User login
Man with COVID finally tests negative after 411 days
according to experts in the United Kingdom.
The man was treated with a mixture of neutralizing monoclonal antibodies, King’s College London said in a news release.
The man, 59, tested positive in December 2020 and tested negative in January 2022. He had a weakened immune system because of a previous kidney transplant. He received three doses of vaccine and his symptoms lessened, but he kept testing positive for COVID.
To find out if the man had a persistent infection or had been infected several times, doctors did a genetic analysis of the virus.
“This revealed that the patient’s infection was a persistent infection with an early COVID variant – a variation of the original Wuhan variant that was dominant in the United Kingdom in the later months of 2020. Analysis found the patient’s virus had multiple mutations since he was first infected,” King’s College said.
The doctors treated him with a Regeneron treatment that is no longer widely used because it’s not effective against newer COVID variants.
“Some new variants of the virus are resistant to all the antibody treatments available in the United Kingdom and Europe. Some people with weakened immune systems are still at risk of severe illness and becoming persistently infected. We are still working to understand the best way to protect and treat them,” Luke Snell, MD, from the King’s College School of Immunology & Microbial Sciences, said in the news release.
This is one of the longest known cases of COVID infection. Another man in England was infected with COVID for 505 days before his death, which King’s College said was the longest known COVID infection.
A version of this article first appeared on WebMD.com.
according to experts in the United Kingdom.
The man was treated with a mixture of neutralizing monoclonal antibodies, King’s College London said in a news release.
The man, 59, tested positive in December 2020 and tested negative in January 2022. He had a weakened immune system because of a previous kidney transplant. He received three doses of vaccine and his symptoms lessened, but he kept testing positive for COVID.
To find out if the man had a persistent infection or had been infected several times, doctors did a genetic analysis of the virus.
“This revealed that the patient’s infection was a persistent infection with an early COVID variant – a variation of the original Wuhan variant that was dominant in the United Kingdom in the later months of 2020. Analysis found the patient’s virus had multiple mutations since he was first infected,” King’s College said.
The doctors treated him with a Regeneron treatment that is no longer widely used because it’s not effective against newer COVID variants.
“Some new variants of the virus are resistant to all the antibody treatments available in the United Kingdom and Europe. Some people with weakened immune systems are still at risk of severe illness and becoming persistently infected. We are still working to understand the best way to protect and treat them,” Luke Snell, MD, from the King’s College School of Immunology & Microbial Sciences, said in the news release.
This is one of the longest known cases of COVID infection. Another man in England was infected with COVID for 505 days before his death, which King’s College said was the longest known COVID infection.
A version of this article first appeared on WebMD.com.
according to experts in the United Kingdom.
The man was treated with a mixture of neutralizing monoclonal antibodies, King’s College London said in a news release.
The man, 59, tested positive in December 2020 and tested negative in January 2022. He had a weakened immune system because of a previous kidney transplant. He received three doses of vaccine and his symptoms lessened, but he kept testing positive for COVID.
To find out if the man had a persistent infection or had been infected several times, doctors did a genetic analysis of the virus.
“This revealed that the patient’s infection was a persistent infection with an early COVID variant – a variation of the original Wuhan variant that was dominant in the United Kingdom in the later months of 2020. Analysis found the patient’s virus had multiple mutations since he was first infected,” King’s College said.
The doctors treated him with a Regeneron treatment that is no longer widely used because it’s not effective against newer COVID variants.
“Some new variants of the virus are resistant to all the antibody treatments available in the United Kingdom and Europe. Some people with weakened immune systems are still at risk of severe illness and becoming persistently infected. We are still working to understand the best way to protect and treat them,” Luke Snell, MD, from the King’s College School of Immunology & Microbial Sciences, said in the news release.
This is one of the longest known cases of COVID infection. Another man in England was infected with COVID for 505 days before his death, which King’s College said was the longest known COVID infection.
A version of this article first appeared on WebMD.com.
COVID bivalent booster better vs. recent Omicron subvariants: Pfizer
the company reported on Nov. 4, supporting calls by public health officials for eligible people to get this booster before a potential COVID-19 surge this winter.
The company’s ongoing phase 2/3 study of their Omicron BA.4 and BA.5 bivalent – which targets both the virus’ original strain and the two subvariants – shows that the vaccine offered the strongest protection in people older than 55 years.
One month after receiving a 30-mcg booster with the bivalent vaccine, those older than 55 had four times more neutralizing antibodies against these Omicron subvariants, compared with people who received the original monovalent vaccine as a booster in the study.
Researchers compared the geometric mean titer (GMT) levels of these antibodies in three groups before and 1 month after boosting. The 36 people older than 55 years in the released study findings had an GMT level of 896 with the bivalent booster, a level 13 times higher than before this immunization.
For the 38 adults ages 18-55 in the study, the GMT level increased to 606 at 1 month after the bivalent booster, an increase of almost 10-fold, compared with baseline. In a comparator group of 40 people receiving the original vaccine as a fourth dose, the GMT level was 236, or threefold higher than before their booster shot.
The newly released data is “very encouraging and consistent now with three studies all showing a substantial 3-4 fold increased level of neutralizing antibodies versus BA.5 as compared with the original booster,” said Eric Topol, MD, director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape Medical News.
Pfizer and BioNTech announced the updated findings in a Nov. 4 press release.
A booster dose of the BA.4/BA.5-adapted bivalent vaccine is authorized for emergency use by the Food and Drug Administration for ages 5 years and older. The safety and tolerability profile of the Pfizer/BioNTech bivalent booster remains favorable and similar to the original COVID-19 vaccine, the company reported.
Until recently, the BA.5 Omicron variant was the dominant strain in the United States, but is now getting elbowed out by the subvariants BQ.1.1, BQ.1, and BA.4.6, which together make up almost 45% of the circulating virus.
Some skepticism
“It is important to note that these data are press-release level, which does not allow a view of the data totality,” Hana El Sahly, MD, professor of molecular virology and microbiology, Baylor College of Medicine, Houston, said in an interview.
“For example, there may be significant differences between the groups, and the release mentions at least one difference that is of importance: the interval since the last vaccination which often affects the response to subsequent boosting,” she said.
Dr. El Sahly added that the findings are not surprising. “In the short term, a variant-specific vaccine produces a higher level of antibody against the variant in the vaccine than the vaccines based on the ancestral strains.”
More researcher results are warranted. “These data do not indicate that these differences between the two vaccines translate into a meaningful clinical benefit at a population level,” Dr. El Sahly said.
An uncertain winter ahead
“As we head into the holiday season, we hope these updated data will encourage people to seek out a COVID-19 bivalent booster as soon as they are eligible in order to maintain high levels of protection against the widely circulating Omicron BA.4 and BA.5 sublineages,” Albert Bourla, Pfizer chairman and CEO, stated in the release.
The updated data from the Pfizer/BioNTech study are “all the more reason to get a booster, with added protection also versus BQ.1.1, which will soon become dominant in the U.S.,” Dr. Topol predicted.
It is unclear when the next surge will happen, as COVID-19 does not always follow a seasonal pattern, at least not yet, Dr. El Sahly said. “Regardless, it is reasonable to recommend additional vaccine doses to immunocompromised and frail or older persons. More importantly, influenza vaccination and being up to date on pneumococcal vaccines are highly recommended as soon as feasible, given the early and intense flu season.”
A version of this article first appeared on Medscape.com.
the company reported on Nov. 4, supporting calls by public health officials for eligible people to get this booster before a potential COVID-19 surge this winter.
The company’s ongoing phase 2/3 study of their Omicron BA.4 and BA.5 bivalent – which targets both the virus’ original strain and the two subvariants – shows that the vaccine offered the strongest protection in people older than 55 years.
One month after receiving a 30-mcg booster with the bivalent vaccine, those older than 55 had four times more neutralizing antibodies against these Omicron subvariants, compared with people who received the original monovalent vaccine as a booster in the study.
Researchers compared the geometric mean titer (GMT) levels of these antibodies in three groups before and 1 month after boosting. The 36 people older than 55 years in the released study findings had an GMT level of 896 with the bivalent booster, a level 13 times higher than before this immunization.
For the 38 adults ages 18-55 in the study, the GMT level increased to 606 at 1 month after the bivalent booster, an increase of almost 10-fold, compared with baseline. In a comparator group of 40 people receiving the original vaccine as a fourth dose, the GMT level was 236, or threefold higher than before their booster shot.
The newly released data is “very encouraging and consistent now with three studies all showing a substantial 3-4 fold increased level of neutralizing antibodies versus BA.5 as compared with the original booster,” said Eric Topol, MD, director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape Medical News.
Pfizer and BioNTech announced the updated findings in a Nov. 4 press release.
A booster dose of the BA.4/BA.5-adapted bivalent vaccine is authorized for emergency use by the Food and Drug Administration for ages 5 years and older. The safety and tolerability profile of the Pfizer/BioNTech bivalent booster remains favorable and similar to the original COVID-19 vaccine, the company reported.
Until recently, the BA.5 Omicron variant was the dominant strain in the United States, but is now getting elbowed out by the subvariants BQ.1.1, BQ.1, and BA.4.6, which together make up almost 45% of the circulating virus.
Some skepticism
“It is important to note that these data are press-release level, which does not allow a view of the data totality,” Hana El Sahly, MD, professor of molecular virology and microbiology, Baylor College of Medicine, Houston, said in an interview.
“For example, there may be significant differences between the groups, and the release mentions at least one difference that is of importance: the interval since the last vaccination which often affects the response to subsequent boosting,” she said.
Dr. El Sahly added that the findings are not surprising. “In the short term, a variant-specific vaccine produces a higher level of antibody against the variant in the vaccine than the vaccines based on the ancestral strains.”
More researcher results are warranted. “These data do not indicate that these differences between the two vaccines translate into a meaningful clinical benefit at a population level,” Dr. El Sahly said.
An uncertain winter ahead
“As we head into the holiday season, we hope these updated data will encourage people to seek out a COVID-19 bivalent booster as soon as they are eligible in order to maintain high levels of protection against the widely circulating Omicron BA.4 and BA.5 sublineages,” Albert Bourla, Pfizer chairman and CEO, stated in the release.
The updated data from the Pfizer/BioNTech study are “all the more reason to get a booster, with added protection also versus BQ.1.1, which will soon become dominant in the U.S.,” Dr. Topol predicted.
It is unclear when the next surge will happen, as COVID-19 does not always follow a seasonal pattern, at least not yet, Dr. El Sahly said. “Regardless, it is reasonable to recommend additional vaccine doses to immunocompromised and frail or older persons. More importantly, influenza vaccination and being up to date on pneumococcal vaccines are highly recommended as soon as feasible, given the early and intense flu season.”
A version of this article first appeared on Medscape.com.
the company reported on Nov. 4, supporting calls by public health officials for eligible people to get this booster before a potential COVID-19 surge this winter.
The company’s ongoing phase 2/3 study of their Omicron BA.4 and BA.5 bivalent – which targets both the virus’ original strain and the two subvariants – shows that the vaccine offered the strongest protection in people older than 55 years.
One month after receiving a 30-mcg booster with the bivalent vaccine, those older than 55 had four times more neutralizing antibodies against these Omicron subvariants, compared with people who received the original monovalent vaccine as a booster in the study.
Researchers compared the geometric mean titer (GMT) levels of these antibodies in three groups before and 1 month after boosting. The 36 people older than 55 years in the released study findings had an GMT level of 896 with the bivalent booster, a level 13 times higher than before this immunization.
