User login
Parents driving the ‘talk’ supports healthy sexual behaviors in GBQ teens and young adults
When it comes to sexual health education in the United States, one thing is abundantly clear: It’s a messy patchwork of programs, topics, and criteria. Only 29 states and the District of Columbia currently mandate sexual health education. Sixteen states have an abstinence-only curriculum, whereas 13 do not require that instruction be age-appropriate, inclusive, medically accurate, or evidence-based/informed. And this is just the tip of the iceberg, according to a 2022 report issued by the Sex Ed for Social Change organization.
Parents should take an inclusive approach to sex communication and create a safe space for discussing sex and sexual orientation, said almost all (96.7%) of male young adults who participated in a qualitative study. This would help reinforce acceptance and parents could possibly serve as a proxy for children who’ve not yet disclosed their sexual orientation. Yet, few parents are equipped or prepared to have these meaningful conversations with gay, bisexual, queer, or gender-diverse children, despite the fact that they are especially vulnerable to poor sexual health outcomes, bullying, abuse, and mental health challenges, as well as high-risk sexual behaviors.
“Parents are sexual socialization agents,” Dalmacio Dennis Flores, PhD, ACRN, assistant professor of nursing at the University of Pennsylvania, Philadelphia, told this news organization. “It’s through the information that they convey, the way that they normalize rituals and expectations, that they inform young people of all of societal expectations or roles they’ll be fulfilling in the future.”
Dr. Flores is lead author of a study published in the Journal of Adolescent Health. He and his colleagues collected perspectives on comprehensive, inclusive, and age-appropriate parent-child sex communications from 30 GBQ adolescent males aged 15-20 years who were already “out” to their parents. Participants were asked to sort through 28 preprinted note cards containing broad sexual health topics (for instance, human anatomy, dating, sexually transmitted infections) as well as topics theoretically specific to GBQ individuals (for example, anal sex), and were asked to add additional topics that they felt were missing. They were then directed to recommend topics along with ideal timing (that is, elementary, middle, or high school) for these conversations.
Study findings also underscored the importance of initiating comprehensive sexuality talks as early as elementary school age – namely to start preparing GBQ children for inevitable adversities that they were likely to encounter later in life, as well as to form building blocks for more mature, in-depth discussions during high school.
Importantly, these recommendations generally align with those aimed at heterosexual youth.
“When we refer to topics for elementary school, they are general parameters of what kids might be interested in or want to hear more about; it’s not planting a seed,” explained Dr. Flores.
Eva Goldfarb, PhD, LHD, MA, professor of public health at Montclair (N.J.) State University, agreed. “We always talk about (in sex education) to follow young people’s lead. If your child is asking you a question, they deserve a response,” said Dr. Goldfarb, who wasn’t involved in the study. “It doesn’t mean you have to give a detailed- level explanation but if they’re asking about it, it means that they are thinking about it. But it’s really important for all young people to know all of this information.”
Along those lines, participants deemed that fundamental issues about bodies (for example, human anatomy, reproduction), different sexual orientations, and an introduction to foundational issues (like privacy, peer or social pressure, sexual abuse) would help elementary-aged children to normalize discussions about sex, anatomy, and sexual orientation.
Middle school conversations were ideally more in-depth to reflect the time when young people are beginning to explore and accept their social and sexual identities. Topics of discussion might include types of sexual intercourse (anal, oral, and vaginal), health promotion strategies (abstinence, condoms, and contraception), possible adverse outcomes of condomless intercourse (HIV, STIs), considerations about engaging in sexual intercourse (including readiness, negotiating boundaries, virginity), and interpersonal safety (for instance, sexting, alcohol/drugs/chemsex, sexual coercion, and partner abuse/violence).
Finally, high school age recommendations focused on socio-relational topics (such as hook-up culture, technology/online dating, and multiple or concurrent sex partners), which are most relevant during a time when adolescents are most prone to experimentation and risk-taking.
Acknowledging that the study approach was novel, Dr. Flores noted that hearing about these topics from the youth perspective allowed parents to prepare. “Communication is better when it’s anticipated vs. reactive,” he said.
Last but not least, clinicians also have an important role in supporting these conversations.
“We’ve always looked at sex communication as a dyadic process, as a parent bestowing wisdom on a child who doesn’t have that knowledge yet. But it can be a triadic model,” said Dr. Flores. “Providers can encourage parents to ask if a child is dating or is familiar with ways to protect themselves or provide consent, and act as a resource exclusively to troubleshoot emergent issues.”
This study was funded by the National Institutes of Health. The study also received supplementary funding from the Surgeon General C. Everett Koop HIV/AIDS Research Award. Dr. Flores and Dr. Goldfarb report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
When it comes to sexual health education in the United States, one thing is abundantly clear: It’s a messy patchwork of programs, topics, and criteria. Only 29 states and the District of Columbia currently mandate sexual health education. Sixteen states have an abstinence-only curriculum, whereas 13 do not require that instruction be age-appropriate, inclusive, medically accurate, or evidence-based/informed. And this is just the tip of the iceberg, according to a 2022 report issued by the Sex Ed for Social Change organization.
Parents should take an inclusive approach to sex communication and create a safe space for discussing sex and sexual orientation, said almost all (96.7%) of male young adults who participated in a qualitative study. This would help reinforce acceptance and parents could possibly serve as a proxy for children who’ve not yet disclosed their sexual orientation. Yet, few parents are equipped or prepared to have these meaningful conversations with gay, bisexual, queer, or gender-diverse children, despite the fact that they are especially vulnerable to poor sexual health outcomes, bullying, abuse, and mental health challenges, as well as high-risk sexual behaviors.
“Parents are sexual socialization agents,” Dalmacio Dennis Flores, PhD, ACRN, assistant professor of nursing at the University of Pennsylvania, Philadelphia, told this news organization. “It’s through the information that they convey, the way that they normalize rituals and expectations, that they inform young people of all of societal expectations or roles they’ll be fulfilling in the future.”
Dr. Flores is lead author of a study published in the Journal of Adolescent Health. He and his colleagues collected perspectives on comprehensive, inclusive, and age-appropriate parent-child sex communications from 30 GBQ adolescent males aged 15-20 years who were already “out” to their parents. Participants were asked to sort through 28 preprinted note cards containing broad sexual health topics (for instance, human anatomy, dating, sexually transmitted infections) as well as topics theoretically specific to GBQ individuals (for example, anal sex), and were asked to add additional topics that they felt were missing. They were then directed to recommend topics along with ideal timing (that is, elementary, middle, or high school) for these conversations.
Study findings also underscored the importance of initiating comprehensive sexuality talks as early as elementary school age – namely to start preparing GBQ children for inevitable adversities that they were likely to encounter later in life, as well as to form building blocks for more mature, in-depth discussions during high school.
Importantly, these recommendations generally align with those aimed at heterosexual youth.
“When we refer to topics for elementary school, they are general parameters of what kids might be interested in or want to hear more about; it’s not planting a seed,” explained Dr. Flores.
Eva Goldfarb, PhD, LHD, MA, professor of public health at Montclair (N.J.) State University, agreed. “We always talk about (in sex education) to follow young people’s lead. If your child is asking you a question, they deserve a response,” said Dr. Goldfarb, who wasn’t involved in the study. “It doesn’t mean you have to give a detailed- level explanation but if they’re asking about it, it means that they are thinking about it. But it’s really important for all young people to know all of this information.”
Along those lines, participants deemed that fundamental issues about bodies (for example, human anatomy, reproduction), different sexual orientations, and an introduction to foundational issues (like privacy, peer or social pressure, sexual abuse) would help elementary-aged children to normalize discussions about sex, anatomy, and sexual orientation.
Middle school conversations were ideally more in-depth to reflect the time when young people are beginning to explore and accept their social and sexual identities. Topics of discussion might include types of sexual intercourse (anal, oral, and vaginal), health promotion strategies (abstinence, condoms, and contraception), possible adverse outcomes of condomless intercourse (HIV, STIs), considerations about engaging in sexual intercourse (including readiness, negotiating boundaries, virginity), and interpersonal safety (for instance, sexting, alcohol/drugs/chemsex, sexual coercion, and partner abuse/violence).
Finally, high school age recommendations focused on socio-relational topics (such as hook-up culture, technology/online dating, and multiple or concurrent sex partners), which are most relevant during a time when adolescents are most prone to experimentation and risk-taking.
Acknowledging that the study approach was novel, Dr. Flores noted that hearing about these topics from the youth perspective allowed parents to prepare. “Communication is better when it’s anticipated vs. reactive,” he said.
Last but not least, clinicians also have an important role in supporting these conversations.
“We’ve always looked at sex communication as a dyadic process, as a parent bestowing wisdom on a child who doesn’t have that knowledge yet. But it can be a triadic model,” said Dr. Flores. “Providers can encourage parents to ask if a child is dating or is familiar with ways to protect themselves or provide consent, and act as a resource exclusively to troubleshoot emergent issues.”
This study was funded by the National Institutes of Health. The study also received supplementary funding from the Surgeon General C. Everett Koop HIV/AIDS Research Award. Dr. Flores and Dr. Goldfarb report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
When it comes to sexual health education in the United States, one thing is abundantly clear: It’s a messy patchwork of programs, topics, and criteria. Only 29 states and the District of Columbia currently mandate sexual health education. Sixteen states have an abstinence-only curriculum, whereas 13 do not require that instruction be age-appropriate, inclusive, medically accurate, or evidence-based/informed. And this is just the tip of the iceberg, according to a 2022 report issued by the Sex Ed for Social Change organization.
Parents should take an inclusive approach to sex communication and create a safe space for discussing sex and sexual orientation, said almost all (96.7%) of male young adults who participated in a qualitative study. This would help reinforce acceptance and parents could possibly serve as a proxy for children who’ve not yet disclosed their sexual orientation. Yet, few parents are equipped or prepared to have these meaningful conversations with gay, bisexual, queer, or gender-diverse children, despite the fact that they are especially vulnerable to poor sexual health outcomes, bullying, abuse, and mental health challenges, as well as high-risk sexual behaviors.
“Parents are sexual socialization agents,” Dalmacio Dennis Flores, PhD, ACRN, assistant professor of nursing at the University of Pennsylvania, Philadelphia, told this news organization. “It’s through the information that they convey, the way that they normalize rituals and expectations, that they inform young people of all of societal expectations or roles they’ll be fulfilling in the future.”
Dr. Flores is lead author of a study published in the Journal of Adolescent Health. He and his colleagues collected perspectives on comprehensive, inclusive, and age-appropriate parent-child sex communications from 30 GBQ adolescent males aged 15-20 years who were already “out” to their parents. Participants were asked to sort through 28 preprinted note cards containing broad sexual health topics (for instance, human anatomy, dating, sexually transmitted infections) as well as topics theoretically specific to GBQ individuals (for example, anal sex), and were asked to add additional topics that they felt were missing. They were then directed to recommend topics along with ideal timing (that is, elementary, middle, or high school) for these conversations.
Study findings also underscored the importance of initiating comprehensive sexuality talks as early as elementary school age – namely to start preparing GBQ children for inevitable adversities that they were likely to encounter later in life, as well as to form building blocks for more mature, in-depth discussions during high school.
Importantly, these recommendations generally align with those aimed at heterosexual youth.
“When we refer to topics for elementary school, they are general parameters of what kids might be interested in or want to hear more about; it’s not planting a seed,” explained Dr. Flores.
Eva Goldfarb, PhD, LHD, MA, professor of public health at Montclair (N.J.) State University, agreed. “We always talk about (in sex education) to follow young people’s lead. If your child is asking you a question, they deserve a response,” said Dr. Goldfarb, who wasn’t involved in the study. “It doesn’t mean you have to give a detailed- level explanation but if they’re asking about it, it means that they are thinking about it. But it’s really important for all young people to know all of this information.”
Along those lines, participants deemed that fundamental issues about bodies (for example, human anatomy, reproduction), different sexual orientations, and an introduction to foundational issues (like privacy, peer or social pressure, sexual abuse) would help elementary-aged children to normalize discussions about sex, anatomy, and sexual orientation.
Middle school conversations were ideally more in-depth to reflect the time when young people are beginning to explore and accept their social and sexual identities. Topics of discussion might include types of sexual intercourse (anal, oral, and vaginal), health promotion strategies (abstinence, condoms, and contraception), possible adverse outcomes of condomless intercourse (HIV, STIs), considerations about engaging in sexual intercourse (including readiness, negotiating boundaries, virginity), and interpersonal safety (for instance, sexting, alcohol/drugs/chemsex, sexual coercion, and partner abuse/violence).
Finally, high school age recommendations focused on socio-relational topics (such as hook-up culture, technology/online dating, and multiple or concurrent sex partners), which are most relevant during a time when adolescents are most prone to experimentation and risk-taking.
Acknowledging that the study approach was novel, Dr. Flores noted that hearing about these topics from the youth perspective allowed parents to prepare. “Communication is better when it’s anticipated vs. reactive,” he said.
Last but not least, clinicians also have an important role in supporting these conversations.
“We’ve always looked at sex communication as a dyadic process, as a parent bestowing wisdom on a child who doesn’t have that knowledge yet. But it can be a triadic model,” said Dr. Flores. “Providers can encourage parents to ask if a child is dating or is familiar with ways to protect themselves or provide consent, and act as a resource exclusively to troubleshoot emergent issues.”
This study was funded by the National Institutes of Health. The study also received supplementary funding from the Surgeon General C. Everett Koop HIV/AIDS Research Award. Dr. Flores and Dr. Goldfarb report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Race and geography tied to breast cancer care delays
suggesting the need to target high-risk geographic regions and patient groups to ensure timely care, new research suggests.
Among nearly 33,000 women from North Carolina with stage I-III breast cancer, Black patients were nearly twice as likely has non-Black patients to experience treatment delays of more than 60 days, researchers found.
“Our findings suggest that treatment delays are alarmingly common in patients at high risk for breast cancer death, including young Black women and patients with stage III disease,” the authors note in their article, which was published online in Cancer.
Research shows that breast cancer treatment delays of 30-60 days can lower survival, and Black patients face a “disproportionate risk of treatment delays across the breast cancer care delivery spectrum,” the authors explain.
However, studies exploring whether or how racial disparities in treatment delays relate to geography are more limited.
In the current analysis, researchers amassed a retrospective cohort of all patients with stage I-III breast cancer between 2004 and 2015 in the North Carolina Central Cancer Registry and explored the risk of treatment delay by race and geographic subregion.
The cohort included 32,626 women, 6,190 (19.0%) of whom were Black. Counties were divided into the nine Area Health Education Center regions for North Carolina.
Compared with non‐Black patients, Black patients were more likely to have stage III disease (15.2% vs. 9.3%), hormone receptor–negative tumors (29.3% vs. 15.6%), Medicaid insurance (46.7% vs. 14.9%), and to live within 5 miles of their treatment site (30.6% vs. 25.2%).
Overall, Black patients were almost two times more likely to experience a treatment delay of more than 60 days (15% vs. 8%).
On average, about one in seven Black women experienced a lengthy delay, but the risk varied depending on geographic location. Patients living in certain regions of the state were more likely to experience delays; those in the highest-risk region were about twice as likely to experience a delay as those in the lowest-risk region (relative risk, 2.1 among Black patients; and RR, 1.9 among non-Black patients).
The magnitude of the racial gap in treatment delay varied by region – from 0% to 9.4%. But overall, of patients who experienced treatment delays, a significantly greater proportion were Black patients in every region except region 2, where only 2.7% (93 of 3,362) of patients were Black.
Notably, two regions with the greatest disparities in treatment delay, as well as the highest absolute risk of treatment delay for Black patients, surround large cities.
“These delays weren’t explained by the patients’ distance from cancer treatment facilities, their specific stage of cancer or type of treatment, or what insurance they had,” lead author Katherine Reeder-Hayes, MD, with the University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill, said in a news release.
Instead, Dr. Reeder-Hayes said, the findings suggest that the structure of local health care systems, rather than patient characteristics, may better explain why some patients experience treatment delays.
In other words, “if cancer care teams in certain areas say, ‘Oh, it’s particularly hard to treat breast cancer in our area because people are poor or have really advanced stages of cancer when they come in,’ our research does not bear out that explanation,” Dr. Reeder-Hayes said in email to this news organization.
This study “highlights the persistent disparities in treatment delays Black women encounter, which often lead to worse outcomes,” said Kathie-Ann Joseph, MD, MPH, who was not involved in the research.
