longer-lasting immunity (at least 2 years), greater ease of administration, and lower cost than immune globulin.15,48
Scabies
Scabies is an infestation of the skin by the mite Sarcoptes scabiei var hominis. Person-to-person transmission typically occurs through direct, prolonged skin-to-skin contact with an infested person (eg, household and sexual contacts). However, crusted scabies can be transmitted after brief skin-to-skin contact or by exposure to bedding, clothing, or furniture used by the infested person.
All potentially infested persons should be treated concomitantly (Table 5).14,49
INFECTIONS TRANSMITTED BY MAMMAL BITES AND INJURIES
Bites and injury wounds account for approximately 1% of all visits to emergency departments.50 Human bites are associated with a risk of infection by blood-borne pathogens, herpes simplex infection, and bacterial infections (eg, skin and soft-tissue infections, bacteremia). Animal bites are associated with a risk of bacterial infections, rabies, tetanus, hepatitis B virus, and monkeypox.50
Rabies
Human rabies (Table 5) is almost always fatal. Essential factors in determining the need for postexposure prophylaxis include knowledge of the epidemiology of animal rabies in the area where the contact occurred and the species of animal involved, availability of the animal for observation or rabies testing, health status of the biting animal, and vaccination history of both the animal and exposed individual.6 Clinicians should seek assistance from public health officials for evaluating exposures and determining the need for postexposure prophylaxis in situations that are not routine.51
High-risk wild animals associated with rabies in North America include bats, raccoons, skunks, foxes, coyotes, bobcats, and woodchucks. Bats are the most common source of human rabies infections in the United States, and transmission can occur from minor, sometimes unnoticed, bites. The types of exposures that require postexposure prophylaxis include bites, abrasions, scratches, and contamination of mucous membranes or open wound with saliva or neural tissue of a suspected rabid animal.
Human-to-human transmission of rabies can rarely occur through exposure of mucous membrane or nonintact skin to an infectious material (saliva, tears, neural tissue), in addition to organ transplantation.6
Animal capture and testing is a strategy for excluding rabies risk and reducing the need for postexposure prophylaxis. A dog, cat, or ferret that bites a person should be confined and observed for 10 days without administering postexposure prophylaxis for rabies, unless the bite or exposure is on the face or neck, in which case this treatment should be given immediately.6 If the observed biting animal lives and remains healthy, postexposure prophylaxis is not recommended. However, if signs suggestive of rabies develop, postexposure prophylaxis should be given and the animal should be euthanized, with testing of brain tissue for rabies virus. Postexposure prophylaxis should be discontinued if rabies testing is negative.
The combination of rabies vaccine and human rabies immunoglobulin is nearly 100% effective in preventing rabies if administered in a timely and accurate fashion after exposure (Table 5).6
Tetanus
Tetanus transmission can occur through injuries ranging from small cuts to severe trauma and through contact with contaminated objects (eg, bites, nails, needles, splinters, neonates whose umbilical cord is cut with contaminated surgical instruments, and during circumcision or piercing with contaminated instruments).5
Tetanus is almost completely preventable with vaccination, and timely administration of postexposure prophylaxis (tetanus toxoid-containing vaccine, tetanus immune globulin) decreases disease severity (Table 5).2,5,52
People who have been exposed to an infectious disease should be evaluated promptly and systematically, whether they are healthcare professionals at work,1 patients, or contacts of patients. The primary goals are to prevent acquisition and transmission of the infection, allay the exposed person’s anxiety, and avoid unnecessary interventions and loss of work days.1,2 Some may need postexposure prophylaxis.
ESSENTIAL ELEMENTS OF POSTEXPOSURE MANAGEMENT
Because postexposure management can be challenging, an experienced clinician or expert consultant (eg, infectious disease specialist, infection control provider, or public health officer) should be involved. Institution-specific policies and procedures for postexposure prophylaxis and testing should be followed.1,2
Postexposure management should include the following elements:
- Immediate care of the wound or other site of exposure in cases of blood-borne exposures and tetanus- and rabies-prone injuries. This includes thoroughly washing with soap and water or cleansing with an antiseptic agent, flushing affected mucous membranes with water, and debridement of devitalized tissue.1–6
- Deciding whether postexposure prophylaxis is indicated and, if so, the type, dose, route, and duration.
- Initiating prophylaxis as soon as possible.
- Determining an appropriate baseline assessment and follow-up plan for the exposed individual.
- Counseling exposed women who are pregnant or breast-feeding about the risks and benefits of postexposure prophylaxis to mother, fetus, and infant.
- Identifying required infection control precautions, including work and school restriction, for exposed and source individuals.
- Counseling and psychological support for exposed individuals, who need to know about the risks of acquiring the infection and transmitting it to others, infection control precautions, benefits, and adverse effects of postexposure prophylaxis, the importance of adhering to the regimen, and the follow-up plan. They must understand that this treatment may not completely prevent the infection, and they should seek medical attention if they develop fever or any symptoms or signs of the infection of concern.1,2
IS POSTEXPOSURE PROPHYLAXIS INDICATED?