For the 38 adults ages 18-55 in the study, the GMT level increased to 606 at 1 month after the bivalent booster, an increase of almost 10-fold, compared with baseline. In a comparator group of 40 people receiving the original vaccine as a fourth dose, the GMT level was 236, or threefold higher than before their booster shot.
The newly released data is “very encouraging and consistent now with three studies all showing a substantial 3-4 fold increased level of neutralizing antibodies versus BA.5 as compared with the original booster,” said Eric Topol, MD, director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape Medical News.
Pfizer and BioNTech announced the updated findings in a Nov. 4 press release.
A booster dose of the BA.4/BA.5-adapted bivalent vaccine is authorized for emergency use by the Food and Drug Administration for ages 5 years and older. The safety and tolerability profile of the Pfizer/BioNTech bivalent booster remains favorable and similar to the original COVID-19 vaccine, the company reported.
Until recently, the BA.5 Omicron variant was the dominant strain in the United States, but is now getting elbowed out by the subvariants BQ.1.1, BQ.1, and BA.4.6, which together make up almost 45% of the circulating virus.
Some skepticism
“It is important to note that these data are press-release level, which does not allow a view of the data totality,” Hana El Sahly, MD, professor of molecular virology and microbiology, Baylor College of Medicine, Houston, said in an interview.
“For example, there may be significant differences between the groups, and the release mentions at least one difference that is of importance: the interval since the last vaccination which often affects the response to subsequent boosting,” she said.
Dr. El Sahly added that the findings are not surprising. “In the short term, a variant-specific vaccine produces a higher level of antibody against the variant in the vaccine than the vaccines based on the ancestral strains.”
More researcher results are warranted. “These data do not indicate that these differences between the two vaccines translate into a meaningful clinical benefit at a population level,” Dr. El Sahly said.
An uncertain winter ahead
“As we head into the holiday season, we hope these updated data will encourage people to seek out a COVID-19 bivalent booster as soon as they are eligible in order to maintain high levels of protection against the widely circulating Omicron BA.4 and BA.5 sublineages,” Albert Bourla, Pfizer chairman and CEO, stated in the release.
The updated data from the Pfizer/BioNTech study are “all the more reason to get a booster, with added protection also versus BQ.1.1, which will soon become dominant in the U.S.,” Dr. Topol predicted.
It is unclear when the next surge will happen, as COVID-19 does not always follow a seasonal pattern, at least not yet, Dr. El Sahly said. “Regardless, it is reasonable to recommend additional vaccine doses to immunocompromised and frail or older persons. More importantly, influenza vaccination and being up to date on pneumococcal vaccines are highly recommended as soon as feasible, given the early and intense flu season.”
A version of this article first appeared on Medscape.com.
Working while sick: Why doctors don’t stay home when ill
The reasons are likely as varied as, “you weren’t feeling bad enough to miss work,” “you couldn’t afford to miss pay,” “you had too many patients to see,” or “too much work to do.”
In Medscape’s Employed Physicians Report: Loving the Focus, Hating the Bureaucracy, 61% of physicians reported that they sometimes or often come to work sick. Only 2% of respondents said they never come to work unwell.
Medscape wanted to know more about how often you call in sick, how often you come to work feeling unwell, what symptoms you have, and the dogma of your workplace culture regarding sick days. Not to mention the brutal ethos that starts in medical school, in which calling in sick shows weakness or is unacceptable.
So, we polled 2,347 physicians in the United States and abroad and asked them about their sniffling, sneezing, cold, flu, and fever symptoms, and, of course, COVID. Results were split about 50-50 among male and female physicians. The poll ran from Sept. 28 through Oct. 11.
Coming to work sick
It’s no surprise that the majority of physicians who were polled (85%) have come to work sick in 2022. In the last prepandemic year (2019), about 70% came to work feeling sick one to five times, and 13% worked while sick six to ten times.
When asked about the symptoms that they’ve previously come to work with, 48% of U.S. physicians said multiple symptoms. They gave high marks for runny nose, cough, congestion, and sore throat. Only 27% have worked with a fever, 22% have worked with other symptoms, and 7% have worked with both strep throat and COVID.
“My workplace, especially in the COVID years, accommodates persons who honestly do not feel well enough to report. Sooner or later, everyone covers for someone else who has to be out,” says Kenneth Abbott, MD, an oncologist in Maryland.
The culture of working while sick
Why doctors come to work when they’re sick is complicated. The overwhelming majority of U.S. respondents cited professional obligations; 73% noted that they feel a professional obligation to their patients, and 72% feel a professional obligation to their co-workers. Half of the polled U.S. physicians said they didn’t feel bad enough to stay home, while 48% said they had too much work to do to stay home.
Some 45% said the expectation at their workplace is to come to work unless seriously ill; 43% had too many patients to see; and 18% didn’t think they were contagious when they headed to work sick. Unfortunately, 15% chose to work while sick because otherwise they would lose pay.
In light of these responses, it’s not surprising that 93% reported they’d seen other medical professionals working when sick.
“My schedule is almost always booked weeks in advance. If someone misses or has to cancel their appointment, they typically have 2-4 weeks to wait to get back in. If I was sick and a full day of patients (or God forbid more than a day) had to be canceled because I called in, it’s so much more work when I return,” says Caitlin Briggs, MD, a psychiatrist in Lexington, Ky.
Doctors’ workplace sick day policy
Most employees’ benefits allow at least a few sick days, but doctors who treat society’s ill patients don’t seem to stay home from work when they’re suffering. So, we asked physicians, official policy aside, whether they thought going to work sick was expected in their workplace. The majority (76%) said yes, while 24% said no.
“Unless I’m dying or extremely contagious, I usually work. At least now, I have the telehealth option. Not saying any of this is right, but it’s the reality we deal with and the choice we must make,” says Dr. Briggs.
Additionally, 58% of polled physicians said their workplace did not have a clearly defined policy against coming to work sick, while 20% said theirs did, and 22% weren’t sure.
“The first thing I heard on the subject as a medical student was that sick people come to the hospital, so if you’re sick, then you come to the hospital too ... to work. If you can’t work, then you will be admitted. Another aphorism was from Churchill, that ‘most of the world’s work is done by people who don’t feel very well,’ ” says Paul Andreason, MD, a psychiatrist in Bethesda, Md.
Working in the time of COVID
Working while ill during ordinary times is one thing, but what about working in the time of COVID? Has the pandemic changed the culture of coming to work sick because medical facilities, such as doctor’s offices and hospitals, don’t want their staff coming in when they have COVID?
Surprisingly, when we asked physicians whether the pandemic has made it more or less acceptable to come to work sick, only 61% thought COVID has made it less acceptable to work while sick, while 16% thought it made it more acceptable, and 23% said there’s no change.
“I draw the line at fevers/chills, feeling like you’ve just been run over, or significant enteritis,” says Dr. Abbott. “Also, if I have to take palliative meds that interfere with alertness, I’m not doing my patients any favors.”
While a minority of physicians may call in sick, most still suffer through their sneezing, coughing, chills, and fever while seeing patients as usual.
A version of this article first appeared on Medscape.com.
The reasons are likely as varied as, “you weren’t feeling bad enough to miss work,” “you couldn’t afford to miss pay,” “you had too many patients to see,” or “too much work to do.”
In Medscape’s Employed Physicians Report: Loving the Focus, Hating the Bureaucracy, 61% of physicians reported that they sometimes or often come to work sick. Only 2% of respondents said they never come to work unwell.
Medscape wanted to know more about how often you call in sick, how often you come to work feeling unwell, what symptoms you have, and the dogma of your workplace culture regarding sick days. Not to mention the brutal ethos that starts in medical school, in which calling in sick shows weakness or is unacceptable.
So, we polled 2,347 physicians in the United States and abroad and asked them about their sniffling, sneezing, cold, flu, and fever symptoms, and, of course, COVID. Results were split about 50-50 among male and female physicians. The poll ran from Sept. 28 through Oct. 11.
Coming to work sick
It’s no surprise that the majority of physicians who were polled (85%) have come to work sick in 2022. In the last prepandemic year (2019), about 70% came to work feeling sick one to five times, and 13% worked while sick six to ten times.
When asked about the symptoms that they’ve previously come to work with, 48% of U.S. physicians said multiple symptoms. They gave high marks for runny nose, cough, congestion, and sore throat. Only 27% have worked with a fever, 22% have worked with other symptoms, and 7% have worked with both strep throat and COVID.
“My workplace, especially in the COVID years, accommodates persons who honestly do not feel well enough to report. Sooner or later, everyone covers for someone else who has to be out,” says Kenneth Abbott, MD, an oncologist in Maryland.
The culture of working while sick
Why doctors come to work when they’re sick is complicated. The overwhelming majority of U.S. respondents cited professional obligations; 73% noted that they feel a professional obligation to their patients, and 72% feel a professional obligation to their co-workers. Half of the polled U.S. physicians said they didn’t feel bad enough to stay home, while 48% said they had too much work to do to stay home.
Some 45% said the expectation at their workplace is to come to work unless seriously ill; 43% had too many patients to see; and 18% didn’t think they were contagious when they headed to work sick. Unfortunately, 15% chose to work while sick because otherwise they would lose pay.
In light of these responses, it’s not surprising that 93% reported they’d seen other medical professionals working when sick.
“My schedule is almost always booked weeks in advance. If someone misses or has to cancel their appointment, they typically have 2-4 weeks to wait to get back in. If I was sick and a full day of patients (or God forbid more than a day) had to be canceled because I called in, it’s so much more work when I return,” says Caitlin Briggs, MD, a psychiatrist in Lexington, Ky.
Doctors’ workplace sick day policy
Most employees’ benefits allow at least a few sick days, but doctors who treat society’s ill patients don’t seem to stay home from work when they’re suffering. So, we asked physicians, official policy aside, whether they thought going to work sick was expected in their workplace. The majority (76%) said yes, while 24% said no.
“Unless I’m dying or extremely contagious, I usually work. At least now, I have the telehealth option. Not saying any of this is right, but it’s the reality we deal with and the choice we must make,” says Dr. Briggs.
Additionally, 58% of polled physicians said their workplace did not have a clearly defined policy against coming to work sick, while 20% said theirs did, and 22% weren’t sure.
“The first thing I heard on the subject as a medical student was that sick people come to the hospital, so if you’re sick, then you come to the hospital too ... to work. If you can’t work, then you will be admitted. Another aphorism was from Churchill, that ‘most of the world’s work is done by people who don’t feel very well,’ ” says Paul Andreason, MD, a psychiatrist in Bethesda, Md.
Working in the time of COVID
Working while ill during ordinary times is one thing, but what about working in the time of COVID? Has the pandemic changed the culture of coming to work sick because medical facilities, such as doctor’s offices and hospitals, don’t want their staff coming in when they have COVID?
Surprisingly, when we asked physicians whether the pandemic has made it more or less acceptable to come to work sick, only 61% thought COVID has made it less acceptable to work while sick, while 16% thought it made it more acceptable, and 23% said there’s no change.
“I draw the line at fevers/chills, feeling like you’ve just been run over, or significant enteritis,” says Dr. Abbott. “Also, if I have to take palliative meds that interfere with alertness, I’m not doing my patients any favors.”
While a minority of physicians may call in sick, most still suffer through their sneezing, coughing, chills, and fever while seeing patients as usual.
A version of this article first appeared on Medscape.com.
The reasons are likely as varied as, “you weren’t feeling bad enough to miss work,” “you couldn’t afford to miss pay,” “you had too many patients to see,” or “too much work to do.”
In Medscape’s Employed Physicians Report: Loving the Focus, Hating the Bureaucracy, 61% of physicians reported that they sometimes or often come to work sick. Only 2% of respondents said they never come to work unwell.
