User login
ACC/AHA issue clinical lexicon for complications of COVID-19
The American College of Cardiology and the American Heart Association have jointly issued a comprehensive set of data standards to help clarify definitions of the cardiovascular (CV) and non-CV complications of COVID-19.
It’s the work of the ACC/AHA Task Force on Clinical Data Standards and has been endorsed by the Heart Failure Society of America and Society for Cardiac Angiography and Interventions.
There is increased importance to understanding the acute and long-term impact of COVID-19 on CV health, the writing group notes. Until now, however, there has not been “clarity or consensus” on definitions of CV conditions related to COVID-19, with different diagnostic terminologies being used for overlapping conditions, such as “myocardial injury,” “myocarditis,” “type Il myocardial infarction,” “stress cardiomyopathy,” and “inflammatory cardiomyopathy,” they point out.
“We, as a research community, did some things right and some things wrong surrounding the COVID pandemic,” Sandeep Das, MD, MPH, vice chair of the writing group, noted in an interview with this news organization.
“The things that we really did right is that everybody responded with enthusiasm, kind of all hands on deck with a massive crisis response, and that was fantastic,” Dr. Das said.
“However, because of the need to hurry, we didn’t structure and organize in the way that we typically would for something that was sort of a slow burn kind of problem rather than an emergency. One of the consequences of that was fragmentation of how things are collected, reported, et cetera, and that leads to confusion,” he added.
The report was published simultaneously June 23 in the Journal of the American College of Cardiology and Circulation: Cardiovascular Quality and Outcomes.
A necessary but not glamorous project
The new data standards for COVID-19 will help standardize definitions and set the framework to capture and better understand how COVID-19 affects CV health.
“It wasn’t exactly a glamorous-type project but, at the same time, it’s super necessary to kind of get everybody on the same page and working together,” Dr. Das said.
Broad agreement on common vocabulary and definitions will help with efforts to pool or compare data from electronic health records, clinical registries, administrative datasets, and other databases, and determine whether these data apply to clinical practice and research endeavors, the writing group says.
They considered data elements relevant to the full range of care provided to COVID-19 patients in all care settings. Among the key items included in the document are:
- Case definitions for confirmed, probable, and suspected acute COVID-19, as well as postacute sequelae of COVID-19.
- Definitions for acute CV complications related to COVID-19, including acute myocardial injury, heart failure, shock, arrhythmia, thromboembolic complications, and .
- Data elements related to COVID-19 vaccination status, comorbidities, and preexisting CV conditions.
- Definitions for postacute CV sequelae of SARS-CoV-2 infection and long-term CV complications of COVID-19.
- Data elements for CV mortality during acute COVID-19.
- Data elements for non-CV complications to help document severity of illness and other competing diagnoses and complications that might affect CV outcomes.
- A list of symptoms and signs related to COVID-19 and CV complications.
- Data elements for diagnostic and therapeutic strategies for COVID-19 and CV conditions.
- A discussion of advanced therapies, including , extracorporeal membrane oxygenation, and end-of-life management strategies.
These data standards will be useful for researchers, registry developers, and clinicians, and they are proposed as a framework for ICD-10 code development of COVID-19–related CV conditions, the writing group says.
The standards are also of “great importance” to patients, clinicians, investigators, scientists, administrators, public health officials, policymakers, and payers, the group says.
Dr. Das said that, although there is no formal plan in place to update the document, he could see sections that might be refined.
“For example, there’s a nice long list of all the various variants, and unfortunately, I suspect that that is going to change and evolve over time,” Dr. Das told this news organization.
“We tried very hard not to include things like specifying specific treatments so we didn’t get proscriptive. We wanted to make it descriptive, so hopefully it will stand the test of time pretty well,” he added.
This research had no commercial funding. The writing group has no relevant disclosures.
A version of this article first appeared on Medscape.com.
The American College of Cardiology and the American Heart Association have jointly issued a comprehensive set of data standards to help clarify definitions of the cardiovascular (CV) and non-CV complications of COVID-19.
It’s the work of the ACC/AHA Task Force on Clinical Data Standards and has been endorsed by the Heart Failure Society of America and Society for Cardiac Angiography and Interventions.
There is increased importance to understanding the acute and long-term impact of COVID-19 on CV health, the writing group notes. Until now, however, there has not been “clarity or consensus” on definitions of CV conditions related to COVID-19, with different diagnostic terminologies being used for overlapping conditions, such as “myocardial injury,” “myocarditis,” “type Il myocardial infarction,” “stress cardiomyopathy,” and “inflammatory cardiomyopathy,” they point out.
“We, as a research community, did some things right and some things wrong surrounding the COVID pandemic,” Sandeep Das, MD, MPH, vice chair of the writing group, noted in an interview with this news organization.
“The things that we really did right is that everybody responded with enthusiasm, kind of all hands on deck with a massive crisis response, and that was fantastic,” Dr. Das said.
“However, because of the need to hurry, we didn’t structure and organize in the way that we typically would for something that was sort of a slow burn kind of problem rather than an emergency. One of the consequences of that was fragmentation of how things are collected, reported, et cetera, and that leads to confusion,” he added.
The report was published simultaneously June 23 in the Journal of the American College of Cardiology and Circulation: Cardiovascular Quality and Outcomes.
A necessary but not glamorous project
The new data standards for COVID-19 will help standardize definitions and set the framework to capture and better understand how COVID-19 affects CV health.
“It wasn’t exactly a glamorous-type project but, at the same time, it’s super necessary to kind of get everybody on the same page and working together,” Dr. Das said.
Broad agreement on common vocabulary and definitions will help with efforts to pool or compare data from electronic health records, clinical registries, administrative datasets, and other databases, and determine whether these data apply to clinical practice and research endeavors, the writing group says.
They considered data elements relevant to the full range of care provided to COVID-19 patients in all care settings. Among the key items included in the document are:
- Case definitions for confirmed, probable, and suspected acute COVID-19, as well as postacute sequelae of COVID-19.
- Definitions for acute CV complications related to COVID-19, including acute myocardial injury, heart failure, shock, arrhythmia, thromboembolic complications, and .
- Data elements related to COVID-19 vaccination status, comorbidities, and preexisting CV conditions.
- Definitions for postacute CV sequelae of SARS-CoV-2 infection and long-term CV complications of COVID-19.
- Data elements for CV mortality during acute COVID-19.
- Data elements for non-CV complications to help document severity of illness and other competing diagnoses and complications that might affect CV outcomes.
- A list of symptoms and signs related to COVID-19 and CV complications.
- Data elements for diagnostic and therapeutic strategies for COVID-19 and CV conditions.
- A discussion of advanced therapies, including , extracorporeal membrane oxygenation, and end-of-life management strategies.
These data standards will be useful for researchers, registry developers, and clinicians, and they are proposed as a framework for ICD-10 code development of COVID-19–related CV conditions, the writing group says.
The standards are also of “great importance” to patients, clinicians, investigators, scientists, administrators, public health officials, policymakers, and payers, the group says.
Dr. Das said that, although there is no formal plan in place to update the document, he could see sections that might be refined.
“For example, there’s a nice long list of all the various variants, and unfortunately, I suspect that that is going to change and evolve over time,” Dr. Das told this news organization.
“We tried very hard not to include things like specifying specific treatments so we didn’t get proscriptive. We wanted to make it descriptive, so hopefully it will stand the test of time pretty well,” he added.
This research had no commercial funding. The writing group has no relevant disclosures.
A version of this article first appeared on Medscape.com.
The American College of Cardiology and the American Heart Association have jointly issued a comprehensive set of data standards to help clarify definitions of the cardiovascular (CV) and non-CV complications of COVID-19.
It’s the work of the ACC/AHA Task Force on Clinical Data Standards and has been endorsed by the Heart Failure Society of America and Society for Cardiac Angiography and Interventions.
There is increased importance to understanding the acute and long-term impact of COVID-19 on CV health, the writing group notes. Until now, however, there has not been “clarity or consensus” on definitions of CV conditions related to COVID-19, with different diagnostic terminologies being used for overlapping conditions, such as “myocardial injury,” “myocarditis,” “type Il myocardial infarction,” “stress cardiomyopathy,” and “inflammatory cardiomyopathy,” they point out.
“We, as a research community, did some things right and some things wrong surrounding the COVID pandemic,” Sandeep Das, MD, MPH, vice chair of the writing group, noted in an interview with this news organization.
“The things that we really did right is that everybody responded with enthusiasm, kind of all hands on deck with a massive crisis response, and that was fantastic,” Dr. Das said.
“However, because of the need to hurry, we didn’t structure and organize in the way that we typically would for something that was sort of a slow burn kind of problem rather than an emergency. One of the consequences of that was fragmentation of how things are collected, reported, et cetera, and that leads to confusion,” he added.
The report was published simultaneously June 23 in the Journal of the American College of Cardiology and Circulation: Cardiovascular Quality and Outcomes.
A necessary but not glamorous project
The new data standards for COVID-19 will help standardize definitions and set the framework to capture and better understand how COVID-19 affects CV health.
“It wasn’t exactly a glamorous-type project but, at the same time, it’s super necessary to kind of get everybody on the same page and working together,” Dr. Das said.
Broad agreement on common vocabulary and definitions will help with efforts to pool or compare data from electronic health records, clinical registries, administrative datasets, and other databases, and determine whether these data apply to clinical practice and research endeavors, the writing group says.
They considered data elements relevant to the full range of care provided to COVID-19 patients in all care settings. Among the key items included in the document are:
- Case definitions for confirmed, probable, and suspected acute COVID-19, as well as postacute sequelae of COVID-19.
- Definitions for acute CV complications related to COVID-19, including acute myocardial injury, heart failure, shock, arrhythmia, thromboembolic complications, and .
- Data elements related to COVID-19 vaccination status, comorbidities, and preexisting CV conditions.
- Definitions for postacute CV sequelae of SARS-CoV-2 infection and long-term CV complications of COVID-19.
- Data elements for CV mortality during acute COVID-19.
- Data elements for non-CV complications to help document severity of illness and other competing diagnoses and complications that might affect CV outcomes.
- A list of symptoms and signs related to COVID-19 and CV complications.
- Data elements for diagnostic and therapeutic strategies for COVID-19 and CV conditions.
- A discussion of advanced therapies, including , extracorporeal membrane oxygenation, and end-of-life management strategies.
These data standards will be useful for researchers, registry developers, and clinicians, and they are proposed as a framework for ICD-10 code development of COVID-19–related CV conditions, the writing group says.
The standards are also of “great importance” to patients, clinicians, investigators, scientists, administrators, public health officials, policymakers, and payers, the group says.
Dr. Das said that, although there is no formal plan in place to update the document, he could see sections that might be refined.
“For example, there’s a nice long list of all the various variants, and unfortunately, I suspect that that is going to change and evolve over time,” Dr. Das told this news organization.
“We tried very hard not to include things like specifying specific treatments so we didn’t get proscriptive. We wanted to make it descriptive, so hopefully it will stand the test of time pretty well,” he added.
This research had no commercial funding. The writing group has no relevant disclosures.
A version of this article first appeared on Medscape.com.
COVID-19 Pandemic stress affected ovulation, not menstruation
ATLANTA – Disturbances in ovulation that didn’t produce any actual changes in the menstrual cycle of women were extremely common during the first year of the COVID-19 pandemic and were linked to emotional stress, according to the findings of an “experiment of nature” that allowed for comparison with women a decade earlier.
Findings from two studies of reproductive-age women, one conducted in 2006-2008 and the other in 2020-2021, were presented by Jerilynn C. Prior, MD, at the annual meeting of the Endocrine Society.
The comparison of the two time periods yielded several novel findings. “I was taught in medical school that when women don’t eat enough they lose their period. But what we now understand is there’s a graded response to various stressors, acting through the hypothalamus in a common pathway. There is a gradation of disturbances, some of which are subclinical or not obvious,” said Dr. Prior, professor of endocrinology and metabolism at the University of British Columbia, Vancouver.
Moreover, women’s menstrual cycle lengths didn’t differ across the two time periods, despite a dramatic 63% decrement in normal ovulatory function related to increased depression, anxiety, and outside stresses that the women reported in diaries.
“Assuming that regular cycles need normal ovulation is something we should just get out of our minds. It changes our concept about what’s normal if we only know about the cycle length,” she observed.
It will be critical going forward to see whether the ovulatory disturbances have resolved as the pandemic has shifted “because there’s strong evidence that ovulatory disturbances, even with normal cycle length, are related to bone loss and some evidence it’s related to early heart attacks, breast and endometrial cancers,” Dr. Prior said during a press conference.
Asked to comment, session moderator Genevieve Neal-Perry, MD, PhD, told this news organization: “I think what we can take away is that stress itself is a modifier of the way the brain and the gonads communicate with each other, and that then has an impact on ovulatory function.”
Dr. Neal-Perry noted that the association of stress and ovulatory disruption has been reported in various ways previously, but “clearly it doesn’t affect everyone. What we don’t know is who is most susceptible. There have been some studies showing a genetic predisposition and a genetic anomaly that actually makes them more susceptible to the impact of stress on the reproductive system.”
But the lack of data on weight change in the study cohorts is a limitation. “To me one of the more important questions was what was going on with weight. Just looking at a static number doesn’t tell you whether there were changes. We know that weight gain or weight loss can stress the reproductive axis,” noted Dr. Neal-Parry of the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill.
‘Experiment of nature’ revealed invisible effect of pandemic stress
The women in both cohorts of the Menstruation Ovulation Study (MOS) were healthy volunteers aged 19-35 years recruited from the metropolitan Vancouver region. All were menstruating monthly and none were taking hormonal birth control. Recruitment for the second cohort had begun just prior to the March 2020 COVID-19 pandemic lockdown.
Interviewer-administered questionnaires (CaMos) covering demographics, socioeconomic status, and reproductive history, and daily diaries kept by the women (menstrual cycle diary) were identical for both cohorts.
Assessments of ovulation differed for the two studies but were cross-validated. For the earlier time period, ovulation was assessed by a threefold increase in follicular-to-luteal urinary progesterone (PdG). For the pandemic-era study, the validated quantitative basal temperature (QBT) method was used.
There were 301 women in the earlier cohort and 125 during the pandemic. Both were an average age of about 29 years and had a body mass index of about 24.3 kg/m2 (within the normal range). The pandemic cohort was more racially/ethnically diverse than the earlier one and more in-line with recent census data.
More of the women were nulliparous during the pandemic than earlier (92.7% vs. 80.4%; P = .002).
The distribution of menstrual cycle lengths didn’t differ, with both cohorts averaging about 30 days (P = .893). However, while 90% of the women in the earlier cohort ovulated normally, only 37% did during the pandemic, a highly significant difference (P < .0001).
Thus, during the pandemic, 63% of women had “silent ovulatory disturbances,” either with short luteal phases after ovulation or no ovulation, compared with just 10% in the earlier cohort, “which is remarkable, unbelievable actually,” Dr. Prior remarked.
The difference wasn’t explained by any of the demographic information collected either, including socioeconomic status, lifestyle, or reproductive history variables.
And it wasn’t because of COVID-19 vaccination, as the vaccine wasn’t available when most of the women were recruited, and of the 79 who were recruited during vaccine availability, only two received a COVID-19 vaccine during the study (and both had normal ovulation).
Employment changes, caring responsibilities, and worry likely causes
The information from the diaries was more revealing. Several diary components were far more common during the pandemic, including negative mood (feeling depressed or anxious, sleep problems, and outside stresses), self-worth, interest in sex, energy level, and appetite. All were significantly different between the two cohorts (P < .001) and between those with and without ovulatory disturbances.
“So menstrual cycle lengths and long cycles didn’t differ, but there was a much higher prevalence of silent or subclinical ovulatory disturbances, and these were related to the increased stresses that women recorded in their diaries. This means that the estrogen levels were pretty close to normal but the progesterone levels were remarkably decreased,” Dr. Prior said.
Interestingly, reported menstrual cramps were also significantly more common during the pandemic and associated with ovulatory disruption.
“That is a new observation because previously we’ve always thought that you needed to ovulate in order to even have cramps,” she commented.
Asked whether COVID-19 itself might have played a role, Dr. Prior said no woman in the study tested positive for the virus or had long COVID.
“As far as I’m aware, it was the changes in employment … and caring for elders and worry about illness in somebody you loved that was related,” she said.
Asked what she thinks the result would be if the study were conducted now, she said: “I don’t know. We’re still in a stressful time with inflation and not complete recovery, so probably the issue is still very present.”
Dr. Prior and Dr. Neal-Perry have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ATLANTA – Disturbances in ovulation that didn’t produce any actual changes in the menstrual cycle of women were extremely common during the first year of the COVID-19 pandemic and were linked to emotional stress, according to the findings of an “experiment of nature” that allowed for comparison with women a decade earlier.
Findings from two studies of reproductive-age women, one conducted in 2006-2008 and the other in 2020-2021, were presented by Jerilynn C. Prior, MD, at the annual meeting of the Endocrine Society.
The comparison of the two time periods yielded several novel findings. “I was taught in medical school that when women don’t eat enough they lose their period. But what we now understand is there’s a graded response to various stressors, acting through the hypothalamus in a common pathway. There is a gradation of disturbances, some of which are subclinical or not obvious,” said Dr. Prior, professor of endocrinology and metabolism at the University of British Columbia, Vancouver.
Moreover, women’s menstrual cycle lengths didn’t differ across the two time periods, despite a dramatic 63% decrement in normal ovulatory function related to increased depression, anxiety, and outside stresses that the women reported in diaries.
“Assuming that regular cycles need normal ovulation is something we should just get out of our minds. It changes our concept about what’s normal if we only know about the cycle length,” she observed.
It will be critical going forward to see whether the ovulatory disturbances have resolved as the pandemic has shifted “because there’s strong evidence that ovulatory disturbances, even with normal cycle length, are related to bone loss and some evidence it’s related to early heart attacks, breast and endometrial cancers,” Dr. Prior said during a press conference.
Asked to comment, session moderator Genevieve Neal-Perry, MD, PhD, told this news organization: “I think what we can take away is that stress itself is a modifier of the way the brain and the gonads communicate with each other, and that then has an impact on ovulatory function.”
Dr. Neal-Perry noted that the association of stress and ovulatory disruption has been reported in various ways previously, but “clearly it doesn’t affect everyone. What we don’t know is who is most susceptible. There have been some studies showing a genetic predisposition and a genetic anomaly that actually makes them more susceptible to the impact of stress on the reproductive system.”
But the lack of data on weight change in the study cohorts is a limitation. “To me one of the more important questions was what was going on with weight. Just looking at a static number doesn’t tell you whether there were changes. We know that weight gain or weight loss can stress the reproductive axis,” noted Dr. Neal-Parry of the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill.
‘Experiment of nature’ revealed invisible effect of pandemic stress
The women in both cohorts of the Menstruation Ovulation Study (MOS) were healthy volunteers aged 19-35 years recruited from the metropolitan Vancouver region. All were menstruating monthly and none were taking hormonal birth control. Recruitment for the second cohort had begun just prior to the March 2020 COVID-19 pandemic lockdown.
Interviewer-administered questionnaires (CaMos) covering demographics, socioeconomic status, and reproductive history, and daily diaries kept by the women (menstrual cycle diary) were identical for both cohorts.
Assessments of ovulation differed for the two studies but were cross-validated. For the earlier time period, ovulation was assessed by a threefold increase in follicular-to-luteal urinary progesterone (PdG). For the pandemic-era study, the validated quantitative basal temperature (QBT) method was used.
There were 301 women in the earlier cohort and 125 during the pandemic. Both were an average age of about 29 years and had a body mass index of about 24.3 kg/m2 (within the normal range). The pandemic cohort was more racially/ethnically diverse than the earlier one and more in-line with recent census data.
More of the women were nulliparous during the pandemic than earlier (92.7% vs. 80.4%; P = .002).
The distribution of menstrual cycle lengths didn’t differ, with both cohorts averaging about 30 days (P = .893). However, while 90% of the women in the earlier cohort ovulated normally, only 37% did during the pandemic, a highly significant difference (P < .0001).
Thus, during the pandemic, 63% of women had “silent ovulatory disturbances,” either with short luteal phases after ovulation or no ovulation, compared with just 10% in the earlier cohort, “which is remarkable, unbelievable actually,” Dr. Prior remarked.
The difference wasn’t explained by any of the demographic information collected either, including socioeconomic status, lifestyle, or reproductive history variables.
And it wasn’t because of COVID-19 vaccination, as the vaccine wasn’t available when most of the women were recruited, and of the 79 who were recruited during vaccine availability, only two received a COVID-19 vaccine during the study (and both had normal ovulation).
Employment changes, caring responsibilities, and worry likely causes
The information from the diaries was more revealing. Several diary components were far more common during the pandemic, including negative mood (feeling depressed or anxious, sleep problems, and outside stresses), self-worth, interest in sex, energy level, and appetite. All were significantly different between the two cohorts (P < .001) and between those with and without ovulatory disturbances.
“So menstrual cycle lengths and long cycles didn’t differ, but there was a much higher prevalence of silent or subclinical ovulatory disturbances, and these were related to the increased stresses that women recorded in their diaries. This means that the estrogen levels were pretty close to normal but the progesterone levels were remarkably decreased,” Dr. Prior said.
Interestingly, reported menstrual cramps were also significantly more common during the pandemic and associated with ovulatory disruption.
“That is a new observation because previously we’ve always thought that you needed to ovulate in order to even have cramps,” she commented.
Asked whether COVID-19 itself might have played a role, Dr. Prior said no woman in the study tested positive for the virus or had long COVID.
“As far as I’m aware, it was the changes in employment … and caring for elders and worry about illness in somebody you loved that was related,” she said.
Asked what she thinks the result would be if the study were conducted now, she said: “I don’t know. We’re still in a stressful time with inflation and not complete recovery, so probably the issue is still very present.”
Dr. Prior and Dr. Neal-Perry have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ATLANTA – Disturbances in ovulation that didn’t produce any actual changes in the menstrual cycle of women were extremely common during the first year of the COVID-19 pandemic and were linked to emotional stress, according to the findings of an “experiment of nature” that allowed for comparison with women a decade earlier.
Findings from two studies of reproductive-age women, one conducted in 2006-2008 and the other in 2020-2021, were presented by Jerilynn C. Prior, MD, at the annual meeting of the Endocrine Society.
The comparison of the two time periods yielded several novel findings. “I was taught in medical school that when women don’t eat enough they lose their period. But what we now understand is there’s a graded response to various stressors, acting through the hypothalamus in a common pathway. There is a gradation of disturbances, some of which are subclinical or not obvious,” said Dr. Prior, professor of endocrinology and metabolism at the University of British Columbia, Vancouver.
Moreover, women’s menstrual cycle lengths didn’t differ across the two time periods, despite a dramatic 63% decrement in normal ovulatory function related to increased depression, anxiety, and outside stresses that the women reported in diaries.
“Assuming that regular cycles need normal ovulation is something we should just get out of our minds. It changes our concept about what’s normal if we only know about the cycle length,” she observed.
It will be critical going forward to see whether the ovulatory disturbances have resolved as the pandemic has shifted “because there’s strong evidence that ovulatory disturbances, even with normal cycle length, are related to bone loss and some evidence it’s related to early heart attacks, breast and endometrial cancers,” Dr. Prior said during a press conference.
Asked to comment, session moderator Genevieve Neal-Perry, MD, PhD, told this news organization: “I think what we can take away is that stress itself is a modifier of the way the brain and the gonads communicate with each other, and that then has an impact on ovulatory function.”
Dr. Neal-Perry noted that the association of stress and ovulatory disruption has been reported in various ways previously, but “clearly it doesn’t affect everyone. What we don’t know is who is most susceptible. There have been some studies showing a genetic predisposition and a genetic anomaly that actually makes them more susceptible to the impact of stress on the reproductive system.”
But the lack of data on weight change in the study cohorts is a limitation. “To me one of the more important questions was what was going on with weight. Just looking at a static number doesn’t tell you whether there were changes. We know that weight gain or weight loss can stress the reproductive axis,” noted Dr. Neal-Parry of the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill.