“Interestingly, the authors could not attribute these delays in treatment to patient-level factors,” said Dr. Joseph, a breast cancer surgeon at NYU Langone Perlmutter Cancer Center, New York. But the authors “did find substantial geographic variation, which suggests the need to address structural barriers contributing to treatment delays in Black women.”
Sara P. Cate, MD, who was not involved with the research, also noted that the study highlights a known issue – “that racial minorities have longer delays in cancer treatment.” And notably, she said, the findings reveal that this disparity persists in areas where access to care is better and more robust.
“The nuances of the delays to care are multifactorial,” said Dr. Cate, a breast cancer surgeon and director of the Breast Surgery Quality Program at Mount Sinai in New York. “We need to do better with this population, and it is a multilevel solution of financial assistance, social work, and patient navigation.”
The study was supported in part by grants from the Susan G. Komen Foundation and the NC State Employees’ Credit Union. Dr. Reeder-Hayes, Dr. Cate, and Dr. Joseph have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
suggesting the need to target high-risk geographic regions and patient groups to ensure timely care, new research suggests.
Among nearly 33,000 women from North Carolina with stage I-III breast cancer, Black patients were nearly twice as likely has non-Black patients to experience treatment delays of more than 60 days, researchers found.
“Our findings suggest that treatment delays are alarmingly common in patients at high risk for breast cancer death, including young Black women and patients with stage III disease,” the authors note in their article, which was published online in Cancer.
Research shows that breast cancer treatment delays of 30-60 days can lower survival, and Black patients face a “disproportionate risk of treatment delays across the breast cancer care delivery spectrum,” the authors explain.
However, studies exploring whether or how racial disparities in treatment delays relate to geography are more limited.
In the current analysis, researchers amassed a retrospective cohort of all patients with stage I-III breast cancer between 2004 and 2015 in the North Carolina Central Cancer Registry and explored the risk of treatment delay by race and geographic subregion.
The cohort included 32,626 women, 6,190 (19.0%) of whom were Black. Counties were divided into the nine Area Health Education Center regions for North Carolina.
Compared with non‐Black patients, Black patients were more likely to have stage III disease (15.2% vs. 9.3%), hormone receptor–negative tumors (29.3% vs. 15.6%), Medicaid insurance (46.7% vs. 14.9%), and to live within 5 miles of their treatment site (30.6% vs. 25.2%).
Overall, Black patients were almost two times more likely to experience a treatment delay of more than 60 days (15% vs. 8%).
On average, about one in seven Black women experienced a lengthy delay, but the risk varied depending on geographic location. Patients living in certain regions of the state were more likely to experience delays; those in the highest-risk region were about twice as likely to experience a delay as those in the lowest-risk region (relative risk, 2.1 among Black patients; and RR, 1.9 among non-Black patients).
The magnitude of the racial gap in treatment delay varied by region – from 0% to 9.4%. But overall, of patients who experienced treatment delays, a significantly greater proportion were Black patients in every region except region 2, where only 2.7% (93 of 3,362) of patients were Black.
Notably, two regions with the greatest disparities in treatment delay, as well as the highest absolute risk of treatment delay for Black patients, surround large cities.
“These delays weren’t explained by the patients’ distance from cancer treatment facilities, their specific stage of cancer or type of treatment, or what insurance they had,” lead author Katherine Reeder-Hayes, MD, with the University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill, said in a news release.
Instead, Dr. Reeder-Hayes said, the findings suggest that the structure of local health care systems, rather than patient characteristics, may better explain why some patients experience treatment delays.
In other words, “if cancer care teams in certain areas say, ‘Oh, it’s particularly hard to treat breast cancer in our area because people are poor or have really advanced stages of cancer when they come in,’ our research does not bear out that explanation,” Dr. Reeder-Hayes said in email to this news organization.
This study “highlights the persistent disparities in treatment delays Black women encounter, which often lead to worse outcomes,” said Kathie-Ann Joseph, MD, MPH, who was not involved in the research.
“Interestingly, the authors could not attribute these delays in treatment to patient-level factors,” said Dr. Joseph, a breast cancer surgeon at NYU Langone Perlmutter Cancer Center, New York. But the authors “did find substantial geographic variation, which suggests the need to address structural barriers contributing to treatment delays in Black women.”
Sara P. Cate, MD, who was not involved with the research, also noted that the study highlights a known issue – “that racial minorities have longer delays in cancer treatment.” And notably, she said, the findings reveal that this disparity persists in areas where access to care is better and more robust.
“The nuances of the delays to care are multifactorial,” said Dr. Cate, a breast cancer surgeon and director of the Breast Surgery Quality Program at Mount Sinai in New York. “We need to do better with this population, and it is a multilevel solution of financial assistance, social work, and patient navigation.”
The study was supported in part by grants from the Susan G. Komen Foundation and the NC State Employees’ Credit Union. Dr. Reeder-Hayes, Dr. Cate, and Dr. Joseph have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
suggesting the need to target high-risk geographic regions and patient groups to ensure timely care, new research suggests.
Among nearly 33,000 women from North Carolina with stage I-III breast cancer, Black patients were nearly twice as likely has non-Black patients to experience treatment delays of more than 60 days, researchers found.
“Our findings suggest that treatment delays are alarmingly common in patients at high risk for breast cancer death, including young Black women and patients with stage III disease,” the authors note in their article, which was published online in Cancer.
Research shows that breast cancer treatment delays of 30-60 days can lower survival, and Black patients face a “disproportionate risk of treatment delays across the breast cancer care delivery spectrum,” the authors explain.
However, studies exploring whether or how racial disparities in treatment delays relate to geography are more limited.
In the current analysis, researchers amassed a retrospective cohort of all patients with stage I-III breast cancer between 2004 and 2015 in the North Carolina Central Cancer Registry and explored the risk of treatment delay by race and geographic subregion.
The cohort included 32,626 women, 6,190 (19.0%) of whom were Black. Counties were divided into the nine Area Health Education Center regions for North Carolina.
Compared with non‐Black patients, Black patients were more likely to have stage III disease (15.2% vs. 9.3%), hormone receptor–negative tumors (29.3% vs. 15.6%), Medicaid insurance (46.7% vs. 14.9%), and to live within 5 miles of their treatment site (30.6% vs. 25.2%).
Overall, Black patients were almost two times more likely to experience a treatment delay of more than 60 days (15% vs. 8%).
On average, about one in seven Black women experienced a lengthy delay, but the risk varied depending on geographic location. Patients living in certain regions of the state were more likely to experience delays; those in the highest-risk region were about twice as likely to experience a delay as those in the lowest-risk region (relative risk, 2.1 among Black patients; and RR, 1.9 among non-Black patients).
The magnitude of the racial gap in treatment delay varied by region – from 0% to 9.4%. But overall, of patients who experienced treatment delays, a significantly greater proportion were Black patients in every region except region 2, where only 2.7% (93 of 3,362) of patients were Black.
Notably, two regions with the greatest disparities in treatment delay, as well as the highest absolute risk of treatment delay for Black patients, surround large cities.
“These delays weren’t explained by the patients’ distance from cancer treatment facilities, their specific stage of cancer or type of treatment, or what insurance they had,” lead author Katherine Reeder-Hayes, MD, with the University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill, said in a news release.
Instead, Dr. Reeder-Hayes said, the findings suggest that the structure of local health care systems, rather than patient characteristics, may better explain why some patients experience treatment delays.
In other words, “if cancer care teams in certain areas say, ‘Oh, it’s particularly hard to treat breast cancer in our area because people are poor or have really advanced stages of cancer when they come in,’ our research does not bear out that explanation,” Dr. Reeder-Hayes said in email to this news organization.
This study “highlights the persistent disparities in treatment delays Black women encounter, which often lead to worse outcomes,” said Kathie-Ann Joseph, MD, MPH, who was not involved in the research.
“Interestingly, the authors could not attribute these delays in treatment to patient-level factors,” said Dr. Joseph, a breast cancer surgeon at NYU Langone Perlmutter Cancer Center, New York. But the authors “did find substantial geographic variation, which suggests the need to address structural barriers contributing to treatment delays in Black women.”
Sara P. Cate, MD, who was not involved with the research, also noted that the study highlights a known issue – “that racial minorities have longer delays in cancer treatment.” And notably, she said, the findings reveal that this disparity persists in areas where access to care is better and more robust.
“The nuances of the delays to care are multifactorial,” said Dr. Cate, a breast cancer surgeon and director of the Breast Surgery Quality Program at Mount Sinai in New York. “We need to do better with this population, and it is a multilevel solution of financial assistance, social work, and patient navigation.”
The study was supported in part by grants from the Susan G. Komen Foundation and the NC State Employees’ Credit Union. Dr. Reeder-Hayes, Dr. Cate, and Dr. Joseph have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CANCER
Black patients less likely to receive opioids for advanced cancer
Opioids are widely regarded as a linchpin in the treatment of moderate to severe cancer-related pain and end-of-life symptoms; however, a new study suggests.
Black patients were more likely to undergo urine drug screening (UDS) despite being less likely to receive any opioids for pain management and receiving lower daily doses of opioids in comparison with White patients, the study found.
The inequities were particularly stark for Black men. “We found that Black men were far less likely to be prescribed reasonable doses than White men were,” said the study’s senior author, Alexi Wright, MD, MPH, a gynecologic oncologist and a researcher in the division of population sciences at Dana-Farber Cancer Institute, Boston. “And Black men were less likely to receive long-acting opioids, which are essential for many patients dying of cancer. Our findings are startling because everyone should agree that cancer patients should have equal access to pain relief at the end of life.”
The study was published on in the Journal of Clinical Oncology.
The researchers gathered data on 318,549 Medicare beneficiaries older than 65 years with poor-prognosis cancers who died between 2007 and 2019. During this time frame, for all groups, access to opioids declined and urine drug testing expanded, owing to the overall opioid epidemic in the United States. Overall, the proportion of patients near end of life (EOL) who received any opioid or long-acting opioids decreased from 42.2% to 32.7% and from 17.9% to 9.4%, respectively.
The investigators used National Drug Codes to identify all Medicare Part D claims for outpatient opioid prescriptions, excluding addiction treatments, cough suppressants, and parenteral opioids. They focused on prescriptions that were filled at least 30 days before death or hospice enrollment.
Among the study participants, the majority (85.5%) of patients were White, 29,555 patients (9.3%) were Black, and 16,636 patients (5.2%) were Hispanic.
Black and Hispanic patients were statistically less likely than White patients to receive opioid prescriptions near EOL (Black, –4.3 percentage points; Hispanic, –3.6 percentage points). They were also less likely to receive long-acting opioid prescriptions (Black, –3.1 percentage points; Hispanic, –2.2 percentage points).
“It’s not just that patients of color are less likely to get opioids, but when they do get them, they get lower doses, and they also are less likely to get long-acting opioids, which a lot of people view as sort of more potential for addiction, which isn’t necessarily true but kind of viewed with heightened concern or suspicion,” the study’s lead author, Andrea Enzinger, MD, a gastrointestinal oncologist and a researcher in Dana-Farber’s division of population sciences, said in an interview.
Dr. Enzinger added that she believes systemic racism and preconceived biases toward minorities and drug addiction may be contributing to these trends.
When Black patients did receive at least one opioid prescription, they received daily doses that were 10.5 morphine milligram equivalents (MMEs) lower than doses given to White patients. Compared with the total opioid dose filled per White decedent near EOL, the total dose filled per Black decedent was 210 MMEs lower.
“We all need to be worried about the potential for misuse or addiction, but this is the one setting that is very low on my priority list when somebody is dying. I mean, we’re looking at the last month of life, so nobody has the potential to become addicted,” Dr. Enzinger commented.
The team also evaluated rates or urine drug screening (UDS), but as these rates were relatively low, they expanded the time frame to 180 days before death or hospice. They found that disparities in UDS disproportionately affected Black men.
From 2007 to 2019, the proportion of patients who underwent UDS increased from 0.6% to 6.7% in the 180 days before death or hospice; however, Black decedents were tested more often than White or Hispanic decedents.
Black decedents were 0.5 percentage points more likely than White decedents to undergo UDS near EOL.
“The disparities in urine drug screening are modest but important, because they hint at underlying systematic racism in recommending patients for screening,” Dr. Wright said. “Screening needs to either be applied uniformly or not at all for patients in this situation.”
The researchers acknowledged that their findings likely do not represent the full spectrum of prescribing disparities and believe that the work should be expanded among younger populations. Nevertheless, the investigators believe the work highlights the persistent racial and ethnic disparities in opioid access.
The study was supported by a grant from the Agency for Healthcare Research and Policy.
A version of this article first appeared on Medscape.com.
Opioids are widely regarded as a linchpin in the treatment of moderate to severe cancer-related pain and end-of-life symptoms; however, a new study suggests.
Black patients were more likely to undergo urine drug screening (UDS) despite being less likely to receive any opioids for pain management and receiving lower daily doses of opioids in comparison with White patients, the study found.
The inequities were particularly stark for Black men. “We found that Black men were far less likely to be prescribed reasonable doses than White men were,” said the study’s senior author, Alexi Wright, MD, MPH, a gynecologic oncologist and a researcher in the division of population sciences at Dana-Farber Cancer Institute, Boston. “And Black men were less likely to receive long-acting opioids, which are essential for many patients dying of cancer. Our findings are startling because everyone should agree that cancer patients should have equal access to pain relief at the end of life.”
The study was published on in the Journal of Clinical Oncology.
The researchers gathered data on 318,549 Medicare beneficiaries older than 65 years with poor-prognosis cancers who died between 2007 and 2019. During this time frame, for all groups, access to opioids declined and urine drug testing expanded, owing to the overall opioid epidemic in the United States. Overall, the proportion of patients near end of life (EOL) who received any opioid or long-acting opioids decreased from 42.2% to 32.7% and from 17.9% to 9.4%, respectively.
The investigators used National Drug Codes to identify all Medicare Part D claims for outpatient opioid prescriptions, excluding addiction treatments, cough suppressants, and parenteral opioids. They focused on prescriptions that were filled at least 30 days before death or hospice enrollment.
Among the study participants, the majority (85.5%) of patients were White, 29,555 patients (9.3%) were Black, and 16,636 patients (5.2%) were Hispanic.
Black and Hispanic patients were statistically less likely than White patients to receive opioid prescriptions near EOL (Black, –4.3 percentage points; Hispanic, –3.6 percentage points). They were also less likely to receive long-acting opioid prescriptions (Black, –3.1 percentage points; Hispanic, –2.2 percentage points).
“It’s not just that patients of color are less likely to get opioids, but when they do get them, they get lower doses, and they also are less likely to get long-acting opioids, which a lot of people view as sort of more potential for addiction, which isn’t necessarily true but kind of viewed with heightened concern or suspicion,” the study’s lead author, Andrea Enzinger, MD, a gastrointestinal oncologist and a researcher in Dana-Farber’s division of population sciences, said in an interview.
Dr. Enzinger added that she believes systemic racism and preconceived biases toward minorities and drug addiction may be contributing to these trends.
When Black patients did receive at least one opioid prescription, they received daily doses that were 10.5 morphine milligram equivalents (MMEs) lower than doses given to White patients. Compared with the total opioid dose filled per White decedent near EOL, the total dose filled per Black decedent was 210 MMEs lower.
“We all need to be worried about the potential for misuse or addiction, but this is the one setting that is very low on my priority list when somebody is dying. I mean, we’re looking at the last month of life, so nobody has the potential to become addicted,” Dr. Enzinger commented.
The team also evaluated rates or urine drug screening (UDS), but as these rates were relatively low, they expanded the time frame to 180 days before death or hospice. They found that disparities in UDS disproportionately affected Black men.
From 2007 to 2019, the proportion of patients who underwent UDS increased from 0.6% to 6.7% in the 180 days before death or hospice; however, Black decedents were tested more often than White or Hispanic decedents.
Black decedents were 0.5 percentage points more likely than White decedents to undergo UDS near EOL.
“The disparities in urine drug screening are modest but important, because they hint at underlying systematic racism in recommending patients for screening,” Dr. Wright said. “Screening needs to either be applied uniformly or not at all for patients in this situation.”
The researchers acknowledged that their findings likely do not represent the full spectrum of prescribing disparities and believe that the work should be expanded among younger populations. Nevertheless, the investigators believe the work highlights the persistent racial and ethnic disparities in opioid access.
