User login
‘Dealing with a different beast’: Why Delta has doctors worried
Catherine O’Neal, MD, an infectious disease physician, took to the podium of the Louisiana governor’s press conference recently and did not mince words.
“The Delta variant is not last year’s virus, and it’s become incredibly apparent to healthcare workers that we are dealing with a different beast,” she said.
Louisiana is one of the least vaccinated states in the country. In the United States as a whole, 48.6% of the population is fully vaccinated. In Louisiana, it’s just 36%, and Delta is bearing down.
Dr. O’Neal spoke about the pressure that rising COVID cases were already putting on her hospital, Our Lady of the Lake Regional Medical Center in Baton Rouge. She talked about watching her peers, 30- and 40-year-olds, become severely ill with the latest iteration of the new coronavirus — the Delta variant — which is sweeping through the United States with astonishing speed, causing new cases, hospitalizations, and deaths to rise again.
Dr. O’Neal talked about parents who might not be alive to see their children go off to college in a few weeks. She talked about increasing hospital admissions for infected kids and pregnant women on ventilators.
“I want to be clear after seeing what we’ve seen the last two weeks. We only have two choices: We are either going to get vaccinated and end the pandemic, or we’re going to accept death and a lot of it,” Dr. O’Neal said, her voice choked by emotion.
Where Delta goes, death follows
Delta was first identified in India, where it caused a devastating surge in the spring. In a population that was largely unvaccinated, researchers think it may have caused as many as three million deaths. In just a few months’ time, it has sped across the globe.
, which was first identified in the United Kingdom).
Where a single infected person might have spread older versions of the virus to two or three others, mathematician and epidemiologist Adam Kucharski, PhD, an associate professor at the London School of Hygiene and Tropical Medicine, thinks that number — called the basic reproduction number — might be around six for Delta, meaning that, on average, each infected person spreads the virus to six others.
“The Delta variant is the most able and fastest and fittest of those viruses,” said Mike Ryan, executive director of the World Health Organization’s Health Emergencies Programme, in a recent press briefing.
Early evidence suggests it may also cause more severe disease in people who are not vaccinated.
“There’s clearly increased risk of ICU admission, hospitalization, and death,” said Ashleigh Tuite, PhD, MPH, an infectious disease epidemiologist at the University of Toronto in Ontario.
In a study published ahead of peer review, Dr. Tuite and her coauthor, David Fisman, MD, MPH, reviewed the health outcomes for more than 200,000 people who tested positive for SARS-CoV-2 in Ontario between February and June of 2021. Starting in February, Ontario began screening all positive COVID tests for mutations in the N501Y region for signs of mutation.
Compared with versions of the coronavirus that circulated in 2020, having an Alpha, Beta, or Gamma variant modestly increased the odds that an infected person would become sicker. The Delta variant raised the risk even higher, more than doubling the odds that an infected person would need to be hospitalized or could die from their infection.
Emerging evidence from England and Scotland, analyzed by Public Health England, also shows an increased risk for hospitalization with Delta. The increases are in line with the Canadian data. Experts caution that the picture may change over time as more evidence is gathered.
“What is causing that? We don’t know,” Dr. Tuite said.
Enhanced virus
The Delta variants (there’s actually more than one in the same viral family) have about 15 different mutations compared with the original virus. Two of these, L452R and E484Q, are mutations to the spike protein that were first flagged as problematic in other variants because they appear to help the virus escape the antibodies we make to fight it.
It has another mutation away from its binding site that’s also getting researchers’ attention — P681R.
This mutation appears to enhance the “springiness” of the parts of the virus that dock onto our cells, said Alexander Greninger, MD, PhD, assistant director of the UW Medicine Clinical Virology Laboratory at the University of Washington in Seattle. So it’s more likely to be in the right position to infect our cells if we come into contact with it.
Another theory is that P681R may also enhance the virus’s ability to fuse cells together into clumps that have several different nuclei. These balls of fused cells are called syncytia.
“So it turns into a big factory for making viruses,” said Kamran Kadkhoda, PhD, medical director of immunopathology at the Cleveland Clinic in Ohio.
This capability is not unique to Delta or even to the new coronavirus. Earlier versions and other viruses can do the same thing, but according to a recent paper in Nature, the syncytia that Delta creates are larger than the ones created by previous variants.
Scientists aren’t sure what these supersized syncytia mean, exactly, but they have some theories. They may help the virus copy itself more quickly, so a person’s viral load builds up quickly. That may enhance the ability of the virus to transmit from person to person.
And at least one recent study from China supports this idea. That study, which was posted ahead of peer review on the website Virological.org, tracked 167 people infected with Delta back to a single index case.
China has used extensive contact tracing to identify people that may have been exposed to the virus and sequester them quickly to tamp down its spread. Once a person is isolated or quarantined, they are tested daily with gold-standard PCR testing to determine whether or not they were infected.
Researchers compared the characteristics of Delta cases with those of people infected in 2020 with previous versions of the virus.
This study found that people infected by Delta tested positive more quickly than their predecessors did. In 2020, it took an average of 6 days for someone to test positive after an exposure. With Delta, it took an average of about 4 days.
When people tested positive, they had more than 1,000 times more virus in their bodies, suggesting that the Delta variant has a higher growth rate in the body.
This gives Delta a big advantage. According to Angie Rasmussen, PhD, a virologist at the Vaccine and Infectious Disease Organization at the University of Saskatchewan in Canada, who posted a thread about the study on Twitter, if people are shedding 1,000 times more virus, it is much more likely that close contacts will be exposed to enough of it to become infected themselves.
And if they’re shedding earlier in the course of their infections, the virus has more opportunity to spread.
This may help explain why Delta is so much more contagious.
Beyond transmission, Delta’s ability to form syncytia may have two other important consequences. It may help the virus hide from our immune system, and it may make the virus more damaging to the body.
Commonly, when a virus infects a cell, it will corrupt the cell’s protein-making machinery to crank out more copies of itself. When the cell dies, these new copies are released into the plasma outside the cell where they can float over and infect new cells. It’s in this extracellular space where a virus can also be attacked by the neutralizing antibodies our immune system makes to fight it off.
“Antibodies don’t penetrate inside the cell. If these viruses are going from one cell to another by just fusing to each other, antibodies become less useful,” Dr. Kadkhoda said.
Escape artist
Recent studies show that Delta is also able to escape antibodies made in response to vaccination more effectively than the Alpha, or B.1.1.7 strain. The effect was more pronounced in older adults, who tend to have weaker responses to vaccines in general.
This evasion of the immune system is particularly problematic for people who are only partially vaccinated. Data from the United Kingdom show that a single dose of vaccine is only about 31% effective at preventing illness with Delta, and 75% effective at preventing hospitalization.
After two doses, the vaccines are still highly effective — even against Delta — reaching 80% protection for illness, and 94% for hospitalization, which is why U.S. officials are begging people to get both doses of their shots, and do it as quickly as possible.
Finally, the virus’s ability to form syncytia may leave greater damage behind in the body’s tissues and organs.
“Especially in the lungs,” Dr. Kadkhoda said. The lungs are very fragile tissues. Their tiny air sacs — the alveoli — are only a single-cell thick. They have to be very thin to exchange oxygen in the blood.
“Any damage like that can severely affect any oxygen exchange and the normal housekeeping activities of that tissue,” he said. “In those vital organs, it may be very problematic.”
The research is still early, but studies in animals and cell lines are backing up what doctors say they are seeing in hospitalized patients.
A recent preprint study from researchers in Japan found that hamsters infected with Delta lost more weight — a proxy for how sick they were — compared with hamsters infected with an older version of the virus. The researchers attribute this to the viruses› ability to fuse cells together to form syncytia.
Another investigation, from researchers in India, infected two groups of hamsters — one with the original “wild type” strain of the virus, the other with the Delta variant of the new coronavirus.
As in the Japanese study, the hamsters infected with Delta lost more weight. When the researchers performed necropsies on the animals, they found more lung damage and bleeding in hamsters infected with Delta. This study was also posted as a preprint ahead of peer review.
German researchers working with pseudotyped versions of the new coronavirus — viruses that have been genetically changed to make them safer to work with — watched what happened after they used these pseudoviruses to infect lung, colon, and kidney cells in the lab.
They, too, found that cells infected with the Delta variant formed more and larger syncytia compared with cells infected with the wild type strain of the virus. The authors write that their findings suggest Delta could “cause more tissue damage, and thus be more pathogenic, than previous variants.”Researchers say it’s important to remember that, while interesting, this research isn’t conclusive. Hamsters and cells aren’t humans. More studies are needed to prove these theories.
Scientists say that what we already know about Delta makes vaccination more important than ever.
“The net effect is really that, you know, this is worrisome in people who are unvaccinated and then people who have breakthrough infections, but it’s not…a reason to panic or to throw up our hands and say you know, this pandemic is never going to end,” Dr. Tuite said, “[b]ecause what we do see is that the vaccines continue to be highly protective.”
A version of this article first appeared on Medscape.com.
Catherine O’Neal, MD, an infectious disease physician, took to the podium of the Louisiana governor’s press conference recently and did not mince words.
“The Delta variant is not last year’s virus, and it’s become incredibly apparent to healthcare workers that we are dealing with a different beast,” she said.
Louisiana is one of the least vaccinated states in the country. In the United States as a whole, 48.6% of the population is fully vaccinated. In Louisiana, it’s just 36%, and Delta is bearing down.
Dr. O’Neal spoke about the pressure that rising COVID cases were already putting on her hospital, Our Lady of the Lake Regional Medical Center in Baton Rouge. She talked about watching her peers, 30- and 40-year-olds, become severely ill with the latest iteration of the new coronavirus — the Delta variant — which is sweeping through the United States with astonishing speed, causing new cases, hospitalizations, and deaths to rise again.
Dr. O’Neal talked about parents who might not be alive to see their children go off to college in a few weeks. She talked about increasing hospital admissions for infected kids and pregnant women on ventilators.
“I want to be clear after seeing what we’ve seen the last two weeks. We only have two choices: We are either going to get vaccinated and end the pandemic, or we’re going to accept death and a lot of it,” Dr. O’Neal said, her voice choked by emotion.
Where Delta goes, death follows
Delta was first identified in India, where it caused a devastating surge in the spring. In a population that was largely unvaccinated, researchers think it may have caused as many as three million deaths. In just a few months’ time, it has sped across the globe.
, which was first identified in the United Kingdom).
Where a single infected person might have spread older versions of the virus to two or three others, mathematician and epidemiologist Adam Kucharski, PhD, an associate professor at the London School of Hygiene and Tropical Medicine, thinks that number — called the basic reproduction number — might be around six for Delta, meaning that, on average, each infected person spreads the virus to six others.
“The Delta variant is the most able and fastest and fittest of those viruses,” said Mike Ryan, executive director of the World Health Organization’s Health Emergencies Programme, in a recent press briefing.
Early evidence suggests it may also cause more severe disease in people who are not vaccinated.
“There’s clearly increased risk of ICU admission, hospitalization, and death,” said Ashleigh Tuite, PhD, MPH, an infectious disease epidemiologist at the University of Toronto in Ontario.
In a study published ahead of peer review, Dr. Tuite and her coauthor, David Fisman, MD, MPH, reviewed the health outcomes for more than 200,000 people who tested positive for SARS-CoV-2 in Ontario between February and June of 2021. Starting in February, Ontario began screening all positive COVID tests for mutations in the N501Y region for signs of mutation.
Compared with versions of the coronavirus that circulated in 2020, having an Alpha, Beta, or Gamma variant modestly increased the odds that an infected person would become sicker. The Delta variant raised the risk even higher, more than doubling the odds that an infected person would need to be hospitalized or could die from their infection.
Emerging evidence from England and Scotland, analyzed by Public Health England, also shows an increased risk for hospitalization with Delta. The increases are in line with the Canadian data. Experts caution that the picture may change over time as more evidence is gathered.
“What is causing that? We don’t know,” Dr. Tuite said.
Enhanced virus
The Delta variants (there’s actually more than one in the same viral family) have about 15 different mutations compared with the original virus. Two of these, L452R and E484Q, are mutations to the spike protein that were first flagged as problematic in other variants because they appear to help the virus escape the antibodies we make to fight it.
It has another mutation away from its binding site that’s also getting researchers’ attention — P681R.
This mutation appears to enhance the “springiness” of the parts of the virus that dock onto our cells, said Alexander Greninger, MD, PhD, assistant director of the UW Medicine Clinical Virology Laboratory at the University of Washington in Seattle. So it’s more likely to be in the right position to infect our cells if we come into contact with it.
Another theory is that P681R may also enhance the virus’s ability to fuse cells together into clumps that have several different nuclei. These balls of fused cells are called syncytia.
“So it turns into a big factory for making viruses,” said Kamran Kadkhoda, PhD, medical director of immunopathology at the Cleveland Clinic in Ohio.
This capability is not unique to Delta or even to the new coronavirus. Earlier versions and other viruses can do the same thing, but according to a recent paper in Nature, the syncytia that Delta creates are larger than the ones created by previous variants.
Scientists aren’t sure what these supersized syncytia mean, exactly, but they have some theories. They may help the virus copy itself more quickly, so a person’s viral load builds up quickly. That may enhance the ability of the virus to transmit from person to person.
And at least one recent study from China supports this idea. That study, which was posted ahead of peer review on the website Virological.org, tracked 167 people infected with Delta back to a single index case.
China has used extensive contact tracing to identify people that may have been exposed to the virus and sequester them quickly to tamp down its spread. Once a person is isolated or quarantined, they are tested daily with gold-standard PCR testing to determine whether or not they were infected.
Researchers compared the characteristics of Delta cases with those of people infected in 2020 with previous versions of the virus.
This study found that people infected by Delta tested positive more quickly than their predecessors did. In 2020, it took an average of 6 days for someone to test positive after an exposure. With Delta, it took an average of about 4 days.
When people tested positive, they had more than 1,000 times more virus in their bodies, suggesting that the Delta variant has a higher growth rate in the body.
This gives Delta a big advantage. According to Angie Rasmussen, PhD, a virologist at the Vaccine and Infectious Disease Organization at the University of Saskatchewan in Canada, who posted a thread about the study on Twitter, if people are shedding 1,000 times more virus, it is much more likely that close contacts will be exposed to enough of it to become infected themselves.
And if they’re shedding earlier in the course of their infections, the virus has more opportunity to spread.
This may help explain why Delta is so much more contagious.
Beyond transmission, Delta’s ability to form syncytia may have two other important consequences. It may help the virus hide from our immune system, and it may make the virus more damaging to the body.
Commonly, when a virus infects a cell, it will corrupt the cell’s protein-making machinery to crank out more copies of itself. When the cell dies, these new copies are released into the plasma outside the cell where they can float over and infect new cells. It’s in this extracellular space where a virus can also be attacked by the neutralizing antibodies our immune system makes to fight it off.
“Antibodies don’t penetrate inside the cell. If these viruses are going from one cell to another by just fusing to each other, antibodies become less useful,” Dr. Kadkhoda said.
Escape artist
Recent studies show that Delta is also able to escape antibodies made in response to vaccination more effectively than the Alpha, or B.1.1.7 strain. The effect was more pronounced in older adults, who tend to have weaker responses to vaccines in general.
This evasion of the immune system is particularly problematic for people who are only partially vaccinated. Data from the United Kingdom show that a single dose of vaccine is only about 31% effective at preventing illness with Delta, and 75% effective at preventing hospitalization.
After two doses, the vaccines are still highly effective — even against Delta — reaching 80% protection for illness, and 94% for hospitalization, which is why U.S. officials are begging people to get both doses of their shots, and do it as quickly as possible.
Finally, the virus’s ability to form syncytia may leave greater damage behind in the body’s tissues and organs.
“Especially in the lungs,” Dr. Kadkhoda said. The lungs are very fragile tissues. Their tiny air sacs — the alveoli — are only a single-cell thick. They have to be very thin to exchange oxygen in the blood.
“Any damage like that can severely affect any oxygen exchange and the normal housekeeping activities of that tissue,” he said. “In those vital organs, it may be very problematic.”
The research is still early, but studies in animals and cell lines are backing up what doctors say they are seeing in hospitalized patients.
A recent preprint study from researchers in Japan found that hamsters infected with Delta lost more weight — a proxy for how sick they were — compared with hamsters infected with an older version of the virus. The researchers attribute this to the viruses› ability to fuse cells together to form syncytia.
Another investigation, from researchers in India, infected two groups of hamsters — one with the original “wild type” strain of the virus, the other with the Delta variant of the new coronavirus.
As in the Japanese study, the hamsters infected with Delta lost more weight. When the researchers performed necropsies on the animals, they found more lung damage and bleeding in hamsters infected with Delta. This study was also posted as a preprint ahead of peer review.
German researchers working with pseudotyped versions of the new coronavirus — viruses that have been genetically changed to make them safer to work with — watched what happened after they used these pseudoviruses to infect lung, colon, and kidney cells in the lab.
They, too, found that cells infected with the Delta variant formed more and larger syncytia compared with cells infected with the wild type strain of the virus. The authors write that their findings suggest Delta could “cause more tissue damage, and thus be more pathogenic, than previous variants.”Researchers say it’s important to remember that, while interesting, this research isn’t conclusive. Hamsters and cells aren’t humans. More studies are needed to prove these theories.
Scientists say that what we already know about Delta makes vaccination more important than ever.
“The net effect is really that, you know, this is worrisome in people who are unvaccinated and then people who have breakthrough infections, but it’s not…a reason to panic or to throw up our hands and say you know, this pandemic is never going to end,” Dr. Tuite said, “[b]ecause what we do see is that the vaccines continue to be highly protective.”
A version of this article first appeared on Medscape.com.
Catherine O’Neal, MD, an infectious disease physician, took to the podium of the Louisiana governor’s press conference recently and did not mince words.
“The Delta variant is not last year’s virus, and it’s become incredibly apparent to healthcare workers that we are dealing with a different beast,” she said.
Louisiana is one of the least vaccinated states in the country. In the United States as a whole, 48.6% of the population is fully vaccinated. In Louisiana, it’s just 36%, and Delta is bearing down.
Dr. O’Neal spoke about the pressure that rising COVID cases were already putting on her hospital, Our Lady of the Lake Regional Medical Center in Baton Rouge. She talked about watching her peers, 30- and 40-year-olds, become severely ill with the latest iteration of the new coronavirus — the Delta variant — which is sweeping through the United States with astonishing speed, causing new cases, hospitalizations, and deaths to rise again.
Dr. O’Neal talked about parents who might not be alive to see their children go off to college in a few weeks. She talked about increasing hospital admissions for infected kids and pregnant women on ventilators.
“I want to be clear after seeing what we’ve seen the last two weeks. We only have two choices: We are either going to get vaccinated and end the pandemic, or we’re going to accept death and a lot of it,” Dr. O’Neal said, her voice choked by emotion.
Where Delta goes, death follows
Delta was first identified in India, where it caused a devastating surge in the spring. In a population that was largely unvaccinated, researchers think it may have caused as many as three million deaths. In just a few months’ time, it has sped across the globe.
, which was first identified in the United Kingdom).
Where a single infected person might have spread older versions of the virus to two or three others, mathematician and epidemiologist Adam Kucharski, PhD, an associate professor at the London School of Hygiene and Tropical Medicine, thinks that number — called the basic reproduction number — might be around six for Delta, meaning that, on average, each infected person spreads the virus to six others.
“The Delta variant is the most able and fastest and fittest of those viruses,” said Mike Ryan, executive director of the World Health Organization’s Health Emergencies Programme, in a recent press briefing.
Early evidence suggests it may also cause more severe disease in people who are not vaccinated.
“There’s clearly increased risk of ICU admission, hospitalization, and death,” said Ashleigh Tuite, PhD, MPH, an infectious disease epidemiologist at the University of Toronto in Ontario.
In a study published ahead of peer review, Dr. Tuite and her coauthor, David Fisman, MD, MPH, reviewed the health outcomes for more than 200,000 people who tested positive for SARS-CoV-2 in Ontario between February and June of 2021. Starting in February, Ontario began screening all positive COVID tests for mutations in the N501Y region for signs of mutation.