Medscape wanted to know more about how often you call in sick, how often you come to work feeling unwell, what symptoms you have, and the dogma of your workplace culture regarding sick days. Not to mention the brutal ethos that starts in medical school, in which calling in sick shows weakness or is unacceptable.
So, we polled 2,347 physicians in the United States and abroad and asked them about their sniffling, sneezing, cold, flu, and fever symptoms, and, of course, COVID. Results were split about 50-50 among male and female physicians. The poll ran from Sept. 28 through Oct. 11.
Coming to work sick
It’s no surprise that the majority of physicians who were polled (85%) have come to work sick in 2022. In the last prepandemic year (2019), about 70% came to work feeling sick one to five times, and 13% worked while sick six to ten times.
When asked about the symptoms that they’ve previously come to work with, 48% of U.S. physicians said multiple symptoms. They gave high marks for runny nose, cough, congestion, and sore throat. Only 27% have worked with a fever, 22% have worked with other symptoms, and 7% have worked with both strep throat and COVID.
“My workplace, especially in the COVID years, accommodates persons who honestly do not feel well enough to report. Sooner or later, everyone covers for someone else who has to be out,” says Kenneth Abbott, MD, an oncologist in Maryland.
The culture of working while sick
Why doctors come to work when they’re sick is complicated. The overwhelming majority of U.S. respondents cited professional obligations; 73% noted that they feel a professional obligation to their patients, and 72% feel a professional obligation to their co-workers. Half of the polled U.S. physicians said they didn’t feel bad enough to stay home, while 48% said they had too much work to do to stay home.
Some 45% said the expectation at their workplace is to come to work unless seriously ill; 43% had too many patients to see; and 18% didn’t think they were contagious when they headed to work sick. Unfortunately, 15% chose to work while sick because otherwise they would lose pay.
In light of these responses, it’s not surprising that 93% reported they’d seen other medical professionals working when sick.
“My schedule is almost always booked weeks in advance. If someone misses or has to cancel their appointment, they typically have 2-4 weeks to wait to get back in. If I was sick and a full day of patients (or God forbid more than a day) had to be canceled because I called in, it’s so much more work when I return,” says Caitlin Briggs, MD, a psychiatrist in Lexington, Ky.
Doctors’ workplace sick day policy
Most employees’ benefits allow at least a few sick days, but doctors who treat society’s ill patients don’t seem to stay home from work when they’re suffering. So, we asked physicians, official policy aside, whether they thought going to work sick was expected in their workplace. The majority (76%) said yes, while 24% said no.
“Unless I’m dying or extremely contagious, I usually work. At least now, I have the telehealth option. Not saying any of this is right, but it’s the reality we deal with and the choice we must make,” says Dr. Briggs.
Additionally, 58% of polled physicians said their workplace did not have a clearly defined policy against coming to work sick, while 20% said theirs did, and 22% weren’t sure.
“The first thing I heard on the subject as a medical student was that sick people come to the hospital, so if you’re sick, then you come to the hospital too ... to work. If you can’t work, then you will be admitted. Another aphorism was from Churchill, that ‘most of the world’s work is done by people who don’t feel very well,’ ” says Paul Andreason, MD, a psychiatrist in Bethesda, Md.
Working in the time of COVID
Working while ill during ordinary times is one thing, but what about working in the time of COVID? Has the pandemic changed the culture of coming to work sick because medical facilities, such as doctor’s offices and hospitals, don’t want their staff coming in when they have COVID?
Surprisingly, when we asked physicians whether the pandemic has made it more or less acceptable to come to work sick, only 61% thought COVID has made it less acceptable to work while sick, while 16% thought it made it more acceptable, and 23% said there’s no change.
“I draw the line at fevers/chills, feeling like you’ve just been run over, or significant enteritis,” says Dr. Abbott. “Also, if I have to take palliative meds that interfere with alertness, I’m not doing my patients any favors.”
While a minority of physicians may call in sick, most still suffer through their sneezing, coughing, chills, and fever while seeing patients as usual.
A version of this article first appeared on Medscape.com.
New research confirms recommendations on COVID-19 boosters in MS
, as currently recommended.
“We have shown that even MS patients whose B cells were depleted from circulation with ocrelizumab can mount immune responses to COVID-19 vaccines,” said lead study author Ilya Kister, MD, of NYU Langone’s Multiple Sclerosis Comprehensive Care Center in New York.
The findings were presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
VIOLA study
The data stem from VIOLA, an ongoing prospective study of humoral and cellular immune responses to COVID-19 vaccines in 60 patients with MS receiving ocrelizumab at MS care centers at NYU Langone and the University of Colorado Denver.
The mean age of participants was 38 years, 73% were women, all had been taking ocrelizumab for a mean of 1.7 years, and 45% had had COVID-19 prior to vaccination.
The researchers examined antibody and cellular responses to the two-dose series of mRNA COVID-19 vaccine (80% received the Pfizer-BioNTech vaccine, 18% the Moderna vaccine, and 2% unknown) over 24 weeks. In addition, 57% of the participants received the third dose/booster.
Results showed that antibody and cellular responses to SARS-CoV-2 spike protein significantly increased after the two-dose mRNA COVID-19 vaccination, though antibody responses tended to peak between 4 and 12 weeks and declined thereafter. There was no significant decline in cellular responses at week 24.
“The third dose ‘booster’ again significantly increased antibody and cellular responses compared with the pre–third dose levels,” Dr. Kister said.
“Importantly, cellular responses remained elevated or even increased from 4 weeks to 12 weeks after third dose/booster. Overall, these data strongly support the need for a third dose in MS patients on ocrelizumab,” Dr. Kister added.
Participants with “hybrid immunity” (those who had been infected with SARS-CoV-2 and who had also been vaccinated for COVID) had markedly higher SARS-CoV-2–specific antibody and cellular responses than those of peers with vaccine-only immunity.
CDC recs
Looking ahead, Dr. Kister said the VIOLA investigators plan to present data on the durability of COVID-19 vaccines in ocrelizumab-treated patients up to 48 weeks after the third dose.
For immunocompromised patients, such as those taking ocrelizumab, the Centers for Disease Control and Prevention considers the third dose of mRNA vaccine not as a “booster” but as part of the regular vaccine series.
“In other words, all these patients should receive three doses as part of their ‘primary’ series,” Dr. Kister noted.
The CDC also recommends receiving the updated booster for COVID-19 that became available in September 2022 (the fourth dose of the vaccine).
“Our study did not evaluate the efficacy of this fourth dose; but based on our results, it is reasonable to suppose that the fourth dose would also lead to a further increase in immune defenses,” Dr. Kister said.
The VIOLA study is an investigator-initiated collaboration supported by F. Hoffmann-La Roche Ltd/Genentech Inc. Dr. Kister has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, as currently recommended.
“We have shown that even MS patients whose B cells were depleted from circulation with ocrelizumab can mount immune responses to COVID-19 vaccines,” said lead study author Ilya Kister, MD, of NYU Langone’s Multiple Sclerosis Comprehensive Care Center in New York.
The findings were presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
VIOLA study
The data stem from VIOLA, an ongoing prospective study of humoral and cellular immune responses to COVID-19 vaccines in 60 patients with MS receiving ocrelizumab at MS care centers at NYU Langone and the University of Colorado Denver.
The mean age of participants was 38 years, 73% were women, all had been taking ocrelizumab for a mean of 1.7 years, and 45% had had COVID-19 prior to vaccination.
The researchers examined antibody and cellular responses to the two-dose series of mRNA COVID-19 vaccine (80% received the Pfizer-BioNTech vaccine, 18% the Moderna vaccine, and 2% unknown) over 24 weeks. In addition, 57% of the participants received the third dose/booster.
Results showed that antibody and cellular responses to SARS-CoV-2 spike protein significantly increased after the two-dose mRNA COVID-19 vaccination, though antibody responses tended to peak between 4 and 12 weeks and declined thereafter. There was no significant decline in cellular responses at week 24.
“The third dose ‘booster’ again significantly increased antibody and cellular responses compared with the pre–third dose levels,” Dr. Kister said.
“Importantly, cellular responses remained elevated or even increased from 4 weeks to 12 weeks after third dose/booster. Overall, these data strongly support the need for a third dose in MS patients on ocrelizumab,” Dr. Kister added.
Participants with “hybrid immunity” (those who had been infected with SARS-CoV-2 and who had also been vaccinated for COVID) had markedly higher SARS-CoV-2–specific antibody and cellular responses than those of peers with vaccine-only immunity.
CDC recs
Looking ahead, Dr. Kister said the VIOLA investigators plan to present data on the durability of COVID-19 vaccines in ocrelizumab-treated patients up to 48 weeks after the third dose.
For immunocompromised patients, such as those taking ocrelizumab, the Centers for Disease Control and Prevention considers the third dose of mRNA vaccine not as a “booster” but as part of the regular vaccine series.
“In other words, all these patients should receive three doses as part of their ‘primary’ series,” Dr. Kister noted.
The CDC also recommends receiving the updated booster for COVID-19 that became available in September 2022 (the fourth dose of the vaccine).
“Our study did not evaluate the efficacy of this fourth dose; but based on our results, it is reasonable to suppose that the fourth dose would also lead to a further increase in immune defenses,” Dr. Kister said.
The VIOLA study is an investigator-initiated collaboration supported by F. Hoffmann-La Roche Ltd/Genentech Inc. Dr. Kister has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, as currently recommended.
“We have shown that even MS patients whose B cells were depleted from circulation with ocrelizumab can mount immune responses to COVID-19 vaccines,” said lead study author Ilya Kister, MD, of NYU Langone’s Multiple Sclerosis Comprehensive Care Center in New York.
The findings were presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
VIOLA study
The data stem from VIOLA, an ongoing prospective study of humoral and cellular immune responses to COVID-19 vaccines in 60 patients with MS receiving ocrelizumab at MS care centers at NYU Langone and the University of Colorado Denver.
The mean age of participants was 38 years, 73% were women, all had been taking ocrelizumab for a mean of 1.7 years, and 45% had had COVID-19 prior to vaccination.
The researchers examined antibody and cellular responses to the two-dose series of mRNA COVID-19 vaccine (80% received the Pfizer-BioNTech vaccine, 18% the Moderna vaccine, and 2% unknown) over 24 weeks. In addition, 57% of the participants received the third dose/booster.
Results showed that antibody and cellular responses to SARS-CoV-2 spike protein significantly increased after the two-dose mRNA COVID-19 vaccination, though antibody responses tended to peak between 4 and 12 weeks and declined thereafter. There was no significant decline in cellular responses at week 24.
“The third dose ‘booster’ again significantly increased antibody and cellular responses compared with the pre–third dose levels,” Dr. Kister said.
“Importantly, cellular responses remained elevated or even increased from 4 weeks to 12 weeks after third dose/booster. Overall, these data strongly support the need for a third dose in MS patients on ocrelizumab,” Dr. Kister added.
Participants with “hybrid immunity” (those who had been infected with SARS-CoV-2 and who had also been vaccinated for COVID) had markedly higher SARS-CoV-2–specific antibody and cellular responses than those of peers with vaccine-only immunity.
CDC recs
Looking ahead, Dr. Kister said the VIOLA investigators plan to present data on the durability of COVID-19 vaccines in ocrelizumab-treated patients up to 48 weeks after the third dose.
For immunocompromised patients, such as those taking ocrelizumab, the Centers for Disease Control and Prevention considers the third dose of mRNA vaccine not as a “booster” but as part of the regular vaccine series.
