User login
COVID attacks DNA in heart, unlike flu, study says
, according to a study published in Immunology.
The study looked at the hearts of patients who died from COVID-19, the flu, and other causes. The findings could provide clues about why coronavirus has led to complications such as ongoing heart issues.
“We found a lot of DNA damage that was unique to the COVID-19 patients, which wasn’t present in the flu patients,” Arutha Kulasinghe, one of the lead study authors and a research fellow at the University of Queensland, Brisbane, Australia, told the Brisbane Times.
“So in this study, COVID-19 and flu look very different in the way they affect the heart,” he said.
Dr. Kulasinghe and colleagues analyzed the hearts of seven COVID-19 patients, two flu patients, and six patients who died from other causes. They used transcriptomic profiling, which looks at the DNA landscape of an organ, to investigate heart tissue from the patients.
Because of previous studies about heart problems associated with COVID-19, he and colleagues expected to find extreme inflammation in the heart. Instead, they found that inflammation signals had been suppressed in the heart, and markers for DNA damage and repair were much higher. They’re still unsure of the underlying cause.
“The indications here are that there’s DNA damage here, it’s not inflammation,” Dr. Kulasinghe said. “There’s something else going on that we need to figure out.”
The damage was similar to the way chronic diseases such as diabetes and cancer appear in the heart, he said, with heart tissue showing DNA damage signals.
Dr. Kulasinghe said he hopes other studies can build on the findings to develop risk models to understand which patients may face a higher risk of serious COVID-19 complications. In turn, this could help doctors provide early treatment. For instance, all seven COVID-19 patients had other chronic diseases, such as diabetes, hypertension, and heart disease.
“Ideally in the future, if you have cardiovascular disease, if you’re obese or have other complications, and you’ve got a signature in your blood that indicates you are at risk of severe disease, then we can risk-stratify patients when they are diagnosed,” he said.
The research is a preliminary step, Dr. Kulasinghe said, because of the small sample size. This type of study is often difficult to conduct because researchers have to wait for the availability of organs, as well as request permission from families for postmortem autopsies and biopsies, to be able to look at the effects on dead tissues.
“Our challenge now is to draw a clinical finding from this, which we can’t at this stage,” he added. “But it’s a really fundamental biological difference we’re observing [between COVID-19 and flu], which we need to validate with larger studies.”
A version of this article first appeared on WebMD.com.
, according to a study published in Immunology.
The study looked at the hearts of patients who died from COVID-19, the flu, and other causes. The findings could provide clues about why coronavirus has led to complications such as ongoing heart issues.
“We found a lot of DNA damage that was unique to the COVID-19 patients, which wasn’t present in the flu patients,” Arutha Kulasinghe, one of the lead study authors and a research fellow at the University of Queensland, Brisbane, Australia, told the Brisbane Times.
“So in this study, COVID-19 and flu look very different in the way they affect the heart,” he said.
Dr. Kulasinghe and colleagues analyzed the hearts of seven COVID-19 patients, two flu patients, and six patients who died from other causes. They used transcriptomic profiling, which looks at the DNA landscape of an organ, to investigate heart tissue from the patients.
Because of previous studies about heart problems associated with COVID-19, he and colleagues expected to find extreme inflammation in the heart. Instead, they found that inflammation signals had been suppressed in the heart, and markers for DNA damage and repair were much higher. They’re still unsure of the underlying cause.
“The indications here are that there’s DNA damage here, it’s not inflammation,” Dr. Kulasinghe said. “There’s something else going on that we need to figure out.”
The damage was similar to the way chronic diseases such as diabetes and cancer appear in the heart, he said, with heart tissue showing DNA damage signals.
Dr. Kulasinghe said he hopes other studies can build on the findings to develop risk models to understand which patients may face a higher risk of serious COVID-19 complications. In turn, this could help doctors provide early treatment. For instance, all seven COVID-19 patients had other chronic diseases, such as diabetes, hypertension, and heart disease.
“Ideally in the future, if you have cardiovascular disease, if you’re obese or have other complications, and you’ve got a signature in your blood that indicates you are at risk of severe disease, then we can risk-stratify patients when they are diagnosed,” he said.
The research is a preliminary step, Dr. Kulasinghe said, because of the small sample size. This type of study is often difficult to conduct because researchers have to wait for the availability of organs, as well as request permission from families for postmortem autopsies and biopsies, to be able to look at the effects on dead tissues.
“Our challenge now is to draw a clinical finding from this, which we can’t at this stage,” he added. “But it’s a really fundamental biological difference we’re observing [between COVID-19 and flu], which we need to validate with larger studies.”
A version of this article first appeared on WebMD.com.
, according to a study published in Immunology.
The study looked at the hearts of patients who died from COVID-19, the flu, and other causes. The findings could provide clues about why coronavirus has led to complications such as ongoing heart issues.
“We found a lot of DNA damage that was unique to the COVID-19 patients, which wasn’t present in the flu patients,” Arutha Kulasinghe, one of the lead study authors and a research fellow at the University of Queensland, Brisbane, Australia, told the Brisbane Times.
“So in this study, COVID-19 and flu look very different in the way they affect the heart,” he said.
Dr. Kulasinghe and colleagues analyzed the hearts of seven COVID-19 patients, two flu patients, and six patients who died from other causes. They used transcriptomic profiling, which looks at the DNA landscape of an organ, to investigate heart tissue from the patients.
Because of previous studies about heart problems associated with COVID-19, he and colleagues expected to find extreme inflammation in the heart. Instead, they found that inflammation signals had been suppressed in the heart, and markers for DNA damage and repair were much higher. They’re still unsure of the underlying cause.
“The indications here are that there’s DNA damage here, it’s not inflammation,” Dr. Kulasinghe said. “There’s something else going on that we need to figure out.”
The damage was similar to the way chronic diseases such as diabetes and cancer appear in the heart, he said, with heart tissue showing DNA damage signals.
Dr. Kulasinghe said he hopes other studies can build on the findings to develop risk models to understand which patients may face a higher risk of serious COVID-19 complications. In turn, this could help doctors provide early treatment. For instance, all seven COVID-19 patients had other chronic diseases, such as diabetes, hypertension, and heart disease.
“Ideally in the future, if you have cardiovascular disease, if you’re obese or have other complications, and you’ve got a signature in your blood that indicates you are at risk of severe disease, then we can risk-stratify patients when they are diagnosed,” he said.
The research is a preliminary step, Dr. Kulasinghe said, because of the small sample size. This type of study is often difficult to conduct because researchers have to wait for the availability of organs, as well as request permission from families for postmortem autopsies and biopsies, to be able to look at the effects on dead tissues.
“Our challenge now is to draw a clinical finding from this, which we can’t at this stage,” he added. “But it’s a really fundamental biological difference we’re observing [between COVID-19 and flu], which we need to validate with larger studies.”
A version of this article first appeared on WebMD.com.
FROM IMMUNOLOGY
CDC: Masking no longer required in health care settings
It’s a “major departure” from the CDC’s previous recommendation of universal masking to fight the COVID-19 pandemic, The Hill says.
“Updates were made to reflect the high levels of vaccine-and infection-induced immunity and the availability of effective treatments and prevention tools,” the CDC’s new guidance says.
The agency now says that facilities in areas without high transmission can decide for themselves whether to require everyone – doctors, patients, and visitors – to wear masks.
Community transmission “is the metric currently recommended to guide select practices in healthcare settings to allow for earlier intervention, before there is strain on the health care system and to better protect the individuals seeking care in these settings,” the CDC said.
About 73% of the country is having “high” rates of transmission, The Hill said.
“Community transmission” is different from the “community level” metric that’s used for non–health care settings.
Community transmission refers to measures of the presence and spread of SARS-CoV-2, the CDC said. “Community levels place an emphasis on measures of the impact of COVID-19 in terms of hospitalizations and health care system strain, while accounting for transmission in the community.”
Just 7% of counties are considered high risk, while nearly 62 percent are low.
The new guidance applies wherever health care is delivered, including nursing homes and home health, the CDC said.
A version of this article first appeared on WebMD.com.
It’s a “major departure” from the CDC’s previous recommendation of universal masking to fight the COVID-19 pandemic, The Hill says.
“Updates were made to reflect the high levels of vaccine-and infection-induced immunity and the availability of effective treatments and prevention tools,” the CDC’s new guidance says.
The agency now says that facilities in areas without high transmission can decide for themselves whether to require everyone – doctors, patients, and visitors – to wear masks.
Community transmission “is the metric currently recommended to guide select practices in healthcare settings to allow for earlier intervention, before there is strain on the health care system and to better protect the individuals seeking care in these settings,” the CDC said.
About 73% of the country is having “high” rates of transmission, The Hill said.
“Community transmission” is different from the “community level” metric that’s used for non–health care settings.
Community transmission refers to measures of the presence and spread of SARS-CoV-2, the CDC said. “Community levels place an emphasis on measures of the impact of COVID-19 in terms of hospitalizations and health care system strain, while accounting for transmission in the community.”
Just 7% of counties are considered high risk, while nearly 62 percent are low.
The new guidance applies wherever health care is delivered, including nursing homes and home health, the CDC said.
A version of this article first appeared on WebMD.com.
It’s a “major departure” from the CDC’s previous recommendation of universal masking to fight the COVID-19 pandemic, The Hill says.
“Updates were made to reflect the high levels of vaccine-and infection-induced immunity and the availability of effective treatments and prevention tools,” the CDC’s new guidance says.
The agency now says that facilities in areas without high transmission can decide for themselves whether to require everyone – doctors, patients, and visitors – to wear masks.
Community transmission “is the metric currently recommended to guide select practices in healthcare settings to allow for earlier intervention, before there is strain on the health care system and to better protect the individuals seeking care in these settings,” the CDC said.
About 73% of the country is having “high” rates of transmission, The Hill said.
“Community transmission” is different from the “community level” metric that’s used for non–health care settings.
Community transmission refers to measures of the presence and spread of SARS-CoV-2, the CDC said. “Community levels place an emphasis on measures of the impact of COVID-19 in terms of hospitalizations and health care system strain, while accounting for transmission in the community.”
Just 7% of counties are considered high risk, while nearly 62 percent are low.
The new guidance applies wherever health care is delivered, including nursing homes and home health, the CDC said.
A version of this article first appeared on WebMD.com.
Why can’t U.K. immunocompromised patients get Evusheld?
This transcript has been edited for clarity.
I’m David Kerr, professor of cancer medicine at Oxford. As I’m gearing up to have my autumnal COVID-19 booster vaccine,
This was developed by AstraZeneca. It’s a combination of two relatively long-acting antibodies (tixagevimab and cilgavimab) that bind to the spike protein on the outside of the SARS-CoV-2 virus, the virus that causes COVID-19. The antibody binds to the spike protein and prevents it from binding to and infecting or damaging cells, so it’s what’s called preexposure prophylaxis.
Although vaccination is still the best approach to protecting against and, one would hope, conferring a degree of herd protection to our population as a whole, there are some people who cannot mount an appropriate immune response and we have to take care of these folks. Because the vaccines don’t work very well for them, the vaccine itself is not sufficient to protect them.
Evusheld, in trials that have been done hitherto, can protect people who can’t mount an immune response from being infected. Between 75% and 80% of patients treated with Evusheld didn’t get COVID-19. The duration of effect seemed to be at least for 6 months, possibly longer, so it’s a really good result. This caused our medicines regulatory authority in the United Kingdom to approve the drug in March of this year. Although the drug has been approved, it’s not yet funded and not yet available for vulnerable patients.
These are patients who, for reasons of inborn genetic diseases, cannot mount an immune response; patients who are pharmacologically immune depleted; patients who have had transplants and are on immunosuppressive drugs; and some of our cancer patients, particularly those with blood or hematologic malignancies, who can receive very heavy treatment that can pound the immune system to bits.
These are, in the population as a whole, relatively small numbers, but an important number of people who are still vulnerable to developing COVID-19 despite vaccination.
Why isn’t the drug available? We have a two-stage process in the United Kingdom. We have the scientists and regulatory authorities looking at the evidence and data and saying, “Yes, it stacks up. This drug is effective and safe to some extent.”
The second phase is a health technology assessment undertaken by NICE, our National Institute for Health and Care Excellence – something that I’ve talked about a number of times before and the sometimes seemingly arbitrary decisions that they make. NICE hasn’t evaluated the drug yet, and the British government has held out because they are arguing that we don’t have enough data.
The trials with Evusheld were done before the Omicron variant dominated, as it does now; therefore, they are looking to try to work with AstraZeneca to generate more real-world data to show that Evusheld would prevent infection from the Omicron variant of the virus. Equally as important, how long does that protection last? Is it as protective against Omicron, and what’s the duration of that protection? Those bits of work are going on now.
Some real-world data are starting to emerge, showing that Evusheld will offer some degree of protection against Omicron, but there are still question marks about duration and the proportion of the population that would benefit.
NICE aren’t due to report on this – although the drug was approved in March of this year – until next year some time. That’s what’s caused a degree of consternation in the community of patients that we serve. Some of my clinical colleagues are beating the drum, saying, “We must have this drug now.” We’re still waiting on NICE to announce.
One obvious way to go around this is the government, which has bent over backwards in the United Kingdom to do as much as it can to protect the population from COVID-19. There was fantastic vaccine rollout and an extraordinary economic package to support individuals during lockdown to maintain the workforce, to support families and people at home. They’ve done a fantastic job.
Wanting to damp down this controversy, perhaps the sensible thing would be to ask NICE to evaluate the data that they have just now, to allow AstraZeneca to present whatever real-world evidence they have, and although it may not be perfect, it may be sufficient – we don’t know – to pass the NICE health technology assessment.
Watch this space. Let’s see what happens. If I were government, that’s what I would do. I would ask NICE to bring their appraisal forward. I would ask them to work with AstraZeneca to go over every ounce and iota of data that they have to see if this drug will be sufficiently effective and sufficiently cost-effective to be used before winter comes. I think the whole world is holding its breath, expecting another COVID-19 winter surge. Now would be the time to act.
What do you think? Here we are in the United Kingdom discussing yet another quasi–”health-rationing” problem. It’s not. This is about collecting more data and being as rational as possible. Can we accelerate that process? Perhaps.