Compared with versions of the coronavirus that circulated in 2020, having an Alpha, Beta, or Gamma variant modestly increased the odds that an infected person would become sicker. The Delta variant raised the risk even higher, more than doubling the odds that an infected person would need to be hospitalized or could die from their infection.
Emerging evidence from England and Scotland, analyzed by Public Health England, also shows an increased risk for hospitalization with Delta. The increases are in line with the Canadian data. Experts caution that the picture may change over time as more evidence is gathered.
“What is causing that? We don’t know,” Dr. Tuite said.
Enhanced virus
The Delta variants (there’s actually more than one in the same viral family) have about 15 different mutations compared with the original virus. Two of these, L452R and E484Q, are mutations to the spike protein that were first flagged as problematic in other variants because they appear to help the virus escape the antibodies we make to fight it.
It has another mutation away from its binding site that’s also getting researchers’ attention — P681R.
This mutation appears to enhance the “springiness” of the parts of the virus that dock onto our cells, said Alexander Greninger, MD, PhD, assistant director of the UW Medicine Clinical Virology Laboratory at the University of Washington in Seattle. So it’s more likely to be in the right position to infect our cells if we come into contact with it.
Another theory is that P681R may also enhance the virus’s ability to fuse cells together into clumps that have several different nuclei. These balls of fused cells are called syncytia.
“So it turns into a big factory for making viruses,” said Kamran Kadkhoda, PhD, medical director of immunopathology at the Cleveland Clinic in Ohio.
This capability is not unique to Delta or even to the new coronavirus. Earlier versions and other viruses can do the same thing, but according to a recent paper in Nature, the syncytia that Delta creates are larger than the ones created by previous variants.
Scientists aren’t sure what these supersized syncytia mean, exactly, but they have some theories. They may help the virus copy itself more quickly, so a person’s viral load builds up quickly. That may enhance the ability of the virus to transmit from person to person.
And at least one recent study from China supports this idea. That study, which was posted ahead of peer review on the website Virological.org, tracked 167 people infected with Delta back to a single index case.
China has used extensive contact tracing to identify people that may have been exposed to the virus and sequester them quickly to tamp down its spread. Once a person is isolated or quarantined, they are tested daily with gold-standard PCR testing to determine whether or not they were infected.
Researchers compared the characteristics of Delta cases with those of people infected in 2020 with previous versions of the virus.
This study found that people infected by Delta tested positive more quickly than their predecessors did. In 2020, it took an average of 6 days for someone to test positive after an exposure. With Delta, it took an average of about 4 days.
When people tested positive, they had more than 1,000 times more virus in their bodies, suggesting that the Delta variant has a higher growth rate in the body.
This gives Delta a big advantage. According to Angie Rasmussen, PhD, a virologist at the Vaccine and Infectious Disease Organization at the University of Saskatchewan in Canada, who posted a thread about the study on Twitter, if people are shedding 1,000 times more virus, it is much more likely that close contacts will be exposed to enough of it to become infected themselves.
And if they’re shedding earlier in the course of their infections, the virus has more opportunity to spread.
This may help explain why Delta is so much more contagious.
Beyond transmission, Delta’s ability to form syncytia may have two other important consequences. It may help the virus hide from our immune system, and it may make the virus more damaging to the body.
Commonly, when a virus infects a cell, it will corrupt the cell’s protein-making machinery to crank out more copies of itself. When the cell dies, these new copies are released into the plasma outside the cell where they can float over and infect new cells. It’s in this extracellular space where a virus can also be attacked by the neutralizing antibodies our immune system makes to fight it off.
“Antibodies don’t penetrate inside the cell. If these viruses are going from one cell to another by just fusing to each other, antibodies become less useful,” Dr. Kadkhoda said.
Escape artist
Recent studies show that Delta is also able to escape antibodies made in response to vaccination more effectively than the Alpha, or B.1.1.7 strain. The effect was more pronounced in older adults, who tend to have weaker responses to vaccines in general.
This evasion of the immune system is particularly problematic for people who are only partially vaccinated. Data from the United Kingdom show that a single dose of vaccine is only about 31% effective at preventing illness with Delta, and 75% effective at preventing hospitalization.
After two doses, the vaccines are still highly effective — even against Delta — reaching 80% protection for illness, and 94% for hospitalization, which is why U.S. officials are begging people to get both doses of their shots, and do it as quickly as possible.
Finally, the virus’s ability to form syncytia may leave greater damage behind in the body’s tissues and organs.
“Especially in the lungs,” Dr. Kadkhoda said. The lungs are very fragile tissues. Their tiny air sacs — the alveoli — are only a single-cell thick. They have to be very thin to exchange oxygen in the blood.
“Any damage like that can severely affect any oxygen exchange and the normal housekeeping activities of that tissue,” he said. “In those vital organs, it may be very problematic.”
The research is still early, but studies in animals and cell lines are backing up what doctors say they are seeing in hospitalized patients.
A recent preprint study from researchers in Japan found that hamsters infected with Delta lost more weight — a proxy for how sick they were — compared with hamsters infected with an older version of the virus. The researchers attribute this to the viruses› ability to fuse cells together to form syncytia.
Another investigation, from researchers in India, infected two groups of hamsters — one with the original “wild type” strain of the virus, the other with the Delta variant of the new coronavirus.
As in the Japanese study, the hamsters infected with Delta lost more weight. When the researchers performed necropsies on the animals, they found more lung damage and bleeding in hamsters infected with Delta. This study was also posted as a preprint ahead of peer review.
German researchers working with pseudotyped versions of the new coronavirus — viruses that have been genetically changed to make them safer to work with — watched what happened after they used these pseudoviruses to infect lung, colon, and kidney cells in the lab.
They, too, found that cells infected with the Delta variant formed more and larger syncytia compared with cells infected with the wild type strain of the virus. The authors write that their findings suggest Delta could “cause more tissue damage, and thus be more pathogenic, than previous variants.”Researchers say it’s important to remember that, while interesting, this research isn’t conclusive. Hamsters and cells aren’t humans. More studies are needed to prove these theories.
Scientists say that what we already know about Delta makes vaccination more important than ever.
“The net effect is really that, you know, this is worrisome in people who are unvaccinated and then people who have breakthrough infections, but it’s not…a reason to panic or to throw up our hands and say you know, this pandemic is never going to end,” Dr. Tuite said, “[b]ecause what we do see is that the vaccines continue to be highly protective.”
A version of this article first appeared on Medscape.com.
COVID-19 vaccine hesitancy still weighs heavy for some rheumatic disease patients
With 49% of the U.S. population fully vaccinated against SARS-CoV-2, a new study highlights the degree of vaccine hesitancy among patients with rheumatic disease to get the vaccine.
The international study, published in May 2021 in Rheumatology, suggests that, of 1,258 patients surveyed worldwide, approximately 40% of patients said they would decline the vaccine.
“Sometimes it’s helpful to talk through their concerns,” said Jeffrey Curtis, MD, MPH, a University of Alabama at Birmingham rheumatologist who leads the American College of Rheumatology COVID-19 vaccine task force. Dr. Curtis recently reviewed the current literature on COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases (RMDs) at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
COVID-19 vaccinations for patients with autoimmune inflammatory rheumatic disease (AIIRD) is not straightforward. The immune response can be blunted by existing treatments and disease flares can occur.
The latest version of COVID-19 vaccination guidance for patients with RMDs from the ACR addresses vaccine use and implementation strategies. The guidance was issued as conditional or provisional because of the lack of evidence. Its principals are largely based on accepted practice for other vaccines. The guidance is routinely updated as new evidence becomes available. In his presentation at GRAPPA, Dr. Curtis reviewed the latest version of the guidance, which he emphasized is a guidance only and not meant to replace clinical judgment or shared decision-making with patients.
“This is a platform for you to start from as you are thinking about and discussing with your patient what might be best for him or her,” he said.
Concerns about impact of disease activity, treatments on effectiveness
Dr. Curtis highlighted some controversial aspects of COVID-19 vaccines, including heterogeneity of rheumatic diseases and treatment. Patients with AIIRD, including psoriatic arthritis, spondyloarthritis, RA, and lupus, are at higher risk for hospitalized COVID-19 and worse outcomes, and as such, they are prioritized for vaccination by the Centers for Disease Control and Prevention.
However, for AIIRD patients, the immune response to COVID-19 vaccination can be “blunted,” according to one study. This may be because of glucocorticoid use or high disease activity. Immunomodulatory therapies, such as methotrexate, rituximab, and abatacept, are known to diminish vaccine response in general. The evidence is less clear for tumor necrosis factor and Janus kinase inhibitors, but they are thought to have the same impact on vaccine effectiveness, Dr. Curtis said. But in these cases, if the effect of a COVID-19 vaccine drops from 90% to 70%, the benefits of vaccination still far outweighs the risk of contracting COVID-19.
“Although we don’t have strong data with clinical outcomes for autoimmune disease or inflammatory disease patients, I’ll run a hypothetical and say: ‘Look, if this vaccine starts 90%-95% effective, even if it’s only 70% effective in somebody with lupus or vasculitis or someone who is taking a higher dose of steroids, I’ll take 70% over nothing if you chose to be vaccinated,’ ” he said.
The benefit of vaccination also outweighs the potential risk of disease flare, he said. The risk is real, but to date, no studies have pointed to a significant risk of disease flare or worsening. However, there have been reported cases of myocardial infarction.
Autoimmune manifestations after vaccination vs. after infection
Researchers writing in the June 29, 2021, issue of JAMA Cardiology described case reports of acute myocarditis in 23 people who received the BNT162b2-mRNA (Pfizer-BioNTech) or mRNA-1273 (Moderna) messenger RNA (mRNA) COVID-19 vaccines. Plus, there been subsequent reports of myocarditis in other patients, wrote David K. Shay, MD, MPH, in an accompanying editorial. Dr. Shay is a member of the CDC COVID-19 Response Team.
“What do we know about this possible association between myocarditis and immunization with mRNA-based COVID-19 vaccines, and what remains unclear? Acute onset of chest pain 3-5 days after vaccine administration, usually after a second dose, is a typical feature of reported cases and suggests an immune-mediated mechanism,” he said.
The cases of myocarditis are concerning, Dr. Curtis said, but the risk is very low with relatively few cases reported among 161 million fully vaccinated people in the United States.
“Certainly, we’re not seeking to minimize that, but the risk of getting COVID and some of the downstream sequelae (autoimmune manifestations) almost certainly outweigh the risks for some of the autoimmune manifestations or worsening [condition],” he said.
A nationwide cohort study from Denmark of 58,052 patients with inflammatory rheumatic disease published in December 2020 in Rheumatology, found that patients with COVID-19 who had an inflammatory rheumatic disease were more likely to be admitted to the hospital, compared with COVID-19 patients without rheumatic disease. Patients with rheumatic disease had a higher risk of a severe COVID-19 outcome, but it was not a statistically significant difference, said Dr. Curtis, adding that the individual factors such as age and treatment currently received largely determines the risk. The strongest associations between hospitalization for COVID-19 and rheumatic disease were found among patients with RA, vasculitis, and connective tissue disease. Dr. Curtis noted that his own new study results show that risk of death from a COVID-19 infection is higher for patients who have RA or psoriatic arthritis.
There have been published case reports of patients who have developed new-onset lupus, vasculitis, Kawasaki disease, multiple sclerosis, autoimmune cytopenias, and other manifestations after a COVID-19 infection. “These authors suggest that perhaps there is a transient influence on the immune system that leads to a loss of self-tolerance to antigens,” Dr. Curtis said. “Some patients may have an underlying predisposition to autoimmunity in which infections just unmask as we sometimes see with other infections – chronic hepatitis for example.”
Antibody tests not recommended
In its COVID-19 guidance, the ACR, like the Food and Drug Administration, recommends health care providers not to routinely order antibody tests for IgM or IgG to assess immunity after a person has been vaccinated or to assess the need for vaccination in an unvaccinated person. More research is needed to determine if antibodies provide protection, and if so, for how long and how much. Plus, the antibody testing process is not clear cut, so ordering the wrong test is possible, Dr. Curtis said. The tests should clearly differentiate between spike proteins or nucleocapsid proteins.
“The bottom line is that you might be ordering the wrong lab test. Even if you’re ordering the right lab test, I would assert that you probably don’t know what to do with the result. I would then ask you, ‘Does it mean they are protected? Does it mean they are not protected? What are you going to do with the results?’ ” he asked.
Kevin Winthrop, MD, MPH, a specialist in infectious diseases at Oregon Health & Science University, Portland, said that, at this point, it’s too early to know what antibody tests mean. “I think it is tempting to test some people, especially patients on B-cell depletion therapy and those on mycophenolate mofetil (MMF). Outside of those two types of [disease-modifying antirheumatic drug] users, I wouldn’t be tempted to test. We don’t know how well protected they are, but we assume they are protected to some extent,” he said. “They’re probably partially protected and as such, they should take the same precautions they were taking a year ago: masking and avoidance. I think that’s just how it’s going to be for those folks for another year until we get this thing sorted out.”
Modifications to existing rheumatic disease therapies
In its COVID-19 vaccine guidance, the ACR issued recommendations for some common rheumatic disease therapeutics before and/or after the COVID-19 vaccine is administered. The modifications are limited to MMF, methotrexate, JAK inhibitors, subcutaneous abatacept, acetaminophen, and NSAIDs. The recommendations include: hold mycophenolate for 1 week after vaccination if disease is stable; for patients with well-controlled disease, hold methotrexate for 1 week after each of the two mRNA vaccine doses; for patients with well-controlled disease receiving the Johnson & Johnson vaccine, hold methotrexate for 2 weeks after receiving the vaccine; hold JAK inhibitors for 1 week after each dose; for abatacept subcutaneous, hold treatment for 1 week before and after the first dose; and in patients with stable disease, hold acetaminophen and NSAIDs for 24 hours before vaccination, because taking either before vaccination could blunt the vaccine response, Dr. Curtis said.
Holding medication, such as methotrexate, could risk having a flare-up of disease. One study showed the rate of disease flare-up because of withholding standard treatment may be up to 11%, compared with 5.1% in patients who did not hold treatment, he said.
“The point is, if you hold some of these therapies, whether methotrexate or tofacitinib, arthritis will get a little bit worse,” Dr. Curtis said.
A study published on the preprint server medRxiv found that immunosuppressive therapies blunted the response of SARS-CoV-2 vaccines in patients with chronic inflammatory diseases, most significantly with glucocorticoids and B-cell therapies.
“That’s what’s led to a lot of the guidance statements about holding treatments for a week or 2 for rituximab. If you’re giving it at 6-month intervals, you want to schedule the vaccine dose or series at about month 5, or a month before the next cycle,” he said.
Talking with patients about COVID-19 vaccination
In talking with patients about vaccine safety, Dr. Curtis recommends addressing a few common misperceptions. First, COVID-19 viruses were not created with a live-attenuated virus (which would be contraindicated for immunosuppressed patients). “You can put patients’ mind at ease that none of the vaccine candidates or platforms – even those that say viral vector – put patients at risk for contracting the infection. These are nonreplicating. So, it’s like you extracted the engine that would allow this virus to replicate,” he said.
Of three COVID-19 vaccinations available in the United States, is one better than the other? The ACR COVID-19 vaccine task force did not reach a consensus on safety profiles of the vaccines because, without head-to-head comparisons, it’s impossible to know, he said.
In talking with patients, review the protocol for continuing with prescribed treatment modalities before the patient receives a COVID-19 vaccine. Safety concerns and concerns about the possibility of having a disease flare-up should be addressed, he said.
With 49% of the U.S. population fully vaccinated against SARS-CoV-2, a new study highlights the degree of vaccine hesitancy among patients with rheumatic disease to get the vaccine.
The international study, published in May 2021 in Rheumatology, suggests that, of 1,258 patients surveyed worldwide, approximately 40% of patients said they would decline the vaccine.
“Sometimes it’s helpful to talk through their concerns,” said Jeffrey Curtis, MD, MPH, a University of Alabama at Birmingham rheumatologist who leads the American College of Rheumatology COVID-19 vaccine task force. Dr. Curtis recently reviewed the current literature on COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases (RMDs) at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
COVID-19 vaccinations for patients with autoimmune inflammatory rheumatic disease (AIIRD) is not straightforward. The immune response can be blunted by existing treatments and disease flares can occur.
The latest version of COVID-19 vaccination guidance for patients with RMDs from the ACR addresses vaccine use and implementation strategies. The guidance was issued as conditional or provisional because of the lack of evidence. Its principals are largely based on accepted practice for other vaccines. The guidance is routinely updated as new evidence becomes available. In his presentation at GRAPPA, Dr. Curtis reviewed the latest version of the guidance, which he emphasized is a guidance only and not meant to replace clinical judgment or shared decision-making with patients.
“This is a platform for you to start from as you are thinking about and discussing with your patient what might be best for him or her,” he said.
Concerns about impact of disease activity, treatments on effectiveness
Dr. Curtis highlighted some controversial aspects of COVID-19 vaccines, including heterogeneity of rheumatic diseases and treatment. Patients with AIIRD, including psoriatic arthritis, spondyloarthritis, RA, and lupus, are at higher risk for hospitalized COVID-19 and worse outcomes, and as such, they are prioritized for vaccination by the Centers for Disease Control and Prevention.
However, for AIIRD patients, the immune response to COVID-19 vaccination can be “blunted,” according to one study. This may be because of glucocorticoid use or high disease activity. Immunomodulatory therapies, such as methotrexate, rituximab, and abatacept, are known to diminish vaccine response in general. The evidence is less clear for tumor necrosis factor and Janus kinase inhibitors, but they are thought to have the same impact on vaccine effectiveness, Dr. Curtis said. But in these cases, if the effect of a COVID-19 vaccine drops from 90% to 70%, the benefits of vaccination still far outweighs the risk of contracting COVID-19.
“Although we don’t have strong data with clinical outcomes for autoimmune disease or inflammatory disease patients, I’ll run a hypothetical and say: ‘Look, if this vaccine starts 90%-95% effective, even if it’s only 70% effective in somebody with lupus or vasculitis or someone who is taking a higher dose of steroids, I’ll take 70% over nothing if you chose to be vaccinated,’ ” he said.
The benefit of vaccination also outweighs the potential risk of disease flare, he said. The risk is real, but to date, no studies have pointed to a significant risk of disease flare or worsening. However, there have been reported cases of myocardial infarction.
Autoimmune manifestations after vaccination vs. after infection
Researchers writing in the June 29, 2021, issue of JAMA Cardiology described case reports of acute myocarditis in 23 people who received the BNT162b2-mRNA (Pfizer-BioNTech) or mRNA-1273 (Moderna) messenger RNA (mRNA) COVID-19 vaccines. Plus, there been subsequent reports of myocarditis in other patients, wrote David K. Shay, MD, MPH, in an accompanying editorial. Dr. Shay is a member of the CDC COVID-19 Response Team.
“What do we know about this possible association between myocarditis and immunization with mRNA-based COVID-19 vaccines, and what remains unclear? Acute onset of chest pain 3-5 days after vaccine administration, usually after a second dose, is a typical feature of reported cases and suggests an immune-mediated mechanism,” he said.
The cases of myocarditis are concerning, Dr. Curtis said, but the risk is very low with relatively few cases reported among 161 million fully vaccinated people in the United States.
“Certainly, we’re not seeking to minimize that, but the risk of getting COVID and some of the downstream sequelae (autoimmune manifestations) almost certainly outweigh the risks for some of the autoimmune manifestations or worsening [condition],” he said.