The study was supported by a grant from the Agency for Healthcare Research and Policy.
A version of this article first appeared on Medscape.com.
Opioids are widely regarded as a linchpin in the treatment of moderate to severe cancer-related pain and end-of-life symptoms; however, a new study suggests.
Black patients were more likely to undergo urine drug screening (UDS) despite being less likely to receive any opioids for pain management and receiving lower daily doses of opioids in comparison with White patients, the study found.
The inequities were particularly stark for Black men. “We found that Black men were far less likely to be prescribed reasonable doses than White men were,” said the study’s senior author, Alexi Wright, MD, MPH, a gynecologic oncologist and a researcher in the division of population sciences at Dana-Farber Cancer Institute, Boston. “And Black men were less likely to receive long-acting opioids, which are essential for many patients dying of cancer. Our findings are startling because everyone should agree that cancer patients should have equal access to pain relief at the end of life.”
The study was published on in the Journal of Clinical Oncology.
The researchers gathered data on 318,549 Medicare beneficiaries older than 65 years with poor-prognosis cancers who died between 2007 and 2019. During this time frame, for all groups, access to opioids declined and urine drug testing expanded, owing to the overall opioid epidemic in the United States. Overall, the proportion of patients near end of life (EOL) who received any opioid or long-acting opioids decreased from 42.2% to 32.7% and from 17.9% to 9.4%, respectively.
The investigators used National Drug Codes to identify all Medicare Part D claims for outpatient opioid prescriptions, excluding addiction treatments, cough suppressants, and parenteral opioids. They focused on prescriptions that were filled at least 30 days before death or hospice enrollment.
Among the study participants, the majority (85.5%) of patients were White, 29,555 patients (9.3%) were Black, and 16,636 patients (5.2%) were Hispanic.
Black and Hispanic patients were statistically less likely than White patients to receive opioid prescriptions near EOL (Black, –4.3 percentage points; Hispanic, –3.6 percentage points). They were also less likely to receive long-acting opioid prescriptions (Black, –3.1 percentage points; Hispanic, –2.2 percentage points).
“It’s not just that patients of color are less likely to get opioids, but when they do get them, they get lower doses, and they also are less likely to get long-acting opioids, which a lot of people view as sort of more potential for addiction, which isn’t necessarily true but kind of viewed with heightened concern or suspicion,” the study’s lead author, Andrea Enzinger, MD, a gastrointestinal oncologist and a researcher in Dana-Farber’s division of population sciences, said in an interview.
Dr. Enzinger added that she believes systemic racism and preconceived biases toward minorities and drug addiction may be contributing to these trends.
When Black patients did receive at least one opioid prescription, they received daily doses that were 10.5 morphine milligram equivalents (MMEs) lower than doses given to White patients. Compared with the total opioid dose filled per White decedent near EOL, the total dose filled per Black decedent was 210 MMEs lower.
“We all need to be worried about the potential for misuse or addiction, but this is the one setting that is very low on my priority list when somebody is dying. I mean, we’re looking at the last month of life, so nobody has the potential to become addicted,” Dr. Enzinger commented.
The team also evaluated rates or urine drug screening (UDS), but as these rates were relatively low, they expanded the time frame to 180 days before death or hospice. They found that disparities in UDS disproportionately affected Black men.
From 2007 to 2019, the proportion of patients who underwent UDS increased from 0.6% to 6.7% in the 180 days before death or hospice; however, Black decedents were tested more often than White or Hispanic decedents.
Black decedents were 0.5 percentage points more likely than White decedents to undergo UDS near EOL.
“The disparities in urine drug screening are modest but important, because they hint at underlying systematic racism in recommending patients for screening,” Dr. Wright said. “Screening needs to either be applied uniformly or not at all for patients in this situation.”
The researchers acknowledged that their findings likely do not represent the full spectrum of prescribing disparities and believe that the work should be expanded among younger populations. Nevertheless, the investigators believe the work highlights the persistent racial and ethnic disparities in opioid access.
The study was supported by a grant from the Agency for Healthcare Research and Policy.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Vitiligo
THE COMPARISON
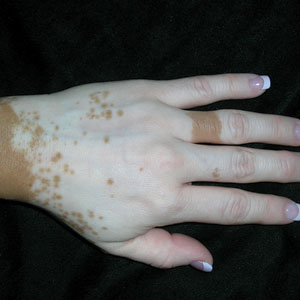
A Vitiligo in a young Hispanic female, which spared the area under a ring. The patient has spotty return of pigment on the hand after narrowband UVB treatment.
B Vitiligo on the hand in a young Hispanic male.

Vitiligo is a chronic autoimmune disorder characterized by areas of depigmented white patches on the skin due to the loss of melanocytes in the epidermis. Various theories on the pathogenesis of vitiligo exist; however, autoimmune destruction of melanocytes remains the leading hypothesis, followed by intrinsic defects in melanocytes.1 Vitiligo is associated with various autoimmune diseases but is most frequently reported in conjunction with thyroid disorders.2
Epidemiology
Vitiligo affects approximately 1% of the US population and up to 8% worldwide.2 There is no difference in prevalence between races or genders. Females typically acquire the disease earlier than males. Onset may occur at any age, although about half of patients will have vitiligo by 20 years of age.1
Key clinical features in people with darker skin tones
Bright white patches are characteristic of vitiligo. The patches typically are asymptomatic and often affect the hands (Figures A and B), perioral skin, feet, and scalp, as well as areas more vulnerable to friction and trauma, such as the elbows and knees.2 Trichrome lesions—consisting of varying zones of white (depigmented), lighter brown (hypopigmented), and normal skin—are most commonly seen in individuals with darker skin. Trichrome vitiligo is considered an actively progressing variant of vitiligo.2
An important distinction when diagnosing vitiligo is evaluating for segmental vs nonsegmental vitiligo. Although nonsegmental vitiligo—the more common subtype—is characterized by symmetric distribution and a less predictable course, segmental vitiligo manifests in a localized and unilateral distribution, often avoiding extension past the midline. Segmental vitiligo typically manifests at a younger age and follows a more rapidly stabilizing course.3
Worth noting
Given that stark contrasts between pigmented and depigmented lesions are more prominent in darker skin tones, vitiligo can be more socially stigmatizing and psychologically devastating in these patients.4,5
Treatment of vitiligo includes narrowband UVB (NB-UVB) light phototherapy, excimer laser, topical corticosteroids, topical calcineurin inhibitors such as tacrolimus and pimecrolimus, and surgical melanocyte transplantation.1 In July 2022, ruxolitinib cream 1.5% was approved by the US Food and Drug Administration (FDA) for nonsegmental vitiligo in patients 12 years and older.6,7 It is the only FDA-approved therapy for vitiligo. It is thought to work by inhibiting the Janus kinase– signal transducers and activators of the transcription pathway.6 However, topical ruxolitinib is expensive, costing more than $2000 for 60 g.8
Health disparity highlight
A 2021 study reviewing the coverage policies of 15 commercial health care insurance companies, 50 BlueCross BlueShield plans, Medicaid, Medicare, and Veterans Affairs plans found inequities in the insurance coverage patterns for therapies used to treat vitiligo. There were 2 commonly cited reasons for denying coverage for therapies: vitiligo was considered cosmetic and therapies were not FDA approved.7 In comparison, NB-UVB light phototherapy for psoriasis is not considered cosmetic and has a much higher insurance coverage rate.9,10 The out-of-pocket cost for a patient to purchase their own NB-UVB light phototherapy is more than $5000.11 Not all patients of color are economically disadvantaged, but in the United States, Black and Hispanic populations experience disproportionately higher rates of poverty (19% and 17%, respectively) compared to their White counterparts (8%).12
Final thoughts
US Food and Drug Administration approval of new drugs or new treatment indications comes after years of research discovery and large-scale trials. This pursuit of new discovery, however, is uneven. Vitiligo has historically been understudied and underfunded for research; this is common among several conditions adversely affecting people of color in the United States.13
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016/j.jaad.2010.11.061
- van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin. 2017; 35:145-150. doi:10.1016/j.det.2016.11.005
- Grimes PE, Miller MM. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4:32-37. doi:10.1016/j.ijwd.2017.11.005
- Ezzedine K, Eleftheriadou V, Jones H, et al. Psychosocial effects of vitiligo: a systematic literature review [published online September 23, 2021]. Am J Clin Dermatol. 2021;22:757-774. doi:10.1007/s40257 -021-00631-6
- FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. News release. US Food and Drug Administration; July 19, 2022. Accessed December 27, 2022. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients -aged-12-and-older
- Blundell A, Sachar M, Gabel CK, et al. The scope of health insurance coverage of vitiligo treatments in the United States: implications for health care outcomes and disparities in children of color [published online July 16, 2021]. Pediatr Dermatol. 2021; 38(suppl 2):79-85. doi:10.1111/pde.14714
- Opzelura prices, coupons, and patient assistance programs. Drugs.com. Accessed January 10, 2023. https://www.drugs.com /price-guide/opzelura#:~:text=Opzelura%20Prices%2C%20 Coupons%20and%20Patient,on%20the%20pharmacy%20you%20visit
- Bhutani T, Liao W. A practical approach to home UVB phototherapy for the treatment of generalized psoriasis. Pract Dermatol. 2010;7:31-35.
- Castro Porto Silva Lopes F, Ahmed A. Insurance coverage for phototherapy for vitiligo in comparison to psoriasis and atopic dermatitis. SKIN The Journal of Cutaneous Medicine. 2022;6:217-224. https://doi.org/10.25251/skin.6.3.6
- Smith MP, Ly K, Thibodeaux Q, et al. Home phototherapy for patients with vitiligo: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:451-459. doi:10.2147/CCID.S185798
- Shrider EA, Kollar M, Chen F, et al. Income and poverty in the United States: 2020. US Census Bureau. September 14, 2021. Accessed December 27, 2022. https://www.census.gov/library/publications/2021/demo/p60-273.html
- Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;(1):CD003263. doi:10.1002/14651858.CD003263.pub4
THE COMPARISON
A Vitiligo in a young Hispanic female, which spared the area under a ring. The patient has spotty return of pigment on the hand after narrowband UVB treatment.
B Vitiligo on the hand in a young Hispanic male.

Vitiligo is a chronic autoimmune disorder characterized by areas of depigmented white patches on the skin due to the loss of melanocytes in the epidermis. Various theories on the pathogenesis of vitiligo exist; however, autoimmune destruction of melanocytes remains the leading hypothesis, followed by intrinsic defects in melanocytes.1 Vitiligo is associated with various autoimmune diseases but is most frequently reported in conjunction with thyroid disorders.2
Epidemiology
Vitiligo affects approximately 1% of the US population and up to 8% worldwide.2 There is no difference in prevalence between races or genders. Females typically acquire the disease earlier than males. Onset may occur at any age, although about half of patients will have vitiligo by 20 years of age.1
Key clinical features in people with darker skin tones
Bright white patches are characteristic of vitiligo. The patches typically are asymptomatic and often affect the hands (Figures A and B), perioral skin, feet, and scalp, as well as areas more vulnerable to friction and trauma, such as the elbows and knees.2 Trichrome lesions—consisting of varying zones of white (depigmented), lighter brown (hypopigmented), and normal skin—are most commonly seen in individuals with darker skin. Trichrome vitiligo is considered an actively progressing variant of vitiligo.2
An important distinction when diagnosing vitiligo is evaluating for segmental vs nonsegmental vitiligo. Although nonsegmental vitiligo—the more common subtype—is characterized by symmetric distribution and a less predictable course, segmental vitiligo manifests in a localized and unilateral distribution, often avoiding extension past the midline. Segmental vitiligo typically manifests at a younger age and follows a more rapidly stabilizing course.3
Worth noting
Given that stark contrasts between pigmented and depigmented lesions are more prominent in darker skin tones, vitiligo can be more socially stigmatizing and psychologically devastating in these patients.4,5
Treatment of vitiligo includes narrowband UVB (NB-UVB) light phototherapy, excimer laser, topical corticosteroids, topical calcineurin inhibitors such as tacrolimus and pimecrolimus, and surgical melanocyte transplantation.1 In July 2022, ruxolitinib cream 1.5% was approved by the US Food and Drug Administration (FDA) for nonsegmental vitiligo in patients 12 years and older.6,7 It is the only FDA-approved therapy for vitiligo. It is thought to work by inhibiting the Janus kinase– signal transducers and activators of the transcription pathway.6 However, topical ruxolitinib is expensive, costing more than $2000 for 60 g.8
Health disparity highlight
A 2021 study reviewing the coverage policies of 15 commercial health care insurance companies, 50 BlueCross BlueShield plans, Medicaid, Medicare, and Veterans Affairs plans found inequities in the insurance coverage patterns for therapies used to treat vitiligo. There were 2 commonly cited reasons for denying coverage for therapies: vitiligo was considered cosmetic and therapies were not FDA approved.7 In comparison, NB-UVB light phototherapy for psoriasis is not considered cosmetic and has a much higher insurance coverage rate.9,10 The out-of-pocket cost for a patient to purchase their own NB-UVB light phototherapy is more than $5000.11 Not all patients of color are economically disadvantaged, but in the United States, Black and Hispanic populations experience disproportionately higher rates of poverty (19% and 17%, respectively) compared to their White counterparts (8%).12
Final thoughts
US Food and Drug Administration approval of new drugs or new treatment indications comes after years of research discovery and large-scale trials. This pursuit of new discovery, however, is uneven. Vitiligo has historically been understudied and underfunded for research; this is common among several conditions adversely affecting people of color in the United States.13
THE COMPARISON
A Vitiligo in a young Hispanic female, which spared the area under a ring. The patient has spotty return of pigment on the hand after narrowband UVB treatment.
B Vitiligo on the hand in a young Hispanic male.

Vitiligo is a chronic autoimmune disorder characterized by areas of depigmented white patches on the skin due to the loss of melanocytes in the epidermis. Various theories on the pathogenesis of vitiligo exist; however, autoimmune destruction of melanocytes remains the leading hypothesis, followed by intrinsic defects in melanocytes.1 Vitiligo is associated with various autoimmune diseases but is most frequently reported in conjunction with thyroid disorders.2
Epidemiology
Vitiligo affects approximately 1% of the US population and up to 8% worldwide.2 There is no difference in prevalence between races or genders. Females typically acquire the disease earlier than males. Onset may occur at any age, although about half of patients will have vitiligo by 20 years of age.1
Key clinical features in people with darker skin tones
Bright white patches are characteristic of vitiligo. The patches typically are asymptomatic and often affect the hands (Figures A and B), perioral skin, feet, and scalp, as well as areas more vulnerable to friction and trauma, such as the elbows and knees.2 Trichrome lesions—consisting of varying zones of white (depigmented), lighter brown (hypopigmented), and normal skin—are most commonly seen in individuals with darker skin. Trichrome vitiligo is considered an actively progressing variant of vitiligo.2
An important distinction when diagnosing vitiligo is evaluating for segmental vs nonsegmental vitiligo. Although nonsegmental vitiligo—the more common subtype—is characterized by symmetric distribution and a less predictable course, segmental vitiligo manifests in a localized and unilateral distribution, often avoiding extension past the midline. Segmental vitiligo typically manifests at a younger age and follows a more rapidly stabilizing course.3
Worth noting
Given that stark contrasts between pigmented and depigmented lesions are more prominent in darker skin tones, vitiligo can be more socially stigmatizing and psychologically devastating in these patients.4,5
Treatment of vitiligo includes narrowband UVB (NB-UVB) light phototherapy, excimer laser, topical corticosteroids, topical calcineurin inhibitors such as tacrolimus and pimecrolimus, and surgical melanocyte transplantation.1 In July 2022, ruxolitinib cream 1.5% was approved by the US Food and Drug Administration (FDA) for nonsegmental vitiligo in patients 12 years and older.6,7 It is the only FDA-approved therapy for vitiligo. It is thought to work by inhibiting the Janus kinase– signal transducers and activators of the transcription pathway.6 However, topical ruxolitinib is expensive, costing more than $2000 for 60 g.8
Health disparity highlight
A 2021 study reviewing the coverage policies of 15 commercial health care insurance companies, 50 BlueCross BlueShield plans, Medicaid, Medicare, and Veterans Affairs plans found inequities in the insurance coverage patterns for therapies used to treat vitiligo. There were 2 commonly cited reasons for denying coverage for therapies: vitiligo was considered cosmetic and therapies were not FDA approved.7 In comparison, NB-UVB light phototherapy for psoriasis is not considered cosmetic and has a much higher insurance coverage rate.9,10 The out-of-pocket cost for a patient to purchase their own NB-UVB light phototherapy is more than $5000.11 Not all patients of color are economically disadvantaged, but in the United States, Black and Hispanic populations experience disproportionately higher rates of poverty (19% and 17%, respectively) compared to their White counterparts (8%).12
Final thoughts
US Food and Drug Administration approval of new drugs or new treatment indications comes after years of research discovery and large-scale trials. This pursuit of new discovery, however, is uneven. Vitiligo has historically been understudied and underfunded for research; this is common among several conditions adversely affecting people of color in the United States.13
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016/j.jaad.2010.11.061
- van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin. 2017; 35:145-150. doi:10.1016/j.det.2016.11.005
- Grimes PE, Miller MM. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4:32-37. doi:10.1016/j.ijwd.2017.11.005
- Ezzedine K, Eleftheriadou V, Jones H, et al. Psychosocial effects of vitiligo: a systematic literature review [published online September 23, 2021]. Am J Clin Dermatol. 2021;22:757-774. doi:10.1007/s40257 -021-00631-6
- FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. News release. US Food and Drug Administration; July 19, 2022. Accessed December 27, 2022. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients -aged-12-and-older
- Blundell A, Sachar M, Gabel CK, et al. The scope of health insurance coverage of vitiligo treatments in the United States: implications for health care outcomes and disparities in children of color [published online July 16, 2021]. Pediatr Dermatol. 2021; 38(suppl 2):79-85. doi:10.1111/pde.14714
- Opzelura prices, coupons, and patient assistance programs. Drugs.com. Accessed January 10, 2023. https://www.drugs.com /price-guide/opzelura#:~:text=Opzelura%20Prices%2C%20 Coupons%20and%20Patient,on%20the%20pharmacy%20you%20visit
- Bhutani T, Liao W. A practical approach to home UVB phototherapy for the treatment of generalized psoriasis. Pract Dermatol. 2010;7:31-35.
