User login
The inappropriate use of antibiotics is associated with an increased risk of antibiotic resistance, health care costs, and risk of adverse drug reactions.1 According to the Centers for Disease Control and Prevention (CDC), a 10% decrease in overall antibiotic use across different wards was associated with a 34% decrease in Clostridioides difficile (C difficile) infections.2 In addition, antimicrobial resistance accounts for > 2.8 million infections and > 35,000 deaths each year.3 The estimated total economic costs of antibiotic resistance to the US economy have ranged as high as $20 billion in excess direct health care costs.4 A main goal of an antimicrobial stewardship program (ASP) is to optimize antibiotic use to prevent the adverse consequences of inappropriate antibiotic prescribing.
During the COVID-19 pandemic, increased use of empiric antibiotic therapy has been observed. According to the CDC, almost 80% of patients hospitalized with COVID-19 received an antibiotic from March 2020 to October 2020.5 Studies were conducted to investigate the prevalence of bacterial coinfection in patients with COVID-19 and whether antibiotics were indicated in this patient population. A United Kingdom multicenter, prospective cohort study showed a high proportion of patients hospitalized with COVID-19 received antimicrobials despite microbiologically confirmed bacterial infections being rare and more likely to be secondary infections.6
Many other studies have reported similar findings. Langord and colleagues found the prevalence of bacterial coinfection in patients with COVID-19 was 3.5% but that 71.9% received antibiotics.7 Coenen and colleagues identified 12.4% of the patients with possible and 1.1% of patients with probable bacterial coinfection, while 81% of the study population and 78% of patients were classified as unlikely bacterial coinfection received antibiotics.8
At Veterans Affairs Southern Nevada Healthcare System (VASNHS), an ASP team consisting of an infectious disease (ID) physician and 2 pharmacists provide daily prospective audits with intervention and feedback along with other interventions, such as providing restricted order menus, institutional treatment guidelines, and staff education to help improve antibiotic prescribing. The ASP pharmacists have a scope of practice to make changes to anti-infective therapies. The purpose of this study was to describe antibiotic prescribing in patients hospitalized with COVID-19 from November 1, 2020, to January 31, 2021, in an ASP setting led by pharmacists.
Methods
This retrospective descriptive study included patients who were hospitalized for the treatment of laboratory-confirmed COVID-19 infection. The Theradoc clinical surveillance system was used to retrieve a list of patients who were admitted to VASNHS from November 1, 2020, to January 31, 2021, and tested positive for COVID-19. Patients with incidental positive COVID-19 test results or those who received antibiotics for extrapulmonary indications on hospital admission were excluded.
Each patient chart was reviewed and data, including clinical presentations, procalcitonin (PCT), the requirement of supplemental oxygen, vital signs, imaging findings, antibiotic orders on admission, ASP interventions such as discontinuation or changes to antibiotic therapy during the first 72 hours of hospital admission, clinical outcomes, culture results, and readmission rate, defined as any hospital admission related to COVID-19 or respiratory tract infection within 30 days from the previous discharge, were collected.
The primary objective of the study was to describe antibiotic prescribing in patients hospitalized with COVID-19. The secondary outcomes included the prevalence of bacterial coinfection and nosocomial bacterial infection in patients hospitalized with COVID-19.
Results
A total of 199 patients were admitted to the hospital for laboratory-confirmed COVID-19 infection from November 1, 2020, to January 31, 2021. Sixty-one patients (31%) received at least 1 antibiotic on hospital admission. Among those patients who received empiric antibiotic treatment, 29 patients (48%) met the Systemic Inflammatory Response Syndrome (SIRS) criteria. Fifty-six patients (92%) had ≥ 1 PCT level obtained, and 26 of those (46%) presented with elevated PCT levels (PCT > 0.25). Fifty patients (82%) required oxygen supplement and 49 (80%) presented with remarkable imaging findings. Of 138 patients who did not receive empiric antibiotic therapy within 72 hours of hospital admission, 56 (41%) met the SIRS criteria, 31 (29%) had elevated PCT levels, 100 (72%) required oxygen supplement, and 79 (59%) presented with remarkable imaging findings.