‘Experiment of nature’ revealed invisible effect of pandemic stress
The women in both cohorts of the Menstruation Ovulation Study (MOS) were healthy volunteers aged 19-35 years recruited from the metropolitan Vancouver region. All were menstruating monthly and none were taking hormonal birth control. Recruitment for the second cohort had begun just prior to the March 2020 COVID-19 pandemic lockdown.
Interviewer-administered questionnaires (CaMos) covering demographics, socioeconomic status, and reproductive history, and daily diaries kept by the women (menstrual cycle diary) were identical for both cohorts.
Assessments of ovulation differed for the two studies but were cross-validated. For the earlier time period, ovulation was assessed by a threefold increase in follicular-to-luteal urinary progesterone (PdG). For the pandemic-era study, the validated quantitative basal temperature (QBT) method was used.
There were 301 women in the earlier cohort and 125 during the pandemic. Both were an average age of about 29 years and had a body mass index of about 24.3 kg/m2 (within the normal range). The pandemic cohort was more racially/ethnically diverse than the earlier one and more in-line with recent census data.
More of the women were nulliparous during the pandemic than earlier (92.7% vs. 80.4%; P = .002).
The distribution of menstrual cycle lengths didn’t differ, with both cohorts averaging about 30 days (P = .893). However, while 90% of the women in the earlier cohort ovulated normally, only 37% did during the pandemic, a highly significant difference (P < .0001).
Thus, during the pandemic, 63% of women had “silent ovulatory disturbances,” either with short luteal phases after ovulation or no ovulation, compared with just 10% in the earlier cohort, “which is remarkable, unbelievable actually,” Dr. Prior remarked.
The difference wasn’t explained by any of the demographic information collected either, including socioeconomic status, lifestyle, or reproductive history variables.
And it wasn’t because of COVID-19 vaccination, as the vaccine wasn’t available when most of the women were recruited, and of the 79 who were recruited during vaccine availability, only two received a COVID-19 vaccine during the study (and both had normal ovulation).
Employment changes, caring responsibilities, and worry likely causes
The information from the diaries was more revealing. Several diary components were far more common during the pandemic, including negative mood (feeling depressed or anxious, sleep problems, and outside stresses), self-worth, interest in sex, energy level, and appetite. All were significantly different between the two cohorts (P < .001) and between those with and without ovulatory disturbances.
“So menstrual cycle lengths and long cycles didn’t differ, but there was a much higher prevalence of silent or subclinical ovulatory disturbances, and these were related to the increased stresses that women recorded in their diaries. This means that the estrogen levels were pretty close to normal but the progesterone levels were remarkably decreased,” Dr. Prior said.
Interestingly, reported menstrual cramps were also significantly more common during the pandemic and associated with ovulatory disruption.
“That is a new observation because previously we’ve always thought that you needed to ovulate in order to even have cramps,” she commented.
Asked whether COVID-19 itself might have played a role, Dr. Prior said no woman in the study tested positive for the virus or had long COVID.
“As far as I’m aware, it was the changes in employment … and caring for elders and worry about illness in somebody you loved that was related,” she said.
Asked what she thinks the result would be if the study were conducted now, she said: “I don’t know. We’re still in a stressful time with inflation and not complete recovery, so probably the issue is still very present.”
Dr. Prior and Dr. Neal-Perry have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ENDO 2022
How to communicate effectively with patients when tension is high
“At my hospital, it was such a big thing to make sure that families are called,” said Dr. Nwankwo, in an interview following a session on compassionate communication at the annual meeting of the American College of Physicians. “So you have 19 patients, and you have to call almost every family to update them. And then you call, and they say, ‘Call this person as well.’ You feel like you’re at your wit’s end a lot of times.”
Sometimes, she has had to dig deep to find the empathy for patients that she knows her patients deserve.
“You really want to care by thinking about where is this patient coming from? What’s going on in their lives? And not just label them a difficult patient,” she said.
Become curious
Auguste Fortin, MD, MPH, offered advice for handling patient interactions under these kinds of circumstances, while serving as a moderator during the session.
“When the going gets tough, turn to wonder.” Become curious about why a patient might be feeling the way they are, he said.
Dr. Fortin, professor of internal medicine at Yale University, New Haven, Conn., said using the ADOBE acronym, has helped him more effectively communicate with his patients. This tool cues him to keep the following in mind: acknowledge, discover, opportunity, boundary setting, and extend.
He went on to explain to the audience why thinking about these terms is useful when interacting with patients.
First, acknowledge the feelings of the patient. Noting that a patient is angry, perhaps counterintuitively, helps, he said. In fact, not acknowledging the anger “throws gasoline on the fire.”
Then, discover the cause of their emotion. Saying "tell me more" and "help me understand" can be powerful tools, he noted.
Next, take this as an opportunity for empathy – especially important to remember when you’re being verbally attacked.
Boundary setting is important, because it lets the patient know that the conversation won’t continue unless they show the same respect the physician is showing, he said.
Finally, physicians can extend the system of support by asking others – such as colleagues or security – for help.
Use the NURS guide to show empathy
Dr. Fortin said he uses the “NURS” guide or calling to mind “name, express, respect, and support” to show empathy:
This involves naming a patient’s emotion; expressing understanding, with phrases like "I can see how you could be …"; showing respect, acknowledging a patient is going through a lot; and offering support, by saying something like, "Let’s see what we can do together to get to the bottom of this," he explained.
“My lived experience in using [these] in this order is that by the end of it, the patient cannot stay mad at me,” Dr. Fortin said.
“It’s really quite remarkable,” he added.
Steps for nonviolent communication
Rebecca Andrews, MD, MS, another moderator for the session, offered these steps for “nonviolent communication”:
- Observing the situation without blame or judgment.
- Telling the person how this situation makes you feel.
- Connecting with a need of the other person.
- Making a request that is specific and based on action, rather than a request not to do something, such as "Would you be willing to … ?"
Dr. Andrews, who is professor of medicine at the University of Connecticut, Farmington, said this approach has worked well for her, both in interactions with patients and in her personal life.
“It is evidence based that compassion actually makes care better,” she noted.
Varun Jain, MD, a member of the audience, expressed gratitude to the session’s speakers for teaching him something that he had not learned in medical school or residency.
“Every week you will have one or two people who will be labeled as ‘difficult,’ ” and it was nice to have some proven advice on how to handle these tough interactions, said the hospitalist at St. Francis Hospital in Hartford, Conn.
“We never got any actual training on this, and we were expected to know this because we are just physicians, and physicians are expected to be compassionate,” Dr. Jain said. “No one taught us how to have compassion.”
Dr. Fortin and Dr. Andrews disclosed no relevant financial relationships.
“At my hospital, it was such a big thing to make sure that families are called,” said Dr. Nwankwo, in an interview following a session on compassionate communication at the annual meeting of the American College of Physicians. “So you have 19 patients, and you have to call almost every family to update them. And then you call, and they say, ‘Call this person as well.’ You feel like you’re at your wit’s end a lot of times.”
Sometimes, she has had to dig deep to find the empathy for patients that she knows her patients deserve.
“You really want to care by thinking about where is this patient coming from? What’s going on in their lives? And not just label them a difficult patient,” she said.
Become curious
Auguste Fortin, MD, MPH, offered advice for handling patient interactions under these kinds of circumstances, while serving as a moderator during the session.
“When the going gets tough, turn to wonder.” Become curious about why a patient might be feeling the way they are, he said.
Dr. Fortin, professor of internal medicine at Yale University, New Haven, Conn., said using the ADOBE acronym, has helped him more effectively communicate with his patients. This tool cues him to keep the following in mind: acknowledge, discover, opportunity, boundary setting, and extend.
He went on to explain to the audience why thinking about these terms is useful when interacting with patients.
First, acknowledge the feelings of the patient. Noting that a patient is angry, perhaps counterintuitively, helps, he said. In fact, not acknowledging the anger “throws gasoline on the fire.”
Then, discover the cause of their emotion. Saying "tell me more" and "help me understand" can be powerful tools, he noted.
Next, take this as an opportunity for empathy – especially important to remember when you’re being verbally attacked.
Boundary setting is important, because it lets the patient know that the conversation won’t continue unless they show the same respect the physician is showing, he said.
Finally, physicians can extend the system of support by asking others – such as colleagues or security – for help.
Use the NURS guide to show empathy
Dr. Fortin said he uses the “NURS” guide or calling to mind “name, express, respect, and support” to show empathy:
This involves naming a patient’s emotion; expressing understanding, with phrases like "I can see how you could be …"; showing respect, acknowledging a patient is going through a lot; and offering support, by saying something like, "Let’s see what we can do together to get to the bottom of this," he explained.
“My lived experience in using [these] in this order is that by the end of it, the patient cannot stay mad at me,” Dr. Fortin said.
“It’s really quite remarkable,” he added.
Steps for nonviolent communication
Rebecca Andrews, MD, MS, another moderator for the session, offered these steps for “nonviolent communication”:
- Observing the situation without blame or judgment.
- Telling the person how this situation makes you feel.
- Connecting with a need of the other person.
- Making a request that is specific and based on action, rather than a request not to do something, such as "Would you be willing to … ?"
Dr. Andrews, who is professor of medicine at the University of Connecticut, Farmington, said this approach has worked well for her, both in interactions with patients and in her personal life.
“It is evidence based that compassion actually makes care better,” she noted.
Varun Jain, MD, a member of the audience, expressed gratitude to the session’s speakers for teaching him something that he had not learned in medical school or residency.
“Every week you will have one or two people who will be labeled as ‘difficult,’ ” and it was nice to have some proven advice on how to handle these tough interactions, said the hospitalist at St. Francis Hospital in Hartford, Conn.
“We never got any actual training on this, and we were expected to know this because we are just physicians, and physicians are expected to be compassionate,” Dr. Jain said. “No one taught us how to have compassion.”
Dr. Fortin and Dr. Andrews disclosed no relevant financial relationships.
“At my hospital, it was such a big thing to make sure that families are called,” said Dr. Nwankwo, in an interview following a session on compassionate communication at the annual meeting of the American College of Physicians. “So you have 19 patients, and you have to call almost every family to update them. And then you call, and they say, ‘Call this person as well.’ You feel like you’re at your wit’s end a lot of times.”
Sometimes, she has had to dig deep to find the empathy for patients that she knows her patients deserve.
“You really want to care by thinking about where is this patient coming from? What’s going on in their lives? And not just label them a difficult patient,” she said.
Become curious
Auguste Fortin, MD, MPH, offered advice for handling patient interactions under these kinds of circumstances, while serving as a moderator during the session.
“When the going gets tough, turn to wonder.” Become curious about why a patient might be feeling the way they are, he said.
Dr. Fortin, professor of internal medicine at Yale University, New Haven, Conn., said using the ADOBE acronym, has helped him more effectively communicate with his patients. This tool cues him to keep the following in mind: acknowledge, discover, opportunity, boundary setting, and extend.
He went on to explain to the audience why thinking about these terms is useful when interacting with patients.
First, acknowledge the feelings of the patient. Noting that a patient is angry, perhaps counterintuitively, helps, he said. In fact, not acknowledging the anger “throws gasoline on the fire.”
Then, discover the cause of their emotion. Saying "tell me more" and "help me understand" can be powerful tools, he noted.
Next, take this as an opportunity for empathy – especially important to remember when you’re being verbally attacked.
Boundary setting is important, because it lets the patient know that the conversation won’t continue unless they show the same respect the physician is showing, he said.
Finally, physicians can extend the system of support by asking others – such as colleagues or security – for help.
Use the NURS guide to show empathy
Dr. Fortin said he uses the “NURS” guide or calling to mind “name, express, respect, and support” to show empathy:
This involves naming a patient’s emotion; expressing understanding, with phrases like "I can see how you could be …"; showing respect, acknowledging a patient is going through a lot; and offering support, by saying something like, "Let’s see what we can do together to get to the bottom of this," he explained.
“My lived experience in using [these] in this order is that by the end of it, the patient cannot stay mad at me,” Dr. Fortin said.
“It’s really quite remarkable,” he added.
Steps for nonviolent communication
Rebecca Andrews, MD, MS, another moderator for the session, offered these steps for “nonviolent communication”:
- Observing the situation without blame or judgment.
- Telling the person how this situation makes you feel.
- Connecting with a need of the other person.
- Making a request that is specific and based on action, rather than a request not to do something, such as "Would you be willing to … ?"
Dr. Andrews, who is professor of medicine at the University of Connecticut, Farmington, said this approach has worked well for her, both in interactions with patients and in her personal life.
“It is evidence based that compassion actually makes care better,” she noted.
Varun Jain, MD, a member of the audience, expressed gratitude to the session’s speakers for teaching him something that he had not learned in medical school or residency.
“Every week you will have one or two people who will be labeled as ‘difficult,’ ” and it was nice to have some proven advice on how to handle these tough interactions, said the hospitalist at St. Francis Hospital in Hartford, Conn.
“We never got any actual training on this, and we were expected to know this because we are just physicians, and physicians are expected to be compassionate,” Dr. Jain said. “No one taught us how to have compassion.”
Dr. Fortin and Dr. Andrews disclosed no relevant financial relationships.
AT INTERNAL MEDICINE 2022
Who doesn’t text in 2022? Most state Medicaid programs
West Virginia will use the U.S. Postal Service and an online account in the summer of 2022 to connect with Medicaid enrollees about the expected end of the COVID public health emergency, which will put many recipients at risk of losing their coverage.
What West Virginia won’t do is use a form of communication that’s ubiquitous worldwide: text messaging.
“West Virginia isn’t set up to text its members,” Allison Adler, the state’s Medicaid spokesperson, wrote to KHN in an email.
Indeed, most states’ Medicaid programs won’t text enrollees despite the urgency to reach them about renewing their coverage. A KFF report published in March found just 11 states said they would use texting to alert Medicaid recipients about the end of the COVID public health emergency. In contrast, 33 states plan to use snail mail and at least 20 will reach out with individual or automated phone calls.
“It doesn’t make any sense when texting is how most people communicate today,” said Kinda Serafi, a partner with the consulting firm Manatt Health.
State Medicaid agencies for months have been preparing for the end of the public health emergency. As part of a COVID relief law approved in March 2020, Congress prohibited states from dropping anyone from Medicaid coverage unless they moved out of state during the public health emergency. When the emergency ends, state Medicaid officials must reevaluate each enrollee’s eligibility. Millions of people could lose their coverage if they earn too much or fail to provide the information needed to verify income or residency.
As of November, about 86 million people were enrolled in Medicaid, according to the Centers for Medicare & Medicaid Services. That’s up from 71 million in February 2020, before COVID began to ravage the nation.
West Virginia has more than 600,000 Medicaid enrollees. Adler said about 100,000 of them could lose their eligibility at the end of the public health emergency because either the state has determined they’re ineligible or they’ve failed to respond to requests that they update their income information.
“It’s frustrating that texting is a means to meet people where they are and that this has not been picked up more by states,” said Jennifer Wagner, director of Medicaid eligibility and enrollment for the Center on Budget and Policy Priorities, a Washington-based research group.
The problem with relying on the Postal Service is that a letter can get hidden in “junk” mail or can fail to reach people who have moved or are homeless, Ms. Serafi said. And email, if people have an account, can end up in spam folders.
In contrast, surveys show lower-income Americans are just as likely to have smartphones and cellphones as the general population. And most people regularly use texting.
In Michigan, Medicaid officials started using text messaging to communicate with enrollees in 2020 after building a system with the help of federal COVID relief funding. They said texting is an economical way to reach enrollees.
“It costs us 2 cents per text message, which is incredibly cheap,” said Steph White, an enrollment coordinator for the Michigan Department of Health and Human Services. “It’s a great return on investment.”
CMS officials have told states they should consider texting, along with other communication methods, when trying to reach enrollees when the public health emergency ends. But many states don’t have the technology or information about enrollees to do it.
Efforts to add texting also face legal barriers, including a federal law that bars texting people without their consent. The Federal Communications Commission ruled in 2021 that state agencies are exempt from the law, but whether counties that handle Medicaid duties for some states and Medicaid managed-care organizations that work in more than 40 states are exempt as well is unclear, said Matt Salo, executive director of the National Association of Medicaid Directors.
CMS spokesperson Beth Lynk said the agency is trying to figure out how Medicaid agencies, counties, and health plans can text enrollees within the constraints of federal law.
Several states told KHN that Medicaid health plans will be helping connect with enrollees and that they expect the plans to use text messaging. But the requirement to get consent from enrollees before texting could limit that effort.
That’s the situation in Virginia, where only about 30,000 Medicaid enrollees – out of more than a million – have agreed to receive text messages directly from the state, said spokesperson Christina Nuckols.
In an effort to boost that number, the state plans to ask enrollees if they want to opt out of receiving text messages, rather than ask them to opt in, she said. This way enrollees would contact the state only if they don’t want to be texted. The state is reviewing its legal options to make that happen.
Meanwhile, Ms. Nuckols added, the state expects Medicaid health plans to contact enrollees about updating their contact information. Four of Virginia’s six Medicaid plans, which serve the bulk of the state’s enrollees, have permission to text about 316,000.
Craig Kennedy, CEO of Medicaid Health Plans of America, a trade group, said that most plans are using texting and that Medicaid officials will use multiple strategies to connect with enrollees. “I do not see this as a detriment, that states are not texting information about reenrollment,” he said. “I know we will be helping with that.”
California officials in March directed Medicaid health plans to use a variety of communication methods, including texting, to ensure that members can retain coverage if they remain eligible. The officials told health plans they could ask for consent through an initial text.
California officials say they also plan to ask enrollees for consent to be texted on the enrollment application, although federal approval for the change is not expected until the fall.
A few state Medicaid programs have experimented in recent years with pilot programs that included texting enrollees.
In 2019, Louisiana worked with the nonprofit group Code for America to send text messages that reminded people about renewing coverage and providing income information for verification. Compared with traditional communication methods, the texts led to a 67% increase in enrollees being renewed for coverage and a 56% increase in enrollees verifying their income in response to inquiries, said Medicaid spokesperson Alyson Neel.
Nonetheless, the state isn’t planning to text Medicaid enrollees about the end of the public health emergency because it hasn’t set up a system for that. “Medicaid has not yet been able to implement a text messaging system of its own due to other agency priorities,” Ms. Neel said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
West Virginia will use the U.S. Postal Service and an online account in the summer of 2022 to connect with Medicaid enrollees about the expected end of the COVID public health emergency, which will put many recipients at risk of losing their coverage.
What West Virginia won’t do is use a form of communication that’s ubiquitous worldwide: text messaging.
“West Virginia isn’t set up to text its members,” Allison Adler, the state’s Medicaid spokesperson, wrote to KHN in an email.
Indeed, most states’ Medicaid programs won’t text enrollees despite the urgency to reach them about renewing their coverage. A KFF report published in March found just 11 states said they would use texting to alert Medicaid recipients about the end of the COVID public health emergency. In contrast, 33 states plan to use snail mail and at least 20 will reach out with individual or automated phone calls.
“It doesn’t make any sense when texting is how most people communicate today,” said Kinda Serafi, a partner with the consulting firm Manatt Health.
State Medicaid agencies for months have been preparing for the end of the public health emergency. As part of a COVID relief law approved in March 2020, Congress prohibited states from dropping anyone from Medicaid coverage unless they moved out of state during the public health emergency. When the emergency ends, state Medicaid officials must reevaluate each enrollee’s eligibility. Millions of people could lose their coverage if they earn too much or fail to provide the information needed to verify income or residency.
As of November, about 86 million people were enrolled in Medicaid, according to the Centers for Medicare & Medicaid Services. That’s up from 71 million in February 2020, before COVID began to ravage the nation.
West Virginia has more than 600,000 Medicaid enrollees. Adler said about 100,000 of them could lose their eligibility at the end of the public health emergency because either the state has determined they’re ineligible or they’ve failed to respond to requests that they update their income information.
“It’s frustrating that texting is a means to meet people where they are and that this has not been picked up more by states,” said Jennifer Wagner, director of Medicaid eligibility and enrollment for the Center on Budget and Policy Priorities, a Washington-based research group.
The problem with relying on the Postal Service is that a letter can get hidden in “junk” mail or can fail to reach people who have moved or are homeless, Ms. Serafi said. And email, if people have an account, can end up in spam folders.
In contrast, surveys show lower-income Americans are just as likely to have smartphones and cellphones as the general population. And most people regularly use texting.
In Michigan, Medicaid officials started using text messaging to communicate with enrollees in 2020 after building a system with the help of federal COVID relief funding. They said texting is an economical way to reach enrollees.
“It costs us 2 cents per text message, which is incredibly cheap,” said Steph White, an enrollment coordinator for the Michigan Department of Health and Human Services. “It’s a great return on investment.”
CMS officials have told states they should consider texting, along with other communication methods, when trying to reach enrollees when the public health emergency ends. But many states don’t have the technology or information about enrollees to do it.
Efforts to add texting also face legal barriers, including a federal law that bars texting people without their consent. The Federal Communications Commission ruled in 2021 that state agencies are exempt from the law, but whether counties that handle Medicaid duties for some states and Medicaid managed-care organizations that work in more than 40 states are exempt as well is unclear, said Matt Salo, executive director of the National Association of Medicaid Directors.
CMS spokesperson Beth Lynk said the agency is trying to figure out how Medicaid agencies, counties, and health plans can text enrollees within the constraints of federal law.
Several states told KHN that Medicaid health plans will be helping connect with enrollees and that they expect the plans to use text messaging. But the requirement to get consent from enrollees before texting could limit that effort.
That’s the situation in Virginia, where only about 30,000 Medicaid enrollees – out of more than a million – have agreed to receive text messages directly from the state, said spokesperson Christina Nuckols.
In an effort to boost that number, the state plans to ask enrollees if they want to opt out of receiving text messages, rather than ask them to opt in, she said. This way enrollees would contact the state only if they don’t want to be texted. The state is reviewing its legal options to make that happen.
Meanwhile, Ms. Nuckols added, the state expects Medicaid health plans to contact enrollees about updating their contact information. Four of Virginia’s six Medicaid plans, which serve the bulk of the state’s enrollees, have permission to text about 316,000.
Craig Kennedy, CEO of Medicaid Health Plans of America, a trade group, said that most plans are using texting and that Medicaid officials will use multiple strategies to connect with enrollees. “I do not see this as a detriment, that states are not texting information about reenrollment,” he said. “I know we will be helping with that.”
California officials in March directed Medicaid health plans to use a variety of communication methods, including texting, to ensure that members can retain coverage if they remain eligible. The officials told health plans they could ask for consent through an initial text.
California officials say they also plan to ask enrollees for consent to be texted on the enrollment application, although federal approval for the change is not expected until the fall.
A few state Medicaid programs have experimented in recent years with pilot programs that included texting enrollees.
In 2019, Louisiana worked with the nonprofit group Code for America to send text messages that reminded people about renewing coverage and providing income information for verification. Compared with traditional communication methods, the texts led to a 67% increase in enrollees being renewed for coverage and a 56% increase in enrollees verifying their income in response to inquiries, said Medicaid spokesperson Alyson Neel.
Nonetheless, the state isn’t planning to text Medicaid enrollees about the end of the public health emergency because it hasn’t set up a system for that. “Medicaid has not yet been able to implement a text messaging system of its own due to other agency priorities,” Ms. Neel said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
West Virginia will use the U.S. Postal Service and an online account in the summer of 2022 to connect with Medicaid enrollees about the expected end of the COVID public health emergency, which will put many recipients at risk of losing their coverage.
What West Virginia won’t do is use a form of communication that’s ubiquitous worldwide: text messaging.
“West Virginia isn’t set up to text its members,” Allison Adler, the state’s Medicaid spokesperson, wrote to KHN in an email.
Indeed, most states’ Medicaid programs won’t text enrollees despite the urgency to reach them about renewing their coverage. A KFF report published in March found just 11 states said they would use texting to alert Medicaid recipients about the end of the COVID public health emergency. In contrast, 33 states plan to use snail mail and at least 20 will reach out with individual or automated phone calls.
