User login
Multidisciplinary Amputation Prevention at the DeBakey VA Hospital: Our First Decade
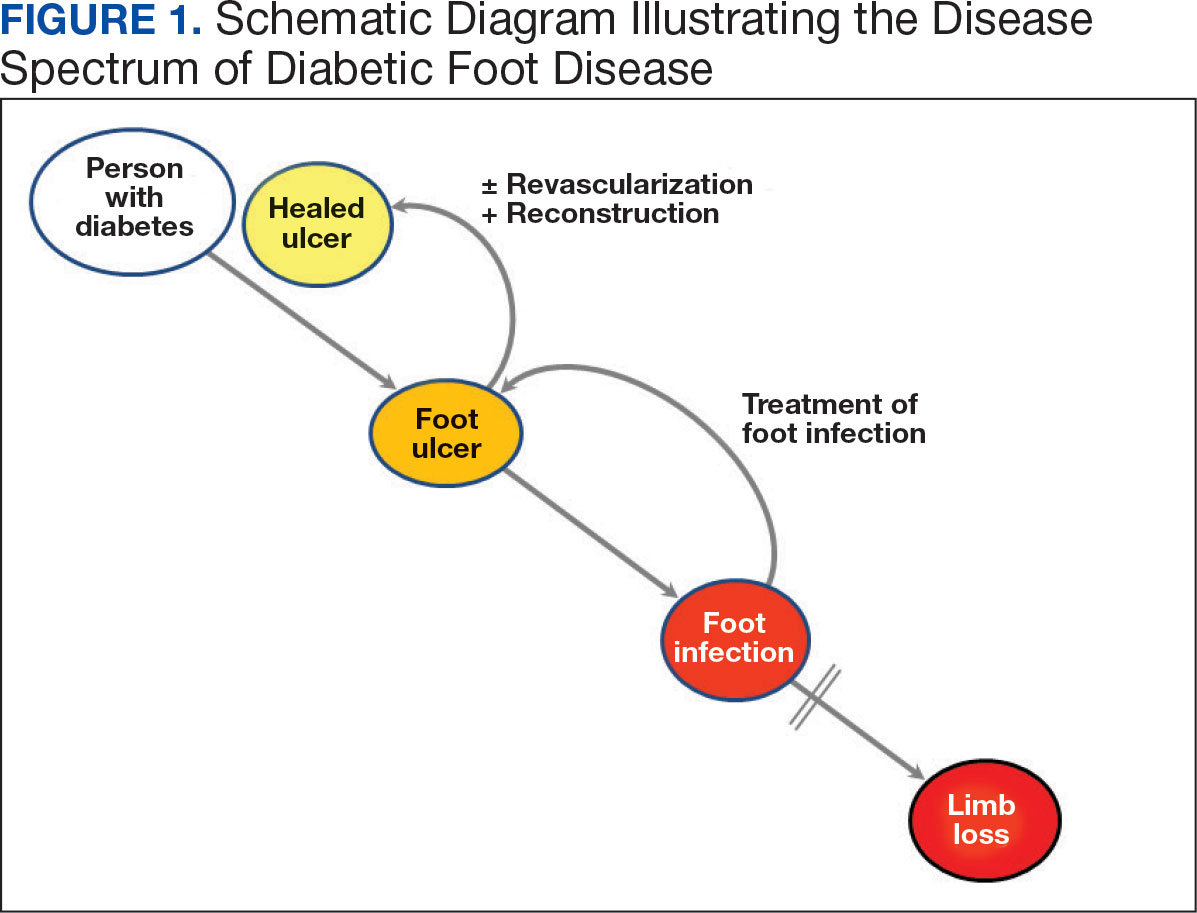
Individuals with diabetes are at risk for developing foot ulcers or full-thickness defects in the epithelium of the foot. These defects can lead to bacterial invasion and foot infection, potentially resulting in leg amputation (Figure 1). Effective treatment to prevent leg amputation, known as limb salvage, requires management across multiple medical specialties including podiatry, vascular surgery, and infectious diseases. The multidisciplinary team approach to limb salvage was introduced in Boston in 1928 and has been the prevailing approach to this cross-specialty medical problem for at least a decade.1,2
The Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) has established an inpatient limb salvage program—a group of dedicated clinicians working collaboratively to provide evidence-guided management of patients hospitalized with foot ulcers, foot gangrene or any superimposed infection with the goal of avoiding leg amputations. We have seen a significant and durable reduction in the incidence of leg amputations among veterans at MEDVAMC.
This article describes the evolution and outcomes of the MEDVAMC limb salvage program over more than a decade. It includes changes to team structure and workflow, as well as past and present successes and challenges. The eAppendix provides a narrative summary with examples of how our clinical practice and research efforts have informed one another and how these findings are applied to clinical management. This process is part of the larger efforts of the Veterans Health Administration (VHA) to create a learning health system in which “internal data and experience are systematically integrated with external evidence, and that knowledge is put into practice.”3
Methods
Data from the VHA Support Service Center were used to obtain monthly major (leg) and minor (toe and partial foot) amputation records at MEDVAMC from October 2000 through May 2023. Yearly totals for the number of persons with diabetes and foot ulcers at MEDVAMC were also obtained from the support service center. Annual patient population sizes and number of persons with foot ulcers were converted to monthly estimates using cubic spline interpolation. Rates were calculated as 12-month rolling averages. Trend lines were created with locally weighted running line smoothing that used a span α of 0.1.
We characterized the patient population using data from cohorts of veterans treated for foot ulcers and foot infections at MEDVAMC. To compare the contemporary veteran population with nonveteran inpatients treated for foot ulcers and foot infections at other hospitals, we created a 2:1 nonveteran to veteran cohort matched by sex and zip code, using publicly available hospital admission data from the Texas Department of Health and State Health Services. Veterans used for this cohort comparison are consistent with the 100 consecutive patients who underwent angiography for limb salvage in 2022.
This research was approved by the Baylor College of Medicine Institutional Review Board (protocol H-34858) and the MEDVAMC Research Committee (IRBNet protocol 15A12. HB). All analyses used deidentified data in the R programming language version 4.2.2 using RStudio version 2022.06.0 Build 421.
Program Description
MEDVAMC is a 350-bed teaching hospital located in central Houston. Its hospital system includes 11 outpatient clinics, ranging from 28 to 126 miles (eAppendix, Supplemental Figure A) from MEDVAMC. MEDVAMC provides vascular, orthopedic, and podiatric surgery services, as well as many other highly specialized services such as liver and heart transplants. The hospital’s risk-adjusted rates of operative morbidity and mortality (observed-to-expected ratios) are significantly lower than expected.
Despite this, the incidence rate of leg amputations at MEDVAMC in early 2011 was nearly 3-times higher than the VHA average. The inpatient management of veterans with infected foot ulcers was fragmented, with the general, orthopedic, and vascular surgery teams separately providing siloed care. Delays in treatment were common. There was much service- and practitioner-level practice heterogeneity. No diagnostic or treatment protocols were used, and standard treatment components were sporadically provided.
Patient Population
Compared to the matched non-VHA patient cohort (Supplemental Table 1), veterans treated at MEDVAMC for limb salvage are older. Nearly half (46%) identify as Black, which is associated with a 2-fold higher riskadjusted rate of leg amputations.4 MEDVAMC patients also have significantly higher rates of diabetes, chronic kidney disease, and systolic heart failure. About 22% travel > 40 miles for treatment at MEDVAMC, double that of the matched cohort (10.7%). Additionally, 35% currently smoke and 37% have moderate to severe peripheral artery disease (PAD).5
Program Design
In late 2011, the MEDVAMC vascular surgery team led limb salvage efforts by implementing a single team model, which involved assuming the primary role of managing foot ulcers for all veterans, both infected and uninfected (eAppendix, Supplemental Figure B). Consultations were directed to a dedicated limb salvage pager. The vascular team provided interdisciplinary limb salvage management across the spectrum of disease, including the surgical treatment of infection, assessment for PAD, open surgical operations and endovascular interventions to treat PAD, and foot reconstruction (debridement, minor or partial foot amputations, and skin grafting). This care was complemented by frequent consultation with the infectious disease, vascular medicine, podiatry, and geriatric wound care teams. This approach streamlined the delivery of consistent multidisciplinary care.
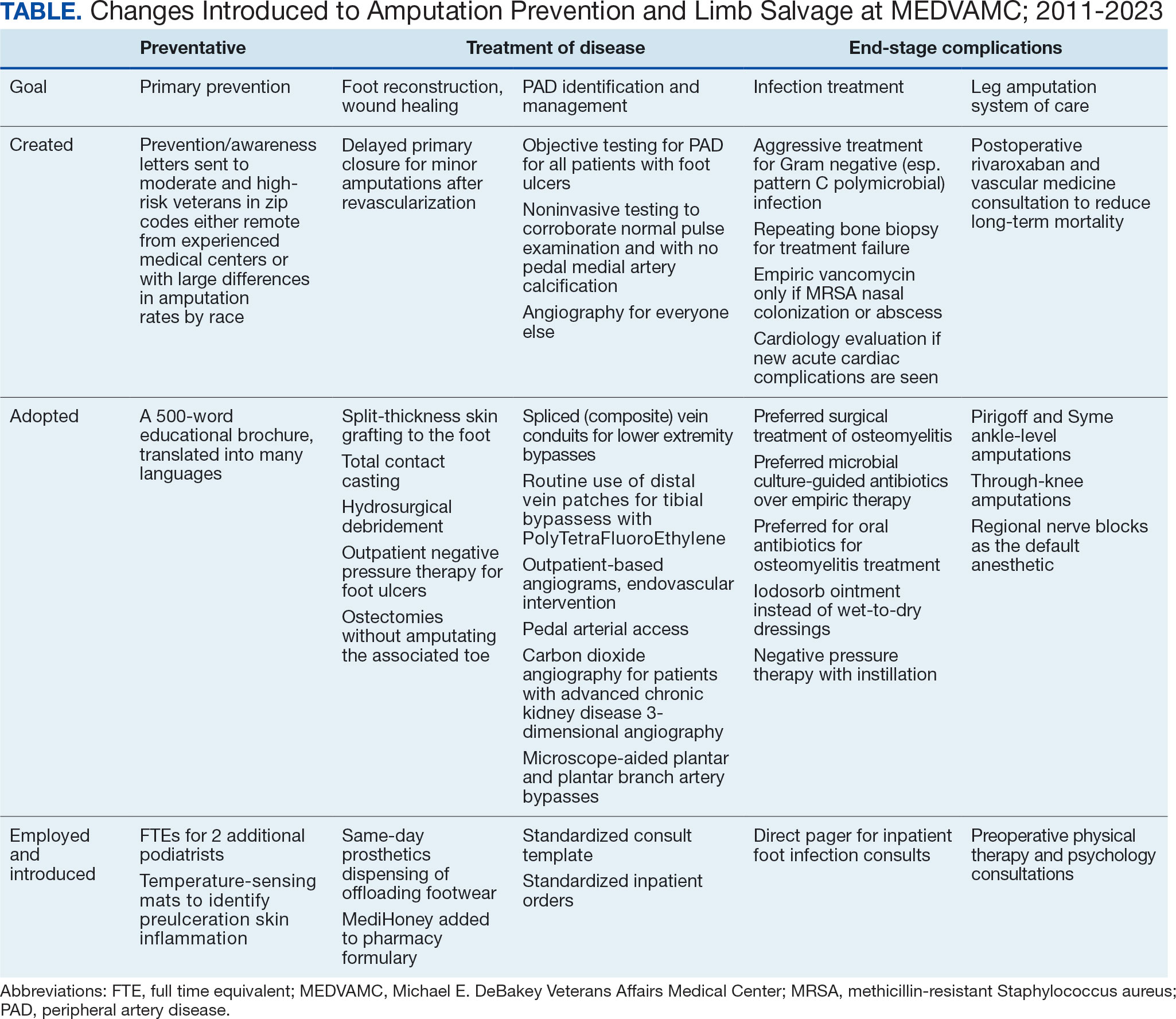
This collaborative effort aimed to develop ideal multidisciplinary care plans through research spanning the spectrum of the diabetic foot infection disease process (eAppendix, Supplemental Table 1). Some of the most impactful practices were: (1) a proclivity towards surgical treatment of foot infections, especially osteomyelitis5; (2) improved identification of PAD6,7; (3) early surgical closure of foot wounds following revascularization8,9; and (4) palliative wound care as an alternative to leg amputation in veterans who are not candidates for revascularization and limb salvage.10 Initally, the vascular surgery team held monthly multidisciplinary limb salvage meetings to coordinate patient management, identify ways to streamline care and avoid waste, discuss research findings, and review the 12-month rolling average of the MEDVAMC leg amputation incidence rate.
During the study period, the MEDVAMC vascular surgery team consisted of 2 to 5 board certified vascular or general surgeons, 2 or 3 nurse practitioners, and 3 vascular ultrasound technologists. Associated specialists included 2 podiatrists, 3 geriatricians with wound care certification, as well as additional infectious diseases, vascular medicine, orthopedics, and general surgery specialists.
Program Assessment
We noted a significant and sustained decrease in the MEDVAMC leg amputation rate after implementing multidisciplinary meetings and a single- team model from early 2012 through 2017 (Figure 2). The amputation incidence rate decreased steadily over the period from a maximum of 160 per 100,000 per year in February 2012 to a nadir of 66 per 100,000 per year in April 2017, an overall 60% decrease. Increases were noted in early 2018 after ceasing the single- team model, and in the summer of 2022, following periods of bed shortages after the onset of the COVID-19 pandemic. Tracking this metric allowed clinicians to make course corrections.
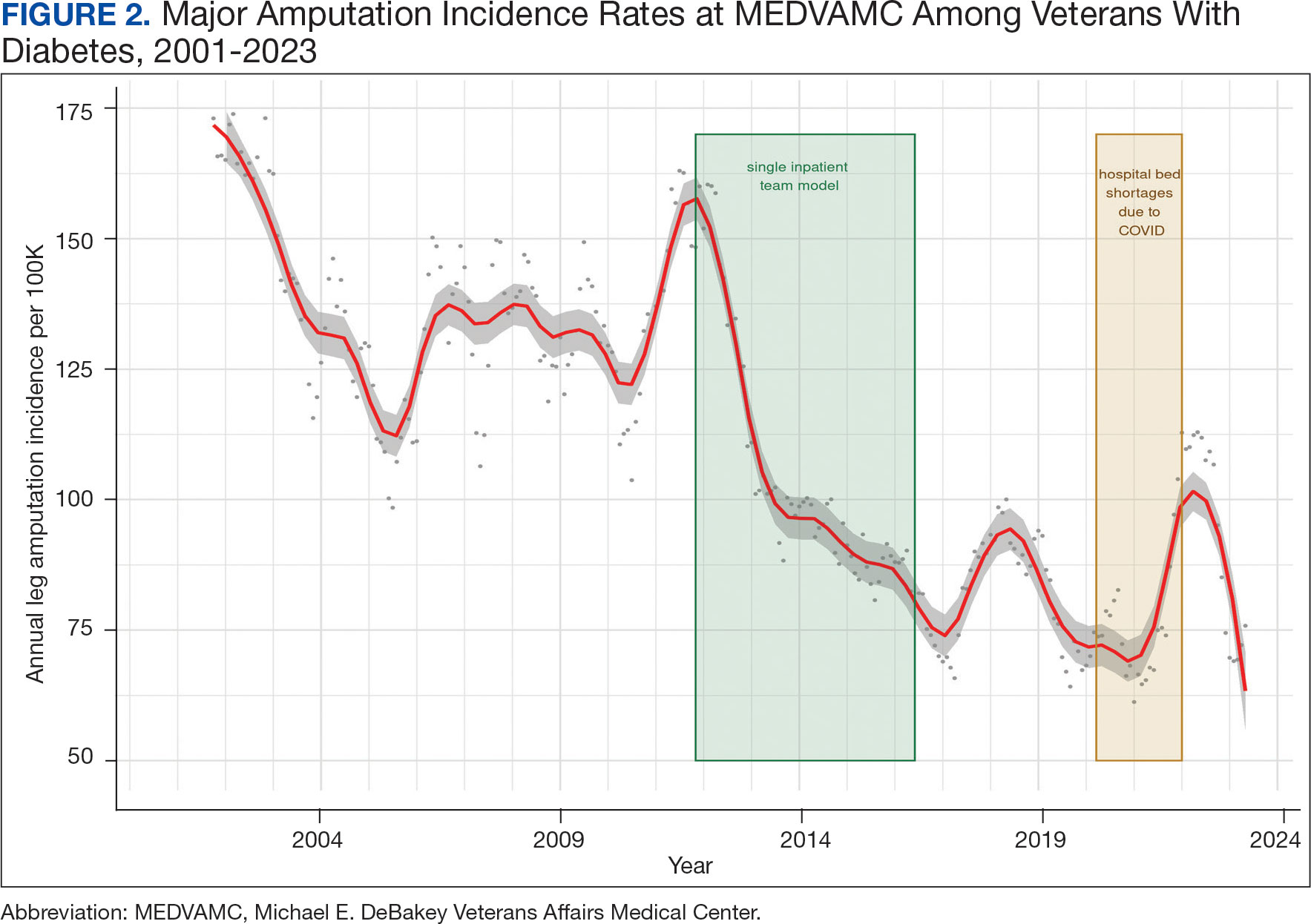
The decreased leg amputation rate at MEDVAMC does not seem to be mirroring national or regional trends. During this 10-year period, the VHA annualized amputation rate decreased minimally, from 58 to 54 per 100,000 (eAppendix Supplemental Figure C). Leg amputation incidence at non-VHA hospitals in Texas slightly increased over the same period.11
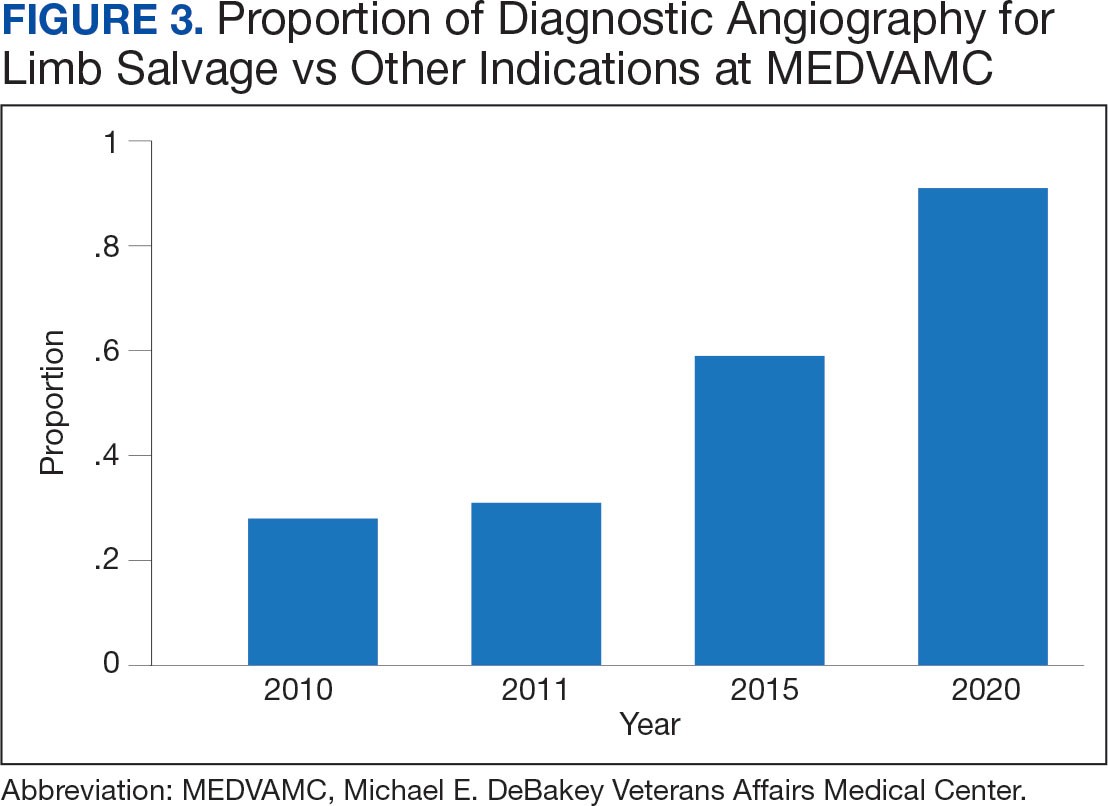
Value was also reflected in other metrics. MEDVAMC improved safety through a bundled strategy that reduced the risk-adjusted rate of surgical wound infections by 95%.12 MEDVAMC prioritized limb salvage when selecting patients for angiography and nearly eliminated using stent-grafts, cryopreserved allogeneic saphenous vein grafts, and expensive surgical and endovascular implants, which were identified as more expensive and less effective than other options (Figure 3).13-15 The MEDVAMC team achieved a > 90% patient trust rating on the Veterans Signals survey in fiscal years 2021 and 2022.
Challenges
A significant increase in the patient-physician ratio occurred 5 years into the program. In 2016, 2 vascular surgeons left MEDVAMC and a planned renovation of 1 of the 2 vascular surgery-assigned hybrid working facilities began even as the number of MEDVAMC patients with diabetes grew 120% (from 89,400 to 107,746 between 2010 and 2016), and the incidence rate of foot ulcers grew 300% (from 392 in 2010 to 1183 in 2016 per 100,000). The net result was a higher clinical workload among the remaining vascular surgeons with less operating room availability.
To stabilize surgeon retention, MEDVAMC reverted from the single team model back to inpatient care being distributed among general surgery, orthopedic surgery, and vascular surgery. After noting an increase in the leg amputation incidence rate, we adjusted the focus from multidisciplinary to interdisciplinary care (ie, majority of limb salvage clinical care can be provided by practitioners of any involved specialties). We worked to establish a local, written, interdisciplinary consensus on evaluating and managing veterans with nonhealing foot ulcers to mitigate the loss of a consolidated inpatient approach. Despite frequent staff turnover, ≥ 1 physician or surgeon from the core specialties of vascular surgery, podiatry, and infectious diseases remained throughout the study period.
The COVID-19 pandemic caused a shortage of hospital beds. This was followed by more bed shortages due to decreased nursing staff. Our health care system also had a period of restricted outpatient encounters early in the pandemic. During this time, we noted a delayed presentation of veterans with advanced infections and another increase in leg amputation incidence rate.
Like many health systems, MEDVAMC pivoted to telephone- and video-based outpatient encounters. Our team also used publicly available Texas hospitalization data to identify zip codes with particularly high leg amputation incidence rates, and > 3500 educational mailings to veterans categorized as moderate and high risk for leg amputation in these zip codes. These mailings provided information on recognizing foot ulcers and infections, emphasized timely evaluation, and named the MEDVAMC vascular surgery team as a point-of-contact. More recently, we have seen a further decrease in the MEDVAMC incidences of leg amputation to its lowest rate in > 20 years.
Discussion
A learning organization that directs its research based on clinical observations and informs its clinical care with research findings can produce palpable improvements in outcomes. Understanding the disease process and trying to better understand management across the entire range of this disease process has allowed our team to make consistent and systematic changes in care (Table). Consolidating inpatient care in a single team model seems to have been effective in reducing amputation rates among veterans with diabetes. The role the MEDVAMC vascular surgery team served for limb salvage patients may have been particularly beneficial because of the large impact untreated or unidentified PAD can have and because of the high prevalence of PAD among the limb salvage population seen at MEDVAMC. To be sustainable, though, a single-team model needs resources. A multiteam model can also be effective if the degree of multidisciplinary involvement for any given veteran is appropriate to the individual's clinical needs, teams are engaged and willing to contribute in a defined role within their specialty, and lines of communication remain open.
The primary challenge at MEDVAMC has been, and will continue to be, the retention of physicians and surgeons. MEDVAMC has excellent leadership and a collegial working environment, but better access to operating rooms for elective and time-sensitive operations, additional clinical staff support, and higher salary at non-VA positions have been the basis for many of physicians— especially surgeons—leaving MEDVAMC. Despite high staff turnover and a constant flow of resident and fellow trainees, MEDVAMC has been able to keep the clinical approach relatively consistent due to the use of written protocols and continuity of care as ≥ 1 physician or surgeon from each of the 4 main teams remained engaged with limb salvage throughout the entire period.
Going forward, we will work to ensure that all requirements of the 2022 Prevention of Amputation in Veterans Everywhere directive are incorporated into care.8 We plan to standardize MEDVAMC management algorithms further, both to streamline care and reduce the opportunity for disparities in treatment. More prophylactic podiatric procedures, surgical forms of offloading, and a shared multidisciplinary clinic space may also further help patients.
Conclusions
The introduction of multidisciplinary limb salvage at MEDVAMC has led to significant and sustained reductions in leg amputation incidence. These reductions do not seem dependent upon a specific team structure for inpatient care. To improve patient outcomes, efforts should focus on making improvements across the entire disease spectrum. For limb salvage, this includes primary prevention of foot ulcers, the treatment of foot infections, identification and management of PAD, surgical reconstruction/optimal wound healing, and care for patients who undergo leg amputation.
- Sanders LJ, Robbins JM, Edmonds ME. History of the team approach to amputation prevention: pioneers and milestones. J Am Podiatr Med Assoc. 2010;100(5):317- 334. doi:10.7547/1000317
- Sumpio BE, Armstrong DG, Lavery LA, Andros G. The role of interdisciplinary team approach in the management of the diabetic foot: a joint statement from the society for vascular surgery and the American podiatric medical association. J Am Podiatr Med Assoc. 2010;100(4):309-311. doi:10.7547/1000309
- About learning health systems. Agency for Healthcare Research and Quality. Published March 2019. Updated May 2019. Accessed October 9, 2024. https://www.ahrq.gov/learning-health-systems/about.html
- Barshes NR, Minc SD. Healthcare disparities in vascular surgery: a critical review. J Vasc Surg. 2021;74(2S):6S-14S.