“In other words, all these patients should receive three doses as part of their ‘primary’ series,” Dr. Kister noted.
The CDC also recommends receiving the updated booster for COVID-19 that became available in September 2022 (the fourth dose of the vaccine).
“Our study did not evaluate the efficacy of this fourth dose; but based on our results, it is reasonable to suppose that the fourth dose would also lead to a further increase in immune defenses,” Dr. Kister said.
The VIOLA study is an investigator-initiated collaboration supported by F. Hoffmann-La Roche Ltd/Genentech Inc. Dr. Kister has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From ECTRIMS 2022
Black Veterans Less Likely to Get COVID-Specific Treatments at VAMCs
Black veterans hospitalized with COVID-19 were less likely to be treated with evidence-based treatments, in a study conducted in 130 US Department of Veterans Affairs (VA) medical centers between March 1, 2020, and February 28, 2022.
The study involved 12,135 Black veterans and 40,717 White veterans. Most patients hospitalized during period 1 (March-September 2020) were Black veterans and the proportion of White patients increased over time. The latter 3 periods, which included the Delta- and Omicron-predominant periods, saw the most admissions.
Controlling for the site of treatment, Black patients were equally likely to be admitted to the intensive care unit (40% vs 43%). However, they were less likely to receive steroids, remdesivir, or immunomodulatory drugs.
The researchers say their data confirm other findings from 41 US health care systems participating in the National Patient-Centered Clinical Research Network (PCORNet), which found lower use of monoclonal antibody treatment for COVID infection for patients who identified as Asian, Black, Hispanic, American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, or multiple races.
The researchers did not observe consistent differences in clinical outcomes between Black and White patients. After adjusting for demographics, chronic health conditions, severity of acute illness, and receipt of COVID-19–specific treatments, there was no association of Black race with hospital mortality or 30-day readmission. Black and White patients had a similar burden of preexisting health conditions. Of 38,782 patients discharged, 14% were readmitted within 30 days; the median time to readmission for both groups was 9 days.
Differences in care were partially explained by within- and between-hospital differences, the researchers say. They also cite research that demonstrated a poorer quality of care for hospitals with higher monthly COVID-19 discharges and hospital size.
The study results contradict the assumptions that differences in inpatient treatment by race and ethnicity may be due to differences in clinical indications for medication use based on age and comorbidities, such as chronic kidney or liver disease, the researchers say. For one thing, the VA issued a systemwide COVID-19 response plan that included specific treatment guidelines and distribution plans. But they also point to recent reports that have suggested that occult hypoxemia not detected by pulse oximetry occurs “far more often in Black patients than White patients,” which could result in delayed or missed opportunities to treat patients with COVID-19.
Black veterans hospitalized with COVID-19 were less likely to be treated with evidence-based treatments, in a study conducted in 130 US Department of Veterans Affairs (VA) medical centers between March 1, 2020, and February 28, 2022.
The study involved 12,135 Black veterans and 40,717 White veterans. Most patients hospitalized during period 1 (March-September 2020) were Black veterans and the proportion of White patients increased over time. The latter 3 periods, which included the Delta- and Omicron-predominant periods, saw the most admissions.
Controlling for the site of treatment, Black patients were equally likely to be admitted to the intensive care unit (40% vs 43%). However, they were less likely to receive steroids, remdesivir, or immunomodulatory drugs.
The researchers say their data confirm other findings from 41 US health care systems participating in the National Patient-Centered Clinical Research Network (PCORNet), which found lower use of monoclonal antibody treatment for COVID infection for patients who identified as Asian, Black, Hispanic, American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, or multiple races.
The researchers did not observe consistent differences in clinical outcomes between Black and White patients. After adjusting for demographics, chronic health conditions, severity of acute illness, and receipt of COVID-19–specific treatments, there was no association of Black race with hospital mortality or 30-day readmission. Black and White patients had a similar burden of preexisting health conditions. Of 38,782 patients discharged, 14% were readmitted within 30 days; the median time to readmission for both groups was 9 days.
Differences in care were partially explained by within- and between-hospital differences, the researchers say. They also cite research that demonstrated a poorer quality of care for hospitals with higher monthly COVID-19 discharges and hospital size.
The study results contradict the assumptions that differences in inpatient treatment by race and ethnicity may be due to differences in clinical indications for medication use based on age and comorbidities, such as chronic kidney or liver disease, the researchers say. For one thing, the VA issued a systemwide COVID-19 response plan that included specific treatment guidelines and distribution plans. But they also point to recent reports that have suggested that occult hypoxemia not detected by pulse oximetry occurs “far more often in Black patients than White patients,” which could result in delayed or missed opportunities to treat patients with COVID-19.
Black veterans hospitalized with COVID-19 were less likely to be treated with evidence-based treatments, in a study conducted in 130 US Department of Veterans Affairs (VA) medical centers between March 1, 2020, and February 28, 2022.
The study involved 12,135 Black veterans and 40,717 White veterans. Most patients hospitalized during period 1 (March-September 2020) were Black veterans and the proportion of White patients increased over time. The latter 3 periods, which included the Delta- and Omicron-predominant periods, saw the most admissions.
Controlling for the site of treatment, Black patients were equally likely to be admitted to the intensive care unit (40% vs 43%). However, they were less likely to receive steroids, remdesivir, or immunomodulatory drugs.
The researchers say their data confirm other findings from 41 US health care systems participating in the National Patient-Centered Clinical Research Network (PCORNet), which found lower use of monoclonal antibody treatment for COVID infection for patients who identified as Asian, Black, Hispanic, American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, or multiple races.
The researchers did not observe consistent differences in clinical outcomes between Black and White patients. After adjusting for demographics, chronic health conditions, severity of acute illness, and receipt of COVID-19–specific treatments, there was no association of Black race with hospital mortality or 30-day readmission. Black and White patients had a similar burden of preexisting health conditions. Of 38,782 patients discharged, 14% were readmitted within 30 days; the median time to readmission for both groups was 9 days.
Differences in care were partially explained by within- and between-hospital differences, the researchers say. They also cite research that demonstrated a poorer quality of care for hospitals with higher monthly COVID-19 discharges and hospital size.
The study results contradict the assumptions that differences in inpatient treatment by race and ethnicity may be due to differences in clinical indications for medication use based on age and comorbidities, such as chronic kidney or liver disease, the researchers say. For one thing, the VA issued a systemwide COVID-19 response plan that included specific treatment guidelines and distribution plans. But they also point to recent reports that have suggested that occult hypoxemia not detected by pulse oximetry occurs “far more often in Black patients than White patients,” which could result in delayed or missed opportunities to treat patients with COVID-19.
Mid-October flulike illness cases higher than past 5 years
Outpatient visits for influenzalike illness (ILI), which includes influenza, SARS-CoV-2, and RSV, were higher after 3 weeks than for any of the previous five flu seasons: 3.3% of visits reported through the CDC’s Outpatient Influenza-like Illness Surveillance Network involved ILI as of Oct. 22. The highest comparable rate in the previous 5 years was the 1.9% recorded in late October of 2021, shortly after the definition of ILI was changed to also include illnesses other than influenza.
This season’s higher flu activity is in contrast to the previous two, which were unusually mild. The change, however, is not unexpected, as William Schaffner, MD, an infectious disease expert and professor of preventive medicine at Vanderbilt University, recently told CNN.
“Here we are in the middle of October – not the middle of November – we’re already seeing scattered influenza cases, even hospitalized influenza cases, around the country,” he said. “So we know that this virus is now spreading out in the community already. It’s gathering speed already. It looks to me to be about a month early.”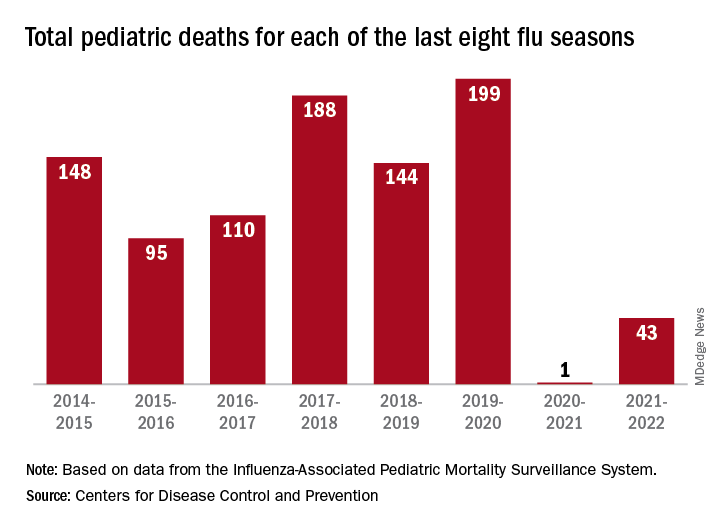
One indication of the mildness of the previous two flu seasons was the number of deaths, both pediatric and overall. Influenza-associated pediatric deaths had averaged about 110 per season over the previous eight seasons, compared with just 1 for 2020-2021 and 43 in 2021-2022. Overall flu deaths never reached 1% of all weekly deaths for either season, well below baseline levels for the flu, which range from 5.5% to 6.8%, CDC data show.
Other indicators of early severity
This season’s early rise in viral activity also can be seen in hospitalizations. The cumulative rate of flu-related admissions was 1.5 per 100,000 population as of Oct. 22, higher than the rate observed in the comparable week of previous seasons going back to 2010-2011, according to the CDC’s Influenza Hospitalization Surveillance Network.
A look at state reports of ILI outpatient visit rates shows that the District of Columbia and South Carolina are already in the very high range of the CDC’s severity scale, while 11 states are in the high range. Again going back to 2010-2011, no jurisdiction has ever been in the very high range this early in the season, based on data from the Outpatient Influenza-like Illness Surveillance Network.
Outpatient visits for influenzalike illness (ILI), which includes influenza, SARS-CoV-2, and RSV, were higher after 3 weeks than for any of the previous five flu seasons: 3.3% of visits reported through the CDC’s Outpatient Influenza-like Illness Surveillance Network involved ILI as of Oct. 22. The highest comparable rate in the previous 5 years was the 1.9% recorded in late October of 2021, shortly after the definition of ILI was changed to also include illnesses other than influenza.
This season’s higher flu activity is in contrast to the previous two, which were unusually mild. The change, however, is not unexpected, as William Schaffner, MD, an infectious disease expert and professor of preventive medicine at Vanderbilt University, recently told CNN.
“Here we are in the middle of October – not the middle of November – we’re already seeing scattered influenza cases, even hospitalized influenza cases, around the country,” he said. “So we know that this virus is now spreading out in the community already. It’s gathering speed already. It looks to me to be about a month early.”
One indication of the mildness of the previous two flu seasons was the number of deaths, both pediatric and overall. Influenza-associated pediatric deaths had averaged about 110 per season over the previous eight seasons, compared with just 1 for 2020-2021 and 43 in 2021-2022. Overall flu deaths never reached 1% of all weekly deaths for either season, well below baseline levels for the flu, which range from 5.5% to 6.8%, CDC data show.
Other indicators of early severity
This season’s early rise in viral activity also can be seen in hospitalizations. The cumulative rate of flu-related admissions was 1.5 per 100,000 population as of Oct. 22, higher than the rate observed in the comparable week of previous seasons going back to 2010-2011, according to the CDC’s Influenza Hospitalization Surveillance Network.
A look at state reports of ILI outpatient visit rates shows that the District of Columbia and South Carolina are already in the very high range of the CDC’s severity scale, while 11 states are in the high range. Again going back to 2010-2011, no jurisdiction has ever been in the very high range this early in the season, based on data from the Outpatient Influenza-like Illness Surveillance Network.