A nationwide cohort study from Denmark of 58,052 patients with inflammatory rheumatic disease published in December 2020 in Rheumatology, found that patients with COVID-19 who had an inflammatory rheumatic disease were more likely to be admitted to the hospital, compared with COVID-19 patients without rheumatic disease. Patients with rheumatic disease had a higher risk of a severe COVID-19 outcome, but it was not a statistically significant difference, said Dr. Curtis, adding that the individual factors such as age and treatment currently received largely determines the risk. The strongest associations between hospitalization for COVID-19 and rheumatic disease were found among patients with RA, vasculitis, and connective tissue disease. Dr. Curtis noted that his own new study results show that risk of death from a COVID-19 infection is higher for patients who have RA or psoriatic arthritis.
There have been published case reports of patients who have developed new-onset lupus, vasculitis, Kawasaki disease, multiple sclerosis, autoimmune cytopenias, and other manifestations after a COVID-19 infection. “These authors suggest that perhaps there is a transient influence on the immune system that leads to a loss of self-tolerance to antigens,” Dr. Curtis said. “Some patients may have an underlying predisposition to autoimmunity in which infections just unmask as we sometimes see with other infections – chronic hepatitis for example.”
Antibody tests not recommended
In its COVID-19 guidance, the ACR, like the Food and Drug Administration, recommends health care providers not to routinely order antibody tests for IgM or IgG to assess immunity after a person has been vaccinated or to assess the need for vaccination in an unvaccinated person. More research is needed to determine if antibodies provide protection, and if so, for how long and how much. Plus, the antibody testing process is not clear cut, so ordering the wrong test is possible, Dr. Curtis said. The tests should clearly differentiate between spike proteins or nucleocapsid proteins.
“The bottom line is that you might be ordering the wrong lab test. Even if you’re ordering the right lab test, I would assert that you probably don’t know what to do with the result. I would then ask you, ‘Does it mean they are protected? Does it mean they are not protected? What are you going to do with the results?’ ” he asked.
Kevin Winthrop, MD, MPH, a specialist in infectious diseases at Oregon Health & Science University, Portland, said that, at this point, it’s too early to know what antibody tests mean. “I think it is tempting to test some people, especially patients on B-cell depletion therapy and those on mycophenolate mofetil (MMF). Outside of those two types of [disease-modifying antirheumatic drug] users, I wouldn’t be tempted to test. We don’t know how well protected they are, but we assume they are protected to some extent,” he said. “They’re probably partially protected and as such, they should take the same precautions they were taking a year ago: masking and avoidance. I think that’s just how it’s going to be for those folks for another year until we get this thing sorted out.”
Modifications to existing rheumatic disease therapies
In its COVID-19 vaccine guidance, the ACR issued recommendations for some common rheumatic disease therapeutics before and/or after the COVID-19 vaccine is administered. The modifications are limited to MMF, methotrexate, JAK inhibitors, subcutaneous abatacept, acetaminophen, and NSAIDs. The recommendations include: hold mycophenolate for 1 week after vaccination if disease is stable; for patients with well-controlled disease, hold methotrexate for 1 week after each of the two mRNA vaccine doses; for patients with well-controlled disease receiving the Johnson & Johnson vaccine, hold methotrexate for 2 weeks after receiving the vaccine; hold JAK inhibitors for 1 week after each dose; for abatacept subcutaneous, hold treatment for 1 week before and after the first dose; and in patients with stable disease, hold acetaminophen and NSAIDs for 24 hours before vaccination, because taking either before vaccination could blunt the vaccine response, Dr. Curtis said.
Holding medication, such as methotrexate, could risk having a flare-up of disease. One study showed the rate of disease flare-up because of withholding standard treatment may be up to 11%, compared with 5.1% in patients who did not hold treatment, he said.
“The point is, if you hold some of these therapies, whether methotrexate or tofacitinib, arthritis will get a little bit worse,” Dr. Curtis said.
A study published on the preprint server medRxiv found that immunosuppressive therapies blunted the response of SARS-CoV-2 vaccines in patients with chronic inflammatory diseases, most significantly with glucocorticoids and B-cell therapies.
“That’s what’s led to a lot of the guidance statements about holding treatments for a week or 2 for rituximab. If you’re giving it at 6-month intervals, you want to schedule the vaccine dose or series at about month 5, or a month before the next cycle,” he said.
Talking with patients about COVID-19 vaccination
In talking with patients about vaccine safety, Dr. Curtis recommends addressing a few common misperceptions. First, COVID-19 viruses were not created with a live-attenuated virus (which would be contraindicated for immunosuppressed patients). “You can put patients’ mind at ease that none of the vaccine candidates or platforms – even those that say viral vector – put patients at risk for contracting the infection. These are nonreplicating. So, it’s like you extracted the engine that would allow this virus to replicate,” he said.
Of three COVID-19 vaccinations available in the United States, is one better than the other? The ACR COVID-19 vaccine task force did not reach a consensus on safety profiles of the vaccines because, without head-to-head comparisons, it’s impossible to know, he said.
In talking with patients, review the protocol for continuing with prescribed treatment modalities before the patient receives a COVID-19 vaccine. Safety concerns and concerns about the possibility of having a disease flare-up should be addressed, he said.
With 49% of the U.S. population fully vaccinated against SARS-CoV-2, a new study highlights the degree of vaccine hesitancy among patients with rheumatic disease to get the vaccine.
The international study, published in May 2021 in Rheumatology, suggests that, of 1,258 patients surveyed worldwide, approximately 40% of patients said they would decline the vaccine.
“Sometimes it’s helpful to talk through their concerns,” said Jeffrey Curtis, MD, MPH, a University of Alabama at Birmingham rheumatologist who leads the American College of Rheumatology COVID-19 vaccine task force. Dr. Curtis recently reviewed the current literature on COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases (RMDs) at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
COVID-19 vaccinations for patients with autoimmune inflammatory rheumatic disease (AIIRD) is not straightforward. The immune response can be blunted by existing treatments and disease flares can occur.
The latest version of COVID-19 vaccination guidance for patients with RMDs from the ACR addresses vaccine use and implementation strategies. The guidance was issued as conditional or provisional because of the lack of evidence. Its principals are largely based on accepted practice for other vaccines. The guidance is routinely updated as new evidence becomes available. In his presentation at GRAPPA, Dr. Curtis reviewed the latest version of the guidance, which he emphasized is a guidance only and not meant to replace clinical judgment or shared decision-making with patients.
“This is a platform for you to start from as you are thinking about and discussing with your patient what might be best for him or her,” he said.
Concerns about impact of disease activity, treatments on effectiveness
Dr. Curtis highlighted some controversial aspects of COVID-19 vaccines, including heterogeneity of rheumatic diseases and treatment. Patients with AIIRD, including psoriatic arthritis, spondyloarthritis, RA, and lupus, are at higher risk for hospitalized COVID-19 and worse outcomes, and as such, they are prioritized for vaccination by the Centers for Disease Control and Prevention.
However, for AIIRD patients, the immune response to COVID-19 vaccination can be “blunted,” according to one study. This may be because of glucocorticoid use or high disease activity. Immunomodulatory therapies, such as methotrexate, rituximab, and abatacept, are known to diminish vaccine response in general. The evidence is less clear for tumor necrosis factor and Janus kinase inhibitors, but they are thought to have the same impact on vaccine effectiveness, Dr. Curtis said. But in these cases, if the effect of a COVID-19 vaccine drops from 90% to 70%, the benefits of vaccination still far outweighs the risk of contracting COVID-19.
“Although we don’t have strong data with clinical outcomes for autoimmune disease or inflammatory disease patients, I’ll run a hypothetical and say: ‘Look, if this vaccine starts 90%-95% effective, even if it’s only 70% effective in somebody with lupus or vasculitis or someone who is taking a higher dose of steroids, I’ll take 70% over nothing if you chose to be vaccinated,’ ” he said.
The benefit of vaccination also outweighs the potential risk of disease flare, he said. The risk is real, but to date, no studies have pointed to a significant risk of disease flare or worsening. However, there have been reported cases of myocardial infarction.
Autoimmune manifestations after vaccination vs. after infection
Researchers writing in the June 29, 2021, issue of JAMA Cardiology described case reports of acute myocarditis in 23 people who received the BNT162b2-mRNA (Pfizer-BioNTech) or mRNA-1273 (Moderna) messenger RNA (mRNA) COVID-19 vaccines. Plus, there been subsequent reports of myocarditis in other patients, wrote David K. Shay, MD, MPH, in an accompanying editorial. Dr. Shay is a member of the CDC COVID-19 Response Team.
“What do we know about this possible association between myocarditis and immunization with mRNA-based COVID-19 vaccines, and what remains unclear? Acute onset of chest pain 3-5 days after vaccine administration, usually after a second dose, is a typical feature of reported cases and suggests an immune-mediated mechanism,” he said.
The cases of myocarditis are concerning, Dr. Curtis said, but the risk is very low with relatively few cases reported among 161 million fully vaccinated people in the United States.
“Certainly, we’re not seeking to minimize that, but the risk of getting COVID and some of the downstream sequelae (autoimmune manifestations) almost certainly outweigh the risks for some of the autoimmune manifestations or worsening [condition],” he said.
A nationwide cohort study from Denmark of 58,052 patients with inflammatory rheumatic disease published in December 2020 in Rheumatology, found that patients with COVID-19 who had an inflammatory rheumatic disease were more likely to be admitted to the hospital, compared with COVID-19 patients without rheumatic disease. Patients with rheumatic disease had a higher risk of a severe COVID-19 outcome, but it was not a statistically significant difference, said Dr. Curtis, adding that the individual factors such as age and treatment currently received largely determines the risk. The strongest associations between hospitalization for COVID-19 and rheumatic disease were found among patients with RA, vasculitis, and connective tissue disease. Dr. Curtis noted that his own new study results show that risk of death from a COVID-19 infection is higher for patients who have RA or psoriatic arthritis.
There have been published case reports of patients who have developed new-onset lupus, vasculitis, Kawasaki disease, multiple sclerosis, autoimmune cytopenias, and other manifestations after a COVID-19 infection. “These authors suggest that perhaps there is a transient influence on the immune system that leads to a loss of self-tolerance to antigens,” Dr. Curtis said. “Some patients may have an underlying predisposition to autoimmunity in which infections just unmask as we sometimes see with other infections – chronic hepatitis for example.”
Antibody tests not recommended
In its COVID-19 guidance, the ACR, like the Food and Drug Administration, recommends health care providers not to routinely order antibody tests for IgM or IgG to assess immunity after a person has been vaccinated or to assess the need for vaccination in an unvaccinated person. More research is needed to determine if antibodies provide protection, and if so, for how long and how much. Plus, the antibody testing process is not clear cut, so ordering the wrong test is possible, Dr. Curtis said. The tests should clearly differentiate between spike proteins or nucleocapsid proteins.
“The bottom line is that you might be ordering the wrong lab test. Even if you’re ordering the right lab test, I would assert that you probably don’t know what to do with the result. I would then ask you, ‘Does it mean they are protected? Does it mean they are not protected? What are you going to do with the results?’ ” he asked.
Kevin Winthrop, MD, MPH, a specialist in infectious diseases at Oregon Health & Science University, Portland, said that, at this point, it’s too early to know what antibody tests mean. “I think it is tempting to test some people, especially patients on B-cell depletion therapy and those on mycophenolate mofetil (MMF). Outside of those two types of [disease-modifying antirheumatic drug] users, I wouldn’t be tempted to test. We don’t know how well protected they are, but we assume they are protected to some extent,” he said. “They’re probably partially protected and as such, they should take the same precautions they were taking a year ago: masking and avoidance. I think that’s just how it’s going to be for those folks for another year until we get this thing sorted out.”
Modifications to existing rheumatic disease therapies
In its COVID-19 vaccine guidance, the ACR issued recommendations for some common rheumatic disease therapeutics before and/or after the COVID-19 vaccine is administered. The modifications are limited to MMF, methotrexate, JAK inhibitors, subcutaneous abatacept, acetaminophen, and NSAIDs. The recommendations include: hold mycophenolate for 1 week after vaccination if disease is stable; for patients with well-controlled disease, hold methotrexate for 1 week after each of the two mRNA vaccine doses; for patients with well-controlled disease receiving the Johnson & Johnson vaccine, hold methotrexate for 2 weeks after receiving the vaccine; hold JAK inhibitors for 1 week after each dose; for abatacept subcutaneous, hold treatment for 1 week before and after the first dose; and in patients with stable disease, hold acetaminophen and NSAIDs for 24 hours before vaccination, because taking either before vaccination could blunt the vaccine response, Dr. Curtis said.
Holding medication, such as methotrexate, could risk having a flare-up of disease. One study showed the rate of disease flare-up because of withholding standard treatment may be up to 11%, compared with 5.1% in patients who did not hold treatment, he said.
“The point is, if you hold some of these therapies, whether methotrexate or tofacitinib, arthritis will get a little bit worse,” Dr. Curtis said.
A study published on the preprint server medRxiv found that immunosuppressive therapies blunted the response of SARS-CoV-2 vaccines in patients with chronic inflammatory diseases, most significantly with glucocorticoids and B-cell therapies.
“That’s what’s led to a lot of the guidance statements about holding treatments for a week or 2 for rituximab. If you’re giving it at 6-month intervals, you want to schedule the vaccine dose or series at about month 5, or a month before the next cycle,” he said.
Talking with patients about COVID-19 vaccination
In talking with patients about vaccine safety, Dr. Curtis recommends addressing a few common misperceptions. First, COVID-19 viruses were not created with a live-attenuated virus (which would be contraindicated for immunosuppressed patients). “You can put patients’ mind at ease that none of the vaccine candidates or platforms – even those that say viral vector – put patients at risk for contracting the infection. These are nonreplicating. So, it’s like you extracted the engine that would allow this virus to replicate,” he said.
Of three COVID-19 vaccinations available in the United States, is one better than the other? The ACR COVID-19 vaccine task force did not reach a consensus on safety profiles of the vaccines because, without head-to-head comparisons, it’s impossible to know, he said.
In talking with patients, review the protocol for continuing with prescribed treatment modalities before the patient receives a COVID-19 vaccine. Safety concerns and concerns about the possibility of having a disease flare-up should be addressed, he said.
FROM THE GRAPPA 2021 ANNUAL MEETING
Children and COVID: New vaccinations increase as cases continue to climb
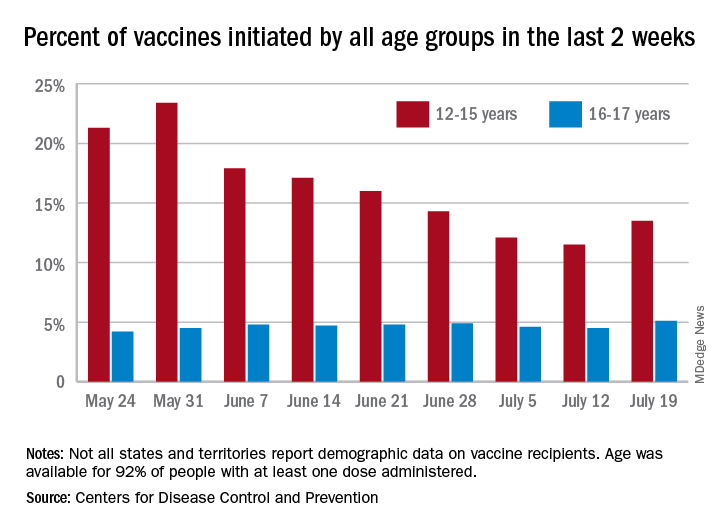
Children aged 12-15 years represented 13.5% of all first vaccinations received during the 2 weeks ending July 19, compared with 11.5% for the 2 weeks ending July 12, marking the first increase since the end of May. First vaccinations in 16- and 17-year-olds, who make up a much smaller share of the U.S. population, also went up, topping 5%, the Centers for Disease Control and Prevention said in its COVID Data Tracker.
The total number of vaccine initiations was almost 250,000 for the week ending July 19, after dropping to a low of 201,000 the previous week. Before that, first vaccinations had fallen in 5 of the previous 6 weeks, going from 1.4 million on May 24 to 307,000 on July 5, the CDC said.
New cases of COVID-19, unfortunately, continued to follow the trend among the larger population: As of July 15, weekly cases in children were up by 179% since dropping to 8,400 on June 24, the American Academy of Pediatrics and the Children’s Hospital Association said in a joint report. The 23,551 new cases in children for the week ending July 15 were 15.9% of all cases reported.
With those new cases, the total number of children infected with COVID-19 comes to almost 4.1 million since the start of the pandemic, the AAP and CHA said. The CDC data indicate that just over 5.35 million children aged 12-15 years and 3.53 million 16- and 17-year-olds have received at least one dose of the COVID-19 vaccine and that 6.8 million children aged 12-17 are fully vaccinated.
Fully vaccinated children represent 26.4% of all 12- to 15-year-olds and 38.3% of the 16- 17-year-olds as of July 19. The corresponding numbers for those who have received at least one dose are 35.2% (ages 12-15) and 46.8% (16-17), the CDC said.
The AAP recently recommended in-person learning with universal masking in schools this fall “because a significant portion of the student population is not yet eligible for vaccines. ... Many schools will not have a system to monitor vaccine status of students, teachers and staff, and some communities overall have low vaccination uptake where the virus may be circulating more prominently.”

Children aged 12-15 years represented 13.5% of all first vaccinations received during the 2 weeks ending July 19, compared with 11.5% for the 2 weeks ending July 12, marking the first increase since the end of May. First vaccinations in 16- and 17-year-olds, who make up a much smaller share of the U.S. population, also went up, topping 5%, the Centers for Disease Control and Prevention said in its COVID Data Tracker.
The total number of vaccine initiations was almost 250,000 for the week ending July 19, after dropping to a low of 201,000 the previous week. Before that, first vaccinations had fallen in 5 of the previous 6 weeks, going from 1.4 million on May 24 to 307,000 on July 5, the CDC said.
New cases of COVID-19, unfortunately, continued to follow the trend among the larger population: As of July 15, weekly cases in children were up by 179% since dropping to 8,400 on June 24, the American Academy of Pediatrics and the Children’s Hospital Association said in a joint report. The 23,551 new cases in children for the week ending July 15 were 15.9% of all cases reported.
With those new cases, the total number of children infected with COVID-19 comes to almost 4.1 million since the start of the pandemic, the AAP and CHA said. The CDC data indicate that just over 5.35 million children aged 12-15 years and 3.53 million 16- and 17-year-olds have received at least one dose of the COVID-19 vaccine and that 6.8 million children aged 12-17 are fully vaccinated.
Fully vaccinated children represent 26.4% of all 12- to 15-year-olds and 38.3% of the 16- 17-year-olds as of July 19. The corresponding numbers for those who have received at least one dose are 35.2% (ages 12-15) and 46.8% (16-17), the CDC said.
The AAP recently recommended in-person learning with universal masking in schools this fall “because a significant portion of the student population is not yet eligible for vaccines. ... Many schools will not have a system to monitor vaccine status of students, teachers and staff, and some communities overall have low vaccination uptake where the virus may be circulating more prominently.”

Children aged 12-15 years represented 13.5% of all first vaccinations received during the 2 weeks ending July 19, compared with 11.5% for the 2 weeks ending July 12, marking the first increase since the end of May. First vaccinations in 16- and 17-year-olds, who make up a much smaller share of the U.S. population, also went up, topping 5%, the Centers for Disease Control and Prevention said in its COVID Data Tracker.
The total number of vaccine initiations was almost 250,000 for the week ending July 19, after dropping to a low of 201,000 the previous week. Before that, first vaccinations had fallen in 5 of the previous 6 weeks, going from 1.4 million on May 24 to 307,000 on July 5, the CDC said.
New cases of COVID-19, unfortunately, continued to follow the trend among the larger population: As of July 15, weekly cases in children were up by 179% since dropping to 8,400 on June 24, the American Academy of Pediatrics and the Children’s Hospital Association said in a joint report. The 23,551 new cases in children for the week ending July 15 were 15.9% of all cases reported.
With those new cases, the total number of children infected with COVID-19 comes to almost 4.1 million since the start of the pandemic, the AAP and CHA said. The CDC data indicate that just over 5.35 million children aged 12-15 years and 3.53 million 16- and 17-year-olds have received at least one dose of the COVID-19 vaccine and that 6.8 million children aged 12-17 are fully vaccinated.
