User login
The family physician’s role in long COVID management
Several years into the pandemic, COVID-19 continues to deeply impact our society; at the time of publication of this review, 98.8 million cases in the United States have been reported to the Centers for Disease Control and Prevention (CDC).1 Although many people recover well from infection, there is mounting concern regarding long-term sequelae of COVID-19. These long-term symptoms have been termed long COVID, among other names.
What exactly is long COVID?
The CDC and National Institutes of Health define long COVID as new or ongoing health problems experienced ≥ 4 weeks after initial infection.2 Evidence suggests that even people who have mild initial COVID-19 symptoms are at risk for long COVID.
Available data about long COVID are imperfect, however; much about the condition remains poorly understood. For example, there is little evidence regarding the effect of vaccination and viral variants on the prevalence of long COVID. A recent study of more than 13 million people from the US Department of Veterans Affairs database did demonstrate that vaccination against SARS-CoV-2 lowered the risk for long COVID by only about 15%.3
Persistent symptoms associated with long COVID often lead to disability and decreased quality of life. Furthermore, long COVID is a challenge to treat because there is a paucity of evidence to guide COVID-19 treatment beyond initial infection.
Because many patients who have ongoing COVID-19 symptoms will be seen in primary care, it is important to understand how to manage and support them. In this article, we discuss current understanding of long COVID epidemiology, symptoms that can persist 4 weeks after initial infection, and potential treatment options.
Prevalence and diagnosis
The prevalence of long COVID is not well defined because many epidemiologic studies rely on self-reporting. The CDC reports that 20% to 25% of COVID-19 survivors experience a new condition that might be attributable to their initial infection.4 Other studies variously cite 5% to 85% of people who have had a diagnosis of COVID-19 as experiencing long COVID, although that rate more consistently appears to be 10% to 30%.5

A study of adult patients in France found that self-reported symptoms of long COVID, 10 to 12 months after the first wave of the pandemic (May through November 2020), were associated with the belief of having had COVID-19 but not necessarily with having tested positive for anti-SARS-CoV-2 antibodies,6 which indicates prior COVID-19. This complicates research on long COVID because, first, there is no specific test to confirm a diagnosis of long COVID and, second, studies often rely on self-reporting of earlier COVID-19.
Continue to: As such, long COVID...
As such, long COVID is diagnosed primarily through a medical history and physical examination. The medical history provides a guide as to whether additional testing is warranted to evaluate for known complications of COVID-19, such as deep vein thrombosis, pulmonary embolism, myocarditis, and pulmonary fibrosis. As of October 1, 2021, a new International Classification of Disease (10th Revision) code went into effect for post COVID condition, unspecified (U09.9).7
The prevalence of long COVID symptoms appears to increase with age. Among patients whose disease was diagnosed using code U09.9, most were 36 to 64 years of age; children and adults ages 22 years or younger constituted only 10.5% of diagnoses.7 Long COVID symptoms might also be more prevalent among women and in people with a preexisting chronic comorbidity.2,7
Symptoms can be numerous, severe or mild, and lasting
Initially, there was no widely accepted definition of long COVID; follow-up in early studies ranged from 21 days to 2 years after initial infection (or from discharge, for hospitalized patients).8 Differences in descriptions that have been used on surveys to self-report symptoms make it a challenge to clearly summarize the frequency of each aspect of long COVID.
Long COVID can be mild or debilitating; severity can fluctuate. Common symptoms include fatigue, dyspnea or other breathing difficulties, headache, and cognitive dysfunction, but as many as 203 lasting symptoms have been reported.2,8-12 From October 1, 2021, through January 31, 2022, the most common accompanying manifestations of long COVID were difficulty breathing, cough, and fatigue.7 Long COVID can affect multiple organ systems,13,14 with symptoms varying by organ system affected. Regardless of the need for hospitalization initially, having had COVID-19 significantly increases the risk for subsequent death at 30 days and at 6 months after initial infection.15
Symptoms of long COVID have been reported as long as 2 years after initial infection.8 When Davis and colleagues studied the onset and progression of reported symptoms of long COVID,9 they determined that, among patients who reported recovery from COVID-19 in < 90 days, symptoms peaked at approximately Week 2 of infection. In comparison, patients who reported not having recovered in < 90 days had (1) symptoms that peaked later (2 months) and (2) on average, more symptoms (mean, 17 reported symptoms, compared to 11 in recovered patients).9
Continue to: Fatigue
Fatigue, including postexertion malaise and impaired daily function and mobility, is the most common symptom of long COVID,8-10,14 reported in 28% to 98%14 of patients after initial COVID-19. This fatigue is more than simply being tired: Patients describe profound exhaustion, in which fatigue is out of proportion to exertion. Fatigue and myalgia are commonly reported among patients with impaired hepatic and pulmonary function as a consequence of long COVID.13 Patients often report that even minor activities result in decreased attention, focus, and energy, for many hours or days afterward. Fatigue has been reported to persist from 2.5 months to as long as 6 months after initial infection or hospitalization.9,16
Postviral fatigue has been seen in other viral outbreaks and seems to share characteristics with myalgic encephalomyelitis/chronic fatigue syndrome, or ME/CFS, which itself has historically been stigmatized and poorly understood.17 Long COVID fatigue might be more common among women and patients who have an existing diagnosis of depression and antidepressant use,10,11,16,18 although the mechanism of this relationship is unclear. Potential mechanisms include damage from systemic inflammation to metabolism in the frontal lobe and cerebellum19 and direct infection by SARS-CoV-2 in skeletal muscle.20 Townsend and colleagues16 found no relationship between long COVID fatigue and markers of inflammation (leukocyte, neutrophil, and lymphocyte counts; the neutrophil-to-lymphocyte ratio; lactate dehydrogenase; C-reactive protein; serum interleukin-6; and soluble CD25).
Neuropsychiatric symptoms are also common in long COVID and can have a significant impact on patients’ quality of life. Studies have reported poor sleep quality or insomnia (38% to 90%), headache (17% to 91.2%), speech and language problems (48% to 50%), confusion (20%), dementia (28.6%), difficulty concentrating (1.9% to 27%), and memory loss or cognitive impairment (5.4% to 73%).9,10,14,15 For some patients, these symptoms persisted for ≥ 6 months, making it difficult for those affected to return to work.9
Isolation and loneliness, a common situation for patients with COVID-19, can have long-term effects on mental health.21 The COVID-19 pandemic itself has had a negative effect on behavioral health, including depression (4.3% to 25% of patients), anxiety (1.9% to 46%), obsessive compulsive disorder (4.9% to 20%), and posttraumatic stress disorder (29%).22 The persistence of symptoms of long COVID has resulted in a great deal of frustration, fear, and confusion for those affected—some of whom report a loss of trust in their community health care providers to address their ongoing struggles.23 Such loss can be accompanied by a reported increase in feelings of anxiety and changes to perceptions of self (ie, “how I used to be” in contrast to “how I am now”).23 These neuropsychiatric symptoms, including mental health conditions, appear to be more common among older adults.4
Other neurologic deficits found in long COVID include olfactory disorders (9% to 27% of patients), altered taste (5% to 18%), numbness or tingling sensations (6%), blurred vision (17.1%), and tinnitus (16.%).14 Dizziness (2.6% to 6%) and lightheadedness or presyncope (7%) have also been reported, although these symptoms appear to be less common than other neurocognitive effects.14
Continue to: The mechanism of action...
The mechanism of action of damage to the nervous system in long COVID is likely multifactorial. COVID-19 can directly infect the central nervous system through a hematogenous route, which can result in direct cytolytic damage to neurons. Infection can also affect the blood–brain barrier.24 Additionally, COVID-19 can invade the central nervous system through peripheral nerves, including the olfactory and vagus nerves.25 Many human respiratory viruses, including SARS-CoV-2, result in an increase in pro-inflammatory and anti-inflammatory cytokines; this so-called cytokine storm is an exaggerated response to infection and can trigger neurodegenerative and psychiatric syndromes.26 It is unclear whether the cytokine storm is different for people with COVID-19, compared to other respiratory viruses.
Respiratory symptoms are very common after COVID-1915: In studies, as many as 87.1% of patients continued to have shortness of breath ≥ 140 days after initial symptom onset, including breathlessness (48% to 60%), wheezing (5.3%), cough (10.5% to 46%), and congestion (32%),14,18 any of which can persist for as long as 6 months.9 Among a sample of previously hospitalized COVID-19 patients in Wuhan, China, 22% to 56% displayed a pulmonary diffusion abnormality 6 months later, with those who required supplemental oxygen during initial COVID-19 having a greater risk for these abnormalities at follow-up, compared to those who did not require supplemental oxygen (odds ratio = 2.42; 95% CI, 1.15-5.08).11
Cardiovascular symptoms. New-onset autonomic dysfunction has been described in multiple case reports and in some larger cohort studies of patients post COVID-19.27 Many common long COVID symptoms, including fatigue and orthostatic intolerance, are commonly seen in postural orthostatic tachycardia syndrome. Emerging evidence indicates that there are likely similar underlying mechanisms and a significant amount of overlap between long COVID and postural orthostatic tachycardia syndrome.27
A study of patients within the US Department of Veterans Affairs population found that, regardless of disease severity, patients who had a positive COVID-19 test had a higher rate of cardiac disease 30 days after diagnosis,28 including stroke, transient ischemic attack, dysrhythmia, inflammatory heart disease, acute coronary disease, myocardial infarction, ischemic cardiopathy, angina, heart failure, nonischemic cardiomyopathy, and cardiac arrest. Patients with COVID-19 were at increased risk for major adverse cardiovascular events (myocardial infarction, stroke, and all-cause mortality).28 Demographics of the VA population (ie, most are White men) might limit the generalizability of these data, but similar findings have been found elsewhere.5,10,15Given that, in general, chest pain is common after the acute phase of an infection and the causes of chest pain are broad, the high rate of cardiac complications post COVID-19 nevertheless highlights the importance of a thorough evaluation and work-up of chest pain in patients who have had COVID-19.
Other symptoms. Body aches and generalized joint pain are another common symptom group of long COVID.9 These include body aches (20%), joint pain (78%), and muscle aches (87.7%).14,18
Continue to: Commonly reported...
Commonly reported gastrointestinal symptoms include diarrhea, loss of appetite, nausea, and abdominal pain.9,15
Other symptoms reported less commonly include dermatologic conditions, such as pruritus and rash; reproductive and endocrine symptoms, including extreme thirst, irregular menstruation, and sexual dysfunction; and new or exacerbated allergic response.9
Does severity of initial disease play a role?
Keep in mind that long COVID is not specific to patients who were hospitalized or had severe initial infection. In fact, 75% of patients who have a diagnosis of a post–COVID-19 condition were not hospitalized for their initial infection.7 However, the severity of initial COVID-19 infection might contribute to the presence or severity of long COVID symptoms2—although findings in current literature are mixed. For example:
- In reporting from Wuhan, China, higher position on a disease severity scale during a hospital stay for COVID-19 was associated with:
- greater likelihood of reporting ≥ 1 symptoms at a 6-month follow-up
- increased risk for pulmonary diffusion abnormalities, fatigue, and mood disorders.11
- After 2 years’ follow-up of the same cohort, 55% of patients continued to report ≥ 1 symptoms of long COVID, and those who had been hospitalized with COVID-19 continued to report reduced health-related quality of life, compared to the control group.8
- Similarly, patients initially hospitalized with COVID-19 were more likely to experience impairment of ≥ 2 organs—in particular, the liver and pancreas—compared to nonhospitalized patients after a median 5 months post initial infection, among a sample in the United Kingdom.13
- In an international cohort, patients who reported a greater number of symptoms during initial COVID-19 were more likely to experience long COVID.12
- Last, long COVID fatigue did not vary by severity of initial COVID-19 infection among a sample of hospitalized and nonhospitalized participants in Dublin, Ireland.16
No specific treatments yet available
There are no specific treatments for long COVID; overall, the emphasis is on providing supportive care and managing preexisting chronic conditions.5 This is where expertise in primary care, relationships with patients and the community, and psychosocial knowledge can help patients recover from ongoing COVID-19 symptoms.
Clinicians should continue to perform a thorough physical assessment of patients with previous or ongoing COVID-19 to identify and monitor new or recurring symptoms after hospital discharge or initial resolution of symptoms.29 This approach includes developing an individualized plan for care and rehabilitation that is specific to presenting symptoms, including psychological support. We encourage family physicians to familiarize themselves with the work of Vance and colleagues,30 who have created a comprehensive tablea to guide treatment and referral for the gamut of long COVID symptoms, including cardiovascular issues (eg, palpitations, edema), chronic cough, headache, pain, and insomnia.
Continue to: This new clinical entity is a formidable challenge
This new clinical entity is a formidable challenge
Long COVID is a new condition that requires comprehensive evaluation to understand the full, often long-term, effects of COVID-19. Our review of this condition substantiated that symptoms of long COVID often affect a variety of organs13,14 and have been observed to persist for ≥ 2 years.8
Some studies that have examined the long-term effects of COVID-19 included only participants who were not hospitalized; others include hospitalized patients exclusively. The literature is mixed in regard to including severity of initial infection as it relates to long COVID. Available research demonstrates that it is common for people with COVID-19 to experience persistent symptoms that can significantly impact daily life and well-being.
Likely, it will be several years before we even begin to understand the full extent of COVID-19. Until research elucidates the relationship between the disease and short- and long-term health outcomes, clinicians should:
- acknowledge and address the reality of long COVID when meeting with persistently symptomatic patients,
- provide support, therapeutic listening, and referral to rehabilitation as appropriate, and
- offer information on the potential for long-term effects of COVID-19 to vaccine-hesitant patients.
a “Systems, symptoms, and treatments for post-COVID patients,” pages 1231-1234 in the source article (www.jabfm.org/content/jabfp/34/6/1229.full.pdf).30
CORRESPONDENCE
Nicole Mayo, PhD, 46 Prince Street, Rochester, NY 14607; [email protected]
1. Centers for Disease Control and Prevention. COVID data tracker. December 6, 2022. Accessed December 7, 2022. https://covid.cdc.gov/covid-data-tracker
2. Centers for Disease Control and Prevention. Long COVID or post-COVID conditions. Updated September 1, 2021. Accessed November 17, 2022. www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html
3. Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022;28:1461-1467. doi: 10.1038/s41591-022-01840-0
4. Bull-Otterson L, Baca S, Saydah S, et al. Post-COVID conditions among adult COVID-19 survivors aged 18-64 and ≥ 65 years—United States, March 2020–November 2021. MMWR Morb Mortal Wkly Rep. 2022;71:713-717. doi: 10.15585/mmwr.mm7121e1
5. Greenhalgh T, Knight M, A’Court C, et al. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026
6. Matta J, Wiernik E, Robineau O, et al; . Association of self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms among French adults during the COVID-19 pandemic. JAMA Intern Med. 2022;182:19-25. doi: 10.1001/jamainternmed.2021.6454
7. FAIR Health. Patients diagnosed with post-COVID conditions: an analysis of private healthcare claims using the official ICD-10 diagnostic code. May 18, 2022. Accessed October 15, 2022. https://s3.amazonaws.com/media2.fairhealth.org/whitepaper/asset/Patients%20Diagnosed%20with%20Post-COVID%20Con ditions%20-%20A%20FAIR%20Health%20White%20Paper.pdf
8. Huang L, Li X, Gu X, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med. 2022;10:863-876. doi: 10.1016/S2213-2600(22)00126-6
9. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
10. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11:16144. doi: 10.1038/s41598-021-95565-8
11. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220-232. doi: 10.1016/S0140-6736(20)32656-8
12. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626-631. doi: 10.1038/s41591-021-01292-y
13. Dennis A, Wamil M, Alberts J, et al; . Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11:e048391. doi: 10.1136/bmjopen-2020-048391
14. Crook H, Raza S, Nowell J, et al.. Long covid—mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648
15. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi: 10.1038/s41586-021-03553-9
16. Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PloS One. 2020;15:e0240784. doi: 10.1371/journal.pone.0240784
17. Wong TL, Weitzer DJ. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)—a systematic review and comparison of clinical presentation and symptomatology. Medicina (Kaunas). 2021;57:418. doi: 10.3390/ medicina57050418
18. Sykes DL, Holdsworth L, Jawad N, et al. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. 2021;199:113-119. doi: 10.1007/s00408-021-00423-z
19. Guedj E, Million M, Dudouet P, et al. 18F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: substrate for persistent/delayed disorders? Euro J Nucl Med Mol Imaging. 2021;48:592-595. doi: 10.1007/s00259-020-04973-x
20. Ferrandi PJ, Alway SE, Mohamed JS. The interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J Appl Physiol (1985). 2020;129:864-867. doi: 10.1152/japplphysiol.00321.2020
21. Leigh-Hunt N, Bagguley D, Bash K, et al. An overview of systematic reviews on the public health consequences of social isolation and loneliness. Public health. 2017;152:157-171.
22. Kathirvel N. Post COVID-19 pandemic mental health challenges. Asian J Psychiatr. 2020;53:102430. doi: 10.1016/j.ajp.2020.102430
23. Macpherson K, Cooper K, Harbour J, et al. Experiences of living with long COVID and of accessing healthcare services: a qualitative systematic review. BMJ Open. 2022;12:e050979. doi: 10.1136/bmjopen-2021-050979
24. Yachou Y, El Idrissi A, Belapasov V, et al. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neuro Sci. 2020;41:2657-2669. doi: 10.1007/s10072-020-04575-3
25. Gialluisi A, de Gaetano G, Iacoviello L. New challenges from Covid-19 pandemic: an unexpected opportunity to enlighten the link between viral infections and brain disorders? Neurol Sci. 2020;41:1349-1350. doi: 10.1007/s10072-020-04444-z
26. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34-39. doi: 10.1016/j.bbi.2020.04.027
27. Bisaccia G, Ricci F, Recce V, et al. Post-acute sequelae of COVID-19 and cardiovascular autonomic dysfunction: what do we know? J Cardiovasc Dev Dis. 2021;8:156. doi: 10.3390/jcdd8110156
28. Xie Y, Xu E, Bowe B, et al. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi: 10.1038/s41591-022-01689-3
29. Gorna R, MacDermott N, Rayner C, et al. Long COVID guidelines need to reflect lived experience. Lancet. 2021;397:455-457. doi: 10.1016/S0140-6736(20)32705-7
30. Vance H, Maslach A, Stoneman E, et al. Addressing post-COVID symptoms: a guide for primary care physicians. J Am Board Fam Med. 2021;34:1229-1242. doi: 10.3122/jabfm.2021.06.210254
Several years into the pandemic, COVID-19 continues to deeply impact our society; at the time of publication of this review, 98.8 million cases in the United States have been reported to the Centers for Disease Control and Prevention (CDC).1 Although many people recover well from infection, there is mounting concern regarding long-term sequelae of COVID-19. These long-term symptoms have been termed long COVID, among other names.
What exactly is long COVID?
The CDC and National Institutes of Health define long COVID as new or ongoing health problems experienced ≥ 4 weeks after initial infection.2 Evidence suggests that even people who have mild initial COVID-19 symptoms are at risk for long COVID.
Available data about long COVID are imperfect, however; much about the condition remains poorly understood. For example, there is little evidence regarding the effect of vaccination and viral variants on the prevalence of long COVID. A recent study of more than 13 million people from the US Department of Veterans Affairs database did demonstrate that vaccination against SARS-CoV-2 lowered the risk for long COVID by only about 15%.3
Persistent symptoms associated with long COVID often lead to disability and decreased quality of life. Furthermore, long COVID is a challenge to treat because there is a paucity of evidence to guide COVID-19 treatment beyond initial infection.
Because many patients who have ongoing COVID-19 symptoms will be seen in primary care, it is important to understand how to manage and support them. In this article, we discuss current understanding of long COVID epidemiology, symptoms that can persist 4 weeks after initial infection, and potential treatment options.
Prevalence and diagnosis
The prevalence of long COVID is not well defined because many epidemiologic studies rely on self-reporting. The CDC reports that 20% to 25% of COVID-19 survivors experience a new condition that might be attributable to their initial infection.4 Other studies variously cite 5% to 85% of people who have had a diagnosis of COVID-19 as experiencing long COVID, although that rate more consistently appears to be 10% to 30%.5

A study of adult patients in France found that self-reported symptoms of long COVID, 10 to 12 months after the first wave of the pandemic (May through November 2020), were associated with the belief of having had COVID-19 but not necessarily with having tested positive for anti-SARS-CoV-2 antibodies,6 which indicates prior COVID-19. This complicates research on long COVID because, first, there is no specific test to confirm a diagnosis of long COVID and, second, studies often rely on self-reporting of earlier COVID-19.
Continue to: As such, long COVID...
As such, long COVID is diagnosed primarily through a medical history and physical examination. The medical history provides a guide as to whether additional testing is warranted to evaluate for known complications of COVID-19, such as deep vein thrombosis, pulmonary embolism, myocarditis, and pulmonary fibrosis. As of October 1, 2021, a new International Classification of Disease (10th Revision) code went into effect for post COVID condition, unspecified (U09.9).7
The prevalence of long COVID symptoms appears to increase with age. Among patients whose disease was diagnosed using code U09.9, most were 36 to 64 years of age; children and adults ages 22 years or younger constituted only 10.5% of diagnoses.7 Long COVID symptoms might also be more prevalent among women and in people with a preexisting chronic comorbidity.2,7
Symptoms can be numerous, severe or mild, and lasting
Initially, there was no widely accepted definition of long COVID; follow-up in early studies ranged from 21 days to 2 years after initial infection (or from discharge, for hospitalized patients).8 Differences in descriptions that have been used on surveys to self-report symptoms make it a challenge to clearly summarize the frequency of each aspect of long COVID.
Long COVID can be mild or debilitating; severity can fluctuate. Common symptoms include fatigue, dyspnea or other breathing difficulties, headache, and cognitive dysfunction, but as many as 203 lasting symptoms have been reported.2,8-12 From October 1, 2021, through January 31, 2022, the most common accompanying manifestations of long COVID were difficulty breathing, cough, and fatigue.7 Long COVID can affect multiple organ systems,13,14 with symptoms varying by organ system affected. Regardless of the need for hospitalization initially, having had COVID-19 significantly increases the risk for subsequent death at 30 days and at 6 months after initial infection.15
Symptoms of long COVID have been reported as long as 2 years after initial infection.8 When Davis and colleagues studied the onset and progression of reported symptoms of long COVID,9 they determined that, among patients who reported recovery from COVID-19 in < 90 days, symptoms peaked at approximately Week 2 of infection. In comparison, patients who reported not having recovered in < 90 days had (1) symptoms that peaked later (2 months) and (2) on average, more symptoms (mean, 17 reported symptoms, compared to 11 in recovered patients).9
Continue to: Fatigue
Fatigue, including postexertion malaise and impaired daily function and mobility, is the most common symptom of long COVID,8-10,14 reported in 28% to 98%14 of patients after initial COVID-19. This fatigue is more than simply being tired: Patients describe profound exhaustion, in which fatigue is out of proportion to exertion. Fatigue and myalgia are commonly reported among patients with impaired hepatic and pulmonary function as a consequence of long COVID.13 Patients often report that even minor activities result in decreased attention, focus, and energy, for many hours or days afterward. Fatigue has been reported to persist from 2.5 months to as long as 6 months after initial infection or hospitalization.9,16
Postviral fatigue has been seen in other viral outbreaks and seems to share characteristics with myalgic encephalomyelitis/chronic fatigue syndrome, or ME/CFS, which itself has historically been stigmatized and poorly understood.17 Long COVID fatigue might be more common among women and patients who have an existing diagnosis of depression and antidepressant use,10,11,16,18 although the mechanism of this relationship is unclear. Potential mechanisms include damage from systemic inflammation to metabolism in the frontal lobe and cerebellum19 and direct infection by SARS-CoV-2 in skeletal muscle.20 Townsend and colleagues16 found no relationship between long COVID fatigue and markers of inflammation (leukocyte, neutrophil, and lymphocyte counts; the neutrophil-to-lymphocyte ratio; lactate dehydrogenase; C-reactive protein; serum interleukin-6; and soluble CD25).
Neuropsychiatric symptoms are also common in long COVID and can have a significant impact on patients’ quality of life. Studies have reported poor sleep quality or insomnia (38% to 90%), headache (17% to 91.2%), speech and language problems (48% to 50%), confusion (20%), dementia (28.6%), difficulty concentrating (1.9% to 27%), and memory loss or cognitive impairment (5.4% to 73%).9,10,14,15 For some patients, these symptoms persisted for ≥ 6 months, making it difficult for those affected to return to work.9
Isolation and loneliness, a common situation for patients with COVID-19, can have long-term effects on mental health.21 The COVID-19 pandemic itself has had a negative effect on behavioral health, including depression (4.3% to 25% of patients), anxiety (1.9% to 46%), obsessive compulsive disorder (4.9% to 20%), and posttraumatic stress disorder (29%).22 The persistence of symptoms of long COVID has resulted in a great deal of frustration, fear, and confusion for those affected—some of whom report a loss of trust in their community health care providers to address their ongoing struggles.23 Such loss can be accompanied by a reported increase in feelings of anxiety and changes to perceptions of self (ie, “how I used to be” in contrast to “how I am now”).23 These neuropsychiatric symptoms, including mental health conditions, appear to be more common among older adults.4
Other neurologic deficits found in long COVID include olfactory disorders (9% to 27% of patients), altered taste (5% to 18%), numbness or tingling sensations (6%), blurred vision (17.1%), and tinnitus (16.%).14 Dizziness (2.6% to 6%) and lightheadedness or presyncope (7%) have also been reported, although these symptoms appear to be less common than other neurocognitive effects.14
Continue to: The mechanism of action...
The mechanism of action of damage to the nervous system in long COVID is likely multifactorial. COVID-19 can directly infect the central nervous system through a hematogenous route, which can result in direct cytolytic damage to neurons. Infection can also affect the blood–brain barrier.24 Additionally, COVID-19 can invade the central nervous system through peripheral nerves, including the olfactory and vagus nerves.25 Many human respiratory viruses, including SARS-CoV-2, result in an increase in pro-inflammatory and anti-inflammatory cytokines; this so-called cytokine storm is an exaggerated response to infection and can trigger neurodegenerative and psychiatric syndromes.26 It is unclear whether the cytokine storm is different for people with COVID-19, compared to other respiratory viruses.
Respiratory symptoms are very common after COVID-1915: In studies, as many as 87.1% of patients continued to have shortness of breath ≥ 140 days after initial symptom onset, including breathlessness (48% to 60%), wheezing (5.3%), cough (10.5% to 46%), and congestion (32%),14,18 any of which can persist for as long as 6 months.9 Among a sample of previously hospitalized COVID-19 patients in Wuhan, China, 22% to 56% displayed a pulmonary diffusion abnormality 6 months later, with those who required supplemental oxygen during initial COVID-19 having a greater risk for these abnormalities at follow-up, compared to those who did not require supplemental oxygen (odds ratio = 2.42; 95% CI, 1.15-5.08).11
Cardiovascular symptoms. New-onset autonomic dysfunction has been described in multiple case reports and in some larger cohort studies of patients post COVID-19.27 Many common long COVID symptoms, including fatigue and orthostatic intolerance, are commonly seen in postural orthostatic tachycardia syndrome. Emerging evidence indicates that there are likely similar underlying mechanisms and a significant amount of overlap between long COVID and postural orthostatic tachycardia syndrome.27
A study of patients within the US Department of Veterans Affairs population found that, regardless of disease severity, patients who had a positive COVID-19 test had a higher rate of cardiac disease 30 days after diagnosis,28 including stroke, transient ischemic attack, dysrhythmia, inflammatory heart disease, acute coronary disease, myocardial infarction, ischemic cardiopathy, angina, heart failure, nonischemic cardiomyopathy, and cardiac arrest. Patients with COVID-19 were at increased risk for major adverse cardiovascular events (myocardial infarction, stroke, and all-cause mortality).28 Demographics of the VA population (ie, most are White men) might limit the generalizability of these data, but similar findings have been found elsewhere.5,10,15Given that, in general, chest pain is common after the acute phase of an infection and the causes of chest pain are broad, the high rate of cardiac complications post COVID-19 nevertheless highlights the importance of a thorough evaluation and work-up of chest pain in patients who have had COVID-19.