Postexposure management begins with an assessment to determine whether the exposure is likely to result in infection; whether the exposed individual is susceptible to the infection of concern or is at greater risk of complications from it than the general population; and whether postexposure prophylaxis is needed. This involves a complete focused history, physical examination, and laboratory testing of the potentially exposed individual and of the source, if possible.1,2
Postexposure prophylaxis should begin as soon as possible to maximize its effects while awaiting the results of further diagnostic tests. However, if the exposed individual seeks care after the recommended period, prophylactic therapy can still be effective for certain infections that have a long incubation period, such as tetanus and rabies.5,6 The choice of regimen should be guided by efficacy, safety, cost, toxicity, ease of adherence, drug interactions, and antimicrobial resistance.1,2
HOW GREAT IS THE RISK OF INFECTION?
Exposed individuals are not all at the same risk of acquiring a given infection. The risk depends on:
- Type and extent of exposure (see below)
- Characteristics of the infectious agent (eg, virulence, infectious dose)
- Status of the infectious source (eg, whether the disease is in its infectious period or is being treated); effective treatment can shorten the duration of microbial shedding and subsequently reduce risk of transmission of certain infections such as tuberculosis, meningococcal infection, invasive group A streptococcal infection, and pertussis7–10
- Immune status of the exposed individual (eg, prior infection or vaccination), since people who are immune to the infection of concern usually do not need postexposure prophylaxis2
- Adherence to infection prevention and control principles; postexposure prophylaxis may not be required if the potentially exposed individual was wearing appropriate personal protective equipment such as a surgical mask, gown, and gloves and was following standard precautions.1
WHO SHOULD BE RESTRICTED FROM WORK OR SCHOOL?
Most people without symptoms who were exposed to most types of infections do not need to stay home from work or school. However, susceptible people, particularly healthcare providers exposed to measles, mumps, rubella, and varicella, should be excluded from work while they are capable of transmitting these diseases, even if they have no symptoms.11,12 Moreover, people with symptoms with infections primarily transmitted via the airborne, droplet, or contact route should be restricted from work until no longer infectious.1,2,7,9–15
Most healthcare institutions have clear protocols for managing occupational exposures to infectious diseases, in particular for blood-borne pathogens such as human immunodeficiency virus (HIV). The protocol should include appropriate evaluation and laboratory testing of the source patient and exposed healthcare provider, as well as procedures for counseling the exposed provider, identifying and procuring an initial prophylactic regimen for timely administration, a mechanism for formal expert consultation (eg, with an in-house infectious diseases consultant), and a plan for outpatient follow-up.
The next section reviews postexposure management of common infections categorized by mode of transmission, including the risk of transmission, initial and follow-up evaluation, and considerations for postexposure prophylaxis.
BLOOD-BORNE INFECTIONS
Blood-borne pathogens can be transmitted by accidental needlesticks or cuts or by exposure of the eyes, mucous membranes, or nonintact skin to blood, tissue, or other potentially infectious body fluids—cerebrospinal, pericardial, pleural, peritoneal, synovial, and amniotic fluid, semen, and vaginal secretions. (Feces, nasal secretions, saliva, sputum, sweat, tears, urine, and vomitus are considered noninfectious for blood-borne pathogens unless they contain blood.16)
Healthcare professionals are commonly exposed to blood-borne pathogens as a result of needlestick injuries, and these exposures tend to be underreported.17
When someone has been exposed to blood or other infectious body fluids, the source individual and the exposed individual should be assessed for risk factors for hepatitis B virus, hepatitis C virus, HIV, and other blood-borne pathogens.3,4,16,18 If the disease status for these viruses is unknown, the source and exposed individual should be tested in accordance with institutional policies regarding consent to testing. Testing of needles or sharp instruments implicated in an exposure is not recommended.3,4,16,18
Determining the need for prophylaxis after exposure to an unknown source such as a disposed needle can be challenging. Assessment should be made on a case-by-case basis, depending on the known prevalence of the infection of concern in the local community. The risk of transmission in most source-unknown exposures is negligible.3,4,18 However, hepatitis B vaccine and hepatitis B immunoglobulin should be used liberally as postexposure prophylaxis for previously unvaccinated healthcare providers exposed to an unknown source.3,4,16,18
Hepatitis B
Hepatitis B virus (Table 1) is the most infectious of the common blood-borne viruses. The risk of transmission after percutaneous exposure to hepatitis B-infected blood ranges from 1% to 30% based on hepatitis Be antigen status and viral load (based on hepatitis B viral DNA).1,2,4,16
Hepatitis B vaccine or immunoglobulin, or both, are recommended for postexposure prophylaxis in pregnant women, based on evidence that perinatal transmission was reduced by 70% to 90% when these were given within 12 to 24 hours of exposure.4,16,19
Hepatitis C
The risk of infection after percutaneous exposure to hepatitis C virus-infected blood is estimated to be 1.8% per exposure.16 The risk is lower with exposure of a mucous membrane or nonintact skin to blood, fluids, or tissues from hepatitis C-infected patients.16,18
Since there is no effective postexposure prophylactic regimen, the goal of postexposure assessment of hepatitis C is early identification of infection (by monitoring the patient to see if he or she seroconverts) and, if infection is present, referral to an experienced clinician for further evaluation (Table 1). However, data supporting the utility of direct-acting anti-hepatitis C antiviral drugs as postexposure prophylaxis after occupational exposure to hepatitis C are lacking.