Antibiotic Prescribing
Forty-six of 61 patients (75%) received antibiotic treatment for community-acquired pneumonia (CAP) that included ceftriaxone and azithromycin. Three patients (5%) received ≥ 1 broad-spectrum antibiotic (4th generation cephalosporin [cefepime] or piperacillin-tazobactam), 2 (3%) received vancomycin, and 1 (2%) received a fluoroquinolone (levofloxacin) on admission.
Among 61 patients who received empiric antibiotics, the readmission rate was 6%. The mortality rate was 20%, and the mean (SD) duration of hospital stay was 13.1 (12.5) days.
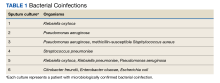
Six of 199 patients (3%) had microbiologically confirmed bacterial coinfection on hospital admission: 3 were Pseudomonas aeruginosa (P aeruginosa) and 2 were Klebsiella oxytoca (Table 1).
Discussion
Prospective audit and feedback and preauthorization are recommended in guidelines as “core components of any stewardship program.”9 At VASNHS, the ASP performs daily prospective audits with intervention and feedback. Efforts have been made to maintain daily ASP activities during the pandemic. This study aimed to describe antibiotic prescribing for patients hospitalized with COVID-19 in a pharmacist-led ASP setting. It was found that up to 31% of the patients received ≥ 1 antibiotic on admission for empiric treatment of bacterial coinfection. About half of these patients met the SIRS criteria. Most of these patients received ceftriaxone and azithromycin for concern of CAP. ASP discontinued antibiotics within 72 hours in most of the patients. Chart review and discussion with ID physicians and/or hospitalists determined the probability of bacterial coinfection as well as any potential complication or patient-specific risk factor. It is important to note that most patients who received antibiotics on admission had ≥ 1 PCT level and up to 46% of them had a PCT level > 0.25. However, according to Relph and colleagues, PCT may not be a reliable indicator of bacterial infection in severe viral diseases with raised interleukin-6 levels.10 An elevated PCT level should not be the sole indicator for empiric antibiotic treatment.
Study findings confirmed the low prevalence of bacterial coinfection in patients hospitalized with COVID-19. The overuse of empiric antibiotics in a patient population unlikely to present with bacterial coinfection is concerning. It is essential to continue promoting antimicrobial stewardship during the COVID-19 pandemic to ensure appropriate and responsible antimicrobial prescribing. A thorough clinical assessment consisting of comorbidities, clinical symptoms, radiologic and microbiologic findings, as well as other relevant workup or biomarker results is crucial to determine whether the antibiotic is strongly indicated in patients hospitalized with COVID-19. Empiric antibiotic therapy should be considered only in patients with clinical findings suggestive of bacterial coinfection.
Limitations
Limitations of our study included the study design (single-center, retrospective review, lack of comparative group) and small sample size with a 3-month study period. In addition, respiratory cultures are not commonly obtained in patients who present with mild-to-moderate CAP. Using culture results solely to confirm bacterial coinfection in patients with COVID-19 could have underestimated the prevalence of bacterial infection. Developing diagnostic criteria that include clinical signs and symptoms, imaging findings, and laboratory results as well as culture results would help to better assess the presence of bacterial coinfection in this patient population.
Conclusions
The study findings showed that up to 30% of patients hospitalized for COVID-19 infection received empiric antibiotic treatment for concern of bacterial coinfection. A pharmacist-led ASP provided interventions, including early discontinuation of antibiotics in 77% of these patients.
A low prevalence of bacterial coinfection (3%) in patients hospitalized with COVID-19 also was reported. A thorough clinical workup to determine the risk of bacterial coinfection in patients with COVID-19 is important before starting empiric antibiotic therapy. Continuing to promote the ASP during the COVID-19 pandemic to ensure responsible antibiotic use and prevent antimicrobial resistance is essential.