“It doesn’t make any sense when texting is how most people communicate today,” said Kinda Serafi, a partner with the consulting firm Manatt Health.
State Medicaid agencies for months have been preparing for the end of the public health emergency. As part of a COVID relief law approved in March 2020, Congress prohibited states from dropping anyone from Medicaid coverage unless they moved out of state during the public health emergency. When the emergency ends, state Medicaid officials must reevaluate each enrollee’s eligibility. Millions of people could lose their coverage if they earn too much or fail to provide the information needed to verify income or residency.
As of November, about 86 million people were enrolled in Medicaid, according to the Centers for Medicare & Medicaid Services. That’s up from 71 million in February 2020, before COVID began to ravage the nation.
West Virginia has more than 600,000 Medicaid enrollees. Adler said about 100,000 of them could lose their eligibility at the end of the public health emergency because either the state has determined they’re ineligible or they’ve failed to respond to requests that they update their income information.
“It’s frustrating that texting is a means to meet people where they are and that this has not been picked up more by states,” said Jennifer Wagner, director of Medicaid eligibility and enrollment for the Center on Budget and Policy Priorities, a Washington-based research group.
The problem with relying on the Postal Service is that a letter can get hidden in “junk” mail or can fail to reach people who have moved or are homeless, Ms. Serafi said. And email, if people have an account, can end up in spam folders.
In contrast, surveys show lower-income Americans are just as likely to have smartphones and cellphones as the general population. And most people regularly use texting.
In Michigan, Medicaid officials started using text messaging to communicate with enrollees in 2020 after building a system with the help of federal COVID relief funding. They said texting is an economical way to reach enrollees.
“It costs us 2 cents per text message, which is incredibly cheap,” said Steph White, an enrollment coordinator for the Michigan Department of Health and Human Services. “It’s a great return on investment.”
CMS officials have told states they should consider texting, along with other communication methods, when trying to reach enrollees when the public health emergency ends. But many states don’t have the technology or information about enrollees to do it.
Efforts to add texting also face legal barriers, including a federal law that bars texting people without their consent. The Federal Communications Commission ruled in 2021 that state agencies are exempt from the law, but whether counties that handle Medicaid duties for some states and Medicaid managed-care organizations that work in more than 40 states are exempt as well is unclear, said Matt Salo, executive director of the National Association of Medicaid Directors.
CMS spokesperson Beth Lynk said the agency is trying to figure out how Medicaid agencies, counties, and health plans can text enrollees within the constraints of federal law.
Several states told KHN that Medicaid health plans will be helping connect with enrollees and that they expect the plans to use text messaging. But the requirement to get consent from enrollees before texting could limit that effort.
That’s the situation in Virginia, where only about 30,000 Medicaid enrollees – out of more than a million – have agreed to receive text messages directly from the state, said spokesperson Christina Nuckols.
In an effort to boost that number, the state plans to ask enrollees if they want to opt out of receiving text messages, rather than ask them to opt in, she said. This way enrollees would contact the state only if they don’t want to be texted. The state is reviewing its legal options to make that happen.
Meanwhile, Ms. Nuckols added, the state expects Medicaid health plans to contact enrollees about updating their contact information. Four of Virginia’s six Medicaid plans, which serve the bulk of the state’s enrollees, have permission to text about 316,000.
Craig Kennedy, CEO of Medicaid Health Plans of America, a trade group, said that most plans are using texting and that Medicaid officials will use multiple strategies to connect with enrollees. “I do not see this as a detriment, that states are not texting information about reenrollment,” he said. “I know we will be helping with that.”
California officials in March directed Medicaid health plans to use a variety of communication methods, including texting, to ensure that members can retain coverage if they remain eligible. The officials told health plans they could ask for consent through an initial text.
California officials say they also plan to ask enrollees for consent to be texted on the enrollment application, although federal approval for the change is not expected until the fall.
A few state Medicaid programs have experimented in recent years with pilot programs that included texting enrollees.
In 2019, Louisiana worked with the nonprofit group Code for America to send text messages that reminded people about renewing coverage and providing income information for verification. Compared with traditional communication methods, the texts led to a 67% increase in enrollees being renewed for coverage and a 56% increase in enrollees verifying their income in response to inquiries, said Medicaid spokesperson Alyson Neel.
Nonetheless, the state isn’t planning to text Medicaid enrollees about the end of the public health emergency because it hasn’t set up a system for that. “Medicaid has not yet been able to implement a text messaging system of its own due to other agency priorities,” Ms. Neel said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Direct-to-Consumer Teledermatology Growth: A Review and Outlook for the Future
In recent years, direct-to-consumer (DTC) teledermatology platforms have gained popularity as telehealth business models, allowing patients to directly initiate visits with physicians and purchase medications from single platforms. A shortage of dermatologists, improved technology, drug patent expirations, and rising health care costs accelerated the growth of DTC dermatology.1 During the COVID-19 pandemic, teledermatology adoption surged due to the need to provide care while social distancing and minimizing viral exposure. These needs prompted additional federal funding and loosened regulatory provisions.2 As the userbase of these companies has grown, so have their valuations.3 Although the DTC model has attracted the attention of patients and investors, its rise provokes many questions about patients acting as consumers in health care. Indeed, DTC telemedicine offers greater autonomy and convenience for patients, but it may impact the quality of care and the nature of physician-patient relationships, perhaps making them more transactional.
Evolution of DTC in Health Care
The DTC model emphasizes individual choice and accessible health care. Although the definition has evolved, the core idea is not new.4 Over decades, pharmaceutical companies have spent billions of dollars on DTC advertising, circumventing physicians by directly reaching patients with campaigns on prescription drugs and laboratory tests and shaping public definitions of diseases.5
The DTC model of care is fundamentally different from traditional care models in that it changes the roles of the patient and physician. Whereas early telehealth models required a health care provider to initiate teleconsultations with specialists, DTC telemedicine bypasses this step (eg, the patient can consult a dermatologist without needing a primary care provider’s input first). This care can then be provided by dermatologists with whom patients may or may not have pre-established relationships.4,6
Dermatology was an early adopter of DTC telemedicine. The shortage of dermatologists in the United States created demand for increasing accessibility to dermatologic care. Additionally, the visual nature of diagnosing dermatologic disease was ideal for platforms supporting image sharing.7 Early DTC providers were primarily individual companies offering teledermatology. However, many dermatologists can now offer DTC capabilities via companies such as Amwell and Teladoc Health.8
Over the last 2 decades, start-ups such as Warby Parker (eyeglasses) and Casper (mattresses) defined the DTC industry using borrowed supply chains, cohesive branding, heavy social media marketing, and web-only retail. Scalability, lack of competition, and abundant venture capital created competition across numerous markets.9 Health care capitalized on this DTC model, creating a $700 billion market for products ranging from hearing aids to over-the-counter medications.10
Borrowing from this DTC playbook, platforms were created to offer delivery of generic prescription drugs to patients’ doorsteps. However, unlike with other products bought online, a consumer cannot simply add prescription drugs to their shopping cart and check out. In all models of American medical practice, physicians still serve as gatekeepers, providing a safeguard for patients to ensure appropriate prescription and avoid negative consequences of unnecessary drug use. This new model effectively streamlines diagnosis, prescription, and drug delivery without the patient ever having to leave home. Combining the prescribing and selling of medications (2 tasks that traditionally have been separated) potentially creates financial conflicts of interest (COIs). Additionally, high utilization of health care, including more prescriptions and visits, does not necessarily equal high quality of care. The companies stand to benefit from extra care regardless of need, and thus these models must be scrutinized for any incentives driving unnecessary care and prescriptions.
Ultimately, DTC has evolved to encompass multiple definitions in health care (Table 1). Although all models provide health care, each offers a different modality of delivery. The primary service may be the sale of prescription drugs or simply telemedicine visits. This review primarily discusses DTC pharmaceutical telemedicine platforms that sell private-label drugs and also offer telemedicine services to streamline care. However, the history, risks, and benefits discussed may apply to all models.
The DTC Landscape
Most DTC companies employ variations on a model with the same 3 main components: a triage questionnaire, telehealth services, and prescription/drug delivery (Figure). The triage questionnaire elicits a history of the patient’s presentation and medical history. Some companies may use artificial intelligence (AI) algorithms to tailor questions to patient needs. There are 2 modalities for patient-provider communication: synchronous and asynchronous. Synchronous communication entails real-time patient-physician conversations via audio only or video call. Asynchronous (or store-and-forward) communication refers to consultations provided via messaging or text-based modality, where a provider may respond to a patient within 24 hours.6 Direct-to-consumer platforms primarily use asynchronous visits (Table 2). However, some also use synchronous modalities if the provider deems it necessary or if state laws require it.
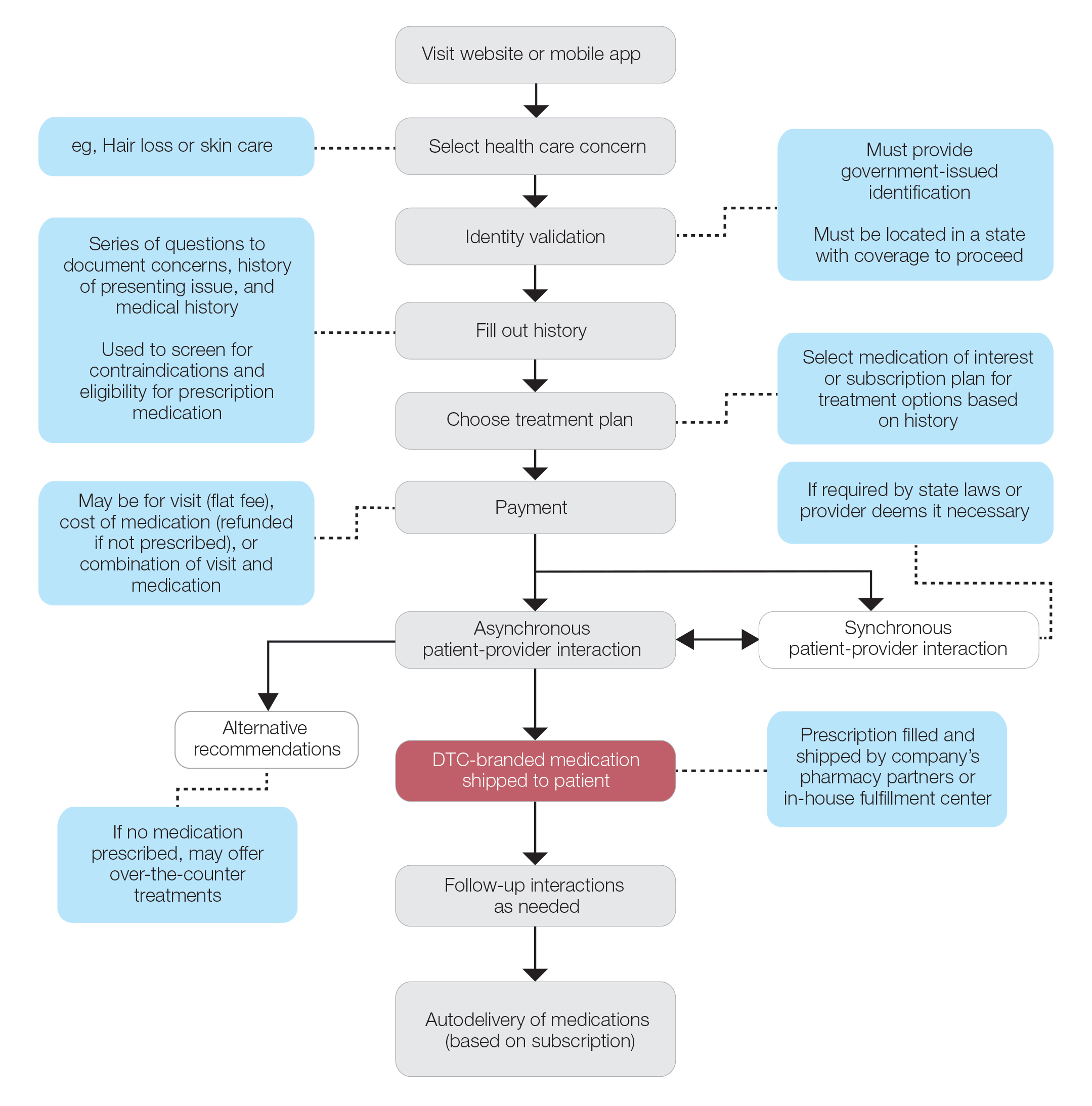
Once a provider has consulted with the patient, they can prescribe medication as needed. In certain cases, with adequate history, a prescription may be issued without a full physician visit. Furthermore, DTC companies require purchase of their custom-branded generic drugs. Prescriptions are fulfilled by the company’s pharmacy network and directly shipped to patients; few will allow patients to transfer a prescription to a pharmacy of their choice. Some platforms also sell supplements and over-the-counter medications.
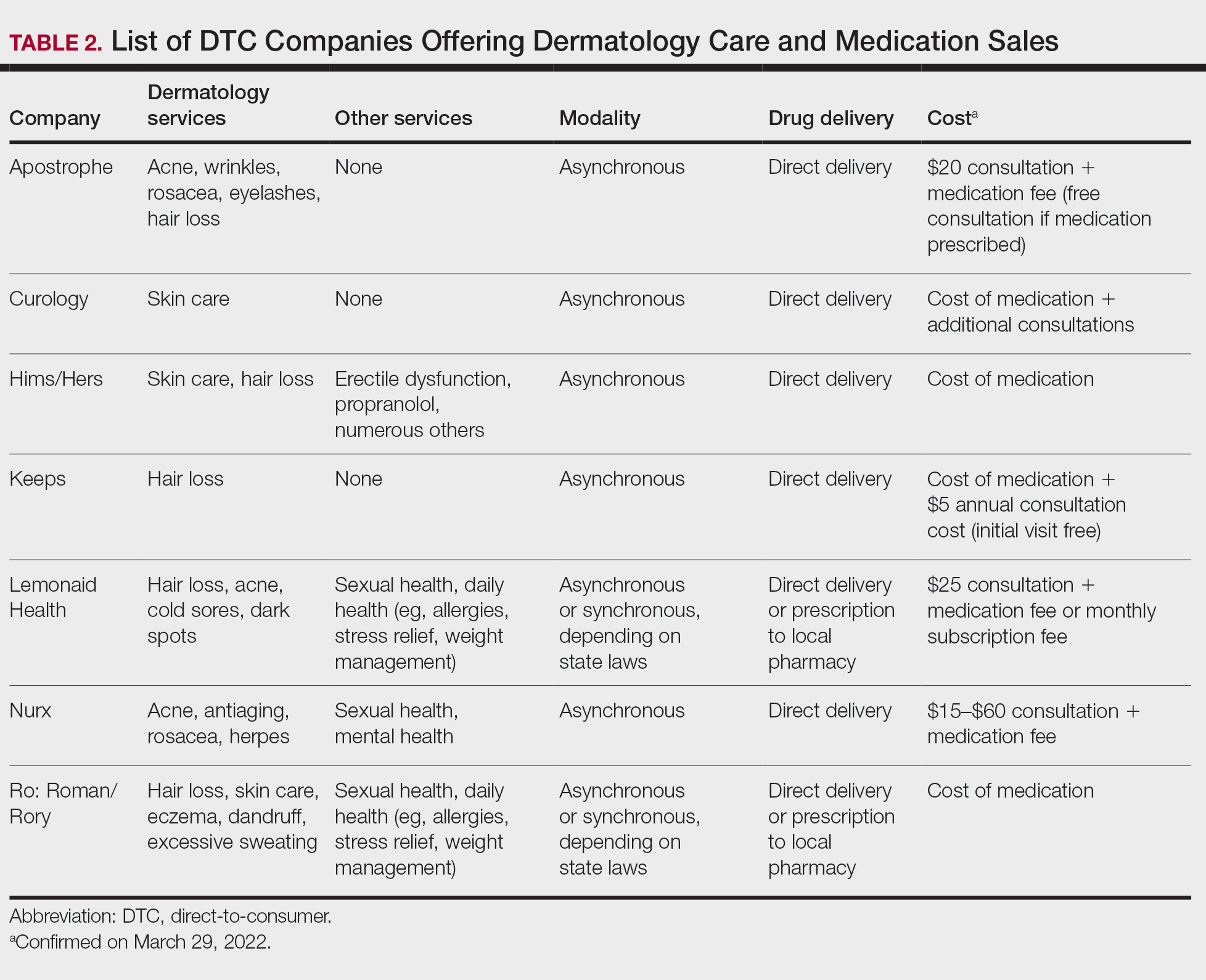
Payment models vary among these companies, and most do not accept insurance (Table 2). Select models may provide free consultations and only require payment for pharmaceuticals. Others charge for consultations but reallocate payment to the cost of medication if prescribed. Another model involves flat rates for consultations and additional charges for drugs but unlimited messaging with providers for the duration of the prescription. Moreover, patients can subscribe to monthly deliveries of their medications.
Foundation of DTC
Technological advances have enabled patients to receive remote treatment from a single platform offering video calls, AI, electronic medical record interoperability, and integration of drug supply chains. Even in its simplest form, AI is increasingly used, as it allows for programs and chatbots to screen and triage patients.11 Technology also has improved at targeted mass marketing through social media platforms and search engines (eg, companies can use age, interests, location, and other parameters to target individuals likely needing acne treatment).
Drug patent expirations are a key catalyst for the rise of DTC companies, creating an attractive business model with generic drugs as the core product. Since 2008, patents for medications treating chronic conditions, such as erectile dysfunction, have expired. These patent expirations are responsible for $198 billion in projected prescription sales between 2019 and 2024.1 Thus, it follows that DTC companies have seized this opportunity to act as middlemen, taking advantage of these generic medications’ lower costs to create platforms focused on personalization and accessibility.
Rising deductibles have led patients to consider cheaper out-of-pocket alternatives that are not covered by insurance.1 For example, insurers typically do not cover finasteride treatment for conditions deemed cosmetic, such as androgenetic alopecia.12 The low cost of generic drugs creates an attractive business model for patients and investors. According to GoodRx, the average retail price for a 30-day supply of brand-name finasteride (Propecia [Merck]) is $135.92, whereas generic finasteride is $75.24.13 Direct-to-consumer pharmaceutical companies offer a 30-day supply of generic finasteride ranging from $8.33 to $30.14 The average wholesale cost for retailers is an estimated $2.31 for 30 days.15 Although profit margins on generic medications may be lower, more affordable drugs increase the size of the total market. These prescriptions are available as subscription plans, resulting in recurring revenue.
Lax US pharmaceutical marketing regulations allow direct advertising to the general public.16 In 1997, the US Food and Drug Administration allowed DTC advertisements to replace summaries of serious and common adverse effects with short statements covering important risks or referrals to other sources for complete information. In 2015, the US Food and Drug Administration guidelines preventing encouragement of self-diagnosis and self-treatment were withdrawn.5 These changes enable DTC companies to launch large advertising campaigns and to accelerate customer acquisition, as the industry often describes it, with ease.
Rapid Growth and Implications
Increasing generic drug availability and improving telemedicine capabilities have the potential to reduce costs and barriers but also have the potential for financial gain. Venture capital funds have recognized this opportunity, reflected by millions of dollars of investments, and accelerated the growth of DTC health care start-ups. For example, Ro has raised $376 million from venture capital, valuing the company at $1.5 billion.3
Direct-to-consumer companies require a heavy focus on marketing campaigns for customer acquisition. Their aesthetically pleasing websites and aggressive campaigns target specific audiences based on demographics, digital use habits, and purchasing behavior.4 Some campaigns celebrate the ease of obtaining prescriptions.17 Companies have been effective in recruiting so-called millennial and Generation Z patients, known to search the internet for remedies prior to seeking physician consultations.18 Recognizing these needs, some platforms offer guides on diseases they treat, creating effective customer-acquisition funnels. Recruitment of these technology-friendly patients has proven effective, especially given the largely positive media coverage of DTC platforms––potentially serving as a surrogate for medical credibility for patients.18
Some DTC companies also market physically; skin care ads may be strategically placed in social media feeds, or even found near mirrors in public bathrooms.19 Marketing campaigns also involve disease awareness; such efforts serve to increase diagnoses and prescribed treatments while destigmatizing diseases. Although DTC companies argue this strategy empowers patients, these marketing habits have the potential to take advantage of uninformed patients. Campaigns could potentially medicalize normal experiences and expand disease definitions resulting in overdiagnosis, overtreatment, and wasted resources.5 For example, off-label propranolol use has been advertised to attract patients who might have “nerves that come creeping before an important presentation.”17 Disease awareness campaigns also may lead people to falsely believe unproven drug benefits.5 According to studies, DTC pharmaceutical advertisements are low in informational quality and result in increased patient visits and prescriptions despite cost-effective alternatives.5,20-22
Fragmentation of the health care system is another possible complication of DTC teledermatology. These companies operate as for-profit organizations separated from the rest of the health care system, raising concerns about care coordination.8 Vital health data may not be conveyed as patients move among different providers and pharmacies. One study found DTC teledermatology rarely offered to provide medical records or facilitate a referral to a local physician.23 Such a lack of communication is concerning, as medication errors are the leading cause of avoidable harm in health care.24
Direct-to-consumer care models also seemingly redefine the physician-patient relationship by turning patients into consumers. Patient interactions may seem transactional and streamlined toward sales. For these platforms, a visit often is set up as an evaluation of a patient’s suitability for a prescription, not necessarily for the best treatment modality for the problem. These companies primarily make money through the sale of prescription drugs, creating a potential COI that may undermine the patient-physician relationship. Although some companies have made it clear that medical care and pharmaceutical sales are provided by legally separate business entities and that they do not pay physicians on commission, a conflict may still exist given the financial importance of physicians prescribing medication to the success of the business.16
Even as DTC models advertise upon expanded access and choice, the companies largely prohibit patients from choosing their own pharmacy. Instead, they encourage patients to fill prescriptions with the company’s pharmacy network by claiming lower costs compared with competitors. One DTC company, Hims, is launching a prescription-fulfillment center to further consolidate their business.17,19,25 The inherent COI of issuing and fulfilling prescriptions raises concerns of patient harm.26 For example, when Dermatology.com launched as a DTC prescription skin medication shop backed by Bausch Health Companies Inc, its model included telemedicine consultation. Although consultations were provided by RxDefine, a third party, only Dermatology.com drugs were prescribed. Given the poor quality of care and obvious financial COI, an uproar in the dermatology community and advocacy by the American Academy of Dermatology led to the shutdown of Dermatology.com’s online prescription services.26
The quality of care among DTC telemedicine platforms has been equivocal. Some studies have reported equivalent care in person and online, while others have reported poor adherence to guidelines, overuse of antibiotics, and misdiagnosis.8,23 A vital portion of the DTC experience is the history questionnaire, which is geared to diagnosis and risk assessment.25 Resneck et al23 found diagnostic quality to be adequate for simple dermatologic clinical scenarios but poor for scenarios requiring more than basic histories. Although Ro has reported leveraging data from millions of interactions to ask the right questions and streamline visits, it is still unclear whether history questionnaires are adequate.17,27 Additionally, consultations may lack sufficient counseling on adverse effects, risks, or pregnancy warnings, as well as discussions on alternative treatments and preventative care.17,23 Finally, patients often are limited in their choice of dermatologist; the lack of a fully developed relationship increases concerns of follow-up and monitoring practices. Although some DTC platforms offer unlimited interactions with physicians for the duration of a prescription, it is unknown how often these services are utilized or how adequate the quality of these interactions is. This potential for lax follow-up is especially concerning for prescriptions that autorenew on a monthly basis and could result in unnecessary overtreatment.
