User login
Post Pandemic Return to Colorectal Cancer Screening
Purpose/Background
Colorectal cancer (CRC) screening was significantly curtailed due to the COVID-19 pandemic and Hines VA Medical Center in Illinois performed 50% fewer screening colonoscopies in 2020 compared to 2019 (pre-pandemic). This quality study aimed to increase use of fecal immunochemical tests (FIT) as an alternative screening method while in-person screening was limited. The primary goal was to return to pre-pandemic rates of screening (colonoscopy + FIT) and the secondary goal was to increase monthly screenings by 10% to address the backlog of patients not screened early in the pandemic.
Methods/Data Analysis
Using Plan-Do-StudyAct (PDSA) quality improvement methodology, a multidisciplinary team led by Primary Care, Gastroenterology and Laboratory/Pathology services, standardized processes for dissemination and processing of FIT tests. The first PDSA cycle implemented utilization of Colorectal Cancer Screening & Surveillance Clinical Reports (CRCS/S) to identify average-risk patients due or overdue for screening, devised plain language patient instructions for FIT-based testing, and formalized a mechanism for tracking FIT test kits.
Results
Baseline number of CRC screenings in 2019 was 2,808 (750 colonoscopy + 2,058 FIT). After the first PDSA cycle, CRC screenings were recorded during the 12-month period from April 2021 to March 2022. Colonoscopy + FIT increased to 3,558, largely due to an increase in completed FIT tests (362 colonoscopy + 3,196 FIT tests). While the number of screening colonoscopies was 52% lower compared to 2019, the number of patients screened with FIT increased by 55% after the intervention. Colonoscopy + FIT in the 12 month period starting in April of 2021 exceeded that of 2019, supporting the fact that stoolbased FIT testing was a feasible approach to screening average risk patients while in-person screening activities were restricted.
Conclusions
This quality improvement study met the primary goal of returning to pre-pandemic rates of colonoscopy + FIT and the secondary goal of increasing average number of monthly screenings by 10% to address the backlog of patients not screened early in the pandemic. Interventions directed at optimizing the FIT test process were associated with an increase in completed FIT tests. Planned PDSA cycle two will implement a mailed FIT Outreach pilot to reach additional patients for CRC screening.
Purpose/Background
Colorectal cancer (CRC) screening was significantly curtailed due to the COVID-19 pandemic and Hines VA Medical Center in Illinois performed 50% fewer screening colonoscopies in 2020 compared to 2019 (pre-pandemic). This quality study aimed to increase use of fecal immunochemical tests (FIT) as an alternative screening method while in-person screening was limited. The primary goal was to return to pre-pandemic rates of screening (colonoscopy + FIT) and the secondary goal was to increase monthly screenings by 10% to address the backlog of patients not screened early in the pandemic.
Methods/Data Analysis
Using Plan-Do-StudyAct (PDSA) quality improvement methodology, a multidisciplinary team led by Primary Care, Gastroenterology and Laboratory/Pathology services, standardized processes for dissemination and processing of FIT tests. The first PDSA cycle implemented utilization of Colorectal Cancer Screening & Surveillance Clinical Reports (CRCS/S) to identify average-risk patients due or overdue for screening, devised plain language patient instructions for FIT-based testing, and formalized a mechanism for tracking FIT test kits.
Results
Baseline number of CRC screenings in 2019 was 2,808 (750 colonoscopy + 2,058 FIT). After the first PDSA cycle, CRC screenings were recorded during the 12-month period from April 2021 to March 2022. Colonoscopy + FIT increased to 3,558, largely due to an increase in completed FIT tests (362 colonoscopy + 3,196 FIT tests). While the number of screening colonoscopies was 52% lower compared to 2019, the number of patients screened with FIT increased by 55% after the intervention. Colonoscopy + FIT in the 12 month period starting in April of 2021 exceeded that of 2019, supporting the fact that stoolbased FIT testing was a feasible approach to screening average risk patients while in-person screening activities were restricted.
Conclusions
This quality improvement study met the primary goal of returning to pre-pandemic rates of colonoscopy + FIT and the secondary goal of increasing average number of monthly screenings by 10% to address the backlog of patients not screened early in the pandemic. Interventions directed at optimizing the FIT test process were associated with an increase in completed FIT tests. Planned PDSA cycle two will implement a mailed FIT Outreach pilot to reach additional patients for CRC screening.
Purpose/Background
Colorectal cancer (CRC) screening was significantly curtailed due to the COVID-19 pandemic and Hines VA Medical Center in Illinois performed 50% fewer screening colonoscopies in 2020 compared to 2019 (pre-pandemic). This quality study aimed to increase use of fecal immunochemical tests (FIT) as an alternative screening method while in-person screening was limited. The primary goal was to return to pre-pandemic rates of screening (colonoscopy + FIT) and the secondary goal was to increase monthly screenings by 10% to address the backlog of patients not screened early in the pandemic.
Methods/Data Analysis
Using Plan-Do-StudyAct (PDSA) quality improvement methodology, a multidisciplinary team led by Primary Care, Gastroenterology and Laboratory/Pathology services, standardized processes for dissemination and processing of FIT tests. The first PDSA cycle implemented utilization of Colorectal Cancer Screening & Surveillance Clinical Reports (CRCS/S) to identify average-risk patients due or overdue for screening, devised plain language patient instructions for FIT-based testing, and formalized a mechanism for tracking FIT test kits.
Results
Baseline number of CRC screenings in 2019 was 2,808 (750 colonoscopy + 2,058 FIT). After the first PDSA cycle, CRC screenings were recorded during the 12-month period from April 2021 to March 2022. Colonoscopy + FIT increased to 3,558, largely due to an increase in completed FIT tests (362 colonoscopy + 3,196 FIT tests). While the number of screening colonoscopies was 52% lower compared to 2019, the number of patients screened with FIT increased by 55% after the intervention. Colonoscopy + FIT in the 12 month period starting in April of 2021 exceeded that of 2019, supporting the fact that stoolbased FIT testing was a feasible approach to screening average risk patients while in-person screening activities were restricted.
Conclusions
This quality improvement study met the primary goal of returning to pre-pandemic rates of colonoscopy + FIT and the secondary goal of increasing average number of monthly screenings by 10% to address the backlog of patients not screened early in the pandemic. Interventions directed at optimizing the FIT test process were associated with an increase in completed FIT tests. Planned PDSA cycle two will implement a mailed FIT Outreach pilot to reach additional patients for CRC screening.
Children and COVID: New cases took a downturn in September
After 2 weeks of increases in the number of new COVID-19 cases in children – a trend that just happened to coincide with the start of a new school year – there were fewer cases reported during the first full week of September, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, the AAP and CHA said in their weekly COVID-19 report, noting also that seven states and the District of Columbia no longer update their online dashboards while others publish new data less often than every week.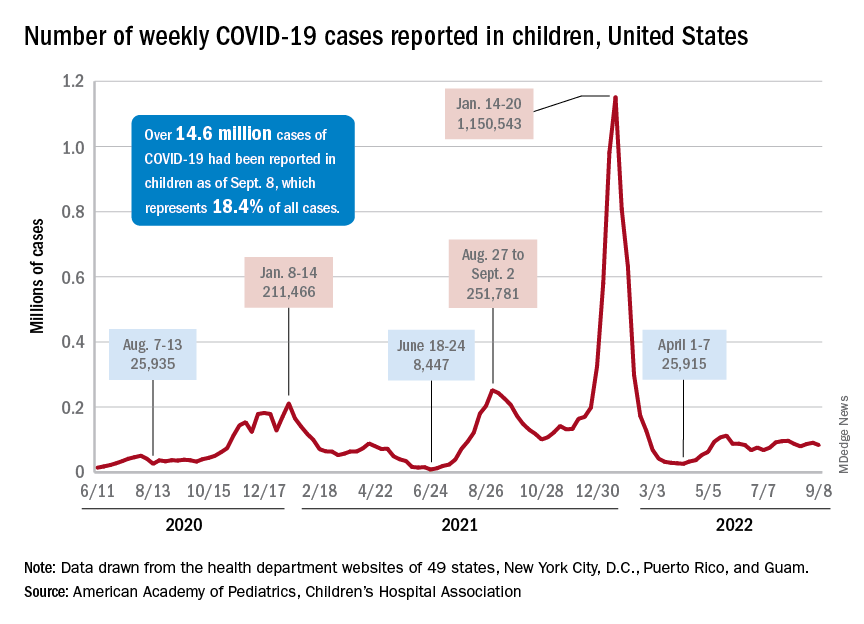
The drop in new cases was accompanied by declines in emergency department visits and hospital admissions, both of which had shown some signs of resurgence in mid- to late August. The brief rise in ED visits seemed to be age-related, occurring in those aged 12 years and older but not in younger children, whose ED visit rate fell steadily through August. Through the first week of September, however, 7-day averages were down for both those aged 12-15 and for 16- to 17-year-olds, the Centers for Disease Control and Prevention reported.
The rate of new hospital admissions of children with confirmed COVID-19, available only for ages 0-17 years, has declined every day since Aug. 28, when it reached 0.44 per 100,000 population after a week of climbing, the CDC said on its COVID Data Tracker.
Cumulatively, about 156,000 children were hospitalized with COVID from Aug. 1, 2020 to Sept. 10, 2022, according to the CDC, which puts the total number of pediatric cases at just over 15 million and deaths at 1,778. Those last two figures represent 17.4% and about 0.4% of all U.S. cases and deaths. The AAP and CHA estimate that about 14.6 million child cases have been reported so far, which is 18.4% of cases in all ages.
Vaccinations are slowly adding up
On the prevention side of the health care system’s response to COVID, the CDC’s cumulative numbers looked like this as of Sept. 6:
- 1.1 million children under age 5 (about 5.8% of the age group) had received at least one dose of vaccine, and 280,000 (1.4%) were fully vaccinated.
- Almost 11 million (38.2%) children aged 5-11 had gotten one dose, and 8.9 million (31.1%) were fully vaccinated.
- 17.9 million (70.8%) children aged 12-17 had received at least one dose, and 15.3 million (60.5%) were fully vaccinated.
Over the 14 days ending Sept. 7, children aged 2-4 years made up the largest group (21.4%) of Americans getting their first vaccine doses, while those aged 5-11 years were the third largest age group at 16.7% of all vaccinees (25- to 49-year-olds were second). The situation was reversed for vaccine completion over the last 2 weeks: Those aged 5-11 were first at 24.7%, and the 2- to 4-year-olds were third at 16.7% (those aged 25-49 were second again), according to the COVID Data Tracker.
After 2 weeks of increases in the number of new COVID-19 cases in children – a trend that just happened to coincide with the start of a new school year – there were fewer cases reported during the first full week of September, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, the AAP and CHA said in their weekly COVID-19 report, noting also that seven states and the District of Columbia no longer update their online dashboards while others publish new data less often than every week.
The drop in new cases was accompanied by declines in emergency department visits and hospital admissions, both of which had shown some signs of resurgence in mid- to late August. The brief rise in ED visits seemed to be age-related, occurring in those aged 12 years and older but not in younger children, whose ED visit rate fell steadily through August. Through the first week of September, however, 7-day averages were down for both those aged 12-15 and for 16- to 17-year-olds, the Centers for Disease Control and Prevention reported.
The rate of new hospital admissions of children with confirmed COVID-19, available only for ages 0-17 years, has declined every day since Aug. 28, when it reached 0.44 per 100,000 population after a week of climbing, the CDC said on its COVID Data Tracker.
Cumulatively, about 156,000 children were hospitalized with COVID from Aug. 1, 2020 to Sept. 10, 2022, according to the CDC, which puts the total number of pediatric cases at just over 15 million and deaths at 1,778. Those last two figures represent 17.4% and about 0.4% of all U.S. cases and deaths. The AAP and CHA estimate that about 14.6 million child cases have been reported so far, which is 18.4% of cases in all ages.
Vaccinations are slowly adding up
On the prevention side of the health care system’s response to COVID, the CDC’s cumulative numbers looked like this as of Sept. 6:
- 1.1 million children under age 5 (about 5.8% of the age group) had received at least one dose of vaccine, and 280,000 (1.4%) were fully vaccinated.
- Almost 11 million (38.2%) children aged 5-11 had gotten one dose, and 8.9 million (31.1%) were fully vaccinated.
- 17.9 million (70.8%) children aged 12-17 had received at least one dose, and 15.3 million (60.5%) were fully vaccinated.
Over the 14 days ending Sept. 7, children aged 2-4 years made up the largest group (21.4%) of Americans getting their first vaccine doses, while those aged 5-11 years were the third largest age group at 16.7% of all vaccinees (25- to 49-year-olds were second). The situation was reversed for vaccine completion over the last 2 weeks: Those aged 5-11 were first at 24.7%, and the 2- to 4-year-olds were third at 16.7% (those aged 25-49 were second again), according to the COVID Data Tracker.
After 2 weeks of increases in the number of new COVID-19 cases in children – a trend that just happened to coincide with the start of a new school year – there were fewer cases reported during the first full week of September, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, the AAP and CHA said in their weekly COVID-19 report, noting also that seven states and the District of Columbia no longer update their online dashboards while others publish new data less often than every week.
The drop in new cases was accompanied by declines in emergency department visits and hospital admissions, both of which had shown some signs of resurgence in mid- to late August. The brief rise in ED visits seemed to be age-related, occurring in those aged 12 years and older but not in younger children, whose ED visit rate fell steadily through August. Through the first week of September, however, 7-day averages were down for both those aged 12-15 and for 16- to 17-year-olds, the Centers for Disease Control and Prevention reported.
The rate of new hospital admissions of children with confirmed COVID-19, available only for ages 0-17 years, has declined every day since Aug. 28, when it reached 0.44 per 100,000 population after a week of climbing, the CDC said on its COVID Data Tracker.
Cumulatively, about 156,000 children were hospitalized with COVID from Aug. 1, 2020 to Sept. 10, 2022, according to the CDC, which puts the total number of pediatric cases at just over 15 million and deaths at 1,778. Those last two figures represent 17.4% and about 0.4% of all U.S. cases and deaths. The AAP and CHA estimate that about 14.6 million child cases have been reported so far, which is 18.4% of cases in all ages.
Vaccinations are slowly adding up
On the prevention side of the health care system’s response to COVID, the CDC’s cumulative numbers looked like this as of Sept. 6:
- 1.1 million children under age 5 (about 5.8% of the age group) had received at least one dose of vaccine, and 280,000 (1.4%) were fully vaccinated.
- Almost 11 million (38.2%) children aged 5-11 had gotten one dose, and 8.9 million (31.1%) were fully vaccinated.
- 17.9 million (70.8%) children aged 12-17 had received at least one dose, and 15.3 million (60.5%) were fully vaccinated.
Over the 14 days ending Sept. 7, children aged 2-4 years made up the largest group (21.4%) of Americans getting their first vaccine doses, while those aged 5-11 years were the third largest age group at 16.7% of all vaccinees (25- to 49-year-olds were second). The situation was reversed for vaccine completion over the last 2 weeks: Those aged 5-11 were first at 24.7%, and the 2- to 4-year-olds were third at 16.7% (those aged 25-49 were second again), according to the COVID Data Tracker.
57-year-old man • type 2 diabetes • neuropathy • bilateral foot blisters • Dx?
THE CASE
A 57-year-old man with type 2 diabetes, hyperlipidemia, and obesity presented to the emergency department (ED) for bilateral foot blisters, both of which appeared 1 day prior to evaluation. The patient’s history also included right-side Charcot foot diagnosed 4 years earlier and right foot osteomyelitis diagnosed 2 years prior. He had ongoing neuropathy in both feet but denied any significant pain.
The patient wore orthotics daily and he’d had new orthotics made 6 months prior; however, a recent COVID-19 diagnosis and prolonged hospital stay resulted in a 30-pound weight loss and decreased swelling in his ankles. He acquired new shoes 2 weeks prior to ED presentation.
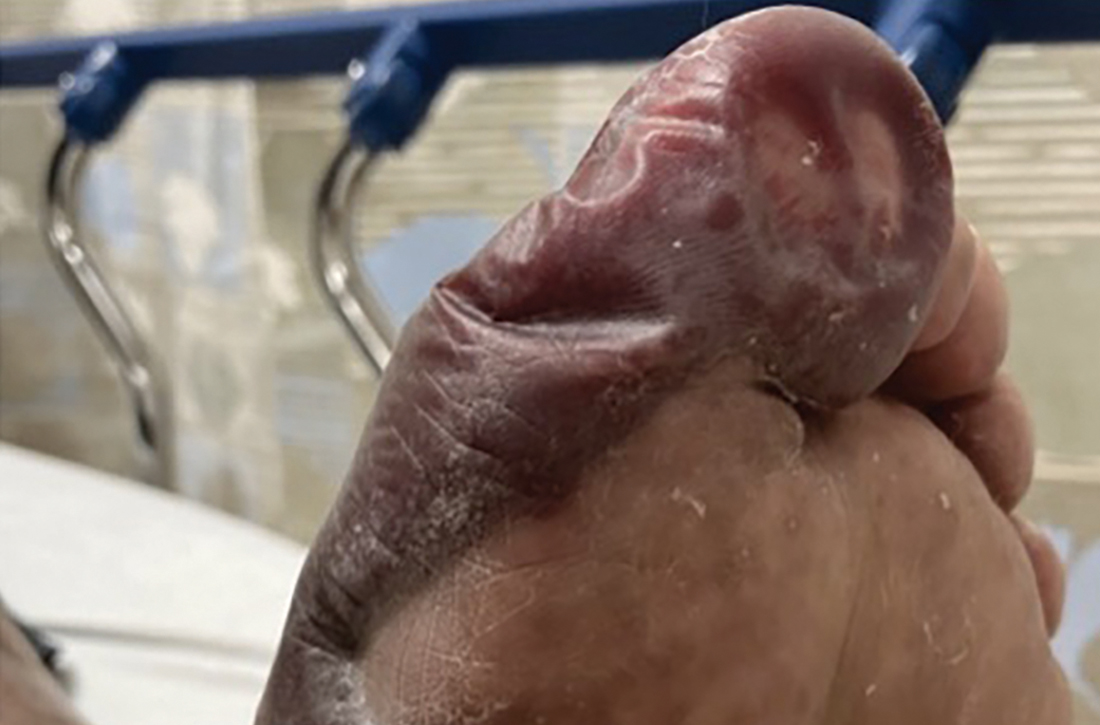
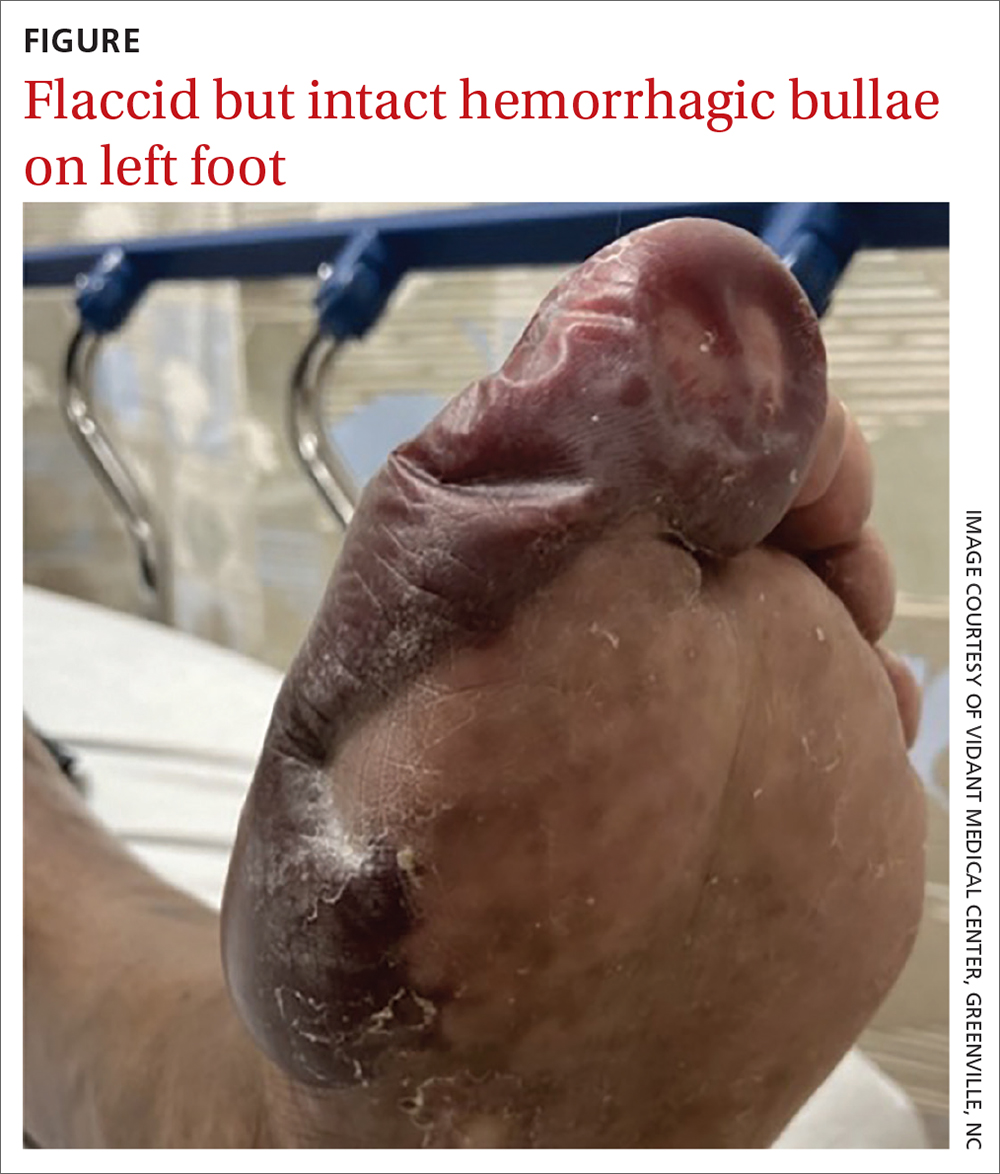
Physical examination revealed large blisters along the medial aspect of the patient’s feet, with both hemorrhagic and serous fluid-filled bullae. The lesions were flaccid but intact, without drainage or surrounding erythema, warmth, or tenderness. The blister on the left foot measured 8 x 5 cm and extended from the great toe to mid-arch (FIGURE), while the one on the right foot measured 8 x 3 cm and extended from the great toe to the base of the proximal arch. Sensation was decreased in the bilateral first and second digits but unchanged from prior documented exams. Bilateral dorsalis pedis pulses were normal.
Work-up included imaging and lab work. The patient’s complete blood count was normal, as were his erythrocyte sedimentation rate and C-reactive protein level. Radiographs of the right foot were normal, but those of the left foot were concerning, although inconclusive, for osteomyelitis. Further evaluation with magnetic resonance imaging of his left foot revealed a deformity of the first digit with some subchondral signal change that was thought to be posttraumatic or degenerative, but unlikely osteomyelitis.
THE DIAGNOSIS
Podiatry was consulted for blister management. Based on atraumatic history, rapid appearance, location of blisters, unremarkable lab work and imaging, and concurrent diabetes, the patient received a diagnosis of bilateral bullous diabeticorum (BD).
DISCUSSION
Roughly one-third of patients with diabetes will experience some cutaneous adverse effect because of the disease.1 Common iterations include acanthosis nigricans, rash, or even infection.2 BD is a rare bullous skin lesion that occurs in patients with diabetes; it has a reported annual incidence of 0.16% and may be underdiagnosed.1
Cases of BD have been described both in patients with longstanding diabetes and in those newly diagnosed, although the former group is more often affected.1 BD is reported more frequently in males than females, at a ratio of 2:1.1,3 Patients ages 17 to 80 years (average age, 55 years) have received a diagnosis of BD.1 Most affected patients will have a concomitant peripheral neuropathy and sometimes nephropathy or retinopathy.1
Continue to: The etiology of BD...
The etiology of BD is unclear but appears to be multifactorial. Hypotheses suggest that there’s a link to neuropathy/nephropathy, excessive exposure to ultraviolet light, or a vascular cause secondary to hyaline deposition in the capillary walls.4,5
What you’ll see at presentation
The typical manifestation of BD is the rapid appearance of tense blisters, which may occur overnight or even within hours.1 They are usually painless; common locations include the feet, distal legs, hands, and forearms.1,5 The bullae can be serous or hemorrhagic.1
Most notable in the patient’s history will be a lack of trauma or injury to the area.1 Although A1C values do not correlate with blister formation, patients with hypoglycemic episodes and highly varying blood glucose values seem to have higher rates of occurrence.1
Other sources of blistering must be ruled out
The diagnosis of BD is clinical and based on history, exam, and exclusion of other bullous diagnoses.6 A key clue in the history is the spontaneous and rapid onset without associated trauma in a patient with diabetes.6 Direct immunofluorescence, although nonspecific, can be helpful to rule out other disorders (such as porphyria cutanea tarda and bullous pemphigoid) if the history and exam are inconclusive. Direct and indirect immunofluorescence is typically negative in BD.4,6
The differential diagnosis includes other conditions that involve bullae—such as frictional bullae, bullous pemphigoid, and bullous systemic lupus erythematosus—as well as porphyria, erythema multiforme, insect bites, or even fixed drug eruption.2,7
Continue to: Porphyria
Porphyria tends to develop on the hands, whereas BD most commonly occurs on the feet.5
Erythema multiforme typically includes inflammatory skin changes.5
Trauma or fixed drug eruption as a cause of blistering lesions would be revealed during history taking.
Considerations for treatment and follow-up
Without treatment, blisters often self-resolve in 2 to 6 weeks, but there is high likelihood of recurrence.6,8 There is no consensus on treatment, although a typical course of action is to deroof the blister and examine the area to rule out infection.6 The wound is then covered with wet-to-dry gauze that is changed regularly. If there is suspicion for or signs of underlying infection, such as an ulcer or skin necrosis, antibiotics should be included in the treatment plan.7
Additional considerations. Patients will often need therapeutic footwear if the blisters are located on the feet. Given the higher prevalence of microvascular complications in patients with diabetes who develop BD, routine ophthalmologic examination and renal function testing to monitor for microalbuminuria are recommended.5
Our patient underwent bedside incision and drainage and was discharged home with appropriate wound care and follow-up.
THE TAKEAWAY
BD cases may be underdiagnosed in clinical practice, perhaps due to patients not seeking help for a seemingly nonthreatening condition or lack of clinician recognition that bullae are related to a patient’s diabetes status. Prompt recognition and proper wound care are important to prevent poor outcomes, such as ulceration or necrosis.
CORRESPONDENCE
Kathleen S. Kinderwater, MD, 101 Heart Drive, Greenville, NC 27834; [email protected]
1. Larsen K, Jensen T, Karlsmark T, et al. Incidence of bullosis diabeticorum—a controversial cause of chronic foot ulceration. Int Wound J. 2008;5:591-596. doi: 10.1111/j.1742-481X.2008.00476.x
2. Lipsky BA, Baker PD, Ahroni JH. Diabetic bullae: 12 cases of a purportedly rare cutaneous disorder. Int J Dermatol. 2000;39:196-200. doi: 10.1046/j.1365-4362.2000.00947.x
3. Gupta V, Gulati N, Bahl J, et al. Bullosis diabeticorum: rare presentation in a common disease. Case Rep Endocrinol. 2014;2014:862912.
4. Sonani H, Abdul Salim S, Garla VV, et al. Bullosis diabeticorum: a rare presentation with immunoglobulin G (IgG) deposition related vasculopathy. Case report and focused review. Am J Case Rep. 2018;19:52-56. doi: 10.12659/ajcr.905452
5. Chouk C, Litaiem N. Bullosis diabeticorum. StatPearls [Internet]. Updated June 5, 2021. Accessed July 14, 2022. www.ncbi.nlm.nih.gov/books/NBK539872/
6. Chatterjee D, Radotra A, Radotra BD, et al. Bullous diabeticorum: a rare blistering manifestation of diabetes. Indian Dermatol Online J. 2017;8:274-275. doi: 10.4103/idoj.IDOJ_340_16
7. Kansal NK, Anuragi RP. Bullous lesions in diabetes mellitus: bullous diabeticorum (diabetic bulla). BMJ Case Rep. 2020;13:e238617. doi: 10.1136/bcr-2020-238617
8. Bello F, Samaila OM, Lawal Y, et al. 2 cases of bullosis diabeticorum following long-distance journeys by road: a report of 2 cases. Case Rep Endocrinol. 2012;2012:367218. doi: 10.1155/2012/367218
THE CASE
A 57-year-old man with type 2 diabetes, hyperlipidemia, and obesity presented to the emergency department (ED) for bilateral foot blisters, both of which appeared 1 day prior to evaluation. The patient’s history also included right-side Charcot foot diagnosed 4 years earlier and right foot osteomyelitis diagnosed 2 years prior. He had ongoing neuropathy in both feet but denied any significant pain.
The patient wore orthotics daily and he’d had new orthotics made 6 months prior; however, a recent COVID-19 diagnosis and prolonged hospital stay resulted in a 30-pound weight loss and decreased swelling in his ankles. He acquired new shoes 2 weeks prior to ED presentation.
Physical examination revealed large blisters along the medial aspect of the patient’s feet, with both hemorrhagic and serous fluid-filled bullae. The lesions were flaccid but intact, without drainage or surrounding erythema, warmth, or tenderness. The blister on the left foot measured 8 x 5 cm and extended from the great toe to mid-arch (FIGURE), while the one on the right foot measured 8 x 3 cm and extended from the great toe to the base of the proximal arch. Sensation was decreased in the bilateral first and second digits but unchanged from prior documented exams. Bilateral dorsalis pedis pulses were normal.

