User login
Sewer data says Ohio person has had COVID for 2 years
The strain of the virus appears to be unique, the researchers said.
The mutated version of the virus was discovered by a team of researchers, led by University of Missouri–Columbia virologist Marc Johnson, PhD, that has been studying standalone mutations identified in wastewater. On Twitter, Dr. Johnson said their work could help warn people of a potential risk.
“If you knew of an exposure of a group of people to a deadly disease, there would be an obligation to inform them,” he wrote.
He believes the infected person lives in Columbus, works at a courthouse in a nearby county, and has gut health problems. The county where the person works has a population of just 15,000 people but had record COVID wastewater levels in May, The Columbus Dispatch reported. The unique COVID strain that Dr. Johnson is researching was the only COVID strain found in Fayette County’s wastewater.
“This person was shedding thousands of times more material than a normal person ever would,” Dr. Johnson told the Dispatch. “I think this person isn’t well. ... I’m guessing they have GI issues.”
Monitoring wastewater for COVID-19 is only used to inform public health officials of community levels and spread of the virus. People with COVID are not tracked down using such information.
The Centers for Disease Control and Prevention told the Dispatch that the findings do not mean there’s a public health threat.
“Unusual or ‘cryptic’ sequences identified in wastewater may represent viruses that can replicate in particular individuals, but not in the general population,” the CDC wrote in a statement to the newspaper. “This can be because of a compromised immune system. CDC and other institutions conduct studies in immunocompromised individuals to understand persistent infection and virus evolution.”
Ohio health officials told the newspaper that they don’t consider the situation a public health threat because the cryptic strain hasn’t spread beyond two sewer sheds for those 2 years.
Dr. Johnson and colleagues have been researching other unique COVID strains found in wastewater. They wrote a paper about a case in Wisconsin currently in preprint.
In the paper, the researchers suggest some people are persistently infected, calling them “prolonged shedders.” The researchers wrote that prolonged shedders could be human or “nonhuman,” and that “increased global monitoring of such lineages in wastewater could help anticipate future circulating mutations and/or variants of concern.”
Earlier in 2023, the CDC announced it was ending its community-level reporting of COVID test data and would rely more heavily on hospitalization reports and wastewater monitoring. COVID hospitalizations dipped to 7,212 nationally for the week of June 1-8, which is a 6% decline from the week prior, according to the CDC. That number of hospitalizations equals about two hospitalizations per 100,000 people.
A version of this article first appeared on WebMD.com.
The strain of the virus appears to be unique, the researchers said.
The mutated version of the virus was discovered by a team of researchers, led by University of Missouri–Columbia virologist Marc Johnson, PhD, that has been studying standalone mutations identified in wastewater. On Twitter, Dr. Johnson said their work could help warn people of a potential risk.
“If you knew of an exposure of a group of people to a deadly disease, there would be an obligation to inform them,” he wrote.
He believes the infected person lives in Columbus, works at a courthouse in a nearby county, and has gut health problems. The county where the person works has a population of just 15,000 people but had record COVID wastewater levels in May, The Columbus Dispatch reported. The unique COVID strain that Dr. Johnson is researching was the only COVID strain found in Fayette County’s wastewater.
“This person was shedding thousands of times more material than a normal person ever would,” Dr. Johnson told the Dispatch. “I think this person isn’t well. ... I’m guessing they have GI issues.”
Monitoring wastewater for COVID-19 is only used to inform public health officials of community levels and spread of the virus. People with COVID are not tracked down using such information.
The Centers for Disease Control and Prevention told the Dispatch that the findings do not mean there’s a public health threat.
“Unusual or ‘cryptic’ sequences identified in wastewater may represent viruses that can replicate in particular individuals, but not in the general population,” the CDC wrote in a statement to the newspaper. “This can be because of a compromised immune system. CDC and other institutions conduct studies in immunocompromised individuals to understand persistent infection and virus evolution.”
Ohio health officials told the newspaper that they don’t consider the situation a public health threat because the cryptic strain hasn’t spread beyond two sewer sheds for those 2 years.
Dr. Johnson and colleagues have been researching other unique COVID strains found in wastewater. They wrote a paper about a case in Wisconsin currently in preprint.
In the paper, the researchers suggest some people are persistently infected, calling them “prolonged shedders.” The researchers wrote that prolonged shedders could be human or “nonhuman,” and that “increased global monitoring of such lineages in wastewater could help anticipate future circulating mutations and/or variants of concern.”
Earlier in 2023, the CDC announced it was ending its community-level reporting of COVID test data and would rely more heavily on hospitalization reports and wastewater monitoring. COVID hospitalizations dipped to 7,212 nationally for the week of June 1-8, which is a 6% decline from the week prior, according to the CDC. That number of hospitalizations equals about two hospitalizations per 100,000 people.
A version of this article first appeared on WebMD.com.
The strain of the virus appears to be unique, the researchers said.
The mutated version of the virus was discovered by a team of researchers, led by University of Missouri–Columbia virologist Marc Johnson, PhD, that has been studying standalone mutations identified in wastewater. On Twitter, Dr. Johnson said their work could help warn people of a potential risk.
“If you knew of an exposure of a group of people to a deadly disease, there would be an obligation to inform them,” he wrote.
He believes the infected person lives in Columbus, works at a courthouse in a nearby county, and has gut health problems. The county where the person works has a population of just 15,000 people but had record COVID wastewater levels in May, The Columbus Dispatch reported. The unique COVID strain that Dr. Johnson is researching was the only COVID strain found in Fayette County’s wastewater.
“This person was shedding thousands of times more material than a normal person ever would,” Dr. Johnson told the Dispatch. “I think this person isn’t well. ... I’m guessing they have GI issues.”
Monitoring wastewater for COVID-19 is only used to inform public health officials of community levels and spread of the virus. People with COVID are not tracked down using such information.
The Centers for Disease Control and Prevention told the Dispatch that the findings do not mean there’s a public health threat.
“Unusual or ‘cryptic’ sequences identified in wastewater may represent viruses that can replicate in particular individuals, but not in the general population,” the CDC wrote in a statement to the newspaper. “This can be because of a compromised immune system. CDC and other institutions conduct studies in immunocompromised individuals to understand persistent infection and virus evolution.”
Ohio health officials told the newspaper that they don’t consider the situation a public health threat because the cryptic strain hasn’t spread beyond two sewer sheds for those 2 years.
Dr. Johnson and colleagues have been researching other unique COVID strains found in wastewater. They wrote a paper about a case in Wisconsin currently in preprint.
In the paper, the researchers suggest some people are persistently infected, calling them “prolonged shedders.” The researchers wrote that prolonged shedders could be human or “nonhuman,” and that “increased global monitoring of such lineages in wastewater could help anticipate future circulating mutations and/or variants of concern.”
Earlier in 2023, the CDC announced it was ending its community-level reporting of COVID test data and would rely more heavily on hospitalization reports and wastewater monitoring. COVID hospitalizations dipped to 7,212 nationally for the week of June 1-8, which is a 6% decline from the week prior, according to the CDC. That number of hospitalizations equals about two hospitalizations per 100,000 people.
A version of this article first appeared on WebMD.com.
Frailty Trends in an Older Veteran Subpopulation 1 Year Prior and Into the COVID-19 Pandemic Using CAN Scores
Frailty is an age-associated, nonspecific vulnerability to adverse health outcomes. Frailty can also be described as a complex of symptoms characterized by impaired stress tolerance due to a decline in the functionality of different organs.1 The prevalence of frailty varies widely depending on the method of measurement and the population studied.2-4 It is a nonconstant factor that increases with age. A deficit accumulation frailty index (FI) is one method used to measure frailty.5 This approach sees frailty as a multidimensional risk state measured by quantity rather than the nature of health concerns. A deficit accumulation FI does not require physical testing but correlates well with other phenotypic FIs.6 It is, however, time consuming, as ≥ 30 deficits need to be measured to offer greater stability to the frailty estimate.
Health care is seeing increasing utilization of big data analytics to derive predictive models and help with resource allocation. There are currently 2 existing automated tools to predict health care utilization and mortality at the US Department of Veterans Affairs (VA): the VA Frailty Index (VA-FI-10) and the Care Assessment Need (CAN). VA-FI-10 is an International Statistical Classification of Diseases, Tenth Revision (ICD-10) update of the VA-FI that was created in March 2021. The VA-FI-10 is a claims-based frailty assessment tool using 31 health deficits. Calculating the VA-FI-10 requires defining an index date and lookback period (typically 3 years) relative to which it will be calculated.7
CAN is a set of risk-stratifying statistical models run on veterans receiving VA primary care services as part of a patient aligned care team (PACT) using electronic health record data.8 Each veteran is stratified based on the individual’s risks of hospitalization, death, and hospitalization or death. These 3 events are predicted for 90-day and 1-year time periods for a total of 6 distinct outcomes. CAN is currently on its third iteration (CAN 2.5) and scores range from 0 (low) to 99 (high). CAN scores are updated weekly. The 1-year hospitalization probabilities for all patients range from 0.8% to 93.1%. For patients with a CAN score of 50, the probability of being hospitalized within a year ranges from 4.5% to 5.2%, which increases to 32.2% to 36% for veterans with a CAN score of 95. The probability range widens significantly (32.2%-93.1%) for patients in the top 5 CAN scores (95-99).
CAN scores are a potential screening tool for frailty among older adults; they are generated automatically and provide acceptable diagnostic accuracy. Hence, the CAN score may be a useful tool for primary care practitioners for the detection of frailty in their patients. The CAN score has shown a moderate positive association with the FRAIL Scale.9,10 The population-based studies that have used the FI approach (differing FIs, depending on the data available) give robust results: People accumulate an average of 0.03 deficits per year after the age of 70 years.11 Interventions to delay or reverse frailty have not been clearly defined with heterogeneity in the definition of frailty and measurement of frailty outcomes.12,13 The prevalence of frailty in the veteran population is substantially higher than the prevalence in community populations with a similar age distribution. There is also mounting evidence that veterans accumulate deficits more rapidly than their civilian counterparts.14
COVID-19 was declared a pandemic in March 2020 and had many impacts on global health that were most marked in the first year. These included reductions in hospital visits for non-COVID-19 health concerns, a reduction in completed screening tests, an initial reduction in other infectious diseases (attributable to quarantines), and an increase or worsening of mental health concerns.15,16
We aimed to investigate whether frailty increased disproportionately in a subset of older veterans in the first year of the COVID-19 pandemic when compared with the previous year using CAN scores. This single institution, longitudinal cohort study was determined to be exempt from institutional review board review but was approved by the Phoenix VA Health Care System (PVAHCS) Research and Development Committee.
Methods
The Office of Clinical Systems Development and Evaluation (CSDE–10E2A) produces a weekly CAN Score Report to help identify the highest-risk patients in a primary care panel or cohort. CAN scores range from 0 (lowest risk) to 99 (highest risk), indicating how likely a patient is to experience hospitalization or death compared with other VA patients. CAN scores are calculated with statistical prediction models that use data elements from the following Corporate Data Warehouse (CDW) domains: demographics, health care utilization, laboratory tests, medical conditions, medications, and vital signs (eAppendix available online at 10.12788/fp.0385).
The CAN Score Report is generated weekly and stored on a CDW server. A patient will receive all 6 distinct CAN scores if they are: (1) assigned to a primary care PACT on the risk date; (2) a veteran; (3) not hospitalized in a VA facility on the risk date; and (4) alive as of the risk date. New to CAN 2.5 is that patients who meet criteria 1, 2, and 4 but are hospitalized in a VA facility on the risk date will receive CAN scores for the 1-year and 90-day mortality models.
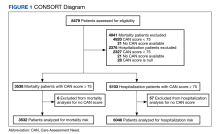
Utilizing VA Informatics and Computing Infrastructure (VA HSR RES 13-457, US Department of Veterans Affairs [2008]), we obtained 2 lists of veterans aged 70 to 75 years on February 8, 2019, with a calculated CAN score of ≥ 75 for 1-year mortality and 1-year hospitalization on that date. A veteran with a CAN score of ≥ 75 is likely to be prefrail or frail.9,10 Veterans who did not have a corresponding calculated CAN score on February 7, 2020, and February 12, 2021, were excluded. COVID-19 was declared a public health emergency in the United States on January 31, 2020, and the World Health Organization declared COVID-19 a pandemic on March 11, 2020.17 We picked February 7, 2020, within this time frame and without any other special significance. We picked additional CAN score calculation dates approximately 1 year prior and 1 year after this date. Veterans had to be alive on February 12, 2021, (the last date of the CAN score) to be included in the cohorts.
Statistical Analyses
The difference in CAN score from one year to the next was calculated for each patient. The difference between 2019 and 2020 was compared with the difference between 2020 to 2021 using a paired t test. Yearly CAN score values were analyzed using repeated measures analysis of variance. The number of patients that showed an increase in CAN score (ie, increased risk of either mortality or hospitalization within the year) or a decrease (lower risk) was compared using the χ2 test. IBM SPSS v26 and GraphPad Prism v18 were used for statistical analysis. P < .05 was considered statistically significant.
Results
There were 3538 veterans at PVAHCS who met the inclusion criteria and had a 1-year mortality CAN score ≥ 75 on February 8, 2019.
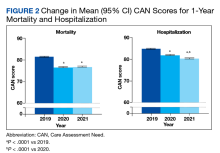
In the hospitalization group, there were 6046 veterans in the analysis; 57 veterans missing a 1-year hospitalization CAN score that were excluded. The mean age was 71.7 (1.3) years and included 5874 male (97.2%) and 172 female (2.8%) veterans. There was a decline in mean 1-year hospitalization CAN scores in our subset of frail older veterans by 2.8 (95% CI, -3.1 to -2.6) in the year preceding the COVID-19 pandemic. This mean decline slowed significantly to 1.5 (95% CI, -1.8 to -1.2; P < .0001) after the first year of the COVID-19 pandemic. Mean CAN scores for 1-year hospitalization were 84.6 (95% CI, 84.4 to 84.8), 81.8 (95% CI, 81.5 to 82.1), and 80.2 (95% CI, 79.9 to 80.6)
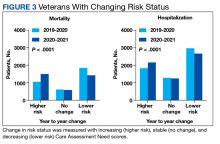
We also calculated the number of veterans with increasing, stable, and decreasing CAN scores across each of our defined periods in both the 1-year mortality and hospitalization groups.
A previous study used a 1-year combined hospitalization or mortality event CAN score as the most all-inclusive measure of frailty but determined that it was possible that 1 of the other 5 CAN risk measures could perform better in predicting frailty.10 We collected and presented data for 1-year mortality and hospitalization CAN scores. There were declines in pandemic-related US hospitalizations for illnesses not related to COVID-19 during the first few months of the pandemic.18 This may or may not have affected the 1-year hospitalization CAN score data; thus, we used the 1-year mortality CAN score data to predict frailty.
Discussion
We studied frailty trends in an older veteran subpopulation enrolled at the PVAHCS 1 year prior and into the COVID-19 pandemic using CAN scores. Frailty is a dynamic state. Previous frailty assessments aimed to identify patients at the highest risk of death. With the advent of advanced therapeutics for several diseases, the number of medical conditions that are now managed as chronic illnesses continues to grow. There is a role for repeated measures of frailty to try to identify frailty trends.19 These trends may assist us in resource allocation, identifying interventions that work and those that do not.
Some studies have shown an overall declining lethality of frailty. This may reflect improvements in the care and management of chronic conditions, screening tests, and increased awareness of healthy lifestyles.20 Another study of frailty trajectories in a veteran population in the 5 years preceding death showed multiple trajectories (stable, gradually increasing, rapidly increasing, and recovering).19
The PACT is a primary care model implemented at VA medical centers in April 2010. It is a patient-centered medical home model (PCMH) with several components. The VA treats a population of socioeconomically vulnerable patients with complex chronic illness management needs. Some of the components of a PACT model relevant to our study include facilitated self-management support for veterans in between practitioner visits via care partners, peer-to-peer and transitional care programs, physical activity and diet programs, primary care mental health, integration between primary and specialty care, and telehealth.21 A previous study has shown that VA primary care clinics with the most PCMH components in place had greater improvements in several chronic disease quality measures than in clinics with a lower number of PCMH components.22
Limitations
Our study is limited by our older veteran population demographics. We chose only a subset of older veterans at a single VA center for this study and cannot extrapolate the results to all older frail veterans or community dwelling older adults. Robust individuals may also transition to prefrailty and frailty over longer periods; our study monitored frailty trends over 2 years.
CAN scores are not quality measures to improve upon. Allocation and utilization of additional resources may clinically benefit a patient but increase their CAN scores. Although our results are statistically significant, we are unable to make any conclusions about clinical significance.
Conclusions
Our study results indicate frailty as determined by 1-year mortality CAN scores significantly increased in a subset of older veterans during the first year of the COVID-19 pandemic when compared with the previous year. Whether this change in frailty is temporary or long lasting remains to be seen. Automated CAN scores can be effectively utilized to monitor frailty trends in certain veteran populations over longer periods.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Phoenix Veterans Affairs Health Care System.
1. Rohrmann S. Epidemiology of frailty in older people. Adv Exp Med Biol. 2020;1216:21-27. doi:10.1007/978-3-030-33330-0_3
2. Bandeen-Roche K, Seplaki CL, Huang J, et al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci. 2015;70(11):1427-1434. doi:10.1093/gerona/glv133
3. Siriwardhana DD, Hardoon S, Rait G, Weerasinghe MC, Walters KR. Prevalence of frailty and prefrailty among community-dwelling older adults in low-income and middle-income countries: a systematic review and meta-analysis. BMJ Open. 2018;8(3):e018195. Published 2018 Mar 1. doi:10.1136/bmjopen-2017-018195
4. Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58(4):681-687. doi:10.1111/j.1532-5415.2010.02764.x
5. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722-727. doi:10.1093/gerona/62.7.722
6. Buta BJ, Walston JD, Godino JG, et al. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev. 2016;26:53-61. doi:10.1016/j.arr.2015.12.003
7. Cheng D, DuMontier C, Yildirim C, et al. Updating and validating the U.S. Veterans Affairs Frailty Index: transitioning From ICD-9 to ICD-10. J Gerontol A Biol Sci Med Sci. 2021;76(7):1318-1325. doi:10.1093/gerona/glab071
8. Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
9. Ruiz JG, Priyadarshni S, Rahaman Z, et al. Validation of an automatically generated screening score for frailty: the care assessment need (CAN) score. BMC Geriatr. 2018;18(1):106. doi:10.1186/s12877-018-0802-7
10. Ruiz JG, Rahaman Z, Dang S, Anam R, Valencia WM, Mintzer MJ. Association of the CAN score with the FRAIL scale in community dwelling older adults. Aging Clin Exp Res. 2018;30(10):1241-1245. doi:10.1007/s40520-018-0910-4
11. Ofori-Asenso R, Chin KL, Mazidi M, et al. Global incidence of frailty and prefrailty among community-dwelling older adults: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(8):e198398. Published 2019 Aug 2. doi:10.1001/jamanetworkopen.2019.8398
12. Marcucci M, Damanti S, Germini F, et al. Interventions to prevent, delay or reverse frailty in older people: a journey towards clinical guidelines. BMC Med. 2019;17(1):193. Published 2019 Oct 29. doi:10.1186/s12916-019-1434-2
13. Travers J, Romero-Ortuno R, Bailey J, Cooney MT. Delaying and reversing frailty: a systematic review of primary care interventions. Br J Gen Pract. 2019;69(678):e61-e69. doi:10.3399/bjgp18X700241
14. Orkaby AR, Nussbaum L, Ho YL, et al. The burden of frailty among U.S. veterans and its association with mortality, 2002-2012. J Gerontol A Biol Sci Med Sci. 2019;74(8):1257-1264. doi:10.1093/gerona/gly232
15. Bakouny Z, Paciotti M, Schmidt AL, Lipsitz SR, Choueiri TK, Trinh QD. Cancer screening tests and cancer diagnoses during the COVID-19 pandemic. JAMA Oncol. 2021;7(3):458-460. doi:10.1001/jamaoncol.2020.7600
16. Steffen R, Lautenschlager S, Fehr J. Travel restrictions and lockdown during the COVID-19 pandemic-impact on notified infectious diseases in Switzerland. J Travel Med. 2020;27(8):taaa180. doi:10.1093/jtm/taaa180
17. CDC Museum COVID-19 Timeline. Centers for Disease Control and Prevention. Updated March 15, 2023. Accessed May 12, 2023. https://www.cdc.gov/museum/timeline/covid19.html18. Nguyen JL, Benigno M, Malhotra D, et al. Pandemic-related declines in hospitalization for non-COVID-19-related illness in the United States from January through July 2020. PLoS One. 2022;17(1):e0262347. Published 2022 Jan 6. doi:10.1371/journal.pone.0262347
19. Ward RE, Orkaby AR, Dumontier C, et al. Trajectories of frailty in the 5 years prior to death among U.S. veterans born 1927-1934. J Gerontol A Biol Sci Med Sci. 2021;76(11):e347-e353. doi:10.1093/gerona/glab196
20. Bäckman K, Joas E, Falk H, Mitnitski A, Rockwood K, Skoog I. Changes in the lethality of frailty over 30 years: evidence from two cohorts of 70-year-olds in Gothenburg Sweden. J Gerontol A Biol Sci Med Sci. 2017;72(7):945-950. doi:10.1093/gerona/glw160
21. Piette JD, Holtz B, Beard AJ, et al. Improving chronic illness care for veterans within the framework of the Patient-Centered Medical Home: experiences from the Ann Arbor Patient-Aligned Care Team Laboratory. Transl Behav Med. 2011;1(4):615-623. doi:10.1007/s13142-011-0065-8
22. Rosland AM, Nelson K, Sun H, et al. The patient-centered medical home in the Veterans Health Administration. Am J Manag Care. 2013;19(7):e263-e272. Published 2013 Jul 1.
Frailty is an age-associated, nonspecific vulnerability to adverse health outcomes. Frailty can also be described as a complex of symptoms characterized by impaired stress tolerance due to a decline in the functionality of different organs.1 The prevalence of frailty varies widely depending on the method of measurement and the population studied.2-4 It is a nonconstant factor that increases with age. A deficit accumulation frailty index (FI) is one method used to measure frailty.5 This approach sees frailty as a multidimensional risk state measured by quantity rather than the nature of health concerns. A deficit accumulation FI does not require physical testing but correlates well with other phenotypic FIs.6 It is, however, time consuming, as ≥ 30 deficits need to be measured to offer greater stability to the frailty estimate.
