User login
Benefits and challenges of caring for international patients
It is much more important to know what sort of a patient has a disease than what sort of a disease a patient has.
—Attributed to Sir William Osler1
Recent years have seen an increase in people traveling away from their home region for healthcare, often for care that is less expensive or unavailable where they live.2–4 Many Americans seek care abroad (engaging in “medical tourism”); conversely, the United States annually receives thousands of foreign travelers for medical evaluations, a trend projected to increase.2,3,5 Additionally, US healthcare providers often see foreign travelers for unexpected ailments that develop during their time here.
Traveling for healthcare can be stressful for patients, and caring for international patients may pose challenges for providers and medical centers. On the other hand, such encounters also provide many mutual benefits. Unfortunately, there is little published guidance addressing these issues.2 In this article, we therefore discuss many of the benefits and challenges, with the hope of improving the quality of care delivered and the clinical experience for both providers and patients.
CHALLENGES FOR INTERNATIONAL PATIENTS AND THEIR PROVIDERS
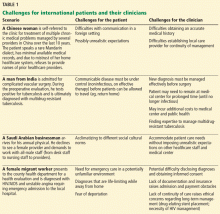
Some scenarios that illustrate challenges faced by international patients and their healthcare providers are presented in Table 1.
For patients, heightened anxiety
Many international patients feel anxious, isolated, and vulnerable, particularly if they have never been away from home before. These feelings arise from multiple factors, including the stress of traveling, lack of family or social support, an unfamiliar environment, contrasting cultural practices, and high expectations.3,4 Language barriers, especially for patients who speak uncommon dialects, and lack of continuously available interpretive services often augment the unsettled emotions of international patients.
Cultural differences
International patients may quickly notice significant differences from their home country in how healthcare is practiced and culturally applied.4,6 Such differences may include dress codes and the comparatively equal role of women vis-à-vis men in the Western medical profession.
For cultural, personal, or religious reasons, some patients feel uncomfortable with healthcare providers of the opposite sex. This discomfort can be heightened if the patient needs a potentially uncomfortable and humiliating procedure such as a gynecologic or rectal examination.
The multidisciplinary team approach to healthcare, which can include trainees, nurses, and pharmacists, may leave patients confused about who their primary health provider is.
Decision-making also has cultural implications. In Western medicine, we respect individual autonomy and expect patients to participate in decisions about their care. However, in many areas of the world, medical decision-making is deferred to extended family members or cultural leaders.2 Additional and often repeated conversations may be needed with both the patient and family members to ensure appropriate understanding and ethical consent for care.
Some international patients may have expectations that are quite different from those of the healthcare provider and that are sometimes unrealistic.2,6
Institutional challenges
Many medical conditions require prolonged treatment and longitudinal care, a notable challenge when that care is delivered outside of one’s home country. Practice models within a clinic may not allow for prolonged subsequent visits, which may be needed to accommodate language-translation services. Complex multidisciplinary plans of care must somehow effectively utilize available appointment slots and be time-efficient.
Criteria for hospitalization differ widely among different countries, often based on resources, and may necessitate additional dialogue between the patient and healthcare provider.
Obtaining, interpreting the patient’s record
Medical records from foreign institutions are often unavailable, incomplete, or illegible. Further, depending on the country, it may be difficult to contact local providers for supplemental information. Differences in time zones, limited access to technology, language barriers, and handwritten notes all pose problems when trying to obtain additional information.
Many under-resourced foreign medical centers cannot duplicate medical records and radiographic films for the patient to bring to the United States. Medical records from foreign laboratories often raise questions about the quality, accuracy, and methodology of the testing platform used.2 Thus, the provider may need to start over and repeat the entire clinical, radiologic, and laboratory evaluation.
Communicating with the patient
Difficulties in communication between patients and providers can hinder the development of a positive and productive relationship, reducing patient autonomy and complicating informed consent.2 Obtaining a medical history from non–English-speaking patients can be arduous and time-consuming. Colloquial language may further alter interpretation and understanding, even for formally trained interpreters. Language differences may make it more difficult to explain differential diagnoses, diagnostic approaches, and management plans.
Many US medical centers provide interpreters for many languages, but the great number of languages spoken around the world ensures that barriers in communication persist. Telephone language lines and other commercial language services are available but may feel less personal to patients or evoke concerns about medical confidentiality. For less commonly spoken languages and dialects, appropriate translation services may not even be available.6
Filling in information gaps
Medical conditions, medications, and treatments may have different names in different countries. The quality of pharmaceuticals in some regions may be questionable, and herbal supplements may be unique to a particular location. Many medications available abroad are not available in the United States, potentially confusing US providers as to medication appropriateness, efficacy, and potential toxicities.
Lacking adequate medical records and trying to obtain a new medical history from patients and their family members, providers may struggle with continued gaps of information, hindering a timely diagnosis and composition of an appropriate management plan.
A culturally sensitive but complete physical examination
Every effort should be made to complete a thorough and comprehensive physical examination, even if the patient’s culture differs on this point. This may require a “chaperone” to be present or, if available, a clinician of the same sex as the patient to perform the examination. A compromised examination will impede making the correct diagnosis.
Religious, cultural, and other patient-specific attitudes and beliefs that may affect a medical evaluation should ideally be addressed before scheduling the appointment. A preexamination discussion with the patient and family can help avert unintentional actions and behavior misperceived as offensive, while strengthening the level of trust between patient and provider.2
Money matters
Foreign patients typically have limited or no medical insurance coverage and thus may be paying out of pocket or through limited governmental subsidies. Many refugees and asylum-seekers have no insurance or money to pay for care. (A full discussion of refugee care is beyond the scope of this article). Thus, it is necessary to ascertain in advance who will pay for the care.
Clinicians must be sensitive to the exorbitant costs of medical care and medications in the United States, particularly from the perspective of foreign patients. We strive to provide the best cost-effective care, but what is considered cost-effective and standard care for a patient with US health insurance may be viewed differently by international patients. For some foreign patients, some tests and treatments may be just too expensive, raising personal and institutional ethical concerns regarding how best to evaluate and manage these patients. Ideally, these issues should also be addressed before the patient’s appointment is scheduled.
Clinicians must optimize diagnostic and medical management while minimizing unnecessary testing. This principle further underscores the importance of obtaining a complete medical history and physical examination within a time-sensitive and well-coordinated plan of care.2,4
Continuity of care after the patient leaves
As the medical evaluation and care plan approach completion, ensuring some form of continued medical care can become challenging. Some foreign patients may have the financial or legal means (eg, through an extended medical visa) to remain for further care and follow-up, but most do not.
Finding an available, willing health provider in the patient’s native country for continued management may be difficult and time-consuming. Most US medical centers have no established system to identify available foreign health providers, and usually the patient and family are responsible for arranging continued healthcare back in their home country.
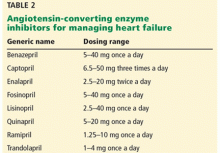
Opportunities for possible improvement of care are noted in Table 2.
ADVANTAGES OF CARING FOR INTERNATIONAL PATIENTS
Despite the possible challenges, there are many benefits of caring for international patients.
Gaining medical knowledge
In US medical centers caring for both regional and referred patients, providers are often exposed to medical conditions that range from common ailments to the rare conditions (or “zebras”) taught during residency training. From the medical education standpoint, international patients provide US health providers heightened opportunities to encounter diseases not commonly seen in the United States (eg, infections such as malaria, schistosomiasis, drug-resistant tuberculosis, and advanced or end-stage forms of noncommunicable diseases). Although not limited to international patients, chronically neglected diseases often give providers first-hand experience in the natural history of select disease progression.
Gaining cultural knowledge
Caring for international patients also enables health providers to learn about different cultures, societal norms, and regional beliefs affecting healthcare. In essence, international patients enable US providers to become more diversified and enlightened with communication skills and assorted managerial strategies on a global scale.
These patients remind us of the stark differences regarding access and quality of medical care globally, particularly in lesser-resourced locations. In a busy domestic medical practice with its own daily challenges, many of us forget these international healthcare disparities, and often take for granted the comparative abundance of healthcare resources available in the United States. Provider frustrations about domestic policies and concerns for a “broken” healthcare system often blind us to the available resources we are fortunate to have at our disposal.
Further, as members of the global community, we have the opportunity to learn from international patients while broadening our view of humanity, thereby enhancing our awareness and empathy toward patients and communities struggling with under-resourced healthcare systems. Healthcare providers are often touched by the gratitude of patients for the opportunity to receive treatments that may otherwise be unavailable. Such experiences may motivate many US health providers to become more engaged in coordinated strategies for global health improvement.
Reimbursement is possible
Caring for international patients should not financially deter US health care centers. Complex, multidisciplinary care evaluations may incur notable expenses; however, alternative and more lucrative payer systems, including government subsidies, can be involved to maintain revenue, reimbursements, and even possibly lead to increased donations.3–5 Given the potential for high costs to be incurred, US providers and institutions need to continually ensure appropriate evidence-based use of resources and cost-effective care without compromising the quality of care provided. The price of certain drugs has been rising astonishingly in the United States, and some patients may therefore prefer to obtain them for long-term use upon return to their home country.
High-quality cost-effective care is satisfying to the patient, provider, and institution, and also may save money that can be reallocated.4 Providers also may find personal fulfillment in striving for and achieving such goals, despite the potential challenges throughout the course of care.
Opportunities for improvement
Regardless of the challenges presented by international patients, participating medical centers often enjoy the prestige and credibility of becoming an “international healthcare center.”4,7 From the standpoint of medical education, these centers have the potential to train providers with increased clinical and cultural competencies along with expanding healthcare services to include clinical, educational and research opportunities abroad.
Research is needed to provide evidence-based guidance on best strategies for patients, clinicians, and healthcare systems to effectively care for international patients.
Suggested opportunities for maximizing advantages are noted in Table 3.
- William Osler. BrainyQuote.com, Xplore Inc, 2016. www.brainyquote.com/quotes/quotes/w/williamosl391388.html. Accessed September 21, 2016.
- Martin DR. Challenges and opportunities in the care of international patients: clinical and health services issues for academic medical centers. Acad Med 2006; 81:189–192.
- Bower LC, Johnson TJ, Hohmann SF, Garman AN, Allen M, Meurer SJ. An evaluation of international patient length of stay. Int J Healthc Manag 2014; 7:200–205.
- Satjapot SP, Johnson TJ, Garman AN. International medical travelers, length of stay, and the continuum of care: inquiry and comparison. Qual Manag Health Care 2011; 20:76–83.
- Donohoe M. Luxury primary care, academic medical centers, and the erosion of science and professional ethics. J Gen Intern Med 2004; 19:90–94.
- Dogan H, Tschudin V, Hot I, Özkan I. Patients’ transcultural needs and carers’ ethical responses. Nurs Ethics 2009; 16:683–696.
- Bauer AM, Alegria M. Impact of patient language proficiency and interpreter service use on the quality of psychiatric care: a systematic review. Psychiatr Serv 2010; 61:765–773.
It is much more important to know what sort of a patient has a disease than what sort of a disease a patient has.
—Attributed to Sir William Osler1
Recent years have seen an increase in people traveling away from their home region for healthcare, often for care that is less expensive or unavailable where they live.2–4 Many Americans seek care abroad (engaging in “medical tourism”); conversely, the United States annually receives thousands of foreign travelers for medical evaluations, a trend projected to increase.2,3,5 Additionally, US healthcare providers often see foreign travelers for unexpected ailments that develop during their time here.
Traveling for healthcare can be stressful for patients, and caring for international patients may pose challenges for providers and medical centers. On the other hand, such encounters also provide many mutual benefits. Unfortunately, there is little published guidance addressing these issues.2 In this article, we therefore discuss many of the benefits and challenges, with the hope of improving the quality of care delivered and the clinical experience for both providers and patients.
CHALLENGES FOR INTERNATIONAL PATIENTS AND THEIR PROVIDERS
Some scenarios that illustrate challenges faced by international patients and their healthcare providers are presented in Table 1.
For patients, heightened anxiety
Many international patients feel anxious, isolated, and vulnerable, particularly if they have never been away from home before. These feelings arise from multiple factors, including the stress of traveling, lack of family or social support, an unfamiliar environment, contrasting cultural practices, and high expectations.3,4 Language barriers, especially for patients who speak uncommon dialects, and lack of continuously available interpretive services often augment the unsettled emotions of international patients.
Cultural differences
International patients may quickly notice significant differences from their home country in how healthcare is practiced and culturally applied.4,6 Such differences may include dress codes and the comparatively equal role of women vis-à-vis men in the Western medical profession.
For cultural, personal, or religious reasons, some patients feel uncomfortable with healthcare providers of the opposite sex. This discomfort can be heightened if the patient needs a potentially uncomfortable and humiliating procedure such as a gynecologic or rectal examination.
The multidisciplinary team approach to healthcare, which can include trainees, nurses, and pharmacists, may leave patients confused about who their primary health provider is.
Decision-making also has cultural implications. In Western medicine, we respect individual autonomy and expect patients to participate in decisions about their care. However, in many areas of the world, medical decision-making is deferred to extended family members or cultural leaders.2 Additional and often repeated conversations may be needed with both the patient and family members to ensure appropriate understanding and ethical consent for care.
Some international patients may have expectations that are quite different from those of the healthcare provider and that are sometimes unrealistic.2,6
Institutional challenges
Many medical conditions require prolonged treatment and longitudinal care, a notable challenge when that care is delivered outside of one’s home country. Practice models within a clinic may not allow for prolonged subsequent visits, which may be needed to accommodate language-translation services. Complex multidisciplinary plans of care must somehow effectively utilize available appointment slots and be time-efficient.
Criteria for hospitalization differ widely among different countries, often based on resources, and may necessitate additional dialogue between the patient and healthcare provider.
Obtaining, interpreting the patient’s record
Medical records from foreign institutions are often unavailable, incomplete, or illegible. Further, depending on the country, it may be difficult to contact local providers for supplemental information. Differences in time zones, limited access to technology, language barriers, and handwritten notes all pose problems when trying to obtain additional information.
Many under-resourced foreign medical centers cannot duplicate medical records and radiographic films for the patient to bring to the United States. Medical records from foreign laboratories often raise questions about the quality, accuracy, and methodology of the testing platform used.2 Thus, the provider may need to start over and repeat the entire clinical, radiologic, and laboratory evaluation.
Communicating with the patient
Difficulties in communication between patients and providers can hinder the development of a positive and productive relationship, reducing patient autonomy and complicating informed consent.2 Obtaining a medical history from non–English-speaking patients can be arduous and time-consuming. Colloquial language may further alter interpretation and understanding, even for formally trained interpreters. Language differences may make it more difficult to explain differential diagnoses, diagnostic approaches, and management plans.
Many US medical centers provide interpreters for many languages, but the great number of languages spoken around the world ensures that barriers in communication persist. Telephone language lines and other commercial language services are available but may feel less personal to patients or evoke concerns about medical confidentiality. For less commonly spoken languages and dialects, appropriate translation services may not even be available.6
Filling in information gaps
Medical conditions, medications, and treatments may have different names in different countries. The quality of pharmaceuticals in some regions may be questionable, and herbal supplements may be unique to a particular location. Many medications available abroad are not available in the United States, potentially confusing US providers as to medication appropriateness, efficacy, and potential toxicities.
Lacking adequate medical records and trying to obtain a new medical history from patients and their family members, providers may struggle with continued gaps of information, hindering a timely diagnosis and composition of an appropriate management plan.
A culturally sensitive but complete physical examination
Every effort should be made to complete a thorough and comprehensive physical examination, even if the patient’s culture differs on this point. This may require a “chaperone” to be present or, if available, a clinician of the same sex as the patient to perform the examination. A compromised examination will impede making the correct diagnosis.
Religious, cultural, and other patient-specific attitudes and beliefs that may affect a medical evaluation should ideally be addressed before scheduling the appointment. A preexamination discussion with the patient and family can help avert unintentional actions and behavior misperceived as offensive, while strengthening the level of trust between patient and provider.2
Money matters
Foreign patients typically have limited or no medical insurance coverage and thus may be paying out of pocket or through limited governmental subsidies. Many refugees and asylum-seekers have no insurance or money to pay for care. (A full discussion of refugee care is beyond the scope of this article). Thus, it is necessary to ascertain in advance who will pay for the care.
Clinicians must be sensitive to the exorbitant costs of medical care and medications in the United States, particularly from the perspective of foreign patients. We strive to provide the best cost-effective care, but what is considered cost-effective and standard care for a patient with US health insurance may be viewed differently by international patients. For some foreign patients, some tests and treatments may be just too expensive, raising personal and institutional ethical concerns regarding how best to evaluate and manage these patients. Ideally, these issues should also be addressed before the patient’s appointment is scheduled.
Clinicians must optimize diagnostic and medical management while minimizing unnecessary testing. This principle further underscores the importance of obtaining a complete medical history and physical examination within a time-sensitive and well-coordinated plan of care.2,4
Continuity of care after the patient leaves
As the medical evaluation and care plan approach completion, ensuring some form of continued medical care can become challenging. Some foreign patients may have the financial or legal means (eg, through an extended medical visa) to remain for further care and follow-up, but most do not.
Finding an available, willing health provider in the patient’s native country for continued management may be difficult and time-consuming. Most US medical centers have no established system to identify available foreign health providers, and usually the patient and family are responsible for arranging continued healthcare back in their home country.
Opportunities for possible improvement of care are noted in Table 2.
ADVANTAGES OF CARING FOR INTERNATIONAL PATIENTS
Despite the possible challenges, there are many benefits of caring for international patients.
Gaining medical knowledge
In US medical centers caring for both regional and referred patients, providers are often exposed to medical conditions that range from common ailments to the rare conditions (or “zebras”) taught during residency training. From the medical education standpoint, international patients provide US health providers heightened opportunities to encounter diseases not commonly seen in the United States (eg, infections such as malaria, schistosomiasis, drug-resistant tuberculosis, and advanced or end-stage forms of noncommunicable diseases). Although not limited to international patients, chronically neglected diseases often give providers first-hand experience in the natural history of select disease progression.
Gaining cultural knowledge
Caring for international patients also enables health providers to learn about different cultures, societal norms, and regional beliefs affecting healthcare. In essence, international patients enable US providers to become more diversified and enlightened with communication skills and assorted managerial strategies on a global scale.
These patients remind us of the stark differences regarding access and quality of medical care globally, particularly in lesser-resourced locations. In a busy domestic medical practice with its own daily challenges, many of us forget these international healthcare disparities, and often take for granted the comparative abundance of healthcare resources available in the United States. Provider frustrations about domestic policies and concerns for a “broken” healthcare system often blind us to the available resources we are fortunate to have at our disposal.
Further, as members of the global community, we have the opportunity to learn from international patients while broadening our view of humanity, thereby enhancing our awareness and empathy toward patients and communities struggling with under-resourced healthcare systems. Healthcare providers are often touched by the gratitude of patients for the opportunity to receive treatments that may otherwise be unavailable. Such experiences may motivate many US health providers to become more engaged in coordinated strategies for global health improvement.
Reimbursement is possible
Caring for international patients should not financially deter US health care centers. Complex, multidisciplinary care evaluations may incur notable expenses; however, alternative and more lucrative payer systems, including government subsidies, can be involved to maintain revenue, reimbursements, and even possibly lead to increased donations.3–5 Given the potential for high costs to be incurred, US providers and institutions need to continually ensure appropriate evidence-based use of resources and cost-effective care without compromising the quality of care provided. The price of certain drugs has been rising astonishingly in the United States, and some patients may therefore prefer to obtain them for long-term use upon return to their home country.
High-quality cost-effective care is satisfying to the patient, provider, and institution, and also may save money that can be reallocated.4 Providers also may find personal fulfillment in striving for and achieving such goals, despite the potential challenges throughout the course of care.
Opportunities for improvement
Regardless of the challenges presented by international patients, participating medical centers often enjoy the prestige and credibility of becoming an “international healthcare center.”4,7 From the standpoint of medical education, these centers have the potential to train providers with increased clinical and cultural competencies along with expanding healthcare services to include clinical, educational and research opportunities abroad.
Research is needed to provide evidence-based guidance on best strategies for patients, clinicians, and healthcare systems to effectively care for international patients.
Suggested opportunities for maximizing advantages are noted in Table 3.
It is much more important to know what sort of a patient has a disease than what sort of a disease a patient has.
—Attributed to Sir William Osler1
Recent years have seen an increase in people traveling away from their home region for healthcare, often for care that is less expensive or unavailable where they live.2–4 Many Americans seek care abroad (engaging in “medical tourism”); conversely, the United States annually receives thousands of foreign travelers for medical evaluations, a trend projected to increase.2,3,5 Additionally, US healthcare providers often see foreign travelers for unexpected ailments that develop during their time here.
Traveling for healthcare can be stressful for patients, and caring for international patients may pose challenges for providers and medical centers. On the other hand, such encounters also provide many mutual benefits. Unfortunately, there is little published guidance addressing these issues.2 In this article, we therefore discuss many of the benefits and challenges, with the hope of improving the quality of care delivered and the clinical experience for both providers and patients.
CHALLENGES FOR INTERNATIONAL PATIENTS AND THEIR PROVIDERS
Some scenarios that illustrate challenges faced by international patients and their healthcare providers are presented in Table 1.
For patients, heightened anxiety
Many international patients feel anxious, isolated, and vulnerable, particularly if they have never been away from home before. These feelings arise from multiple factors, including the stress of traveling, lack of family or social support, an unfamiliar environment, contrasting cultural practices, and high expectations.3,4 Language barriers, especially for patients who speak uncommon dialects, and lack of continuously available interpretive services often augment the unsettled emotions of international patients.
Cultural differences
International patients may quickly notice significant differences from their home country in how healthcare is practiced and culturally applied.4,6 Such differences may include dress codes and the comparatively equal role of women vis-à-vis men in the Western medical profession.
For cultural, personal, or religious reasons, some patients feel uncomfortable with healthcare providers of the opposite sex. This discomfort can be heightened if the patient needs a potentially uncomfortable and humiliating procedure such as a gynecologic or rectal examination.
The multidisciplinary team approach to healthcare, which can include trainees, nurses, and pharmacists, may leave patients confused about who their primary health provider is.
Decision-making also has cultural implications. In Western medicine, we respect individual autonomy and expect patients to participate in decisions about their care. However, in many areas of the world, medical decision-making is deferred to extended family members or cultural leaders.2 Additional and often repeated conversations may be needed with both the patient and family members to ensure appropriate understanding and ethical consent for care.
Some international patients may have expectations that are quite different from those of the healthcare provider and that are sometimes unrealistic.2,6
Institutional challenges
Many medical conditions require prolonged treatment and longitudinal care, a notable challenge when that care is delivered outside of one’s home country. Practice models within a clinic may not allow for prolonged subsequent visits, which may be needed to accommodate language-translation services. Complex multidisciplinary plans of care must somehow effectively utilize available appointment slots and be time-efficient.
Criteria for hospitalization differ widely among different countries, often based on resources, and may necessitate additional dialogue between the patient and healthcare provider.
Obtaining, interpreting the patient’s record
Medical records from foreign institutions are often unavailable, incomplete, or illegible. Further, depending on the country, it may be difficult to contact local providers for supplemental information. Differences in time zones, limited access to technology, language barriers, and handwritten notes all pose problems when trying to obtain additional information.
Many under-resourced foreign medical centers cannot duplicate medical records and radiographic films for the patient to bring to the United States. Medical records from foreign laboratories often raise questions about the quality, accuracy, and methodology of the testing platform used.2 Thus, the provider may need to start over and repeat the entire clinical, radiologic, and laboratory evaluation.
Communicating with the patient
Difficulties in communication between patients and providers can hinder the development of a positive and productive relationship, reducing patient autonomy and complicating informed consent.2 Obtaining a medical history from non–English-speaking patients can be arduous and time-consuming. Colloquial language may further alter interpretation and understanding, even for formally trained interpreters. Language differences may make it more difficult to explain differential diagnoses, diagnostic approaches, and management plans.
Many US medical centers provide interpreters for many languages, but the great number of languages spoken around the world ensures that barriers in communication persist. Telephone language lines and other commercial language services are available but may feel less personal to patients or evoke concerns about medical confidentiality. For less commonly spoken languages and dialects, appropriate translation services may not even be available.6
Filling in information gaps
Medical conditions, medications, and treatments may have different names in different countries. The quality of pharmaceuticals in some regions may be questionable, and herbal supplements may be unique to a particular location. Many medications available abroad are not available in the United States, potentially confusing US providers as to medication appropriateness, efficacy, and potential toxicities.
Lacking adequate medical records and trying to obtain a new medical history from patients and their family members, providers may struggle with continued gaps of information, hindering a timely diagnosis and composition of an appropriate management plan.
A culturally sensitive but complete physical examination
Every effort should be made to complete a thorough and comprehensive physical examination, even if the patient’s culture differs on this point. This may require a “chaperone” to be present or, if available, a clinician of the same sex as the patient to perform the examination. A compromised examination will impede making the correct diagnosis.
Religious, cultural, and other patient-specific attitudes and beliefs that may affect a medical evaluation should ideally be addressed before scheduling the appointment. A preexamination discussion with the patient and family can help avert unintentional actions and behavior misperceived as offensive, while strengthening the level of trust between patient and provider.2
Money matters
Foreign patients typically have limited or no medical insurance coverage and thus may be paying out of pocket or through limited governmental subsidies. Many refugees and asylum-seekers have no insurance or money to pay for care. (A full discussion of refugee care is beyond the scope of this article). Thus, it is necessary to ascertain in advance who will pay for the care.
Clinicians must be sensitive to the exorbitant costs of medical care and medications in the United States, particularly from the perspective of foreign patients. We strive to provide the best cost-effective care, but what is considered cost-effective and standard care for a patient with US health insurance may be viewed differently by international patients. For some foreign patients, some tests and treatments may be just too expensive, raising personal and institutional ethical concerns regarding how best to evaluate and manage these patients. Ideally, these issues should also be addressed before the patient’s appointment is scheduled.
Clinicians must optimize diagnostic and medical management while minimizing unnecessary testing. This principle further underscores the importance of obtaining a complete medical history and physical examination within a time-sensitive and well-coordinated plan of care.2,4
Continuity of care after the patient leaves
As the medical evaluation and care plan approach completion, ensuring some form of continued medical care can become challenging. Some foreign patients may have the financial or legal means (eg, through an extended medical visa) to remain for further care and follow-up, but most do not.
Finding an available, willing health provider in the patient’s native country for continued management may be difficult and time-consuming. Most US medical centers have no established system to identify available foreign health providers, and usually the patient and family are responsible for arranging continued healthcare back in their home country.
Opportunities for possible improvement of care are noted in Table 2.
ADVANTAGES OF CARING FOR INTERNATIONAL PATIENTS
Despite the possible challenges, there are many benefits of caring for international patients.
Gaining medical knowledge
In US medical centers caring for both regional and referred patients, providers are often exposed to medical conditions that range from common ailments to the rare conditions (or “zebras”) taught during residency training. From the medical education standpoint, international patients provide US health providers heightened opportunities to encounter diseases not commonly seen in the United States (eg, infections such as malaria, schistosomiasis, drug-resistant tuberculosis, and advanced or end-stage forms of noncommunicable diseases). Although not limited to international patients, chronically neglected diseases often give providers first-hand experience in the natural history of select disease progression.
Gaining cultural knowledge
Caring for international patients also enables health providers to learn about different cultures, societal norms, and regional beliefs affecting healthcare. In essence, international patients enable US providers to become more diversified and enlightened with communication skills and assorted managerial strategies on a global scale.
These patients remind us of the stark differences regarding access and quality of medical care globally, particularly in lesser-resourced locations. In a busy domestic medical practice with its own daily challenges, many of us forget these international healthcare disparities, and often take for granted the comparative abundance of healthcare resources available in the United States. Provider frustrations about domestic policies and concerns for a “broken” healthcare system often blind us to the available resources we are fortunate to have at our disposal.
Further, as members of the global community, we have the opportunity to learn from international patients while broadening our view of humanity, thereby enhancing our awareness and empathy toward patients and communities struggling with under-resourced healthcare systems. Healthcare providers are often touched by the gratitude of patients for the opportunity to receive treatments that may otherwise be unavailable. Such experiences may motivate many US health providers to become more engaged in coordinated strategies for global health improvement.
Reimbursement is possible
Caring for international patients should not financially deter US health care centers. Complex, multidisciplinary care evaluations may incur notable expenses; however, alternative and more lucrative payer systems, including government subsidies, can be involved to maintain revenue, reimbursements, and even possibly lead to increased donations.3–5 Given the potential for high costs to be incurred, US providers and institutions need to continually ensure appropriate evidence-based use of resources and cost-effective care without compromising the quality of care provided. The price of certain drugs has been rising astonishingly in the United States, and some patients may therefore prefer to obtain them for long-term use upon return to their home country.
High-quality cost-effective care is satisfying to the patient, provider, and institution, and also may save money that can be reallocated.4 Providers also may find personal fulfillment in striving for and achieving such goals, despite the potential challenges throughout the course of care.
Opportunities for improvement
Regardless of the challenges presented by international patients, participating medical centers often enjoy the prestige and credibility of becoming an “international healthcare center.”4,7 From the standpoint of medical education, these centers have the potential to train providers with increased clinical and cultural competencies along with expanding healthcare services to include clinical, educational and research opportunities abroad.
Research is needed to provide evidence-based guidance on best strategies for patients, clinicians, and healthcare systems to effectively care for international patients.
Suggested opportunities for maximizing advantages are noted in Table 3.
- William Osler. BrainyQuote.com, Xplore Inc, 2016. www.brainyquote.com/quotes/quotes/w/williamosl391388.html. Accessed September 21, 2016.
- Martin DR. Challenges and opportunities in the care of international patients: clinical and health services issues for academic medical centers. Acad Med 2006; 81:189–192.
- Bower LC, Johnson TJ, Hohmann SF, Garman AN, Allen M, Meurer SJ. An evaluation of international patient length of stay. Int J Healthc Manag 2014; 7:200–205.
- Satjapot SP, Johnson TJ, Garman AN. International medical travelers, length of stay, and the continuum of care: inquiry and comparison. Qual Manag Health Care 2011; 20:76–83.
- Donohoe M. Luxury primary care, academic medical centers, and the erosion of science and professional ethics. J Gen Intern Med 2004; 19:90–94.
- Dogan H, Tschudin V, Hot I, Özkan I. Patients’ transcultural needs and carers’ ethical responses. Nurs Ethics 2009; 16:683–696.
- Bauer AM, Alegria M. Impact of patient language proficiency and interpreter service use on the quality of psychiatric care: a systematic review. Psychiatr Serv 2010; 61:765–773.
- William Osler. BrainyQuote.com, Xplore Inc, 2016. www.brainyquote.com/quotes/quotes/w/williamosl391388.html. Accessed September 21, 2016.
- Martin DR. Challenges and opportunities in the care of international patients: clinical and health services issues for academic medical centers. Acad Med 2006; 81:189–192.
- Bower LC, Johnson TJ, Hohmann SF, Garman AN, Allen M, Meurer SJ. An evaluation of international patient length of stay. Int J Healthc Manag 2014; 7:200–205.
- Satjapot SP, Johnson TJ, Garman AN. International medical travelers, length of stay, and the continuum of care: inquiry and comparison. Qual Manag Health Care 2011; 20:76–83.
- Donohoe M. Luxury primary care, academic medical centers, and the erosion of science and professional ethics. J Gen Intern Med 2004; 19:90–94.
- Dogan H, Tschudin V, Hot I, Özkan I. Patients’ transcultural needs and carers’ ethical responses. Nurs Ethics 2009; 16:683–696.
- Bauer AM, Alegria M. Impact of patient language proficiency and interpreter service use on the quality of psychiatric care: a systematic review. Psychiatr Serv 2010; 61:765–773.
KEY POINTS
- Challenges in caring for international patients include cultural differences, institutional barriers, communication difficulties, sparse medical records, and financial considerations.
- Understanding should be reached beforehand on potentially sensitive issues such as physical examinations, payment, tests, and treatment.
- Benefits to the provider and institution include enhanced medical skills, cultural competency, personal satisfaction, and institutional prestige.
Seeking medical care abroad: A challenge to empathy
On an otherwise pleasant evening during the first week of July 2016, a businessman who was a citizen of the United Arab Emirates visiting Cleveland for medical treatment was falsely accused of links to a terror organization. Officers stormed his hotel with assault rifles and handcuffed and arrested him—all this, apparently, because the man was dressed in traditional Emirati clothing.
This case highlights a level of complexity in providing medical care to foreigners far beyond language interpreting services and outside the borders of the institution where medical care is provided. In the current issue of the Journal, Cawcutt and Wilson1 review their experiences in the care of international patients and the unique challenges associated with it.
FROM THE TEMPLE OF AESCULAPIUS TO CLEVELAND CLINIC
In 2015, patients from more than 100 countries traveled to Cleveland seeking care at Cleveland Clinic. But medical travel was part of the practice of medicine long before major US hospitals became destinations for international patients, and it has been refined over the years.
Ancient cultures had a thriving tradition of patients traveling long distances for the best and most advanced medical treatment.2–4 In ancient Greece, people from all around the Mediterranean came to the city of Epidaurus to be cured in its famous temple of Aesculapius, built as a medical center.
Similarly, early Islamic cultures established a healthcare system that catered to foreigners. A noted example is the Mansuri hospital in Cairo, built in 1248 ce and considered the most advanced hospital of its time. Accommodating nearly 8,000 patients, the Mansuri hospital became a healthcare destination for foreigners regardless of race or religion.2–4
Europe also had a great tradition of providing medical care to foreign patients. Between the 15th and 17th centuries, belief in the healing power of mineral water led to the establishment of spas and the rise of spa towns, particularly in the south of France near mineral springs. The poor sanitary conditions of Europe at the time may have prompted the interest in the healing effect of mineral spas, but wealthy individuals from all over the world traveled to these destinations, creating local prosperity due to medical tourism.2–4
The city of Bath, in England, is a great example. In the 1720s, Bath was a popular destination for those traveling for healthcare. It became the first city in England to build a covered sewage system, ahead of London by several years. It also had paved roads, lights, hotels, and restaurants in much greater numbers than other cities in England, a likely result of prosperity associated with medical tourism.
ALL PATIENTS WANT TO BE TREATED WITH RESPECT AND KINDNESS
While medical knowledge and health delivery models have changed over the years, caring for foreign patients is perhaps as old as medicine itself. The central focus of restoring health is certainly not unique to international patients, but understanding their unique needs is important in order to achieve the best outcomes, something that Cawcutt and Wilson highlight well.1
A number of studies have addressed the question of what patients really want. Responses were surprisingly consistent: they want to be treated with respect and kindness.5,6 In other words, they want empathy, and this is true of all patients regardless of ethnicity or background. Empathy is a tremendous therapeutic force and can narrow what may look like an unbridgeable gap between patient and physician.7,8
EMPATHY REQUIRES EFFECTIVE COMMUNICATION
Empathy, though sometimes innate, requires effective communication and shared experiences. Neither of these two requirements is easily achievable in the care of foreign patients.
Communication is hampered by language barriers, although it can be enhanced significantly by language translating services and the work of certified medical interpreters. These often-invisible heroes should be recognized as essential members of the medical team. Their work requires cultural sensitivity and formal training to avoid miscommunication and medical errors. Codes of ethics for medical interpreters include confidentiality, accuracy in conveying the content and spirit of the message, freedom from personal biases, cultural training, and professional boundaries.9
TOWARD CULTURAL COMPETENCY
Lack of shared experiences between the foreign patient and care provider is an even greater obstacle to overcome in eliminating any empathy deficit. Shared experiences, whether cultural, religious, or social, help us to see the world through the eyes of the patient.
International patients may differ from us in background, ethnicity, religion, dress, expectations, and other areas. Cultural and religious backgrounds often dictate certain behaviors in the event of critical illness or death. Even in routine and less acute medical care, the background of a foreign patient may lead to logistical quandaries such as the need for same-sex caregivers or a private room.
A paradox currently exists in our efforts to meet patients’ need and desire for empathy. While culturally empathic care is necessary to achieve the best medical outcomes, this topic is not yet part of the curriculum for physicians or other healthcare providers in training. A culturally sensitive institution has many business advantages.10 Thorough and focused cultural training of medical staff is essential. Shared experiences can potentially be fashioned through a well-designed cultural competency training program to enhance empathy for foreign patients.
A SERVICE-ORIENTED APPROACH
Besides cultural competency and language training, a service-oriented approach to accommodate the needs of medical travelers and their family members is of paramount importance. Many of the complaints and burdens of medical visitors concern services that are not medical in nature, such as daily living necessities. Transportation, religious services, banking, extended-stay facilities, cell phone service, legal services, shopping, dining, and entertainment are among many other living needs for those receiving medical care abroad. These services are inconsistently provided throughout medical institutions in the United States, which provide care to thousands of international patients annually.
Unique challenges of providing medical care to international patients have direct effects on medical outcomes. A population-based cohort study of US-born and foreign-born adults with lung or colorectal cancer suggested disparities in quality and type of care.11 Foreign-born patients reported lower-quality care and were less likely to receive complex cancer treatments recommended by clinical guidelines. The authors proposed that quality of care and outcomes may be improved with greater emphasis on coordination of care and improving communication. Similar findings were reported in foreign-born patients with breast cancer.12
‘WHAT WOULD YOU THINK TO BE USED THUS?’
Four hundred years ago, in the play Sir Thomas More (a collaboration between several Elizabethan playwrights),13 the title character confronts a mob of anti-immigrant rioters, and in a speech believed to have been written by William Shakespeare (Act 2, Scene 4), asks them to imagine themselves banished to a foreign country and subjected to hostility such as they were meting out:
To be used thus?”
Empathy for foreigners seeking medical care is not merely an act of kindness; rather, it is a central piece of healing. Medical institutions interested in providing healthcare to this unique group of patients should take these principles into account and carefully examine their ability to deliver compassionate care collectively to local and foreign-born patients alike.
- Cawcutt KA, Wilson JW. The benefits and challenges of caring for international patients. Cleve Clin J Med 2016; 83:794–800.
- Health-Tourism.com. The history of medical tourism. Health-Tourism.com. www.health-tourism.com/medical-tourism/history/. Accessed September 21, 2016.
- Chen LH, Hochberg NS, Magill AJ. The pre-travel consultation. US Centers for Disease Control and Prevention. wwwnc.cdc.gov/travel/yellowbook/2016/the-pre-travel-consultation/the-pre-travel-consultation. Accessed September 21, 2016.
- Rogers K. Medical tourism. Encyclopedia Britannica. www.britannica.com/topic/medical-tourism. Accessed September 21, 2016.
- Detsky AS. What do patients really want from healthcare? JAMA 2011; 306:2500–2501.
- Shaywitz D. What do patients really want from healthcare? Forbes Dec 24, 2011. www.forbes.com/sites/davidshaywitz/2011/12/24/what-do-patients-really-want-from-health-care/print/. Accessed September 21, 2016.
- Lee TH. How to spread empathy in healthcare. Harvard Business Review July 17, 2014.
- Friedman R. Understanding empathy: can you feel my pain? New York Times April 24, 2007.
- National Council on Interpreting in Health Care. A national code of ethics for interpreters in healthcare. July 2004. www.ncihc.org/assets/documents/publications/NCIHC%20National%20Code%20of%20Ethics.pdf. Accessed September 21, 2016.
- Minguet L. Creating a culturally sensitive corporation. Harvard Business Review, September 2014.
- Nielsen SS, He Y, Ayanian JZ, Gomez SL, Khan KL, West DW, et al. Quality of cancer care among foreign-born patients with lung or colorectal cancer. Cancer 2010; 116:5497–5506.
- Kouri EM, He Y, Winer EP, Keating NL. Influence of birthplace on breast cancer diagnosis and treatment for Hispanic women. Breast Cancer Res Treat 2009; 121:743–751.
- Dyce A, editor. Sir Thomas More, a play. London: The Shakespeare Society, 1844. https://archive.org/details/sirthomasmorepla00mund. Accessed September 21, 2016.
On an otherwise pleasant evening during the first week of July 2016, a businessman who was a citizen of the United Arab Emirates visiting Cleveland for medical treatment was falsely accused of links to a terror organization. Officers stormed his hotel with assault rifles and handcuffed and arrested him—all this, apparently, because the man was dressed in traditional Emirati clothing.
This case highlights a level of complexity in providing medical care to foreigners far beyond language interpreting services and outside the borders of the institution where medical care is provided. In the current issue of the Journal, Cawcutt and Wilson1 review their experiences in the care of international patients and the unique challenges associated with it.
FROM THE TEMPLE OF AESCULAPIUS TO CLEVELAND CLINIC
In 2015, patients from more than 100 countries traveled to Cleveland seeking care at Cleveland Clinic. But medical travel was part of the practice of medicine long before major US hospitals became destinations for international patients, and it has been refined over the years.
Ancient cultures had a thriving tradition of patients traveling long distances for the best and most advanced medical treatment.2–4 In ancient Greece, people from all around the Mediterranean came to the city of Epidaurus to be cured in its famous temple of Aesculapius, built as a medical center.
Similarly, early Islamic cultures established a healthcare system that catered to foreigners. A noted example is the Mansuri hospital in Cairo, built in 1248 ce and considered the most advanced hospital of its time. Accommodating nearly 8,000 patients, the Mansuri hospital became a healthcare destination for foreigners regardless of race or religion.2–4
Europe also had a great tradition of providing medical care to foreign patients. Between the 15th and 17th centuries, belief in the healing power of mineral water led to the establishment of spas and the rise of spa towns, particularly in the south of France near mineral springs. The poor sanitary conditions of Europe at the time may have prompted the interest in the healing effect of mineral spas, but wealthy individuals from all over the world traveled to these destinations, creating local prosperity due to medical tourism.2–4
The city of Bath, in England, is a great example. In the 1720s, Bath was a popular destination for those traveling for healthcare. It became the first city in England to build a covered sewage system, ahead of London by several years. It also had paved roads, lights, hotels, and restaurants in much greater numbers than other cities in England, a likely result of prosperity associated with medical tourism.
ALL PATIENTS WANT TO BE TREATED WITH RESPECT AND KINDNESS
While medical knowledge and health delivery models have changed over the years, caring for foreign patients is perhaps as old as medicine itself. The central focus of restoring health is certainly not unique to international patients, but understanding their unique needs is important in order to achieve the best outcomes, something that Cawcutt and Wilson highlight well.1
A number of studies have addressed the question of what patients really want. Responses were surprisingly consistent: they want to be treated with respect and kindness.5,6 In other words, they want empathy, and this is true of all patients regardless of ethnicity or background. Empathy is a tremendous therapeutic force and can narrow what may look like an unbridgeable gap between patient and physician.7,8
EMPATHY REQUIRES EFFECTIVE COMMUNICATION
Empathy, though sometimes innate, requires effective communication and shared experiences. Neither of these two requirements is easily achievable in the care of foreign patients.
Communication is hampered by language barriers, although it can be enhanced significantly by language translating services and the work of certified medical interpreters. These often-invisible heroes should be recognized as essential members of the medical team. Their work requires cultural sensitivity and formal training to avoid miscommunication and medical errors. Codes of ethics for medical interpreters include confidentiality, accuracy in conveying the content and spirit of the message, freedom from personal biases, cultural training, and professional boundaries.9
TOWARD CULTURAL COMPETENCY
Lack of shared experiences between the foreign patient and care provider is an even greater obstacle to overcome in eliminating any empathy deficit. Shared experiences, whether cultural, religious, or social, help us to see the world through the eyes of the patient.
International patients may differ from us in background, ethnicity, religion, dress, expectations, and other areas. Cultural and religious backgrounds often dictate certain behaviors in the event of critical illness or death. Even in routine and less acute medical care, the background of a foreign patient may lead to logistical quandaries such as the need for same-sex caregivers or a private room.
A paradox currently exists in our efforts to meet patients’ need and desire for empathy. While culturally empathic care is necessary to achieve the best medical outcomes, this topic is not yet part of the curriculum for physicians or other healthcare providers in training. A culturally sensitive institution has many business advantages.10 Thorough and focused cultural training of medical staff is essential. Shared experiences can potentially be fashioned through a well-designed cultural competency training program to enhance empathy for foreign patients.
A SERVICE-ORIENTED APPROACH
Besides cultural competency and language training, a service-oriented approach to accommodate the needs of medical travelers and their family members is of paramount importance. Many of the complaints and burdens of medical visitors concern services that are not medical in nature, such as daily living necessities. Transportation, religious services, banking, extended-stay facilities, cell phone service, legal services, shopping, dining, and entertainment are among many other living needs for those receiving medical care abroad. These services are inconsistently provided throughout medical institutions in the United States, which provide care to thousands of international patients annually.
Unique challenges of providing medical care to international patients have direct effects on medical outcomes. A population-based cohort study of US-born and foreign-born adults with lung or colorectal cancer suggested disparities in quality and type of care.11 Foreign-born patients reported lower-quality care and were less likely to receive complex cancer treatments recommended by clinical guidelines. The authors proposed that quality of care and outcomes may be improved with greater emphasis on coordination of care and improving communication. Similar findings were reported in foreign-born patients with breast cancer.12
‘WHAT WOULD YOU THINK TO BE USED THUS?’
Four hundred years ago, in the play Sir Thomas More (a collaboration between several Elizabethan playwrights),13 the title character confronts a mob of anti-immigrant rioters, and in a speech believed to have been written by William Shakespeare (Act 2, Scene 4), asks them to imagine themselves banished to a foreign country and subjected to hostility such as they were meting out:
To be used thus?”
Empathy for foreigners seeking medical care is not merely an act of kindness; rather, it is a central piece of healing. Medical institutions interested in providing healthcare to this unique group of patients should take these principles into account and carefully examine their ability to deliver compassionate care collectively to local and foreign-born patients alike.
On an otherwise pleasant evening during the first week of July 2016, a businessman who was a citizen of the United Arab Emirates visiting Cleveland for medical treatment was falsely accused of links to a terror organization. Officers stormed his hotel with assault rifles and handcuffed and arrested him—all this, apparently, because the man was dressed in traditional Emirati clothing.
This case highlights a level of complexity in providing medical care to foreigners far beyond language interpreting services and outside the borders of the institution where medical care is provided. In the current issue of the Journal, Cawcutt and Wilson1 review their experiences in the care of international patients and the unique challenges associated with it.
FROM THE TEMPLE OF AESCULAPIUS TO CLEVELAND CLINIC
In 2015, patients from more than 100 countries traveled to Cleveland seeking care at Cleveland Clinic. But medical travel was part of the practice of medicine long before major US hospitals became destinations for international patients, and it has been refined over the years.
Ancient cultures had a thriving tradition of patients traveling long distances for the best and most advanced medical treatment.2–4 In ancient Greece, people from all around the Mediterranean came to the city of Epidaurus to be cured in its famous temple of Aesculapius, built as a medical center.
Similarly, early Islamic cultures established a healthcare system that catered to foreigners. A noted example is the Mansuri hospital in Cairo, built in 1248 ce and considered the most advanced hospital of its time. Accommodating nearly 8,000 patients, the Mansuri hospital became a healthcare destination for foreigners regardless of race or religion.2–4
Europe also had a great tradition of providing medical care to foreign patients. Between the 15th and 17th centuries, belief in the healing power of mineral water led to the establishment of spas and the rise of spa towns, particularly in the south of France near mineral springs. The poor sanitary conditions of Europe at the time may have prompted the interest in the healing effect of mineral spas, but wealthy individuals from all over the world traveled to these destinations, creating local prosperity due to medical tourism.2–4
The city of Bath, in England, is a great example. In the 1720s, Bath was a popular destination for those traveling for healthcare. It became the first city in England to build a covered sewage system, ahead of London by several years. It also had paved roads, lights, hotels, and restaurants in much greater numbers than other cities in England, a likely result of prosperity associated with medical tourism.
ALL PATIENTS WANT TO BE TREATED WITH RESPECT AND KINDNESS
While medical knowledge and health delivery models have changed over the years, caring for foreign patients is perhaps as old as medicine itself. The central focus of restoring health is certainly not unique to international patients, but understanding their unique needs is important in order to achieve the best outcomes, something that Cawcutt and Wilson highlight well.1
A number of studies have addressed the question of what patients really want. Responses were surprisingly consistent: they want to be treated with respect and kindness.5,6 In other words, they want empathy, and this is true of all patients regardless of ethnicity or background. Empathy is a tremendous therapeutic force and can narrow what may look like an unbridgeable gap between patient and physician.7,8
EMPATHY REQUIRES EFFECTIVE COMMUNICATION
Empathy, though sometimes innate, requires effective communication and shared experiences. Neither of these two requirements is easily achievable in the care of foreign patients.
Communication is hampered by language barriers, although it can be enhanced significantly by language translating services and the work of certified medical interpreters. These often-invisible heroes should be recognized as essential members of the medical team. Their work requires cultural sensitivity and formal training to avoid miscommunication and medical errors. Codes of ethics for medical interpreters include confidentiality, accuracy in conveying the content and spirit of the message, freedom from personal biases, cultural training, and professional boundaries.9
TOWARD CULTURAL COMPETENCY
Lack of shared experiences between the foreign patient and care provider is an even greater obstacle to overcome in eliminating any empathy deficit. Shared experiences, whether cultural, religious, or social, help us to see the world through the eyes of the patient.
International patients may differ from us in background, ethnicity, religion, dress, expectations, and other areas. Cultural and religious backgrounds often dictate certain behaviors in the event of critical illness or death. Even in routine and less acute medical care, the background of a foreign patient may lead to logistical quandaries such as the need for same-sex caregivers or a private room.
A paradox currently exists in our efforts to meet patients’ need and desire for empathy. While culturally empathic care is necessary to achieve the best medical outcomes, this topic is not yet part of the curriculum for physicians or other healthcare providers in training. A culturally sensitive institution has many business advantages.10 Thorough and focused cultural training of medical staff is essential. Shared experiences can potentially be fashioned through a well-designed cultural competency training program to enhance empathy for foreign patients.
A SERVICE-ORIENTED APPROACH
Besides cultural competency and language training, a service-oriented approach to accommodate the needs of medical travelers and their family members is of paramount importance. Many of the complaints and burdens of medical visitors concern services that are not medical in nature, such as daily living necessities. Transportation, religious services, banking, extended-stay facilities, cell phone service, legal services, shopping, dining, and entertainment are among many other living needs for those receiving medical care abroad. These services are inconsistently provided throughout medical institutions in the United States, which provide care to thousands of international patients annually.
Unique challenges of providing medical care to international patients have direct effects on medical outcomes. A population-based cohort study of US-born and foreign-born adults with lung or colorectal cancer suggested disparities in quality and type of care.11 Foreign-born patients reported lower-quality care and were less likely to receive complex cancer treatments recommended by clinical guidelines. The authors proposed that quality of care and outcomes may be improved with greater emphasis on coordination of care and improving communication. Similar findings were reported in foreign-born patients with breast cancer.12
‘WHAT WOULD YOU THINK TO BE USED THUS?’
Four hundred years ago, in the play Sir Thomas More (a collaboration between several Elizabethan playwrights),13 the title character confronts a mob of anti-immigrant rioters, and in a speech believed to have been written by William Shakespeare (Act 2, Scene 4), asks them to imagine themselves banished to a foreign country and subjected to hostility such as they were meting out:
To be used thus?”
Empathy for foreigners seeking medical care is not merely an act of kindness; rather, it is a central piece of healing. Medical institutions interested in providing healthcare to this unique group of patients should take these principles into account and carefully examine their ability to deliver compassionate care collectively to local and foreign-born patients alike.
- Cawcutt KA, Wilson JW. The benefits and challenges of caring for international patients. Cleve Clin J Med 2016; 83:794–800.
- Health-Tourism.com. The history of medical tourism. Health-Tourism.com. www.health-tourism.com/medical-tourism/history/. Accessed September 21, 2016.
- Chen LH, Hochberg NS, Magill AJ. The pre-travel consultation. US Centers for Disease Control and Prevention. wwwnc.cdc.gov/travel/yellowbook/2016/the-pre-travel-consultation/the-pre-travel-consultation. Accessed September 21, 2016.
- Rogers K. Medical tourism. Encyclopedia Britannica. www.britannica.com/topic/medical-tourism. Accessed September 21, 2016.
- Detsky AS. What do patients really want from healthcare? JAMA 2011; 306:2500–2501.
- Shaywitz D. What do patients really want from healthcare? Forbes Dec 24, 2011. www.forbes.com/sites/davidshaywitz/2011/12/24/what-do-patients-really-want-from-health-care/print/. Accessed September 21, 2016.
- Lee TH. How to spread empathy in healthcare. Harvard Business Review July 17, 2014.
- Friedman R. Understanding empathy: can you feel my pain? New York Times April 24, 2007.
- National Council on Interpreting in Health Care. A national code of ethics for interpreters in healthcare. July 2004. www.ncihc.org/assets/documents/publications/NCIHC%20National%20Code%20of%20Ethics.pdf. Accessed September 21, 2016.
- Minguet L. Creating a culturally sensitive corporation. Harvard Business Review, September 2014.
- Nielsen SS, He Y, Ayanian JZ, Gomez SL, Khan KL, West DW, et al. Quality of cancer care among foreign-born patients with lung or colorectal cancer. Cancer 2010; 116:5497–5506.
- Kouri EM, He Y, Winer EP, Keating NL. Influence of birthplace on breast cancer diagnosis and treatment for Hispanic women. Breast Cancer Res Treat 2009; 121:743–751.
- Dyce A, editor. Sir Thomas More, a play. London: The Shakespeare Society, 1844. https://archive.org/details/sirthomasmorepla00mund. Accessed September 21, 2016.
- Cawcutt KA, Wilson JW. The benefits and challenges of caring for international patients. Cleve Clin J Med 2016; 83:794–800.
- Health-Tourism.com. The history of medical tourism. Health-Tourism.com. www.health-tourism.com/medical-tourism/history/. Accessed September 21, 2016.
- Chen LH, Hochberg NS, Magill AJ. The pre-travel consultation. US Centers for Disease Control and Prevention. wwwnc.cdc.gov/travel/yellowbook/2016/the-pre-travel-consultation/the-pre-travel-consultation. Accessed September 21, 2016.
- Rogers K. Medical tourism. Encyclopedia Britannica. www.britannica.com/topic/medical-tourism. Accessed September 21, 2016.
- Detsky AS. What do patients really want from healthcare? JAMA 2011; 306:2500–2501.
- Shaywitz D. What do patients really want from healthcare? Forbes Dec 24, 2011. www.forbes.com/sites/davidshaywitz/2011/12/24/what-do-patients-really-want-from-health-care/print/. Accessed September 21, 2016.
- Lee TH. How to spread empathy in healthcare. Harvard Business Review July 17, 2014.
- Friedman R. Understanding empathy: can you feel my pain? New York Times April 24, 2007.
- National Council on Interpreting in Health Care. A national code of ethics for interpreters in healthcare. July 2004. www.ncihc.org/assets/documents/publications/NCIHC%20National%20Code%20of%20Ethics.pdf. Accessed September 21, 2016.
- Minguet L. Creating a culturally sensitive corporation. Harvard Business Review, September 2014.
- Nielsen SS, He Y, Ayanian JZ, Gomez SL, Khan KL, West DW, et al. Quality of cancer care among foreign-born patients with lung or colorectal cancer. Cancer 2010; 116:5497–5506.
- Kouri EM, He Y, Winer EP, Keating NL. Influence of birthplace on breast cancer diagnosis and treatment for Hispanic women. Breast Cancer Res Treat 2009; 121:743–751.
- Dyce A, editor. Sir Thomas More, a play. London: The Shakespeare Society, 1844. https://archive.org/details/sirthomasmorepla00mund. Accessed September 21, 2016.
The peacock and the doctor
Of the seven deadly sins, the worst is said to be pride, often represented in allegorical form as a peacock. In this month’s Journal, Kelly A. Cawcutt, MD and John W. Wilson, MD, and Nizar N. Zein, MD, note the rewards and challenges of caring for international patients. Pride, it seems to me, can get in the way of a successful relationship with these patients.
In the United States, we encounter a wide range of international patients, but there are two distinct categories: medical tourists, who come here by choice and often have significant financial means, and immigrants, who come here by choice or necessity and run the gamut of economic status.
The former group generally seeks care at major academic medical centers such as Cleveland Clinic and Mayo Clinic, which have built infrastructures to accommodate them, including paying special attention to the social aspects of the visit. For the medical center, there are immediate financial gains as well as potential long-term benefits, including international networking and philanthropy.
On the other hand, new immigrants, including refugees, generally seek care as needed where they have settled, mostly hoping that medical issues will not arise in the midst of the challenges of resettlement. They deserve and should expect to be able to establish a comfortable therapeutic relationship with a physician in a local medical practice, although one likely dissimilar from what they previously encountered.
For all of these patients, the focal point of interaction is us, the physician next to the examination table. Dr. Zein emphasizes the power of empathy and how our demeanor and choice of words are critical in building the therapeutic relationship. But pride can slip in, and the peacock subtly fans his tail.
While medical practice in the United States is technologically advanced in terms of tests and procedures, we are not the world leaders in outcomes or cost-effective care. We most certainly do not have a monopoly on delivering compassionate and empathic care or forging one-on-one doctor-patient relationships. We must be careful not to express a demeaning or dismissive attitude about the care our patients’ physicians provided in their home countries. That the laboratory and imaging reports are written in a different language, and perhaps reported in different units, should not imply any lower standard. We should also recognize that many of our physical examination skills have atrophied as we have come to over-rely on imaging studies. The apparent omission of an echocardiogram may in fact be an act of commission—a careful and confident physical examination may have resulted in a thoughtful decision to save the patient money. Careless words or a casually chauvinistic attitude can be disruptive to building a comfortable ongoing doctor-patient relationship.
At the same time, international patients come to see us with significant expectations (they may even have read our hospital’s marketing materials). But they may not be accustomed to their physician openly expressing a lack of certainty about a diagnosis. They may never have heard their at-home doctor say, “I don’t know.” The concept of patient involvement in the treatment plan may be totally foreign and discomforting to some, while others will expect that the entire family entourage (filling the exam room) will have an active role in decision-making.
Cultural awareness is critical as we sort these issues out so they do not stand in the way of successfully caring for the patient in front of us. We should avoid being too self-confident in our entrenched approach to healthcare delivery in the exam room (as well as in the redesign of our healthcare system). The peacock can be an attractive impediment.
Of the seven deadly sins, the worst is said to be pride, often represented in allegorical form as a peacock. In this month’s Journal, Kelly A. Cawcutt, MD and John W. Wilson, MD, and Nizar N. Zein, MD, note the rewards and challenges of caring for international patients. Pride, it seems to me, can get in the way of a successful relationship with these patients.
In the United States, we encounter a wide range of international patients, but there are two distinct categories: medical tourists, who come here by choice and often have significant financial means, and immigrants, who come here by choice or necessity and run the gamut of economic status.
The former group generally seeks care at major academic medical centers such as Cleveland Clinic and Mayo Clinic, which have built infrastructures to accommodate them, including paying special attention to the social aspects of the visit. For the medical center, there are immediate financial gains as well as potential long-term benefits, including international networking and philanthropy.
On the other hand, new immigrants, including refugees, generally seek care as needed where they have settled, mostly hoping that medical issues will not arise in the midst of the challenges of resettlement. They deserve and should expect to be able to establish a comfortable therapeutic relationship with a physician in a local medical practice, although one likely dissimilar from what they previously encountered.
For all of these patients, the focal point of interaction is us, the physician next to the examination table. Dr. Zein emphasizes the power of empathy and how our demeanor and choice of words are critical in building the therapeutic relationship. But pride can slip in, and the peacock subtly fans his tail.
While medical practice in the United States is technologically advanced in terms of tests and procedures, we are not the world leaders in outcomes or cost-effective care. We most certainly do not have a monopoly on delivering compassionate and empathic care or forging one-on-one doctor-patient relationships. We must be careful not to express a demeaning or dismissive attitude about the care our patients’ physicians provided in their home countries. That the laboratory and imaging reports are written in a different language, and perhaps reported in different units, should not imply any lower standard. We should also recognize that many of our physical examination skills have atrophied as we have come to over-rely on imaging studies. The apparent omission of an echocardiogram may in fact be an act of commission—a careful and confident physical examination may have resulted in a thoughtful decision to save the patient money. Careless words or a casually chauvinistic attitude can be disruptive to building a comfortable ongoing doctor-patient relationship.
At the same time, international patients come to see us with significant expectations (they may even have read our hospital’s marketing materials). But they may not be accustomed to their physician openly expressing a lack of certainty about a diagnosis. They may never have heard their at-home doctor say, “I don’t know.” The concept of patient involvement in the treatment plan may be totally foreign and discomforting to some, while others will expect that the entire family entourage (filling the exam room) will have an active role in decision-making.
Cultural awareness is critical as we sort these issues out so they do not stand in the way of successfully caring for the patient in front of us. We should avoid being too self-confident in our entrenched approach to healthcare delivery in the exam room (as well as in the redesign of our healthcare system). The peacock can be an attractive impediment.
Of the seven deadly sins, the worst is said to be pride, often represented in allegorical form as a peacock. In this month’s Journal, Kelly A. Cawcutt, MD and John W. Wilson, MD, and Nizar N. Zein, MD, note the rewards and challenges of caring for international patients. Pride, it seems to me, can get in the way of a successful relationship with these patients.
In the United States, we encounter a wide range of international patients, but there are two distinct categories: medical tourists, who come here by choice and often have significant financial means, and immigrants, who come here by choice or necessity and run the gamut of economic status.
The former group generally seeks care at major academic medical centers such as Cleveland Clinic and Mayo Clinic, which have built infrastructures to accommodate them, including paying special attention to the social aspects of the visit. For the medical center, there are immediate financial gains as well as potential long-term benefits, including international networking and philanthropy.
On the other hand, new immigrants, including refugees, generally seek care as needed where they have settled, mostly hoping that medical issues will not arise in the midst of the challenges of resettlement. They deserve and should expect to be able to establish a comfortable therapeutic relationship with a physician in a local medical practice, although one likely dissimilar from what they previously encountered.
