User login
Perceived Benefits of a Research Fellowship for Dermatology Residency Applicants: Outcomes of a Faculty-Reported Survey
Dermatology residency positions continue to be highly coveted among applicants in the match. In 2019, dermatology proved to be the most competitive specialty, with 36.3% of US medical school seniors and independent applicants going unmatched.1 Prior to the transition to a pass/fail system, the mean US Medical Licensing Examination (USMLE) Step 1 score for matched applicants increased from 247 in 2014 to 251 in 2019. The growing number of scholarly activities reported by applicants has contributed to the competitiveness of the specialty. In 2018, the mean number of abstracts, presentations, and publications reported by matched applicants was 14.71, which was higher than other competitive specialties, including orthopedic surgery and otolaryngology (11.5 and 10.4, respectively). Dermatology applicants who did not match in 2018 reported a mean of 8.6 abstracts, presentations, and publications, which was on par with successful applicants in many other specialties.1 In 2011, Stratman and Ness2 found that publishing manuscripts and listing research experience were factors strongly associated with matching into dermatology for reapplicants. These trends in reported research have added pressure for applicants to increase their publications.
Given that many students do not choose a career in dermatology until later in medical school, some students choose to take a gap year between their third and fourth years of medical school to pursue a research fellowship (RF) and produce publications, in theory to increase the chances of matching in dermatology. A survey of dermatology applicants conducted by Costello et al3 in 2021 found that, of the students who completed a gap year (n=90; 31.25%), 78.7% (n=71) of them completed an RF, and those who completed RFs were more likely to match at top dermatology residency programs (P<.01). The authors also reported that there was no significant difference in overall match rates between gap-year and non–gap-year applicants.3 Another survey of 328 medical students found that the most common reason students take years off for research during medical school is to increase competitiveness for residency application.4 Although it is clear that students completing an RF often find success in the match, there are limited published data on how those involved in selecting dermatology residents view this additional year. We surveyed faculty members participating in the resident selection process to assess their viewpoints on how RFs factored into an applicant’s odds of matching into dermatology residency and performance as a resident.
Materials and Methods
An institutional review board application was submitted through the Geisinger Health System (Danville, Pennsylvania), and an exemption to complete the survey was granted. The survey consisted of 16 questions via REDCap electronic data capture and was sent to a listserve of dermatology program directors who were asked to distribute the survey to program chairs and faculty members within their department. Survey questions evaluated the participants’ involvement in medical student advising and the residency selection process. Questions relating to the respondents’ opinions were based on a 5-point Likert scale on level of agreement (1=strongly agree; 5=strongly disagree) or importance (1=a great deal; 5=not at all). All responses were collected anonymously. Data points were compiled and analyzed using REDCap. Statistical analysis via χ2 tests were conducted when appropriate.
Results
The survey was sent to 142 individuals and distributed to faculty members within those departments between August 16, 2019, and September 24, 2019. The survey elicited a total of 110 respondents. Demographic information is shown in eTable 1. Of these respondents, 35.5% were program directors, 23.6% were program chairs, 3.6% were both program director and program chair, and 37.3% were core faculty members. Although respondents’ roles were varied, 96.4% indicated that they were involved in both advising medical students and in selecting residents.
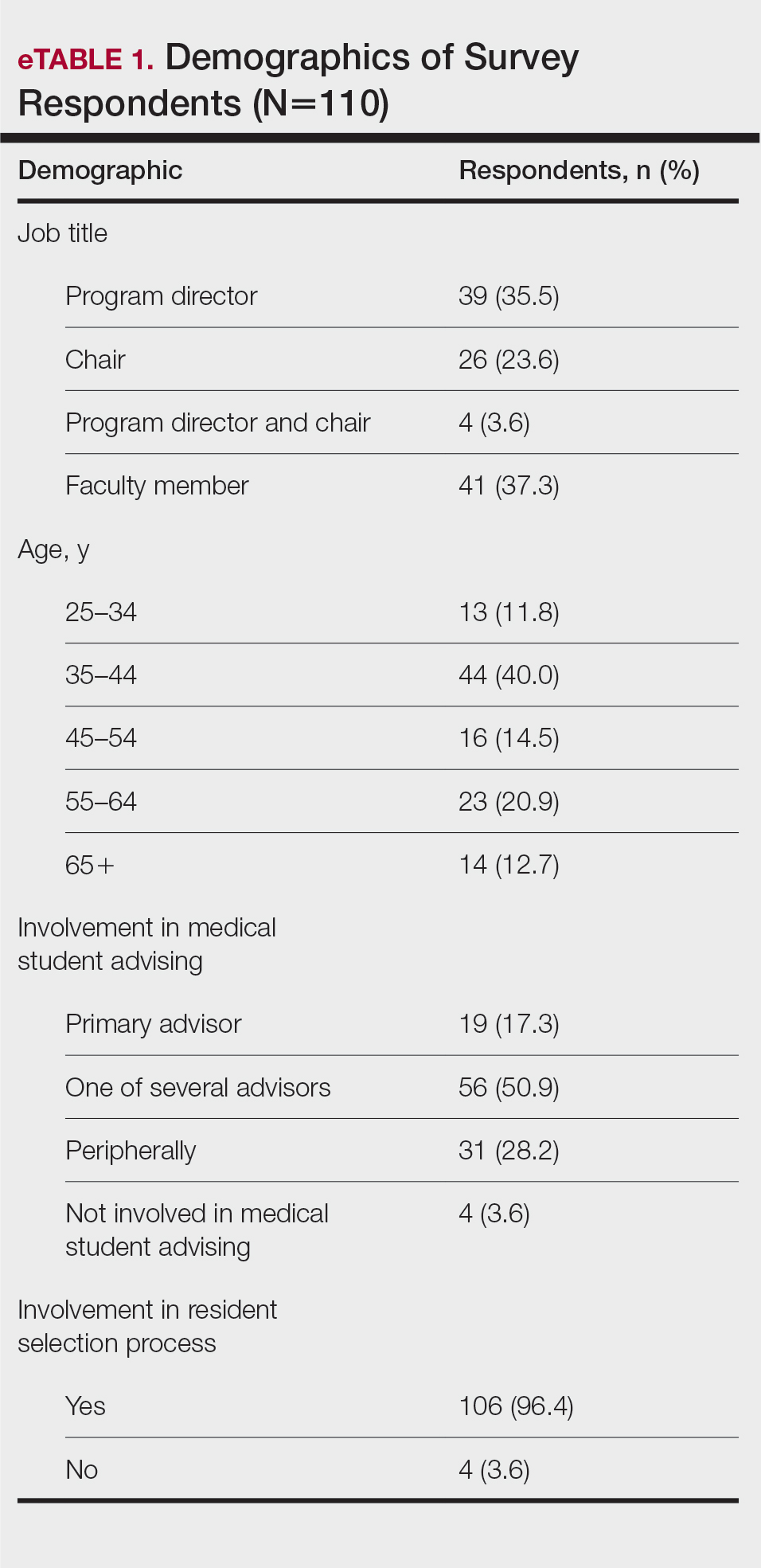
None of the respondents indicated that they always recommend that students complete an RF, and only 4.5% indicated that they usually recommend it; 40% of respondents rarely or never recommend an RF, while 55.5% sometimes recommend it. Although there was a variety of responses to how frequently faculty members recommend an RF, almost all respondents (98.2%) agreed that the reason medical students pursued an RF prior to residency application was to increase the competitiveness of their residency application. However, 20% of respondents believed that students in this cohort were seeking to gain a deeper understanding of the specialty, and 27.3% thought that this cohort had genuine interest in research. Interestingly, despite the medical students’ intentions of choosing an RF, most respondents (67.3%) agreed or strongly agreed that the publications produced by fellows make an impact on the dermatologic scientific community.
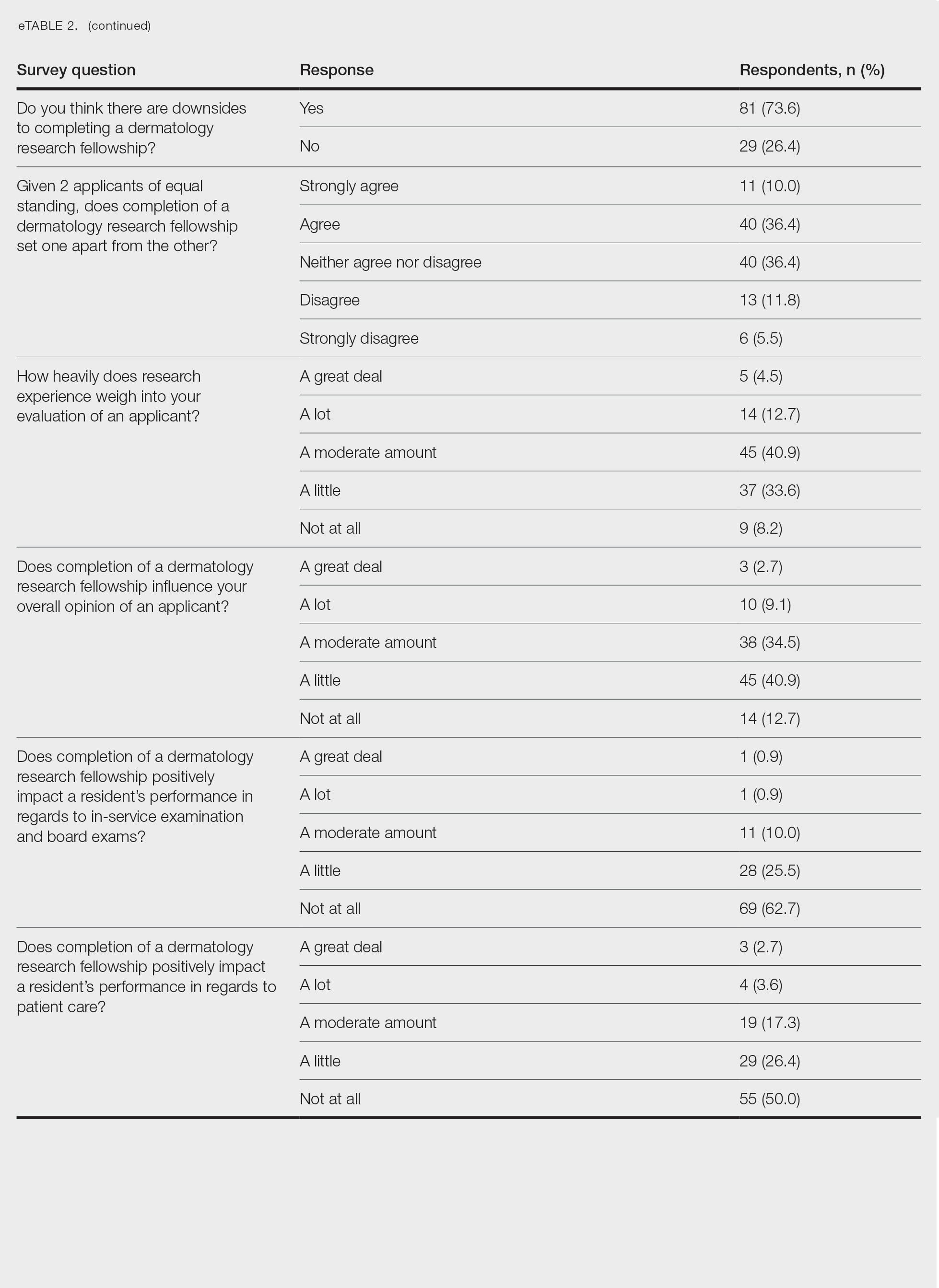
Although some respondents indicated that completion of an RF positively impacts resident performance with regard to patient care, most indicated that the impact was a little (26.4%) or not at all (50%). Additionally, a minority of respondents (11.8%) believed that RFs positively impact resident performance on in-service and board examinations at least a moderate amount, with 62.7% indicating no positive impact at all. Only 12.7% of participants agreed or strongly agreed that completion of an RF led to increased applicant involvement in research throughout their career, and most (73.6%) believed there were downsides to completing an RF. Finally, only 20% agreed or strongly agreed that students who completed an RF were more dedicated to the field of dermatology (eTable 2).
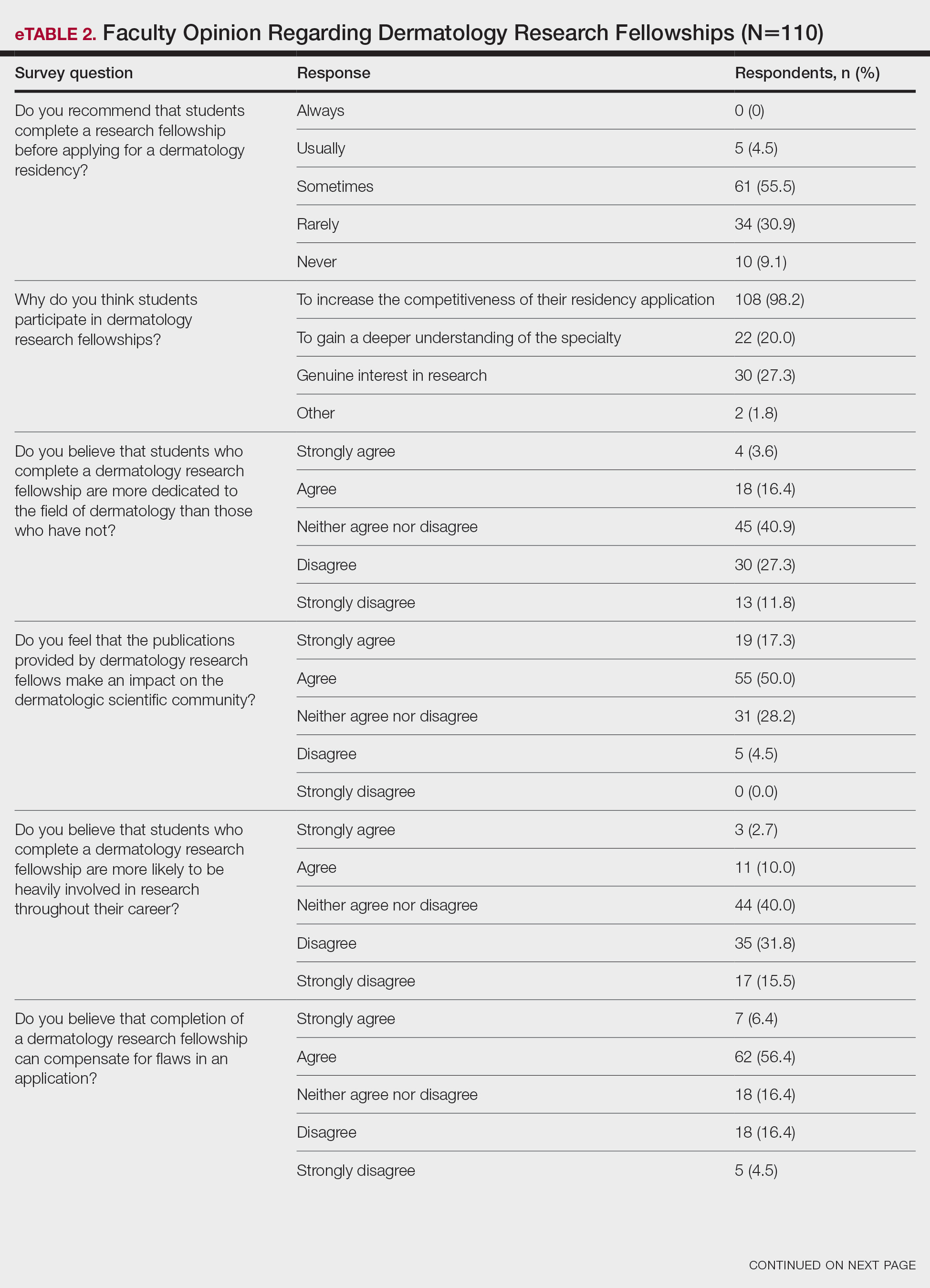
Further evaluation of the data indicated that the perceived utility of RFs did not affect respondents’ recommendation on whether to pursue an RF or not. For example, of the 4.5% of respondents who indicated that they always or usually recommended RFs, only 1 respondent believed that students who completed an RF were more dedicated to the field of dermatology than those who did not. Although 55.5% of respondents answered that they sometimes recommended completion of an RF, less than a quarter of this group believed that students who completed an RF were more likely to be heavily involved in research throughout their career (P=.99).
Overall, 11.8% of respondents indicated that completion of a dermatology RF influenced the evaluation of an applicant a great deal or a lot, while 53.6% of respondents indicated a little or no influence at all. Most respondents (62.8%) agreed or strongly agreed that completion of an RF can compensate for flaws in a residency application. Furthermore, when asked if completion of an RF could set 2 otherwise equivocal applicants apart from one another, 46.4% of respondents agreed or strongly agreed with the statement, while only 17.3% disagreed or strongly disagreed (eTable 2).
Comment
This study characterized how completion of an RF is viewed by those involved in advising medical students and selecting dermatology residents. The growing pressure for applicants to increase the number of publications combined with the competitiveness of applying for a dermatology residency position has led to increased participation in RFs. However, studies have found that students who completed an RF often did so despite a lack of interest.4 Nonetheless, little is known about how this is perceived by those involved in choosing residents.
We found that few respondents always or usually advised applicants to complete an RF, but the majority sometimes recommended them, demonstrating the complexity of this issue. Completion of an RF impacted 11.8% of respondents’ overall opinion of an applicant a lot or a great deal, while most respondents (53.6%) were influenced a little or not at all. However, 46.4% of respondents indicated that completion of a dermatology RF would set apart 2 applicants of otherwise equal standing, and 62.8% agreed or strongly agreed that completion of an RF would compensate for flaws in an application. These responses align with the findings of a study conducted by Kaffenberger et al,5 who surveyed members of the Association of Professors of Dermatology and found that 74.5% (73/98) of mentors almost always or sometimes recommended a research gap year for reasons that included low grades, low USMLE Step scores, and little research. These data suggest that completion of an RF can give a competitive advantage to applicants despite most advisors acknowledging that these applicants are not likely to be involved in research throughout their careers, perform better on standardized examinations, or provide better patient care.
Given the complexity of this issue, respondents may not have been able to accurately answer the question about how much an RF influenced their overall opinion of an applicant because of subconscious bias. Furthermore, respondents likely tailored their recommendations to complete an RF based on individual applicant strengths and weaknesses, and the specific reasons why one may recommend an RF need to be further investigated.
Although there may be other perceived advantages to RFs that were not captured by our survey, completion of a dermatology RF is not without disadvantages. Fellowships often are unfunded and offered in cities with high costs of living. Additionally, students are forced to delay graduation from medical school by a year at minimum and continue to accrue interest on medical school loans during this time. The financial burdens of completing an RF may exclude students of lower socioeconomic status and contribute to a decrease in diversity within the field. Dermatology has been found to be the second least diverse specialty, behind orthopedics.6 Soliman et al7 found that racial minorities and low-income students were more likely to cite socioeconomic barriers as factors involved in their decision not to pursue a career in dermatology. This notion was supported by Rinderknecht et al,8 who found that Black and Latinx dermatology applicants were more likely to come from disadvantaged backgrounds, and Black applicants were more likely to indicate financial concerns as their primary reason for not pursuing an RF. The impact of accumulated student debt and decreased access should be carefully weighed against the potential benefits of an RF. However, as the USMLE transitions their Step 1 score reporting from numerical to a pass/fail system, it also is possible that dermatology programs will place more emphasis on research productivity when evaluating applications for residency. Overall, the decision to recommend an RF represents an extremely complex topic, as indicated by the results of this study.
Limitations—Our survey-based study is limited by response rate and response bias. Despite the large number of responses, the overall response rate cannot be determined because it is unknown how many total faculty members actually received the survey. Moreover, data collected from current dermatology residents who have completed RFs vs those who have not as they pertain to resident performance and preparedness for the rigors of a dermatology residency would be useful.
- National Resident Matching Program. Results and Data: 2019 Main Residency Match. National Resident Matching Program; 2019. Accessed September 13, 2023. https://www.nrmp.org/wp-content/uploads/2021/07/NRMP-Results-and-Data-2019_04112019_final.pdf
- Stratman EJ, Ness RM. Factors associated with successful matching to dermatology residency programs by reapplicants and other applicants who previously graduated from medical school. Arch Dermatol. 2011;147:196-202.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap-years play in a successful dermatology match. J Am Acad Dermatol. 2021;85:AB22.
- Pathipati AS, Taleghani N. Research in medical school: a survey evaluating why medical students take research years. Cureus. 2016;8:E741.
- Kaffenberger J, Lee B, Ahmed AM. How to advise medical students interested in dermatology: a survey of academic dermatology mentors. Cutis. 2023;111:124-127.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254.
- Rinderknecht FA, Brumfiel CM, Jefferson IS, et al. Differences in underrepresented in medicine applicant backgrounds and outcomes in the 2020-2021 dermatology residency match. Cutis. 2022;110:76-79.
Dermatology residency positions continue to be highly coveted among applicants in the match. In 2019, dermatology proved to be the most competitive specialty, with 36.3% of US medical school seniors and independent applicants going unmatched.1 Prior to the transition to a pass/fail system, the mean US Medical Licensing Examination (USMLE) Step 1 score for matched applicants increased from 247 in 2014 to 251 in 2019. The growing number of scholarly activities reported by applicants has contributed to the competitiveness of the specialty. In 2018, the mean number of abstracts, presentations, and publications reported by matched applicants was 14.71, which was higher than other competitive specialties, including orthopedic surgery and otolaryngology (11.5 and 10.4, respectively). Dermatology applicants who did not match in 2018 reported a mean of 8.6 abstracts, presentations, and publications, which was on par with successful applicants in many other specialties.1 In 2011, Stratman and Ness2 found that publishing manuscripts and listing research experience were factors strongly associated with matching into dermatology for reapplicants. These trends in reported research have added pressure for applicants to increase their publications.
Given that many students do not choose a career in dermatology until later in medical school, some students choose to take a gap year between their third and fourth years of medical school to pursue a research fellowship (RF) and produce publications, in theory to increase the chances of matching in dermatology. A survey of dermatology applicants conducted by Costello et al3 in 2021 found that, of the students who completed a gap year (n=90; 31.25%), 78.7% (n=71) of them completed an RF, and those who completed RFs were more likely to match at top dermatology residency programs (P<.01). The authors also reported that there was no significant difference in overall match rates between gap-year and non–gap-year applicants.3 Another survey of 328 medical students found that the most common reason students take years off for research during medical school is to increase competitiveness for residency application.4 Although it is clear that students completing an RF often find success in the match, there are limited published data on how those involved in selecting dermatology residents view this additional year. We surveyed faculty members participating in the resident selection process to assess their viewpoints on how RFs factored into an applicant’s odds of matching into dermatology residency and performance as a resident.
Materials and Methods
An institutional review board application was submitted through the Geisinger Health System (Danville, Pennsylvania), and an exemption to complete the survey was granted. The survey consisted of 16 questions via REDCap electronic data capture and was sent to a listserve of dermatology program directors who were asked to distribute the survey to program chairs and faculty members within their department. Survey questions evaluated the participants’ involvement in medical student advising and the residency selection process. Questions relating to the respondents’ opinions were based on a 5-point Likert scale on level of agreement (1=strongly agree; 5=strongly disagree) or importance (1=a great deal; 5=not at all). All responses were collected anonymously. Data points were compiled and analyzed using REDCap. Statistical analysis via χ2 tests were conducted when appropriate.
Results
The survey was sent to 142 individuals and distributed to faculty members within those departments between August 16, 2019, and September 24, 2019. The survey elicited a total of 110 respondents. Demographic information is shown in eTable 1. Of these respondents, 35.5% were program directors, 23.6% were program chairs, 3.6% were both program director and program chair, and 37.3% were core faculty members. Although respondents’ roles were varied, 96.4% indicated that they were involved in both advising medical students and in selecting residents.

None of the respondents indicated that they always recommend that students complete an RF, and only 4.5% indicated that they usually recommend it; 40% of respondents rarely or never recommend an RF, while 55.5% sometimes recommend it. Although there was a variety of responses to how frequently faculty members recommend an RF, almost all respondents (98.2%) agreed that the reason medical students pursued an RF prior to residency application was to increase the competitiveness of their residency application. However, 20% of respondents believed that students in this cohort were seeking to gain a deeper understanding of the specialty, and 27.3% thought that this cohort had genuine interest in research. Interestingly, despite the medical students’ intentions of choosing an RF, most respondents (67.3%) agreed or strongly agreed that the publications produced by fellows make an impact on the dermatologic scientific community.
Although some respondents indicated that completion of an RF positively impacts resident performance with regard to patient care, most indicated that the impact was a little (26.4%) or not at all (50%). Additionally, a minority of respondents (11.8%) believed that RFs positively impact resident performance on in-service and board examinations at least a moderate amount, with 62.7% indicating no positive impact at all. Only 12.7% of participants agreed or strongly agreed that completion of an RF led to increased applicant involvement in research throughout their career, and most (73.6%) believed there were downsides to completing an RF. Finally, only 20% agreed or strongly agreed that students who completed an RF were more dedicated to the field of dermatology (eTable 2).


Further evaluation of the data indicated that the perceived utility of RFs did not affect respondents’ recommendation on whether to pursue an RF or not. For example, of the 4.5% of respondents who indicated that they always or usually recommended RFs, only 1 respondent believed that students who completed an RF were more dedicated to the field of dermatology than those who did not. Although 55.5% of respondents answered that they sometimes recommended completion of an RF, less than a quarter of this group believed that students who completed an RF were more likely to be heavily involved in research throughout their career (P=.99).
Overall, 11.8% of respondents indicated that completion of a dermatology RF influenced the evaluation of an applicant a great deal or a lot, while 53.6% of respondents indicated a little or no influence at all. Most respondents (62.8%) agreed or strongly agreed that completion of an RF can compensate for flaws in a residency application. Furthermore, when asked if completion of an RF could set 2 otherwise equivocal applicants apart from one another, 46.4% of respondents agreed or strongly agreed with the statement, while only 17.3% disagreed or strongly disagreed (eTable 2).
Comment
This study characterized how completion of an RF is viewed by those involved in advising medical students and selecting dermatology residents. The growing pressure for applicants to increase the number of publications combined with the competitiveness of applying for a dermatology residency position has led to increased participation in RFs. However, studies have found that students who completed an RF often did so despite a lack of interest.4 Nonetheless, little is known about how this is perceived by those involved in choosing residents.
We found that few respondents always or usually advised applicants to complete an RF, but the majority sometimes recommended them, demonstrating the complexity of this issue. Completion of an RF impacted 11.8% of respondents’ overall opinion of an applicant a lot or a great deal, while most respondents (53.6%) were influenced a little or not at all. However, 46.4% of respondents indicated that completion of a dermatology RF would set apart 2 applicants of otherwise equal standing, and 62.8% agreed or strongly agreed that completion of an RF would compensate for flaws in an application. These responses align with the findings of a study conducted by Kaffenberger et al,5 who surveyed members of the Association of Professors of Dermatology and found that 74.5% (73/98) of mentors almost always or sometimes recommended a research gap year for reasons that included low grades, low USMLE Step scores, and little research. These data suggest that completion of an RF can give a competitive advantage to applicants despite most advisors acknowledging that these applicants are not likely to be involved in research throughout their careers, perform better on standardized examinations, or provide better patient care.
Given the complexity of this issue, respondents may not have been able to accurately answer the question about how much an RF influenced their overall opinion of an applicant because of subconscious bias. Furthermore, respondents likely tailored their recommendations to complete an RF based on individual applicant strengths and weaknesses, and the specific reasons why one may recommend an RF need to be further investigated.
Although there may be other perceived advantages to RFs that were not captured by our survey, completion of a dermatology RF is not without disadvantages. Fellowships often are unfunded and offered in cities with high costs of living. Additionally, students are forced to delay graduation from medical school by a year at minimum and continue to accrue interest on medical school loans during this time. The financial burdens of completing an RF may exclude students of lower socioeconomic status and contribute to a decrease in diversity within the field. Dermatology has been found to be the second least diverse specialty, behind orthopedics.6 Soliman et al7 found that racial minorities and low-income students were more likely to cite socioeconomic barriers as factors involved in their decision not to pursue a career in dermatology. This notion was supported by Rinderknecht et al,8 who found that Black and Latinx dermatology applicants were more likely to come from disadvantaged backgrounds, and Black applicants were more likely to indicate financial concerns as their primary reason for not pursuing an RF. The impact of accumulated student debt and decreased access should be carefully weighed against the potential benefits of an RF. However, as the USMLE transitions their Step 1 score reporting from numerical to a pass/fail system, it also is possible that dermatology programs will place more emphasis on research productivity when evaluating applications for residency. Overall, the decision to recommend an RF represents an extremely complex topic, as indicated by the results of this study.
Limitations—Our survey-based study is limited by response rate and response bias. Despite the large number of responses, the overall response rate cannot be determined because it is unknown how many total faculty members actually received the survey. Moreover, data collected from current dermatology residents who have completed RFs vs those who have not as they pertain to resident performance and preparedness for the rigors of a dermatology residency would be useful.