Thanks for listening. I’d be very grateful for any comments that you might choose to make.
David J. Kerr, MD, DSc, is a professor of cancer medicine at the University of Oxford (England). He disclosed financial relationships with Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Bayer HealthCare Pharmaceuticals, Genomic Health, Merck Serono, Roche, and Celleron Therapeutics.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m David Kerr, professor of cancer medicine at Oxford. As I’m gearing up to have my autumnal COVID-19 booster vaccine,
This was developed by AstraZeneca. It’s a combination of two relatively long-acting antibodies (tixagevimab and cilgavimab) that bind to the spike protein on the outside of the SARS-CoV-2 virus, the virus that causes COVID-19. The antibody binds to the spike protein and prevents it from binding to and infecting or damaging cells, so it’s what’s called preexposure prophylaxis.
Although vaccination is still the best approach to protecting against and, one would hope, conferring a degree of herd protection to our population as a whole, there are some people who cannot mount an appropriate immune response and we have to take care of these folks. Because the vaccines don’t work very well for them, the vaccine itself is not sufficient to protect them.
Evusheld, in trials that have been done hitherto, can protect people who can’t mount an immune response from being infected. Between 75% and 80% of patients treated with Evusheld didn’t get COVID-19. The duration of effect seemed to be at least for 6 months, possibly longer, so it’s a really good result. This caused our medicines regulatory authority in the United Kingdom to approve the drug in March of this year. Although the drug has been approved, it’s not yet funded and not yet available for vulnerable patients.
These are patients who, for reasons of inborn genetic diseases, cannot mount an immune response; patients who are pharmacologically immune depleted; patients who have had transplants and are on immunosuppressive drugs; and some of our cancer patients, particularly those with blood or hematologic malignancies, who can receive very heavy treatment that can pound the immune system to bits.
These are, in the population as a whole, relatively small numbers, but an important number of people who are still vulnerable to developing COVID-19 despite vaccination.
Why isn’t the drug available? We have a two-stage process in the United Kingdom. We have the scientists and regulatory authorities looking at the evidence and data and saying, “Yes, it stacks up. This drug is effective and safe to some extent.”
The second phase is a health technology assessment undertaken by NICE, our National Institute for Health and Care Excellence – something that I’ve talked about a number of times before and the sometimes seemingly arbitrary decisions that they make. NICE hasn’t evaluated the drug yet, and the British government has held out because they are arguing that we don’t have enough data.
The trials with Evusheld were done before the Omicron variant dominated, as it does now; therefore, they are looking to try to work with AstraZeneca to generate more real-world data to show that Evusheld would prevent infection from the Omicron variant of the virus. Equally as important, how long does that protection last? Is it as protective against Omicron, and what’s the duration of that protection? Those bits of work are going on now.
Some real-world data are starting to emerge, showing that Evusheld will offer some degree of protection against Omicron, but there are still question marks about duration and the proportion of the population that would benefit.
NICE aren’t due to report on this – although the drug was approved in March of this year – until next year some time. That’s what’s caused a degree of consternation in the community of patients that we serve. Some of my clinical colleagues are beating the drum, saying, “We must have this drug now.” We’re still waiting on NICE to announce.
One obvious way to go around this is the government, which has bent over backwards in the United Kingdom to do as much as it can to protect the population from COVID-19. There was fantastic vaccine rollout and an extraordinary economic package to support individuals during lockdown to maintain the workforce, to support families and people at home. They’ve done a fantastic job.
Wanting to damp down this controversy, perhaps the sensible thing would be to ask NICE to evaluate the data that they have just now, to allow AstraZeneca to present whatever real-world evidence they have, and although it may not be perfect, it may be sufficient – we don’t know – to pass the NICE health technology assessment.
Watch this space. Let’s see what happens. If I were government, that’s what I would do. I would ask NICE to bring their appraisal forward. I would ask them to work with AstraZeneca to go over every ounce and iota of data that they have to see if this drug will be sufficiently effective and sufficiently cost-effective to be used before winter comes. I think the whole world is holding its breath, expecting another COVID-19 winter surge. Now would be the time to act.
What do you think? Here we are in the United Kingdom discussing yet another quasi–”health-rationing” problem. It’s not. This is about collecting more data and being as rational as possible. Can we accelerate that process? Perhaps.
Thanks for listening. I’d be very grateful for any comments that you might choose to make.
David J. Kerr, MD, DSc, is a professor of cancer medicine at the University of Oxford (England). He disclosed financial relationships with Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Bayer HealthCare Pharmaceuticals, Genomic Health, Merck Serono, Roche, and Celleron Therapeutics.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m David Kerr, professor of cancer medicine at Oxford. As I’m gearing up to have my autumnal COVID-19 booster vaccine,
This was developed by AstraZeneca. It’s a combination of two relatively long-acting antibodies (tixagevimab and cilgavimab) that bind to the spike protein on the outside of the SARS-CoV-2 virus, the virus that causes COVID-19. The antibody binds to the spike protein and prevents it from binding to and infecting or damaging cells, so it’s what’s called preexposure prophylaxis.
Although vaccination is still the best approach to protecting against and, one would hope, conferring a degree of herd protection to our population as a whole, there are some people who cannot mount an appropriate immune response and we have to take care of these folks. Because the vaccines don’t work very well for them, the vaccine itself is not sufficient to protect them.
Evusheld, in trials that have been done hitherto, can protect people who can’t mount an immune response from being infected. Between 75% and 80% of patients treated with Evusheld didn’t get COVID-19. The duration of effect seemed to be at least for 6 months, possibly longer, so it’s a really good result. This caused our medicines regulatory authority in the United Kingdom to approve the drug in March of this year. Although the drug has been approved, it’s not yet funded and not yet available for vulnerable patients.
These are patients who, for reasons of inborn genetic diseases, cannot mount an immune response; patients who are pharmacologically immune depleted; patients who have had transplants and are on immunosuppressive drugs; and some of our cancer patients, particularly those with blood or hematologic malignancies, who can receive very heavy treatment that can pound the immune system to bits.
These are, in the population as a whole, relatively small numbers, but an important number of people who are still vulnerable to developing COVID-19 despite vaccination.
Why isn’t the drug available? We have a two-stage process in the United Kingdom. We have the scientists and regulatory authorities looking at the evidence and data and saying, “Yes, it stacks up. This drug is effective and safe to some extent.”
The second phase is a health technology assessment undertaken by NICE, our National Institute for Health and Care Excellence – something that I’ve talked about a number of times before and the sometimes seemingly arbitrary decisions that they make. NICE hasn’t evaluated the drug yet, and the British government has held out because they are arguing that we don’t have enough data.
The trials with Evusheld were done before the Omicron variant dominated, as it does now; therefore, they are looking to try to work with AstraZeneca to generate more real-world data to show that Evusheld would prevent infection from the Omicron variant of the virus. Equally as important, how long does that protection last? Is it as protective against Omicron, and what’s the duration of that protection? Those bits of work are going on now.
Some real-world data are starting to emerge, showing that Evusheld will offer some degree of protection against Omicron, but there are still question marks about duration and the proportion of the population that would benefit.
NICE aren’t due to report on this – although the drug was approved in March of this year – until next year some time. That’s what’s caused a degree of consternation in the community of patients that we serve. Some of my clinical colleagues are beating the drum, saying, “We must have this drug now.” We’re still waiting on NICE to announce.
One obvious way to go around this is the government, which has bent over backwards in the United Kingdom to do as much as it can to protect the population from COVID-19. There was fantastic vaccine rollout and an extraordinary economic package to support individuals during lockdown to maintain the workforce, to support families and people at home. They’ve done a fantastic job.
Wanting to damp down this controversy, perhaps the sensible thing would be to ask NICE to evaluate the data that they have just now, to allow AstraZeneca to present whatever real-world evidence they have, and although it may not be perfect, it may be sufficient – we don’t know – to pass the NICE health technology assessment.
Watch this space. Let’s see what happens. If I were government, that’s what I would do. I would ask NICE to bring their appraisal forward. I would ask them to work with AstraZeneca to go over every ounce and iota of data that they have to see if this drug will be sufficiently effective and sufficiently cost-effective to be used before winter comes. I think the whole world is holding its breath, expecting another COVID-19 winter surge. Now would be the time to act.
What do you think? Here we are in the United Kingdom discussing yet another quasi–”health-rationing” problem. It’s not. This is about collecting more data and being as rational as possible. Can we accelerate that process? Perhaps.
Thanks for listening. I’d be very grateful for any comments that you might choose to make.
David J. Kerr, MD, DSc, is a professor of cancer medicine at the University of Oxford (England). He disclosed financial relationships with Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Bayer HealthCare Pharmaceuticals, Genomic Health, Merck Serono, Roche, and Celleron Therapeutics.
A version of this article first appeared on Medscape.com.
Does COVID-19 cause type 1 diabetes in children? Time will tell
STOCKHOLM – It remains inconclusive whether SARS-CoV-2 infection predisposes children and adolescents to a higher risk of type 1 diabetes. Data from two new studies and a recently published research letter add to the growing body of knowledge on the subject, but still can’t draw any definitive conclusions.
The latest results from a Norwegian and a Scottish study both examine incidence of type 1 diabetes in young people with a history of SARS-CoV-2 infection and were reported at the annual meeting of the European Association for the Study of Diabetes.
A 60% increased risk for type 1 diabetes at least 31 days after SARS-CoV-2 infection (adjusted hazard ratio, 1.63) was found in the Norwegian study, while in contrast, the Scottish study only found an increased risk in the first few months of the pandemic, in 2020, but importantly, no association over a much longer time period (March 2020–November 2021).
In a comment on Twitter on the two studies presented at EASD, session moderator Kamlesh Khunti, MD, professor of primary care diabetes and vascular medicine at the University of Leicester, (England), said: “In summary, two studies showing no or weak association of type 1 diabetes with COVID.”
But new data in the research letter published in JAMA Network Open, based on U.S. figures, also found an almost doubling of type 1 diabetes in children in the first few months after COVID-19 infection relative to infection with other respiratory viruses.
Lead author of the Scottish study, Helen Colhoun, PhD, honorary public health consultant at Public Health Scotland, commented: “Data in children are variable year on year, which emphasizes the need to be cautious over taking a tiny snapshot.”
Nevertheless, this is “a hugely important question and we must not drop the ball. [We must] keep looking at it and maintain scientific equipoise. ... [This] reinforces the need to carry on this analysis into the future to obtain an unequivocal picture,” she emphasized.
Norwegian study: If there is an association, the risk is small
German Tapia, PhD, from the Norwegian Institute of Public Health, Oslo, presented the results of a study of SARS-CoV-2 infection and subsequent risk of type 1 diabetes in 1.2 million children in Norway.
Of these, 424,354 children had been infected with SARS-CoV-2, and there were 990 incident cases of type 1 diabetes.
“What we do know about COVID-19 in children is that the symptoms are mild and only a small proportion are hospitalized with more serious symptoms. But we do not know the long-term effects of COVID-19 infection because this requires a longer follow-up period,” remarked Dr. Tapia, adding that other viral infections are thought to be linked to the development of type 1 diabetes, in particular, respiratory infections.
The data were sourced from the Norwegian Emergency Preparedness Register for COVID-19, which gathers daily data updates including infections (positive and negative results for free-of-charge testing), diagnoses (primary and secondary care), vaccinations (also free of charge), prescribed medications, and basic demographics.
“We link these data using the personal identification number that every Norwegian citizen has,” explained Dr. Tapia.
He presented results from two cohorts: firstly, results in children only, including those tested for SARS-CoV-2 infection, and secondly, a full national Norwegian population cohort.
Regarding the first cohort, those under 18 years who tested positive for SARS-CoV-2 infection, from March 2020 to March 2022, had a significantly increased risk of type 1 diabetes at least 31 days after infection, with an adjusted hazard ratio of 1.63 (95% confidence interval, 1.08-2.47; P = .02). Adjustments were made for age, sex, non-Nordic country of origin, geographic area, and socioeconomic factors.
For children who developed type 1 diabetes within 30 days of a SARS-CoV-2 infection, the HR was 1.26 (95% CI, 0.72-2.19; P = .42), which did not reach statistical significance.
“The fact that fewer people developed type 1 diabetes within 30 days is not surprising because we know that type 1 diabetes develops over a long period of time,” Dr. Tapia said.
“For this reason, we would not expect to find new cases of those people who develop type 1 diabetes within 30 days of COVID-19 infection,” he explained. In these cases, “it is most likely that they already had [type 1 diabetes], and the infection probably triggered clinical symptoms, so their type 1 diabetes was discovered.”
Turning to the full population cohort and diagnoses of type 1 diabetes over 30 days after SARS-CoV-2 infection, the Norwegian researchers found an association, with an HR of 1.57 (95% CI, 1.06-2.33; P = .03), while diagnosis of type 1 diabetes at 30 days or less generated a hazard ratio of 1.22 (95% CI, 0.72-2.19; P = .42).
“So very similar results were found, and after adjustment for confounders, results were still similar,” reported Dr. Tapia.
He also conducted a similar analysis with vaccination as an exposure but found no association between vaccination against SARS-CoV-2 and diagnosis of type 1 diabetes.
“From these results, we conclude that this suggests an increase in diagnosis of type 1 diabetes after SARS-CoV-2 infection, but it must be noted that the absolute risk of developing type 1 diabetes after infection in children is low, with most children not developing the disease,” he emphasized. “There are nearly half a million children who have been infected with SARS-CoV-2 in Norway, but only a very small proportion develop type 1 diabetes.”
Scottish study: No association found over longer term
Dr. Colhoun and colleagues looked at the relationship between incident type 1 diabetes and SARS-CoV-2 infection in children in Scotland using e-health record linkage.
The study involved 1.8 million people under 35 years of age and found very weak, if any, evidence of an association between incident type 1 diabetes and SARS-CoV-2.
Examining data between March 2020 and November 2021, Dr. Colhoun and colleagues identified 365,080 individuals up to age 35 with at least one detected SARS-CoV-2 infection during follow-up and 1,074 who developed type 1 diabetes.