Postpandemic and Future Outlook
The COVID-19 pandemic dramatically impacted the use of telemedicine. To minimize COVID-19 transmission, the Centers for Medicare & Medicaid Services and private payers expanded telehealth coverage and eliminated reimbursement and licensing barriers.28 A decade’s worth of regulatory changes and consumer adoption was accelerated to weeks, resulting in telemedicine companies reaching record-high visit numbers.29 McKinsey & Company estimated that telehealth visit numbers surged 50- to 175-fold compared with pre–COVID-19 numbers. Additionally, 76% of patients were interested in future telehealth use, and 64% of providers were more comfortable using telehealth than before the pandemic.30 For their part, US dermatologists reported an increase in telemedicine use from 14.1% to 96.9% since COVID-19.31
Exactly how much DTC pharmaceutical telemedicine companies are growing is unclear, but private investments may be an indication. A record $14.7 billion was invested in the digital health sector in the first half of 2021; the majority went to telehealth companies.30 Ro, which reported $230 million in revenue in 2020 and has served 6 million visits, raised $200 milllion in July 2020 and $500 million in March 2021.32 Although post–COVID-19 health care will certainly involve increased telemedicine, the extent remains unclear, as telehealth vendors saw decreased usage upon reopening of state economies. Ultimately, the postpandemic regulatory landscape is hard to predict.30
Although COVID-19 appears to have caused rapid growth for DTC platforms, it also may have spurred competition. Telemedicine providers have given independent dermatologists and health care systems the infrastructure to implement custom DTC services.33 Although systems do not directly sell prescription drugs, the target market is essentially the same: patients looking for instant virtual dermatologic care. Therefore, sustained telemedicine services offered by traditional practices and systems may prove detrimental to DTC companies. However, unlike most telemedicine services, DTC models are less affected by certain changes in regulation since they do not rely on insurance. If regulations are tightened and reimbursements for telehealth are not attractive for dermatologists, teledermatology services may see an overall decrease. If so, patients who appreciate teledermatology may shift to using DTC platforms, even if their insurance does not cover them. Still, a nationwide survey found 56% of respondents felt an established relationship with a physician prior to a telemedicine visit is important, which may create a barrier for DTC adoption.34
Conclusion
Direct-to-consumer teledermatology represents a growing for-profit model of health care that provides patients with seemingly affordable and convenient care. However, there is potential for overtreatment, misdiagnosis, and fragmentation of health care. It will be important to monitor and evaluate the quality of care that DTC teledermatology offers and advocate for appropriate regulations and oversight. Eventually, more patients will have medications prescribed and dermatologic care administered through DTC companies. Dermatologists will benefit from this knowledge of DTC models to properly counsel patients on the risks and benefits of their use.
- Vennare J. The DTC healthcare report. Fitt Insider. September 15, 2019. Accessed February 23, 2022. https://insider.fitt.co/direct-to-consumer-healthcare-startups/
- Kannampallil T, Ma J. Digital translucence: adapting telemedicine delivery post-COVID-19. Telemed J E Health. 2020;26:1120-1122.
- Farr C. Ro, a 3-year-old online health provider, just raised a new round that values it at $1.5 billion. CNBC. July 27, 2020. Accessed February 23, 2022. https://www.cnbc.com/2020/07/27/ro-raises-200-million-at-1point5-billion-valuation-250-million-sales.html
- Elliott T, Shih J. Direct to consumer telemedicine. Curr Allergy Asthma Rep. 2019;19:1.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Coates SJ, Kvedar J, Granstein RD. Teledermatology: from historical perspective to emerging techniques of the modern era. J Am Acad Dermatol. 2015;72:563-574.
- Rheuban KS, Krupinski EA, eds. Understanding Telehealth. McGraw-Hill Education; 2017.
- Schlesinger LA, Higgins M, Roseman S. Reinventing the direct-to-consumer business model. Harvard Business Review. March 31, 2020. Accessed February 23, 2022. https://hbr.org/2020/03/reinventing-the-direct-to-consumer-business-model
- Cohen AB, Mathews SC, Dorsey ER, et al. Direct-to-consumer digital health. Lancet Digit Health. 2020;2:E163-E165.
- 6 telehealth trends for 2020. Wolters Kluwer. Published January 27, 2021. Accessed February 23, 2022. https://www.wolterskluwer.com/en/expert-insights/6-telehealth-trends-for-2020
- Jadoo SA, Lipoff JB. Prescribing to save patients money: ethical considerations. J Am Acad Dermatol. 2018;78:826-828.
- Propecia. GoodRx. Accessed February 23, 2022. https://www.goodrx.com/propecia
- Lauer A. The truth about online hair-loss treatments like Roman and Hims, according to a dermatologist. InsideHook. January 13, 2020. Accessed February 23, 2022. https://www.insidehook.com/article/grooming/men-hair-loss-treatments-dermatologist-review
- Friedman Y. Drug price trends for NDC 16729-0089. DrugPatentWatch. Accessed February 23, 2022. https://www.drugpatentwatch.com/p/drug-price/ndc/index.php?query=16729-0089
- Curtis H, Milner J. Ethical concerns with online direct-to-consumer pharmaceutical companies. J Med Ethics. 2020;46:168-171.
- Jain T, Lu RJ, Mehrotra A. Prescriptions on demand: the growth of direct-to-consumer telemedicine companies. JAMA. 2019;322:925-926.
- Shahinyan RH, Amighi A, Carey AN, et al. Direct-to-consumer internet prescription platforms overlook crucial pathology found during traditional office evaluation of young men with erectile dysfunction. Urology. 2020;143:165-172.
- Ali M. Andrew Dudum—bold strategies that propelled Hims & Hers into unicorn status. Exit Strategy with Moiz Ali. Published April 2020. Accessed February 23, 2022. https://open.spotify.com/episode/6DtaJxwZDjvZSJI88DTf24?si=b3FHQiUIQY62YjfRHmnJBQ
- Klara K, Kim J, Ross JS. Direct-to-consumer broadcast advertisements for pharmaceuticals: off-label promotion and adherence to FDA guidelines. J Gen Intern Med. 2018;33:651-658.
- Sullivan HW, Aikin KJ, Poehlman J. Communicating risk information in direct-to-consumer prescription drug television ads: a content analysis. Health Commun. 2019;34:212-219.
- Applequist J, Ball JG. An updated analysis of direct-to-consumer television advertisements for prescription drugs. Ann Fam Med. 2018;16:211-216.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Patient safety. World Health Organization. Published September 13, 2019. Accessed February 1, 2022. https://www.who.int/news-room/fact-sheets/detail/patient-safety
- Bollmeier SG, Stevenson E, Finnegan P, et al. Direct to consumer telemedicine: is healthcare from home best? Mo Med. 2020;117:303-309.
26. Court E. Bausch yanked online prescribing after dermatologist backlash. Bloomberg.com. Published March 11, 2020. Accessed September 25, 2020. https://www.bloomberg.com/news/articles/2020-03-11/bausch-yanked-online-prescribing-after-dermatologist-backlash
27. Reitano Z. The future of healthcare: how Ro helps providers treat patients 2 minutes, 2 days, 2 weeks, and 2 years at a time. Medium. Published March 4, 2019. Accessed February 1, 2022. https://medium.com/ro-co/the-future-of-healthcare-how-ro-helps-providers-treat-patients-2-mins-2-days-2-weeks-and-2-10efc0679d7
28. Lee I, Kovarik C, Tejasvi T, et al. Telehealth: helping your patients and practice survive and thrive during the COVID-19 crisis with rapid quality implementation. J Am Acad Dermatol. 2020;82:1213-1214.
29. Pifer R. “Weeks where decades happen”: telehealth 6 months into COVID-19. Healthcare Dive. Published July 27, 2020. Accessed February 23, 2022. https://www.healthcaredive.com/news/telehealth-6-months-coronavirus/581447/
30. Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. Updated July 9, 2021. Accessed February 23, 2022. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality
31. Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
32. Jennings K. Digital health startup Ro raised $500 million at $5 billion valuation. Forbes. March 22, 2021. Accessed March 29, 2022. https://www.forbes.com/sites/katiejennings/2021/03/22/digital-health-startup-ro-raised-500-million-at-5-billion-valuation/?sh=695be0e462f5
33. Hollander JE, Carr BG. Virtually perfect? telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
34. Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
In recent years, direct-to-consumer (DTC) teledermatology platforms have gained popularity as telehealth business models, allowing patients to directly initiate visits with physicians and purchase medications from single platforms. A shortage of dermatologists, improved technology, drug patent expirations, and rising health care costs accelerated the growth of DTC dermatology.1 During the COVID-19 pandemic, teledermatology adoption surged due to the need to provide care while social distancing and minimizing viral exposure. These needs prompted additional federal funding and loosened regulatory provisions.2 As the userbase of these companies has grown, so have their valuations.3 Although the DTC model has attracted the attention of patients and investors, its rise provokes many questions about patients acting as consumers in health care. Indeed, DTC telemedicine offers greater autonomy and convenience for patients, but it may impact the quality of care and the nature of physician-patient relationships, perhaps making them more transactional.
Evolution of DTC in Health Care
The DTC model emphasizes individual choice and accessible health care. Although the definition has evolved, the core idea is not new.4 Over decades, pharmaceutical companies have spent billions of dollars on DTC advertising, circumventing physicians by directly reaching patients with campaigns on prescription drugs and laboratory tests and shaping public definitions of diseases.5
The DTC model of care is fundamentally different from traditional care models in that it changes the roles of the patient and physician. Whereas early telehealth models required a health care provider to initiate teleconsultations with specialists, DTC telemedicine bypasses this step (eg, the patient can consult a dermatologist without needing a primary care provider’s input first). This care can then be provided by dermatologists with whom patients may or may not have pre-established relationships.4,6
Dermatology was an early adopter of DTC telemedicine. The shortage of dermatologists in the United States created demand for increasing accessibility to dermatologic care. Additionally, the visual nature of diagnosing dermatologic disease was ideal for platforms supporting image sharing.7 Early DTC providers were primarily individual companies offering teledermatology. However, many dermatologists can now offer DTC capabilities via companies such as Amwell and Teladoc Health.8
Over the last 2 decades, start-ups such as Warby Parker (eyeglasses) and Casper (mattresses) defined the DTC industry using borrowed supply chains, cohesive branding, heavy social media marketing, and web-only retail. Scalability, lack of competition, and abundant venture capital created competition across numerous markets.9 Health care capitalized on this DTC model, creating a $700 billion market for products ranging from hearing aids to over-the-counter medications.10
Borrowing from this DTC playbook, platforms were created to offer delivery of generic prescription drugs to patients’ doorsteps. However, unlike with other products bought online, a consumer cannot simply add prescription drugs to their shopping cart and check out. In all models of American medical practice, physicians still serve as gatekeepers, providing a safeguard for patients to ensure appropriate prescription and avoid negative consequences of unnecessary drug use. This new model effectively streamlines diagnosis, prescription, and drug delivery without the patient ever having to leave home. Combining the prescribing and selling of medications (2 tasks that traditionally have been separated) potentially creates financial conflicts of interest (COIs). Additionally, high utilization of health care, including more prescriptions and visits, does not necessarily equal high quality of care. The companies stand to benefit from extra care regardless of need, and thus these models must be scrutinized for any incentives driving unnecessary care and prescriptions.
Ultimately, DTC has evolved to encompass multiple definitions in health care (Table 1). Although all models provide health care, each offers a different modality of delivery. The primary service may be the sale of prescription drugs or simply telemedicine visits. This review primarily discusses DTC pharmaceutical telemedicine platforms that sell private-label drugs and also offer telemedicine services to streamline care. However, the history, risks, and benefits discussed may apply to all models.

The DTC Landscape
Most DTC companies employ variations on a model with the same 3 main components: a triage questionnaire, telehealth services, and prescription/drug delivery (Figure). The triage questionnaire elicits a history of the patient’s presentation and medical history. Some companies may use artificial intelligence (AI) algorithms to tailor questions to patient needs. There are 2 modalities for patient-provider communication: synchronous and asynchronous. Synchronous communication entails real-time patient-physician conversations via audio only or video call. Asynchronous (or store-and-forward) communication refers to consultations provided via messaging or text-based modality, where a provider may respond to a patient within 24 hours.6 Direct-to-consumer platforms primarily use asynchronous visits (Table 2). However, some also use synchronous modalities if the provider deems it necessary or if state laws require it.

Once a provider has consulted with the patient, they can prescribe medication as needed. In certain cases, with adequate history, a prescription may be issued without a full physician visit. Furthermore, DTC companies require purchase of their custom-branded generic drugs. Prescriptions are fulfilled by the company’s pharmacy network and directly shipped to patients; few will allow patients to transfer a prescription to a pharmacy of their choice. Some platforms also sell supplements and over-the-counter medications.

Payment models vary among these companies, and most do not accept insurance (Table 2). Select models may provide free consultations and only require payment for pharmaceuticals. Others charge for consultations but reallocate payment to the cost of medication if prescribed. Another model involves flat rates for consultations and additional charges for drugs but unlimited messaging with providers for the duration of the prescription. Moreover, patients can subscribe to monthly deliveries of their medications.
Foundation of DTC
Technological advances have enabled patients to receive remote treatment from a single platform offering video calls, AI, electronic medical record interoperability, and integration of drug supply chains. Even in its simplest form, AI is increasingly used, as it allows for programs and chatbots to screen and triage patients.11 Technology also has improved at targeted mass marketing through social media platforms and search engines (eg, companies can use age, interests, location, and other parameters to target individuals likely needing acne treatment).
Drug patent expirations are a key catalyst for the rise of DTC companies, creating an attractive business model with generic drugs as the core product. Since 2008, patents for medications treating chronic conditions, such as erectile dysfunction, have expired. These patent expirations are responsible for $198 billion in projected prescription sales between 2019 and 2024.1 Thus, it follows that DTC companies have seized this opportunity to act as middlemen, taking advantage of these generic medications’ lower costs to create platforms focused on personalization and accessibility.
Rising deductibles have led patients to consider cheaper out-of-pocket alternatives that are not covered by insurance.1 For example, insurers typically do not cover finasteride treatment for conditions deemed cosmetic, such as androgenetic alopecia.12 The low cost of generic drugs creates an attractive business model for patients and investors. According to GoodRx, the average retail price for a 30-day supply of brand-name finasteride (Propecia [Merck]) is $135.92, whereas generic finasteride is $75.24.13 Direct-to-consumer pharmaceutical companies offer a 30-day supply of generic finasteride ranging from $8.33 to $30.14 The average wholesale cost for retailers is an estimated $2.31 for 30 days.15 Although profit margins on generic medications may be lower, more affordable drugs increase the size of the total market. These prescriptions are available as subscription plans, resulting in recurring revenue.
Lax US pharmaceutical marketing regulations allow direct advertising to the general public.16 In 1997, the US Food and Drug Administration allowed DTC advertisements to replace summaries of serious and common adverse effects with short statements covering important risks or referrals to other sources for complete information. In 2015, the US Food and Drug Administration guidelines preventing encouragement of self-diagnosis and self-treatment were withdrawn.5 These changes enable DTC companies to launch large advertising campaigns and to accelerate customer acquisition, as the industry often describes it, with ease.
Rapid Growth and Implications
Increasing generic drug availability and improving telemedicine capabilities have the potential to reduce costs and barriers but also have the potential for financial gain. Venture capital funds have recognized this opportunity, reflected by millions of dollars of investments, and accelerated the growth of DTC health care start-ups. For example, Ro has raised $376 million from venture capital, valuing the company at $1.5 billion.3
Direct-to-consumer companies require a heavy focus on marketing campaigns for customer acquisition. Their aesthetically pleasing websites and aggressive campaigns target specific audiences based on demographics, digital use habits, and purchasing behavior.4 Some campaigns celebrate the ease of obtaining prescriptions.17 Companies have been effective in recruiting so-called millennial and Generation Z patients, known to search the internet for remedies prior to seeking physician consultations.18 Recognizing these needs, some platforms offer guides on diseases they treat, creating effective customer-acquisition funnels. Recruitment of these technology-friendly patients has proven effective, especially given the largely positive media coverage of DTC platforms––potentially serving as a surrogate for medical credibility for patients.18
Some DTC companies also market physically; skin care ads may be strategically placed in social media feeds, or even found near mirrors in public bathrooms.19 Marketing campaigns also involve disease awareness; such efforts serve to increase diagnoses and prescribed treatments while destigmatizing diseases. Although DTC companies argue this strategy empowers patients, these marketing habits have the potential to take advantage of uninformed patients. Campaigns could potentially medicalize normal experiences and expand disease definitions resulting in overdiagnosis, overtreatment, and wasted resources.5 For example, off-label propranolol use has been advertised to attract patients who might have “nerves that come creeping before an important presentation.”17 Disease awareness campaigns also may lead people to falsely believe unproven drug benefits.5 According to studies, DTC pharmaceutical advertisements are low in informational quality and result in increased patient visits and prescriptions despite cost-effective alternatives.5,20-22
Fragmentation of the health care system is another possible complication of DTC teledermatology. These companies operate as for-profit organizations separated from the rest of the health care system, raising concerns about care coordination.8 Vital health data may not be conveyed as patients move among different providers and pharmacies. One study found DTC teledermatology rarely offered to provide medical records or facilitate a referral to a local physician.23 Such a lack of communication is concerning, as medication errors are the leading cause of avoidable harm in health care.24
Direct-to-consumer care models also seemingly redefine the physician-patient relationship by turning patients into consumers. Patient interactions may seem transactional and streamlined toward sales. For these platforms, a visit often is set up as an evaluation of a patient’s suitability for a prescription, not necessarily for the best treatment modality for the problem. These companies primarily make money through the sale of prescription drugs, creating a potential COI that may undermine the patient-physician relationship. Although some companies have made it clear that medical care and pharmaceutical sales are provided by legally separate business entities and that they do not pay physicians on commission, a conflict may still exist given the financial importance of physicians prescribing medication to the success of the business.16
Even as DTC models advertise upon expanded access and choice, the companies largely prohibit patients from choosing their own pharmacy. Instead, they encourage patients to fill prescriptions with the company’s pharmacy network by claiming lower costs compared with competitors. One DTC company, Hims, is launching a prescription-fulfillment center to further consolidate their business.17,19,25 The inherent COI of issuing and fulfilling prescriptions raises concerns of patient harm.26 For example, when Dermatology.com launched as a DTC prescription skin medication shop backed by Bausch Health Companies Inc, its model included telemedicine consultation. Although consultations were provided by RxDefine, a third party, only Dermatology.com drugs were prescribed. Given the poor quality of care and obvious financial COI, an uproar in the dermatology community and advocacy by the American Academy of Dermatology led to the shutdown of Dermatology.com’s online prescription services.26
The quality of care among DTC telemedicine platforms has been equivocal. Some studies have reported equivalent care in person and online, while others have reported poor adherence to guidelines, overuse of antibiotics, and misdiagnosis.8,23 A vital portion of the DTC experience is the history questionnaire, which is geared to diagnosis and risk assessment.25 Resneck et al23 found diagnostic quality to be adequate for simple dermatologic clinical scenarios but poor for scenarios requiring more than basic histories. Although Ro has reported leveraging data from millions of interactions to ask the right questions and streamline visits, it is still unclear whether history questionnaires are adequate.17,27 Additionally, consultations may lack sufficient counseling on adverse effects, risks, or pregnancy warnings, as well as discussions on alternative treatments and preventative care.17,23 Finally, patients often are limited in their choice of dermatologist; the lack of a fully developed relationship increases concerns of follow-up and monitoring practices. Although some DTC platforms offer unlimited interactions with physicians for the duration of a prescription, it is unknown how often these services are utilized or how adequate the quality of these interactions is. This potential for lax follow-up is especially concerning for prescriptions that autorenew on a monthly basis and could result in unnecessary overtreatment.
Postpandemic and Future Outlook
The COVID-19 pandemic dramatically impacted the use of telemedicine. To minimize COVID-19 transmission, the Centers for Medicare & Medicaid Services and private payers expanded telehealth coverage and eliminated reimbursement and licensing barriers.28 A decade’s worth of regulatory changes and consumer adoption was accelerated to weeks, resulting in telemedicine companies reaching record-high visit numbers.29 McKinsey & Company estimated that telehealth visit numbers surged 50- to 175-fold compared with pre–COVID-19 numbers. Additionally, 76% of patients were interested in future telehealth use, and 64% of providers were more comfortable using telehealth than before the pandemic.30 For their part, US dermatologists reported an increase in telemedicine use from 14.1% to 96.9% since COVID-19.31
Exactly how much DTC pharmaceutical telemedicine companies are growing is unclear, but private investments may be an indication. A record $14.7 billion was invested in the digital health sector in the first half of 2021; the majority went to telehealth companies.30 Ro, which reported $230 million in revenue in 2020 and has served 6 million visits, raised $200 milllion in July 2020 and $500 million in March 2021.32 Although post–COVID-19 health care will certainly involve increased telemedicine, the extent remains unclear, as telehealth vendors saw decreased usage upon reopening of state economies. Ultimately, the postpandemic regulatory landscape is hard to predict.30
Although COVID-19 appears to have caused rapid growth for DTC platforms, it also may have spurred competition. Telemedicine providers have given independent dermatologists and health care systems the infrastructure to implement custom DTC services.33 Although systems do not directly sell prescription drugs, the target market is essentially the same: patients looking for instant virtual dermatologic care. Therefore, sustained telemedicine services offered by traditional practices and systems may prove detrimental to DTC companies. However, unlike most telemedicine services, DTC models are less affected by certain changes in regulation since they do not rely on insurance. If regulations are tightened and reimbursements for telehealth are not attractive for dermatologists, teledermatology services may see an overall decrease. If so, patients who appreciate teledermatology may shift to using DTC platforms, even if their insurance does not cover them. Still, a nationwide survey found 56% of respondents felt an established relationship with a physician prior to a telemedicine visit is important, which may create a barrier for DTC adoption.34
Conclusion
Direct-to-consumer teledermatology represents a growing for-profit model of health care that provides patients with seemingly affordable and convenient care. However, there is potential for overtreatment, misdiagnosis, and fragmentation of health care. It will be important to monitor and evaluate the quality of care that DTC teledermatology offers and advocate for appropriate regulations and oversight. Eventually, more patients will have medications prescribed and dermatologic care administered through DTC companies. Dermatologists will benefit from this knowledge of DTC models to properly counsel patients on the risks and benefits of their use.
In recent years, direct-to-consumer (DTC) teledermatology platforms have gained popularity as telehealth business models, allowing patients to directly initiate visits with physicians and purchase medications from single platforms. A shortage of dermatologists, improved technology, drug patent expirations, and rising health care costs accelerated the growth of DTC dermatology.1 During the COVID-19 pandemic, teledermatology adoption surged due to the need to provide care while social distancing and minimizing viral exposure. These needs prompted additional federal funding and loosened regulatory provisions.2 As the userbase of these companies has grown, so have their valuations.3 Although the DTC model has attracted the attention of patients and investors, its rise provokes many questions about patients acting as consumers in health care. Indeed, DTC telemedicine offers greater autonomy and convenience for patients, but it may impact the quality of care and the nature of physician-patient relationships, perhaps making them more transactional.
Evolution of DTC in Health Care
The DTC model emphasizes individual choice and accessible health care. Although the definition has evolved, the core idea is not new.4 Over decades, pharmaceutical companies have spent billions of dollars on DTC advertising, circumventing physicians by directly reaching patients with campaigns on prescription drugs and laboratory tests and shaping public definitions of diseases.5
The DTC model of care is fundamentally different from traditional care models in that it changes the roles of the patient and physician. Whereas early telehealth models required a health care provider to initiate teleconsultations with specialists, DTC telemedicine bypasses this step (eg, the patient can consult a dermatologist without needing a primary care provider’s input first). This care can then be provided by dermatologists with whom patients may or may not have pre-established relationships.4,6
Dermatology was an early adopter of DTC telemedicine. The shortage of dermatologists in the United States created demand for increasing accessibility to dermatologic care. Additionally, the visual nature of diagnosing dermatologic disease was ideal for platforms supporting image sharing.7 Early DTC providers were primarily individual companies offering teledermatology. However, many dermatologists can now offer DTC capabilities via companies such as Amwell and Teladoc Health.8
Over the last 2 decades, start-ups such as Warby Parker (eyeglasses) and Casper (mattresses) defined the DTC industry using borrowed supply chains, cohesive branding, heavy social media marketing, and web-only retail. Scalability, lack of competition, and abundant venture capital created competition across numerous markets.9 Health care capitalized on this DTC model, creating a $700 billion market for products ranging from hearing aids to over-the-counter medications.10
Borrowing from this DTC playbook, platforms were created to offer delivery of generic prescription drugs to patients’ doorsteps. However, unlike with other products bought online, a consumer cannot simply add prescription drugs to their shopping cart and check out. In all models of American medical practice, physicians still serve as gatekeepers, providing a safeguard for patients to ensure appropriate prescription and avoid negative consequences of unnecessary drug use. This new model effectively streamlines diagnosis, prescription, and drug delivery without the patient ever having to leave home. Combining the prescribing and selling of medications (2 tasks that traditionally have been separated) potentially creates financial conflicts of interest (COIs). Additionally, high utilization of health care, including more prescriptions and visits, does not necessarily equal high quality of care. The companies stand to benefit from extra care regardless of need, and thus these models must be scrutinized for any incentives driving unnecessary care and prescriptions.