Health care is seeing increasing utilization of big data analytics to derive predictive models and help with resource allocation. There are currently 2 existing automated tools to predict health care utilization and mortality at the US Department of Veterans Affairs (VA): the VA Frailty Index (VA-FI-10) and the Care Assessment Need (CAN). VA-FI-10 is an International Statistical Classification of Diseases, Tenth Revision (ICD-10) update of the VA-FI that was created in March 2021. The VA-FI-10 is a claims-based frailty assessment tool using 31 health deficits. Calculating the VA-FI-10 requires defining an index date and lookback period (typically 3 years) relative to which it will be calculated.7
CAN is a set of risk-stratifying statistical models run on veterans receiving VA primary care services as part of a patient aligned care team (PACT) using electronic health record data.8 Each veteran is stratified based on the individual’s risks of hospitalization, death, and hospitalization or death. These 3 events are predicted for 90-day and 1-year time periods for a total of 6 distinct outcomes. CAN is currently on its third iteration (CAN 2.5) and scores range from 0 (low) to 99 (high). CAN scores are updated weekly. The 1-year hospitalization probabilities for all patients range from 0.8% to 93.1%. For patients with a CAN score of 50, the probability of being hospitalized within a year ranges from 4.5% to 5.2%, which increases to 32.2% to 36% for veterans with a CAN score of 95. The probability range widens significantly (32.2%-93.1%) for patients in the top 5 CAN scores (95-99).
CAN scores are a potential screening tool for frailty among older adults; they are generated automatically and provide acceptable diagnostic accuracy. Hence, the CAN score may be a useful tool for primary care practitioners for the detection of frailty in their patients. The CAN score has shown a moderate positive association with the FRAIL Scale.9,10 The population-based studies that have used the FI approach (differing FIs, depending on the data available) give robust results: People accumulate an average of 0.03 deficits per year after the age of 70 years.11 Interventions to delay or reverse frailty have not been clearly defined with heterogeneity in the definition of frailty and measurement of frailty outcomes.12,13 The prevalence of frailty in the veteran population is substantially higher than the prevalence in community populations with a similar age distribution. There is also mounting evidence that veterans accumulate deficits more rapidly than their civilian counterparts.14
COVID-19 was declared a pandemic in March 2020 and had many impacts on global health that were most marked in the first year. These included reductions in hospital visits for non-COVID-19 health concerns, a reduction in completed screening tests, an initial reduction in other infectious diseases (attributable to quarantines), and an increase or worsening of mental health concerns.15,16
We aimed to investigate whether frailty increased disproportionately in a subset of older veterans in the first year of the COVID-19 pandemic when compared with the previous year using CAN scores. This single institution, longitudinal cohort study was determined to be exempt from institutional review board review but was approved by the Phoenix VA Health Care System (PVAHCS) Research and Development Committee.
Methods
The Office of Clinical Systems Development and Evaluation (CSDE–10E2A) produces a weekly CAN Score Report to help identify the highest-risk patients in a primary care panel or cohort. CAN scores range from 0 (lowest risk) to 99 (highest risk), indicating how likely a patient is to experience hospitalization or death compared with other VA patients. CAN scores are calculated with statistical prediction models that use data elements from the following Corporate Data Warehouse (CDW) domains: demographics, health care utilization, laboratory tests, medical conditions, medications, and vital signs (eAppendix available online at 10.12788/fp.0385).
The CAN Score Report is generated weekly and stored on a CDW server. A patient will receive all 6 distinct CAN scores if they are: (1) assigned to a primary care PACT on the risk date; (2) a veteran; (3) not hospitalized in a VA facility on the risk date; and (4) alive as of the risk date. New to CAN 2.5 is that patients who meet criteria 1, 2, and 4 but are hospitalized in a VA facility on the risk date will receive CAN scores for the 1-year and 90-day mortality models.
Utilizing VA Informatics and Computing Infrastructure (VA HSR RES 13-457, US Department of Veterans Affairs [2008]), we obtained 2 lists of veterans aged 70 to 75 years on February 8, 2019, with a calculated CAN score of ≥ 75 for 1-year mortality and 1-year hospitalization on that date. A veteran with a CAN score of ≥ 75 is likely to be prefrail or frail.9,10 Veterans who did not have a corresponding calculated CAN score on February 7, 2020, and February 12, 2021, were excluded. COVID-19 was declared a public health emergency in the United States on January 31, 2020, and the World Health Organization declared COVID-19 a pandemic on March 11, 2020.17 We picked February 7, 2020, within this time frame and without any other special significance. We picked additional CAN score calculation dates approximately 1 year prior and 1 year after this date. Veterans had to be alive on February 12, 2021, (the last date of the CAN score) to be included in the cohorts.
Statistical Analyses
The difference in CAN score from one year to the next was calculated for each patient. The difference between 2019 and 2020 was compared with the difference between 2020 to 2021 using a paired t test. Yearly CAN score values were analyzed using repeated measures analysis of variance. The number of patients that showed an increase in CAN score (ie, increased risk of either mortality or hospitalization within the year) or a decrease (lower risk) was compared using the χ2 test. IBM SPSS v26 and GraphPad Prism v18 were used for statistical analysis. P < .05 was considered statistically significant.
Results
There were 3538 veterans at PVAHCS who met the inclusion criteria and had a 1-year mortality CAN score ≥ 75 on February 8, 2019.
In the hospitalization group, there were 6046 veterans in the analysis; 57 veterans missing a 1-year hospitalization CAN score that were excluded. The mean age was 71.7 (1.3) years and included 5874 male (97.2%) and 172 female (2.8%) veterans. There was a decline in mean 1-year hospitalization CAN scores in our subset of frail older veterans by 2.8 (95% CI, -3.1 to -2.6) in the year preceding the COVID-19 pandemic. This mean decline slowed significantly to 1.5 (95% CI, -1.8 to -1.2; P < .0001) after the first year of the COVID-19 pandemic. Mean CAN scores for 1-year hospitalization were 84.6 (95% CI, 84.4 to 84.8), 81.8 (95% CI, 81.5 to 82.1), and 80.2 (95% CI, 79.9 to 80.6)
We also calculated the number of veterans with increasing, stable, and decreasing CAN scores across each of our defined periods in both the 1-year mortality and hospitalization groups.
A previous study used a 1-year combined hospitalization or mortality event CAN score as the most all-inclusive measure of frailty but determined that it was possible that 1 of the other 5 CAN risk measures could perform better in predicting frailty.10 We collected and presented data for 1-year mortality and hospitalization CAN scores. There were declines in pandemic-related US hospitalizations for illnesses not related to COVID-19 during the first few months of the pandemic.18 This may or may not have affected the 1-year hospitalization CAN score data; thus, we used the 1-year mortality CAN score data to predict frailty.
Discussion
We studied frailty trends in an older veteran subpopulation enrolled at the PVAHCS 1 year prior and into the COVID-19 pandemic using CAN scores. Frailty is a dynamic state. Previous frailty assessments aimed to identify patients at the highest risk of death. With the advent of advanced therapeutics for several diseases, the number of medical conditions that are now managed as chronic illnesses continues to grow. There is a role for repeated measures of frailty to try to identify frailty trends.19 These trends may assist us in resource allocation, identifying interventions that work and those that do not.
Some studies have shown an overall declining lethality of frailty. This may reflect improvements in the care and management of chronic conditions, screening tests, and increased awareness of healthy lifestyles.20 Another study of frailty trajectories in a veteran population in the 5 years preceding death showed multiple trajectories (stable, gradually increasing, rapidly increasing, and recovering).19
The PACT is a primary care model implemented at VA medical centers in April 2010. It is a patient-centered medical home model (PCMH) with several components. The VA treats a population of socioeconomically vulnerable patients with complex chronic illness management needs. Some of the components of a PACT model relevant to our study include facilitated self-management support for veterans in between practitioner visits via care partners, peer-to-peer and transitional care programs, physical activity and diet programs, primary care mental health, integration between primary and specialty care, and telehealth.21 A previous study has shown that VA primary care clinics with the most PCMH components in place had greater improvements in several chronic disease quality measures than in clinics with a lower number of PCMH components.22
Limitations
Our study is limited by our older veteran population demographics. We chose only a subset of older veterans at a single VA center for this study and cannot extrapolate the results to all older frail veterans or community dwelling older adults. Robust individuals may also transition to prefrailty and frailty over longer periods; our study monitored frailty trends over 2 years.
CAN scores are not quality measures to improve upon. Allocation and utilization of additional resources may clinically benefit a patient but increase their CAN scores. Although our results are statistically significant, we are unable to make any conclusions about clinical significance.
Conclusions
Our study results indicate frailty as determined by 1-year mortality CAN scores significantly increased in a subset of older veterans during the first year of the COVID-19 pandemic when compared with the previous year. Whether this change in frailty is temporary or long lasting remains to be seen. Automated CAN scores can be effectively utilized to monitor frailty trends in certain veteran populations over longer periods.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Phoenix Veterans Affairs Health Care System.
Frailty is an age-associated, nonspecific vulnerability to adverse health outcomes. Frailty can also be described as a complex of symptoms characterized by impaired stress tolerance due to a decline in the functionality of different organs.1 The prevalence of frailty varies widely depending on the method of measurement and the population studied.2-4 It is a nonconstant factor that increases with age. A deficit accumulation frailty index (FI) is one method used to measure frailty.5 This approach sees frailty as a multidimensional risk state measured by quantity rather than the nature of health concerns. A deficit accumulation FI does not require physical testing but correlates well with other phenotypic FIs.6 It is, however, time consuming, as ≥ 30 deficits need to be measured to offer greater stability to the frailty estimate.
Health care is seeing increasing utilization of big data analytics to derive predictive models and help with resource allocation. There are currently 2 existing automated tools to predict health care utilization and mortality at the US Department of Veterans Affairs (VA): the VA Frailty Index (VA-FI-10) and the Care Assessment Need (CAN). VA-FI-10 is an International Statistical Classification of Diseases, Tenth Revision (ICD-10) update of the VA-FI that was created in March 2021. The VA-FI-10 is a claims-based frailty assessment tool using 31 health deficits. Calculating the VA-FI-10 requires defining an index date and lookback period (typically 3 years) relative to which it will be calculated.7
CAN is a set of risk-stratifying statistical models run on veterans receiving VA primary care services as part of a patient aligned care team (PACT) using electronic health record data.8 Each veteran is stratified based on the individual’s risks of hospitalization, death, and hospitalization or death. These 3 events are predicted for 90-day and 1-year time periods for a total of 6 distinct outcomes. CAN is currently on its third iteration (CAN 2.5) and scores range from 0 (low) to 99 (high). CAN scores are updated weekly. The 1-year hospitalization probabilities for all patients range from 0.8% to 93.1%. For patients with a CAN score of 50, the probability of being hospitalized within a year ranges from 4.5% to 5.2%, which increases to 32.2% to 36% for veterans with a CAN score of 95. The probability range widens significantly (32.2%-93.1%) for patients in the top 5 CAN scores (95-99).
CAN scores are a potential screening tool for frailty among older adults; they are generated automatically and provide acceptable diagnostic accuracy. Hence, the CAN score may be a useful tool for primary care practitioners for the detection of frailty in their patients. The CAN score has shown a moderate positive association with the FRAIL Scale.9,10 The population-based studies that have used the FI approach (differing FIs, depending on the data available) give robust results: People accumulate an average of 0.03 deficits per year after the age of 70 years.11 Interventions to delay or reverse frailty have not been clearly defined with heterogeneity in the definition of frailty and measurement of frailty outcomes.12,13 The prevalence of frailty in the veteran population is substantially higher than the prevalence in community populations with a similar age distribution. There is also mounting evidence that veterans accumulate deficits more rapidly than their civilian counterparts.14
COVID-19 was declared a pandemic in March 2020 and had many impacts on global health that were most marked in the first year. These included reductions in hospital visits for non-COVID-19 health concerns, a reduction in completed screening tests, an initial reduction in other infectious diseases (attributable to quarantines), and an increase or worsening of mental health concerns.15,16
We aimed to investigate whether frailty increased disproportionately in a subset of older veterans in the first year of the COVID-19 pandemic when compared with the previous year using CAN scores. This single institution, longitudinal cohort study was determined to be exempt from institutional review board review but was approved by the Phoenix VA Health Care System (PVAHCS) Research and Development Committee.
Methods
The Office of Clinical Systems Development and Evaluation (CSDE–10E2A) produces a weekly CAN Score Report to help identify the highest-risk patients in a primary care panel or cohort. CAN scores range from 0 (lowest risk) to 99 (highest risk), indicating how likely a patient is to experience hospitalization or death compared with other VA patients. CAN scores are calculated with statistical prediction models that use data elements from the following Corporate Data Warehouse (CDW) domains: demographics, health care utilization, laboratory tests, medical conditions, medications, and vital signs (eAppendix available online at 10.12788/fp.0385).
The CAN Score Report is generated weekly and stored on a CDW server. A patient will receive all 6 distinct CAN scores if they are: (1) assigned to a primary care PACT on the risk date; (2) a veteran; (3) not hospitalized in a VA facility on the risk date; and (4) alive as of the risk date. New to CAN 2.5 is that patients who meet criteria 1, 2, and 4 but are hospitalized in a VA facility on the risk date will receive CAN scores for the 1-year and 90-day mortality models.
Utilizing VA Informatics and Computing Infrastructure (VA HSR RES 13-457, US Department of Veterans Affairs [2008]), we obtained 2 lists of veterans aged 70 to 75 years on February 8, 2019, with a calculated CAN score of ≥ 75 for 1-year mortality and 1-year hospitalization on that date. A veteran with a CAN score of ≥ 75 is likely to be prefrail or frail.9,10 Veterans who did not have a corresponding calculated CAN score on February 7, 2020, and February 12, 2021, were excluded. COVID-19 was declared a public health emergency in the United States on January 31, 2020, and the World Health Organization declared COVID-19 a pandemic on March 11, 2020.17 We picked February 7, 2020, within this time frame and without any other special significance. We picked additional CAN score calculation dates approximately 1 year prior and 1 year after this date. Veterans had to be alive on February 12, 2021, (the last date of the CAN score) to be included in the cohorts.
Statistical Analyses
The difference in CAN score from one year to the next was calculated for each patient. The difference between 2019 and 2020 was compared with the difference between 2020 to 2021 using a paired t test. Yearly CAN score values were analyzed using repeated measures analysis of variance. The number of patients that showed an increase in CAN score (ie, increased risk of either mortality or hospitalization within the year) or a decrease (lower risk) was compared using the χ2 test. IBM SPSS v26 and GraphPad Prism v18 were used for statistical analysis. P < .05 was considered statistically significant.
Results
There were 3538 veterans at PVAHCS who met the inclusion criteria and had a 1-year mortality CAN score ≥ 75 on February 8, 2019.
In the hospitalization group, there were 6046 veterans in the analysis; 57 veterans missing a 1-year hospitalization CAN score that were excluded. The mean age was 71.7 (1.3) years and included 5874 male (97.2%) and 172 female (2.8%) veterans. There was a decline in mean 1-year hospitalization CAN scores in our subset of frail older veterans by 2.8 (95% CI, -3.1 to -2.6) in the year preceding the COVID-19 pandemic. This mean decline slowed significantly to 1.5 (95% CI, -1.8 to -1.2; P < .0001) after the first year of the COVID-19 pandemic. Mean CAN scores for 1-year hospitalization were 84.6 (95% CI, 84.4 to 84.8), 81.8 (95% CI, 81.5 to 82.1), and 80.2 (95% CI, 79.9 to 80.6)
We also calculated the number of veterans with increasing, stable, and decreasing CAN scores across each of our defined periods in both the 1-year mortality and hospitalization groups.
A previous study used a 1-year combined hospitalization or mortality event CAN score as the most all-inclusive measure of frailty but determined that it was possible that 1 of the other 5 CAN risk measures could perform better in predicting frailty.10 We collected and presented data for 1-year mortality and hospitalization CAN scores. There were declines in pandemic-related US hospitalizations for illnesses not related to COVID-19 during the first few months of the pandemic.18 This may or may not have affected the 1-year hospitalization CAN score data; thus, we used the 1-year mortality CAN score data to predict frailty.
Discussion
We studied frailty trends in an older veteran subpopulation enrolled at the PVAHCS 1 year prior and into the COVID-19 pandemic using CAN scores. Frailty is a dynamic state. Previous frailty assessments aimed to identify patients at the highest risk of death. With the advent of advanced therapeutics for several diseases, the number of medical conditions that are now managed as chronic illnesses continues to grow. There is a role for repeated measures of frailty to try to identify frailty trends.19 These trends may assist us in resource allocation, identifying interventions that work and those that do not.
Some studies have shown an overall declining lethality of frailty. This may reflect improvements in the care and management of chronic conditions, screening tests, and increased awareness of healthy lifestyles.20 Another study of frailty trajectories in a veteran population in the 5 years preceding death showed multiple trajectories (stable, gradually increasing, rapidly increasing, and recovering).19
The PACT is a primary care model implemented at VA medical centers in April 2010. It is a patient-centered medical home model (PCMH) with several components. The VA treats a population of socioeconomically vulnerable patients with complex chronic illness management needs. Some of the components of a PACT model relevant to our study include facilitated self-management support for veterans in between practitioner visits via care partners, peer-to-peer and transitional care programs, physical activity and diet programs, primary care mental health, integration between primary and specialty care, and telehealth.21 A previous study has shown that VA primary care clinics with the most PCMH components in place had greater improvements in several chronic disease quality measures than in clinics with a lower number of PCMH components.22
Limitations
Our study is limited by our older veteran population demographics. We chose only a subset of older veterans at a single VA center for this study and cannot extrapolate the results to all older frail veterans or community dwelling older adults. Robust individuals may also transition to prefrailty and frailty over longer periods; our study monitored frailty trends over 2 years.
CAN scores are not quality measures to improve upon. Allocation and utilization of additional resources may clinically benefit a patient but increase their CAN scores. Although our results are statistically significant, we are unable to make any conclusions about clinical significance.
Conclusions
Our study results indicate frailty as determined by 1-year mortality CAN scores significantly increased in a subset of older veterans during the first year of the COVID-19 pandemic when compared with the previous year. Whether this change in frailty is temporary or long lasting remains to be seen. Automated CAN scores can be effectively utilized to monitor frailty trends in certain veteran populations over longer periods.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Phoenix Veterans Affairs Health Care System.
1. Rohrmann S. Epidemiology of frailty in older people. Adv Exp Med Biol. 2020;1216:21-27. doi:10.1007/978-3-030-33330-0_3
2. Bandeen-Roche K, Seplaki CL, Huang J, et al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci. 2015;70(11):1427-1434. doi:10.1093/gerona/glv133
3. Siriwardhana DD, Hardoon S, Rait G, Weerasinghe MC, Walters KR. Prevalence of frailty and prefrailty among community-dwelling older adults in low-income and middle-income countries: a systematic review and meta-analysis. BMJ Open. 2018;8(3):e018195. Published 2018 Mar 1. doi:10.1136/bmjopen-2017-018195
4. Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58(4):681-687. doi:10.1111/j.1532-5415.2010.02764.x
5. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722-727. doi:10.1093/gerona/62.7.722
6. Buta BJ, Walston JD, Godino JG, et al. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev. 2016;26:53-61. doi:10.1016/j.arr.2015.12.003
7. Cheng D, DuMontier C, Yildirim C, et al. Updating and validating the U.S. Veterans Affairs Frailty Index: transitioning From ICD-9 to ICD-10. J Gerontol A Biol Sci Med Sci. 2021;76(7):1318-1325. doi:10.1093/gerona/glab071
8. Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
9. Ruiz JG, Priyadarshni S, Rahaman Z, et al. Validation of an automatically generated screening score for frailty: the care assessment need (CAN) score. BMC Geriatr. 2018;18(1):106. doi:10.1186/s12877-018-0802-7
10. Ruiz JG, Rahaman Z, Dang S, Anam R, Valencia WM, Mintzer MJ. Association of the CAN score with the FRAIL scale in community dwelling older adults. Aging Clin Exp Res. 2018;30(10):1241-1245. doi:10.1007/s40520-018-0910-4
11. Ofori-Asenso R, Chin KL, Mazidi M, et al. Global incidence of frailty and prefrailty among community-dwelling older adults: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(8):e198398. Published 2019 Aug 2. doi:10.1001/jamanetworkopen.2019.8398
12. Marcucci M, Damanti S, Germini F, et al. Interventions to prevent, delay or reverse frailty in older people: a journey towards clinical guidelines. BMC Med. 2019;17(1):193. Published 2019 Oct 29. doi:10.1186/s12916-019-1434-2
13. Travers J, Romero-Ortuno R, Bailey J, Cooney MT. Delaying and reversing frailty: a systematic review of primary care interventions. Br J Gen Pract. 2019;69(678):e61-e69. doi:10.3399/bjgp18X700241
14. Orkaby AR, Nussbaum L, Ho YL, et al. The burden of frailty among U.S. veterans and its association with mortality, 2002-2012. J Gerontol A Biol Sci Med Sci. 2019;74(8):1257-1264. doi:10.1093/gerona/gly232
15. Bakouny Z, Paciotti M, Schmidt AL, Lipsitz SR, Choueiri TK, Trinh QD. Cancer screening tests and cancer diagnoses during the COVID-19 pandemic. JAMA Oncol. 2021;7(3):458-460. doi:10.1001/jamaoncol.2020.7600
16. Steffen R, Lautenschlager S, Fehr J. Travel restrictions and lockdown during the COVID-19 pandemic-impact on notified infectious diseases in Switzerland. J Travel Med. 2020;27(8):taaa180. doi:10.1093/jtm/taaa180
17. CDC Museum COVID-19 Timeline. Centers for Disease Control and Prevention. Updated March 15, 2023. Accessed May 12, 2023. https://www.cdc.gov/museum/timeline/covid19.html18. Nguyen JL, Benigno M, Malhotra D, et al. Pandemic-related declines in hospitalization for non-COVID-19-related illness in the United States from January through July 2020. PLoS One. 2022;17(1):e0262347. Published 2022 Jan 6. doi:10.1371/journal.pone.0262347
19. Ward RE, Orkaby AR, Dumontier C, et al. Trajectories of frailty in the 5 years prior to death among U.S. veterans born 1927-1934. J Gerontol A Biol Sci Med Sci. 2021;76(11):e347-e353. doi:10.1093/gerona/glab196
20. Bäckman K, Joas E, Falk H, Mitnitski A, Rockwood K, Skoog I. Changes in the lethality of frailty over 30 years: evidence from two cohorts of 70-year-olds in Gothenburg Sweden. J Gerontol A Biol Sci Med Sci. 2017;72(7):945-950. doi:10.1093/gerona/glw160
21. Piette JD, Holtz B, Beard AJ, et al. Improving chronic illness care for veterans within the framework of the Patient-Centered Medical Home: experiences from the Ann Arbor Patient-Aligned Care Team Laboratory. Transl Behav Med. 2011;1(4):615-623. doi:10.1007/s13142-011-0065-8
22. Rosland AM, Nelson K, Sun H, et al. The patient-centered medical home in the Veterans Health Administration. Am J Manag Care. 2013;19(7):e263-e272. Published 2013 Jul 1.