“In children under 16 years, suspected cases of type 1 diabetes are admitted to hospital, and 97% of diagnosis dates are recorded in the Scottish Care Information – Diabetes Collaboration register [SCI-Diabetes] prior to or within 2 days of the first hospital admission for type 1 diabetes,” Dr. Colhoun said, stressing the timeliness of the data.
“We found the incidence of type 1 diabetes diagnosis increased 1.2-fold in those aged 0-14 years, but we did not find any association at an individual level of COVID-19 infection over 30 days prior to a type 1 diabetes diagnosis, in this particular dataset,” she reported. In young people aged 15-34, there was a linear increase in incident type 1 diabetes from 2015 to 2021 with no pandemic increase.
Referring to the 1.2-fold increase soon after the pandemic started, she explained that, in 0- to 14-year-olds, the increase followed a drop in the preceding months prepandemic in 2019. They also found that the seasonal pattern of type 1 diabetes diagnoses remained roughly the same across the pandemic months, with typical peaks in February and September.
In the cohort of under 35s, researchers also found a rate ratio of 2.62 (95% CI, 1.81-3.78) within a 30-day window of SARS-CoV-2 infection, but beyond 30 days, no evidence was seen of an association, with a RR of 0.86 (95% CI, 0.62-1.21; P = .40), she reported.
She explained her reasons for not considering diagnoses within 30 days of COVID-19 as causative. Echoing Dr. Tapia, Dr. Colhoun said the median time from symptom onset to diagnosis of type 1 diabetes is 25 days. “This suggests that 50% have had symptoms for over 25 days at diagnosis.”
She also stressed that when they compared the timing of SARS-CoV-2 testing with diagnosis, they found a much higher rate of COVID-19 testing around diagnosis. “This was not least because everyone admitted to hospital had to have a COVID-19 test.”
Latest U.S. data point to a link
Meanwhile, for the new data reported in JAMA Network Open, medical student Ellen K. Kendall of Case Western Reserve University, Cleveland, matched 571,256 pediatric patients: 285,628 with COVID-19 and 285,628 with non–COVID-19 respiratory infections.
By 6 months after COVID-19, 123 patients (0.043%) had received a new diagnosis of type 1 diabetes, but only 72 (0.025%) were diagnosed with type 1 diabetes within 6 months after non–COVID-19 respiratory infection.
At 1, 3, and 6 months after infection, risk of diagnosis of type 1 diabetes was greater among those infected with SARS-CoV-2, compared with those with non–COVID-19 respiratory infection (1 month: HR, 1.96; 3 months: HR, 2.10; and 6 months: HR, 1.83), and in subgroups of patients aged 0-9 years, a group unlikely to develop type 2 diabetes.
“In this study, new type 1 diabetes diagnoses were more likely to occur among pediatric patients with prior COVID-19 than among those with other respiratory infections (or with other encounters with health systems),” noted Ms. Kendall and coauthors. “Respiratory infections have previously been associated with onset of type 1 diabetes, but this risk was even higher among those with COVID-19 in our study, raising concern for long-term, post–COVID-19 autoimmune complications among youths.”
“The increased risk of new-onset type 1 diabetes after COVID-19 adds an important consideration for risk–benefit discussions for prevention and treatment of SARS-CoV-2 infection in pediatric populations,” they concluded.
A study from the Centers for Disease Control and Prevention published in January 2022, also concluded there was a link between COVID-19 and diabetes in children, but not with other acute respiratory infections. Children were 2.5 times more likely to be diagnosed with diabetes following a SARS-CoV-2 infection, it found.
However, the study has been criticized because it pooled all types of diabetes together and did not account for other health conditions, medications that can increase blood glucose levels, race, obesity, and other social determinants of health that might influence a child’s risk of acquiring COVID-19 or diabetes.
“I’ve no doubt that the CDC data were incorrect because the incidence rate for ... diabetes, even in those never exposed to COVID-19 infection, was 10 times the rate ever reported in the U.S.,” Dr. Colhoun said. “There’s no way these data are correct. I believe there was a confusion between incidence and prevalence of diabetes.”
“This paper caused a great deal of panic, especially among those who have a child with type 1diabetes, so we need to be very careful not to cause undue alarm until we have more definitive evidence in this arena,” she stressed.
However, she also acknowledged that the new Norwegian study was well conducted, and she has no methodological concerns about it, so “I think we just have to wait and see.”
Given the inconclusiveness on the issue, there is an ongoing CoviDiab registry collecting data on this very subject.
Dr. Tapia presented on behalf of lead author Dr. Gulseth, who has reported no relevant financial relationships. Dr. Colhoun also reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – It remains inconclusive whether SARS-CoV-2 infection predisposes children and adolescents to a higher risk of type 1 diabetes. Data from two new studies and a recently published research letter add to the growing body of knowledge on the subject, but still can’t draw any definitive conclusions.
The latest results from a Norwegian and a Scottish study both examine incidence of type 1 diabetes in young people with a history of SARS-CoV-2 infection and were reported at the annual meeting of the European Association for the Study of Diabetes.
A 60% increased risk for type 1 diabetes at least 31 days after SARS-CoV-2 infection (adjusted hazard ratio, 1.63) was found in the Norwegian study, while in contrast, the Scottish study only found an increased risk in the first few months of the pandemic, in 2020, but importantly, no association over a much longer time period (March 2020–November 2021).
In a comment on Twitter on the two studies presented at EASD, session moderator Kamlesh Khunti, MD, professor of primary care diabetes and vascular medicine at the University of Leicester, (England), said: “In summary, two studies showing no or weak association of type 1 diabetes with COVID.”
But new data in the research letter published in JAMA Network Open, based on U.S. figures, also found an almost doubling of type 1 diabetes in children in the first few months after COVID-19 infection relative to infection with other respiratory viruses.
Lead author of the Scottish study, Helen Colhoun, PhD, honorary public health consultant at Public Health Scotland, commented: “Data in children are variable year on year, which emphasizes the need to be cautious over taking a tiny snapshot.”
Nevertheless, this is “a hugely important question and we must not drop the ball. [We must] keep looking at it and maintain scientific equipoise. ... [This] reinforces the need to carry on this analysis into the future to obtain an unequivocal picture,” she emphasized.
Norwegian study: If there is an association, the risk is small
German Tapia, PhD, from the Norwegian Institute of Public Health, Oslo, presented the results of a study of SARS-CoV-2 infection and subsequent risk of type 1 diabetes in 1.2 million children in Norway.
Of these, 424,354 children had been infected with SARS-CoV-2, and there were 990 incident cases of type 1 diabetes.
“What we do know about COVID-19 in children is that the symptoms are mild and only a small proportion are hospitalized with more serious symptoms. But we do not know the long-term effects of COVID-19 infection because this requires a longer follow-up period,” remarked Dr. Tapia, adding that other viral infections are thought to be linked to the development of type 1 diabetes, in particular, respiratory infections.
The data were sourced from the Norwegian Emergency Preparedness Register for COVID-19, which gathers daily data updates including infections (positive and negative results for free-of-charge testing), diagnoses (primary and secondary care), vaccinations (also free of charge), prescribed medications, and basic demographics.
“We link these data using the personal identification number that every Norwegian citizen has,” explained Dr. Tapia.
He presented results from two cohorts: firstly, results in children only, including those tested for SARS-CoV-2 infection, and secondly, a full national Norwegian population cohort.
Regarding the first cohort, those under 18 years who tested positive for SARS-CoV-2 infection, from March 2020 to March 2022, had a significantly increased risk of type 1 diabetes at least 31 days after infection, with an adjusted hazard ratio of 1.63 (95% confidence interval, 1.08-2.47; P = .02). Adjustments were made for age, sex, non-Nordic country of origin, geographic area, and socioeconomic factors.
For children who developed type 1 diabetes within 30 days of a SARS-CoV-2 infection, the HR was 1.26 (95% CI, 0.72-2.19; P = .42), which did not reach statistical significance.
“The fact that fewer people developed type 1 diabetes within 30 days is not surprising because we know that type 1 diabetes develops over a long period of time,” Dr. Tapia said.
“For this reason, we would not expect to find new cases of those people who develop type 1 diabetes within 30 days of COVID-19 infection,” he explained. In these cases, “it is most likely that they already had [type 1 diabetes], and the infection probably triggered clinical symptoms, so their type 1 diabetes was discovered.”
Turning to the full population cohort and diagnoses of type 1 diabetes over 30 days after SARS-CoV-2 infection, the Norwegian researchers found an association, with an HR of 1.57 (95% CI, 1.06-2.33; P = .03), while diagnosis of type 1 diabetes at 30 days or less generated a hazard ratio of 1.22 (95% CI, 0.72-2.19; P = .42).
“So very similar results were found, and after adjustment for confounders, results were still similar,” reported Dr. Tapia.
He also conducted a similar analysis with vaccination as an exposure but found no association between vaccination against SARS-CoV-2 and diagnosis of type 1 diabetes.
“From these results, we conclude that this suggests an increase in diagnosis of type 1 diabetes after SARS-CoV-2 infection, but it must be noted that the absolute risk of developing type 1 diabetes after infection in children is low, with most children not developing the disease,” he emphasized. “There are nearly half a million children who have been infected with SARS-CoV-2 in Norway, but only a very small proportion develop type 1 diabetes.”
Scottish study: No association found over longer term
Dr. Colhoun and colleagues looked at the relationship between incident type 1 diabetes and SARS-CoV-2 infection in children in Scotland using e-health record linkage.
The study involved 1.8 million people under 35 years of age and found very weak, if any, evidence of an association between incident type 1 diabetes and SARS-CoV-2.
Examining data between March 2020 and November 2021, Dr. Colhoun and colleagues identified 365,080 individuals up to age 35 with at least one detected SARS-CoV-2 infection during follow-up and 1,074 who developed type 1 diabetes.
“In children under 16 years, suspected cases of type 1 diabetes are admitted to hospital, and 97% of diagnosis dates are recorded in the Scottish Care Information – Diabetes Collaboration register [SCI-Diabetes] prior to or within 2 days of the first hospital admission for type 1 diabetes,” Dr. Colhoun said, stressing the timeliness of the data.
“We found the incidence of type 1 diabetes diagnosis increased 1.2-fold in those aged 0-14 years, but we did not find any association at an individual level of COVID-19 infection over 30 days prior to a type 1 diabetes diagnosis, in this particular dataset,” she reported. In young people aged 15-34, there was a linear increase in incident type 1 diabetes from 2015 to 2021 with no pandemic increase.
Referring to the 1.2-fold increase soon after the pandemic started, she explained that, in 0- to 14-year-olds, the increase followed a drop in the preceding months prepandemic in 2019. They also found that the seasonal pattern of type 1 diabetes diagnoses remained roughly the same across the pandemic months, with typical peaks in February and September.
In the cohort of under 35s, researchers also found a rate ratio of 2.62 (95% CI, 1.81-3.78) within a 30-day window of SARS-CoV-2 infection, but beyond 30 days, no evidence was seen of an association, with a RR of 0.86 (95% CI, 0.62-1.21; P = .40), she reported.
She explained her reasons for not considering diagnoses within 30 days of COVID-19 as causative. Echoing Dr. Tapia, Dr. Colhoun said the median time from symptom onset to diagnosis of type 1 diabetes is 25 days. “This suggests that 50% have had symptoms for over 25 days at diagnosis.”
She also stressed that when they compared the timing of SARS-CoV-2 testing with diagnosis, they found a much higher rate of COVID-19 testing around diagnosis. “This was not least because everyone admitted to hospital had to have a COVID-19 test.”
Latest U.S. data point to a link
Meanwhile, for the new data reported in JAMA Network Open, medical student Ellen K. Kendall of Case Western Reserve University, Cleveland, matched 571,256 pediatric patients: 285,628 with COVID-19 and 285,628 with non–COVID-19 respiratory infections.
By 6 months after COVID-19, 123 patients (0.043%) had received a new diagnosis of type 1 diabetes, but only 72 (0.025%) were diagnosed with type 1 diabetes within 6 months after non–COVID-19 respiratory infection.
At 1, 3, and 6 months after infection, risk of diagnosis of type 1 diabetes was greater among those infected with SARS-CoV-2, compared with those with non–COVID-19 respiratory infection (1 month: HR, 1.96; 3 months: HR, 2.10; and 6 months: HR, 1.83), and in subgroups of patients aged 0-9 years, a group unlikely to develop type 2 diabetes.
“In this study, new type 1 diabetes diagnoses were more likely to occur among pediatric patients with prior COVID-19 than among those with other respiratory infections (or with other encounters with health systems),” noted Ms. Kendall and coauthors. “Respiratory infections have previously been associated with onset of type 1 diabetes, but this risk was even higher among those with COVID-19 in our study, raising concern for long-term, post–COVID-19 autoimmune complications among youths.”
“The increased risk of new-onset type 1 diabetes after COVID-19 adds an important consideration for risk–benefit discussions for prevention and treatment of SARS-CoV-2 infection in pediatric populations,” they concluded.
A study from the Centers for Disease Control and Prevention published in January 2022, also concluded there was a link between COVID-19 and diabetes in children, but not with other acute respiratory infections. Children were 2.5 times more likely to be diagnosed with diabetes following a SARS-CoV-2 infection, it found.
However, the study has been criticized because it pooled all types of diabetes together and did not account for other health conditions, medications that can increase blood glucose levels, race, obesity, and other social determinants of health that might influence a child’s risk of acquiring COVID-19 or diabetes.
“I’ve no doubt that the CDC data were incorrect because the incidence rate for ... diabetes, even in those never exposed to COVID-19 infection, was 10 times the rate ever reported in the U.S.,” Dr. Colhoun said. “There’s no way these data are correct. I believe there was a confusion between incidence and prevalence of diabetes.”
“This paper caused a great deal of panic, especially among those who have a child with type 1diabetes, so we need to be very careful not to cause undue alarm until we have more definitive evidence in this arena,” she stressed.
However, she also acknowledged that the new Norwegian study was well conducted, and she has no methodological concerns about it, so “I think we just have to wait and see.”
Given the inconclusiveness on the issue, there is an ongoing CoviDiab registry collecting data on this very subject.
