User login
FDA authorizes Pfizer antiviral pill for COVID-19
The Food and Drug Administration on Dec. 22, 2021, granted emergency use authorization (EUA) for a new antiviral pill to treat people with symptomatic COVID-19.
Pfizer’s ritonavir, name brand Paxlovid, can now be taken by patients ages 12 and up who weigh at least 88 pounds.
The antiviral is only for people who test positive for the coronavirus and who are at high risk for severe COVID-19, including hospitalization or death. It is available by prescription only and should be taken as soon as possible after diagnosis and within 5 days of the start of symptoms.
Paxlovid is taken as three tablets together orally twice a day for 5 days, for a total of 30 tablets.
Possible side effects include a reduced sense of taste, diarrhea, high blood pressure, and muscle aches.
The authorization arrives as U.S. cases of the Omicron variant are surging, some monoclonal antibody treatments are becoming less effective, and Americans struggle to maintain some sense of tradition and normalcy around the holidays.
Paxlovid joins remdesivir as an available antiviral to treat COVID-19. Remdesivir is fully approved by the FDA but is given only intravenously in a hospital.
The COVID-19 antiviral pills come with some obvious advantages, including greater convenience for consumers – such as home use – and the potential to expand treatment for people in low- and middle-income countries.
‘An exciting step forward’
The EUA for Pfizer’s new drug has been highly anticipated, and news of its impending authorization circulated on social media on Tuesday. Eric Topol, MD, called the development an “exciting step forward.” Dr. Topol is editor in chief of Medscape, the parent company of MDedge.
He and many others also expected the FDA to grant emergency use authorization for an antiviral from Merck. But there was no immediate word Wednesday if that was still going to happen.
An accelerated authorization?
The FDA’s authorization for Pfizer’s antiviral comes about 5 weeks after the company submitted an application to the agency. In its submission, the company said a study showed the pill reduced by 89% the rate of hospitalization and death for people with mild to moderate COVID-19 illness.
In April 2021, Pfizer announced its antiviral pill for COVID-19 could be available by year’s end. In September, an official at the National Institutes of Allergy and Infectious Diseases seconded the prediction.
Merck filed its EUA application with the FDA in October. The company included results of its phase 3 study showing the treatment was linked to a 50% reduction in COVID-19 hospitalizations.
Interestingly, in September, Merck announced the findings of laboratory studies suggesting that molnupiravir would work against variants of the coronavirus because the agent does not target the virus’s spike protein. At the time, Delta was the dominant variant in the United States.
Faith-based purchasing
The U.S. government has already recognized the potential of these oral therapies, at least in terms of preorders.
Last month, it announced intentions to purchase $1 billion worth of Merck’s molnupiravir, adding to the $1.2 billion worth of the pills the U.S. ordered in June 2021. Also in November, the government announced it would purchase 10 million courses of the Pfizer pill at an estimated cost of $5.3 billion.
The government preorders of the antiviral pills for COVID-19 are separate from the orders for COVID-19 vaccines. Most recently, the Biden administration announced it will make 500 million tests for coronavirus infection available to Americans for free in early 2022.
A version of this article first appeared on WebMD.com.
The Food and Drug Administration on Dec. 22, 2021, granted emergency use authorization (EUA) for a new antiviral pill to treat people with symptomatic COVID-19.
Pfizer’s ritonavir, name brand Paxlovid, can now be taken by patients ages 12 and up who weigh at least 88 pounds.
The antiviral is only for people who test positive for the coronavirus and who are at high risk for severe COVID-19, including hospitalization or death. It is available by prescription only and should be taken as soon as possible after diagnosis and within 5 days of the start of symptoms.
Paxlovid is taken as three tablets together orally twice a day for 5 days, for a total of 30 tablets.
Possible side effects include a reduced sense of taste, diarrhea, high blood pressure, and muscle aches.
The authorization arrives as U.S. cases of the Omicron variant are surging, some monoclonal antibody treatments are becoming less effective, and Americans struggle to maintain some sense of tradition and normalcy around the holidays.
Paxlovid joins remdesivir as an available antiviral to treat COVID-19. Remdesivir is fully approved by the FDA but is given only intravenously in a hospital.
The COVID-19 antiviral pills come with some obvious advantages, including greater convenience for consumers – such as home use – and the potential to expand treatment for people in low- and middle-income countries.
‘An exciting step forward’
The EUA for Pfizer’s new drug has been highly anticipated, and news of its impending authorization circulated on social media on Tuesday. Eric Topol, MD, called the development an “exciting step forward.” Dr. Topol is editor in chief of Medscape, the parent company of MDedge.
He and many others also expected the FDA to grant emergency use authorization for an antiviral from Merck. But there was no immediate word Wednesday if that was still going to happen.
An accelerated authorization?
The FDA’s authorization for Pfizer’s antiviral comes about 5 weeks after the company submitted an application to the agency. In its submission, the company said a study showed the pill reduced by 89% the rate of hospitalization and death for people with mild to moderate COVID-19 illness.
In April 2021, Pfizer announced its antiviral pill for COVID-19 could be available by year’s end. In September, an official at the National Institutes of Allergy and Infectious Diseases seconded the prediction.
Merck filed its EUA application with the FDA in October. The company included results of its phase 3 study showing the treatment was linked to a 50% reduction in COVID-19 hospitalizations.
Interestingly, in September, Merck announced the findings of laboratory studies suggesting that molnupiravir would work against variants of the coronavirus because the agent does not target the virus’s spike protein. At the time, Delta was the dominant variant in the United States.
Faith-based purchasing
The U.S. government has already recognized the potential of these oral therapies, at least in terms of preorders.
Last month, it announced intentions to purchase $1 billion worth of Merck’s molnupiravir, adding to the $1.2 billion worth of the pills the U.S. ordered in June 2021. Also in November, the government announced it would purchase 10 million courses of the Pfizer pill at an estimated cost of $5.3 billion.
The government preorders of the antiviral pills for COVID-19 are separate from the orders for COVID-19 vaccines. Most recently, the Biden administration announced it will make 500 million tests for coronavirus infection available to Americans for free in early 2022.
A version of this article first appeared on WebMD.com.
The Food and Drug Administration on Dec. 22, 2021, granted emergency use authorization (EUA) for a new antiviral pill to treat people with symptomatic COVID-19.
Pfizer’s ritonavir, name brand Paxlovid, can now be taken by patients ages 12 and up who weigh at least 88 pounds.
The antiviral is only for people who test positive for the coronavirus and who are at high risk for severe COVID-19, including hospitalization or death. It is available by prescription only and should be taken as soon as possible after diagnosis and within 5 days of the start of symptoms.
Paxlovid is taken as three tablets together orally twice a day for 5 days, for a total of 30 tablets.
Possible side effects include a reduced sense of taste, diarrhea, high blood pressure, and muscle aches.
The authorization arrives as U.S. cases of the Omicron variant are surging, some monoclonal antibody treatments are becoming less effective, and Americans struggle to maintain some sense of tradition and normalcy around the holidays.
Paxlovid joins remdesivir as an available antiviral to treat COVID-19. Remdesivir is fully approved by the FDA but is given only intravenously in a hospital.
The COVID-19 antiviral pills come with some obvious advantages, including greater convenience for consumers – such as home use – and the potential to expand treatment for people in low- and middle-income countries.
‘An exciting step forward’
The EUA for Pfizer’s new drug has been highly anticipated, and news of its impending authorization circulated on social media on Tuesday. Eric Topol, MD, called the development an “exciting step forward.” Dr. Topol is editor in chief of Medscape, the parent company of MDedge.
He and many others also expected the FDA to grant emergency use authorization for an antiviral from Merck. But there was no immediate word Wednesday if that was still going to happen.
An accelerated authorization?
The FDA’s authorization for Pfizer’s antiviral comes about 5 weeks after the company submitted an application to the agency. In its submission, the company said a study showed the pill reduced by 89% the rate of hospitalization and death for people with mild to moderate COVID-19 illness.
In April 2021, Pfizer announced its antiviral pill for COVID-19 could be available by year’s end. In September, an official at the National Institutes of Allergy and Infectious Diseases seconded the prediction.
Merck filed its EUA application with the FDA in October. The company included results of its phase 3 study showing the treatment was linked to a 50% reduction in COVID-19 hospitalizations.
Interestingly, in September, Merck announced the findings of laboratory studies suggesting that molnupiravir would work against variants of the coronavirus because the agent does not target the virus’s spike protein. At the time, Delta was the dominant variant in the United States.
Faith-based purchasing
The U.S. government has already recognized the potential of these oral therapies, at least in terms of preorders.
Last month, it announced intentions to purchase $1 billion worth of Merck’s molnupiravir, adding to the $1.2 billion worth of the pills the U.S. ordered in June 2021. Also in November, the government announced it would purchase 10 million courses of the Pfizer pill at an estimated cost of $5.3 billion.
The government preorders of the antiviral pills for COVID-19 are separate from the orders for COVID-19 vaccines. Most recently, the Biden administration announced it will make 500 million tests for coronavirus infection available to Americans for free in early 2022.
A version of this article first appeared on WebMD.com.
Pandemic poses short- and long-term risks to babies, especially boys
The pandemic has created a hostile environment for pregnant people and their babies.
Stress levels among expectant mothers have soared. Pregnant women with COVID are 5 times as likely as uninfected pregnant people to require intensive care and 22 times as likely to die. Infected moms are four times as likely to have a stillborn child.
Yet some of the pandemic’s greatest threats to infants’ health may not be apparent for years or even decades.
That’s because babies of COVID-infected moms are 60% more likely to be born very prematurely, which increases the danger of infant mortality and long-term disabilities such as cerebral palsy, asthma, and hearing loss, as well as a child’s risk of adult disease, including depression, anxiety, heart disease, and kidney disease.
Studies have linked fever and infection during pregnancy to developmental and psychiatric conditions such as autism, depression, and schizophrenia.
“Some of these conditions do not show up until middle childhood or early adult life, but they have their origins in fetal life,” said Evdokia Anagnostou, MD, a child neurologist at Holland Bloorview Kids Rehabilitation Hospital and a pediatrics professor at the University of Toronto.
For fetuses exposed to COVID, the greatest danger is usually not the coronavirus itself, but the mother’s immune system.
Both severe COVID infections and the strain of the pandemic can expose fetuses to harmful inflammation, which can occur when a mother’s immune system is fighting a virus or when stress hormones send nonstop alarm signals.
Prenatal inflammation “changes the way the brain develops and, depending on the timing of the infection, it can change the way the heart or kidneys develop,” Dr. Anagnostou said.
Although health officials have strongly recommended COVID vaccines for pregnant people, only 35% are fully vaccinated.
At least 150,000 pregnant women have been diagnosed with COVID; more than 25,000 of them have been hospitalized, and 249 have died, according to the Centers for Disease Control and Prevention.
Although most babies will be fine, even a small increase in the percentage of children with special medical or educational needs could have a large effect on the population, given the huge number of COVID infections, Dr. Anagnostou said.
“If someone has a baby who is doing well, that is what they should focus on,” Dr. Anagnostou said. “But from a public health point of view, we need to follow women who experienced severe COVID and their babies to understand the impact.”
Learning from history
Researchers in the United States and other countries are already studying “the COVID generation” to see whether these children have more health issues than those conceived or born before 2020.
Previous crises have shown that the challenges fetuses face in the womb – such as maternal infections, hunger, stress, and hormone-disrupting chemicals – can leave a lasting imprint on their health, as well as that of their children and grandchildren, said Frederick Kaskel, MD, director of pediatric nephrology at the Children’s Hospital at Montefiore, New York.
People whose mothers were pregnant during surges in the 1918 influenza pandemic, for example, had poorer health throughout their lives, compared with Americans born at other times, said John McCarthy, who is a medical student at Albert Einstein College of Medicine, New York, and cowrote a recent review in JAMA Pediatrics with Dr. Kaskel.
Researchers don’t know exactly which moms were infected with pandemic flu, Mr. McCarthy said. But women who were pregnant during major surges – when infection was widespread – had children with higher rates of heart disease or diabetes. These children were also less successful in school, less economically productive, and more likely to live with a disability.
Because organ systems develop during different periods of pregnancy, fetuses exposed during the first trimester may face different risks than those exposed toward the end of pregnancy, Mr. McCarthy said. For example, people born in the fall of 1918 were 50% more likely than others to develop kidney disease; that may reflect an exposure to the pandemic in the third trimester, while the kidneys were still developing.
Nearly 2 years into the COVID pandemic, researchers have begun to publish preliminary observations of infants exposed to COVID infections and stress before birth.
Although Dr. Anagnostou noted that it’s too early to reach definitive conclusions, “there is evidence that babies born to moms with severe COVID infections have changes to their immune system,” she said. “It’s enough to make us worry a little bit.”
Damaging a fetal security system
The good news about the coronavirus is that it seldom crosses the placenta, the organ tasked with protecting a developing fetus from infections and providing it with oxygen. So moms with COVID rarely give the virus to their children before birth.
That’s important, because some viruses that directly infect the fetus – such as Zika – can cause devastating birth defects, said Karin Nielsen-Saines, MD, a specialist in pediatric infectious diseases at University of California, Los Angeles.
But studies also suggest that inflammation from a mother’s COVID infection can injure the placenta, said Jeffery Goldstein, MD, an assistant professor of pathology at Northwestern University, Chicago. In a study published in American Journal of Clinical Pathology , Dr. Goldstein and his coauthors found that placentas from COVID-infected moms had more abnormal blood vessels than placentas from patients without COVID, making it harder for them to deliver sufficient oxygen to the fetus.
Placental damage can also lead to preeclampsia, a serious complication of pregnancy that can cause a mother’s blood pressure to spike.
Preeclampsia occurs when blood vessels in the placenta don’t develop or function properly, forcing the mother’s heart to work harder to get blood to the fetus, which may not receive enough oxygen and nutrients. Preeclampsia also predisposes women to heart attacks and strokes later in life.
Rewiring the immune system
In some cases, COVID also appears to rewire a baby’s immune response, Dr. Nielsen-Saines said.
In an October study in the journal Cell Reports Medicine, Dr. Nielsen-Saines and her coauthors found that infants born to people with severe COVID infections had a different mix of immune cells and proteins than other babies. None of the newborns tested positive for the coronavirus.
The immune changes are concerning, Dr. Nielsen-Saines said, because this pattern of immune cells and proteins has previously been found in infants with respiratory problems and in some cases poor neurodevelopment.
Notably, all the babies in her study appear healthy, said Dr. Nielsen-Saines, who plans to follow them for 3 years to see whether these early signals translate into developmental delays, such as problems talking, walking, or interacting with others.
“How big of a difference does any of this make in the baby?” asked Dr. Anagnostou. “We won’t know for a few years. All we can do is try to be as prepared as possible.”
Increasing the risk for boys
Boys could face higher risks from COVID, even before birth.
Males are generally more vulnerable than females as fetuses and newborns; they’re more likely to be born prematurely and to die as infants. Preterm boys also have a higher risk of disability and death.
But coronavirus infection poses special dangers, said Sabra Klein, PhD, a professor of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health, Baltimore.
That’s because boys are disproportionately affected by conditions linked to maternal infections. Boys are four times as likely as girls to be diagnosed with autism or attention-deficit/hyperactivity disorder, for example, while men are 75% more likely than women to develop schizophrenia.
Scientists don’t fully understand why boys appear more fragile in the womb, although testosterone – which can dampen immune response – may play a role, said Kristina Adams Waldorf, MD, a professor of obstetrics and gynecology at the University of Washington.
Men generally mount weaker immune responses than women and more often develop severe COVID infections. Recent research suggests boys with COVID are more likely than girls to become seriously ill or develop a rare inflammatory condition called multisystem inflammatory syndrome.
New research on COVID could help illuminate this vulnerability.
In a study published in October, researchers found that the sex of a fetus influences the way its placenta responds to COVID, as well as how its mother’s immune system responds.
Pregnant people infected with COVID made fewer antibodies against the coronavirus if they were carrying male fetuses than if they were carrying females. Mothers also transferred fewer antibodies to boys than to girls, said Andrea Edlow, MD, senior author of the study and a maternal-fetal medicine specialist at Massachusetts General Hospital, Boston.
When examining the placentas of male fetuses after delivery, researchers found changes that could leave boys less protected against damaging inflammation.
The sex of a fetus can influence its mother’s response to other illnesses, as well.
For example, research shows that pregnant women with asthma have worse symptoms if they’re carrying a female. Women carrying males are slightly more likely to develop gestational diabetes.
Dr. Edlow said her findings raise questions about the “cross talk” between mother and baby. “The mom’s immune system is sensing there is a male fetus,” Dr. Edlow said. “And the fetus is actively communicating with the mom’s immune system.”
Boosting toxic stress
Rates of depression and stress among pregnant women have increased dramatically during the pandemic.
That’s concerning because chronic stress can lead to inflammation, affecting the babies of both infected and uninfected women, Dr. Anagnostou said.
Studies consistently show that infants born to mothers who experience significant stress during pregnancy have higher rates of short- and long-term health damage – including heart defects and obesity – than babies born to women with less stress.
“We know that inflammation directly influences the way a baby’s brain develops,” said Elinor Sullivan, PhD, an associate professor in psychiatry at Oregon Health & Science University, Portland.
Lockdowns, travel restrictions and physical distancing left many pregnant women without the support of family and friends. The stress of losing a loved one, a job, or a home further heightens the risks to moms and babies, said Dr. Sullivan, who is following children born during the pandemic for 5 years.
In research that has not yet been published, Dr. Sullivan found that babies of women who were pregnant during the pandemic showed more sadness and negative emotions in the first year of life, compared with infants of women who were pregnant before the pandemic.
The findings show the importance of helping and protecting pregnant people before and after delivery, said Dr. Sullivan, who conducted a separate study that found women who received more social support were less depressed.
Italian researchers are also studying the effect of maternal stress on infants’ behavior, as well as the way their genes are regulated.
Although stress-related inflammation doesn’t alter the structure of a baby’s genes, it can influence whether they’re turned on and off, said Livio Provenzi, PhD, a psychologist at the C. Mondino National Institute of Neurology Foundation in Pavia, Italy.
In Dr. Provenzi’s study of 163 mother-baby pairs he found differences in how genes that regulate the stress response were activated. Genes that help people respond to stress were more likely to be turned off in babies whose moms reported the most stress during pregnancy. The same moms also reported that their babies cried more and were fussier when they were 3 months old.
Researchers usually prefer to make in-person observations of babies as they interact with their mothers, Dr. Provenzi said. But because of the pandemic, Dr. Provenzi asked mothers to fill out questionnaires about infant behavior. He plans to observe mothers and babies in person when the children are 12 months old.
While vaccinating pregnant people is the best way to protect them and their fetuses from the virus, Dr. Anagnostou said, society needs to do more to preserve expectant mothers’ mental health.
“We can’t escape the fact that we’ve lived through 2 years of a pandemic,” Dr. Anagnostou said. “But we can think about opportunities for reducing the risk.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The pandemic has created a hostile environment for pregnant people and their babies.
Stress levels among expectant mothers have soared. Pregnant women with COVID are 5 times as likely as uninfected pregnant people to require intensive care and 22 times as likely to die. Infected moms are four times as likely to have a stillborn child.
Yet some of the pandemic’s greatest threats to infants’ health may not be apparent for years or even decades.
That’s because babies of COVID-infected moms are 60% more likely to be born very prematurely, which increases the danger of infant mortality and long-term disabilities such as cerebral palsy, asthma, and hearing loss, as well as a child’s risk of adult disease, including depression, anxiety, heart disease, and kidney disease.
Studies have linked fever and infection during pregnancy to developmental and psychiatric conditions such as autism, depression, and schizophrenia.
“Some of these conditions do not show up until middle childhood or early adult life, but they have their origins in fetal life,” said Evdokia Anagnostou, MD, a child neurologist at Holland Bloorview Kids Rehabilitation Hospital and a pediatrics professor at the University of Toronto.
