User login
Church-based services may help close gaps in mental health care
Black individuals who received mental health services through a church-based program reported high levels of satisfaction, data from a small, qualitative study show.
“This model of providing mental health services adjacent to or supported by a trusted institution, with providers who may have a more nuanced and intimate knowledge of the experiences of and perceptions held by community members, may facilitate important therapy-mediating factors, such as trust,” wrote Angela Coombs, MD, of Columbia University, New York, and colleagues.
Black Americans continue to face barriers to mental health services, and fewer than one-third of Black Americans with a mental health condition receive formal mental health care, Dr. Coombs and colleagues reported. Barriers to treatment include stigma and distrust of medical institutions, and strategies are needed to address these barriers to improve access. Consequently, “one approach includes the development of mental health programming and supports with trusted institutions, such as churches,” they said. Data are limited, however, on the perspectives of individuals who have used church-based services.
In the study, published in Psychiatric Services, Dr. Coombs and colleagues recruited 15 adults aged 27-69 years who were receiving or had received mental health services at the HOPE (Healing On Purpose and Evolving) Center, a freestanding mental health clinic affiliated with the First Corinthian Baptist Church in Harlem, New York. At the time of the study in 2019, those attending the center (referred to as “innovators” rather than patients or clients to reduce stigma) received 10 free sessions of evidence-based psychotherapy.
Treatment included cognitive-behavioral therapy (CBT), religiously integrated CBT, and interpersonal psychotherapy (IPT) to individuals, couples, and families. Group psychotherapy also was an option. Clinicians at the HOPE Center included licensed social workers with doctoral and master’s-level degrees, as well as supervised social work student interns.
Study participants took part in a 30-minute interview, in person or by phone, with a female psychiatrist who was not employed by the HOPE Center or involved in treating the patients. There were 15 participants: 13 women and 2 men, with mean ages of 48 and 51 years, respectively; 14 identified as Black, non-Hispanic. Most (13 individuals) identified as heterosexual, 11 had never married, and 14 had some college or technical school education.
Notably, 11 participants reported attending church once a week, and 13 said they considered religion or spirituality highly important. Participants “reported that services that could integrate their spiritual beliefs with their current mental health challenges enhanced the therapeutic experience,” the researchers said.
Positive messaging about mental health care from the church and senior pastor also encouraged the participants to take advantage of the HOPE Center services.
As one participant said, “I’ve always believed that I can handle my own issues ... but listening to the pastor always talking about the [HOPE] Center and not to be ashamed if you have weaknesses, that’s when I said, ‘You know what, let me just start seeking mental health services because I really need [them].’ ”
, including recognizing cycles of unproductive behavior, processing traumatic experiences and learning self-love, and embracing meditation at home.
“A common theme among participants was that the HOPE Center provided them with tools to destress, process trauma, and manage anxiety,” the researchers wrote. In particular, several participants cited group sessions on teaching and practicing mindfulness as their favorite services. They described the HOPE Center as a positive, peaceful, and welcoming environment where they felt safe.
Cost issues were important as well. Participants noted that the HOPE Center’s ability to provide services that were free made it easier for them to attend. “Although participants said that it was helpful that the HOPE Center provided referrals to external providers and agencies for additional services, some said they wished that the HOPE Center would provide long-term therapy,” the researchers noted.
Overall, “most participants said that establishing more mental health resources within faith-based spaces could accelerate normalization of seeking and receiving mental health care within religious Black communities,” they said.
The study findings were limited by the absence of clinical data – and data on participants’ frequency and location of church attendance, the researchers noted. In addition, the positive results could be tied to selection bias, Dr. Coombs and colleagues said. Another possible limitation is the overrepresentation of cisgender women among the participants. Still, “the perspectives shared by participants suggest that this model of care may address several important barriers to care faced by some Black American populations,” the researchers wrote.
Bridging gap between spirituality and mental health
In an interview, Atasha Jordan, MD, said Black Americans with mental illnesses have long lacked equal access to mental health services. “However, in light of the COVID-19 pandemic, published studies have shown that rates of mental illness increased concurrently with a rise in spirituality and faith. That said, we currently live in a time where mental health and spirituality are more likely to intersect,” noted Dr. Jordan, of the University of Pennsylvania, Philadelphia.
She said it is not surprising that the study participants felt more comfortable receiving mental health services at a clinic that was church affiliated.
“We have known for years that people of faith are more likely to seek comfort for psychological distress from clergy, rather than mental health professionals. Providing a more familiar entry point to mental health services through a church-affiliated mental health clinic helps to bridge the existing gap between spirituality and mental health,” Dr. Jordan said. “For many Black Americans, spirituality is a central component of culturally-informed mental health care.
“Mental health providers may find improved service utilization and outcomes for their patients by collaborating with faith-based organizations or investing time to learn spiritually-based psychotherapies.”
Recently published data, notably a study published May 1, 2021, in Psychiatric Services, continue to support the existing knowledge “that many patients with psychiatric illnesses want increased attention paid to spirituality during their mental health care,” Dr. Jordan noted. “Moreover, they showed that nonreligious clinicians may be more apt than religious clinicians to provide objective, spiritually-oriented mental health care. In this vein, further research aimed at understanding the most effective methods to address spiritual health in times of mental distress can help all mental health providers better meet their patients’ psychiatric and psychological needs.”
Overcoming stigma, mistrust
During the pandemic, clinicians have seen an increase in mental health distress in the form of anxiety, depression, and trauma symptoms, Lorenzo Norris, MD, of George Washington University, Washington, said in an interview.
“Historically, African Americans have faced numerous barriers to mental health care, including stigma and mistrust of medical institutions,” Dr. Norris said. “At this time, perhaps more than in recent decades, novel ways of eliminating and navigating these barriers must be explored in an evidence-based fashion that will inform future interventions.”
Dr. Norris also found that the study findings make sense.
“Historically, the Black church has been a central institution in the community,” he said. “In my personal experience, the church served in a variety of roles, including but not limited to advocacy, employment, social services, peer support, and notably a trusted source of advice pertaining to health. In addition, Black churches may be in an ideal position to serve as culturally sensitive facilitators to build trust,” he said.
The study’s message for clinicians, according to Dr. Norris, is to “carefully consider partnering with faith-based organizations and community leaders if you want to supplement your efforts at decreasing mental health care disparities in the African American community.”
He pointed out, however, that in addition to the small number of participants, the study did not examine clinical outcomes. “So we must be careful how much we take from the initial conclusions,” Dr. Norris said.
Additional research is needed on a much larger scale to add support to the study findings, he said. “This study focused on one church and its particular program,” Dr. Norris noted. “There is likely a great deal of heterogeneity with Black churches and definitely among church members they serve,” he said. “Although it may be tempting to go with an ‘of course it will work’ approach, it is best to have additional qualitative and quantitative research of a much larger scale, with clinical controls that examine the ability of Black churches to address barriers African Americans face in receiving and utilizing mental health services,” he concluded.
Dr. Jordan disclosed receiving a 2021-2022 American Psychiatric Association/Substance Abuse and Mental Health Services Administration Minority Fellowship Program grant to study mental health literacy in the Black church. Dr. Norris disclosed serving as CEO of the Cleveland Clergy Alliance, a nonprofit organization providing outreach assistance as a mechanism to help seniors and the disabled population through community programming. The study authors reported no disclosures.
Black individuals who received mental health services through a church-based program reported high levels of satisfaction, data from a small, qualitative study show.
“This model of providing mental health services adjacent to or supported by a trusted institution, with providers who may have a more nuanced and intimate knowledge of the experiences of and perceptions held by community members, may facilitate important therapy-mediating factors, such as trust,” wrote Angela Coombs, MD, of Columbia University, New York, and colleagues.
Black Americans continue to face barriers to mental health services, and fewer than one-third of Black Americans with a mental health condition receive formal mental health care, Dr. Coombs and colleagues reported. Barriers to treatment include stigma and distrust of medical institutions, and strategies are needed to address these barriers to improve access. Consequently, “one approach includes the development of mental health programming and supports with trusted institutions, such as churches,” they said. Data are limited, however, on the perspectives of individuals who have used church-based services.
In the study, published in Psychiatric Services, Dr. Coombs and colleagues recruited 15 adults aged 27-69 years who were receiving or had received mental health services at the HOPE (Healing On Purpose and Evolving) Center, a freestanding mental health clinic affiliated with the First Corinthian Baptist Church in Harlem, New York. At the time of the study in 2019, those attending the center (referred to as “innovators” rather than patients or clients to reduce stigma) received 10 free sessions of evidence-based psychotherapy.
Treatment included cognitive-behavioral therapy (CBT), religiously integrated CBT, and interpersonal psychotherapy (IPT) to individuals, couples, and families. Group psychotherapy also was an option. Clinicians at the HOPE Center included licensed social workers with doctoral and master’s-level degrees, as well as supervised social work student interns.
Study participants took part in a 30-minute interview, in person or by phone, with a female psychiatrist who was not employed by the HOPE Center or involved in treating the patients. There were 15 participants: 13 women and 2 men, with mean ages of 48 and 51 years, respectively; 14 identified as Black, non-Hispanic. Most (13 individuals) identified as heterosexual, 11 had never married, and 14 had some college or technical school education.
Notably, 11 participants reported attending church once a week, and 13 said they considered religion or spirituality highly important. Participants “reported that services that could integrate their spiritual beliefs with their current mental health challenges enhanced the therapeutic experience,” the researchers said.
Positive messaging about mental health care from the church and senior pastor also encouraged the participants to take advantage of the HOPE Center services.
As one participant said, “I’ve always believed that I can handle my own issues ... but listening to the pastor always talking about the [HOPE] Center and not to be ashamed if you have weaknesses, that’s when I said, ‘You know what, let me just start seeking mental health services because I really need [them].’ ”
, including recognizing cycles of unproductive behavior, processing traumatic experiences and learning self-love, and embracing meditation at home.
“A common theme among participants was that the HOPE Center provided them with tools to destress, process trauma, and manage anxiety,” the researchers wrote. In particular, several participants cited group sessions on teaching and practicing mindfulness as their favorite services. They described the HOPE Center as a positive, peaceful, and welcoming environment where they felt safe.
Cost issues were important as well. Participants noted that the HOPE Center’s ability to provide services that were free made it easier for them to attend. “Although participants said that it was helpful that the HOPE Center provided referrals to external providers and agencies for additional services, some said they wished that the HOPE Center would provide long-term therapy,” the researchers noted.
Overall, “most participants said that establishing more mental health resources within faith-based spaces could accelerate normalization of seeking and receiving mental health care within religious Black communities,” they said.
The study findings were limited by the absence of clinical data – and data on participants’ frequency and location of church attendance, the researchers noted. In addition, the positive results could be tied to selection bias, Dr. Coombs and colleagues said. Another possible limitation is the overrepresentation of cisgender women among the participants. Still, “the perspectives shared by participants suggest that this model of care may address several important barriers to care faced by some Black American populations,” the researchers wrote.
Bridging gap between spirituality and mental health
In an interview, Atasha Jordan, MD, said Black Americans with mental illnesses have long lacked equal access to mental health services. “However, in light of the COVID-19 pandemic, published studies have shown that rates of mental illness increased concurrently with a rise in spirituality and faith. That said, we currently live in a time where mental health and spirituality are more likely to intersect,” noted Dr. Jordan, of the University of Pennsylvania, Philadelphia.
She said it is not surprising that the study participants felt more comfortable receiving mental health services at a clinic that was church affiliated.
“We have known for years that people of faith are more likely to seek comfort for psychological distress from clergy, rather than mental health professionals. Providing a more familiar entry point to mental health services through a church-affiliated mental health clinic helps to bridge the existing gap between spirituality and mental health,” Dr. Jordan said. “For many Black Americans, spirituality is a central component of culturally-informed mental health care.
“Mental health providers may find improved service utilization and outcomes for their patients by collaborating with faith-based organizations or investing time to learn spiritually-based psychotherapies.”
Recently published data, notably a study published May 1, 2021, in Psychiatric Services, continue to support the existing knowledge “that many patients with psychiatric illnesses want increased attention paid to spirituality during their mental health care,” Dr. Jordan noted. “Moreover, they showed that nonreligious clinicians may be more apt than religious clinicians to provide objective, spiritually-oriented mental health care. In this vein, further research aimed at understanding the most effective methods to address spiritual health in times of mental distress can help all mental health providers better meet their patients’ psychiatric and psychological needs.”
Overcoming stigma, mistrust
During the pandemic, clinicians have seen an increase in mental health distress in the form of anxiety, depression, and trauma symptoms, Lorenzo Norris, MD, of George Washington University, Washington, said in an interview.
“Historically, African Americans have faced numerous barriers to mental health care, including stigma and mistrust of medical institutions,” Dr. Norris said. “At this time, perhaps more than in recent decades, novel ways of eliminating and navigating these barriers must be explored in an evidence-based fashion that will inform future interventions.”
Dr. Norris also found that the study findings make sense.
“Historically, the Black church has been a central institution in the community,” he said. “In my personal experience, the church served in a variety of roles, including but not limited to advocacy, employment, social services, peer support, and notably a trusted source of advice pertaining to health. In addition, Black churches may be in an ideal position to serve as culturally sensitive facilitators to build trust,” he said.
The study’s message for clinicians, according to Dr. Norris, is to “carefully consider partnering with faith-based organizations and community leaders if you want to supplement your efforts at decreasing mental health care disparities in the African American community.”
He pointed out, however, that in addition to the small number of participants, the study did not examine clinical outcomes. “So we must be careful how much we take from the initial conclusions,” Dr. Norris said.
Additional research is needed on a much larger scale to add support to the study findings, he said. “This study focused on one church and its particular program,” Dr. Norris noted. “There is likely a great deal of heterogeneity with Black churches and definitely among church members they serve,” he said. “Although it may be tempting to go with an ‘of course it will work’ approach, it is best to have additional qualitative and quantitative research of a much larger scale, with clinical controls that examine the ability of Black churches to address barriers African Americans face in receiving and utilizing mental health services,” he concluded.
Dr. Jordan disclosed receiving a 2021-2022 American Psychiatric Association/Substance Abuse and Mental Health Services Administration Minority Fellowship Program grant to study mental health literacy in the Black church. Dr. Norris disclosed serving as CEO of the Cleveland Clergy Alliance, a nonprofit organization providing outreach assistance as a mechanism to help seniors and the disabled population through community programming. The study authors reported no disclosures.
Black individuals who received mental health services through a church-based program reported high levels of satisfaction, data from a small, qualitative study show.
“This model of providing mental health services adjacent to or supported by a trusted institution, with providers who may have a more nuanced and intimate knowledge of the experiences of and perceptions held by community members, may facilitate important therapy-mediating factors, such as trust,” wrote Angela Coombs, MD, of Columbia University, New York, and colleagues.
Black Americans continue to face barriers to mental health services, and fewer than one-third of Black Americans with a mental health condition receive formal mental health care, Dr. Coombs and colleagues reported. Barriers to treatment include stigma and distrust of medical institutions, and strategies are needed to address these barriers to improve access. Consequently, “one approach includes the development of mental health programming and supports with trusted institutions, such as churches,” they said. Data are limited, however, on the perspectives of individuals who have used church-based services.
In the study, published in Psychiatric Services, Dr. Coombs and colleagues recruited 15 adults aged 27-69 years who were receiving or had received mental health services at the HOPE (Healing On Purpose and Evolving) Center, a freestanding mental health clinic affiliated with the First Corinthian Baptist Church in Harlem, New York. At the time of the study in 2019, those attending the center (referred to as “innovators” rather than patients or clients to reduce stigma) received 10 free sessions of evidence-based psychotherapy.
Treatment included cognitive-behavioral therapy (CBT), religiously integrated CBT, and interpersonal psychotherapy (IPT) to individuals, couples, and families. Group psychotherapy also was an option. Clinicians at the HOPE Center included licensed social workers with doctoral and master’s-level degrees, as well as supervised social work student interns.
Study participants took part in a 30-minute interview, in person or by phone, with a female psychiatrist who was not employed by the HOPE Center or involved in treating the patients. There were 15 participants: 13 women and 2 men, with mean ages of 48 and 51 years, respectively; 14 identified as Black, non-Hispanic. Most (13 individuals) identified as heterosexual, 11 had never married, and 14 had some college or technical school education.
Notably, 11 participants reported attending church once a week, and 13 said they considered religion or spirituality highly important. Participants “reported that services that could integrate their spiritual beliefs with their current mental health challenges enhanced the therapeutic experience,” the researchers said.
Positive messaging about mental health care from the church and senior pastor also encouraged the participants to take advantage of the HOPE Center services.
As one participant said, “I’ve always believed that I can handle my own issues ... but listening to the pastor always talking about the [HOPE] Center and not to be ashamed if you have weaknesses, that’s when I said, ‘You know what, let me just start seeking mental health services because I really need [them].’ ”
, including recognizing cycles of unproductive behavior, processing traumatic experiences and learning self-love, and embracing meditation at home.
“A common theme among participants was that the HOPE Center provided them with tools to destress, process trauma, and manage anxiety,” the researchers wrote. In particular, several participants cited group sessions on teaching and practicing mindfulness as their favorite services. They described the HOPE Center as a positive, peaceful, and welcoming environment where they felt safe.
Cost issues were important as well. Participants noted that the HOPE Center’s ability to provide services that were free made it easier for them to attend. “Although participants said that it was helpful that the HOPE Center provided referrals to external providers and agencies for additional services, some said they wished that the HOPE Center would provide long-term therapy,” the researchers noted.