- Barshes NR, Mindru C, Ashong C, Rodriguez-Barradas M, Trautner BW. Treatment failure and leg amputation among patients with foot osteomyelitis. Int J Low Extrem Wounds. 2016;15(4):303-312. doi:10.1177/1534734616661058
- Barshes NR, Flores E, Belkin M, Kougias P, Armstrong DG, Mills JL Sr. The accuracy and cost-effectiveness of strategies used to identify peripheral artery disease among patients with diabetic foot ulcers. J Vasc Surg. 2016;64(6):1682-1690.e3. doi:10.1016/j.jvs.2016.04.056 e1. doi:10.1016/j.jvs.2021.03.055
- Choi JC, Miranda J, Greenleaf E, et al. Lower-extremity pressure, staging, and grading thresholds to identify chronic limb-threatening ischemia. Vasc Med. 2023;28(1):45-53. doi:10.1177/1358863X221147945
- Barshes NR, Chambers JD, Cohen J, Belkin M; Model To Optimize Healthcare Value in Ischemic Extremities 1 (MOVIE) Study Collaborators. Cost-effectiveness in the contemporary management of critical limb ischemia with tissue loss. J Vasc Surg. 2012;56(4):1015-24.e1. doi:10.1016/j.jvs.2012.02.069
- Barshes NR, Bechara CF, Pisimisis G, Kougias P. Preliminary experiences with early primary closure of foot wounds after lower extremity revascularization. Ann Vasc Surg. 2014;28(1):48-52. doi:10.1016/j.avsg.2013.06.012
- Barshes NR, Gold B, Garcia A, Bechara CF, Pisimisis G, Kougias P. Minor amputation and palliative wound care as a strategy to avoid major amputation in patients with foot infections and severe peripheral arterial disease. Int J Low Extrem Wounds. 2014;13(3):211-219. doi:10.1177/1534734614543663
- Garcia M, Hernandez B, Ellington TG, et al. A lack of decline in major nontraumatic amputations in Texas: contemporary trends, risk factor associations, and impact of revascularization. Diabetes Care. 2019;42(6):1061-1066. doi:10.2337/dc19-0078
- Zamani N, Sharath SE, Vo E, Awad SS, Kougias P, Barshes NR. A multi-component strategy to decrease wound complications after open infra-inguinal re-vascularization. Surg Infect (Larchmt). 2018;19(1):87-94. doi:10.1089/sur.2017.193
- Barshes NR, Ozaki CK, Kougias P, Belkin M. A costeffectiveness analysis of infrainguinal bypass in the absence of great saphenous vein conduit. J Vasc Surg. 2013;57(6):1466-1470. doi:10.1016/j.jvs.2012.11.115
- Zamani N, Sharath S, Browder R, et al. PC158 longterm outcomes after endovascular stent placement for symptomatic, long-segment superficial femoral artery lesions. J Vasc Surg. 2017;65(6):182S-183S. doi:10.1016/j.jvs.2017.03.344
- Zamani N, Sharath SE, Browder RC, et al. Outcomes after endovascular stent placement for long-segment superficial femoral artery lesions. Ann Vasc Surg. 2021;71:298-307. doi:10.1016/j.avsg.2020.08.124
Individuals with diabetes are at risk for developing foot ulcers or full-thickness defects in the epithelium of the foot. These defects can lead to bacterial invasion and foot infection, potentially resulting in leg amputation (Figure 1). Effective treatment to prevent leg amputation, known as limb salvage, requires management across multiple medical specialties including podiatry, vascular surgery, and infectious diseases. The multidisciplinary team approach to limb salvage was introduced in Boston in 1928 and has been the prevailing approach to this cross-specialty medical problem for at least a decade.1,2

The Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) has established an inpatient limb salvage program—a group of dedicated clinicians working collaboratively to provide evidence-guided management of patients hospitalized with foot ulcers, foot gangrene or any superimposed infection with the goal of avoiding leg amputations. We have seen a significant and durable reduction in the incidence of leg amputations among veterans at MEDVAMC.
This article describes the evolution and outcomes of the MEDVAMC limb salvage program over more than a decade. It includes changes to team structure and workflow, as well as past and present successes and challenges. The eAppendix provides a narrative summary with examples of how our clinical practice and research efforts have informed one another and how these findings are applied to clinical management. This process is part of the larger efforts of the Veterans Health Administration (VHA) to create a learning health system in which “internal data and experience are systematically integrated with external evidence, and that knowledge is put into practice.”3
Methods
Data from the VHA Support Service Center were used to obtain monthly major (leg) and minor (toe and partial foot) amputation records at MEDVAMC from October 2000 through May 2023. Yearly totals for the number of persons with diabetes and foot ulcers at MEDVAMC were also obtained from the support service center. Annual patient population sizes and number of persons with foot ulcers were converted to monthly estimates using cubic spline interpolation. Rates were calculated as 12-month rolling averages. Trend lines were created with locally weighted running line smoothing that used a span α of 0.1.
We characterized the patient population using data from cohorts of veterans treated for foot ulcers and foot infections at MEDVAMC. To compare the contemporary veteran population with nonveteran inpatients treated for foot ulcers and foot infections at other hospitals, we created a 2:1 nonveteran to veteran cohort matched by sex and zip code, using publicly available hospital admission data from the Texas Department of Health and State Health Services. Veterans used for this cohort comparison are consistent with the 100 consecutive patients who underwent angiography for limb salvage in 2022.
This research was approved by the Baylor College of Medicine Institutional Review Board (protocol H-34858) and the MEDVAMC Research Committee (IRBNet protocol 15A12. HB). All analyses used deidentified data in the R programming language version 4.2.2 using RStudio version 2022.06.0 Build 421.
Program Description
MEDVAMC is a 350-bed teaching hospital located in central Houston. Its hospital system includes 11 outpatient clinics, ranging from 28 to 126 miles (eAppendix, Supplemental Figure A) from MEDVAMC. MEDVAMC provides vascular, orthopedic, and podiatric surgery services, as well as many other highly specialized services such as liver and heart transplants. The hospital’s risk-adjusted rates of operative morbidity and mortality (observed-to-expected ratios) are significantly lower than expected.
Despite this, the incidence rate of leg amputations at MEDVAMC in early 2011 was nearly 3-times higher than the VHA average. The inpatient management of veterans with infected foot ulcers was fragmented, with the general, orthopedic, and vascular surgery teams separately providing siloed care. Delays in treatment were common. There was much service- and practitioner-level practice heterogeneity. No diagnostic or treatment protocols were used, and standard treatment components were sporadically provided.
Patient Population
Compared to the matched non-VHA patient cohort (Supplemental Table 1), veterans treated at MEDVAMC for limb salvage are older. Nearly half (46%) identify as Black, which is associated with a 2-fold higher riskadjusted rate of leg amputations.4 MEDVAMC patients also have significantly higher rates of diabetes, chronic kidney disease, and systolic heart failure. About 22% travel > 40 miles for treatment at MEDVAMC, double that of the matched cohort (10.7%). Additionally, 35% currently smoke and 37% have moderate to severe peripheral artery disease (PAD).5
Program Design
In late 2011, the MEDVAMC vascular surgery team led limb salvage efforts by implementing a single team model, which involved assuming the primary role of managing foot ulcers for all veterans, both infected and uninfected (eAppendix, Supplemental Figure B). Consultations were directed to a dedicated limb salvage pager. The vascular team provided interdisciplinary limb salvage management across the spectrum of disease, including the surgical treatment of infection, assessment for PAD, open surgical operations and endovascular interventions to treat PAD, and foot reconstruction (debridement, minor or partial foot amputations, and skin grafting). This care was complemented by frequent consultation with the infectious disease, vascular medicine, podiatry, and geriatric wound care teams. This approach streamlined the delivery of consistent multidisciplinary care.
This collaborative effort aimed to develop ideal multidisciplinary care plans through research spanning the spectrum of the diabetic foot infection disease process (eAppendix, Supplemental Table 1). Some of the most impactful practices were: (1) a proclivity towards surgical treatment of foot infections, especially osteomyelitis5; (2) improved identification of PAD6,7; (3) early surgical closure of foot wounds following revascularization8,9; and (4) palliative wound care as an alternative to leg amputation in veterans who are not candidates for revascularization and limb salvage.10 Initally, the vascular surgery team held monthly multidisciplinary limb salvage meetings to coordinate patient management, identify ways to streamline care and avoid waste, discuss research findings, and review the 12-month rolling average of the MEDVAMC leg amputation incidence rate.
During the study period, the MEDVAMC vascular surgery team consisted of 2 to 5 board certified vascular or general surgeons, 2 or 3 nurse practitioners, and 3 vascular ultrasound technologists. Associated specialists included 2 podiatrists, 3 geriatricians with wound care certification, as well as additional infectious diseases, vascular medicine, orthopedics, and general surgery specialists.
Program Assessment
We noted a significant and sustained decrease in the MEDVAMC leg amputation rate after implementing multidisciplinary meetings and a single- team model from early 2012 through 2017 (Figure 2). The amputation incidence rate decreased steadily over the period from a maximum of 160 per 100,000 per year in February 2012 to a nadir of 66 per 100,000 per year in April 2017, an overall 60% decrease. Increases were noted in early 2018 after ceasing the single- team model, and in the summer of 2022, following periods of bed shortages after the onset of the COVID-19 pandemic. Tracking this metric allowed clinicians to make course corrections.

The decreased leg amputation rate at MEDVAMC does not seem to be mirroring national or regional trends. During this 10-year period, the VHA annualized amputation rate decreased minimally, from 58 to 54 per 100,000 (eAppendix Supplemental Figure C). Leg amputation incidence at non-VHA hospitals in Texas slightly increased over the same period.11
Value was also reflected in other metrics. MEDVAMC improved safety through a bundled strategy that reduced the risk-adjusted rate of surgical wound infections by 95%.12 MEDVAMC prioritized limb salvage when selecting patients for angiography and nearly eliminated using stent-grafts, cryopreserved allogeneic saphenous vein grafts, and expensive surgical and endovascular implants, which were identified as more expensive and less effective than other options (Figure 3).13-15 The MEDVAMC team achieved a > 90% patient trust rating on the Veterans Signals survey in fiscal years 2021 and 2022.

Challenges
A significant increase in the patient-physician ratio occurred 5 years into the program. In 2016, 2 vascular surgeons left MEDVAMC and a planned renovation of 1 of the 2 vascular surgery-assigned hybrid working facilities began even as the number of MEDVAMC patients with diabetes grew 120% (from 89,400 to 107,746 between 2010 and 2016), and the incidence rate of foot ulcers grew 300% (from 392 in 2010 to 1183 in 2016 per 100,000). The net result was a higher clinical workload among the remaining vascular surgeons with less operating room availability.
To stabilize surgeon retention, MEDVAMC reverted from the single team model back to inpatient care being distributed among general surgery, orthopedic surgery, and vascular surgery. After noting an increase in the leg amputation incidence rate, we adjusted the focus from multidisciplinary to interdisciplinary care (ie, majority of limb salvage clinical care can be provided by practitioners of any involved specialties). We worked to establish a local, written, interdisciplinary consensus on evaluating and managing veterans with nonhealing foot ulcers to mitigate the loss of a consolidated inpatient approach. Despite frequent staff turnover, ≥ 1 physician or surgeon from the core specialties of vascular surgery, podiatry, and infectious diseases remained throughout the study period.
The COVID-19 pandemic caused a shortage of hospital beds. This was followed by more bed shortages due to decreased nursing staff. Our health care system also had a period of restricted outpatient encounters early in the pandemic. During this time, we noted a delayed presentation of veterans with advanced infections and another increase in leg amputation incidence rate.
Like many health systems, MEDVAMC pivoted to telephone- and video-based outpatient encounters. Our team also used publicly available Texas hospitalization data to identify zip codes with particularly high leg amputation incidence rates, and > 3500 educational mailings to veterans categorized as moderate and high risk for leg amputation in these zip codes. These mailings provided information on recognizing foot ulcers and infections, emphasized timely evaluation, and named the MEDVAMC vascular surgery team as a point-of-contact. More recently, we have seen a further decrease in the MEDVAMC incidences of leg amputation to its lowest rate in > 20 years.
Discussion
A learning organization that directs its research based on clinical observations and informs its clinical care with research findings can produce palpable improvements in outcomes. Understanding the disease process and trying to better understand management across the entire range of this disease process has allowed our team to make consistent and systematic changes in care (Table). Consolidating inpatient care in a single team model seems to have been effective in reducing amputation rates among veterans with diabetes. The role the MEDVAMC vascular surgery team served for limb salvage patients may have been particularly beneficial because of the large impact untreated or unidentified PAD can have and because of the high prevalence of PAD among the limb salvage population seen at MEDVAMC. To be sustainable, though, a single-team model needs resources. A multiteam model can also be effective if the degree of multidisciplinary involvement for any given veteran is appropriate to the individual's clinical needs, teams are engaged and willing to contribute in a defined role within their specialty, and lines of communication remain open.

The primary challenge at MEDVAMC has been, and will continue to be, the retention of physicians and surgeons. MEDVAMC has excellent leadership and a collegial working environment, but better access to operating rooms for elective and time-sensitive operations, additional clinical staff support, and higher salary at non-VA positions have been the basis for many of physicians— especially surgeons—leaving MEDVAMC. Despite high staff turnover and a constant flow of resident and fellow trainees, MEDVAMC has been able to keep the clinical approach relatively consistent due to the use of written protocols and continuity of care as ≥ 1 physician or surgeon from each of the 4 main teams remained engaged with limb salvage throughout the entire period.
Going forward, we will work to ensure that all requirements of the 2022 Prevention of Amputation in Veterans Everywhere directive are incorporated into care.8 We plan to standardize MEDVAMC management algorithms further, both to streamline care and reduce the opportunity for disparities in treatment. More prophylactic podiatric procedures, surgical forms of offloading, and a shared multidisciplinary clinic space may also further help patients.
Conclusions
The introduction of multidisciplinary limb salvage at MEDVAMC has led to significant and sustained reductions in leg amputation incidence. These reductions do not seem dependent upon a specific team structure for inpatient care. To improve patient outcomes, efforts should focus on making improvements across the entire disease spectrum. For limb salvage, this includes primary prevention of foot ulcers, the treatment of foot infections, identification and management of PAD, surgical reconstruction/optimal wound healing, and care for patients who undergo leg amputation.
Individuals with diabetes are at risk for developing foot ulcers or full-thickness defects in the epithelium of the foot. These defects can lead to bacterial invasion and foot infection, potentially resulting in leg amputation (Figure 1). Effective treatment to prevent leg amputation, known as limb salvage, requires management across multiple medical specialties including podiatry, vascular surgery, and infectious diseases. The multidisciplinary team approach to limb salvage was introduced in Boston in 1928 and has been the prevailing approach to this cross-specialty medical problem for at least a decade.1,2

The Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) has established an inpatient limb salvage program—a group of dedicated clinicians working collaboratively to provide evidence-guided management of patients hospitalized with foot ulcers, foot gangrene or any superimposed infection with the goal of avoiding leg amputations. We have seen a significant and durable reduction in the incidence of leg amputations among veterans at MEDVAMC.
This article describes the evolution and outcomes of the MEDVAMC limb salvage program over more than a decade. It includes changes to team structure and workflow, as well as past and present successes and challenges. The eAppendix provides a narrative summary with examples of how our clinical practice and research efforts have informed one another and how these findings are applied to clinical management. This process is part of the larger efforts of the Veterans Health Administration (VHA) to create a learning health system in which “internal data and experience are systematically integrated with external evidence, and that knowledge is put into practice.”3
Methods
Data from the VHA Support Service Center were used to obtain monthly major (leg) and minor (toe and partial foot) amputation records at MEDVAMC from October 2000 through May 2023. Yearly totals for the number of persons with diabetes and foot ulcers at MEDVAMC were also obtained from the support service center. Annual patient population sizes and number of persons with foot ulcers were converted to monthly estimates using cubic spline interpolation. Rates were calculated as 12-month rolling averages. Trend lines were created with locally weighted running line smoothing that used a span α of 0.1.
We characterized the patient population using data from cohorts of veterans treated for foot ulcers and foot infections at MEDVAMC. To compare the contemporary veteran population with nonveteran inpatients treated for foot ulcers and foot infections at other hospitals, we created a 2:1 nonveteran to veteran cohort matched by sex and zip code, using publicly available hospital admission data from the Texas Department of Health and State Health Services. Veterans used for this cohort comparison are consistent with the 100 consecutive patients who underwent angiography for limb salvage in 2022.
This research was approved by the Baylor College of Medicine Institutional Review Board (protocol H-34858) and the MEDVAMC Research Committee (IRBNet protocol 15A12. HB). All analyses used deidentified data in the R programming language version 4.2.2 using RStudio version 2022.06.0 Build 421.
Program Description
MEDVAMC is a 350-bed teaching hospital located in central Houston. Its hospital system includes 11 outpatient clinics, ranging from 28 to 126 miles (eAppendix, Supplemental Figure A) from MEDVAMC. MEDVAMC provides vascular, orthopedic, and podiatric surgery services, as well as many other highly specialized services such as liver and heart transplants. The hospital’s risk-adjusted rates of operative morbidity and mortality (observed-to-expected ratios) are significantly lower than expected.
Despite this, the incidence rate of leg amputations at MEDVAMC in early 2011 was nearly 3-times higher than the VHA average. The inpatient management of veterans with infected foot ulcers was fragmented, with the general, orthopedic, and vascular surgery teams separately providing siloed care. Delays in treatment were common. There was much service- and practitioner-level practice heterogeneity. No diagnostic or treatment protocols were used, and standard treatment components were sporadically provided.
Patient Population
Compared to the matched non-VHA patient cohort (Supplemental Table 1), veterans treated at MEDVAMC for limb salvage are older. Nearly half (46%) identify as Black, which is associated with a 2-fold higher riskadjusted rate of leg amputations.4 MEDVAMC patients also have significantly higher rates of diabetes, chronic kidney disease, and systolic heart failure. About 22% travel > 40 miles for treatment at MEDVAMC, double that of the matched cohort (10.7%). Additionally, 35% currently smoke and 37% have moderate to severe peripheral artery disease (PAD).5
Program Design
In late 2011, the MEDVAMC vascular surgery team led limb salvage efforts by implementing a single team model, which involved assuming the primary role of managing foot ulcers for all veterans, both infected and uninfected (eAppendix, Supplemental Figure B). Consultations were directed to a dedicated limb salvage pager. The vascular team provided interdisciplinary limb salvage management across the spectrum of disease, including the surgical treatment of infection, assessment for PAD, open surgical operations and endovascular interventions to treat PAD, and foot reconstruction (debridement, minor or partial foot amputations, and skin grafting). This care was complemented by frequent consultation with the infectious disease, vascular medicine, podiatry, and geriatric wound care teams. This approach streamlined the delivery of consistent multidisciplinary care.
This collaborative effort aimed to develop ideal multidisciplinary care plans through research spanning the spectrum of the diabetic foot infection disease process (eAppendix, Supplemental Table 1). Some of the most impactful practices were: (1) a proclivity towards surgical treatment of foot infections, especially osteomyelitis5; (2) improved identification of PAD6,7; (3) early surgical closure of foot wounds following revascularization8,9; and (4) palliative wound care as an alternative to leg amputation in veterans who are not candidates for revascularization and limb salvage.10 Initally, the vascular surgery team held monthly multidisciplinary limb salvage meetings to coordinate patient management, identify ways to streamline care and avoid waste, discuss research findings, and review the 12-month rolling average of the MEDVAMC leg amputation incidence rate.
During the study period, the MEDVAMC vascular surgery team consisted of 2 to 5 board certified vascular or general surgeons, 2 or 3 nurse practitioners, and 3 vascular ultrasound technologists. Associated specialists included 2 podiatrists, 3 geriatricians with wound care certification, as well as additional infectious diseases, vascular medicine, orthopedics, and general surgery specialists.
Program Assessment
We noted a significant and sustained decrease in the MEDVAMC leg amputation rate after implementing multidisciplinary meetings and a single- team model from early 2012 through 2017 (Figure 2). The amputation incidence rate decreased steadily over the period from a maximum of 160 per 100,000 per year in February 2012 to a nadir of 66 per 100,000 per year in April 2017, an overall 60% decrease. Increases were noted in early 2018 after ceasing the single- team model, and in the summer of 2022, following periods of bed shortages after the onset of the COVID-19 pandemic. Tracking this metric allowed clinicians to make course corrections.

The decreased leg amputation rate at MEDVAMC does not seem to be mirroring national or regional trends. During this 10-year period, the VHA annualized amputation rate decreased minimally, from 58 to 54 per 100,000 (eAppendix Supplemental Figure C). Leg amputation incidence at non-VHA hospitals in Texas slightly increased over the same period.11
Value was also reflected in other metrics. MEDVAMC improved safety through a bundled strategy that reduced the risk-adjusted rate of surgical wound infections by 95%.12 MEDVAMC prioritized limb salvage when selecting patients for angiography and nearly eliminated using stent-grafts, cryopreserved allogeneic saphenous vein grafts, and expensive surgical and endovascular implants, which were identified as more expensive and less effective than other options (Figure 3).13-15 The MEDVAMC team achieved a > 90% patient trust rating on the Veterans Signals survey in fiscal years 2021 and 2022.

Challenges
A significant increase in the patient-physician ratio occurred 5 years into the program. In 2016, 2 vascular surgeons left MEDVAMC and a planned renovation of 1 of the 2 vascular surgery-assigned hybrid working facilities began even as the number of MEDVAMC patients with diabetes grew 120% (from 89,400 to 107,746 between 2010 and 2016), and the incidence rate of foot ulcers grew 300% (from 392 in 2010 to 1183 in 2016 per 100,000). The net result was a higher clinical workload among the remaining vascular surgeons with less operating room availability.
To stabilize surgeon retention, MEDVAMC reverted from the single team model back to inpatient care being distributed among general surgery, orthopedic surgery, and vascular surgery. After noting an increase in the leg amputation incidence rate, we adjusted the focus from multidisciplinary to interdisciplinary care (ie, majority of limb salvage clinical care can be provided by practitioners of any involved specialties). We worked to establish a local, written, interdisciplinary consensus on evaluating and managing veterans with nonhealing foot ulcers to mitigate the loss of a consolidated inpatient approach. Despite frequent staff turnover, ≥ 1 physician or surgeon from the core specialties of vascular surgery, podiatry, and infectious diseases remained throughout the study period.
The COVID-19 pandemic caused a shortage of hospital beds. This was followed by more bed shortages due to decreased nursing staff. Our health care system also had a period of restricted outpatient encounters early in the pandemic. During this time, we noted a delayed presentation of veterans with advanced infections and another increase in leg amputation incidence rate.
Like many health systems, MEDVAMC pivoted to telephone- and video-based outpatient encounters. Our team also used publicly available Texas hospitalization data to identify zip codes with particularly high leg amputation incidence rates, and > 3500 educational mailings to veterans categorized as moderate and high risk for leg amputation in these zip codes. These mailings provided information on recognizing foot ulcers and infections, emphasized timely evaluation, and named the MEDVAMC vascular surgery team as a point-of-contact. More recently, we have seen a further decrease in the MEDVAMC incidences of leg amputation to its lowest rate in > 20 years.
Discussion
A learning organization that directs its research based on clinical observations and informs its clinical care with research findings can produce palpable improvements in outcomes. Understanding the disease process and trying to better understand management across the entire range of this disease process has allowed our team to make consistent and systematic changes in care (Table). Consolidating inpatient care in a single team model seems to have been effective in reducing amputation rates among veterans with diabetes. The role the MEDVAMC vascular surgery team served for limb salvage patients may have been particularly beneficial because of the large impact untreated or unidentified PAD can have and because of the high prevalence of PAD among the limb salvage population seen at MEDVAMC. To be sustainable, though, a single-team model needs resources. A multiteam model can also be effective if the degree of multidisciplinary involvement for any given veteran is appropriate to the individual's clinical needs, teams are engaged and willing to contribute in a defined role within their specialty, and lines of communication remain open.

The primary challenge at MEDVAMC has been, and will continue to be, the retention of physicians and surgeons. MEDVAMC has excellent leadership and a collegial working environment, but better access to operating rooms for elective and time-sensitive operations, additional clinical staff support, and higher salary at non-VA positions have been the basis for many of physicians— especially surgeons—leaving MEDVAMC. Despite high staff turnover and a constant flow of resident and fellow trainees, MEDVAMC has been able to keep the clinical approach relatively consistent due to the use of written protocols and continuity of care as ≥ 1 physician or surgeon from each of the 4 main teams remained engaged with limb salvage throughout the entire period.
Going forward, we will work to ensure that all requirements of the 2022 Prevention of Amputation in Veterans Everywhere directive are incorporated into care.8 We plan to standardize MEDVAMC management algorithms further, both to streamline care and reduce the opportunity for disparities in treatment. More prophylactic podiatric procedures, surgical forms of offloading, and a shared multidisciplinary clinic space may also further help patients.
Conclusions
The introduction of multidisciplinary limb salvage at MEDVAMC has led to significant and sustained reductions in leg amputation incidence. These reductions do not seem dependent upon a specific team structure for inpatient care. To improve patient outcomes, efforts should focus on making improvements across the entire disease spectrum. For limb salvage, this includes primary prevention of foot ulcers, the treatment of foot infections, identification and management of PAD, surgical reconstruction/optimal wound healing, and care for patients who undergo leg amputation.
- Sanders LJ, Robbins JM, Edmonds ME. History of the team approach to amputation prevention: pioneers and milestones. J Am Podiatr Med Assoc. 2010;100(5):317- 334. doi:10.7547/1000317
- Sumpio BE, Armstrong DG, Lavery LA, Andros G. The role of interdisciplinary team approach in the management of the diabetic foot: a joint statement from the society for vascular surgery and the American podiatric medical association. J Am Podiatr Med Assoc. 2010;100(4):309-311. doi:10.7547/1000309
- About learning health systems. Agency for Healthcare Research and Quality. Published March 2019. Updated May 2019. Accessed October 9, 2024. https://www.ahrq.gov/learning-health-systems/about.html
- Barshes NR, Minc SD. Healthcare disparities in vascular surgery: a critical review. J Vasc Surg. 2021;74(2S):6S-14S.
- Barshes NR, Mindru C, Ashong C, Rodriguez-Barradas M, Trautner BW. Treatment failure and leg amputation among patients with foot osteomyelitis. Int J Low Extrem Wounds. 2016;15(4):303-312. doi:10.1177/1534734616661058
- Barshes NR, Flores E, Belkin M, Kougias P, Armstrong DG, Mills JL Sr. The accuracy and cost-effectiveness of strategies used to identify peripheral artery disease among patients with diabetic foot ulcers. J Vasc Surg. 2016;64(6):1682-1690.e3. doi:10.1016/j.jvs.2016.04.056 e1. doi:10.1016/j.jvs.2021.03.055
- Choi JC, Miranda J, Greenleaf E, et al. Lower-extremity pressure, staging, and grading thresholds to identify chronic limb-threatening ischemia. Vasc Med. 2023;28(1):45-53. doi:10.1177/1358863X221147945
- Barshes NR, Chambers JD, Cohen J, Belkin M; Model To Optimize Healthcare Value in Ischemic Extremities 1 (MOVIE) Study Collaborators. Cost-effectiveness in the contemporary management of critical limb ischemia with tissue loss. J Vasc Surg. 2012;56(4):1015-24.e1. doi:10.1016/j.jvs.2012.02.069
- Barshes NR, Bechara CF, Pisimisis G, Kougias P. Preliminary experiences with early primary closure of foot wounds after lower extremity revascularization. Ann Vasc Surg. 2014;28(1):48-52. doi:10.1016/j.avsg.2013.06.012
- Barshes NR, Gold B, Garcia A, Bechara CF, Pisimisis G, Kougias P. Minor amputation and palliative wound care as a strategy to avoid major amputation in patients with foot infections and severe peripheral arterial disease. Int J Low Extrem Wounds. 2014;13(3):211-219. doi:10.1177/1534734614543663
- Garcia M, Hernandez B, Ellington TG, et al. A lack of decline in major nontraumatic amputations in Texas: contemporary trends, risk factor associations, and impact of revascularization. Diabetes Care. 2019;42(6):1061-1066. doi:10.2337/dc19-0078
- Zamani N, Sharath SE, Vo E, Awad SS, Kougias P, Barshes NR. A multi-component strategy to decrease wound complications after open infra-inguinal re-vascularization. Surg Infect (Larchmt). 2018;19(1):87-94. doi:10.1089/sur.2017.193
- Barshes NR, Ozaki CK, Kougias P, Belkin M. A costeffectiveness analysis of infrainguinal bypass in the absence of great saphenous vein conduit. J Vasc Surg. 2013;57(6):1466-1470. doi:10.1016/j.jvs.2012.11.115
- Zamani N, Sharath S, Browder R, et al. PC158 longterm outcomes after endovascular stent placement for symptomatic, long-segment superficial femoral artery lesions. J Vasc Surg. 2017;65(6):182S-183S. doi:10.1016/j.jvs.2017.03.344
- Zamani N, Sharath SE, Browder RC, et al. Outcomes after endovascular stent placement for long-segment superficial femoral artery lesions. Ann Vasc Surg. 2021;71:298-307. doi:10.1016/j.avsg.2020.08.124
- Sanders LJ, Robbins JM, Edmonds ME. History of the team approach to amputation prevention: pioneers and milestones. J Am Podiatr Med Assoc. 2010;100(5):317- 334. doi:10.7547/1000317
- Sumpio BE, Armstrong DG, Lavery LA, Andros G. The role of interdisciplinary team approach in the management of the diabetic foot: a joint statement from the society for vascular surgery and the American podiatric medical association. J Am Podiatr Med Assoc. 2010;100(4):309-311. doi:10.7547/1000309
- About learning health systems. Agency for Healthcare Research and Quality. Published March 2019. Updated May 2019. Accessed October 9, 2024. https://www.ahrq.gov/learning-health-systems/about.html
- Barshes NR, Minc SD. Healthcare disparities in vascular surgery: a critical review. J Vasc Surg. 2021;74(2S):6S-14S.