- Castro Porto Silva Lopes F, Ahmed A. Insurance coverage for phototherapy for vitiligo in comparison to psoriasis and atopic dermatitis. SKIN The Journal of Cutaneous Medicine. 2022;6:217-224. https://doi.org/10.25251/skin.6.3.6
- Smith MP, Ly K, Thibodeaux Q, et al. Home phototherapy for patients with vitiligo: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:451-459. doi:10.2147/CCID.S185798
- Shrider EA, Kollar M, Chen F, et al. Income and poverty in the United States: 2020. US Census Bureau. September 14, 2021. Accessed December 27, 2022. https://www.census.gov/library/publications/2021/demo/p60-273.html
- Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;(1):CD003263. doi:10.1002/14651858.CD003263.pub4
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016/j.jaad.2010.11.061
- van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin. 2017; 35:145-150. doi:10.1016/j.det.2016.11.005
- Grimes PE, Miller MM. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4:32-37. doi:10.1016/j.ijwd.2017.11.005
- Ezzedine K, Eleftheriadou V, Jones H, et al. Psychosocial effects of vitiligo: a systematic literature review [published online September 23, 2021]. Am J Clin Dermatol. 2021;22:757-774. doi:10.1007/s40257 -021-00631-6
- FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. News release. US Food and Drug Administration; July 19, 2022. Accessed December 27, 2022. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients -aged-12-and-older
- Blundell A, Sachar M, Gabel CK, et al. The scope of health insurance coverage of vitiligo treatments in the United States: implications for health care outcomes and disparities in children of color [published online July 16, 2021]. Pediatr Dermatol. 2021; 38(suppl 2):79-85. doi:10.1111/pde.14714
- Opzelura prices, coupons, and patient assistance programs. Drugs.com. Accessed January 10, 2023. https://www.drugs.com /price-guide/opzelura#:~:text=Opzelura%20Prices%2C%20 Coupons%20and%20Patient,on%20the%20pharmacy%20you%20visit
- Bhutani T, Liao W. A practical approach to home UVB phototherapy for the treatment of generalized psoriasis. Pract Dermatol. 2010;7:31-35.
- Castro Porto Silva Lopes F, Ahmed A. Insurance coverage for phototherapy for vitiligo in comparison to psoriasis and atopic dermatitis. SKIN The Journal of Cutaneous Medicine. 2022;6:217-224. https://doi.org/10.25251/skin.6.3.6
- Smith MP, Ly K, Thibodeaux Q, et al. Home phototherapy for patients with vitiligo: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:451-459. doi:10.2147/CCID.S185798
- Shrider EA, Kollar M, Chen F, et al. Income and poverty in the United States: 2020. US Census Bureau. September 14, 2021. Accessed December 27, 2022. https://www.census.gov/library/publications/2021/demo/p60-273.html
- Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;(1):CD003263. doi:10.1002/14651858.CD003263.pub4
Brain scans show effect of poverty, stress on Black children
Childhood stress can change the brain negatively, according to a new study that says Black children are affected more because they experience more poverty and adversity.
“The researchers analyzed MRI scans to identify small differences in the volume of certain brain structures, and said these could accumulate as children age and play a role in the later development of mental health problems,” STAT News reported. “The finding, part of an emerging research field looking at how racism and other social factors may affect the physical architecture of the brain, may help explain longstanding racial disparities in the prevalence of psychiatric disorders such as PTSD.”
The study was published in The American Journal of Psychiatry.
Brain development is affected by “disparities faced by certain groups of people,” even among children as young as 9 years old, said Nathaniel Harnett, an assistant professor of psychiatry at Harvard Medical School, Boston, and the study’s senior author. “If we’re going to treat the world as colorblind, we’re not going to create mental health solutions that are effective for all people.”
The study used evidence from the Adolescent Brain Cognitive Development Study, which the National Institutes of Health established in 2015 to study the brains and experiences of thousands of American children through early adulthood.
Brain scans revealed that Black children had less gray matter in 11 of 14 brain areas that were examined. Disparities in 8 of the 14 brain areas were affected by childhood adversity, particularly poverty.
A version of this article first appeared on WebMD.com.
Childhood stress can change the brain negatively, according to a new study that says Black children are affected more because they experience more poverty and adversity.
“The researchers analyzed MRI scans to identify small differences in the volume of certain brain structures, and said these could accumulate as children age and play a role in the later development of mental health problems,” STAT News reported. “The finding, part of an emerging research field looking at how racism and other social factors may affect the physical architecture of the brain, may help explain longstanding racial disparities in the prevalence of psychiatric disorders such as PTSD.”
The study was published in The American Journal of Psychiatry.
Brain development is affected by “disparities faced by certain groups of people,” even among children as young as 9 years old, said Nathaniel Harnett, an assistant professor of psychiatry at Harvard Medical School, Boston, and the study’s senior author. “If we’re going to treat the world as colorblind, we’re not going to create mental health solutions that are effective for all people.”
The study used evidence from the Adolescent Brain Cognitive Development Study, which the National Institutes of Health established in 2015 to study the brains and experiences of thousands of American children through early adulthood.
Brain scans revealed that Black children had less gray matter in 11 of 14 brain areas that were examined. Disparities in 8 of the 14 brain areas were affected by childhood adversity, particularly poverty.
A version of this article first appeared on WebMD.com.
Childhood stress can change the brain negatively, according to a new study that says Black children are affected more because they experience more poverty and adversity.
“The researchers analyzed MRI scans to identify small differences in the volume of certain brain structures, and said these could accumulate as children age and play a role in the later development of mental health problems,” STAT News reported. “The finding, part of an emerging research field looking at how racism and other social factors may affect the physical architecture of the brain, may help explain longstanding racial disparities in the prevalence of psychiatric disorders such as PTSD.”
The study was published in The American Journal of Psychiatry.
Brain development is affected by “disparities faced by certain groups of people,” even among children as young as 9 years old, said Nathaniel Harnett, an assistant professor of psychiatry at Harvard Medical School, Boston, and the study’s senior author. “If we’re going to treat the world as colorblind, we’re not going to create mental health solutions that are effective for all people.”
The study used evidence from the Adolescent Brain Cognitive Development Study, which the National Institutes of Health established in 2015 to study the brains and experiences of thousands of American children through early adulthood.
Brain scans revealed that Black children had less gray matter in 11 of 14 brain areas that were examined. Disparities in 8 of the 14 brain areas were affected by childhood adversity, particularly poverty.
A version of this article first appeared on WebMD.com.
FROM THE AMERICAN JOURNAL OF PSYCHIATRY
CV deaths jumped in 2020, reflecting pandemic toll
Cardiovascular-related deaths increased dramatically in 2020, marking the largest single-year increase since 2015 and surpassing the previous record from 2003, according to the American Heart Association’s 2023 Statistical Update.
During the first year of the COVID-19 pandemic, the largest increases in cardiovascular disease (CVD) deaths were seen among Asian, Black, and Hispanic people.
“We thought we had been improving as a country with respect to CVD deaths over the past few decades,” Connie Tsao, MD, chair of the AHA Statistical Update writing committee, told this news organization.
Since 2020, however, those trends have changed. Dr. Tsao, a staff cardiologist at Beth Israel Deaconess Medical Center and assistant professor of medicine at Harvard Medical School, both in Boston, noted the firsthand experience that many clinicians had in seeing the shift.
“We observed this sharp rise in age-adjusted CVD deaths, which corresponds to the COVID-19 pandemic,” she said. “Those of us health care providers knew from the overfull hospitals and ICUs that clearly COVID took a toll, particularly in those with cardiovascular risk factors.”
The AHA Statistical Update was published online in the journal Circulation.
Data on deaths
Each year, the American Heart Association and National Institutes of Health report the latest statistics related to heart disease, stroke, and cardiovascular risk factors. The 2023 update includes additional information about pandemic-related data.
Overall, the number of people who died from cardiovascular disease increased during the first year of the pandemic, rising from 876,613 in 2019 to 928,741 in 2020. This topped the previous high of 910,000 in 2003.
In addition, the age-adjusted mortality rate increased for the first time in several years, Dr. Tsao said, by a “fairly substantial” 4.6%. The age-adjusted mortality rate incorporates the variability in the aging population from year to year, accounting for higher death rates among older people.
“Even though our total number of deaths has been slowly increasing over the past decade, we have seen a decline each year in our age-adjusted rates – until 2020,” she said. “I think that is very indicative of what has been going on within our country – and the world – in light of people of all ages being impacted by the COVID-19 pandemic, especially before vaccines were available to slow the spread.”
The largest increases in CVD-related deaths occurred among Asian, Black, and Hispanic people, who were most heavily affected during the first year of the pandemic.
“People from communities of color were among those most highly impacted, especially early on, often due to a disproportionate burden of cardiovascular risk factors, such as hypertension and obesity,” Michelle Albert, MD, MPH, president of AHA and a professor of medicine at the University of California, San Francisco, said in a statement.
Dr. Albert, who is also the director of UCSF’s Center for the Study of Adversity and Cardiovascular Disease, does research on health equity and noted the disparities seen in the 2020 numbers. “Additionally, there are socioeconomic considerations, as well as the ongoing impact of structural racism on multiple factors, including limiting the ability to access quality health care,” she said.
Additional considerations
In a special commentary, the Statistical Update writing committee pointed to the need to track data for other underrepresented communities, including LGBTQ people and those living in rural or urban areas. The authors outlined several ways to better understand the effects of identity and social determinants of health, as well as strategies to reduce cardiovascular-related disparities.
“This year’s writing group made a concerted effort to gather information on specific social factors related to health risk and outcomes, including sexual orientation, gender identity, urbanization, and socioeconomic position,” Dr. Tsao said. “However, the data are lacking because these communities are grossly underrepresented in clinical and epidemiological research.”
For the next several years, the AHA Statistical Update will likely include more insights about the effects of the COVID-19 pandemic, as well as ongoing disparities.
“For sure, we will be continuing to see the effects of the pandemic for years to come,” Dr. Tsao said. “Recognition of the disparities in outcomes among vulnerable groups should be a call to action among health care providers and researchers, administration, and policy leaders to investigate the reasons and make changes to reverse these trends.”
The statistical update was prepared by a volunteer writing group on behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee.
A version of this article first appeared on Medscape.com.
Cardiovascular-related deaths increased dramatically in 2020, marking the largest single-year increase since 2015 and surpassing the previous record from 2003, according to the American Heart Association’s 2023 Statistical Update.
During the first year of the COVID-19 pandemic, the largest increases in cardiovascular disease (CVD) deaths were seen among Asian, Black, and Hispanic people.
“We thought we had been improving as a country with respect to CVD deaths over the past few decades,” Connie Tsao, MD, chair of the AHA Statistical Update writing committee, told this news organization.
Since 2020, however, those trends have changed. Dr. Tsao, a staff cardiologist at Beth Israel Deaconess Medical Center and assistant professor of medicine at Harvard Medical School, both in Boston, noted the firsthand experience that many clinicians had in seeing the shift.
“We observed this sharp rise in age-adjusted CVD deaths, which corresponds to the COVID-19 pandemic,” she said. “Those of us health care providers knew from the overfull hospitals and ICUs that clearly COVID took a toll, particularly in those with cardiovascular risk factors.”
The AHA Statistical Update was published online in the journal Circulation.
Data on deaths
Each year, the American Heart Association and National Institutes of Health report the latest statistics related to heart disease, stroke, and cardiovascular risk factors. The 2023 update includes additional information about pandemic-related data.
Overall, the number of people who died from cardiovascular disease increased during the first year of the pandemic, rising from 876,613 in 2019 to 928,741 in 2020. This topped the previous high of 910,000 in 2003.
In addition, the age-adjusted mortality rate increased for the first time in several years, Dr. Tsao said, by a “fairly substantial” 4.6%. The age-adjusted mortality rate incorporates the variability in the aging population from year to year, accounting for higher death rates among older people.
“Even though our total number of deaths has been slowly increasing over the past decade, we have seen a decline each year in our age-adjusted rates – until 2020,” she said. “I think that is very indicative of what has been going on within our country – and the world – in light of people of all ages being impacted by the COVID-19 pandemic, especially before vaccines were available to slow the spread.”
The largest increases in CVD-related deaths occurred among Asian, Black, and Hispanic people, who were most heavily affected during the first year of the pandemic.
“People from communities of color were among those most highly impacted, especially early on, often due to a disproportionate burden of cardiovascular risk factors, such as hypertension and obesity,” Michelle Albert, MD, MPH, president of AHA and a professor of medicine at the University of California, San Francisco, said in a statement.
Dr. Albert, who is also the director of UCSF’s Center for the Study of Adversity and Cardiovascular Disease, does research on health equity and noted the disparities seen in the 2020 numbers. “Additionally, there are socioeconomic considerations, as well as the ongoing impact of structural racism on multiple factors, including limiting the ability to access quality health care,” she said.
Additional considerations
In a special commentary, the Statistical Update writing committee pointed to the need to track data for other underrepresented communities, including LGBTQ people and those living in rural or urban areas. The authors outlined several ways to better understand the effects of identity and social determinants of health, as well as strategies to reduce cardiovascular-related disparities.
“This year’s writing group made a concerted effort to gather information on specific social factors related to health risk and outcomes, including sexual orientation, gender identity, urbanization, and socioeconomic position,” Dr. Tsao said. “However, the data are lacking because these communities are grossly underrepresented in clinical and epidemiological research.”
For the next several years, the AHA Statistical Update will likely include more insights about the effects of the COVID-19 pandemic, as well as ongoing disparities.
“For sure, we will be continuing to see the effects of the pandemic for years to come,” Dr. Tsao said. “Recognition of the disparities in outcomes among vulnerable groups should be a call to action among health care providers and researchers, administration, and policy leaders to investigate the reasons and make changes to reverse these trends.”
The statistical update was prepared by a volunteer writing group on behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee.
A version of this article first appeared on Medscape.com.
Cardiovascular-related deaths increased dramatically in 2020, marking the largest single-year increase since 2015 and surpassing the previous record from 2003, according to the American Heart Association’s 2023 Statistical Update.
During the first year of the COVID-19 pandemic, the largest increases in cardiovascular disease (CVD) deaths were seen among Asian, Black, and Hispanic people.