For fetuses exposed to COVID, the greatest danger is usually not the coronavirus itself, but the mother’s immune system.
Both severe COVID infections and the strain of the pandemic can expose fetuses to harmful inflammation, which can occur when a mother’s immune system is fighting a virus or when stress hormones send nonstop alarm signals.
Prenatal inflammation “changes the way the brain develops and, depending on the timing of the infection, it can change the way the heart or kidneys develop,” Dr. Anagnostou said.
Although health officials have strongly recommended COVID vaccines for pregnant people, only 35% are fully vaccinated.
At least 150,000 pregnant women have been diagnosed with COVID; more than 25,000 of them have been hospitalized, and 249 have died, according to the Centers for Disease Control and Prevention.
Although most babies will be fine, even a small increase in the percentage of children with special medical or educational needs could have a large effect on the population, given the huge number of COVID infections, Dr. Anagnostou said.
“If someone has a baby who is doing well, that is what they should focus on,” Dr. Anagnostou said. “But from a public health point of view, we need to follow women who experienced severe COVID and their babies to understand the impact.”
Learning from history
Researchers in the United States and other countries are already studying “the COVID generation” to see whether these children have more health issues than those conceived or born before 2020.
Previous crises have shown that the challenges fetuses face in the womb – such as maternal infections, hunger, stress, and hormone-disrupting chemicals – can leave a lasting imprint on their health, as well as that of their children and grandchildren, said Frederick Kaskel, MD, director of pediatric nephrology at the Children’s Hospital at Montefiore, New York.
People whose mothers were pregnant during surges in the 1918 influenza pandemic, for example, had poorer health throughout their lives, compared with Americans born at other times, said John McCarthy, who is a medical student at Albert Einstein College of Medicine, New York, and cowrote a recent review in JAMA Pediatrics with Dr. Kaskel.
Researchers don’t know exactly which moms were infected with pandemic flu, Mr. McCarthy said. But women who were pregnant during major surges – when infection was widespread – had children with higher rates of heart disease or diabetes. These children were also less successful in school, less economically productive, and more likely to live with a disability.
Because organ systems develop during different periods of pregnancy, fetuses exposed during the first trimester may face different risks than those exposed toward the end of pregnancy, Mr. McCarthy said. For example, people born in the fall of 1918 were 50% more likely than others to develop kidney disease; that may reflect an exposure to the pandemic in the third trimester, while the kidneys were still developing.
Nearly 2 years into the COVID pandemic, researchers have begun to publish preliminary observations of infants exposed to COVID infections and stress before birth.
Although Dr. Anagnostou noted that it’s too early to reach definitive conclusions, “there is evidence that babies born to moms with severe COVID infections have changes to their immune system,” she said. “It’s enough to make us worry a little bit.”
Damaging a fetal security system
The good news about the coronavirus is that it seldom crosses the placenta, the organ tasked with protecting a developing fetus from infections and providing it with oxygen. So moms with COVID rarely give the virus to their children before birth.
That’s important, because some viruses that directly infect the fetus – such as Zika – can cause devastating birth defects, said Karin Nielsen-Saines, MD, a specialist in pediatric infectious diseases at University of California, Los Angeles.
But studies also suggest that inflammation from a mother’s COVID infection can injure the placenta, said Jeffery Goldstein, MD, an assistant professor of pathology at Northwestern University, Chicago. In a study published in American Journal of Clinical Pathology , Dr. Goldstein and his coauthors found that placentas from COVID-infected moms had more abnormal blood vessels than placentas from patients without COVID, making it harder for them to deliver sufficient oxygen to the fetus.
Placental damage can also lead to preeclampsia, a serious complication of pregnancy that can cause a mother’s blood pressure to spike.
Preeclampsia occurs when blood vessels in the placenta don’t develop or function properly, forcing the mother’s heart to work harder to get blood to the fetus, which may not receive enough oxygen and nutrients. Preeclampsia also predisposes women to heart attacks and strokes later in life.
Rewiring the immune system
In some cases, COVID also appears to rewire a baby’s immune response, Dr. Nielsen-Saines said.
In an October study in the journal Cell Reports Medicine, Dr. Nielsen-Saines and her coauthors found that infants born to people with severe COVID infections had a different mix of immune cells and proteins than other babies. None of the newborns tested positive for the coronavirus.
The immune changes are concerning, Dr. Nielsen-Saines said, because this pattern of immune cells and proteins has previously been found in infants with respiratory problems and in some cases poor neurodevelopment.
Notably, all the babies in her study appear healthy, said Dr. Nielsen-Saines, who plans to follow them for 3 years to see whether these early signals translate into developmental delays, such as problems talking, walking, or interacting with others.
“How big of a difference does any of this make in the baby?” asked Dr. Anagnostou. “We won’t know for a few years. All we can do is try to be as prepared as possible.”
Increasing the risk for boys
Boys could face higher risks from COVID, even before birth.
Males are generally more vulnerable than females as fetuses and newborns; they’re more likely to be born prematurely and to die as infants. Preterm boys also have a higher risk of disability and death.
But coronavirus infection poses special dangers, said Sabra Klein, PhD, a professor of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health, Baltimore.
That’s because boys are disproportionately affected by conditions linked to maternal infections. Boys are four times as likely as girls to be diagnosed with autism or attention-deficit/hyperactivity disorder, for example, while men are 75% more likely than women to develop schizophrenia.
Scientists don’t fully understand why boys appear more fragile in the womb, although testosterone – which can dampen immune response – may play a role, said Kristina Adams Waldorf, MD, a professor of obstetrics and gynecology at the University of Washington.
Men generally mount weaker immune responses than women and more often develop severe COVID infections. Recent research suggests boys with COVID are more likely than girls to become seriously ill or develop a rare inflammatory condition called multisystem inflammatory syndrome.
New research on COVID could help illuminate this vulnerability.
In a study published in October, researchers found that the sex of a fetus influences the way its placenta responds to COVID, as well as how its mother’s immune system responds.
Pregnant people infected with COVID made fewer antibodies against the coronavirus if they were carrying male fetuses than if they were carrying females. Mothers also transferred fewer antibodies to boys than to girls, said Andrea Edlow, MD, senior author of the study and a maternal-fetal medicine specialist at Massachusetts General Hospital, Boston.
When examining the placentas of male fetuses after delivery, researchers found changes that could leave boys less protected against damaging inflammation.
The sex of a fetus can influence its mother’s response to other illnesses, as well.
For example, research shows that pregnant women with asthma have worse symptoms if they’re carrying a female. Women carrying males are slightly more likely to develop gestational diabetes.
Dr. Edlow said her findings raise questions about the “cross talk” between mother and baby. “The mom’s immune system is sensing there is a male fetus,” Dr. Edlow said. “And the fetus is actively communicating with the mom’s immune system.”
Boosting toxic stress
Rates of depression and stress among pregnant women have increased dramatically during the pandemic.
That’s concerning because chronic stress can lead to inflammation, affecting the babies of both infected and uninfected women, Dr. Anagnostou said.
Studies consistently show that infants born to mothers who experience significant stress during pregnancy have higher rates of short- and long-term health damage – including heart defects and obesity – than babies born to women with less stress.
“We know that inflammation directly influences the way a baby’s brain develops,” said Elinor Sullivan, PhD, an associate professor in psychiatry at Oregon Health & Science University, Portland.
Lockdowns, travel restrictions and physical distancing left many pregnant women without the support of family and friends. The stress of losing a loved one, a job, or a home further heightens the risks to moms and babies, said Dr. Sullivan, who is following children born during the pandemic for 5 years.
In research that has not yet been published, Dr. Sullivan found that babies of women who were pregnant during the pandemic showed more sadness and negative emotions in the first year of life, compared with infants of women who were pregnant before the pandemic.
The findings show the importance of helping and protecting pregnant people before and after delivery, said Dr. Sullivan, who conducted a separate study that found women who received more social support were less depressed.
Italian researchers are also studying the effect of maternal stress on infants’ behavior, as well as the way their genes are regulated.
Although stress-related inflammation doesn’t alter the structure of a baby’s genes, it can influence whether they’re turned on and off, said Livio Provenzi, PhD, a psychologist at the C. Mondino National Institute of Neurology Foundation in Pavia, Italy.
In Dr. Provenzi’s study of 163 mother-baby pairs he found differences in how genes that regulate the stress response were activated. Genes that help people respond to stress were more likely to be turned off in babies whose moms reported the most stress during pregnancy. The same moms also reported that their babies cried more and were fussier when they were 3 months old.
Researchers usually prefer to make in-person observations of babies as they interact with their mothers, Dr. Provenzi said. But because of the pandemic, Dr. Provenzi asked mothers to fill out questionnaires about infant behavior. He plans to observe mothers and babies in person when the children are 12 months old.
While vaccinating pregnant people is the best way to protect them and their fetuses from the virus, Dr. Anagnostou said, society needs to do more to preserve expectant mothers’ mental health.
“We can’t escape the fact that we’ve lived through 2 years of a pandemic,” Dr. Anagnostou said. “But we can think about opportunities for reducing the risk.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The pandemic has created a hostile environment for pregnant people and their babies.
Stress levels among expectant mothers have soared. Pregnant women with COVID are 5 times as likely as uninfected pregnant people to require intensive care and 22 times as likely to die. Infected moms are four times as likely to have a stillborn child.
Yet some of the pandemic’s greatest threats to infants’ health may not be apparent for years or even decades.
That’s because babies of COVID-infected moms are 60% more likely to be born very prematurely, which increases the danger of infant mortality and long-term disabilities such as cerebral palsy, asthma, and hearing loss, as well as a child’s risk of adult disease, including depression, anxiety, heart disease, and kidney disease.
Studies have linked fever and infection during pregnancy to developmental and psychiatric conditions such as autism, depression, and schizophrenia.
“Some of these conditions do not show up until middle childhood or early adult life, but they have their origins in fetal life,” said Evdokia Anagnostou, MD, a child neurologist at Holland Bloorview Kids Rehabilitation Hospital and a pediatrics professor at the University of Toronto.
For fetuses exposed to COVID, the greatest danger is usually not the coronavirus itself, but the mother’s immune system.
Both severe COVID infections and the strain of the pandemic can expose fetuses to harmful inflammation, which can occur when a mother’s immune system is fighting a virus or when stress hormones send nonstop alarm signals.
Prenatal inflammation “changes the way the brain develops and, depending on the timing of the infection, it can change the way the heart or kidneys develop,” Dr. Anagnostou said.
Although health officials have strongly recommended COVID vaccines for pregnant people, only 35% are fully vaccinated.
At least 150,000 pregnant women have been diagnosed with COVID; more than 25,000 of them have been hospitalized, and 249 have died, according to the Centers for Disease Control and Prevention.
Although most babies will be fine, even a small increase in the percentage of children with special medical or educational needs could have a large effect on the population, given the huge number of COVID infections, Dr. Anagnostou said.
“If someone has a baby who is doing well, that is what they should focus on,” Dr. Anagnostou said. “But from a public health point of view, we need to follow women who experienced severe COVID and their babies to understand the impact.”
Learning from history
Researchers in the United States and other countries are already studying “the COVID generation” to see whether these children have more health issues than those conceived or born before 2020.
Previous crises have shown that the challenges fetuses face in the womb – such as maternal infections, hunger, stress, and hormone-disrupting chemicals – can leave a lasting imprint on their health, as well as that of their children and grandchildren, said Frederick Kaskel, MD, director of pediatric nephrology at the Children’s Hospital at Montefiore, New York.
People whose mothers were pregnant during surges in the 1918 influenza pandemic, for example, had poorer health throughout their lives, compared with Americans born at other times, said John McCarthy, who is a medical student at Albert Einstein College of Medicine, New York, and cowrote a recent review in JAMA Pediatrics with Dr. Kaskel.
Researchers don’t know exactly which moms were infected with pandemic flu, Mr. McCarthy said. But women who were pregnant during major surges – when infection was widespread – had children with higher rates of heart disease or diabetes. These children were also less successful in school, less economically productive, and more likely to live with a disability.
Because organ systems develop during different periods of pregnancy, fetuses exposed during the first trimester may face different risks than those exposed toward the end of pregnancy, Mr. McCarthy said. For example, people born in the fall of 1918 were 50% more likely than others to develop kidney disease; that may reflect an exposure to the pandemic in the third trimester, while the kidneys were still developing.
Nearly 2 years into the COVID pandemic, researchers have begun to publish preliminary observations of infants exposed to COVID infections and stress before birth.
Although Dr. Anagnostou noted that it’s too early to reach definitive conclusions, “there is evidence that babies born to moms with severe COVID infections have changes to their immune system,” she said. “It’s enough to make us worry a little bit.”
Damaging a fetal security system
The good news about the coronavirus is that it seldom crosses the placenta, the organ tasked with protecting a developing fetus from infections and providing it with oxygen. So moms with COVID rarely give the virus to their children before birth.
That’s important, because some viruses that directly infect the fetus – such as Zika – can cause devastating birth defects, said Karin Nielsen-Saines, MD, a specialist in pediatric infectious diseases at University of California, Los Angeles.
But studies also suggest that inflammation from a mother’s COVID infection can injure the placenta, said Jeffery Goldstein, MD, an assistant professor of pathology at Northwestern University, Chicago. In a study published in American Journal of Clinical Pathology , Dr. Goldstein and his coauthors found that placentas from COVID-infected moms had more abnormal blood vessels than placentas from patients without COVID, making it harder for them to deliver sufficient oxygen to the fetus.
Placental damage can also lead to preeclampsia, a serious complication of pregnancy that can cause a mother’s blood pressure to spike.
Preeclampsia occurs when blood vessels in the placenta don’t develop or function properly, forcing the mother’s heart to work harder to get blood to the fetus, which may not receive enough oxygen and nutrients. Preeclampsia also predisposes women to heart attacks and strokes later in life.
Rewiring the immune system
In some cases, COVID also appears to rewire a baby’s immune response, Dr. Nielsen-Saines said.
In an October study in the journal Cell Reports Medicine, Dr. Nielsen-Saines and her coauthors found that infants born to people with severe COVID infections had a different mix of immune cells and proteins than other babies. None of the newborns tested positive for the coronavirus.
The immune changes are concerning, Dr. Nielsen-Saines said, because this pattern of immune cells and proteins has previously been found in infants with respiratory problems and in some cases poor neurodevelopment.
Notably, all the babies in her study appear healthy, said Dr. Nielsen-Saines, who plans to follow them for 3 years to see whether these early signals translate into developmental delays, such as problems talking, walking, or interacting with others.
“How big of a difference does any of this make in the baby?” asked Dr. Anagnostou. “We won’t know for a few years. All we can do is try to be as prepared as possible.”
Increasing the risk for boys
Boys could face higher risks from COVID, even before birth.
Males are generally more vulnerable than females as fetuses and newborns; they’re more likely to be born prematurely and to die as infants. Preterm boys also have a higher risk of disability and death.
But coronavirus infection poses special dangers, said Sabra Klein, PhD, a professor of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health, Baltimore.
That’s because boys are disproportionately affected by conditions linked to maternal infections. Boys are four times as likely as girls to be diagnosed with autism or attention-deficit/hyperactivity disorder, for example, while men are 75% more likely than women to develop schizophrenia.
Scientists don’t fully understand why boys appear more fragile in the womb, although testosterone – which can dampen immune response – may play a role, said Kristina Adams Waldorf, MD, a professor of obstetrics and gynecology at the University of Washington.
Men generally mount weaker immune responses than women and more often develop severe COVID infections. Recent research suggests boys with COVID are more likely than girls to become seriously ill or develop a rare inflammatory condition called multisystem inflammatory syndrome.
New research on COVID could help illuminate this vulnerability.
In a study published in October, researchers found that the sex of a fetus influences the way its placenta responds to COVID, as well as how its mother’s immune system responds.
Pregnant people infected with COVID made fewer antibodies against the coronavirus if they were carrying male fetuses than if they were carrying females. Mothers also transferred fewer antibodies to boys than to girls, said Andrea Edlow, MD, senior author of the study and a maternal-fetal medicine specialist at Massachusetts General Hospital, Boston.
When examining the placentas of male fetuses after delivery, researchers found changes that could leave boys less protected against damaging inflammation.
The sex of a fetus can influence its mother’s response to other illnesses, as well.
For example, research shows that pregnant women with asthma have worse symptoms if they’re carrying a female. Women carrying males are slightly more likely to develop gestational diabetes.
Dr. Edlow said her findings raise questions about the “cross talk” between mother and baby. “The mom’s immune system is sensing there is a male fetus,” Dr. Edlow said. “And the fetus is actively communicating with the mom’s immune system.”
Boosting toxic stress
Rates of depression and stress among pregnant women have increased dramatically during the pandemic.
That’s concerning because chronic stress can lead to inflammation, affecting the babies of both infected and uninfected women, Dr. Anagnostou said.
Studies consistently show that infants born to mothers who experience significant stress during pregnancy have higher rates of short- and long-term health damage – including heart defects and obesity – than babies born to women with less stress.
“We know that inflammation directly influences the way a baby’s brain develops,” said Elinor Sullivan, PhD, an associate professor in psychiatry at Oregon Health & Science University, Portland.
Lockdowns, travel restrictions and physical distancing left many pregnant women without the support of family and friends. The stress of losing a loved one, a job, or a home further heightens the risks to moms and babies, said Dr. Sullivan, who is following children born during the pandemic for 5 years.
In research that has not yet been published, Dr. Sullivan found that babies of women who were pregnant during the pandemic showed more sadness and negative emotions in the first year of life, compared with infants of women who were pregnant before the pandemic.
The findings show the importance of helping and protecting pregnant people before and after delivery, said Dr. Sullivan, who conducted a separate study that found women who received more social support were less depressed.
Italian researchers are also studying the effect of maternal stress on infants’ behavior, as well as the way their genes are regulated.
Although stress-related inflammation doesn’t alter the structure of a baby’s genes, it can influence whether they’re turned on and off, said Livio Provenzi, PhD, a psychologist at the C. Mondino National Institute of Neurology Foundation in Pavia, Italy.
In Dr. Provenzi’s study of 163 mother-baby pairs he found differences in how genes that regulate the stress response were activated. Genes that help people respond to stress were more likely to be turned off in babies whose moms reported the most stress during pregnancy. The same moms also reported that their babies cried more and were fussier when they were 3 months old.
Researchers usually prefer to make in-person observations of babies as they interact with their mothers, Dr. Provenzi said. But because of the pandemic, Dr. Provenzi asked mothers to fill out questionnaires about infant behavior. He plans to observe mothers and babies in person when the children are 12 months old.
While vaccinating pregnant people is the best way to protect them and their fetuses from the virus, Dr. Anagnostou said, society needs to do more to preserve expectant mothers’ mental health.
“We can’t escape the fact that we’ve lived through 2 years of a pandemic,” Dr. Anagnostou said. “But we can think about opportunities for reducing the risk.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
GI symptoms in kids with COVID may predict severe outcomes
Severe gastrointestinal involvement can be common in children who have had COVID-19, a new study shows.
Andrea Lo Veccio, MD, PhD, with the department of translational medical sciences, section of pediatrics, University of Naples (Italy) Federico II, and colleagues retrospectively analyzed data from a large cohort of children aged 18 years and younger who had been diagnosed with COVID-19 between Feb. 25, 2020, and Jan. 20, 2021, in 54 Italian institutions.
Overall, 685 Italian children (56.4% boys; average age, 7 years) were included in the study. Of these, 628 (91.7%) were diagnosed with acute SARS-CoV-2 infection and 57 (8.3%) with multisystem inflammatory syndrome in children (MIS-C).
When children had GI symptoms, the authors found a higher risk of hospitalization (odds ratio, 2.64; 95% confidence interval, 1.89-3.69) and nearly four times the risk of ICU admission (OR, 3.90; 95% CI, 1.98-7.68).
