User login
Scientists use mRNA technology for universal flu vaccine
Two years ago, when the first COVID-19 vaccines were administered, marked a game-changing moment in the fight against the pandemic. But it also was a significant moment for messenger RNA (mRNA) technology, which up until then had shown promise but had never quite broken through.
It’s the latest advance in a new age of vaccinology, where vaccines are easier and faster to produce, as well as more flexible and customizable.
“It’s all about covering the different flavors of flu in a way the current vaccines cannot do,” says Ofer Levy, MD, PhD, director of the Precision Vaccines Program at Boston Children’s Hospital, who is not involved with the UPenn research. “The mRNA platform is attractive here given its scalability and modularity, where you can mix and match different mRNAs.”
A recent paper, published in Science, reports successful animal tests of the experimental vaccine, which, like the Pfizer-BioNTech and Moderna COVID vaccines, relies on mRNA. But the idea is not to replace the annual flu shot. It’s to develop a primer that could be administered in childhood, readying the body’s B cells and T cells to react quickly if faced with a flu virus.
It’s all part of a National Institutes of Health–funded effort to develop a universal flu vaccine, with hopes of heading off future flu pandemics. Annual shots protect against flu subtypes known to spread in humans. But many subtypes circulate in animals, like birds and pigs, and occasionally jump to humans, causing pandemics.
“The current vaccines provide very little protection against these other subtypes,” says lead study author Scott Hensley, PhD, a professor of microbiology at UPenn. “We set out to make a vaccine that would provide some level of immunity against essentially every influenza subtype we know about.”
That’s 20 subtypes altogether. The unique properties of mRNA vaccines make immune responses against all those antigens possible, Dr. Hensley says.
Old-school vaccines introduce a weakened or dead bacteria or virus into the body, but mRNA vaccines use mRNA encoded with a protein from the virus. That’s the “spike” protein for COVID, and for the experimental vaccine, it’s hemagglutinin, the major protein found on the surface of all flu viruses.
Mice and ferrets that had never been exposed to the flu were given the vaccine and produced high levels of antibodies against all 20 flu subtypes. Vaccinated mice exposed to the exact strains in the vaccine stayed pretty healthy, while those exposed to strains not found in the vaccine got sick but recovered quickly and survived. Unvaccinated mice exposed to the flu strain died.
The vaccine seems to be able to “induce broad immunity against all the different influenza subtypes,” Dr. Hensley says, preventing severe illness if not infection overall.
Still, whether it could truly stave off a pandemic that hasn’t happened yet is hard to say, Dr. Levy cautions.
“We are going to need to better learn the molecular rules by which these vaccines protect,” he says.
But the UPenn team is forging ahead, with plans to test their vaccine in human adults in 2023 to determine safety, dosing, and antibody response.
A version of this article first appeared on WebMD.com.
Two years ago, when the first COVID-19 vaccines were administered, marked a game-changing moment in the fight against the pandemic. But it also was a significant moment for messenger RNA (mRNA) technology, which up until then had shown promise but had never quite broken through.
It’s the latest advance in a new age of vaccinology, where vaccines are easier and faster to produce, as well as more flexible and customizable.
“It’s all about covering the different flavors of flu in a way the current vaccines cannot do,” says Ofer Levy, MD, PhD, director of the Precision Vaccines Program at Boston Children’s Hospital, who is not involved with the UPenn research. “The mRNA platform is attractive here given its scalability and modularity, where you can mix and match different mRNAs.”
A recent paper, published in Science, reports successful animal tests of the experimental vaccine, which, like the Pfizer-BioNTech and Moderna COVID vaccines, relies on mRNA. But the idea is not to replace the annual flu shot. It’s to develop a primer that could be administered in childhood, readying the body’s B cells and T cells to react quickly if faced with a flu virus.
It’s all part of a National Institutes of Health–funded effort to develop a universal flu vaccine, with hopes of heading off future flu pandemics. Annual shots protect against flu subtypes known to spread in humans. But many subtypes circulate in animals, like birds and pigs, and occasionally jump to humans, causing pandemics.
“The current vaccines provide very little protection against these other subtypes,” says lead study author Scott Hensley, PhD, a professor of microbiology at UPenn. “We set out to make a vaccine that would provide some level of immunity against essentially every influenza subtype we know about.”
That’s 20 subtypes altogether. The unique properties of mRNA vaccines make immune responses against all those antigens possible, Dr. Hensley says.
Old-school vaccines introduce a weakened or dead bacteria or virus into the body, but mRNA vaccines use mRNA encoded with a protein from the virus. That’s the “spike” protein for COVID, and for the experimental vaccine, it’s hemagglutinin, the major protein found on the surface of all flu viruses.
Mice and ferrets that had never been exposed to the flu were given the vaccine and produced high levels of antibodies against all 20 flu subtypes. Vaccinated mice exposed to the exact strains in the vaccine stayed pretty healthy, while those exposed to strains not found in the vaccine got sick but recovered quickly and survived. Unvaccinated mice exposed to the flu strain died.
The vaccine seems to be able to “induce broad immunity against all the different influenza subtypes,” Dr. Hensley says, preventing severe illness if not infection overall.
Still, whether it could truly stave off a pandemic that hasn’t happened yet is hard to say, Dr. Levy cautions.
“We are going to need to better learn the molecular rules by which these vaccines protect,” he says.
But the UPenn team is forging ahead, with plans to test their vaccine in human adults in 2023 to determine safety, dosing, and antibody response.
A version of this article first appeared on WebMD.com.
Two years ago, when the first COVID-19 vaccines were administered, marked a game-changing moment in the fight against the pandemic. But it also was a significant moment for messenger RNA (mRNA) technology, which up until then had shown promise but had never quite broken through.
It’s the latest advance in a new age of vaccinology, where vaccines are easier and faster to produce, as well as more flexible and customizable.
“It’s all about covering the different flavors of flu in a way the current vaccines cannot do,” says Ofer Levy, MD, PhD, director of the Precision Vaccines Program at Boston Children’s Hospital, who is not involved with the UPenn research. “The mRNA platform is attractive here given its scalability and modularity, where you can mix and match different mRNAs.”
A recent paper, published in Science, reports successful animal tests of the experimental vaccine, which, like the Pfizer-BioNTech and Moderna COVID vaccines, relies on mRNA. But the idea is not to replace the annual flu shot. It’s to develop a primer that could be administered in childhood, readying the body’s B cells and T cells to react quickly if faced with a flu virus.
It’s all part of a National Institutes of Health–funded effort to develop a universal flu vaccine, with hopes of heading off future flu pandemics. Annual shots protect against flu subtypes known to spread in humans. But many subtypes circulate in animals, like birds and pigs, and occasionally jump to humans, causing pandemics.
“The current vaccines provide very little protection against these other subtypes,” says lead study author Scott Hensley, PhD, a professor of microbiology at UPenn. “We set out to make a vaccine that would provide some level of immunity against essentially every influenza subtype we know about.”
That’s 20 subtypes altogether. The unique properties of mRNA vaccines make immune responses against all those antigens possible, Dr. Hensley says.
Old-school vaccines introduce a weakened or dead bacteria or virus into the body, but mRNA vaccines use mRNA encoded with a protein from the virus. That’s the “spike” protein for COVID, and for the experimental vaccine, it’s hemagglutinin, the major protein found on the surface of all flu viruses.
Mice and ferrets that had never been exposed to the flu were given the vaccine and produced high levels of antibodies against all 20 flu subtypes. Vaccinated mice exposed to the exact strains in the vaccine stayed pretty healthy, while those exposed to strains not found in the vaccine got sick but recovered quickly and survived. Unvaccinated mice exposed to the flu strain died.
The vaccine seems to be able to “induce broad immunity against all the different influenza subtypes,” Dr. Hensley says, preventing severe illness if not infection overall.
Still, whether it could truly stave off a pandemic that hasn’t happened yet is hard to say, Dr. Levy cautions.
“We are going to need to better learn the molecular rules by which these vaccines protect,” he says.
But the UPenn team is forging ahead, with plans to test their vaccine in human adults in 2023 to determine safety, dosing, and antibody response.
A version of this article first appeared on WebMD.com.
FROM SCIENCE
COVID booster shot poll: People ‘don’t think they need one’
Now, a new poll shows why so few people are willing to roll up their sleeves again.
The most common reasons people give for not getting the latest booster shot is that they “don’t think they need one” (44%) and they “don’t think the benefits are worth it” (37%), according to poll results from the Kaiser Family Foundation.
The data comes amid announcements by the Centers for Disease Control and Prevention that boosters reduced COVID-19 hospitalizations by up to 57% for U.S. adults and by up to 84% for people age 65 and older. Those figures are just the latest in a mountain of research reporting the public health benefits of COVID-19 vaccines.
Despite all of the statistical data, health officials’ recent vaccination campaigns have proven far from compelling.
So far, just 15% of people age 12 and older have gotten the latest booster, and 36% of people age 65 and older have gotten it, the CDC’s vaccination trackershows.
Since the start of the pandemic, 1.1 million people in the U.S. have died from COVID-19, with the number of deaths currently rising by 400 per day, The New York Times COVID tracker shows.
Many experts continue to note the need for everyone to get booster shots regularly, but some advocate that perhaps a change in strategy is in order.
“What the administration should do is push for vaccinating people in high-risk groups, including those who are older, those who are immunocompromised and those who have comorbidities,” Paul Offitt, MD, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, told CNN.
Federal regulators have announced they will meet Jan. 26 with a panel of vaccine advisors to examine the current recommended vaccination schedule as well as look at the effectiveness and composition of current vaccines and boosters, with an eye toward the make-up of next-generation shots.
Vaccines are the “best available protection” against hospitalization and death caused by COVID-19, said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in a statement announcing the planned meeting.
“Since the initial authorizations of these vaccines, we have learned that protection wanes over time, especially as the virus rapidly mutates and new variants and subvariants emerge,” he said. “Therefore, it’s important to continue discussions about the optimal composition of COVID-19 vaccines for primary and booster vaccination, as well as the optimal interval for booster vaccination.”
A version of this article first appeared on WebMD.com.
Now, a new poll shows why so few people are willing to roll up their sleeves again.
The most common reasons people give for not getting the latest booster shot is that they “don’t think they need one” (44%) and they “don’t think the benefits are worth it” (37%), according to poll results from the Kaiser Family Foundation.
The data comes amid announcements by the Centers for Disease Control and Prevention that boosters reduced COVID-19 hospitalizations by up to 57% for U.S. adults and by up to 84% for people age 65 and older. Those figures are just the latest in a mountain of research reporting the public health benefits of COVID-19 vaccines.
Despite all of the statistical data, health officials’ recent vaccination campaigns have proven far from compelling.
So far, just 15% of people age 12 and older have gotten the latest booster, and 36% of people age 65 and older have gotten it, the CDC’s vaccination trackershows.
Since the start of the pandemic, 1.1 million people in the U.S. have died from COVID-19, with the number of deaths currently rising by 400 per day, The New York Times COVID tracker shows.
Many experts continue to note the need for everyone to get booster shots regularly, but some advocate that perhaps a change in strategy is in order.
“What the administration should do is push for vaccinating people in high-risk groups, including those who are older, those who are immunocompromised and those who have comorbidities,” Paul Offitt, MD, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, told CNN.
Federal regulators have announced they will meet Jan. 26 with a panel of vaccine advisors to examine the current recommended vaccination schedule as well as look at the effectiveness and composition of current vaccines and boosters, with an eye toward the make-up of next-generation shots.
Vaccines are the “best available protection” against hospitalization and death caused by COVID-19, said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in a statement announcing the planned meeting.
“Since the initial authorizations of these vaccines, we have learned that protection wanes over time, especially as the virus rapidly mutates and new variants and subvariants emerge,” he said. “Therefore, it’s important to continue discussions about the optimal composition of COVID-19 vaccines for primary and booster vaccination, as well as the optimal interval for booster vaccination.”
A version of this article first appeared on WebMD.com.
Now, a new poll shows why so few people are willing to roll up their sleeves again.
The most common reasons people give for not getting the latest booster shot is that they “don’t think they need one” (44%) and they “don’t think the benefits are worth it” (37%), according to poll results from the Kaiser Family Foundation.
The data comes amid announcements by the Centers for Disease Control and Prevention that boosters reduced COVID-19 hospitalizations by up to 57% for U.S. adults and by up to 84% for people age 65 and older. Those figures are just the latest in a mountain of research reporting the public health benefits of COVID-19 vaccines.
Despite all of the statistical data, health officials’ recent vaccination campaigns have proven far from compelling.
So far, just 15% of people age 12 and older have gotten the latest booster, and 36% of people age 65 and older have gotten it, the CDC’s vaccination trackershows.
Since the start of the pandemic, 1.1 million people in the U.S. have died from COVID-19, with the number of deaths currently rising by 400 per day, The New York Times COVID tracker shows.
Many experts continue to note the need for everyone to get booster shots regularly, but some advocate that perhaps a change in strategy is in order.
“What the administration should do is push for vaccinating people in high-risk groups, including those who are older, those who are immunocompromised and those who have comorbidities,” Paul Offitt, MD, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, told CNN.
Federal regulators have announced they will meet Jan. 26 with a panel of vaccine advisors to examine the current recommended vaccination schedule as well as look at the effectiveness and composition of current vaccines and boosters, with an eye toward the make-up of next-generation shots.
Vaccines are the “best available protection” against hospitalization and death caused by COVID-19, said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in a statement announcing the planned meeting.
“Since the initial authorizations of these vaccines, we have learned that protection wanes over time, especially as the virus rapidly mutates and new variants and subvariants emerge,” he said. “Therefore, it’s important to continue discussions about the optimal composition of COVID-19 vaccines for primary and booster vaccination, as well as the optimal interval for booster vaccination.”
A version of this article first appeared on WebMD.com.