Fully vaccinated children represent 26.4% of all 12- to 15-year-olds and 38.3% of the 16- 17-year-olds as of July 19. The corresponding numbers for those who have received at least one dose are 35.2% (ages 12-15) and 46.8% (16-17), the CDC said.
The AAP recently recommended in-person learning with universal masking in schools this fall “because a significant portion of the student population is not yet eligible for vaccines. ... Many schools will not have a system to monitor vaccine status of students, teachers and staff, and some communities overall have low vaccination uptake where the virus may be circulating more prominently.”
HIV increases risk for severe COVID-19
according to a report from the World Health Organization on COVID-19 outcomes among people living with HIV. The study primarily included people from South Africa but also some data from other parts of the world, including the United States.
However, the report, presented at the 11th IAS Conference on HIV Science (IAS 2021), couldn’t answer some crucial questions clinicians have been wondering about since the COVID-19 pandemic began. For example, was the increase in COVID risk a result of the presence of HIV or because of the immune compromise caused by untreated HIV?
The report didn’t include data on viral load or CD counts, both used to evaluate the health of a person’s immune system. On effective treatment, people living with HIV have a lifespan close to their HIV-negative peers. And effective treatment causes undetectable viral loads which, when maintained for 6 months or more, eliminates transmission of HIV to sexual partners.
What’s clear is that in people with HIV, as in people without HIV, older people, men, and people with diabetes, hypertension, or obesity had the worst outcomes and were most likely to die from COVID-19.
For David Malebranche, MD, MPH, an internal medicine doctor who provides primary care for people in Atlanta, and who was not involved in the study, the WHO study didn’t add anything new. He already recommends the COVID-19 vaccine for all of his patients, HIV-positive or not.
“We don’t have any information from this about the T-cell counts [or] the rates of viral suppression, which I think is tremendously important,” he told this news organization. “To bypass that and not include that in any of the discussion puts the results in a questionable place for me.”
The results come from the WHO Clinical Platform, which culls data from WHO member country surveillance as well as manual case reports from all over the world. By April 29, data on 268,412 people hospitalized with COVID-19 from 37 countries were reported to the platform. Of those, 22,640 people are from the U.S.
A total of 15,522 participants worldwide were living with HIV, 664 in the United States. All U.S. cases were reported from the New York City Health and Hospitals system, Henry Ford Hospital in Detroit, and BronxCare Health System in New York City. Almost all of the remaining participants lived in South Africa – 14,682 of the 15,522, or 94.5%.
Of the 15,522 people living with HIV in the overall group, 37.1% of participants were male, and their median age was 45 years. More than 1 in 3 (36.2%) were admitted with severe or critical COVID-19, and nearly one quarter – 23.1% – with a known outcome died. More than half had one or more chronic conditions, including those that themselves are associated with worse COVID-19 outcomes, such as hypertension (in 33.2% of the participants), diabetes (22.7%), and BMIs above 30 (16.9%). In addition, 8.9% were smokers, 6.6% had chronic pulmonary disease, and 4.3% had chronic heart disease.
After adjusting for those chronic conditions, age, and sex, people living with HIV had a 6% higher rate of severe or critical COVID-19 illness. When investigators adjusted the analysis additionally to differentiate outcomes based on not just the presence of comorbid conditions but the number of them a person had, that increased risk rose to 13%. HIV itself is a comorbid condition, though it wasn’t counted as one in this adjusted analysis.
It didn’t matter whether researchers looked at risk for severe outcomes or deaths after removing the significant co-occurring conditions or if they looked at number of chronic illnesses (aside from HIV), said Silvia Bertagnolio, MD, medical officer at the World Health Organization and co-author of the analysis.
“Both models show almost identical [adjusted odds ratios], meaning that HIV was independently significantly associated with severe/critical presentation,” she told this news organization.
As for death, the analysis showed that, overall, people living with HIV were 30% more likely to die of COVID-19 compared with those not living with HIV. And while this held true even when they adjusted the data for comorbidities, people with HIV were more likely to die if they were over age 65 (risk increased by 82%), male (risk increased by 21%), had diabetes (risk increased by 50%), or had hypertension (risk increased by 26%).
When they broke down the data by WHO region – Africa, Europe, the Americas – investigators found that the increased risk for death held true in Africa. But there were not enough data from the other regions to model mortality risk. What’s more, when they broke the data down by country and excluded South Africa, they found that the elevated risk for death in people living with HIV did not reach statistical significance. Dr. Bertagnolio said she suspects that the small sample sizes from other regions made it impossible to detect a difference, but one could still be present.
One thing conspicuously absent from the analysis was information on viral load, CD4 T-cell count, progression of HIV to AIDS, and whether individuals were in HIV care. The first three factors were not reported in the platform, and the fourth was available for 60% of participants but was not included in the analysis. Dr. Bertagnolio pointed out that, for those 60% of participants, 91.8% were on antiretroviral treatment (ART).
“The majority of patients come from South Africa, and we know that in South Africa, over 90% of people receiving ART are virologically suppressed,” she told this news organization. “So we could speculate that this effect persists despite the use of ART, in a population likely to be virally suppressed, although we cannot assess this with certainty through the data set we had.”
A much smaller study of 749 people living with HIV and diagnosed with SARS-CoV-2, also presented at the conference, found that detectable HIV viral load was significantly associated with a slightly higher risk of severe outcomes (P < .039), but CD4 counts less than 200 cells/mm3 was not (P = .15).
And although both Dr. Bertagnolio and conference organizers presented this data as proof that HIV increases the risk for poor COVID-19 outcomes, Dr. Malebranche isn’t so sure. He estimates that only about half his patients have received the COVID-19 vaccine. But this study is unlikely to make him forcefully recommend a COVID-19 vaccination with young, otherwise healthy, and undetectable people in his care who express particular concern about long-term effects of the vaccine. He also manages a lot of people with HIV who have undetectable viral loads and CD4 counts of up to 1,200 but are older, with diabetes, obesity, and high blood pressure. Those are the people he will target with stronger messages regarding the vaccine.
“The young patients who are healthy, virally suppressed, and doing well may very much argue with me, ‘I’m not going to push it,’ but I will bring it up on the next visit,” he said. The analysis “just helps reinforce in me that I need to have these conversations and be a little bit more persuasive to my older patients with comorbid conditions.”
Dr. Bertagnolio has disclosed no relevant financial relationships. Dr. Malebranche serves on the pre-exposure prophylaxis (PrEP) speakers bureau for Gilead Sciences and has consulted and advised for ViiV Healthcare. This study was funded by the World Health Organization.
A version of this article first appeared on Medscape.com.
according to a report from the World Health Organization on COVID-19 outcomes among people living with HIV. The study primarily included people from South Africa but also some data from other parts of the world, including the United States.
However, the report, presented at the 11th IAS Conference on HIV Science (IAS 2021), couldn’t answer some crucial questions clinicians have been wondering about since the COVID-19 pandemic began. For example, was the increase in COVID risk a result of the presence of HIV or because of the immune compromise caused by untreated HIV?
The report didn’t include data on viral load or CD counts, both used to evaluate the health of a person’s immune system. On effective treatment, people living with HIV have a lifespan close to their HIV-negative peers. And effective treatment causes undetectable viral loads which, when maintained for 6 months or more, eliminates transmission of HIV to sexual partners.
What’s clear is that in people with HIV, as in people without HIV, older people, men, and people with diabetes, hypertension, or obesity had the worst outcomes and were most likely to die from COVID-19.
For David Malebranche, MD, MPH, an internal medicine doctor who provides primary care for people in Atlanta, and who was not involved in the study, the WHO study didn’t add anything new. He already recommends the COVID-19 vaccine for all of his patients, HIV-positive or not.
“We don’t have any information from this about the T-cell counts [or] the rates of viral suppression, which I think is tremendously important,” he told this news organization. “To bypass that and not include that in any of the discussion puts the results in a questionable place for me.”
The results come from the WHO Clinical Platform, which culls data from WHO member country surveillance as well as manual case reports from all over the world. By April 29, data on 268,412 people hospitalized with COVID-19 from 37 countries were reported to the platform. Of those, 22,640 people are from the U.S.
A total of 15,522 participants worldwide were living with HIV, 664 in the United States. All U.S. cases were reported from the New York City Health and Hospitals system, Henry Ford Hospital in Detroit, and BronxCare Health System in New York City. Almost all of the remaining participants lived in South Africa – 14,682 of the 15,522, or 94.5%.
Of the 15,522 people living with HIV in the overall group, 37.1% of participants were male, and their median age was 45 years. More than 1 in 3 (36.2%) were admitted with severe or critical COVID-19, and nearly one quarter – 23.1% – with a known outcome died. More than half had one or more chronic conditions, including those that themselves are associated with worse COVID-19 outcomes, such as hypertension (in 33.2% of the participants), diabetes (22.7%), and BMIs above 30 (16.9%). In addition, 8.9% were smokers, 6.6% had chronic pulmonary disease, and 4.3% had chronic heart disease.
After adjusting for those chronic conditions, age, and sex, people living with HIV had a 6% higher rate of severe or critical COVID-19 illness. When investigators adjusted the analysis additionally to differentiate outcomes based on not just the presence of comorbid conditions but the number of them a person had, that increased risk rose to 13%. HIV itself is a comorbid condition, though it wasn’t counted as one in this adjusted analysis.
It didn’t matter whether researchers looked at risk for severe outcomes or deaths after removing the significant co-occurring conditions or if they looked at number of chronic illnesses (aside from HIV), said Silvia Bertagnolio, MD, medical officer at the World Health Organization and co-author of the analysis.
“Both models show almost identical [adjusted odds ratios], meaning that HIV was independently significantly associated with severe/critical presentation,” she told this news organization.
As for death, the analysis showed that, overall, people living with HIV were 30% more likely to die of COVID-19 compared with those not living with HIV. And while this held true even when they adjusted the data for comorbidities, people with HIV were more likely to die if they were over age 65 (risk increased by 82%), male (risk increased by 21%), had diabetes (risk increased by 50%), or had hypertension (risk increased by 26%).
When they broke down the data by WHO region – Africa, Europe, the Americas – investigators found that the increased risk for death held true in Africa. But there were not enough data from the other regions to model mortality risk. What’s more, when they broke the data down by country and excluded South Africa, they found that the elevated risk for death in people living with HIV did not reach statistical significance. Dr. Bertagnolio said she suspects that the small sample sizes from other regions made it impossible to detect a difference, but one could still be present.
One thing conspicuously absent from the analysis was information on viral load, CD4 T-cell count, progression of HIV to AIDS, and whether individuals were in HIV care. The first three factors were not reported in the platform, and the fourth was available for 60% of participants but was not included in the analysis. Dr. Bertagnolio pointed out that, for those 60% of participants, 91.8% were on antiretroviral treatment (ART).
“The majority of patients come from South Africa, and we know that in South Africa, over 90% of people receiving ART are virologically suppressed,” she told this news organization. “So we could speculate that this effect persists despite the use of ART, in a population likely to be virally suppressed, although we cannot assess this with certainty through the data set we had.”
A much smaller study of 749 people living with HIV and diagnosed with SARS-CoV-2, also presented at the conference, found that detectable HIV viral load was significantly associated with a slightly higher risk of severe outcomes (P < .039), but CD4 counts less than 200 cells/mm3 was not (P = .15).
And although both Dr. Bertagnolio and conference organizers presented this data as proof that HIV increases the risk for poor COVID-19 outcomes, Dr. Malebranche isn’t so sure. He estimates that only about half his patients have received the COVID-19 vaccine. But this study is unlikely to make him forcefully recommend a COVID-19 vaccination with young, otherwise healthy, and undetectable people in his care who express particular concern about long-term effects of the vaccine. He also manages a lot of people with HIV who have undetectable viral loads and CD4 counts of up to 1,200 but are older, with diabetes, obesity, and high blood pressure. Those are the people he will target with stronger messages regarding the vaccine.
“The young patients who are healthy, virally suppressed, and doing well may very much argue with me, ‘I’m not going to push it,’ but I will bring it up on the next visit,” he said. The analysis “just helps reinforce in me that I need to have these conversations and be a little bit more persuasive to my older patients with comorbid conditions.”
Dr. Bertagnolio has disclosed no relevant financial relationships. Dr. Malebranche serves on the pre-exposure prophylaxis (PrEP) speakers bureau for Gilead Sciences and has consulted and advised for ViiV Healthcare. This study was funded by the World Health Organization.
A version of this article first appeared on Medscape.com.
according to a report from the World Health Organization on COVID-19 outcomes among people living with HIV. The study primarily included people from South Africa but also some data from other parts of the world, including the United States.
However, the report, presented at the 11th IAS Conference on HIV Science (IAS 2021), couldn’t answer some crucial questions clinicians have been wondering about since the COVID-19 pandemic began. For example, was the increase in COVID risk a result of the presence of HIV or because of the immune compromise caused by untreated HIV?
The report didn’t include data on viral load or CD counts, both used to evaluate the health of a person’s immune system. On effective treatment, people living with HIV have a lifespan close to their HIV-negative peers. And effective treatment causes undetectable viral loads which, when maintained for 6 months or more, eliminates transmission of HIV to sexual partners.
What’s clear is that in people with HIV, as in people without HIV, older people, men, and people with diabetes, hypertension, or obesity had the worst outcomes and were most likely to die from COVID-19.
For David Malebranche, MD, MPH, an internal medicine doctor who provides primary care for people in Atlanta, and who was not involved in the study, the WHO study didn’t add anything new. He already recommends the COVID-19 vaccine for all of his patients, HIV-positive or not.
“We don’t have any information from this about the T-cell counts [or] the rates of viral suppression, which I think is tremendously important,” he told this news organization. “To bypass that and not include that in any of the discussion puts the results in a questionable place for me.”
The results come from the WHO Clinical Platform, which culls data from WHO member country surveillance as well as manual case reports from all over the world. By April 29, data on 268,412 people hospitalized with COVID-19 from 37 countries were reported to the platform. Of those, 22,640 people are from the U.S.
A total of 15,522 participants worldwide were living with HIV, 664 in the United States. All U.S. cases were reported from the New York City Health and Hospitals system, Henry Ford Hospital in Detroit, and BronxCare Health System in New York City. Almost all of the remaining participants lived in South Africa – 14,682 of the 15,522, or 94.5%.
Of the 15,522 people living with HIV in the overall group, 37.1% of participants were male, and their median age was 45 years. More than 1 in 3 (36.2%) were admitted with severe or critical COVID-19, and nearly one quarter – 23.1% – with a known outcome died. More than half had one or more chronic conditions, including those that themselves are associated with worse COVID-19 outcomes, such as hypertension (in 33.2% of the participants), diabetes (22.7%), and BMIs above 30 (16.9%). In addition, 8.9% were smokers, 6.6% had chronic pulmonary disease, and 4.3% had chronic heart disease.
After adjusting for those chronic conditions, age, and sex, people living with HIV had a 6% higher rate of severe or critical COVID-19 illness. When investigators adjusted the analysis additionally to differentiate outcomes based on not just the presence of comorbid conditions but the number of them a person had, that increased risk rose to 13%. HIV itself is a comorbid condition, though it wasn’t counted as one in this adjusted analysis.
It didn’t matter whether researchers looked at risk for severe outcomes or deaths after removing the significant co-occurring conditions or if they looked at number of chronic illnesses (aside from HIV), said Silvia Bertagnolio, MD, medical officer at the World Health Organization and co-author of the analysis.
“Both models show almost identical [adjusted odds ratios], meaning that HIV was independently significantly associated with severe/critical presentation,” she told this news organization.
As for death, the analysis showed that, overall, people living with HIV were 30% more likely to die of COVID-19 compared with those not living with HIV. And while this held true even when they adjusted the data for comorbidities, people with HIV were more likely to die if they were over age 65 (risk increased by 82%), male (risk increased by 21%), had diabetes (risk increased by 50%), or had hypertension (risk increased by 26%).
When they broke down the data by WHO region – Africa, Europe, the Americas – investigators found that the increased risk for death held true in Africa. But there were not enough data from the other regions to model mortality risk. What’s more, when they broke the data down by country and excluded South Africa, they found that the elevated risk for death in people living with HIV did not reach statistical significance. Dr. Bertagnolio said she suspects that the small sample sizes from other regions made it impossible to detect a difference, but one could still be present.
One thing conspicuously absent from the analysis was information on viral load, CD4 T-cell count, progression of HIV to AIDS, and whether individuals were in HIV care. The first three factors were not reported in the platform, and the fourth was available for 60% of participants but was not included in the analysis. Dr. Bertagnolio pointed out that, for those 60% of participants, 91.8% were on antiretroviral treatment (ART).
“The majority of patients come from South Africa, and we know that in South Africa, over 90% of people receiving ART are virologically suppressed,” she told this news organization. “So we could speculate that this effect persists despite the use of ART, in a population likely to be virally suppressed, although we cannot assess this with certainty through the data set we had.”
A much smaller study of 749 people living with HIV and diagnosed with SARS-CoV-2, also presented at the conference, found that detectable HIV viral load was significantly associated with a slightly higher risk of severe outcomes (P < .039), but CD4 counts less than 200 cells/mm3 was not (P = .15).
And although both Dr. Bertagnolio and conference organizers presented this data as proof that HIV increases the risk for poor COVID-19 outcomes, Dr. Malebranche isn’t so sure. He estimates that only about half his patients have received the COVID-19 vaccine. But this study is unlikely to make him forcefully recommend a COVID-19 vaccination with young, otherwise healthy, and undetectable people in his care who express particular concern about long-term effects of the vaccine. He also manages a lot of people with HIV who have undetectable viral loads and CD4 counts of up to 1,200 but are older, with diabetes, obesity, and high blood pressure. Those are the people he will target with stronger messages regarding the vaccine.
“The young patients who are healthy, virally suppressed, and doing well may very much argue with me, ‘I’m not going to push it,’ but I will bring it up on the next visit,” he said. The analysis “just helps reinforce in me that I need to have these conversations and be a little bit more persuasive to my older patients with comorbid conditions.”
Dr. Bertagnolio has disclosed no relevant financial relationships. Dr. Malebranche serves on the pre-exposure prophylaxis (PrEP) speakers bureau for Gilead Sciences and has consulted and advised for ViiV Healthcare. This study was funded by the World Health Organization.
A version of this article first appeared on Medscape.com.
Pandemic drives drop in prescription drugs for children
The amount of prescription drugs given to children in the United States decreased by 27.1% between April and December 2020, compared with the same period in 2019, based on data from a national database.
Overall, dispensing of prescription drugs to all patients in the United States decreased in the wake of COVID-19 but has since rebounded, wrote Kao-Ping Chua, MD, of the University of Michigan, Ann Arbor, and colleagues. “However, whether these same trends occurred for children is unknown.”
In a study published in Pediatrics, the researchers used the IQVIA National Prescription Audit, a database that contains monthly dispensing details from 92% of retail pharmacies in the United States. They compared changes in the dispensing of prescriptions with children aged 0-19 years during 2018-2020.
In the April 2020–December 2020 time period, prescriptions for children aged 1-2 years, 3-9 years, and 10-19 years decreased by 48.7%, 40.6%, and 16.8%, respectively, compared with the same time period in 2019.
The overall dispensing total for children from April 2020 to December 2020 was 160,630,406, representing a 27.1% reduction, compared with the 220,284,613 total from April 2019 to December 2019.
By drug class, prescriptions for antibiotics, ADHD medications, and antidepressants decreased by 55.6%, 11.8%, and 0.1%, respectively, in comparing the two time periods. Prescriptions for drug classes used typically for acute infections decreased by 51.3%, and those used for chronic diseases decreased by 17.4%.
From January 2018 to February 2020, a median of 25,744,758 prescriptions were dispensed to children aged 0-19 years each month. The total prescriptions decreased from 25,684,219 in March 2020 to 16,742,568 in April 2020, increased to 19,657,289 in October 2020, and decreased again to 15,821,914 during December 2020.
