User login
Under 2% of eligible have gotten newest COVID booster shot
The newest booster became available to the public around Labor Day weekend, and about 4.4 million people have gotten it as of Sept. 21, according to Centers for Disease Control and Prevention data. That figure represents about 1.5% of the people eligible to receive the booster, NBC News reported.
The White House has said the total is probably closer to 5 million people. The CDC totals don’t yet include Texas and Idaho, which use an aggregate vaccination record reporting method for the Pfizer vaccine.
Scott Roberts, MD, a Yale Medicine infectious disease specialist in New Haven, Conn., told NBC News the low numbers are “demoralizing.”
“I would expect a much higher proportion of Americans to have gotten the booster by this point,” he said. “The fact that this booster came out days before Biden said the pandemic is over is a huge mixed message. Now it’s going to be that much harder to convince those at risk who are on the fence to get a booster.”
White House COVID-19 coordinator Ashish Jha, MD, says he thinks demand will pick up in the coming weeks.
“We’ve been thinking and talking about this as an annual vaccine like the flu vaccine. Flu vaccine season picks up in late September and early October. We’re just getting our education campaign going. So we expect to see, despite the fact that this was a strong start, we actually expect this to ramp up stronger,” Dr. Jha said.
The new booster is the third one authorized by the federal government and was redesigned to protect against the currently circulating subvariants BA.4 and BA.5 of the Omicron strain. People who have received a primary vaccine series or a booster at least 2 months before can receive it.
The new Pfizer booster is available for people 12 and up and the Moderna version for people 18 and up. The vaccines can be mixed and matched.
A version of this article first appeared on WebMD.com.
The newest booster became available to the public around Labor Day weekend, and about 4.4 million people have gotten it as of Sept. 21, according to Centers for Disease Control and Prevention data. That figure represents about 1.5% of the people eligible to receive the booster, NBC News reported.
The White House has said the total is probably closer to 5 million people. The CDC totals don’t yet include Texas and Idaho, which use an aggregate vaccination record reporting method for the Pfizer vaccine.
Scott Roberts, MD, a Yale Medicine infectious disease specialist in New Haven, Conn., told NBC News the low numbers are “demoralizing.”
“I would expect a much higher proportion of Americans to have gotten the booster by this point,” he said. “The fact that this booster came out days before Biden said the pandemic is over is a huge mixed message. Now it’s going to be that much harder to convince those at risk who are on the fence to get a booster.”
White House COVID-19 coordinator Ashish Jha, MD, says he thinks demand will pick up in the coming weeks.
“We’ve been thinking and talking about this as an annual vaccine like the flu vaccine. Flu vaccine season picks up in late September and early October. We’re just getting our education campaign going. So we expect to see, despite the fact that this was a strong start, we actually expect this to ramp up stronger,” Dr. Jha said.
The new booster is the third one authorized by the federal government and was redesigned to protect against the currently circulating subvariants BA.4 and BA.5 of the Omicron strain. People who have received a primary vaccine series or a booster at least 2 months before can receive it.
The new Pfizer booster is available for people 12 and up and the Moderna version for people 18 and up. The vaccines can be mixed and matched.
A version of this article first appeared on WebMD.com.
The newest booster became available to the public around Labor Day weekend, and about 4.4 million people have gotten it as of Sept. 21, according to Centers for Disease Control and Prevention data. That figure represents about 1.5% of the people eligible to receive the booster, NBC News reported.
The White House has said the total is probably closer to 5 million people. The CDC totals don’t yet include Texas and Idaho, which use an aggregate vaccination record reporting method for the Pfizer vaccine.
Scott Roberts, MD, a Yale Medicine infectious disease specialist in New Haven, Conn., told NBC News the low numbers are “demoralizing.”
“I would expect a much higher proportion of Americans to have gotten the booster by this point,” he said. “The fact that this booster came out days before Biden said the pandemic is over is a huge mixed message. Now it’s going to be that much harder to convince those at risk who are on the fence to get a booster.”
White House COVID-19 coordinator Ashish Jha, MD, says he thinks demand will pick up in the coming weeks.
“We’ve been thinking and talking about this as an annual vaccine like the flu vaccine. Flu vaccine season picks up in late September and early October. We’re just getting our education campaign going. So we expect to see, despite the fact that this was a strong start, we actually expect this to ramp up stronger,” Dr. Jha said.
The new booster is the third one authorized by the federal government and was redesigned to protect against the currently circulating subvariants BA.4 and BA.5 of the Omicron strain. People who have received a primary vaccine series or a booster at least 2 months before can receive it.
The new Pfizer booster is available for people 12 and up and the Moderna version for people 18 and up. The vaccines can be mixed and matched.
A version of this article first appeared on WebMD.com.
Limiting antibiotic overprescription in pandemics: New guidelines
A statement by the Society for Healthcare Epidemiology of America, published online in Infection Control & Hospital Epidemiology, offers health care providers guidelines on how to prevent inappropriate antibiotic use in future pandemics and to avoid some of the negative scenarios that have been seen with COVID-19.
According to the U.S. Centers of Disease Control and Prevention,
The culprit might be the widespread antibiotic overprescription during the current pandemic. A 2022 meta-analysis revealed that in high-income countries, 58% of patients with COVID-19 were given antibiotics, whereas in lower- and middle-income countries, 89% of patients were put on such drugs. Some hospitals in Europe and the United States reported similarly elevated numbers, sometimes approaching 100%.
“We’ve lost control,” Natasha Pettit, PharmD, pharmacy director at University of Chicago Medicine, told this news organization. Dr. Pettit was not involved in the SHEA study. “Even if CDC didn’t come out with that data, I can tell you right now more of my time is spent trying to figure out how to manage these multi-drug–resistant infections, and we are running out of options for these patients,”
“Dealing with uncertainty, exhaustion, [and] critical illness in often young, otherwise healthy patients meant doctors wanted to do something for their patients,” said Tamar Barlam, MD, an infectious diseases expert at the Boston Medical Center who led the development of the SHEA white paper, in an interview.
That something often was a prescription for antibiotics, even without a clear indication that they were actually needed. A British study revealed that in times of pandemic uncertainty, clinicians often reached for antibiotics “just in case” and referred to conservative prescribing as “bravery.”
Studies have shown, however, that bacterial co-infections in COVID-19 are rare. A 2020 meta-analysis of 24 studies concluded that only 3.5% of patients had a bacterial co-infection on presentation, and 14.3% had a secondary infection. Similar patterns had previously been observed in other viral outbreaks. Research on MERS-CoV, for example, documented only 1% of patients with a bacterial co-infection on admission. During the 2009 H1N1 influenza pandemic, that number was 12% of non–ICU hospitalized patients.
Yet, according to Dr. Pettit, even when such data became available, it didn’t necessarily change prescribing patterns. “Information was coming at us so quickly, I think the providers didn’t have a moment to see the data, to understand what it meant for their prescribing. Having external guidance earlier on would have been hugely helpful,” she told this news organization.
That’s where the newly published SHEA statement comes in: It outlines recommendations on when to prescribe antibiotics during a respiratory viral pandemic, what tests to order, and when to de-escalate or discontinue the treatment. These recommendations include, for instance, advice to not trust inflammatory markers as reliable indicators of bacterial or fungal infection and to not use procalcitonin routinely to aid in the decision to initiate antibiotics.
According to Dr. Barlam, one of the crucial lessons here is that if clinicians see patients with symptoms that are consistent with the current pandemic, they should trust their own impressions and avoid reaching for antimicrobials “just in case.”
Another important lesson is that antibiotic stewardship programs have a huge role to play during pandemics. They should not only monitor prescribing but also compile new information on bacterial co-infections as it gets released and make sure it reaches the clinicians in a clear form.
Evidence suggests that such programs and guidelines do work to limit unnecessary antibiotic use. In one medical center in Chicago, for example, before recommendations on when to initiate and discontinue antimicrobials were released, over 74% of COVID-19 patients received antibiotics. After guidelines were put in place, the use of such drugs fell to 42%.
Dr. Pettit believes, however, that it’s important not to leave each medical center to its own devices. “Hindsight is always twenty-twenty,” she said, “but I think it would be great that, if we start hearing about a pathogen that might lead to another pandemic, we should have a mechanism in place to call together an expert body to get guidance for how antimicrobial stewardship programs should get involved.”
One of the authors of the SHEA statement, Susan Seo, reports an investigator-initiated Merck grant on cost-effectiveness of letermovir in hematopoietic stem cell transplant patients. Another author, Graeme Forrest, reports a clinical study grant from Regeneron for inpatient monoclonals against SARS-CoV-2. All other authors report no conflicts of interest. The study was independently supported.
A version of this article first appeared on Medscape.com.
A statement by the Society for Healthcare Epidemiology of America, published online in Infection Control & Hospital Epidemiology, offers health care providers guidelines on how to prevent inappropriate antibiotic use in future pandemics and to avoid some of the negative scenarios that have been seen with COVID-19.
According to the U.S. Centers of Disease Control and Prevention,
The culprit might be the widespread antibiotic overprescription during the current pandemic. A 2022 meta-analysis revealed that in high-income countries, 58% of patients with COVID-19 were given antibiotics, whereas in lower- and middle-income countries, 89% of patients were put on such drugs. Some hospitals in Europe and the United States reported similarly elevated numbers, sometimes approaching 100%.
“We’ve lost control,” Natasha Pettit, PharmD, pharmacy director at University of Chicago Medicine, told this news organization. Dr. Pettit was not involved in the SHEA study. “Even if CDC didn’t come out with that data, I can tell you right now more of my time is spent trying to figure out how to manage these multi-drug–resistant infections, and we are running out of options for these patients,”
“Dealing with uncertainty, exhaustion, [and] critical illness in often young, otherwise healthy patients meant doctors wanted to do something for their patients,” said Tamar Barlam, MD, an infectious diseases expert at the Boston Medical Center who led the development of the SHEA white paper, in an interview.
That something often was a prescription for antibiotics, even without a clear indication that they were actually needed. A British study revealed that in times of pandemic uncertainty, clinicians often reached for antibiotics “just in case” and referred to conservative prescribing as “bravery.”
Studies have shown, however, that bacterial co-infections in COVID-19 are rare. A 2020 meta-analysis of 24 studies concluded that only 3.5% of patients had a bacterial co-infection on presentation, and 14.3% had a secondary infection. Similar patterns had previously been observed in other viral outbreaks. Research on MERS-CoV, for example, documented only 1% of patients with a bacterial co-infection on admission. During the 2009 H1N1 influenza pandemic, that number was 12% of non–ICU hospitalized patients.
Yet, according to Dr. Pettit, even when such data became available, it didn’t necessarily change prescribing patterns. “Information was coming at us so quickly, I think the providers didn’t have a moment to see the data, to understand what it meant for their prescribing. Having external guidance earlier on would have been hugely helpful,” she told this news organization.
That’s where the newly published SHEA statement comes in: It outlines recommendations on when to prescribe antibiotics during a respiratory viral pandemic, what tests to order, and when to de-escalate or discontinue the treatment. These recommendations include, for instance, advice to not trust inflammatory markers as reliable indicators of bacterial or fungal infection and to not use procalcitonin routinely to aid in the decision to initiate antibiotics.
According to Dr. Barlam, one of the crucial lessons here is that if clinicians see patients with symptoms that are consistent with the current pandemic, they should trust their own impressions and avoid reaching for antimicrobials “just in case.”
Another important lesson is that antibiotic stewardship programs have a huge role to play during pandemics. They should not only monitor prescribing but also compile new information on bacterial co-infections as it gets released and make sure it reaches the clinicians in a clear form.
Evidence suggests that such programs and guidelines do work to limit unnecessary antibiotic use. In one medical center in Chicago, for example, before recommendations on when to initiate and discontinue antimicrobials were released, over 74% of COVID-19 patients received antibiotics. After guidelines were put in place, the use of such drugs fell to 42%.
Dr. Pettit believes, however, that it’s important not to leave each medical center to its own devices. “Hindsight is always twenty-twenty,” she said, “but I think it would be great that, if we start hearing about a pathogen that might lead to another pandemic, we should have a mechanism in place to call together an expert body to get guidance for how antimicrobial stewardship programs should get involved.”
One of the authors of the SHEA statement, Susan Seo, reports an investigator-initiated Merck grant on cost-effectiveness of letermovir in hematopoietic stem cell transplant patients. Another author, Graeme Forrest, reports a clinical study grant from Regeneron for inpatient monoclonals against SARS-CoV-2. All other authors report no conflicts of interest. The study was independently supported.
A version of this article first appeared on Medscape.com.
A statement by the Society for Healthcare Epidemiology of America, published online in Infection Control & Hospital Epidemiology, offers health care providers guidelines on how to prevent inappropriate antibiotic use in future pandemics and to avoid some of the negative scenarios that have been seen with COVID-19.
According to the U.S. Centers of Disease Control and Prevention,
The culprit might be the widespread antibiotic overprescription during the current pandemic. A 2022 meta-analysis revealed that in high-income countries, 58% of patients with COVID-19 were given antibiotics, whereas in lower- and middle-income countries, 89% of patients were put on such drugs. Some hospitals in Europe and the United States reported similarly elevated numbers, sometimes approaching 100%.
“We’ve lost control,” Natasha Pettit, PharmD, pharmacy director at University of Chicago Medicine, told this news organization. Dr. Pettit was not involved in the SHEA study. “Even if CDC didn’t come out with that data, I can tell you right now more of my time is spent trying to figure out how to manage these multi-drug–resistant infections, and we are running out of options for these patients,”
“Dealing with uncertainty, exhaustion, [and] critical illness in often young, otherwise healthy patients meant doctors wanted to do something for their patients,” said Tamar Barlam, MD, an infectious diseases expert at the Boston Medical Center who led the development of the SHEA white paper, in an interview.
That something often was a prescription for antibiotics, even without a clear indication that they were actually needed. A British study revealed that in times of pandemic uncertainty, clinicians often reached for antibiotics “just in case” and referred to conservative prescribing as “bravery.”
Studies have shown, however, that bacterial co-infections in COVID-19 are rare. A 2020 meta-analysis of 24 studies concluded that only 3.5% of patients had a bacterial co-infection on presentation, and 14.3% had a secondary infection. Similar patterns had previously been observed in other viral outbreaks. Research on MERS-CoV, for example, documented only 1% of patients with a bacterial co-infection on admission. During the 2009 H1N1 influenza pandemic, that number was 12% of non–ICU hospitalized patients.
Yet, according to Dr. Pettit, even when such data became available, it didn’t necessarily change prescribing patterns. “Information was coming at us so quickly, I think the providers didn’t have a moment to see the data, to understand what it meant for their prescribing. Having external guidance earlier on would have been hugely helpful,” she told this news organization.
That’s where the newly published SHEA statement comes in: It outlines recommendations on when to prescribe antibiotics during a respiratory viral pandemic, what tests to order, and when to de-escalate or discontinue the treatment. These recommendations include, for instance, advice to not trust inflammatory markers as reliable indicators of bacterial or fungal infection and to not use procalcitonin routinely to aid in the decision to initiate antibiotics.
According to Dr. Barlam, one of the crucial lessons here is that if clinicians see patients with symptoms that are consistent with the current pandemic, they should trust their own impressions and avoid reaching for antimicrobials “just in case.”
Another important lesson is that antibiotic stewardship programs have a huge role to play during pandemics. They should not only monitor prescribing but also compile new information on bacterial co-infections as it gets released and make sure it reaches the clinicians in a clear form.
Evidence suggests that such programs and guidelines do work to limit unnecessary antibiotic use. In one medical center in Chicago, for example, before recommendations on when to initiate and discontinue antimicrobials were released, over 74% of COVID-19 patients received antibiotics. After guidelines were put in place, the use of such drugs fell to 42%.
Dr. Pettit believes, however, that it’s important not to leave each medical center to its own devices. “Hindsight is always twenty-twenty,” she said, “but I think it would be great that, if we start hearing about a pathogen that might lead to another pandemic, we should have a mechanism in place to call together an expert body to get guidance for how antimicrobial stewardship programs should get involved.”
One of the authors of the SHEA statement, Susan Seo, reports an investigator-initiated Merck grant on cost-effectiveness of letermovir in hematopoietic stem cell transplant patients. Another author, Graeme Forrest, reports a clinical study grant from Regeneron for inpatient monoclonals against SARS-CoV-2. All other authors report no conflicts of interest. The study was independently supported.
A version of this article first appeared on Medscape.com.
FROM INFECTION CONTROL & HOSPITAL EPIDEMIOLOGY
COVID vaccination does not appear to worsen symptoms of Parkinson’s disease
Nonmotor symptoms seemed to improve after SARS-CoV-2 vaccination, although the investigators could not verify a causal relationship.
Vaccination programs should continue for patients with Parkinson’s disease, they said, reporting their clinical results at the International Congress of Parkinson’s Disease and Movement Disorders.
The International Parkinson and Movement Disorder Society has recommended vaccining patients with Parkinson’s disease. “All approved mRNA-based and viral vector vaccines are not expected to interact with Parkinson’s disease, but patients [still] report concern with regard to the benefits, risks, and safeness in Parkinson’s disease,” Mayela Rodríguez-Violante, MD, MSc, and colleagues wrote in an abstract of their findings.
Social isolation may be contributing to these beliefs and concerns, though this is inconclusive.
Investigators from Mexico City conducted a retrospective study of patients with Parkinson’s disease to see how COVID-19 vaccination affected motor and nonmotor symptoms. They enlisted 60 patients (66.7% were male; aged 65.7 ± 11.35 years) who received either a vector-viral vaccine (Vaxzevria Coronavirus) or an mRNA vaccine (BNT162b2).
A Wilcoxon signed-rank test assessed scale differences before and after vaccination, measuring motor involvement (Unified Parkinson’s Disease Rating Scale), nonmotor involvement (Non-Motor Rating Scale [NMSS]), cognitive impairment (Montreal Cognitive Assessment), and quality of life (8-item Parkinson’s Disease Questionnaire index).
Investigators found no significant difference between scales, although they did notice a marked improvement in non-motor symptoms.
“The main takeaway is that vaccination against COVID-19 does not appear to worsen motor or nonmotor symptoms in persons with Parkinson’s disease. The benefits outweigh the risks,” said Dr. Rodríguez-Violante, the study’s lead author and a movement disorder specialist at the National Institute of Neurology and Neurosurgery, Mexico City.
Next steps are to increase the sample size to see if it’s possible to have a similar number in terms of type of vaccine, said Dr. Rodríguez-Violante. “Also, the data presented refers to primary series doses so booster effects will also be studied.”
Few studies have looked at vaccines and their possible effects on this patient population. However, a 2021 study of 181 patients with Parkinson’s disease reported that 2 (1.1%) had adverse effects after receiving the BNT162b2 mRNA vaccine. One of the patients, a 61-year-old woman with a decade-long history of Parkinson’s disease, developed severe, continuous, generalized dyskinesia 6 hours after a first dose of vaccine. The second patient was 79 years old and had Parkinson’s disease for 5 years. She developed fever, confusion, delusions, and continuous severe dyskinesia for 3 days following her vaccination.
“This highlights that there is a variability in the response triggered by the vaccine that might likely depend on individual immunological profiles … clinicians should be aware of this possibility and monitor their patients after they receive their vaccination,” Roberto Erro, MD, PhD and colleagues wrote in the Movement Disorders journal.
Nonmotor symptoms seemed to improve after SARS-CoV-2 vaccination, although the investigators could not verify a causal relationship.
Vaccination programs should continue for patients with Parkinson’s disease, they said, reporting their clinical results at the International Congress of Parkinson’s Disease and Movement Disorders.
The International Parkinson and Movement Disorder Society has recommended vaccining patients with Parkinson’s disease. “All approved mRNA-based and viral vector vaccines are not expected to interact with Parkinson’s disease, but patients [still] report concern with regard to the benefits, risks, and safeness in Parkinson’s disease,” Mayela Rodríguez-Violante, MD, MSc, and colleagues wrote in an abstract of their findings.
Social isolation may be contributing to these beliefs and concerns, though this is inconclusive.
Investigators from Mexico City conducted a retrospective study of patients with Parkinson’s disease to see how COVID-19 vaccination affected motor and nonmotor symptoms. They enlisted 60 patients (66.7% were male; aged 65.7 ± 11.35 years) who received either a vector-viral vaccine (Vaxzevria Coronavirus) or an mRNA vaccine (BNT162b2).
A Wilcoxon signed-rank test assessed scale differences before and after vaccination, measuring motor involvement (Unified Parkinson’s Disease Rating Scale), nonmotor involvement (Non-Motor Rating Scale [NMSS]), cognitive impairment (Montreal Cognitive Assessment), and quality of life (8-item Parkinson’s Disease Questionnaire index).
Investigators found no significant difference between scales, although they did notice a marked improvement in non-motor symptoms.
“The main takeaway is that vaccination against COVID-19 does not appear to worsen motor or nonmotor symptoms in persons with Parkinson’s disease. The benefits outweigh the risks,” said Dr. Rodríguez-Violante, the study’s lead author and a movement disorder specialist at the National Institute of Neurology and Neurosurgery, Mexico City.
Next steps are to increase the sample size to see if it’s possible to have a similar number in terms of type of vaccine, said Dr. Rodríguez-Violante. “Also, the data presented refers to primary series doses so booster effects will also be studied.”
Few studies have looked at vaccines and their possible effects on this patient population. However, a 2021 study of 181 patients with Parkinson’s disease reported that 2 (1.1%) had adverse effects after receiving the BNT162b2 mRNA vaccine. One of the patients, a 61-year-old woman with a decade-long history of Parkinson’s disease, developed severe, continuous, generalized dyskinesia 6 hours after a first dose of vaccine. The second patient was 79 years old and had Parkinson’s disease for 5 years. She developed fever, confusion, delusions, and continuous severe dyskinesia for 3 days following her vaccination.
“This highlights that there is a variability in the response triggered by the vaccine that might likely depend on individual immunological profiles … clinicians should be aware of this possibility and monitor their patients after they receive their vaccination,” Roberto Erro, MD, PhD and colleagues wrote in the Movement Disorders journal.
Nonmotor symptoms seemed to improve after SARS-CoV-2 vaccination, although the investigators could not verify a causal relationship.
Vaccination programs should continue for patients with Parkinson’s disease, they said, reporting their clinical results at the International Congress of Parkinson’s Disease and Movement Disorders.
The International Parkinson and Movement Disorder Society has recommended vaccining patients with Parkinson’s disease. “All approved mRNA-based and viral vector vaccines are not expected to interact with Parkinson’s disease, but patients [still] report concern with regard to the benefits, risks, and safeness in Parkinson’s disease,” Mayela Rodríguez-Violante, MD, MSc, and colleagues wrote in an abstract of their findings.
Social isolation may be contributing to these beliefs and concerns, though this is inconclusive.
Investigators from Mexico City conducted a retrospective study of patients with Parkinson’s disease to see how COVID-19 vaccination affected motor and nonmotor symptoms. They enlisted 60 patients (66.7% were male; aged 65.7 ± 11.35 years) who received either a vector-viral vaccine (Vaxzevria Coronavirus) or an mRNA vaccine (BNT162b2).
A Wilcoxon signed-rank test assessed scale differences before and after vaccination, measuring motor involvement (Unified Parkinson’s Disease Rating Scale), nonmotor involvement (Non-Motor Rating Scale [NMSS]), cognitive impairment (Montreal Cognitive Assessment), and quality of life (8-item Parkinson’s Disease Questionnaire index).
Investigators found no significant difference between scales, although they did notice a marked improvement in non-motor symptoms.
“The main takeaway is that vaccination against COVID-19 does not appear to worsen motor or nonmotor symptoms in persons with Parkinson’s disease. The benefits outweigh the risks,” said Dr. Rodríguez-Violante, the study’s lead author and a movement disorder specialist at the National Institute of Neurology and Neurosurgery, Mexico City.
Next steps are to increase the sample size to see if it’s possible to have a similar number in terms of type of vaccine, said Dr. Rodríguez-Violante. “Also, the data presented refers to primary series doses so booster effects will also be studied.”
Few studies have looked at vaccines and their possible effects on this patient population. However, a 2021 study of 181 patients with Parkinson’s disease reported that 2 (1.1%) had adverse effects after receiving the BNT162b2 mRNA vaccine. One of the patients, a 61-year-old woman with a decade-long history of Parkinson’s disease, developed severe, continuous, generalized dyskinesia 6 hours after a first dose of vaccine. The second patient was 79 years old and had Parkinson’s disease for 5 years. She developed fever, confusion, delusions, and continuous severe dyskinesia for 3 days following her vaccination.
“This highlights that there is a variability in the response triggered by the vaccine that might likely depend on individual immunological profiles … clinicians should be aware of this possibility and monitor their patients after they receive their vaccination,” Roberto Erro, MD, PhD and colleagues wrote in the Movement Disorders journal.