Human immunodeficiency virus
The estimated risk of HIV transmission from a known infected source after percutaneous exposure is 0.3%, and after mucosal exposures it is 0.09%.20
If postexposure prophylaxis is indicated, it should be a three-drug regimen (Table 1).3,18 The recommended antiretroviral therapies have been proven effective in clinical trials of HIV treatment, not for postexposure prophylaxis per se, but they are recommended because they are effective, safe, tolerable, and associated with high adherence rates.3,16,18,21 If the source individual is known to have HIV infection, information about his or her stage of infection, CD4+ T-cell count, results of viral load testing, current and previous antiretroviral therapy, and results of any genotypic viral resistance testing will guide the choice of postexposure prophylactic regimen.3,18
The clinician should give the exposed patient a starter pack of 5 to 7 days of medication, give the first dose then and there, and arrange follow-up with an experienced clinician within a few days of the exposure to determine whether a complete 30-day course is needed.3,16,18
SEXUALLY TRANSMITTED INFECTIONS
In the case of sexually transmitted infections, “exposure” means unprotected sexual contact with someone who has a sexually transmitted infection.22 People with sexually transmitted infections often have no symptoms but can still transmit the infection. Thus, people at risk should be identified and screened for all suspected sexually transmitted infections.23–25
Patients with sexually transmitted infections should be instructed to refer their sex partners for evaluation and treatment to prevent further transmission and reinfection. Assessment of exposed partners includes a medical history, physical examination, microbiologic testing for all potential sexually transmitted infections, and eligibility for hepatitis A virus, hepatitis B virus, and human papillomavirus vaccines.22 Ideally, exposed partners should be reassessed within 1 to 2 weeks to follow up testing results and to monitor for side effects of and adherence to postexposure prophylaxis, if applicable.
Public health departments should be notified of sexually transmitted infections such as gonorrhea, chlamydia, chancroid, and syphilis.22
Expedited partner therapy, in which index patients deliver the medication or a prescription for it directly to their partners, is an alternative for partner management where legally allowed by state and local health departments (see www.cdc.gov/std/ept/legal/).22
Recommended postexposure prophylactic regimens for sexually transmitted infections (Table 2) are based on their efficacy in the treatment of these infections.22,26–28 The regimen for HIV prophylaxis is the same as in Table 1.3,18,26
Chlamydia
Chlamydia is the most commonly reported communicable disease in the United States. The risk of transmission after sexual intercourse with a person who has an active infection is approximately 65% and increases with the number of exposures.22,29
Gonorrhea
Infection with Neisseria gonorrhoeae is the second most commonly reported communicable disease in the United States. The transmission rate of gonorrhea after sex with someone who has it ranges from 50% to 93%.22 When prescribing postexposure prophylaxis for gonorrhea, it is essential to consider the risk of antimicrobial resistance and local susceptibility data.22
Human immunodeficiency virus
Risk of HIV transmission through sexual contact varies depending on the nature of the exposure, ranging from 0.05% to 0.5%.30
Syphilis
The risk of transmission of syphilis in its early stages (primary and secondary) after sexual exposure is approximately 30%. Transmission requires open lesions such as chancres in primary syphilis and mucocutaneous lesions (mucous patches, condyloma lata) in secondary syphilis.22
After sexual assault
In cases of sexual assault, the risk of sexually transmitted infections may be increased due to trauma and bleeding. Testing for all sexually transmitted infections, including HIV, should be considered on a case-by-case basis.22
Survivors of sexual assault have been shown to be poorly compliant with follow-up visits, and thus provision of postexposure prophylaxis at the time of initial assessment is preferable to deferred treatment.22 The recommended regimen should cover chlamydia, gonorrhea, and trichomoniasis (a single dose of intramuscular ceftriaxone 250 mg, oral azithromycin 1 g, and either oral metronidazole 2 g or tinidazole 2 g), in addition to HIV if the victim presents within 72 hours of exposure (Table 2).22,26
Hepatitis B virus vaccine, not immunoglobulin, should be given if the hepatitis status of the assailant is unknown and the survivor has not been previously vaccinated. Both hepatitis B vaccine and immunoglobulin should be given to unvaccinated survivors if the assailant is known to be hepatitis B surface antigen-positive.22
Human papillomavirus vaccination is recommended for female survivors ages 9 to 26 and male survivors ages 9 to 21.
Emergency contraception should be given if there is a risk of pregnancy.22,26
In many jurisdictions, sexual assault centers provide trained examiners through Sexual Assault Nurse Examiners to perform evidence collection and to provide initial contact with the aftercare resources of the center.
Advice on medical management of sexual assault can be obtained by calling National PEPline (888–448–4911).