1. Rohrmann S. Epidemiology of frailty in older people. Adv Exp Med Biol. 2020;1216:21-27. doi:10.1007/978-3-030-33330-0_3
2. Bandeen-Roche K, Seplaki CL, Huang J, et al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci. 2015;70(11):1427-1434. doi:10.1093/gerona/glv133
3. Siriwardhana DD, Hardoon S, Rait G, Weerasinghe MC, Walters KR. Prevalence of frailty and prefrailty among community-dwelling older adults in low-income and middle-income countries: a systematic review and meta-analysis. BMJ Open. 2018;8(3):e018195. Published 2018 Mar 1. doi:10.1136/bmjopen-2017-018195
4. Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58(4):681-687. doi:10.1111/j.1532-5415.2010.02764.x
5. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722-727. doi:10.1093/gerona/62.7.722
6. Buta BJ, Walston JD, Godino JG, et al. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev. 2016;26:53-61. doi:10.1016/j.arr.2015.12.003
7. Cheng D, DuMontier C, Yildirim C, et al. Updating and validating the U.S. Veterans Affairs Frailty Index: transitioning From ICD-9 to ICD-10. J Gerontol A Biol Sci Med Sci. 2021;76(7):1318-1325. doi:10.1093/gerona/glab071
8. Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
9. Ruiz JG, Priyadarshni S, Rahaman Z, et al. Validation of an automatically generated screening score for frailty: the care assessment need (CAN) score. BMC Geriatr. 2018;18(1):106. doi:10.1186/s12877-018-0802-7
10. Ruiz JG, Rahaman Z, Dang S, Anam R, Valencia WM, Mintzer MJ. Association of the CAN score with the FRAIL scale in community dwelling older adults. Aging Clin Exp Res. 2018;30(10):1241-1245. doi:10.1007/s40520-018-0910-4
11. Ofori-Asenso R, Chin KL, Mazidi M, et al. Global incidence of frailty and prefrailty among community-dwelling older adults: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(8):e198398. Published 2019 Aug 2. doi:10.1001/jamanetworkopen.2019.8398
12. Marcucci M, Damanti S, Germini F, et al. Interventions to prevent, delay or reverse frailty in older people: a journey towards clinical guidelines. BMC Med. 2019;17(1):193. Published 2019 Oct 29. doi:10.1186/s12916-019-1434-2
13. Travers J, Romero-Ortuno R, Bailey J, Cooney MT. Delaying and reversing frailty: a systematic review of primary care interventions. Br J Gen Pract. 2019;69(678):e61-e69. doi:10.3399/bjgp18X700241
14. Orkaby AR, Nussbaum L, Ho YL, et al. The burden of frailty among U.S. veterans and its association with mortality, 2002-2012. J Gerontol A Biol Sci Med Sci. 2019;74(8):1257-1264. doi:10.1093/gerona/gly232
15. Bakouny Z, Paciotti M, Schmidt AL, Lipsitz SR, Choueiri TK, Trinh QD. Cancer screening tests and cancer diagnoses during the COVID-19 pandemic. JAMA Oncol. 2021;7(3):458-460. doi:10.1001/jamaoncol.2020.7600
16. Steffen R, Lautenschlager S, Fehr J. Travel restrictions and lockdown during the COVID-19 pandemic-impact on notified infectious diseases in Switzerland. J Travel Med. 2020;27(8):taaa180. doi:10.1093/jtm/taaa180
17. CDC Museum COVID-19 Timeline. Centers for Disease Control and Prevention. Updated March 15, 2023. Accessed May 12, 2023. https://www.cdc.gov/museum/timeline/covid19.html18. Nguyen JL, Benigno M, Malhotra D, et al. Pandemic-related declines in hospitalization for non-COVID-19-related illness in the United States from January through July 2020. PLoS One. 2022;17(1):e0262347. Published 2022 Jan 6. doi:10.1371/journal.pone.0262347
19. Ward RE, Orkaby AR, Dumontier C, et al. Trajectories of frailty in the 5 years prior to death among U.S. veterans born 1927-1934. J Gerontol A Biol Sci Med Sci. 2021;76(11):e347-e353. doi:10.1093/gerona/glab196
20. Bäckman K, Joas E, Falk H, Mitnitski A, Rockwood K, Skoog I. Changes in the lethality of frailty over 30 years: evidence from two cohorts of 70-year-olds in Gothenburg Sweden. J Gerontol A Biol Sci Med Sci. 2017;72(7):945-950. doi:10.1093/gerona/glw160
21. Piette JD, Holtz B, Beard AJ, et al. Improving chronic illness care for veterans within the framework of the Patient-Centered Medical Home: experiences from the Ann Arbor Patient-Aligned Care Team Laboratory. Transl Behav Med. 2011;1(4):615-623. doi:10.1007/s13142-011-0065-8
22. Rosland AM, Nelson K, Sun H, et al. The patient-centered medical home in the Veterans Health Administration. Am J Manag Care. 2013;19(7):e263-e272. Published 2013 Jul 1.
Pyogenic Hepatic Abscess in an Immunocompetent Patient With Poor Oral Health and COVID-19 Infection
Pyogenic hepatic abscess (PHA) is a collection of pus in the liver caused by bacterial infection of the liver parenchyma. This potentially life-threatening condition has a mortality rate reported to be as high as 47%.1 The incidence of PHA is reported to be 2.3 per 100,000 individuals and is more common in immunosuppressed individuals and those with diabetes mellitus, cancer, and liver transplant.2,3 PHA infections are usually polymicrobial and most commonly include enteric organisms like Escherichia coli and Klebsiella pneumoniae.4
We present a rare cause of PHA with Fusobacterium nucleatum (F nucleatum) in an immunocompetent patient with poor oral health, history of diverticulitis, and recent COVID-19 infection whose only symptoms were chest pain and a 4-week history of fever and malaise.
Case Presentation
A 52-year-old man initially presented to the C.W. Bill Young Veterans Affairs Medical Center (CWBYVAMC) emergency department in Bay Pines, Florida, for fever, malaise, and right-sided chest pain on inspiration. The fever and malaise began while he was on vacation 4 weeks prior. He originally presented to an outside hospital where he tested positive for COVID-19 and was recommended ibuprofen and rest. His symptoms did not improve, and he returned a second time to the outside hospital 2 weeks later and was diagnosed with pneumonia and placed on outpatient antibiotics. The patient subsequently returned to CWBYVAMC 2 weeks after starting antibiotics when he began to develop right-sided inspiratory chest pain. He reported no other recent travel and no abdominal pain. The patient’s history was significant for diverticulitis 2 years before. A colonoscopy was performed during that time and showed no masses.
On presentation, the patient was febrile with a temperature of 100.8 °F; otherwise, his vital signs were stable. Physical examinations, including abdominal, respiratory, and cardiovascular, were unremarkable. The initial laboratory workup revealed a white blood cell (WBC) count of 18.7 K/μL (reference range, 5-10 K/μL) and microcytic anemia with a hemoglobin level of 8.8 g/dL. The comprehensive metabolic panel revealed normal aspartate transaminase, alanine transaminase, and total bilirubin levels and elevated alkaline phosphatase of 215 U/L (reference range, 44-147 U/L), revealing possible mild intrahepatic cholestasis. Urinalysis showed trace proteinuria and urobilinogen. Coagulation studies showed elevated D-dimer and procalcitonin levels at 1.9 ng/mL (reference range, < 0.1 ng/mL) and 1.21 ng/mL (reference range, < 0.5 ng/mL), respectively, with normal prothrombin and partial thromboplastin times. The patient had a normal troponin, fecal, and blood culture; entamoeba serology was negative.
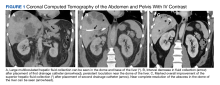
A computed tomograph (CT) angiography of the chest was performed to rule out pulmonary embolism, revealing liver lesions suspicious for abscess or metastatic disease. Minimal pleural effusion was detected bilaterally. A subsequent CT
Following the procedure, the patient developed shaking chills, hypertension, fever, and acute hypoxic respiratory failure. He improved with oxygen and was transferred to the intensive care unit (ICU) where he had an increase in temperature and became septic without shock. A repeat blood culture was negative. An echocardiogram revealed no vegetation. Vancomycin was added for empiric coverage of potentially resistant organisms. The patient clinically improved and was able to leave the ICU 2 days later on hospital day 4.
The patient’s renal function worsened on day 5, and piperacillin-tazobactam and vancomycin were discontinued due to possible acute interstitial nephritis and renal toxicity. He started cefepime and continued metronidazole, and his renal function returned to normal 2 days later. Vancomycin was then re-administered. The results of the culture taken from the abscess came back positive for monomicrobial growth of F nucleatum on hospital day 9.
Due to the patient’s persisting fever and WBC count, a repeat CT of the abdomen on hospital day 10 revealed a partial decrease in the abscess with a persistent collection superior to the location of the initial pigtail catheter placement. A second pigtail catheter was then placed near the dome of the liver 1 day later on hospital day 11. Following the procedure, the patient improved significantly. The repeat CT after 1 week showed marked overall resolution of the abscess, and the repeat culture of the abscess did not reveal any organism growth. Vancomycin was discontinued on day 19, and the drains were removed on hospital day 20. He was discharged home in stable condition on metronidazole and cefdinir for 21 days with follow-up appointments for CT of the abdomen and with primary care, infectious disease, and a dental specialist.
Discussion
F nucleatum is a gram-negative, nonmotile, spindle-shaped rod found in dental plaques.5 The incidence of F nucleatum bacteremia is 0.34 per 100,000 people and increases with age, with the median age being 53.5 years.6 Although our patient did not present with F nucleatum bacteremia, it is possible that bacteremia was present before hospitalization but resolved by the time the sample was drawn for culture. F nucleatum bacteremia can lead to a variety of presentations. The most common primary diagnoses are intra-abdominal infections (eg, PHA, respiratory tract infections, and hematological disorders).1,6
PHA Presentation
The most common presenting symptoms of PHA are fever (88%), abdominal pain (79%), and vomiting (50%).4 The patient’s presentation of inspiratory right-sided chest pain is likely due to irritation of the diaphragmatic pleura of the right lung secondary to the abscess formation. The patient did not experience abdominal pain throughout the course of this disease or on palpation of his right upper quadrant. To our knowledge, this is the only case of PHA in the literature of a patient with inspiratory chest pain without respiratory infection, abdominal pain, and cardiac abnormalities. There was no radiologic evidence or signs of hypoxia on admission to CWBYVAMC, which makes respiratory infection an unlikely cause of the chest pain. Moreover, the patient presented with new-onset chest pain 2 weeks after the diagnosis of pneumonia.
Common laboratory findings of PHA include transaminitis, leukocytosis, and bilirubinemia.4 Of note, increased procalcitonin has also been associated with PHA and extreme elevation (> 200 μg/L) may be a useful biomarker to identify F nucleatum infections before the presence of leukocytosis.3 CT of PHA usually reveals right lobe involvement, and F nucleatum infection usually demonstrates multiple abscesses.4,7
Contributing Factors in F nucleatum PHA
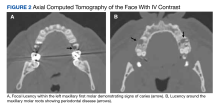
F nucleatum is associated with several oral diseases, such as periodontitis and gingivitis.8 It is important to do an oral inspection on patients with F nucleatum infections because it can spread from oral cavities to different body parts.
F nucleatum is also found in the gut.9 Any disease that can cause a break in the gastrointestinal mucosa may result in F nucleatum bacteremia and PHA. This may be why F nucleatum has been associated with a variety of different diseases, such as diverticulitis, inflammatory bowel disease, appendicitis, and colorectal cancer.10,11 Our patient had a history of diverticulosis with diverticulitis. Bawa and colleagues described a patient with recurrent diverticulitis who developed F nucleatum bacteremia and PHA.11 Our patient did not have any signs of diverticulitis.
Our patient’s COVID-19 infection also had a role in delaying the appropriate treatment of PHA. Without any symptoms of PHA, a diagnosis is difficult in a patient with a positive COVID-19 test, and treatment was delayed 1 month. Moreover, COVID-19 has been reported to delay the diagnosis of PHA even in the absence of a positive COVID-19 test. Collins and Diamond presented a patient during the COVID-19 pandemic who developed a periodontal abscess, which resulted in F nucleatum bacteremia and PHA due to delayed hospital presentation after the patient’s practitioners recommended self-isolation, despite a negative COVID-19 test.12 This highlights the impact that COVID-19 may have on the timely diagnosis and treatment of patients with PHA.
Malignancy has been associated with F nucleatum bacteremia.1,13 Possibly the association is due to gastrointestinal mucosa malignancy’s ability to cause micro-abrasions, resulting in F nucleatum bacteremia.10 Additionally, F nucleatum may promote the development of colorectal neoplasms.8 Due to this association, screening for colorectal cancer in patients with F nucleatum infection is important. In our patient, a colonoscopy was performed during the patient’s hospitalization for diverticulitis 2 years prior. No signs of colorectal neoplasm were noted
Conclusions
PHA due to F nucleatum is a rare but potentially life-threatening condition that must be diagnosed and treated promptly. It usually presents with fever, abdominal pain, and vomiting but can present with chest pain in the absence of a respiratory infection, cardiac abnormalities, and abdominal pain, as in our patient. A wide spectrum of infections can occur with F nucleatum, including PHA.
Suspicion for infection with this organism should be kept high in middle-aged and older individuals who present with an indolent disease course and have risk factors, such as poor oral health and comorbidities. Suspicion should be kept high even in the event of COVID-19 infection, especially in individuals with prolonged fever without other signs indicating respiratory infection. We believe that the most likely causes of this patient’s infection were his dental caries and periodontal disease. The timing of his symptoms is not consistent with his previous episode of diverticulitis. Due to the mortality of PHA, diagnosis and treatment must be prompt. Initial treatment with drainage and empiric anaerobic coverage is recommended, followed by a tailored antibiotic regiment if indicated by culture, and further drainage if suggested by imaging.
1. Yang CC, Ye JJ, Hsu PC, et al. Characteristics and outcomes of Fusobacterium nucleatum bacteremia—a 6-year experience at a tertiary care hospital in northern Taiwan. Diagn Microbiol Infect Dis. 2011;70(2):167-174. doi:10.1016/j.diagmicrobio.2010.12.017
2. Kaplan GG, Gregson DB, Laupland KB. Population-based study of the epidemiology of and the risk factors for pyogenic liver abscess. Clin Gastroenterol Hepatol. 2004;2(11):1032-1038. doi:10.1016/s1542-3565(04)00459-8
3. Cao SA, Hinchey S. Identification and management of fusobacterium nucleatum liver abscess and bacteremia in a young healthy man. Cureus. 2020;12(12):e12303. doi:10.7759/cureus.12303
4. Abbas MT, Khan FY, Muhsin SA, Al-Dehwe B, Abukamar M, Elzouki AN. Epidemiology, clinical features and outcome of liver abscess: a single reference center experience in Qatar. Oman Med J. 2014;29(4):260-263. doi:10.5001/omj.2014.69
5. Bolstad AI, Jensen HB, Bakken V. Taxonomy, biology, and periodontal aspects of Fusobacterium nucleatum. Clin Microbiol Rev. 1996;9(1):55-71. doi:10.1128/CMR.9.1.55
6. Afra K, Laupland K, Leal J, Lloyd T, Gregson D. Incidence, risk factors, and outcomes of Fusobacterium species bacteremia. BMC Infect Dis. 2013;13:264. doi:10.1186/1471-2334-13-264
7. Crippin JS, Wang KK. An unrecognized etiology for pyogenic hepatic abscesses in normal hosts: dental disease. Am J Gastroenterol. 1992;87(12):1740-1743.
8. Shang FM, Liu HL. Fusobacterium nucleatum and colorectal cancer: a review. World J Gastrointest Oncol. 2018;10(3):71-81. doi:10.4251/wjgo.v10.i3.71
9. Allen-Vercoe E, Strauss J, Chadee K. Fusobacterium nucleatum: an emerging gut pathogen? Gut Microbes. 2011;2(5):294-298. doi:10.4161/gmic.2.5.18603
10. Han YW. Fusobacterium nucleatum: a commensal-turned pathogen. Curr Opin Microbiol. 2015;23:141-147. doi:10.1016/j.mib.2014.11.013
11. Bawa A, Kainat A, Raza H, George TB, Omer H, Pillai AC. Fusobacterium bacteremia causing hepatic abscess in a patient with diverticulitis. Cureus. 2022;14(7):e26938. doi:10.7759/cureus.26938
12. Collins L, Diamond T. Fusobacterium nucleatum causing a pyogenic liver abscess: a rare complication of periodontal disease that occurred during the COVID-19 pandemic. BMJ Case Rep. 2021;14(1):e240080. doi:10.1136/bcr-2020-240080
13. Nohrstrom E, Mattila T, Pettila V, et al. Clinical spectrum of bacteraemic Fusobacterium infections: from septic shock to nosocomial bacteraemia. Scand J Infect Dis. 2011;43(6-7):463-470. doi:10.3109/00365548.2011.565071
Pyogenic hepatic abscess (PHA) is a collection of pus in the liver caused by bacterial infection of the liver parenchyma. This potentially life-threatening condition has a mortality rate reported to be as high as 47%.1 The incidence of PHA is reported to be 2.3 per 100,000 individuals and is more common in immunosuppressed individuals and those with diabetes mellitus, cancer, and liver transplant.2,3 PHA infections are usually polymicrobial and most commonly include enteric organisms like Escherichia coli and Klebsiella pneumoniae.4
We present a rare cause of PHA with Fusobacterium nucleatum (F nucleatum) in an immunocompetent patient with poor oral health, history of diverticulitis, and recent COVID-19 infection whose only symptoms were chest pain and a 4-week history of fever and malaise.
Case Presentation
A 52-year-old man initially presented to the C.W. Bill Young Veterans Affairs Medical Center (CWBYVAMC) emergency department in Bay Pines, Florida, for fever, malaise, and right-sided chest pain on inspiration. The fever and malaise began while he was on vacation 4 weeks prior. He originally presented to an outside hospital where he tested positive for COVID-19 and was recommended ibuprofen and rest. His symptoms did not improve, and he returned a second time to the outside hospital 2 weeks later and was diagnosed with pneumonia and placed on outpatient antibiotics. The patient subsequently returned to CWBYVAMC 2 weeks after starting antibiotics when he began to develop right-sided inspiratory chest pain. He reported no other recent travel and no abdominal pain. The patient’s history was significant for diverticulitis 2 years before. A colonoscopy was performed during that time and showed no masses.
On presentation, the patient was febrile with a temperature of 100.8 °F; otherwise, his vital signs were stable. Physical examinations, including abdominal, respiratory, and cardiovascular, were unremarkable. The initial laboratory workup revealed a white blood cell (WBC) count of 18.7 K/μL (reference range, 5-10 K/μL) and microcytic anemia with a hemoglobin level of 8.8 g/dL. The comprehensive metabolic panel revealed normal aspartate transaminase, alanine transaminase, and total bilirubin levels and elevated alkaline phosphatase of 215 U/L (reference range, 44-147 U/L), revealing possible mild intrahepatic cholestasis. Urinalysis showed trace proteinuria and urobilinogen. Coagulation studies showed elevated D-dimer and procalcitonin levels at 1.9 ng/mL (reference range, < 0.1 ng/mL) and 1.21 ng/mL (reference range, < 0.5 ng/mL), respectively, with normal prothrombin and partial thromboplastin times. The patient had a normal troponin, fecal, and blood culture; entamoeba serology was negative.
A computed tomograph (CT) angiography of the chest was performed to rule out pulmonary embolism, revealing liver lesions suspicious for abscess or metastatic disease. Minimal pleural effusion was detected bilaterally. A subsequent CT
Following the procedure, the patient developed shaking chills, hypertension, fever, and acute hypoxic respiratory failure. He improved with oxygen and was transferred to the intensive care unit (ICU) where he had an increase in temperature and became septic without shock. A repeat blood culture was negative. An echocardiogram revealed no vegetation. Vancomycin was added for empiric coverage of potentially resistant organisms. The patient clinically improved and was able to leave the ICU 2 days later on hospital day 4.
The patient’s renal function worsened on day 5, and piperacillin-tazobactam and vancomycin were discontinued due to possible acute interstitial nephritis and renal toxicity. He started cefepime and continued metronidazole, and his renal function returned to normal 2 days later. Vancomycin was then re-administered. The results of the culture taken from the abscess came back positive for monomicrobial growth of F nucleatum on hospital day 9.
Due to the patient’s persisting fever and WBC count, a repeat CT of the abdomen on hospital day 10 revealed a partial decrease in the abscess with a persistent collection superior to the location of the initial pigtail catheter placement. A second pigtail catheter was then placed near the dome of the liver 1 day later on hospital day 11. Following the procedure, the patient improved significantly. The repeat CT after 1 week showed marked overall resolution of the abscess, and the repeat culture of the abscess did not reveal any organism growth. Vancomycin was discontinued on day 19, and the drains were removed on hospital day 20. He was discharged home in stable condition on metronidazole and cefdinir for 21 days with follow-up appointments for CT of the abdomen and with primary care, infectious disease, and a dental specialist.
Discussion
F nucleatum is a gram-negative, nonmotile, spindle-shaped rod found in dental plaques.5 The incidence of F nucleatum bacteremia is 0.34 per 100,000 people and increases with age, with the median age being 53.5 years.6 Although our patient did not present with F nucleatum bacteremia, it is possible that bacteremia was present before hospitalization but resolved by the time the sample was drawn for culture. F nucleatum bacteremia can lead to a variety of presentations. The most common primary diagnoses are intra-abdominal infections (eg, PHA, respiratory tract infections, and hematological disorders).1,6
PHA Presentation
The most common presenting symptoms of PHA are fever (88%), abdominal pain (79%), and vomiting (50%).4 The patient’s presentation of inspiratory right-sided chest pain is likely due to irritation of the diaphragmatic pleura of the right lung secondary to the abscess formation. The patient did not experience abdominal pain throughout the course of this disease or on palpation of his right upper quadrant. To our knowledge, this is the only case of PHA in the literature of a patient with inspiratory chest pain without respiratory infection, abdominal pain, and cardiac abnormalities. There was no radiologic evidence or signs of hypoxia on admission to CWBYVAMC, which makes respiratory infection an unlikely cause of the chest pain. Moreover, the patient presented with new-onset chest pain 2 weeks after the diagnosis of pneumonia.