Other symptoms. Body aches and generalized joint pain are another common symptom group of long COVID.9 These include body aches (20%), joint pain (78%), and muscle aches (87.7%).14,18
Continue to: Commonly reported...
Commonly reported gastrointestinal symptoms include diarrhea, loss of appetite, nausea, and abdominal pain.9,15
Other symptoms reported less commonly include dermatologic conditions, such as pruritus and rash; reproductive and endocrine symptoms, including extreme thirst, irregular menstruation, and sexual dysfunction; and new or exacerbated allergic response.9
Does severity of initial disease play a role?
Keep in mind that long COVID is not specific to patients who were hospitalized or had severe initial infection. In fact, 75% of patients who have a diagnosis of a post–COVID-19 condition were not hospitalized for their initial infection.7 However, the severity of initial COVID-19 infection might contribute to the presence or severity of long COVID symptoms2—although findings in current literature are mixed. For example:
- In reporting from Wuhan, China, higher position on a disease severity scale during a hospital stay for COVID-19 was associated with:
- greater likelihood of reporting ≥ 1 symptoms at a 6-month follow-up
- increased risk for pulmonary diffusion abnormalities, fatigue, and mood disorders.11
- After 2 years’ follow-up of the same cohort, 55% of patients continued to report ≥ 1 symptoms of long COVID, and those who had been hospitalized with COVID-19 continued to report reduced health-related quality of life, compared to the control group.8
- Similarly, patients initially hospitalized with COVID-19 were more likely to experience impairment of ≥ 2 organs—in particular, the liver and pancreas—compared to nonhospitalized patients after a median 5 months post initial infection, among a sample in the United Kingdom.13
- In an international cohort, patients who reported a greater number of symptoms during initial COVID-19 were more likely to experience long COVID.12
- Last, long COVID fatigue did not vary by severity of initial COVID-19 infection among a sample of hospitalized and nonhospitalized participants in Dublin, Ireland.16
No specific treatments yet available
There are no specific treatments for long COVID; overall, the emphasis is on providing supportive care and managing preexisting chronic conditions.5 This is where expertise in primary care, relationships with patients and the community, and psychosocial knowledge can help patients recover from ongoing COVID-19 symptoms.
Clinicians should continue to perform a thorough physical assessment of patients with previous or ongoing COVID-19 to identify and monitor new or recurring symptoms after hospital discharge or initial resolution of symptoms.29 This approach includes developing an individualized plan for care and rehabilitation that is specific to presenting symptoms, including psychological support. We encourage family physicians to familiarize themselves with the work of Vance and colleagues,30 who have created a comprehensive tablea to guide treatment and referral for the gamut of long COVID symptoms, including cardiovascular issues (eg, palpitations, edema), chronic cough, headache, pain, and insomnia.
Continue to: This new clinical entity is a formidable challenge
This new clinical entity is a formidable challenge
Long COVID is a new condition that requires comprehensive evaluation to understand the full, often long-term, effects of COVID-19. Our review of this condition substantiated that symptoms of long COVID often affect a variety of organs13,14 and have been observed to persist for ≥ 2 years.8
Some studies that have examined the long-term effects of COVID-19 included only participants who were not hospitalized; others include hospitalized patients exclusively. The literature is mixed in regard to including severity of initial infection as it relates to long COVID. Available research demonstrates that it is common for people with COVID-19 to experience persistent symptoms that can significantly impact daily life and well-being.
Likely, it will be several years before we even begin to understand the full extent of COVID-19. Until research elucidates the relationship between the disease and short- and long-term health outcomes, clinicians should:
- acknowledge and address the reality of long COVID when meeting with persistently symptomatic patients,
- provide support, therapeutic listening, and referral to rehabilitation as appropriate, and
- offer information on the potential for long-term effects of COVID-19 to vaccine-hesitant patients.
a “Systems, symptoms, and treatments for post-COVID patients,” pages 1231-1234 in the source article (www.jabfm.org/content/jabfp/34/6/1229.full.pdf).30
CORRESPONDENCE
Nicole Mayo, PhD, 46 Prince Street, Rochester, NY 14607; [email protected]
Several years into the pandemic, COVID-19 continues to deeply impact our society; at the time of publication of this review, 98.8 million cases in the United States have been reported to the Centers for Disease Control and Prevention (CDC).1 Although many people recover well from infection, there is mounting concern regarding long-term sequelae of COVID-19. These long-term symptoms have been termed long COVID, among other names.
What exactly is long COVID?
The CDC and National Institutes of Health define long COVID as new or ongoing health problems experienced ≥ 4 weeks after initial infection.2 Evidence suggests that even people who have mild initial COVID-19 symptoms are at risk for long COVID.
Available data about long COVID are imperfect, however; much about the condition remains poorly understood. For example, there is little evidence regarding the effect of vaccination and viral variants on the prevalence of long COVID. A recent study of more than 13 million people from the US Department of Veterans Affairs database did demonstrate that vaccination against SARS-CoV-2 lowered the risk for long COVID by only about 15%.3
Persistent symptoms associated with long COVID often lead to disability and decreased quality of life. Furthermore, long COVID is a challenge to treat because there is a paucity of evidence to guide COVID-19 treatment beyond initial infection.
Because many patients who have ongoing COVID-19 symptoms will be seen in primary care, it is important to understand how to manage and support them. In this article, we discuss current understanding of long COVID epidemiology, symptoms that can persist 4 weeks after initial infection, and potential treatment options.
Prevalence and diagnosis
The prevalence of long COVID is not well defined because many epidemiologic studies rely on self-reporting. The CDC reports that 20% to 25% of COVID-19 survivors experience a new condition that might be attributable to their initial infection.4 Other studies variously cite 5% to 85% of people who have had a diagnosis of COVID-19 as experiencing long COVID, although that rate more consistently appears to be 10% to 30%.5

A study of adult patients in France found that self-reported symptoms of long COVID, 10 to 12 months after the first wave of the pandemic (May through November 2020), were associated with the belief of having had COVID-19 but not necessarily with having tested positive for anti-SARS-CoV-2 antibodies,6 which indicates prior COVID-19. This complicates research on long COVID because, first, there is no specific test to confirm a diagnosis of long COVID and, second, studies often rely on self-reporting of earlier COVID-19.
Continue to: As such, long COVID...
As such, long COVID is diagnosed primarily through a medical history and physical examination. The medical history provides a guide as to whether additional testing is warranted to evaluate for known complications of COVID-19, such as deep vein thrombosis, pulmonary embolism, myocarditis, and pulmonary fibrosis. As of October 1, 2021, a new International Classification of Disease (10th Revision) code went into effect for post COVID condition, unspecified (U09.9).7
The prevalence of long COVID symptoms appears to increase with age. Among patients whose disease was diagnosed using code U09.9, most were 36 to 64 years of age; children and adults ages 22 years or younger constituted only 10.5% of diagnoses.7 Long COVID symptoms might also be more prevalent among women and in people with a preexisting chronic comorbidity.2,7
Symptoms can be numerous, severe or mild, and lasting
Initially, there was no widely accepted definition of long COVID; follow-up in early studies ranged from 21 days to 2 years after initial infection (or from discharge, for hospitalized patients).8 Differences in descriptions that have been used on surveys to self-report symptoms make it a challenge to clearly summarize the frequency of each aspect of long COVID.
Long COVID can be mild or debilitating; severity can fluctuate. Common symptoms include fatigue, dyspnea or other breathing difficulties, headache, and cognitive dysfunction, but as many as 203 lasting symptoms have been reported.2,8-12 From October 1, 2021, through January 31, 2022, the most common accompanying manifestations of long COVID were difficulty breathing, cough, and fatigue.7 Long COVID can affect multiple organ systems,13,14 with symptoms varying by organ system affected. Regardless of the need for hospitalization initially, having had COVID-19 significantly increases the risk for subsequent death at 30 days and at 6 months after initial infection.15
Symptoms of long COVID have been reported as long as 2 years after initial infection.8 When Davis and colleagues studied the onset and progression of reported symptoms of long COVID,9 they determined that, among patients who reported recovery from COVID-19 in < 90 days, symptoms peaked at approximately Week 2 of infection. In comparison, patients who reported not having recovered in < 90 days had (1) symptoms that peaked later (2 months) and (2) on average, more symptoms (mean, 17 reported symptoms, compared to 11 in recovered patients).9
Continue to: Fatigue
Fatigue, including postexertion malaise and impaired daily function and mobility, is the most common symptom of long COVID,8-10,14 reported in 28% to 98%14 of patients after initial COVID-19. This fatigue is more than simply being tired: Patients describe profound exhaustion, in which fatigue is out of proportion to exertion. Fatigue and myalgia are commonly reported among patients with impaired hepatic and pulmonary function as a consequence of long COVID.13 Patients often report that even minor activities result in decreased attention, focus, and energy, for many hours or days afterward. Fatigue has been reported to persist from 2.5 months to as long as 6 months after initial infection or hospitalization.9,16
Postviral fatigue has been seen in other viral outbreaks and seems to share characteristics with myalgic encephalomyelitis/chronic fatigue syndrome, or ME/CFS, which itself has historically been stigmatized and poorly understood.17 Long COVID fatigue might be more common among women and patients who have an existing diagnosis of depression and antidepressant use,10,11,16,18 although the mechanism of this relationship is unclear. Potential mechanisms include damage from systemic inflammation to metabolism in the frontal lobe and cerebellum19 and direct infection by SARS-CoV-2 in skeletal muscle.20 Townsend and colleagues16 found no relationship between long COVID fatigue and markers of inflammation (leukocyte, neutrophil, and lymphocyte counts; the neutrophil-to-lymphocyte ratio; lactate dehydrogenase; C-reactive protein; serum interleukin-6; and soluble CD25).
Neuropsychiatric symptoms are also common in long COVID and can have a significant impact on patients’ quality of life. Studies have reported poor sleep quality or insomnia (38% to 90%), headache (17% to 91.2%), speech and language problems (48% to 50%), confusion (20%), dementia (28.6%), difficulty concentrating (1.9% to 27%), and memory loss or cognitive impairment (5.4% to 73%).9,10,14,15 For some patients, these symptoms persisted for ≥ 6 months, making it difficult for those affected to return to work.9
Isolation and loneliness, a common situation for patients with COVID-19, can have long-term effects on mental health.21 The COVID-19 pandemic itself has had a negative effect on behavioral health, including depression (4.3% to 25% of patients), anxiety (1.9% to 46%), obsessive compulsive disorder (4.9% to 20%), and posttraumatic stress disorder (29%).22 The persistence of symptoms of long COVID has resulted in a great deal of frustration, fear, and confusion for those affected—some of whom report a loss of trust in their community health care providers to address their ongoing struggles.23 Such loss can be accompanied by a reported increase in feelings of anxiety and changes to perceptions of self (ie, “how I used to be” in contrast to “how I am now”).23 These neuropsychiatric symptoms, including mental health conditions, appear to be more common among older adults.4
Other neurologic deficits found in long COVID include olfactory disorders (9% to 27% of patients), altered taste (5% to 18%), numbness or tingling sensations (6%), blurred vision (17.1%), and tinnitus (16.%).14 Dizziness (2.6% to 6%) and lightheadedness or presyncope (7%) have also been reported, although these symptoms appear to be less common than other neurocognitive effects.14
Continue to: The mechanism of action...
The mechanism of action of damage to the nervous system in long COVID is likely multifactorial. COVID-19 can directly infect the central nervous system through a hematogenous route, which can result in direct cytolytic damage to neurons. Infection can also affect the blood–brain barrier.24 Additionally, COVID-19 can invade the central nervous system through peripheral nerves, including the olfactory and vagus nerves.25 Many human respiratory viruses, including SARS-CoV-2, result in an increase in pro-inflammatory and anti-inflammatory cytokines; this so-called cytokine storm is an exaggerated response to infection and can trigger neurodegenerative and psychiatric syndromes.26 It is unclear whether the cytokine storm is different for people with COVID-19, compared to other respiratory viruses.
Respiratory symptoms are very common after COVID-1915: In studies, as many as 87.1% of patients continued to have shortness of breath ≥ 140 days after initial symptom onset, including breathlessness (48% to 60%), wheezing (5.3%), cough (10.5% to 46%), and congestion (32%),14,18 any of which can persist for as long as 6 months.9 Among a sample of previously hospitalized COVID-19 patients in Wuhan, China, 22% to 56% displayed a pulmonary diffusion abnormality 6 months later, with those who required supplemental oxygen during initial COVID-19 having a greater risk for these abnormalities at follow-up, compared to those who did not require supplemental oxygen (odds ratio = 2.42; 95% CI, 1.15-5.08).11
Cardiovascular symptoms. New-onset autonomic dysfunction has been described in multiple case reports and in some larger cohort studies of patients post COVID-19.27 Many common long COVID symptoms, including fatigue and orthostatic intolerance, are commonly seen in postural orthostatic tachycardia syndrome. Emerging evidence indicates that there are likely similar underlying mechanisms and a significant amount of overlap between long COVID and postural orthostatic tachycardia syndrome.27
A study of patients within the US Department of Veterans Affairs population found that, regardless of disease severity, patients who had a positive COVID-19 test had a higher rate of cardiac disease 30 days after diagnosis,28 including stroke, transient ischemic attack, dysrhythmia, inflammatory heart disease, acute coronary disease, myocardial infarction, ischemic cardiopathy, angina, heart failure, nonischemic cardiomyopathy, and cardiac arrest. Patients with COVID-19 were at increased risk for major adverse cardiovascular events (myocardial infarction, stroke, and all-cause mortality).28 Demographics of the VA population (ie, most are White men) might limit the generalizability of these data, but similar findings have been found elsewhere.5,10,15Given that, in general, chest pain is common after the acute phase of an infection and the causes of chest pain are broad, the high rate of cardiac complications post COVID-19 nevertheless highlights the importance of a thorough evaluation and work-up of chest pain in patients who have had COVID-19.
Other symptoms. Body aches and generalized joint pain are another common symptom group of long COVID.9 These include body aches (20%), joint pain (78%), and muscle aches (87.7%).14,18
Continue to: Commonly reported...
Commonly reported gastrointestinal symptoms include diarrhea, loss of appetite, nausea, and abdominal pain.9,15
Other symptoms reported less commonly include dermatologic conditions, such as pruritus and rash; reproductive and endocrine symptoms, including extreme thirst, irregular menstruation, and sexual dysfunction; and new or exacerbated allergic response.9
Does severity of initial disease play a role?
Keep in mind that long COVID is not specific to patients who were hospitalized or had severe initial infection. In fact, 75% of patients who have a diagnosis of a post–COVID-19 condition were not hospitalized for their initial infection.7 However, the severity of initial COVID-19 infection might contribute to the presence or severity of long COVID symptoms2—although findings in current literature are mixed. For example:
- In reporting from Wuhan, China, higher position on a disease severity scale during a hospital stay for COVID-19 was associated with:
- greater likelihood of reporting ≥ 1 symptoms at a 6-month follow-up
- increased risk for pulmonary diffusion abnormalities, fatigue, and mood disorders.11
- After 2 years’ follow-up of the same cohort, 55% of patients continued to report ≥ 1 symptoms of long COVID, and those who had been hospitalized with COVID-19 continued to report reduced health-related quality of life, compared to the control group.8
- Similarly, patients initially hospitalized with COVID-19 were more likely to experience impairment of ≥ 2 organs—in particular, the liver and pancreas—compared to nonhospitalized patients after a median 5 months post initial infection, among a sample in the United Kingdom.13
- In an international cohort, patients who reported a greater number of symptoms during initial COVID-19 were more likely to experience long COVID.12
- Last, long COVID fatigue did not vary by severity of initial COVID-19 infection among a sample of hospitalized and nonhospitalized participants in Dublin, Ireland.16
No specific treatments yet available
There are no specific treatments for long COVID; overall, the emphasis is on providing supportive care and managing preexisting chronic conditions.5 This is where expertise in primary care, relationships with patients and the community, and psychosocial knowledge can help patients recover from ongoing COVID-19 symptoms.
Clinicians should continue to perform a thorough physical assessment of patients with previous or ongoing COVID-19 to identify and monitor new or recurring symptoms after hospital discharge or initial resolution of symptoms.29 This approach includes developing an individualized plan for care and rehabilitation that is specific to presenting symptoms, including psychological support. We encourage family physicians to familiarize themselves with the work of Vance and colleagues,30 who have created a comprehensive tablea to guide treatment and referral for the gamut of long COVID symptoms, including cardiovascular issues (eg, palpitations, edema), chronic cough, headache, pain, and insomnia.
Continue to: This new clinical entity is a formidable challenge
This new clinical entity is a formidable challenge
Long COVID is a new condition that requires comprehensive evaluation to understand the full, often long-term, effects of COVID-19. Our review of this condition substantiated that symptoms of long COVID often affect a variety of organs13,14 and have been observed to persist for ≥ 2 years.8
Some studies that have examined the long-term effects of COVID-19 included only participants who were not hospitalized; others include hospitalized patients exclusively. The literature is mixed in regard to including severity of initial infection as it relates to long COVID. Available research demonstrates that it is common for people with COVID-19 to experience persistent symptoms that can significantly impact daily life and well-being.
Likely, it will be several years before we even begin to understand the full extent of COVID-19. Until research elucidates the relationship between the disease and short- and long-term health outcomes, clinicians should:
- acknowledge and address the reality of long COVID when meeting with persistently symptomatic patients,
- provide support, therapeutic listening, and referral to rehabilitation as appropriate, and
- offer information on the potential for long-term effects of COVID-19 to vaccine-hesitant patients.
a “Systems, symptoms, and treatments for post-COVID patients,” pages 1231-1234 in the source article (www.jabfm.org/content/jabfp/34/6/1229.full.pdf).30
CORRESPONDENCE
Nicole Mayo, PhD, 46 Prince Street, Rochester, NY 14607; [email protected]
1. Centers for Disease Control and Prevention. COVID data tracker. December 6, 2022. Accessed December 7, 2022. https://covid.cdc.gov/covid-data-tracker
2. Centers for Disease Control and Prevention. Long COVID or post-COVID conditions. Updated September 1, 2021. Accessed November 17, 2022. www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html
3. Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022;28:1461-1467. doi: 10.1038/s41591-022-01840-0
4. Bull-Otterson L, Baca S, Saydah S, et al. Post-COVID conditions among adult COVID-19 survivors aged 18-64 and ≥ 65 years—United States, March 2020–November 2021. MMWR Morb Mortal Wkly Rep. 2022;71:713-717. doi: 10.15585/mmwr.mm7121e1
5. Greenhalgh T, Knight M, A’Court C, et al. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026
6. Matta J, Wiernik E, Robineau O, et al; . Association of self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms among French adults during the COVID-19 pandemic. JAMA Intern Med. 2022;182:19-25. doi: 10.1001/jamainternmed.2021.6454
7. FAIR Health. Patients diagnosed with post-COVID conditions: an analysis of private healthcare claims using the official ICD-10 diagnostic code. May 18, 2022. Accessed October 15, 2022. https://s3.amazonaws.com/media2.fairhealth.org/whitepaper/asset/Patients%20Diagnosed%20with%20Post-COVID%20Con ditions%20-%20A%20FAIR%20Health%20White%20Paper.pdf
8. Huang L, Li X, Gu X, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med. 2022;10:863-876. doi: 10.1016/S2213-2600(22)00126-6
9. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
10. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11:16144. doi: 10.1038/s41598-021-95565-8
11. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220-232. doi: 10.1016/S0140-6736(20)32656-8
12. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626-631. doi: 10.1038/s41591-021-01292-y
13. Dennis A, Wamil M, Alberts J, et al; . Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11:e048391. doi: 10.1136/bmjopen-2020-048391
14. Crook H, Raza S, Nowell J, et al.. Long covid—mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648
15. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi: 10.1038/s41586-021-03553-9
16. Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PloS One. 2020;15:e0240784. doi: 10.1371/journal.pone.0240784
17. Wong TL, Weitzer DJ. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)—a systematic review and comparison of clinical presentation and symptomatology. Medicina (Kaunas). 2021;57:418. doi: 10.3390/ medicina57050418
18. Sykes DL, Holdsworth L, Jawad N, et al. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. 2021;199:113-119. doi: 10.1007/s00408-021-00423-z
19. Guedj E, Million M, Dudouet P, et al. 18F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: substrate for persistent/delayed disorders? Euro J Nucl Med Mol Imaging. 2021;48:592-595. doi: 10.1007/s00259-020-04973-x
20. Ferrandi PJ, Alway SE, Mohamed JS. The interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J Appl Physiol (1985). 2020;129:864-867. doi: 10.1152/japplphysiol.00321.2020
21. Leigh-Hunt N, Bagguley D, Bash K, et al. An overview of systematic reviews on the public health consequences of social isolation and loneliness. Public health. 2017;152:157-171.
22. Kathirvel N. Post COVID-19 pandemic mental health challenges. Asian J Psychiatr. 2020;53:102430. doi: 10.1016/j.ajp.2020.102430
23. Macpherson K, Cooper K, Harbour J, et al. Experiences of living with long COVID and of accessing healthcare services: a qualitative systematic review. BMJ Open. 2022;12:e050979. doi: 10.1136/bmjopen-2021-050979
24. Yachou Y, El Idrissi A, Belapasov V, et al. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neuro Sci. 2020;41:2657-2669. doi: 10.1007/s10072-020-04575-3
25. Gialluisi A, de Gaetano G, Iacoviello L. New challenges from Covid-19 pandemic: an unexpected opportunity to enlighten the link between viral infections and brain disorders? Neurol Sci. 2020;41:1349-1350. doi: 10.1007/s10072-020-04444-z
26. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34-39. doi: 10.1016/j.bbi.2020.04.027
27. Bisaccia G, Ricci F, Recce V, et al. Post-acute sequelae of COVID-19 and cardiovascular autonomic dysfunction: what do we know? J Cardiovasc Dev Dis. 2021;8:156. doi: 10.3390/jcdd8110156
28. Xie Y, Xu E, Bowe B, et al. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi: 10.1038/s41591-022-01689-3
29. Gorna R, MacDermott N, Rayner C, et al. Long COVID guidelines need to reflect lived experience. Lancet. 2021;397:455-457. doi: 10.1016/S0140-6736(20)32705-7
30. Vance H, Maslach A, Stoneman E, et al. Addressing post-COVID symptoms: a guide for primary care physicians. J Am Board Fam Med. 2021;34:1229-1242. doi: 10.3122/jabfm.2021.06.210254
1. Centers for Disease Control and Prevention. COVID data tracker. December 6, 2022. Accessed December 7, 2022. https://covid.cdc.gov/covid-data-tracker
2. Centers for Disease Control and Prevention. Long COVID or post-COVID conditions. Updated September 1, 2021. Accessed November 17, 2022. www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html
3. Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022;28:1461-1467. doi: 10.1038/s41591-022-01840-0
4. Bull-Otterson L, Baca S, Saydah S, et al. Post-COVID conditions among adult COVID-19 survivors aged 18-64 and ≥ 65 years—United States, March 2020–November 2021. MMWR Morb Mortal Wkly Rep. 2022;71:713-717. doi: 10.15585/mmwr.mm7121e1
5. Greenhalgh T, Knight M, A’Court C, et al. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026
6. Matta J, Wiernik E, Robineau O, et al; . Association of self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms among French adults during the COVID-19 pandemic. JAMA Intern Med. 2022;182:19-25. doi: 10.1001/jamainternmed.2021.6454
7. FAIR Health. Patients diagnosed with post-COVID conditions: an analysis of private healthcare claims using the official ICD-10 diagnostic code. May 18, 2022. Accessed October 15, 2022. https://s3.amazonaws.com/media2.fairhealth.org/whitepaper/asset/Patients%20Diagnosed%20with%20Post-COVID%20Con ditions%20-%20A%20FAIR%20Health%20White%20Paper.pdf
8. Huang L, Li X, Gu X, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med. 2022;10:863-876. doi: 10.1016/S2213-2600(22)00126-6
9. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
10. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11:16144. doi: 10.1038/s41598-021-95565-8
11. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220-232. doi: 10.1016/S0140-6736(20)32656-8
12. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626-631. doi: 10.1038/s41591-021-01292-y
13. Dennis A, Wamil M, Alberts J, et al; . Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11:e048391. doi: 10.1136/bmjopen-2020-048391
14. Crook H, Raza S, Nowell J, et al.. Long covid—mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648
15. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi: 10.1038/s41586-021-03553-9
16. Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PloS One. 2020;15:e0240784. doi: 10.1371/journal.pone.0240784
17. Wong TL, Weitzer DJ. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)—a systematic review and comparison of clinical presentation and symptomatology. Medicina (Kaunas). 2021;57:418. doi: 10.3390/ medicina57050418
18. Sykes DL, Holdsworth L, Jawad N, et al. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. 2021;199:113-119. doi: 10.1007/s00408-021-00423-z
19. Guedj E, Million M, Dudouet P, et al. 18F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: substrate for persistent/delayed disorders? Euro J Nucl Med Mol Imaging. 2021;48:592-595. doi: 10.1007/s00259-020-04973-x
20. Ferrandi PJ, Alway SE, Mohamed JS. The interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J Appl Physiol (1985). 2020;129:864-867. doi: 10.1152/japplphysiol.00321.2020
21. Leigh-Hunt N, Bagguley D, Bash K, et al. An overview of systematic reviews on the public health consequences of social isolation and loneliness. Public health. 2017;152:157-171.