Overall, “most participants said that establishing more mental health resources within faith-based spaces could accelerate normalization of seeking and receiving mental health care within religious Black communities,” they said.
The study findings were limited by the absence of clinical data – and data on participants’ frequency and location of church attendance, the researchers noted. In addition, the positive results could be tied to selection bias, Dr. Coombs and colleagues said. Another possible limitation is the overrepresentation of cisgender women among the participants. Still, “the perspectives shared by participants suggest that this model of care may address several important barriers to care faced by some Black American populations,” the researchers wrote.
Bridging gap between spirituality and mental health
In an interview, Atasha Jordan, MD, said Black Americans with mental illnesses have long lacked equal access to mental health services. “However, in light of the COVID-19 pandemic, published studies have shown that rates of mental illness increased concurrently with a rise in spirituality and faith. That said, we currently live in a time where mental health and spirituality are more likely to intersect,” noted Dr. Jordan, of the University of Pennsylvania, Philadelphia.
She said it is not surprising that the study participants felt more comfortable receiving mental health services at a clinic that was church affiliated.
“We have known for years that people of faith are more likely to seek comfort for psychological distress from clergy, rather than mental health professionals. Providing a more familiar entry point to mental health services through a church-affiliated mental health clinic helps to bridge the existing gap between spirituality and mental health,” Dr. Jordan said. “For many Black Americans, spirituality is a central component of culturally-informed mental health care.
“Mental health providers may find improved service utilization and outcomes for their patients by collaborating with faith-based organizations or investing time to learn spiritually-based psychotherapies.”
Recently published data, notably a study published May 1, 2021, in Psychiatric Services, continue to support the existing knowledge “that many patients with psychiatric illnesses want increased attention paid to spirituality during their mental health care,” Dr. Jordan noted. “Moreover, they showed that nonreligious clinicians may be more apt than religious clinicians to provide objective, spiritually-oriented mental health care. In this vein, further research aimed at understanding the most effective methods to address spiritual health in times of mental distress can help all mental health providers better meet their patients’ psychiatric and psychological needs.”
Overcoming stigma, mistrust
During the pandemic, clinicians have seen an increase in mental health distress in the form of anxiety, depression, and trauma symptoms, Lorenzo Norris, MD, of George Washington University, Washington, said in an interview.
“Historically, African Americans have faced numerous barriers to mental health care, including stigma and mistrust of medical institutions,” Dr. Norris said. “At this time, perhaps more than in recent decades, novel ways of eliminating and navigating these barriers must be explored in an evidence-based fashion that will inform future interventions.”
Dr. Norris also found that the study findings make sense.
“Historically, the Black church has been a central institution in the community,” he said. “In my personal experience, the church served in a variety of roles, including but not limited to advocacy, employment, social services, peer support, and notably a trusted source of advice pertaining to health. In addition, Black churches may be in an ideal position to serve as culturally sensitive facilitators to build trust,” he said.
The study’s message for clinicians, according to Dr. Norris, is to “carefully consider partnering with faith-based organizations and community leaders if you want to supplement your efforts at decreasing mental health care disparities in the African American community.”
He pointed out, however, that in addition to the small number of participants, the study did not examine clinical outcomes. “So we must be careful how much we take from the initial conclusions,” Dr. Norris said.
Additional research is needed on a much larger scale to add support to the study findings, he said. “This study focused on one church and its particular program,” Dr. Norris noted. “There is likely a great deal of heterogeneity with Black churches and definitely among church members they serve,” he said. “Although it may be tempting to go with an ‘of course it will work’ approach, it is best to have additional qualitative and quantitative research of a much larger scale, with clinical controls that examine the ability of Black churches to address barriers African Americans face in receiving and utilizing mental health services,” he concluded.
Dr. Jordan disclosed receiving a 2021-2022 American Psychiatric Association/Substance Abuse and Mental Health Services Administration Minority Fellowship Program grant to study mental health literacy in the Black church. Dr. Norris disclosed serving as CEO of the Cleveland Clergy Alliance, a nonprofit organization providing outreach assistance as a mechanism to help seniors and the disabled population through community programming. The study authors reported no disclosures.
FROM PSYCHIATRIC SERVICES
CDC panel updates info on rare side effect after J&J vaccine
Despite recent reports of Guillain-Barré Syndrome (GBS) after the Johnson & Johnson vaccine,
The company also presented new data suggesting that the shots generate strong immune responses against circulating variants and that antibodies generated by the vaccine stay elevated for at least 8 months.
Members of the Advisory Committee on Immunization Practices (ACIP) did not vote, but discussed and affirmed their support for recent decisions by the Food and Drug Administration and CDC to update patient information about the very low risk of GBS that appears to be associated with the vaccine, but to continue offering the vaccine to people in the United States.
The Johnson & Johnson shot has been a minor player in the U.S. vaccination campaign, accounting for less than 4% of all vaccine doses given in this country. Still, the single-dose inoculation, which doesn’t require ultra-cold storage, has been important for reaching people in rural areas, through mobile clinics, at colleges and primary care offices, and in vulnerable populations – those who are incarcerated or homeless.
The FDA says it has received reports of 100 cases of GBS after the Johnson & Johnson vaccine in its Vaccine Adverse Event Reporting System database through the end of June. The cases are still under investigation.
To date, more than 12 million doses of the vaccine have been administered, making the rate of GBS 8.1 cases for every million doses administered.
Although it is still extremely rare, that’s above the expected background rate of GBS of 1.6 cases for every million people, said Grace Lee, MD, a Stanford, Calif., pediatrician who chairs the ACIP’s Vaccine Safety Technical Work Group.
So far, most GBS cases (61%) have been among men. The midpoint age of the cases was 57 years. The average time to onset was 14 days, and 98% of cases occurred within 42 days of the shot. Facial paralysis has been associated with an estimated 30%-50% of cases. One person, who had heart failure, high blood pressure, and diabetes, has died.
Still, the benefits of the vaccine far outweigh its risks. For every million doses given to people over age 50, the vaccine prevents nearly 7,500 COVID-19 hospitalizations and nearly 100 deaths in women, and more than 13,000 COVID-19 hospitalizations and more than 2,400 deaths in men.
Rates of GBS after the mRNA vaccines made by Pfizer and Moderna were around 1 case for every 1 million doses given, which is not above the rate that would be expected without vaccination.
The link to the Johnson & Johnson vaccine prompted the FDA to add a warning to the vaccine’s patient safety information on July 12.
Also in July, the European Medicines Agency recommended a similar warning for the product information of the AstraZeneca vaccine Vaxzevria, which relies on similar technology.
Good against variants
Johnson & Johnson also presented new information showing its vaccine maintained high levels of neutralizing antibodies against four of the so-called “variants of concern” – Alpha, Gamma, Beta, and Delta. The protection generated by the vaccine lasted for at least 8 months after the shot, the company said.
“We’re still learning about the duration of protection and the breadth of coverage against this evolving variant landscape for each of the authorized vaccines,” said Mathai Mammen, MD, PhD, global head of research and development at Janssen, the company that makes the vaccine for J&J.
The company also said that its vaccine generated very strong T-cell responses. T cells destroy infected cells and, along with antibodies, are an important part of the body’s immune response.
Antibody levels and T-cell responses are markers for immunity. Measuring these levels isn’t the same as proving that shots can fend off an infection.
It’s still unclear exactly which component of the immune response is most important for fighting off COVID-19.
Dr. Mammen said the companies are still gathering that clinical data, and would present it soon.
“We will have a better view of the clinical efficacy in the coming weeks,” he said.
A version of this article first appeared on Medscape.com.
Despite recent reports of Guillain-Barré Syndrome (GBS) after the Johnson & Johnson vaccine,
The company also presented new data suggesting that the shots generate strong immune responses against circulating variants and that antibodies generated by the vaccine stay elevated for at least 8 months.
Members of the Advisory Committee on Immunization Practices (ACIP) did not vote, but discussed and affirmed their support for recent decisions by the Food and Drug Administration and CDC to update patient information about the very low risk of GBS that appears to be associated with the vaccine, but to continue offering the vaccine to people in the United States.
The Johnson & Johnson shot has been a minor player in the U.S. vaccination campaign, accounting for less than 4% of all vaccine doses given in this country. Still, the single-dose inoculation, which doesn’t require ultra-cold storage, has been important for reaching people in rural areas, through mobile clinics, at colleges and primary care offices, and in vulnerable populations – those who are incarcerated or homeless.
The FDA says it has received reports of 100 cases of GBS after the Johnson & Johnson vaccine in its Vaccine Adverse Event Reporting System database through the end of June. The cases are still under investigation.
To date, more than 12 million doses of the vaccine have been administered, making the rate of GBS 8.1 cases for every million doses administered.
Although it is still extremely rare, that’s above the expected background rate of GBS of 1.6 cases for every million people, said Grace Lee, MD, a Stanford, Calif., pediatrician who chairs the ACIP’s Vaccine Safety Technical Work Group.
So far, most GBS cases (61%) have been among men. The midpoint age of the cases was 57 years. The average time to onset was 14 days, and 98% of cases occurred within 42 days of the shot. Facial paralysis has been associated with an estimated 30%-50% of cases. One person, who had heart failure, high blood pressure, and diabetes, has died.
Still, the benefits of the vaccine far outweigh its risks. For every million doses given to people over age 50, the vaccine prevents nearly 7,500 COVID-19 hospitalizations and nearly 100 deaths in women, and more than 13,000 COVID-19 hospitalizations and more than 2,400 deaths in men.
Rates of GBS after the mRNA vaccines made by Pfizer and Moderna were around 1 case for every 1 million doses given, which is not above the rate that would be expected without vaccination.
The link to the Johnson & Johnson vaccine prompted the FDA to add a warning to the vaccine’s patient safety information on July 12.
Also in July, the European Medicines Agency recommended a similar warning for the product information of the AstraZeneca vaccine Vaxzevria, which relies on similar technology.
Good against variants
Johnson & Johnson also presented new information showing its vaccine maintained high levels of neutralizing antibodies against four of the so-called “variants of concern” – Alpha, Gamma, Beta, and Delta. The protection generated by the vaccine lasted for at least 8 months after the shot, the company said.
“We’re still learning about the duration of protection and the breadth of coverage against this evolving variant landscape for each of the authorized vaccines,” said Mathai Mammen, MD, PhD, global head of research and development at Janssen, the company that makes the vaccine for J&J.
The company also said that its vaccine generated very strong T-cell responses. T cells destroy infected cells and, along with antibodies, are an important part of the body’s immune response.
Antibody levels and T-cell responses are markers for immunity. Measuring these levels isn’t the same as proving that shots can fend off an infection.
It’s still unclear exactly which component of the immune response is most important for fighting off COVID-19.
Dr. Mammen said the companies are still gathering that clinical data, and would present it soon.
“We will have a better view of the clinical efficacy in the coming weeks,” he said.
A version of this article first appeared on Medscape.com.
Despite recent reports of Guillain-Barré Syndrome (GBS) after the Johnson & Johnson vaccine,
The company also presented new data suggesting that the shots generate strong immune responses against circulating variants and that antibodies generated by the vaccine stay elevated for at least 8 months.
Members of the Advisory Committee on Immunization Practices (ACIP) did not vote, but discussed and affirmed their support for recent decisions by the Food and Drug Administration and CDC to update patient information about the very low risk of GBS that appears to be associated with the vaccine, but to continue offering the vaccine to people in the United States.
The Johnson & Johnson shot has been a minor player in the U.S. vaccination campaign, accounting for less than 4% of all vaccine doses given in this country. Still, the single-dose inoculation, which doesn’t require ultra-cold storage, has been important for reaching people in rural areas, through mobile clinics, at colleges and primary care offices, and in vulnerable populations – those who are incarcerated or homeless.
The FDA says it has received reports of 100 cases of GBS after the Johnson & Johnson vaccine in its Vaccine Adverse Event Reporting System database through the end of June. The cases are still under investigation.
To date, more than 12 million doses of the vaccine have been administered, making the rate of GBS 8.1 cases for every million doses administered.
Although it is still extremely rare, that’s above the expected background rate of GBS of 1.6 cases for every million people, said Grace Lee, MD, a Stanford, Calif., pediatrician who chairs the ACIP’s Vaccine Safety Technical Work Group.
So far, most GBS cases (61%) have been among men. The midpoint age of the cases was 57 years. The average time to onset was 14 days, and 98% of cases occurred within 42 days of the shot. Facial paralysis has been associated with an estimated 30%-50% of cases. One person, who had heart failure, high blood pressure, and diabetes, has died.
Still, the benefits of the vaccine far outweigh its risks. For every million doses given to people over age 50, the vaccine prevents nearly 7,500 COVID-19 hospitalizations and nearly 100 deaths in women, and more than 13,000 COVID-19 hospitalizations and more than 2,400 deaths in men.
Rates of GBS after the mRNA vaccines made by Pfizer and Moderna were around 1 case for every 1 million doses given, which is not above the rate that would be expected without vaccination.
The link to the Johnson & Johnson vaccine prompted the FDA to add a warning to the vaccine’s patient safety information on July 12.
Also in July, the European Medicines Agency recommended a similar warning for the product information of the AstraZeneca vaccine Vaxzevria, which relies on similar technology.
Good against variants
Johnson & Johnson also presented new information showing its vaccine maintained high levels of neutralizing antibodies against four of the so-called “variants of concern” – Alpha, Gamma, Beta, and Delta. The protection generated by the vaccine lasted for at least 8 months after the shot, the company said.
“We’re still learning about the duration of protection and the breadth of coverage against this evolving variant landscape for each of the authorized vaccines,” said Mathai Mammen, MD, PhD, global head of research and development at Janssen, the company that makes the vaccine for J&J.
The company also said that its vaccine generated very strong T-cell responses. T cells destroy infected cells and, along with antibodies, are an important part of the body’s immune response.
Antibody levels and T-cell responses are markers for immunity. Measuring these levels isn’t the same as proving that shots can fend off an infection.
It’s still unclear exactly which component of the immune response is most important for fighting off COVID-19.
Dr. Mammen said the companies are still gathering that clinical data, and would present it soon.
“We will have a better view of the clinical efficacy in the coming weeks,” he said.
A version of this article first appeared on Medscape.com.
Exploring your fishpond: Steps toward managing anxiety in the age of COVID
COVID-19’s ever-changing trajectory has led to a notable rise in anxiety-related disorders in the United States. The average share of U.S. adults reporting symptoms of anxiety and or depressive disorder rose from 11% in 2019 to more than 41% in January 2021, according to a report from the Kaiser Family Foundation.
With the arrival of vaccines, Elspeth Cameron Ritchie, MD, MPH, chair of psychiatry at Medstar Washington (D.C.) Hospital Center, has noticed a shift in patients’ fears and concerns. In an interview, she explained how anxiety in patients has evolved along with the pandemic. She also offered strategies for gaining control, engaging with community, and managing anxiety.
Question: When you see patients at this point in the pandemic, what do you ask them?
Answer: I ask them how the pandemic has affected them. Responses have changed over time. In the beginning, I saw a lot of fear, dread of the unknown, a lot of frustration about being in lockdown. As the vaccines have come in and taken hold, there is both a sense of relief, but still a lot of anxiety. Part of that is we’re getting different messages and very much changing messages over time. Then there’s the people who are unvaccinated, and we’re also seeing the Delta variant taking hold in the rest of the world. There’s a lot of anxiety, fear, and some depression, although that’s gotten better with the vaccine.
Q: How do we distinguish between reasonable or rational anxiety and excessive or irrational anxiety?
A: There’s not a bright line between them. What’s rational for one person is not rational for another. What we’ve seen is a spectrum. A rational anxiety is: “I’m not ready to go to a party.” Irrational represents all these crazy theories that are made up, such as putting a microchip into your arm with the vaccine so that the government can track you.
Q: How do you talk to these people thinking irrational thoughts?
A: You must listen to them and not just shut them down. Work with them. Many people with irrational thoughts, or believe in conspiracy theories, may not want to go near a psychiatrist. But there’s also the patients in the psychiatric ward who believe COVID doesn’t exist and there’s government plots. Like any other delusional material, we work with this by talking to these patients and using medication as appropriate.
Q: Do you support prescribing medication for those patients who continue to experience anxiety that is irrational?
A: Patients based in inpatient psychiatry are usually delusional. The medication we usually prescribe for these patients is antipsychotics. If it’s an outpatient who’s anxious about COVID, but has rational anxiety, we usually use antidepressants or antianxiety agents such as Zoloft, Paxil, or Lexapro.
Q: What other strategies can psychiatrists share with patients?
A: What I’ve seen throughout COVID is often an overwhelming sense of dread and inability to control the situation. I tell patients to do things they can control. You can go out and get exercise. Especially during the winter, I recommend that people take a walk and get some sunshine.
It also helps with anxiety to reach out and help someone else. Is there a neighbor you’re concerned about? By and large, this is something many communities have done well. The challenge is we’ve been avoiding each other physically for a long time. So, some of the standard ways of helping each other out, like volunteering at a food bank, have been a little problematic. But there are ways to have minimal people on staff to reduce exposure.
One thing I recommend with any type of anxiety is to learn how to control your breathing. Take breaths through the nose several times a day and teach yourself how to slow down. Another thing that helps many people is contact with animals – especially horses, dogs, and cats. You may not be able to adopt an animal, but you could work at a rescue shelter or other facilities. People can benefit from the nonverbal cues of an animal. A friend of mine got a shelter cat. It sleeps with her and licks her when she feels anxious.