FROM MDS 2022
Children and COVID: Weekly cases drop to lowest level since April
A hefty decline in new COVID-19 cases among children resulted in the lowest weekly total since late April, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
, making for 2 consecutive weeks of declines after almost 91,000 cases were recorded for the week ending Sept. 1, the AAP and CHA said in their latest COVID report of state-level data.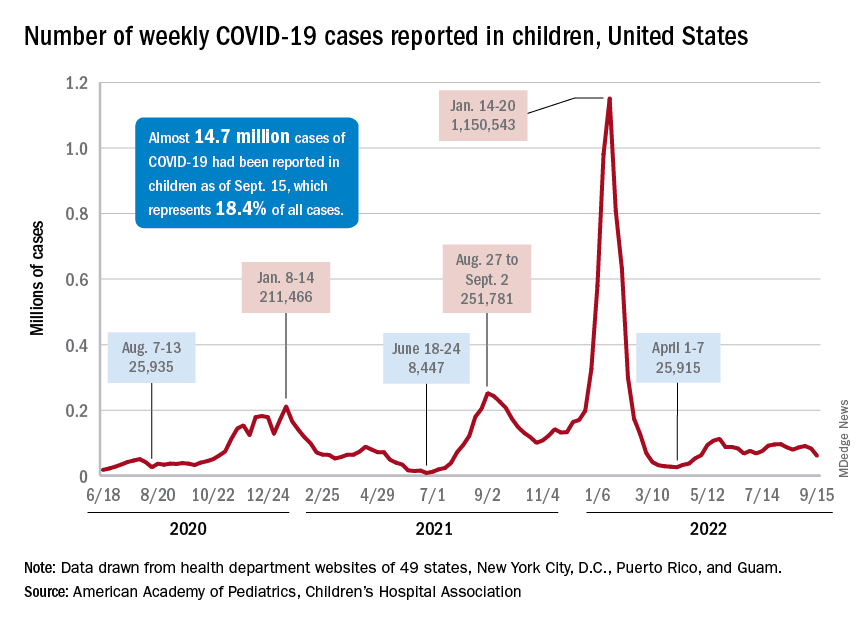
The last time the weekly count was under 60,000 came during the week of April 22-28, when 53,000 were reported by state and territorial health departments in the midst of a 7-week stretch of rising cases. Since that streak ended in mid-May, however, “reported weekly cases have plateaued, fluctuating between a low, now of 60,300 cases and a high of about 112,000,” the AAP noted.
Emergency department visits and hospital admissions, which showed less fluctuation over the summer and more steady rise and fall, have both dropped in recent weeks and are now approaching late May/early June rates, according to data from the Centers for Disease Control and Prevention.
On Sept. 15, for example, ED visits for children under 12 years with diagnosed COVID were just 2.2% of all visits, lower than at any time since May 19 and down from a summer high of 6.8% in late July. Hospital admissions for children aged 0-17 years also rose steadily through June and July, reaching 0.46 per 100,000 population on July 30, but have since slipped to 0.29 per 100,000 as of Sept. 17, the CDC said on its COVID Data Tracker.
Vaccination continues to be a tough sell
Vaccination activity among the most recently eligible age group, in the meantime, remains tepid. Just 6.0% of children under age 5 had received at least one dose of COVID-19 vaccine as of Sept. 13, about 3 months since its final approval in June, and 1.6% were fully vaccinated. For the two older groups of children with separate vaccine approvals, 31.5% of those aged 5-11 years and 43.3% of those aged 12-15 had received at least one dose 3 months after their vaccinations began, the CDC data show.
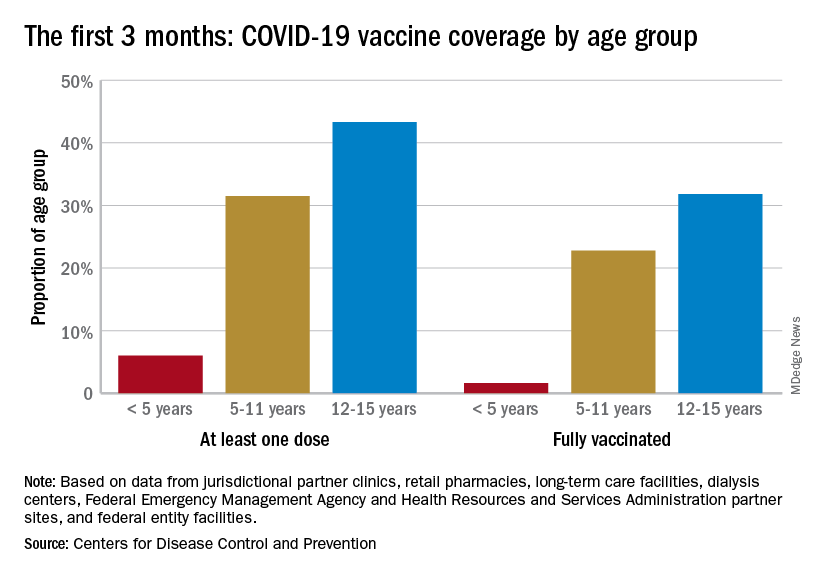
In the 2 weeks ending Sept. 14, almost 59,000 children under age 5 received their initial COVID-19 vaccine dose, as did 28,000 5- to 11-year-olds and 14,000 children aged 12-17. Children under age 5 years represented almost 20% of all Americans getting a first dose during Sept. 1-14, compared with 9.7% for those aged 5-11 and 4.8% for the 12- to 17-year-olds, the CDC said.
At the state level, children under age 5 years in the District of Columbia, where 28% have received at least one dose, and Vermont, at 24%, are the most likely to be vaccinated. The states with the lowest rates in this age group are Alabama, Louisiana, and Mississippi, all of which are at 2%. Vermont and D.C. have the highest rates for ages 5-11 at 70% each, and Alabama (17%) is the lowest, while D.C. (100%), Rhode Island (99%), and Massachusetts (99%) are highest for children aged 12-17 years and Wyoming (41%) is the lowest, the AAP said in a separate report.
A hefty decline in new COVID-19 cases among children resulted in the lowest weekly total since late April, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
, making for 2 consecutive weeks of declines after almost 91,000 cases were recorded for the week ending Sept. 1, the AAP and CHA said in their latest COVID report of state-level data.
The last time the weekly count was under 60,000 came during the week of April 22-28, when 53,000 were reported by state and territorial health departments in the midst of a 7-week stretch of rising cases. Since that streak ended in mid-May, however, “reported weekly cases have plateaued, fluctuating between a low, now of 60,300 cases and a high of about 112,000,” the AAP noted.
Emergency department visits and hospital admissions, which showed less fluctuation over the summer and more steady rise and fall, have both dropped in recent weeks and are now approaching late May/early June rates, according to data from the Centers for Disease Control and Prevention.
On Sept. 15, for example, ED visits for children under 12 years with diagnosed COVID were just 2.2% of all visits, lower than at any time since May 19 and down from a summer high of 6.8% in late July. Hospital admissions for children aged 0-17 years also rose steadily through June and July, reaching 0.46 per 100,000 population on July 30, but have since slipped to 0.29 per 100,000 as of Sept. 17, the CDC said on its COVID Data Tracker.
Vaccination continues to be a tough sell
Vaccination activity among the most recently eligible age group, in the meantime, remains tepid. Just 6.0% of children under age 5 had received at least one dose of COVID-19 vaccine as of Sept. 13, about 3 months since its final approval in June, and 1.6% were fully vaccinated. For the two older groups of children with separate vaccine approvals, 31.5% of those aged 5-11 years and 43.3% of those aged 12-15 had received at least one dose 3 months after their vaccinations began, the CDC data show.

In the 2 weeks ending Sept. 14, almost 59,000 children under age 5 received their initial COVID-19 vaccine dose, as did 28,000 5- to 11-year-olds and 14,000 children aged 12-17. Children under age 5 years represented almost 20% of all Americans getting a first dose during Sept. 1-14, compared with 9.7% for those aged 5-11 and 4.8% for the 12- to 17-year-olds, the CDC said.
At the state level, children under age 5 years in the District of Columbia, where 28% have received at least one dose, and Vermont, at 24%, are the most likely to be vaccinated. The states with the lowest rates in this age group are Alabama, Louisiana, and Mississippi, all of which are at 2%. Vermont and D.C. have the highest rates for ages 5-11 at 70% each, and Alabama (17%) is the lowest, while D.C. (100%), Rhode Island (99%), and Massachusetts (99%) are highest for children aged 12-17 years and Wyoming (41%) is the lowest, the AAP said in a separate report.
A hefty decline in new COVID-19 cases among children resulted in the lowest weekly total since late April, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
, making for 2 consecutive weeks of declines after almost 91,000 cases were recorded for the week ending Sept. 1, the AAP and CHA said in their latest COVID report of state-level data.
The last time the weekly count was under 60,000 came during the week of April 22-28, when 53,000 were reported by state and territorial health departments in the midst of a 7-week stretch of rising cases. Since that streak ended in mid-May, however, “reported weekly cases have plateaued, fluctuating between a low, now of 60,300 cases and a high of about 112,000,” the AAP noted.
Emergency department visits and hospital admissions, which showed less fluctuation over the summer and more steady rise and fall, have both dropped in recent weeks and are now approaching late May/early June rates, according to data from the Centers for Disease Control and Prevention.
On Sept. 15, for example, ED visits for children under 12 years with diagnosed COVID were just 2.2% of all visits, lower than at any time since May 19 and down from a summer high of 6.8% in late July. Hospital admissions for children aged 0-17 years also rose steadily through June and July, reaching 0.46 per 100,000 population on July 30, but have since slipped to 0.29 per 100,000 as of Sept. 17, the CDC said on its COVID Data Tracker.
Vaccination continues to be a tough sell
Vaccination activity among the most recently eligible age group, in the meantime, remains tepid. Just 6.0% of children under age 5 had received at least one dose of COVID-19 vaccine as of Sept. 13, about 3 months since its final approval in June, and 1.6% were fully vaccinated. For the two older groups of children with separate vaccine approvals, 31.5% of those aged 5-11 years and 43.3% of those aged 12-15 had received at least one dose 3 months after their vaccinations began, the CDC data show.

In the 2 weeks ending Sept. 14, almost 59,000 children under age 5 received their initial COVID-19 vaccine dose, as did 28,000 5- to 11-year-olds and 14,000 children aged 12-17. Children under age 5 years represented almost 20% of all Americans getting a first dose during Sept. 1-14, compared with 9.7% for those aged 5-11 and 4.8% for the 12- to 17-year-olds, the CDC said.
At the state level, children under age 5 years in the District of Columbia, where 28% have received at least one dose, and Vermont, at 24%, are the most likely to be vaccinated. The states with the lowest rates in this age group are Alabama, Louisiana, and Mississippi, all of which are at 2%. Vermont and D.C. have the highest rates for ages 5-11 at 70% each, and Alabama (17%) is the lowest, while D.C. (100%), Rhode Island (99%), and Massachusetts (99%) are highest for children aged 12-17 years and Wyoming (41%) is the lowest, the AAP said in a separate report.
Me, my spouse, and COVID
Managing family conflict and cohesion
I watched you in the garage, with your wipes and your mask, your gloves and bottles of sprays and potions. I admired your fealty to CNN’s Dr. Sanjay Gupta as he demonstrated the proper technique for disinfecting groceries. I watched sterile protocol being broken and quietly closed the garage door.
I listened to your descriptions of the agility of the virus with each exhalation of breath, and how far the virus could travel with a tailwind and in cold dry air. I listen as closely and with the same intention as I listen to my yoga teacher’s explication of the benefits of attention to the breath.
Relatives and friends came prepared to be entertained outdoors. Even masked, you eschewed the world. Your version of science clashes with my laissez-faire attitude. We blow up as a couple. Then we settle down and learn how to cope with the stress, as a team, together.
The COVID factor
In the first few months of any stressor, family and couple functioning must reorganize to manage well.
During lockdown, social scientists accessed an eager public ready to participate in their studies. With nowhere to go, many people, especially women, completed online COVID surveys. Community-based tools such as the Centers for Disease Control and Prevention’s Social Vulnerability Index identified populations of high social vulnerability (as caused by external stresses on human health, such as unemployment, overcrowding, presence of an individual with caregiving needs, and low educational attainment). It is assumed that such populations will experience more stress and have more difficulty coping and adjusting.
In a study by a team at the University of Miami, social vulnerability was associated with more disrupted family functioning, except when households with children (n = 2,666) were compared to households without children (n = 1,456).1 What allowed these families with children to enjoy better functioning?
Looking more closely at the Miami study, what can we find? It is a large survey study (n = 4,122), disseminated through professional networks and social media via purchased Facebook and Instagram ads. Data were logged in REDCap, and participants had the option of taking the survey in English or Spanish. Most participants were female (93.5%), 55.7% responded in English, and 44.3% in Spanish. There were few differences between the women who had and did not have children, in terms of their age, employment status, and education level. The number of children in the household did not affect the results.
This study used a new tool called the COVID-19 Household Environment Scale. This tool has 25 items measuring individual and household characteristics, and associated COVID-19 stressors. This tool also includes two family functioning measures: conflict and cohesion, asking the respondent to reflect on the change in “conflict” or “togetherness,” as it relates to household experiences and activities, compared with the period before social distancing.
The surprising finding was that even though households with children reported more conflict than before the start of the pandemic, they also reported more cohesion. This syncs with my experience. My niece and nephew found that having their teenage children at home brought them closer as a family, cut down on some of the extracurricular activities they did not support, and generally “slowed the world down.”
However, in a study in Germany, survey respondents (n = 1,042) noted that having children up to 17 years old was associated with decreases in satisfaction with family life, although this was not related to changes in family demands. The study assessed changes over 6 months and underscores the fact that perceptions of family demands and family well-being are independent of each other.2
These findings also resonate with prior research that measured burden and reward in couples. High burden is not associated with low reward; these two constructs are independent of each other.3
What about couples?
It is no surprise that poor relationships begat poor coping. In an online Belgian survey of 1,491 cohabiting couples during the shutdown, both men and women felt significantly more stress than before, because they felt restricted in their relationship.4
However, only women reported significantly more stress during the lockdown than before, because of relationship conflicts, such as feeling neglected by their partner. These feelings had predated lockdown.
In another lockdown online survey of 782 U.S. adults (89.8% White, 84.5% female), cohabitating intimate partners reported that there were higher thoughts of separation if the participants were younger, or if there was higher verbal aggression, higher relationship invalidation, and lower relationship satisfaction. Higher relationship satisfaction was reported when there was lower money stress, higher sexual fulfillment, lower relationship invalidation, and higher perceived fairness of relationship power. High relationship satisfaction was also reported where there were no children in the home.5
It should be noted that none of these relationship variables was measured in the Miami study discussed above, and this study did not measure perceived conflict or perceived cohesion, so we know less about these aspects of the family unit.
What about teens?
The COVID-19 lockdown had a positive effect on the dynamics in some families, according to a naturalistic study of adolescents (n = 155) who completed surveys at two time periods (initial and 8 weeks).6
These adolescents reported a reduction in perceived psychological control by their mothers, and no change in autonomy support. The changes did not vary according to gender or the mother’s employment situation. The decrease in psychological control was greater with higher initial levels of satisfaction with the mother, and lower levels of the teens disobeying their parents.
What about hospital settings?
The worst of the COVID experience was in the hospital. The pain was displayed on the faces of the staff as they labored to figure out how to care for the dying patients who had no contact with their families. Hospitals, out of fear of contamination and viral dissemination, excluded visitors. In those early days of uncertainty, the stress among staff, patients, and family members was high.
In response to family members feeling disconnected from the health care team and the psychological and moral distress of the staff, Nadine J. Kaslow and colleagues revised policies and procedures at Emory University, Atlanta, facilities to reprioritize patient- and family-centered care.7
The guiding principles focus on providing safe yet compassionate and ethical care, balancing community health and the mitigation of viral transmission, while appreciating family members as essential partners in care; fostering communication between patients and their families; and promoting interactions and decision-making among health care providers, patients, and families.
COVID continues to intrude in many of our lives. Many people are mourning family members and friends who died after contracting the disease. Many people choose to ignore their risk and live their lives as before. Many people, like my spouse and me, continue to debate the merits of venturing into public spaces. Personally, COVID has given me time to read many more books than I could ever have imagined and allowed my spouse to explore the delicate nuances of cooking.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at [email protected].
References
1. Chavez JV et al. Assessing the impact of COVID-19 social distancing and social vulnerability on family functioning in an international sample of households with and without children. Couple Fam Psychol: Res Pract. 2021 Dec;10(4): 233-48. doi: 10.1037/cfp0000166.
2. Rudolph CW, Zacher H. Family demands and satisfaction with family life during the COVID-19 pandemic. Couple Fam Psychol: Res Pract. 2021 Dec;10(4): 249-59. doi: 10.1037/cfp0000170.
3. Heru AM et al. Family functioning in the caregivers of patients with dementia. Int J Geriatr Psychiatry. 2004 Jun;19(6):533-7. doi: 10.1002/gps.1119.
4. Schokkenbroek JM et al. Partners in lockdown: Relationship stress in men and women during the COVID-19 pandemic. Couple Fam Psychol: Res Pract. 2021 Sept;10(3): 149-57. doi: 10.1037/cfp0000172.
5. Eubanks Fleming CJ, Franzese AT. Should I stay or should I go? Evaluating intimate relationship outcomes during the 2020 pandemic shutdown. Couple Fam Psychol: Res Pract. 2021 Sept;10(3): 158-67. doi: 10.1037/cfp0000169.
6. Bacikova-Sleskova M,et al. Did perceived parenting in adolescence change as a result of the COVID-19 lockdown? A natural experiment. Couple Fam Psychol: Res Pract. 2021 Dec;10(4): 271-80. doi: 10.1037/cfp0000167.
7. Kaslow NJ et al. A roadmap for patient- and family-centered care during the pandemic. Couple Fam Psychol: Res Pract. 2021 Sept;10(3): 223-32. doi: 10.1037/cfp0000176.
Managing family conflict and cohesion
Managing family conflict and cohesion
I watched you in the garage, with your wipes and your mask, your gloves and bottles of sprays and potions. I admired your fealty to CNN’s Dr. Sanjay Gupta as he demonstrated the proper technique for disinfecting groceries. I watched sterile protocol being broken and quietly closed the garage door.
I listened to your descriptions of the agility of the virus with each exhalation of breath, and how far the virus could travel with a tailwind and in cold dry air. I listen as closely and with the same intention as I listen to my yoga teacher’s explication of the benefits of attention to the breath.
Relatives and friends came prepared to be entertained outdoors. Even masked, you eschewed the world. Your version of science clashes with my laissez-faire attitude. We blow up as a couple. Then we settle down and learn how to cope with the stress, as a team, together.
The COVID factor
In the first few months of any stressor, family and couple functioning must reorganize to manage well.
During lockdown, social scientists accessed an eager public ready to participate in their studies. With nowhere to go, many people, especially women, completed online COVID surveys. Community-based tools such as the Centers for Disease Control and Prevention’s Social Vulnerability Index identified populations of high social vulnerability (as caused by external stresses on human health, such as unemployment, overcrowding, presence of an individual with caregiving needs, and low educational attainment). It is assumed that such populations will experience more stress and have more difficulty coping and adjusting.
In a study by a team at the University of Miami, social vulnerability was associated with more disrupted family functioning, except when households with children (n = 2,666) were compared to households without children (n = 1,456).1 What allowed these families with children to enjoy better functioning?
Looking more closely at the Miami study, what can we find? It is a large survey study (n = 4,122), disseminated through professional networks and social media via purchased Facebook and Instagram ads. Data were logged in REDCap, and participants had the option of taking the survey in English or Spanish. Most participants were female (93.5%), 55.7% responded in English, and 44.3% in Spanish. There were few differences between the women who had and did not have children, in terms of their age, employment status, and education level. The number of children in the household did not affect the results.
This study used a new tool called the COVID-19 Household Environment Scale. This tool has 25 items measuring individual and household characteristics, and associated COVID-19 stressors. This tool also includes two family functioning measures: conflict and cohesion, asking the respondent to reflect on the change in “conflict” or “togetherness,” as it relates to household experiences and activities, compared with the period before social distancing.
The surprising finding was that even though households with children reported more conflict than before the start of the pandemic, they also reported more cohesion. This syncs with my experience. My niece and nephew found that having their teenage children at home brought them closer as a family, cut down on some of the extracurricular activities they did not support, and generally “slowed the world down.”
However, in a study in Germany, survey respondents (n = 1,042) noted that having children up to 17 years old was associated with decreases in satisfaction with family life, although this was not related to changes in family demands. The study assessed changes over 6 months and underscores the fact that perceptions of family demands and family well-being are independent of each other.2
These findings also resonate with prior research that measured burden and reward in couples. High burden is not associated with low reward; these two constructs are independent of each other.3
What about couples?
It is no surprise that poor relationships begat poor coping. In an online Belgian survey of 1,491 cohabiting couples during the shutdown, both men and women felt significantly more stress than before, because they felt restricted in their relationship.4
However, only women reported significantly more stress during the lockdown than before, because of relationship conflicts, such as feeling neglected by their partner. These feelings had predated lockdown.
In another lockdown online survey of 782 U.S. adults (89.8% White, 84.5% female), cohabitating intimate partners reported that there were higher thoughts of separation if the participants were younger, or if there was higher verbal aggression, higher relationship invalidation, and lower relationship satisfaction. Higher relationship satisfaction was reported when there was lower money stress, higher sexual fulfillment, lower relationship invalidation, and higher perceived fairness of relationship power. High relationship satisfaction was also reported where there were no children in the home.5
It should be noted that none of these relationship variables was measured in the Miami study discussed above, and this study did not measure perceived conflict or perceived cohesion, so we know less about these aspects of the family unit.
What about teens?
The COVID-19 lockdown had a positive effect on the dynamics in some families, according to a naturalistic study of adolescents (n = 155) who completed surveys at two time periods (initial and 8 weeks).6
These adolescents reported a reduction in perceived psychological control by their mothers, and no change in autonomy support. The changes did not vary according to gender or the mother’s employment situation. The decrease in psychological control was greater with higher initial levels of satisfaction with the mother, and lower levels of the teens disobeying their parents.
What about hospital settings?
The worst of the COVID experience was in the hospital. The pain was displayed on the faces of the staff as they labored to figure out how to care for the dying patients who had no contact with their families. Hospitals, out of fear of contamination and viral dissemination, excluded visitors. In those early days of uncertainty, the stress among staff, patients, and family members was high.
In response to family members feeling disconnected from the health care team and the psychological and moral distress of the staff, Nadine J. Kaslow and colleagues revised policies and procedures at Emory University, Atlanta, facilities to reprioritize patient- and family-centered care.7
The guiding principles focus on providing safe yet compassionate and ethical care, balancing community health and the mitigation of viral transmission, while appreciating family members as essential partners in care; fostering communication between patients and their families; and promoting interactions and decision-making among health care providers, patients, and families.
COVID continues to intrude in many of our lives. Many people are mourning family members and friends who died after contracting the disease. Many people choose to ignore their risk and live their lives as before. Many people, like my spouse and me, continue to debate the merits of venturing into public spaces. Personally, COVID has given me time to read many more books than I could ever have imagined and allowed my spouse to explore the delicate nuances of cooking.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at [email protected].
References
1. Chavez JV et al. Assessing the impact of COVID-19 social distancing and social vulnerability on family functioning in an international sample of households with and without children. Couple Fam Psychol: Res Pract. 2021 Dec;10(4): 233-48. doi: 10.1037/cfp0000166.
2. Rudolph CW, Zacher H. Family demands and satisfaction with family life during the COVID-19 pandemic. Couple Fam Psychol: Res Pract. 2021 Dec;10(4): 249-59. doi: 10.1037/cfp0000170.
3. Heru AM et al. Family functioning in the caregivers of patients with dementia. Int J Geriatr Psychiatry. 2004 Jun;19(6):533-7. doi: 10.1002/gps.1119.
4. Schokkenbroek JM et al. Partners in lockdown: Relationship stress in men and women during the COVID-19 pandemic. Couple Fam Psychol: Res Pract. 2021 Sept;10(3): 149-57. doi: 10.1037/cfp0000172.
5. Eubanks Fleming CJ, Franzese AT. Should I stay or should I go? Evaluating intimate relationship outcomes during the 2020 pandemic shutdown. Couple Fam Psychol: Res Pract. 2021 Sept;10(3): 158-67. doi: 10.1037/cfp0000169.
6. Bacikova-Sleskova M,et al. Did perceived parenting in adolescence change as a result of the COVID-19 lockdown? A natural experiment. Couple Fam Psychol: Res Pract. 2021 Dec;10(4): 271-80. doi: 10.1037/cfp0000167.
7. Kaslow NJ et al. A roadmap for patient- and family-centered care during the pandemic. Couple Fam Psychol: Res Pract. 2021 Sept;10(3): 223-32. doi: 10.1037/cfp0000176.
I watched you in the garage, with your wipes and your mask, your gloves and bottles of sprays and potions. I admired your fealty to CNN’s Dr. Sanjay Gupta as he demonstrated the proper technique for disinfecting groceries. I watched sterile protocol being broken and quietly closed the garage door.
I listened to your descriptions of the agility of the virus with each exhalation of breath, and how far the virus could travel with a tailwind and in cold dry air. I listen as closely and with the same intention as I listen to my yoga teacher’s explication of the benefits of attention to the breath.
Relatives and friends came prepared to be entertained outdoors. Even masked, you eschewed the world. Your version of science clashes with my laissez-faire attitude. We blow up as a couple. Then we settle down and learn how to cope with the stress, as a team, together.
The COVID factor
In the first few months of any stressor, family and couple functioning must reorganize to manage well.
During lockdown, social scientists accessed an eager public ready to participate in their studies. With nowhere to go, many people, especially women, completed online COVID surveys. Community-based tools such as the Centers for Disease Control and Prevention’s Social Vulnerability Index identified populations of high social vulnerability (as caused by external stresses on human health, such as unemployment, overcrowding, presence of an individual with caregiving needs, and low educational attainment). It is assumed that such populations will experience more stress and have more difficulty coping and adjusting.