Work-up included imaging and lab work. The patient’s complete blood count was normal, as were his erythrocyte sedimentation rate and C-reactive protein level. Radiographs of the right foot were normal, but those of the left foot were concerning, although inconclusive, for osteomyelitis. Further evaluation with magnetic resonance imaging of his left foot revealed a deformity of the first digit with some subchondral signal change that was thought to be posttraumatic or degenerative, but unlikely osteomyelitis.
THE DIAGNOSIS
Podiatry was consulted for blister management. Based on atraumatic history, rapid appearance, location of blisters, unremarkable lab work and imaging, and concurrent diabetes, the patient received a diagnosis of bilateral bullous diabeticorum (BD).
DISCUSSION
Roughly one-third of patients with diabetes will experience some cutaneous adverse effect because of the disease.1 Common iterations include acanthosis nigricans, rash, or even infection.2 BD is a rare bullous skin lesion that occurs in patients with diabetes; it has a reported annual incidence of 0.16% and may be underdiagnosed.1
Cases of BD have been described both in patients with longstanding diabetes and in those newly diagnosed, although the former group is more often affected.1 BD is reported more frequently in males than females, at a ratio of 2:1.1,3 Patients ages 17 to 80 years (average age, 55 years) have received a diagnosis of BD.1 Most affected patients will have a concomitant peripheral neuropathy and sometimes nephropathy or retinopathy.1
Continue to: The etiology of BD...
The etiology of BD is unclear but appears to be multifactorial. Hypotheses suggest that there’s a link to neuropathy/nephropathy, excessive exposure to ultraviolet light, or a vascular cause secondary to hyaline deposition in the capillary walls.4,5
What you’ll see at presentation
The typical manifestation of BD is the rapid appearance of tense blisters, which may occur overnight or even within hours.1 They are usually painless; common locations include the feet, distal legs, hands, and forearms.1,5 The bullae can be serous or hemorrhagic.1
Most notable in the patient’s history will be a lack of trauma or injury to the area.1 Although A1C values do not correlate with blister formation, patients with hypoglycemic episodes and highly varying blood glucose values seem to have higher rates of occurrence.1
Other sources of blistering must be ruled out
The diagnosis of BD is clinical and based on history, exam, and exclusion of other bullous diagnoses.6 A key clue in the history is the spontaneous and rapid onset without associated trauma in a patient with diabetes.6 Direct immunofluorescence, although nonspecific, can be helpful to rule out other disorders (such as porphyria cutanea tarda and bullous pemphigoid) if the history and exam are inconclusive. Direct and indirect immunofluorescence is typically negative in BD.4,6
The differential diagnosis includes other conditions that involve bullae—such as frictional bullae, bullous pemphigoid, and bullous systemic lupus erythematosus—as well as porphyria, erythema multiforme, insect bites, or even fixed drug eruption.2,7
Continue to: Porphyria
Porphyria tends to develop on the hands, whereas BD most commonly occurs on the feet.5
Erythema multiforme typically includes inflammatory skin changes.5
Trauma or fixed drug eruption as a cause of blistering lesions would be revealed during history taking.
Considerations for treatment and follow-up
Without treatment, blisters often self-resolve in 2 to 6 weeks, but there is high likelihood of recurrence.6,8 There is no consensus on treatment, although a typical course of action is to deroof the blister and examine the area to rule out infection.6 The wound is then covered with wet-to-dry gauze that is changed regularly. If there is suspicion for or signs of underlying infection, such as an ulcer or skin necrosis, antibiotics should be included in the treatment plan.7
Additional considerations. Patients will often need therapeutic footwear if the blisters are located on the feet. Given the higher prevalence of microvascular complications in patients with diabetes who develop BD, routine ophthalmologic examination and renal function testing to monitor for microalbuminuria are recommended.5
Our patient underwent bedside incision and drainage and was discharged home with appropriate wound care and follow-up.
THE TAKEAWAY
BD cases may be underdiagnosed in clinical practice, perhaps due to patients not seeking help for a seemingly nonthreatening condition or lack of clinician recognition that bullae are related to a patient’s diabetes status. Prompt recognition and proper wound care are important to prevent poor outcomes, such as ulceration or necrosis.
CORRESPONDENCE
Kathleen S. Kinderwater, MD, 101 Heart Drive, Greenville, NC 27834; [email protected]
THE CASE
A 57-year-old man with type 2 diabetes, hyperlipidemia, and obesity presented to the emergency department (ED) for bilateral foot blisters, both of which appeared 1 day prior to evaluation. The patient’s history also included right-side Charcot foot diagnosed 4 years earlier and right foot osteomyelitis diagnosed 2 years prior. He had ongoing neuropathy in both feet but denied any significant pain.
The patient wore orthotics daily and he’d had new orthotics made 6 months prior; however, a recent COVID-19 diagnosis and prolonged hospital stay resulted in a 30-pound weight loss and decreased swelling in his ankles. He acquired new shoes 2 weeks prior to ED presentation.
Physical examination revealed large blisters along the medial aspect of the patient’s feet, with both hemorrhagic and serous fluid-filled bullae. The lesions were flaccid but intact, without drainage or surrounding erythema, warmth, or tenderness. The blister on the left foot measured 8 x 5 cm and extended from the great toe to mid-arch (FIGURE), while the one on the right foot measured 8 x 3 cm and extended from the great toe to the base of the proximal arch. Sensation was decreased in the bilateral first and second digits but unchanged from prior documented exams. Bilateral dorsalis pedis pulses were normal.

