User login
Injectable monoclonal antibodies prevent COVID-19 in trial
according to results of a randomized, double-blind, placebo-controlled clinical trial published online August 4, 2021, in the New England Journal of Medicine.
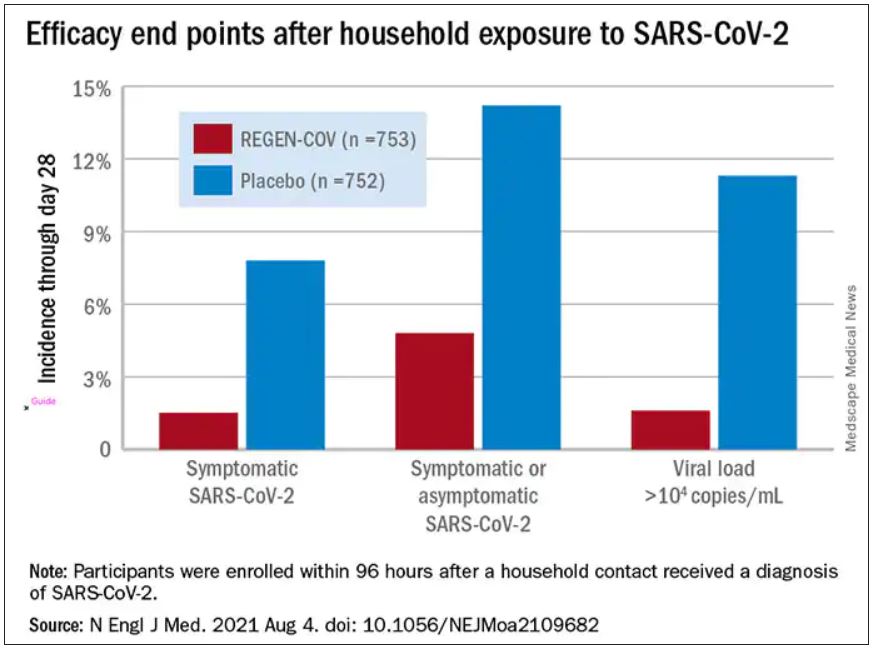
The cocktail of the monoclonal antibodies casirivimab and imdevimab (REGEN-COV, Regeneron Pharmaceuticals) reduced participants’ relative risk of infection by 72%, compared with placebo within the first week. After the first week, risk reduction increased to 93%.
“Long after you would be exposed by your household, there is an enduring effect that prevents you from community spread,” said David Wohl, MD, professor of medicine in the division of infectious diseases at the University of North Carolina at Chapel Hill, who was a site investigator for the trial but not a study author.
Participants were enrolled within 96 hours after someone in their household tested positive for SARS-CoV-2. Participants were randomly assigned to receive 1,200 mg of REGEN-COV subcutaneously or a placebo. Based on serologic testing, study participants showed no evidence of current or previous SARS-CoV-2 infection. The median age of participants was 42.9, but 45% were male teenagers (ages 12-17).
In the group that received REGEN-COV, 11 out of 753 participants developed symptomatic COVID-19, compared with 59 out of 752 participants who received placebo. The relative risk reduction for the study’s 4-week period was 81.4% (P < .001). Of the participants that did develop a SARS-CoV-2 infection, those that received REGEN-COV were less likely to be symptomatic. Asymptomatic infections developed in 25 participants who received REGEN-COV versus 48 in the placebo group. The relative risk of developing any SARS-CoV-2 infection, symptomatic or asymptomatic, was reduced by 66.4% with REGEN-COV (P < .001).
Among the patients who were symptomatic, symptoms subsided within a median of 1.2 weeks for the group that received REGEN-COV, 2 weeks earlier than the placebo group. These patients also had a shorter duration of a high viral load (>104 copies/mL). Few adverse events were reported in the treatment or placebo groups. Monoclonal antibodies “seem to be incredibly safe,” Dr. Wohl said.
“These monoclonal antibodies have proven they can reduce the viral replication in the nose,” said study author Myron Cohen, MD, an infectious disease specialist and professor of epidemiology at the University of North Carolina.
The Food and Drug Administration first granted REGEN-COV emergency use authorization (EUA) in November 2020 for use in patients with mild or moderate COVID-19 who were also at high risk for progressing to severe COVID-19. At that time, the cocktail of monoclonal antibodies was delivered by a single intravenous infusion.
In January, Regeneron first announced the success of this trial of the subcutaneous injection for exposed household contacts based on early results, and in June of 2021, the FDA expanded the EUA to include a subcutaneous delivery when IV is not feasible. On July 30, the EUA was expanded again to include prophylactic use in exposed patients based on these trial results.
The U.S. government has purchased approximately 1.5 million doses of REGEN-COV from Regeneron and has agreed to make the treatments free of charge to patients.
But despite being free, available, and backed by promising data, monoclonal antibodies as a therapeutic answer to COVID-19 still hasn’t really taken off. “The problem is, it first requires knowledge and awareness,” Dr. Wohl said. “A lot [of people] don’t know this exists. To be honest, vaccination has taken up all the oxygen in the room.”
Dr. Cohen agreed. One reason for the slow uptake may be because the drug supply is owned by the government and not a pharmaceutical company. There hasn’t been a typical marketing push to make physicians and consumers aware. Additionally, “the logistics are daunting,” Dr. Cohen said. The office spaces where many physicians care for patients “often aren’t appropriate for patients who think they have SARS-CoV-2.”
“Right now, there’s not a mechanism” to administer the drug to people who could benefit from it, Dr. Wohl said. Eligible patients are either immunocompromised and unlikely to mount a sufficient immune response with vaccination, or not fully vaccinated. They should have been exposed to an infected individual or have a high likelihood of exposure due to where they live, such as in a prison or nursing home. Local doctors are unlikely to be the primary administrators of the drug, Dr. Wohl added. “How do we operationalize this for people who fit the criteria?”
There’s also an issue of timing. REGEN-COV is most effective when given early, Dr. Cohen said. “[Monoclonal antibodies] really only work well in the replication phase.” Many patients who would be eligible delay care until they’ve had symptoms for several days, when REGEN-COV would no longer have the desired effect.
Eventually, Dr. Wohl suspects demand will increase when people realize REGEN-COV can help those with COVID-19 and those who have been exposed. But before then, “we do have to think about how to integrate this into a workflow people can access without being confused.”