Dermatology residency positions continue to be highly coveted among applicants in the match. In 2019, dermatology proved to be the most competitive specialty, with 36.3% of US medical school seniors and independent applicants going unmatched.1 Prior to the transition to a pass/fail system, the mean US Medical Licensing Examination (USMLE) Step 1 score for matched applicants increased from 247 in 2014 to 251 in 2019. The growing number of scholarly activities reported by applicants has contributed to the competitiveness of the specialty. In 2018, the mean number of abstracts, presentations, and publications reported by matched applicants was 14.71, which was higher than other competitive specialties, including orthopedic surgery and otolaryngology (11.5 and 10.4, respectively). Dermatology applicants who did not match in 2018 reported a mean of 8.6 abstracts, presentations, and publications, which was on par with successful applicants in many other specialties.1 In 2011, Stratman and Ness2 found that publishing manuscripts and listing research experience were factors strongly associated with matching into dermatology for reapplicants. These trends in reported research have added pressure for applicants to increase their publications.
Given that many students do not choose a career in dermatology until later in medical school, some students choose to take a gap year between their third and fourth years of medical school to pursue a research fellowship (RF) and produce publications, in theory to increase the chances of matching in dermatology. A survey of dermatology applicants conducted by Costello et al3 in 2021 found that, of the students who completed a gap year (n=90; 31.25%), 78.7% (n=71) of them completed an RF, and those who completed RFs were more likely to match at top dermatology residency programs (P<.01). The authors also reported that there was no significant difference in overall match rates between gap-year and non–gap-year applicants.3 Another survey of 328 medical students found that the most common reason students take years off for research during medical school is to increase competitiveness for residency application.4 Although it is clear that students completing an RF often find success in the match, there are limited published data on how those involved in selecting dermatology residents view this additional year. We surveyed faculty members participating in the resident selection process to assess their viewpoints on how RFs factored into an applicant’s odds of matching into dermatology residency and performance as a resident.
Materials and Methods
An institutional review board application was submitted through the Geisinger Health System (Danville, Pennsylvania), and an exemption to complete the survey was granted. The survey consisted of 16 questions via REDCap electronic data capture and was sent to a listserve of dermatology program directors who were asked to distribute the survey to program chairs and faculty members within their department. Survey questions evaluated the participants’ involvement in medical student advising and the residency selection process. Questions relating to the respondents’ opinions were based on a 5-point Likert scale on level of agreement (1=strongly agree; 5=strongly disagree) or importance (1=a great deal; 5=not at all). All responses were collected anonymously. Data points were compiled and analyzed using REDCap. Statistical analysis via χ2 tests were conducted when appropriate.
Results
The survey was sent to 142 individuals and distributed to faculty members within those departments between August 16, 2019, and September 24, 2019. The survey elicited a total of 110 respondents. Demographic information is shown in eTable 1. Of these respondents, 35.5% were program directors, 23.6% were program chairs, 3.6% were both program director and program chair, and 37.3% were core faculty members. Although respondents’ roles were varied, 96.4% indicated that they were involved in both advising medical students and in selecting residents.

None of the respondents indicated that they always recommend that students complete an RF, and only 4.5% indicated that they usually recommend it; 40% of respondents rarely or never recommend an RF, while 55.5% sometimes recommend it. Although there was a variety of responses to how frequently faculty members recommend an RF, almost all respondents (98.2%) agreed that the reason medical students pursued an RF prior to residency application was to increase the competitiveness of their residency application. However, 20% of respondents believed that students in this cohort were seeking to gain a deeper understanding of the specialty, and 27.3% thought that this cohort had genuine interest in research. Interestingly, despite the medical students’ intentions of choosing an RF, most respondents (67.3%) agreed or strongly agreed that the publications produced by fellows make an impact on the dermatologic scientific community.
Although some respondents indicated that completion of an RF positively impacts resident performance with regard to patient care, most indicated that the impact was a little (26.4%) or not at all (50%). Additionally, a minority of respondents (11.8%) believed that RFs positively impact resident performance on in-service and board examinations at least a moderate amount, with 62.7% indicating no positive impact at all. Only 12.7% of participants agreed or strongly agreed that completion of an RF led to increased applicant involvement in research throughout their career, and most (73.6%) believed there were downsides to completing an RF. Finally, only 20% agreed or strongly agreed that students who completed an RF were more dedicated to the field of dermatology (eTable 2).


Further evaluation of the data indicated that the perceived utility of RFs did not affect respondents’ recommendation on whether to pursue an RF or not. For example, of the 4.5% of respondents who indicated that they always or usually recommended RFs, only 1 respondent believed that students who completed an RF were more dedicated to the field of dermatology than those who did not. Although 55.5% of respondents answered that they sometimes recommended completion of an RF, less than a quarter of this group believed that students who completed an RF were more likely to be heavily involved in research throughout their career (P=.99).
Overall, 11.8% of respondents indicated that completion of a dermatology RF influenced the evaluation of an applicant a great deal or a lot, while 53.6% of respondents indicated a little or no influence at all. Most respondents (62.8%) agreed or strongly agreed that completion of an RF can compensate for flaws in a residency application. Furthermore, when asked if completion of an RF could set 2 otherwise equivocal applicants apart from one another, 46.4% of respondents agreed or strongly agreed with the statement, while only 17.3% disagreed or strongly disagreed (eTable 2).
Comment
This study characterized how completion of an RF is viewed by those involved in advising medical students and selecting dermatology residents. The growing pressure for applicants to increase the number of publications combined with the competitiveness of applying for a dermatology residency position has led to increased participation in RFs. However, studies have found that students who completed an RF often did so despite a lack of interest.4 Nonetheless, little is known about how this is perceived by those involved in choosing residents.
We found that few respondents always or usually advised applicants to complete an RF, but the majority sometimes recommended them, demonstrating the complexity of this issue. Completion of an RF impacted 11.8% of respondents’ overall opinion of an applicant a lot or a great deal, while most respondents (53.6%) were influenced a little or not at all. However, 46.4% of respondents indicated that completion of a dermatology RF would set apart 2 applicants of otherwise equal standing, and 62.8% agreed or strongly agreed that completion of an RF would compensate for flaws in an application. These responses align with the findings of a study conducted by Kaffenberger et al,5 who surveyed members of the Association of Professors of Dermatology and found that 74.5% (73/98) of mentors almost always or sometimes recommended a research gap year for reasons that included low grades, low USMLE Step scores, and little research. These data suggest that completion of an RF can give a competitive advantage to applicants despite most advisors acknowledging that these applicants are not likely to be involved in research throughout their careers, perform better on standardized examinations, or provide better patient care.
Given the complexity of this issue, respondents may not have been able to accurately answer the question about how much an RF influenced their overall opinion of an applicant because of subconscious bias. Furthermore, respondents likely tailored their recommendations to complete an RF based on individual applicant strengths and weaknesses, and the specific reasons why one may recommend an RF need to be further investigated.
Although there may be other perceived advantages to RFs that were not captured by our survey, completion of a dermatology RF is not without disadvantages. Fellowships often are unfunded and offered in cities with high costs of living. Additionally, students are forced to delay graduation from medical school by a year at minimum and continue to accrue interest on medical school loans during this time. The financial burdens of completing an RF may exclude students of lower socioeconomic status and contribute to a decrease in diversity within the field. Dermatology has been found to be the second least diverse specialty, behind orthopedics.6 Soliman et al7 found that racial minorities and low-income students were more likely to cite socioeconomic barriers as factors involved in their decision not to pursue a career in dermatology. This notion was supported by Rinderknecht et al,8 who found that Black and Latinx dermatology applicants were more likely to come from disadvantaged backgrounds, and Black applicants were more likely to indicate financial concerns as their primary reason for not pursuing an RF. The impact of accumulated student debt and decreased access should be carefully weighed against the potential benefits of an RF. However, as the USMLE transitions their Step 1 score reporting from numerical to a pass/fail system, it also is possible that dermatology programs will place more emphasis on research productivity when evaluating applications for residency. Overall, the decision to recommend an RF represents an extremely complex topic, as indicated by the results of this study.
Limitations—Our survey-based study is limited by response rate and response bias. Despite the large number of responses, the overall response rate cannot be determined because it is unknown how many total faculty members actually received the survey. Moreover, data collected from current dermatology residents who have completed RFs vs those who have not as they pertain to resident performance and preparedness for the rigors of a dermatology residency would be useful.
- National Resident Matching Program. Results and Data: 2019 Main Residency Match. National Resident Matching Program; 2019. Accessed September 13, 2023. https://www.nrmp.org/wp-content/uploads/2021/07/NRMP-Results-and-Data-2019_04112019_final.pdf
- Stratman EJ, Ness RM. Factors associated with successful matching to dermatology residency programs by reapplicants and other applicants who previously graduated from medical school. Arch Dermatol. 2011;147:196-202.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap-years play in a successful dermatology match. J Am Acad Dermatol. 2021;85:AB22.
- Pathipati AS, Taleghani N. Research in medical school: a survey evaluating why medical students take research years. Cureus. 2016;8:E741.
- Kaffenberger J, Lee B, Ahmed AM. How to advise medical students interested in dermatology: a survey of academic dermatology mentors. Cutis. 2023;111:124-127.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254.
- Rinderknecht FA, Brumfiel CM, Jefferson IS, et al. Differences in underrepresented in medicine applicant backgrounds and outcomes in the 2020-2021 dermatology residency match. Cutis. 2022;110:76-79.
- National Resident Matching Program. Results and Data: 2019 Main Residency Match. National Resident Matching Program; 2019. Accessed September 13, 2023. https://www.nrmp.org/wp-content/uploads/2021/07/NRMP-Results-and-Data-2019_04112019_final.pdf
- Stratman EJ, Ness RM. Factors associated with successful matching to dermatology residency programs by reapplicants and other applicants who previously graduated from medical school. Arch Dermatol. 2011;147:196-202.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap-years play in a successful dermatology match. J Am Acad Dermatol. 2021;85:AB22.
- Pathipati AS, Taleghani N. Research in medical school: a survey evaluating why medical students take research years. Cureus. 2016;8:E741.
- Kaffenberger J, Lee B, Ahmed AM. How to advise medical students interested in dermatology: a survey of academic dermatology mentors. Cutis. 2023;111:124-127.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254.
- Rinderknecht FA, Brumfiel CM, Jefferson IS, et al. Differences in underrepresented in medicine applicant backgrounds and outcomes in the 2020-2021 dermatology residency match. Cutis. 2022;110:76-79.
PRACTICE POINTS
- Many medical students seeking to match into a dermatology residency program complete a research fellowship (RF).
- Completion of an RF can give a competitive advantage to applicants even though most advisors acknowledge that these applicants are not likely to be involved in research throughout their career, perform better on standardized examinations, or provide better patient care.
- The decision to recommend an RF represents an extremely complex topic and should be tailored to each individual applicant.
Assessment of the Efficacy of Tranexamic Acid Solution 5% in the Treatment of Melasma in Patients of South Asian Descent
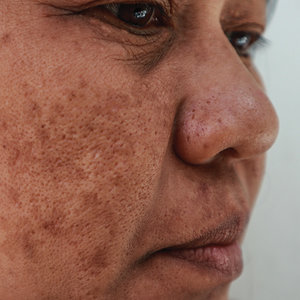
Melasma is a complex, long-lasting, acquired dermatologic pigmentation disorder resulting in grey-brown patches that last for more than 3 months. Sun-exposed areas including the nose, cheeks, forehead, and forearms are most likely to be affected.1 In Southeast Asia, 0.25% to 4% of the population affected by melasma is aged 30 to 40 years.2 In particular, melasma is a concern among pregnant women due to increased levels of melanocyte-stimulating hormones (MSHs) and is impacted by genetics, hormonal influence, and exposure to UV light.3,4 In Pakistan, approximately 46% of women are affected by melasma during pregnancy.2,5 Although few studies have focused on the clinical approaches to melasma in darker skin types, it continues to disproportionately affect the skin of color population.4
The areas of hyperpigmentation seen in melasma exhibit increased deposition of melanin in the epidermis and dermis, but melanocytes are not elevated. However, in areas of hyperpigmentation, the melanocytes are larger and more dendritic and demonstrate an increased level of melanogenesis.6 During pregnancy, especially in the third trimester, elevated levels of estrogen, progesterone, and MSH often are found in association with melasma.7 Tyrosinase (TYR) activity increases and cellular proliferation is reduced after treatment of melanocytes in culture with β-estradiol.8 Sex steroids increase transcription of genes encoding melanogenic enzymes in normal human melanocytes, especially TYR.9 These results are consistent with the notable increases in melanin synthesis and TYR activity reported for normal human melanocytes under similar conditions in culture.10 Because melanocytes contain both cytosolic and nuclear estrogen receptors, melanocytes in patients with melasma may be inherently more sensitive to the stimulatory effects of estrogens and possibly other steroid hormones.11
The current treatment options for melasma have varying levels of success and include topical depigmenting agents such as hydroquinone, tretinoin, azelaic acid, kojic acid, and corticosteroids; dermabrasion; and chemical peels.12-14 Chemical peels with glycolic acid, salicylic acid, lactic acid, trichloroacetic acid, and phenol, as well as laser therapy, are reliable management options.13,14 Traditionally, melasma has been treated with a combination of modalities along with photoprotection and trigger avoidance.12
The efficacy and safety of the available therapies for melasma are still controversial and require further exploration. In recent years, off-label tranexamic acid (TA) has emerged as a potential therapy for melasma. Although the mechanism of action remains unclear, TA may inhibit melanin synthesis by blocking the interaction between melanocytes and keratinocytes.15 Tranexamic acid also may reverse the abnormal dermal changes associated with melasma by inhibiting melanogenesis and angiogenesis.16
Although various therapeutic options exist for melasma, the search for a reliable option in patients with darker skin types continues.13 We sought to evaluate the efficacy of TA solution 5% in reducing the severity of melasma in South Asian patients, thereby improving patient outcomes and maximizing patient satisfaction. Topical TA is inexpensive and readily accessible and does not cause systemic side effects. These qualities make it a promising treatment compared to traditional therapies.
Methods
We conducted a randomized controlled trial at Rawalpindi Medical Institute (Punjab, Pakistan). The researchers obtained informed consent for all enrolled patients. Cases were sampled from the original patient population seen at the office using nonprobability consecutive sampling. The sample size was calculated with a 95% CI, margin of error of 9%, and expected percentage of efficacy of 86.1% by using TA solution 5%. South Asian male and female patients aged 20 to 45 years with melasma were included in the analysis. Patients were excluded if they were already taking TA, oral contraceptive pills, or photosensitizing drugs (eg, nonsteroidal anti-inflammatory drugs, tetracyclines, phenytoin, carbamazepine); were pregnant; had chronic kidney disease (creatinine >2.0 mg/dL); had cardiac abnormalities (abnormal electrocardiogram); had hematologic disorders (international normalized ratio >2); or had received another melasma treatment within the last 3 to 6 months.
All enrolled patients underwent a detailed history and physical examination. Patient demographics were subsequently noted, including age, sex, history of diabetes mellitus or hypertension, and duration of melasma. The melasma area and severity index (MASI) score of each patient was calculated at baseline, and a corresponding photograph was taken.
The topical solution was prepared with 5 g of TA dissolved in 10 cc of ethanol at 96 °F, 10 cc of 1,3-butanediol, and distilled water up to 100 cc. The TA solution was applied to the affected areas once daily by the patient for 12 weeks. Each application covered the affected areas completely. Patients were instructed to apply sunscreen with sun protection factor 60 to those same areas for UV protection after 15 minutes of TA application. Biweekly follow-ups were scheduled during the trial, and the MASI score was recorded at these visits. If the mean MASI score was reduced by half after 12 weeks of treatment, then the treatment was considered efficacious with a 95% CI.
The percentage reduction from baseline was calculated as follows: percentage reduction=(baseline score– follow-up score)/baseline score×100.
Statistical Analysis—Data were analyzed in SPSS Statistics 25 (IBM). The quantitative variables of age, duration of melasma, and body mass index were presented as mean (SD). Qualitative variables such as sex, history of diabetes mellitus or hypertension, site of melasma, and efficacy were presented as frequencies and percentages. Mean MASI scores at baseline and 12 weeks posttreatment were compared using a paired t test (P≤.05). Data were stratified for age, sex, history of diabetes mellitus or hypertension, site of melasma, and duration of melasma, and a χ2 test was applied to compare efficacy in stratified groups (P≤.05).
Results
Sixty patients were enrolled in the study. Of them, 17 (28.33%) were male, and 43 (71.67%) were female (2:5 ratio). They ranged in age from 20 to 45 years (mean [SD], 31.93 [6.26] years). Thirty-seven patients (61.67%) were aged 31 to 45 years of age (Table 1). The mean (SD) duration of disease was 10.18 (2.10) months. The response to TA was recorded based on patient distribution according to the site of melasma as well as history of diabetes mellitus and hypertension.

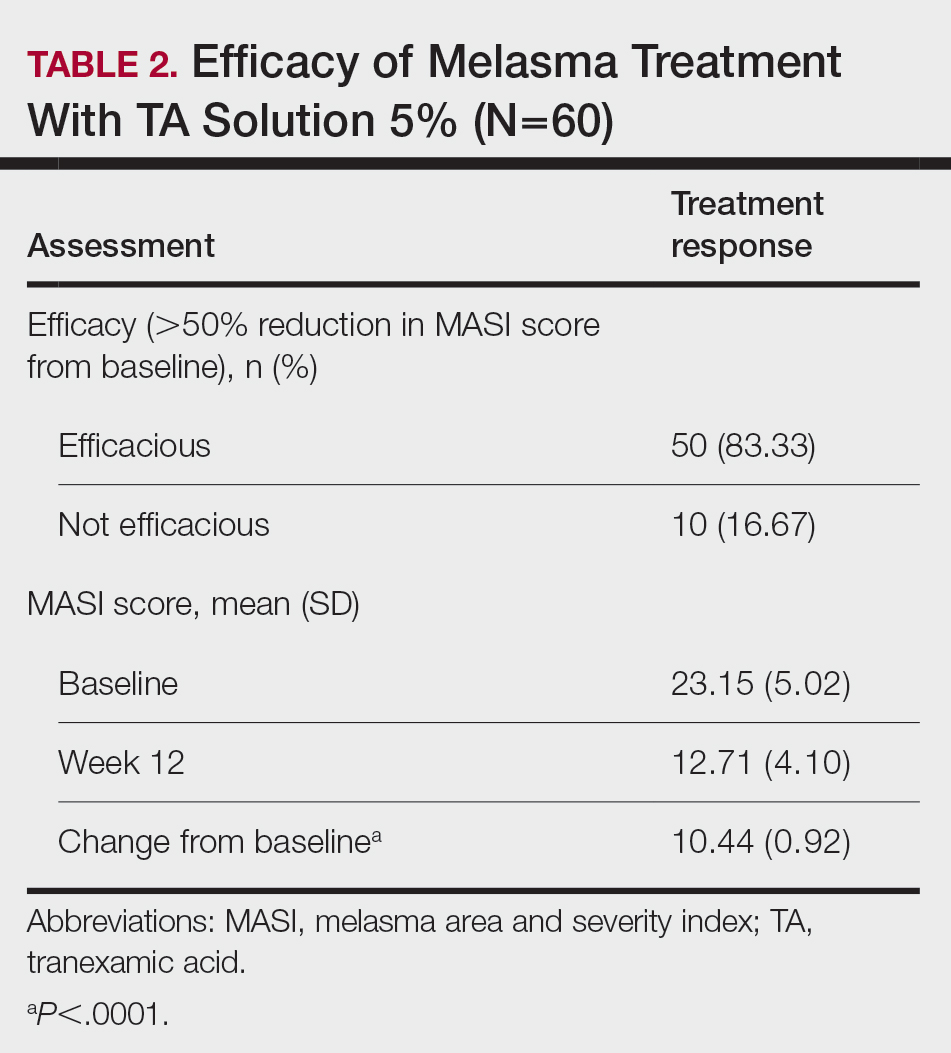
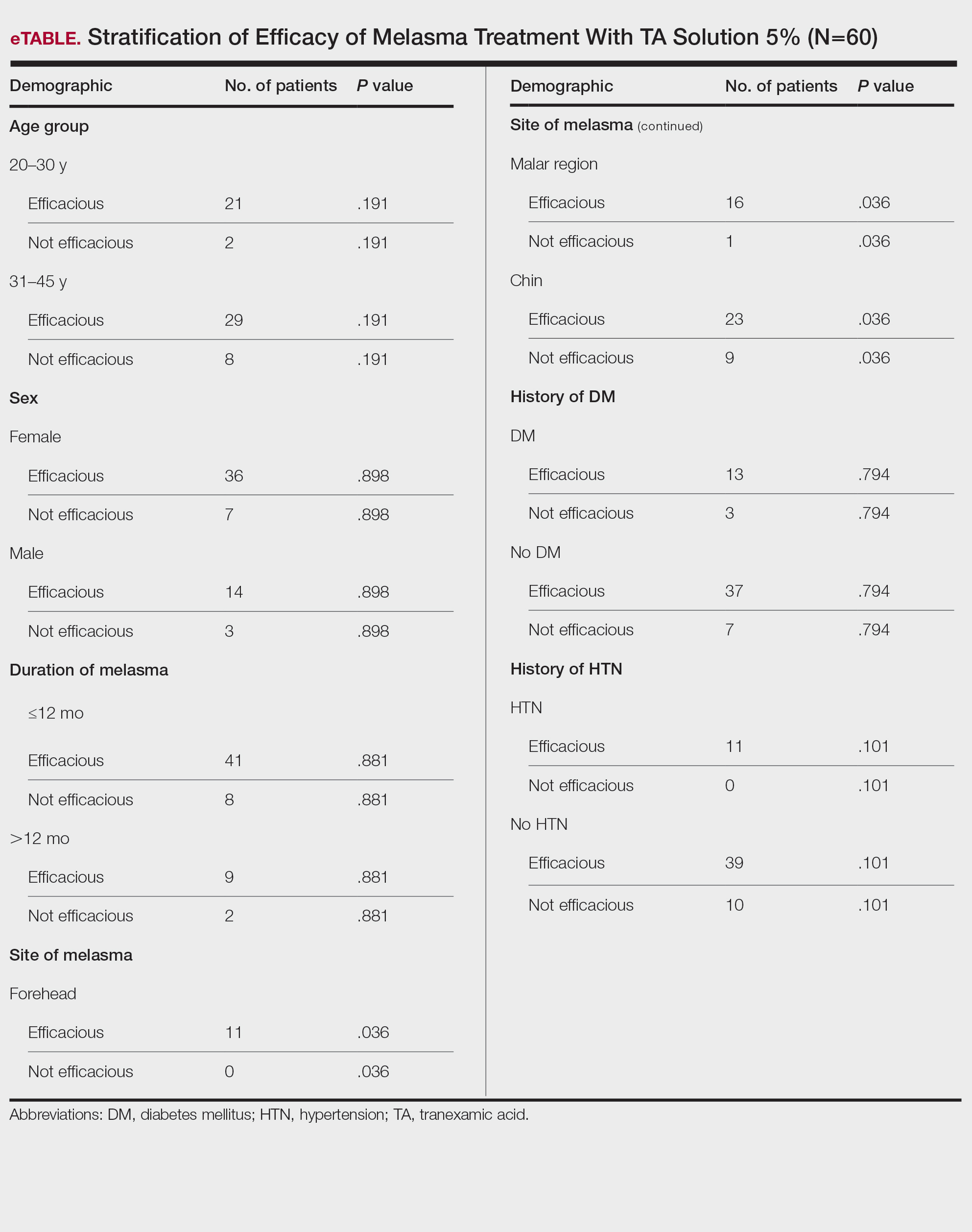
Topical TA was found to be efficacious for melasma in 50 (83.33%) patients. The mean (SD) baseline and week 12 MASI scores were 23.15 (5.02) and 12.71 (4.10)(P<.0001), respectively (Table 2). The stratification of efficacy with respect to age, sex, duration of melasma, site of melasma, and history of diabetes mellitus or hypertension is shown in the eTable. The site of melasma was significant with respect to stratification of efficacy. On the forehead, TA was found to be efficacious in 11 patients and nonefficacious in 0 patients (P=.036). In the malar region, it was efficacious in 16 patients and nonefficacious in 1 patient (P=.036). Finally, on the chin, it was efficacious in 23 patients and nonefficacious in 9 patients (P=.036).
Comment
Melasma Presentation and Development—Melasma is a chronic skin condition that more often affects patients with darker skin types. This condition is characterized by hyperpigmentation of skin that is directly exposed to the sun, such as the cheek, nose, forehead, and above the upper lip.17 Although the mechanism behind how melasma develops is unknown, one theory suggests that UV light can lead to increased plasmin in keratinocytes.18 This increased plasmin will thereby increase the arachidonic acid and α-MSH, leading to the observed uneven hyperpigmentation that is notable in melasma. Melasma is common in patients using oral contraceptives or expired cosmetic drugs; in those who are pregnant; and in those with liver dysfunction.18 Melasma has a negative impact on patients’ quality of life because of substantial psychological and social distress. Thus, finding an accessible treatment is imperative.19
Melasma Management—The most common treatments for melasma have been topical bleaching agents and photoprotection. Combination therapy options include chemical peels, dermabrasion, and laser treatments, though they present with limited efficacy.17,20 Because melasma focuses on pigmentation correction, topical treatments work to disturb melanocyte pigment production at the enzymatic level.21 Tyrosinase is rate limiting in melanin production, as it converts L-tyrosinase to L-3,4-dihydroxyphenylalanine, using copper to interact with L-3,4-dihydroxyphenylalanine as a cofactor in the active site.22 Therefore, tyrosine is a major target for many drugs that have been developed for melasma to decrease melaninization.21
Recently, research has focused on the effects of topical, intradermal, and oral TA for melasma.17 Tranexamic acid most commonly has been used in medicine as a fibrinolytic agent because of its antiplasmin properties. It has been hypothesized that TA can inhibit the release of paracrine melanogenic factors that normally act to stimulate melanocytes.17 Although studies have supported the safety and efficacy of TA, there remains a lack of clinical studies that are sufficiently powered. No definitive consensus on the use of TA for melasma currently exists, which indicates the need for large-scale, randomized, controlled trials.23
One trial (N=25) found that TA solution 5% achieved efficacy (>50% reduction in MASI score from baseline) in 86.1% of patients with melasma.24 In another study (N=18), topical TA 5% achieved efficacy (>50% reduction in MASI score) in 86% of patients with melasma.25
Melasma Comorbidities—To determine if certain comorbidities, such as diabetes mellitus or hypertension, influenced the progression of melasma, we stratified the efficacy results for patients with these 2 comorbidities, which showed no significant difference (P=.794 and P=.101, respectively). Thus, the relatively higher prevalence of diabetes mellitus (16 patients) and hypertension (11 patients) did not contribute to the efficacy of TA in lowering MASI scores over the 12-week period, which supports the findings of Doolan and Gupta,26 who investigated the endocrinologic conditions associated with melasma and found no such association with diabetes mellitus or hypertension.
TA Formulations for Melasma—The efficacy of topical TA has been explored in several studies. Six studies with sample sizes of 13 to 50 patients each showed statistically significant differences in MASI scores between baseline and following TA treatment (P<.001).27-32 Several formulations and regimens were utilized, including TA cream 3% for 12 weeks, TA gel 5% for 12 weeks, TA solution 3% for 12 weeks, TA liposome 5% for 12 weeks, and TA solution 2% for 12 weeks.18 Additionally, these studies found TA to be effective in limiting dyschromia and decreasing MASI scores. There were no statistically significant differences between formulations and method of application. Topical TA has been found to be just as effective as other treatments for melasma, including intradermal TA injections, topical hydroquinone, and a combination of topical hydroquinone and dexamethasone.18
Further study of the efficacy of intradermal TA is necessary because many human trials have lacked statistical significance or a control group. Lee et al32 conducted a trial of 100 female patients who received weekly intradermal TA microinjections for 12 weeks. After 8 and 12 weeks, MASI scores decreased significantly (P<.01).32 Similarly, Badran et al33 observed 60 female patients in 3 trial groups: group A received TA (4 mg/mL) intradermal injections every 2 weeks, group B received TA (10 mg/mL) intradermal injections every 2 weeks, and group C received TA cream 10% twice daily. Although all groups showed improvement in MASI, group B, which had the highest intradermal TA concentration, exhibited the most improvement. Thus, it was determined that intradermal application led to better results, but the cream was still effective.33
Saki et al34 conducted a randomized, split-face trial of 37 patients comparing the efficacy of intradermal TA and topical hydroquinone. Each group was treated with either monthly intradermal TA injections or nightly hydroquinone for 3 months. After 4 weeks of treatment, TA initially had a greater improvement. However, after 20 weeks, the overall changes were not significant between the 2 groups.34 Pazyar et al35 conducted a randomized, split-face trial of 49 patients comparing the efficacy of intradermal TA and hydroquinone cream. After 24 weeks of biweekly TA injections or twice-daily hydroquinone, there were no statistically significant differences in the decreased MASI scores between treatments.35 Additional large, double-blind, controlled trials are needed to thoroughly assess the role of intradermal TA in comparison to its treatment counterpart of hydroquinone.
Ebrahimi and Naeini29 conducted a 12-week, double-blind, split-phase trial of 50 Iranian melasma patients, which showed that 27.3% of patients rated the improvement in melasma as excellent, 42.4% as good, and 30.3% as fair after using TA solution 3%. Wu et al36 also showed a total melasma improvement rate of 80.9% in 256 patients with long-term oral use of TA. In a study by Kim et al31 (N=245), the mean MASI score considerably decreased after topical TA use, with a total response rate of 95.6%. In another study, Atefi et al37 presented significantly increased levels of satisfaction in patients treated with topical TA 5% vs hydroquinone (P=.015).