In a study by a team at the University of Miami, social vulnerability was associated with more disrupted family functioning, except when households with children (n = 2,666) were compared to households without children (n = 1,456).1 What allowed these families with children to enjoy better functioning?
Looking more closely at the Miami study, what can we find? It is a large survey study (n = 4,122), disseminated through professional networks and social media via purchased Facebook and Instagram ads. Data were logged in REDCap, and participants had the option of taking the survey in English or Spanish. Most participants were female (93.5%), 55.7% responded in English, and 44.3% in Spanish. There were few differences between the women who had and did not have children, in terms of their age, employment status, and education level. The number of children in the household did not affect the results.
This study used a new tool called the COVID-19 Household Environment Scale. This tool has 25 items measuring individual and household characteristics, and associated COVID-19 stressors. This tool also includes two family functioning measures: conflict and cohesion, asking the respondent to reflect on the change in “conflict” or “togetherness,” as it relates to household experiences and activities, compared with the period before social distancing.
The surprising finding was that even though households with children reported more conflict than before the start of the pandemic, they also reported more cohesion. This syncs with my experience. My niece and nephew found that having their teenage children at home brought them closer as a family, cut down on some of the extracurricular activities they did not support, and generally “slowed the world down.”
However, in a study in Germany, survey respondents (n = 1,042) noted that having children up to 17 years old was associated with decreases in satisfaction with family life, although this was not related to changes in family demands. The study assessed changes over 6 months and underscores the fact that perceptions of family demands and family well-being are independent of each other.2
These findings also resonate with prior research that measured burden and reward in couples. High burden is not associated with low reward; these two constructs are independent of each other.3
What about couples?
It is no surprise that poor relationships begat poor coping. In an online Belgian survey of 1,491 cohabiting couples during the shutdown, both men and women felt significantly more stress than before, because they felt restricted in their relationship.4
However, only women reported significantly more stress during the lockdown than before, because of relationship conflicts, such as feeling neglected by their partner. These feelings had predated lockdown.
In another lockdown online survey of 782 U.S. adults (89.8% White, 84.5% female), cohabitating intimate partners reported that there were higher thoughts of separation if the participants were younger, or if there was higher verbal aggression, higher relationship invalidation, and lower relationship satisfaction. Higher relationship satisfaction was reported when there was lower money stress, higher sexual fulfillment, lower relationship invalidation, and higher perceived fairness of relationship power. High relationship satisfaction was also reported where there were no children in the home.5
It should be noted that none of these relationship variables was measured in the Miami study discussed above, and this study did not measure perceived conflict or perceived cohesion, so we know less about these aspects of the family unit.
What about teens?
The COVID-19 lockdown had a positive effect on the dynamics in some families, according to a naturalistic study of adolescents (n = 155) who completed surveys at two time periods (initial and 8 weeks).6
These adolescents reported a reduction in perceived psychological control by their mothers, and no change in autonomy support. The changes did not vary according to gender or the mother’s employment situation. The decrease in psychological control was greater with higher initial levels of satisfaction with the mother, and lower levels of the teens disobeying their parents.
What about hospital settings?
The worst of the COVID experience was in the hospital. The pain was displayed on the faces of the staff as they labored to figure out how to care for the dying patients who had no contact with their families. Hospitals, out of fear of contamination and viral dissemination, excluded visitors. In those early days of uncertainty, the stress among staff, patients, and family members was high.
In response to family members feeling disconnected from the health care team and the psychological and moral distress of the staff, Nadine J. Kaslow and colleagues revised policies and procedures at Emory University, Atlanta, facilities to reprioritize patient- and family-centered care.7
The guiding principles focus on providing safe yet compassionate and ethical care, balancing community health and the mitigation of viral transmission, while appreciating family members as essential partners in care; fostering communication between patients and their families; and promoting interactions and decision-making among health care providers, patients, and families.
COVID continues to intrude in many of our lives. Many people are mourning family members and friends who died after contracting the disease. Many people choose to ignore their risk and live their lives as before. Many people, like my spouse and me, continue to debate the merits of venturing into public spaces. Personally, COVID has given me time to read many more books than I could ever have imagined and allowed my spouse to explore the delicate nuances of cooking.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at [email protected].
References
1. Chavez JV et al. Assessing the impact of COVID-19 social distancing and social vulnerability on family functioning in an international sample of households with and without children. Couple Fam Psychol: Res Pract. 2021 Dec;10(4): 233-48. doi: 10.1037/cfp0000166.
2. Rudolph CW, Zacher H. Family demands and satisfaction with family life during the COVID-19 pandemic. Couple Fam Psychol: Res Pract. 2021 Dec;10(4): 249-59. doi: 10.1037/cfp0000170.
3. Heru AM et al. Family functioning in the caregivers of patients with dementia. Int J Geriatr Psychiatry. 2004 Jun;19(6):533-7. doi: 10.1002/gps.1119.
4. Schokkenbroek JM et al. Partners in lockdown: Relationship stress in men and women during the COVID-19 pandemic. Couple Fam Psychol: Res Pract. 2021 Sept;10(3): 149-57. doi: 10.1037/cfp0000172.
5. Eubanks Fleming CJ, Franzese AT. Should I stay or should I go? Evaluating intimate relationship outcomes during the 2020 pandemic shutdown. Couple Fam Psychol: Res Pract. 2021 Sept;10(3): 158-67. doi: 10.1037/cfp0000169.
6. Bacikova-Sleskova M,et al. Did perceived parenting in adolescence change as a result of the COVID-19 lockdown? A natural experiment. Couple Fam Psychol: Res Pract. 2021 Dec;10(4): 271-80. doi: 10.1037/cfp0000167.
7. Kaslow NJ et al. A roadmap for patient- and family-centered care during the pandemic. Couple Fam Psychol: Res Pract. 2021 Sept;10(3): 223-32. doi: 10.1037/cfp0000176.
COVID-19 linked to increased Alzheimer’s risk
The study of more than 6 million people aged 65 years or older found a 50%-80% increased risk for AD in the year after COVID-19; the risk was especially high for women older than 85 years.
However, the investigators were quick to point out that the observational retrospective study offers no evidence that COVID-19 causes AD. There could be a viral etiology at play, or the connection could be related to inflammation in neural tissue from the SARS-CoV-2 infection. Or it could simply be that exposure to the health care system for COVID-19 increased the odds of detection of existing undiagnosed AD cases.
Whatever the case, these findings point to a potential spike in AD cases, which is a cause for concern, study investigator Pamela Davis, MD, PhD, a professor in the Center for Community Health Integration at Case Western Reserve University, Cleveland, said in an interview.
“COVID may be giving us a legacy of ongoing medical difficulties,” Dr. Davis said. “We were already concerned about having a very large care burden and cost burden from Alzheimer’s disease. If this is another burden that’s increased by COVID, this is something we’re really going to have to prepare for.”
The findings were published online in Journal of Alzheimer’s Disease.
Increased risk
Earlier research points to a potential link between COVID-19 and increased risk for AD and Parkinson’s disease.
For the current study, researchers analyzed anonymous electronic health records of 6.2 million adults aged 65 years or older who received medical treatment between February 2020 and May 2021 and had no prior diagnosis of AD. The database includes information on almost 30% of the entire U.S. population.
Overall, there were 410,748 cases of COVID-19 during the study period.
The overall risk for new diagnosis of AD in the COVID-19 cohort was close to double that of those who did not have COVID-19 (0.68% vs. 0.35%, respectively).
After propensity-score matching, those who have had COVID-19 had a significantly higher risk for an AD diagnosis compared with those who were not infected (hazard ratio [HR], 1.69; 95% confidence interval [CI],1.53-1.72).
Risk for AD was elevated in all age groups, regardless of gender or ethnicity. Researchers did not collect data on COVID-19 severity, and the medical codes for long COVID were not published until after the study had ended.
Those with the highest risk were individuals older than 85 years (HR, 1.89; 95% CI, 1.73-2.07) and women (HR, 1.82; 95% CI, 1.69-1.97).
“We expected to see some impact, but I was surprised that it was as potent as it was,” Dr. Davis said.
Association, not causation
Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, who commented on the findings for this article, called the study interesting but emphasized caution in interpreting the results.
“Because this study only showed an association through medical records, we cannot know what the underlying mechanisms driving this association are without more research,” Dr. Snyder said. “If you have had COVID-19, it doesn’t mean you’re going to get dementia. But if you have had COVID-19 and are experiencing long-term symptoms including cognitive difficulties, talk to your doctor.”
Dr. Davis agreed, noting that this type of study offers information on association, but not causation. “I do think that this makes it imperative that we continue to follow the population for what’s going on in various neurodegenerative diseases,” Dr. Davis said.
The study was funded by the National Institute of Aging, National Institute on Alcohol Abuse and Alcoholism, the Clinical and Translational Science Collaborative of Cleveland, and the National Cancer Institute. Dr. Synder reports no relevant financial conflicts.
A version of this article first appeared on Medscape.com.
The study of more than 6 million people aged 65 years or older found a 50%-80% increased risk for AD in the year after COVID-19; the risk was especially high for women older than 85 years.
However, the investigators were quick to point out that the observational retrospective study offers no evidence that COVID-19 causes AD. There could be a viral etiology at play, or the connection could be related to inflammation in neural tissue from the SARS-CoV-2 infection. Or it could simply be that exposure to the health care system for COVID-19 increased the odds of detection of existing undiagnosed AD cases.
Whatever the case, these findings point to a potential spike in AD cases, which is a cause for concern, study investigator Pamela Davis, MD, PhD, a professor in the Center for Community Health Integration at Case Western Reserve University, Cleveland, said in an interview.
“COVID may be giving us a legacy of ongoing medical difficulties,” Dr. Davis said. “We were already concerned about having a very large care burden and cost burden from Alzheimer’s disease. If this is another burden that’s increased by COVID, this is something we’re really going to have to prepare for.”
The findings were published online in Journal of Alzheimer’s Disease.
Increased risk
Earlier research points to a potential link between COVID-19 and increased risk for AD and Parkinson’s disease.
For the current study, researchers analyzed anonymous electronic health records of 6.2 million adults aged 65 years or older who received medical treatment between February 2020 and May 2021 and had no prior diagnosis of AD. The database includes information on almost 30% of the entire U.S. population.
Overall, there were 410,748 cases of COVID-19 during the study period.
The overall risk for new diagnosis of AD in the COVID-19 cohort was close to double that of those who did not have COVID-19 (0.68% vs. 0.35%, respectively).
After propensity-score matching, those who have had COVID-19 had a significantly higher risk for an AD diagnosis compared with those who were not infected (hazard ratio [HR], 1.69; 95% confidence interval [CI],1.53-1.72).
Risk for AD was elevated in all age groups, regardless of gender or ethnicity. Researchers did not collect data on COVID-19 severity, and the medical codes for long COVID were not published until after the study had ended.
Those with the highest risk were individuals older than 85 years (HR, 1.89; 95% CI, 1.73-2.07) and women (HR, 1.82; 95% CI, 1.69-1.97).
“We expected to see some impact, but I was surprised that it was as potent as it was,” Dr. Davis said.
Association, not causation
Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, who commented on the findings for this article, called the study interesting but emphasized caution in interpreting the results.
“Because this study only showed an association through medical records, we cannot know what the underlying mechanisms driving this association are without more research,” Dr. Snyder said. “If you have had COVID-19, it doesn’t mean you’re going to get dementia. But if you have had COVID-19 and are experiencing long-term symptoms including cognitive difficulties, talk to your doctor.”
Dr. Davis agreed, noting that this type of study offers information on association, but not causation. “I do think that this makes it imperative that we continue to follow the population for what’s going on in various neurodegenerative diseases,” Dr. Davis said.
The study was funded by the National Institute of Aging, National Institute on Alcohol Abuse and Alcoholism, the Clinical and Translational Science Collaborative of Cleveland, and the National Cancer Institute. Dr. Synder reports no relevant financial conflicts.
A version of this article first appeared on Medscape.com.
The study of more than 6 million people aged 65 years or older found a 50%-80% increased risk for AD in the year after COVID-19; the risk was especially high for women older than 85 years.
However, the investigators were quick to point out that the observational retrospective study offers no evidence that COVID-19 causes AD. There could be a viral etiology at play, or the connection could be related to inflammation in neural tissue from the SARS-CoV-2 infection. Or it could simply be that exposure to the health care system for COVID-19 increased the odds of detection of existing undiagnosed AD cases.
Whatever the case, these findings point to a potential spike in AD cases, which is a cause for concern, study investigator Pamela Davis, MD, PhD, a professor in the Center for Community Health Integration at Case Western Reserve University, Cleveland, said in an interview.
“COVID may be giving us a legacy of ongoing medical difficulties,” Dr. Davis said. “We were already concerned about having a very large care burden and cost burden from Alzheimer’s disease. If this is another burden that’s increased by COVID, this is something we’re really going to have to prepare for.”
The findings were published online in Journal of Alzheimer’s Disease.
Increased risk
Earlier research points to a potential link between COVID-19 and increased risk for AD and Parkinson’s disease.
For the current study, researchers analyzed anonymous electronic health records of 6.2 million adults aged 65 years or older who received medical treatment between February 2020 and May 2021 and had no prior diagnosis of AD. The database includes information on almost 30% of the entire U.S. population.
Overall, there were 410,748 cases of COVID-19 during the study period.
The overall risk for new diagnosis of AD in the COVID-19 cohort was close to double that of those who did not have COVID-19 (0.68% vs. 0.35%, respectively).
After propensity-score matching, those who have had COVID-19 had a significantly higher risk for an AD diagnosis compared with those who were not infected (hazard ratio [HR], 1.69; 95% confidence interval [CI],1.53-1.72).
Risk for AD was elevated in all age groups, regardless of gender or ethnicity. Researchers did not collect data on COVID-19 severity, and the medical codes for long COVID were not published until after the study had ended.
Those with the highest risk were individuals older than 85 years (HR, 1.89; 95% CI, 1.73-2.07) and women (HR, 1.82; 95% CI, 1.69-1.97).
“We expected to see some impact, but I was surprised that it was as potent as it was,” Dr. Davis said.
Association, not causation
Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, who commented on the findings for this article, called the study interesting but emphasized caution in interpreting the results.
“Because this study only showed an association through medical records, we cannot know what the underlying mechanisms driving this association are without more research,” Dr. Snyder said. “If you have had COVID-19, it doesn’t mean you’re going to get dementia. But if you have had COVID-19 and are experiencing long-term symptoms including cognitive difficulties, talk to your doctor.”
Dr. Davis agreed, noting that this type of study offers information on association, but not causation. “I do think that this makes it imperative that we continue to follow the population for what’s going on in various neurodegenerative diseases,” Dr. Davis said.
The study was funded by the National Institute of Aging, National Institute on Alcohol Abuse and Alcoholism, the Clinical and Translational Science Collaborative of Cleveland, and the National Cancer Institute. Dr. Synder reports no relevant financial conflicts.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ALZHEIMER’S DISEASE
Pandemic has helped clinicians to gain better insight on pernio, expert says
PORTLAND, ORE. – while others are not, according to Lindy P. Fox, MD, professor of dermatology and director of the hospital consultation service at the University of California, San Francisco.
“We’re learning a lot about pernio because of COVID,” Dr. Fox, a member of the American Academy of Dermatology’s Ad Hoc Task Force on COVID-19, said at the annual meeting of the Pacific Dermatologic Association. “Patients with pernio tend to either have bright red or purple individual lesions or an erythromelalgia-like presentation, often waking up in the middle of the night saying ‘my feet hurt. I can’t put sheets over my feet.’ In my experience, the patients with an erythromelalgia-like presentation tend to be a lot harder to treat.”
Establishing terminology to describe pernio-like lesions was a challenge in the early stages of the COVID-19 pandemic, Dr. Fox added, with clinicians using terms like erythema multiforme-like, coxsackie-like, or even necrotic to describe the lesions. “I don’t think pernio is truly necrotic; I think it’s really inflammatory and purpuric,” she said.
Early in the pandemic, studies suggesting a link with these cases and COVID-19 infection include a case series of 318 patients with pernio-like skin lesions who had confirmed or suspected COVID-19. Most of these patients were generally young and healthy and most had relatively mild COVID-19; 7% were laboratory-confirmed COVID-19 positive, and 6% were close contacts of patients with confirmed COVID-19. Pernio-like lesions were the only symptoms in 55% of the patients.
In another study, researchers in France evaluated the clinical, laboratory, and pathologic characteristics of 40 patients who developed chilblain-like lesions (mostly involving the toes) during the COVID-19 pandemic and were seen as outpatients in April 2020 . All were polymerase chain reaction (PCR) negative, 30% were SARS-CoV-2 serology positive, and 60% had elevated D-dimers. Histology obtained from 19 of the patients revealed lymphocytic inflammation and vascular damage, and 8 had IgA positivity.
In a retrospective analysis of seven pediatric chilblains cases during the pandemic, researchers examined the skin biopsies to evaluate histopathological features and explored the presence of SARS-CoV-2 in the tissue. All patients were PCR negative. The authors observed cytoplasmic granular positivity for SARS-CoV-2 spike protein in endothelial cells, a feature that they said showed coronavirus-like particles, consistent with SARS-CoV-2.
Not all studies in the medical literature have demonstrated an association between pernio-like/chilblains-like lesions and COVID-19, though. An analysis of 23 patients, with skin eruptions considered associated with SARS-CoV-2 infections (including 21 cases of chilblains) during the first wave of the pandemic found that the antibody and T-cell response in patients with pandemic chilblains was the same as in negative controls.
“What’s remarkably interesting about this study is that they did autopsies of samples from patients who had died prepandemic, so there was no such thing as COVID-19,” said Dr. Fox, who was not involved with the study. “They stained for viral particles in those patients, and they were positive in a subset of patients. This makes me wonder about what the significance of that staining positivity is.”
Yet another group of investigators looked at what was happening with pernio during the waves of COVID in a study of chilblains cases in children in Spain, and found a stronger association between lockdown and cold temperature, which argues against a direct association between pernio and COVID infection.
In Dr. Fox’s experience, COVID toes can recur, especially upon exposure to cold. “What taught me this in real life is a patient who I saw remotely by video,” she recalled. “It was early on in the pandemic. I could not prove he had COVID no matter how hard I tried, but I do think he had COVID toes at that time.” When he later was confirmed to have COVID, “he got pernio in the same exact location as his original suspected COVID toes.”
According to an analysis of long COVID in the skin, based on cases reported to the American Academy of Dermatology–International League of Dermatological Societies registry from April 8 to Oct. 8, 2020, pernio-like lesions lasted a median of 12 days in patients with lab-confirmed COVID-19 and a median of 15 days in those with suspected COVID-19. But almost 7% of the 103 pernio cases were long-haulers, defined as those with dermatologic signs of COVID that lasted beyond 60 days.
“There are some patients who are resistant to treatment,” Dr. Fox said. “In addition, recurrent lesions make me think that maybe all pernio is triggered by some viral cause. This causes an immunologic phenomenon that’s responding to a viral trigger you’re trying to deal with. That may be the better way to think about COVID toes.”
Different variants of COVID also appear to be changing the characteristics of dermatologic manifestations associated with infection. Results from a large retrospective analysis of nearly 350,000 users of a COVID study App in the United Kingdom found that skin lesions were more predictive of a positive test in the Delta wave, compared with the Omicron wave, while pernio-like lesions were predictive of infection in the Delta wave but not in the Omicron wave.
“And, whether you were vaccinated or unvaccinated really did not influence whether or not you were going to have a skin rash as a presenting sign of COVID, except for the burning rash, which was less in vaccinated patients,” said Dr. Fox, who was not involved with the study.
Dr. Fox reported having no relevant disclosures.
PORTLAND, ORE. – while others are not, according to Lindy P. Fox, MD, professor of dermatology and director of the hospital consultation service at the University of California, San Francisco.
“We’re learning a lot about pernio because of COVID,” Dr. Fox, a member of the American Academy of Dermatology’s Ad Hoc Task Force on COVID-19, said at the annual meeting of the Pacific Dermatologic Association. “Patients with pernio tend to either have bright red or purple individual lesions or an erythromelalgia-like presentation, often waking up in the middle of the night saying ‘my feet hurt. I can’t put sheets over my feet.’ In my experience, the patients with an erythromelalgia-like presentation tend to be a lot harder to treat.”
Establishing terminology to describe pernio-like lesions was a challenge in the early stages of the COVID-19 pandemic, Dr. Fox added, with clinicians using terms like erythema multiforme-like, coxsackie-like, or even necrotic to describe the lesions. “I don’t think pernio is truly necrotic; I think it’s really inflammatory and purpuric,” she said.
Early in the pandemic, studies suggesting a link with these cases and COVID-19 infection include a case series of 318 patients with pernio-like skin lesions who had confirmed or suspected COVID-19. Most of these patients were generally young and healthy and most had relatively mild COVID-19; 7% were laboratory-confirmed COVID-19 positive, and 6% were close contacts of patients with confirmed COVID-19. Pernio-like lesions were the only symptoms in 55% of the patients.
In another study, researchers in France evaluated the clinical, laboratory, and pathologic characteristics of 40 patients who developed chilblain-like lesions (mostly involving the toes) during the COVID-19 pandemic and were seen as outpatients in April 2020 . All were polymerase chain reaction (PCR) negative, 30% were SARS-CoV-2 serology positive, and 60% had elevated D-dimers. Histology obtained from 19 of the patients revealed lymphocytic inflammation and vascular damage, and 8 had IgA positivity.
In a retrospective analysis of seven pediatric chilblains cases during the pandemic, researchers examined the skin biopsies to evaluate histopathological features and explored the presence of SARS-CoV-2 in the tissue. All patients were PCR negative. The authors observed cytoplasmic granular positivity for SARS-CoV-2 spike protein in endothelial cells, a feature that they said showed coronavirus-like particles, consistent with SARS-CoV-2.
Not all studies in the medical literature have demonstrated an association between pernio-like/chilblains-like lesions and COVID-19, though. An analysis of 23 patients, with skin eruptions considered associated with SARS-CoV-2 infections (including 21 cases of chilblains) during the first wave of the pandemic found that the antibody and T-cell response in patients with pandemic chilblains was the same as in negative controls.
“What’s remarkably interesting about this study is that they did autopsies of samples from patients who had died prepandemic, so there was no such thing as COVID-19,” said Dr. Fox, who was not involved with the study. “They stained for viral particles in those patients, and they were positive in a subset of patients. This makes me wonder about what the significance of that staining positivity is.”
Yet another group of investigators looked at what was happening with pernio during the waves of COVID in a study of chilblains cases in children in Spain, and found a stronger association between lockdown and cold temperature, which argues against a direct association between pernio and COVID infection.
In Dr. Fox’s experience, COVID toes can recur, especially upon exposure to cold. “What taught me this in real life is a patient who I saw remotely by video,” she recalled. “It was early on in the pandemic. I could not prove he had COVID no matter how hard I tried, but I do think he had COVID toes at that time.” When he later was confirmed to have COVID, “he got pernio in the same exact location as his original suspected COVID toes.”
According to an analysis of long COVID in the skin, based on cases reported to the American Academy of Dermatology–International League of Dermatological Societies registry from April 8 to Oct. 8, 2020, pernio-like lesions lasted a median of 12 days in patients with lab-confirmed COVID-19 and a median of 15 days in those with suspected COVID-19. But almost 7% of the 103 pernio cases were long-haulers, defined as those with dermatologic signs of COVID that lasted beyond 60 days.
“There are some patients who are resistant to treatment,” Dr. Fox said. “In addition, recurrent lesions make me think that maybe all pernio is triggered by some viral cause. This causes an immunologic phenomenon that’s responding to a viral trigger you’re trying to deal with. That may be the better way to think about COVID toes.”
Different variants of COVID also appear to be changing the characteristics of dermatologic manifestations associated with infection. Results from a large retrospective analysis of nearly 350,000 users of a COVID study App in the United Kingdom found that skin lesions were more predictive of a positive test in the Delta wave, compared with the Omicron wave, while pernio-like lesions were predictive of infection in the Delta wave but not in the Omicron wave.
“And, whether you were vaccinated or unvaccinated really did not influence whether or not you were going to have a skin rash as a presenting sign of COVID, except for the burning rash, which was less in vaccinated patients,” said Dr. Fox, who was not involved with the study.
Dr. Fox reported having no relevant disclosures.
PORTLAND, ORE. – while others are not, according to Lindy P. Fox, MD, professor of dermatology and director of the hospital consultation service at the University of California, San Francisco.
“We’re learning a lot about pernio because of COVID,” Dr. Fox, a member of the American Academy of Dermatology’s Ad Hoc Task Force on COVID-19, said at the annual meeting of the Pacific Dermatologic Association. “Patients with pernio tend to either have bright red or purple individual lesions or an erythromelalgia-like presentation, often waking up in the middle of the night saying ‘my feet hurt. I can’t put sheets over my feet.’ In my experience, the patients with an erythromelalgia-like presentation tend to be a lot harder to treat.”
Establishing terminology to describe pernio-like lesions was a challenge in the early stages of the COVID-19 pandemic, Dr. Fox added, with clinicians using terms like erythema multiforme-like, coxsackie-like, or even necrotic to describe the lesions. “I don’t think pernio is truly necrotic; I think it’s really inflammatory and purpuric,” she said.
Early in the pandemic, studies suggesting a link with these cases and COVID-19 infection include a case series of 318 patients with pernio-like skin lesions who had confirmed or suspected COVID-19. Most of these patients were generally young and healthy and most had relatively mild COVID-19; 7% were laboratory-confirmed COVID-19 positive, and 6% were close contacts of patients with confirmed COVID-19. Pernio-like lesions were the only symptoms in 55% of the patients.