The trial was done before there was widespread vaccination, so it’s unclear what the results mean for people who have been vaccinated. Dr. Cohen and Dr. Wohl said there are ongoing conversations about whether monoclonal antibodies could be complementary to vaccination and if there’s potential for continued monthly use of these therapies.
Cohen and Wohl reported no relevant financial relationships. The trial was supported by Regeneron Pharmaceuticals, F. Hoffmann–La Roche, the National Institute of Allergy and Infectious Diseases, NIH, and the COVID-19 Prevention Network.
A version of this article first appeared on Medscape.com.
according to results of a randomized, double-blind, placebo-controlled clinical trial published online August 4, 2021, in the New England Journal of Medicine.
The cocktail of the monoclonal antibodies casirivimab and imdevimab (REGEN-COV, Regeneron Pharmaceuticals) reduced participants’ relative risk of infection by 72%, compared with placebo within the first week. After the first week, risk reduction increased to 93%.
“Long after you would be exposed by your household, there is an enduring effect that prevents you from community spread,” said David Wohl, MD, professor of medicine in the division of infectious diseases at the University of North Carolina at Chapel Hill, who was a site investigator for the trial but not a study author.
Participants were enrolled within 96 hours after someone in their household tested positive for SARS-CoV-2. Participants were randomly assigned to receive 1,200 mg of REGEN-COV subcutaneously or a placebo. Based on serologic testing, study participants showed no evidence of current or previous SARS-CoV-2 infection. The median age of participants was 42.9, but 45% were male teenagers (ages 12-17).
In the group that received REGEN-COV, 11 out of 753 participants developed symptomatic COVID-19, compared with 59 out of 752 participants who received placebo. The relative risk reduction for the study’s 4-week period was 81.4% (P < .001). Of the participants that did develop a SARS-CoV-2 infection, those that received REGEN-COV were less likely to be symptomatic. Asymptomatic infections developed in 25 participants who received REGEN-COV versus 48 in the placebo group. The relative risk of developing any SARS-CoV-2 infection, symptomatic or asymptomatic, was reduced by 66.4% with REGEN-COV (P < .001).
Among the patients who were symptomatic, symptoms subsided within a median of 1.2 weeks for the group that received REGEN-COV, 2 weeks earlier than the placebo group. These patients also had a shorter duration of a high viral load (>104 copies/mL). Few adverse events were reported in the treatment or placebo groups. Monoclonal antibodies “seem to be incredibly safe,” Dr. Wohl said.
“These monoclonal antibodies have proven they can reduce the viral replication in the nose,” said study author Myron Cohen, MD, an infectious disease specialist and professor of epidemiology at the University of North Carolina.
The Food and Drug Administration first granted REGEN-COV emergency use authorization (EUA) in November 2020 for use in patients with mild or moderate COVID-19 who were also at high risk for progressing to severe COVID-19. At that time, the cocktail of monoclonal antibodies was delivered by a single intravenous infusion.
In January, Regeneron first announced the success of this trial of the subcutaneous injection for exposed household contacts based on early results, and in June of 2021, the FDA expanded the EUA to include a subcutaneous delivery when IV is not feasible. On July 30, the EUA was expanded again to include prophylactic use in exposed patients based on these trial results.
The U.S. government has purchased approximately 1.5 million doses of REGEN-COV from Regeneron and has agreed to make the treatments free of charge to patients.
But despite being free, available, and backed by promising data, monoclonal antibodies as a therapeutic answer to COVID-19 still hasn’t really taken off. “The problem is, it first requires knowledge and awareness,” Dr. Wohl said. “A lot [of people] don’t know this exists. To be honest, vaccination has taken up all the oxygen in the room.”
Dr. Cohen agreed. One reason for the slow uptake may be because the drug supply is owned by the government and not a pharmaceutical company. There hasn’t been a typical marketing push to make physicians and consumers aware. Additionally, “the logistics are daunting,” Dr. Cohen said. The office spaces where many physicians care for patients “often aren’t appropriate for patients who think they have SARS-CoV-2.”
“Right now, there’s not a mechanism” to administer the drug to people who could benefit from it, Dr. Wohl said. Eligible patients are either immunocompromised and unlikely to mount a sufficient immune response with vaccination, or not fully vaccinated. They should have been exposed to an infected individual or have a high likelihood of exposure due to where they live, such as in a prison or nursing home. Local doctors are unlikely to be the primary administrators of the drug, Dr. Wohl added. “How do we operationalize this for people who fit the criteria?”
There’s also an issue of timing. REGEN-COV is most effective when given early, Dr. Cohen said. “[Monoclonal antibodies] really only work well in the replication phase.” Many patients who would be eligible delay care until they’ve had symptoms for several days, when REGEN-COV would no longer have the desired effect.
Eventually, Dr. Wohl suspects demand will increase when people realize REGEN-COV can help those with COVID-19 and those who have been exposed. But before then, “we do have to think about how to integrate this into a workflow people can access without being confused.”
The trial was done before there was widespread vaccination, so it’s unclear what the results mean for people who have been vaccinated. Dr. Cohen and Dr. Wohl said there are ongoing conversations about whether monoclonal antibodies could be complementary to vaccination and if there’s potential for continued monthly use of these therapies.
Cohen and Wohl reported no relevant financial relationships. The trial was supported by Regeneron Pharmaceuticals, F. Hoffmann–La Roche, the National Institute of Allergy and Infectious Diseases, NIH, and the COVID-19 Prevention Network.
A version of this article first appeared on Medscape.com.
according to results of a randomized, double-blind, placebo-controlled clinical trial published online August 4, 2021, in the New England Journal of Medicine.
The cocktail of the monoclonal antibodies casirivimab and imdevimab (REGEN-COV, Regeneron Pharmaceuticals) reduced participants’ relative risk of infection by 72%, compared with placebo within the first week. After the first week, risk reduction increased to 93%.
“Long after you would be exposed by your household, there is an enduring effect that prevents you from community spread,” said David Wohl, MD, professor of medicine in the division of infectious diseases at the University of North Carolina at Chapel Hill, who was a site investigator for the trial but not a study author.
Participants were enrolled within 96 hours after someone in their household tested positive for SARS-CoV-2. Participants were randomly assigned to receive 1,200 mg of REGEN-COV subcutaneously or a placebo. Based on serologic testing, study participants showed no evidence of current or previous SARS-CoV-2 infection. The median age of participants was 42.9, but 45% were male teenagers (ages 12-17).