- Barshes NR, Mindru C, Ashong C, Rodriguez-Barradas M, Trautner BW. Treatment failure and leg amputation among patients with foot osteomyelitis. Int J Low Extrem Wounds. 2016;15(4):303-312. doi:10.1177/1534734616661058
- Barshes NR, Flores E, Belkin M, Kougias P, Armstrong DG, Mills JL Sr. The accuracy and cost-effectiveness of strategies used to identify peripheral artery disease among patients with diabetic foot ulcers. J Vasc Surg. 2016;64(6):1682-1690.e3. doi:10.1016/j.jvs.2016.04.056 e1. doi:10.1016/j.jvs.2021.03.055
- Choi JC, Miranda J, Greenleaf E, et al. Lower-extremity pressure, staging, and grading thresholds to identify chronic limb-threatening ischemia. Vasc Med. 2023;28(1):45-53. doi:10.1177/1358863X221147945
- Barshes NR, Chambers JD, Cohen J, Belkin M; Model To Optimize Healthcare Value in Ischemic Extremities 1 (MOVIE) Study Collaborators. Cost-effectiveness in the contemporary management of critical limb ischemia with tissue loss. J Vasc Surg. 2012;56(4):1015-24.e1. doi:10.1016/j.jvs.2012.02.069
- Barshes NR, Bechara CF, Pisimisis G, Kougias P. Preliminary experiences with early primary closure of foot wounds after lower extremity revascularization. Ann Vasc Surg. 2014;28(1):48-52. doi:10.1016/j.avsg.2013.06.012
- Barshes NR, Gold B, Garcia A, Bechara CF, Pisimisis G, Kougias P. Minor amputation and palliative wound care as a strategy to avoid major amputation in patients with foot infections and severe peripheral arterial disease. Int J Low Extrem Wounds. 2014;13(3):211-219. doi:10.1177/1534734614543663
- Garcia M, Hernandez B, Ellington TG, et al. A lack of decline in major nontraumatic amputations in Texas: contemporary trends, risk factor associations, and impact of revascularization. Diabetes Care. 2019;42(6):1061-1066. doi:10.2337/dc19-0078
- Zamani N, Sharath SE, Vo E, Awad SS, Kougias P, Barshes NR. A multi-component strategy to decrease wound complications after open infra-inguinal re-vascularization. Surg Infect (Larchmt). 2018;19(1):87-94. doi:10.1089/sur.2017.193
- Barshes NR, Ozaki CK, Kougias P, Belkin M. A costeffectiveness analysis of infrainguinal bypass in the absence of great saphenous vein conduit. J Vasc Surg. 2013;57(6):1466-1470. doi:10.1016/j.jvs.2012.11.115
- Zamani N, Sharath S, Browder R, et al. PC158 longterm outcomes after endovascular stent placement for symptomatic, long-segment superficial femoral artery lesions. J Vasc Surg. 2017;65(6):182S-183S. doi:10.1016/j.jvs.2017.03.344
- Zamani N, Sharath SE, Browder RC, et al. Outcomes after endovascular stent placement for long-segment superficial femoral artery lesions. Ann Vasc Surg. 2021;71:298-307. doi:10.1016/j.avsg.2020.08.124
Evaluating Use of Empagliflozin for Diabetes Management in Veterans With Chronic Kidney Disease
More than 37 million Americans have diabetes mellitus (DM), and approximately 90% have type 2 DM (T2DM), including about 25% of veterans.1,2 The current guidelines suggest that therapy depends on a patient's comorbidities, management needs, and patient-centered treatment factors.3 About 1 in 3 adults with DM have chronic kidney disease (CKD), defined as the presence of kidney damage or an estimated glomerular filtration rate (eGFR) < 60 mL/min per 1.73 m2, persisting for ≥ 3 months.4
Sodium-glucose cotransporter-2 (SGLT-2) inhibitors are a class of antihyperglycemic agents acting on the SGLT-2 proteins expressed in the renal proximal convoluted tubules. They exert their effects by preventing the reabsorption of filtered glucose from the tubular lumen. There are 4 SGLT-2 inhibitors approved by the US Food and Drug Administration: canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin. Empagliflozin is currently the preferred SGLT-2 inhibitor on the US Department of Veterans Affairs (VA) formulary.
According to the American Diabetes Association guidelines, empagliflozin is considered when an individual has or is at risk for atherosclerotic cardiovascular disease, heart failure, and CKD.3 SGLT-2 inhibitors are a favorable option due to their low risk for hypoglycemia while also promoting weight loss. The EMPEROR-Reduced trial demonstrated that, in addition to benefits for patients with heart failure, empagliflozin also slowed the progressive decline in kidney function in those with and without DM.5 The purpose of this study was to evaluate the effectiveness of empagliflozin on hemoglobin A1c (HbA1c) levels in patients with CKD at the Hershel “Woody” Williams VA Medical Center (HWWVAMC) in Huntington, West Virginia, along with other laboratory test markers.
Methods
The Marshall University Institutional Review Board #1 (Medical) and the HWWVAMC institutional review board and research and development committee each reviewed and approved this study. A retrospective chart review was conducted on patients diagnosed with T2DM and stage 3 CKD who were prescribed empagliflozin for DM management between January 1, 2015, and October 1, 2022, yielding 1771 patients. Data were obtained through the VHA Corporate Data Warehouse (CDW) and stored on the VA Informatics and Computing Infrastructure (VINCI) research server.
Patients were included if they were aged 18 to 89 years, prescribed empagliflozin by a VA clinician for the treatment of T2DM, had an eGFR between 30 and 59 mL/min/1.73 m2, and had an initial HbA1c between 7% and 10%. Using further random sampling, patients were either excluded or divided into, those with stage 3a CKD and those with stage 3b CKD. The primary endpoint of this study was the change in HbA1c levels in patients with stage 3b CKD (eGFR 30-44 mL/min/1.73 m2) compared with stage 3a (eGFR 45-59 mL/min/1.73 m2) after 12 months. The secondary endpoints included effects on renal function, weight, blood pressure, incidence of adverse drug events, and cardiovascular events. Of the excluded, 38 had HbA1c < 7%, 30 had HbA1c ≥ 10%, 21 did not have data at 1-year mark, 15 had the medication discontinued due to decline in renal function, 14 discontinued their medication without documented reason, 10 discontinued their medication due to adverse drug reactions (ADRs), 12 had eGFR > 60 mL/ min/1.73 m2, 9 died within 1 year of initiation, 4 had eGFR < 30 mL/min/1.73 m2, 1 had no baseline eGFR, and 1 was the spouse of a veteran.
Statistical Analysis
All statistical analyses were performed using STATA v.15. We used t tests to examine changes within each group, along with paired t tests to compare the 2 groups. Two-sample t tests were used to analyze the continuous data at both the primary and secondary endpoints.
Results
Of the 1771 patients included in the initial data set, a randomized sample of 255 charts were reviewed, 155 were excluded, and 100 were included. Fifty patients, had stage 3a CKD and 50 had stage 3b CKD. Baseline demographics were similar between the stage 3a and 3b groups (Table 1). Both groups were predominantly White and male, with mean age > 70 years.
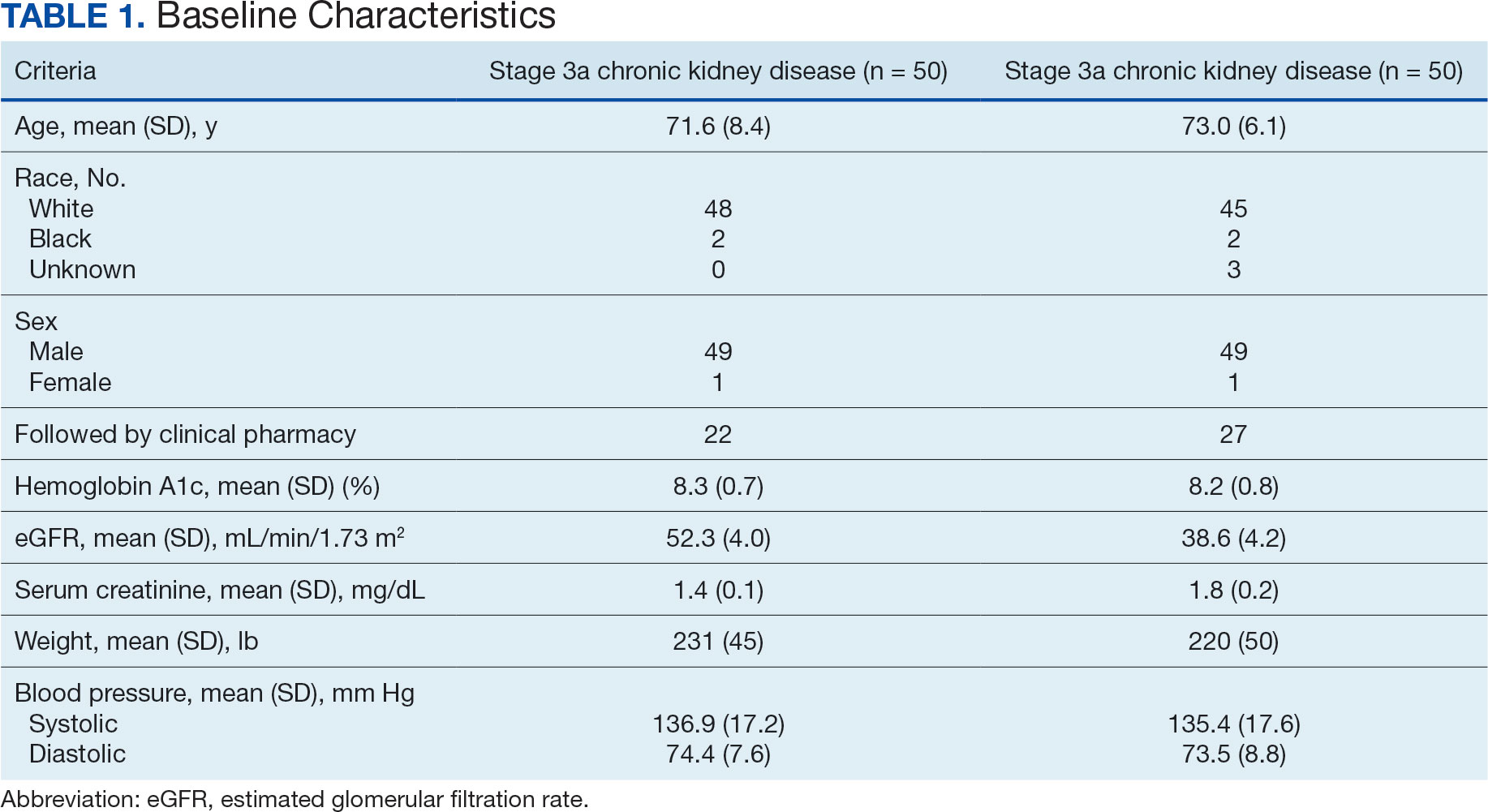
The primary endpoint was the differences in HbA1c levels over time and between groups for patients with stage 3a and stage 3b CKD 1 year after initiation of empagliflozin. The starting doses of empagliflozin were either 12.5 mg or 25.0 mg. For both groups, the changes in HbA1c levels were statistically significant (Table 2). HbA1c levels dropped 0.65% for the stage 3a group and 0.48% for the 3b group. When compared to one another, the results were not statistically significant (P = .51).
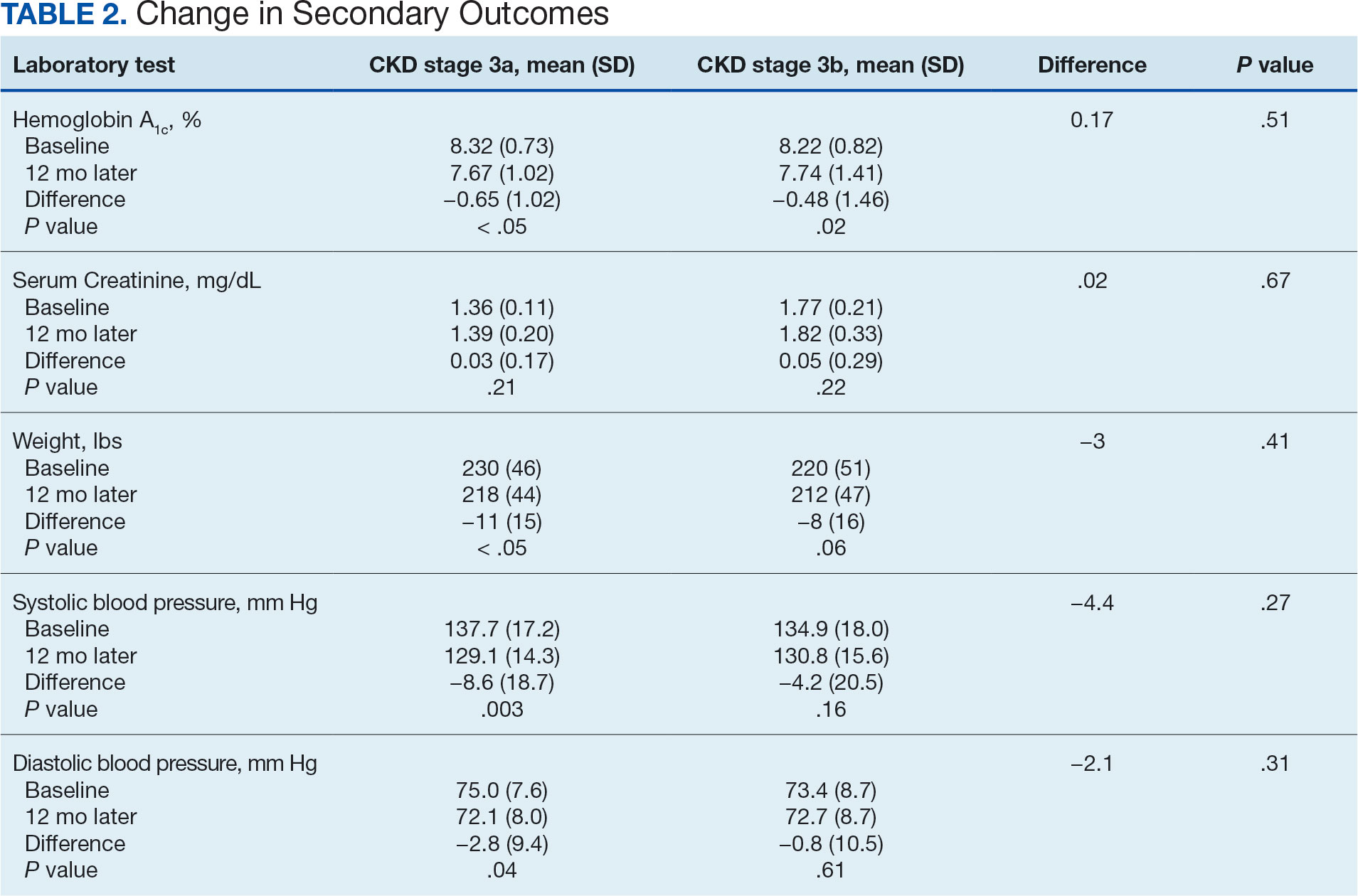
Secondary Endpoint
There was no statistically significant difference in serum creatinine levels within each group between baselines and 1 year later for the stage 3a (P = .21) and stage 3b (P = .22) groups, or when compared to each other (P = .67). There were statistically significant changes in weight for patients in the stage 3a group (P < .05), but not for stage 3b group (P = .06) or when compared to each other (P = .41). A statistically significant change in systolic blood pressure was observed for the stage 3a group (P = .003), but not the stage 3b group (P = .16) or when compared to each other (P = .27). There were statistically significant changes in diastolic blood pressure within the stage 3a group (P = .04), but not within the stage 3b group (P = .61) or when compared to each other (P = .31).
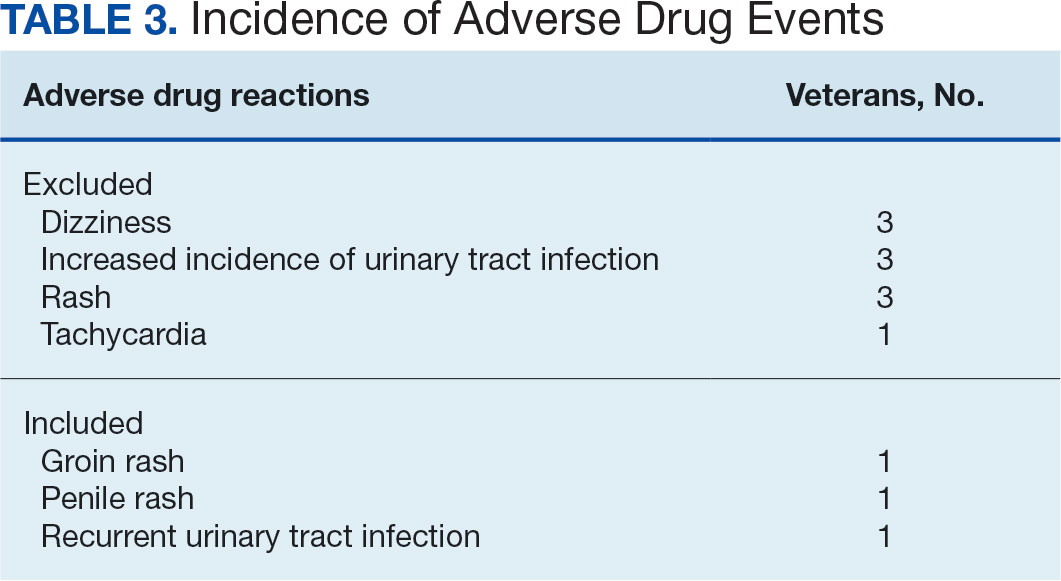
Ten patients discontinued empagliflozin before the 1-year mark due to ADRs, including dizziness, increased incidence of urinary tract infections, rash, and tachycardia (Table 3). Additionally, 3 ADRs resulted in the empagliflozin discontinuation after 1 year (Table 3).
Discussion
This study showed a statistically significant change in HbA1c levels for patients with stage 3a and stage 3b CKD. With eGFR levels in these 2 groups > 30 mL/min/1.73 m2, patients were able to achieve glycemic benefits. There were no significant changes to the serum creatinine levels. Both groups saw statistically significant changes in weight loss within their own group; however, there were no statistically significant changes when compared to each other. With both systolic and diastolic blood pressure, the stage 3a group had statistically significant changes.
The EMPA-REG BP study demonstrated that empagliflozin was associated with significant and clinically meaningful reductions in blood pressure and HbA1c levels compared with placebo and was well tolerated in patients with T2DM and hypertension.6,7,8
Limitations
This study had a retrospective study design, which resulted in missing information for many patients and higher rates of exclusion. The population was predominantly older, White, and male and may not reflect other populations. The starting doses of empagliflozin varied between the groups. The VA employs tablet splitting for some patients, and the available doses were either 10.0 mg, 12.5 mg, or 25.0 mg. Some prescribers start veterans at lower doses and gradually increase to the higher dose of 25.0 mg, adding to the variability in starting doses.
Patients with eGFR < 30 mL/min/1.73 m2 make it difficult to determine any potential benefit in this population. The EMPA-KIDNEY trial demonstrated that the benefits of empagliflozin treatment were consistent among patients with or without DM and regardless of eGFR at randomization.9 Furthermore, many veterans had an initial HbA1c levels outside the inclusion criteria range, which was a factor in the smaller sample size.
Conclusions
While the reduction in HbA1c levels was less in patients with stage 3b CKD compared to patients stage 3a CKD, all patients experienced a benefit. The overall incidence of ADRs was low in the study population, showing empagliflozin as a favorable choice for those with T2DM and CKD. Based on the findings of this study, empagliflozin is a potentially beneficial option for reducing HbA1c levels in patients with CKD.
- Centers for Disease Control and Prevention. Type 2 diabetes. Updated May 25, 2024. Accessed September 27, 2024. https://www.cdc.gov/diabetes/about/about-type-2-diabetes.html?CDC_AAref_Val
- US Department of Veterans Affairs, VA research on diabetes. Updated September 2019. Accessed September 27, 2024. https://www.research.va.gov/pubs/docs/va_factsheets/Diabetes.pdf
- American Diabetes Association. Standards of Medical Care in Diabetes-2022 Abridged for Primary Care Providers. Clin Diabetes. 2022;40(1):10-38. doi:10.2337/cd22-as01
- Centers for Disease Control and Prevention. Diabetes, chronic kidney disease. Updated May 15, 2024. Accessed September 27, 2024. https://www.cdc.gov/diabetes/diabetes-complications/diabetes-and-chronic-kidney-disease.html
- Packer M, Anker SD, Butler J, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020;383(15):1413-1424. doi:10.1056/NEJMoa2022190
- Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420-428. doi:10.2337/dc14-1096
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/NEJMoa1504720
- Chilton R, Tikkanen I, Cannon CP, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17(12):1180-1193. doi:10.1111/dom.12572
- The EMPA-KIDNEY Collaborative Group, Herrington WG, Staplin N, et al. Empagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2023;388(2):117-127. doi:10.1056/NEJMoa2204233
More than 37 million Americans have diabetes mellitus (DM), and approximately 90% have type 2 DM (T2DM), including about 25% of veterans.1,2 The current guidelines suggest that therapy depends on a patient's comorbidities, management needs, and patient-centered treatment factors.3 About 1 in 3 adults with DM have chronic kidney disease (CKD), defined as the presence of kidney damage or an estimated glomerular filtration rate (eGFR) < 60 mL/min per 1.73 m2, persisting for ≥ 3 months.4
Sodium-glucose cotransporter-2 (SGLT-2) inhibitors are a class of antihyperglycemic agents acting on the SGLT-2 proteins expressed in the renal proximal convoluted tubules. They exert their effects by preventing the reabsorption of filtered glucose from the tubular lumen. There are 4 SGLT-2 inhibitors approved by the US Food and Drug Administration: canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin. Empagliflozin is currently the preferred SGLT-2 inhibitor on the US Department of Veterans Affairs (VA) formulary.
According to the American Diabetes Association guidelines, empagliflozin is considered when an individual has or is at risk for atherosclerotic cardiovascular disease, heart failure, and CKD.3 SGLT-2 inhibitors are a favorable option due to their low risk for hypoglycemia while also promoting weight loss. The EMPEROR-Reduced trial demonstrated that, in addition to benefits for patients with heart failure, empagliflozin also slowed the progressive decline in kidney function in those with and without DM.5 The purpose of this study was to evaluate the effectiveness of empagliflozin on hemoglobin A1c (HbA1c) levels in patients with CKD at the Hershel “Woody” Williams VA Medical Center (HWWVAMC) in Huntington, West Virginia, along with other laboratory test markers.
Methods
The Marshall University Institutional Review Board #1 (Medical) and the HWWVAMC institutional review board and research and development committee each reviewed and approved this study. A retrospective chart review was conducted on patients diagnosed with T2DM and stage 3 CKD who were prescribed empagliflozin for DM management between January 1, 2015, and October 1, 2022, yielding 1771 patients. Data were obtained through the VHA Corporate Data Warehouse (CDW) and stored on the VA Informatics and Computing Infrastructure (VINCI) research server.
Patients were included if they were aged 18 to 89 years, prescribed empagliflozin by a VA clinician for the treatment of T2DM, had an eGFR between 30 and 59 mL/min/1.73 m2, and had an initial HbA1c between 7% and 10%. Using further random sampling, patients were either excluded or divided into, those with stage 3a CKD and those with stage 3b CKD. The primary endpoint of this study was the change in HbA1c levels in patients with stage 3b CKD (eGFR 30-44 mL/min/1.73 m2) compared with stage 3a (eGFR 45-59 mL/min/1.73 m2) after 12 months. The secondary endpoints included effects on renal function, weight, blood pressure, incidence of adverse drug events, and cardiovascular events. Of the excluded, 38 had HbA1c < 7%, 30 had HbA1c ≥ 10%, 21 did not have data at 1-year mark, 15 had the medication discontinued due to decline in renal function, 14 discontinued their medication without documented reason, 10 discontinued their medication due to adverse drug reactions (ADRs), 12 had eGFR > 60 mL/ min/1.73 m2, 9 died within 1 year of initiation, 4 had eGFR < 30 mL/min/1.73 m2, 1 had no baseline eGFR, and 1 was the spouse of a veteran.
Statistical Analysis
All statistical analyses were performed using STATA v.15. We used t tests to examine changes within each group, along with paired t tests to compare the 2 groups. Two-sample t tests were used to analyze the continuous data at both the primary and secondary endpoints.
Results
Of the 1771 patients included in the initial data set, a randomized sample of 255 charts were reviewed, 155 were excluded, and 100 were included. Fifty patients, had stage 3a CKD and 50 had stage 3b CKD. Baseline demographics were similar between the stage 3a and 3b groups (Table 1). Both groups were predominantly White and male, with mean age > 70 years.

The primary endpoint was the differences in HbA1c levels over time and between groups for patients with stage 3a and stage 3b CKD 1 year after initiation of empagliflozin. The starting doses of empagliflozin were either 12.5 mg or 25.0 mg. For both groups, the changes in HbA1c levels were statistically significant (Table 2). HbA1c levels dropped 0.65% for the stage 3a group and 0.48% for the 3b group. When compared to one another, the results were not statistically significant (P = .51).