Common laboratory findings of PHA include transaminitis, leukocytosis, and bilirubinemia.4 Of note, increased procalcitonin has also been associated with PHA and extreme elevation (> 200 μg/L) may be a useful biomarker to identify F nucleatum infections before the presence of leukocytosis.3 CT of PHA usually reveals right lobe involvement, and F nucleatum infection usually demonstrates multiple abscesses.4,7
Contributing Factors in F nucleatum PHA
F nucleatum is associated with several oral diseases, such as periodontitis and gingivitis.8 It is important to do an oral inspection on patients with F nucleatum infections because it can spread from oral cavities to different body parts.
F nucleatum is also found in the gut.9 Any disease that can cause a break in the gastrointestinal mucosa may result in F nucleatum bacteremia and PHA. This may be why F nucleatum has been associated with a variety of different diseases, such as diverticulitis, inflammatory bowel disease, appendicitis, and colorectal cancer.10,11 Our patient had a history of diverticulosis with diverticulitis. Bawa and colleagues described a patient with recurrent diverticulitis who developed F nucleatum bacteremia and PHA.11 Our patient did not have any signs of diverticulitis.
Our patient’s COVID-19 infection also had a role in delaying the appropriate treatment of PHA. Without any symptoms of PHA, a diagnosis is difficult in a patient with a positive COVID-19 test, and treatment was delayed 1 month. Moreover, COVID-19 has been reported to delay the diagnosis of PHA even in the absence of a positive COVID-19 test. Collins and Diamond presented a patient during the COVID-19 pandemic who developed a periodontal abscess, which resulted in F nucleatum bacteremia and PHA due to delayed hospital presentation after the patient’s practitioners recommended self-isolation, despite a negative COVID-19 test.12 This highlights the impact that COVID-19 may have on the timely diagnosis and treatment of patients with PHA.
Malignancy has been associated with F nucleatum bacteremia.1,13 Possibly the association is due to gastrointestinal mucosa malignancy’s ability to cause micro-abrasions, resulting in F nucleatum bacteremia.10 Additionally, F nucleatum may promote the development of colorectal neoplasms.8 Due to this association, screening for colorectal cancer in patients with F nucleatum infection is important. In our patient, a colonoscopy was performed during the patient’s hospitalization for diverticulitis 2 years prior. No signs of colorectal neoplasm were noted
Conclusions
PHA due to F nucleatum is a rare but potentially life-threatening condition that must be diagnosed and treated promptly. It usually presents with fever, abdominal pain, and vomiting but can present with chest pain in the absence of a respiratory infection, cardiac abnormalities, and abdominal pain, as in our patient. A wide spectrum of infections can occur with F nucleatum, including PHA.
Suspicion for infection with this organism should be kept high in middle-aged and older individuals who present with an indolent disease course and have risk factors, such as poor oral health and comorbidities. Suspicion should be kept high even in the event of COVID-19 infection, especially in individuals with prolonged fever without other signs indicating respiratory infection. We believe that the most likely causes of this patient’s infection were his dental caries and periodontal disease. The timing of his symptoms is not consistent with his previous episode of diverticulitis. Due to the mortality of PHA, diagnosis and treatment must be prompt. Initial treatment with drainage and empiric anaerobic coverage is recommended, followed by a tailored antibiotic regiment if indicated by culture, and further drainage if suggested by imaging.
Pyogenic hepatic abscess (PHA) is a collection of pus in the liver caused by bacterial infection of the liver parenchyma. This potentially life-threatening condition has a mortality rate reported to be as high as 47%.1 The incidence of PHA is reported to be 2.3 per 100,000 individuals and is more common in immunosuppressed individuals and those with diabetes mellitus, cancer, and liver transplant.2,3 PHA infections are usually polymicrobial and most commonly include enteric organisms like Escherichia coli and Klebsiella pneumoniae.4
We present a rare cause of PHA with Fusobacterium nucleatum (F nucleatum) in an immunocompetent patient with poor oral health, history of diverticulitis, and recent COVID-19 infection whose only symptoms were chest pain and a 4-week history of fever and malaise.
Case Presentation
A 52-year-old man initially presented to the C.W. Bill Young Veterans Affairs Medical Center (CWBYVAMC) emergency department in Bay Pines, Florida, for fever, malaise, and right-sided chest pain on inspiration. The fever and malaise began while he was on vacation 4 weeks prior. He originally presented to an outside hospital where he tested positive for COVID-19 and was recommended ibuprofen and rest. His symptoms did not improve, and he returned a second time to the outside hospital 2 weeks later and was diagnosed with pneumonia and placed on outpatient antibiotics. The patient subsequently returned to CWBYVAMC 2 weeks after starting antibiotics when he began to develop right-sided inspiratory chest pain. He reported no other recent travel and no abdominal pain. The patient’s history was significant for diverticulitis 2 years before. A colonoscopy was performed during that time and showed no masses.
On presentation, the patient was febrile with a temperature of 100.8 °F; otherwise, his vital signs were stable. Physical examinations, including abdominal, respiratory, and cardiovascular, were unremarkable. The initial laboratory workup revealed a white blood cell (WBC) count of 18.7 K/μL (reference range, 5-10 K/μL) and microcytic anemia with a hemoglobin level of 8.8 g/dL. The comprehensive metabolic panel revealed normal aspartate transaminase, alanine transaminase, and total bilirubin levels and elevated alkaline phosphatase of 215 U/L (reference range, 44-147 U/L), revealing possible mild intrahepatic cholestasis. Urinalysis showed trace proteinuria and urobilinogen. Coagulation studies showed elevated D-dimer and procalcitonin levels at 1.9 ng/mL (reference range, < 0.1 ng/mL) and 1.21 ng/mL (reference range, < 0.5 ng/mL), respectively, with normal prothrombin and partial thromboplastin times. The patient had a normal troponin, fecal, and blood culture; entamoeba serology was negative.
A computed tomograph (CT) angiography of the chest was performed to rule out pulmonary embolism, revealing liver lesions suspicious for abscess or metastatic disease. Minimal pleural effusion was detected bilaterally. A subsequent CT
Following the procedure, the patient developed shaking chills, hypertension, fever, and acute hypoxic respiratory failure. He improved with oxygen and was transferred to the intensive care unit (ICU) where he had an increase in temperature and became septic without shock. A repeat blood culture was negative. An echocardiogram revealed no vegetation. Vancomycin was added for empiric coverage of potentially resistant organisms. The patient clinically improved and was able to leave the ICU 2 days later on hospital day 4.
The patient’s renal function worsened on day 5, and piperacillin-tazobactam and vancomycin were discontinued due to possible acute interstitial nephritis and renal toxicity. He started cefepime and continued metronidazole, and his renal function returned to normal 2 days later. Vancomycin was then re-administered. The results of the culture taken from the abscess came back positive for monomicrobial growth of F nucleatum on hospital day 9.
Due to the patient’s persisting fever and WBC count, a repeat CT of the abdomen on hospital day 10 revealed a partial decrease in the abscess with a persistent collection superior to the location of the initial pigtail catheter placement. A second pigtail catheter was then placed near the dome of the liver 1 day later on hospital day 11. Following the procedure, the patient improved significantly. The repeat CT after 1 week showed marked overall resolution of the abscess, and the repeat culture of the abscess did not reveal any organism growth. Vancomycin was discontinued on day 19, and the drains were removed on hospital day 20. He was discharged home in stable condition on metronidazole and cefdinir for 21 days with follow-up appointments for CT of the abdomen and with primary care, infectious disease, and a dental specialist.
Discussion
F nucleatum is a gram-negative, nonmotile, spindle-shaped rod found in dental plaques.5 The incidence of F nucleatum bacteremia is 0.34 per 100,000 people and increases with age, with the median age being 53.5 years.6 Although our patient did not present with F nucleatum bacteremia, it is possible that bacteremia was present before hospitalization but resolved by the time the sample was drawn for culture. F nucleatum bacteremia can lead to a variety of presentations. The most common primary diagnoses are intra-abdominal infections (eg, PHA, respiratory tract infections, and hematological disorders).1,6
PHA Presentation
The most common presenting symptoms of PHA are fever (88%), abdominal pain (79%), and vomiting (50%).4 The patient’s presentation of inspiratory right-sided chest pain is likely due to irritation of the diaphragmatic pleura of the right lung secondary to the abscess formation. The patient did not experience abdominal pain throughout the course of this disease or on palpation of his right upper quadrant. To our knowledge, this is the only case of PHA in the literature of a patient with inspiratory chest pain without respiratory infection, abdominal pain, and cardiac abnormalities. There was no radiologic evidence or signs of hypoxia on admission to CWBYVAMC, which makes respiratory infection an unlikely cause of the chest pain. Moreover, the patient presented with new-onset chest pain 2 weeks after the diagnosis of pneumonia.
Common laboratory findings of PHA include transaminitis, leukocytosis, and bilirubinemia.4 Of note, increased procalcitonin has also been associated with PHA and extreme elevation (> 200 μg/L) may be a useful biomarker to identify F nucleatum infections before the presence of leukocytosis.3 CT of PHA usually reveals right lobe involvement, and F nucleatum infection usually demonstrates multiple abscesses.4,7
Contributing Factors in F nucleatum PHA
F nucleatum is associated with several oral diseases, such as periodontitis and gingivitis.8 It is important to do an oral inspection on patients with F nucleatum infections because it can spread from oral cavities to different body parts.
F nucleatum is also found in the gut.9 Any disease that can cause a break in the gastrointestinal mucosa may result in F nucleatum bacteremia and PHA. This may be why F nucleatum has been associated with a variety of different diseases, such as diverticulitis, inflammatory bowel disease, appendicitis, and colorectal cancer.10,11 Our patient had a history of diverticulosis with diverticulitis. Bawa and colleagues described a patient with recurrent diverticulitis who developed F nucleatum bacteremia and PHA.11 Our patient did not have any signs of diverticulitis.
Our patient’s COVID-19 infection also had a role in delaying the appropriate treatment of PHA. Without any symptoms of PHA, a diagnosis is difficult in a patient with a positive COVID-19 test, and treatment was delayed 1 month. Moreover, COVID-19 has been reported to delay the diagnosis of PHA even in the absence of a positive COVID-19 test. Collins and Diamond presented a patient during the COVID-19 pandemic who developed a periodontal abscess, which resulted in F nucleatum bacteremia and PHA due to delayed hospital presentation after the patient’s practitioners recommended self-isolation, despite a negative COVID-19 test.12 This highlights the impact that COVID-19 may have on the timely diagnosis and treatment of patients with PHA.
Malignancy has been associated with F nucleatum bacteremia.1,13 Possibly the association is due to gastrointestinal mucosa malignancy’s ability to cause micro-abrasions, resulting in F nucleatum bacteremia.10 Additionally, F nucleatum may promote the development of colorectal neoplasms.8 Due to this association, screening for colorectal cancer in patients with F nucleatum infection is important. In our patient, a colonoscopy was performed during the patient’s hospitalization for diverticulitis 2 years prior. No signs of colorectal neoplasm were noted
Conclusions
PHA due to F nucleatum is a rare but potentially life-threatening condition that must be diagnosed and treated promptly. It usually presents with fever, abdominal pain, and vomiting but can present with chest pain in the absence of a respiratory infection, cardiac abnormalities, and abdominal pain, as in our patient. A wide spectrum of infections can occur with F nucleatum, including PHA.
Suspicion for infection with this organism should be kept high in middle-aged and older individuals who present with an indolent disease course and have risk factors, such as poor oral health and comorbidities. Suspicion should be kept high even in the event of COVID-19 infection, especially in individuals with prolonged fever without other signs indicating respiratory infection. We believe that the most likely causes of this patient’s infection were his dental caries and periodontal disease. The timing of his symptoms is not consistent with his previous episode of diverticulitis. Due to the mortality of PHA, diagnosis and treatment must be prompt. Initial treatment with drainage and empiric anaerobic coverage is recommended, followed by a tailored antibiotic regiment if indicated by culture, and further drainage if suggested by imaging.
1. Yang CC, Ye JJ, Hsu PC, et al. Characteristics and outcomes of Fusobacterium nucleatum bacteremia—a 6-year experience at a tertiary care hospital in northern Taiwan. Diagn Microbiol Infect Dis. 2011;70(2):167-174. doi:10.1016/j.diagmicrobio.2010.12.017
2. Kaplan GG, Gregson DB, Laupland KB. Population-based study of the epidemiology of and the risk factors for pyogenic liver abscess. Clin Gastroenterol Hepatol. 2004;2(11):1032-1038. doi:10.1016/s1542-3565(04)00459-8
3. Cao SA, Hinchey S. Identification and management of fusobacterium nucleatum liver abscess and bacteremia in a young healthy man. Cureus. 2020;12(12):e12303. doi:10.7759/cureus.12303
4. Abbas MT, Khan FY, Muhsin SA, Al-Dehwe B, Abukamar M, Elzouki AN. Epidemiology, clinical features and outcome of liver abscess: a single reference center experience in Qatar. Oman Med J. 2014;29(4):260-263. doi:10.5001/omj.2014.69
5. Bolstad AI, Jensen HB, Bakken V. Taxonomy, biology, and periodontal aspects of Fusobacterium nucleatum. Clin Microbiol Rev. 1996;9(1):55-71. doi:10.1128/CMR.9.1.55
6. Afra K, Laupland K, Leal J, Lloyd T, Gregson D. Incidence, risk factors, and outcomes of Fusobacterium species bacteremia. BMC Infect Dis. 2013;13:264. doi:10.1186/1471-2334-13-264
7. Crippin JS, Wang KK. An unrecognized etiology for pyogenic hepatic abscesses in normal hosts: dental disease. Am J Gastroenterol. 1992;87(12):1740-1743.
8. Shang FM, Liu HL. Fusobacterium nucleatum and colorectal cancer: a review. World J Gastrointest Oncol. 2018;10(3):71-81. doi:10.4251/wjgo.v10.i3.71
9. Allen-Vercoe E, Strauss J, Chadee K. Fusobacterium nucleatum: an emerging gut pathogen? Gut Microbes. 2011;2(5):294-298. doi:10.4161/gmic.2.5.18603
10. Han YW. Fusobacterium nucleatum: a commensal-turned pathogen. Curr Opin Microbiol. 2015;23:141-147. doi:10.1016/j.mib.2014.11.013
11. Bawa A, Kainat A, Raza H, George TB, Omer H, Pillai AC. Fusobacterium bacteremia causing hepatic abscess in a patient with diverticulitis. Cureus. 2022;14(7):e26938. doi:10.7759/cureus.26938
12. Collins L, Diamond T. Fusobacterium nucleatum causing a pyogenic liver abscess: a rare complication of periodontal disease that occurred during the COVID-19 pandemic. BMJ Case Rep. 2021;14(1):e240080. doi:10.1136/bcr-2020-240080
13. Nohrstrom E, Mattila T, Pettila V, et al. Clinical spectrum of bacteraemic Fusobacterium infections: from septic shock to nosocomial bacteraemia. Scand J Infect Dis. 2011;43(6-7):463-470. doi:10.3109/00365548.2011.565071
1. Yang CC, Ye JJ, Hsu PC, et al. Characteristics and outcomes of Fusobacterium nucleatum bacteremia—a 6-year experience at a tertiary care hospital in northern Taiwan. Diagn Microbiol Infect Dis. 2011;70(2):167-174. doi:10.1016/j.diagmicrobio.2010.12.017
2. Kaplan GG, Gregson DB, Laupland KB. Population-based study of the epidemiology of and the risk factors for pyogenic liver abscess. Clin Gastroenterol Hepatol. 2004;2(11):1032-1038. doi:10.1016/s1542-3565(04)00459-8
3. Cao SA, Hinchey S. Identification and management of fusobacterium nucleatum liver abscess and bacteremia in a young healthy man. Cureus. 2020;12(12):e12303. doi:10.7759/cureus.12303
4. Abbas MT, Khan FY, Muhsin SA, Al-Dehwe B, Abukamar M, Elzouki AN. Epidemiology, clinical features and outcome of liver abscess: a single reference center experience in Qatar. Oman Med J. 2014;29(4):260-263. doi:10.5001/omj.2014.69
5. Bolstad AI, Jensen HB, Bakken V. Taxonomy, biology, and periodontal aspects of Fusobacterium nucleatum. Clin Microbiol Rev. 1996;9(1):55-71. doi:10.1128/CMR.9.1.55
6. Afra K, Laupland K, Leal J, Lloyd T, Gregson D. Incidence, risk factors, and outcomes of Fusobacterium species bacteremia. BMC Infect Dis. 2013;13:264. doi:10.1186/1471-2334-13-264
7. Crippin JS, Wang KK. An unrecognized etiology for pyogenic hepatic abscesses in normal hosts: dental disease. Am J Gastroenterol. 1992;87(12):1740-1743.
8. Shang FM, Liu HL. Fusobacterium nucleatum and colorectal cancer: a review. World J Gastrointest Oncol. 2018;10(3):71-81. doi:10.4251/wjgo.v10.i3.71
9. Allen-Vercoe E, Strauss J, Chadee K. Fusobacterium nucleatum: an emerging gut pathogen? Gut Microbes. 2011;2(5):294-298. doi:10.4161/gmic.2.5.18603
10. Han YW. Fusobacterium nucleatum: a commensal-turned pathogen. Curr Opin Microbiol. 2015;23:141-147. doi:10.1016/j.mib.2014.11.013
11. Bawa A, Kainat A, Raza H, George TB, Omer H, Pillai AC. Fusobacterium bacteremia causing hepatic abscess in a patient with diverticulitis. Cureus. 2022;14(7):e26938. doi:10.7759/cureus.26938
12. Collins L, Diamond T. Fusobacterium nucleatum causing a pyogenic liver abscess: a rare complication of periodontal disease that occurred during the COVID-19 pandemic. BMJ Case Rep. 2021;14(1):e240080. doi:10.1136/bcr-2020-240080
13. Nohrstrom E, Mattila T, Pettila V, et al. Clinical spectrum of bacteraemic Fusobacterium infections: from septic shock to nosocomial bacteraemia. Scand J Infect Dis. 2011;43(6-7):463-470. doi:10.3109/00365548.2011.565071
Anxiety, your brain, and long COVID: What the research says
Having anxiety and depression before a COVID infection increases the risk of developing long COVID, researchers have found.
Those with long COVID who develop anxiety and depression after an infection may have brain shrinkage in areas that regulate memory, emotion, and other functions as well as disruption of brain connectivity.
While many questions remain about these intertwined relationships, the associations aren’t a complete surprise. Experts already know that depression and anxiety are associated with inflammation and immune dysfunction, perhaps helping to explain the link between these mental health conditions, the risk of long COVID, and the changes in the brain.
Brain changes accompanying a COVID infection have concerned researchers since earlier in the pandemic, when U.K. Biobank researchers found brain atrophy, loss of grey matter, and decline in cognition in those infected with COVID, compared with those not infected.
Common conditions
The ramifications of the research linking anxiety, depression, and long COVID are far-reaching. According to the Centers for Disease Control and Prevention, 12.5% of U.S. adults have regular feelings of anxiety (as well as nervousness and worry), and the latest Gallup Poll found that nearly 18% of adults currently have or are being treated for depression.
As of May 8, 10% of infected adults in the United States have long COVID, according to the CDC, and among U.S. adults ever infected, 27% have reported long COVID. Long COVID has been defined by the CDC as symptoms such as fatigue, brain fog, and cough that persist longer than 4 weeks and by the World Health Organization as symptoms persisting for 3 months or more.
Here’s a roundup of what the research shows about mental health and long COVID risk – along with other research finding that paying attention to health habits may reduce that risk.
Pre-existing depression, anxiety, and long COVID risk
A history of mental health issues – including depression, anxiety, worry, perceived stress, and loneliness – raises the risk of long COVID if infection occurs, Harvard researchers have found.
The researchers evaluated data from three large, ongoing studies including nearly 55,000 participants to determine the effects of high levels of psychological distress before a COVID infection.
“Our study was purely survey based,” said Siwen Wang, MD, the study’s lead author and a research fellow at Harvard School of Public Health, Boston.
At the start of the survey in April 2020, none of the participants reported a current or previous COVID infection. They answered surveys about psychological distress at the start of the study, at 6 monthly time points, then quarterly until November 2021.
Over the follow up, 3,193 people reported a positive COVID test and 43% of those, or 1,403, developed long COVID. That number may seem high, but 38% of the 55,000 were active health care workers. On the final questionnaire, they reported whether their symptoms persisted for 4 weeks or longer and thus had long COVID by the standard CDC definition.
Dr. Wang’s team then looked at the infected participants’ psychological status. Anxiety raised the risk of long COVID by 42%, depression by 32%, worry about COVID by 37%, perceived stress, 46%, and loneliness, 32%.
COVID patients with a history of depression or anxiety are also more likely than others to report trouble with cognition in the weeks after a COVID infection and to develop brain fog and long COVID, UCLA researchers found. They evaluated 766 people with a confirmed COVID infection; 36% said their thinking was affected within 4 weeks of the infection. Those with anxiety and depression were more likely to report those difficulties.
Long COVID, then anxiety, depression, brain changes
Even mild cases of COVID infection can lead to long COVID and brain changes in those who suffer anxiety or depression after the infection, according to Clarissa Yasuda, MD, PhD, assistant professor of neurology at the University of Campinas in Sao Paulo. She has researched long COVID’s effects on the brain, even as she is coping with being a long COVID patient.
In one of her studies, presented at the 2023 annual meeting of the American Academy of Neurology, she found brain changes in people with anxiety, depression, and COVID but not in those infected who did not have either mental health issue. She evaluated 254 people, median age 41, after about 82 days from their positive PCR test for COVID. Everyone completed a standard questionnaire for depression (the Beck Depression Inventory) and another for anxiety (the Beck Anxiety Inventory). She further divided them into two groups – the 102 with symptoms and the 152 who had no symptoms of either depression or anxiety.
Brain scans showed those with COVID who also had anxiety and depression had shrinkage in the limbic area of the brain (which helps process emotion and memory), while those infected who didn’t have anxiety or depression did not. The researchers then scanned the brains of 148 healthy people without COVID and found no shrinkage.
The atrophy, Dr. Yasuda said, “is not something you can see with your eyes. It was only detected with computer analysis. Visualization on an MRI is normal.”
The number of people in this study with mental health issues was surprisingly high, Dr. Yasuda said. “It was intriguing for us that we noticed many individuals have both symptoms, anxiety and depression. We were not expecting it at that proportion.”
The researchers found a pattern of change not only in brain structure but in brain communication. They found those changes by using specialized software to analyze brain networks in some of the participants. Those with anxiety and depression had widespread functional changes in each of 12 networks tested. The participants without mental health symptoms showed changes in just five networks. These changes are enough to lead to problems with thinking skills and memory, Dr. Yasuda said.