In the group that received REGEN-COV, 11 out of 753 participants developed symptomatic COVID-19, compared with 59 out of 752 participants who received placebo. The relative risk reduction for the study’s 4-week period was 81.4% (P < .001). Of the participants that did develop a SARS-CoV-2 infection, those that received REGEN-COV were less likely to be symptomatic. Asymptomatic infections developed in 25 participants who received REGEN-COV versus 48 in the placebo group. The relative risk of developing any SARS-CoV-2 infection, symptomatic or asymptomatic, was reduced by 66.4% with REGEN-COV (P < .001).
Among the patients who were symptomatic, symptoms subsided within a median of 1.2 weeks for the group that received REGEN-COV, 2 weeks earlier than the placebo group. These patients also had a shorter duration of a high viral load (>104 copies/mL). Few adverse events were reported in the treatment or placebo groups. Monoclonal antibodies “seem to be incredibly safe,” Dr. Wohl said.
“These monoclonal antibodies have proven they can reduce the viral replication in the nose,” said study author Myron Cohen, MD, an infectious disease specialist and professor of epidemiology at the University of North Carolina.
The Food and Drug Administration first granted REGEN-COV emergency use authorization (EUA) in November 2020 for use in patients with mild or moderate COVID-19 who were also at high risk for progressing to severe COVID-19. At that time, the cocktail of monoclonal antibodies was delivered by a single intravenous infusion.
In January, Regeneron first announced the success of this trial of the subcutaneous injection for exposed household contacts based on early results, and in June of 2021, the FDA expanded the EUA to include a subcutaneous delivery when IV is not feasible. On July 30, the EUA was expanded again to include prophylactic use in exposed patients based on these trial results.
The U.S. government has purchased approximately 1.5 million doses of REGEN-COV from Regeneron and has agreed to make the treatments free of charge to patients.
But despite being free, available, and backed by promising data, monoclonal antibodies as a therapeutic answer to COVID-19 still hasn’t really taken off. “The problem is, it first requires knowledge and awareness,” Dr. Wohl said. “A lot [of people] don’t know this exists. To be honest, vaccination has taken up all the oxygen in the room.”
Dr. Cohen agreed. One reason for the slow uptake may be because the drug supply is owned by the government and not a pharmaceutical company. There hasn’t been a typical marketing push to make physicians and consumers aware. Additionally, “the logistics are daunting,” Dr. Cohen said. The office spaces where many physicians care for patients “often aren’t appropriate for patients who think they have SARS-CoV-2.”
“Right now, there’s not a mechanism” to administer the drug to people who could benefit from it, Dr. Wohl said. Eligible patients are either immunocompromised and unlikely to mount a sufficient immune response with vaccination, or not fully vaccinated. They should have been exposed to an infected individual or have a high likelihood of exposure due to where they live, such as in a prison or nursing home. Local doctors are unlikely to be the primary administrators of the drug, Dr. Wohl added. “How do we operationalize this for people who fit the criteria?”
There’s also an issue of timing. REGEN-COV is most effective when given early, Dr. Cohen said. “[Monoclonal antibodies] really only work well in the replication phase.” Many patients who would be eligible delay care until they’ve had symptoms for several days, when REGEN-COV would no longer have the desired effect.
Eventually, Dr. Wohl suspects demand will increase when people realize REGEN-COV can help those with COVID-19 and those who have been exposed. But before then, “we do have to think about how to integrate this into a workflow people can access without being confused.”
The trial was done before there was widespread vaccination, so it’s unclear what the results mean for people who have been vaccinated. Dr. Cohen and Dr. Wohl said there are ongoing conversations about whether monoclonal antibodies could be complementary to vaccination and if there’s potential for continued monthly use of these therapies.
Cohen and Wohl reported no relevant financial relationships. The trial was supported by Regeneron Pharmaceuticals, F. Hoffmann–La Roche, the National Institute of Allergy and Infectious Diseases, NIH, and the COVID-19 Prevention Network.
A version of this article first appeared on Medscape.com.
Moderna says boosters may be needed after 6 months
Moderna says neutralizing antibodies generated by its COVID-19 vaccine against three variants of the virus that causes the disease waned substantially 6 months after the second dose.
Because of this, the company expects an increase in breakthrough infections with a need for boosters before winter.
In an experiment, a 50-mg dose of the vaccine, given as a third shot, boosted levels of antibodies in 20 previously vaccinated people by 32 times against the Beta variant, by 44 times against the Gamma variant, and by 42 times against Delta.
The new data was presented in an earnings call to investors and is based on a small study that hasn’t yet been published in medical literature.
The company also said its vaccine remained highly effective at preventing severe COVID outcomes through 6 months.
Last week, Pfizer released early data suggesting a similar drop in protection from its vaccine. The company also showed a third dose substantially boosted protection, including against the Delta variant.
The new results come just 1 day after the World Health Organization implored wealthy nations to hold off on third doses until more of the world’s population could get a first dose.
More than 80% of the 4 billion vaccine doses given around the world have been distributed to high-income countries.
A version of this article first appeared on WebMD.com.
Moderna says neutralizing antibodies generated by its COVID-19 vaccine against three variants of the virus that causes the disease waned substantially 6 months after the second dose.
Because of this, the company expects an increase in breakthrough infections with a need for boosters before winter.
In an experiment, a 50-mg dose of the vaccine, given as a third shot, boosted levels of antibodies in 20 previously vaccinated people by 32 times against the Beta variant, by 44 times against the Gamma variant, and by 42 times against Delta.
The new data was presented in an earnings call to investors and is based on a small study that hasn’t yet been published in medical literature.
The company also said its vaccine remained highly effective at preventing severe COVID outcomes through 6 months.
Last week, Pfizer released early data suggesting a similar drop in protection from its vaccine. The company also showed a third dose substantially boosted protection, including against the Delta variant.
The new results come just 1 day after the World Health Organization implored wealthy nations to hold off on third doses until more of the world’s population could get a first dose.
More than 80% of the 4 billion vaccine doses given around the world have been distributed to high-income countries.
A version of this article first appeared on WebMD.com.
Moderna says neutralizing antibodies generated by its COVID-19 vaccine against three variants of the virus that causes the disease waned substantially 6 months after the second dose.
Because of this, the company expects an increase in breakthrough infections with a need for boosters before winter.