Meditation and yoga are also useful. This is not for everyone, but it’s a way to turn down the level of “buzz” or anxiety. Don’t overdo it on caffeine or other things that increase anxiety. I would stay away from illicit drugs, as they increase anxiety.
Q: What do you say to patients to give them a sense of hope?
A: A lot of people aren’t ready to return to normal; they want to keep the social isolation, the masks, the working from home. We need to show patients what they have control over to minimize their own risk. For example, if they want to wear a mask, then they should wear one. Patients also really like the option of telehealth appointments.
Another way to cope is to identify what’s better about the way things are now and concentrate on those improvements. Here in Maryland, the traffic is so much better in the morning than it once was. There are things I don’t miss, like going to the airport and waiting 5 hours for a flight.
Q: What advice can you give psychiatrists who are experiencing anxiety?
A: We must manage our own anxiety so we can help our patients. Strategies I’ve mentioned are also helpful to psychiatrists or other health care professionals (such as) taking a walk, getting exercise, controlling what you can control. For me, it’s getting dressed, going to work, seeing patients. Having a daily structure, a routine, is important. Many people struggled with this at first. They were working from home and didn’t get much done; they did too much videogaming. It helps to set regular appointments if you’re working from home.
Pre-COVID, many of us got a lot out of our professional meetings. We saw friends there. Now they’re either canceled or we’re doing them virtually, which isn’t the same thing. I think our profession could do a better job of reaching out to each other. We’re used to seeing each other once or twice a year at conventions. I’ve since found it hard to reach out to my colleagues via email. And everyone is tired of Zoom.
If they’re local, ask them to do a safe outdoor activity, a happy hour, a walk. If they’re not, maybe engage with them through a postcard or a phone call.
My colleagues and I go for walks at lunch. There’s a fishpond nearby and we talk to the fish and get a little silly. We sometimes take fish nets with us. People ask what the fish nets are for and we’ll say, “we’re chasing COVID away.”
Dr. Ritchie reported no conflicts of interest.
COVID-19’s ever-changing trajectory has led to a notable rise in anxiety-related disorders in the United States. The average share of U.S. adults reporting symptoms of anxiety and or depressive disorder rose from 11% in 2019 to more than 41% in January 2021, according to a report from the Kaiser Family Foundation.
With the arrival of vaccines, Elspeth Cameron Ritchie, MD, MPH, chair of psychiatry at Medstar Washington (D.C.) Hospital Center, has noticed a shift in patients’ fears and concerns. In an interview, she explained how anxiety in patients has evolved along with the pandemic. She also offered strategies for gaining control, engaging with community, and managing anxiety.
Question: When you see patients at this point in the pandemic, what do you ask them?
Answer: I ask them how the pandemic has affected them. Responses have changed over time. In the beginning, I saw a lot of fear, dread of the unknown, a lot of frustration about being in lockdown. As the vaccines have come in and taken hold, there is both a sense of relief, but still a lot of anxiety. Part of that is we’re getting different messages and very much changing messages over time. Then there’s the people who are unvaccinated, and we’re also seeing the Delta variant taking hold in the rest of the world. There’s a lot of anxiety, fear, and some depression, although that’s gotten better with the vaccine.
Q: How do we distinguish between reasonable or rational anxiety and excessive or irrational anxiety?
A: There’s not a bright line between them. What’s rational for one person is not rational for another. What we’ve seen is a spectrum. A rational anxiety is: “I’m not ready to go to a party.” Irrational represents all these crazy theories that are made up, such as putting a microchip into your arm with the vaccine so that the government can track you.
Q: How do you talk to these people thinking irrational thoughts?
A: You must listen to them and not just shut them down. Work with them. Many people with irrational thoughts, or believe in conspiracy theories, may not want to go near a psychiatrist. But there’s also the patients in the psychiatric ward who believe COVID doesn’t exist and there’s government plots. Like any other delusional material, we work with this by talking to these patients and using medication as appropriate.
Q: Do you support prescribing medication for those patients who continue to experience anxiety that is irrational?
A: Patients based in inpatient psychiatry are usually delusional. The medication we usually prescribe for these patients is antipsychotics. If it’s an outpatient who’s anxious about COVID, but has rational anxiety, we usually use antidepressants or antianxiety agents such as Zoloft, Paxil, or Lexapro.
Q: What other strategies can psychiatrists share with patients?
A: What I’ve seen throughout COVID is often an overwhelming sense of dread and inability to control the situation. I tell patients to do things they can control. You can go out and get exercise. Especially during the winter, I recommend that people take a walk and get some sunshine.
It also helps with anxiety to reach out and help someone else. Is there a neighbor you’re concerned about? By and large, this is something many communities have done well. The challenge is we’ve been avoiding each other physically for a long time. So, some of the standard ways of helping each other out, like volunteering at a food bank, have been a little problematic. But there are ways to have minimal people on staff to reduce exposure.
One thing I recommend with any type of anxiety is to learn how to control your breathing. Take breaths through the nose several times a day and teach yourself how to slow down. Another thing that helps many people is contact with animals – especially horses, dogs, and cats. You may not be able to adopt an animal, but you could work at a rescue shelter or other facilities. People can benefit from the nonverbal cues of an animal. A friend of mine got a shelter cat. It sleeps with her and licks her when she feels anxious.
Meditation and yoga are also useful. This is not for everyone, but it’s a way to turn down the level of “buzz” or anxiety. Don’t overdo it on caffeine or other things that increase anxiety. I would stay away from illicit drugs, as they increase anxiety.
Q: What do you say to patients to give them a sense of hope?
A: A lot of people aren’t ready to return to normal; they want to keep the social isolation, the masks, the working from home. We need to show patients what they have control over to minimize their own risk. For example, if they want to wear a mask, then they should wear one. Patients also really like the option of telehealth appointments.
Another way to cope is to identify what’s better about the way things are now and concentrate on those improvements. Here in Maryland, the traffic is so much better in the morning than it once was. There are things I don’t miss, like going to the airport and waiting 5 hours for a flight.
Q: What advice can you give psychiatrists who are experiencing anxiety?
A: We must manage our own anxiety so we can help our patients. Strategies I’ve mentioned are also helpful to psychiatrists or other health care professionals (such as) taking a walk, getting exercise, controlling what you can control. For me, it’s getting dressed, going to work, seeing patients. Having a daily structure, a routine, is important. Many people struggled with this at first. They were working from home and didn’t get much done; they did too much videogaming. It helps to set regular appointments if you’re working from home.
Pre-COVID, many of us got a lot out of our professional meetings. We saw friends there. Now they’re either canceled or we’re doing them virtually, which isn’t the same thing. I think our profession could do a better job of reaching out to each other. We’re used to seeing each other once or twice a year at conventions. I’ve since found it hard to reach out to my colleagues via email. And everyone is tired of Zoom.
If they’re local, ask them to do a safe outdoor activity, a happy hour, a walk. If they’re not, maybe engage with them through a postcard or a phone call.
My colleagues and I go for walks at lunch. There’s a fishpond nearby and we talk to the fish and get a little silly. We sometimes take fish nets with us. People ask what the fish nets are for and we’ll say, “we’re chasing COVID away.”
Dr. Ritchie reported no conflicts of interest.
COVID-19’s ever-changing trajectory has led to a notable rise in anxiety-related disorders in the United States. The average share of U.S. adults reporting symptoms of anxiety and or depressive disorder rose from 11% in 2019 to more than 41% in January 2021, according to a report from the Kaiser Family Foundation.
With the arrival of vaccines, Elspeth Cameron Ritchie, MD, MPH, chair of psychiatry at Medstar Washington (D.C.) Hospital Center, has noticed a shift in patients’ fears and concerns. In an interview, she explained how anxiety in patients has evolved along with the pandemic. She also offered strategies for gaining control, engaging with community, and managing anxiety.
Question: When you see patients at this point in the pandemic, what do you ask them?
Answer: I ask them how the pandemic has affected them. Responses have changed over time. In the beginning, I saw a lot of fear, dread of the unknown, a lot of frustration about being in lockdown. As the vaccines have come in and taken hold, there is both a sense of relief, but still a lot of anxiety. Part of that is we’re getting different messages and very much changing messages over time. Then there’s the people who are unvaccinated, and we’re also seeing the Delta variant taking hold in the rest of the world. There’s a lot of anxiety, fear, and some depression, although that’s gotten better with the vaccine.
Q: How do we distinguish between reasonable or rational anxiety and excessive or irrational anxiety?
A: There’s not a bright line between them. What’s rational for one person is not rational for another. What we’ve seen is a spectrum. A rational anxiety is: “I’m not ready to go to a party.” Irrational represents all these crazy theories that are made up, such as putting a microchip into your arm with the vaccine so that the government can track you.
Q: How do you talk to these people thinking irrational thoughts?
A: You must listen to them and not just shut them down. Work with them. Many people with irrational thoughts, or believe in conspiracy theories, may not want to go near a psychiatrist. But there’s also the patients in the psychiatric ward who believe COVID doesn’t exist and there’s government plots. Like any other delusional material, we work with this by talking to these patients and using medication as appropriate.
Q: Do you support prescribing medication for those patients who continue to experience anxiety that is irrational?
A: Patients based in inpatient psychiatry are usually delusional. The medication we usually prescribe for these patients is antipsychotics. If it’s an outpatient who’s anxious about COVID, but has rational anxiety, we usually use antidepressants or antianxiety agents such as Zoloft, Paxil, or Lexapro.
Q: What other strategies can psychiatrists share with patients?
A: What I’ve seen throughout COVID is often an overwhelming sense of dread and inability to control the situation. I tell patients to do things they can control. You can go out and get exercise. Especially during the winter, I recommend that people take a walk and get some sunshine.
It also helps with anxiety to reach out and help someone else. Is there a neighbor you’re concerned about? By and large, this is something many communities have done well. The challenge is we’ve been avoiding each other physically for a long time. So, some of the standard ways of helping each other out, like volunteering at a food bank, have been a little problematic. But there are ways to have minimal people on staff to reduce exposure.
One thing I recommend with any type of anxiety is to learn how to control your breathing. Take breaths through the nose several times a day and teach yourself how to slow down. Another thing that helps many people is contact with animals – especially horses, dogs, and cats. You may not be able to adopt an animal, but you could work at a rescue shelter or other facilities. People can benefit from the nonverbal cues of an animal. A friend of mine got a shelter cat. It sleeps with her and licks her when she feels anxious.
Meditation and yoga are also useful. This is not for everyone, but it’s a way to turn down the level of “buzz” or anxiety. Don’t overdo it on caffeine or other things that increase anxiety. I would stay away from illicit drugs, as they increase anxiety.
Q: What do you say to patients to give them a sense of hope?
A: A lot of people aren’t ready to return to normal; they want to keep the social isolation, the masks, the working from home. We need to show patients what they have control over to minimize their own risk. For example, if they want to wear a mask, then they should wear one. Patients also really like the option of telehealth appointments.
Another way to cope is to identify what’s better about the way things are now and concentrate on those improvements. Here in Maryland, the traffic is so much better in the morning than it once was. There are things I don’t miss, like going to the airport and waiting 5 hours for a flight.
Q: What advice can you give psychiatrists who are experiencing anxiety?
A: We must manage our own anxiety so we can help our patients. Strategies I’ve mentioned are also helpful to psychiatrists or other health care professionals (such as) taking a walk, getting exercise, controlling what you can control. For me, it’s getting dressed, going to work, seeing patients. Having a daily structure, a routine, is important. Many people struggled with this at first. They were working from home and didn’t get much done; they did too much videogaming. It helps to set regular appointments if you’re working from home.
Pre-COVID, many of us got a lot out of our professional meetings. We saw friends there. Now they’re either canceled or we’re doing them virtually, which isn’t the same thing. I think our profession could do a better job of reaching out to each other. We’re used to seeing each other once or twice a year at conventions. I’ve since found it hard to reach out to my colleagues via email. And everyone is tired of Zoom.
If they’re local, ask them to do a safe outdoor activity, a happy hour, a walk. If they’re not, maybe engage with them through a postcard or a phone call.
My colleagues and I go for walks at lunch. There’s a fishpond nearby and we talk to the fish and get a little silly. We sometimes take fish nets with us. People ask what the fish nets are for and we’ll say, “we’re chasing COVID away.”
Dr. Ritchie reported no conflicts of interest.
Face mask–related injuries rose dramatically in 2020
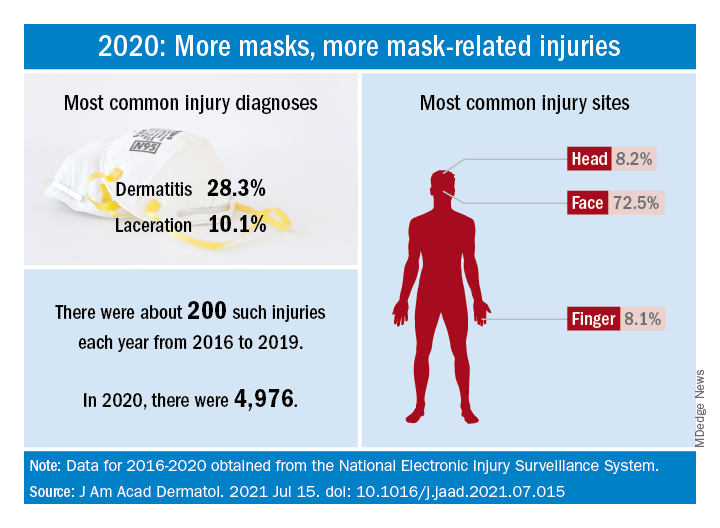
How dramatic? The number of mask-related injuries treated in U.S. emergency departments averaged about 200 per year from 2016 to 2019. In 2020, that figure soared to 4,976 – an increase of almost 2,400%, Gerald McGwin Jr., PhD, and associates said in a research letter published in the Journal to the American Academy of Dermatology.
“Prior to the COVID-19 pandemic the use of respiratory protection equipment was largely limited to healthcare and industrial settings. As [face mask] use by the general population increased, so too have reports of dermatologic reactions,” said Dr. McGwin and associates of the department of epidemiology at the University of Alabama at Birmingham.
Dermatitis was the most common mask-related injury treated last year, affecting 28.3% of those presenting to EDs, followed by lacerations at 10.1%. Injuries were more common in women than men, but while and black patients “were equally represented,” they noted, based on data from the National Electronic Injury Surveillance System, which includes about 100 hospitals and EDs.
Most injuries were caused by rashes/allergic reactions (38%) from prolonged use, poorly fitting masks (19%), and obscured vision (14%). “There was a small (5%) but meaningful number of injuries, all among children, attributable to consuming pieces of a mask or inserting dismantled pieces of a mask into body orifices,” the investigators said.
Guidance from the Centers for Disease Control and Prevention is available “to aid in the choice and proper fit of face masks,” they wrote, and “increased awareness of these resources [could] minimize the future occurrence of mask-related injuries.”
There was no funding source for the study, and the investigators did not declare any conflicts of interest.

How dramatic? The number of mask-related injuries treated in U.S. emergency departments averaged about 200 per year from 2016 to 2019. In 2020, that figure soared to 4,976 – an increase of almost 2,400%, Gerald McGwin Jr., PhD, and associates said in a research letter published in the Journal to the American Academy of Dermatology.
“Prior to the COVID-19 pandemic the use of respiratory protection equipment was largely limited to healthcare and industrial settings. As [face mask] use by the general population increased, so too have reports of dermatologic reactions,” said Dr. McGwin and associates of the department of epidemiology at the University of Alabama at Birmingham.
Dermatitis was the most common mask-related injury treated last year, affecting 28.3% of those presenting to EDs, followed by lacerations at 10.1%. Injuries were more common in women than men, but while and black patients “were equally represented,” they noted, based on data from the National Electronic Injury Surveillance System, which includes about 100 hospitals and EDs.
Most injuries were caused by rashes/allergic reactions (38%) from prolonged use, poorly fitting masks (19%), and obscured vision (14%). “There was a small (5%) but meaningful number of injuries, all among children, attributable to consuming pieces of a mask or inserting dismantled pieces of a mask into body orifices,” the investigators said.
Guidance from the Centers for Disease Control and Prevention is available “to aid in the choice and proper fit of face masks,” they wrote, and “increased awareness of these resources [could] minimize the future occurrence of mask-related injuries.”
There was no funding source for the study, and the investigators did not declare any conflicts of interest.

How dramatic? The number of mask-related injuries treated in U.S. emergency departments averaged about 200 per year from 2016 to 2019. In 2020, that figure soared to 4,976 – an increase of almost 2,400%, Gerald McGwin Jr., PhD, and associates said in a research letter published in the Journal to the American Academy of Dermatology.
“Prior to the COVID-19 pandemic the use of respiratory protection equipment was largely limited to healthcare and industrial settings. As [face mask] use by the general population increased, so too have reports of dermatologic reactions,” said Dr. McGwin and associates of the department of epidemiology at the University of Alabama at Birmingham.
Dermatitis was the most common mask-related injury treated last year, affecting 28.3% of those presenting to EDs, followed by lacerations at 10.1%. Injuries were more common in women than men, but while and black patients “were equally represented,” they noted, based on data from the National Electronic Injury Surveillance System, which includes about 100 hospitals and EDs.