Secondary Endpoint
There was no statistically significant difference in serum creatinine levels within each group between baselines and 1 year later for the stage 3a (P = .21) and stage 3b (P = .22) groups, or when compared to each other (P = .67). There were statistically significant changes in weight for patients in the stage 3a group (P < .05), but not for stage 3b group (P = .06) or when compared to each other (P = .41). A statistically significant change in systolic blood pressure was observed for the stage 3a group (P = .003), but not the stage 3b group (P = .16) or when compared to each other (P = .27). There were statistically significant changes in diastolic blood pressure within the stage 3a group (P = .04), but not within the stage 3b group (P = .61) or when compared to each other (P = .31).
Ten patients discontinued empagliflozin before the 1-year mark due to ADRs, including dizziness, increased incidence of urinary tract infections, rash, and tachycardia (Table 3). Additionally, 3 ADRs resulted in the empagliflozin discontinuation after 1 year (Table 3).

Discussion
This study showed a statistically significant change in HbA1c levels for patients with stage 3a and stage 3b CKD. With eGFR levels in these 2 groups > 30 mL/min/1.73 m2, patients were able to achieve glycemic benefits. There were no significant changes to the serum creatinine levels. Both groups saw statistically significant changes in weight loss within their own group; however, there were no statistically significant changes when compared to each other. With both systolic and diastolic blood pressure, the stage 3a group had statistically significant changes.
The EMPA-REG BP study demonstrated that empagliflozin was associated with significant and clinically meaningful reductions in blood pressure and HbA1c levels compared with placebo and was well tolerated in patients with T2DM and hypertension.6,7,8
Limitations
This study had a retrospective study design, which resulted in missing information for many patients and higher rates of exclusion. The population was predominantly older, White, and male and may not reflect other populations. The starting doses of empagliflozin varied between the groups. The VA employs tablet splitting for some patients, and the available doses were either 10.0 mg, 12.5 mg, or 25.0 mg. Some prescribers start veterans at lower doses and gradually increase to the higher dose of 25.0 mg, adding to the variability in starting doses.
Patients with eGFR < 30 mL/min/1.73 m2 make it difficult to determine any potential benefit in this population. The EMPA-KIDNEY trial demonstrated that the benefits of empagliflozin treatment were consistent among patients with or without DM and regardless of eGFR at randomization.9 Furthermore, many veterans had an initial HbA1c levels outside the inclusion criteria range, which was a factor in the smaller sample size.
Conclusions
While the reduction in HbA1c levels was less in patients with stage 3b CKD compared to patients stage 3a CKD, all patients experienced a benefit. The overall incidence of ADRs was low in the study population, showing empagliflozin as a favorable choice for those with T2DM and CKD. Based on the findings of this study, empagliflozin is a potentially beneficial option for reducing HbA1c levels in patients with CKD.
More than 37 million Americans have diabetes mellitus (DM), and approximately 90% have type 2 DM (T2DM), including about 25% of veterans.1,2 The current guidelines suggest that therapy depends on a patient's comorbidities, management needs, and patient-centered treatment factors.3 About 1 in 3 adults with DM have chronic kidney disease (CKD), defined as the presence of kidney damage or an estimated glomerular filtration rate (eGFR) < 60 mL/min per 1.73 m2, persisting for ≥ 3 months.4
Sodium-glucose cotransporter-2 (SGLT-2) inhibitors are a class of antihyperglycemic agents acting on the SGLT-2 proteins expressed in the renal proximal convoluted tubules. They exert their effects by preventing the reabsorption of filtered glucose from the tubular lumen. There are 4 SGLT-2 inhibitors approved by the US Food and Drug Administration: canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin. Empagliflozin is currently the preferred SGLT-2 inhibitor on the US Department of Veterans Affairs (VA) formulary.
According to the American Diabetes Association guidelines, empagliflozin is considered when an individual has or is at risk for atherosclerotic cardiovascular disease, heart failure, and CKD.3 SGLT-2 inhibitors are a favorable option due to their low risk for hypoglycemia while also promoting weight loss. The EMPEROR-Reduced trial demonstrated that, in addition to benefits for patients with heart failure, empagliflozin also slowed the progressive decline in kidney function in those with and without DM.5 The purpose of this study was to evaluate the effectiveness of empagliflozin on hemoglobin A1c (HbA1c) levels in patients with CKD at the Hershel “Woody” Williams VA Medical Center (HWWVAMC) in Huntington, West Virginia, along with other laboratory test markers.
Methods
The Marshall University Institutional Review Board #1 (Medical) and the HWWVAMC institutional review board and research and development committee each reviewed and approved this study. A retrospective chart review was conducted on patients diagnosed with T2DM and stage 3 CKD who were prescribed empagliflozin for DM management between January 1, 2015, and October 1, 2022, yielding 1771 patients. Data were obtained through the VHA Corporate Data Warehouse (CDW) and stored on the VA Informatics and Computing Infrastructure (VINCI) research server.
Patients were included if they were aged 18 to 89 years, prescribed empagliflozin by a VA clinician for the treatment of T2DM, had an eGFR between 30 and 59 mL/min/1.73 m2, and had an initial HbA1c between 7% and 10%. Using further random sampling, patients were either excluded or divided into, those with stage 3a CKD and those with stage 3b CKD. The primary endpoint of this study was the change in HbA1c levels in patients with stage 3b CKD (eGFR 30-44 mL/min/1.73 m2) compared with stage 3a (eGFR 45-59 mL/min/1.73 m2) after 12 months. The secondary endpoints included effects on renal function, weight, blood pressure, incidence of adverse drug events, and cardiovascular events. Of the excluded, 38 had HbA1c < 7%, 30 had HbA1c ≥ 10%, 21 did not have data at 1-year mark, 15 had the medication discontinued due to decline in renal function, 14 discontinued their medication without documented reason, 10 discontinued their medication due to adverse drug reactions (ADRs), 12 had eGFR > 60 mL/ min/1.73 m2, 9 died within 1 year of initiation, 4 had eGFR < 30 mL/min/1.73 m2, 1 had no baseline eGFR, and 1 was the spouse of a veteran.
Statistical Analysis
All statistical analyses were performed using STATA v.15. We used t tests to examine changes within each group, along with paired t tests to compare the 2 groups. Two-sample t tests were used to analyze the continuous data at both the primary and secondary endpoints.
Results
Of the 1771 patients included in the initial data set, a randomized sample of 255 charts were reviewed, 155 were excluded, and 100 were included. Fifty patients, had stage 3a CKD and 50 had stage 3b CKD. Baseline demographics were similar between the stage 3a and 3b groups (Table 1). Both groups were predominantly White and male, with mean age > 70 years.

The primary endpoint was the differences in HbA1c levels over time and between groups for patients with stage 3a and stage 3b CKD 1 year after initiation of empagliflozin. The starting doses of empagliflozin were either 12.5 mg or 25.0 mg. For both groups, the changes in HbA1c levels were statistically significant (Table 2). HbA1c levels dropped 0.65% for the stage 3a group and 0.48% for the 3b group. When compared to one another, the results were not statistically significant (P = .51).

Secondary Endpoint
There was no statistically significant difference in serum creatinine levels within each group between baselines and 1 year later for the stage 3a (P = .21) and stage 3b (P = .22) groups, or when compared to each other (P = .67). There were statistically significant changes in weight for patients in the stage 3a group (P < .05), but not for stage 3b group (P = .06) or when compared to each other (P = .41). A statistically significant change in systolic blood pressure was observed for the stage 3a group (P = .003), but not the stage 3b group (P = .16) or when compared to each other (P = .27). There were statistically significant changes in diastolic blood pressure within the stage 3a group (P = .04), but not within the stage 3b group (P = .61) or when compared to each other (P = .31).
Ten patients discontinued empagliflozin before the 1-year mark due to ADRs, including dizziness, increased incidence of urinary tract infections, rash, and tachycardia (Table 3). Additionally, 3 ADRs resulted in the empagliflozin discontinuation after 1 year (Table 3).

Discussion
This study showed a statistically significant change in HbA1c levels for patients with stage 3a and stage 3b CKD. With eGFR levels in these 2 groups > 30 mL/min/1.73 m2, patients were able to achieve glycemic benefits. There were no significant changes to the serum creatinine levels. Both groups saw statistically significant changes in weight loss within their own group; however, there were no statistically significant changes when compared to each other. With both systolic and diastolic blood pressure, the stage 3a group had statistically significant changes.
The EMPA-REG BP study demonstrated that empagliflozin was associated with significant and clinically meaningful reductions in blood pressure and HbA1c levels compared with placebo and was well tolerated in patients with T2DM and hypertension.6,7,8
Limitations
This study had a retrospective study design, which resulted in missing information for many patients and higher rates of exclusion. The population was predominantly older, White, and male and may not reflect other populations. The starting doses of empagliflozin varied between the groups. The VA employs tablet splitting for some patients, and the available doses were either 10.0 mg, 12.5 mg, or 25.0 mg. Some prescribers start veterans at lower doses and gradually increase to the higher dose of 25.0 mg, adding to the variability in starting doses.
Patients with eGFR < 30 mL/min/1.73 m2 make it difficult to determine any potential benefit in this population. The EMPA-KIDNEY trial demonstrated that the benefits of empagliflozin treatment were consistent among patients with or without DM and regardless of eGFR at randomization.9 Furthermore, many veterans had an initial HbA1c levels outside the inclusion criteria range, which was a factor in the smaller sample size.
Conclusions
While the reduction in HbA1c levels was less in patients with stage 3b CKD compared to patients stage 3a CKD, all patients experienced a benefit. The overall incidence of ADRs was low in the study population, showing empagliflozin as a favorable choice for those with T2DM and CKD. Based on the findings of this study, empagliflozin is a potentially beneficial option for reducing HbA1c levels in patients with CKD.
- Centers for Disease Control and Prevention. Type 2 diabetes. Updated May 25, 2024. Accessed September 27, 2024. https://www.cdc.gov/diabetes/about/about-type-2-diabetes.html?CDC_AAref_Val
- US Department of Veterans Affairs, VA research on diabetes. Updated September 2019. Accessed September 27, 2024. https://www.research.va.gov/pubs/docs/va_factsheets/Diabetes.pdf
- American Diabetes Association. Standards of Medical Care in Diabetes-2022 Abridged for Primary Care Providers. Clin Diabetes. 2022;40(1):10-38. doi:10.2337/cd22-as01
- Centers for Disease Control and Prevention. Diabetes, chronic kidney disease. Updated May 15, 2024. Accessed September 27, 2024. https://www.cdc.gov/diabetes/diabetes-complications/diabetes-and-chronic-kidney-disease.html
- Packer M, Anker SD, Butler J, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020;383(15):1413-1424. doi:10.1056/NEJMoa2022190
- Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420-428. doi:10.2337/dc14-1096
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/NEJMoa1504720
- Chilton R, Tikkanen I, Cannon CP, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17(12):1180-1193. doi:10.1111/dom.12572
- The EMPA-KIDNEY Collaborative Group, Herrington WG, Staplin N, et al. Empagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2023;388(2):117-127. doi:10.1056/NEJMoa2204233
- Centers for Disease Control and Prevention. Type 2 diabetes. Updated May 25, 2024. Accessed September 27, 2024. https://www.cdc.gov/diabetes/about/about-type-2-diabetes.html?CDC_AAref_Val
- US Department of Veterans Affairs, VA research on diabetes. Updated September 2019. Accessed September 27, 2024. https://www.research.va.gov/pubs/docs/va_factsheets/Diabetes.pdf
- American Diabetes Association. Standards of Medical Care in Diabetes-2022 Abridged for Primary Care Providers. Clin Diabetes. 2022;40(1):10-38. doi:10.2337/cd22-as01
- Centers for Disease Control and Prevention. Diabetes, chronic kidney disease. Updated May 15, 2024. Accessed September 27, 2024. https://www.cdc.gov/diabetes/diabetes-complications/diabetes-and-chronic-kidney-disease.html
- Packer M, Anker SD, Butler J, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020;383(15):1413-1424. doi:10.1056/NEJMoa2022190
- Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420-428. doi:10.2337/dc14-1096
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/NEJMoa1504720
- Chilton R, Tikkanen I, Cannon CP, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17(12):1180-1193. doi:10.1111/dom.12572
- The EMPA-KIDNEY Collaborative Group, Herrington WG, Staplin N, et al. Empagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2023;388(2):117-127. doi:10.1056/NEJMoa2204233
Patients With IBD More Likely to Develop, or Have Prior, T1D
VIENNA —
Their findings showed that patients with IBD had a moderately increased risk for T1D and higher odds of having prior T1D than the general population. These bidirectional associations were partially independent of shared familial factors.
Although the absolute risk for T1D is low in patients with IBD, these findings suggest that if there are nonspecific symptoms, such as weight loss and fatigue, which are typical of T1D but not of IBD, then it might be reasonable to test for diabetes, lead researcher Jiangwei Sun, PhD, postdoctoral researcher at the Karolinska Institutet, Stockholm, Sweden, told this news organization.
“Patients with IBD and T1D also tend to have worse disease outcomes for both diseases, but these two diseases are not recognized as comorbidities in the clinical guidelines,” he said.
Anecdotally, “many clinicians believe there is a higher risk of autoimmune disease in patients with IBD but not much attention is paid to type 1 diabetes,” he added.
Sun presented the study at United European Gastroenterology (UEG) Week 2024. It was also published recently in The Lancet.
Exploring the Bidirectional Relationship
Prior research in the form of a systematic review found no association between IBD and T1D, which was surprising, Sun said. Further studies found an association between IBD and incident T1D; however, these studies did not explore bidirectionality between the two diseases.
These studies also did not take shared genetic and environmental factors into consideration, though “there is known to be familial co-aggregation of IBD and T1D based on previous findings,” he said.
In this current study, Sun and colleagues compared patients with IBD with the general population, as well as with siblings without IBD to consider the potential influence of shared genetics and earlier environmental factors.
The research used two approaches to look for a bidirectional association: A nationwide matched cohort study (IBD and incident T1D) and a case-control study (IBD and prior T1D).
The cohort study included 20,314 patients with IBD aged ≤ 28 years, who were identified between 1987 and 2017. Of these, 7277 had Crohn’s disease, 10,112 had ulcerative colitis, and 2925 had unclassified IBD. There were 99,200 individually matched reference individuals.
The case-control study included 87,001 patients with IBD (without age restriction) and 431,054 matched control individuals.
Risk ratios were calculated using an adjusted hazard ratio (aHR) of incident T1D in the cohort study and an adjusted odds ratio (aOR) of prior T1D in the case-control study.
In the cohort study, the median follow-up was 14 years. Over that time, 116 patients with IBD and 353 reference individuals developed T1D. The aHR for a patient with IBD developing T1D was 1.58 (95% CI, 1.27-1.95). For patients with ulcerative colitis, the aHR of developing T1D increased to 2.02 (95% CI, 1.51-2.70); however, the association was not found for Crohn’s disease or unclassified IBD possibly because of the sample size of these latter categories, noted Sun.
In the case-control study, Sun and colleagues identified 1018 (1.2%) patients with IBD and 3496 (0.8%) control individuals who had been previously diagnosed with T1D. Patients with IBD had higher odds of having prior T1D than those without IBD (aOR, 1.36; 95% CI, 1.26-1.46). This positive association was observed in all IBD subtypes, said Sun, who added that the sample size was larger in this analysis than in the cohort analysis.
Upon comparing patients with IBD with their siblings without IBD, analyses showed similar associations between IBD and T1D; the aHR was 1.44 (95% CI, 0.97-2.15) for developing T1D, and the aOR was 1.32 (95% CI, 1.18-1.49) for prior T1D.
That these positive associations between IBD and T1D exist even when comparing patients with IBD with their siblings without IBD suggests genetics and shared environmental factors do not fully explain the association, and that later environmental factors might play a role, said Sun.
“I’m not surprised with these results,” he added. “They make sense because we know that both IBD and T1D are immunity-related diseases and have some shared pathways.”
Commenting on the study, Tine Jess, MD, director, Center for Molecular Prediction of Inflammatory Bowel Disease, PREDICT, Aalborg University in Copenhagen, Denmark, said: “The really interesting finding here is that type 1 diabetes may precede IBD, which points toward common etiologies rather than one disease leading to the other.”
“This is in line with mounting evidence that IBD is measurable at the molecular level years prior to diagnosis,” she added.
Awareness of the bidirectional association may facilitate early detection of both conditions, Sun and his colleagues noted.
Sun reported no relevant financial relationships. Jess reported receiving consultancy fees from Ferring and Pfizer.
A version of this article appeared on Medscape.com.
VIENNA —
Their findings showed that patients with IBD had a moderately increased risk for T1D and higher odds of having prior T1D than the general population. These bidirectional associations were partially independent of shared familial factors.
Although the absolute risk for T1D is low in patients with IBD, these findings suggest that if there are nonspecific symptoms, such as weight loss and fatigue, which are typical of T1D but not of IBD, then it might be reasonable to test for diabetes, lead researcher Jiangwei Sun, PhD, postdoctoral researcher at the Karolinska Institutet, Stockholm, Sweden, told this news organization.
“Patients with IBD and T1D also tend to have worse disease outcomes for both diseases, but these two diseases are not recognized as comorbidities in the clinical guidelines,” he said.
Anecdotally, “many clinicians believe there is a higher risk of autoimmune disease in patients with IBD but not much attention is paid to type 1 diabetes,” he added.
Sun presented the study at United European Gastroenterology (UEG) Week 2024. It was also published recently in The Lancet.
Exploring the Bidirectional Relationship
Prior research in the form of a systematic review found no association between IBD and T1D, which was surprising, Sun said. Further studies found an association between IBD and incident T1D; however, these studies did not explore bidirectionality between the two diseases.
These studies also did not take shared genetic and environmental factors into consideration, though “there is known to be familial co-aggregation of IBD and T1D based on previous findings,” he said.
In this current study, Sun and colleagues compared patients with IBD with the general population, as well as with siblings without IBD to consider the potential influence of shared genetics and earlier environmental factors.
The research used two approaches to look for a bidirectional association: A nationwide matched cohort study (IBD and incident T1D) and a case-control study (IBD and prior T1D).
The cohort study included 20,314 patients with IBD aged ≤ 28 years, who were identified between 1987 and 2017. Of these, 7277 had Crohn’s disease, 10,112 had ulcerative colitis, and 2925 had unclassified IBD. There were 99,200 individually matched reference individuals.
The case-control study included 87,001 patients with IBD (without age restriction) and 431,054 matched control individuals.
Risk ratios were calculated using an adjusted hazard ratio (aHR) of incident T1D in the cohort study and an adjusted odds ratio (aOR) of prior T1D in the case-control study.
In the cohort study, the median follow-up was 14 years. Over that time, 116 patients with IBD and 353 reference individuals developed T1D. The aHR for a patient with IBD developing T1D was 1.58 (95% CI, 1.27-1.95). For patients with ulcerative colitis, the aHR of developing T1D increased to 2.02 (95% CI, 1.51-2.70); however, the association was not found for Crohn’s disease or unclassified IBD possibly because of the sample size of these latter categories, noted Sun.
In the case-control study, Sun and colleagues identified 1018 (1.2%) patients with IBD and 3496 (0.8%) control individuals who had been previously diagnosed with T1D. Patients with IBD had higher odds of having prior T1D than those without IBD (aOR, 1.36; 95% CI, 1.26-1.46). This positive association was observed in all IBD subtypes, said Sun, who added that the sample size was larger in this analysis than in the cohort analysis.
Upon comparing patients with IBD with their siblings without IBD, analyses showed similar associations between IBD and T1D; the aHR was 1.44 (95% CI, 0.97-2.15) for developing T1D, and the aOR was 1.32 (95% CI, 1.18-1.49) for prior T1D.
That these positive associations between IBD and T1D exist even when comparing patients with IBD with their siblings without IBD suggests genetics and shared environmental factors do not fully explain the association, and that later environmental factors might play a role, said Sun.
“I’m not surprised with these results,” he added. “They make sense because we know that both IBD and T1D are immunity-related diseases and have some shared pathways.”
Commenting on the study, Tine Jess, MD, director, Center for Molecular Prediction of Inflammatory Bowel Disease, PREDICT, Aalborg University in Copenhagen, Denmark, said: “The really interesting finding here is that type 1 diabetes may precede IBD, which points toward common etiologies rather than one disease leading to the other.”
“This is in line with mounting evidence that IBD is measurable at the molecular level years prior to diagnosis,” she added.
Awareness of the bidirectional association may facilitate early detection of both conditions, Sun and his colleagues noted.
Sun reported no relevant financial relationships. Jess reported receiving consultancy fees from Ferring and Pfizer.
A version of this article appeared on Medscape.com.
VIENNA —
Their findings showed that patients with IBD had a moderately increased risk for T1D and higher odds of having prior T1D than the general population. These bidirectional associations were partially independent of shared familial factors.
Although the absolute risk for T1D is low in patients with IBD, these findings suggest that if there are nonspecific symptoms, such as weight loss and fatigue, which are typical of T1D but not of IBD, then it might be reasonable to test for diabetes, lead researcher Jiangwei Sun, PhD, postdoctoral researcher at the Karolinska Institutet, Stockholm, Sweden, told this news organization.
“Patients with IBD and T1D also tend to have worse disease outcomes for both diseases, but these two diseases are not recognized as comorbidities in the clinical guidelines,” he said.
Anecdotally, “many clinicians believe there is a higher risk of autoimmune disease in patients with IBD but not much attention is paid to type 1 diabetes,” he added.
Sun presented the study at United European Gastroenterology (UEG) Week 2024. It was also published recently in The Lancet.
Exploring the Bidirectional Relationship
Prior research in the form of a systematic review found no association between IBD and T1D, which was surprising, Sun said. Further studies found an association between IBD and incident T1D; however, these studies did not explore bidirectionality between the two diseases.
These studies also did not take shared genetic and environmental factors into consideration, though “there is known to be familial co-aggregation of IBD and T1D based on previous findings,” he said.
In this current study, Sun and colleagues compared patients with IBD with the general population, as well as with siblings without IBD to consider the potential influence of shared genetics and earlier environmental factors.
The research used two approaches to look for a bidirectional association: A nationwide matched cohort study (IBD and incident T1D) and a case-control study (IBD and prior T1D).
The cohort study included 20,314 patients with IBD aged ≤ 28 years, who were identified between 1987 and 2017. Of these, 7277 had Crohn’s disease, 10,112 had ulcerative colitis, and 2925 had unclassified IBD. There were 99,200 individually matched reference individuals.
The case-control study included 87,001 patients with IBD (without age restriction) and 431,054 matched control individuals.
Risk ratios were calculated using an adjusted hazard ratio (aHR) of incident T1D in the cohort study and an adjusted odds ratio (aOR) of prior T1D in the case-control study.
In the cohort study, the median follow-up was 14 years. Over that time, 116 patients with IBD and 353 reference individuals developed T1D. The aHR for a patient with IBD developing T1D was 1.58 (95% CI, 1.27-1.95). For patients with ulcerative colitis, the aHR of developing T1D increased to 2.02 (95% CI, 1.51-2.70); however, the association was not found for Crohn’s disease or unclassified IBD possibly because of the sample size of these latter categories, noted Sun.
In the case-control study, Sun and colleagues identified 1018 (1.2%) patients with IBD and 3496 (0.8%) control individuals who had been previously diagnosed with T1D. Patients with IBD had higher odds of having prior T1D than those without IBD (aOR, 1.36; 95% CI, 1.26-1.46). This positive association was observed in all IBD subtypes, said Sun, who added that the sample size was larger in this analysis than in the cohort analysis.
Upon comparing patients with IBD with their siblings without IBD, analyses showed similar associations between IBD and T1D; the aHR was 1.44 (95% CI, 0.97-2.15) for developing T1D, and the aOR was 1.32 (95% CI, 1.18-1.49) for prior T1D.
That these positive associations between IBD and T1D exist even when comparing patients with IBD with their siblings without IBD suggests genetics and shared environmental factors do not fully explain the association, and that later environmental factors might play a role, said Sun.
“I’m not surprised with these results,” he added. “They make sense because we know that both IBD and T1D are immunity-related diseases and have some shared pathways.”
Commenting on the study, Tine Jess, MD, director, Center for Molecular Prediction of Inflammatory Bowel Disease, PREDICT, Aalborg University in Copenhagen, Denmark, said: “The really interesting finding here is that type 1 diabetes may precede IBD, which points toward common etiologies rather than one disease leading to the other.”
“This is in line with mounting evidence that IBD is measurable at the molecular level years prior to diagnosis,” she added.
Awareness of the bidirectional association may facilitate early detection of both conditions, Sun and his colleagues noted.
Sun reported no relevant financial relationships. Jess reported receiving consultancy fees from Ferring and Pfizer.
A version of this article appeared on Medscape.com.
FROM UEG 2024
Coming Soon: A New Disease Definition, ‘Clinical Obesity’
SAN ANTONIO, TEXAS —
The authors of the new framework are a Lancet Commission of 56 of the world’s leading obesity experts, including academic clinicians, scientists, public health experts, patient representatives, and officers from the World Health Organization. Following peer review, it will be launched via livestream and published in Lancet Diabetes & Endocrinology in mid-January 2025, with formal endorsement from more than 75 medical societies and other relevant stakeholder organizations.
On November 4, 2024, at the Obesity Society’s Obesity Week meeting, the publication’s lead author, Francesco Rubino, MD, Chair of Bariatric and Metabolic Surgery at King’s College London in England, gave a preview. He began by noting that, despite the declaration of obesity as a chronic disease over a decade ago, the concept is still debated and not widely accepted by the public or even by all in the medical community.
“The idea of obesity as a disease remains highly controversial,” Rubino noted, adding that the current body mass index (BMI)–based definition contributes to this because it doesn’t distinguish between people whose excess adiposity place them at excess risk for disease but they’re currently healthy vs those who already have undergone bodily harm from that adiposity.
“Having a framework that distinguishes at an individual level when you are in a condition of risk and when you have a condition of disease is fundamentally important. You don’t want to blur the picture in either direction, because obviously the consequence would be quite significant. ... So, the commission focused exactly on that point,” he said.
The new paper will propose a two-part clinical approach: First, assess whether the patient has excess adiposity, with methods that will be outlined. Next, assess on an organ-by-organ basis for the presence of abnormalities related to excess adiposity, or “clinical obesity.” The document will also provide those specific criteria, Rubino said, noting that those details are under embargo until January.
However, he did say that “We are going to propose a pragmatic approach to say that BMI alone is not enough in the clinic. It’s okay as a screening tool, but when somebody potentially has obesity, then you have to add additional measures of adiposity that makes sure you decrease the level of risk… Once you have obesity, then you need to establish if it’s clinical or nonclinical.”
Asked to comment, session moderator John D. Clark, MD, PhD, Chief Population Health Officer at Sharp Rees-Stealy Medical Group, San Diego, California, said in an interview, “I think it’ll help explain and move medicine as a whole in a direction to a greater understanding of obesity actually being a disease, how to define it, and how to identify it. And will, I think, lead to a greater understanding of the underlying disease.”
And, Clark said, it should also help target individuals with preventive vs therapeutic approaches. “I would describe it as matching the right tool to the right patient. If a person has clinical obesity, they likely can and would benefit from either different or additional tools, as opposed to otherwise healthy obesity.”