“We thought we had been improving as a country with respect to CVD deaths over the past few decades,” Connie Tsao, MD, chair of the AHA Statistical Update writing committee, told this news organization.
Since 2020, however, those trends have changed. Dr. Tsao, a staff cardiologist at Beth Israel Deaconess Medical Center and assistant professor of medicine at Harvard Medical School, both in Boston, noted the firsthand experience that many clinicians had in seeing the shift.
“We observed this sharp rise in age-adjusted CVD deaths, which corresponds to the COVID-19 pandemic,” she said. “Those of us health care providers knew from the overfull hospitals and ICUs that clearly COVID took a toll, particularly in those with cardiovascular risk factors.”
The AHA Statistical Update was published online in the journal Circulation.
Data on deaths
Each year, the American Heart Association and National Institutes of Health report the latest statistics related to heart disease, stroke, and cardiovascular risk factors. The 2023 update includes additional information about pandemic-related data.
Overall, the number of people who died from cardiovascular disease increased during the first year of the pandemic, rising from 876,613 in 2019 to 928,741 in 2020. This topped the previous high of 910,000 in 2003.
In addition, the age-adjusted mortality rate increased for the first time in several years, Dr. Tsao said, by a “fairly substantial” 4.6%. The age-adjusted mortality rate incorporates the variability in the aging population from year to year, accounting for higher death rates among older people.
“Even though our total number of deaths has been slowly increasing over the past decade, we have seen a decline each year in our age-adjusted rates – until 2020,” she said. “I think that is very indicative of what has been going on within our country – and the world – in light of people of all ages being impacted by the COVID-19 pandemic, especially before vaccines were available to slow the spread.”
The largest increases in CVD-related deaths occurred among Asian, Black, and Hispanic people, who were most heavily affected during the first year of the pandemic.
“People from communities of color were among those most highly impacted, especially early on, often due to a disproportionate burden of cardiovascular risk factors, such as hypertension and obesity,” Michelle Albert, MD, MPH, president of AHA and a professor of medicine at the University of California, San Francisco, said in a statement.
Dr. Albert, who is also the director of UCSF’s Center for the Study of Adversity and Cardiovascular Disease, does research on health equity and noted the disparities seen in the 2020 numbers. “Additionally, there are socioeconomic considerations, as well as the ongoing impact of structural racism on multiple factors, including limiting the ability to access quality health care,” she said.
Additional considerations
In a special commentary, the Statistical Update writing committee pointed to the need to track data for other underrepresented communities, including LGBTQ people and those living in rural or urban areas. The authors outlined several ways to better understand the effects of identity and social determinants of health, as well as strategies to reduce cardiovascular-related disparities.
“This year’s writing group made a concerted effort to gather information on specific social factors related to health risk and outcomes, including sexual orientation, gender identity, urbanization, and socioeconomic position,” Dr. Tsao said. “However, the data are lacking because these communities are grossly underrepresented in clinical and epidemiological research.”
For the next several years, the AHA Statistical Update will likely include more insights about the effects of the COVID-19 pandemic, as well as ongoing disparities.
“For sure, we will be continuing to see the effects of the pandemic for years to come,” Dr. Tsao said. “Recognition of the disparities in outcomes among vulnerable groups should be a call to action among health care providers and researchers, administration, and policy leaders to investigate the reasons and make changes to reverse these trends.”
The statistical update was prepared by a volunteer writing group on behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
Pandemic pregnancy-linked deaths up 35% from 2019
Pregnancy-associated deaths, including drug-related deaths and homicide, were up 35% in 2020, compared with prepandemic 2019, new research indicates.
The data also show a 7.1% decrease in pregnancy-related suicides in 2020 from 2019.
The study, led by Claire E. Margerison, PhD, with the department of epidemiology and biostatistics at Michigan State University, East Lansing, included 4,528 pregnancy-associated deaths. The rate of deaths per 100,000 live births from April to December 2020 was 66.9 (95% confidence interval, 63.9-70.1). The comparative rate from April to December 2019 was 49.6. Researchers looked at that time period because the pandemic started in March 2020.
The findings were published online in JAMA Open Network.
Drug-related deaths up 55.3%
During the study period, drug deaths increased 55.3% and deaths from homicide increased 41.2%. Deaths from obstetric and other causes (mainly vehicle crashes) increased 28.4% and 56.7%, respectively, according to Dr. Margerison's group.
“Although pregnancy-associated deaths increased over time, increases from 2019 to 2020 were substantially larger than increases from 2018 to 2019,” the authors wrote.
The findings align with deaths in the general population in that time frame, they added.
Another study – this one looking at all-cause and cause-specific mortality from 2019 to 2020 in recently pregnant women, also published in JAMA Network Open, found significant racial and ethnic disparities in rates and cause of death.
According to the study, “Compared with non-Hispanic White women, mortality rates were three- to fivefold higher among American Indian or Alaska Native women for every cause, including suicide. Likewise, these findings suggest that non-Hispanic Black women experienced significantly higher mortality rates across causes, with the highest rates for homicide.”
Dr. Margerison and colleagues did not try to answer what caused the increases but pointed to the fentanyl epidemic, the murder of George Floyd, and COVID-19–related economic strain as potential stressors. They also suggest fewer screenings during the pandemic may have played a role.
Prevention opportunities missed
“Although pregnancy is considered an opportunity for screening and prevention related to physical, mental, and behavioral health, our data suggest that such opportunities were missed for hundreds of pregnant people during the pandemic,” the authors wrote.
Researchers analyzed cross-sectional U.S. death certificates from Jan. 1, 2018, to Dec. 31, 2020, for female U.S. residents ages 15-44 years. They then obtained the count for live births for the same population and time frame from the Centers for Disease Control and Prevention WONDER database.
They were able to identify pregnancy-associated deaths as the 2003 Revised Death Certificate contains a standardized pregnancy checkbox that asks whether the person was pregnant at the time of death, within 42 days of death, or within 43 days to 1 year of death.
Researchers also included deaths with ICD-10 codes linked with death from obstetric causes.
Deaths from overdose, suicide, and homicide are making up large and growing proportions of all deaths during pregnancy and in the first year postpartum, the authors report.
Dr. Margerison and coauthors, in research published in 2022, reported that these causes account for more than one-fifth of all pregnancy-related deaths. They also reported that drug-related deaths and homicides in this population have increased over the past 10 years.
“Substantial racial and ethnic inequities in these deaths exist,” they wrote in that paper.
The authors concluded in the current research: “Our study findings suggest that there is a need for prevention and intervention efforts, including harm-reduction strategies, tailored to pregnant and postpartum women, particularly during times of population stress and decreased utilization of preventive care, such as a pandemic.”
Dr. Margerison and coauthors reported receiving grant support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development during the study. One coauthor received personal fees from the World Health Organization and Population Reference Bureau outside the submitted work. One coauthor reported receiving grant support from the National Institutes of Mental Health during the study.
*This story was updated on 2/1.
Pregnancy-associated deaths, including drug-related deaths and homicide, were up 35% in 2020, compared with prepandemic 2019, new research indicates.
The data also show a 7.1% decrease in pregnancy-related suicides in 2020 from 2019.
The study, led by Claire E. Margerison, PhD, with the department of epidemiology and biostatistics at Michigan State University, East Lansing, included 4,528 pregnancy-associated deaths. The rate of deaths per 100,000 live births from April to December 2020 was 66.9 (95% confidence interval, 63.9-70.1). The comparative rate from April to December 2019 was 49.6. Researchers looked at that time period because the pandemic started in March 2020.
The findings were published online in JAMA Open Network.
Drug-related deaths up 55.3%
During the study period, drug deaths increased 55.3% and deaths from homicide increased 41.2%. Deaths from obstetric and other causes (mainly vehicle crashes) increased 28.4% and 56.7%, respectively, according to Dr. Margerison's group.
“Although pregnancy-associated deaths increased over time, increases from 2019 to 2020 were substantially larger than increases from 2018 to 2019,” the authors wrote.
The findings align with deaths in the general population in that time frame, they added.
Another study – this one looking at all-cause and cause-specific mortality from 2019 to 2020 in recently pregnant women, also published in JAMA Network Open, found significant racial and ethnic disparities in rates and cause of death.
According to the study, “Compared with non-Hispanic White women, mortality rates were three- to fivefold higher among American Indian or Alaska Native women for every cause, including suicide. Likewise, these findings suggest that non-Hispanic Black women experienced significantly higher mortality rates across causes, with the highest rates for homicide.”
Dr. Margerison and colleagues did not try to answer what caused the increases but pointed to the fentanyl epidemic, the murder of George Floyd, and COVID-19–related economic strain as potential stressors. They also suggest fewer screenings during the pandemic may have played a role.
Prevention opportunities missed
“Although pregnancy is considered an opportunity for screening and prevention related to physical, mental, and behavioral health, our data suggest that such opportunities were missed for hundreds of pregnant people during the pandemic,” the authors wrote.
Researchers analyzed cross-sectional U.S. death certificates from Jan. 1, 2018, to Dec. 31, 2020, for female U.S. residents ages 15-44 years. They then obtained the count for live births for the same population and time frame from the Centers for Disease Control and Prevention WONDER database.
They were able to identify pregnancy-associated deaths as the 2003 Revised Death Certificate contains a standardized pregnancy checkbox that asks whether the person was pregnant at the time of death, within 42 days of death, or within 43 days to 1 year of death.
Researchers also included deaths with ICD-10 codes linked with death from obstetric causes.
Deaths from overdose, suicide, and homicide are making up large and growing proportions of all deaths during pregnancy and in the first year postpartum, the authors report.
Dr. Margerison and coauthors, in research published in 2022, reported that these causes account for more than one-fifth of all pregnancy-related deaths. They also reported that drug-related deaths and homicides in this population have increased over the past 10 years.
“Substantial racial and ethnic inequities in these deaths exist,” they wrote in that paper.
The authors concluded in the current research: “Our study findings suggest that there is a need for prevention and intervention efforts, including harm-reduction strategies, tailored to pregnant and postpartum women, particularly during times of population stress and decreased utilization of preventive care, such as a pandemic.”
Dr. Margerison and coauthors reported receiving grant support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development during the study. One coauthor received personal fees from the World Health Organization and Population Reference Bureau outside the submitted work. One coauthor reported receiving grant support from the National Institutes of Mental Health during the study.
*This story was updated on 2/1.
Pregnancy-associated deaths, including drug-related deaths and homicide, were up 35% in 2020, compared with prepandemic 2019, new research indicates.
The data also show a 7.1% decrease in pregnancy-related suicides in 2020 from 2019.
The study, led by Claire E. Margerison, PhD, with the department of epidemiology and biostatistics at Michigan State University, East Lansing, included 4,528 pregnancy-associated deaths. The rate of deaths per 100,000 live births from April to December 2020 was 66.9 (95% confidence interval, 63.9-70.1). The comparative rate from April to December 2019 was 49.6. Researchers looked at that time period because the pandemic started in March 2020.
The findings were published online in JAMA Open Network.
Drug-related deaths up 55.3%
During the study period, drug deaths increased 55.3% and deaths from homicide increased 41.2%. Deaths from obstetric and other causes (mainly vehicle crashes) increased 28.4% and 56.7%, respectively, according to Dr. Margerison's group.
“Although pregnancy-associated deaths increased over time, increases from 2019 to 2020 were substantially larger than increases from 2018 to 2019,” the authors wrote.
The findings align with deaths in the general population in that time frame, they added.
Another study – this one looking at all-cause and cause-specific mortality from 2019 to 2020 in recently pregnant women, also published in JAMA Network Open, found significant racial and ethnic disparities in rates and cause of death.
According to the study, “Compared with non-Hispanic White women, mortality rates were three- to fivefold higher among American Indian or Alaska Native women for every cause, including suicide. Likewise, these findings suggest that non-Hispanic Black women experienced significantly higher mortality rates across causes, with the highest rates for homicide.”
Dr. Margerison and colleagues did not try to answer what caused the increases but pointed to the fentanyl epidemic, the murder of George Floyd, and COVID-19–related economic strain as potential stressors. They also suggest fewer screenings during the pandemic may have played a role.
Prevention opportunities missed
“Although pregnancy is considered an opportunity for screening and prevention related to physical, mental, and behavioral health, our data suggest that such opportunities were missed for hundreds of pregnant people during the pandemic,” the authors wrote.
Researchers analyzed cross-sectional U.S. death certificates from Jan. 1, 2018, to Dec. 31, 2020, for female U.S. residents ages 15-44 years. They then obtained the count for live births for the same population and time frame from the Centers for Disease Control and Prevention WONDER database.
They were able to identify pregnancy-associated deaths as the 2003 Revised Death Certificate contains a standardized pregnancy checkbox that asks whether the person was pregnant at the time of death, within 42 days of death, or within 43 days to 1 year of death.
Researchers also included deaths with ICD-10 codes linked with death from obstetric causes.
Deaths from overdose, suicide, and homicide are making up large and growing proportions of all deaths during pregnancy and in the first year postpartum, the authors report.
Dr. Margerison and coauthors, in research published in 2022, reported that these causes account for more than one-fifth of all pregnancy-related deaths. They also reported that drug-related deaths and homicides in this population have increased over the past 10 years.
“Substantial racial and ethnic inequities in these deaths exist,” they wrote in that paper.
The authors concluded in the current research: “Our study findings suggest that there is a need for prevention and intervention efforts, including harm-reduction strategies, tailored to pregnant and postpartum women, particularly during times of population stress and decreased utilization of preventive care, such as a pandemic.”
Dr. Margerison and coauthors reported receiving grant support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development during the study. One coauthor received personal fees from the World Health Organization and Population Reference Bureau outside the submitted work. One coauthor reported receiving grant support from the National Institutes of Mental Health during the study.
*This story was updated on 2/1.
FROM JAMA NETWORK OPEN
Female doctors have higher infertility rates and riskier pregnancies: What can be done?
In 2021, Eugene Kim, MD, division director of pediatric surgery and vice chair in the department of surgery at Cedars-Sinai Medical Center, Los Angeles, gave his presidential address to the Association for Academic Surgery.
“Presidents tend to give a message of hope or inspiration; I probably took it in a different way,” he said.
Dr. Kim told the story of one of his clinical partners, Eveline Shue, who, after five rounds of in vitro fertilization (IVF), became pregnant with twins. A high-achiever in her field, Ms. Shue continued working the grueling hours required by her job throughout pregnancy until she noticed concerning symptoms – musculoskeletal issues, extreme swelling, and more. She and her group decided that she should step back from work in her third trimester. A few days later, Ms. Shue suffered a stroke. She was rushed to the hospital where her babies were delivered by emergency C-section. Ms. Shue underwent brain surgery but later recovered and is still practicing in Southern California.
“I remember being at her bedside thinking, ‘How could we have let this happen? How could we have prevented this?’ ”
Dr. Kim’s speech kicked off a firestorm of awareness about pregnancy complications among physicians. “I got scores of emails from women around the country, surgeons in particular, who felt like their issues had been seen. The conversation was long overdue,” he said.
Family planning issues, pregnancy complications, infertility, and pregnancy loss are common, pervasive, and often silent issues in medicine. In July 2021, Dr. Kim and a group of other researchers published a study in JAMA Surgery. It revealed staggering truths: When compared to non-surgeons, female surgeons were more likely to delay pregnancy, use assisted reproductive technology such as IVF, have non-elective C-sections, and suffer pregnancy loss. In the study, 42% of surgeons had experienced pregnancy loss – more than double the rate of the general population. Almost half had serious pregnancy complications.
Research has found that female physicians in general have a significantly greater incidence of miscarriage, infertility, and pregnancy complications than the general population. According to a 2016 survey in the Journal of Women’s Health, the infertility rate for physicians is nearly 1 in 4, about double the rate of the general public.
The barriers to starting a family
Physicians face significant professional barriers that impact family planning. Demanding jobs with exhausting and often unpredictable hours contribute to a culture that, traditionally, has been far from family friendly. As a result, many physicians start families later. “For a pediatric surgeon, you finish training at age 35 – minimum,” says Dr. Kim. “Simply being a surgeon makes you a high-risk pregnancy candidate just because of the career.”
In 2020, Ariela L. Marshall, MD, an associate professor of clinical medicine at the University of Pennsylvania’s Perelman school of medicine, co-authored a commentary article in Academic Medicine titled “Physician Fertility: A Call to Action” which was based on her own experiences with infertility. Dr. Marshall was 34 when she and her husband decided to start a family, and she says her infertility diagnosis “came as a shock.”