In another study, researchers in France evaluated the clinical, laboratory, and pathologic characteristics of 40 patients who developed chilblain-like lesions (mostly involving the toes) during the COVID-19 pandemic and were seen as outpatients in April 2020 . All were polymerase chain reaction (PCR) negative, 30% were SARS-CoV-2 serology positive, and 60% had elevated D-dimers. Histology obtained from 19 of the patients revealed lymphocytic inflammation and vascular damage, and 8 had IgA positivity.
In a retrospective analysis of seven pediatric chilblains cases during the pandemic, researchers examined the skin biopsies to evaluate histopathological features and explored the presence of SARS-CoV-2 in the tissue. All patients were PCR negative. The authors observed cytoplasmic granular positivity for SARS-CoV-2 spike protein in endothelial cells, a feature that they said showed coronavirus-like particles, consistent with SARS-CoV-2.
Not all studies in the medical literature have demonstrated an association between pernio-like/chilblains-like lesions and COVID-19, though. An analysis of 23 patients, with skin eruptions considered associated with SARS-CoV-2 infections (including 21 cases of chilblains) during the first wave of the pandemic found that the antibody and T-cell response in patients with pandemic chilblains was the same as in negative controls.
“What’s remarkably interesting about this study is that they did autopsies of samples from patients who had died prepandemic, so there was no such thing as COVID-19,” said Dr. Fox, who was not involved with the study. “They stained for viral particles in those patients, and they were positive in a subset of patients. This makes me wonder about what the significance of that staining positivity is.”
Yet another group of investigators looked at what was happening with pernio during the waves of COVID in a study of chilblains cases in children in Spain, and found a stronger association between lockdown and cold temperature, which argues against a direct association between pernio and COVID infection.
In Dr. Fox’s experience, COVID toes can recur, especially upon exposure to cold. “What taught me this in real life is a patient who I saw remotely by video,” she recalled. “It was early on in the pandemic. I could not prove he had COVID no matter how hard I tried, but I do think he had COVID toes at that time.” When he later was confirmed to have COVID, “he got pernio in the same exact location as his original suspected COVID toes.”
According to an analysis of long COVID in the skin, based on cases reported to the American Academy of Dermatology–International League of Dermatological Societies registry from April 8 to Oct. 8, 2020, pernio-like lesions lasted a median of 12 days in patients with lab-confirmed COVID-19 and a median of 15 days in those with suspected COVID-19. But almost 7% of the 103 pernio cases were long-haulers, defined as those with dermatologic signs of COVID that lasted beyond 60 days.
“There are some patients who are resistant to treatment,” Dr. Fox said. “In addition, recurrent lesions make me think that maybe all pernio is triggered by some viral cause. This causes an immunologic phenomenon that’s responding to a viral trigger you’re trying to deal with. That may be the better way to think about COVID toes.”
Different variants of COVID also appear to be changing the characteristics of dermatologic manifestations associated with infection. Results from a large retrospective analysis of nearly 350,000 users of a COVID study App in the United Kingdom found that skin lesions were more predictive of a positive test in the Delta wave, compared with the Omicron wave, while pernio-like lesions were predictive of infection in the Delta wave but not in the Omicron wave.
“And, whether you were vaccinated or unvaccinated really did not influence whether or not you were going to have a skin rash as a presenting sign of COVID, except for the burning rash, which was less in vaccinated patients,” said Dr. Fox, who was not involved with the study.
Dr. Fox reported having no relevant disclosures.
AT PDA 2022
Improving Inpatient COVID-19 Vaccination Rates Among Adult Patients at a Tertiary Academic Medical Center
From the Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC.
Abstract
Objective: Inpatient vaccination initiatives are well described in the literature. During the COVID-19 pandemic, hospitals began administering COVID-19 vaccines to hospitalized patients. Although vaccination rates increased, there remained many unvaccinated patients despite community efforts. This quality improvement project aimed to increase the COVID-19 vaccination rates of hospitalized patients on the medicine service at the George Washington University Hospital (GWUH).
Methods: From November 2021 through February 2022, we conducted a Plan-Do-Study-Act (PDSA) cycle with 3 phases. Initial steps included gathering baseline data from the electronic health record and consulting stakeholders. The first 2 phases focused on educating housestaff on the availability, ordering process, and administration of the Pfizer vaccine. The third phase consisted of developing educational pamphlets for patients to be included in their admission packets.
Results: The baseline mean COVID-19 vaccination rate (August to October 2021) of eligible patients on the medicine service was 10.7%. In the months after we implemented the PDSA cycle (November 2021 to February 2022), the mean vaccination rate increased to 15.4%.
Conclusion: This quality improvement project implemented measures to increase administration of the Pfizer vaccine to eligible patients admitted to the medicine service at GWUH. The mean vaccination rate increased from 10.7% in the 3 months prior to implementation to 15.4% during the 4 months post implementation. Other measures to consider in the future include increasing the availability of other COVID-19 vaccines at our hospital and incorporating the vaccine into the admission order set to help facilitate vaccination early in the hospital course.
Keywords: housestaff, quality improvement, PDSA, COVID-19, BNT162b2 vaccine, patient education
Throughout the COVID-19 pandemic, case rates in the United States have fluctuated considerably, corresponding to epidemic waves. In 2021, US daily cases of COVID-19 peaked at nearly 300,000 in early January and reached a nadir of 8000 cases in mid-June.1 In September 2021, new cases had increased to 200,000 per day due to the prevalence of the Delta variant.1 Particularly with the emergence of new variants of SARS-CoV-2, vaccination efforts to limit the spread of infection and severity of illness are critical. Data have shown that 2 doses of the BNT162b2 vaccine (Pfizer-BioNTech) were largely protective against severe infection for approximately 6 months.2,3 When we began this quality improvement (QI) project in September 2021, only 179 million Americans had been fully vaccinated, according to data from the Centers for Disease Control and Prevention, which is just over half of the US population.4 An electronic survey conducted in the United States with more than 5 million responses found that, of those who were hesitant about receiving the vaccine, 49% reported a fear of adverse effects and 48% reported a lack of trust in the vaccine.5
This QI project sought to target unvaccinated individuals admitted to the internal medicine inpatient service. Vaccinating hospitalized patients is especially important since they are sicker than the general population and at higher risk of having poor outcomes from COVID-19. Inpatient vaccine initiatives, such as administering influenza vaccine prior to discharge, have been successfully implemented in the past.6 One large COVID-19 vaccination program featured an admission order set to increase the rates of vaccination among hospitalized patients.7 Our QI project piloted a multidisciplinary approach involving the nursing staff, pharmacy, information technology (IT) department, and internal medicine housestaff to increase COVID-19 vaccination rates among hospitalized patients on the medical service. This project aimed to increase inpatient vaccination rates through interventions targeting both primary providers as well as the patients themselves.
Methods
Setting and Interventions
This project was conducted at the George Washington University Hospital (GWUH) in Washington, DC. The clinicians involved in the study were the internal medicine housestaff, and the patients included were adults admitted to the resident medicine ward teams. The project was exempt by the institutional review board and did not require informed consent.
The quality improvement initiative had 3 phases, each featuring a different intervention (Table 1). The first phase involved sending a weekly announcement (via email and a secure health care messaging app) to current residents rotating on the inpatient medicine service. The announcement contained information regarding COVID-19 vaccine availability at the hospital, instructions on ordering the vaccine, and the process of coordinating with pharmacy to facilitate vaccine administration. Thereafter, residents were educated on the process of giving a COVID-19 vaccine to a patient from start to finish. Due to the nature of the residency schedule, different housestaff members rotated in and out of the medicine wards during the intervention periods. The weekly email was sent to the entire internal medicine housestaff, informing all residents about the QI project, while the weekly secure messages served as reminders and were only sent to residents currently on the medicine wards.
In the second phase, we posted paper flyers throughout the hospital to remind housestaff to give the vaccine and again educate them on the process of ordering the vaccine. For the third intervention, a COVID-19 vaccine educational pamphlet was developed for distribution to inpatients at GWUH. The pamphlet included information on vaccine efficacy, safety, side effects, and eligibility. The pamphlet was incorporated in the admission packet that every patient receives upon admission to the hospital. The patients reviewed the pamphlets with nursing staff, who would answer any questions, with residents available to discuss any outstanding concerns.
Measures and Data Gathering
The primary endpoint of the study was inpatient vaccination rate, defined as the number of COVID-19 vaccines administered divided by the number of patients eligible to receive a vaccine (not fully vaccinated). During initial triage, nursing staff documented vaccination status in the electronic health record (EHR), checking a box in a data entry form if a patient had received 0, 1, or 2 doses of the COVID-19 vaccine. The GWUH IT department generated data from this form to determine the number of patients eligible to receive a COVID-19 vaccine. Data were extracted from the medication administration record in the EHR to determine the number of vaccines that were administered to patients during their hospitalization on the inpatient medical service. Each month, the IT department extracted data for the number of eligible patients and the number of vaccines administered. This yielded the monthly vaccination rates. The monthly vaccination rates in the period prior to starting the QI initiative were compared to the rates in the period after the interventions were implemented.
Of note, during the course of this project, patients became eligible for a third COVID-19 vaccine (booster). We decided to continue with the original aim of vaccinating adults who had only received 0 or 1 dose of the vaccine. Therefore, the eligibility criteria remained the same throughout the study. We obtained retrospective data to ensure that the vaccines being counted toward the vaccination rate were vaccines given to patients not yet fully vaccinated and not vaccines given as boosters.
Results
From August to October 2021, the baseline average monthly vaccination rate of patients on the medicine service who were eligible to receive a COVID-19 vaccine was 10.7%. After the first intervention, the vaccination rate increased to 19.7% in November 2021 (Table 2). The second intervention yielded vaccination rates of 11.4% and 11.8% in December 2021 and January 2022, respectively. During the final phase in February 2022, the vaccination rate was 19.0%. At the conclusion of the study, the mean vaccination rate for the intervention months was 15.4% (Figure 1). Process stability and variation are demonstrated with a statistical process control chart (Figure 2).
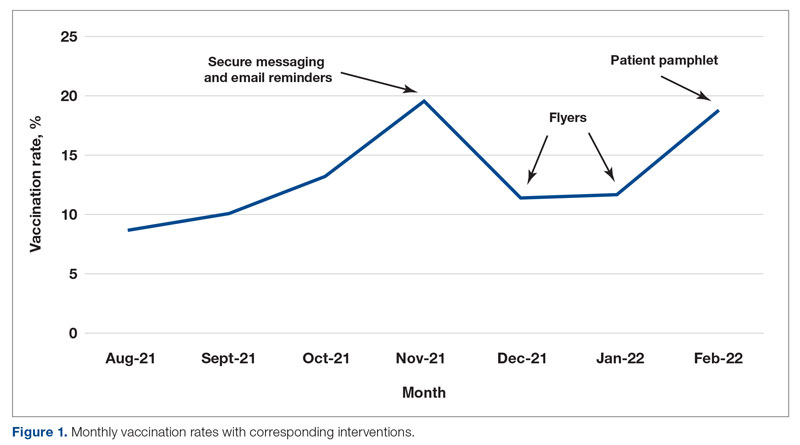
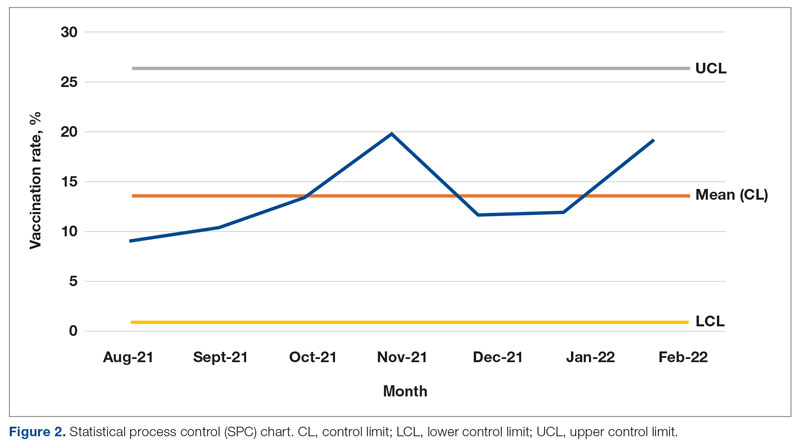
Discussion
For this housestaff-driven QI project, we implemented an inpatient COVID-19 vaccination campaign consisting of 3 phases that targeted both providers and patients. During the intervention period, we observed an increased vaccination rate compared to the period just prior to implementation of the QI project. While our interventions may certainly have boosted vaccination rates, we understand other variables could have contributed to increased rates as well. The emergence of variants in the United States, such as omicron in December 2021,8 could have precipitated a demand for vaccinations among patients. Holidays in November and December may also have increased patients’ desire to get vaccinated before travel.
We encountered a number of roadblocks that challenged our project, including difficulty identifying patients who were eligible for the vaccine, logistical vaccine administration challenges, and hesitancy among the inpatient population. Accurately identifying patients who were eligible for a vaccine in the EHR was especially challenging in the setting of rapidly changing guidelines regarding COVID-19 vaccination. In September 2021, the US Food and Drug Administration authorized the Pfizer booster for certain populations and later, in November 2021, for all adults. This meant that some fully vaccinated hospitalized patients (those with 2 doses) then qualified for an additional dose of the vaccine and received a dose during hospitalization. To determine the true vaccination rate, we obtained retrospective data that allowed us to track each vaccine administered. If a patient had already received 2 doses of the COVID-19 vaccine, the vaccine administered was counted as a booster and excluded from the calculation of the vaccination rate. Future PDSA cycles could include updating the EHR to capture the whole range of COVID-19 vaccination status (unvaccinated, partially vaccinated, fully vaccinated, fully vaccinated with 1 booster, fully vaccinated with 2 boosters).
We also encountered logistical challenges with the administration of the COVID-19 vaccine to hospitalized patients. During the intervention period, our pharmacy department required 5 COVID-19 vaccination orders before opening a vial and administering the vaccine doses in order to reduce waste. This policy may have limited our ability to vaccinate eligible inpatients because we were not always able to identify 5 patients simultaneously on the service who were eligible and consented to the vaccine.
The majority of patients who were interested in receiving COVID-19 vaccination had already been vaccinated in the outpatient setting. This fact made the inpatient internal medicine subset of patients a particularly challenging population to target, given their possible hesitancy regarding vaccination. By utilizing a multidisciplinary team and increasing communication of providers and nursing staff, we helped to increase the COVID-19 vaccination rates at our hospital from 10.7% to 15.4%.
Future Directions
Future interventions to consider include increasing the availability of other approved COVID-19 vaccines at our hospital besides the Pfizer-BioNTech vaccine. Furthermore, incorporating the vaccine into the admission order set would help initiate the vaccination process early in the hospital course. We encourage other institutions to utilize similar approaches to not only remind providers about inpatient vaccination, but also educate and encourage patients to receive the vaccine. These measures will help institutions increase inpatient COVID-19 vaccination rates in a high-risk population.
Corresponding author: Anna Rubin, MD, Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC; [email protected]
Disclosures: None reported.
1. Trends in number of COVID-19 cases and deaths in the US reported to CDC, by state/territory. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#trends_dailycases
2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162B2 MRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/nejmoa2034577
3. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-COV-2 after covid-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207-1220. doi:10.1056/nejmoa2118691
4. Trends in number of COVID-19 vaccinations in the US. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#vaccination-trends_vacctrends-fully-cum
5. King WC, Rubinstein M, Reinhart A, Mejia R. Time trends, factors associated with, and reasons for covid-19 vaccine hesitancy: A massive online survey of US adults from January-May 2021. PLOS ONE. 2021;16(12). doi:10.1371/journal.pone.0260731
6. Cohen ES, Ogrinc G, Taylor T, et al. Influenza vaccination rates for hospitalised patients: A multiyear quality improvement effort. BMJ Qual Saf. 2015;24(3):221-227. doi:10.1136/bmjqs-2014-003556
7. Berger RE, Diaz DC, Chacko S, et al. Implementation of an inpatient covid-19 vaccination program. NEJM Catalyst. 2021;2(10). doi:10.1056/cat.21.0235
8. CDC COVID-19 Response Team. SARS-CoV-2 B.1.1.529 (Omicron) Variant - United States, December 1-8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(50):1731-1734. doi:10.15585/mmwr.mm7050e1
From the Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC.
Abstract
Objective: Inpatient vaccination initiatives are well described in the literature. During the COVID-19 pandemic, hospitals began administering COVID-19 vaccines to hospitalized patients. Although vaccination rates increased, there remained many unvaccinated patients despite community efforts. This quality improvement project aimed to increase the COVID-19 vaccination rates of hospitalized patients on the medicine service at the George Washington University Hospital (GWUH).
Methods: From November 2021 through February 2022, we conducted a Plan-Do-Study-Act (PDSA) cycle with 3 phases. Initial steps included gathering baseline data from the electronic health record and consulting stakeholders. The first 2 phases focused on educating housestaff on the availability, ordering process, and administration of the Pfizer vaccine. The third phase consisted of developing educational pamphlets for patients to be included in their admission packets.
Results: The baseline mean COVID-19 vaccination rate (August to October 2021) of eligible patients on the medicine service was 10.7%. In the months after we implemented the PDSA cycle (November 2021 to February 2022), the mean vaccination rate increased to 15.4%.
Conclusion: This quality improvement project implemented measures to increase administration of the Pfizer vaccine to eligible patients admitted to the medicine service at GWUH. The mean vaccination rate increased from 10.7% in the 3 months prior to implementation to 15.4% during the 4 months post implementation. Other measures to consider in the future include increasing the availability of other COVID-19 vaccines at our hospital and incorporating the vaccine into the admission order set to help facilitate vaccination early in the hospital course.
Keywords: housestaff, quality improvement, PDSA, COVID-19, BNT162b2 vaccine, patient education
Throughout the COVID-19 pandemic, case rates in the United States have fluctuated considerably, corresponding to epidemic waves. In 2021, US daily cases of COVID-19 peaked at nearly 300,000 in early January and reached a nadir of 8000 cases in mid-June.1 In September 2021, new cases had increased to 200,000 per day due to the prevalence of the Delta variant.1 Particularly with the emergence of new variants of SARS-CoV-2, vaccination efforts to limit the spread of infection and severity of illness are critical. Data have shown that 2 doses of the BNT162b2 vaccine (Pfizer-BioNTech) were largely protective against severe infection for approximately 6 months.2,3 When we began this quality improvement (QI) project in September 2021, only 179 million Americans had been fully vaccinated, according to data from the Centers for Disease Control and Prevention, which is just over half of the US population.4 An electronic survey conducted in the United States with more than 5 million responses found that, of those who were hesitant about receiving the vaccine, 49% reported a fear of adverse effects and 48% reported a lack of trust in the vaccine.5
This QI project sought to target unvaccinated individuals admitted to the internal medicine inpatient service. Vaccinating hospitalized patients is especially important since they are sicker than the general population and at higher risk of having poor outcomes from COVID-19. Inpatient vaccine initiatives, such as administering influenza vaccine prior to discharge, have been successfully implemented in the past.6 One large COVID-19 vaccination program featured an admission order set to increase the rates of vaccination among hospitalized patients.7 Our QI project piloted a multidisciplinary approach involving the nursing staff, pharmacy, information technology (IT) department, and internal medicine housestaff to increase COVID-19 vaccination rates among hospitalized patients on the medical service. This project aimed to increase inpatient vaccination rates through interventions targeting both primary providers as well as the patients themselves.
Methods
Setting and Interventions
This project was conducted at the George Washington University Hospital (GWUH) in Washington, DC. The clinicians involved in the study were the internal medicine housestaff, and the patients included were adults admitted to the resident medicine ward teams. The project was exempt by the institutional review board and did not require informed consent.
The quality improvement initiative had 3 phases, each featuring a different intervention (Table 1). The first phase involved sending a weekly announcement (via email and a secure health care messaging app) to current residents rotating on the inpatient medicine service. The announcement contained information regarding COVID-19 vaccine availability at the hospital, instructions on ordering the vaccine, and the process of coordinating with pharmacy to facilitate vaccine administration. Thereafter, residents were educated on the process of giving a COVID-19 vaccine to a patient from start to finish. Due to the nature of the residency schedule, different housestaff members rotated in and out of the medicine wards during the intervention periods. The weekly email was sent to the entire internal medicine housestaff, informing all residents about the QI project, while the weekly secure messages served as reminders and were only sent to residents currently on the medicine wards.

In the second phase, we posted paper flyers throughout the hospital to remind housestaff to give the vaccine and again educate them on the process of ordering the vaccine. For the third intervention, a COVID-19 vaccine educational pamphlet was developed for distribution to inpatients at GWUH. The pamphlet included information on vaccine efficacy, safety, side effects, and eligibility. The pamphlet was incorporated in the admission packet that every patient receives upon admission to the hospital. The patients reviewed the pamphlets with nursing staff, who would answer any questions, with residents available to discuss any outstanding concerns.
Measures and Data Gathering
The primary endpoint of the study was inpatient vaccination rate, defined as the number of COVID-19 vaccines administered divided by the number of patients eligible to receive a vaccine (not fully vaccinated). During initial triage, nursing staff documented vaccination status in the electronic health record (EHR), checking a box in a data entry form if a patient had received 0, 1, or 2 doses of the COVID-19 vaccine. The GWUH IT department generated data from this form to determine the number of patients eligible to receive a COVID-19 vaccine. Data were extracted from the medication administration record in the EHR to determine the number of vaccines that were administered to patients during their hospitalization on the inpatient medical service. Each month, the IT department extracted data for the number of eligible patients and the number of vaccines administered. This yielded the monthly vaccination rates. The monthly vaccination rates in the period prior to starting the QI initiative were compared to the rates in the period after the interventions were implemented.
Of note, during the course of this project, patients became eligible for a third COVID-19 vaccine (booster). We decided to continue with the original aim of vaccinating adults who had only received 0 or 1 dose of the vaccine. Therefore, the eligibility criteria remained the same throughout the study. We obtained retrospective data to ensure that the vaccines being counted toward the vaccination rate were vaccines given to patients not yet fully vaccinated and not vaccines given as boosters.

Results
From August to October 2021, the baseline average monthly vaccination rate of patients on the medicine service who were eligible to receive a COVID-19 vaccine was 10.7%. After the first intervention, the vaccination rate increased to 19.7% in November 2021 (Table 2). The second intervention yielded vaccination rates of 11.4% and 11.8% in December 2021 and January 2022, respectively. During the final phase in February 2022, the vaccination rate was 19.0%. At the conclusion of the study, the mean vaccination rate for the intervention months was 15.4% (Figure 1). Process stability and variation are demonstrated with a statistical process control chart (Figure 2).


Discussion
For this housestaff-driven QI project, we implemented an inpatient COVID-19 vaccination campaign consisting of 3 phases that targeted both providers and patients. During the intervention period, we observed an increased vaccination rate compared to the period just prior to implementation of the QI project. While our interventions may certainly have boosted vaccination rates, we understand other variables could have contributed to increased rates as well. The emergence of variants in the United States, such as omicron in December 2021,8 could have precipitated a demand for vaccinations among patients. Holidays in November and December may also have increased patients’ desire to get vaccinated before travel.
We encountered a number of roadblocks that challenged our project, including difficulty identifying patients who were eligible for the vaccine, logistical vaccine administration challenges, and hesitancy among the inpatient population. Accurately identifying patients who were eligible for a vaccine in the EHR was especially challenging in the setting of rapidly changing guidelines regarding COVID-19 vaccination. In September 2021, the US Food and Drug Administration authorized the Pfizer booster for certain populations and later, in November 2021, for all adults. This meant that some fully vaccinated hospitalized patients (those with 2 doses) then qualified for an additional dose of the vaccine and received a dose during hospitalization. To determine the true vaccination rate, we obtained retrospective data that allowed us to track each vaccine administered. If a patient had already received 2 doses of the COVID-19 vaccine, the vaccine administered was counted as a booster and excluded from the calculation of the vaccination rate. Future PDSA cycles could include updating the EHR to capture the whole range of COVID-19 vaccination status (unvaccinated, partially vaccinated, fully vaccinated, fully vaccinated with 1 booster, fully vaccinated with 2 boosters).
We also encountered logistical challenges with the administration of the COVID-19 vaccine to hospitalized patients. During the intervention period, our pharmacy department required 5 COVID-19 vaccination orders before opening a vial and administering the vaccine doses in order to reduce waste. This policy may have limited our ability to vaccinate eligible inpatients because we were not always able to identify 5 patients simultaneously on the service who were eligible and consented to the vaccine.
The majority of patients who were interested in receiving COVID-19 vaccination had already been vaccinated in the outpatient setting. This fact made the inpatient internal medicine subset of patients a particularly challenging population to target, given their possible hesitancy regarding vaccination. By utilizing a multidisciplinary team and increasing communication of providers and nursing staff, we helped to increase the COVID-19 vaccination rates at our hospital from 10.7% to 15.4%.
Future Directions
Future interventions to consider include increasing the availability of other approved COVID-19 vaccines at our hospital besides the Pfizer-BioNTech vaccine. Furthermore, incorporating the vaccine into the admission order set would help initiate the vaccination process early in the hospital course. We encourage other institutions to utilize similar approaches to not only remind providers about inpatient vaccination, but also educate and encourage patients to receive the vaccine. These measures will help institutions increase inpatient COVID-19 vaccination rates in a high-risk population.
Corresponding author: Anna Rubin, MD, Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC; [email protected]
Disclosures: None reported.
From the Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC.