Severe GI involvement occurred in 65 children (9.5%). The authors included the following within that category: disseminated adenomesenteritis (39.6%), appendicitis (33.5%), abdominal fluid collection (21.3%), pancreatitis (6.9%), or intussusception (4.6%). Additionally, out of these 65 children, 27 (41.5%) underwent surgery.
Older children were much more likely than preschoolers to have severe GI symptoms. Children aged 5-10 years were eight times more likely than preschoolers to show severe symptoms (OR, 8.33; 95% CI, 2.62-26.5). In those older than age 10 years, severe symptoms were six times more likely (OR, 6.37; 95% CI, 2.12-19.1).
Awareness about its frequency and presentation may help practitioners to appropriately manage children at risk of severe outcomes, the authors wrote.
The findings of this study were published online Dec. 20 in JAMA Network Open.
Study highlights the GI link
Reached for comment, William Balistreri, MD, with the division of gastroenterology, hepatology, and nutrition at Cincinnati Children’s Hospital Medical Center, said that it has been known that children are more likely than adults to present with GI symptoms, and also that these symptoms are especially common in children with MIS-C.
“The symptoms most commonly cited in the literature to date include diarrhea, nausea, vomiting, or abdominal pain,” he said. “What [has not been known] is the frequency, predictive markers, and clinical course of the severe GI manifestations of COVID-19.”
The findings of this study are important to clinicians to help recognize the potential for severe GI involvement, Dr. Balistreri said, adding that “the occurrence of abdominal pain, leukopenia, and elevated inflammatory markers or MIS-C should raise suspicion and lead to early evaluation.”
Margaret E. Thew, APNP, medical director in adolescent medicine and a family nurse practitioner with Medical College of Wisconsin, Milwaukee, said that news reports typically emphasize the respiratory involvement, but this study provides a detailed analysis of the link between GI symptoms and COVID-19.
“Their data show that there may be less respiratory illness with children, regardless of whether they are generally healthy kids,” she said. “They may have more GI symptoms.
“We know that COVID-19 causes a lot of inflammation, and a large percentage of these kids had inflammation in their stomach or an inflamed bowel,” she added.
Dr. Thew said primary care doctors and urgent and emergency care clinicians will benefit from the findings of this study and should be on alert when kids come in with belly pain or vomiting.
Parents will benefit too, she said, if they are waiting for respiratory illness before they suspect COVID.
“You have to have a high suspicion this is going to be COVID positive,” she said. “You have to have that as part of your thought process.”
Though the study was done in Italy, Dr. Thew added that their experiences mimic those she’s seen locally.
Dr. Lo Vecchio reported receiving fees from Pfizer as an advisory board member outside the submitted work. A coauthor reported speaker’s fees from Angelini, Sobi, and X4 Pharma outside the submitted work. No other disclosures were reported. Dr. Balistreri and Dr. Thew reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Severe gastrointestinal involvement can be common in children who have had COVID-19, a new study shows.
Andrea Lo Veccio, MD, PhD, with the department of translational medical sciences, section of pediatrics, University of Naples (Italy) Federico II, and colleagues retrospectively analyzed data from a large cohort of children aged 18 years and younger who had been diagnosed with COVID-19 between Feb. 25, 2020, and Jan. 20, 2021, in 54 Italian institutions.
Overall, 685 Italian children (56.4% boys; average age, 7 years) were included in the study. Of these, 628 (91.7%) were diagnosed with acute SARS-CoV-2 infection and 57 (8.3%) with multisystem inflammatory syndrome in children (MIS-C).
When children had GI symptoms, the authors found a higher risk of hospitalization (odds ratio, 2.64; 95% confidence interval, 1.89-3.69) and nearly four times the risk of ICU admission (OR, 3.90; 95% CI, 1.98-7.68).
Severe GI involvement occurred in 65 children (9.5%). The authors included the following within that category: disseminated adenomesenteritis (39.6%), appendicitis (33.5%), abdominal fluid collection (21.3%), pancreatitis (6.9%), or intussusception (4.6%). Additionally, out of these 65 children, 27 (41.5%) underwent surgery.
Older children were much more likely than preschoolers to have severe GI symptoms. Children aged 5-10 years were eight times more likely than preschoolers to show severe symptoms (OR, 8.33; 95% CI, 2.62-26.5). In those older than age 10 years, severe symptoms were six times more likely (OR, 6.37; 95% CI, 2.12-19.1).
Awareness about its frequency and presentation may help practitioners to appropriately manage children at risk of severe outcomes, the authors wrote.
The findings of this study were published online Dec. 20 in JAMA Network Open.
Study highlights the GI link
Reached for comment, William Balistreri, MD, with the division of gastroenterology, hepatology, and nutrition at Cincinnati Children’s Hospital Medical Center, said that it has been known that children are more likely than adults to present with GI symptoms, and also that these symptoms are especially common in children with MIS-C.
“The symptoms most commonly cited in the literature to date include diarrhea, nausea, vomiting, or abdominal pain,” he said. “What [has not been known] is the frequency, predictive markers, and clinical course of the severe GI manifestations of COVID-19.”
The findings of this study are important to clinicians to help recognize the potential for severe GI involvement, Dr. Balistreri said, adding that “the occurrence of abdominal pain, leukopenia, and elevated inflammatory markers or MIS-C should raise suspicion and lead to early evaluation.”
Margaret E. Thew, APNP, medical director in adolescent medicine and a family nurse practitioner with Medical College of Wisconsin, Milwaukee, said that news reports typically emphasize the respiratory involvement, but this study provides a detailed analysis of the link between GI symptoms and COVID-19.
“Their data show that there may be less respiratory illness with children, regardless of whether they are generally healthy kids,” she said. “They may have more GI symptoms.
“We know that COVID-19 causes a lot of inflammation, and a large percentage of these kids had inflammation in their stomach or an inflamed bowel,” she added.
Dr. Thew said primary care doctors and urgent and emergency care clinicians will benefit from the findings of this study and should be on alert when kids come in with belly pain or vomiting.
Parents will benefit too, she said, if they are waiting for respiratory illness before they suspect COVID.
“You have to have a high suspicion this is going to be COVID positive,” she said. “You have to have that as part of your thought process.”
Though the study was done in Italy, Dr. Thew added that their experiences mimic those she’s seen locally.
Dr. Lo Vecchio reported receiving fees from Pfizer as an advisory board member outside the submitted work. A coauthor reported speaker’s fees from Angelini, Sobi, and X4 Pharma outside the submitted work. No other disclosures were reported. Dr. Balistreri and Dr. Thew reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Severe gastrointestinal involvement can be common in children who have had COVID-19, a new study shows.
Andrea Lo Veccio, MD, PhD, with the department of translational medical sciences, section of pediatrics, University of Naples (Italy) Federico II, and colleagues retrospectively analyzed data from a large cohort of children aged 18 years and younger who had been diagnosed with COVID-19 between Feb. 25, 2020, and Jan. 20, 2021, in 54 Italian institutions.
Overall, 685 Italian children (56.4% boys; average age, 7 years) were included in the study. Of these, 628 (91.7%) were diagnosed with acute SARS-CoV-2 infection and 57 (8.3%) with multisystem inflammatory syndrome in children (MIS-C).
When children had GI symptoms, the authors found a higher risk of hospitalization (odds ratio, 2.64; 95% confidence interval, 1.89-3.69) and nearly four times the risk of ICU admission (OR, 3.90; 95% CI, 1.98-7.68).
Severe GI involvement occurred in 65 children (9.5%). The authors included the following within that category: disseminated adenomesenteritis (39.6%), appendicitis (33.5%), abdominal fluid collection (21.3%), pancreatitis (6.9%), or intussusception (4.6%). Additionally, out of these 65 children, 27 (41.5%) underwent surgery.
Older children were much more likely than preschoolers to have severe GI symptoms. Children aged 5-10 years were eight times more likely than preschoolers to show severe symptoms (OR, 8.33; 95% CI, 2.62-26.5). In those older than age 10 years, severe symptoms were six times more likely (OR, 6.37; 95% CI, 2.12-19.1).
Awareness about its frequency and presentation may help practitioners to appropriately manage children at risk of severe outcomes, the authors wrote.
The findings of this study were published online Dec. 20 in JAMA Network Open.
Study highlights the GI link
Reached for comment, William Balistreri, MD, with the division of gastroenterology, hepatology, and nutrition at Cincinnati Children’s Hospital Medical Center, said that it has been known that children are more likely than adults to present with GI symptoms, and also that these symptoms are especially common in children with MIS-C.
“The symptoms most commonly cited in the literature to date include diarrhea, nausea, vomiting, or abdominal pain,” he said. “What [has not been known] is the frequency, predictive markers, and clinical course of the severe GI manifestations of COVID-19.”
The findings of this study are important to clinicians to help recognize the potential for severe GI involvement, Dr. Balistreri said, adding that “the occurrence of abdominal pain, leukopenia, and elevated inflammatory markers or MIS-C should raise suspicion and lead to early evaluation.”
Margaret E. Thew, APNP, medical director in adolescent medicine and a family nurse practitioner with Medical College of Wisconsin, Milwaukee, said that news reports typically emphasize the respiratory involvement, but this study provides a detailed analysis of the link between GI symptoms and COVID-19.
“Their data show that there may be less respiratory illness with children, regardless of whether they are generally healthy kids,” she said. “They may have more GI symptoms.
“We know that COVID-19 causes a lot of inflammation, and a large percentage of these kids had inflammation in their stomach or an inflamed bowel,” she added.
Dr. Thew said primary care doctors and urgent and emergency care clinicians will benefit from the findings of this study and should be on alert when kids come in with belly pain or vomiting.
Parents will benefit too, she said, if they are waiting for respiratory illness before they suspect COVID.
“You have to have a high suspicion this is going to be COVID positive,” she said. “You have to have that as part of your thought process.”
Though the study was done in Italy, Dr. Thew added that their experiences mimic those she’s seen locally.
Dr. Lo Vecchio reported receiving fees from Pfizer as an advisory board member outside the submitted work. A coauthor reported speaker’s fees from Angelini, Sobi, and X4 Pharma outside the submitted work. No other disclosures were reported. Dr. Balistreri and Dr. Thew reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Convalescent plasma cuts COVID-19 hospitalizations in half: Study
A “definitive study” from Johns Hopkins University researchers and others shows that convalescent plasma can cut hospital admissions for COVID-19 by 54% if therapy is administered within 8 days of symptom onset.
In the study of 1,181 adults randomly assigned to high-titer convalescent plasma or placebo, 2.9% of people receiving the therapy were hospitalized, compared with 6.3% who received placebo control plasma.
This translates to a 54% risk reduction for hospitalization with convalescent plasma.
“We have a clear difference,” principal investigator David Sullivan, MD, a professor at Johns Hopkins University, Baltimore, said during a Dec. 21 media briefing.
“This is very good news since we are in the midst of the Omicron surge, which has defeated [some of] our major monocular antibody therapies,” said Arturo Casadevall, MD, chair of the department of molecular microbiology and immunology at Johns Hopkins.
“So we have a new tool to keep people from progressing in their disease and to reduce progression or hospitalization,” Dr. Casadevall said.
The findings were published as a preprint study on Dec. 21, 2021, on medRxiv. The study has not yet been peer reviewed.
Whereas many convalescent plasma studies were done in hospitalized patients, this is one of only a handful performed in outpatients, the researchers noted.
There is a regulatory catch. The Food and Drug Administration restricted emergency use authorization (EUA) for convalescent plasma in February 2021 to include only high-dose titer plasma and to limit the therapy to hospitalized patients with early disease or for immunocompromised people who cannot mount an adequate antibody response.
Dr. Sullivan and colleagues hoped their findings will prompt the FDA to expand the EUA to include outpatients.
“We have shared this data with both the World Health Organization and the FDA,” study coauthor Kelly Gebo, MD, MPH, said during the media briefing.
“We do believe that this could be scaled up quickly,” added Dr. Gebo, professor of medicine at Johns Hopkins University. Convalescent plasma “could be used as a potential treatment as variants continue to evolve, such as we’ve seen with Omicron.”
Pre-Omicron results
The study was conducted at Johns Hopkins University and 23 other sites nationwide between June 2020 and October 2021. This means researchers enrolled symptomatic adults during circulation of the SARS-CoV-2 ancestral strain and the Alpha and Delta variants.
However, Dr. Sullivan said, “we think that ... plasma with high levels of antibodies can adapt faster to Omicron, although it will take us longer to get an Omicron-specific supply.”
Because of the timing of the study, 80% of participants were unvaccinated. Mean age was 44 years and 57% were women. Black and Hispanic participants each accounted for more than 12% of the study population.
On average, participants received a transfusion within 6 days of the start of symptoms.
In the study, 37 people out of 589 control group participants were hospitalized, compared with 17 of the 592 who received the convalescent plasma.
“We know antibodies work against SARS-CoV-2. The vaccines have been spectacular – producing antibodies that reduce hospitalizations and prevent transmission,” Dr. Sullivan said. “Convalescent plasma provides much of the same antibodies instantly.”
Convalescent and controversial
Convalescent plasma has been one of the controversial treatments for people with COVID-19 – with studies going back and forth on the potential benefits and efficacy. A National Institutes of Health–funded study published in August 2021, for example, showed no significant benefit.
“As you know, convalescent plasma has had a rocky ride,” Dr. Casadevall said.
“It was deployed with great excitement in the terrible, early days of the pandemic. Unfortunately, the early excitement and optimism was dampened with some of the randomized control trials appearing to show no benefit in reducing mortality and hospitalized patients,” he added.
In contrast, the current study shows “where convalescent plasma works using the latest, most rigorous clinical investigation tools available: a double-blinded, randomized, placebo-control trial,” Dr. Casadevall said.
Why a preprint, and why now?
The researchers decided to release their data in recognition of the lag time between reporting of COVID-19 cases and hospitalizations, Dr. Sullivan said. “That’s part of the reason we decided to act now with this knowledge – that it does take a couple of weeks – with cases of Omicron going up.”
Furthermore, “we thought this was actionable data for decision-makers,” he added.
A reporter asked why the Johns Hopkins researchers chose to hold a media briefing for a preprint study.
A preprint is “not so unusual given the SARS-CoV-2 pandemic,” said study senior author Daniel Hanley, MD, division director of brain injury outcomes at Johns Hopkins University.
“The data are the data,” Dr. Casadevall added. “This is not going to change from peer review.”
Peer review may change some of the wording of the manuscript, but not the numbers, he added.
“Now with the Omicron crisis and the fact that we have lost some more main monoclonal antibodies, it is essential to get this information out,” Dr. Casadevall said.
Plasma therapy nothing new
Donation and transfusion of convalescent plasma is highly regulated with strict criteria, said Evan Bloch, MBChB, associate director of the transfusion medicine division at Johns Hopkins University.
If the FDA opts to expand the EUA based on this or other evidence, administration of convalescent plasma could be rolled out fairly quickly, the researchers noted.
Plasma transfusion takes place in hospitals and at infusion centers every day. The infrastructure is in place in many countries, even low- and middle-resource nations, around the world to provide convalescent plasma therapy. The major difference between traditional plasma and SARS-CoV-2 convalescent plasma is the indication, Dr. Bloch added.
In addition, convalescent plasma has a polyclonal composition – a benefit compared with monoclonal antibodies, he added. “It’s more durable or adaptive [compared with] some of the targeted therapies, such as monoclonal antibodies, where we’ve witnessed this diminished efficacy with viral evolution.”
A version of this article first appeared on Medscape.com.
A “definitive study” from Johns Hopkins University researchers and others shows that convalescent plasma can cut hospital admissions for COVID-19 by 54% if therapy is administered within 8 days of symptom onset.
In the study of 1,181 adults randomly assigned to high-titer convalescent plasma or placebo, 2.9% of people receiving the therapy were hospitalized, compared with 6.3% who received placebo control plasma.
This translates to a 54% risk reduction for hospitalization with convalescent plasma.
“We have a clear difference,” principal investigator David Sullivan, MD, a professor at Johns Hopkins University, Baltimore, said during a Dec. 21 media briefing.
“This is very good news since we are in the midst of the Omicron surge, which has defeated [some of] our major monocular antibody therapies,” said Arturo Casadevall, MD, chair of the department of molecular microbiology and immunology at Johns Hopkins.
“So we have a new tool to keep people from progressing in their disease and to reduce progression or hospitalization,” Dr. Casadevall said.
The findings were published as a preprint study on Dec. 21, 2021, on medRxiv. The study has not yet been peer reviewed.
Whereas many convalescent plasma studies were done in hospitalized patients, this is one of only a handful performed in outpatients, the researchers noted.
There is a regulatory catch. The Food and Drug Administration restricted emergency use authorization (EUA) for convalescent plasma in February 2021 to include only high-dose titer plasma and to limit the therapy to hospitalized patients with early disease or for immunocompromised people who cannot mount an adequate antibody response.
Dr. Sullivan and colleagues hoped their findings will prompt the FDA to expand the EUA to include outpatients.
“We have shared this data with both the World Health Organization and the FDA,” study coauthor Kelly Gebo, MD, MPH, said during the media briefing.
“We do believe that this could be scaled up quickly,” added Dr. Gebo, professor of medicine at Johns Hopkins University. Convalescent plasma “could be used as a potential treatment as variants continue to evolve, such as we’ve seen with Omicron.”
Pre-Omicron results
The study was conducted at Johns Hopkins University and 23 other sites nationwide between June 2020 and October 2021. This means researchers enrolled symptomatic adults during circulation of the SARS-CoV-2 ancestral strain and the Alpha and Delta variants.
However, Dr. Sullivan said, “we think that ... plasma with high levels of antibodies can adapt faster to Omicron, although it will take us longer to get an Omicron-specific supply.”
Because of the timing of the study, 80% of participants were unvaccinated. Mean age was 44 years and 57% were women. Black and Hispanic participants each accounted for more than 12% of the study population.
On average, participants received a transfusion within 6 days of the start of symptoms.
In the study, 37 people out of 589 control group participants were hospitalized, compared with 17 of the 592 who received the convalescent plasma.
“We know antibodies work against SARS-CoV-2. The vaccines have been spectacular – producing antibodies that reduce hospitalizations and prevent transmission,” Dr. Sullivan said. “Convalescent plasma provides much of the same antibodies instantly.”
Convalescent and controversial
Convalescent plasma has been one of the controversial treatments for people with COVID-19 – with studies going back and forth on the potential benefits and efficacy. A National Institutes of Health–funded study published in August 2021, for example, showed no significant benefit.
“As you know, convalescent plasma has had a rocky ride,” Dr. Casadevall said.
“It was deployed with great excitement in the terrible, early days of the pandemic. Unfortunately, the early excitement and optimism was dampened with some of the randomized control trials appearing to show no benefit in reducing mortality and hospitalized patients,” he added.
In contrast, the current study shows “where convalescent plasma works using the latest, most rigorous clinical investigation tools available: a double-blinded, randomized, placebo-control trial,” Dr. Casadevall said.
Why a preprint, and why now?
The researchers decided to release their data in recognition of the lag time between reporting of COVID-19 cases and hospitalizations, Dr. Sullivan said. “That’s part of the reason we decided to act now with this knowledge – that it does take a couple of weeks – with cases of Omicron going up.”
Furthermore, “we thought this was actionable data for decision-makers,” he added.
A reporter asked why the Johns Hopkins researchers chose to hold a media briefing for a preprint study.
A preprint is “not so unusual given the SARS-CoV-2 pandemic,” said study senior author Daniel Hanley, MD, division director of brain injury outcomes at Johns Hopkins University.
“The data are the data,” Dr. Casadevall added. “This is not going to change from peer review.”
Peer review may change some of the wording of the manuscript, but not the numbers, he added.
“Now with the Omicron crisis and the fact that we have lost some more main monoclonal antibodies, it is essential to get this information out,” Dr. Casadevall said.