Most injuries were caused by rashes/allergic reactions (38%) from prolonged use, poorly fitting masks (19%), and obscured vision (14%). “There was a small (5%) but meaningful number of injuries, all among children, attributable to consuming pieces of a mask or inserting dismantled pieces of a mask into body orifices,” the investigators said.
Guidance from the Centers for Disease Control and Prevention is available “to aid in the choice and proper fit of face masks,” they wrote, and “increased awareness of these resources [could] minimize the future occurrence of mask-related injuries.”
There was no funding source for the study, and the investigators did not declare any conflicts of interest.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Twofold increased risk for death from COVID-19 in psych patients
compared with those without a psychiatric diagnosis, according to the results of the largest study of its kind to date.
These findings, the investigators noted, highlight the need to prioritize vaccination in patients with preexisting mental health disorders.
“We have proven beyond a shadow of a doubt that there are increased risks” among psychiatric patients who get COVID-19, study investigator Livia De Picker, MD, PhD, psychiatrist and postdoctoral researcher, University Psychiatric Hospital Campus Duffel and University of Antwerp (Belgium), told this news organization.
“Doctors need to look at these patients the same way they would other high-risk people, for example those with diabetes or chronic obstructive pulmonary disease,” all of whom should be protected against COVID-19, Dr. De Picker added.
The study was published online July 15, 2021, in Lancet Psychiatry.
Risk by mental illness type
The systematic review included 33 studies from 22 countries that reported risk estimates for mortality, hospitalization, and ICU admission in patients with confirmed SARS-CoV-2 infection. The meta-analysis included 23 of these studies with a total of 1.47 million participants. Of these, 43,938 had a psychiatric disorder.
The primary outcome was mortality after COVID-19. Secondary outcomes included hospitalization and ICU admission after COVID-19. Researchers adjusted for age, sex, and other covariates.
Results showed the presence of any comorbid mental illness was associated with an increased risk for death after SARS-CoV-2 infection (odds ratio, 2.00; 95% confidence interval, 1.58-2.54; P < .0001).
When researchers stratified mortality risk by psychiatric disorder type, the most robust associations were for psychotic and mood disorders. Substance use disorders, intellectual disabilities, and developmental disorders were associated with higher mortality only in crude estimates. There was no increased death risk associated with anxiety disorders.
“That there are differences between the various types of disorders was an interesting finding,” said Dr. De Picker, adding that previous research “just lumped together all diagnostic categories.”
Potential mechanisms
The study did not explore why psychiatric illness raise the risk for death in the setting of COVID-19, so potential mechanisms are purely speculative. However, the investigators believe it may reflect biological processes such as immune-inflammatory alterations.
Psychotic disorders and mood disorders in particular, are associated with immune changes, including immunogenetic abnormalities, raised cytokine concentrations, autoantibodies, acute-phase proteins, and aberrant counts of leukocyte cell types, said Dr. De Picker.
She likened this to elderly people being at increased risk following COVID-19 because their immune system is compromised and less able to fight infection.
There are likely other factors at play, said Dr. De Picker. These could include social isolation and lifestyle factors like poor diet, physical inactivity, high alcohol and tobacco use, and sleep disturbances.
In addition, psychiatric patients have a higher prevalence of comorbidities including diabetes, cardiovascular disease, and respiratory disease, which could also play a role.
The increased mortality might also reflect reduced access to care. “Some of these patients may be living in difficult socioeconomic conditions,” said Dr. De Picker.
She noted that, while the in-hospital mortality was not increased, the risk was significantly increased in samples that were outside of the hospital. This reinforces the need for providing close monitoring and early referral to hospital for psychiatric patients with COVID-19.
Mortality varied significantly among countries, with the lowest risk in Europe and the United States. This difference might be attributable to differences in health care systems and access to care, said Dr. De Picker.
Overall, the risk for hospitalization was about double for COVID patients with a mental illness, but when stratified by disorder, there was only a significantly increased risk for substance use and mood disorders. “But mood disorders were not even significant any more after adjusting for age, sex, and comorbid conditions, and we don’t see an increased risk for psychotic disorders whereas they had the highest mortality risks,” said Dr. De Picker.
Psych meds a risk factor?
The studies were primarily based on electronic medical records, so investigators were unable to carry out “a fine grain analysis” into clinical factors affecting outcomes, she noted.
Antipsychotics were consistently associated with an increased risk for mortality (adjusted OR, 2.43; 95% CI, 1.81-3.25), as were anxiolytics (aOR, 1.47; 95% CI, 1.15-1.88).
“There are some theoretical reasons why we believe there could be a risk associated with these drugs,” said Dr. De Picker. For example, antipsychotics can increase the risk for cardiac arrhythmias and thromboembolic events, and cause interactions with drugs used to treat COVID-19.
As for anxiolytics, especially benzodiazepines, these drugs are associated with respiratory risk and with all-cause mortality. “So you could imagine that someone who is infected with a respiratory virus and [is] then using these drugs on top of that would have a worse outcome,” said Dr. De Picker.
In contrast to antipsychotics and anxiolytics, antidepressants did not increase mortality risk.
Dr. De Picker noted a new study by French researchers showing a protective effect of certain serotonergic antidepressants on COVID outcomes, including mortality.
There was no robust evidence of an increased risk for ICU admission for patients with mental disorders. However, the authors noted some studies included small samples of patients with psychiatric disorders, “contributing to a low certainty of evidence for ICU admission.”
Dr. De Picker criticized COVID vaccine policies that don’t prioritize patients with psychiatric disorders. In many countries, groups that were initially green-lighted for the vaccine included health care workers, the elderly, and those with underlying conditions such as diabetes, obesity and even mild hypertension – but not mental illness, which is also an underlying risk.
‘Outstanding’ research
Commenting on the study for this news organization, Jonathan E. Alpert, MD, PhD, department of psychiatry and behavioral sciences, Montefiore Medical Center, New York, and chair of the American Psychiatric Association Council on Research, called it “outstanding” and the largest of its kind.
“There have been a number of studies that have come to similar conclusions, that people with psychiatric illness are at greater risk for poorer outcomes, but because any given study had a relatively limited sample, perhaps from one health system or one country, there were some inconsistencies,” said Dr. Alpert.
“This is the strongest report so far that has made the point that people with psychiatric illness are a vulnerable population for a negative outcome from COVID, including the most worrisome – mortality.”
The study helps drive home a “very important public health lesson” that applies to COVID-19 but goes “beyond,” said Dr. Alpert.
“As a society, we need to keep in mind that people with serious mental disorders are a vulnerable population for poorer outcomes in most general medical conditions,” he stressed, “whether it’s cancer or heart disease or diabetes, and special efforts need to be made to reach out to those populations.”
Dr. Alpert agreed that, at the start of the pandemic, psychiatric patients in the United States were not prioritized for vaccination, and although psychiatric patients may initially have found it difficult to navigate the health care system to learn where and how to get a COVID shot, today that barrier has mostly been removed.
“Our patients are at least as willing as any other subgroup to get the vaccine, and that includes people with psychotic disorders,” he said.
The study was supported by the European College of Neuropsychopharmacology Immuno-NeuroPsychiatry network and Fondazione Centro San Raffaele (Milan). Dr. De Picker reported receiving grants from Boehringer Ingelheim and Janssen outside the submitted work. She is a member of the European College of Neuropsychopharmacology Immuno-NeuroPsychiatry Thematic Working Group.
A version of this article first appeared on Medscape.com.
compared with those without a psychiatric diagnosis, according to the results of the largest study of its kind to date.
These findings, the investigators noted, highlight the need to prioritize vaccination in patients with preexisting mental health disorders.
“We have proven beyond a shadow of a doubt that there are increased risks” among psychiatric patients who get COVID-19, study investigator Livia De Picker, MD, PhD, psychiatrist and postdoctoral researcher, University Psychiatric Hospital Campus Duffel and University of Antwerp (Belgium), told this news organization.
“Doctors need to look at these patients the same way they would other high-risk people, for example those with diabetes or chronic obstructive pulmonary disease,” all of whom should be protected against COVID-19, Dr. De Picker added.
The study was published online July 15, 2021, in Lancet Psychiatry.
Risk by mental illness type
The systematic review included 33 studies from 22 countries that reported risk estimates for mortality, hospitalization, and ICU admission in patients with confirmed SARS-CoV-2 infection. The meta-analysis included 23 of these studies with a total of 1.47 million participants. Of these, 43,938 had a psychiatric disorder.
The primary outcome was mortality after COVID-19. Secondary outcomes included hospitalization and ICU admission after COVID-19. Researchers adjusted for age, sex, and other covariates.
Results showed the presence of any comorbid mental illness was associated with an increased risk for death after SARS-CoV-2 infection (odds ratio, 2.00; 95% confidence interval, 1.58-2.54; P < .0001).
When researchers stratified mortality risk by psychiatric disorder type, the most robust associations were for psychotic and mood disorders. Substance use disorders, intellectual disabilities, and developmental disorders were associated with higher mortality only in crude estimates. There was no increased death risk associated with anxiety disorders.
“That there are differences between the various types of disorders was an interesting finding,” said Dr. De Picker, adding that previous research “just lumped together all diagnostic categories.”
Potential mechanisms
The study did not explore why psychiatric illness raise the risk for death in the setting of COVID-19, so potential mechanisms are purely speculative. However, the investigators believe it may reflect biological processes such as immune-inflammatory alterations.
Psychotic disorders and mood disorders in particular, are associated with immune changes, including immunogenetic abnormalities, raised cytokine concentrations, autoantibodies, acute-phase proteins, and aberrant counts of leukocyte cell types, said Dr. De Picker.
She likened this to elderly people being at increased risk following COVID-19 because their immune system is compromised and less able to fight infection.
There are likely other factors at play, said Dr. De Picker. These could include social isolation and lifestyle factors like poor diet, physical inactivity, high alcohol and tobacco use, and sleep disturbances.
In addition, psychiatric patients have a higher prevalence of comorbidities including diabetes, cardiovascular disease, and respiratory disease, which could also play a role.
The increased mortality might also reflect reduced access to care. “Some of these patients may be living in difficult socioeconomic conditions,” said Dr. De Picker.
She noted that, while the in-hospital mortality was not increased, the risk was significantly increased in samples that were outside of the hospital. This reinforces the need for providing close monitoring and early referral to hospital for psychiatric patients with COVID-19.
Mortality varied significantly among countries, with the lowest risk in Europe and the United States. This difference might be attributable to differences in health care systems and access to care, said Dr. De Picker.
Overall, the risk for hospitalization was about double for COVID patients with a mental illness, but when stratified by disorder, there was only a significantly increased risk for substance use and mood disorders. “But mood disorders were not even significant any more after adjusting for age, sex, and comorbid conditions, and we don’t see an increased risk for psychotic disorders whereas they had the highest mortality risks,” said Dr. De Picker.
Psych meds a risk factor?
The studies were primarily based on electronic medical records, so investigators were unable to carry out “a fine grain analysis” into clinical factors affecting outcomes, she noted.
Antipsychotics were consistently associated with an increased risk for mortality (adjusted OR, 2.43; 95% CI, 1.81-3.25), as were anxiolytics (aOR, 1.47; 95% CI, 1.15-1.88).
“There are some theoretical reasons why we believe there could be a risk associated with these drugs,” said Dr. De Picker. For example, antipsychotics can increase the risk for cardiac arrhythmias and thromboembolic events, and cause interactions with drugs used to treat COVID-19.
As for anxiolytics, especially benzodiazepines, these drugs are associated with respiratory risk and with all-cause mortality. “So you could imagine that someone who is infected with a respiratory virus and [is] then using these drugs on top of that would have a worse outcome,” said Dr. De Picker.
In contrast to antipsychotics and anxiolytics, antidepressants did not increase mortality risk.
Dr. De Picker noted a new study by French researchers showing a protective effect of certain serotonergic antidepressants on COVID outcomes, including mortality.
There was no robust evidence of an increased risk for ICU admission for patients with mental disorders. However, the authors noted some studies included small samples of patients with psychiatric disorders, “contributing to a low certainty of evidence for ICU admission.”
Dr. De Picker criticized COVID vaccine policies that don’t prioritize patients with psychiatric disorders. In many countries, groups that were initially green-lighted for the vaccine included health care workers, the elderly, and those with underlying conditions such as diabetes, obesity and even mild hypertension – but not mental illness, which is also an underlying risk.
‘Outstanding’ research
Commenting on the study for this news organization, Jonathan E. Alpert, MD, PhD, department of psychiatry and behavioral sciences, Montefiore Medical Center, New York, and chair of the American Psychiatric Association Council on Research, called it “outstanding” and the largest of its kind.
“There have been a number of studies that have come to similar conclusions, that people with psychiatric illness are at greater risk for poorer outcomes, but because any given study had a relatively limited sample, perhaps from one health system or one country, there were some inconsistencies,” said Dr. Alpert.
“This is the strongest report so far that has made the point that people with psychiatric illness are a vulnerable population for a negative outcome from COVID, including the most worrisome – mortality.”
The study helps drive home a “very important public health lesson” that applies to COVID-19 but goes “beyond,” said Dr. Alpert.
“As a society, we need to keep in mind that people with serious mental disorders are a vulnerable population for poorer outcomes in most general medical conditions,” he stressed, “whether it’s cancer or heart disease or diabetes, and special efforts need to be made to reach out to those populations.”
Dr. Alpert agreed that, at the start of the pandemic, psychiatric patients in the United States were not prioritized for vaccination, and although psychiatric patients may initially have found it difficult to navigate the health care system to learn where and how to get a COVID shot, today that barrier has mostly been removed.
“Our patients are at least as willing as any other subgroup to get the vaccine, and that includes people with psychotic disorders,” he said.
The study was supported by the European College of Neuropsychopharmacology Immuno-NeuroPsychiatry network and Fondazione Centro San Raffaele (Milan). Dr. De Picker reported receiving grants from Boehringer Ingelheim and Janssen outside the submitted work. She is a member of the European College of Neuropsychopharmacology Immuno-NeuroPsychiatry Thematic Working Group.
A version of this article first appeared on Medscape.com.
compared with those without a psychiatric diagnosis, according to the results of the largest study of its kind to date.
These findings, the investigators noted, highlight the need to prioritize vaccination in patients with preexisting mental health disorders.
“We have proven beyond a shadow of a doubt that there are increased risks” among psychiatric patients who get COVID-19, study investigator Livia De Picker, MD, PhD, psychiatrist and postdoctoral researcher, University Psychiatric Hospital Campus Duffel and University of Antwerp (Belgium), told this news organization.
“Doctors need to look at these patients the same way they would other high-risk people, for example those with diabetes or chronic obstructive pulmonary disease,” all of whom should be protected against COVID-19, Dr. De Picker added.
The study was published online July 15, 2021, in Lancet Psychiatry.
Risk by mental illness type
The systematic review included 33 studies from 22 countries that reported risk estimates for mortality, hospitalization, and ICU admission in patients with confirmed SARS-CoV-2 infection. The meta-analysis included 23 of these studies with a total of 1.47 million participants. Of these, 43,938 had a psychiatric disorder.
The primary outcome was mortality after COVID-19. Secondary outcomes included hospitalization and ICU admission after COVID-19. Researchers adjusted for age, sex, and other covariates.
Results showed the presence of any comorbid mental illness was associated with an increased risk for death after SARS-CoV-2 infection (odds ratio, 2.00; 95% confidence interval, 1.58-2.54; P < .0001).
When researchers stratified mortality risk by psychiatric disorder type, the most robust associations were for psychotic and mood disorders. Substance use disorders, intellectual disabilities, and developmental disorders were associated with higher mortality only in crude estimates. There was no increased death risk associated with anxiety disorders.
“That there are differences between the various types of disorders was an interesting finding,” said Dr. De Picker, adding that previous research “just lumped together all diagnostic categories.”
Potential mechanisms
The study did not explore why psychiatric illness raise the risk for death in the setting of COVID-19, so potential mechanisms are purely speculative. However, the investigators believe it may reflect biological processes such as immune-inflammatory alterations.
Psychotic disorders and mood disorders in particular, are associated with immune changes, including immunogenetic abnormalities, raised cytokine concentrations, autoantibodies, acute-phase proteins, and aberrant counts of leukocyte cell types, said Dr. De Picker.
She likened this to elderly people being at increased risk following COVID-19 because their immune system is compromised and less able to fight infection.
There are likely other factors at play, said Dr. De Picker. These could include social isolation and lifestyle factors like poor diet, physical inactivity, high alcohol and tobacco use, and sleep disturbances.
In addition, psychiatric patients have a higher prevalence of comorbidities including diabetes, cardiovascular disease, and respiratory disease, which could also play a role.
The increased mortality might also reflect reduced access to care. “Some of these patients may be living in difficult socioeconomic conditions,” said Dr. De Picker.
She noted that, while the in-hospital mortality was not increased, the risk was significantly increased in samples that were outside of the hospital. This reinforces the need for providing close monitoring and early referral to hospital for psychiatric patients with COVID-19.
Mortality varied significantly among countries, with the lowest risk in Europe and the United States. This difference might be attributable to differences in health care systems and access to care, said Dr. De Picker.
Overall, the risk for hospitalization was about double for COVID patients with a mental illness, but when stratified by disorder, there was only a significantly increased risk for substance use and mood disorders. “But mood disorders were not even significant any more after adjusting for age, sex, and comorbid conditions, and we don’t see an increased risk for psychotic disorders whereas they had the highest mortality risks,” said Dr. De Picker.
Psych meds a risk factor?