Rubino said he hopes the new framework will prompt improvements in reimbursement and public policy. “Policymakers scratch their heads when they have limited resources and you need to prioritize things. Having an obesity definition that is blurry doesn’t allow you to have a fair, human, and meaningful prioritization. ... Now that we have drugs that cannot be given to 100% of people, how do you decide who gets them first? I hope this will make it easier for people to access treatment. At the moment, it is not only difficult, but it’s also unfair. It’s random. Somebody gets access, while somebody else who is very, very sick has no access. I don’t think that’s what we want.”
A version of this article appeared on Medscape.com.
SAN ANTONIO, TEXAS —
The authors of the new framework are a Lancet Commission of 56 of the world’s leading obesity experts, including academic clinicians, scientists, public health experts, patient representatives, and officers from the World Health Organization. Following peer review, it will be launched via livestream and published in Lancet Diabetes & Endocrinology in mid-January 2025, with formal endorsement from more than 75 medical societies and other relevant stakeholder organizations.
On November 4, 2024, at the Obesity Society’s Obesity Week meeting, the publication’s lead author, Francesco Rubino, MD, Chair of Bariatric and Metabolic Surgery at King’s College London in England, gave a preview. He began by noting that, despite the declaration of obesity as a chronic disease over a decade ago, the concept is still debated and not widely accepted by the public or even by all in the medical community.
“The idea of obesity as a disease remains highly controversial,” Rubino noted, adding that the current body mass index (BMI)–based definition contributes to this because it doesn’t distinguish between people whose excess adiposity place them at excess risk for disease but they’re currently healthy vs those who already have undergone bodily harm from that adiposity.
“Having a framework that distinguishes at an individual level when you are in a condition of risk and when you have a condition of disease is fundamentally important. You don’t want to blur the picture in either direction, because obviously the consequence would be quite significant. ... So, the commission focused exactly on that point,” he said.
The new paper will propose a two-part clinical approach: First, assess whether the patient has excess adiposity, with methods that will be outlined. Next, assess on an organ-by-organ basis for the presence of abnormalities related to excess adiposity, or “clinical obesity.” The document will also provide those specific criteria, Rubino said, noting that those details are under embargo until January.
However, he did say that “We are going to propose a pragmatic approach to say that BMI alone is not enough in the clinic. It’s okay as a screening tool, but when somebody potentially has obesity, then you have to add additional measures of adiposity that makes sure you decrease the level of risk… Once you have obesity, then you need to establish if it’s clinical or nonclinical.”
Asked to comment, session moderator John D. Clark, MD, PhD, Chief Population Health Officer at Sharp Rees-Stealy Medical Group, San Diego, California, said in an interview, “I think it’ll help explain and move medicine as a whole in a direction to a greater understanding of obesity actually being a disease, how to define it, and how to identify it. And will, I think, lead to a greater understanding of the underlying disease.”
And, Clark said, it should also help target individuals with preventive vs therapeutic approaches. “I would describe it as matching the right tool to the right patient. If a person has clinical obesity, they likely can and would benefit from either different or additional tools, as opposed to otherwise healthy obesity.”
Rubino said he hopes the new framework will prompt improvements in reimbursement and public policy. “Policymakers scratch their heads when they have limited resources and you need to prioritize things. Having an obesity definition that is blurry doesn’t allow you to have a fair, human, and meaningful prioritization. ... Now that we have drugs that cannot be given to 100% of people, how do you decide who gets them first? I hope this will make it easier for people to access treatment. At the moment, it is not only difficult, but it’s also unfair. It’s random. Somebody gets access, while somebody else who is very, very sick has no access. I don’t think that’s what we want.”
A version of this article appeared on Medscape.com.
SAN ANTONIO, TEXAS —
The authors of the new framework are a Lancet Commission of 56 of the world’s leading obesity experts, including academic clinicians, scientists, public health experts, patient representatives, and officers from the World Health Organization. Following peer review, it will be launched via livestream and published in Lancet Diabetes & Endocrinology in mid-January 2025, with formal endorsement from more than 75 medical societies and other relevant stakeholder organizations.
On November 4, 2024, at the Obesity Society’s Obesity Week meeting, the publication’s lead author, Francesco Rubino, MD, Chair of Bariatric and Metabolic Surgery at King’s College London in England, gave a preview. He began by noting that, despite the declaration of obesity as a chronic disease over a decade ago, the concept is still debated and not widely accepted by the public or even by all in the medical community.
“The idea of obesity as a disease remains highly controversial,” Rubino noted, adding that the current body mass index (BMI)–based definition contributes to this because it doesn’t distinguish between people whose excess adiposity place them at excess risk for disease but they’re currently healthy vs those who already have undergone bodily harm from that adiposity.
“Having a framework that distinguishes at an individual level when you are in a condition of risk and when you have a condition of disease is fundamentally important. You don’t want to blur the picture in either direction, because obviously the consequence would be quite significant. ... So, the commission focused exactly on that point,” he said.
The new paper will propose a two-part clinical approach: First, assess whether the patient has excess adiposity, with methods that will be outlined. Next, assess on an organ-by-organ basis for the presence of abnormalities related to excess adiposity, or “clinical obesity.” The document will also provide those specific criteria, Rubino said, noting that those details are under embargo until January.
However, he did say that “We are going to propose a pragmatic approach to say that BMI alone is not enough in the clinic. It’s okay as a screening tool, but when somebody potentially has obesity, then you have to add additional measures of adiposity that makes sure you decrease the level of risk… Once you have obesity, then you need to establish if it’s clinical or nonclinical.”
Asked to comment, session moderator John D. Clark, MD, PhD, Chief Population Health Officer at Sharp Rees-Stealy Medical Group, San Diego, California, said in an interview, “I think it’ll help explain and move medicine as a whole in a direction to a greater understanding of obesity actually being a disease, how to define it, and how to identify it. And will, I think, lead to a greater understanding of the underlying disease.”
And, Clark said, it should also help target individuals with preventive vs therapeutic approaches. “I would describe it as matching the right tool to the right patient. If a person has clinical obesity, they likely can and would benefit from either different or additional tools, as opposed to otherwise healthy obesity.”
Rubino said he hopes the new framework will prompt improvements in reimbursement and public policy. “Policymakers scratch their heads when they have limited resources and you need to prioritize things. Having an obesity definition that is blurry doesn’t allow you to have a fair, human, and meaningful prioritization. ... Now that we have drugs that cannot be given to 100% of people, how do you decide who gets them first? I hope this will make it easier for people to access treatment. At the moment, it is not only difficult, but it’s also unfair. It’s random. Somebody gets access, while somebody else who is very, very sick has no access. I don’t think that’s what we want.”
A version of this article appeared on Medscape.com.
FROM OBESITY WEEK
Can We Repurpose Obesity Drugs to Reverse Liver Disease?
Metabolic dysfunction–associated steatotic liver disease (MASLD) has become the most common liver disease worldwide, with a global prevalence of 32.4%. Its growth over the past three decades has occurred in tandem with increasing rates of obesity and type 2 diabetes — two cornerstones of MASLD.
Higher rates of MASLD and metabolic dysfunction–associated steatohepatitis (MASH) with fibrosis are present in adults with obesity and diabetes, noted Arun Sanyal, MD, professor and director of the Stravitz-Sanyal Institute for Liver Disease and Metabolic Health, Virginia Commonwealth University, Richmond, Virginia.
The success surrounding the medications for obesity and type 2 diabetes, including glucagon-like peptide 1 receptor agonists (GLP-1 RAs), has sparked studies investigating whether they could also be an effective treatment for liver disease.
In particular, GLP-1 RAs help patients lose weight and/or control diabetes by mimicking the function of the gut hormone GLP-1, released in response to nutrient intake, and are able to increase insulin secretion and reduce glucagon secretion, delay gastric emptying, and reduce appetite and caloric intake.
The studies for MASLD are testing whether these functions will also work against liver disease, either directly or indirectly, through obesity and diabetes control. The early results are promising.
More Than One Risk Factor in Play
MASLD is defined by the presence of hepatic steatosis and at least one of five cardiometabolic risk factors: Overweight/obesity, hypertension, hyperglycemia, dyslipidemia with either low-plasma high-density lipoprotein cholesterol or high triglycerides, or treatment for these conditions.
It is a grim trajectory if the disease progresses to MASH, as the patient may accumulate hepatic fibrosis and go on to develop cirrhosis and/or hepatocellular carcinoma.
Typically, more than one risk factor is at play in MASLD, noted Adnan Said, MD, chief of the Division of Gastroenterology and Hepatology at the William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin.
“It most commonly occurs in the setting of weight gain and obesity, which are epidemics in the United States and worldwide, as well as the associated condition — metabolic syndrome — which goes along with obesity and includes type 2 diabetes, hyperlipidemia, hypertension, and sleep apnea,” Said, a hepatology and gastroenterology professor at the University of Wisconsin–Madison, told this news organization.
The research surrounding MASLD is investigating GLP-1 RAs as single agents and in combination with other drugs.
Finding treatment is critical, as there is only one drug — resmetirom — approved for the treatment of MASH with moderate to advanced fibrosis. But because it’s not approved for earlier stages, a treatment gap exists. The drug also doesn’t produce weight loss, which is key to treating MASLD. And while GLP-1 RAs help patients with the weight loss that is critical to MASLD, they are only approved by the US Food and Drug Administration (FDA) for obesity and type 2 diabetes.
Single Agents
The GLP-1 RAs liraglutide and semaglutide, both approved for diabetes and weight loss, are being studied as single agents against liver disease, Said said.
“Their action in the setting of MASLD and MASH is primarily indirect, through systemic pathways, improving these conditions via weight loss, as well as by improving insulin sensitivity and reducing lipotoxicity,” he added.
One of the first trials of these agents for liver disease was in 2016. In that double-blind, randomized, 48-week clinical trial of liraglutide in patients with MASH and overweight, 39% of patients who received liraglutide had a resolution of MASH compared with only 9% of those who received placebo. Moreover, only 9% vs 36% of patients in the treatment vs placebo group had progression of fibrosis.
Since then, a 72-week phase 2 trial in patients with MASH, liver fibrosis (stages F1-F3), and overweight or obesity found that once-daily subcutaneous semaglutide (0.1, 0.2, or 0.4 mg) outperformed placebo on MASH resolution without worsening of fibrosis (36%-59% vs 17%) and on weight loss (5%-13% vs 1%), with the greatest benefits at the largest dose. However, neoplasms were reported in 15% of patients receiving semaglutide vs 8% of those receiving placebo.
A phase 1 trial involving patients with liver stiffness, steatosis, and overweight or obesity found significantly greater reductions in liver fat at 48 weeks with semaglutide vs placebo, as well as decreases in liver enzymes, body weight, and A1c. There was no significant difference in liver stiffness.
Furthermore, a meta-analysis of eight studies found that treatment with 24 weeks of semaglutide significantly improved liver enzymes, reduced liver stiffness, and improved metabolic parameters in patients with MASLD/MASH. The authors cautioned that gastrointestinal adverse effects “could be a major concern.”
Several studies have found other GLP-1 RAs, including exenatide and dulaglutide, have a beneficial impact on liver injury indices and liver steatosis.
A new retrospective observational study offers evidence that GLP-1 RAs may have a direct impact on MASLD, independent of weight loss. Among the 28% of patients with type 2 diabetes and MASLD who received a GLP-1 RA, there was a significant reduction not only in body mass index but also in A1c, liver enzymes, and controlled attenuation parameter scores. A beneficial impact on liver parameters was observed even in patients who didn’t lose weight. While there was no difference in liver stiffness measurement, the median 60-month follow-up time may not have been long enough to capture such changes.
Another study indicated that the apparent benefits of GLP-1 RAs, in this case semaglutide, may not extend to patients whose disease has progressed to cirrhosis.
Dual and Triple Mechanisms of Action
Newer agents with double or triple mechanisms of action appear to have a more direct effect on the liver.
“Dual agents may have an added effect by improving MASLD directly through adipose regulation and thermogenesis, thereby improving fibrosis,” Said said.
An example is tirzepatide, a GLP-1 RA and an agonist of glucose-dependent insulinotropic polypeptide (GIP). Like GLP-1, GIP is an incretin. When used together as co-agonists, GLP-1 and GIP have been shown to increase insulin and glucagonostatic response and may work synergistically.
A new phase 2 trial that randomly assigned patients with biopsy-confirmed MASH and moderate or severe fibrosis to receive either once-weekly subcutaneous tirzepatide at one of three doses (5, 10, or 15 mg) or placebo found that tirzepatide at each dosage outperformed placebo in resolution of MASH without worsening of fibrosis.
“These findings were encouraging,” Said said. “We’ll see if the results continue into phase 3 trials.”
The combination of GLP-1 RAs with glucagon (GCG) receptor agonists also has garnered interest.
In a phase 2 trial, adults with biopsy-confirmed MASH and fibrosis stages F1-F3 were randomly assigned to receive either one of three doses of the GLP-1/GCG RA survodutide (2.4, 4.8, or 6 mg) or placebo. Survodutide at each dose was found to be superior to placebo in improving MASH without the worsening of fibrosis, reducing liver fat content by at least 30%, and decreasing liver fibrosis by at least one stage, with the 4.8-mg dose showing the best performance for each measure. However, adverse events, including nausea, diarrhea, and vomiting, were more frequent with survodutide than with placebo.
Trials of triple-action agents (GLP-1/GIP/GCG RAs) are underway too.
The hope is the triple agonists could deliver greater reduction in hepatic fat in patients with MASLD, Sanyal said.
Sanyal further noted that a reduction in liver fat is important, citing a meta-analysis that showed ≥ 30% relative decline in liver fat is associated with higher odds of histologic response and MASH resolution.
Sanyal pointed to efocipegtrutide (HM15211), a GLP-1/GIP/GCG RA, which demonstrated significant liver fat reduction after 12 weeks in patients with MASLD in a phase 1b/2a randomized, placebo-controlled trial and is now in phase 2 development.
Another example is retatrutide (LY3437943), a once-weekly injectable, that was associated with up to a 24.2% reduction in body weight at 48 weeks, compared with 2.1% with placebo, in a phase 2 trial involving patients with obesity.
A sub-study assessed the mean relative change from baseline in liver fat at 24 weeks. These participants, who also had MASLD and ≥ 10% of liver fat content, were randomly assigned to receive either retatrutide in one of four doses (1, 4, 8, or 12 mg) or placebo for 48 weeks. All doses of retatrutide showed significantly greater reduction in liver fat content compared with placebo in weeks 24-48, with a mean relative liver fat reduction > 80% at the two higher doses. Moreover, ≥ 80% of participants on the higher retatrutide doses experienced ≥ 70% reduction in liver fat at 48 weeks, compared with 0% reduction in those on placebo, and hepatic steatosis resolved in > 85% of these participants.
This space “continues to evolve at a rapid rate,” Sanyal said. For example, oral dual-action agents are under development.
Obstacles and Warnings
Sanyal warned that GLP-1 RAs can cause nausea, so they have to be introduced at a low dose and slowly titrated upward. They should be used with caution in people with a history of multiple endocrine neoplasia. There is also a small but increased risk for gallstone formation and gallstone-induced pancreatitis with rapid weight loss.
GLP-1 RAs may increase the risk for suicidal ideation, with the authors of a recent study calling for “urgent clarification” regarding this possibility.
Following reports of suicidality submitted through its Adverse Events Reporting System, the FDA concluded that it could find no causal relationship between these agents and increased risk for suicidal ideation but also that it could not “definitively rule out that a small risk may exist” and would continue to investigate.
Access to GLP-1 RAs is an obstacle as well. Semaglutide continues to be on the FDA’s shortage list.
“This is improving, but there are still issues around getting approval from insurance companies,” Sanyal said.
Many patients discontinue use because of tolerability or access issues, which is problematic because most regain the weight they had lost while on the medication.
“Right now, we see GLP-1 RAs as a long-term therapeutic commitment, but there is a lot of research interest in figuring out if there’s a more modest benefit — almost an induction-remission maintenance approach to weight loss,” Sanyal said. These are “evolving trends,” and it’s unclear how they will unfold.
“As of now, you have to decide that if you’re putting your patient on these medications, they will have to take them on a long-term basis and include that consideration in your risk-benefit analysis, together with any concerns about adverse effects,” he said.
Sanyal reported consulting for Boehringer Ingelheim, Eli Lilly, and Novo Nordisk. Said received research support from Exact Sciences, Boehringer Ingelheim, and Mallinckrodt.
A version of this article first appeared on Medscape.com.
Metabolic dysfunction–associated steatotic liver disease (MASLD) has become the most common liver disease worldwide, with a global prevalence of 32.4%. Its growth over the past three decades has occurred in tandem with increasing rates of obesity and type 2 diabetes — two cornerstones of MASLD.
Higher rates of MASLD and metabolic dysfunction–associated steatohepatitis (MASH) with fibrosis are present in adults with obesity and diabetes, noted Arun Sanyal, MD, professor and director of the Stravitz-Sanyal Institute for Liver Disease and Metabolic Health, Virginia Commonwealth University, Richmond, Virginia.
The success surrounding the medications for obesity and type 2 diabetes, including glucagon-like peptide 1 receptor agonists (GLP-1 RAs), has sparked studies investigating whether they could also be an effective treatment for liver disease.
In particular, GLP-1 RAs help patients lose weight and/or control diabetes by mimicking the function of the gut hormone GLP-1, released in response to nutrient intake, and are able to increase insulin secretion and reduce glucagon secretion, delay gastric emptying, and reduce appetite and caloric intake.
The studies for MASLD are testing whether these functions will also work against liver disease, either directly or indirectly, through obesity and diabetes control. The early results are promising.
More Than One Risk Factor in Play
MASLD is defined by the presence of hepatic steatosis and at least one of five cardiometabolic risk factors: Overweight/obesity, hypertension, hyperglycemia, dyslipidemia with either low-plasma high-density lipoprotein cholesterol or high triglycerides, or treatment for these conditions.
It is a grim trajectory if the disease progresses to MASH, as the patient may accumulate hepatic fibrosis and go on to develop cirrhosis and/or hepatocellular carcinoma.
Typically, more than one risk factor is at play in MASLD, noted Adnan Said, MD, chief of the Division of Gastroenterology and Hepatology at the William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin.
“It most commonly occurs in the setting of weight gain and obesity, which are epidemics in the United States and worldwide, as well as the associated condition — metabolic syndrome — which goes along with obesity and includes type 2 diabetes, hyperlipidemia, hypertension, and sleep apnea,” Said, a hepatology and gastroenterology professor at the University of Wisconsin–Madison, told this news organization.
The research surrounding MASLD is investigating GLP-1 RAs as single agents and in combination with other drugs.
Finding treatment is critical, as there is only one drug — resmetirom — approved for the treatment of MASH with moderate to advanced fibrosis. But because it’s not approved for earlier stages, a treatment gap exists. The drug also doesn’t produce weight loss, which is key to treating MASLD. And while GLP-1 RAs help patients with the weight loss that is critical to MASLD, they are only approved by the US Food and Drug Administration (FDA) for obesity and type 2 diabetes.
Single Agents
The GLP-1 RAs liraglutide and semaglutide, both approved for diabetes and weight loss, are being studied as single agents against liver disease, Said said.
“Their action in the setting of MASLD and MASH is primarily indirect, through systemic pathways, improving these conditions via weight loss, as well as by improving insulin sensitivity and reducing lipotoxicity,” he added.
One of the first trials of these agents for liver disease was in 2016. In that double-blind, randomized, 48-week clinical trial of liraglutide in patients with MASH and overweight, 39% of patients who received liraglutide had a resolution of MASH compared with only 9% of those who received placebo. Moreover, only 9% vs 36% of patients in the treatment vs placebo group had progression of fibrosis.
Since then, a 72-week phase 2 trial in patients with MASH, liver fibrosis (stages F1-F3), and overweight or obesity found that once-daily subcutaneous semaglutide (0.1, 0.2, or 0.4 mg) outperformed placebo on MASH resolution without worsening of fibrosis (36%-59% vs 17%) and on weight loss (5%-13% vs 1%), with the greatest benefits at the largest dose. However, neoplasms were reported in 15% of patients receiving semaglutide vs 8% of those receiving placebo.
A phase 1 trial involving patients with liver stiffness, steatosis, and overweight or obesity found significantly greater reductions in liver fat at 48 weeks with semaglutide vs placebo, as well as decreases in liver enzymes, body weight, and A1c. There was no significant difference in liver stiffness.
Furthermore, a meta-analysis of eight studies found that treatment with 24 weeks of semaglutide significantly improved liver enzymes, reduced liver stiffness, and improved metabolic parameters in patients with MASLD/MASH. The authors cautioned that gastrointestinal adverse effects “could be a major concern.”
Several studies have found other GLP-1 RAs, including exenatide and dulaglutide, have a beneficial impact on liver injury indices and liver steatosis.
A new retrospective observational study offers evidence that GLP-1 RAs may have a direct impact on MASLD, independent of weight loss. Among the 28% of patients with type 2 diabetes and MASLD who received a GLP-1 RA, there was a significant reduction not only in body mass index but also in A1c, liver enzymes, and controlled attenuation parameter scores. A beneficial impact on liver parameters was observed even in patients who didn’t lose weight. While there was no difference in liver stiffness measurement, the median 60-month follow-up time may not have been long enough to capture such changes.
Another study indicated that the apparent benefits of GLP-1 RAs, in this case semaglutide, may not extend to patients whose disease has progressed to cirrhosis.
Dual and Triple Mechanisms of Action
Newer agents with double or triple mechanisms of action appear to have a more direct effect on the liver.
“Dual agents may have an added effect by improving MASLD directly through adipose regulation and thermogenesis, thereby improving fibrosis,” Said said.
An example is tirzepatide, a GLP-1 RA and an agonist of glucose-dependent insulinotropic polypeptide (GIP). Like GLP-1, GIP is an incretin. When used together as co-agonists, GLP-1 and GIP have been shown to increase insulin and glucagonostatic response and may work synergistically.
A new phase 2 trial that randomly assigned patients with biopsy-confirmed MASH and moderate or severe fibrosis to receive either once-weekly subcutaneous tirzepatide at one of three doses (5, 10, or 15 mg) or placebo found that tirzepatide at each dosage outperformed placebo in resolution of MASH without worsening of fibrosis.
“These findings were encouraging,” Said said. “We’ll see if the results continue into phase 3 trials.”
The combination of GLP-1 RAs with glucagon (GCG) receptor agonists also has garnered interest.
In a phase 2 trial, adults with biopsy-confirmed MASH and fibrosis stages F1-F3 were randomly assigned to receive either one of three doses of the GLP-1/GCG RA survodutide (2.4, 4.8, or 6 mg) or placebo. Survodutide at each dose was found to be superior to placebo in improving MASH without the worsening of fibrosis, reducing liver fat content by at least 30%, and decreasing liver fibrosis by at least one stage, with the 4.8-mg dose showing the best performance for each measure. However, adverse events, including nausea, diarrhea, and vomiting, were more frequent with survodutide than with placebo.
Trials of triple-action agents (GLP-1/GIP/GCG RAs) are underway too.
The hope is the triple agonists could deliver greater reduction in hepatic fat in patients with MASLD, Sanyal said.
Sanyal further noted that a reduction in liver fat is important, citing a meta-analysis that showed ≥ 30% relative decline in liver fat is associated with higher odds of histologic response and MASH resolution.
Sanyal pointed to efocipegtrutide (HM15211), a GLP-1/GIP/GCG RA, which demonstrated significant liver fat reduction after 12 weeks in patients with MASLD in a phase 1b/2a randomized, placebo-controlled trial and is now in phase 2 development.
Another example is retatrutide (LY3437943), a once-weekly injectable, that was associated with up to a 24.2% reduction in body weight at 48 weeks, compared with 2.1% with placebo, in a phase 2 trial involving patients with obesity.
A sub-study assessed the mean relative change from baseline in liver fat at 24 weeks. These participants, who also had MASLD and ≥ 10% of liver fat content, were randomly assigned to receive either retatrutide in one of four doses (1, 4, 8, or 12 mg) or placebo for 48 weeks. All doses of retatrutide showed significantly greater reduction in liver fat content compared with placebo in weeks 24-48, with a mean relative liver fat reduction > 80% at the two higher doses. Moreover, ≥ 80% of participants on the higher retatrutide doses experienced ≥ 70% reduction in liver fat at 48 weeks, compared with 0% reduction in those on placebo, and hepatic steatosis resolved in > 85% of these participants.
This space “continues to evolve at a rapid rate,” Sanyal said. For example, oral dual-action agents are under development.
Obstacles and Warnings
Sanyal warned that GLP-1 RAs can cause nausea, so they have to be introduced at a low dose and slowly titrated upward. They should be used with caution in people with a history of multiple endocrine neoplasia. There is also a small but increased risk for gallstone formation and gallstone-induced pancreatitis with rapid weight loss.
GLP-1 RAs may increase the risk for suicidal ideation, with the authors of a recent study calling for “urgent clarification” regarding this possibility.
Following reports of suicidality submitted through its Adverse Events Reporting System, the FDA concluded that it could find no causal relationship between these agents and increased risk for suicidal ideation but also that it could not “definitively rule out that a small risk may exist” and would continue to investigate.
Access to GLP-1 RAs is an obstacle as well. Semaglutide continues to be on the FDA’s shortage list.
“This is improving, but there are still issues around getting approval from insurance companies,” Sanyal said.
Many patients discontinue use because of tolerability or access issues, which is problematic because most regain the weight they had lost while on the medication.
“Right now, we see GLP-1 RAs as a long-term therapeutic commitment, but there is a lot of research interest in figuring out if there’s a more modest benefit — almost an induction-remission maintenance approach to weight loss,” Sanyal said. These are “evolving trends,” and it’s unclear how they will unfold.
“As of now, you have to decide that if you’re putting your patient on these medications, they will have to take them on a long-term basis and include that consideration in your risk-benefit analysis, together with any concerns about adverse effects,” he said.
Sanyal reported consulting for Boehringer Ingelheim, Eli Lilly, and Novo Nordisk. Said received research support from Exact Sciences, Boehringer Ingelheim, and Mallinckrodt.
A version of this article first appeared on Medscape.com.
Metabolic dysfunction–associated steatotic liver disease (MASLD) has become the most common liver disease worldwide, with a global prevalence of 32.4%. Its growth over the past three decades has occurred in tandem with increasing rates of obesity and type 2 diabetes — two cornerstones of MASLD.
Higher rates of MASLD and metabolic dysfunction–associated steatohepatitis (MASH) with fibrosis are present in adults with obesity and diabetes, noted Arun Sanyal, MD, professor and director of the Stravitz-Sanyal Institute for Liver Disease and Metabolic Health, Virginia Commonwealth University, Richmond, Virginia.
The success surrounding the medications for obesity and type 2 diabetes, including glucagon-like peptide 1 receptor agonists (GLP-1 RAs), has sparked studies investigating whether they could also be an effective treatment for liver disease.
In particular, GLP-1 RAs help patients lose weight and/or control diabetes by mimicking the function of the gut hormone GLP-1, released in response to nutrient intake, and are able to increase insulin secretion and reduce glucagon secretion, delay gastric emptying, and reduce appetite and caloric intake.