Plasma therapy nothing new
Donation and transfusion of convalescent plasma is highly regulated with strict criteria, said Evan Bloch, MBChB, associate director of the transfusion medicine division at Johns Hopkins University.
If the FDA opts to expand the EUA based on this or other evidence, administration of convalescent plasma could be rolled out fairly quickly, the researchers noted.
Plasma transfusion takes place in hospitals and at infusion centers every day. The infrastructure is in place in many countries, even low- and middle-resource nations, around the world to provide convalescent plasma therapy. The major difference between traditional plasma and SARS-CoV-2 convalescent plasma is the indication, Dr. Bloch added.
In addition, convalescent plasma has a polyclonal composition – a benefit compared with monoclonal antibodies, he added. “It’s more durable or adaptive [compared with] some of the targeted therapies, such as monoclonal antibodies, where we’ve witnessed this diminished efficacy with viral evolution.”
A version of this article first appeared on Medscape.com.
A “definitive study” from Johns Hopkins University researchers and others shows that convalescent plasma can cut hospital admissions for COVID-19 by 54% if therapy is administered within 8 days of symptom onset.
In the study of 1,181 adults randomly assigned to high-titer convalescent plasma or placebo, 2.9% of people receiving the therapy were hospitalized, compared with 6.3% who received placebo control plasma.
This translates to a 54% risk reduction for hospitalization with convalescent plasma.
“We have a clear difference,” principal investigator David Sullivan, MD, a professor at Johns Hopkins University, Baltimore, said during a Dec. 21 media briefing.
“This is very good news since we are in the midst of the Omicron surge, which has defeated [some of] our major monocular antibody therapies,” said Arturo Casadevall, MD, chair of the department of molecular microbiology and immunology at Johns Hopkins.
“So we have a new tool to keep people from progressing in their disease and to reduce progression or hospitalization,” Dr. Casadevall said.
The findings were published as a preprint study on Dec. 21, 2021, on medRxiv. The study has not yet been peer reviewed.
Whereas many convalescent plasma studies were done in hospitalized patients, this is one of only a handful performed in outpatients, the researchers noted.
There is a regulatory catch. The Food and Drug Administration restricted emergency use authorization (EUA) for convalescent plasma in February 2021 to include only high-dose titer plasma and to limit the therapy to hospitalized patients with early disease or for immunocompromised people who cannot mount an adequate antibody response.
Dr. Sullivan and colleagues hoped their findings will prompt the FDA to expand the EUA to include outpatients.
“We have shared this data with both the World Health Organization and the FDA,” study coauthor Kelly Gebo, MD, MPH, said during the media briefing.
“We do believe that this could be scaled up quickly,” added Dr. Gebo, professor of medicine at Johns Hopkins University. Convalescent plasma “could be used as a potential treatment as variants continue to evolve, such as we’ve seen with Omicron.”
Pre-Omicron results
The study was conducted at Johns Hopkins University and 23 other sites nationwide between June 2020 and October 2021. This means researchers enrolled symptomatic adults during circulation of the SARS-CoV-2 ancestral strain and the Alpha and Delta variants.
However, Dr. Sullivan said, “we think that ... plasma with high levels of antibodies can adapt faster to Omicron, although it will take us longer to get an Omicron-specific supply.”
Because of the timing of the study, 80% of participants were unvaccinated. Mean age was 44 years and 57% were women. Black and Hispanic participants each accounted for more than 12% of the study population.
On average, participants received a transfusion within 6 days of the start of symptoms.
In the study, 37 people out of 589 control group participants were hospitalized, compared with 17 of the 592 who received the convalescent plasma.
“We know antibodies work against SARS-CoV-2. The vaccines have been spectacular – producing antibodies that reduce hospitalizations and prevent transmission,” Dr. Sullivan said. “Convalescent plasma provides much of the same antibodies instantly.”
Convalescent and controversial
Convalescent plasma has been one of the controversial treatments for people with COVID-19 – with studies going back and forth on the potential benefits and efficacy. A National Institutes of Health–funded study published in August 2021, for example, showed no significant benefit.
“As you know, convalescent plasma has had a rocky ride,” Dr. Casadevall said.
“It was deployed with great excitement in the terrible, early days of the pandemic. Unfortunately, the early excitement and optimism was dampened with some of the randomized control trials appearing to show no benefit in reducing mortality and hospitalized patients,” he added.
In contrast, the current study shows “where convalescent plasma works using the latest, most rigorous clinical investigation tools available: a double-blinded, randomized, placebo-control trial,” Dr. Casadevall said.
Why a preprint, and why now?
The researchers decided to release their data in recognition of the lag time between reporting of COVID-19 cases and hospitalizations, Dr. Sullivan said. “That’s part of the reason we decided to act now with this knowledge – that it does take a couple of weeks – with cases of Omicron going up.”
Furthermore, “we thought this was actionable data for decision-makers,” he added.
A reporter asked why the Johns Hopkins researchers chose to hold a media briefing for a preprint study.
A preprint is “not so unusual given the SARS-CoV-2 pandemic,” said study senior author Daniel Hanley, MD, division director of brain injury outcomes at Johns Hopkins University.
“The data are the data,” Dr. Casadevall added. “This is not going to change from peer review.”
Peer review may change some of the wording of the manuscript, but not the numbers, he added.
“Now with the Omicron crisis and the fact that we have lost some more main monoclonal antibodies, it is essential to get this information out,” Dr. Casadevall said.
Plasma therapy nothing new
Donation and transfusion of convalescent plasma is highly regulated with strict criteria, said Evan Bloch, MBChB, associate director of the transfusion medicine division at Johns Hopkins University.
If the FDA opts to expand the EUA based on this or other evidence, administration of convalescent plasma could be rolled out fairly quickly, the researchers noted.
Plasma transfusion takes place in hospitals and at infusion centers every day. The infrastructure is in place in many countries, even low- and middle-resource nations, around the world to provide convalescent plasma therapy. The major difference between traditional plasma and SARS-CoV-2 convalescent plasma is the indication, Dr. Bloch added.
In addition, convalescent plasma has a polyclonal composition – a benefit compared with monoclonal antibodies, he added. “It’s more durable or adaptive [compared with] some of the targeted therapies, such as monoclonal antibodies, where we’ve witnessed this diminished efficacy with viral evolution.”
A version of this article first appeared on Medscape.com.
FROM MEDRXIV
Bamlanivimab’s effects in COVID-19 depend on antibodies
In the randomized controlled trial, in both the group who received bamlanivimab and the group who received placebo, higher antigen and viral RNA levels were associated with a lower proportion of patients achieving recovery.
Other studies have shown that the use of monoclonal antibodies reduces hospitalization risk in outpatients with early COVID-19, and appears to promote viral load decline in the nasopharynx, wrote Jens D. Lundgren, MD, of the University of Copenhagen and colleagues in their article published in the Annals of Internal Medicine. What had been missing prior to this new research was final results from hospitalized patients, the authors said.
In the new study, the researchers randomized 314 adults hospitalized with COVID-19 but without end-organ failure to receive 7,000 mg bamlanivimab (163 patients) or a placebo (151 patients). All patients received study-supplied remdesivir unless contraindicated. The researchers compared the efficacy of bamlanivimab versus placebo, but considered remdesivir the standard of care in this study.
At baseline, 50% of patients overall had antispike endogenous neutralizing antibodies (nAbs), and 50% had SARS-CoV-2 nucleocapsid plasma antigen levels of at least 1,000 ng/L.
The median time to sustained recovery, 19 days, was not significantly different between the bamlanivimab and placebo groups (subhazard ratio, 0.99).
“As hypothesized, among those who were negative for nAb, the difference between bamlanivimab and placebo was more evident if levels of plasma antigen or nasal-swab viral RNA were above the median entry levels,” with subhazard ratios of 1.48 and 1.89, respectively, the researchers explained.
However, the hazard ratio for death for bamlanivimab vs. placebo was 0.45 for patients negative for nAb vs. 3.53 for those positive for nAb. These differences with respect to nAb status were similar across all 90 elements of a composite safety outcome, the researchers said.
Potential benefits remain unclear
The use of neutralizing monoclonal antibodies has been extensively documented as an effective treatment for COVID-19 among ambulatory patients, corresponding author Dr. Lundgren said in an interview.
“Conversely, among admitted patients with COVID-19 pneumonia, the benefit has been questionable,” he said.
The researchers examined a hypothesis that the null finding in hospitalized patients may stem from differences in underlying mechanisms, “either from uncontrolled viral replication – which would be predicted to occur in particular among those not yet been able to mount an endogenous immune response – or from hyperinflammation among those that have mounted such a response,” Dr. Lundgren said.
The study findings supported the stated hypothesis, said Dr. Lundgren. “However, it was surprising that not only was the neutralizing antibody without any benefit among those that had mounted an endogenous immune response, but it actually may have been harmful,” he said.
Bamlanivimab was effective against the viral strain that circulated at the time of enrollment in the study, but subsequent viral strains have appeared to be unaffected by the neutralizing activity of the antibody, said Dr. Lundgren.
From a practical standpoint, “the findings would suggest that use of neutralizing monoclonal antibodies for patients admitted to a hospital with COVID pneumonia should be restricted to those that have not yet mounted an endogenous immune response, as determined by lack of detectable neutralizing antibodies at the time of admission,” Dr. Lundgren said.
Looking ahead, studies are currently underway to examine how the findings translate to vaccinated patients, he added. Other questions to be addressed include whether the benefits and harms apply to some or all neutralizing antibody products, he said.
In addition, “our research consortium is currently doing field testing of several point-of-care test candidates to examine their reliability and functionality,” for how quickly they might identify an endogenous neutralizing antibody response in an admitted COVID pneumonia patient,” Dr. Lundgren noted.
Findings show bamlanivimab’s limits
“Based on the findings of the current study, no clear subgroup of patients could be identified who would benefit from bamlanivimab when hospitalized with COVID-19,” said Suman Pal, MD, of the University of New Mexico, Albuquerque, in an interview.
“The study findings also show possible harm of using bamlanivimab in hospitalized COVID-19 patients who were seropositive for neutralizing antibodies prior to receiving therapy,” Dr. Pal emphasized. “Moreover, the study did not include participants with COVID-19 from variant strains, such as delta and omicron, which currently account for a large number of cases.” “Therefore, the results of this study do not support the use of bamlanivimab in the clinical setting until further evidence is available to guide the selection of patients who may benefit from therapy,” he explained.
“The possible benefit of bamlanivimab does not outweigh the risks in patients hospitalized with COVID-19,” he concluded.
Dr. Pal emphasized the need for larger prospective studies to establish whether bamlanivimab may have benefits in a subgroup of patients, but “well-validated point-of-care tests to identify such patients need to be readily available before this therapy can be considered by clinicians at the bedside,” he concluded.
Diligent screening required before use
Monoclonal antibody treatment has been administered to individuals with diagnosis of COVID-19 infection as outpatients as well as for hospitalized inpatients, said Noel Deep, MD, an internist in Antigo, Wisc., in an interview. “This study is important because it helps physicians and health care institutions to evaluate whether continued use of the monoclonal antibodies would be beneficial and, if so, in what patient populations,” he said.
The findings present interesting implications for the care of COVID-19 patients, said Dr. Deep. “This study indicates that bamlanivimab does not provide the benefit that was initially envisioned when the monoclonal antibody infusions were initially initiated in the treatment of COVID-19 infections. “Serological screening of the patients would help to identify that subgroup of individuals who could benefit from this monoclonal antibody rather than administering it to every COVID-19–positive individual,” he explained.
However, “it is important to note that the emergency use authorization (EUA) for single-agent bamlanivimab has been revoked,” Dr. Deep said.
“The potential benefits of bamlanivimab can be realized only if adequate attention is paid to identifying the appropriate candidates based on serological screening, and administering bamlanivimab to those who are already producing endogenous antibodies could lead to increased risk to those individuals,” he said. Dr. Deep added that he would favor administration of bamlanivimab “in those appropriately screened and eligible candidates, and it is my opinion that the benefits outweigh the risks in those individuals.”
Although the EUA for single-agent bamlanivimab has been revoked, “alternative monoclonal antibody therapies remain available under EUA, including REGEN-COV (casirivimab and imdevimab, administered together), and bamlanivimab and etesevimab administered together, for the same uses as previously authorized for bamlanivimab alone,” Dr. Deep said. “The FDA believes that these alternative monoclonal antibody therapies remain appropriate to treat patients with COVID-19, and I would like to see some data about the benefits and risks of these agents,” he noted.
Limitations, funding, and disclosures
The main limitation of the study was the small size and the fact that it was a subgroup analysis of a trial that ended early because of futility, the researchers wrote. However, the Therapeutics for Inpatients With COVID-19 (TICO) platform will proceed with clinical evaluation of additional COVID-19 treatments, they said.
The study was supported primarily by the U.S. government Operation Warp Speed and the National Institute of Allergy and Infectious Diseases. Other funding sources included the Division of Clinical Research and Leidos Biomedical Research for the INSIGHT (International Network for Strategic Initiatives in Global HIV Trials) Network, as well as an agreement between the National Heart, Lung, and Blood Institute and the Research Triangle Institute for the PETAL (Prevention & Early Treatment of Acute Lung Injury) Network and CTSN (Cardiothoracic Surgical Trials Network). Other support came from the U.S. Department of Veterans Affairs and the governments of Denmark (National Research Foundation), Australia (National Health and Medical Research Council), and the United Kingdom (Medical Research Council).
The medications used in the study were donated by Gilead Sciences and Eli Lilly.
The researchers had no financial conflicts do disclose. Dr. Deep and Dr. Pal had no relevant financial conflicts to disclose.
In the randomized controlled trial, in both the group who received bamlanivimab and the group who received placebo, higher antigen and viral RNA levels were associated with a lower proportion of patients achieving recovery.
Other studies have shown that the use of monoclonal antibodies reduces hospitalization risk in outpatients with early COVID-19, and appears to promote viral load decline in the nasopharynx, wrote Jens D. Lundgren, MD, of the University of Copenhagen and colleagues in their article published in the Annals of Internal Medicine. What had been missing prior to this new research was final results from hospitalized patients, the authors said.
In the new study, the researchers randomized 314 adults hospitalized with COVID-19 but without end-organ failure to receive 7,000 mg bamlanivimab (163 patients) or a placebo (151 patients). All patients received study-supplied remdesivir unless contraindicated. The researchers compared the efficacy of bamlanivimab versus placebo, but considered remdesivir the standard of care in this study.
At baseline, 50% of patients overall had antispike endogenous neutralizing antibodies (nAbs), and 50% had SARS-CoV-2 nucleocapsid plasma antigen levels of at least 1,000 ng/L.
The median time to sustained recovery, 19 days, was not significantly different between the bamlanivimab and placebo groups (subhazard ratio, 0.99).
“As hypothesized, among those who were negative for nAb, the difference between bamlanivimab and placebo was more evident if levels of plasma antigen or nasal-swab viral RNA were above the median entry levels,” with subhazard ratios of 1.48 and 1.89, respectively, the researchers explained.
However, the hazard ratio for death for bamlanivimab vs. placebo was 0.45 for patients negative for nAb vs. 3.53 for those positive for nAb. These differences with respect to nAb status were similar across all 90 elements of a composite safety outcome, the researchers said.
Potential benefits remain unclear
The use of neutralizing monoclonal antibodies has been extensively documented as an effective treatment for COVID-19 among ambulatory patients, corresponding author Dr. Lundgren said in an interview.
“Conversely, among admitted patients with COVID-19 pneumonia, the benefit has been questionable,” he said.
The researchers examined a hypothesis that the null finding in hospitalized patients may stem from differences in underlying mechanisms, “either from uncontrolled viral replication – which would be predicted to occur in particular among those not yet been able to mount an endogenous immune response – or from hyperinflammation among those that have mounted such a response,” Dr. Lundgren said.
The study findings supported the stated hypothesis, said Dr. Lundgren. “However, it was surprising that not only was the neutralizing antibody without any benefit among those that had mounted an endogenous immune response, but it actually may have been harmful,” he said.
Bamlanivimab was effective against the viral strain that circulated at the time of enrollment in the study, but subsequent viral strains have appeared to be unaffected by the neutralizing activity of the antibody, said Dr. Lundgren.
From a practical standpoint, “the findings would suggest that use of neutralizing monoclonal antibodies for patients admitted to a hospital with COVID pneumonia should be restricted to those that have not yet mounted an endogenous immune response, as determined by lack of detectable neutralizing antibodies at the time of admission,” Dr. Lundgren said.
Looking ahead, studies are currently underway to examine how the findings translate to vaccinated patients, he added. Other questions to be addressed include whether the benefits and harms apply to some or all neutralizing antibody products, he said.
In addition, “our research consortium is currently doing field testing of several point-of-care test candidates to examine their reliability and functionality,” for how quickly they might identify an endogenous neutralizing antibody response in an admitted COVID pneumonia patient,” Dr. Lundgren noted.
Findings show bamlanivimab’s limits
“Based on the findings of the current study, no clear subgroup of patients could be identified who would benefit from bamlanivimab when hospitalized with COVID-19,” said Suman Pal, MD, of the University of New Mexico, Albuquerque, in an interview.
“The study findings also show possible harm of using bamlanivimab in hospitalized COVID-19 patients who were seropositive for neutralizing antibodies prior to receiving therapy,” Dr. Pal emphasized. “Moreover, the study did not include participants with COVID-19 from variant strains, such as delta and omicron, which currently account for a large number of cases.” “Therefore, the results of this study do not support the use of bamlanivimab in the clinical setting until further evidence is available to guide the selection of patients who may benefit from therapy,” he explained.
“The possible benefit of bamlanivimab does not outweigh the risks in patients hospitalized with COVID-19,” he concluded.
Dr. Pal emphasized the need for larger prospective studies to establish whether bamlanivimab may have benefits in a subgroup of patients, but “well-validated point-of-care tests to identify such patients need to be readily available before this therapy can be considered by clinicians at the bedside,” he concluded.
Diligent screening required before use
Monoclonal antibody treatment has been administered to individuals with diagnosis of COVID-19 infection as outpatients as well as for hospitalized inpatients, said Noel Deep, MD, an internist in Antigo, Wisc., in an interview. “This study is important because it helps physicians and health care institutions to evaluate whether continued use of the monoclonal antibodies would be beneficial and, if so, in what patient populations,” he said.
The findings present interesting implications for the care of COVID-19 patients, said Dr. Deep. “This study indicates that bamlanivimab does not provide the benefit that was initially envisioned when the monoclonal antibody infusions were initially initiated in the treatment of COVID-19 infections. “Serological screening of the patients would help to identify that subgroup of individuals who could benefit from this monoclonal antibody rather than administering it to every COVID-19–positive individual,” he explained.
However, “it is important to note that the emergency use authorization (EUA) for single-agent bamlanivimab has been revoked,” Dr. Deep said.
“The potential benefits of bamlanivimab can be realized only if adequate attention is paid to identifying the appropriate candidates based on serological screening, and administering bamlanivimab to those who are already producing endogenous antibodies could lead to increased risk to those individuals,” he said. Dr. Deep added that he would favor administration of bamlanivimab “in those appropriately screened and eligible candidates, and it is my opinion that the benefits outweigh the risks in those individuals.”
Although the EUA for single-agent bamlanivimab has been revoked, “alternative monoclonal antibody therapies remain available under EUA, including REGEN-COV (casirivimab and imdevimab, administered together), and bamlanivimab and etesevimab administered together, for the same uses as previously authorized for bamlanivimab alone,” Dr. Deep said. “The FDA believes that these alternative monoclonal antibody therapies remain appropriate to treat patients with COVID-19, and I would like to see some data about the benefits and risks of these agents,” he noted.
Limitations, funding, and disclosures
The main limitation of the study was the small size and the fact that it was a subgroup analysis of a trial that ended early because of futility, the researchers wrote. However, the Therapeutics for Inpatients With COVID-19 (TICO) platform will proceed with clinical evaluation of additional COVID-19 treatments, they said.