The studies were primarily based on electronic medical records, so investigators were unable to carry out “a fine grain analysis” into clinical factors affecting outcomes, she noted.
Antipsychotics were consistently associated with an increased risk for mortality (adjusted OR, 2.43; 95% CI, 1.81-3.25), as were anxiolytics (aOR, 1.47; 95% CI, 1.15-1.88).
“There are some theoretical reasons why we believe there could be a risk associated with these drugs,” said Dr. De Picker. For example, antipsychotics can increase the risk for cardiac arrhythmias and thromboembolic events, and cause interactions with drugs used to treat COVID-19.
As for anxiolytics, especially benzodiazepines, these drugs are associated with respiratory risk and with all-cause mortality. “So you could imagine that someone who is infected with a respiratory virus and [is] then using these drugs on top of that would have a worse outcome,” said Dr. De Picker.
In contrast to antipsychotics and anxiolytics, antidepressants did not increase mortality risk.
Dr. De Picker noted a new study by French researchers showing a protective effect of certain serotonergic antidepressants on COVID outcomes, including mortality.
There was no robust evidence of an increased risk for ICU admission for patients with mental disorders. However, the authors noted some studies included small samples of patients with psychiatric disorders, “contributing to a low certainty of evidence for ICU admission.”
Dr. De Picker criticized COVID vaccine policies that don’t prioritize patients with psychiatric disorders. In many countries, groups that were initially green-lighted for the vaccine included health care workers, the elderly, and those with underlying conditions such as diabetes, obesity and even mild hypertension – but not mental illness, which is also an underlying risk.
‘Outstanding’ research
Commenting on the study for this news organization, Jonathan E. Alpert, MD, PhD, department of psychiatry and behavioral sciences, Montefiore Medical Center, New York, and chair of the American Psychiatric Association Council on Research, called it “outstanding” and the largest of its kind.
“There have been a number of studies that have come to similar conclusions, that people with psychiatric illness are at greater risk for poorer outcomes, but because any given study had a relatively limited sample, perhaps from one health system or one country, there were some inconsistencies,” said Dr. Alpert.
“This is the strongest report so far that has made the point that people with psychiatric illness are a vulnerable population for a negative outcome from COVID, including the most worrisome – mortality.”
The study helps drive home a “very important public health lesson” that applies to COVID-19 but goes “beyond,” said Dr. Alpert.
“As a society, we need to keep in mind that people with serious mental disorders are a vulnerable population for poorer outcomes in most general medical conditions,” he stressed, “whether it’s cancer or heart disease or diabetes, and special efforts need to be made to reach out to those populations.”
Dr. Alpert agreed that, at the start of the pandemic, psychiatric patients in the United States were not prioritized for vaccination, and although psychiatric patients may initially have found it difficult to navigate the health care system to learn where and how to get a COVID shot, today that barrier has mostly been removed.
“Our patients are at least as willing as any other subgroup to get the vaccine, and that includes people with psychotic disorders,” he said.
The study was supported by the European College of Neuropsychopharmacology Immuno-NeuroPsychiatry network and Fondazione Centro San Raffaele (Milan). Dr. De Picker reported receiving grants from Boehringer Ingelheim and Janssen outside the submitted work. She is a member of the European College of Neuropsychopharmacology Immuno-NeuroPsychiatry Thematic Working Group.
A version of this article first appeared on Medscape.com.
Necessary or not, COVID booster shots are probably on the horizon
The drug maker Pfizer recently announced that vaccinated people are likely to need a booster shot to be effectively protected against new variants of COVID-19 and that the company would apply for Food and Drug Administration emergency use authorization for the shot. Top government health officials immediately and emphatically announced that the booster isn’t needed right now – and held firm to that position even after Pfizer’s top scientist made his case and shared preliminary data with them on July 12.
This has led to confusion. Is the protection that has allowed them to see loved ones and go out to dinner fading?
Ultimately, the question of whether a booster is needed is unlikely to determine the FDA’s decision. If recent history is predictive, booster shots will be here before long. That’s because of the outdated, 60-year-old basic standard the FDA uses to authorize medicines for sale: Is a new drug “safe and effective?”
The FDA, using that standard, will very likely have to authorize Pfizer’s booster for emergency use, as it did the company’s prior COVID shot. The booster is likely to be safe – hundreds of millions have taken the earlier shots – and Pfizer reported that it dramatically increases a vaccinated person’s antibodies against SARS-CoV-2. From that perspective, it may also be considered very effective.
But does that kind of efficacy matter? Is a higher level of antibodies needed to protect vaccinated Americans? Though antibody levels may wane some over time, the current vaccines deliver perfectly good immunity so far.
What if a booster is safe and effective in one sense but simply not needed – at least for now?
Reliance on the simple “safe and effective” standard – which certainly sounds reasonable – is a relic of a time when there were far fewer and simpler medicines available to treat diseases and before pharmaceutical manufacturing became one of the world’s biggest businesses.
The FDA’s 1938 landmark legislation focused primarily on safety after more than 100 Americans died from a raspberry-flavored liquid form of an early antibiotic because one of its ingredients was used as antifreeze. The 1962 Kefauver-Harris Amendments to the Federal Food, Drug, and Cosmetic Act set out more specific requirements for drug approval: Companies must scientifically prove a drug’s effectiveness through “adequate and well-controlled studies.”
In today’s pharmaceutical universe, a simple “safe and effective” determination is not always an adequate bar, and it can be manipulated to sell drugs of questionable value. There’s also big money involved: Pfizer is already projecting $26 billion in COVID revenue in 2021.
The United States’ continued use of this standard to let drugs into the market has led to the approval of expensive, not necessarily very effective drugs. In 2014, for example, the FDA approved a toenail fungus drug that can cost up to $1,500 a month and that studies showed cured fewer than 10% of patients after a year of treatment. That’s more effective than doing nothing but less effective and more costly than a number of other treatments for this bothersome malady.
It has also led to a plethora of high-priced drugs to treat diseases like cancers, multiple sclerosis and type 2 diabetes that are all more effective than a placebo but have often not been tested very much against one another to determine which are most effective.
In today’s complex world, clarification is needed to determine just what kind of effectiveness the FDA should demand. And should that be the job of the FDA alone?
For example, should drugmakers prove a drug is significantly more effective than products already on the market? Or demonstrate cost-effectiveness – the health value of a product relative to its price – a metric used by Britain’s health system? And in which cases is effectiveness against a surrogate marker – like an antibody level – a good enough stand-in for whether a drug will have a significant impact on a patient’s health?
In most industrialized countries, broad access to the national market is a two-step process, said Aaron Kesselheim, a professor of medicine at Harvard Medical School, Boston, who studies drug development, marketing and law and recently served on an FDA advisory committee. The first part certifies that a drug is sufficiently safe and effective. That is immediately followed by an independent health technology assessment to see where it fits in the treatment armamentarium, including, in some countries, whether it is useful enough to be sold at all at the stated price. But there’s no such automatic process in the United States.
When Pfizer applies for authorization, the FDA may well clear a booster for the U.S. market. The Centers for Disease Control and Prevention, likely with advice from National Institutes of Health experts, will then have to decide whether to recommend it and for whom. This judgment call usually determines whether insurers will cover it. Pfizer is likely to profit handsomely from a government authorization, and the company will gain some revenue even if only the worried well, who can pay out of pocket, decide to get the shot.
To make any recommendation on a booster, government experts say they need more data. They could, for example, as Anthony S. Fauci, MD, has suggested, eventually green-light the additional vaccine shot only for a small group of patients at high risk for a deadly infection, such as the very old or transplant recipients who take immunosuppressant drugs, as some other countries have done.
But until the United States refines the FDA’s “safe and effective” standard or adds a second layer of vetting, when new products hit the market and manufacturers promote them, Americans will be left to decipher whose version of effective and necessary matters to them.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The drug maker Pfizer recently announced that vaccinated people are likely to need a booster shot to be effectively protected against new variants of COVID-19 and that the company would apply for Food and Drug Administration emergency use authorization for the shot. Top government health officials immediately and emphatically announced that the booster isn’t needed right now – and held firm to that position even after Pfizer’s top scientist made his case and shared preliminary data with them on July 12.
This has led to confusion. Is the protection that has allowed them to see loved ones and go out to dinner fading?
Ultimately, the question of whether a booster is needed is unlikely to determine the FDA’s decision. If recent history is predictive, booster shots will be here before long. That’s because of the outdated, 60-year-old basic standard the FDA uses to authorize medicines for sale: Is a new drug “safe and effective?”
The FDA, using that standard, will very likely have to authorize Pfizer’s booster for emergency use, as it did the company’s prior COVID shot. The booster is likely to be safe – hundreds of millions have taken the earlier shots – and Pfizer reported that it dramatically increases a vaccinated person’s antibodies against SARS-CoV-2. From that perspective, it may also be considered very effective.
But does that kind of efficacy matter? Is a higher level of antibodies needed to protect vaccinated Americans? Though antibody levels may wane some over time, the current vaccines deliver perfectly good immunity so far.
What if a booster is safe and effective in one sense but simply not needed – at least for now?
Reliance on the simple “safe and effective” standard – which certainly sounds reasonable – is a relic of a time when there were far fewer and simpler medicines available to treat diseases and before pharmaceutical manufacturing became one of the world’s biggest businesses.
The FDA’s 1938 landmark legislation focused primarily on safety after more than 100 Americans died from a raspberry-flavored liquid form of an early antibiotic because one of its ingredients was used as antifreeze. The 1962 Kefauver-Harris Amendments to the Federal Food, Drug, and Cosmetic Act set out more specific requirements for drug approval: Companies must scientifically prove a drug’s effectiveness through “adequate and well-controlled studies.”
In today’s pharmaceutical universe, a simple “safe and effective” determination is not always an adequate bar, and it can be manipulated to sell drugs of questionable value. There’s also big money involved: Pfizer is already projecting $26 billion in COVID revenue in 2021.
The United States’ continued use of this standard to let drugs into the market has led to the approval of expensive, not necessarily very effective drugs. In 2014, for example, the FDA approved a toenail fungus drug that can cost up to $1,500 a month and that studies showed cured fewer than 10% of patients after a year of treatment. That’s more effective than doing nothing but less effective and more costly than a number of other treatments for this bothersome malady.
It has also led to a plethora of high-priced drugs to treat diseases like cancers, multiple sclerosis and type 2 diabetes that are all more effective than a placebo but have often not been tested very much against one another to determine which are most effective.
In today’s complex world, clarification is needed to determine just what kind of effectiveness the FDA should demand. And should that be the job of the FDA alone?
For example, should drugmakers prove a drug is significantly more effective than products already on the market? Or demonstrate cost-effectiveness – the health value of a product relative to its price – a metric used by Britain’s health system? And in which cases is effectiveness against a surrogate marker – like an antibody level – a good enough stand-in for whether a drug will have a significant impact on a patient’s health?
In most industrialized countries, broad access to the national market is a two-step process, said Aaron Kesselheim, a professor of medicine at Harvard Medical School, Boston, who studies drug development, marketing and law and recently served on an FDA advisory committee. The first part certifies that a drug is sufficiently safe and effective. That is immediately followed by an independent health technology assessment to see where it fits in the treatment armamentarium, including, in some countries, whether it is useful enough to be sold at all at the stated price. But there’s no such automatic process in the United States.
When Pfizer applies for authorization, the FDA may well clear a booster for the U.S. market. The Centers for Disease Control and Prevention, likely with advice from National Institutes of Health experts, will then have to decide whether to recommend it and for whom. This judgment call usually determines whether insurers will cover it. Pfizer is likely to profit handsomely from a government authorization, and the company will gain some revenue even if only the worried well, who can pay out of pocket, decide to get the shot.
To make any recommendation on a booster, government experts say they need more data. They could, for example, as Anthony S. Fauci, MD, has suggested, eventually green-light the additional vaccine shot only for a small group of patients at high risk for a deadly infection, such as the very old or transplant recipients who take immunosuppressant drugs, as some other countries have done.
But until the United States refines the FDA’s “safe and effective” standard or adds a second layer of vetting, when new products hit the market and manufacturers promote them, Americans will be left to decipher whose version of effective and necessary matters to them.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The drug maker Pfizer recently announced that vaccinated people are likely to need a booster shot to be effectively protected against new variants of COVID-19 and that the company would apply for Food and Drug Administration emergency use authorization for the shot. Top government health officials immediately and emphatically announced that the booster isn’t needed right now – and held firm to that position even after Pfizer’s top scientist made his case and shared preliminary data with them on July 12.
This has led to confusion. Is the protection that has allowed them to see loved ones and go out to dinner fading?
Ultimately, the question of whether a booster is needed is unlikely to determine the FDA’s decision. If recent history is predictive, booster shots will be here before long. That’s because of the outdated, 60-year-old basic standard the FDA uses to authorize medicines for sale: Is a new drug “safe and effective?”
The FDA, using that standard, will very likely have to authorize Pfizer’s booster for emergency use, as it did the company’s prior COVID shot. The booster is likely to be safe – hundreds of millions have taken the earlier shots – and Pfizer reported that it dramatically increases a vaccinated person’s antibodies against SARS-CoV-2. From that perspective, it may also be considered very effective.
But does that kind of efficacy matter? Is a higher level of antibodies needed to protect vaccinated Americans? Though antibody levels may wane some over time, the current vaccines deliver perfectly good immunity so far.
What if a booster is safe and effective in one sense but simply not needed – at least for now?
Reliance on the simple “safe and effective” standard – which certainly sounds reasonable – is a relic of a time when there were far fewer and simpler medicines available to treat diseases and before pharmaceutical manufacturing became one of the world’s biggest businesses.
The FDA’s 1938 landmark legislation focused primarily on safety after more than 100 Americans died from a raspberry-flavored liquid form of an early antibiotic because one of its ingredients was used as antifreeze. The 1962 Kefauver-Harris Amendments to the Federal Food, Drug, and Cosmetic Act set out more specific requirements for drug approval: Companies must scientifically prove a drug’s effectiveness through “adequate and well-controlled studies.”
In today’s pharmaceutical universe, a simple “safe and effective” determination is not always an adequate bar, and it can be manipulated to sell drugs of questionable value. There’s also big money involved: Pfizer is already projecting $26 billion in COVID revenue in 2021.
The United States’ continued use of this standard to let drugs into the market has led to the approval of expensive, not necessarily very effective drugs. In 2014, for example, the FDA approved a toenail fungus drug that can cost up to $1,500 a month and that studies showed cured fewer than 10% of patients after a year of treatment. That’s more effective than doing nothing but less effective and more costly than a number of other treatments for this bothersome malady.
It has also led to a plethora of high-priced drugs to treat diseases like cancers, multiple sclerosis and type 2 diabetes that are all more effective than a placebo but have often not been tested very much against one another to determine which are most effective.
In today’s complex world, clarification is needed to determine just what kind of effectiveness the FDA should demand. And should that be the job of the FDA alone?
For example, should drugmakers prove a drug is significantly more effective than products already on the market? Or demonstrate cost-effectiveness – the health value of a product relative to its price – a metric used by Britain’s health system? And in which cases is effectiveness against a surrogate marker – like an antibody level – a good enough stand-in for whether a drug will have a significant impact on a patient’s health?
In most industrialized countries, broad access to the national market is a two-step process, said Aaron Kesselheim, a professor of medicine at Harvard Medical School, Boston, who studies drug development, marketing and law and recently served on an FDA advisory committee. The first part certifies that a drug is sufficiently safe and effective. That is immediately followed by an independent health technology assessment to see where it fits in the treatment armamentarium, including, in some countries, whether it is useful enough to be sold at all at the stated price. But there’s no such automatic process in the United States.
When Pfizer applies for authorization, the FDA may well clear a booster for the U.S. market. The Centers for Disease Control and Prevention, likely with advice from National Institutes of Health experts, will then have to decide whether to recommend it and for whom. This judgment call usually determines whether insurers will cover it. Pfizer is likely to profit handsomely from a government authorization, and the company will gain some revenue even if only the worried well, who can pay out of pocket, decide to get the shot.
To make any recommendation on a booster, government experts say they need more data. They could, for example, as Anthony S. Fauci, MD, has suggested, eventually green-light the additional vaccine shot only for a small group of patients at high risk for a deadly infection, such as the very old or transplant recipients who take immunosuppressant drugs, as some other countries have done.
But until the United States refines the FDA’s “safe and effective” standard or adds a second layer of vetting, when new products hit the market and manufacturers promote them, Americans will be left to decipher whose version of effective and necessary matters to them.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
More post–COVID-19 GI symptoms: Malnutrition, weight loss
After acute SARS-CoV-2 infection, patients report lingering malnutrition, loss of appetite, and failure to regain lost weight long after other gastrointestinal and non-GI symptoms have resolved, according to the results of a new study.
In the large, multicenter retrospective study published online in Clinical Gastroenterology and Hepatology, Anam Rizvi, MD, and colleagues at Long Island Jewish Medical Center, in New Hyde Park (N.Y.), report a high prevalence of GI symptoms among patients with COVID-19.
They followed 17,462 adult patients who were hospitalized for severe COVID-19 between March 2020 and January 2021. Of these, 3,229 (18.5%) also had GI symptoms.
The median age of the patients was 66 years, and 46.9% were women. The diverse population included White (46%), Black (23%), and Hispanic (17%) patients admitted to 12 medical centers of the Northwell Health System in Manhattan, Queens, Long Island, and Staten Island. The researchers followed patients for 3 months (88.7%) and 6 months (56.5%).