Melasma in Patients With Darker Skin Types—Special attention must be given to choosing the appropriate medication in melasma patients with darker skin types, as there is an increased risk for postinflammatory hyperpigmentation. Currently, few randomized controlled trials exist that fulfill the criteria of evaluating pharmacologic options for patients with melasma, and even fewer studies solely focus on patients with darker skin types.38 In addition to treatment advances, patients must be educated on the need to avoid sun exposure when possible or to use photoprotection, especially in the South Asian region, where these practices rarely are taught. Our study provided a unique analysis regarding the efficacy of TA solution 5% for the treatment of melasma in patients of South Asian descent. Clinicians can use these findings as a foundation for treating all patients with melasma but particularly those with darker skin types.
Study Limitations—Our study consisted of 60 patients; although our study had more patients than similar trials, larger studies are needed. Additionally, other variables were excluded from our analysis, such as comorbidities beyond diabetes mellitus and hypertension.
Conclusion
This study contributes to the growing field of melasma therapeutics by evaluating the efficacy of using TA solution 5% for the treatment of melasma in South Asian patients with darker skin types. Clinicians may use our study to broaden their treatment options for a common condition while also addressing the lack of clinical options for patients with darker skin types. Further studies investigating the effectiveness of TA in large clinical trials in humans are warranted to understand the efficacy and the risk for any complications.
- Espósito ACC, Brianezi G, De Souza NP, et al. Exploratory study of epidermis, basement membrane zone, upper dermis alterations and Wnt pathway activation in melasma compared to adjacent and retroauricular skin. Ann Dermatol. 2020;32:101-108.
- Janney MS, Subramaniyan R, Dabas R, et al. A randomized controlled study comparing the efficacy of topical 5% tranexamic acid solution versus 3% hydroquinone cream in melasma. J Cutan Aesthet Surg. 2019;12:63-67.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasmaJ Cosmet Laser Ther. 2018;20:134-139.
- Grimes PE, Ijaz S, Nashawati R, et al. New oral and topical approaches for the treatment of melasma. Int J Womens Dermatol. 2019;5:30-36.
- Handel AC, Miot LDB, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Barankin B, Silver SG, Carruthers A. The skin in pregnancy. J Cutan Med Surg. 2002;6:236-240.
- Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- Smith AG, Shuster S, Thody AJ, et al. Chloasma, oral contraceptives, and plasma immunoreactive beta-melanocyte-stimulating hormone. J Invest Dermatol. 1977;68:169-170.
- Ranson M, Posen S, Mason RS. Human melanocytes as a target tissue for hormones: in vitro studies with 1 alpha-25, dihydroxyvitamin D3, alpha-melanocyte stimulating hormone, and beta-estradiol. J Invest Dermatol. 1988;91:593-598.
- Kippenberger S, Loitsch S, Solano F, et al. Quantification of tyrosinase, TRP-1, and Trp-2 transcripts in human melanocytes by reverse transcriptase-competitive multiplex PCR—regulation by steroid hormones. J Invest Dermatol. 1998;110:364-367.
- McLeod SD, Ranson M, Mason RS. Effects of estrogens on human melanocytes in vitro. J Steroid Biochem Mol Biol. 1994;49:9-14.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasma. J Cosmet Laser Ther. 2018;20:134-139.
- Sheu SL. Treatment of melasma using tranexamic acid: what’s known and what’s next. Cutis. 2018;101:E7-E8.
- Tian B. The Asian problem of frequent laser toning for melasma. J Clin Aesthet Dermatol. 2017;10:40-42.
- Zhang L, Tan WQ, Fang QQ, et al. Tranexamic acid for adults with melasma: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:1683414.
- Zhu JW, Ni YJ, Tong XY, et al. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting VEGF receptors. Int J Med Sci. 2020;17:903-911.
- Colferai MMT, Miquelin GM, Steiner D. Evaluation of oral tranexamic acid in the treatment of melasma. J Cosmet Dermatol. 2019;18:1495-1501.
- Taraz M, Niknam S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol Ther. 2017;30:19-26.
- Yalamanchili R, Shastry V, Betkerur J. Clinico-epidemiological study and quality of life assessment in melasma. Indian J Dermatol. 2015;60:519.
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781.
- Kim YJ, Kim MJ, Kweon DK, et al. Quantification of hypopigmentation activity in vitro. J Vis Exp. 2019;145:20-25.
- Cardoso R, Valente R, Souza da Costa CH, et al. Analysis of kojic acid derivatives as competitive inhibitors of tyrosinase: a molecular modeling approach. Molecules. 2021;26:2875.
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
- Khuraiya S, Kachhawa D, Chouhan B, et al. A comparative study of topical 5% tranexamic acid and triple combination therapy for the treatment of melasma in Indian population. Pigment International. 2019;6:18-23.
- Steiner D, Feola C, Bialeski N, et al. Study evaluating the efficacy of topical and injected tranexamic acid in treatment of melasma. Surg Cosmet Dermatol. 2009;1:174-177.
- Doolan B, Gupta M. Melasma. Aust J Gen Pract. 2021;50:880-885.
- Banihashemi M, Zabolinejad N, Jaafari MR, et al. Comparison of therapeutic effects of liposomal tranexamic acid and conventional hydroquinone on melasma. J Cosmet Dermatol. 2015;14:174-177.
- Chung JY, Lee JH, Lee JH. Topical tranexamic acid as an adjuvant treatment in melasma: side-by-side comparison clinical study. J Dermatolog Treat. 2016;27:373-377.
- Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci. 2014;19:753-757.
- Kanechorn Na Ayuthaya P, Niumphradit N, Manosroi A, et al. Topical 5% tranexamic acid for the treatment of melasma in Asians: a double-blind randomized controlled clinical trial. J Cosmet Laser Ther. 2012;14:150-154.
- Kim SJ, Park JY, Shibata T, et al. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol. 2016;41:480-485.
- Lee JH, Park JG, Lim SH, et al. Localized intradermal microinjection of tranexamic acid for treatment of melasma in Asian patients: a preliminary clinical trial. Dermatol Surg. 2006;32:626-631.
- Badran AY, Ali AU, Gomaa AS. Efficacy of topical versus intradermal injection of tranexamic acid in Egyptian melasma patients: a randomised clinical trial. Australas J Dermatol. 2021;62:E373-E379.
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial. J Dermatolog Treat. 2018;29:405-410.
- Pazyar N, Yaghoobi R, Zeynalie M, et al. Comparison of the efficacy of intradermal injected tranexamic acid vs hydroquinone cream in the treatment of melasma. Clin Cosmet Investig Dermatol. 2019;12:115-122.
- Wu S, Shi H, Wu H, et al. Treatment of melasma with oral administration of tranexamic acid. Aesthetic Plast Surg. 2012;36:964-970.
- Atefi N, Dalvand B, Ghassemi M, et al. Therapeutic effects of topical tranexamic acid in comparison with hydroquinone in treatment of women with melasma. Dermatol Ther (Heidelb). 2017;7:417-424.
- Cestari T, Arellano I, Hexsel D, et al. Melasma in Latin America: options for therapy and treatment algorithm. J Eur Acad Dermatol Venereol. 2009;23:760-772.
Melasma is a complex, long-lasting, acquired dermatologic pigmentation disorder resulting in grey-brown patches that last for more than 3 months. Sun-exposed areas including the nose, cheeks, forehead, and forearms are most likely to be affected.1 In Southeast Asia, 0.25% to 4% of the population affected by melasma is aged 30 to 40 years.2 In particular, melasma is a concern among pregnant women due to increased levels of melanocyte-stimulating hormones (MSHs) and is impacted by genetics, hormonal influence, and exposure to UV light.3,4 In Pakistan, approximately 46% of women are affected by melasma during pregnancy.2,5 Although few studies have focused on the clinical approaches to melasma in darker skin types, it continues to disproportionately affect the skin of color population.4
The areas of hyperpigmentation seen in melasma exhibit increased deposition of melanin in the epidermis and dermis, but melanocytes are not elevated. However, in areas of hyperpigmentation, the melanocytes are larger and more dendritic and demonstrate an increased level of melanogenesis.6 During pregnancy, especially in the third trimester, elevated levels of estrogen, progesterone, and MSH often are found in association with melasma.7 Tyrosinase (TYR) activity increases and cellular proliferation is reduced after treatment of melanocytes in culture with β-estradiol.8 Sex steroids increase transcription of genes encoding melanogenic enzymes in normal human melanocytes, especially TYR.9 These results are consistent with the notable increases in melanin synthesis and TYR activity reported for normal human melanocytes under similar conditions in culture.10 Because melanocytes contain both cytosolic and nuclear estrogen receptors, melanocytes in patients with melasma may be inherently more sensitive to the stimulatory effects of estrogens and possibly other steroid hormones.11
The current treatment options for melasma have varying levels of success and include topical depigmenting agents such as hydroquinone, tretinoin, azelaic acid, kojic acid, and corticosteroids; dermabrasion; and chemical peels.12-14 Chemical peels with glycolic acid, salicylic acid, lactic acid, trichloroacetic acid, and phenol, as well as laser therapy, are reliable management options.13,14 Traditionally, melasma has been treated with a combination of modalities along with photoprotection and trigger avoidance.12
The efficacy and safety of the available therapies for melasma are still controversial and require further exploration. In recent years, off-label tranexamic acid (TA) has emerged as a potential therapy for melasma. Although the mechanism of action remains unclear, TA may inhibit melanin synthesis by blocking the interaction between melanocytes and keratinocytes.15 Tranexamic acid also may reverse the abnormal dermal changes associated with melasma by inhibiting melanogenesis and angiogenesis.16
Although various therapeutic options exist for melasma, the search for a reliable option in patients with darker skin types continues.13 We sought to evaluate the efficacy of TA solution 5% in reducing the severity of melasma in South Asian patients, thereby improving patient outcomes and maximizing patient satisfaction. Topical TA is inexpensive and readily accessible and does not cause systemic side effects. These qualities make it a promising treatment compared to traditional therapies.
Methods
We conducted a randomized controlled trial at Rawalpindi Medical Institute (Punjab, Pakistan). The researchers obtained informed consent for all enrolled patients. Cases were sampled from the original patient population seen at the office using nonprobability consecutive sampling. The sample size was calculated with a 95% CI, margin of error of 9%, and expected percentage of efficacy of 86.1% by using TA solution 5%. South Asian male and female patients aged 20 to 45 years with melasma were included in the analysis. Patients were excluded if they were already taking TA, oral contraceptive pills, or photosensitizing drugs (eg, nonsteroidal anti-inflammatory drugs, tetracyclines, phenytoin, carbamazepine); were pregnant; had chronic kidney disease (creatinine >2.0 mg/dL); had cardiac abnormalities (abnormal electrocardiogram); had hematologic disorders (international normalized ratio >2); or had received another melasma treatment within the last 3 to 6 months.
All enrolled patients underwent a detailed history and physical examination. Patient demographics were subsequently noted, including age, sex, history of diabetes mellitus or hypertension, and duration of melasma. The melasma area and severity index (MASI) score of each patient was calculated at baseline, and a corresponding photograph was taken.
The topical solution was prepared with 5 g of TA dissolved in 10 cc of ethanol at 96 °F, 10 cc of 1,3-butanediol, and distilled water up to 100 cc. The TA solution was applied to the affected areas once daily by the patient for 12 weeks. Each application covered the affected areas completely. Patients were instructed to apply sunscreen with sun protection factor 60 to those same areas for UV protection after 15 minutes of TA application. Biweekly follow-ups were scheduled during the trial, and the MASI score was recorded at these visits. If the mean MASI score was reduced by half after 12 weeks of treatment, then the treatment was considered efficacious with a 95% CI.
The percentage reduction from baseline was calculated as follows: percentage reduction=(baseline score– follow-up score)/baseline score×100.
Statistical Analysis—Data were analyzed in SPSS Statistics 25 (IBM). The quantitative variables of age, duration of melasma, and body mass index were presented as mean (SD). Qualitative variables such as sex, history of diabetes mellitus or hypertension, site of melasma, and efficacy were presented as frequencies and percentages. Mean MASI scores at baseline and 12 weeks posttreatment were compared using a paired t test (P≤.05). Data were stratified for age, sex, history of diabetes mellitus or hypertension, site of melasma, and duration of melasma, and a χ2 test was applied to compare efficacy in stratified groups (P≤.05).
Results
Sixty patients were enrolled in the study. Of them, 17 (28.33%) were male, and 43 (71.67%) were female (2:5 ratio). They ranged in age from 20 to 45 years (mean [SD], 31.93 [6.26] years). Thirty-seven patients (61.67%) were aged 31 to 45 years of age (Table 1). The mean (SD) duration of disease was 10.18 (2.10) months. The response to TA was recorded based on patient distribution according to the site of melasma as well as history of diabetes mellitus and hypertension.

Topical TA was found to be efficacious for melasma in 50 (83.33%) patients. The mean (SD) baseline and week 12 MASI scores were 23.15 (5.02) and 12.71 (4.10)(P<.0001), respectively (Table 2). The stratification of efficacy with respect to age, sex, duration of melasma, site of melasma, and history of diabetes mellitus or hypertension is shown in the eTable. The site of melasma was significant with respect to stratification of efficacy. On the forehead, TA was found to be efficacious in 11 patients and nonefficacious in 0 patients (P=.036). In the malar region, it was efficacious in 16 patients and nonefficacious in 1 patient (P=.036). Finally, on the chin, it was efficacious in 23 patients and nonefficacious in 9 patients (P=.036).

Comment
Melasma Presentation and Development—Melasma is a chronic skin condition that more often affects patients with darker skin types. This condition is characterized by hyperpigmentation of skin that is directly exposed to the sun, such as the cheek, nose, forehead, and above the upper lip.17 Although the mechanism behind how melasma develops is unknown, one theory suggests that UV light can lead to increased plasmin in keratinocytes.18 This increased plasmin will thereby increase the arachidonic acid and α-MSH, leading to the observed uneven hyperpigmentation that is notable in melasma. Melasma is common in patients using oral contraceptives or expired cosmetic drugs; in those who are pregnant; and in those with liver dysfunction.18 Melasma has a negative impact on patients’ quality of life because of substantial psychological and social distress. Thus, finding an accessible treatment is imperative.19

Melasma Management—The most common treatments for melasma have been topical bleaching agents and photoprotection. Combination therapy options include chemical peels, dermabrasion, and laser treatments, though they present with limited efficacy.17,20 Because melasma focuses on pigmentation correction, topical treatments work to disturb melanocyte pigment production at the enzymatic level.21 Tyrosinase is rate limiting in melanin production, as it converts L-tyrosinase to L-3,4-dihydroxyphenylalanine, using copper to interact with L-3,4-dihydroxyphenylalanine as a cofactor in the active site.22 Therefore, tyrosine is a major target for many drugs that have been developed for melasma to decrease melaninization.21
Recently, research has focused on the effects of topical, intradermal, and oral TA for melasma.17 Tranexamic acid most commonly has been used in medicine as a fibrinolytic agent because of its antiplasmin properties. It has been hypothesized that TA can inhibit the release of paracrine melanogenic factors that normally act to stimulate melanocytes.17 Although studies have supported the safety and efficacy of TA, there remains a lack of clinical studies that are sufficiently powered. No definitive consensus on the use of TA for melasma currently exists, which indicates the need for large-scale, randomized, controlled trials.23
One trial (N=25) found that TA solution 5% achieved efficacy (>50% reduction in MASI score from baseline) in 86.1% of patients with melasma.24 In another study (N=18), topical TA 5% achieved efficacy (>50% reduction in MASI score) in 86% of patients with melasma.25
Melasma Comorbidities—To determine if certain comorbidities, such as diabetes mellitus or hypertension, influenced the progression of melasma, we stratified the efficacy results for patients with these 2 comorbidities, which showed no significant difference (P=.794 and P=.101, respectively). Thus, the relatively higher prevalence of diabetes mellitus (16 patients) and hypertension (11 patients) did not contribute to the efficacy of TA in lowering MASI scores over the 12-week period, which supports the findings of Doolan and Gupta,26 who investigated the endocrinologic conditions associated with melasma and found no such association with diabetes mellitus or hypertension.
TA Formulations for Melasma—The efficacy of topical TA has been explored in several studies. Six studies with sample sizes of 13 to 50 patients each showed statistically significant differences in MASI scores between baseline and following TA treatment (P<.001).27-32 Several formulations and regimens were utilized, including TA cream 3% for 12 weeks, TA gel 5% for 12 weeks, TA solution 3% for 12 weeks, TA liposome 5% for 12 weeks, and TA solution 2% for 12 weeks.18 Additionally, these studies found TA to be effective in limiting dyschromia and decreasing MASI scores. There were no statistically significant differences between formulations and method of application. Topical TA has been found to be just as effective as other treatments for melasma, including intradermal TA injections, topical hydroquinone, and a combination of topical hydroquinone and dexamethasone.18
Further study of the efficacy of intradermal TA is necessary because many human trials have lacked statistical significance or a control group. Lee et al32 conducted a trial of 100 female patients who received weekly intradermal TA microinjections for 12 weeks. After 8 and 12 weeks, MASI scores decreased significantly (P<.01).32 Similarly, Badran et al33 observed 60 female patients in 3 trial groups: group A received TA (4 mg/mL) intradermal injections every 2 weeks, group B received TA (10 mg/mL) intradermal injections every 2 weeks, and group C received TA cream 10% twice daily. Although all groups showed improvement in MASI, group B, which had the highest intradermal TA concentration, exhibited the most improvement. Thus, it was determined that intradermal application led to better results, but the cream was still effective.33
Saki et al34 conducted a randomized, split-face trial of 37 patients comparing the efficacy of intradermal TA and topical hydroquinone. Each group was treated with either monthly intradermal TA injections or nightly hydroquinone for 3 months. After 4 weeks of treatment, TA initially had a greater improvement. However, after 20 weeks, the overall changes were not significant between the 2 groups.34 Pazyar et al35 conducted a randomized, split-face trial of 49 patients comparing the efficacy of intradermal TA and hydroquinone cream. After 24 weeks of biweekly TA injections or twice-daily hydroquinone, there were no statistically significant differences in the decreased MASI scores between treatments.35 Additional large, double-blind, controlled trials are needed to thoroughly assess the role of intradermal TA in comparison to its treatment counterpart of hydroquinone.
Ebrahimi and Naeini29 conducted a 12-week, double-blind, split-phase trial of 50 Iranian melasma patients, which showed that 27.3% of patients rated the improvement in melasma as excellent, 42.4% as good, and 30.3% as fair after using TA solution 3%. Wu et al36 also showed a total melasma improvement rate of 80.9% in 256 patients with long-term oral use of TA. In a study by Kim et al31 (N=245), the mean MASI score considerably decreased after topical TA use, with a total response rate of 95.6%. In another study, Atefi et al37 presented significantly increased levels of satisfaction in patients treated with topical TA 5% vs hydroquinone (P=.015).
Melasma in Patients With Darker Skin Types—Special attention must be given to choosing the appropriate medication in melasma patients with darker skin types, as there is an increased risk for postinflammatory hyperpigmentation. Currently, few randomized controlled trials exist that fulfill the criteria of evaluating pharmacologic options for patients with melasma, and even fewer studies solely focus on patients with darker skin types.38 In addition to treatment advances, patients must be educated on the need to avoid sun exposure when possible or to use photoprotection, especially in the South Asian region, where these practices rarely are taught. Our study provided a unique analysis regarding the efficacy of TA solution 5% for the treatment of melasma in patients of South Asian descent. Clinicians can use these findings as a foundation for treating all patients with melasma but particularly those with darker skin types.
Study Limitations—Our study consisted of 60 patients; although our study had more patients than similar trials, larger studies are needed. Additionally, other variables were excluded from our analysis, such as comorbidities beyond diabetes mellitus and hypertension.
Conclusion
This study contributes to the growing field of melasma therapeutics by evaluating the efficacy of using TA solution 5% for the treatment of melasma in South Asian patients with darker skin types. Clinicians may use our study to broaden their treatment options for a common condition while also addressing the lack of clinical options for patients with darker skin types. Further studies investigating the effectiveness of TA in large clinical trials in humans are warranted to understand the efficacy and the risk for any complications.
Melasma is a complex, long-lasting, acquired dermatologic pigmentation disorder resulting in grey-brown patches that last for more than 3 months. Sun-exposed areas including the nose, cheeks, forehead, and forearms are most likely to be affected.1 In Southeast Asia, 0.25% to 4% of the population affected by melasma is aged 30 to 40 years.2 In particular, melasma is a concern among pregnant women due to increased levels of melanocyte-stimulating hormones (MSHs) and is impacted by genetics, hormonal influence, and exposure to UV light.3,4 In Pakistan, approximately 46% of women are affected by melasma during pregnancy.2,5 Although few studies have focused on the clinical approaches to melasma in darker skin types, it continues to disproportionately affect the skin of color population.4
The areas of hyperpigmentation seen in melasma exhibit increased deposition of melanin in the epidermis and dermis, but melanocytes are not elevated. However, in areas of hyperpigmentation, the melanocytes are larger and more dendritic and demonstrate an increased level of melanogenesis.6 During pregnancy, especially in the third trimester, elevated levels of estrogen, progesterone, and MSH often are found in association with melasma.7 Tyrosinase (TYR) activity increases and cellular proliferation is reduced after treatment of melanocytes in culture with β-estradiol.8 Sex steroids increase transcription of genes encoding melanogenic enzymes in normal human melanocytes, especially TYR.9 These results are consistent with the notable increases in melanin synthesis and TYR activity reported for normal human melanocytes under similar conditions in culture.10 Because melanocytes contain both cytosolic and nuclear estrogen receptors, melanocytes in patients with melasma may be inherently more sensitive to the stimulatory effects of estrogens and possibly other steroid hormones.11
The current treatment options for melasma have varying levels of success and include topical depigmenting agents such as hydroquinone, tretinoin, azelaic acid, kojic acid, and corticosteroids; dermabrasion; and chemical peels.12-14 Chemical peels with glycolic acid, salicylic acid, lactic acid, trichloroacetic acid, and phenol, as well as laser therapy, are reliable management options.13,14 Traditionally, melasma has been treated with a combination of modalities along with photoprotection and trigger avoidance.12
The efficacy and safety of the available therapies for melasma are still controversial and require further exploration. In recent years, off-label tranexamic acid (TA) has emerged as a potential therapy for melasma. Although the mechanism of action remains unclear, TA may inhibit melanin synthesis by blocking the interaction between melanocytes and keratinocytes.15 Tranexamic acid also may reverse the abnormal dermal changes associated with melasma by inhibiting melanogenesis and angiogenesis.16
Although various therapeutic options exist for melasma, the search for a reliable option in patients with darker skin types continues.13 We sought to evaluate the efficacy of TA solution 5% in reducing the severity of melasma in South Asian patients, thereby improving patient outcomes and maximizing patient satisfaction. Topical TA is inexpensive and readily accessible and does not cause systemic side effects. These qualities make it a promising treatment compared to traditional therapies.
Methods
We conducted a randomized controlled trial at Rawalpindi Medical Institute (Punjab, Pakistan). The researchers obtained informed consent for all enrolled patients. Cases were sampled from the original patient population seen at the office using nonprobability consecutive sampling. The sample size was calculated with a 95% CI, margin of error of 9%, and expected percentage of efficacy of 86.1% by using TA solution 5%. South Asian male and female patients aged 20 to 45 years with melasma were included in the analysis. Patients were excluded if they were already taking TA, oral contraceptive pills, or photosensitizing drugs (eg, nonsteroidal anti-inflammatory drugs, tetracyclines, phenytoin, carbamazepine); were pregnant; had chronic kidney disease (creatinine >2.0 mg/dL); had cardiac abnormalities (abnormal electrocardiogram); had hematologic disorders (international normalized ratio >2); or had received another melasma treatment within the last 3 to 6 months.
All enrolled patients underwent a detailed history and physical examination. Patient demographics were subsequently noted, including age, sex, history of diabetes mellitus or hypertension, and duration of melasma. The melasma area and severity index (MASI) score of each patient was calculated at baseline, and a corresponding photograph was taken.
The topical solution was prepared with 5 g of TA dissolved in 10 cc of ethanol at 96 °F, 10 cc of 1,3-butanediol, and distilled water up to 100 cc. The TA solution was applied to the affected areas once daily by the patient for 12 weeks. Each application covered the affected areas completely. Patients were instructed to apply sunscreen with sun protection factor 60 to those same areas for UV protection after 15 minutes of TA application. Biweekly follow-ups were scheduled during the trial, and the MASI score was recorded at these visits. If the mean MASI score was reduced by half after 12 weeks of treatment, then the treatment was considered efficacious with a 95% CI.
The percentage reduction from baseline was calculated as follows: percentage reduction=(baseline score– follow-up score)/baseline score×100.
Statistical Analysis—Data were analyzed in SPSS Statistics 25 (IBM). The quantitative variables of age, duration of melasma, and body mass index were presented as mean (SD). Qualitative variables such as sex, history of diabetes mellitus or hypertension, site of melasma, and efficacy were presented as frequencies and percentages. Mean MASI scores at baseline and 12 weeks posttreatment were compared using a paired t test (P≤.05). Data were stratified for age, sex, history of diabetes mellitus or hypertension, site of melasma, and duration of melasma, and a χ2 test was applied to compare efficacy in stratified groups (P≤.05).
Results
Sixty patients were enrolled in the study. Of them, 17 (28.33%) were male, and 43 (71.67%) were female (2:5 ratio). They ranged in age from 20 to 45 years (mean [SD], 31.93 [6.26] years). Thirty-seven patients (61.67%) were aged 31 to 45 years of age (Table 1). The mean (SD) duration of disease was 10.18 (2.10) months. The response to TA was recorded based on patient distribution according to the site of melasma as well as history of diabetes mellitus and hypertension.

Topical TA was found to be efficacious for melasma in 50 (83.33%) patients. The mean (SD) baseline and week 12 MASI scores were 23.15 (5.02) and 12.71 (4.10)(P<.0001), respectively (Table 2). The stratification of efficacy with respect to age, sex, duration of melasma, site of melasma, and history of diabetes mellitus or hypertension is shown in the eTable. The site of melasma was significant with respect to stratification of efficacy. On the forehead, TA was found to be efficacious in 11 patients and nonefficacious in 0 patients (P=.036). In the malar region, it was efficacious in 16 patients and nonefficacious in 1 patient (P=.036). Finally, on the chin, it was efficacious in 23 patients and nonefficacious in 9 patients (P=.036).

Comment
Melasma Presentation and Development—Melasma is a chronic skin condition that more often affects patients with darker skin types. This condition is characterized by hyperpigmentation of skin that is directly exposed to the sun, such as the cheek, nose, forehead, and above the upper lip.17 Although the mechanism behind how melasma develops is unknown, one theory suggests that UV light can lead to increased plasmin in keratinocytes.18 This increased plasmin will thereby increase the arachidonic acid and α-MSH, leading to the observed uneven hyperpigmentation that is notable in melasma. Melasma is common in patients using oral contraceptives or expired cosmetic drugs; in those who are pregnant; and in those with liver dysfunction.18 Melasma has a negative impact on patients’ quality of life because of substantial psychological and social distress. Thus, finding an accessible treatment is imperative.19

Melasma Management—The most common treatments for melasma have been topical bleaching agents and photoprotection. Combination therapy options include chemical peels, dermabrasion, and laser treatments, though they present with limited efficacy.17,20 Because melasma focuses on pigmentation correction, topical treatments work to disturb melanocyte pigment production at the enzymatic level.21 Tyrosinase is rate limiting in melanin production, as it converts L-tyrosinase to L-3,4-dihydroxyphenylalanine, using copper to interact with L-3,4-dihydroxyphenylalanine as a cofactor in the active site.22 Therefore, tyrosine is a major target for many drugs that have been developed for melasma to decrease melaninization.21
Recently, research has focused on the effects of topical, intradermal, and oral TA for melasma.17 Tranexamic acid most commonly has been used in medicine as a fibrinolytic agent because of its antiplasmin properties. It has been hypothesized that TA can inhibit the release of paracrine melanogenic factors that normally act to stimulate melanocytes.17 Although studies have supported the safety and efficacy of TA, there remains a lack of clinical studies that are sufficiently powered. No definitive consensus on the use of TA for melasma currently exists, which indicates the need for large-scale, randomized, controlled trials.23
One trial (N=25) found that TA solution 5% achieved efficacy (>50% reduction in MASI score from baseline) in 86.1% of patients with melasma.24 In another study (N=18), topical TA 5% achieved efficacy (>50% reduction in MASI score) in 86% of patients with melasma.25
Melasma Comorbidities—To determine if certain comorbidities, such as diabetes mellitus or hypertension, influenced the progression of melasma, we stratified the efficacy results for patients with these 2 comorbidities, which showed no significant difference (P=.794 and P=.101, respectively). Thus, the relatively higher prevalence of diabetes mellitus (16 patients) and hypertension (11 patients) did not contribute to the efficacy of TA in lowering MASI scores over the 12-week period, which supports the findings of Doolan and Gupta,26 who investigated the endocrinologic conditions associated with melasma and found no such association with diabetes mellitus or hypertension.