Abstract
Objective: Inpatient vaccination initiatives are well described in the literature. During the COVID-19 pandemic, hospitals began administering COVID-19 vaccines to hospitalized patients. Although vaccination rates increased, there remained many unvaccinated patients despite community efforts. This quality improvement project aimed to increase the COVID-19 vaccination rates of hospitalized patients on the medicine service at the George Washington University Hospital (GWUH).
Methods: From November 2021 through February 2022, we conducted a Plan-Do-Study-Act (PDSA) cycle with 3 phases. Initial steps included gathering baseline data from the electronic health record and consulting stakeholders. The first 2 phases focused on educating housestaff on the availability, ordering process, and administration of the Pfizer vaccine. The third phase consisted of developing educational pamphlets for patients to be included in their admission packets.
Results: The baseline mean COVID-19 vaccination rate (August to October 2021) of eligible patients on the medicine service was 10.7%. In the months after we implemented the PDSA cycle (November 2021 to February 2022), the mean vaccination rate increased to 15.4%.
Conclusion: This quality improvement project implemented measures to increase administration of the Pfizer vaccine to eligible patients admitted to the medicine service at GWUH. The mean vaccination rate increased from 10.7% in the 3 months prior to implementation to 15.4% during the 4 months post implementation. Other measures to consider in the future include increasing the availability of other COVID-19 vaccines at our hospital and incorporating the vaccine into the admission order set to help facilitate vaccination early in the hospital course.
Keywords: housestaff, quality improvement, PDSA, COVID-19, BNT162b2 vaccine, patient education
Throughout the COVID-19 pandemic, case rates in the United States have fluctuated considerably, corresponding to epidemic waves. In 2021, US daily cases of COVID-19 peaked at nearly 300,000 in early January and reached a nadir of 8000 cases in mid-June.1 In September 2021, new cases had increased to 200,000 per day due to the prevalence of the Delta variant.1 Particularly with the emergence of new variants of SARS-CoV-2, vaccination efforts to limit the spread of infection and severity of illness are critical. Data have shown that 2 doses of the BNT162b2 vaccine (Pfizer-BioNTech) were largely protective against severe infection for approximately 6 months.2,3 When we began this quality improvement (QI) project in September 2021, only 179 million Americans had been fully vaccinated, according to data from the Centers for Disease Control and Prevention, which is just over half of the US population.4 An electronic survey conducted in the United States with more than 5 million responses found that, of those who were hesitant about receiving the vaccine, 49% reported a fear of adverse effects and 48% reported a lack of trust in the vaccine.5
This QI project sought to target unvaccinated individuals admitted to the internal medicine inpatient service. Vaccinating hospitalized patients is especially important since they are sicker than the general population and at higher risk of having poor outcomes from COVID-19. Inpatient vaccine initiatives, such as administering influenza vaccine prior to discharge, have been successfully implemented in the past.6 One large COVID-19 vaccination program featured an admission order set to increase the rates of vaccination among hospitalized patients.7 Our QI project piloted a multidisciplinary approach involving the nursing staff, pharmacy, information technology (IT) department, and internal medicine housestaff to increase COVID-19 vaccination rates among hospitalized patients on the medical service. This project aimed to increase inpatient vaccination rates through interventions targeting both primary providers as well as the patients themselves.
Methods
Setting and Interventions
This project was conducted at the George Washington University Hospital (GWUH) in Washington, DC. The clinicians involved in the study were the internal medicine housestaff, and the patients included were adults admitted to the resident medicine ward teams. The project was exempt by the institutional review board and did not require informed consent.
The quality improvement initiative had 3 phases, each featuring a different intervention (Table 1). The first phase involved sending a weekly announcement (via email and a secure health care messaging app) to current residents rotating on the inpatient medicine service. The announcement contained information regarding COVID-19 vaccine availability at the hospital, instructions on ordering the vaccine, and the process of coordinating with pharmacy to facilitate vaccine administration. Thereafter, residents were educated on the process of giving a COVID-19 vaccine to a patient from start to finish. Due to the nature of the residency schedule, different housestaff members rotated in and out of the medicine wards during the intervention periods. The weekly email was sent to the entire internal medicine housestaff, informing all residents about the QI project, while the weekly secure messages served as reminders and were only sent to residents currently on the medicine wards.

In the second phase, we posted paper flyers throughout the hospital to remind housestaff to give the vaccine and again educate them on the process of ordering the vaccine. For the third intervention, a COVID-19 vaccine educational pamphlet was developed for distribution to inpatients at GWUH. The pamphlet included information on vaccine efficacy, safety, side effects, and eligibility. The pamphlet was incorporated in the admission packet that every patient receives upon admission to the hospital. The patients reviewed the pamphlets with nursing staff, who would answer any questions, with residents available to discuss any outstanding concerns.
Measures and Data Gathering
The primary endpoint of the study was inpatient vaccination rate, defined as the number of COVID-19 vaccines administered divided by the number of patients eligible to receive a vaccine (not fully vaccinated). During initial triage, nursing staff documented vaccination status in the electronic health record (EHR), checking a box in a data entry form if a patient had received 0, 1, or 2 doses of the COVID-19 vaccine. The GWUH IT department generated data from this form to determine the number of patients eligible to receive a COVID-19 vaccine. Data were extracted from the medication administration record in the EHR to determine the number of vaccines that were administered to patients during their hospitalization on the inpatient medical service. Each month, the IT department extracted data for the number of eligible patients and the number of vaccines administered. This yielded the monthly vaccination rates. The monthly vaccination rates in the period prior to starting the QI initiative were compared to the rates in the period after the interventions were implemented.
Of note, during the course of this project, patients became eligible for a third COVID-19 vaccine (booster). We decided to continue with the original aim of vaccinating adults who had only received 0 or 1 dose of the vaccine. Therefore, the eligibility criteria remained the same throughout the study. We obtained retrospective data to ensure that the vaccines being counted toward the vaccination rate were vaccines given to patients not yet fully vaccinated and not vaccines given as boosters.

Results
From August to October 2021, the baseline average monthly vaccination rate of patients on the medicine service who were eligible to receive a COVID-19 vaccine was 10.7%. After the first intervention, the vaccination rate increased to 19.7% in November 2021 (Table 2). The second intervention yielded vaccination rates of 11.4% and 11.8% in December 2021 and January 2022, respectively. During the final phase in February 2022, the vaccination rate was 19.0%. At the conclusion of the study, the mean vaccination rate for the intervention months was 15.4% (Figure 1). Process stability and variation are demonstrated with a statistical process control chart (Figure 2).


Discussion
For this housestaff-driven QI project, we implemented an inpatient COVID-19 vaccination campaign consisting of 3 phases that targeted both providers and patients. During the intervention period, we observed an increased vaccination rate compared to the period just prior to implementation of the QI project. While our interventions may certainly have boosted vaccination rates, we understand other variables could have contributed to increased rates as well. The emergence of variants in the United States, such as omicron in December 2021,8 could have precipitated a demand for vaccinations among patients. Holidays in November and December may also have increased patients’ desire to get vaccinated before travel.
We encountered a number of roadblocks that challenged our project, including difficulty identifying patients who were eligible for the vaccine, logistical vaccine administration challenges, and hesitancy among the inpatient population. Accurately identifying patients who were eligible for a vaccine in the EHR was especially challenging in the setting of rapidly changing guidelines regarding COVID-19 vaccination. In September 2021, the US Food and Drug Administration authorized the Pfizer booster for certain populations and later, in November 2021, for all adults. This meant that some fully vaccinated hospitalized patients (those with 2 doses) then qualified for an additional dose of the vaccine and received a dose during hospitalization. To determine the true vaccination rate, we obtained retrospective data that allowed us to track each vaccine administered. If a patient had already received 2 doses of the COVID-19 vaccine, the vaccine administered was counted as a booster and excluded from the calculation of the vaccination rate. Future PDSA cycles could include updating the EHR to capture the whole range of COVID-19 vaccination status (unvaccinated, partially vaccinated, fully vaccinated, fully vaccinated with 1 booster, fully vaccinated with 2 boosters).
We also encountered logistical challenges with the administration of the COVID-19 vaccine to hospitalized patients. During the intervention period, our pharmacy department required 5 COVID-19 vaccination orders before opening a vial and administering the vaccine doses in order to reduce waste. This policy may have limited our ability to vaccinate eligible inpatients because we were not always able to identify 5 patients simultaneously on the service who were eligible and consented to the vaccine.
The majority of patients who were interested in receiving COVID-19 vaccination had already been vaccinated in the outpatient setting. This fact made the inpatient internal medicine subset of patients a particularly challenging population to target, given their possible hesitancy regarding vaccination. By utilizing a multidisciplinary team and increasing communication of providers and nursing staff, we helped to increase the COVID-19 vaccination rates at our hospital from 10.7% to 15.4%.
Future Directions
Future interventions to consider include increasing the availability of other approved COVID-19 vaccines at our hospital besides the Pfizer-BioNTech vaccine. Furthermore, incorporating the vaccine into the admission order set would help initiate the vaccination process early in the hospital course. We encourage other institutions to utilize similar approaches to not only remind providers about inpatient vaccination, but also educate and encourage patients to receive the vaccine. These measures will help institutions increase inpatient COVID-19 vaccination rates in a high-risk population.
Corresponding author: Anna Rubin, MD, Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC; [email protected]
Disclosures: None reported.
1. Trends in number of COVID-19 cases and deaths in the US reported to CDC, by state/territory. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#trends_dailycases
2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162B2 MRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/nejmoa2034577
3. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-COV-2 after covid-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207-1220. doi:10.1056/nejmoa2118691
4. Trends in number of COVID-19 vaccinations in the US. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#vaccination-trends_vacctrends-fully-cum
5. King WC, Rubinstein M, Reinhart A, Mejia R. Time trends, factors associated with, and reasons for covid-19 vaccine hesitancy: A massive online survey of US adults from January-May 2021. PLOS ONE. 2021;16(12). doi:10.1371/journal.pone.0260731
6. Cohen ES, Ogrinc G, Taylor T, et al. Influenza vaccination rates for hospitalised patients: A multiyear quality improvement effort. BMJ Qual Saf. 2015;24(3):221-227. doi:10.1136/bmjqs-2014-003556
7. Berger RE, Diaz DC, Chacko S, et al. Implementation of an inpatient covid-19 vaccination program. NEJM Catalyst. 2021;2(10). doi:10.1056/cat.21.0235
8. CDC COVID-19 Response Team. SARS-CoV-2 B.1.1.529 (Omicron) Variant - United States, December 1-8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(50):1731-1734. doi:10.15585/mmwr.mm7050e1
1. Trends in number of COVID-19 cases and deaths in the US reported to CDC, by state/territory. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#trends_dailycases
2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162B2 MRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/nejmoa2034577
3. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-COV-2 after covid-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207-1220. doi:10.1056/nejmoa2118691
4. Trends in number of COVID-19 vaccinations in the US. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#vaccination-trends_vacctrends-fully-cum
5. King WC, Rubinstein M, Reinhart A, Mejia R. Time trends, factors associated with, and reasons for covid-19 vaccine hesitancy: A massive online survey of US adults from January-May 2021. PLOS ONE. 2021;16(12). doi:10.1371/journal.pone.0260731
6. Cohen ES, Ogrinc G, Taylor T, et al. Influenza vaccination rates for hospitalised patients: A multiyear quality improvement effort. BMJ Qual Saf. 2015;24(3):221-227. doi:10.1136/bmjqs-2014-003556
7. Berger RE, Diaz DC, Chacko S, et al. Implementation of an inpatient covid-19 vaccination program. NEJM Catalyst. 2021;2(10). doi:10.1056/cat.21.0235
8. CDC COVID-19 Response Team. SARS-CoV-2 B.1.1.529 (Omicron) Variant - United States, December 1-8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(50):1731-1734. doi:10.15585/mmwr.mm7050e1
Diabetes Population Health Innovations in the Age of COVID-19: Insights From the T1D Exchange Quality Improvement Collaborative
From the T1D Exchange, Boston, MA (Ann Mungmode, Nicole Rioles, Jesse Cases, Dr. Ebekozien); The Leona M. and Harry B. Hemsley Charitable Trust, New York, NY (Laurel Koester); and the University of Mississippi School of Population Health, Jackson, MS (Dr. Ebekozien).
Abstract
There have been remarkable innovations in diabetes management since the start of the COVID-19 pandemic, but these groundbreaking innovations are drawing limited focus as the field focuses on the adverse impact of the pandemic on patients with diabetes. This article reviews select population health innovations in diabetes management that have become available over the past 2 years of the COVID-19 pandemic from the perspective of the T1D Exchange Quality Improvement Collaborative, a learning health network that focuses on improving care and outcomes for individuals with type 1 diabetes (T1D). Such innovations include expanded telemedicine access, collection of real-world data, machine learning and artificial intelligence, and new diabetes medications and devices. In addition, multiple innovative studies have been undertaken to explore contributors to health inequities in diabetes, and advocacy efforts for specific populations have been successful. Looking to the future, work is required to explore additional health equity successes that do not further exacerbate inequities and to look for additional innovative ways to engage people with T1D in their health care through conversations on social determinants of health and societal structures.
Keywords: type 1 diabetes, learning health network, continuous glucose monitoring, health equity
One in 10 people in the United States has diabetes.1 Diabetes is the nation’s second leading cause of death, costing the US health system more than $300 billion annually.2 The COVID-19 pandemic presented additional health burdens for people living with diabetes. For example, preexisting diabetes was identified as a risk factor for COVID-19–associated morbidity and mortality.3,4 Over the past 2 years, there have been remarkable innovations in diabetes management, including stem cell therapy and new medication options. Additionally, improved technology solutions have aided in diabetes management through continuous glucose monitors (CGM), smart insulin pens, advanced hybrid closed-loop systems, and continuous subcutaneous insulin injections.5,6 Unfortunately, these groundbreaking innovations are drawing limited focus, as the field is rightfully focused on the adverse impact of the pandemic on patients with diabetes.
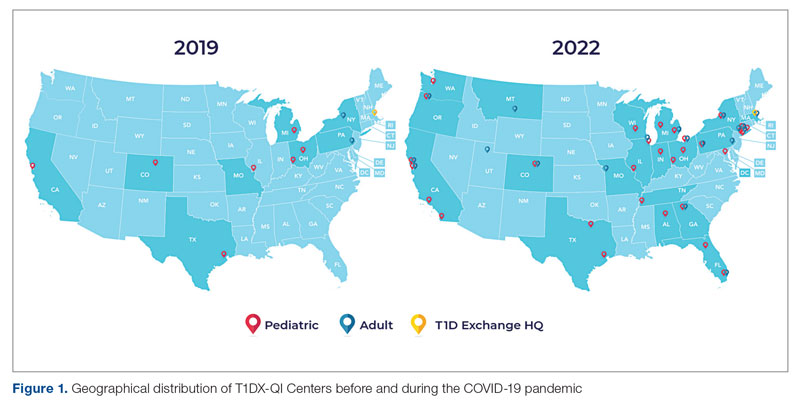
Learning health networks like the T1D Exchange Quality Improvement Collaborative (T1DX-QI) have implemented some of these innovative solutions to improve care for people with diabetes.7 T1DX-QI has more than 50 data-sharing endocrinology centers that care for over 75,000 people with diabetes across the United States (Figure 1). Centers participating in the T1DX-QI use quality improvement (QI) and implementation science methods to quickly translate research into evidence-based clinical practice. T1DX-QI leads diabetes population health and health system research and supports widespread transferability across health care organizations through regular collaborative calls, conferences, and case study documentation.8
In this review, we summarize impactful population health innovations in diabetes management that have become available over the past 2 years of the COVID-19 pandemic from the perspective of T1DX-QI (see Figure 2 for relevant definitions). This review is limited in scope and is not meant to be an exhaustive list of innovations. The review also reflects significant changes from the perspective of academic diabetes centers, which may not apply to rural or primary care diabetes practices.
Methods
The first (A.M.), second (H.H.), and senior (O.E.) authors conducted a scoping review of published literature using terms related to diabetes, population health, and innovation on PubMed Central and Google Scholar for the period March 2020 to June 2022. To complement the review, A.M. and O.E. also reviewed abstracts from presentations at major international diabetes conferences, including the American Diabetes Association (ADA), the International Society for Pediatric and Adolescent Diabetes (ISPAD), the T1DX-QI Learning Session Conference, and the Advanced Technologies & Treatments for Diabetes (ATTD) 2020 to 2022 conferences.9-14 The authors also searched FDA.gov and ClinicalTrials.gov for relevant insights. A.M. and O.E. sorted the reviewed literature into major themes (Figure 3) from the population health improvement perspective of the T1DX-QI.
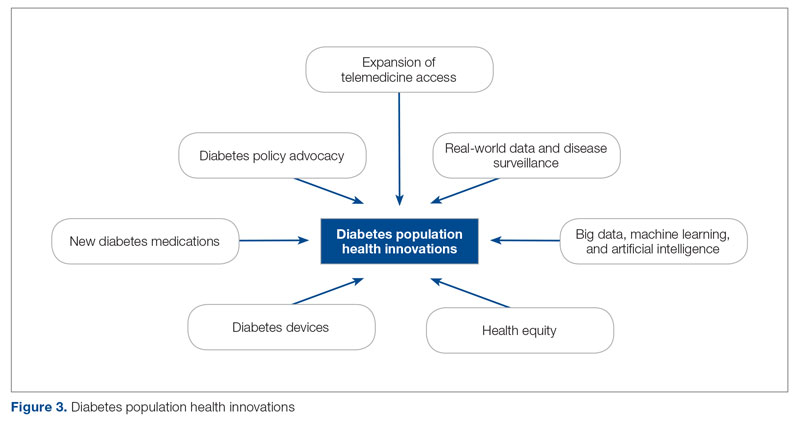
Population Health Innovations in Diabetes Management
Expansion of Telemedicine Access
Telemedicine is cost-effective for patients with diabetes,15 including those with complex cases.16 Before the COVID-19 pandemic, telemedicine and virtual care were rare in diabetes management. However, the pandemic offered a new opportunity to expand the practice of telemedicine in diabetes management. A study from the T1DX-QI showed that telemedicine visits grew from comprising <1% of visits pre-pandemic (December 2019) to 95.2% during the pandemic (August 2020).17 Additional studies, like those conducted by Phillip et al,18 confirmed the noninferiority of telemedicine practice for patients with diabetes.Telemedicine was also found to be an effective strategy to educate patients on the use of diabetes technologies.19
Real-World Data and Disease Surveillance
As the COVID-19 pandemic exacerbated outcomes for people with type 1 diabetes (T1D), a need arose to understand the immediate effects of the pandemic on people with T1D through real-world data and disease surveillance. In April 2020, the T1DX-QI initiated a multicenter surveillance study to collect data and analyze the impact of COVID-19 on people with T1D. The existing health collaborative served as a springboard for robust surveillance study, documenting numerous works on the effects of COVID-19.3,4,20-28 Other investigators also embraced the power of real-world surveillance and real-world data.29,30
Big Data, Machine Learning, and Artificial Intelligence
The past 2 years have seen a shift toward embracing the incredible opportunity to tap the large volume of data generated from routine care for practical insights.31 In particular, researchers have demonstrated the widespread application of machine learning and artificial intelligence to improve diabetes management.32 The T1DX-QI also harnessed the growing power of big data by expanding the functionality of innovative benchmarking software. The T1DX QI Portal uses electronic medical record data of diabetes patients for clinic-to-clinic benchmarking and data analysis, using business intelligence solutions.33
Health Equity
While inequities across various health outcomes have been well documented for years,34 the COVID-19 pandemic further exaggerated racial/ethnic health inequities in T1D.23,35 In response, several organizations have outlined specific strategies to address these health inequities. Emboldened by the pandemic, the T1DX-QI announced a multipronged approach to address health inequities among patients with T1D through the Health Equity Advancement Lab (HEAL).36 One of HEAL’s main components is using real-world data to champion population-level insights and demonstrate progress in QI efforts.
Multiple innovative studies have been undertaken to explore contributors to health inequities in diabetes, and these studies are expanding our understanding of the chasm.37 There have also been innovative solutions to addressing these inequities, with multiple studies published over the past 2 years.38 A source of inequity among patients with T1D is the lack of representation of racial/ethnic minorities with T1D in clinical trials.39 The T1DX-QI suggests that the equity-adapted framework for QI can be applied by research leaders to support trial diversity and representation, ensuring future device innovations are meaningful for all people with T1D.40
Diabetes Devices
Glucose monitoring and insulin therapy are vital tools to support individuals living with T1D, and devices such as CGM and insulin pumps have become the standard of care for diabetes management (Table).41 Innovations in diabetes technology and device access are imperative for a chronic disease with no cure.
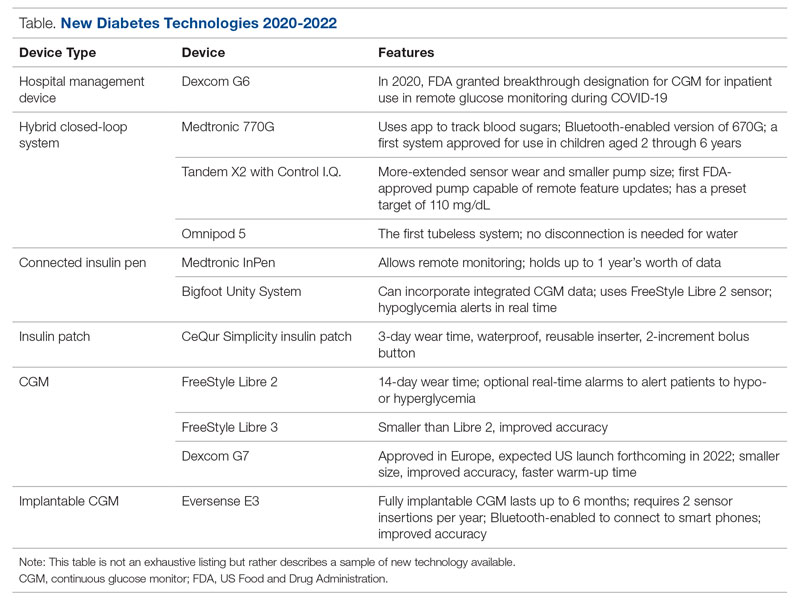
The COVID-19 pandemic created an opportunity to increase access to diabetes devices in inpatient settings. In 2020, the US Food and Drug Administration expanded the use of CGM to support remote monitoring of patients in inpatient hospital settings, simultaneously supporting the glucose monitoring needs of patients with T1D and reducing COVID-19 transmission through reduced patient-clinician contact.42 This effort has been expanded and will continue in 2022 and beyond,43 and aligns with the growing consensus that supports patients wearing both CGMs and insulin pumps in ambulatory settings to improve patient health outcomes.44
Since 2020, innovations in diabetes technology have improved and increased the variety of options available to people with T1D and made them easier to use (Table). New, advanced hybrid closed-loop systems have progressed to offer Bluetooth features, including automatic software upgrades, tubeless systems, and the ability to allow parents to use their smartphones to bolus for children.45-47 The next big step in insulin delivery innovation is the release of functioning, fully closed loop systems, of which several are currently in clinical trials.48 These systems support reduced hypoglycemia and improved time in range.49
Additional innovations in insulin delivery have improved the user experience and expanded therapeutic options, including a variety of smart insulin pens complete with dosing logs50,51 and even a patch to deliver insulin without the burden of injections.52 As barriers to diabetes technology persist,53 innovations in alternate insulin delivery provide people with T1D more options to align with their personal access and technology preferences.
Innovations in CGM address cited barriers to their use, including size or overall wear.53-55 CGMs released in the past few years are smaller in physical size, have longer durations of time between changings, are more accurate, and do not require calibrations for accuracy.
New Diabetes Medications
Many new medications and therapeutic advances have become available in the past 2 years.56 Additionally, more medications are being tested as adjunct therapies to support glycemic management in patients with T1D, including metformin, sodium-glucose cotransporter 1 and 2 inhibitors, pramlintide, glucagon-like polypeptide-1 analogs, and glucagon receptor agonists.57 Other recent advances include stem cell replacement therapy for patients with T1D.58 The ultra-long-acting biosimilar insulins are one medical innovation that has been stalled, rather than propelled, during the COVID-19 pandemic.59
Diabetes Policy Advocacy
People with T1D require insulin to survive. The cost of insulin has increased in recent years, with some studies citing a 64% to 100% increase in the past decade.60,61 In fact, 1 in 4 insulin users report that cost has impacted their insulin use, including rationing their insulin.62 Lockdowns during the COVID-19 pandemic stressed US families financially, increasing the urgency for insulin cost caps.
Although the COVID-19 pandemic halted national conversations on drug financing,63 advocacy efforts have succeeded for specific populations. The new Medicare Part D Senior Savings Model will cap the cost of insulin at $35 for a 30-day supply,64 and 20 states passed legislation capping insulin pricing.62 Efforts to codify national cost caps are under debate, including the passage of the Affordable Insulin Now Act, which passed the House in March 2022 and is currently under review in the Senate.65
Perspective: The Role of Private Philanthropy in Supporting Population Health Innovations
Funders and industry partners play a crucial role in leading and supporting innovations that improve the lives of people with T1D and reduce society’s costs of living with the disease. Data infrastructure is critical to supporting population health. While building the data infrastructure to support population health is both time- and resource-intensive, private foundations such as Helmsley are uniquely positioned—and have a responsibility—to take large, informed risks to help reach all communities with T1D.