A reason for hope in the face of long COVID
In this issue, Mayo and colleagues1 summarize what we know about patients with long COVID. The report made me pause and realize that it has been 3 years since we heard the very first reports of patients infected with SARS-CoV-2, which would eventually cause the COVID-19 pandemic. I suspect that I am not alone in having been fascinated by the rapid communication of information (of variable quality and veracity) via peer-reviewed papers, pre-print servers, the media, and social media.
The early studies were largely descriptive, focusing on symptom constellations and outbreak data. Much of what we had by way of treatment was supportive and “let’s try anything”—whether reasonable or, in some cases, not. In relatively short order, though, we developed effective vaccines to help protect people from getting seriously ill, being hospitalized, and dying; we also identified targeted therapies for those who became ill.2 But variants continued—or rather, continue—to emerge, and we remain committed to meeting the demands of the day.
The Centers for Disease Control and Prevention reports that more than 98 million Americans have contracted COVID, and more than 1 million have died.3 Besides the astonishingly high total mortality, the ravages of COVID-19 include new-onset respiratory, cardiovascular, neurologic, and psychiatric illnesses.4,5 As many as half of adults hospitalized for COVID report having persistent symptoms.6
As described in this issue, what we know about long COVID appears to be following the early course of its parent illness. As was true then, we are learning about the symptoms, etiology, and best ways to manage our patients. As in the early days of the pandemic, treatment is supportive, and we await definitive therapies.
I am optimistic, though. Why? Because shortly after the first reports of COVID-19, the virus’ DNA sequence was shared online. Based on that information, diagnostic assays were developed. Within 2 years of the outbreak, we had effective vaccines and specific therapies.
Another call to action. If 5% of patients contracting COVID (a very low estimate) develop long COVID, that would translate to 4.9 million people with long COVID in the United States. That is an astounding burden of suffering that I have no doubt will motivate innovation.
Innovation is a strength of the US health care system. I believe we will rise to the next challenge that COVID-19 has put before us. We have reason to be hopeful.
1. Mayo NL, Ellenbogen RL, Mendoza MD, et al. The family physician’s role in long COVID management. J Fam Pract. 2022;71:426-431. doi: 10.12788/jfp.0517
2. Kulshreshtha A, Sizemore S, Barry HC. COVID-19 therapy: What works? What doesn’t? And what’s on the horizon? J Fam Pract. 2022;71:E3-E16. doi: 10.12788/jfp.0474
3. CDC. COVID data tracker. Accessed December 5, 2022. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
4. Taquet M, Geddes JR, Husain M, et al. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416-427. doi: 10.1016/s2215-0366(21) 00084-5
5. Ayoubkhani D, Khunti K, Nafilyan V, et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021;372:n693. doi: 10.1136/bmj.n693
6. Writing Committee for the Comebac Study Group, Morin L, Savale L, Pham T, et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325:1525-1534. doi: 10.1001/jama.2021.3331
In this issue, Mayo and colleagues1 summarize what we know about patients with long COVID. The report made me pause and realize that it has been 3 years since we heard the very first reports of patients infected with SARS-CoV-2, which would eventually cause the COVID-19 pandemic. I suspect that I am not alone in having been fascinated by the rapid communication of information (of variable quality and veracity) via peer-reviewed papers, pre-print servers, the media, and social media.
The early studies were largely descriptive, focusing on symptom constellations and outbreak data. Much of what we had by way of treatment was supportive and “let’s try anything”—whether reasonable or, in some cases, not. In relatively short order, though, we developed effective vaccines to help protect people from getting seriously ill, being hospitalized, and dying; we also identified targeted therapies for those who became ill.2 But variants continued—or rather, continue—to emerge, and we remain committed to meeting the demands of the day.
The Centers for Disease Control and Prevention reports that more than 98 million Americans have contracted COVID, and more than 1 million have died.3 Besides the astonishingly high total mortality, the ravages of COVID-19 include new-onset respiratory, cardiovascular, neurologic, and psychiatric illnesses.4,5 As many as half of adults hospitalized for COVID report having persistent symptoms.6
As described in this issue, what we know about long COVID appears to be following the early course of its parent illness. As was true then, we are learning about the symptoms, etiology, and best ways to manage our patients. As in the early days of the pandemic, treatment is supportive, and we await definitive therapies.
I am optimistic, though. Why? Because shortly after the first reports of COVID-19, the virus’ DNA sequence was shared online. Based on that information, diagnostic assays were developed. Within 2 years of the outbreak, we had effective vaccines and specific therapies.
Another call to action. If 5% of patients contracting COVID (a very low estimate) develop long COVID, that would translate to 4.9 million people with long COVID in the United States. That is an astounding burden of suffering that I have no doubt will motivate innovation.
Innovation is a strength of the US health care system. I believe we will rise to the next challenge that COVID-19 has put before us. We have reason to be hopeful.
In this issue, Mayo and colleagues1 summarize what we know about patients with long COVID. The report made me pause and realize that it has been 3 years since we heard the very first reports of patients infected with SARS-CoV-2, which would eventually cause the COVID-19 pandemic. I suspect that I am not alone in having been fascinated by the rapid communication of information (of variable quality and veracity) via peer-reviewed papers, pre-print servers, the media, and social media.
The early studies were largely descriptive, focusing on symptom constellations and outbreak data. Much of what we had by way of treatment was supportive and “let’s try anything”—whether reasonable or, in some cases, not. In relatively short order, though, we developed effective vaccines to help protect people from getting seriously ill, being hospitalized, and dying; we also identified targeted therapies for those who became ill.2 But variants continued—or rather, continue—to emerge, and we remain committed to meeting the demands of the day.
The Centers for Disease Control and Prevention reports that more than 98 million Americans have contracted COVID, and more than 1 million have died.3 Besides the astonishingly high total mortality, the ravages of COVID-19 include new-onset respiratory, cardiovascular, neurologic, and psychiatric illnesses.4,5 As many as half of adults hospitalized for COVID report having persistent symptoms.6
As described in this issue, what we know about long COVID appears to be following the early course of its parent illness. As was true then, we are learning about the symptoms, etiology, and best ways to manage our patients. As in the early days of the pandemic, treatment is supportive, and we await definitive therapies.
I am optimistic, though. Why? Because shortly after the first reports of COVID-19, the virus’ DNA sequence was shared online. Based on that information, diagnostic assays were developed. Within 2 years of the outbreak, we had effective vaccines and specific therapies.
Another call to action. If 5% of patients contracting COVID (a very low estimate) develop long COVID, that would translate to 4.9 million people with long COVID in the United States. That is an astounding burden of suffering that I have no doubt will motivate innovation.
Innovation is a strength of the US health care system. I believe we will rise to the next challenge that COVID-19 has put before us. We have reason to be hopeful.
1. Mayo NL, Ellenbogen RL, Mendoza MD, et al. The family physician’s role in long COVID management. J Fam Pract. 2022;71:426-431. doi: 10.12788/jfp.0517
2. Kulshreshtha A, Sizemore S, Barry HC. COVID-19 therapy: What works? What doesn’t? And what’s on the horizon? J Fam Pract. 2022;71:E3-E16. doi: 10.12788/jfp.0474
3. CDC. COVID data tracker. Accessed December 5, 2022. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
4. Taquet M, Geddes JR, Husain M, et al. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416-427. doi: 10.1016/s2215-0366(21) 00084-5
5. Ayoubkhani D, Khunti K, Nafilyan V, et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021;372:n693. doi: 10.1136/bmj.n693
6. Writing Committee for the Comebac Study Group, Morin L, Savale L, Pham T, et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325:1525-1534. doi: 10.1001/jama.2021.3331
1. Mayo NL, Ellenbogen RL, Mendoza MD, et al. The family physician’s role in long COVID management. J Fam Pract. 2022;71:426-431. doi: 10.12788/jfp.0517
2. Kulshreshtha A, Sizemore S, Barry HC. COVID-19 therapy: What works? What doesn’t? And what’s on the horizon? J Fam Pract. 2022;71:E3-E16. doi: 10.12788/jfp.0474
3. CDC. COVID data tracker. Accessed December 5, 2022. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
4. Taquet M, Geddes JR, Husain M, et al. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416-427. doi: 10.1016/s2215-0366(21) 00084-5
5. Ayoubkhani D, Khunti K, Nafilyan V, et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021;372:n693. doi: 10.1136/bmj.n693
6. Writing Committee for the Comebac Study Group, Morin L, Savale L, Pham T, et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325:1525-1534. doi: 10.1001/jama.2021.3331
Multiple myeloma diagnosed more via emergency care during COVID
The study covered in this summary was published on Research Square as a preprint and has not yet been peer reviewed.
Key takeaway
Why this matters
While trying to avoid COVID-19 infection, patients ultimately diagnosed with multiple myeloma may have delayed interactions with healthcare professionals and consequently delayed their cancer diagnosis.
Study design
Researchers collected data on newly diagnosed patients with multiple myeloma from January 2019 until July 2021 across five institutions (three universities and two hospitals) in England. In total, 323 patients with multiple myeloma were identified.
Patients were divided into two groups: those diagnosed between Jan. 1, 2019, until Jan. 31, 2020, or pre-COVID, and those diagnosed from Feb. 1, 2020, to July 31, 2021, or post COVID.
Key results
Among all patients, 80 (24.8%) were diagnosed with smoldering multiple myeloma and 243 (75.2%) were diagnosed with multiple myeloma requiring treatment.
Significantly more patients in the post-COVID group were diagnosed with myeloma through the emergency route (45.5% post COVID vs. 32.7% pre-COVID; P = .03).
Clinical complications leading to emergency admission prior to a myeloma diagnosis also differed between the two cohorts: Acute kidney injury accounted for most emergency admissions in the pre-COVID cohort while skeletal-related events, including spinal cord compression, were the major causes for diagnosis through the emergency route in the post-COVID cohort.
Patients who were diagnosed with symptomatic myeloma pre-COVID were more likely to be treated with a triplet rather than doublet combination compared with those diagnosed in the post-COVID period (triplet pre-COVID 79.1%, post COVID 63.75%; P = .014).
Overall survival at 1 year was not significantly different between the pre-COVID and post-COVID groups: 88.2% pre-COVID, compared with 87.8% post COVID.
Overall, the authors concluded that the COVID pandemic “resulted in a shift in the symptomatology, disease burden, and routes of diagnosis of patients presenting with myeloma” and “this may have significant consequences” over the long term.
Limitations
The study does not provide a clear time frame of delays in diagnosis.
Disclosures
The study authors did not report any conflicts of interest.
A version of this article first appeared on Medscape.com .
The study covered in this summary was published on Research Square as a preprint and has not yet been peer reviewed.
Key takeaway
Why this matters
While trying to avoid COVID-19 infection, patients ultimately diagnosed with multiple myeloma may have delayed interactions with healthcare professionals and consequently delayed their cancer diagnosis.
Study design
Researchers collected data on newly diagnosed patients with multiple myeloma from January 2019 until July 2021 across five institutions (three universities and two hospitals) in England. In total, 323 patients with multiple myeloma were identified.
Patients were divided into two groups: those diagnosed between Jan. 1, 2019, until Jan. 31, 2020, or pre-COVID, and those diagnosed from Feb. 1, 2020, to July 31, 2021, or post COVID.
Key results
Among all patients, 80 (24.8%) were diagnosed with smoldering multiple myeloma and 243 (75.2%) were diagnosed with multiple myeloma requiring treatment.
Significantly more patients in the post-COVID group were diagnosed with myeloma through the emergency route (45.5% post COVID vs. 32.7% pre-COVID; P = .03).
Clinical complications leading to emergency admission prior to a myeloma diagnosis also differed between the two cohorts: Acute kidney injury accounted for most emergency admissions in the pre-COVID cohort while skeletal-related events, including spinal cord compression, were the major causes for diagnosis through the emergency route in the post-COVID cohort.
Patients who were diagnosed with symptomatic myeloma pre-COVID were more likely to be treated with a triplet rather than doublet combination compared with those diagnosed in the post-COVID period (triplet pre-COVID 79.1%, post COVID 63.75%; P = .014).
Overall survival at 1 year was not significantly different between the pre-COVID and post-COVID groups: 88.2% pre-COVID, compared with 87.8% post COVID.
Overall, the authors concluded that the COVID pandemic “resulted in a shift in the symptomatology, disease burden, and routes of diagnosis of patients presenting with myeloma” and “this may have significant consequences” over the long term.
Limitations
The study does not provide a clear time frame of delays in diagnosis.
Disclosures
The study authors did not report any conflicts of interest.
A version of this article first appeared on Medscape.com .
The study covered in this summary was published on Research Square as a preprint and has not yet been peer reviewed.
Key takeaway
Why this matters
While trying to avoid COVID-19 infection, patients ultimately diagnosed with multiple myeloma may have delayed interactions with healthcare professionals and consequently delayed their cancer diagnosis.
Study design
Researchers collected data on newly diagnosed patients with multiple myeloma from January 2019 until July 2021 across five institutions (three universities and two hospitals) in England. In total, 323 patients with multiple myeloma were identified.
Patients were divided into two groups: those diagnosed between Jan. 1, 2019, until Jan. 31, 2020, or pre-COVID, and those diagnosed from Feb. 1, 2020, to July 31, 2021, or post COVID.
Key results
Among all patients, 80 (24.8%) were diagnosed with smoldering multiple myeloma and 243 (75.2%) were diagnosed with multiple myeloma requiring treatment.
Significantly more patients in the post-COVID group were diagnosed with myeloma through the emergency route (45.5% post COVID vs. 32.7% pre-COVID; P = .03).
Clinical complications leading to emergency admission prior to a myeloma diagnosis also differed between the two cohorts: Acute kidney injury accounted for most emergency admissions in the pre-COVID cohort while skeletal-related events, including spinal cord compression, were the major causes for diagnosis through the emergency route in the post-COVID cohort.
Patients who were diagnosed with symptomatic myeloma pre-COVID were more likely to be treated with a triplet rather than doublet combination compared with those diagnosed in the post-COVID period (triplet pre-COVID 79.1%, post COVID 63.75%; P = .014).