For all of these patients, the focal point of interaction is us, the physician next to the examination table. Dr. Zein emphasizes the power of empathy and how our demeanor and choice of words are critical in building the therapeutic relationship. But pride can slip in, and the peacock subtly fans his tail.
While medical practice in the United States is technologically advanced in terms of tests and procedures, we are not the world leaders in outcomes or cost-effective care. We most certainly do not have a monopoly on delivering compassionate and empathic care or forging one-on-one doctor-patient relationships. We must be careful not to express a demeaning or dismissive attitude about the care our patients’ physicians provided in their home countries. That the laboratory and imaging reports are written in a different language, and perhaps reported in different units, should not imply any lower standard. We should also recognize that many of our physical examination skills have atrophied as we have come to over-rely on imaging studies. The apparent omission of an echocardiogram may in fact be an act of commission—a careful and confident physical examination may have resulted in a thoughtful decision to save the patient money. Careless words or a casually chauvinistic attitude can be disruptive to building a comfortable ongoing doctor-patient relationship.
At the same time, international patients come to see us with significant expectations (they may even have read our hospital’s marketing materials). But they may not be accustomed to their physician openly expressing a lack of certainty about a diagnosis. They may never have heard their at-home doctor say, “I don’t know.” The concept of patient involvement in the treatment plan may be totally foreign and discomforting to some, while others will expect that the entire family entourage (filling the exam room) will have an active role in decision-making.
Cultural awareness is critical as we sort these issues out so they do not stand in the way of successfully caring for the patient in front of us. We should avoid being too self-confident in our entrenched approach to healthcare delivery in the exam room (as well as in the redesign of our healthcare system). The peacock can be an attractive impediment.
Update in perioperative cardiac medicine
Perioperative medicine is an evolving field with a rapidly growing body of literature. Because physicians and patients are often concerned about cardiac risk, we focus this review on perioperative cardiology.
The information we present here is derived from presentations at the Perioperative Medicine Summit and the annual meetings of the Society of Hospital Medicine and Society of General Internal Medicine in 2016. We surveyed perioperative literature from January 2015 through March 2016 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
We have divided this review into four sections:
- Preoperative cardiac risk assessment
- Medical therapy to reduce postoperative cardiac complications (beta-blockers, statins, and angiotensin II receptor blockers [ARBs])
- Perioperative management of patients with a coronary stent on antiplatelet therapy
- Perioperative bridging anticoagulation.
PREOPERATIVE ASSESSMENT OF CARDIAC RISK
Functionally independent patients do better
Visnjevac O, Davari-Farid S, Lee J, et al. The effect of adding functional classification to ASA status for predicting 30-day mortality. Anesth Analg 2015; 121:110–116.
Functional capacity is an independent predictor of perioperative death and is included in the algorithm of the current joint American College of Cardiology/American Heart Association (ACC/AHA) guidelines,1 but it is not in the Revised Cardiac Risk Index2 or the American Society of Anesthesiologists (ASA) classification.3
The study. Visnjevac et al4 performed a retrospective, observational cohort study of 12,324 patients who underwent noncardiac surgery, stratifying rates of all-cause mortality and 30-day postoperative complications based on ASA class and functional capacity.
The ASA physical status classification is defined as:
- 1—Normal healthy patient
- 2—Patient with mild systemic disease
- 3—Patient with severe systemic disease
- 4—Patient with severe systemic disease that is a constant threat to life
- 5—Moribund patient not expected to survive without surgery.
Functional capacity was defined as the ability to perform all activities of daily living. It was prospectively assessed during the patient interview by pre-anesthesia personnel and entered into the database of the Veterans Affairs Surgical Quality Improvement Program.
Results. Within each ASA class, the mortality rate was significantly lower for functionally independent patients than for partially or fully dependent patients:
- In class 2—odds ratio (OR) 0.14 for functionally independent patients
- In class 3—OR 0.29 for functionally independent patients
- In class 4—OR 0.5 for functionally independent patients.
The mortality rate was higher for dependent patients than for independent patients who were one ASA class higher, despite the higher class having greater rates of comorbidity.
Adding functional capacity to the ASA classification improved the area under the receiver operating curve from 0.811 to 0.848 (a perfect test would have a value of 1.0), suggesting that physicians should incorporate functional capacity into their preoperative evaluation, perhaps by increasing a patient’s ASA class to the next higher class if he or she is functionally dependent.
Angina portends poor outcomes
Pandey A, Sood A, Sammon JD, et al. Effect of preoperative angina pectoris on cardiac outcomes in patients with previous myocardial infarction undergoing major noncardiac surgery (data from ACS-NSQIP). Am J Cardiol 2015; 115:1080–1084.
Coronary artery disease is a risk factor for adverse perioperative outcomes, but the risk varies depending on whether the patient has had a myocardial infarction (and how long ago) and whether he or she has anginal symptoms (and how severe they are).
The study. Pandey et al5 used data from the American College of Surgeons National Surgical Quality Improvement Program to evaluate the impact of stable angina in 1,568 patients who underwent noncardiac surgery after a myocardial infarction.
Results. Postoperative myocardial infarction or cardiac arrest occurred in 5.5% of patients. The incidence was significantly greater in those who had anginal symptoms before surgery than in those without symptoms (8.4% vs 5%, P = .035); reintervention rates and length of stay were also higher in this group. In multivariate analysis, preoperative angina remained a significant predictor of postoperative myocardial infarction (OR 2.49, 95% confidence interval [CI] 1.20–5.81) and reintervention (OR 2.4, 95% CI 1.44–3.82.
The authors cautioned against relying on predictive tools such as the Revised Cardiac Risk Index that do not consider stable angina and previous myocardial infarction as separate independent risk factors.
Implications for clinical practice. While functional capacity is an integral part of the ACC/AHA guideline algorithm,1 the findings of these two studies suggest that other current tools to calculate perioperative risk (ASA class and Revised Cardiac Risk Index) could be improved by including functional capacity and stable angina.
PERIOPERATIVE MEDICAL THERAPY
Beta-blockers help only those at high risk and may harm others
Friedell ML, Van Way CW 3rd, Freyberg RW, Almenoff PL. ß-blockade and operative mortality in noncardiac surgery: harmful or helpful? JAMA Surg 2015; 150:658–663.
Beta-blockers have been used perioperatively for nearly 2 decades to try to reduce rates of postoperative major adverse cardiovascular events. However, in view of recent trials, fewer patients are likely to benefit from this intervention than has been thought.
The study. Friedell et al6 retrospectively analyzed data from 343,645 patients in Veterans Affairs hospitals to determine the effect of beta-blockers on major adverse cardiac event rates after major noncardiac surgery. Beta-blockers were considered to have been used perioperatively if given any time between 8 hours before and 24 hours after surgery. The outcome studied was the mortality rate at 30 days.
The authors derived a novel risk score and used multivariate analysis to attempt to adjust for confounding factors. The risk score was based on four risk factors identified a priori:
- Serum creatinine level > 2.0 mg/dL
- Coronary artery disease
- Diabetes
- Surgery in a major body cavity (abdomen or chest).
Results. In this cohort, 43.2% of patients had received a beta-blocker. The unadjusted mortality rates by risk category for patients receiving or not receiving a beta-blocker were:
- No risk factors: 1.0% with a beta-blocker vs 0.6% without
- One or two risk factors: 1.7% vs 1.5%
- Three or four risk factors: 2.3% vs 4.5%.
After adjustment for confounding factors, the 30-day mortality rate was higher in low-risk patients and lower in high-risk patients who received beta-blockers. Odds ratios for death in beta-blocker users (entire cohort) by risk category were:
- No risk factors: 1.19
- One or two risk factors 0.97
- Three or four risk factors 0.76.
In the 3.8% of the total cohort who underwent cardiac surgery, beta-blockers had no significant effect—beneficial or harmful—in any risk group.
Jørgensen ME, Hlatky MA, Køber L, et al. ß-blocker-associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med 2015; 175:1923–1931.
The study. Jørgensen et al7 investigated the association between chronic beta-blocker use for the treatment of hypertension and 30-day rates of mortality and major adverse cardiac events. Eligible patients (N = 55,320) were at least 20 years old and were undergoing any type of noncardiac surgery. The authors established that hypertension was present through use of an algorithm based on the International Classification of Diseases (10th edition). Patients with existing cardiovascular disease and renal disease were excluded. The authors used multivariate analysis to adjust for confounding factors.
Results. Twenty-six percent of the patients were on chronic beta-blocker therapy for hypertension. The mortality rate at 30 days was 1.93% in patients treated with a beta-blocker alone or in combination with other antihypertensive drugs; the rate was 1.32% for patients receiving any combination of renin-angiotensin system inhibitor, calcium antagonist, or thiazide, but no beta-blocker. Similarly, the 30-day major adverse cardiac event rates were 1.32% with beta-blockers and 0.84% without beta-blockers.
In subgroup analysis, each medication combination that included a beta-blocker was associated with higher rates of death and major adverse cardiac events than the same combination without a beta-blocker. Odds ratios for major adverse cardiac events with beta-blocker combinations ranged from 1.22 to 2.16 compared with regimens with no beta-blocker.
Implications for clinical practice. These two studies added to a growing chorus of concerns about the value and safety of beta-blockers in surgical patients. Friedell et al6 made an observation that was remarkably similar to one reported by Lindenauer et al8 in 2005: when patients were stratified by baseline risk of death, only those with the highest baseline risk benefited from beta-blocker therapy. Those in the lowest risk group actually were harmed by beta-blocker use, ie, the mortality rate was higher.
More interesting is the novel observation by Jørgensen et al7 that even in patients with no known cardiovascular disease who are on chronic beta-blocker therapy—presumably on stable doses and not solely for perioperative risk reduction—rates of mortality and major adverse cardiac events were higher than for patients not on chronic beta-blocker therapy.
The current studies support a cautious, selective approach to the perioperative use of beta-blockers—they should be used only in high-risk patients undergoing high-risk surgery, as has been proposed by the ACC/AHA.1
Statins protect
Antoniou GA, Hajibandeh S, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg 2015; 61:519–532.
The study9 was a comprehensive meta-analysis of randomized controlled trials and observational studies of the effects of HMG-CoA reductase inhibitors (statins) on perioperative outcomes in patients undergoing vascular surgery (but not for intracranial or coronary artery disease). Twenty-four studies were included, 4 randomized controlled trials and 20 observational studies (including 16 cohort and 4 case-controlled studies), with a total of 22,536 patients, 8,052 receiving statins and 15,484 not receiving statins.
Results. Although there was no significant difference in cardiovascular mortality rates, patients receiving statins had significantly lower rates of all-cause mortality, myocardial infarction, stroke, and a composite of myocardial infarction, stroke, and death at 30 days postoperatively than patients not receiving statins. Additionally, there was no difference in the incidence of kidney injury between groups. The possibility of publication bias was thought to be low for all of these outcomes.
Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
The study. The Vascular Events in Non-cardiac Surgery Patients Cohort Evaluation (VISION) study10,11 was an international prospective cohort study of more than 40,000 patients age 45 and older undergoing major noncardiac surgery with either general or regional anesthesia. Postoperative troponin measurements were obtained in all patients 6 to 12 hours after surgery and for the first 3 postoperative days. The authors evaluated the effect of preoperative statin use on cardiovascular outcomes at 30 days after surgery using a multivariate logistic model and propensity score analysis to correct for confounding factors. Statin use was defined as exposure within 7 days before surgery or 3 days after.
Results. In the 15,478 patients included in the analysis, statin use conferred a significant reduction in the primary outcome (composite of all-cause mortality, myocardial injury after noncardiac surgery, or stroke); the absolute risk reduction was 2.0%. Statin users also had a significantly lower risk of all-cause mortality, cardiovascular mortality, and myocardial injury after noncardiac surgery, but not of postoperative myocardial infarction or stroke. This analysis did not address the type of statin, dosing, or safety markers such as liver and muscle function.
Implications for clinical practice. With largely observational data and a few small randomized trials, these meta-analyses provide important information with respect to perioperative cardiovascular protection by statins. Starting a statin before surgery and continuing it perioperatively seems appropriate in patients at high risk (as recommended by the ACC/AHA guidelines1). Based on other data, the benefit may be evident in as little as 5 days, as this is when statins appear to reach their plateau with regard to their vascular pleiotropic effects.12 The incidence of adverse effects of statins, including muscle and liver injury, appears to be low in the perioperative setting.13
Given the inconsistent data regarding perioperative beta-blocker therapy, statins may very well be the most important perioperative medication with respect to cardiovascular risk reduction. However, a large randomized trial would help to confirm this belief.
Restart angiotensin II receptor blockers soon after surgery
Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology 2015; 123:288–306.
A concern about perioperative use of ARBs is that they impair the renin-angiotensin-aldosterone system, which maintains blood pressure under general anesthesia. ARB-induced intraoperative hypotension is particularly difficult to control, as it is often refractory to treatment with conventional adrenergic vasopressors.
The study. Lee et al14 conducted a retrospective cohort trial to evaluate the effects of continuing to withhold ARBs postoperatively. Of the 30,173 patients admitted for surgery in the Veterans Affairs system from 1999 through 2011 who were taking an ARB before surgery and who met the inclusion criteria, 10,205 (33.8%) were not restarted on their medication by postoperative day 2.
Results. The mortality rate at 30 days was higher in those whose ARBs were withheld than in those in whom it was resumed, with a multivariable-adjusted hazard ratio of 1.74 (95% CI 1.47–2.06; P < .001). The risk of withholding ARBs was more pronounced in younger patients (hazard ratio 2.52; 95% CI 1.69–3.76 in those under age 60) than in older patients (hazard ratio 1.42, 95% CI 1.09–1.85 in those over age 75).
Implications for clinical practice. While not addressing whether to continue or withhold ARBs preoperatively, this retrospective study presented evidence that delay in resuming chronic ARB therapy after surgery was common and appeared to be associated with a higher 30-day mortality rate. The ACC/AHA guidelines1 state:
- Continuing angiotensin-converting enzyme (ACE) inhibitors or ARBs perioperatively is reasonable (class IIa recommendation, level of evidence B) (Table 1).
- If an ACE inhibitor or ARB is withheld before surgery, it is reasonable to restart it postoperatively as soon as clinically feasible (class IIa recommendation, level of evidence C).
Close attention to medication reconciliation in the postoperative period is necessary to facilitate early resumption of ARBs.
CORONARY STENTS AND ANTIPLATELET THERAPY IN NONCARDIAC SURGERY PATIENTS
Considerations in the management of noncardiac surgery patients with stents include risks of stent thrombosis, bleeding, and potentially delaying procedures to continue uninterrupted dual antiplatelet therapy. Evidence is evolving regarding the risks of perioperative complications in patients with bare-metal stents and drug-eluting stents, as well as the optimal timing before noncardiac surgery.
Bare-metal vs drug-eluting stents
Bangalore S, Silbaugh TS, Normand SL, Lovett AF, Welt FG, Resnic FS. Drug-eluting stents versus bare metal stents prior to noncardiac surgery. Catheter Cardiovasc Interv 2015; 85:533–541.
The study. Bangalore et al15 compared the safety of drug-eluting vs bare-metal stents in noncardiac surgery patients and investigated adverse events stratified by time since stent placement. This was a retrospective observational study of 8,415 patients in the Massachusetts claims database who underwent noncardiac surgery 1 year or less after percutaneous coronary intervention.
Results. There was no significant difference in the incidence of the primary outcome (composite of death, myocardial infarction, and bleeding) between the two groups.
With drug-eluting stents, patients had lower 30-day postoperative mortality rates, and their rate of the primary outcome decreased with time from percutaneous coronary intervention to surgery, being lowest beyond 90 days:
- 8.6% in days 1–30
- 7.5% in days 31–90
- 5.2% in days 91–180
- 5.8% in days 181–365 (P = .02).
With bare-metal stents, the event rate remained high over time:
- 8.2% in days 1–30
- 6.6% in days 31–90
- 8.1% in days 91–180
- 8.8% in days 181–365 (P = .60).
This study did not report information about perioperative antiplatelet management and was limited to first-generation drug-eluting stents.
Saia F, Belotti LM, Guastaroba P, et al. Risk of adverse cardiac and bleeding events following cardiac and noncardiac surgery in patients with coronary stents: how important is the interplay between stent type and time from stenting to surgery? Circ Cardiovasc Qual Outcomes 2015; 9:39–47.
The study. Saia et al16 retrospectively examined predictors of periprocedural ischemic and bleeding events among cardiac and noncardiac surgical patients who had previously undergone percutaneous coronary intervention. They also assessed the risks associated with stent type and time from percutaneous coronary intervention to surgery.
Of 39,362 patients, 13,128 underwent procedures during the 5-year study period. The cumulative incidence of surgery was 3.6% at 30 days, 14% at 1 year, and 40% at 5 years after percutaneous coronary intervention. Almost 30% of the procedures were done urgently.
Results. The 30-day rate of postoperative cardiac death was 2.5%, nonfatal myocardial infarction 1.5%, and serious bleeding events 6.5%. Older drug-eluting stents were associated with higher risks of adverse events than newer drug-eluting stents at any time point (odds ratio 2.1 at 0–180 days, 1.9 at 6–12 months, and 1.45 after 12 months). Surgery performed 6 to 12 months after percutaneous coronary intervention had lower rates of adverse outcomes than surgery performed within 6 months. Beyond 6 months from percutaneous coronary intervention, bare-metal stents and newer drug-eluting stents did not have significantly different adverse event rates; however, newer drug-eluting stents appeared safer than bare-metal stents from 0 to 180 days.
Limitations of this study included lack of information regarding periprocedural antiplatelet management and a relatively small subset of newer drug-eluting stent patients.
Implications for clinical practice. These studies added to earlier work that demonstrated that the risk of perioperative adverse events differs by both the stent type and the time from percutaneous coronary intervention to noncardiac surgery. In patients with a drug-eluting stent, the risk levels off 90 days after percutaneous coronary intervention, suggesting that the previously recommended 12 months of uninterrupted dual antiplatelet therapy (per the 2014 ACC/AHA guidelines1) may not be needed, particularly with newer-generation drug-eluting stents. Based on new evidence, the ACC/AHA guidelines regarding perioperative management of dual antiplatelet therapy in noncardiac surgery patients were updated,17 as noted below.
An update to the ACC/AHA guidelines on dual antiplatelet therapy
Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016 Mar 29. DOI: 10.1161/CIR.0000000000000404. [Epub ahead of print]
The 2016 update17 provides the following recommendations for patients with coronary stents who undergo noncardiac surgery:
- Delay elective surgery for 30 days after placement of a bare-metal stent (class I recommendation, level of evidence B).
- It is optimal to delay elective surgery 6 months after drug-eluting stent placement (class I recommendation, level of evidence B).
- If dual antiplatelet therapy must be discontinued, then continue aspirin if possible and restart the P2Y12 inhibitor as soon as possible postoperatively (class I recommendation, level of evidence C ).
- A consensus decision among treating clinicians is useful regarding the risks of surgery and discontinuation or continuation of antiplatelet therapy (class IIa recommendation, level of evidence C).
- If dual antiplatelet therapy must be discontinued, then elective surgery should not be performed less than 30 days after bare-metal stent placement, or less than 3 months after drug-eluting stent placement (class III recommendation, level of evidence B).
- Elective surgery after drug-eluting stent placement when the P2Y12 inhibitor must be discontinued may be considered 3 months after drug-eluting stent placement if the risk of surgical delay is greater than the risk of stent thrombosis (class IIb recommendation, level of evidence C).
The basic differences are the new recommendations for a minimum of 6 months of dual antiplatelet therapy as opposed to 12 months after drug-eluting stent placement before elective noncardiac surgery, and to allow surgery after 3 months (as opposed to 6 months) if the risk of delaying surgery outweighs the risk of stent thrombosis or myocardial infarction.
PERIOPERATIVE ANTICOAGULATION
The optimal perioperative management of patients with atrial fibrillation who are on warfarin is uncertain. The American College of Chest Physicians guidelines18 categorized patients with atrial fibrillation into low, moderate, and high thromboembolic risk. Based primarily on observational data, these guidelines recommended perioperative bridging anticoagulation for those at high risk but not for those at low risk. For intermediate-risk patients, there were insufficient data to make any recommendation.
Bridging may not benefit those at intermediate risk
Douketis JD, Spyropoulos AC, Kaatz S, et al; BRIDGE Investigators. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
The study. The Bridging Anticoagulation in Patients Who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery (BRIDGE) trial19 was the first randomized controlled trial to examine the effects of perioperative bridging anticoagulation in patients with atrial fibrillation without mechanical heart valves.
Results. In 1,884 patients undergoing elective surgery, the incidence of arterial thromboembolism was 0.4% in the no-bridging group and 0.3% in the bridging group (95% CI −0.6 to 0.8; P = .01 for noninferiority). Major bleeding occurred in 1.3% of patients in the no-bridging group and 3.2% in the bridging group (95% CI 0.20–0.78; P = .005 for superiority).
These results suggest that the risks of bridging therapy are greater than the benefits. Of note, the mean CHADS2 score (1 point each for congestive heart failure, hypertension, age ≥ 75 years, and diabetes mellitus; 2 points for previous stroke or transient ischemic attack; a total score > 2 indicates significant risk of stroke) for patients enrolled in this trial was 2.3, and it may be difficult to extrapolate these results to the limited number of patients at highest risk, ie, who have a CHADS2 score of 5 or 6. Also, this study did not address patients with arterial or venous thromboembolism.
Implications for clinical practice. Despite the limitations noted above, this study does provide guidance for management of the intermediate-risk group with atrial fibrillation as defined by the American College of Chest Physicians: a no-bridging strategy is the best option.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 64:e77–e137.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Dripps RD, Lamont A, Eckenhoff JE. The role of anesthesia in surgical mortality. JAMA 1961; 178:261–266.
- Visnjevac O, Davari-Farid S, Lee J, et al. The effect of adding functional classification to ASA status for predicting 30-day mortality. Anesth Analg 2015; 121:110–116.
- Pandey A, Sood A, Sammon JD, et al. Effect of preoperative angina pectoris on cardiac outcomes in patients with previous myocardial infarction undergoing major noncardiac surgery (data from ACS-NSQIP). Am J Cardiol 2015; 115:1080–1084.
- Friedell ML, Van Way CW 3rd, Freyberg RW, Almenoff PL. ß-blockade and operative mortality in noncardiac surgery: harmful or helpful? JAMA Surg 2015; 150:658–663.
- Jørgensen ME, Hlatky MA, Køber L, et al. ß-blocker-associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med 2015; 175:1923–1931.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med 2005; 353:349–361.
- Antoniou GA, Hajibandeh S, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg 2015; 61:519–532.
- Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study I; Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307:2295–2304.
- Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
- Laufs U, Wassmann S, Hilgers S, Ribaudo N, Bohm M, Nickenig G. Rapid effects on vascular function after initiation and withdrawal of atorvastatin in healthy, normocholesterolemic men. Am J Cardiol 2001; 88:1306–1307.
- Schouten O, Kertai MD, Bax JJ, et al. Safety of perioperative statin use in high-risk patients undergoing major vascular surgery. Am J Cardiol 2005; 95:658–660.
- Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology 2015; 123:288–306.
- Bangalore S, Silbaugh TS, Normand SL, Lovett AF, Welt FG, Resnic FS. Drug-eluting stents versus bare metal stents prior to noncardiac surgery. Catheter Cardiovasc Interv 2015; 85:533–541.
- Saia F, Belotti LM, Guastaroba P, et al. Risk of adverse cardiac and bleeding events following cardiac and noncardiac surgery in patients with coronary stents: how important is the interplay between stent type and time from stenting to surgery? Circ Cardiovasc Qual Outcomes 2015; 9:39–47.
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016 Mar 29 DOI: 10.1161/CIR.0000000000000404. [Epub ahead of print]. Accessed August 16, 2016.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S. Erratum in Chest 2012; 141:1129.
- Douketis JD, Spyropoulos AC, Kaatz S, et al; BRIDGE Investigators. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
Perioperative medicine is an evolving field with a rapidly growing body of literature. Because physicians and patients are often concerned about cardiac risk, we focus this review on perioperative cardiology.
The information we present here is derived from presentations at the Perioperative Medicine Summit and the annual meetings of the Society of Hospital Medicine and Society of General Internal Medicine in 2016. We surveyed perioperative literature from January 2015 through March 2016 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
We have divided this review into four sections:
- Preoperative cardiac risk assessment
- Medical therapy to reduce postoperative cardiac complications (beta-blockers, statins, and angiotensin II receptor blockers [ARBs])
- Perioperative management of patients with a coronary stent on antiplatelet therapy
- Perioperative bridging anticoagulation.
PREOPERATIVE ASSESSMENT OF CARDIAC RISK
Functionally independent patients do better
Visnjevac O, Davari-Farid S, Lee J, et al. The effect of adding functional classification to ASA status for predicting 30-day mortality. Anesth Analg 2015; 121:110–116.
Functional capacity is an independent predictor of perioperative death and is included in the algorithm of the current joint American College of Cardiology/American Heart Association (ACC/AHA) guidelines,1 but it is not in the Revised Cardiac Risk Index2 or the American Society of Anesthesiologists (ASA) classification.3
The study. Visnjevac et al4 performed a retrospective, observational cohort study of 12,324 patients who underwent noncardiac surgery, stratifying rates of all-cause mortality and 30-day postoperative complications based on ASA class and functional capacity.
The ASA physical status classification is defined as:
- 1—Normal healthy patient
- 2—Patient with mild systemic disease
- 3—Patient with severe systemic disease
- 4—Patient with severe systemic disease that is a constant threat to life
- 5—Moribund patient not expected to survive without surgery.
Functional capacity was defined as the ability to perform all activities of daily living. It was prospectively assessed during the patient interview by pre-anesthesia personnel and entered into the database of the Veterans Affairs Surgical Quality Improvement Program.
Results. Within each ASA class, the mortality rate was significantly lower for functionally independent patients than for partially or fully dependent patients:
- In class 2—odds ratio (OR) 0.14 for functionally independent patients
- In class 3—OR 0.29 for functionally independent patients
- In class 4—OR 0.5 for functionally independent patients.
The mortality rate was higher for dependent patients than for independent patients who were one ASA class higher, despite the higher class having greater rates of comorbidity.
Adding functional capacity to the ASA classification improved the area under the receiver operating curve from 0.811 to 0.848 (a perfect test would have a value of 1.0), suggesting that physicians should incorporate functional capacity into their preoperative evaluation, perhaps by increasing a patient’s ASA class to the next higher class if he or she is functionally dependent.
Angina portends poor outcomes
Pandey A, Sood A, Sammon JD, et al. Effect of preoperative angina pectoris on cardiac outcomes in patients with previous myocardial infarction undergoing major noncardiac surgery (data from ACS-NSQIP). Am J Cardiol 2015; 115:1080–1084.
Coronary artery disease is a risk factor for adverse perioperative outcomes, but the risk varies depending on whether the patient has had a myocardial infarction (and how long ago) and whether he or she has anginal symptoms (and how severe they are).
The study. Pandey et al5 used data from the American College of Surgeons National Surgical Quality Improvement Program to evaluate the impact of stable angina in 1,568 patients who underwent noncardiac surgery after a myocardial infarction.
Results. Postoperative myocardial infarction or cardiac arrest occurred in 5.5% of patients. The incidence was significantly greater in those who had anginal symptoms before surgery than in those without symptoms (8.4% vs 5%, P = .035); reintervention rates and length of stay were also higher in this group. In multivariate analysis, preoperative angina remained a significant predictor of postoperative myocardial infarction (OR 2.49, 95% confidence interval [CI] 1.20–5.81) and reintervention (OR 2.4, 95% CI 1.44–3.82.
The authors cautioned against relying on predictive tools such as the Revised Cardiac Risk Index that do not consider stable angina and previous myocardial infarction as separate independent risk factors.
Implications for clinical practice. While functional capacity is an integral part of the ACC/AHA guideline algorithm,1 the findings of these two studies suggest that other current tools to calculate perioperative risk (ASA class and Revised Cardiac Risk Index) could be improved by including functional capacity and stable angina.
PERIOPERATIVE MEDICAL THERAPY
Beta-blockers help only those at high risk and may harm others
Friedell ML, Van Way CW 3rd, Freyberg RW, Almenoff PL. ß-blockade and operative mortality in noncardiac surgery: harmful or helpful? JAMA Surg 2015; 150:658–663.
Beta-blockers have been used perioperatively for nearly 2 decades to try to reduce rates of postoperative major adverse cardiovascular events. However, in view of recent trials, fewer patients are likely to benefit from this intervention than has been thought.
The study. Friedell et al6 retrospectively analyzed data from 343,645 patients in Veterans Affairs hospitals to determine the effect of beta-blockers on major adverse cardiac event rates after major noncardiac surgery. Beta-blockers were considered to have been used perioperatively if given any time between 8 hours before and 24 hours after surgery. The outcome studied was the mortality rate at 30 days.
The authors derived a novel risk score and used multivariate analysis to attempt to adjust for confounding factors. The risk score was based on four risk factors identified a priori:
- Serum creatinine level > 2.0 mg/dL
- Coronary artery disease
- Diabetes
- Surgery in a major body cavity (abdomen or chest).
Results. In this cohort, 43.2% of patients had received a beta-blocker. The unadjusted mortality rates by risk category for patients receiving or not receiving a beta-blocker were:
- No risk factors: 1.0% with a beta-blocker vs 0.6% without
- One or two risk factors: 1.7% vs 1.5%
- Three or four risk factors: 2.3% vs 4.5%.
After adjustment for confounding factors, the 30-day mortality rate was higher in low-risk patients and lower in high-risk patients who received beta-blockers. Odds ratios for death in beta-blocker users (entire cohort) by risk category were:
- No risk factors: 1.19
- One or two risk factors 0.97
- Three or four risk factors 0.76.
In the 3.8% of the total cohort who underwent cardiac surgery, beta-blockers had no significant effect—beneficial or harmful—in any risk group.
Jørgensen ME, Hlatky MA, Køber L, et al. ß-blocker-associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med 2015; 175:1923–1931.
The study. Jørgensen et al7 investigated the association between chronic beta-blocker use for the treatment of hypertension and 30-day rates of mortality and major adverse cardiac events. Eligible patients (N = 55,320) were at least 20 years old and were undergoing any type of noncardiac surgery. The authors established that hypertension was present through use of an algorithm based on the International Classification of Diseases (10th edition). Patients with existing cardiovascular disease and renal disease were excluded. The authors used multivariate analysis to adjust for confounding factors.
Results. Twenty-six percent of the patients were on chronic beta-blocker therapy for hypertension. The mortality rate at 30 days was 1.93% in patients treated with a beta-blocker alone or in combination with other antihypertensive drugs; the rate was 1.32% for patients receiving any combination of renin-angiotensin system inhibitor, calcium antagonist, or thiazide, but no beta-blocker. Similarly, the 30-day major adverse cardiac event rates were 1.32% with beta-blockers and 0.84% without beta-blockers.
In subgroup analysis, each medication combination that included a beta-blocker was associated with higher rates of death and major adverse cardiac events than the same combination without a beta-blocker. Odds ratios for major adverse cardiac events with beta-blocker combinations ranged from 1.22 to 2.16 compared with regimens with no beta-blocker.
Implications for clinical practice. These two studies added to a growing chorus of concerns about the value and safety of beta-blockers in surgical patients. Friedell et al6 made an observation that was remarkably similar to one reported by Lindenauer et al8 in 2005: when patients were stratified by baseline risk of death, only those with the highest baseline risk benefited from beta-blocker therapy. Those in the lowest risk group actually were harmed by beta-blocker use, ie, the mortality rate was higher.
More interesting is the novel observation by Jørgensen et al7 that even in patients with no known cardiovascular disease who are on chronic beta-blocker therapy—presumably on stable doses and not solely for perioperative risk reduction—rates of mortality and major adverse cardiac events were higher than for patients not on chronic beta-blocker therapy.
The current studies support a cautious, selective approach to the perioperative use of beta-blockers—they should be used only in high-risk patients undergoing high-risk surgery, as has been proposed by the ACC/AHA.1
Statins protect
Antoniou GA, Hajibandeh S, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg 2015; 61:519–532.
The study9 was a comprehensive meta-analysis of randomized controlled trials and observational studies of the effects of HMG-CoA reductase inhibitors (statins) on perioperative outcomes in patients undergoing vascular surgery (but not for intracranial or coronary artery disease). Twenty-four studies were included, 4 randomized controlled trials and 20 observational studies (including 16 cohort and 4 case-controlled studies), with a total of 22,536 patients, 8,052 receiving statins and 15,484 not receiving statins.
Results. Although there was no significant difference in cardiovascular mortality rates, patients receiving statins had significantly lower rates of all-cause mortality, myocardial infarction, stroke, and a composite of myocardial infarction, stroke, and death at 30 days postoperatively than patients not receiving statins. Additionally, there was no difference in the incidence of kidney injury between groups. The possibility of publication bias was thought to be low for all of these outcomes.
Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
The study. The Vascular Events in Non-cardiac Surgery Patients Cohort Evaluation (VISION) study10,11 was an international prospective cohort study of more than 40,000 patients age 45 and older undergoing major noncardiac surgery with either general or regional anesthesia. Postoperative troponin measurements were obtained in all patients 6 to 12 hours after surgery and for the first 3 postoperative days. The authors evaluated the effect of preoperative statin use on cardiovascular outcomes at 30 days after surgery using a multivariate logistic model and propensity score analysis to correct for confounding factors. Statin use was defined as exposure within 7 days before surgery or 3 days after.
Results. In the 15,478 patients included in the analysis, statin use conferred a significant reduction in the primary outcome (composite of all-cause mortality, myocardial injury after noncardiac surgery, or stroke); the absolute risk reduction was 2.0%. Statin users also had a significantly lower risk of all-cause mortality, cardiovascular mortality, and myocardial injury after noncardiac surgery, but not of postoperative myocardial infarction or stroke. This analysis did not address the type of statin, dosing, or safety markers such as liver and muscle function.
Implications for clinical practice. With largely observational data and a few small randomized trials, these meta-analyses provide important information with respect to perioperative cardiovascular protection by statins. Starting a statin before surgery and continuing it perioperatively seems appropriate in patients at high risk (as recommended by the ACC/AHA guidelines1). Based on other data, the benefit may be evident in as little as 5 days, as this is when statins appear to reach their plateau with regard to their vascular pleiotropic effects.12 The incidence of adverse effects of statins, including muscle and liver injury, appears to be low in the perioperative setting.13
Given the inconsistent data regarding perioperative beta-blocker therapy, statins may very well be the most important perioperative medication with respect to cardiovascular risk reduction. However, a large randomized trial would help to confirm this belief.
Restart angiotensin II receptor blockers soon after surgery
Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology 2015; 123:288–306.
A concern about perioperative use of ARBs is that they impair the renin-angiotensin-aldosterone system, which maintains blood pressure under general anesthesia. ARB-induced intraoperative hypotension is particularly difficult to control, as it is often refractory to treatment with conventional adrenergic vasopressors.
The study. Lee et al14 conducted a retrospective cohort trial to evaluate the effects of continuing to withhold ARBs postoperatively. Of the 30,173 patients admitted for surgery in the Veterans Affairs system from 1999 through 2011 who were taking an ARB before surgery and who met the inclusion criteria, 10,205 (33.8%) were not restarted on their medication by postoperative day 2.
Results. The mortality rate at 30 days was higher in those whose ARBs were withheld than in those in whom it was resumed, with a multivariable-adjusted hazard ratio of 1.74 (95% CI 1.47–2.06; P < .001). The risk of withholding ARBs was more pronounced in younger patients (hazard ratio 2.52; 95% CI 1.69–3.76 in those under age 60) than in older patients (hazard ratio 1.42, 95% CI 1.09–1.85 in those over age 75).
Implications for clinical practice. While not addressing whether to continue or withhold ARBs preoperatively, this retrospective study presented evidence that delay in resuming chronic ARB therapy after surgery was common and appeared to be associated with a higher 30-day mortality rate. The ACC/AHA guidelines1 state:
- Continuing angiotensin-converting enzyme (ACE) inhibitors or ARBs perioperatively is reasonable (class IIa recommendation, level of evidence B) (Table 1).
- If an ACE inhibitor or ARB is withheld before surgery, it is reasonable to restart it postoperatively as soon as clinically feasible (class IIa recommendation, level of evidence C).
Close attention to medication reconciliation in the postoperative period is necessary to facilitate early resumption of ARBs.
CORONARY STENTS AND ANTIPLATELET THERAPY IN NONCARDIAC SURGERY PATIENTS
Considerations in the management of noncardiac surgery patients with stents include risks of stent thrombosis, bleeding, and potentially delaying procedures to continue uninterrupted dual antiplatelet therapy. Evidence is evolving regarding the risks of perioperative complications in patients with bare-metal stents and drug-eluting stents, as well as the optimal timing before noncardiac surgery.
Bare-metal vs drug-eluting stents
Bangalore S, Silbaugh TS, Normand SL, Lovett AF, Welt FG, Resnic FS. Drug-eluting stents versus bare metal stents prior to noncardiac surgery. Catheter Cardiovasc Interv 2015; 85:533–541.
The study. Bangalore et al15 compared the safety of drug-eluting vs bare-metal stents in noncardiac surgery patients and investigated adverse events stratified by time since stent placement. This was a retrospective observational study of 8,415 patients in the Massachusetts claims database who underwent noncardiac surgery 1 year or less after percutaneous coronary intervention.
Results. There was no significant difference in the incidence of the primary outcome (composite of death, myocardial infarction, and bleeding) between the two groups.
With drug-eluting stents, patients had lower 30-day postoperative mortality rates, and their rate of the primary outcome decreased with time from percutaneous coronary intervention to surgery, being lowest beyond 90 days:
- 8.6% in days 1–30
- 7.5% in days 31–90
- 5.2% in days 91–180
- 5.8% in days 181–365 (P = .02).
With bare-metal stents, the event rate remained high over time:
- 8.2% in days 1–30
- 6.6% in days 31–90
- 8.1% in days 91–180
- 8.8% in days 181–365 (P = .60).
This study did not report information about perioperative antiplatelet management and was limited to first-generation drug-eluting stents.
Saia F, Belotti LM, Guastaroba P, et al. Risk of adverse cardiac and bleeding events following cardiac and noncardiac surgery in patients with coronary stents: how important is the interplay between stent type and time from stenting to surgery? Circ Cardiovasc Qual Outcomes 2015; 9:39–47.
The study. Saia et al16 retrospectively examined predictors of periprocedural ischemic and bleeding events among cardiac and noncardiac surgical patients who had previously undergone percutaneous coronary intervention. They also assessed the risks associated with stent type and time from percutaneous coronary intervention to surgery.
Of 39,362 patients, 13,128 underwent procedures during the 5-year study period. The cumulative incidence of surgery was 3.6% at 30 days, 14% at 1 year, and 40% at 5 years after percutaneous coronary intervention. Almost 30% of the procedures were done urgently.
Results. The 30-day rate of postoperative cardiac death was 2.5%, nonfatal myocardial infarction 1.5%, and serious bleeding events 6.5%. Older drug-eluting stents were associated with higher risks of adverse events than newer drug-eluting stents at any time point (odds ratio 2.1 at 0–180 days, 1.9 at 6–12 months, and 1.45 after 12 months). Surgery performed 6 to 12 months after percutaneous coronary intervention had lower rates of adverse outcomes than surgery performed within 6 months. Beyond 6 months from percutaneous coronary intervention, bare-metal stents and newer drug-eluting stents did not have significantly different adverse event rates; however, newer drug-eluting stents appeared safer than bare-metal stents from 0 to 180 days.
Limitations of this study included lack of information regarding periprocedural antiplatelet management and a relatively small subset of newer drug-eluting stent patients.
Implications for clinical practice. These studies added to earlier work that demonstrated that the risk of perioperative adverse events differs by both the stent type and the time from percutaneous coronary intervention to noncardiac surgery. In patients with a drug-eluting stent, the risk levels off 90 days after percutaneous coronary intervention, suggesting that the previously recommended 12 months of uninterrupted dual antiplatelet therapy (per the 2014 ACC/AHA guidelines1) may not be needed, particularly with newer-generation drug-eluting stents. Based on new evidence, the ACC/AHA guidelines regarding perioperative management of dual antiplatelet therapy in noncardiac surgery patients were updated,17 as noted below.
An update to the ACC/AHA guidelines on dual antiplatelet therapy
Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016 Mar 29. DOI: 10.1161/CIR.0000000000000404. [Epub ahead of print]
The 2016 update17 provides the following recommendations for patients with coronary stents who undergo noncardiac surgery:
- Delay elective surgery for 30 days after placement of a bare-metal stent (class I recommendation, level of evidence B).
- It is optimal to delay elective surgery 6 months after drug-eluting stent placement (class I recommendation, level of evidence B).
- If dual antiplatelet therapy must be discontinued, then continue aspirin if possible and restart the P2Y12 inhibitor as soon as possible postoperatively (class I recommendation, level of evidence C ).
- A consensus decision among treating clinicians is useful regarding the risks of surgery and discontinuation or continuation of antiplatelet therapy (class IIa recommendation, level of evidence C).
- If dual antiplatelet therapy must be discontinued, then elective surgery should not be performed less than 30 days after bare-metal stent placement, or less than 3 months after drug-eluting stent placement (class III recommendation, level of evidence B).
- Elective surgery after drug-eluting stent placement when the P2Y12 inhibitor must be discontinued may be considered 3 months after drug-eluting stent placement if the risk of surgical delay is greater than the risk of stent thrombosis (class IIb recommendation, level of evidence C).
The basic differences are the new recommendations for a minimum of 6 months of dual antiplatelet therapy as opposed to 12 months after drug-eluting stent placement before elective noncardiac surgery, and to allow surgery after 3 months (as opposed to 6 months) if the risk of delaying surgery outweighs the risk of stent thrombosis or myocardial infarction.
PERIOPERATIVE ANTICOAGULATION
The optimal perioperative management of patients with atrial fibrillation who are on warfarin is uncertain. The American College of Chest Physicians guidelines18 categorized patients with atrial fibrillation into low, moderate, and high thromboembolic risk. Based primarily on observational data, these guidelines recommended perioperative bridging anticoagulation for those at high risk but not for those at low risk. For intermediate-risk patients, there were insufficient data to make any recommendation.
Bridging may not benefit those at intermediate risk
Douketis JD, Spyropoulos AC, Kaatz S, et al; BRIDGE Investigators. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
The study. The Bridging Anticoagulation in Patients Who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery (BRIDGE) trial19 was the first randomized controlled trial to examine the effects of perioperative bridging anticoagulation in patients with atrial fibrillation without mechanical heart valves.
Results. In 1,884 patients undergoing elective surgery, the incidence of arterial thromboembolism was 0.4% in the no-bridging group and 0.3% in the bridging group (95% CI −0.6 to 0.8; P = .01 for noninferiority). Major bleeding occurred in 1.3% of patients in the no-bridging group and 3.2% in the bridging group (95% CI 0.20–0.78; P = .005 for superiority).
These results suggest that the risks of bridging therapy are greater than the benefits. Of note, the mean CHADS2 score (1 point each for congestive heart failure, hypertension, age ≥ 75 years, and diabetes mellitus; 2 points for previous stroke or transient ischemic attack; a total score > 2 indicates significant risk of stroke) for patients enrolled in this trial was 2.3, and it may be difficult to extrapolate these results to the limited number of patients at highest risk, ie, who have a CHADS2 score of 5 or 6. Also, this study did not address patients with arterial or venous thromboembolism.
Implications for clinical practice. Despite the limitations noted above, this study does provide guidance for management of the intermediate-risk group with atrial fibrillation as defined by the American College of Chest Physicians: a no-bridging strategy is the best option.
Perioperative medicine is an evolving field with a rapidly growing body of literature. Because physicians and patients are often concerned about cardiac risk, we focus this review on perioperative cardiology.
The information we present here is derived from presentations at the Perioperative Medicine Summit and the annual meetings of the Society of Hospital Medicine and Society of General Internal Medicine in 2016. We surveyed perioperative literature from January 2015 through March 2016 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
We have divided this review into four sections:
- Preoperative cardiac risk assessment
- Medical therapy to reduce postoperative cardiac complications (beta-blockers, statins, and angiotensin II receptor blockers [ARBs])
- Perioperative management of patients with a coronary stent on antiplatelet therapy
- Perioperative bridging anticoagulation.
PREOPERATIVE ASSESSMENT OF CARDIAC RISK
Functionally independent patients do better
Visnjevac O, Davari-Farid S, Lee J, et al. The effect of adding functional classification to ASA status for predicting 30-day mortality. Anesth Analg 2015; 121:110–116.
Functional capacity is an independent predictor of perioperative death and is included in the algorithm of the current joint American College of Cardiology/American Heart Association (ACC/AHA) guidelines,1 but it is not in the Revised Cardiac Risk Index2 or the American Society of Anesthesiologists (ASA) classification.3
The study. Visnjevac et al4 performed a retrospective, observational cohort study of 12,324 patients who underwent noncardiac surgery, stratifying rates of all-cause mortality and 30-day postoperative complications based on ASA class and functional capacity.
The ASA physical status classification is defined as:
- 1—Normal healthy patient
- 2—Patient with mild systemic disease
- 3—Patient with severe systemic disease
- 4—Patient with severe systemic disease that is a constant threat to life
- 5—Moribund patient not expected to survive without surgery.
Functional capacity was defined as the ability to perform all activities of daily living. It was prospectively assessed during the patient interview by pre-anesthesia personnel and entered into the database of the Veterans Affairs Surgical Quality Improvement Program.
Results. Within each ASA class, the mortality rate was significantly lower for functionally independent patients than for partially or fully dependent patients:
- In class 2—odds ratio (OR) 0.14 for functionally independent patients
- In class 3—OR 0.29 for functionally independent patients
- In class 4—OR 0.5 for functionally independent patients.
The mortality rate was higher for dependent patients than for independent patients who were one ASA class higher, despite the higher class having greater rates of comorbidity.
Adding functional capacity to the ASA classification improved the area under the receiver operating curve from 0.811 to 0.848 (a perfect test would have a value of 1.0), suggesting that physicians should incorporate functional capacity into their preoperative evaluation, perhaps by increasing a patient’s ASA class to the next higher class if he or she is functionally dependent.
Angina portends poor outcomes
Pandey A, Sood A, Sammon JD, et al. Effect of preoperative angina pectoris on cardiac outcomes in patients with previous myocardial infarction undergoing major noncardiac surgery (data from ACS-NSQIP). Am J Cardiol 2015; 115:1080–1084.
Coronary artery disease is a risk factor for adverse perioperative outcomes, but the risk varies depending on whether the patient has had a myocardial infarction (and how long ago) and whether he or she has anginal symptoms (and how severe they are).
The study. Pandey et al5 used data from the American College of Surgeons National Surgical Quality Improvement Program to evaluate the impact of stable angina in 1,568 patients who underwent noncardiac surgery after a myocardial infarction.
Results. Postoperative myocardial infarction or cardiac arrest occurred in 5.5% of patients. The incidence was significantly greater in those who had anginal symptoms before surgery than in those without symptoms (8.4% vs 5%, P = .035); reintervention rates and length of stay were also higher in this group. In multivariate analysis, preoperative angina remained a significant predictor of postoperative myocardial infarction (OR 2.49, 95% confidence interval [CI] 1.20–5.81) and reintervention (OR 2.4, 95% CI 1.44–3.82.
The authors cautioned against relying on predictive tools such as the Revised Cardiac Risk Index that do not consider stable angina and previous myocardial infarction as separate independent risk factors.
Implications for clinical practice. While functional capacity is an integral part of the ACC/AHA guideline algorithm,1 the findings of these two studies suggest that other current tools to calculate perioperative risk (ASA class and Revised Cardiac Risk Index) could be improved by including functional capacity and stable angina.
PERIOPERATIVE MEDICAL THERAPY
Beta-blockers help only those at high risk and may harm others
Friedell ML, Van Way CW 3rd, Freyberg RW, Almenoff PL. ß-blockade and operative mortality in noncardiac surgery: harmful or helpful? JAMA Surg 2015; 150:658–663.
Beta-blockers have been used perioperatively for nearly 2 decades to try to reduce rates of postoperative major adverse cardiovascular events. However, in view of recent trials, fewer patients are likely to benefit from this intervention than has been thought.
The study. Friedell et al6 retrospectively analyzed data from 343,645 patients in Veterans Affairs hospitals to determine the effect of beta-blockers on major adverse cardiac event rates after major noncardiac surgery. Beta-blockers were considered to have been used perioperatively if given any time between 8 hours before and 24 hours after surgery. The outcome studied was the mortality rate at 30 days.
The authors derived a novel risk score and used multivariate analysis to attempt to adjust for confounding factors. The risk score was based on four risk factors identified a priori:
- Serum creatinine level > 2.0 mg/dL
- Coronary artery disease
- Diabetes
- Surgery in a major body cavity (abdomen or chest).
Results. In this cohort, 43.2% of patients had received a beta-blocker. The unadjusted mortality rates by risk category for patients receiving or not receiving a beta-blocker were:
- No risk factors: 1.0% with a beta-blocker vs 0.6% without
- One or two risk factors: 1.7% vs 1.5%
- Three or four risk factors: 2.3% vs 4.5%.
After adjustment for confounding factors, the 30-day mortality rate was higher in low-risk patients and lower in high-risk patients who received beta-blockers. Odds ratios for death in beta-blocker users (entire cohort) by risk category were:
- No risk factors: 1.19
- One or two risk factors 0.97
- Three or four risk factors 0.76.
In the 3.8% of the total cohort who underwent cardiac surgery, beta-blockers had no significant effect—beneficial or harmful—in any risk group.
Jørgensen ME, Hlatky MA, Køber L, et al. ß-blocker-associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med 2015; 175:1923–1931.
The study. Jørgensen et al7 investigated the association between chronic beta-blocker use for the treatment of hypertension and 30-day rates of mortality and major adverse cardiac events. Eligible patients (N = 55,320) were at least 20 years old and were undergoing any type of noncardiac surgery. The authors established that hypertension was present through use of an algorithm based on the International Classification of Diseases (10th edition). Patients with existing cardiovascular disease and renal disease were excluded. The authors used multivariate analysis to adjust for confounding factors.
Results. Twenty-six percent of the patients were on chronic beta-blocker therapy for hypertension. The mortality rate at 30 days was 1.93% in patients treated with a beta-blocker alone or in combination with other antihypertensive drugs; the rate was 1.32% for patients receiving any combination of renin-angiotensin system inhibitor, calcium antagonist, or thiazide, but no beta-blocker. Similarly, the 30-day major adverse cardiac event rates were 1.32% with beta-blockers and 0.84% without beta-blockers.
In subgroup analysis, each medication combination that included a beta-blocker was associated with higher rates of death and major adverse cardiac events than the same combination without a beta-blocker. Odds ratios for major adverse cardiac events with beta-blocker combinations ranged from 1.22 to 2.16 compared with regimens with no beta-blocker.
Implications for clinical practice. These two studies added to a growing chorus of concerns about the value and safety of beta-blockers in surgical patients. Friedell et al6 made an observation that was remarkably similar to one reported by Lindenauer et al8 in 2005: when patients were stratified by baseline risk of death, only those with the highest baseline risk benefited from beta-blocker therapy. Those in the lowest risk group actually were harmed by beta-blocker use, ie, the mortality rate was higher.
More interesting is the novel observation by Jørgensen et al7 that even in patients with no known cardiovascular disease who are on chronic beta-blocker therapy—presumably on stable doses and not solely for perioperative risk reduction—rates of mortality and major adverse cardiac events were higher than for patients not on chronic beta-blocker therapy.
The current studies support a cautious, selective approach to the perioperative use of beta-blockers—they should be used only in high-risk patients undergoing high-risk surgery, as has been proposed by the ACC/AHA.1
Statins protect
Antoniou GA, Hajibandeh S, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg 2015; 61:519–532.
The study9 was a comprehensive meta-analysis of randomized controlled trials and observational studies of the effects of HMG-CoA reductase inhibitors (statins) on perioperative outcomes in patients undergoing vascular surgery (but not for intracranial or coronary artery disease). Twenty-four studies were included, 4 randomized controlled trials and 20 observational studies (including 16 cohort and 4 case-controlled studies), with a total of 22,536 patients, 8,052 receiving statins and 15,484 not receiving statins.
Results. Although there was no significant difference in cardiovascular mortality rates, patients receiving statins had significantly lower rates of all-cause mortality, myocardial infarction, stroke, and a composite of myocardial infarction, stroke, and death at 30 days postoperatively than patients not receiving statins. Additionally, there was no difference in the incidence of kidney injury between groups. The possibility of publication bias was thought to be low for all of these outcomes.
Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
The study. The Vascular Events in Non-cardiac Surgery Patients Cohort Evaluation (VISION) study10,11 was an international prospective cohort study of more than 40,000 patients age 45 and older undergoing major noncardiac surgery with either general or regional anesthesia. Postoperative troponin measurements were obtained in all patients 6 to 12 hours after surgery and for the first 3 postoperative days. The authors evaluated the effect of preoperative statin use on cardiovascular outcomes at 30 days after surgery using a multivariate logistic model and propensity score analysis to correct for confounding factors. Statin use was defined as exposure within 7 days before surgery or 3 days after.
Results. In the 15,478 patients included in the analysis, statin use conferred a significant reduction in the primary outcome (composite of all-cause mortality, myocardial injury after noncardiac surgery, or stroke); the absolute risk reduction was 2.0%. Statin users also had a significantly lower risk of all-cause mortality, cardiovascular mortality, and myocardial injury after noncardiac surgery, but not of postoperative myocardial infarction or stroke. This analysis did not address the type of statin, dosing, or safety markers such as liver and muscle function.
Implications for clinical practice. With largely observational data and a few small randomized trials, these meta-analyses provide important information with respect to perioperative cardiovascular protection by statins. Starting a statin before surgery and continuing it perioperatively seems appropriate in patients at high risk (as recommended by the ACC/AHA guidelines1). Based on other data, the benefit may be evident in as little as 5 days, as this is when statins appear to reach their plateau with regard to their vascular pleiotropic effects.12 The incidence of adverse effects of statins, including muscle and liver injury, appears to be low in the perioperative setting.13
Given the inconsistent data regarding perioperative beta-blocker therapy, statins may very well be the most important perioperative medication with respect to cardiovascular risk reduction. However, a large randomized trial would help to confirm this belief.
Restart angiotensin II receptor blockers soon after surgery
Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology 2015; 123:288–306.
A concern about perioperative use of ARBs is that they impair the renin-angiotensin-aldosterone system, which maintains blood pressure under general anesthesia. ARB-induced intraoperative hypotension is particularly difficult to control, as it is often refractory to treatment with conventional adrenergic vasopressors.
The study. Lee et al14 conducted a retrospective cohort trial to evaluate the effects of continuing to withhold ARBs postoperatively. Of the 30,173 patients admitted for surgery in the Veterans Affairs system from 1999 through 2011 who were taking an ARB before surgery and who met the inclusion criteria, 10,205 (33.8%) were not restarted on their medication by postoperative day 2.
Results. The mortality rate at 30 days was higher in those whose ARBs were withheld than in those in whom it was resumed, with a multivariable-adjusted hazard ratio of 1.74 (95% CI 1.47–2.06; P < .001). The risk of withholding ARBs was more pronounced in younger patients (hazard ratio 2.52; 95% CI 1.69–3.76 in those under age 60) than in older patients (hazard ratio 1.42, 95% CI 1.09–1.85 in those over age 75).
Implications for clinical practice. While not addressing whether to continue or withhold ARBs preoperatively, this retrospective study presented evidence that delay in resuming chronic ARB therapy after surgery was common and appeared to be associated with a higher 30-day mortality rate. The ACC/AHA guidelines1 state:
- Continuing angiotensin-converting enzyme (ACE) inhibitors or ARBs perioperatively is reasonable (class IIa recommendation, level of evidence B) (Table 1).
- If an ACE inhibitor or ARB is withheld before surgery, it is reasonable to restart it postoperatively as soon as clinically feasible (class IIa recommendation, level of evidence C).
Close attention to medication reconciliation in the postoperative period is necessary to facilitate early resumption of ARBs.
CORONARY STENTS AND ANTIPLATELET THERAPY IN NONCARDIAC SURGERY PATIENTS
Considerations in the management of noncardiac surgery patients with stents include risks of stent thrombosis, bleeding, and potentially delaying procedures to continue uninterrupted dual antiplatelet therapy. Evidence is evolving regarding the risks of perioperative complications in patients with bare-metal stents and drug-eluting stents, as well as the optimal timing before noncardiac surgery.
Bare-metal vs drug-eluting stents
Bangalore S, Silbaugh TS, Normand SL, Lovett AF, Welt FG, Resnic FS. Drug-eluting stents versus bare metal stents prior to noncardiac surgery. Catheter Cardiovasc Interv 2015; 85:533–541.
The study. Bangalore et al15 compared the safety of drug-eluting vs bare-metal stents in noncardiac surgery patients and investigated adverse events stratified by time since stent placement. This was a retrospective observational study of 8,415 patients in the Massachusetts claims database who underwent noncardiac surgery 1 year or less after percutaneous coronary intervention.
Results. There was no significant difference in the incidence of the primary outcome (composite of death, myocardial infarction, and bleeding) between the two groups.
With drug-eluting stents, patients had lower 30-day postoperative mortality rates, and their rate of the primary outcome decreased with time from percutaneous coronary intervention to surgery, being lowest beyond 90 days:
- 8.6% in days 1–30
- 7.5% in days 31–90
- 5.2% in days 91–180
- 5.8% in days 181–365 (P = .02).
With bare-metal stents, the event rate remained high over time:
- 8.2% in days 1–30
- 6.6% in days 31–90
- 8.1% in days 91–180
- 8.8% in days 181–365 (P = .60).
This study did not report information about perioperative antiplatelet management and was limited to first-generation drug-eluting stents.
Saia F, Belotti LM, Guastaroba P, et al. Risk of adverse cardiac and bleeding events following cardiac and noncardiac surgery in patients with coronary stents: how important is the interplay between stent type and time from stenting to surgery? Circ Cardiovasc Qual Outcomes 2015; 9:39–47.
The study. Saia et al16 retrospectively examined predictors of periprocedural ischemic and bleeding events among cardiac and noncardiac surgical patients who had previously undergone percutaneous coronary intervention. They also assessed the risks associated with stent type and time from percutaneous coronary intervention to surgery.
Of 39,362 patients, 13,128 underwent procedures during the 5-year study period. The cumulative incidence of surgery was 3.6% at 30 days, 14% at 1 year, and 40% at 5 years after percutaneous coronary intervention. Almost 30% of the procedures were done urgently.
Results. The 30-day rate of postoperative cardiac death was 2.5%, nonfatal myocardial infarction 1.5%, and serious bleeding events 6.5%. Older drug-eluting stents were associated with higher risks of adverse events than newer drug-eluting stents at any time point (odds ratio 2.1 at 0–180 days, 1.9 at 6–12 months, and 1.45 after 12 months). Surgery performed 6 to 12 months after percutaneous coronary intervention had lower rates of adverse outcomes than surgery performed within 6 months. Beyond 6 months from percutaneous coronary intervention, bare-metal stents and newer drug-eluting stents did not have significantly different adverse event rates; however, newer drug-eluting stents appeared safer than bare-metal stents from 0 to 180 days.
Limitations of this study included lack of information regarding periprocedural antiplatelet management and a relatively small subset of newer drug-eluting stent patients.
Implications for clinical practice. These studies added to earlier work that demonstrated that the risk of perioperative adverse events differs by both the stent type and the time from percutaneous coronary intervention to noncardiac surgery. In patients with a drug-eluting stent, the risk levels off 90 days after percutaneous coronary intervention, suggesting that the previously recommended 12 months of uninterrupted dual antiplatelet therapy (per the 2014 ACC/AHA guidelines1) may not be needed, particularly with newer-generation drug-eluting stents. Based on new evidence, the ACC/AHA guidelines regarding perioperative management of dual antiplatelet therapy in noncardiac surgery patients were updated,17 as noted below.
An update to the ACC/AHA guidelines on dual antiplatelet therapy
Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016 Mar 29. DOI: 10.1161/CIR.0000000000000404. [Epub ahead of print]
The 2016 update17 provides the following recommendations for patients with coronary stents who undergo noncardiac surgery:
- Delay elective surgery for 30 days after placement of a bare-metal stent (class I recommendation, level of evidence B).
- It is optimal to delay elective surgery 6 months after drug-eluting stent placement (class I recommendation, level of evidence B).
- If dual antiplatelet therapy must be discontinued, then continue aspirin if possible and restart the P2Y12 inhibitor as soon as possible postoperatively (class I recommendation, level of evidence C ).
- A consensus decision among treating clinicians is useful regarding the risks of surgery and discontinuation or continuation of antiplatelet therapy (class IIa recommendation, level of evidence C).
- If dual antiplatelet therapy must be discontinued, then elective surgery should not be performed less than 30 days after bare-metal stent placement, or less than 3 months after drug-eluting stent placement (class III recommendation, level of evidence B).
- Elective surgery after drug-eluting stent placement when the P2Y12 inhibitor must be discontinued may be considered 3 months after drug-eluting stent placement if the risk of surgical delay is greater than the risk of stent thrombosis (class IIb recommendation, level of evidence C).
The basic differences are the new recommendations for a minimum of 6 months of dual antiplatelet therapy as opposed to 12 months after drug-eluting stent placement before elective noncardiac surgery, and to allow surgery after 3 months (as opposed to 6 months) if the risk of delaying surgery outweighs the risk of stent thrombosis or myocardial infarction.
PERIOPERATIVE ANTICOAGULATION
The optimal perioperative management of patients with atrial fibrillation who are on warfarin is uncertain. The American College of Chest Physicians guidelines18 categorized patients with atrial fibrillation into low, moderate, and high thromboembolic risk. Based primarily on observational data, these guidelines recommended perioperative bridging anticoagulation for those at high risk but not for those at low risk. For intermediate-risk patients, there were insufficient data to make any recommendation.
Bridging may not benefit those at intermediate risk
Douketis JD, Spyropoulos AC, Kaatz S, et al; BRIDGE Investigators. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
The study. The Bridging Anticoagulation in Patients Who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery (BRIDGE) trial19 was the first randomized controlled trial to examine the effects of perioperative bridging anticoagulation in patients with atrial fibrillation without mechanical heart valves.
Results. In 1,884 patients undergoing elective surgery, the incidence of arterial thromboembolism was 0.4% in the no-bridging group and 0.3% in the bridging group (95% CI −0.6 to 0.8; P = .01 for noninferiority). Major bleeding occurred in 1.3% of patients in the no-bridging group and 3.2% in the bridging group (95% CI 0.20–0.78; P = .005 for superiority).
These results suggest that the risks of bridging therapy are greater than the benefits. Of note, the mean CHADS2 score (1 point each for congestive heart failure, hypertension, age ≥ 75 years, and diabetes mellitus; 2 points for previous stroke or transient ischemic attack; a total score > 2 indicates significant risk of stroke) for patients enrolled in this trial was 2.3, and it may be difficult to extrapolate these results to the limited number of patients at highest risk, ie, who have a CHADS2 score of 5 or 6. Also, this study did not address patients with arterial or venous thromboembolism.
Implications for clinical practice. Despite the limitations noted above, this study does provide guidance for management of the intermediate-risk group with atrial fibrillation as defined by the American College of Chest Physicians: a no-bridging strategy is the best option.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 64:e77–e137.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Dripps RD, Lamont A, Eckenhoff JE. The role of anesthesia in surgical mortality. JAMA 1961; 178:261–266.
- Visnjevac O, Davari-Farid S, Lee J, et al. The effect of adding functional classification to ASA status for predicting 30-day mortality. Anesth Analg 2015; 121:110–116.
- Pandey A, Sood A, Sammon JD, et al. Effect of preoperative angina pectoris on cardiac outcomes in patients with previous myocardial infarction undergoing major noncardiac surgery (data from ACS-NSQIP). Am J Cardiol 2015; 115:1080–1084.
- Friedell ML, Van Way CW 3rd, Freyberg RW, Almenoff PL. ß-blockade and operative mortality in noncardiac surgery: harmful or helpful? JAMA Surg 2015; 150:658–663.
- Jørgensen ME, Hlatky MA, Køber L, et al. ß-blocker-associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med 2015; 175:1923–1931.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med 2005; 353:349–361.
- Antoniou GA, Hajibandeh S, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg 2015; 61:519–532.
- Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study I; Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307:2295–2304.
- Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
- Laufs U, Wassmann S, Hilgers S, Ribaudo N, Bohm M, Nickenig G. Rapid effects on vascular function after initiation and withdrawal of atorvastatin in healthy, normocholesterolemic men. Am J Cardiol 2001; 88:1306–1307.
- Schouten O, Kertai MD, Bax JJ, et al. Safety of perioperative statin use in high-risk patients undergoing major vascular surgery. Am J Cardiol 2005; 95:658–660.
- Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology 2015; 123:288–306.
- Bangalore S, Silbaugh TS, Normand SL, Lovett AF, Welt FG, Resnic FS. Drug-eluting stents versus bare metal stents prior to noncardiac surgery. Catheter Cardiovasc Interv 2015; 85:533–541.
- Saia F, Belotti LM, Guastaroba P, et al. Risk of adverse cardiac and bleeding events following cardiac and noncardiac surgery in patients with coronary stents: how important is the interplay between stent type and time from stenting to surgery? Circ Cardiovasc Qual Outcomes 2015; 9:39–47.
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016 Mar 29 DOI: 10.1161/CIR.0000000000000404. [Epub ahead of print]. Accessed August 16, 2016.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S. Erratum in Chest 2012; 141:1129.
- Douketis JD, Spyropoulos AC, Kaatz S, et al; BRIDGE Investigators. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 64:e77–e137.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Dripps RD, Lamont A, Eckenhoff JE. The role of anesthesia in surgical mortality. JAMA 1961; 178:261–266.
- Visnjevac O, Davari-Farid S, Lee J, et al. The effect of adding functional classification to ASA status for predicting 30-day mortality. Anesth Analg 2015; 121:110–116.
- Pandey A, Sood A, Sammon JD, et al. Effect of preoperative angina pectoris on cardiac outcomes in patients with previous myocardial infarction undergoing major noncardiac surgery (data from ACS-NSQIP). Am J Cardiol 2015; 115:1080–1084.
- Friedell ML, Van Way CW 3rd, Freyberg RW, Almenoff PL. ß-blockade and operative mortality in noncardiac surgery: harmful or helpful? JAMA Surg 2015; 150:658–663.
- Jørgensen ME, Hlatky MA, Køber L, et al. ß-blocker-associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med 2015; 175:1923–1931.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med 2005; 353:349–361.
- Antoniou GA, Hajibandeh S, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg 2015; 61:519–532.
- Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study I; Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307:2295–2304.
- Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
- Laufs U, Wassmann S, Hilgers S, Ribaudo N, Bohm M, Nickenig G. Rapid effects on vascular function after initiation and withdrawal of atorvastatin in healthy, normocholesterolemic men. Am J Cardiol 2001; 88:1306–1307.
- Schouten O, Kertai MD, Bax JJ, et al. Safety of perioperative statin use in high-risk patients undergoing major vascular surgery. Am J Cardiol 2005; 95:658–660.
- Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology 2015; 123:288–306.
- Bangalore S, Silbaugh TS, Normand SL, Lovett AF, Welt FG, Resnic FS. Drug-eluting stents versus bare metal stents prior to noncardiac surgery. Catheter Cardiovasc Interv 2015; 85:533–541.
- Saia F, Belotti LM, Guastaroba P, et al. Risk of adverse cardiac and bleeding events following cardiac and noncardiac surgery in patients with coronary stents: how important is the interplay between stent type and time from stenting to surgery? Circ Cardiovasc Qual Outcomes 2015; 9:39–47.
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016 Mar 29 DOI: 10.1161/CIR.0000000000000404. [Epub ahead of print]. Accessed August 16, 2016.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S. Erratum in Chest 2012; 141:1129.
- Douketis JD, Spyropoulos AC, Kaatz S, et al; BRIDGE Investigators. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
KEY POINTS
- Outcomes are worse in patients with poor functional capacity or stable angina, and these factors should be considered in preoperative risk assessment.
- Perioperative use of beta-blockers may benefit only patients at highest risk and may harm other patients.
- Statins seem to provide perioperative protection.
- If an ARB is withheld for surgery, it should be restarted soon after.
- For patients with a coronary stent, the type of stent and duration of dual antiplatelet therapy need to be considered before noncardiac surgery.
- Bridging anticoagulant therapy should not be used in patients at intermediate or low risk of thromboembolism.
The ABCs of managing systolic heart failure: Past, present, and future
Managing heart failure is a challenge. To aid clinicians in this task, the American College of Cardiology Foundation (ACC) and the American Heart Association (AHA) publish evidence-based guidelines, most recently in 2013.1 Since then, new drugs and devices have been shown to improve survival and reduce hospitalizations.
This paper reviews the ABCs of outpatient management of systolic heart failure (or heart failure with reduced ejection fraction), including the results of major trials and recommendations.