Ultimately, DTC has evolved to encompass multiple definitions in health care (Table 1). Although all models provide health care, each offers a different modality of delivery. The primary service may be the sale of prescription drugs or simply telemedicine visits. This review primarily discusses DTC pharmaceutical telemedicine platforms that sell private-label drugs and also offer telemedicine services to streamline care. However, the history, risks, and benefits discussed may apply to all models.

The DTC Landscape
Most DTC companies employ variations on a model with the same 3 main components: a triage questionnaire, telehealth services, and prescription/drug delivery (Figure). The triage questionnaire elicits a history of the patient’s presentation and medical history. Some companies may use artificial intelligence (AI) algorithms to tailor questions to patient needs. There are 2 modalities for patient-provider communication: synchronous and asynchronous. Synchronous communication entails real-time patient-physician conversations via audio only or video call. Asynchronous (or store-and-forward) communication refers to consultations provided via messaging or text-based modality, where a provider may respond to a patient within 24 hours.6 Direct-to-consumer platforms primarily use asynchronous visits (Table 2). However, some also use synchronous modalities if the provider deems it necessary or if state laws require it.

Once a provider has consulted with the patient, they can prescribe medication as needed. In certain cases, with adequate history, a prescription may be issued without a full physician visit. Furthermore, DTC companies require purchase of their custom-branded generic drugs. Prescriptions are fulfilled by the company’s pharmacy network and directly shipped to patients; few will allow patients to transfer a prescription to a pharmacy of their choice. Some platforms also sell supplements and over-the-counter medications.

Payment models vary among these companies, and most do not accept insurance (Table 2). Select models may provide free consultations and only require payment for pharmaceuticals. Others charge for consultations but reallocate payment to the cost of medication if prescribed. Another model involves flat rates for consultations and additional charges for drugs but unlimited messaging with providers for the duration of the prescription. Moreover, patients can subscribe to monthly deliveries of their medications.
Foundation of DTC
Technological advances have enabled patients to receive remote treatment from a single platform offering video calls, AI, electronic medical record interoperability, and integration of drug supply chains. Even in its simplest form, AI is increasingly used, as it allows for programs and chatbots to screen and triage patients.11 Technology also has improved at targeted mass marketing through social media platforms and search engines (eg, companies can use age, interests, location, and other parameters to target individuals likely needing acne treatment).
Drug patent expirations are a key catalyst for the rise of DTC companies, creating an attractive business model with generic drugs as the core product. Since 2008, patents for medications treating chronic conditions, such as erectile dysfunction, have expired. These patent expirations are responsible for $198 billion in projected prescription sales between 2019 and 2024.1 Thus, it follows that DTC companies have seized this opportunity to act as middlemen, taking advantage of these generic medications’ lower costs to create platforms focused on personalization and accessibility.
Rising deductibles have led patients to consider cheaper out-of-pocket alternatives that are not covered by insurance.1 For example, insurers typically do not cover finasteride treatment for conditions deemed cosmetic, such as androgenetic alopecia.12 The low cost of generic drugs creates an attractive business model for patients and investors. According to GoodRx, the average retail price for a 30-day supply of brand-name finasteride (Propecia [Merck]) is $135.92, whereas generic finasteride is $75.24.13 Direct-to-consumer pharmaceutical companies offer a 30-day supply of generic finasteride ranging from $8.33 to $30.14 The average wholesale cost for retailers is an estimated $2.31 for 30 days.15 Although profit margins on generic medications may be lower, more affordable drugs increase the size of the total market. These prescriptions are available as subscription plans, resulting in recurring revenue.
Lax US pharmaceutical marketing regulations allow direct advertising to the general public.16 In 1997, the US Food and Drug Administration allowed DTC advertisements to replace summaries of serious and common adverse effects with short statements covering important risks or referrals to other sources for complete information. In 2015, the US Food and Drug Administration guidelines preventing encouragement of self-diagnosis and self-treatment were withdrawn.5 These changes enable DTC companies to launch large advertising campaigns and to accelerate customer acquisition, as the industry often describes it, with ease.
Rapid Growth and Implications
Increasing generic drug availability and improving telemedicine capabilities have the potential to reduce costs and barriers but also have the potential for financial gain. Venture capital funds have recognized this opportunity, reflected by millions of dollars of investments, and accelerated the growth of DTC health care start-ups. For example, Ro has raised $376 million from venture capital, valuing the company at $1.5 billion.3
Direct-to-consumer companies require a heavy focus on marketing campaigns for customer acquisition. Their aesthetically pleasing websites and aggressive campaigns target specific audiences based on demographics, digital use habits, and purchasing behavior.4 Some campaigns celebrate the ease of obtaining prescriptions.17 Companies have been effective in recruiting so-called millennial and Generation Z patients, known to search the internet for remedies prior to seeking physician consultations.18 Recognizing these needs, some platforms offer guides on diseases they treat, creating effective customer-acquisition funnels. Recruitment of these technology-friendly patients has proven effective, especially given the largely positive media coverage of DTC platforms––potentially serving as a surrogate for medical credibility for patients.18
Some DTC companies also market physically; skin care ads may be strategically placed in social media feeds, or even found near mirrors in public bathrooms.19 Marketing campaigns also involve disease awareness; such efforts serve to increase diagnoses and prescribed treatments while destigmatizing diseases. Although DTC companies argue this strategy empowers patients, these marketing habits have the potential to take advantage of uninformed patients. Campaigns could potentially medicalize normal experiences and expand disease definitions resulting in overdiagnosis, overtreatment, and wasted resources.5 For example, off-label propranolol use has been advertised to attract patients who might have “nerves that come creeping before an important presentation.”17 Disease awareness campaigns also may lead people to falsely believe unproven drug benefits.5 According to studies, DTC pharmaceutical advertisements are low in informational quality and result in increased patient visits and prescriptions despite cost-effective alternatives.5,20-22
Fragmentation of the health care system is another possible complication of DTC teledermatology. These companies operate as for-profit organizations separated from the rest of the health care system, raising concerns about care coordination.8 Vital health data may not be conveyed as patients move among different providers and pharmacies. One study found DTC teledermatology rarely offered to provide medical records or facilitate a referral to a local physician.23 Such a lack of communication is concerning, as medication errors are the leading cause of avoidable harm in health care.24
Direct-to-consumer care models also seemingly redefine the physician-patient relationship by turning patients into consumers. Patient interactions may seem transactional and streamlined toward sales. For these platforms, a visit often is set up as an evaluation of a patient’s suitability for a prescription, not necessarily for the best treatment modality for the problem. These companies primarily make money through the sale of prescription drugs, creating a potential COI that may undermine the patient-physician relationship. Although some companies have made it clear that medical care and pharmaceutical sales are provided by legally separate business entities and that they do not pay physicians on commission, a conflict may still exist given the financial importance of physicians prescribing medication to the success of the business.16
Even as DTC models advertise upon expanded access and choice, the companies largely prohibit patients from choosing their own pharmacy. Instead, they encourage patients to fill prescriptions with the company’s pharmacy network by claiming lower costs compared with competitors. One DTC company, Hims, is launching a prescription-fulfillment center to further consolidate their business.17,19,25 The inherent COI of issuing and fulfilling prescriptions raises concerns of patient harm.26 For example, when Dermatology.com launched as a DTC prescription skin medication shop backed by Bausch Health Companies Inc, its model included telemedicine consultation. Although consultations were provided by RxDefine, a third party, only Dermatology.com drugs were prescribed. Given the poor quality of care and obvious financial COI, an uproar in the dermatology community and advocacy by the American Academy of Dermatology led to the shutdown of Dermatology.com’s online prescription services.26
The quality of care among DTC telemedicine platforms has been equivocal. Some studies have reported equivalent care in person and online, while others have reported poor adherence to guidelines, overuse of antibiotics, and misdiagnosis.8,23 A vital portion of the DTC experience is the history questionnaire, which is geared to diagnosis and risk assessment.25 Resneck et al23 found diagnostic quality to be adequate for simple dermatologic clinical scenarios but poor for scenarios requiring more than basic histories. Although Ro has reported leveraging data from millions of interactions to ask the right questions and streamline visits, it is still unclear whether history questionnaires are adequate.17,27 Additionally, consultations may lack sufficient counseling on adverse effects, risks, or pregnancy warnings, as well as discussions on alternative treatments and preventative care.17,23 Finally, patients often are limited in their choice of dermatologist; the lack of a fully developed relationship increases concerns of follow-up and monitoring practices. Although some DTC platforms offer unlimited interactions with physicians for the duration of a prescription, it is unknown how often these services are utilized or how adequate the quality of these interactions is. This potential for lax follow-up is especially concerning for prescriptions that autorenew on a monthly basis and could result in unnecessary overtreatment.
Postpandemic and Future Outlook
The COVID-19 pandemic dramatically impacted the use of telemedicine. To minimize COVID-19 transmission, the Centers for Medicare & Medicaid Services and private payers expanded telehealth coverage and eliminated reimbursement and licensing barriers.28 A decade’s worth of regulatory changes and consumer adoption was accelerated to weeks, resulting in telemedicine companies reaching record-high visit numbers.29 McKinsey & Company estimated that telehealth visit numbers surged 50- to 175-fold compared with pre–COVID-19 numbers. Additionally, 76% of patients were interested in future telehealth use, and 64% of providers were more comfortable using telehealth than before the pandemic.30 For their part, US dermatologists reported an increase in telemedicine use from 14.1% to 96.9% since COVID-19.31
Exactly how much DTC pharmaceutical telemedicine companies are growing is unclear, but private investments may be an indication. A record $14.7 billion was invested in the digital health sector in the first half of 2021; the majority went to telehealth companies.30 Ro, which reported $230 million in revenue in 2020 and has served 6 million visits, raised $200 milllion in July 2020 and $500 million in March 2021.32 Although post–COVID-19 health care will certainly involve increased telemedicine, the extent remains unclear, as telehealth vendors saw decreased usage upon reopening of state economies. Ultimately, the postpandemic regulatory landscape is hard to predict.30
Although COVID-19 appears to have caused rapid growth for DTC platforms, it also may have spurred competition. Telemedicine providers have given independent dermatologists and health care systems the infrastructure to implement custom DTC services.33 Although systems do not directly sell prescription drugs, the target market is essentially the same: patients looking for instant virtual dermatologic care. Therefore, sustained telemedicine services offered by traditional practices and systems may prove detrimental to DTC companies. However, unlike most telemedicine services, DTC models are less affected by certain changes in regulation since they do not rely on insurance. If regulations are tightened and reimbursements for telehealth are not attractive for dermatologists, teledermatology services may see an overall decrease. If so, patients who appreciate teledermatology may shift to using DTC platforms, even if their insurance does not cover them. Still, a nationwide survey found 56% of respondents felt an established relationship with a physician prior to a telemedicine visit is important, which may create a barrier for DTC adoption.34
Conclusion
Direct-to-consumer teledermatology represents a growing for-profit model of health care that provides patients with seemingly affordable and convenient care. However, there is potential for overtreatment, misdiagnosis, and fragmentation of health care. It will be important to monitor and evaluate the quality of care that DTC teledermatology offers and advocate for appropriate regulations and oversight. Eventually, more patients will have medications prescribed and dermatologic care administered through DTC companies. Dermatologists will benefit from this knowledge of DTC models to properly counsel patients on the risks and benefits of their use.
- Vennare J. The DTC healthcare report. Fitt Insider. September 15, 2019. Accessed February 23, 2022. https://insider.fitt.co/direct-to-consumer-healthcare-startups/
- Kannampallil T, Ma J. Digital translucence: adapting telemedicine delivery post-COVID-19. Telemed J E Health. 2020;26:1120-1122.
- Farr C. Ro, a 3-year-old online health provider, just raised a new round that values it at $1.5 billion. CNBC. July 27, 2020. Accessed February 23, 2022. https://www.cnbc.com/2020/07/27/ro-raises-200-million-at-1point5-billion-valuation-250-million-sales.html
- Elliott T, Shih J. Direct to consumer telemedicine. Curr Allergy Asthma Rep. 2019;19:1.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Coates SJ, Kvedar J, Granstein RD. Teledermatology: from historical perspective to emerging techniques of the modern era. J Am Acad Dermatol. 2015;72:563-574.
- Rheuban KS, Krupinski EA, eds. Understanding Telehealth. McGraw-Hill Education; 2017.
- Schlesinger LA, Higgins M, Roseman S. Reinventing the direct-to-consumer business model. Harvard Business Review. March 31, 2020. Accessed February 23, 2022. https://hbr.org/2020/03/reinventing-the-direct-to-consumer-business-model
- Cohen AB, Mathews SC, Dorsey ER, et al. Direct-to-consumer digital health. Lancet Digit Health. 2020;2:E163-E165.
- 6 telehealth trends for 2020. Wolters Kluwer. Published January 27, 2021. Accessed February 23, 2022. https://www.wolterskluwer.com/en/expert-insights/6-telehealth-trends-for-2020
- Jadoo SA, Lipoff JB. Prescribing to save patients money: ethical considerations. J Am Acad Dermatol. 2018;78:826-828.
- Propecia. GoodRx. Accessed February 23, 2022. https://www.goodrx.com/propecia
- Lauer A. The truth about online hair-loss treatments like Roman and Hims, according to a dermatologist. InsideHook. January 13, 2020. Accessed February 23, 2022. https://www.insidehook.com/article/grooming/men-hair-loss-treatments-dermatologist-review
- Friedman Y. Drug price trends for NDC 16729-0089. DrugPatentWatch. Accessed February 23, 2022. https://www.drugpatentwatch.com/p/drug-price/ndc/index.php?query=16729-0089
- Curtis H, Milner J. Ethical concerns with online direct-to-consumer pharmaceutical companies. J Med Ethics. 2020;46:168-171.
- Jain T, Lu RJ, Mehrotra A. Prescriptions on demand: the growth of direct-to-consumer telemedicine companies. JAMA. 2019;322:925-926.
- Shahinyan RH, Amighi A, Carey AN, et al. Direct-to-consumer internet prescription platforms overlook crucial pathology found during traditional office evaluation of young men with erectile dysfunction. Urology. 2020;143:165-172.
- Ali M. Andrew Dudum—bold strategies that propelled Hims & Hers into unicorn status. Exit Strategy with Moiz Ali. Published April 2020. Accessed February 23, 2022. https://open.spotify.com/episode/6DtaJxwZDjvZSJI88DTf24?si=b3FHQiUIQY62YjfRHmnJBQ
- Klara K, Kim J, Ross JS. Direct-to-consumer broadcast advertisements for pharmaceuticals: off-label promotion and adherence to FDA guidelines. J Gen Intern Med. 2018;33:651-658.
- Sullivan HW, Aikin KJ, Poehlman J. Communicating risk information in direct-to-consumer prescription drug television ads: a content analysis. Health Commun. 2019;34:212-219.
- Applequist J, Ball JG. An updated analysis of direct-to-consumer television advertisements for prescription drugs. Ann Fam Med. 2018;16:211-216.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Patient safety. World Health Organization. Published September 13, 2019. Accessed February 1, 2022. https://www.who.int/news-room/fact-sheets/detail/patient-safety
- Bollmeier SG, Stevenson E, Finnegan P, et al. Direct to consumer telemedicine: is healthcare from home best? Mo Med. 2020;117:303-309.
26. Court E. Bausch yanked online prescribing after dermatologist backlash. Bloomberg.com. Published March 11, 2020. Accessed September 25, 2020. https://www.bloomberg.com/news/articles/2020-03-11/bausch-yanked-online-prescribing-after-dermatologist-backlash
27. Reitano Z. The future of healthcare: how Ro helps providers treat patients 2 minutes, 2 days, 2 weeks, and 2 years at a time. Medium. Published March 4, 2019. Accessed February 1, 2022. https://medium.com/ro-co/the-future-of-healthcare-how-ro-helps-providers-treat-patients-2-mins-2-days-2-weeks-and-2-10efc0679d7
28. Lee I, Kovarik C, Tejasvi T, et al. Telehealth: helping your patients and practice survive and thrive during the COVID-19 crisis with rapid quality implementation. J Am Acad Dermatol. 2020;82:1213-1214.
29. Pifer R. “Weeks where decades happen”: telehealth 6 months into COVID-19. Healthcare Dive. Published July 27, 2020. Accessed February 23, 2022. https://www.healthcaredive.com/news/telehealth-6-months-coronavirus/581447/
30. Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. Updated July 9, 2021. Accessed February 23, 2022. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality
31. Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
32. Jennings K. Digital health startup Ro raised $500 million at $5 billion valuation. Forbes. March 22, 2021. Accessed March 29, 2022. https://www.forbes.com/sites/katiejennings/2021/03/22/digital-health-startup-ro-raised-500-million-at-5-billion-valuation/?sh=695be0e462f5
33. Hollander JE, Carr BG. Virtually perfect? telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
34. Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
- Vennare J. The DTC healthcare report. Fitt Insider. September 15, 2019. Accessed February 23, 2022. https://insider.fitt.co/direct-to-consumer-healthcare-startups/
- Kannampallil T, Ma J. Digital translucence: adapting telemedicine delivery post-COVID-19. Telemed J E Health. 2020;26:1120-1122.
- Farr C. Ro, a 3-year-old online health provider, just raised a new round that values it at $1.5 billion. CNBC. July 27, 2020. Accessed February 23, 2022. https://www.cnbc.com/2020/07/27/ro-raises-200-million-at-1point5-billion-valuation-250-million-sales.html
- Elliott T, Shih J. Direct to consumer telemedicine. Curr Allergy Asthma Rep. 2019;19:1.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Coates SJ, Kvedar J, Granstein RD. Teledermatology: from historical perspective to emerging techniques of the modern era. J Am Acad Dermatol. 2015;72:563-574.
- Rheuban KS, Krupinski EA, eds. Understanding Telehealth. McGraw-Hill Education; 2017.
- Schlesinger LA, Higgins M, Roseman S. Reinventing the direct-to-consumer business model. Harvard Business Review. March 31, 2020. Accessed February 23, 2022. https://hbr.org/2020/03/reinventing-the-direct-to-consumer-business-model
- Cohen AB, Mathews SC, Dorsey ER, et al. Direct-to-consumer digital health. Lancet Digit Health. 2020;2:E163-E165.
- 6 telehealth trends for 2020. Wolters Kluwer. Published January 27, 2021. Accessed February 23, 2022. https://www.wolterskluwer.com/en/expert-insights/6-telehealth-trends-for-2020
- Jadoo SA, Lipoff JB. Prescribing to save patients money: ethical considerations. J Am Acad Dermatol. 2018;78:826-828.
- Propecia. GoodRx. Accessed February 23, 2022. https://www.goodrx.com/propecia
- Lauer A. The truth about online hair-loss treatments like Roman and Hims, according to a dermatologist. InsideHook. January 13, 2020. Accessed February 23, 2022. https://www.insidehook.com/article/grooming/men-hair-loss-treatments-dermatologist-review
- Friedman Y. Drug price trends for NDC 16729-0089. DrugPatentWatch. Accessed February 23, 2022. https://www.drugpatentwatch.com/p/drug-price/ndc/index.php?query=16729-0089
- Curtis H, Milner J. Ethical concerns with online direct-to-consumer pharmaceutical companies. J Med Ethics. 2020;46:168-171.
- Jain T, Lu RJ, Mehrotra A. Prescriptions on demand: the growth of direct-to-consumer telemedicine companies. JAMA. 2019;322:925-926.
- Shahinyan RH, Amighi A, Carey AN, et al. Direct-to-consumer internet prescription platforms overlook crucial pathology found during traditional office evaluation of young men with erectile dysfunction. Urology. 2020;143:165-172.
- Ali M. Andrew Dudum—bold strategies that propelled Hims & Hers into unicorn status. Exit Strategy with Moiz Ali. Published April 2020. Accessed February 23, 2022. https://open.spotify.com/episode/6DtaJxwZDjvZSJI88DTf24?si=b3FHQiUIQY62YjfRHmnJBQ
- Klara K, Kim J, Ross JS. Direct-to-consumer broadcast advertisements for pharmaceuticals: off-label promotion and adherence to FDA guidelines. J Gen Intern Med. 2018;33:651-658.
- Sullivan HW, Aikin KJ, Poehlman J. Communicating risk information in direct-to-consumer prescription drug television ads: a content analysis. Health Commun. 2019;34:212-219.
- Applequist J, Ball JG. An updated analysis of direct-to-consumer television advertisements for prescription drugs. Ann Fam Med. 2018;16:211-216.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Patient safety. World Health Organization. Published September 13, 2019. Accessed February 1, 2022. https://www.who.int/news-room/fact-sheets/detail/patient-safety
- Bollmeier SG, Stevenson E, Finnegan P, et al. Direct to consumer telemedicine: is healthcare from home best? Mo Med. 2020;117:303-309.
26. Court E. Bausch yanked online prescribing after dermatologist backlash. Bloomberg.com. Published March 11, 2020. Accessed September 25, 2020. https://www.bloomberg.com/news/articles/2020-03-11/bausch-yanked-online-prescribing-after-dermatologist-backlash
27. Reitano Z. The future of healthcare: how Ro helps providers treat patients 2 minutes, 2 days, 2 weeks, and 2 years at a time. Medium. Published March 4, 2019. Accessed February 1, 2022. https://medium.com/ro-co/the-future-of-healthcare-how-ro-helps-providers-treat-patients-2-mins-2-days-2-weeks-and-2-10efc0679d7
28. Lee I, Kovarik C, Tejasvi T, et al. Telehealth: helping your patients and practice survive and thrive during the COVID-19 crisis with rapid quality implementation. J Am Acad Dermatol. 2020;82:1213-1214.
29. Pifer R. “Weeks where decades happen”: telehealth 6 months into COVID-19. Healthcare Dive. Published July 27, 2020. Accessed February 23, 2022. https://www.healthcaredive.com/news/telehealth-6-months-coronavirus/581447/
30. Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. Updated July 9, 2021. Accessed February 23, 2022. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality
31. Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
32. Jennings K. Digital health startup Ro raised $500 million at $5 billion valuation. Forbes. March 22, 2021. Accessed March 29, 2022. https://www.forbes.com/sites/katiejennings/2021/03/22/digital-health-startup-ro-raised-500-million-at-5-billion-valuation/?sh=695be0e462f5
33. Hollander JE, Carr BG. Virtually perfect? telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
34. Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
Practice Points
- Direct-to-consumer (DTC) teledermatology platforms are for-profit companies that provide telemedicine visits and sell prescription drugs directly to patients.
- Although they are growing in popularity, DTC teledermatology platforms may lead to overdiagnosis, overtreatment, and fragmentation of health care. Knowledge of teledermatology will be vital to counsel patients on the risks and benefits of these platforms.
COVID vaccines open rifts between parents, children
The picture of rebellious teenagers sneaking “shots” has widened beyond breaking into Mom and Dad’s liquor cabinet. For some teens now, it means getting a COVID-19 vaccination without their parents’ consent – and, unlike the cabinet raids for the booze, they have adults willing to endorse the practice.