Work-up included imaging and lab work. The patient’s complete blood count was normal, as were his erythrocyte sedimentation rate and C-reactive protein level. Radiographs of the right foot were normal, but those of the left foot were concerning, although inconclusive, for osteomyelitis. Further evaluation with magnetic resonance imaging of his left foot revealed a deformity of the first digit with some subchondral signal change that was thought to be posttraumatic or degenerative, but unlikely osteomyelitis.
THE DIAGNOSIS
Podiatry was consulted for blister management. Based on atraumatic history, rapid appearance, location of blisters, unremarkable lab work and imaging, and concurrent diabetes, the patient received a diagnosis of bilateral bullous diabeticorum (BD).
DISCUSSION
Roughly one-third of patients with diabetes will experience some cutaneous adverse effect because of the disease.1 Common iterations include acanthosis nigricans, rash, or even infection.2 BD is a rare bullous skin lesion that occurs in patients with diabetes; it has a reported annual incidence of 0.16% and may be underdiagnosed.1
Cases of BD have been described both in patients with longstanding diabetes and in those newly diagnosed, although the former group is more often affected.1 BD is reported more frequently in males than females, at a ratio of 2:1.1,3 Patients ages 17 to 80 years (average age, 55 years) have received a diagnosis of BD.1 Most affected patients will have a concomitant peripheral neuropathy and sometimes nephropathy or retinopathy.1
Continue to: The etiology of BD...
The etiology of BD is unclear but appears to be multifactorial. Hypotheses suggest that there’s a link to neuropathy/nephropathy, excessive exposure to ultraviolet light, or a vascular cause secondary to hyaline deposition in the capillary walls.4,5
What you’ll see at presentation
The typical manifestation of BD is the rapid appearance of tense blisters, which may occur overnight or even within hours.1 They are usually painless; common locations include the feet, distal legs, hands, and forearms.1,5 The bullae can be serous or hemorrhagic.1
Most notable in the patient’s history will be a lack of trauma or injury to the area.1 Although A1C values do not correlate with blister formation, patients with hypoglycemic episodes and highly varying blood glucose values seem to have higher rates of occurrence.1
Other sources of blistering must be ruled out
The diagnosis of BD is clinical and based on history, exam, and exclusion of other bullous diagnoses.6 A key clue in the history is the spontaneous and rapid onset without associated trauma in a patient with diabetes.6 Direct immunofluorescence, although nonspecific, can be helpful to rule out other disorders (such as porphyria cutanea tarda and bullous pemphigoid) if the history and exam are inconclusive. Direct and indirect immunofluorescence is typically negative in BD.4,6
The differential diagnosis includes other conditions that involve bullae—such as frictional bullae, bullous pemphigoid, and bullous systemic lupus erythematosus—as well as porphyria, erythema multiforme, insect bites, or even fixed drug eruption.2,7
Continue to: Porphyria
Porphyria tends to develop on the hands, whereas BD most commonly occurs on the feet.5
Erythema multiforme typically includes inflammatory skin changes.5
Trauma or fixed drug eruption as a cause of blistering lesions would be revealed during history taking.
Considerations for treatment and follow-up
Without treatment, blisters often self-resolve in 2 to 6 weeks, but there is high likelihood of recurrence.6,8 There is no consensus on treatment, although a typical course of action is to deroof the blister and examine the area to rule out infection.6 The wound is then covered with wet-to-dry gauze that is changed regularly. If there is suspicion for or signs of underlying infection, such as an ulcer or skin necrosis, antibiotics should be included in the treatment plan.7
Additional considerations. Patients will often need therapeutic footwear if the blisters are located on the feet. Given the higher prevalence of microvascular complications in patients with diabetes who develop BD, routine ophthalmologic examination and renal function testing to monitor for microalbuminuria are recommended.5
Our patient underwent bedside incision and drainage and was discharged home with appropriate wound care and follow-up.
THE TAKEAWAY
BD cases may be underdiagnosed in clinical practice, perhaps due to patients not seeking help for a seemingly nonthreatening condition or lack of clinician recognition that bullae are related to a patient’s diabetes status. Prompt recognition and proper wound care are important to prevent poor outcomes, such as ulceration or necrosis.
CORRESPONDENCE
Kathleen S. Kinderwater, MD, 101 Heart Drive, Greenville, NC 27834; [email protected]
1. Larsen K, Jensen T, Karlsmark T, et al. Incidence of bullosis diabeticorum—a controversial cause of chronic foot ulceration. Int Wound J. 2008;5:591-596. doi: 10.1111/j.1742-481X.2008.00476.x
2. Lipsky BA, Baker PD, Ahroni JH. Diabetic bullae: 12 cases of a purportedly rare cutaneous disorder. Int J Dermatol. 2000;39:196-200. doi: 10.1046/j.1365-4362.2000.00947.x
3. Gupta V, Gulati N, Bahl J, et al. Bullosis diabeticorum: rare presentation in a common disease. Case Rep Endocrinol. 2014;2014:862912.
4. Sonani H, Abdul Salim S, Garla VV, et al. Bullosis diabeticorum: a rare presentation with immunoglobulin G (IgG) deposition related vasculopathy. Case report and focused review. Am J Case Rep. 2018;19:52-56. doi: 10.12659/ajcr.905452
5. Chouk C, Litaiem N. Bullosis diabeticorum. StatPearls [Internet]. Updated June 5, 2021. Accessed July 14, 2022. www.ncbi.nlm.nih.gov/books/NBK539872/
6. Chatterjee D, Radotra A, Radotra BD, et al. Bullous diabeticorum: a rare blistering manifestation of diabetes. Indian Dermatol Online J. 2017;8:274-275. doi: 10.4103/idoj.IDOJ_340_16
7. Kansal NK, Anuragi RP. Bullous lesions in diabetes mellitus: bullous diabeticorum (diabetic bulla). BMJ Case Rep. 2020;13:e238617. doi: 10.1136/bcr-2020-238617
8. Bello F, Samaila OM, Lawal Y, et al. 2 cases of bullosis diabeticorum following long-distance journeys by road: a report of 2 cases. Case Rep Endocrinol. 2012;2012:367218. doi: 10.1155/2012/367218
1. Larsen K, Jensen T, Karlsmark T, et al. Incidence of bullosis diabeticorum—a controversial cause of chronic foot ulceration. Int Wound J. 2008;5:591-596. doi: 10.1111/j.1742-481X.2008.00476.x
2. Lipsky BA, Baker PD, Ahroni JH. Diabetic bullae: 12 cases of a purportedly rare cutaneous disorder. Int J Dermatol. 2000;39:196-200. doi: 10.1046/j.1365-4362.2000.00947.x
3. Gupta V, Gulati N, Bahl J, et al. Bullosis diabeticorum: rare presentation in a common disease. Case Rep Endocrinol. 2014;2014:862912.
4. Sonani H, Abdul Salim S, Garla VV, et al. Bullosis diabeticorum: a rare presentation with immunoglobulin G (IgG) deposition related vasculopathy. Case report and focused review. Am J Case Rep. 2018;19:52-56. doi: 10.12659/ajcr.905452
5. Chouk C, Litaiem N. Bullosis diabeticorum. StatPearls [Internet]. Updated June 5, 2021. Accessed July 14, 2022. www.ncbi.nlm.nih.gov/books/NBK539872/
6. Chatterjee D, Radotra A, Radotra BD, et al. Bullous diabeticorum: a rare blistering manifestation of diabetes. Indian Dermatol Online J. 2017;8:274-275. doi: 10.4103/idoj.IDOJ_340_16
7. Kansal NK, Anuragi RP. Bullous lesions in diabetes mellitus: bullous diabeticorum (diabetic bulla). BMJ Case Rep. 2020;13:e238617. doi: 10.1136/bcr-2020-238617
8. Bello F, Samaila OM, Lawal Y, et al. 2 cases of bullosis diabeticorum following long-distance journeys by road: a report of 2 cases. Case Rep Endocrinol. 2012;2012:367218. doi: 10.1155/2012/367218
COVID-19 therapy: What works? What doesn’t? And what’s on the horizon?
The ongoing COVID-19 pandemic has caused more than 1 million deaths in the United States and continues to be a major public health challenge. Cases can be asymptomatic, or symptoms can range from a mild respiratory tract infection to acute respiratory distress and multiorgan failure.
Three strategies can successfully contain the pandemic and its consequences:
- Public health measures, such as masking and social distancing
- Prophylactic vaccines to reduce transmission
- Safe and effective drugs for reducing morbidity and mortality among infected patients.
Optimal treatment strategies for patients in ambulatory and hospital settings continue to evolve as new studies are reported and new strains of the virus arise. Many medical and scientific organizations, including the National Institutes of Health (NIH) COVID-19 treatment panel,1 Infectious Diseases Society of America (IDSA),2 World Health Organization (WHO),3 and Centers for Disease Control and Prevention,4 provide recommendations for managing patients with COVID-19. Their guidance is based on the strongest research available and is updated intermittently; nevertheless, a plethora of new data emerges weekly and controversies surround several treatments.
In this article, we
We encourage clinicians, in planning treatment, to consider:
- The availability of medications (ie, use the COVID-19 Public Therapeutic Locatora)
- The local COVID-19 situation
- Patient factors and preferences
- Evolving evidence regarding new and existing treatments.
Most evidence about the treatment of COVID-19 comes from studies conducted when the Omicron variant of SARS-CoV-2 was not the dominant variant, as it is today in the United States. As such, drugs authorized or approved by the US Food and Drug Administration (FDA) to treat COVID-19 or used off-label for that purpose might not be as efficacious today as they were almost a year ago. Furthermore, many trials of potential therapies against new viral variants are ongoing; if your patient is interested in enrolling in a clinical trial of an investigational COVID-19 treatment, refer them to www.clinicaltrials.gov.
General managementof COVID-19
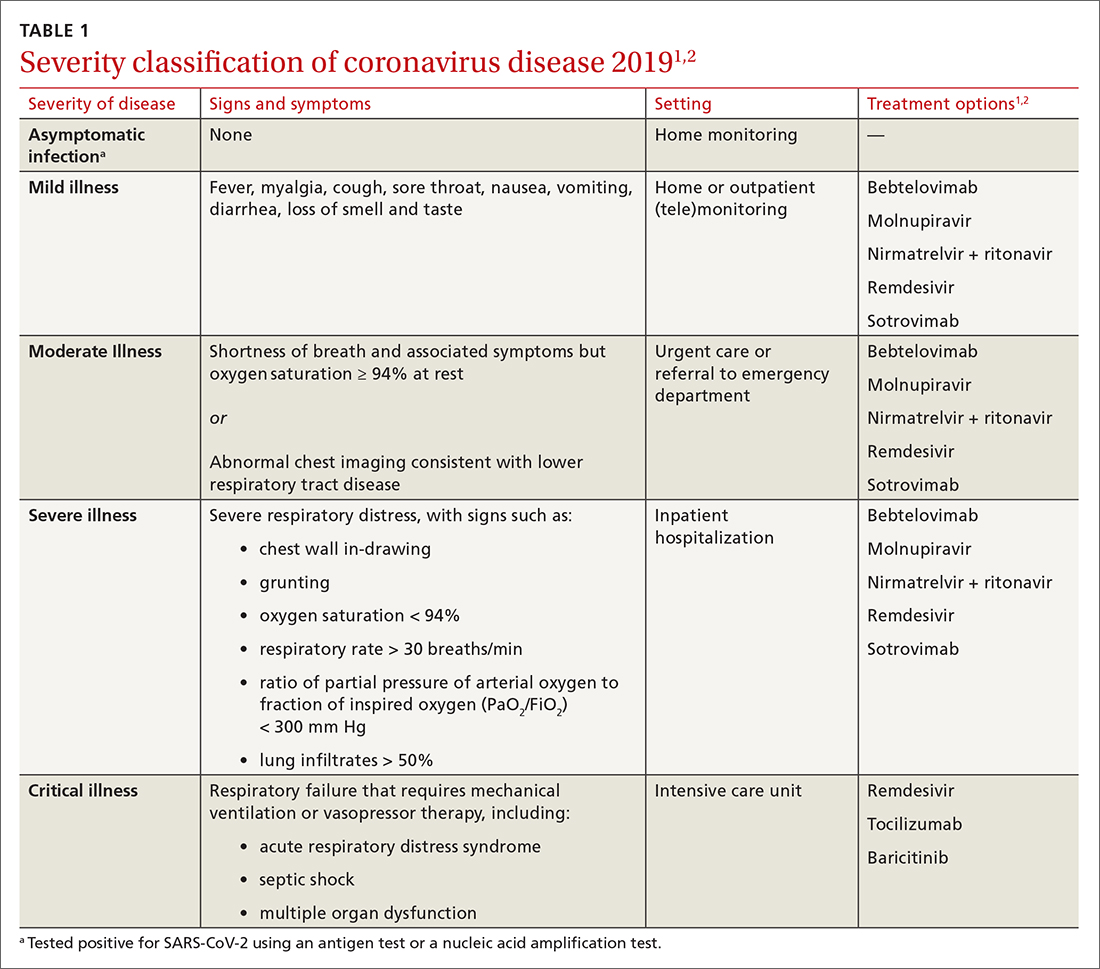
Patients with COVID-19 experience a range of illness severity—from asymptomatic to mild symptoms, such as fever and myalgia, to critical illness requiring intensive care (TABLE 11,2). Patients with COVID-19 should therefore be monitored for progression, remotely or in person, until full recovery is achieved. Key concepts of general management include:
Assess and monitor patients’ oxygenation status by pulse oximetry; identify those with low or declining oxygen saturation before further clinical deterioration.
Continue to: Consider the patient's age and general health
Consider the patient’s age and general health. Patients are at higher risk of severe disease if they are > 65 years or have an underlying comorbidity.4
Emphasize self-isolation and supportive care, including rest, hydration, and over-the-counter medications to relieve cough, reduce fever, and alleviate other symptoms.
Drugs: Few approved, some under study
The antiviral remdesivir is the only drug fully approved for clinical use by the FDA to treat COVID-19 in patients > 12 years.5,6
In addition, the FDA has issued an emergency use authorization (EUA) for several monoclonal antibodies as prophylaxis and treatment: tixagevimab packaged with cilgavimab (Evusheld) is the first antibody combination for pre-exposure prophylaxis (PrEP) against COVID-19; the separately packaged injectables are recommended for patients who have a history of severe allergy that prevents them from being vaccinated or those with moderate or severe immune-compromising disorders.7
In the pipeline. Several treatments are being tested in clinical trials to evaluate their effectiveness and safety in combating COVID-19, including:
- Antivirals, which prevent viruses from multiplying
- Immunomodulators, which reduce the body’s immune reaction to the virus
- Antibody therapies, which are manufactured antibodies against the virus
- Anti-inflammatory drugs, which reduce systemic inflammation and prevent organ dysfunction
- Cell therapies and gene therapies, which alter the expression of cells and genes.
Continue to: Outpatient treatment
Outpatient treatment
Several assessment tools that take into account patients’ age, respiratory status, and comorbidities are available for triage of patients infected with COVID-19.8
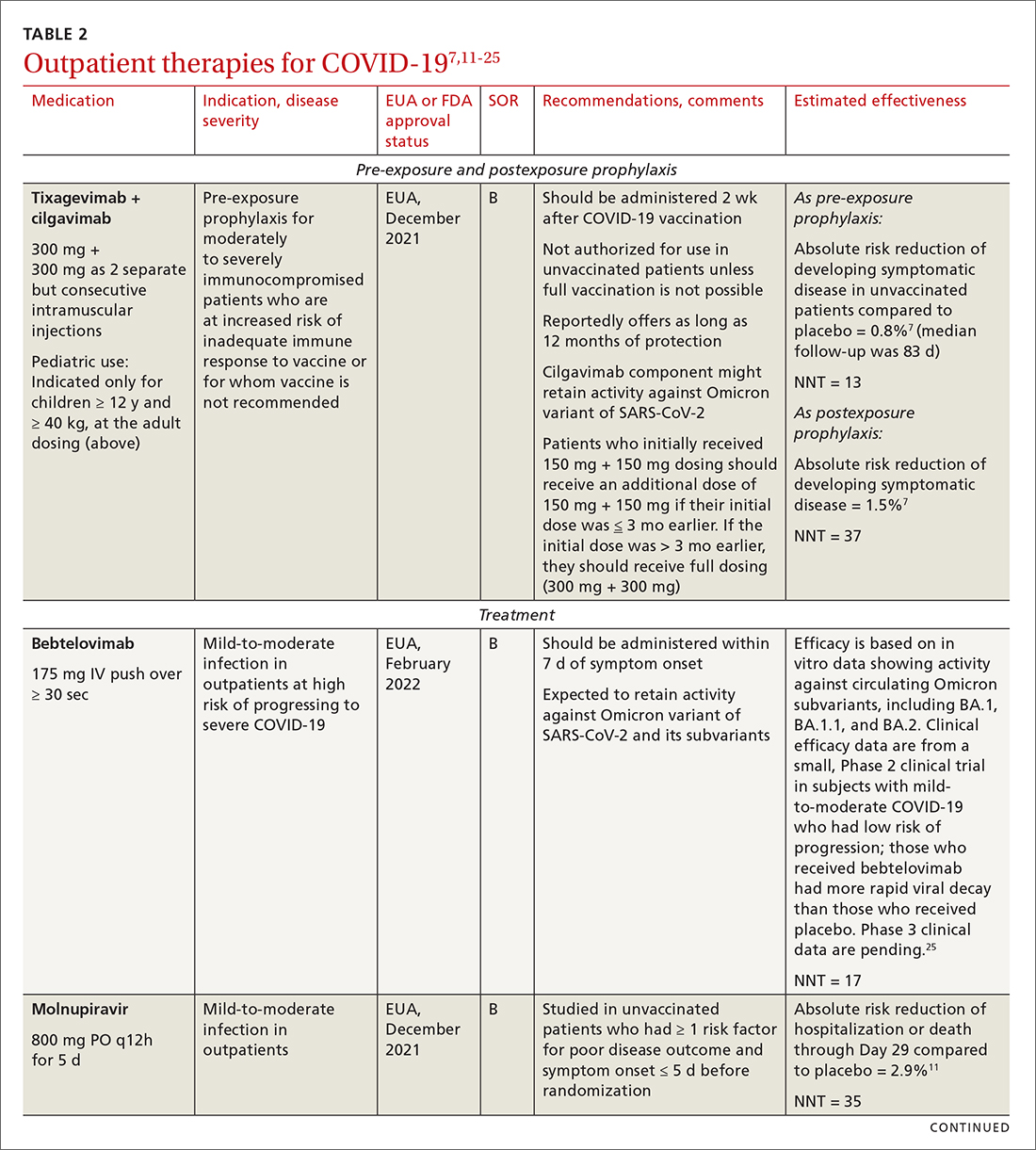
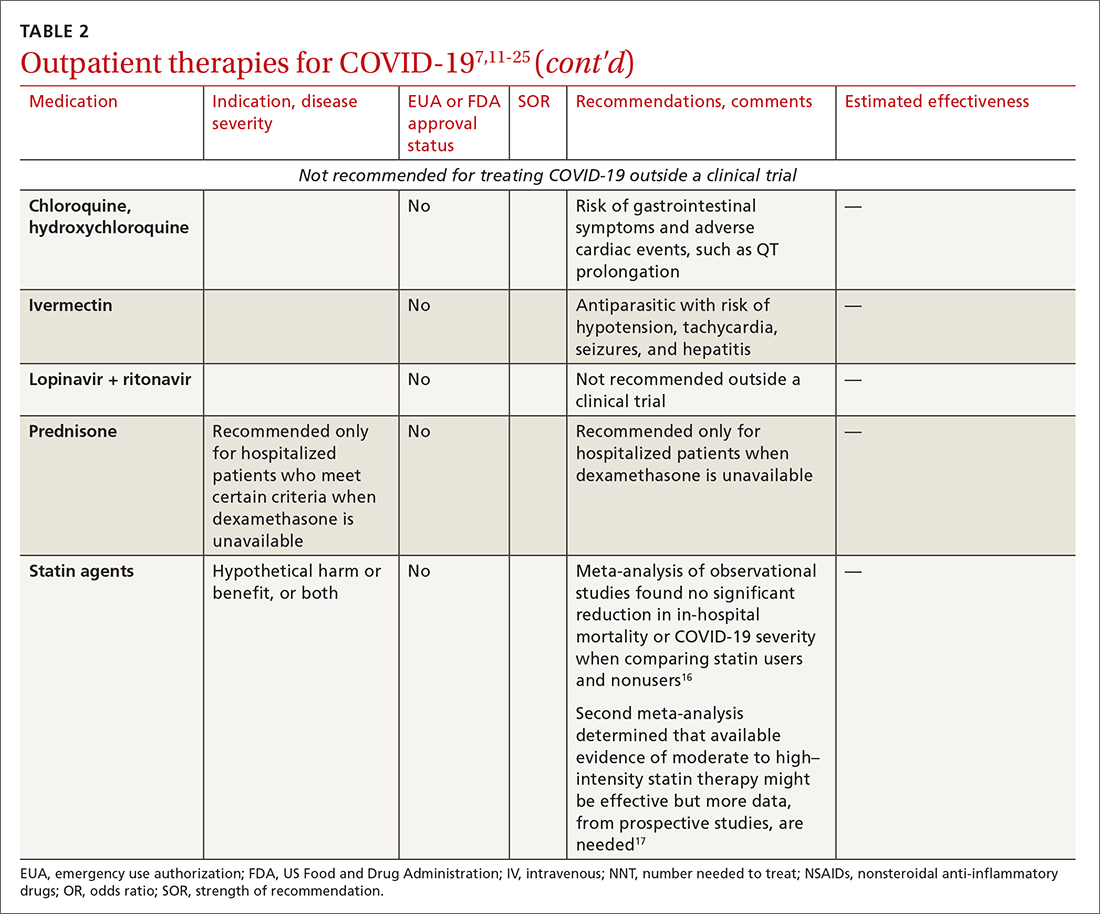
Most (> 80%) patients with COVID-19 have mild infection and are safely managed as outpatients or at home.9,10 For patients at high risk of severe disease, a few options are recommended for patients who do not require hospitalization or supplemental oxygen; guidelines on treatment of COVID-19 in outpatient settings that have been developed by various organizations are summarized in TABLE 2.7,11-25
Antiviral drugs target different stages of the SARS-CoV-2 replication cycle. They should be used early in the course of infection, particularly in patients at high risk of severe disease.
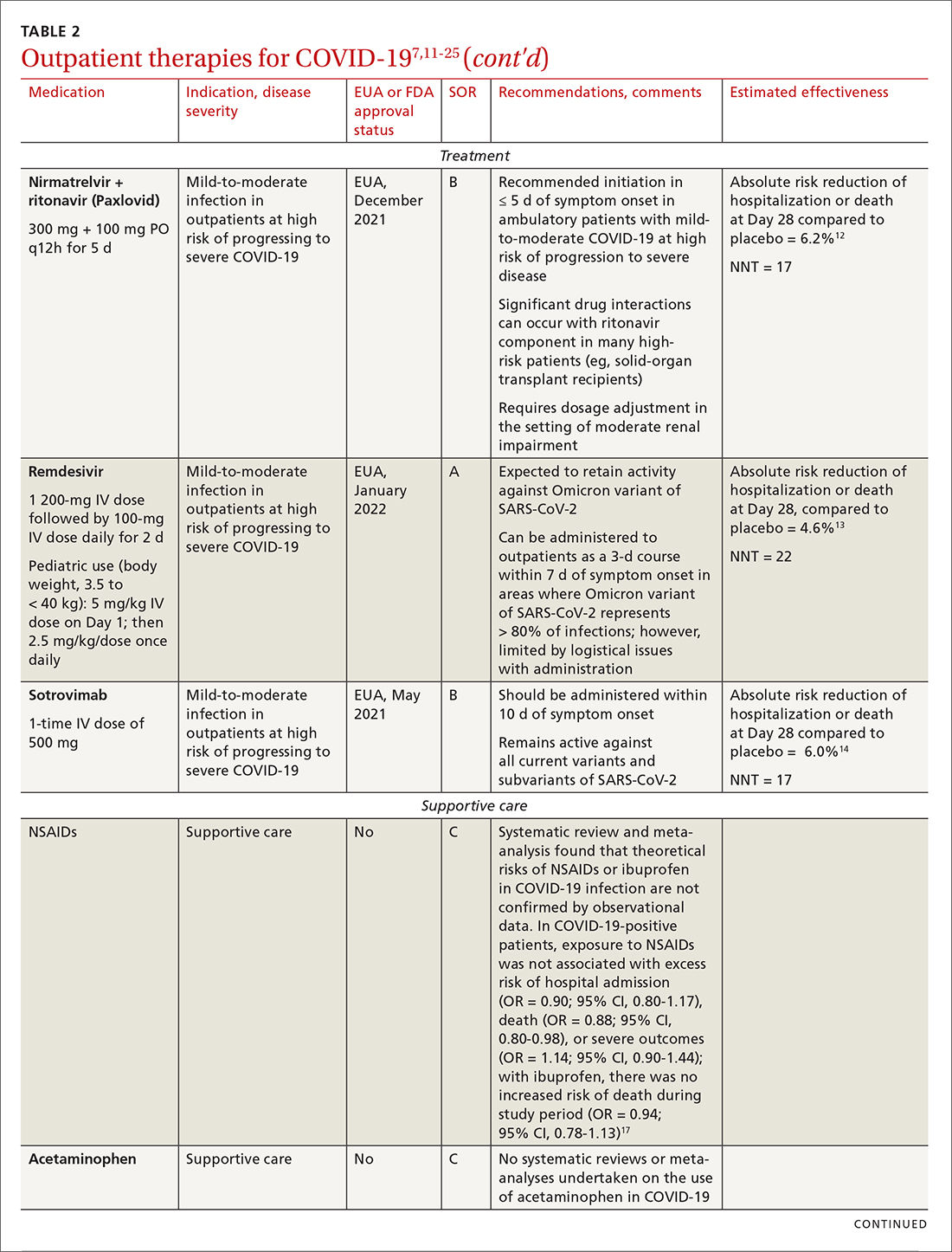
IDSA recommends antiviral therapy with molnupiravir, nirmatrelvir + ritonavir packaged together (Paxlovid), or remdesivir.11,12,26,27 Remdesivir requires intravenous (IV) infusion on 3 consecutive days, which can be difficult in some clinic settings.13,28 Nirmatrelvir + ritonavir should be initiated within 5 days after symptom onset. Overall, for most patients, nirmatrelvir + ritonavir is preferred because of oral dosing and higher efficacy in comparison to other antivirals. With nirmatrelvir + ritonavir, carefully consider drug–drug interactions and the need to adjust dosing in the presence of renal disease.28,29 There are no data on the efficacy of any combination treatments with these agents (other than co-packaged Paxlovid).
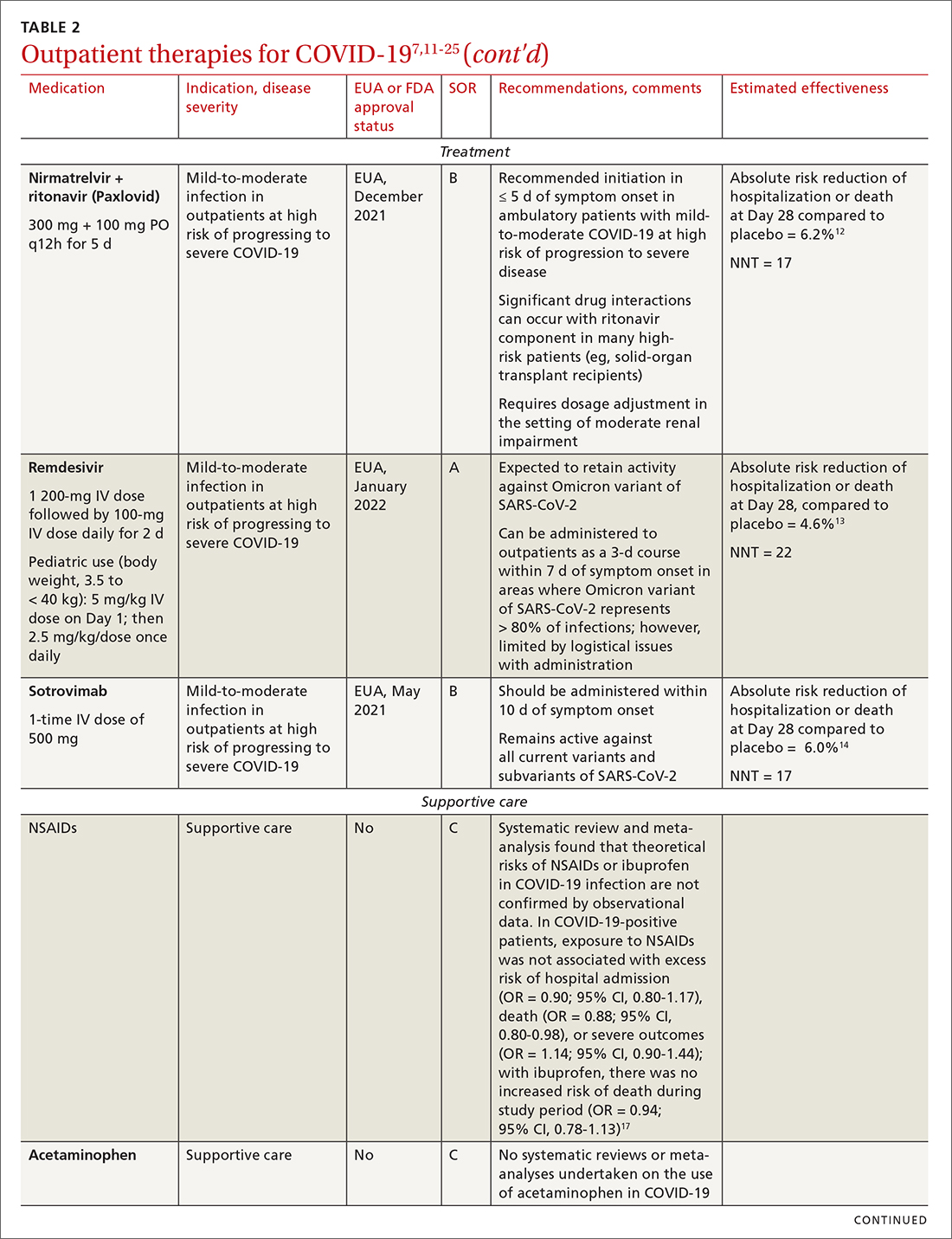
Monoclonal antibodies for COVID-19 are given primarily intravenously. They bind to the viral spike protein, thus preventing SARS-CoV-2 from attaching to and entering cells. Bamlanivimab + etesevimab and bebtelovimab are available under an EUA for outpatient treatment.14b Treatment should be initiated as early as possible in the course of infection—ideally, within 7 to 10 days after onset of symptoms.
Continue to: Bebtelovimab was recently given...
Bebtelovimab was recently given an EUA. It is a next-generation antibody that neutralizes all currently known variants and is the most potent monoclonal antibody against the Omicron variant, including its BA.2 subvariant.31 However, data about its activity against the BA.2 subvariant are based on laboratory testing and have not been confirmed in clinical trials. Clinical data were similar for this agent alone and for its use in combination with other monoclonal antibodies, but those trials were conducted before the emergence of Omicron.
In your decision-making about the most appropriate therapy, consider (1) the requirement that monoclonal antibodies be administered parenterally and (2) the susceptibility of the locally predominating viral variant.
Other monoclonal antibody agents are in the investigative pipeline; however, data about them have been largely presented through press releases or selectively reported in applications to the FDA for EUA. For example, preliminary reports show cilgavimab coverage against the Omicron variant14; so far, cilgavimab is not approved for treatment but is used in combination with tixagevimab for PreP—reportedly providing as long as 12 months of protection for patients who are less likely to respond to a vaccine.32
Corticosteroids. Guidelines recommend against dexamethasone and other systemic corticosteroids in outpatient settings. For patients with moderate-to-severe symptoms but for whom hospitalization is not possible (eg, beds are unavailable), the NIH panel recommends dexamethasone, 6 mg/d, for the duration of supplemental oxygen, not to exceed 10 days of treatment.1
Patients who were recently discharged after COVID-19 hospitalization should not continue remdesivir, dexamethasone, or baricitinib at home, even if they still require supplemental oxygen.
Continue to: Some treatments should not be in your COVID-19 toolbox
Some treatments should not be in your COVID-19 toolbox
High-quality studies are lacking for several other potential COVID-19 treatments. Some of these drugs are under investigation, with unclear benefit and with the potential risk of toxicity—and therefore should not be prescribed or used outside a clinical trial. See “Treatments not recommended for COVID-19,” page E14. 1-4,15-19,33-41
SIDEBAR
Treatments not recommended for COVID-191-4,15-19,33-41
Fluvoxamine. A few studies suggest that the selective serotonin reuptake inhibitor fluvoxamine reduces progression to severe disease; however, those studies have methodologic challenges.33 The drug is not FDA approved for treating COVID.33
Convalescent plasma, given to high-risk outpatients early in the course of disease, can reduce progression to severe disease,34,35 but it remains investigational for COVID-19 because trials have yielded mixed results.34-36
Ivermectin. The effect of ivermectin in patients with COVID-19 is unclear because high-quality studies do not exist and cases of ivermectin toxicity have occurred with incorrect administration.39
Hydroxychloroquine showed potential in a few observational studies, but randomized clinical trials have not shown any benefit.15
Azithromycin likewise showed potential in a few observational studies; randomized clinical trials have not shown any benefit, however.15
Statins. A few meta-analyses, based on observational studies, reported benefit from statins, but recent studies have shown that this class of drugs does not provide clinical benefit in alleviating COVID-19 symptoms.16,17,37
Inhaled corticosteroids. A systematic review reported no benefit or harm from using an inhaled corticosteroid.18 More recent studies show that the inhaled corticosteroid budesonide used in early COVID-19 might reduce the need for urgent care38 and, in patients who are at higher risk of COVID-19-related complications, shorten time to recovery.19
Vitamins and minerals. Limited observational studies suggest an association between vitamin and mineral deficiency (eg, vitamin C, zinc, and vitamin D) and risk of severe disease, but high-quality data about this finding do not exist.40,41
Casirivimab + imdevimab [REGEN-COV2]. This unapproved investigational combination treatment was granted an EUA in 2020 for postexposure prophylaxis. The EUA was withdrawn in January 2022 because of the limited efficacy of casirivimab + imdevimab against the Omicron variant of SARS-CoV-2.
Postexposure prophylaxis. National guidelines1-4 recommend against postexposure prophylaxis with hydroxychloroquine, colchicine, inhaled corticosteroids, or azithromycin.