A common and serious condition
Heart failure is a debilitating syndrome that takes a significant physical and mental toll on those affected.
And it is common. An American age 40 or older faces a 20% lifetime risk of heart failure.1 An estimated 5.1 million Americans have clinical signs and symptoms of heart failure, and 900,000 new cases are diagnosed each year.2 By 2030 the prevalence of heart failure is projected to increase by 46%, and 9 million Americans will have been diagnosed with it.2
The severity of heart failure can be described using either the functional classification devised by the New York Heart Association (NYHA; Table 1) or the stages defined by the ACC and AHA.1,3 Though survival rates have improved, there is a direct correlation between worsening symptoms and death.4
Heart failure is the leading cause of hospitalizations annually. It accounts for $30 billion in healthcare costs, with direct medical costs accounting for 68% and another $1.8 billion associated with clinic visits, most often with primary care providers. By 2030, the cost is projected to increase by 127% to $69.7 billion—$244 per person in the United States.2
ACE inhibitors
The renin-angiotensin-aldosterone system has been studied for over 100 years.5
In heart failure with reduced ejection fraction, this system is upregulated as an adaptive mechanism to maintain hemodynamic homeostasis.6–8 However, prolonged activation of the renin-angiotensin-aldosterone system can lead to deleterious cardiovascular effects such as myocyte hypertrophy, myocardial fibrosis, sodium conservation, and fluid overload.8,9 Angiotensin II is a potent vasoconstrictor and plays a role in cardiovascular remodeling, leading to worsening progression of heart failure.6
CONSENSUS (the Cooperative North Scandinavian Enalapril Survival Study) examined the effect of the angiotensin-converting enzyme (ACE) inhibitor enalapril on survival in 253 patients with NYHA class IV heart failure. Participants were randomized to receive either enalapril or placebo. At 6 months, the mortality rate was 26% in the enalapril group vs 44% in the placebo group, an 18% absolute risk reduction and a 41% relative risk reduction (P = .002). At 12 months, the relative risk reduction in mortality was 30% (P = .001).10
SOLVD (the Study of Left Ventricular Dysfunction) extended the use of ACE inhibitors to all patients with heart failure, not just those in NYHA class IV. It randomized 1,284 patients with heart failure of any NYHA class and an ejection fraction less than 35% to receive either enalapril or placebo, and demonstrated a 16% relative risk reduction in mortality in the enalapril group, with mortality rates of 36% vs 39.7% (P = .0036).11
Recommendations. The benefits of ACE inhibition have been demonstrated in patients with mild, moderate, and severe heart failure. Thus, the guidelines recommend ACE inhibitors (Table 2) for all patients with heart failure with reduced ejection fraction.1
Angiotensin II receptor blockers
Angiotensin II receptor blockers (ARBs) (Table 3) have been proven to be suitable alternatives for patients with heart failure with reduced ejection fraction who cannot tolerate ACE inhibitors.
Val-HefT (the Valsartan HF Trial)12 randomized 5,010 patients in a double-blind fashion to receive either valsartan or placebo, with background therapy that included beta-blockers, digoxin, diuretics, and ACE inhibitors. There was a 13% reduction of the combined primary end point of mortality and morbidity and a 24% reduction in heart failure hospitalizations in the valsartan group.12
Subgroup analysis compared patients on the basis of use of ACE inhibitors and beta-blockers at study entry. Valsartan had a favorable effect in the subgroups using beta-blockers alone, ACE inhibitors alone, and neither drug. However, when patients received all three (a beta-blocker, an ACE inhibitor, and valsartan), the mortality rate was significantly increased (P = .009).12 This finding conflicted with those of other studies, which found a small benefit of combining an ACE inhibitor and an ARB.
CHARM-Added (the Candesartan in HF Assessment of Reduction in Mortality and Morbidity trial)13 investigated whether adding the ARB candesartan to an ACE inhibitor would improve clinical outcomes. In the study, 2,548 patients in NYHA class II, III, or IV with a left ventricular ejection fraction of less than 40% who were receiving ACE inhibitors were randomized to either candesartan or placebo. The addition of candesartan resulted in a significant reduction in cardiovascular mortality and heart failure hospitalizations, but with the downside of higher rates of hyperkalemia and serum creatinine elevation.13
Recommendations. The 2013 guidelines recommend that ARBs be used in patients who cannot tolerate an ACE inhibitor due to cough. However, routine combined use of ARBs, ACE inhibitors, and aldosterone antagonists is not recommended and may cause harm.1
Aldosterone receptor antagonists
Elevated levels of aldosterone lead to fluid retention, loss of magnesium and potassium, and myocardial fibrosis.
RALES (the Randomized Aldactone Evaluation Study)14 tested the hypothesis that the aldosterone receptor antagonist spironolactone (25 mg daily) would reduce deaths from all causes in patients with severe heart failure receiving standard medications including an ACE inhibitor. RALES included 1,663 patients in NYHA class III or IV with a left ventricular ejection fraction of 35% or less, randomized to receive 25 mg of spironolactone or matching placebo. This study found a 30% relative risk reduction and an 11% absolute risk reduction in all-cause mortality, a 31% relative risk reduction and a 10% absolute risk reduction in cardiac mortality, and 30% fewer cardiac-related hospitalizations in the spironolactone group.14
Eplerenone, an aldosterone receptor antagonist that lacks the antiandrogenic side effects of spironolactone, has also been shown to be beneficial. Its efficacy in patients with left ventricular systolic dysfunction was first established in postmyocardial infarction patients.15
EMPHASIS-HF (the Eplerenone in Mild Patients Hospitalized and Survival Study in Heart Failure)16 broadened the application of eplerenone (and aldosterone antagonists in general), investigating the effects of eplerenone in 2,737 NYHA class II patients, regardless of ischemic etiology. The composite end point of cardiovascular death or heart failure hospitalization occurred in 18.3% of the eplerenone group vs 25.9% of the placebo group (P < .001). A total of 12.5% of patients in the eplerenone group died, compared with 15.5% in the placebo group (P = .008). Hospitalizations were also fewer in the eplerenone group.
Recommendations. The 2013 guidelines recommend aldosterone receptor antagonists (Table 4) for patients with NYHA class II, III, or IV heart failure who have an ejection fraction of 35% or less, to reduce morbidity and mortality (class IA recommendation).1 The guidelines also recommend that these agents not be used in patients with renal insufficiency (serum creatinine > 2.5 mg/dL in men or > 2.0 mg/dL in women; an estimated glomerular filtration rate < 30 mL/min/1.73 m2); or a serum potassium level above 5 mmol/L.1
Angiotensin-neprilysin inhibitor (the future)
Research has identified neprilysin as another potential target in the treatment of heart failure and has sought to combine inhibition of angiotensin and neprilysin.
Neprilysin, a neutral endopeptidase, is associated with degradation of several natural vasoactive peptides such as natriuretic peptide, bradykinin, and adrenomedullin. Neprilysin inhibition increases these substances and counters the neurohormonal overactivation that leads to vasoconstriction, sodium retention, and cardiac remodeling.17
The ARB valsartan has been combined with the neprilysin inhibitor sacubitril to create the first angiotensin-neprilysin inhibitor (ARNI) (Table 5). The combination was selected to minimize the potential for angioedema.
PARADIGM-HF (the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure trial)17 examined whether combined angiotensin-neprilysin inhibition was superior to ACE inhibition alone with enalapril in patients with chronic heart failure.
In PARADIGM-HF, 10,521 patients with NYHA class II, III, or IV heart failure were randomized to receive either sacubitril-valsartan or enalapril. The group receiving sacubitril-valsartan had significantly fewer deaths from cardiovascular causes and heart failure hospitalizations.17 An improvement in quality of life and NYHA functional class was also observed in the sacubitril-valsartan group.17
Sacubitril-valsartan underwent priority review by the US Food and Drug Administration and has been approved. Currently, it is indicated for the treatment of heart failure with reduced ejection fraction and NYHA class II, III, or IV symptoms. It should be avoided in patients who have previously experienced angioedema with an ACE inhibitor or ARB, in patients receiving aliskiren for diabetes, and in patients with hypersensitivity reactions to either of its components. Simultaneous use of sacubitril-valsartan and an ACE inhibitor should be avoided, and a washout period is recommended when transitioning from an ACE inhibitor to this combined agent.