Outpatient visits for influenzalike illness (ILI), which includes influenza, SARS-CoV-2, and RSV, were higher after 3 weeks than for any of the previous five flu seasons: 3.3% of visits reported through the CDC’s Outpatient Influenza-like Illness Surveillance Network involved ILI as of Oct. 22. The highest comparable rate in the previous 5 years was the 1.9% recorded in late October of 2021, shortly after the definition of ILI was changed to also include illnesses other than influenza.
This season’s higher flu activity is in contrast to the previous two, which were unusually mild. The change, however, is not unexpected, as William Schaffner, MD, an infectious disease expert and professor of preventive medicine at Vanderbilt University, recently told CNN.
“Here we are in the middle of October – not the middle of November – we’re already seeing scattered influenza cases, even hospitalized influenza cases, around the country,” he said. “So we know that this virus is now spreading out in the community already. It’s gathering speed already. It looks to me to be about a month early.”
One indication of the mildness of the previous two flu seasons was the number of deaths, both pediatric and overall. Influenza-associated pediatric deaths had averaged about 110 per season over the previous eight seasons, compared with just 1 for 2020-2021 and 43 in 2021-2022. Overall flu deaths never reached 1% of all weekly deaths for either season, well below baseline levels for the flu, which range from 5.5% to 6.8%, CDC data show.
Other indicators of early severity
This season’s early rise in viral activity also can be seen in hospitalizations. The cumulative rate of flu-related admissions was 1.5 per 100,000 population as of Oct. 22, higher than the rate observed in the comparable week of previous seasons going back to 2010-2011, according to the CDC’s Influenza Hospitalization Surveillance Network.
A look at state reports of ILI outpatient visit rates shows that the District of Columbia and South Carolina are already in the very high range of the CDC’s severity scale, while 11 states are in the high range. Again going back to 2010-2011, no jurisdiction has ever been in the very high range this early in the season, based on data from the Outpatient Influenza-like Illness Surveillance Network.
‘Unappreciated’ ties between COVID and gut dysbiosis
(BSIs), new research suggests.
“Collectively, these results reveal an unappreciated link between SARS-CoV-2 infection, gut microbiome dysbiosis, and a severe complication of COVID-19, BSIs,” the study team reported in Nature Communications.
“Our findings suggest that coronavirus infection directly interferes with the healthy balance of microbes in the gut, further endangering patients in the process,” microbiologist and co–senior author Ken Cadwell, PhD, New York University, added in a news release. “Now that we have uncovered the source of this bacterial imbalance, physicians can better identify those coronavirus patients most at risk of a secondary bloodstream infection.”
In a mouse model, the researchers first demonstrated that the SARS-CoV-2 infection alone induces gut microbiome dysbiosis and gut epithelial cell alterations, which correlate with markers of gut barrier permeability.
Next, they analyzed the bacterial composition of stool samples from 96 adults hospitalized with COVID-19 in 2020 in New York and New Haven, Conn.
In line with their observations in mice, they found that the SARS-CoV-2 infection is associated with “severe microbiome injury,” characterized by the loss of gut microbiome diversity.
They also observed an increase in populations of several microbes known to include antibiotic-resistant species. An analysis of stool samples paired with blood cultures found that antibiotic-resistant bacteria in the gut migrated to the bloodstream in 20% of patients.
This migration could be caused by a combination of the immune-compromising effects of the viral infection and the antibiotic-driven depletion of commensal gut microbes, the researchers said.
However, COVID-19 patients are also uniquely exposed to other potential factors predisposing them to bacteremia, including immunosuppressive drugs, long hospital stays, and catheters, the investigators noted. The study is limited in its ability to investigate the individual effects of these factors.
“Our findings support a scenario in which gut-to-blood translocation of microorganisms following microbiome dysbiosis leads to dangerous BSIs during COVID-19, a complication seen in other immunocompromised patients, including patients with cancer, acute respiratory distress syndrome, and in ICU patients receiving probiotics,” the researchers wrote.
Investigating the underlying mechanism behind their observations could help inform “the judicious application of antibiotics and immunosuppressives in patients with respiratory viral infections and increase our resilience to pandemics,” they added.
Funding for the study was provided by the National Institutes of Health, the Yale School of Public Health, and numerous other sources. Dr. Cadwell has received research support from Pfizer, Takeda, Pacific Biosciences, Genentech, and AbbVie; consulted for or received an honoraria from PureTech Health, Genentech, and AbbVie; and is named as an inventor on US patent 10,722,600 and provisional patents 62/935,035 and 63/157,225.
A version of this article first appeared on Medscape.com.
(BSIs), new research suggests.
“Collectively, these results reveal an unappreciated link between SARS-CoV-2 infection, gut microbiome dysbiosis, and a severe complication of COVID-19, BSIs,” the study team reported in Nature Communications.
“Our findings suggest that coronavirus infection directly interferes with the healthy balance of microbes in the gut, further endangering patients in the process,” microbiologist and co–senior author Ken Cadwell, PhD, New York University, added in a news release. “Now that we have uncovered the source of this bacterial imbalance, physicians can better identify those coronavirus patients most at risk of a secondary bloodstream infection.”
In a mouse model, the researchers first demonstrated that the SARS-CoV-2 infection alone induces gut microbiome dysbiosis and gut epithelial cell alterations, which correlate with markers of gut barrier permeability.
Next, they analyzed the bacterial composition of stool samples from 96 adults hospitalized with COVID-19 in 2020 in New York and New Haven, Conn.
In line with their observations in mice, they found that the SARS-CoV-2 infection is associated with “severe microbiome injury,” characterized by the loss of gut microbiome diversity.
They also observed an increase in populations of several microbes known to include antibiotic-resistant species. An analysis of stool samples paired with blood cultures found that antibiotic-resistant bacteria in the gut migrated to the bloodstream in 20% of patients.
This migration could be caused by a combination of the immune-compromising effects of the viral infection and the antibiotic-driven depletion of commensal gut microbes, the researchers said.
However, COVID-19 patients are also uniquely exposed to other potential factors predisposing them to bacteremia, including immunosuppressive drugs, long hospital stays, and catheters, the investigators noted. The study is limited in its ability to investigate the individual effects of these factors.
“Our findings support a scenario in which gut-to-blood translocation of microorganisms following microbiome dysbiosis leads to dangerous BSIs during COVID-19, a complication seen in other immunocompromised patients, including patients with cancer, acute respiratory distress syndrome, and in ICU patients receiving probiotics,” the researchers wrote.
Investigating the underlying mechanism behind their observations could help inform “the judicious application of antibiotics and immunosuppressives in patients with respiratory viral infections and increase our resilience to pandemics,” they added.
Funding for the study was provided by the National Institutes of Health, the Yale School of Public Health, and numerous other sources. Dr. Cadwell has received research support from Pfizer, Takeda, Pacific Biosciences, Genentech, and AbbVie; consulted for or received an honoraria from PureTech Health, Genentech, and AbbVie; and is named as an inventor on US patent 10,722,600 and provisional patents 62/935,035 and 63/157,225.
A version of this article first appeared on Medscape.com.
(BSIs), new research suggests.
“Collectively, these results reveal an unappreciated link between SARS-CoV-2 infection, gut microbiome dysbiosis, and a severe complication of COVID-19, BSIs,” the study team reported in Nature Communications.
“Our findings suggest that coronavirus infection directly interferes with the healthy balance of microbes in the gut, further endangering patients in the process,” microbiologist and co–senior author Ken Cadwell, PhD, New York University, added in a news release. “Now that we have uncovered the source of this bacterial imbalance, physicians can better identify those coronavirus patients most at risk of a secondary bloodstream infection.”
In a mouse model, the researchers first demonstrated that the SARS-CoV-2 infection alone induces gut microbiome dysbiosis and gut epithelial cell alterations, which correlate with markers of gut barrier permeability.
Next, they analyzed the bacterial composition of stool samples from 96 adults hospitalized with COVID-19 in 2020 in New York and New Haven, Conn.
In line with their observations in mice, they found that the SARS-CoV-2 infection is associated with “severe microbiome injury,” characterized by the loss of gut microbiome diversity.
They also observed an increase in populations of several microbes known to include antibiotic-resistant species. An analysis of stool samples paired with blood cultures found that antibiotic-resistant bacteria in the gut migrated to the bloodstream in 20% of patients.
This migration could be caused by a combination of the immune-compromising effects of the viral infection and the antibiotic-driven depletion of commensal gut microbes, the researchers said.
However, COVID-19 patients are also uniquely exposed to other potential factors predisposing them to bacteremia, including immunosuppressive drugs, long hospital stays, and catheters, the investigators noted. The study is limited in its ability to investigate the individual effects of these factors.
“Our findings support a scenario in which gut-to-blood translocation of microorganisms following microbiome dysbiosis leads to dangerous BSIs during COVID-19, a complication seen in other immunocompromised patients, including patients with cancer, acute respiratory distress syndrome, and in ICU patients receiving probiotics,” the researchers wrote.
Investigating the underlying mechanism behind their observations could help inform “the judicious application of antibiotics and immunosuppressives in patients with respiratory viral infections and increase our resilience to pandemics,” they added.
Funding for the study was provided by the National Institutes of Health, the Yale School of Public Health, and numerous other sources. Dr. Cadwell has received research support from Pfizer, Takeda, Pacific Biosciences, Genentech, and AbbVie; consulted for or received an honoraria from PureTech Health, Genentech, and AbbVie; and is named as an inventor on US patent 10,722,600 and provisional patents 62/935,035 and 63/157,225.
A version of this article first appeared on Medscape.com.
FROM NATURE COMMUNICATIONS
Children and COVID: Weekly cases can’t sustain downward trend
New COVID-19 cases in children inched up in late October, just 1 week after dipping to their lowest level in more than a year, and some measures of pediatric emergency visits and hospital admissions rose as well.
There was an 8% increase in the number of cases for the week of Oct. 21-27, compared with the previous week, but this week’s total was still below 25,000, and the overall trend since the beginning of September is still one of decline, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.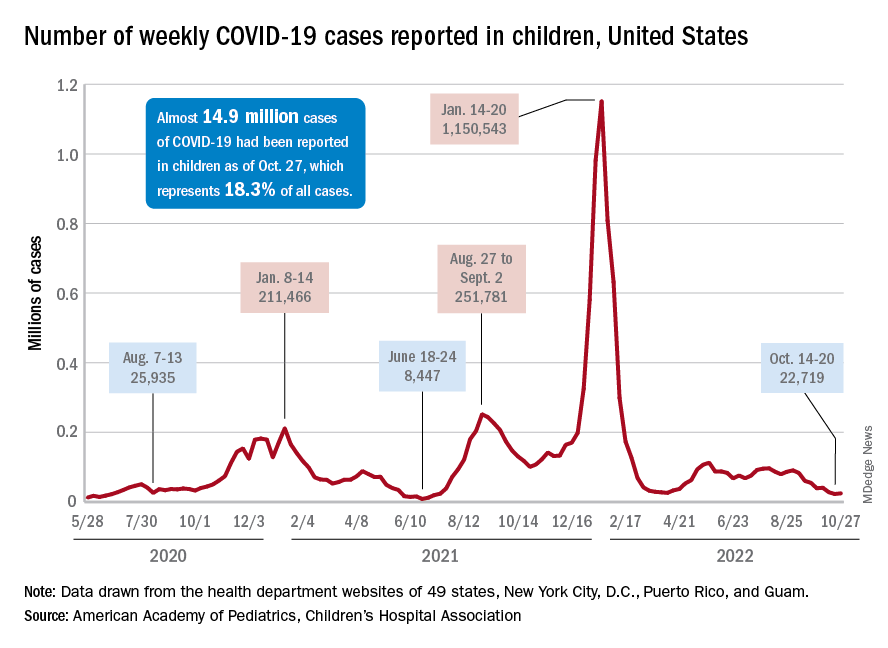
A similar increase can be seen for hospitalizations with confirmed COVID. The rate for children aged 0-17 years fell from 0.44 admissions per 100,000 population at the end of August to 0.16 per 100,000 on Oct. 23. Hospitalizations have since ticked up to 0.17 per 100,000, according to the Centers for Disease Control and Prevention.