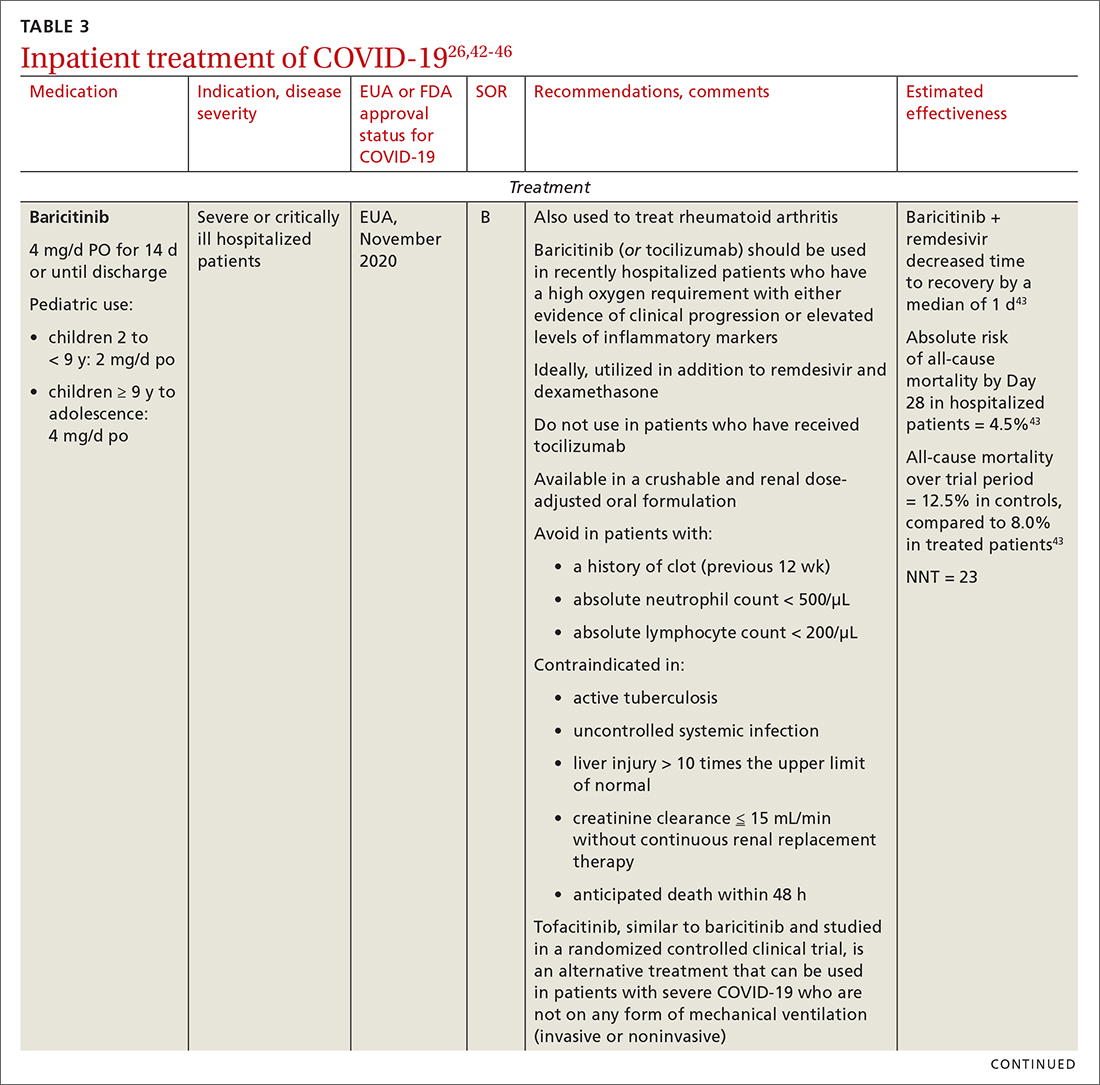
TABLE 27,11-25 and TABLE 326,42-46 provide additional information on treatments not recommended outside trials, or not recommended at all, for COVID-19.
Treatment during hospitalization
The NIH COVID-19 treatment panel recommends hospitalization for patients who have any of the following findings1:
- Oxygen saturation < 94% while breathing room air
- Respiratory rate > 30 breaths/min
- A ratio of partial pressure of arterial O2 to fraction of inspired O2 (PaO2/FiO2) < 300 mm Hg
- Lung infiltrates > 50%.
General guidance for the care of hospitalized patients:
- Treatments that target the virus have the greatest efficacy when given early in the course of disease.
- Anti-inflammatory and immunosuppressive agents help prevent tissue damage from a dysregulated immune system. (See TABLE 326,42-46)
- The NIH panel,1 IDSA,2 and WHO3 recommend against dexamethasone and other corticosteroids for hospitalized patients who do not require supplemental oxygen.
- Prone positioning distributes oxygen more evenly in the lungs and improves overall oxygenation, thus reducing the need for mechanical ventilation.
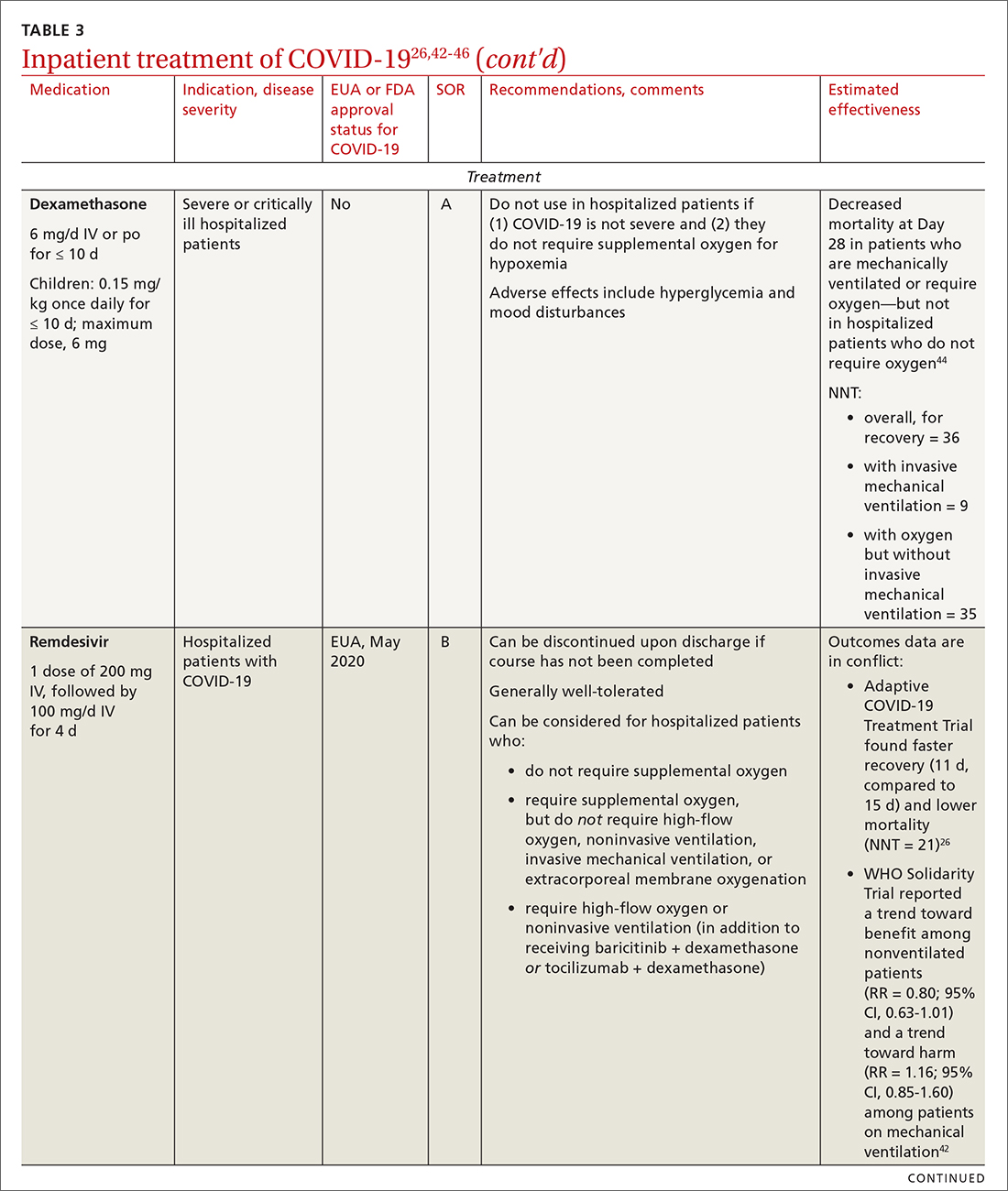
Remdesivir. Once a hospitalized patient does require supplemental oxygen, the NIH panel,1 IDSA,2 and WHO3 recommend remdesivir; however, remdesivir is not recommended in many other countries because WHO has noted its limited efficacy.42 Dexamethasone is recommended alone, or in combination with remdesivir for patients who require increasing supplemental oxygen and those on mechanical ventilation.
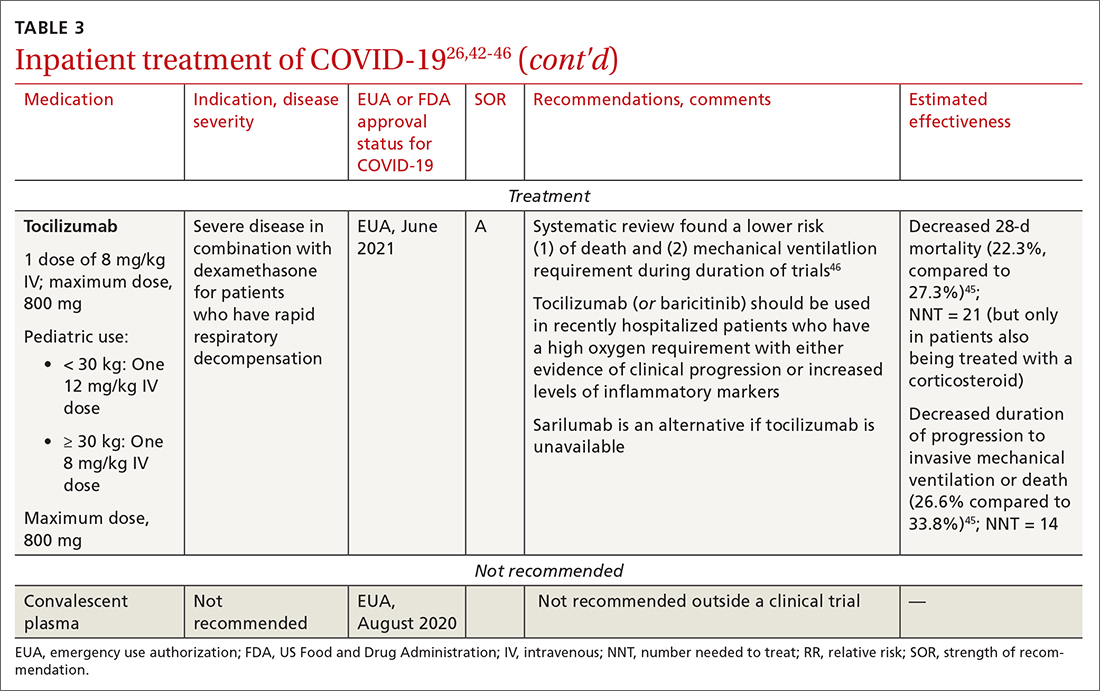
Baricitinib. For patients with rapidly increasing oxygen requirements, invasive mechanical ventilation, and systemic inflammation, baricitinib, a Janus kinase inhibitor, can be administered, in addition to dexamethasone, with or without remdesivir.47
Continue to: Tocilizumab
Tocilizumab. A monoclonal antibody and interleukin (IL)-6 inhibitor, tocilizumab is also recommended in addition to dexamethasone, with or without remdesivir.48 Tocilizumab should be given only in combination with dexamethasone.49 Patients should receive baricitinib or tocilizumab—not both. IDSA recommends tofacitinib, with a prophylactic dose of an anticoagulant, for patients who are hospitalized with severe COVID-19 but who are not on any form of ventilation.50
Care of special populations
Special patient populations often seek primary care. Although many questions remain regarding the appropriate care of these populations, it is useful to summarize existing evidence and recommendations from current guidelines.
Children. COVID-19 is generally milder in children than in adults; many infected children are asymptomatic. However, infants and children who have an underlying medical condition are at risk of severe disease, including multisystem inflammatory syndrome.51
The NIH panel recommends supportive care alone for most children with mild-to-moderate disease.1 Remdesivir is recommended for hospitalized children ≥ 12 years who weigh ≥ 40 kg, have risk factors for severe disease, and have an emergent or increasing need for supplemental oxygen. Dexamethasone is recommended for hospitalized children requiring high-flow oxygen, noninvasive ventilation, invasive mechanical ventilation, or extracorporeal membrane oxygenation. Molnupiravir is not authorized for patients < 18 years because it can impede bone and cartilage growth.
There is insufficient evidence for or against the use of monoclonal antibody products for children with COVID-19 in an ambulatory setting. For hospitalized children, there is insufficient evidence for or against use of baricitinib and tocilizumab.
Continue to: Patients who are pregnant
Patients who are pregnant are at increased risk of severe COVID-19.52,53 The NIH states that, in general, treatment and vaccination of pregnant patients with COVID-19 should be the same as for nonpregnant patients.1
Pregnant subjects were excluded from several trials of COVID-19 treatments.54 Because Janus kinase inhibitors, such as baricitinib, are associated with an increased risk of thromboembolism, they are not recommended in pregnant patients who are already at risk of thromboembolic complications. Molnupiravir is not recommended for pregnant patients because of its potential for teratogenic effects.
The Society for Maternal-Fetal Medicine states that there are no absolute contraindications to the use of monoclonal antibodies in appropriate pregnant patients with COVID-19.55 Remdesivir has no known fetal toxicity and is recommended as a treatment that can be offered to pregnant patients. Dexamethasone can also be administered to pregnant patients who require oxygen; however, if dexamethasone is also being used to accelerate fetal lung maturity, more frequent initial dosing is needed.
Older people. COVID-19 treatments for older patients are the same as for the general adult population. However, because older people are more likely to have impaired renal function, renal function should be monitored when an older patient is being treated with COVID-19 medications that are eliminated renally (eg, remdesivir, baricitinib). Furthermore, drug–drug interactions have been reported in older patients treated with nirmatrelvir + ritonavir, primarily because of the effects of ritonavir. Review all of a patient’s medications, including over-the-counter drugs and herbal supplements, when prescribing treatment for COVID-19, and adjust the dosage by following guidance in FDA-approved prescribing information—ideally, in consultation with a pharmacist.
Immunocompromised patients. The combination product tixagevimab + cilgavimab [Evusheld] is FDA approved for COVID-19 PrEP, under an EUA, in patients who are not infected with SARS-CoV-2 who have an immune-compromising condition, who are unlikely to mount an adequate immune response to the COVID-19 vaccine, or those in whom vaccination is not recommended because of their history of a severe adverse reaction to a COVID-19 vaccine or one of its components.7
Continue to: Summing up
Summing up
With a growing need for effective and readily available COVID-19 treatments, there are an unprecedented number of clinical trials in process. Besides antivirals, immunomodulators, and antibody therapies, some novel mechanisms being tested include Janus kinase inhibitors, IL-6-receptor blockers, and drugs that target adult respiratory distress syndrome and cytokine release.
Once larger trials are completed, we can expect stronger evidence of potential treatment options and of safety and efficacy in children, pregnant women, and vulnerable populations. During the pandemic, the FDA’s EUA program has brought emerging treatments rapidly to clinicians; nevertheless, high-quality evidence, with thorough peer review, remains critical to inform COVID-19 treatment guidelines.
a https://healthdata.gov/Health/COVID-19-PublicTherapeutic-Locator/rxn6-qnx8/data
b Sotrovimab was effective against the Omicron variant of SARS-CoV-2—the dominant variant in early 2022— but is currently not FDA authorized in any region of the United States because of the prevalence of the Omicron BA.2 subvariant.30
CORRESPONDENCE
Ambar Kulshreshtha, MD, PhD, Department of Epidemiology, Emory Rollins School of Public Health, 4500 North Shallowford Road, Suite 134, Atlanta, GA 30338; [email protected]
1. COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. July 19, 2022. Accessed July 21, 2022. www.covid19treatmentguidelines.nih.gov
2. IDSA guidelines on the treatment and management of patients with COVID-19. Infectious Diseases Society of America. Updated June 29, 2022. Accessed July 21, 2022. www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/#toc-23
3. Therapeutics and COVID-19: living guideline. World Health Organization. July 14, 2022. Accessed July 21, 2022. https://apps.who.int/iris/rest/bitstreams/1449398/retrieve
4. Centers for Disease Control and Prevention. Clinical care considerations. Updated May 27, 2022. Accessed July 21, 2022. www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html
5. Coronavirus (COVID-19) update: FDA approves first COVID-19 treatment for young children. Press release. US Food and Drug Administration. April 25, 2022. Accessed August 11, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-approves-first-covid-19-treatment-young-children
6. Know your treatment options for COVID-19. US Food and Drug Administration. Updated August 15, 2022. Accessed July 21, 2022. www.fda.gov/consumers/consumer-updates/know-your-treatment-options-covid-19
7. Tixagevimab and cilgavimab (Evusheld) for pre-exposure prophylaxis of COVID-19. JAMA. 2022;327:384-385. doi: 10.1001/jama.2021.24931
8. Judson TJ, Odisho AY, Neinstein AB, et al. Rapid design and implementation of an integrated patient self-triage and self-scheduling tool for COVID-19. J Am Med Inform Assoc. 2020;27:860-866. doi: 10.1093/jamia/ocaa051
9. Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383:1757-1766. doi: 10.1056/NEJMcp2009249
10. Greenhalgh T, Koh GCH, Car J. Covid-19: a remote assessment in primary care. BMJ. 2020;368:m1182. doi: 10.1136/bmj.m1182
11. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al; . Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386:509-520. doi: 10.1056/NEJMoa2116044
12. Hammond J, Leister-Tebbe H, Gardner A, et al; . Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386:1397-1408. doi: 10.1056/NEJMoa2118542
13. Gottlieb RL, Vaca CE, Paredes R, et al; . Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 2022;386:305-315. doi: 10.1056/NEJMoa2116846
14. Gupta A, Gonzalez-Rojas Y, Juarez E, et al; . Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941-1950. doi: 10.1056/NEJMoa2107934
15. Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Intern Med. 2020;173:623-631. doi: 10.7326/M20-4207
16. Scheen AJ. Statins and clinical outcomes with COVID-19: meta-analyses of observational studies. Diabetes Metab. 2021;47:101220. doi: 10.1016/j.diabet.2020.101220
17. Kow CS, Hasan SS. Meta-analysis of effect of statins in patients with COVID-19. Am J Cardiol. 2020;134:153-155. doi: 10.1016/j.amjcard.2020.08.004
18. Halpin DMG, Singh D, Hadfield RM. Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective. Eur Respir J. 2020;55:2001009. doi: 10.1183/13993003.01009-2020
19. Yu L-M, Bafadhel M, Dorward J, et al; . Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;398:843-855. doi: 10.1016/S0140-6736(21)01744-X
20. Siemieniuk RA, Bartoszko JJ, JP, et al. Antibody and cellular therapies for treatment of covid-19: a living systematic review and network meta-analysis. BMJ. 2021;374:n2231. doi: 10.1136/bmj.n2231
21. Siemieniuk RA, Bartoszko JJ, Zeraatkar D, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. doi: 10.1136/bmj.m2980
22. Agarwal A, Rochwerg B, Lamontagne F, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379. doi: 10.1136/bmj.m3379
23. Goldstein KM, Ghadimi K, Mystakelis H, et al. Risk of transmitting coronavirus disease 2019 during nebulizer treatment: a systematic review. J Aerosol Med Pulm Drug Deliv. 2021;34:155-170. doi: 10.1089/jamp.2020.1659
24. Schultze A, Walker AJ, MacKenna B, et al; . Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med. 2020;8:1106-1120. doi: 10.1016/S2213-2600(20)30415-X
25. What are the safety and efficacy results of bebtelovimab from BLAZE-4? Lilly USA. January 12, 2022. Accessed August 17, 2022. www.lillymedical.com/en-us/answers/what-are-the-safety-and-efficacy-results-of-bebtelovimab-from-blaze-4-159290
26. Beigel JH, Tomashek KM, Dodd LE, et al; . Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. doi: 10.1056/NEJMoa2007764
27. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787-1799. doi: 10.1056/NEJMoa2001282
28. Gandhi RT, Malani PN, Del Rio C. COVID-19 therapeutics for nonhospitalized patients. JAMA. 2022;327:617-618. doi: 10.1001/jama.2022.0335
29. Wen W, Chen C, Tang J, et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis. Ann Med. 2022;54:516-523. doi: 10.1080/07853890.2022.2034936
30. Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022:602:671-675. doi: 10.1038/s41586-021-04389-z
31. Emergency use authorization (EUA) for bebtelovimab (LY-CoV1404): Center for Drug Evaluation and Research (CDER) review. US Food and Drug Administration. Updated February 11, 2022. Accessed July 21, 2022. www.fda.gov/media/156396/download
32. Bartoszko JJ, Siemieniuk RAC, Kum E, et al. Prophylaxis against covid-19: living systematic review and network meta-analysis. BMJ. 2021;373:n949. doi: 10.1136/bmj.n949
33. Reis G, Dos Santos Moreira-Silva EA, Silva DCM, et al; TOGETHER Investigators. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health. 2022;10:e42-e51. doi: 10.1016/S2214-109X(21)00448-4
34. RECOVERY Collaborative Group. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet. 2021;397:2049-2059. doi: 10.1016/S0140-6736(21)00897-7
35. Simonovich VA, Burgos Pratx LD, Scibona P, et al; . A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021;384:619-629. doi: 10.1056/NEJMoa2031304
36. Joyner MJ, Carter RE, Senefeld JW, et al. Convalescent plasma antibody levels and the risk of death from Covid-19. N Engl J Med. 2021;384:1015-1027. doi: 10.1056/NEJMoa2031893
37. Ayeh SK, Abbey EJ, Khalifa BAA, et al. Statins use and COVID-19 outcomes in hospitalized patients. PLoS One. 2021;16:e0256899. doi: 10.1371/journal.pone.0256899
38. Ramakrishnan S, Nicolau DV Jr, Langford B, et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med. 2021;9:763-772. doi: 10.1016/S2213-2600(21)00160-0
39. Temple C, Hoang R, Hendrickson RG. Toxic effects from ivermectin use associated with prevention and treatment of Covid-19. N Engl J Med. 2021;385:2197-2198. doi: 10.1056/NEJMc2114907
40. Thomas S, Patel D, Bittel B, et al. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: the COVID A to Z randomized clinical trial. JAMA Netw Open. 2021;4:e210369. doi: 10.1001/jamanetworkopen.2021.0369
41. Adams KK, Baker WL, Sobieraj DM. Myth busters: dietary supplements and COVID-19. Ann Pharmacother. 2020;54:820-826. doi: 10.1177/1060028020928052
42. Pan H, Peto R, Henao-Restrepo A-M, et al. Repurposed antiviral drugs for Covid-19—interim WHO Solidarity trial results. N Engl J Med. 2021;384:497-511. doi: 10.1056/NEJMoa2023184
43. Kalil AC, Patterson TF, Mehta AK, et al; . Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384:795-807. doi: 10.1056/NEJMoa2031994
44. ; Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330-1341. doi: 10.1001/jama.2020.17023
45. ; Shankar-Hari M, Vale CL, Godolphin PJ, et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA. 2021;326:499-518. doi: 10.1001/jama.2021.11330
46. Wei QW, Lin H, Wei R-G, et al. Tocilizumab treatment for COVID-19 patients: a systematic review and meta-analysis. Infect Dis Poverty. 2021;10:71. doi: 10.1186/s40249-021-00857-w
47. Zhang X, Shang L, Fan G, et al. The efficacy and safety of Janus kinase inhibitors for patients with COVID-19: a living systematic review and meta-analysis. Front Med (Lausanne). 2021;8:800492. doi: 10.3389/fmed.2021.800492
48. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637-1645. doi: 10.1016/S0140-6736(21)00676-0
49. Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384:1491-1502. doi: 10.1056/NEJMoa2100433
50. Guimaraes PO, Quirk D, Furtado RH, et al; . Tofacitinib in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;385:406-415. doi: 10.1056/NEJMoa2101643
51. Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334-346. doi: 10.1056/NEJMoa2021680
52. Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320
53. Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175:817-826. doi: 10.1001/jamapediatrics.2021.1050
54. Jorgensen SCJ, Davis MR, Lapinsky SE. A review of remdesivir for COVID-19 in pregnancy and lactation. J Antimicrob Chemother. 2021;77:24-30. doi: 10.1093/jac/dkab311
55. Management considerations for pregnant patients with COVID-19. Society for Maternal-Fetal Medicine. Accessed July 21, 2022. https://s3.amazonaws.com/cdn.smfm.org/media/2336/SMFM_COVID_Management_of_COVID_pos_preg_patients_4-30-20_final.pdf
The ongoing COVID-19 pandemic has caused more than 1 million deaths in the United States and continues to be a major public health challenge. Cases can be asymptomatic, or symptoms can range from a mild respiratory tract infection to acute respiratory distress and multiorgan failure.
Three strategies can successfully contain the pandemic and its consequences:
- Public health measures, such as masking and social distancing
- Prophylactic vaccines to reduce transmission
- Safe and effective drugs for reducing morbidity and mortality among infected patients.
Optimal treatment strategies for patients in ambulatory and hospital settings continue to evolve as new studies are reported and new strains of the virus arise. Many medical and scientific organizations, including the National Institutes of Health (NIH) COVID-19 treatment panel,1 Infectious Diseases Society of America (IDSA),2 World Health Organization (WHO),3 and Centers for Disease Control and Prevention,4 provide recommendations for managing patients with COVID-19. Their guidance is based on the strongest research available and is updated intermittently; nevertheless, a plethora of new data emerges weekly and controversies surround several treatments.
In this article, we
We encourage clinicians, in planning treatment, to consider:
- The availability of medications (ie, use the COVID-19 Public Therapeutic Locatora)
- The local COVID-19 situation
- Patient factors and preferences
- Evolving evidence regarding new and existing treatments.
Most evidence about the treatment of COVID-19 comes from studies conducted when the Omicron variant of SARS-CoV-2 was not the dominant variant, as it is today in the United States. As such, drugs authorized or approved by the US Food and Drug Administration (FDA) to treat COVID-19 or used off-label for that purpose might not be as efficacious today as they were almost a year ago. Furthermore, many trials of potential therapies against new viral variants are ongoing; if your patient is interested in enrolling in a clinical trial of an investigational COVID-19 treatment, refer them to www.clinicaltrials.gov.
General managementof COVID-19
Patients with COVID-19 experience a range of illness severity—from asymptomatic to mild symptoms, such as fever and myalgia, to critical illness requiring intensive care (TABLE 11,2). Patients with COVID-19 should therefore be monitored for progression, remotely or in person, until full recovery is achieved. Key concepts of general management include:
Assess and monitor patients’ oxygenation status by pulse oximetry; identify those with low or declining oxygen saturation before further clinical deterioration.