The studies for MASLD are testing whether these functions will also work against liver disease, either directly or indirectly, through obesity and diabetes control. The early results are promising.
More Than One Risk Factor in Play
MASLD is defined by the presence of hepatic steatosis and at least one of five cardiometabolic risk factors: Overweight/obesity, hypertension, hyperglycemia, dyslipidemia with either low-plasma high-density lipoprotein cholesterol or high triglycerides, or treatment for these conditions.
It is a grim trajectory if the disease progresses to MASH, as the patient may accumulate hepatic fibrosis and go on to develop cirrhosis and/or hepatocellular carcinoma.
Typically, more than one risk factor is at play in MASLD, noted Adnan Said, MD, chief of the Division of Gastroenterology and Hepatology at the William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin.
“It most commonly occurs in the setting of weight gain and obesity, which are epidemics in the United States and worldwide, as well as the associated condition — metabolic syndrome — which goes along with obesity and includes type 2 diabetes, hyperlipidemia, hypertension, and sleep apnea,” Said, a hepatology and gastroenterology professor at the University of Wisconsin–Madison, told this news organization.
The research surrounding MASLD is investigating GLP-1 RAs as single agents and in combination with other drugs.
Finding treatment is critical, as there is only one drug — resmetirom — approved for the treatment of MASH with moderate to advanced fibrosis. But because it’s not approved for earlier stages, a treatment gap exists. The drug also doesn’t produce weight loss, which is key to treating MASLD. And while GLP-1 RAs help patients with the weight loss that is critical to MASLD, they are only approved by the US Food and Drug Administration (FDA) for obesity and type 2 diabetes.
Single Agents
The GLP-1 RAs liraglutide and semaglutide, both approved for diabetes and weight loss, are being studied as single agents against liver disease, Said said.
“Their action in the setting of MASLD and MASH is primarily indirect, through systemic pathways, improving these conditions via weight loss, as well as by improving insulin sensitivity and reducing lipotoxicity,” he added.
One of the first trials of these agents for liver disease was in 2016. In that double-blind, randomized, 48-week clinical trial of liraglutide in patients with MASH and overweight, 39% of patients who received liraglutide had a resolution of MASH compared with only 9% of those who received placebo. Moreover, only 9% vs 36% of patients in the treatment vs placebo group had progression of fibrosis.
Since then, a 72-week phase 2 trial in patients with MASH, liver fibrosis (stages F1-F3), and overweight or obesity found that once-daily subcutaneous semaglutide (0.1, 0.2, or 0.4 mg) outperformed placebo on MASH resolution without worsening of fibrosis (36%-59% vs 17%) and on weight loss (5%-13% vs 1%), with the greatest benefits at the largest dose. However, neoplasms were reported in 15% of patients receiving semaglutide vs 8% of those receiving placebo.
A phase 1 trial involving patients with liver stiffness, steatosis, and overweight or obesity found significantly greater reductions in liver fat at 48 weeks with semaglutide vs placebo, as well as decreases in liver enzymes, body weight, and A1c. There was no significant difference in liver stiffness.
Furthermore, a meta-analysis of eight studies found that treatment with 24 weeks of semaglutide significantly improved liver enzymes, reduced liver stiffness, and improved metabolic parameters in patients with MASLD/MASH. The authors cautioned that gastrointestinal adverse effects “could be a major concern.”
Several studies have found other GLP-1 RAs, including exenatide and dulaglutide, have a beneficial impact on liver injury indices and liver steatosis.
A new retrospective observational study offers evidence that GLP-1 RAs may have a direct impact on MASLD, independent of weight loss. Among the 28% of patients with type 2 diabetes and MASLD who received a GLP-1 RA, there was a significant reduction not only in body mass index but also in A1c, liver enzymes, and controlled attenuation parameter scores. A beneficial impact on liver parameters was observed even in patients who didn’t lose weight. While there was no difference in liver stiffness measurement, the median 60-month follow-up time may not have been long enough to capture such changes.
Another study indicated that the apparent benefits of GLP-1 RAs, in this case semaglutide, may not extend to patients whose disease has progressed to cirrhosis.
Dual and Triple Mechanisms of Action
Newer agents with double or triple mechanisms of action appear to have a more direct effect on the liver.
“Dual agents may have an added effect by improving MASLD directly through adipose regulation and thermogenesis, thereby improving fibrosis,” Said said.
An example is tirzepatide, a GLP-1 RA and an agonist of glucose-dependent insulinotropic polypeptide (GIP). Like GLP-1, GIP is an incretin. When used together as co-agonists, GLP-1 and GIP have been shown to increase insulin and glucagonostatic response and may work synergistically.
A new phase 2 trial that randomly assigned patients with biopsy-confirmed MASH and moderate or severe fibrosis to receive either once-weekly subcutaneous tirzepatide at one of three doses (5, 10, or 15 mg) or placebo found that tirzepatide at each dosage outperformed placebo in resolution of MASH without worsening of fibrosis.
“These findings were encouraging,” Said said. “We’ll see if the results continue into phase 3 trials.”
The combination of GLP-1 RAs with glucagon (GCG) receptor agonists also has garnered interest.
In a phase 2 trial, adults with biopsy-confirmed MASH and fibrosis stages F1-F3 were randomly assigned to receive either one of three doses of the GLP-1/GCG RA survodutide (2.4, 4.8, or 6 mg) or placebo. Survodutide at each dose was found to be superior to placebo in improving MASH without the worsening of fibrosis, reducing liver fat content by at least 30%, and decreasing liver fibrosis by at least one stage, with the 4.8-mg dose showing the best performance for each measure. However, adverse events, including nausea, diarrhea, and vomiting, were more frequent with survodutide than with placebo.
Trials of triple-action agents (GLP-1/GIP/GCG RAs) are underway too.
The hope is the triple agonists could deliver greater reduction in hepatic fat in patients with MASLD, Sanyal said.
Sanyal further noted that a reduction in liver fat is important, citing a meta-analysis that showed ≥ 30% relative decline in liver fat is associated with higher odds of histologic response and MASH resolution.
Sanyal pointed to efocipegtrutide (HM15211), a GLP-1/GIP/GCG RA, which demonstrated significant liver fat reduction after 12 weeks in patients with MASLD in a phase 1b/2a randomized, placebo-controlled trial and is now in phase 2 development.
Another example is retatrutide (LY3437943), a once-weekly injectable, that was associated with up to a 24.2% reduction in body weight at 48 weeks, compared with 2.1% with placebo, in a phase 2 trial involving patients with obesity.
A sub-study assessed the mean relative change from baseline in liver fat at 24 weeks. These participants, who also had MASLD and ≥ 10% of liver fat content, were randomly assigned to receive either retatrutide in one of four doses (1, 4, 8, or 12 mg) or placebo for 48 weeks. All doses of retatrutide showed significantly greater reduction in liver fat content compared with placebo in weeks 24-48, with a mean relative liver fat reduction > 80% at the two higher doses. Moreover, ≥ 80% of participants on the higher retatrutide doses experienced ≥ 70% reduction in liver fat at 48 weeks, compared with 0% reduction in those on placebo, and hepatic steatosis resolved in > 85% of these participants.
This space “continues to evolve at a rapid rate,” Sanyal said. For example, oral dual-action agents are under development.
Obstacles and Warnings
Sanyal warned that GLP-1 RAs can cause nausea, so they have to be introduced at a low dose and slowly titrated upward. They should be used with caution in people with a history of multiple endocrine neoplasia. There is also a small but increased risk for gallstone formation and gallstone-induced pancreatitis with rapid weight loss.
GLP-1 RAs may increase the risk for suicidal ideation, with the authors of a recent study calling for “urgent clarification” regarding this possibility.
Following reports of suicidality submitted through its Adverse Events Reporting System, the FDA concluded that it could find no causal relationship between these agents and increased risk for suicidal ideation but also that it could not “definitively rule out that a small risk may exist” and would continue to investigate.
Access to GLP-1 RAs is an obstacle as well. Semaglutide continues to be on the FDA’s shortage list.
“This is improving, but there are still issues around getting approval from insurance companies,” Sanyal said.
Many patients discontinue use because of tolerability or access issues, which is problematic because most regain the weight they had lost while on the medication.
“Right now, we see GLP-1 RAs as a long-term therapeutic commitment, but there is a lot of research interest in figuring out if there’s a more modest benefit — almost an induction-remission maintenance approach to weight loss,” Sanyal said. These are “evolving trends,” and it’s unclear how they will unfold.
“As of now, you have to decide that if you’re putting your patient on these medications, they will have to take them on a long-term basis and include that consideration in your risk-benefit analysis, together with any concerns about adverse effects,” he said.
Sanyal reported consulting for Boehringer Ingelheim, Eli Lilly, and Novo Nordisk. Said received research support from Exact Sciences, Boehringer Ingelheim, and Mallinckrodt.
A version of this article first appeared on Medscape.com.
ATA: Updates on Risk, Diagnosis, and Treatment of Thyroid Cancer
The study, presented by Juan Brito Campana, MBBS, of the Mayo Clinic in Rochester, Minnesota, used Medicare records to perform a secondary analysis of 41,000 adults with type 2 diabetes and moderate cardiovascular risk who were new users of GLP-1 receptor agonists, compared to users of other diabetes medications.
“We took the innovative approach of applying the methodological rigor of a randomized clinical trial to the very large dataset of observational studies,” said Brito Campana.
The results showed a low absolute risk of thyroid cancer, with only 0.17% of patients in the GLP-1 group developing the disease. However, the data also showed a potential relative increase in risk during the first year of GLP-1 receptor agonist use.
“This is likely due to increased detection rather than true incidence, as the latency period for thyroid cancer development is typically longer,” Brito Campana said.
“We also note the limitations of the observational study design, including the short follow-up period and lack of detailed histological data. However, we believe the benefits of GLP-1 receptor agonists likely outweigh the risk of thyroid cancer.”
Malignancy in Bethesda III and IV Thyroid Nodules
At the same ATA session, Sapir Nachum Goldberg, MD, of the University of Pennsylvania, Philadelphia, presented the results of a retrospective record review that examined the prevalence of malignancy in Bethesda III and IV thyroid nodules with negative Thyrogen Receptor Signaling (ThyroSeq) version 3 molecular testing results.
Goldberg reported that 87% of patients with ThyroSeq negative subtype results were managed nonoperatively. “Based on our data, the true prevalence of malignancy likely lies between our low and high estimates of 3% and 23%,” she said. “We believe that the prevalence of malignancy may be higher in real-world practice than validation studies.”
Additionally, nodules with “currently negative” or “negative but limited” ThyroSeq results had a higher prevalence of malignancy (7%), compared with those with a “negative” result (2%). Factors like immediate vs delayed surgery, nodule size, and ultrasound pattern did not significantly impact malignancy prevalence.
The study results also indicated that surveillance ultrasonography is not routinely performed in up to one-third of patients, Goldberg said.
She closed by suggesting that colleagues consider the negative subtype in clinical decision-making. For “negative but limited” nodules, repeat the fine needle aspiration and, for “negative” and “currently negative” nodules, consider ultrasound follow-up as per ATA guidelines for Bethesda II cytology, she said.
RET-Mutated Medullary Thyroid Cancer
For patients with RET-mutated medullary thyroid cancer, Julien Hadoux, MD, PhD, of Institut de Cancérologie Gustave Roussy, Villejuif, France, presented a combined analysis of the efficacy of the RET inhibitor selpercatinib from the phase 1/2 LIBRETTO-001 and phase 3 LIBRETTO-531 trials.
This post hoc analysis used a combined cohort of 509 patients with RET-mutated advanced or metastatic medullary thyroid cancer who had received selpercatinib in the two trials.
Hadoux reported that robust and durable responses were seen across all mutation groups, including M918T, extracellular cysteine, and an “other” group composed of various uncommon RET mutations. “The median [progression-free survival] PFS was not reached for either the M918T or extracellular groups and it was 51.4 months for the Other group,” he said.
“Selpercatinib showed superior median PFS vs control, regardless of the RET mutation. This analysis constitutes the largest catalog of RET mutations in medullary thyroid cancers treated with RET-specific inhibitors.”
TRK-Fusion Differentiated Thyroid Cancer
Steven Waguespack, MD, of the University of Texas MD Anderson Cancer Center, Houston, shared updated efficacy and safety data from three phase 1/2 pooled clinical trials of the tropomyosin kinase receptor (TRK) inhibitor larotrectinib in thyroid cancer. These data updated results initially published in 2022.
“Larotrectinib continues to demonstrate rapid and durable responses, extended survival, and offers a favorable safety profile in patients with TRK fusion differentiated thyroid cancer, with limited activity in anaplastic thyroid cancer,” Waguespack said.
“Additionally, in a subset of patients, we identified some acquired on-target NTRK mutations and off-target GNAS and TP53 mutations that may give further insight into mechanisms of resistance.”
The primary endpoint was the investigator-assessed objective response rate (ORR); at 48 months, the ORR was 79% by independent review. The median PFS in patients with TRK fusion differentiated thyroid cancer was 44 months, while the median duration of response was 41 months. The 4-year overall survival rate was 86%.
Waguespack closed with a cautionary note to colleagues: “While circulating tumor DNA next-generation sequencing (NGS) analysis can be used to test for NTRK gene fusions, negative results should be followed up with tissue-based NGS,” he said.
Brito Campana and Goldberg disclosed no relevant financial relationships. Hadoux reported receiving honoraria for speaker engagements, advisory roles, or funding for CME from Eli Lilly, AAA, IPSEN, Roche, Pharma Mar, and EISAI, and research grants from Novartis, Sanofi, and Eli Lilly.
A version of this article appeared on Medscape.com.
The study, presented by Juan Brito Campana, MBBS, of the Mayo Clinic in Rochester, Minnesota, used Medicare records to perform a secondary analysis of 41,000 adults with type 2 diabetes and moderate cardiovascular risk who were new users of GLP-1 receptor agonists, compared to users of other diabetes medications.
“We took the innovative approach of applying the methodological rigor of a randomized clinical trial to the very large dataset of observational studies,” said Brito Campana.
The results showed a low absolute risk of thyroid cancer, with only 0.17% of patients in the GLP-1 group developing the disease. However, the data also showed a potential relative increase in risk during the first year of GLP-1 receptor agonist use.
“This is likely due to increased detection rather than true incidence, as the latency period for thyroid cancer development is typically longer,” Brito Campana said.
“We also note the limitations of the observational study design, including the short follow-up period and lack of detailed histological data. However, we believe the benefits of GLP-1 receptor agonists likely outweigh the risk of thyroid cancer.”
Malignancy in Bethesda III and IV Thyroid Nodules
At the same ATA session, Sapir Nachum Goldberg, MD, of the University of Pennsylvania, Philadelphia, presented the results of a retrospective record review that examined the prevalence of malignancy in Bethesda III and IV thyroid nodules with negative Thyrogen Receptor Signaling (ThyroSeq) version 3 molecular testing results.
Goldberg reported that 87% of patients with ThyroSeq negative subtype results were managed nonoperatively. “Based on our data, the true prevalence of malignancy likely lies between our low and high estimates of 3% and 23%,” she said. “We believe that the prevalence of malignancy may be higher in real-world practice than validation studies.”
Additionally, nodules with “currently negative” or “negative but limited” ThyroSeq results had a higher prevalence of malignancy (7%), compared with those with a “negative” result (2%). Factors like immediate vs delayed surgery, nodule size, and ultrasound pattern did not significantly impact malignancy prevalence.
The study results also indicated that surveillance ultrasonography is not routinely performed in up to one-third of patients, Goldberg said.
She closed by suggesting that colleagues consider the negative subtype in clinical decision-making. For “negative but limited” nodules, repeat the fine needle aspiration and, for “negative” and “currently negative” nodules, consider ultrasound follow-up as per ATA guidelines for Bethesda II cytology, she said.
RET-Mutated Medullary Thyroid Cancer
For patients with RET-mutated medullary thyroid cancer, Julien Hadoux, MD, PhD, of Institut de Cancérologie Gustave Roussy, Villejuif, France, presented a combined analysis of the efficacy of the RET inhibitor selpercatinib from the phase 1/2 LIBRETTO-001 and phase 3 LIBRETTO-531 trials.
This post hoc analysis used a combined cohort of 509 patients with RET-mutated advanced or metastatic medullary thyroid cancer who had received selpercatinib in the two trials.
Hadoux reported that robust and durable responses were seen across all mutation groups, including M918T, extracellular cysteine, and an “other” group composed of various uncommon RET mutations. “The median [progression-free survival] PFS was not reached for either the M918T or extracellular groups and it was 51.4 months for the Other group,” he said.
“Selpercatinib showed superior median PFS vs control, regardless of the RET mutation. This analysis constitutes the largest catalog of RET mutations in medullary thyroid cancers treated with RET-specific inhibitors.”
TRK-Fusion Differentiated Thyroid Cancer
Steven Waguespack, MD, of the University of Texas MD Anderson Cancer Center, Houston, shared updated efficacy and safety data from three phase 1/2 pooled clinical trials of the tropomyosin kinase receptor (TRK) inhibitor larotrectinib in thyroid cancer. These data updated results initially published in 2022.
“Larotrectinib continues to demonstrate rapid and durable responses, extended survival, and offers a favorable safety profile in patients with TRK fusion differentiated thyroid cancer, with limited activity in anaplastic thyroid cancer,” Waguespack said.
“Additionally, in a subset of patients, we identified some acquired on-target NTRK mutations and off-target GNAS and TP53 mutations that may give further insight into mechanisms of resistance.”
The primary endpoint was the investigator-assessed objective response rate (ORR); at 48 months, the ORR was 79% by independent review. The median PFS in patients with TRK fusion differentiated thyroid cancer was 44 months, while the median duration of response was 41 months. The 4-year overall survival rate was 86%.
Waguespack closed with a cautionary note to colleagues: “While circulating tumor DNA next-generation sequencing (NGS) analysis can be used to test for NTRK gene fusions, negative results should be followed up with tissue-based NGS,” he said.
Brito Campana and Goldberg disclosed no relevant financial relationships. Hadoux reported receiving honoraria for speaker engagements, advisory roles, or funding for CME from Eli Lilly, AAA, IPSEN, Roche, Pharma Mar, and EISAI, and research grants from Novartis, Sanofi, and Eli Lilly.
A version of this article appeared on Medscape.com.
The study, presented by Juan Brito Campana, MBBS, of the Mayo Clinic in Rochester, Minnesota, used Medicare records to perform a secondary analysis of 41,000 adults with type 2 diabetes and moderate cardiovascular risk who were new users of GLP-1 receptor agonists, compared to users of other diabetes medications.
“We took the innovative approach of applying the methodological rigor of a randomized clinical trial to the very large dataset of observational studies,” said Brito Campana.
The results showed a low absolute risk of thyroid cancer, with only 0.17% of patients in the GLP-1 group developing the disease. However, the data also showed a potential relative increase in risk during the first year of GLP-1 receptor agonist use.
“This is likely due to increased detection rather than true incidence, as the latency period for thyroid cancer development is typically longer,” Brito Campana said.
“We also note the limitations of the observational study design, including the short follow-up period and lack of detailed histological data. However, we believe the benefits of GLP-1 receptor agonists likely outweigh the risk of thyroid cancer.”
Malignancy in Bethesda III and IV Thyroid Nodules
At the same ATA session, Sapir Nachum Goldberg, MD, of the University of Pennsylvania, Philadelphia, presented the results of a retrospective record review that examined the prevalence of malignancy in Bethesda III and IV thyroid nodules with negative Thyrogen Receptor Signaling (ThyroSeq) version 3 molecular testing results.
Goldberg reported that 87% of patients with ThyroSeq negative subtype results were managed nonoperatively. “Based on our data, the true prevalence of malignancy likely lies between our low and high estimates of 3% and 23%,” she said. “We believe that the prevalence of malignancy may be higher in real-world practice than validation studies.”
Additionally, nodules with “currently negative” or “negative but limited” ThyroSeq results had a higher prevalence of malignancy (7%), compared with those with a “negative” result (2%). Factors like immediate vs delayed surgery, nodule size, and ultrasound pattern did not significantly impact malignancy prevalence.
The study results also indicated that surveillance ultrasonography is not routinely performed in up to one-third of patients, Goldberg said.
She closed by suggesting that colleagues consider the negative subtype in clinical decision-making. For “negative but limited” nodules, repeat the fine needle aspiration and, for “negative” and “currently negative” nodules, consider ultrasound follow-up as per ATA guidelines for Bethesda II cytology, she said.
RET-Mutated Medullary Thyroid Cancer
For patients with RET-mutated medullary thyroid cancer, Julien Hadoux, MD, PhD, of Institut de Cancérologie Gustave Roussy, Villejuif, France, presented a combined analysis of the efficacy of the RET inhibitor selpercatinib from the phase 1/2 LIBRETTO-001 and phase 3 LIBRETTO-531 trials.
This post hoc analysis used a combined cohort of 509 patients with RET-mutated advanced or metastatic medullary thyroid cancer who had received selpercatinib in the two trials.
Hadoux reported that robust and durable responses were seen across all mutation groups, including M918T, extracellular cysteine, and an “other” group composed of various uncommon RET mutations. “The median [progression-free survival] PFS was not reached for either the M918T or extracellular groups and it was 51.4 months for the Other group,” he said.
“Selpercatinib showed superior median PFS vs control, regardless of the RET mutation. This analysis constitutes the largest catalog of RET mutations in medullary thyroid cancers treated with RET-specific inhibitors.”
TRK-Fusion Differentiated Thyroid Cancer
Steven Waguespack, MD, of the University of Texas MD Anderson Cancer Center, Houston, shared updated efficacy and safety data from three phase 1/2 pooled clinical trials of the tropomyosin kinase receptor (TRK) inhibitor larotrectinib in thyroid cancer. These data updated results initially published in 2022.
“Larotrectinib continues to demonstrate rapid and durable responses, extended survival, and offers a favorable safety profile in patients with TRK fusion differentiated thyroid cancer, with limited activity in anaplastic thyroid cancer,” Waguespack said.
“Additionally, in a subset of patients, we identified some acquired on-target NTRK mutations and off-target GNAS and TP53 mutations that may give further insight into mechanisms of resistance.”
The primary endpoint was the investigator-assessed objective response rate (ORR); at 48 months, the ORR was 79% by independent review. The median PFS in patients with TRK fusion differentiated thyroid cancer was 44 months, while the median duration of response was 41 months. The 4-year overall survival rate was 86%.
Waguespack closed with a cautionary note to colleagues: “While circulating tumor DNA next-generation sequencing (NGS) analysis can be used to test for NTRK gene fusions, negative results should be followed up with tissue-based NGS,” he said.
Brito Campana and Goldberg disclosed no relevant financial relationships. Hadoux reported receiving honoraria for speaker engagements, advisory roles, or funding for CME from Eli Lilly, AAA, IPSEN, Roche, Pharma Mar, and EISAI, and research grants from Novartis, Sanofi, and Eli Lilly.
A version of this article appeared on Medscape.com.
FROM ATA 2024
Lifestyle Medicine Trends to Keep an Eye On
Our current healthcare system, which is a costly and unending cycle of merely managing chronic disease symptoms, is failing us. What we truly need is a patient-centered approach that restores health by addressing not just diagnoses but also the physical, emotional, and social needs of each individual. This is the essence of whole-person health, and transformation toward this model of care is already underway.
This shift underscores why clinicians like me support placing lifestyle medicine at the foundation of health and healthcare. Evidence-based lifestyle medicine — which applies interventions in nutrition, physical activity, restorative sleep, stress management, positive social connections, and avoidance of risky substances to prevent, treat, and when used intensively, even reverse lifestyle-related chronic disease — is a medical specialty equipped to successfully address patients’ whole-person health in an effective, high-value clinical care delivery model.
As this transformation continues, here are four key lifestyle medicine trends for 2025.
Lifestyle Medicine Becomes More Ingrained in Primary Care
The 2021 National Academies of Science, Engineering, and Medicine report, “Implementing High-Quality Primary Care” sounded the alarm about the state of primary care and outlined a comprehensive approach to transform it. Lifestyle medicine emerged as a solution as clinicians found innovative ways to integrate lifestyle behavior interventions into existing care models in a financially sustainable, scalable manner. Examples include Blue Zones Health, a new delivery model that aligns lifestyle medicine–certified clinicians with community and payers in California, and the University of Pittsburgh Medical Center lifestyle medicine program, where primary care patients are referred to virtual group coaching, a teaching kitchen, and classes on food as medicine, obesity, type 2 diabetes, and more.
Organizations dedicated to advancing primary care are paying close attention to the potential of lifestyle medicine. Currently, The Primary Care Collaborative has launched a new multi-year initiative on whole-person care and lifestyle medicine. This initiative aims to broaden the primary care community’s understanding of whole health and lifestyle medicine concepts and the evidence behind them, as well as lay the groundwork for future work to promote whole-person primary care and lifestyle medicine among an engaged and committed community of members.
Digital Tools and AI Spark Lifestyle Medicine Innovations
American College of Lifestyle Medicine partner organizations are increasingly utilizing digital tools, such as health apps tailored to lifestyle behavior interventions, to expand access to care and support behavior change. One of the biggest challenges in lifestyle interventions is the limited time during patient encounters. But artificial intelligence (AI) tools can record (with patient permission) and summarize encounters, enabling clinicians to turn away from their keyboards and be more present to learn about the unique living, environmental, and societal factors that impact every individual’s lifestyle choices. AI tools can create individualized whole-food, plant-predominant meal plans or physical activity schedules for patients in just a few seconds. The potential for AI in lifestyle medicine is vast, and its applications were further explored at the American College of Lifestyle Medicine’s annual conference in October.
Behavior Change and Sustainability of the Food-as-Medicine Movement
Significant investments have been made in food as medicine to address diet-related chronic diseases. But merely providing medically tailored meals or produce prescriptions is not enough because once the prescriptions end, so will the health benefits. Clinicians certified in lifestyle medicine are prepared to coach patients into long-term behavior change, supporting them with education and information to shop for and prepare tasty, nutritious, and affordable food. The same applies to the use of glucagon-like peptide 1 drugs. Although the initial weight loss offers motivation, lifestyle changes are necessary to sustain long-term health benefits beyond medications.
Lifestyle Medicine Emerges as a Strategy to Achieve Health Equity
Lifestyle behavior interventions have the unique ability to address health status and social drivers of health. For example, food as medicine affects an individual’s health while also addressing nutrition security. Certainly, no medication can both improve health status and feed someone. The addition of payment for the screening of social drivers of health to the 2024 Medicare Physician Fee Schedule is an important step toward connecting clinicians with community health–based organizations that can address factors that influence patients’ ability to adhere to lifestyle behavior care plans. Lifestyle medicine clinicians are poised to lead this effort because they are already having conversations with patients about their environment, living conditions, and access to nutritious food.
The changes coming to our healthcare system are exciting and long overdue. Lifestyle medicine is positioned to be at the forefront of this transformation now and in the future.
Dr. Patel, president of the American College of Lifestyle Medicine in St. Louis, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Our current healthcare system, which is a costly and unending cycle of merely managing chronic disease symptoms, is failing us. What we truly need is a patient-centered approach that restores health by addressing not just diagnoses but also the physical, emotional, and social needs of each individual. This is the essence of whole-person health, and transformation toward this model of care is already underway.