In a subgroup analysis, the decline in prescriptions was greater in children aged 0-9 years, compared with those aged 10-19 years. “Because young children have a higher rate of antibiotic use than older children, declines in antibiotic dispensing might affect overall dispensing totals to a greater degree in young children,” the researchers said.
The study findings were limited by several factors including the lack of information on clinical outcomes, disease severity, and details of new versus ongoing prescriptions, as well as the possible heterogeneity in indications within drug classes, and lack of data from small pharmacies, the researchers noted. However, the results were strengthened by the use of a national all-payer database that including most prescriptions dispensed in the United States, and the use of objective measurements of prescribing practices rather than self-reports.
Despite concerns for the decreased dispensing of chronic disease drugs to children during the pandemic, “declines in dispensing of infection-related drugs, such as antitussives and antibiotics, may be welcome developments,” the researchers said. “These declines reveal that substantial reductions in prescribing of these drugs are possible,” and ongoing monitoring is needed to follow whether the reductions continue long term.
COVID precautions contributed to prescription declines
The mask-wearing and social distancing imposed by the COVID-19 pandemic has contributed to reduced rates of other illnesses, Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview.
“On the surface, with masks and social isolation, we have seen a drastic reduction in infectious disease,” she said. Fewer infections mean a reduced need for prescriptions to treat them. However, Dr. Kinsella expects the situation to change as more venues and activities open. “I expect that, as things continue to open, we will continue to see more infectious disease,” which will likely lead to more prescription drug use.
Part of the study data were provided through the IQVIA Institute’s Human Data Science Research Collaborative. Lead author Dr. Chua was supported by a career development award from the National Institute on Drug Abuse, but had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts to disclose, but serves as a member of the Pediatric News editorial advisory board.
The amount of prescription drugs given to children in the United States decreased by 27.1% between April and December 2020, compared with the same period in 2019, based on data from a national database.
Overall, dispensing of prescription drugs to all patients in the United States decreased in the wake of COVID-19 but has since rebounded, wrote Kao-Ping Chua, MD, of the University of Michigan, Ann Arbor, and colleagues. “However, whether these same trends occurred for children is unknown.”
In a study published in Pediatrics, the researchers used the IQVIA National Prescription Audit, a database that contains monthly dispensing details from 92% of retail pharmacies in the United States. They compared changes in the dispensing of prescriptions with children aged 0-19 years during 2018-2020.
In the April 2020–December 2020 time period, prescriptions for children aged 1-2 years, 3-9 years, and 10-19 years decreased by 48.7%, 40.6%, and 16.8%, respectively, compared with the same time period in 2019.
The overall dispensing total for children from April 2020 to December 2020 was 160,630,406, representing a 27.1% reduction, compared with the 220,284,613 total from April 2019 to December 2019.
By drug class, prescriptions for antibiotics, ADHD medications, and antidepressants decreased by 55.6%, 11.8%, and 0.1%, respectively, in comparing the two time periods. Prescriptions for drug classes used typically for acute infections decreased by 51.3%, and those used for chronic diseases decreased by 17.4%.
From January 2018 to February 2020, a median of 25,744,758 prescriptions were dispensed to children aged 0-19 years each month. The total prescriptions decreased from 25,684,219 in March 2020 to 16,742,568 in April 2020, increased to 19,657,289 in October 2020, and decreased again to 15,821,914 during December 2020.
In a subgroup analysis, the decline in prescriptions was greater in children aged 0-9 years, compared with those aged 10-19 years. “Because young children have a higher rate of antibiotic use than older children, declines in antibiotic dispensing might affect overall dispensing totals to a greater degree in young children,” the researchers said.
The study findings were limited by several factors including the lack of information on clinical outcomes, disease severity, and details of new versus ongoing prescriptions, as well as the possible heterogeneity in indications within drug classes, and lack of data from small pharmacies, the researchers noted. However, the results were strengthened by the use of a national all-payer database that including most prescriptions dispensed in the United States, and the use of objective measurements of prescribing practices rather than self-reports.
Despite concerns for the decreased dispensing of chronic disease drugs to children during the pandemic, “declines in dispensing of infection-related drugs, such as antitussives and antibiotics, may be welcome developments,” the researchers said. “These declines reveal that substantial reductions in prescribing of these drugs are possible,” and ongoing monitoring is needed to follow whether the reductions continue long term.
COVID precautions contributed to prescription declines
The mask-wearing and social distancing imposed by the COVID-19 pandemic has contributed to reduced rates of other illnesses, Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview.
“On the surface, with masks and social isolation, we have seen a drastic reduction in infectious disease,” she said. Fewer infections mean a reduced need for prescriptions to treat them. However, Dr. Kinsella expects the situation to change as more venues and activities open. “I expect that, as things continue to open, we will continue to see more infectious disease,” which will likely lead to more prescription drug use.
Part of the study data were provided through the IQVIA Institute’s Human Data Science Research Collaborative. Lead author Dr. Chua was supported by a career development award from the National Institute on Drug Abuse, but had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts to disclose, but serves as a member of the Pediatric News editorial advisory board.
The amount of prescription drugs given to children in the United States decreased by 27.1% between April and December 2020, compared with the same period in 2019, based on data from a national database.
Overall, dispensing of prescription drugs to all patients in the United States decreased in the wake of COVID-19 but has since rebounded, wrote Kao-Ping Chua, MD, of the University of Michigan, Ann Arbor, and colleagues. “However, whether these same trends occurred for children is unknown.”
In a study published in Pediatrics, the researchers used the IQVIA National Prescription Audit, a database that contains monthly dispensing details from 92% of retail pharmacies in the United States. They compared changes in the dispensing of prescriptions with children aged 0-19 years during 2018-2020.
In the April 2020–December 2020 time period, prescriptions for children aged 1-2 years, 3-9 years, and 10-19 years decreased by 48.7%, 40.6%, and 16.8%, respectively, compared with the same time period in 2019.
The overall dispensing total for children from April 2020 to December 2020 was 160,630,406, representing a 27.1% reduction, compared with the 220,284,613 total from April 2019 to December 2019.
By drug class, prescriptions for antibiotics, ADHD medications, and antidepressants decreased by 55.6%, 11.8%, and 0.1%, respectively, in comparing the two time periods. Prescriptions for drug classes used typically for acute infections decreased by 51.3%, and those used for chronic diseases decreased by 17.4%.
From January 2018 to February 2020, a median of 25,744,758 prescriptions were dispensed to children aged 0-19 years each month. The total prescriptions decreased from 25,684,219 in March 2020 to 16,742,568 in April 2020, increased to 19,657,289 in October 2020, and decreased again to 15,821,914 during December 2020.
In a subgroup analysis, the decline in prescriptions was greater in children aged 0-9 years, compared with those aged 10-19 years. “Because young children have a higher rate of antibiotic use than older children, declines in antibiotic dispensing might affect overall dispensing totals to a greater degree in young children,” the researchers said.
The study findings were limited by several factors including the lack of information on clinical outcomes, disease severity, and details of new versus ongoing prescriptions, as well as the possible heterogeneity in indications within drug classes, and lack of data from small pharmacies, the researchers noted. However, the results were strengthened by the use of a national all-payer database that including most prescriptions dispensed in the United States, and the use of objective measurements of prescribing practices rather than self-reports.
Despite concerns for the decreased dispensing of chronic disease drugs to children during the pandemic, “declines in dispensing of infection-related drugs, such as antitussives and antibiotics, may be welcome developments,” the researchers said. “These declines reveal that substantial reductions in prescribing of these drugs are possible,” and ongoing monitoring is needed to follow whether the reductions continue long term.
COVID precautions contributed to prescription declines
The mask-wearing and social distancing imposed by the COVID-19 pandemic has contributed to reduced rates of other illnesses, Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview.
“On the surface, with masks and social isolation, we have seen a drastic reduction in infectious disease,” she said. Fewer infections mean a reduced need for prescriptions to treat them. However, Dr. Kinsella expects the situation to change as more venues and activities open. “I expect that, as things continue to open, we will continue to see more infectious disease,” which will likely lead to more prescription drug use.
Part of the study data were provided through the IQVIA Institute’s Human Data Science Research Collaborative. Lead author Dr. Chua was supported by a career development award from the National Institute on Drug Abuse, but had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts to disclose, but serves as a member of the Pediatric News editorial advisory board.
FROM PEDIATRICS
Long COVID seen in patients with severe and mild disease
Findings from the cohort, composed of 113 COVID-19 survivors who developed ARDS after admission to a single center before to April 16, 2020, were presented online at the 31st European Congress of Clinical Microbiology & Infectious Diseases by Judit Aranda, MD, from Complex Hospitalari Moisés Broggi in Barcelona.
Median age of the participants was 64 years, and 70% were male. At least one persistent symptom was experienced during follow-up by 81% of the cohort, with 45% reporting shortness of breath, 50% reporting muscle pain, 43% reporting memory impairment, and 46% reporting physical weakness of at least 5 on a 10-point scale.
Of the 104 participants who completed a 6-minute walk test, 30% had a decrease in oxygen saturation level of at least 4%, and 5% had an initial or final level below 88%. Of the 46 participants who underwent a pulmonary function test, 15% had a forced expiratory volume in 1 second below 70%.
And of the 49% of participants with pathologic findings on chest x-ray, most were bilateral interstitial infiltrates (88%).
In addition, more than 90% of participants developed depression, anxiety, or PTSD, Dr. Aranda reported.
Not the whole picture
This study shows that sicker people – “those in intensive care units with acute respiratory distress syndrome” – are “more likely to be struggling with more severe symptoms,” said Christopher Terndrup, MD, from the division of general internal medicine and geriatrics at Oregon Health & Science University, Portland.
But a Swiss study, also presented at the meeting, “shows how even mild COVID cases can lead to debilitating symptoms,” Dr. Terndrup said in an interview.
The investigation of long-term COVID symptoms in outpatients was presented online by Florian Desgranges, MD, from Lausanne (Switzerland) University Hospital. He and his colleagues found that more than half of those with a mild to moderate disease had persistent symptoms at least 3 months after diagnosis.
The prevalence of long COVID has varied in previous research, from 15% in a study of health care workers, to 46% in a study of patients with mild COVID, 52% in a study of young COVID outpatients, and 76% in a study of patients hospitalized with COVID.
Dr. Desgranges and colleagues evaluated patients seen in an ED or outpatient clinic from February to April 2020.
The 418 patients with a confirmed COVID-19 diagnosis were compared with a control group of 89 patients who presented to the same centers during the same time frame with similar symptoms – cough, shortness of breath, or fever – but had a negative SARS-CoV-2 test.
The number of patients with comorbidities was similar in the COVID and control groups (34% vs. 36%), as was median age (41 vs. 36 years) and the prevalence of women (62% vs 64%), but the proportion of health care workers was lower in the COVID group (64% vs 82%; P =.006).
Symptoms that persisted for at least 3 months were more common in the COVID than in the control group (53% vs. 37%). And patients in the COVID group reported more symptoms than those in the control group after adjustment for age, gender, smoking status, comorbidities, and timing of the survey phone call.
Levels of sleeping problems and headache were similar in the two groups.
“We have to remember that with COVID-19 came the psychosocial changes of the pandemic situation” Dr. Desgranges said.
This study suggests that some long-COVID symptoms – such as the fatigue, headache, and sleep disorders reported in the control group – could be related to the pandemic itself, which has caused psychosocial distress, Dr. Terndrup said.
Another study that looked at outpatients “has some fantastic long-term follow-up data, and shows that many patients are still engaging in rehabilitation programs nearly a year after their diagnosis,” he explained.
The COVID HOME study
That prospective longitudinal COVID HOME study, which assessed long-term symptoms in people who were never hospitalized for COVID, was presented online by Adriana Tami, MD, PhD, from the University Medical Center Groningen (the Netherlands).
The researchers visited the homes of patients to collect data, blood samples, and perform polymerase chain reaction (PCR) testing 1, 2, and 3 weeks after a diagnosis of COVID-19. If their PCR test was still positive, testing continued until week 6 or a negative test. In addition, participants completed questionnaires at week 2 and at months 3, 6 and 12 to assess fatigue, quality of life, and symptoms of depression and anxiety.
Three-month follow-up data were available for 134 of the 276 people initially enrolled in the study. Questionnaires were completed by 85 participants at 3 months, 62 participants at 6 months, and 10 participants at 12 months.
At least 40% of participants reported long-lasting symptoms at some point during follow-up, and at least 30% said they didn’t feel fully recovered at 12 months. The most common symptom was persistent fatigue, reported at 3, 6, and 12 months by at least 44% of participants. Other common symptoms – reported by at least 20% of respondents at 3, 6, and 12 months – were headache, mental or neurologic symptoms, and sleep disorders, shortness of breath, lack of smell or taste, and severe fatigue.
“We have a high proportion of nonhospitalized individuals who suffer from long COVID after more than 12 months,” Dr. Tami concluded, adding that the study is ongoing. “We have other variables that we want to look at, including duration viral shedding and serological results and variants.”
“These cohort studies are very helpful, but they can lead to inaccurate conclusions,” Dr. Terndrup cautioned.
They only provide pieces of the big picture, but they “do add to a growing body of knowledge about a significant portion of COVID patients still struggling with symptoms long after their initial infection. The symptoms can be quite variable but are dominated by both physical and mental fatigue, and tend to be worse in patients who were sicker at initial infection,” he said in an interview.
As a whole, these studies reinforce the need for treatment programs to help patients who suffer from long COVID, he added, but “I advise caution to folks suffering out there who seek ‘miracle cures’; across the world, we are collaborating to find solutions that are safe and effective.”
We are in desperate need of an equity lens in these studies.
“There is still a great deal to learn about long COVID,” said Dr. Terndrup. Data on underrepresented populations – such as Black, Indigenous, and people of color – are lacking from these and others studies, he explained. “We are in desperate need of an equity lens in these studies,” particularly in the United States, where there are “significant disparities” in the treatment of different populations.
However, “I do hope that this work can lead to a better understanding of how other viral infections can cause long-lasting symptoms,” said Dr. Terndrup.
“We have long proposed that after acute presentation, some microbes can cause chronic symptoms, like fatigue and widespread pain. Perhaps we can learn how to better care for these patients after learning from COVID’s significant impact on our societies across the globe.”
Dr. Aranda and Dr. Desgranges have disclosed no relevant financial relationships or study funding. The study by Dr. Tami’s team was funded by the University Medical Center Groningen Organization for Health Research and Development, and Connecting European Cohorts to Increase Common and Effective Response to SARS-CoV-2 Pandemic. Dr. Terndrup disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Findings from the cohort, composed of 113 COVID-19 survivors who developed ARDS after admission to a single center before to April 16, 2020, were presented online at the 31st European Congress of Clinical Microbiology & Infectious Diseases by Judit Aranda, MD, from Complex Hospitalari Moisés Broggi in Barcelona.
Median age of the participants was 64 years, and 70% were male. At least one persistent symptom was experienced during follow-up by 81% of the cohort, with 45% reporting shortness of breath, 50% reporting muscle pain, 43% reporting memory impairment, and 46% reporting physical weakness of at least 5 on a 10-point scale.
Of the 104 participants who completed a 6-minute walk test, 30% had a decrease in oxygen saturation level of at least 4%, and 5% had an initial or final level below 88%. Of the 46 participants who underwent a pulmonary function test, 15% had a forced expiratory volume in 1 second below 70%.
And of the 49% of participants with pathologic findings on chest x-ray, most were bilateral interstitial infiltrates (88%).
In addition, more than 90% of participants developed depression, anxiety, or PTSD, Dr. Aranda reported.
Not the whole picture
This study shows that sicker people – “those in intensive care units with acute respiratory distress syndrome” – are “more likely to be struggling with more severe symptoms,” said Christopher Terndrup, MD, from the division of general internal medicine and geriatrics at Oregon Health & Science University, Portland.
But a Swiss study, also presented at the meeting, “shows how even mild COVID cases can lead to debilitating symptoms,” Dr. Terndrup said in an interview.
The investigation of long-term COVID symptoms in outpatients was presented online by Florian Desgranges, MD, from Lausanne (Switzerland) University Hospital. He and his colleagues found that more than half of those with a mild to moderate disease had persistent symptoms at least 3 months after diagnosis.
The prevalence of long COVID has varied in previous research, from 15% in a study of health care workers, to 46% in a study of patients with mild COVID, 52% in a study of young COVID outpatients, and 76% in a study of patients hospitalized with COVID.
Dr. Desgranges and colleagues evaluated patients seen in an ED or outpatient clinic from February to April 2020.
The 418 patients with a confirmed COVID-19 diagnosis were compared with a control group of 89 patients who presented to the same centers during the same time frame with similar symptoms – cough, shortness of breath, or fever – but had a negative SARS-CoV-2 test.
The number of patients with comorbidities was similar in the COVID and control groups (34% vs. 36%), as was median age (41 vs. 36 years) and the prevalence of women (62% vs 64%), but the proportion of health care workers was lower in the COVID group (64% vs 82%; P =.006).
Symptoms that persisted for at least 3 months were more common in the COVID than in the control group (53% vs. 37%). And patients in the COVID group reported more symptoms than those in the control group after adjustment for age, gender, smoking status, comorbidities, and timing of the survey phone call.
Levels of sleeping problems and headache were similar in the two groups.
“We have to remember that with COVID-19 came the psychosocial changes of the pandemic situation” Dr. Desgranges said.
This study suggests that some long-COVID symptoms – such as the fatigue, headache, and sleep disorders reported in the control group – could be related to the pandemic itself, which has caused psychosocial distress, Dr. Terndrup said.
Another study that looked at outpatients “has some fantastic long-term follow-up data, and shows that many patients are still engaging in rehabilitation programs nearly a year after their diagnosis,” he explained.
The COVID HOME study
That prospective longitudinal COVID HOME study, which assessed long-term symptoms in people who were never hospitalized for COVID, was presented online by Adriana Tami, MD, PhD, from the University Medical Center Groningen (the Netherlands).
The researchers visited the homes of patients to collect data, blood samples, and perform polymerase chain reaction (PCR) testing 1, 2, and 3 weeks after a diagnosis of COVID-19. If their PCR test was still positive, testing continued until week 6 or a negative test. In addition, participants completed questionnaires at week 2 and at months 3, 6 and 12 to assess fatigue, quality of life, and symptoms of depression and anxiety.
Three-month follow-up data were available for 134 of the 276 people initially enrolled in the study. Questionnaires were completed by 85 participants at 3 months, 62 participants at 6 months, and 10 participants at 12 months.
At least 40% of participants reported long-lasting symptoms at some point during follow-up, and at least 30% said they didn’t feel fully recovered at 12 months. The most common symptom was persistent fatigue, reported at 3, 6, and 12 months by at least 44% of participants. Other common symptoms – reported by at least 20% of respondents at 3, 6, and 12 months – were headache, mental or neurologic symptoms, and sleep disorders, shortness of breath, lack of smell or taste, and severe fatigue.
“We have a high proportion of nonhospitalized individuals who suffer from long COVID after more than 12 months,” Dr. Tami concluded, adding that the study is ongoing. “We have other variables that we want to look at, including duration viral shedding and serological results and variants.”
“These cohort studies are very helpful, but they can lead to inaccurate conclusions,” Dr. Terndrup cautioned.
They only provide pieces of the big picture, but they “do add to a growing body of knowledge about a significant portion of COVID patients still struggling with symptoms long after their initial infection. The symptoms can be quite variable but are dominated by both physical and mental fatigue, and tend to be worse in patients who were sicker at initial infection,” he said in an interview.
As a whole, these studies reinforce the need for treatment programs to help patients who suffer from long COVID, he added, but “I advise caution to folks suffering out there who seek ‘miracle cures’; across the world, we are collaborating to find solutions that are safe and effective.”
We are in desperate need of an equity lens in these studies.
“There is still a great deal to learn about long COVID,” said Dr. Terndrup. Data on underrepresented populations – such as Black, Indigenous, and people of color – are lacking from these and others studies, he explained. “We are in desperate need of an equity lens in these studies,” particularly in the United States, where there are “significant disparities” in the treatment of different populations.