Continue to: Consider the patient's age and general health
Consider the patient’s age and general health. Patients are at higher risk of severe disease if they are > 65 years or have an underlying comorbidity.4
Emphasize self-isolation and supportive care, including rest, hydration, and over-the-counter medications to relieve cough, reduce fever, and alleviate other symptoms.
Drugs: Few approved, some under study
The antiviral remdesivir is the only drug fully approved for clinical use by the FDA to treat COVID-19 in patients > 12 years.5,6
In addition, the FDA has issued an emergency use authorization (EUA) for several monoclonal antibodies as prophylaxis and treatment: tixagevimab packaged with cilgavimab (Evusheld) is the first antibody combination for pre-exposure prophylaxis (PrEP) against COVID-19; the separately packaged injectables are recommended for patients who have a history of severe allergy that prevents them from being vaccinated or those with moderate or severe immune-compromising disorders.7
In the pipeline. Several treatments are being tested in clinical trials to evaluate their effectiveness and safety in combating COVID-19, including:
- Antivirals, which prevent viruses from multiplying
- Immunomodulators, which reduce the body’s immune reaction to the virus
- Antibody therapies, which are manufactured antibodies against the virus
- Anti-inflammatory drugs, which reduce systemic inflammation and prevent organ dysfunction
- Cell therapies and gene therapies, which alter the expression of cells and genes.
Continue to: Outpatient treatment
Outpatient treatment
Several assessment tools that take into account patients’ age, respiratory status, and comorbidities are available for triage of patients infected with COVID-19.8
Most (> 80%) patients with COVID-19 have mild infection and are safely managed as outpatients or at home.9,10 For patients at high risk of severe disease, a few options are recommended for patients who do not require hospitalization or supplemental oxygen; guidelines on treatment of COVID-19 in outpatient settings that have been developed by various organizations are summarized in TABLE 2.7,11-25

Antiviral drugs target different stages of the SARS-CoV-2 replication cycle. They should be used early in the course of infection, particularly in patients at high risk of severe disease.

IDSA recommends antiviral therapy with molnupiravir, nirmatrelvir + ritonavir packaged together (Paxlovid), or remdesivir.11,12,26,27 Remdesivir requires intravenous (IV) infusion on 3 consecutive days, which can be difficult in some clinic settings.13,28 Nirmatrelvir + ritonavir should be initiated within 5 days after symptom onset. Overall, for most patients, nirmatrelvir + ritonavir is preferred because of oral dosing and higher efficacy in comparison to other antivirals. With nirmatrelvir + ritonavir, carefully consider drug–drug interactions and the need to adjust dosing in the presence of renal disease.28,29 There are no data on the efficacy of any combination treatments with these agents (other than co-packaged Paxlovid).

Monoclonal antibodies for COVID-19 are given primarily intravenously. They bind to the viral spike protein, thus preventing SARS-CoV-2 from attaching to and entering cells. Bamlanivimab + etesevimab and bebtelovimab are available under an EUA for outpatient treatment.14b Treatment should be initiated as early as possible in the course of infection—ideally, within 7 to 10 days after onset of symptoms.

Continue to: Bebtelovimab was recently given...
Bebtelovimab was recently given an EUA. It is a next-generation antibody that neutralizes all currently known variants and is the most potent monoclonal antibody against the Omicron variant, including its BA.2 subvariant.31 However, data about its activity against the BA.2 subvariant are based on laboratory testing and have not been confirmed in clinical trials. Clinical data were similar for this agent alone and for its use in combination with other monoclonal antibodies, but those trials were conducted before the emergence of Omicron.
In your decision-making about the most appropriate therapy, consider (1) the requirement that monoclonal antibodies be administered parenterally and (2) the susceptibility of the locally predominating viral variant.
Other monoclonal antibody agents are in the investigative pipeline; however, data about them have been largely presented through press releases or selectively reported in applications to the FDA for EUA. For example, preliminary reports show cilgavimab coverage against the Omicron variant14; so far, cilgavimab is not approved for treatment but is used in combination with tixagevimab for PreP—reportedly providing as long as 12 months of protection for patients who are less likely to respond to a vaccine.32
Corticosteroids. Guidelines recommend against dexamethasone and other systemic corticosteroids in outpatient settings. For patients with moderate-to-severe symptoms but for whom hospitalization is not possible (eg, beds are unavailable), the NIH panel recommends dexamethasone, 6 mg/d, for the duration of supplemental oxygen, not to exceed 10 days of treatment.1
Patients who were recently discharged after COVID-19 hospitalization should not continue remdesivir, dexamethasone, or baricitinib at home, even if they still require supplemental oxygen.
Continue to: Some treatments should not be in your COVID-19 toolbox
Some treatments should not be in your COVID-19 toolbox
High-quality studies are lacking for several other potential COVID-19 treatments. Some of these drugs are under investigation, with unclear benefit and with the potential risk of toxicity—and therefore should not be prescribed or used outside a clinical trial. See “Treatments not recommended for COVID-19,” page E14. 1-4,15-19,33-41
SIDEBAR
Treatments not recommended for COVID-191-4,15-19,33-41
Fluvoxamine. A few studies suggest that the selective serotonin reuptake inhibitor fluvoxamine reduces progression to severe disease; however, those studies have methodologic challenges.33 The drug is not FDA approved for treating COVID.33
Convalescent plasma, given to high-risk outpatients early in the course of disease, can reduce progression to severe disease,34,35 but it remains investigational for COVID-19 because trials have yielded mixed results.34-36
Ivermectin. The effect of ivermectin in patients with COVID-19 is unclear because high-quality studies do not exist and cases of ivermectin toxicity have occurred with incorrect administration.39
Hydroxychloroquine showed potential in a few observational studies, but randomized clinical trials have not shown any benefit.15
Azithromycin likewise showed potential in a few observational studies; randomized clinical trials have not shown any benefit, however.15
Statins. A few meta-analyses, based on observational studies, reported benefit from statins, but recent studies have shown that this class of drugs does not provide clinical benefit in alleviating COVID-19 symptoms.16,17,37
Inhaled corticosteroids. A systematic review reported no benefit or harm from using an inhaled corticosteroid.18 More recent studies show that the inhaled corticosteroid budesonide used in early COVID-19 might reduce the need for urgent care38 and, in patients who are at higher risk of COVID-19-related complications, shorten time to recovery.19
Vitamins and minerals. Limited observational studies suggest an association between vitamin and mineral deficiency (eg, vitamin C, zinc, and vitamin D) and risk of severe disease, but high-quality data about this finding do not exist.40,41
Casirivimab + imdevimab [REGEN-COV2]. This unapproved investigational combination treatment was granted an EUA in 2020 for postexposure prophylaxis. The EUA was withdrawn in January 2022 because of the limited efficacy of casirivimab + imdevimab against the Omicron variant of SARS-CoV-2.
Postexposure prophylaxis. National guidelines1-4 recommend against postexposure prophylaxis with hydroxychloroquine, colchicine, inhaled corticosteroids, or azithromycin.
TABLE 27,11-25 and TABLE 326,42-46 provide additional information on treatments not recommended outside trials, or not recommended at all, for COVID-19.
Treatment during hospitalization
The NIH COVID-19 treatment panel recommends hospitalization for patients who have any of the following findings1:
- Oxygen saturation < 94% while breathing room air
- Respiratory rate > 30 breaths/min
- A ratio of partial pressure of arterial O2 to fraction of inspired O2 (PaO2/FiO2) < 300 mm Hg
- Lung infiltrates > 50%.

General guidance for the care of hospitalized patients:
- Treatments that target the virus have the greatest efficacy when given early in the course of disease.
- Anti-inflammatory and immunosuppressive agents help prevent tissue damage from a dysregulated immune system. (See TABLE 326,42-46)
- The NIH panel,1 IDSA,2 and WHO3 recommend against dexamethasone and other corticosteroids for hospitalized patients who do not require supplemental oxygen.
- Prone positioning distributes oxygen more evenly in the lungs and improves overall oxygenation, thus reducing the need for mechanical ventilation.

Remdesivir. Once a hospitalized patient does require supplemental oxygen, the NIH panel,1 IDSA,2 and WHO3 recommend remdesivir; however, remdesivir is not recommended in many other countries because WHO has noted its limited efficacy.42 Dexamethasone is recommended alone, or in combination with remdesivir for patients who require increasing supplemental oxygen and those on mechanical ventilation.

Baricitinib. For patients with rapidly increasing oxygen requirements, invasive mechanical ventilation, and systemic inflammation, baricitinib, a Janus kinase inhibitor, can be administered, in addition to dexamethasone, with or without remdesivir.47
Continue to: Tocilizumab
Tocilizumab. A monoclonal antibody and interleukin (IL)-6 inhibitor, tocilizumab is also recommended in addition to dexamethasone, with or without remdesivir.48 Tocilizumab should be given only in combination with dexamethasone.49 Patients should receive baricitinib or tocilizumab—not both. IDSA recommends tofacitinib, with a prophylactic dose of an anticoagulant, for patients who are hospitalized with severe COVID-19 but who are not on any form of ventilation.50
Care of special populations
Special patient populations often seek primary care. Although many questions remain regarding the appropriate care of these populations, it is useful to summarize existing evidence and recommendations from current guidelines.
Children. COVID-19 is generally milder in children than in adults; many infected children are asymptomatic. However, infants and children who have an underlying medical condition are at risk of severe disease, including multisystem inflammatory syndrome.51
The NIH panel recommends supportive care alone for most children with mild-to-moderate disease.1 Remdesivir is recommended for hospitalized children ≥ 12 years who weigh ≥ 40 kg, have risk factors for severe disease, and have an emergent or increasing need for supplemental oxygen. Dexamethasone is recommended for hospitalized children requiring high-flow oxygen, noninvasive ventilation, invasive mechanical ventilation, or extracorporeal membrane oxygenation. Molnupiravir is not authorized for patients < 18 years because it can impede bone and cartilage growth.
There is insufficient evidence for or against the use of monoclonal antibody products for children with COVID-19 in an ambulatory setting. For hospitalized children, there is insufficient evidence for or against use of baricitinib and tocilizumab.
Continue to: Patients who are pregnant
Patients who are pregnant are at increased risk of severe COVID-19.52,53 The NIH states that, in general, treatment and vaccination of pregnant patients with COVID-19 should be the same as for nonpregnant patients.1
Pregnant subjects were excluded from several trials of COVID-19 treatments.54 Because Janus kinase inhibitors, such as baricitinib, are associated with an increased risk of thromboembolism, they are not recommended in pregnant patients who are already at risk of thromboembolic complications. Molnupiravir is not recommended for pregnant patients because of its potential for teratogenic effects.
The Society for Maternal-Fetal Medicine states that there are no absolute contraindications to the use of monoclonal antibodies in appropriate pregnant patients with COVID-19.55 Remdesivir has no known fetal toxicity and is recommended as a treatment that can be offered to pregnant patients. Dexamethasone can also be administered to pregnant patients who require oxygen; however, if dexamethasone is also being used to accelerate fetal lung maturity, more frequent initial dosing is needed.
Older people. COVID-19 treatments for older patients are the same as for the general adult population. However, because older people are more likely to have impaired renal function, renal function should be monitored when an older patient is being treated with COVID-19 medications that are eliminated renally (eg, remdesivir, baricitinib). Furthermore, drug–drug interactions have been reported in older patients treated with nirmatrelvir + ritonavir, primarily because of the effects of ritonavir. Review all of a patient’s medications, including over-the-counter drugs and herbal supplements, when prescribing treatment for COVID-19, and adjust the dosage by following guidance in FDA-approved prescribing information—ideally, in consultation with a pharmacist.
Immunocompromised patients. The combination product tixagevimab + cilgavimab [Evusheld] is FDA approved for COVID-19 PrEP, under an EUA, in patients who are not infected with SARS-CoV-2 who have an immune-compromising condition, who are unlikely to mount an adequate immune response to the COVID-19 vaccine, or those in whom vaccination is not recommended because of their history of a severe adverse reaction to a COVID-19 vaccine or one of its components.7
Continue to: Summing up
Summing up
With a growing need for effective and readily available COVID-19 treatments, there are an unprecedented number of clinical trials in process. Besides antivirals, immunomodulators, and antibody therapies, some novel mechanisms being tested include Janus kinase inhibitors, IL-6-receptor blockers, and drugs that target adult respiratory distress syndrome and cytokine release.
Once larger trials are completed, we can expect stronger evidence of potential treatment options and of safety and efficacy in children, pregnant women, and vulnerable populations. During the pandemic, the FDA’s EUA program has brought emerging treatments rapidly to clinicians; nevertheless, high-quality evidence, with thorough peer review, remains critical to inform COVID-19 treatment guidelines.
a https://healthdata.gov/Health/COVID-19-PublicTherapeutic-Locator/rxn6-qnx8/data
b Sotrovimab was effective against the Omicron variant of SARS-CoV-2—the dominant variant in early 2022— but is currently not FDA authorized in any region of the United States because of the prevalence of the Omicron BA.2 subvariant.30
CORRESPONDENCE
Ambar Kulshreshtha, MD, PhD, Department of Epidemiology, Emory Rollins School of Public Health, 4500 North Shallowford Road, Suite 134, Atlanta, GA 30338; [email protected]
The ongoing COVID-19 pandemic has caused more than 1 million deaths in the United States and continues to be a major public health challenge. Cases can be asymptomatic, or symptoms can range from a mild respiratory tract infection to acute respiratory distress and multiorgan failure.
Three strategies can successfully contain the pandemic and its consequences:
- Public health measures, such as masking and social distancing
- Prophylactic vaccines to reduce transmission
- Safe and effective drugs for reducing morbidity and mortality among infected patients.
Optimal treatment strategies for patients in ambulatory and hospital settings continue to evolve as new studies are reported and new strains of the virus arise. Many medical and scientific organizations, including the National Institutes of Health (NIH) COVID-19 treatment panel,1 Infectious Diseases Society of America (IDSA),2 World Health Organization (WHO),3 and Centers for Disease Control and Prevention,4 provide recommendations for managing patients with COVID-19. Their guidance is based on the strongest research available and is updated intermittently; nevertheless, a plethora of new data emerges weekly and controversies surround several treatments.
In this article, we
We encourage clinicians, in planning treatment, to consider:
- The availability of medications (ie, use the COVID-19 Public Therapeutic Locatora)
- The local COVID-19 situation
- Patient factors and preferences
- Evolving evidence regarding new and existing treatments.
Most evidence about the treatment of COVID-19 comes from studies conducted when the Omicron variant of SARS-CoV-2 was not the dominant variant, as it is today in the United States. As such, drugs authorized or approved by the US Food and Drug Administration (FDA) to treat COVID-19 or used off-label for that purpose might not be as efficacious today as they were almost a year ago. Furthermore, many trials of potential therapies against new viral variants are ongoing; if your patient is interested in enrolling in a clinical trial of an investigational COVID-19 treatment, refer them to www.clinicaltrials.gov.
General managementof COVID-19
Patients with COVID-19 experience a range of illness severity—from asymptomatic to mild symptoms, such as fever and myalgia, to critical illness requiring intensive care (TABLE 11,2). Patients with COVID-19 should therefore be monitored for progression, remotely or in person, until full recovery is achieved. Key concepts of general management include:
Assess and monitor patients’ oxygenation status by pulse oximetry; identify those with low or declining oxygen saturation before further clinical deterioration.

Continue to: Consider the patient's age and general health
Consider the patient’s age and general health. Patients are at higher risk of severe disease if they are > 65 years or have an underlying comorbidity.4
Emphasize self-isolation and supportive care, including rest, hydration, and over-the-counter medications to relieve cough, reduce fever, and alleviate other symptoms.
Drugs: Few approved, some under study
The antiviral remdesivir is the only drug fully approved for clinical use by the FDA to treat COVID-19 in patients > 12 years.5,6
In addition, the FDA has issued an emergency use authorization (EUA) for several monoclonal antibodies as prophylaxis and treatment: tixagevimab packaged with cilgavimab (Evusheld) is the first antibody combination for pre-exposure prophylaxis (PrEP) against COVID-19; the separately packaged injectables are recommended for patients who have a history of severe allergy that prevents them from being vaccinated or those with moderate or severe immune-compromising disorders.7
In the pipeline. Several treatments are being tested in clinical trials to evaluate their effectiveness and safety in combating COVID-19, including:
- Antivirals, which prevent viruses from multiplying
- Immunomodulators, which reduce the body’s immune reaction to the virus
- Antibody therapies, which are manufactured antibodies against the virus
- Anti-inflammatory drugs, which reduce systemic inflammation and prevent organ dysfunction
- Cell therapies and gene therapies, which alter the expression of cells and genes.
Continue to: Outpatient treatment
Outpatient treatment
Several assessment tools that take into account patients’ age, respiratory status, and comorbidities are available for triage of patients infected with COVID-19.8
Most (> 80%) patients with COVID-19 have mild infection and are safely managed as outpatients or at home.9,10 For patients at high risk of severe disease, a few options are recommended for patients who do not require hospitalization or supplemental oxygen; guidelines on treatment of COVID-19 in outpatient settings that have been developed by various organizations are summarized in TABLE 2.7,11-25

Antiviral drugs target different stages of the SARS-CoV-2 replication cycle. They should be used early in the course of infection, particularly in patients at high risk of severe disease.

IDSA recommends antiviral therapy with molnupiravir, nirmatrelvir + ritonavir packaged together (Paxlovid), or remdesivir.11,12,26,27 Remdesivir requires intravenous (IV) infusion on 3 consecutive days, which can be difficult in some clinic settings.13,28 Nirmatrelvir + ritonavir should be initiated within 5 days after symptom onset. Overall, for most patients, nirmatrelvir + ritonavir is preferred because of oral dosing and higher efficacy in comparison to other antivirals. With nirmatrelvir + ritonavir, carefully consider drug–drug interactions and the need to adjust dosing in the presence of renal disease.28,29 There are no data on the efficacy of any combination treatments with these agents (other than co-packaged Paxlovid).

Monoclonal antibodies for COVID-19 are given primarily intravenously. They bind to the viral spike protein, thus preventing SARS-CoV-2 from attaching to and entering cells. Bamlanivimab + etesevimab and bebtelovimab are available under an EUA for outpatient treatment.14b Treatment should be initiated as early as possible in the course of infection—ideally, within 7 to 10 days after onset of symptoms.

Continue to: Bebtelovimab was recently given...
Bebtelovimab was recently given an EUA. It is a next-generation antibody that neutralizes all currently known variants and is the most potent monoclonal antibody against the Omicron variant, including its BA.2 subvariant.31 However, data about its activity against the BA.2 subvariant are based on laboratory testing and have not been confirmed in clinical trials. Clinical data were similar for this agent alone and for its use in combination with other monoclonal antibodies, but those trials were conducted before the emergence of Omicron.
In your decision-making about the most appropriate therapy, consider (1) the requirement that monoclonal antibodies be administered parenterally and (2) the susceptibility of the locally predominating viral variant.
Other monoclonal antibody agents are in the investigative pipeline; however, data about them have been largely presented through press releases or selectively reported in applications to the FDA for EUA. For example, preliminary reports show cilgavimab coverage against the Omicron variant14; so far, cilgavimab is not approved for treatment but is used in combination with tixagevimab for PreP—reportedly providing as long as 12 months of protection for patients who are less likely to respond to a vaccine.32
Corticosteroids. Guidelines recommend against dexamethasone and other systemic corticosteroids in outpatient settings. For patients with moderate-to-severe symptoms but for whom hospitalization is not possible (eg, beds are unavailable), the NIH panel recommends dexamethasone, 6 mg/d, for the duration of supplemental oxygen, not to exceed 10 days of treatment.1
Patients who were recently discharged after COVID-19 hospitalization should not continue remdesivir, dexamethasone, or baricitinib at home, even if they still require supplemental oxygen.
Continue to: Some treatments should not be in your COVID-19 toolbox
Some treatments should not be in your COVID-19 toolbox
High-quality studies are lacking for several other potential COVID-19 treatments. Some of these drugs are under investigation, with unclear benefit and with the potential risk of toxicity—and therefore should not be prescribed or used outside a clinical trial. See “Treatments not recommended for COVID-19,” page E14. 1-4,15-19,33-41
SIDEBAR
Treatments not recommended for COVID-191-4,15-19,33-41
Fluvoxamine. A few studies suggest that the selective serotonin reuptake inhibitor fluvoxamine reduces progression to severe disease; however, those studies have methodologic challenges.33 The drug is not FDA approved for treating COVID.33
Convalescent plasma, given to high-risk outpatients early in the course of disease, can reduce progression to severe disease,34,35 but it remains investigational for COVID-19 because trials have yielded mixed results.34-36
Ivermectin. The effect of ivermectin in patients with COVID-19 is unclear because high-quality studies do not exist and cases of ivermectin toxicity have occurred with incorrect administration.39
Hydroxychloroquine showed potential in a few observational studies, but randomized clinical trials have not shown any benefit.15
Azithromycin likewise showed potential in a few observational studies; randomized clinical trials have not shown any benefit, however.15
Statins. A few meta-analyses, based on observational studies, reported benefit from statins, but recent studies have shown that this class of drugs does not provide clinical benefit in alleviating COVID-19 symptoms.16,17,37
Inhaled corticosteroids. A systematic review reported no benefit or harm from using an inhaled corticosteroid.18 More recent studies show that the inhaled corticosteroid budesonide used in early COVID-19 might reduce the need for urgent care38 and, in patients who are at higher risk of COVID-19-related complications, shorten time to recovery.19
Vitamins and minerals. Limited observational studies suggest an association between vitamin and mineral deficiency (eg, vitamin C, zinc, and vitamin D) and risk of severe disease, but high-quality data about this finding do not exist.40,41
Casirivimab + imdevimab [REGEN-COV2]. This unapproved investigational combination treatment was granted an EUA in 2020 for postexposure prophylaxis. The EUA was withdrawn in January 2022 because of the limited efficacy of casirivimab + imdevimab against the Omicron variant of SARS-CoV-2.
Postexposure prophylaxis. National guidelines1-4 recommend against postexposure prophylaxis with hydroxychloroquine, colchicine, inhaled corticosteroids, or azithromycin.
TABLE 27,11-25 and TABLE 326,42-46 provide additional information on treatments not recommended outside trials, or not recommended at all, for COVID-19.
Treatment during hospitalization
The NIH COVID-19 treatment panel recommends hospitalization for patients who have any of the following findings1:
- Oxygen saturation < 94% while breathing room air
- Respiratory rate > 30 breaths/min
- A ratio of partial pressure of arterial O2 to fraction of inspired O2 (PaO2/FiO2) < 300 mm Hg
- Lung infiltrates > 50%.