Overall survival at 1 year was not significantly different between the pre-COVID and post-COVID groups: 88.2% pre-COVID, compared with 87.8% post COVID.
Overall, the authors concluded that the COVID pandemic “resulted in a shift in the symptomatology, disease burden, and routes of diagnosis of patients presenting with myeloma” and “this may have significant consequences” over the long term.
Limitations
The study does not provide a clear time frame of delays in diagnosis.
Disclosures
The study authors did not report any conflicts of interest.
A version of this article first appeared on Medscape.com .
Rise of ‘alarming’ subvariants of COVID ‘worrisome’ for winter
It’s a story perhaps more appropriate for Halloween than for the festive holiday season, given its scary implications.
Not too dire so far, until the researchers’ other findings are considered.
The BQ.1, BQ1.1, XBB, and XBB.1 subvariants are the most resistant to neutralizing antibodies, researcher Qian Wang, PhD, and colleagues wrote in a study published online in the journal Cell. This means people have no or “markedly reduced” protection against infection from these four strains, even if they’ve already had COVID-19 or are vaccinated and boosted multiple times, including with a bivalent vaccine.
On top of that, all available monoclonal antibody treatments are mostly or completely ineffective against these subvariants.
What does that mean for the immediate future? The findings are definitely “worrisome,” said Eric Topol, MD, founder and director of the Scripps Translational Research Institute in La Jolla, Calif.
But evidence from other countries, specifically Singapore and France, show that at least two of these variants turned out not to be as damaging as expected, likely because of high numbers of people vaccinated or who survived previous infections, he said.
Still, there is little to celebrate in the new findings, except that COVID-19 vaccinations and prior infections can still reduce the risk for serious outcomes such as hospitalization and death, the researchers wrote.
In fact, Centers for Disease Control and Prevention data released on Dec. 16 shows that people who have received four shots of the original COVID-19 vaccines as well as the bivalent booster were 57% less likely to visit an urgent care clinic or emergency room, regardless of age.
It comes at a time when BQ.1 and BQ.1.1 account for about 70% of the circulating variants, data show. In addition, hospitalizations are up 18% over the past 2 weeks and COVID-19 deaths are up 50% nationwide, The New York Times reported.
Globally, in many places, an “immunity wall” that has been built, Dr. Topol said. That may not be the case in the United States.
“The problem in the United States, making it harder to predict, is that we have a very low rate of recent boosters, in the past 6 months, especially in seniors,” he said. For example, only 36% of Americans aged 65 years and older, the group with highest risk, have received an updated bivalent booster.
An evolving virus
The subvariants are successfully replacing BA.5, which reigned as one of the most common Omicron variants over the past year. The latest CDC data show that BA.5 now accounts for only about 10% of the circulating virus. The researchers wrote: “This rapid replacement of virus strains is raising the specter of yet another wave of infections in the coming months.”
BQ.1 and BQ.1.1 evolved directly from BA.5 – adding more and some novel mutations to the SARS-CoV-2 virus. XBB and XBB.1 are the “offspring” of a combination of two other strains, known as BJ.1 and BA.2.75.
The story sounds familiar to the researchers. “The rapid rise of these subvariants and their extensive array of spike mutations are reminiscent of the appearance of the first Omicron variant last year, thus raising concerns that they may further compromise the efficacy of current COVID-19 vaccines and monoclonal antibody therapeutics,” they wrote. “We now report findings that indicate that such concerns are, sadly, justified, especially so for the XBB and XBB.1 subvariants.”
To figure out how effective existing antibodies could be against these newer subvariants, Dr. Wang and colleagues used blood samples from five groups of people. They tested serum from people who had three doses of the original COVID-19 vaccine, four doses of the original vaccine, those who received a bivalent booster, people who experienced a breakthrough infection with the BA.2 Omicron variant, and those who had a breakthrough with a BA.4 or BA.5 variant.
Adding the new subvariants to these serum samples revealed that the existing antibodies in the blood were ineffective at wiping out or neutralizing BQ.1, BQ.1.1, XBB, and XBB.1.
The BQ.1 subvariant was six times more resistant to antibodies than BA.5, its parent strain, and XBB.1 was 63 times more resistant compared with its predecessor, BA.2.
This shift in the ability of vaccines to stop the subvariants “is particularly concerning,” the researchers wrote.
Wiping out treatments too
Dr. Wang and colleagues also tested how well a panel of 23 different monoclonal antibody drugs might work against the four subvariants. The therapies all worked well against the original Omicron variant and included some approved for use through the Food and Drug Administration emergency use authorization (EUA) program at the time of the study.
They found that 19 of these 23 monoclonal antibodies lost effectiveness “greatly or completely” against XBB and XBB.1, for example.
This is not the first time that monoclonal antibody therapies have gone from effective to ineffective. Previous variants have come out that no longer responded to treatment with bamlanivimab, etesevimab, imdevimab, casirivimab, tixagevimab, cilgavimab, and sotrovimab. Bebtelovimab now joins this list and is no longer available from Eli Lilly under EUA because of this lack of effectiveness.
The lack of an effective monoclonal antibody treatment “poses a serious problem for millions of immunocompromised individuals who do not respond robustly to COVID-19 vaccines,” the researchers wrote, adding that “the urgent need to develop active monoclonal antibodies for clinical use is obvious.”
A limitation of the study is that the work is done in blood samples. The effectiveness of COVID-19 vaccination against the BQ and XBB subvariants should be evaluated in people in clinical studies, the authors noted.
Also, the current study looked at how well antibodies could neutralize the viral strains, but future research, they added, should look at how well “cellular immunity” or other aspects of the immune system might protect people.
Going forward, the challenge remains to develop vaccines and treatments that offer broad protection as the coronavirus continues to evolve.
In an alarming ending, the researchers wrote: “We have collectively chased after SARS-CoV-2 variants for over 2 years, and yet, the virus continues to evolve and evade.”
A version of this article first appeared on Medscape.com.
It’s a story perhaps more appropriate for Halloween than for the festive holiday season, given its scary implications.
Not too dire so far, until the researchers’ other findings are considered.
The BQ.1, BQ1.1, XBB, and XBB.1 subvariants are the most resistant to neutralizing antibodies, researcher Qian Wang, PhD, and colleagues wrote in a study published online in the journal Cell. This means people have no or “markedly reduced” protection against infection from these four strains, even if they’ve already had COVID-19 or are vaccinated and boosted multiple times, including with a bivalent vaccine.
On top of that, all available monoclonal antibody treatments are mostly or completely ineffective against these subvariants.
What does that mean for the immediate future? The findings are definitely “worrisome,” said Eric Topol, MD, founder and director of the Scripps Translational Research Institute in La Jolla, Calif.
But evidence from other countries, specifically Singapore and France, show that at least two of these variants turned out not to be as damaging as expected, likely because of high numbers of people vaccinated or who survived previous infections, he said.
Still, there is little to celebrate in the new findings, except that COVID-19 vaccinations and prior infections can still reduce the risk for serious outcomes such as hospitalization and death, the researchers wrote.
In fact, Centers for Disease Control and Prevention data released on Dec. 16 shows that people who have received four shots of the original COVID-19 vaccines as well as the bivalent booster were 57% less likely to visit an urgent care clinic or emergency room, regardless of age.
It comes at a time when BQ.1 and BQ.1.1 account for about 70% of the circulating variants, data show. In addition, hospitalizations are up 18% over the past 2 weeks and COVID-19 deaths are up 50% nationwide, The New York Times reported.
Globally, in many places, an “immunity wall” that has been built, Dr. Topol said. That may not be the case in the United States.
“The problem in the United States, making it harder to predict, is that we have a very low rate of recent boosters, in the past 6 months, especially in seniors,” he said. For example, only 36% of Americans aged 65 years and older, the group with highest risk, have received an updated bivalent booster.
An evolving virus
The subvariants are successfully replacing BA.5, which reigned as one of the most common Omicron variants over the past year. The latest CDC data show that BA.5 now accounts for only about 10% of the circulating virus. The researchers wrote: “This rapid replacement of virus strains is raising the specter of yet another wave of infections in the coming months.”
BQ.1 and BQ.1.1 evolved directly from BA.5 – adding more and some novel mutations to the SARS-CoV-2 virus. XBB and XBB.1 are the “offspring” of a combination of two other strains, known as BJ.1 and BA.2.75.
The story sounds familiar to the researchers. “The rapid rise of these subvariants and their extensive array of spike mutations are reminiscent of the appearance of the first Omicron variant last year, thus raising concerns that they may further compromise the efficacy of current COVID-19 vaccines and monoclonal antibody therapeutics,” they wrote. “We now report findings that indicate that such concerns are, sadly, justified, especially so for the XBB and XBB.1 subvariants.”
To figure out how effective existing antibodies could be against these newer subvariants, Dr. Wang and colleagues used blood samples from five groups of people. They tested serum from people who had three doses of the original COVID-19 vaccine, four doses of the original vaccine, those who received a bivalent booster, people who experienced a breakthrough infection with the BA.2 Omicron variant, and those who had a breakthrough with a BA.4 or BA.5 variant.
Adding the new subvariants to these serum samples revealed that the existing antibodies in the blood were ineffective at wiping out or neutralizing BQ.1, BQ.1.1, XBB, and XBB.1.
The BQ.1 subvariant was six times more resistant to antibodies than BA.5, its parent strain, and XBB.1 was 63 times more resistant compared with its predecessor, BA.2.
This shift in the ability of vaccines to stop the subvariants “is particularly concerning,” the researchers wrote.
Wiping out treatments too
Dr. Wang and colleagues also tested how well a panel of 23 different monoclonal antibody drugs might work against the four subvariants. The therapies all worked well against the original Omicron variant and included some approved for use through the Food and Drug Administration emergency use authorization (EUA) program at the time of the study.
They found that 19 of these 23 monoclonal antibodies lost effectiveness “greatly or completely” against XBB and XBB.1, for example.
This is not the first time that monoclonal antibody therapies have gone from effective to ineffective. Previous variants have come out that no longer responded to treatment with bamlanivimab, etesevimab, imdevimab, casirivimab, tixagevimab, cilgavimab, and sotrovimab. Bebtelovimab now joins this list and is no longer available from Eli Lilly under EUA because of this lack of effectiveness.
The lack of an effective monoclonal antibody treatment “poses a serious problem for millions of immunocompromised individuals who do not respond robustly to COVID-19 vaccines,” the researchers wrote, adding that “the urgent need to develop active monoclonal antibodies for clinical use is obvious.”
A limitation of the study is that the work is done in blood samples. The effectiveness of COVID-19 vaccination against the BQ and XBB subvariants should be evaluated in people in clinical studies, the authors noted.
Also, the current study looked at how well antibodies could neutralize the viral strains, but future research, they added, should look at how well “cellular immunity” or other aspects of the immune system might protect people.
Going forward, the challenge remains to develop vaccines and treatments that offer broad protection as the coronavirus continues to evolve.
In an alarming ending, the researchers wrote: “We have collectively chased after SARS-CoV-2 variants for over 2 years, and yet, the virus continues to evolve and evade.”
A version of this article first appeared on Medscape.com.
It’s a story perhaps more appropriate for Halloween than for the festive holiday season, given its scary implications.
Not too dire so far, until the researchers’ other findings are considered.
The BQ.1, BQ1.1, XBB, and XBB.1 subvariants are the most resistant to neutralizing antibodies, researcher Qian Wang, PhD, and colleagues wrote in a study published online in the journal Cell. This means people have no or “markedly reduced” protection against infection from these four strains, even if they’ve already had COVID-19 or are vaccinated and boosted multiple times, including with a bivalent vaccine.
On top of that, all available monoclonal antibody treatments are mostly or completely ineffective against these subvariants.
What does that mean for the immediate future? The findings are definitely “worrisome,” said Eric Topol, MD, founder and director of the Scripps Translational Research Institute in La Jolla, Calif.
But evidence from other countries, specifically Singapore and France, show that at least two of these variants turned out not to be as damaging as expected, likely because of high numbers of people vaccinated or who survived previous infections, he said.
Still, there is little to celebrate in the new findings, except that COVID-19 vaccinations and prior infections can still reduce the risk for serious outcomes such as hospitalization and death, the researchers wrote.
In fact, Centers for Disease Control and Prevention data released on Dec. 16 shows that people who have received four shots of the original COVID-19 vaccines as well as the bivalent booster were 57% less likely to visit an urgent care clinic or emergency room, regardless of age.
It comes at a time when BQ.1 and BQ.1.1 account for about 70% of the circulating variants, data show. In addition, hospitalizations are up 18% over the past 2 weeks and COVID-19 deaths are up 50% nationwide, The New York Times reported.
Globally, in many places, an “immunity wall” that has been built, Dr. Topol said. That may not be the case in the United States.
“The problem in the United States, making it harder to predict, is that we have a very low rate of recent boosters, in the past 6 months, especially in seniors,” he said. For example, only 36% of Americans aged 65 years and older, the group with highest risk, have received an updated bivalent booster.
An evolving virus
The subvariants are successfully replacing BA.5, which reigned as one of the most common Omicron variants over the past year. The latest CDC data show that BA.5 now accounts for only about 10% of the circulating virus. The researchers wrote: “This rapid replacement of virus strains is raising the specter of yet another wave of infections in the coming months.”
BQ.1 and BQ.1.1 evolved directly from BA.5 – adding more and some novel mutations to the SARS-CoV-2 virus. XBB and XBB.1 are the “offspring” of a combination of two other strains, known as BJ.1 and BA.2.75.
The story sounds familiar to the researchers. “The rapid rise of these subvariants and their extensive array of spike mutations are reminiscent of the appearance of the first Omicron variant last year, thus raising concerns that they may further compromise the efficacy of current COVID-19 vaccines and monoclonal antibody therapeutics,” they wrote. “We now report findings that indicate that such concerns are, sadly, justified, especially so for the XBB and XBB.1 subvariants.”