Beta-blockers
In heart failure, there is increased sympathetic activation and associated elevations in norepinephrine levels, which may lead to deleterious long-term effects on cardiac function and structure. Beta-adrenergic receptor blockade is now known to be cardioprotective, but it was not always so; beta-blockers used to be contraindicated in patients with heart failure.
An early experience using beta-blockers in heart failure was described in 1975.18,19 The first study to report a survival benefit of treating systolic heart failure with a beta-blocker was published in 1979.20 Later, small controlled trials demonstrated a reduction in heart failure symptoms and improvement in left ventricular function and in NYHA functional class.21 Larger clinical trials have demonstrated a tremendous survival benefit with beta-blockers in heart failure, specifically carvedilol, extended-release metoprolol, and bisoprolol.
The US Carvedilol Heart Failure Study Group trial22 evaluated whether beta-blocker use in heart failure patients would reduce the rates of morbidity and mortality.22 The trial included 1,094 patients with symptomatic heart failure for at least 3 months and a left ventricular ejection fraction of 35% or less on background therapy including vasodilators, ACE inhibitors, and digoxin. Patients were randomized to receive either carvedilol or placebo. Carvedilol use was associated with a dramatic 65% risk reduction in mortality (7.8% with placebo vs 3.2% with carvedilol, P < .001) and a 27% risk reduction in hospitalizations (19.6% vs 14.1%, P = .036), leading to early trial termination.
CIBIS-II (the Cardiac Insufficiency Bisoprolol Study II)23 investigated the effects of beta-blockers on survival and morbidity. CIBIS-II included 2,647 NYHA class III or IV patients with a left ventricular ejection fraction less than 35% on background medical therapy that included diuretics and ACE inhibitors. This trial was also terminated early, after demonstrating a significant survival benefit with bisoprolol.
MERIT-HF (the Metoprolol Extended Release Randomized Intervention Trial in Congestive Heart Failure)24 evaluated if once-daily metoprolol would lower mortality rates in patients with symptomatic heart failure. The study enrolled 3,991 NYHA class II–IV patients with chronic heart failure and a left ventricular ejection fraction of 40% or less. Like the previous two beta-blocker trials, MERIT-HF was terminated early, as it demonstrated a 34% reduction in all-cause mortality (7.2% risk of death per patient-year vs 11.0%, P = .00009).
The beta-blocker trials have shown that when added to background therapy, beta-blockers improve survival and reduce hospitalizations. However, when prescribing a beta-blocker, it is important to understand that not all beta-blockers are equal in the treatment of heart failure.
COMET (the Carvedilol or Metoprolol European Trial)25 was the only head-to-head randomized control trial evaluating clinical outcomes in patients receiving carvedilol or metoprolol tartrate (not metoprolol succinate). In COMET, 1,511 patients with NYHA class II, III, or IV heart failure with a left ventricular ejection fraction of 35% or less were randomized to carvedilol or metoprolol tartrate. The primary end point of all-cause mortality occurred in 34% of the carvedilol group and 40% of the metoprolol tartrate group (P = .0017). There was no significant difference with regard to the composite end point of mortality and all-cause admissions.
Recommendations. The 2013 guidelines give a class IA recommendation for starting a beta-blocker (carvedilol, bisoprolol, or metoprolol succinate, Table 6) in patients with current or prior symptoms of heart failure.1 Beta-blockers should be initiated with caution or avoided in patients with acutely decompensated heart failure with evidence of fluid overload.
Brain-type natriuretic peptide
Brain-type natriuretic peptide (BNP) or its amino-terminal cleavage product (NT-proBNP) originates in cardiomyocytes and is released by several triggers, most commonly cardiomyocyte stretch in the setting of volume or pressure overload.26 The biologic significance of BNP includes natriuresis and vasodilation, renin-angiotensin system inhibition, and sympathetic nervous system modulation.26
TIME-CHF (the Trial of Intensified vs. Standard Medical Therapy in Elderly Patients With Congestive HF)27 investigated whether 18-month outcomes would be better if treatment were guided by N-terminal BNP levels rather than by symptoms. The BNP-guided strategy was not associated with a reduction in hospitalization or a survival benefit.
BATTLESCARRED (the NT-proBNP-Assisted Treatment to Lessen Serial Cardiac Readmissions and Death trial)28 in 2009 showed that a BNP-guided management strategy significantly reduced mortality rates in patients under age 75 compared with standard medical therapy.
PROTECT (the Use of NT-proBNP Testing to Guide HF Therapy in the Outpatient Setting study)29 also showed that a BNP-guided strategy was superior to usual care and was associated with reduced cardiovascular events and improved quality of life.29
GUIDE IT-HF (the Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure study), currently ongoing, is designed to assess the safety, efficacy and cost-effectiveness of a biomarker-guided strategy in 1,100 high-risk patients with heart failure with reduced ejection fraction.
Recommendations. The 2013 ACC/AHA guidelines give a class IA recommendation for the use of BNP to support clinical decision-making, particularly in cases of clinical uncertainty.1 BNP can also be used to establish prognosis or disease severity in chronic heart failure and to achieve optimal dosage of goal-directed medical therapy for euvolemic patients followed in a structured heart failure program.1