“I never stopped to think about the consequences of a career path where I’m not going to be established until my 30s,” Dr. Marshall says. “I never thought about how long hours, overnight shifts, or working all the time could impact my fertility.”
It would take four cycles of IVF egg retrieval to create embryos and one failed implantation before Dr. Marshall became pregnant with her son.
When it comes to the timing of pregnancy, medical culture also plays a role. “There’s a lot of messaging around when it’s appropriate to carry a baby – and it’s not until after training is done,” says Arghavan Salles, MD, PhD, a clinical associate professor and special advisor for DEI programs at Stanford (Calif.) University’s department of medicine.
There are always exceptions. Some institutions are more flexible than others about pregnancy during residency. But Dr. Salles notes that this attitude is “not universal,” partly because of the lack of a comprehensive approach to pregnancy or parenthood in the United States. “There’s no federal paid parental leave in this country,” reminds Dr. Salles. “That signals that we don’t value parenting.”
The trickle-down effect of this in medicine is more like a waterfall. Some physicians complain when other physicians are out on leave. There’s an additional burden of work when people take time away, and there are often no support structures in place for backup or fill-in care. Dr. Salles said doctors often tell her that they were responsible for finding coverage for any time off during pregnancy or after becoming a parent. A paper of hers published in JAMA Surgery found that, for physicians, a fear of burdening others was a major barrier to getting pregnant during residency in the first place.
The physical consequences
Although research supports the benefits of physical activity throughout pregnancy, a job such as surgery that requires being on your feet for long periods of time “is not the same as exercise,” explains Erika Lu Rangel, MD, a gastrointestinal surgeon at Brigham and Women’s Hospital, Boston, and Dr. Kim’s lead author on the JAMA Surgery article.
Surgeons operating for more than 12 hours a week are at higher risk for pregnancy complications, the study found. Dr. Rangel also cites data suggesting that night shifts or swing shifts (the hours between day and night) put women at higher risk for pregnancy complications.
Equally alarming: Medical trainees appear to have “almost as high a rate of pregnancy complications as surgeons who have already completed their training,” said Dr. Rangel. It is a concerning finding since, as a younger cohort, they should have lower complication rates based on their age. But doctors in training may be on their feet even more than surgeons during long shifts.
Like Dr. Salles, Dr. Rangel sees these issues as part of a pervasive culture of “presenteeism” in medicine, and she points out that many surgeons don’t even take time off to grieve pregnancy loss or physically recover from it. “We work even when we’re sick and even when it’s not good for our health,” she said. “I think that’s an unhealthy behavior that we cultivate from the time that we’re trainees, and we carry it on through when we’re in practice.”
Penn Medicine’s Dr. Marshall remembers that her own maternity leave was “not an easy process to navigate.” From her hospital room on a magnesium drip for preeclampsia, she still attended Zoom meetings with her colleagues. “Nobody says, ‘Oh, you have to do this,’ ” Dr. Marshall explains, “but you wind up feeling guilty if you’re not there at all moments for everyone. That’s also something that needs to change.”
Dr. Rangel was pregnant with her oldest son as a fourth-year surgery resident. The day she gave birth to him she remembers waking up with a flu-like illness and a fever. She went to work anyway, because “you don’t call in sick as a resident.” She was barely able to complete her rounds and then had to lie down between cases. A co-resident found her and took her to labor and delivery. She had gone into premature labor at 37 weeks, and her son went into the NICU with complications.
“I remember feeling this enormous guilt,” says Dr. Rangel. “I’d been a mom for just a few minutes, and I felt like I had already failed him because I had prioritized what the residency thought of me above what I knew was necessary for his health.”
Hope for the future
Disturbed by the status quo, many physicians are pushing for change. “I think there’s a really important and positive conversation going on in the medical community right now about ways that we need to support new parent physicians,” said Dr. Rangel.
Parental leave is a key part of that support. Last year, The American Board of Medical Specialties enacted a mandate that all specialty boards 2 years or more in duration must provide at least 6 weeks of parental and caregiver leave. In 2023, the Accreditation Council for Graduate Medical Education (ACGME) required that all training programs match that policy. “This sends a message to policymakers and leaders in American medicine that this is a priority,” said Dr. Rangel.
In January 2022, a group from the University of Michigan also published an article in the Annals of Surgery called “Safe and Supported Pregnancy: A Call to Action for Surgery Chairs and Program Directors”. The essay urged leading groups such as the ACGME and the American Board of Surgery to “directly address the health and safety of pregnant trainees” and specifically, to “allow for further flexibility during training for pregnancy and peripartum periods,” calling these “fundamental necessities for cultural progress.”
Others have recommended allowing pregnant trainees more flexibility in their schedules or front-loading certain parts of the training that may be more difficult as a pregnancy progresses. Insurance coverage for fertility preservation and reproductive endocrinology services, and support for reentry (including lactation and childcare) are also issues that must be addressed, says Dr. Salles.
A new paper of Dr. Rangel’s, published in JAMA Surgery, suggests that things like mentorship for residents from faculty can also be important pieces of the puzzle.
Education about reproductive health must start earlier, too – as early as medical school. Research suggests only 8% of physicians receive education on the risks of delaying pregnancy. Those who do are significantly less likely to experience pregnancy loss or seek infertility treatment.
Dr. Salles recalls sitting in a classroom learning about advanced maternal age at a time when age 35 seemed unimaginably distant. “It was never taught – at least to my recollection – in a way that was like, ‘this could be your future,’ ” Dr. Salles says.” It was more like this abstract patient who might have advanced maternal age and what the consequences would be. Maybe some of my colleagues put two and two together, but I definitely didn’t.”
Dr. Marshall is the curriculum chair for the IGNITEMed Initiative, which aims to educate medical students about issues not discussed in traditional medical school curricula. Dr. Marshall and her colleague Julia Files, MD, talk with IGNITEMed students about reproductive life planning.
“Raising awareness is a very big thing. That’s not just true for medical students but for professionals at every level of medicine,” Dr. Marshall said. “Residency and fellowship training program directors, department chairs, and hospital CEOs all need to understand that these issues are very common in the people they oversee – and that they are medical issues, like any other medical issue, where people need time off and support.”
A version of this article first appeared on Medscape.com.
In 2021, Eugene Kim, MD, division director of pediatric surgery and vice chair in the department of surgery at Cedars-Sinai Medical Center, Los Angeles, gave his presidential address to the Association for Academic Surgery.
“Presidents tend to give a message of hope or inspiration; I probably took it in a different way,” he said.
Dr. Kim told the story of one of his clinical partners, Eveline Shue, who, after five rounds of in vitro fertilization (IVF), became pregnant with twins. A high-achiever in her field, Ms. Shue continued working the grueling hours required by her job throughout pregnancy until she noticed concerning symptoms – musculoskeletal issues, extreme swelling, and more. She and her group decided that she should step back from work in her third trimester. A few days later, Ms. Shue suffered a stroke. She was rushed to the hospital where her babies were delivered by emergency C-section. Ms. Shue underwent brain surgery but later recovered and is still practicing in Southern California.
“I remember being at her bedside thinking, ‘How could we have let this happen? How could we have prevented this?’ ”
Dr. Kim’s speech kicked off a firestorm of awareness about pregnancy complications among physicians. “I got scores of emails from women around the country, surgeons in particular, who felt like their issues had been seen. The conversation was long overdue,” he said.
Family planning issues, pregnancy complications, infertility, and pregnancy loss are common, pervasive, and often silent issues in medicine. In July 2021, Dr. Kim and a group of other researchers published a study in JAMA Surgery. It revealed staggering truths: When compared to non-surgeons, female surgeons were more likely to delay pregnancy, use assisted reproductive technology such as IVF, have non-elective C-sections, and suffer pregnancy loss. In the study, 42% of surgeons had experienced pregnancy loss – more than double the rate of the general population. Almost half had serious pregnancy complications.
Research has found that female physicians in general have a significantly greater incidence of miscarriage, infertility, and pregnancy complications than the general population. According to a 2016 survey in the Journal of Women’s Health, the infertility rate for physicians is nearly 1 in 4, about double the rate of the general public.
The barriers to starting a family
Physicians face significant professional barriers that impact family planning. Demanding jobs with exhausting and often unpredictable hours contribute to a culture that, traditionally, has been far from family friendly. As a result, many physicians start families later. “For a pediatric surgeon, you finish training at age 35 – minimum,” says Dr. Kim. “Simply being a surgeon makes you a high-risk pregnancy candidate just because of the career.”
In 2020, Ariela L. Marshall, MD, an associate professor of clinical medicine at the University of Pennsylvania’s Perelman school of medicine, co-authored a commentary article in Academic Medicine titled “Physician Fertility: A Call to Action” which was based on her own experiences with infertility. Dr. Marshall was 34 when she and her husband decided to start a family, and she says her infertility diagnosis “came as a shock.”
“I never stopped to think about the consequences of a career path where I’m not going to be established until my 30s,” Dr. Marshall says. “I never thought about how long hours, overnight shifts, or working all the time could impact my fertility.”
It would take four cycles of IVF egg retrieval to create embryos and one failed implantation before Dr. Marshall became pregnant with her son.
When it comes to the timing of pregnancy, medical culture also plays a role. “There’s a lot of messaging around when it’s appropriate to carry a baby – and it’s not until after training is done,” says Arghavan Salles, MD, PhD, a clinical associate professor and special advisor for DEI programs at Stanford (Calif.) University’s department of medicine.
There are always exceptions. Some institutions are more flexible than others about pregnancy during residency. But Dr. Salles notes that this attitude is “not universal,” partly because of the lack of a comprehensive approach to pregnancy or parenthood in the United States. “There’s no federal paid parental leave in this country,” reminds Dr. Salles. “That signals that we don’t value parenting.”
The trickle-down effect of this in medicine is more like a waterfall. Some physicians complain when other physicians are out on leave. There’s an additional burden of work when people take time away, and there are often no support structures in place for backup or fill-in care. Dr. Salles said doctors often tell her that they were responsible for finding coverage for any time off during pregnancy or after becoming a parent. A paper of hers published in JAMA Surgery found that, for physicians, a fear of burdening others was a major barrier to getting pregnant during residency in the first place.
The physical consequences
Although research supports the benefits of physical activity throughout pregnancy, a job such as surgery that requires being on your feet for long periods of time “is not the same as exercise,” explains Erika Lu Rangel, MD, a gastrointestinal surgeon at Brigham and Women’s Hospital, Boston, and Dr. Kim’s lead author on the JAMA Surgery article.
Surgeons operating for more than 12 hours a week are at higher risk for pregnancy complications, the study found. Dr. Rangel also cites data suggesting that night shifts or swing shifts (the hours between day and night) put women at higher risk for pregnancy complications.
Equally alarming: Medical trainees appear to have “almost as high a rate of pregnancy complications as surgeons who have already completed their training,” said Dr. Rangel. It is a concerning finding since, as a younger cohort, they should have lower complication rates based on their age. But doctors in training may be on their feet even more than surgeons during long shifts.
Like Dr. Salles, Dr. Rangel sees these issues as part of a pervasive culture of “presenteeism” in medicine, and she points out that many surgeons don’t even take time off to grieve pregnancy loss or physically recover from it. “We work even when we’re sick and even when it’s not good for our health,” she said. “I think that’s an unhealthy behavior that we cultivate from the time that we’re trainees, and we carry it on through when we’re in practice.”
Penn Medicine’s Dr. Marshall remembers that her own maternity leave was “not an easy process to navigate.” From her hospital room on a magnesium drip for preeclampsia, she still attended Zoom meetings with her colleagues. “Nobody says, ‘Oh, you have to do this,’ ” Dr. Marshall explains, “but you wind up feeling guilty if you’re not there at all moments for everyone. That’s also something that needs to change.”
Dr. Rangel was pregnant with her oldest son as a fourth-year surgery resident. The day she gave birth to him she remembers waking up with a flu-like illness and a fever. She went to work anyway, because “you don’t call in sick as a resident.” She was barely able to complete her rounds and then had to lie down between cases. A co-resident found her and took her to labor and delivery. She had gone into premature labor at 37 weeks, and her son went into the NICU with complications.
“I remember feeling this enormous guilt,” says Dr. Rangel. “I’d been a mom for just a few minutes, and I felt like I had already failed him because I had prioritized what the residency thought of me above what I knew was necessary for his health.”
Hope for the future
Disturbed by the status quo, many physicians are pushing for change. “I think there’s a really important and positive conversation going on in the medical community right now about ways that we need to support new parent physicians,” said Dr. Rangel.
Parental leave is a key part of that support. Last year, The American Board of Medical Specialties enacted a mandate that all specialty boards 2 years or more in duration must provide at least 6 weeks of parental and caregiver leave. In 2023, the Accreditation Council for Graduate Medical Education (ACGME) required that all training programs match that policy. “This sends a message to policymakers and leaders in American medicine that this is a priority,” said Dr. Rangel.
In January 2022, a group from the University of Michigan also published an article in the Annals of Surgery called “Safe and Supported Pregnancy: A Call to Action for Surgery Chairs and Program Directors”. The essay urged leading groups such as the ACGME and the American Board of Surgery to “directly address the health and safety of pregnant trainees” and specifically, to “allow for further flexibility during training for pregnancy and peripartum periods,” calling these “fundamental necessities for cultural progress.”
Others have recommended allowing pregnant trainees more flexibility in their schedules or front-loading certain parts of the training that may be more difficult as a pregnancy progresses. Insurance coverage for fertility preservation and reproductive endocrinology services, and support for reentry (including lactation and childcare) are also issues that must be addressed, says Dr. Salles.
A new paper of Dr. Rangel’s, published in JAMA Surgery, suggests that things like mentorship for residents from faculty can also be important pieces of the puzzle.
Education about reproductive health must start earlier, too – as early as medical school. Research suggests only 8% of physicians receive education on the risks of delaying pregnancy. Those who do are significantly less likely to experience pregnancy loss or seek infertility treatment.
Dr. Salles recalls sitting in a classroom learning about advanced maternal age at a time when age 35 seemed unimaginably distant. “It was never taught – at least to my recollection – in a way that was like, ‘this could be your future,’ ” Dr. Salles says.” It was more like this abstract patient who might have advanced maternal age and what the consequences would be. Maybe some of my colleagues put two and two together, but I definitely didn’t.”
Dr. Marshall is the curriculum chair for the IGNITEMed Initiative, which aims to educate medical students about issues not discussed in traditional medical school curricula. Dr. Marshall and her colleague Julia Files, MD, talk with IGNITEMed students about reproductive life planning.
“Raising awareness is a very big thing. That’s not just true for medical students but for professionals at every level of medicine,” Dr. Marshall said. “Residency and fellowship training program directors, department chairs, and hospital CEOs all need to understand that these issues are very common in the people they oversee – and that they are medical issues, like any other medical issue, where people need time off and support.”
A version of this article first appeared on Medscape.com.
In 2021, Eugene Kim, MD, division director of pediatric surgery and vice chair in the department of surgery at Cedars-Sinai Medical Center, Los Angeles, gave his presidential address to the Association for Academic Surgery.
“Presidents tend to give a message of hope or inspiration; I probably took it in a different way,” he said.
Dr. Kim told the story of one of his clinical partners, Eveline Shue, who, after five rounds of in vitro fertilization (IVF), became pregnant with twins. A high-achiever in her field, Ms. Shue continued working the grueling hours required by her job throughout pregnancy until she noticed concerning symptoms – musculoskeletal issues, extreme swelling, and more. She and her group decided that she should step back from work in her third trimester. A few days later, Ms. Shue suffered a stroke. She was rushed to the hospital where her babies were delivered by emergency C-section. Ms. Shue underwent brain surgery but later recovered and is still practicing in Southern California.
“I remember being at her bedside thinking, ‘How could we have let this happen? How could we have prevented this?’ ”
Dr. Kim’s speech kicked off a firestorm of awareness about pregnancy complications among physicians. “I got scores of emails from women around the country, surgeons in particular, who felt like their issues had been seen. The conversation was long overdue,” he said.