However, “I do hope that this work can lead to a better understanding of how other viral infections can cause long-lasting symptoms,” said Dr. Terndrup.
“We have long proposed that after acute presentation, some microbes can cause chronic symptoms, like fatigue and widespread pain. Perhaps we can learn how to better care for these patients after learning from COVID’s significant impact on our societies across the globe.”
Dr. Aranda and Dr. Desgranges have disclosed no relevant financial relationships or study funding. The study by Dr. Tami’s team was funded by the University Medical Center Groningen Organization for Health Research and Development, and Connecting European Cohorts to Increase Common and Effective Response to SARS-CoV-2 Pandemic. Dr. Terndrup disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Findings from the cohort, composed of 113 COVID-19 survivors who developed ARDS after admission to a single center before to April 16, 2020, were presented online at the 31st European Congress of Clinical Microbiology & Infectious Diseases by Judit Aranda, MD, from Complex Hospitalari Moisés Broggi in Barcelona.
Median age of the participants was 64 years, and 70% were male. At least one persistent symptom was experienced during follow-up by 81% of the cohort, with 45% reporting shortness of breath, 50% reporting muscle pain, 43% reporting memory impairment, and 46% reporting physical weakness of at least 5 on a 10-point scale.
Of the 104 participants who completed a 6-minute walk test, 30% had a decrease in oxygen saturation level of at least 4%, and 5% had an initial or final level below 88%. Of the 46 participants who underwent a pulmonary function test, 15% had a forced expiratory volume in 1 second below 70%.
And of the 49% of participants with pathologic findings on chest x-ray, most were bilateral interstitial infiltrates (88%).
In addition, more than 90% of participants developed depression, anxiety, or PTSD, Dr. Aranda reported.
Not the whole picture
This study shows that sicker people – “those in intensive care units with acute respiratory distress syndrome” – are “more likely to be struggling with more severe symptoms,” said Christopher Terndrup, MD, from the division of general internal medicine and geriatrics at Oregon Health & Science University, Portland.
But a Swiss study, also presented at the meeting, “shows how even mild COVID cases can lead to debilitating symptoms,” Dr. Terndrup said in an interview.
The investigation of long-term COVID symptoms in outpatients was presented online by Florian Desgranges, MD, from Lausanne (Switzerland) University Hospital. He and his colleagues found that more than half of those with a mild to moderate disease had persistent symptoms at least 3 months after diagnosis.
The prevalence of long COVID has varied in previous research, from 15% in a study of health care workers, to 46% in a study of patients with mild COVID, 52% in a study of young COVID outpatients, and 76% in a study of patients hospitalized with COVID.
Dr. Desgranges and colleagues evaluated patients seen in an ED or outpatient clinic from February to April 2020.
The 418 patients with a confirmed COVID-19 diagnosis were compared with a control group of 89 patients who presented to the same centers during the same time frame with similar symptoms – cough, shortness of breath, or fever – but had a negative SARS-CoV-2 test.
The number of patients with comorbidities was similar in the COVID and control groups (34% vs. 36%), as was median age (41 vs. 36 years) and the prevalence of women (62% vs 64%), but the proportion of health care workers was lower in the COVID group (64% vs 82%; P =.006).
Symptoms that persisted for at least 3 months were more common in the COVID than in the control group (53% vs. 37%). And patients in the COVID group reported more symptoms than those in the control group after adjustment for age, gender, smoking status, comorbidities, and timing of the survey phone call.
Levels of sleeping problems and headache were similar in the two groups.
“We have to remember that with COVID-19 came the psychosocial changes of the pandemic situation” Dr. Desgranges said.
This study suggests that some long-COVID symptoms – such as the fatigue, headache, and sleep disorders reported in the control group – could be related to the pandemic itself, which has caused psychosocial distress, Dr. Terndrup said.
Another study that looked at outpatients “has some fantastic long-term follow-up data, and shows that many patients are still engaging in rehabilitation programs nearly a year after their diagnosis,” he explained.
The COVID HOME study
That prospective longitudinal COVID HOME study, which assessed long-term symptoms in people who were never hospitalized for COVID, was presented online by Adriana Tami, MD, PhD, from the University Medical Center Groningen (the Netherlands).
The researchers visited the homes of patients to collect data, blood samples, and perform polymerase chain reaction (PCR) testing 1, 2, and 3 weeks after a diagnosis of COVID-19. If their PCR test was still positive, testing continued until week 6 or a negative test. In addition, participants completed questionnaires at week 2 and at months 3, 6 and 12 to assess fatigue, quality of life, and symptoms of depression and anxiety.
Three-month follow-up data were available for 134 of the 276 people initially enrolled in the study. Questionnaires were completed by 85 participants at 3 months, 62 participants at 6 months, and 10 participants at 12 months.
At least 40% of participants reported long-lasting symptoms at some point during follow-up, and at least 30% said they didn’t feel fully recovered at 12 months. The most common symptom was persistent fatigue, reported at 3, 6, and 12 months by at least 44% of participants. Other common symptoms – reported by at least 20% of respondents at 3, 6, and 12 months – were headache, mental or neurologic symptoms, and sleep disorders, shortness of breath, lack of smell or taste, and severe fatigue.
“We have a high proportion of nonhospitalized individuals who suffer from long COVID after more than 12 months,” Dr. Tami concluded, adding that the study is ongoing. “We have other variables that we want to look at, including duration viral shedding and serological results and variants.”
“These cohort studies are very helpful, but they can lead to inaccurate conclusions,” Dr. Terndrup cautioned.
They only provide pieces of the big picture, but they “do add to a growing body of knowledge about a significant portion of COVID patients still struggling with symptoms long after their initial infection. The symptoms can be quite variable but are dominated by both physical and mental fatigue, and tend to be worse in patients who were sicker at initial infection,” he said in an interview.
As a whole, these studies reinforce the need for treatment programs to help patients who suffer from long COVID, he added, but “I advise caution to folks suffering out there who seek ‘miracle cures’; across the world, we are collaborating to find solutions that are safe and effective.”
We are in desperate need of an equity lens in these studies.
“There is still a great deal to learn about long COVID,” said Dr. Terndrup. Data on underrepresented populations – such as Black, Indigenous, and people of color – are lacking from these and others studies, he explained. “We are in desperate need of an equity lens in these studies,” particularly in the United States, where there are “significant disparities” in the treatment of different populations.
However, “I do hope that this work can lead to a better understanding of how other viral infections can cause long-lasting symptoms,” said Dr. Terndrup.
“We have long proposed that after acute presentation, some microbes can cause chronic symptoms, like fatigue and widespread pain. Perhaps we can learn how to better care for these patients after learning from COVID’s significant impact on our societies across the globe.”
Dr. Aranda and Dr. Desgranges have disclosed no relevant financial relationships or study funding. The study by Dr. Tami’s team was funded by the University Medical Center Groningen Organization for Health Research and Development, and Connecting European Cohorts to Increase Common and Effective Response to SARS-CoV-2 Pandemic. Dr. Terndrup disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EUROPEAN CONGRESS OF CLINICAL MICROBIOLOGY & INFECTIOUS DISEASES
Targeted outreach increases Black COVID-19 vaccination rates
Thoughtful, targeted approaches are needed to increase COVID-19 vaccination rates in Black and Latinx communities, which often distrust the health care system and face barriers to vaccine access, new data show.
“Black, Latinx, and Native American individuals represent about a combined 60% of COVID-19 deaths, despite comprising significantly less of the United States population,” said Jacinda C. Abdul-Mutakabbir, PharmD, from Loma Linda (Calif.) University.
“To put this into perspective, Black individuals represent 13.4% of the United States population, while Native Americans represent 1.6%, clearly showing the disproportionate outcomes here,” she explained during her online presentation at the 31st European Congress of Clinical Microbiology & Infectious Diseases.
The vaccine creates an opportunity to change the disproportionate way COVID-19 has affected racial and ethnic communities, said Dr. Abdul-Mutakabbir, but “a long history of mistreatment within the U.S. health care system decreases their trust for the system to use fair practices when delivering these vaccines.”
For people in minority communities, often “the fear of cost associated with health care keeps them from being vaccinated,” she said. “Also, there is a lack of vaccines actually allocated to these communities, or inconsistent computer-based sign-ups that make WiFi mandatory, which in turn has created additional barriers for vaccination access.”
Loma Linda University maintains the largest mass-vaccination site in San Bernardino County, the fourth-largest county in southern California. However, only 3.0% of the people vaccinated there have been Black. And although 8% of the state’s population is Black, only 2.7% of the Black population has been vaccinated.
In contrast, Black Californians have accounted for about 20% of COVID-19 cases in the state, and 20%-30% of COVID-19 deaths.
To promote equitable access to COVID-19 vaccines, Dr. Abdul-Mutakabbir and colleagues developed a “three-tiered approach.” First, they had local Black faith leaders hold summits ahead of the vaccination clinics. Next, at those summits, they had a Black pharmacist educate attendees about the vaccines. And finally, they held a low-barrier community vaccination clinic in a Black community, where the pharmacist oversaw the transport and preparation of the vaccines.
Because access, transportation, and internet are all barriers to vaccination, the clinic used paper-based registration and was held as a pop-up clinic at a local Black church. The team held two clinics for the first Moderna dose, one clinic for the second Moderna dose, and one clinic for the Johnson & Johnson one-dose vaccine.
During the Moderna first-dose clinics, 673 vaccinations were administered, and during the second-dose clinic, 366 were administered. Early data showed a return rate of 87%, but the team has yet to update the final numbers, Dr. Abdul-Mutakabbir reported. During the Johnson & Johnson clinic, 314 vaccinations were administered, nearly half to Black people. After the community vaccination clinics, the mass vaccination site saw a 0.6% increase in vaccinations for Black people.
Dr. Abdul-Mutakabbir’s team also held three community clinics in Latinx communities. During the first-dose Moderna pop-up clinic, 258 vaccinations were administered, and during the second-dose clinic, 253 were, for a 98% return rate. Approximately 92% of those vaccinated were Latinx.
The study findings are not surprising, said Rhea Boyd, MD, director of equity and justice for California Children’s Trust.
“The barriers to vaccination are known and clear,” she said in an interview. “Mobile clinics with paper appointments address a number of those barriers head on, like transportation, internet access, and accessibility. Having Black providers leading the effort and church leaders involved also has been shown to increase confidence in the operations and process.”
Information campaigns can help counter online disinformation. Ultimately, however, “the main barrier to vaccination is access,” Dr. Boyd said. “Address access and rates will increase.”
The health inequities seen in vaccination rates among Black and Latinx people “are a product of structural and systemic racism,” Dr. Abdul-Mutakabbir said. “To create equitable processes, it is essential that we evaluate how we approach each of these different minoritized groups.”
Dr. Abdul-Mutakabbir disclosed no relevant financial relationships. Dr. Boyd codeveloped THE CONVERSATION, a national campaign to bring credible information about the COVID vaccines to Black and Latinx communities in partnership with KFF, BCAC, and Unidos US.
A version of this article first appeared on Medscape.com.
Thoughtful, targeted approaches are needed to increase COVID-19 vaccination rates in Black and Latinx communities, which often distrust the health care system and face barriers to vaccine access, new data show.
“Black, Latinx, and Native American individuals represent about a combined 60% of COVID-19 deaths, despite comprising significantly less of the United States population,” said Jacinda C. Abdul-Mutakabbir, PharmD, from Loma Linda (Calif.) University.
“To put this into perspective, Black individuals represent 13.4% of the United States population, while Native Americans represent 1.6%, clearly showing the disproportionate outcomes here,” she explained during her online presentation at the 31st European Congress of Clinical Microbiology & Infectious Diseases.
The vaccine creates an opportunity to change the disproportionate way COVID-19 has affected racial and ethnic communities, said Dr. Abdul-Mutakabbir, but “a long history of mistreatment within the U.S. health care system decreases their trust for the system to use fair practices when delivering these vaccines.”
For people in minority communities, often “the fear of cost associated with health care keeps them from being vaccinated,” she said. “Also, there is a lack of vaccines actually allocated to these communities, or inconsistent computer-based sign-ups that make WiFi mandatory, which in turn has created additional barriers for vaccination access.”
Loma Linda University maintains the largest mass-vaccination site in San Bernardino County, the fourth-largest county in southern California. However, only 3.0% of the people vaccinated there have been Black. And although 8% of the state’s population is Black, only 2.7% of the Black population has been vaccinated.
In contrast, Black Californians have accounted for about 20% of COVID-19 cases in the state, and 20%-30% of COVID-19 deaths.
To promote equitable access to COVID-19 vaccines, Dr. Abdul-Mutakabbir and colleagues developed a “three-tiered approach.” First, they had local Black faith leaders hold summits ahead of the vaccination clinics. Next, at those summits, they had a Black pharmacist educate attendees about the vaccines. And finally, they held a low-barrier community vaccination clinic in a Black community, where the pharmacist oversaw the transport and preparation of the vaccines.
Because access, transportation, and internet are all barriers to vaccination, the clinic used paper-based registration and was held as a pop-up clinic at a local Black church. The team held two clinics for the first Moderna dose, one clinic for the second Moderna dose, and one clinic for the Johnson & Johnson one-dose vaccine.
During the Moderna first-dose clinics, 673 vaccinations were administered, and during the second-dose clinic, 366 were administered. Early data showed a return rate of 87%, but the team has yet to update the final numbers, Dr. Abdul-Mutakabbir reported. During the Johnson & Johnson clinic, 314 vaccinations were administered, nearly half to Black people. After the community vaccination clinics, the mass vaccination site saw a 0.6% increase in vaccinations for Black people.
Dr. Abdul-Mutakabbir’s team also held three community clinics in Latinx communities. During the first-dose Moderna pop-up clinic, 258 vaccinations were administered, and during the second-dose clinic, 253 were, for a 98% return rate. Approximately 92% of those vaccinated were Latinx.
The study findings are not surprising, said Rhea Boyd, MD, director of equity and justice for California Children’s Trust.
“The barriers to vaccination are known and clear,” she said in an interview. “Mobile clinics with paper appointments address a number of those barriers head on, like transportation, internet access, and accessibility. Having Black providers leading the effort and church leaders involved also has been shown to increase confidence in the operations and process.”
Information campaigns can help counter online disinformation. Ultimately, however, “the main barrier to vaccination is access,” Dr. Boyd said. “Address access and rates will increase.”
The health inequities seen in vaccination rates among Black and Latinx people “are a product of structural and systemic racism,” Dr. Abdul-Mutakabbir said. “To create equitable processes, it is essential that we evaluate how we approach each of these different minoritized groups.”
Dr. Abdul-Mutakabbir disclosed no relevant financial relationships. Dr. Boyd codeveloped THE CONVERSATION, a national campaign to bring credible information about the COVID vaccines to Black and Latinx communities in partnership with KFF, BCAC, and Unidos US.
A version of this article first appeared on Medscape.com.
Thoughtful, targeted approaches are needed to increase COVID-19 vaccination rates in Black and Latinx communities, which often distrust the health care system and face barriers to vaccine access, new data show.
“Black, Latinx, and Native American individuals represent about a combined 60% of COVID-19 deaths, despite comprising significantly less of the United States population,” said Jacinda C. Abdul-Mutakabbir, PharmD, from Loma Linda (Calif.) University.
“To put this into perspective, Black individuals represent 13.4% of the United States population, while Native Americans represent 1.6%, clearly showing the disproportionate outcomes here,” she explained during her online presentation at the 31st European Congress of Clinical Microbiology & Infectious Diseases.
The vaccine creates an opportunity to change the disproportionate way COVID-19 has affected racial and ethnic communities, said Dr. Abdul-Mutakabbir, but “a long history of mistreatment within the U.S. health care system decreases their trust for the system to use fair practices when delivering these vaccines.”
For people in minority communities, often “the fear of cost associated with health care keeps them from being vaccinated,” she said. “Also, there is a lack of vaccines actually allocated to these communities, or inconsistent computer-based sign-ups that make WiFi mandatory, which in turn has created additional barriers for vaccination access.”
Loma Linda University maintains the largest mass-vaccination site in San Bernardino County, the fourth-largest county in southern California. However, only 3.0% of the people vaccinated there have been Black. And although 8% of the state’s population is Black, only 2.7% of the Black population has been vaccinated.
In contrast, Black Californians have accounted for about 20% of COVID-19 cases in the state, and 20%-30% of COVID-19 deaths.
To promote equitable access to COVID-19 vaccines, Dr. Abdul-Mutakabbir and colleagues developed a “three-tiered approach.” First, they had local Black faith leaders hold summits ahead of the vaccination clinics. Next, at those summits, they had a Black pharmacist educate attendees about the vaccines. And finally, they held a low-barrier community vaccination clinic in a Black community, where the pharmacist oversaw the transport and preparation of the vaccines.
Because access, transportation, and internet are all barriers to vaccination, the clinic used paper-based registration and was held as a pop-up clinic at a local Black church. The team held two clinics for the first Moderna dose, one clinic for the second Moderna dose, and one clinic for the Johnson & Johnson one-dose vaccine.
During the Moderna first-dose clinics, 673 vaccinations were administered, and during the second-dose clinic, 366 were administered. Early data showed a return rate of 87%, but the team has yet to update the final numbers, Dr. Abdul-Mutakabbir reported. During the Johnson & Johnson clinic, 314 vaccinations were administered, nearly half to Black people. After the community vaccination clinics, the mass vaccination site saw a 0.6% increase in vaccinations for Black people.
Dr. Abdul-Mutakabbir’s team also held three community clinics in Latinx communities. During the first-dose Moderna pop-up clinic, 258 vaccinations were administered, and during the second-dose clinic, 253 were, for a 98% return rate. Approximately 92% of those vaccinated were Latinx.
The study findings are not surprising, said Rhea Boyd, MD, director of equity and justice for California Children’s Trust.
“The barriers to vaccination are known and clear,” she said in an interview. “Mobile clinics with paper appointments address a number of those barriers head on, like transportation, internet access, and accessibility. Having Black providers leading the effort and church leaders involved also has been shown to increase confidence in the operations and process.”
Information campaigns can help counter online disinformation. Ultimately, however, “the main barrier to vaccination is access,” Dr. Boyd said. “Address access and rates will increase.”
The health inequities seen in vaccination rates among Black and Latinx people “are a product of structural and systemic racism,” Dr. Abdul-Mutakabbir said. “To create equitable processes, it is essential that we evaluate how we approach each of these different minoritized groups.”
Dr. Abdul-Mutakabbir disclosed no relevant financial relationships. Dr. Boyd codeveloped THE CONVERSATION, a national campaign to bring credible information about the COVID vaccines to Black and Latinx communities in partnership with KFF, BCAC, and Unidos US.
A version of this article first appeared on Medscape.com.
Large remdesivir study finds no COVID-19 survival benefit
A lack of consensus in the evidence regarding the antiviral remdesivir (Veklury) to treat people with COVID-19 continues, leaving clinicians without clear direction on one of the few treatments for the illness approved under U.S. Food and Drug Administration emergency use authorization.
The latest research comes from Michael Ohl, MD, MSPH, and colleagues, who studied a large group of VA patients hospitalized with COVID-19. Compared with a matched group of veterans who did not receive the antiviral, remdesivir did not significantly improve survival.
The percentages were close: 12.2% of patients in the remdesivir group died within 30 days compared with 10.6% of those in the control group.
At the same time, the retrospective cohort study showed remdesivir was associated with more days in the hospital.
“There is still uncertainty about the role of remdesivir in treatment for people hospitalized with COVID-19,” Dr. Ohl told this news organization.
“It is reasonable to follow the CDC and Infectious Diseases Society of America guidelines for remdesivir use, “but clinicians should avoid admitting people or keeping people in the hospital solely to receive remdesivir if they do not meet other criteria for hospitalization,” said Dr. Ohl, lead author and an infectious disease specialist at the Center for Access & Delivery Research and Evaluation, Iowa City Veterans Affairs (VA) Health Care System.