The most frequent initial GI symptoms were gastroenteritis (52.5%), malnutrition (23%), GI bleeding (20.4%), and idiopathic pancreatitis (0.5%). Notably, 50.6% of those with GI manifestations reported an inability to regain lost weight at 3 months; 32.4% reported failure to regain lost weight at 6 months.
These percentages rose among patients with malnutrition, as determined by a board-certified in-hospital nutritionist; 56.4% failed to gain weight at 6 months. A median 14.7-lb weight loss persisted at the half-year mark.
In contrast to these lingering symptoms, gastroenteritis, GI bleeding, and pancreatitis all resolved by 3 months post hospitalization.
“We were somewhat shocked that the prevalence of these symptoms was so high, but it’s overall reassuring that most GI symptoms of COVID-19 resolve,” study author Arvind J. Trindade, MD, told this news organization. “In some COVID patients, we’re seeing an inability to gain weight without diarrhea or postinfectious irritable bowel syndrome.
“Patients with an inability to regain weight should consider follow-up with a nutritionist,” continued Dr. Trindade, who is the center’s director of endoscopy and an associate professor at the Feinstein Institutes for Medical Research, in Manhasset (N.Y.). His group also recommends developing malnutrition screening assessments for COVID-19 patients who recover from the acute infection.
The study was prompted by clinical observations during follow-up.
“We saw that a lot of these patients had trouble regaining weight, but we still don’t know why,” Dr. Trindade said. There were no discriminating clinical features apart from malnutrition that indicated an increased risk, and no socioeconomic or demographic characteristics. “We also looked at whether any factors predicted malnutrition, and there weren’t any that would predispose to malnutrition,” he added.
“We’re now reaching out to nonclinical investigators to see if there’s an interest in studying the underlying science behind these symptoms,” Dr. Trindade said.
His group plans to release 12-month follow-up data from the second wave of the pandemic in January 2022.
Initial GI symptoms are thought to be due to the virus’s S1 spike protein’s binding to the angiotensin-converting enzyme 2 receptors, which are abundant in GI epithelial cells. “But why patients have long-term GI sequelae is probably a whole different physiological mechanism,” Dr. Trindade said. “The thought is that there has to be some hormone or pathway that doesn’t allow them to regain weight.”
“The hospital cohort by [Dr. Rizvi] and colleagues is unique and helpful in that patients with GI symptoms are less likely to be hospitalized, and perhaps those patients who are sick enough for admission to the hospital who also have GI symptoms need specific attention paid to their appetite, weight, and nutritional status,” said Jordan M. Shapiro, MD, who commented on the study but was not involved in it.
The constellations of GI symptoms are difficult to distinguish from other postinfectious GI syndromes, such as irritable bowel syndrome and gastroparesis, added Dr. Shapiro, an assistant professor of medicine in gastroenterology and hepatology at Baylor College of Medicine, Houston. “We’re still unpacking what is and is not specific to post–COVID-19 GI symptoms. Prospective studies are necessary to further study this phenomenon.”
Last year, a small Italian study documented significant weight loss and malnutrition in a hospital cohort of 213 discharged COVID-19 patients. In that study, the duration of disease was predictive of weight loss.
The authors note several study limitations, including that the cohort was limited to hospitalized patients from New York and that the 6-month follow-up period was short.
The study received no funding. Dr. Trindade serves as a consultant to Pentax Medical. All other authors and Dr. Shapiro have disclosed no relevant financial relationships.
For the latest clinical guidance, education, research and physician resources about coronavirus, visit the AGA COVID-19 Resource Center at www.gastro.org/COVID.
A version of this article first appeared on Medscape.com.
After acute SARS-CoV-2 infection, patients report lingering malnutrition, loss of appetite, and failure to regain lost weight long after other gastrointestinal and non-GI symptoms have resolved, according to the results of a new study.
In the large, multicenter retrospective study published online in Clinical Gastroenterology and Hepatology, Anam Rizvi, MD, and colleagues at Long Island Jewish Medical Center, in New Hyde Park (N.Y.), report a high prevalence of GI symptoms among patients with COVID-19.
They followed 17,462 adult patients who were hospitalized for severe COVID-19 between March 2020 and January 2021. Of these, 3,229 (18.5%) also had GI symptoms.
The median age of the patients was 66 years, and 46.9% were women. The diverse population included White (46%), Black (23%), and Hispanic (17%) patients admitted to 12 medical centers of the Northwell Health System in Manhattan, Queens, Long Island, and Staten Island. The researchers followed patients for 3 months (88.7%) and 6 months (56.5%).
The most frequent initial GI symptoms were gastroenteritis (52.5%), malnutrition (23%), GI bleeding (20.4%), and idiopathic pancreatitis (0.5%). Notably, 50.6% of those with GI manifestations reported an inability to regain lost weight at 3 months; 32.4% reported failure to regain lost weight at 6 months.
These percentages rose among patients with malnutrition, as determined by a board-certified in-hospital nutritionist; 56.4% failed to gain weight at 6 months. A median 14.7-lb weight loss persisted at the half-year mark.
In contrast to these lingering symptoms, gastroenteritis, GI bleeding, and pancreatitis all resolved by 3 months post hospitalization.
“We were somewhat shocked that the prevalence of these symptoms was so high, but it’s overall reassuring that most GI symptoms of COVID-19 resolve,” study author Arvind J. Trindade, MD, told this news organization. “In some COVID patients, we’re seeing an inability to gain weight without diarrhea or postinfectious irritable bowel syndrome.
“Patients with an inability to regain weight should consider follow-up with a nutritionist,” continued Dr. Trindade, who is the center’s director of endoscopy and an associate professor at the Feinstein Institutes for Medical Research, in Manhasset (N.Y.). His group also recommends developing malnutrition screening assessments for COVID-19 patients who recover from the acute infection.
The study was prompted by clinical observations during follow-up.
“We saw that a lot of these patients had trouble regaining weight, but we still don’t know why,” Dr. Trindade said. There were no discriminating clinical features apart from malnutrition that indicated an increased risk, and no socioeconomic or demographic characteristics. “We also looked at whether any factors predicted malnutrition, and there weren’t any that would predispose to malnutrition,” he added.
“We’re now reaching out to nonclinical investigators to see if there’s an interest in studying the underlying science behind these symptoms,” Dr. Trindade said.
His group plans to release 12-month follow-up data from the second wave of the pandemic in January 2022.
Initial GI symptoms are thought to be due to the virus’s S1 spike protein’s binding to the angiotensin-converting enzyme 2 receptors, which are abundant in GI epithelial cells. “But why patients have long-term GI sequelae is probably a whole different physiological mechanism,” Dr. Trindade said. “The thought is that there has to be some hormone or pathway that doesn’t allow them to regain weight.”
“The hospital cohort by [Dr. Rizvi] and colleagues is unique and helpful in that patients with GI symptoms are less likely to be hospitalized, and perhaps those patients who are sick enough for admission to the hospital who also have GI symptoms need specific attention paid to their appetite, weight, and nutritional status,” said Jordan M. Shapiro, MD, who commented on the study but was not involved in it.
The constellations of GI symptoms are difficult to distinguish from other postinfectious GI syndromes, such as irritable bowel syndrome and gastroparesis, added Dr. Shapiro, an assistant professor of medicine in gastroenterology and hepatology at Baylor College of Medicine, Houston. “We’re still unpacking what is and is not specific to post–COVID-19 GI symptoms. Prospective studies are necessary to further study this phenomenon.”
Last year, a small Italian study documented significant weight loss and malnutrition in a hospital cohort of 213 discharged COVID-19 patients. In that study, the duration of disease was predictive of weight loss.
The authors note several study limitations, including that the cohort was limited to hospitalized patients from New York and that the 6-month follow-up period was short.
The study received no funding. Dr. Trindade serves as a consultant to Pentax Medical. All other authors and Dr. Shapiro have disclosed no relevant financial relationships.
For the latest clinical guidance, education, research and physician resources about coronavirus, visit the AGA COVID-19 Resource Center at www.gastro.org/COVID.
A version of this article first appeared on Medscape.com.
After acute SARS-CoV-2 infection, patients report lingering malnutrition, loss of appetite, and failure to regain lost weight long after other gastrointestinal and non-GI symptoms have resolved, according to the results of a new study.
In the large, multicenter retrospective study published online in Clinical Gastroenterology and Hepatology, Anam Rizvi, MD, and colleagues at Long Island Jewish Medical Center, in New Hyde Park (N.Y.), report a high prevalence of GI symptoms among patients with COVID-19.
They followed 17,462 adult patients who were hospitalized for severe COVID-19 between March 2020 and January 2021. Of these, 3,229 (18.5%) also had GI symptoms.
The median age of the patients was 66 years, and 46.9% were women. The diverse population included White (46%), Black (23%), and Hispanic (17%) patients admitted to 12 medical centers of the Northwell Health System in Manhattan, Queens, Long Island, and Staten Island. The researchers followed patients for 3 months (88.7%) and 6 months (56.5%).
The most frequent initial GI symptoms were gastroenteritis (52.5%), malnutrition (23%), GI bleeding (20.4%), and idiopathic pancreatitis (0.5%). Notably, 50.6% of those with GI manifestations reported an inability to regain lost weight at 3 months; 32.4% reported failure to regain lost weight at 6 months.
These percentages rose among patients with malnutrition, as determined by a board-certified in-hospital nutritionist; 56.4% failed to gain weight at 6 months. A median 14.7-lb weight loss persisted at the half-year mark.
In contrast to these lingering symptoms, gastroenteritis, GI bleeding, and pancreatitis all resolved by 3 months post hospitalization.
“We were somewhat shocked that the prevalence of these symptoms was so high, but it’s overall reassuring that most GI symptoms of COVID-19 resolve,” study author Arvind J. Trindade, MD, told this news organization. “In some COVID patients, we’re seeing an inability to gain weight without diarrhea or postinfectious irritable bowel syndrome.
“Patients with an inability to regain weight should consider follow-up with a nutritionist,” continued Dr. Trindade, who is the center’s director of endoscopy and an associate professor at the Feinstein Institutes for Medical Research, in Manhasset (N.Y.). His group also recommends developing malnutrition screening assessments for COVID-19 patients who recover from the acute infection.
The study was prompted by clinical observations during follow-up.
“We saw that a lot of these patients had trouble regaining weight, but we still don’t know why,” Dr. Trindade said. There were no discriminating clinical features apart from malnutrition that indicated an increased risk, and no socioeconomic or demographic characteristics. “We also looked at whether any factors predicted malnutrition, and there weren’t any that would predispose to malnutrition,” he added.
“We’re now reaching out to nonclinical investigators to see if there’s an interest in studying the underlying science behind these symptoms,” Dr. Trindade said.
His group plans to release 12-month follow-up data from the second wave of the pandemic in January 2022.
Initial GI symptoms are thought to be due to the virus’s S1 spike protein’s binding to the angiotensin-converting enzyme 2 receptors, which are abundant in GI epithelial cells. “But why patients have long-term GI sequelae is probably a whole different physiological mechanism,” Dr. Trindade said. “The thought is that there has to be some hormone or pathway that doesn’t allow them to regain weight.”
“The hospital cohort by [Dr. Rizvi] and colleagues is unique and helpful in that patients with GI symptoms are less likely to be hospitalized, and perhaps those patients who are sick enough for admission to the hospital who also have GI symptoms need specific attention paid to their appetite, weight, and nutritional status,” said Jordan M. Shapiro, MD, who commented on the study but was not involved in it.
The constellations of GI symptoms are difficult to distinguish from other postinfectious GI syndromes, such as irritable bowel syndrome and gastroparesis, added Dr. Shapiro, an assistant professor of medicine in gastroenterology and hepatology at Baylor College of Medicine, Houston. “We’re still unpacking what is and is not specific to post–COVID-19 GI symptoms. Prospective studies are necessary to further study this phenomenon.”
Last year, a small Italian study documented significant weight loss and malnutrition in a hospital cohort of 213 discharged COVID-19 patients. In that study, the duration of disease was predictive of weight loss.
The authors note several study limitations, including that the cohort was limited to hospitalized patients from New York and that the 6-month follow-up period was short.
The study received no funding. Dr. Trindade serves as a consultant to Pentax Medical. All other authors and Dr. Shapiro have disclosed no relevant financial relationships.
For the latest clinical guidance, education, research and physician resources about coronavirus, visit the AGA COVID-19 Resource Center at www.gastro.org/COVID.
A version of this article first appeared on Medscape.com.
Delta variant among the most infectious respiratory viruses, CDC says
.
“Today, I want to speak about our need to come together against a common enemy. SARS-CoV-2 and the Delta variant is spreading with incredible efficiency, and now represents more than 83% of the virus circulating in the U.S.,” Dr. Walensky said at a news briefing July 22. “It is one of the most infectious respiratory viruses we know of and that I have seen in my 20-year career.”
Dr. Walensky said there were 46,318 cases of COVID-19 reported July 21, with a 7-day average of 37,700 cases per day -- up 53% from the previous week. Hospital admissions average about 3,500 per day, an increase of 32%. The 7-day average of deaths is 237 -- a 19% increase from the previous week.
Meanwhile, there are now 162 million Americans who are fully vaccinated against COVID-19.
Areas with low vaccination coverage continue to have the highest case numbers, she reported, with unvaccinated people accounting for 97% of hospitalizations and deaths.
But there may be early signs of progress. The four states with the highest case rates -- Arkansas, Florida, Louisiana, and Nevada -- had a higher rate of new vaccinations, compared with the national average over the past week, White House COVID-19 Response Coordinator Jeff Zients said.
He also announced that the administration will send $100 million to nearly 2,000 rural health clinics to support vaccine education and outreach efforts.
Dr. Walensky said despite the rising numbers, the CDC mask guidance remains the same, but she encouraged vaccinated people to wear masks if they choose.
“Whether you are vaccinated or not, please know we together are not out of the woods yet,” she said. “We are yet at another pivotal moment in this pandemic, with cases rising again and hospitals reaching their capacity in some areas.”
A version of this article first appeared on WebMD.com.
.
“Today, I want to speak about our need to come together against a common enemy. SARS-CoV-2 and the Delta variant is spreading with incredible efficiency, and now represents more than 83% of the virus circulating in the U.S.,” Dr. Walensky said at a news briefing July 22. “It is one of the most infectious respiratory viruses we know of and that I have seen in my 20-year career.”
Dr. Walensky said there were 46,318 cases of COVID-19 reported July 21, with a 7-day average of 37,700 cases per day -- up 53% from the previous week. Hospital admissions average about 3,500 per day, an increase of 32%. The 7-day average of deaths is 237 -- a 19% increase from the previous week.
Meanwhile, there are now 162 million Americans who are fully vaccinated against COVID-19.
Areas with low vaccination coverage continue to have the highest case numbers, she reported, with unvaccinated people accounting for 97% of hospitalizations and deaths.
But there may be early signs of progress. The four states with the highest case rates -- Arkansas, Florida, Louisiana, and Nevada -- had a higher rate of new vaccinations, compared with the national average over the past week, White House COVID-19 Response Coordinator Jeff Zients said.
He also announced that the administration will send $100 million to nearly 2,000 rural health clinics to support vaccine education and outreach efforts.
Dr. Walensky said despite the rising numbers, the CDC mask guidance remains the same, but she encouraged vaccinated people to wear masks if they choose.
“Whether you are vaccinated or not, please know we together are not out of the woods yet,” she said. “We are yet at another pivotal moment in this pandemic, with cases rising again and hospitals reaching their capacity in some areas.”
A version of this article first appeared on WebMD.com.
.
“Today, I want to speak about our need to come together against a common enemy. SARS-CoV-2 and the Delta variant is spreading with incredible efficiency, and now represents more than 83% of the virus circulating in the U.S.,” Dr. Walensky said at a news briefing July 22. “It is one of the most infectious respiratory viruses we know of and that I have seen in my 20-year career.”
Dr. Walensky said there were 46,318 cases of COVID-19 reported July 21, with a 7-day average of 37,700 cases per day -- up 53% from the previous week. Hospital admissions average about 3,500 per day, an increase of 32%. The 7-day average of deaths is 237 -- a 19% increase from the previous week.
Meanwhile, there are now 162 million Americans who are fully vaccinated against COVID-19.
Areas with low vaccination coverage continue to have the highest case numbers, she reported, with unvaccinated people accounting for 97% of hospitalizations and deaths.
But there may be early signs of progress. The four states with the highest case rates -- Arkansas, Florida, Louisiana, and Nevada -- had a higher rate of new vaccinations, compared with the national average over the past week, White House COVID-19 Response Coordinator Jeff Zients said.
He also announced that the administration will send $100 million to nearly 2,000 rural health clinics to support vaccine education and outreach efforts.
Dr. Walensky said despite the rising numbers, the CDC mask guidance remains the same, but she encouraged vaccinated people to wear masks if they choose.
“Whether you are vaccinated or not, please know we together are not out of the woods yet,” she said. “We are yet at another pivotal moment in this pandemic, with cases rising again and hospitals reaching their capacity in some areas.”
A version of this article first appeared on WebMD.com.
Sickle cell disease, trait may up risk for poor COVID outcomes
Sickle cell disease (SCD) was associated with a greater than fourfold excess risk for COVID-19–related hospitalization and a greater than twofold risk for COVID-19–related death, according to a big-data analysis from the United Kingdom.