The study was supported primarily by the U.S. government Operation Warp Speed and the National Institute of Allergy and Infectious Diseases. Other funding sources included the Division of Clinical Research and Leidos Biomedical Research for the INSIGHT (International Network for Strategic Initiatives in Global HIV Trials) Network, as well as an agreement between the National Heart, Lung, and Blood Institute and the Research Triangle Institute for the PETAL (Prevention & Early Treatment of Acute Lung Injury) Network and CTSN (Cardiothoracic Surgical Trials Network). Other support came from the U.S. Department of Veterans Affairs and the governments of Denmark (National Research Foundation), Australia (National Health and Medical Research Council), and the United Kingdom (Medical Research Council).
The medications used in the study were donated by Gilead Sciences and Eli Lilly.
The researchers had no financial conflicts do disclose. Dr. Deep and Dr. Pal had no relevant financial conflicts to disclose.
In the randomized controlled trial, in both the group who received bamlanivimab and the group who received placebo, higher antigen and viral RNA levels were associated with a lower proportion of patients achieving recovery.
Other studies have shown that the use of monoclonal antibodies reduces hospitalization risk in outpatients with early COVID-19, and appears to promote viral load decline in the nasopharynx, wrote Jens D. Lundgren, MD, of the University of Copenhagen and colleagues in their article published in the Annals of Internal Medicine. What had been missing prior to this new research was final results from hospitalized patients, the authors said.
In the new study, the researchers randomized 314 adults hospitalized with COVID-19 but without end-organ failure to receive 7,000 mg bamlanivimab (163 patients) or a placebo (151 patients). All patients received study-supplied remdesivir unless contraindicated. The researchers compared the efficacy of bamlanivimab versus placebo, but considered remdesivir the standard of care in this study.
At baseline, 50% of patients overall had antispike endogenous neutralizing antibodies (nAbs), and 50% had SARS-CoV-2 nucleocapsid plasma antigen levels of at least 1,000 ng/L.
The median time to sustained recovery, 19 days, was not significantly different between the bamlanivimab and placebo groups (subhazard ratio, 0.99).
“As hypothesized, among those who were negative for nAb, the difference between bamlanivimab and placebo was more evident if levels of plasma antigen or nasal-swab viral RNA were above the median entry levels,” with subhazard ratios of 1.48 and 1.89, respectively, the researchers explained.
However, the hazard ratio for death for bamlanivimab vs. placebo was 0.45 for patients negative for nAb vs. 3.53 for those positive for nAb. These differences with respect to nAb status were similar across all 90 elements of a composite safety outcome, the researchers said.
Potential benefits remain unclear
The use of neutralizing monoclonal antibodies has been extensively documented as an effective treatment for COVID-19 among ambulatory patients, corresponding author Dr. Lundgren said in an interview.
“Conversely, among admitted patients with COVID-19 pneumonia, the benefit has been questionable,” he said.
The researchers examined a hypothesis that the null finding in hospitalized patients may stem from differences in underlying mechanisms, “either from uncontrolled viral replication – which would be predicted to occur in particular among those not yet been able to mount an endogenous immune response – or from hyperinflammation among those that have mounted such a response,” Dr. Lundgren said.
The study findings supported the stated hypothesis, said Dr. Lundgren. “However, it was surprising that not only was the neutralizing antibody without any benefit among those that had mounted an endogenous immune response, but it actually may have been harmful,” he said.
Bamlanivimab was effective against the viral strain that circulated at the time of enrollment in the study, but subsequent viral strains have appeared to be unaffected by the neutralizing activity of the antibody, said Dr. Lundgren.
From a practical standpoint, “the findings would suggest that use of neutralizing monoclonal antibodies for patients admitted to a hospital with COVID pneumonia should be restricted to those that have not yet mounted an endogenous immune response, as determined by lack of detectable neutralizing antibodies at the time of admission,” Dr. Lundgren said.
Looking ahead, studies are currently underway to examine how the findings translate to vaccinated patients, he added. Other questions to be addressed include whether the benefits and harms apply to some or all neutralizing antibody products, he said.
In addition, “our research consortium is currently doing field testing of several point-of-care test candidates to examine their reliability and functionality,” for how quickly they might identify an endogenous neutralizing antibody response in an admitted COVID pneumonia patient,” Dr. Lundgren noted.
Findings show bamlanivimab’s limits
“Based on the findings of the current study, no clear subgroup of patients could be identified who would benefit from bamlanivimab when hospitalized with COVID-19,” said Suman Pal, MD, of the University of New Mexico, Albuquerque, in an interview.
“The study findings also show possible harm of using bamlanivimab in hospitalized COVID-19 patients who were seropositive for neutralizing antibodies prior to receiving therapy,” Dr. Pal emphasized. “Moreover, the study did not include participants with COVID-19 from variant strains, such as delta and omicron, which currently account for a large number of cases.” “Therefore, the results of this study do not support the use of bamlanivimab in the clinical setting until further evidence is available to guide the selection of patients who may benefit from therapy,” he explained.
“The possible benefit of bamlanivimab does not outweigh the risks in patients hospitalized with COVID-19,” he concluded.
Dr. Pal emphasized the need for larger prospective studies to establish whether bamlanivimab may have benefits in a subgroup of patients, but “well-validated point-of-care tests to identify such patients need to be readily available before this therapy can be considered by clinicians at the bedside,” he concluded.
Diligent screening required before use
Monoclonal antibody treatment has been administered to individuals with diagnosis of COVID-19 infection as outpatients as well as for hospitalized inpatients, said Noel Deep, MD, an internist in Antigo, Wisc., in an interview. “This study is important because it helps physicians and health care institutions to evaluate whether continued use of the monoclonal antibodies would be beneficial and, if so, in what patient populations,” he said.
The findings present interesting implications for the care of COVID-19 patients, said Dr. Deep. “This study indicates that bamlanivimab does not provide the benefit that was initially envisioned when the monoclonal antibody infusions were initially initiated in the treatment of COVID-19 infections. “Serological screening of the patients would help to identify that subgroup of individuals who could benefit from this monoclonal antibody rather than administering it to every COVID-19–positive individual,” he explained.
However, “it is important to note that the emergency use authorization (EUA) for single-agent bamlanivimab has been revoked,” Dr. Deep said.
“The potential benefits of bamlanivimab can be realized only if adequate attention is paid to identifying the appropriate candidates based on serological screening, and administering bamlanivimab to those who are already producing endogenous antibodies could lead to increased risk to those individuals,” he said. Dr. Deep added that he would favor administration of bamlanivimab “in those appropriately screened and eligible candidates, and it is my opinion that the benefits outweigh the risks in those individuals.”
Although the EUA for single-agent bamlanivimab has been revoked, “alternative monoclonal antibody therapies remain available under EUA, including REGEN-COV (casirivimab and imdevimab, administered together), and bamlanivimab and etesevimab administered together, for the same uses as previously authorized for bamlanivimab alone,” Dr. Deep said. “The FDA believes that these alternative monoclonal antibody therapies remain appropriate to treat patients with COVID-19, and I would like to see some data about the benefits and risks of these agents,” he noted.
Limitations, funding, and disclosures
The main limitation of the study was the small size and the fact that it was a subgroup analysis of a trial that ended early because of futility, the researchers wrote. However, the Therapeutics for Inpatients With COVID-19 (TICO) platform will proceed with clinical evaluation of additional COVID-19 treatments, they said.
The study was supported primarily by the U.S. government Operation Warp Speed and the National Institute of Allergy and Infectious Diseases. Other funding sources included the Division of Clinical Research and Leidos Biomedical Research for the INSIGHT (International Network for Strategic Initiatives in Global HIV Trials) Network, as well as an agreement between the National Heart, Lung, and Blood Institute and the Research Triangle Institute for the PETAL (Prevention & Early Treatment of Acute Lung Injury) Network and CTSN (Cardiothoracic Surgical Trials Network). Other support came from the U.S. Department of Veterans Affairs and the governments of Denmark (National Research Foundation), Australia (National Health and Medical Research Council), and the United Kingdom (Medical Research Council).
The medications used in the study were donated by Gilead Sciences and Eli Lilly.
The researchers had no financial conflicts do disclose. Dr. Deep and Dr. Pal had no relevant financial conflicts to disclose.
FROM ANNALS OF INTERNAL MEDICINE
Children and COVID: New cases up slightly, vaccinations continue to slow
New COVID-19 vaccinations in children were down by almost 24% in the last week as new cases rose by just 3.5%, based on new data.
That fairly low number suggests the latest case count from the American Academy of Pediatrics and the Children’s Hospital Association has not caught up yet to the reality of the Omicron variant, which has sent new cases climbing among all ages and now represents the majority of COVID-19 infections nationwide, the Centers for Disease Control and Prevention said.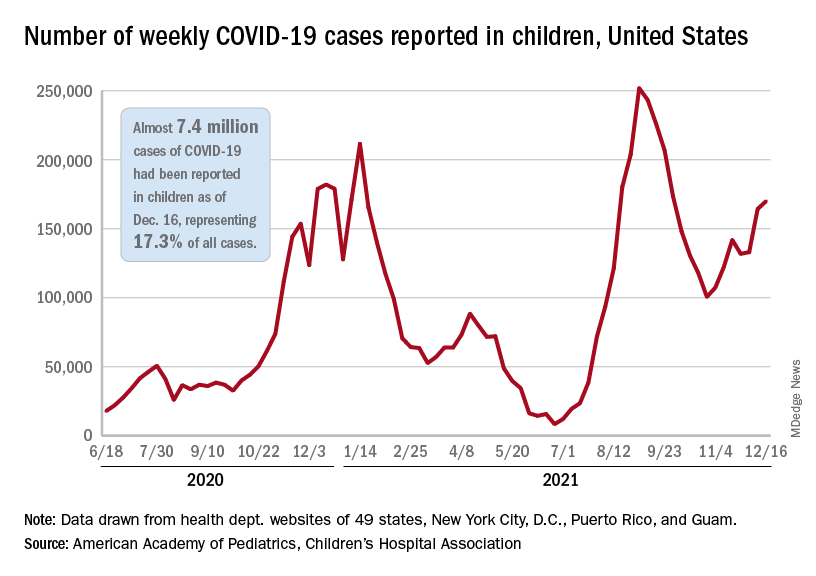
Meanwhile, in the midst of the latest surge, the United States just passed yet another sobering COVID milestone: 1,000 deaths in children aged 17 and under. The total as of Dec. 20 was 1,015, according to the CDC, with the largest share, almost 32%, occurring in children less than 5 years of age.
Regionally, the majority of that increase came in the Northeast, with a small rise in the South and decreases in the Midwest and West, the AAP and CHA said in their weekly COVID report.
At the state level, the largest percent increases in cases over the past 2 weeks were seen in Maine and New Hampshire, as well as Vermont, which has the nation’s highest vaccination rates for children aged 5-11 (51%) and 12-17 (84%), the AAP said in its vaccination trends report.
Nationally, new COVID vaccinations in children continue to trend downward. The number of children aged 5-17 years who had received at least one dose increased by about 498,000 for the week of Dec. 13-19, down from 654,000 (–23.9%) the previous week. Children aged 5-11 years still represented the largest share (22.7%) of all vaccine initiators in the last 2 weeks, but that proportion was 42.8% just before Thanksgiving, according to data from the CDC.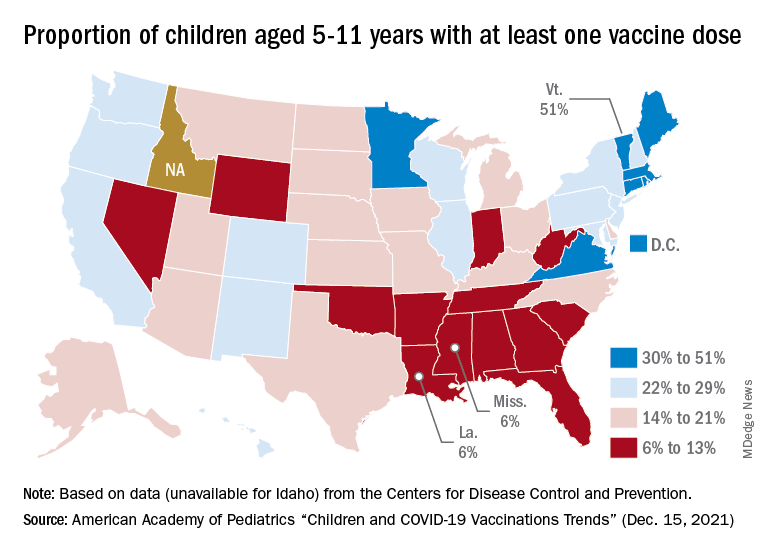
On a more positive note, children aged 5-11 made up 51% of all Americans who completed the vaccine regimen during the 2 weeks ending Dec. 20. The cumulative completion count is 3.6 million in that age group, along with almost 13.4 million children aged 12-17, and the CDC data show that 6.1 million children aged 5-11 and 15.9 million children aged 12-17 have received at least one dose.
On a less positive note, however, that means almost half (47%) of 12- to 17-year-olds still are not fully vaccinated and that over a third (37%) have received no vaccine at all, according to the COVID Data Tracker.
New COVID-19 vaccinations in children were down by almost 24% in the last week as new cases rose by just 3.5%, based on new data.
That fairly low number suggests the latest case count from the American Academy of Pediatrics and the Children’s Hospital Association has not caught up yet to the reality of the Omicron variant, which has sent new cases climbing among all ages and now represents the majority of COVID-19 infections nationwide, the Centers for Disease Control and Prevention said.
Meanwhile, in the midst of the latest surge, the United States just passed yet another sobering COVID milestone: 1,000 deaths in children aged 17 and under. The total as of Dec. 20 was 1,015, according to the CDC, with the largest share, almost 32%, occurring in children less than 5 years of age.
Regionally, the majority of that increase came in the Northeast, with a small rise in the South and decreases in the Midwest and West, the AAP and CHA said in their weekly COVID report.
At the state level, the largest percent increases in cases over the past 2 weeks were seen in Maine and New Hampshire, as well as Vermont, which has the nation’s highest vaccination rates for children aged 5-11 (51%) and 12-17 (84%), the AAP said in its vaccination trends report.
Nationally, new COVID vaccinations in children continue to trend downward. The number of children aged 5-17 years who had received at least one dose increased by about 498,000 for the week of Dec. 13-19, down from 654,000 (–23.9%) the previous week. Children aged 5-11 years still represented the largest share (22.7%) of all vaccine initiators in the last 2 weeks, but that proportion was 42.8% just before Thanksgiving, according to data from the CDC.
On a more positive note, children aged 5-11 made up 51% of all Americans who completed the vaccine regimen during the 2 weeks ending Dec. 20. The cumulative completion count is 3.6 million in that age group, along with almost 13.4 million children aged 12-17, and the CDC data show that 6.1 million children aged 5-11 and 15.9 million children aged 12-17 have received at least one dose.
On a less positive note, however, that means almost half (47%) of 12- to 17-year-olds still are not fully vaccinated and that over a third (37%) have received no vaccine at all, according to the COVID Data Tracker.
New COVID-19 vaccinations in children were down by almost 24% in the last week as new cases rose by just 3.5%, based on new data.
That fairly low number suggests the latest case count from the American Academy of Pediatrics and the Children’s Hospital Association has not caught up yet to the reality of the Omicron variant, which has sent new cases climbing among all ages and now represents the majority of COVID-19 infections nationwide, the Centers for Disease Control and Prevention said.
Meanwhile, in the midst of the latest surge, the United States just passed yet another sobering COVID milestone: 1,000 deaths in children aged 17 and under. The total as of Dec. 20 was 1,015, according to the CDC, with the largest share, almost 32%, occurring in children less than 5 years of age.
Regionally, the majority of that increase came in the Northeast, with a small rise in the South and decreases in the Midwest and West, the AAP and CHA said in their weekly COVID report.
At the state level, the largest percent increases in cases over the past 2 weeks were seen in Maine and New Hampshire, as well as Vermont, which has the nation’s highest vaccination rates for children aged 5-11 (51%) and 12-17 (84%), the AAP said in its vaccination trends report.
Nationally, new COVID vaccinations in children continue to trend downward. The number of children aged 5-17 years who had received at least one dose increased by about 498,000 for the week of Dec. 13-19, down from 654,000 (–23.9%) the previous week. Children aged 5-11 years still represented the largest share (22.7%) of all vaccine initiators in the last 2 weeks, but that proportion was 42.8% just before Thanksgiving, according to data from the CDC.
On a more positive note, children aged 5-11 made up 51% of all Americans who completed the vaccine regimen during the 2 weeks ending Dec. 20. The cumulative completion count is 3.6 million in that age group, along with almost 13.4 million children aged 12-17, and the CDC data show that 6.1 million children aged 5-11 and 15.9 million children aged 12-17 have received at least one dose.
On a less positive note, however, that means almost half (47%) of 12- to 17-year-olds still are not fully vaccinated and that over a third (37%) have received no vaccine at all, according to the COVID Data Tracker.
RSV resurgence likely in wake of COVID-19
The impact of respiratory syncytial virus (RSV)will likely be greater in 2021 and 2022 in the United States than in previous years as a result of the ongoing COVID-19 pandemic, based on data from a simulation-modeling study involving approximately 19 million individuals.
Although RSV usually follows consistent patterns of timing and duration, the disease all but disappeared starting in March 2020 after the introduction of measures to mitigate the spread of COVID-19, Zhe Zheng, MBBS, of Yale University, New Haven, Conn., and colleagues wrote.
However, lifting of mitigation measures has resulted in emergence of RSV in various parts of the world in early 2021, and trends may be similar in the United States, but data are needed to plan for prophylaxis and hospital use, they noted.
In a study published in JAMA Network Open, the researchers developed a simulation model for epidemics of RSV based on historical data. They acquired inpatient records from New York during 2005-2014 and from California during 2003-2011. The primary clinical outcome was the estimated monthly hospitalizations for RSV.
The simulated study population was 19.45 million individuals. After evaluating several scenarios including continued low transmission associated with social distancing and other mitigation measures, the researchers focused on the likely scenario that introduction of RSV from other regions would likely spark RSV epidemics in the United States.
They determined that spring and summer 2021 would show an increase in hospitalizations for RSV. Overall, higher rates of virus introduction from other regions were associated with more intense spring and summer RSV epidemics, with the trade-off of smaller winter epidemics. In the model, the expected RSV epidemic in spring and summer 2021 in New York was small, with a peak incidence of 419 hospitalizations per 100,000 people in April; by contrast, for states with less seasonal variability, such as Florida, the model predicted a larger summer epidemic.
In the model, the mean age of hospitalization for children younger than 5 years for January 2022 was expected to be 1.17 years, compared with 0.84 years in January 2019, the researchers noted.
Across all age groups, the greatest relative increase in the incidence of RSV infection was predicted for children aged 1-4 years (ranging from 82% to 86%), as were lower respiratory infections (87%-101%) and hospitalization (99%-119%), compared with prepandemic levels.
Hospitalizations for children aged 1 year were predicted to double compared with prepandemic seasons; 707 per 100,000 children per year for 2021 and 2022 versus 355 per 100,000 children per year in a typical prepandemic season. However, the largest incidence of lower respiratory infections (30,075 per 100,000) was predicted for infants aged 3-5 months, and the largest incidence of hospitalizations (3,116 per 100,000) was predicted for infants younger than 3 months.
“Without virus importation, the risk of RSV infections across all age groups in the winter of 2021 and 2022 would be greater, as more susceptible individuals were spared from infections in the absence of summer epidemics,” the researchers noted.
The older mean hospitalization age seen in the model was similar to the reported median patient age in Australia both before the pandemic and during the reemergent RSV epidemic.