TA Formulations for Melasma—The efficacy of topical TA has been explored in several studies. Six studies with sample sizes of 13 to 50 patients each showed statistically significant differences in MASI scores between baseline and following TA treatment (P<.001).27-32 Several formulations and regimens were utilized, including TA cream 3% for 12 weeks, TA gel 5% for 12 weeks, TA solution 3% for 12 weeks, TA liposome 5% for 12 weeks, and TA solution 2% for 12 weeks.18 Additionally, these studies found TA to be effective in limiting dyschromia and decreasing MASI scores. There were no statistically significant differences between formulations and method of application. Topical TA has been found to be just as effective as other treatments for melasma, including intradermal TA injections, topical hydroquinone, and a combination of topical hydroquinone and dexamethasone.18
Further study of the efficacy of intradermal TA is necessary because many human trials have lacked statistical significance or a control group. Lee et al32 conducted a trial of 100 female patients who received weekly intradermal TA microinjections for 12 weeks. After 8 and 12 weeks, MASI scores decreased significantly (P<.01).32 Similarly, Badran et al33 observed 60 female patients in 3 trial groups: group A received TA (4 mg/mL) intradermal injections every 2 weeks, group B received TA (10 mg/mL) intradermal injections every 2 weeks, and group C received TA cream 10% twice daily. Although all groups showed improvement in MASI, group B, which had the highest intradermal TA concentration, exhibited the most improvement. Thus, it was determined that intradermal application led to better results, but the cream was still effective.33
Saki et al34 conducted a randomized, split-face trial of 37 patients comparing the efficacy of intradermal TA and topical hydroquinone. Each group was treated with either monthly intradermal TA injections or nightly hydroquinone for 3 months. After 4 weeks of treatment, TA initially had a greater improvement. However, after 20 weeks, the overall changes were not significant between the 2 groups.34 Pazyar et al35 conducted a randomized, split-face trial of 49 patients comparing the efficacy of intradermal TA and hydroquinone cream. After 24 weeks of biweekly TA injections or twice-daily hydroquinone, there were no statistically significant differences in the decreased MASI scores between treatments.35 Additional large, double-blind, controlled trials are needed to thoroughly assess the role of intradermal TA in comparison to its treatment counterpart of hydroquinone.
Ebrahimi and Naeini29 conducted a 12-week, double-blind, split-phase trial of 50 Iranian melasma patients, which showed that 27.3% of patients rated the improvement in melasma as excellent, 42.4% as good, and 30.3% as fair after using TA solution 3%. Wu et al36 also showed a total melasma improvement rate of 80.9% in 256 patients with long-term oral use of TA. In a study by Kim et al31 (N=245), the mean MASI score considerably decreased after topical TA use, with a total response rate of 95.6%. In another study, Atefi et al37 presented significantly increased levels of satisfaction in patients treated with topical TA 5% vs hydroquinone (P=.015).
Melasma in Patients With Darker Skin Types—Special attention must be given to choosing the appropriate medication in melasma patients with darker skin types, as there is an increased risk for postinflammatory hyperpigmentation. Currently, few randomized controlled trials exist that fulfill the criteria of evaluating pharmacologic options for patients with melasma, and even fewer studies solely focus on patients with darker skin types.38 In addition to treatment advances, patients must be educated on the need to avoid sun exposure when possible or to use photoprotection, especially in the South Asian region, where these practices rarely are taught. Our study provided a unique analysis regarding the efficacy of TA solution 5% for the treatment of melasma in patients of South Asian descent. Clinicians can use these findings as a foundation for treating all patients with melasma but particularly those with darker skin types.
Study Limitations—Our study consisted of 60 patients; although our study had more patients than similar trials, larger studies are needed. Additionally, other variables were excluded from our analysis, such as comorbidities beyond diabetes mellitus and hypertension.
Conclusion
This study contributes to the growing field of melasma therapeutics by evaluating the efficacy of using TA solution 5% for the treatment of melasma in South Asian patients with darker skin types. Clinicians may use our study to broaden their treatment options for a common condition while also addressing the lack of clinical options for patients with darker skin types. Further studies investigating the effectiveness of TA in large clinical trials in humans are warranted to understand the efficacy and the risk for any complications.
- Espósito ACC, Brianezi G, De Souza NP, et al. Exploratory study of epidermis, basement membrane zone, upper dermis alterations and Wnt pathway activation in melasma compared to adjacent and retroauricular skin. Ann Dermatol. 2020;32:101-108.
- Janney MS, Subramaniyan R, Dabas R, et al. A randomized controlled study comparing the efficacy of topical 5% tranexamic acid solution versus 3% hydroquinone cream in melasma. J Cutan Aesthet Surg. 2019;12:63-67.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasmaJ Cosmet Laser Ther. 2018;20:134-139.
- Grimes PE, Ijaz S, Nashawati R, et al. New oral and topical approaches for the treatment of melasma. Int J Womens Dermatol. 2019;5:30-36.
- Handel AC, Miot LDB, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Barankin B, Silver SG, Carruthers A. The skin in pregnancy. J Cutan Med Surg. 2002;6:236-240.
- Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- Smith AG, Shuster S, Thody AJ, et al. Chloasma, oral contraceptives, and plasma immunoreactive beta-melanocyte-stimulating hormone. J Invest Dermatol. 1977;68:169-170.
- Ranson M, Posen S, Mason RS. Human melanocytes as a target tissue for hormones: in vitro studies with 1 alpha-25, dihydroxyvitamin D3, alpha-melanocyte stimulating hormone, and beta-estradiol. J Invest Dermatol. 1988;91:593-598.
- Kippenberger S, Loitsch S, Solano F, et al. Quantification of tyrosinase, TRP-1, and Trp-2 transcripts in human melanocytes by reverse transcriptase-competitive multiplex PCR—regulation by steroid hormones. J Invest Dermatol. 1998;110:364-367.
- McLeod SD, Ranson M, Mason RS. Effects of estrogens on human melanocytes in vitro. J Steroid Biochem Mol Biol. 1994;49:9-14.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasma. J Cosmet Laser Ther. 2018;20:134-139.
- Sheu SL. Treatment of melasma using tranexamic acid: what’s known and what’s next. Cutis. 2018;101:E7-E8.
- Tian B. The Asian problem of frequent laser toning for melasma. J Clin Aesthet Dermatol. 2017;10:40-42.
- Zhang L, Tan WQ, Fang QQ, et al. Tranexamic acid for adults with melasma: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:1683414.
- Zhu JW, Ni YJ, Tong XY, et al. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting VEGF receptors. Int J Med Sci. 2020;17:903-911.
- Colferai MMT, Miquelin GM, Steiner D. Evaluation of oral tranexamic acid in the treatment of melasma. J Cosmet Dermatol. 2019;18:1495-1501.
- Taraz M, Niknam S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol Ther. 2017;30:19-26.
- Yalamanchili R, Shastry V, Betkerur J. Clinico-epidemiological study and quality of life assessment in melasma. Indian J Dermatol. 2015;60:519.
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781.
- Kim YJ, Kim MJ, Kweon DK, et al. Quantification of hypopigmentation activity in vitro. J Vis Exp. 2019;145:20-25.
- Cardoso R, Valente R, Souza da Costa CH, et al. Analysis of kojic acid derivatives as competitive inhibitors of tyrosinase: a molecular modeling approach. Molecules. 2021;26:2875.
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
- Khuraiya S, Kachhawa D, Chouhan B, et al. A comparative study of topical 5% tranexamic acid and triple combination therapy for the treatment of melasma in Indian population. Pigment International. 2019;6:18-23.
- Steiner D, Feola C, Bialeski N, et al. Study evaluating the efficacy of topical and injected tranexamic acid in treatment of melasma. Surg Cosmet Dermatol. 2009;1:174-177.
- Doolan B, Gupta M. Melasma. Aust J Gen Pract. 2021;50:880-885.
- Banihashemi M, Zabolinejad N, Jaafari MR, et al. Comparison of therapeutic effects of liposomal tranexamic acid and conventional hydroquinone on melasma. J Cosmet Dermatol. 2015;14:174-177.
- Chung JY, Lee JH, Lee JH. Topical tranexamic acid as an adjuvant treatment in melasma: side-by-side comparison clinical study. J Dermatolog Treat. 2016;27:373-377.
- Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci. 2014;19:753-757.
- Kanechorn Na Ayuthaya P, Niumphradit N, Manosroi A, et al. Topical 5% tranexamic acid for the treatment of melasma in Asians: a double-blind randomized controlled clinical trial. J Cosmet Laser Ther. 2012;14:150-154.
- Kim SJ, Park JY, Shibata T, et al. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol. 2016;41:480-485.
- Lee JH, Park JG, Lim SH, et al. Localized intradermal microinjection of tranexamic acid for treatment of melasma in Asian patients: a preliminary clinical trial. Dermatol Surg. 2006;32:626-631.
- Badran AY, Ali AU, Gomaa AS. Efficacy of topical versus intradermal injection of tranexamic acid in Egyptian melasma patients: a randomised clinical trial. Australas J Dermatol. 2021;62:E373-E379.
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial. J Dermatolog Treat. 2018;29:405-410.
- Pazyar N, Yaghoobi R, Zeynalie M, et al. Comparison of the efficacy of intradermal injected tranexamic acid vs hydroquinone cream in the treatment of melasma. Clin Cosmet Investig Dermatol. 2019;12:115-122.
- Wu S, Shi H, Wu H, et al. Treatment of melasma with oral administration of tranexamic acid. Aesthetic Plast Surg. 2012;36:964-970.
- Atefi N, Dalvand B, Ghassemi M, et al. Therapeutic effects of topical tranexamic acid in comparison with hydroquinone in treatment of women with melasma. Dermatol Ther (Heidelb). 2017;7:417-424.
- Cestari T, Arellano I, Hexsel D, et al. Melasma in Latin America: options for therapy and treatment algorithm. J Eur Acad Dermatol Venereol. 2009;23:760-772.
- Espósito ACC, Brianezi G, De Souza NP, et al. Exploratory study of epidermis, basement membrane zone, upper dermis alterations and Wnt pathway activation in melasma compared to adjacent and retroauricular skin. Ann Dermatol. 2020;32:101-108.
- Janney MS, Subramaniyan R, Dabas R, et al. A randomized controlled study comparing the efficacy of topical 5% tranexamic acid solution versus 3% hydroquinone cream in melasma. J Cutan Aesthet Surg. 2019;12:63-67.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasmaJ Cosmet Laser Ther. 2018;20:134-139.
- Grimes PE, Ijaz S, Nashawati R, et al. New oral and topical approaches for the treatment of melasma. Int J Womens Dermatol. 2019;5:30-36.
- Handel AC, Miot LDB, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Barankin B, Silver SG, Carruthers A. The skin in pregnancy. J Cutan Med Surg. 2002;6:236-240.
- Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- Smith AG, Shuster S, Thody AJ, et al. Chloasma, oral contraceptives, and plasma immunoreactive beta-melanocyte-stimulating hormone. J Invest Dermatol. 1977;68:169-170.
- Ranson M, Posen S, Mason RS. Human melanocytes as a target tissue for hormones: in vitro studies with 1 alpha-25, dihydroxyvitamin D3, alpha-melanocyte stimulating hormone, and beta-estradiol. J Invest Dermatol. 1988;91:593-598.
- Kippenberger S, Loitsch S, Solano F, et al. Quantification of tyrosinase, TRP-1, and Trp-2 transcripts in human melanocytes by reverse transcriptase-competitive multiplex PCR—regulation by steroid hormones. J Invest Dermatol. 1998;110:364-367.
- McLeod SD, Ranson M, Mason RS. Effects of estrogens on human melanocytes in vitro. J Steroid Biochem Mol Biol. 1994;49:9-14.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasma. J Cosmet Laser Ther. 2018;20:134-139.
- Sheu SL. Treatment of melasma using tranexamic acid: what’s known and what’s next. Cutis. 2018;101:E7-E8.
- Tian B. The Asian problem of frequent laser toning for melasma. J Clin Aesthet Dermatol. 2017;10:40-42.
- Zhang L, Tan WQ, Fang QQ, et al. Tranexamic acid for adults with melasma: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:1683414.
- Zhu JW, Ni YJ, Tong XY, et al. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting VEGF receptors. Int J Med Sci. 2020;17:903-911.
- Colferai MMT, Miquelin GM, Steiner D. Evaluation of oral tranexamic acid in the treatment of melasma. J Cosmet Dermatol. 2019;18:1495-1501.
- Taraz M, Niknam S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol Ther. 2017;30:19-26.
- Yalamanchili R, Shastry V, Betkerur J. Clinico-epidemiological study and quality of life assessment in melasma. Indian J Dermatol. 2015;60:519.
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781.
- Kim YJ, Kim MJ, Kweon DK, et al. Quantification of hypopigmentation activity in vitro. J Vis Exp. 2019;145:20-25.
- Cardoso R, Valente R, Souza da Costa CH, et al. Analysis of kojic acid derivatives as competitive inhibitors of tyrosinase: a molecular modeling approach. Molecules. 2021;26:2875.
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
- Khuraiya S, Kachhawa D, Chouhan B, et al. A comparative study of topical 5% tranexamic acid and triple combination therapy for the treatment of melasma in Indian population. Pigment International. 2019;6:18-23.
- Steiner D, Feola C, Bialeski N, et al. Study evaluating the efficacy of topical and injected tranexamic acid in treatment of melasma. Surg Cosmet Dermatol. 2009;1:174-177.
- Doolan B, Gupta M. Melasma. Aust J Gen Pract. 2021;50:880-885.
- Banihashemi M, Zabolinejad N, Jaafari MR, et al. Comparison of therapeutic effects of liposomal tranexamic acid and conventional hydroquinone on melasma. J Cosmet Dermatol. 2015;14:174-177.
- Chung JY, Lee JH, Lee JH. Topical tranexamic acid as an adjuvant treatment in melasma: side-by-side comparison clinical study. J Dermatolog Treat. 2016;27:373-377.
- Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci. 2014;19:753-757.
- Kanechorn Na Ayuthaya P, Niumphradit N, Manosroi A, et al. Topical 5% tranexamic acid for the treatment of melasma in Asians: a double-blind randomized controlled clinical trial. J Cosmet Laser Ther. 2012;14:150-154.
- Kim SJ, Park JY, Shibata T, et al. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol. 2016;41:480-485.
- Lee JH, Park JG, Lim SH, et al. Localized intradermal microinjection of tranexamic acid for treatment of melasma in Asian patients: a preliminary clinical trial. Dermatol Surg. 2006;32:626-631.
- Badran AY, Ali AU, Gomaa AS. Efficacy of topical versus intradermal injection of tranexamic acid in Egyptian melasma patients: a randomised clinical trial. Australas J Dermatol. 2021;62:E373-E379.
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial. J Dermatolog Treat. 2018;29:405-410.
- Pazyar N, Yaghoobi R, Zeynalie M, et al. Comparison of the efficacy of intradermal injected tranexamic acid vs hydroquinone cream in the treatment of melasma. Clin Cosmet Investig Dermatol. 2019;12:115-122.
- Wu S, Shi H, Wu H, et al. Treatment of melasma with oral administration of tranexamic acid. Aesthetic Plast Surg. 2012;36:964-970.
- Atefi N, Dalvand B, Ghassemi M, et al. Therapeutic effects of topical tranexamic acid in comparison with hydroquinone in treatment of women with melasma. Dermatol Ther (Heidelb). 2017;7:417-424.
- Cestari T, Arellano I, Hexsel D, et al. Melasma in Latin America: options for therapy and treatment algorithm. J Eur Acad Dermatol Venereol. 2009;23:760-772.
PRATICE POINTS
- Tranexamic acid (TA) solution 5% is an efficacious treatment for skin of color patients with melasma.
- Topical TA is a treatment alternative for patients who may not be able to tolerate oral TA.
- Our study revealed the greatest efficacy for TA solution 5% was seen on the forehead and malar region, with less efficacy on the chin.
Menopausal hormone therapy less prescribed for Black women
PHILADELPHIA – , according to a review of published studies presented at the annual meeting of the Menopause Society (formerly The North American Menopause Society).
“Gaps in treatment can be used to inform health care providers about menopausal HT prescribing disparities, with the goal of improving equitable and advanced patient care among disadvantaged populations,” wrote Danette Conklin, PhD, an assistant professor of psychiatry and reproductive biology at Case Western Reserve University, Cleveland, and a psychologist at University Hospitals Cleveland Medical Center; Sally MacPhedran, MD, an associate professor of reproductive biology at Case Western Reserve University and an ob.gyn at MetroHealth Medical Center, also in Cleveland; and their colleagues.
The researchers combed through PubMed, CINAHL, Cochrane Library, Web of Science and PsychInfo databases to identify all studies conducted in the United States since 1940 that contained data on patient demographics and prescribing patterns for hormone therapy to treat menopausal symptoms. In addition to excluding men, children, teens, trans men, and women who had contraindications for HT, the investigators excluded randomized clinical trials so that prescribing patterns would not be based on protocols or RCT participatory criteria.
The researchers identified 20 studies, ranging from 1973 through 2015, including 9 national studies and the others across different U.S. regions. They then analyzed differences in HT prescribing according to age, race/ethnicity, education, income, insurance type, body mass index, and mental health, including alcohol or substance use.
Seven of the studies assessed HT use based on patient surveys, seven used medical or medication records showing an HT prescription, two studies used insurance claims to show an HT prescription, and one study surveyed patients about whether they received an HT prescription. Another four studies used surveys that asked patients whether they received HT counseling but did not indicate if the patients received a prescription.
Half of the studies showed racial disparities in HT prescribing. In all of them, Black women used or were prescribed or counseled on using HT less than white, Hispanic, or Asian women. White women had greater use, prescribing, or counseling than all other races/ethnicities except one study in which Hispanic women were prescribed vaginal estrogen more often than white women.
Six of the studies showed education disparities in which menopausal women with lower education levels used less HT or were prescribed or counseled on HT less than women with higher education.
Complex reasons
Monica Christmas, MD, an associate professor of obstetrics and gynecology at the University of Chicago and director of the Menopause Program and the Center for Women’s Integrated Health, said the study’s findings were not surprising, but the reasons for the racial disparities are likely complex.
Implicit bias in providers is likely one contributing factor, with some providers not thinking of offering HT to certain patients or not expecting the patients to be interested in it. Providers may also hesitate to prescribe HT to patients with more comorbidities because of concerns about HT risks, so if Black patients have more comorbidities, that could play a role in how many are offered or counseled on HT, she said.
“Probably the biggest take home is that it is important to be asking all of our patients about their symptoms and being proactive about talking about it,” Dr. Christmas said in an interview.
At the same time, in her anecdotal experience at a previous institution, Dr. Christmas noticed that her Black patients were less receptive to using hormone therapy than her White patients even though her Black patients tended to exhibit or report greater or more severe symptoms. But there’s been a “paradigm shift” more recently, Dr. Christmas said. With awareness about menopause growing in the media and particularly on social media, and with greater awareness about racial disparities in menopausal symptoms and care – including that shown in Dr. Christmas’s work in the SWAN Study – Dr. Christmas has had more Black patients asking about HT and other treatments for their menopausal symptoms more recently.
“Just 10 years ago, I was trying to talk to people about hormones, and I’ve been giving them to people that need them for a long time, and I couldn’t,” Dr. Christmas said. “Now people are coming in, saying ‘no one’s ever talked to me about it’ or ‘I deserve this.’ It shows you the persuasion that social media and the Internet have on our thinking too, and I think that’s going to be interesting to look at, to see how that impacts people’s perception about wanting treatment.”
Dr. Conklin agreed that reasons for the disparities likely involve a combination of factors, including providers’ assumptions about different racial groups’ knowledge and receptiveness toward different treatments. One of the studies in their review also reported provider barriers to prescribing HT, which included lack of time, lack of adequate knowledge, and concern about risks to patients’ health.
“Medical providers tend to have less time with their patients compared to PhDs, and that time factor really makes a big difference in terms of what the focus is going to be in that [short] appointment,” Dr. Conklin said in an interview. “Perhaps from a provider point of view, they are prioritizing what they think is more important to their patient and not really listening deeply to what their patient is saying.”
Educating clinicians
Potentially supporting that possibility, Dr. Conklin and Dr. MacPhedran also had a poster at the conference that looked at prescribing of HT in both Black and White women with a diagnosis of depression, anxiety, or bipolar disorder.
“In a population with a high percentage of Black patients known to have more menopause symptoms, the data demonstrated a surprisingly low rate of documented menopause symptoms (11%) compared to prior reports of up to 80%,” the researchers reported. “This low rate may be related to patient reporting, physician inquiry, or physician documentation of menopause symptoms.” They further found that White women with menopause symptoms and one of those psychiatric diagnosis were 40% more likely to receive an HT prescription for menopausal symptoms than Black women with the same diagnoses and symptoms.
Dr. Conklin emphasized the importance of providers not overlooking women who have mental health disorders when it comes to treating menopausal symptoms, particularly since mental health conditions and menopausal symptoms can exacerbate each other.
“Their depression could worsen irritability, and anxiety can worsen during the transition, and it could be overlooked or thought of as another [psychiatric] episode,” Dr. Conklin said. Providers may need to “dig a little deeper,” especially if patients are reporting having hot flashes or brain fog.
A key way to help overcome the racial disparities – whether they result from systemic issues, implicit bias or assumptions, or patients’ own reticence – is education, Dr. Conklin said. She recommended that providers have educational material about menopause and treatments for menopausal symptoms in the waiting room and then ask patients about their symptoms and invite patients to ask questions.
Dr. MacPhedran added that education for clinicians is key as well.
“Now is a great time – menopause is hot, menopause is interesting, and it’s getting a little bit of a push in terms of research dollars,” Dr. MacPhedran said. “That will trickle down to more emphasis in medical education, whether that’s nurse practitioners, physicians, PAs, or midwives. Everybody needs more education on menopause so they can be more comfortable asking and answering these questions.”
Dr. Conklin said she would like to see expanded education on menopause for medical residents and in health psychology curricula as well.
Among the 13 studies that found disparities in prescribing patterns by age, seven studies showed that older women used or were prescribed or counseled on HT more often than younger women. Four studies found the opposite, with older women less likely to use or be prescribed or counseled about HT. One study had mixed results, and one study had expected prescribing patterns.
Five studies found income disparities and five studies found disparities by medical conditions in terms of HT use, prescribing, or counseling. Other disparities identified in smaller numbers of studies (four or fewer) included natural versus surgical menopause, insurance coverage, body mass index, geographic region, smoking and alcohol use.
The two biggest limitations of the research were its heterogeneity and the small number of studies included, which points to how scarce research on racial disparities in HT use really are, Dr. Conklin said.
The research did not use any external funding. The authors had no industry disclosures. Dr. Christmas has done an educational video for FertilityIQ.
PHILADELPHIA – , according to a review of published studies presented at the annual meeting of the Menopause Society (formerly The North American Menopause Society).
“Gaps in treatment can be used to inform health care providers about menopausal HT prescribing disparities, with the goal of improving equitable and advanced patient care among disadvantaged populations,” wrote Danette Conklin, PhD, an assistant professor of psychiatry and reproductive biology at Case Western Reserve University, Cleveland, and a psychologist at University Hospitals Cleveland Medical Center; Sally MacPhedran, MD, an associate professor of reproductive biology at Case Western Reserve University and an ob.gyn at MetroHealth Medical Center, also in Cleveland; and their colleagues.
The researchers combed through PubMed, CINAHL, Cochrane Library, Web of Science and PsychInfo databases to identify all studies conducted in the United States since 1940 that contained data on patient demographics and prescribing patterns for hormone therapy to treat menopausal symptoms. In addition to excluding men, children, teens, trans men, and women who had contraindications for HT, the investigators excluded randomized clinical trials so that prescribing patterns would not be based on protocols or RCT participatory criteria.
The researchers identified 20 studies, ranging from 1973 through 2015, including 9 national studies and the others across different U.S. regions. They then analyzed differences in HT prescribing according to age, race/ethnicity, education, income, insurance type, body mass index, and mental health, including alcohol or substance use.
Seven of the studies assessed HT use based on patient surveys, seven used medical or medication records showing an HT prescription, two studies used insurance claims to show an HT prescription, and one study surveyed patients about whether they received an HT prescription. Another four studies used surveys that asked patients whether they received HT counseling but did not indicate if the patients received a prescription.
Half of the studies showed racial disparities in HT prescribing. In all of them, Black women used or were prescribed or counseled on using HT less than white, Hispanic, or Asian women. White women had greater use, prescribing, or counseling than all other races/ethnicities except one study in which Hispanic women were prescribed vaginal estrogen more often than white women.
Six of the studies showed education disparities in which menopausal women with lower education levels used less HT or were prescribed or counseled on HT less than women with higher education.
Complex reasons
Monica Christmas, MD, an associate professor of obstetrics and gynecology at the University of Chicago and director of the Menopause Program and the Center for Women’s Integrated Health, said the study’s findings were not surprising, but the reasons for the racial disparities are likely complex.
Implicit bias in providers is likely one contributing factor, with some providers not thinking of offering HT to certain patients or not expecting the patients to be interested in it. Providers may also hesitate to prescribe HT to patients with more comorbidities because of concerns about HT risks, so if Black patients have more comorbidities, that could play a role in how many are offered or counseled on HT, she said.
“Probably the biggest take home is that it is important to be asking all of our patients about their symptoms and being proactive about talking about it,” Dr. Christmas said in an interview.
At the same time, in her anecdotal experience at a previous institution, Dr. Christmas noticed that her Black patients were less receptive to using hormone therapy than her White patients even though her Black patients tended to exhibit or report greater or more severe symptoms. But there’s been a “paradigm shift” more recently, Dr. Christmas said. With awareness about menopause growing in the media and particularly on social media, and with greater awareness about racial disparities in menopausal symptoms and care – including that shown in Dr. Christmas’s work in the SWAN Study – Dr. Christmas has had more Black patients asking about HT and other treatments for their menopausal symptoms more recently.
“Just 10 years ago, I was trying to talk to people about hormones, and I’ve been giving them to people that need them for a long time, and I couldn’t,” Dr. Christmas said. “Now people are coming in, saying ‘no one’s ever talked to me about it’ or ‘I deserve this.’ It shows you the persuasion that social media and the Internet have on our thinking too, and I think that’s going to be interesting to look at, to see how that impacts people’s perception about wanting treatment.”
Dr. Conklin agreed that reasons for the disparities likely involve a combination of factors, including providers’ assumptions about different racial groups’ knowledge and receptiveness toward different treatments. One of the studies in their review also reported provider barriers to prescribing HT, which included lack of time, lack of adequate knowledge, and concern about risks to patients’ health.
“Medical providers tend to have less time with their patients compared to PhDs, and that time factor really makes a big difference in terms of what the focus is going to be in that [short] appointment,” Dr. Conklin said in an interview. “Perhaps from a provider point of view, they are prioritizing what they think is more important to their patient and not really listening deeply to what their patient is saying.”
Educating clinicians
Potentially supporting that possibility, Dr. Conklin and Dr. MacPhedran also had a poster at the conference that looked at prescribing of HT in both Black and White women with a diagnosis of depression, anxiety, or bipolar disorder.
“In a population with a high percentage of Black patients known to have more menopause symptoms, the data demonstrated a surprisingly low rate of documented menopause symptoms (11%) compared to prior reports of up to 80%,” the researchers reported. “This low rate may be related to patient reporting, physician inquiry, or physician documentation of menopause symptoms.” They further found that White women with menopause symptoms and one of those psychiatric diagnosis were 40% more likely to receive an HT prescription for menopausal symptoms than Black women with the same diagnoses and symptoms.
Dr. Conklin emphasized the importance of providers not overlooking women who have mental health disorders when it comes to treating menopausal symptoms, particularly since mental health conditions and menopausal symptoms can exacerbate each other.
“Their depression could worsen irritability, and anxiety can worsen during the transition, and it could be overlooked or thought of as another [psychiatric] episode,” Dr. Conklin said. Providers may need to “dig a little deeper,” especially if patients are reporting having hot flashes or brain fog.
A key way to help overcome the racial disparities – whether they result from systemic issues, implicit bias or assumptions, or patients’ own reticence – is education, Dr. Conklin said. She recommended that providers have educational material about menopause and treatments for menopausal symptoms in the waiting room and then ask patients about their symptoms and invite patients to ask questions.
Dr. MacPhedran added that education for clinicians is key as well.
“Now is a great time – menopause is hot, menopause is interesting, and it’s getting a little bit of a push in terms of research dollars,” Dr. MacPhedran said. “That will trickle down to more emphasis in medical education, whether that’s nurse practitioners, physicians, PAs, or midwives. Everybody needs more education on menopause so they can be more comfortable asking and answering these questions.”
Dr. Conklin said she would like to see expanded education on menopause for medical residents and in health psychology curricula as well.
Among the 13 studies that found disparities in prescribing patterns by age, seven studies showed that older women used or were prescribed or counseled on HT more often than younger women. Four studies found the opposite, with older women less likely to use or be prescribed or counseled about HT. One study had mixed results, and one study had expected prescribing patterns.
Five studies found income disparities and five studies found disparities by medical conditions in terms of HT use, prescribing, or counseling. Other disparities identified in smaller numbers of studies (four or fewer) included natural versus surgical menopause, insurance coverage, body mass index, geographic region, smoking and alcohol use.
The two biggest limitations of the research were its heterogeneity and the small number of studies included, which points to how scarce research on racial disparities in HT use really are, Dr. Conklin said.
The research did not use any external funding. The authors had no industry disclosures. Dr. Christmas has done an educational video for FertilityIQ.
PHILADELPHIA – , according to a review of published studies presented at the annual meeting of the Menopause Society (formerly The North American Menopause Society).