Emergency department visits with diagnosed COVID among children aged 16-17 years, as a percentage of all ED visits, rose from 0.6% on Oct. 21 to 0.8% on Oct. 26. ED visits for 12- to 15-year-olds rose from 0.6% to 0.7% at about the same time, with both increases coming after declines that started in late August. No such increase has occurred yet among children aged 0-11 years, the CDC reported on its COVID Data Tracker.
One small milestone reached in the past week involved the proportion of all COVID cases that have occurred in children. The total number of child cases as of Oct. 27 was almost 14.9 million, which represents 18.3% of cases in all Americans, according to the AAP and CHA. That figure had been sitting at 18.4% since mid-August after reaching as high as 19.0% during the spring.
The CDC puts total COVID-related hospital admissions for children aged 0-17 at 163,588 since Aug. 1, 2020, which is 3.0% of all U.S. admissions. Total pediatric deaths number 1,843, or just about 0.2% of all COVID-related fatalities since the start of the pandemic, the CDC data show.
The latest vaccination figures show that 71.3% of children aged 12-17 years have received at least one dose, as have 38.8% of 5- to 11-year-olds, 8.4% of 2- to 4-year-olds, and 5.5% of those under age 2. Full vaccination by age group looks like this: 60.9% (12-17 years), 31.7% (5-11 years), 3.7% (2-4 years), and 2.1% (<2 years), the CDC reported. Almost 30% of children aged 12-17 have gotten a first booster dose, as have 16% of 5- to 11-year-olds.
New COVID-19 cases in children inched up in late October, just 1 week after dipping to their lowest level in more than a year, and some measures of pediatric emergency visits and hospital admissions rose as well.
There was an 8% increase in the number of cases for the week of Oct. 21-27, compared with the previous week, but this week’s total was still below 25,000, and the overall trend since the beginning of September is still one of decline, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
A similar increase can be seen for hospitalizations with confirmed COVID. The rate for children aged 0-17 years fell from 0.44 admissions per 100,000 population at the end of August to 0.16 per 100,000 on Oct. 23. Hospitalizations have since ticked up to 0.17 per 100,000, according to the Centers for Disease Control and Prevention.
Emergency department visits with diagnosed COVID among children aged 16-17 years, as a percentage of all ED visits, rose from 0.6% on Oct. 21 to 0.8% on Oct. 26. ED visits for 12- to 15-year-olds rose from 0.6% to 0.7% at about the same time, with both increases coming after declines that started in late August. No such increase has occurred yet among children aged 0-11 years, the CDC reported on its COVID Data Tracker.
One small milestone reached in the past week involved the proportion of all COVID cases that have occurred in children. The total number of child cases as of Oct. 27 was almost 14.9 million, which represents 18.3% of cases in all Americans, according to the AAP and CHA. That figure had been sitting at 18.4% since mid-August after reaching as high as 19.0% during the spring.
The CDC puts total COVID-related hospital admissions for children aged 0-17 at 163,588 since Aug. 1, 2020, which is 3.0% of all U.S. admissions. Total pediatric deaths number 1,843, or just about 0.2% of all COVID-related fatalities since the start of the pandemic, the CDC data show.
The latest vaccination figures show that 71.3% of children aged 12-17 years have received at least one dose, as have 38.8% of 5- to 11-year-olds, 8.4% of 2- to 4-year-olds, and 5.5% of those under age 2. Full vaccination by age group looks like this: 60.9% (12-17 years), 31.7% (5-11 years), 3.7% (2-4 years), and 2.1% (<2 years), the CDC reported. Almost 30% of children aged 12-17 have gotten a first booster dose, as have 16% of 5- to 11-year-olds.
New COVID-19 cases in children inched up in late October, just 1 week after dipping to their lowest level in more than a year, and some measures of pediatric emergency visits and hospital admissions rose as well.
There was an 8% increase in the number of cases for the week of Oct. 21-27, compared with the previous week, but this week’s total was still below 25,000, and the overall trend since the beginning of September is still one of decline, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
A similar increase can be seen for hospitalizations with confirmed COVID. The rate for children aged 0-17 years fell from 0.44 admissions per 100,000 population at the end of August to 0.16 per 100,000 on Oct. 23. Hospitalizations have since ticked up to 0.17 per 100,000, according to the Centers for Disease Control and Prevention.
Emergency department visits with diagnosed COVID among children aged 16-17 years, as a percentage of all ED visits, rose from 0.6% on Oct. 21 to 0.8% on Oct. 26. ED visits for 12- to 15-year-olds rose from 0.6% to 0.7% at about the same time, with both increases coming after declines that started in late August. No such increase has occurred yet among children aged 0-11 years, the CDC reported on its COVID Data Tracker.
One small milestone reached in the past week involved the proportion of all COVID cases that have occurred in children. The total number of child cases as of Oct. 27 was almost 14.9 million, which represents 18.3% of cases in all Americans, according to the AAP and CHA. That figure had been sitting at 18.4% since mid-August after reaching as high as 19.0% during the spring.
The CDC puts total COVID-related hospital admissions for children aged 0-17 at 163,588 since Aug. 1, 2020, which is 3.0% of all U.S. admissions. Total pediatric deaths number 1,843, or just about 0.2% of all COVID-related fatalities since the start of the pandemic, the CDC data show.
The latest vaccination figures show that 71.3% of children aged 12-17 years have received at least one dose, as have 38.8% of 5- to 11-year-olds, 8.4% of 2- to 4-year-olds, and 5.5% of those under age 2. Full vaccination by age group looks like this: 60.9% (12-17 years), 31.7% (5-11 years), 3.7% (2-4 years), and 2.1% (<2 years), the CDC reported. Almost 30% of children aged 12-17 have gotten a first booster dose, as have 16% of 5- to 11-year-olds.
Original COVID-19 vaccines fall short against Omicron subvariants for the immunocompromised
The effectiveness of up to three doses of COVID-19 vaccine was moderate overall and significantly lower among individuals with immunocompromising conditions, compared with the general population during the period of Omicron dominance, according to an analysis of data from more than 34,000 hospitalizations.
Previous studies have suggested lower COVID-19 vaccine effectiveness among immunocompromised individuals, compared with healthy individuals from the general population, but data from the period in which Omicron subvariants have been dominant are limited, wrote Amadea Britton, MD, of the Centers for Disease Control and Prevention’s COVID-19 Emergency Response Team, and colleagues.
The CDC currently recommends an expanded primary vaccine series of three doses of an mRNA vaccine, and the Advisory Committee on Immunization Practices has recommended a fourth dose with the new bivalent booster that contains elements of the Omicron variant, the researchers noted.
In a study published in the CDC’s Morbidity and Mortality Weekly Report, the researchers identified 34,220 adults with immunocompromising conditions who were hospitalized for COVID-19–like illness between Dec. 16, 2021, and Aug. 20, 2022. These conditions included solid malignancy (40.5%), hematologic malignancy (14.6%), rheumatologic or inflammatory disorder (24.4%), other intrinsic immune condition or immunodeficiency (38.5%), or organ or stem cell transplant (8.6%). They used data from the CDC’s VISION Network, a multistate database. The data include spring and summer 2022, when the BA.4 and BA.5 Omicron subvariants dominated other strains, and adults with immunocompromising conditions were eligible for a total of four vaccine doses (two primary doses and two boosters). The median age of the study population was 69 years, and 25.7%, 41.7%, and 7.0% had received two, three, and four doses, respectively, of COVID-19 vaccine.
Overall, vaccine effectiveness (VE) among immunocompromised patients was 34% after two vaccine doses, increasing to 71% during days 7-89 after a third dose, then declining to 41% 90 days or more after that dose.
During the full Omicron period, VE was 36% for 14 or more days after dose two, 69% for 7-89 days after dose three, and 44% for 90 or more days after dose three.
When VE was stratified by sublineage period, VE was higher 7 or more days after dose three during the predominance of BA.1 (67%), compared with VE during the dominant periods of BA.2/BA.2.12.1 (32%) and BA.4/BA.5 (35%).
In the later periods when Omicron BA.2/BA.2.12.1 and BA.4/BA.5 variants dominated, and individuals who had received three doses of vaccine were eligible for a fourth, VE against these variants was 32% 90 or more days after dose three and 43% 7 or more days after dose four.
VE was lowest among individuals with potentially more severe immunocompromising conditions, notably solid organ or stem cell transplants, the researchers wrote in their discussion.
The study findings were limited by several factors including the use of ICD-9 and -10 discharge diagnosis codes for immunocompromising conditions, potential confounding in VE models, lack of data on outpatient treatments such as nirmatelvir/ritonavir (Paxlovid), and lack of COVID-19 genomic sequencing data that may have affected which sublineage was identified, the researchers noted.
However, “this study confirms that even with boosters, immunocompromised adults, because of their weakened immune systems, are still at high risk of moderate to severe COVID,” said coauthor Brian Dixon, PhD, of the Regenstrief Institute and Indiana University Richard M. Fairbanks School of Public Health, Indianapolis, in a press release about the study.
“Given the incomplete protection against hospitalization afforded by monovalent COVID-19 vaccines, persons with immunocompromising conditions might benefit from updated bivalent vaccine booster doses that target recently circulating Omicron sublineages, in line with ACIP [Advisory Committee on Immunization Practices] recommendations,” the researchers concluded in the study.
The study was funded by the CDC. The researchers had no financial conflicts to disclose. The VISION Network is a collaboration between the CDC, the Regenstrief Institute, and seven health care systems across the United States: Columbia University Irving Medical Center (New York), HealthPartners (Wisconsin), Intermountain Healthcare (Utah), Kaiser Permanente Northern California, Kaiser Permanente Northwest (Washington State), the University of Colorado, and Paso Del Norte Health Information Exchange (Texas).
The effectiveness of up to three doses of COVID-19 vaccine was moderate overall and significantly lower among individuals with immunocompromising conditions, compared with the general population during the period of Omicron dominance, according to an analysis of data from more than 34,000 hospitalizations.
Previous studies have suggested lower COVID-19 vaccine effectiveness among immunocompromised individuals, compared with healthy individuals from the general population, but data from the period in which Omicron subvariants have been dominant are limited, wrote Amadea Britton, MD, of the Centers for Disease Control and Prevention’s COVID-19 Emergency Response Team, and colleagues.
The CDC currently recommends an expanded primary vaccine series of three doses of an mRNA vaccine, and the Advisory Committee on Immunization Practices has recommended a fourth dose with the new bivalent booster that contains elements of the Omicron variant, the researchers noted.