This shift underscores why clinicians like me support placing lifestyle medicine at the foundation of health and healthcare. Evidence-based lifestyle medicine — which applies interventions in nutrition, physical activity, restorative sleep, stress management, positive social connections, and avoidance of risky substances to prevent, treat, and when used intensively, even reverse lifestyle-related chronic disease — is a medical specialty equipped to successfully address patients’ whole-person health in an effective, high-value clinical care delivery model.
As this transformation continues, here are four key lifestyle medicine trends for 2025.
Lifestyle Medicine Becomes More Ingrained in Primary Care
The 2021 National Academies of Science, Engineering, and Medicine report, “Implementing High-Quality Primary Care” sounded the alarm about the state of primary care and outlined a comprehensive approach to transform it. Lifestyle medicine emerged as a solution as clinicians found innovative ways to integrate lifestyle behavior interventions into existing care models in a financially sustainable, scalable manner. Examples include Blue Zones Health, a new delivery model that aligns lifestyle medicine–certified clinicians with community and payers in California, and the University of Pittsburgh Medical Center lifestyle medicine program, where primary care patients are referred to virtual group coaching, a teaching kitchen, and classes on food as medicine, obesity, type 2 diabetes, and more.
Organizations dedicated to advancing primary care are paying close attention to the potential of lifestyle medicine. Currently, The Primary Care Collaborative has launched a new multi-year initiative on whole-person care and lifestyle medicine. This initiative aims to broaden the primary care community’s understanding of whole health and lifestyle medicine concepts and the evidence behind them, as well as lay the groundwork for future work to promote whole-person primary care and lifestyle medicine among an engaged and committed community of members.
Digital Tools and AI Spark Lifestyle Medicine Innovations
American College of Lifestyle Medicine partner organizations are increasingly utilizing digital tools, such as health apps tailored to lifestyle behavior interventions, to expand access to care and support behavior change. One of the biggest challenges in lifestyle interventions is the limited time during patient encounters. But artificial intelligence (AI) tools can record (with patient permission) and summarize encounters, enabling clinicians to turn away from their keyboards and be more present to learn about the unique living, environmental, and societal factors that impact every individual’s lifestyle choices. AI tools can create individualized whole-food, plant-predominant meal plans or physical activity schedules for patients in just a few seconds. The potential for AI in lifestyle medicine is vast, and its applications were further explored at the American College of Lifestyle Medicine’s annual conference in October.
Behavior Change and Sustainability of the Food-as-Medicine Movement
Significant investments have been made in food as medicine to address diet-related chronic diseases. But merely providing medically tailored meals or produce prescriptions is not enough because once the prescriptions end, so will the health benefits. Clinicians certified in lifestyle medicine are prepared to coach patients into long-term behavior change, supporting them with education and information to shop for and prepare tasty, nutritious, and affordable food. The same applies to the use of glucagon-like peptide 1 drugs. Although the initial weight loss offers motivation, lifestyle changes are necessary to sustain long-term health benefits beyond medications.
Lifestyle Medicine Emerges as a Strategy to Achieve Health Equity
Lifestyle behavior interventions have the unique ability to address health status and social drivers of health. For example, food as medicine affects an individual’s health while also addressing nutrition security. Certainly, no medication can both improve health status and feed someone. The addition of payment for the screening of social drivers of health to the 2024 Medicare Physician Fee Schedule is an important step toward connecting clinicians with community health–based organizations that can address factors that influence patients’ ability to adhere to lifestyle behavior care plans. Lifestyle medicine clinicians are poised to lead this effort because they are already having conversations with patients about their environment, living conditions, and access to nutritious food.
The changes coming to our healthcare system are exciting and long overdue. Lifestyle medicine is positioned to be at the forefront of this transformation now and in the future.
Dr. Patel, president of the American College of Lifestyle Medicine in St. Louis, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Our current healthcare system, which is a costly and unending cycle of merely managing chronic disease symptoms, is failing us. What we truly need is a patient-centered approach that restores health by addressing not just diagnoses but also the physical, emotional, and social needs of each individual. This is the essence of whole-person health, and transformation toward this model of care is already underway.
This shift underscores why clinicians like me support placing lifestyle medicine at the foundation of health and healthcare. Evidence-based lifestyle medicine — which applies interventions in nutrition, physical activity, restorative sleep, stress management, positive social connections, and avoidance of risky substances to prevent, treat, and when used intensively, even reverse lifestyle-related chronic disease — is a medical specialty equipped to successfully address patients’ whole-person health in an effective, high-value clinical care delivery model.
As this transformation continues, here are four key lifestyle medicine trends for 2025.
Lifestyle Medicine Becomes More Ingrained in Primary Care
The 2021 National Academies of Science, Engineering, and Medicine report, “Implementing High-Quality Primary Care” sounded the alarm about the state of primary care and outlined a comprehensive approach to transform it. Lifestyle medicine emerged as a solution as clinicians found innovative ways to integrate lifestyle behavior interventions into existing care models in a financially sustainable, scalable manner. Examples include Blue Zones Health, a new delivery model that aligns lifestyle medicine–certified clinicians with community and payers in California, and the University of Pittsburgh Medical Center lifestyle medicine program, where primary care patients are referred to virtual group coaching, a teaching kitchen, and classes on food as medicine, obesity, type 2 diabetes, and more.
Organizations dedicated to advancing primary care are paying close attention to the potential of lifestyle medicine. Currently, The Primary Care Collaborative has launched a new multi-year initiative on whole-person care and lifestyle medicine. This initiative aims to broaden the primary care community’s understanding of whole health and lifestyle medicine concepts and the evidence behind them, as well as lay the groundwork for future work to promote whole-person primary care and lifestyle medicine among an engaged and committed community of members.
Digital Tools and AI Spark Lifestyle Medicine Innovations
American College of Lifestyle Medicine partner organizations are increasingly utilizing digital tools, such as health apps tailored to lifestyle behavior interventions, to expand access to care and support behavior change. One of the biggest challenges in lifestyle interventions is the limited time during patient encounters. But artificial intelligence (AI) tools can record (with patient permission) and summarize encounters, enabling clinicians to turn away from their keyboards and be more present to learn about the unique living, environmental, and societal factors that impact every individual’s lifestyle choices. AI tools can create individualized whole-food, plant-predominant meal plans or physical activity schedules for patients in just a few seconds. The potential for AI in lifestyle medicine is vast, and its applications were further explored at the American College of Lifestyle Medicine’s annual conference in October.
Behavior Change and Sustainability of the Food-as-Medicine Movement
Significant investments have been made in food as medicine to address diet-related chronic diseases. But merely providing medically tailored meals or produce prescriptions is not enough because once the prescriptions end, so will the health benefits. Clinicians certified in lifestyle medicine are prepared to coach patients into long-term behavior change, supporting them with education and information to shop for and prepare tasty, nutritious, and affordable food. The same applies to the use of glucagon-like peptide 1 drugs. Although the initial weight loss offers motivation, lifestyle changes are necessary to sustain long-term health benefits beyond medications.
Lifestyle Medicine Emerges as a Strategy to Achieve Health Equity
Lifestyle behavior interventions have the unique ability to address health status and social drivers of health. For example, food as medicine affects an individual’s health while also addressing nutrition security. Certainly, no medication can both improve health status and feed someone. The addition of payment for the screening of social drivers of health to the 2024 Medicare Physician Fee Schedule is an important step toward connecting clinicians with community health–based organizations that can address factors that influence patients’ ability to adhere to lifestyle behavior care plans. Lifestyle medicine clinicians are poised to lead this effort because they are already having conversations with patients about their environment, living conditions, and access to nutritious food.
The changes coming to our healthcare system are exciting and long overdue. Lifestyle medicine is positioned to be at the forefront of this transformation now and in the future.
Dr. Patel, president of the American College of Lifestyle Medicine in St. Louis, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Can Fish Skin Grafts Heal Diabetic Foot Ulcers?
TOPLINE:
METHODOLOGY:
- Standard wound care for diabetic foot ulcers involves vascular assessment, surgical debridement, use of appropriate dressings, infection management, and glycemic control; however, standard care is typically associated with poor outcomes.
- Researchers conducted a multicenter clinical trial in 15 tertiary care centers with diabetic foot units across France, Italy, Germany, and Sweden to evaluate the efficacy and safety of intact fish skin grafts over standard-of-care practices in treating complex diabetic foot ulcers.
- A total of 255 patients aged 18 years or older with diabetes and lower limb wounds penetrating to the tendon, capsule, bone, or joint were randomly assigned to receive either an intact fish skin graft or standard wound care for 14 weeks.
- The primary endpoint was the percentage of wounds achieving complete closure by 16 weeks.
- Wound healing was also assessed at 20 and 24 weeks.
TAKEAWAY:
- The proportion of wounds healed at 16 weeks was higher with intact fish skin grafts than with standard-of-care (44.0% vs 26.4% adjusted odds ratio [aOR], 2.58; 95% CI, 1.48-4.56).
- The fish skin grafts continued to be more effective than standard wound care practices at weeks 20 (aOR, 2.15; 95% CI, 1.27–3.70) and 24 (aOR, 2.19; 95% CI, 1.31–3.70).
- The mean time to healing was 17.31 weeks for the intact fish skin graft group and 19.37 weeks for the standard-of-care group; intact fish skin grafts were also associated with faster healing times than standard wound care (hazard ratio, 1.59; 95% CI, 1.07-2.36).
- Target wound infections were the most common adverse events, occurring in a similar number of patients in both the groups.
IN PRACTICE:
“Our trial demonstrated treatment of complex diabetic foot ulcers with intact fish skin grafts achieved a significantly greater proportion of diabetic foot ulcers healed at 16 weeks than standard of care, and was associated with increased healing at 20 and 24 weeks. That these results were achieved in non-superficial UT [University of Texas diabetic wound classification system] grade 2 and 3 diabetic foot ulcers and included ischemic and/or infected diabetic foot ulcers is of importance,” the authors wrote.
SOURCE:
The study was led by Dured Dardari, MD, PhD, Center Hospitalier Sud Francilien, Corbeil-Essonnes, France, and was published online in NEJM Evidence.
LIMITATIONS:
No limitations were discussed for this study.
DISCLOSURES:
The study was funded by European Commission Fast Track to Innovation Horizon 2020 and Kerecis. Two authors reported being employees with or without stock options at Kerecis, and other authors reported having ties with many sources including Kerecis.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Standard wound care for diabetic foot ulcers involves vascular assessment, surgical debridement, use of appropriate dressings, infection management, and glycemic control; however, standard care is typically associated with poor outcomes.
- Researchers conducted a multicenter clinical trial in 15 tertiary care centers with diabetic foot units across France, Italy, Germany, and Sweden to evaluate the efficacy and safety of intact fish skin grafts over standard-of-care practices in treating complex diabetic foot ulcers.
- A total of 255 patients aged 18 years or older with diabetes and lower limb wounds penetrating to the tendon, capsule, bone, or joint were randomly assigned to receive either an intact fish skin graft or standard wound care for 14 weeks.
- The primary endpoint was the percentage of wounds achieving complete closure by 16 weeks.
- Wound healing was also assessed at 20 and 24 weeks.
TAKEAWAY:
- The proportion of wounds healed at 16 weeks was higher with intact fish skin grafts than with standard-of-care (44.0% vs 26.4% adjusted odds ratio [aOR], 2.58; 95% CI, 1.48-4.56).
- The fish skin grafts continued to be more effective than standard wound care practices at weeks 20 (aOR, 2.15; 95% CI, 1.27–3.70) and 24 (aOR, 2.19; 95% CI, 1.31–3.70).
- The mean time to healing was 17.31 weeks for the intact fish skin graft group and 19.37 weeks for the standard-of-care group; intact fish skin grafts were also associated with faster healing times than standard wound care (hazard ratio, 1.59; 95% CI, 1.07-2.36).
- Target wound infections were the most common adverse events, occurring in a similar number of patients in both the groups.
IN PRACTICE:
“Our trial demonstrated treatment of complex diabetic foot ulcers with intact fish skin grafts achieved a significantly greater proportion of diabetic foot ulcers healed at 16 weeks than standard of care, and was associated with increased healing at 20 and 24 weeks. That these results were achieved in non-superficial UT [University of Texas diabetic wound classification system] grade 2 and 3 diabetic foot ulcers and included ischemic and/or infected diabetic foot ulcers is of importance,” the authors wrote.
SOURCE:
The study was led by Dured Dardari, MD, PhD, Center Hospitalier Sud Francilien, Corbeil-Essonnes, France, and was published online in NEJM Evidence.
LIMITATIONS:
No limitations were discussed for this study.
DISCLOSURES:
The study was funded by European Commission Fast Track to Innovation Horizon 2020 and Kerecis. Two authors reported being employees with or without stock options at Kerecis, and other authors reported having ties with many sources including Kerecis.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Standard wound care for diabetic foot ulcers involves vascular assessment, surgical debridement, use of appropriate dressings, infection management, and glycemic control; however, standard care is typically associated with poor outcomes.
- Researchers conducted a multicenter clinical trial in 15 tertiary care centers with diabetic foot units across France, Italy, Germany, and Sweden to evaluate the efficacy and safety of intact fish skin grafts over standard-of-care practices in treating complex diabetic foot ulcers.
- A total of 255 patients aged 18 years or older with diabetes and lower limb wounds penetrating to the tendon, capsule, bone, or joint were randomly assigned to receive either an intact fish skin graft or standard wound care for 14 weeks.
- The primary endpoint was the percentage of wounds achieving complete closure by 16 weeks.
- Wound healing was also assessed at 20 and 24 weeks.
TAKEAWAY:
- The proportion of wounds healed at 16 weeks was higher with intact fish skin grafts than with standard-of-care (44.0% vs 26.4% adjusted odds ratio [aOR], 2.58; 95% CI, 1.48-4.56).
- The fish skin grafts continued to be more effective than standard wound care practices at weeks 20 (aOR, 2.15; 95% CI, 1.27–3.70) and 24 (aOR, 2.19; 95% CI, 1.31–3.70).
- The mean time to healing was 17.31 weeks for the intact fish skin graft group and 19.37 weeks for the standard-of-care group; intact fish skin grafts were also associated with faster healing times than standard wound care (hazard ratio, 1.59; 95% CI, 1.07-2.36).
- Target wound infections were the most common adverse events, occurring in a similar number of patients in both the groups.
IN PRACTICE:
“Our trial demonstrated treatment of complex diabetic foot ulcers with intact fish skin grafts achieved a significantly greater proportion of diabetic foot ulcers healed at 16 weeks than standard of care, and was associated with increased healing at 20 and 24 weeks. That these results were achieved in non-superficial UT [University of Texas diabetic wound classification system] grade 2 and 3 diabetic foot ulcers and included ischemic and/or infected diabetic foot ulcers is of importance,” the authors wrote.
SOURCE:
The study was led by Dured Dardari, MD, PhD, Center Hospitalier Sud Francilien, Corbeil-Essonnes, France, and was published online in NEJM Evidence.
LIMITATIONS:
No limitations were discussed for this study.
DISCLOSURES:
The study was funded by European Commission Fast Track to Innovation Horizon 2020 and Kerecis. Two authors reported being employees with or without stock options at Kerecis, and other authors reported having ties with many sources including Kerecis.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Open Clinical Trials for Patients With Diabetes
Actively Recruiting
→Continuous Glucose Monitoring Devices in Hospitalized Veterans With Diabetes
More than 25% of the patients admitted in the general wards have a history of diabetes mellitus. Up to 30% of the hospitalized diabetics develop hypoglycemia (low glucose values); a condition that is associated with seizures, cardiac arrhythmias, and even death. In veterans, the prevalence is disproportionally higher. It is estimated that 40% to 50% of hospitalized veterans are diabetics. In this clinical trial the investigators describe the development of a novel system, the Glucose Telemetry System, with which glucose values can be wirelessly transmitted from the patient’s bedside to a monitor device at the nursing station. The goal of this work is to develop a more effective glucose surveillance system at the general wards, which can decrease hypoglycemia in the hospital and improve clinical outcomes.
ID: NCT03508934
Sponsor; Investigator: VA Office of Research and Development; Ilias Spanakis, MD
Locations: Baltimore VA Medical Center, Maryland
→Optimizing Gait Rehabilitation for Veterans With Non-Traumatic Lower Limb Amputation (GEM)
The population of older veterans with nontraumatic lower limb amputation is growing. Following lower limb amputation, asymmetrical movements persist during walking and likely contribute to disabling sequelae including secondary pain conditions, poor gait efficiency, impaired physical function, and compromised skin integrity of the residual limb. This study seeks to address chronic gait asymmetry by evaluating the efficacy of two error-manipulation gait training programs to improve gait symmetry for veterans with nontraumatic lower limb amputation. Additionally, this study will evaluate the potential of error-manipulation training programs to improve secondary measures of disability and residual limb skin health. Ultimately, this study aims to improve conventional prosthetic rehabilitation for veterans with nontraumatic amputation through gait training programs based in motor learning principles, resulting in improved.
ID: NCT003995238
Sponsor; Investigator: VA Office of Research and Development; Cory L. Christiansen, PhD
Locations: Rocky Mountain Regional VA Medical Center, Aurora, Colorado; Hunter Holmes McGuire VA Medical Center, Richmond, Virginia
→Empowering Veterans to Actively Communicate and Engage in Shared Decision Making in Medical Visits, A Randomized Controlled Trial (ACTIVet-2)
Type 2 diabetes is a significant condition in VA affecting 20% of VA patients. Adherence to medication regimens and lifestyle factors is important to achieve care goals for these patients. Patients who use active participatory communication behaviors with their providers have better adherence to treatment and better biomedical outcomes, yet many patients are not prepared to engage in active communication with their providers. Existing coaching interventions have not been adopted in practice because of the cost of trained personnel. The investigators haveshown the efficacy of a low-cost video that did not require trained personnel. This proposal proposes to test implementation strategies to deliver that video in VA primary care clinics and to test the effectiveness of the video to improve outcomes in a hybrid type 2 effectiveness implementation trial using a cluster randomized stepped wedgedesign at eight sites. This proposal will test feasibility of implementing the video and if successful will generate the evidence to justify widespread dissemination of the video.
ID: NCT05169359
Sponsor; Investigator: VA Office of Research and Development; Howard S. Gordon, MD
Location: 8 locations, including Jesse Brown VA Medical Center, Chicago; Edward Hines Jr. VA Hopsital, Hines, Illinois
→The Diabetes Staging System in Patient Aligned Care Teams
The purpose of this study is to examine the feasibility/acceptability of the Diabetes Staging System in patient aligned care teams and its ability to increase sodium-glucosecotransporter-2 inhibitor and glucagon-like-1 peptide use in veteran patients with type 2 diabetes and cardiovascular disease and/or chronic kidney disease. A novel type 2 diabetes staging system patterned after Tumor Node Metastasis cancer staging that uses the number of macrovascular and microvascular complications and most recent hemoglobin A1c and glomerular filtration rate to determine Diabetes Staging System stage which reflects disease severity.
ID: NCT06142006
Sponsor; Collaborator: Durham VA Medical Center; Moahad Dar, MD
Location: Greenville VA Health Care Center, North Carolina
→Enhancing Mental and Physical Health of Women Veterans (EMPOWER)
Women veterans are the fastest growing segment of VA users. This dramatic growth has created challenges for VA to ensure that appropriate services are available to meet women veterans’ needs, and that they will want and be able to use those services. The EMPOWER QUERI 2.0 Program is a cluster randomized type 3 hybrid implementation effectiveness trial testing 2 strategies designed to support implementation and sustainment of evidence-based practices for women veterans in at least 20 VA facilities from 4 regions.
ID: NCT05050266
Sponsor; Investigator: VA Office of Research and Development; Alison B. Hamilton, PhD, MPH
Location: VA Greater Los Angeles Healthcare System
→Investigation of Rifampin to Reduce Pedal Amputations for Osteomyelitis in Diabetics (VA INTREPID)
The purpose of this research study is to determine if rifampin, an antibiotic (a medicine that treats infections), is effective in treating osteomyelitis (infection of the bone) of the foot in patients with diabetes. Despite use of powerful antibiotics prescribed over a long period of time, many diabetic patients remain at a high risk for needing an amputation of part of the foot or lower leg because the osteomyelitis is not cured. Some small research studies have shown that addition of rifampin to other antibiotics is effective in treating osteomyelitis. However, because few diabetics with osteomyelitis have been studied, there is no definite proof that it is better than the usual treatments for diabetic patients. If this study finds that adding rifampin to the usual antibiotics prescribed for osteomyelitis reduces the risk for amputations, doctors will be able to more effectively treat many veteran patients with this serious infection. Improving treatment outcomes is an important healthcare goal of the VA.
ID: NCT03012529
Sponsor; Investigator: VA Office of Research and Development; Paul A. Monach, MD, PhD
Location: 30 VA locations, including South Texas Health Care System, San Antonio; Cincinnati VA Medical Center, Ohio; VA Northern California Health Care System, Mather; Washington DC VA Medical Center; William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin
→Effectiveness of Remote Foot Temperature Monitoring (STOP)
Diabetic foot ulcers are common, debilitating, and costly complications of diabetes, disproportionately impacting Black and rural veterans. Forty percent of individuals have an ulcer recurrence within a year of ulcer healing and 65% within 5 years. Monitoring plantar foot temperatures is one of the few interventions that reduces the risk of ulcer recurrence. Despite the evidence, adoption has been poorbecause the original procedures, including the use of handheld thermometers, were burdensome and time-consuming. Podimetrics, a private company, has developed a temperature monitoring system involving a “smart” mat that can wirelessly transmit data and a remote monitoring team that works with VA providers to assist with triage and monitoring. This care model has incredible promise, but has been untested in VA. The investigators propose to conduct a randomized trial to evaluate effectiveness of remote temperature monitoring as well as costs. Additionally, the investigators will evaluate the implementation process, including barriers and facilitators to use among key stakeholders.
ID: NCT05728411
Sponsor; Investigator: VA Office of Research and Development; Rachel M. Thomas
Location: Edward Hines Jr. VA Hospital, Hines, Illinois; Hunter Holmes McGuire VA Medical Center, Richmond, Virginia; VA Puget Sound Health Care System, Seattle, Washington
→Continuous Glucose Monitoring for Hyperglycemia in Critically Ill Patients
The investigators intend to conduct a single-center, prospective, randomized comparative trial of patients admitted to the intensive care unit who received continuous glucose monitoring vs point of care glucose monitoring. The study will examine relevant outcomes for patients in the intensive care unit with diabetes mellitus and/or hyperglycemia. The primary outcome of the study will be the proportion of time in target range (blood glucose 70-180 mg/dL).
ID: NCT05442853
Sponsor; Investigator: Malcom Randall VA Medical Center; Andrew J. Franck, PharmD
Location: Malcolm Randall VA Medical Center, Gainesville, Florida
→Investigation of Metformin in Pre-Diabetes on Atherosclerotic Cardiovascular OuTcomes (VA-IMPACT)
CSP #2002 is a multicenter, prospective, randomized, double blind, secondary prevention trial to test the hypothesis that treatment with metformin, compared with placebo, reduces mortality and cardiovascular morbidity in veterans with pre-diabetes and established atherosclerotic cardiovascular disease. Qualifying patients have pre-diabetes defined by HbA 1c , fasting blood glucose, or oral glucose tolerance test criteria; clinically evident coronary, cerebrovascular, or peripheral arterial atherosclerotic cardiovascular disease; and estimated glomerular filtration rate of at least 45 mL/min/1.73 m 2 ; and do not fulfill any exclusion criteria.
ID: NCT04838392
Sponsor; Investigator: VA Office of Research and Development; Gregory G. Schwartz, PhD, MD
Locations: 40 locations
Actively Recruiting
→Continuous Glucose Monitoring Devices in Hospitalized Veterans With Diabetes
More than 25% of the patients admitted in the general wards have a history of diabetes mellitus. Up to 30% of the hospitalized diabetics develop hypoglycemia (low glucose values); a condition that is associated with seizures, cardiac arrhythmias, and even death. In veterans, the prevalence is disproportionally higher. It is estimated that 40% to 50% of hospitalized veterans are diabetics. In this clinical trial the investigators describe the development of a novel system, the Glucose Telemetry System, with which glucose values can be wirelessly transmitted from the patient’s bedside to a monitor device at the nursing station. The goal of this work is to develop a more effective glucose surveillance system at the general wards, which can decrease hypoglycemia in the hospital and improve clinical outcomes.
ID: NCT03508934
Sponsor; Investigator: VA Office of Research and Development; Ilias Spanakis, MD
Locations: Baltimore VA Medical Center, Maryland
→Optimizing Gait Rehabilitation for Veterans With Non-Traumatic Lower Limb Amputation (GEM)
The population of older veterans with nontraumatic lower limb amputation is growing. Following lower limb amputation, asymmetrical movements persist during walking and likely contribute to disabling sequelae including secondary pain conditions, poor gait efficiency, impaired physical function, and compromised skin integrity of the residual limb. This study seeks to address chronic gait asymmetry by evaluating the efficacy of two error-manipulation gait training programs to improve gait symmetry for veterans with nontraumatic lower limb amputation. Additionally, this study will evaluate the potential of error-manipulation training programs to improve secondary measures of disability and residual limb skin health. Ultimately, this study aims to improve conventional prosthetic rehabilitation for veterans with nontraumatic amputation through gait training programs based in motor learning principles, resulting in improved.
ID: NCT003995238
Sponsor; Investigator: VA Office of Research and Development; Cory L. Christiansen, PhD
Locations: Rocky Mountain Regional VA Medical Center, Aurora, Colorado; Hunter Holmes McGuire VA Medical Center, Richmond, Virginia
→Empowering Veterans to Actively Communicate and Engage in Shared Decision Making in Medical Visits, A Randomized Controlled Trial (ACTIVet-2)
Type 2 diabetes is a significant condition in VA affecting 20% of VA patients. Adherence to medication regimens and lifestyle factors is important to achieve care goals for these patients. Patients who use active participatory communication behaviors with their providers have better adherence to treatment and better biomedical outcomes, yet many patients are not prepared to engage in active communication with their providers. Existing coaching interventions have not been adopted in practice because of the cost of trained personnel. The investigators haveshown the efficacy of a low-cost video that did not require trained personnel. This proposal proposes to test implementation strategies to deliver that video in VA primary care clinics and to test the effectiveness of the video to improve outcomes in a hybrid type 2 effectiveness implementation trial using a cluster randomized stepped wedgedesign at eight sites. This proposal will test feasibility of implementing the video and if successful will generate the evidence to justify widespread dissemination of the video.
ID: NCT05169359
Sponsor; Investigator: VA Office of Research and Development; Howard S. Gordon, MD
Location: 8 locations, including Jesse Brown VA Medical Center, Chicago; Edward Hines Jr. VA Hopsital, Hines, Illinois
→The Diabetes Staging System in Patient Aligned Care Teams
The purpose of this study is to examine the feasibility/acceptability of the Diabetes Staging System in patient aligned care teams and its ability to increase sodium-glucosecotransporter-2 inhibitor and glucagon-like-1 peptide use in veteran patients with type 2 diabetes and cardiovascular disease and/or chronic kidney disease. A novel type 2 diabetes staging system patterned after Tumor Node Metastasis cancer staging that uses the number of macrovascular and microvascular complications and most recent hemoglobin A1c and glomerular filtration rate to determine Diabetes Staging System stage which reflects disease severity.