Family planning issues, pregnancy complications, infertility, and pregnancy loss are common, pervasive, and often silent issues in medicine. In July 2021, Dr. Kim and a group of other researchers published a study in JAMA Surgery. It revealed staggering truths: When compared to non-surgeons, female surgeons were more likely to delay pregnancy, use assisted reproductive technology such as IVF, have non-elective C-sections, and suffer pregnancy loss. In the study, 42% of surgeons had experienced pregnancy loss – more than double the rate of the general population. Almost half had serious pregnancy complications.
Research has found that female physicians in general have a significantly greater incidence of miscarriage, infertility, and pregnancy complications than the general population. According to a 2016 survey in the Journal of Women’s Health, the infertility rate for physicians is nearly 1 in 4, about double the rate of the general public.
The barriers to starting a family
Physicians face significant professional barriers that impact family planning. Demanding jobs with exhausting and often unpredictable hours contribute to a culture that, traditionally, has been far from family friendly. As a result, many physicians start families later. “For a pediatric surgeon, you finish training at age 35 – minimum,” says Dr. Kim. “Simply being a surgeon makes you a high-risk pregnancy candidate just because of the career.”
In 2020, Ariela L. Marshall, MD, an associate professor of clinical medicine at the University of Pennsylvania’s Perelman school of medicine, co-authored a commentary article in Academic Medicine titled “Physician Fertility: A Call to Action” which was based on her own experiences with infertility. Dr. Marshall was 34 when she and her husband decided to start a family, and she says her infertility diagnosis “came as a shock.”
“I never stopped to think about the consequences of a career path where I’m not going to be established until my 30s,” Dr. Marshall says. “I never thought about how long hours, overnight shifts, or working all the time could impact my fertility.”
It would take four cycles of IVF egg retrieval to create embryos and one failed implantation before Dr. Marshall became pregnant with her son.
When it comes to the timing of pregnancy, medical culture also plays a role. “There’s a lot of messaging around when it’s appropriate to carry a baby – and it’s not until after training is done,” says Arghavan Salles, MD, PhD, a clinical associate professor and special advisor for DEI programs at Stanford (Calif.) University’s department of medicine.
There are always exceptions. Some institutions are more flexible than others about pregnancy during residency. But Dr. Salles notes that this attitude is “not universal,” partly because of the lack of a comprehensive approach to pregnancy or parenthood in the United States. “There’s no federal paid parental leave in this country,” reminds Dr. Salles. “That signals that we don’t value parenting.”
The trickle-down effect of this in medicine is more like a waterfall. Some physicians complain when other physicians are out on leave. There’s an additional burden of work when people take time away, and there are often no support structures in place for backup or fill-in care. Dr. Salles said doctors often tell her that they were responsible for finding coverage for any time off during pregnancy or after becoming a parent. A paper of hers published in JAMA Surgery found that, for physicians, a fear of burdening others was a major barrier to getting pregnant during residency in the first place.
The physical consequences
Although research supports the benefits of physical activity throughout pregnancy, a job such as surgery that requires being on your feet for long periods of time “is not the same as exercise,” explains Erika Lu Rangel, MD, a gastrointestinal surgeon at Brigham and Women’s Hospital, Boston, and Dr. Kim’s lead author on the JAMA Surgery article.
Surgeons operating for more than 12 hours a week are at higher risk for pregnancy complications, the study found. Dr. Rangel also cites data suggesting that night shifts or swing shifts (the hours between day and night) put women at higher risk for pregnancy complications.
Equally alarming: Medical trainees appear to have “almost as high a rate of pregnancy complications as surgeons who have already completed their training,” said Dr. Rangel. It is a concerning finding since, as a younger cohort, they should have lower complication rates based on their age. But doctors in training may be on their feet even more than surgeons during long shifts.
Like Dr. Salles, Dr. Rangel sees these issues as part of a pervasive culture of “presenteeism” in medicine, and she points out that many surgeons don’t even take time off to grieve pregnancy loss or physically recover from it. “We work even when we’re sick and even when it’s not good for our health,” she said. “I think that’s an unhealthy behavior that we cultivate from the time that we’re trainees, and we carry it on through when we’re in practice.”
Penn Medicine’s Dr. Marshall remembers that her own maternity leave was “not an easy process to navigate.” From her hospital room on a magnesium drip for preeclampsia, she still attended Zoom meetings with her colleagues. “Nobody says, ‘Oh, you have to do this,’ ” Dr. Marshall explains, “but you wind up feeling guilty if you’re not there at all moments for everyone. That’s also something that needs to change.”
Dr. Rangel was pregnant with her oldest son as a fourth-year surgery resident. The day she gave birth to him she remembers waking up with a flu-like illness and a fever. She went to work anyway, because “you don’t call in sick as a resident.” She was barely able to complete her rounds and then had to lie down between cases. A co-resident found her and took her to labor and delivery. She had gone into premature labor at 37 weeks, and her son went into the NICU with complications.
“I remember feeling this enormous guilt,” says Dr. Rangel. “I’d been a mom for just a few minutes, and I felt like I had already failed him because I had prioritized what the residency thought of me above what I knew was necessary for his health.”
Hope for the future
Disturbed by the status quo, many physicians are pushing for change. “I think there’s a really important and positive conversation going on in the medical community right now about ways that we need to support new parent physicians,” said Dr. Rangel.
Parental leave is a key part of that support. Last year, The American Board of Medical Specialties enacted a mandate that all specialty boards 2 years or more in duration must provide at least 6 weeks of parental and caregiver leave. In 2023, the Accreditation Council for Graduate Medical Education (ACGME) required that all training programs match that policy. “This sends a message to policymakers and leaders in American medicine that this is a priority,” said Dr. Rangel.
In January 2022, a group from the University of Michigan also published an article in the Annals of Surgery called “Safe and Supported Pregnancy: A Call to Action for Surgery Chairs and Program Directors”. The essay urged leading groups such as the ACGME and the American Board of Surgery to “directly address the health and safety of pregnant trainees” and specifically, to “allow for further flexibility during training for pregnancy and peripartum periods,” calling these “fundamental necessities for cultural progress.”
Others have recommended allowing pregnant trainees more flexibility in their schedules or front-loading certain parts of the training that may be more difficult as a pregnancy progresses. Insurance coverage for fertility preservation and reproductive endocrinology services, and support for reentry (including lactation and childcare) are also issues that must be addressed, says Dr. Salles.
A new paper of Dr. Rangel’s, published in JAMA Surgery, suggests that things like mentorship for residents from faculty can also be important pieces of the puzzle.
Education about reproductive health must start earlier, too – as early as medical school. Research suggests only 8% of physicians receive education on the risks of delaying pregnancy. Those who do are significantly less likely to experience pregnancy loss or seek infertility treatment.
Dr. Salles recalls sitting in a classroom learning about advanced maternal age at a time when age 35 seemed unimaginably distant. “It was never taught – at least to my recollection – in a way that was like, ‘this could be your future,’ ” Dr. Salles says.” It was more like this abstract patient who might have advanced maternal age and what the consequences would be. Maybe some of my colleagues put two and two together, but I definitely didn’t.”
Dr. Marshall is the curriculum chair for the IGNITEMed Initiative, which aims to educate medical students about issues not discussed in traditional medical school curricula. Dr. Marshall and her colleague Julia Files, MD, talk with IGNITEMed students about reproductive life planning.
“Raising awareness is a very big thing. That’s not just true for medical students but for professionals at every level of medicine,” Dr. Marshall said. “Residency and fellowship training program directors, department chairs, and hospital CEOs all need to understand that these issues are very common in the people they oversee – and that they are medical issues, like any other medical issue, where people need time off and support.”
A version of this article first appeared on Medscape.com.
Dermatologists address cultural competence and unconscious biases in the specialty
ORLANDO – When he was applying for residency, Omar N. Qutub, MD, eagerly arrived at his first interview of the day. But he was quickly thrown off his game.
The interviewer, he said, spent a surprising amount of time asking about his ethnicity and his last name. “I think I spent about 3-5 minutes in the first interview talking about my last name,” said Dr. Qutub, who practices in Portland, Ore., during a session titled “unconscious bias and microaggressions in dermatology” at the ODAC Dermatology, Aesthetic and Surgical Conference. “I really would have rather talked about my research interests.” The interaction threw him off for the rest of the interview process, he said.
The experience is an example of how the field has a ways to go in acquiring cultural competence and in overcoming unconscious biases, said Dr. Qutub. In 2020, a review in Clinics in Dermatology referred to a report that dermatology was the second-least diverse medical specialty, only behind orthopedic surgery, because of its low numbers of residents and faculty from groups underrepresented in medicine.
“We really need to put cultural competency at the forefront in order to do better for our patients,” he said.
Adam Friedman, MD, professor and chair of dermatology and director of the residency program at George Washington University, Washington, who also spoke during the session, said that the process of diversifying the field has to go deeper than the resident interviewing process. “If we just focus on trying to increase the diversity of our applicant pool for residents, it’s too late.”
Nada Elbuluk, MD, associate professor of dermatology at the University of Southern California, Los Angeles, pointed to USC’s Derm RISES initiative, a service program that aims to reach inner-city students through education in the sciences, starting from kindergarten to 12th grade. The program also includes premed undergraduate and medical students, “with the goal of increasing exposure to the sciences, medicine, and dermatology,” according to the USC website. “It’s crucial to begin the process early to get a high yield of students who reach medical school and eventually dermatology, she said, because of the inevitable attrition at each level of the education process.
“It’s incredibly rewarding,” added Dr. Elbuluk, who is also director of the dermatology diversity and inclusion program at USC. “And we get these thank-you letters back from students who [say], ‘I didn’t know I could be a doctor.’ ”
In another presentation, Kavita Mariwalla, MD, who practices in West Islip, N.Y., provided tips on boosting cultural competence during aesthetic consults.
One was to “know your fillers,” she said, noting that fillers have different effects on different skin tones, because of differences in fibroblast content, and fat cells will interact with fillers in different ways across skin tones.
Another is to “understand the shortfall of facial canons,” the idea that you can divide a face into sections that can be viewed and enhanced discretely. This concept was based on a White European model and has to be expanded when considering other ethnicities, Dr. Mariwalla said.
Overgeneralizing categories is another pitfall, she said. “Asian” is a term that covers countries from India to Japan, but within that category are a multitude of notions and nuances about aesthetics, and dermatologists have to be sensitive to all of them.
When meeting with a patient, Dr. Mariwalla said, asking the typical “Where are you from?” is not a helpful question. Instead, she suggested asking: “What is your cultural background? Can you tell me more about what your expectations are?”
“I ask for pictures,” she said. “I want to know what they looked like as a kid. I want to know what their family looks like. And I always hand patients a mirror. Patients will say to me: ‘I want to do what you think.’ It’s not about what I think, because what I see, and what you see in your magnifying mirror, are totally different things.”
After the session ended, a member of the audience, Sharon Stokes, MD, a dermatologist in the Orlando area, provided her view of the presentations, noting that it was an important discussion.
“I think it’s past time in medicine for cultural diversity training and awareness for physicians to understand their patients better and getting to know them – and how to even approach the patient and not to offensively and microaggressively approach the patient,” she said.
Dr. Elbuluk reported relevant relationships with Avita, Incyte, Beiersdorf, and other companies. Dr. Friedman reported financial relationships with Sanova, Pfizer, Novartis and other companies. Dr. Mariwalla reported relevant financial relationships with Abbvie, Sanofi, Regeneron and other companies. Dr. Qutub reported no relevant financial relationships. Dr. Qutub is the ODAC director of equity, diversity, and inclusion.
ORLANDO – When he was applying for residency, Omar N. Qutub, MD, eagerly arrived at his first interview of the day. But he was quickly thrown off his game.
The interviewer, he said, spent a surprising amount of time asking about his ethnicity and his last name. “I think I spent about 3-5 minutes in the first interview talking about my last name,” said Dr. Qutub, who practices in Portland, Ore., during a session titled “unconscious bias and microaggressions in dermatology” at the ODAC Dermatology, Aesthetic and Surgical Conference. “I really would have rather talked about my research interests.” The interaction threw him off for the rest of the interview process, he said.
The experience is an example of how the field has a ways to go in acquiring cultural competence and in overcoming unconscious biases, said Dr. Qutub. In 2020, a review in Clinics in Dermatology referred to a report that dermatology was the second-least diverse medical specialty, only behind orthopedic surgery, because of its low numbers of residents and faculty from groups underrepresented in medicine.
“We really need to put cultural competency at the forefront in order to do better for our patients,” he said.
Adam Friedman, MD, professor and chair of dermatology and director of the residency program at George Washington University, Washington, who also spoke during the session, said that the process of diversifying the field has to go deeper than the resident interviewing process. “If we just focus on trying to increase the diversity of our applicant pool for residents, it’s too late.”
Nada Elbuluk, MD, associate professor of dermatology at the University of Southern California, Los Angeles, pointed to USC’s Derm RISES initiative, a service program that aims to reach inner-city students through education in the sciences, starting from kindergarten to 12th grade. The program also includes premed undergraduate and medical students, “with the goal of increasing exposure to the sciences, medicine, and dermatology,” according to the USC website. “It’s crucial to begin the process early to get a high yield of students who reach medical school and eventually dermatology, she said, because of the inevitable attrition at each level of the education process.
“It’s incredibly rewarding,” added Dr. Elbuluk, who is also director of the dermatology diversity and inclusion program at USC. “And we get these thank-you letters back from students who [say], ‘I didn’t know I could be a doctor.’ ”
In another presentation, Kavita Mariwalla, MD, who practices in West Islip, N.Y., provided tips on boosting cultural competence during aesthetic consults.
One was to “know your fillers,” she said, noting that fillers have different effects on different skin tones, because of differences in fibroblast content, and fat cells will interact with fillers in different ways across skin tones.
Another is to “understand the shortfall of facial canons,” the idea that you can divide a face into sections that can be viewed and enhanced discretely. This concept was based on a White European model and has to be expanded when considering other ethnicities, Dr. Mariwalla said.
Overgeneralizing categories is another pitfall, she said. “Asian” is a term that covers countries from India to Japan, but within that category are a multitude of notions and nuances about aesthetics, and dermatologists have to be sensitive to all of them.
When meeting with a patient, Dr. Mariwalla said, asking the typical “Where are you from?” is not a helpful question. Instead, she suggested asking: “What is your cultural background? Can you tell me more about what your expectations are?”
“I ask for pictures,” she said. “I want to know what they looked like as a kid. I want to know what their family looks like. And I always hand patients a mirror. Patients will say to me: ‘I want to do what you think.’ It’s not about what I think, because what I see, and what you see in your magnifying mirror, are totally different things.”
After the session ended, a member of the audience, Sharon Stokes, MD, a dermatologist in the Orlando area, provided her view of the presentations, noting that it was an important discussion.
“I think it’s past time in medicine for cultural diversity training and awareness for physicians to understand their patients better and getting to know them – and how to even approach the patient and not to offensively and microaggressively approach the patient,” she said.
Dr. Elbuluk reported relevant relationships with Avita, Incyte, Beiersdorf, and other companies. Dr. Friedman reported financial relationships with Sanova, Pfizer, Novartis and other companies. Dr. Mariwalla reported relevant financial relationships with Abbvie, Sanofi, Regeneron and other companies. Dr. Qutub reported no relevant financial relationships. Dr. Qutub is the ODAC director of equity, diversity, and inclusion.
ORLANDO – When he was applying for residency, Omar N. Qutub, MD, eagerly arrived at his first interview of the day. But he was quickly thrown off his game.
The interviewer, he said, spent a surprising amount of time asking about his ethnicity and his last name. “I think I spent about 3-5 minutes in the first interview talking about my last name,” said Dr. Qutub, who practices in Portland, Ore., during a session titled “unconscious bias and microaggressions in dermatology” at the ODAC Dermatology, Aesthetic and Surgical Conference. “I really would have rather talked about my research interests.” The interaction threw him off for the rest of the interview process, he said.
The experience is an example of how the field has a ways to go in acquiring cultural competence and in overcoming unconscious biases, said Dr. Qutub. In 2020, a review in Clinics in Dermatology referred to a report that dermatology was the second-least diverse medical specialty, only behind orthopedic surgery, because of its low numbers of residents and faculty from groups underrepresented in medicine.
“We really need to put cultural competency at the forefront in order to do better for our patients,” he said.
Adam Friedman, MD, professor and chair of dermatology and director of the residency program at George Washington University, Washington, who also spoke during the session, said that the process of diversifying the field has to go deeper than the resident interviewing process. “If we just focus on trying to increase the diversity of our applicant pool for residents, it’s too late.”