22. Kathirvel N. Post COVID-19 pandemic mental health challenges. Asian J Psychiatr. 2020;53:102430. doi: 10.1016/j.ajp.2020.102430
23. Macpherson K, Cooper K, Harbour J, et al. Experiences of living with long COVID and of accessing healthcare services: a qualitative systematic review. BMJ Open. 2022;12:e050979. doi: 10.1136/bmjopen-2021-050979
24. Yachou Y, El Idrissi A, Belapasov V, et al. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neuro Sci. 2020;41:2657-2669. doi: 10.1007/s10072-020-04575-3
25. Gialluisi A, de Gaetano G, Iacoviello L. New challenges from Covid-19 pandemic: an unexpected opportunity to enlighten the link between viral infections and brain disorders? Neurol Sci. 2020;41:1349-1350. doi: 10.1007/s10072-020-04444-z
26. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34-39. doi: 10.1016/j.bbi.2020.04.027
27. Bisaccia G, Ricci F, Recce V, et al. Post-acute sequelae of COVID-19 and cardiovascular autonomic dysfunction: what do we know? J Cardiovasc Dev Dis. 2021;8:156. doi: 10.3390/jcdd8110156
28. Xie Y, Xu E, Bowe B, et al. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi: 10.1038/s41591-022-01689-3
29. Gorna R, MacDermott N, Rayner C, et al. Long COVID guidelines need to reflect lived experience. Lancet. 2021;397:455-457. doi: 10.1016/S0140-6736(20)32705-7
30. Vance H, Maslach A, Stoneman E, et al. Addressing post-COVID symptoms: a guide for primary care physicians. J Am Board Fam Med. 2021;34:1229-1242. doi: 10.3122/jabfm.2021.06.210254
PRACTICE RECOMMENDATIONS
› Acknowledge and address the persistence of COVID-19 symptoms when meeting with patients. C
› Continue to monitor persistent, fluctuating symptoms of COVID-19 well after hospital discharge or apparent resolution of initial symptoms. C
› Provide psychological support and resources for mental health care to patients regarding their ongoing fears and frustrations with persistent COVID-19 symptoms. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
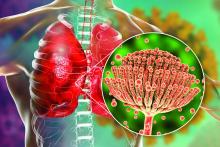
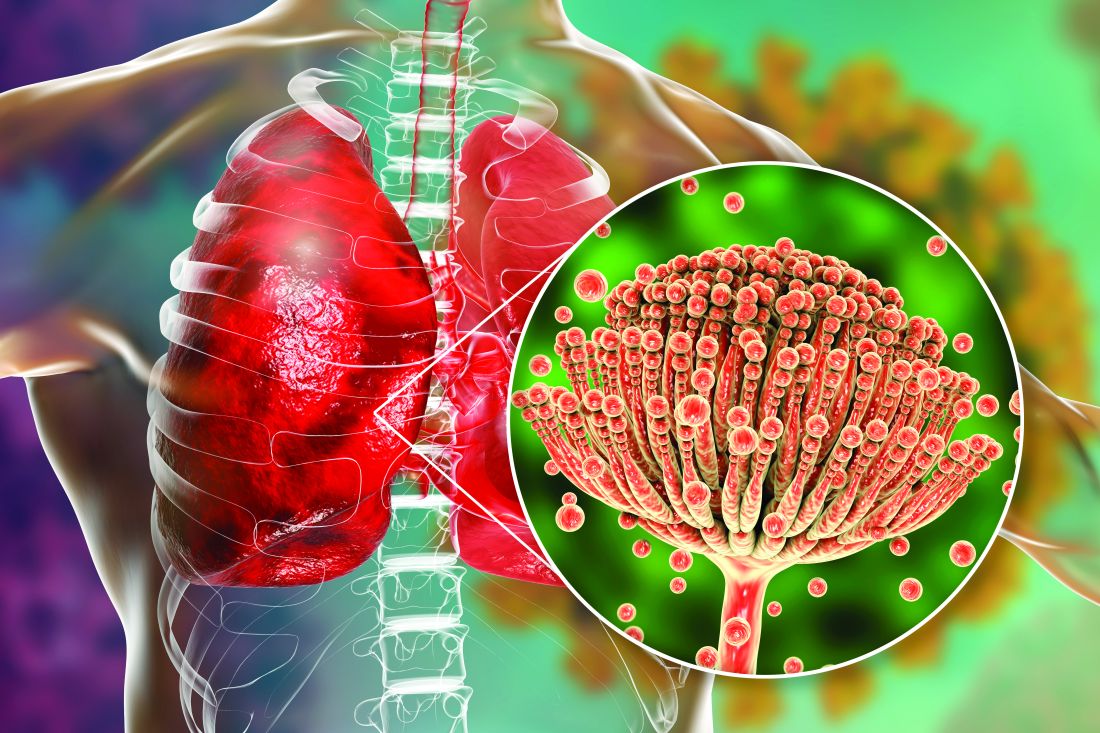
Rise of the fungi: Pandemic tied to increasing fungal infections
COVID-19 has lifted the lid on the risks of secondary pulmonary fungal infections in patients with severe respiratory viral illness – even previously immunocompetent individuals – and highlighted the importance of vigilant investigation to achieve early diagnoses, leading experts say.
Most fungi are not under surveillance in the United States, leaving experts without a national picture of the true burden of infection through the pandemic. However, a collection of published case series, cohort studies, and reviews from Europe, the United States, and throughout the world – mainly pre-Omicron – show that fungal disease has affected a significant portion of critically ill patients with COVID-19, with concerning excess mortality, these experts say.
COVID-associated pulmonary aspergillosis (CAPA) has been the predominant fungal coinfection in the United States and internationally. But COVID-associated mucormycosis (CAM) – the infection that surged in India in early 2021 – has also affected some patients in the United States, published data show. So have Pneumocystitis pneumonia, cryptococcosis, histoplasmosis, and Candida infections (which mainly affect the bloodstream and abdomen), say the experts who were interviewed.
“We had predicted [a rise in] aspergillosis, but we saw more than we thought we’d see. Most fungal infections became more common with COVID-19,” said George Thompson, MD, professor of clinical medicine at the University of California, Davis, and cochair of the University of Alabama–based Mycoses Study Group Education Committee, a group of experts in medical mycology. Pneumocystitis, for instance, “has historically been associated with AIDS or different types of leukemia or lymphoma, and is not an infection we’ve typically seen in our otherwise healthy ICU patients,” he noted. “But we did see more of it [with COVID-19].”
More recently, with fewer patients during the Omicron phase in intensive care units with acute respiratory failure, the profile of fungal disease secondary to COVID-19 has changed. Increasing proportions of patients have traditional risk factors for aspergillosis, such as hematologic malignancies and longer-term, pre-COVID use of systemic corticosteroids – a change that makes the contribution of the viral illness harder to distinguish.
Moving forward, the lessons of the COVID era – the fungal risks to patients with serious viral infections and the persistence needed to diagnose aspergillosis and other pulmonary fungal infections using bronchoscopy and imperfect noninvasive tests – should be taken to heart, experts say.
“Fungal diseases are not rare. They’re just not diagnosed because no one thinks to look for them,” said Dr. Thompson, a contributor to a recently released World Health Organization report naming a “fungal priority pathogens” list.
“We’re going to continue to see [secondary fungal infections] with other respiratory viruses,” he said. And overall, given environmental and other changes, “we’re going to see more and more fungal disease in the patients we take care of.”
CAPA not a surprise
CAPA is “not an unfamiliar story” in the world of fungal disease, given a history of influenza-associated pulmonary aspergillosis (IAPA), said Kieren A. Marr, MD, MBA, adjunct professor of medicine and past director of the transplant and oncology infectious diseases program at Johns Hopkins University, Baltimore, who has long researched invasive fungal disease.
European researchers, she said, have led the way in describing a high incidence of IAPA in patients admitted to ICUs with influenza. In a retrospective multicenter cohort study reported in 2018 by the Dutch-Belgian Mycosis Study group, for instance, almost 20% of 432 influenza patients admitted to the ICU, including patients who were otherwise healthy and not immunocompromised, had the diagnosis a median of 3 days after ICU admission. (Across other cohort studies, rates of IAPA have ranged from 7% to 30%.)
Mortality was significant: 51% of patients with influenza and invasive pulmonary aspergillosis died within 90 days, compared with 28% of patients with influenza and no invasive pulmonary aspergillosis.
Reports from Europe early in the pandemic indicated that CAPA was a similarly serious problem, prompting establishment at Johns Hopkins University of an aggressive screening program utilizing biomarker-based testing of blood and bronchoalveolar lavage (BAL) fluid. Of 396 mechanically ventilated COVID-19 patients admitted to Johns Hopkins University hospitals between March and August 2020, 39 met the institution’s criteria for CAPA, Dr. Marr and her colleagues reported this year in what might be the largest U.S. cohort study of CAPA published to date.
“We now know definitively that people with severe influenza and with severe COVID also have high risks for both invasive and airway disease caused by airborne fungi, most commonly aspergilliosis,” Dr. Marr said.
More recent unpublished analyses of patients from the start of the pandemic to June 2021 show persistent risk, said Nitipong Permpalung, MD, MPH, assistant professor in transplant and oncology infectious diseases at Johns Hopkins University and lead author of the cohort study. Among 832 patients with COVID-19 who were mechanically ventilated in Johns Hopkins University hospitals, 11.8% had CAPA, he said. (Also, 3.2% had invasive candidiasis, and 1.1% had other invasive fungal infections.)
Other sources said in interviews that these CAPA prevalence rates generally mirror reports from Europe, though some investigators in Europe have reported CAPA rates more toward 15%.
(The Mycoses Study Group recently collected data from its consortium of U.S. medical centers on the prevalence of CAPA, with funding support from the CDC, but at press time the data had not yet been released. Dr. Thompson said he suspected the prevalence will be lower than earlier papers have suggested, “but still will reflect a significant burden of disease.”)
Patients in the published Johns Hopkins University study who had CAPA were more likely than those with COVID-19 but no CAPA to have underlying pulmonary disease, liver disease, coagulopathy, solid tumors, multiple myeloma, and COVID-19–directed corticosteroids. And they had uniformly worse outcomes with regards to severity of illness and length of intubation.
How much of CAPA is driven by the SARS-CoV-2 virus itself and how much is a consequence of COVID-19 treatments is a topic of active discussion and research. Martin Hoenigl, MD, of the University of Graz, Austria, a leading researcher in medical mycology, said research shows corticosteroids and anti–IL-6 treatments, such as tocilizumab, used to treat COVID-19–driven acute respiratory failure clearly have contributed to CAPA. But he contends that “a number of other mechanisms” are involved as well.
“The immunologic mechanisms are definitely different in these patients with viral illness than in other ICU patients [who develop aspergilliosis]. It’s not just the corticosteroids. The more we learn, we see the virus plays a role as well, suppressing the interferon pathway,” for example, said Dr. Hoenigl, associate professor in the division of infectious diseases and the European Confederation of Medical Mycology (ECMM) Center of Excellence at the university. The earliest reports of CAPA came “when ICUs weren’t using dexamethasone or tocilizumab,” he noted.
In a paper published recently in Lancet Respiratory Medicine that Dr. Hoenigl and others point to, Belgian researchers reported a “three-level breach” in innate antifungal immunity in both IAPA and CAPA, affecting the integrity of the epithelial barrier, the capacity to phagocytose and kill Aspergillus spores, and the ability to destroy Aspergillus hyphae, which is mainly mediated by neutrophils.
The researchers ran a host of genetic and protein analyses on lung samples (most collected via BAL) of 169 patients with influenza or COVID-19, with and without aspergillosis. They found that patients with CAPA had significantly lower neutrophil cell fractions than patients with COVID-19 only, and patients with IAPA or CAPA had reduced type II IFN signaling and increased concentrations of fibrosis-associated growth factors in the lower respiratory tracts (Lancet Respir Med. 2022 Aug 24).
Tom Chiller, MD, MPH, chief of the Center for Disease Control and Prevention’s Mycotic Disease Branch, said he’s watching such research with interest. For now, he said, it’s important to also consider that “data on COVID show that almost all patients going into the ICUs with pneumonia and COVID are getting broad-spectrum antibiotics” in addition to corticosteroids.
By wiping out good bacteria, the antibiotics could be “creating a perfect niche for fungi to grow,” he said.
Diagnostic challenges
Aspergillus that has invaded the lung tissue in patients with COVID-19 appears to grow there for some time – around 8-10 days, much longer than in IAPA – before becoming angioinvasive, said Dr. Hoenigl. Such a pathophysiology “implicates that we should try to diagnose it while it’s in the lung tissue, using the BAL fluid, and not yet in the blood,” he said.
Some multicenter studies, including one from Europe on Aspergillus test profiles in critically ill COVID-19 patients, have shown mortality rates of close to 90% in patients with CAPA who have positive serum biomarkers, despite appropriate antifungal therapy. “If diagnosed while confined to the lung, however, mortality rates are more like 40%-50% with antifungal therapy,” Dr. Hoenigl said. (Cohort studies published thus far have fairly consistently reported mortality rates in patients with CAPA greater than 40%, he said.)
Bronchoscopy isn’t always pragmatic or possible, however, and is variably used. Some patients with severe COVID-19 may be too unstable for any invasive procedure, said Dr. Permpalung.
Dr. Permpalung looks for CAPA using serum (1-3) beta-D-glucan (BDG, a generic fungal test not specific to Aspergillus), serum galactomannan (GM, specific for Aspergillus), and respiratory cultures (sputum or endotracheal aspirate if intubated) as initial screening tests in the ICU. If there are concerns for CAPA – based on these tests and/or the clinical picture – “a thoughtful risk-benefit discussion is required to determine if patients would benefit from a bronchoscopy or if we should just start them on empiric antifungal therapy.”
Unfortunately, the sensitivity of serum GM is relatively low in CAPA – lower than with classic invasive aspergillosis in the nonviral setting, sources said. BDG, on the other hand, can be falsely positive in the setting of antimicrobials and within the ICU. And the utility of imaging for CAPA is limited. Both the clinical picture and radiological findings of CAPA have resembled those of severe COVID – with the caveat of cavitary lung lesions visible on imaging.
“Cavities or nodules are a highly suspicious finding that could indicate possible fungal infection,” said pulmonologist Amir A. Zeki, MD, MAS, professor of medicine at the University of California, Davis, and codirector of the UC Davis Asthma Network Clinic, who has cared for patients with CAPA.
Cavitation has been described in only a proportion of patients with CAPA, however. So in patients not doing well, “your suspicion has to be raised if you’re not seeing cavities,” he said.
Early in the pandemic, when patients worsened or failed to progress on mechanical ventilation, clinicians at the University of California, Davis, quickly learned not to pin blame too quickly on COVID-19 alone. This remains good advice today, Dr. Zeki said.
“If you have a patient who’s not doing well on a ventilator, not getting better [over weeks], has to be reintubated, has infiltrates or lung nodules that are evolving, or certainly, if they have a cavity, you have to suspect fungal infection,” said Dr. Zeki, who also practices at the Veterans Affairs Medical Center in San Diego. “Think about it for those patients who just aren’t moving forward and are continuing to struggle. Have a high index of suspicion, and consult with your infectious disease colleagues.”
Empiric treatment is warranted in some cases if a patient is doing poorly and suspicion for fungal infection is high based on clinical, radiographic, and/or laboratory evidence, he said.
The CDC’s Dr. Chiller said that screening and diagnostic algorithms currently vary from institution to institution, and that diagnostic challenges likely dissuade clinicians from thinking about fungi. “Clinicians often don’t want to deal with fungi – they’re difficult to diagnose, the treatments are limited and can be toxic. But fungi get pushed back until it’s too late,” he said.
“Fungal diagnostics is an area we all need a lot more help with,” and new diagnostics are in the pipeline, he said. In the meantime, he said, “there are tools out there, and we just need to use them more, and improve how they’re used.”
While reported CAPA thus far has typically occurred in the setting of ICU care and mechanical ventilation, it’s not always the case, Dr. Permpalung said. Lung and other solid organ transplant (SOT) recipients with COVID-19 are developing CAPA and other invasive secondary invasive fungal infections despite not being intubated, he said.
Of 276 SOT recipients with COVID-19 who required inpatient treatment at Johns Hopkins University hospitals from the beginning of the pandemic to March 2022, 23 patients developed invasive fungal infections (13 CAPA). Only a fraction – 38 of the 276 – had been intubated, he said.
Mucormycosis resistance
After CAPA, candidiasis and COVID-19-associated mucormycosis (CAM) – most frequently, rhino-orbital-cerebral disease or pulmonary disease – have been the leading reported fungal coinfections in COVID-19, said Dr. Hoenigl, who described the incidence, timeline, risk factors, and pathogenesis of these infections in a review published this year in Nature Microbiology. .
In India, where there has long been high exposure to Mucorales spores and a greater burden of invasive fungal disease, the rate of mucormycosis doubled in 2021, with rhino-orbital-cerebral disease reported almost exclusively, he said. Pulmonary disease has occurred almost exclusively in the ICU setting and has been present in about 50% of cases outside of India, including Europe and the United States.
A preprint meta-analysis of CAM cases posted by the Lancet in July 2022, in which investigators analyzed individual data of 556 reported cases of COVID-19–associated CAM, shows diabetes and history of corticosteroid use present in most patients, and an overall mortality rate of 44.4%, most of which stems from cases of pulmonary or disseminated disease. Thirteen of the 556 reported cases were from the United States.
An important take-away from the analysis, Dr. Hoenigl said, is that Aspergillus coinfection was seen in 7% of patients and was associated with higher mortality. “It’s important to consider that coinfections [of Aspergillus and Mucorales] can exist,” Dr. Hoenigl said, noting that like CAPA, pulmonary CAM is likely underdiagnosed and underreported.
As with CAPA, the clinical and radiological features of pulmonary CAM largely overlap with those associated with COVID-19, and bronchoscopy plays a central role in definitive diagnosis. In the United States, a Mucorales PCR test for blood and BAL fluid is commercially available and used at some centers, Dr. Hoenigl said.
“Mucormycosis is always difficult to treat ... a lot of the treatments don’t work particularly well,” said Dr. Thompson. “With aspergillosis, we have better treatment options.”
Dr. Thompson worries, however, about treatment resistance becoming widespread. Resistance to azole antifungal agents “is already pretty widespread in northern Europe, particularly in the Netherlands and part of the U.K.” because of injudicious use of antifungals in agriculture, he said. “We’ve started to see a few cases [of azole-resistant aspergillosis in the United States] and know it will be more widespread soon.”
Treatment resistance is a focus of the new WHO fungal priority pathogens list – the first such report from the organization. Of the 19 fungi on the list, 4 were ranked as critical: Cryptococcus neoformans, Candida auris, Aspergillus fumigatus, and Candida albicans. Like Dr. Thompson, Dr. Hoenigl contributed to the WHO report.
Dr. Hoenigl reported grant/research support from Astellas, Merck, F2G, Gilread, Pfizer, and Scynexis. Dr. Marr disclosed employment and equity in Pearl Diagnostics and Sfunga Therapeutics. Dr. Thompson, Dr. Permpalung, and Dr. Zeki reported that they have no relevant financial disclosures.
COVID-19 has lifted the lid on the risks of secondary pulmonary fungal infections in patients with severe respiratory viral illness – even previously immunocompetent individuals – and highlighted the importance of vigilant investigation to achieve early diagnoses, leading experts say.
Most fungi are not under surveillance in the United States, leaving experts without a national picture of the true burden of infection through the pandemic. However, a collection of published case series, cohort studies, and reviews from Europe, the United States, and throughout the world – mainly pre-Omicron – show that fungal disease has affected a significant portion of critically ill patients with COVID-19, with concerning excess mortality, these experts say.
COVID-associated pulmonary aspergillosis (CAPA) has been the predominant fungal coinfection in the United States and internationally. But COVID-associated mucormycosis (CAM) – the infection that surged in India in early 2021 – has also affected some patients in the United States, published data show. So have Pneumocystitis pneumonia, cryptococcosis, histoplasmosis, and Candida infections (which mainly affect the bloodstream and abdomen), say the experts who were interviewed.
“We had predicted [a rise in] aspergillosis, but we saw more than we thought we’d see. Most fungal infections became more common with COVID-19,” said George Thompson, MD, professor of clinical medicine at the University of California, Davis, and cochair of the University of Alabama–based Mycoses Study Group Education Committee, a group of experts in medical mycology. Pneumocystitis, for instance, “has historically been associated with AIDS or different types of leukemia or lymphoma, and is not an infection we’ve typically seen in our otherwise healthy ICU patients,” he noted. “But we did see more of it [with COVID-19].”
More recently, with fewer patients during the Omicron phase in intensive care units with acute respiratory failure, the profile of fungal disease secondary to COVID-19 has changed. Increasing proportions of patients have traditional risk factors for aspergillosis, such as hematologic malignancies and longer-term, pre-COVID use of systemic corticosteroids – a change that makes the contribution of the viral illness harder to distinguish.
Moving forward, the lessons of the COVID era – the fungal risks to patients with serious viral infections and the persistence needed to diagnose aspergillosis and other pulmonary fungal infections using bronchoscopy and imperfect noninvasive tests – should be taken to heart, experts say.
“Fungal diseases are not rare. They’re just not diagnosed because no one thinks to look for them,” said Dr. Thompson, a contributor to a recently released World Health Organization report naming a “fungal priority pathogens” list.
“We’re going to continue to see [secondary fungal infections] with other respiratory viruses,” he said. And overall, given environmental and other changes, “we’re going to see more and more fungal disease in the patients we take care of.”
CAPA not a surprise
CAPA is “not an unfamiliar story” in the world of fungal disease, given a history of influenza-associated pulmonary aspergillosis (IAPA), said Kieren A. Marr, MD, MBA, adjunct professor of medicine and past director of the transplant and oncology infectious diseases program at Johns Hopkins University, Baltimore, who has long researched invasive fungal disease.
European researchers, she said, have led the way in describing a high incidence of IAPA in patients admitted to ICUs with influenza. In a retrospective multicenter cohort study reported in 2018 by the Dutch-Belgian Mycosis Study group, for instance, almost 20% of 432 influenza patients admitted to the ICU, including patients who were otherwise healthy and not immunocompromised, had the diagnosis a median of 3 days after ICU admission. (Across other cohort studies, rates of IAPA have ranged from 7% to 30%.)
Mortality was significant: 51% of patients with influenza and invasive pulmonary aspergillosis died within 90 days, compared with 28% of patients with influenza and no invasive pulmonary aspergillosis.
Reports from Europe early in the pandemic indicated that CAPA was a similarly serious problem, prompting establishment at Johns Hopkins University of an aggressive screening program utilizing biomarker-based testing of blood and bronchoalveolar lavage (BAL) fluid. Of 396 mechanically ventilated COVID-19 patients admitted to Johns Hopkins University hospitals between March and August 2020, 39 met the institution’s criteria for CAPA, Dr. Marr and her colleagues reported this year in what might be the largest U.S. cohort study of CAPA published to date.
“We now know definitively that people with severe influenza and with severe COVID also have high risks for both invasive and airway disease caused by airborne fungi, most commonly aspergilliosis,” Dr. Marr said.
More recent unpublished analyses of patients from the start of the pandemic to June 2021 show persistent risk, said Nitipong Permpalung, MD, MPH, assistant professor in transplant and oncology infectious diseases at Johns Hopkins University and lead author of the cohort study. Among 832 patients with COVID-19 who were mechanically ventilated in Johns Hopkins University hospitals, 11.8% had CAPA, he said. (Also, 3.2% had invasive candidiasis, and 1.1% had other invasive fungal infections.)
Other sources said in interviews that these CAPA prevalence rates generally mirror reports from Europe, though some investigators in Europe have reported CAPA rates more toward 15%.
(The Mycoses Study Group recently collected data from its consortium of U.S. medical centers on the prevalence of CAPA, with funding support from the CDC, but at press time the data had not yet been released. Dr. Thompson said he suspected the prevalence will be lower than earlier papers have suggested, “but still will reflect a significant burden of disease.”)
Patients in the published Johns Hopkins University study who had CAPA were more likely than those with COVID-19 but no CAPA to have underlying pulmonary disease, liver disease, coagulopathy, solid tumors, multiple myeloma, and COVID-19–directed corticosteroids. And they had uniformly worse outcomes with regards to severity of illness and length of intubation.
How much of CAPA is driven by the SARS-CoV-2 virus itself and how much is a consequence of COVID-19 treatments is a topic of active discussion and research. Martin Hoenigl, MD, of the University of Graz, Austria, a leading researcher in medical mycology, said research shows corticosteroids and anti–IL-6 treatments, such as tocilizumab, used to treat COVID-19–driven acute respiratory failure clearly have contributed to CAPA. But he contends that “a number of other mechanisms” are involved as well.
“The immunologic mechanisms are definitely different in these patients with viral illness than in other ICU patients [who develop aspergilliosis]. It’s not just the corticosteroids. The more we learn, we see the virus plays a role as well, suppressing the interferon pathway,” for example, said Dr. Hoenigl, associate professor in the division of infectious diseases and the European Confederation of Medical Mycology (ECMM) Center of Excellence at the university. The earliest reports of CAPA came “when ICUs weren’t using dexamethasone or tocilizumab,” he noted.
In a paper published recently in Lancet Respiratory Medicine that Dr. Hoenigl and others point to, Belgian researchers reported a “three-level breach” in innate antifungal immunity in both IAPA and CAPA, affecting the integrity of the epithelial barrier, the capacity to phagocytose and kill Aspergillus spores, and the ability to destroy Aspergillus hyphae, which is mainly mediated by neutrophils.
The researchers ran a host of genetic and protein analyses on lung samples (most collected via BAL) of 169 patients with influenza or COVID-19, with and without aspergillosis. They found that patients with CAPA had significantly lower neutrophil cell fractions than patients with COVID-19 only, and patients with IAPA or CAPA had reduced type II IFN signaling and increased concentrations of fibrosis-associated growth factors in the lower respiratory tracts (Lancet Respir Med. 2022 Aug 24).
Tom Chiller, MD, MPH, chief of the Center for Disease Control and Prevention’s Mycotic Disease Branch, said he’s watching such research with interest. For now, he said, it’s important to also consider that “data on COVID show that almost all patients going into the ICUs with pneumonia and COVID are getting broad-spectrum antibiotics” in addition to corticosteroids.
By wiping out good bacteria, the antibiotics could be “creating a perfect niche for fungi to grow,” he said.
Diagnostic challenges
Aspergillus that has invaded the lung tissue in patients with COVID-19 appears to grow there for some time – around 8-10 days, much longer than in IAPA – before becoming angioinvasive, said Dr. Hoenigl. Such a pathophysiology “implicates that we should try to diagnose it while it’s in the lung tissue, using the BAL fluid, and not yet in the blood,” he said.
Some multicenter studies, including one from Europe on Aspergillus test profiles in critically ill COVID-19 patients, have shown mortality rates of close to 90% in patients with CAPA who have positive serum biomarkers, despite appropriate antifungal therapy. “If diagnosed while confined to the lung, however, mortality rates are more like 40%-50% with antifungal therapy,” Dr. Hoenigl said. (Cohort studies published thus far have fairly consistently reported mortality rates in patients with CAPA greater than 40%, he said.)
Bronchoscopy isn’t always pragmatic or possible, however, and is variably used. Some patients with severe COVID-19 may be too unstable for any invasive procedure, said Dr. Permpalung.
Dr. Permpalung looks for CAPA using serum (1-3) beta-D-glucan (BDG, a generic fungal test not specific to Aspergillus), serum galactomannan (GM, specific for Aspergillus), and respiratory cultures (sputum or endotracheal aspirate if intubated) as initial screening tests in the ICU. If there are concerns for CAPA – based on these tests and/or the clinical picture – “a thoughtful risk-benefit discussion is required to determine if patients would benefit from a bronchoscopy or if we should just start them on empiric antifungal therapy.”
Unfortunately, the sensitivity of serum GM is relatively low in CAPA – lower than with classic invasive aspergillosis in the nonviral setting, sources said. BDG, on the other hand, can be falsely positive in the setting of antimicrobials and within the ICU. And the utility of imaging for CAPA is limited. Both the clinical picture and radiological findings of CAPA have resembled those of severe COVID – with the caveat of cavitary lung lesions visible on imaging.