1. Demirjian A, Sanchez GV, Finkelstein JA, et al. CDC grand rounds: getting smart about antibiotics. MMWR Morb Mortal Wkly Rep. 2015;64(32):871-873. doi:10.15585/mmwr.mm6432a3
2. Nearly half a million Americans suffered from Clostridium difficile infections in a single year. Centers for Disease Control and Prevention. Updated March 22, 2017. Accessed March 21, 2023. https://www.cdc.gov/media/releases/2015/p0225-clostridium-difficile.html
3. Centers for Disease Control and Prevention. About antimicrobial resistance. Updated October 5, 2022. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/about.html
4. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf
5. Centers for Disease Control and Prevention. COVID-19 & antibiotic resistance. Updated February 25, 2022. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/covid19.html
6. Russell CD, Fairfield CJ, Drake TM, et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe. 2021;2(8):e354-e365. doi:10.1016/S2666-5247(21)00090-2
7. Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622-1629. doi:10.1016/j.cmi.2020.07.016
8. Coenen S, de la Court JR, Buis DTP, et al. Low frequency of community-acquired bacterial co-infection in patients hospitalized for COVID-19 based on clinical, radiological and microbiological criteria: a retrospective cohort study. Antimicrob Resist Infect Control. 2021;10(1):155. doi:10.1186/s13756-021-01024-4
9. Centers for Disease Control and Prevention. The core elements of hospital antibiotic stewardship programs: 2019. Accessed March 21, 2023. https://www.cdc.gov/antibiotic-use/healthcare/pdfs/hospital-core-elements-H.pdf
10. Relph KA, Russell CD, Fairfield CJ, et al; International Severe Acute Respiratory and Emerging Infections Consortium; Coronavirus Clinical Characterisation Consortium (ISARIC4C) Investigators. Procalcitonin is not a reliable biomarker of bacterial coinfection in people with Coronavirus Disease 2019 undergoing microbiological investigation at the time of hospital admission. Open Forum Infect Dis. 2022;9(5):ofac179. doi:10.1093/ofid/ofac179
The inappropriate use of antibiotics is associated with an increased risk of antibiotic resistance, health care costs, and risk of adverse drug reactions.1 According to the Centers for Disease Control and Prevention (CDC), a 10% decrease in overall antibiotic use across different wards was associated with a 34% decrease in Clostridioides difficile (C difficile) infections.2 In addition, antimicrobial resistance accounts for > 2.8 million infections and > 35,000 deaths each year.3 The estimated total economic costs of antibiotic resistance to the US economy have ranged as high as $20 billion in excess direct health care costs.4 A main goal of an antimicrobial stewardship program (ASP) is to optimize antibiotic use to prevent the adverse consequences of inappropriate antibiotic prescribing.
During the COVID-19 pandemic, increased use of empiric antibiotic therapy has been observed. According to the CDC, almost 80% of patients hospitalized with COVID-19 received an antibiotic from March 2020 to October 2020.5 Studies were conducted to investigate the prevalence of bacterial coinfection in patients with COVID-19 and whether antibiotics were indicated in this patient population. A United Kingdom multicenter, prospective cohort study showed a high proportion of patients hospitalized with COVID-19 received antimicrobials despite microbiologically confirmed bacterial infections being rare and more likely to be secondary infections.6
Many other studies have reported similar findings. Langord and colleagues found the prevalence of bacterial coinfection in patients with COVID-19 was 3.5% but that 71.9% received antibiotics.7 Coenen and colleagues identified 12.4% of the patients with possible and 1.1% of patients with probable bacterial coinfection, while 81% of the study population and 78% of patients were classified as unlikely bacterial coinfection received antibiotics.8
At Veterans Affairs Southern Nevada Healthcare System (VASNHS), an ASP team consisting of an infectious disease (ID) physician and 2 pharmacists provide daily prospective audits with intervention and feedback along with other interventions, such as providing restricted order menus, institutional treatment guidelines, and staff education to help improve antibiotic prescribing. The ASP pharmacists have a scope of practice to make changes to anti-infective therapies. The purpose of this study was to describe antibiotic prescribing in patients hospitalized with COVID-19 from November 1, 2020, to January 31, 2021, in an ASP setting led by pharmacists.
Methods
This retrospective descriptive study included patients who were hospitalized for the treatment of laboratory-confirmed COVID-19 infection. The Theradoc clinical surveillance system was used to retrieve a list of patients who were admitted to VASNHS from November 1, 2020, to January 31, 2021, and tested positive for COVID-19. Patients with incidental positive COVID-19 test results or those who received antibiotics for extrapulmonary indications on hospital admission were excluded.