“Gaps in treatment can be used to inform health care providers about menopausal HT prescribing disparities, with the goal of improving equitable and advanced patient care among disadvantaged populations,” wrote Danette Conklin, PhD, an assistant professor of psychiatry and reproductive biology at Case Western Reserve University, Cleveland, and a psychologist at University Hospitals Cleveland Medical Center; Sally MacPhedran, MD, an associate professor of reproductive biology at Case Western Reserve University and an ob.gyn at MetroHealth Medical Center, also in Cleveland; and their colleagues.
The researchers combed through PubMed, CINAHL, Cochrane Library, Web of Science and PsychInfo databases to identify all studies conducted in the United States since 1940 that contained data on patient demographics and prescribing patterns for hormone therapy to treat menopausal symptoms. In addition to excluding men, children, teens, trans men, and women who had contraindications for HT, the investigators excluded randomized clinical trials so that prescribing patterns would not be based on protocols or RCT participatory criteria.
The researchers identified 20 studies, ranging from 1973 through 2015, including 9 national studies and the others across different U.S. regions. They then analyzed differences in HT prescribing according to age, race/ethnicity, education, income, insurance type, body mass index, and mental health, including alcohol or substance use.
Seven of the studies assessed HT use based on patient surveys, seven used medical or medication records showing an HT prescription, two studies used insurance claims to show an HT prescription, and one study surveyed patients about whether they received an HT prescription. Another four studies used surveys that asked patients whether they received HT counseling but did not indicate if the patients received a prescription.
Half of the studies showed racial disparities in HT prescribing. In all of them, Black women used or were prescribed or counseled on using HT less than white, Hispanic, or Asian women. White women had greater use, prescribing, or counseling than all other races/ethnicities except one study in which Hispanic women were prescribed vaginal estrogen more often than white women.
Six of the studies showed education disparities in which menopausal women with lower education levels used less HT or were prescribed or counseled on HT less than women with higher education.
Complex reasons
Monica Christmas, MD, an associate professor of obstetrics and gynecology at the University of Chicago and director of the Menopause Program and the Center for Women’s Integrated Health, said the study’s findings were not surprising, but the reasons for the racial disparities are likely complex.
Implicit bias in providers is likely one contributing factor, with some providers not thinking of offering HT to certain patients or not expecting the patients to be interested in it. Providers may also hesitate to prescribe HT to patients with more comorbidities because of concerns about HT risks, so if Black patients have more comorbidities, that could play a role in how many are offered or counseled on HT, she said.
“Probably the biggest take home is that it is important to be asking all of our patients about their symptoms and being proactive about talking about it,” Dr. Christmas said in an interview.
At the same time, in her anecdotal experience at a previous institution, Dr. Christmas noticed that her Black patients were less receptive to using hormone therapy than her White patients even though her Black patients tended to exhibit or report greater or more severe symptoms. But there’s been a “paradigm shift” more recently, Dr. Christmas said. With awareness about menopause growing in the media and particularly on social media, and with greater awareness about racial disparities in menopausal symptoms and care – including that shown in Dr. Christmas’s work in the SWAN Study – Dr. Christmas has had more Black patients asking about HT and other treatments for their menopausal symptoms more recently.
“Just 10 years ago, I was trying to talk to people about hormones, and I’ve been giving them to people that need them for a long time, and I couldn’t,” Dr. Christmas said. “Now people are coming in, saying ‘no one’s ever talked to me about it’ or ‘I deserve this.’ It shows you the persuasion that social media and the Internet have on our thinking too, and I think that’s going to be interesting to look at, to see how that impacts people’s perception about wanting treatment.”
Dr. Conklin agreed that reasons for the disparities likely involve a combination of factors, including providers’ assumptions about different racial groups’ knowledge and receptiveness toward different treatments. One of the studies in their review also reported provider barriers to prescribing HT, which included lack of time, lack of adequate knowledge, and concern about risks to patients’ health.
“Medical providers tend to have less time with their patients compared to PhDs, and that time factor really makes a big difference in terms of what the focus is going to be in that [short] appointment,” Dr. Conklin said in an interview. “Perhaps from a provider point of view, they are prioritizing what they think is more important to their patient and not really listening deeply to what their patient is saying.”
Educating clinicians
Potentially supporting that possibility, Dr. Conklin and Dr. MacPhedran also had a poster at the conference that looked at prescribing of HT in both Black and White women with a diagnosis of depression, anxiety, or bipolar disorder.
“In a population with a high percentage of Black patients known to have more menopause symptoms, the data demonstrated a surprisingly low rate of documented menopause symptoms (11%) compared to prior reports of up to 80%,” the researchers reported. “This low rate may be related to patient reporting, physician inquiry, or physician documentation of menopause symptoms.” They further found that White women with menopause symptoms and one of those psychiatric diagnosis were 40% more likely to receive an HT prescription for menopausal symptoms than Black women with the same diagnoses and symptoms.
Dr. Conklin emphasized the importance of providers not overlooking women who have mental health disorders when it comes to treating menopausal symptoms, particularly since mental health conditions and menopausal symptoms can exacerbate each other.
“Their depression could worsen irritability, and anxiety can worsen during the transition, and it could be overlooked or thought of as another [psychiatric] episode,” Dr. Conklin said. Providers may need to “dig a little deeper,” especially if patients are reporting having hot flashes or brain fog.
A key way to help overcome the racial disparities – whether they result from systemic issues, implicit bias or assumptions, or patients’ own reticence – is education, Dr. Conklin said. She recommended that providers have educational material about menopause and treatments for menopausal symptoms in the waiting room and then ask patients about their symptoms and invite patients to ask questions.
Dr. MacPhedran added that education for clinicians is key as well.
“Now is a great time – menopause is hot, menopause is interesting, and it’s getting a little bit of a push in terms of research dollars,” Dr. MacPhedran said. “That will trickle down to more emphasis in medical education, whether that’s nurse practitioners, physicians, PAs, or midwives. Everybody needs more education on menopause so they can be more comfortable asking and answering these questions.”
Dr. Conklin said she would like to see expanded education on menopause for medical residents and in health psychology curricula as well.
Among the 13 studies that found disparities in prescribing patterns by age, seven studies showed that older women used or were prescribed or counseled on HT more often than younger women. Four studies found the opposite, with older women less likely to use or be prescribed or counseled about HT. One study had mixed results, and one study had expected prescribing patterns.
Five studies found income disparities and five studies found disparities by medical conditions in terms of HT use, prescribing, or counseling. Other disparities identified in smaller numbers of studies (four or fewer) included natural versus surgical menopause, insurance coverage, body mass index, geographic region, smoking and alcohol use.
The two biggest limitations of the research were its heterogeneity and the small number of studies included, which points to how scarce research on racial disparities in HT use really are, Dr. Conklin said.
The research did not use any external funding. The authors had no industry disclosures. Dr. Christmas has done an educational video for FertilityIQ.
AT NAMS 2023
Study spotlights paucity of black dermatologists in academia
TOPLINE:
METHODOLOGY:
- To assess the prevalence of Black dermatologists in academic dermatology programs, researchers obtained an inventory of all 142 U.S.-accredited dermatology residency programs from the Accreditation Council for Graduate Medical Education.
- The researchers drew from institutional websites, the Black Derm Directory (an online repository of Black dermatologists), and other sources to identify full- and part-time Black faculty.
- Variables of interest for each Black dermatologist included gender, institution, department title, academic and nonacademic leadership roles, publication number, National Institutes of Health grant funding, degrees, subspecialties, medical school attended, place of residency, and fellowship training.
- The researchers used Pearson’s chi-squared testing to calculate associations.
TAKEAWAY:
- Of the 86 Black faculty identified, 81.4% were female; most (42.4%) were in the southern United States, followed by the Midwest (23.5%); and 83% held full-time positions.
- Slightly more than one-quarter (26.7%) of the Black faculty attended a top 10 medical school, 16.3% graduated from a historically Black college and university medical school, and 43.5% of those with 25 or more research publications had attended a top 10 medical school.
- Only three dermatology department chairs were Black, and all were female. In addition, more than half of Black faculty (59.2%) were assistant professors, 37.7% held leadership positions at their institutions, and 32.6% held outside leadership roles in dermatology (such as leadership titles at professional dermatology organizations or editorial positions at a journal).
IN PRACTICE:
“Greater efforts are needed to recruit Black dermatology graduates into academic faculty positions,” and “faculty development programs offered by academic institutions and dermatologic associations ... should continue to be expanded,” the authors conclude.
SOURCE:
Corresponding author Nada Elbuluk, MD, MSc, director of the skin of color and pigmentary disorders program and the diversity and inclusion program in the department of dermatology at the University of Southern California, Los Angeles, led the research. The study was published in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The process for identifying Black faculty and insufficient or outdated information on department websites were limitations.
DISCLOSURES:
Dr. Elbuluk disclosed that she has served as a consultant for Avita, Scientis, Incyte, VisualDx, La Roche Posay, Beiersdorf, and Unilever. She has served on advisory boards for Allergan, Eli Lilly, Galderma, Incyte, Pfizer, Janssen, La Roche Posay, L’Oreal, McGraw Hill, and Dior. She has been a speaker for La Roche Posay, Scientis, Medscape, Beiersdorf, and Dior, and has served as investigator for Avita. Another author is an investigator and speaker for Castle Biosciences.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- To assess the prevalence of Black dermatologists in academic dermatology programs, researchers obtained an inventory of all 142 U.S.-accredited dermatology residency programs from the Accreditation Council for Graduate Medical Education.
- The researchers drew from institutional websites, the Black Derm Directory (an online repository of Black dermatologists), and other sources to identify full- and part-time Black faculty.
- Variables of interest for each Black dermatologist included gender, institution, department title, academic and nonacademic leadership roles, publication number, National Institutes of Health grant funding, degrees, subspecialties, medical school attended, place of residency, and fellowship training.
- The researchers used Pearson’s chi-squared testing to calculate associations.
TAKEAWAY:
- Of the 86 Black faculty identified, 81.4% were female; most (42.4%) were in the southern United States, followed by the Midwest (23.5%); and 83% held full-time positions.
- Slightly more than one-quarter (26.7%) of the Black faculty attended a top 10 medical school, 16.3% graduated from a historically Black college and university medical school, and 43.5% of those with 25 or more research publications had attended a top 10 medical school.
- Only three dermatology department chairs were Black, and all were female. In addition, more than half of Black faculty (59.2%) were assistant professors, 37.7% held leadership positions at their institutions, and 32.6% held outside leadership roles in dermatology (such as leadership titles at professional dermatology organizations or editorial positions at a journal).
IN PRACTICE:
“Greater efforts are needed to recruit Black dermatology graduates into academic faculty positions,” and “faculty development programs offered by academic institutions and dermatologic associations ... should continue to be expanded,” the authors conclude.
SOURCE:
Corresponding author Nada Elbuluk, MD, MSc, director of the skin of color and pigmentary disorders program and the diversity and inclusion program in the department of dermatology at the University of Southern California, Los Angeles, led the research. The study was published in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The process for identifying Black faculty and insufficient or outdated information on department websites were limitations.
DISCLOSURES:
Dr. Elbuluk disclosed that she has served as a consultant for Avita, Scientis, Incyte, VisualDx, La Roche Posay, Beiersdorf, and Unilever. She has served on advisory boards for Allergan, Eli Lilly, Galderma, Incyte, Pfizer, Janssen, La Roche Posay, L’Oreal, McGraw Hill, and Dior. She has been a speaker for La Roche Posay, Scientis, Medscape, Beiersdorf, and Dior, and has served as investigator for Avita. Another author is an investigator and speaker for Castle Biosciences.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- To assess the prevalence of Black dermatologists in academic dermatology programs, researchers obtained an inventory of all 142 U.S.-accredited dermatology residency programs from the Accreditation Council for Graduate Medical Education.
- The researchers drew from institutional websites, the Black Derm Directory (an online repository of Black dermatologists), and other sources to identify full- and part-time Black faculty.
- Variables of interest for each Black dermatologist included gender, institution, department title, academic and nonacademic leadership roles, publication number, National Institutes of Health grant funding, degrees, subspecialties, medical school attended, place of residency, and fellowship training.
- The researchers used Pearson’s chi-squared testing to calculate associations.
TAKEAWAY:
- Of the 86 Black faculty identified, 81.4% were female; most (42.4%) were in the southern United States, followed by the Midwest (23.5%); and 83% held full-time positions.
- Slightly more than one-quarter (26.7%) of the Black faculty attended a top 10 medical school, 16.3% graduated from a historically Black college and university medical school, and 43.5% of those with 25 or more research publications had attended a top 10 medical school.
- Only three dermatology department chairs were Black, and all were female. In addition, more than half of Black faculty (59.2%) were assistant professors, 37.7% held leadership positions at their institutions, and 32.6% held outside leadership roles in dermatology (such as leadership titles at professional dermatology organizations or editorial positions at a journal).
IN PRACTICE:
“Greater efforts are needed to recruit Black dermatology graduates into academic faculty positions,” and “faculty development programs offered by academic institutions and dermatologic associations ... should continue to be expanded,” the authors conclude.
SOURCE:
Corresponding author Nada Elbuluk, MD, MSc, director of the skin of color and pigmentary disorders program and the diversity and inclusion program in the department of dermatology at the University of Southern California, Los Angeles, led the research. The study was published in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The process for identifying Black faculty and insufficient or outdated information on department websites were limitations.
DISCLOSURES:
Dr. Elbuluk disclosed that she has served as a consultant for Avita, Scientis, Incyte, VisualDx, La Roche Posay, Beiersdorf, and Unilever. She has served on advisory boards for Allergan, Eli Lilly, Galderma, Incyte, Pfizer, Janssen, La Roche Posay, L’Oreal, McGraw Hill, and Dior. She has been a speaker for La Roche Posay, Scientis, Medscape, Beiersdorf, and Dior, and has served as investigator for Avita. Another author is an investigator and speaker for Castle Biosciences.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
MDMA effective in diverse patients with PTSD
TOPLINE:
METHODOLOGY:
Trauma-focused psychotherapies are the gold standard treatment for PTSD, which affects about 5% of Americans each year. However, many patients have persistent symptoms, and up to 47% don’t respond to the SSRIs sertraline and paroxetine, which are approved for PTSD by the Food and Drug Administration.
Mounting evidence suggests 3,4-methylenedioxymethamphetamine-assisted therapy (MDMA-AT), which promotes monoamine reuptake inhibition and release, simultaneously inducing prosocial feelings and softening responses to emotionally challenging and fearful stimuli, could be an alternative treatment for PTSD, possibly enhancing the benefits of psychotherapy.
A phase 3 study (MAPP1) showed MDMA-AT was generally well-tolerated and met the primary and secondary endpoints of reduced PTSD symptom severity and decreased functional impairment.
This new confirmatory phase 3 study (MAPP2) included 104 patients with PTSD who were randomized to MDMA-AT or placebo with therapy. Participants were a mean age of about 39 years, 71.2% were assigned female sex at birth, 33.7% identified as non-White, and 26.9% identified as Hispanic/Latino.
The mean Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) score at baseline was 39.0 and was similar between groups. Overall, 26.9% and 73.1% of patients had moderate or severe PTSD, respectively.
TAKEAWAY:
Among the 94 participants who completed the study, the least-squares mean change in CAPS-5 total score at 18 weeks was −23.7 (95% confidence interval, −26.9 to −20.4) for MDMA-AT versus −14.8 (95% CI, −18.3 to −11.3) for placebo with therapy (treatment difference: −8.9; 95% CI, −13.7 to −4.1; P < .001).
MDMA-AT significantly mitigated the secondary outcome of clinician-rated functional impairment, as measured by a reduction in the Sheehan Disability Scale score.
About 86.5% of participants treated with MDMA-AT achieved a clinically meaningful benefit, and 71.2% no longer met criteria for PTSD by study end.
Treatment-emergent adverse events were mostly transient and mild or moderate in severity. Although suicidal ideation was reported in both groups, MDMA did not appear to increase the risk, and there were no reports of problematic MDMA abuse or dependence.
IN PRACTICE:
“This confirmatory phase 3 trial showed consistent benefits of MDMA-AT in an ethnoracially diverse group of individuals with long-standing moderate to severe PTSD and numerous comorbidities,” write the authors, noting the dropout rate was low and treatment was generally well tolerated.
SOURCE:
The study was conducted by Jennifer M. Mitchell, PhD, department of neurology and department of psychiatry and behavioral sciences, University of California, San Francisco, and colleagues. It was published online in Nature Medicine.
LIMITATIONS:
The study excluded participants with high suicide risk, comorbid personality disorders, and underlying cardiovascular disease. Effect sizes for MDMA-AT were similar to MAPP1 and, although higher than those observed in SSRI studies, the superiority of MDMA-AT over SSRIs cannot be assumed without a direct comparison.
DISCLOSURES:
The study was funded by the Multidisciplinary Association for Psychedelic Studies, with support from the Steven and Alexandra Cohen Foundation, and organized by the MAPS Public Benefit Corporation. Dr. Mitchell has reported receiving research support from MAPS; grants/contracts from the Veterans Administration and FDA; royalties/licenses from the University of California, Los Angeles; and payment/honoraria from Stanford University and Johns Hopkins. She has been a reviewer for the National Institute on Drug Abuse Clinical Trials Network, a member of the Research Advisory Panel for the California Department of Justice, and a grant reviewer for the Australian National Health and Medical Research Council.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
Trauma-focused psychotherapies are the gold standard treatment for PTSD, which affects about 5% of Americans each year. However, many patients have persistent symptoms, and up to 47% don’t respond to the SSRIs sertraline and paroxetine, which are approved for PTSD by the Food and Drug Administration.
Mounting evidence suggests 3,4-methylenedioxymethamphetamine-assisted therapy (MDMA-AT), which promotes monoamine reuptake inhibition and release, simultaneously inducing prosocial feelings and softening responses to emotionally challenging and fearful stimuli, could be an alternative treatment for PTSD, possibly enhancing the benefits of psychotherapy.
A phase 3 study (MAPP1) showed MDMA-AT was generally well-tolerated and met the primary and secondary endpoints of reduced PTSD symptom severity and decreased functional impairment.
This new confirmatory phase 3 study (MAPP2) included 104 patients with PTSD who were randomized to MDMA-AT or placebo with therapy. Participants were a mean age of about 39 years, 71.2% were assigned female sex at birth, 33.7% identified as non-White, and 26.9% identified as Hispanic/Latino.
The mean Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) score at baseline was 39.0 and was similar between groups. Overall, 26.9% and 73.1% of patients had moderate or severe PTSD, respectively.
TAKEAWAY:
Among the 94 participants who completed the study, the least-squares mean change in CAPS-5 total score at 18 weeks was −23.7 (95% confidence interval, −26.9 to −20.4) for MDMA-AT versus −14.8 (95% CI, −18.3 to −11.3) for placebo with therapy (treatment difference: −8.9; 95% CI, −13.7 to −4.1; P < .001).
MDMA-AT significantly mitigated the secondary outcome of clinician-rated functional impairment, as measured by a reduction in the Sheehan Disability Scale score.
About 86.5% of participants treated with MDMA-AT achieved a clinically meaningful benefit, and 71.2% no longer met criteria for PTSD by study end.
Treatment-emergent adverse events were mostly transient and mild or moderate in severity. Although suicidal ideation was reported in both groups, MDMA did not appear to increase the risk, and there were no reports of problematic MDMA abuse or dependence.
IN PRACTICE:
“This confirmatory phase 3 trial showed consistent benefits of MDMA-AT in an ethnoracially diverse group of individuals with long-standing moderate to severe PTSD and numerous comorbidities,” write the authors, noting the dropout rate was low and treatment was generally well tolerated.
SOURCE:
The study was conducted by Jennifer M. Mitchell, PhD, department of neurology and department of psychiatry and behavioral sciences, University of California, San Francisco, and colleagues. It was published online in Nature Medicine.
LIMITATIONS:
The study excluded participants with high suicide risk, comorbid personality disorders, and underlying cardiovascular disease. Effect sizes for MDMA-AT were similar to MAPP1 and, although higher than those observed in SSRI studies, the superiority of MDMA-AT over SSRIs cannot be assumed without a direct comparison.
DISCLOSURES:
The study was funded by the Multidisciplinary Association for Psychedelic Studies, with support from the Steven and Alexandra Cohen Foundation, and organized by the MAPS Public Benefit Corporation. Dr. Mitchell has reported receiving research support from MAPS; grants/contracts from the Veterans Administration and FDA; royalties/licenses from the University of California, Los Angeles; and payment/honoraria from Stanford University and Johns Hopkins. She has been a reviewer for the National Institute on Drug Abuse Clinical Trials Network, a member of the Research Advisory Panel for the California Department of Justice, and a grant reviewer for the Australian National Health and Medical Research Council.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
Trauma-focused psychotherapies are the gold standard treatment for PTSD, which affects about 5% of Americans each year. However, many patients have persistent symptoms, and up to 47% don’t respond to the SSRIs sertraline and paroxetine, which are approved for PTSD by the Food and Drug Administration.
Mounting evidence suggests 3,4-methylenedioxymethamphetamine-assisted therapy (MDMA-AT), which promotes monoamine reuptake inhibition and release, simultaneously inducing prosocial feelings and softening responses to emotionally challenging and fearful stimuli, could be an alternative treatment for PTSD, possibly enhancing the benefits of psychotherapy.
A phase 3 study (MAPP1) showed MDMA-AT was generally well-tolerated and met the primary and secondary endpoints of reduced PTSD symptom severity and decreased functional impairment.
This new confirmatory phase 3 study (MAPP2) included 104 patients with PTSD who were randomized to MDMA-AT or placebo with therapy. Participants were a mean age of about 39 years, 71.2% were assigned female sex at birth, 33.7% identified as non-White, and 26.9% identified as Hispanic/Latino.
The mean Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) score at baseline was 39.0 and was similar between groups. Overall, 26.9% and 73.1% of patients had moderate or severe PTSD, respectively.
TAKEAWAY:
Among the 94 participants who completed the study, the least-squares mean change in CAPS-5 total score at 18 weeks was −23.7 (95% confidence interval, −26.9 to −20.4) for MDMA-AT versus −14.8 (95% CI, −18.3 to −11.3) for placebo with therapy (treatment difference: −8.9; 95% CI, −13.7 to −4.1; P < .001).
MDMA-AT significantly mitigated the secondary outcome of clinician-rated functional impairment, as measured by a reduction in the Sheehan Disability Scale score.
About 86.5% of participants treated with MDMA-AT achieved a clinically meaningful benefit, and 71.2% no longer met criteria for PTSD by study end.
Treatment-emergent adverse events were mostly transient and mild or moderate in severity. Although suicidal ideation was reported in both groups, MDMA did not appear to increase the risk, and there were no reports of problematic MDMA abuse or dependence.
IN PRACTICE:
“This confirmatory phase 3 trial showed consistent benefits of MDMA-AT in an ethnoracially diverse group of individuals with long-standing moderate to severe PTSD and numerous comorbidities,” write the authors, noting the dropout rate was low and treatment was generally well tolerated.
SOURCE:
The study was conducted by Jennifer M. Mitchell, PhD, department of neurology and department of psychiatry and behavioral sciences, University of California, San Francisco, and colleagues. It was published online in Nature Medicine.
LIMITATIONS:
The study excluded participants with high suicide risk, comorbid personality disorders, and underlying cardiovascular disease. Effect sizes for MDMA-AT were similar to MAPP1 and, although higher than those observed in SSRI studies, the superiority of MDMA-AT over SSRIs cannot be assumed without a direct comparison.
DISCLOSURES:
The study was funded by the Multidisciplinary Association for Psychedelic Studies, with support from the Steven and Alexandra Cohen Foundation, and organized by the MAPS Public Benefit Corporation. Dr. Mitchell has reported receiving research support from MAPS; grants/contracts from the Veterans Administration and FDA; royalties/licenses from the University of California, Los Angeles; and payment/honoraria from Stanford University and Johns Hopkins. She has been a reviewer for the National Institute on Drug Abuse Clinical Trials Network, a member of the Research Advisory Panel for the California Department of Justice, and a grant reviewer for the Australian National Health and Medical Research Council.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE
New risk factors for cardiovascular disease in women emerging
Multiple emerging risk factors for cardiovascular disease in women must be recognized and assessed to provide timely diagnosis and treatment, according to Dipti N. Itchhaporia, MD, an interventional cardiologist in southern California. These risk factors include pregnancy complications, autoimmune diseases, depression, breast cancer, and breast arterial calcification.
During the session titled “Cardiac Care in Women: Emerging Risk Factors” at CardioAcademic 2023, the former president of the American College of Cardiology emphasized that gender equity in care for cardiovascular disease will be achieved only when risk factors are evaluated from a gender-dependent perspective and when assessments are broadened to include novel and unrecognized risk factors, not just traditional risk factors.
Dr. Itchhaporia also remarked that
“Cardiovascular disease remains the leading cause of death in women, at least in the United States, and globally the outlook is similar,” she explained. “That’s why we need to provide our patients with guidance and carefully investigate when they experience chest pain. We need to remember that smoking and obesity pose a higher risk for cardiovascular disease in women than in men. Taking these risk factors into account will really make a difference by allowing us to provide more timely and targeted care.”
In her presentation, Dr. Itchhaporia noted that cardiovascular disease accounts for 35% of deaths in women worldwide. She reminded her audience that, according to The Lancet Women and Cardiovascular Disease Commission, heart diseases in this population remain “understudied, underrecognized, underdiagnosed, and undertreated. Furthermore, women are underrepresented in cardiovascular [clinical practice].”
She mentioned this because, despite U.S. legislation enacted between 1980 and 1990 that mandated the inclusion of women in clinical trials, women accounted for less than 39% of participants in cardiovascular clinical trials between 2010 and 2017. According to Dr. Itchhaporia, this situation limits the potential for developing tailored strategies and recommendations to treat the cardiovascular diseases affecting women.
Emerging risk factors
Dr. Itchhaporia pointed out that traditional risk factors have been known for many years. For example, 80% of women aged 75 years or younger have arterial hypertension. Only 29% receive adequate blood pressure control, those living with diabetes have a 45% greater risk of suffering ischemic heart disease, and obesity confers a 64% higher risk of developing ischemic heart disease in women versus 46% in men.
In addition to these factors, she noted that emerging factors must be assessed carefully. For example, women who experience pregnancy complications like gestational diabetes have a higher risk for ischemic heart disease and type 2 diabetes. Women with hypertension and preeclampsia are at a threefold higher risk of developing ischemic heart disease.
“Pregnancy can really be a major stress test for the heart, and I believe that, as health care professionals, we should all be asking women if they have had pregnancy-related complications. I don’t think that’s something we’ve been doing on a regular basis. Statistically, we know that 10%-20% of pregnant women report complications during pregnancy, and strong associations have been shown between gestational hypertension [and] preeclampsia.”
Dr. Itchhaporia explained that depression, a condition that globally affects women twice as much as men, is another emerging factor (though it has received some increased recognition). She explained that, in women, depression is a significant risk factor for developing a major adverse cardiovascular event or a combined event of cardiac death and myocardial infarction related to the target lesion and revascularization of the target lesion because of ischemia. Furthermore, women who have experienced a cardiac-related event are more likely to have depression than men.
“If we look into it in more detail, depression leads to changes in behavioral habits and physiological mechanisms,” she said. “Women living with depression are at higher risk of smoking, not exercising as much, are perhaps less careful with their hygiene, are not likely to adhere to their medications, and don’t sleep as well. All this moves them in the direction of heart disease.”
Added to these factors are autoimmune diseases like rheumatoid arthritis and systemic lupus erythematosus, where the female-to-male ratio for rheumatoid arthritis is 2½:1 and for lupus it’s 9:1. Dr. Itchhaporia explained that patients with rheumatoid arthritis are at two- to threefold greater risk for myocardial infarction and have a 50% higher risk for stroke. In the case of systemic lupus, the risk of myocardial infarction is 7-50 times greater than in the general population. She noted that cardiovascular risk calculators underestimate the burden of risk in patients with these diseases.
Lastly, she brought up breast cancer and breast arterial calcification as additional emerging risk factors. She explained that women with breast cancer are more likely to develop hypertension and diabetes, compared with women without this diagnosis. Women with hypertension or diabetes before developing breast cancer have twice the risk for heart problems after cancer.
She added that 12.7% of women screened for breast cancer have some degree of breast calcification. She explained that this occurs when calcium accumulates in the middle layer of artery walls in the breast, which is linked to aging, type 2 diabetes, or arterial hypertension and may be a marker of arterial stiffening, which is a cardiovascular disease.
“It’s extremely important to take into consideration data suggesting a strong association between breast calcifications and cardiovascular disease, independent of other known risk factors of cardiovascular disease. We need to improve our tests for detecting cardiovascular disease in women and we need to ask specific questions and not overlook these emerging factors,” she noted.
Improving health outcomes
Panelist María Guadalupe Parra Machuca, MD, a cardiologist in Guadalajara, Mexico, specializing in women’s heart disease, agreed that it is high time that clinical practice reflect public health policies, so that efforts to diagnose and treat cardiovascular diseases in women more effectively can transition from theory to reality.
“As physicians, we cannot allow public policy to remain outside of the reality we face,” she stressed. “We need to let it impact the decisions we make. Everything we see day to day, the things we learn at these conferences – let’s put it into practice. Otherwise, all our discussions and all the steps taken to improve care, from primary to highly specialized care and to detect and treat cardiovascular disease in women, will be nothing but rhetoric.”
Clinical cardiology specialist Victor Leal, MD, noted that, according to preliminary results from the national survey of cardiovascular risk factors in Mexican women, Mexico is no exception to these emerging risk factors for cardiovascular disease in women. More than 50% of women in Mexico have traditional risk factors, most notably hypertension, obesity, and diabetes, while hypertensive disorders of pregnancy top the list of other sex-specific risk factors.