“This makes intuitive sense, since many children born in 2020 were spared from RSV infection due to the low virus activity; these children will be older when they get infected for the first time during the reemergent epidemics,” the researchers wrote. “Consequently, stakeholders should consider modifying prophylaxis guidelines to include high-risk infants less than 2 years of age for the 2021-2022 season.”
The study findings were limited by several factors including the lack of data on level of virus introduction or on the impact of lack of boosting on infants with only transplacentally acquired RSV antibodies, the researchers noted. Other limitations include the use of historical data and the lack of data on values outside those included in the model, as well as the inability to control for other factors that could influence RSV, such as vaccines or long-lasting antibodies.
However, the results suggest that the rate of imported infections is associated with RSV hospitalizations, and the model effectively captured the RSV epidemics in the United States in spring and summer 2021.
Models can guide clinical preparations
“Health care simulation modeling is a growing field, with very exciting implications,” Lenore Jarvis, MD, of George Washington University, Washington, said in an interview. The field has the potential ability to influence health care in a data-driven way, including, but not limited to, staffing and other hospital operations, as well as patient care decision-making. “In short, accurate modeling and predictions can help us to make informed health care decisions that can lead to increased quality of care, potential cost savings, and even to help save lives,” she said.
Although the details of transmission modeling were not mentioned in the study, the authors evaluated the performances of several models and scenarios. “Scenario 4, for example, was focused on in particular because it best captured the observed dynamics [for RSV] that emerged during the spring and summer of 2021,” Dr. Jarvis said.
“Pediatricians can speak to these trends firsthand. A decrease in expected RSV infections and hospitalizations in 2020, followed by an unprecedented and early increase in RSV infections and severity in 2021, and the factors that the authors account for make sense, such as reintroduction of RSV from other regions and low immunity in the population,” she said. “It also makes sense that, in these transmission modeling scenarios, the expected mean age of hospitalization because of RSV increased with a temporary (hopefully) increase in RSV hospitalizations in the 2021 season, and potentially the 2022 RSV season.”
As for additional research, Dr. Jarvis said she would like to see follow-up data on the RSV transmission modeling. “For example, with scenario 4, does this scenario continue to perform well in other time periods, such as the winter? If the modeling continues to be accurate during other periods of evaluation and reevaluation, this modeling could be very useful in helping pediatric clinics and hospitals to prepare for RSV care and hospital capacity management.”
The study was supported by grants to various researchers from the National Institute of Allergy and Infectious Diseases/National Institutes of Health, the National Center for Advancing Translational Science at the National Institutes of Health, and NIH Roadmap for Medical Research. Lead author Ms. Zheng had no financial conflicts to disclose. Her study coauthors disclosed relationships with companies including AbbVie, Merck, Pfizer, GlaxoSmithKline, MedImmune, and Janssen. Dr. Jarvis had no financial conflicts to disclose and serves on the Pediatric News editorial advisory board.
The impact of respiratory syncytial virus (RSV)will likely be greater in 2021 and 2022 in the United States than in previous years as a result of the ongoing COVID-19 pandemic, based on data from a simulation-modeling study involving approximately 19 million individuals.
Although RSV usually follows consistent patterns of timing and duration, the disease all but disappeared starting in March 2020 after the introduction of measures to mitigate the spread of COVID-19, Zhe Zheng, MBBS, of Yale University, New Haven, Conn., and colleagues wrote.
However, lifting of mitigation measures has resulted in emergence of RSV in various parts of the world in early 2021, and trends may be similar in the United States, but data are needed to plan for prophylaxis and hospital use, they noted.
In a study published in JAMA Network Open, the researchers developed a simulation model for epidemics of RSV based on historical data. They acquired inpatient records from New York during 2005-2014 and from California during 2003-2011. The primary clinical outcome was the estimated monthly hospitalizations for RSV.
The simulated study population was 19.45 million individuals. After evaluating several scenarios including continued low transmission associated with social distancing and other mitigation measures, the researchers focused on the likely scenario that introduction of RSV from other regions would likely spark RSV epidemics in the United States.
They determined that spring and summer 2021 would show an increase in hospitalizations for RSV. Overall, higher rates of virus introduction from other regions were associated with more intense spring and summer RSV epidemics, with the trade-off of smaller winter epidemics. In the model, the expected RSV epidemic in spring and summer 2021 in New York was small, with a peak incidence of 419 hospitalizations per 100,000 people in April; by contrast, for states with less seasonal variability, such as Florida, the model predicted a larger summer epidemic.
In the model, the mean age of hospitalization for children younger than 5 years for January 2022 was expected to be 1.17 years, compared with 0.84 years in January 2019, the researchers noted.
Across all age groups, the greatest relative increase in the incidence of RSV infection was predicted for children aged 1-4 years (ranging from 82% to 86%), as were lower respiratory infections (87%-101%) and hospitalization (99%-119%), compared with prepandemic levels.
Hospitalizations for children aged 1 year were predicted to double compared with prepandemic seasons; 707 per 100,000 children per year for 2021 and 2022 versus 355 per 100,000 children per year in a typical prepandemic season. However, the largest incidence of lower respiratory infections (30,075 per 100,000) was predicted for infants aged 3-5 months, and the largest incidence of hospitalizations (3,116 per 100,000) was predicted for infants younger than 3 months.
“Without virus importation, the risk of RSV infections across all age groups in the winter of 2021 and 2022 would be greater, as more susceptible individuals were spared from infections in the absence of summer epidemics,” the researchers noted.
The older mean hospitalization age seen in the model was similar to the reported median patient age in Australia both before the pandemic and during the reemergent RSV epidemic.
“This makes intuitive sense, since many children born in 2020 were spared from RSV infection due to the low virus activity; these children will be older when they get infected for the first time during the reemergent epidemics,” the researchers wrote. “Consequently, stakeholders should consider modifying prophylaxis guidelines to include high-risk infants less than 2 years of age for the 2021-2022 season.”
The study findings were limited by several factors including the lack of data on level of virus introduction or on the impact of lack of boosting on infants with only transplacentally acquired RSV antibodies, the researchers noted. Other limitations include the use of historical data and the lack of data on values outside those included in the model, as well as the inability to control for other factors that could influence RSV, such as vaccines or long-lasting antibodies.
However, the results suggest that the rate of imported infections is associated with RSV hospitalizations, and the model effectively captured the RSV epidemics in the United States in spring and summer 2021.
Models can guide clinical preparations
“Health care simulation modeling is a growing field, with very exciting implications,” Lenore Jarvis, MD, of George Washington University, Washington, said in an interview. The field has the potential ability to influence health care in a data-driven way, including, but not limited to, staffing and other hospital operations, as well as patient care decision-making. “In short, accurate modeling and predictions can help us to make informed health care decisions that can lead to increased quality of care, potential cost savings, and even to help save lives,” she said.
Although the details of transmission modeling were not mentioned in the study, the authors evaluated the performances of several models and scenarios. “Scenario 4, for example, was focused on in particular because it best captured the observed dynamics [for RSV] that emerged during the spring and summer of 2021,” Dr. Jarvis said.
“Pediatricians can speak to these trends firsthand. A decrease in expected RSV infections and hospitalizations in 2020, followed by an unprecedented and early increase in RSV infections and severity in 2021, and the factors that the authors account for make sense, such as reintroduction of RSV from other regions and low immunity in the population,” she said. “It also makes sense that, in these transmission modeling scenarios, the expected mean age of hospitalization because of RSV increased with a temporary (hopefully) increase in RSV hospitalizations in the 2021 season, and potentially the 2022 RSV season.”
As for additional research, Dr. Jarvis said she would like to see follow-up data on the RSV transmission modeling. “For example, with scenario 4, does this scenario continue to perform well in other time periods, such as the winter? If the modeling continues to be accurate during other periods of evaluation and reevaluation, this modeling could be very useful in helping pediatric clinics and hospitals to prepare for RSV care and hospital capacity management.”
The study was supported by grants to various researchers from the National Institute of Allergy and Infectious Diseases/National Institutes of Health, the National Center for Advancing Translational Science at the National Institutes of Health, and NIH Roadmap for Medical Research. Lead author Ms. Zheng had no financial conflicts to disclose. Her study coauthors disclosed relationships with companies including AbbVie, Merck, Pfizer, GlaxoSmithKline, MedImmune, and Janssen. Dr. Jarvis had no financial conflicts to disclose and serves on the Pediatric News editorial advisory board.
The impact of respiratory syncytial virus (RSV)will likely be greater in 2021 and 2022 in the United States than in previous years as a result of the ongoing COVID-19 pandemic, based on data from a simulation-modeling study involving approximately 19 million individuals.
Although RSV usually follows consistent patterns of timing and duration, the disease all but disappeared starting in March 2020 after the introduction of measures to mitigate the spread of COVID-19, Zhe Zheng, MBBS, of Yale University, New Haven, Conn., and colleagues wrote.
However, lifting of mitigation measures has resulted in emergence of RSV in various parts of the world in early 2021, and trends may be similar in the United States, but data are needed to plan for prophylaxis and hospital use, they noted.
In a study published in JAMA Network Open, the researchers developed a simulation model for epidemics of RSV based on historical data. They acquired inpatient records from New York during 2005-2014 and from California during 2003-2011. The primary clinical outcome was the estimated monthly hospitalizations for RSV.
The simulated study population was 19.45 million individuals. After evaluating several scenarios including continued low transmission associated with social distancing and other mitigation measures, the researchers focused on the likely scenario that introduction of RSV from other regions would likely spark RSV epidemics in the United States.
They determined that spring and summer 2021 would show an increase in hospitalizations for RSV. Overall, higher rates of virus introduction from other regions were associated with more intense spring and summer RSV epidemics, with the trade-off of smaller winter epidemics. In the model, the expected RSV epidemic in spring and summer 2021 in New York was small, with a peak incidence of 419 hospitalizations per 100,000 people in April; by contrast, for states with less seasonal variability, such as Florida, the model predicted a larger summer epidemic.
In the model, the mean age of hospitalization for children younger than 5 years for January 2022 was expected to be 1.17 years, compared with 0.84 years in January 2019, the researchers noted.
Across all age groups, the greatest relative increase in the incidence of RSV infection was predicted for children aged 1-4 years (ranging from 82% to 86%), as were lower respiratory infections (87%-101%) and hospitalization (99%-119%), compared with prepandemic levels.
Hospitalizations for children aged 1 year were predicted to double compared with prepandemic seasons; 707 per 100,000 children per year for 2021 and 2022 versus 355 per 100,000 children per year in a typical prepandemic season. However, the largest incidence of lower respiratory infections (30,075 per 100,000) was predicted for infants aged 3-5 months, and the largest incidence of hospitalizations (3,116 per 100,000) was predicted for infants younger than 3 months.
“Without virus importation, the risk of RSV infections across all age groups in the winter of 2021 and 2022 would be greater, as more susceptible individuals were spared from infections in the absence of summer epidemics,” the researchers noted.
The older mean hospitalization age seen in the model was similar to the reported median patient age in Australia both before the pandemic and during the reemergent RSV epidemic.
“This makes intuitive sense, since many children born in 2020 were spared from RSV infection due to the low virus activity; these children will be older when they get infected for the first time during the reemergent epidemics,” the researchers wrote. “Consequently, stakeholders should consider modifying prophylaxis guidelines to include high-risk infants less than 2 years of age for the 2021-2022 season.”
The study findings were limited by several factors including the lack of data on level of virus introduction or on the impact of lack of boosting on infants with only transplacentally acquired RSV antibodies, the researchers noted. Other limitations include the use of historical data and the lack of data on values outside those included in the model, as well as the inability to control for other factors that could influence RSV, such as vaccines or long-lasting antibodies.
However, the results suggest that the rate of imported infections is associated with RSV hospitalizations, and the model effectively captured the RSV epidemics in the United States in spring and summer 2021.
Models can guide clinical preparations
“Health care simulation modeling is a growing field, with very exciting implications,” Lenore Jarvis, MD, of George Washington University, Washington, said in an interview. The field has the potential ability to influence health care in a data-driven way, including, but not limited to, staffing and other hospital operations, as well as patient care decision-making. “In short, accurate modeling and predictions can help us to make informed health care decisions that can lead to increased quality of care, potential cost savings, and even to help save lives,” she said.
Although the details of transmission modeling were not mentioned in the study, the authors evaluated the performances of several models and scenarios. “Scenario 4, for example, was focused on in particular because it best captured the observed dynamics [for RSV] that emerged during the spring and summer of 2021,” Dr. Jarvis said.
“Pediatricians can speak to these trends firsthand. A decrease in expected RSV infections and hospitalizations in 2020, followed by an unprecedented and early increase in RSV infections and severity in 2021, and the factors that the authors account for make sense, such as reintroduction of RSV from other regions and low immunity in the population,” she said. “It also makes sense that, in these transmission modeling scenarios, the expected mean age of hospitalization because of RSV increased with a temporary (hopefully) increase in RSV hospitalizations in the 2021 season, and potentially the 2022 RSV season.”
As for additional research, Dr. Jarvis said she would like to see follow-up data on the RSV transmission modeling. “For example, with scenario 4, does this scenario continue to perform well in other time periods, such as the winter? If the modeling continues to be accurate during other periods of evaluation and reevaluation, this modeling could be very useful in helping pediatric clinics and hospitals to prepare for RSV care and hospital capacity management.”
The study was supported by grants to various researchers from the National Institute of Allergy and Infectious Diseases/National Institutes of Health, the National Center for Advancing Translational Science at the National Institutes of Health, and NIH Roadmap for Medical Research. Lead author Ms. Zheng had no financial conflicts to disclose. Her study coauthors disclosed relationships with companies including AbbVie, Merck, Pfizer, GlaxoSmithKline, MedImmune, and Janssen. Dr. Jarvis had no financial conflicts to disclose and serves on the Pediatric News editorial advisory board.
FROM JAMA NETWORK OPEN
BMJ slams ‘incompetent’ Facebook fact-checking of vaccine article
According to an open letter written by outgoing BMJ editor-in-chief Fiona Godlee, MD, and incoming editor-in-chief Kamran Abbasi, MD, Facebook hired a third-party contractor to evaluate the article’s findings. This resulted in “inaccurate, incompetent, and irresponsible” conclusions that “should be of concern to anyone who values and relies on sources such as the BMJ for reliable medical information.”
The article in question investigated data integrity concerns at Pfizer vaccine clinical trial sites. In September 2020, the letter states, a former employee of the research group involved in Pfizer’s main vaccine trials, Ventavia, reached out to the BMJ and “began providing ... dozens of internal company documents, photos, audio recordings, and emails.” According to the company’s website, Ventavia “played a significant part in [COVID-19 clinical trial] recruitment” and “has received recognition by Pfizer for their contribution to vaccine trials.”
It was previously reported that the whistle-blower is a former regional director who was involved in Pfizer’s vaccine trials in Texas during the fall of 2020. She alleges “the company falsified data, unblinded patients, employed inadequately trained vaccinators, and was slow to follow up on adverse events reported in Pfizer’s pivotal phase 3 trial.”
The images provided to the BMJ “showed needles discarded in a plastic biohazard bag instead of a sharps container box” and another displayed “vaccine packaging materials with trial participants’ identification numbers written on them left out in the open, potentially unblinding participants.”
Despite informing Ventavia, the director’s concerns went unaddressed. She then filed a complaint with the Food and Drug Administration and was subsequently fired the same day. The FDA did not investigate the director’s allegations, said Dr. Godlee and Dr. Abbasi, even though the evidence “revealed a host of poor clinical trial research practices occurring at Ventavia that could impact data integrity and patient safety.”
Article labeled as ‘hoax,’ without pointing out errors
The BMJ hired an investigative reporter to follow up on the clinical trial claims. The findings were published in an article on Nov. 2, 2021, after the article “went through ... the usual high-level legal and editorial oversight and peer review,” according to the journal.
However, by Nov. 10, the journal began receiving complaints from readers unable to share the article on social media. Others had their posts flagged with warnings, such as “missing context ... independent fact-checkers say this information could mislead people.” Administrators of various Facebook groups were notified that posts containing the article were “partly false.”
Readers were informed that Facebook contractor Lead Stories performed the article’s “fact check.” Lead Stories is “an award-winning innovative fact checking and debunking website” and “an active part of Facebook’s partnership with third-party fact checkers” – with the latter granting them “access to listings of content that has been flagged as potentially false by Facebook’s systems or its users.” The company said they “decide independently if we want to fact check it or not.”
Lead Stories stated that they “can enter our fact checks into a tool provided by Facebook and Facebook then uses our data to help slow down the spread of false information on its platform.” Although the contractor is compensated, Lead Stories claims they have “no say or influence over what we fact check or what our conclusions are.”
Both editors question the validity of the fact check performed by Lead Stories, as it failed to provide any “assertions of fact” as to what the BMJ got wrong. Moreover, the editors take issue with Lead Stories referring to the journal as a “news blog” and using the phrase “hoax-alert” in the URL when publishing the story on its site.
The BMJ has reached out to Lead Stories and Facebook, said the letter, but Lead Stories refuses to “change anything about their article or actions that have led to Facebook flagging our article.” Requests for Facebook to remove the “fact-checking” label and allow “readers to freely share the article on [Facebook’s] platform” have been unfruitful.
Dr. Godlee and Dr. Abbasi expressed concern that other “high quality information provider[s] have been affected by the incompetence of Meta’s fact checking regime.” In November, Instagram censored Cochrane, an international provider of independent systematic medical reviews. Instagram, also owned by Meta, prohibited users from tagging Cochrane because the organization “repeatedly posted ... false content about COVID-19 or vaccines.” Cochrane refuted the allegations.
While “fact checking has been a staple of good journalism for decades,” said the editors, Meta has “apparently delegated responsibility to people incompetent in carrying out this crucial task.” They urged the company to reconsider its fact-checking strategy and review the issues that contributed to the error.
This news organization reached out to Meta for comment but did not receive a response at press time.
Lead Stories has posted a reply (Lead Stories’ Response To BMJ Open Letter Objecting To A Lead Stories Fact Check) to the BMJ’s complaint on its website.
A version of this article first appeared on Medscape.com.
According to an open letter written by outgoing BMJ editor-in-chief Fiona Godlee, MD, and incoming editor-in-chief Kamran Abbasi, MD, Facebook hired a third-party contractor to evaluate the article’s findings. This resulted in “inaccurate, incompetent, and irresponsible” conclusions that “should be of concern to anyone who values and relies on sources such as the BMJ for reliable medical information.”
The article in question investigated data integrity concerns at Pfizer vaccine clinical trial sites. In September 2020, the letter states, a former employee of the research group involved in Pfizer’s main vaccine trials, Ventavia, reached out to the BMJ and “began providing ... dozens of internal company documents, photos, audio recordings, and emails.” According to the company’s website, Ventavia “played a significant part in [COVID-19 clinical trial] recruitment” and “has received recognition by Pfizer for their contribution to vaccine trials.”
It was previously reported that the whistle-blower is a former regional director who was involved in Pfizer’s vaccine trials in Texas during the fall of 2020. She alleges “the company falsified data, unblinded patients, employed inadequately trained vaccinators, and was slow to follow up on adverse events reported in Pfizer’s pivotal phase 3 trial.”
The images provided to the BMJ “showed needles discarded in a plastic biohazard bag instead of a sharps container box” and another displayed “vaccine packaging materials with trial participants’ identification numbers written on them left out in the open, potentially unblinding participants.”
Despite informing Ventavia, the director’s concerns went unaddressed. She then filed a complaint with the Food and Drug Administration and was subsequently fired the same day. The FDA did not investigate the director’s allegations, said Dr. Godlee and Dr. Abbasi, even though the evidence “revealed a host of poor clinical trial research practices occurring at Ventavia that could impact data integrity and patient safety.”
Article labeled as ‘hoax,’ without pointing out errors
The BMJ hired an investigative reporter to follow up on the clinical trial claims. The findings were published in an article on Nov. 2, 2021, after the article “went through ... the usual high-level legal and editorial oversight and peer review,” according to the journal.