Explaining the links
Several ideas have been proposed to explain the link between psychological distress and long COVID risk, Dr. Wang said. “The first and most mainstream mechanism for long COVID is chronic inflammation and immune dysregulation. Several mental health conditions, such as anxiety and depression, are associated with inflammation and dysfunction and that might be the link between depression, anxiety, and long COVID.”
Another less mainstream hypothesis, she said, is that “those with long COVID have more autoantibodies and they are more likely to have blood clotting issues. These have also been found in people with anxiety, depression, or other psychological distress.”
Other researchers are looking more broadly at how COVID infections affect the brain. When German researchers evaluated the brain and other body parts of 20 patients who died from non-COVID causes but had documented COVID infections, they found that 12 had accumulations of the SARS-CoV-2 spike protein in the brain tissue as well as the skull and meninges, the membranes that line the skull and spinal cord. Healthy controls did not.
The findings suggest the persistence of the spike protein may contribute to the long-term neurologic symptoms of long COVID and may also lead to understanding of the molecular mechanisms as well as therapies for long COVID, the researchers said in their preprint report, which has not yet been peer reviewed.
In another recent study, researchers from Germany performed neuroimaging and neuropsychological assessments of 223 people who were not vaccinated and recovered from mild to moderate COVID infections, comparing them with 223 matched healthy controls who had the same testing. In those infected, they found alterations in the cerebral white matter but no worse cognitive function in the first year after recovering. They conclude that the infection triggers a prolonged neuroinflammatory response.
Can the brain changes reverse? “We don’t have an answer right now, but we are working on that,” Dr. Yasuda said. For now, she speculates about the return of brain volume: “I think for most it will. But I think we need to treat the symptoms. We can’t disregard the symptoms of long COVID. People are suffering a lot, and this suffering is causing some brain damage.”
Lifestyle habits and risk of long COVID
Meanwhile, healthy lifestyle habits in those infected can reduce the risk of long COVID, research by Dr. Wang and colleagues found. They followed nearly 2,000 women with a positive COVID test over 19 months. Of these, 44%, or 871, developed long COVID. Compared with women who followed none of the healthy lifestyle habits evaluated, those with five to six of the habits had a 49% lower risk of long COVID.
The habits included: a healthy body mass index (18.5-24.9 kg/m2), never smoking, at least 150 minutes weekly of moderate to vigorous physical activity, moderate alcohol intake (5-15 grams a day), high diet quality, and good sleep (7-9 hours nightly).
Long-term solutions
Dr. Yasuda hopes that mental health care – of those infected and those not – will be taken more seriously. In a commentary on her own long COVID experience, she wrote, in part: “I fear for the numerous survivors of COVID-19 who do not have access to medical attention for their post-COVID symptoms. ... The mental health system needs to become prepared to receive survivors with different neuropsychiatric symptoms, including anxiety and depression.”
A version of this article originally appeared on Medscape.com.
Having anxiety and depression before a COVID infection increases the risk of developing long COVID, researchers have found.
Those with long COVID who develop anxiety and depression after an infection may have brain shrinkage in areas that regulate memory, emotion, and other functions as well as disruption of brain connectivity.
While many questions remain about these intertwined relationships, the associations aren’t a complete surprise. Experts already know that depression and anxiety are associated with inflammation and immune dysfunction, perhaps helping to explain the link between these mental health conditions, the risk of long COVID, and the changes in the brain.
Brain changes accompanying a COVID infection have concerned researchers since earlier in the pandemic, when U.K. Biobank researchers found brain atrophy, loss of grey matter, and decline in cognition in those infected with COVID, compared with those not infected.
Common conditions
The ramifications of the research linking anxiety, depression, and long COVID are far-reaching. According to the Centers for Disease Control and Prevention, 12.5% of U.S. adults have regular feelings of anxiety (as well as nervousness and worry), and the latest Gallup Poll found that nearly 18% of adults currently have or are being treated for depression.
As of May 8, 10% of infected adults in the United States have long COVID, according to the CDC, and among U.S. adults ever infected, 27% have reported long COVID. Long COVID has been defined by the CDC as symptoms such as fatigue, brain fog, and cough that persist longer than 4 weeks and by the World Health Organization as symptoms persisting for 3 months or more.
Here’s a roundup of what the research shows about mental health and long COVID risk – along with other research finding that paying attention to health habits may reduce that risk.
Pre-existing depression, anxiety, and long COVID risk
A history of mental health issues – including depression, anxiety, worry, perceived stress, and loneliness – raises the risk of long COVID if infection occurs, Harvard researchers have found.
The researchers evaluated data from three large, ongoing studies including nearly 55,000 participants to determine the effects of high levels of psychological distress before a COVID infection.
“Our study was purely survey based,” said Siwen Wang, MD, the study’s lead author and a research fellow at Harvard School of Public Health, Boston.
At the start of the survey in April 2020, none of the participants reported a current or previous COVID infection. They answered surveys about psychological distress at the start of the study, at 6 monthly time points, then quarterly until November 2021.
Over the follow up, 3,193 people reported a positive COVID test and 43% of those, or 1,403, developed long COVID. That number may seem high, but 38% of the 55,000 were active health care workers. On the final questionnaire, they reported whether their symptoms persisted for 4 weeks or longer and thus had long COVID by the standard CDC definition.
Dr. Wang’s team then looked at the infected participants’ psychological status. Anxiety raised the risk of long COVID by 42%, depression by 32%, worry about COVID by 37%, perceived stress, 46%, and loneliness, 32%.
COVID patients with a history of depression or anxiety are also more likely than others to report trouble with cognition in the weeks after a COVID infection and to develop brain fog and long COVID, UCLA researchers found. They evaluated 766 people with a confirmed COVID infection; 36% said their thinking was affected within 4 weeks of the infection. Those with anxiety and depression were more likely to report those difficulties.
Long COVID, then anxiety, depression, brain changes
Even mild cases of COVID infection can lead to long COVID and brain changes in those who suffer anxiety or depression after the infection, according to Clarissa Yasuda, MD, PhD, assistant professor of neurology at the University of Campinas in Sao Paulo. She has researched long COVID’s effects on the brain, even as she is coping with being a long COVID patient.
In one of her studies, presented at the 2023 annual meeting of the American Academy of Neurology, she found brain changes in people with anxiety, depression, and COVID but not in those infected who did not have either mental health issue. She evaluated 254 people, median age 41, after about 82 days from their positive PCR test for COVID. Everyone completed a standard questionnaire for depression (the Beck Depression Inventory) and another for anxiety (the Beck Anxiety Inventory). She further divided them into two groups – the 102 with symptoms and the 152 who had no symptoms of either depression or anxiety.
Brain scans showed those with COVID who also had anxiety and depression had shrinkage in the limbic area of the brain (which helps process emotion and memory), while those infected who didn’t have anxiety or depression did not. The researchers then scanned the brains of 148 healthy people without COVID and found no shrinkage.
The atrophy, Dr. Yasuda said, “is not something you can see with your eyes. It was only detected with computer analysis. Visualization on an MRI is normal.”
The number of people in this study with mental health issues was surprisingly high, Dr. Yasuda said. “It was intriguing for us that we noticed many individuals have both symptoms, anxiety and depression. We were not expecting it at that proportion.”
The researchers found a pattern of change not only in brain structure but in brain communication. They found those changes by using specialized software to analyze brain networks in some of the participants. Those with anxiety and depression had widespread functional changes in each of 12 networks tested. The participants without mental health symptoms showed changes in just five networks. These changes are enough to lead to problems with thinking skills and memory, Dr. Yasuda said.
Explaining the links
Several ideas have been proposed to explain the link between psychological distress and long COVID risk, Dr. Wang said. “The first and most mainstream mechanism for long COVID is chronic inflammation and immune dysregulation. Several mental health conditions, such as anxiety and depression, are associated with inflammation and dysfunction and that might be the link between depression, anxiety, and long COVID.”
Another less mainstream hypothesis, she said, is that “those with long COVID have more autoantibodies and they are more likely to have blood clotting issues. These have also been found in people with anxiety, depression, or other psychological distress.”
Other researchers are looking more broadly at how COVID infections affect the brain. When German researchers evaluated the brain and other body parts of 20 patients who died from non-COVID causes but had documented COVID infections, they found that 12 had accumulations of the SARS-CoV-2 spike protein in the brain tissue as well as the skull and meninges, the membranes that line the skull and spinal cord. Healthy controls did not.
The findings suggest the persistence of the spike protein may contribute to the long-term neurologic symptoms of long COVID and may also lead to understanding of the molecular mechanisms as well as therapies for long COVID, the researchers said in their preprint report, which has not yet been peer reviewed.
In another recent study, researchers from Germany performed neuroimaging and neuropsychological assessments of 223 people who were not vaccinated and recovered from mild to moderate COVID infections, comparing them with 223 matched healthy controls who had the same testing. In those infected, they found alterations in the cerebral white matter but no worse cognitive function in the first year after recovering. They conclude that the infection triggers a prolonged neuroinflammatory response.
Can the brain changes reverse? “We don’t have an answer right now, but we are working on that,” Dr. Yasuda said. For now, she speculates about the return of brain volume: “I think for most it will. But I think we need to treat the symptoms. We can’t disregard the symptoms of long COVID. People are suffering a lot, and this suffering is causing some brain damage.”
Lifestyle habits and risk of long COVID
Meanwhile, healthy lifestyle habits in those infected can reduce the risk of long COVID, research by Dr. Wang and colleagues found. They followed nearly 2,000 women with a positive COVID test over 19 months. Of these, 44%, or 871, developed long COVID. Compared with women who followed none of the healthy lifestyle habits evaluated, those with five to six of the habits had a 49% lower risk of long COVID.
The habits included: a healthy body mass index (18.5-24.9 kg/m2), never smoking, at least 150 minutes weekly of moderate to vigorous physical activity, moderate alcohol intake (5-15 grams a day), high diet quality, and good sleep (7-9 hours nightly).
Long-term solutions
Dr. Yasuda hopes that mental health care – of those infected and those not – will be taken more seriously. In a commentary on her own long COVID experience, she wrote, in part: “I fear for the numerous survivors of COVID-19 who do not have access to medical attention for their post-COVID symptoms. ... The mental health system needs to become prepared to receive survivors with different neuropsychiatric symptoms, including anxiety and depression.”
A version of this article originally appeared on Medscape.com.
Having anxiety and depression before a COVID infection increases the risk of developing long COVID, researchers have found.
Those with long COVID who develop anxiety and depression after an infection may have brain shrinkage in areas that regulate memory, emotion, and other functions as well as disruption of brain connectivity.
While many questions remain about these intertwined relationships, the associations aren’t a complete surprise. Experts already know that depression and anxiety are associated with inflammation and immune dysfunction, perhaps helping to explain the link between these mental health conditions, the risk of long COVID, and the changes in the brain.
Brain changes accompanying a COVID infection have concerned researchers since earlier in the pandemic, when U.K. Biobank researchers found brain atrophy, loss of grey matter, and decline in cognition in those infected with COVID, compared with those not infected.
Common conditions
The ramifications of the research linking anxiety, depression, and long COVID are far-reaching. According to the Centers for Disease Control and Prevention, 12.5% of U.S. adults have regular feelings of anxiety (as well as nervousness and worry), and the latest Gallup Poll found that nearly 18% of adults currently have or are being treated for depression.
As of May 8, 10% of infected adults in the United States have long COVID, according to the CDC, and among U.S. adults ever infected, 27% have reported long COVID. Long COVID has been defined by the CDC as symptoms such as fatigue, brain fog, and cough that persist longer than 4 weeks and by the World Health Organization as symptoms persisting for 3 months or more.
Here’s a roundup of what the research shows about mental health and long COVID risk – along with other research finding that paying attention to health habits may reduce that risk.
Pre-existing depression, anxiety, and long COVID risk
A history of mental health issues – including depression, anxiety, worry, perceived stress, and loneliness – raises the risk of long COVID if infection occurs, Harvard researchers have found.
The researchers evaluated data from three large, ongoing studies including nearly 55,000 participants to determine the effects of high levels of psychological distress before a COVID infection.
“Our study was purely survey based,” said Siwen Wang, MD, the study’s lead author and a research fellow at Harvard School of Public Health, Boston.
At the start of the survey in April 2020, none of the participants reported a current or previous COVID infection. They answered surveys about psychological distress at the start of the study, at 6 monthly time points, then quarterly until November 2021.
Over the follow up, 3,193 people reported a positive COVID test and 43% of those, or 1,403, developed long COVID. That number may seem high, but 38% of the 55,000 were active health care workers. On the final questionnaire, they reported whether their symptoms persisted for 4 weeks or longer and thus had long COVID by the standard CDC definition.
Dr. Wang’s team then looked at the infected participants’ psychological status. Anxiety raised the risk of long COVID by 42%, depression by 32%, worry about COVID by 37%, perceived stress, 46%, and loneliness, 32%.
COVID patients with a history of depression or anxiety are also more likely than others to report trouble with cognition in the weeks after a COVID infection and to develop brain fog and long COVID, UCLA researchers found. They evaluated 766 people with a confirmed COVID infection; 36% said their thinking was affected within 4 weeks of the infection. Those with anxiety and depression were more likely to report those difficulties.
Long COVID, then anxiety, depression, brain changes
Even mild cases of COVID infection can lead to long COVID and brain changes in those who suffer anxiety or depression after the infection, according to Clarissa Yasuda, MD, PhD, assistant professor of neurology at the University of Campinas in Sao Paulo. She has researched long COVID’s effects on the brain, even as she is coping with being a long COVID patient.
In one of her studies, presented at the 2023 annual meeting of the American Academy of Neurology, she found brain changes in people with anxiety, depression, and COVID but not in those infected who did not have either mental health issue. She evaluated 254 people, median age 41, after about 82 days from their positive PCR test for COVID. Everyone completed a standard questionnaire for depression (the Beck Depression Inventory) and another for anxiety (the Beck Anxiety Inventory). She further divided them into two groups – the 102 with symptoms and the 152 who had no symptoms of either depression or anxiety.
Brain scans showed those with COVID who also had anxiety and depression had shrinkage in the limbic area of the brain (which helps process emotion and memory), while those infected who didn’t have anxiety or depression did not. The researchers then scanned the brains of 148 healthy people without COVID and found no shrinkage.
The atrophy, Dr. Yasuda said, “is not something you can see with your eyes. It was only detected with computer analysis. Visualization on an MRI is normal.”
The number of people in this study with mental health issues was surprisingly high, Dr. Yasuda said. “It was intriguing for us that we noticed many individuals have both symptoms, anxiety and depression. We were not expecting it at that proportion.”
The researchers found a pattern of change not only in brain structure but in brain communication. They found those changes by using specialized software to analyze brain networks in some of the participants. Those with anxiety and depression had widespread functional changes in each of 12 networks tested. The participants without mental health symptoms showed changes in just five networks. These changes are enough to lead to problems with thinking skills and memory, Dr. Yasuda said.
Explaining the links
Several ideas have been proposed to explain the link between psychological distress and long COVID risk, Dr. Wang said. “The first and most mainstream mechanism for long COVID is chronic inflammation and immune dysregulation. Several mental health conditions, such as anxiety and depression, are associated with inflammation and dysfunction and that might be the link between depression, anxiety, and long COVID.”
Another less mainstream hypothesis, she said, is that “those with long COVID have more autoantibodies and they are more likely to have blood clotting issues. These have also been found in people with anxiety, depression, or other psychological distress.”
Other researchers are looking more broadly at how COVID infections affect the brain. When German researchers evaluated the brain and other body parts of 20 patients who died from non-COVID causes but had documented COVID infections, they found that 12 had accumulations of the SARS-CoV-2 spike protein in the brain tissue as well as the skull and meninges, the membranes that line the skull and spinal cord. Healthy controls did not.
The findings suggest the persistence of the spike protein may contribute to the long-term neurologic symptoms of long COVID and may also lead to understanding of the molecular mechanisms as well as therapies for long COVID, the researchers said in their preprint report, which has not yet been peer reviewed.
In another recent study, researchers from Germany performed neuroimaging and neuropsychological assessments of 223 people who were not vaccinated and recovered from mild to moderate COVID infections, comparing them with 223 matched healthy controls who had the same testing. In those infected, they found alterations in the cerebral white matter but no worse cognitive function in the first year after recovering. They conclude that the infection triggers a prolonged neuroinflammatory response.
Can the brain changes reverse? “We don’t have an answer right now, but we are working on that,” Dr. Yasuda said. For now, she speculates about the return of brain volume: “I think for most it will. But I think we need to treat the symptoms. We can’t disregard the symptoms of long COVID. People are suffering a lot, and this suffering is causing some brain damage.”
Lifestyle habits and risk of long COVID
Meanwhile, healthy lifestyle habits in those infected can reduce the risk of long COVID, research by Dr. Wang and colleagues found. They followed nearly 2,000 women with a positive COVID test over 19 months. Of these, 44%, or 871, developed long COVID. Compared with women who followed none of the healthy lifestyle habits evaluated, those with five to six of the habits had a 49% lower risk of long COVID.
The habits included: a healthy body mass index (18.5-24.9 kg/m2), never smoking, at least 150 minutes weekly of moderate to vigorous physical activity, moderate alcohol intake (5-15 grams a day), high diet quality, and good sleep (7-9 hours nightly).
Long-term solutions
Dr. Yasuda hopes that mental health care – of those infected and those not – will be taken more seriously. In a commentary on her own long COVID experience, she wrote, in part: “I fear for the numerous survivors of COVID-19 who do not have access to medical attention for their post-COVID symptoms. ... The mental health system needs to become prepared to receive survivors with different neuropsychiatric symptoms, including anxiety and depression.”
A version of this article originally appeared on Medscape.com.
COVID vaccines safe for young children, study finds
TOPLINE:
COVID-19 vaccines from Moderna and Pfizer-BioNTech are safe for children under age 5 years, according to findings from a study funded by the Centers for Disease Control and Prevention.
METHODOLOGY:
- Data came from the Vaccine Safety Datalink, which gathers information from eight health systems in the United States.
- Analyzed data from 135,005 doses given to children age 4 and younger who received the Pfizer-BioNTech , and 112,006 doses given to children aged 5 and younger who received the Moderna version.
- Assessed for 23 safety outcomes, including myocarditis, pericarditis, and seizures.
TAKEAWAY:
- One case of hemorrhagic stroke and one case of pulmonary embolism occurred after vaccination but these were linked to preexisting congenital abnormalities.
IN PRACTICE:
“These results can provide reassurance to clinicians, parents, and policymakers alike.”
STUDY DETAILS:
The study was led by Kristin Goddard, MPH, a researcher at the Kaiser Permanente Vaccine Study Center in Oakland, Calif., and was funded by the Centers for Disease Control and Prevention.
LIMITATIONS:
The researchers reported low statistical power for early analysis, especially for rare outcomes. In addition, fewer than 25% of children in the database had received a vaccine at the time of analysis.
DISCLOSURES:
A coauthor reported receiving funding from Janssen Vaccines and Prevention for a study unrelated to COVID-19 vaccines. Another coauthor reported receiving grants from Pfizer in 2019 for clinical trials for coronavirus vaccines, and from Merck, GSK, and Sanofi Pasteur for unrelated research.
A version of this article first appeared on Medscape.com.
TOPLINE:
COVID-19 vaccines from Moderna and Pfizer-BioNTech are safe for children under age 5 years, according to findings from a study funded by the Centers for Disease Control and Prevention.
METHODOLOGY:
- Data came from the Vaccine Safety Datalink, which gathers information from eight health systems in the United States.
- Analyzed data from 135,005 doses given to children age 4 and younger who received the Pfizer-BioNTech , and 112,006 doses given to children aged 5 and younger who received the Moderna version.
- Assessed for 23 safety outcomes, including myocarditis, pericarditis, and seizures.
TAKEAWAY:
- One case of hemorrhagic stroke and one case of pulmonary embolism occurred after vaccination but these were linked to preexisting congenital abnormalities.
IN PRACTICE:
“These results can provide reassurance to clinicians, parents, and policymakers alike.”
STUDY DETAILS:
The study was led by Kristin Goddard, MPH, a researcher at the Kaiser Permanente Vaccine Study Center in Oakland, Calif., and was funded by the Centers for Disease Control and Prevention.
LIMITATIONS:
The researchers reported low statistical power for early analysis, especially for rare outcomes. In addition, fewer than 25% of children in the database had received a vaccine at the time of analysis.
DISCLOSURES:
A coauthor reported receiving funding from Janssen Vaccines and Prevention for a study unrelated to COVID-19 vaccines. Another coauthor reported receiving grants from Pfizer in 2019 for clinical trials for coronavirus vaccines, and from Merck, GSK, and Sanofi Pasteur for unrelated research.
A version of this article first appeared on Medscape.com.
TOPLINE:
COVID-19 vaccines from Moderna and Pfizer-BioNTech are safe for children under age 5 years, according to findings from a study funded by the Centers for Disease Control and Prevention.
METHODOLOGY:
- Data came from the Vaccine Safety Datalink, which gathers information from eight health systems in the United States.
- Analyzed data from 135,005 doses given to children age 4 and younger who received the Pfizer-BioNTech , and 112,006 doses given to children aged 5 and younger who received the Moderna version.
- Assessed for 23 safety outcomes, including myocarditis, pericarditis, and seizures.
TAKEAWAY:
- One case of hemorrhagic stroke and one case of pulmonary embolism occurred after vaccination but these were linked to preexisting congenital abnormalities.
IN PRACTICE:
“These results can provide reassurance to clinicians, parents, and policymakers alike.”
STUDY DETAILS:
The study was led by Kristin Goddard, MPH, a researcher at the Kaiser Permanente Vaccine Study Center in Oakland, Calif., and was funded by the Centers for Disease Control and Prevention.
LIMITATIONS:
The researchers reported low statistical power for early analysis, especially for rare outcomes. In addition, fewer than 25% of children in the database had received a vaccine at the time of analysis.
DISCLOSURES:
A coauthor reported receiving funding from Janssen Vaccines and Prevention for a study unrelated to COVID-19 vaccines. Another coauthor reported receiving grants from Pfizer in 2019 for clinical trials for coronavirus vaccines, and from Merck, GSK, and Sanofi Pasteur for unrelated research.
A version of this article first appeared on Medscape.com.
FROM PEDIATRICS
We may need a new defense against new COVID variants
At the end of 2022, the European Medicines Agency’s Emergency Task Force warned European regulatory bodies, governments, and doctors that Antiviral drugs remain available but have many limitations. And, of course, there are still vaccines, which can significantly reduce (but not remove) the risk of severe cases and decrease the number of deaths, although they have lost the efficacy that they once had in countering the original virus.