SCD was associated with an adjusted hazard ratio (HR) of 4.11 (95% confidence interval, 2.98-5.66) for admission to hospital and an HR of 2.55 (95% CI, 1.36-4.75) for death, report Ashley K. Clift, MBBS, a clinical research fellow at the University of Oxford, and colleagues. The results were published online July 20 in Annals of Internal Medicine.
Even those who carry just one copy of the sickle cell gene – the carrier status for sickle cell disease – appeared to be at heightened risk for these outcomes (HR for hospitalization, 1.38; 95% CI, 1.12-1.70; HR for death, 1.51; 95% CI, 1.13-2.00).
“Given the well-known ethnic patterning of sickle cell disorders, the predisposition they pose to other infections, and early evidence from smaller registries, we thought this would be an important analysis to run at the population level,” Dr. Clift said in an interview.
in terms of vaccination strategies and advice on nonpharmacological interventions,” he said.
“The best course of action for managing risk in this group is vaccination,” said Enrico M. Novelli, MD, director of the adult sickle cell program at the University of Pittsburgh Medical Center. Dr. Novelli, who is also section chief of benign hematology in the university’s School of Medicine, was not involved in the study. “To date, there are no specific studies of the effect of COVID-19 vaccination in patients with SCD, but there is no reason to believe it would be less effective or more risky in this patient population,” he said.
In addition, common-sense measures, such as masking and physical distancing, particularly at large, indoor gatherings, should be encouraged, Dr. Novelli added. Keeping SCD under good control with available treatments is also important. “Any patient with SCD who contracts COVID-19 should undergo close, outpatient monitoring with pulse oxygen measurements. If sick, they should be hospitalized in a center familiar with the care of SCD patients.”
The U.K. results are in line with and expand on earlier evidence from specialist centers and registries, but the association with sickle cell trait has been unclear and is notable in these findings, Dr. Clift said.
“The finding of the association with sickle cell trait is somewhat unexpected,” pediatric hematologist/oncologist Rabi Hanna, MD, director of pediatric bone marrow transplantation at Cleveland Clinic Children’s, told this news organization. “But I would question the accuracy of the numbers, since not all people with the trait realize they have it. In other respects, the study confirms earlier hypotheses and data from single-center studies.” Dr. Hanna did not participate in the U.K. study.
Study details
The SCD cohort consisted of 5,059 persons with SCD and 25,682 carriers, those with just one copy of the trait. Data were drawn from the United Kingdom’s large primary-care QResearch database. Follow-up for hospitalizations was conducted from Jan. 24, 2020 to Sept. 30, 2020; follow-up for deaths was conducted from Jan. 24, 2020 to Jan. 18, 2021. Among adults with SCD, there were 40 hospitalizations and 10 deaths. Among those with sickle cell trait, there were 98 hospitalizations and 50 deaths. No children died, and only a few (<5) required hospitalization.
Previous registry research showed similarly elevated risks for severe disease and fatality among patients with SCD who were infected with SARS-CoV-2.
Because SCD affects 8 to 12 million people globally – 100,000 in the United States – the authors say their results are important for policymakers and for prioritizing vaccination. They also note that trait carriers may be underdiagnosed.
“While SCD is part of newborn screening, there may be undiagnosed older people with the trait in the general population, but it’s difficult to quantify how much this is undiagnosed,” Dr. Clift said. “But now we have these results, it’s not that surprising that sickle cell trait is also associated with increased risk, albeit to a lower extent. This could suggest an almost dose-like effect of the sickle mutations on COVID hospitalization risk.”
Neonatal screening for the most common form of SCD is currently mandatory in the United States, but the Centers for Disease Control and Prevention has no clear data on how many people are aware they are carriers, Dr. Hanna said. “The states didn’t all begin screening at the same time – some started in the 1990s, others started in the 2000s – so many young adults may be unaware they have the trait,” he said.
Dr. Clift said the multiorgan complications of SCD, such as cardiac and immune problems, may be contributing to the heightened risk in individuals infected with SARS-CoV-2. “For example, we know that people with sickle cell disease are more susceptible to other viral infections. There is also some pathophysiological overlap between SCD disease and severe COVID, such as clotting dysfunction, so that may be worth further exploration,” he said.
The overlapping clotting problems associated with both COVID-19 and SCD could increase the risk for severe venous thromboembolism. In addition, experts noted that patients with SCD often have pre-COVID endothelial damage and baseline inflammation and are very sensitive to hypoxia; as well, a sizable proportion have lung disease.
The message to patients and physicians counseling patients is twofold, said Dr. Hanna: “SCD patients are at higher risk of COVID complications, and these are preventable with vaccination.”
The study was supported by the UK Medical Research Council. Dr. Clift is supported by Cancer Research UK. Coauthor Dr. Hippisley-Cox has received fees from ClinRisk and nonfinancial support from QResearch outside of the submitted work. Dr. Hanna has disclosed no relevant financial relationships. Dr. Novelli is a consultant for Novartis.
A version of this article first appeared on Medscape.com.
Sickle cell disease (SCD) was associated with a greater than fourfold excess risk for COVID-19–related hospitalization and a greater than twofold risk for COVID-19–related death, according to a big-data analysis from the United Kingdom.
SCD was associated with an adjusted hazard ratio (HR) of 4.11 (95% confidence interval, 2.98-5.66) for admission to hospital and an HR of 2.55 (95% CI, 1.36-4.75) for death, report Ashley K. Clift, MBBS, a clinical research fellow at the University of Oxford, and colleagues. The results were published online July 20 in Annals of Internal Medicine.
Even those who carry just one copy of the sickle cell gene – the carrier status for sickle cell disease – appeared to be at heightened risk for these outcomes (HR for hospitalization, 1.38; 95% CI, 1.12-1.70; HR for death, 1.51; 95% CI, 1.13-2.00).
“Given the well-known ethnic patterning of sickle cell disorders, the predisposition they pose to other infections, and early evidence from smaller registries, we thought this would be an important analysis to run at the population level,” Dr. Clift said in an interview.
in terms of vaccination strategies and advice on nonpharmacological interventions,” he said.
“The best course of action for managing risk in this group is vaccination,” said Enrico M. Novelli, MD, director of the adult sickle cell program at the University of Pittsburgh Medical Center. Dr. Novelli, who is also section chief of benign hematology in the university’s School of Medicine, was not involved in the study. “To date, there are no specific studies of the effect of COVID-19 vaccination in patients with SCD, but there is no reason to believe it would be less effective or more risky in this patient population,” he said.
In addition, common-sense measures, such as masking and physical distancing, particularly at large, indoor gatherings, should be encouraged, Dr. Novelli added. Keeping SCD under good control with available treatments is also important. “Any patient with SCD who contracts COVID-19 should undergo close, outpatient monitoring with pulse oxygen measurements. If sick, they should be hospitalized in a center familiar with the care of SCD patients.”
The U.K. results are in line with and expand on earlier evidence from specialist centers and registries, but the association with sickle cell trait has been unclear and is notable in these findings, Dr. Clift said.
“The finding of the association with sickle cell trait is somewhat unexpected,” pediatric hematologist/oncologist Rabi Hanna, MD, director of pediatric bone marrow transplantation at Cleveland Clinic Children’s, told this news organization. “But I would question the accuracy of the numbers, since not all people with the trait realize they have it. In other respects, the study confirms earlier hypotheses and data from single-center studies.” Dr. Hanna did not participate in the U.K. study.
Study details
The SCD cohort consisted of 5,059 persons with SCD and 25,682 carriers, those with just one copy of the trait. Data were drawn from the United Kingdom’s large primary-care QResearch database. Follow-up for hospitalizations was conducted from Jan. 24, 2020 to Sept. 30, 2020; follow-up for deaths was conducted from Jan. 24, 2020 to Jan. 18, 2021. Among adults with SCD, there were 40 hospitalizations and 10 deaths. Among those with sickle cell trait, there were 98 hospitalizations and 50 deaths. No children died, and only a few (<5) required hospitalization.
Previous registry research showed similarly elevated risks for severe disease and fatality among patients with SCD who were infected with SARS-CoV-2.
Because SCD affects 8 to 12 million people globally – 100,000 in the United States – the authors say their results are important for policymakers and for prioritizing vaccination. They also note that trait carriers may be underdiagnosed.
“While SCD is part of newborn screening, there may be undiagnosed older people with the trait in the general population, but it’s difficult to quantify how much this is undiagnosed,” Dr. Clift said. “But now we have these results, it’s not that surprising that sickle cell trait is also associated with increased risk, albeit to a lower extent. This could suggest an almost dose-like effect of the sickle mutations on COVID hospitalization risk.”
Neonatal screening for the most common form of SCD is currently mandatory in the United States, but the Centers for Disease Control and Prevention has no clear data on how many people are aware they are carriers, Dr. Hanna said. “The states didn’t all begin screening at the same time – some started in the 1990s, others started in the 2000s – so many young adults may be unaware they have the trait,” he said.
Dr. Clift said the multiorgan complications of SCD, such as cardiac and immune problems, may be contributing to the heightened risk in individuals infected with SARS-CoV-2. “For example, we know that people with sickle cell disease are more susceptible to other viral infections. There is also some pathophysiological overlap between SCD disease and severe COVID, such as clotting dysfunction, so that may be worth further exploration,” he said.
The overlapping clotting problems associated with both COVID-19 and SCD could increase the risk for severe venous thromboembolism. In addition, experts noted that patients with SCD often have pre-COVID endothelial damage and baseline inflammation and are very sensitive to hypoxia; as well, a sizable proportion have lung disease.
The message to patients and physicians counseling patients is twofold, said Dr. Hanna: “SCD patients are at higher risk of COVID complications, and these are preventable with vaccination.”
The study was supported by the UK Medical Research Council. Dr. Clift is supported by Cancer Research UK. Coauthor Dr. Hippisley-Cox has received fees from ClinRisk and nonfinancial support from QResearch outside of the submitted work. Dr. Hanna has disclosed no relevant financial relationships. Dr. Novelli is a consultant for Novartis.
A version of this article first appeared on Medscape.com.
Sickle cell disease (SCD) was associated with a greater than fourfold excess risk for COVID-19–related hospitalization and a greater than twofold risk for COVID-19–related death, according to a big-data analysis from the United Kingdom.
SCD was associated with an adjusted hazard ratio (HR) of 4.11 (95% confidence interval, 2.98-5.66) for admission to hospital and an HR of 2.55 (95% CI, 1.36-4.75) for death, report Ashley K. Clift, MBBS, a clinical research fellow at the University of Oxford, and colleagues. The results were published online July 20 in Annals of Internal Medicine.
Even those who carry just one copy of the sickle cell gene – the carrier status for sickle cell disease – appeared to be at heightened risk for these outcomes (HR for hospitalization, 1.38; 95% CI, 1.12-1.70; HR for death, 1.51; 95% CI, 1.13-2.00).
“Given the well-known ethnic patterning of sickle cell disorders, the predisposition they pose to other infections, and early evidence from smaller registries, we thought this would be an important analysis to run at the population level,” Dr. Clift said in an interview.
in terms of vaccination strategies and advice on nonpharmacological interventions,” he said.
“The best course of action for managing risk in this group is vaccination,” said Enrico M. Novelli, MD, director of the adult sickle cell program at the University of Pittsburgh Medical Center. Dr. Novelli, who is also section chief of benign hematology in the university’s School of Medicine, was not involved in the study. “To date, there are no specific studies of the effect of COVID-19 vaccination in patients with SCD, but there is no reason to believe it would be less effective or more risky in this patient population,” he said.
In addition, common-sense measures, such as masking and physical distancing, particularly at large, indoor gatherings, should be encouraged, Dr. Novelli added. Keeping SCD under good control with available treatments is also important. “Any patient with SCD who contracts COVID-19 should undergo close, outpatient monitoring with pulse oxygen measurements. If sick, they should be hospitalized in a center familiar with the care of SCD patients.”
The U.K. results are in line with and expand on earlier evidence from specialist centers and registries, but the association with sickle cell trait has been unclear and is notable in these findings, Dr. Clift said.
“The finding of the association with sickle cell trait is somewhat unexpected,” pediatric hematologist/oncologist Rabi Hanna, MD, director of pediatric bone marrow transplantation at Cleveland Clinic Children’s, told this news organization. “But I would question the accuracy of the numbers, since not all people with the trait realize they have it. In other respects, the study confirms earlier hypotheses and data from single-center studies.” Dr. Hanna did not participate in the U.K. study.
Study details
The SCD cohort consisted of 5,059 persons with SCD and 25,682 carriers, those with just one copy of the trait. Data were drawn from the United Kingdom’s large primary-care QResearch database. Follow-up for hospitalizations was conducted from Jan. 24, 2020 to Sept. 30, 2020; follow-up for deaths was conducted from Jan. 24, 2020 to Jan. 18, 2021. Among adults with SCD, there were 40 hospitalizations and 10 deaths. Among those with sickle cell trait, there were 98 hospitalizations and 50 deaths. No children died, and only a few (<5) required hospitalization.
Previous registry research showed similarly elevated risks for severe disease and fatality among patients with SCD who were infected with SARS-CoV-2.
Because SCD affects 8 to 12 million people globally – 100,000 in the United States – the authors say their results are important for policymakers and for prioritizing vaccination. They also note that trait carriers may be underdiagnosed.
“While SCD is part of newborn screening, there may be undiagnosed older people with the trait in the general population, but it’s difficult to quantify how much this is undiagnosed,” Dr. Clift said. “But now we have these results, it’s not that surprising that sickle cell trait is also associated with increased risk, albeit to a lower extent. This could suggest an almost dose-like effect of the sickle mutations on COVID hospitalization risk.”
Neonatal screening for the most common form of SCD is currently mandatory in the United States, but the Centers for Disease Control and Prevention has no clear data on how many people are aware they are carriers, Dr. Hanna said. “The states didn’t all begin screening at the same time – some started in the 1990s, others started in the 2000s – so many young adults may be unaware they have the trait,” he said.
Dr. Clift said the multiorgan complications of SCD, such as cardiac and immune problems, may be contributing to the heightened risk in individuals infected with SARS-CoV-2. “For example, we know that people with sickle cell disease are more susceptible to other viral infections. There is also some pathophysiological overlap between SCD disease and severe COVID, such as clotting dysfunction, so that may be worth further exploration,” he said.
The overlapping clotting problems associated with both COVID-19 and SCD could increase the risk for severe venous thromboembolism. In addition, experts noted that patients with SCD often have pre-COVID endothelial damage and baseline inflammation and are very sensitive to hypoxia; as well, a sizable proportion have lung disease.
The message to patients and physicians counseling patients is twofold, said Dr. Hanna: “SCD patients are at higher risk of COVID complications, and these are preventable with vaccination.”
The study was supported by the UK Medical Research Council. Dr. Clift is supported by Cancer Research UK. Coauthor Dr. Hippisley-Cox has received fees from ClinRisk and nonfinancial support from QResearch outside of the submitted work. Dr. Hanna has disclosed no relevant financial relationships. Dr. Novelli is a consultant for Novartis.
A version of this article first appeared on Medscape.com.
Statins again linked to lower COVID-19 mortality
Among patients hospitalized for COVID-19, those who had been taking statins had a substantially lower risk of death in a new large observational study.
Results showed that use of statins prior to admission was linked to a greater than 40% reduction in mortality and a greater than 25% reduction in risk of developing a severe outcome.
The findings come an analysis of data from the American Heart Association’s COVID-19 Cardiovascular Disease Registry on more than 10,000 patients hospitalized with COVID-19 at 104 hospitals across the United States published in PLoS One.
While several other studies have suggested benefits of statins in COVID-19, this is by far the largest study so far on this topic.
“I would say this is the most reliable study on statins in COVID-19 to date, with the results adjusted for many confounders, including socioeconomic factors and insurance type,” lead author Lori B. Daniels, MD, told this news organization. “However, it still an observational study and therefore falls short of a randomized study. But I would think a randomized study of statins in COVID-19 is probably not feasible, so this study provides excellent data at an observational level.”
After propensity matching for cardiovascular disease, results showed that most of the benefit of statins occurred in patients with known cardiovascular disease.
“While most patients taking statins will have cardiovascular disease, there are also many patients who take these drugs who don’t have heart disease but do have cardiovascular risk factors, such as those with raised cholesterol, or a family history of cardiovascular disease. For [such patients], the effect of statins was also in the same direction but it was not significant. This doesn’t exclude an effect,” noted Dr. Daniels, who is professor of medicine and director of cardiovascular intensive care at the University of California, San Diego.
“We are not saying that everyone should rush out and take a statin if they do not have risk factors for cardiovascular in order to lower their risk of dying from COVID. But if individuals do have an indication for a statin and are not taking one of these dugs this is another good reason to start taking them now,” she added.
The investigators embarked on the study because, although previous observational studies have found that statins may reduce the severity of COVID-19 infection, these studies have been limited in size with mostly single-center or regional studies, and some results have been conflicting. They therefore conducted the current, much larger analysis, in the AHA COVID-19 CVD Registry which systematically collected hospitalized patient–level data in a broad and diverse hospital and patient population across the United States.
For the analysis, the researchers analyzed data from 10,541 patients hospitalized with COVID-19 through September 2020 at 104 U.S. hospitals enrolled in the AHA registry to evaluate the associations between statin use and outcomes.
Most patients (71%) had either cardiovascular disease, hypertension, or both. Prior to admission, 42% of subjects used statins, with 7% being on statins alone and 35% on statins plus antihypertensives. Death (or discharge to hospice) occurred in 2,212 subjects (21%).