The T1DX-QI is the largest source of population health data on T1D in the United States and is becoming the premiere data authority on its incidence, prevalence, and outcomes. The T1DX-QI enables a robust understanding of T1D-related health trends at the population level, as well as trends among clinics and providers. Pilot centers in the T1DX-QI have reported reductions in patients’ A1c and acute diabetes-related events, as well as improvements in device usage and depression screening. The ability to capture changes speaks to the promise and power of these data to demonstrate the clinical impact of QI interventions and to support the spread of best practices and learnings across health systems.
Additional philanthropic efforts have supported innovation in the last 2 years. For example, the JDRF, a nonprofit philanthropic equity firm, has supported efforts in developing artificial pancreas systems and cell therapies currently in clinical trials like teplizumab, a drug that has demonstrated delayed onset of T1D through JDRF’s T1D Fund.66 Industry partners also have an opportunity for significant influence in this area, as they continue to fund meaningful projects to advance care for people with T1D.67
Conclusion
We are optimistic that the innovations summarized here describe a shift in the tide of equitable T1D outcomes; however, future work is required to explore additional health equity successes that do not further exacerbate inequities. We also see further opportunities for innovative ways to engage people with T1D in their health care through conversations on social determinants of health and societal structures.
Corresponding author: Ann Mungmode, MPH, T1D Exchange, 11 Avenue de Lafayette, Boston, MA 02111; Email: [email protected]
Disclosures: Dr. Ebekozien serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for the Medtronic Advisory Board and received research grants from Medtronic Diabetes, Eli Lilly, and Dexcom.
Funding: The T1DX-QI is funded by The Leona M. and Harry B. Hemsley Charitable Trust.
1. Centers for Disease Control and Prevention. National diabetes statistics report. Accessed August 30, 2022. www.cdc.gov/diabetes/data/statistics-report/index.html
2. Centers for Disease Control and Prevention. Diabetes fast facts. Accessed August 30, 2022. www.cdc.gov/diabetes/basics/quick-facts.html
3. O’Malley G, Ebekozien O, Desimone M, et al. COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D Exchange Multicenter Surveillance Study. J Clin Endocrinol Metab. 2020;106(2):e936-e942. doi:10.1210/clinem/dgaa825
4. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care. 2020;43(8):e83-e85. doi:10.2337/dc20-1088
5. Zimmerman C, Albanese-O’Neill A, Haller MJ. Advances in type 1 diabetes technology over the last decade. Eur Endocrinol. 2019;15(2):70-76. doi:10.17925/ee.2019.15.2.70
6. Wake DJ, Gibb FW, Kar P, et al. Endocrinology in the time of COVID-19: remodelling diabetes services and emerging innovation. Eur J Endocrinol. 2020;183(2):G67-G77. doi:10.1530/eje-20-0377
7. Alonso GT, Corathers S, Shah A, et al. Establishment of the T1D Exchange Quality Improvement Collaborative (T1DX-QI). Clin Diabetes. 2020;38(2):141-151. doi:10.2337/cd19-0032
8. Ginnard OZB, Alonso GT, Corathers SD, et al. Quality improvement in diabetes care: a review of initiatives and outcomes in the T1D Exchange Quality Improvement Collaborative. Clin Diabetes. 2021;39(3):256-263. doi:10.2337/cd21-0029
9. ATTD 2021 invited speaker abstracts. Diabetes Technol Ther. 2021;23(S2):A1-A206. doi:10.1089/dia.2021.2525.abstracts
10. Rompicherla SN, Edelen N, Gallagher R, et al. Children and adolescent patients with pre-existing type 1 diabetes and additional comorbidities have an increased risk of hospitalization from COVID-19; data from the T1D Exchange COVID Registry. Pediatr Diabetes. 2021;22(S30):3-32. doi:10.1111/pedi.13268
11. Abstracts for the T1D Exchange QI Collaborative (T1DX-QI) Learning Session 2021. November 8-9, 2021. J Diabetes. 2021;13(S1):3-17. doi:10.1111/1753-0407.13227
12. The Official Journal of ATTD Advanced Technologies & Treatments for Diabetes conference 27-30 April 2022. Barcelona and online. Diabetes Technol Ther. 2022;24(S1):A1-A237. doi:10.1089/dia.2022.2525.abstracts
13. Ebekozien ON, Kamboj N, Odugbesan MK, et al. Inequities in glycemic outcomes for patients with type 1 diabetes: six-year (2016-2021) longitudinal follow-up by race and ethnicity of 36,390 patients in the T1DX-QI Collaborative. Diabetes. 2022;71(suppl 1). doi:10.2337/db22-167-OR
14. Narayan KA, Noor M, Rompicherla N, et al. No BMI increase during the COVID-pandemic in children and adults with T1D in three continents: joint analysis of ADDN, T1DX, and DPV registries. Diabetes. 2022;71(suppl 1). doi:10.2337/db22-269-OR
15. Lee JY, Lee SWH. Telemedicine cost-effectiveness for diabetes management: a systematic review. Diabetes Technol Ther. 2018;20(7):492-500. doi:10.1089/dia.2018.0098
16. McDonnell ME. Telemedicine in complex diabetes management. Curr Diab Rep. 2018;18(7):42. doi:10.1007/s11892-018-1015-3
17. Lee JM, Carlson E, Albanese-O’Neill A, et al. Adoption of telemedicine for type 1 diabetes care during the COVID-19 pandemic. Diabetes Technol Ther. 2021;23(9):642-651. doi:10.1089/dia.2021.0080
18. Phillip M, Bergenstal RM, Close KL, et al. The digital/virtual diabetes clinic: the future is now–recommendations from an international panel on diabetes digital technologies introduction. Diabetes Technol Ther. 2021;23(2):146-154. doi:10.1089/dia.2020.0375
19. Garg SK, Rodriguez E. COVID‐19 pandemic and diabetes care. Diabetes Technol Ther. 2022;24(S1):S2-S20. doi:10.1089/dia.2022.2501
20. Beliard K, Ebekozien O, Demeterco-Berggren C, et al. Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: data from a multi-site surveillance registry. J Diabetes. 2021;13(3):270-272. doi:10.1111/1753-0407.13141
21. Ebekozien O, Agarwal S, Noor N, et al. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab. 2020;106(4):1755-1762. doi:10.1210/clinem/dgaa920
22. Alonso GT, Ebekozien O, Gallagher MP, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes. 2021;13(8):681-687. doi:10.1111/1753-0407.13184
23. Noor N, Ebekozien O, Levin L, et al. Diabetes technology use for management of type 1 diabetes is associated with fewer adverse COVID-19 outcomes: findings from the T1D Exchange COVID-19 Surveillance Registry. Diabetes Care. 2021;44(8):e160-e162. doi:10.2337/dc21-0074
24. Demeterco-Berggren C, Ebekozien O, Rompicherla S, et al. Age and hospitalization risk in people with type 1 diabetes and COVID-19: data from the T1D Exchange Surveillance Study. J Clin Endocrinol Metab. 2021;107(2):410-418. doi:10.1210/clinem/dgab668
25. DeSalvo DJ, Noor N, Xie C, et al. Patient demographics and clinical outcomes among type 1 diabetes patients using continuous glucose monitors: data from T1D Exchange real-world observational study. J Diabetes Sci Technol. 2021 Oct 9. [Epub ahead of print] doi:10.1177/19322968211049783
26. Gallagher MP, Rompicherla S, Ebekozien O, et al. Differences in COVID-19 outcomes among patients with type 1 diabetes: first vs later surges. J Clin Outcomes Manage. 2022;29(1):27-31. doi:10.12788/jcom.0084
27. Wolf RM, Noor N, Izquierdo R, et al. Increase in newly diagnosed type 1 diabetes in youth during the COVID-19 pandemic in the United States: a multi-center analysis. Pediatr Diabetes. 2022;23(4):433-438. doi:10.1111/pedi.13328
28. Lavik AR, Ebekozien O, Noor N, et al. Trends in type 1 diabetic ketoacidosis during COVID-19 surges at 7 US centers: highest burden on non-Hispanic Black patients. J Clin Endocrinol Metab. 2022;107(7):1948-1955. doi:10.1210/clinem/dgac158
29. van der Linden J, Welsh JB, Hirsch IB, Garg SK. Real-time continuous glucose monitoring during the coronavirus disease 2019 pandemic and its impact on time in range. Diabetes Technol Ther. 2021;23(S1):S1-S7. doi:10.1089/dia.2020.0649
30. Nwosu BU, Al-Halbouni L, Parajuli S, et al. COVID-19 pandemic and pediatric type 1 diabetes: no significant change in glycemic control during the pandemic lockdown of 2020. Front Endocrinol (Lausanne). 2021;12:703905. doi:10.3389/fendo.2021.703905
31. Ellahham S. Artificial intelligence: the future for diabetes care. Am J Med. 2020;133(8):895-900. doi:10.1016/j.amjmed.2020.03.033
32. Nomura A, Noguchi M, Kometani M, et al. Artificial intelligence in current diabetes management and prediction. Curr Diab Rep. 2021;21(12):61. doi:10.1007/s11892-021-01423-2
33. Mungmode A, Noor N, Weinstock RS, et al. Making diabetes electronic medical record data actionable: promoting benchmarking and population health using the T1D Exchange Quality Improvement Portal. Clin Diabetes. Forthcoming 2022.
34. Lavizzo-Mourey RJ, Besser RE, Williams DR. Understanding and mitigating health inequities—past, current, and future directions. N Engl J Med. 2021;384(18):1681-1684. doi:10.1056/NEJMp2008628
35. Majidi S, Ebekozien O, Noor N, et al. Inequities in health outcomes in children and adults with type 1 diabetes: data from the T1D Exchange Quality Improvement Collaborative. Clin Diabetes. 2021;39(3):278-283. doi:10.2337/cd21-0028
36. Ebekozien O, Mungmode A, Odugbesan O, et al. Addressing type 1 diabetes health inequities in the United States: approaches from the T1D Exchange QI Collaborative. J Diabetes. 2022;14(1):79-82. doi:10.1111/1753-0407.13235
37. Odugbesan O, Addala A, Nelson G, et al. Implicit racial-ethnic and insurance-mediated bias to recommending diabetes technology: insights from T1D Exchange multicenter pediatric and adult diabetes provider cohort. Diabetes Technol Ther. 2022 Jun 13. [Epub ahead of print] doi:10.1089/dia.2022.0042
38. Schmitt J, Fogle K, Scott ML, Iyer P. Improving equitable access to continuous glucose monitors for Alabama’s children with type 1 diabetes: a quality improvement project. Diabetes Technol Ther. 2022;24(7):481-491. doi:10.1089/dia.2021.0511
39. Akturk HK, Agarwal S, Hoffecker L, Shah VN. Inequity in racial-ethnic representation in randomized controlled trials of diabetes technologies in type 1 diabetes: critical need for new standards. Diabetes Care. 2021;44(6):e121-e123. doi:10.2337/dc20-3063
40. Ebekozien O, Mungmode A, Buckingham D, et al. Achieving equity in diabetes research: borrowing from the field of quality improvement using a practical framework and improvement tools. Diabetes Spectr. 2022;35(3):304-312. doi:10.2237/dsi22-0002
41. Zhang J, Xu J, Lim J, et al. Wearable glucose monitoring and implantable drug delivery systems for diabetes management. Adv Healthc Mater. 2021;10(17):e2100194. doi:10.1002/adhm.202100194
42. FDA expands remote patient monitoring in hospitals for people with diabetes during COVID-19; manufacturers donate CGM supplies. News release. April 21, 2020. Accessed August 30, 2022. https://www.diabetes.org/newsroom/press-releases/2020/fda-remote-patient-monitoring-cgm
43. Campbell P. FDA grants Dexcom CGM breakthrough designation for in-hospital use. March 2, 2022. Accessed August 30, 2022. https://www.endocrinologynetwork.com/view/fda-grants-dexcom-cgm-breakthrough-designation-for-in-hospital-use
44. Yeh T, Yeung M, Mendelsohn Curanaj FA. Managing patients with insulin pumps and continuous glucose monitors in the hospital: to wear or not to wear. Curr Diab Rep. 2021;21(2):7. doi:10.1007/s11892-021-01375-7
45. Medtronic announces FDA approval for MiniMed 770G insulin pump system. News release. September 21, 2020. Accessed August 30, 2022. https://bit.ly/3TyEna4
46. Tandem Diabetes Care announces commercial launch of the t:slim X2 insulin pump with Control-IQ technology in the United States. News release. January 15, 2020. Accessed August 30, 2022. https://investor.tandemdiabetes.com/news-releases/news-release-details/tandem-diabetes-care-announces-commercial-launch-tslim-x2-0
47. Garza M, Gutow H, Mahoney K. Omnipod 5 cleared by the FDA. Updated August 22, 2022. Accessed August 30, 2022.https://diatribe.org/omnipod-5-approved-fda
48. Boughton CK. Fully closed-loop insulin delivery—are we nearly there yet? Lancet Digit Health. 2021;3(11):e689-e690. doi:10.1016/s2589-7500(21)00218-1
49. Noor N, Kamboj MK, Triolo T, et al. Hybrid closed-loop systems and glycemic outcomes in children and adults with type 1 diabetes: real-world evidence from a U.S.-based multicenter collaborative. Diabetes Care. 2022;45(8):e118-e119. doi:10.2337/dc22-0329
50. Medtronic launches InPen with real-time Guardian Connect CGM data--the first integrated smart insulin pen for people with diabetes on MDI. News release. November 12, 2020. Accessed August 30, 2022. https://bit.ly/3CTSWPL
51. Bigfoot Biomedical receives FDA clearance for Bigfoot Unity Diabetes Management System, featuring first-of-its-kind smart pen caps for insulin pens used to treat type 1 and type 2 diabetes. News release. May 10, 2021. Accessed August 30, 2022. https://bit.ly/3BeyoAh
52. Vieira G. All about the CeQur Simplicity insulin patch. Updated May 24, 2022. Accessed August 30, 2022. https://beyondtype1.org/cequr-simplicity-insulin-patch/.
53. Messer LH, Tanenbaum ML, Cook PF, et al. Cost, hassle, and on-body experience: barriers to diabetes device use in adolescents and potential intervention targets. Diabetes Technol Ther. 2020;22(10):760-767. doi:10.1089/dia.2019.0509
54. Hilliard ME, Levy W, Anderson BJ, et al. Benefits and barriers of continuous glucose monitoring in young children with type 1 diabetes. Diabetes Technol Ther. 2019;21(9):493-498. doi:10.1089/dia.2019.0142
55. Dexcom G7 Release Delayed Until Late 2022. News release. August 8, 2022. Accessed September 7, 2022. https://diatribe.org/dexcom-g7-release-delayed-until-late-2022
56. Drucker DJ. Transforming type 1 diabetes: the next wave of innovation. Diabetologia. 2021;64(5):1059-1065. doi:10.1007/s00125-021-05396-5
57. Garg SK, Rodriguez E, Shah VN, Hirsch IB. New medications for the treatment of diabetes. Diabetes Technol Ther. 2022;24(S1):S190-S208. doi:10.1089/dia.2022.2513
58. Melton D. The promise of stem cell-derived islet replacement therapy. Diabetologia. 2021;64(5):1030-1036. doi:10.1007/s00125-020-05367-2
59. Danne T, Heinemann L, Bolinder J. New insulins, biosimilars, and insulin therapy. Diabetes Technol Ther. 2022;24(S1):S35-S57. doi:10.1089/dia.2022.2503
60. Kenney J. Insulin copay caps–a path to affordability. July 6, 2021. Accessed August 30, 2022.https://diatribechange.org/news/insulin-copay-caps-path-affordability
61. Glied SA, Zhu B. Not so sweet: insulin affordability over time. September 25, 2020. Accessed August 30, 2022. https://www.commonwealthfund.org/publications/issue-briefs/2020/sep/not-so-sweet-insulin-affordability-over-time
62. American Diabetes Association. Insulin and drug affordability. Accessed August 30, 2022. https://www.diabetes.org/advocacy/insulin-and-drug-affordability
63. Sullivan P. Chances for drug pricing, surprise billing action fade until November. March 24, 2020. Accessed August 30, 2022. https://thehill.com/policy/healthcare/489334-chances-for-drug-pricing-surprise-billing-action-fade-until-november/
64. Brown TD. How Medicare’s new Senior Savings Model makes insulin more affordable. June 4, 2020. Accessed August 30, 2022. https://www.diabetes.org/blog/how-medicares-new-senior-savings-model-makes-insulin-more-affordable
65. American Diabetes Association. ADA applauds the U.S. House of Representatives passage of the Affordable Insulin Now Act. News release. April 1, 2022. https://www.diabetes.org/newsroom/official-statement/2022/ada-applauds-us-house-of-representatives-passage-of-the-affordable-insulin-now-act
66. JDRF. Driving T1D cures during challenging times. 2022.
67. Medtronic announces ongoing initiatives to address health equity for people of color living with diabetes. News release. April 7, 2021. Access August 30, 2022. https://bit.ly/3KGTOZU
From the T1D Exchange, Boston, MA (Ann Mungmode, Nicole Rioles, Jesse Cases, Dr. Ebekozien); The Leona M. and Harry B. Hemsley Charitable Trust, New York, NY (Laurel Koester); and the University of Mississippi School of Population Health, Jackson, MS (Dr. Ebekozien).
Abstract
There have been remarkable innovations in diabetes management since the start of the COVID-19 pandemic, but these groundbreaking innovations are drawing limited focus as the field focuses on the adverse impact of the pandemic on patients with diabetes. This article reviews select population health innovations in diabetes management that have become available over the past 2 years of the COVID-19 pandemic from the perspective of the T1D Exchange Quality Improvement Collaborative, a learning health network that focuses on improving care and outcomes for individuals with type 1 diabetes (T1D). Such innovations include expanded telemedicine access, collection of real-world data, machine learning and artificial intelligence, and new diabetes medications and devices. In addition, multiple innovative studies have been undertaken to explore contributors to health inequities in diabetes, and advocacy efforts for specific populations have been successful. Looking to the future, work is required to explore additional health equity successes that do not further exacerbate inequities and to look for additional innovative ways to engage people with T1D in their health care through conversations on social determinants of health and societal structures.
Keywords: type 1 diabetes, learning health network, continuous glucose monitoring, health equity
One in 10 people in the United States has diabetes.1 Diabetes is the nation’s second leading cause of death, costing the US health system more than $300 billion annually.2 The COVID-19 pandemic presented additional health burdens for people living with diabetes. For example, preexisting diabetes was identified as a risk factor for COVID-19–associated morbidity and mortality.3,4 Over the past 2 years, there have been remarkable innovations in diabetes management, including stem cell therapy and new medication options. Additionally, improved technology solutions have aided in diabetes management through continuous glucose monitors (CGM), smart insulin pens, advanced hybrid closed-loop systems, and continuous subcutaneous insulin injections.5,6 Unfortunately, these groundbreaking innovations are drawing limited focus, as the field is rightfully focused on the adverse impact of the pandemic on patients with diabetes.

Learning health networks like the T1D Exchange Quality Improvement Collaborative (T1DX-QI) have implemented some of these innovative solutions to improve care for people with diabetes.7 T1DX-QI has more than 50 data-sharing endocrinology centers that care for over 75,000 people with diabetes across the United States (Figure 1). Centers participating in the T1DX-QI use quality improvement (QI) and implementation science methods to quickly translate research into evidence-based clinical practice. T1DX-QI leads diabetes population health and health system research and supports widespread transferability across health care organizations through regular collaborative calls, conferences, and case study documentation.8
In this review, we summarize impactful population health innovations in diabetes management that have become available over the past 2 years of the COVID-19 pandemic from the perspective of T1DX-QI (see Figure 2 for relevant definitions). This review is limited in scope and is not meant to be an exhaustive list of innovations. The review also reflects significant changes from the perspective of academic diabetes centers, which may not apply to rural or primary care diabetes practices.
Methods
The first (A.M.), second (H.H.), and senior (O.E.) authors conducted a scoping review of published literature using terms related to diabetes, population health, and innovation on PubMed Central and Google Scholar for the period March 2020 to June 2022. To complement the review, A.M. and O.E. also reviewed abstracts from presentations at major international diabetes conferences, including the American Diabetes Association (ADA), the International Society for Pediatric and Adolescent Diabetes (ISPAD), the T1DX-QI Learning Session Conference, and the Advanced Technologies & Treatments for Diabetes (ATTD) 2020 to 2022 conferences.9-14 The authors also searched FDA.gov and ClinicalTrials.gov for relevant insights. A.M. and O.E. sorted the reviewed literature into major themes (Figure 3) from the population health improvement perspective of the T1DX-QI.

Population Health Innovations in Diabetes Management
Expansion of Telemedicine Access
Telemedicine is cost-effective for patients with diabetes,15 including those with complex cases.16 Before the COVID-19 pandemic, telemedicine and virtual care were rare in diabetes management. However, the pandemic offered a new opportunity to expand the practice of telemedicine in diabetes management. A study from the T1DX-QI showed that telemedicine visits grew from comprising <1% of visits pre-pandemic (December 2019) to 95.2% during the pandemic (August 2020).17 Additional studies, like those conducted by Phillip et al,18 confirmed the noninferiority of telemedicine practice for patients with diabetes.Telemedicine was also found to be an effective strategy to educate patients on the use of diabetes technologies.19
Real-World Data and Disease Surveillance
As the COVID-19 pandemic exacerbated outcomes for people with type 1 diabetes (T1D), a need arose to understand the immediate effects of the pandemic on people with T1D through real-world data and disease surveillance. In April 2020, the T1DX-QI initiated a multicenter surveillance study to collect data and analyze the impact of COVID-19 on people with T1D. The existing health collaborative served as a springboard for robust surveillance study, documenting numerous works on the effects of COVID-19.3,4,20-28 Other investigators also embraced the power of real-world surveillance and real-world data.29,30
Big Data, Machine Learning, and Artificial Intelligence
The past 2 years have seen a shift toward embracing the incredible opportunity to tap the large volume of data generated from routine care for practical insights.31 In particular, researchers have demonstrated the widespread application of machine learning and artificial intelligence to improve diabetes management.32 The T1DX-QI also harnessed the growing power of big data by expanding the functionality of innovative benchmarking software. The T1DX QI Portal uses electronic medical record data of diabetes patients for clinic-to-clinic benchmarking and data analysis, using business intelligence solutions.33
Health Equity
While inequities across various health outcomes have been well documented for years,34 the COVID-19 pandemic further exaggerated racial/ethnic health inequities in T1D.23,35 In response, several organizations have outlined specific strategies to address these health inequities. Emboldened by the pandemic, the T1DX-QI announced a multipronged approach to address health inequities among patients with T1D through the Health Equity Advancement Lab (HEAL).36 One of HEAL’s main components is using real-world data to champion population-level insights and demonstrate progress in QI efforts.
Multiple innovative studies have been undertaken to explore contributors to health inequities in diabetes, and these studies are expanding our understanding of the chasm.37 There have also been innovative solutions to addressing these inequities, with multiple studies published over the past 2 years.38 A source of inequity among patients with T1D is the lack of representation of racial/ethnic minorities with T1D in clinical trials.39 The T1DX-QI suggests that the equity-adapted framework for QI can be applied by research leaders to support trial diversity and representation, ensuring future device innovations are meaningful for all people with T1D.40
Diabetes Devices
Glucose monitoring and insulin therapy are vital tools to support individuals living with T1D, and devices such as CGM and insulin pumps have become the standard of care for diabetes management (Table).41 Innovations in diabetes technology and device access are imperative for a chronic disease with no cure.

The COVID-19 pandemic created an opportunity to increase access to diabetes devices in inpatient settings. In 2020, the US Food and Drug Administration expanded the use of CGM to support remote monitoring of patients in inpatient hospital settings, simultaneously supporting the glucose monitoring needs of patients with T1D and reducing COVID-19 transmission through reduced patient-clinician contact.42 This effort has been expanded and will continue in 2022 and beyond,43 and aligns with the growing consensus that supports patients wearing both CGMs and insulin pumps in ambulatory settings to improve patient health outcomes.44
Since 2020, innovations in diabetes technology have improved and increased the variety of options available to people with T1D and made them easier to use (Table). New, advanced hybrid closed-loop systems have progressed to offer Bluetooth features, including automatic software upgrades, tubeless systems, and the ability to allow parents to use their smartphones to bolus for children.45-47 The next big step in insulin delivery innovation is the release of functioning, fully closed loop systems, of which several are currently in clinical trials.48 These systems support reduced hypoglycemia and improved time in range.49
Additional innovations in insulin delivery have improved the user experience and expanded therapeutic options, including a variety of smart insulin pens complete with dosing logs50,51 and even a patch to deliver insulin without the burden of injections.52 As barriers to diabetes technology persist,53 innovations in alternate insulin delivery provide people with T1D more options to align with their personal access and technology preferences.
Innovations in CGM address cited barriers to their use, including size or overall wear.53-55 CGMs released in the past few years are smaller in physical size, have longer durations of time between changings, are more accurate, and do not require calibrations for accuracy.
New Diabetes Medications
Many new medications and therapeutic advances have become available in the past 2 years.56 Additionally, more medications are being tested as adjunct therapies to support glycemic management in patients with T1D, including metformin, sodium-glucose cotransporter 1 and 2 inhibitors, pramlintide, glucagon-like polypeptide-1 analogs, and glucagon receptor agonists.57 Other recent advances include stem cell replacement therapy for patients with T1D.58 The ultra-long-acting biosimilar insulins are one medical innovation that has been stalled, rather than propelled, during the COVID-19 pandemic.59
Diabetes Policy Advocacy
People with T1D require insulin to survive. The cost of insulin has increased in recent years, with some studies citing a 64% to 100% increase in the past decade.60,61 In fact, 1 in 4 insulin users report that cost has impacted their insulin use, including rationing their insulin.62 Lockdowns during the COVID-19 pandemic stressed US families financially, increasing the urgency for insulin cost caps.