In a study published in the CDC’s Morbidity and Mortality Weekly Report, the researchers identified 34,220 adults with immunocompromising conditions who were hospitalized for COVID-19–like illness between Dec. 16, 2021, and Aug. 20, 2022. These conditions included solid malignancy (40.5%), hematologic malignancy (14.6%), rheumatologic or inflammatory disorder (24.4%), other intrinsic immune condition or immunodeficiency (38.5%), or organ or stem cell transplant (8.6%). They used data from the CDC’s VISION Network, a multistate database. The data include spring and summer 2022, when the BA.4 and BA.5 Omicron subvariants dominated other strains, and adults with immunocompromising conditions were eligible for a total of four vaccine doses (two primary doses and two boosters). The median age of the study population was 69 years, and 25.7%, 41.7%, and 7.0% had received two, three, and four doses, respectively, of COVID-19 vaccine.
Overall, vaccine effectiveness (VE) among immunocompromised patients was 34% after two vaccine doses, increasing to 71% during days 7-89 after a third dose, then declining to 41% 90 days or more after that dose.
During the full Omicron period, VE was 36% for 14 or more days after dose two, 69% for 7-89 days after dose three, and 44% for 90 or more days after dose three.
When VE was stratified by sublineage period, VE was higher 7 or more days after dose three during the predominance of BA.1 (67%), compared with VE during the dominant periods of BA.2/BA.2.12.1 (32%) and BA.4/BA.5 (35%).
In the later periods when Omicron BA.2/BA.2.12.1 and BA.4/BA.5 variants dominated, and individuals who had received three doses of vaccine were eligible for a fourth, VE against these variants was 32% 90 or more days after dose three and 43% 7 or more days after dose four.
VE was lowest among individuals with potentially more severe immunocompromising conditions, notably solid organ or stem cell transplants, the researchers wrote in their discussion.
The study findings were limited by several factors including the use of ICD-9 and -10 discharge diagnosis codes for immunocompromising conditions, potential confounding in VE models, lack of data on outpatient treatments such as nirmatelvir/ritonavir (Paxlovid), and lack of COVID-19 genomic sequencing data that may have affected which sublineage was identified, the researchers noted.
However, “this study confirms that even with boosters, immunocompromised adults, because of their weakened immune systems, are still at high risk of moderate to severe COVID,” said coauthor Brian Dixon, PhD, of the Regenstrief Institute and Indiana University Richard M. Fairbanks School of Public Health, Indianapolis, in a press release about the study.
“Given the incomplete protection against hospitalization afforded by monovalent COVID-19 vaccines, persons with immunocompromising conditions might benefit from updated bivalent vaccine booster doses that target recently circulating Omicron sublineages, in line with ACIP [Advisory Committee on Immunization Practices] recommendations,” the researchers concluded in the study.
The study was funded by the CDC. The researchers had no financial conflicts to disclose. The VISION Network is a collaboration between the CDC, the Regenstrief Institute, and seven health care systems across the United States: Columbia University Irving Medical Center (New York), HealthPartners (Wisconsin), Intermountain Healthcare (Utah), Kaiser Permanente Northern California, Kaiser Permanente Northwest (Washington State), the University of Colorado, and Paso Del Norte Health Information Exchange (Texas).
The effectiveness of up to three doses of COVID-19 vaccine was moderate overall and significantly lower among individuals with immunocompromising conditions, compared with the general population during the period of Omicron dominance, according to an analysis of data from more than 34,000 hospitalizations.
Previous studies have suggested lower COVID-19 vaccine effectiveness among immunocompromised individuals, compared with healthy individuals from the general population, but data from the period in which Omicron subvariants have been dominant are limited, wrote Amadea Britton, MD, of the Centers for Disease Control and Prevention’s COVID-19 Emergency Response Team, and colleagues.
The CDC currently recommends an expanded primary vaccine series of three doses of an mRNA vaccine, and the Advisory Committee on Immunization Practices has recommended a fourth dose with the new bivalent booster that contains elements of the Omicron variant, the researchers noted.
In a study published in the CDC’s Morbidity and Mortality Weekly Report, the researchers identified 34,220 adults with immunocompromising conditions who were hospitalized for COVID-19–like illness between Dec. 16, 2021, and Aug. 20, 2022. These conditions included solid malignancy (40.5%), hematologic malignancy (14.6%), rheumatologic or inflammatory disorder (24.4%), other intrinsic immune condition or immunodeficiency (38.5%), or organ or stem cell transplant (8.6%). They used data from the CDC’s VISION Network, a multistate database. The data include spring and summer 2022, when the BA.4 and BA.5 Omicron subvariants dominated other strains, and adults with immunocompromising conditions were eligible for a total of four vaccine doses (two primary doses and two boosters). The median age of the study population was 69 years, and 25.7%, 41.7%, and 7.0% had received two, three, and four doses, respectively, of COVID-19 vaccine.
Overall, vaccine effectiveness (VE) among immunocompromised patients was 34% after two vaccine doses, increasing to 71% during days 7-89 after a third dose, then declining to 41% 90 days or more after that dose.
During the full Omicron period, VE was 36% for 14 or more days after dose two, 69% for 7-89 days after dose three, and 44% for 90 or more days after dose three.
When VE was stratified by sublineage period, VE was higher 7 or more days after dose three during the predominance of BA.1 (67%), compared with VE during the dominant periods of BA.2/BA.2.12.1 (32%) and BA.4/BA.5 (35%).
In the later periods when Omicron BA.2/BA.2.12.1 and BA.4/BA.5 variants dominated, and individuals who had received three doses of vaccine were eligible for a fourth, VE against these variants was 32% 90 or more days after dose three and 43% 7 or more days after dose four.
VE was lowest among individuals with potentially more severe immunocompromising conditions, notably solid organ or stem cell transplants, the researchers wrote in their discussion.
The study findings were limited by several factors including the use of ICD-9 and -10 discharge diagnosis codes for immunocompromising conditions, potential confounding in VE models, lack of data on outpatient treatments such as nirmatelvir/ritonavir (Paxlovid), and lack of COVID-19 genomic sequencing data that may have affected which sublineage was identified, the researchers noted.
However, “this study confirms that even with boosters, immunocompromised adults, because of their weakened immune systems, are still at high risk of moderate to severe COVID,” said coauthor Brian Dixon, PhD, of the Regenstrief Institute and Indiana University Richard M. Fairbanks School of Public Health, Indianapolis, in a press release about the study.
“Given the incomplete protection against hospitalization afforded by monovalent COVID-19 vaccines, persons with immunocompromising conditions might benefit from updated bivalent vaccine booster doses that target recently circulating Omicron sublineages, in line with ACIP [Advisory Committee on Immunization Practices] recommendations,” the researchers concluded in the study.
The study was funded by the CDC. The researchers had no financial conflicts to disclose. The VISION Network is a collaboration between the CDC, the Regenstrief Institute, and seven health care systems across the United States: Columbia University Irving Medical Center (New York), HealthPartners (Wisconsin), Intermountain Healthcare (Utah), Kaiser Permanente Northern California, Kaiser Permanente Northwest (Washington State), the University of Colorado, and Paso Del Norte Health Information Exchange (Texas).
FROM MMWR
Thyroid dysfunction may linger a year after severe COVID-19
MONTREAL – Patients hospitalized with severe COVID-19 and no prior history of thyroid dysfunction show signs of thyroiditis that, although asymptomatic, continue to persist for up to a year after infection, according to research that adds to evidence on the complex involvement of the thyroid in COVID-19.
“To our knowledge these findings are novel,” first author Ilaria Muller, MD, PhD, an assistant professor in endocrinology in the department of clinical sciences and community health, University of Milan, told this news organization.
“Little has been written about the long-term follow-up of thyroid function after severe COVID-19 disease, and we have followed patients up to 1 year after infection.”
The effects are seen in about 10%-15% of patients, and “[while] the thyroid dysfunction is transient, ultrasound areas of thyroiditis may persist after 1 year, even if they progressively shrink,” said Dr. Muller, who presented the findings at the American Thyroid Association annual meeting.
Immunological scars? Clinical implications unclear
The nature and implications of the persistent thyroiditis areas are uncertain, Dr. Muller noted. “These areas of thyroiditis are likely a sort of ‘immunologic scar’ of the previous SARS-CoV-2 infection,” she explained. “We still don’t know if there are clinical implications, even if they seem unlikely.”
Of note, increases in autoimmune processes or a higher incidence of thyroid dysfunction after COVID-19 have not been observed, and the shrinkage of the areas of thyroiditis over time is encouraging, she said.
The reasons why some patients develop atypical thyroiditis and others don’t are also unclear, with Dr. Muller’s team investigating further. Importantly, similar effects have been associated with other severe infections, not just COVID-19. “It is well known that in classic subacute thyroiditis due to other viral infections, the areas of thyroiditis persist for months, so this phenomenon might not be unique to COVID-19,” she explained.
Commenting on the story, Jeffrey R. Garber, MD, also noted that such thyroiditis areas stemming from other types of infection may persist – but go unnoticed.
“Resolution is the clinical rule, [and] we generally do not restudy in detail those who clinically recover,” he said in an interview. “However, there is evidence of impaired thyroid reserve in those who recover from viral thyroiditis due to other sources.”
“Thyroid symptoms are often not specific, so ‘atypical’ [cases] are common, [and] resolution with restoring thyroid status to normal is mixed,” noted Dr. Garber, an associate professor of medicine at Harvard Medical School and chief of the division of endocrinology at Atrius Health, Boston.
In terms of clinical practice, while such issues should be kept in mind when evaluating abnormal thyroid tests during severe COVID-19, “it is not a call for routinely checking it in the absence of clinical suspicion,” he observed.
Study details
Dr. Muller and her team previously observed that patients hospitalized in intensive care with COVID-19 often had low or suppressed serum thyroid-stimulating hormone (TSH) levels, with and without elevated free thyroxine (FT4) concentrations, suggestive of thyrotoxicosis.
Upon investigating those cases, they found, as in their previous study reported by this news organization, that a painless, atypical thyroiditis occurs with nonthyroidal illness syndrome among patients hospitalized with severe COVID-19. The atypical thyroiditis was slightly more common in men and was associated with lymphopenia.
To further investigate those cases and follow patients up to 1 year, the team conducted a longitudinal study of 183 patients hospitalized with severe COVID-19 in Italy. The patients, who had no known prior history of thyroid dysfunction, were assessed for serum thyroid function, autoantibodies, and inflammatory markers.
At baseline, 10% of the patients were found to have thyrotoxicosis, and ultrasound performed within 2-3 months postinfection on 65 patients showed that 18 (28%) had areas of thyroiditis.
Importantly, 60% of those patients with the areas of thyroiditis had low TSH levels, while 25% had normal TSH levels (P = .034).
In addition, those showing the presence of thyroiditis on ultrasound at 23 months were more likely to have elevated serum concentrations of FT4 (P = .018) and higher levels of interleukin-26 (P = .016), compared with those with normal ultrasound readings.
In a longitudinal analysis further following patients post infection, among 15 patients who were evaluated at 6 months, most, 13 (87%), still had areas of thyroiditis, and 6 of 12 (50%) had thyroiditis areas that, though reduced in size, still persisted even at 12 months.
In terms of thyroid uptake, at 3 months, 14 of 17 patients (82%) had diffused or focal areas of a reduction of uptake. After 6 months, there was a recovery, with a median of 28% of thyroid uptake recovered, however, 67% of patients still had some focal or diffused reduction in thyroid uptake.
Of note, the indications of thyroiditis on imaging persisted even though patients’ TSH levels had quickly normalized at the end of infection and remained normal up to 1 year of follow-up.
The patients showed no apparent development of thyroglobulin antibody, thyroid peroxidase antibodies, or TSH receptor antibodies.