Research therefore continues. Immunologists continue to search for new targets to synthesize broadly neutralizing monoclonal antibodies for treating or preventing the infection. These results could also lead to new vaccines that induce longer-lasting immunity not only against the thousands of subvariants and recombinant versions of SARS-CoV-2 being identified around the world, but also possibly against other coronaviruses that could emerge in the coming years. A study conducted at Stanford (Calif.) University and published in the journal Science Translational Medicine has afforded a glimmer of hope by discovering the broadly neutralizing efficacy of some antibodies produced by macaque monkeys in response to vaccination with AS03 (squalene) adjuvanted monovalent subunit vaccines.
The speed with which the virus continues to evolve has rendered the plan for annual vaccine updates, which initially was envisioned early in the pandemic, unfeasible for the time being. In 2020, scientists were considering updating vaccines annually based on the prevalent variants of the disease, similar to the approach to the flu. Perhaps that day will come, but in the meantime, laboratories are pursuing other routes: finding spike epitopes that are preserved more than others each time the virus evolves or focusing on other virus proteins that still manage to induce a neutralizing antibody response.
Eventually, artificial intelligence might be able to custom design monoclonal antibodies that are even more effective than natural ones. Or researchers could completely change tack and shift their attention to the host, rather than the virus itself.
This is the approach taken by one study published in Nature Microbiology, which starts from a simple assumption: SARS-CoV-2 continues to modify its spike protein because of the evolutionary pressure of the antibodies produced by millions of infected people, but all these variants and subvariants, both present and future, enter cells by binding – not solely, but mostly – to the ACE2 receptor. Instead of neutralizing the virus, why not try to block its access to the cells occupying its route in? In this way, we could also be ready for future emerging sarbecoviruses that will have a spike sequence that cannot yet be predicted.
Researchers at Rockefeller University, New York, have generated six human monoclonal antibodies that bind to the ACE2 receptor, rather than to the spike, preventing infection by all sarbecoviruses tested, even at low concentrations, including the virus that originated in Wuhan, China; the aggressive Delta variant; and various forms of Omicron.
The monoclonal antibodies bind to the ACE2 receptor at a part of the protein that is distal to the active enzyme portion that converts angiotensin and does not modify its expression on the cell surface. Therefore, no adverse effects are expected at this level. In animal models, these monoclonal antibodies succeed in stopping the infection. Moving into the clinical phase will be needed to find out if it will be possible to create products adapted to preventing and treating all SARS-CoV-2 variants, and perhaps also the next coronavirus large enough to spill over into a new epidemic that threatens the human race.
This article was translated from Univadis Italy. A version appeared on Medscape.com.
At the end of 2022, the European Medicines Agency’s Emergency Task Force warned European regulatory bodies, governments, and doctors that Antiviral drugs remain available but have many limitations. And, of course, there are still vaccines, which can significantly reduce (but not remove) the risk of severe cases and decrease the number of deaths, although they have lost the efficacy that they once had in countering the original virus.
Research therefore continues. Immunologists continue to search for new targets to synthesize broadly neutralizing monoclonal antibodies for treating or preventing the infection. These results could also lead to new vaccines that induce longer-lasting immunity not only against the thousands of subvariants and recombinant versions of SARS-CoV-2 being identified around the world, but also possibly against other coronaviruses that could emerge in the coming years. A study conducted at Stanford (Calif.) University and published in the journal Science Translational Medicine has afforded a glimmer of hope by discovering the broadly neutralizing efficacy of some antibodies produced by macaque monkeys in response to vaccination with AS03 (squalene) adjuvanted monovalent subunit vaccines.
The speed with which the virus continues to evolve has rendered the plan for annual vaccine updates, which initially was envisioned early in the pandemic, unfeasible for the time being. In 2020, scientists were considering updating vaccines annually based on the prevalent variants of the disease, similar to the approach to the flu. Perhaps that day will come, but in the meantime, laboratories are pursuing other routes: finding spike epitopes that are preserved more than others each time the virus evolves or focusing on other virus proteins that still manage to induce a neutralizing antibody response.
Eventually, artificial intelligence might be able to custom design monoclonal antibodies that are even more effective than natural ones. Or researchers could completely change tack and shift their attention to the host, rather than the virus itself.
This is the approach taken by one study published in Nature Microbiology, which starts from a simple assumption: SARS-CoV-2 continues to modify its spike protein because of the evolutionary pressure of the antibodies produced by millions of infected people, but all these variants and subvariants, both present and future, enter cells by binding – not solely, but mostly – to the ACE2 receptor. Instead of neutralizing the virus, why not try to block its access to the cells occupying its route in? In this way, we could also be ready for future emerging sarbecoviruses that will have a spike sequence that cannot yet be predicted.
Researchers at Rockefeller University, New York, have generated six human monoclonal antibodies that bind to the ACE2 receptor, rather than to the spike, preventing infection by all sarbecoviruses tested, even at low concentrations, including the virus that originated in Wuhan, China; the aggressive Delta variant; and various forms of Omicron.
The monoclonal antibodies bind to the ACE2 receptor at a part of the protein that is distal to the active enzyme portion that converts angiotensin and does not modify its expression on the cell surface. Therefore, no adverse effects are expected at this level. In animal models, these monoclonal antibodies succeed in stopping the infection. Moving into the clinical phase will be needed to find out if it will be possible to create products adapted to preventing and treating all SARS-CoV-2 variants, and perhaps also the next coronavirus large enough to spill over into a new epidemic that threatens the human race.
This article was translated from Univadis Italy. A version appeared on Medscape.com.
At the end of 2022, the European Medicines Agency’s Emergency Task Force warned European regulatory bodies, governments, and doctors that Antiviral drugs remain available but have many limitations. And, of course, there are still vaccines, which can significantly reduce (but not remove) the risk of severe cases and decrease the number of deaths, although they have lost the efficacy that they once had in countering the original virus.
Research therefore continues. Immunologists continue to search for new targets to synthesize broadly neutralizing monoclonal antibodies for treating or preventing the infection. These results could also lead to new vaccines that induce longer-lasting immunity not only against the thousands of subvariants and recombinant versions of SARS-CoV-2 being identified around the world, but also possibly against other coronaviruses that could emerge in the coming years. A study conducted at Stanford (Calif.) University and published in the journal Science Translational Medicine has afforded a glimmer of hope by discovering the broadly neutralizing efficacy of some antibodies produced by macaque monkeys in response to vaccination with AS03 (squalene) adjuvanted monovalent subunit vaccines.
The speed with which the virus continues to evolve has rendered the plan for annual vaccine updates, which initially was envisioned early in the pandemic, unfeasible for the time being. In 2020, scientists were considering updating vaccines annually based on the prevalent variants of the disease, similar to the approach to the flu. Perhaps that day will come, but in the meantime, laboratories are pursuing other routes: finding spike epitopes that are preserved more than others each time the virus evolves or focusing on other virus proteins that still manage to induce a neutralizing antibody response.
Eventually, artificial intelligence might be able to custom design monoclonal antibodies that are even more effective than natural ones. Or researchers could completely change tack and shift their attention to the host, rather than the virus itself.
This is the approach taken by one study published in Nature Microbiology, which starts from a simple assumption: SARS-CoV-2 continues to modify its spike protein because of the evolutionary pressure of the antibodies produced by millions of infected people, but all these variants and subvariants, both present and future, enter cells by binding – not solely, but mostly – to the ACE2 receptor. Instead of neutralizing the virus, why not try to block its access to the cells occupying its route in? In this way, we could also be ready for future emerging sarbecoviruses that will have a spike sequence that cannot yet be predicted.
Researchers at Rockefeller University, New York, have generated six human monoclonal antibodies that bind to the ACE2 receptor, rather than to the spike, preventing infection by all sarbecoviruses tested, even at low concentrations, including the virus that originated in Wuhan, China; the aggressive Delta variant; and various forms of Omicron.
The monoclonal antibodies bind to the ACE2 receptor at a part of the protein that is distal to the active enzyme portion that converts angiotensin and does not modify its expression on the cell surface. Therefore, no adverse effects are expected at this level. In animal models, these monoclonal antibodies succeed in stopping the infection. Moving into the clinical phase will be needed to find out if it will be possible to create products adapted to preventing and treating all SARS-CoV-2 variants, and perhaps also the next coronavirus large enough to spill over into a new epidemic that threatens the human race.
This article was translated from Univadis Italy. A version appeared on Medscape.com.
COVID nonvaccination linked with avoidable hospitalizations
A retrospective, population-based cohort study in Alberta, Edmonton, found that between late September 2021 and late January 2022, eligible unvaccinated patients with COVID-19 had a nearly 10-fold higher risk for hospitalization than did patients who were fully vaccinated with two doses. Unvaccinated patients had a nearly 21-fold higher risk than did patients who were boosted with three doses.
“We have shown that eligible nonvaccinated persons, especially in the age strata 50-79 years, accounted for 3,000-4,000 potentially avoidable hospitalizations, 35,000-40,000 avoidable bed-days, and $100–$110 million [Canadian dollars] in avoidable health care costs during a 120-day period coinciding with the fourth (Delta) and fifth (Omicron) COVID-19 waves, respectively,” wrote Sean M. Bagshaw, MD, chair of critical care medicine at the University of Alberta, Edmonton, and colleagues.
The findings were published in the Canadian Journal of Public Health.
‘Unsatisfactory’ vaccine uptake
While a previous study by Dr. Bagshaw and colleagues recently showed that higher vaccine uptake could have avoided significant intensive care unit admissions and costs, the researchers sought to expand their analysis to include non-ICU use.
The current study examined data from the government of Alberta and the Discharge Abstract Database to assess vaccination status and hospitalization with confirmed SARS-CoV-2. Secondary outcomes included avoidable hospitalizations, avoidable hospital bed-days, and the potential cost avoidance related to COVID-19.
During the study period, “societal factors contributed to an unsatisfactory voluntary vaccine uptake, particularly in the province of Alberta,” wrote the authors, adding that “only 63.7% and 2.7% of the eligible population in Alberta [had] received two (full) and three (boosted) COVID-19 vaccine doses as of September 27, 2021.”
The analysis found the highest number of hospitalizations among unvaccinated patients (n = 3,835), compared with vaccinated (n = 1,907) and boosted patients (n = 481). This finding yielded a risk ratio (RR) of hospitalization of 9.7 for unvaccinated patients, compared with fully vaccinated patients, and an RR of 20.6, compared with patients who were boosted. Unvaccinated patients aged 60-69 years had the highest RR for hospitalization, compared with vaccinated (RR, 16.4) and boosted patients (RR, 151.9).
The estimated number of avoidable hospitalizations for unvaccinated patients was 3,439 (total of 36,331 bed-days), compared with vaccinated patients, and 3,764 (total of 40,185 bed-days), compared with boosted patients.
The avoidable hospitalization-related costs for unvaccinated patients totaled $101.4 million (Canadian dollars) if they had been vaccinated and $110.24 million if they had been boosted.
“Moreover, strained hospital systems and the widespread adoption of crisis standards of care in response to surges in COVID-19 hospitalizations have contributed to unnecessary excess deaths,” wrote the authors. “These are preventable and missed public health opportunities that provoked massive health system disruptions and resource diversions, including deferral of routine health services (e.g., cancer and chronic disease screening and monitoring and scheduled vaccinations), postponement of scheduled procedures and surgeries, and redeployment of health care professionals.”
Dr. Bagshaw said in an interview that he was not surprised by the findings. “However, I wonder whether the public and those who direct policy and make decisions about the health system would be interested in better understanding the scope and sheer disruption the health system suffered due to COVID-19,” he said.
The current study suggests that “at least some of this could have been avoided,” said Dr. Bagshaw. “I hope we – that is the public, users of the health system, decision-makers and health care professionals – can learn from our experiences.” Studies such as the current analysis “will reinforce the importance of timely and clearly articulated public health promotion, education, and policy,” he added.
Economic benefit underestimated
Commenting on the study, David Fisman, MD, MPH, an epidemiologist and professor at the University of Toronto, said: “The approach these investigators have taken is clear and straightforward. It is easy to reproduce. It is also entirely consistent with what other scientific groups have been demonstrating for a couple of years now.” Dr. Fisman was not involved with the study.
A group led by Dr. Fisman as senior author has just completed a study examining the effectiveness of the Canadian pandemic response, compared with responses in four peer countries. In the as-yet unpublished paper, the researchers concluded that “relative to the United States, United Kingdom, and France, the Canadian pandemic response was estimated to have averted 94,492, 64,306, and 13,641 deaths, respectively, with more than 480,000 hospitalizations averted and 1 million QALY [quality-adjusted life-years] saved, relative to the United States. A United States pandemic response applied to Canada would have resulted in more than $40 billion in economic losses due to healthcare expenditures and lost QALY; losses relative to the United Kingdom and France would have been $21 billion and $5 billion, respectively. By contrast, an Australian pandemic response would have averted over 28,000 additional deaths and averted nearly $9 billion in costs in Canada.”
Dr. Fisman added that while the current researchers focused their study on the direct protective effects of vaccines, “we know that, even with initial waves of Omicron, vaccinated individuals continued to be protected against infection as well as disease, and even if they were infected, we know from household contact studies that they were less infectious to others. That means that even though the implicit estimate of cost savings that could have been achieved through better coverage are pretty high in this paper, the economic benefit of vaccination is underestimated in this analysis, because we can’t quantify the infections that never happened because of vaccination.”
The study was supported by the Strategic Clinical Networks, Alberta Health Services. Dr. Bagshaw declared no relevant financial relationships. Dr. Fisman has taken part in advisory boards for Seqirus, Pfizer, AstraZeneca, Sanofi, and Merck vaccines during the past 3 years.
A version of this article first appeared on Medscape.com.
A retrospective, population-based cohort study in Alberta, Edmonton, found that between late September 2021 and late January 2022, eligible unvaccinated patients with COVID-19 had a nearly 10-fold higher risk for hospitalization than did patients who were fully vaccinated with two doses. Unvaccinated patients had a nearly 21-fold higher risk than did patients who were boosted with three doses.
“We have shown that eligible nonvaccinated persons, especially in the age strata 50-79 years, accounted for 3,000-4,000 potentially avoidable hospitalizations, 35,000-40,000 avoidable bed-days, and $100–$110 million [Canadian dollars] in avoidable health care costs during a 120-day period coinciding with the fourth (Delta) and fifth (Omicron) COVID-19 waves, respectively,” wrote Sean M. Bagshaw, MD, chair of critical care medicine at the University of Alberta, Edmonton, and colleagues.
The findings were published in the Canadian Journal of Public Health.
‘Unsatisfactory’ vaccine uptake
While a previous study by Dr. Bagshaw and colleagues recently showed that higher vaccine uptake could have avoided significant intensive care unit admissions and costs, the researchers sought to expand their analysis to include non-ICU use.
The current study examined data from the government of Alberta and the Discharge Abstract Database to assess vaccination status and hospitalization with confirmed SARS-CoV-2. Secondary outcomes included avoidable hospitalizations, avoidable hospital bed-days, and the potential cost avoidance related to COVID-19.
During the study period, “societal factors contributed to an unsatisfactory voluntary vaccine uptake, particularly in the province of Alberta,” wrote the authors, adding that “only 63.7% and 2.7% of the eligible population in Alberta [had] received two (full) and three (boosted) COVID-19 vaccine doses as of September 27, 2021.”
The analysis found the highest number of hospitalizations among unvaccinated patients (n = 3,835), compared with vaccinated (n = 1,907) and boosted patients (n = 481). This finding yielded a risk ratio (RR) of hospitalization of 9.7 for unvaccinated patients, compared with fully vaccinated patients, and an RR of 20.6, compared with patients who were boosted. Unvaccinated patients aged 60-69 years had the highest RR for hospitalization, compared with vaccinated (RR, 16.4) and boosted patients (RR, 151.9).
The estimated number of avoidable hospitalizations for unvaccinated patients was 3,439 (total of 36,331 bed-days), compared with vaccinated patients, and 3,764 (total of 40,185 bed-days), compared with boosted patients.
The avoidable hospitalization-related costs for unvaccinated patients totaled $101.4 million (Canadian dollars) if they had been vaccinated and $110.24 million if they had been boosted.
“Moreover, strained hospital systems and the widespread adoption of crisis standards of care in response to surges in COVID-19 hospitalizations have contributed to unnecessary excess deaths,” wrote the authors. “These are preventable and missed public health opportunities that provoked massive health system disruptions and resource diversions, including deferral of routine health services (e.g., cancer and chronic disease screening and monitoring and scheduled vaccinations), postponement of scheduled procedures and surgeries, and redeployment of health care professionals.”
Dr. Bagshaw said in an interview that he was not surprised by the findings. “However, I wonder whether the public and those who direct policy and make decisions about the health system would be interested in better understanding the scope and sheer disruption the health system suffered due to COVID-19,” he said.
The current study suggests that “at least some of this could have been avoided,” said Dr. Bagshaw. “I hope we – that is the public, users of the health system, decision-makers and health care professionals – can learn from our experiences.” Studies such as the current analysis “will reinforce the importance of timely and clearly articulated public health promotion, education, and policy,” he added.
Economic benefit underestimated
Commenting on the study, David Fisman, MD, MPH, an epidemiologist and professor at the University of Toronto, said: “The approach these investigators have taken is clear and straightforward. It is easy to reproduce. It is also entirely consistent with what other scientific groups have been demonstrating for a couple of years now.” Dr. Fisman was not involved with the study.
A group led by Dr. Fisman as senior author has just completed a study examining the effectiveness of the Canadian pandemic response, compared with responses in four peer countries. In the as-yet unpublished paper, the researchers concluded that “relative to the United States, United Kingdom, and France, the Canadian pandemic response was estimated to have averted 94,492, 64,306, and 13,641 deaths, respectively, with more than 480,000 hospitalizations averted and 1 million QALY [quality-adjusted life-years] saved, relative to the United States. A United States pandemic response applied to Canada would have resulted in more than $40 billion in economic losses due to healthcare expenditures and lost QALY; losses relative to the United Kingdom and France would have been $21 billion and $5 billion, respectively. By contrast, an Australian pandemic response would have averted over 28,000 additional deaths and averted nearly $9 billion in costs in Canada.”
Dr. Fisman added that while the current researchers focused their study on the direct protective effects of vaccines, “we know that, even with initial waves of Omicron, vaccinated individuals continued to be protected against infection as well as disease, and even if they were infected, we know from household contact studies that they were less infectious to others. That means that even though the implicit estimate of cost savings that could have been achieved through better coverage are pretty high in this paper, the economic benefit of vaccination is underestimated in this analysis, because we can’t quantify the infections that never happened because of vaccination.”
The study was supported by the Strategic Clinical Networks, Alberta Health Services. Dr. Bagshaw declared no relevant financial relationships. Dr. Fisman has taken part in advisory boards for Seqirus, Pfizer, AstraZeneca, Sanofi, and Merck vaccines during the past 3 years.
A version of this article first appeared on Medscape.com.
A retrospective, population-based cohort study in Alberta, Edmonton, found that between late September 2021 and late January 2022, eligible unvaccinated patients with COVID-19 had a nearly 10-fold higher risk for hospitalization than did patients who were fully vaccinated with two doses. Unvaccinated patients had a nearly 21-fold higher risk than did patients who were boosted with three doses.
“We have shown that eligible nonvaccinated persons, especially in the age strata 50-79 years, accounted for 3,000-4,000 potentially avoidable hospitalizations, 35,000-40,000 avoidable bed-days, and $100–$110 million [Canadian dollars] in avoidable health care costs during a 120-day period coinciding with the fourth (Delta) and fifth (Omicron) COVID-19 waves, respectively,” wrote Sean M. Bagshaw, MD, chair of critical care medicine at the University of Alberta, Edmonton, and colleagues.
The findings were published in the Canadian Journal of Public Health.
‘Unsatisfactory’ vaccine uptake
While a previous study by Dr. Bagshaw and colleagues recently showed that higher vaccine uptake could have avoided significant intensive care unit admissions and costs, the researchers sought to expand their analysis to include non-ICU use.
The current study examined data from the government of Alberta and the Discharge Abstract Database to assess vaccination status and hospitalization with confirmed SARS-CoV-2. Secondary outcomes included avoidable hospitalizations, avoidable hospital bed-days, and the potential cost avoidance related to COVID-19.
During the study period, “societal factors contributed to an unsatisfactory voluntary vaccine uptake, particularly in the province of Alberta,” wrote the authors, adding that “only 63.7% and 2.7% of the eligible population in Alberta [had] received two (full) and three (boosted) COVID-19 vaccine doses as of September 27, 2021.”
The analysis found the highest number of hospitalizations among unvaccinated patients (n = 3,835), compared with vaccinated (n = 1,907) and boosted patients (n = 481). This finding yielded a risk ratio (RR) of hospitalization of 9.7 for unvaccinated patients, compared with fully vaccinated patients, and an RR of 20.6, compared with patients who were boosted. Unvaccinated patients aged 60-69 years had the highest RR for hospitalization, compared with vaccinated (RR, 16.4) and boosted patients (RR, 151.9).
The estimated number of avoidable hospitalizations for unvaccinated patients was 3,439 (total of 36,331 bed-days), compared with vaccinated patients, and 3,764 (total of 40,185 bed-days), compared with boosted patients.
The avoidable hospitalization-related costs for unvaccinated patients totaled $101.4 million (Canadian dollars) if they had been vaccinated and $110.24 million if they had been boosted.
“Moreover, strained hospital systems and the widespread adoption of crisis standards of care in response to surges in COVID-19 hospitalizations have contributed to unnecessary excess deaths,” wrote the authors. “These are preventable and missed public health opportunities that provoked massive health system disruptions and resource diversions, including deferral of routine health services (e.g., cancer and chronic disease screening and monitoring and scheduled vaccinations), postponement of scheduled procedures and surgeries, and redeployment of health care professionals.”
Dr. Bagshaw said in an interview that he was not surprised by the findings. “However, I wonder whether the public and those who direct policy and make decisions about the health system would be interested in better understanding the scope and sheer disruption the health system suffered due to COVID-19,” he said.
The current study suggests that “at least some of this could have been avoided,” said Dr. Bagshaw. “I hope we – that is the public, users of the health system, decision-makers and health care professionals – can learn from our experiences.” Studies such as the current analysis “will reinforce the importance of timely and clearly articulated public health promotion, education, and policy,” he added.
Economic benefit underestimated
Commenting on the study, David Fisman, MD, MPH, an epidemiologist and professor at the University of Toronto, said: “The approach these investigators have taken is clear and straightforward. It is easy to reproduce. It is also entirely consistent with what other scientific groups have been demonstrating for a couple of years now.” Dr. Fisman was not involved with the study.