Results showed that outpatient use of statins, either alone or with antihypertensives, was associated with a 41% reduced risk of death (odds ratio, 0.59; 95% confidence interval, 0.50-0.69), after adjusting for demographic characteristics, underlying conditions, insurance status, hospital site, and concurrent medications. Statin use was also associated with a roughly 25% lower adjusted odds of developing severe disease.
Noting that patients on statins are also likely to be on antihypertensive medication, the researchers found that the statin benefit on mortality was seen in both patients taking a statin alone (OR, 0.54) and in those taking statins with an antihypertensive medication (OR, 0.60).
Use of antihypertensive drugs was associated with a smaller, albeit still substantial, 27% lower odds of death (OR, 0.73; 95% CI, 0.62-0.87).
In propensity-matched analyses, use of statins and/or antihypertensives was tied to a 32% reduced risk of death among those with a history of CVD and/or hypertension (OR, 0.68; 95% CI, 0.58-0.81). An observed 16% reduction in odds of death with statins and/or antihypertensive drugs among those without cardiovascular disease and/or hypertension was not statistically significant (OR, 0.84; 95% CI, 0.58-1.22).
Stabilizing the underlying disease
The researchers pointed out that the results of the propensity matching analysis are consistent with the hypothesis that the major benefit of these medications accrues from treating and/or stabilizing underlying disease.
“Although it is well known that statins improve long-term outcomes among patients with or at elevated risk for cardiovascular disease, the association with a large short-term benefit which accrues in the setting of hospitalization for COVID-19 is a new and intriguing finding,” they said.
They cited several “plausible mechanisms whereby statins could directly mitigate outcomes in COVID-19 beyond treating underlying disease conditions,” including anti-inflammatory effects and a direct inhibitory effect on the SARS-CoV-2 virus.
Dr. Daniels elaborated more on the potential mechanism at play in an interview: “I think what is happening is that the statin is stabilizing the coronary disease so patients are less likely to die from MI or stroke, and this gives them more time and strength to recover from COVID-19.”
She added: “Statins may also have some direct anti-COVID effects such as an anti-inflammatory actions, but I would guess that this is probably not the primary effect behind what we’re seeing here.”
‘Important clinical implications’
The authors say their findings have “important clinical implications.”
They noted that early in the pandemic there was speculation that certain medications, including statins, and the ACE inhibitor/angiotensin receptor blocker (ARB) classes of antihypertensives may confer an increased susceptibility to COVID-19 positivity and/or severity.
“Our study reinforces the AHA and others’ recommendations that not only is it safe to remain on these medications, but they may substantially reduce risk of severe COVID-19 and especially death from COVID-19, particularly statins, and particularly among those with associated underlying conditions,” the authors stressed.
Dr. Daniels added that, although statins are very safe drugs, there are always some patients who prefer not to take medication even if indicated, and others who may have borderline indications and decide not to take a statin at present.
“This study may persuade these patients that taking a statin is the right thing to do. It may give those patients on the cusp of thinking about taking one of these drugs a reason to go ahead,” she said.
‘Provocative but not definitive’
Commenting on the study, Robert Harrington, MD, professor of medicine and chair of the department of medicine at Stanford (Calif.) University, said: “These are interesting observational data but as such have all the limitations of nonrandomized comparisons despite the best attempts to adjust for a variety of potential confounders. For example, is this an effect of statins (perhaps through some anti-inflammatory mechanism) or is it more an effect that can be attributed to the patients who are prescribed and taking a statin, compared with those who are not?”
He added: “The primary clinical benefit of statins, based on many large randomized clinical trials, seems to be derived from their LDL lowering effect. Observational studies have suggested potential benefits from anti-inflammatory effects of statins, but the randomized trials have not confirmed these observations. So, the current data are interesting, even provocative, but ultimately hypothesis generating rather than definitive.”
Also commenting on the study, Steven Nissen, MD, professor of medicine at the Cleveland Clinic, said: “While statins have many established benefits, their role in preventing COVID-19 complications is very speculative. Like all observational studies, the current study must be viewed as hypothesis generating, not definitive evidence of benefit. There are many potential confounders. I’m skeptical.”
The authors of this study received no specific funding for this work and report no competing interests. Dr. Harrington was AHA president when the COVID registry was created and he is still a member of the AHA board, which has oversight over the project.
Among patients hospitalized for COVID-19, those who had been taking statins had a substantially lower risk of death in a new large observational study.
Results showed that use of statins prior to admission was linked to a greater than 40% reduction in mortality and a greater than 25% reduction in risk of developing a severe outcome.
The findings come an analysis of data from the American Heart Association’s COVID-19 Cardiovascular Disease Registry on more than 10,000 patients hospitalized with COVID-19 at 104 hospitals across the United States published in PLoS One.
While several other studies have suggested benefits of statins in COVID-19, this is by far the largest study so far on this topic.
“I would say this is the most reliable study on statins in COVID-19 to date, with the results adjusted for many confounders, including socioeconomic factors and insurance type,” lead author Lori B. Daniels, MD, told this news organization. “However, it still an observational study and therefore falls short of a randomized study. But I would think a randomized study of statins in COVID-19 is probably not feasible, so this study provides excellent data at an observational level.”
After propensity matching for cardiovascular disease, results showed that most of the benefit of statins occurred in patients with known cardiovascular disease.
“While most patients taking statins will have cardiovascular disease, there are also many patients who take these drugs who don’t have heart disease but do have cardiovascular risk factors, such as those with raised cholesterol, or a family history of cardiovascular disease. For [such patients], the effect of statins was also in the same direction but it was not significant. This doesn’t exclude an effect,” noted Dr. Daniels, who is professor of medicine and director of cardiovascular intensive care at the University of California, San Diego.
“We are not saying that everyone should rush out and take a statin if they do not have risk factors for cardiovascular in order to lower their risk of dying from COVID. But if individuals do have an indication for a statin and are not taking one of these dugs this is another good reason to start taking them now,” she added.
The investigators embarked on the study because, although previous observational studies have found that statins may reduce the severity of COVID-19 infection, these studies have been limited in size with mostly single-center or regional studies, and some results have been conflicting. They therefore conducted the current, much larger analysis, in the AHA COVID-19 CVD Registry which systematically collected hospitalized patient–level data in a broad and diverse hospital and patient population across the United States.
For the analysis, the researchers analyzed data from 10,541 patients hospitalized with COVID-19 through September 2020 at 104 U.S. hospitals enrolled in the AHA registry to evaluate the associations between statin use and outcomes.
Most patients (71%) had either cardiovascular disease, hypertension, or both. Prior to admission, 42% of subjects used statins, with 7% being on statins alone and 35% on statins plus antihypertensives. Death (or discharge to hospice) occurred in 2,212 subjects (21%).
Results showed that outpatient use of statins, either alone or with antihypertensives, was associated with a 41% reduced risk of death (odds ratio, 0.59; 95% confidence interval, 0.50-0.69), after adjusting for demographic characteristics, underlying conditions, insurance status, hospital site, and concurrent medications. Statin use was also associated with a roughly 25% lower adjusted odds of developing severe disease.
Noting that patients on statins are also likely to be on antihypertensive medication, the researchers found that the statin benefit on mortality was seen in both patients taking a statin alone (OR, 0.54) and in those taking statins with an antihypertensive medication (OR, 0.60).
Use of antihypertensive drugs was associated with a smaller, albeit still substantial, 27% lower odds of death (OR, 0.73; 95% CI, 0.62-0.87).
In propensity-matched analyses, use of statins and/or antihypertensives was tied to a 32% reduced risk of death among those with a history of CVD and/or hypertension (OR, 0.68; 95% CI, 0.58-0.81). An observed 16% reduction in odds of death with statins and/or antihypertensive drugs among those without cardiovascular disease and/or hypertension was not statistically significant (OR, 0.84; 95% CI, 0.58-1.22).
Stabilizing the underlying disease
The researchers pointed out that the results of the propensity matching analysis are consistent with the hypothesis that the major benefit of these medications accrues from treating and/or stabilizing underlying disease.
“Although it is well known that statins improve long-term outcomes among patients with or at elevated risk for cardiovascular disease, the association with a large short-term benefit which accrues in the setting of hospitalization for COVID-19 is a new and intriguing finding,” they said.
They cited several “plausible mechanisms whereby statins could directly mitigate outcomes in COVID-19 beyond treating underlying disease conditions,” including anti-inflammatory effects and a direct inhibitory effect on the SARS-CoV-2 virus.
Dr. Daniels elaborated more on the potential mechanism at play in an interview: “I think what is happening is that the statin is stabilizing the coronary disease so patients are less likely to die from MI or stroke, and this gives them more time and strength to recover from COVID-19.”
She added: “Statins may also have some direct anti-COVID effects such as an anti-inflammatory actions, but I would guess that this is probably not the primary effect behind what we’re seeing here.”
‘Important clinical implications’
The authors say their findings have “important clinical implications.”
They noted that early in the pandemic there was speculation that certain medications, including statins, and the ACE inhibitor/angiotensin receptor blocker (ARB) classes of antihypertensives may confer an increased susceptibility to COVID-19 positivity and/or severity.
“Our study reinforces the AHA and others’ recommendations that not only is it safe to remain on these medications, but they may substantially reduce risk of severe COVID-19 and especially death from COVID-19, particularly statins, and particularly among those with associated underlying conditions,” the authors stressed.
Dr. Daniels added that, although statins are very safe drugs, there are always some patients who prefer not to take medication even if indicated, and others who may have borderline indications and decide not to take a statin at present.
“This study may persuade these patients that taking a statin is the right thing to do. It may give those patients on the cusp of thinking about taking one of these drugs a reason to go ahead,” she said.
‘Provocative but not definitive’
Commenting on the study, Robert Harrington, MD, professor of medicine and chair of the department of medicine at Stanford (Calif.) University, said: “These are interesting observational data but as such have all the limitations of nonrandomized comparisons despite the best attempts to adjust for a variety of potential confounders. For example, is this an effect of statins (perhaps through some anti-inflammatory mechanism) or is it more an effect that can be attributed to the patients who are prescribed and taking a statin, compared with those who are not?”
He added: “The primary clinical benefit of statins, based on many large randomized clinical trials, seems to be derived from their LDL lowering effect. Observational studies have suggested potential benefits from anti-inflammatory effects of statins, but the randomized trials have not confirmed these observations. So, the current data are interesting, even provocative, but ultimately hypothesis generating rather than definitive.”
Also commenting on the study, Steven Nissen, MD, professor of medicine at the Cleveland Clinic, said: “While statins have many established benefits, their role in preventing COVID-19 complications is very speculative. Like all observational studies, the current study must be viewed as hypothesis generating, not definitive evidence of benefit. There are many potential confounders. I’m skeptical.”
The authors of this study received no specific funding for this work and report no competing interests. Dr. Harrington was AHA president when the COVID registry was created and he is still a member of the AHA board, which has oversight over the project.
Among patients hospitalized for COVID-19, those who had been taking statins had a substantially lower risk of death in a new large observational study.
Results showed that use of statins prior to admission was linked to a greater than 40% reduction in mortality and a greater than 25% reduction in risk of developing a severe outcome.
The findings come an analysis of data from the American Heart Association’s COVID-19 Cardiovascular Disease Registry on more than 10,000 patients hospitalized with COVID-19 at 104 hospitals across the United States published in PLoS One.
While several other studies have suggested benefits of statins in COVID-19, this is by far the largest study so far on this topic.
“I would say this is the most reliable study on statins in COVID-19 to date, with the results adjusted for many confounders, including socioeconomic factors and insurance type,” lead author Lori B. Daniels, MD, told this news organization. “However, it still an observational study and therefore falls short of a randomized study. But I would think a randomized study of statins in COVID-19 is probably not feasible, so this study provides excellent data at an observational level.”
After propensity matching for cardiovascular disease, results showed that most of the benefit of statins occurred in patients with known cardiovascular disease.
“While most patients taking statins will have cardiovascular disease, there are also many patients who take these drugs who don’t have heart disease but do have cardiovascular risk factors, such as those with raised cholesterol, or a family history of cardiovascular disease. For [such patients], the effect of statins was also in the same direction but it was not significant. This doesn’t exclude an effect,” noted Dr. Daniels, who is professor of medicine and director of cardiovascular intensive care at the University of California, San Diego.
“We are not saying that everyone should rush out and take a statin if they do not have risk factors for cardiovascular in order to lower their risk of dying from COVID. But if individuals do have an indication for a statin and are not taking one of these dugs this is another good reason to start taking them now,” she added.
The investigators embarked on the study because, although previous observational studies have found that statins may reduce the severity of COVID-19 infection, these studies have been limited in size with mostly single-center or regional studies, and some results have been conflicting. They therefore conducted the current, much larger analysis, in the AHA COVID-19 CVD Registry which systematically collected hospitalized patient–level data in a broad and diverse hospital and patient population across the United States.
For the analysis, the researchers analyzed data from 10,541 patients hospitalized with COVID-19 through September 2020 at 104 U.S. hospitals enrolled in the AHA registry to evaluate the associations between statin use and outcomes.
Most patients (71%) had either cardiovascular disease, hypertension, or both. Prior to admission, 42% of subjects used statins, with 7% being on statins alone and 35% on statins plus antihypertensives. Death (or discharge to hospice) occurred in 2,212 subjects (21%).
Results showed that outpatient use of statins, either alone or with antihypertensives, was associated with a 41% reduced risk of death (odds ratio, 0.59; 95% confidence interval, 0.50-0.69), after adjusting for demographic characteristics, underlying conditions, insurance status, hospital site, and concurrent medications. Statin use was also associated with a roughly 25% lower adjusted odds of developing severe disease.
Noting that patients on statins are also likely to be on antihypertensive medication, the researchers found that the statin benefit on mortality was seen in both patients taking a statin alone (OR, 0.54) and in those taking statins with an antihypertensive medication (OR, 0.60).
Use of antihypertensive drugs was associated with a smaller, albeit still substantial, 27% lower odds of death (OR, 0.73; 95% CI, 0.62-0.87).
In propensity-matched analyses, use of statins and/or antihypertensives was tied to a 32% reduced risk of death among those with a history of CVD and/or hypertension (OR, 0.68; 95% CI, 0.58-0.81). An observed 16% reduction in odds of death with statins and/or antihypertensive drugs among those without cardiovascular disease and/or hypertension was not statistically significant (OR, 0.84; 95% CI, 0.58-1.22).
Stabilizing the underlying disease
The researchers pointed out that the results of the propensity matching analysis are consistent with the hypothesis that the major benefit of these medications accrues from treating and/or stabilizing underlying disease.
“Although it is well known that statins improve long-term outcomes among patients with or at elevated risk for cardiovascular disease, the association with a large short-term benefit which accrues in the setting of hospitalization for COVID-19 is a new and intriguing finding,” they said.
They cited several “plausible mechanisms whereby statins could directly mitigate outcomes in COVID-19 beyond treating underlying disease conditions,” including anti-inflammatory effects and a direct inhibitory effect on the SARS-CoV-2 virus.
Dr. Daniels elaborated more on the potential mechanism at play in an interview: “I think what is happening is that the statin is stabilizing the coronary disease so patients are less likely to die from MI or stroke, and this gives them more time and strength to recover from COVID-19.”
She added: “Statins may also have some direct anti-COVID effects such as an anti-inflammatory actions, but I would guess that this is probably not the primary effect behind what we’re seeing here.”
‘Important clinical implications’
The authors say their findings have “important clinical implications.”
They noted that early in the pandemic there was speculation that certain medications, including statins, and the ACE inhibitor/angiotensin receptor blocker (ARB) classes of antihypertensives may confer an increased susceptibility to COVID-19 positivity and/or severity.
“Our study reinforces the AHA and others’ recommendations that not only is it safe to remain on these medications, but they may substantially reduce risk of severe COVID-19 and especially death from COVID-19, particularly statins, and particularly among those with associated underlying conditions,” the authors stressed.
Dr. Daniels added that, although statins are very safe drugs, there are always some patients who prefer not to take medication even if indicated, and others who may have borderline indications and decide not to take a statin at present.
“This study may persuade these patients that taking a statin is the right thing to do. It may give those patients on the cusp of thinking about taking one of these drugs a reason to go ahead,” she said.
‘Provocative but not definitive’
Commenting on the study, Robert Harrington, MD, professor of medicine and chair of the department of medicine at Stanford (Calif.) University, said: “These are interesting observational data but as such have all the limitations of nonrandomized comparisons despite the best attempts to adjust for a variety of potential confounders. For example, is this an effect of statins (perhaps through some anti-inflammatory mechanism) or is it more an effect that can be attributed to the patients who are prescribed and taking a statin, compared with those who are not?”
He added: “The primary clinical benefit of statins, based on many large randomized clinical trials, seems to be derived from their LDL lowering effect. Observational studies have suggested potential benefits from anti-inflammatory effects of statins, but the randomized trials have not confirmed these observations. So, the current data are interesting, even provocative, but ultimately hypothesis generating rather than definitive.”
Also commenting on the study, Steven Nissen, MD, professor of medicine at the Cleveland Clinic, said: “While statins have many established benefits, their role in preventing COVID-19 complications is very speculative. Like all observational studies, the current study must be viewed as hypothesis generating, not definitive evidence of benefit. There are many potential confounders. I’m skeptical.”
The authors of this study received no specific funding for this work and report no competing interests. Dr. Harrington was AHA president when the COVID registry was created and he is still a member of the AHA board, which has oversight over the project.
FROM PLOS ONE