However, by Nov. 10, the journal began receiving complaints from readers unable to share the article on social media. Others had their posts flagged with warnings, such as “missing context ... independent fact-checkers say this information could mislead people.” Administrators of various Facebook groups were notified that posts containing the article were “partly false.”
Readers were informed that Facebook contractor Lead Stories performed the article’s “fact check.” Lead Stories is “an award-winning innovative fact checking and debunking website” and “an active part of Facebook’s partnership with third-party fact checkers” – with the latter granting them “access to listings of content that has been flagged as potentially false by Facebook’s systems or its users.” The company said they “decide independently if we want to fact check it or not.”
Lead Stories stated that they “can enter our fact checks into a tool provided by Facebook and Facebook then uses our data to help slow down the spread of false information on its platform.” Although the contractor is compensated, Lead Stories claims they have “no say or influence over what we fact check or what our conclusions are.”
Both editors question the validity of the fact check performed by Lead Stories, as it failed to provide any “assertions of fact” as to what the BMJ got wrong. Moreover, the editors take issue with Lead Stories referring to the journal as a “news blog” and using the phrase “hoax-alert” in the URL when publishing the story on its site.
The BMJ has reached out to Lead Stories and Facebook, said the letter, but Lead Stories refuses to “change anything about their article or actions that have led to Facebook flagging our article.” Requests for Facebook to remove the “fact-checking” label and allow “readers to freely share the article on [Facebook’s] platform” have been unfruitful.
Dr. Godlee and Dr. Abbasi expressed concern that other “high quality information provider[s] have been affected by the incompetence of Meta’s fact checking regime.” In November, Instagram censored Cochrane, an international provider of independent systematic medical reviews. Instagram, also owned by Meta, prohibited users from tagging Cochrane because the organization “repeatedly posted ... false content about COVID-19 or vaccines.” Cochrane refuted the allegations.
While “fact checking has been a staple of good journalism for decades,” said the editors, Meta has “apparently delegated responsibility to people incompetent in carrying out this crucial task.” They urged the company to reconsider its fact-checking strategy and review the issues that contributed to the error.
This news organization reached out to Meta for comment but did not receive a response at press time.
Lead Stories has posted a reply (Lead Stories’ Response To BMJ Open Letter Objecting To A Lead Stories Fact Check) to the BMJ’s complaint on its website.
A version of this article first appeared on Medscape.com.
According to an open letter written by outgoing BMJ editor-in-chief Fiona Godlee, MD, and incoming editor-in-chief Kamran Abbasi, MD, Facebook hired a third-party contractor to evaluate the article’s findings. This resulted in “inaccurate, incompetent, and irresponsible” conclusions that “should be of concern to anyone who values and relies on sources such as the BMJ for reliable medical information.”
The article in question investigated data integrity concerns at Pfizer vaccine clinical trial sites. In September 2020, the letter states, a former employee of the research group involved in Pfizer’s main vaccine trials, Ventavia, reached out to the BMJ and “began providing ... dozens of internal company documents, photos, audio recordings, and emails.” According to the company’s website, Ventavia “played a significant part in [COVID-19 clinical trial] recruitment” and “has received recognition by Pfizer for their contribution to vaccine trials.”
It was previously reported that the whistle-blower is a former regional director who was involved in Pfizer’s vaccine trials in Texas during the fall of 2020. She alleges “the company falsified data, unblinded patients, employed inadequately trained vaccinators, and was slow to follow up on adverse events reported in Pfizer’s pivotal phase 3 trial.”
The images provided to the BMJ “showed needles discarded in a plastic biohazard bag instead of a sharps container box” and another displayed “vaccine packaging materials with trial participants’ identification numbers written on them left out in the open, potentially unblinding participants.”
Despite informing Ventavia, the director’s concerns went unaddressed. She then filed a complaint with the Food and Drug Administration and was subsequently fired the same day. The FDA did not investigate the director’s allegations, said Dr. Godlee and Dr. Abbasi, even though the evidence “revealed a host of poor clinical trial research practices occurring at Ventavia that could impact data integrity and patient safety.”
Article labeled as ‘hoax,’ without pointing out errors
The BMJ hired an investigative reporter to follow up on the clinical trial claims. The findings were published in an article on Nov. 2, 2021, after the article “went through ... the usual high-level legal and editorial oversight and peer review,” according to the journal.
However, by Nov. 10, the journal began receiving complaints from readers unable to share the article on social media. Others had their posts flagged with warnings, such as “missing context ... independent fact-checkers say this information could mislead people.” Administrators of various Facebook groups were notified that posts containing the article were “partly false.”
Readers were informed that Facebook contractor Lead Stories performed the article’s “fact check.” Lead Stories is “an award-winning innovative fact checking and debunking website” and “an active part of Facebook’s partnership with third-party fact checkers” – with the latter granting them “access to listings of content that has been flagged as potentially false by Facebook’s systems or its users.” The company said they “decide independently if we want to fact check it or not.”
Lead Stories stated that they “can enter our fact checks into a tool provided by Facebook and Facebook then uses our data to help slow down the spread of false information on its platform.” Although the contractor is compensated, Lead Stories claims they have “no say or influence over what we fact check or what our conclusions are.”
Both editors question the validity of the fact check performed by Lead Stories, as it failed to provide any “assertions of fact” as to what the BMJ got wrong. Moreover, the editors take issue with Lead Stories referring to the journal as a “news blog” and using the phrase “hoax-alert” in the URL when publishing the story on its site.
The BMJ has reached out to Lead Stories and Facebook, said the letter, but Lead Stories refuses to “change anything about their article or actions that have led to Facebook flagging our article.” Requests for Facebook to remove the “fact-checking” label and allow “readers to freely share the article on [Facebook’s] platform” have been unfruitful.
Dr. Godlee and Dr. Abbasi expressed concern that other “high quality information provider[s] have been affected by the incompetence of Meta’s fact checking regime.” In November, Instagram censored Cochrane, an international provider of independent systematic medical reviews. Instagram, also owned by Meta, prohibited users from tagging Cochrane because the organization “repeatedly posted ... false content about COVID-19 or vaccines.” Cochrane refuted the allegations.
While “fact checking has been a staple of good journalism for decades,” said the editors, Meta has “apparently delegated responsibility to people incompetent in carrying out this crucial task.” They urged the company to reconsider its fact-checking strategy and review the issues that contributed to the error.
This news organization reached out to Meta for comment but did not receive a response at press time.
Lead Stories has posted a reply (Lead Stories’ Response To BMJ Open Letter Objecting To A Lead Stories Fact Check) to the BMJ’s complaint on its website.
A version of this article first appeared on Medscape.com.
COVID-19 and coping with superimposed traumas
While 2022 is lurking around the corner, many of us still have 2020 on our minds. Social media posts are already emerging: “No new years resolutions. It is the circumstances turn to improve [sic],” one post declares. Others proclaim that it is difficult coming to terms with the idea that 2022 is actually pronounced “2020 too.” A critical difference exists between then and now – we have experienced months of living in limbo and rolling with the punches of pandemic life.
In some ways, it has become easy to think of the early pandemic days as a distant memory, yet respect that the impact of 2020 has been indelible for virtually all of us and feels palpable as if it were yesterday.
The year 2020 was marked by the beginning of the COVID-19 pandemic, which was accompanied by extreme uncertainty, loss of all kinds, and emotional turmoil. The early pandemic had a profound economic and social impact, with added stress tethered to political and race-related division in America that created divides among families and friends, and yielded ceaseless discourse related to divergent perspectives. This only exacerbated the stress that came with the pandemic, given that providing support and leaning on one another was more important than ever. All of this was compounded by natural disasters that have plagued the country.
So much was unprecedented. There was a collective sense of feeling “worn down,” and the burnout that was felt was quite profound. Enormous amounts of mental and physical effort were allocated to simply surviving, getting basic needs met, having enough food and supplies, and completing basic tasks. Ordinary relating felt taxing. At this stage of the pandemic, the COVID-19 experience can be conceived of as a traumatic stressor capable of eliciting a traumatic response and exacerbating other mental health symptoms. Our capacity to cope has been diminished. Anxiety rates have soared, as have rates of clinical depression. Those most affected have had lower household incomes, are unmarried, and have experienced pandemic-related stressors. The links between the impact of the pandemic on mental health have been clear.
The pandemic has forced the landscape of social support to dramatically change. Initially, we felt pulled to connect and we leaned into the use of virtual platforms to connect for all matters (simple social gatherings, big birthday events, family reunions, celebration of holidays, work duties, and academic work). However, “Zoom fatigue” began to set in, and our screen time was maxed out. There has been the added dynamic of frontline workers who did not have the option to work virtually or from home. This group largely has felt disconnected from others who didn’t understand the depth of their anxiety and loneliness of their experience. Health care workers have had to make challenging, life-and-death, patient-related decisions that called into question personal morals and ethics all while their own lives were at risk.
Fast-forward to the present, and support systems have either strengthened or worn down – which has yielded a unique dichotomy. Maintaining friendships has either felt of utmost importance given the impact of the disconnect and physical distance or has felt challenging given the mental energy expended from working and connecting virtually. Empathy burnout is also a real and important facet in the equation. We begin to ask the question: Are we checking in with others in the spirit of authentic relating, to cultivate real connection, or to check a box?
Impact of layered traumas
It is interesting to think about the pandemic’s traumatic impact being “superimposed” on top of the “ordinary traumas” experienced outside of the pandemic. We are essentially at the 2-year mark, in some ways have cultivated a sense of resilience and found ways to adapt, and in other ways at times feel right back where we were in early 2020. There were moments that felt hopeful, glimmers of normalcy, and setbacks that all ebbed and flowed – but even so, there have not been many “mental breaks,” only temporary and transient reprieves. Some got sick and died; some recovered; and others are still experiencing long-hauler syndrome and have lingering sequelae. Despite adaptation and resilience, one can’t help but wonder the impact of superimposed traumas on top of this collective trauma. Many of us have not even rebounded from the pandemic, and then are faced with loss, grief, challenges, illness, hard and big life decisions. We are challenged to answer the question: How do we endure in the face of this trauma inception?
It has been a challenging time for all, including those who are ordinarily happy-go-lucky, resilient, and see the glass half-full and are struggling with the idea of struggling. I am no “resilience expert” but gleaned much wisdom from responding to the Surfside, Fla., building collapse. This was a collective trauma that took place in the summer of 2021, and the wisdom of this event highlighted the value of collective healing and unification even in spite of the times. What happened in Surfside was a shock, and the loss was felt by those directly affected, the surrounding community, and those who were part of the disaster response efforts. All of those parties had been processing losses prior to this – loss of normalcy because of the pandemic, loss of people we loved as a result, other personal losses – and this community tragedy was yet another loss to disentangle on top of a period in U.S. history demarcated by a great lack of unity, divisiveness, anger, and hatred. The collapse highlighted the small size yet interconnectedness of the community and the power of connection and authentic relating. It was overwhelming in the moment but extremely heartening and beautiful to see the amount of willingness to drop everything and help. Despite feeling worn down from the pandemic, people drew upon their internal resources, natural goodness, and kindness “reserves” to provide support.
Responding to the collapse highlighted that resilience in the context of collective trauma requires flexibility, embracing uncertainty, cultivating unity, and paying attention to meeting basic needs/self-care. The role of kindness cannot be overemphasized. In the realm of reflecting on the notion of kindness, it is worth noting how much power there is to bearing witness to someone’s experience, especially when they are in pain. People often diminish the role or at the very least do not recognize the power of showing up for someone and just listening. Pandemic resilience, and coping with coalescing traumas, is likely composed of these same facets that were essential in the context of coping with the collapse.
It is not only the immediate impact of a trauma as much as the aftermath that needs to processed and worked through. In one sense, people feel that they should be adjusted to and accustomed to this new reality, and at the same time, one has to remember and reflect on how unnatural this experience has been. There is an impact of a cumulative onslaught of negative events, and it is hard to imagine not being phased, remaining unchanged, or not feeling affected. We may feel hardened and that there are limits to the compassion we have to offer others. We may be feel empathic. There can be desensitization and an apathy to others’ suffering when our patience is worn down and we have limited bandwidth. There are data to support the idea that a level of habituation occurs to individuals who experience multiple traumas, which yields a level of “sensitization” to the negative impact of subsequent events. It becomes easy to make comparisons of suffering. The challenge will be to rise above these and make a conscious effort to connect with who and how we were before we were worn down.
I am still in awe about how much I learned from the victims’ families, survivors, and my colleagues at Surfside – about pain, suffering, loss, resilience, coping, fortitude, and meaning making. We were all forced to think beyond ourselves, show up for others, and unify in a way that remedied this period of fragmentation. With respect to the pandemic and “where we are at now,” some elements of our lives are stabilizing; other aspects feel volatile from the fatigue of what we have been experiencing. This pandemic has not fully abated, but we can find some clarity in the value of setting boundaries and knowing our limits – but not overlooking the power of unity and kindness and the value of the reciprocating those qualities.
Dr. Feldman is a licensed clinical psychologist in private practice in Miami. She is an adjunct professor in the college of psychology at Nova Southeastern University, Fort Lauderdale, Fla., where she teaches clinical psychology doctoral students. She also serves on the board of directors of the Southeast Florida Association for Psychoanalytic Psychology. Dr. Feldman has no disclosures.
While 2022 is lurking around the corner, many of us still have 2020 on our minds. Social media posts are already emerging: “No new years resolutions. It is the circumstances turn to improve [sic],” one post declares. Others proclaim that it is difficult coming to terms with the idea that 2022 is actually pronounced “2020 too.” A critical difference exists between then and now – we have experienced months of living in limbo and rolling with the punches of pandemic life.
In some ways, it has become easy to think of the early pandemic days as a distant memory, yet respect that the impact of 2020 has been indelible for virtually all of us and feels palpable as if it were yesterday.
The year 2020 was marked by the beginning of the COVID-19 pandemic, which was accompanied by extreme uncertainty, loss of all kinds, and emotional turmoil. The early pandemic had a profound economic and social impact, with added stress tethered to political and race-related division in America that created divides among families and friends, and yielded ceaseless discourse related to divergent perspectives. This only exacerbated the stress that came with the pandemic, given that providing support and leaning on one another was more important than ever. All of this was compounded by natural disasters that have plagued the country.
So much was unprecedented. There was a collective sense of feeling “worn down,” and the burnout that was felt was quite profound. Enormous amounts of mental and physical effort were allocated to simply surviving, getting basic needs met, having enough food and supplies, and completing basic tasks. Ordinary relating felt taxing. At this stage of the pandemic, the COVID-19 experience can be conceived of as a traumatic stressor capable of eliciting a traumatic response and exacerbating other mental health symptoms. Our capacity to cope has been diminished. Anxiety rates have soared, as have rates of clinical depression. Those most affected have had lower household incomes, are unmarried, and have experienced pandemic-related stressors. The links between the impact of the pandemic on mental health have been clear.
The pandemic has forced the landscape of social support to dramatically change. Initially, we felt pulled to connect and we leaned into the use of virtual platforms to connect for all matters (simple social gatherings, big birthday events, family reunions, celebration of holidays, work duties, and academic work). However, “Zoom fatigue” began to set in, and our screen time was maxed out. There has been the added dynamic of frontline workers who did not have the option to work virtually or from home. This group largely has felt disconnected from others who didn’t understand the depth of their anxiety and loneliness of their experience. Health care workers have had to make challenging, life-and-death, patient-related decisions that called into question personal morals and ethics all while their own lives were at risk.
Fast-forward to the present, and support systems have either strengthened or worn down – which has yielded a unique dichotomy. Maintaining friendships has either felt of utmost importance given the impact of the disconnect and physical distance or has felt challenging given the mental energy expended from working and connecting virtually. Empathy burnout is also a real and important facet in the equation. We begin to ask the question: Are we checking in with others in the spirit of authentic relating, to cultivate real connection, or to check a box?
Impact of layered traumas
It is interesting to think about the pandemic’s traumatic impact being “superimposed” on top of the “ordinary traumas” experienced outside of the pandemic. We are essentially at the 2-year mark, in some ways have cultivated a sense of resilience and found ways to adapt, and in other ways at times feel right back where we were in early 2020. There were moments that felt hopeful, glimmers of normalcy, and setbacks that all ebbed and flowed – but even so, there have not been many “mental breaks,” only temporary and transient reprieves. Some got sick and died; some recovered; and others are still experiencing long-hauler syndrome and have lingering sequelae. Despite adaptation and resilience, one can’t help but wonder the impact of superimposed traumas on top of this collective trauma. Many of us have not even rebounded from the pandemic, and then are faced with loss, grief, challenges, illness, hard and big life decisions. We are challenged to answer the question: How do we endure in the face of this trauma inception?
It has been a challenging time for all, including those who are ordinarily happy-go-lucky, resilient, and see the glass half-full and are struggling with the idea of struggling. I am no “resilience expert” but gleaned much wisdom from responding to the Surfside, Fla., building collapse. This was a collective trauma that took place in the summer of 2021, and the wisdom of this event highlighted the value of collective healing and unification even in spite of the times. What happened in Surfside was a shock, and the loss was felt by those directly affected, the surrounding community, and those who were part of the disaster response efforts. All of those parties had been processing losses prior to this – loss of normalcy because of the pandemic, loss of people we loved as a result, other personal losses – and this community tragedy was yet another loss to disentangle on top of a period in U.S. history demarcated by a great lack of unity, divisiveness, anger, and hatred. The collapse highlighted the small size yet interconnectedness of the community and the power of connection and authentic relating. It was overwhelming in the moment but extremely heartening and beautiful to see the amount of willingness to drop everything and help. Despite feeling worn down from the pandemic, people drew upon their internal resources, natural goodness, and kindness “reserves” to provide support.
Responding to the collapse highlighted that resilience in the context of collective trauma requires flexibility, embracing uncertainty, cultivating unity, and paying attention to meeting basic needs/self-care. The role of kindness cannot be overemphasized. In the realm of reflecting on the notion of kindness, it is worth noting how much power there is to bearing witness to someone’s experience, especially when they are in pain. People often diminish the role or at the very least do not recognize the power of showing up for someone and just listening. Pandemic resilience, and coping with coalescing traumas, is likely composed of these same facets that were essential in the context of coping with the collapse.
It is not only the immediate impact of a trauma as much as the aftermath that needs to processed and worked through. In one sense, people feel that they should be adjusted to and accustomed to this new reality, and at the same time, one has to remember and reflect on how unnatural this experience has been. There is an impact of a cumulative onslaught of negative events, and it is hard to imagine not being phased, remaining unchanged, or not feeling affected. We may feel hardened and that there are limits to the compassion we have to offer others. We may be feel empathic. There can be desensitization and an apathy to others’ suffering when our patience is worn down and we have limited bandwidth. There are data to support the idea that a level of habituation occurs to individuals who experience multiple traumas, which yields a level of “sensitization” to the negative impact of subsequent events. It becomes easy to make comparisons of suffering. The challenge will be to rise above these and make a conscious effort to connect with who and how we were before we were worn down.
I am still in awe about how much I learned from the victims’ families, survivors, and my colleagues at Surfside – about pain, suffering, loss, resilience, coping, fortitude, and meaning making. We were all forced to think beyond ourselves, show up for others, and unify in a way that remedied this period of fragmentation. With respect to the pandemic and “where we are at now,” some elements of our lives are stabilizing; other aspects feel volatile from the fatigue of what we have been experiencing. This pandemic has not fully abated, but we can find some clarity in the value of setting boundaries and knowing our limits – but not overlooking the power of unity and kindness and the value of the reciprocating those qualities.
Dr. Feldman is a licensed clinical psychologist in private practice in Miami. She is an adjunct professor in the college of psychology at Nova Southeastern University, Fort Lauderdale, Fla., where she teaches clinical psychology doctoral students. She also serves on the board of directors of the Southeast Florida Association for Psychoanalytic Psychology. Dr. Feldman has no disclosures.