Heart failure clinics
Continuity of care upon discharge from the hospital is currently in a state of evolution. Those diagnosed with heart failure can now experience more comprehensive posthospital care by virtue of disease management clinics. The name may vary by institution, but whether it is called a “diuresis clinic,” “bridge clinic,” or “heart failure clinic,” the goal is to improve guideline-driven care, educate the patient, and reduce heart failure hospitalizations. Heart failure clinics are designed to provide a smooth transition from inpatient to outpatient care and to encourage patient self-accountability in health maintenance thereafter.
Studies have shown that heart failure clinics are associated with better medication dosing, fewer hospitalizations, and lower healthcare costs.30–32
Chronotropy: If inhibition
An elevated resting heart rate has been shown to be associated with increased cardiovascular morbidity and mortality.33 Studies have shown that slowing the heart rate improves myocardial contraction and energy supply and reduces energy expenditure.34 Ivabradine, a selective If (the f is for “funny”) channel inhibitor, slows the heart rate without other known cardiovascular effects.
SHIFT (the Systolic Heart Failure Treatment With the If Inhibitor Ivabradine Trial)35 investigated whether isolated heart rate reduction with ivabradine would reduce adverse clinical outcomes in patients with symptomatic heart failure. SHIFT randomized 6,505 patients with a left ventricular ejection fraction of 35% or less, in sinus rhythm, with a heart rate of at least 70 beats per minute, on optimal medical therapy, and hospitalized within 12 months of enrollment to receive ivabradine or placebo. The primary end point was a composite of cardiovascular mortality and hospital admission for worsening heart failure. Outcomes varied by heart rates achieved, with the best outcomes in those with the lowest heart rates at trial conclusion.
Ivabradine (Table 7) is indicated for patients with symptomatic heart failure with a left ventricular ejection fraction less than 35%, in sinus rhythm, with a resting heart rate of at least 70 beats per minute, and either on a maximally tolerated beta-blocker or with a contraindication to beta-blockers.
Ivabradine should be avoided in patients who are in acute decompensated heart failure or are hypotensive (blood pressure < 90/50 mm Hg), as well as in patients with a significant conduction abnormality (sick sinus syndrome, sinoatrial block, third-degree atrioventricular block), hepatic impairment, or bradycardia (resting heart rate < 60 beats per minute).

Digoxin
Digoxin has been used in treating systolic heart failure for more than 70 years.36,37
DIG (Digoxin Investigative Group trial)38 evaluated the long-term effect of digoxin on rates of mortality and hospitalization for heart failure over a 3-year period. In patients with a left ventricular ejection fraction less than 45%, digoxin had no effect on overall mortality when combined with diuretics and ACE inhibitors. However, the risk of hospitalization for worsening heart failure was significantly reduced with digoxin treatment.38
Recommendations. Digoxin should be considered when patients are on guideline-recommended therapy but heart failure symptoms persist. It is commonly initiated at a dose of 0.125 to 0.25 mg. The target therapeutic range for digoxin is 0.5 to 0.9 ng/mL.1 Digoxin toxicity can occur in patients with renal impairment, hypokalemia, hypomagnesemia, and hypothyroidism.
The 2013 ACC/AHA guidelines give a class IIA recommendation (treatment is “reasonable”) for digoxin in patients with heart failure with reduced ejection fraction unless contraindicated, to decrease hospitalizations for heart failure.1
Diuretics
Clinical manifestations of volume overload in patients with heart failure are from excess salt and water retention leading to inappropriate volume expansion in both the vascular and extravascular space. Diuretics (Table 8) are the foundation of heart failure treatment. Most patients are first initiated on a combination of a loop diuretic and a low-sodium diet to improve symptoms.
The 2013 ACC/AHA guidelines give a class I recommendation for diuretics in patients with heart failure with reduced ejection fraction who have evidence of fluid retention, unless contraindicated, to improve symptoms.1
Devices: ICDs
Patients with heart failure are at increased risk of sudden death and ventricular arrhythmias.39 Previously, antiarrhythmic drugs were considered the standard of care for nonsustained ventricular tachycardia after myocardial infarction.
MADIT (the Multicenter Automatic Defibrillator Implantation Trial) investigated whether prophylactic implantation of an internal cardiac defibrillator would improve 5-year survival rates in patients with heart failure. Eligible patients had had a Q-wave or enzyme-positive myocardial infarction within 3 weeks of study entry. They also had had an episode of asymptomatic nonsustained ventricular tachycardia unrelated to an acute myocardial infarction. Additionally, the patients had a left ventricular ejection fraction less than 35%, and inducible, sustained, nonsuppressible ventricular tachyarrhythmia on electrophysiologic testing.40
During the study, 15 patients in the defibrillator group died vs 39 in the conventional therapy group (P = .009).40
MADIT II evaluated the potential survival benefit of a prophylactically implanted defibrillator in the absence of electrophysiologic testing to induce arrhythmias.41 MADIT II included 1,232 patients with prior myocardial infarctions and a left ventricular ejection fracton of 30% or less. Patients were randomized to receive an implanted cardioverter-defibrillator or conventional medical therapy. The primary end point was death from any cause.41
The mortality rate was 19.8% in the conventional therapy group vs 14.2% in the defibrillator group (hazard ratio 0.69, P = .016).41 Thus, MADIT-II confirmed the benefits of prophylactic implantable cardioverter-defibrillator therapy seen in the original MADIT, and additionally eliminated the need for an electrophysiology test prior to device implantation.
SCD-HeFT (the Sudden Cardiac Death in Heart Failure Trial) evaluated whether amiodarone or a conservatively programmed shock-only, single-lead implanted cardioverter-defibrillator would decrease the risk of death (all-cause) in a population with mild to moderate heart failure with ischemic and nonischemic causes.42 In this trial, 2,521 patients with an ejection fraction of 35% or less, in NYHA class II or III, and with stable heart failure were randomized to receive a single-chamber implantable cardioverter-defibrillator, amiodarone, or placebo.
There were 244 deaths in the placebo group, 240 deaths in the amiodarone group (P = .53 compared with placebo), and 182 deaths in the defibrillator group (P = .007 compared with placebo).42
Recommendations. The 2013 ACC/AHA guideline1 gives implantable defibrillator therapy a class IA recommendation for the primary prevention of sudden cardiac death in selected patients with nonischemic cardiomyopathy or ischemic cardiomyopathy at least 40 days after a myocardial infarction and 90 days after percutaneous coronary intervention or coronary artery bypass grafting; with a left ventricular ejection fraction of 35% or less; and NYHA class II or III symptoms on chronic goal-directed medical management.
This therapy receives a class IB recommendation for primary prevention of sudden cardiac death to reduce total mortality in selected patients at least 40 days after myocardial infarction with a left ventricular ejection fraction of 30% or less and NYHA class I symptoms while receiving goal-directed medical therapy.
Implantable cardioverter-defibrillators are not recommended in patients who otherwise have a life expectancy of less than 1 year.
Devices: Cardiac resynchronization therapy
From 25% to 30% of heart failure patients have an intraventricular conduction abnormality,43,44 which can result in abnormalities of systolic and diastolic function. Biventricular pacing, in which a pacing lead is placed in the coronary sinus in addition to the right atrium and right ventricle, optimizes synchronization of ventricular contraction.43,44
MUSTIC (the Multisite Stimulation in Cardiomyopathies study) was a randomized trial designed to assess the efficacy of biventricular pacing (also known as cardiac resynchronization therapy) in heart failure patients.44 Entry criteria included NYHA class III heart failure for at least 1 month, left ventricular ejection fraction less than 35%, left ventricular end-diastolic diameter greater than 60 mm, and QRS duration longer than 150 ms. Patients were followed up at 9 and 12 months with 6-minute walking distance, peak oxygen consumption, changes in NYHA class, and left ventricular systolic function by echocardiography or radionuclide testing. Quality of life was assessed by the Minnesota Living With Heart Failure Questionnaire.
At 12 months, patients could walk significantly farther in 6 minutes, and their peak oxygen consumption had increased. They also reported significant improvement in quality of life, and NYHA class improved by 25%. MUSTIC was the first study to show a benefit in exercise tolerance, quality of life, improvement in cardiac performance, and reduction in heart failure symptoms with the use of biventricular pacing at 1 year.
MIRACLE (the Multicenter InSync Randomized Clinical Evaluation) validated the findings seen in MUSTIC by using a larger population size and a double-blinded method.45 Compared with a control group, patients who underwent cardiac resynchronization therapy could walk farther in 6 minutes and scored better in NYHA class, quality of life, and left ventricular ejection fraction.45
Recommendations. The 2013 ACC/AHA guidelines1 give cardiac resynchronization therapy a class IA/B indication for NYHA class II, III, or IV patients on goal-directed medical therapy in sinus rhythm with left ventricular ejection fraction 35% or less, left bundle branch block, and QRS duration of 150 ms or more.1
Devices: Implantable sensors
The future of ambulatory heart failure management may include implantable pulmonary artery pressure sensors.
The CardioMEMS is a permanently implantable pressure measurement system designed to provide daily pulmonary artery pressure measurements in an ambulatory setting with a goal of reducing heart failure-related hospitalizations. Through a transvenous delivery system, an implantable, battery-free sensor is positioned in the distal pulmonary artery.46,47
CHAMPION (the CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Patients trial) was one of the first major trials to assess the safety and efficacy of implantable pulmonary artery pressure monitoring systems.46 The study device was associated with a significant reduction in mean pulmonary artery pressures, fewer heart failure hospitalizations, and better quality of life. The length of stay for heart failure-related hospitalizations was also significantly shorter in the CardioMEMs group.46

Exercise
Patients with heart failure routinely experience a decline in functional capacity. This decline manifests as reduced exercise tolerance and poor quality of life, usually resulting in a physician recommendation to rest and paradoxical deconditioning and possible progression of symptoms.
Several studies have shown that cardiac rehabilitation has improved outcomes in heart failure patients.48 Cardiac rehabilitation is a supervised program that helps patients with exercise training, healthy living, education, and psychosocial counseling.
HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) is the largest randomized trial performed to determine whether aerobic exercise training reduces all-cause mortality or all-cause hospitalization and improves quality of life in patients with stable heart failure.49 Although the reduction in end points was initially not statistically significant, after adjusting for highly prognostic predictors of poor outcomes (cardiopulmonary exercise time, left ventricular ejection fraction, atrial fibrillation, and depression), exercise training was found to reduce the incidence of all-cause mortality or all-cause hospitalization by 11% (P = .03).49
Recommendations. Based on the results of HF-ACTION and several smaller studies, the ACC/AHA guidelines give exercise training a class IA recommendation as a safe and effective activity for patients with heart failure who are able to participate, to improve functional status.1 A class IIA recommendation is given to cardiac rehabilitation for the improvement of functional capacity, exercise duration, quality of life, and mortality rates.1
End-stage heart failure: Recognition
Despite adequate titration of goal-directed medical therapy, a portion of patients with heart failure with reduced ejection fraction ultimately progress to stage D, also termed “advanced” heart failure. The 5-year survival rate for patients with heart failure overall is 50%, but the 1-year mortality rate for those with advanced heart failure exceeds 50%.50
Because the high rates of morbidity and mortality can potentially be lowered, recognition of heart failure disease progression is imperative so that patients can be promptly referred for therapies such as inotropic infusion, mechanical circulatory support, and cardiac transplant, as well as end-of-life care such as hospice.1
The ACC/AHA1 have published clinical events and findings useful in identifying patients with advanced heart failure:
- Two or more hospitalizations or emergency department visits for heart failure in the past year
- Progressive deterioration in renal function (eg, elevation in creatinine or blood urea nitrogen)
- Weight loss without other cause
- Intolerance to ACE inhibitors due to hypotension or worsening renal function
- Inability to tolerate beta-blockers due to worsening heart failure or hypotension
- Systolic blood pressure often below 90 mm Hg
- Persistent dyspnea with dressing or bathing requiring rest
- Inability to walk one block on level ground due to dyspnea or fatigue
- Recent need to escalate diuretics to maintain volume status, often reaching daily dose equivalent to furosemide more than 160 mg/day or use of supplemental metolazone
- Progressive decline in serum sodium, usually to below 133 mmol/L
- Frequent shocks from implanted cardiac defibrillator.
End-stage heart failure: Left ventricular assist devices
For patients with refractory heart failure despite optimal medical management, advanced therapies such as heart transplant or ventricular assist devices have been proven to be durable options. These mechanical circulatory support devices “unload” the diseased ventricle and maintain cardiac output to vital organs.51 They were initially designed as temporary support to allow ventricular recovery or as a bridge to cardiac transplant. However, they have also evolved into permanent (“destination”) therapy.52
REMATCH (the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive HF trial) was the landmark study that showed that left ventricular assist device implantation resulted in a survival benefit and an improved quality of life in patients with advanced heart failure ineligible for cardiac transplant, compared with medical management.50 Implantation of a left ventricular assist device was associated with a 27% absolute reduction in the 1-year mortality rate.50
Since the National Institutes of Health’s artificial heart program was launched in 1964, there has been tremendous progress in the development of mechanical circulatory devices.50 The results of REMATCH were promising, but the 2-year survival rate was still only 23%, leaving a lot to be desired.
The HeartMate II (Thoratec) trial compared an axial continuous-flow device vs the previously established pulsatile left ventricular assist device, and noted a 2-year survival of 58% with the continuous flow device vs 24% with the pulsatile device (P = .008).53
ADVANCE (Evaluation of the HeartWare Left Ventricular Assist Device for the Treatment of Advanced Heart Failure) showed similar efficacy of the HVAD (Heartware), a centrifugal continuous-flow LVAD currently in use.54
The next generation of continuous-flow left ventricular assist devices are currently in clinical trials in the United States and include the axial flow MVAD (Heartware) and centrifugal flow Heartmate III (Thoratec).
We emphasize the importance of early identification of patients with advanced disease who may qualify for and benefit from such therapies.