Dr. Tapia presented on behalf of lead author Dr. Gulseth, who has reported no relevant financial relationships. Dr. Colhoun also reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – It remains inconclusive whether SARS-CoV-2 infection predisposes children and adolescents to a higher risk of type 1 diabetes. Data from two new studies and a recently published research letter add to the growing body of knowledge on the subject, but still can’t draw any definitive conclusions.
The latest results from a Norwegian and a Scottish study both examine incidence of type 1 diabetes in young people with a history of SARS-CoV-2 infection and were reported at the annual meeting of the European Association for the Study of Diabetes.
A 60% increased risk for type 1 diabetes at least 31 days after SARS-CoV-2 infection (adjusted hazard ratio, 1.63) was found in the Norwegian study, while in contrast, the Scottish study only found an increased risk in the first few months of the pandemic, in 2020, but importantly, no association over a much longer time period (March 2020–November 2021).
In a comment on Twitter on the two studies presented at EASD, session moderator Kamlesh Khunti, MD, professor of primary care diabetes and vascular medicine at the University of Leicester, (England), said: “In summary, two studies showing no or weak association of type 1 diabetes with COVID.”
But new data in the research letter published in JAMA Network Open, based on U.S. figures, also found an almost doubling of type 1 diabetes in children in the first few months after COVID-19 infection relative to infection with other respiratory viruses.
Lead author of the Scottish study, Helen Colhoun, PhD, honorary public health consultant at Public Health Scotland, commented: “Data in children are variable year on year, which emphasizes the need to be cautious over taking a tiny snapshot.”
Nevertheless, this is “a hugely important question and we must not drop the ball. [We must] keep looking at it and maintain scientific equipoise. ... [This] reinforces the need to carry on this analysis into the future to obtain an unequivocal picture,” she emphasized.
Norwegian study: If there is an association, the risk is small
German Tapia, PhD, from the Norwegian Institute of Public Health, Oslo, presented the results of a study of SARS-CoV-2 infection and subsequent risk of type 1 diabetes in 1.2 million children in Norway.
Of these, 424,354 children had been infected with SARS-CoV-2, and there were 990 incident cases of type 1 diabetes.
“What we do know about COVID-19 in children is that the symptoms are mild and only a small proportion are hospitalized with more serious symptoms. But we do not know the long-term effects of COVID-19 infection because this requires a longer follow-up period,” remarked Dr. Tapia, adding that other viral infections are thought to be linked to the development of type 1 diabetes, in particular, respiratory infections.
The data were sourced from the Norwegian Emergency Preparedness Register for COVID-19, which gathers daily data updates including infections (positive and negative results for free-of-charge testing), diagnoses (primary and secondary care), vaccinations (also free of charge), prescribed medications, and basic demographics.
“We link these data using the personal identification number that every Norwegian citizen has,” explained Dr. Tapia.
He presented results from two cohorts: firstly, results in children only, including those tested for SARS-CoV-2 infection, and secondly, a full national Norwegian population cohort.
Regarding the first cohort, those under 18 years who tested positive for SARS-CoV-2 infection, from March 2020 to March 2022, had a significantly increased risk of type 1 diabetes at least 31 days after infection, with an adjusted hazard ratio of 1.63 (95% confidence interval, 1.08-2.47; P = .02). Adjustments were made for age, sex, non-Nordic country of origin, geographic area, and socioeconomic factors.
For children who developed type 1 diabetes within 30 days of a SARS-CoV-2 infection, the HR was 1.26 (95% CI, 0.72-2.19; P = .42), which did not reach statistical significance.
“The fact that fewer people developed type 1 diabetes within 30 days is not surprising because we know that type 1 diabetes develops over a long period of time,” Dr. Tapia said.
“For this reason, we would not expect to find new cases of those people who develop type 1 diabetes within 30 days of COVID-19 infection,” he explained. In these cases, “it is most likely that they already had [type 1 diabetes], and the infection probably triggered clinical symptoms, so their type 1 diabetes was discovered.”
Turning to the full population cohort and diagnoses of type 1 diabetes over 30 days after SARS-CoV-2 infection, the Norwegian researchers found an association, with an HR of 1.57 (95% CI, 1.06-2.33; P = .03), while diagnosis of type 1 diabetes at 30 days or less generated a hazard ratio of 1.22 (95% CI, 0.72-2.19; P = .42).
“So very similar results were found, and after adjustment for confounders, results were still similar,” reported Dr. Tapia.
He also conducted a similar analysis with vaccination as an exposure but found no association between vaccination against SARS-CoV-2 and diagnosis of type 1 diabetes.
“From these results, we conclude that this suggests an increase in diagnosis of type 1 diabetes after SARS-CoV-2 infection, but it must be noted that the absolute risk of developing type 1 diabetes after infection in children is low, with most children not developing the disease,” he emphasized. “There are nearly half a million children who have been infected with SARS-CoV-2 in Norway, but only a very small proportion develop type 1 diabetes.”
Scottish study: No association found over longer term
Dr. Colhoun and colleagues looked at the relationship between incident type 1 diabetes and SARS-CoV-2 infection in children in Scotland using e-health record linkage.
The study involved 1.8 million people under 35 years of age and found very weak, if any, evidence of an association between incident type 1 diabetes and SARS-CoV-2.
Examining data between March 2020 and November 2021, Dr. Colhoun and colleagues identified 365,080 individuals up to age 35 with at least one detected SARS-CoV-2 infection during follow-up and 1,074 who developed type 1 diabetes.
“In children under 16 years, suspected cases of type 1 diabetes are admitted to hospital, and 97% of diagnosis dates are recorded in the Scottish Care Information – Diabetes Collaboration register [SCI-Diabetes] prior to or within 2 days of the first hospital admission for type 1 diabetes,” Dr. Colhoun said, stressing the timeliness of the data.
“We found the incidence of type 1 diabetes diagnosis increased 1.2-fold in those aged 0-14 years, but we did not find any association at an individual level of COVID-19 infection over 30 days prior to a type 1 diabetes diagnosis, in this particular dataset,” she reported. In young people aged 15-34, there was a linear increase in incident type 1 diabetes from 2015 to 2021 with no pandemic increase.
Referring to the 1.2-fold increase soon after the pandemic started, she explained that, in 0- to 14-year-olds, the increase followed a drop in the preceding months prepandemic in 2019. They also found that the seasonal pattern of type 1 diabetes diagnoses remained roughly the same across the pandemic months, with typical peaks in February and September.
In the cohort of under 35s, researchers also found a rate ratio of 2.62 (95% CI, 1.81-3.78) within a 30-day window of SARS-CoV-2 infection, but beyond 30 days, no evidence was seen of an association, with a RR of 0.86 (95% CI, 0.62-1.21; P = .40), she reported.
She explained her reasons for not considering diagnoses within 30 days of COVID-19 as causative. Echoing Dr. Tapia, Dr. Colhoun said the median time from symptom onset to diagnosis of type 1 diabetes is 25 days. “This suggests that 50% have had symptoms for over 25 days at diagnosis.”
She also stressed that when they compared the timing of SARS-CoV-2 testing with diagnosis, they found a much higher rate of COVID-19 testing around diagnosis. “This was not least because everyone admitted to hospital had to have a COVID-19 test.”
Latest U.S. data point to a link
Meanwhile, for the new data reported in JAMA Network Open, medical student Ellen K. Kendall of Case Western Reserve University, Cleveland, matched 571,256 pediatric patients: 285,628 with COVID-19 and 285,628 with non–COVID-19 respiratory infections.
By 6 months after COVID-19, 123 patients (0.043%) had received a new diagnosis of type 1 diabetes, but only 72 (0.025%) were diagnosed with type 1 diabetes within 6 months after non–COVID-19 respiratory infection.
At 1, 3, and 6 months after infection, risk of diagnosis of type 1 diabetes was greater among those infected with SARS-CoV-2, compared with those with non–COVID-19 respiratory infection (1 month: HR, 1.96; 3 months: HR, 2.10; and 6 months: HR, 1.83), and in subgroups of patients aged 0-9 years, a group unlikely to develop type 2 diabetes.
“In this study, new type 1 diabetes diagnoses were more likely to occur among pediatric patients with prior COVID-19 than among those with other respiratory infections (or with other encounters with health systems),” noted Ms. Kendall and coauthors. “Respiratory infections have previously been associated with onset of type 1 diabetes, but this risk was even higher among those with COVID-19 in our study, raising concern for long-term, post–COVID-19 autoimmune complications among youths.”
“The increased risk of new-onset type 1 diabetes after COVID-19 adds an important consideration for risk–benefit discussions for prevention and treatment of SARS-CoV-2 infection in pediatric populations,” they concluded.
A study from the Centers for Disease Control and Prevention published in January 2022, also concluded there was a link between COVID-19 and diabetes in children, but not with other acute respiratory infections. Children were 2.5 times more likely to be diagnosed with diabetes following a SARS-CoV-2 infection, it found.
However, the study has been criticized because it pooled all types of diabetes together and did not account for other health conditions, medications that can increase blood glucose levels, race, obesity, and other social determinants of health that might influence a child’s risk of acquiring COVID-19 or diabetes.
“I’ve no doubt that the CDC data were incorrect because the incidence rate for ... diabetes, even in those never exposed to COVID-19 infection, was 10 times the rate ever reported in the U.S.,” Dr. Colhoun said. “There’s no way these data are correct. I believe there was a confusion between incidence and prevalence of diabetes.”
“This paper caused a great deal of panic, especially among those who have a child with type 1diabetes, so we need to be very careful not to cause undue alarm until we have more definitive evidence in this arena,” she stressed.
However, she also acknowledged that the new Norwegian study was well conducted, and she has no methodological concerns about it, so “I think we just have to wait and see.”
Given the inconclusiveness on the issue, there is an ongoing CoviDiab registry collecting data on this very subject.
Dr. Tapia presented on behalf of lead author Dr. Gulseth, who has reported no relevant financial relationships. Dr. Colhoun also reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT EASD 2022
Severe COVID-19–related outcomes found worse in men with RA
A retrospective study that analyzed sex disparities in patients with COVID-19 and rheumatoid arthritis found that men had more baseline comorbidities and increased risk of COVID-19–related outcomes, compared with women.
“Differences in genetics between sex and sex steroid hormones may play a role in predisposition to COVID-19 infection as well as modulating the disease progression,” according to Xiaofeng Zhou, PhD, senior director at Pfizer, New York, and the study’s lead author.
Dr. Zhou presented her findings at The Lancet Summit on Sex and Gender in Rheumatology.
Patients with chronic rheumatic diseases treated with immunomodulatory therapies may be at higher risk for more severe COVID-19 outcomes, including hospitalization, complications, and death. Research on sex-based disparities in RA patients with COVID-19 in the United States is limited, said Dr. Zhou, who embarked on a retrospective cohort study to examine the demographic and clinical characteristics of RA patients with COVID-19 and estimate the risk of possible COVID-19 outcomes by sex.
Dr. Zhou and colleagues used U.S. COVID-19 data collected through electronic health records by Optum during 2020 to June 2021. The study included adult patients with RA and a COVID-19 diagnosis (≥ 1 diagnosis code or positive SARS-CoV-2 laboratory test) and greater than or equal to 183 days of database enrollment who received treatment with immunomodulatory therapies prior to the diagnosis date. They were stratified by sex.
Investigators used logistic regression to estimate the risk of 11 possible COVID-19–related outcomes within 30 days of the COVID-19 diagnosis (hospitalization, ICU admission, pneumonia, kidney failure, thrombotic event, heart failure, acute respiratory distress syndrome [ARDS], sepsis/septic shock, mechanical ventilation/extracorporeal membrane oxygenation [ECMO], in-hospital death, and all-cause mortality), adjusting for demographics and baseline clinical covariates.
A total of 4,476 COVID-19 patients with RA (78% female) took part in the study. Male patients trended older (64 vs. 60 years) and had lower African American representation and Medicaid enrollment than female patients, but they had more baseline comorbidities such as hypertension (55% vs. 45%), hyperlipidemia (45% vs. 33%), diabetes (25% vs. 20%), coronary artery disease (28% vs. 12%), and chronic kidney disease (20% vs. 15%).
Eight of the eleven COVID-19 outcomes were significantly more likely to occur in men than women (hospitalization: odds ratio, 1.32 [95% confidence interval (CI), 1.11-1.56]; ICU admission: OR, 1.80 [95% CI, 1.36-2.40]; mechanical ventilation/ECMO: OR, 1.48 [95% CI, 1.04-2.11]; in-hospital death: OR, 1.53 [95% CI, 1.13-2.07]; all-cause mortality: OR, 1.42 [95% CI, 1.09-1.86]; sepsis: OR, 1.55 [95% CI, 1.20-2.02]; kidney failure: OR, 1.46 [95% CI, 1.15-1.85]; ARDS: OR, 1.39 [95% CI, 1.15-1.69]).
Sex hormones factor into risk
The data illustrated that men with RA had more baseline comorbidities and increased risk of COVID-19 outcomes than women.
Sex hormones regulate virus entry into host cells, respiratory function, immune response, the cardiovascular system, and coagulation, explained Dr. Zhou.
Estrogen and progesterone in women could help develop stronger and efficient immune responses to viruses and reduce virus entry into the host cells. Also, “[the] larger number of copies of ACE2 genes in women, [which] is linked with protection in the lungs against edema, permeability, and pulmonary damage, could be associated with lower incidence of severe COVID-19 outcomes, such as respiratory-related mortality and mortality,” Dr. Zhou said.
By comparison, androgens in men may increase virus entry into the host cells and promote unfavorable immune response through the induction of cytokine production and reducing the antibody response to the virus. This could lead to severe infection, Dr. Zhou said.
Sex-based differences in steroid hormones may also explain the higher incidence of morbidity and fatality that’s been observed in other studies of male patients with other infectious diseases, such as severe acute respiratory syndrome and Middle East respiratory syndrome.
Study bolsters evidence on sex disparities
The results add real-world evidence to the limited literature on sex disparities in COVID-19 outcomes among patients with RA in the United States, Dr. Zhou said. “The differential role in sex steroid hormones among women and men may shed light on clinical management of COVID-19 patients and the need to consider sex-specific approaches in clinical trials in preventing and treating COVID-19 patients,” she said.