“Cavities or nodules are a highly suspicious finding that could indicate possible fungal infection,” said pulmonologist Amir A. Zeki, MD, MAS, professor of medicine at the University of California, Davis, and codirector of the UC Davis Asthma Network Clinic, who has cared for patients with CAPA.
Cavitation has been described in only a proportion of patients with CAPA, however. So in patients not doing well, “your suspicion has to be raised if you’re not seeing cavities,” he said.
Early in the pandemic, when patients worsened or failed to progress on mechanical ventilation, clinicians at the University of California, Davis, quickly learned not to pin blame too quickly on COVID-19 alone. This remains good advice today, Dr. Zeki said.
“If you have a patient who’s not doing well on a ventilator, not getting better [over weeks], has to be reintubated, has infiltrates or lung nodules that are evolving, or certainly, if they have a cavity, you have to suspect fungal infection,” said Dr. Zeki, who also practices at the Veterans Affairs Medical Center in San Diego. “Think about it for those patients who just aren’t moving forward and are continuing to struggle. Have a high index of suspicion, and consult with your infectious disease colleagues.”
Empiric treatment is warranted in some cases if a patient is doing poorly and suspicion for fungal infection is high based on clinical, radiographic, and/or laboratory evidence, he said.
The CDC’s Dr. Chiller said that screening and diagnostic algorithms currently vary from institution to institution, and that diagnostic challenges likely dissuade clinicians from thinking about fungi. “Clinicians often don’t want to deal with fungi – they’re difficult to diagnose, the treatments are limited and can be toxic. But fungi get pushed back until it’s too late,” he said.
“Fungal diagnostics is an area we all need a lot more help with,” and new diagnostics are in the pipeline, he said. In the meantime, he said, “there are tools out there, and we just need to use them more, and improve how they’re used.”
While reported CAPA thus far has typically occurred in the setting of ICU care and mechanical ventilation, it’s not always the case, Dr. Permpalung said. Lung and other solid organ transplant (SOT) recipients with COVID-19 are developing CAPA and other invasive secondary invasive fungal infections despite not being intubated, he said.
Of 276 SOT recipients with COVID-19 who required inpatient treatment at Johns Hopkins University hospitals from the beginning of the pandemic to March 2022, 23 patients developed invasive fungal infections (13 CAPA). Only a fraction – 38 of the 276 – had been intubated, he said.
Mucormycosis resistance
After CAPA, candidiasis and COVID-19-associated mucormycosis (CAM) – most frequently, rhino-orbital-cerebral disease or pulmonary disease – have been the leading reported fungal coinfections in COVID-19, said Dr. Hoenigl, who described the incidence, timeline, risk factors, and pathogenesis of these infections in a review published this year in Nature Microbiology. .
In India, where there has long been high exposure to Mucorales spores and a greater burden of invasive fungal disease, the rate of mucormycosis doubled in 2021, with rhino-orbital-cerebral disease reported almost exclusively, he said. Pulmonary disease has occurred almost exclusively in the ICU setting and has been present in about 50% of cases outside of India, including Europe and the United States.
A preprint meta-analysis of CAM cases posted by the Lancet in July 2022, in which investigators analyzed individual data of 556 reported cases of COVID-19–associated CAM, shows diabetes and history of corticosteroid use present in most patients, and an overall mortality rate of 44.4%, most of which stems from cases of pulmonary or disseminated disease. Thirteen of the 556 reported cases were from the United States.
An important take-away from the analysis, Dr. Hoenigl said, is that Aspergillus coinfection was seen in 7% of patients and was associated with higher mortality. “It’s important to consider that coinfections [of Aspergillus and Mucorales] can exist,” Dr. Hoenigl said, noting that like CAPA, pulmonary CAM is likely underdiagnosed and underreported.
As with CAPA, the clinical and radiological features of pulmonary CAM largely overlap with those associated with COVID-19, and bronchoscopy plays a central role in definitive diagnosis. In the United States, a Mucorales PCR test for blood and BAL fluid is commercially available and used at some centers, Dr. Hoenigl said.
“Mucormycosis is always difficult to treat ... a lot of the treatments don’t work particularly well,” said Dr. Thompson. “With aspergillosis, we have better treatment options.”
Dr. Thompson worries, however, about treatment resistance becoming widespread. Resistance to azole antifungal agents “is already pretty widespread in northern Europe, particularly in the Netherlands and part of the U.K.” because of injudicious use of antifungals in agriculture, he said. “We’ve started to see a few cases [of azole-resistant aspergillosis in the United States] and know it will be more widespread soon.”
Treatment resistance is a focus of the new WHO fungal priority pathogens list – the first such report from the organization. Of the 19 fungi on the list, 4 were ranked as critical: Cryptococcus neoformans, Candida auris, Aspergillus fumigatus, and Candida albicans. Like Dr. Thompson, Dr. Hoenigl contributed to the WHO report.
Dr. Hoenigl reported grant/research support from Astellas, Merck, F2G, Gilread, Pfizer, and Scynexis. Dr. Marr disclosed employment and equity in Pearl Diagnostics and Sfunga Therapeutics. Dr. Thompson, Dr. Permpalung, and Dr. Zeki reported that they have no relevant financial disclosures.
COVID-19 has lifted the lid on the risks of secondary pulmonary fungal infections in patients with severe respiratory viral illness – even previously immunocompetent individuals – and highlighted the importance of vigilant investigation to achieve early diagnoses, leading experts say.
Most fungi are not under surveillance in the United States, leaving experts without a national picture of the true burden of infection through the pandemic. However, a collection of published case series, cohort studies, and reviews from Europe, the United States, and throughout the world – mainly pre-Omicron – show that fungal disease has affected a significant portion of critically ill patients with COVID-19, with concerning excess mortality, these experts say.
COVID-associated pulmonary aspergillosis (CAPA) has been the predominant fungal coinfection in the United States and internationally. But COVID-associated mucormycosis (CAM) – the infection that surged in India in early 2021 – has also affected some patients in the United States, published data show. So have Pneumocystitis pneumonia, cryptococcosis, histoplasmosis, and Candida infections (which mainly affect the bloodstream and abdomen), say the experts who were interviewed.
“We had predicted [a rise in] aspergillosis, but we saw more than we thought we’d see. Most fungal infections became more common with COVID-19,” said George Thompson, MD, professor of clinical medicine at the University of California, Davis, and cochair of the University of Alabama–based Mycoses Study Group Education Committee, a group of experts in medical mycology. Pneumocystitis, for instance, “has historically been associated with AIDS or different types of leukemia or lymphoma, and is not an infection we’ve typically seen in our otherwise healthy ICU patients,” he noted. “But we did see more of it [with COVID-19].”
More recently, with fewer patients during the Omicron phase in intensive care units with acute respiratory failure, the profile of fungal disease secondary to COVID-19 has changed. Increasing proportions of patients have traditional risk factors for aspergillosis, such as hematologic malignancies and longer-term, pre-COVID use of systemic corticosteroids – a change that makes the contribution of the viral illness harder to distinguish.
Moving forward, the lessons of the COVID era – the fungal risks to patients with serious viral infections and the persistence needed to diagnose aspergillosis and other pulmonary fungal infections using bronchoscopy and imperfect noninvasive tests – should be taken to heart, experts say.
“Fungal diseases are not rare. They’re just not diagnosed because no one thinks to look for them,” said Dr. Thompson, a contributor to a recently released World Health Organization report naming a “fungal priority pathogens” list.
“We’re going to continue to see [secondary fungal infections] with other respiratory viruses,” he said. And overall, given environmental and other changes, “we’re going to see more and more fungal disease in the patients we take care of.”
CAPA not a surprise
CAPA is “not an unfamiliar story” in the world of fungal disease, given a history of influenza-associated pulmonary aspergillosis (IAPA), said Kieren A. Marr, MD, MBA, adjunct professor of medicine and past director of the transplant and oncology infectious diseases program at Johns Hopkins University, Baltimore, who has long researched invasive fungal disease.
European researchers, she said, have led the way in describing a high incidence of IAPA in patients admitted to ICUs with influenza. In a retrospective multicenter cohort study reported in 2018 by the Dutch-Belgian Mycosis Study group, for instance, almost 20% of 432 influenza patients admitted to the ICU, including patients who were otherwise healthy and not immunocompromised, had the diagnosis a median of 3 days after ICU admission. (Across other cohort studies, rates of IAPA have ranged from 7% to 30%.)
Mortality was significant: 51% of patients with influenza and invasive pulmonary aspergillosis died within 90 days, compared with 28% of patients with influenza and no invasive pulmonary aspergillosis.
Reports from Europe early in the pandemic indicated that CAPA was a similarly serious problem, prompting establishment at Johns Hopkins University of an aggressive screening program utilizing biomarker-based testing of blood and bronchoalveolar lavage (BAL) fluid. Of 396 mechanically ventilated COVID-19 patients admitted to Johns Hopkins University hospitals between March and August 2020, 39 met the institution’s criteria for CAPA, Dr. Marr and her colleagues reported this year in what might be the largest U.S. cohort study of CAPA published to date.
“We now know definitively that people with severe influenza and with severe COVID also have high risks for both invasive and airway disease caused by airborne fungi, most commonly aspergilliosis,” Dr. Marr said.
More recent unpublished analyses of patients from the start of the pandemic to June 2021 show persistent risk, said Nitipong Permpalung, MD, MPH, assistant professor in transplant and oncology infectious diseases at Johns Hopkins University and lead author of the cohort study. Among 832 patients with COVID-19 who were mechanically ventilated in Johns Hopkins University hospitals, 11.8% had CAPA, he said. (Also, 3.2% had invasive candidiasis, and 1.1% had other invasive fungal infections.)
Other sources said in interviews that these CAPA prevalence rates generally mirror reports from Europe, though some investigators in Europe have reported CAPA rates more toward 15%.
(The Mycoses Study Group recently collected data from its consortium of U.S. medical centers on the prevalence of CAPA, with funding support from the CDC, but at press time the data had not yet been released. Dr. Thompson said he suspected the prevalence will be lower than earlier papers have suggested, “but still will reflect a significant burden of disease.”)
Patients in the published Johns Hopkins University study who had CAPA were more likely than those with COVID-19 but no CAPA to have underlying pulmonary disease, liver disease, coagulopathy, solid tumors, multiple myeloma, and COVID-19–directed corticosteroids. And they had uniformly worse outcomes with regards to severity of illness and length of intubation.
How much of CAPA is driven by the SARS-CoV-2 virus itself and how much is a consequence of COVID-19 treatments is a topic of active discussion and research. Martin Hoenigl, MD, of the University of Graz, Austria, a leading researcher in medical mycology, said research shows corticosteroids and anti–IL-6 treatments, such as tocilizumab, used to treat COVID-19–driven acute respiratory failure clearly have contributed to CAPA. But he contends that “a number of other mechanisms” are involved as well.
“The immunologic mechanisms are definitely different in these patients with viral illness than in other ICU patients [who develop aspergilliosis]. It’s not just the corticosteroids. The more we learn, we see the virus plays a role as well, suppressing the interferon pathway,” for example, said Dr. Hoenigl, associate professor in the division of infectious diseases and the European Confederation of Medical Mycology (ECMM) Center of Excellence at the university. The earliest reports of CAPA came “when ICUs weren’t using dexamethasone or tocilizumab,” he noted.
In a paper published recently in Lancet Respiratory Medicine that Dr. Hoenigl and others point to, Belgian researchers reported a “three-level breach” in innate antifungal immunity in both IAPA and CAPA, affecting the integrity of the epithelial barrier, the capacity to phagocytose and kill Aspergillus spores, and the ability to destroy Aspergillus hyphae, which is mainly mediated by neutrophils.
The researchers ran a host of genetic and protein analyses on lung samples (most collected via BAL) of 169 patients with influenza or COVID-19, with and without aspergillosis. They found that patients with CAPA had significantly lower neutrophil cell fractions than patients with COVID-19 only, and patients with IAPA or CAPA had reduced type II IFN signaling and increased concentrations of fibrosis-associated growth factors in the lower respiratory tracts (Lancet Respir Med. 2022 Aug 24).
Tom Chiller, MD, MPH, chief of the Center for Disease Control and Prevention’s Mycotic Disease Branch, said he’s watching such research with interest. For now, he said, it’s important to also consider that “data on COVID show that almost all patients going into the ICUs with pneumonia and COVID are getting broad-spectrum antibiotics” in addition to corticosteroids.
By wiping out good bacteria, the antibiotics could be “creating a perfect niche for fungi to grow,” he said.
Diagnostic challenges
Aspergillus that has invaded the lung tissue in patients with COVID-19 appears to grow there for some time – around 8-10 days, much longer than in IAPA – before becoming angioinvasive, said Dr. Hoenigl. Such a pathophysiology “implicates that we should try to diagnose it while it’s in the lung tissue, using the BAL fluid, and not yet in the blood,” he said.
Some multicenter studies, including one from Europe on Aspergillus test profiles in critically ill COVID-19 patients, have shown mortality rates of close to 90% in patients with CAPA who have positive serum biomarkers, despite appropriate antifungal therapy. “If diagnosed while confined to the lung, however, mortality rates are more like 40%-50% with antifungal therapy,” Dr. Hoenigl said. (Cohort studies published thus far have fairly consistently reported mortality rates in patients with CAPA greater than 40%, he said.)
Bronchoscopy isn’t always pragmatic or possible, however, and is variably used. Some patients with severe COVID-19 may be too unstable for any invasive procedure, said Dr. Permpalung.
Dr. Permpalung looks for CAPA using serum (1-3) beta-D-glucan (BDG, a generic fungal test not specific to Aspergillus), serum galactomannan (GM, specific for Aspergillus), and respiratory cultures (sputum or endotracheal aspirate if intubated) as initial screening tests in the ICU. If there are concerns for CAPA – based on these tests and/or the clinical picture – “a thoughtful risk-benefit discussion is required to determine if patients would benefit from a bronchoscopy or if we should just start them on empiric antifungal therapy.”
Unfortunately, the sensitivity of serum GM is relatively low in CAPA – lower than with classic invasive aspergillosis in the nonviral setting, sources said. BDG, on the other hand, can be falsely positive in the setting of antimicrobials and within the ICU. And the utility of imaging for CAPA is limited. Both the clinical picture and radiological findings of CAPA have resembled those of severe COVID – with the caveat of cavitary lung lesions visible on imaging.
“Cavities or nodules are a highly suspicious finding that could indicate possible fungal infection,” said pulmonologist Amir A. Zeki, MD, MAS, professor of medicine at the University of California, Davis, and codirector of the UC Davis Asthma Network Clinic, who has cared for patients with CAPA.
Cavitation has been described in only a proportion of patients with CAPA, however. So in patients not doing well, “your suspicion has to be raised if you’re not seeing cavities,” he said.
Early in the pandemic, when patients worsened or failed to progress on mechanical ventilation, clinicians at the University of California, Davis, quickly learned not to pin blame too quickly on COVID-19 alone. This remains good advice today, Dr. Zeki said.
“If you have a patient who’s not doing well on a ventilator, not getting better [over weeks], has to be reintubated, has infiltrates or lung nodules that are evolving, or certainly, if they have a cavity, you have to suspect fungal infection,” said Dr. Zeki, who also practices at the Veterans Affairs Medical Center in San Diego. “Think about it for those patients who just aren’t moving forward and are continuing to struggle. Have a high index of suspicion, and consult with your infectious disease colleagues.”
Empiric treatment is warranted in some cases if a patient is doing poorly and suspicion for fungal infection is high based on clinical, radiographic, and/or laboratory evidence, he said.
The CDC’s Dr. Chiller said that screening and diagnostic algorithms currently vary from institution to institution, and that diagnostic challenges likely dissuade clinicians from thinking about fungi. “Clinicians often don’t want to deal with fungi – they’re difficult to diagnose, the treatments are limited and can be toxic. But fungi get pushed back until it’s too late,” he said.
“Fungal diagnostics is an area we all need a lot more help with,” and new diagnostics are in the pipeline, he said. In the meantime, he said, “there are tools out there, and we just need to use them more, and improve how they’re used.”
While reported CAPA thus far has typically occurred in the setting of ICU care and mechanical ventilation, it’s not always the case, Dr. Permpalung said. Lung and other solid organ transplant (SOT) recipients with COVID-19 are developing CAPA and other invasive secondary invasive fungal infections despite not being intubated, he said.
Of 276 SOT recipients with COVID-19 who required inpatient treatment at Johns Hopkins University hospitals from the beginning of the pandemic to March 2022, 23 patients developed invasive fungal infections (13 CAPA). Only a fraction – 38 of the 276 – had been intubated, he said.
Mucormycosis resistance
After CAPA, candidiasis and COVID-19-associated mucormycosis (CAM) – most frequently, rhino-orbital-cerebral disease or pulmonary disease – have been the leading reported fungal coinfections in COVID-19, said Dr. Hoenigl, who described the incidence, timeline, risk factors, and pathogenesis of these infections in a review published this year in Nature Microbiology. .
In India, where there has long been high exposure to Mucorales spores and a greater burden of invasive fungal disease, the rate of mucormycosis doubled in 2021, with rhino-orbital-cerebral disease reported almost exclusively, he said. Pulmonary disease has occurred almost exclusively in the ICU setting and has been present in about 50% of cases outside of India, including Europe and the United States.
A preprint meta-analysis of CAM cases posted by the Lancet in July 2022, in which investigators analyzed individual data of 556 reported cases of COVID-19–associated CAM, shows diabetes and history of corticosteroid use present in most patients, and an overall mortality rate of 44.4%, most of which stems from cases of pulmonary or disseminated disease. Thirteen of the 556 reported cases were from the United States.
An important take-away from the analysis, Dr. Hoenigl said, is that Aspergillus coinfection was seen in 7% of patients and was associated with higher mortality. “It’s important to consider that coinfections [of Aspergillus and Mucorales] can exist,” Dr. Hoenigl said, noting that like CAPA, pulmonary CAM is likely underdiagnosed and underreported.
As with CAPA, the clinical and radiological features of pulmonary CAM largely overlap with those associated with COVID-19, and bronchoscopy plays a central role in definitive diagnosis. In the United States, a Mucorales PCR test for blood and BAL fluid is commercially available and used at some centers, Dr. Hoenigl said.
“Mucormycosis is always difficult to treat ... a lot of the treatments don’t work particularly well,” said Dr. Thompson. “With aspergillosis, we have better treatment options.”
Dr. Thompson worries, however, about treatment resistance becoming widespread. Resistance to azole antifungal agents “is already pretty widespread in northern Europe, particularly in the Netherlands and part of the U.K.” because of injudicious use of antifungals in agriculture, he said. “We’ve started to see a few cases [of azole-resistant aspergillosis in the United States] and know it will be more widespread soon.”
Treatment resistance is a focus of the new WHO fungal priority pathogens list – the first such report from the organization. Of the 19 fungi on the list, 4 were ranked as critical: Cryptococcus neoformans, Candida auris, Aspergillus fumigatus, and Candida albicans. Like Dr. Thompson, Dr. Hoenigl contributed to the WHO report.
Dr. Hoenigl reported grant/research support from Astellas, Merck, F2G, Gilread, Pfizer, and Scynexis. Dr. Marr disclosed employment and equity in Pearl Diagnostics and Sfunga Therapeutics. Dr. Thompson, Dr. Permpalung, and Dr. Zeki reported that they have no relevant financial disclosures.
How a cheap liver drug may be the key to preventing COVID
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
As soon as the pandemic started, the search was on for a medication that could stave off infection, or at least the worst consequences of infection.
One that would be cheap to make, safe, easy to distribute, and, ideally, was already available. The search had a quest-like quality, like something from a fairy tale. Society, poisoned by COVID, would find the antidote out there, somewhere, if we looked hard enough.
You know the story. There were some pretty dramatic failures: hydroxychloroquine, ivermectin. There were some successes, like dexamethasone.
I’m not here today to tell you that the antidote has been found – no, it takes large randomized trials to figure that out. But
How do you make a case that an existing drug – UDCA, in this case – might be useful to prevent or treat COVID? In contrast to prior basic-science studies, like the original ivermectin study, which essentially took a bunch of cells and virus in a tube filled with varying concentrations of the antiparasitic agent, the authors of this paper appearing in Nature give us multiple, complementary lines of evidence. Let me walk you through it.
All good science starts with a biologically plausible hypothesis. In this case, the authors recognized that SARS-CoV-2, in all its variants, requires the presence of the ACE2 receptor on the surface of cells to bind.
That is the doorway to infection. Vaccines and antibodies block the key to this door, the spike protein and its receptor binding domain. But what if you could get rid of the doors altogether?
The authors first showed that ACE2 expression is controlled by a certain transcription factor known as the farnesoid X receptor, or FXR. Reducing the binding of FXR should therefore reduce ACE2 expression.
As luck would have it, UDCA – Actigall – reduces the levels of FXR and thus the expression of ACE2 in cells.
Okay. So we have a drug that can reduce ACE2, and we know that ACE2 is necessary for the virus to infect cells. Would UDCA prevent viral infection?
They started with test tubes, showing that cells were less likely to be infected by SARS-CoV-2 in the presence of UDCA at concentrations similar to what humans achieve in their blood after standard dosing. The red staining here is spike protein; you can see that it is markedly lower in the cells exposed to UDCA.
So far, so good. But test tubes aren’t people. So they moved up to mice and Syrian golden hamsters. These cute fellows are quite susceptible to human COVID and have been a model organism in countless studies
Mice and hamsters treated with UDCA in the presence of littermates with COVID infections were less likely to become infected themselves compared with mice not so treated. They also showed that mice and hamsters treated with UDCA had lower levels of ACE2 in their nasal passages.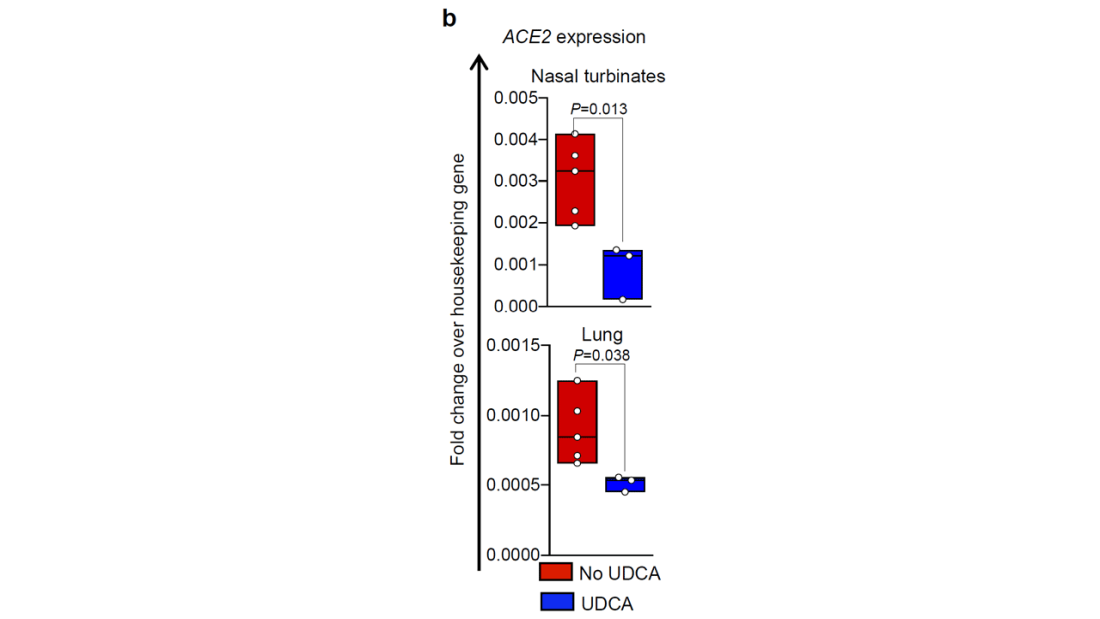
Of course, mice aren’t humans either. So the researchers didn’t stop there.
To determine the effects of UDCA on human tissue, they utilized perfused human lungs that had been declined for transplantation. The lungs were perfused with a special fluid to keep them viable, and were mechanically ventilated. One lung was exposed to UDCA and the other served as a control. The authors were able to show that ACE2 levels went down in the exposed lung. And, importantly, when samples of tissue from both lungs were exposed to SARS-CoV-2, the lung tissue exposed to UDCA had lower levels of viral infection.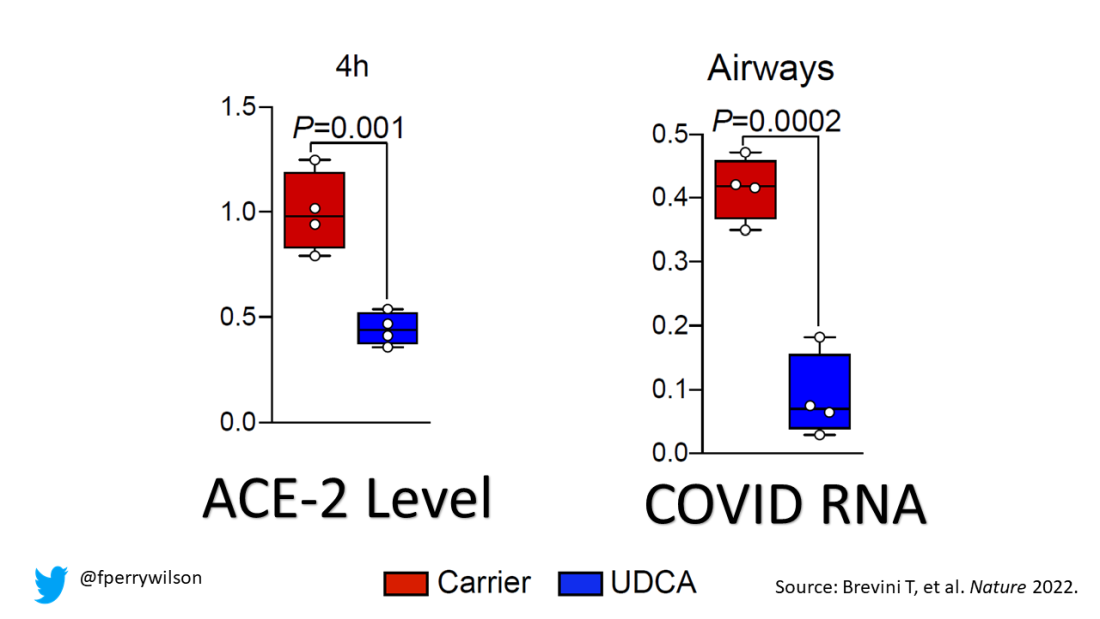
They didn’t stop there.