“Not only are these factors increasing, but also having them increases the risk of a worse prognosis, leaving us with a very challenging scenario,” said Dr. Leal. “Not only do we need to educate patients about the traditional risk factors, but also about factors that might not be on our radar. We need to get women to link these factors to cardiovascular disease and to the possibility of developing much more adverse outcomes. This will reinforce our diagnosis and treatment.”
In an interview, Dr. Itchhaporia emphasized the changing face of cardiovascular disease for women, who have worse short- and long-term outcomes than men because they are not asked sex-specific questions during initial encounters and they experience greater prehospital delays.
She noted that, while experts need to raise awareness of the emerging risk factors among health care professionals, they also need to use information campaigns to make women aware of what the risks are. Then, if they experience any of the emerging risk factors, they can discuss it with their treating physicians.
“We need to assess both the traditional risk factors and the novel ones, those that are underrecognized. We need to include the history of pregnancy and complications during this period and we need to educate women about symptoms of heart disease like chest pain, difficulty breathing, and increasing fatigue,” she emphasized. “We must also provide guidance as to lifestyle, diet, and levels of physical activity and be aware of stress and symptoms of depression. Only then will we bring greater awareness to the fact that cardiovascular disease is the leading cause of death among women, and then we can reverse these trends.”
Dr. Itchhaporia, Dr. Parra, and Dr. Leal reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Multiple emerging risk factors for cardiovascular disease in women must be recognized and assessed to provide timely diagnosis and treatment, according to Dipti N. Itchhaporia, MD, an interventional cardiologist in southern California. These risk factors include pregnancy complications, autoimmune diseases, depression, breast cancer, and breast arterial calcification.
During the session titled “Cardiac Care in Women: Emerging Risk Factors” at CardioAcademic 2023, the former president of the American College of Cardiology emphasized that gender equity in care for cardiovascular disease will be achieved only when risk factors are evaluated from a gender-dependent perspective and when assessments are broadened to include novel and unrecognized risk factors, not just traditional risk factors.
Dr. Itchhaporia also remarked that
“Cardiovascular disease remains the leading cause of death in women, at least in the United States, and globally the outlook is similar,” she explained. “That’s why we need to provide our patients with guidance and carefully investigate when they experience chest pain. We need to remember that smoking and obesity pose a higher risk for cardiovascular disease in women than in men. Taking these risk factors into account will really make a difference by allowing us to provide more timely and targeted care.”
In her presentation, Dr. Itchhaporia noted that cardiovascular disease accounts for 35% of deaths in women worldwide. She reminded her audience that, according to The Lancet Women and Cardiovascular Disease Commission, heart diseases in this population remain “understudied, underrecognized, underdiagnosed, and undertreated. Furthermore, women are underrepresented in cardiovascular [clinical practice].”
She mentioned this because, despite U.S. legislation enacted between 1980 and 1990 that mandated the inclusion of women in clinical trials, women accounted for less than 39% of participants in cardiovascular clinical trials between 2010 and 2017. According to Dr. Itchhaporia, this situation limits the potential for developing tailored strategies and recommendations to treat the cardiovascular diseases affecting women.
Emerging risk factors
Dr. Itchhaporia pointed out that traditional risk factors have been known for many years. For example, 80% of women aged 75 years or younger have arterial hypertension. Only 29% receive adequate blood pressure control, those living with diabetes have a 45% greater risk of suffering ischemic heart disease, and obesity confers a 64% higher risk of developing ischemic heart disease in women versus 46% in men.
In addition to these factors, she noted that emerging factors must be assessed carefully. For example, women who experience pregnancy complications like gestational diabetes have a higher risk for ischemic heart disease and type 2 diabetes. Women with hypertension and preeclampsia are at a threefold higher risk of developing ischemic heart disease.
“Pregnancy can really be a major stress test for the heart, and I believe that, as health care professionals, we should all be asking women if they have had pregnancy-related complications. I don’t think that’s something we’ve been doing on a regular basis. Statistically, we know that 10%-20% of pregnant women report complications during pregnancy, and strong associations have been shown between gestational hypertension [and] preeclampsia.”
Dr. Itchhaporia explained that depression, a condition that globally affects women twice as much as men, is another emerging factor (though it has received some increased recognition). She explained that, in women, depression is a significant risk factor for developing a major adverse cardiovascular event or a combined event of cardiac death and myocardial infarction related to the target lesion and revascularization of the target lesion because of ischemia. Furthermore, women who have experienced a cardiac-related event are more likely to have depression than men.
“If we look into it in more detail, depression leads to changes in behavioral habits and physiological mechanisms,” she said. “Women living with depression are at higher risk of smoking, not exercising as much, are perhaps less careful with their hygiene, are not likely to adhere to their medications, and don’t sleep as well. All this moves them in the direction of heart disease.”
Added to these factors are autoimmune diseases like rheumatoid arthritis and systemic lupus erythematosus, where the female-to-male ratio for rheumatoid arthritis is 2½:1 and for lupus it’s 9:1. Dr. Itchhaporia explained that patients with rheumatoid arthritis are at two- to threefold greater risk for myocardial infarction and have a 50% higher risk for stroke. In the case of systemic lupus, the risk of myocardial infarction is 7-50 times greater than in the general population. She noted that cardiovascular risk calculators underestimate the burden of risk in patients with these diseases.
Lastly, she brought up breast cancer and breast arterial calcification as additional emerging risk factors. She explained that women with breast cancer are more likely to develop hypertension and diabetes, compared with women without this diagnosis. Women with hypertension or diabetes before developing breast cancer have twice the risk for heart problems after cancer.
She added that 12.7% of women screened for breast cancer have some degree of breast calcification. She explained that this occurs when calcium accumulates in the middle layer of artery walls in the breast, which is linked to aging, type 2 diabetes, or arterial hypertension and may be a marker of arterial stiffening, which is a cardiovascular disease.
“It’s extremely important to take into consideration data suggesting a strong association between breast calcifications and cardiovascular disease, independent of other known risk factors of cardiovascular disease. We need to improve our tests for detecting cardiovascular disease in women and we need to ask specific questions and not overlook these emerging factors,” she noted.
Improving health outcomes
Panelist María Guadalupe Parra Machuca, MD, a cardiologist in Guadalajara, Mexico, specializing in women’s heart disease, agreed that it is high time that clinical practice reflect public health policies, so that efforts to diagnose and treat cardiovascular diseases in women more effectively can transition from theory to reality.
“As physicians, we cannot allow public policy to remain outside of the reality we face,” she stressed. “We need to let it impact the decisions we make. Everything we see day to day, the things we learn at these conferences – let’s put it into practice. Otherwise, all our discussions and all the steps taken to improve care, from primary to highly specialized care and to detect and treat cardiovascular disease in women, will be nothing but rhetoric.”
Clinical cardiology specialist Victor Leal, MD, noted that, according to preliminary results from the national survey of cardiovascular risk factors in Mexican women, Mexico is no exception to these emerging risk factors for cardiovascular disease in women. More than 50% of women in Mexico have traditional risk factors, most notably hypertension, obesity, and diabetes, while hypertensive disorders of pregnancy top the list of other sex-specific risk factors.
“Not only are these factors increasing, but also having them increases the risk of a worse prognosis, leaving us with a very challenging scenario,” said Dr. Leal. “Not only do we need to educate patients about the traditional risk factors, but also about factors that might not be on our radar. We need to get women to link these factors to cardiovascular disease and to the possibility of developing much more adverse outcomes. This will reinforce our diagnosis and treatment.”
In an interview, Dr. Itchhaporia emphasized the changing face of cardiovascular disease for women, who have worse short- and long-term outcomes than men because they are not asked sex-specific questions during initial encounters and they experience greater prehospital delays.
She noted that, while experts need to raise awareness of the emerging risk factors among health care professionals, they also need to use information campaigns to make women aware of what the risks are. Then, if they experience any of the emerging risk factors, they can discuss it with their treating physicians.
“We need to assess both the traditional risk factors and the novel ones, those that are underrecognized. We need to include the history of pregnancy and complications during this period and we need to educate women about symptoms of heart disease like chest pain, difficulty breathing, and increasing fatigue,” she emphasized. “We must also provide guidance as to lifestyle, diet, and levels of physical activity and be aware of stress and symptoms of depression. Only then will we bring greater awareness to the fact that cardiovascular disease is the leading cause of death among women, and then we can reverse these trends.”
Dr. Itchhaporia, Dr. Parra, and Dr. Leal reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Multiple emerging risk factors for cardiovascular disease in women must be recognized and assessed to provide timely diagnosis and treatment, according to Dipti N. Itchhaporia, MD, an interventional cardiologist in southern California. These risk factors include pregnancy complications, autoimmune diseases, depression, breast cancer, and breast arterial calcification.
During the session titled “Cardiac Care in Women: Emerging Risk Factors” at CardioAcademic 2023, the former president of the American College of Cardiology emphasized that gender equity in care for cardiovascular disease will be achieved only when risk factors are evaluated from a gender-dependent perspective and when assessments are broadened to include novel and unrecognized risk factors, not just traditional risk factors.
Dr. Itchhaporia also remarked that
“Cardiovascular disease remains the leading cause of death in women, at least in the United States, and globally the outlook is similar,” she explained. “That’s why we need to provide our patients with guidance and carefully investigate when they experience chest pain. We need to remember that smoking and obesity pose a higher risk for cardiovascular disease in women than in men. Taking these risk factors into account will really make a difference by allowing us to provide more timely and targeted care.”
In her presentation, Dr. Itchhaporia noted that cardiovascular disease accounts for 35% of deaths in women worldwide. She reminded her audience that, according to The Lancet Women and Cardiovascular Disease Commission, heart diseases in this population remain “understudied, underrecognized, underdiagnosed, and undertreated. Furthermore, women are underrepresented in cardiovascular [clinical practice].”
She mentioned this because, despite U.S. legislation enacted between 1980 and 1990 that mandated the inclusion of women in clinical trials, women accounted for less than 39% of participants in cardiovascular clinical trials between 2010 and 2017. According to Dr. Itchhaporia, this situation limits the potential for developing tailored strategies and recommendations to treat the cardiovascular diseases affecting women.
Emerging risk factors
Dr. Itchhaporia pointed out that traditional risk factors have been known for many years. For example, 80% of women aged 75 years or younger have arterial hypertension. Only 29% receive adequate blood pressure control, those living with diabetes have a 45% greater risk of suffering ischemic heart disease, and obesity confers a 64% higher risk of developing ischemic heart disease in women versus 46% in men.
In addition to these factors, she noted that emerging factors must be assessed carefully. For example, women who experience pregnancy complications like gestational diabetes have a higher risk for ischemic heart disease and type 2 diabetes. Women with hypertension and preeclampsia are at a threefold higher risk of developing ischemic heart disease.
“Pregnancy can really be a major stress test for the heart, and I believe that, as health care professionals, we should all be asking women if they have had pregnancy-related complications. I don’t think that’s something we’ve been doing on a regular basis. Statistically, we know that 10%-20% of pregnant women report complications during pregnancy, and strong associations have been shown between gestational hypertension [and] preeclampsia.”
Dr. Itchhaporia explained that depression, a condition that globally affects women twice as much as men, is another emerging factor (though it has received some increased recognition). She explained that, in women, depression is a significant risk factor for developing a major adverse cardiovascular event or a combined event of cardiac death and myocardial infarction related to the target lesion and revascularization of the target lesion because of ischemia. Furthermore, women who have experienced a cardiac-related event are more likely to have depression than men.
“If we look into it in more detail, depression leads to changes in behavioral habits and physiological mechanisms,” she said. “Women living with depression are at higher risk of smoking, not exercising as much, are perhaps less careful with their hygiene, are not likely to adhere to their medications, and don’t sleep as well. All this moves them in the direction of heart disease.”
Added to these factors are autoimmune diseases like rheumatoid arthritis and systemic lupus erythematosus, where the female-to-male ratio for rheumatoid arthritis is 2½:1 and for lupus it’s 9:1. Dr. Itchhaporia explained that patients with rheumatoid arthritis are at two- to threefold greater risk for myocardial infarction and have a 50% higher risk for stroke. In the case of systemic lupus, the risk of myocardial infarction is 7-50 times greater than in the general population. She noted that cardiovascular risk calculators underestimate the burden of risk in patients with these diseases.
Lastly, she brought up breast cancer and breast arterial calcification as additional emerging risk factors. She explained that women with breast cancer are more likely to develop hypertension and diabetes, compared with women without this diagnosis. Women with hypertension or diabetes before developing breast cancer have twice the risk for heart problems after cancer.
She added that 12.7% of women screened for breast cancer have some degree of breast calcification. She explained that this occurs when calcium accumulates in the middle layer of artery walls in the breast, which is linked to aging, type 2 diabetes, or arterial hypertension and may be a marker of arterial stiffening, which is a cardiovascular disease.
“It’s extremely important to take into consideration data suggesting a strong association between breast calcifications and cardiovascular disease, independent of other known risk factors of cardiovascular disease. We need to improve our tests for detecting cardiovascular disease in women and we need to ask specific questions and not overlook these emerging factors,” she noted.
Improving health outcomes
Panelist María Guadalupe Parra Machuca, MD, a cardiologist in Guadalajara, Mexico, specializing in women’s heart disease, agreed that it is high time that clinical practice reflect public health policies, so that efforts to diagnose and treat cardiovascular diseases in women more effectively can transition from theory to reality.
“As physicians, we cannot allow public policy to remain outside of the reality we face,” she stressed. “We need to let it impact the decisions we make. Everything we see day to day, the things we learn at these conferences – let’s put it into practice. Otherwise, all our discussions and all the steps taken to improve care, from primary to highly specialized care and to detect and treat cardiovascular disease in women, will be nothing but rhetoric.”
Clinical cardiology specialist Victor Leal, MD, noted that, according to preliminary results from the national survey of cardiovascular risk factors in Mexican women, Mexico is no exception to these emerging risk factors for cardiovascular disease in women. More than 50% of women in Mexico have traditional risk factors, most notably hypertension, obesity, and diabetes, while hypertensive disorders of pregnancy top the list of other sex-specific risk factors.
“Not only are these factors increasing, but also having them increases the risk of a worse prognosis, leaving us with a very challenging scenario,” said Dr. Leal. “Not only do we need to educate patients about the traditional risk factors, but also about factors that might not be on our radar. We need to get women to link these factors to cardiovascular disease and to the possibility of developing much more adverse outcomes. This will reinforce our diagnosis and treatment.”
In an interview, Dr. Itchhaporia emphasized the changing face of cardiovascular disease for women, who have worse short- and long-term outcomes than men because they are not asked sex-specific questions during initial encounters and they experience greater prehospital delays.
She noted that, while experts need to raise awareness of the emerging risk factors among health care professionals, they also need to use information campaigns to make women aware of what the risks are. Then, if they experience any of the emerging risk factors, they can discuss it with their treating physicians.
“We need to assess both the traditional risk factors and the novel ones, those that are underrecognized. We need to include the history of pregnancy and complications during this period and we need to educate women about symptoms of heart disease like chest pain, difficulty breathing, and increasing fatigue,” she emphasized. “We must also provide guidance as to lifestyle, diet, and levels of physical activity and be aware of stress and symptoms of depression. Only then will we bring greater awareness to the fact that cardiovascular disease is the leading cause of death among women, and then we can reverse these trends.”
Dr. Itchhaporia, Dr. Parra, and Dr. Leal reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CARDIOACADEMIC 2023
Fighting disparities in palliative and end-of-life care
Palliative care has been shown to improve quality of life, receipt of goal-concordant care, end-of-life decision-making, and improvement in pain and symptoms in individuals with serious illness. However, palliative and end-of-life care remain underutilized in racial and ethnic minorities.1 Health disparities such as access, quality of care, and health outcomes among minority groups exist in delivery and receipt of care within the health care system, and this includes the care of individuals with serious illness and at the end of life.1
Racial and ethnic minorities are less likely to receive goal-concordant care, participate in advance care planning, and have access to palliative care or hospice.2-4 They are more likely to die in a hospital, have inadequate pain and symptom management, and experience poor provider-patient communication.5-7 Other contributing factors include lack of knowledge of hospice and palliative care services, mistrust of the health care system, spiritual and religious beliefs, provider bias, and cultural beliefs.1
Despite these disparities, interventions have had limited success,8 and there are gaps in content, methods, and inclusion of racial and ethnic groups within palliative care research.7
Efforts to improve health equity for people with serious illness have been identified as an “urgent call to action.”1
A few recommended actionable items include delivering culturally competent care by ensuring availability of culturally and linguistically appropriate materials and information, education, and training for providers, and practicing cultural humility; contributing to workforce diversity by hiring and training diverse staff; and partnering with community organizations to build trust and to facilitate dissemination of culturally and linguistically appropriate information to providers in caring for their diverse patient populations.1,9
One of the first steps identified is to recognize that there is a problem and prioritize efforts to understand its “multifaceted nature.”10 This should occur on multiple levels including the individual (patient and caregiver), interpersonal (health care team), organization, and policy levels,10 and be done through clinical, research, and educational platforms.
At the interpersonal level, we as the health care team can start by reflecting, acknowledging biases, seeking educational and training opportunities on cross-cultural interactions, learning about cultural and spiritual beliefs, and developing skills in culturally and linguistically appropriate communication regarding goals of care and advance care planning.1,10
For those seeking resources, organizations such as the Center to Advance Palliative Care’s Project Equity and the American Academy of Hospice and Palliative Medicine have ongoing efforts to educate and train physicians and health care professionals to improve and understand health equity in palliative care by providing resource portals, toolkits, training, and general information.
It is imperative to move forward in actionable ways to address not only racial and ethnic disparities, but advance equity in serious illness care for health care organizations, providers, and policymakers.1
Dr. Kang is in the division of gerontology and geriatric medicine at the University of Washington, Seattle.
References
1. Barrett NJ et al. N C Med J. 2020;81:254-6.
2. Johnson KS et al. J Am Geriatr Soc. 2011;59:732-7.
3. Sharma RK et al. J Clin Oncol. 2015;33:3802-8.
4. Muni S et al. Chest. 2011;139:1025-33.
5. Anderson KO et al. J Pain. 2009;10:1187-204.
6. Mack JW et al. Arch Intern Med. 2010;170:1533-40.
7. Johnson KS. J Palliat Med. 2013;16(11):1329-34.
8. Brown CE et al. J Pain Symptom Manage. 2021;63(5):e465-e71.
9. Chambers B. Center for Advancing Palliative Care. July 9, 2020.
10. Koffman J et al. BMC Palliat Care. 2023;22(64):1-3.
Palliative care has been shown to improve quality of life, receipt of goal-concordant care, end-of-life decision-making, and improvement in pain and symptoms in individuals with serious illness. However, palliative and end-of-life care remain underutilized in racial and ethnic minorities.1 Health disparities such as access, quality of care, and health outcomes among minority groups exist in delivery and receipt of care within the health care system, and this includes the care of individuals with serious illness and at the end of life.1
Racial and ethnic minorities are less likely to receive goal-concordant care, participate in advance care planning, and have access to palliative care or hospice.2-4 They are more likely to die in a hospital, have inadequate pain and symptom management, and experience poor provider-patient communication.5-7 Other contributing factors include lack of knowledge of hospice and palliative care services, mistrust of the health care system, spiritual and religious beliefs, provider bias, and cultural beliefs.1
Despite these disparities, interventions have had limited success,8 and there are gaps in content, methods, and inclusion of racial and ethnic groups within palliative care research.7
Efforts to improve health equity for people with serious illness have been identified as an “urgent call to action.”1
A few recommended actionable items include delivering culturally competent care by ensuring availability of culturally and linguistically appropriate materials and information, education, and training for providers, and practicing cultural humility; contributing to workforce diversity by hiring and training diverse staff; and partnering with community organizations to build trust and to facilitate dissemination of culturally and linguistically appropriate information to providers in caring for their diverse patient populations.1,9
One of the first steps identified is to recognize that there is a problem and prioritize efforts to understand its “multifaceted nature.”10 This should occur on multiple levels including the individual (patient and caregiver), interpersonal (health care team), organization, and policy levels,10 and be done through clinical, research, and educational platforms.
At the interpersonal level, we as the health care team can start by reflecting, acknowledging biases, seeking educational and training opportunities on cross-cultural interactions, learning about cultural and spiritual beliefs, and developing skills in culturally and linguistically appropriate communication regarding goals of care and advance care planning.1,10
For those seeking resources, organizations such as the Center to Advance Palliative Care’s Project Equity and the American Academy of Hospice and Palliative Medicine have ongoing efforts to educate and train physicians and health care professionals to improve and understand health equity in palliative care by providing resource portals, toolkits, training, and general information.
It is imperative to move forward in actionable ways to address not only racial and ethnic disparities, but advance equity in serious illness care for health care organizations, providers, and policymakers.1
Dr. Kang is in the division of gerontology and geriatric medicine at the University of Washington, Seattle.
References
1. Barrett NJ et al. N C Med J. 2020;81:254-6.
2. Johnson KS et al. J Am Geriatr Soc. 2011;59:732-7.
3. Sharma RK et al. J Clin Oncol. 2015;33:3802-8.
4. Muni S et al. Chest. 2011;139:1025-33.
5. Anderson KO et al. J Pain. 2009;10:1187-204.
6. Mack JW et al. Arch Intern Med. 2010;170:1533-40.
7. Johnson KS. J Palliat Med. 2013;16(11):1329-34.
8. Brown CE et al. J Pain Symptom Manage. 2021;63(5):e465-e71.
9. Chambers B. Center for Advancing Palliative Care. July 9, 2020.
10. Koffman J et al. BMC Palliat Care. 2023;22(64):1-3.
Palliative care has been shown to improve quality of life, receipt of goal-concordant care, end-of-life decision-making, and improvement in pain and symptoms in individuals with serious illness. However, palliative and end-of-life care remain underutilized in racial and ethnic minorities.1 Health disparities such as access, quality of care, and health outcomes among minority groups exist in delivery and receipt of care within the health care system, and this includes the care of individuals with serious illness and at the end of life.1
Racial and ethnic minorities are less likely to receive goal-concordant care, participate in advance care planning, and have access to palliative care or hospice.2-4 They are more likely to die in a hospital, have inadequate pain and symptom management, and experience poor provider-patient communication.5-7 Other contributing factors include lack of knowledge of hospice and palliative care services, mistrust of the health care system, spiritual and religious beliefs, provider bias, and cultural beliefs.1
Despite these disparities, interventions have had limited success,8 and there are gaps in content, methods, and inclusion of racial and ethnic groups within palliative care research.7
Efforts to improve health equity for people with serious illness have been identified as an “urgent call to action.”1
A few recommended actionable items include delivering culturally competent care by ensuring availability of culturally and linguistically appropriate materials and information, education, and training for providers, and practicing cultural humility; contributing to workforce diversity by hiring and training diverse staff; and partnering with community organizations to build trust and to facilitate dissemination of culturally and linguistically appropriate information to providers in caring for their diverse patient populations.1,9
One of the first steps identified is to recognize that there is a problem and prioritize efforts to understand its “multifaceted nature.”10 This should occur on multiple levels including the individual (patient and caregiver), interpersonal (health care team), organization, and policy levels,10 and be done through clinical, research, and educational platforms.
At the interpersonal level, we as the health care team can start by reflecting, acknowledging biases, seeking educational and training opportunities on cross-cultural interactions, learning about cultural and spiritual beliefs, and developing skills in culturally and linguistically appropriate communication regarding goals of care and advance care planning.1,10
For those seeking resources, organizations such as the Center to Advance Palliative Care’s Project Equity and the American Academy of Hospice and Palliative Medicine have ongoing efforts to educate and train physicians and health care professionals to improve and understand health equity in palliative care by providing resource portals, toolkits, training, and general information.
It is imperative to move forward in actionable ways to address not only racial and ethnic disparities, but advance equity in serious illness care for health care organizations, providers, and policymakers.1
Dr. Kang is in the division of gerontology and geriatric medicine at the University of Washington, Seattle.
References
1. Barrett NJ et al. N C Med J. 2020;81:254-6.
2. Johnson KS et al. J Am Geriatr Soc. 2011;59:732-7.
3. Sharma RK et al. J Clin Oncol. 2015;33:3802-8.
4. Muni S et al. Chest. 2011;139:1025-33.
5. Anderson KO et al. J Pain. 2009;10:1187-204.
6. Mack JW et al. Arch Intern Med. 2010;170:1533-40.
7. Johnson KS. J Palliat Med. 2013;16(11):1329-34.
8. Brown CE et al. J Pain Symptom Manage. 2021;63(5):e465-e71.
9. Chambers B. Center for Advancing Palliative Care. July 9, 2020.
10. Koffman J et al. BMC Palliat Care. 2023;22(64):1-3.
The new normal in body temperature
This transcript has been edited for clarity.
Every branch of science has its constants. Physics has the speed of light, the gravitational constant, the Planck constant. Chemistry gives us Avogadro’s number, Faraday’s constant, the charge of an electron. Medicine isn’t quite as reliable as physics when it comes to these things, but insofar as there are any constants in medicine, might I suggest normal body temperature: 37° Celsius, 98.6° Fahrenheit.
Sure, serum sodium may be less variable and lactate concentration more clinically relevant, but even my 7-year-old knows that normal body temperature is 98.6°.
Except, as it turns out, 98.6° isn’t normal at all.
How did we arrive at 37.0° C for normal body temperature? We got it from this guy – German physician Carl Reinhold August Wunderlich, who, in addition to looking eerily like Luciano Pavarotti, was the first to realize that fever was not itself a disease but a symptom of one.
In 1851, Dr. Wunderlich released his measurements of more than 1 million body temperatures taken from 25,000 Germans – a painstaking process at the time, which employed a foot-long thermometer and took 20 minutes to obtain a measurement.
The average temperature measured, of course, was 37° C.
We’re more than 150 years post-Wunderlich right now, and the average person in the United States might be quite a bit different from the average German in 1850. Moreover, we can do a lot better than just measuring a ton of people and taking the average, because we have statistics. The problem with measuring a bunch of people and taking the average temperature as normal is that you can’t be sure that the people you are measuring are normal. There are obvious causes of elevated temperature that you could exclude. Let’s not take people with a respiratory infection or who are taking Tylenol, for example. But as highlighted in this paper in JAMA Internal Medicine, we can do a lot better than that.
The study leverages the fact that body temperature is typically measured during all medical office visits and recorded in the ever-present electronic medical record.
Researchers from Stanford identified 724,199 patient encounters with outpatient temperature data. They excluded extreme temperatures – less than 34° C or greater than 40° C – excluded patients under 20 or above 80 years, and excluded those with extremes of height, weight, or body mass index.
You end up with a distribution like this. Note that the peak is clearly lower than 37° C.
But we’re still not at “normal.” Some people would be seeing their doctor for conditions that affect body temperature, such as infection. You could use diagnosis codes to flag these individuals and drop them, but that feels a bit arbitrary.