In an experiment, a 50-mg dose of the vaccine, given as a third shot, boosted levels of antibodies in 20 previously vaccinated people by 32 times against the Beta variant, by 44 times against the Gamma variant, and by 42 times against Delta.
The new data was presented in an earnings call to investors and is based on a small study that hasn’t yet been published in medical literature.
The company also said its vaccine remained highly effective at preventing severe COVID outcomes through 6 months.
Last week, Pfizer released early data suggesting a similar drop in protection from its vaccine. The company also showed a third dose substantially boosted protection, including against the Delta variant.
The new results come just 1 day after the World Health Organization implored wealthy nations to hold off on third doses until more of the world’s population could get a first dose.
More than 80% of the 4 billion vaccine doses given around the world have been distributed to high-income countries.
A version of this article first appeared on WebMD.com.
Despite retraction, study using fraudulent Surgisphere data still cited
A retracted study on the safety of blood pressure medications in patients with COVID-19 continues to be cited nearly a year later, new research shows.
The study in question, published on May 1, 2020, in the New England Journal of Medicine, showed no increased risk for in-hospital death with the use of ACE inhibitors or angiotensin-receptor blockers (ARBs) in hospitalized patients with COVID-19.
Concerns about the veracity of the Surgisphere database used for the study, however, led to a June 4 retraction and to the June 13 retraction of a second study, published in the Lancet, that focused on hydroxychloroquine as a COVID-19 treatment.
Although the Surgisphere scandal caused a global reckoning of COVID-19 scientific studies, the new analysis identified 652 citations of the NEJM article as of May 31.
More than a third of the citations occurred in the first 2 months after the retraction, 54% were at least 3 months later, and 2.8% at least 6 months later. In May, 11 months after the article was retracted, it was cited 21 times, senior author Emily G. McDonald, MD, MSc, McGill University, Montreal, and colleagues reported in a research letter in JAMA Internal Medicine.
“In early May and June there were already more than 200 citations in one of the world’s leading scientific journals, so I do believe it was a highly influential article early on and had an impact on different types of studies or research taking place,” she said in an interview.
Dr. McDonald said she’s also “certain that it impacted patient care,” observing that when there are no guidelines available on how to manage patients, physicians will turn to the most recent evidence in the most reputable journals.
“In the case of ACE [inhibitors] and ARBs, although the study was based on fraudulent data, we were lucky that the overall message was in the end probably correct, but that might not have been the case for another study or dataset,” she said.
Early in the pandemic, concerns existed that ACE inhibitors and ARBs could be harmful, increasing the expression of ACE2 receptors, which the SARS-CoV-2 virus uses to gain entry into cells. The first randomized trial to examine the issue, BRACE CORONA, showed no clinical benefit to interrupting use of the agents in hospitalized patients. An observational study suggested ACE inhibitors may even be protective.
Of two high-profile retractions, McDonald said they chose to bypass the hydroxychloroquine study, which had an eye-popping Altmetric attention score of 23,084, compared with 3,727 for the NEJM paper, because it may have been cited for “other” reasons. “We wanted to focus less on the politics and more on the problem of retracted work.”
The team found that researchers across the globe were citing the retracted ACE/ARB paper (18.7% in the United States, 8.1% in Italy, and 44% other countries). Most citations were used to support a statement in the main text of a study, but in nearly 3% of cases, the data were incorporated into new analyses.
Just 17.6% of the studies cited or noted the retraction. “For sure, that was surprising to us. We suspected it, but our study confirmed it,” Dr. McDonald said.
Although retracted articles can be identified by a watermark or line of text, in some cases that can be easily missed, she noted. What’s more, not all citation software points out when a study has been retracted, a fate shared by the copyediting process.
“There are a lot of mechanisms in place and, in general, what’s happening is rare but there isn’t a perfect automated system solution to absolutely prevent this from happening,” she said. “It’s still subject to human error.”
The findings also have to be taken in the context of a rapidly emerging pandemic and the unprecedented torrent of scientific papers released over the past year.
“That might have contributed to why this happened, but the takeaway message is that this can happen despite our best efforts, and we need to challenge ourselves to come up with a system solution to prevent this from happening in the future,” Dr. McDonald said. “Current mechanisms are probably capturing 95% of it, but we need to do better.”
Limitations of the present analysis are that it was limited to the single retracted study; used only a single search engine, Google Scholar, to identify the citing works; and that additional citations may have been missed, the authors noted.
McDonald and coauthor Todd C. Lee, MD, report being signatories on a public letter calling for the retraction of the Surgisphere papers. Dr. Lee also reported receiving research support from Fonds De Recherche du Quebec-Sante during the conduct of the study.
A version of this article first appeared on Medscape.com.
A retracted study on the safety of blood pressure medications in patients with COVID-19 continues to be cited nearly a year later, new research shows.
The study in question, published on May 1, 2020, in the New England Journal of Medicine, showed no increased risk for in-hospital death with the use of ACE inhibitors or angiotensin-receptor blockers (ARBs) in hospitalized patients with COVID-19.
Concerns about the veracity of the Surgisphere database used for the study, however, led to a June 4 retraction and to the June 13 retraction of a second study, published in the Lancet, that focused on hydroxychloroquine as a COVID-19 treatment.
Although the Surgisphere scandal caused a global reckoning of COVID-19 scientific studies, the new analysis identified 652 citations of the NEJM article as of May 31.
More than a third of the citations occurred in the first 2 months after the retraction, 54% were at least 3 months later, and 2.8% at least 6 months later. In May, 11 months after the article was retracted, it was cited 21 times, senior author Emily G. McDonald, MD, MSc, McGill University, Montreal, and colleagues reported in a research letter in JAMA Internal Medicine.
“In early May and June there were already more than 200 citations in one of the world’s leading scientific journals, so I do believe it was a highly influential article early on and had an impact on different types of studies or research taking place,” she said in an interview.
Dr. McDonald said she’s also “certain that it impacted patient care,” observing that when there are no guidelines available on how to manage patients, physicians will turn to the most recent evidence in the most reputable journals.
“In the case of ACE [inhibitors] and ARBs, although the study was based on fraudulent data, we were lucky that the overall message was in the end probably correct, but that might not have been the case for another study or dataset,” she said.