Eight human volunteers were recruited to take UDCA for 5 days. ACE2 levels in the nasal passages went down over the course of treatment. They confirmed those results from a proteomics dataset with several hundred people who had received UDCA for clinical reasons. Treated individuals had lower ACE2 levels.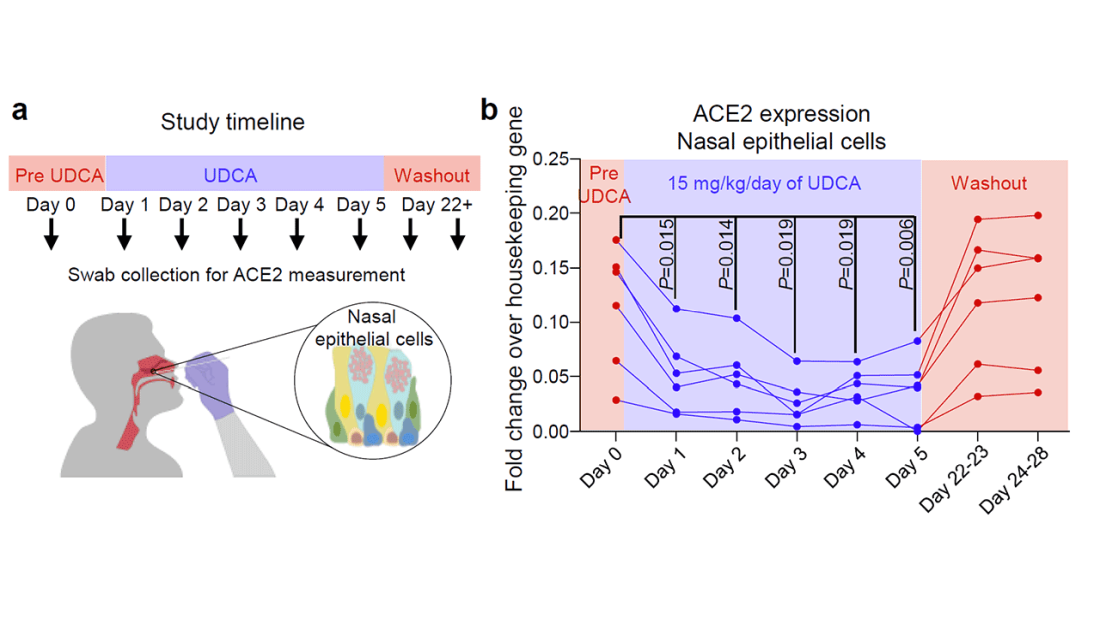
Finally, they looked at the epidemiologic effect. They examined a dataset that contained information on over 1,000 patients with liver disease who had contracted COVID-19, 31 of whom had been receiving UDCA. Even after adjustment for baseline differences, those receiving UDCA were less likely to be hospitalized, require an ICU, or die.
Okay, we’ll stop there. Reading this study, all I could think was, Yes! This is how you generate evidence that you have a drug that might work – step by careful step.
But let’s be careful as well. Does this study show that taking Actigall will prevent COVID? Of course not. It doesn’t show that it will treat COVID either. But I bring it up because the rigor of this study stands in contrast to those that generated huge enthusiasm earlier in the pandemic only to let us down in randomized trials. If there has been a drug out there this whole time which will prevent or treat COVID, this is how we’ll find it. The next step? Test it in a randomized trial.
For Medscape, I’m Perry Wilson.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant financial relationships.
A version of this video transcript first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
As soon as the pandemic started, the search was on for a medication that could stave off infection, or at least the worst consequences of infection.
One that would be cheap to make, safe, easy to distribute, and, ideally, was already available. The search had a quest-like quality, like something from a fairy tale. Society, poisoned by COVID, would find the antidote out there, somewhere, if we looked hard enough.
You know the story. There were some pretty dramatic failures: hydroxychloroquine, ivermectin. There were some successes, like dexamethasone.
I’m not here today to tell you that the antidote has been found – no, it takes large randomized trials to figure that out. But
How do you make a case that an existing drug – UDCA, in this case – might be useful to prevent or treat COVID? In contrast to prior basic-science studies, like the original ivermectin study, which essentially took a bunch of cells and virus in a tube filled with varying concentrations of the antiparasitic agent, the authors of this paper appearing in Nature give us multiple, complementary lines of evidence. Let me walk you through it.
All good science starts with a biologically plausible hypothesis. In this case, the authors recognized that SARS-CoV-2, in all its variants, requires the presence of the ACE2 receptor on the surface of cells to bind.
That is the doorway to infection. Vaccines and antibodies block the key to this door, the spike protein and its receptor binding domain. But what if you could get rid of the doors altogether?
The authors first showed that ACE2 expression is controlled by a certain transcription factor known as the farnesoid X receptor, or FXR. Reducing the binding of FXR should therefore reduce ACE2 expression.
As luck would have it, UDCA – Actigall – reduces the levels of FXR and thus the expression of ACE2 in cells.
Okay. So we have a drug that can reduce ACE2, and we know that ACE2 is necessary for the virus to infect cells. Would UDCA prevent viral infection?
They started with test tubes, showing that cells were less likely to be infected by SARS-CoV-2 in the presence of UDCA at concentrations similar to what humans achieve in their blood after standard dosing. The red staining here is spike protein; you can see that it is markedly lower in the cells exposed to UDCA.
So far, so good. But test tubes aren’t people. So they moved up to mice and Syrian golden hamsters. These cute fellows are quite susceptible to human COVID and have been a model organism in countless studies
Mice and hamsters treated with UDCA in the presence of littermates with COVID infections were less likely to become infected themselves compared with mice not so treated. They also showed that mice and hamsters treated with UDCA had lower levels of ACE2 in their nasal passages.
Of course, mice aren’t humans either. So the researchers didn’t stop there.
To determine the effects of UDCA on human tissue, they utilized perfused human lungs that had been declined for transplantation. The lungs were perfused with a special fluid to keep them viable, and were mechanically ventilated. One lung was exposed to UDCA and the other served as a control. The authors were able to show that ACE2 levels went down in the exposed lung. And, importantly, when samples of tissue from both lungs were exposed to SARS-CoV-2, the lung tissue exposed to UDCA had lower levels of viral infection.
They didn’t stop there.
Eight human volunteers were recruited to take UDCA for 5 days. ACE2 levels in the nasal passages went down over the course of treatment. They confirmed those results from a proteomics dataset with several hundred people who had received UDCA for clinical reasons. Treated individuals had lower ACE2 levels.
Finally, they looked at the epidemiologic effect. They examined a dataset that contained information on over 1,000 patients with liver disease who had contracted COVID-19, 31 of whom had been receiving UDCA. Even after adjustment for baseline differences, those receiving UDCA were less likely to be hospitalized, require an ICU, or die.
Okay, we’ll stop there. Reading this study, all I could think was, Yes! This is how you generate evidence that you have a drug that might work – step by careful step.
But let’s be careful as well. Does this study show that taking Actigall will prevent COVID? Of course not. It doesn’t show that it will treat COVID either. But I bring it up because the rigor of this study stands in contrast to those that generated huge enthusiasm earlier in the pandemic only to let us down in randomized trials. If there has been a drug out there this whole time which will prevent or treat COVID, this is how we’ll find it. The next step? Test it in a randomized trial.
For Medscape, I’m Perry Wilson.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant financial relationships.
A version of this video transcript first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
As soon as the pandemic started, the search was on for a medication that could stave off infection, or at least the worst consequences of infection.
One that would be cheap to make, safe, easy to distribute, and, ideally, was already available. The search had a quest-like quality, like something from a fairy tale. Society, poisoned by COVID, would find the antidote out there, somewhere, if we looked hard enough.
You know the story. There were some pretty dramatic failures: hydroxychloroquine, ivermectin. There were some successes, like dexamethasone.
I’m not here today to tell you that the antidote has been found – no, it takes large randomized trials to figure that out. But
How do you make a case that an existing drug – UDCA, in this case – might be useful to prevent or treat COVID? In contrast to prior basic-science studies, like the original ivermectin study, which essentially took a bunch of cells and virus in a tube filled with varying concentrations of the antiparasitic agent, the authors of this paper appearing in Nature give us multiple, complementary lines of evidence. Let me walk you through it.
All good science starts with a biologically plausible hypothesis. In this case, the authors recognized that SARS-CoV-2, in all its variants, requires the presence of the ACE2 receptor on the surface of cells to bind.
That is the doorway to infection. Vaccines and antibodies block the key to this door, the spike protein and its receptor binding domain. But what if you could get rid of the doors altogether?
The authors first showed that ACE2 expression is controlled by a certain transcription factor known as the farnesoid X receptor, or FXR. Reducing the binding of FXR should therefore reduce ACE2 expression.
As luck would have it, UDCA – Actigall – reduces the levels of FXR and thus the expression of ACE2 in cells.
Okay. So we have a drug that can reduce ACE2, and we know that ACE2 is necessary for the virus to infect cells. Would UDCA prevent viral infection?
They started with test tubes, showing that cells were less likely to be infected by SARS-CoV-2 in the presence of UDCA at concentrations similar to what humans achieve in their blood after standard dosing. The red staining here is spike protein; you can see that it is markedly lower in the cells exposed to UDCA.
So far, so good. But test tubes aren’t people. So they moved up to mice and Syrian golden hamsters. These cute fellows are quite susceptible to human COVID and have been a model organism in countless studies
Mice and hamsters treated with UDCA in the presence of littermates with COVID infections were less likely to become infected themselves compared with mice not so treated. They also showed that mice and hamsters treated with UDCA had lower levels of ACE2 in their nasal passages.
Of course, mice aren’t humans either. So the researchers didn’t stop there.
To determine the effects of UDCA on human tissue, they utilized perfused human lungs that had been declined for transplantation. The lungs were perfused with a special fluid to keep them viable, and were mechanically ventilated. One lung was exposed to UDCA and the other served as a control. The authors were able to show that ACE2 levels went down in the exposed lung. And, importantly, when samples of tissue from both lungs were exposed to SARS-CoV-2, the lung tissue exposed to UDCA had lower levels of viral infection.
They didn’t stop there.
Eight human volunteers were recruited to take UDCA for 5 days. ACE2 levels in the nasal passages went down over the course of treatment. They confirmed those results from a proteomics dataset with several hundred people who had received UDCA for clinical reasons. Treated individuals had lower ACE2 levels.
Finally, they looked at the epidemiologic effect. They examined a dataset that contained information on over 1,000 patients with liver disease who had contracted COVID-19, 31 of whom had been receiving UDCA. Even after adjustment for baseline differences, those receiving UDCA were less likely to be hospitalized, require an ICU, or die.
Okay, we’ll stop there. Reading this study, all I could think was, Yes! This is how you generate evidence that you have a drug that might work – step by careful step.
But let’s be careful as well. Does this study show that taking Actigall will prevent COVID? Of course not. It doesn’t show that it will treat COVID either. But I bring it up because the rigor of this study stands in contrast to those that generated huge enthusiasm earlier in the pandemic only to let us down in randomized trials. If there has been a drug out there this whole time which will prevent or treat COVID, this is how we’ll find it. The next step? Test it in a randomized trial.
For Medscape, I’m Perry Wilson.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant financial relationships.
A version of this video transcript first appeared on Medscape.com.
Paxlovid has been free so far. Next year, sticker shock awaits
Nearly 6 million Americans have taken Paxlovid for free, courtesy of the federal government. The Pfizer pill has helped prevent many people infected with COVID-19 from being hospitalized or dying, and it may even reduce the risk of developing long COVID.
And that means fewer people will get the potentially lifesaving treatments, experts said.
“I think the numbers will go way down,” said Jill Rosenthal, director of public health policy at the Center for American Progress, a left-leaning think tank. A bill for several hundred dollars or more would lead many people to decide the medication isn’t worth the price, she said.
In response to the unprecedented public health crisis caused by COVID, the federal government spent billions of dollars on developing new vaccines and treatments, to swift success: Less than a year after the pandemic was declared, medical workers got their first vaccines. But as many people have refused the shots and stopped wearing masks, the virus still rages and mutates. In 2022 alone, 250,000 Americans have died from COVID, more than from strokes or diabetes.
But soon the Department of Health & Human Services will stop supplying COVID treatments, and pharmacies will purchase and bill for them the same way they do for antibiotic pills or asthma inhalers. Paxlovid is expected to hit the private market in mid-2023, according to HHS plans shared in an October meeting with state health officials and clinicians. Merck’s Lagevrio, a less-effective COVID treatment pill, and AstraZeneca’s Evusheld, a preventive therapy for the immunocompromised, are on track to be commercialized sooner, sometime in the winter.
The U.S. government has so far purchased 20 million courses of Paxlovid, priced at about $530 each, a discount for buying in bulk that Pfizer CEO Albert Bourla called “really very attractive” to the federal government in a July earnings call. The drug will cost far more on the private market, although in a statement to Kaiser Health News, Pfizer declined to share the planned price. The government will also stop paying for the company’s COVID vaccine next year – those shots will quadruple in price, from the discount rate the government pays of $30 to about $120.
Mr. Bourla told investors in November that he expects the move will make Paxlovid and its COVID vaccine “a multibillion-dollars franchise.”
Nearly 9 in 10 people dying from the virus now are 65 or older. Yet federal law restricts Medicare Part D – the prescription drug program that covers nearly 50 million seniors – from covering the COVID treatment pills. The medications are meant for those most at risk of serious illness, including seniors.
Paxlovid and the other treatments are currently available under an emergency use authorization from the FDA, a fast-track review used in extraordinary situations. Although Pfizer applied for full approval in June, the process can take anywhere from several months to years. And Medicare Part D can’t cover any medications without that full stamp of approval.
Paying out-of-pocket would be “a substantial barrier” for seniors on Medicare – the very people who would benefit most from the drug, wrote federal health experts.
“From a public health perspective, and even from a health care capacity and cost perspective, it would just defy reason to not continue to make these drugs readily available,” said Dr. Larry Madoff, medical director of Massachusetts’s Bureau of Infectious Disease and Laboratory Sciences. He’s hopeful that the federal health agency will find a way to set aside unused doses for seniors and people without insurance.
In mid-November, the White House requested that Congress approve an additional $2.5 billion for COVID therapeutics and vaccines to make sure people can afford the medications when they’re no longer free. But there’s little hope it will be approved – the Senate voted that same day to end the public health emergency and denied similar requests in recent months.
Many Americans have already faced hurdles just getting a prescription for COVID treatment. Although the federal government doesn’t track who’s gotten the drug, a Centers for Disease Control and Prevention study using data from 30 medical centers found that Black and Hispanic patients with COVID were much less likely to receive Paxlovid than White patients. (Hispanic people can be of any race or combination of races.) And when the government is no longer picking up the tab, experts predict that these gaps by race, income, and geography will widen.
People in Northeastern states used the drug far more often than those in the rest of the country, according to a KHN analysis of Paxlovid use in September and October. But it wasn’t because people in the region were getting sick from COVID at much higher rates – instead, many of those states offered better access to health care to begin with and created special programs to get Paxlovid to their residents.
About 10 mostly Democratic states and several large counties in the Northeast and elsewhere created free “test-to-treat” programs that allow their residents to get an immediate doctor visit and prescription for treatment after testing positive for COVID. In Massachusetts, more than 20,000 residents have used the state’s video and phone hotline, which is available 7 days a week in 13 languages. Massachusetts, which has the highest insurance rate in the country and relatively low travel times to pharmacies, had the second-highest Paxlovid usage rate among states this fall.
States with higher COVID death rates, like Florida and Kentucky, where residents must travel farther for health care and are more likely to be uninsured, used the drug less often. Without no-cost test-to-treat options, residents have struggled to get prescriptions even though the drug itself is still free.
“If you look at access to medications for people who are uninsured, I think that there’s no question that will widen those disparities,” Ms. Rosenthal said.
People who get insurance through their jobs could face high copays at the register, too, just as they do for insulin and other expensive or brand-name drugs.
Most private insurance companies will end up covering COVID therapeutics to some extent, said Sabrina Corlette, a research professor at Georgetown University’s Center on Health Insurance Reforms. After all, the pills are cheaper than a hospital stay. But for most people who get insurance through their jobs, there are “really no rules at all,” she said. Some insurers could take months to add the drugs to their plans or decide not to pay for them.
And the additional cost means many people will go without the medication. “We know from lots of research that when people face cost sharing for these drugs that they need to take, they will often forgo or cut back,” Ms. Corlette said.
One group doesn’t need to worry about sticker shock. Medicaid, the public insurance program for low-income adults and children, will cover the treatments in full until at least early 2024.
HHS officials could set aside any leftover taxpayer-funded medication for people who can’t afford to pay the full cost, but they haven’t shared any concrete plans to do so. The government purchased 20 million courses of Paxlovid and 3 million of Lagevrio. Fewer than a third have been used, and usage has fallen in recent months, according to KHN’s analysis of the data from HHS.
Sixty percent of the government’s supply of Evusheld is also still available, although the COVID prevention therapy is less effective against new strains of the virus. The health department in one state, New Mexico, has recommended against using it.
HHS did not make officials available for an interview or answer written questions about the commercialization plans.
The government created a potential workaround when they moved bebtelovimab, another COVID treatment, to the private market this summer. It now retails for $2,100 per patient. The agency set aside the remaining 60,000 government-purchased doses that hospitals could use to treat uninsured patients in a convoluted dose-replacement process. But it’s hard to tell how well that setup would work for Paxlovid: Bebtelovimab was already much less popular, and the FDA halted its use on Nov. 30 because it’s less effective against current strains of the virus.
Federal officials and insurance companies would have good reason to make sure patients can continue to afford COVID drugs: They’re far cheaper than if patients land in the emergency room.
“The medications are so worthwhile,” said Dr. Madoff, the Massachusetts health official. “They’re not expensive in the grand scheme of health care costs.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Nearly 6 million Americans have taken Paxlovid for free, courtesy of the federal government. The Pfizer pill has helped prevent many people infected with COVID-19 from being hospitalized or dying, and it may even reduce the risk of developing long COVID.
And that means fewer people will get the potentially lifesaving treatments, experts said.
“I think the numbers will go way down,” said Jill Rosenthal, director of public health policy at the Center for American Progress, a left-leaning think tank. A bill for several hundred dollars or more would lead many people to decide the medication isn’t worth the price, she said.
In response to the unprecedented public health crisis caused by COVID, the federal government spent billions of dollars on developing new vaccines and treatments, to swift success: Less than a year after the pandemic was declared, medical workers got their first vaccines. But as many people have refused the shots and stopped wearing masks, the virus still rages and mutates. In 2022 alone, 250,000 Americans have died from COVID, more than from strokes or diabetes.
But soon the Department of Health & Human Services will stop supplying COVID treatments, and pharmacies will purchase and bill for them the same way they do for antibiotic pills or asthma inhalers. Paxlovid is expected to hit the private market in mid-2023, according to HHS plans shared in an October meeting with state health officials and clinicians. Merck’s Lagevrio, a less-effective COVID treatment pill, and AstraZeneca’s Evusheld, a preventive therapy for the immunocompromised, are on track to be commercialized sooner, sometime in the winter.
The U.S. government has so far purchased 20 million courses of Paxlovid, priced at about $530 each, a discount for buying in bulk that Pfizer CEO Albert Bourla called “really very attractive” to the federal government in a July earnings call. The drug will cost far more on the private market, although in a statement to Kaiser Health News, Pfizer declined to share the planned price. The government will also stop paying for the company’s COVID vaccine next year – those shots will quadruple in price, from the discount rate the government pays of $30 to about $120.
Mr. Bourla told investors in November that he expects the move will make Paxlovid and its COVID vaccine “a multibillion-dollars franchise.”
Nearly 9 in 10 people dying from the virus now are 65 or older. Yet federal law restricts Medicare Part D – the prescription drug program that covers nearly 50 million seniors – from covering the COVID treatment pills. The medications are meant for those most at risk of serious illness, including seniors.
Paxlovid and the other treatments are currently available under an emergency use authorization from the FDA, a fast-track review used in extraordinary situations. Although Pfizer applied for full approval in June, the process can take anywhere from several months to years. And Medicare Part D can’t cover any medications without that full stamp of approval.
Paying out-of-pocket would be “a substantial barrier” for seniors on Medicare – the very people who would benefit most from the drug, wrote federal health experts.
“From a public health perspective, and even from a health care capacity and cost perspective, it would just defy reason to not continue to make these drugs readily available,” said Dr. Larry Madoff, medical director of Massachusetts’s Bureau of Infectious Disease and Laboratory Sciences. He’s hopeful that the federal health agency will find a way to set aside unused doses for seniors and people without insurance.
In mid-November, the White House requested that Congress approve an additional $2.5 billion for COVID therapeutics and vaccines to make sure people can afford the medications when they’re no longer free. But there’s little hope it will be approved – the Senate voted that same day to end the public health emergency and denied similar requests in recent months.
Many Americans have already faced hurdles just getting a prescription for COVID treatment. Although the federal government doesn’t track who’s gotten the drug, a Centers for Disease Control and Prevention study using data from 30 medical centers found that Black and Hispanic patients with COVID were much less likely to receive Paxlovid than White patients. (Hispanic people can be of any race or combination of races.) And when the government is no longer picking up the tab, experts predict that these gaps by race, income, and geography will widen.
People in Northeastern states used the drug far more often than those in the rest of the country, according to a KHN analysis of Paxlovid use in September and October. But it wasn’t because people in the region were getting sick from COVID at much higher rates – instead, many of those states offered better access to health care to begin with and created special programs to get Paxlovid to their residents.
About 10 mostly Democratic states and several large counties in the Northeast and elsewhere created free “test-to-treat” programs that allow their residents to get an immediate doctor visit and prescription for treatment after testing positive for COVID. In Massachusetts, more than 20,000 residents have used the state’s video and phone hotline, which is available 7 days a week in 13 languages. Massachusetts, which has the highest insurance rate in the country and relatively low travel times to pharmacies, had the second-highest Paxlovid usage rate among states this fall.
States with higher COVID death rates, like Florida and Kentucky, where residents must travel farther for health care and are more likely to be uninsured, used the drug less often. Without no-cost test-to-treat options, residents have struggled to get prescriptions even though the drug itself is still free.
“If you look at access to medications for people who are uninsured, I think that there’s no question that will widen those disparities,” Ms. Rosenthal said.
People who get insurance through their jobs could face high copays at the register, too, just as they do for insulin and other expensive or brand-name drugs.
Most private insurance companies will end up covering COVID therapeutics to some extent, said Sabrina Corlette, a research professor at Georgetown University’s Center on Health Insurance Reforms. After all, the pills are cheaper than a hospital stay. But for most people who get insurance through their jobs, there are “really no rules at all,” she said. Some insurers could take months to add the drugs to their plans or decide not to pay for them.
And the additional cost means many people will go without the medication. “We know from lots of research that when people face cost sharing for these drugs that they need to take, they will often forgo or cut back,” Ms. Corlette said.
One group doesn’t need to worry about sticker shock. Medicaid, the public insurance program for low-income adults and children, will cover the treatments in full until at least early 2024.
HHS officials could set aside any leftover taxpayer-funded medication for people who can’t afford to pay the full cost, but they haven’t shared any concrete plans to do so. The government purchased 20 million courses of Paxlovid and 3 million of Lagevrio. Fewer than a third have been used, and usage has fallen in recent months, according to KHN’s analysis of the data from HHS.
Sixty percent of the government’s supply of Evusheld is also still available, although the COVID prevention therapy is less effective against new strains of the virus. The health department in one state, New Mexico, has recommended against using it.
HHS did not make officials available for an interview or answer written questions about the commercialization plans.
The government created a potential workaround when they moved bebtelovimab, another COVID treatment, to the private market this summer. It now retails for $2,100 per patient. The agency set aside the remaining 60,000 government-purchased doses that hospitals could use to treat uninsured patients in a convoluted dose-replacement process. But it’s hard to tell how well that setup would work for Paxlovid: Bebtelovimab was already much less popular, and the FDA halted its use on Nov. 30 because it’s less effective against current strains of the virus.
Federal officials and insurance companies would have good reason to make sure patients can continue to afford COVID drugs: They’re far cheaper than if patients land in the emergency room.
“The medications are so worthwhile,” said Dr. Madoff, the Massachusetts health official. “They’re not expensive in the grand scheme of health care costs.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Nearly 6 million Americans have taken Paxlovid for free, courtesy of the federal government. The Pfizer pill has helped prevent many people infected with COVID-19 from being hospitalized or dying, and it may even reduce the risk of developing long COVID.
And that means fewer people will get the potentially lifesaving treatments, experts said.
“I think the numbers will go way down,” said Jill Rosenthal, director of public health policy at the Center for American Progress, a left-leaning think tank. A bill for several hundred dollars or more would lead many people to decide the medication isn’t worth the price, she said.
In response to the unprecedented public health crisis caused by COVID, the federal government spent billions of dollars on developing new vaccines and treatments, to swift success: Less than a year after the pandemic was declared, medical workers got their first vaccines. But as many people have refused the shots and stopped wearing masks, the virus still rages and mutates. In 2022 alone, 250,000 Americans have died from COVID, more than from strokes or diabetes.
But soon the Department of Health & Human Services will stop supplying COVID treatments, and pharmacies will purchase and bill for them the same way they do for antibiotic pills or asthma inhalers. Paxlovid is expected to hit the private market in mid-2023, according to HHS plans shared in an October meeting with state health officials and clinicians. Merck’s Lagevrio, a less-effective COVID treatment pill, and AstraZeneca’s Evusheld, a preventive therapy for the immunocompromised, are on track to be commercialized sooner, sometime in the winter.
The U.S. government has so far purchased 20 million courses of Paxlovid, priced at about $530 each, a discount for buying in bulk that Pfizer CEO Albert Bourla called “really very attractive” to the federal government in a July earnings call. The drug will cost far more on the private market, although in a statement to Kaiser Health News, Pfizer declined to share the planned price. The government will also stop paying for the company’s COVID vaccine next year – those shots will quadruple in price, from the discount rate the government pays of $30 to about $120.
Mr. Bourla told investors in November that he expects the move will make Paxlovid and its COVID vaccine “a multibillion-dollars franchise.”
Nearly 9 in 10 people dying from the virus now are 65 or older. Yet federal law restricts Medicare Part D – the prescription drug program that covers nearly 50 million seniors – from covering the COVID treatment pills. The medications are meant for those most at risk of serious illness, including seniors.
Paxlovid and the other treatments are currently available under an emergency use authorization from the FDA, a fast-track review used in extraordinary situations. Although Pfizer applied for full approval in June, the process can take anywhere from several months to years. And Medicare Part D can’t cover any medications without that full stamp of approval.
Paying out-of-pocket would be “a substantial barrier” for seniors on Medicare – the very people who would benefit most from the drug, wrote federal health experts.
“From a public health perspective, and even from a health care capacity and cost perspective, it would just defy reason to not continue to make these drugs readily available,” said Dr. Larry Madoff, medical director of Massachusetts’s Bureau of Infectious Disease and Laboratory Sciences. He’s hopeful that the federal health agency will find a way to set aside unused doses for seniors and people without insurance.
In mid-November, the White House requested that Congress approve an additional $2.5 billion for COVID therapeutics and vaccines to make sure people can afford the medications when they’re no longer free. But there’s little hope it will be approved – the Senate voted that same day to end the public health emergency and denied similar requests in recent months.
Many Americans have already faced hurdles just getting a prescription for COVID treatment. Although the federal government doesn’t track who’s gotten the drug, a Centers for Disease Control and Prevention study using data from 30 medical centers found that Black and Hispanic patients with COVID were much less likely to receive Paxlovid than White patients. (Hispanic people can be of any race or combination of races.) And when the government is no longer picking up the tab, experts predict that these gaps by race, income, and geography will widen.
People in Northeastern states used the drug far more often than those in the rest of the country, according to a KHN analysis of Paxlovid use in September and October. But it wasn’t because people in the region were getting sick from COVID at much higher rates – instead, many of those states offered better access to health care to begin with and created special programs to get Paxlovid to their residents.