Each patient chart was reviewed and data, including clinical presentations, procalcitonin (PCT), the requirement of supplemental oxygen, vital signs, imaging findings, antibiotic orders on admission, ASP interventions such as discontinuation or changes to antibiotic therapy during the first 72 hours of hospital admission, clinical outcomes, culture results, and readmission rate, defined as any hospital admission related to COVID-19 or respiratory tract infection within 30 days from the previous discharge, were collected.
The primary objective of the study was to describe antibiotic prescribing in patients hospitalized with COVID-19. The secondary outcomes included the prevalence of bacterial coinfection and nosocomial bacterial infection in patients hospitalized with COVID-19.
Results
A total of 199 patients were admitted to the hospital for laboratory-confirmed COVID-19 infection from November 1, 2020, to January 31, 2021. Sixty-one patients (31%) received at least 1 antibiotic on hospital admission. Among those patients who received empiric antibiotic treatment, 29 patients (48%) met the Systemic Inflammatory Response Syndrome (SIRS) criteria. Fifty-six patients (92%) had ≥ 1 PCT level obtained, and 26 of those (46%) presented with elevated PCT levels (PCT > 0.25). Fifty patients (82%) required oxygen supplement and 49 (80%) presented with remarkable imaging findings. Of 138 patients who did not receive empiric antibiotic therapy within 72 hours of hospital admission, 56 (41%) met the SIRS criteria, 31 (29%) had elevated PCT levels, 100 (72%) required oxygen supplement, and 79 (59%) presented with remarkable imaging findings.
Antibiotic Prescribing
Forty-six of 61 patients (75%) received antibiotic treatment for community-acquired pneumonia (CAP) that included ceftriaxone and azithromycin. Three patients (5%) received ≥ 1 broad-spectrum antibiotic (4th generation cephalosporin [cefepime] or piperacillin-tazobactam), 2 (3%) received vancomycin, and 1 (2%) received a fluoroquinolone (levofloxacin) on admission.
Among 61 patients who received empiric antibiotics, the readmission rate was 6%. The mortality rate was 20%, and the mean (SD) duration of hospital stay was 13.1 (12.5) days.
Six of 199 patients (3%) had microbiologically confirmed bacterial coinfection on hospital admission: 3 were Pseudomonas aeruginosa (P aeruginosa) and 2 were Klebsiella oxytoca (Table 1).
Discussion
Prospective audit and feedback and preauthorization are recommended in guidelines as “core components of any stewardship program.”9 At VASNHS, the ASP performs daily prospective audits with intervention and feedback. Efforts have been made to maintain daily ASP activities during the pandemic. This study aimed to describe antibiotic prescribing for patients hospitalized with COVID-19 in a pharmacist-led ASP setting. It was found that up to 31% of the patients received ≥ 1 antibiotic on admission for empiric treatment of bacterial coinfection. About half of these patients met the SIRS criteria. Most of these patients received ceftriaxone and azithromycin for concern of CAP. ASP discontinued antibiotics within 72 hours in most of the patients. Chart review and discussion with ID physicians and/or hospitalists determined the probability of bacterial coinfection as well as any potential complication or patient-specific risk factor. It is important to note that most patients who received antibiotics on admission had ≥ 1 PCT level and up to 46% of them had a PCT level > 0.25. However, according to Relph and colleagues, PCT may not be a reliable indicator of bacterial infection in severe viral diseases with raised interleukin-6 levels.10 An elevated PCT level should not be the sole indicator for empiric antibiotic treatment.
Study findings confirmed the low prevalence of bacterial coinfection in patients hospitalized with COVID-19. The overuse of empiric antibiotics in a patient population unlikely to present with bacterial coinfection is concerning. It is essential to continue promoting antimicrobial stewardship during the COVID-19 pandemic to ensure appropriate and responsible antimicrobial prescribing. A thorough clinical assessment consisting of comorbidities, clinical symptoms, radiologic and microbiologic findings, as well as other relevant workup or biomarker results is crucial to determine whether the antibiotic is strongly indicated in patients hospitalized with COVID-19. Empiric antibiotic therapy should be considered only in patients with clinical findings suggestive of bacterial coinfection.
Limitations
Limitations of our study included the study design (single-center, retrospective review, lack of comparative group) and small sample size with a 3-month study period. In addition, respiratory cultures are not commonly obtained in patients who present with mild-to-moderate CAP. Using culture results solely to confirm bacterial coinfection in patients with COVID-19 could have underestimated the prevalence of bacterial infection. Developing diagnostic criteria that include clinical signs and symptoms, imaging findings, and laboratory results as well as culture results would help to better assess the presence of bacterial coinfection in this patient population.