The management of heart failure is evolving. In the 1960s, the standard heart failure medical regimen included digoxin, diuretics, and the recommendation of rest. This contrasts with the current era, in which medical regimens include neurohormonal blockade, diuretics, and the promotion of physical activity.55 Since the publication of the 2013 heart failure guidelines, new medical and device options have emerged that have been proven to either improve survival or reduce hospitalizations. The development of clinical guidelines promotes evidence-based practice and overcomes the inertia of practice patterns based on anecdotal evidence.
Several approaches to the management of heart failure have been recommended. A major effort should be made to identify those at risk for heart failure (stage A) and to implement risk factor modification. Treatment of hypertension, diabetes mellitus, and dyslipidemia decreases the risk of heart failure.1
For patients with evidence of structural heart disease with and without symptoms, Figure 1 summarizes a guideline approach to the management of heart failure. It should be stressed that guidelines are meant to guide management, but do not serve as a substitute for sound clinical judgment.
Heart failure is the common final pathway of all cardiac pathology, and understanding the neurohormonal response and maladaptive physiology has led to the development of novel therapeutics and devices. At present, the field of cardiology may not be able to remove the “failure” from heart failure, but we can make every effort to prevent failure of treatment delivery and reduce resource utilization and morbidity associated with this syndrome.
Acknowledgments: We would like to thank Chankya Dahagam and Cynthia Obenwa for their valuable contribution in the preparation of this manuscript.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128:e240–e327.
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 2015; 133:e38–e360.
- Goldberg LR, Jessup M. Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction. Circulation 2006; 113:2851–2860.
- Ammar KA, Jacobsen SJ, Mahoney DW, et al. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation 2007; 115:1563–1570.
- Tigerstedt R, Bergman PQ. Niere und Kreislauf. Skand Arch Physiol 1898; 8:223–271.
- Unger T, Li J. The role of the renin-angiotensin-aldosterone system in heart failure. J Renin Angiotensin Aldosterone Syst 2004; 5(suppl 1):S7–S10.
- Cohn JN, Levine TB, Francis GS, Goldsmith S. Neurohumoral control mechanisms in congestive heart failure. Am Heart J 1981; 102:509–514.
- von Lueder TG, Sangaralingham SJ, Wang BH, et al. Renin-angiotensin blockade combined with natriuretic peptide system augmentation: novel therapeutic concepts to combat heart failure. Circ Heart Fail 2013; 6:594–605.
- Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation 1991; 83:1849–1865.
- The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987; 316:1429–1435.
- The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325:293–302.
- Cohn JN, Tognoni G, Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345:1667–1675.
- McMurray JJ, Ostergren J, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 2003; 362:767–771.
- Pitt B, Zannad F, Remme WJ, et al.The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341:709–717.
- Pitt B, Remme W, Zannad F, et al; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348:1309–1321.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2010; 364:11–21.
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371:993–1004.
- Waagstein F, Hjalmarson A, Varnauskas E, Wallentin I. Effect of chronic beta-adrenergic receptor blockade in congestive cardiomyopathy. Br Heart J 1975; 37:1022–1036.
- Gheorghiade M, Colucci WS, Swedberg K. Beta-blockers in chronic heart failure. Circulation 2003; 107:1570–1575.
- Swedberg K, Hjalmarson A, Waagstein F, Wallentin I. Prolongation of survival in congestive cardiomyopathy by beta-receptor blockade. Lancet 1979; 1:1374–1376.
- Klapholz M. Beta-blocker use for the stages of heart failure. Mayo Clin Proc 2009; 84:718–729.
- Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med 1996; 334:1349–1355.
- The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999; 353:9–13.
- MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353:2001–2007.
- Poole-Wilson PA, Swedberg K, Cleland JG, et al; Carvedilol Or Metoprolol European Trial Investigators. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet 2003; 362:7–13.
- Kim H-N, Januzzi JL Jr. Natriuretic peptide testing in heart failure. Circulation 2011; 123:2015–2019.
- Pfisterer M, Buser P, Rickli H, et al; TIME-CHF Investigators. BNP-guided vs symptom-guided heart failure therapy: the Trial of Intensified vs Standard Medical Therapy in Elderly Patients with Congestive Heart Failure (TIME-CHF) randomized trial. JAMA 2009; 301:383–392.
- Lainchbury JG, Troughton RW, Strangman KM, et al. N-terminal pro–B-type natriuretic peptide-guided treatment for chronic heart failure: results From the BATTLESCARRED (NT-proBNP–Assisted Treatment To Lessen Serial Cardiac Readmissions and Death) trial. J Am Coll Cardiol 2009; 55:53–60.
- Januzzi JL Jr, Rehman SU, Mohammed AA, et al. Use of amino-terminal pro–B-type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J Am Coll Cardiol 2011; 58:1881-1889.
- Whellan DJ, Gaulden L, Gattis WA, et al. The benefit of implementing a heart failure disease management program. Arch Intern Med 2001; 161:2223–2228.
- Fonarow GC, Stevenson LW, Walden JA, et al. Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure. J Am Coll Cardiol 1997; 30:725–732.
- Grady KL, Dracup K, Kennedy G, et al. Team management of patients with heart filure: a statement for healthcare professionals from the Cardiovascular Nursing Council of the American Heart Association. Circulation 2000; 102:2443–2456.
- Kannel WB, Kannel C, Paffenbarger RS Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J 1987; 113:1489-1494.
- Colin P, Ghaleh B, Monnet X, Hittinger L, Berdeaux A. Effect of graded heart rate reduction with ivabradine on myocardial oxygen consumption and diastolic time in exercising dogs. J Pharmacol Exper Ther 2004; 308:236–240.
- Böhm M, Swedberg K, Komajda M, et al; SHIFT Investigators. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet 2010; 376:886–894.
- Batterman RC, DeGraff AC. Comparative study on the use of the purified digitalis glycosides, digoxin, digitoxin, and lanatoside C, for the management of ambulatory patients with congestive heart failure. Am Heart J 1947; 34:663–673.
- Ouyang AJ, Lv YN, Zhong HL, et al. Meta-analysis of digoxin use and risk of mortality in patients with atrial fibrillation. Am J Cardiol 2015; 115:901–906.
- Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997; 336:525–533.
- Aleong RG, Mulvahill MJ, Halder I, et al. Left ventricular dilatation increases the risk of ventricular arrhythmias in patients with reduced systolic function. J Am Heart Assoc 2015; 4:e001566.
- Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med 1996; 335:1933–1940.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Bardy GH, Lee KL, Mark DB, et al; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005; 352:225–237.
- Greenberg B, Mehra MR. All patients with heart failure and intraventricular conduction defect or dyssynchrony should not receive cardiac resynchronization therapy. Circulation 2006; 114:2685–2691.
- Linde C, Leclercq C, Rex S, et al. Long-term benefits of biventricular pacing in congestive heart failure: results from the MUltisite STimulation in cardiomyopathy (MUSTIC) study. J Am Coll Cardiol 2002; 40:111–118.
- Abraham WT, Fisher WG, Smith AL, et al; MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002; 346:1845–1853.
- Abraham WT, Adamson PB, Bourge RC, et al; CHAMPION Study Group. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011; 377:658–666.
- Loh JP, Barbash IM, Waksman R. Overview of the 2011 Food and Drug Administration Circulatory System Devices Panel of the Medical Devices Advisory Committee Meeting on the CardioMEMS Champion Heart Failure Monitoring System. J Am Coll Cardiol 2013; 61:1571–1576.
- Ades PA, Keteyian SJ, Balady GJ, et al. Cardiac rehabilitation exercise and self-care for chronic heart failure. JACC Heart Fail 2013; 1:540–547.
- O’Connor CM, Whellan DJ, Lee KL, et al; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009; 301:1439–1450.
- Rose EA, Gelijns AC, Moskowitz AJ, et al; Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001; 345:1435–1443.
- Givertz MM. Ventricular assist devices: important information for patients and families. Circulation 2011; 124:e305–e311.
- Daneshmand MA, Rajagopal K, Lima B, et al. Left ventricular assist device destination therapy versus extended criteria cardiac transplant. Ann Thorac Surg 2010; 89:1205–1210.
- Slaughter MS, Rogers JG, Milano CA, et al; HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009; 361:2241–2251.
- Aaronson KD, Slaughter MS, Miller LW, et al; HeartWare Ventricular Assist Device (HVAD) Bridge to Transplant ADVANCE Trial Investigators. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation 2012; 125:3191–3200.
- Katz AM. The “modern” view of heart failure: how did we get here? Circ Heart Fail 2008; 1:63–71.
Managing heart failure is a challenge. To aid clinicians in this task, the American College of Cardiology Foundation (ACC) and the American Heart Association (AHA) publish evidence-based guidelines, most recently in 2013.1 Since then, new drugs and devices have been shown to improve survival and reduce hospitalizations.
This paper reviews the ABCs of outpatient management of systolic heart failure (or heart failure with reduced ejection fraction), including the results of major trials and recommendations.

A common and serious condition
Heart failure is a debilitating syndrome that takes a significant physical and mental toll on those affected.
And it is common. An American age 40 or older faces a 20% lifetime risk of heart failure.1 An estimated 5.1 million Americans have clinical signs and symptoms of heart failure, and 900,000 new cases are diagnosed each year.2 By 2030 the prevalence of heart failure is projected to increase by 46%, and 9 million Americans will have been diagnosed with it.2
The severity of heart failure can be described using either the functional classification devised by the New York Heart Association (NYHA; Table 1) or the stages defined by the ACC and AHA.1,3 Though survival rates have improved, there is a direct correlation between worsening symptoms and death.4
Heart failure is the leading cause of hospitalizations annually. It accounts for $30 billion in healthcare costs, with direct medical costs accounting for 68% and another $1.8 billion associated with clinic visits, most often with primary care providers. By 2030, the cost is projected to increase by 127% to $69.7 billion—$244 per person in the United States.2
ACE inhibitors
The renin-angiotensin-aldosterone system has been studied for over 100 years.5
In heart failure with reduced ejection fraction, this system is upregulated as an adaptive mechanism to maintain hemodynamic homeostasis.6–8 However, prolonged activation of the renin-angiotensin-aldosterone system can lead to deleterious cardiovascular effects such as myocyte hypertrophy, myocardial fibrosis, sodium conservation, and fluid overload.8,9 Angiotensin II is a potent vasoconstrictor and plays a role in cardiovascular remodeling, leading to worsening progression of heart failure.6
CONSENSUS (the Cooperative North Scandinavian Enalapril Survival Study) examined the effect of the angiotensin-converting enzyme (ACE) inhibitor enalapril on survival in 253 patients with NYHA class IV heart failure. Participants were randomized to receive either enalapril or placebo. At 6 months, the mortality rate was 26% in the enalapril group vs 44% in the placebo group, an 18% absolute risk reduction and a 41% relative risk reduction (P = .002). At 12 months, the relative risk reduction in mortality was 30% (P = .001).10
SOLVD (the Study of Left Ventricular Dysfunction) extended the use of ACE inhibitors to all patients with heart failure, not just those in NYHA class IV. It randomized 1,284 patients with heart failure of any NYHA class and an ejection fraction less than 35% to receive either enalapril or placebo, and demonstrated a 16% relative risk reduction in mortality in the enalapril group, with mortality rates of 36% vs 39.7% (P = .0036).11
Recommendations. The benefits of ACE inhibition have been demonstrated in patients with mild, moderate, and severe heart failure. Thus, the guidelines recommend ACE inhibitors (Table 2) for all patients with heart failure with reduced ejection fraction.1
Angiotensin II receptor blockers
Angiotensin II receptor blockers (ARBs) (Table 3) have been proven to be suitable alternatives for patients with heart failure with reduced ejection fraction who cannot tolerate ACE inhibitors.
Val-HefT (the Valsartan HF Trial)12 randomized 5,010 patients in a double-blind fashion to receive either valsartan or placebo, with background therapy that included beta-blockers, digoxin, diuretics, and ACE inhibitors. There was a 13% reduction of the combined primary end point of mortality and morbidity and a 24% reduction in heart failure hospitalizations in the valsartan group.12
Subgroup analysis compared patients on the basis of use of ACE inhibitors and beta-blockers at study entry. Valsartan had a favorable effect in the subgroups using beta-blockers alone, ACE inhibitors alone, and neither drug. However, when patients received all three (a beta-blocker, an ACE inhibitor, and valsartan), the mortality rate was significantly increased (P = .009).12 This finding conflicted with those of other studies, which found a small benefit of combining an ACE inhibitor and an ARB.
CHARM-Added (the Candesartan in HF Assessment of Reduction in Mortality and Morbidity trial)13 investigated whether adding the ARB candesartan to an ACE inhibitor would improve clinical outcomes. In the study, 2,548 patients in NYHA class II, III, or IV with a left ventricular ejection fraction of less than 40% who were receiving ACE inhibitors were randomized to either candesartan or placebo. The addition of candesartan resulted in a significant reduction in cardiovascular mortality and heart failure hospitalizations, but with the downside of higher rates of hyperkalemia and serum creatinine elevation.13
Recommendations. The 2013 guidelines recommend that ARBs be used in patients who cannot tolerate an ACE inhibitor due to cough. However, routine combined use of ARBs, ACE inhibitors, and aldosterone antagonists is not recommended and may cause harm.1
Aldosterone receptor antagonists
Elevated levels of aldosterone lead to fluid retention, loss of magnesium and potassium, and myocardial fibrosis.
RALES (the Randomized Aldactone Evaluation Study)14 tested the hypothesis that the aldosterone receptor antagonist spironolactone (25 mg daily) would reduce deaths from all causes in patients with severe heart failure receiving standard medications including an ACE inhibitor. RALES included 1,663 patients in NYHA class III or IV with a left ventricular ejection fraction of 35% or less, randomized to receive 25 mg of spironolactone or matching placebo. This study found a 30% relative risk reduction and an 11% absolute risk reduction in all-cause mortality, a 31% relative risk reduction and a 10% absolute risk reduction in cardiac mortality, and 30% fewer cardiac-related hospitalizations in the spironolactone group.14
Eplerenone, an aldosterone receptor antagonist that lacks the antiandrogenic side effects of spironolactone, has also been shown to be beneficial. Its efficacy in patients with left ventricular systolic dysfunction was first established in postmyocardial infarction patients.15
EMPHASIS-HF (the Eplerenone in Mild Patients Hospitalized and Survival Study in Heart Failure)16 broadened the application of eplerenone (and aldosterone antagonists in general), investigating the effects of eplerenone in 2,737 NYHA class II patients, regardless of ischemic etiology. The composite end point of cardiovascular death or heart failure hospitalization occurred in 18.3% of the eplerenone group vs 25.9% of the placebo group (P < .001). A total of 12.5% of patients in the eplerenone group died, compared with 15.5% in the placebo group (P = .008). Hospitalizations were also fewer in the eplerenone group.
Recommendations. The 2013 guidelines recommend aldosterone receptor antagonists (Table 4) for patients with NYHA class II, III, or IV heart failure who have an ejection fraction of 35% or less, to reduce morbidity and mortality (class IA recommendation).1 The guidelines also recommend that these agents not be used in patients with renal insufficiency (serum creatinine > 2.5 mg/dL in men or > 2.0 mg/dL in women; an estimated glomerular filtration rate < 30 mL/min/1.73 m2); or a serum potassium level above 5 mmol/L.1
Angiotensin-neprilysin inhibitor (the future)
Research has identified neprilysin as another potential target in the treatment of heart failure and has sought to combine inhibition of angiotensin and neprilysin.
Neprilysin, a neutral endopeptidase, is associated with degradation of several natural vasoactive peptides such as natriuretic peptide, bradykinin, and adrenomedullin. Neprilysin inhibition increases these substances and counters the neurohormonal overactivation that leads to vasoconstriction, sodium retention, and cardiac remodeling.17
The ARB valsartan has been combined with the neprilysin inhibitor sacubitril to create the first angiotensin-neprilysin inhibitor (ARNI) (Table 5). The combination was selected to minimize the potential for angioedema.
PARADIGM-HF (the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure trial)17 examined whether combined angiotensin-neprilysin inhibition was superior to ACE inhibition alone with enalapril in patients with chronic heart failure.
In PARADIGM-HF, 10,521 patients with NYHA class II, III, or IV heart failure were randomized to receive either sacubitril-valsartan or enalapril. The group receiving sacubitril-valsartan had significantly fewer deaths from cardiovascular causes and heart failure hospitalizations.17 An improvement in quality of life and NYHA functional class was also observed in the sacubitril-valsartan group.17
Sacubitril-valsartan underwent priority review by the US Food and Drug Administration and has been approved. Currently, it is indicated for the treatment of heart failure with reduced ejection fraction and NYHA class II, III, or IV symptoms. It should be avoided in patients who have previously experienced angioedema with an ACE inhibitor or ARB, in patients receiving aliskiren for diabetes, and in patients with hypersensitivity reactions to either of its components. Simultaneous use of sacubitril-valsartan and an ACE inhibitor should be avoided, and a washout period is recommended when transitioning from an ACE inhibitor to this combined agent.

Beta-blockers
In heart failure, there is increased sympathetic activation and associated elevations in norepinephrine levels, which may lead to deleterious long-term effects on cardiac function and structure. Beta-adrenergic receptor blockade is now known to be cardioprotective, but it was not always so; beta-blockers used to be contraindicated in patients with heart failure.
An early experience using beta-blockers in heart failure was described in 1975.18,19 The first study to report a survival benefit of treating systolic heart failure with a beta-blocker was published in 1979.20 Later, small controlled trials demonstrated a reduction in heart failure symptoms and improvement in left ventricular function and in NYHA functional class.21 Larger clinical trials have demonstrated a tremendous survival benefit with beta-blockers in heart failure, specifically carvedilol, extended-release metoprolol, and bisoprolol.
The US Carvedilol Heart Failure Study Group trial22 evaluated whether beta-blocker use in heart failure patients would reduce the rates of morbidity and mortality.22 The trial included 1,094 patients with symptomatic heart failure for at least 3 months and a left ventricular ejection fraction of 35% or less on background therapy including vasodilators, ACE inhibitors, and digoxin. Patients were randomized to receive either carvedilol or placebo. Carvedilol use was associated with a dramatic 65% risk reduction in mortality (7.8% with placebo vs 3.2% with carvedilol, P < .001) and a 27% risk reduction in hospitalizations (19.6% vs 14.1%, P = .036), leading to early trial termination.
CIBIS-II (the Cardiac Insufficiency Bisoprolol Study II)23 investigated the effects of beta-blockers on survival and morbidity. CIBIS-II included 2,647 NYHA class III or IV patients with a left ventricular ejection fraction less than 35% on background medical therapy that included diuretics and ACE inhibitors. This trial was also terminated early, after demonstrating a significant survival benefit with bisoprolol.
MERIT-HF (the Metoprolol Extended Release Randomized Intervention Trial in Congestive Heart Failure)24 evaluated if once-daily metoprolol would lower mortality rates in patients with symptomatic heart failure. The study enrolled 3,991 NYHA class II–IV patients with chronic heart failure and a left ventricular ejection fraction of 40% or less. Like the previous two beta-blocker trials, MERIT-HF was terminated early, as it demonstrated a 34% reduction in all-cause mortality (7.2% risk of death per patient-year vs 11.0%, P = .00009).
The beta-blocker trials have shown that when added to background therapy, beta-blockers improve survival and reduce hospitalizations. However, when prescribing a beta-blocker, it is important to understand that not all beta-blockers are equal in the treatment of heart failure.
COMET (the Carvedilol or Metoprolol European Trial)25 was the only head-to-head randomized control trial evaluating clinical outcomes in patients receiving carvedilol or metoprolol tartrate (not metoprolol succinate). In COMET, 1,511 patients with NYHA class II, III, or IV heart failure with a left ventricular ejection fraction of 35% or less were randomized to carvedilol or metoprolol tartrate. The primary end point of all-cause mortality occurred in 34% of the carvedilol group and 40% of the metoprolol tartrate group (P = .0017). There was no significant difference with regard to the composite end point of mortality and all-cause admissions.
Recommendations. The 2013 guidelines give a class IA recommendation for starting a beta-blocker (carvedilol, bisoprolol, or metoprolol succinate, Table 6) in patients with current or prior symptoms of heart failure.1 Beta-blockers should be initiated with caution or avoided in patients with acutely decompensated heart failure with evidence of fluid overload.
Brain-type natriuretic peptide
Brain-type natriuretic peptide (BNP) or its amino-terminal cleavage product (NT-proBNP) originates in cardiomyocytes and is released by several triggers, most commonly cardiomyocyte stretch in the setting of volume or pressure overload.26 The biologic significance of BNP includes natriuresis and vasodilation, renin-angiotensin system inhibition, and sympathetic nervous system modulation.26
TIME-CHF (the Trial of Intensified vs. Standard Medical Therapy in Elderly Patients With Congestive HF)27 investigated whether 18-month outcomes would be better if treatment were guided by N-terminal BNP levels rather than by symptoms. The BNP-guided strategy was not associated with a reduction in hospitalization or a survival benefit.
BATTLESCARRED (the NT-proBNP-Assisted Treatment to Lessen Serial Cardiac Readmissions and Death trial)28 in 2009 showed that a BNP-guided management strategy significantly reduced mortality rates in patients under age 75 compared with standard medical therapy.
PROTECT (the Use of NT-proBNP Testing to Guide HF Therapy in the Outpatient Setting study)29 also showed that a BNP-guided strategy was superior to usual care and was associated with reduced cardiovascular events and improved quality of life.29
GUIDE IT-HF (the Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure study), currently ongoing, is designed to assess the safety, efficacy and cost-effectiveness of a biomarker-guided strategy in 1,100 high-risk patients with heart failure with reduced ejection fraction.
Recommendations. The 2013 ACC/AHA guidelines give a class IA recommendation for the use of BNP to support clinical decision-making, particularly in cases of clinical uncertainty.1 BNP can also be used to establish prognosis or disease severity in chronic heart failure and to achieve optimal dosage of goal-directed medical therapy for euvolemic patients followed in a structured heart failure program.1

Heart failure clinics
Continuity of care upon discharge from the hospital is currently in a state of evolution. Those diagnosed with heart failure can now experience more comprehensive posthospital care by virtue of disease management clinics. The name may vary by institution, but whether it is called a “diuresis clinic,” “bridge clinic,” or “heart failure clinic,” the goal is to improve guideline-driven care, educate the patient, and reduce heart failure hospitalizations. Heart failure clinics are designed to provide a smooth transition from inpatient to outpatient care and to encourage patient self-accountability in health maintenance thereafter.
Studies have shown that heart failure clinics are associated with better medication dosing, fewer hospitalizations, and lower healthcare costs.30–32
Chronotropy: If inhibition
An elevated resting heart rate has been shown to be associated with increased cardiovascular morbidity and mortality.33 Studies have shown that slowing the heart rate improves myocardial contraction and energy supply and reduces energy expenditure.34 Ivabradine, a selective If (the f is for “funny”) channel inhibitor, slows the heart rate without other known cardiovascular effects.
SHIFT (the Systolic Heart Failure Treatment With the If Inhibitor Ivabradine Trial)35 investigated whether isolated heart rate reduction with ivabradine would reduce adverse clinical outcomes in patients with symptomatic heart failure. SHIFT randomized 6,505 patients with a left ventricular ejection fraction of 35% or less, in sinus rhythm, with a heart rate of at least 70 beats per minute, on optimal medical therapy, and hospitalized within 12 months of enrollment to receive ivabradine or placebo. The primary end point was a composite of cardiovascular mortality and hospital admission for worsening heart failure. Outcomes varied by heart rates achieved, with the best outcomes in those with the lowest heart rates at trial conclusion.
Ivabradine (Table 7) is indicated for patients with symptomatic heart failure with a left ventricular ejection fraction less than 35%, in sinus rhythm, with a resting heart rate of at least 70 beats per minute, and either on a maximally tolerated beta-blocker or with a contraindication to beta-blockers.
Ivabradine should be avoided in patients who are in acute decompensated heart failure or are hypotensive (blood pressure < 90/50 mm Hg), as well as in patients with a significant conduction abnormality (sick sinus syndrome, sinoatrial block, third-degree atrioventricular block), hepatic impairment, or bradycardia (resting heart rate < 60 beats per minute).

Digoxin
Digoxin has been used in treating systolic heart failure for more than 70 years.36,37
DIG (Digoxin Investigative Group trial)38 evaluated the long-term effect of digoxin on rates of mortality and hospitalization for heart failure over a 3-year period. In patients with a left ventricular ejection fraction less than 45%, digoxin had no effect on overall mortality when combined with diuretics and ACE inhibitors. However, the risk of hospitalization for worsening heart failure was significantly reduced with digoxin treatment.38
Recommendations. Digoxin should be considered when patients are on guideline-recommended therapy but heart failure symptoms persist. It is commonly initiated at a dose of 0.125 to 0.25 mg. The target therapeutic range for digoxin is 0.5 to 0.9 ng/mL.1 Digoxin toxicity can occur in patients with renal impairment, hypokalemia, hypomagnesemia, and hypothyroidism.
The 2013 ACC/AHA guidelines give a class IIA recommendation (treatment is “reasonable”) for digoxin in patients with heart failure with reduced ejection fraction unless contraindicated, to decrease hospitalizations for heart failure.1
Diuretics
Clinical manifestations of volume overload in patients with heart failure are from excess salt and water retention leading to inappropriate volume expansion in both the vascular and extravascular space. Diuretics (Table 8) are the foundation of heart failure treatment. Most patients are first initiated on a combination of a loop diuretic and a low-sodium diet to improve symptoms.
The 2013 ACC/AHA guidelines give a class I recommendation for diuretics in patients with heart failure with reduced ejection fraction who have evidence of fluid retention, unless contraindicated, to improve symptoms.1
Devices: ICDs
Patients with heart failure are at increased risk of sudden death and ventricular arrhythmias.39 Previously, antiarrhythmic drugs were considered the standard of care for nonsustained ventricular tachycardia after myocardial infarction.
MADIT (the Multicenter Automatic Defibrillator Implantation Trial) investigated whether prophylactic implantation of an internal cardiac defibrillator would improve 5-year survival rates in patients with heart failure. Eligible patients had had a Q-wave or enzyme-positive myocardial infarction within 3 weeks of study entry. They also had had an episode of asymptomatic nonsustained ventricular tachycardia unrelated to an acute myocardial infarction. Additionally, the patients had a left ventricular ejection fraction less than 35%, and inducible, sustained, nonsuppressible ventricular tachyarrhythmia on electrophysiologic testing.40
During the study, 15 patients in the defibrillator group died vs 39 in the conventional therapy group (P = .009).40
MADIT II evaluated the potential survival benefit of a prophylactically implanted defibrillator in the absence of electrophysiologic testing to induce arrhythmias.41 MADIT II included 1,232 patients with prior myocardial infarctions and a left ventricular ejection fracton of 30% or less. Patients were randomized to receive an implanted cardioverter-defibrillator or conventional medical therapy. The primary end point was death from any cause.41
The mortality rate was 19.8% in the conventional therapy group vs 14.2% in the defibrillator group (hazard ratio 0.69, P = .016).41 Thus, MADIT-II confirmed the benefits of prophylactic implantable cardioverter-defibrillator therapy seen in the original MADIT, and additionally eliminated the need for an electrophysiology test prior to device implantation.
SCD-HeFT (the Sudden Cardiac Death in Heart Failure Trial) evaluated whether amiodarone or a conservatively programmed shock-only, single-lead implanted cardioverter-defibrillator would decrease the risk of death (all-cause) in a population with mild to moderate heart failure with ischemic and nonischemic causes.42 In this trial, 2,521 patients with an ejection fraction of 35% or less, in NYHA class II or III, and with stable heart failure were randomized to receive a single-chamber implantable cardioverter-defibrillator, amiodarone, or placebo.
There were 244 deaths in the placebo group, 240 deaths in the amiodarone group (P = .53 compared with placebo), and 182 deaths in the defibrillator group (P = .007 compared with placebo).42
Recommendations. The 2013 ACC/AHA guideline1 gives implantable defibrillator therapy a class IA recommendation for the primary prevention of sudden cardiac death in selected patients with nonischemic cardiomyopathy or ischemic cardiomyopathy at least 40 days after a myocardial infarction and 90 days after percutaneous coronary intervention or coronary artery bypass grafting; with a left ventricular ejection fraction of 35% or less; and NYHA class II or III symptoms on chronic goal-directed medical management.
This therapy receives a class IB recommendation for primary prevention of sudden cardiac death to reduce total mortality in selected patients at least 40 days after myocardial infarction with a left ventricular ejection fraction of 30% or less and NYHA class I symptoms while receiving goal-directed medical therapy.
Implantable cardioverter-defibrillators are not recommended in patients who otherwise have a life expectancy of less than 1 year.
Devices: Cardiac resynchronization therapy
From 25% to 30% of heart failure patients have an intraventricular conduction abnormality,43,44 which can result in abnormalities of systolic and diastolic function. Biventricular pacing, in which a pacing lead is placed in the coronary sinus in addition to the right atrium and right ventricle, optimizes synchronization of ventricular contraction.43,44
MUSTIC (the Multisite Stimulation in Cardiomyopathies study) was a randomized trial designed to assess the efficacy of biventricular pacing (also known as cardiac resynchronization therapy) in heart failure patients.44 Entry criteria included NYHA class III heart failure for at least 1 month, left ventricular ejection fraction less than 35%, left ventricular end-diastolic diameter greater than 60 mm, and QRS duration longer than 150 ms. Patients were followed up at 9 and 12 months with 6-minute walking distance, peak oxygen consumption, changes in NYHA class, and left ventricular systolic function by echocardiography or radionuclide testing. Quality of life was assessed by the Minnesota Living With Heart Failure Questionnaire.
At 12 months, patients could walk significantly farther in 6 minutes, and their peak oxygen consumption had increased. They also reported significant improvement in quality of life, and NYHA class improved by 25%. MUSTIC was the first study to show a benefit in exercise tolerance, quality of life, improvement in cardiac performance, and reduction in heart failure symptoms with the use of biventricular pacing at 1 year.
MIRACLE (the Multicenter InSync Randomized Clinical Evaluation) validated the findings seen in MUSTIC by using a larger population size and a double-blinded method.45 Compared with a control group, patients who underwent cardiac resynchronization therapy could walk farther in 6 minutes and scored better in NYHA class, quality of life, and left ventricular ejection fraction.45
Recommendations. The 2013 ACC/AHA guidelines1 give cardiac resynchronization therapy a class IA/B indication for NYHA class II, III, or IV patients on goal-directed medical therapy in sinus rhythm with left ventricular ejection fraction 35% or less, left bundle branch block, and QRS duration of 150 ms or more.1
Devices: Implantable sensors
The future of ambulatory heart failure management may include implantable pulmonary artery pressure sensors.
The CardioMEMS is a permanently implantable pressure measurement system designed to provide daily pulmonary artery pressure measurements in an ambulatory setting with a goal of reducing heart failure-related hospitalizations. Through a transvenous delivery system, an implantable, battery-free sensor is positioned in the distal pulmonary artery.46,47
CHAMPION (the CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Patients trial) was one of the first major trials to assess the safety and efficacy of implantable pulmonary artery pressure monitoring systems.46 The study device was associated with a significant reduction in mean pulmonary artery pressures, fewer heart failure hospitalizations, and better quality of life. The length of stay for heart failure-related hospitalizations was also significantly shorter in the CardioMEMs group.46

Exercise
Patients with heart failure routinely experience a decline in functional capacity. This decline manifests as reduced exercise tolerance and poor quality of life, usually resulting in a physician recommendation to rest and paradoxical deconditioning and possible progression of symptoms.
Several studies have shown that cardiac rehabilitation has improved outcomes in heart failure patients.48 Cardiac rehabilitation is a supervised program that helps patients with exercise training, healthy living, education, and psychosocial counseling.
HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) is the largest randomized trial performed to determine whether aerobic exercise training reduces all-cause mortality or all-cause hospitalization and improves quality of life in patients with stable heart failure.49 Although the reduction in end points was initially not statistically significant, after adjusting for highly prognostic predictors of poor outcomes (cardiopulmonary exercise time, left ventricular ejection fraction, atrial fibrillation, and depression), exercise training was found to reduce the incidence of all-cause mortality or all-cause hospitalization by 11% (P = .03).49
Recommendations. Based on the results of HF-ACTION and several smaller studies, the ACC/AHA guidelines give exercise training a class IA recommendation as a safe and effective activity for patients with heart failure who are able to participate, to improve functional status.1 A class IIA recommendation is given to cardiac rehabilitation for the improvement of functional capacity, exercise duration, quality of life, and mortality rates.1
End-stage heart failure: Recognition
Despite adequate titration of goal-directed medical therapy, a portion of patients with heart failure with reduced ejection fraction ultimately progress to stage D, also termed “advanced” heart failure. The 5-year survival rate for patients with heart failure overall is 50%, but the 1-year mortality rate for those with advanced heart failure exceeds 50%.50
Because the high rates of morbidity and mortality can potentially be lowered, recognition of heart failure disease progression is imperative so that patients can be promptly referred for therapies such as inotropic infusion, mechanical circulatory support, and cardiac transplant, as well as end-of-life care such as hospice.1
The ACC/AHA1 have published clinical events and findings useful in identifying patients with advanced heart failure:
- Two or more hospitalizations or emergency department visits for heart failure in the past year
- Progressive deterioration in renal function (eg, elevation in creatinine or blood urea nitrogen)
- Weight loss without other cause
- Intolerance to ACE inhibitors due to hypotension or worsening renal function
- Inability to tolerate beta-blockers due to worsening heart failure or hypotension
- Systolic blood pressure often below 90 mm Hg
- Persistent dyspnea with dressing or bathing requiring rest
- Inability to walk one block on level ground due to dyspnea or fatigue
- Recent need to escalate diuretics to maintain volume status, often reaching daily dose equivalent to furosemide more than 160 mg/day or use of supplemental metolazone
- Progressive decline in serum sodium, usually to below 133 mmol/L
- Frequent shocks from implanted cardiac defibrillator.
End-stage heart failure: Left ventricular assist devices
For patients with refractory heart failure despite optimal medical management, advanced therapies such as heart transplant or ventricular assist devices have been proven to be durable options. These mechanical circulatory support devices “unload” the diseased ventricle and maintain cardiac output to vital organs.51 They were initially designed as temporary support to allow ventricular recovery or as a bridge to cardiac transplant. However, they have also evolved into permanent (“destination”) therapy.52
REMATCH (the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive HF trial) was the landmark study that showed that left ventricular assist device implantation resulted in a survival benefit and an improved quality of life in patients with advanced heart failure ineligible for cardiac transplant, compared with medical management.50 Implantation of a left ventricular assist device was associated with a 27% absolute reduction in the 1-year mortality rate.50
Since the National Institutes of Health’s artificial heart program was launched in 1964, there has been tremendous progress in the development of mechanical circulatory devices.50 The results of REMATCH were promising, but the 2-year survival rate was still only 23%, leaving a lot to be desired.
The HeartMate II (Thoratec) trial compared an axial continuous-flow device vs the previously established pulsatile left ventricular assist device, and noted a 2-year survival of 58% with the continuous flow device vs 24% with the pulsatile device (P = .008).53
ADVANCE (Evaluation of the HeartWare Left Ventricular Assist Device for the Treatment of Advanced Heart Failure) showed similar efficacy of the HVAD (Heartware), a centrifugal continuous-flow LVAD currently in use.54
The next generation of continuous-flow left ventricular assist devices are currently in clinical trials in the United States and include the axial flow MVAD (Heartware) and centrifugal flow Heartmate III (Thoratec).
We emphasize the importance of early identification of patients with advanced disease who may qualify for and benefit from such therapies.

The management of heart failure is evolving. In the 1960s, the standard heart failure medical regimen included digoxin, diuretics, and the recommendation of rest. This contrasts with the current era, in which medical regimens include neurohormonal blockade, diuretics, and the promotion of physical activity.55 Since the publication of the 2013 heart failure guidelines, new medical and device options have emerged that have been proven to either improve survival or reduce hospitalizations. The development of clinical guidelines promotes evidence-based practice and overcomes the inertia of practice patterns based on anecdotal evidence.
Several approaches to the management of heart failure have been recommended. A major effort should be made to identify those at risk for heart failure (stage A) and to implement risk factor modification. Treatment of hypertension, diabetes mellitus, and dyslipidemia decreases the risk of heart failure.1
For patients with evidence of structural heart disease with and without symptoms, Figure 1 summarizes a guideline approach to the management of heart failure. It should be stressed that guidelines are meant to guide management, but do not serve as a substitute for sound clinical judgment.
Heart failure is the common final pathway of all cardiac pathology, and understanding the neurohormonal response and maladaptive physiology has led to the development of novel therapeutics and devices. At present, the field of cardiology may not be able to remove the “failure” from heart failure, but we can make every effort to prevent failure of treatment delivery and reduce resource utilization and morbidity associated with this syndrome.
Acknowledgments: We would like to thank Chankya Dahagam and Cynthia Obenwa for their valuable contribution in the preparation of this manuscript.
Managing heart failure is a challenge. To aid clinicians in this task, the American College of Cardiology Foundation (ACC) and the American Heart Association (AHA) publish evidence-based guidelines, most recently in 2013.1 Since then, new drugs and devices have been shown to improve survival and reduce hospitalizations.
This paper reviews the ABCs of outpatient management of systolic heart failure (or heart failure with reduced ejection fraction), including the results of major trials and recommendations.

A common and serious condition
Heart failure is a debilitating syndrome that takes a significant physical and mental toll on those affected.
And it is common. An American age 40 or older faces a 20% lifetime risk of heart failure.1 An estimated 5.1 million Americans have clinical signs and symptoms of heart failure, and 900,000 new cases are diagnosed each year.2 By 2030 the prevalence of heart failure is projected to increase by 46%, and 9 million Americans will have been diagnosed with it.2
The severity of heart failure can be described using either the functional classification devised by the New York Heart Association (NYHA; Table 1) or the stages defined by the ACC and AHA.1,3 Though survival rates have improved, there is a direct correlation between worsening symptoms and death.4
Heart failure is the leading cause of hospitalizations annually. It accounts for $30 billion in healthcare costs, with direct medical costs accounting for 68% and another $1.8 billion associated with clinic visits, most often with primary care providers. By 2030, the cost is projected to increase by 127% to $69.7 billion—$244 per person in the United States.2
ACE inhibitors
The renin-angiotensin-aldosterone system has been studied for over 100 years.5
In heart failure with reduced ejection fraction, this system is upregulated as an adaptive mechanism to maintain hemodynamic homeostasis.6–8 However, prolonged activation of the renin-angiotensin-aldosterone system can lead to deleterious cardiovascular effects such as myocyte hypertrophy, myocardial fibrosis, sodium conservation, and fluid overload.8,9 Angiotensin II is a potent vasoconstrictor and plays a role in cardiovascular remodeling, leading to worsening progression of heart failure.6
CONSENSUS (the Cooperative North Scandinavian Enalapril Survival Study) examined the effect of the angiotensin-converting enzyme (ACE) inhibitor enalapril on survival in 253 patients with NYHA class IV heart failure. Participants were randomized to receive either enalapril or placebo. At 6 months, the mortality rate was 26% in the enalapril group vs 44% in the placebo group, an 18% absolute risk reduction and a 41% relative risk reduction (P = .002). At 12 months, the relative risk reduction in mortality was 30% (P = .001).10
SOLVD (the Study of Left Ventricular Dysfunction) extended the use of ACE inhibitors to all patients with heart failure, not just those in NYHA class IV. It randomized 1,284 patients with heart failure of any NYHA class and an ejection fraction less than 35% to receive either enalapril or placebo, and demonstrated a 16% relative risk reduction in mortality in the enalapril group, with mortality rates of 36% vs 39.7% (P = .0036).11
Recommendations. The benefits of ACE inhibition have been demonstrated in patients with mild, moderate, and severe heart failure. Thus, the guidelines recommend ACE inhibitors (Table 2) for all patients with heart failure with reduced ejection fraction.1
Angiotensin II receptor blockers
Angiotensin II receptor blockers (ARBs) (Table 3) have been proven to be suitable alternatives for patients with heart failure with reduced ejection fraction who cannot tolerate ACE inhibitors.
Val-HefT (the Valsartan HF Trial)12 randomized 5,010 patients in a double-blind fashion to receive either valsartan or placebo, with background therapy that included beta-blockers, digoxin, diuretics, and ACE inhibitors. There was a 13% reduction of the combined primary end point of mortality and morbidity and a 24% reduction in heart failure hospitalizations in the valsartan group.12
Subgroup analysis compared patients on the basis of use of ACE inhibitors and beta-blockers at study entry. Valsartan had a favorable effect in the subgroups using beta-blockers alone, ACE inhibitors alone, and neither drug. However, when patients received all three (a beta-blocker, an ACE inhibitor, and valsartan), the mortality rate was significantly increased (P = .009).12 This finding conflicted with those of other studies, which found a small benefit of combining an ACE inhibitor and an ARB.
CHARM-Added (the Candesartan in HF Assessment of Reduction in Mortality and Morbidity trial)13 investigated whether adding the ARB candesartan to an ACE inhibitor would improve clinical outcomes. In the study, 2,548 patients in NYHA class II, III, or IV with a left ventricular ejection fraction of less than 40% who were receiving ACE inhibitors were randomized to either candesartan or placebo. The addition of candesartan resulted in a significant reduction in cardiovascular mortality and heart failure hospitalizations, but with the downside of higher rates of hyperkalemia and serum creatinine elevation.13
Recommendations. The 2013 guidelines recommend that ARBs be used in patients who cannot tolerate an ACE inhibitor due to cough. However, routine combined use of ARBs, ACE inhibitors, and aldosterone antagonists is not recommended and may cause harm.1
Aldosterone receptor antagonists
Elevated levels of aldosterone lead to fluid retention, loss of magnesium and potassium, and myocardial fibrosis.
RALES (the Randomized Aldactone Evaluation Study)14 tested the hypothesis that the aldosterone receptor antagonist spironolactone (25 mg daily) would reduce deaths from all causes in patients with severe heart failure receiving standard medications including an ACE inhibitor. RALES included 1,663 patients in NYHA class III or IV with a left ventricular ejection fraction of 35% or less, randomized to receive 25 mg of spironolactone or matching placebo. This study found a 30% relative risk reduction and an 11% absolute risk reduction in all-cause mortality, a 31% relative risk reduction and a 10% absolute risk reduction in cardiac mortality, and 30% fewer cardiac-related hospitalizations in the spironolactone group.14
Eplerenone, an aldosterone receptor antagonist that lacks the antiandrogenic side effects of spironolactone, has also been shown to be beneficial. Its efficacy in patients with left ventricular systolic dysfunction was first established in postmyocardial infarction patients.15
EMPHASIS-HF (the Eplerenone in Mild Patients Hospitalized and Survival Study in Heart Failure)16 broadened the application of eplerenone (and aldosterone antagonists in general), investigating the effects of eplerenone in 2,737 NYHA class II patients, regardless of ischemic etiology. The composite end point of cardiovascular death or heart failure hospitalization occurred in 18.3% of the eplerenone group vs 25.9% of the placebo group (P < .001). A total of 12.5% of patients in the eplerenone group died, compared with 15.5% in the placebo group (P = .008). Hospitalizations were also fewer in the eplerenone group.
Recommendations. The 2013 guidelines recommend aldosterone receptor antagonists (Table 4) for patients with NYHA class II, III, or IV heart failure who have an ejection fraction of 35% or less, to reduce morbidity and mortality (class IA recommendation).1 The guidelines also recommend that these agents not be used in patients with renal insufficiency (serum creatinine > 2.5 mg/dL in men or > 2.0 mg/dL in women; an estimated glomerular filtration rate < 30 mL/min/1.73 m2); or a serum potassium level above 5 mmol/L.1
Angiotensin-neprilysin inhibitor (the future)
Research has identified neprilysin as another potential target in the treatment of heart failure and has sought to combine inhibition of angiotensin and neprilysin.
Neprilysin, a neutral endopeptidase, is associated with degradation of several natural vasoactive peptides such as natriuretic peptide, bradykinin, and adrenomedullin. Neprilysin inhibition increases these substances and counters the neurohormonal overactivation that leads to vasoconstriction, sodium retention, and cardiac remodeling.17
The ARB valsartan has been combined with the neprilysin inhibitor sacubitril to create the first angiotensin-neprilysin inhibitor (ARNI) (Table 5). The combination was selected to minimize the potential for angioedema.
PARADIGM-HF (the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure trial)17 examined whether combined angiotensin-neprilysin inhibition was superior to ACE inhibition alone with enalapril in patients with chronic heart failure.
In PARADIGM-HF, 10,521 patients with NYHA class II, III, or IV heart failure were randomized to receive either sacubitril-valsartan or enalapril. The group receiving sacubitril-valsartan had significantly fewer deaths from cardiovascular causes and heart failure hospitalizations.17 An improvement in quality of life and NYHA functional class was also observed in the sacubitril-valsartan group.17
Sacubitril-valsartan underwent priority review by the US Food and Drug Administration and has been approved. Currently, it is indicated for the treatment of heart failure with reduced ejection fraction and NYHA class II, III, or IV symptoms. It should be avoided in patients who have previously experienced angioedema with an ACE inhibitor or ARB, in patients receiving aliskiren for diabetes, and in patients with hypersensitivity reactions to either of its components. Simultaneous use of sacubitril-valsartan and an ACE inhibitor should be avoided, and a washout period is recommended when transitioning from an ACE inhibitor to this combined agent.