I really love how the researchers used data to fix this problem. They used a technique called LIMIT (Laboratory Information Mining for Individualized Thresholds). It works like this:
Take all the temperature measurements and then identify the outliers – the very tails of the distribution.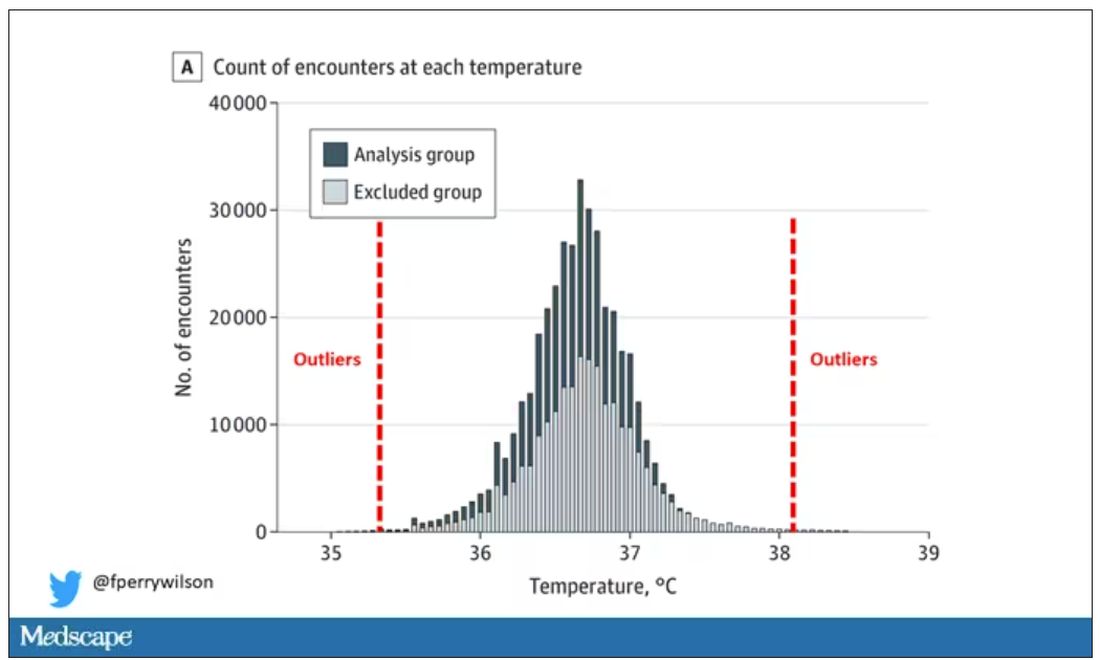
Look at all the diagnosis codes in those distributions. Determine which diagnosis codes are overrepresented in those distributions. Now you have a data-driven way to say that yes, these diagnoses are associated with weird temperatures. Next, eliminate everyone with those diagnoses from the dataset. What you are left with is a normal population, or at least a population that doesn’t have a condition that seems to meaningfully affect temperature.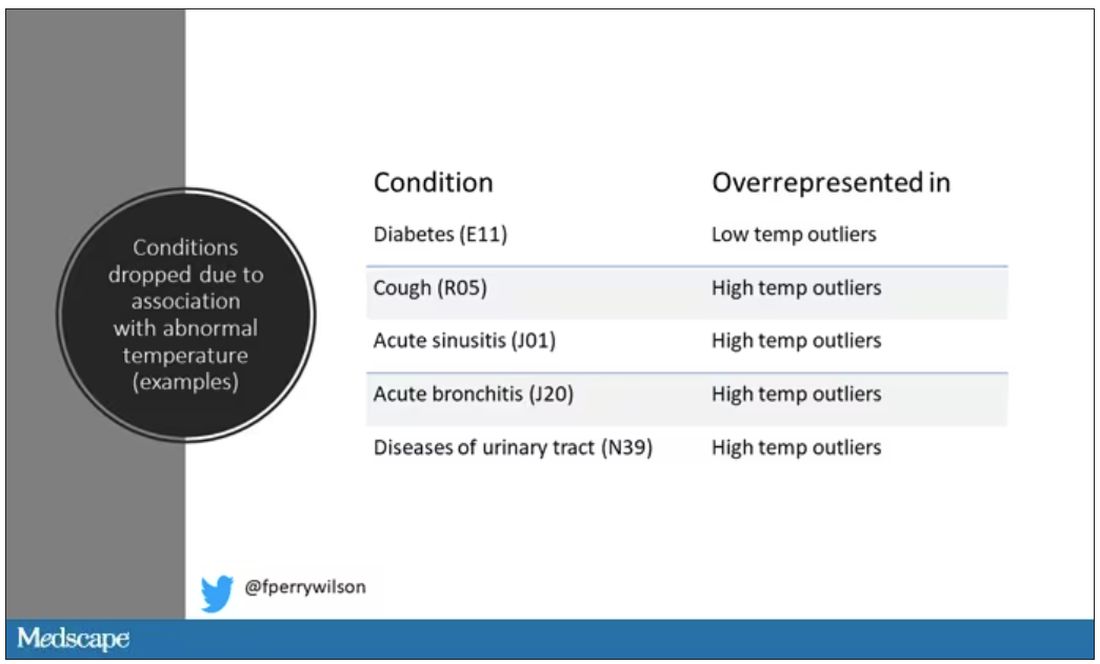
So, who was dropped? Well, a lot of people, actually. It turned out that diabetes was way overrepresented in the outlier group. Although 9.2% of the population had diabetes, 26% of people with very low temperatures did, so everyone with diabetes is removed from the dataset. While 5% of the population had a cough at their encounter, 7% of the people with very high temperature and 7% of the people with very low temperature had a cough, so everyone with cough gets thrown out.
The algorithm excluded people on antibiotics or who had sinusitis, urinary tract infections, pneumonia, and, yes, a diagnosis of “fever.” The list makes sense, which is always nice when you have a purely algorithmic classification system.
What do we have left? What is the real normal temperature? Ready?
It’s 36.64° C, or about 98.0° F.
Of course, normal temperature varied depending on the time of day it was measured – higher in the afternoon.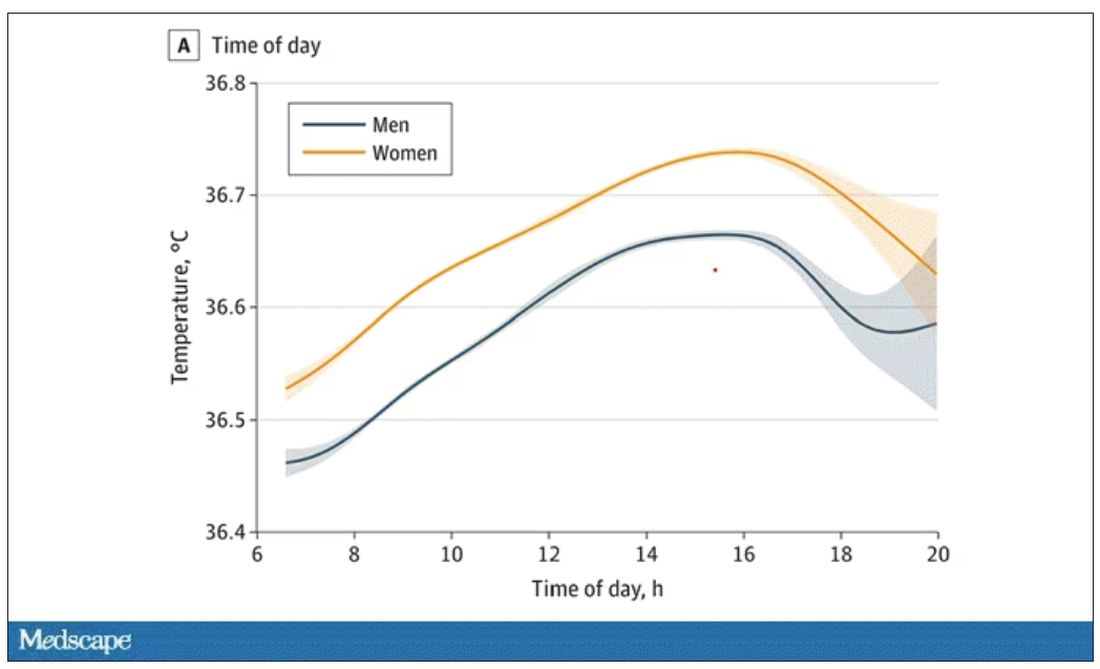
The normal temperature in women tended to be higher than in men. The normal temperature declined with age as well.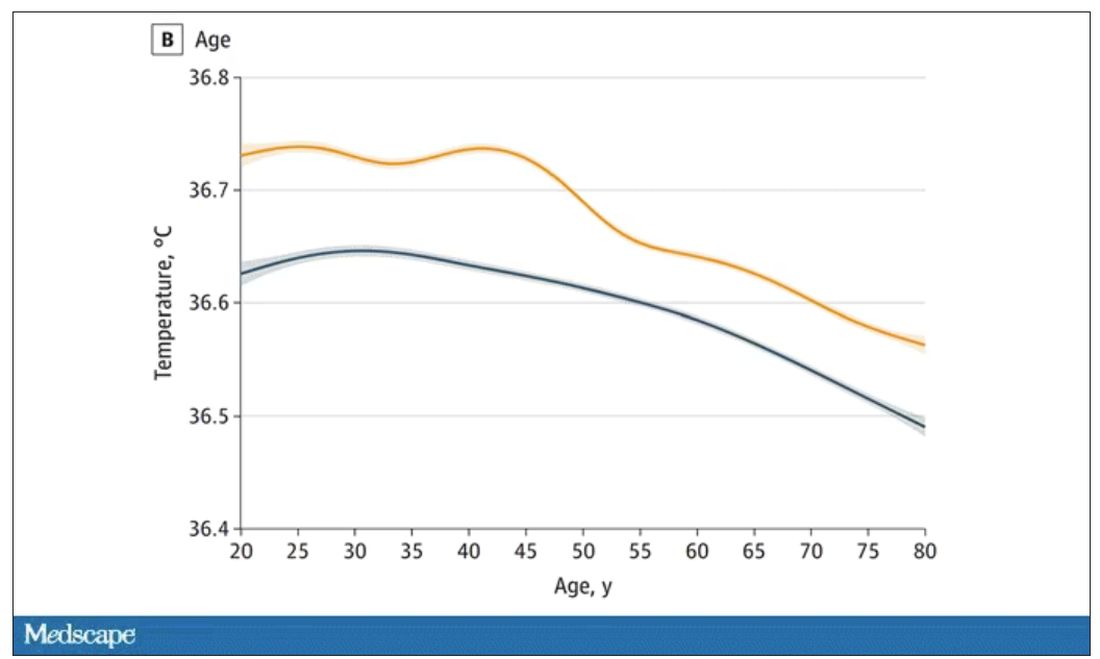
In fact, the researchers built a nice online calculator where you can enter your own, or your patient’s, parameters and calculate a normal body temperature for them. Here’s mine. My normal temperature at around 2 p.m. should be 36.7° C.
So, we’re all more cold-blooded than we thought. Is this just because of better methods? Maybe. But studies have actually shown that body temperature may be decreasing over time in humans, possibly because of the lower levels of inflammation we face in modern life (thanks to improvements in hygiene and antibiotics).
Of course, I’m sure some of you are asking yourselves whether any of this really matters. Is 37° C close enough?
Sure, this may be sort of puttering around the edges of physical diagnosis, but I think the methodology is really interesting and can obviously be applied to other broadly collected data points. But these data show us that thin, older individuals really do run cooler, and that we may need to pay more attention to a low-grade fever in that population than we otherwise would.
In any case, it’s time for a little re-education. If someone asks you what normal body temperature is, just say 36.6° C, 98.0° F. For his work in this area, I suggest we call it Wunderlich’s constant.
Dr. Wilson is associate professor of medicine and public health at Yale University, New Haven, Conn., and director of Yale’s Clinical and Translational Research Accelerator. He has no disclosures.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Every branch of science has its constants. Physics has the speed of light, the gravitational constant, the Planck constant. Chemistry gives us Avogadro’s number, Faraday’s constant, the charge of an electron. Medicine isn’t quite as reliable as physics when it comes to these things, but insofar as there are any constants in medicine, might I suggest normal body temperature: 37° Celsius, 98.6° Fahrenheit.
Sure, serum sodium may be less variable and lactate concentration more clinically relevant, but even my 7-year-old knows that normal body temperature is 98.6°.
Except, as it turns out, 98.6° isn’t normal at all.
How did we arrive at 37.0° C for normal body temperature? We got it from this guy – German physician Carl Reinhold August Wunderlich, who, in addition to looking eerily like Luciano Pavarotti, was the first to realize that fever was not itself a disease but a symptom of one.
In 1851, Dr. Wunderlich released his measurements of more than 1 million body temperatures taken from 25,000 Germans – a painstaking process at the time, which employed a foot-long thermometer and took 20 minutes to obtain a measurement.
The average temperature measured, of course, was 37° C.
We’re more than 150 years post-Wunderlich right now, and the average person in the United States might be quite a bit different from the average German in 1850. Moreover, we can do a lot better than just measuring a ton of people and taking the average, because we have statistics. The problem with measuring a bunch of people and taking the average temperature as normal is that you can’t be sure that the people you are measuring are normal. There are obvious causes of elevated temperature that you could exclude. Let’s not take people with a respiratory infection or who are taking Tylenol, for example. But as highlighted in this paper in JAMA Internal Medicine, we can do a lot better than that.
The study leverages the fact that body temperature is typically measured during all medical office visits and recorded in the ever-present electronic medical record.
Researchers from Stanford identified 724,199 patient encounters with outpatient temperature data. They excluded extreme temperatures – less than 34° C or greater than 40° C – excluded patients under 20 or above 80 years, and excluded those with extremes of height, weight, or body mass index.
You end up with a distribution like this. Note that the peak is clearly lower than 37° C.
But we’re still not at “normal.” Some people would be seeing their doctor for conditions that affect body temperature, such as infection. You could use diagnosis codes to flag these individuals and drop them, but that feels a bit arbitrary.
I really love how the researchers used data to fix this problem. They used a technique called LIMIT (Laboratory Information Mining for Individualized Thresholds). It works like this:
Take all the temperature measurements and then identify the outliers – the very tails of the distribution.
Look at all the diagnosis codes in those distributions. Determine which diagnosis codes are overrepresented in those distributions. Now you have a data-driven way to say that yes, these diagnoses are associated with weird temperatures. Next, eliminate everyone with those diagnoses from the dataset. What you are left with is a normal population, or at least a population that doesn’t have a condition that seems to meaningfully affect temperature.
So, who was dropped? Well, a lot of people, actually. It turned out that diabetes was way overrepresented in the outlier group. Although 9.2% of the population had diabetes, 26% of people with very low temperatures did, so everyone with diabetes is removed from the dataset. While 5% of the population had a cough at their encounter, 7% of the people with very high temperature and 7% of the people with very low temperature had a cough, so everyone with cough gets thrown out.
The algorithm excluded people on antibiotics or who had sinusitis, urinary tract infections, pneumonia, and, yes, a diagnosis of “fever.” The list makes sense, which is always nice when you have a purely algorithmic classification system.
What do we have left? What is the real normal temperature? Ready?
It’s 36.64° C, or about 98.0° F.
Of course, normal temperature varied depending on the time of day it was measured – higher in the afternoon.
The normal temperature in women tended to be higher than in men. The normal temperature declined with age as well.
In fact, the researchers built a nice online calculator where you can enter your own, or your patient’s, parameters and calculate a normal body temperature for them. Here’s mine. My normal temperature at around 2 p.m. should be 36.7° C.
So, we’re all more cold-blooded than we thought. Is this just because of better methods? Maybe. But studies have actually shown that body temperature may be decreasing over time in humans, possibly because of the lower levels of inflammation we face in modern life (thanks to improvements in hygiene and antibiotics).
Of course, I’m sure some of you are asking yourselves whether any of this really matters. Is 37° C close enough?
Sure, this may be sort of puttering around the edges of physical diagnosis, but I think the methodology is really interesting and can obviously be applied to other broadly collected data points. But these data show us that thin, older individuals really do run cooler, and that we may need to pay more attention to a low-grade fever in that population than we otherwise would.
In any case, it’s time for a little re-education. If someone asks you what normal body temperature is, just say 36.6° C, 98.0° F. For his work in this area, I suggest we call it Wunderlich’s constant.
Dr. Wilson is associate professor of medicine and public health at Yale University, New Haven, Conn., and director of Yale’s Clinical and Translational Research Accelerator. He has no disclosures.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Every branch of science has its constants. Physics has the speed of light, the gravitational constant, the Planck constant. Chemistry gives us Avogadro’s number, Faraday’s constant, the charge of an electron. Medicine isn’t quite as reliable as physics when it comes to these things, but insofar as there are any constants in medicine, might I suggest normal body temperature: 37° Celsius, 98.6° Fahrenheit.
Sure, serum sodium may be less variable and lactate concentration more clinically relevant, but even my 7-year-old knows that normal body temperature is 98.6°.
Except, as it turns out, 98.6° isn’t normal at all.
How did we arrive at 37.0° C for normal body temperature? We got it from this guy – German physician Carl Reinhold August Wunderlich, who, in addition to looking eerily like Luciano Pavarotti, was the first to realize that fever was not itself a disease but a symptom of one.
In 1851, Dr. Wunderlich released his measurements of more than 1 million body temperatures taken from 25,000 Germans – a painstaking process at the time, which employed a foot-long thermometer and took 20 minutes to obtain a measurement.
The average temperature measured, of course, was 37° C.
We’re more than 150 years post-Wunderlich right now, and the average person in the United States might be quite a bit different from the average German in 1850. Moreover, we can do a lot better than just measuring a ton of people and taking the average, because we have statistics. The problem with measuring a bunch of people and taking the average temperature as normal is that you can’t be sure that the people you are measuring are normal. There are obvious causes of elevated temperature that you could exclude. Let’s not take people with a respiratory infection or who are taking Tylenol, for example. But as highlighted in this paper in JAMA Internal Medicine, we can do a lot better than that.
The study leverages the fact that body temperature is typically measured during all medical office visits and recorded in the ever-present electronic medical record.
Researchers from Stanford identified 724,199 patient encounters with outpatient temperature data. They excluded extreme temperatures – less than 34° C or greater than 40° C – excluded patients under 20 or above 80 years, and excluded those with extremes of height, weight, or body mass index.
You end up with a distribution like this. Note that the peak is clearly lower than 37° C.
But we’re still not at “normal.” Some people would be seeing their doctor for conditions that affect body temperature, such as infection. You could use diagnosis codes to flag these individuals and drop them, but that feels a bit arbitrary.
I really love how the researchers used data to fix this problem. They used a technique called LIMIT (Laboratory Information Mining for Individualized Thresholds). It works like this:
Take all the temperature measurements and then identify the outliers – the very tails of the distribution.
Look at all the diagnosis codes in those distributions. Determine which diagnosis codes are overrepresented in those distributions. Now you have a data-driven way to say that yes, these diagnoses are associated with weird temperatures. Next, eliminate everyone with those diagnoses from the dataset. What you are left with is a normal population, or at least a population that doesn’t have a condition that seems to meaningfully affect temperature.
So, who was dropped? Well, a lot of people, actually. It turned out that diabetes was way overrepresented in the outlier group. Although 9.2% of the population had diabetes, 26% of people with very low temperatures did, so everyone with diabetes is removed from the dataset. While 5% of the population had a cough at their encounter, 7% of the people with very high temperature and 7% of the people with very low temperature had a cough, so everyone with cough gets thrown out.
The algorithm excluded people on antibiotics or who had sinusitis, urinary tract infections, pneumonia, and, yes, a diagnosis of “fever.” The list makes sense, which is always nice when you have a purely algorithmic classification system.
What do we have left? What is the real normal temperature? Ready?
It’s 36.64° C, or about 98.0° F.
Of course, normal temperature varied depending on the time of day it was measured – higher in the afternoon.
The normal temperature in women tended to be higher than in men. The normal temperature declined with age as well.
In fact, the researchers built a nice online calculator where you can enter your own, or your patient’s, parameters and calculate a normal body temperature for them. Here’s mine. My normal temperature at around 2 p.m. should be 36.7° C.
So, we’re all more cold-blooded than we thought. Is this just because of better methods? Maybe. But studies have actually shown that body temperature may be decreasing over time in humans, possibly because of the lower levels of inflammation we face in modern life (thanks to improvements in hygiene and antibiotics).
Of course, I’m sure some of you are asking yourselves whether any of this really matters. Is 37° C close enough?
Sure, this may be sort of puttering around the edges of physical diagnosis, but I think the methodology is really interesting and can obviously be applied to other broadly collected data points. But these data show us that thin, older individuals really do run cooler, and that we may need to pay more attention to a low-grade fever in that population than we otherwise would.
In any case, it’s time for a little re-education. If someone asks you what normal body temperature is, just say 36.6° C, 98.0° F. For his work in this area, I suggest we call it Wunderlich’s constant.
Dr. Wilson is associate professor of medicine and public health at Yale University, New Haven, Conn., and director of Yale’s Clinical and Translational Research Accelerator. He has no disclosures.
A version of this article appeared on Medscape.com.
Treatments for Hidradenitis Suppurativa Comorbidities Help With Pain Management
Hidradenitis suppurativa (HS) has an unpredictable disease course and poses substantial therapeutic challenges. It carries an increased risk for adverse cardiovascular outcomes and all-cause mortality. It also is associated with comorbidities including mood disorders, tobacco smoking, obesity, diabetes mellitus, sleep disorders, sexual dysfunction, and autoimmune diseases, which can complicate its management and considerably affect patients’ quality of life (QOL).1 Hidradenitis suppurativa also disproportionately affects minority groups and has far-reaching inequities; for example, the condition has a notable economic impact on patients, including higher unemployment and disability rates, lower-paying jobs, less paid time off, and other indirect costs.2,3 Race can impact how pain itself is treated. In one study (N = 217), Black patients with extremity fractures presenting to anemergency department were significantly less likely to receive analgesia compared to White patients despite reporting similar pain (57% vs 74%, respectively; P = .01).4 In another study, Hispanic patients were 7-times less likely to be treated with opioids compared to non-Hispanic patients with long-bone fractures.5 Herein, we highlight pain management disparities in HS patients.
Treating HS Comorbidities Helps Improve Pain
Pain is reported by almost all HS patients and is the symptom most associated with QOL impairment.6,7 Pain in HS is multifactorial, with other symptoms and comorbidities affecting its severity. Treatment of acute flares often is painful and procedural, including intralesional steroid injections or incision and drainage.8 Algorithms for addressing pain through the treatment of comorbidities also have been developed.6 Although there are few studies on the medications that treat related comorbidities in HS, there is evidence of their benefits in similar diseases; for example, treating depression in patients with irritable bowel disease (IBD) improved pain perception, cognitive function, and sexual dysfunction.9
Depression exacerbates pain, and higher levels of depression have been observed in severe HS.10,11 Additionally, more than 80% of individuals with HS report tobacco smoking.1 Nicotine not only increases pain sensitivity and decreases pain tolerance but also worsens neuropathic, nociceptive, and psychosocial pain, as well as mood disorders and sleep disturbances.12 Given the higher prevalence of depression and smoking in HS patients and the impact on pain, addressing these comorbidities is crucial. Additionally, poor sleep amplifies pain sensitivity and affects neurologic pain modulation.13 Chronic pain also is associated with obesity and sleep dysfunction.14
Treatments Targeting Pain and Comorbidities
Treatments that target comorbidities and other symptoms of HS also may improve pain. Bupropion is a well-studied antidepressant and first-line option to aid in smoking cessation. It provides acute and chronic pain relief associated with IBD and may perform similarly in patients with HS.15-18 Bupropion also demonstrated dose-dependent weight reduction in obese and overweight individuals.19,20 Additionally, varenicline is a first-line option to aid in smoking cessation and can be combined with bupropion to increase long-term efficacy.21,22
Other antidepressants may alleviate HS pain. The selective norepinephrine reuptake inhibitors duloxetine and venlafaxine are recommended for chronic pain in HS.6 Selective serotonin reuptake inhibitors such as citalopram, escitalopram, and paroxetine are inexpensive and widely available antidepressants. Citalopram is as efficacious as duloxetine for chronic pain with fewer side effects.23 Paroxetine has been shown to improve pain and pruritus, QOL, and depression in patients with IBD.24 Benefits such as improved weight and sexual dysfunction also have been reported.25
Metformin is well studied in Black patients, and greater glycemic response supports its efficacy for diabetes as well as HS, which disproportionately affects individuals with skin of color.26 Metformin also targets other comorbidities of HS, such as improving insulin resistance, polycystic ovary syndrome, acne vulgaris, weight loss, hyperlipidemia, cardiovascular risk, and neuropsychologic conditions.27 Growing evidence supports the use of metformin as a new agent in chronic pain management, specifically for patients with HS.28,29
Final Thoughts
Hidradenitis suppurativa is a complex medical condition seen disproportionately in minority groups. Understanding common comorbidities as well as the biases associated with pain management will allow providers to treat HS patients more effectively. Dermatologists who see many HS patients should become more familiar with treating these associated comorbidities to provide patient care that is more holistic and effective.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Tzellos T, Yang H, Mu F, et al. Impact of hidradenitis suppurativa on work loss, indirect costs and income. Br J Dermatol. 2019;181:147-154. doi:10.1111/bjd.17101
- Udechukwu NS, Fleischer AB. Higher risk of care for hidradenitis suppurativa in African American and non-Hispanic patients in the United States. J Natl Med Assoc. 2017;109:44-48. doi:10.1016/j.jnma.2016.09.002
- Todd KH, Deaton C, D’Adamo AP, et al. Ethnicity and analgesic practice. Ann Emerg Med. 2000;35:11-16. doi:10.1016/s0196-0644(00)70099-0
- Todd KH, Samaroo N, Hoffman JR. Ethnicity as a risk factor for inadequate emergency department analgesia. JAMA. 1993;269:1537-1539.
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Matusiak Ł, Szcze˛ch J, Kaaz K, et al. Clinical characteristics of pruritus and pain in patients with hidradenitis suppurativa. Acta Derm Venereol. 2018;98:191-194. doi:10.2340/00015555-2815
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016/j.jaad.2019.02.067
- Walker EA, Gelfand MD, Gelfand AN, et al. The relationship of current psychiatric disorder to functional disability and distress in patients with inflammatory bowel disease. Gen Hosp Psychiatry. 1996;18:220-229. doi:10.1016/0163-8343(96)00036-9
- Phan K, Huo YR, Smith SD. Hidradenitis suppurativa and psychiatric comorbidities, suicides and substance abuse: systematic review and meta-analysis. Ann Transl Med. 2020;8:821. doi:10.21037/atm-20-1028
- Woo AK. Depression and anxiety in pain. Rev Pain. 2010;4:8-12. doi:10.1177/204946371000400103
- Iida H, Yamaguchi S, Goyagi T, et al. Consensus statement on smoking cessation in patients with pain. J Anesth. 2022;36:671-687. doi:10.1007/s00540-022-03097-w
- Krause AJ, Prather AA, Wager TD, et al. The pain of sleep loss: a brain characterization in humans. J Neurosci. 2019;39:2291-2300. doi:10.1523/JNEUROSCI.2408-18.2018
- Mundal I, Gråwe RW, Bjørngaard JH, et al. Prevalence and long-term predictors of persistent chronic widespread pain in the general population in an 11-year prospective study: the HUNT study. BMC Musculoskelet Disord. 2014;15:213. doi:10.1186/1471-2474-15-213
- Aubin H-J. Tolerability and safety of sustained-release bupropion in the management of smoking cessation. Drugs. 2002;(62 suppl 2):45-52. doi:10.2165/00003495-200262002-00005
- Shah TH, Moradimehr A. Bupropion for the treatment of neuropathic pain. Am J Hosp Palliat Care. 2010;27:333-336. doi:10.1177/1049909110361229
- Baune BT, Renger L. Pharmacological and non-pharmacological interventions to improve cognitive dysfunction and functional ability in clinical depression—a systematic review. Psychiatry Res. 2014;219:25-50. doi:10.1016/j.psychres.2014.05.013
- Walker PW, Cole JO, Gardner EA, et al. Improvement in fluoxetine-associated sexual dysfunction in patients switched to bupropion. J Clin Psychiatry. 1993;54:459-465.
- Sherman MM, Ungureanu S, Rey JA. Naltrexone/bupropion ER (contrave): newly approved treatment option for chronic weight management in obese adults. P T. 2016;41:164-172.
- Anderson JW, Greenway FL, Fujioka K, et al. Bupropion SR enhances weight loss: a 48-week double-blind, placebo-controlled trial. Obes Res. 2002;10:633-641. doi:10.1038/oby.2002.86
- Kalkhoran S, Benowitz NL, Rigotti NA. Prevention and treatment of tobacco use: JACC health promotion series. J Am Coll Cardiol. 2018;72:1030-1045. doi:10.1016/j.jacc.2018.06.036
- Singh D, Saadabadi A. Varenicline. StatPearls Publishing; 2023.
- Mazza M, Mazza O, Pazzaglia C, et al. Escitalopram 20 mg versus duloxetine 60 mg for the treatment of chronic low back pain. Expert Opin Pharmacother. 2010;11:1049-1052. doi:10.1517/14656561003730413
- Docherty MJ, Jones RCW, Wallace MS. Managing pain in inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2011;7:592-601.
- Shrestha P, Fariba KA, Abdijadid S. Paroxetine. StatPearls Publishing; 2022.
- Williams LK, Padhukasahasram B, Ahmedani BK, et al. Differing effects of metformin on glycemic control by race-ethnicity. J Clin Endocrinol Metab. 2014;99:3160-3168. doi:10.1210/jc.2014-1539
- Sharma S, Mathur DK, Paliwal V, et al. Efficacy of metformin in the treatment of acne in women with polycystic ovarian syndrome: a newer approach to acne therapy. J Clin Aesthet Dermatol. 2019;12:34-38.
- Scheinfeld N. Hidradenitis suppurativa: a practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol Online J. 2013;19:1. doi:10.5070/D35VW402NF
- Baeza-Flores GDC, Guzmán-Priego CG, Parra-Flores LI, et al. Metformin: a prospective alternative for the treatment of chronic pain. Front Pharmacol. 2020;11:558474. doi:10.3389/fphar.2020.558474
Hidradenitis suppurativa (HS) has an unpredictable disease course and poses substantial therapeutic challenges. It carries an increased risk for adverse cardiovascular outcomes and all-cause mortality. It also is associated with comorbidities including mood disorders, tobacco smoking, obesity, diabetes mellitus, sleep disorders, sexual dysfunction, and autoimmune diseases, which can complicate its management and considerably affect patients’ quality of life (QOL).1 Hidradenitis suppurativa also disproportionately affects minority groups and has far-reaching inequities; for example, the condition has a notable economic impact on patients, including higher unemployment and disability rates, lower-paying jobs, less paid time off, and other indirect costs.2,3 Race can impact how pain itself is treated. In one study (N = 217), Black patients with extremity fractures presenting to anemergency department were significantly less likely to receive analgesia compared to White patients despite reporting similar pain (57% vs 74%, respectively; P = .01).4 In another study, Hispanic patients were 7-times less likely to be treated with opioids compared to non-Hispanic patients with long-bone fractures.5 Herein, we highlight pain management disparities in HS patients.