Conclusions
The study findings showed that up to 30% of patients hospitalized for COVID-19 infection received empiric antibiotic treatment for concern of bacterial coinfection. A pharmacist-led ASP provided interventions, including early discontinuation of antibiotics in 77% of these patients.
A low prevalence of bacterial coinfection (3%) in patients hospitalized with COVID-19 also was reported. A thorough clinical workup to determine the risk of bacterial coinfection in patients with COVID-19 is important before starting empiric antibiotic therapy. Continuing to promote the ASP during the COVID-19 pandemic to ensure responsible antibiotic use and prevent antimicrobial resistance is essential.
The inappropriate use of antibiotics is associated with an increased risk of antibiotic resistance, health care costs, and risk of adverse drug reactions.1 According to the Centers for Disease Control and Prevention (CDC), a 10% decrease in overall antibiotic use across different wards was associated with a 34% decrease in Clostridioides difficile (C difficile) infections.2 In addition, antimicrobial resistance accounts for > 2.8 million infections and > 35,000 deaths each year.3 The estimated total economic costs of antibiotic resistance to the US economy have ranged as high as $20 billion in excess direct health care costs.4 A main goal of an antimicrobial stewardship program (ASP) is to optimize antibiotic use to prevent the adverse consequences of inappropriate antibiotic prescribing.
During the COVID-19 pandemic, increased use of empiric antibiotic therapy has been observed. According to the CDC, almost 80% of patients hospitalized with COVID-19 received an antibiotic from March 2020 to October 2020.5 Studies were conducted to investigate the prevalence of bacterial coinfection in patients with COVID-19 and whether antibiotics were indicated in this patient population. A United Kingdom multicenter, prospective cohort study showed a high proportion of patients hospitalized with COVID-19 received antimicrobials despite microbiologically confirmed bacterial infections being rare and more likely to be secondary infections.6
Many other studies have reported similar findings. Langord and colleagues found the prevalence of bacterial coinfection in patients with COVID-19 was 3.5% but that 71.9% received antibiotics.7 Coenen and colleagues identified 12.4% of the patients with possible and 1.1% of patients with probable bacterial coinfection, while 81% of the study population and 78% of patients were classified as unlikely bacterial coinfection received antibiotics.8
At Veterans Affairs Southern Nevada Healthcare System (VASNHS), an ASP team consisting of an infectious disease (ID) physician and 2 pharmacists provide daily prospective audits with intervention and feedback along with other interventions, such as providing restricted order menus, institutional treatment guidelines, and staff education to help improve antibiotic prescribing. The ASP pharmacists have a scope of practice to make changes to anti-infective therapies. The purpose of this study was to describe antibiotic prescribing in patients hospitalized with COVID-19 from November 1, 2020, to January 31, 2021, in an ASP setting led by pharmacists.
Methods
This retrospective descriptive study included patients who were hospitalized for the treatment of laboratory-confirmed COVID-19 infection. The Theradoc clinical surveillance system was used to retrieve a list of patients who were admitted to VASNHS from November 1, 2020, to January 31, 2021, and tested positive for COVID-19. Patients with incidental positive COVID-19 test results or those who received antibiotics for extrapulmonary indications on hospital admission were excluded.
Each patient chart was reviewed and data, including clinical presentations, procalcitonin (PCT), the requirement of supplemental oxygen, vital signs, imaging findings, antibiotic orders on admission, ASP interventions such as discontinuation or changes to antibiotic therapy during the first 72 hours of hospital admission, clinical outcomes, culture results, and readmission rate, defined as any hospital admission related to COVID-19 or respiratory tract infection within 30 days from the previous discharge, were collected.
The primary objective of the study was to describe antibiotic prescribing in patients hospitalized with COVID-19. The secondary outcomes included the prevalence of bacterial coinfection and nosocomial bacterial infection in patients hospitalized with COVID-19.