Considering that all patients are recommended to get COVID-19 vaccinations, “it is difficult to say how this impacts clinical practice,” said Janet Pope, MD, MPH, professor of medicine in the division of rheumatology at the University of Western Ontario, London, who was not involved with the study.
Sharing results with some patients may help to encourage vaccination, thus reducing risk of poor COVID-19 outcomes, Dr. Pope said.
In future studies, Dr. Zhou suggests using multiple databases and considering other geographies beyond the United States to further understand the etiology of sexual dimorphism in COVID-19 and expand generalizability. “In addition, future research will seek to provide insights into health equity gaps in the management of COVID-19. This may inform development of precision medicines and vaccines, especially among patients on immunosuppressive treatments,” she said.
The study was sponsored by Pfizer. Dr. Zhou and other study authors are Pfizer employees and hold Pfizer stock.
A version of this article first appeared on Medscape.com.
A retrospective study that analyzed sex disparities in patients with COVID-19 and rheumatoid arthritis found that men had more baseline comorbidities and increased risk of COVID-19–related outcomes, compared with women.
“Differences in genetics between sex and sex steroid hormones may play a role in predisposition to COVID-19 infection as well as modulating the disease progression,” according to Xiaofeng Zhou, PhD, senior director at Pfizer, New York, and the study’s lead author.
Dr. Zhou presented her findings at The Lancet Summit on Sex and Gender in Rheumatology.
Patients with chronic rheumatic diseases treated with immunomodulatory therapies may be at higher risk for more severe COVID-19 outcomes, including hospitalization, complications, and death. Research on sex-based disparities in RA patients with COVID-19 in the United States is limited, said Dr. Zhou, who embarked on a retrospective cohort study to examine the demographic and clinical characteristics of RA patients with COVID-19 and estimate the risk of possible COVID-19 outcomes by sex.
Dr. Zhou and colleagues used U.S. COVID-19 data collected through electronic health records by Optum during 2020 to June 2021. The study included adult patients with RA and a COVID-19 diagnosis (≥ 1 diagnosis code or positive SARS-CoV-2 laboratory test) and greater than or equal to 183 days of database enrollment who received treatment with immunomodulatory therapies prior to the diagnosis date. They were stratified by sex.
Investigators used logistic regression to estimate the risk of 11 possible COVID-19–related outcomes within 30 days of the COVID-19 diagnosis (hospitalization, ICU admission, pneumonia, kidney failure, thrombotic event, heart failure, acute respiratory distress syndrome [ARDS], sepsis/septic shock, mechanical ventilation/extracorporeal membrane oxygenation [ECMO], in-hospital death, and all-cause mortality), adjusting for demographics and baseline clinical covariates.
A total of 4,476 COVID-19 patients with RA (78% female) took part in the study. Male patients trended older (64 vs. 60 years) and had lower African American representation and Medicaid enrollment than female patients, but they had more baseline comorbidities such as hypertension (55% vs. 45%), hyperlipidemia (45% vs. 33%), diabetes (25% vs. 20%), coronary artery disease (28% vs. 12%), and chronic kidney disease (20% vs. 15%).
Eight of the eleven COVID-19 outcomes were significantly more likely to occur in men than women (hospitalization: odds ratio, 1.32 [95% confidence interval (CI), 1.11-1.56]; ICU admission: OR, 1.80 [95% CI, 1.36-2.40]; mechanical ventilation/ECMO: OR, 1.48 [95% CI, 1.04-2.11]; in-hospital death: OR, 1.53 [95% CI, 1.13-2.07]; all-cause mortality: OR, 1.42 [95% CI, 1.09-1.86]; sepsis: OR, 1.55 [95% CI, 1.20-2.02]; kidney failure: OR, 1.46 [95% CI, 1.15-1.85]; ARDS: OR, 1.39 [95% CI, 1.15-1.69]).
Sex hormones factor into risk
The data illustrated that men with RA had more baseline comorbidities and increased risk of COVID-19 outcomes than women.
Sex hormones regulate virus entry into host cells, respiratory function, immune response, the cardiovascular system, and coagulation, explained Dr. Zhou.
Estrogen and progesterone in women could help develop stronger and efficient immune responses to viruses and reduce virus entry into the host cells. Also, “[the] larger number of copies of ACE2 genes in women, [which] is linked with protection in the lungs against edema, permeability, and pulmonary damage, could be associated with lower incidence of severe COVID-19 outcomes, such as respiratory-related mortality and mortality,” Dr. Zhou said.
By comparison, androgens in men may increase virus entry into the host cells and promote unfavorable immune response through the induction of cytokine production and reducing the antibody response to the virus. This could lead to severe infection, Dr. Zhou said.
Sex-based differences in steroid hormones may also explain the higher incidence of morbidity and fatality that’s been observed in other studies of male patients with other infectious diseases, such as severe acute respiratory syndrome and Middle East respiratory syndrome.
Study bolsters evidence on sex disparities
The results add real-world evidence to the limited literature on sex disparities in COVID-19 outcomes among patients with RA in the United States, Dr. Zhou said. “The differential role in sex steroid hormones among women and men may shed light on clinical management of COVID-19 patients and the need to consider sex-specific approaches in clinical trials in preventing and treating COVID-19 patients,” she said.
Considering that all patients are recommended to get COVID-19 vaccinations, “it is difficult to say how this impacts clinical practice,” said Janet Pope, MD, MPH, professor of medicine in the division of rheumatology at the University of Western Ontario, London, who was not involved with the study.
Sharing results with some patients may help to encourage vaccination, thus reducing risk of poor COVID-19 outcomes, Dr. Pope said.
In future studies, Dr. Zhou suggests using multiple databases and considering other geographies beyond the United States to further understand the etiology of sexual dimorphism in COVID-19 and expand generalizability. “In addition, future research will seek to provide insights into health equity gaps in the management of COVID-19. This may inform development of precision medicines and vaccines, especially among patients on immunosuppressive treatments,” she said.
The study was sponsored by Pfizer. Dr. Zhou and other study authors are Pfizer employees and hold Pfizer stock.
A version of this article first appeared on Medscape.com.
A retrospective study that analyzed sex disparities in patients with COVID-19 and rheumatoid arthritis found that men had more baseline comorbidities and increased risk of COVID-19–related outcomes, compared with women.
“Differences in genetics between sex and sex steroid hormones may play a role in predisposition to COVID-19 infection as well as modulating the disease progression,” according to Xiaofeng Zhou, PhD, senior director at Pfizer, New York, and the study’s lead author.
Dr. Zhou presented her findings at The Lancet Summit on Sex and Gender in Rheumatology.
Patients with chronic rheumatic diseases treated with immunomodulatory therapies may be at higher risk for more severe COVID-19 outcomes, including hospitalization, complications, and death. Research on sex-based disparities in RA patients with COVID-19 in the United States is limited, said Dr. Zhou, who embarked on a retrospective cohort study to examine the demographic and clinical characteristics of RA patients with COVID-19 and estimate the risk of possible COVID-19 outcomes by sex.
Dr. Zhou and colleagues used U.S. COVID-19 data collected through electronic health records by Optum during 2020 to June 2021. The study included adult patients with RA and a COVID-19 diagnosis (≥ 1 diagnosis code or positive SARS-CoV-2 laboratory test) and greater than or equal to 183 days of database enrollment who received treatment with immunomodulatory therapies prior to the diagnosis date. They were stratified by sex.
Investigators used logistic regression to estimate the risk of 11 possible COVID-19–related outcomes within 30 days of the COVID-19 diagnosis (hospitalization, ICU admission, pneumonia, kidney failure, thrombotic event, heart failure, acute respiratory distress syndrome [ARDS], sepsis/septic shock, mechanical ventilation/extracorporeal membrane oxygenation [ECMO], in-hospital death, and all-cause mortality), adjusting for demographics and baseline clinical covariates.
A total of 4,476 COVID-19 patients with RA (78% female) took part in the study. Male patients trended older (64 vs. 60 years) and had lower African American representation and Medicaid enrollment than female patients, but they had more baseline comorbidities such as hypertension (55% vs. 45%), hyperlipidemia (45% vs. 33%), diabetes (25% vs. 20%), coronary artery disease (28% vs. 12%), and chronic kidney disease (20% vs. 15%).
Eight of the eleven COVID-19 outcomes were significantly more likely to occur in men than women (hospitalization: odds ratio, 1.32 [95% confidence interval (CI), 1.11-1.56]; ICU admission: OR, 1.80 [95% CI, 1.36-2.40]; mechanical ventilation/ECMO: OR, 1.48 [95% CI, 1.04-2.11]; in-hospital death: OR, 1.53 [95% CI, 1.13-2.07]; all-cause mortality: OR, 1.42 [95% CI, 1.09-1.86]; sepsis: OR, 1.55 [95% CI, 1.20-2.02]; kidney failure: OR, 1.46 [95% CI, 1.15-1.85]; ARDS: OR, 1.39 [95% CI, 1.15-1.69]).
Sex hormones factor into risk
The data illustrated that men with RA had more baseline comorbidities and increased risk of COVID-19 outcomes than women.
Sex hormones regulate virus entry into host cells, respiratory function, immune response, the cardiovascular system, and coagulation, explained Dr. Zhou.
Estrogen and progesterone in women could help develop stronger and efficient immune responses to viruses and reduce virus entry into the host cells. Also, “[the] larger number of copies of ACE2 genes in women, [which] is linked with protection in the lungs against edema, permeability, and pulmonary damage, could be associated with lower incidence of severe COVID-19 outcomes, such as respiratory-related mortality and mortality,” Dr. Zhou said.
By comparison, androgens in men may increase virus entry into the host cells and promote unfavorable immune response through the induction of cytokine production and reducing the antibody response to the virus. This could lead to severe infection, Dr. Zhou said.
Sex-based differences in steroid hormones may also explain the higher incidence of morbidity and fatality that’s been observed in other studies of male patients with other infectious diseases, such as severe acute respiratory syndrome and Middle East respiratory syndrome.
Study bolsters evidence on sex disparities
The results add real-world evidence to the limited literature on sex disparities in COVID-19 outcomes among patients with RA in the United States, Dr. Zhou said. “The differential role in sex steroid hormones among women and men may shed light on clinical management of COVID-19 patients and the need to consider sex-specific approaches in clinical trials in preventing and treating COVID-19 patients,” she said.
Considering that all patients are recommended to get COVID-19 vaccinations, “it is difficult to say how this impacts clinical practice,” said Janet Pope, MD, MPH, professor of medicine in the division of rheumatology at the University of Western Ontario, London, who was not involved with the study.
Sharing results with some patients may help to encourage vaccination, thus reducing risk of poor COVID-19 outcomes, Dr. Pope said.
In future studies, Dr. Zhou suggests using multiple databases and considering other geographies beyond the United States to further understand the etiology of sexual dimorphism in COVID-19 and expand generalizability. “In addition, future research will seek to provide insights into health equity gaps in the management of COVID-19. This may inform development of precision medicines and vaccines, especially among patients on immunosuppressive treatments,” she said.
The study was sponsored by Pfizer. Dr. Zhou and other study authors are Pfizer employees and hold Pfizer stock.
A version of this article first appeared on Medscape.com.
FROM THE LANCET SUMMIT ON SEX AND GENDER IN RHEUMATOLOGY
COVID pandemic associated with anorexia in Canadian youth
, data suggest.
Preliminary results of the Canadian Paediatric Surveillance Program (CPSP) indicate that the pandemic has been a precipitating factor in the development of anorexia nervosa in almost half of children and adolescents studied. The pandemic also has precipitated hospitalizations for anorexia in more than one-third of cases.
“Data globally, and certainly our data here in Canada, have shown a real increase in health care utilization with the onset of the COVID-19 pandemic,” study author Debra Katzman, MD, professor of pediatrics at the Hospital for Sick Children in Toronto and the University of Toronto, said in an interview. “And when I talk about health care utilization, I’m talking about hospitalizations for eating disorders.”
The data were included in the 2021 results of the CPSP.
Focus on appearance
CPSP is a collaboration between the Public Health Agency of Canada and the Canadian Pediatric Society that consists of a network of 2,800 pediatricians and pediatric subspecialists across Canada. The latest results include surveillance studies on 14 diseases and conditions, with data collected during various periods.
From April 2020 to May 2021, researchers identified 1,800 COVID-19 cases in children and collected detailed information on 1,456 of them, including 405 cases hospitalized with pediatric inflammatory multisystem syndrome (PIMS). The median age of hospitalized cases was 3.2 years for SARS-CoV-2 infection and 5.4 years for PIMS.
Dr. Katzman and colleagues observed 118 first-time hospitalizations for anorexia nervosa between Sept. 1 and Dec. 31, 2021. More than 90% of reported cases were female, with 66% of verified cases in teens aged 14-17 years and the remainder in adolescents aged 11-13 years.
In 49% of cases, the reporting physician identified the COVID-19 pandemic as a precipitating factor in the development of anorexia nervosa. In 37% of cases, the reporting physician identified the pandemic as having precipitated the anorexia-related hospitalization.
Last year, a cross-sectional analysis of children in Canada reported that monthly hospitalizations for anorexia nervosa increased from 7.5 to 20 from March through November 2020. The monthly rate in the CPSP study was closer to 30 for first-time hospitalizations.
Dr. Katzman said that the findings about anorexia nervosa didn’t surprise her. “There was so much disruption and [so many] restrictions to young peoples’ daily routines – closures of schools and recreational activities – they lost regular connection with their peers, and they lost extracurricular and social activities,” she said. “That led to heightened anxiety and depression and really a lack of control.”
Adolescents and teens were also spending more time on social media than they were before the pandemic, she noted. “They were looking at themselves all the time, so they were getting preoccupied with their body image. There was a heightened focus on appearance, and I think that things like public-health mitigation strategies – things like hand washing, social distancing, mask wearing – may have impacted the psychological well-being of young people.”
The closure of outpatient facilities, long waiting lists to get into facilities that were opened, and “coronaphobia” about going to physicians’ offices and emergency departments compounded the problem, Dr. Katzman added.