Treating HS Comorbidities Helps Improve Pain
Pain is reported by almost all HS patients and is the symptom most associated with QOL impairment.6,7 Pain in HS is multifactorial, with other symptoms and comorbidities affecting its severity. Treatment of acute flares often is painful and procedural, including intralesional steroid injections or incision and drainage.8 Algorithms for addressing pain through the treatment of comorbidities also have been developed.6 Although there are few studies on the medications that treat related comorbidities in HS, there is evidence of their benefits in similar diseases; for example, treating depression in patients with irritable bowel disease (IBD) improved pain perception, cognitive function, and sexual dysfunction.9
Depression exacerbates pain, and higher levels of depression have been observed in severe HS.10,11 Additionally, more than 80% of individuals with HS report tobacco smoking.1 Nicotine not only increases pain sensitivity and decreases pain tolerance but also worsens neuropathic, nociceptive, and psychosocial pain, as well as mood disorders and sleep disturbances.12 Given the higher prevalence of depression and smoking in HS patients and the impact on pain, addressing these comorbidities is crucial. Additionally, poor sleep amplifies pain sensitivity and affects neurologic pain modulation.13 Chronic pain also is associated with obesity and sleep dysfunction.14
Treatments Targeting Pain and Comorbidities
Treatments that target comorbidities and other symptoms of HS also may improve pain. Bupropion is a well-studied antidepressant and first-line option to aid in smoking cessation. It provides acute and chronic pain relief associated with IBD and may perform similarly in patients with HS.15-18 Bupropion also demonstrated dose-dependent weight reduction in obese and overweight individuals.19,20 Additionally, varenicline is a first-line option to aid in smoking cessation and can be combined with bupropion to increase long-term efficacy.21,22
Other antidepressants may alleviate HS pain. The selective norepinephrine reuptake inhibitors duloxetine and venlafaxine are recommended for chronic pain in HS.6 Selective serotonin reuptake inhibitors such as citalopram, escitalopram, and paroxetine are inexpensive and widely available antidepressants. Citalopram is as efficacious as duloxetine for chronic pain with fewer side effects.23 Paroxetine has been shown to improve pain and pruritus, QOL, and depression in patients with IBD.24 Benefits such as improved weight and sexual dysfunction also have been reported.25
Metformin is well studied in Black patients, and greater glycemic response supports its efficacy for diabetes as well as HS, which disproportionately affects individuals with skin of color.26 Metformin also targets other comorbidities of HS, such as improving insulin resistance, polycystic ovary syndrome, acne vulgaris, weight loss, hyperlipidemia, cardiovascular risk, and neuropsychologic conditions.27 Growing evidence supports the use of metformin as a new agent in chronic pain management, specifically for patients with HS.28,29
Final Thoughts
Hidradenitis suppurativa is a complex medical condition seen disproportionately in minority groups. Understanding common comorbidities as well as the biases associated with pain management will allow providers to treat HS patients more effectively. Dermatologists who see many HS patients should become more familiar with treating these associated comorbidities to provide patient care that is more holistic and effective.
Hidradenitis suppurativa (HS) has an unpredictable disease course and poses substantial therapeutic challenges. It carries an increased risk for adverse cardiovascular outcomes and all-cause mortality. It also is associated with comorbidities including mood disorders, tobacco smoking, obesity, diabetes mellitus, sleep disorders, sexual dysfunction, and autoimmune diseases, which can complicate its management and considerably affect patients’ quality of life (QOL).1 Hidradenitis suppurativa also disproportionately affects minority groups and has far-reaching inequities; for example, the condition has a notable economic impact on patients, including higher unemployment and disability rates, lower-paying jobs, less paid time off, and other indirect costs.2,3 Race can impact how pain itself is treated. In one study (N = 217), Black patients with extremity fractures presenting to anemergency department were significantly less likely to receive analgesia compared to White patients despite reporting similar pain (57% vs 74%, respectively; P = .01).4 In another study, Hispanic patients were 7-times less likely to be treated with opioids compared to non-Hispanic patients with long-bone fractures.5 Herein, we highlight pain management disparities in HS patients.
Treating HS Comorbidities Helps Improve Pain
Pain is reported by almost all HS patients and is the symptom most associated with QOL impairment.6,7 Pain in HS is multifactorial, with other symptoms and comorbidities affecting its severity. Treatment of acute flares often is painful and procedural, including intralesional steroid injections or incision and drainage.8 Algorithms for addressing pain through the treatment of comorbidities also have been developed.6 Although there are few studies on the medications that treat related comorbidities in HS, there is evidence of their benefits in similar diseases; for example, treating depression in patients with irritable bowel disease (IBD) improved pain perception, cognitive function, and sexual dysfunction.9
Depression exacerbates pain, and higher levels of depression have been observed in severe HS.10,11 Additionally, more than 80% of individuals with HS report tobacco smoking.1 Nicotine not only increases pain sensitivity and decreases pain tolerance but also worsens neuropathic, nociceptive, and psychosocial pain, as well as mood disorders and sleep disturbances.12 Given the higher prevalence of depression and smoking in HS patients and the impact on pain, addressing these comorbidities is crucial. Additionally, poor sleep amplifies pain sensitivity and affects neurologic pain modulation.13 Chronic pain also is associated with obesity and sleep dysfunction.14
Treatments Targeting Pain and Comorbidities
Treatments that target comorbidities and other symptoms of HS also may improve pain. Bupropion is a well-studied antidepressant and first-line option to aid in smoking cessation. It provides acute and chronic pain relief associated with IBD and may perform similarly in patients with HS.15-18 Bupropion also demonstrated dose-dependent weight reduction in obese and overweight individuals.19,20 Additionally, varenicline is a first-line option to aid in smoking cessation and can be combined with bupropion to increase long-term efficacy.21,22
Other antidepressants may alleviate HS pain. The selective norepinephrine reuptake inhibitors duloxetine and venlafaxine are recommended for chronic pain in HS.6 Selective serotonin reuptake inhibitors such as citalopram, escitalopram, and paroxetine are inexpensive and widely available antidepressants. Citalopram is as efficacious as duloxetine for chronic pain with fewer side effects.23 Paroxetine has been shown to improve pain and pruritus, QOL, and depression in patients with IBD.24 Benefits such as improved weight and sexual dysfunction also have been reported.25
Metformin is well studied in Black patients, and greater glycemic response supports its efficacy for diabetes as well as HS, which disproportionately affects individuals with skin of color.26 Metformin also targets other comorbidities of HS, such as improving insulin resistance, polycystic ovary syndrome, acne vulgaris, weight loss, hyperlipidemia, cardiovascular risk, and neuropsychologic conditions.27 Growing evidence supports the use of metformin as a new agent in chronic pain management, specifically for patients with HS.28,29
Final Thoughts
Hidradenitis suppurativa is a complex medical condition seen disproportionately in minority groups. Understanding common comorbidities as well as the biases associated with pain management will allow providers to treat HS patients more effectively. Dermatologists who see many HS patients should become more familiar with treating these associated comorbidities to provide patient care that is more holistic and effective.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Tzellos T, Yang H, Mu F, et al. Impact of hidradenitis suppurativa on work loss, indirect costs and income. Br J Dermatol. 2019;181:147-154. doi:10.1111/bjd.17101
- Udechukwu NS, Fleischer AB. Higher risk of care for hidradenitis suppurativa in African American and non-Hispanic patients in the United States. J Natl Med Assoc. 2017;109:44-48. doi:10.1016/j.jnma.2016.09.002
- Todd KH, Deaton C, D’Adamo AP, et al. Ethnicity and analgesic practice. Ann Emerg Med. 2000;35:11-16. doi:10.1016/s0196-0644(00)70099-0
- Todd KH, Samaroo N, Hoffman JR. Ethnicity as a risk factor for inadequate emergency department analgesia. JAMA. 1993;269:1537-1539.
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Matusiak Ł, Szcze˛ch J, Kaaz K, et al. Clinical characteristics of pruritus and pain in patients with hidradenitis suppurativa. Acta Derm Venereol. 2018;98:191-194. doi:10.2340/00015555-2815
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016/j.jaad.2019.02.067
- Walker EA, Gelfand MD, Gelfand AN, et al. The relationship of current psychiatric disorder to functional disability and distress in patients with inflammatory bowel disease. Gen Hosp Psychiatry. 1996;18:220-229. doi:10.1016/0163-8343(96)00036-9
- Phan K, Huo YR, Smith SD. Hidradenitis suppurativa and psychiatric comorbidities, suicides and substance abuse: systematic review and meta-analysis. Ann Transl Med. 2020;8:821. doi:10.21037/atm-20-1028
- Woo AK. Depression and anxiety in pain. Rev Pain. 2010;4:8-12. doi:10.1177/204946371000400103
- Iida H, Yamaguchi S, Goyagi T, et al. Consensus statement on smoking cessation in patients with pain. J Anesth. 2022;36:671-687. doi:10.1007/s00540-022-03097-w
- Krause AJ, Prather AA, Wager TD, et al. The pain of sleep loss: a brain characterization in humans. J Neurosci. 2019;39:2291-2300. doi:10.1523/JNEUROSCI.2408-18.2018
- Mundal I, Gråwe RW, Bjørngaard JH, et al. Prevalence and long-term predictors of persistent chronic widespread pain in the general population in an 11-year prospective study: the HUNT study. BMC Musculoskelet Disord. 2014;15:213. doi:10.1186/1471-2474-15-213
- Aubin H-J. Tolerability and safety of sustained-release bupropion in the management of smoking cessation. Drugs. 2002;(62 suppl 2):45-52. doi:10.2165/00003495-200262002-00005
- Shah TH, Moradimehr A. Bupropion for the treatment of neuropathic pain. Am J Hosp Palliat Care. 2010;27:333-336. doi:10.1177/1049909110361229
- Baune BT, Renger L. Pharmacological and non-pharmacological interventions to improve cognitive dysfunction and functional ability in clinical depression—a systematic review. Psychiatry Res. 2014;219:25-50. doi:10.1016/j.psychres.2014.05.013
- Walker PW, Cole JO, Gardner EA, et al. Improvement in fluoxetine-associated sexual dysfunction in patients switched to bupropion. J Clin Psychiatry. 1993;54:459-465.
- Sherman MM, Ungureanu S, Rey JA. Naltrexone/bupropion ER (contrave): newly approved treatment option for chronic weight management in obese adults. P T. 2016;41:164-172.
- Anderson JW, Greenway FL, Fujioka K, et al. Bupropion SR enhances weight loss: a 48-week double-blind, placebo-controlled trial. Obes Res. 2002;10:633-641. doi:10.1038/oby.2002.86
- Kalkhoran S, Benowitz NL, Rigotti NA. Prevention and treatment of tobacco use: JACC health promotion series. J Am Coll Cardiol. 2018;72:1030-1045. doi:10.1016/j.jacc.2018.06.036
- Singh D, Saadabadi A. Varenicline. StatPearls Publishing; 2023.
- Mazza M, Mazza O, Pazzaglia C, et al. Escitalopram 20 mg versus duloxetine 60 mg for the treatment of chronic low back pain. Expert Opin Pharmacother. 2010;11:1049-1052. doi:10.1517/14656561003730413
- Docherty MJ, Jones RCW, Wallace MS. Managing pain in inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2011;7:592-601.
- Shrestha P, Fariba KA, Abdijadid S. Paroxetine. StatPearls Publishing; 2022.
- Williams LK, Padhukasahasram B, Ahmedani BK, et al. Differing effects of metformin on glycemic control by race-ethnicity. J Clin Endocrinol Metab. 2014;99:3160-3168. doi:10.1210/jc.2014-1539
- Sharma S, Mathur DK, Paliwal V, et al. Efficacy of metformin in the treatment of acne in women with polycystic ovarian syndrome: a newer approach to acne therapy. J Clin Aesthet Dermatol. 2019;12:34-38.
- Scheinfeld N. Hidradenitis suppurativa: a practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol Online J. 2013;19:1. doi:10.5070/D35VW402NF
- Baeza-Flores GDC, Guzmán-Priego CG, Parra-Flores LI, et al. Metformin: a prospective alternative for the treatment of chronic pain. Front Pharmacol. 2020;11:558474. doi:10.3389/fphar.2020.558474
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Tzellos T, Yang H, Mu F, et al. Impact of hidradenitis suppurativa on work loss, indirect costs and income. Br J Dermatol. 2019;181:147-154. doi:10.1111/bjd.17101
- Udechukwu NS, Fleischer AB. Higher risk of care for hidradenitis suppurativa in African American and non-Hispanic patients in the United States. J Natl Med Assoc. 2017;109:44-48. doi:10.1016/j.jnma.2016.09.002
- Todd KH, Deaton C, D’Adamo AP, et al. Ethnicity and analgesic practice. Ann Emerg Med. 2000;35:11-16. doi:10.1016/s0196-0644(00)70099-0
- Todd KH, Samaroo N, Hoffman JR. Ethnicity as a risk factor for inadequate emergency department analgesia. JAMA. 1993;269:1537-1539.
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Matusiak Ł, Szcze˛ch J, Kaaz K, et al. Clinical characteristics of pruritus and pain in patients with hidradenitis suppurativa. Acta Derm Venereol. 2018;98:191-194. doi:10.2340/00015555-2815
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016/j.jaad.2019.02.067
- Walker EA, Gelfand MD, Gelfand AN, et al. The relationship of current psychiatric disorder to functional disability and distress in patients with inflammatory bowel disease. Gen Hosp Psychiatry. 1996;18:220-229. doi:10.1016/0163-8343(96)00036-9
- Phan K, Huo YR, Smith SD. Hidradenitis suppurativa and psychiatric comorbidities, suicides and substance abuse: systematic review and meta-analysis. Ann Transl Med. 2020;8:821. doi:10.21037/atm-20-1028
- Woo AK. Depression and anxiety in pain. Rev Pain. 2010;4:8-12. doi:10.1177/204946371000400103
- Iida H, Yamaguchi S, Goyagi T, et al. Consensus statement on smoking cessation in patients with pain. J Anesth. 2022;36:671-687. doi:10.1007/s00540-022-03097-w
- Krause AJ, Prather AA, Wager TD, et al. The pain of sleep loss: a brain characterization in humans. J Neurosci. 2019;39:2291-2300. doi:10.1523/JNEUROSCI.2408-18.2018
- Mundal I, Gråwe RW, Bjørngaard JH, et al. Prevalence and long-term predictors of persistent chronic widespread pain in the general population in an 11-year prospective study: the HUNT study. BMC Musculoskelet Disord. 2014;15:213. doi:10.1186/1471-2474-15-213
- Aubin H-J. Tolerability and safety of sustained-release bupropion in the management of smoking cessation. Drugs. 2002;(62 suppl 2):45-52. doi:10.2165/00003495-200262002-00005
- Shah TH, Moradimehr A. Bupropion for the treatment of neuropathic pain. Am J Hosp Palliat Care. 2010;27:333-336. doi:10.1177/1049909110361229
- Baune BT, Renger L. Pharmacological and non-pharmacological interventions to improve cognitive dysfunction and functional ability in clinical depression—a systematic review. Psychiatry Res. 2014;219:25-50. doi:10.1016/j.psychres.2014.05.013
- Walker PW, Cole JO, Gardner EA, et al. Improvement in fluoxetine-associated sexual dysfunction in patients switched to bupropion. J Clin Psychiatry. 1993;54:459-465.
- Sherman MM, Ungureanu S, Rey JA. Naltrexone/bupropion ER (contrave): newly approved treatment option for chronic weight management in obese adults. P T. 2016;41:164-172.
- Anderson JW, Greenway FL, Fujioka K, et al. Bupropion SR enhances weight loss: a 48-week double-blind, placebo-controlled trial. Obes Res. 2002;10:633-641. doi:10.1038/oby.2002.86
- Kalkhoran S, Benowitz NL, Rigotti NA. Prevention and treatment of tobacco use: JACC health promotion series. J Am Coll Cardiol. 2018;72:1030-1045. doi:10.1016/j.jacc.2018.06.036
- Singh D, Saadabadi A. Varenicline. StatPearls Publishing; 2023.
- Mazza M, Mazza O, Pazzaglia C, et al. Escitalopram 20 mg versus duloxetine 60 mg for the treatment of chronic low back pain. Expert Opin Pharmacother. 2010;11:1049-1052. doi:10.1517/14656561003730413
- Docherty MJ, Jones RCW, Wallace MS. Managing pain in inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2011;7:592-601.
- Shrestha P, Fariba KA, Abdijadid S. Paroxetine. StatPearls Publishing; 2022.
- Williams LK, Padhukasahasram B, Ahmedani BK, et al. Differing effects of metformin on glycemic control by race-ethnicity. J Clin Endocrinol Metab. 2014;99:3160-3168. doi:10.1210/jc.2014-1539
- Sharma S, Mathur DK, Paliwal V, et al. Efficacy of metformin in the treatment of acne in women with polycystic ovarian syndrome: a newer approach to acne therapy. J Clin Aesthet Dermatol. 2019;12:34-38.
- Scheinfeld N. Hidradenitis suppurativa: a practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol Online J. 2013;19:1. doi:10.5070/D35VW402NF
- Baeza-Flores GDC, Guzmán-Priego CG, Parra-Flores LI, et al. Metformin: a prospective alternative for the treatment of chronic pain. Front Pharmacol. 2020;11:558474. doi:10.3389/fphar.2020.558474
One in five doctors with long COVID can no longer work: Survey
Crippling symptoms, lost careers, and eroded incomes: This is the harsh reality for doctors suffering with long COVID, according to the first major survey of physicians with the condition.
The survey, conducted by the British Medical Association and the Long COVID Doctors for Action support group, sheds light on the lingering effects of long COVID on more than 600 chronically ill and disabled doctors with the condition. It also spotlights what they describe as a lack of medical and financial support from their government and employers at the National Health Service.
“We feel betrayed and abandoned,” said Kelly Fearnley, MBChB, chair and cofounder of Long COVID Doctors for Action. “At a time of national crisis, when health care workers were asked to step up, we did. When the nation needed us, we stepped up. We put our lives on the line. We put our families’ lives on the line. And now that we are injured after knowingly being unprotected and deliberately and repeatedly exposed to a level 3 biohazard, we now find ourselves in this position.”
Dr. Fearnley fell ill while working in a hospital’s COVID ward in November 2020. She is one of an estimated 2 million people in the United Kingdom – including thousands of NHS employees – with long COVID. She hasn’t been able to return to work in nearly 3 years.
Long COVID affects more than 65 million people worldwide. It is estimated that 1 in 10 people infected with the virus develop long-term symptoms. In the United Kingdom, health care and social care workers are seven times more likely to have had severe COVID-19 than other types of employees.
Doctors responding to the BMA survey reported a wide range of long COVID symptoms, including fatigue, headaches, muscular pain, nerve damage, joint pain, and respiratory problems.
Among the survey’s key findings, 60% of doctors said long COVID has affected their ability to carry out day-to-day tasks on a regular basis. Almost one in five (18%) said they were no longer able to work, while fewer than one in three (31%) were working full time. This compares with more than half (57%) of respondents working full time before the onset of their COVID illness – a decline of 46%.
Nearly half (48%) of respondents said they have experienced some form of loss of earnings as a result of long COVID, and almost half of the doctors were never referred to an NHS long COVID clinic. The survey included the following first-person accounts from doctors living with the condition.
- One doctor said: “I nearly lost my life, my home, my partner and my career. I have received little support to help keep these. The impact on my mental health nearly cost [me] my life again.”
- A senior consulting physician commented: “Life is absolutely miserable. Every day is a struggle. I wake up exhausted, the insomnia and night terrors are horrendous as I live through my worst fears every night. Any activity such as eating meals, washing, etc., will mean I have to go to bed for a few hours. I am unable to look after myself or my child, exercise or maintain social relationships. I have no financial security. Long COVID has totally destroyed my life.”
- A salaried general practitioner said: “I can no longer work, finances are ruined. I didn’t have employment protection so am now unemployed and penniless.”
Calls for action from the BMA include the following:
- Financial support for doctors and health care staff with long COVID.
- The recognition of long COVID as an occupational disease among health care workers, along with a definition of the condition that covers all of the debilitating disease’s symptoms.
- Improved access to physical and mental health services to help comprehensive assessment, investigations, and treatment.
- Greater workplace protection for health care staff who risk their lives for others.
- Better support for long COVID sufferers to return to work safely if they can, including a flexible approach to the use of workplace adjustments.
“One would think, given the circumstances under which we fell ill and current workforce shortages, NHS employers would be eager to do everything to facilitate the return to work of people with long COVID,” said Dr. Fearnley. “However, NHS employers are legally required to implement only ‘reasonable adjustments,’ and so things such as extended phased return or adjustments to shift patterns are not always being facilitated. Instead, an increasing number of employers are choosing to terminate contracts.”
Raymond Agius, the BMA’s occupational medicine committee cochair, also put the blame on inadequate safety measures for doctors. Those inadequate measures persist to this day, inasmuch as U.K. hospitals have dropped masking requirements.
“During the COVID-19 pandemic, doctors were left exposed and unprotected at work,” he said in a BMA press release. “They often did not have access to the right PPE. ... Too many risk assessments of workplaces and especially of vulnerable doctors were not undertaken.”
A small minority of doctors who were surveyed said they had access to respiratory protective equipment about the time they contracted COVID-19. Only 11% had access to an FFP2 respirator (the equivalent of an N95 mask); 16% had an FFP3 respirator (the equivalent of an N99 mask).
To date, the British government hasn’t issued much of a response to the survey, saying only that it has invested more than ₤50 million to better understand long COVID.
A version of this article first appeared on Medscape.com.
Crippling symptoms, lost careers, and eroded incomes: This is the harsh reality for doctors suffering with long COVID, according to the first major survey of physicians with the condition.
The survey, conducted by the British Medical Association and the Long COVID Doctors for Action support group, sheds light on the lingering effects of long COVID on more than 600 chronically ill and disabled doctors with the condition. It also spotlights what they describe as a lack of medical and financial support from their government and employers at the National Health Service.
“We feel betrayed and abandoned,” said Kelly Fearnley, MBChB, chair and cofounder of Long COVID Doctors for Action. “At a time of national crisis, when health care workers were asked to step up, we did. When the nation needed us, we stepped up. We put our lives on the line. We put our families’ lives on the line. And now that we are injured after knowingly being unprotected and deliberately and repeatedly exposed to a level 3 biohazard, we now find ourselves in this position.”
Dr. Fearnley fell ill while working in a hospital’s COVID ward in November 2020. She is one of an estimated 2 million people in the United Kingdom – including thousands of NHS employees – with long COVID. She hasn’t been able to return to work in nearly 3 years.
Long COVID affects more than 65 million people worldwide. It is estimated that 1 in 10 people infected with the virus develop long-term symptoms. In the United Kingdom, health care and social care workers are seven times more likely to have had severe COVID-19 than other types of employees.
Doctors responding to the BMA survey reported a wide range of long COVID symptoms, including fatigue, headaches, muscular pain, nerve damage, joint pain, and respiratory problems.
Among the survey’s key findings, 60% of doctors said long COVID has affected their ability to carry out day-to-day tasks on a regular basis. Almost one in five (18%) said they were no longer able to work, while fewer than one in three (31%) were working full time. This compares with more than half (57%) of respondents working full time before the onset of their COVID illness – a decline of 46%.
Nearly half (48%) of respondents said they have experienced some form of loss of earnings as a result of long COVID, and almost half of the doctors were never referred to an NHS long COVID clinic. The survey included the following first-person accounts from doctors living with the condition.
- One doctor said: “I nearly lost my life, my home, my partner and my career. I have received little support to help keep these. The impact on my mental health nearly cost [me] my life again.”
- A senior consulting physician commented: “Life is absolutely miserable. Every day is a struggle. I wake up exhausted, the insomnia and night terrors are horrendous as I live through my worst fears every night. Any activity such as eating meals, washing, etc., will mean I have to go to bed for a few hours. I am unable to look after myself or my child, exercise or maintain social relationships. I have no financial security. Long COVID has totally destroyed my life.”
- A salaried general practitioner said: “I can no longer work, finances are ruined. I didn’t have employment protection so am now unemployed and penniless.”
Calls for action from the BMA include the following:
- Financial support for doctors and health care staff with long COVID.
- The recognition of long COVID as an occupational disease among health care workers, along with a definition of the condition that covers all of the debilitating disease’s symptoms.
- Improved access to physical and mental health services to help comprehensive assessment, investigations, and treatment.
- Greater workplace protection for health care staff who risk their lives for others.
- Better support for long COVID sufferers to return to work safely if they can, including a flexible approach to the use of workplace adjustments.
“One would think, given the circumstances under which we fell ill and current workforce shortages, NHS employers would be eager to do everything to facilitate the return to work of people with long COVID,” said Dr. Fearnley. “However, NHS employers are legally required to implement only ‘reasonable adjustments,’ and so things such as extended phased return or adjustments to shift patterns are not always being facilitated. Instead, an increasing number of employers are choosing to terminate contracts.”
Raymond Agius, the BMA’s occupational medicine committee cochair, also put the blame on inadequate safety measures for doctors. Those inadequate measures persist to this day, inasmuch as U.K. hospitals have dropped masking requirements.
“During the COVID-19 pandemic, doctors were left exposed and unprotected at work,” he said in a BMA press release. “They often did not have access to the right PPE. ... Too many risk assessments of workplaces and especially of vulnerable doctors were not undertaken.”
A small minority of doctors who were surveyed said they had access to respiratory protective equipment about the time they contracted COVID-19. Only 11% had access to an FFP2 respirator (the equivalent of an N95 mask); 16% had an FFP3 respirator (the equivalent of an N99 mask).
To date, the British government hasn’t issued much of a response to the survey, saying only that it has invested more than ₤50 million to better understand long COVID.
A version of this article first appeared on Medscape.com.
Crippling symptoms, lost careers, and eroded incomes: This is the harsh reality for doctors suffering with long COVID, according to the first major survey of physicians with the condition.
The survey, conducted by the British Medical Association and the Long COVID Doctors for Action support group, sheds light on the lingering effects of long COVID on more than 600 chronically ill and disabled doctors with the condition. It also spotlights what they describe as a lack of medical and financial support from their government and employers at the National Health Service.
“We feel betrayed and abandoned,” said Kelly Fearnley, MBChB, chair and cofounder of Long COVID Doctors for Action. “At a time of national crisis, when health care workers were asked to step up, we did. When the nation needed us, we stepped up. We put our lives on the line. We put our families’ lives on the line. And now that we are injured after knowingly being unprotected and deliberately and repeatedly exposed to a level 3 biohazard, we now find ourselves in this position.”
Dr. Fearnley fell ill while working in a hospital’s COVID ward in November 2020. She is one of an estimated 2 million people in the United Kingdom – including thousands of NHS employees – with long COVID. She hasn’t been able to return to work in nearly 3 years.
Long COVID affects more than 65 million people worldwide. It is estimated that 1 in 10 people infected with the virus develop long-term symptoms. In the United Kingdom, health care and social care workers are seven times more likely to have had severe COVID-19 than other types of employees.
Doctors responding to the BMA survey reported a wide range of long COVID symptoms, including fatigue, headaches, muscular pain, nerve damage, joint pain, and respiratory problems.
Among the survey’s key findings, 60% of doctors said long COVID has affected their ability to carry out day-to-day tasks on a regular basis. Almost one in five (18%) said they were no longer able to work, while fewer than one in three (31%) were working full time. This compares with more than half (57%) of respondents working full time before the onset of their COVID illness – a decline of 46%.
Nearly half (48%) of respondents said they have experienced some form of loss of earnings as a result of long COVID, and almost half of the doctors were never referred to an NHS long COVID clinic. The survey included the following first-person accounts from doctors living with the condition.
- One doctor said: “I nearly lost my life, my home, my partner and my career. I have received little support to help keep these. The impact on my mental health nearly cost [me] my life again.”
- A senior consulting physician commented: “Life is absolutely miserable. Every day is a struggle. I wake up exhausted, the insomnia and night terrors are horrendous as I live through my worst fears every night. Any activity such as eating meals, washing, etc., will mean I have to go to bed for a few hours. I am unable to look after myself or my child, exercise or maintain social relationships. I have no financial security. Long COVID has totally destroyed my life.”
- A salaried general practitioner said: “I can no longer work, finances are ruined. I didn’t have employment protection so am now unemployed and penniless.”
Calls for action from the BMA include the following:
- Financial support for doctors and health care staff with long COVID.
- The recognition of long COVID as an occupational disease among health care workers, along with a definition of the condition that covers all of the debilitating disease’s symptoms.
- Improved access to physical and mental health services to help comprehensive assessment, investigations, and treatment.
- Greater workplace protection for health care staff who risk their lives for others.
- Better support for long COVID sufferers to return to work safely if they can, including a flexible approach to the use of workplace adjustments.
“One would think, given the circumstances under which we fell ill and current workforce shortages, NHS employers would be eager to do everything to facilitate the return to work of people with long COVID,” said Dr. Fearnley. “However, NHS employers are legally required to implement only ‘reasonable adjustments,’ and so things such as extended phased return or adjustments to shift patterns are not always being facilitated. Instead, an increasing number of employers are choosing to terminate contracts.”
Raymond Agius, the BMA’s occupational medicine committee cochair, also put the blame on inadequate safety measures for doctors. Those inadequate measures persist to this day, inasmuch as U.K. hospitals have dropped masking requirements.
“During the COVID-19 pandemic, doctors were left exposed and unprotected at work,” he said in a BMA press release. “They often did not have access to the right PPE. ... Too many risk assessments of workplaces and especially of vulnerable doctors were not undertaken.”
A small minority of doctors who were surveyed said they had access to respiratory protective equipment about the time they contracted COVID-19. Only 11% had access to an FFP2 respirator (the equivalent of an N95 mask); 16% had an FFP3 respirator (the equivalent of an N99 mask).
To date, the British government hasn’t issued much of a response to the survey, saying only that it has invested more than ₤50 million to better understand long COVID.
A version of this article first appeared on Medscape.com.