Results
A total of 199 patients were admitted to the hospital for laboratory-confirmed COVID-19 infection from November 1, 2020, to January 31, 2021. Sixty-one patients (31%) received at least 1 antibiotic on hospital admission. Among those patients who received empiric antibiotic treatment, 29 patients (48%) met the Systemic Inflammatory Response Syndrome (SIRS) criteria. Fifty-six patients (92%) had ≥ 1 PCT level obtained, and 26 of those (46%) presented with elevated PCT levels (PCT > 0.25). Fifty patients (82%) required oxygen supplement and 49 (80%) presented with remarkable imaging findings. Of 138 patients who did not receive empiric antibiotic therapy within 72 hours of hospital admission, 56 (41%) met the SIRS criteria, 31 (29%) had elevated PCT levels, 100 (72%) required oxygen supplement, and 79 (59%) presented with remarkable imaging findings.
Antibiotic Prescribing
Forty-six of 61 patients (75%) received antibiotic treatment for community-acquired pneumonia (CAP) that included ceftriaxone and azithromycin. Three patients (5%) received ≥ 1 broad-spectrum antibiotic (4th generation cephalosporin [cefepime] or piperacillin-tazobactam), 2 (3%) received vancomycin, and 1 (2%) received a fluoroquinolone (levofloxacin) on admission.
Among 61 patients who received empiric antibiotics, the readmission rate was 6%. The mortality rate was 20%, and the mean (SD) duration of hospital stay was 13.1 (12.5) days.
Six of 199 patients (3%) had microbiologically confirmed bacterial coinfection on hospital admission: 3 were Pseudomonas aeruginosa (P aeruginosa) and 2 were Klebsiella oxytoca (Table 1).
Discussion
Prospective audit and feedback and preauthorization are recommended in guidelines as “core components of any stewardship program.”9 At VASNHS, the ASP performs daily prospective audits with intervention and feedback. Efforts have been made to maintain daily ASP activities during the pandemic. This study aimed to describe antibiotic prescribing for patients hospitalized with COVID-19 in a pharmacist-led ASP setting. It was found that up to 31% of the patients received ≥ 1 antibiotic on admission for empiric treatment of bacterial coinfection. About half of these patients met the SIRS criteria. Most of these patients received ceftriaxone and azithromycin for concern of CAP. ASP discontinued antibiotics within 72 hours in most of the patients. Chart review and discussion with ID physicians and/or hospitalists determined the probability of bacterial coinfection as well as any potential complication or patient-specific risk factor. It is important to note that most patients who received antibiotics on admission had ≥ 1 PCT level and up to 46% of them had a PCT level > 0.25. However, according to Relph and colleagues, PCT may not be a reliable indicator of bacterial infection in severe viral diseases with raised interleukin-6 levels.10 An elevated PCT level should not be the sole indicator for empiric antibiotic treatment.
Study findings confirmed the low prevalence of bacterial coinfection in patients hospitalized with COVID-19. The overuse of empiric antibiotics in a patient population unlikely to present with bacterial coinfection is concerning. It is essential to continue promoting antimicrobial stewardship during the COVID-19 pandemic to ensure appropriate and responsible antimicrobial prescribing. A thorough clinical assessment consisting of comorbidities, clinical symptoms, radiologic and microbiologic findings, as well as other relevant workup or biomarker results is crucial to determine whether the antibiotic is strongly indicated in patients hospitalized with COVID-19. Empiric antibiotic therapy should be considered only in patients with clinical findings suggestive of bacterial coinfection.
Limitations
Limitations of our study included the study design (single-center, retrospective review, lack of comparative group) and small sample size with a 3-month study period. In addition, respiratory cultures are not commonly obtained in patients who present with mild-to-moderate CAP. Using culture results solely to confirm bacterial coinfection in patients with COVID-19 could have underestimated the prevalence of bacterial infection. Developing diagnostic criteria that include clinical signs and symptoms, imaging findings, and laboratory results as well as culture results would help to better assess the presence of bacterial coinfection in this patient population.
Conclusions
The study findings showed that up to 30% of patients hospitalized for COVID-19 infection received empiric antibiotic treatment for concern of bacterial coinfection. A pharmacist-led ASP provided interventions, including early discontinuation of antibiotics in 77% of these patients.
A low prevalence of bacterial coinfection (3%) in patients hospitalized with COVID-19 also was reported. A thorough clinical workup to determine the risk of bacterial coinfection in patients with COVID-19 is important before starting empiric antibiotic therapy. Continuing to promote the ASP during the COVID-19 pandemic to ensure responsible antibiotic use and prevent antimicrobial resistance is essential.