Since the U.S. Food and Drug Administration first granted emergency use authorization to Pfizer’s COVID-19 vaccine for teenagers in mid-2021, health officials have had to deal with a small subset of vaccine hesitancy where minors want the shot over the objections of their reluctant parents. The split has buoyed groups that were formed initially to convince teenagers to get vaccinated against other diseases.
When 14-year-old Arin Parsa of San Jose, California founded Teens for Vaccines in 2019 after a measles outbreak among unvaccinated children, “hardly anyone was interested,” he said. “Many teens were into climate change and other causes. Then, when the pandemic hit, so many were suddenly aware.”
Heavy toll on teens
Mr. Parsa’s parents fully supported Teens for Vaccines, he said, but he quickly found out how “politicized” COVID shots had become.
“We find people who are sad, angry, and frustrated at this stage of the pandemic,” he told this news organization. “The anti-vax lobby is riding the coat-tails of other movements. It has a very severe effect on their mental health. They can’t go out with their friends and socialize.”
In the pandemic’s initial stages, children were less likely to fall sick with COVID, but the Omicron variant led to a dramatic increase in illnesses among young people. The American Academy of Pediatrics has found that 3.5 million of the 11.4 million pediatric cases of the virus in the United States were reported in January 2022 alone. Meanwhile, vaccination rates for children aged 12-17, which were only 34% in June 2021 and lagged through the fall, are now at about 61% thanks to a sharp uptick during the Omicron surge, according to polling by the Kaiser Family Foundation.
No statistics are available on how many minors have received a COVID vaccine against their parents’ wishes.
“It’s not like there’s a big movement,” said Arthur Caplan, PhD, who heads the Division of Medical Ethics at the NYU Grossman School of Medicine. He said he noticed a divide around the HPV and hepatitis B vaccines. “They were tied up with sexual behavior,” he said, but “there were also some kids whose parents were really antivaxxers.”
Mr. Parsa said his and similar teen-oriented groups, such as VaxTeen, seek to educate their teen cohort, convince family members of the vaccines’ benefits, and to connect them with resources to get a shot. They also strive to change laws to make it easier for teenagers to receive the vaccine.
Consent laws vary from state to state (and within states), and proposed changes are afoot – some to loosen the laws and some to tighten them. Currently a 14-year-old in Alabama may get a COVID shot without parental permission, according to VaxTeen. In California, minors may receive the HPV shot without parental consent but not a COVID vaccine, although groups like Teens for Vaccines are pushing to change that. A bill now before the state legislature, the Teens Choose Vaccines Act (Senate Bill 866), would allow adolescents aged 12 and older to be able receive any FDA-approved vaccine – including COVID vaccines – without parental consent.
A second bill in California, the Keep Schools Open and Safe Act, would add the COVID-19 vaccines to the required list of immunizations needed to attend school in the state as well as eliminate the “personal belief” exemption against immunization.
California Sen. Richard Pan, MD (D-6th District), cowrote both bills with fellow Democrat Sen. Scott Wiener (D-11th District) and teen advocates from Teens for Vaccines and Generation Up, who helped draft the language in consultation with the lawmakers.
“As a pediatrician, I have seen all manner of situations where the requirement for a signed form has prevented teens from being able to get a vaccine that otherwise they and their guardians approved of them getting,” Dr. Pan told this news organization. “As a father, I don’t want to see my kids or any teen that wishes to protect themselves from deadly diseases unable to do so, particularly as we continue to fight off the dangers of the COVID-19 pandemic. I always encourage parents or teens that have questions about vaccines to speak directly with their pediatrician.”
Lawmakers in Philadelphia passed a provision last year to allow anyone age 11 or over to get the COVID vaccine without parental permission, keeping it in line with other vaccinations like hepatitis or HPV. “People from surrounding counties have come into the city, but it hasn’t been a huge rush,” says James Garrow, MPH, a spokesman for the city’s Department of Health.
Strive for collaboration, but listen to the children
Experts say the best solution is to for a doctor to meet with minors and their reluctant parents to get them on board for a COVID shot.
“Physicians are still the trusted messengers,” said Emma Olivera, MD, a pediatrician in suburban Chicago who advises groups that combat COVID misinformation.
Dr. Olivera said she often finds that internet-savvy teenagers have access to more information than older people, including their parents.
Thanks to COVID policies, office meetings are “difficult to do,” NYU’s Dr. Caplan added. In such a meeting, Dr. Caplan said he would try to convince the parents that the shots are needed for their children to stay in school or play sports. In the end, he said minors should get the shot but would also notify the parents before that happens: “My duty is to them.”
If parents take opposite stances, the pro-vaccine side is likely to prevail, even in California, said Patrick Baghdaserians, JD, a family law attorney in Pasadena. Mr. Baghdaserians said he is now representing a father who wants his teenager to get vaccinated but the mother doesn’t. “The court will fall on our side,” he predicted.
A version of this article first appeared on Medscape.com.
The picture of rebellious teenagers sneaking “shots” has widened beyond breaking into Mom and Dad’s liquor cabinet. For some teens now, it means getting a COVID-19 vaccination without their parents’ consent – and, unlike the cabinet raids for the booze, they have adults willing to endorse the practice.
Since the U.S. Food and Drug Administration first granted emergency use authorization to Pfizer’s COVID-19 vaccine for teenagers in mid-2021, health officials have had to deal with a small subset of vaccine hesitancy where minors want the shot over the objections of their reluctant parents. The split has buoyed groups that were formed initially to convince teenagers to get vaccinated against other diseases.
When 14-year-old Arin Parsa of San Jose, California founded Teens for Vaccines in 2019 after a measles outbreak among unvaccinated children, “hardly anyone was interested,” he said. “Many teens were into climate change and other causes. Then, when the pandemic hit, so many were suddenly aware.”
Heavy toll on teens
Mr. Parsa’s parents fully supported Teens for Vaccines, he said, but he quickly found out how “politicized” COVID shots had become.
“We find people who are sad, angry, and frustrated at this stage of the pandemic,” he told this news organization. “The anti-vax lobby is riding the coat-tails of other movements. It has a very severe effect on their mental health. They can’t go out with their friends and socialize.”
In the pandemic’s initial stages, children were less likely to fall sick with COVID, but the Omicron variant led to a dramatic increase in illnesses among young people. The American Academy of Pediatrics has found that 3.5 million of the 11.4 million pediatric cases of the virus in the United States were reported in January 2022 alone. Meanwhile, vaccination rates for children aged 12-17, which were only 34% in June 2021 and lagged through the fall, are now at about 61% thanks to a sharp uptick during the Omicron surge, according to polling by the Kaiser Family Foundation.
No statistics are available on how many minors have received a COVID vaccine against their parents’ wishes.
“It’s not like there’s a big movement,” said Arthur Caplan, PhD, who heads the Division of Medical Ethics at the NYU Grossman School of Medicine. He said he noticed a divide around the HPV and hepatitis B vaccines. “They were tied up with sexual behavior,” he said, but “there were also some kids whose parents were really antivaxxers.”
Mr. Parsa said his and similar teen-oriented groups, such as VaxTeen, seek to educate their teen cohort, convince family members of the vaccines’ benefits, and to connect them with resources to get a shot. They also strive to change laws to make it easier for teenagers to receive the vaccine.
Consent laws vary from state to state (and within states), and proposed changes are afoot – some to loosen the laws and some to tighten them. Currently a 14-year-old in Alabama may get a COVID shot without parental permission, according to VaxTeen. In California, minors may receive the HPV shot without parental consent but not a COVID vaccine, although groups like Teens for Vaccines are pushing to change that. A bill now before the state legislature, the Teens Choose Vaccines Act (Senate Bill 866), would allow adolescents aged 12 and older to be able receive any FDA-approved vaccine – including COVID vaccines – without parental consent.
A second bill in California, the Keep Schools Open and Safe Act, would add the COVID-19 vaccines to the required list of immunizations needed to attend school in the state as well as eliminate the “personal belief” exemption against immunization.
California Sen. Richard Pan, MD (D-6th District), cowrote both bills with fellow Democrat Sen. Scott Wiener (D-11th District) and teen advocates from Teens for Vaccines and Generation Up, who helped draft the language in consultation with the lawmakers.
“As a pediatrician, I have seen all manner of situations where the requirement for a signed form has prevented teens from being able to get a vaccine that otherwise they and their guardians approved of them getting,” Dr. Pan told this news organization. “As a father, I don’t want to see my kids or any teen that wishes to protect themselves from deadly diseases unable to do so, particularly as we continue to fight off the dangers of the COVID-19 pandemic. I always encourage parents or teens that have questions about vaccines to speak directly with their pediatrician.”
Lawmakers in Philadelphia passed a provision last year to allow anyone age 11 or over to get the COVID vaccine without parental permission, keeping it in line with other vaccinations like hepatitis or HPV. “People from surrounding counties have come into the city, but it hasn’t been a huge rush,” says James Garrow, MPH, a spokesman for the city’s Department of Health.
Strive for collaboration, but listen to the children
Experts say the best solution is to for a doctor to meet with minors and their reluctant parents to get them on board for a COVID shot.
“Physicians are still the trusted messengers,” said Emma Olivera, MD, a pediatrician in suburban Chicago who advises groups that combat COVID misinformation.
Dr. Olivera said she often finds that internet-savvy teenagers have access to more information than older people, including their parents.
Thanks to COVID policies, office meetings are “difficult to do,” NYU’s Dr. Caplan added. In such a meeting, Dr. Caplan said he would try to convince the parents that the shots are needed for their children to stay in school or play sports. In the end, he said minors should get the shot but would also notify the parents before that happens: “My duty is to them.”
If parents take opposite stances, the pro-vaccine side is likely to prevail, even in California, said Patrick Baghdaserians, JD, a family law attorney in Pasadena. Mr. Baghdaserians said he is now representing a father who wants his teenager to get vaccinated but the mother doesn’t. “The court will fall on our side,” he predicted.
A version of this article first appeared on Medscape.com.
The picture of rebellious teenagers sneaking “shots” has widened beyond breaking into Mom and Dad’s liquor cabinet. For some teens now, it means getting a COVID-19 vaccination without their parents’ consent – and, unlike the cabinet raids for the booze, they have adults willing to endorse the practice.
Since the U.S. Food and Drug Administration first granted emergency use authorization to Pfizer’s COVID-19 vaccine for teenagers in mid-2021, health officials have had to deal with a small subset of vaccine hesitancy where minors want the shot over the objections of their reluctant parents. The split has buoyed groups that were formed initially to convince teenagers to get vaccinated against other diseases.
When 14-year-old Arin Parsa of San Jose, California founded Teens for Vaccines in 2019 after a measles outbreak among unvaccinated children, “hardly anyone was interested,” he said. “Many teens were into climate change and other causes. Then, when the pandemic hit, so many were suddenly aware.”
Heavy toll on teens
Mr. Parsa’s parents fully supported Teens for Vaccines, he said, but he quickly found out how “politicized” COVID shots had become.
“We find people who are sad, angry, and frustrated at this stage of the pandemic,” he told this news organization. “The anti-vax lobby is riding the coat-tails of other movements. It has a very severe effect on their mental health. They can’t go out with their friends and socialize.”
In the pandemic’s initial stages, children were less likely to fall sick with COVID, but the Omicron variant led to a dramatic increase in illnesses among young people. The American Academy of Pediatrics has found that 3.5 million of the 11.4 million pediatric cases of the virus in the United States were reported in January 2022 alone. Meanwhile, vaccination rates for children aged 12-17, which were only 34% in June 2021 and lagged through the fall, are now at about 61% thanks to a sharp uptick during the Omicron surge, according to polling by the Kaiser Family Foundation.
No statistics are available on how many minors have received a COVID vaccine against their parents’ wishes.
“It’s not like there’s a big movement,” said Arthur Caplan, PhD, who heads the Division of Medical Ethics at the NYU Grossman School of Medicine. He said he noticed a divide around the HPV and hepatitis B vaccines. “They were tied up with sexual behavior,” he said, but “there were also some kids whose parents were really antivaxxers.”
Mr. Parsa said his and similar teen-oriented groups, such as VaxTeen, seek to educate their teen cohort, convince family members of the vaccines’ benefits, and to connect them with resources to get a shot. They also strive to change laws to make it easier for teenagers to receive the vaccine.
Consent laws vary from state to state (and within states), and proposed changes are afoot – some to loosen the laws and some to tighten them. Currently a 14-year-old in Alabama may get a COVID shot without parental permission, according to VaxTeen. In California, minors may receive the HPV shot without parental consent but not a COVID vaccine, although groups like Teens for Vaccines are pushing to change that. A bill now before the state legislature, the Teens Choose Vaccines Act (Senate Bill 866), would allow adolescents aged 12 and older to be able receive any FDA-approved vaccine – including COVID vaccines – without parental consent.
A second bill in California, the Keep Schools Open and Safe Act, would add the COVID-19 vaccines to the required list of immunizations needed to attend school in the state as well as eliminate the “personal belief” exemption against immunization.
California Sen. Richard Pan, MD (D-6th District), cowrote both bills with fellow Democrat Sen. Scott Wiener (D-11th District) and teen advocates from Teens for Vaccines and Generation Up, who helped draft the language in consultation with the lawmakers.
“As a pediatrician, I have seen all manner of situations where the requirement for a signed form has prevented teens from being able to get a vaccine that otherwise they and their guardians approved of them getting,” Dr. Pan told this news organization. “As a father, I don’t want to see my kids or any teen that wishes to protect themselves from deadly diseases unable to do so, particularly as we continue to fight off the dangers of the COVID-19 pandemic. I always encourage parents or teens that have questions about vaccines to speak directly with their pediatrician.”
Lawmakers in Philadelphia passed a provision last year to allow anyone age 11 or over to get the COVID vaccine without parental permission, keeping it in line with other vaccinations like hepatitis or HPV. “People from surrounding counties have come into the city, but it hasn’t been a huge rush,” says James Garrow, MPH, a spokesman for the city’s Department of Health.
Strive for collaboration, but listen to the children
Experts say the best solution is to for a doctor to meet with minors and their reluctant parents to get them on board for a COVID shot.
“Physicians are still the trusted messengers,” said Emma Olivera, MD, a pediatrician in suburban Chicago who advises groups that combat COVID misinformation.
Dr. Olivera said she often finds that internet-savvy teenagers have access to more information than older people, including their parents.
Thanks to COVID policies, office meetings are “difficult to do,” NYU’s Dr. Caplan added. In such a meeting, Dr. Caplan said he would try to convince the parents that the shots are needed for their children to stay in school or play sports. In the end, he said minors should get the shot but would also notify the parents before that happens: “My duty is to them.”
If parents take opposite stances, the pro-vaccine side is likely to prevail, even in California, said Patrick Baghdaserians, JD, a family law attorney in Pasadena. Mr. Baghdaserians said he is now representing a father who wants his teenager to get vaccinated but the mother doesn’t. “The court will fall on our side,” he predicted.
A version of this article first appeared on Medscape.com.
Long COVID associated with risk of metabolic liver disease
Postacute COVID syndrome (PACS), an ongoing inflammatory state following infection with SARS-CoV-2, is associated with greater risk of metabolic-associated fatty liver disease (MAFLD), according to an analysis of patients at a single clinic in Canada published in Open Forum Infectious Diseases.
MAFLD, also known as nonalcoholic fatty liver disease (NAFLD), is considered an indicator of general health and is in turn linked to greater risk of cardiovascular complications and mortality. It may be a multisystem disorder with various underlying causes.
PACS includes symptoms that affect various organ systems, with neurocognitive, autonomic, gastrointestinal, respiratory, musculoskeletal, psychological, sensory, and dermatologic clusters. An estimated 50%-80% of COVID-19 patients experience one or more clusters of symptoms 3 months after leaving the hospital.
But liver problems also appear in the acute phase, said Paul Martin, MD, who was asked to comment on the study. “Up to about half the patients during the acute illness may have elevated liver tests, but there seems to be a subset of patients in whom the abnormality persists. And then there are some reports in the literature of patients developing injury to their bile ducts in the liver over the long term, apparently as a consequence of COVID infection. What this paper suggests is that there may be some metabolic derangements associated with COVID infection, which in turn can accentuate or possibly cause fatty liver,” said Dr. Martin in an interview. He is chief of digestive health and liver diseases and a professor of medicine at the University of Miami.
“It highlights the need to get vaccinated against COVID and to take appropriate precautions because contracting the infection may lead to all sorts of consequences quite apart from having a respiratory illness,” said Dr. Martin.
The researchers retrospectively identified 235 patients hospitalized with COVID-19 between July 2020 and April 2021. Overall, 69% were men, and the median age was 61 years; 19.2% underwent mechanical ventilation and the mean duration of hospitalization was 11.7 days. They were seen for PACS symptoms a median 143 days after COVID-19 symptoms began, with 77.5% having symptoms of at least one PACS cluster. Of these clusters, 34.9% were neurocognitive, 53.2% were respiratory, 26.4% were musculoskeletal, 29.4% were psychological, 25.1% were dermatologic, and 17.5% were sensory.
At the later clinical visit for PACS symptoms, all patients underwent screening for MAFLD, which was defined as the presence of liver steatosis plus overweight/obesity or type 2 diabetes. Hepatic steatosis was determined from controlled attenuation parameter using transient elastrography. The analysis excluded patients with significant alcohol intake or hepatitis B or C. All patients with liver steatosis also had MAFLD, and this included 55.3% of the study population.
The hospital was able to obtain hepatic steatosis index (HSI) scores for 103 of 235 patients. Of these, 50% had MAFLD on admission for acute COVID-19, and 48.1% had MAFLD upon discharge based on this criterion. At the PACS follow-up visit, 71.3% were diagnosed with MAFLD. There was no statistically significant difference in the use of glucocorticoids or tocilizumab during hospitalization between those with and without MAFLD, and remdesivir use was insignificant in the patient population.
Given that the prevalence of MAFLD among the study population is more than double that in the general population, the authors suggest that MAFLD may be a new PACS cluster phenotype that could lead to long-term metabolic and cardiovascular complications. A potential explanation is loss of lean body mass during COVID-19 hospitalization followed by liver fat accumulation during recovery.
Other infections have also shown an association with increased MAFLD incidence, including HIV, Heliobacter pylori, and viral hepatitis. The authors worry that COVID-19 infection could exacerbate underlying conditions to a more severe MAFLD disease state.
The study is limited by a small sample size, limited follow-up, and the lack of a control group. Its retrospective nature leaves it vulnerable to biases.
“The natural history of MAFLD in the context of PACS is unknown at this time, and careful follow-up of these patients is needed to understand the clinical implications of this syndrome in the context of long COVID,” the authors wrote. “We speculate that [MAFLD] may be considered as an independent PACS-cluster phenotype, potentially affecting the metabolic and cardiovascular health of patients with PACS.”
One author has relationships with several pharmaceutical companies, but the remaining authors reported no conflicts of interest. Dr. Martin has no relevant financial disclosures.
Postacute COVID syndrome (PACS), an ongoing inflammatory state following infection with SARS-CoV-2, is associated with greater risk of metabolic-associated fatty liver disease (MAFLD), according to an analysis of patients at a single clinic in Canada published in Open Forum Infectious Diseases.
MAFLD, also known as nonalcoholic fatty liver disease (NAFLD), is considered an indicator of general health and is in turn linked to greater risk of cardiovascular complications and mortality. It may be a multisystem disorder with various underlying causes.
PACS includes symptoms that affect various organ systems, with neurocognitive, autonomic, gastrointestinal, respiratory, musculoskeletal, psychological, sensory, and dermatologic clusters. An estimated 50%-80% of COVID-19 patients experience one or more clusters of symptoms 3 months after leaving the hospital.
But liver problems also appear in the acute phase, said Paul Martin, MD, who was asked to comment on the study. “Up to about half the patients during the acute illness may have elevated liver tests, but there seems to be a subset of patients in whom the abnormality persists. And then there are some reports in the literature of patients developing injury to their bile ducts in the liver over the long term, apparently as a consequence of COVID infection. What this paper suggests is that there may be some metabolic derangements associated with COVID infection, which in turn can accentuate or possibly cause fatty liver,” said Dr. Martin in an interview. He is chief of digestive health and liver diseases and a professor of medicine at the University of Miami.
“It highlights the need to get vaccinated against COVID and to take appropriate precautions because contracting the infection may lead to all sorts of consequences quite apart from having a respiratory illness,” said Dr. Martin.
The researchers retrospectively identified 235 patients hospitalized with COVID-19 between July 2020 and April 2021. Overall, 69% were men, and the median age was 61 years; 19.2% underwent mechanical ventilation and the mean duration of hospitalization was 11.7 days. They were seen for PACS symptoms a median 143 days after COVID-19 symptoms began, with 77.5% having symptoms of at least one PACS cluster. Of these clusters, 34.9% were neurocognitive, 53.2% were respiratory, 26.4% were musculoskeletal, 29.4% were psychological, 25.1% were dermatologic, and 17.5% were sensory.
At the later clinical visit for PACS symptoms, all patients underwent screening for MAFLD, which was defined as the presence of liver steatosis plus overweight/obesity or type 2 diabetes. Hepatic steatosis was determined from controlled attenuation parameter using transient elastrography. The analysis excluded patients with significant alcohol intake or hepatitis B or C. All patients with liver steatosis also had MAFLD, and this included 55.3% of the study population.
The hospital was able to obtain hepatic steatosis index (HSI) scores for 103 of 235 patients. Of these, 50% had MAFLD on admission for acute COVID-19, and 48.1% had MAFLD upon discharge based on this criterion. At the PACS follow-up visit, 71.3% were diagnosed with MAFLD. There was no statistically significant difference in the use of glucocorticoids or tocilizumab during hospitalization between those with and without MAFLD, and remdesivir use was insignificant in the patient population.
Given that the prevalence of MAFLD among the study population is more than double that in the general population, the authors suggest that MAFLD may be a new PACS cluster phenotype that could lead to long-term metabolic and cardiovascular complications. A potential explanation is loss of lean body mass during COVID-19 hospitalization followed by liver fat accumulation during recovery.
Other infections have also shown an association with increased MAFLD incidence, including HIV, Heliobacter pylori, and viral hepatitis. The authors worry that COVID-19 infection could exacerbate underlying conditions to a more severe MAFLD disease state.
The study is limited by a small sample size, limited follow-up, and the lack of a control group. Its retrospective nature leaves it vulnerable to biases.
“The natural history of MAFLD in the context of PACS is unknown at this time, and careful follow-up of these patients is needed to understand the clinical implications of this syndrome in the context of long COVID,” the authors wrote. “We speculate that [MAFLD] may be considered as an independent PACS-cluster phenotype, potentially affecting the metabolic and cardiovascular health of patients with PACS.”
One author has relationships with several pharmaceutical companies, but the remaining authors reported no conflicts of interest. Dr. Martin has no relevant financial disclosures.
Postacute COVID syndrome (PACS), an ongoing inflammatory state following infection with SARS-CoV-2, is associated with greater risk of metabolic-associated fatty liver disease (MAFLD), according to an analysis of patients at a single clinic in Canada published in Open Forum Infectious Diseases.
MAFLD, also known as nonalcoholic fatty liver disease (NAFLD), is considered an indicator of general health and is in turn linked to greater risk of cardiovascular complications and mortality. It may be a multisystem disorder with various underlying causes.
PACS includes symptoms that affect various organ systems, with neurocognitive, autonomic, gastrointestinal, respiratory, musculoskeletal, psychological, sensory, and dermatologic clusters. An estimated 50%-80% of COVID-19 patients experience one or more clusters of symptoms 3 months after leaving the hospital.
But liver problems also appear in the acute phase, said Paul Martin, MD, who was asked to comment on the study. “Up to about half the patients during the acute illness may have elevated liver tests, but there seems to be a subset of patients in whom the abnormality persists. And then there are some reports in the literature of patients developing injury to their bile ducts in the liver over the long term, apparently as a consequence of COVID infection. What this paper suggests is that there may be some metabolic derangements associated with COVID infection, which in turn can accentuate or possibly cause fatty liver,” said Dr. Martin in an interview. He is chief of digestive health and liver diseases and a professor of medicine at the University of Miami.