Although the COVID-19 pandemic halted national conversations on drug financing,63 advocacy efforts have succeeded for specific populations. The new Medicare Part D Senior Savings Model will cap the cost of insulin at $35 for a 30-day supply,64 and 20 states passed legislation capping insulin pricing.62 Efforts to codify national cost caps are under debate, including the passage of the Affordable Insulin Now Act, which passed the House in March 2022 and is currently under review in the Senate.65
Perspective: The Role of Private Philanthropy in Supporting Population Health Innovations
Funders and industry partners play a crucial role in leading and supporting innovations that improve the lives of people with T1D and reduce society’s costs of living with the disease. Data infrastructure is critical to supporting population health. While building the data infrastructure to support population health is both time- and resource-intensive, private foundations such as Helmsley are uniquely positioned—and have a responsibility—to take large, informed risks to help reach all communities with T1D.
The T1DX-QI is the largest source of population health data on T1D in the United States and is becoming the premiere data authority on its incidence, prevalence, and outcomes. The T1DX-QI enables a robust understanding of T1D-related health trends at the population level, as well as trends among clinics and providers. Pilot centers in the T1DX-QI have reported reductions in patients’ A1c and acute diabetes-related events, as well as improvements in device usage and depression screening. The ability to capture changes speaks to the promise and power of these data to demonstrate the clinical impact of QI interventions and to support the spread of best practices and learnings across health systems.
Additional philanthropic efforts have supported innovation in the last 2 years. For example, the JDRF, a nonprofit philanthropic equity firm, has supported efforts in developing artificial pancreas systems and cell therapies currently in clinical trials like teplizumab, a drug that has demonstrated delayed onset of T1D through JDRF’s T1D Fund.66 Industry partners also have an opportunity for significant influence in this area, as they continue to fund meaningful projects to advance care for people with T1D.67
Conclusion
We are optimistic that the innovations summarized here describe a shift in the tide of equitable T1D outcomes; however, future work is required to explore additional health equity successes that do not further exacerbate inequities. We also see further opportunities for innovative ways to engage people with T1D in their health care through conversations on social determinants of health and societal structures.
Corresponding author: Ann Mungmode, MPH, T1D Exchange, 11 Avenue de Lafayette, Boston, MA 02111; Email: [email protected]
Disclosures: Dr. Ebekozien serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for the Medtronic Advisory Board and received research grants from Medtronic Diabetes, Eli Lilly, and Dexcom.
Funding: The T1DX-QI is funded by The Leona M. and Harry B. Hemsley Charitable Trust.
From the T1D Exchange, Boston, MA (Ann Mungmode, Nicole Rioles, Jesse Cases, Dr. Ebekozien); The Leona M. and Harry B. Hemsley Charitable Trust, New York, NY (Laurel Koester); and the University of Mississippi School of Population Health, Jackson, MS (Dr. Ebekozien).
Abstract
There have been remarkable innovations in diabetes management since the start of the COVID-19 pandemic, but these groundbreaking innovations are drawing limited focus as the field focuses on the adverse impact of the pandemic on patients with diabetes. This article reviews select population health innovations in diabetes management that have become available over the past 2 years of the COVID-19 pandemic from the perspective of the T1D Exchange Quality Improvement Collaborative, a learning health network that focuses on improving care and outcomes for individuals with type 1 diabetes (T1D). Such innovations include expanded telemedicine access, collection of real-world data, machine learning and artificial intelligence, and new diabetes medications and devices. In addition, multiple innovative studies have been undertaken to explore contributors to health inequities in diabetes, and advocacy efforts for specific populations have been successful. Looking to the future, work is required to explore additional health equity successes that do not further exacerbate inequities and to look for additional innovative ways to engage people with T1D in their health care through conversations on social determinants of health and societal structures.
Keywords: type 1 diabetes, learning health network, continuous glucose monitoring, health equity
One in 10 people in the United States has diabetes.1 Diabetes is the nation’s second leading cause of death, costing the US health system more than $300 billion annually.2 The COVID-19 pandemic presented additional health burdens for people living with diabetes. For example, preexisting diabetes was identified as a risk factor for COVID-19–associated morbidity and mortality.3,4 Over the past 2 years, there have been remarkable innovations in diabetes management, including stem cell therapy and new medication options. Additionally, improved technology solutions have aided in diabetes management through continuous glucose monitors (CGM), smart insulin pens, advanced hybrid closed-loop systems, and continuous subcutaneous insulin injections.5,6 Unfortunately, these groundbreaking innovations are drawing limited focus, as the field is rightfully focused on the adverse impact of the pandemic on patients with diabetes.

Learning health networks like the T1D Exchange Quality Improvement Collaborative (T1DX-QI) have implemented some of these innovative solutions to improve care for people with diabetes.7 T1DX-QI has more than 50 data-sharing endocrinology centers that care for over 75,000 people with diabetes across the United States (Figure 1). Centers participating in the T1DX-QI use quality improvement (QI) and implementation science methods to quickly translate research into evidence-based clinical practice. T1DX-QI leads diabetes population health and health system research and supports widespread transferability across health care organizations through regular collaborative calls, conferences, and case study documentation.8
In this review, we summarize impactful population health innovations in diabetes management that have become available over the past 2 years of the COVID-19 pandemic from the perspective of T1DX-QI (see Figure 2 for relevant definitions). This review is limited in scope and is not meant to be an exhaustive list of innovations. The review also reflects significant changes from the perspective of academic diabetes centers, which may not apply to rural or primary care diabetes practices.
Methods
The first (A.M.), second (H.H.), and senior (O.E.) authors conducted a scoping review of published literature using terms related to diabetes, population health, and innovation on PubMed Central and Google Scholar for the period March 2020 to June 2022. To complement the review, A.M. and O.E. also reviewed abstracts from presentations at major international diabetes conferences, including the American Diabetes Association (ADA), the International Society for Pediatric and Adolescent Diabetes (ISPAD), the T1DX-QI Learning Session Conference, and the Advanced Technologies & Treatments for Diabetes (ATTD) 2020 to 2022 conferences.9-14 The authors also searched FDA.gov and ClinicalTrials.gov for relevant insights. A.M. and O.E. sorted the reviewed literature into major themes (Figure 3) from the population health improvement perspective of the T1DX-QI.

Population Health Innovations in Diabetes Management
Expansion of Telemedicine Access
Telemedicine is cost-effective for patients with diabetes,15 including those with complex cases.16 Before the COVID-19 pandemic, telemedicine and virtual care were rare in diabetes management. However, the pandemic offered a new opportunity to expand the practice of telemedicine in diabetes management. A study from the T1DX-QI showed that telemedicine visits grew from comprising <1% of visits pre-pandemic (December 2019) to 95.2% during the pandemic (August 2020).17 Additional studies, like those conducted by Phillip et al,18 confirmed the noninferiority of telemedicine practice for patients with diabetes.Telemedicine was also found to be an effective strategy to educate patients on the use of diabetes technologies.19
Real-World Data and Disease Surveillance
As the COVID-19 pandemic exacerbated outcomes for people with type 1 diabetes (T1D), a need arose to understand the immediate effects of the pandemic on people with T1D through real-world data and disease surveillance. In April 2020, the T1DX-QI initiated a multicenter surveillance study to collect data and analyze the impact of COVID-19 on people with T1D. The existing health collaborative served as a springboard for robust surveillance study, documenting numerous works on the effects of COVID-19.3,4,20-28 Other investigators also embraced the power of real-world surveillance and real-world data.29,30
Big Data, Machine Learning, and Artificial Intelligence
The past 2 years have seen a shift toward embracing the incredible opportunity to tap the large volume of data generated from routine care for practical insights.31 In particular, researchers have demonstrated the widespread application of machine learning and artificial intelligence to improve diabetes management.32 The T1DX-QI also harnessed the growing power of big data by expanding the functionality of innovative benchmarking software. The T1DX QI Portal uses electronic medical record data of diabetes patients for clinic-to-clinic benchmarking and data analysis, using business intelligence solutions.33
Health Equity
While inequities across various health outcomes have been well documented for years,34 the COVID-19 pandemic further exaggerated racial/ethnic health inequities in T1D.23,35 In response, several organizations have outlined specific strategies to address these health inequities. Emboldened by the pandemic, the T1DX-QI announced a multipronged approach to address health inequities among patients with T1D through the Health Equity Advancement Lab (HEAL).36 One of HEAL’s main components is using real-world data to champion population-level insights and demonstrate progress in QI efforts.
Multiple innovative studies have been undertaken to explore contributors to health inequities in diabetes, and these studies are expanding our understanding of the chasm.37 There have also been innovative solutions to addressing these inequities, with multiple studies published over the past 2 years.38 A source of inequity among patients with T1D is the lack of representation of racial/ethnic minorities with T1D in clinical trials.39 The T1DX-QI suggests that the equity-adapted framework for QI can be applied by research leaders to support trial diversity and representation, ensuring future device innovations are meaningful for all people with T1D.40
Diabetes Devices
Glucose monitoring and insulin therapy are vital tools to support individuals living with T1D, and devices such as CGM and insulin pumps have become the standard of care for diabetes management (Table).41 Innovations in diabetes technology and device access are imperative for a chronic disease with no cure.

The COVID-19 pandemic created an opportunity to increase access to diabetes devices in inpatient settings. In 2020, the US Food and Drug Administration expanded the use of CGM to support remote monitoring of patients in inpatient hospital settings, simultaneously supporting the glucose monitoring needs of patients with T1D and reducing COVID-19 transmission through reduced patient-clinician contact.42 This effort has been expanded and will continue in 2022 and beyond,43 and aligns with the growing consensus that supports patients wearing both CGMs and insulin pumps in ambulatory settings to improve patient health outcomes.44
Since 2020, innovations in diabetes technology have improved and increased the variety of options available to people with T1D and made them easier to use (Table). New, advanced hybrid closed-loop systems have progressed to offer Bluetooth features, including automatic software upgrades, tubeless systems, and the ability to allow parents to use their smartphones to bolus for children.45-47 The next big step in insulin delivery innovation is the release of functioning, fully closed loop systems, of which several are currently in clinical trials.48 These systems support reduced hypoglycemia and improved time in range.49
Additional innovations in insulin delivery have improved the user experience and expanded therapeutic options, including a variety of smart insulin pens complete with dosing logs50,51 and even a patch to deliver insulin without the burden of injections.52 As barriers to diabetes technology persist,53 innovations in alternate insulin delivery provide people with T1D more options to align with their personal access and technology preferences.
Innovations in CGM address cited barriers to their use, including size or overall wear.53-55 CGMs released in the past few years are smaller in physical size, have longer durations of time between changings, are more accurate, and do not require calibrations for accuracy.
New Diabetes Medications
Many new medications and therapeutic advances have become available in the past 2 years.56 Additionally, more medications are being tested as adjunct therapies to support glycemic management in patients with T1D, including metformin, sodium-glucose cotransporter 1 and 2 inhibitors, pramlintide, glucagon-like polypeptide-1 analogs, and glucagon receptor agonists.57 Other recent advances include stem cell replacement therapy for patients with T1D.58 The ultra-long-acting biosimilar insulins are one medical innovation that has been stalled, rather than propelled, during the COVID-19 pandemic.59
Diabetes Policy Advocacy
People with T1D require insulin to survive. The cost of insulin has increased in recent years, with some studies citing a 64% to 100% increase in the past decade.60,61 In fact, 1 in 4 insulin users report that cost has impacted their insulin use, including rationing their insulin.62 Lockdowns during the COVID-19 pandemic stressed US families financially, increasing the urgency for insulin cost caps.
Although the COVID-19 pandemic halted national conversations on drug financing,63 advocacy efforts have succeeded for specific populations. The new Medicare Part D Senior Savings Model will cap the cost of insulin at $35 for a 30-day supply,64 and 20 states passed legislation capping insulin pricing.62 Efforts to codify national cost caps are under debate, including the passage of the Affordable Insulin Now Act, which passed the House in March 2022 and is currently under review in the Senate.65
Perspective: The Role of Private Philanthropy in Supporting Population Health Innovations
Funders and industry partners play a crucial role in leading and supporting innovations that improve the lives of people with T1D and reduce society’s costs of living with the disease. Data infrastructure is critical to supporting population health. While building the data infrastructure to support population health is both time- and resource-intensive, private foundations such as Helmsley are uniquely positioned—and have a responsibility—to take large, informed risks to help reach all communities with T1D.
The T1DX-QI is the largest source of population health data on T1D in the United States and is becoming the premiere data authority on its incidence, prevalence, and outcomes. The T1DX-QI enables a robust understanding of T1D-related health trends at the population level, as well as trends among clinics and providers. Pilot centers in the T1DX-QI have reported reductions in patients’ A1c and acute diabetes-related events, as well as improvements in device usage and depression screening. The ability to capture changes speaks to the promise and power of these data to demonstrate the clinical impact of QI interventions and to support the spread of best practices and learnings across health systems.
Additional philanthropic efforts have supported innovation in the last 2 years. For example, the JDRF, a nonprofit philanthropic equity firm, has supported efforts in developing artificial pancreas systems and cell therapies currently in clinical trials like teplizumab, a drug that has demonstrated delayed onset of T1D through JDRF’s T1D Fund.66 Industry partners also have an opportunity for significant influence in this area, as they continue to fund meaningful projects to advance care for people with T1D.67
Conclusion
We are optimistic that the innovations summarized here describe a shift in the tide of equitable T1D outcomes; however, future work is required to explore additional health equity successes that do not further exacerbate inequities. We also see further opportunities for innovative ways to engage people with T1D in their health care through conversations on social determinants of health and societal structures.
Corresponding author: Ann Mungmode, MPH, T1D Exchange, 11 Avenue de Lafayette, Boston, MA 02111; Email: [email protected]
Disclosures: Dr. Ebekozien serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for the Medtronic Advisory Board and received research grants from Medtronic Diabetes, Eli Lilly, and Dexcom.
Funding: The T1DX-QI is funded by The Leona M. and Harry B. Hemsley Charitable Trust.
1. Centers for Disease Control and Prevention. National diabetes statistics report. Accessed August 30, 2022. www.cdc.gov/diabetes/data/statistics-report/index.html
2. Centers for Disease Control and Prevention. Diabetes fast facts. Accessed August 30, 2022. www.cdc.gov/diabetes/basics/quick-facts.html
3. O’Malley G, Ebekozien O, Desimone M, et al. COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D Exchange Multicenter Surveillance Study. J Clin Endocrinol Metab. 2020;106(2):e936-e942. doi:10.1210/clinem/dgaa825
4. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care. 2020;43(8):e83-e85. doi:10.2337/dc20-1088
5. Zimmerman C, Albanese-O’Neill A, Haller MJ. Advances in type 1 diabetes technology over the last decade. Eur Endocrinol. 2019;15(2):70-76. doi:10.17925/ee.2019.15.2.70
6. Wake DJ, Gibb FW, Kar P, et al. Endocrinology in the time of COVID-19: remodelling diabetes services and emerging innovation. Eur J Endocrinol. 2020;183(2):G67-G77. doi:10.1530/eje-20-0377
7. Alonso GT, Corathers S, Shah A, et al. Establishment of the T1D Exchange Quality Improvement Collaborative (T1DX-QI). Clin Diabetes. 2020;38(2):141-151. doi:10.2337/cd19-0032
8. Ginnard OZB, Alonso GT, Corathers SD, et al. Quality improvement in diabetes care: a review of initiatives and outcomes in the T1D Exchange Quality Improvement Collaborative. Clin Diabetes. 2021;39(3):256-263. doi:10.2337/cd21-0029
9. ATTD 2021 invited speaker abstracts. Diabetes Technol Ther. 2021;23(S2):A1-A206. doi:10.1089/dia.2021.2525.abstracts
10. Rompicherla SN, Edelen N, Gallagher R, et al. Children and adolescent patients with pre-existing type 1 diabetes and additional comorbidities have an increased risk of hospitalization from COVID-19; data from the T1D Exchange COVID Registry. Pediatr Diabetes. 2021;22(S30):3-32. doi:10.1111/pedi.13268
11. Abstracts for the T1D Exchange QI Collaborative (T1DX-QI) Learning Session 2021. November 8-9, 2021. J Diabetes. 2021;13(S1):3-17. doi:10.1111/1753-0407.13227
12. The Official Journal of ATTD Advanced Technologies & Treatments for Diabetes conference 27-30 April 2022. Barcelona and online. Diabetes Technol Ther. 2022;24(S1):A1-A237. doi:10.1089/dia.2022.2525.abstracts
13. Ebekozien ON, Kamboj N, Odugbesan MK, et al. Inequities in glycemic outcomes for patients with type 1 diabetes: six-year (2016-2021) longitudinal follow-up by race and ethnicity of 36,390 patients in the T1DX-QI Collaborative. Diabetes. 2022;71(suppl 1). doi:10.2337/db22-167-OR
14. Narayan KA, Noor M, Rompicherla N, et al. No BMI increase during the COVID-pandemic in children and adults with T1D in three continents: joint analysis of ADDN, T1DX, and DPV registries. Diabetes. 2022;71(suppl 1). doi:10.2337/db22-269-OR
15. Lee JY, Lee SWH. Telemedicine cost-effectiveness for diabetes management: a systematic review. Diabetes Technol Ther. 2018;20(7):492-500. doi:10.1089/dia.2018.0098
16. McDonnell ME. Telemedicine in complex diabetes management. Curr Diab Rep. 2018;18(7):42. doi:10.1007/s11892-018-1015-3
17. Lee JM, Carlson E, Albanese-O’Neill A, et al. Adoption of telemedicine for type 1 diabetes care during the COVID-19 pandemic. Diabetes Technol Ther. 2021;23(9):642-651. doi:10.1089/dia.2021.0080
18. Phillip M, Bergenstal RM, Close KL, et al. The digital/virtual diabetes clinic: the future is now–recommendations from an international panel on diabetes digital technologies introduction. Diabetes Technol Ther. 2021;23(2):146-154. doi:10.1089/dia.2020.0375
19. Garg SK, Rodriguez E. COVID‐19 pandemic and diabetes care. Diabetes Technol Ther. 2022;24(S1):S2-S20. doi:10.1089/dia.2022.2501
20. Beliard K, Ebekozien O, Demeterco-Berggren C, et al. Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: data from a multi-site surveillance registry. J Diabetes. 2021;13(3):270-272. doi:10.1111/1753-0407.13141
21. Ebekozien O, Agarwal S, Noor N, et al. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab. 2020;106(4):1755-1762. doi:10.1210/clinem/dgaa920
22. Alonso GT, Ebekozien O, Gallagher MP, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes. 2021;13(8):681-687. doi:10.1111/1753-0407.13184
23. Noor N, Ebekozien O, Levin L, et al. Diabetes technology use for management of type 1 diabetes is associated with fewer adverse COVID-19 outcomes: findings from the T1D Exchange COVID-19 Surveillance Registry. Diabetes Care. 2021;44(8):e160-e162. doi:10.2337/dc21-0074
24. Demeterco-Berggren C, Ebekozien O, Rompicherla S, et al. Age and hospitalization risk in people with type 1 diabetes and COVID-19: data from the T1D Exchange Surveillance Study. J Clin Endocrinol Metab. 2021;107(2):410-418. doi:10.1210/clinem/dgab668
25. DeSalvo DJ, Noor N, Xie C, et al. Patient demographics and clinical outcomes among type 1 diabetes patients using continuous glucose monitors: data from T1D Exchange real-world observational study. J Diabetes Sci Technol. 2021 Oct 9. [Epub ahead of print] doi:10.1177/19322968211049783
26. Gallagher MP, Rompicherla S, Ebekozien O, et al. Differences in COVID-19 outcomes among patients with type 1 diabetes: first vs later surges. J Clin Outcomes Manage. 2022;29(1):27-31. doi:10.12788/jcom.0084
27. Wolf RM, Noor N, Izquierdo R, et al. Increase in newly diagnosed type 1 diabetes in youth during the COVID-19 pandemic in the United States: a multi-center analysis. Pediatr Diabetes. 2022;23(4):433-438. doi:10.1111/pedi.13328
28. Lavik AR, Ebekozien O, Noor N, et al. Trends in type 1 diabetic ketoacidosis during COVID-19 surges at 7 US centers: highest burden on non-Hispanic Black patients. J Clin Endocrinol Metab. 2022;107(7):1948-1955. doi:10.1210/clinem/dgac158
29. van der Linden J, Welsh JB, Hirsch IB, Garg SK. Real-time continuous glucose monitoring during the coronavirus disease 2019 pandemic and its impact on time in range. Diabetes Technol Ther. 2021;23(S1):S1-S7. doi:10.1089/dia.2020.0649
30. Nwosu BU, Al-Halbouni L, Parajuli S, et al. COVID-19 pandemic and pediatric type 1 diabetes: no significant change in glycemic control during the pandemic lockdown of 2020. Front Endocrinol (Lausanne). 2021;12:703905. doi:10.3389/fendo.2021.703905
31. Ellahham S. Artificial intelligence: the future for diabetes care. Am J Med. 2020;133(8):895-900. doi:10.1016/j.amjmed.2020.03.033
32. Nomura A, Noguchi M, Kometani M, et al. Artificial intelligence in current diabetes management and prediction. Curr Diab Rep. 2021;21(12):61. doi:10.1007/s11892-021-01423-2
33. Mungmode A, Noor N, Weinstock RS, et al. Making diabetes electronic medical record data actionable: promoting benchmarking and population health using the T1D Exchange Quality Improvement Portal. Clin Diabetes. Forthcoming 2022.
34. Lavizzo-Mourey RJ, Besser RE, Williams DR. Understanding and mitigating health inequities—past, current, and future directions. N Engl J Med. 2021;384(18):1681-1684. doi:10.1056/NEJMp2008628
35. Majidi S, Ebekozien O, Noor N, et al. Inequities in health outcomes in children and adults with type 1 diabetes: data from the T1D Exchange Quality Improvement Collaborative. Clin Diabetes. 2021;39(3):278-283. doi:10.2337/cd21-0028
36. Ebekozien O, Mungmode A, Odugbesan O, et al. Addressing type 1 diabetes health inequities in the United States: approaches from the T1D Exchange QI Collaborative. J Diabetes. 2022;14(1):79-82. doi:10.1111/1753-0407.13235
37. Odugbesan O, Addala A, Nelson G, et al. Implicit racial-ethnic and insurance-mediated bias to recommending diabetes technology: insights from T1D Exchange multicenter pediatric and adult diabetes provider cohort. Diabetes Technol Ther. 2022 Jun 13. [Epub ahead of print] doi:10.1089/dia.2022.0042
38. Schmitt J, Fogle K, Scott ML, Iyer P. Improving equitable access to continuous glucose monitors for Alabama’s children with type 1 diabetes: a quality improvement project. Diabetes Technol Ther. 2022;24(7):481-491. doi:10.1089/dia.2021.0511
39. Akturk HK, Agarwal S, Hoffecker L, Shah VN. Inequity in racial-ethnic representation in randomized controlled trials of diabetes technologies in type 1 diabetes: critical need for new standards. Diabetes Care. 2021;44(6):e121-e123. doi:10.2337/dc20-3063
40. Ebekozien O, Mungmode A, Buckingham D, et al. Achieving equity in diabetes research: borrowing from the field of quality improvement using a practical framework and improvement tools. Diabetes Spectr. 2022;35(3):304-312. doi:10.2237/dsi22-0002
41. Zhang J, Xu J, Lim J, et al. Wearable glucose monitoring and implantable drug delivery systems for diabetes management. Adv Healthc Mater. 2021;10(17):e2100194. doi:10.1002/adhm.202100194
42. FDA expands remote patient monitoring in hospitals for people with diabetes during COVID-19; manufacturers donate CGM supplies. News release. April 21, 2020. Accessed August 30, 2022. https://www.diabetes.org/newsroom/press-releases/2020/fda-remote-patient-monitoring-cgm
43. Campbell P. FDA grants Dexcom CGM breakthrough designation for in-hospital use. March 2, 2022. Accessed August 30, 2022. https://www.endocrinologynetwork.com/view/fda-grants-dexcom-cgm-breakthrough-designation-for-in-hospital-use
44. Yeh T, Yeung M, Mendelsohn Curanaj FA. Managing patients with insulin pumps and continuous glucose monitors in the hospital: to wear or not to wear. Curr Diab Rep. 2021;21(2):7. doi:10.1007/s11892-021-01375-7
45. Medtronic announces FDA approval for MiniMed 770G insulin pump system. News release. September 21, 2020. Accessed August 30, 2022. https://bit.ly/3TyEna4
46. Tandem Diabetes Care announces commercial launch of the t:slim X2 insulin pump with Control-IQ technology in the United States. News release. January 15, 2020. Accessed August 30, 2022. https://investor.tandemdiabetes.com/news-releases/news-release-details/tandem-diabetes-care-announces-commercial-launch-tslim-x2-0
47. Garza M, Gutow H, Mahoney K. Omnipod 5 cleared by the FDA. Updated August 22, 2022. Accessed August 30, 2022.https://diatribe.org/omnipod-5-approved-fda
48. Boughton CK. Fully closed-loop insulin delivery—are we nearly there yet? Lancet Digit Health. 2021;3(11):e689-e690. doi:10.1016/s2589-7500(21)00218-1
49. Noor N, Kamboj MK, Triolo T, et al. Hybrid closed-loop systems and glycemic outcomes in children and adults with type 1 diabetes: real-world evidence from a U.S.-based multicenter collaborative. Diabetes Care. 2022;45(8):e118-e119. doi:10.2337/dc22-0329
50. Medtronic launches InPen with real-time Guardian Connect CGM data--the first integrated smart insulin pen for people with diabetes on MDI. News release. November 12, 2020. Accessed August 30, 2022. https://bit.ly/3CTSWPL
51. Bigfoot Biomedical receives FDA clearance for Bigfoot Unity Diabetes Management System, featuring first-of-its-kind smart pen caps for insulin pens used to treat type 1 and type 2 diabetes. News release. May 10, 2021. Accessed August 30, 2022. https://bit.ly/3BeyoAh
52. Vieira G. All about the CeQur Simplicity insulin patch. Updated May 24, 2022. Accessed August 30, 2022. https://beyondtype1.org/cequr-simplicity-insulin-patch/.