A group led by Dr. Fisman as senior author has just completed a study examining the effectiveness of the Canadian pandemic response, compared with responses in four peer countries. In the as-yet unpublished paper, the researchers concluded that “relative to the United States, United Kingdom, and France, the Canadian pandemic response was estimated to have averted 94,492, 64,306, and 13,641 deaths, respectively, with more than 480,000 hospitalizations averted and 1 million QALY [quality-adjusted life-years] saved, relative to the United States. A United States pandemic response applied to Canada would have resulted in more than $40 billion in economic losses due to healthcare expenditures and lost QALY; losses relative to the United Kingdom and France would have been $21 billion and $5 billion, respectively. By contrast, an Australian pandemic response would have averted over 28,000 additional deaths and averted nearly $9 billion in costs in Canada.”
Dr. Fisman added that while the current researchers focused their study on the direct protective effects of vaccines, “we know that, even with initial waves of Omicron, vaccinated individuals continued to be protected against infection as well as disease, and even if they were infected, we know from household contact studies that they were less infectious to others. That means that even though the implicit estimate of cost savings that could have been achieved through better coverage are pretty high in this paper, the economic benefit of vaccination is underestimated in this analysis, because we can’t quantify the infections that never happened because of vaccination.”
The study was supported by the Strategic Clinical Networks, Alberta Health Services. Dr. Bagshaw declared no relevant financial relationships. Dr. Fisman has taken part in advisory boards for Seqirus, Pfizer, AstraZeneca, Sanofi, and Merck vaccines during the past 3 years.
A version of this article first appeared on Medscape.com.
FROM CANADIAN JOURNAL OF PUBLIC HEALTH
One in 10 people who had Omicron got long COVID: Study
About 10% of people infected with Omicron reported having long COVID, a lower percentage than estimated for people infected with earlier strains of the coronavirus, according to a study published in JAMA.
The research team looked at data from 8,646 adults infected with COVID-19 at different times of the pandemic and 1,118 who did not have COVID.
“Based on a subset of 2,231 patients in this analysis who had a first COVID-19 infection the National Institutes of Health said in a news release.
People who were unvaccinated or got COVID before Omicron were more likely to have long COVID and had more severe cases, the NIH said.
Previous studies have come up with higher figures than 10% for people who have long COVID.
For instance, in June 2022 the CDC said one in five Americans who had COVID reported having long COVID. And a University of Oxford study published in September 2021 found more than a third of patients had long COVID symptoms.
The scientists in the most recent study identified 12 symptoms that distinguished people who did and didn’t have COVID. The scientists developed a scoring system for the symptoms to set a threshold to identify people who had long COVID, the NIH said.
The symptoms were fatigue, brain fog, dizziness, stomach upset, heart palpitations, issues with sexual desire or capacity, loss of smell or taste, thirst, chronic coughing, chest pain, and abnormal movements. Another symptom was postexertional malaise, or worse symptoms after mental or physical exertion.
Scientists still have many questions about long COVID, such as how many people get it and why some people get it and others don’t.
The study was coordinated through the NIH’s RECOVER (Researching COVID to Enhance Recovery) initiative, which aims to find out how to define, detect, and treat long COVID.
“The researchers hope this study is the next step toward potential treatments for long COVID, which affects the health and wellbeing of millions of Americans,” the NIH said.
A version of this article first appeared on WebMD.com.
About 10% of people infected with Omicron reported having long COVID, a lower percentage than estimated for people infected with earlier strains of the coronavirus, according to a study published in JAMA.
The research team looked at data from 8,646 adults infected with COVID-19 at different times of the pandemic and 1,118 who did not have COVID.
“Based on a subset of 2,231 patients in this analysis who had a first COVID-19 infection the National Institutes of Health said in a news release.
People who were unvaccinated or got COVID before Omicron were more likely to have long COVID and had more severe cases, the NIH said.
Previous studies have come up with higher figures than 10% for people who have long COVID.
For instance, in June 2022 the CDC said one in five Americans who had COVID reported having long COVID. And a University of Oxford study published in September 2021 found more than a third of patients had long COVID symptoms.
The scientists in the most recent study identified 12 symptoms that distinguished people who did and didn’t have COVID. The scientists developed a scoring system for the symptoms to set a threshold to identify people who had long COVID, the NIH said.
The symptoms were fatigue, brain fog, dizziness, stomach upset, heart palpitations, issues with sexual desire or capacity, loss of smell or taste, thirst, chronic coughing, chest pain, and abnormal movements. Another symptom was postexertional malaise, or worse symptoms after mental or physical exertion.
Scientists still have many questions about long COVID, such as how many people get it and why some people get it and others don’t.
The study was coordinated through the NIH’s RECOVER (Researching COVID to Enhance Recovery) initiative, which aims to find out how to define, detect, and treat long COVID.
“The researchers hope this study is the next step toward potential treatments for long COVID, which affects the health and wellbeing of millions of Americans,” the NIH said.
A version of this article first appeared on WebMD.com.
About 10% of people infected with Omicron reported having long COVID, a lower percentage than estimated for people infected with earlier strains of the coronavirus, according to a study published in JAMA.
The research team looked at data from 8,646 adults infected with COVID-19 at different times of the pandemic and 1,118 who did not have COVID.
“Based on a subset of 2,231 patients in this analysis who had a first COVID-19 infection the National Institutes of Health said in a news release.
People who were unvaccinated or got COVID before Omicron were more likely to have long COVID and had more severe cases, the NIH said.
Previous studies have come up with higher figures than 10% for people who have long COVID.
For instance, in June 2022 the CDC said one in five Americans who had COVID reported having long COVID. And a University of Oxford study published in September 2021 found more than a third of patients had long COVID symptoms.
The scientists in the most recent study identified 12 symptoms that distinguished people who did and didn’t have COVID. The scientists developed a scoring system for the symptoms to set a threshold to identify people who had long COVID, the NIH said.
The symptoms were fatigue, brain fog, dizziness, stomach upset, heart palpitations, issues with sexual desire or capacity, loss of smell or taste, thirst, chronic coughing, chest pain, and abnormal movements. Another symptom was postexertional malaise, or worse symptoms after mental or physical exertion.
Scientists still have many questions about long COVID, such as how many people get it and why some people get it and others don’t.
The study was coordinated through the NIH’s RECOVER (Researching COVID to Enhance Recovery) initiative, which aims to find out how to define, detect, and treat long COVID.
“The researchers hope this study is the next step toward potential treatments for long COVID, which affects the health and wellbeing of millions of Americans,” the NIH said.
A version of this article first appeared on WebMD.com.
FROM JAMA
Study finds COVID-19 boosters don’t increase miscarriage risk
COVID-19 boosters are not linked to an increased chance of miscarriage, according to a new study in JAMA Network Open.
Researchers were seeking to learn whether a booster in early pregnancy, before 20 weeks, was associated with greater likelihood of spontaneous abortion.
They examined more than 100,000 pregnancies at 6-19 weeks from eight health systems in the Vaccine Safety Datalink (VSD). They found that receiving a COVID-19 booster shot in a 28-day or 42-day exposure window did not increase the chances of miscarriage.
The VSD is a collaboration between the Centers for Disease Control and Prevention’s Immunization Safety Office and large health care systems. The “observational, case-control, surveillance study” was conducted from Nov. 1, 2021, to June 12, 2022.
“COVID infection during pregnancy increases risk of poor outcomes, yet many people who are pregnant or thinking about getting pregnant are hesitant to get a booster dose because of questions about safety,” said Elyse Kharbanda, MD, senior investigator at HealthPartners Institute and lead author of the study in a press release.
The University of Minnesota reported that “previous studies have shown COIVD-19 primary vaccination is safe in pregnancy and not associated with an increased risk for miscarriage. Several studies have also shown COVID-19 can be more severe in pregnancy and lead to worse outcomes for the mother.”
The study was funded by the CDC. Five study authors reported conflicts of interest with Pfizer, Merck, GlaxoSmithKline, AbbVie, and Sanofi Pasteur.
A version of this article first appeared on Medscape.com.
COVID-19 boosters are not linked to an increased chance of miscarriage, according to a new study in JAMA Network Open.
Researchers were seeking to learn whether a booster in early pregnancy, before 20 weeks, was associated with greater likelihood of spontaneous abortion.
They examined more than 100,000 pregnancies at 6-19 weeks from eight health systems in the Vaccine Safety Datalink (VSD). They found that receiving a COVID-19 booster shot in a 28-day or 42-day exposure window did not increase the chances of miscarriage.
The VSD is a collaboration between the Centers for Disease Control and Prevention’s Immunization Safety Office and large health care systems. The “observational, case-control, surveillance study” was conducted from Nov. 1, 2021, to June 12, 2022.
“COVID infection during pregnancy increases risk of poor outcomes, yet many people who are pregnant or thinking about getting pregnant are hesitant to get a booster dose because of questions about safety,” said Elyse Kharbanda, MD, senior investigator at HealthPartners Institute and lead author of the study in a press release.
The University of Minnesota reported that “previous studies have shown COIVD-19 primary vaccination is safe in pregnancy and not associated with an increased risk for miscarriage. Several studies have also shown COVID-19 can be more severe in pregnancy and lead to worse outcomes for the mother.”
The study was funded by the CDC. Five study authors reported conflicts of interest with Pfizer, Merck, GlaxoSmithKline, AbbVie, and Sanofi Pasteur.
A version of this article first appeared on Medscape.com.
COVID-19 boosters are not linked to an increased chance of miscarriage, according to a new study in JAMA Network Open.
Researchers were seeking to learn whether a booster in early pregnancy, before 20 weeks, was associated with greater likelihood of spontaneous abortion.
They examined more than 100,000 pregnancies at 6-19 weeks from eight health systems in the Vaccine Safety Datalink (VSD). They found that receiving a COVID-19 booster shot in a 28-day or 42-day exposure window did not increase the chances of miscarriage.
The VSD is a collaboration between the Centers for Disease Control and Prevention’s Immunization Safety Office and large health care systems. The “observational, case-control, surveillance study” was conducted from Nov. 1, 2021, to June 12, 2022.
“COVID infection during pregnancy increases risk of poor outcomes, yet many people who are pregnant or thinking about getting pregnant are hesitant to get a booster dose because of questions about safety,” said Elyse Kharbanda, MD, senior investigator at HealthPartners Institute and lead author of the study in a press release.
The University of Minnesota reported that “previous studies have shown COIVD-19 primary vaccination is safe in pregnancy and not associated with an increased risk for miscarriage. Several studies have also shown COVID-19 can be more severe in pregnancy and lead to worse outcomes for the mother.”
The study was funded by the CDC. Five study authors reported conflicts of interest with Pfizer, Merck, GlaxoSmithKline, AbbVie, and Sanofi Pasteur.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
COVID boosters effective, but not for long
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
I am here today to talk about the effectiveness of COVID vaccine boosters in the midst of 2023. The reason I want to talk about this isn’t necessarily to dig into exactly how effective vaccines are. This is an area that’s been trod upon multiple times. But it does give me an opportunity to talk about a neat study design called the “test-negative case-control” design, which has some unique properties when you’re trying to evaluate the effect of something outside of the context of a randomized trial.
So, just a little bit of background to remind everyone where we are. These are the number of doses of COVID vaccines administered over time throughout the pandemic.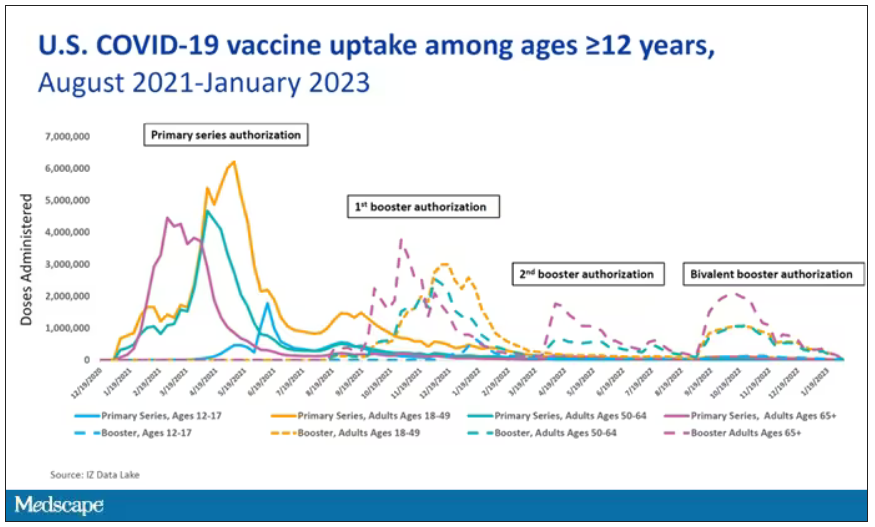
You can see that it’s stratified by age. The orange lines are adults ages 18-49, for example. You can see a big wave of vaccination when the vaccine first came out at the start of 2021. Then subsequently, you can see smaller waves after the first and second booster authorizations, and maybe a bit of a pickup, particularly among older adults, when the bivalent boosters were authorized. But still very little overall pickup of the bivalent booster, compared with the monovalent vaccines, which might suggest vaccine fatigue going on this far into the pandemic. But it’s important to try to understand exactly how effective those new boosters are, at least at this point in time.
I’m talking about Early Estimates of Bivalent mRNA Booster Dose Vaccine Effectiveness in Preventing Symptomatic SARS-CoV-2 Infection Attributable to Omicron BA.5– and XBB/XBB.1.5–Related Sublineages Among Immunocompetent Adults – Increasing Community Access to Testing Program, United States, December 2022–January 2023, which came out in the Morbidity and Mortality Weekly Report very recently, which uses this test-negative case-control design to evaluate the ability of bivalent mRNA vaccines to prevent hospitalization.
The question is: Does receipt of a bivalent COVID vaccine booster prevent hospitalizations, ICU stay, or death? That may not be the question that is of interest to everyone. I know people are interested in symptoms, missed work, and transmission, but this paper was looking at hospitalization, ICU stay, and death.
What’s kind of tricky here is that the data they’re using are in people who are hospitalized with various diseases. You might look at that on the surface and say: “Well, you can’t – that’s impossible.” But you can, actually, with this cool test-negative case-control design.
Here’s basically how it works. You take a population of people who are hospitalized and confirmed to have COVID. Some of them will be vaccinated and some of them will be unvaccinated. And the proportion of vaccinated and unvaccinated people doesn’t tell you very much because it depends on how that compares with the rates in the general population, for instance. Let me clarify this. If 100% of the population were vaccinated, then 100% of the people hospitalized with COVID would be vaccinated. That doesn’t mean vaccines are bad. Put another way, if 90% of the population were vaccinated and 60% of people hospitalized with COVID were vaccinated, that would actually show that the vaccines were working to some extent, all else being equal. So it’s not just the raw percentages that tell you anything. Some people are vaccinated, some people aren’t. You need to understand what the baseline rate is.
The test-negative case-control design looks at people who are hospitalized without COVID. Now who those people are (who the controls are, in this case) is something you really need to think about. In the case of this CDC study, they used people who were hospitalized with COVID-like illnesses – flu-like illnesses, respiratory illnesses, pneumonia, influenza, etc. This is a pretty good idea because it standardizes a little bit for people who have access to healthcare. They can get to a hospital and they’re the type of person who would go to a hospital when they’re feeling sick. That’s a better control than the general population overall, which is something I like about this design.
Some of those people who don’t have COVID (they’re in the hospital for flu or whatever) will have been vaccinated for COVID, and some will not have been vaccinated for COVID. And of course, we don’t expect COVID vaccines necessarily to protect against the flu or pneumonia, but that gives us a way to standardize.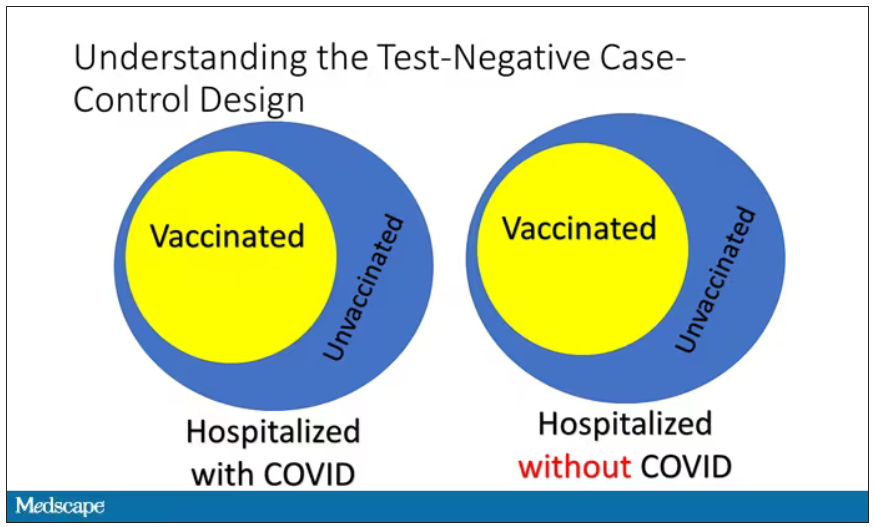
If you look at these Venn diagrams, I’ve got vaccinated/unvaccinated being exactly the same proportion, which would suggest that you’re just as likely to be hospitalized with COVID if you’re vaccinated as you are to be hospitalized with some other respiratory illness, which suggests that the vaccine isn’t particularly effective.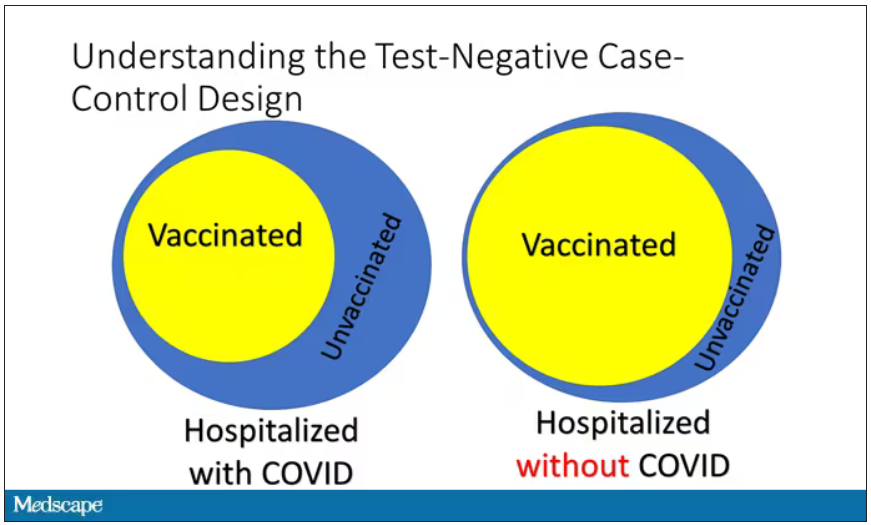
However, if you saw something like this, looking at all those patients with flu and other non-COVID illnesses, a lot more of them had been vaccinated for COVID. What that tells you is that we’re seeing fewer vaccinated people hospitalized with COVID than we would expect because we have this standardization from other respiratory infections. We expect this many vaccinated people because that’s how many vaccinated people there are who show up with flu. But in the COVID population, there are fewer, and that would suggest that the vaccines are effective. So that is the test-negative case-control design. You can do the same thing with ICU stays and death.
There are some assumptions here which you might already be thinking about. The most important one is that vaccination status is not associated with the risk for the disease. I always think of older people in this context. During the pandemic, at least in the United States, older people were much more likely to be vaccinated but were also much more likely to contract COVID and be hospitalized with COVID. The test-negative design actually accounts for this in some sense, because older people are also more likely to be hospitalized for things like flu and pneumonia. So there’s some control there.
But to the extent that older people are uniquely susceptible to COVID compared with other respiratory illnesses, that would bias your results to make the vaccines look worse. So the standard approach here is to adjust for these things. I think the CDC adjusted for age, sex, race, ethnicity, and a few other things to settle down and see how effective the vaccines were.
Let’s get to a worked example.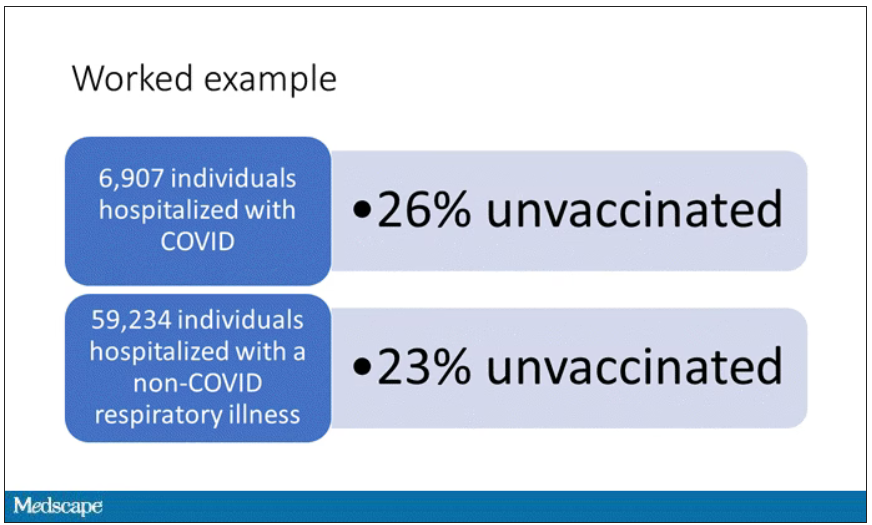
This is the actual data from the CDC paper. They had 6,907 individuals who were hospitalized with COVID, and 26% of them were unvaccinated. What’s the baseline rate that we would expect to be unvaccinated? A total of 59,234 individuals were hospitalized with a non-COVID respiratory illness, and 23% of them were unvaccinated. So you can see that there were more unvaccinated people than you would think in the COVID group. In other words, fewer vaccinated people, which suggests that the vaccine works to some degree because it’s keeping some people out of the hospital.