To figure out how effective existing antibodies could be against these newer subvariants, Dr. Wang and colleagues used blood samples from five groups of people. They tested serum from people who had three doses of the original COVID-19 vaccine, four doses of the original vaccine, those who received a bivalent booster, people who experienced a breakthrough infection with the BA.2 Omicron variant, and those who had a breakthrough with a BA.4 or BA.5 variant.
Adding the new subvariants to these serum samples revealed that the existing antibodies in the blood were ineffective at wiping out or neutralizing BQ.1, BQ.1.1, XBB, and XBB.1.
The BQ.1 subvariant was six times more resistant to antibodies than BA.5, its parent strain, and XBB.1 was 63 times more resistant compared with its predecessor, BA.2.
This shift in the ability of vaccines to stop the subvariants “is particularly concerning,” the researchers wrote.
Wiping out treatments too
Dr. Wang and colleagues also tested how well a panel of 23 different monoclonal antibody drugs might work against the four subvariants. The therapies all worked well against the original Omicron variant and included some approved for use through the Food and Drug Administration emergency use authorization (EUA) program at the time of the study.
They found that 19 of these 23 monoclonal antibodies lost effectiveness “greatly or completely” against XBB and XBB.1, for example.
This is not the first time that monoclonal antibody therapies have gone from effective to ineffective. Previous variants have come out that no longer responded to treatment with bamlanivimab, etesevimab, imdevimab, casirivimab, tixagevimab, cilgavimab, and sotrovimab. Bebtelovimab now joins this list and is no longer available from Eli Lilly under EUA because of this lack of effectiveness.
The lack of an effective monoclonal antibody treatment “poses a serious problem for millions of immunocompromised individuals who do not respond robustly to COVID-19 vaccines,” the researchers wrote, adding that “the urgent need to develop active monoclonal antibodies for clinical use is obvious.”
A limitation of the study is that the work is done in blood samples. The effectiveness of COVID-19 vaccination against the BQ and XBB subvariants should be evaluated in people in clinical studies, the authors noted.
Also, the current study looked at how well antibodies could neutralize the viral strains, but future research, they added, should look at how well “cellular immunity” or other aspects of the immune system might protect people.
Going forward, the challenge remains to develop vaccines and treatments that offer broad protection as the coronavirus continues to evolve.
In an alarming ending, the researchers wrote: “We have collectively chased after SARS-CoV-2 variants for over 2 years, and yet, the virus continues to evolve and evade.”
A version of this article first appeared on Medscape.com.
FROM CELL
Most women with breast cancer elude serious COVID-19 vaccine side effects
Findings from the LymphVAX study recently presented at the San Antonio Breast Cancer Symposium show that relatively
Lymph node swelling can be a particularly troubling side effect, since it could be mistaken for breast cancer progression. In this study, of 621 women who received the first dose of an mRNA COVID-19 vaccine, 9.8% developed lymph node swelling as compared with 12.9% of 621 women who received the second dose, and 11.3% of 469 women who received the third dose. The findings were comparable to those of studies conducted of the general population, said study author Brooke C. Juhel, BS, a clinical research coordinator in the lymphedema research program at Massachusetts General Hospital and a student at Harvard Medical School, both in Boston. In the general population, 10.2% experienced lymph node swelling after the first dose and 14% after the second dose, according to the Centers for Disease Control and studies of the Pfizer and Moderna vaccines.
“This is consistent with the hypothesis that, after repeated vaccine doses, the immune system already has the antigens ready to fight the virus, thus the side effects may worsen as the immune response has increased,” she said. “Having screened over 6,500 women for breast cancer–related lymphedema, and with our patients reaching out with concerns about vaccine side effects, we were in a unique position to conduct this study.”
The study also confirmed that the most common side effects of receiving mRNA COVID-19 vaccines for women treated for breast cancer included injection site soreness, fatigue, muscle soreness, headache and chills lasting an average of 48 hours, which are symptoms comparable with those experienced by the general population.
“The side-effect profiles reported in this study for a cohort of women treated for breast cancer can be used to provide evidence-based patient education regarding future COVID-19 vaccine administration. The effect of the COVID-19 vaccines on breast cancer–related lymphedema risk is currently unknown and more research is required. In the interim, we would recommend vaccination away from the side of lymph node removal, either in the contralateral arm or in the thigh,” Ms. Juhel said.
The median duration of lymph node swelling was less than 1 week. In cases where lymph node swelling occurred after the first dose, 54.1% had swelling in ipsilateral axillary lymph nodes, and 45.9% in contralateral axillary lymph nodes. About 29.5% experienced swelling in ipsilateral supraclavicular lymph nodes, and 18.0% in contralateral supraclavicular lymph nodes.
Injection-site soreness, fatigue, GMS, headache, and chills occurred less often among older individuals (P < .001), and fatigue, muscle soreness, headache, and chills occurred more frequently after the second dose than the first (P < .001). The median duration of all side effects was 48 hours or less.
“The informed education that can be produced based on these results will hopefully ease the fears of women treated for breast cancer and empower them to make informed decisions regarding future vaccine doses,” Ms. Juhel said.
Ms. Juhel has no relevant financial disclosures.
Findings from the LymphVAX study recently presented at the San Antonio Breast Cancer Symposium show that relatively
Lymph node swelling can be a particularly troubling side effect, since it could be mistaken for breast cancer progression. In this study, of 621 women who received the first dose of an mRNA COVID-19 vaccine, 9.8% developed lymph node swelling as compared with 12.9% of 621 women who received the second dose, and 11.3% of 469 women who received the third dose. The findings were comparable to those of studies conducted of the general population, said study author Brooke C. Juhel, BS, a clinical research coordinator in the lymphedema research program at Massachusetts General Hospital and a student at Harvard Medical School, both in Boston. In the general population, 10.2% experienced lymph node swelling after the first dose and 14% after the second dose, according to the Centers for Disease Control and studies of the Pfizer and Moderna vaccines.
“This is consistent with the hypothesis that, after repeated vaccine doses, the immune system already has the antigens ready to fight the virus, thus the side effects may worsen as the immune response has increased,” she said. “Having screened over 6,500 women for breast cancer–related lymphedema, and with our patients reaching out with concerns about vaccine side effects, we were in a unique position to conduct this study.”
The study also confirmed that the most common side effects of receiving mRNA COVID-19 vaccines for women treated for breast cancer included injection site soreness, fatigue, muscle soreness, headache and chills lasting an average of 48 hours, which are symptoms comparable with those experienced by the general population.
“The side-effect profiles reported in this study for a cohort of women treated for breast cancer can be used to provide evidence-based patient education regarding future COVID-19 vaccine administration. The effect of the COVID-19 vaccines on breast cancer–related lymphedema risk is currently unknown and more research is required. In the interim, we would recommend vaccination away from the side of lymph node removal, either in the contralateral arm or in the thigh,” Ms. Juhel said.
The median duration of lymph node swelling was less than 1 week. In cases where lymph node swelling occurred after the first dose, 54.1% had swelling in ipsilateral axillary lymph nodes, and 45.9% in contralateral axillary lymph nodes. About 29.5% experienced swelling in ipsilateral supraclavicular lymph nodes, and 18.0% in contralateral supraclavicular lymph nodes.
Injection-site soreness, fatigue, GMS, headache, and chills occurred less often among older individuals (P < .001), and fatigue, muscle soreness, headache, and chills occurred more frequently after the second dose than the first (P < .001). The median duration of all side effects was 48 hours or less.
“The informed education that can be produced based on these results will hopefully ease the fears of women treated for breast cancer and empower them to make informed decisions regarding future vaccine doses,” Ms. Juhel said.
Ms. Juhel has no relevant financial disclosures.
Findings from the LymphVAX study recently presented at the San Antonio Breast Cancer Symposium show that relatively
Lymph node swelling can be a particularly troubling side effect, since it could be mistaken for breast cancer progression. In this study, of 621 women who received the first dose of an mRNA COVID-19 vaccine, 9.8% developed lymph node swelling as compared with 12.9% of 621 women who received the second dose, and 11.3% of 469 women who received the third dose. The findings were comparable to those of studies conducted of the general population, said study author Brooke C. Juhel, BS, a clinical research coordinator in the lymphedema research program at Massachusetts General Hospital and a student at Harvard Medical School, both in Boston. In the general population, 10.2% experienced lymph node swelling after the first dose and 14% after the second dose, according to the Centers for Disease Control and studies of the Pfizer and Moderna vaccines.
“This is consistent with the hypothesis that, after repeated vaccine doses, the immune system already has the antigens ready to fight the virus, thus the side effects may worsen as the immune response has increased,” she said. “Having screened over 6,500 women for breast cancer–related lymphedema, and with our patients reaching out with concerns about vaccine side effects, we were in a unique position to conduct this study.”
The study also confirmed that the most common side effects of receiving mRNA COVID-19 vaccines for women treated for breast cancer included injection site soreness, fatigue, muscle soreness, headache and chills lasting an average of 48 hours, which are symptoms comparable with those experienced by the general population.
“The side-effect profiles reported in this study for a cohort of women treated for breast cancer can be used to provide evidence-based patient education regarding future COVID-19 vaccine administration. The effect of the COVID-19 vaccines on breast cancer–related lymphedema risk is currently unknown and more research is required. In the interim, we would recommend vaccination away from the side of lymph node removal, either in the contralateral arm or in the thigh,” Ms. Juhel said.
The median duration of lymph node swelling was less than 1 week. In cases where lymph node swelling occurred after the first dose, 54.1% had swelling in ipsilateral axillary lymph nodes, and 45.9% in contralateral axillary lymph nodes. About 29.5% experienced swelling in ipsilateral supraclavicular lymph nodes, and 18.0% in contralateral supraclavicular lymph nodes.
Injection-site soreness, fatigue, GMS, headache, and chills occurred less often among older individuals (P < .001), and fatigue, muscle soreness, headache, and chills occurred more frequently after the second dose than the first (P < .001). The median duration of all side effects was 48 hours or less.
“The informed education that can be produced based on these results will hopefully ease the fears of women treated for breast cancer and empower them to make informed decisions regarding future vaccine doses,” Ms. Juhel said.
Ms. Juhel has no relevant financial disclosures.
FROM SABCS 2022
Breast cancer diagnoses worse among Hispanics during COVID-19 pandemic
In a series of studies recently presented at the San Antonio Breast Cancer Symposium that examine the effects of the COVID-19 pandemic on women with breast cancer, researchers report that ethnicity played a role in later diagnoses, Hispanics presented with more advanced and aggressive disease, and a focus on a single hospital in San Antonio finds a statistical difference between stage at diagnosis prior to the pandemic, compared with the postvaccine era.
Patients treated at the Mays Cancer Center, a cancer hospital of University of Texas Health and MD Anderson Cancer Center in San Antonio, during the pandemic were found to more likely present with advanced disease between March and December 2020, according to Marcela Mazo, MD, an oncologist with UT Health, San Antonio, and an author of each of three studies.
“We learned that Hispanic patients were presenting with more aggressive histologies such as HER2-positive and triple-negative disease. We also confirmed what we were suspecting, which is that Latina women had less access to medical coverage. We had a higher proportion of Hispanic patients presenting to us without medical coverage, which of course made the treatment extremely challenging,” said Dr. Mazo.
Hispanics are one of the fastest-growing minority groups in the United States, and understanding the factors that affect their healthcare is critical to formulating health policies.
And I’m sad to say that, even after everything opened up and people could get vaccinated, I still saw some patients who, for whatever reason, did not get a mammogram – which led to [more] clinical presentations of advanced cancer by the time they were seen by us,” she said.
Dr. Mazo said that underscreened women could also be considered victims of the pandemic. “I tell my patients to get their vaccines so they’re protected and they can feel more comfortable going to the doctor where there is a higher proportion of people who could potentially have COVID.”
Other studies have shown that patients in general, regardless of race or ethnicity, have been diagnosed with later-stage breast cancer diagnoses during the pandemic.
The three studies are based on an analysis of 696 patients treated at Mays Cancer Center. Of these, 264 were diagnosed before the pandemic (cohort A), 171 during the lockdown (Apr. 1 to Dec. 31, 2020, cohort B) and 261 after vaccines were introduced (Jan. 1 to Dec. 31, 2021, cohort C). Overall, there was a slight trend toward a higher incidence of HER2-positive disease during the lockdown period (odds ratio, 1.45) and in the postvaccine period (OR, 1.40), though neither relationship was statistically significant (P = .2). No relationships were seen between time period and incidence of triple-negative breast cancer.
The researchers found that Hispanic patients were more likely to be diagnosed with advanced disease in the pandemic years, compared with pre-COVID times. For example, the likelihood of being diagnosed with carcinoma in situ (Tis) versus T1 disease was lower in the postvaccine era than the pre-COVID era (OR, 0.38; P < .001), although there was no significant difference in Tis versus T1 during the lockdown period, compared with the pre-COVID era. The researchers concluded the difference was likely caused by the latency period of breast cancer.
The postvaccine era saw a 15% increase in patients diagnosed with HER2-positive disease, compared with the pre-COVID era. Patients diagnosed in the COVID era (cohorts B and C) were more likely to require neoadjuvant therapy than patients diagnosed in the pre-COVID era (OR, 1.78; P = .009).
They also found significant disparities in health insurance coverage. 91% of non-Hispanic patients were covered by insurance, compared with 70% of Hispanic patients.
Overall, the findings hint at the depth of health care inequities faced by Hispanic women in the region, and should be a call for action, Dr. Mazo said. “I wish that we as physicians would take the lead to do the best we can to support legislative changes that could help all of our patients get treated – independent of where they come from.”
Dr. Mazo has no relevant financial disclosures.
In a series of studies recently presented at the San Antonio Breast Cancer Symposium that examine the effects of the COVID-19 pandemic on women with breast cancer, researchers report that ethnicity played a role in later diagnoses, Hispanics presented with more advanced and aggressive disease, and a focus on a single hospital in San Antonio finds a statistical difference between stage at diagnosis prior to the pandemic, compared with the postvaccine era.