53. Messer LH, Tanenbaum ML, Cook PF, et al. Cost, hassle, and on-body experience: barriers to diabetes device use in adolescents and potential intervention targets. Diabetes Technol Ther. 2020;22(10):760-767. doi:10.1089/dia.2019.0509
54. Hilliard ME, Levy W, Anderson BJ, et al. Benefits and barriers of continuous glucose monitoring in young children with type 1 diabetes. Diabetes Technol Ther. 2019;21(9):493-498. doi:10.1089/dia.2019.0142
55. Dexcom G7 Release Delayed Until Late 2022. News release. August 8, 2022. Accessed September 7, 2022. https://diatribe.org/dexcom-g7-release-delayed-until-late-2022
56. Drucker DJ. Transforming type 1 diabetes: the next wave of innovation. Diabetologia. 2021;64(5):1059-1065. doi:10.1007/s00125-021-05396-5
57. Garg SK, Rodriguez E, Shah VN, Hirsch IB. New medications for the treatment of diabetes. Diabetes Technol Ther. 2022;24(S1):S190-S208. doi:10.1089/dia.2022.2513
58. Melton D. The promise of stem cell-derived islet replacement therapy. Diabetologia. 2021;64(5):1030-1036. doi:10.1007/s00125-020-05367-2
59. Danne T, Heinemann L, Bolinder J. New insulins, biosimilars, and insulin therapy. Diabetes Technol Ther. 2022;24(S1):S35-S57. doi:10.1089/dia.2022.2503
60. Kenney J. Insulin copay caps–a path to affordability. July 6, 2021. Accessed August 30, 2022.https://diatribechange.org/news/insulin-copay-caps-path-affordability
61. Glied SA, Zhu B. Not so sweet: insulin affordability over time. September 25, 2020. Accessed August 30, 2022. https://www.commonwealthfund.org/publications/issue-briefs/2020/sep/not-so-sweet-insulin-affordability-over-time
62. American Diabetes Association. Insulin and drug affordability. Accessed August 30, 2022. https://www.diabetes.org/advocacy/insulin-and-drug-affordability
63. Sullivan P. Chances for drug pricing, surprise billing action fade until November. March 24, 2020. Accessed August 30, 2022. https://thehill.com/policy/healthcare/489334-chances-for-drug-pricing-surprise-billing-action-fade-until-november/
64. Brown TD. How Medicare’s new Senior Savings Model makes insulin more affordable. June 4, 2020. Accessed August 30, 2022. https://www.diabetes.org/blog/how-medicares-new-senior-savings-model-makes-insulin-more-affordable
65. American Diabetes Association. ADA applauds the U.S. House of Representatives passage of the Affordable Insulin Now Act. News release. April 1, 2022. https://www.diabetes.org/newsroom/official-statement/2022/ada-applauds-us-house-of-representatives-passage-of-the-affordable-insulin-now-act
66. JDRF. Driving T1D cures during challenging times. 2022.
67. Medtronic announces ongoing initiatives to address health equity for people of color living with diabetes. News release. April 7, 2021. Access August 30, 2022. https://bit.ly/3KGTOZU
1. Centers for Disease Control and Prevention. National diabetes statistics report. Accessed August 30, 2022. www.cdc.gov/diabetes/data/statistics-report/index.html
2. Centers for Disease Control and Prevention. Diabetes fast facts. Accessed August 30, 2022. www.cdc.gov/diabetes/basics/quick-facts.html
3. O’Malley G, Ebekozien O, Desimone M, et al. COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D Exchange Multicenter Surveillance Study. J Clin Endocrinol Metab. 2020;106(2):e936-e942. doi:10.1210/clinem/dgaa825
4. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care. 2020;43(8):e83-e85. doi:10.2337/dc20-1088
5. Zimmerman C, Albanese-O’Neill A, Haller MJ. Advances in type 1 diabetes technology over the last decade. Eur Endocrinol. 2019;15(2):70-76. doi:10.17925/ee.2019.15.2.70
6. Wake DJ, Gibb FW, Kar P, et al. Endocrinology in the time of COVID-19: remodelling diabetes services and emerging innovation. Eur J Endocrinol. 2020;183(2):G67-G77. doi:10.1530/eje-20-0377
7. Alonso GT, Corathers S, Shah A, et al. Establishment of the T1D Exchange Quality Improvement Collaborative (T1DX-QI). Clin Diabetes. 2020;38(2):141-151. doi:10.2337/cd19-0032
8. Ginnard OZB, Alonso GT, Corathers SD, et al. Quality improvement in diabetes care: a review of initiatives and outcomes in the T1D Exchange Quality Improvement Collaborative. Clin Diabetes. 2021;39(3):256-263. doi:10.2337/cd21-0029
9. ATTD 2021 invited speaker abstracts. Diabetes Technol Ther. 2021;23(S2):A1-A206. doi:10.1089/dia.2021.2525.abstracts
10. Rompicherla SN, Edelen N, Gallagher R, et al. Children and adolescent patients with pre-existing type 1 diabetes and additional comorbidities have an increased risk of hospitalization from COVID-19; data from the T1D Exchange COVID Registry. Pediatr Diabetes. 2021;22(S30):3-32. doi:10.1111/pedi.13268
11. Abstracts for the T1D Exchange QI Collaborative (T1DX-QI) Learning Session 2021. November 8-9, 2021. J Diabetes. 2021;13(S1):3-17. doi:10.1111/1753-0407.13227
12. The Official Journal of ATTD Advanced Technologies & Treatments for Diabetes conference 27-30 April 2022. Barcelona and online. Diabetes Technol Ther. 2022;24(S1):A1-A237. doi:10.1089/dia.2022.2525.abstracts
13. Ebekozien ON, Kamboj N, Odugbesan MK, et al. Inequities in glycemic outcomes for patients with type 1 diabetes: six-year (2016-2021) longitudinal follow-up by race and ethnicity of 36,390 patients in the T1DX-QI Collaborative. Diabetes. 2022;71(suppl 1). doi:10.2337/db22-167-OR
14. Narayan KA, Noor M, Rompicherla N, et al. No BMI increase during the COVID-pandemic in children and adults with T1D in three continents: joint analysis of ADDN, T1DX, and DPV registries. Diabetes. 2022;71(suppl 1). doi:10.2337/db22-269-OR
15. Lee JY, Lee SWH. Telemedicine cost-effectiveness for diabetes management: a systematic review. Diabetes Technol Ther. 2018;20(7):492-500. doi:10.1089/dia.2018.0098
16. McDonnell ME. Telemedicine in complex diabetes management. Curr Diab Rep. 2018;18(7):42. doi:10.1007/s11892-018-1015-3
17. Lee JM, Carlson E, Albanese-O’Neill A, et al. Adoption of telemedicine for type 1 diabetes care during the COVID-19 pandemic. Diabetes Technol Ther. 2021;23(9):642-651. doi:10.1089/dia.2021.0080
18. Phillip M, Bergenstal RM, Close KL, et al. The digital/virtual diabetes clinic: the future is now–recommendations from an international panel on diabetes digital technologies introduction. Diabetes Technol Ther. 2021;23(2):146-154. doi:10.1089/dia.2020.0375
19. Garg SK, Rodriguez E. COVID‐19 pandemic and diabetes care. Diabetes Technol Ther. 2022;24(S1):S2-S20. doi:10.1089/dia.2022.2501
20. Beliard K, Ebekozien O, Demeterco-Berggren C, et al. Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: data from a multi-site surveillance registry. J Diabetes. 2021;13(3):270-272. doi:10.1111/1753-0407.13141
21. Ebekozien O, Agarwal S, Noor N, et al. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab. 2020;106(4):1755-1762. doi:10.1210/clinem/dgaa920
22. Alonso GT, Ebekozien O, Gallagher MP, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes. 2021;13(8):681-687. doi:10.1111/1753-0407.13184
23. Noor N, Ebekozien O, Levin L, et al. Diabetes technology use for management of type 1 diabetes is associated with fewer adverse COVID-19 outcomes: findings from the T1D Exchange COVID-19 Surveillance Registry. Diabetes Care. 2021;44(8):e160-e162. doi:10.2337/dc21-0074
24. Demeterco-Berggren C, Ebekozien O, Rompicherla S, et al. Age and hospitalization risk in people with type 1 diabetes and COVID-19: data from the T1D Exchange Surveillance Study. J Clin Endocrinol Metab. 2021;107(2):410-418. doi:10.1210/clinem/dgab668
25. DeSalvo DJ, Noor N, Xie C, et al. Patient demographics and clinical outcomes among type 1 diabetes patients using continuous glucose monitors: data from T1D Exchange real-world observational study. J Diabetes Sci Technol. 2021 Oct 9. [Epub ahead of print] doi:10.1177/19322968211049783
26. Gallagher MP, Rompicherla S, Ebekozien O, et al. Differences in COVID-19 outcomes among patients with type 1 diabetes: first vs later surges. J Clin Outcomes Manage. 2022;29(1):27-31. doi:10.12788/jcom.0084
27. Wolf RM, Noor N, Izquierdo R, et al. Increase in newly diagnosed type 1 diabetes in youth during the COVID-19 pandemic in the United States: a multi-center analysis. Pediatr Diabetes. 2022;23(4):433-438. doi:10.1111/pedi.13328
28. Lavik AR, Ebekozien O, Noor N, et al. Trends in type 1 diabetic ketoacidosis during COVID-19 surges at 7 US centers: highest burden on non-Hispanic Black patients. J Clin Endocrinol Metab. 2022;107(7):1948-1955. doi:10.1210/clinem/dgac158
29. van der Linden J, Welsh JB, Hirsch IB, Garg SK. Real-time continuous glucose monitoring during the coronavirus disease 2019 pandemic and its impact on time in range. Diabetes Technol Ther. 2021;23(S1):S1-S7. doi:10.1089/dia.2020.0649
30. Nwosu BU, Al-Halbouni L, Parajuli S, et al. COVID-19 pandemic and pediatric type 1 diabetes: no significant change in glycemic control during the pandemic lockdown of 2020. Front Endocrinol (Lausanne). 2021;12:703905. doi:10.3389/fendo.2021.703905
31. Ellahham S. Artificial intelligence: the future for diabetes care. Am J Med. 2020;133(8):895-900. doi:10.1016/j.amjmed.2020.03.033
32. Nomura A, Noguchi M, Kometani M, et al. Artificial intelligence in current diabetes management and prediction. Curr Diab Rep. 2021;21(12):61. doi:10.1007/s11892-021-01423-2
33. Mungmode A, Noor N, Weinstock RS, et al. Making diabetes electronic medical record data actionable: promoting benchmarking and population health using the T1D Exchange Quality Improvement Portal. Clin Diabetes. Forthcoming 2022.
34. Lavizzo-Mourey RJ, Besser RE, Williams DR. Understanding and mitigating health inequities—past, current, and future directions. N Engl J Med. 2021;384(18):1681-1684. doi:10.1056/NEJMp2008628
35. Majidi S, Ebekozien O, Noor N, et al. Inequities in health outcomes in children and adults with type 1 diabetes: data from the T1D Exchange Quality Improvement Collaborative. Clin Diabetes. 2021;39(3):278-283. doi:10.2337/cd21-0028
36. Ebekozien O, Mungmode A, Odugbesan O, et al. Addressing type 1 diabetes health inequities in the United States: approaches from the T1D Exchange QI Collaborative. J Diabetes. 2022;14(1):79-82. doi:10.1111/1753-0407.13235
37. Odugbesan O, Addala A, Nelson G, et al. Implicit racial-ethnic and insurance-mediated bias to recommending diabetes technology: insights from T1D Exchange multicenter pediatric and adult diabetes provider cohort. Diabetes Technol Ther. 2022 Jun 13. [Epub ahead of print] doi:10.1089/dia.2022.0042
38. Schmitt J, Fogle K, Scott ML, Iyer P. Improving equitable access to continuous glucose monitors for Alabama’s children with type 1 diabetes: a quality improvement project. Diabetes Technol Ther. 2022;24(7):481-491. doi:10.1089/dia.2021.0511
39. Akturk HK, Agarwal S, Hoffecker L, Shah VN. Inequity in racial-ethnic representation in randomized controlled trials of diabetes technologies in type 1 diabetes: critical need for new standards. Diabetes Care. 2021;44(6):e121-e123. doi:10.2337/dc20-3063
40. Ebekozien O, Mungmode A, Buckingham D, et al. Achieving equity in diabetes research: borrowing from the field of quality improvement using a practical framework and improvement tools. Diabetes Spectr. 2022;35(3):304-312. doi:10.2237/dsi22-0002
41. Zhang J, Xu J, Lim J, et al. Wearable glucose monitoring and implantable drug delivery systems for diabetes management. Adv Healthc Mater. 2021;10(17):e2100194. doi:10.1002/adhm.202100194
42. FDA expands remote patient monitoring in hospitals for people with diabetes during COVID-19; manufacturers donate CGM supplies. News release. April 21, 2020. Accessed August 30, 2022. https://www.diabetes.org/newsroom/press-releases/2020/fda-remote-patient-monitoring-cgm
43. Campbell P. FDA grants Dexcom CGM breakthrough designation for in-hospital use. March 2, 2022. Accessed August 30, 2022. https://www.endocrinologynetwork.com/view/fda-grants-dexcom-cgm-breakthrough-designation-for-in-hospital-use
44. Yeh T, Yeung M, Mendelsohn Curanaj FA. Managing patients with insulin pumps and continuous glucose monitors in the hospital: to wear or not to wear. Curr Diab Rep. 2021;21(2):7. doi:10.1007/s11892-021-01375-7
45. Medtronic announces FDA approval for MiniMed 770G insulin pump system. News release. September 21, 2020. Accessed August 30, 2022. https://bit.ly/3TyEna4
46. Tandem Diabetes Care announces commercial launch of the t:slim X2 insulin pump with Control-IQ technology in the United States. News release. January 15, 2020. Accessed August 30, 2022. https://investor.tandemdiabetes.com/news-releases/news-release-details/tandem-diabetes-care-announces-commercial-launch-tslim-x2-0
47. Garza M, Gutow H, Mahoney K. Omnipod 5 cleared by the FDA. Updated August 22, 2022. Accessed August 30, 2022.https://diatribe.org/omnipod-5-approved-fda
48. Boughton CK. Fully closed-loop insulin delivery—are we nearly there yet? Lancet Digit Health. 2021;3(11):e689-e690. doi:10.1016/s2589-7500(21)00218-1
49. Noor N, Kamboj MK, Triolo T, et al. Hybrid closed-loop systems and glycemic outcomes in children and adults with type 1 diabetes: real-world evidence from a U.S.-based multicenter collaborative. Diabetes Care. 2022;45(8):e118-e119. doi:10.2337/dc22-0329
50. Medtronic launches InPen with real-time Guardian Connect CGM data--the first integrated smart insulin pen for people with diabetes on MDI. News release. November 12, 2020. Accessed August 30, 2022. https://bit.ly/3CTSWPL
51. Bigfoot Biomedical receives FDA clearance for Bigfoot Unity Diabetes Management System, featuring first-of-its-kind smart pen caps for insulin pens used to treat type 1 and type 2 diabetes. News release. May 10, 2021. Accessed August 30, 2022. https://bit.ly/3BeyoAh
52. Vieira G. All about the CeQur Simplicity insulin patch. Updated May 24, 2022. Accessed August 30, 2022. https://beyondtype1.org/cequr-simplicity-insulin-patch/.
53. Messer LH, Tanenbaum ML, Cook PF, et al. Cost, hassle, and on-body experience: barriers to diabetes device use in adolescents and potential intervention targets. Diabetes Technol Ther. 2020;22(10):760-767. doi:10.1089/dia.2019.0509
54. Hilliard ME, Levy W, Anderson BJ, et al. Benefits and barriers of continuous glucose monitoring in young children with type 1 diabetes. Diabetes Technol Ther. 2019;21(9):493-498. doi:10.1089/dia.2019.0142
55. Dexcom G7 Release Delayed Until Late 2022. News release. August 8, 2022. Accessed September 7, 2022. https://diatribe.org/dexcom-g7-release-delayed-until-late-2022
56. Drucker DJ. Transforming type 1 diabetes: the next wave of innovation. Diabetologia. 2021;64(5):1059-1065. doi:10.1007/s00125-021-05396-5
57. Garg SK, Rodriguez E, Shah VN, Hirsch IB. New medications for the treatment of diabetes. Diabetes Technol Ther. 2022;24(S1):S190-S208. doi:10.1089/dia.2022.2513
58. Melton D. The promise of stem cell-derived islet replacement therapy. Diabetologia. 2021;64(5):1030-1036. doi:10.1007/s00125-020-05367-2
59. Danne T, Heinemann L, Bolinder J. New insulins, biosimilars, and insulin therapy. Diabetes Technol Ther. 2022;24(S1):S35-S57. doi:10.1089/dia.2022.2503
60. Kenney J. Insulin copay caps–a path to affordability. July 6, 2021. Accessed August 30, 2022.https://diatribechange.org/news/insulin-copay-caps-path-affordability
61. Glied SA, Zhu B. Not so sweet: insulin affordability over time. September 25, 2020. Accessed August 30, 2022. https://www.commonwealthfund.org/publications/issue-briefs/2020/sep/not-so-sweet-insulin-affordability-over-time
62. American Diabetes Association. Insulin and drug affordability. Accessed August 30, 2022. https://www.diabetes.org/advocacy/insulin-and-drug-affordability
63. Sullivan P. Chances for drug pricing, surprise billing action fade until November. March 24, 2020. Accessed August 30, 2022. https://thehill.com/policy/healthcare/489334-chances-for-drug-pricing-surprise-billing-action-fade-until-november/
64. Brown TD. How Medicare’s new Senior Savings Model makes insulin more affordable. June 4, 2020. Accessed August 30, 2022. https://www.diabetes.org/blog/how-medicares-new-senior-savings-model-makes-insulin-more-affordable
65. American Diabetes Association. ADA applauds the U.S. House of Representatives passage of the Affordable Insulin Now Act. News release. April 1, 2022. https://www.diabetes.org/newsroom/official-statement/2022/ada-applauds-us-house-of-representatives-passage-of-the-affordable-insulin-now-act
66. JDRF. Driving T1D cures during challenging times. 2022.
67. Medtronic announces ongoing initiatives to address health equity for people of color living with diabetes. News release. April 7, 2021. Access August 30, 2022. https://bit.ly/3KGTOZU
Single Institution Retrospective Review of Patterns of Care and Disease Presentation in Female Veterans With Breast Cancer During the COVID-19 Pandemic
Background
Delays in care can impact patient satisfaction and survival outcomes. There are no studies in the literature evaluating the care continuum in veterans with breast cancer. A study of this predominantly African American female veteran population will help us understand barriers to care in this population.
Methods
A retrospective review of 87 patients diagnosed with breast cancer in the year 2021 at the Atlanta VA Medical Center was conducted to assess current care patterns as well as disease characteristics. Patients were included if their initial diagnostic evaluation and therapy for stage I-III breast cancer was at the Atlanta VA. Patients with a history of noncompliance causing delays in care were excluded from analysis. A total of 20 patients were identified for final analysis.
Results
Veterans were predominately African American (85%). Median age was 61 years. Stage at presentation was as follows: stage 1(35%) stage II (30%) and stage III (35%). Receptor status was as follows: hormone receptor positive (35%), Triple negative (35%), and HER-2/neu positive (30%). Genetic testing and genomic assays were completed in 100% of eligible patients per NCCN guidelines. Lumpectomy was performed in 44% of cases and mastectomy in 55% of cases. 40% of cases where mastectomy was performed were done for patient preference alone. Median time for various phases of care were as follows: symptomatic presentation to diagnostic imaging 48 days (range, 7-146), abnormal screening mammogram to diagnostic mammogram 6 days (range, 0-74), diagnostic imaging to diagnostic biopsy 15.5 days (range, 0-43), diagnostic biopsy to initiation of neoadjuvant systemic therapy 22 days (range, 14-31), diagnosis or completion of neoadjuvant systemic therapy to breast cancer surgery 58 days (range, 15-113), and surgery to initiation of adjuvant chemotherapy 33 days (range, 14-44).
Conclusions
In comparison to national statistics there was a higher incidence of HER-2/neu positivity (15% vs 30%) and triple negative (12% vs 35%) subtypes, highlighting the need for quicker diagnostic testing. The delay from symptomatic presentation to diagnostic mammogram and biopsy necessitates a response given that high-risk presentations account for 75% of the cases. These findings demonstrate the need for in-house mammography to care for this high-risk minority veteran population.
Background
Delays in care can impact patient satisfaction and survival outcomes. There are no studies in the literature evaluating the care continuum in veterans with breast cancer. A study of this predominantly African American female veteran population will help us understand barriers to care in this population.
Methods
A retrospective review of 87 patients diagnosed with breast cancer in the year 2021 at the Atlanta VA Medical Center was conducted to assess current care patterns as well as disease characteristics. Patients were included if their initial diagnostic evaluation and therapy for stage I-III breast cancer was at the Atlanta VA. Patients with a history of noncompliance causing delays in care were excluded from analysis. A total of 20 patients were identified for final analysis.
Results
Veterans were predominately African American (85%). Median age was 61 years. Stage at presentation was as follows: stage 1(35%) stage II (30%) and stage III (35%). Receptor status was as follows: hormone receptor positive (35%), Triple negative (35%), and HER-2/neu positive (30%). Genetic testing and genomic assays were completed in 100% of eligible patients per NCCN guidelines. Lumpectomy was performed in 44% of cases and mastectomy in 55% of cases. 40% of cases where mastectomy was performed were done for patient preference alone. Median time for various phases of care were as follows: symptomatic presentation to diagnostic imaging 48 days (range, 7-146), abnormal screening mammogram to diagnostic mammogram 6 days (range, 0-74), diagnostic imaging to diagnostic biopsy 15.5 days (range, 0-43), diagnostic biopsy to initiation of neoadjuvant systemic therapy 22 days (range, 14-31), diagnosis or completion of neoadjuvant systemic therapy to breast cancer surgery 58 days (range, 15-113), and surgery to initiation of adjuvant chemotherapy 33 days (range, 14-44).
Conclusions
In comparison to national statistics there was a higher incidence of HER-2/neu positivity (15% vs 30%) and triple negative (12% vs 35%) subtypes, highlighting the need for quicker diagnostic testing. The delay from symptomatic presentation to diagnostic mammogram and biopsy necessitates a response given that high-risk presentations account for 75% of the cases. These findings demonstrate the need for in-house mammography to care for this high-risk minority veteran population.
Background
Delays in care can impact patient satisfaction and survival outcomes. There are no studies in the literature evaluating the care continuum in veterans with breast cancer. A study of this predominantly African American female veteran population will help us understand barriers to care in this population.
Methods
A retrospective review of 87 patients diagnosed with breast cancer in the year 2021 at the Atlanta VA Medical Center was conducted to assess current care patterns as well as disease characteristics. Patients were included if their initial diagnostic evaluation and therapy for stage I-III breast cancer was at the Atlanta VA. Patients with a history of noncompliance causing delays in care were excluded from analysis. A total of 20 patients were identified for final analysis.
Results
Veterans were predominately African American (85%). Median age was 61 years. Stage at presentation was as follows: stage 1(35%) stage II (30%) and stage III (35%). Receptor status was as follows: hormone receptor positive (35%), Triple negative (35%), and HER-2/neu positive (30%). Genetic testing and genomic assays were completed in 100% of eligible patients per NCCN guidelines. Lumpectomy was performed in 44% of cases and mastectomy in 55% of cases. 40% of cases where mastectomy was performed were done for patient preference alone. Median time for various phases of care were as follows: symptomatic presentation to diagnostic imaging 48 days (range, 7-146), abnormal screening mammogram to diagnostic mammogram 6 days (range, 0-74), diagnostic imaging to diagnostic biopsy 15.5 days (range, 0-43), diagnostic biopsy to initiation of neoadjuvant systemic therapy 22 days (range, 14-31), diagnosis or completion of neoadjuvant systemic therapy to breast cancer surgery 58 days (range, 15-113), and surgery to initiation of adjuvant chemotherapy 33 days (range, 14-44).
Conclusions
In comparison to national statistics there was a higher incidence of HER-2/neu positivity (15% vs 30%) and triple negative (12% vs 35%) subtypes, highlighting the need for quicker diagnostic testing. The delay from symptomatic presentation to diagnostic mammogram and biopsy necessitates a response given that high-risk presentations account for 75% of the cases. These findings demonstrate the need for in-house mammography to care for this high-risk minority veteran population.