INFECTIONS TRANSMITTED BY THE AIRBORNE ROUTE
Airborne transmission of infections occurs by inhalation of droplet nuclei (diameter ≤ 5 μm) generated by coughing and sneezing. Certain procedures (eg, administration of nebulized medication, sputum induction, bronchoscopy) also generate droplets and aerosols, which can transmit organisms.1
Measles
Measles (Table 3) is highly contagious; up to 90% of susceptible individuals develop measles after exposure. The virus is transmitted by direct contact with infectious droplets and by the airborne route. It remains infectious in the air and on surfaces for up to 2 hours; therefore, any type of exposure, even transient, is an indication for postexposure prophylaxis in susceptible individuals.11
Both the measles, mumps, rubella (MMR) vaccine and immune globulin may prevent or modify disease severity in susceptible exposed individuals if given within 3 days of exposure (for the vaccine) or within 6 days of exposure (for immune globulin).31,32
Tuberculosis
Mycobacterium tuberculosis is transmitted from patients with pulmonary or laryngeal tuberculosis, particularly if patients cough and are sputum-positive for acid-fast bacilli. Patients with extrapulmonary tuberculosis or latent tuberculosis infection are not infectious.1,7
Postexposure management of tuberculosis occurs through contact investigation of a newly diagnosed index case of tuberculosis disease. Contacts are categorized as household contacts, close nonhousehold contacts (those having regular, extensive contact with the index case), casual contacts, and transient community contacts. The highest priority for contact investigations should be household contacts, close nonhousehold or casual contacts at high risk of progressing to tuberculosis disease (eg, those with HIV, those on dialysis, or transplant recipients), and unprotected healthcare providers exposed during aerosol-generating procedures.7,33
Postexposure management includes screening exposed individuals for tuberculosis symptoms and performing tuberculin skin testing or interferon-gamma release assay (blood testing) for those who had previously negative results (Table 3). Chest radiography is recommended for exposed immunocompromised individuals, due to high risk of tuberculosis disease and low sensitivity of skin or blood testing, and for those with a documented history of tuberculosis or previous positive skin or blood test.7,33,34
A positive tuberculin skin test for persons with recent contact with tuberculosis is defined as a wheal 5 mm or larger on baseline or follow-up screening. Prior bacillus Calmette-Guérin vaccination status should not be used in the interpretation of tuberculin skin testing in the setting of contact investigation.7,33
All exposed asymptomatic people with a positive result on testing should be treated for latent tuberculosis infection, since treatment reduces the risk of progression to tuberculosis disease by 60% to 90% .7,33,35–37
Varicella and disseminated herpes zoster
Varicella zoster virus is transmitted by direct contact with vesicular fluid of skin lesions and inhalation of aerosols from vesicular fluid or respiratory tract secretions. Varicella (chickenpox) is highly contagious, with a secondary attack rate in susceptible household contacts of 85%.12 Herpes zoster is less contagious than varicella.38
Postexposure prophylaxis against varicella is recommended for susceptible individuals who had household exposure, had face-to-face contact with an infectious patient while indoors, or shared the same hospital room with the patient.12
Postexposure prophylactic options for varicella and herpes zoster include varicella vaccine (not zoster vaccine) and varicella zoster immune globulin (Table 3).12,38–40
Varicella vaccine is approximately 90% effective if given within 3 days of exposure, and 70% effective if given within 5 days.12,39
Antiviral agents should be given if the exposed individual develops manifestations of varicella or herpes zoster.12,38
INFECTIONS TRANSMITTED BY THE DROPLET ROUTE
Droplet transmission occurs when respiratory droplets carrying infectious agents travel directly across short distances (3–6 feet) from the respiratory tract of the infected to mucosal surfaces of the susceptible exposed individual. Droplets are generated during coughing, sneezing, talking, and aerosol-generating procedures. Indirect contact with droplets can also transmit infection.1
Group A streptococcal infection
Although group A streptococcal infection (Table 4) may spread to close contacts of the index case and in closed populations (eg, military recruit camps, schools, institutions), secondary cases of invasive group A streptococcal infection rarely occur in family and institutional contacts.9,41,42
Postexposure prophylaxis for contacts of people with invasive group A streptococcal infection is debated, because it is unknown if antibiotic therapy will decrease the risk of acquiring the infection. It is generally agreed that it should not be routinely given to all contacts. The decision should be based on the clinician’s assessment of each individual’s risk and guidance from the local institution. If indicated, postexposure prophylaxis should be given to household and close contacts, particularly in high-risk groups (eg, Native Americans and those with risk factors such as old age, HIV infection, diabetes mellitus, heart disease, chickenpox, cancer, systemic corticosteroid therapy, other immunosuppressive medications, intravenous drug use, recent surgery or childbirth).9,41,42
Influenza
Influenza (Table 4) causes a significant burden in healthcare settings, given its prevalence and potential to cause outbreaks of severe respiratory illness in hospitalized patients and residents of long-term-care facilities.13,43
Neuraminidase inhibitors are effective as prophylaxis after unprotected exposure to influenza, particularly in outbreak situations. However, their use is not widely recommended, since overuse could lead to antiviral resistance. In selected cases, postexposure prophylaxis may be indicated for close contacts who are at high risk of complications of influenza (eg, age 65 or older, in third trimester of pregnancy or 2 weeks postpartum, morbid obesity, chronic comorbid conditions such as a cardiopulmonary and renal disorder, immunocompromising condition) or who are in close contact with persons at high risk of influenza-related complications.13,44,45
Meningococcal disease
N meningitidis is transmitted from individuals with meningococcal disease or from asymptomatic carriers.8
Postexposure prophylaxis is effective in eradicating N meningiditis and is recommended for all close contacts of patients with invasive meningococcal disease (Table 4).46 Close contacts include household contacts, childcare and preschool contacts, contacts exposed in dormitories or military training centers, those who had direct contact with the index case’s respiratory secretions (eg, intimate kissing, mouth-to-mouth resuscitation, unprotected contact during endotracheal intubation or endotracheal tube management), and passengers seated directly next to an index case on airplane flights of longer than 8 hours.