Beta-blockers
In heart failure, there is increased sympathetic activation and associated elevations in norepinephrine levels, which may lead to deleterious long-term effects on cardiac function and structure. Beta-adrenergic receptor blockade is now known to be cardioprotective, but it was not always so; beta-blockers used to be contraindicated in patients with heart failure.
An early experience using beta-blockers in heart failure was described in 1975.18,19 The first study to report a survival benefit of treating systolic heart failure with a beta-blocker was published in 1979.20 Later, small controlled trials demonstrated a reduction in heart failure symptoms and improvement in left ventricular function and in NYHA functional class.21 Larger clinical trials have demonstrated a tremendous survival benefit with beta-blockers in heart failure, specifically carvedilol, extended-release metoprolol, and bisoprolol.
The US Carvedilol Heart Failure Study Group trial22 evaluated whether beta-blocker use in heart failure patients would reduce the rates of morbidity and mortality.22 The trial included 1,094 patients with symptomatic heart failure for at least 3 months and a left ventricular ejection fraction of 35% or less on background therapy including vasodilators, ACE inhibitors, and digoxin. Patients were randomized to receive either carvedilol or placebo. Carvedilol use was associated with a dramatic 65% risk reduction in mortality (7.8% with placebo vs 3.2% with carvedilol, P < .001) and a 27% risk reduction in hospitalizations (19.6% vs 14.1%, P = .036), leading to early trial termination.
CIBIS-II (the Cardiac Insufficiency Bisoprolol Study II)23 investigated the effects of beta-blockers on survival and morbidity. CIBIS-II included 2,647 NYHA class III or IV patients with a left ventricular ejection fraction less than 35% on background medical therapy that included diuretics and ACE inhibitors. This trial was also terminated early, after demonstrating a significant survival benefit with bisoprolol.
MERIT-HF (the Metoprolol Extended Release Randomized Intervention Trial in Congestive Heart Failure)24 evaluated if once-daily metoprolol would lower mortality rates in patients with symptomatic heart failure. The study enrolled 3,991 NYHA class II–IV patients with chronic heart failure and a left ventricular ejection fraction of 40% or less. Like the previous two beta-blocker trials, MERIT-HF was terminated early, as it demonstrated a 34% reduction in all-cause mortality (7.2% risk of death per patient-year vs 11.0%, P = .00009).
The beta-blocker trials have shown that when added to background therapy, beta-blockers improve survival and reduce hospitalizations. However, when prescribing a beta-blocker, it is important to understand that not all beta-blockers are equal in the treatment of heart failure.
COMET (the Carvedilol or Metoprolol European Trial)25 was the only head-to-head randomized control trial evaluating clinical outcomes in patients receiving carvedilol or metoprolol tartrate (not metoprolol succinate). In COMET, 1,511 patients with NYHA class II, III, or IV heart failure with a left ventricular ejection fraction of 35% or less were randomized to carvedilol or metoprolol tartrate. The primary end point of all-cause mortality occurred in 34% of the carvedilol group and 40% of the metoprolol tartrate group (P = .0017). There was no significant difference with regard to the composite end point of mortality and all-cause admissions.
Recommendations. The 2013 guidelines give a class IA recommendation for starting a beta-blocker (carvedilol, bisoprolol, or metoprolol succinate, Table 6) in patients with current or prior symptoms of heart failure.1 Beta-blockers should be initiated with caution or avoided in patients with acutely decompensated heart failure with evidence of fluid overload.
Brain-type natriuretic peptide
Brain-type natriuretic peptide (BNP) or its amino-terminal cleavage product (NT-proBNP) originates in cardiomyocytes and is released by several triggers, most commonly cardiomyocyte stretch in the setting of volume or pressure overload.26 The biologic significance of BNP includes natriuresis and vasodilation, renin-angiotensin system inhibition, and sympathetic nervous system modulation.26
TIME-CHF (the Trial of Intensified vs. Standard Medical Therapy in Elderly Patients With Congestive HF)27 investigated whether 18-month outcomes would be better if treatment were guided by N-terminal BNP levels rather than by symptoms. The BNP-guided strategy was not associated with a reduction in hospitalization or a survival benefit.
BATTLESCARRED (the NT-proBNP-Assisted Treatment to Lessen Serial Cardiac Readmissions and Death trial)28 in 2009 showed that a BNP-guided management strategy significantly reduced mortality rates in patients under age 75 compared with standard medical therapy.
PROTECT (the Use of NT-proBNP Testing to Guide HF Therapy in the Outpatient Setting study)29 also showed that a BNP-guided strategy was superior to usual care and was associated with reduced cardiovascular events and improved quality of life.29
GUIDE IT-HF (the Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure study), currently ongoing, is designed to assess the safety, efficacy and cost-effectiveness of a biomarker-guided strategy in 1,100 high-risk patients with heart failure with reduced ejection fraction.
Recommendations. The 2013 ACC/AHA guidelines give a class IA recommendation for the use of BNP to support clinical decision-making, particularly in cases of clinical uncertainty.1 BNP can also be used to establish prognosis or disease severity in chronic heart failure and to achieve optimal dosage of goal-directed medical therapy for euvolemic patients followed in a structured heart failure program.1

Heart failure clinics
Continuity of care upon discharge from the hospital is currently in a state of evolution. Those diagnosed with heart failure can now experience more comprehensive posthospital care by virtue of disease management clinics. The name may vary by institution, but whether it is called a “diuresis clinic,” “bridge clinic,” or “heart failure clinic,” the goal is to improve guideline-driven care, educate the patient, and reduce heart failure hospitalizations. Heart failure clinics are designed to provide a smooth transition from inpatient to outpatient care and to encourage patient self-accountability in health maintenance thereafter.
Studies have shown that heart failure clinics are associated with better medication dosing, fewer hospitalizations, and lower healthcare costs.30–32
Chronotropy: If inhibition
An elevated resting heart rate has been shown to be associated with increased cardiovascular morbidity and mortality.33 Studies have shown that slowing the heart rate improves myocardial contraction and energy supply and reduces energy expenditure.34 Ivabradine, a selective If (the f is for “funny”) channel inhibitor, slows the heart rate without other known cardiovascular effects.
SHIFT (the Systolic Heart Failure Treatment With the If Inhibitor Ivabradine Trial)35 investigated whether isolated heart rate reduction with ivabradine would reduce adverse clinical outcomes in patients with symptomatic heart failure. SHIFT randomized 6,505 patients with a left ventricular ejection fraction of 35% or less, in sinus rhythm, with a heart rate of at least 70 beats per minute, on optimal medical therapy, and hospitalized within 12 months of enrollment to receive ivabradine or placebo. The primary end point was a composite of cardiovascular mortality and hospital admission for worsening heart failure. Outcomes varied by heart rates achieved, with the best outcomes in those with the lowest heart rates at trial conclusion.
Ivabradine (Table 7) is indicated for patients with symptomatic heart failure with a left ventricular ejection fraction less than 35%, in sinus rhythm, with a resting heart rate of at least 70 beats per minute, and either on a maximally tolerated beta-blocker or with a contraindication to beta-blockers.
Ivabradine should be avoided in patients who are in acute decompensated heart failure or are hypotensive (blood pressure < 90/50 mm Hg), as well as in patients with a significant conduction abnormality (sick sinus syndrome, sinoatrial block, third-degree atrioventricular block), hepatic impairment, or bradycardia (resting heart rate < 60 beats per minute).

Digoxin
Digoxin has been used in treating systolic heart failure for more than 70 years.36,37
DIG (Digoxin Investigative Group trial)38 evaluated the long-term effect of digoxin on rates of mortality and hospitalization for heart failure over a 3-year period. In patients with a left ventricular ejection fraction less than 45%, digoxin had no effect on overall mortality when combined with diuretics and ACE inhibitors. However, the risk of hospitalization for worsening heart failure was significantly reduced with digoxin treatment.38
Recommendations. Digoxin should be considered when patients are on guideline-recommended therapy but heart failure symptoms persist. It is commonly initiated at a dose of 0.125 to 0.25 mg. The target therapeutic range for digoxin is 0.5 to 0.9 ng/mL.1 Digoxin toxicity can occur in patients with renal impairment, hypokalemia, hypomagnesemia, and hypothyroidism.
The 2013 ACC/AHA guidelines give a class IIA recommendation (treatment is “reasonable”) for digoxin in patients with heart failure with reduced ejection fraction unless contraindicated, to decrease hospitalizations for heart failure.1
Diuretics
Clinical manifestations of volume overload in patients with heart failure are from excess salt and water retention leading to inappropriate volume expansion in both the vascular and extravascular space. Diuretics (Table 8) are the foundation of heart failure treatment. Most patients are first initiated on a combination of a loop diuretic and a low-sodium diet to improve symptoms.
The 2013 ACC/AHA guidelines give a class I recommendation for diuretics in patients with heart failure with reduced ejection fraction who have evidence of fluid retention, unless contraindicated, to improve symptoms.1
Devices: ICDs
Patients with heart failure are at increased risk of sudden death and ventricular arrhythmias.39 Previously, antiarrhythmic drugs were considered the standard of care for nonsustained ventricular tachycardia after myocardial infarction.
MADIT (the Multicenter Automatic Defibrillator Implantation Trial) investigated whether prophylactic implantation of an internal cardiac defibrillator would improve 5-year survival rates in patients with heart failure. Eligible patients had had a Q-wave or enzyme-positive myocardial infarction within 3 weeks of study entry. They also had had an episode of asymptomatic nonsustained ventricular tachycardia unrelated to an acute myocardial infarction. Additionally, the patients had a left ventricular ejection fraction less than 35%, and inducible, sustained, nonsuppressible ventricular tachyarrhythmia on electrophysiologic testing.40
During the study, 15 patients in the defibrillator group died vs 39 in the conventional therapy group (P = .009).40
MADIT II evaluated the potential survival benefit of a prophylactically implanted defibrillator in the absence of electrophysiologic testing to induce arrhythmias.41 MADIT II included 1,232 patients with prior myocardial infarctions and a left ventricular ejection fracton of 30% or less. Patients were randomized to receive an implanted cardioverter-defibrillator or conventional medical therapy. The primary end point was death from any cause.41
The mortality rate was 19.8% in the conventional therapy group vs 14.2% in the defibrillator group (hazard ratio 0.69, P = .016).41 Thus, MADIT-II confirmed the benefits of prophylactic implantable cardioverter-defibrillator therapy seen in the original MADIT, and additionally eliminated the need for an electrophysiology test prior to device implantation.
SCD-HeFT (the Sudden Cardiac Death in Heart Failure Trial) evaluated whether amiodarone or a conservatively programmed shock-only, single-lead implanted cardioverter-defibrillator would decrease the risk of death (all-cause) in a population with mild to moderate heart failure with ischemic and nonischemic causes.42 In this trial, 2,521 patients with an ejection fraction of 35% or less, in NYHA class II or III, and with stable heart failure were randomized to receive a single-chamber implantable cardioverter-defibrillator, amiodarone, or placebo.
There were 244 deaths in the placebo group, 240 deaths in the amiodarone group (P = .53 compared with placebo), and 182 deaths in the defibrillator group (P = .007 compared with placebo).42
Recommendations. The 2013 ACC/AHA guideline1 gives implantable defibrillator therapy a class IA recommendation for the primary prevention of sudden cardiac death in selected patients with nonischemic cardiomyopathy or ischemic cardiomyopathy at least 40 days after a myocardial infarction and 90 days after percutaneous coronary intervention or coronary artery bypass grafting; with a left ventricular ejection fraction of 35% or less; and NYHA class II or III symptoms on chronic goal-directed medical management.
This therapy receives a class IB recommendation for primary prevention of sudden cardiac death to reduce total mortality in selected patients at least 40 days after myocardial infarction with a left ventricular ejection fraction of 30% or less and NYHA class I symptoms while receiving goal-directed medical therapy.
Implantable cardioverter-defibrillators are not recommended in patients who otherwise have a life expectancy of less than 1 year.
Devices: Cardiac resynchronization therapy
From 25% to 30% of heart failure patients have an intraventricular conduction abnormality,43,44 which can result in abnormalities of systolic and diastolic function. Biventricular pacing, in which a pacing lead is placed in the coronary sinus in addition to the right atrium and right ventricle, optimizes synchronization of ventricular contraction.43,44
MUSTIC (the Multisite Stimulation in Cardiomyopathies study) was a randomized trial designed to assess the efficacy of biventricular pacing (also known as cardiac resynchronization therapy) in heart failure patients.44 Entry criteria included NYHA class III heart failure for at least 1 month, left ventricular ejection fraction less than 35%, left ventricular end-diastolic diameter greater than 60 mm, and QRS duration longer than 150 ms. Patients were followed up at 9 and 12 months with 6-minute walking distance, peak oxygen consumption, changes in NYHA class, and left ventricular systolic function by echocardiography or radionuclide testing. Quality of life was assessed by the Minnesota Living With Heart Failure Questionnaire.
At 12 months, patients could walk significantly farther in 6 minutes, and their peak oxygen consumption had increased. They also reported significant improvement in quality of life, and NYHA class improved by 25%. MUSTIC was the first study to show a benefit in exercise tolerance, quality of life, improvement in cardiac performance, and reduction in heart failure symptoms with the use of biventricular pacing at 1 year.
MIRACLE (the Multicenter InSync Randomized Clinical Evaluation) validated the findings seen in MUSTIC by using a larger population size and a double-blinded method.45 Compared with a control group, patients who underwent cardiac resynchronization therapy could walk farther in 6 minutes and scored better in NYHA class, quality of life, and left ventricular ejection fraction.45
Recommendations. The 2013 ACC/AHA guidelines1 give cardiac resynchronization therapy a class IA/B indication for NYHA class II, III, or IV patients on goal-directed medical therapy in sinus rhythm with left ventricular ejection fraction 35% or less, left bundle branch block, and QRS duration of 150 ms or more.1
Devices: Implantable sensors
The future of ambulatory heart failure management may include implantable pulmonary artery pressure sensors.
The CardioMEMS is a permanently implantable pressure measurement system designed to provide daily pulmonary artery pressure measurements in an ambulatory setting with a goal of reducing heart failure-related hospitalizations. Through a transvenous delivery system, an implantable, battery-free sensor is positioned in the distal pulmonary artery.46,47
CHAMPION (the CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Patients trial) was one of the first major trials to assess the safety and efficacy of implantable pulmonary artery pressure monitoring systems.46 The study device was associated with a significant reduction in mean pulmonary artery pressures, fewer heart failure hospitalizations, and better quality of life. The length of stay for heart failure-related hospitalizations was also significantly shorter in the CardioMEMs group.46

Exercise
Patients with heart failure routinely experience a decline in functional capacity. This decline manifests as reduced exercise tolerance and poor quality of life, usually resulting in a physician recommendation to rest and paradoxical deconditioning and possible progression of symptoms.
Several studies have shown that cardiac rehabilitation has improved outcomes in heart failure patients.48 Cardiac rehabilitation is a supervised program that helps patients with exercise training, healthy living, education, and psychosocial counseling.
HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) is the largest randomized trial performed to determine whether aerobic exercise training reduces all-cause mortality or all-cause hospitalization and improves quality of life in patients with stable heart failure.49 Although the reduction in end points was initially not statistically significant, after adjusting for highly prognostic predictors of poor outcomes (cardiopulmonary exercise time, left ventricular ejection fraction, atrial fibrillation, and depression), exercise training was found to reduce the incidence of all-cause mortality or all-cause hospitalization by 11% (P = .03).49
Recommendations. Based on the results of HF-ACTION and several smaller studies, the ACC/AHA guidelines give exercise training a class IA recommendation as a safe and effective activity for patients with heart failure who are able to participate, to improve functional status.1 A class IIA recommendation is given to cardiac rehabilitation for the improvement of functional capacity, exercise duration, quality of life, and mortality rates.1
End-stage heart failure: Recognition
Despite adequate titration of goal-directed medical therapy, a portion of patients with heart failure with reduced ejection fraction ultimately progress to stage D, also termed “advanced” heart failure. The 5-year survival rate for patients with heart failure overall is 50%, but the 1-year mortality rate for those with advanced heart failure exceeds 50%.50
Because the high rates of morbidity and mortality can potentially be lowered, recognition of heart failure disease progression is imperative so that patients can be promptly referred for therapies such as inotropic infusion, mechanical circulatory support, and cardiac transplant, as well as end-of-life care such as hospice.1
The ACC/AHA1 have published clinical events and findings useful in identifying patients with advanced heart failure:
- Two or more hospitalizations or emergency department visits for heart failure in the past year
- Progressive deterioration in renal function (eg, elevation in creatinine or blood urea nitrogen)
- Weight loss without other cause
- Intolerance to ACE inhibitors due to hypotension or worsening renal function
- Inability to tolerate beta-blockers due to worsening heart failure or hypotension
- Systolic blood pressure often below 90 mm Hg
- Persistent dyspnea with dressing or bathing requiring rest
- Inability to walk one block on level ground due to dyspnea or fatigue
- Recent need to escalate diuretics to maintain volume status, often reaching daily dose equivalent to furosemide more than 160 mg/day or use of supplemental metolazone
- Progressive decline in serum sodium, usually to below 133 mmol/L
- Frequent shocks from implanted cardiac defibrillator.
End-stage heart failure: Left ventricular assist devices
For patients with refractory heart failure despite optimal medical management, advanced therapies such as heart transplant or ventricular assist devices have been proven to be durable options. These mechanical circulatory support devices “unload” the diseased ventricle and maintain cardiac output to vital organs.51 They were initially designed as temporary support to allow ventricular recovery or as a bridge to cardiac transplant. However, they have also evolved into permanent (“destination”) therapy.52
REMATCH (the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive HF trial) was the landmark study that showed that left ventricular assist device implantation resulted in a survival benefit and an improved quality of life in patients with advanced heart failure ineligible for cardiac transplant, compared with medical management.50 Implantation of a left ventricular assist device was associated with a 27% absolute reduction in the 1-year mortality rate.50
Since the National Institutes of Health’s artificial heart program was launched in 1964, there has been tremendous progress in the development of mechanical circulatory devices.50 The results of REMATCH were promising, but the 2-year survival rate was still only 23%, leaving a lot to be desired.
The HeartMate II (Thoratec) trial compared an axial continuous-flow device vs the previously established pulsatile left ventricular assist device, and noted a 2-year survival of 58% with the continuous flow device vs 24% with the pulsatile device (P = .008).53
ADVANCE (Evaluation of the HeartWare Left Ventricular Assist Device for the Treatment of Advanced Heart Failure) showed similar efficacy of the HVAD (Heartware), a centrifugal continuous-flow LVAD currently in use.54
The next generation of continuous-flow left ventricular assist devices are currently in clinical trials in the United States and include the axial flow MVAD (Heartware) and centrifugal flow Heartmate III (Thoratec).
We emphasize the importance of early identification of patients with advanced disease who may qualify for and benefit from such therapies.