Early in the pandemic, concerns existed that ACE inhibitors and ARBs could be harmful, increasing the expression of ACE2 receptors, which the SARS-CoV-2 virus uses to gain entry into cells. The first randomized trial to examine the issue, BRACE CORONA, showed no clinical benefit to interrupting use of the agents in hospitalized patients. An observational study suggested ACE inhibitors may even be protective.
Of two high-profile retractions, McDonald said they chose to bypass the hydroxychloroquine study, which had an eye-popping Altmetric attention score of 23,084, compared with 3,727 for the NEJM paper, because it may have been cited for “other” reasons. “We wanted to focus less on the politics and more on the problem of retracted work.”
The team found that researchers across the globe were citing the retracted ACE/ARB paper (18.7% in the United States, 8.1% in Italy, and 44% other countries). Most citations were used to support a statement in the main text of a study, but in nearly 3% of cases, the data were incorporated into new analyses.
Just 17.6% of the studies cited or noted the retraction. “For sure, that was surprising to us. We suspected it, but our study confirmed it,” Dr. McDonald said.
Although retracted articles can be identified by a watermark or line of text, in some cases that can be easily missed, she noted. What’s more, not all citation software points out when a study has been retracted, a fate shared by the copyediting process.
“There are a lot of mechanisms in place and, in general, what’s happening is rare but there isn’t a perfect automated system solution to absolutely prevent this from happening,” she said. “It’s still subject to human error.”
The findings also have to be taken in the context of a rapidly emerging pandemic and the unprecedented torrent of scientific papers released over the past year.
“That might have contributed to why this happened, but the takeaway message is that this can happen despite our best efforts, and we need to challenge ourselves to come up with a system solution to prevent this from happening in the future,” Dr. McDonald said. “Current mechanisms are probably capturing 95% of it, but we need to do better.”
Limitations of the present analysis are that it was limited to the single retracted study; used only a single search engine, Google Scholar, to identify the citing works; and that additional citations may have been missed, the authors noted.
McDonald and coauthor Todd C. Lee, MD, report being signatories on a public letter calling for the retraction of the Surgisphere papers. Dr. Lee also reported receiving research support from Fonds De Recherche du Quebec-Sante during the conduct of the study.
A version of this article first appeared on Medscape.com.
A retracted study on the safety of blood pressure medications in patients with COVID-19 continues to be cited nearly a year later, new research shows.
The study in question, published on May 1, 2020, in the New England Journal of Medicine, showed no increased risk for in-hospital death with the use of ACE inhibitors or angiotensin-receptor blockers (ARBs) in hospitalized patients with COVID-19.
Concerns about the veracity of the Surgisphere database used for the study, however, led to a June 4 retraction and to the June 13 retraction of a second study, published in the Lancet, that focused on hydroxychloroquine as a COVID-19 treatment.
Although the Surgisphere scandal caused a global reckoning of COVID-19 scientific studies, the new analysis identified 652 citations of the NEJM article as of May 31.
More than a third of the citations occurred in the first 2 months after the retraction, 54% were at least 3 months later, and 2.8% at least 6 months later. In May, 11 months after the article was retracted, it was cited 21 times, senior author Emily G. McDonald, MD, MSc, McGill University, Montreal, and colleagues reported in a research letter in JAMA Internal Medicine.
“In early May and June there were already more than 200 citations in one of the world’s leading scientific journals, so I do believe it was a highly influential article early on and had an impact on different types of studies or research taking place,” she said in an interview.
Dr. McDonald said she’s also “certain that it impacted patient care,” observing that when there are no guidelines available on how to manage patients, physicians will turn to the most recent evidence in the most reputable journals.
“In the case of ACE [inhibitors] and ARBs, although the study was based on fraudulent data, we were lucky that the overall message was in the end probably correct, but that might not have been the case for another study or dataset,” she said.
Early in the pandemic, concerns existed that ACE inhibitors and ARBs could be harmful, increasing the expression of ACE2 receptors, which the SARS-CoV-2 virus uses to gain entry into cells. The first randomized trial to examine the issue, BRACE CORONA, showed no clinical benefit to interrupting use of the agents in hospitalized patients. An observational study suggested ACE inhibitors may even be protective.
Of two high-profile retractions, McDonald said they chose to bypass the hydroxychloroquine study, which had an eye-popping Altmetric attention score of 23,084, compared with 3,727 for the NEJM paper, because it may have been cited for “other” reasons. “We wanted to focus less on the politics and more on the problem of retracted work.”
The team found that researchers across the globe were citing the retracted ACE/ARB paper (18.7% in the United States, 8.1% in Italy, and 44% other countries). Most citations were used to support a statement in the main text of a study, but in nearly 3% of cases, the data were incorporated into new analyses.
Just 17.6% of the studies cited or noted the retraction. “For sure, that was surprising to us. We suspected it, but our study confirmed it,” Dr. McDonald said.
Although retracted articles can be identified by a watermark or line of text, in some cases that can be easily missed, she noted. What’s more, not all citation software points out when a study has been retracted, a fate shared by the copyediting process.
“There are a lot of mechanisms in place and, in general, what’s happening is rare but there isn’t a perfect automated system solution to absolutely prevent this from happening,” she said. “It’s still subject to human error.”
The findings also have to be taken in the context of a rapidly emerging pandemic and the unprecedented torrent of scientific papers released over the past year.
“That might have contributed to why this happened, but the takeaway message is that this can happen despite our best efforts, and we need to challenge ourselves to come up with a system solution to prevent this from happening in the future,” Dr. McDonald said. “Current mechanisms are probably capturing 95% of it, but we need to do better.”
Limitations of the present analysis are that it was limited to the single retracted study; used only a single search engine, Google Scholar, to identify the citing works; and that additional citations may have been missed, the authors noted.
McDonald and coauthor Todd C. Lee, MD, report being signatories on a public letter calling for the retraction of the Surgisphere papers. Dr. Lee also reported receiving research support from Fonds De Recherche du Quebec-Sante during the conduct of the study.
A version of this article first appeared on Medscape.com.
Myocarditis tied to COVID-19 shots more common than reported?
While cases of pericarditis or myocarditis temporally linked to COVID-19 vaccination remain rare, they may happen more often than reported, according to a large review of electronic medical records (EMRs).
They also appear to represent two “distinct syndromes,” George Diaz, MD, Providence Regional Medical Center Everett (Washington), said in an interview.
Myocarditis typically occurs soon after vaccination in younger patients and mostly after the second dose, while pericarditis occurs later in older patients, after the first or second dose.