Postexposure prophylaxis is not indicated for those who had brief contact, those who had contact that did not involve exposure to oral or respiratory secretions, or for close contacts of patients with N meningitidis isolated in nonsterile sites only (eg, oropharynyx, trachea, conjunctiva).8,46
Pertussis
Pertussis is highly contagious, with a secondary attack rate of approximately 80% in susceptible individuals. Approximately one-third of susceptible household contacts develop pertussis after exposure.10
Postexposure prophylaxis for pertussis should be given to all household and close contacts (Table 4).10,47
Rubella
Transmission occurs through droplets or direct contact with nasopharyngeal secretions of an infectious case. Neither MMR vaccine nor immunoglobulin has been shown to prevent rubella in exposed contacts, and they are not recommended.11
INFECTIONS TRANSMITTED BY DIRECT CONTACT
Direct contact transmission includes infectious agents transmitted from an infected or colonized individual to another, whereas indirect contact transmission involves a contaminated intermediate object or person (eg, hands of healthcare providers, electronic thermometers, surgical instruments).1
There are no available postexposure prophylactic regimens for the organisms most commonly transmitted by this route (eg, methicillin-resistant Staphylococcus aureus, Clostridium difficile), but transmission can be prevented with adherence to standard precautions, including hand hygiene.1
Hepatitis A
Person-to-person transmission of hepatitis A virus occurs via the fecal-oral route. Common-source outbreaks and sporadic cases can occur from exposure to food or water contaminated with feces.1,15
Postexposure prophylaxis is indicated only for nonimmune close contacts (eg, household and sexual contacts) (Table 5). Without this treatment, secondary attack rates of 15% to 30% have been reported among households.15,48 Both hepatitis A vaccine and immune globulin are effective in preventing and ameliorating symptomatic hepatitis A infection. Advantages of vaccination include induction of longer-lasting immunity (at least 2 years), greater ease of administration, and lower cost than immune globulin.15,48
Scabies
Scabies is an infestation of the skin by the mite Sarcoptes scabiei var hominis. Person-to-person transmission typically occurs through direct, prolonged skin-to-skin contact with an infested person (eg, household and sexual contacts). However, crusted scabies can be transmitted after brief skin-to-skin contact or by exposure to bedding, clothing, or furniture used by the infested person.
All potentially infested persons should be treated concomitantly (Table 5).14,49
INFECTIONS TRANSMITTED BY MAMMAL BITES AND INJURIES
Bites and injury wounds account for approximately 1% of all visits to emergency departments.50 Human bites are associated with a risk of infection by blood-borne pathogens, herpes simplex infection, and bacterial infections (eg, skin and soft-tissue infections, bacteremia). Animal bites are associated with a risk of bacterial infections, rabies, tetanus, hepatitis B virus, and monkeypox.50
Rabies
Human rabies (Table 5) is almost always fatal. Essential factors in determining the need for postexposure prophylaxis include knowledge of the epidemiology of animal rabies in the area where the contact occurred and the species of animal involved, availability of the animal for observation or rabies testing, health status of the biting animal, and vaccination history of both the animal and exposed individual.6 Clinicians should seek assistance from public health officials for evaluating exposures and determining the need for postexposure prophylaxis in situations that are not routine.51
High-risk wild animals associated with rabies in North America include bats, raccoons, skunks, foxes, coyotes, bobcats, and woodchucks. Bats are the most common source of human rabies infections in the United States, and transmission can occur from minor, sometimes unnoticed, bites. The types of exposures that require postexposure prophylaxis include bites, abrasions, scratches, and contamination of mucous membranes or open wound with saliva or neural tissue of a suspected rabid animal.
Human-to-human transmission of rabies can rarely occur through exposure of mucous membrane or nonintact skin to an infectious material (saliva, tears, neural tissue), in addition to organ transplantation.6
Animal capture and testing is a strategy for excluding rabies risk and reducing the need for postexposure prophylaxis. A dog, cat, or ferret that bites a person should be confined and observed for 10 days without administering postexposure prophylaxis for rabies, unless the bite or exposure is on the face or neck, in which case this treatment should be given immediately.6 If the observed biting animal lives and remains healthy, postexposure prophylaxis is not recommended. However, if signs suggestive of rabies develop, postexposure prophylaxis should be given and the animal should be euthanized, with testing of brain tissue for rabies virus. Postexposure prophylaxis should be discontinued if rabies testing is negative.
The combination of rabies vaccine and human rabies immunoglobulin is nearly 100% effective in preventing rabies if administered in a timely and accurate fashion after exposure (Table 5).6
Tetanus
Tetanus transmission can occur through injuries ranging from small cuts to severe trauma and through contact with contaminated objects (eg, bites, nails, needles, splinters, neonates whose umbilical cord is cut with contaminated surgical instruments, and during circumcision or piercing with contaminated instruments).5
Tetanus is almost completely preventable with vaccination, and timely administration of postexposure prophylaxis (tetanus toxoid-containing vaccine, tetanus immune globulin) decreases disease severity (Table 5).2,5,52
- Siegel JD, Rhinehart E, Jackson M, Chiarello L; Health Care Infection Control Practices Advisory Committee. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control 2007; 35(suppl 2): S65–S164.