While 2022 is lurking around the corner, many of us still have 2020 on our minds. Social media posts are already emerging: “No new years resolutions. It is the circumstances turn to improve [sic],” one post declares. Others proclaim that it is difficult coming to terms with the idea that 2022 is actually pronounced “2020 too.” A critical difference exists between then and now – we have experienced months of living in limbo and rolling with the punches of pandemic life.
In some ways, it has become easy to think of the early pandemic days as a distant memory, yet respect that the impact of 2020 has been indelible for virtually all of us and feels palpable as if it were yesterday.
The year 2020 was marked by the beginning of the COVID-19 pandemic, which was accompanied by extreme uncertainty, loss of all kinds, and emotional turmoil. The early pandemic had a profound economic and social impact, with added stress tethered to political and race-related division in America that created divides among families and friends, and yielded ceaseless discourse related to divergent perspectives. This only exacerbated the stress that came with the pandemic, given that providing support and leaning on one another was more important than ever. All of this was compounded by natural disasters that have plagued the country.
So much was unprecedented. There was a collective sense of feeling “worn down,” and the burnout that was felt was quite profound. Enormous amounts of mental and physical effort were allocated to simply surviving, getting basic needs met, having enough food and supplies, and completing basic tasks. Ordinary relating felt taxing. At this stage of the pandemic, the COVID-19 experience can be conceived of as a traumatic stressor capable of eliciting a traumatic response and exacerbating other mental health symptoms. Our capacity to cope has been diminished. Anxiety rates have soared, as have rates of clinical depression. Those most affected have had lower household incomes, are unmarried, and have experienced pandemic-related stressors. The links between the impact of the pandemic on mental health have been clear.
The pandemic has forced the landscape of social support to dramatically change. Initially, we felt pulled to connect and we leaned into the use of virtual platforms to connect for all matters (simple social gatherings, big birthday events, family reunions, celebration of holidays, work duties, and academic work). However, “Zoom fatigue” began to set in, and our screen time was maxed out. There has been the added dynamic of frontline workers who did not have the option to work virtually or from home. This group largely has felt disconnected from others who didn’t understand the depth of their anxiety and loneliness of their experience. Health care workers have had to make challenging, life-and-death, patient-related decisions that called into question personal morals and ethics all while their own lives were at risk.
Fast-forward to the present, and support systems have either strengthened or worn down – which has yielded a unique dichotomy. Maintaining friendships has either felt of utmost importance given the impact of the disconnect and physical distance or has felt challenging given the mental energy expended from working and connecting virtually. Empathy burnout is also a real and important facet in the equation. We begin to ask the question: Are we checking in with others in the spirit of authentic relating, to cultivate real connection, or to check a box?
Impact of layered traumas
It is interesting to think about the pandemic’s traumatic impact being “superimposed” on top of the “ordinary traumas” experienced outside of the pandemic. We are essentially at the 2-year mark, in some ways have cultivated a sense of resilience and found ways to adapt, and in other ways at times feel right back where we were in early 2020. There were moments that felt hopeful, glimmers of normalcy, and setbacks that all ebbed and flowed – but even so, there have not been many “mental breaks,” only temporary and transient reprieves. Some got sick and died; some recovered; and others are still experiencing long-hauler syndrome and have lingering sequelae. Despite adaptation and resilience, one can’t help but wonder the impact of superimposed traumas on top of this collective trauma. Many of us have not even rebounded from the pandemic, and then are faced with loss, grief, challenges, illness, hard and big life decisions. We are challenged to answer the question: How do we endure in the face of this trauma inception?
It has been a challenging time for all, including those who are ordinarily happy-go-lucky, resilient, and see the glass half-full and are struggling with the idea of struggling. I am no “resilience expert” but gleaned much wisdom from responding to the Surfside, Fla., building collapse. This was a collective trauma that took place in the summer of 2021, and the wisdom of this event highlighted the value of collective healing and unification even in spite of the times. What happened in Surfside was a shock, and the loss was felt by those directly affected, the surrounding community, and those who were part of the disaster response efforts. All of those parties had been processing losses prior to this – loss of normalcy because of the pandemic, loss of people we loved as a result, other personal losses – and this community tragedy was yet another loss to disentangle on top of a period in U.S. history demarcated by a great lack of unity, divisiveness, anger, and hatred. The collapse highlighted the small size yet interconnectedness of the community and the power of connection and authentic relating. It was overwhelming in the moment but extremely heartening and beautiful to see the amount of willingness to drop everything and help. Despite feeling worn down from the pandemic, people drew upon their internal resources, natural goodness, and kindness “reserves” to provide support.
Responding to the collapse highlighted that resilience in the context of collective trauma requires flexibility, embracing uncertainty, cultivating unity, and paying attention to meeting basic needs/self-care. The role of kindness cannot be overemphasized. In the realm of reflecting on the notion of kindness, it is worth noting how much power there is to bearing witness to someone’s experience, especially when they are in pain. People often diminish the role or at the very least do not recognize the power of showing up for someone and just listening. Pandemic resilience, and coping with coalescing traumas, is likely composed of these same facets that were essential in the context of coping with the collapse.
It is not only the immediate impact of a trauma as much as the aftermath that needs to processed and worked through. In one sense, people feel that they should be adjusted to and accustomed to this new reality, and at the same time, one has to remember and reflect on how unnatural this experience has been. There is an impact of a cumulative onslaught of negative events, and it is hard to imagine not being phased, remaining unchanged, or not feeling affected. We may feel hardened and that there are limits to the compassion we have to offer others. We may be feel empathic. There can be desensitization and an apathy to others’ suffering when our patience is worn down and we have limited bandwidth. There are data to support the idea that a level of habituation occurs to individuals who experience multiple traumas, which yields a level of “sensitization” to the negative impact of subsequent events. It becomes easy to make comparisons of suffering. The challenge will be to rise above these and make a conscious effort to connect with who and how we were before we were worn down.
I am still in awe about how much I learned from the victims’ families, survivors, and my colleagues at Surfside – about pain, suffering, loss, resilience, coping, fortitude, and meaning making. We were all forced to think beyond ourselves, show up for others, and unify in a way that remedied this period of fragmentation. With respect to the pandemic and “where we are at now,” some elements of our lives are stabilizing; other aspects feel volatile from the fatigue of what we have been experiencing. This pandemic has not fully abated, but we can find some clarity in the value of setting boundaries and knowing our limits – but not overlooking the power of unity and kindness and the value of the reciprocating those qualities.
Dr. Feldman is a licensed clinical psychologist in private practice in Miami. She is an adjunct professor in the college of psychology at Nova Southeastern University, Fort Lauderdale, Fla., where she teaches clinical psychology doctoral students. She also serves on the board of directors of the Southeast Florida Association for Psychoanalytic Psychology. Dr. Feldman has no disclosures.
Axilla swelling after COVID booster puts focus on mammogram timing
This inflammation is caused by the enlargement of lymph nodes and can show up as an abnormal finding on mammograms and other types of chest scans, causing concern and even the need for additional imaging and follow up, wrote Constance D. Lehman, MD, PhD, and colleagues in an article published in Journal of the American College of Radiology.
Lymph node swelling is a normal immune system reaction to vaccination, and “COVID-19 vaccinations in the arm are a well-documented cause of inflammatory unilateral axillary adenopathy,” noted Dr. Lehman, in an interview. The side effect will occur on the side of the body where the patient received a vaccine, and it is not always noticeable to the woman experiencing it, she said.
“We’re finding that the patients’ bodies are responding to the booster in many ways that are similar to the initial COVID vaccines, with lymph node swelling, muscle aches and pains, headaches, and so on,” said Dr. Lehman, who is chief of breast imaging at the Massachusetts General Hospital, Boston. There have been no real differences in reactions between the Moderna and Pfizer vaccines, she added.
Because axillary lymph node swelling can obscure mammogram results, staff of at least a few imaging centers, including Penn State Breast Center in Hershey, Pa., and Providence Women’s Imaging Center in Torrance, Calif., told this news organization that they are asking women to delay mammogram imaging either 6 weeks or 4-6 weeks after getting a COVID-19 booster.
Experts’ suggestions on mammograms, boosters timing
Other experts, including Jessica Leung, MD, acknowledged that vaccine-related reactive adenopathy is seen after the booster dose and provided recommendations for the timing of getting mammograms and the booster with this in mind.
“I would recommend getting the screening mammogram first, which can be followed immediately by vaccination, even on the same day,” said Jessica Leung, MD, a professor of diagnostic radiology at the University of Texas MD Anderson Cancer Center in Houston, Tex.
“If this is not possible from the scheduling perspective, then the patient should consult her health care provider regarding whether it is okay to wait a bit after receiving the vaccine before getting her screening mammogram.”
The answer to that question will likely depend on the time interval since the prior mammogram and the patient’s personal risk factors for developing breast cancer. Dr. Leung noted. “This is all predicated on the assumption that the patient is asymptomatic. If she has any symptoms, for example a palpable breast lump, then she should seek medical attention regardless of timing of vaccination.”
The same holds true for boosters, she said.
She emphasized that careful consideration should be given before delaying the mammogram. “The medical community has a great deal more knowledge at this time than in the early days of COVID-19 vaccination, so we are often able to identify reactive adenopathy related to vaccination. If patients were to delay the mammogram, any reactive adenopathy may persist, on average, for 4-6 weeks.”
Debra Patt, MD, PhD, MBA, executive vice president at Texas Oncology, professor at the University of Texas at Austin, provided a specific example of when a patient should not delay the diagnostic imaging, which is “in the event that there is an abnormal mass in the breast that requires evaluation.”
Providers are now prepared to address these issues, she added.
Dr. Lehman’s nuanced recommendations
“It’s easy to get both a mammogram and booster, and just a matter of timing them – so that the reaction doesn’t interfere with the mammography results,” Dr. Lehman said.
But she emphasized that women should not be choosing between their mammograms or a booster. “We are now saying the same thing that we did with the initial vaccine,” said Dr. Lehman. “We don’t want patients delaying their mammograms, and we don’t want them delaying their boosters – both are critical to staying healthy.”
In her center, a model was developed to navigate vaccine-associated adenopathy. While this approach was developed for the primary vaccine series, the same applies for the booster, which is essentially a third dose of the same vaccine, explained Dr. Lehman.
When patients present for mammography, ultrasound, or MRI, the technologist will document their COVID-19 vaccination status (first or second dose or booster), the date it was given, and the location. Adding vaccination documentation to intake forms helps to support appropriate management of patients who undergo imaging after COVID-19 vaccination. Six weeks is used as the cutoff point for defining “recent” vaccination.
For patients who are getting a screening mammography or MRI, and who have no symptoms beyond unilateral axillary adenopathy on the same side of the body where they received the COVID-19 vaccination (given in the arm) within a 6-week period, the following is included in the screening mammography or screening MRI report: “In the specific setting of a patient with documented recent (within the past 6 weeks) COVID-19 vaccination in the ipsilateral arm, axillary adenopathy is a benign imaging finding. No further imaging is indicated at this time. If there is clinical concern that persists more than 6 weeks after the patient received the final vaccine dose, axillary ultrasound is recommended.”
The experts interviewed reported no conflicts of interest.
This inflammation is caused by the enlargement of lymph nodes and can show up as an abnormal finding on mammograms and other types of chest scans, causing concern and even the need for additional imaging and follow up, wrote Constance D. Lehman, MD, PhD, and colleagues in an article published in Journal of the American College of Radiology.
Lymph node swelling is a normal immune system reaction to vaccination, and “COVID-19 vaccinations in the arm are a well-documented cause of inflammatory unilateral axillary adenopathy,” noted Dr. Lehman, in an interview. The side effect will occur on the side of the body where the patient received a vaccine, and it is not always noticeable to the woman experiencing it, she said.
“We’re finding that the patients’ bodies are responding to the booster in many ways that are similar to the initial COVID vaccines, with lymph node swelling, muscle aches and pains, headaches, and so on,” said Dr. Lehman, who is chief of breast imaging at the Massachusetts General Hospital, Boston. There have been no real differences in reactions between the Moderna and Pfizer vaccines, she added.
Because axillary lymph node swelling can obscure mammogram results, staff of at least a few imaging centers, including Penn State Breast Center in Hershey, Pa., and Providence Women’s Imaging Center in Torrance, Calif., told this news organization that they are asking women to delay mammogram imaging either 6 weeks or 4-6 weeks after getting a COVID-19 booster.
Experts’ suggestions on mammograms, boosters timing
Other experts, including Jessica Leung, MD, acknowledged that vaccine-related reactive adenopathy is seen after the booster dose and provided recommendations for the timing of getting mammograms and the booster with this in mind.
“I would recommend getting the screening mammogram first, which can be followed immediately by vaccination, even on the same day,” said Jessica Leung, MD, a professor of diagnostic radiology at the University of Texas MD Anderson Cancer Center in Houston, Tex.
“If this is not possible from the scheduling perspective, then the patient should consult her health care provider regarding whether it is okay to wait a bit after receiving the vaccine before getting her screening mammogram.”
The answer to that question will likely depend on the time interval since the prior mammogram and the patient’s personal risk factors for developing breast cancer. Dr. Leung noted. “This is all predicated on the assumption that the patient is asymptomatic. If she has any symptoms, for example a palpable breast lump, then she should seek medical attention regardless of timing of vaccination.”
The same holds true for boosters, she said.
She emphasized that careful consideration should be given before delaying the mammogram. “The medical community has a great deal more knowledge at this time than in the early days of COVID-19 vaccination, so we are often able to identify reactive adenopathy related to vaccination. If patients were to delay the mammogram, any reactive adenopathy may persist, on average, for 4-6 weeks.”
Debra Patt, MD, PhD, MBA, executive vice president at Texas Oncology, professor at the University of Texas at Austin, provided a specific example of when a patient should not delay the diagnostic imaging, which is “in the event that there is an abnormal mass in the breast that requires evaluation.”
Providers are now prepared to address these issues, she added.
Dr. Lehman’s nuanced recommendations
“It’s easy to get both a mammogram and booster, and just a matter of timing them – so that the reaction doesn’t interfere with the mammography results,” Dr. Lehman said.
But she emphasized that women should not be choosing between their mammograms or a booster. “We are now saying the same thing that we did with the initial vaccine,” said Dr. Lehman. “We don’t want patients delaying their mammograms, and we don’t want them delaying their boosters – both are critical to staying healthy.”
In her center, a model was developed to navigate vaccine-associated adenopathy. While this approach was developed for the primary vaccine series, the same applies for the booster, which is essentially a third dose of the same vaccine, explained Dr. Lehman.
When patients present for mammography, ultrasound, or MRI, the technologist will document their COVID-19 vaccination status (first or second dose or booster), the date it was given, and the location. Adding vaccination documentation to intake forms helps to support appropriate management of patients who undergo imaging after COVID-19 vaccination. Six weeks is used as the cutoff point for defining “recent” vaccination.
For patients who are getting a screening mammography or MRI, and who have no symptoms beyond unilateral axillary adenopathy on the same side of the body where they received the COVID-19 vaccination (given in the arm) within a 6-week period, the following is included in the screening mammography or screening MRI report: “In the specific setting of a patient with documented recent (within the past 6 weeks) COVID-19 vaccination in the ipsilateral arm, axillary adenopathy is a benign imaging finding. No further imaging is indicated at this time. If there is clinical concern that persists more than 6 weeks after the patient received the final vaccine dose, axillary ultrasound is recommended.”
The experts interviewed reported no conflicts of interest.
This inflammation is caused by the enlargement of lymph nodes and can show up as an abnormal finding on mammograms and other types of chest scans, causing concern and even the need for additional imaging and follow up, wrote Constance D. Lehman, MD, PhD, and colleagues in an article published in Journal of the American College of Radiology.
Lymph node swelling is a normal immune system reaction to vaccination, and “COVID-19 vaccinations in the arm are a well-documented cause of inflammatory unilateral axillary adenopathy,” noted Dr. Lehman, in an interview. The side effect will occur on the side of the body where the patient received a vaccine, and it is not always noticeable to the woman experiencing it, she said.
“We’re finding that the patients’ bodies are responding to the booster in many ways that are similar to the initial COVID vaccines, with lymph node swelling, muscle aches and pains, headaches, and so on,” said Dr. Lehman, who is chief of breast imaging at the Massachusetts General Hospital, Boston. There have been no real differences in reactions between the Moderna and Pfizer vaccines, she added.
Because axillary lymph node swelling can obscure mammogram results, staff of at least a few imaging centers, including Penn State Breast Center in Hershey, Pa., and Providence Women’s Imaging Center in Torrance, Calif., told this news organization that they are asking women to delay mammogram imaging either 6 weeks or 4-6 weeks after getting a COVID-19 booster.
Experts’ suggestions on mammograms, boosters timing
Other experts, including Jessica Leung, MD, acknowledged that vaccine-related reactive adenopathy is seen after the booster dose and provided recommendations for the timing of getting mammograms and the booster with this in mind.
“I would recommend getting the screening mammogram first, which can be followed immediately by vaccination, even on the same day,” said Jessica Leung, MD, a professor of diagnostic radiology at the University of Texas MD Anderson Cancer Center in Houston, Tex.
“If this is not possible from the scheduling perspective, then the patient should consult her health care provider regarding whether it is okay to wait a bit after receiving the vaccine before getting her screening mammogram.”
The answer to that question will likely depend on the time interval since the prior mammogram and the patient’s personal risk factors for developing breast cancer. Dr. Leung noted. “This is all predicated on the assumption that the patient is asymptomatic. If she has any symptoms, for example a palpable breast lump, then she should seek medical attention regardless of timing of vaccination.”
The same holds true for boosters, she said.
She emphasized that careful consideration should be given before delaying the mammogram. “The medical community has a great deal more knowledge at this time than in the early days of COVID-19 vaccination, so we are often able to identify reactive adenopathy related to vaccination. If patients were to delay the mammogram, any reactive adenopathy may persist, on average, for 4-6 weeks.”
Debra Patt, MD, PhD, MBA, executive vice president at Texas Oncology, professor at the University of Texas at Austin, provided a specific example of when a patient should not delay the diagnostic imaging, which is “in the event that there is an abnormal mass in the breast that requires evaluation.”
Providers are now prepared to address these issues, she added.
Dr. Lehman’s nuanced recommendations
“It’s easy to get both a mammogram and booster, and just a matter of timing them – so that the reaction doesn’t interfere with the mammography results,” Dr. Lehman said.
But she emphasized that women should not be choosing between their mammograms or a booster. “We are now saying the same thing that we did with the initial vaccine,” said Dr. Lehman. “We don’t want patients delaying their mammograms, and we don’t want them delaying their boosters – both are critical to staying healthy.”
In her center, a model was developed to navigate vaccine-associated adenopathy. While this approach was developed for the primary vaccine series, the same applies for the booster, which is essentially a third dose of the same vaccine, explained Dr. Lehman.
When patients present for mammography, ultrasound, or MRI, the technologist will document their COVID-19 vaccination status (first or second dose or booster), the date it was given, and the location. Adding vaccination documentation to intake forms helps to support appropriate management of patients who undergo imaging after COVID-19 vaccination. Six weeks is used as the cutoff point for defining “recent” vaccination.
For patients who are getting a screening mammography or MRI, and who have no symptoms beyond unilateral axillary adenopathy on the same side of the body where they received the COVID-19 vaccination (given in the arm) within a 6-week period, the following is included in the screening mammography or screening MRI report: “In the specific setting of a patient with documented recent (within the past 6 weeks) COVID-19 vaccination in the ipsilateral arm, axillary adenopathy is a benign imaging finding. No further imaging is indicated at this time. If there is clinical concern that persists more than 6 weeks after the patient received the final vaccine dose, axillary ultrasound is recommended.”
The experts interviewed reported no conflicts of interest.