General guidance for the care of hospitalized patients:
- Treatments that target the virus have the greatest efficacy when given early in the course of disease.
- Anti-inflammatory and immunosuppressive agents help prevent tissue damage from a dysregulated immune system. (See TABLE 326,42-46)
- The NIH panel,1 IDSA,2 and WHO3 recommend against dexamethasone and other corticosteroids for hospitalized patients who do not require supplemental oxygen.
- Prone positioning distributes oxygen more evenly in the lungs and improves overall oxygenation, thus reducing the need for mechanical ventilation.

Remdesivir. Once a hospitalized patient does require supplemental oxygen, the NIH panel,1 IDSA,2 and WHO3 recommend remdesivir; however, remdesivir is not recommended in many other countries because WHO has noted its limited efficacy.42 Dexamethasone is recommended alone, or in combination with remdesivir for patients who require increasing supplemental oxygen and those on mechanical ventilation.

Baricitinib. For patients with rapidly increasing oxygen requirements, invasive mechanical ventilation, and systemic inflammation, baricitinib, a Janus kinase inhibitor, can be administered, in addition to dexamethasone, with or without remdesivir.47
Continue to: Tocilizumab
Tocilizumab. A monoclonal antibody and interleukin (IL)-6 inhibitor, tocilizumab is also recommended in addition to dexamethasone, with or without remdesivir.48 Tocilizumab should be given only in combination with dexamethasone.49 Patients should receive baricitinib or tocilizumab—not both. IDSA recommends tofacitinib, with a prophylactic dose of an anticoagulant, for patients who are hospitalized with severe COVID-19 but who are not on any form of ventilation.50
Care of special populations
Special patient populations often seek primary care. Although many questions remain regarding the appropriate care of these populations, it is useful to summarize existing evidence and recommendations from current guidelines.
Children. COVID-19 is generally milder in children than in adults; many infected children are asymptomatic. However, infants and children who have an underlying medical condition are at risk of severe disease, including multisystem inflammatory syndrome.51
The NIH panel recommends supportive care alone for most children with mild-to-moderate disease.1 Remdesivir is recommended for hospitalized children ≥ 12 years who weigh ≥ 40 kg, have risk factors for severe disease, and have an emergent or increasing need for supplemental oxygen. Dexamethasone is recommended for hospitalized children requiring high-flow oxygen, noninvasive ventilation, invasive mechanical ventilation, or extracorporeal membrane oxygenation. Molnupiravir is not authorized for patients < 18 years because it can impede bone and cartilage growth.
There is insufficient evidence for or against the use of monoclonal antibody products for children with COVID-19 in an ambulatory setting. For hospitalized children, there is insufficient evidence for or against use of baricitinib and tocilizumab.
Continue to: Patients who are pregnant
Patients who are pregnant are at increased risk of severe COVID-19.52,53 The NIH states that, in general, treatment and vaccination of pregnant patients with COVID-19 should be the same as for nonpregnant patients.1
Pregnant subjects were excluded from several trials of COVID-19 treatments.54 Because Janus kinase inhibitors, such as baricitinib, are associated with an increased risk of thromboembolism, they are not recommended in pregnant patients who are already at risk of thromboembolic complications. Molnupiravir is not recommended for pregnant patients because of its potential for teratogenic effects.
The Society for Maternal-Fetal Medicine states that there are no absolute contraindications to the use of monoclonal antibodies in appropriate pregnant patients with COVID-19.55 Remdesivir has no known fetal toxicity and is recommended as a treatment that can be offered to pregnant patients. Dexamethasone can also be administered to pregnant patients who require oxygen; however, if dexamethasone is also being used to accelerate fetal lung maturity, more frequent initial dosing is needed.
Older people. COVID-19 treatments for older patients are the same as for the general adult population. However, because older people are more likely to have impaired renal function, renal function should be monitored when an older patient is being treated with COVID-19 medications that are eliminated renally (eg, remdesivir, baricitinib). Furthermore, drug–drug interactions have been reported in older patients treated with nirmatrelvir + ritonavir, primarily because of the effects of ritonavir. Review all of a patient’s medications, including over-the-counter drugs and herbal supplements, when prescribing treatment for COVID-19, and adjust the dosage by following guidance in FDA-approved prescribing information—ideally, in consultation with a pharmacist.
Immunocompromised patients. The combination product tixagevimab + cilgavimab [Evusheld] is FDA approved for COVID-19 PrEP, under an EUA, in patients who are not infected with SARS-CoV-2 who have an immune-compromising condition, who are unlikely to mount an adequate immune response to the COVID-19 vaccine, or those in whom vaccination is not recommended because of their history of a severe adverse reaction to a COVID-19 vaccine or one of its components.7
Continue to: Summing up
Summing up
With a growing need for effective and readily available COVID-19 treatments, there are an unprecedented number of clinical trials in process. Besides antivirals, immunomodulators, and antibody therapies, some novel mechanisms being tested include Janus kinase inhibitors, IL-6-receptor blockers, and drugs that target adult respiratory distress syndrome and cytokine release.
Once larger trials are completed, we can expect stronger evidence of potential treatment options and of safety and efficacy in children, pregnant women, and vulnerable populations. During the pandemic, the FDA’s EUA program has brought emerging treatments rapidly to clinicians; nevertheless, high-quality evidence, with thorough peer review, remains critical to inform COVID-19 treatment guidelines.
a https://healthdata.gov/Health/COVID-19-PublicTherapeutic-Locator/rxn6-qnx8/data
b Sotrovimab was effective against the Omicron variant of SARS-CoV-2—the dominant variant in early 2022— but is currently not FDA authorized in any region of the United States because of the prevalence of the Omicron BA.2 subvariant.30
CORRESPONDENCE
Ambar Kulshreshtha, MD, PhD, Department of Epidemiology, Emory Rollins School of Public Health, 4500 North Shallowford Road, Suite 134, Atlanta, GA 30338; [email protected]
1. COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. July 19, 2022. Accessed July 21, 2022. www.covid19treatmentguidelines.nih.gov
2. IDSA guidelines on the treatment and management of patients with COVID-19. Infectious Diseases Society of America. Updated June 29, 2022. Accessed July 21, 2022. www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/#toc-23
3. Therapeutics and COVID-19: living guideline. World Health Organization. July 14, 2022. Accessed July 21, 2022. https://apps.who.int/iris/rest/bitstreams/1449398/retrieve
4. Centers for Disease Control and Prevention. Clinical care considerations. Updated May 27, 2022. Accessed July 21, 2022. www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html
5. Coronavirus (COVID-19) update: FDA approves first COVID-19 treatment for young children. Press release. US Food and Drug Administration. April 25, 2022. Accessed August 11, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-approves-first-covid-19-treatment-young-children
6. Know your treatment options for COVID-19. US Food and Drug Administration. Updated August 15, 2022. Accessed July 21, 2022. www.fda.gov/consumers/consumer-updates/know-your-treatment-options-covid-19
7. Tixagevimab and cilgavimab (Evusheld) for pre-exposure prophylaxis of COVID-19. JAMA. 2022;327:384-385. doi: 10.1001/jama.2021.24931
8. Judson TJ, Odisho AY, Neinstein AB, et al. Rapid design and implementation of an integrated patient self-triage and self-scheduling tool for COVID-19. J Am Med Inform Assoc. 2020;27:860-866. doi: 10.1093/jamia/ocaa051
9. Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383:1757-1766. doi: 10.1056/NEJMcp2009249
10. Greenhalgh T, Koh GCH, Car J. Covid-19: a remote assessment in primary care. BMJ. 2020;368:m1182. doi: 10.1136/bmj.m1182
11. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al; . Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386:509-520. doi: 10.1056/NEJMoa2116044
12. Hammond J, Leister-Tebbe H, Gardner A, et al; . Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386:1397-1408. doi: 10.1056/NEJMoa2118542
13. Gottlieb RL, Vaca CE, Paredes R, et al; . Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 2022;386:305-315. doi: 10.1056/NEJMoa2116846
14. Gupta A, Gonzalez-Rojas Y, Juarez E, et al; . Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941-1950. doi: 10.1056/NEJMoa2107934
15. Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Intern Med. 2020;173:623-631. doi: 10.7326/M20-4207
16. Scheen AJ. Statins and clinical outcomes with COVID-19: meta-analyses of observational studies. Diabetes Metab. 2021;47:101220. doi: 10.1016/j.diabet.2020.101220
17. Kow CS, Hasan SS. Meta-analysis of effect of statins in patients with COVID-19. Am J Cardiol. 2020;134:153-155. doi: 10.1016/j.amjcard.2020.08.004
18. Halpin DMG, Singh D, Hadfield RM. Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective. Eur Respir J. 2020;55:2001009. doi: 10.1183/13993003.01009-2020
19. Yu L-M, Bafadhel M, Dorward J, et al; . Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;398:843-855. doi: 10.1016/S0140-6736(21)01744-X
20. Siemieniuk RA, Bartoszko JJ, JP, et al. Antibody and cellular therapies for treatment of covid-19: a living systematic review and network meta-analysis. BMJ. 2021;374:n2231. doi: 10.1136/bmj.n2231
21. Siemieniuk RA, Bartoszko JJ, Zeraatkar D, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. doi: 10.1136/bmj.m2980
22. Agarwal A, Rochwerg B, Lamontagne F, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379. doi: 10.1136/bmj.m3379
23. Goldstein KM, Ghadimi K, Mystakelis H, et al. Risk of transmitting coronavirus disease 2019 during nebulizer treatment: a systematic review. J Aerosol Med Pulm Drug Deliv. 2021;34:155-170. doi: 10.1089/jamp.2020.1659
24. Schultze A, Walker AJ, MacKenna B, et al; . Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med. 2020;8:1106-1120. doi: 10.1016/S2213-2600(20)30415-X
25. What are the safety and efficacy results of bebtelovimab from BLAZE-4? Lilly USA. January 12, 2022. Accessed August 17, 2022. www.lillymedical.com/en-us/answers/what-are-the-safety-and-efficacy-results-of-bebtelovimab-from-blaze-4-159290
26. Beigel JH, Tomashek KM, Dodd LE, et al; . Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. doi: 10.1056/NEJMoa2007764
27. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787-1799. doi: 10.1056/NEJMoa2001282
28. Gandhi RT, Malani PN, Del Rio C. COVID-19 therapeutics for nonhospitalized patients. JAMA. 2022;327:617-618. doi: 10.1001/jama.2022.0335
29. Wen W, Chen C, Tang J, et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis. Ann Med. 2022;54:516-523. doi: 10.1080/07853890.2022.2034936
30. Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022:602:671-675. doi: 10.1038/s41586-021-04389-z
31. Emergency use authorization (EUA) for bebtelovimab (LY-CoV1404): Center for Drug Evaluation and Research (CDER) review. US Food and Drug Administration. Updated February 11, 2022. Accessed July 21, 2022. www.fda.gov/media/156396/download
32. Bartoszko JJ, Siemieniuk RAC, Kum E, et al. Prophylaxis against covid-19: living systematic review and network meta-analysis. BMJ. 2021;373:n949. doi: 10.1136/bmj.n949
33. Reis G, Dos Santos Moreira-Silva EA, Silva DCM, et al; TOGETHER Investigators. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health. 2022;10:e42-e51. doi: 10.1016/S2214-109X(21)00448-4
34. RECOVERY Collaborative Group. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet. 2021;397:2049-2059. doi: 10.1016/S0140-6736(21)00897-7
35. Simonovich VA, Burgos Pratx LD, Scibona P, et al; . A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021;384:619-629. doi: 10.1056/NEJMoa2031304
36. Joyner MJ, Carter RE, Senefeld JW, et al. Convalescent plasma antibody levels and the risk of death from Covid-19. N Engl J Med. 2021;384:1015-1027. doi: 10.1056/NEJMoa2031893
37. Ayeh SK, Abbey EJ, Khalifa BAA, et al. Statins use and COVID-19 outcomes in hospitalized patients. PLoS One. 2021;16:e0256899. doi: 10.1371/journal.pone.0256899
38. Ramakrishnan S, Nicolau DV Jr, Langford B, et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med. 2021;9:763-772. doi: 10.1016/S2213-2600(21)00160-0
39. Temple C, Hoang R, Hendrickson RG. Toxic effects from ivermectin use associated with prevention and treatment of Covid-19. N Engl J Med. 2021;385:2197-2198. doi: 10.1056/NEJMc2114907
40. Thomas S, Patel D, Bittel B, et al. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: the COVID A to Z randomized clinical trial. JAMA Netw Open. 2021;4:e210369. doi: 10.1001/jamanetworkopen.2021.0369
41. Adams KK, Baker WL, Sobieraj DM. Myth busters: dietary supplements and COVID-19. Ann Pharmacother. 2020;54:820-826. doi: 10.1177/1060028020928052
42. Pan H, Peto R, Henao-Restrepo A-M, et al. Repurposed antiviral drugs for Covid-19—interim WHO Solidarity trial results. N Engl J Med. 2021;384:497-511. doi: 10.1056/NEJMoa2023184
43. Kalil AC, Patterson TF, Mehta AK, et al; . Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384:795-807. doi: 10.1056/NEJMoa2031994
44. ; Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330-1341. doi: 10.1001/jama.2020.17023
45. ; Shankar-Hari M, Vale CL, Godolphin PJ, et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA. 2021;326:499-518. doi: 10.1001/jama.2021.11330
46. Wei QW, Lin H, Wei R-G, et al. Tocilizumab treatment for COVID-19 patients: a systematic review and meta-analysis. Infect Dis Poverty. 2021;10:71. doi: 10.1186/s40249-021-00857-w
47. Zhang X, Shang L, Fan G, et al. The efficacy and safety of Janus kinase inhibitors for patients with COVID-19: a living systematic review and meta-analysis. Front Med (Lausanne). 2021;8:800492. doi: 10.3389/fmed.2021.800492
48. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637-1645. doi: 10.1016/S0140-6736(21)00676-0
49. Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384:1491-1502. doi: 10.1056/NEJMoa2100433
50. Guimaraes PO, Quirk D, Furtado RH, et al; . Tofacitinib in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;385:406-415. doi: 10.1056/NEJMoa2101643
51. Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334-346. doi: 10.1056/NEJMoa2021680
52. Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320
53. Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175:817-826. doi: 10.1001/jamapediatrics.2021.1050
54. Jorgensen SCJ, Davis MR, Lapinsky SE. A review of remdesivir for COVID-19 in pregnancy and lactation. J Antimicrob Chemother. 2021;77:24-30. doi: 10.1093/jac/dkab311
55. Management considerations for pregnant patients with COVID-19. Society for Maternal-Fetal Medicine. Accessed July 21, 2022. https://s3.amazonaws.com/cdn.smfm.org/media/2336/SMFM_COVID_Management_of_COVID_pos_preg_patients_4-30-20_final.pdf
1. COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. July 19, 2022. Accessed July 21, 2022. www.covid19treatmentguidelines.nih.gov
2. IDSA guidelines on the treatment and management of patients with COVID-19. Infectious Diseases Society of America. Updated June 29, 2022. Accessed July 21, 2022. www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/#toc-23
3. Therapeutics and COVID-19: living guideline. World Health Organization. July 14, 2022. Accessed July 21, 2022. https://apps.who.int/iris/rest/bitstreams/1449398/retrieve
4. Centers for Disease Control and Prevention. Clinical care considerations. Updated May 27, 2022. Accessed July 21, 2022. www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html
5. Coronavirus (COVID-19) update: FDA approves first COVID-19 treatment for young children. Press release. US Food and Drug Administration. April 25, 2022. Accessed August 11, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-approves-first-covid-19-treatment-young-children
6. Know your treatment options for COVID-19. US Food and Drug Administration. Updated August 15, 2022. Accessed July 21, 2022. www.fda.gov/consumers/consumer-updates/know-your-treatment-options-covid-19
7. Tixagevimab and cilgavimab (Evusheld) for pre-exposure prophylaxis of COVID-19. JAMA. 2022;327:384-385. doi: 10.1001/jama.2021.24931
8. Judson TJ, Odisho AY, Neinstein AB, et al. Rapid design and implementation of an integrated patient self-triage and self-scheduling tool for COVID-19. J Am Med Inform Assoc. 2020;27:860-866. doi: 10.1093/jamia/ocaa051
9. Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383:1757-1766. doi: 10.1056/NEJMcp2009249
10. Greenhalgh T, Koh GCH, Car J. Covid-19: a remote assessment in primary care. BMJ. 2020;368:m1182. doi: 10.1136/bmj.m1182
11. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al; . Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386:509-520. doi: 10.1056/NEJMoa2116044
12. Hammond J, Leister-Tebbe H, Gardner A, et al; . Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386:1397-1408. doi: 10.1056/NEJMoa2118542
13. Gottlieb RL, Vaca CE, Paredes R, et al; . Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 2022;386:305-315. doi: 10.1056/NEJMoa2116846
14. Gupta A, Gonzalez-Rojas Y, Juarez E, et al; . Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941-1950. doi: 10.1056/NEJMoa2107934
15. Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Intern Med. 2020;173:623-631. doi: 10.7326/M20-4207
16. Scheen AJ. Statins and clinical outcomes with COVID-19: meta-analyses of observational studies. Diabetes Metab. 2021;47:101220. doi: 10.1016/j.diabet.2020.101220
17. Kow CS, Hasan SS. Meta-analysis of effect of statins in patients with COVID-19. Am J Cardiol. 2020;134:153-155. doi: 10.1016/j.amjcard.2020.08.004
18. Halpin DMG, Singh D, Hadfield RM. Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective. Eur Respir J. 2020;55:2001009. doi: 10.1183/13993003.01009-2020
19. Yu L-M, Bafadhel M, Dorward J, et al; . Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;398:843-855. doi: 10.1016/S0140-6736(21)01744-X
20. Siemieniuk RA, Bartoszko JJ, JP, et al. Antibody and cellular therapies for treatment of covid-19: a living systematic review and network meta-analysis. BMJ. 2021;374:n2231. doi: 10.1136/bmj.n2231
21. Siemieniuk RA, Bartoszko JJ, Zeraatkar D, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. doi: 10.1136/bmj.m2980
22. Agarwal A, Rochwerg B, Lamontagne F, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379. doi: 10.1136/bmj.m3379
23. Goldstein KM, Ghadimi K, Mystakelis H, et al. Risk of transmitting coronavirus disease 2019 during nebulizer treatment: a systematic review. J Aerosol Med Pulm Drug Deliv. 2021;34:155-170. doi: 10.1089/jamp.2020.1659
24. Schultze A, Walker AJ, MacKenna B, et al; . Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med. 2020;8:1106-1120. doi: 10.1016/S2213-2600(20)30415-X
25. What are the safety and efficacy results of bebtelovimab from BLAZE-4? Lilly USA. January 12, 2022. Accessed August 17, 2022. www.lillymedical.com/en-us/answers/what-are-the-safety-and-efficacy-results-of-bebtelovimab-from-blaze-4-159290
26. Beigel JH, Tomashek KM, Dodd LE, et al; . Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. doi: 10.1056/NEJMoa2007764
27. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787-1799. doi: 10.1056/NEJMoa2001282
28. Gandhi RT, Malani PN, Del Rio C. COVID-19 therapeutics for nonhospitalized patients. JAMA. 2022;327:617-618. doi: 10.1001/jama.2022.0335
29. Wen W, Chen C, Tang J, et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis. Ann Med. 2022;54:516-523. doi: 10.1080/07853890.2022.2034936
30. Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022:602:671-675. doi: 10.1038/s41586-021-04389-z
31. Emergency use authorization (EUA) for bebtelovimab (LY-CoV1404): Center for Drug Evaluation and Research (CDER) review. US Food and Drug Administration. Updated February 11, 2022. Accessed July 21, 2022. www.fda.gov/media/156396/download
32. Bartoszko JJ, Siemieniuk RAC, Kum E, et al. Prophylaxis against covid-19: living systematic review and network meta-analysis. BMJ. 2021;373:n949. doi: 10.1136/bmj.n949
33. Reis G, Dos Santos Moreira-Silva EA, Silva DCM, et al; TOGETHER Investigators. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health. 2022;10:e42-e51. doi: 10.1016/S2214-109X(21)00448-4
34. RECOVERY Collaborative Group. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet. 2021;397:2049-2059. doi: 10.1016/S0140-6736(21)00897-7
35. Simonovich VA, Burgos Pratx LD, Scibona P, et al; . A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021;384:619-629. doi: 10.1056/NEJMoa2031304
36. Joyner MJ, Carter RE, Senefeld JW, et al. Convalescent plasma antibody levels and the risk of death from Covid-19. N Engl J Med. 2021;384:1015-1027. doi: 10.1056/NEJMoa2031893
37. Ayeh SK, Abbey EJ, Khalifa BAA, et al. Statins use and COVID-19 outcomes in hospitalized patients. PLoS One. 2021;16:e0256899. doi: 10.1371/journal.pone.0256899
38. Ramakrishnan S, Nicolau DV Jr, Langford B, et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med. 2021;9:763-772. doi: 10.1016/S2213-2600(21)00160-0
39. Temple C, Hoang R, Hendrickson RG. Toxic effects from ivermectin use associated with prevention and treatment of Covid-19. N Engl J Med. 2021;385:2197-2198. doi: 10.1056/NEJMc2114907
40. Thomas S, Patel D, Bittel B, et al. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: the COVID A to Z randomized clinical trial. JAMA Netw Open. 2021;4:e210369. doi: 10.1001/jamanetworkopen.2021.0369
41. Adams KK, Baker WL, Sobieraj DM. Myth busters: dietary supplements and COVID-19. Ann Pharmacother. 2020;54:820-826. doi: 10.1177/1060028020928052
42. Pan H, Peto R, Henao-Restrepo A-M, et al. Repurposed antiviral drugs for Covid-19—interim WHO Solidarity trial results. N Engl J Med. 2021;384:497-511. doi: 10.1056/NEJMoa2023184
43. Kalil AC, Patterson TF, Mehta AK, et al; . Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384:795-807. doi: 10.1056/NEJMoa2031994
44. ; Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330-1341. doi: 10.1001/jama.2020.17023
45. ; Shankar-Hari M, Vale CL, Godolphin PJ, et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA. 2021;326:499-518. doi: 10.1001/jama.2021.11330
46. Wei QW, Lin H, Wei R-G, et al. Tocilizumab treatment for COVID-19 patients: a systematic review and meta-analysis. Infect Dis Poverty. 2021;10:71. doi: 10.1186/s40249-021-00857-w
47. Zhang X, Shang L, Fan G, et al. The efficacy and safety of Janus kinase inhibitors for patients with COVID-19: a living systematic review and meta-analysis. Front Med (Lausanne). 2021;8:800492. doi: 10.3389/fmed.2021.800492
48. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637-1645. doi: 10.1016/S0140-6736(21)00676-0
49. Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384:1491-1502. doi: 10.1056/NEJMoa2100433
50. Guimaraes PO, Quirk D, Furtado RH, et al; . Tofacitinib in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;385:406-415. doi: 10.1056/NEJMoa2101643
51. Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334-346. doi: 10.1056/NEJMoa2021680
52. Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320
53. Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175:817-826. doi: 10.1001/jamapediatrics.2021.1050
54. Jorgensen SCJ, Davis MR, Lapinsky SE. A review of remdesivir for COVID-19 in pregnancy and lactation. J Antimicrob Chemother. 2021;77:24-30. doi: 10.1093/jac/dkab311
55. Management considerations for pregnant patients with COVID-19. Society for Maternal-Fetal Medicine. Accessed July 21, 2022. https://s3.amazonaws.com/cdn.smfm.org/media/2336/SMFM_COVID_Management_of_COVID_pos_preg_patients_4-30-20_final.pdf
PRACTICE RECOMMENDATIONS
› Use antivirals (eg, molnupiravir, nirmatrelvir packaged with ritonavir [Paxlovid], and remdesivir) and monoclonal antibody agents (eg, bebtelovimab) effective against the circulating Omicron variant, to treat symptoms of mild-to-moderate COVID-19 illness. C
› Treat severely ill hospitalized COVID-19 patients who require supplemental oxygen with dexamethasone, alone or in combination with remdesivir, to produce better outcomes. B
› Consider administering baricitinib or tocilizumab, in addition to dexamethasone with or without remdesivir, to COVID-19 patients with rapidly increasing oxygen requirements. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
FAQ: New COVID Omicron boosters
Here are answers to frequently asked questions about the shots produced by Moderna and Pfizer/BioNTech, based on information provided by the CDC and Keri Althoff, PhD, and virologist Andrew Pekosz, PhD, Johns Hopkins Bloomberg School of Public Health epidemiologists.
Question: Who is eligible for the new bivalent boosters?
Answer: The CDC greenlighted the upgraded Pfizer/BioNTech shots for Americans 12 and older and the Moderna booster for those 18 and over, if they have received a primary vaccine series or a booster at least 2 months before.
The boosters have been redesigned to protect against the predominant BA.4 and BA.5 strains of the virus. The Biden administration is making 160 million of the booster shots available free of charge through pharmacies, doctor’s offices, clinics, and state health departments.
Q: What about children under 12?
A: The new boosters are not approved for children under 12. Additional testing and trials need to be conducted for safety and effectiveness. But officials recommend that children 5 and above receive the primary vaccine series and be boosted with one shot. Children 6 months to under 5 years are not yet eligible for boosters.
Pfizer said it hopes to ask the Food and Drug Administration for authorization in 5- to 11-year-olds in October.
Q: How do the new bivalent boosters differ from previous shots?
A: The new shots use the same mRNA technology as the prior Moderna and Pfizer/BioNTech vaccines and boosters but have been upgraded to target the newer Omicron strains. The shots use mRNA created in a lab to teach our cells to produce a specific protein that triggers an immune-system response and make antibodies that help protect us from SARS-CoV-2, the virus that causes COVID.
The recipe for the new shots incorporates the so-called “spike protein” of both the original (ancestral) strain of the virus and more highly transmissible Omicron strains (BA.4, BA.5). Once your body produces these proteins, your immune system kicks into gear to mount a response.
It’s also possible – but yet to be determined – that the new bivalent boosters will offer protection against newer but less common strains known as BA.4.6 and BA.2.75.
Q: Are there any new risks or side effects associated with these boosters?
A: Health experts don’t expect to see anything beyond what has already been noted with prior mRNA vaccines, with the vast majority of recipients experiencing only mild issues such as redness from the shot, soreness, and fatigue.
Q: Do I need one of the new shots if I’ve already had past boosters or had COVID?
A: Yes. Even if you’ve been infected with COVID in the past year and/or received the prior series of primary vaccines and boosters, you should get a bivalent Omicron shot.
Doing so will give you broader immunity against COVID and also help limit the emergence of other variants. The more Americans with high immunity, the better; it makes it less likely other variants will emerge that can escape the immunity provided by vaccines and COVID infections.
Q: How long should I wait, from the time of my last shot, before getting a new booster?
A: The bivalent boosters are most effective when given after a period of time has passed between your last shot and the new one. A 2- to 3-month waiting period is the minimum, but some evidence suggests extending it out to 4-6 months might be good timing.
To determine when you should get a new booster, check out the CDC’s Stay Up to Date with COVID-19 Vaccines Including Boosters website.
Q: What if I’ve recently had COVID?
A: There are no specific rules about a waiting period after COVID infection. But if you have been infected with the virus in the last 8 weeks, you may want to wait for 8 weeks to pass before receiving the bivalent booster to allow your immune system to get greater benefit from the shot.
Q: If I never got the original vaccines, do I need to get those shots first?
A: Yes. The bivalent vaccine has a lower dose of mRNA than the vaccines used in the primary series of vaccines, rolled out in late 2020. The bivalent vaccine is authorized for use as a booster dose and not a primary vaccine series dose.
Q: Do the Omicron-specific boosters entirely replace the other boosters?
A: Yes. The new booster shots, which target the original strain and the Omicron subvariants, are now the only available boosters for people ages 12 and older. The FDA no longer authorizes the previous booster doses for people in the approved age groups.
Q: What if I received a non-mRNA vaccine produced by Novavax or Johnson & Johnson? Should I still get an mRNA booster?
A: You can mix and match COVID vaccines, and you are eligible to get the bivalent booster 8 weeks after completing the primary COVID vaccination series – whether that was two doses of mRNA or Novavax, or one shot of J&J.
Q: How effective are the new boosters?
A: Scientists don’t have complete effectiveness data from the bivalent vaccines yet. But because the new boosters contain mRNA from the Omicron and the original strains, they are believed to offer greater protection against COVID overall.
Cellular-level data support this, with studies showing the bivalent vaccines increase neutralizing antibodies to BA.4/BA.5 strains. Scientists regard these kinds of studies as surrogate stand-ins for clinical trials. But officials will be studying the effectiveness of the new boosters, examining to what degree they reduce hospitalizations and deaths.
Q: How long will the boosters’ protection last?
A: Research shows that vaccine effectiveness eventually wanes, which is why we have the boosters. Scientists will be monitoring to see how long the protection lasts from the bivalent boosters through studies of antibody levels as well as assessments of severe COVID illnesses over time, throughout the fall and winter.
Q: Is it OK to get a flu shot and a COVID booster at the same time?
A: Yes. In fact, it’s important to get a flu shot this year because some experts believe we could see overlapping COVID-influenza surges this fall – a phenomenon some have fancifully called a “twindemic.” Getting a flu shot and COVID booster – simultaneously, if possible – is particularly important if you’re in a high-risk group.
People who are susceptible to severe complications from COVID – such as older people, people with weakened immune systems, and those with chronic health conditions – are also especially vulnerable to severe influenza complications.
Q: Will a new booster mean I can stop wearing a mask, social distancing, avoiding crowded indoor spaces, and taking other precautions to avoid COVID?
A: No. It’s still a good idea to mask up, keep your distance from others, avoid indoor spaces with people whose vaccine status is unknown, and take other precautions against COVID.
Although the new boosters are front of mind, it’s a good idea to also use other tools in the toolbox, as well, particularly if you have contact with someone who is older, immune-suppressed, or has a chronic condition that puts them at higher risk from COVID.
Keep in mind: The community risk of infection nationwide is still high today, with about 67,400 new cases and nearly 320 deaths reported each day in the United States, according to the latest CDC reports.A version of this article first appeared on WebMD.
Here are answers to frequently asked questions about the shots produced by Moderna and Pfizer/BioNTech, based on information provided by the CDC and Keri Althoff, PhD, and virologist Andrew Pekosz, PhD, Johns Hopkins Bloomberg School of Public Health epidemiologists.
Question: Who is eligible for the new bivalent boosters?
Answer: The CDC greenlighted the upgraded Pfizer/BioNTech shots for Americans 12 and older and the Moderna booster for those 18 and over, if they have received a primary vaccine series or a booster at least 2 months before.
The boosters have been redesigned to protect against the predominant BA.4 and BA.5 strains of the virus. The Biden administration is making 160 million of the booster shots available free of charge through pharmacies, doctor’s offices, clinics, and state health departments.
Q: What about children under 12?
A: The new boosters are not approved for children under 12. Additional testing and trials need to be conducted for safety and effectiveness. But officials recommend that children 5 and above receive the primary vaccine series and be boosted with one shot. Children 6 months to under 5 years are not yet eligible for boosters.
Pfizer said it hopes to ask the Food and Drug Administration for authorization in 5- to 11-year-olds in October.
Q: How do the new bivalent boosters differ from previous shots?
A: The new shots use the same mRNA technology as the prior Moderna and Pfizer/BioNTech vaccines and boosters but have been upgraded to target the newer Omicron strains. The shots use mRNA created in a lab to teach our cells to produce a specific protein that triggers an immune-system response and make antibodies that help protect us from SARS-CoV-2, the virus that causes COVID.
The recipe for the new shots incorporates the so-called “spike protein” of both the original (ancestral) strain of the virus and more highly transmissible Omicron strains (BA.4, BA.5). Once your body produces these proteins, your immune system kicks into gear to mount a response.
It’s also possible – but yet to be determined – that the new bivalent boosters will offer protection against newer but less common strains known as BA.4.6 and BA.2.75.
Q: Are there any new risks or side effects associated with these boosters?
A: Health experts don’t expect to see anything beyond what has already been noted with prior mRNA vaccines, with the vast majority of recipients experiencing only mild issues such as redness from the shot, soreness, and fatigue.
Q: Do I need one of the new shots if I’ve already had past boosters or had COVID?
A: Yes. Even if you’ve been infected with COVID in the past year and/or received the prior series of primary vaccines and boosters, you should get a bivalent Omicron shot.
Doing so will give you broader immunity against COVID and also help limit the emergence of other variants. The more Americans with high immunity, the better; it makes it less likely other variants will emerge that can escape the immunity provided by vaccines and COVID infections.
Q: How long should I wait, from the time of my last shot, before getting a new booster?
A: The bivalent boosters are most effective when given after a period of time has passed between your last shot and the new one. A 2- to 3-month waiting period is the minimum, but some evidence suggests extending it out to 4-6 months might be good timing.
To determine when you should get a new booster, check out the CDC’s Stay Up to Date with COVID-19 Vaccines Including Boosters website.
Q: What if I’ve recently had COVID?
A: There are no specific rules about a waiting period after COVID infection. But if you have been infected with the virus in the last 8 weeks, you may want to wait for 8 weeks to pass before receiving the bivalent booster to allow your immune system to get greater benefit from the shot.
Q: If I never got the original vaccines, do I need to get those shots first?
A: Yes. The bivalent vaccine has a lower dose of mRNA than the vaccines used in the primary series of vaccines, rolled out in late 2020. The bivalent vaccine is authorized for use as a booster dose and not a primary vaccine series dose.
Q: Do the Omicron-specific boosters entirely replace the other boosters?
A: Yes. The new booster shots, which target the original strain and the Omicron subvariants, are now the only available boosters for people ages 12 and older. The FDA no longer authorizes the previous booster doses for people in the approved age groups.
Q: What if I received a non-mRNA vaccine produced by Novavax or Johnson & Johnson? Should I still get an mRNA booster?
A: You can mix and match COVID vaccines, and you are eligible to get the bivalent booster 8 weeks after completing the primary COVID vaccination series – whether that was two doses of mRNA or Novavax, or one shot of J&J.
Q: How effective are the new boosters?
A: Scientists don’t have complete effectiveness data from the bivalent vaccines yet. But because the new boosters contain mRNA from the Omicron and the original strains, they are believed to offer greater protection against COVID overall.
Cellular-level data support this, with studies showing the bivalent vaccines increase neutralizing antibodies to BA.4/BA.5 strains. Scientists regard these kinds of studies as surrogate stand-ins for clinical trials. But officials will be studying the effectiveness of the new boosters, examining to what degree they reduce hospitalizations and deaths.
Q: How long will the boosters’ protection last?
A: Research shows that vaccine effectiveness eventually wanes, which is why we have the boosters. Scientists will be monitoring to see how long the protection lasts from the bivalent boosters through studies of antibody levels as well as assessments of severe COVID illnesses over time, throughout the fall and winter.
Q: Is it OK to get a flu shot and a COVID booster at the same time?
A: Yes. In fact, it’s important to get a flu shot this year because some experts believe we could see overlapping COVID-influenza surges this fall – a phenomenon some have fancifully called a “twindemic.” Getting a flu shot and COVID booster – simultaneously, if possible – is particularly important if you’re in a high-risk group.
People who are susceptible to severe complications from COVID – such as older people, people with weakened immune systems, and those with chronic health conditions – are also especially vulnerable to severe influenza complications.
Q: Will a new booster mean I can stop wearing a mask, social distancing, avoiding crowded indoor spaces, and taking other precautions to avoid COVID?
A: No. It’s still a good idea to mask up, keep your distance from others, avoid indoor spaces with people whose vaccine status is unknown, and take other precautions against COVID.
Although the new boosters are front of mind, it’s a good idea to also use other tools in the toolbox, as well, particularly if you have contact with someone who is older, immune-suppressed, or has a chronic condition that puts them at higher risk from COVID.
Keep in mind: The community risk of infection nationwide is still high today, with about 67,400 new cases and nearly 320 deaths reported each day in the United States, according to the latest CDC reports.A version of this article first appeared on WebMD.
Here are answers to frequently asked questions about the shots produced by Moderna and Pfizer/BioNTech, based on information provided by the CDC and Keri Althoff, PhD, and virologist Andrew Pekosz, PhD, Johns Hopkins Bloomberg School of Public Health epidemiologists.
Question: Who is eligible for the new bivalent boosters?
Answer: The CDC greenlighted the upgraded Pfizer/BioNTech shots for Americans 12 and older and the Moderna booster for those 18 and over, if they have received a primary vaccine series or a booster at least 2 months before.
The boosters have been redesigned to protect against the predominant BA.4 and BA.5 strains of the virus. The Biden administration is making 160 million of the booster shots available free of charge through pharmacies, doctor’s offices, clinics, and state health departments.
Q: What about children under 12?
A: The new boosters are not approved for children under 12. Additional testing and trials need to be conducted for safety and effectiveness. But officials recommend that children 5 and above receive the primary vaccine series and be boosted with one shot. Children 6 months to under 5 years are not yet eligible for boosters.
Pfizer said it hopes to ask the Food and Drug Administration for authorization in 5- to 11-year-olds in October.
Q: How do the new bivalent boosters differ from previous shots?
A: The new shots use the same mRNA technology as the prior Moderna and Pfizer/BioNTech vaccines and boosters but have been upgraded to target the newer Omicron strains. The shots use mRNA created in a lab to teach our cells to produce a specific protein that triggers an immune-system response and make antibodies that help protect us from SARS-CoV-2, the virus that causes COVID.
The recipe for the new shots incorporates the so-called “spike protein” of both the original (ancestral) strain of the virus and more highly transmissible Omicron strains (BA.4, BA.5). Once your body produces these proteins, your immune system kicks into gear to mount a response.
It’s also possible – but yet to be determined – that the new bivalent boosters will offer protection against newer but less common strains known as BA.4.6 and BA.2.75.
Q: Are there any new risks or side effects associated with these boosters?
A: Health experts don’t expect to see anything beyond what has already been noted with prior mRNA vaccines, with the vast majority of recipients experiencing only mild issues such as redness from the shot, soreness, and fatigue.
Q: Do I need one of the new shots if I’ve already had past boosters or had COVID?
A: Yes. Even if you’ve been infected with COVID in the past year and/or received the prior series of primary vaccines and boosters, you should get a bivalent Omicron shot.
Doing so will give you broader immunity against COVID and also help limit the emergence of other variants. The more Americans with high immunity, the better; it makes it less likely other variants will emerge that can escape the immunity provided by vaccines and COVID infections.
Q: How long should I wait, from the time of my last shot, before getting a new booster?
A: The bivalent boosters are most effective when given after a period of time has passed between your last shot and the new one. A 2- to 3-month waiting period is the minimum, but some evidence suggests extending it out to 4-6 months might be good timing.
To determine when you should get a new booster, check out the CDC’s Stay Up to Date with COVID-19 Vaccines Including Boosters website.
Q: What if I’ve recently had COVID?
A: There are no specific rules about a waiting period after COVID infection. But if you have been infected with the virus in the last 8 weeks, you may want to wait for 8 weeks to pass before receiving the bivalent booster to allow your immune system to get greater benefit from the shot.
Q: If I never got the original vaccines, do I need to get those shots first?
A: Yes. The bivalent vaccine has a lower dose of mRNA than the vaccines used in the primary series of vaccines, rolled out in late 2020. The bivalent vaccine is authorized for use as a booster dose and not a primary vaccine series dose.
Q: Do the Omicron-specific boosters entirely replace the other boosters?
A: Yes. The new booster shots, which target the original strain and the Omicron subvariants, are now the only available boosters for people ages 12 and older. The FDA no longer authorizes the previous booster doses for people in the approved age groups.
Q: What if I received a non-mRNA vaccine produced by Novavax or Johnson & Johnson? Should I still get an mRNA booster?
A: You can mix and match COVID vaccines, and you are eligible to get the bivalent booster 8 weeks after completing the primary COVID vaccination series – whether that was two doses of mRNA or Novavax, or one shot of J&J.
Q: How effective are the new boosters?
A: Scientists don’t have complete effectiveness data from the bivalent vaccines yet. But because the new boosters contain mRNA from the Omicron and the original strains, they are believed to offer greater protection against COVID overall.
Cellular-level data support this, with studies showing the bivalent vaccines increase neutralizing antibodies to BA.4/BA.5 strains. Scientists regard these kinds of studies as surrogate stand-ins for clinical trials. But officials will be studying the effectiveness of the new boosters, examining to what degree they reduce hospitalizations and deaths.
Q: How long will the boosters’ protection last?
A: Research shows that vaccine effectiveness eventually wanes, which is why we have the boosters. Scientists will be monitoring to see how long the protection lasts from the bivalent boosters through studies of antibody levels as well as assessments of severe COVID illnesses over time, throughout the fall and winter.
Q: Is it OK to get a flu shot and a COVID booster at the same time?
A: Yes. In fact, it’s important to get a flu shot this year because some experts believe we could see overlapping COVID-influenza surges this fall – a phenomenon some have fancifully called a “twindemic.” Getting a flu shot and COVID booster – simultaneously, if possible – is particularly important if you’re in a high-risk group.
People who are susceptible to severe complications from COVID – such as older people, people with weakened immune systems, and those with chronic health conditions – are also especially vulnerable to severe influenza complications.
Q: Will a new booster mean I can stop wearing a mask, social distancing, avoiding crowded indoor spaces, and taking other precautions to avoid COVID?
A: No. It’s still a good idea to mask up, keep your distance from others, avoid indoor spaces with people whose vaccine status is unknown, and take other precautions against COVID.
Although the new boosters are front of mind, it’s a good idea to also use other tools in the toolbox, as well, particularly if you have contact with someone who is older, immune-suppressed, or has a chronic condition that puts them at higher risk from COVID.
Keep in mind: The community risk of infection nationwide is still high today, with about 67,400 new cases and nearly 320 deaths reported each day in the United States, according to the latest CDC reports.A version of this article first appeared on WebMD.
Even mild COVID tied to vascular impairment
In a small prospective study, participants who previously had COVID-19, even those with mild illness, had significantly decreased CVR, compared with never-infected individuals.
Results also showed cerebral blood flow (CBF) was greater in never-infected versus previously infected participants, and whole-brain CVR was lower in previously infected versus never-infected participants. Although CVR was also smaller in those with versus those without post-COVID neurologic conditions, the difference was not considered significant.