The study was published online July 15 in JAMA Network Open.
Sticking with the official protocol?
The longer a hospital stays associated with remdesivir, a median 6 days versus 3 days, could be a result of treating people for 5 or 10 days with the antiviral agent. In other words, it is “possible that clinicians were not discharging patients who otherwise met the criteria for hospital discharge until the remdesivir course was completed,” Dr. Ohl and colleagues note.
Not doing so, they add, could have resulted in “increased used of scarce hospital beds during the pandemic.”
“The recommended remdesivir treatment course is a somewhat arbitrary 5 or 10 days depending on illness severity, and remdesivir is currently available only as an intravenous formulation for use in health care settings,” they add.
This is the “most likely explanation,” notes Gio J. Baracco, MD, in an invited commentary accompanying the study.
At the time of the study, use of remdesivir also required patient consent, close adverse event monitoring, and ongoing testing, Dr. Baracco notes.
He added that an option to discharge patients earlier if they responded to treatment might have been lost in translation from clinical trial protocol to real-world use in the VA system.
While a large clinical trial protocol called for the remdesivir infusions to be stopped early if the patient met the primary outcome and was ready to be discharged, “this detail was not adequately translated to the clinicians treating these patients,” added Dr. Baracco, who’s with the Division of Infectious Diseases at the University of Miami Miller School of Medicine and the Miami VA Healthcare System.
Conflicting evidence
Another large study, the World Health Organization Solidarity Trial, found remdesivir was not associated with shorter hospital stays or improved survival compared with standard of care. For this reason, the WHO recommends against use of remdesivir.
In contrast, the double-blind, randomized Adaptive COVID-19 Treatment Trial (ACTT-1) linked remdesivir treatment to shorter stays in the hospital, a median 10 days versus 15 days in a placebo group.
The FDA included the 2020 ACTT-1 in its consideration for remdesivir emergency use authorization. The FDA issued the EUA in May 2020, followed by full approval as the first treatment indicated for COVID-19 in October.
ACTT-1 lead author John H. Beigel, MD, and colleagues also looked at the death rates for remdesivir versus placebo.
By day 15, the proportion of people who died was 6.7% in the remdesivir group versus 11%. By day 29, the rate was 11.4% among those who received the antiviral versus 15.2% among those who did not.
When asked why the VA and ACTT-1 studies yielded different results, Dr. Beigel cited two reasons. The timing was different, with the VA study starting after the remdesivir EUA was issued, and ACTT-1 findings were announced.
“So at that point, clinicians understood those populations most likely to benefit from remdesivir. The use of remdesivir likely did not occur at random; it was likely to be more commonly used in those who were sicker or at higher risk for poor outcomes,” said Dr. Beigel, associate director for clinical research in the Division of Microbiology and Infectious Diseases at the National Institute of Allergy and Infectious Diseases (NIAID).
In addition, the studies evaluated very different populations, he said. The differences in median duration of hospitalization between the trials reflects this, Dr. Beigel added.
Furthermore, when asked if he thinks the new evidence should affect clinical use of remdesivir, Dr. Beigel replied, “No. Observational studies, even with adjustments such as propensity score matching, are not equivalent levels of proof compared to randomized trials.”
Study details
Dr. Ohl and colleagues identified patients admitted to one of 123 VA hospitals for the first time for COVID-19 from May 1 to Oct. 8, 2020. Each had a PCR-confirmed SARS-CoV-2 infection. The researchers then compared 1,172 patients receiving remdesivir to another 1,172 patients not receiving the agent.
Those receiving remdesivir were more likely to be older, White, have chronic obstructive pulmonary disease and have more severe COVID-19. A total 94% of the remdesivir group were men.
“Over 90% of the people included in VA study were men, mostly over the age of 60,” Dr. Ohl said when asked how generalizable the findings would be to a non-VA population.
“There is no obvious biological reason that remdesivir should have different effects in men and women, but we should be cautious about extrapolating study findings to women and younger individuals,” he added.
Limitations of the study include its observational design, which makes unadjusted confounding based on illness severity a possibility. In addition, the investigators were unable to identify specific subgroups that might benefit from remdesivir treatment.
The data did suggest that remdesivir was more effective earlier in the course of disease when patients required supplemental oxygen and before need for mechanical ventilation.
Dr. Baracco pointed out the contradictory findings in his commentary: “The real-life application of a drug promising to hasten discharge from the hospital as its primary beneficial outcome must include an assessment of how easy it is to do so and make it clear that once a patient reaches that point, they can discontinue the drug.”
“The paradoxical findings in the study by Dr. Ohl et al. compared with the study used for its authorization illustrate this point very clearly,” he adds.
Dr. Ohl reported receiving grants from Veterans Affairs Health Services Research and Development during the conduct of the study and consulting for Gilead Pharmaceuticals outside the submitted work. Dr. Baracco reported receiving salary support from the U.S. Department of Veterans Affairs. Dr. Beigel has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A lack of consensus in the evidence regarding the antiviral remdesivir (Veklury) to treat people with COVID-19 continues, leaving clinicians without clear direction on one of the few treatments for the illness approved under U.S. Food and Drug Administration emergency use authorization.
The latest research comes from Michael Ohl, MD, MSPH, and colleagues, who studied a large group of VA patients hospitalized with COVID-19. Compared with a matched group of veterans who did not receive the antiviral, remdesivir did not significantly improve survival.
The percentages were close: 12.2% of patients in the remdesivir group died within 30 days compared with 10.6% of those in the control group.
At the same time, the retrospective cohort study showed remdesivir was associated with more days in the hospital.
“There is still uncertainty about the role of remdesivir in treatment for people hospitalized with COVID-19,” Dr. Ohl told this news organization.
“It is reasonable to follow the CDC and Infectious Diseases Society of America guidelines for remdesivir use, “but clinicians should avoid admitting people or keeping people in the hospital solely to receive remdesivir if they do not meet other criteria for hospitalization,” said Dr. Ohl, lead author and an infectious disease specialist at the Center for Access & Delivery Research and Evaluation, Iowa City Veterans Affairs (VA) Health Care System.
The study was published online July 15 in JAMA Network Open.
Sticking with the official protocol?
The longer a hospital stays associated with remdesivir, a median 6 days versus 3 days, could be a result of treating people for 5 or 10 days with the antiviral agent. In other words, it is “possible that clinicians were not discharging patients who otherwise met the criteria for hospital discharge until the remdesivir course was completed,” Dr. Ohl and colleagues note.
Not doing so, they add, could have resulted in “increased used of scarce hospital beds during the pandemic.”
“The recommended remdesivir treatment course is a somewhat arbitrary 5 or 10 days depending on illness severity, and remdesivir is currently available only as an intravenous formulation for use in health care settings,” they add.
This is the “most likely explanation,” notes Gio J. Baracco, MD, in an invited commentary accompanying the study.
At the time of the study, use of remdesivir also required patient consent, close adverse event monitoring, and ongoing testing, Dr. Baracco notes.
He added that an option to discharge patients earlier if they responded to treatment might have been lost in translation from clinical trial protocol to real-world use in the VA system.
While a large clinical trial protocol called for the remdesivir infusions to be stopped early if the patient met the primary outcome and was ready to be discharged, “this detail was not adequately translated to the clinicians treating these patients,” added Dr. Baracco, who’s with the Division of Infectious Diseases at the University of Miami Miller School of Medicine and the Miami VA Healthcare System.
Conflicting evidence
Another large study, the World Health Organization Solidarity Trial, found remdesivir was not associated with shorter hospital stays or improved survival compared with standard of care. For this reason, the WHO recommends against use of remdesivir.
In contrast, the double-blind, randomized Adaptive COVID-19 Treatment Trial (ACTT-1) linked remdesivir treatment to shorter stays in the hospital, a median 10 days versus 15 days in a placebo group.
The FDA included the 2020 ACTT-1 in its consideration for remdesivir emergency use authorization. The FDA issued the EUA in May 2020, followed by full approval as the first treatment indicated for COVID-19 in October.
ACTT-1 lead author John H. Beigel, MD, and colleagues also looked at the death rates for remdesivir versus placebo.
By day 15, the proportion of people who died was 6.7% in the remdesivir group versus 11%. By day 29, the rate was 11.4% among those who received the antiviral versus 15.2% among those who did not.
When asked why the VA and ACTT-1 studies yielded different results, Dr. Beigel cited two reasons. The timing was different, with the VA study starting after the remdesivir EUA was issued, and ACTT-1 findings were announced.
“So at that point, clinicians understood those populations most likely to benefit from remdesivir. The use of remdesivir likely did not occur at random; it was likely to be more commonly used in those who were sicker or at higher risk for poor outcomes,” said Dr. Beigel, associate director for clinical research in the Division of Microbiology and Infectious Diseases at the National Institute of Allergy and Infectious Diseases (NIAID).
In addition, the studies evaluated very different populations, he said. The differences in median duration of hospitalization between the trials reflects this, Dr. Beigel added.
Furthermore, when asked if he thinks the new evidence should affect clinical use of remdesivir, Dr. Beigel replied, “No. Observational studies, even with adjustments such as propensity score matching, are not equivalent levels of proof compared to randomized trials.”
Study details
Dr. Ohl and colleagues identified patients admitted to one of 123 VA hospitals for the first time for COVID-19 from May 1 to Oct. 8, 2020. Each had a PCR-confirmed SARS-CoV-2 infection. The researchers then compared 1,172 patients receiving remdesivir to another 1,172 patients not receiving the agent.
Those receiving remdesivir were more likely to be older, White, have chronic obstructive pulmonary disease and have more severe COVID-19. A total 94% of the remdesivir group were men.
“Over 90% of the people included in VA study were men, mostly over the age of 60,” Dr. Ohl said when asked how generalizable the findings would be to a non-VA population.
“There is no obvious biological reason that remdesivir should have different effects in men and women, but we should be cautious about extrapolating study findings to women and younger individuals,” he added.
Limitations of the study include its observational design, which makes unadjusted confounding based on illness severity a possibility. In addition, the investigators were unable to identify specific subgroups that might benefit from remdesivir treatment.
The data did suggest that remdesivir was more effective earlier in the course of disease when patients required supplemental oxygen and before need for mechanical ventilation.
Dr. Baracco pointed out the contradictory findings in his commentary: “The real-life application of a drug promising to hasten discharge from the hospital as its primary beneficial outcome must include an assessment of how easy it is to do so and make it clear that once a patient reaches that point, they can discontinue the drug.”
“The paradoxical findings in the study by Dr. Ohl et al. compared with the study used for its authorization illustrate this point very clearly,” he adds.
Dr. Ohl reported receiving grants from Veterans Affairs Health Services Research and Development during the conduct of the study and consulting for Gilead Pharmaceuticals outside the submitted work. Dr. Baracco reported receiving salary support from the U.S. Department of Veterans Affairs. Dr. Beigel has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A lack of consensus in the evidence regarding the antiviral remdesivir (Veklury) to treat people with COVID-19 continues, leaving clinicians without clear direction on one of the few treatments for the illness approved under U.S. Food and Drug Administration emergency use authorization.
The latest research comes from Michael Ohl, MD, MSPH, and colleagues, who studied a large group of VA patients hospitalized with COVID-19. Compared with a matched group of veterans who did not receive the antiviral, remdesivir did not significantly improve survival.
The percentages were close: 12.2% of patients in the remdesivir group died within 30 days compared with 10.6% of those in the control group.
At the same time, the retrospective cohort study showed remdesivir was associated with more days in the hospital.
“There is still uncertainty about the role of remdesivir in treatment for people hospitalized with COVID-19,” Dr. Ohl told this news organization.
“It is reasonable to follow the CDC and Infectious Diseases Society of America guidelines for remdesivir use, “but clinicians should avoid admitting people or keeping people in the hospital solely to receive remdesivir if they do not meet other criteria for hospitalization,” said Dr. Ohl, lead author and an infectious disease specialist at the Center for Access & Delivery Research and Evaluation, Iowa City Veterans Affairs (VA) Health Care System.
The study was published online July 15 in JAMA Network Open.
Sticking with the official protocol?
The longer a hospital stays associated with remdesivir, a median 6 days versus 3 days, could be a result of treating people for 5 or 10 days with the antiviral agent. In other words, it is “possible that clinicians were not discharging patients who otherwise met the criteria for hospital discharge until the remdesivir course was completed,” Dr. Ohl and colleagues note.
Not doing so, they add, could have resulted in “increased used of scarce hospital beds during the pandemic.”
“The recommended remdesivir treatment course is a somewhat arbitrary 5 or 10 days depending on illness severity, and remdesivir is currently available only as an intravenous formulation for use in health care settings,” they add.
This is the “most likely explanation,” notes Gio J. Baracco, MD, in an invited commentary accompanying the study.
At the time of the study, use of remdesivir also required patient consent, close adverse event monitoring, and ongoing testing, Dr. Baracco notes.
He added that an option to discharge patients earlier if they responded to treatment might have been lost in translation from clinical trial protocol to real-world use in the VA system.
While a large clinical trial protocol called for the remdesivir infusions to be stopped early if the patient met the primary outcome and was ready to be discharged, “this detail was not adequately translated to the clinicians treating these patients,” added Dr. Baracco, who’s with the Division of Infectious Diseases at the University of Miami Miller School of Medicine and the Miami VA Healthcare System.
Conflicting evidence
Another large study, the World Health Organization Solidarity Trial, found remdesivir was not associated with shorter hospital stays or improved survival compared with standard of care. For this reason, the WHO recommends against use of remdesivir.
In contrast, the double-blind, randomized Adaptive COVID-19 Treatment Trial (ACTT-1) linked remdesivir treatment to shorter stays in the hospital, a median 10 days versus 15 days in a placebo group.
The FDA included the 2020 ACTT-1 in its consideration for remdesivir emergency use authorization. The FDA issued the EUA in May 2020, followed by full approval as the first treatment indicated for COVID-19 in October.
ACTT-1 lead author John H. Beigel, MD, and colleagues also looked at the death rates for remdesivir versus placebo.
By day 15, the proportion of people who died was 6.7% in the remdesivir group versus 11%. By day 29, the rate was 11.4% among those who received the antiviral versus 15.2% among those who did not.
When asked why the VA and ACTT-1 studies yielded different results, Dr. Beigel cited two reasons. The timing was different, with the VA study starting after the remdesivir EUA was issued, and ACTT-1 findings were announced.
“So at that point, clinicians understood those populations most likely to benefit from remdesivir. The use of remdesivir likely did not occur at random; it was likely to be more commonly used in those who were sicker or at higher risk for poor outcomes,” said Dr. Beigel, associate director for clinical research in the Division of Microbiology and Infectious Diseases at the National Institute of Allergy and Infectious Diseases (NIAID).
In addition, the studies evaluated very different populations, he said. The differences in median duration of hospitalization between the trials reflects this, Dr. Beigel added.
Furthermore, when asked if he thinks the new evidence should affect clinical use of remdesivir, Dr. Beigel replied, “No. Observational studies, even with adjustments such as propensity score matching, are not equivalent levels of proof compared to randomized trials.”
Study details
Dr. Ohl and colleagues identified patients admitted to one of 123 VA hospitals for the first time for COVID-19 from May 1 to Oct. 8, 2020. Each had a PCR-confirmed SARS-CoV-2 infection. The researchers then compared 1,172 patients receiving remdesivir to another 1,172 patients not receiving the agent.
Those receiving remdesivir were more likely to be older, White, have chronic obstructive pulmonary disease and have more severe COVID-19. A total 94% of the remdesivir group were men.
“Over 90% of the people included in VA study were men, mostly over the age of 60,” Dr. Ohl said when asked how generalizable the findings would be to a non-VA population.
“There is no obvious biological reason that remdesivir should have different effects in men and women, but we should be cautious about extrapolating study findings to women and younger individuals,” he added.
Limitations of the study include its observational design, which makes unadjusted confounding based on illness severity a possibility. In addition, the investigators were unable to identify specific subgroups that might benefit from remdesivir treatment.
The data did suggest that remdesivir was more effective earlier in the course of disease when patients required supplemental oxygen and before need for mechanical ventilation.
Dr. Baracco pointed out the contradictory findings in his commentary: “The real-life application of a drug promising to hasten discharge from the hospital as its primary beneficial outcome must include an assessment of how easy it is to do so and make it clear that once a patient reaches that point, they can discontinue the drug.”
“The paradoxical findings in the study by Dr. Ohl et al. compared with the study used for its authorization illustrate this point very clearly,” he adds.
Dr. Ohl reported receiving grants from Veterans Affairs Health Services Research and Development during the conduct of the study and consulting for Gilead Pharmaceuticals outside the submitted work. Dr. Baracco reported receiving salary support from the U.S. Department of Veterans Affairs. Dr. Beigel has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Homeopath arrested for fake COVID immunization, vaccine card scheme
A homeopathic doctor licensed in California was arrested July 14 and charged with a scheme to sell homeoprophylaxis immunization pellets and to falsify COVID-19 vaccination cards by making it appear that her customers had received the Moderna vaccine, according to the U.S. Department of Justice.
Juli A. Mazi, 41, of Napa, is charged with one count of wire fraud and one count of false statements related to health care matters. The case is the first federal criminal fraud prosecution related to homeoprophylaxis immunizations and fraudulent vaccination record cards, the DOJ said in a news release.
In April, according to federal authorities, an individual submitted a complaint to the Department of Health and Human Services Office of Inspector General, stating that family members had purchased the immunization pellets from Ms. Mazi. The complainant stated that the family members had told her/him that Ms. Mazi had said the pellets contained the COVID-19 virus and would create an antibody response in the immune system.
The affidavit noted that none of the family members had received injections of any of the COVID-19 vaccines authorized by the Food and Drug Administration.
However, the complainant said, Ms. Mazi sent COVID-19 vaccination cards listing Moderna to the complainant family. Ms. Mazi allegedly instructed the family members to mark the cards to falsely state that they had received the Moderna vaccine on the date that they ingested the homeoprophylaxis immunization pellets.
She also allegedly provided instructions on how to fraudulently complete the cards to make it appear that a customer had received two doses of the Moderna vaccine. She even supplied Moderna lot numbers to enter on the cards.
In addition, Ms. Mazi allegedly offered homeoprophylaxis immunizations for childhood illnesses that she falsely claimed would satisfy the immunization requirements for California schools, and falsified immunization cards that were submitted by parents to California schools.
Ms. Mazi further stated that her customers could provide the pellets to children for COVID-19 immunity, and that “the dose is actually the same for babies,” the news release said.
Ms. Mazi is alleged to have falsely claimed that ingesting the pellets would result in full lifelong immunity from COVID-19. In addition, she exploited the disinformation and fear surrounding COVID-19 vaccination by falsely claiming that the FDA-authorized vaccines contain “toxic ingredients,” the DOJ said.
Homeopathic preparations
According to the DOJ, “Homeophrophylaxis involves the exposure of an individual to dilute amounts of a disease, purportedly to stimulate the immune system and confer immunity.”
According to Australia’s National Centre for Immunisation Research & Surveillance (NCIRS), a private organization funded by the Australian and New South Wales governments, there is no high-quality research showing that homeopathic preparations are effective in preventing infectious disease.
Typical homeopathic preparations dilute a disease, tissue, or plant extract in water “to the point where none of the original material is contained within the preparation by the end of the process,” an NCIRS fact sheet says.
Referring to Ms. Mazi, Deputy Attorney General Lisa Monaco said in the news release, “This defendant allegedly defrauded and endangered the public by preying on fears and spreading misinformation about FDA-authorized vaccinations, while also peddling fake treatments that put people’s lives at risk.
“Even worse, the defendant allegedly created counterfeit COVID-19 vaccination cards and instructed her customers to falsely mark that they had received a vaccine, allowing them to circumvent efforts to contain the spread of the disease.”
The case against Ms. Mazi was brought in coordination with the DOJ Health Care Fraud Unit’s COVID-19 Interagency Working Group, which organizes efforts to address illegal activity involving health care programs during the pandemic.