1. Demirjian A, Sanchez GV, Finkelstein JA, et al. CDC grand rounds: getting smart about antibiotics. MMWR Morb Mortal Wkly Rep. 2015;64(32):871-873. doi:10.15585/mmwr.mm6432a3
2. Nearly half a million Americans suffered from Clostridium difficile infections in a single year. Centers for Disease Control and Prevention. Updated March 22, 2017. Accessed March 21, 2023. https://www.cdc.gov/media/releases/2015/p0225-clostridium-difficile.html
3. Centers for Disease Control and Prevention. About antimicrobial resistance. Updated October 5, 2022. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/about.html
4. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf
5. Centers for Disease Control and Prevention. COVID-19 & antibiotic resistance. Updated February 25, 2022. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/covid19.html
6. Russell CD, Fairfield CJ, Drake TM, et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe. 2021;2(8):e354-e365. doi:10.1016/S2666-5247(21)00090-2
7. Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622-1629. doi:10.1016/j.cmi.2020.07.016
8. Coenen S, de la Court JR, Buis DTP, et al. Low frequency of community-acquired bacterial co-infection in patients hospitalized for COVID-19 based on clinical, radiological and microbiological criteria: a retrospective cohort study. Antimicrob Resist Infect Control. 2021;10(1):155. doi:10.1186/s13756-021-01024-4
9. Centers for Disease Control and Prevention. The core elements of hospital antibiotic stewardship programs: 2019. Accessed March 21, 2023. https://www.cdc.gov/antibiotic-use/healthcare/pdfs/hospital-core-elements-H.pdf
10. Relph KA, Russell CD, Fairfield CJ, et al; International Severe Acute Respiratory and Emerging Infections Consortium; Coronavirus Clinical Characterisation Consortium (ISARIC4C) Investigators. Procalcitonin is not a reliable biomarker of bacterial coinfection in people with Coronavirus Disease 2019 undergoing microbiological investigation at the time of hospital admission. Open Forum Infect Dis. 2022;9(5):ofac179. doi:10.1093/ofid/ofac179
1. Demirjian A, Sanchez GV, Finkelstein JA, et al. CDC grand rounds: getting smart about antibiotics. MMWR Morb Mortal Wkly Rep. 2015;64(32):871-873. doi:10.15585/mmwr.mm6432a3
2. Nearly half a million Americans suffered from Clostridium difficile infections in a single year. Centers for Disease Control and Prevention. Updated March 22, 2017. Accessed March 21, 2023. https://www.cdc.gov/media/releases/2015/p0225-clostridium-difficile.html
3. Centers for Disease Control and Prevention. About antimicrobial resistance. Updated October 5, 2022. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/about.html
4. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf
5. Centers for Disease Control and Prevention. COVID-19 & antibiotic resistance. Updated February 25, 2022. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/covid19.html
6. Russell CD, Fairfield CJ, Drake TM, et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe. 2021;2(8):e354-e365. doi:10.1016/S2666-5247(21)00090-2
7. Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622-1629. doi:10.1016/j.cmi.2020.07.016
8. Coenen S, de la Court JR, Buis DTP, et al. Low frequency of community-acquired bacterial co-infection in patients hospitalized for COVID-19 based on clinical, radiological and microbiological criteria: a retrospective cohort study. Antimicrob Resist Infect Control. 2021;10(1):155. doi:10.1186/s13756-021-01024-4
9. Centers for Disease Control and Prevention. The core elements of hospital antibiotic stewardship programs: 2019. Accessed March 21, 2023. https://www.cdc.gov/antibiotic-use/healthcare/pdfs/hospital-core-elements-H.pdf
10. Relph KA, Russell CD, Fairfield CJ, et al; International Severe Acute Respiratory and Emerging Infections Consortium; Coronavirus Clinical Characterisation Consortium (ISARIC4C) Investigators. Procalcitonin is not a reliable biomarker of bacterial coinfection in people with Coronavirus Disease 2019 undergoing microbiological investigation at the time of hospital admission. Open Forum Infect Dis. 2022;9(5):ofac179. doi:10.1093/ofid/ofac179