Now, 26% versus 23% is not a very impressive difference. But it gets more interesting when you break it down by the type of vaccine and how long ago the individual was vaccinated.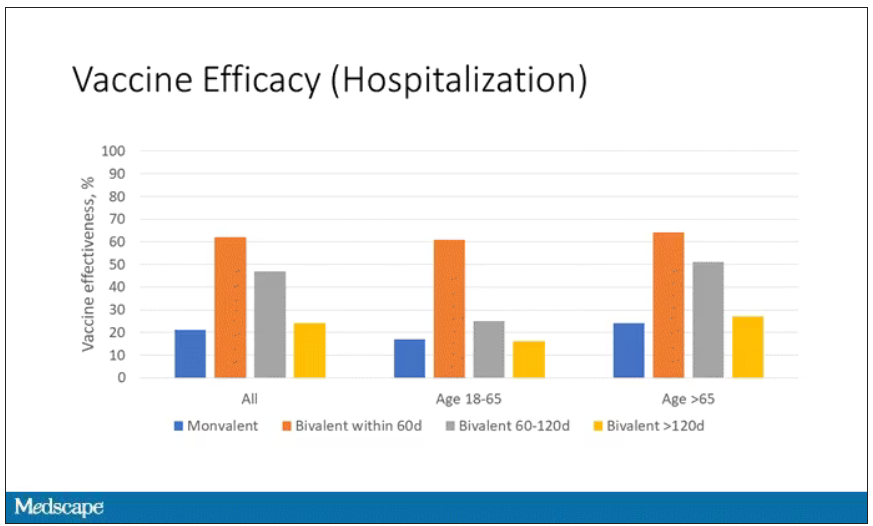
Let’s walk through the “all” group on this figure. What you can see is the calculated vaccine effectiveness. If you look at just the monovalent vaccine here, we see a 20% vaccine effectiveness. This means that you’re preventing 20% of hospitalizations basically due to COVID by people getting vaccinated. That’s okay but it’s certainly not anything to write home about. But we see much better vaccine effectiveness with the bivalent vaccine if it had been received within 60 days.
This compares people who received the bivalent vaccine within 60 days in the COVID group and the non-COVID group. The concern that the vaccine was given very recently affects both groups equally so it shouldn’t result in bias there. You see a step-off in vaccine effectiveness from 60 days, 60-120 days, and greater than 120 days. This is 4 months, and you’ve gone from 60% to 20%. When you break that down by age, you can see a similar pattern in the 18-to-65 group and potentially some more protection the greater than 65 age group.
Why is vaccine efficacy going down? The study doesn’t tell us, but we can hypothesize that this might be an immunologic effect – the antibodies or the protective T cells are waning over time. This could also reflect changes in the virus in the environment as the virus seeks to evade certain immune responses. But overall, this suggests that waiting a year between booster doses may leave you exposed for quite some time, although the take-home here is that bivalent vaccines in general are probably a good idea for the proportion of people who haven’t gotten them.
When we look at critical illness and death, the numbers look a little bit better.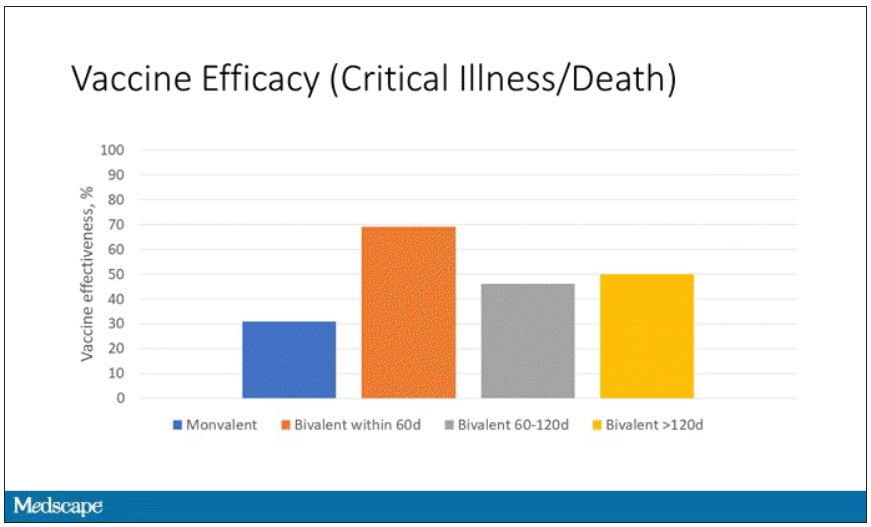
You can see that bivalent is better than monovalent – certainly pretty good if you’ve received it within 60 days. It does tend to wane a little bit, but not nearly as much. You’ve still got about 50% vaccine efficacy beyond 120 days when we’re looking at critical illness, which is stays in the ICU and death.
The overriding thing to think about when we think about vaccine policy is that the way you get immunized against COVID is either by vaccine or by getting infected with COVID, or both.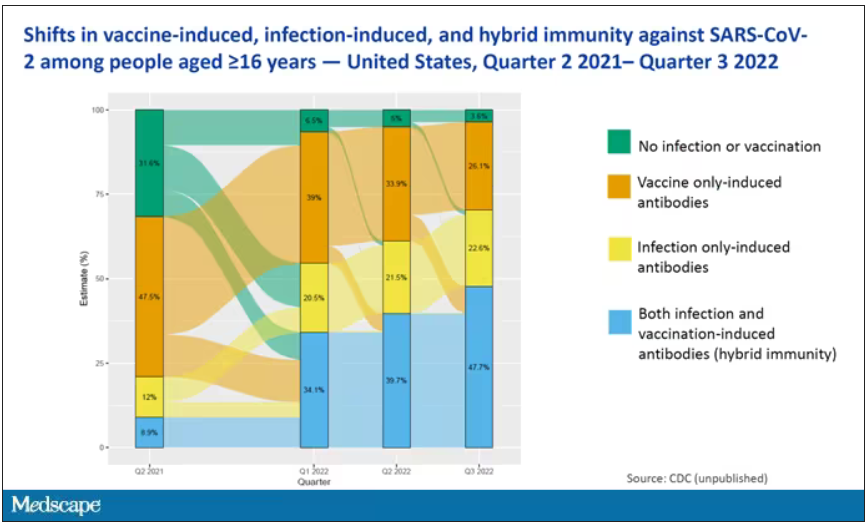
This really interesting graph from the CDC (although it’s updated only through quarter three of 2022) shows the proportion of Americans, based on routine lab tests, who have varying degrees of protection against COVID. What you can see is that, by quarter three of 2022, just 3.6% of people who had blood drawn at a commercial laboratory had no evidence of infection or vaccination. In other words, almost no one was totally naive. Then 26% of people had never been infected – they only have vaccine antibodies – plus 22% of people had only been infected but had never been vaccinated. And then 50% of people had both. So there’s a tremendous amount of existing immunity out there.
The really interesting question about future vaccination and future booster doses is, how does it work on the background of this pattern? The CDC study doesn’t tell us, and I don’t think they have the data to tell us the vaccine efficacy in these different groups. Is it more effective in people who have only had an infection, for example? Is it more effective in people who have only had vaccination versus people who had both, or people who have no protection whatsoever? Those are the really interesting questions that need to be answered going forward as vaccine policy gets developed in the future.
I hope this was a helpful primer on how the test-negative case-control design can answer questions that seem a little bit unanswerable.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
I am here today to talk about the effectiveness of COVID vaccine boosters in the midst of 2023. The reason I want to talk about this isn’t necessarily to dig into exactly how effective vaccines are. This is an area that’s been trod upon multiple times. But it does give me an opportunity to talk about a neat study design called the “test-negative case-control” design, which has some unique properties when you’re trying to evaluate the effect of something outside of the context of a randomized trial.
So, just a little bit of background to remind everyone where we are. These are the number of doses of COVID vaccines administered over time throughout the pandemic.
You can see that it’s stratified by age. The orange lines are adults ages 18-49, for example. You can see a big wave of vaccination when the vaccine first came out at the start of 2021. Then subsequently, you can see smaller waves after the first and second booster authorizations, and maybe a bit of a pickup, particularly among older adults, when the bivalent boosters were authorized. But still very little overall pickup of the bivalent booster, compared with the monovalent vaccines, which might suggest vaccine fatigue going on this far into the pandemic. But it’s important to try to understand exactly how effective those new boosters are, at least at this point in time.
I’m talking about Early Estimates of Bivalent mRNA Booster Dose Vaccine Effectiveness in Preventing Symptomatic SARS-CoV-2 Infection Attributable to Omicron BA.5– and XBB/XBB.1.5–Related Sublineages Among Immunocompetent Adults – Increasing Community Access to Testing Program, United States, December 2022–January 2023, which came out in the Morbidity and Mortality Weekly Report very recently, which uses this test-negative case-control design to evaluate the ability of bivalent mRNA vaccines to prevent hospitalization.
The question is: Does receipt of a bivalent COVID vaccine booster prevent hospitalizations, ICU stay, or death? That may not be the question that is of interest to everyone. I know people are interested in symptoms, missed work, and transmission, but this paper was looking at hospitalization, ICU stay, and death.
What’s kind of tricky here is that the data they’re using are in people who are hospitalized with various diseases. You might look at that on the surface and say: “Well, you can’t – that’s impossible.” But you can, actually, with this cool test-negative case-control design.
Here’s basically how it works. You take a population of people who are hospitalized and confirmed to have COVID. Some of them will be vaccinated and some of them will be unvaccinated. And the proportion of vaccinated and unvaccinated people doesn’t tell you very much because it depends on how that compares with the rates in the general population, for instance. Let me clarify this. If 100% of the population were vaccinated, then 100% of the people hospitalized with COVID would be vaccinated. That doesn’t mean vaccines are bad. Put another way, if 90% of the population were vaccinated and 60% of people hospitalized with COVID were vaccinated, that would actually show that the vaccines were working to some extent, all else being equal. So it’s not just the raw percentages that tell you anything. Some people are vaccinated, some people aren’t. You need to understand what the baseline rate is.
The test-negative case-control design looks at people who are hospitalized without COVID. Now who those people are (who the controls are, in this case) is something you really need to think about. In the case of this CDC study, they used people who were hospitalized with COVID-like illnesses – flu-like illnesses, respiratory illnesses, pneumonia, influenza, etc. This is a pretty good idea because it standardizes a little bit for people who have access to healthcare. They can get to a hospital and they’re the type of person who would go to a hospital when they’re feeling sick. That’s a better control than the general population overall, which is something I like about this design.
Some of those people who don’t have COVID (they’re in the hospital for flu or whatever) will have been vaccinated for COVID, and some will not have been vaccinated for COVID. And of course, we don’t expect COVID vaccines necessarily to protect against the flu or pneumonia, but that gives us a way to standardize.
If you look at these Venn diagrams, I’ve got vaccinated/unvaccinated being exactly the same proportion, which would suggest that you’re just as likely to be hospitalized with COVID if you’re vaccinated as you are to be hospitalized with some other respiratory illness, which suggests that the vaccine isn’t particularly effective.
However, if you saw something like this, looking at all those patients with flu and other non-COVID illnesses, a lot more of them had been vaccinated for COVID. What that tells you is that we’re seeing fewer vaccinated people hospitalized with COVID than we would expect because we have this standardization from other respiratory infections. We expect this many vaccinated people because that’s how many vaccinated people there are who show up with flu. But in the COVID population, there are fewer, and that would suggest that the vaccines are effective. So that is the test-negative case-control design. You can do the same thing with ICU stays and death.
There are some assumptions here which you might already be thinking about. The most important one is that vaccination status is not associated with the risk for the disease. I always think of older people in this context. During the pandemic, at least in the United States, older people were much more likely to be vaccinated but were also much more likely to contract COVID and be hospitalized with COVID. The test-negative design actually accounts for this in some sense, because older people are also more likely to be hospitalized for things like flu and pneumonia. So there’s some control there.
But to the extent that older people are uniquely susceptible to COVID compared with other respiratory illnesses, that would bias your results to make the vaccines look worse. So the standard approach here is to adjust for these things. I think the CDC adjusted for age, sex, race, ethnicity, and a few other things to settle down and see how effective the vaccines were.
Let’s get to a worked example.
This is the actual data from the CDC paper. They had 6,907 individuals who were hospitalized with COVID, and 26% of them were unvaccinated. What’s the baseline rate that we would expect to be unvaccinated? A total of 59,234 individuals were hospitalized with a non-COVID respiratory illness, and 23% of them were unvaccinated. So you can see that there were more unvaccinated people than you would think in the COVID group. In other words, fewer vaccinated people, which suggests that the vaccine works to some degree because it’s keeping some people out of the hospital.
Now, 26% versus 23% is not a very impressive difference. But it gets more interesting when you break it down by the type of vaccine and how long ago the individual was vaccinated.
Let’s walk through the “all” group on this figure. What you can see is the calculated vaccine effectiveness. If you look at just the monovalent vaccine here, we see a 20% vaccine effectiveness. This means that you’re preventing 20% of hospitalizations basically due to COVID by people getting vaccinated. That’s okay but it’s certainly not anything to write home about. But we see much better vaccine effectiveness with the bivalent vaccine if it had been received within 60 days.
This compares people who received the bivalent vaccine within 60 days in the COVID group and the non-COVID group. The concern that the vaccine was given very recently affects both groups equally so it shouldn’t result in bias there. You see a step-off in vaccine effectiveness from 60 days, 60-120 days, and greater than 120 days. This is 4 months, and you’ve gone from 60% to 20%. When you break that down by age, you can see a similar pattern in the 18-to-65 group and potentially some more protection the greater than 65 age group.
Why is vaccine efficacy going down? The study doesn’t tell us, but we can hypothesize that this might be an immunologic effect – the antibodies or the protective T cells are waning over time. This could also reflect changes in the virus in the environment as the virus seeks to evade certain immune responses. But overall, this suggests that waiting a year between booster doses may leave you exposed for quite some time, although the take-home here is that bivalent vaccines in general are probably a good idea for the proportion of people who haven’t gotten them.
When we look at critical illness and death, the numbers look a little bit better.
You can see that bivalent is better than monovalent – certainly pretty good if you’ve received it within 60 days. It does tend to wane a little bit, but not nearly as much. You’ve still got about 50% vaccine efficacy beyond 120 days when we’re looking at critical illness, which is stays in the ICU and death.
The overriding thing to think about when we think about vaccine policy is that the way you get immunized against COVID is either by vaccine or by getting infected with COVID, or both.
This really interesting graph from the CDC (although it’s updated only through quarter three of 2022) shows the proportion of Americans, based on routine lab tests, who have varying degrees of protection against COVID. What you can see is that, by quarter three of 2022, just 3.6% of people who had blood drawn at a commercial laboratory had no evidence of infection or vaccination. In other words, almost no one was totally naive. Then 26% of people had never been infected – they only have vaccine antibodies – plus 22% of people had only been infected but had never been vaccinated. And then 50% of people had both. So there’s a tremendous amount of existing immunity out there.
The really interesting question about future vaccination and future booster doses is, how does it work on the background of this pattern? The CDC study doesn’t tell us, and I don’t think they have the data to tell us the vaccine efficacy in these different groups. Is it more effective in people who have only had an infection, for example? Is it more effective in people who have only had vaccination versus people who had both, or people who have no protection whatsoever? Those are the really interesting questions that need to be answered going forward as vaccine policy gets developed in the future.
I hope this was a helpful primer on how the test-negative case-control design can answer questions that seem a little bit unanswerable.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
I am here today to talk about the effectiveness of COVID vaccine boosters in the midst of 2023. The reason I want to talk about this isn’t necessarily to dig into exactly how effective vaccines are. This is an area that’s been trod upon multiple times. But it does give me an opportunity to talk about a neat study design called the “test-negative case-control” design, which has some unique properties when you’re trying to evaluate the effect of something outside of the context of a randomized trial.
So, just a little bit of background to remind everyone where we are. These are the number of doses of COVID vaccines administered over time throughout the pandemic.
You can see that it’s stratified by age. The orange lines are adults ages 18-49, for example. You can see a big wave of vaccination when the vaccine first came out at the start of 2021. Then subsequently, you can see smaller waves after the first and second booster authorizations, and maybe a bit of a pickup, particularly among older adults, when the bivalent boosters were authorized. But still very little overall pickup of the bivalent booster, compared with the monovalent vaccines, which might suggest vaccine fatigue going on this far into the pandemic. But it’s important to try to understand exactly how effective those new boosters are, at least at this point in time.
I’m talking about Early Estimates of Bivalent mRNA Booster Dose Vaccine Effectiveness in Preventing Symptomatic SARS-CoV-2 Infection Attributable to Omicron BA.5– and XBB/XBB.1.5–Related Sublineages Among Immunocompetent Adults – Increasing Community Access to Testing Program, United States, December 2022–January 2023, which came out in the Morbidity and Mortality Weekly Report very recently, which uses this test-negative case-control design to evaluate the ability of bivalent mRNA vaccines to prevent hospitalization.
The question is: Does receipt of a bivalent COVID vaccine booster prevent hospitalizations, ICU stay, or death? That may not be the question that is of interest to everyone. I know people are interested in symptoms, missed work, and transmission, but this paper was looking at hospitalization, ICU stay, and death.
What’s kind of tricky here is that the data they’re using are in people who are hospitalized with various diseases. You might look at that on the surface and say: “Well, you can’t – that’s impossible.” But you can, actually, with this cool test-negative case-control design.
Here’s basically how it works. You take a population of people who are hospitalized and confirmed to have COVID. Some of them will be vaccinated and some of them will be unvaccinated. And the proportion of vaccinated and unvaccinated people doesn’t tell you very much because it depends on how that compares with the rates in the general population, for instance. Let me clarify this. If 100% of the population were vaccinated, then 100% of the people hospitalized with COVID would be vaccinated. That doesn’t mean vaccines are bad. Put another way, if 90% of the population were vaccinated and 60% of people hospitalized with COVID were vaccinated, that would actually show that the vaccines were working to some extent, all else being equal. So it’s not just the raw percentages that tell you anything. Some people are vaccinated, some people aren’t. You need to understand what the baseline rate is.
The test-negative case-control design looks at people who are hospitalized without COVID. Now who those people are (who the controls are, in this case) is something you really need to think about. In the case of this CDC study, they used people who were hospitalized with COVID-like illnesses – flu-like illnesses, respiratory illnesses, pneumonia, influenza, etc. This is a pretty good idea because it standardizes a little bit for people who have access to healthcare. They can get to a hospital and they’re the type of person who would go to a hospital when they’re feeling sick. That’s a better control than the general population overall, which is something I like about this design.
Some of those people who don’t have COVID (they’re in the hospital for flu or whatever) will have been vaccinated for COVID, and some will not have been vaccinated for COVID. And of course, we don’t expect COVID vaccines necessarily to protect against the flu or pneumonia, but that gives us a way to standardize.
If you look at these Venn diagrams, I’ve got vaccinated/unvaccinated being exactly the same proportion, which would suggest that you’re just as likely to be hospitalized with COVID if you’re vaccinated as you are to be hospitalized with some other respiratory illness, which suggests that the vaccine isn’t particularly effective.
However, if you saw something like this, looking at all those patients with flu and other non-COVID illnesses, a lot more of them had been vaccinated for COVID. What that tells you is that we’re seeing fewer vaccinated people hospitalized with COVID than we would expect because we have this standardization from other respiratory infections. We expect this many vaccinated people because that’s how many vaccinated people there are who show up with flu. But in the COVID population, there are fewer, and that would suggest that the vaccines are effective. So that is the test-negative case-control design. You can do the same thing with ICU stays and death.
There are some assumptions here which you might already be thinking about. The most important one is that vaccination status is not associated with the risk for the disease. I always think of older people in this context. During the pandemic, at least in the United States, older people were much more likely to be vaccinated but were also much more likely to contract COVID and be hospitalized with COVID. The test-negative design actually accounts for this in some sense, because older people are also more likely to be hospitalized for things like flu and pneumonia. So there’s some control there.
But to the extent that older people are uniquely susceptible to COVID compared with other respiratory illnesses, that would bias your results to make the vaccines look worse. So the standard approach here is to adjust for these things. I think the CDC adjusted for age, sex, race, ethnicity, and a few other things to settle down and see how effective the vaccines were.
Let’s get to a worked example.
This is the actual data from the CDC paper. They had 6,907 individuals who were hospitalized with COVID, and 26% of them were unvaccinated. What’s the baseline rate that we would expect to be unvaccinated? A total of 59,234 individuals were hospitalized with a non-COVID respiratory illness, and 23% of them were unvaccinated. So you can see that there were more unvaccinated people than you would think in the COVID group. In other words, fewer vaccinated people, which suggests that the vaccine works to some degree because it’s keeping some people out of the hospital.
Now, 26% versus 23% is not a very impressive difference. But it gets more interesting when you break it down by the type of vaccine and how long ago the individual was vaccinated.
Let’s walk through the “all” group on this figure. What you can see is the calculated vaccine effectiveness. If you look at just the monovalent vaccine here, we see a 20% vaccine effectiveness. This means that you’re preventing 20% of hospitalizations basically due to COVID by people getting vaccinated. That’s okay but it’s certainly not anything to write home about. But we see much better vaccine effectiveness with the bivalent vaccine if it had been received within 60 days.
This compares people who received the bivalent vaccine within 60 days in the COVID group and the non-COVID group. The concern that the vaccine was given very recently affects both groups equally so it shouldn’t result in bias there. You see a step-off in vaccine effectiveness from 60 days, 60-120 days, and greater than 120 days. This is 4 months, and you’ve gone from 60% to 20%. When you break that down by age, you can see a similar pattern in the 18-to-65 group and potentially some more protection the greater than 65 age group.
Why is vaccine efficacy going down? The study doesn’t tell us, but we can hypothesize that this might be an immunologic effect – the antibodies or the protective T cells are waning over time. This could also reflect changes in the virus in the environment as the virus seeks to evade certain immune responses. But overall, this suggests that waiting a year between booster doses may leave you exposed for quite some time, although the take-home here is that bivalent vaccines in general are probably a good idea for the proportion of people who haven’t gotten them.
When we look at critical illness and death, the numbers look a little bit better.
You can see that bivalent is better than monovalent – certainly pretty good if you’ve received it within 60 days. It does tend to wane a little bit, but not nearly as much. You’ve still got about 50% vaccine efficacy beyond 120 days when we’re looking at critical illness, which is stays in the ICU and death.
The overriding thing to think about when we think about vaccine policy is that the way you get immunized against COVID is either by vaccine or by getting infected with COVID, or both.
This really interesting graph from the CDC (although it’s updated only through quarter three of 2022) shows the proportion of Americans, based on routine lab tests, who have varying degrees of protection against COVID. What you can see is that, by quarter three of 2022, just 3.6% of people who had blood drawn at a commercial laboratory had no evidence of infection or vaccination. In other words, almost no one was totally naive. Then 26% of people had never been infected – they only have vaccine antibodies – plus 22% of people had only been infected but had never been vaccinated. And then 50% of people had both. So there’s a tremendous amount of existing immunity out there.
The really interesting question about future vaccination and future booster doses is, how does it work on the background of this pattern? The CDC study doesn’t tell us, and I don’t think they have the data to tell us the vaccine efficacy in these different groups. Is it more effective in people who have only had an infection, for example? Is it more effective in people who have only had vaccination versus people who had both, or people who have no protection whatsoever? Those are the really interesting questions that need to be answered going forward as vaccine policy gets developed in the future.
I hope this was a helpful primer on how the test-negative case-control design can answer questions that seem a little bit unanswerable.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.