The long-term effects of COVID and eating disorders in children are unknown, Dr. Katzman said. “This is sort of a wake-up call for the health care system that during times of stress or pandemics or crises, these kinds of things can happen, and we need to be prepared to provide the resources for vulnerable populations moving forward,” she said.
Heightened anxiety
Commenting on the data, Margaret Thew, APNP, director of the eating disorders program at Children’s Wisconsin in Milwaukee, said that isolation due to school closures and negative social media messages created the “perfect storm” for eating disorders in adolescents and teenagers because of higher rates of anxiety and depression. Ms. Thew was not involved in the research.
The storm is not over yet, she said. “What everyone needs to keep in mind is that we still have this very heightened state of anxiety and depression ... for adolescents, teenagers, and preteens alike,” Ms. Thew said in an interview, “and we know that many of them are not coping with their anxiety very well.”
In her experience, since the start of the pandemic, the average age of pediatric patients with eating disorders declined from 16 to 15 years, and the youngest age declined from 12 to 11 years.
Overall, the CPSP results show that children are affected by mental health issues at an earlier age than before the pandemic, said Ms. Thew. “Years ago, we wouldn’t have thought that an 8-year-old needed to be screened for some of these risk factors, but now we’re definitely getting more younger children who are struggling, and I think it’s taking too long for them to get the care they need because it’s being overlooked,” she said.
The report was funded by the Public Health Agency of Canada, Health Canada, Alberta Children’s Hospital Research Institute, Bethanys Hope Foundation, CHEO Research Institute, and Children’s Hospital Research Institute of Manitoba. Dr. Katzman and Ms. Thew have no relevant disclosures.
A version of this article first appeared on Medscape.com.
, data suggest.
Preliminary results of the Canadian Paediatric Surveillance Program (CPSP) indicate that the pandemic has been a precipitating factor in the development of anorexia nervosa in almost half of children and adolescents studied. The pandemic also has precipitated hospitalizations for anorexia in more than one-third of cases.
“Data globally, and certainly our data here in Canada, have shown a real increase in health care utilization with the onset of the COVID-19 pandemic,” study author Debra Katzman, MD, professor of pediatrics at the Hospital for Sick Children in Toronto and the University of Toronto, said in an interview. “And when I talk about health care utilization, I’m talking about hospitalizations for eating disorders.”
The data were included in the 2021 results of the CPSP.
Focus on appearance
CPSP is a collaboration between the Public Health Agency of Canada and the Canadian Pediatric Society that consists of a network of 2,800 pediatricians and pediatric subspecialists across Canada. The latest results include surveillance studies on 14 diseases and conditions, with data collected during various periods.
From April 2020 to May 2021, researchers identified 1,800 COVID-19 cases in children and collected detailed information on 1,456 of them, including 405 cases hospitalized with pediatric inflammatory multisystem syndrome (PIMS). The median age of hospitalized cases was 3.2 years for SARS-CoV-2 infection and 5.4 years for PIMS.
Dr. Katzman and colleagues observed 118 first-time hospitalizations for anorexia nervosa between Sept. 1 and Dec. 31, 2021. More than 90% of reported cases were female, with 66% of verified cases in teens aged 14-17 years and the remainder in adolescents aged 11-13 years.
In 49% of cases, the reporting physician identified the COVID-19 pandemic as a precipitating factor in the development of anorexia nervosa. In 37% of cases, the reporting physician identified the pandemic as having precipitated the anorexia-related hospitalization.
Last year, a cross-sectional analysis of children in Canada reported that monthly hospitalizations for anorexia nervosa increased from 7.5 to 20 from March through November 2020. The monthly rate in the CPSP study was closer to 30 for first-time hospitalizations.
Dr. Katzman said that the findings about anorexia nervosa didn’t surprise her. “There was so much disruption and [so many] restrictions to young peoples’ daily routines – closures of schools and recreational activities – they lost regular connection with their peers, and they lost extracurricular and social activities,” she said. “That led to heightened anxiety and depression and really a lack of control.”
Adolescents and teens were also spending more time on social media than they were before the pandemic, she noted. “They were looking at themselves all the time, so they were getting preoccupied with their body image. There was a heightened focus on appearance, and I think that things like public-health mitigation strategies – things like hand washing, social distancing, mask wearing – may have impacted the psychological well-being of young people.”
The closure of outpatient facilities, long waiting lists to get into facilities that were opened, and “coronaphobia” about going to physicians’ offices and emergency departments compounded the problem, Dr. Katzman added.
The long-term effects of COVID and eating disorders in children are unknown, Dr. Katzman said. “This is sort of a wake-up call for the health care system that during times of stress or pandemics or crises, these kinds of things can happen, and we need to be prepared to provide the resources for vulnerable populations moving forward,” she said.
Heightened anxiety
Commenting on the data, Margaret Thew, APNP, director of the eating disorders program at Children’s Wisconsin in Milwaukee, said that isolation due to school closures and negative social media messages created the “perfect storm” for eating disorders in adolescents and teenagers because of higher rates of anxiety and depression. Ms. Thew was not involved in the research.
The storm is not over yet, she said. “What everyone needs to keep in mind is that we still have this very heightened state of anxiety and depression ... for adolescents, teenagers, and preteens alike,” Ms. Thew said in an interview, “and we know that many of them are not coping with their anxiety very well.”
In her experience, since the start of the pandemic, the average age of pediatric patients with eating disorders declined from 16 to 15 years, and the youngest age declined from 12 to 11 years.
Overall, the CPSP results show that children are affected by mental health issues at an earlier age than before the pandemic, said Ms. Thew. “Years ago, we wouldn’t have thought that an 8-year-old needed to be screened for some of these risk factors, but now we’re definitely getting more younger children who are struggling, and I think it’s taking too long for them to get the care they need because it’s being overlooked,” she said.
The report was funded by the Public Health Agency of Canada, Health Canada, Alberta Children’s Hospital Research Institute, Bethanys Hope Foundation, CHEO Research Institute, and Children’s Hospital Research Institute of Manitoba. Dr. Katzman and Ms. Thew have no relevant disclosures.
A version of this article first appeared on Medscape.com.
, data suggest.
Preliminary results of the Canadian Paediatric Surveillance Program (CPSP) indicate that the pandemic has been a precipitating factor in the development of anorexia nervosa in almost half of children and adolescents studied. The pandemic also has precipitated hospitalizations for anorexia in more than one-third of cases.
“Data globally, and certainly our data here in Canada, have shown a real increase in health care utilization with the onset of the COVID-19 pandemic,” study author Debra Katzman, MD, professor of pediatrics at the Hospital for Sick Children in Toronto and the University of Toronto, said in an interview. “And when I talk about health care utilization, I’m talking about hospitalizations for eating disorders.”
The data were included in the 2021 results of the CPSP.
Focus on appearance
CPSP is a collaboration between the Public Health Agency of Canada and the Canadian Pediatric Society that consists of a network of 2,800 pediatricians and pediatric subspecialists across Canada. The latest results include surveillance studies on 14 diseases and conditions, with data collected during various periods.
From April 2020 to May 2021, researchers identified 1,800 COVID-19 cases in children and collected detailed information on 1,456 of them, including 405 cases hospitalized with pediatric inflammatory multisystem syndrome (PIMS). The median age of hospitalized cases was 3.2 years for SARS-CoV-2 infection and 5.4 years for PIMS.
Dr. Katzman and colleagues observed 118 first-time hospitalizations for anorexia nervosa between Sept. 1 and Dec. 31, 2021. More than 90% of reported cases were female, with 66% of verified cases in teens aged 14-17 years and the remainder in adolescents aged 11-13 years.
In 49% of cases, the reporting physician identified the COVID-19 pandemic as a precipitating factor in the development of anorexia nervosa. In 37% of cases, the reporting physician identified the pandemic as having precipitated the anorexia-related hospitalization.
Last year, a cross-sectional analysis of children in Canada reported that monthly hospitalizations for anorexia nervosa increased from 7.5 to 20 from March through November 2020. The monthly rate in the CPSP study was closer to 30 for first-time hospitalizations.
Dr. Katzman said that the findings about anorexia nervosa didn’t surprise her. “There was so much disruption and [so many] restrictions to young peoples’ daily routines – closures of schools and recreational activities – they lost regular connection with their peers, and they lost extracurricular and social activities,” she said. “That led to heightened anxiety and depression and really a lack of control.”
Adolescents and teens were also spending more time on social media than they were before the pandemic, she noted. “They were looking at themselves all the time, so they were getting preoccupied with their body image. There was a heightened focus on appearance, and I think that things like public-health mitigation strategies – things like hand washing, social distancing, mask wearing – may have impacted the psychological well-being of young people.”
The closure of outpatient facilities, long waiting lists to get into facilities that were opened, and “coronaphobia” about going to physicians’ offices and emergency departments compounded the problem, Dr. Katzman added.
The long-term effects of COVID and eating disorders in children are unknown, Dr. Katzman said. “This is sort of a wake-up call for the health care system that during times of stress or pandemics or crises, these kinds of things can happen, and we need to be prepared to provide the resources for vulnerable populations moving forward,” she said.
Heightened anxiety
Commenting on the data, Margaret Thew, APNP, director of the eating disorders program at Children’s Wisconsin in Milwaukee, said that isolation due to school closures and negative social media messages created the “perfect storm” for eating disorders in adolescents and teenagers because of higher rates of anxiety and depression. Ms. Thew was not involved in the research.
The storm is not over yet, she said. “What everyone needs to keep in mind is that we still have this very heightened state of anxiety and depression ... for adolescents, teenagers, and preteens alike,” Ms. Thew said in an interview, “and we know that many of them are not coping with their anxiety very well.”
In her experience, since the start of the pandemic, the average age of pediatric patients with eating disorders declined from 16 to 15 years, and the youngest age declined from 12 to 11 years.
Overall, the CPSP results show that children are affected by mental health issues at an earlier age than before the pandemic, said Ms. Thew. “Years ago, we wouldn’t have thought that an 8-year-old needed to be screened for some of these risk factors, but now we’re definitely getting more younger children who are struggling, and I think it’s taking too long for them to get the care they need because it’s being overlooked,” she said.
The report was funded by the Public Health Agency of Canada, Health Canada, Alberta Children’s Hospital Research Institute, Bethanys Hope Foundation, CHEO Research Institute, and Children’s Hospital Research Institute of Manitoba. Dr. Katzman and Ms. Thew have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Long COVID could cost the economy trillions, experts predict
from restaurants struggling to replace low-wage workers, to airlines scrambling to replace crew, to overwhelmed hospitals, experts are predicting.
“There’s a lot we need to do to understand what it takes to enable disabled people to participate more in the economy,” said Katie Bach, a senior fellow with Brookings Institution and the author of a study looking into long COVID’s impact on the labor market.
Data from June 2022 from the Centers for Disease Control and Prevention shows that, of the 40% of American adults who contracted COVID-19, nearly one in five still have long COVID symptoms. That works out to 1 in 13, or 7.5%, of the overall U.S. adult population.
Drawing from the CDC data, Ms. Bach estimates in her August 2022 report that as many as 4 million working-age Americans are too sick with long COVID to perform their jobs. That works out to as much as $230 billion in lost wages, or almost 1% of the U.S. GDP.
“This is a big deal,” she said. “We’re talking potentially hundreds of billions of dollars a year and that this is big enough to have a measurable impact on the labor market.”
Other sources have suggested lower figures, but the conclusions are the same: Long COVID is an urgent issue that will cost tens of billions of dollars a year in lost wages alone, Ms. Bach said. But it’s not just lost income for workers. There is a cost for businesses and the public.
Throughout the pandemic, COVID-19’s crippling force could be felt across multiple industries. While business has picked up again, staffing shortages remain a challenge. At some airports this summer, air passengers spent hours in security lines; were stranded for days as flights were canceled, rebooked, and canceled again on short notice; and waited weeks for lost luggage. Restaurants have had to cut back their hours. Those seeking medical care had longer than usual wait times in EDs and urgent care clinics. Some EDs temporarily closed.
These challenges have been attributed in part to the “great resignation” and in part because so many infected workers were out, especially during the Omicron waves. But increasingly, economists and health care professionals alike worry about long COVID’s impact on employers and the broader economy.
David Cutler, PhD, a professor of economics at Harvard University, Cambridge, Mass., believes the total economic loss could be as high as $3.7 trillion, when factoring in the lost quality of life, the cost in lost earnings, and the cost of higher spending on medical care. His estimate is more than a trillion dollars higher than a previous projection he and fellow economist Lawrence Summers, PhD, made in 2020. The reason? Long COVID.
“The higher estimate is largely a result of the greater prevalence of long COVID than we had guessed at the time,” Dr. Cutler wrote in a paper released in July.
“There are about 10 times the number of people with long COVID as have died of COVID. Because long COVID is so new, there is uncertainty about all of the numbers involved in the calculations. Still, the costs here are conservative, based on only cases to date.”
In Ms. Bach’s Brookings report, she projected that, if recovery from long COVID does not pick up and the population of Americans with long COVID were to grow by 10% a year, the annual cost of lost wages alone could reach half a trillion dollars in a decade.
Meanwhile, a working paper by the National Bureau of Economic Research found that workers who missed an entire week of work because of probable COVID-19 illnesses were roughly 7 percentage points less likely to be working a year later, compared with those who did not miss work for health reasons.
“It’s not just individuals with long COVID who are suffering from this. It impacts their families, their livelihoods, and the economy on a global scale. So, we have to raise awareness about those ripple effects,” said Linda Geng, MD, a clinical assistant professor of medicine with Stanford (Calif.) University’s Primary Care and Population Health.
“I think it’s hard for the public to grasp ... and understand the scale of this public health crisis.”
Debilitating fatigue
Long COVID is roughly defined; the CDC defines it as symptoms that linger 3 or more months after a patient first catches the virus.
The symptoms vary and include profound fatigue and brain issues.
“It’s a new degree of extreme and debilitating fatigue and exhaustion, to the point where you can’t do your daily tasks,” said Dr. Geng, who is also the codirector of Stanford’s Post-Acute COVID-19 Syndrome Clinic.