“It highlights the need to get vaccinated against COVID and to take appropriate precautions because contracting the infection may lead to all sorts of consequences quite apart from having a respiratory illness,” said Dr. Martin.
The researchers retrospectively identified 235 patients hospitalized with COVID-19 between July 2020 and April 2021. Overall, 69% were men, and the median age was 61 years; 19.2% underwent mechanical ventilation and the mean duration of hospitalization was 11.7 days. They were seen for PACS symptoms a median 143 days after COVID-19 symptoms began, with 77.5% having symptoms of at least one PACS cluster. Of these clusters, 34.9% were neurocognitive, 53.2% were respiratory, 26.4% were musculoskeletal, 29.4% were psychological, 25.1% were dermatologic, and 17.5% were sensory.
At the later clinical visit for PACS symptoms, all patients underwent screening for MAFLD, which was defined as the presence of liver steatosis plus overweight/obesity or type 2 diabetes. Hepatic steatosis was determined from controlled attenuation parameter using transient elastrography. The analysis excluded patients with significant alcohol intake or hepatitis B or C. All patients with liver steatosis also had MAFLD, and this included 55.3% of the study population.
The hospital was able to obtain hepatic steatosis index (HSI) scores for 103 of 235 patients. Of these, 50% had MAFLD on admission for acute COVID-19, and 48.1% had MAFLD upon discharge based on this criterion. At the PACS follow-up visit, 71.3% were diagnosed with MAFLD. There was no statistically significant difference in the use of glucocorticoids or tocilizumab during hospitalization between those with and without MAFLD, and remdesivir use was insignificant in the patient population.
Given that the prevalence of MAFLD among the study population is more than double that in the general population, the authors suggest that MAFLD may be a new PACS cluster phenotype that could lead to long-term metabolic and cardiovascular complications. A potential explanation is loss of lean body mass during COVID-19 hospitalization followed by liver fat accumulation during recovery.
Other infections have also shown an association with increased MAFLD incidence, including HIV, Heliobacter pylori, and viral hepatitis. The authors worry that COVID-19 infection could exacerbate underlying conditions to a more severe MAFLD disease state.
The study is limited by a small sample size, limited follow-up, and the lack of a control group. Its retrospective nature leaves it vulnerable to biases.
“The natural history of MAFLD in the context of PACS is unknown at this time, and careful follow-up of these patients is needed to understand the clinical implications of this syndrome in the context of long COVID,” the authors wrote. “We speculate that [MAFLD] may be considered as an independent PACS-cluster phenotype, potentially affecting the metabolic and cardiovascular health of patients with PACS.”
One author has relationships with several pharmaceutical companies, but the remaining authors reported no conflicts of interest. Dr. Martin has no relevant financial disclosures.
FROM OPEN FORUM INFECTIOUS DISEASES
SARS-Co-V2 Deserves Our Respect, Can We Provide It Before the Next Variant Arrives?
Health care’s modern-day version of the Greek chorus is growing louder and more persistent. My colleagues and I have long been among them.
In news conferences, journal articles, and podcasts, this chorus is pleading with the public to pay attention to its message: SARs-CoV-2 is not done with us. Omicron can kill; it can infect the vaccinated.
We have found, like everyone else, that Omicron runs on its rules; with Delta, two vaccine shots were able to lower the positivity rate. With Omicron, two shots have not been enough.
The WHO used the word “surprise” in its November announcement that Omicron was a variant of “concern.”
So did Trevor Bedford, a computational biologist and infectious disease scientist at the Fred Hutchinson Cancer Center in Seattle, who said that Omicron “took me and everyone by surprise.”1 Speaking on KevinMD’s podcast, he told his host that with the degree of immunity in the global population, he was expecting subsequent evolving strains to have minor, subtle genetic mutations, akin to how flu varies from year to year. What was a surprise was the giant leap in evolution that occurred with Omicron, which contains 36 mutations in the spike protein and approximately 50 mutations in total. Because of these mutations, the original two-shot mRNA COVID vaccines becomes only 40% effective against symptomatic disease after several months (thankfully a booster shot increases this to ~80%).2 But the decreased vaccine protection without a booster, along with relaxation of mitigation measures, brought us to where we are now.
In Chicago, we knew the Omicron variant would move quickly, considering how it moved through South Africa and the United Kingdom. What we didn’t anticipate was that in one week’s time, our hospital would need to add another 100 dedicated COVID ICU beds. Nor did we anticipate the extent that Omicron would affect staffing levels in the same amount of time.
At our hospital, we have eliminated elective surgeries that require a hospital stay, which includes surgeries for cancer. One of my colleagues, Ryan Merkow, MD, a surgical oncologist, remarked recently he had to cancel half of his scheduled surgeries because of a lack of hospital space.3
Dispelling Myths
What is concerning about this current wave is how many unvaccinated are hospitalized. Because Omicron is so infectious and because of lower vaccination rates in younger adults and children, we have a younger group of adults and children admitted with COVID, who had been uninfected by previous surges.
A major myth that makes health care workers so frustrated is the tale that Omicron is milder. Unvaccinated people infected with this variant are seriously ill and are dying. Despite its “mild” label, once a patient is hospitalized, Omicron can be just as severe as its predecessors.4 For many, getting vaccinated is the difference between staying at home with some symptoms and being in the ICU.
As of January 10, according to the CDC, although 88% of people over the age of 65 are vaccinated, only 37.5% have gotten boosters which are key to restoring protection against Omicron. And among children, only 54% between 12- and 17-years-old are fully vaccinated, and a mere 17% of children aged 5 to 11 have gotten both of their shots.
Remember the conversations regarding natural immunity? Omicron has muffled that conversation. Those who have been infected with SARS-Co-V2 before can still get infected and very ill with Omicron. So now is the time to get vaccinated.
Transmissibility
We knew SARS-CoV-2 could spread 1 of 2 ways: large virus-carrying droplets that enter through the nose, mouth, and eyes, as well as miniscule airborne droplets of virus that float in the air and travel further than 6 feet. However, prior to Omicron, transmission of these smaller droplets via the air was not as frequent. But with Omicron, while it still travels by larger respiratory droplets, it appears to have more airborne spread.
In late December, The Lancet Regional Health published results of research conducted one month earlier at a designated quarantine hotel in Hong Kong.5 The index case was housed in the room across a hallway from the second case, who developed their case 8 days into quarantine. Testing showed the Omicron variant in both cases. Environmental testing of the walls and ceiling suggested airborne spread of the virus in places unreachable by large respiratory droplets.
Now with Omicron, people need to wear high-filtering masks that fit tight against the face, such as a N95, KN-95, or KF-94 if possible. And when removing the mask to eat and drink, one should be in well-ventilated areas, away from others.
People should avoid getting Omicron, regardless of vaccination status. This variant is so infectious that, compared to the Delta variant, people are twice as likely to infect others that live with them. And infecting others leads to a chain of transmission that can close schools, take over hospital beds, and disable or kill the most vulnerable in our communities.
Public and private behavior, and public policy
In July, months before that WHO announced Omicron’s existence, Rella and colleagues reported in Scientific Reports on the outcome of a new model designed to show how a vaccine-resistant strain could rapidly transmit through a highly vaccinated population if transmission mitigation interventions are dropped too soon.6
The authors wrote that the success of a vaccine-resistant strain making inroads into a population depends on the obvious – it finds populations with a low rate of vaccination. What is not so obvious, the authors wrote, is that a vaccine-resistant virus does its worst when transmission is not well controlled in a highly vaccinated population. What can prevent a surge like this are social behaviors and public policy that decrease the chain of transmission of SARS-CoV-2, such as vaccination, masking, and testing.
It is people’s behavior, and ineffective public policy, that are so frustrating to us. The WHO’s secretary general warned against relying solely on vaccines in December. “Vaccines alone will not get any country out of this crisis.”
Omicron takes a new mindset. What we were doing before is not protecting now. Unchecked spread is overwhelming our health care systems and putting the vulnerable in our population at risk. The ramification of this unchecked spread reaches everywhere – into the economy, our educational system, and our nation’s mental health.
When the pandemic started, the policies to control its spread rested on local government and public agencies; we all would have been better served had there been a unified, national response to an infectious threat that does not obey municipal or state boundaries.
The universal sentiment among health care workers is frustration with local and state governments that are either dictating policy that can harm the public we are trying to protect.
As of September, at least 23 state legislatures have passed laws changing a governor’s executive power reach. Many have taken it away. Others are fighting in the courts over mask mandates.
As for when the pandemic will subside, that appears to be up to the public and public policy makers. They will determine how long this will last and how many will die or be disabled before its end.
References
- KevinMD.com. Trevor Bedford on Omicron and what about Covid keeps him up at night. Dec. 17 podcast. https://www.kevinmd.com/blog/post-author/the-podcast-by-kevinmd/page/2
- Andrews N, Stowe J, Kirsebom F, et al. Effectiveness of COVID-19 vaccines against the Omicron (B.1.1.529) variant of concern. MedRxiv. Preprint. doi: https://doi.org/10.1101/2021.12.14.21267615
- Weise E and Shamus KJ. (January 13, 2022). As COVID-19 surges, there are no hospital beds for others in need of care. USA Today. As COVID-19 surges, there are no hospital beds for others in need of care (yahoo.com)
- Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 Omicron variant in South Africa. MedRxiv. Preprint. doi: https://doi.org/10.1101/2021.12.21.21268116
- Shuk-Ching Wong, Albert Ka-Wing Au, Hong Chen et al. Transmission of Omicron (B.1.1.529) - SARS-CoV-2 Variant of Concern in a designated quarantine hotel for travelers: a challenge of elimination strategy of COVID-19. The Lancet Regional Health - Western Pacific. Available online 23 December 2021
- Rella SA, Kulikova YA, Dermitzakis ET, et al. Rates of SARS-CoV-2 transmission and vaccination impact the fate of vaccine-resistant strains. Sci Rep 11, 15729 (2021).
- WHO press conference on coronavirus disease (COVID-19) - 14 December 2021.
- Telebriefing on Covid-19 Update. https://www.cdc.gov/media/releases/2022/t0107-Covid-update.html
- Shuk-Ching Wong, Albert Ka-Wing Au, Hong Chen et al. Transmission of Omicron (B.1.1.529) - SARS-CoV-2 Variant of Concern in a designated quarantine hotel for travelers: a challenge of elimination strategy of COVID-1 The Lancet Regional Health - Western Pacific. Available online 23 December 2021
- CDC. Interim Guidance for Managing Healthcare Personnel with SARS-CoV-2 Infection or Exposure to SARS-CoV-2. Dec. 23, 2021.
- Mariah Timms. Tennessee appeals federal court order temporarily blocking new state law on school masks. Nashville Tennessean. Jan. 3, 2022.
- Statewide Number of Covid-19 Hospitalized Pediatric Patients. Jan. 4, 2022.
- National Conference of State Legislatures. Legislative Oversight of Emergency Executive Powers. Jan. 4, 2022.
https://news.yahoo.com/Covid-surges-others-care-theres-105949790.html
Elizabeth Weise and Kristen Jordan Shamus. As COVID-19 surges, there are no hospital beds for others in need of care. USA Today. Jan. 13, 2022.
Health care’s modern-day version of the Greek chorus is growing louder and more persistent. My colleagues and I have long been among them.
In news conferences, journal articles, and podcasts, this chorus is pleading with the public to pay attention to its message: SARs-CoV-2 is not done with us. Omicron can kill; it can infect the vaccinated.
We have found, like everyone else, that Omicron runs on its rules; with Delta, two vaccine shots were able to lower the positivity rate. With Omicron, two shots have not been enough.
The WHO used the word “surprise” in its November announcement that Omicron was a variant of “concern.”
So did Trevor Bedford, a computational biologist and infectious disease scientist at the Fred Hutchinson Cancer Center in Seattle, who said that Omicron “took me and everyone by surprise.”1 Speaking on KevinMD’s podcast, he told his host that with the degree of immunity in the global population, he was expecting subsequent evolving strains to have minor, subtle genetic mutations, akin to how flu varies from year to year. What was a surprise was the giant leap in evolution that occurred with Omicron, which contains 36 mutations in the spike protein and approximately 50 mutations in total. Because of these mutations, the original two-shot mRNA COVID vaccines becomes only 40% effective against symptomatic disease after several months (thankfully a booster shot increases this to ~80%).2 But the decreased vaccine protection without a booster, along with relaxation of mitigation measures, brought us to where we are now.
In Chicago, we knew the Omicron variant would move quickly, considering how it moved through South Africa and the United Kingdom. What we didn’t anticipate was that in one week’s time, our hospital would need to add another 100 dedicated COVID ICU beds. Nor did we anticipate the extent that Omicron would affect staffing levels in the same amount of time.
At our hospital, we have eliminated elective surgeries that require a hospital stay, which includes surgeries for cancer. One of my colleagues, Ryan Merkow, MD, a surgical oncologist, remarked recently he had to cancel half of his scheduled surgeries because of a lack of hospital space.3
Dispelling Myths
What is concerning about this current wave is how many unvaccinated are hospitalized. Because Omicron is so infectious and because of lower vaccination rates in younger adults and children, we have a younger group of adults and children admitted with COVID, who had been uninfected by previous surges.
A major myth that makes health care workers so frustrated is the tale that Omicron is milder. Unvaccinated people infected with this variant are seriously ill and are dying. Despite its “mild” label, once a patient is hospitalized, Omicron can be just as severe as its predecessors.4 For many, getting vaccinated is the difference between staying at home with some symptoms and being in the ICU.
As of January 10, according to the CDC, although 88% of people over the age of 65 are vaccinated, only 37.5% have gotten boosters which are key to restoring protection against Omicron. And among children, only 54% between 12- and 17-years-old are fully vaccinated, and a mere 17% of children aged 5 to 11 have gotten both of their shots.
Remember the conversations regarding natural immunity? Omicron has muffled that conversation. Those who have been infected with SARS-Co-V2 before can still get infected and very ill with Omicron. So now is the time to get vaccinated.
Transmissibility
We knew SARS-CoV-2 could spread 1 of 2 ways: large virus-carrying droplets that enter through the nose, mouth, and eyes, as well as miniscule airborne droplets of virus that float in the air and travel further than 6 feet. However, prior to Omicron, transmission of these smaller droplets via the air was not as frequent. But with Omicron, while it still travels by larger respiratory droplets, it appears to have more airborne spread.
In late December, The Lancet Regional Health published results of research conducted one month earlier at a designated quarantine hotel in Hong Kong.5 The index case was housed in the room across a hallway from the second case, who developed their case 8 days into quarantine. Testing showed the Omicron variant in both cases. Environmental testing of the walls and ceiling suggested airborne spread of the virus in places unreachable by large respiratory droplets.
Now with Omicron, people need to wear high-filtering masks that fit tight against the face, such as a N95, KN-95, or KF-94 if possible. And when removing the mask to eat and drink, one should be in well-ventilated areas, away from others.
People should avoid getting Omicron, regardless of vaccination status. This variant is so infectious that, compared to the Delta variant, people are twice as likely to infect others that live with them. And infecting others leads to a chain of transmission that can close schools, take over hospital beds, and disable or kill the most vulnerable in our communities.
Public and private behavior, and public policy
In July, months before that WHO announced Omicron’s existence, Rella and colleagues reported in Scientific Reports on the outcome of a new model designed to show how a vaccine-resistant strain could rapidly transmit through a highly vaccinated population if transmission mitigation interventions are dropped too soon.6
The authors wrote that the success of a vaccine-resistant strain making inroads into a population depends on the obvious – it finds populations with a low rate of vaccination. What is not so obvious, the authors wrote, is that a vaccine-resistant virus does its worst when transmission is not well controlled in a highly vaccinated population. What can prevent a surge like this are social behaviors and public policy that decrease the chain of transmission of SARS-CoV-2, such as vaccination, masking, and testing.
It is people’s behavior, and ineffective public policy, that are so frustrating to us. The WHO’s secretary general warned against relying solely on vaccines in December. “Vaccines alone will not get any country out of this crisis.”
Omicron takes a new mindset. What we were doing before is not protecting now. Unchecked spread is overwhelming our health care systems and putting the vulnerable in our population at risk. The ramification of this unchecked spread reaches everywhere – into the economy, our educational system, and our nation’s mental health.
When the pandemic started, the policies to control its spread rested on local government and public agencies; we all would have been better served had there been a unified, national response to an infectious threat that does not obey municipal or state boundaries.
The universal sentiment among health care workers is frustration with local and state governments that are either dictating policy that can harm the public we are trying to protect.
As of September, at least 23 state legislatures have passed laws changing a governor’s executive power reach. Many have taken it away. Others are fighting in the courts over mask mandates.
As for when the pandemic will subside, that appears to be up to the public and public policy makers. They will determine how long this will last and how many will die or be disabled before its end.
Health care’s modern-day version of the Greek chorus is growing louder and more persistent. My colleagues and I have long been among them.
In news conferences, journal articles, and podcasts, this chorus is pleading with the public to pay attention to its message: SARs-CoV-2 is not done with us. Omicron can kill; it can infect the vaccinated.
We have found, like everyone else, that Omicron runs on its rules; with Delta, two vaccine shots were able to lower the positivity rate. With Omicron, two shots have not been enough.
The WHO used the word “surprise” in its November announcement that Omicron was a variant of “concern.”
So did Trevor Bedford, a computational biologist and infectious disease scientist at the Fred Hutchinson Cancer Center in Seattle, who said that Omicron “took me and everyone by surprise.”1 Speaking on KevinMD’s podcast, he told his host that with the degree of immunity in the global population, he was expecting subsequent evolving strains to have minor, subtle genetic mutations, akin to how flu varies from year to year. What was a surprise was the giant leap in evolution that occurred with Omicron, which contains 36 mutations in the spike protein and approximately 50 mutations in total. Because of these mutations, the original two-shot mRNA COVID vaccines becomes only 40% effective against symptomatic disease after several months (thankfully a booster shot increases this to ~80%).2 But the decreased vaccine protection without a booster, along with relaxation of mitigation measures, brought us to where we are now.
In Chicago, we knew the Omicron variant would move quickly, considering how it moved through South Africa and the United Kingdom. What we didn’t anticipate was that in one week’s time, our hospital would need to add another 100 dedicated COVID ICU beds. Nor did we anticipate the extent that Omicron would affect staffing levels in the same amount of time.
At our hospital, we have eliminated elective surgeries that require a hospital stay, which includes surgeries for cancer. One of my colleagues, Ryan Merkow, MD, a surgical oncologist, remarked recently he had to cancel half of his scheduled surgeries because of a lack of hospital space.3
Dispelling Myths
What is concerning about this current wave is how many unvaccinated are hospitalized. Because Omicron is so infectious and because of lower vaccination rates in younger adults and children, we have a younger group of adults and children admitted with COVID, who had been uninfected by previous surges.
A major myth that makes health care workers so frustrated is the tale that Omicron is milder. Unvaccinated people infected with this variant are seriously ill and are dying. Despite its “mild” label, once a patient is hospitalized, Omicron can be just as severe as its predecessors.4 For many, getting vaccinated is the difference between staying at home with some symptoms and being in the ICU.
As of January 10, according to the CDC, although 88% of people over the age of 65 are vaccinated, only 37.5% have gotten boosters which are key to restoring protection against Omicron. And among children, only 54% between 12- and 17-years-old are fully vaccinated, and a mere 17% of children aged 5 to 11 have gotten both of their shots.
Remember the conversations regarding natural immunity? Omicron has muffled that conversation. Those who have been infected with SARS-Co-V2 before can still get infected and very ill with Omicron. So now is the time to get vaccinated.
Transmissibility
We knew SARS-CoV-2 could spread 1 of 2 ways: large virus-carrying droplets that enter through the nose, mouth, and eyes, as well as miniscule airborne droplets of virus that float in the air and travel further than 6 feet. However, prior to Omicron, transmission of these smaller droplets via the air was not as frequent. But with Omicron, while it still travels by larger respiratory droplets, it appears to have more airborne spread.
In late December, The Lancet Regional Health published results of research conducted one month earlier at a designated quarantine hotel in Hong Kong.5 The index case was housed in the room across a hallway from the second case, who developed their case 8 days into quarantine. Testing showed the Omicron variant in both cases. Environmental testing of the walls and ceiling suggested airborne spread of the virus in places unreachable by large respiratory droplets.
Now with Omicron, people need to wear high-filtering masks that fit tight against the face, such as a N95, KN-95, or KF-94 if possible. And when removing the mask to eat and drink, one should be in well-ventilated areas, away from others.
People should avoid getting Omicron, regardless of vaccination status. This variant is so infectious that, compared to the Delta variant, people are twice as likely to infect others that live with them. And infecting others leads to a chain of transmission that can close schools, take over hospital beds, and disable or kill the most vulnerable in our communities.
Public and private behavior, and public policy
In July, months before that WHO announced Omicron’s existence, Rella and colleagues reported in Scientific Reports on the outcome of a new model designed to show how a vaccine-resistant strain could rapidly transmit through a highly vaccinated population if transmission mitigation interventions are dropped too soon.6
The authors wrote that the success of a vaccine-resistant strain making inroads into a population depends on the obvious – it finds populations with a low rate of vaccination. What is not so obvious, the authors wrote, is that a vaccine-resistant virus does its worst when transmission is not well controlled in a highly vaccinated population. What can prevent a surge like this are social behaviors and public policy that decrease the chain of transmission of SARS-CoV-2, such as vaccination, masking, and testing.
It is people’s behavior, and ineffective public policy, that are so frustrating to us. The WHO’s secretary general warned against relying solely on vaccines in December. “Vaccines alone will not get any country out of this crisis.”
Omicron takes a new mindset. What we were doing before is not protecting now. Unchecked spread is overwhelming our health care systems and putting the vulnerable in our population at risk. The ramification of this unchecked spread reaches everywhere – into the economy, our educational system, and our nation’s mental health.
When the pandemic started, the policies to control its spread rested on local government and public agencies; we all would have been better served had there been a unified, national response to an infectious threat that does not obey municipal or state boundaries.
The universal sentiment among health care workers is frustration with local and state governments that are either dictating policy that can harm the public we are trying to protect.
As of September, at least 23 state legislatures have passed laws changing a governor’s executive power reach. Many have taken it away. Others are fighting in the courts over mask mandates.
As for when the pandemic will subside, that appears to be up to the public and public policy makers. They will determine how long this will last and how many will die or be disabled before its end.
References
- KevinMD.com. Trevor Bedford on Omicron and what about Covid keeps him up at night. Dec. 17 podcast. https://www.kevinmd.com/blog/post-author/the-podcast-by-kevinmd/page/2
- Andrews N, Stowe J, Kirsebom F, et al. Effectiveness of COVID-19 vaccines against the Omicron (B.1.1.529) variant of concern. MedRxiv. Preprint. doi: https://doi.org/10.1101/2021.12.14.21267615
- Weise E and Shamus KJ. (January 13, 2022). As COVID-19 surges, there are no hospital beds for others in need of care. USA Today. As COVID-19 surges, there are no hospital beds for others in need of care (yahoo.com)
- Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 Omicron variant in South Africa. MedRxiv. Preprint. doi: https://doi.org/10.1101/2021.12.21.21268116
- Shuk-Ching Wong, Albert Ka-Wing Au, Hong Chen et al. Transmission of Omicron (B.1.1.529) - SARS-CoV-2 Variant of Concern in a designated quarantine hotel for travelers: a challenge of elimination strategy of COVID-19. The Lancet Regional Health - Western Pacific. Available online 23 December 2021
- Rella SA, Kulikova YA, Dermitzakis ET, et al. Rates of SARS-CoV-2 transmission and vaccination impact the fate of vaccine-resistant strains. Sci Rep 11, 15729 (2021).
- WHO press conference on coronavirus disease (COVID-19) - 14 December 2021.