Nada Elbuluk, MD, associate professor of dermatology at the University of Southern California, Los Angeles, pointed to USC’s Derm RISES initiative, a service program that aims to reach inner-city students through education in the sciences, starting from kindergarten to 12th grade. The program also includes premed undergraduate and medical students, “with the goal of increasing exposure to the sciences, medicine, and dermatology,” according to the USC website. “It’s crucial to begin the process early to get a high yield of students who reach medical school and eventually dermatology, she said, because of the inevitable attrition at each level of the education process.
“It’s incredibly rewarding,” added Dr. Elbuluk, who is also director of the dermatology diversity and inclusion program at USC. “And we get these thank-you letters back from students who [say], ‘I didn’t know I could be a doctor.’ ”
In another presentation, Kavita Mariwalla, MD, who practices in West Islip, N.Y., provided tips on boosting cultural competence during aesthetic consults.
One was to “know your fillers,” she said, noting that fillers have different effects on different skin tones, because of differences in fibroblast content, and fat cells will interact with fillers in different ways across skin tones.
Another is to “understand the shortfall of facial canons,” the idea that you can divide a face into sections that can be viewed and enhanced discretely. This concept was based on a White European model and has to be expanded when considering other ethnicities, Dr. Mariwalla said.
Overgeneralizing categories is another pitfall, she said. “Asian” is a term that covers countries from India to Japan, but within that category are a multitude of notions and nuances about aesthetics, and dermatologists have to be sensitive to all of them.
When meeting with a patient, Dr. Mariwalla said, asking the typical “Where are you from?” is not a helpful question. Instead, she suggested asking: “What is your cultural background? Can you tell me more about what your expectations are?”
“I ask for pictures,” she said. “I want to know what they looked like as a kid. I want to know what their family looks like. And I always hand patients a mirror. Patients will say to me: ‘I want to do what you think.’ It’s not about what I think, because what I see, and what you see in your magnifying mirror, are totally different things.”
After the session ended, a member of the audience, Sharon Stokes, MD, a dermatologist in the Orlando area, provided her view of the presentations, noting that it was an important discussion.
“I think it’s past time in medicine for cultural diversity training and awareness for physicians to understand their patients better and getting to know them – and how to even approach the patient and not to offensively and microaggressively approach the patient,” she said.
Dr. Elbuluk reported relevant relationships with Avita, Incyte, Beiersdorf, and other companies. Dr. Friedman reported financial relationships with Sanova, Pfizer, Novartis and other companies. Dr. Mariwalla reported relevant financial relationships with Abbvie, Sanofi, Regeneron and other companies. Dr. Qutub reported no relevant financial relationships. Dr. Qutub is the ODAC director of equity, diversity, and inclusion.
AT ODAC 2023
Minorities with epilepsy blocked from receiving ‘highest quality of care’
, new research shows.
Even after controlling for epilepsy severity, comorbid conditions, and other factors that might affect medication choice, researchers found that newer medication use was 29% less likely in Black patients, 23% less likely in Native Hawaiian and other Pacific Islander patients, and 7% less likely in Hispanic patients, compared with White individuals.
“I hope that clinicians will see from our findings that minoritized patients with epilepsy face a myriad of barriers in receiving the highest quality of care, including ASM use,” said lead investigator Wyatt P. Bensken, PhD, adjunct assistant professor of Population and Quantitative Health Sciences at Case Western Reserve University, Cleveland. “Considering your patients’ barriers, and how that influences their care – including ASM selection – will be critical to helping reduce these population-level inequities.”
The study was published online in Neurology Clinical Practice.
A prompt for practice change
For the study, researchers used Medicaid claims for more than 78,000 people who had filled at least two prescriptions for an ASM between 2010 and 2014.
Most patients were White (53.4%); 22.6% were Black; 11.9% were Hispanic; 1.6% were Asian; 1.5% were Native Hawaiian or other Pacific Islander; 0.6% American Indian or Alaskan Native; and 8.3% were classified as “other.”
One-quarter of participants were taking an older ASM, such as carbamazepine, phenytoin, and valproate. About 65% were taking second-generation ASMs, including gabapentin, levetiracetam, and zonisamide. A little less than 10% were taking lacosamide, perampenel, or another third-generation ASM.
Compared with White patients, newer medication prescriptions were significantly less likely in Black individuals (adjusted odds ratio, 0.71; 95% confidence interval, 0.68-0.75), Native Hawaiian or other Pacific Islanders (aOR, 0.77; 95% CI, 0.67-0.88), and Hispanic patients (aOR, 0.93; 95% CI, 0.88-0.99).
Third-generation ASMs were used by 10.7% of White patients versus 6% of Black individuals and 5.1% of American Indian or Alaskan Native patients.
Researchers also found that taking a second-generation ASM was associated with better treatment adherence (aOR, 1.17; 95% CI, 1.11-1.23) and that patients on newer ASMs were more than three times as likely to be under the care of a neurologist (aOR, 3.26; 95% CI, 3.13-3.41).
The findings draw attention to racial inequities surrounding access to medication and specialists and subspecialists, Dr. Bensken said. Identifying specific barriers and developing solutions is the long-range goal, he added.
“In the interim, increasing the attention to these inequities will, we hope, prompt changes across practices,” Dr. Bensken said.
A ‘wake-up call’
Commenting on the findings, Joseph Sirven, MD, professor of neurology at the Mayo Clinic Florida, Jacksonville, said the results were “striking” because newer ASMs are generally the go-to for most physicians who treat epilepsy.
“Use of first-generation ASMs is typically reserved [for] if one runs out of options,” Dr. Sirven said.
This study and others like it should serve as a “wake-up call” for clinicians, Dr. Sirven added.
“This study is important because it shows that whether we realize it or not, race and ethnicities are playing a role in ASM, and this is related to financial access to newer-generation drugs,” he said. “Similar findings are seen in impoverished countries where first-generation ASM drugs are routinely used because of drug pricing.”
More to explore
Also commenting on the study, Scott Mintzer, MD, a professor and director of the Epilepsy Monitoring Unit at Thomas Jefferson University, Philadelphia, said using first-generation ASMs as a proxy for quality of care is “a very innovative concept.”
“From that perspective, the finding that racial minority patients are more likely to be on a first-generation drug is not surprising. But after that it gets far more complicated to interpret,” he added.
Neither adherence nor care by a neurologist was different in a consistent direction within the various minority populations, Dr. Mintzer noted. In addition, Black patients were as likely to see a neurologist as White patients but still more likely to be on a first-generation drug.
There are also a few caveats to the findings that should be considered, Dr. Mintzer added. First, the sample included only Medicaid recipients, nearly 35% of whom had a comorbid psychosis. Those and other characteristics of the study pool suggest participants aren’t representative of the United States population as a whole. Second, significant shifts in ASM use have occurred since the study data cutoff in 2014, none of which are reflected in these findings.
“So, I don’t think we can really say how to address this yet,” Dr. Mintzer said. “There’s a lot to explore about whether this is still occurring, how generalizable these findings are, and what they might be due to, as there are a host of potential explanations, which the authors themselves acknowledge.”
The study was funded by the U.S. Centers for Disease Control and Prevention and the National Institute on Minority Health and Health Disparities. Dr. Bensken has received support for this work from NIMHD and serves on the Editorial Board of the journal Neurology. Dr. Sirven and Mintzer report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, new research shows.
Even after controlling for epilepsy severity, comorbid conditions, and other factors that might affect medication choice, researchers found that newer medication use was 29% less likely in Black patients, 23% less likely in Native Hawaiian and other Pacific Islander patients, and 7% less likely in Hispanic patients, compared with White individuals.
“I hope that clinicians will see from our findings that minoritized patients with epilepsy face a myriad of barriers in receiving the highest quality of care, including ASM use,” said lead investigator Wyatt P. Bensken, PhD, adjunct assistant professor of Population and Quantitative Health Sciences at Case Western Reserve University, Cleveland. “Considering your patients’ barriers, and how that influences their care – including ASM selection – will be critical to helping reduce these population-level inequities.”
The study was published online in Neurology Clinical Practice.
A prompt for practice change
For the study, researchers used Medicaid claims for more than 78,000 people who had filled at least two prescriptions for an ASM between 2010 and 2014.
Most patients were White (53.4%); 22.6% were Black; 11.9% were Hispanic; 1.6% were Asian; 1.5% were Native Hawaiian or other Pacific Islander; 0.6% American Indian or Alaskan Native; and 8.3% were classified as “other.”
One-quarter of participants were taking an older ASM, such as carbamazepine, phenytoin, and valproate. About 65% were taking second-generation ASMs, including gabapentin, levetiracetam, and zonisamide. A little less than 10% were taking lacosamide, perampenel, or another third-generation ASM.
Compared with White patients, newer medication prescriptions were significantly less likely in Black individuals (adjusted odds ratio, 0.71; 95% confidence interval, 0.68-0.75), Native Hawaiian or other Pacific Islanders (aOR, 0.77; 95% CI, 0.67-0.88), and Hispanic patients (aOR, 0.93; 95% CI, 0.88-0.99).
Third-generation ASMs were used by 10.7% of White patients versus 6% of Black individuals and 5.1% of American Indian or Alaskan Native patients.
Researchers also found that taking a second-generation ASM was associated with better treatment adherence (aOR, 1.17; 95% CI, 1.11-1.23) and that patients on newer ASMs were more than three times as likely to be under the care of a neurologist (aOR, 3.26; 95% CI, 3.13-3.41).
The findings draw attention to racial inequities surrounding access to medication and specialists and subspecialists, Dr. Bensken said. Identifying specific barriers and developing solutions is the long-range goal, he added.
“In the interim, increasing the attention to these inequities will, we hope, prompt changes across practices,” Dr. Bensken said.
A ‘wake-up call’
Commenting on the findings, Joseph Sirven, MD, professor of neurology at the Mayo Clinic Florida, Jacksonville, said the results were “striking” because newer ASMs are generally the go-to for most physicians who treat epilepsy.
“Use of first-generation ASMs is typically reserved [for] if one runs out of options,” Dr. Sirven said.
This study and others like it should serve as a “wake-up call” for clinicians, Dr. Sirven added.
“This study is important because it shows that whether we realize it or not, race and ethnicities are playing a role in ASM, and this is related to financial access to newer-generation drugs,” he said. “Similar findings are seen in impoverished countries where first-generation ASM drugs are routinely used because of drug pricing.”
More to explore
Also commenting on the study, Scott Mintzer, MD, a professor and director of the Epilepsy Monitoring Unit at Thomas Jefferson University, Philadelphia, said using first-generation ASMs as a proxy for quality of care is “a very innovative concept.”
“From that perspective, the finding that racial minority patients are more likely to be on a first-generation drug is not surprising. But after that it gets far more complicated to interpret,” he added.
Neither adherence nor care by a neurologist was different in a consistent direction within the various minority populations, Dr. Mintzer noted. In addition, Black patients were as likely to see a neurologist as White patients but still more likely to be on a first-generation drug.
There are also a few caveats to the findings that should be considered, Dr. Mintzer added. First, the sample included only Medicaid recipients, nearly 35% of whom had a comorbid psychosis. Those and other characteristics of the study pool suggest participants aren’t representative of the United States population as a whole. Second, significant shifts in ASM use have occurred since the study data cutoff in 2014, none of which are reflected in these findings.
“So, I don’t think we can really say how to address this yet,” Dr. Mintzer said. “There’s a lot to explore about whether this is still occurring, how generalizable these findings are, and what they might be due to, as there are a host of potential explanations, which the authors themselves acknowledge.”
The study was funded by the U.S. Centers for Disease Control and Prevention and the National Institute on Minority Health and Health Disparities. Dr. Bensken has received support for this work from NIMHD and serves on the Editorial Board of the journal Neurology. Dr. Sirven and Mintzer report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, new research shows.
Even after controlling for epilepsy severity, comorbid conditions, and other factors that might affect medication choice, researchers found that newer medication use was 29% less likely in Black patients, 23% less likely in Native Hawaiian and other Pacific Islander patients, and 7% less likely in Hispanic patients, compared with White individuals.
“I hope that clinicians will see from our findings that minoritized patients with epilepsy face a myriad of barriers in receiving the highest quality of care, including ASM use,” said lead investigator Wyatt P. Bensken, PhD, adjunct assistant professor of Population and Quantitative Health Sciences at Case Western Reserve University, Cleveland. “Considering your patients’ barriers, and how that influences their care – including ASM selection – will be critical to helping reduce these population-level inequities.”
The study was published online in Neurology Clinical Practice.
A prompt for practice change
For the study, researchers used Medicaid claims for more than 78,000 people who had filled at least two prescriptions for an ASM between 2010 and 2014.
Most patients were White (53.4%); 22.6% were Black; 11.9% were Hispanic; 1.6% were Asian; 1.5% were Native Hawaiian or other Pacific Islander; 0.6% American Indian or Alaskan Native; and 8.3% were classified as “other.”
One-quarter of participants were taking an older ASM, such as carbamazepine, phenytoin, and valproate. About 65% were taking second-generation ASMs, including gabapentin, levetiracetam, and zonisamide. A little less than 10% were taking lacosamide, perampenel, or another third-generation ASM.
Compared with White patients, newer medication prescriptions were significantly less likely in Black individuals (adjusted odds ratio, 0.71; 95% confidence interval, 0.68-0.75), Native Hawaiian or other Pacific Islanders (aOR, 0.77; 95% CI, 0.67-0.88), and Hispanic patients (aOR, 0.93; 95% CI, 0.88-0.99).
Third-generation ASMs were used by 10.7% of White patients versus 6% of Black individuals and 5.1% of American Indian or Alaskan Native patients.
Researchers also found that taking a second-generation ASM was associated with better treatment adherence (aOR, 1.17; 95% CI, 1.11-1.23) and that patients on newer ASMs were more than three times as likely to be under the care of a neurologist (aOR, 3.26; 95% CI, 3.13-3.41).
The findings draw attention to racial inequities surrounding access to medication and specialists and subspecialists, Dr. Bensken said. Identifying specific barriers and developing solutions is the long-range goal, he added.
“In the interim, increasing the attention to these inequities will, we hope, prompt changes across practices,” Dr. Bensken said.
A ‘wake-up call’
Commenting on the findings, Joseph Sirven, MD, professor of neurology at the Mayo Clinic Florida, Jacksonville, said the results were “striking” because newer ASMs are generally the go-to for most physicians who treat epilepsy.
“Use of first-generation ASMs is typically reserved [for] if one runs out of options,” Dr. Sirven said.
This study and others like it should serve as a “wake-up call” for clinicians, Dr. Sirven added.
“This study is important because it shows that whether we realize it or not, race and ethnicities are playing a role in ASM, and this is related to financial access to newer-generation drugs,” he said. “Similar findings are seen in impoverished countries where first-generation ASM drugs are routinely used because of drug pricing.”
More to explore
Also commenting on the study, Scott Mintzer, MD, a professor and director of the Epilepsy Monitoring Unit at Thomas Jefferson University, Philadelphia, said using first-generation ASMs as a proxy for quality of care is “a very innovative concept.”
“From that perspective, the finding that racial minority patients are more likely to be on a first-generation drug is not surprising. But after that it gets far more complicated to interpret,” he added.
Neither adherence nor care by a neurologist was different in a consistent direction within the various minority populations, Dr. Mintzer noted. In addition, Black patients were as likely to see a neurologist as White patients but still more likely to be on a first-generation drug.
There are also a few caveats to the findings that should be considered, Dr. Mintzer added. First, the sample included only Medicaid recipients, nearly 35% of whom had a comorbid psychosis. Those and other characteristics of the study pool suggest participants aren’t representative of the United States population as a whole. Second, significant shifts in ASM use have occurred since the study data cutoff in 2014, none of which are reflected in these findings.
“So, I don’t think we can really say how to address this yet,” Dr. Mintzer said. “There’s a lot to explore about whether this is still occurring, how generalizable these findings are, and what they might be due to, as there are a host of potential explanations, which the authors themselves acknowledge.”
The study was funded by the U.S. Centers for Disease Control and Prevention and the National Institute on Minority Health and Health Disparities. Dr. Bensken has received support for this work from NIMHD and serves on the Editorial Board of the journal Neurology. Dr. Sirven and Mintzer report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM NEUROLOGY CLINICAL PRACTICE