Patients treated at the Mays Cancer Center, a cancer hospital of University of Texas Health and MD Anderson Cancer Center in San Antonio, during the pandemic were found to more likely present with advanced disease between March and December 2020, according to Marcela Mazo, MD, an oncologist with UT Health, San Antonio, and an author of each of three studies.
“We learned that Hispanic patients were presenting with more aggressive histologies such as HER2-positive and triple-negative disease. We also confirmed what we were suspecting, which is that Latina women had less access to medical coverage. We had a higher proportion of Hispanic patients presenting to us without medical coverage, which of course made the treatment extremely challenging,” said Dr. Mazo.
Hispanics are one of the fastest-growing minority groups in the United States, and understanding the factors that affect their healthcare is critical to formulating health policies.
And I’m sad to say that, even after everything opened up and people could get vaccinated, I still saw some patients who, for whatever reason, did not get a mammogram – which led to [more] clinical presentations of advanced cancer by the time they were seen by us,” she said.
Dr. Mazo said that underscreened women could also be considered victims of the pandemic. “I tell my patients to get their vaccines so they’re protected and they can feel more comfortable going to the doctor where there is a higher proportion of people who could potentially have COVID.”
Other studies have shown that patients in general, regardless of race or ethnicity, have been diagnosed with later-stage breast cancer diagnoses during the pandemic.
The three studies are based on an analysis of 696 patients treated at Mays Cancer Center. Of these, 264 were diagnosed before the pandemic (cohort A), 171 during the lockdown (Apr. 1 to Dec. 31, 2020, cohort B) and 261 after vaccines were introduced (Jan. 1 to Dec. 31, 2021, cohort C). Overall, there was a slight trend toward a higher incidence of HER2-positive disease during the lockdown period (odds ratio, 1.45) and in the postvaccine period (OR, 1.40), though neither relationship was statistically significant (P = .2). No relationships were seen between time period and incidence of triple-negative breast cancer.
The researchers found that Hispanic patients were more likely to be diagnosed with advanced disease in the pandemic years, compared with pre-COVID times. For example, the likelihood of being diagnosed with carcinoma in situ (Tis) versus T1 disease was lower in the postvaccine era than the pre-COVID era (OR, 0.38; P < .001), although there was no significant difference in Tis versus T1 during the lockdown period, compared with the pre-COVID era. The researchers concluded the difference was likely caused by the latency period of breast cancer.
The postvaccine era saw a 15% increase in patients diagnosed with HER2-positive disease, compared with the pre-COVID era. Patients diagnosed in the COVID era (cohorts B and C) were more likely to require neoadjuvant therapy than patients diagnosed in the pre-COVID era (OR, 1.78; P = .009).
They also found significant disparities in health insurance coverage. 91% of non-Hispanic patients were covered by insurance, compared with 70% of Hispanic patients.
Overall, the findings hint at the depth of health care inequities faced by Hispanic women in the region, and should be a call for action, Dr. Mazo said. “I wish that we as physicians would take the lead to do the best we can to support legislative changes that could help all of our patients get treated – independent of where they come from.”
Dr. Mazo has no relevant financial disclosures.
In a series of studies recently presented at the San Antonio Breast Cancer Symposium that examine the effects of the COVID-19 pandemic on women with breast cancer, researchers report that ethnicity played a role in later diagnoses, Hispanics presented with more advanced and aggressive disease, and a focus on a single hospital in San Antonio finds a statistical difference between stage at diagnosis prior to the pandemic, compared with the postvaccine era.
Patients treated at the Mays Cancer Center, a cancer hospital of University of Texas Health and MD Anderson Cancer Center in San Antonio, during the pandemic were found to more likely present with advanced disease between March and December 2020, according to Marcela Mazo, MD, an oncologist with UT Health, San Antonio, and an author of each of three studies.
“We learned that Hispanic patients were presenting with more aggressive histologies such as HER2-positive and triple-negative disease. We also confirmed what we were suspecting, which is that Latina women had less access to medical coverage. We had a higher proportion of Hispanic patients presenting to us without medical coverage, which of course made the treatment extremely challenging,” said Dr. Mazo.
Hispanics are one of the fastest-growing minority groups in the United States, and understanding the factors that affect their healthcare is critical to formulating health policies.
And I’m sad to say that, even after everything opened up and people could get vaccinated, I still saw some patients who, for whatever reason, did not get a mammogram – which led to [more] clinical presentations of advanced cancer by the time they were seen by us,” she said.
Dr. Mazo said that underscreened women could also be considered victims of the pandemic. “I tell my patients to get their vaccines so they’re protected and they can feel more comfortable going to the doctor where there is a higher proportion of people who could potentially have COVID.”
Other studies have shown that patients in general, regardless of race or ethnicity, have been diagnosed with later-stage breast cancer diagnoses during the pandemic.
The three studies are based on an analysis of 696 patients treated at Mays Cancer Center. Of these, 264 were diagnosed before the pandemic (cohort A), 171 during the lockdown (Apr. 1 to Dec. 31, 2020, cohort B) and 261 after vaccines were introduced (Jan. 1 to Dec. 31, 2021, cohort C). Overall, there was a slight trend toward a higher incidence of HER2-positive disease during the lockdown period (odds ratio, 1.45) and in the postvaccine period (OR, 1.40), though neither relationship was statistically significant (P = .2). No relationships were seen between time period and incidence of triple-negative breast cancer.
The researchers found that Hispanic patients were more likely to be diagnosed with advanced disease in the pandemic years, compared with pre-COVID times. For example, the likelihood of being diagnosed with carcinoma in situ (Tis) versus T1 disease was lower in the postvaccine era than the pre-COVID era (OR, 0.38; P < .001), although there was no significant difference in Tis versus T1 during the lockdown period, compared with the pre-COVID era. The researchers concluded the difference was likely caused by the latency period of breast cancer.
The postvaccine era saw a 15% increase in patients diagnosed with HER2-positive disease, compared with the pre-COVID era. Patients diagnosed in the COVID era (cohorts B and C) were more likely to require neoadjuvant therapy than patients diagnosed in the pre-COVID era (OR, 1.78; P = .009).
They also found significant disparities in health insurance coverage. 91% of non-Hispanic patients were covered by insurance, compared with 70% of Hispanic patients.
Overall, the findings hint at the depth of health care inequities faced by Hispanic women in the region, and should be a call for action, Dr. Mazo said. “I wish that we as physicians would take the lead to do the best we can to support legislative changes that could help all of our patients get treated – independent of where they come from.”
Dr. Mazo has no relevant financial disclosures.
FROM SABCS 2022
U.S. sees most flu hospitalizations in a decade
But the number of deaths and outpatient visits for flu or flu-like illnesses was down slightly from the week before, the CDC said in its weekly FluView report.
There were almost 26,000 new hospital admissions involving laboratory-confirmed influenza over those 7 days, up by over 31% from the previous week, based on data from 5,000 hospitals in the HHS Protect system, which tracks and shares COVID-19 data.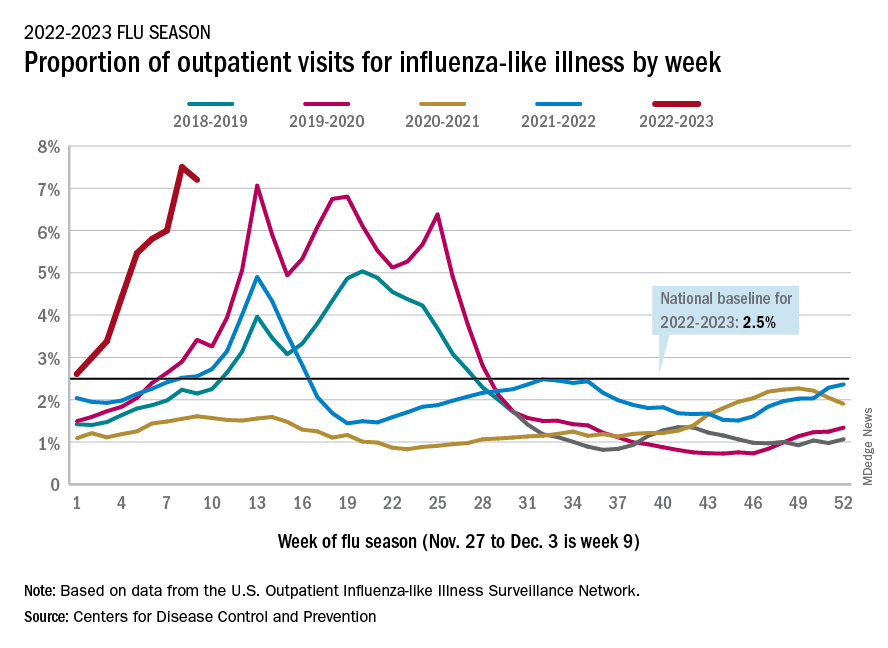
The cumulative hospitalization rate for the 2022-2023 season is 26.0 per 100,000 people, the highest seen at this time of year since 2010-2011, the CDC said, based on data from its Influenza Hospitalization Surveillance Network, which includes hospitals in select counties in 13 states.
At this point in the 2019-2020 season, just before the COVID-19 pandemic began, the cumulative rate was 3.1 per 100,000 people, the CDC’s data show.
On the positive side, the proportion of outpatient visits for influenza-like illness dropped slightly to 7.2%, from 7.5% the week before. But these cases from the CDC’s Outpatient Influenza-like Illness Surveillance Network are not laboratory confirmed, so the data could include people with the flu, COVID-19, or respiratory syncytial virus.
The number of confirmed flu deaths for the week of Nov. 27 to Dec. 3 also fell slightly from the last full week of November, 246 vs. 255, but the number of pediatric deaths rose from 2 to 7, and total deaths in children are already up to 21 for 2022-2023. That’s compared to 44 that were reported during all of the 2021-2022 season, the CDC said.
“So far this season, there have been at least 13 million illnesses, 120,000 hospitalizations, and 7,300 deaths from flu,” the agency estimated.
A version of this article first appeared on Medscape.com.
But the number of deaths and outpatient visits for flu or flu-like illnesses was down slightly from the week before, the CDC said in its weekly FluView report.
There were almost 26,000 new hospital admissions involving laboratory-confirmed influenza over those 7 days, up by over 31% from the previous week, based on data from 5,000 hospitals in the HHS Protect system, which tracks and shares COVID-19 data.
The cumulative hospitalization rate for the 2022-2023 season is 26.0 per 100,000 people, the highest seen at this time of year since 2010-2011, the CDC said, based on data from its Influenza Hospitalization Surveillance Network, which includes hospitals in select counties in 13 states.
At this point in the 2019-2020 season, just before the COVID-19 pandemic began, the cumulative rate was 3.1 per 100,000 people, the CDC’s data show.
On the positive side, the proportion of outpatient visits for influenza-like illness dropped slightly to 7.2%, from 7.5% the week before. But these cases from the CDC’s Outpatient Influenza-like Illness Surveillance Network are not laboratory confirmed, so the data could include people with the flu, COVID-19, or respiratory syncytial virus.
The number of confirmed flu deaths for the week of Nov. 27 to Dec. 3 also fell slightly from the last full week of November, 246 vs. 255, but the number of pediatric deaths rose from 2 to 7, and total deaths in children are already up to 21 for 2022-2023. That’s compared to 44 that were reported during all of the 2021-2022 season, the CDC said.
“So far this season, there have been at least 13 million illnesses, 120,000 hospitalizations, and 7,300 deaths from flu,” the agency estimated.
A version of this article first appeared on Medscape.com.
But the number of deaths and outpatient visits for flu or flu-like illnesses was down slightly from the week before, the CDC said in its weekly FluView report.
There were almost 26,000 new hospital admissions involving laboratory-confirmed influenza over those 7 days, up by over 31% from the previous week, based on data from 5,000 hospitals in the HHS Protect system, which tracks and shares COVID-19 data.
The cumulative hospitalization rate for the 2022-2023 season is 26.0 per 100,000 people, the highest seen at this time of year since 2010-2011, the CDC said, based on data from its Influenza Hospitalization Surveillance Network, which includes hospitals in select counties in 13 states.
At this point in the 2019-2020 season, just before the COVID-19 pandemic began, the cumulative rate was 3.1 per 100,000 people, the CDC’s data show.
On the positive side, the proportion of outpatient visits for influenza-like illness dropped slightly to 7.2%, from 7.5% the week before. But these cases from the CDC’s Outpatient Influenza-like Illness Surveillance Network are not laboratory confirmed, so the data could include people with the flu, COVID-19, or respiratory syncytial virus.
The number of confirmed flu deaths for the week of Nov. 27 to Dec. 3 also fell slightly from the last full week of November, 246 vs. 255, but the number of pediatric deaths rose from 2 to 7, and total deaths in children are already up to 21 for 2022-2023. That’s compared to 44 that were reported during all of the 2021-2022 season, the CDC said.
“So far this season, there have been at least 13 million illnesses, 120,000 hospitalizations, and 7,300 deaths from flu,” the agency estimated.
A version of this article first appeared on Medscape.com.
As COVID treatments dwindle, are new ones waiting in the wings?
It was the last monoclonal antibody treatment standing. But less than 10 months after the U.S. Food and Drug Administration gave bebtelovimab its emergency use authorization (EUA) to fight COVID-19, it earlier this month de-authorized it, just as it had for other monoclonal antibody treatments, and for the same reason:
Bebtelovimab couldn’t neutralize the Omicron subvariants BQ.1 and BQ.1.1, the cause of nearly 60% of COVID cases nationally as of November 30.
Next on the chopping block, some predict, will be Evusheld, the combination of tixagevimab and cilgavimab given as a preventive monoclonal antibody to people who are immunocompromised and at high risk of contracting COVID and to those who can’t take the vaccine. In October, the FDA warned that Evusheld was not neutralizing circulating COVID variants.