Dr. Diaz and colleagues reported their analysis in a research letter published online August 4 in JAMA.
They reviewed the records of 2,000,287 people who received at least one COVID-19 vaccination at 40 hospitals in Washington, Oregon, Montana, and California that are part of the Providence health care system and use the same EMRs.
The median age of the cohort was 57 years and 59% were women.
A little more than three quarters (77%) received more than one dose; most received the mRNA vaccines made by Pfizer (53%) and Moderna (44%); 3% received the Johnson & Johnson vaccine.
The records showed that 20 people had vaccine-related myocarditis (1.0 per 100,000) and 37 had pericarditis (1.8 per 100,000).
A recent report, based on data from the Centers for Disease Control and Prevention’s Vaccine Adverse Events Reporting System, suggested an incidence of myocarditis of about 4.8 cases per 1 million following receipt of mRNA COVID-19 vaccine.
The new study shows a “similar pattern, although at higher incidence, suggesting vaccine adverse event underreporting. In addition, pericarditis may be more common than myocarditis among older patients,” the study team wrote.
“Our study resulted in higher numbers of cases probably because we searched the EMR, and VAERS requires doctors to report suspected cases voluntarily,” Dr. Diaz said in an interview.
Also, in the governments’ statistics, pericarditis and myocarditis were “lumped together,” he noted.
Myocarditis cases
The 20 myocarditis cases occurred a median of 3.5 days after vaccination (11 after the Moderna vaccine and 9 after the Pfizer vaccine), 15 of the patients (75%) were men, and the median age was 36 years.
Four individuals (20%) developed myocarditis symptoms after the first vaccination and 16 (80%) after the second dose. Nineteen of the patients (95%) were admitted to the hospital and all were discharged after a median of 2 days.
None of the 20 patients were readmitted or died. Two received a second vaccination after onset of myocarditis; neither had worsening of symptoms. At last available follow-up (median, 23.5 days after symptom onset), 13 patients (65%) had a resolution of their myocarditis symptoms and seven (35%) were improving.
Pericarditis cases
The 37 pericarditis cases occurred a median of 20 days after the most recent COVID-19 vaccination: 23 (62%) with Pfizer, 12 (32%) with Moderna, and 2 (5%) with the J&J vaccine. Fifteen developed pericarditis after the first vaccine dose (41%) and 22 (59%) after the second.
Twenty-seven (73%) of the cases occurred in men; the median age was 59 years.
Thirteen patients (35%) were admitted to the hospital, none to intensive care. The median hospital stay was 1 day. Seven patients with pericarditis received a second vaccination. No patient died.
At last available follow-up (median, 28 days), 7 patients (19%) had resolved symptoms and 23 (62%) were improving.
The researchers also calculate that the average monthly number of cases of myocarditis or myopericarditis during the prevaccine period of January 2019 through January 2021 was 16.9 (95% confidence interval, 15.3-18.6) compared with 27.3 (95% CI, 22.4-32.9) during the vaccine period of February through May 2021 (P < .001).
The mean numbers of pericarditis cases during the same periods were 49.1 (95% CI, 46.4-51.9) and 78.8 (95% CI, 70.3-87.9), respectively (P < .001).
The authors say limitations of their analysis include potential missed cases outside care settings and missed diagnoses of myocarditis or pericarditis, which would underestimate the incidence, as well as inaccurate EMR vaccination information.
“Temporal association does not prove causation, although the short span between vaccination and myocarditis onset and the elevated incidence of myocarditis and pericarditis in the study hospitals lend support to a possible relationship,” they wrote.
In late June, the Food and Drug Administration added a warning to the fact sheets accompanying the Pfizer and Moderna mRNA COVID-19 vaccines, flagging the rare risk of heart inflammation after their use.
Dr. Diaz cautioned that myocarditis and pericarditis events remain “a rare occurrence” after COVID-19 vaccination.
“When discussing vaccination with patients, [health care providers] can advise them that patients generally recover in the rare event they get pericarditis or myocarditis and no deaths were found, and that the vaccines are safe and effective,” Dr. Diaz said.
The study had no specific funding. Dr. Diaz reported receipt of clinical trial research support from Gilead Sciences, Regeneron, Roche, Boehringer Ingelheim, and Edesa Biotech and scientific advisory board membership for Safeology.
A version of this article first appeared on Medscape.com.
While cases of pericarditis or myocarditis temporally linked to COVID-19 vaccination remain rare, they may happen more often than reported, according to a large review of electronic medical records (EMRs).
They also appear to represent two “distinct syndromes,” George Diaz, MD, Providence Regional Medical Center Everett (Washington), said in an interview.
Myocarditis typically occurs soon after vaccination in younger patients and mostly after the second dose, while pericarditis occurs later in older patients, after the first or second dose.
Dr. Diaz and colleagues reported their analysis in a research letter published online August 4 in JAMA.
They reviewed the records of 2,000,287 people who received at least one COVID-19 vaccination at 40 hospitals in Washington, Oregon, Montana, and California that are part of the Providence health care system and use the same EMRs.
The median age of the cohort was 57 years and 59% were women.
A little more than three quarters (77%) received more than one dose; most received the mRNA vaccines made by Pfizer (53%) and Moderna (44%); 3% received the Johnson & Johnson vaccine.
The records showed that 20 people had vaccine-related myocarditis (1.0 per 100,000) and 37 had pericarditis (1.8 per 100,000).
A recent report, based on data from the Centers for Disease Control and Prevention’s Vaccine Adverse Events Reporting System, suggested an incidence of myocarditis of about 4.8 cases per 1 million following receipt of mRNA COVID-19 vaccine.
The new study shows a “similar pattern, although at higher incidence, suggesting vaccine adverse event underreporting. In addition, pericarditis may be more common than myocarditis among older patients,” the study team wrote.
“Our study resulted in higher numbers of cases probably because we searched the EMR, and VAERS requires doctors to report suspected cases voluntarily,” Dr. Diaz said in an interview.
Also, in the governments’ statistics, pericarditis and myocarditis were “lumped together,” he noted.
Myocarditis cases
The 20 myocarditis cases occurred a median of 3.5 days after vaccination (11 after the Moderna vaccine and 9 after the Pfizer vaccine), 15 of the patients (75%) were men, and the median age was 36 years.