- Advisory Committee on Immunization Practices; Centers for Disease Control and Prevention. Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:1–45.
- Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol 2013; 34: 875–892.
- Schille S, Murphy TV, Sawyer M, et al; Centers for Disease Control and Prevention (CDC). CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR Recomm Rep 2013; 62:1–19.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:13–15.
- Manning SE, Rupprecht CE, Fishbein D, et al; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Human rabies prevention—United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2008; 57:1–28.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon R, Centers for Disease Control and Prevention (CDC). Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Morb Mortal Wkly Rep 2005; 54:1–141.
- Cohn AC, MacNeil JR, Clark TA, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2013; 62:1–28.
- Prevention of Invasive Group A Streptococcal Infections Workshop Participants. Prevention of invasive group A streptococcal disease among household contacts of case patients and among postpartum and postsurgical patients: recommendations from the Centers for Disease Control and Prevention. Clin Infect Dis 2002; 35:950–959.
- Tiwari T, Murphy TV, Moran J; National Immunization Program, Centers for Disease Control and Prevention (CDC). Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis: 2005 CDC Guidelines. MMWR Morb Mortal Wkly Rep 2005; 54:1–16.
- McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS; Centers for Disease Control and Prevention (CDC). Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2013; 62:1–34.
- Marin M, Guris D, Chaves SS, Schmid S, Seward JF; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56:1–40.
- Harper SA, Bradley JS, Englund JA, et al; Expert Panel of the Infectious Diseases Society of America. Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1003–1032.
- Centers for Disease Control and Prevention (CDC). Scabies. www.cdc.gov/parasites/scabies/. Accessed November 4, 2016.
- Advisory Committee on Immunization Practices (ACIP); Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2006; 55:1–23.
- US Public Health Service. Updated US Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep 2001; 50:1–52.
- Treakle AM, Schultz M, Giannakos GP, Joyce PC, Gordin FM. Evaluating a decade of exposures to blood and body fluids in an inner-city teaching hospital. Infect Control Hosp Epidemiol 2011; 32:903–907.
- New York State Department of Health AIDS Institute. Update: HIV prophylaxis following non-occupational exposure. www.hivguidelines.org/clinical-guidelines/post-exposure-prophylaxis/hiv-prophylaxis-following-non-occupational-exposure/. Accessed November 4, 2016.
- Beasley RP, Hwang LY, Lee GC, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet 1983; 2:1099–1102.
- Baggaley RF, Boily MC, White RG, Alary M. Risk of HIV-1 transmission for parenteral exposure and blood transfusion: a systematic review and meta-analysis. AIDS 2006; 20:805–812.
- McAllister J, Read P, McNulty A, Tong WW, Ingersoll A, Carr A. Raltegravir-emtricitabine-tenofovir as HIV nonoccupational post-exposure prophylaxis in men who have sex with men: safety, tolerability and adherence. HIV Med 2014; 15:13–22.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137.
- US Preventive Services Task Force (USPSTF). Final recommendation statement: chlamydia and gonorrhea: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/chlamydia-and-gonorrhea-screening. Accessed November 4, 2016.
- US Preventive Services Task Force (USPSTF). Human immunodeficiency virus (HIV) infection: screening. www.uspreventiveservicestaskforce.org/uspstf/uspshivi.htm. Accessed November 4, 2016.
- US Preventive Services Task Force (USPSTF). Screening for syphilis. www.uspreventiveservicestaskforce.org/uspstf/uspssyph.htm#update. Accessed November 4, 2016.
- Smith DK, Grohskopf LA, Black RJ, et al; US Department of Health and Human Services. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the US Department of Health and Human Services. MMWR Recomm Rep 2005; 54:1–20.
- Lin JS, Donegan SP, Heeren TC, et al. Transmission of Chlamydia trachomatis and Neisseria gonorrhoeae among men with urethritis and their female sex partners. J Infect Dis 1998; 178:1707–1712.
- Varghese B, Maher JE, Peterman TA, Branson BM, Steketee RW. Reducing the risk of sexual HIV transmission: quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use. Sex Transm Dis 2002; 29:38–43.
- Gülmezoglu AM, Azhar M. Interventions for trichomoniasis in pregnancy. Cochrane Database Syst Rev 2011; (5):CD000220.
- Forna F, Gülmezoglu AM. Interventions for treating trichomoniasis in women. Cochrane Database Syst Rev 2003; (2):CD000218.
- Rice P, Young Y, Cohen B, Ramsay M. MMR immunization after contact with measles virus. Lancet 2004; 363:569–570.
- Young MK, Nimmo GR, Cripps AW, Jones MA. Post-exposure passive immunization for preventing measles. Cochrane Database Syst Rev 2014; 4:CD010056.
- National Tuberculosis Controllers Association; Centers for Disease Control and Prevention (CDC). Guidelines for the investigation of contacts of persons with infectious tuberculosis. Recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Recomm Rep 2005; 54:1–47.
- Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Morb Mortal Wkly Rep 2010; 59:1–25.
- Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Morb Mortal Wkly Rep 2000; 49:1–51.
- Stagg HR, Zenner D, Harris RJ, Munoz L, Lipman MC, Abubakar I. Treatment of latent tuberculosis infection: a network meta-analysis. Ann Intern Med 2014; 161:419–428.
- Centers for Disease Control and Prevention (CDC). Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep 2011; 60:1650–1653.
- Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2008; 57:1–30.
- Macartney K, Heywood A, McIntyre P. Vaccines for post-exposure prophylaxis against varicella (chickenpox) in children and adults. Cochrane Database Syst Rev 2014; 6:CD001833.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of VariZIG—United States, 2013. MMWR Morb Mortal Wkly Rep 2013; 62: 574–576.
- Public Health Agency of Canada. Guidelines for the prevention and control of invasive group A streptococcal disease. Can Commun Dis Rep 2006; 32(suppl 2):1–26.
- Steer JA, Lamagni T, Healy B, et al. Guidelines for prevention and control of group A streptococcal infection in acute healthcare and maternity settings in the UK. J Infect 2012; 64:1–18.
- Grohskopf LA, Olsen SJ, Sokolow LZ, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2014–15 influenza season. MMWR Morb Mortal Wkly Rep 2014; 63: 691–697.
- Fiore AE, Fry A, Shay D, et al; Centers for Disease Control and Prevention (CDC). Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2011; 60:1–24.
- Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev 2014; 4:CD008965.
- Zalmanovici Trestioreanu A, Fraser A, Gafter-Gvili A, Paul M, Leibovici L. Antibiotics for preventing meningococcal infections. Cochrane Database Syst Rev 2013; 10:CD004785.
- Altunaiji S, Kukuruzovic R, Curtis N, Massie J. Antibiotics for whooping cough (pertussis). Cochrane Database Syst Rev 2007: CD004404.
- Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56:1080–1084.
- FitzGerald D, Grainger RJ, Reid A. Interventions for preventing the spread of infestation in close contacts of people with scabies. Cochrane Database Syst Rev 2014; 2:CD009943.
- Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:e10–e52.
- Rupprecht CE, Briggs D, Brown CM, et al; Centers for Disease Control and Prevention (CDC). Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies—recommendations of the Advisory Committee on Immunization Practice. MMWR Recomm Rep 2010; 59:1–9.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine in adults aged 65 years and older—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep 2012; 61:468–470.
- Siegel JD, Rhinehart E, Jackson M, Chiarello L; Health Care Infection Control Practices Advisory Committee. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control 2007; 35(suppl 2): S65–S164.
- Advisory Committee on Immunization Practices; Centers for Disease Control and Prevention. Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:1–45.
- Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol 2013; 34: 875–892.
- Schille S, Murphy TV, Sawyer M, et al; Centers for Disease Control and Prevention (CDC). CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR Recomm Rep 2013; 62:1–19.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:13–15.
- Manning SE, Rupprecht CE, Fishbein D, et al; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Human rabies prevention—United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2008; 57:1–28.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon R, Centers for Disease Control and Prevention (CDC). Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Morb Mortal Wkly Rep 2005; 54:1–141.
- Cohn AC, MacNeil JR, Clark TA, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2013; 62:1–28.
- Prevention of Invasive Group A Streptococcal Infections Workshop Participants. Prevention of invasive group A streptococcal disease among household contacts of case patients and among postpartum and postsurgical patients: recommendations from the Centers for Disease Control and Prevention. Clin Infect Dis 2002; 35:950–959.
- Tiwari T, Murphy TV, Moran J; National Immunization Program, Centers for Disease Control and Prevention (CDC). Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis: 2005 CDC Guidelines. MMWR Morb Mortal Wkly Rep 2005; 54:1–16.
- McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS; Centers for Disease Control and Prevention (CDC). Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2013; 62:1–34.
- Marin M, Guris D, Chaves SS, Schmid S, Seward JF; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56:1–40.
- Harper SA, Bradley JS, Englund JA, et al; Expert Panel of the Infectious Diseases Society of America. Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1003–1032.
- Centers for Disease Control and Prevention (CDC). Scabies. www.cdc.gov/parasites/scabies/. Accessed November 4, 2016.
- Advisory Committee on Immunization Practices (ACIP); Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2006; 55:1–23.
- US Public Health Service. Updated US Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep 2001; 50:1–52.
- Treakle AM, Schultz M, Giannakos GP, Joyce PC, Gordin FM. Evaluating a decade of exposures to blood and body fluids in an inner-city teaching hospital. Infect Control Hosp Epidemiol 2011; 32:903–907.
- New York State Department of Health AIDS Institute. Update: HIV prophylaxis following non-occupational exposure. www.hivguidelines.org/clinical-guidelines/post-exposure-prophylaxis/hiv-prophylaxis-following-non-occupational-exposure/. Accessed November 4, 2016.
- Beasley RP, Hwang LY, Lee GC, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet 1983; 2:1099–1102.
- Baggaley RF, Boily MC, White RG, Alary M. Risk of HIV-1 transmission for parenteral exposure and blood transfusion: a systematic review and meta-analysis. AIDS 2006; 20:805–812.