As the options for treating and preventing COVID decline, will companies rally quickly to develop new ones, or cut their losses in developing treatments that may work for only a few months, given the speed of viral mutations?
But although monoclonal antibody treatments are off the table, at least for now, antiviral drugs – including Paxlovid – are still very much available, and some say underused.
Others suggest it’s time to resurrect interest in convalescent plasma, a treatment used early in the pandemic before drugs or vaccines were here and still authorized for use in those who are immunosuppressed or receiving immunosuppressive treatment.
And on the prevention front, staying up to date with booster vaccines, masking, and taking other precautions should be stressed more, others say, regardless of the number of treatment options, and especially now, as cases rise and people gather for the winter holidays.
‘A major setback’
The bebtelovimab de-authorization was “a major setback,” but an understandable one, said Arturo Casadevall, MD, PhD, professor and chair of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health in Baltimore. “Monoclonal antibodies are great drugs. We are in an unfortunate situation in that they are vulnerable to changes in the virus” and can’t offer long-lasting protection.
Supplies of bebtelovimab will be retained, according to the FDA, in case variants susceptible to it return.
“What happened to bebtelovimab is no surprise,” agreed Amesh Adalja, MD, senior scholar at Johns Hopkins Center for Health Security. “This is what is going to happen when you are targeting a virus that mutates a lot.”
Monoclonal antibodies work by binding to the spike protein on the virus surface to prevent it from entering cells.
However, Dr. Adalja doesn’t view the disappearance of monoclonal antibody treatments as a major setback. Monoclonal antibodies were not the primary way COVID was treated, he said.
While he does believe it’s important that more monoclonal antibody treatments be developed, “I think it’s important to remember we still have Paxlovid while everyone is lamenting the loss of bebtelovimab.’’
Antivirals: What’s here, what’s coming
Compared with monoclonal antibodies, “Paxlovid remains a much easier drug to give,” Dr. Adalja told this news organization, because it is taken orally, not intravenously.
And it’s effective. In a recent study, researchers found that adults diagnosed with COVID given Paxlovid within 5 days of diagnosis had a 51% lower hospitalization rate within the next 30 days than those not given it. Another study shows it could also reduce a person’s risk of developing long COVID by 26%.
Paxlovid is underused, Dr. Adalja said, partly because the rebound potential got more press than the effectiveness. When a celebrity got rebound from Paxlovid, he said, that would make the news, overshadowing the research on its effectiveness.
Besides Paxlovid, the antivirals remdesivir (Veklury), given intravenously for 3 days, and molnupiravir (Lagevrio), taken orally, are also still available. Antivirals work by targeting specific parts of the virus to prevent it from multiplying.
In the lab, remdesivir, molnupiravir, and another antiviral, nirmatrelvir, all appear to be effective against both BQ.1.1 (a BA.5 subvariant) and XBB (a BA.2 subvariant), both rapidly rising in the United States, according to a report last week in the New England Journal of Medicine.
The researchers also tested several monoclonal antibodies and found they did not neutralize either of the subvariants BQ.1.1 and XBB.
A new oral antiviral, Xocova (ensitrelvir fumaric acid), from Japanese manufacturer Shionogi, received emergency approval in Japan on November 22. It’s taken once a day for 5 days. The goal is to expand access to it globally, according to the company.
Pardes Biosciences launched a phase 2 trial in September for its oral antiviral drug (PBI-0451), under study as a treatment and preventive for COVID. It expects data by the first quarter of 2023.
Pfizer, which makes Paxlovid, has partnered with Clear Creek Bio to develop another oral antiviral COVID drug.
Other approaches
A receptor protein known as ACE2 (angiotensin-converting enzyme 2) is the main “doorway” that SARS-CoV-2 uses to enter and infect cells.
Dana-Farber Cancer Institute scientists are developing a “decoy” drug that works by mimicking the ACE2 receptor on the surface of cells; when the virus tries to bind to it, the spike protein is destroyed. Human trials have not yet started.
Other researchers are investigating whether an already-approved drug used to treat a liver disease, Actigall (UDCA/ursodeoxycholic acid), could protect against COVID infection by reducing ACE2.
So far, the researchers have found in early research that people taking UDCA for liver conditions were less likely than those not taking the drug to have severe COVID. They also found that UDCA reduced SARS-CoV-2 infection in human lungs maintained outside the body.
Monoclonal antibody treatments?
After the FDA decision to withdraw the bebtelovimab EUA, which Eli Lilly said it agreed with, the company issued a statement, promising it wasn’t giving up on monoclonal antibody treatments.
“Lilly will continue to search and evaluate monoclonal antibodies to identify potential candidates for clinical development against new variants,” it read in part.
AstraZeneca, which makes Evusheld, is also continuing to work on monoclonal antibody development. According to a spokesperson, “We are also developing a new long-acting antibody combination – AZD5156 – which has been shown in the lab to neutralize emerging new variants and all known variants to date. We are working to accelerate the development of AZD5156 to make it available at the end of 2023.”
The AstraZeneca spokesperson said he could share no more information about what the combination would include.
A convalescent plasma comeback?
Although Paxlovid can help, there are many contraindications to it, such as drug-drug interactions, Dr. Casadevall told this news organization. And now that the monoclonal antibody treatments have been paused, convalescent plasma “is the only antibody-based therapy that is reliably available. Convalescent plasma includes thousands of different antibodies.”
With his colleagues, Dr. Casadevall evaluated plasma samples from 740 patients. Some had received booster vaccines and been infected with Omicron, others had received boosters and not been infected, and still others had not been vaccinated and became infected.
In a report (not yet peer-reviewed), they found the plasma from those who had been infected or boosted within the past 6 months neutralized the new Omicron variants BQ.1.1, XBB.1, and BF.7.
A push for boosters, masks
To get through the coming months, taking precautions like masking and distancing and staying up to date on booster vaccinations, especially for older adults, can make a difference, other experts say.
In a Twitter thread in early December, Peter Hotez, MD, PhD, professor of pediatrics and molecular virology and microbiology at Baylor College of Medicine, Houston, urged people to take COVID seriously as holiday parties and gatherings occur.
“The single most impactful thing you can do is get your bivalent booster,” he tweeted, as well as give your kids the booster, citing preliminary research that the bivalent mRNA booster broadens immunity against the Omicron subvariants.
For seniors, he said, ‘‘if you get breakthrough COVID, [it’s] really important to get Paxlovid.” Masks will help not only for COVID but also influenza, respiratory syncytial virus (RSV), and other conditions.
Mitigation measures have largely been abandoned, according to Eric Topol, MD, director of the Scripps Research Translational Institute, La Jolla, Calif., and editor-in-chief of Medscape. In an op-ed in the Los Angeles Times, and on his Twitter feed, he reminds people about masking and urges people to get the bivalent booster.
According to the Centers for Disease Control and Prevention, as of Dec. 8, only 13.5% of people aged 5 and older have gotten an updated booster, despite research that shows an increase in antibodies to BQ.1.1. Recent research has found that the bivalent booster increases antibodies to BQ.1.1 by up to 10-fold, Dr. Topol said.
Dr. Adalja is on advisory boards for Shionogi, GSK, and Pardes. Dr. Casadevall reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It was the last monoclonal antibody treatment standing. But less than 10 months after the U.S. Food and Drug Administration gave bebtelovimab its emergency use authorization (EUA) to fight COVID-19, it earlier this month de-authorized it, just as it had for other monoclonal antibody treatments, and for the same reason:
Bebtelovimab couldn’t neutralize the Omicron subvariants BQ.1 and BQ.1.1, the cause of nearly 60% of COVID cases nationally as of November 30.
Next on the chopping block, some predict, will be Evusheld, the combination of tixagevimab and cilgavimab given as a preventive monoclonal antibody to people who are immunocompromised and at high risk of contracting COVID and to those who can’t take the vaccine. In October, the FDA warned that Evusheld was not neutralizing circulating COVID variants.
As the options for treating and preventing COVID decline, will companies rally quickly to develop new ones, or cut their losses in developing treatments that may work for only a few months, given the speed of viral mutations?
But although monoclonal antibody treatments are off the table, at least for now, antiviral drugs – including Paxlovid – are still very much available, and some say underused.
Others suggest it’s time to resurrect interest in convalescent plasma, a treatment used early in the pandemic before drugs or vaccines were here and still authorized for use in those who are immunosuppressed or receiving immunosuppressive treatment.
And on the prevention front, staying up to date with booster vaccines, masking, and taking other precautions should be stressed more, others say, regardless of the number of treatment options, and especially now, as cases rise and people gather for the winter holidays.
‘A major setback’
The bebtelovimab de-authorization was “a major setback,” but an understandable one, said Arturo Casadevall, MD, PhD, professor and chair of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health in Baltimore. “Monoclonal antibodies are great drugs. We are in an unfortunate situation in that they are vulnerable to changes in the virus” and can’t offer long-lasting protection.
Supplies of bebtelovimab will be retained, according to the FDA, in case variants susceptible to it return.
“What happened to bebtelovimab is no surprise,” agreed Amesh Adalja, MD, senior scholar at Johns Hopkins Center for Health Security. “This is what is going to happen when you are targeting a virus that mutates a lot.”
Monoclonal antibodies work by binding to the spike protein on the virus surface to prevent it from entering cells.
However, Dr. Adalja doesn’t view the disappearance of monoclonal antibody treatments as a major setback. Monoclonal antibodies were not the primary way COVID was treated, he said.
While he does believe it’s important that more monoclonal antibody treatments be developed, “I think it’s important to remember we still have Paxlovid while everyone is lamenting the loss of bebtelovimab.’’
Antivirals: What’s here, what’s coming
Compared with monoclonal antibodies, “Paxlovid remains a much easier drug to give,” Dr. Adalja told this news organization, because it is taken orally, not intravenously.
And it’s effective. In a recent study, researchers found that adults diagnosed with COVID given Paxlovid within 5 days of diagnosis had a 51% lower hospitalization rate within the next 30 days than those not given it. Another study shows it could also reduce a person’s risk of developing long COVID by 26%.
Paxlovid is underused, Dr. Adalja said, partly because the rebound potential got more press than the effectiveness. When a celebrity got rebound from Paxlovid, he said, that would make the news, overshadowing the research on its effectiveness.
Besides Paxlovid, the antivirals remdesivir (Veklury), given intravenously for 3 days, and molnupiravir (Lagevrio), taken orally, are also still available. Antivirals work by targeting specific parts of the virus to prevent it from multiplying.
In the lab, remdesivir, molnupiravir, and another antiviral, nirmatrelvir, all appear to be effective against both BQ.1.1 (a BA.5 subvariant) and XBB (a BA.2 subvariant), both rapidly rising in the United States, according to a report last week in the New England Journal of Medicine.
The researchers also tested several monoclonal antibodies and found they did not neutralize either of the subvariants BQ.1.1 and XBB.
A new oral antiviral, Xocova (ensitrelvir fumaric acid), from Japanese manufacturer Shionogi, received emergency approval in Japan on November 22. It’s taken once a day for 5 days. The goal is to expand access to it globally, according to the company.
Pardes Biosciences launched a phase 2 trial in September for its oral antiviral drug (PBI-0451), under study as a treatment and preventive for COVID. It expects data by the first quarter of 2023.
Pfizer, which makes Paxlovid, has partnered with Clear Creek Bio to develop another oral antiviral COVID drug.
Other approaches
A receptor protein known as ACE2 (angiotensin-converting enzyme 2) is the main “doorway” that SARS-CoV-2 uses to enter and infect cells.
Dana-Farber Cancer Institute scientists are developing a “decoy” drug that works by mimicking the ACE2 receptor on the surface of cells; when the virus tries to bind to it, the spike protein is destroyed. Human trials have not yet started.
Other researchers are investigating whether an already-approved drug used to treat a liver disease, Actigall (UDCA/ursodeoxycholic acid), could protect against COVID infection by reducing ACE2.
So far, the researchers have found in early research that people taking UDCA for liver conditions were less likely than those not taking the drug to have severe COVID. They also found that UDCA reduced SARS-CoV-2 infection in human lungs maintained outside the body.
Monoclonal antibody treatments?
After the FDA decision to withdraw the bebtelovimab EUA, which Eli Lilly said it agreed with, the company issued a statement, promising it wasn’t giving up on monoclonal antibody treatments.
“Lilly will continue to search and evaluate monoclonal antibodies to identify potential candidates for clinical development against new variants,” it read in part.
AstraZeneca, which makes Evusheld, is also continuing to work on monoclonal antibody development. According to a spokesperson, “We are also developing a new long-acting antibody combination – AZD5156 – which has been shown in the lab to neutralize emerging new variants and all known variants to date. We are working to accelerate the development of AZD5156 to make it available at the end of 2023.”
The AstraZeneca spokesperson said he could share no more information about what the combination would include.
A convalescent plasma comeback?
Although Paxlovid can help, there are many contraindications to it, such as drug-drug interactions, Dr. Casadevall told this news organization. And now that the monoclonal antibody treatments have been paused, convalescent plasma “is the only antibody-based therapy that is reliably available. Convalescent plasma includes thousands of different antibodies.”
With his colleagues, Dr. Casadevall evaluated plasma samples from 740 patients. Some had received booster vaccines and been infected with Omicron, others had received boosters and not been infected, and still others had not been vaccinated and became infected.
In a report (not yet peer-reviewed), they found the plasma from those who had been infected or boosted within the past 6 months neutralized the new Omicron variants BQ.1.1, XBB.1, and BF.7.
A push for boosters, masks
To get through the coming months, taking precautions like masking and distancing and staying up to date on booster vaccinations, especially for older adults, can make a difference, other experts say.
In a Twitter thread in early December, Peter Hotez, MD, PhD, professor of pediatrics and molecular virology and microbiology at Baylor College of Medicine, Houston, urged people to take COVID seriously as holiday parties and gatherings occur.
“The single most impactful thing you can do is get your bivalent booster,” he tweeted, as well as give your kids the booster, citing preliminary research that the bivalent mRNA booster broadens immunity against the Omicron subvariants.