Four individuals (20%) developed myocarditis symptoms after the first vaccination and 16 (80%) after the second dose. Nineteen of the patients (95%) were admitted to the hospital and all were discharged after a median of 2 days.
None of the 20 patients were readmitted or died. Two received a second vaccination after onset of myocarditis; neither had worsening of symptoms. At last available follow-up (median, 23.5 days after symptom onset), 13 patients (65%) had a resolution of their myocarditis symptoms and seven (35%) were improving.
Pericarditis cases
The 37 pericarditis cases occurred a median of 20 days after the most recent COVID-19 vaccination: 23 (62%) with Pfizer, 12 (32%) with Moderna, and 2 (5%) with the J&J vaccine. Fifteen developed pericarditis after the first vaccine dose (41%) and 22 (59%) after the second.
Twenty-seven (73%) of the cases occurred in men; the median age was 59 years.
Thirteen patients (35%) were admitted to the hospital, none to intensive care. The median hospital stay was 1 day. Seven patients with pericarditis received a second vaccination. No patient died.
At last available follow-up (median, 28 days), 7 patients (19%) had resolved symptoms and 23 (62%) were improving.
The researchers also calculate that the average monthly number of cases of myocarditis or myopericarditis during the prevaccine period of January 2019 through January 2021 was 16.9 (95% confidence interval, 15.3-18.6) compared with 27.3 (95% CI, 22.4-32.9) during the vaccine period of February through May 2021 (P < .001).
The mean numbers of pericarditis cases during the same periods were 49.1 (95% CI, 46.4-51.9) and 78.8 (95% CI, 70.3-87.9), respectively (P < .001).
The authors say limitations of their analysis include potential missed cases outside care settings and missed diagnoses of myocarditis or pericarditis, which would underestimate the incidence, as well as inaccurate EMR vaccination information.
“Temporal association does not prove causation, although the short span between vaccination and myocarditis onset and the elevated incidence of myocarditis and pericarditis in the study hospitals lend support to a possible relationship,” they wrote.
In late June, the Food and Drug Administration added a warning to the fact sheets accompanying the Pfizer and Moderna mRNA COVID-19 vaccines, flagging the rare risk of heart inflammation after their use.
Dr. Diaz cautioned that myocarditis and pericarditis events remain “a rare occurrence” after COVID-19 vaccination.
“When discussing vaccination with patients, [health care providers] can advise them that patients generally recover in the rare event they get pericarditis or myocarditis and no deaths were found, and that the vaccines are safe and effective,” Dr. Diaz said.
The study had no specific funding. Dr. Diaz reported receipt of clinical trial research support from Gilead Sciences, Regeneron, Roche, Boehringer Ingelheim, and Edesa Biotech and scientific advisory board membership for Safeology.
A version of this article first appeared on Medscape.com.
While cases of pericarditis or myocarditis temporally linked to COVID-19 vaccination remain rare, they may happen more often than reported, according to a large review of electronic medical records (EMRs).
They also appear to represent two “distinct syndromes,” George Diaz, MD, Providence Regional Medical Center Everett (Washington), said in an interview.
Myocarditis typically occurs soon after vaccination in younger patients and mostly after the second dose, while pericarditis occurs later in older patients, after the first or second dose.
Dr. Diaz and colleagues reported their analysis in a research letter published online August 4 in JAMA.
They reviewed the records of 2,000,287 people who received at least one COVID-19 vaccination at 40 hospitals in Washington, Oregon, Montana, and California that are part of the Providence health care system and use the same EMRs.
The median age of the cohort was 57 years and 59% were women.
A little more than three quarters (77%) received more than one dose; most received the mRNA vaccines made by Pfizer (53%) and Moderna (44%); 3% received the Johnson & Johnson vaccine.
The records showed that 20 people had vaccine-related myocarditis (1.0 per 100,000) and 37 had pericarditis (1.8 per 100,000).
A recent report, based on data from the Centers for Disease Control and Prevention’s Vaccine Adverse Events Reporting System, suggested an incidence of myocarditis of about 4.8 cases per 1 million following receipt of mRNA COVID-19 vaccine.
The new study shows a “similar pattern, although at higher incidence, suggesting vaccine adverse event underreporting. In addition, pericarditis may be more common than myocarditis among older patients,” the study team wrote.
“Our study resulted in higher numbers of cases probably because we searched the EMR, and VAERS requires doctors to report suspected cases voluntarily,” Dr. Diaz said in an interview.
Also, in the governments’ statistics, pericarditis and myocarditis were “lumped together,” he noted.
Myocarditis cases
The 20 myocarditis cases occurred a median of 3.5 days after vaccination (11 after the Moderna vaccine and 9 after the Pfizer vaccine), 15 of the patients (75%) were men, and the median age was 36 years.
Four individuals (20%) developed myocarditis symptoms after the first vaccination and 16 (80%) after the second dose. Nineteen of the patients (95%) were admitted to the hospital and all were discharged after a median of 2 days.
None of the 20 patients were readmitted or died. Two received a second vaccination after onset of myocarditis; neither had worsening of symptoms. At last available follow-up (median, 23.5 days after symptom onset), 13 patients (65%) had a resolution of their myocarditis symptoms and seven (35%) were improving.
Pericarditis cases
The 37 pericarditis cases occurred a median of 20 days after the most recent COVID-19 vaccination: 23 (62%) with Pfizer, 12 (32%) with Moderna, and 2 (5%) with the J&J vaccine. Fifteen developed pericarditis after the first vaccine dose (41%) and 22 (59%) after the second.
Twenty-seven (73%) of the cases occurred in men; the median age was 59 years.
Thirteen patients (35%) were admitted to the hospital, none to intensive care. The median hospital stay was 1 day. Seven patients with pericarditis received a second vaccination. No patient died.
At last available follow-up (median, 28 days), 7 patients (19%) had resolved symptoms and 23 (62%) were improving.
The researchers also calculate that the average monthly number of cases of myocarditis or myopericarditis during the prevaccine period of January 2019 through January 2021 was 16.9 (95% confidence interval, 15.3-18.6) compared with 27.3 (95% CI, 22.4-32.9) during the vaccine period of February through May 2021 (P < .001).
The mean numbers of pericarditis cases during the same periods were 49.1 (95% CI, 46.4-51.9) and 78.8 (95% CI, 70.3-87.9), respectively (P < .001).