“It is important to remember that while our findings were statistically significant, we had a relatively small sample size – 25 total participants – and so we encourage future larger studies in this domain to see if these results are reproducible at a larger scale,” lead author Andrew Callen, MD, assistant professor of radiology, Neuroradiology Section, University of Colorado at Denver, Aurora, said in an interview.
“In a practical sense, it may encourage treating clinicians to be more aggressive with preventative neurovascular and cardiovascular health measures and/or screening in this patient population,” Dr. Callen said.
The findings were published online in the American Journal of Roentgenology.
Endothelial dysfunction
The acute phase SARS-CoV-2 infection “is associated with strokes that have features of both vascular inflammation and thromboembolism,” the investigators note.
Moreover, following the acute phase of infection, up to three-quarters of patients “experience persistent neurologic symptoms not attributable to another diagnosis, including headache, difficulty concentrating, vision changes, disequilibrium, and fatigue,” they write.
Preliminary studies “suggest a potential role for endothelial and circulatory dysfunction” in these symptoms, they add.
The researchers note that vessel wall imaging is an MRI technique that can detect and characterize arterial vascular inflammation and may differentiate vasculitic arterial pathology from atherosclerotic pathology.
Dr. Callen conducted previous research assessing cerebral vasoreactivity in women living with HIV. He noted that this is a population at a much higher risk of stroke, compared with uninfected individuals with otherwise similar cardiovascular risk factors, even when their viral load is controlled with antiretroviral therapies.
Evidence has pointed to chronic endothelial dysfunction in these individuals, and endothelial function and dysfunction can be measured through vasoreactivity testing, Dr. Callen said.
“As the COVID pandemic progressed, not only did we observe an increased rate of stroke in individuals acutely infected with COVID, but histopathological evidence began to emerge which suggested that the COVID-19 virus had tropism to and often damaged the vascular endothelium,” he noted.
This emerging evidence prompted Dr. Callen to wonder whether “individuals previously infected with COVID might also demonstrate long-term impairment in cerebral vasoreactivity or if we might see abnormalities using high resolution vessel wall imaging.”
In the current study, 15 individuals with prior SARS-CoV-2 infection (11 women, 4 men; mean age, 43 years) were compared with 10 never-infected individuals (8 women, 2 men; mean age, 43 years) who functioned as the control group.
The previously infected individuals, of whom three had prior critical infection and 12 had prior mild infection, were assessed, on average, about 8 months after infection. Of this group, seven had various post-COVID neurologic conditions, including headache, memory impairment, insomnia, depression, disequilibrium, fatigue, personality change, phantosmias (detecting smells that aren’t present), dysgeusia (taste disorder), and tinnitus.
All participants underwent MRI and vessel wall imaging. The MRI included arterial spin labeling perfusion imaging with acetazolamide stimulus to measure CBF and calculate CVR. The vessel wall imaging examinations used a contrast-enhanced black-blood 3D T1-weighted sequence.
Imaging data
Prior to acetazolamide administration, the mean whole-cortex CBF did not differ significantly between never-infected and previously infected participants. However, following the acetazolamide administration, the mean whole-cortex CBF was greater in never-infected participants (73.8 mL/100 g/min vs. 60.5 mL/100 g/min, respectively; P = .04).
Moreover, the mean whole-brain CVR was greater in never-infected participants, compared with previously infected participants (27.8 mL/100 g/min vs. 19.1 mL/100 g/min; P < .001).
After adjusting for age and sex, researchers found that prior infection was associated with a lower whole-brain CVR (–8.9 mL/100 g/min; 95% confidence interval, 4.6-13.3 ml/100g/min; P < .001).
Previously infected individuals also showed significantly lower CVR, even after the researchers excluded those with prior critical illness.
A nonsignificant difference was found in previously infected participants, with smaller CVR in participants with versus without post-COVID neurologic symptoms (16.9 vs. 21.0 mL/100 g/min; P = .22).
In addition, 40% of the previously infected participants versus 10% of the never-infected participants had at least one vessel wall imaging abnormality – but the difference was not deemed significant (P = .18). Notably, “all detected vessel wall imaging abnormalities were morphologically consistent with atherosclerosis rather than vasculitis,” the investigators said.
Dr. Callen said it is “unknown whether the lack of statistical significance in the differences in vasoreactivity impairment with those living with long COVID symptoms is due to a lack of a biomechanistic correlation or due to statistical underpowering.”
If it is the latter, “it may emphasize the role of vascular health in those living with long COVID symptoms and potentially all individuals living with COVID,” he added.
Independent risk factor?
Commenting on the study for this article, Jared Narvid, MD, associate professor of neuroradiology, University of California, San Francisco, said it “adds to the literature suggesting a correlation between COVID-19 infection and measures of cerebrovascular abnormality.”
Dr. Narvid, who was not involved with the research, added that “although it is a small case-control study, it is well executed and should encourage scientists to further study whether COVID-19 infection represents an independent risk factor for cerebrovascular disease.”
The investigators agree. “Future studies are needed to determine the clinical implications arising from SARS-CoV-2–associated CVR impairment,” they write.
The study was funded by a University of Colorado department of radiology Faculty Development Seed Grant. The investigators and Dr. Narvid report no relevant financial relationships.
A version of this article first appeared on Medscape.com .
In a small prospective study, participants who previously had COVID-19, even those with mild illness, had significantly decreased CVR, compared with never-infected individuals.
Results also showed cerebral blood flow (CBF) was greater in never-infected versus previously infected participants, and whole-brain CVR was lower in previously infected versus never-infected participants. Although CVR was also smaller in those with versus those without post-COVID neurologic conditions, the difference was not considered significant.
“It is important to remember that while our findings were statistically significant, we had a relatively small sample size – 25 total participants – and so we encourage future larger studies in this domain to see if these results are reproducible at a larger scale,” lead author Andrew Callen, MD, assistant professor of radiology, Neuroradiology Section, University of Colorado at Denver, Aurora, said in an interview.
“In a practical sense, it may encourage treating clinicians to be more aggressive with preventative neurovascular and cardiovascular health measures and/or screening in this patient population,” Dr. Callen said.
The findings were published online in the American Journal of Roentgenology.
Endothelial dysfunction
The acute phase SARS-CoV-2 infection “is associated with strokes that have features of both vascular inflammation and thromboembolism,” the investigators note.
Moreover, following the acute phase of infection, up to three-quarters of patients “experience persistent neurologic symptoms not attributable to another diagnosis, including headache, difficulty concentrating, vision changes, disequilibrium, and fatigue,” they write.
Preliminary studies “suggest a potential role for endothelial and circulatory dysfunction” in these symptoms, they add.
The researchers note that vessel wall imaging is an MRI technique that can detect and characterize arterial vascular inflammation and may differentiate vasculitic arterial pathology from atherosclerotic pathology.
Dr. Callen conducted previous research assessing cerebral vasoreactivity in women living with HIV. He noted that this is a population at a much higher risk of stroke, compared with uninfected individuals with otherwise similar cardiovascular risk factors, even when their viral load is controlled with antiretroviral therapies.
Evidence has pointed to chronic endothelial dysfunction in these individuals, and endothelial function and dysfunction can be measured through vasoreactivity testing, Dr. Callen said.
“As the COVID pandemic progressed, not only did we observe an increased rate of stroke in individuals acutely infected with COVID, but histopathological evidence began to emerge which suggested that the COVID-19 virus had tropism to and often damaged the vascular endothelium,” he noted.
This emerging evidence prompted Dr. Callen to wonder whether “individuals previously infected with COVID might also demonstrate long-term impairment in cerebral vasoreactivity or if we might see abnormalities using high resolution vessel wall imaging.”
In the current study, 15 individuals with prior SARS-CoV-2 infection (11 women, 4 men; mean age, 43 years) were compared with 10 never-infected individuals (8 women, 2 men; mean age, 43 years) who functioned as the control group.
The previously infected individuals, of whom three had prior critical infection and 12 had prior mild infection, were assessed, on average, about 8 months after infection. Of this group, seven had various post-COVID neurologic conditions, including headache, memory impairment, insomnia, depression, disequilibrium, fatigue, personality change, phantosmias (detecting smells that aren’t present), dysgeusia (taste disorder), and tinnitus.
All participants underwent MRI and vessel wall imaging. The MRI included arterial spin labeling perfusion imaging with acetazolamide stimulus to measure CBF and calculate CVR. The vessel wall imaging examinations used a contrast-enhanced black-blood 3D T1-weighted sequence.
Imaging data
Prior to acetazolamide administration, the mean whole-cortex CBF did not differ significantly between never-infected and previously infected participants. However, following the acetazolamide administration, the mean whole-cortex CBF was greater in never-infected participants (73.8 mL/100 g/min vs. 60.5 mL/100 g/min, respectively; P = .04).
Moreover, the mean whole-brain CVR was greater in never-infected participants, compared with previously infected participants (27.8 mL/100 g/min vs. 19.1 mL/100 g/min; P < .001).
After adjusting for age and sex, researchers found that prior infection was associated with a lower whole-brain CVR (–8.9 mL/100 g/min; 95% confidence interval, 4.6-13.3 ml/100g/min; P < .001).
Previously infected individuals also showed significantly lower CVR, even after the researchers excluded those with prior critical illness.
A nonsignificant difference was found in previously infected participants, with smaller CVR in participants with versus without post-COVID neurologic symptoms (16.9 vs. 21.0 mL/100 g/min; P = .22).
In addition, 40% of the previously infected participants versus 10% of the never-infected participants had at least one vessel wall imaging abnormality – but the difference was not deemed significant (P = .18). Notably, “all detected vessel wall imaging abnormalities were morphologically consistent with atherosclerosis rather than vasculitis,” the investigators said.
Dr. Callen said it is “unknown whether the lack of statistical significance in the differences in vasoreactivity impairment with those living with long COVID symptoms is due to a lack of a biomechanistic correlation or due to statistical underpowering.”
If it is the latter, “it may emphasize the role of vascular health in those living with long COVID symptoms and potentially all individuals living with COVID,” he added.
Independent risk factor?
Commenting on the study for this article, Jared Narvid, MD, associate professor of neuroradiology, University of California, San Francisco, said it “adds to the literature suggesting a correlation between COVID-19 infection and measures of cerebrovascular abnormality.”
Dr. Narvid, who was not involved with the research, added that “although it is a small case-control study, it is well executed and should encourage scientists to further study whether COVID-19 infection represents an independent risk factor for cerebrovascular disease.”
The investigators agree. “Future studies are needed to determine the clinical implications arising from SARS-CoV-2–associated CVR impairment,” they write.
The study was funded by a University of Colorado department of radiology Faculty Development Seed Grant. The investigators and Dr. Narvid report no relevant financial relationships.
A version of this article first appeared on Medscape.com .
In a small prospective study, participants who previously had COVID-19, even those with mild illness, had significantly decreased CVR, compared with never-infected individuals.
Results also showed cerebral blood flow (CBF) was greater in never-infected versus previously infected participants, and whole-brain CVR was lower in previously infected versus never-infected participants. Although CVR was also smaller in those with versus those without post-COVID neurologic conditions, the difference was not considered significant.
“It is important to remember that while our findings were statistically significant, we had a relatively small sample size – 25 total participants – and so we encourage future larger studies in this domain to see if these results are reproducible at a larger scale,” lead author Andrew Callen, MD, assistant professor of radiology, Neuroradiology Section, University of Colorado at Denver, Aurora, said in an interview.
“In a practical sense, it may encourage treating clinicians to be more aggressive with preventative neurovascular and cardiovascular health measures and/or screening in this patient population,” Dr. Callen said.
The findings were published online in the American Journal of Roentgenology.
Endothelial dysfunction
The acute phase SARS-CoV-2 infection “is associated with strokes that have features of both vascular inflammation and thromboembolism,” the investigators note.
Moreover, following the acute phase of infection, up to three-quarters of patients “experience persistent neurologic symptoms not attributable to another diagnosis, including headache, difficulty concentrating, vision changes, disequilibrium, and fatigue,” they write.
Preliminary studies “suggest a potential role for endothelial and circulatory dysfunction” in these symptoms, they add.
The researchers note that vessel wall imaging is an MRI technique that can detect and characterize arterial vascular inflammation and may differentiate vasculitic arterial pathology from atherosclerotic pathology.
Dr. Callen conducted previous research assessing cerebral vasoreactivity in women living with HIV. He noted that this is a population at a much higher risk of stroke, compared with uninfected individuals with otherwise similar cardiovascular risk factors, even when their viral load is controlled with antiretroviral therapies.
Evidence has pointed to chronic endothelial dysfunction in these individuals, and endothelial function and dysfunction can be measured through vasoreactivity testing, Dr. Callen said.
“As the COVID pandemic progressed, not only did we observe an increased rate of stroke in individuals acutely infected with COVID, but histopathological evidence began to emerge which suggested that the COVID-19 virus had tropism to and often damaged the vascular endothelium,” he noted.
This emerging evidence prompted Dr. Callen to wonder whether “individuals previously infected with COVID might also demonstrate long-term impairment in cerebral vasoreactivity or if we might see abnormalities using high resolution vessel wall imaging.”
In the current study, 15 individuals with prior SARS-CoV-2 infection (11 women, 4 men; mean age, 43 years) were compared with 10 never-infected individuals (8 women, 2 men; mean age, 43 years) who functioned as the control group.
The previously infected individuals, of whom three had prior critical infection and 12 had prior mild infection, were assessed, on average, about 8 months after infection. Of this group, seven had various post-COVID neurologic conditions, including headache, memory impairment, insomnia, depression, disequilibrium, fatigue, personality change, phantosmias (detecting smells that aren’t present), dysgeusia (taste disorder), and tinnitus.
All participants underwent MRI and vessel wall imaging. The MRI included arterial spin labeling perfusion imaging with acetazolamide stimulus to measure CBF and calculate CVR. The vessel wall imaging examinations used a contrast-enhanced black-blood 3D T1-weighted sequence.
Imaging data
Prior to acetazolamide administration, the mean whole-cortex CBF did not differ significantly between never-infected and previously infected participants. However, following the acetazolamide administration, the mean whole-cortex CBF was greater in never-infected participants (73.8 mL/100 g/min vs. 60.5 mL/100 g/min, respectively; P = .04).
Moreover, the mean whole-brain CVR was greater in never-infected participants, compared with previously infected participants (27.8 mL/100 g/min vs. 19.1 mL/100 g/min; P < .001).
After adjusting for age and sex, researchers found that prior infection was associated with a lower whole-brain CVR (–8.9 mL/100 g/min; 95% confidence interval, 4.6-13.3 ml/100g/min; P < .001).
Previously infected individuals also showed significantly lower CVR, even after the researchers excluded those with prior critical illness.
A nonsignificant difference was found in previously infected participants, with smaller CVR in participants with versus without post-COVID neurologic symptoms (16.9 vs. 21.0 mL/100 g/min; P = .22).
In addition, 40% of the previously infected participants versus 10% of the never-infected participants had at least one vessel wall imaging abnormality – but the difference was not deemed significant (P = .18). Notably, “all detected vessel wall imaging abnormalities were morphologically consistent with atherosclerosis rather than vasculitis,” the investigators said.
Dr. Callen said it is “unknown whether the lack of statistical significance in the differences in vasoreactivity impairment with those living with long COVID symptoms is due to a lack of a biomechanistic correlation or due to statistical underpowering.”
If it is the latter, “it may emphasize the role of vascular health in those living with long COVID symptoms and potentially all individuals living with COVID,” he added.
Independent risk factor?
Commenting on the study for this article, Jared Narvid, MD, associate professor of neuroradiology, University of California, San Francisco, said it “adds to the literature suggesting a correlation between COVID-19 infection and measures of cerebrovascular abnormality.”
Dr. Narvid, who was not involved with the research, added that “although it is a small case-control study, it is well executed and should encourage scientists to further study whether COVID-19 infection represents an independent risk factor for cerebrovascular disease.”
The investigators agree. “Future studies are needed to determine the clinical implications arising from SARS-CoV-2–associated CVR impairment,” they write.
The study was funded by a University of Colorado department of radiology Faculty Development Seed Grant. The investigators and Dr. Narvid report no relevant financial relationships.
A version of this article first appeared on Medscape.com .
Dermatoses often occur in people who wear face masks
according to a recently published systematic review and meta-analysis.
“This report finds the most statistically significant risk factor for developing a facial dermatosis under a face mask is how long one wears the mask. Specifically, wearing a mask for more than 4 to 6 hours correlated most strongly with the development of a facial skin problem,” Jami L. Miller, MD, associate professor of dermatology, Vanderbilt University Medical Center, Nashville, Tenn., told this news organization. Dr. Miller was not involved in the study.
“The type of mask and the environment were of less significance,” she added.
Mask wearing for infection control has been common during the COVID-19 pandemic and will likely continue for some time, study coauthors Lim Yi Shen Justin, MBBS, and Yik Weng Yew*, MBBS, MPH, PhD, Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, write in Contact Dermatitis. And cross-sectional studies have suggested a link between mask wearing and various facial dermatoses.
To evaluate this link, as well as potential risk factors for facial dermatoses, the researchers reviewed 37 studies published between 2004 and 2022 involving 29,557 adult participants self-reporting regular use of any face mask type across 17 countries in Europe and Asia. The mask types commonly studied in the papers they analyzed included surgical masks and respirators.
Facial dermatoses were self-reported in 30 studies (81.1%) and were diagnosed by trained dermatologists in seven studies (18.9%).
Dr. Justin and Dr. Yew found that:
- The overall prevalence of facial dermatoses was 55%
- Individually, facial dermatitis, itch, acne, and pressure injuries were consistently reported as facial dermatoses, with pooled prevalence rates of 24%, 30%, 31%, and 31%, respectively
- The duration of mask wearing was the most significant risk factor for facial dermatoses (P < .001)
- Respirators, including N95 masks, were not more likely than surgical masks to be linked with facial dermatoses
“Understanding risk factors of mask wearing, including situation, duration, and type of mask, may allow for targeted interventions to mitigate problems,” Dr. Yew told this news organization.
He advised taking a break from mask wearing after 4 to 6 hours to improve outcomes.
Dr. Yew acknowledged limitations, including that most of the reviewed studies relied on self-reported symptoms.
“Patient factors were not investigated in most studies; therefore, we were not able to ascertain their contributory role in the development of facial dermatoses from mask wearing,” he said. “We were also unable to prove causation between risk factors and outcome.”
Four dermatologists welcome the findings
Dr. Miller called this an “interesting, and certainly relevant” study, now that mask wearing is common and facial skin problems are fairly common complaints in medical visits.
“As the authors say, irritants or contact allergens with longer exposures can be expected to cause a more severe dermatitis than short contact,” she said. “Longer duration also can cause occlusion of pores and hair follicles, which can be expected to worsen acne and folliculitis.”
“I was surprised that the type of mask did not seem to matter significantly,” she added. “Patients wearing N95 masks may be relieved to know N95s do not cause more skin problems than lighter masks.”
Still, Dr. Miller had several questions, including if the materials and chemical finishes that vary by manufacturer may affect skin conditions.
Olga Bunimovich, MD, assistant professor, department of dermatology, University of Pittsburgh School of Medicine, Pennsylvania, called this study “an excellent step towards characterizing the role masks play in facial dermatoses.”
“The study provides a window into the prevalence of these conditions, as well as some understanding of the factors that may be contributing to it,” Dr. Bunimovich, who was not part of the study, added. But “we can also utilize this information to alter behavior in the work environment, allowing ‘mask-free’ breaks to decrease the risk of facial dermatoses.”
Elma Baron, MD, professor and director, Skin Study Center, department of dermatology, Case Western Reserve University School of Medicine, Cleveland, expected skin problems to be linked with mask wearing but didn’t expect the prevalence to be as high as 55%, which she called “very significant.”
“Mask wearing is an important means to prevent transmission of communicable infections, and the practice will most likely continue,” she said.
“Given the data, it is reasonable to advise patients who are already prone to these specific dermatoses to be proactive,” she added. “Early intervention with proper topical medications, preferably prescribed by a dermatologist or other health care provider, and changing masks frequently before they get soaked with moisture, will hopefully lessen the severity of skin rashes and minimize the negative impact on quality of life.”
Also commenting on the study, Susan Massick, MD, dermatologist and clinical associate professor of internal medicine, The Ohio State University Wexner Medical Center, Westerville, said in an interview that she urges people to wear masks, despite these risks.
“The majority of concerns are straightforward, manageable, and overall benign,” she said. “We have a multitude of treatments that can help control, address, or improve symptoms.”
“Masks are an effective and easy way to protect yourself from infection, and they remain one of the most reliable preventions we have,” Dr. Massick noted. “The findings in this article should not preclude anyone from wearing a mask, nor should facial dermatoses be a cause for people to stop wearing their masks.”
The study received no funding. The authors, as well as Dr. Baron, Dr. Miller, Dr. Bunimovich, and Dr. Massick, who were not involved in the study, reported no relevant financial relationships. All experts commented by email.
A version of this article first appeared on Medscape.com.
Correction, 9/22/22: An earlier version of this article misstated the name of Dr. Yik Weng Yew.
according to a recently published systematic review and meta-analysis.
“This report finds the most statistically significant risk factor for developing a facial dermatosis under a face mask is how long one wears the mask. Specifically, wearing a mask for more than 4 to 6 hours correlated most strongly with the development of a facial skin problem,” Jami L. Miller, MD, associate professor of dermatology, Vanderbilt University Medical Center, Nashville, Tenn., told this news organization. Dr. Miller was not involved in the study.
“The type of mask and the environment were of less significance,” she added.
Mask wearing for infection control has been common during the COVID-19 pandemic and will likely continue for some time, study coauthors Lim Yi Shen Justin, MBBS, and Yik Weng Yew*, MBBS, MPH, PhD, Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, write in Contact Dermatitis. And cross-sectional studies have suggested a link between mask wearing and various facial dermatoses.
To evaluate this link, as well as potential risk factors for facial dermatoses, the researchers reviewed 37 studies published between 2004 and 2022 involving 29,557 adult participants self-reporting regular use of any face mask type across 17 countries in Europe and Asia. The mask types commonly studied in the papers they analyzed included surgical masks and respirators.
Facial dermatoses were self-reported in 30 studies (81.1%) and were diagnosed by trained dermatologists in seven studies (18.9%).
Dr. Justin and Dr. Yew found that:
- The overall prevalence of facial dermatoses was 55%
- Individually, facial dermatitis, itch, acne, and pressure injuries were consistently reported as facial dermatoses, with pooled prevalence rates of 24%, 30%, 31%, and 31%, respectively
- The duration of mask wearing was the most significant risk factor for facial dermatoses (P < .001)
- Respirators, including N95 masks, were not more likely than surgical masks to be linked with facial dermatoses
“Understanding risk factors of mask wearing, including situation, duration, and type of mask, may allow for targeted interventions to mitigate problems,” Dr. Yew told this news organization.
He advised taking a break from mask wearing after 4 to 6 hours to improve outcomes.
Dr. Yew acknowledged limitations, including that most of the reviewed studies relied on self-reported symptoms.
“Patient factors were not investigated in most studies; therefore, we were not able to ascertain their contributory role in the development of facial dermatoses from mask wearing,” he said. “We were also unable to prove causation between risk factors and outcome.”
Four dermatologists welcome the findings
Dr. Miller called this an “interesting, and certainly relevant” study, now that mask wearing is common and facial skin problems are fairly common complaints in medical visits.
“As the authors say, irritants or contact allergens with longer exposures can be expected to cause a more severe dermatitis than short contact,” she said. “Longer duration also can cause occlusion of pores and hair follicles, which can be expected to worsen acne and folliculitis.”
“I was surprised that the type of mask did not seem to matter significantly,” she added. “Patients wearing N95 masks may be relieved to know N95s do not cause more skin problems than lighter masks.”
Still, Dr. Miller had several questions, including if the materials and chemical finishes that vary by manufacturer may affect skin conditions.
Olga Bunimovich, MD, assistant professor, department of dermatology, University of Pittsburgh School of Medicine, Pennsylvania, called this study “an excellent step towards characterizing the role masks play in facial dermatoses.”
“The study provides a window into the prevalence of these conditions, as well as some understanding of the factors that may be contributing to it,” Dr. Bunimovich, who was not part of the study, added. But “we can also utilize this information to alter behavior in the work environment, allowing ‘mask-free’ breaks to decrease the risk of facial dermatoses.”
Elma Baron, MD, professor and director, Skin Study Center, department of dermatology, Case Western Reserve University School of Medicine, Cleveland, expected skin problems to be linked with mask wearing but didn’t expect the prevalence to be as high as 55%, which she called “very significant.”
“Mask wearing is an important means to prevent transmission of communicable infections, and the practice will most likely continue,” she said.
“Given the data, it is reasonable to advise patients who are already prone to these specific dermatoses to be proactive,” she added. “Early intervention with proper topical medications, preferably prescribed by a dermatologist or other health care provider, and changing masks frequently before they get soaked with moisture, will hopefully lessen the severity of skin rashes and minimize the negative impact on quality of life.”
Also commenting on the study, Susan Massick, MD, dermatologist and clinical associate professor of internal medicine, The Ohio State University Wexner Medical Center, Westerville, said in an interview that she urges people to wear masks, despite these risks.
“The majority of concerns are straightforward, manageable, and overall benign,” she said. “We have a multitude of treatments that can help control, address, or improve symptoms.”
“Masks are an effective and easy way to protect yourself from infection, and they remain one of the most reliable preventions we have,” Dr. Massick noted. “The findings in this article should not preclude anyone from wearing a mask, nor should facial dermatoses be a cause for people to stop wearing their masks.”
The study received no funding. The authors, as well as Dr. Baron, Dr. Miller, Dr. Bunimovich, and Dr. Massick, who were not involved in the study, reported no relevant financial relationships. All experts commented by email.
A version of this article first appeared on Medscape.com.
Correction, 9/22/22: An earlier version of this article misstated the name of Dr. Yik Weng Yew.
according to a recently published systematic review and meta-analysis.
“This report finds the most statistically significant risk factor for developing a facial dermatosis under a face mask is how long one wears the mask. Specifically, wearing a mask for more than 4 to 6 hours correlated most strongly with the development of a facial skin problem,” Jami L. Miller, MD, associate professor of dermatology, Vanderbilt University Medical Center, Nashville, Tenn., told this news organization. Dr. Miller was not involved in the study.
“The type of mask and the environment were of less significance,” she added.
Mask wearing for infection control has been common during the COVID-19 pandemic and will likely continue for some time, study coauthors Lim Yi Shen Justin, MBBS, and Yik Weng Yew*, MBBS, MPH, PhD, Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, write in Contact Dermatitis. And cross-sectional studies have suggested a link between mask wearing and various facial dermatoses.
To evaluate this link, as well as potential risk factors for facial dermatoses, the researchers reviewed 37 studies published between 2004 and 2022 involving 29,557 adult participants self-reporting regular use of any face mask type across 17 countries in Europe and Asia. The mask types commonly studied in the papers they analyzed included surgical masks and respirators.
Facial dermatoses were self-reported in 30 studies (81.1%) and were diagnosed by trained dermatologists in seven studies (18.9%).
Dr. Justin and Dr. Yew found that:
- The overall prevalence of facial dermatoses was 55%
- Individually, facial dermatitis, itch, acne, and pressure injuries were consistently reported as facial dermatoses, with pooled prevalence rates of 24%, 30%, 31%, and 31%, respectively
- The duration of mask wearing was the most significant risk factor for facial dermatoses (P < .001)
- Respirators, including N95 masks, were not more likely than surgical masks to be linked with facial dermatoses
“Understanding risk factors of mask wearing, including situation, duration, and type of mask, may allow for targeted interventions to mitigate problems,” Dr. Yew told this news organization.
He advised taking a break from mask wearing after 4 to 6 hours to improve outcomes.
Dr. Yew acknowledged limitations, including that most of the reviewed studies relied on self-reported symptoms.
“Patient factors were not investigated in most studies; therefore, we were not able to ascertain their contributory role in the development of facial dermatoses from mask wearing,” he said. “We were also unable to prove causation between risk factors and outcome.”
Four dermatologists welcome the findings
Dr. Miller called this an “interesting, and certainly relevant” study, now that mask wearing is common and facial skin problems are fairly common complaints in medical visits.
“As the authors say, irritants or contact allergens with longer exposures can be expected to cause a more severe dermatitis than short contact,” she said. “Longer duration also can cause occlusion of pores and hair follicles, which can be expected to worsen acne and folliculitis.”
“I was surprised that the type of mask did not seem to matter significantly,” she added. “Patients wearing N95 masks may be relieved to know N95s do not cause more skin problems than lighter masks.”
Still, Dr. Miller had several questions, including if the materials and chemical finishes that vary by manufacturer may affect skin conditions.
Olga Bunimovich, MD, assistant professor, department of dermatology, University of Pittsburgh School of Medicine, Pennsylvania, called this study “an excellent step towards characterizing the role masks play in facial dermatoses.”
“The study provides a window into the prevalence of these conditions, as well as some understanding of the factors that may be contributing to it,” Dr. Bunimovich, who was not part of the study, added. But “we can also utilize this information to alter behavior in the work environment, allowing ‘mask-free’ breaks to decrease the risk of facial dermatoses.”
Elma Baron, MD, professor and director, Skin Study Center, department of dermatology, Case Western Reserve University School of Medicine, Cleveland, expected skin problems to be linked with mask wearing but didn’t expect the prevalence to be as high as 55%, which she called “very significant.”
“Mask wearing is an important means to prevent transmission of communicable infections, and the practice will most likely continue,” she said.
“Given the data, it is reasonable to advise patients who are already prone to these specific dermatoses to be proactive,” she added. “Early intervention with proper topical medications, preferably prescribed by a dermatologist or other health care provider, and changing masks frequently before they get soaked with moisture, will hopefully lessen the severity of skin rashes and minimize the negative impact on quality of life.”
Also commenting on the study, Susan Massick, MD, dermatologist and clinical associate professor of internal medicine, The Ohio State University Wexner Medical Center, Westerville, said in an interview that she urges people to wear masks, despite these risks.
“The majority of concerns are straightforward, manageable, and overall benign,” she said. “We have a multitude of treatments that can help control, address, or improve symptoms.”
“Masks are an effective and easy way to protect yourself from infection, and they remain one of the most reliable preventions we have,” Dr. Massick noted. “The findings in this article should not preclude anyone from wearing a mask, nor should facial dermatoses be a cause for people to stop wearing their masks.”
The study received no funding. The authors, as well as Dr. Baron, Dr. Miller, Dr. Bunimovich, and Dr. Massick, who were not involved in the study, reported no relevant financial relationships. All experts commented by email.
A version of this article first appeared on Medscape.com.
Correction, 9/22/22: An earlier version of this article misstated the name of Dr. Yik Weng Yew.
Risk factors linked to post–COVID vaccination death identified
The researchers have identified factors that put a person at greater risk of COVID-related death after they have completed both doses of the primary COVID vaccination schedule and a booster dose.
For their research, published in JAMA Network Open, researchers from the Office for National Statistics (ONS); Public Health Scotland; the University of Strathclyde, Glasgow; and the University of Edinburgh used data from the ONS Public linked data set combining the 2011 Census of England and covering 80% of the population of England. The study population included 19,473,570 individuals aged 18-100 years (mean age 60.8 years, 45.2% men, 92.0% White individuals) living in England who had completed both doses of their primary vaccination schedule and had received their mRNA booster 14 days or more prior to Dec. 31, 2021. The outcome of interest was time to death involving COVID-19 occurring between Jan. 1 and March 16, 2022.
Prioritization of booster doses and COVID-19 treatments
The authors highlighted how it had become “critical” to identify risk factors associated with COVID-19 death in those who had been vaccinated and pointed out that existing evidence was “based on people who have received one or two doses of a COVID-19 vaccine and were infected by the Alpha or Delta variant”. They emphasized that establishing which groups are at increased risk of COVID-19 death after receiving a booster is crucial for the “prioritization of further booster doses and access to COVID-19 therapeutics.”
During the study period the authors found that there were 4,781 (0.02%) deaths involving COVID-19 and 58,020 (0.3%) deaths from other causes. Of those who died of coronavirus, the mean age was 83.3 years, and the authors highlighted how “age was the most important characteristic” associated with the risk of postbooster COVID-19 death. They added that, compared with a 50-year-old, the HR for an 80-year-old individual was 31.3 (95% confidence interval, 26.1-37.6).
They found that women were at lower risk than men with an HR of 0.52 (95% CI, 0.49-0.55). An increased risk of COVID-19 death was also associated with living in a care home or in a socioeconomically deprived area.
Of note, they said that “there was no association between the risk of COVID-19 death and ethnicity, except for those of Indian background”, who they explained were at slightly elevated risk, compared with White individuals. However, they explained how the association with ethnicity was “unclear and differed from previous studies”, with their findings likely to be due “largely to the pronounced differences in vaccination uptake” between ethnic groups in previous studies.
Dementia concern
With regard to existing health conditions the authors commented that “most of the QCovid risk groups were associated with an increased HR of postbooster breakthrough death, except for of congenital heart disease, asthma, and prior fracture.”
Risk was particularly elevated, they said, for people with severe combined immunodeficiency (HR, 6.2; 95% CI, 3.3-11.5), and they also identified several conditions associated with HRs of greater than 3, including dementia.
In July, Alzheimer’s Research UK urged the Government to boost the development and deployment of new dementia treatments having found that a significant proportion of people who died of COVID-19 in 2020 and 2021 were living with the condition. At the time, data published by the ONS of deaths caused by coronavirus in England and Wales in 2021 showed dementia to be the second-most common pre-existing condition.
David Thomas, head of policy at Alzheimer’s Research UK, said: “We’ve known for some time that people with dementia have been hit disproportionately hard during the pandemic, but this new data serves as a stark reminder of the growing challenge we face in tackling the condition, and the urgent need to address it.”
The authors of the new research acknowledged the study’s limitations, notably that only data for the population living in England who were enumerated in the 2011 Census of England and Wales was included.
However, subpopulations “remain at increased risk of COVID-19 fatality” after receiving a booster vaccine during the Omicron wave, they pointed out.
“The subpopulations with the highest risk should be considered a priority for COVID-19 therapeutics and further booster doses,” they urged.
A version of this article first appeared on Medscape UK.
The researchers have identified factors that put a person at greater risk of COVID-related death after they have completed both doses of the primary COVID vaccination schedule and a booster dose.
For their research, published in JAMA Network Open, researchers from the Office for National Statistics (ONS); Public Health Scotland; the University of Strathclyde, Glasgow; and the University of Edinburgh used data from the ONS Public linked data set combining the 2011 Census of England and covering 80% of the population of England. The study population included 19,473,570 individuals aged 18-100 years (mean age 60.8 years, 45.2% men, 92.0% White individuals) living in England who had completed both doses of their primary vaccination schedule and had received their mRNA booster 14 days or more prior to Dec. 31, 2021. The outcome of interest was time to death involving COVID-19 occurring between Jan. 1 and March 16, 2022.
Prioritization of booster doses and COVID-19 treatments
The authors highlighted how it had become “critical” to identify risk factors associated with COVID-19 death in those who had been vaccinated and pointed out that existing evidence was “based on people who have received one or two doses of a COVID-19 vaccine and were infected by the Alpha or Delta variant”. They emphasized that establishing which groups are at increased risk of COVID-19 death after receiving a booster is crucial for the “prioritization of further booster doses and access to COVID-19 therapeutics.”
During the study period the authors found that there were 4,781 (0.02%) deaths involving COVID-19 and 58,020 (0.3%) deaths from other causes. Of those who died of coronavirus, the mean age was 83.3 years, and the authors highlighted how “age was the most important characteristic” associated with the risk of postbooster COVID-19 death. They added that, compared with a 50-year-old, the HR for an 80-year-old individual was 31.3 (95% confidence interval, 26.1-37.6).
They found that women were at lower risk than men with an HR of 0.52 (95% CI, 0.49-0.55). An increased risk of COVID-19 death was also associated with living in a care home or in a socioeconomically deprived area.
Of note, they said that “there was no association between the risk of COVID-19 death and ethnicity, except for those of Indian background”, who they explained were at slightly elevated risk, compared with White individuals. However, they explained how the association with ethnicity was “unclear and differed from previous studies”, with their findings likely to be due “largely to the pronounced differences in vaccination uptake” between ethnic groups in previous studies.
Dementia concern
With regard to existing health conditions the authors commented that “most of the QCovid risk groups were associated with an increased HR of postbooster breakthrough death, except for of congenital heart disease, asthma, and prior fracture.”
Risk was particularly elevated, they said, for people with severe combined immunodeficiency (HR, 6.2; 95% CI, 3.3-11.5), and they also identified several conditions associated with HRs of greater than 3, including dementia.
In July, Alzheimer’s Research UK urged the Government to boost the development and deployment of new dementia treatments having found that a significant proportion of people who died of COVID-19 in 2020 and 2021 were living with the condition. At the time, data published by the ONS of deaths caused by coronavirus in England and Wales in 2021 showed dementia to be the second-most common pre-existing condition.
David Thomas, head of policy at Alzheimer’s Research UK, said: “We’ve known for some time that people with dementia have been hit disproportionately hard during the pandemic, but this new data serves as a stark reminder of the growing challenge we face in tackling the condition, and the urgent need to address it.”
The authors of the new research acknowledged the study’s limitations, notably that only data for the population living in England who were enumerated in the 2011 Census of England and Wales was included.
However, subpopulations “remain at increased risk of COVID-19 fatality” after receiving a booster vaccine during the Omicron wave, they pointed out.
“The subpopulations with the highest risk should be considered a priority for COVID-19 therapeutics and further booster doses,” they urged.