About 10 mostly Democratic states and several large counties in the Northeast and elsewhere created free “test-to-treat” programs that allow their residents to get an immediate doctor visit and prescription for treatment after testing positive for COVID. In Massachusetts, more than 20,000 residents have used the state’s video and phone hotline, which is available 7 days a week in 13 languages. Massachusetts, which has the highest insurance rate in the country and relatively low travel times to pharmacies, had the second-highest Paxlovid usage rate among states this fall.
States with higher COVID death rates, like Florida and Kentucky, where residents must travel farther for health care and are more likely to be uninsured, used the drug less often. Without no-cost test-to-treat options, residents have struggled to get prescriptions even though the drug itself is still free.
“If you look at access to medications for people who are uninsured, I think that there’s no question that will widen those disparities,” Ms. Rosenthal said.
People who get insurance through their jobs could face high copays at the register, too, just as they do for insulin and other expensive or brand-name drugs.
Most private insurance companies will end up covering COVID therapeutics to some extent, said Sabrina Corlette, a research professor at Georgetown University’s Center on Health Insurance Reforms. After all, the pills are cheaper than a hospital stay. But for most people who get insurance through their jobs, there are “really no rules at all,” she said. Some insurers could take months to add the drugs to their plans or decide not to pay for them.
And the additional cost means many people will go without the medication. “We know from lots of research that when people face cost sharing for these drugs that they need to take, they will often forgo or cut back,” Ms. Corlette said.
One group doesn’t need to worry about sticker shock. Medicaid, the public insurance program for low-income adults and children, will cover the treatments in full until at least early 2024.
HHS officials could set aside any leftover taxpayer-funded medication for people who can’t afford to pay the full cost, but they haven’t shared any concrete plans to do so. The government purchased 20 million courses of Paxlovid and 3 million of Lagevrio. Fewer than a third have been used, and usage has fallen in recent months, according to KHN’s analysis of the data from HHS.
Sixty percent of the government’s supply of Evusheld is also still available, although the COVID prevention therapy is less effective against new strains of the virus. The health department in one state, New Mexico, has recommended against using it.
HHS did not make officials available for an interview or answer written questions about the commercialization plans.
The government created a potential workaround when they moved bebtelovimab, another COVID treatment, to the private market this summer. It now retails for $2,100 per patient. The agency set aside the remaining 60,000 government-purchased doses that hospitals could use to treat uninsured patients in a convoluted dose-replacement process. But it’s hard to tell how well that setup would work for Paxlovid: Bebtelovimab was already much less popular, and the FDA halted its use on Nov. 30 because it’s less effective against current strains of the virus.
Federal officials and insurance companies would have good reason to make sure patients can continue to afford COVID drugs: They’re far cheaper than if patients land in the emergency room.
“The medications are so worthwhile,” said Dr. Madoff, the Massachusetts health official. “They’re not expensive in the grand scheme of health care costs.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Immunity debt and the tripledemic
Respiratory syncytial virus (RSV) and influenza cases are surging to record numbers this winter in the wake of the COVID-19 pandemic when children were sheltering in the home, receiving virtual education, masking, and hand sanitizing, and when other precautionary health measures were in place.
RSV and flu illness in children now have hospital emergency rooms and pediatric ICUs and wards over capacity. As these respiratory infections increase and variants of SARS-CoV-2 come to dominate, we may expect the full impact of a tripledemic (RSV + flu + SARS-CoV-2).
It has been estimated that RSV causes 33 million lower respiratory infections and 3.6 million hospitalizations annually worldwide in children younger than 5 years old (Lancet. 2022 May 19. doi: 10.1016/S0140-6736(22)00478-0). RSV is typically a seasonal respiratory infection occurring in late fall through early winter, when it gives way to dominance by flu. Thus, we have experienced an out-of-season surge in RSV since it began in early fall 2022, and it persists. A likely explanation for the early and persisting surge in RSV is immunity debt (Infect Dis Now. 2021 Aug. doi: 10.1016/j.idnow.2021.05.004).
Immunity debt is an unintended consequence of prevention of infections that occurred because of public health measures to prevent spread of SARS-CoV-2 infections. The COVID-19 lockdown undoubtedly saved many lives. However, while we were sheltering from SARS-CoV-2 infections, we also were avoiding other infections, especially other respiratory infections such as RSV and flu.
Our group studied this in community-based pediatric practices in Rochester, N.Y. Physician-diagnosed, medically attended infectious disease illness visits were assessed in two child cohorts, age 6-36 months from March 15 to Dec. 31, 2020 (the pandemic period), compared with the same months in 2019 (prepandemic). One hundred forty-four children were included in the pandemic cohort and 215 in the prepandemic cohort. Visits for bronchiolitis were 7.4-fold lower (P = .04), acute otitis media 3.7-fold lower (P < .0001), viral upper respiratory infections (URI) 3.8-fold lower (P < .0001), and croup 27.5-fold lower (P < .0001) in the pandemic than the prepandemic cohort (Front Pediatr. 2021 Sep 13. doi: 10.3389/fped.2021.72248).
The significant reduction in respiratory illness during the COVID-19 epidemic we and others observed resulted in a large pool of children who did not experience RSV or flu infections for an entire year or more. Herd immunity dropped. The susceptible child population increased, including children older than typically seen. We had an immunity debt that had to be repaid, and the repayment is occurring now.
As a consequence of the surge in RSV, interest in prevention has gained more attention. In 1966, tragically, two infant deaths and hospitalization of 80% of the participating infants occurred during a clinical trial of an experimental candidate RSV vaccine, which contained an inactivated version of the entire virus. The severe side effect was later found to be caused by both an antibody and a T-cell problem. The antibody produced in response to the inactivated whole virus didn’t have very good functional activity at blocking or neutralizing the virus. That led to deposition of immune complexes and activation of complement that damaged the airways. The vaccine also triggered a T-cell response with inflammatory cytokine release that added to airway obstruction and lack of clearance of the virus. RSV vaccine development was halted and the bar for further studies was raised very high to ensure safety of any future RSV vaccines. Now, 55 years later, two RSV vaccines and a new preventive monoclonal antibody are nearing licensure.
GlaxoSmithKline (GSK) and Pfizer are in phase 3 clinical trials of a safer RSV vaccine that contains only the RSV surface protein known as protein F. Protein F changes its structure when the virus infects and fuses with human respiratory epithelial cells. The GSK and Pfizer vaccines use a molecular strategy developed at the National Institutes of Health to lock protein F into its original, prefusion configuration. A similar strategy was used by Pfizer/BioNTech and Moderna in their design of mRNA vaccines to the SARS-CoV-2 spike surface protein.
A vaccine with the F protein in its prefusion form takes care of the antibody problem that caused the severe side-effects from the 1966 version of inactivated whole virus vaccine because it induces very high-efficiency, high-potency antibodies that neutralize the RSV. The T-cell response is not as well understood and that is why studies are being done in adults first and then moving to young infants.
The new RSV vaccines are being developed for use in adults over age 60, adults with comorbidities, maternal immunization, and infants. Encouraging results were recently reported by GSK and Pfizer from adult trials. In an interim analysis, Pfizer also recently reported that maternal immunization in the late second or third trimester with their vaccine had an efficacy of 82% within a newborn’s first 90 days of life against severe lower respiratory tract illness. At age 6 months, the efficacy was sustained at 69%. So far, both the GSK and Pfizer RSV vaccines have shown a favorable safety profile.
Another strategy in the RSV prevention field has been a monoclonal antibody. Palivizumab (Synagis, AstraZeneca) is used to prevent severe RSV infections in prematurely born and other infants who are at higher risk of mortality and severe morbidity. Soon there will likely be another monoclonal antibody, called nirsevimab (Beyfortus, AstraZeneca and Sanofi). It is approved in Europe but not yet approved in the United States as I prepare this column. Nirsevimab may be even better than palivizumab – based on phase 3 trial data – and a single injection lasts through an entire normal RSV season while palivizumab requires monthly injections.
Similar to the situation with RSV, the flu season started earlier than usual in fall 2022 and has been picking up steam, likely also because of immunity debt. The WHO estimates that annual epidemics of influenza cause 1 billion infections, 3 million to 5 million severe cases, and 300,000-500,000 deaths. Seasonal flu vaccines provide modest protection. Current flu vaccine formulations consist of the hemagglutinin (H) and neuraminidase (N) proteins but those proteins change sufficiently (called antigenic drift) such that production of the vaccines based on a best guess each year often is not correct for the influenza A or influenza B strains that circulate in a given year (antigenic mismatch).
Public health authorities have long worried about a major change in the composition of the H and N proteins of the influenza virus (called antigenic shift). Preparedness and response to the COVID-19 pandemic was based on preparedness and response to an anticipated influenza pandemic similar to the 1918 flu pandemic. For flu, new “universal” vaccines are in development. Among the candidate vaccines are mRNA vaccines, building on the success of the SARS-CoV-2 mRNA vaccines (Science. 2022 Nov 24. doi: 10.1126/science.abm0271).
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
Respiratory syncytial virus (RSV) and influenza cases are surging to record numbers this winter in the wake of the COVID-19 pandemic when children were sheltering in the home, receiving virtual education, masking, and hand sanitizing, and when other precautionary health measures were in place.
RSV and flu illness in children now have hospital emergency rooms and pediatric ICUs and wards over capacity. As these respiratory infections increase and variants of SARS-CoV-2 come to dominate, we may expect the full impact of a tripledemic (RSV + flu + SARS-CoV-2).
It has been estimated that RSV causes 33 million lower respiratory infections and 3.6 million hospitalizations annually worldwide in children younger than 5 years old (Lancet. 2022 May 19. doi: 10.1016/S0140-6736(22)00478-0). RSV is typically a seasonal respiratory infection occurring in late fall through early winter, when it gives way to dominance by flu. Thus, we have experienced an out-of-season surge in RSV since it began in early fall 2022, and it persists. A likely explanation for the early and persisting surge in RSV is immunity debt (Infect Dis Now. 2021 Aug. doi: 10.1016/j.idnow.2021.05.004).
Immunity debt is an unintended consequence of prevention of infections that occurred because of public health measures to prevent spread of SARS-CoV-2 infections. The COVID-19 lockdown undoubtedly saved many lives. However, while we were sheltering from SARS-CoV-2 infections, we also were avoiding other infections, especially other respiratory infections such as RSV and flu.
Our group studied this in community-based pediatric practices in Rochester, N.Y. Physician-diagnosed, medically attended infectious disease illness visits were assessed in two child cohorts, age 6-36 months from March 15 to Dec. 31, 2020 (the pandemic period), compared with the same months in 2019 (prepandemic). One hundred forty-four children were included in the pandemic cohort and 215 in the prepandemic cohort. Visits for bronchiolitis were 7.4-fold lower (P = .04), acute otitis media 3.7-fold lower (P < .0001), viral upper respiratory infections (URI) 3.8-fold lower (P < .0001), and croup 27.5-fold lower (P < .0001) in the pandemic than the prepandemic cohort (Front Pediatr. 2021 Sep 13. doi: 10.3389/fped.2021.72248).
The significant reduction in respiratory illness during the COVID-19 epidemic we and others observed resulted in a large pool of children who did not experience RSV or flu infections for an entire year or more. Herd immunity dropped. The susceptible child population increased, including children older than typically seen. We had an immunity debt that had to be repaid, and the repayment is occurring now.
As a consequence of the surge in RSV, interest in prevention has gained more attention. In 1966, tragically, two infant deaths and hospitalization of 80% of the participating infants occurred during a clinical trial of an experimental candidate RSV vaccine, which contained an inactivated version of the entire virus. The severe side effect was later found to be caused by both an antibody and a T-cell problem. The antibody produced in response to the inactivated whole virus didn’t have very good functional activity at blocking or neutralizing the virus. That led to deposition of immune complexes and activation of complement that damaged the airways. The vaccine also triggered a T-cell response with inflammatory cytokine release that added to airway obstruction and lack of clearance of the virus. RSV vaccine development was halted and the bar for further studies was raised very high to ensure safety of any future RSV vaccines. Now, 55 years later, two RSV vaccines and a new preventive monoclonal antibody are nearing licensure.
GlaxoSmithKline (GSK) and Pfizer are in phase 3 clinical trials of a safer RSV vaccine that contains only the RSV surface protein known as protein F. Protein F changes its structure when the virus infects and fuses with human respiratory epithelial cells. The GSK and Pfizer vaccines use a molecular strategy developed at the National Institutes of Health to lock protein F into its original, prefusion configuration. A similar strategy was used by Pfizer/BioNTech and Moderna in their design of mRNA vaccines to the SARS-CoV-2 spike surface protein.
A vaccine with the F protein in its prefusion form takes care of the antibody problem that caused the severe side-effects from the 1966 version of inactivated whole virus vaccine because it induces very high-efficiency, high-potency antibodies that neutralize the RSV. The T-cell response is not as well understood and that is why studies are being done in adults first and then moving to young infants.
The new RSV vaccines are being developed for use in adults over age 60, adults with comorbidities, maternal immunization, and infants. Encouraging results were recently reported by GSK and Pfizer from adult trials. In an interim analysis, Pfizer also recently reported that maternal immunization in the late second or third trimester with their vaccine had an efficacy of 82% within a newborn’s first 90 days of life against severe lower respiratory tract illness. At age 6 months, the efficacy was sustained at 69%. So far, both the GSK and Pfizer RSV vaccines have shown a favorable safety profile.
Another strategy in the RSV prevention field has been a monoclonal antibody. Palivizumab (Synagis, AstraZeneca) is used to prevent severe RSV infections in prematurely born and other infants who are at higher risk of mortality and severe morbidity. Soon there will likely be another monoclonal antibody, called nirsevimab (Beyfortus, AstraZeneca and Sanofi). It is approved in Europe but not yet approved in the United States as I prepare this column. Nirsevimab may be even better than palivizumab – based on phase 3 trial data – and a single injection lasts through an entire normal RSV season while palivizumab requires monthly injections.
Similar to the situation with RSV, the flu season started earlier than usual in fall 2022 and has been picking up steam, likely also because of immunity debt. The WHO estimates that annual epidemics of influenza cause 1 billion infections, 3 million to 5 million severe cases, and 300,000-500,000 deaths. Seasonal flu vaccines provide modest protection. Current flu vaccine formulations consist of the hemagglutinin (H) and neuraminidase (N) proteins but those proteins change sufficiently (called antigenic drift) such that production of the vaccines based on a best guess each year often is not correct for the influenza A or influenza B strains that circulate in a given year (antigenic mismatch).
Public health authorities have long worried about a major change in the composition of the H and N proteins of the influenza virus (called antigenic shift). Preparedness and response to the COVID-19 pandemic was based on preparedness and response to an anticipated influenza pandemic similar to the 1918 flu pandemic. For flu, new “universal” vaccines are in development. Among the candidate vaccines are mRNA vaccines, building on the success of the SARS-CoV-2 mRNA vaccines (Science. 2022 Nov 24. doi: 10.1126/science.abm0271).
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
Respiratory syncytial virus (RSV) and influenza cases are surging to record numbers this winter in the wake of the COVID-19 pandemic when children were sheltering in the home, receiving virtual education, masking, and hand sanitizing, and when other precautionary health measures were in place.
RSV and flu illness in children now have hospital emergency rooms and pediatric ICUs and wards over capacity. As these respiratory infections increase and variants of SARS-CoV-2 come to dominate, we may expect the full impact of a tripledemic (RSV + flu + SARS-CoV-2).
It has been estimated that RSV causes 33 million lower respiratory infections and 3.6 million hospitalizations annually worldwide in children younger than 5 years old (Lancet. 2022 May 19. doi: 10.1016/S0140-6736(22)00478-0). RSV is typically a seasonal respiratory infection occurring in late fall through early winter, when it gives way to dominance by flu. Thus, we have experienced an out-of-season surge in RSV since it began in early fall 2022, and it persists. A likely explanation for the early and persisting surge in RSV is immunity debt (Infect Dis Now. 2021 Aug. doi: 10.1016/j.idnow.2021.05.004).
Immunity debt is an unintended consequence of prevention of infections that occurred because of public health measures to prevent spread of SARS-CoV-2 infections. The COVID-19 lockdown undoubtedly saved many lives. However, while we were sheltering from SARS-CoV-2 infections, we also were avoiding other infections, especially other respiratory infections such as RSV and flu.
Our group studied this in community-based pediatric practices in Rochester, N.Y. Physician-diagnosed, medically attended infectious disease illness visits were assessed in two child cohorts, age 6-36 months from March 15 to Dec. 31, 2020 (the pandemic period), compared with the same months in 2019 (prepandemic). One hundred forty-four children were included in the pandemic cohort and 215 in the prepandemic cohort. Visits for bronchiolitis were 7.4-fold lower (P = .04), acute otitis media 3.7-fold lower (P < .0001), viral upper respiratory infections (URI) 3.8-fold lower (P < .0001), and croup 27.5-fold lower (P < .0001) in the pandemic than the prepandemic cohort (Front Pediatr. 2021 Sep 13. doi: 10.3389/fped.2021.72248).
The significant reduction in respiratory illness during the COVID-19 epidemic we and others observed resulted in a large pool of children who did not experience RSV or flu infections for an entire year or more. Herd immunity dropped. The susceptible child population increased, including children older than typically seen. We had an immunity debt that had to be repaid, and the repayment is occurring now.
As a consequence of the surge in RSV, interest in prevention has gained more attention. In 1966, tragically, two infant deaths and hospitalization of 80% of the participating infants occurred during a clinical trial of an experimental candidate RSV vaccine, which contained an inactivated version of the entire virus. The severe side effect was later found to be caused by both an antibody and a T-cell problem. The antibody produced in response to the inactivated whole virus didn’t have very good functional activity at blocking or neutralizing the virus. That led to deposition of immune complexes and activation of complement that damaged the airways. The vaccine also triggered a T-cell response with inflammatory cytokine release that added to airway obstruction and lack of clearance of the virus. RSV vaccine development was halted and the bar for further studies was raised very high to ensure safety of any future RSV vaccines. Now, 55 years later, two RSV vaccines and a new preventive monoclonal antibody are nearing licensure.
GlaxoSmithKline (GSK) and Pfizer are in phase 3 clinical trials of a safer RSV vaccine that contains only the RSV surface protein known as protein F. Protein F changes its structure when the virus infects and fuses with human respiratory epithelial cells. The GSK and Pfizer vaccines use a molecular strategy developed at the National Institutes of Health to lock protein F into its original, prefusion configuration. A similar strategy was used by Pfizer/BioNTech and Moderna in their design of mRNA vaccines to the SARS-CoV-2 spike surface protein.
A vaccine with the F protein in its prefusion form takes care of the antibody problem that caused the severe side-effects from the 1966 version of inactivated whole virus vaccine because it induces very high-efficiency, high-potency antibodies that neutralize the RSV. The T-cell response is not as well understood and that is why studies are being done in adults first and then moving to young infants.
The new RSV vaccines are being developed for use in adults over age 60, adults with comorbidities, maternal immunization, and infants. Encouraging results were recently reported by GSK and Pfizer from adult trials. In an interim analysis, Pfizer also recently reported that maternal immunization in the late second or third trimester with their vaccine had an efficacy of 82% within a newborn’s first 90 days of life against severe lower respiratory tract illness. At age 6 months, the efficacy was sustained at 69%. So far, both the GSK and Pfizer RSV vaccines have shown a favorable safety profile.
Another strategy in the RSV prevention field has been a monoclonal antibody. Palivizumab (Synagis, AstraZeneca) is used to prevent severe RSV infections in prematurely born and other infants who are at higher risk of mortality and severe morbidity. Soon there will likely be another monoclonal antibody, called nirsevimab (Beyfortus, AstraZeneca and Sanofi). It is approved in Europe but not yet approved in the United States as I prepare this column. Nirsevimab may be even better than palivizumab – based on phase 3 trial data – and a single injection lasts through an entire normal RSV season while palivizumab requires monthly injections.
Similar to the situation with RSV, the flu season started earlier than usual in fall 2022 and has been picking up steam, likely also because of immunity debt. The WHO estimates that annual epidemics of influenza cause 1 billion infections, 3 million to 5 million severe cases, and 300,000-500,000 deaths. Seasonal flu vaccines provide modest protection. Current flu vaccine formulations consist of the hemagglutinin (H) and neuraminidase (N) proteins but those proteins change sufficiently (called antigenic drift) such that production of the vaccines based on a best guess each year often is not correct for the influenza A or influenza B strains that circulate in a given year (antigenic mismatch).
Public health authorities have long worried about a major change in the composition of the H and N proteins of the influenza virus (called antigenic shift). Preparedness and response to the COVID-19 pandemic was based on preparedness and response to an anticipated influenza pandemic similar to the 1918 flu pandemic. For flu, new “universal” vaccines are in development. Among the candidate vaccines are mRNA vaccines, building on the success of the SARS-CoV-2 mRNA vaccines (Science. 2022 Nov 24. doi: 10.1126/science.abm0271).
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
Children and COVID: Hospitalizations provide a tale of two sources
New cases of COVID-19 in children largely held steady over the Thanksgiving holiday, but hospital admissions are telling a somewhat different story.
New pediatric COVID cases for the week ending on Thanksgiving (11/18-11/24) were up by 5.3% over the previous week, but in the most recent week (11/25-12/1) new cases dropped by 2.6%, according to state data collected by the American Academy of Pediatrics and the Children’s Hospital Association.
In both weeks, though, the total case count stayed below 30,000 – a streak that has now lasted 8 weeks – so the actual number of weekly cases remained fairly low, the AAP/CHA weekly report indicates.
The nation’s emergency departments also experienced a small Thanksgiving bump, as the proportion of visits with diagnosed COVID went from 1.0% of all ED visits for children aged 0-11 years on Nov. 14 to 2.0% on Nov. 27, just 3 days after the official holiday, based on data from the Centers for Disease Control and Prevention. The rate was down to 1.5% on Dec. 1, and similar patterns can be seen for children aged 12-15 and 16-17 years.
New hospital admissions, on the other hand, seem to be following a different path, at least according to the CDC. The hospitalization rate for children aged 0-17 years bottomed out at 0.16 new admissions per 100,000 population back on Oct. 21 and has climbed fairly steadily since then. It was up to 0.20 per 100,000 by Nov. 14, had reached 0.22 per 100,000 on Thanksgiving day (11/24), and then continued to 0.26 per 100,000 by Dec. 2, the latest date for which CDC data are available.
The hospitalization story, however, offers yet another twist. The New York Times, using data from the U.S. Department of Health & Human Services, reports that new COVID-related admissions have held steady at 1.0 per 100,000 since Nov. 18. The rate is much higher than has been reported by the CDC, but no increase can be seen in recent weeks among children, which is not the case for Americans overall, Medscape recently reported.
New cases of COVID-19 in children largely held steady over the Thanksgiving holiday, but hospital admissions are telling a somewhat different story.
New pediatric COVID cases for the week ending on Thanksgiving (11/18-11/24) were up by 5.3% over the previous week, but in the most recent week (11/25-12/1) new cases dropped by 2.6%, according to state data collected by the American Academy of Pediatrics and the Children’s Hospital Association.
In both weeks, though, the total case count stayed below 30,000 – a streak that has now lasted 8 weeks – so the actual number of weekly cases remained fairly low, the AAP/CHA weekly report indicates.
The nation’s emergency departments also experienced a small Thanksgiving bump, as the proportion of visits with diagnosed COVID went from 1.0% of all ED visits for children aged 0-11 years on Nov. 14 to 2.0% on Nov. 27, just 3 days after the official holiday, based on data from the Centers for Disease Control and Prevention. The rate was down to 1.5% on Dec. 1, and similar patterns can be seen for children aged 12-15 and 16-17 years.
New hospital admissions, on the other hand, seem to be following a different path, at least according to the CDC. The hospitalization rate for children aged 0-17 years bottomed out at 0.16 new admissions per 100,000 population back on Oct. 21 and has climbed fairly steadily since then. It was up to 0.20 per 100,000 by Nov. 14, had reached 0.22 per 100,000 on Thanksgiving day (11/24), and then continued to 0.26 per 100,000 by Dec. 2, the latest date for which CDC data are available.
The hospitalization story, however, offers yet another twist. The New York Times, using data from the U.S. Department of Health & Human Services, reports that new COVID-related admissions have held steady at 1.0 per 100,000 since Nov. 18. The rate is much higher than has been reported by the CDC, but no increase can be seen in recent weeks among children, which is not the case for Americans overall, Medscape recently reported.
New cases of COVID-19 in children largely held steady over the Thanksgiving holiday, but hospital admissions are telling a somewhat different story.
New pediatric COVID cases for the week ending on Thanksgiving (11/18-11/24) were up by 5.3% over the previous week, but in the most recent week (11/25-12/1) new cases dropped by 2.6%, according to state data collected by the American Academy of Pediatrics and the Children’s Hospital Association.
In both weeks, though, the total case count stayed below 30,000 – a streak that has now lasted 8 weeks – so the actual number of weekly cases remained fairly low, the AAP/CHA weekly report indicates.
The nation’s emergency departments also experienced a small Thanksgiving bump, as the proportion of visits with diagnosed COVID went from 1.0% of all ED visits for children aged 0-11 years on Nov. 14 to 2.0% on Nov. 27, just 3 days after the official holiday, based on data from the Centers for Disease Control and Prevention. The rate was down to 1.5% on Dec. 1, and similar patterns can be seen for children aged 12-15 and 16-17 years.
New hospital admissions, on the other hand, seem to be following a different path, at least according to the CDC. The hospitalization rate for children aged 0-17 years bottomed out at 0.16 new admissions per 100,000 population back on Oct. 21 and has climbed fairly steadily since then. It was up to 0.20 per 100,000 by Nov. 14, had reached 0.22 per 100,000 on Thanksgiving day (11/24), and then continued to 0.26 per 100,000 by Dec. 2, the latest date for which CDC data are available.
The hospitalization story, however, offers yet another twist. The New York Times, using data from the U.S. Department of Health & Human Services, reports that new COVID-related admissions have held steady at 1.0 per 100,000 since Nov. 18. The rate is much higher than has been reported by the CDC, but no increase can be seen in recent weeks among children, which is not the case for Americans overall, Medscape recently reported.
Study comparing surgical and N95 masks sparks concern
The study’s senior author is John Conly, MD, an infectious disease specialist and professor at the University of Calgary (Alta.), and Alberta Health Services. The findings are not consistent with those of many other studies on this topic.
Commenting about Dr. Conly’s study, Eric Topol, MD, editor-in-chief of Medscape, wrote: “It’s woefully underpowered but ruled out a doubling of hazard for use of medical masks.”
The study, which was partially funded by the World Health Organization, was published online in Annals of Internal Medicine.
This is not the first time that Dr. Conly, who also advises the WHO, has been the subject of controversy. He previously denied that COVID-19 is airborne – a position that is contradicted by strong evidence. In 2021, Dr. Conly made headlines with his controversial claim that N95 respirators can cause harms, including oxygen depletion and carbon dioxide retention.
A detailed examination by the Center for Infectious Disease Research and Policy (CIDRAP) at the University of Minnesota, Minneapolis, pointed out numerous scientific flaws in the study, including inconsistent use of both types of masks. The study also examined health care workers in four very different countries (Canada, Israel, Egypt, and Pakistan) during different periods of the pandemic, which may have affected the results. Furthermore, the study did not account for vaccination status and lacked a control group. CIDRAP receives funding from 3M, which makes N95 respirators.