A further fine needle aspiration analysis of eight patients with atypical thyroiditis at 3 months after infection showed that those patients had tissue resident memory T cells (CD4+/CD8+/CD103+/CD69+) within the thyroid, but not in the blood as expected.
Additional assessments at 8 months after infection showed those tissue resident memory T cells continued to be present on imaging.
The results showed “SARS-CoV-2–specific T cells were enriched within the thyroid compared with the blood, many with a tissue resident phenotype,” Dr. Muller explained.
The findings are notable in that “such an in-depth characterization of areas of thyroiditis triggered by SARS-CoV-2 infection combining ultrasound, scintigraphy, and immunological phenotyping has not been performed so far,” she said.
“In particular, SARS-CoV-2–specific tissue-resident memory T lymphocytes have not been described before in the thyroid gland.”
Dr. Muller and Dr. Garber have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MONTREAL – Patients hospitalized with severe COVID-19 and no prior history of thyroid dysfunction show signs of thyroiditis that, although asymptomatic, continue to persist for up to a year after infection, according to research that adds to evidence on the complex involvement of the thyroid in COVID-19.
“To our knowledge these findings are novel,” first author Ilaria Muller, MD, PhD, an assistant professor in endocrinology in the department of clinical sciences and community health, University of Milan, told this news organization.
“Little has been written about the long-term follow-up of thyroid function after severe COVID-19 disease, and we have followed patients up to 1 year after infection.”
The effects are seen in about 10%-15% of patients, and “[while] the thyroid dysfunction is transient, ultrasound areas of thyroiditis may persist after 1 year, even if they progressively shrink,” said Dr. Muller, who presented the findings at the American Thyroid Association annual meeting.
Immunological scars? Clinical implications unclear
The nature and implications of the persistent thyroiditis areas are uncertain, Dr. Muller noted. “These areas of thyroiditis are likely a sort of ‘immunologic scar’ of the previous SARS-CoV-2 infection,” she explained. “We still don’t know if there are clinical implications, even if they seem unlikely.”
Of note, increases in autoimmune processes or a higher incidence of thyroid dysfunction after COVID-19 have not been observed, and the shrinkage of the areas of thyroiditis over time is encouraging, she said.
The reasons why some patients develop atypical thyroiditis and others don’t are also unclear, with Dr. Muller’s team investigating further. Importantly, similar effects have been associated with other severe infections, not just COVID-19. “It is well known that in classic subacute thyroiditis due to other viral infections, the areas of thyroiditis persist for months, so this phenomenon might not be unique to COVID-19,” she explained.
Commenting on the story, Jeffrey R. Garber, MD, also noted that such thyroiditis areas stemming from other types of infection may persist – but go unnoticed.
“Resolution is the clinical rule, [and] we generally do not restudy in detail those who clinically recover,” he said in an interview. “However, there is evidence of impaired thyroid reserve in those who recover from viral thyroiditis due to other sources.”
“Thyroid symptoms are often not specific, so ‘atypical’ [cases] are common, [and] resolution with restoring thyroid status to normal is mixed,” noted Dr. Garber, an associate professor of medicine at Harvard Medical School and chief of the division of endocrinology at Atrius Health, Boston.
In terms of clinical practice, while such issues should be kept in mind when evaluating abnormal thyroid tests during severe COVID-19, “it is not a call for routinely checking it in the absence of clinical suspicion,” he observed.
Study details
Dr. Muller and her team previously observed that patients hospitalized in intensive care with COVID-19 often had low or suppressed serum thyroid-stimulating hormone (TSH) levels, with and without elevated free thyroxine (FT4) concentrations, suggestive of thyrotoxicosis.
Upon investigating those cases, they found, as in their previous study reported by this news organization, that a painless, atypical thyroiditis occurs with nonthyroidal illness syndrome among patients hospitalized with severe COVID-19. The atypical thyroiditis was slightly more common in men and was associated with lymphopenia.
To further investigate those cases and follow patients up to 1 year, the team conducted a longitudinal study of 183 patients hospitalized with severe COVID-19 in Italy. The patients, who had no known prior history of thyroid dysfunction, were assessed for serum thyroid function, autoantibodies, and inflammatory markers.
At baseline, 10% of the patients were found to have thyrotoxicosis, and ultrasound performed within 2-3 months postinfection on 65 patients showed that 18 (28%) had areas of thyroiditis.
Importantly, 60% of those patients with the areas of thyroiditis had low TSH levels, while 25% had normal TSH levels (P = .034).
In addition, those showing the presence of thyroiditis on ultrasound at 23 months were more likely to have elevated serum concentrations of FT4 (P = .018) and higher levels of interleukin-26 (P = .016), compared with those with normal ultrasound readings.
In a longitudinal analysis further following patients post infection, among 15 patients who were evaluated at 6 months, most, 13 (87%), still had areas of thyroiditis, and 6 of 12 (50%) had thyroiditis areas that, though reduced in size, still persisted even at 12 months.
In terms of thyroid uptake, at 3 months, 14 of 17 patients (82%) had diffused or focal areas of a reduction of uptake. After 6 months, there was a recovery, with a median of 28% of thyroid uptake recovered, however, 67% of patients still had some focal or diffused reduction in thyroid uptake.
Of note, the indications of thyroiditis on imaging persisted even though patients’ TSH levels had quickly normalized at the end of infection and remained normal up to 1 year of follow-up.
The patients showed no apparent development of thyroglobulin antibody, thyroid peroxidase antibodies, or TSH receptor antibodies.
A further fine needle aspiration analysis of eight patients with atypical thyroiditis at 3 months after infection showed that those patients had tissue resident memory T cells (CD4+/CD8+/CD103+/CD69+) within the thyroid, but not in the blood as expected.
Additional assessments at 8 months after infection showed those tissue resident memory T cells continued to be present on imaging.
The results showed “SARS-CoV-2–specific T cells were enriched within the thyroid compared with the blood, many with a tissue resident phenotype,” Dr. Muller explained.
The findings are notable in that “such an in-depth characterization of areas of thyroiditis triggered by SARS-CoV-2 infection combining ultrasound, scintigraphy, and immunological phenotyping has not been performed so far,” she said.
“In particular, SARS-CoV-2–specific tissue-resident memory T lymphocytes have not been described before in the thyroid gland.”
Dr. Muller and Dr. Garber have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MONTREAL – Patients hospitalized with severe COVID-19 and no prior history of thyroid dysfunction show signs of thyroiditis that, although asymptomatic, continue to persist for up to a year after infection, according to research that adds to evidence on the complex involvement of the thyroid in COVID-19.
“To our knowledge these findings are novel,” first author Ilaria Muller, MD, PhD, an assistant professor in endocrinology in the department of clinical sciences and community health, University of Milan, told this news organization.
“Little has been written about the long-term follow-up of thyroid function after severe COVID-19 disease, and we have followed patients up to 1 year after infection.”
The effects are seen in about 10%-15% of patients, and “[while] the thyroid dysfunction is transient, ultrasound areas of thyroiditis may persist after 1 year, even if they progressively shrink,” said Dr. Muller, who presented the findings at the American Thyroid Association annual meeting.
Immunological scars? Clinical implications unclear
The nature and implications of the persistent thyroiditis areas are uncertain, Dr. Muller noted. “These areas of thyroiditis are likely a sort of ‘immunologic scar’ of the previous SARS-CoV-2 infection,” she explained. “We still don’t know if there are clinical implications, even if they seem unlikely.”
Of note, increases in autoimmune processes or a higher incidence of thyroid dysfunction after COVID-19 have not been observed, and the shrinkage of the areas of thyroiditis over time is encouraging, she said.
The reasons why some patients develop atypical thyroiditis and others don’t are also unclear, with Dr. Muller’s team investigating further. Importantly, similar effects have been associated with other severe infections, not just COVID-19. “It is well known that in classic subacute thyroiditis due to other viral infections, the areas of thyroiditis persist for months, so this phenomenon might not be unique to COVID-19,” she explained.
Commenting on the story, Jeffrey R. Garber, MD, also noted that such thyroiditis areas stemming from other types of infection may persist – but go unnoticed.
“Resolution is the clinical rule, [and] we generally do not restudy in detail those who clinically recover,” he said in an interview. “However, there is evidence of impaired thyroid reserve in those who recover from viral thyroiditis due to other sources.”
“Thyroid symptoms are often not specific, so ‘atypical’ [cases] are common, [and] resolution with restoring thyroid status to normal is mixed,” noted Dr. Garber, an associate professor of medicine at Harvard Medical School and chief of the division of endocrinology at Atrius Health, Boston.
In terms of clinical practice, while such issues should be kept in mind when evaluating abnormal thyroid tests during severe COVID-19, “it is not a call for routinely checking it in the absence of clinical suspicion,” he observed.
Study details
Dr. Muller and her team previously observed that patients hospitalized in intensive care with COVID-19 often had low or suppressed serum thyroid-stimulating hormone (TSH) levels, with and without elevated free thyroxine (FT4) concentrations, suggestive of thyrotoxicosis.
Upon investigating those cases, they found, as in their previous study reported by this news organization, that a painless, atypical thyroiditis occurs with nonthyroidal illness syndrome among patients hospitalized with severe COVID-19. The atypical thyroiditis was slightly more common in men and was associated with lymphopenia.
To further investigate those cases and follow patients up to 1 year, the team conducted a longitudinal study of 183 patients hospitalized with severe COVID-19 in Italy. The patients, who had no known prior history of thyroid dysfunction, were assessed for serum thyroid function, autoantibodies, and inflammatory markers.
At baseline, 10% of the patients were found to have thyrotoxicosis, and ultrasound performed within 2-3 months postinfection on 65 patients showed that 18 (28%) had areas of thyroiditis.
Importantly, 60% of those patients with the areas of thyroiditis had low TSH levels, while 25% had normal TSH levels (P = .034).
In addition, those showing the presence of thyroiditis on ultrasound at 23 months were more likely to have elevated serum concentrations of FT4 (P = .018) and higher levels of interleukin-26 (P = .016), compared with those with normal ultrasound readings.
In a longitudinal analysis further following patients post infection, among 15 patients who were evaluated at 6 months, most, 13 (87%), still had areas of thyroiditis, and 6 of 12 (50%) had thyroiditis areas that, though reduced in size, still persisted even at 12 months.
In terms of thyroid uptake, at 3 months, 14 of 17 patients (82%) had diffused or focal areas of a reduction of uptake. After 6 months, there was a recovery, with a median of 28% of thyroid uptake recovered, however, 67% of patients still had some focal or diffused reduction in thyroid uptake.
Of note, the indications of thyroiditis on imaging persisted even though patients’ TSH levels had quickly normalized at the end of infection and remained normal up to 1 year of follow-up.
The patients showed no apparent development of thyroglobulin antibody, thyroid peroxidase antibodies, or TSH receptor antibodies.
A further fine needle aspiration analysis of eight patients with atypical thyroiditis at 3 months after infection showed that those patients had tissue resident memory T cells (CD4+/CD8+/CD103+/CD69+) within the thyroid, but not in the blood as expected.
Additional assessments at 8 months after infection showed those tissue resident memory T cells continued to be present on imaging.
The results showed “SARS-CoV-2–specific T cells were enriched within the thyroid compared with the blood, many with a tissue resident phenotype,” Dr. Muller explained.
The findings are notable in that “such an in-depth characterization of areas of thyroiditis triggered by SARS-CoV-2 infection combining ultrasound, scintigraphy, and immunological phenotyping has not been performed so far,” she said.
“In particular, SARS-CoV-2–specific tissue-resident memory T lymphocytes have not been described before in the thyroid gland.”
Dr. Muller and Dr. Garber have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ATA 2022