“People can be so debilitated, they can’t even do basic things, like the activities of daily living, let alone do their job, particularly if it’s physically or mentally demanding.”
Patients can also have postexertional malaise, where they feel especially bad and symptoms worsen when they exert themselves physically or mentally, Dr. Geng said. Compounding the issue for many long COVID patients is their trouble getting restful sleep. Those with brain fog have issues with memory, processing information, focused concentration, confusion, making mistakes, and multitasking. Pain is another debilitating symptom that can disrupt daily life and ability to work.
Even people with relatively mild infections can end up with long COVID, Dr. Geng said, noting that many of the patients at the Stanford clinic were never hospitalized with their initial infections. While existing research and Dr. Geng’s clinical experience show that long COVID can hit any age, she most commonly sees patients from ages 20 to their 60s, with an average age in the 40s – people in their prime working ages.
Jason Furman, PhD, a former White House economic adviser who is now a professor at Harvard University, noted in August that the labor force participation rate was far below what could be explained by standard demographic changes like an aging population, with the decline evident across all age groups. Dr. Furman does not speculate about why, but others have.
“We are pessimistic: Both the aging of the population and the impact of long COVID imply that the participation rate will be slow to return to its prepandemic level,” Anna Wong, Yelena Shulyatyeva, Andrew Husby, and Eliza Winger, economists with Bloomberg Economics, wrote in a research note.
Supportive policies
There is some evidence that vaccination reduces the risk of long COVID, but not completely, and it is too early to know if repeat infections increase long COVID risks. There is also no definitive data on how fast or how many people are recovering. Economists often assume that those with long COVID will recover at some point, Ms. Bach noted, but she is careful not to make assumptions.
“If people aren’t recovering, then this group keeps getting bigger,” she said. “We’re still adding, and if people aren’t coming out of that group, this becomes a bigger and bigger problem.”
For now, the number of new people being diagnosed with long COVID appears to have slowed, Ms. Bach said, but it remains to be seen whether the trend can be sustained.
“If people are impaired longer than we think and if the impairment turns out to be severe, then we can have a lot of people who need services like disability insurance,” Dr. Cutler said.
“That could put a really big strain on public sector programs and our ability to meet those needs.”
Policies that support the research and clinical work necessary to prevent and treat long COVID are essential, experts say.
“To me, that is the biggest economic imperative, to say nothing of human suffering,” said Ms. Bach.
Employers also have a role, and experts say there are a number of accommodations businesses should consider. What happens when an employee has long COVID? Can accommodations be made that allow them to continue working productively? If they spend a great deal of time commuting, can they work from home? What can employers do so that family members do not have to drop out of the workforce to take care of loved ones with long COVID?
Disability insurance
To be sure, there is one piece of the puzzle that does not quite fit, according to Dr. Cutler and Ms. Bach. There is no sign yet of a large increase in federal disability insurance applications, and no one quite knows why. Publicly available government data shows that online applications actually dipped by about 4% each year between 2019 and 2021. Applications in 2022 appear on track to remain slightly below prepandemic levels.
To qualify for Social Security Disability Insurance (SSDI), people need to have a disability that lasts at least a year.
“If you’re disabled with long COVID, who knows, right? You don’t know,” said Ms. Bach. “Two of the most dominant symptoms of long COVID are fatigue and brain fog. So, I’ve heard from people that the process of going through an SSDI application is really hard.”
Some long COVID patients told Ms. Bach they simply assumed they would not get SSDI and did not even bother applying. She stressed that working Americans with debilitating long COVID should be aware that their condition is protected by the Americans with Disabilities Act. But the challenge, based on guidance issued by the government, is that not all cases of long COVID qualify as a disability and that individual assessments are necessary.
While more long COVID data are needed, Ms. Bach believes there is enough information for decisionmakers to go after the issue more aggressively. She pointed to the $1.15 billion in funding that Congress earmarked for the National Institutes of Health over the course of 4 years in support of research into the long-term health effects of COVID-19.
“Now, $250 million a year sounds like a lot of money until you start talking about the cost of lost wages – just lost wages,” Ms. Bach said. “That’s not lost productivity. That’s not the cost of people whose family members are sick. Who have to reduce their own labor force participation. That’s not medical costs. Suddenly, $250 million doesn’t really sound like that much.”
A version of this article first appeared on WebMD.com.
from restaurants struggling to replace low-wage workers, to airlines scrambling to replace crew, to overwhelmed hospitals, experts are predicting.
“There’s a lot we need to do to understand what it takes to enable disabled people to participate more in the economy,” said Katie Bach, a senior fellow with Brookings Institution and the author of a study looking into long COVID’s impact on the labor market.
Data from June 2022 from the Centers for Disease Control and Prevention shows that, of the 40% of American adults who contracted COVID-19, nearly one in five still have long COVID symptoms. That works out to 1 in 13, or 7.5%, of the overall U.S. adult population.
Drawing from the CDC data, Ms. Bach estimates in her August 2022 report that as many as 4 million working-age Americans are too sick with long COVID to perform their jobs. That works out to as much as $230 billion in lost wages, or almost 1% of the U.S. GDP.
“This is a big deal,” she said. “We’re talking potentially hundreds of billions of dollars a year and that this is big enough to have a measurable impact on the labor market.”
Other sources have suggested lower figures, but the conclusions are the same: Long COVID is an urgent issue that will cost tens of billions of dollars a year in lost wages alone, Ms. Bach said. But it’s not just lost income for workers. There is a cost for businesses and the public.
Throughout the pandemic, COVID-19’s crippling force could be felt across multiple industries. While business has picked up again, staffing shortages remain a challenge. At some airports this summer, air passengers spent hours in security lines; were stranded for days as flights were canceled, rebooked, and canceled again on short notice; and waited weeks for lost luggage. Restaurants have had to cut back their hours. Those seeking medical care had longer than usual wait times in EDs and urgent care clinics. Some EDs temporarily closed.
These challenges have been attributed in part to the “great resignation” and in part because so many infected workers were out, especially during the Omicron waves. But increasingly, economists and health care professionals alike worry about long COVID’s impact on employers and the broader economy.
David Cutler, PhD, a professor of economics at Harvard University, Cambridge, Mass., believes the total economic loss could be as high as $3.7 trillion, when factoring in the lost quality of life, the cost in lost earnings, and the cost of higher spending on medical care. His estimate is more than a trillion dollars higher than a previous projection he and fellow economist Lawrence Summers, PhD, made in 2020. The reason? Long COVID.
“The higher estimate is largely a result of the greater prevalence of long COVID than we had guessed at the time,” Dr. Cutler wrote in a paper released in July.
“There are about 10 times the number of people with long COVID as have died of COVID. Because long COVID is so new, there is uncertainty about all of the numbers involved in the calculations. Still, the costs here are conservative, based on only cases to date.”
In Ms. Bach’s Brookings report, she projected that, if recovery from long COVID does not pick up and the population of Americans with long COVID were to grow by 10% a year, the annual cost of lost wages alone could reach half a trillion dollars in a decade.
Meanwhile, a working paper by the National Bureau of Economic Research found that workers who missed an entire week of work because of probable COVID-19 illnesses were roughly 7 percentage points less likely to be working a year later, compared with those who did not miss work for health reasons.
“It’s not just individuals with long COVID who are suffering from this. It impacts their families, their livelihoods, and the economy on a global scale. So, we have to raise awareness about those ripple effects,” said Linda Geng, MD, a clinical assistant professor of medicine with Stanford (Calif.) University’s Primary Care and Population Health.
“I think it’s hard for the public to grasp ... and understand the scale of this public health crisis.”
Debilitating fatigue
Long COVID is roughly defined; the CDC defines it as symptoms that linger 3 or more months after a patient first catches the virus.
The symptoms vary and include profound fatigue and brain issues.
“It’s a new degree of extreme and debilitating fatigue and exhaustion, to the point where you can’t do your daily tasks,” said Dr. Geng, who is also the codirector of Stanford’s Post-Acute COVID-19 Syndrome Clinic.
“People can be so debilitated, they can’t even do basic things, like the activities of daily living, let alone do their job, particularly if it’s physically or mentally demanding.”
Patients can also have postexertional malaise, where they feel especially bad and symptoms worsen when they exert themselves physically or mentally, Dr. Geng said. Compounding the issue for many long COVID patients is their trouble getting restful sleep. Those with brain fog have issues with memory, processing information, focused concentration, confusion, making mistakes, and multitasking. Pain is another debilitating symptom that can disrupt daily life and ability to work.
Even people with relatively mild infections can end up with long COVID, Dr. Geng said, noting that many of the patients at the Stanford clinic were never hospitalized with their initial infections. While existing research and Dr. Geng’s clinical experience show that long COVID can hit any age, she most commonly sees patients from ages 20 to their 60s, with an average age in the 40s – people in their prime working ages.
Jason Furman, PhD, a former White House economic adviser who is now a professor at Harvard University, noted in August that the labor force participation rate was far below what could be explained by standard demographic changes like an aging population, with the decline evident across all age groups. Dr. Furman does not speculate about why, but others have.
“We are pessimistic: Both the aging of the population and the impact of long COVID imply that the participation rate will be slow to return to its prepandemic level,” Anna Wong, Yelena Shulyatyeva, Andrew Husby, and Eliza Winger, economists with Bloomberg Economics, wrote in a research note.
Supportive policies
There is some evidence that vaccination reduces the risk of long COVID, but not completely, and it is too early to know if repeat infections increase long COVID risks. There is also no definitive data on how fast or how many people are recovering. Economists often assume that those with long COVID will recover at some point, Ms. Bach noted, but she is careful not to make assumptions.
“If people aren’t recovering, then this group keeps getting bigger,” she said. “We’re still adding, and if people aren’t coming out of that group, this becomes a bigger and bigger problem.”
For now, the number of new people being diagnosed with long COVID appears to have slowed, Ms. Bach said, but it remains to be seen whether the trend can be sustained.
“If people are impaired longer than we think and if the impairment turns out to be severe, then we can have a lot of people who need services like disability insurance,” Dr. Cutler said.
“That could put a really big strain on public sector programs and our ability to meet those needs.”
Policies that support the research and clinical work necessary to prevent and treat long COVID are essential, experts say.
“To me, that is the biggest economic imperative, to say nothing of human suffering,” said Ms. Bach.
Employers also have a role, and experts say there are a number of accommodations businesses should consider. What happens when an employee has long COVID? Can accommodations be made that allow them to continue working productively? If they spend a great deal of time commuting, can they work from home? What can employers do so that family members do not have to drop out of the workforce to take care of loved ones with long COVID?
Disability insurance
To be sure, there is one piece of the puzzle that does not quite fit, according to Dr. Cutler and Ms. Bach. There is no sign yet of a large increase in federal disability insurance applications, and no one quite knows why. Publicly available government data shows that online applications actually dipped by about 4% each year between 2019 and 2021. Applications in 2022 appear on track to remain slightly below prepandemic levels.
To qualify for Social Security Disability Insurance (SSDI), people need to have a disability that lasts at least a year.
“If you’re disabled with long COVID, who knows, right? You don’t know,” said Ms. Bach. “Two of the most dominant symptoms of long COVID are fatigue and brain fog. So, I’ve heard from people that the process of going through an SSDI application is really hard.”
Some long COVID patients told Ms. Bach they simply assumed they would not get SSDI and did not even bother applying. She stressed that working Americans with debilitating long COVID should be aware that their condition is protected by the Americans with Disabilities Act. But the challenge, based on guidance issued by the government, is that not all cases of long COVID qualify as a disability and that individual assessments are necessary.
While more long COVID data are needed, Ms. Bach believes there is enough information for decisionmakers to go after the issue more aggressively. She pointed to the $1.15 billion in funding that Congress earmarked for the National Institutes of Health over the course of 4 years in support of research into the long-term health effects of COVID-19.
“Now, $250 million a year sounds like a lot of money until you start talking about the cost of lost wages – just lost wages,” Ms. Bach said. “That’s not lost productivity. That’s not the cost of people whose family members are sick. Who have to reduce their own labor force participation. That’s not medical costs. Suddenly, $250 million doesn’t really sound like that much.”
A version of this article first appeared on WebMD.com.
from restaurants struggling to replace low-wage workers, to airlines scrambling to replace crew, to overwhelmed hospitals, experts are predicting.
“There’s a lot we need to do to understand what it takes to enable disabled people to participate more in the economy,” said Katie Bach, a senior fellow with Brookings Institution and the author of a study looking into long COVID’s impact on the labor market.
Data from June 2022 from the Centers for Disease Control and Prevention shows that, of the 40% of American adults who contracted COVID-19, nearly one in five still have long COVID symptoms. That works out to 1 in 13, or 7.5%, of the overall U.S. adult population.
Drawing from the CDC data, Ms. Bach estimates in her August 2022 report that as many as 4 million working-age Americans are too sick with long COVID to perform their jobs. That works out to as much as $230 billion in lost wages, or almost 1% of the U.S. GDP.
“This is a big deal,” she said. “We’re talking potentially hundreds of billions of dollars a year and that this is big enough to have a measurable impact on the labor market.”
Other sources have suggested lower figures, but the conclusions are the same: Long COVID is an urgent issue that will cost tens of billions of dollars a year in lost wages alone, Ms. Bach said. But it’s not just lost income for workers. There is a cost for businesses and the public.
Throughout the pandemic, COVID-19’s crippling force could be felt across multiple industries. While business has picked up again, staffing shortages remain a challenge. At some airports this summer, air passengers spent hours in security lines; were stranded for days as flights were canceled, rebooked, and canceled again on short notice; and waited weeks for lost luggage. Restaurants have had to cut back their hours. Those seeking medical care had longer than usual wait times in EDs and urgent care clinics. Some EDs temporarily closed.
These challenges have been attributed in part to the “great resignation” and in part because so many infected workers were out, especially during the Omicron waves. But increasingly, economists and health care professionals alike worry about long COVID’s impact on employers and the broader economy.