For seniors, he said, ‘‘if you get breakthrough COVID, [it’s] really important to get Paxlovid.” Masks will help not only for COVID but also influenza, respiratory syncytial virus (RSV), and other conditions.
Mitigation measures have largely been abandoned, according to Eric Topol, MD, director of the Scripps Research Translational Institute, La Jolla, Calif., and editor-in-chief of Medscape. In an op-ed in the Los Angeles Times, and on his Twitter feed, he reminds people about masking and urges people to get the bivalent booster.
According to the Centers for Disease Control and Prevention, as of Dec. 8, only 13.5% of people aged 5 and older have gotten an updated booster, despite research that shows an increase in antibodies to BQ.1.1. Recent research has found that the bivalent booster increases antibodies to BQ.1.1 by up to 10-fold, Dr. Topol said.
Dr. Adalja is on advisory boards for Shionogi, GSK, and Pardes. Dr. Casadevall reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It was the last monoclonal antibody treatment standing. But less than 10 months after the U.S. Food and Drug Administration gave bebtelovimab its emergency use authorization (EUA) to fight COVID-19, it earlier this month de-authorized it, just as it had for other monoclonal antibody treatments, and for the same reason:
Bebtelovimab couldn’t neutralize the Omicron subvariants BQ.1 and BQ.1.1, the cause of nearly 60% of COVID cases nationally as of November 30.
Next on the chopping block, some predict, will be Evusheld, the combination of tixagevimab and cilgavimab given as a preventive monoclonal antibody to people who are immunocompromised and at high risk of contracting COVID and to those who can’t take the vaccine. In October, the FDA warned that Evusheld was not neutralizing circulating COVID variants.
As the options for treating and preventing COVID decline, will companies rally quickly to develop new ones, or cut their losses in developing treatments that may work for only a few months, given the speed of viral mutations?
But although monoclonal antibody treatments are off the table, at least for now, antiviral drugs – including Paxlovid – are still very much available, and some say underused.
Others suggest it’s time to resurrect interest in convalescent plasma, a treatment used early in the pandemic before drugs or vaccines were here and still authorized for use in those who are immunosuppressed or receiving immunosuppressive treatment.
And on the prevention front, staying up to date with booster vaccines, masking, and taking other precautions should be stressed more, others say, regardless of the number of treatment options, and especially now, as cases rise and people gather for the winter holidays.
‘A major setback’
The bebtelovimab de-authorization was “a major setback,” but an understandable one, said Arturo Casadevall, MD, PhD, professor and chair of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health in Baltimore. “Monoclonal antibodies are great drugs. We are in an unfortunate situation in that they are vulnerable to changes in the virus” and can’t offer long-lasting protection.
Supplies of bebtelovimab will be retained, according to the FDA, in case variants susceptible to it return.
“What happened to bebtelovimab is no surprise,” agreed Amesh Adalja, MD, senior scholar at Johns Hopkins Center for Health Security. “This is what is going to happen when you are targeting a virus that mutates a lot.”
Monoclonal antibodies work by binding to the spike protein on the virus surface to prevent it from entering cells.
However, Dr. Adalja doesn’t view the disappearance of monoclonal antibody treatments as a major setback. Monoclonal antibodies were not the primary way COVID was treated, he said.
While he does believe it’s important that more monoclonal antibody treatments be developed, “I think it’s important to remember we still have Paxlovid while everyone is lamenting the loss of bebtelovimab.’’
Antivirals: What’s here, what’s coming
Compared with monoclonal antibodies, “Paxlovid remains a much easier drug to give,” Dr. Adalja told this news organization, because it is taken orally, not intravenously.
And it’s effective. In a recent study, researchers found that adults diagnosed with COVID given Paxlovid within 5 days of diagnosis had a 51% lower hospitalization rate within the next 30 days than those not given it. Another study shows it could also reduce a person’s risk of developing long COVID by 26%.
Paxlovid is underused, Dr. Adalja said, partly because the rebound potential got more press than the effectiveness. When a celebrity got rebound from Paxlovid, he said, that would make the news, overshadowing the research on its effectiveness.
Besides Paxlovid, the antivirals remdesivir (Veklury), given intravenously for 3 days, and molnupiravir (Lagevrio), taken orally, are also still available. Antivirals work by targeting specific parts of the virus to prevent it from multiplying.
In the lab, remdesivir, molnupiravir, and another antiviral, nirmatrelvir, all appear to be effective against both BQ.1.1 (a BA.5 subvariant) and XBB (a BA.2 subvariant), both rapidly rising in the United States, according to a report last week in the New England Journal of Medicine.
The researchers also tested several monoclonal antibodies and found they did not neutralize either of the subvariants BQ.1.1 and XBB.
A new oral antiviral, Xocova (ensitrelvir fumaric acid), from Japanese manufacturer Shionogi, received emergency approval in Japan on November 22. It’s taken once a day for 5 days. The goal is to expand access to it globally, according to the company.
Pardes Biosciences launched a phase 2 trial in September for its oral antiviral drug (PBI-0451), under study as a treatment and preventive for COVID. It expects data by the first quarter of 2023.
Pfizer, which makes Paxlovid, has partnered with Clear Creek Bio to develop another oral antiviral COVID drug.
Other approaches
A receptor protein known as ACE2 (angiotensin-converting enzyme 2) is the main “doorway” that SARS-CoV-2 uses to enter and infect cells.
Dana-Farber Cancer Institute scientists are developing a “decoy” drug that works by mimicking the ACE2 receptor on the surface of cells; when the virus tries to bind to it, the spike protein is destroyed. Human trials have not yet started.
Other researchers are investigating whether an already-approved drug used to treat a liver disease, Actigall (UDCA/ursodeoxycholic acid), could protect against COVID infection by reducing ACE2.
So far, the researchers have found in early research that people taking UDCA for liver conditions were less likely than those not taking the drug to have severe COVID. They also found that UDCA reduced SARS-CoV-2 infection in human lungs maintained outside the body.
Monoclonal antibody treatments?
After the FDA decision to withdraw the bebtelovimab EUA, which Eli Lilly said it agreed with, the company issued a statement, promising it wasn’t giving up on monoclonal antibody treatments.
“Lilly will continue to search and evaluate monoclonal antibodies to identify potential candidates for clinical development against new variants,” it read in part.
AstraZeneca, which makes Evusheld, is also continuing to work on monoclonal antibody development. According to a spokesperson, “We are also developing a new long-acting antibody combination – AZD5156 – which has been shown in the lab to neutralize emerging new variants and all known variants to date. We are working to accelerate the development of AZD5156 to make it available at the end of 2023.”
The AstraZeneca spokesperson said he could share no more information about what the combination would include.
A convalescent plasma comeback?
Although Paxlovid can help, there are many contraindications to it, such as drug-drug interactions, Dr. Casadevall told this news organization. And now that the monoclonal antibody treatments have been paused, convalescent plasma “is the only antibody-based therapy that is reliably available. Convalescent plasma includes thousands of different antibodies.”
With his colleagues, Dr. Casadevall evaluated plasma samples from 740 patients. Some had received booster vaccines and been infected with Omicron, others had received boosters and not been infected, and still others had not been vaccinated and became infected.
In a report (not yet peer-reviewed), they found the plasma from those who had been infected or boosted within the past 6 months neutralized the new Omicron variants BQ.1.1, XBB.1, and BF.7.
A push for boosters, masks
To get through the coming months, taking precautions like masking and distancing and staying up to date on booster vaccinations, especially for older adults, can make a difference, other experts say.
In a Twitter thread in early December, Peter Hotez, MD, PhD, professor of pediatrics and molecular virology and microbiology at Baylor College of Medicine, Houston, urged people to take COVID seriously as holiday parties and gatherings occur.
“The single most impactful thing you can do is get your bivalent booster,” he tweeted, as well as give your kids the booster, citing preliminary research that the bivalent mRNA booster broadens immunity against the Omicron subvariants.
For seniors, he said, ‘‘if you get breakthrough COVID, [it’s] really important to get Paxlovid.” Masks will help not only for COVID but also influenza, respiratory syncytial virus (RSV), and other conditions.
Mitigation measures have largely been abandoned, according to Eric Topol, MD, director of the Scripps Research Translational Institute, La Jolla, Calif., and editor-in-chief of Medscape. In an op-ed in the Los Angeles Times, and on his Twitter feed, he reminds people about masking and urges people to get the bivalent booster.
According to the Centers for Disease Control and Prevention, as of Dec. 8, only 13.5% of people aged 5 and older have gotten an updated booster, despite research that shows an increase in antibodies to BQ.1.1. Recent research has found that the bivalent booster increases antibodies to BQ.1.1 by up to 10-fold, Dr. Topol said.
Dr. Adalja is on advisory boards for Shionogi, GSK, and Pardes. Dr. Casadevall reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hospital financial decisions play a role in the critical shortage of pediatric beds for RSV patients
The dire shortage of pediatric hospital beds plaguing the nation in the fall of 2022 is a byproduct of financial decisions made by hospitals over the past decade, as they shuttered children’s wards, which often operate in the red, and expanded the number of beds available for more profitable endeavors like joint replacements and cancer care.
To cope with the flood of young patients sickened by a sweeping convergence of nasty bugs – especially respiratory syncytial virus, influenza, and coronavirus – medical centers nationwide have deployed triage tents, delayed elective surgeries, and transferred critically ill children out of state.
A major factor in the bed shortage is a years-long trend among hospitals of eliminating pediatric units, which tend to be less profitable than adult units, said Mark Wietecha, MS, MBA, CEO of the Children’s Hospital Association. Hospitals optimize revenue by striving to keep their beds 100% full – and filled with patients whose conditions command generous insurance reimbursements.
“It really has to do with dollars,” said Scott Krugman, MD, MS, vice chair of pediatrics at the Herman and Walter Samuelson Children’s Hospital at Sinai in Baltimore. “Hospitals rely on high-volume, high-reimbursement procedures from good payers to make money. There’s no incentive for hospitals to provide money-losing services.”
The number of pediatric inpatient units in hospitals fell 19% from 2008 to 2018, according to a study published in 2021 in the journal Pediatrics. Just this year, hospitals have closed pediatric units in Boston and Springfield, Mass.; Richmond, Va.; and Tulsa, Okla.
The current surge in dangerous respiratory illnesses among children is yet another example of how COVID-19 has upended the health care system. The lockdowns and isolation that marked the first years of the pandemic left kids largely unexposed – and still vulnerable – to viruses other than COVID for two winters, and doctors are now essentially treating multiple years’ worth of respiratory ailments.
The pandemic also accelerated changes in the health care industry that have left many communities with fewer hospital beds available for children who are acutely ill, along with fewer doctors and nurses to care for them.
When intensive care units were flooded with older COVID patients in 2020, some hospitals began using children’s beds to treat adults. Many of those pediatric beds haven’t been restored, said Daniel Rauch, MD, chair of the American Academy of Pediatrics’ committee on hospital care.
In addition, the relentless pace of the pandemic has spurred more than 230,000 health care providers – including doctors, nurses, and physician assistants – to quit. Before the pandemic, about 10% of nurses left their jobs every year; the rate has risen to about 20%, Dr. Wietecha said. He estimates that pediatric hospitals are unable to maintain as many as 10% of their beds because of staffing shortages.
“There is just not enough space for all the kids who need beds,” said Megan Ranney, MD, MPH, who works in several emergency departments in Providence, R.I., including Hasbro Children’s Hospital. The number of children seeking emergency care in recent weeks was 25% higher than the hospital’s previous record.
“We have doctors who are cleaning beds so we can get children into them faster,” said Dr. Ranney, a deputy dean at Brown University’s School of Public Health.
There’s not great money in treating kids. About 40% of U.S. children are covered by Medicaid, a joint federal-state program for low-income patients and people with disabilities. Base Medicaid rates are typically more than 20% below those paid by Medicare, the government insurance program for older adults, and are even lower when compared with private insurance. While specialty care for a range of common adult procedures, from knee and hip replacements to heart surgeries and cancer treatments, generates major profits for medical centers, hospitals complain they typically lose money on inpatient pediatric care.
When Tufts Children’s Hospital closed 41 pediatric beds this summer, hospital officials assured residents that young patients could receive care at nearby Boston Children’s Hospital. Now, Boston Children’s is delaying some elective surgeries to make room for kids who are acutely ill.
Dr. Rauch noted that children’s hospitals, which specialize in treating rare and serious conditions such as pediatric cancer, cystic fibrosis, and heart defects, simply aren’t designed to handle this season’s crush of kids acutely ill with respiratory bugs.
Even before the autumn’s viral trifecta, pediatric units were straining to absorb rising numbers of young people in acute mental distress. Stories abound of children in mental crises being marooned for weeks in emergency departments while awaiting transfer to a pediatric psychiatric unit. On a good day, Dr. Ranney said, 20% of pediatric emergency room beds at Hasbro Children’s Hospital are occupied by children experiencing mental health issues.
In hopes of adding pediatric capacity, the American Academy of Pediatrics joined the Children’s Hospital Association last month in calling on the White House to declare a national emergency due to child respiratory infections and provide additional resources to help cover the costs of care. The Biden administration has said that the flexibility hospital systems and providers have been given during the pandemic to sidestep certain staffing requirements also applies to RSV and flu.
Doernbecher Children’s Hospital at Oregon Health & Science University has shifted to “crisis standards of care,” enabling intensive care nurses to treat more patients than they’re usually assigned. Hospitals in Atlanta, Pittsburgh, and Aurora, Colorado, meanwhile, have resorted to treating young patients in overflow tents in parking lots.
Alex Kon, MD, a pediatric critical care physician at Community Medical Center in Missoula, Mont., said providers there have made plans to care for older kids in the adult intensive care unit, and to divert ambulances to other facilities when necessary. With only three pediatric ICUs in the state, that means young patients may be flown as far as Seattle or Spokane, Wash., or Idaho.