In a commentary published alongside the study, Roger Chou, MD, professor of medicine at Oregon Health & Science University, Portland, said that the results were “not definitive,” with “a generous noninferiority threshold” that is actually “consistent with up to a relative 70% increased risk ... which may be unacceptable to many health workers.”
Lead study author Mark Loeb, MD, professor of infectious diseases at McMaster University, Hamilton, Ont., defended the findings. “The confidence intervals around this, that is, what the possible results could be if the trial was repeated many times, range from −2.5% to 4.9%,” he told this news organization. “This means that the risk of a COVID-19 infection in those using the medical masks could have ranged from anywhere from 2.5% reduction in risk to a 4.9% increase in risk. Readers and policy makers can decide for themselves about this.”
“There is no point continuing to run underpowered, poorly designed studies that are designed to confirm existing biases,” Raina MacIntyre, PhD, professor of global biosecurity and head of the Biosecurity Program at the Kirby Institute, Sydney, said in an interview. “The new study in Annals of Internal Medicine is entirely consistent with our finding that to prevent infection, you need an N95, and it needs to be worn throughout the whole shift. A surgical mask and intermittent use of N95 are equally ineffective. This should not surprise anyone, given a surgical mask is not designed as respiratory protection but is designed to prevent splash or spray of liquid on the face. Only a respirator is designed as respiratory protection through both the seal around the face and the filter of the face piece to prevent inhalation of virus laden aerosols, but you need to wear it continually in a high-risk environment like a hospital.”
“It makes zero sense to do a randomized trial on something you can measure directly,” said Kimberly Prather, PhD, an atmospheric chemist, professor, and director of the NSF Center for Aerosol Impacts on Chemistry of the Environment at the University of California, San Diego. “In fact, many studies have shown aerosols leaking out of surgical masks. Surgical masks are designed to block large spray droplets. Aerosols (0.5-3 mcm), which have been shown to contain infectious SARS-CoV-2 virus, travel with the air flow, and escape.”
“This study ... will be used to justify policies of supplying health care workers, and perhaps patients and visitors, too, with inadequate protection,” Trish Greenhalgh, MD, professor of primary care health sciences at the University of Oxford (England), told this news organization.
“These authors have been pushing back against treating COVID as airborne for 3 years,” David Fisman, MD, an epidemiologist and infectious disease specialist at the University of Toronto, said in an interview. “So, you’ll see these folks brandishing this very flawed trial to justify continuing the infection control practices that have been so disastrous throughout the pandemic.”
The study was funded by the World Health Organization, the Canadian Institutes of Health Research, and the Juravinski Research Institute. Dr. Conly reported receiving grants from the Canadian Institutes for Health Research, Pfizer, and the WHO. Dr. Chou disclosed being a methodologist for WHO guidelines on infection prevention and control measures for COVID-19. Dr. Loeb disclosed payment for expert testimony on personal protective equipment from the government of Manitoba and the Peel District School Board. Dr. MacIntyre has led a large body of research on masks and respirators in health workers, including four randomized clinical trials. She is the author of a book, “Dark Winter: An insider’s guide to pandemics and biosecurity” (Syndey: NewSouth Publishing, 2022), which covers the history and politics of the controversies around N95 and masks. Dr. Prather reported no disclosures. Dr. Greenhalgh is a member of Independent SAGE and an unpaid adviser to the philanthropic fund Balvi. Dr. Fisman has served as a paid legal expert for the Ontario Nurses’ Association in their challenge to Directive 5, which restricted access to N95 masks in health care. He also served as a paid legal expert for the Elementary Teachers’ Federation of Ontario in its efforts to make schools safer in Ontario.
A version of this article first appeared on Medscape.com.
The study’s senior author is John Conly, MD, an infectious disease specialist and professor at the University of Calgary (Alta.), and Alberta Health Services. The findings are not consistent with those of many other studies on this topic.
Commenting about Dr. Conly’s study, Eric Topol, MD, editor-in-chief of Medscape, wrote: “It’s woefully underpowered but ruled out a doubling of hazard for use of medical masks.”
The study, which was partially funded by the World Health Organization, was published online in Annals of Internal Medicine.
This is not the first time that Dr. Conly, who also advises the WHO, has been the subject of controversy. He previously denied that COVID-19 is airborne – a position that is contradicted by strong evidence. In 2021, Dr. Conly made headlines with his controversial claim that N95 respirators can cause harms, including oxygen depletion and carbon dioxide retention.
A detailed examination by the Center for Infectious Disease Research and Policy (CIDRAP) at the University of Minnesota, Minneapolis, pointed out numerous scientific flaws in the study, including inconsistent use of both types of masks. The study also examined health care workers in four very different countries (Canada, Israel, Egypt, and Pakistan) during different periods of the pandemic, which may have affected the results. Furthermore, the study did not account for vaccination status and lacked a control group. CIDRAP receives funding from 3M, which makes N95 respirators.
In a commentary published alongside the study, Roger Chou, MD, professor of medicine at Oregon Health & Science University, Portland, said that the results were “not definitive,” with “a generous noninferiority threshold” that is actually “consistent with up to a relative 70% increased risk ... which may be unacceptable to many health workers.”
Lead study author Mark Loeb, MD, professor of infectious diseases at McMaster University, Hamilton, Ont., defended the findings. “The confidence intervals around this, that is, what the possible results could be if the trial was repeated many times, range from −2.5% to 4.9%,” he told this news organization. “This means that the risk of a COVID-19 infection in those using the medical masks could have ranged from anywhere from 2.5% reduction in risk to a 4.9% increase in risk. Readers and policy makers can decide for themselves about this.”
“There is no point continuing to run underpowered, poorly designed studies that are designed to confirm existing biases,” Raina MacIntyre, PhD, professor of global biosecurity and head of the Biosecurity Program at the Kirby Institute, Sydney, said in an interview. “The new study in Annals of Internal Medicine is entirely consistent with our finding that to prevent infection, you need an N95, and it needs to be worn throughout the whole shift. A surgical mask and intermittent use of N95 are equally ineffective. This should not surprise anyone, given a surgical mask is not designed as respiratory protection but is designed to prevent splash or spray of liquid on the face. Only a respirator is designed as respiratory protection through both the seal around the face and the filter of the face piece to prevent inhalation of virus laden aerosols, but you need to wear it continually in a high-risk environment like a hospital.”
“It makes zero sense to do a randomized trial on something you can measure directly,” said Kimberly Prather, PhD, an atmospheric chemist, professor, and director of the NSF Center for Aerosol Impacts on Chemistry of the Environment at the University of California, San Diego. “In fact, many studies have shown aerosols leaking out of surgical masks. Surgical masks are designed to block large spray droplets. Aerosols (0.5-3 mcm), which have been shown to contain infectious SARS-CoV-2 virus, travel with the air flow, and escape.”
“This study ... will be used to justify policies of supplying health care workers, and perhaps patients and visitors, too, with inadequate protection,” Trish Greenhalgh, MD, professor of primary care health sciences at the University of Oxford (England), told this news organization.
“These authors have been pushing back against treating COVID as airborne for 3 years,” David Fisman, MD, an epidemiologist and infectious disease specialist at the University of Toronto, said in an interview. “So, you’ll see these folks brandishing this very flawed trial to justify continuing the infection control practices that have been so disastrous throughout the pandemic.”
The study was funded by the World Health Organization, the Canadian Institutes of Health Research, and the Juravinski Research Institute. Dr. Conly reported receiving grants from the Canadian Institutes for Health Research, Pfizer, and the WHO. Dr. Chou disclosed being a methodologist for WHO guidelines on infection prevention and control measures for COVID-19. Dr. Loeb disclosed payment for expert testimony on personal protective equipment from the government of Manitoba and the Peel District School Board. Dr. MacIntyre has led a large body of research on masks and respirators in health workers, including four randomized clinical trials. She is the author of a book, “Dark Winter: An insider’s guide to pandemics and biosecurity” (Syndey: NewSouth Publishing, 2022), which covers the history and politics of the controversies around N95 and masks. Dr. Prather reported no disclosures. Dr. Greenhalgh is a member of Independent SAGE and an unpaid adviser to the philanthropic fund Balvi. Dr. Fisman has served as a paid legal expert for the Ontario Nurses’ Association in their challenge to Directive 5, which restricted access to N95 masks in health care. He also served as a paid legal expert for the Elementary Teachers’ Federation of Ontario in its efforts to make schools safer in Ontario.
A version of this article first appeared on Medscape.com.
The study’s senior author is John Conly, MD, an infectious disease specialist and professor at the University of Calgary (Alta.), and Alberta Health Services. The findings are not consistent with those of many other studies on this topic.
Commenting about Dr. Conly’s study, Eric Topol, MD, editor-in-chief of Medscape, wrote: “It’s woefully underpowered but ruled out a doubling of hazard for use of medical masks.”
The study, which was partially funded by the World Health Organization, was published online in Annals of Internal Medicine.
This is not the first time that Dr. Conly, who also advises the WHO, has been the subject of controversy. He previously denied that COVID-19 is airborne – a position that is contradicted by strong evidence. In 2021, Dr. Conly made headlines with his controversial claim that N95 respirators can cause harms, including oxygen depletion and carbon dioxide retention.
A detailed examination by the Center for Infectious Disease Research and Policy (CIDRAP) at the University of Minnesota, Minneapolis, pointed out numerous scientific flaws in the study, including inconsistent use of both types of masks. The study also examined health care workers in four very different countries (Canada, Israel, Egypt, and Pakistan) during different periods of the pandemic, which may have affected the results. Furthermore, the study did not account for vaccination status and lacked a control group. CIDRAP receives funding from 3M, which makes N95 respirators.
In a commentary published alongside the study, Roger Chou, MD, professor of medicine at Oregon Health & Science University, Portland, said that the results were “not definitive,” with “a generous noninferiority threshold” that is actually “consistent with up to a relative 70% increased risk ... which may be unacceptable to many health workers.”
Lead study author Mark Loeb, MD, professor of infectious diseases at McMaster University, Hamilton, Ont., defended the findings. “The confidence intervals around this, that is, what the possible results could be if the trial was repeated many times, range from −2.5% to 4.9%,” he told this news organization. “This means that the risk of a COVID-19 infection in those using the medical masks could have ranged from anywhere from 2.5% reduction in risk to a 4.9% increase in risk. Readers and policy makers can decide for themselves about this.”
“There is no point continuing to run underpowered, poorly designed studies that are designed to confirm existing biases,” Raina MacIntyre, PhD, professor of global biosecurity and head of the Biosecurity Program at the Kirby Institute, Sydney, said in an interview. “The new study in Annals of Internal Medicine is entirely consistent with our finding that to prevent infection, you need an N95, and it needs to be worn throughout the whole shift. A surgical mask and intermittent use of N95 are equally ineffective. This should not surprise anyone, given a surgical mask is not designed as respiratory protection but is designed to prevent splash or spray of liquid on the face. Only a respirator is designed as respiratory protection through both the seal around the face and the filter of the face piece to prevent inhalation of virus laden aerosols, but you need to wear it continually in a high-risk environment like a hospital.”
“It makes zero sense to do a randomized trial on something you can measure directly,” said Kimberly Prather, PhD, an atmospheric chemist, professor, and director of the NSF Center for Aerosol Impacts on Chemistry of the Environment at the University of California, San Diego. “In fact, many studies have shown aerosols leaking out of surgical masks. Surgical masks are designed to block large spray droplets. Aerosols (0.5-3 mcm), which have been shown to contain infectious SARS-CoV-2 virus, travel with the air flow, and escape.”
“This study ... will be used to justify policies of supplying health care workers, and perhaps patients and visitors, too, with inadequate protection,” Trish Greenhalgh, MD, professor of primary care health sciences at the University of Oxford (England), told this news organization.
“These authors have been pushing back against treating COVID as airborne for 3 years,” David Fisman, MD, an epidemiologist and infectious disease specialist at the University of Toronto, said in an interview. “So, you’ll see these folks brandishing this very flawed trial to justify continuing the infection control practices that have been so disastrous throughout the pandemic.”
The study was funded by the World Health Organization, the Canadian Institutes of Health Research, and the Juravinski Research Institute. Dr. Conly reported receiving grants from the Canadian Institutes for Health Research, Pfizer, and the WHO. Dr. Chou disclosed being a methodologist for WHO guidelines on infection prevention and control measures for COVID-19. Dr. Loeb disclosed payment for expert testimony on personal protective equipment from the government of Manitoba and the Peel District School Board. Dr. MacIntyre has led a large body of research on masks and respirators in health workers, including four randomized clinical trials. She is the author of a book, “Dark Winter: An insider’s guide to pandemics and biosecurity” (Syndey: NewSouth Publishing, 2022), which covers the history and politics of the controversies around N95 and masks. Dr. Prather reported no disclosures. Dr. Greenhalgh is a member of Independent SAGE and an unpaid adviser to the philanthropic fund Balvi. Dr. Fisman has served as a paid legal expert for the Ontario Nurses’ Association in their challenge to Directive 5, which restricted access to N95 masks in health care. He also served as a paid legal expert for the Elementary Teachers’ Federation of Ontario in its efforts to make schools safer in Ontario.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
Have long COVID? Newest booster vaccines may help you
Yet at 58, the Arizona writer is in no hurry to get the latest vaccine booster. “I just don’t want to risk getting any sicker,” she said.
Ms. Dishner has had two doses of vaccine plus two boosters. Each time, she had what regulators consider to be mild reactions, including a sore arm, slight fever, nausea, and body aches. Still, there’s some evidence that the newest booster, which protects against some of the later variants, could help people like Ms. Dishner in several ways, said Ziyad Al-Aly, MD, a clinical epidemiologist and prolific long COVID researcher at Washington University in St. Louis.
“A bivalent booster might actually [help with] your long COVID,” he said.
There may be other benefits. “What vaccines or current vaccine boosters do is reduce your risk of progression to severe COVID-19 illness,” Dr. Al-Aly said. “You are avoiding hospital stays or even worse; you’re avoiding potentially fatal outcomes after infection. And that’s really worth it. Who wants to be in the hospital this Christmas holiday?”
Each time people are infected with SARS-CoV-2, the virus that causes COVID-19, they have a fresh risk of not only getting severely ill or dying, but of developing long COVID, Dr. Al-Aly and colleagues found in a study published in Nature Medicine. “If you dodged the bullet the first time and did not get long COVID after the first infection, if you get reinfected, you’re trying your luck again,” Dr. Al-Aly said. “I would advise people not to get reinfected, which is another reason to get the booster.”
In a recent review in The Lancet eClinicalMedicine, an international team of researchers looked at 11 studies that sought to find out if vaccines affected long COVID symptoms. Seven of those studies found that people’s symptoms improved after they were vaccinated, and four found that symptoms mostly remained the same. One found symptoms got worse in some patients.
A study of 28,000 people published in the British Medical Journal found more evidence that vaccination may help ease symptoms. “Vaccination may contribute to a reduction in the population health burden of long COVID,” the team at the United Kingdom’s Office for National Statistics concluded. Most studies found vaccination reduced the risk of getting long COVID in the first place.
Vaccines prompt the body to produce antibodies, which stop a microbe from infecting cells. They also prompt the production of immune cells called T cells, which continue to hunt down and attack a pathogen even after infection.
A booster dose could help rev up that immune response in a patient with long COVID, said Stephen J. Thomas, MD, an infectious disease specialist at Upstate Medical Center in Syracuse, N.Y., and the center’s lead principal investigator for Pfizer/BioNTech’s COVID-19 2020 vaccine trial.
Some scientists believe long COVID might be caused when the virus persists in parts of the body where the immune system isn’t particularly active. Although they don’t fully understand the workings of the many and varied long COVID symptoms, they have a good idea about why people with long COVID often do better after receiving a vaccine or booster.
“The theory is that by boosting, the immune system may be able to ‘mop up’ those virus stragglers that have remained behind after your first cleanup attempt,” Dr. Thomas said.
“The vaccine is almost lending a hand or helping your immune response to clear that virus,” Dr. Al-Aly said.
It could be difficult for long COVID patients to make an informed decision about boosters, given the lack of studies that focus exclusively on the relationship between long COVID and boosters, according to Scott Roberts, MD, associate medical director for infection prevention at Yale New Haven (Conn.) Hospital.
Dr. Roberts recommended that patients speak with their health care providers and read about the bivalent booster on trusted sites such as those sponsored by the Food and Drug Administration and the Centers for Disease Control and Prevention. Long COVID patients should get the latest boosters, especially as there’s no evidence they are unsafe for them. “The antibody response is appropriately boosted, and there is a decent chance this will help reduce the impact of long COVID as well,” he said. “Waiting will only increase the risk of getting infected and increase the chances of long COVID.”
Only 12% of Americans 5 years and older have received the updated booster, according to the CDC, although it’s recommended for everyone. Just over 80% of Americans have gotten at least one vaccine dose. Dr. Thomas understands why the uptake has been so low: Along with people like Ms. Dishner, who fear more side effects or worse symptoms, there are those who believe that hybrid immunity – vaccination immunity plus natural infection – is superior to vaccination alone and that they don’t need a booster.
Studies show that the bivalent boosters, which protect against older and newer variants, can target even the new, predominant COVID-19 strains. Whether that is enough to convince people in the no-booster camp who lost faith when their vaccinated peers started getting COVID-19 is unclear, although, as Dr. Al-Aly has pointed out, vaccinations help keep people from getting so sick that they wind up in the hospital. And, with most of the population having received at least one dose of vaccine, most of those getting infected will naturally come from among the vaccinated.
Thomas describes the expectation that vaccines would prevent everyone from getting sick as “one of the major fails” of the pandemic.
Counting on a vaccine to confer 100% immunity is “a very high bar,” he said. “I think that’s what people expected, and when they weren’t seeing it, they kind of said: ‘Well, what’s the point? You know, things are getting better. I’d rather take my chances than keep going and getting boosted.’ ”
One point – and it’s a critical one – is that vaccination immunity wanes. Plus new variants arise that can evade at least some of the immunity provided by vaccination. That’s why boosters are built into the COVID vaccination program.
While it’s not clear why some long COVID patients see improvements in their symptoms after being vaccinated or boosted and others do not, Dr. Al-Aly said there’s little evidence vaccines can make long COVID worse. “There are some reports out there that some people with long COVID, when they got a vaccine or booster, their symptoms got worse. You’ll read anecdotes on this side,” he said, adding that efforts to see if this is really happening have been inconclusive.
“The general consensus is that vaccines really save lives,” Dr. Al-Aly said. “Getting vaccinated, even if you are a long COVID patient, is better than not getting vaccinated.”
A version of this article first appeared on WebMD.com.
Yet at 58, the Arizona writer is in no hurry to get the latest vaccine booster. “I just don’t want to risk getting any sicker,” she said.
Ms. Dishner has had two doses of vaccine plus two boosters. Each time, she had what regulators consider to be mild reactions, including a sore arm, slight fever, nausea, and body aches. Still, there’s some evidence that the newest booster, which protects against some of the later variants, could help people like Ms. Dishner in several ways, said Ziyad Al-Aly, MD, a clinical epidemiologist and prolific long COVID researcher at Washington University in St. Louis.
“A bivalent booster might actually [help with] your long COVID,” he said.
There may be other benefits. “What vaccines or current vaccine boosters do is reduce your risk of progression to severe COVID-19 illness,” Dr. Al-Aly said. “You are avoiding hospital stays or even worse; you’re avoiding potentially fatal outcomes after infection. And that’s really worth it. Who wants to be in the hospital this Christmas holiday?”
Each time people are infected with SARS-CoV-2, the virus that causes COVID-19, they have a fresh risk of not only getting severely ill or dying, but of developing long COVID, Dr. Al-Aly and colleagues found in a study published in Nature Medicine. “If you dodged the bullet the first time and did not get long COVID after the first infection, if you get reinfected, you’re trying your luck again,” Dr. Al-Aly said. “I would advise people not to get reinfected, which is another reason to get the booster.”
In a recent review in The Lancet eClinicalMedicine, an international team of researchers looked at 11 studies that sought to find out if vaccines affected long COVID symptoms. Seven of those studies found that people’s symptoms improved after they were vaccinated, and four found that symptoms mostly remained the same. One found symptoms got worse in some patients.
A study of 28,000 people published in the British Medical Journal found more evidence that vaccination may help ease symptoms. “Vaccination may contribute to a reduction in the population health burden of long COVID,” the team at the United Kingdom’s Office for National Statistics concluded. Most studies found vaccination reduced the risk of getting long COVID in the first place.
Vaccines prompt the body to produce antibodies, which stop a microbe from infecting cells. They also prompt the production of immune cells called T cells, which continue to hunt down and attack a pathogen even after infection.
A booster dose could help rev up that immune response in a patient with long COVID, said Stephen J. Thomas, MD, an infectious disease specialist at Upstate Medical Center in Syracuse, N.Y., and the center’s lead principal investigator for Pfizer/BioNTech’s COVID-19 2020 vaccine trial.
Some scientists believe long COVID might be caused when the virus persists in parts of the body where the immune system isn’t particularly active. Although they don’t fully understand the workings of the many and varied long COVID symptoms, they have a good idea about why people with long COVID often do better after receiving a vaccine or booster.
“The theory is that by boosting, the immune system may be able to ‘mop up’ those virus stragglers that have remained behind after your first cleanup attempt,” Dr. Thomas said.
“The vaccine is almost lending a hand or helping your immune response to clear that virus,” Dr. Al-Aly said.
It could be difficult for long COVID patients to make an informed decision about boosters, given the lack of studies that focus exclusively on the relationship between long COVID and boosters, according to Scott Roberts, MD, associate medical director for infection prevention at Yale New Haven (Conn.) Hospital.
Dr. Roberts recommended that patients speak with their health care providers and read about the bivalent booster on trusted sites such as those sponsored by the Food and Drug Administration and the Centers for Disease Control and Prevention. Long COVID patients should get the latest boosters, especially as there’s no evidence they are unsafe for them. “The antibody response is appropriately boosted, and there is a decent chance this will help reduce the impact of long COVID as well,” he said. “Waiting will only increase the risk of getting infected and increase the chances of long COVID.”
Only 12% of Americans 5 years and older have received the updated booster, according to the CDC, although it’s recommended for everyone. Just over 80% of Americans have gotten at least one vaccine dose. Dr. Thomas understands why the uptake has been so low: Along with people like Ms. Dishner, who fear more side effects or worse symptoms, there are those who believe that hybrid immunity – vaccination immunity plus natural infection – is superior to vaccination alone and that they don’t need a booster.
Studies show that the bivalent boosters, which protect against older and newer variants, can target even the new, predominant COVID-19 strains. Whether that is enough to convince people in the no-booster camp who lost faith when their vaccinated peers started getting COVID-19 is unclear, although, as Dr. Al-Aly has pointed out, vaccinations help keep people from getting so sick that they wind up in the hospital. And, with most of the population having received at least one dose of vaccine, most of those getting infected will naturally come from among the vaccinated.
Thomas describes the expectation that vaccines would prevent everyone from getting sick as “one of the major fails” of the pandemic.
Counting on a vaccine to confer 100% immunity is “a very high bar,” he said. “I think that’s what people expected, and when they weren’t seeing it, they kind of said: ‘Well, what’s the point? You know, things are getting better. I’d rather take my chances than keep going and getting boosted.’ ”
One point – and it’s a critical one – is that vaccination immunity wanes. Plus new variants arise that can evade at least some of the immunity provided by vaccination. That’s why boosters are built into the COVID vaccination program.
While it’s not clear why some long COVID patients see improvements in their symptoms after being vaccinated or boosted and others do not, Dr. Al-Aly said there’s little evidence vaccines can make long COVID worse. “There are some reports out there that some people with long COVID, when they got a vaccine or booster, their symptoms got worse. You’ll read anecdotes on this side,” he said, adding that efforts to see if this is really happening have been inconclusive.
“The general consensus is that vaccines really save lives,” Dr. Al-Aly said. “Getting vaccinated, even if you are a long COVID patient, is better than not getting vaccinated.”
A version of this article first appeared on WebMD.com.
Yet at 58, the Arizona writer is in no hurry to get the latest vaccine booster. “I just don’t want to risk getting any sicker,” she said.
Ms. Dishner has had two doses of vaccine plus two boosters. Each time, she had what regulators consider to be mild reactions, including a sore arm, slight fever, nausea, and body aches. Still, there’s some evidence that the newest booster, which protects against some of the later variants, could help people like Ms. Dishner in several ways, said Ziyad Al-Aly, MD, a clinical epidemiologist and prolific long COVID researcher at Washington University in St. Louis.
“A bivalent booster might actually [help with] your long COVID,” he said.
There may be other benefits. “What vaccines or current vaccine boosters do is reduce your risk of progression to severe COVID-19 illness,” Dr. Al-Aly said. “You are avoiding hospital stays or even worse; you’re avoiding potentially fatal outcomes after infection. And that’s really worth it. Who wants to be in the hospital this Christmas holiday?”
Each time people are infected with SARS-CoV-2, the virus that causes COVID-19, they have a fresh risk of not only getting severely ill or dying, but of developing long COVID, Dr. Al-Aly and colleagues found in a study published in Nature Medicine. “If you dodged the bullet the first time and did not get long COVID after the first infection, if you get reinfected, you’re trying your luck again,” Dr. Al-Aly said. “I would advise people not to get reinfected, which is another reason to get the booster.”
In a recent review in The Lancet eClinicalMedicine, an international team of researchers looked at 11 studies that sought to find out if vaccines affected long COVID symptoms. Seven of those studies found that people’s symptoms improved after they were vaccinated, and four found that symptoms mostly remained the same. One found symptoms got worse in some patients.
A study of 28,000 people published in the British Medical Journal found more evidence that vaccination may help ease symptoms. “Vaccination may contribute to a reduction in the population health burden of long COVID,” the team at the United Kingdom’s Office for National Statistics concluded. Most studies found vaccination reduced the risk of getting long COVID in the first place.
Vaccines prompt the body to produce antibodies, which stop a microbe from infecting cells. They also prompt the production of immune cells called T cells, which continue to hunt down and attack a pathogen even after infection.
A booster dose could help rev up that immune response in a patient with long COVID, said Stephen J. Thomas, MD, an infectious disease specialist at Upstate Medical Center in Syracuse, N.Y., and the center’s lead principal investigator for Pfizer/BioNTech’s COVID-19 2020 vaccine trial.
Some scientists believe long COVID might be caused when the virus persists in parts of the body where the immune system isn’t particularly active. Although they don’t fully understand the workings of the many and varied long COVID symptoms, they have a good idea about why people with long COVID often do better after receiving a vaccine or booster.
“The theory is that by boosting, the immune system may be able to ‘mop up’ those virus stragglers that have remained behind after your first cleanup attempt,” Dr. Thomas said.
“The vaccine is almost lending a hand or helping your immune response to clear that virus,” Dr. Al-Aly said.
It could be difficult for long COVID patients to make an informed decision about boosters, given the lack of studies that focus exclusively on the relationship between long COVID and boosters, according to Scott Roberts, MD, associate medical director for infection prevention at Yale New Haven (Conn.) Hospital.
Dr. Roberts recommended that patients speak with their health care providers and read about the bivalent booster on trusted sites such as those sponsored by the Food and Drug Administration and the Centers for Disease Control and Prevention. Long COVID patients should get the latest boosters, especially as there’s no evidence they are unsafe for them. “The antibody response is appropriately boosted, and there is a decent chance this will help reduce the impact of long COVID as well,” he said. “Waiting will only increase the risk of getting infected and increase the chances of long COVID.”