David Cutler, PhD, a professor of economics at Harvard University, Cambridge, Mass., believes the total economic loss could be as high as $3.7 trillion, when factoring in the lost quality of life, the cost in lost earnings, and the cost of higher spending on medical care. His estimate is more than a trillion dollars higher than a previous projection he and fellow economist Lawrence Summers, PhD, made in 2020. The reason? Long COVID.
“The higher estimate is largely a result of the greater prevalence of long COVID than we had guessed at the time,” Dr. Cutler wrote in a paper released in July.
“There are about 10 times the number of people with long COVID as have died of COVID. Because long COVID is so new, there is uncertainty about all of the numbers involved in the calculations. Still, the costs here are conservative, based on only cases to date.”
In Ms. Bach’s Brookings report, she projected that, if recovery from long COVID does not pick up and the population of Americans with long COVID were to grow by 10% a year, the annual cost of lost wages alone could reach half a trillion dollars in a decade.
Meanwhile, a working paper by the National Bureau of Economic Research found that workers who missed an entire week of work because of probable COVID-19 illnesses were roughly 7 percentage points less likely to be working a year later, compared with those who did not miss work for health reasons.
“It’s not just individuals with long COVID who are suffering from this. It impacts their families, their livelihoods, and the economy on a global scale. So, we have to raise awareness about those ripple effects,” said Linda Geng, MD, a clinical assistant professor of medicine with Stanford (Calif.) University’s Primary Care and Population Health.
“I think it’s hard for the public to grasp ... and understand the scale of this public health crisis.”
Debilitating fatigue
Long COVID is roughly defined; the CDC defines it as symptoms that linger 3 or more months after a patient first catches the virus.
The symptoms vary and include profound fatigue and brain issues.
“It’s a new degree of extreme and debilitating fatigue and exhaustion, to the point where you can’t do your daily tasks,” said Dr. Geng, who is also the codirector of Stanford’s Post-Acute COVID-19 Syndrome Clinic.
“People can be so debilitated, they can’t even do basic things, like the activities of daily living, let alone do their job, particularly if it’s physically or mentally demanding.”
Patients can also have postexertional malaise, where they feel especially bad and symptoms worsen when they exert themselves physically or mentally, Dr. Geng said. Compounding the issue for many long COVID patients is their trouble getting restful sleep. Those with brain fog have issues with memory, processing information, focused concentration, confusion, making mistakes, and multitasking. Pain is another debilitating symptom that can disrupt daily life and ability to work.
Even people with relatively mild infections can end up with long COVID, Dr. Geng said, noting that many of the patients at the Stanford clinic were never hospitalized with their initial infections. While existing research and Dr. Geng’s clinical experience show that long COVID can hit any age, she most commonly sees patients from ages 20 to their 60s, with an average age in the 40s – people in their prime working ages.
Jason Furman, PhD, a former White House economic adviser who is now a professor at Harvard University, noted in August that the labor force participation rate was far below what could be explained by standard demographic changes like an aging population, with the decline evident across all age groups. Dr. Furman does not speculate about why, but others have.
“We are pessimistic: Both the aging of the population and the impact of long COVID imply that the participation rate will be slow to return to its prepandemic level,” Anna Wong, Yelena Shulyatyeva, Andrew Husby, and Eliza Winger, economists with Bloomberg Economics, wrote in a research note.
Supportive policies
There is some evidence that vaccination reduces the risk of long COVID, but not completely, and it is too early to know if repeat infections increase long COVID risks. There is also no definitive data on how fast or how many people are recovering. Economists often assume that those with long COVID will recover at some point, Ms. Bach noted, but she is careful not to make assumptions.
“If people aren’t recovering, then this group keeps getting bigger,” she said. “We’re still adding, and if people aren’t coming out of that group, this becomes a bigger and bigger problem.”
For now, the number of new people being diagnosed with long COVID appears to have slowed, Ms. Bach said, but it remains to be seen whether the trend can be sustained.
“If people are impaired longer than we think and if the impairment turns out to be severe, then we can have a lot of people who need services like disability insurance,” Dr. Cutler said.
“That could put a really big strain on public sector programs and our ability to meet those needs.”
Policies that support the research and clinical work necessary to prevent and treat long COVID are essential, experts say.
“To me, that is the biggest economic imperative, to say nothing of human suffering,” said Ms. Bach.
Employers also have a role, and experts say there are a number of accommodations businesses should consider. What happens when an employee has long COVID? Can accommodations be made that allow them to continue working productively? If they spend a great deal of time commuting, can they work from home? What can employers do so that family members do not have to drop out of the workforce to take care of loved ones with long COVID?
Disability insurance
To be sure, there is one piece of the puzzle that does not quite fit, according to Dr. Cutler and Ms. Bach. There is no sign yet of a large increase in federal disability insurance applications, and no one quite knows why. Publicly available government data shows that online applications actually dipped by about 4% each year between 2019 and 2021. Applications in 2022 appear on track to remain slightly below prepandemic levels.
To qualify for Social Security Disability Insurance (SSDI), people need to have a disability that lasts at least a year.
“If you’re disabled with long COVID, who knows, right? You don’t know,” said Ms. Bach. “Two of the most dominant symptoms of long COVID are fatigue and brain fog. So, I’ve heard from people that the process of going through an SSDI application is really hard.”
Some long COVID patients told Ms. Bach they simply assumed they would not get SSDI and did not even bother applying. She stressed that working Americans with debilitating long COVID should be aware that their condition is protected by the Americans with Disabilities Act. But the challenge, based on guidance issued by the government, is that not all cases of long COVID qualify as a disability and that individual assessments are necessary.
While more long COVID data are needed, Ms. Bach believes there is enough information for decisionmakers to go after the issue more aggressively. She pointed to the $1.15 billion in funding that Congress earmarked for the National Institutes of Health over the course of 4 years in support of research into the long-term health effects of COVID-19.
“Now, $250 million a year sounds like a lot of money until you start talking about the cost of lost wages – just lost wages,” Ms. Bach said. “That’s not lost productivity. That’s not the cost of people whose family members are sick. Who have to reduce their own labor force participation. That’s not medical costs. Suddenly, $250 million doesn’t really sound like that much.”
A version of this article first appeared on WebMD.com.
Meet the JCOM Author with Dr. Barkoudah: Improving Inpatient COVID-19 Vaccination Rates
Children and COVID: September slowdown continues
New COVID-19 cases and hospital admissions in children continue to decline, while the slow pace of vaccinations has not deterred manufacturers from seeking new emergency authorizations.
Since reaching a post-Omicron peak of 112,000 in late May, the number of weekly cases has fluctuated, with no stretch of increases or decreases lasting more than 4 weeks or the weekly count rising above 97,000 or falling lower than the current 55,000, according to state-level data collected by the American Academy of Pediatrics and the Children’s Hospital Association.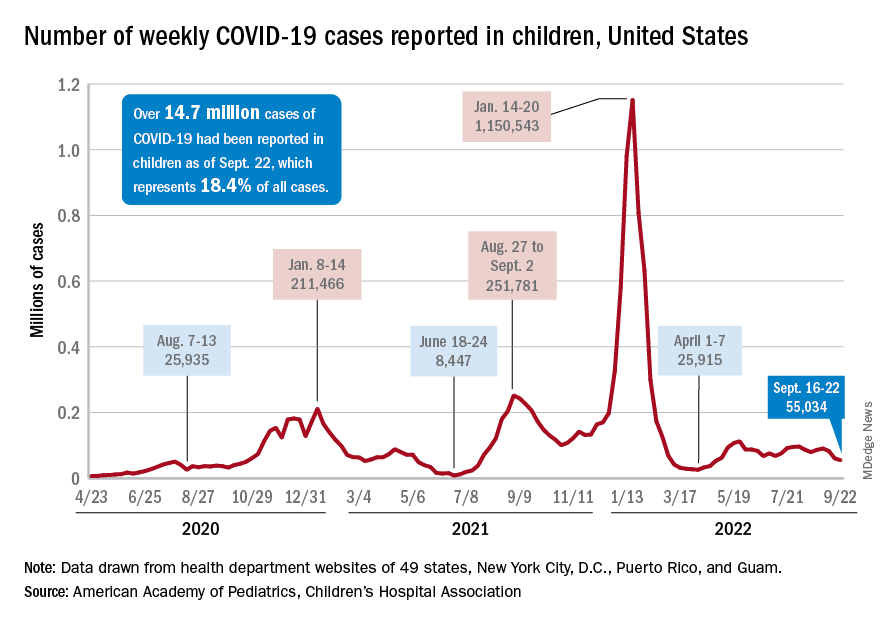
New admissions with confirmed COVID for children aged 0-17 years, which did not follow that pattern and instead continued to rise through the spring and early summer, have been largely decreasing in recent weeks and had fallen to 0.27 per 100,000 population as of Sept. 21 after peaking at 0.46 per 100,000 in late July, the Centers for Disease Control and Prevention reported. A similar decline has been seen for emergency department visits since late August.
The biggest vaccination news of the week came from Moderna and Pfizer and BioNTech, which are each seeking emergency authorization from the Food and Drug Administration for bivalent vaccine boosters that target both the original COVID strain and the BA.4 and BA.5 strains of Omicron.
“Pfizer’s booster would be for children 5 to 11 who have completed a primary vaccination series [and] Moderna’s updated boosters would be for children ages 6 to 17 who have completed a primary vaccination series,” WebMD said.
Although almost 61% of children aged 12-17 years are already fully vaccinated, that is not the case among those aged 5-11, of whom only 31.4% have completed the initial vaccine regimen. Since becoming eligible in June, just 1.9% of children under 5 years of age have been fully vaccinated and 6.3% have received at least one dose, the CDC said on its COVID Data Tracker. The latest data put the already boosted child populations at 28.8% for 12- to 17-year-olds and 14.8% in those aged 5-11.
About 51,000 children under age 5 years received their initial COVID vaccination during the week of Sept. 15-21, and the trend for that measure is one of gradual decline since July. Among the older children that same week, there were 28,000 initial vaccinations in the 5- to 11-year-olds and 18,000 for those aged 12-17, and activity in both age groups has largely stagnated since the spring, according to a separate AAP report based on CDC data.
New COVID-19 cases and hospital admissions in children continue to decline, while the slow pace of vaccinations has not deterred manufacturers from seeking new emergency authorizations.
Since reaching a post-Omicron peak of 112,000 in late May, the number of weekly cases has fluctuated, with no stretch of increases or decreases lasting more than 4 weeks or the weekly count rising above 97,000 or falling lower than the current 55,000, according to state-level data collected by the American Academy of Pediatrics and the Children’s Hospital Association.
New admissions with confirmed COVID for children aged 0-17 years, which did not follow that pattern and instead continued to rise through the spring and early summer, have been largely decreasing in recent weeks and had fallen to 0.27 per 100,000 population as of Sept. 21 after peaking at 0.46 per 100,000 in late July, the Centers for Disease Control and Prevention reported. A similar decline has been seen for emergency department visits since late August.
The biggest vaccination news of the week came from Moderna and Pfizer and BioNTech, which are each seeking emergency authorization from the Food and Drug Administration for bivalent vaccine boosters that target both the original COVID strain and the BA.4 and BA.5 strains of Omicron.
“Pfizer’s booster would be for children 5 to 11 who have completed a primary vaccination series [and] Moderna’s updated boosters would be for children ages 6 to 17 who have completed a primary vaccination series,” WebMD said.
Although almost 61% of children aged 12-17 years are already fully vaccinated, that is not the case among those aged 5-11, of whom only 31.4% have completed the initial vaccine regimen. Since becoming eligible in June, just 1.9% of children under 5 years of age have been fully vaccinated and 6.3% have received at least one dose, the CDC said on its COVID Data Tracker. The latest data put the already boosted child populations at 28.8% for 12- to 17-year-olds and 14.8% in those aged 5-11.
About 51,000 children under age 5 years received their initial COVID vaccination during the week of Sept. 15-21, and the trend for that measure is one of gradual decline since July. Among the older children that same week, there were 28,000 initial vaccinations in the 5- to 11-year-olds and 18,000 for those aged 12-17, and activity in both age groups has largely stagnated since the spring, according to a separate AAP report based on CDC data.
New COVID-19 cases and hospital admissions in children continue to decline, while the slow pace of vaccinations has not deterred manufacturers from seeking new emergency authorizations.
Since reaching a post-Omicron peak of 112,000 in late May, the number of weekly cases has fluctuated, with no stretch of increases or decreases lasting more than 4 weeks or the weekly count rising above 97,000 or falling lower than the current 55,000, according to state-level data collected by the American Academy of Pediatrics and the Children’s Hospital Association.
New admissions with confirmed COVID for children aged 0-17 years, which did not follow that pattern and instead continued to rise through the spring and early summer, have been largely decreasing in recent weeks and had fallen to 0.27 per 100,000 population as of Sept. 21 after peaking at 0.46 per 100,000 in late July, the Centers for Disease Control and Prevention reported. A similar decline has been seen for emergency department visits since late August.
The biggest vaccination news of the week came from Moderna and Pfizer and BioNTech, which are each seeking emergency authorization from the Food and Drug Administration for bivalent vaccine boosters that target both the original COVID strain and the BA.4 and BA.5 strains of Omicron.
“Pfizer’s booster would be for children 5 to 11 who have completed a primary vaccination series [and] Moderna’s updated boosters would be for children ages 6 to 17 who have completed a primary vaccination series,” WebMD said.
Although almost 61% of children aged 12-17 years are already fully vaccinated, that is not the case among those aged 5-11, of whom only 31.4% have completed the initial vaccine regimen. Since becoming eligible in June, just 1.9% of children under 5 years of age have been fully vaccinated and 6.3% have received at least one dose, the CDC said on its COVID Data Tracker. The latest data put the already boosted child populations at 28.8% for 12- to 17-year-olds and 14.8% in those aged 5-11.
About 51,000 children under age 5 years received their initial COVID vaccination during the week of Sept. 15-21, and the trend for that measure is one of gradual decline since July. Among the older children that same week, there were 28,000 initial vaccinations in the 5- to 11-year-olds and 18,000 for those aged 12-17, and activity in both age groups has largely stagnated since the spring, according to a separate AAP report based on CDC data.
Meet the JCOM Author with Dr. Barkoudah: Diabetes Population Health Innovations