- McAllister J, Read P, McNulty A, Tong WW, Ingersoll A, Carr A. Raltegravir-emtricitabine-tenofovir as HIV nonoccupational post-exposure prophylaxis in men who have sex with men: safety, tolerability and adherence. HIV Med 2014; 15:13–22.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137.
- US Preventive Services Task Force (USPSTF). Final recommendation statement: chlamydia and gonorrhea: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/chlamydia-and-gonorrhea-screening. Accessed November 4, 2016.
- US Preventive Services Task Force (USPSTF). Human immunodeficiency virus (HIV) infection: screening. www.uspreventiveservicestaskforce.org/uspstf/uspshivi.htm. Accessed November 4, 2016.
- US Preventive Services Task Force (USPSTF). Screening for syphilis. www.uspreventiveservicestaskforce.org/uspstf/uspssyph.htm#update. Accessed November 4, 2016.
- Smith DK, Grohskopf LA, Black RJ, et al; US Department of Health and Human Services. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the US Department of Health and Human Services. MMWR Recomm Rep 2005; 54:1–20.
- Lin JS, Donegan SP, Heeren TC, et al. Transmission of Chlamydia trachomatis and Neisseria gonorrhoeae among men with urethritis and their female sex partners. J Infect Dis 1998; 178:1707–1712.
- Varghese B, Maher JE, Peterman TA, Branson BM, Steketee RW. Reducing the risk of sexual HIV transmission: quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use. Sex Transm Dis 2002; 29:38–43.
- Gülmezoglu AM, Azhar M. Interventions for trichomoniasis in pregnancy. Cochrane Database Syst Rev 2011; (5):CD000220.
- Forna F, Gülmezoglu AM. Interventions for treating trichomoniasis in women. Cochrane Database Syst Rev 2003; (2):CD000218.
- Rice P, Young Y, Cohen B, Ramsay M. MMR immunization after contact with measles virus. Lancet 2004; 363:569–570.
- Young MK, Nimmo GR, Cripps AW, Jones MA. Post-exposure passive immunization for preventing measles. Cochrane Database Syst Rev 2014; 4:CD010056.
- National Tuberculosis Controllers Association; Centers for Disease Control and Prevention (CDC). Guidelines for the investigation of contacts of persons with infectious tuberculosis. Recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Recomm Rep 2005; 54:1–47.
- Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Morb Mortal Wkly Rep 2010; 59:1–25.
- Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Morb Mortal Wkly Rep 2000; 49:1–51.
- Stagg HR, Zenner D, Harris RJ, Munoz L, Lipman MC, Abubakar I. Treatment of latent tuberculosis infection: a network meta-analysis. Ann Intern Med 2014; 161:419–428.
- Centers for Disease Control and Prevention (CDC). Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep 2011; 60:1650–1653.
- Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2008; 57:1–30.
- Macartney K, Heywood A, McIntyre P. Vaccines for post-exposure prophylaxis against varicella (chickenpox) in children and adults. Cochrane Database Syst Rev 2014; 6:CD001833.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of VariZIG—United States, 2013. MMWR Morb Mortal Wkly Rep 2013; 62: 574–576.
- Public Health Agency of Canada. Guidelines for the prevention and control of invasive group A streptococcal disease. Can Commun Dis Rep 2006; 32(suppl 2):1–26.
- Steer JA, Lamagni T, Healy B, et al. Guidelines for prevention and control of group A streptococcal infection in acute healthcare and maternity settings in the UK. J Infect 2012; 64:1–18.
- Grohskopf LA, Olsen SJ, Sokolow LZ, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2014–15 influenza season. MMWR Morb Mortal Wkly Rep 2014; 63: 691–697.
- Fiore AE, Fry A, Shay D, et al; Centers for Disease Control and Prevention (CDC). Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2011; 60:1–24.
- Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev 2014; 4:CD008965.
- Zalmanovici Trestioreanu A, Fraser A, Gafter-Gvili A, Paul M, Leibovici L. Antibiotics for preventing meningococcal infections. Cochrane Database Syst Rev 2013; 10:CD004785.
- Altunaiji S, Kukuruzovic R, Curtis N, Massie J. Antibiotics for whooping cough (pertussis). Cochrane Database Syst Rev 2007: CD004404.
- Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56:1080–1084.
- FitzGerald D, Grainger RJ, Reid A. Interventions for preventing the spread of infestation in close contacts of people with scabies. Cochrane Database Syst Rev 2014; 2:CD009943.
- Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:e10–e52.
- Rupprecht CE, Briggs D, Brown CM, et al; Centers for Disease Control and Prevention (CDC). Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies—recommendations of the Advisory Committee on Immunization Practice. MMWR Recomm Rep 2010; 59:1–9.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine in adults aged 65 years and older—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep 2012; 61:468–470.
KEY POINTS
- Whether to give prophylactic therapy depends on the transmissibility of the infection, the susceptibility of the exposed individual, and the risk of infection-related complications.
- Postexposure prophylactic therapy should begin as soon as possible, while awaiting results of further diagnostic tests, to maximize the chances of preventing or ameliorating the infection.
- Keeping up-to-date with current institutional policies and national guidelines is essential. Sources include US Public Health Service guidelines and reports from the US Centers for Disease Control and Prevention, as well as consultation with an expert healthcare provider (eg, infectious diseases physician, infection control provider, public health officer).