The management of heart failure is evolving. In the 1960s, the standard heart failure medical regimen included digoxin, diuretics, and the recommendation of rest. This contrasts with the current era, in which medical regimens include neurohormonal blockade, diuretics, and the promotion of physical activity.55 Since the publication of the 2013 heart failure guidelines, new medical and device options have emerged that have been proven to either improve survival or reduce hospitalizations. The development of clinical guidelines promotes evidence-based practice and overcomes the inertia of practice patterns based on anecdotal evidence.
Several approaches to the management of heart failure have been recommended. A major effort should be made to identify those at risk for heart failure (stage A) and to implement risk factor modification. Treatment of hypertension, diabetes mellitus, and dyslipidemia decreases the risk of heart failure.1
For patients with evidence of structural heart disease with and without symptoms, Figure 1 summarizes a guideline approach to the management of heart failure. It should be stressed that guidelines are meant to guide management, but do not serve as a substitute for sound clinical judgment.
Heart failure is the common final pathway of all cardiac pathology, and understanding the neurohormonal response and maladaptive physiology has led to the development of novel therapeutics and devices. At present, the field of cardiology may not be able to remove the “failure” from heart failure, but we can make every effort to prevent failure of treatment delivery and reduce resource utilization and morbidity associated with this syndrome.
Acknowledgments: We would like to thank Chankya Dahagam and Cynthia Obenwa for their valuable contribution in the preparation of this manuscript.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128:e240–e327.
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 2015; 133:e38–e360.
- Goldberg LR, Jessup M. Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction. Circulation 2006; 113:2851–2860.
- Ammar KA, Jacobsen SJ, Mahoney DW, et al. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation 2007; 115:1563–1570.
- Tigerstedt R, Bergman PQ. Niere und Kreislauf. Skand Arch Physiol 1898; 8:223–271.
- Unger T, Li J. The role of the renin-angiotensin-aldosterone system in heart failure. J Renin Angiotensin Aldosterone Syst 2004; 5(suppl 1):S7–S10.
- Cohn JN, Levine TB, Francis GS, Goldsmith S. Neurohumoral control mechanisms in congestive heart failure. Am Heart J 1981; 102:509–514.
- von Lueder TG, Sangaralingham SJ, Wang BH, et al. Renin-angiotensin blockade combined with natriuretic peptide system augmentation: novel therapeutic concepts to combat heart failure. Circ Heart Fail 2013; 6:594–605.
- Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation 1991; 83:1849–1865.
- The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987; 316:1429–1435.
- The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325:293–302.
- Cohn JN, Tognoni G, Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345:1667–1675.
- McMurray JJ, Ostergren J, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 2003; 362:767–771.
- Pitt B, Zannad F, Remme WJ, et al.The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341:709–717.
- Pitt B, Remme W, Zannad F, et al; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348:1309–1321.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2010; 364:11–21.
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371:993–1004.
- Waagstein F, Hjalmarson A, Varnauskas E, Wallentin I. Effect of chronic beta-adrenergic receptor blockade in congestive cardiomyopathy. Br Heart J 1975; 37:1022–1036.
- Gheorghiade M, Colucci WS, Swedberg K. Beta-blockers in chronic heart failure. Circulation 2003; 107:1570–1575.
- Swedberg K, Hjalmarson A, Waagstein F, Wallentin I. Prolongation of survival in congestive cardiomyopathy by beta-receptor blockade. Lancet 1979; 1:1374–1376.
- Klapholz M. Beta-blocker use for the stages of heart failure. Mayo Clin Proc 2009; 84:718–729.
- Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med 1996; 334:1349–1355.
- The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999; 353:9–13.
- MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353:2001–2007.
- Poole-Wilson PA, Swedberg K, Cleland JG, et al; Carvedilol Or Metoprolol European Trial Investigators. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet 2003; 362:7–13.
- Kim H-N, Januzzi JL Jr. Natriuretic peptide testing in heart failure. Circulation 2011; 123:2015–2019.
- Pfisterer M, Buser P, Rickli H, et al; TIME-CHF Investigators. BNP-guided vs symptom-guided heart failure therapy: the Trial of Intensified vs Standard Medical Therapy in Elderly Patients with Congestive Heart Failure (TIME-CHF) randomized trial. JAMA 2009; 301:383–392.
- Lainchbury JG, Troughton RW, Strangman KM, et al. N-terminal pro–B-type natriuretic peptide-guided treatment for chronic heart failure: results From the BATTLESCARRED (NT-proBNP–Assisted Treatment To Lessen Serial Cardiac Readmissions and Death) trial. J Am Coll Cardiol 2009; 55:53–60.
- Januzzi JL Jr, Rehman SU, Mohammed AA, et al. Use of amino-terminal pro–B-type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J Am Coll Cardiol 2011; 58:1881-1889.
- Whellan DJ, Gaulden L, Gattis WA, et al. The benefit of implementing a heart failure disease management program. Arch Intern Med 2001; 161:2223–2228.
- Fonarow GC, Stevenson LW, Walden JA, et al. Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure. J Am Coll Cardiol 1997; 30:725–732.
- Grady KL, Dracup K, Kennedy G, et al. Team management of patients with heart filure: a statement for healthcare professionals from the Cardiovascular Nursing Council of the American Heart Association. Circulation 2000; 102:2443–2456.
- Kannel WB, Kannel C, Paffenbarger RS Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J 1987; 113:1489-1494.
- Colin P, Ghaleh B, Monnet X, Hittinger L, Berdeaux A. Effect of graded heart rate reduction with ivabradine on myocardial oxygen consumption and diastolic time in exercising dogs. J Pharmacol Exper Ther 2004; 308:236–240.
- Böhm M, Swedberg K, Komajda M, et al; SHIFT Investigators. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet 2010; 376:886–894.
- Batterman RC, DeGraff AC. Comparative study on the use of the purified digitalis glycosides, digoxin, digitoxin, and lanatoside C, for the management of ambulatory patients with congestive heart failure. Am Heart J 1947; 34:663–673.
- Ouyang AJ, Lv YN, Zhong HL, et al. Meta-analysis of digoxin use and risk of mortality in patients with atrial fibrillation. Am J Cardiol 2015; 115:901–906.
- Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997; 336:525–533.
- Aleong RG, Mulvahill MJ, Halder I, et al. Left ventricular dilatation increases the risk of ventricular arrhythmias in patients with reduced systolic function. J Am Heart Assoc 2015; 4:e001566.
- Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med 1996; 335:1933–1940.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Bardy GH, Lee KL, Mark DB, et al; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005; 352:225–237.
- Greenberg B, Mehra MR. All patients with heart failure and intraventricular conduction defect or dyssynchrony should not receive cardiac resynchronization therapy. Circulation 2006; 114:2685–2691.
- Linde C, Leclercq C, Rex S, et al. Long-term benefits of biventricular pacing in congestive heart failure: results from the MUltisite STimulation in cardiomyopathy (MUSTIC) study. J Am Coll Cardiol 2002; 40:111–118.
- Abraham WT, Fisher WG, Smith AL, et al; MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002; 346:1845–1853.
- Abraham WT, Adamson PB, Bourge RC, et al; CHAMPION Study Group. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011; 377:658–666.
- Loh JP, Barbash IM, Waksman R. Overview of the 2011 Food and Drug Administration Circulatory System Devices Panel of the Medical Devices Advisory Committee Meeting on the CardioMEMS Champion Heart Failure Monitoring System. J Am Coll Cardiol 2013; 61:1571–1576.
- Ades PA, Keteyian SJ, Balady GJ, et al. Cardiac rehabilitation exercise and self-care for chronic heart failure. JACC Heart Fail 2013; 1:540–547.
- O’Connor CM, Whellan DJ, Lee KL, et al; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009; 301:1439–1450.
- Rose EA, Gelijns AC, Moskowitz AJ, et al; Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001; 345:1435–1443.
- Givertz MM. Ventricular assist devices: important information for patients and families. Circulation 2011; 124:e305–e311.
- Daneshmand MA, Rajagopal K, Lima B, et al. Left ventricular assist device destination therapy versus extended criteria cardiac transplant. Ann Thorac Surg 2010; 89:1205–1210.
- Slaughter MS, Rogers JG, Milano CA, et al; HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009; 361:2241–2251.
- Aaronson KD, Slaughter MS, Miller LW, et al; HeartWare Ventricular Assist Device (HVAD) Bridge to Transplant ADVANCE Trial Investigators. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation 2012; 125:3191–3200.
- Katz AM. The “modern” view of heart failure: how did we get here? Circ Heart Fail 2008; 1:63–71.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128:e240–e327.
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 2015; 133:e38–e360.
- Goldberg LR, Jessup M. Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction. Circulation 2006; 113:2851–2860.
- Ammar KA, Jacobsen SJ, Mahoney DW, et al. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation 2007; 115:1563–1570.
- Tigerstedt R, Bergman PQ. Niere und Kreislauf. Skand Arch Physiol 1898; 8:223–271.
- Unger T, Li J. The role of the renin-angiotensin-aldosterone system in heart failure. J Renin Angiotensin Aldosterone Syst 2004; 5(suppl 1):S7–S10.
- Cohn JN, Levine TB, Francis GS, Goldsmith S. Neurohumoral control mechanisms in congestive heart failure. Am Heart J 1981; 102:509–514.
- von Lueder TG, Sangaralingham SJ, Wang BH, et al. Renin-angiotensin blockade combined with natriuretic peptide system augmentation: novel therapeutic concepts to combat heart failure. Circ Heart Fail 2013; 6:594–605.
- Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation 1991; 83:1849–1865.
- The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987; 316:1429–1435.
- The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325:293–302.
- Cohn JN, Tognoni G, Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345:1667–1675.
- McMurray JJ, Ostergren J, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 2003; 362:767–771.
- Pitt B, Zannad F, Remme WJ, et al.The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341:709–717.
- Pitt B, Remme W, Zannad F, et al; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348:1309–1321.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2010; 364:11–21.
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371:993–1004.
- Waagstein F, Hjalmarson A, Varnauskas E, Wallentin I. Effect of chronic beta-adrenergic receptor blockade in congestive cardiomyopathy. Br Heart J 1975; 37:1022–1036.
- Gheorghiade M, Colucci WS, Swedberg K. Beta-blockers in chronic heart failure. Circulation 2003; 107:1570–1575.
- Swedberg K, Hjalmarson A, Waagstein F, Wallentin I. Prolongation of survival in congestive cardiomyopathy by beta-receptor blockade. Lancet 1979; 1:1374–1376.
- Klapholz M. Beta-blocker use for the stages of heart failure. Mayo Clin Proc 2009; 84:718–729.
- Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med 1996; 334:1349–1355.
- The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999; 353:9–13.
- MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353:2001–2007.
- Poole-Wilson PA, Swedberg K, Cleland JG, et al; Carvedilol Or Metoprolol European Trial Investigators. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet 2003; 362:7–13.
- Kim H-N, Januzzi JL Jr. Natriuretic peptide testing in heart failure. Circulation 2011; 123:2015–2019.
- Pfisterer M, Buser P, Rickli H, et al; TIME-CHF Investigators. BNP-guided vs symptom-guided heart failure therapy: the Trial of Intensified vs Standard Medical Therapy in Elderly Patients with Congestive Heart Failure (TIME-CHF) randomized trial. JAMA 2009; 301:383–392.
- Lainchbury JG, Troughton RW, Strangman KM, et al. N-terminal pro–B-type natriuretic peptide-guided treatment for chronic heart failure: results From the BATTLESCARRED (NT-proBNP–Assisted Treatment To Lessen Serial Cardiac Readmissions and Death) trial. J Am Coll Cardiol 2009; 55:53–60.
- Januzzi JL Jr, Rehman SU, Mohammed AA, et al. Use of amino-terminal pro–B-type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J Am Coll Cardiol 2011; 58:1881-1889.
- Whellan DJ, Gaulden L, Gattis WA, et al. The benefit of implementing a heart failure disease management program. Arch Intern Med 2001; 161:2223–2228.
- Fonarow GC, Stevenson LW, Walden JA, et al. Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure. J Am Coll Cardiol 1997; 30:725–732.
- Grady KL, Dracup K, Kennedy G, et al. Team management of patients with heart filure: a statement for healthcare professionals from the Cardiovascular Nursing Council of the American Heart Association. Circulation 2000; 102:2443–2456.
- Kannel WB, Kannel C, Paffenbarger RS Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J 1987; 113:1489-1494.
- Colin P, Ghaleh B, Monnet X, Hittinger L, Berdeaux A. Effect of graded heart rate reduction with ivabradine on myocardial oxygen consumption and diastolic time in exercising dogs. J Pharmacol Exper Ther 2004; 308:236–240.
- Böhm M, Swedberg K, Komajda M, et al; SHIFT Investigators. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet 2010; 376:886–894.
- Batterman RC, DeGraff AC. Comparative study on the use of the purified digitalis glycosides, digoxin, digitoxin, and lanatoside C, for the management of ambulatory patients with congestive heart failure. Am Heart J 1947; 34:663–673.
- Ouyang AJ, Lv YN, Zhong HL, et al. Meta-analysis of digoxin use and risk of mortality in patients with atrial fibrillation. Am J Cardiol 2015; 115:901–906.
- Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997; 336:525–533.
- Aleong RG, Mulvahill MJ, Halder I, et al. Left ventricular dilatation increases the risk of ventricular arrhythmias in patients with reduced systolic function. J Am Heart Assoc 2015; 4:e001566.
- Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med 1996; 335:1933–1940.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Bardy GH, Lee KL, Mark DB, et al; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005; 352:225–237.
- Greenberg B, Mehra MR. All patients with heart failure and intraventricular conduction defect or dyssynchrony should not receive cardiac resynchronization therapy. Circulation 2006; 114:2685–2691.
- Linde C, Leclercq C, Rex S, et al. Long-term benefits of biventricular pacing in congestive heart failure: results from the MUltisite STimulation in cardiomyopathy (MUSTIC) study. J Am Coll Cardiol 2002; 40:111–118.
- Abraham WT, Fisher WG, Smith AL, et al; MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002; 346:1845–1853.
- Abraham WT, Adamson PB, Bourge RC, et al; CHAMPION Study Group. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011; 377:658–666.
- Loh JP, Barbash IM, Waksman R. Overview of the 2011 Food and Drug Administration Circulatory System Devices Panel of the Medical Devices Advisory Committee Meeting on the CardioMEMS Champion Heart Failure Monitoring System. J Am Coll Cardiol 2013; 61:1571–1576.
- Ades PA, Keteyian SJ, Balady GJ, et al. Cardiac rehabilitation exercise and self-care for chronic heart failure. JACC Heart Fail 2013; 1:540–547.
- O’Connor CM, Whellan DJ, Lee KL, et al; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009; 301:1439–1450.
- Rose EA, Gelijns AC, Moskowitz AJ, et al; Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001; 345:1435–1443.
- Givertz MM. Ventricular assist devices: important information for patients and families. Circulation 2011; 124:e305–e311.
- Daneshmand MA, Rajagopal K, Lima B, et al. Left ventricular assist device destination therapy versus extended criteria cardiac transplant. Ann Thorac Surg 2010; 89:1205–1210.
- Slaughter MS, Rogers JG, Milano CA, et al; HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009; 361:2241–2251.
- Aaronson KD, Slaughter MS, Miller LW, et al; HeartWare Ventricular Assist Device (HVAD) Bridge to Transplant ADVANCE Trial Investigators. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation 2012; 125:3191–3200.
- Katz AM. The “modern” view of heart failure: how did we get here? Circ Heart Fail 2008; 1:63–71.
KEY POINTS
- Most patients with systolic heart failure (also called heart failure with reduced ejection fraction) should receive either an angiotensin-converting enzyme inhibitor or an angiotensin II receptor blocker. Most should also receive a beta-blocker (carvedilol, metoprolol succinate, or bisoprolol).
- If symptoms persist or progress despite these treatments, an aldosterone receptor antagonist (spironolactone or eplerenone) is recommended.
- Since the publication of the ACC/AHA guidelines in 2013, the combination of sacubitril and valsartan has been approved, as has ivabradine.
- Patients with advanced heart failure should be identified early for consideration of resynchronization therapy, an implantable cardiac defibrillator, digoxin, a left ventricular assist device, or heart transplant.
- B-type natriuretic peptide levels can be used to guide therapy.
Evolution of heart failure management: Miles to go
The woods are lovely, dark and deep,
But I have promises to keep,
And miles to go before I sleep,
And miles to go before I sleep.
—Robert Frost, “Stopping by Woods on a Snowy Evening”1
Frost's words are simple yet elegant. They can be interpreted many ways. I see the allegory of life as a journey in this poem. The passage, like the woods, is beautiful, but there is a long, long way to go.
And so it is with the treatment of heart failure. There is beauty in our understanding of the syndrome’s physiologic complexities and natural history, and of effective treatments uncovered. Still, we’ve a monstrous climb ahead to get to the summit of this clinical challenge in order to start a real descent.
THE PAST, PRESENT, AND FUTURE OF HEART FAILURE THERAPY
Okwuosa et al,2 in this issue of the Journal, have capably summarized the ABCs of treating heart failure with reduced ejection fraction (also called systolic heart failure), approaching the subject from a perspective on past, present, and future therapies. They summarize heart failure interventions with a guideline-based philosophy, pointing out that these care paths are supposed to be evidence-based. They observe that in the 1960s the standard of care was digitalis, diuretics (furosemide first became available in 1967), and rest. That was about all we had for this problem.
There are now many drugs, devices, and operations that help patients with heart failure. But they never really cure the disease or, more aptly, the syndrome—and therapies are supposed to cure. This limitation of present therapies is important, given the disturbing epidemiology of heart failure, its economic cost, and the suffering of patients. That burden is well detailed.
In addition to curing, the overarching goals of treatment generally are to ameliorate distressing symptoms and to prevent comorbidities. In heart failure with reduced ejection fraction, we want to prevent premature death, stroke, myocardial infarction, congestive states, hospitalization, renal insufficiency, renal failure, cachexia, inanition, feebleness, and respiratory distress, among others.
The ABC mnemonic of Okwuosa et al will help caregivers remember the basics. It is important, however, to put algorithms into proper perspective and to look toward the future.
PROBLEMS WITH EVIDENCE-BASED MEDICINE
Several problems with our current heart failure treatments are rooted in how we perform clinical trials, arguably the premier method of determining truth in clinical practice and the foundation of evidence-based medicine.3,4
Do the trials represent real-world practice?
Were the clinical trials that led to regulatory approval and professional society endorsement of the therapies that we prescribe in our offices done in the same sorts of patients as those in our waiting rooms asking for help? Perhaps, for the most part, they have been. And thus, Okwuosa et al have crafted a work relevant to all of us and every patient.
But I believe there are major gaps in the types of participants enrolled in trials, eg, underrepresentation of certain racial and ethnic groups, not to mention the relative paucity of women. The very elderly (a rapidly growing population) have largely been ignored as well, and participants with significant renal insufficiency, anemia, and diabetes mellitus seem far fewer than what we deal with in a busy clinic.
In addition, Okwuosa et al focus only on patients with reduced left ventricular ejection fraction, a group that makes up only about half of the heart failure crowd.
What about quality of life and other important outcomes?
Clinical trials in heart failure with reduced ejection fraction have generally focused on major clinical end points (primarily, but not exclusively, mortality), to the exclusion of quality of life. Though sometimes included in trials, quality-of-life metrics generally get relegated to second-class seats or ‘tween-deck steerage. Perhaps that is because measuring quality of life can be time-consuming and difficult.
Yet, in the words of sociologist William Bruce Cameron, not everything that counts can be counted, and not everything that can be counted counts. That goes for quality of life.
Lies, damned lies, and P values
Quandaries in data management and analysis include what to do about trial dropouts, study power, precision of statistical analysis, intention-to-treat principles, and choice of the P value that defines significance (or not) for any end point observation. Of course, there are myriad sophisticated mathematical and statistical reasons to justify why we don’t simply count on-treatment participants or allow imputation of results when patients or results drop out, forcing us to worship at the altar of P < .05.
A review of the P value concept5 recently appeared with an accompanying editorial by Kyriacou6 that concluded that “the automatic application of dichotomized hypothesis testing based on prearranged levels of statistical significance should be substituted with a more complex process using effect estimates, confidence intervals, and even P values, thereby permitting scientists, statisticians, and clinicians to use their own inferential capabilities to assign scientific significance.”6
How many great treatments have we tossed out because of rigid reliance on old-fashioned approaches to determining therapeutic evidence? Many treatments studied have had great results in a minority of patients in clinical trials but did not have a major positive (or negative) impact on the overall cohort (with lack of primary end point statistical significance). And what to do when the primary end point is a neutral or negative one but secondary end points are positive? Why not focus more attention on those patients benefiting from an intervention despite the overall results of any trial?
Dilemmas of trials
Other issues are that clinical trials cost too much, and that recruitment and follow-up take too long. Intercurrent therapies (and guidelines) can emerge that jeopardize the trial itself or make observations untimely. The dilemma of stacking therapies one on top of another, often making patient compliance impossible, is another problem with clinical trials. Yet this is how we get to the ABCs.
A NEW WAY TO DO TRIALS
The information provided by Okwuosa et al is useful and encouraging, but too many gaps exist in our heart failure therapies to permit us to celebrate with exuberance. Too many patients still suffer, too many die too young, and the costs are still too great.
Perhaps the future of therapeutic development should embrace different and better ways to demonstrate real value (relying on the equation of value equals outcomes meaningful to patients, divided by cost) of therapies, including the old, the new, the trashed and the underdeveloped. More creative data analysis to reexamine the current tools on the shelf and the ones tried but discarded is essential.
A position paper from the Cardiovascular Round Table of the European Society of Cardiology concluded that “a coordinated effort involving academia, regulators, industry and payors will help to foster better and more effective conduct of clinical cardiovascular trials, supporting earlier availability of innovative therapies and better management of cardiovascular diseases.”7
Lauer and D’Agostino,8 also in an editorial, argued for innovative methods of doing clinical trials and discovering truth about therapies that are applicable to the future of developing treatments for heart failure with reduced ejection fraction. They noted that “the randomized registry trial represents a disruptive technology” and wondered if it will be “given serious consideration as a way to resolve the recognized limitations of current clinical-trial design.”8
Indeed, conducting megatrials with existing megadatabases using a registry format could help. Registries emerging from early adaptive trial design efforts, particularly when Bayesian analysis theory is applied, might help inform clinical experience faster and more efficiently. Bayesian analysis is a statistical approach that attempts to estimate parameters of an underlying distribution of events in an ongoing fashion based on the observed distribution. A clinical trial of stem cell therapies could, at the end of the trial, be turned into a multicenter registry that would continue to inform us about the more real-world application of newer treatment approaches.
Though the therapeutic cupboard for heart failure is certainly not bare, as Okwuosa et al point out, it is wanting. Let’s look for new therapeutic ABCs differently. We should be attacking the real challenge—curing the disease processes that cause the syndrome. Yes, there are miles to go before we sleep.
- Frost R. Stopping by Woods on a Snowy Evening. In: New Hampshire. New York, Henry Holt, 1923.
- Okwuoso IS, Ojeifo O, Nwabueze C, et al. The ABCs of managing systolic heart failure: the past, present and future. Cleve Clin J Med 2016; 83:753–765.
- Samman Tahhan A, Vaduganathan M, Kelkar A, et al. Trends in heart failure clinical trials from 2001–2012. J Card Fail 2016; 22:171–179.
- Cohn JN. Trials and tribulations. J Card Fail 2016; 22:180–181.
- Chavalarias D, Wallach JD, Li AH, Ioannidis JP. Evolution of reporting P values in the biomedical literature, 1990–2015. JAMA 2016; 315:1141–1148.
- Kyriacou DN. The enduring evolution of the P value. JAMA 2016; 315:1113–1115.
- Jackson N, Atar D, Borentain M, et al. Improving clinical trials for cardiovascular diseases: a position paper from the Cardiovascular Round Table of the European Society of Cardiology. Eur Heart J 2016; 37:747–754.
- Lauer MS, D’Agostino RB Sr. The randomized registry trial—the next disruptive technology in clinical research? N Engl J Med 2013; 369:1579–1581.
The woods are lovely, dark and deep,
But I have promises to keep,
And miles to go before I sleep,
And miles to go before I sleep.
—Robert Frost, “Stopping by Woods on a Snowy Evening”1
Frost's words are simple yet elegant. They can be interpreted many ways. I see the allegory of life as a journey in this poem. The passage, like the woods, is beautiful, but there is a long, long way to go.
And so it is with the treatment of heart failure. There is beauty in our understanding of the syndrome’s physiologic complexities and natural history, and of effective treatments uncovered. Still, we’ve a monstrous climb ahead to get to the summit of this clinical challenge in order to start a real descent.
THE PAST, PRESENT, AND FUTURE OF HEART FAILURE THERAPY
Okwuosa et al,2 in this issue of the Journal, have capably summarized the ABCs of treating heart failure with reduced ejection fraction (also called systolic heart failure), approaching the subject from a perspective on past, present, and future therapies. They summarize heart failure interventions with a guideline-based philosophy, pointing out that these care paths are supposed to be evidence-based. They observe that in the 1960s the standard of care was digitalis, diuretics (furosemide first became available in 1967), and rest. That was about all we had for this problem.
There are now many drugs, devices, and operations that help patients with heart failure. But they never really cure the disease or, more aptly, the syndrome—and therapies are supposed to cure. This limitation of present therapies is important, given the disturbing epidemiology of heart failure, its economic cost, and the suffering of patients. That burden is well detailed.
In addition to curing, the overarching goals of treatment generally are to ameliorate distressing symptoms and to prevent comorbidities. In heart failure with reduced ejection fraction, we want to prevent premature death, stroke, myocardial infarction, congestive states, hospitalization, renal insufficiency, renal failure, cachexia, inanition, feebleness, and respiratory distress, among others.
The ABC mnemonic of Okwuosa et al will help caregivers remember the basics. It is important, however, to put algorithms into proper perspective and to look toward the future.
PROBLEMS WITH EVIDENCE-BASED MEDICINE
Several problems with our current heart failure treatments are rooted in how we perform clinical trials, arguably the premier method of determining truth in clinical practice and the foundation of evidence-based medicine.3,4
Do the trials represent real-world practice?
Were the clinical trials that led to regulatory approval and professional society endorsement of the therapies that we prescribe in our offices done in the same sorts of patients as those in our waiting rooms asking for help? Perhaps, for the most part, they have been. And thus, Okwuosa et al have crafted a work relevant to all of us and every patient.
But I believe there are major gaps in the types of participants enrolled in trials, eg, underrepresentation of certain racial and ethnic groups, not to mention the relative paucity of women. The very elderly (a rapidly growing population) have largely been ignored as well, and participants with significant renal insufficiency, anemia, and diabetes mellitus seem far fewer than what we deal with in a busy clinic.
In addition, Okwuosa et al focus only on patients with reduced left ventricular ejection fraction, a group that makes up only about half of the heart failure crowd.
What about quality of life and other important outcomes?
Clinical trials in heart failure with reduced ejection fraction have generally focused on major clinical end points (primarily, but not exclusively, mortality), to the exclusion of quality of life. Though sometimes included in trials, quality-of-life metrics generally get relegated to second-class seats or ‘tween-deck steerage. Perhaps that is because measuring quality of life can be time-consuming and difficult.
Yet, in the words of sociologist William Bruce Cameron, not everything that counts can be counted, and not everything that can be counted counts. That goes for quality of life.
Lies, damned lies, and P values
Quandaries in data management and analysis include what to do about trial dropouts, study power, precision of statistical analysis, intention-to-treat principles, and choice of the P value that defines significance (or not) for any end point observation. Of course, there are myriad sophisticated mathematical and statistical reasons to justify why we don’t simply count on-treatment participants or allow imputation of results when patients or results drop out, forcing us to worship at the altar of P < .05.
A review of the P value concept5 recently appeared with an accompanying editorial by Kyriacou6 that concluded that “the automatic application of dichotomized hypothesis testing based on prearranged levels of statistical significance should be substituted with a more complex process using effect estimates, confidence intervals, and even P values, thereby permitting scientists, statisticians, and clinicians to use their own inferential capabilities to assign scientific significance.”6
How many great treatments have we tossed out because of rigid reliance on old-fashioned approaches to determining therapeutic evidence? Many treatments studied have had great results in a minority of patients in clinical trials but did not have a major positive (or negative) impact on the overall cohort (with lack of primary end point statistical significance). And what to do when the primary end point is a neutral or negative one but secondary end points are positive? Why not focus more attention on those patients benefiting from an intervention despite the overall results of any trial?
Dilemmas of trials
Other issues are that clinical trials cost too much, and that recruitment and follow-up take too long. Intercurrent therapies (and guidelines) can emerge that jeopardize the trial itself or make observations untimely. The dilemma of stacking therapies one on top of another, often making patient compliance impossible, is another problem with clinical trials. Yet this is how we get to the ABCs.
A NEW WAY TO DO TRIALS
The information provided by Okwuosa et al is useful and encouraging, but too many gaps exist in our heart failure therapies to permit us to celebrate with exuberance. Too many patients still suffer, too many die too young, and the costs are still too great.
Perhaps the future of therapeutic development should embrace different and better ways to demonstrate real value (relying on the equation of value equals outcomes meaningful to patients, divided by cost) of therapies, including the old, the new, the trashed and the underdeveloped. More creative data analysis to reexamine the current tools on the shelf and the ones tried but discarded is essential.
A position paper from the Cardiovascular Round Table of the European Society of Cardiology concluded that “a coordinated effort involving academia, regulators, industry and payors will help to foster better and more effective conduct of clinical cardiovascular trials, supporting earlier availability of innovative therapies and better management of cardiovascular diseases.”7
Lauer and D’Agostino,8 also in an editorial, argued for innovative methods of doing clinical trials and discovering truth about therapies that are applicable to the future of developing treatments for heart failure with reduced ejection fraction. They noted that “the randomized registry trial represents a disruptive technology” and wondered if it will be “given serious consideration as a way to resolve the recognized limitations of current clinical-trial design.”8
Indeed, conducting megatrials with existing megadatabases using a registry format could help. Registries emerging from early adaptive trial design efforts, particularly when Bayesian analysis theory is applied, might help inform clinical experience faster and more efficiently. Bayesian analysis is a statistical approach that attempts to estimate parameters of an underlying distribution of events in an ongoing fashion based on the observed distribution. A clinical trial of stem cell therapies could, at the end of the trial, be turned into a multicenter registry that would continue to inform us about the more real-world application of newer treatment approaches.
Though the therapeutic cupboard for heart failure is certainly not bare, as Okwuosa et al point out, it is wanting. Let’s look for new therapeutic ABCs differently. We should be attacking the real challenge—curing the disease processes that cause the syndrome. Yes, there are miles to go before we sleep.
The woods are lovely, dark and deep,
But I have promises to keep,
And miles to go before I sleep,
And miles to go before I sleep.
—Robert Frost, “Stopping by Woods on a Snowy Evening”1
Frost's words are simple yet elegant. They can be interpreted many ways. I see the allegory of life as a journey in this poem. The passage, like the woods, is beautiful, but there is a long, long way to go.
And so it is with the treatment of heart failure. There is beauty in our understanding of the syndrome’s physiologic complexities and natural history, and of effective treatments uncovered. Still, we’ve a monstrous climb ahead to get to the summit of this clinical challenge in order to start a real descent.
THE PAST, PRESENT, AND FUTURE OF HEART FAILURE THERAPY
Okwuosa et al,2 in this issue of the Journal, have capably summarized the ABCs of treating heart failure with reduced ejection fraction (also called systolic heart failure), approaching the subject from a perspective on past, present, and future therapies. They summarize heart failure interventions with a guideline-based philosophy, pointing out that these care paths are supposed to be evidence-based. They observe that in the 1960s the standard of care was digitalis, diuretics (furosemide first became available in 1967), and rest. That was about all we had for this problem.
There are now many drugs, devices, and operations that help patients with heart failure. But they never really cure the disease or, more aptly, the syndrome—and therapies are supposed to cure. This limitation of present therapies is important, given the disturbing epidemiology of heart failure, its economic cost, and the suffering of patients. That burden is well detailed.
In addition to curing, the overarching goals of treatment generally are to ameliorate distressing symptoms and to prevent comorbidities. In heart failure with reduced ejection fraction, we want to prevent premature death, stroke, myocardial infarction, congestive states, hospitalization, renal insufficiency, renal failure, cachexia, inanition, feebleness, and respiratory distress, among others.
The ABC mnemonic of Okwuosa et al will help caregivers remember the basics. It is important, however, to put algorithms into proper perspective and to look toward the future.
PROBLEMS WITH EVIDENCE-BASED MEDICINE
Several problems with our current heart failure treatments are rooted in how we perform clinical trials, arguably the premier method of determining truth in clinical practice and the foundation of evidence-based medicine.3,4
Do the trials represent real-world practice?
Were the clinical trials that led to regulatory approval and professional society endorsement of the therapies that we prescribe in our offices done in the same sorts of patients as those in our waiting rooms asking for help? Perhaps, for the most part, they have been. And thus, Okwuosa et al have crafted a work relevant to all of us and every patient.
But I believe there are major gaps in the types of participants enrolled in trials, eg, underrepresentation of certain racial and ethnic groups, not to mention the relative paucity of women. The very elderly (a rapidly growing population) have largely been ignored as well, and participants with significant renal insufficiency, anemia, and diabetes mellitus seem far fewer than what we deal with in a busy clinic.
In addition, Okwuosa et al focus only on patients with reduced left ventricular ejection fraction, a group that makes up only about half of the heart failure crowd.
What about quality of life and other important outcomes?
Clinical trials in heart failure with reduced ejection fraction have generally focused on major clinical end points (primarily, but not exclusively, mortality), to the exclusion of quality of life. Though sometimes included in trials, quality-of-life metrics generally get relegated to second-class seats or ‘tween-deck steerage. Perhaps that is because measuring quality of life can be time-consuming and difficult.
Yet, in the words of sociologist William Bruce Cameron, not everything that counts can be counted, and not everything that can be counted counts. That goes for quality of life.
Lies, damned lies, and P values
Quandaries in data management and analysis include what to do about trial dropouts, study power, precision of statistical analysis, intention-to-treat principles, and choice of the P value that defines significance (or not) for any end point observation. Of course, there are myriad sophisticated mathematical and statistical reasons to justify why we don’t simply count on-treatment participants or allow imputation of results when patients or results drop out, forcing us to worship at the altar of P < .05.
A review of the P value concept5 recently appeared with an accompanying editorial by Kyriacou6 that concluded that “the automatic application of dichotomized hypothesis testing based on prearranged levels of statistical significance should be substituted with a more complex process using effect estimates, confidence intervals, and even P values, thereby permitting scientists, statisticians, and clinicians to use their own inferential capabilities to assign scientific significance.”6
How many great treatments have we tossed out because of rigid reliance on old-fashioned approaches to determining therapeutic evidence? Many treatments studied have had great results in a minority of patients in clinical trials but did not have a major positive (or negative) impact on the overall cohort (with lack of primary end point statistical significance). And what to do when the primary end point is a neutral or negative one but secondary end points are positive? Why not focus more attention on those patients benefiting from an intervention despite the overall results of any trial?
Dilemmas of trials
Other issues are that clinical trials cost too much, and that recruitment and follow-up take too long. Intercurrent therapies (and guidelines) can emerge that jeopardize the trial itself or make observations untimely. The dilemma of stacking therapies one on top of another, often making patient compliance impossible, is another problem with clinical trials. Yet this is how we get to the ABCs.
A NEW WAY TO DO TRIALS
The information provided by Okwuosa et al is useful and encouraging, but too many gaps exist in our heart failure therapies to permit us to celebrate with exuberance. Too many patients still suffer, too many die too young, and the costs are still too great.
Perhaps the future of therapeutic development should embrace different and better ways to demonstrate real value (relying on the equation of value equals outcomes meaningful to patients, divided by cost) of therapies, including the old, the new, the trashed and the underdeveloped. More creative data analysis to reexamine the current tools on the shelf and the ones tried but discarded is essential.
A position paper from the Cardiovascular Round Table of the European Society of Cardiology concluded that “a coordinated effort involving academia, regulators, industry and payors will help to foster better and more effective conduct of clinical cardiovascular trials, supporting earlier availability of innovative therapies and better management of cardiovascular diseases.”7
Lauer and D’Agostino,8 also in an editorial, argued for innovative methods of doing clinical trials and discovering truth about therapies that are applicable to the future of developing treatments for heart failure with reduced ejection fraction. They noted that “the randomized registry trial represents a disruptive technology” and wondered if it will be “given serious consideration as a way to resolve the recognized limitations of current clinical-trial design.”8
Indeed, conducting megatrials with existing megadatabases using a registry format could help. Registries emerging from early adaptive trial design efforts, particularly when Bayesian analysis theory is applied, might help inform clinical experience faster and more efficiently. Bayesian analysis is a statistical approach that attempts to estimate parameters of an underlying distribution of events in an ongoing fashion based on the observed distribution. A clinical trial of stem cell therapies could, at the end of the trial, be turned into a multicenter registry that would continue to inform us about the more real-world application of newer treatment approaches.
Though the therapeutic cupboard for heart failure is certainly not bare, as Okwuosa et al point out, it is wanting. Let’s look for new therapeutic ABCs differently. We should be attacking the real challenge—curing the disease processes that cause the syndrome. Yes, there are miles to go before we sleep.
- Frost R. Stopping by Woods on a Snowy Evening. In: New Hampshire. New York, Henry Holt, 1923.
- Okwuoso IS, Ojeifo O, Nwabueze C, et al. The ABCs of managing systolic heart failure: the past, present and future. Cleve Clin J Med 2016; 83:753–765.
- Samman Tahhan A, Vaduganathan M, Kelkar A, et al. Trends in heart failure clinical trials from 2001–2012. J Card Fail 2016; 22:171–179.
- Cohn JN. Trials and tribulations. J Card Fail 2016; 22:180–181.
- Chavalarias D, Wallach JD, Li AH, Ioannidis JP. Evolution of reporting P values in the biomedical literature, 1990–2015. JAMA 2016; 315:1141–1148.
- Kyriacou DN. The enduring evolution of the P value. JAMA 2016; 315:1113–1115.
- Jackson N, Atar D, Borentain M, et al. Improving clinical trials for cardiovascular diseases: a position paper from the Cardiovascular Round Table of the European Society of Cardiology. Eur Heart J 2016; 37:747–754.
- Lauer MS, D’Agostino RB Sr. The randomized registry trial—the next disruptive technology in clinical research? N Engl J Med 2013; 369:1579–1581.
- Frost R. Stopping by Woods on a Snowy Evening. In: New Hampshire. New York, Henry Holt, 1923.
- Okwuoso IS, Ojeifo O, Nwabueze C, et al. The ABCs of managing systolic heart failure: the past, present and future. Cleve Clin J Med 2016; 83:753–765.
- Samman Tahhan A, Vaduganathan M, Kelkar A, et al. Trends in heart failure clinical trials from 2001–2012. J Card Fail 2016; 22:171–179.
- Cohn JN. Trials and tribulations. J Card Fail 2016; 22:180–181.
- Chavalarias D, Wallach JD, Li AH, Ioannidis JP. Evolution of reporting P values in the biomedical literature, 1990–2015. JAMA 2016; 315:1141–1148.
- Kyriacou DN. The enduring evolution of the P value. JAMA 2016; 315:1113–1115.
- Jackson N, Atar D, Borentain M, et al. Improving clinical trials for cardiovascular diseases: a position paper from the Cardiovascular Round Table of the European Society of Cardiology. Eur Heart J 2016; 37:747–754.
- Lauer MS, D’Agostino RB Sr. The randomized registry trial—the next disruptive technology in clinical research? N Engl J Med 2013; 369:1579–1581.
The evolution of office notes and the electronic medical record: The CAPS note
Until the advent of the electronic medical record (EMR), patient charts were filled with handwritten notes documenting visits to the office and read in linear fashion, starting with the patient’s perspective of the problem, then the objective findings of the physical examination, supporting objective data, and finally, the physician’s assessment and treatment plan.
The reliable subjective, objective, assessment, plan (SOAP) approach to notes first advocated by Lawrence Weed in the 1960s did a remarkable job of conveying the physician’s thought process, supporting data, and conclusions.1,2 The notes were brief by necessity, as the physician did not want to spend time writing extraneous information.
In the age of the EMR, large quantities of data are included in the patient notes that have no connection to or do not clearly convey the physician’s thought process. In 2013, 78% of office-based physicians were using EMRs, an increase from 18% in 2001 and an adoption rate accelerated by federal government policies.3,4 But many physicians still do not feel competent reading or writing notes in an EMR and still prefer to read succinct narrative notes.5
This problem is not unique to seasoned physicians. Medical students are also failing to learn how to appropriately document office visits in the EMR, as 52% of medical schools prohibit them from writing in patient charts.6
As a result, we believed that a reassessment of Dr. Weed’s problem-oriented approach to the medical record was required to streamline the EMR and facilitate the way information is conveyed between providers of the patient’s care. Too often, large quantities of laboratory, radiographic, and pathology results are dumped into the record, burying pertinent information about the physician’s thought process, assessment, and evaluation and treatment plan and making it difficult to quickly and efficiently determine the plan.
We recently adopted an approach to office notes that is a modification of the SOAP note. While physicians often gather subjective, objective, and laboratory information to deductively formulate a diagnosis, it is not necessary to document it in the traditional deductive format in the EMR when the information is readily accessible in other areas of the record. Furthermore, a deductive format in the modern EMR produces excessively lengthy notes that require pages of screen scrolling to find the key elements required for effective patient care. This is time-consuming and is a daily obstruction to patient care.
The format that we have been using for almost 10 years still allows the physician to adhere to the problem-oriented medical note philosophy. We call it the CAPS note, which stands for concern, assessment, plan, and supporting data. This approach allows others involved in the patient’s care to efficiently extract critical components (assessment and plan for a specifically stated problem) while still allowing the inclusion of supporting data for reference and for coding and billing.
The structure of the CAPS note is:
- Concern: The primary purpose of the patient’s visit, including the history of the present illness, as conveyed by the patient, and the current status of the concern.
- Assessment: A succinct definition of the patient’s concern along with an accompanying medical diagnosis.
- Plan: The clinician’s immediate and long-term intentions for addressing the patient’s concern or condition.
- Supporting objective and subjective information: All supporting objective data, starting with the physical examination, then the results of laboratory and radiographic tests, and any other information that contributed to the clinician’s medical reasoning. Then, subjective information is included, such as the patient’s past medical, surgical, family, and social histories; current medications; allergies; and a comprehensive review of systems.
This structure keeps the most important information at the top when the encounter is opened on the computer screen and eliminates the need for unnecessary scrolling and searching, not to mention frustration and delays in patient care. Other less pertinent information appears toward the bottom of the record.
THE APSO NOTE VS THE SOAP NOTE
Frustration over the difficulty of finding the most pertinent information in the EMR—the assessment and the plan—has led others to propose a rearrangement of the traditional SOAP note. The APSO (assessment, plan, subjective, objective) note7,8 was created for inpatient daily progress notes, a situation in which the patient’s concern is unlikely to change dramatically on a daily basis and was not intended for use in outpatient clinics.8 While the APSO format does allow colleagues rapid access to the physician’s assessment and plan, it abandons the patient-centered approach of Dr. Weed’s problem-oriented medical record in that it makes it more difficult to find why the patient initially sought care, how long the patient has had the problem, or if there were prior attempts to treat it. These critical details are buried in the bowels of the note.
The advantage of the CAPS note (Table 1) is that it retains the patient-centered, problem-oriented spirit of the SOAP format, while moving potentially supportive yet distracting data fields to later in the note. Thus it is applicable to inpatient and outpatient settings.
In the inpatient setting, the fields remain in the same order, but the chief complaint is often the admitting diagnosis or surgical procedure, followed by a quick line on the interval history. The assessment and plan can then follow in much the same way as it would in the outpatient setting, and below that are the patient’s daily laboratory results, radiographic studies, physical examination findings, and any other relevant supporting data. This format allows rapid access to critical information needed by either consultants or cross-covering practitioners who primarily want to know why the patient was admitted, the status, and the primary team’s plan.
ANY TEMPLATE HAS LIMITATIONS
Any standardized template for progress notes in the EMR has limitations. The CAPS format would be easier for a hospital-based physician, who typically addresses one or a small number of concerns, than for an office-based general practitioner who may have to address a multitude of comorbidities in a single visit.
Also, different physicians use the EMR differently. For example, a survey of 1,088 physicians found that 60% of primary care physicians used templates (60%) vs only 34% of specialists, and that 38% of specialists relied mainly on dictation.9
The CAPS approach to the office visit note offers a blend of a template and free text, either typed or dictated, while keeping a structured format that permits others participating in the patient’s care to easily extract desired information. The template can easily be brought up in the patient’s chart, then by either typing or using voice-recognition software, the patient’s chief complaint, history of the present illness, assessment, and plan can be easily completed.
The CAPS format should continue to allow notes to fulfill medicolegal and billing obligations, but without cluttering true clinical reasoning. As more institutions adopt an open-notes policy, permitting patients to freely browse their own medical records, patients will benefit from a clearly structured clinical note that focuses on their problem and the practitioner’s solution. This provides patients a sense of validation and reassurance that the note starts with their concern and history, followed by the practitioner’s assessment and plan, so they can easily affirm that they were accurately heard and can identify the diagnosis given to them by the medical practitioner and the plan moving forward.
Since a return to succinct, albeit often illegible, handwritten clinic notes is impossible, our proposed method of documenting a clinic visit embraces the EMR with a concise yet comprehensive clinic note.
- Jacobs L. Interview with Lawrence Weed, MD—the father of the problem-oriented medical record looks ahead. Perm J 2009; 13:84–89.
- Cameron S, Turtle-Son I. Learning to write case notes using the SOAP format. JCD 2002; 80:286–292.
- Hsiao CJ, Hing E. Use and characteristics of electronic health record systems among office-based physician practices: United States, 2001-2013. NCHS Data Brief 2014; 143:1–8.
- Centers for Medicare & Medicaid Services (CMS). EHR incentive program. www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/Basics.html. Accessed April 28, 2016.
- Han H, Lopp L. Writing and reading in the electronic health record: an entirely new world. Med Educ Online 2013; 18:1–7.
- Hammoud MM, Dalrymple JL, Christner JG, et al. Medical student documentation in electronic health records: a collaborative statement from the Alliance for Clinical Education. Teach Learn Med 2012; 24:257–266.
- Shoolin J, Ozeran L, Hamann C, Bria W 2nd. Association of Medical Directors of Information Systems consensus on inpatient electronic health record documentation. Appl Clin Inform 2013; 4:293–303.
- Hahn JS, Bernstein JA, McKenzie RB, King BJ, Longhurst CA. Rapid implementation of inpatient electronic physician documentation at an academic hospital. Appl Clin Inform 2012; 3:175–185.
- Pollard SE, Neri PM, Wilcox AR, et al. How physicians document outpatient visit notes in an electronic health record. Int J Med Inform 2013; 82:39–46.
Until the advent of the electronic medical record (EMR), patient charts were filled with handwritten notes documenting visits to the office and read in linear fashion, starting with the patient’s perspective of the problem, then the objective findings of the physical examination, supporting objective data, and finally, the physician’s assessment and treatment plan.
The reliable subjective, objective, assessment, plan (SOAP) approach to notes first advocated by Lawrence Weed in the 1960s did a remarkable job of conveying the physician’s thought process, supporting data, and conclusions.1,2 The notes were brief by necessity, as the physician did not want to spend time writing extraneous information.
In the age of the EMR, large quantities of data are included in the patient notes that have no connection to or do not clearly convey the physician’s thought process. In 2013, 78% of office-based physicians were using EMRs, an increase from 18% in 2001 and an adoption rate accelerated by federal government policies.3,4 But many physicians still do not feel competent reading or writing notes in an EMR and still prefer to read succinct narrative notes.5
This problem is not unique to seasoned physicians. Medical students are also failing to learn how to appropriately document office visits in the EMR, as 52% of medical schools prohibit them from writing in patient charts.6
As a result, we believed that a reassessment of Dr. Weed’s problem-oriented approach to the medical record was required to streamline the EMR and facilitate the way information is conveyed between providers of the patient’s care. Too often, large quantities of laboratory, radiographic, and pathology results are dumped into the record, burying pertinent information about the physician’s thought process, assessment, and evaluation and treatment plan and making it difficult to quickly and efficiently determine the plan.
We recently adopted an approach to office notes that is a modification of the SOAP note. While physicians often gather subjective, objective, and laboratory information to deductively formulate a diagnosis, it is not necessary to document it in the traditional deductive format in the EMR when the information is readily accessible in other areas of the record. Furthermore, a deductive format in the modern EMR produces excessively lengthy notes that require pages of screen scrolling to find the key elements required for effective patient care. This is time-consuming and is a daily obstruction to patient care.
The format that we have been using for almost 10 years still allows the physician to adhere to the problem-oriented medical note philosophy. We call it the CAPS note, which stands for concern, assessment, plan, and supporting data. This approach allows others involved in the patient’s care to efficiently extract critical components (assessment and plan for a specifically stated problem) while still allowing the inclusion of supporting data for reference and for coding and billing.
The structure of the CAPS note is:
- Concern: The primary purpose of the patient’s visit, including the history of the present illness, as conveyed by the patient, and the current status of the concern.
- Assessment: A succinct definition of the patient’s concern along with an accompanying medical diagnosis.
- Plan: The clinician’s immediate and long-term intentions for addressing the patient’s concern or condition.
- Supporting objective and subjective information: All supporting objective data, starting with the physical examination, then the results of laboratory and radiographic tests, and any other information that contributed to the clinician’s medical reasoning. Then, subjective information is included, such as the patient’s past medical, surgical, family, and social histories; current medications; allergies; and a comprehensive review of systems.
This structure keeps the most important information at the top when the encounter is opened on the computer screen and eliminates the need for unnecessary scrolling and searching, not to mention frustration and delays in patient care. Other less pertinent information appears toward the bottom of the record.
THE APSO NOTE VS THE SOAP NOTE
Frustration over the difficulty of finding the most pertinent information in the EMR—the assessment and the plan—has led others to propose a rearrangement of the traditional SOAP note. The APSO (assessment, plan, subjective, objective) note7,8 was created for inpatient daily progress notes, a situation in which the patient’s concern is unlikely to change dramatically on a daily basis and was not intended for use in outpatient clinics.8 While the APSO format does allow colleagues rapid access to the physician’s assessment and plan, it abandons the patient-centered approach of Dr. Weed’s problem-oriented medical record in that it makes it more difficult to find why the patient initially sought care, how long the patient has had the problem, or if there were prior attempts to treat it. These critical details are buried in the bowels of the note.
The advantage of the CAPS note (Table 1) is that it retains the patient-centered, problem-oriented spirit of the SOAP format, while moving potentially supportive yet distracting data fields to later in the note. Thus it is applicable to inpatient and outpatient settings.
In the inpatient setting, the fields remain in the same order, but the chief complaint is often the admitting diagnosis or surgical procedure, followed by a quick line on the interval history. The assessment and plan can then follow in much the same way as it would in the outpatient setting, and below that are the patient’s daily laboratory results, radiographic studies, physical examination findings, and any other relevant supporting data. This format allows rapid access to critical information needed by either consultants or cross-covering practitioners who primarily want to know why the patient was admitted, the status, and the primary team’s plan.
ANY TEMPLATE HAS LIMITATIONS
Any standardized template for progress notes in the EMR has limitations. The CAPS format would be easier for a hospital-based physician, who typically addresses one or a small number of concerns, than for an office-based general practitioner who may have to address a multitude of comorbidities in a single visit.
Also, different physicians use the EMR differently. For example, a survey of 1,088 physicians found that 60% of primary care physicians used templates (60%) vs only 34% of specialists, and that 38% of specialists relied mainly on dictation.9
The CAPS approach to the office visit note offers a blend of a template and free text, either typed or dictated, while keeping a structured format that permits others participating in the patient’s care to easily extract desired information. The template can easily be brought up in the patient’s chart, then by either typing or using voice-recognition software, the patient’s chief complaint, history of the present illness, assessment, and plan can be easily completed.
The CAPS format should continue to allow notes to fulfill medicolegal and billing obligations, but without cluttering true clinical reasoning. As more institutions adopt an open-notes policy, permitting patients to freely browse their own medical records, patients will benefit from a clearly structured clinical note that focuses on their problem and the practitioner’s solution. This provides patients a sense of validation and reassurance that the note starts with their concern and history, followed by the practitioner’s assessment and plan, so they can easily affirm that they were accurately heard and can identify the diagnosis given to them by the medical practitioner and the plan moving forward.
Since a return to succinct, albeit often illegible, handwritten clinic notes is impossible, our proposed method of documenting a clinic visit embraces the EMR with a concise yet comprehensive clinic note.
Until the advent of the electronic medical record (EMR), patient charts were filled with handwritten notes documenting visits to the office and read in linear fashion, starting with the patient’s perspective of the problem, then the objective findings of the physical examination, supporting objective data, and finally, the physician’s assessment and treatment plan.
The reliable subjective, objective, assessment, plan (SOAP) approach to notes first advocated by Lawrence Weed in the 1960s did a remarkable job of conveying the physician’s thought process, supporting data, and conclusions.1,2 The notes were brief by necessity, as the physician did not want to spend time writing extraneous information.
In the age of the EMR, large quantities of data are included in the patient notes that have no connection to or do not clearly convey the physician’s thought process. In 2013, 78% of office-based physicians were using EMRs, an increase from 18% in 2001 and an adoption rate accelerated by federal government policies.3,4 But many physicians still do not feel competent reading or writing notes in an EMR and still prefer to read succinct narrative notes.5
This problem is not unique to seasoned physicians. Medical students are also failing to learn how to appropriately document office visits in the EMR, as 52% of medical schools prohibit them from writing in patient charts.6
As a result, we believed that a reassessment of Dr. Weed’s problem-oriented approach to the medical record was required to streamline the EMR and facilitate the way information is conveyed between providers of the patient’s care. Too often, large quantities of laboratory, radiographic, and pathology results are dumped into the record, burying pertinent information about the physician’s thought process, assessment, and evaluation and treatment plan and making it difficult to quickly and efficiently determine the plan.
We recently adopted an approach to office notes that is a modification of the SOAP note. While physicians often gather subjective, objective, and laboratory information to deductively formulate a diagnosis, it is not necessary to document it in the traditional deductive format in the EMR when the information is readily accessible in other areas of the record. Furthermore, a deductive format in the modern EMR produces excessively lengthy notes that require pages of screen scrolling to find the key elements required for effective patient care. This is time-consuming and is a daily obstruction to patient care.
The format that we have been using for almost 10 years still allows the physician to adhere to the problem-oriented medical note philosophy. We call it the CAPS note, which stands for concern, assessment, plan, and supporting data. This approach allows others involved in the patient’s care to efficiently extract critical components (assessment and plan for a specifically stated problem) while still allowing the inclusion of supporting data for reference and for coding and billing.
The structure of the CAPS note is:
- Concern: The primary purpose of the patient’s visit, including the history of the present illness, as conveyed by the patient, and the current status of the concern.
- Assessment: A succinct definition of the patient’s concern along with an accompanying medical diagnosis.
- Plan: The clinician’s immediate and long-term intentions for addressing the patient’s concern or condition.
- Supporting objective and subjective information: All supporting objective data, starting with the physical examination, then the results of laboratory and radiographic tests, and any other information that contributed to the clinician’s medical reasoning. Then, subjective information is included, such as the patient’s past medical, surgical, family, and social histories; current medications; allergies; and a comprehensive review of systems.
This structure keeps the most important information at the top when the encounter is opened on the computer screen and eliminates the need for unnecessary scrolling and searching, not to mention frustration and delays in patient care. Other less pertinent information appears toward the bottom of the record.
THE APSO NOTE VS THE SOAP NOTE
Frustration over the difficulty of finding the most pertinent information in the EMR—the assessment and the plan—has led others to propose a rearrangement of the traditional SOAP note. The APSO (assessment, plan, subjective, objective) note7,8 was created for inpatient daily progress notes, a situation in which the patient’s concern is unlikely to change dramatically on a daily basis and was not intended for use in outpatient clinics.8 While the APSO format does allow colleagues rapid access to the physician’s assessment and plan, it abandons the patient-centered approach of Dr. Weed’s problem-oriented medical record in that it makes it more difficult to find why the patient initially sought care, how long the patient has had the problem, or if there were prior attempts to treat it. These critical details are buried in the bowels of the note.
The advantage of the CAPS note (Table 1) is that it retains the patient-centered, problem-oriented spirit of the SOAP format, while moving potentially supportive yet distracting data fields to later in the note. Thus it is applicable to inpatient and outpatient settings.
In the inpatient setting, the fields remain in the same order, but the chief complaint is often the admitting diagnosis or surgical procedure, followed by a quick line on the interval history. The assessment and plan can then follow in much the same way as it would in the outpatient setting, and below that are the patient’s daily laboratory results, radiographic studies, physical examination findings, and any other relevant supporting data. This format allows rapid access to critical information needed by either consultants or cross-covering practitioners who primarily want to know why the patient was admitted, the status, and the primary team’s plan.
ANY TEMPLATE HAS LIMITATIONS
Any standardized template for progress notes in the EMR has limitations. The CAPS format would be easier for a hospital-based physician, who typically addresses one or a small number of concerns, than for an office-based general practitioner who may have to address a multitude of comorbidities in a single visit.
Also, different physicians use the EMR differently. For example, a survey of 1,088 physicians found that 60% of primary care physicians used templates (60%) vs only 34% of specialists, and that 38% of specialists relied mainly on dictation.9
The CAPS approach to the office visit note offers a blend of a template and free text, either typed or dictated, while keeping a structured format that permits others participating in the patient’s care to easily extract desired information. The template can easily be brought up in the patient’s chart, then by either typing or using voice-recognition software, the patient’s chief complaint, history of the present illness, assessment, and plan can be easily completed.
The CAPS format should continue to allow notes to fulfill medicolegal and billing obligations, but without cluttering true clinical reasoning. As more institutions adopt an open-notes policy, permitting patients to freely browse their own medical records, patients will benefit from a clearly structured clinical note that focuses on their problem and the practitioner’s solution. This provides patients a sense of validation and reassurance that the note starts with their concern and history, followed by the practitioner’s assessment and plan, so they can easily affirm that they were accurately heard and can identify the diagnosis given to them by the medical practitioner and the plan moving forward.
Since a return to succinct, albeit often illegible, handwritten clinic notes is impossible, our proposed method of documenting a clinic visit embraces the EMR with a concise yet comprehensive clinic note.
- Jacobs L. Interview with Lawrence Weed, MD—the father of the problem-oriented medical record looks ahead. Perm J 2009; 13:84–89.
- Cameron S, Turtle-Son I. Learning to write case notes using the SOAP format. JCD 2002; 80:286–292.
- Hsiao CJ, Hing E. Use and characteristics of electronic health record systems among office-based physician practices: United States, 2001-2013. NCHS Data Brief 2014; 143:1–8.
- Centers for Medicare & Medicaid Services (CMS). EHR incentive program. www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/Basics.html. Accessed April 28, 2016.
- Han H, Lopp L. Writing and reading in the electronic health record: an entirely new world. Med Educ Online 2013; 18:1–7.
- Hammoud MM, Dalrymple JL, Christner JG, et al. Medical student documentation in electronic health records: a collaborative statement from the Alliance for Clinical Education. Teach Learn Med 2012; 24:257–266.
- Shoolin J, Ozeran L, Hamann C, Bria W 2nd. Association of Medical Directors of Information Systems consensus on inpatient electronic health record documentation. Appl Clin Inform 2013; 4:293–303.
- Hahn JS, Bernstein JA, McKenzie RB, King BJ, Longhurst CA. Rapid implementation of inpatient electronic physician documentation at an academic hospital. Appl Clin Inform 2012; 3:175–185.
- Pollard SE, Neri PM, Wilcox AR, et al. How physicians document outpatient visit notes in an electronic health record. Int J Med Inform 2013; 82:39–46.
- Jacobs L. Interview with Lawrence Weed, MD—the father of the problem-oriented medical record looks ahead. Perm J 2009; 13:84–89.
- Cameron S, Turtle-Son I. Learning to write case notes using the SOAP format. JCD 2002; 80:286–292.
- Hsiao CJ, Hing E. Use and characteristics of electronic health record systems among office-based physician practices: United States, 2001-2013. NCHS Data Brief 2014; 143:1–8.
- Centers for Medicare & Medicaid Services (CMS). EHR incentive program. www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/Basics.html. Accessed April 28, 2016.
- Han H, Lopp L. Writing and reading in the electronic health record: an entirely new world. Med Educ Online 2013; 18:1–7.
- Hammoud MM, Dalrymple JL, Christner JG, et al. Medical student documentation in electronic health records: a collaborative statement from the Alliance for Clinical Education. Teach Learn Med 2012; 24:257–266.
- Shoolin J, Ozeran L, Hamann C, Bria W 2nd. Association of Medical Directors of Information Systems consensus on inpatient electronic health record documentation. Appl Clin Inform 2013; 4:293–303.
- Hahn JS, Bernstein JA, McKenzie RB, King BJ, Longhurst CA. Rapid implementation of inpatient electronic physician documentation at an academic hospital. Appl Clin Inform 2012; 3:175–185.
- Pollard SE, Neri PM, Wilcox AR, et al. How physicians document outpatient visit notes in an electronic health record. Int J Med Inform 2013; 82:39–46.
KEY POINTS
- The CAPS format provides an advantage over the traditional approach by transferring potentially note-cluttering data that is available elsewhere in the EMR to the bottom of the note, allowing more efficient communication of the true purpose for the patient’s visit, the diagnosis, and the physician’s approach to resolving the patient’s concern.
- As healthcare systems allow patients to browse their electronic charts, the CAPS format shows them that their concern was heard accurately and clearly states the diagnosis and plan of care.
Are we causing anemia by ordering unnecessary blood tests?
A 68-year-old woman is admitted for community-acquired pneumonia. She receives antibiotics, and her condition begins to improve after 2 days. She has her blood drawn daily throughout her admission.
On hospital day 3, she complains of fatigue, and on day 4, laboratory results show that her hemoglobin and hematocrit values have fallen. To make sure this result is not spurious, her blood is drawn again to repeat the test. On day 5, her hemoglobin level has dropped to 7.0 g/dL, which is 2 g/dL lower than at admission, and she receives a transfusion.
On day 7, her hemoglobin level is stable at 8.5 g/dL, and her physicians decide to discharge her. The morning of her discharge, as a nurse is about to draw her blood, the patient asks, “Are all these blood tests really necessary?”
DO WE DRAW TOO MUCH BLOOD?
This case portrays a common occurrence. Significant amounts of blood are drawn from patients, especially in critical care. Clinical uncertainty drives most laboratory testing ordered by physicians. Too often, however, these tests lead to more testing and interventions, without a clear benefit to the patient.1
When blood testing leads to more testing, a patient’s hemoglobin and hematocrit can fall. Symptomatic iatrogenic anemia is associated with significant morbidity for patients with preexisting cardiopulmonary disease.
We draw much larger volumes of blood than most testing guidelines say are necessary. One author2 has noted that 50 to 60 mL of blood is removed for each set of tests, owing to the size of collection tubes, multiple reagents needed for each test, and the possibility that tests may need to be rerun. Yet about 3 mL of blood is sufficient to perform most laboratory tests even if the test needs to be rerun.2
CAN BLOOD DRAWS CAUSE ANEMIA?
A relationship between the volume of blood drawn and iatrogenic anemia was first described in 2005, when Thavendiranathan et al3 found that in adult patients on general medicine floors, the volume of blood drawn strongly predicted decreased hemoglobin and hematocrit levels. For every 100 mL of blood drawn, hemoglobin levels fell by an average of 0.7 g/dL, and 13.9% of the patients in the study had iron studies and fecal occult blood tests performed to investigate anemia.
Kurniali et al4 reported that during an average admission, 65% of patients experienced a drop in hemoglobin of 1.0 g/dL or more, and 49% developed anemia.
Salisbury et al,5 in 2011, studied 17,676 patients with acute myocardial infarction across 57 centers and found a correlation between the volume of blood taken and the development of anemia. On average, for every 50 mL of blood drawn, the risk of moderate to severe iatrogenic anemia increased by 18%. They also found significant variation in blood loss from testing in patients who developed moderate or severe anemia. The authors believed this indicated that moderate to severe anemia was more frequent at centers with higher than average diagnostic blood loss.5
This relationship has also been described in patients in intensive care, where it contributes to anemia of chronic disease. While anemia of critical illness is multifactorial, phlebotomy contributes to anemia in both short- and long-term stays in the intensive care unit.6
CHOOSING WISELY GUIDELINES
The Choosing Wisely initiative of the American Board of Internal Medicine Foundation collects recommendations by a number of medical specialty societies to reduce overuse of healthcare resources.7 The Critical Care Societies Collaborative recommends ordering diagnostic tests only when they answer specific clinical questions rather than routinely. The Society of Hospital Medicine also recommends against repeat complete blood cell count and blood chemistry testing because it may contribute to anemia, which is of particular concern in patients with cardiorespiratory disease.
POSSIBLE HARM
The Critical Care Societies Collaborative, in its Choosing Wisely Guidelines, specifically cites anemia as a potential harm of unnecessary phlebotomy, noting it may result in transfusion, with its associated risks and costs. In addition, aggressive investigation of incidental and nonpathologic results of routine studies is wasteful and exposes the patient to additional risks.
REDUCING PHLEBOTOMY DECREASES IATROGENIC ANEMIA
Since the relationship between excessive phlebotomy and iatrogenic anemia was described, hospitals have attempted to address the problem.
In 2011, Stuebing and Miner8 described an intervention in which the house staff and attending physicians on non-intensive care surgical services were given weekly reports of the cost of the laboratory services for the previous week. They found that simply making providers aware of the cost of their tests reduced the number of tests ordered and resulted in significant hospital savings.
Another strategy is to use pediatric collection tubes in adult patients. A 2008 study in which all blood samples were drawn using pediatric tubes reduced the blood volume removed per patient by almost 75% in inpatient and critical care patients, without the need for repeat blood draws.9 However, Kurniali et al found that the use of pediatric collection tubes did not significantly change hemoglobin fluctuations throughout patient hospital stays.4
Corson et al10 in 2015 described an intervention involving detailing, auditing, and giving feedback regarding the frequency of laboratory tests commonly ordered by a group of hospitalists. The intervention resulted in a modest reduction in the number of common laboratory tests ordered per patient day and in hospital costs, without any changes in the length of hospital stay, mortality rate, or readmission rate.10
THE CLINICAL BOTTOM LINE
As a general principle, diagnostic testing should be done to answer specific diagnostic questions and to guide management. Ordering of diagnostic tests should be decided on a day-to-day basis rather than scheduled automatically or done reflexively. In the case of blood draws, the volume of blood drawn is significantly increased by unnecessary testing, resulting in higher rates of hospital-acquired anemia.
- Ezzie ME, Aberegg SK, O’Brien JM Jr. Laboratory testing in the intensive care unit. Crit Care Clin 2007; 23:435–465.
- Stefanini M. Iatrogenic anemia (can it be prevented?). J Thromb Haemost 2014; 12:1591.
- Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med 2005; 20:520–524.
- Kurniali PC, Curry S, Brennan KW, et al. A retrospective study investigating the incidence and predisposing factors of hospital-acquired anemia. Anemia 2014; 2014:634582.
- Salisbury AC, Reid KJ, Alexander KP, et al. Diagnostic blood loss from phlebotomy and hospital-acquired anemia during acute myocardial infarction. Arch Intern Med 2011; 171:1646–1653.
- Walsh TS, Lee RJ, Maciver CR, et al. Anemia during and at discharge from intensive care: the impact of restrictive blood transfusion practice. Intensive Care Med 2006; 32:100–109.
- American Board of Internal Medicine Foundation. Choosing Wisely. www.abimfoundation.org/Initiatives/Choosing-Wisely.aspx. Accessed April 19, 2016.
- Stuebing EA, Miner TJ. Surgical vampires and rising health care expenditure: reducing the cost of daily phlebotomy. Arch Surg 2011; 146:524–527.
- Sanchez-Giron F, Alvarez-Mora F. Reduction of blood loss from laboratory testing in hospitalized adult patients using small-volume (pediatric) tubes. Arch Pathol Lab Med 2008; 132:1916–1919.
- Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: decreased frequency of common labs. J Hosp Med 2015; 10:390–395.
A 68-year-old woman is admitted for community-acquired pneumonia. She receives antibiotics, and her condition begins to improve after 2 days. She has her blood drawn daily throughout her admission.
On hospital day 3, she complains of fatigue, and on day 4, laboratory results show that her hemoglobin and hematocrit values have fallen. To make sure this result is not spurious, her blood is drawn again to repeat the test. On day 5, her hemoglobin level has dropped to 7.0 g/dL, which is 2 g/dL lower than at admission, and she receives a transfusion.
On day 7, her hemoglobin level is stable at 8.5 g/dL, and her physicians decide to discharge her. The morning of her discharge, as a nurse is about to draw her blood, the patient asks, “Are all these blood tests really necessary?”
DO WE DRAW TOO MUCH BLOOD?
This case portrays a common occurrence. Significant amounts of blood are drawn from patients, especially in critical care. Clinical uncertainty drives most laboratory testing ordered by physicians. Too often, however, these tests lead to more testing and interventions, without a clear benefit to the patient.1
When blood testing leads to more testing, a patient’s hemoglobin and hematocrit can fall. Symptomatic iatrogenic anemia is associated with significant morbidity for patients with preexisting cardiopulmonary disease.
We draw much larger volumes of blood than most testing guidelines say are necessary. One author2 has noted that 50 to 60 mL of blood is removed for each set of tests, owing to the size of collection tubes, multiple reagents needed for each test, and the possibility that tests may need to be rerun. Yet about 3 mL of blood is sufficient to perform most laboratory tests even if the test needs to be rerun.2
CAN BLOOD DRAWS CAUSE ANEMIA?
A relationship between the volume of blood drawn and iatrogenic anemia was first described in 2005, when Thavendiranathan et al3 found that in adult patients on general medicine floors, the volume of blood drawn strongly predicted decreased hemoglobin and hematocrit levels. For every 100 mL of blood drawn, hemoglobin levels fell by an average of 0.7 g/dL, and 13.9% of the patients in the study had iron studies and fecal occult blood tests performed to investigate anemia.
Kurniali et al4 reported that during an average admission, 65% of patients experienced a drop in hemoglobin of 1.0 g/dL or more, and 49% developed anemia.
Salisbury et al,5 in 2011, studied 17,676 patients with acute myocardial infarction across 57 centers and found a correlation between the volume of blood taken and the development of anemia. On average, for every 50 mL of blood drawn, the risk of moderate to severe iatrogenic anemia increased by 18%. They also found significant variation in blood loss from testing in patients who developed moderate or severe anemia. The authors believed this indicated that moderate to severe anemia was more frequent at centers with higher than average diagnostic blood loss.5
This relationship has also been described in patients in intensive care, where it contributes to anemia of chronic disease. While anemia of critical illness is multifactorial, phlebotomy contributes to anemia in both short- and long-term stays in the intensive care unit.6
CHOOSING WISELY GUIDELINES
The Choosing Wisely initiative of the American Board of Internal Medicine Foundation collects recommendations by a number of medical specialty societies to reduce overuse of healthcare resources.7 The Critical Care Societies Collaborative recommends ordering diagnostic tests only when they answer specific clinical questions rather than routinely. The Society of Hospital Medicine also recommends against repeat complete blood cell count and blood chemistry testing because it may contribute to anemia, which is of particular concern in patients with cardiorespiratory disease.
POSSIBLE HARM
The Critical Care Societies Collaborative, in its Choosing Wisely Guidelines, specifically cites anemia as a potential harm of unnecessary phlebotomy, noting it may result in transfusion, with its associated risks and costs. In addition, aggressive investigation of incidental and nonpathologic results of routine studies is wasteful and exposes the patient to additional risks.
REDUCING PHLEBOTOMY DECREASES IATROGENIC ANEMIA
Since the relationship between excessive phlebotomy and iatrogenic anemia was described, hospitals have attempted to address the problem.
In 2011, Stuebing and Miner8 described an intervention in which the house staff and attending physicians on non-intensive care surgical services were given weekly reports of the cost of the laboratory services for the previous week. They found that simply making providers aware of the cost of their tests reduced the number of tests ordered and resulted in significant hospital savings.
Another strategy is to use pediatric collection tubes in adult patients. A 2008 study in which all blood samples were drawn using pediatric tubes reduced the blood volume removed per patient by almost 75% in inpatient and critical care patients, without the need for repeat blood draws.9 However, Kurniali et al found that the use of pediatric collection tubes did not significantly change hemoglobin fluctuations throughout patient hospital stays.4
Corson et al10 in 2015 described an intervention involving detailing, auditing, and giving feedback regarding the frequency of laboratory tests commonly ordered by a group of hospitalists. The intervention resulted in a modest reduction in the number of common laboratory tests ordered per patient day and in hospital costs, without any changes in the length of hospital stay, mortality rate, or readmission rate.10
THE CLINICAL BOTTOM LINE
As a general principle, diagnostic testing should be done to answer specific diagnostic questions and to guide management. Ordering of diagnostic tests should be decided on a day-to-day basis rather than scheduled automatically or done reflexively. In the case of blood draws, the volume of blood drawn is significantly increased by unnecessary testing, resulting in higher rates of hospital-acquired anemia.
A 68-year-old woman is admitted for community-acquired pneumonia. She receives antibiotics, and her condition begins to improve after 2 days. She has her blood drawn daily throughout her admission.
On hospital day 3, she complains of fatigue, and on day 4, laboratory results show that her hemoglobin and hematocrit values have fallen. To make sure this result is not spurious, her blood is drawn again to repeat the test. On day 5, her hemoglobin level has dropped to 7.0 g/dL, which is 2 g/dL lower than at admission, and she receives a transfusion.
On day 7, her hemoglobin level is stable at 8.5 g/dL, and her physicians decide to discharge her. The morning of her discharge, as a nurse is about to draw her blood, the patient asks, “Are all these blood tests really necessary?”
DO WE DRAW TOO MUCH BLOOD?
This case portrays a common occurrence. Significant amounts of blood are drawn from patients, especially in critical care. Clinical uncertainty drives most laboratory testing ordered by physicians. Too often, however, these tests lead to more testing and interventions, without a clear benefit to the patient.1
When blood testing leads to more testing, a patient’s hemoglobin and hematocrit can fall. Symptomatic iatrogenic anemia is associated with significant morbidity for patients with preexisting cardiopulmonary disease.
We draw much larger volumes of blood than most testing guidelines say are necessary. One author2 has noted that 50 to 60 mL of blood is removed for each set of tests, owing to the size of collection tubes, multiple reagents needed for each test, and the possibility that tests may need to be rerun. Yet about 3 mL of blood is sufficient to perform most laboratory tests even if the test needs to be rerun.2
CAN BLOOD DRAWS CAUSE ANEMIA?
A relationship between the volume of blood drawn and iatrogenic anemia was first described in 2005, when Thavendiranathan et al3 found that in adult patients on general medicine floors, the volume of blood drawn strongly predicted decreased hemoglobin and hematocrit levels. For every 100 mL of blood drawn, hemoglobin levels fell by an average of 0.7 g/dL, and 13.9% of the patients in the study had iron studies and fecal occult blood tests performed to investigate anemia.
Kurniali et al4 reported that during an average admission, 65% of patients experienced a drop in hemoglobin of 1.0 g/dL or more, and 49% developed anemia.
Salisbury et al,5 in 2011, studied 17,676 patients with acute myocardial infarction across 57 centers and found a correlation between the volume of blood taken and the development of anemia. On average, for every 50 mL of blood drawn, the risk of moderate to severe iatrogenic anemia increased by 18%. They also found significant variation in blood loss from testing in patients who developed moderate or severe anemia. The authors believed this indicated that moderate to severe anemia was more frequent at centers with higher than average diagnostic blood loss.5
This relationship has also been described in patients in intensive care, where it contributes to anemia of chronic disease. While anemia of critical illness is multifactorial, phlebotomy contributes to anemia in both short- and long-term stays in the intensive care unit.6
CHOOSING WISELY GUIDELINES
The Choosing Wisely initiative of the American Board of Internal Medicine Foundation collects recommendations by a number of medical specialty societies to reduce overuse of healthcare resources.7 The Critical Care Societies Collaborative recommends ordering diagnostic tests only when they answer specific clinical questions rather than routinely. The Society of Hospital Medicine also recommends against repeat complete blood cell count and blood chemistry testing because it may contribute to anemia, which is of particular concern in patients with cardiorespiratory disease.
POSSIBLE HARM
The Critical Care Societies Collaborative, in its Choosing Wisely Guidelines, specifically cites anemia as a potential harm of unnecessary phlebotomy, noting it may result in transfusion, with its associated risks and costs. In addition, aggressive investigation of incidental and nonpathologic results of routine studies is wasteful and exposes the patient to additional risks.
REDUCING PHLEBOTOMY DECREASES IATROGENIC ANEMIA
Since the relationship between excessive phlebotomy and iatrogenic anemia was described, hospitals have attempted to address the problem.
In 2011, Stuebing and Miner8 described an intervention in which the house staff and attending physicians on non-intensive care surgical services were given weekly reports of the cost of the laboratory services for the previous week. They found that simply making providers aware of the cost of their tests reduced the number of tests ordered and resulted in significant hospital savings.
Another strategy is to use pediatric collection tubes in adult patients. A 2008 study in which all blood samples were drawn using pediatric tubes reduced the blood volume removed per patient by almost 75% in inpatient and critical care patients, without the need for repeat blood draws.9 However, Kurniali et al found that the use of pediatric collection tubes did not significantly change hemoglobin fluctuations throughout patient hospital stays.4
Corson et al10 in 2015 described an intervention involving detailing, auditing, and giving feedback regarding the frequency of laboratory tests commonly ordered by a group of hospitalists. The intervention resulted in a modest reduction in the number of common laboratory tests ordered per patient day and in hospital costs, without any changes in the length of hospital stay, mortality rate, or readmission rate.10
THE CLINICAL BOTTOM LINE
As a general principle, diagnostic testing should be done to answer specific diagnostic questions and to guide management. Ordering of diagnostic tests should be decided on a day-to-day basis rather than scheduled automatically or done reflexively. In the case of blood draws, the volume of blood drawn is significantly increased by unnecessary testing, resulting in higher rates of hospital-acquired anemia.
- Ezzie ME, Aberegg SK, O’Brien JM Jr. Laboratory testing in the intensive care unit. Crit Care Clin 2007; 23:435–465.
- Stefanini M. Iatrogenic anemia (can it be prevented?). J Thromb Haemost 2014; 12:1591.
- Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med 2005; 20:520–524.
- Kurniali PC, Curry S, Brennan KW, et al. A retrospective study investigating the incidence and predisposing factors of hospital-acquired anemia. Anemia 2014; 2014:634582.
- Salisbury AC, Reid KJ, Alexander KP, et al. Diagnostic blood loss from phlebotomy and hospital-acquired anemia during acute myocardial infarction. Arch Intern Med 2011; 171:1646–1653.
- Walsh TS, Lee RJ, Maciver CR, et al. Anemia during and at discharge from intensive care: the impact of restrictive blood transfusion practice. Intensive Care Med 2006; 32:100–109.
- American Board of Internal Medicine Foundation. Choosing Wisely. www.abimfoundation.org/Initiatives/Choosing-Wisely.aspx. Accessed April 19, 2016.
- Stuebing EA, Miner TJ. Surgical vampires and rising health care expenditure: reducing the cost of daily phlebotomy. Arch Surg 2011; 146:524–527.
- Sanchez-Giron F, Alvarez-Mora F. Reduction of blood loss from laboratory testing in hospitalized adult patients using small-volume (pediatric) tubes. Arch Pathol Lab Med 2008; 132:1916–1919.
- Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: decreased frequency of common labs. J Hosp Med 2015; 10:390–395.
- Ezzie ME, Aberegg SK, O’Brien JM Jr. Laboratory testing in the intensive care unit. Crit Care Clin 2007; 23:435–465.
- Stefanini M. Iatrogenic anemia (can it be prevented?). J Thromb Haemost 2014; 12:1591.
- Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med 2005; 20:520–524.
- Kurniali PC, Curry S, Brennan KW, et al. A retrospective study investigating the incidence and predisposing factors of hospital-acquired anemia. Anemia 2014; 2014:634582.
- Salisbury AC, Reid KJ, Alexander KP, et al. Diagnostic blood loss from phlebotomy and hospital-acquired anemia during acute myocardial infarction. Arch Intern Med 2011; 171:1646–1653.
- Walsh TS, Lee RJ, Maciver CR, et al. Anemia during and at discharge from intensive care: the impact of restrictive blood transfusion practice. Intensive Care Med 2006; 32:100–109.
- American Board of Internal Medicine Foundation. Choosing Wisely. www.abimfoundation.org/Initiatives/Choosing-Wisely.aspx. Accessed April 19, 2016.
- Stuebing EA, Miner TJ. Surgical vampires and rising health care expenditure: reducing the cost of daily phlebotomy. Arch Surg 2011; 146:524–527.
- Sanchez-Giron F, Alvarez-Mora F. Reduction of blood loss from laboratory testing in hospitalized adult patients using small-volume (pediatric) tubes. Arch Pathol Lab Med 2008; 132:1916–1919.
- Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: decreased frequency of common labs. J Hosp Med 2015; 10:390–395.
Cognitive bias and diagnostic error
To the Editor: I appreciated the article on cognitive biases and diagnostic error by Mull et al in the November 2015 issue.1 They presented an excellent description of the pitfalls of diagnosis as reflected in a case of a patient misdiagnosed with heart failure who ultimately died of pulmonary tuberculosis complicated by pulmonary embolism (the latter possibly from using the wrong form of heparin). To the points they raised, I would like to add a few of my own about diagnosis in general and heart failure in particular.
First, any initial diagnosis not confirmed objectively within the first 24 hours should be questioned, and other possibilities should be investigated. I have found this to be essential for every day’s stay in the hospital and for every outpatient visit. The authors mention checklists as part of the solution to the problem of misdiagnosis, and I would suggest that confirmation of initial diagnoses be built into these checklists.
In the case of a presumptive diagnosis of an acute exacerbation of heart failure treated empirically with diuretics, the diagnosis should be confirmed by the next day’s response to the diuretics, ie, increased urine output, a lower respiratory rate, and a fall in the pro-B-type natriuretic peptide level. Moreover, a change in the radiographic appearance should be seen, and respiratory and pulmonary function should improve after the first 24 hours on oxygen supplementation plus diuretics. Daily patient weights are also critical in determining response to a diuretic, and are rarely done accurately. I order weights and review them daily for patients like this.
Second, it is good to look at things yourself, including the patient, medication lists, laboratory values, and radiographic films. The attending physician should look at the radiographs together with a senior radiologist. Seeing no improvement or change on the second hospital day, or seeing signs incompatible with heart failure, one could order computed tomography of the chest and begin to entertain pulmonary diagnoses.
Even vital signs can be questionable. For example, in the case presented here, with a temperature of 99°F, a heart rate of 105, and a pulse oxygenation saturation of 89%, a respiratory rate of 24 seems unbelievably low. In my experience, the respiratory rate is recorded erroneously most of the time unless it is recorded electronically or checked at the bedside by the physician using a timepiece with a sweep second-hand.
Additionally, I have found that ordering several days’ laboratory tests (eg, complete blood cell counts, chemistry panels) in advance, in many cases, risks missing important findings and wastes time, energy, and the patient’s blood. I have learned to evaluate each patient daily and to order the most pertinent laboratory tests. With electronic medical records, I can check laboratory results as soon as they are available.
- Mull N, Reilly JB, Myers JS. An elderly woman with ‘heart failure’: cognitive biases and diagnostic error. Cleve Clin J Med 2015; 82:745–753.
To the Editor: I appreciated the article on cognitive biases and diagnostic error by Mull et al in the November 2015 issue.1 They presented an excellent description of the pitfalls of diagnosis as reflected in a case of a patient misdiagnosed with heart failure who ultimately died of pulmonary tuberculosis complicated by pulmonary embolism (the latter possibly from using the wrong form of heparin). To the points they raised, I would like to add a few of my own about diagnosis in general and heart failure in particular.
First, any initial diagnosis not confirmed objectively within the first 24 hours should be questioned, and other possibilities should be investigated. I have found this to be essential for every day’s stay in the hospital and for every outpatient visit. The authors mention checklists as part of the solution to the problem of misdiagnosis, and I would suggest that confirmation of initial diagnoses be built into these checklists.
In the case of a presumptive diagnosis of an acute exacerbation of heart failure treated empirically with diuretics, the diagnosis should be confirmed by the next day’s response to the diuretics, ie, increased urine output, a lower respiratory rate, and a fall in the pro-B-type natriuretic peptide level. Moreover, a change in the radiographic appearance should be seen, and respiratory and pulmonary function should improve after the first 24 hours on oxygen supplementation plus diuretics. Daily patient weights are also critical in determining response to a diuretic, and are rarely done accurately. I order weights and review them daily for patients like this.
Second, it is good to look at things yourself, including the patient, medication lists, laboratory values, and radiographic films. The attending physician should look at the radiographs together with a senior radiologist. Seeing no improvement or change on the second hospital day, or seeing signs incompatible with heart failure, one could order computed tomography of the chest and begin to entertain pulmonary diagnoses.
Even vital signs can be questionable. For example, in the case presented here, with a temperature of 99°F, a heart rate of 105, and a pulse oxygenation saturation of 89%, a respiratory rate of 24 seems unbelievably low. In my experience, the respiratory rate is recorded erroneously most of the time unless it is recorded electronically or checked at the bedside by the physician using a timepiece with a sweep second-hand.
Additionally, I have found that ordering several days’ laboratory tests (eg, complete blood cell counts, chemistry panels) in advance, in many cases, risks missing important findings and wastes time, energy, and the patient’s blood. I have learned to evaluate each patient daily and to order the most pertinent laboratory tests. With electronic medical records, I can check laboratory results as soon as they are available.
To the Editor: I appreciated the article on cognitive biases and diagnostic error by Mull et al in the November 2015 issue.1 They presented an excellent description of the pitfalls of diagnosis as reflected in a case of a patient misdiagnosed with heart failure who ultimately died of pulmonary tuberculosis complicated by pulmonary embolism (the latter possibly from using the wrong form of heparin). To the points they raised, I would like to add a few of my own about diagnosis in general and heart failure in particular.
First, any initial diagnosis not confirmed objectively within the first 24 hours should be questioned, and other possibilities should be investigated. I have found this to be essential for every day’s stay in the hospital and for every outpatient visit. The authors mention checklists as part of the solution to the problem of misdiagnosis, and I would suggest that confirmation of initial diagnoses be built into these checklists.
In the case of a presumptive diagnosis of an acute exacerbation of heart failure treated empirically with diuretics, the diagnosis should be confirmed by the next day’s response to the diuretics, ie, increased urine output, a lower respiratory rate, and a fall in the pro-B-type natriuretic peptide level. Moreover, a change in the radiographic appearance should be seen, and respiratory and pulmonary function should improve after the first 24 hours on oxygen supplementation plus diuretics. Daily patient weights are also critical in determining response to a diuretic, and are rarely done accurately. I order weights and review them daily for patients like this.
Second, it is good to look at things yourself, including the patient, medication lists, laboratory values, and radiographic films. The attending physician should look at the radiographs together with a senior radiologist. Seeing no improvement or change on the second hospital day, or seeing signs incompatible with heart failure, one could order computed tomography of the chest and begin to entertain pulmonary diagnoses.
Even vital signs can be questionable. For example, in the case presented here, with a temperature of 99°F, a heart rate of 105, and a pulse oxygenation saturation of 89%, a respiratory rate of 24 seems unbelievably low. In my experience, the respiratory rate is recorded erroneously most of the time unless it is recorded electronically or checked at the bedside by the physician using a timepiece with a sweep second-hand.
Additionally, I have found that ordering several days’ laboratory tests (eg, complete blood cell counts, chemistry panels) in advance, in many cases, risks missing important findings and wastes time, energy, and the patient’s blood. I have learned to evaluate each patient daily and to order the most pertinent laboratory tests. With electronic medical records, I can check laboratory results as soon as they are available.
- Mull N, Reilly JB, Myers JS. An elderly woman with ‘heart failure’: cognitive biases and diagnostic error. Cleve Clin J Med 2015; 82:745–753.
- Mull N, Reilly JB, Myers JS. An elderly woman with ‘heart failure’: cognitive biases and diagnostic error. Cleve Clin J Med 2015; 82:745–753.
In reply: Cognitive bias and diagnostic error
In Reply: We thank Dr. Field for his insights and personal observations related to diagnosis and biases that contribute to diagnostic errors.
Dr. Field’s comment about the importance of revisiting one’s initial working diagnosis is consistent with our proposed diagnostic time out. A diagnostic time out can incorporate a short checklist and aid in debiasing clinicians when findings do not fit the case presentation, such as lack of response to diuretic therapy. Being mindful of slowing down and not necessarily rushing to judgment is another important component.1 Of note, the residents in our case did revisit their initial working diagnosis, as suggested by Dr. Field. Questions from learners have great potential to serve as debiasing instruments and should always be encouraged. Those who do not work with students can do the same by speaking with nurses or other members of the healthcare team, who offer observations that busy physicians might miss.
Our case highlights the problem that we lack objective criteria to diagnose symptomatic heart failure. While B-type natriuretic factor (BNP) has a strong negative predictive value, serial BNP measurements have not been established to be helpful in the management of heart failure.2 Although certain findings on chest radiography have strong positive and negative likelihood associations, the role of serial chest radiographs is less clear.3 Thus, heart failure remains a clinical diagnosis in current practice.
As Dr. Field points out, the accuracy and performance characteristics of diagnostic testing, such as the respiratory rate, need to be considered in conjunction with debiasing strategies to achieve higher diagnostic accuracy. Multiple factors can contribute to low-performing or misinterpreted diagnostic tests, and inaccurate vital signs have been shown to be similarly prone to potential error.4
Finally, we wholeheartedly agree with Dr. Field’s comment on unnecessary testing. High-value care is appropriate care. Using Bayesian reasoning to guide testing, monitoring the treatment course appropriately, and eliminating waste is highly likely to improve both value and diagnostic accuracy. Automated, ritual ordering of daily tests can indicate that thinking has been shut off, leaving clinicians susceptible to premature closure of the diagnostic process as well as the potential for “incidentalomas” to distract them from the right diagnosis, all the while leading to low-value care such as wasteful spending, patient dissatisfaction, and hospital-acquired anemia.5 We believe that deciding on a daily basis what the next day’s tests will be can be another powerful debiasing habit, one with benefits beyond diagnosis.
- Schiff GD. Minimizing diagnostic error: the importance of follow-up and feedback. Am J Med 2008; 121(suppl):S38–S42.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure. Circulation 2013; 128:e240–e327.
- Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA 2005; 294:1944–1956.
- Philip KE, Pack E, Cambiano V, Rollmann H, Weil S, O’Beirne J. The accuracy of respiratory rate assessment by doctors in a London teaching hospital: a cross-sectional study. J Clin Monit Comput 2015; 29:455–460.
- Koch CG, Li L, Sun Z, et al. Hospital-acquired anemia: prevalence, outcomes, and healthcare implications. J Hosp Med 2013; 8:506–512.
In Reply: We thank Dr. Field for his insights and personal observations related to diagnosis and biases that contribute to diagnostic errors.
Dr. Field’s comment about the importance of revisiting one’s initial working diagnosis is consistent with our proposed diagnostic time out. A diagnostic time out can incorporate a short checklist and aid in debiasing clinicians when findings do not fit the case presentation, such as lack of response to diuretic therapy. Being mindful of slowing down and not necessarily rushing to judgment is another important component.1 Of note, the residents in our case did revisit their initial working diagnosis, as suggested by Dr. Field. Questions from learners have great potential to serve as debiasing instruments and should always be encouraged. Those who do not work with students can do the same by speaking with nurses or other members of the healthcare team, who offer observations that busy physicians might miss.
Our case highlights the problem that we lack objective criteria to diagnose symptomatic heart failure. While B-type natriuretic factor (BNP) has a strong negative predictive value, serial BNP measurements have not been established to be helpful in the management of heart failure.2 Although certain findings on chest radiography have strong positive and negative likelihood associations, the role of serial chest radiographs is less clear.3 Thus, heart failure remains a clinical diagnosis in current practice.
As Dr. Field points out, the accuracy and performance characteristics of diagnostic testing, such as the respiratory rate, need to be considered in conjunction with debiasing strategies to achieve higher diagnostic accuracy. Multiple factors can contribute to low-performing or misinterpreted diagnostic tests, and inaccurate vital signs have been shown to be similarly prone to potential error.4
Finally, we wholeheartedly agree with Dr. Field’s comment on unnecessary testing. High-value care is appropriate care. Using Bayesian reasoning to guide testing, monitoring the treatment course appropriately, and eliminating waste is highly likely to improve both value and diagnostic accuracy. Automated, ritual ordering of daily tests can indicate that thinking has been shut off, leaving clinicians susceptible to premature closure of the diagnostic process as well as the potential for “incidentalomas” to distract them from the right diagnosis, all the while leading to low-value care such as wasteful spending, patient dissatisfaction, and hospital-acquired anemia.5 We believe that deciding on a daily basis what the next day’s tests will be can be another powerful debiasing habit, one with benefits beyond diagnosis.
In Reply: We thank Dr. Field for his insights and personal observations related to diagnosis and biases that contribute to diagnostic errors.
Dr. Field’s comment about the importance of revisiting one’s initial working diagnosis is consistent with our proposed diagnostic time out. A diagnostic time out can incorporate a short checklist and aid in debiasing clinicians when findings do not fit the case presentation, such as lack of response to diuretic therapy. Being mindful of slowing down and not necessarily rushing to judgment is another important component.1 Of note, the residents in our case did revisit their initial working diagnosis, as suggested by Dr. Field. Questions from learners have great potential to serve as debiasing instruments and should always be encouraged. Those who do not work with students can do the same by speaking with nurses or other members of the healthcare team, who offer observations that busy physicians might miss.
Our case highlights the problem that we lack objective criteria to diagnose symptomatic heart failure. While B-type natriuretic factor (BNP) has a strong negative predictive value, serial BNP measurements have not been established to be helpful in the management of heart failure.2 Although certain findings on chest radiography have strong positive and negative likelihood associations, the role of serial chest radiographs is less clear.3 Thus, heart failure remains a clinical diagnosis in current practice.
As Dr. Field points out, the accuracy and performance characteristics of diagnostic testing, such as the respiratory rate, need to be considered in conjunction with debiasing strategies to achieve higher diagnostic accuracy. Multiple factors can contribute to low-performing or misinterpreted diagnostic tests, and inaccurate vital signs have been shown to be similarly prone to potential error.4
Finally, we wholeheartedly agree with Dr. Field’s comment on unnecessary testing. High-value care is appropriate care. Using Bayesian reasoning to guide testing, monitoring the treatment course appropriately, and eliminating waste is highly likely to improve both value and diagnostic accuracy. Automated, ritual ordering of daily tests can indicate that thinking has been shut off, leaving clinicians susceptible to premature closure of the diagnostic process as well as the potential for “incidentalomas” to distract them from the right diagnosis, all the while leading to low-value care such as wasteful spending, patient dissatisfaction, and hospital-acquired anemia.5 We believe that deciding on a daily basis what the next day’s tests will be can be another powerful debiasing habit, one with benefits beyond diagnosis.
- Schiff GD. Minimizing diagnostic error: the importance of follow-up and feedback. Am J Med 2008; 121(suppl):S38–S42.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure. Circulation 2013; 128:e240–e327.
- Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA 2005; 294:1944–1956.
- Philip KE, Pack E, Cambiano V, Rollmann H, Weil S, O’Beirne J. The accuracy of respiratory rate assessment by doctors in a London teaching hospital: a cross-sectional study. J Clin Monit Comput 2015; 29:455–460.
- Koch CG, Li L, Sun Z, et al. Hospital-acquired anemia: prevalence, outcomes, and healthcare implications. J Hosp Med 2013; 8:506–512.
- Schiff GD. Minimizing diagnostic error: the importance of follow-up and feedback. Am J Med 2008; 121(suppl):S38–S42.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure. Circulation 2013; 128:e240–e327.
- Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA 2005; 294:1944–1956.
- Philip KE, Pack E, Cambiano V, Rollmann H, Weil S, O’Beirne J. The accuracy of respiratory rate assessment by doctors in a London teaching hospital: a cross-sectional study. J Clin Monit Comput 2015; 29:455–460.
- Koch CG, Li L, Sun Z, et al. Hospital-acquired anemia: prevalence, outcomes, and healthcare implications. J Hosp Med 2013; 8:506–512.