Hollis Lillard took her 1-year-old son, Calder, to an Army hospital in Northern Virginia last month after he experienced several days of fever, coughing, and labored breathing. They spent 7 anguished hours in the emergency room before the hospital found an open bed and transferred them by ambulance to Walter Reed National Military Medical Center in Maryland.
With proper therapy and instructions for home care, Calder’s virus was readily treatable: He recovered after he was given oxygen and treated with steroids, which fight inflammation, and albuterol, which counteracts bronchospasms. He was discharged the next day.
Although hospitalizations for RSV are falling, rates remain well above the norm for this time of year. And hospitals may not get much relief.
People can be infected with RSV more than once a year, and Dr. Krugman worries about a resurgence in the months to come. Because of the coronavirus, which competes with other viruses, “the usual seasonal pattern of viruses has gone out the window,” he said.
Like RSV, influenza arrived early this season. Both viruses usually peak around January. Three strains of flu are circulating and have caused an estimated 8.7 million illnesses, 78,000 hospitalizations, and 4,500 deaths, according to the Centers for Disease Control and Prevention.
Dr. Krugman doubts the health care industry will learn any quick lessons from the current crisis. “Unless there is a radical change in how we pay for pediatric hospital care,” Dr. Krugman said, “the bed shortage is only going to get worse.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The dire shortage of pediatric hospital beds plaguing the nation in the fall of 2022 is a byproduct of financial decisions made by hospitals over the past decade, as they shuttered children’s wards, which often operate in the red, and expanded the number of beds available for more profitable endeavors like joint replacements and cancer care.
To cope with the flood of young patients sickened by a sweeping convergence of nasty bugs – especially respiratory syncytial virus, influenza, and coronavirus – medical centers nationwide have deployed triage tents, delayed elective surgeries, and transferred critically ill children out of state.
A major factor in the bed shortage is a years-long trend among hospitals of eliminating pediatric units, which tend to be less profitable than adult units, said Mark Wietecha, MS, MBA, CEO of the Children’s Hospital Association. Hospitals optimize revenue by striving to keep their beds 100% full – and filled with patients whose conditions command generous insurance reimbursements.
“It really has to do with dollars,” said Scott Krugman, MD, MS, vice chair of pediatrics at the Herman and Walter Samuelson Children’s Hospital at Sinai in Baltimore. “Hospitals rely on high-volume, high-reimbursement procedures from good payers to make money. There’s no incentive for hospitals to provide money-losing services.”
The number of pediatric inpatient units in hospitals fell 19% from 2008 to 2018, according to a study published in 2021 in the journal Pediatrics. Just this year, hospitals have closed pediatric units in Boston and Springfield, Mass.; Richmond, Va.; and Tulsa, Okla.
The current surge in dangerous respiratory illnesses among children is yet another example of how COVID-19 has upended the health care system. The lockdowns and isolation that marked the first years of the pandemic left kids largely unexposed – and still vulnerable – to viruses other than COVID for two winters, and doctors are now essentially treating multiple years’ worth of respiratory ailments.
The pandemic also accelerated changes in the health care industry that have left many communities with fewer hospital beds available for children who are acutely ill, along with fewer doctors and nurses to care for them.
When intensive care units were flooded with older COVID patients in 2020, some hospitals began using children’s beds to treat adults. Many of those pediatric beds haven’t been restored, said Daniel Rauch, MD, chair of the American Academy of Pediatrics’ committee on hospital care.
In addition, the relentless pace of the pandemic has spurred more than 230,000 health care providers – including doctors, nurses, and physician assistants – to quit. Before the pandemic, about 10% of nurses left their jobs every year; the rate has risen to about 20%, Dr. Wietecha said. He estimates that pediatric hospitals are unable to maintain as many as 10% of their beds because of staffing shortages.
“There is just not enough space for all the kids who need beds,” said Megan Ranney, MD, MPH, who works in several emergency departments in Providence, R.I., including Hasbro Children’s Hospital. The number of children seeking emergency care in recent weeks was 25% higher than the hospital’s previous record.
“We have doctors who are cleaning beds so we can get children into them faster,” said Dr. Ranney, a deputy dean at Brown University’s School of Public Health.
There’s not great money in treating kids. About 40% of U.S. children are covered by Medicaid, a joint federal-state program for low-income patients and people with disabilities. Base Medicaid rates are typically more than 20% below those paid by Medicare, the government insurance program for older adults, and are even lower when compared with private insurance. While specialty care for a range of common adult procedures, from knee and hip replacements to heart surgeries and cancer treatments, generates major profits for medical centers, hospitals complain they typically lose money on inpatient pediatric care.
When Tufts Children’s Hospital closed 41 pediatric beds this summer, hospital officials assured residents that young patients could receive care at nearby Boston Children’s Hospital. Now, Boston Children’s is delaying some elective surgeries to make room for kids who are acutely ill.
Dr. Rauch noted that children’s hospitals, which specialize in treating rare and serious conditions such as pediatric cancer, cystic fibrosis, and heart defects, simply aren’t designed to handle this season’s crush of kids acutely ill with respiratory bugs.
Even before the autumn’s viral trifecta, pediatric units were straining to absorb rising numbers of young people in acute mental distress. Stories abound of children in mental crises being marooned for weeks in emergency departments while awaiting transfer to a pediatric psychiatric unit. On a good day, Dr. Ranney said, 20% of pediatric emergency room beds at Hasbro Children’s Hospital are occupied by children experiencing mental health issues.
In hopes of adding pediatric capacity, the American Academy of Pediatrics joined the Children’s Hospital Association last month in calling on the White House to declare a national emergency due to child respiratory infections and provide additional resources to help cover the costs of care. The Biden administration has said that the flexibility hospital systems and providers have been given during the pandemic to sidestep certain staffing requirements also applies to RSV and flu.
Doernbecher Children’s Hospital at Oregon Health & Science University has shifted to “crisis standards of care,” enabling intensive care nurses to treat more patients than they’re usually assigned. Hospitals in Atlanta, Pittsburgh, and Aurora, Colorado, meanwhile, have resorted to treating young patients in overflow tents in parking lots.
Alex Kon, MD, a pediatric critical care physician at Community Medical Center in Missoula, Mont., said providers there have made plans to care for older kids in the adult intensive care unit, and to divert ambulances to other facilities when necessary. With only three pediatric ICUs in the state, that means young patients may be flown as far as Seattle or Spokane, Wash., or Idaho.
Hollis Lillard took her 1-year-old son, Calder, to an Army hospital in Northern Virginia last month after he experienced several days of fever, coughing, and labored breathing. They spent 7 anguished hours in the emergency room before the hospital found an open bed and transferred them by ambulance to Walter Reed National Military Medical Center in Maryland.
With proper therapy and instructions for home care, Calder’s virus was readily treatable: He recovered after he was given oxygen and treated with steroids, which fight inflammation, and albuterol, which counteracts bronchospasms. He was discharged the next day.
Although hospitalizations for RSV are falling, rates remain well above the norm for this time of year. And hospitals may not get much relief.
People can be infected with RSV more than once a year, and Dr. Krugman worries about a resurgence in the months to come. Because of the coronavirus, which competes with other viruses, “the usual seasonal pattern of viruses has gone out the window,” he said.
Like RSV, influenza arrived early this season. Both viruses usually peak around January. Three strains of flu are circulating and have caused an estimated 8.7 million illnesses, 78,000 hospitalizations, and 4,500 deaths, according to the Centers for Disease Control and Prevention.
Dr. Krugman doubts the health care industry will learn any quick lessons from the current crisis. “Unless there is a radical change in how we pay for pediatric hospital care,” Dr. Krugman said, “the bed shortage is only going to get worse.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The dire shortage of pediatric hospital beds plaguing the nation in the fall of 2022 is a byproduct of financial decisions made by hospitals over the past decade, as they shuttered children’s wards, which often operate in the red, and expanded the number of beds available for more profitable endeavors like joint replacements and cancer care.
To cope with the flood of young patients sickened by a sweeping convergence of nasty bugs – especially respiratory syncytial virus, influenza, and coronavirus – medical centers nationwide have deployed triage tents, delayed elective surgeries, and transferred critically ill children out of state.
A major factor in the bed shortage is a years-long trend among hospitals of eliminating pediatric units, which tend to be less profitable than adult units, said Mark Wietecha, MS, MBA, CEO of the Children’s Hospital Association. Hospitals optimize revenue by striving to keep their beds 100% full – and filled with patients whose conditions command generous insurance reimbursements.
“It really has to do with dollars,” said Scott Krugman, MD, MS, vice chair of pediatrics at the Herman and Walter Samuelson Children’s Hospital at Sinai in Baltimore. “Hospitals rely on high-volume, high-reimbursement procedures from good payers to make money. There’s no incentive for hospitals to provide money-losing services.”
The number of pediatric inpatient units in hospitals fell 19% from 2008 to 2018, according to a study published in 2021 in the journal Pediatrics. Just this year, hospitals have closed pediatric units in Boston and Springfield, Mass.; Richmond, Va.; and Tulsa, Okla.
The current surge in dangerous respiratory illnesses among children is yet another example of how COVID-19 has upended the health care system. The lockdowns and isolation that marked the first years of the pandemic left kids largely unexposed – and still vulnerable – to viruses other than COVID for two winters, and doctors are now essentially treating multiple years’ worth of respiratory ailments.
The pandemic also accelerated changes in the health care industry that have left many communities with fewer hospital beds available for children who are acutely ill, along with fewer doctors and nurses to care for them.
When intensive care units were flooded with older COVID patients in 2020, some hospitals began using children’s beds to treat adults. Many of those pediatric beds haven’t been restored, said Daniel Rauch, MD, chair of the American Academy of Pediatrics’ committee on hospital care.
In addition, the relentless pace of the pandemic has spurred more than 230,000 health care providers – including doctors, nurses, and physician assistants – to quit. Before the pandemic, about 10% of nurses left their jobs every year; the rate has risen to about 20%, Dr. Wietecha said. He estimates that pediatric hospitals are unable to maintain as many as 10% of their beds because of staffing shortages.
“There is just not enough space for all the kids who need beds,” said Megan Ranney, MD, MPH, who works in several emergency departments in Providence, R.I., including Hasbro Children’s Hospital. The number of children seeking emergency care in recent weeks was 25% higher than the hospital’s previous record.
“We have doctors who are cleaning beds so we can get children into them faster,” said Dr. Ranney, a deputy dean at Brown University’s School of Public Health.
There’s not great money in treating kids. About 40% of U.S. children are covered by Medicaid, a joint federal-state program for low-income patients and people with disabilities. Base Medicaid rates are typically more than 20% below those paid by Medicare, the government insurance program for older adults, and are even lower when compared with private insurance. While specialty care for a range of common adult procedures, from knee and hip replacements to heart surgeries and cancer treatments, generates major profits for medical centers, hospitals complain they typically lose money on inpatient pediatric care.
When Tufts Children’s Hospital closed 41 pediatric beds this summer, hospital officials assured residents that young patients could receive care at nearby Boston Children’s Hospital. Now, Boston Children’s is delaying some elective surgeries to make room for kids who are acutely ill.
Dr. Rauch noted that children’s hospitals, which specialize in treating rare and serious conditions such as pediatric cancer, cystic fibrosis, and heart defects, simply aren’t designed to handle this season’s crush of kids acutely ill with respiratory bugs.
Even before the autumn’s viral trifecta, pediatric units were straining to absorb rising numbers of young people in acute mental distress. Stories abound of children in mental crises being marooned for weeks in emergency departments while awaiting transfer to a pediatric psychiatric unit. On a good day, Dr. Ranney said, 20% of pediatric emergency room beds at Hasbro Children’s Hospital are occupied by children experiencing mental health issues.
In hopes of adding pediatric capacity, the American Academy of Pediatrics joined the Children’s Hospital Association last month in calling on the White House to declare a national emergency due to child respiratory infections and provide additional resources to help cover the costs of care. The Biden administration has said that the flexibility hospital systems and providers have been given during the pandemic to sidestep certain staffing requirements also applies to RSV and flu.
Doernbecher Children’s Hospital at Oregon Health & Science University has shifted to “crisis standards of care,” enabling intensive care nurses to treat more patients than they’re usually assigned. Hospitals in Atlanta, Pittsburgh, and Aurora, Colorado, meanwhile, have resorted to treating young patients in overflow tents in parking lots.
Alex Kon, MD, a pediatric critical care physician at Community Medical Center in Missoula, Mont., said providers there have made plans to care for older kids in the adult intensive care unit, and to divert ambulances to other facilities when necessary. With only three pediatric ICUs in the state, that means young patients may be flown as far as Seattle or Spokane, Wash., or Idaho.
Hollis Lillard took her 1-year-old son, Calder, to an Army hospital in Northern Virginia last month after he experienced several days of fever, coughing, and labored breathing. They spent 7 anguished hours in the emergency room before the hospital found an open bed and transferred them by ambulance to Walter Reed National Military Medical Center in Maryland.
With proper therapy and instructions for home care, Calder’s virus was readily treatable: He recovered after he was given oxygen and treated with steroids, which fight inflammation, and albuterol, which counteracts bronchospasms. He was discharged the next day.
Although hospitalizations for RSV are falling, rates remain well above the norm for this time of year. And hospitals may not get much relief.
People can be infected with RSV more than once a year, and Dr. Krugman worries about a resurgence in the months to come. Because of the coronavirus, which competes with other viruses, “the usual seasonal pattern of viruses has gone out the window,” he said.
Like RSV, influenza arrived early this season. Both viruses usually peak around January. Three strains of flu are circulating and have caused an estimated 8.7 million illnesses, 78,000 hospitalizations, and 4,500 deaths, according to the Centers for Disease Control and Prevention.
Dr. Krugman doubts the health care industry will learn any quick lessons from the current crisis. “Unless there is a radical change in how we pay for pediatric hospital care,” Dr. Krugman said, “the bed shortage is only going to get worse.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.