A version of this article first appeared on Medscape UK.
The researchers have identified factors that put a person at greater risk of COVID-related death after they have completed both doses of the primary COVID vaccination schedule and a booster dose.
For their research, published in JAMA Network Open, researchers from the Office for National Statistics (ONS); Public Health Scotland; the University of Strathclyde, Glasgow; and the University of Edinburgh used data from the ONS Public linked data set combining the 2011 Census of England and covering 80% of the population of England. The study population included 19,473,570 individuals aged 18-100 years (mean age 60.8 years, 45.2% men, 92.0% White individuals) living in England who had completed both doses of their primary vaccination schedule and had received their mRNA booster 14 days or more prior to Dec. 31, 2021. The outcome of interest was time to death involving COVID-19 occurring between Jan. 1 and March 16, 2022.
Prioritization of booster doses and COVID-19 treatments
The authors highlighted how it had become “critical” to identify risk factors associated with COVID-19 death in those who had been vaccinated and pointed out that existing evidence was “based on people who have received one or two doses of a COVID-19 vaccine and were infected by the Alpha or Delta variant”. They emphasized that establishing which groups are at increased risk of COVID-19 death after receiving a booster is crucial for the “prioritization of further booster doses and access to COVID-19 therapeutics.”
During the study period the authors found that there were 4,781 (0.02%) deaths involving COVID-19 and 58,020 (0.3%) deaths from other causes. Of those who died of coronavirus, the mean age was 83.3 years, and the authors highlighted how “age was the most important characteristic” associated with the risk of postbooster COVID-19 death. They added that, compared with a 50-year-old, the HR for an 80-year-old individual was 31.3 (95% confidence interval, 26.1-37.6).
They found that women were at lower risk than men with an HR of 0.52 (95% CI, 0.49-0.55). An increased risk of COVID-19 death was also associated with living in a care home or in a socioeconomically deprived area.
Of note, they said that “there was no association between the risk of COVID-19 death and ethnicity, except for those of Indian background”, who they explained were at slightly elevated risk, compared with White individuals. However, they explained how the association with ethnicity was “unclear and differed from previous studies”, with their findings likely to be due “largely to the pronounced differences in vaccination uptake” between ethnic groups in previous studies.
Dementia concern
With regard to existing health conditions the authors commented that “most of the QCovid risk groups were associated with an increased HR of postbooster breakthrough death, except for of congenital heart disease, asthma, and prior fracture.”
Risk was particularly elevated, they said, for people with severe combined immunodeficiency (HR, 6.2; 95% CI, 3.3-11.5), and they also identified several conditions associated with HRs of greater than 3, including dementia.
In July, Alzheimer’s Research UK urged the Government to boost the development and deployment of new dementia treatments having found that a significant proportion of people who died of COVID-19 in 2020 and 2021 were living with the condition. At the time, data published by the ONS of deaths caused by coronavirus in England and Wales in 2021 showed dementia to be the second-most common pre-existing condition.
David Thomas, head of policy at Alzheimer’s Research UK, said: “We’ve known for some time that people with dementia have been hit disproportionately hard during the pandemic, but this new data serves as a stark reminder of the growing challenge we face in tackling the condition, and the urgent need to address it.”
The authors of the new research acknowledged the study’s limitations, notably that only data for the population living in England who were enumerated in the 2011 Census of England and Wales was included.
However, subpopulations “remain at increased risk of COVID-19 fatality” after receiving a booster vaccine during the Omicron wave, they pointed out.
“The subpopulations with the highest risk should be considered a priority for COVID-19 therapeutics and further booster doses,” they urged.
A version of this article first appeared on Medscape UK.
FROM JAMA NETWORK OPEN
The potential problem(s) with a once-a-year COVID vaccine
Comments from the White House this week suggesting a once-a-year COVID-19 shot for most Americans, “just like your annual flu shot,” were met with backlash from many who say COVID and influenza come from different viruses and need different schedules.
Remarks, from “capitulation” to too few data, hit the airwaves and social media.
Some, however, agree with the White House vision and say that asking people to get one shot in the fall instead of periodic pushes for boosters will raise public confidence and buy-in and reduce consumer confusion.
Health leaders, including Bob Wachter, MD, chair of the department of medicine at the University of California, San Francisco, say they like the framing of the concept – that people who are not high-risk should plan each year for a COVID shot and a flu shot.
& we need strategy to bump uptake,” Dr. Wachter tweeted this week.
But the numbers of Americans seeking boosters remain low. Only one-third of all eligible people 50 years and older have gotten a second COVID booster, according to the Centers for Disease Control and Prevention. About half of those who got the original two shots got a first booster.
Meanwhile, the United States is still averaging about 70,000 new COVID cases and more than 300 deaths every day.
The suggested change in approach comes as Pfizer/BioNTech and Moderna roll out their new boosters that target Omicron subvariants BA.4 and BA.5 after the CDC recommended their use and the U.S. Food and Drug Administration approved emergency use authorization.
“As the virus continues to change, we will now be able to update our vaccines annually to target the dominant variant,” President Joe Biden said in a statement promoting the yearly approach.
Some say annual shot premature
Other experts say it’s too soon to tell whether an annual approach will work.
“We have no data to support that current vaccines, including the new BA.5 booster, will provide durable protection beyond 4-6 months. It would be good to aspire to this objective, and much longer duration or protection, but that will likely require next generation and nasal vaccines,” said Eric Topol, MD, Medscape’s editor-in-chief and founder and director of the Scripps Research Translational Institute.
A report in Nature Reviews Immunology states, “Mucosal vaccines offer the potential to trigger robust protective immune responses at the predominant sites of pathogen infection” and potentially “can prevent an infection from becoming established in the first place, rather than only curtailing infection and protecting against the development of disease symptoms.”
Dr. Topol tweeted after the White House statements, “[An annual vaccine] has the ring of Covid capitulation.”
William Schaffner, MD, an infectious disease expert at Vanderbilt University, Nashville, Tenn., told this news organization that he cautions against interpreting the White House comments as official policy.
“This is the difficulty of having public health announcements come out of Washington,” he said. “They ought to come out of the CDC.”
He says there is a reasonable analogy between COVID and influenza, but warns, “don’t push the analogy.”
They are both serious respiratory viruses that can cause much illness and death in essentially the same populations, he notes. These are the older, frail people, people who have underlying illnesses or are immunocompromised.
Both viruses also mutate. But there the paths diverge.
“We’ve gotten into a pattern of annually updating the influenza vaccine because it is such a singularly seasonal virus,” Dr. Schaffner said. “Basically it disappears during the summer. We’ve had plenty of COVID during the summers.”
For COVID, he said, “We will need a periodic booster. Could this be annually? That would certainly make it easier.” But it’s too soon to tell, he said.
Dr. Schaffner noted that several manufacturers are working on a combined flu/COVID vaccine.
Just a ‘first step’ toward annual shot
The currently updated COVID vaccine may be the first step toward an annual vaccine, but it’s only the first step, Dr. Schaffner said. “We haven’t committed to further steps yet because we’re watching this virus.”
Syra Madad, DHSc, MSc, an infectious disease epidemiologist at Harvard University’s Belfer Center for Science and International Affairs, Cambridge, Mass., and the New York City hospital system, told this news organization that arguments on both sides make sense.
Having a single message once a year can help eliminate the considerable confusion involving people on individual timelines with different levels of immunity and separate campaigns for COVID and flu shots coming at different times of the year.
“Communication around vaccines is very muddled and that shows in our overall vaccination rates, particularly booster rates,” she says. “The overall strategy is hopeful and makes sense if we’re going to progress that way based on data.”
However, she said that the data are just not there yet to show it’s time for an annual vaccine. First, scientists will need to see how long protection lasts with the Omicron-specific vaccine and how well and how long it protects against severe disease and death as well as infection.
COVID is less predictable than influenza and the influenza vaccine has been around for decades, Dr. Madad noted. With influenza, the patterns are more easily anticipated with their “ladder-like pattern,” she said. “COVID-19 is not like that.”
What is hopeful, she said, “is that we’ve been in the Omicron dynasty since November of 2021. I’m hopeful that we’ll stick with that particular variant.”
Dr. Topol, Dr. Schaffner, and Dr. Madad declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Comments from the White House this week suggesting a once-a-year COVID-19 shot for most Americans, “just like your annual flu shot,” were met with backlash from many who say COVID and influenza come from different viruses and need different schedules.
Remarks, from “capitulation” to too few data, hit the airwaves and social media.
Some, however, agree with the White House vision and say that asking people to get one shot in the fall instead of periodic pushes for boosters will raise public confidence and buy-in and reduce consumer confusion.
Health leaders, including Bob Wachter, MD, chair of the department of medicine at the University of California, San Francisco, say they like the framing of the concept – that people who are not high-risk should plan each year for a COVID shot and a flu shot.
& we need strategy to bump uptake,” Dr. Wachter tweeted this week.
But the numbers of Americans seeking boosters remain low. Only one-third of all eligible people 50 years and older have gotten a second COVID booster, according to the Centers for Disease Control and Prevention. About half of those who got the original two shots got a first booster.
Meanwhile, the United States is still averaging about 70,000 new COVID cases and more than 300 deaths every day.
The suggested change in approach comes as Pfizer/BioNTech and Moderna roll out their new boosters that target Omicron subvariants BA.4 and BA.5 after the CDC recommended their use and the U.S. Food and Drug Administration approved emergency use authorization.
“As the virus continues to change, we will now be able to update our vaccines annually to target the dominant variant,” President Joe Biden said in a statement promoting the yearly approach.
Some say annual shot premature
Other experts say it’s too soon to tell whether an annual approach will work.
“We have no data to support that current vaccines, including the new BA.5 booster, will provide durable protection beyond 4-6 months. It would be good to aspire to this objective, and much longer duration or protection, but that will likely require next generation and nasal vaccines,” said Eric Topol, MD, Medscape’s editor-in-chief and founder and director of the Scripps Research Translational Institute.
A report in Nature Reviews Immunology states, “Mucosal vaccines offer the potential to trigger robust protective immune responses at the predominant sites of pathogen infection” and potentially “can prevent an infection from becoming established in the first place, rather than only curtailing infection and protecting against the development of disease symptoms.”
Dr. Topol tweeted after the White House statements, “[An annual vaccine] has the ring of Covid capitulation.”
William Schaffner, MD, an infectious disease expert at Vanderbilt University, Nashville, Tenn., told this news organization that he cautions against interpreting the White House comments as official policy.
“This is the difficulty of having public health announcements come out of Washington,” he said. “They ought to come out of the CDC.”
He says there is a reasonable analogy between COVID and influenza, but warns, “don’t push the analogy.”
They are both serious respiratory viruses that can cause much illness and death in essentially the same populations, he notes. These are the older, frail people, people who have underlying illnesses or are immunocompromised.
Both viruses also mutate. But there the paths diverge.
“We’ve gotten into a pattern of annually updating the influenza vaccine because it is such a singularly seasonal virus,” Dr. Schaffner said. “Basically it disappears during the summer. We’ve had plenty of COVID during the summers.”
For COVID, he said, “We will need a periodic booster. Could this be annually? That would certainly make it easier.” But it’s too soon to tell, he said.
Dr. Schaffner noted that several manufacturers are working on a combined flu/COVID vaccine.
Just a ‘first step’ toward annual shot
The currently updated COVID vaccine may be the first step toward an annual vaccine, but it’s only the first step, Dr. Schaffner said. “We haven’t committed to further steps yet because we’re watching this virus.”
Syra Madad, DHSc, MSc, an infectious disease epidemiologist at Harvard University’s Belfer Center for Science and International Affairs, Cambridge, Mass., and the New York City hospital system, told this news organization that arguments on both sides make sense.
Having a single message once a year can help eliminate the considerable confusion involving people on individual timelines with different levels of immunity and separate campaigns for COVID and flu shots coming at different times of the year.
“Communication around vaccines is very muddled and that shows in our overall vaccination rates, particularly booster rates,” she says. “The overall strategy is hopeful and makes sense if we’re going to progress that way based on data.”
However, she said that the data are just not there yet to show it’s time for an annual vaccine. First, scientists will need to see how long protection lasts with the Omicron-specific vaccine and how well and how long it protects against severe disease and death as well as infection.
COVID is less predictable than influenza and the influenza vaccine has been around for decades, Dr. Madad noted. With influenza, the patterns are more easily anticipated with their “ladder-like pattern,” she said. “COVID-19 is not like that.”
What is hopeful, she said, “is that we’ve been in the Omicron dynasty since November of 2021. I’m hopeful that we’ll stick with that particular variant.”
Dr. Topol, Dr. Schaffner, and Dr. Madad declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Comments from the White House this week suggesting a once-a-year COVID-19 shot for most Americans, “just like your annual flu shot,” were met with backlash from many who say COVID and influenza come from different viruses and need different schedules.
Remarks, from “capitulation” to too few data, hit the airwaves and social media.
Some, however, agree with the White House vision and say that asking people to get one shot in the fall instead of periodic pushes for boosters will raise public confidence and buy-in and reduce consumer confusion.
Health leaders, including Bob Wachter, MD, chair of the department of medicine at the University of California, San Francisco, say they like the framing of the concept – that people who are not high-risk should plan each year for a COVID shot and a flu shot.
& we need strategy to bump uptake,” Dr. Wachter tweeted this week.
But the numbers of Americans seeking boosters remain low. Only one-third of all eligible people 50 years and older have gotten a second COVID booster, according to the Centers for Disease Control and Prevention. About half of those who got the original two shots got a first booster.
Meanwhile, the United States is still averaging about 70,000 new COVID cases and more than 300 deaths every day.
The suggested change in approach comes as Pfizer/BioNTech and Moderna roll out their new boosters that target Omicron subvariants BA.4 and BA.5 after the CDC recommended their use and the U.S. Food and Drug Administration approved emergency use authorization.
“As the virus continues to change, we will now be able to update our vaccines annually to target the dominant variant,” President Joe Biden said in a statement promoting the yearly approach.
Some say annual shot premature
Other experts say it’s too soon to tell whether an annual approach will work.
“We have no data to support that current vaccines, including the new BA.5 booster, will provide durable protection beyond 4-6 months. It would be good to aspire to this objective, and much longer duration or protection, but that will likely require next generation and nasal vaccines,” said Eric Topol, MD, Medscape’s editor-in-chief and founder and director of the Scripps Research Translational Institute.
A report in Nature Reviews Immunology states, “Mucosal vaccines offer the potential to trigger robust protective immune responses at the predominant sites of pathogen infection” and potentially “can prevent an infection from becoming established in the first place, rather than only curtailing infection and protecting against the development of disease symptoms.”
Dr. Topol tweeted after the White House statements, “[An annual vaccine] has the ring of Covid capitulation.”
William Schaffner, MD, an infectious disease expert at Vanderbilt University, Nashville, Tenn., told this news organization that he cautions against interpreting the White House comments as official policy.
“This is the difficulty of having public health announcements come out of Washington,” he said. “They ought to come out of the CDC.”
He says there is a reasonable analogy between COVID and influenza, but warns, “don’t push the analogy.”
They are both serious respiratory viruses that can cause much illness and death in essentially the same populations, he notes. These are the older, frail people, people who have underlying illnesses or are immunocompromised.
Both viruses also mutate. But there the paths diverge.
“We’ve gotten into a pattern of annually updating the influenza vaccine because it is such a singularly seasonal virus,” Dr. Schaffner said. “Basically it disappears during the summer. We’ve had plenty of COVID during the summers.”
For COVID, he said, “We will need a periodic booster. Could this be annually? That would certainly make it easier.” But it’s too soon to tell, he said.
Dr. Schaffner noted that several manufacturers are working on a combined flu/COVID vaccine.
Just a ‘first step’ toward annual shot
The currently updated COVID vaccine may be the first step toward an annual vaccine, but it’s only the first step, Dr. Schaffner said. “We haven’t committed to further steps yet because we’re watching this virus.”
Syra Madad, DHSc, MSc, an infectious disease epidemiologist at Harvard University’s Belfer Center for Science and International Affairs, Cambridge, Mass., and the New York City hospital system, told this news organization that arguments on both sides make sense.
Having a single message once a year can help eliminate the considerable confusion involving people on individual timelines with different levels of immunity and separate campaigns for COVID and flu shots coming at different times of the year.
“Communication around vaccines is very muddled and that shows in our overall vaccination rates, particularly booster rates,” she says. “The overall strategy is hopeful and makes sense if we’re going to progress that way based on data.”
However, she said that the data are just not there yet to show it’s time for an annual vaccine. First, scientists will need to see how long protection lasts with the Omicron-specific vaccine and how well and how long it protects against severe disease and death as well as infection.
COVID is less predictable than influenza and the influenza vaccine has been around for decades, Dr. Madad noted. With influenza, the patterns are more easily anticipated with their “ladder-like pattern,” she said. “COVID-19 is not like that.”
What is hopeful, she said, “is that we’ve been in the Omicron dynasty since November of 2021. I’m hopeful that we’ll stick with that particular variant.”
Dr. Topol, Dr. Schaffner, and Dr. Madad declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Full-dose antithrombotic aids selected COVID-19 ICU patients
BARCELONA – Hospitalized patients in the ICU because of an acute COVID-19 infection had significantly fewer thrombotic events and complications when treated with full-dose anticoagulation, compared with patients who received standard-dose anticoagulation prophylaxis, but full-dose anticoagulation also triggered an excess of moderate and severe bleeding events, randomized trial results show.
The new findings from the COVID-PACT trial in an exclusively U.S.-based cohort of 382 on-treatment patients in the ICU with COVID-19 infection may lead to a change in existing guidelines, which currently recommend standard-dose prophylaxis based on results from prior head-to-head comparisons, such as guidelines posted March 2022 from the American Society of Hematology.
” after weighing an individual patient’s risk for both thrombotic events and bleeding, David D. Berg, MD, said at the annual congress of the European Society of Cardiology. Simultaneous with his report at the congress, the results also appeared online in the journal Circulation.
“What the results tell us is that full-dose anticoagulation in critically ill patients with COVID-19 is highly effective for reducing thrombotic complications,” said Dr. Berg, a cardiologist and critical care physician at Brigham and Women’s Hospital, Boston.
The report’s designated discussant agreed with Dr. Berg’s conclusions.
‘Need to replace the guidelines’
“We probably need to replace the guidelines,” said Eduardo Ramacciotti, MD, PhD, MPH, a professor of vascular surgery at Santa Casa School of Medicine, São Paulo. Dr. Ramacciotti praised the study’s design, the endpoints, and the fact that the design excluded patients at high risk for bleeding complications, particularly those with a fibrinogen level below 200 mg/dL (2 g/L).
But other experts questioned the significance of the COVID-PACT results given that the outcomes did not show that full-dose anticoagulation produced incremental improvement in patient survival.
“We should abandon the thought that intensified anticoagulation should be routine, because it did not overall increase the number of patients discharged from the hospital alive,” commented John W. Eikelboom, MBBS, a professor of hematology and thromboembolism at McMaster University, Hamilton, Ont.
“Preventing venous thrombosis is a good thing, but the money is in saving lives and stopping need for ventilation, and we haven’t been successful doing that with an antithrombotic strategy,” said Dr. Eikelboom. “It is useful to prevent venous thrombosis, but we need to look elsewhere to improve the outcomes of [critically ill] patients with COVID-19.”
Reducing thromboembolism is a ‘valid goal’
Dr. Berg took a different view. “It’s a valid goal to try to reduce venous thromboembolism complications,” the major benefit seen in his study, he said. “There is clinical significance to reducing thrombotic events in terms of how people feel, their functional status, and their complications. There are a lot of clinically relevant consequences of thrombosis beyond mortality.”
COVID-PACT ran at 34 U.S. centers from August 2020 to March 2022 but stopped short of its enrollment goal of 750 patients because of waning numbers of patients with COVID-19 admitted to ICUs. In addition to randomly assigning patients within 96 hours of their ICU admission to full-dose anticoagulation or to standard-dose antithrombotic prophylaxis, the study included a second, concurrent randomization to the antiplatelet agent clopidogrel (Plavix) or to no antiplatelet drug. Both randomizations used an open-label design.
The results failed to show a discernable effect from adding clopidogrel on both the primary efficacy and primary safety endpoints, adding to accumulated evidence that treatment with an antiplatelet agent, including aspirin, confers no antithrombotic benefit in patients with COVID-19.
The trial’s participants averaged 61 years old, 68% were obese, 59% had hypertension, and 32% had diabetes. The median time after ICU admission when randomized treatment began was 2.1 days, and researchers followed patients for a median of 13 days, including a median time on anticoagulation of 10.6 days.
The trial design allowed clinicians to use either low molecular weight heparin or unfractionated heparin for anticoagulation, and 82% of patients received low molecular weight heparin as their initial treatment. The prespecified design called for an on-treatment analysis because of an anticipated high crossover rate. During the trial, 34% of patients who started on the prophylactic dose switched to full dose, and 17% had the reverse crossover.
95% increased win ratio with full dose
The study’s primary efficacy endpoint used a win-ratio analysis that included seven different adverse outcomes that ranged from death from venous or arterial thrombosis to clinically silent deep vein thrombosis. Treatment with full-dose anticoagulation led to a significant 95% increase in win ratio.
Researchers also applied a more conventional time-to-first-event secondary efficacy analysis, which showed that full-dose anticoagulation cut the incidence of an adverse outcome by a significant 44% relative to prophylactic dosing.
The two study groups showed no difference in all-cause death rates. The efficacy advantage of the full-dose regimen was driven by reduced rates of venous thrombotic events, especially a reduction in clinically evident deep vein thrombotic events.
The primary safety endpoint was the rate of fatal or life-threatening bleeding episodes, and while life-threatening bleeds were numerically more common among the full-dose recipients (four events, compared with one event on prophylaxis dosing) the difference was not significant, and no patients died from a bleeding event.
More secondary safety bleeds
The safety difference showed up in a secondary measure of bleeding severity, the rate of GUSTO moderate or severe bleeds. These occurred in 15 of the full-dose recipients, compared with 1 patient on the prophylactic dose.
Dr. Berg highlighted that several prior studies have assessed various anticoagulation regimens in critically ill (ICU-admitted and on respiratory or cardiovascular support) patients with COVID-19. For example, two influential reports published in 2021 by the same team of investigators in the New England Journal of Medicine had sharply divergent results.
One multicenter study, which tested full-dose heparin against prophylactic treatment in more than 1,000 critically ill patients, was stopped prematurely because it had not shown a significant difference between the treatment arms. The second study, in more than 2,000 multicenter patients with COVID-19 who did not require critical-level organ support, showed clear superiority of the full-dose heparin regimen.
Notably, both previous studies used a different primary efficacy endpoint than the COVID-PACT study. The earlier reports both measured efficacy in terms of patients being alive and off organ support by 21 days from randomization.
Patients to exclude
Although Dr. Berg stressed the clear positive result, he also cautioned that they should not apply to patients excluded from the study: those with severe coagulopathies, those with severe thrombocytopenia, and patients already maintained on dual antiplatelet therapy. He also cautioned against using the full-dose strategy in elderly patients, because in COVID-PACT, those who developed bleeding complications tended to be older.
Dr. Berg also noted that heparin prophylaxis is a well-established intervention for ICU-admitted patients without COVID-19 for the purpose of preventing venous thromboembolisms without evidence that this approach reduces deaths or organ failure.
But he conceded that “the priority of treatment depends on whether it saves lives, so anticoagulation is probably not as high a priority as other effective treatments” that reduce mortality. “Preventing venous thromboembolism has rarely been shown to have a mortality benefit,” Dr. Berg noted.
COVID-PACT received no direct commercial funding. Dr. Berg has been a consultant to AstraZeneca, Mobility Bio, and Youngene Therapeutics, and he participated in a trial sponsored by Kowa. Dr. Ramacciotti has been a consultant to or speaker on behalf of Aspen, Bayer, Daiichi Sankyo, Mylan, Pfizer, and Sanofi, and he has received research support from Bayer, Esperon, Novartis, and Pfizer. Dr. Eikelboom has received honoraria and research support from Bayer.
A version of this article first appeared on Medscape.com.
BARCELONA – Hospitalized patients in the ICU because of an acute COVID-19 infection had significantly fewer thrombotic events and complications when treated with full-dose anticoagulation, compared with patients who received standard-dose anticoagulation prophylaxis, but full-dose anticoagulation also triggered an excess of moderate and severe bleeding events, randomized trial results show.
The new findings from the COVID-PACT trial in an exclusively U.S.-based cohort of 382 on-treatment patients in the ICU with COVID-19 infection may lead to a change in existing guidelines, which currently recommend standard-dose prophylaxis based on results from prior head-to-head comparisons, such as guidelines posted March 2022 from the American Society of Hematology.
” after weighing an individual patient’s risk for both thrombotic events and bleeding, David D. Berg, MD, said at the annual congress of the European Society of Cardiology. Simultaneous with his report at the congress, the results also appeared online in the journal Circulation.
“What the results tell us is that full-dose anticoagulation in critically ill patients with COVID-19 is highly effective for reducing thrombotic complications,” said Dr. Berg, a cardiologist and critical care physician at Brigham and Women’s Hospital, Boston.
The report’s designated discussant agreed with Dr. Berg’s conclusions.
‘Need to replace the guidelines’
“We probably need to replace the guidelines,” said Eduardo Ramacciotti, MD, PhD, MPH, a professor of vascular surgery at Santa Casa School of Medicine, São Paulo. Dr. Ramacciotti praised the study’s design, the endpoints, and the fact that the design excluded patients at high risk for bleeding complications, particularly those with a fibrinogen level below 200 mg/dL (2 g/L).
But other experts questioned the significance of the COVID-PACT results given that the outcomes did not show that full-dose anticoagulation produced incremental improvement in patient survival.
“We should abandon the thought that intensified anticoagulation should be routine, because it did not overall increase the number of patients discharged from the hospital alive,” commented John W. Eikelboom, MBBS, a professor of hematology and thromboembolism at McMaster University, Hamilton, Ont.
“Preventing venous thrombosis is a good thing, but the money is in saving lives and stopping need for ventilation, and we haven’t been successful doing that with an antithrombotic strategy,” said Dr. Eikelboom. “It is useful to prevent venous thrombosis, but we need to look elsewhere to improve the outcomes of [critically ill] patients with COVID-19.”
Reducing thromboembolism is a ‘valid goal’
Dr. Berg took a different view. “It’s a valid goal to try to reduce venous thromboembolism complications,” the major benefit seen in his study, he said. “There is clinical significance to reducing thrombotic events in terms of how people feel, their functional status, and their complications. There are a lot of clinically relevant consequences of thrombosis beyond mortality.”
COVID-PACT ran at 34 U.S. centers from August 2020 to March 2022 but stopped short of its enrollment goal of 750 patients because of waning numbers of patients with COVID-19 admitted to ICUs. In addition to randomly assigning patients within 96 hours of their ICU admission to full-dose anticoagulation or to standard-dose antithrombotic prophylaxis, the study included a second, concurrent randomization to the antiplatelet agent clopidogrel (Plavix) or to no antiplatelet drug. Both randomizations used an open-label design.
The results failed to show a discernable effect from adding clopidogrel on both the primary efficacy and primary safety endpoints, adding to accumulated evidence that treatment with an antiplatelet agent, including aspirin, confers no antithrombotic benefit in patients with COVID-19.
The trial’s participants averaged 61 years old, 68% were obese, 59% had hypertension, and 32% had diabetes. The median time after ICU admission when randomized treatment began was 2.1 days, and researchers followed patients for a median of 13 days, including a median time on anticoagulation of 10.6 days.
The trial design allowed clinicians to use either low molecular weight heparin or unfractionated heparin for anticoagulation, and 82% of patients received low molecular weight heparin as their initial treatment. The prespecified design called for an on-treatment analysis because of an anticipated high crossover rate. During the trial, 34% of patients who started on the prophylactic dose switched to full dose, and 17% had the reverse crossover.
95% increased win ratio with full dose
The study’s primary efficacy endpoint used a win-ratio analysis that included seven different adverse outcomes that ranged from death from venous or arterial thrombosis to clinically silent deep vein thrombosis. Treatment with full-dose anticoagulation led to a significant 95% increase in win ratio.
Researchers also applied a more conventional time-to-first-event secondary efficacy analysis, which showed that full-dose anticoagulation cut the incidence of an adverse outcome by a significant 44% relative to prophylactic dosing.
The two study groups showed no difference in all-cause death rates. The efficacy advantage of the full-dose regimen was driven by reduced rates of venous thrombotic events, especially a reduction in clinically evident deep vein thrombotic events.
The primary safety endpoint was the rate of fatal or life-threatening bleeding episodes, and while life-threatening bleeds were numerically more common among the full-dose recipients (four events, compared with one event on prophylaxis dosing) the difference was not significant, and no patients died from a bleeding event.
More secondary safety bleeds
The safety difference showed up in a secondary measure of bleeding severity, the rate of GUSTO moderate or severe bleeds. These occurred in 15 of the full-dose recipients, compared with 1 patient on the prophylactic dose.
Dr. Berg highlighted that several prior studies have assessed various anticoagulation regimens in critically ill (ICU-admitted and on respiratory or cardiovascular support) patients with COVID-19. For example, two influential reports published in 2021 by the same team of investigators in the New England Journal of Medicine had sharply divergent results.
One multicenter study, which tested full-dose heparin against prophylactic treatment in more than 1,000 critically ill patients, was stopped prematurely because it had not shown a significant difference between the treatment arms. The second study, in more than 2,000 multicenter patients with COVID-19 who did not require critical-level organ support, showed clear superiority of the full-dose heparin regimen.
Notably, both previous studies used a different primary efficacy endpoint than the COVID-PACT study. The earlier reports both measured efficacy in terms of patients being alive and off organ support by 21 days from randomization.
Patients to exclude
Although Dr. Berg stressed the clear positive result, he also cautioned that they should not apply to patients excluded from the study: those with severe coagulopathies, those with severe thrombocytopenia, and patients already maintained on dual antiplatelet therapy. He also cautioned against using the full-dose strategy in elderly patients, because in COVID-PACT, those who developed bleeding complications tended to be older.
Dr. Berg also noted that heparin prophylaxis is a well-established intervention for ICU-admitted patients without COVID-19 for the purpose of preventing venous thromboembolisms without evidence that this approach reduces deaths or organ failure.
But he conceded that “the priority of treatment depends on whether it saves lives, so anticoagulation is probably not as high a priority as other effective treatments” that reduce mortality. “Preventing venous thromboembolism has rarely been shown to have a mortality benefit,” Dr. Berg noted.
COVID-PACT received no direct commercial funding. Dr. Berg has been a consultant to AstraZeneca, Mobility Bio, and Youngene Therapeutics, and he participated in a trial sponsored by Kowa. Dr. Ramacciotti has been a consultant to or speaker on behalf of Aspen, Bayer, Daiichi Sankyo, Mylan, Pfizer, and Sanofi, and he has received research support from Bayer, Esperon, Novartis, and Pfizer. Dr. Eikelboom has received honoraria and research support from Bayer.
A version of this article first appeared on Medscape.com.
BARCELONA – Hospitalized patients in the ICU because of an acute COVID-19 infection had significantly fewer thrombotic events and complications when treated with full-dose anticoagulation, compared with patients who received standard-dose anticoagulation prophylaxis, but full-dose anticoagulation also triggered an excess of moderate and severe bleeding events, randomized trial results show.
The new findings from the COVID-PACT trial in an exclusively U.S.-based cohort of 382 on-treatment patients in the ICU with COVID-19 infection may lead to a change in existing guidelines, which currently recommend standard-dose prophylaxis based on results from prior head-to-head comparisons, such as guidelines posted March 2022 from the American Society of Hematology.
” after weighing an individual patient’s risk for both thrombotic events and bleeding, David D. Berg, MD, said at the annual congress of the European Society of Cardiology. Simultaneous with his report at the congress, the results also appeared online in the journal Circulation.
“What the results tell us is that full-dose anticoagulation in critically ill patients with COVID-19 is highly effective for reducing thrombotic complications,” said Dr. Berg, a cardiologist and critical care physician at Brigham and Women’s Hospital, Boston.
The report’s designated discussant agreed with Dr. Berg’s conclusions.
‘Need to replace the guidelines’
“We probably need to replace the guidelines,” said Eduardo Ramacciotti, MD, PhD, MPH, a professor of vascular surgery at Santa Casa School of Medicine, São Paulo. Dr. Ramacciotti praised the study’s design, the endpoints, and the fact that the design excluded patients at high risk for bleeding complications, particularly those with a fibrinogen level below 200 mg/dL (2 g/L).
But other experts questioned the significance of the COVID-PACT results given that the outcomes did not show that full-dose anticoagulation produced incremental improvement in patient survival.
“We should abandon the thought that intensified anticoagulation should be routine, because it did not overall increase the number of patients discharged from the hospital alive,” commented John W. Eikelboom, MBBS, a professor of hematology and thromboembolism at McMaster University, Hamilton, Ont.
“Preventing venous thrombosis is a good thing, but the money is in saving lives and stopping need for ventilation, and we haven’t been successful doing that with an antithrombotic strategy,” said Dr. Eikelboom. “It is useful to prevent venous thrombosis, but we need to look elsewhere to improve the outcomes of [critically ill] patients with COVID-19.”
Reducing thromboembolism is a ‘valid goal’
Dr. Berg took a different view. “It’s a valid goal to try to reduce venous thromboembolism complications,” the major benefit seen in his study, he said. “There is clinical significance to reducing thrombotic events in terms of how people feel, their functional status, and their complications. There are a lot of clinically relevant consequences of thrombosis beyond mortality.”
COVID-PACT ran at 34 U.S. centers from August 2020 to March 2022 but stopped short of its enrollment goal of 750 patients because of waning numbers of patients with COVID-19 admitted to ICUs. In addition to randomly assigning patients within 96 hours of their ICU admission to full-dose anticoagulation or to standard-dose antithrombotic prophylaxis, the study included a second, concurrent randomization to the antiplatelet agent clopidogrel (Plavix) or to no antiplatelet drug. Both randomizations used an open-label design.
The results failed to show a discernable effect from adding clopidogrel on both the primary efficacy and primary safety endpoints, adding to accumulated evidence that treatment with an antiplatelet agent, including aspirin, confers no antithrombotic benefit in patients with COVID-19.
The trial’s participants averaged 61 years old, 68% were obese, 59% had hypertension, and 32% had diabetes. The median time after ICU admission when randomized treatment began was 2.1 days, and researchers followed patients for a median of 13 days, including a median time on anticoagulation of 10.6 days.
The trial design allowed clinicians to use either low molecular weight heparin or unfractionated heparin for anticoagulation, and 82% of patients received low molecular weight heparin as their initial treatment. The prespecified design called for an on-treatment analysis because of an anticipated high crossover rate. During the trial, 34% of patients who started on the prophylactic dose switched to full dose, and 17% had the reverse crossover.
95% increased win ratio with full dose
The study’s primary efficacy endpoint used a win-ratio analysis that included seven different adverse outcomes that ranged from death from venous or arterial thrombosis to clinically silent deep vein thrombosis. Treatment with full-dose anticoagulation led to a significant 95% increase in win ratio.
Researchers also applied a more conventional time-to-first-event secondary efficacy analysis, which showed that full-dose anticoagulation cut the incidence of an adverse outcome by a significant 44% relative to prophylactic dosing.
The two study groups showed no difference in all-cause death rates. The efficacy advantage of the full-dose regimen was driven by reduced rates of venous thrombotic events, especially a reduction in clinically evident deep vein thrombotic events.
The primary safety endpoint was the rate of fatal or life-threatening bleeding episodes, and while life-threatening bleeds were numerically more common among the full-dose recipients (four events, compared with one event on prophylaxis dosing) the difference was not significant, and no patients died from a bleeding event.
More secondary safety bleeds
The safety difference showed up in a secondary measure of bleeding severity, the rate of GUSTO moderate or severe bleeds. These occurred in 15 of the full-dose recipients, compared with 1 patient on the prophylactic dose.
Dr. Berg highlighted that several prior studies have assessed various anticoagulation regimens in critically ill (ICU-admitted and on respiratory or cardiovascular support) patients with COVID-19. For example, two influential reports published in 2021 by the same team of investigators in the New England Journal of Medicine had sharply divergent results.
One multicenter study, which tested full-dose heparin against prophylactic treatment in more than 1,000 critically ill patients, was stopped prematurely because it had not shown a significant difference between the treatment arms. The second study, in more than 2,000 multicenter patients with COVID-19 who did not require critical-level organ support, showed clear superiority of the full-dose heparin regimen.
Notably, both previous studies used a different primary efficacy endpoint than the COVID-PACT study. The earlier reports both measured efficacy in terms of patients being alive and off organ support by 21 days from randomization.
Patients to exclude
Although Dr. Berg stressed the clear positive result, he also cautioned that they should not apply to patients excluded from the study: those with severe coagulopathies, those with severe thrombocytopenia, and patients already maintained on dual antiplatelet therapy. He also cautioned against using the full-dose strategy in elderly patients, because in COVID-PACT, those who developed bleeding complications tended to be older.
Dr. Berg also noted that heparin prophylaxis is a well-established intervention for ICU-admitted patients without COVID-19 for the purpose of preventing venous thromboembolisms without evidence that this approach reduces deaths or organ failure.
But he conceded that “the priority of treatment depends on whether it saves lives, so anticoagulation is probably not as high a priority as other effective treatments” that reduce mortality. “Preventing venous thromboembolism has rarely been shown to have a mortality benefit,” Dr. Berg noted.
COVID-PACT received no direct commercial funding. Dr. Berg has been a consultant to AstraZeneca, Mobility Bio, and Youngene Therapeutics, and he participated in a trial sponsored by Kowa. Dr. Ramacciotti has been a consultant to or speaker on behalf of Aspen, Bayer, Daiichi Sankyo, Mylan, Pfizer, and Sanofi, and he has received research support from Bayer, Esperon, Novartis, and Pfizer. Dr. Eikelboom has received honoraria and research support from Bayer.
A version of this article first appeared on Medscape.com.