ID: NCT06142006
Sponsor; Collaborator: Durham VA Medical Center; Moahad Dar, MD
Location: Greenville VA Health Care Center, North Carolina
→Enhancing Mental and Physical Health of Women Veterans (EMPOWER)
Women veterans are the fastest growing segment of VA users. This dramatic growth has created challenges for VA to ensure that appropriate services are available to meet women veterans’ needs, and that they will want and be able to use those services. The EMPOWER QUERI 2.0 Program is a cluster randomized type 3 hybrid implementation effectiveness trial testing 2 strategies designed to support implementation and sustainment of evidence-based practices for women veterans in at least 20 VA facilities from 4 regions.
ID: NCT05050266
Sponsor; Investigator: VA Office of Research and Development; Alison B. Hamilton, PhD, MPH
Location: VA Greater Los Angeles Healthcare System
→Investigation of Rifampin to Reduce Pedal Amputations for Osteomyelitis in Diabetics (VA INTREPID)
The purpose of this research study is to determine if rifampin, an antibiotic (a medicine that treats infections), is effective in treating osteomyelitis (infection of the bone) of the foot in patients with diabetes. Despite use of powerful antibiotics prescribed over a long period of time, many diabetic patients remain at a high risk for needing an amputation of part of the foot or lower leg because the osteomyelitis is not cured. Some small research studies have shown that addition of rifampin to other antibiotics is effective in treating osteomyelitis. However, because few diabetics with osteomyelitis have been studied, there is no definite proof that it is better than the usual treatments for diabetic patients. If this study finds that adding rifampin to the usual antibiotics prescribed for osteomyelitis reduces the risk for amputations, doctors will be able to more effectively treat many veteran patients with this serious infection. Improving treatment outcomes is an important healthcare goal of the VA.
ID: NCT03012529
Sponsor; Investigator: VA Office of Research and Development; Paul A. Monach, MD, PhD
Location: 30 VA locations, including South Texas Health Care System, San Antonio; Cincinnati VA Medical Center, Ohio; VA Northern California Health Care System, Mather; Washington DC VA Medical Center; William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin
→Effectiveness of Remote Foot Temperature Monitoring (STOP)
Diabetic foot ulcers are common, debilitating, and costly complications of diabetes, disproportionately impacting Black and rural veterans. Forty percent of individuals have an ulcer recurrence within a year of ulcer healing and 65% within 5 years. Monitoring plantar foot temperatures is one of the few interventions that reduces the risk of ulcer recurrence. Despite the evidence, adoption has been poorbecause the original procedures, including the use of handheld thermometers, were burdensome and time-consuming. Podimetrics, a private company, has developed a temperature monitoring system involving a “smart” mat that can wirelessly transmit data and a remote monitoring team that works with VA providers to assist with triage and monitoring. This care model has incredible promise, but has been untested in VA. The investigators propose to conduct a randomized trial to evaluate effectiveness of remote temperature monitoring as well as costs. Additionally, the investigators will evaluate the implementation process, including barriers and facilitators to use among key stakeholders.
ID: NCT05728411
Sponsor; Investigator: VA Office of Research and Development; Rachel M. Thomas
Location: Edward Hines Jr. VA Hospital, Hines, Illinois; Hunter Holmes McGuire VA Medical Center, Richmond, Virginia; VA Puget Sound Health Care System, Seattle, Washington
→Continuous Glucose Monitoring for Hyperglycemia in Critically Ill Patients
The investigators intend to conduct a single-center, prospective, randomized comparative trial of patients admitted to the intensive care unit who received continuous glucose monitoring vs point of care glucose monitoring. The study will examine relevant outcomes for patients in the intensive care unit with diabetes mellitus and/or hyperglycemia. The primary outcome of the study will be the proportion of time in target range (blood glucose 70-180 mg/dL).
ID: NCT05442853
Sponsor; Investigator: Malcom Randall VA Medical Center; Andrew J. Franck, PharmD
Location: Malcolm Randall VA Medical Center, Gainesville, Florida
→Investigation of Metformin in Pre-Diabetes on Atherosclerotic Cardiovascular OuTcomes (VA-IMPACT)
CSP #2002 is a multicenter, prospective, randomized, double blind, secondary prevention trial to test the hypothesis that treatment with metformin, compared with placebo, reduces mortality and cardiovascular morbidity in veterans with pre-diabetes and established atherosclerotic cardiovascular disease. Qualifying patients have pre-diabetes defined by HbA 1c , fasting blood glucose, or oral glucose tolerance test criteria; clinically evident coronary, cerebrovascular, or peripheral arterial atherosclerotic cardiovascular disease; and estimated glomerular filtration rate of at least 45 mL/min/1.73 m 2 ; and do not fulfill any exclusion criteria.
ID: NCT04838392
Sponsor; Investigator: VA Office of Research and Development; Gregory G. Schwartz, PhD, MD
Locations: 40 locations
Actively Recruiting
→Continuous Glucose Monitoring Devices in Hospitalized Veterans With Diabetes
More than 25% of the patients admitted in the general wards have a history of diabetes mellitus. Up to 30% of the hospitalized diabetics develop hypoglycemia (low glucose values); a condition that is associated with seizures, cardiac arrhythmias, and even death. In veterans, the prevalence is disproportionally higher. It is estimated that 40% to 50% of hospitalized veterans are diabetics. In this clinical trial the investigators describe the development of a novel system, the Glucose Telemetry System, with which glucose values can be wirelessly transmitted from the patient’s bedside to a monitor device at the nursing station. The goal of this work is to develop a more effective glucose surveillance system at the general wards, which can decrease hypoglycemia in the hospital and improve clinical outcomes.
ID: NCT03508934
Sponsor; Investigator: VA Office of Research and Development; Ilias Spanakis, MD
Locations: Baltimore VA Medical Center, Maryland
→Optimizing Gait Rehabilitation for Veterans With Non-Traumatic Lower Limb Amputation (GEM)
The population of older veterans with nontraumatic lower limb amputation is growing. Following lower limb amputation, asymmetrical movements persist during walking and likely contribute to disabling sequelae including secondary pain conditions, poor gait efficiency, impaired physical function, and compromised skin integrity of the residual limb. This study seeks to address chronic gait asymmetry by evaluating the efficacy of two error-manipulation gait training programs to improve gait symmetry for veterans with nontraumatic lower limb amputation. Additionally, this study will evaluate the potential of error-manipulation training programs to improve secondary measures of disability and residual limb skin health. Ultimately, this study aims to improve conventional prosthetic rehabilitation for veterans with nontraumatic amputation through gait training programs based in motor learning principles, resulting in improved.
ID: NCT003995238
Sponsor; Investigator: VA Office of Research and Development; Cory L. Christiansen, PhD
Locations: Rocky Mountain Regional VA Medical Center, Aurora, Colorado; Hunter Holmes McGuire VA Medical Center, Richmond, Virginia
→Empowering Veterans to Actively Communicate and Engage in Shared Decision Making in Medical Visits, A Randomized Controlled Trial (ACTIVet-2)
Type 2 diabetes is a significant condition in VA affecting 20% of VA patients. Adherence to medication regimens and lifestyle factors is important to achieve care goals for these patients. Patients who use active participatory communication behaviors with their providers have better adherence to treatment and better biomedical outcomes, yet many patients are not prepared to engage in active communication with their providers. Existing coaching interventions have not been adopted in practice because of the cost of trained personnel. The investigators haveshown the efficacy of a low-cost video that did not require trained personnel. This proposal proposes to test implementation strategies to deliver that video in VA primary care clinics and to test the effectiveness of the video to improve outcomes in a hybrid type 2 effectiveness implementation trial using a cluster randomized stepped wedgedesign at eight sites. This proposal will test feasibility of implementing the video and if successful will generate the evidence to justify widespread dissemination of the video.
ID: NCT05169359
Sponsor; Investigator: VA Office of Research and Development; Howard S. Gordon, MD
Location: 8 locations, including Jesse Brown VA Medical Center, Chicago; Edward Hines Jr. VA Hopsital, Hines, Illinois
→The Diabetes Staging System in Patient Aligned Care Teams
The purpose of this study is to examine the feasibility/acceptability of the Diabetes Staging System in patient aligned care teams and its ability to increase sodium-glucosecotransporter-2 inhibitor and glucagon-like-1 peptide use in veteran patients with type 2 diabetes and cardiovascular disease and/or chronic kidney disease. A novel type 2 diabetes staging system patterned after Tumor Node Metastasis cancer staging that uses the number of macrovascular and microvascular complications and most recent hemoglobin A1c and glomerular filtration rate to determine Diabetes Staging System stage which reflects disease severity.
ID: NCT06142006
Sponsor; Collaborator: Durham VA Medical Center; Moahad Dar, MD
Location: Greenville VA Health Care Center, North Carolina
→Enhancing Mental and Physical Health of Women Veterans (EMPOWER)
Women veterans are the fastest growing segment of VA users. This dramatic growth has created challenges for VA to ensure that appropriate services are available to meet women veterans’ needs, and that they will want and be able to use those services. The EMPOWER QUERI 2.0 Program is a cluster randomized type 3 hybrid implementation effectiveness trial testing 2 strategies designed to support implementation and sustainment of evidence-based practices for women veterans in at least 20 VA facilities from 4 regions.
ID: NCT05050266
Sponsor; Investigator: VA Office of Research and Development; Alison B. Hamilton, PhD, MPH
Location: VA Greater Los Angeles Healthcare System
→Investigation of Rifampin to Reduce Pedal Amputations for Osteomyelitis in Diabetics (VA INTREPID)
The purpose of this research study is to determine if rifampin, an antibiotic (a medicine that treats infections), is effective in treating osteomyelitis (infection of the bone) of the foot in patients with diabetes. Despite use of powerful antibiotics prescribed over a long period of time, many diabetic patients remain at a high risk for needing an amputation of part of the foot or lower leg because the osteomyelitis is not cured. Some small research studies have shown that addition of rifampin to other antibiotics is effective in treating osteomyelitis. However, because few diabetics with osteomyelitis have been studied, there is no definite proof that it is better than the usual treatments for diabetic patients. If this study finds that adding rifampin to the usual antibiotics prescribed for osteomyelitis reduces the risk for amputations, doctors will be able to more effectively treat many veteran patients with this serious infection. Improving treatment outcomes is an important healthcare goal of the VA.
ID: NCT03012529
Sponsor; Investigator: VA Office of Research and Development; Paul A. Monach, MD, PhD
Location: 30 VA locations, including South Texas Health Care System, San Antonio; Cincinnati VA Medical Center, Ohio; VA Northern California Health Care System, Mather; Washington DC VA Medical Center; William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin
→Effectiveness of Remote Foot Temperature Monitoring (STOP)
Diabetic foot ulcers are common, debilitating, and costly complications of diabetes, disproportionately impacting Black and rural veterans. Forty percent of individuals have an ulcer recurrence within a year of ulcer healing and 65% within 5 years. Monitoring plantar foot temperatures is one of the few interventions that reduces the risk of ulcer recurrence. Despite the evidence, adoption has been poorbecause the original procedures, including the use of handheld thermometers, were burdensome and time-consuming. Podimetrics, a private company, has developed a temperature monitoring system involving a “smart” mat that can wirelessly transmit data and a remote monitoring team that works with VA providers to assist with triage and monitoring. This care model has incredible promise, but has been untested in VA. The investigators propose to conduct a randomized trial to evaluate effectiveness of remote temperature monitoring as well as costs. Additionally, the investigators will evaluate the implementation process, including barriers and facilitators to use among key stakeholders.
ID: NCT05728411
Sponsor; Investigator: VA Office of Research and Development; Rachel M. Thomas
Location: Edward Hines Jr. VA Hospital, Hines, Illinois; Hunter Holmes McGuire VA Medical Center, Richmond, Virginia; VA Puget Sound Health Care System, Seattle, Washington
→Continuous Glucose Monitoring for Hyperglycemia in Critically Ill Patients
The investigators intend to conduct a single-center, prospective, randomized comparative trial of patients admitted to the intensive care unit who received continuous glucose monitoring vs point of care glucose monitoring. The study will examine relevant outcomes for patients in the intensive care unit with diabetes mellitus and/or hyperglycemia. The primary outcome of the study will be the proportion of time in target range (blood glucose 70-180 mg/dL).
ID: NCT05442853
Sponsor; Investigator: Malcom Randall VA Medical Center; Andrew J. Franck, PharmD
Location: Malcolm Randall VA Medical Center, Gainesville, Florida
→Investigation of Metformin in Pre-Diabetes on Atherosclerotic Cardiovascular OuTcomes (VA-IMPACT)
CSP #2002 is a multicenter, prospective, randomized, double blind, secondary prevention trial to test the hypothesis that treatment with metformin, compared with placebo, reduces mortality and cardiovascular morbidity in veterans with pre-diabetes and established atherosclerotic cardiovascular disease. Qualifying patients have pre-diabetes defined by HbA 1c , fasting blood glucose, or oral glucose tolerance test criteria; clinically evident coronary, cerebrovascular, or peripheral arterial atherosclerotic cardiovascular disease; and estimated glomerular filtration rate of at least 45 mL/min/1.73 m 2 ; and do not fulfill any exclusion criteria.
ID: NCT04838392
Sponsor; Investigator: VA Office of Research and Development; Gregory G. Schwartz, PhD, MD
Locations: 40 locations
GLP-1 RAs Reduce Early-Onset CRC Risk in Patients With Type 2 Diabetes
PHILADELPHIA — according to the results of a retrospective study.
“This is the first large study to investigate the impact of GLP-1 RA use on EO-CRC risk,” principal investigator Temitope Olasehinde, MD, resident physician at the University Hospitals Cleveland Medical Center, Case Western Reserve University in Cleveland, Ohio, said in an interview.
The results indicate the GLP-1 RAs have a potentially protective role to play in combating EO-CRC, the incidence of which is notably rising in younger adults, with a corresponding increase in associated mortality.
Previous studies investigating the link between GLP-1 RAs and CRC did not capture patients aged younger than 50 years; thus, it was unknown if these results could be extrapolated to a younger age group, said Olasehinde.
The researcher presented the findings at the annual meeting of the American College of Gastroenterology.
Retrospective Database Analysis
Olasehinde and colleagues analyzed data from TriNetX, a large federated deidentified health research network, to identify patients (age ≤ 49 years) with diagnosed T2D subsequently prescribed antidiabetic medications who had not received a prior diagnosis of CRC. Additionally, patients were stratified on the basis of first-time GLP-1 RA use.
They identified 2,025,034 drug-naive patients with T2D; of these, 284,685 were subsequently prescribed GLP-1 RAs, and 1,740,349 remained in the non–GLP-1 RA cohort. Following propensity score matching, there were 86,186 patients in each cohort.
Patients who received GLP-1 RAs had significantly lower odds of developing EO-CRC than those who received non–GLP-1 RAs (0.6% vs 0.9%; P < .001; odds ratio [OR], 0.61; 95% CI, 0.54-068).
Furthermore, a sub-analysis revealed that patients who were obese and taking GLP-1 RAs had significantly lower odds of developing EO-CRC than patients who were obese but not taking GLP-1 RAs (0.7% vs 1.1%; P < .001; OR, 0.58; 95% CI, 0.50-067).
A Proposed Protective Effect
Although GLP-1 RAs are indicated for the treatment of T2D and obesity, recent evidence suggests that they may play a role in reducing the risk for CRC as well. This protective effect may be produced not only by addressing T2D and obesity — both important risk factors for CRC — but also via cellular mechanisms, Olasehinde noted.
“GLP-1 receptors are widely expressed throughout the gastrointestinal tract, with various effects on tissues in the stomach, small intestine, and colon,” she explained. Specifically, activation of these receptors in the proximal and distal colon promotes the release of “important factors that protect and facilitate healing of the intestinal epithelium” and “regulate the gut microbiome.”
This is particularly relevant in EO-CRC, she added, given its greater association with T2D and obesity, both factors that “have been shown to create dysbiosis in the gut microbiome and low-grade inflammation via release of free radicals/inflammatory cytokines.”
These results provide more evidence that EO-CRC “is clinically and molecularly distinct from late-onset colorectal cancer,” which is important for both clinicians and patients to understand, said Olasehinde.
“It is imperative that we are all aware of the specific signs and symptoms this population presents with and the implications of this diagnosis in younger age groups,” she added. “Patients should continue making informed dietary and lifestyle modifications/choices to help reduce the burden of EO-CRC.”
Hypothesis-Generating Results
Aasma Shaukat, MD, MPH, who was not affiliated with the research, called the results promising but — at this stage — primarily useful for stimulating future research.
"We do need more studies such as this to generate hypotheses that can be studied prospectively," Shaukat, professor of medicine and population health, and director of GI Outcomes Research at NYU Langone Health in New York City, told Medscape Medical News.
She referred to another study, published in JAMA Oncology, that also used the TriNetX research network, which showed that GLP-1 RAs were associated with reduced CRC risk in drug-naive patients with T2D.
Shaukat also noted that the current analysis has limitations that should be considered. "The study is retrospective, and confounding is a possibility,” she said.
“How the groups that did and did not receive GLP-1 RAs differ in other risk factors that could be the drivers of the cancers is not known. Whether cancers were detected through screening or symptoms, stage, and other features that may differ are not known. Finally, since we don’t know who did or did not have colonoscopy, undiagnosed cancers are not known," she explained.
Shaukat, who was the lead author of the ACG 2021 Colorectal Cancer Screening Guidelines, added that the field would benefit from studies providing "biological plausibility information, such as animal studies to understand how GLP-1 RAs may modulate risk of colon cancer; other population-based cohort studies on the incidence of colon cancer among GLP-1 RA users and non-users; and prospective trials on chemoprevention."
The study had no specific funding. Olasehinde reported no relevant financial relationships. Shaukat reported serving as a consultant for Freenome, Medtronic, and Motus GI, as well as an advisory board member for Iterative Scopes Inc.
A version of this article appeared on Medscape.com.
PHILADELPHIA — according to the results of a retrospective study.
“This is the first large study to investigate the impact of GLP-1 RA use on EO-CRC risk,” principal investigator Temitope Olasehinde, MD, resident physician at the University Hospitals Cleveland Medical Center, Case Western Reserve University in Cleveland, Ohio, said in an interview.
The results indicate the GLP-1 RAs have a potentially protective role to play in combating EO-CRC, the incidence of which is notably rising in younger adults, with a corresponding increase in associated mortality.
Previous studies investigating the link between GLP-1 RAs and CRC did not capture patients aged younger than 50 years; thus, it was unknown if these results could be extrapolated to a younger age group, said Olasehinde.
The researcher presented the findings at the annual meeting of the American College of Gastroenterology.
Retrospective Database Analysis
Olasehinde and colleagues analyzed data from TriNetX, a large federated deidentified health research network, to identify patients (age ≤ 49 years) with diagnosed T2D subsequently prescribed antidiabetic medications who had not received a prior diagnosis of CRC. Additionally, patients were stratified on the basis of first-time GLP-1 RA use.
They identified 2,025,034 drug-naive patients with T2D; of these, 284,685 were subsequently prescribed GLP-1 RAs, and 1,740,349 remained in the non–GLP-1 RA cohort. Following propensity score matching, there were 86,186 patients in each cohort.
Patients who received GLP-1 RAs had significantly lower odds of developing EO-CRC than those who received non–GLP-1 RAs (0.6% vs 0.9%; P < .001; odds ratio [OR], 0.61; 95% CI, 0.54-068).
Furthermore, a sub-analysis revealed that patients who were obese and taking GLP-1 RAs had significantly lower odds of developing EO-CRC than patients who were obese but not taking GLP-1 RAs (0.7% vs 1.1%; P < .001; OR, 0.58; 95% CI, 0.50-067).
A Proposed Protective Effect
Although GLP-1 RAs are indicated for the treatment of T2D and obesity, recent evidence suggests that they may play a role in reducing the risk for CRC as well. This protective effect may be produced not only by addressing T2D and obesity — both important risk factors for CRC — but also via cellular mechanisms, Olasehinde noted.
“GLP-1 receptors are widely expressed throughout the gastrointestinal tract, with various effects on tissues in the stomach, small intestine, and colon,” she explained. Specifically, activation of these receptors in the proximal and distal colon promotes the release of “important factors that protect and facilitate healing of the intestinal epithelium” and “regulate the gut microbiome.”
This is particularly relevant in EO-CRC, she added, given its greater association with T2D and obesity, both factors that “have been shown to create dysbiosis in the gut microbiome and low-grade inflammation via release of free radicals/inflammatory cytokines.”
These results provide more evidence that EO-CRC “is clinically and molecularly distinct from late-onset colorectal cancer,” which is important for both clinicians and patients to understand, said Olasehinde.
“It is imperative that we are all aware of the specific signs and symptoms this population presents with and the implications of this diagnosis in younger age groups,” she added. “Patients should continue making informed dietary and lifestyle modifications/choices to help reduce the burden of EO-CRC.”
Hypothesis-Generating Results
Aasma Shaukat, MD, MPH, who was not affiliated with the research, called the results promising but — at this stage — primarily useful for stimulating future research.
"We do need more studies such as this to generate hypotheses that can be studied prospectively," Shaukat, professor of medicine and population health, and director of GI Outcomes Research at NYU Langone Health in New York City, told Medscape Medical News.
She referred to another study, published in JAMA Oncology, that also used the TriNetX research network, which showed that GLP-1 RAs were associated with reduced CRC risk in drug-naive patients with T2D.
Shaukat also noted that the current analysis has limitations that should be considered. "The study is retrospective, and confounding is a possibility,” she said.
“How the groups that did and did not receive GLP-1 RAs differ in other risk factors that could be the drivers of the cancers is not known. Whether cancers were detected through screening or symptoms, stage, and other features that may differ are not known. Finally, since we don’t know who did or did not have colonoscopy, undiagnosed cancers are not known," she explained.
Shaukat, who was the lead author of the ACG 2021 Colorectal Cancer Screening Guidelines, added that the field would benefit from studies providing "biological plausibility information, such as animal studies to understand how GLP-1 RAs may modulate risk of colon cancer; other population-based cohort studies on the incidence of colon cancer among GLP-1 RA users and non-users; and prospective trials on chemoprevention."
The study had no specific funding. Olasehinde reported no relevant financial relationships. Shaukat reported serving as a consultant for Freenome, Medtronic, and Motus GI, as well as an advisory board member for Iterative Scopes Inc.
A version of this article appeared on Medscape.com.
PHILADELPHIA — according to the results of a retrospective study.
“This is the first large study to investigate the impact of GLP-1 RA use on EO-CRC risk,” principal investigator Temitope Olasehinde, MD, resident physician at the University Hospitals Cleveland Medical Center, Case Western Reserve University in Cleveland, Ohio, said in an interview.
The results indicate the GLP-1 RAs have a potentially protective role to play in combating EO-CRC, the incidence of which is notably rising in younger adults, with a corresponding increase in associated mortality.
Previous studies investigating the link between GLP-1 RAs and CRC did not capture patients aged younger than 50 years; thus, it was unknown if these results could be extrapolated to a younger age group, said Olasehinde.
The researcher presented the findings at the annual meeting of the American College of Gastroenterology.
Retrospective Database Analysis
Olasehinde and colleagues analyzed data from TriNetX, a large federated deidentified health research network, to identify patients (age ≤ 49 years) with diagnosed T2D subsequently prescribed antidiabetic medications who had not received a prior diagnosis of CRC. Additionally, patients were stratified on the basis of first-time GLP-1 RA use.
They identified 2,025,034 drug-naive patients with T2D; of these, 284,685 were subsequently prescribed GLP-1 RAs, and 1,740,349 remained in the non–GLP-1 RA cohort. Following propensity score matching, there were 86,186 patients in each cohort.
Patients who received GLP-1 RAs had significantly lower odds of developing EO-CRC than those who received non–GLP-1 RAs (0.6% vs 0.9%; P < .001; odds ratio [OR], 0.61; 95% CI, 0.54-068).
Furthermore, a sub-analysis revealed that patients who were obese and taking GLP-1 RAs had significantly lower odds of developing EO-CRC than patients who were obese but not taking GLP-1 RAs (0.7% vs 1.1%; P < .001; OR, 0.58; 95% CI, 0.50-067).
A Proposed Protective Effect
Although GLP-1 RAs are indicated for the treatment of T2D and obesity, recent evidence suggests that they may play a role in reducing the risk for CRC as well. This protective effect may be produced not only by addressing T2D and obesity — both important risk factors for CRC — but also via cellular mechanisms, Olasehinde noted.
“GLP-1 receptors are widely expressed throughout the gastrointestinal tract, with various effects on tissues in the stomach, small intestine, and colon,” she explained. Specifically, activation of these receptors in the proximal and distal colon promotes the release of “important factors that protect and facilitate healing of the intestinal epithelium” and “regulate the gut microbiome.”
This is particularly relevant in EO-CRC, she added, given its greater association with T2D and obesity, both factors that “have been shown to create dysbiosis in the gut microbiome and low-grade inflammation via release of free radicals/inflammatory cytokines.”
These results provide more evidence that EO-CRC “is clinically and molecularly distinct from late-onset colorectal cancer,” which is important for both clinicians and patients to understand, said Olasehinde.
“It is imperative that we are all aware of the specific signs and symptoms this population presents with and the implications of this diagnosis in younger age groups,” she added. “Patients should continue making informed dietary and lifestyle modifications/choices to help reduce the burden of EO-CRC.”
Hypothesis-Generating Results
Aasma Shaukat, MD, MPH, who was not affiliated with the research, called the results promising but — at this stage — primarily useful for stimulating future research.
"We do need more studies such as this to generate hypotheses that can be studied prospectively," Shaukat, professor of medicine and population health, and director of GI Outcomes Research at NYU Langone Health in New York City, told Medscape Medical News.
She referred to another study, published in JAMA Oncology, that also used the TriNetX research network, which showed that GLP-1 RAs were associated with reduced CRC risk in drug-naive patients with T2D.
Shaukat also noted that the current analysis has limitations that should be considered. "The study is retrospective, and confounding is a possibility,” she said.
“How the groups that did and did not receive GLP-1 RAs differ in other risk factors that could be the drivers of the cancers is not known. Whether cancers were detected through screening or symptoms, stage, and other features that may differ are not known. Finally, since we don’t know who did or did not have colonoscopy, undiagnosed cancers are not known," she explained.
Shaukat, who was the lead author of the ACG 2021 Colorectal Cancer Screening Guidelines, added that the field would benefit from studies providing "biological plausibility information, such as animal studies to understand how GLP-1 RAs may modulate risk of colon cancer; other population-based cohort studies on the incidence of colon cancer among GLP-1 RA users and non-users; and prospective trials on chemoprevention."
The study had no specific funding. Olasehinde reported no relevant financial relationships. Shaukat reported serving as a consultant for Freenome, Medtronic, and Motus GI, as well as an advisory board member for Iterative Scopes Inc.
A version of this article appeared on Medscape.com.
FROM ACG 2024