Only 12% of Americans 5 years and older have received the updated booster, according to the CDC, although it’s recommended for everyone. Just over 80% of Americans have gotten at least one vaccine dose. Dr. Thomas understands why the uptake has been so low: Along with people like Ms. Dishner, who fear more side effects or worse symptoms, there are those who believe that hybrid immunity – vaccination immunity plus natural infection – is superior to vaccination alone and that they don’t need a booster.
Studies show that the bivalent boosters, which protect against older and newer variants, can target even the new, predominant COVID-19 strains. Whether that is enough to convince people in the no-booster camp who lost faith when their vaccinated peers started getting COVID-19 is unclear, although, as Dr. Al-Aly has pointed out, vaccinations help keep people from getting so sick that they wind up in the hospital. And, with most of the population having received at least one dose of vaccine, most of those getting infected will naturally come from among the vaccinated.
Thomas describes the expectation that vaccines would prevent everyone from getting sick as “one of the major fails” of the pandemic.
Counting on a vaccine to confer 100% immunity is “a very high bar,” he said. “I think that’s what people expected, and when they weren’t seeing it, they kind of said: ‘Well, what’s the point? You know, things are getting better. I’d rather take my chances than keep going and getting boosted.’ ”
One point – and it’s a critical one – is that vaccination immunity wanes. Plus new variants arise that can evade at least some of the immunity provided by vaccination. That’s why boosters are built into the COVID vaccination program.
While it’s not clear why some long COVID patients see improvements in their symptoms after being vaccinated or boosted and others do not, Dr. Al-Aly said there’s little evidence vaccines can make long COVID worse. “There are some reports out there that some people with long COVID, when they got a vaccine or booster, their symptoms got worse. You’ll read anecdotes on this side,” he said, adding that efforts to see if this is really happening have been inconclusive.
“The general consensus is that vaccines really save lives,” Dr. Al-Aly said. “Getting vaccinated, even if you are a long COVID patient, is better than not getting vaccinated.”
A version of this article first appeared on WebMD.com.
FROM NATURE MEDICINE
FDA pulls U.S. authorization for Eli Lilly’s COVID drug bebtelovimab
the Food and Drug Administration said, citing it is not expected to neutralize the dominant BQ.1 and BQ.1.1 subvariants of Omicron.
The announcement on Nov. 30 takes away authorization from the last COVID-19 monoclonal antibody treatment, leaving Pfizer’s antiviral drug Paxlovid, Merck’s Lagevrio, and Gilead Sciences’ Veklury as treatments for the disease, besides convalescent plasma for some patients.
AstraZeneca’s monoclonal antibody Evusheld is also authorized for protection against COVID-19 infection in some people.
Eli Lilly and its authorized distributors have paused commercial distribution of the monoclonal antibody until further notice from the agency, while the U.S. government has also paused fulfillment of any pending requests under its scheme to help uninsured and underinsured Americans access the drug.
The drug, which was discovered by Abcellera and commercialized by Eli Lilly, received an authorization from the FDA in February.
BQ.1 and BQ.1.1 have become the dominant strains in the United States after a steady increase in prevalence over the last 2 months, surpassing Omicron’s BA.5 subvariant, which had driven cases earlier in the year.
The subvariants accounted for around 57% of the cases nationally, as per government data last week.
Reuters Health Information © 2022
the Food and Drug Administration said, citing it is not expected to neutralize the dominant BQ.1 and BQ.1.1 subvariants of Omicron.
The announcement on Nov. 30 takes away authorization from the last COVID-19 monoclonal antibody treatment, leaving Pfizer’s antiviral drug Paxlovid, Merck’s Lagevrio, and Gilead Sciences’ Veklury as treatments for the disease, besides convalescent plasma for some patients.
AstraZeneca’s monoclonal antibody Evusheld is also authorized for protection against COVID-19 infection in some people.
Eli Lilly and its authorized distributors have paused commercial distribution of the monoclonal antibody until further notice from the agency, while the U.S. government has also paused fulfillment of any pending requests under its scheme to help uninsured and underinsured Americans access the drug.
The drug, which was discovered by Abcellera and commercialized by Eli Lilly, received an authorization from the FDA in February.
BQ.1 and BQ.1.1 have become the dominant strains in the United States after a steady increase in prevalence over the last 2 months, surpassing Omicron’s BA.5 subvariant, which had driven cases earlier in the year.
The subvariants accounted for around 57% of the cases nationally, as per government data last week.
Reuters Health Information © 2022
the Food and Drug Administration said, citing it is not expected to neutralize the dominant BQ.1 and BQ.1.1 subvariants of Omicron.
The announcement on Nov. 30 takes away authorization from the last COVID-19 monoclonal antibody treatment, leaving Pfizer’s antiviral drug Paxlovid, Merck’s Lagevrio, and Gilead Sciences’ Veklury as treatments for the disease, besides convalescent plasma for some patients.
AstraZeneca’s monoclonal antibody Evusheld is also authorized for protection against COVID-19 infection in some people.
Eli Lilly and its authorized distributors have paused commercial distribution of the monoclonal antibody until further notice from the agency, while the U.S. government has also paused fulfillment of any pending requests under its scheme to help uninsured and underinsured Americans access the drug.
The drug, which was discovered by Abcellera and commercialized by Eli Lilly, received an authorization from the FDA in February.
BQ.1 and BQ.1.1 have become the dominant strains in the United States after a steady increase in prevalence over the last 2 months, surpassing Omicron’s BA.5 subvariant, which had driven cases earlier in the year.
The subvariants accounted for around 57% of the cases nationally, as per government data last week.
Reuters Health Information © 2022
RSV surge stuns parents and strains providers, but doctors offer help
RSV cases peaked in mid-November, according to the latest Centers for Disease Control and Prevention data, with RSV-associated hospitalizations in the United States among patients 0-4 years having maxed out five times higher than they were at the same time in 2021. These surges strained providers and left parents scrambling for care. Fortunately, pediatric hospitalizations appear to be subsiding.
In interviews, the parents of the child who had a severe case of RSV reflected on their son’s bout with the illness, and doctors described challenges to dealing with the surge in RSV cases this season. The physicians also offered advice on how recognize and respond to future cases of the virus.
Sebastian Witt’s story
“I didn’t even know what RSV was,” said Malte Witt, whose son, Sebastian, 2, was recently hospitalized for RSV in Denver.
Mr. Witt and his wife, Emily Witt, both 32, thought they were dealing with a typical cold until Sebastian’s condition dramatically deteriorated about 36 hours after symptom onset.
“He basically just slumped over and collapsed, coughing uncontrollably,” Mr. Witt said in an interview. “He couldn’t catch his breath.”
The Witts rushed Sebastian to the ED at Children’s Hospital Colorado, expecting to see a doctor immediately. Instead, they spent the night in an overcrowded waiting room alongside many other families in the same situation.
“There was no room for anyone to sit anywhere,” Mr. Witt said. “There were people sitting on the floor. I counted maybe six children hooked up to oxygen when we walked in.”
After waiting approximately 45 minutes, a nurse checked Sebastian’s oxygen saturation. The readings were 79%-83%. This range is significantly below thresholds for supplemental oxygen described by most pediatric guidelines, which range from 90 to 94%.
The nurse connected Sebastian to bottled oxygen in the waiting room, and a recheck 4 hours later showed that his oxygen saturation had improved.
But the improvement didn’t last.
“At roughly hour 10 in the waiting room – it was 4 in the morning – you could tell that Seb was exhausted, really not acting like himself,” Mr. Witt said. “We thought maybe it’s just late at night, he hasn’t really slept. But then Emily noticed that his oxygen tank had run out.”
Mr. Witt told a nurse, and after another check revealed low oxygen saturation, Sebastian was finally admitted.
Early RSV surge strains pediatric providers
With RSV-associated hospitalizations peaking at 48 per 100,000 children, Colorado has been among the states hardest hit by the virus. New Mexico – where hospitalizations peaked at 56.4 per 100,000 children – comes in second. Even in states like California, where hospitalization rates have been almost 10-fold lower than New Mexico, pediatric providers have been stretched to their limits.
“Many hospitals are really being overwhelmed with admissions for RSV, both routine RSV – relatively mild hospitalizations with bronchiolitis – as well as kids in the ICU with more severe cases,” said Dean Blumberg, MD, chief of the division of pediatric infectious diseases at UC Davis Health, Sacramento, said in an interview.
Dr. Blumberg believes the severity of the 2022-2023 RSV season is likely COVID related.
“All community-associated respiratory viral infections are out of whack because of the pandemic, and all the masking and social distancing that was occurring,” he said.
This may also explain why older kids are coming down with more severe cases of RSV.
“Some children are getting RSV for the first time as older children,” Dr. Blumberg said, noting that, historically, most children were infected in the first 2 years of life. “There are reports of children 3 or 4 years of age being admitted with their first episode of RSV because of the [COVID] pandemic.”
This year’s RSV season is also notable for arriving early, potentially catching the community off guard, according to Jennifer D. Kusma, MD, a primary care pediatrician at Ann & Robert H. Lurie Children’s Hospital of Chicago.
“People who should have been protected often weren’t protected yet,” Dr. Kusma said in an interview.
Treatments new, old, and unproven
On Nov. 17, in the midst of the RSV surge, the American Academy of Pediatrics issued updated guidance for palivizumab, an RSV-targeting monoclonal antibody labeled for children at risk of severe RSV, including those with pre-existing lung or heart conditions, and infants with a history of premature birth (less than or equal to 35 weeks’ gestational age).
“If RSV disease activity persists at high levels in a given region through the fall and winter, the AAP supports providing more than five consecutive doses of palivizumab to eligible children,” the update stated.
Insurance companies appear to be responding in kind, covering additional doses for children in need.
“[Payers] have agreed that, if [palivizumab] needs to be given for an additional month or 2 or 3, then they’re making a commitment that they’ll reimburse hospitals for providing that,” Dr. Blumberg said.
For ineligible patients, such as Sebastian, who was born prematurely at 36 weeks – 1 week shy of the label requirement – treatment relies upon supportive care with oxygen and IV fluids.
At home, parents are left with simpler options.
Dr. Blumberg and Dr. Kusma recommended keeping children hydrated, maintaining humidified air, and using saline nose drops with bulb suction to clear mucus.
In the Witts’ experience, that last step may be easier said than done.
“Every time a nurse would walk into the room, Sebastian would yell: ‘Go away, doctor! I don’t want snot sucker!’” Mr. Witt said.
“If you over snot-suck, that’s really uncomfortable for the kid, and really hard for you,” Ms. Witt said. “And it doesn’t make much of a difference. It’s just very hard to find a middle ground, where you’re helping and keeping them comfortable.”
Some parents are turning to novel strategies, such as nebulized hypertonic saline, currently marketed on Amazon for children with RSV.
Although the AAP offers a weak recommendation for nebulized hypertonic saline in children hospitalized more than 72 hours, they advise against it in the emergency setting, citing inconsistent findings in clinical trials.
To any parents tempted by thousands of positive Amazon reviews, Dr. Blumberg said, “I wouldn’t waste my money on that.”
Dr. Kusma agreed.
“[Nebulized hypertonic saline] can be irritating,” she said. “It’s saltwater, essentially. If a parent is in the position where they’re worried about their child’s breathing to the point that they think they need to use it, I would err on the side of calling your pediatrician and being seen.”
Going in, coming home
Dr. Kusma said parents should seek medical attention if a child is breathing faster and working harder to get air. Increased work of breathing is characterized by pulling of the skin at the notch where the throat meets the chest bone (tracheal tugging), and flattening of the belly that makes the ribcage more prominent.
Mr. Witt saw these signs in Sebastian. He knew they were significant, because a friend who is a nurse had previously shown him some examples of children who exhibited these symptoms online.
“That’s how I knew that things were actually really dangerous,” Mr. Witt said. “Had she not shown me those videos a month and a half before this happened, I don’t know that we would have hit the alarm bell as quickly as we did.”
After spending their second night and the following day in a cramped preoperative room converted to manage overflow from the emergency department, Sebastian’s condition improved, and he was discharged. The Witts are relieved to be home, but frustrations from their ordeal remain, especially considering the estimated $5,000 in out-of-pocket costs they expect to pay.
“How is this our health care system?” Ms. Witt asked. “This is unbelievable.”
An optimistic outlook
RSV seasons typically demonstrate a clear peak, followed by a decline through the rest of the season, suggesting better times lie ahead; however, this season has been anything but typical.
“I’m hopeful that it will just go away and stay away,” Dr. Kusma said, citing this trend. “But I can’t know for sure.”
To anxious parents, Dr. Blumberg offered an optimistic view of RSV seasons to come.
“There’s hope,” he said. “There are vaccines that are being developed that are very close to FDA approval. So, it’s possible that this time next year, we might have widespread RSV vaccination available for children so that we don’t have to go through this nightmare again.”
Dr. Blumberg and Dr. Kusma disclosed no relevant conflicts of interest.
RSV cases peaked in mid-November, according to the latest Centers for Disease Control and Prevention data, with RSV-associated hospitalizations in the United States among patients 0-4 years having maxed out five times higher than they were at the same time in 2021. These surges strained providers and left parents scrambling for care. Fortunately, pediatric hospitalizations appear to be subsiding.
In interviews, the parents of the child who had a severe case of RSV reflected on their son’s bout with the illness, and doctors described challenges to dealing with the surge in RSV cases this season. The physicians also offered advice on how recognize and respond to future cases of the virus.
Sebastian Witt’s story
“I didn’t even know what RSV was,” said Malte Witt, whose son, Sebastian, 2, was recently hospitalized for RSV in Denver.
Mr. Witt and his wife, Emily Witt, both 32, thought they were dealing with a typical cold until Sebastian’s condition dramatically deteriorated about 36 hours after symptom onset.
“He basically just slumped over and collapsed, coughing uncontrollably,” Mr. Witt said in an interview. “He couldn’t catch his breath.”
The Witts rushed Sebastian to the ED at Children’s Hospital Colorado, expecting to see a doctor immediately. Instead, they spent the night in an overcrowded waiting room alongside many other families in the same situation.
“There was no room for anyone to sit anywhere,” Mr. Witt said. “There were people sitting on the floor. I counted maybe six children hooked up to oxygen when we walked in.”
After waiting approximately 45 minutes, a nurse checked Sebastian’s oxygen saturation. The readings were 79%-83%. This range is significantly below thresholds for supplemental oxygen described by most pediatric guidelines, which range from 90 to 94%.
The nurse connected Sebastian to bottled oxygen in the waiting room, and a recheck 4 hours later showed that his oxygen saturation had improved.
But the improvement didn’t last.
“At roughly hour 10 in the waiting room – it was 4 in the morning – you could tell that Seb was exhausted, really not acting like himself,” Mr. Witt said. “We thought maybe it’s just late at night, he hasn’t really slept. But then Emily noticed that his oxygen tank had run out.”
Mr. Witt told a nurse, and after another check revealed low oxygen saturation, Sebastian was finally admitted.
Early RSV surge strains pediatric providers
With RSV-associated hospitalizations peaking at 48 per 100,000 children, Colorado has been among the states hardest hit by the virus. New Mexico – where hospitalizations peaked at 56.4 per 100,000 children – comes in second. Even in states like California, where hospitalization rates have been almost 10-fold lower than New Mexico, pediatric providers have been stretched to their limits.
“Many hospitals are really being overwhelmed with admissions for RSV, both routine RSV – relatively mild hospitalizations with bronchiolitis – as well as kids in the ICU with more severe cases,” said Dean Blumberg, MD, chief of the division of pediatric infectious diseases at UC Davis Health, Sacramento, said in an interview.
Dr. Blumberg believes the severity of the 2022-2023 RSV season is likely COVID related.
“All community-associated respiratory viral infections are out of whack because of the pandemic, and all the masking and social distancing that was occurring,” he said.
This may also explain why older kids are coming down with more severe cases of RSV.
“Some children are getting RSV for the first time as older children,” Dr. Blumberg said, noting that, historically, most children were infected in the first 2 years of life. “There are reports of children 3 or 4 years of age being admitted with their first episode of RSV because of the [COVID] pandemic.”
This year’s RSV season is also notable for arriving early, potentially catching the community off guard, according to Jennifer D. Kusma, MD, a primary care pediatrician at Ann & Robert H. Lurie Children’s Hospital of Chicago.
“People who should have been protected often weren’t protected yet,” Dr. Kusma said in an interview.
Treatments new, old, and unproven
On Nov. 17, in the midst of the RSV surge, the American Academy of Pediatrics issued updated guidance for palivizumab, an RSV-targeting monoclonal antibody labeled for children at risk of severe RSV, including those with pre-existing lung or heart conditions, and infants with a history of premature birth (less than or equal to 35 weeks’ gestational age).
“If RSV disease activity persists at high levels in a given region through the fall and winter, the AAP supports providing more than five consecutive doses of palivizumab to eligible children,” the update stated.
Insurance companies appear to be responding in kind, covering additional doses for children in need.
“[Payers] have agreed that, if [palivizumab] needs to be given for an additional month or 2 or 3, then they’re making a commitment that they’ll reimburse hospitals for providing that,” Dr. Blumberg said.
For ineligible patients, such as Sebastian, who was born prematurely at 36 weeks – 1 week shy of the label requirement – treatment relies upon supportive care with oxygen and IV fluids.
At home, parents are left with simpler options.
Dr. Blumberg and Dr. Kusma recommended keeping children hydrated, maintaining humidified air, and using saline nose drops with bulb suction to clear mucus.
In the Witts’ experience, that last step may be easier said than done.
“Every time a nurse would walk into the room, Sebastian would yell: ‘Go away, doctor! I don’t want snot sucker!’” Mr. Witt said.
“If you over snot-suck, that’s really uncomfortable for the kid, and really hard for you,” Ms. Witt said. “And it doesn’t make much of a difference. It’s just very hard to find a middle ground, where you’re helping and keeping them comfortable.”
Some parents are turning to novel strategies, such as nebulized hypertonic saline, currently marketed on Amazon for children with RSV.
Although the AAP offers a weak recommendation for nebulized hypertonic saline in children hospitalized more than 72 hours, they advise against it in the emergency setting, citing inconsistent findings in clinical trials.
To any parents tempted by thousands of positive Amazon reviews, Dr. Blumberg said, “I wouldn’t waste my money on that.”
Dr. Kusma agreed.
“[Nebulized hypertonic saline] can be irritating,” she said. “It’s saltwater, essentially. If a parent is in the position where they’re worried about their child’s breathing to the point that they think they need to use it, I would err on the side of calling your pediatrician and being seen.”
Going in, coming home
Dr. Kusma said parents should seek medical attention if a child is breathing faster and working harder to get air. Increased work of breathing is characterized by pulling of the skin at the notch where the throat meets the chest bone (tracheal tugging), and flattening of the belly that makes the ribcage more prominent.
Mr. Witt saw these signs in Sebastian. He knew they were significant, because a friend who is a nurse had previously shown him some examples of children who exhibited these symptoms online.
“That’s how I knew that things were actually really dangerous,” Mr. Witt said. “Had she not shown me those videos a month and a half before this happened, I don’t know that we would have hit the alarm bell as quickly as we did.”
After spending their second night and the following day in a cramped preoperative room converted to manage overflow from the emergency department, Sebastian’s condition improved, and he was discharged. The Witts are relieved to be home, but frustrations from their ordeal remain, especially considering the estimated $5,000 in out-of-pocket costs they expect to pay.
“How is this our health care system?” Ms. Witt asked. “This is unbelievable.”
An optimistic outlook
RSV seasons typically demonstrate a clear peak, followed by a decline through the rest of the season, suggesting better times lie ahead; however, this season has been anything but typical.
“I’m hopeful that it will just go away and stay away,” Dr. Kusma said, citing this trend. “But I can’t know for sure.”
To anxious parents, Dr. Blumberg offered an optimistic view of RSV seasons to come.
“There’s hope,” he said. “There are vaccines that are being developed that are very close to FDA approval. So, it’s possible that this time next year, we might have widespread RSV vaccination available for children so that we don’t have to go through this nightmare again.”
Dr. Blumberg and Dr. Kusma disclosed no relevant conflicts of interest.
RSV cases peaked in mid-November, according to the latest Centers for Disease Control and Prevention data, with RSV-associated hospitalizations in the United States among patients 0-4 years having maxed out five times higher than they were at the same time in 2021. These surges strained providers and left parents scrambling for care. Fortunately, pediatric hospitalizations appear to be subsiding.
In interviews, the parents of the child who had a severe case of RSV reflected on their son’s bout with the illness, and doctors described challenges to dealing with the surge in RSV cases this season. The physicians also offered advice on how recognize and respond to future cases of the virus.
Sebastian Witt’s story
“I didn’t even know what RSV was,” said Malte Witt, whose son, Sebastian, 2, was recently hospitalized for RSV in Denver.
Mr. Witt and his wife, Emily Witt, both 32, thought they were dealing with a typical cold until Sebastian’s condition dramatically deteriorated about 36 hours after symptom onset.
“He basically just slumped over and collapsed, coughing uncontrollably,” Mr. Witt said in an interview. “He couldn’t catch his breath.”
The Witts rushed Sebastian to the ED at Children’s Hospital Colorado, expecting to see a doctor immediately. Instead, they spent the night in an overcrowded waiting room alongside many other families in the same situation.
“There was no room for anyone to sit anywhere,” Mr. Witt said. “There were people sitting on the floor. I counted maybe six children hooked up to oxygen when we walked in.”
After waiting approximately 45 minutes, a nurse checked Sebastian’s oxygen saturation. The readings were 79%-83%. This range is significantly below thresholds for supplemental oxygen described by most pediatric guidelines, which range from 90 to 94%.
The nurse connected Sebastian to bottled oxygen in the waiting room, and a recheck 4 hours later showed that his oxygen saturation had improved.
But the improvement didn’t last.
“At roughly hour 10 in the waiting room – it was 4 in the morning – you could tell that Seb was exhausted, really not acting like himself,” Mr. Witt said. “We thought maybe it’s just late at night, he hasn’t really slept. But then Emily noticed that his oxygen tank had run out.”
Mr. Witt told a nurse, and after another check revealed low oxygen saturation, Sebastian was finally admitted.
Early RSV surge strains pediatric providers
With RSV-associated hospitalizations peaking at 48 per 100,000 children, Colorado has been among the states hardest hit by the virus. New Mexico – where hospitalizations peaked at 56.4 per 100,000 children – comes in second. Even in states like California, where hospitalization rates have been almost 10-fold lower than New Mexico, pediatric providers have been stretched to their limits.
“Many hospitals are really being overwhelmed with admissions for RSV, both routine RSV – relatively mild hospitalizations with bronchiolitis – as well as kids in the ICU with more severe cases,” said Dean Blumberg, MD, chief of the division of pediatric infectious diseases at UC Davis Health, Sacramento, said in an interview.
Dr. Blumberg believes the severity of the 2022-2023 RSV season is likely COVID related.
“All community-associated respiratory viral infections are out of whack because of the pandemic, and all the masking and social distancing that was occurring,” he said.
This may also explain why older kids are coming down with more severe cases of RSV.
“Some children are getting RSV for the first time as older children,” Dr. Blumberg said, noting that, historically, most children were infected in the first 2 years of life. “There are reports of children 3 or 4 years of age being admitted with their first episode of RSV because of the [COVID] pandemic.”
This year’s RSV season is also notable for arriving early, potentially catching the community off guard, according to Jennifer D. Kusma, MD, a primary care pediatrician at Ann & Robert H. Lurie Children’s Hospital of Chicago.
“People who should have been protected often weren’t protected yet,” Dr. Kusma said in an interview.
Treatments new, old, and unproven
On Nov. 17, in the midst of the RSV surge, the American Academy of Pediatrics issued updated guidance for palivizumab, an RSV-targeting monoclonal antibody labeled for children at risk of severe RSV, including those with pre-existing lung or heart conditions, and infants with a history of premature birth (less than or equal to 35 weeks’ gestational age).
“If RSV disease activity persists at high levels in a given region through the fall and winter, the AAP supports providing more than five consecutive doses of palivizumab to eligible children,” the update stated.
Insurance companies appear to be responding in kind, covering additional doses for children in need.
“[Payers] have agreed that, if [palivizumab] needs to be given for an additional month or 2 or 3, then they’re making a commitment that they’ll reimburse hospitals for providing that,” Dr. Blumberg said.
For ineligible patients, such as Sebastian, who was born prematurely at 36 weeks – 1 week shy of the label requirement – treatment relies upon supportive care with oxygen and IV fluids.
At home, parents are left with simpler options.
Dr. Blumberg and Dr. Kusma recommended keeping children hydrated, maintaining humidified air, and using saline nose drops with bulb suction to clear mucus.
In the Witts’ experience, that last step may be easier said than done.
“Every time a nurse would walk into the room, Sebastian would yell: ‘Go away, doctor! I don’t want snot sucker!’” Mr. Witt said.
“If you over snot-suck, that’s really uncomfortable for the kid, and really hard for you,” Ms. Witt said. “And it doesn’t make much of a difference. It’s just very hard to find a middle ground, where you’re helping and keeping them comfortable.”
Some parents are turning to novel strategies, such as nebulized hypertonic saline, currently marketed on Amazon for children with RSV.
Although the AAP offers a weak recommendation for nebulized hypertonic saline in children hospitalized more than 72 hours, they advise against it in the emergency setting, citing inconsistent findings in clinical trials.
To any parents tempted by thousands of positive Amazon reviews, Dr. Blumberg said, “I wouldn’t waste my money on that.”
Dr. Kusma agreed.
“[Nebulized hypertonic saline] can be irritating,” she said. “It’s saltwater, essentially. If a parent is in the position where they’re worried about their child’s breathing to the point that they think they need to use it, I would err on the side of calling your pediatrician and being seen.”
Going in, coming home
Dr. Kusma said parents should seek medical attention if a child is breathing faster and working harder to get air. Increased work of breathing is characterized by pulling of the skin at the notch where the throat meets the chest bone (tracheal tugging), and flattening of the belly that makes the ribcage more prominent.
Mr. Witt saw these signs in Sebastian. He knew they were significant, because a friend who is a nurse had previously shown him some examples of children who exhibited these symptoms online.
“That’s how I knew that things were actually really dangerous,” Mr. Witt said. “Had she not shown me those videos a month and a half before this happened, I don’t know that we would have hit the alarm bell as quickly as we did.”
After spending their second night and the following day in a cramped preoperative room converted to manage overflow from the emergency department, Sebastian’s condition improved, and he was discharged. The Witts are relieved to be home, but frustrations from their ordeal remain, especially considering the estimated $5,000 in out-of-pocket costs they expect to pay.
“How is this our health care system?” Ms. Witt asked. “This is unbelievable.”
An optimistic outlook
RSV seasons typically demonstrate a clear peak, followed by a decline through the rest of the season, suggesting better times lie ahead; however, this season has been anything but typical.
“I’m hopeful that it will just go away and stay away,” Dr. Kusma said, citing this trend. “But I can’t know for sure.”
To anxious parents, Dr. Blumberg offered an optimistic view of RSV seasons to come.
“There’s hope,” he said. “There are vaccines that are being developed that are very close to FDA approval. So, it’s possible that this time next year, we might have widespread RSV vaccination available for children so that we don’t have to go through this nightmare again.”
Dr. Blumberg and Dr. Kusma disclosed no relevant conflicts of interest.