- Telebriefing on Covid-19 Update. https://www.cdc.gov/media/releases/2022/t0107-Covid-update.html
- Shuk-Ching Wong, Albert Ka-Wing Au, Hong Chen et al. Transmission of Omicron (B.1.1.529) - SARS-CoV-2 Variant of Concern in a designated quarantine hotel for travelers: a challenge of elimination strategy of COVID-1 The Lancet Regional Health - Western Pacific. Available online 23 December 2021
- CDC. Interim Guidance for Managing Healthcare Personnel with SARS-CoV-2 Infection or Exposure to SARS-CoV-2. Dec. 23, 2021.
- Mariah Timms. Tennessee appeals federal court order temporarily blocking new state law on school masks. Nashville Tennessean. Jan. 3, 2022.
- Statewide Number of Covid-19 Hospitalized Pediatric Patients. Jan. 4, 2022.
- National Conference of State Legislatures. Legislative Oversight of Emergency Executive Powers. Jan. 4, 2022.
https://news.yahoo.com/Covid-surges-others-care-theres-105949790.html
Elizabeth Weise and Kristen Jordan Shamus. As COVID-19 surges, there are no hospital beds for others in need of care. USA Today. Jan. 13, 2022.
References
- KevinMD.com. Trevor Bedford on Omicron and what about Covid keeps him up at night. Dec. 17 podcast. https://www.kevinmd.com/blog/post-author/the-podcast-by-kevinmd/page/2
- Andrews N, Stowe J, Kirsebom F, et al. Effectiveness of COVID-19 vaccines against the Omicron (B.1.1.529) variant of concern. MedRxiv. Preprint. doi: https://doi.org/10.1101/2021.12.14.21267615
- Weise E and Shamus KJ. (January 13, 2022). As COVID-19 surges, there are no hospital beds for others in need of care. USA Today. As COVID-19 surges, there are no hospital beds for others in need of care (yahoo.com)
- Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 Omicron variant in South Africa. MedRxiv. Preprint. doi: https://doi.org/10.1101/2021.12.21.21268116
- Shuk-Ching Wong, Albert Ka-Wing Au, Hong Chen et al. Transmission of Omicron (B.1.1.529) - SARS-CoV-2 Variant of Concern in a designated quarantine hotel for travelers: a challenge of elimination strategy of COVID-19. The Lancet Regional Health - Western Pacific. Available online 23 December 2021
- Rella SA, Kulikova YA, Dermitzakis ET, et al. Rates of SARS-CoV-2 transmission and vaccination impact the fate of vaccine-resistant strains. Sci Rep 11, 15729 (2021).
- WHO press conference on coronavirus disease (COVID-19) - 14 December 2021.
- Telebriefing on Covid-19 Update. https://www.cdc.gov/media/releases/2022/t0107-Covid-update.html
- Shuk-Ching Wong, Albert Ka-Wing Au, Hong Chen et al. Transmission of Omicron (B.1.1.529) - SARS-CoV-2 Variant of Concern in a designated quarantine hotel for travelers: a challenge of elimination strategy of COVID-1 The Lancet Regional Health - Western Pacific. Available online 23 December 2021
- CDC. Interim Guidance for Managing Healthcare Personnel with SARS-CoV-2 Infection or Exposure to SARS-CoV-2. Dec. 23, 2021.
- Mariah Timms. Tennessee appeals federal court order temporarily blocking new state law on school masks. Nashville Tennessean. Jan. 3, 2022.
- Statewide Number of Covid-19 Hospitalized Pediatric Patients. Jan. 4, 2022.
- National Conference of State Legislatures. Legislative Oversight of Emergency Executive Powers. Jan. 4, 2022.
https://news.yahoo.com/Covid-surges-others-care-theres-105949790.html
Elizabeth Weise and Kristen Jordan Shamus. As COVID-19 surges, there are no hospital beds for others in need of care. USA Today. Jan. 13, 2022.
Axilla swelling after COVID booster puts focus on mammogram timing
This inflammation is caused by the enlargement of lymph nodes and can show up as an abnormal finding on mammograms and other types of chest scans, causing concern and even the need for additional imaging and follow up, wrote Constance D. Lehman, MD, PhD, and colleagues in an article published in Journal of the American College of Radiology.
Lymph node swelling is a normal immune system reaction to vaccination, and “COVID-19 vaccinations in the arm are a well-documented cause of inflammatory unilateral axillary adenopathy,” noted Dr. Lehman, in an interview. The side effect will occur on the side of the body where the patient received a vaccine, and it is not always noticeable to the woman experiencing it, she said.
“We’re finding that the patients’ bodies are responding to the booster in many ways that are similar to the initial COVID vaccines, with lymph node swelling, muscle aches and pains, headaches, and so on,” said Dr. Lehman, who is chief of breast imaging at the Massachusetts General Hospital, Boston. There have been no real differences in reactions between the Moderna and Pfizer vaccines, she added.
Because axillary lymph node swelling can obscure mammogram results, staff of at least a few imaging centers, including Penn State Breast Center in Hershey, Pa., and Providence Women’s Imaging Center in Torrance, Calif., told this news organization that they are asking women to delay mammogram imaging either 6 weeks or 4-6 weeks after getting a COVID-19 booster.
Experts’ suggestions on mammograms, boosters timing
Other experts, including Jessica Leung, MD, acknowledged that vaccine-related reactive adenopathy is seen after the booster dose and provided recommendations for the timing of getting mammograms and the booster with this in mind.
“I would recommend getting the screening mammogram first, which can be followed immediately by vaccination, even on the same day,” said Jessica Leung, MD, a professor of diagnostic radiology at the University of Texas MD Anderson Cancer Center in Houston, Tex.
“If this is not possible from the scheduling perspective, then the patient should consult her health care provider regarding whether it is okay to wait a bit after receiving the vaccine before getting her screening mammogram.”
The answer to that question will likely depend on the time interval since the prior mammogram and the patient’s personal risk factors for developing breast cancer. Dr. Leung noted. “This is all predicated on the assumption that the patient is asymptomatic. If she has any symptoms, for example a palpable breast lump, then she should seek medical attention regardless of timing of vaccination.”
The same holds true for boosters, she said.
She emphasized that careful consideration should be given before delaying the mammogram. “The medical community has a great deal more knowledge at this time than in the early days of COVID-19 vaccination, so we are often able to identify reactive adenopathy related to vaccination. If patients were to delay the mammogram, any reactive adenopathy may persist, on average, for 4-6 weeks.”
Debra Patt, MD, PhD, MBA, executive vice president at Texas Oncology, professor at the University of Texas at Austin, provided a specific example of when a patient should not delay the diagnostic imaging, which is “in the event that there is an abnormal mass in the breast that requires evaluation.”
Providers are now prepared to address these issues, she added.
Dr. Lehman’s nuanced recommendations
“It’s easy to get both a mammogram and booster, and just a matter of timing them – so that the reaction doesn’t interfere with the mammography results,” Dr. Lehman said.
But she emphasized that women should not be choosing between their mammograms or a booster. “We are now saying the same thing that we did with the initial vaccine,” said Dr. Lehman. “We don’t want patients delaying their mammograms, and we don’t want them delaying their boosters – both are critical to staying healthy.”
In her center, a model was developed to navigate vaccine-associated adenopathy. While this approach was developed for the primary vaccine series, the same applies for the booster, which is essentially a third dose of the same vaccine, explained Dr. Lehman.
When patients present for mammography, ultrasound, or MRI, the technologist will document their COVID-19 vaccination status (first or second dose or booster), the date it was given, and the location. Adding vaccination documentation to intake forms helps to support appropriate management of patients who undergo imaging after COVID-19 vaccination. Six weeks is used as the cutoff point for defining “recent” vaccination.
For patients who are getting a screening mammography or MRI, and who have no symptoms beyond unilateral axillary adenopathy on the same side of the body where they received the COVID-19 vaccination (given in the arm) within a 6-week period, the following is included in the screening mammography or screening MRI report: “In the specific setting of a patient with documented recent (within the past 6 weeks) COVID-19 vaccination in the ipsilateral arm, axillary adenopathy is a benign imaging finding. No further imaging is indicated at this time. If there is clinical concern that persists more than 6 weeks after the patient received the final vaccine dose, axillary ultrasound is recommended.”
The experts interviewed reported no conflicts of interest.
This inflammation is caused by the enlargement of lymph nodes and can show up as an abnormal finding on mammograms and other types of chest scans, causing concern and even the need for additional imaging and follow up, wrote Constance D. Lehman, MD, PhD, and colleagues in an article published in Journal of the American College of Radiology.
Lymph node swelling is a normal immune system reaction to vaccination, and “COVID-19 vaccinations in the arm are a well-documented cause of inflammatory unilateral axillary adenopathy,” noted Dr. Lehman, in an interview. The side effect will occur on the side of the body where the patient received a vaccine, and it is not always noticeable to the woman experiencing it, she said.
“We’re finding that the patients’ bodies are responding to the booster in many ways that are similar to the initial COVID vaccines, with lymph node swelling, muscle aches and pains, headaches, and so on,” said Dr. Lehman, who is chief of breast imaging at the Massachusetts General Hospital, Boston. There have been no real differences in reactions between the Moderna and Pfizer vaccines, she added.
Because axillary lymph node swelling can obscure mammogram results, staff of at least a few imaging centers, including Penn State Breast Center in Hershey, Pa., and Providence Women’s Imaging Center in Torrance, Calif., told this news organization that they are asking women to delay mammogram imaging either 6 weeks or 4-6 weeks after getting a COVID-19 booster.
Experts’ suggestions on mammograms, boosters timing
Other experts, including Jessica Leung, MD, acknowledged that vaccine-related reactive adenopathy is seen after the booster dose and provided recommendations for the timing of getting mammograms and the booster with this in mind.
“I would recommend getting the screening mammogram first, which can be followed immediately by vaccination, even on the same day,” said Jessica Leung, MD, a professor of diagnostic radiology at the University of Texas MD Anderson Cancer Center in Houston, Tex.
“If this is not possible from the scheduling perspective, then the patient should consult her health care provider regarding whether it is okay to wait a bit after receiving the vaccine before getting her screening mammogram.”
The answer to that question will likely depend on the time interval since the prior mammogram and the patient’s personal risk factors for developing breast cancer. Dr. Leung noted. “This is all predicated on the assumption that the patient is asymptomatic. If she has any symptoms, for example a palpable breast lump, then she should seek medical attention regardless of timing of vaccination.”
The same holds true for boosters, she said.
She emphasized that careful consideration should be given before delaying the mammogram. “The medical community has a great deal more knowledge at this time than in the early days of COVID-19 vaccination, so we are often able to identify reactive adenopathy related to vaccination. If patients were to delay the mammogram, any reactive adenopathy may persist, on average, for 4-6 weeks.”
Debra Patt, MD, PhD, MBA, executive vice president at Texas Oncology, professor at the University of Texas at Austin, provided a specific example of when a patient should not delay the diagnostic imaging, which is “in the event that there is an abnormal mass in the breast that requires evaluation.”
Providers are now prepared to address these issues, she added.
Dr. Lehman’s nuanced recommendations
“It’s easy to get both a mammogram and booster, and just a matter of timing them – so that the reaction doesn’t interfere with the mammography results,” Dr. Lehman said.
But she emphasized that women should not be choosing between their mammograms or a booster. “We are now saying the same thing that we did with the initial vaccine,” said Dr. Lehman. “We don’t want patients delaying their mammograms, and we don’t want them delaying their boosters – both are critical to staying healthy.”
In her center, a model was developed to navigate vaccine-associated adenopathy. While this approach was developed for the primary vaccine series, the same applies for the booster, which is essentially a third dose of the same vaccine, explained Dr. Lehman.
When patients present for mammography, ultrasound, or MRI, the technologist will document their COVID-19 vaccination status (first or second dose or booster), the date it was given, and the location. Adding vaccination documentation to intake forms helps to support appropriate management of patients who undergo imaging after COVID-19 vaccination. Six weeks is used as the cutoff point for defining “recent” vaccination.
For patients who are getting a screening mammography or MRI, and who have no symptoms beyond unilateral axillary adenopathy on the same side of the body where they received the COVID-19 vaccination (given in the arm) within a 6-week period, the following is included in the screening mammography or screening MRI report: “In the specific setting of a patient with documented recent (within the past 6 weeks) COVID-19 vaccination in the ipsilateral arm, axillary adenopathy is a benign imaging finding. No further imaging is indicated at this time. If there is clinical concern that persists more than 6 weeks after the patient received the final vaccine dose, axillary ultrasound is recommended.”
The experts interviewed reported no conflicts of interest.
This inflammation is caused by the enlargement of lymph nodes and can show up as an abnormal finding on mammograms and other types of chest scans, causing concern and even the need for additional imaging and follow up, wrote Constance D. Lehman, MD, PhD, and colleagues in an article published in Journal of the American College of Radiology.
Lymph node swelling is a normal immune system reaction to vaccination, and “COVID-19 vaccinations in the arm are a well-documented cause of inflammatory unilateral axillary adenopathy,” noted Dr. Lehman, in an interview. The side effect will occur on the side of the body where the patient received a vaccine, and it is not always noticeable to the woman experiencing it, she said.
“We’re finding that the patients’ bodies are responding to the booster in many ways that are similar to the initial COVID vaccines, with lymph node swelling, muscle aches and pains, headaches, and so on,” said Dr. Lehman, who is chief of breast imaging at the Massachusetts General Hospital, Boston. There have been no real differences in reactions between the Moderna and Pfizer vaccines, she added.
Because axillary lymph node swelling can obscure mammogram results, staff of at least a few imaging centers, including Penn State Breast Center in Hershey, Pa., and Providence Women’s Imaging Center in Torrance, Calif., told this news organization that they are asking women to delay mammogram imaging either 6 weeks or 4-6 weeks after getting a COVID-19 booster.
Experts’ suggestions on mammograms, boosters timing
Other experts, including Jessica Leung, MD, acknowledged that vaccine-related reactive adenopathy is seen after the booster dose and provided recommendations for the timing of getting mammograms and the booster with this in mind.
“I would recommend getting the screening mammogram first, which can be followed immediately by vaccination, even on the same day,” said Jessica Leung, MD, a professor of diagnostic radiology at the University of Texas MD Anderson Cancer Center in Houston, Tex.
“If this is not possible from the scheduling perspective, then the patient should consult her health care provider regarding whether it is okay to wait a bit after receiving the vaccine before getting her screening mammogram.”
The answer to that question will likely depend on the time interval since the prior mammogram and the patient’s personal risk factors for developing breast cancer. Dr. Leung noted. “This is all predicated on the assumption that the patient is asymptomatic. If she has any symptoms, for example a palpable breast lump, then she should seek medical attention regardless of timing of vaccination.”
The same holds true for boosters, she said.
She emphasized that careful consideration should be given before delaying the mammogram. “The medical community has a great deal more knowledge at this time than in the early days of COVID-19 vaccination, so we are often able to identify reactive adenopathy related to vaccination. If patients were to delay the mammogram, any reactive adenopathy may persist, on average, for 4-6 weeks.”
Debra Patt, MD, PhD, MBA, executive vice president at Texas Oncology, professor at the University of Texas at Austin, provided a specific example of when a patient should not delay the diagnostic imaging, which is “in the event that there is an abnormal mass in the breast that requires evaluation.”
Providers are now prepared to address these issues, she added.
Dr. Lehman’s nuanced recommendations
“It’s easy to get both a mammogram and booster, and just a matter of timing them – so that the reaction doesn’t interfere with the mammography results,” Dr. Lehman said.
But she emphasized that women should not be choosing between their mammograms or a booster. “We are now saying the same thing that we did with the initial vaccine,” said Dr. Lehman. “We don’t want patients delaying their mammograms, and we don’t want them delaying their boosters – both are critical to staying healthy.”
In her center, a model was developed to navigate vaccine-associated adenopathy. While this approach was developed for the primary vaccine series, the same applies for the booster, which is essentially a third dose of the same vaccine, explained Dr. Lehman.
When patients present for mammography, ultrasound, or MRI, the technologist will document their COVID-19 vaccination status (first or second dose or booster), the date it was given, and the location. Adding vaccination documentation to intake forms helps to support appropriate management of patients who undergo imaging after COVID-19 vaccination. Six weeks is used as the cutoff point for defining “recent” vaccination.
For patients who are getting a screening mammography or MRI, and who have no symptoms beyond unilateral axillary adenopathy on the same side of the body where they received the COVID-19 vaccination (given in the arm) within a 6-week period, the following is included in the screening mammography or screening MRI report: “In the specific setting of a patient with documented recent (within the past 6 weeks) COVID-19 vaccination in the ipsilateral arm, axillary adenopathy is a benign imaging finding. No further imaging is indicated at this time. If there is clinical concern that persists more than 6 weeks after the patient received the final vaccine dose, axillary ultrasound is recommended.”
The experts interviewed reported no conflicts of interest.
COVID-19 hospital data: New-onset seizures more common than breakthrough seizures
An analysis of hospitalized patients with COVID-19 finds that those with no history of epilepsy had more than 3 times the odds of suffering a new-onset seizure than patients with epilepsy were to have breakthrough seizures (odds radio [OR] 3.15, P < .0001), researchers reported at the annual meeting of the American Epilepsy Society.
study lead author Neeraj Singh, MD, a neurologist at the New York-based Northwell Health system, said in an interview. “That’s new. We don’t normally see that when someone has a bacterial or viral infection. It’s demonstrating that this infection is having direct effect on the brain and brain signals.”
According to Dr. Singh, there’s little data about seizures in patients with COVID-19 because doctors have focused on other symptoms. A 2021 multicenter study found that electrographic seizures were detected in 9.6% of 197 patients with COVID-19 who were referred for cEEG.
For the new study, Dr. Singh and a colleague tracked 917 patients with COVID-19 in the Northwell Health system who were treated from Feb. 14 to June 14, 2020, with antiepileptic medication. Of the patients, 451 had a history of epilepsy, and 466 did not.
According to Dr. Singh, 27.6% of the patients without a history of epilepsy had new-onset seizures, while 10.1% of the patients with history of epilepsy had breakthrough seizures. The difference in odds was more than threefold after adjustment. (Among all COVID-19 patients, he said, perhaps 8%-16% had seizures).
The researchers also found that patients with new-onset seizures stayed in the hospital much longer (average, 26.9 days) than any patients with a known history of epilepsy (12.8 days, P < .0001, for those who had breakthrough seizures and 10.9 days, P < .0001, for those who didn’t).
In addition, the researchers found that having any seizures – new-onset or breakthrough – was linked to higher risk of death (OR 1.41, P = .03).
Antiseizure medications are key treatments for these patients, Dr. Singh said. As for the patients with new-onset seizures who recover from COVID-19, Dr. Singh said, “it’s suspected that these people are going to have a new diagnosis of epilepsy, not just a one-time seizure.”
The findings suggest that some patients with epilepsy are protected against COVID-19-related seizures because they take antiepileptic medications that “protect the brain from getting a trigger for an abnormal signal that leads to a seizure,” he said. “That’s one possibility.”
What can neurologists learn from the study? Dr. Singh recommends a “lower threshold” to recommend or approve EEGs in patients with COVID-19 who are confused/altered when they come in, especially if this is not normal. “They may actually be having silent seizures that no one’s noticing,” he said.
No study funding was reported. The authors reported no relevant disclosures.
An analysis of hospitalized patients with COVID-19 finds that those with no history of epilepsy had more than 3 times the odds of suffering a new-onset seizure than patients with epilepsy were to have breakthrough seizures (odds radio [OR] 3.15, P < .0001), researchers reported at the annual meeting of the American Epilepsy Society.
study lead author Neeraj Singh, MD, a neurologist at the New York-based Northwell Health system, said in an interview. “That’s new. We don’t normally see that when someone has a bacterial or viral infection. It’s demonstrating that this infection is having direct effect on the brain and brain signals.”
According to Dr. Singh, there’s little data about seizures in patients with COVID-19 because doctors have focused on other symptoms. A 2021 multicenter study found that electrographic seizures were detected in 9.6% of 197 patients with COVID-19 who were referred for cEEG.
For the new study, Dr. Singh and a colleague tracked 917 patients with COVID-19 in the Northwell Health system who were treated from Feb. 14 to June 14, 2020, with antiepileptic medication. Of the patients, 451 had a history of epilepsy, and 466 did not.
According to Dr. Singh, 27.6% of the patients without a history of epilepsy had new-onset seizures, while 10.1% of the patients with history of epilepsy had breakthrough seizures. The difference in odds was more than threefold after adjustment. (Among all COVID-19 patients, he said, perhaps 8%-16% had seizures).
The researchers also found that patients with new-onset seizures stayed in the hospital much longer (average, 26.9 days) than any patients with a known history of epilepsy (12.8 days, P < .0001, for those who had breakthrough seizures and 10.9 days, P < .0001, for those who didn’t).
In addition, the researchers found that having any seizures – new-onset or breakthrough – was linked to higher risk of death (OR 1.41, P = .03).
Antiseizure medications are key treatments for these patients, Dr. Singh said. As for the patients with new-onset seizures who recover from COVID-19, Dr. Singh said, “it’s suspected that these people are going to have a new diagnosis of epilepsy, not just a one-time seizure.”
The findings suggest that some patients with epilepsy are protected against COVID-19-related seizures because they take antiepileptic medications that “protect the brain from getting a trigger for an abnormal signal that leads to a seizure,” he said. “That’s one possibility.”
What can neurologists learn from the study? Dr. Singh recommends a “lower threshold” to recommend or approve EEGs in patients with COVID-19 who are confused/altered when they come in, especially if this is not normal. “They may actually be having silent seizures that no one’s noticing,” he said.
No study funding was reported. The authors reported no relevant disclosures.
An analysis of hospitalized patients with COVID-19 finds that those with no history of epilepsy had more than 3 times the odds of suffering a new-onset seizure than patients with epilepsy were to have breakthrough seizures (odds radio [OR] 3.15, P < .0001), researchers reported at the annual meeting of the American Epilepsy Society.
study lead author Neeraj Singh, MD, a neurologist at the New York-based Northwell Health system, said in an interview. “That’s new. We don’t normally see that when someone has a bacterial or viral infection. It’s demonstrating that this infection is having direct effect on the brain and brain signals.”
According to Dr. Singh, there’s little data about seizures in patients with COVID-19 because doctors have focused on other symptoms. A 2021 multicenter study found that electrographic seizures were detected in 9.6% of 197 patients with COVID-19 who were referred for cEEG.
For the new study, Dr. Singh and a colleague tracked 917 patients with COVID-19 in the Northwell Health system who were treated from Feb. 14 to June 14, 2020, with antiepileptic medication. Of the patients, 451 had a history of epilepsy, and 466 did not.
According to Dr. Singh, 27.6% of the patients without a history of epilepsy had new-onset seizures, while 10.1% of the patients with history of epilepsy had breakthrough seizures. The difference in odds was more than threefold after adjustment. (Among all COVID-19 patients, he said, perhaps 8%-16% had seizures).
The researchers also found that patients with new-onset seizures stayed in the hospital much longer (average, 26.9 days) than any patients with a known history of epilepsy (12.8 days, P < .0001, for those who had breakthrough seizures and 10.9 days, P < .0001, for those who didn’t).
In addition, the researchers found that having any seizures – new-onset or breakthrough – was linked to higher risk of death (OR 1.41, P = .03).
Antiseizure medications are key treatments for these patients, Dr. Singh said. As for the patients with new-onset seizures who recover from COVID-19, Dr. Singh said, “it’s suspected that these people are going to have a new diagnosis of epilepsy, not just a one-time seizure.”
The findings suggest that some patients with epilepsy are protected against COVID-19-related seizures because they take antiepileptic medications that “protect the brain from getting a trigger for an abnormal signal that leads to a seizure,” he said. “That’s one possibility.”
What can neurologists learn from the study? Dr. Singh recommends a “lower threshold” to recommend or approve EEGs in patients with COVID-19 who are confused/altered when they come in, especially if this is not normal. “They may actually be having silent seizures that no one’s noticing,” he said.
No study funding was reported. The authors reported no relevant disclosures.
FROM AES 2021