The fraud unit leads the department’s Health Care Fraud Strike Force, which has existed since 2007. In May, U.S. Attorney General Merrick Garland established the COVID-19 Fraud Enforcement Task Force in partnership with other government agencies to combat and prevent pandemic-related fraud.
A version of this article first appeared on Medscape.com.
A homeopathic doctor licensed in California was arrested July 14 and charged with a scheme to sell homeoprophylaxis immunization pellets and to falsify COVID-19 vaccination cards by making it appear that her customers had received the Moderna vaccine, according to the U.S. Department of Justice.
Juli A. Mazi, 41, of Napa, is charged with one count of wire fraud and one count of false statements related to health care matters. The case is the first federal criminal fraud prosecution related to homeoprophylaxis immunizations and fraudulent vaccination record cards, the DOJ said in a news release.
In April, according to federal authorities, an individual submitted a complaint to the Department of Health and Human Services Office of Inspector General, stating that family members had purchased the immunization pellets from Ms. Mazi. The complainant stated that the family members had told her/him that Ms. Mazi had said the pellets contained the COVID-19 virus and would create an antibody response in the immune system.
The affidavit noted that none of the family members had received injections of any of the COVID-19 vaccines authorized by the Food and Drug Administration.
However, the complainant said, Ms. Mazi sent COVID-19 vaccination cards listing Moderna to the complainant family. Ms. Mazi allegedly instructed the family members to mark the cards to falsely state that they had received the Moderna vaccine on the date that they ingested the homeoprophylaxis immunization pellets.
She also allegedly provided instructions on how to fraudulently complete the cards to make it appear that a customer had received two doses of the Moderna vaccine. She even supplied Moderna lot numbers to enter on the cards.
In addition, Ms. Mazi allegedly offered homeoprophylaxis immunizations for childhood illnesses that she falsely claimed would satisfy the immunization requirements for California schools, and falsified immunization cards that were submitted by parents to California schools.
Ms. Mazi further stated that her customers could provide the pellets to children for COVID-19 immunity, and that “the dose is actually the same for babies,” the news release said.
Ms. Mazi is alleged to have falsely claimed that ingesting the pellets would result in full lifelong immunity from COVID-19. In addition, she exploited the disinformation and fear surrounding COVID-19 vaccination by falsely claiming that the FDA-authorized vaccines contain “toxic ingredients,” the DOJ said.
Homeopathic preparations
According to the DOJ, “Homeophrophylaxis involves the exposure of an individual to dilute amounts of a disease, purportedly to stimulate the immune system and confer immunity.”
According to Australia’s National Centre for Immunisation Research & Surveillance (NCIRS), a private organization funded by the Australian and New South Wales governments, there is no high-quality research showing that homeopathic preparations are effective in preventing infectious disease.
Typical homeopathic preparations dilute a disease, tissue, or plant extract in water “to the point where none of the original material is contained within the preparation by the end of the process,” an NCIRS fact sheet says.
Referring to Ms. Mazi, Deputy Attorney General Lisa Monaco said in the news release, “This defendant allegedly defrauded and endangered the public by preying on fears and spreading misinformation about FDA-authorized vaccinations, while also peddling fake treatments that put people’s lives at risk.
“Even worse, the defendant allegedly created counterfeit COVID-19 vaccination cards and instructed her customers to falsely mark that they had received a vaccine, allowing them to circumvent efforts to contain the spread of the disease.”
The case against Ms. Mazi was brought in coordination with the DOJ Health Care Fraud Unit’s COVID-19 Interagency Working Group, which organizes efforts to address illegal activity involving health care programs during the pandemic.
The fraud unit leads the department’s Health Care Fraud Strike Force, which has existed since 2007. In May, U.S. Attorney General Merrick Garland established the COVID-19 Fraud Enforcement Task Force in partnership with other government agencies to combat and prevent pandemic-related fraud.
A version of this article first appeared on Medscape.com.
A homeopathic doctor licensed in California was arrested July 14 and charged with a scheme to sell homeoprophylaxis immunization pellets and to falsify COVID-19 vaccination cards by making it appear that her customers had received the Moderna vaccine, according to the U.S. Department of Justice.
Juli A. Mazi, 41, of Napa, is charged with one count of wire fraud and one count of false statements related to health care matters. The case is the first federal criminal fraud prosecution related to homeoprophylaxis immunizations and fraudulent vaccination record cards, the DOJ said in a news release.
In April, according to federal authorities, an individual submitted a complaint to the Department of Health and Human Services Office of Inspector General, stating that family members had purchased the immunization pellets from Ms. Mazi. The complainant stated that the family members had told her/him that Ms. Mazi had said the pellets contained the COVID-19 virus and would create an antibody response in the immune system.
The affidavit noted that none of the family members had received injections of any of the COVID-19 vaccines authorized by the Food and Drug Administration.
However, the complainant said, Ms. Mazi sent COVID-19 vaccination cards listing Moderna to the complainant family. Ms. Mazi allegedly instructed the family members to mark the cards to falsely state that they had received the Moderna vaccine on the date that they ingested the homeoprophylaxis immunization pellets.
She also allegedly provided instructions on how to fraudulently complete the cards to make it appear that a customer had received two doses of the Moderna vaccine. She even supplied Moderna lot numbers to enter on the cards.
In addition, Ms. Mazi allegedly offered homeoprophylaxis immunizations for childhood illnesses that she falsely claimed would satisfy the immunization requirements for California schools, and falsified immunization cards that were submitted by parents to California schools.
Ms. Mazi further stated that her customers could provide the pellets to children for COVID-19 immunity, and that “the dose is actually the same for babies,” the news release said.
Ms. Mazi is alleged to have falsely claimed that ingesting the pellets would result in full lifelong immunity from COVID-19. In addition, she exploited the disinformation and fear surrounding COVID-19 vaccination by falsely claiming that the FDA-authorized vaccines contain “toxic ingredients,” the DOJ said.
Homeopathic preparations
According to the DOJ, “Homeophrophylaxis involves the exposure of an individual to dilute amounts of a disease, purportedly to stimulate the immune system and confer immunity.”
According to Australia’s National Centre for Immunisation Research & Surveillance (NCIRS), a private organization funded by the Australian and New South Wales governments, there is no high-quality research showing that homeopathic preparations are effective in preventing infectious disease.
Typical homeopathic preparations dilute a disease, tissue, or plant extract in water “to the point where none of the original material is contained within the preparation by the end of the process,” an NCIRS fact sheet says.
Referring to Ms. Mazi, Deputy Attorney General Lisa Monaco said in the news release, “This defendant allegedly defrauded and endangered the public by preying on fears and spreading misinformation about FDA-authorized vaccinations, while also peddling fake treatments that put people’s lives at risk.
“Even worse, the defendant allegedly created counterfeit COVID-19 vaccination cards and instructed her customers to falsely mark that they had received a vaccine, allowing them to circumvent efforts to contain the spread of the disease.”
The case against Ms. Mazi was brought in coordination with the DOJ Health Care Fraud Unit’s COVID-19 Interagency Working Group, which organizes efforts to address illegal activity involving health care programs during the pandemic.
The fraud unit leads the department’s Health Care Fraud Strike Force, which has existed since 2007. In May, U.S. Attorney General Merrick Garland established the COVID-19 Fraud Enforcement Task Force in partnership with other government agencies to combat and prevent pandemic-related fraud.
A version of this article first appeared on Medscape.com.
More post–COVID-19 GI symptoms: Malnutrition, weight loss
After acute SARS-CoV-2 infection, patients report lingering malnutrition, loss of appetite, and failure to regain lost weight long after other gastrointestinal and non-GI symptoms have resolved, according to the results of a new study.
In the large, multicenter retrospective study published online in Clinical Gastroenterology and Hepatology, Anam Rizvi, MD, and colleagues at Long Island Jewish Medical Center, in New Hyde Park (N.Y.), report a high prevalence of GI symptoms among patients with COVID-19.
They followed 17,462 adult patients who were hospitalized for severe COVID-19 between March 2020 and January 2021. Of these, 3,229 (18.5%) also had GI symptoms.
The median age of the patients was 66 years, and 46.9% were women. The diverse population included White (46%), Black (23%), and Hispanic (17%) patients admitted to 12 medical centers of the Northwell Health System in Manhattan, Queens, Long Island, and Staten Island. The researchers followed patients for 3 months (88.7%) and 6 months (56.5%).
The most frequent initial GI symptoms were gastroenteritis (52.5%), malnutrition (23%), GI bleeding (20.4%), and idiopathic pancreatitis (0.5%). Notably, 50.6% of those with GI manifestations reported an inability to regain lost weight at 3 months; 32.4% reported failure to regain lost weight at 6 months.
These percentages rose among patients with malnutrition, as determined by a board-certified in-hospital nutritionist; 56.4% failed to gain weight at 6 months. A median 14.7-lb weight loss persisted at the half-year mark.
In contrast to these lingering symptoms, gastroenteritis, GI bleeding, and pancreatitis all resolved by 3 months post hospitalization.
“We were somewhat shocked that the prevalence of these symptoms was so high, but it’s overall reassuring that most GI symptoms of COVID-19 resolve,” study author Arvind J. Trindade, MD, told this news organization. “In some COVID patients, we’re seeing an inability to gain weight without diarrhea or postinfectious irritable bowel syndrome.
“Patients with an inability to regain weight should consider follow-up with a nutritionist,” continued Dr. Trindade, who is the center’s director of endoscopy and an associate professor at the Feinstein Institutes for Medical Research, in Manhasset (N.Y.). His group also recommends developing malnutrition screening assessments for COVID-19 patients who recover from the acute infection.
The study was prompted by clinical observations during follow-up.
“We saw that a lot of these patients had trouble regaining weight, but we still don’t know why,” Dr. Trindade said. There were no discriminating clinical features apart from malnutrition that indicated an increased risk, and no socioeconomic or demographic characteristics. “We also looked at whether any factors predicted malnutrition, and there weren’t any that would predispose to malnutrition,” he added.
“We’re now reaching out to nonclinical investigators to see if there’s an interest in studying the underlying science behind these symptoms,” Dr. Trindade said.
His group plans to release 12-month follow-up data from the second wave of the pandemic in January 2022.
Initial GI symptoms are thought to be due to the virus’s S1 spike protein’s binding to the angiotensin-converting enzyme 2 receptors, which are abundant in GI epithelial cells. “But why patients have long-term GI sequelae is probably a whole different physiological mechanism,” Dr. Trindade said. “The thought is that there has to be some hormone or pathway that doesn’t allow them to regain weight.”
“The hospital cohort by [Dr. Rizvi] and colleagues is unique and helpful in that patients with GI symptoms are less likely to be hospitalized, and perhaps those patients who are sick enough for admission to the hospital who also have GI symptoms need specific attention paid to their appetite, weight, and nutritional status,” said Jordan M. Shapiro, MD, who commented on the study but was not involved in it.
The constellations of GI symptoms are difficult to distinguish from other postinfectious GI syndromes, such as irritable bowel syndrome and gastroparesis, added Dr. Shapiro, an assistant professor of medicine in gastroenterology and hepatology at Baylor College of Medicine, Houston. “We’re still unpacking what is and is not specific to post–COVID-19 GI symptoms. Prospective studies are necessary to further study this phenomenon.”
Last year, a small Italian study documented significant weight loss and malnutrition in a hospital cohort of 213 discharged COVID-19 patients. In that study, the duration of disease was predictive of weight loss.
The authors note several study limitations, including that the cohort was limited to hospitalized patients from New York and that the 6-month follow-up period was short.
The study received no funding. Dr. Trindade serves as a consultant to Pentax Medical. All other authors and Dr. Shapiro have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
After acute SARS-CoV-2 infection, patients report lingering malnutrition, loss of appetite, and failure to regain lost weight long after other gastrointestinal and non-GI symptoms have resolved, according to the results of a new study.
In the large, multicenter retrospective study published online in Clinical Gastroenterology and Hepatology, Anam Rizvi, MD, and colleagues at Long Island Jewish Medical Center, in New Hyde Park (N.Y.), report a high prevalence of GI symptoms among patients with COVID-19.
They followed 17,462 adult patients who were hospitalized for severe COVID-19 between March 2020 and January 2021. Of these, 3,229 (18.5%) also had GI symptoms.
The median age of the patients was 66 years, and 46.9% were women. The diverse population included White (46%), Black (23%), and Hispanic (17%) patients admitted to 12 medical centers of the Northwell Health System in Manhattan, Queens, Long Island, and Staten Island. The researchers followed patients for 3 months (88.7%) and 6 months (56.5%).
The most frequent initial GI symptoms were gastroenteritis (52.5%), malnutrition (23%), GI bleeding (20.4%), and idiopathic pancreatitis (0.5%). Notably, 50.6% of those with GI manifestations reported an inability to regain lost weight at 3 months; 32.4% reported failure to regain lost weight at 6 months.
These percentages rose among patients with malnutrition, as determined by a board-certified in-hospital nutritionist; 56.4% failed to gain weight at 6 months. A median 14.7-lb weight loss persisted at the half-year mark.
In contrast to these lingering symptoms, gastroenteritis, GI bleeding, and pancreatitis all resolved by 3 months post hospitalization.
“We were somewhat shocked that the prevalence of these symptoms was so high, but it’s overall reassuring that most GI symptoms of COVID-19 resolve,” study author Arvind J. Trindade, MD, told this news organization. “In some COVID patients, we’re seeing an inability to gain weight without diarrhea or postinfectious irritable bowel syndrome.
“Patients with an inability to regain weight should consider follow-up with a nutritionist,” continued Dr. Trindade, who is the center’s director of endoscopy and an associate professor at the Feinstein Institutes for Medical Research, in Manhasset (N.Y.). His group also recommends developing malnutrition screening assessments for COVID-19 patients who recover from the acute infection.
The study was prompted by clinical observations during follow-up.
“We saw that a lot of these patients had trouble regaining weight, but we still don’t know why,” Dr. Trindade said. There were no discriminating clinical features apart from malnutrition that indicated an increased risk, and no socioeconomic or demographic characteristics. “We also looked at whether any factors predicted malnutrition, and there weren’t any that would predispose to malnutrition,” he added.
“We’re now reaching out to nonclinical investigators to see if there’s an interest in studying the underlying science behind these symptoms,” Dr. Trindade said.
His group plans to release 12-month follow-up data from the second wave of the pandemic in January 2022.
Initial GI symptoms are thought to be due to the virus’s S1 spike protein’s binding to the angiotensin-converting enzyme 2 receptors, which are abundant in GI epithelial cells. “But why patients have long-term GI sequelae is probably a whole different physiological mechanism,” Dr. Trindade said. “The thought is that there has to be some hormone or pathway that doesn’t allow them to regain weight.”
“The hospital cohort by [Dr. Rizvi] and colleagues is unique and helpful in that patients with GI symptoms are less likely to be hospitalized, and perhaps those patients who are sick enough for admission to the hospital who also have GI symptoms need specific attention paid to their appetite, weight, and nutritional status,” said Jordan M. Shapiro, MD, who commented on the study but was not involved in it.
The constellations of GI symptoms are difficult to distinguish from other postinfectious GI syndromes, such as irritable bowel syndrome and gastroparesis, added Dr. Shapiro, an assistant professor of medicine in gastroenterology and hepatology at Baylor College of Medicine, Houston. “We’re still unpacking what is and is not specific to post–COVID-19 GI symptoms. Prospective studies are necessary to further study this phenomenon.”
Last year, a small Italian study documented significant weight loss and malnutrition in a hospital cohort of 213 discharged COVID-19 patients. In that study, the duration of disease was predictive of weight loss.
The authors note several study limitations, including that the cohort was limited to hospitalized patients from New York and that the 6-month follow-up period was short.
The study received no funding. Dr. Trindade serves as a consultant to Pentax Medical. All other authors and Dr. Shapiro have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
After acute SARS-CoV-2 infection, patients report lingering malnutrition, loss of appetite, and failure to regain lost weight long after other gastrointestinal and non-GI symptoms have resolved, according to the results of a new study.
In the large, multicenter retrospective study published online in Clinical Gastroenterology and Hepatology, Anam Rizvi, MD, and colleagues at Long Island Jewish Medical Center, in New Hyde Park (N.Y.), report a high prevalence of GI symptoms among patients with COVID-19.
They followed 17,462 adult patients who were hospitalized for severe COVID-19 between March 2020 and January 2021. Of these, 3,229 (18.5%) also had GI symptoms.
The median age of the patients was 66 years, and 46.9% were women. The diverse population included White (46%), Black (23%), and Hispanic (17%) patients admitted to 12 medical centers of the Northwell Health System in Manhattan, Queens, Long Island, and Staten Island. The researchers followed patients for 3 months (88.7%) and 6 months (56.5%).
The most frequent initial GI symptoms were gastroenteritis (52.5%), malnutrition (23%), GI bleeding (20.4%), and idiopathic pancreatitis (0.5%). Notably, 50.6% of those with GI manifestations reported an inability to regain lost weight at 3 months; 32.4% reported failure to regain lost weight at 6 months.
These percentages rose among patients with malnutrition, as determined by a board-certified in-hospital nutritionist; 56.4% failed to gain weight at 6 months. A median 14.7-lb weight loss persisted at the half-year mark.
In contrast to these lingering symptoms, gastroenteritis, GI bleeding, and pancreatitis all resolved by 3 months post hospitalization.
“We were somewhat shocked that the prevalence of these symptoms was so high, but it’s overall reassuring that most GI symptoms of COVID-19 resolve,” study author Arvind J. Trindade, MD, told this news organization. “In some COVID patients, we’re seeing an inability to gain weight without diarrhea or postinfectious irritable bowel syndrome.
“Patients with an inability to regain weight should consider follow-up with a nutritionist,” continued Dr. Trindade, who is the center’s director of endoscopy and an associate professor at the Feinstein Institutes for Medical Research, in Manhasset (N.Y.). His group also recommends developing malnutrition screening assessments for COVID-19 patients who recover from the acute infection.
The study was prompted by clinical observations during follow-up.
“We saw that a lot of these patients had trouble regaining weight, but we still don’t know why,” Dr. Trindade said. There were no discriminating clinical features apart from malnutrition that indicated an increased risk, and no socioeconomic or demographic characteristics. “We also looked at whether any factors predicted malnutrition, and there weren’t any that would predispose to malnutrition,” he added.
“We’re now reaching out to nonclinical investigators to see if there’s an interest in studying the underlying science behind these symptoms,” Dr. Trindade said.
His group plans to release 12-month follow-up data from the second wave of the pandemic in January 2022.
Initial GI symptoms are thought to be due to the virus’s S1 spike protein’s binding to the angiotensin-converting enzyme 2 receptors, which are abundant in GI epithelial cells. “But why patients have long-term GI sequelae is probably a whole different physiological mechanism,” Dr. Trindade said. “The thought is that there has to be some hormone or pathway that doesn’t allow them to regain weight.”
“The hospital cohort by [Dr. Rizvi] and colleagues is unique and helpful in that patients with GI symptoms are less likely to be hospitalized, and perhaps those patients who are sick enough for admission to the hospital who also have GI symptoms need specific attention paid to their appetite, weight, and nutritional status,” said Jordan M. Shapiro, MD, who commented on the study but was not involved in it.
The constellations of GI symptoms are difficult to distinguish from other postinfectious GI syndromes, such as irritable bowel syndrome and gastroparesis, added Dr. Shapiro, an assistant professor of medicine in gastroenterology and hepatology at Baylor College of Medicine, Houston. “We’re still unpacking what is and is not specific to post–COVID-19 GI symptoms. Prospective studies are necessary to further study this phenomenon.”
Last year, a small Italian study documented significant weight loss and malnutrition in a hospital cohort of 213 discharged COVID-19 patients. In that study, the duration of disease was predictive of weight loss.
The authors note several study limitations, including that the cohort was limited to hospitalized patients from New York and that the 6-month follow-up period was short.
The study received no funding. Dr. Trindade serves as a consultant to Pentax Medical. All other authors and Dr. Shapiro have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.