The authors say limitations of their analysis include potential missed cases outside care settings and missed diagnoses of myocarditis or pericarditis, which would underestimate the incidence, as well as inaccurate EMR vaccination information.
“Temporal association does not prove causation, although the short span between vaccination and myocarditis onset and the elevated incidence of myocarditis and pericarditis in the study hospitals lend support to a possible relationship,” they wrote.
In late June, the Food and Drug Administration added a warning to the fact sheets accompanying the Pfizer and Moderna mRNA COVID-19 vaccines, flagging the rare risk of heart inflammation after their use.
Dr. Diaz cautioned that myocarditis and pericarditis events remain “a rare occurrence” after COVID-19 vaccination.
“When discussing vaccination with patients, [health care providers] can advise them that patients generally recover in the rare event they get pericarditis or myocarditis and no deaths were found, and that the vaccines are safe and effective,” Dr. Diaz said.
The study had no specific funding. Dr. Diaz reported receipt of clinical trial research support from Gilead Sciences, Regeneron, Roche, Boehringer Ingelheim, and Edesa Biotech and scientific advisory board membership for Safeology.
A version of this article first appeared on Medscape.com.
COVID-19 tied to acceleration of Alzheimer’s disease pathology
, a new study shows.
These results suggest that COVID-19 may accelerate Alzheimer’s disease symptoms and pathology, said study investigator Thomas Wisniewski, MD, professor of neurology, pathology, and psychiatry at New York University.
The findings were presented here at the Alzheimer’s Association International Conference (AAIC) 2021.
Strong correlation
There’s a clear association between SARS-CoV-2 infection and Alzheimer’s disease-related dementia. Patients with Alzheimer’s disease are at threefold higher risk for the infection and have a twofold higher risk for death, Dr. Wisniewski told meeting delegates.
He and his colleagues conducted a prospective study of patients who had tested positive for SARS-CoV-2 and who experienced neurologic sequelae and SARS-CoV-2 patients who were without neurologic sequelae. All patients were hospitalized from March 10 to May 20, 2020. This was during a period when New York City was overwhelmed by COVID: About 35% of hospitalized patients had COVID.
Of those who experienced neurologic events, the most common “by far and away” (51%) was toxic metabolic encephalopathy (TME), said Dr. Wisniewski. Other associations included seizures, hypoxic/anoxic injury, and ischemic stroke.
The most common TMEs were septic and hypoxic ischemia. In most patients (78%), TME had more than one cause.
Researchers followed 196 patients with COVID and neurologic complications (case patients) and 186 matched control patients who had no neurologic complications over a period of 6 months.
“Unfortunately, both groups had poor outcomes,” said Dr. Wisniewski. About 50% had impaired cognition, and 56% experienced limitations in activities of daily living.
However, those patients with COVID-19 who had neurologic sequelae “fared even worse,” said Dr. Wisniewski. Compared with control patients, they had twofold worse Modified Rankin Scale scores and worse scores on activity of daily living, and they were much less likely to return to work.
Mechanisms by which COVID-19 affects longer-term cognitive dysfunction are unclear, but inflammation likely plays a role.
The research team compared a number of Alzheimer’s disease plasma biomarkers in 158 patients with COVID-19 who had neurologic symptoms and 152 COVID patients with COVID but no neurologic symptoms. They found marked elevations of neurofilament light, a marker of neuronal injury, in those with symptoms (P = .0003) as well as increased glial fibrillary acid protein, a marker of neuroinflammation (P = .0098).
Ubiquitin carboxyl-terminal hydrolase L1, another marker of neuronal injury, was also elevated in those with neurologic symptoms. Regarding Alzheimer’s disease pathology, total tau (t-tau) and phosphorylated tau “also tracked with neurological sequelae,” said Dr. Wisniewski.
There was no difference in levels of amyloid beta 40 (A beta 40) between groups. However, A beta 42 plasma levels were significantly lower in those with neurologic effects, suggesting higher levels in the brain. In addition, the ratio of t-tau to A beta 42 “clearly differentiated the two groups,” he said.
“Serum biomarkers of neuroinflammation and neuronal injury and Alzheimer’s disease correlate strongly, perhaps suggesting that folks with COVID infection and neurological sequelae may have an acceleration of Alzheimer’s disease symptoms and pathology,” he said. “That’s something that needs longer follow-up.”
Important differentiation
Commenting on the research, Rebecca Edelmayer, PhD, senior director of scientific engagement, Alzheimer’s Association, said the study provides important information. The inclusion of plasma biomarkers in this research is “really critical to tease out what’s the impact of COVID itself on the brain,” said Dr. Edelmayer.
“We’re in an era of biomarkers when it comes to Alzheimer’s disease and other dementias, and being able to define those changes that are happening in the brain over time is going to be really critical and aid in early detection and accurate diagnoses,” she said.
What is still to be learned is what these biomarkers reveal long term, said Dr. Edelmayer. “Do those biological markers change? Do they go back to normal? A lot of that is still unknown,” she said.
She noted that many diseases that are linked to inflammation produce similar biomarkers in the brain – for example, neurofilament light.
With other viral infections, such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), similar associations between the infection and cognition have been reported, said Dr. Edelmayer.
“But there are still a lot of questions around cause and effect. Is it really a direct effect of the virus on the brain itself? Is it an effect of having an enormous amount of inflammation going on in the body? A lot of that still needs to be teased out,” she commented.
The study was supported by the National Institutes of Health, the Alzheimer’s Association, and the State of New York. Dr. Wisniewski has consulted for Grifols, Amylon Pharmaceuticals, and Alzamed Neuro; 30 NYU patents are related to AD therapeutics.
A version of this article first appeared on Medscape.com.
, a new study shows.
These results suggest that COVID-19 may accelerate Alzheimer’s disease symptoms and pathology, said study investigator Thomas Wisniewski, MD, professor of neurology, pathology, and psychiatry at New York University.
The findings were presented here at the Alzheimer’s Association International Conference (AAIC) 2021.
Strong correlation
There’s a clear association between SARS-CoV-2 infection and Alzheimer’s disease-related dementia. Patients with Alzheimer’s disease are at threefold higher risk for the infection and have a twofold higher risk for death, Dr. Wisniewski told meeting delegates.
He and his colleagues conducted a prospective study of patients who had tested positive for SARS-CoV-2 and who experienced neurologic sequelae and SARS-CoV-2 patients who were without neurologic sequelae. All patients were hospitalized from March 10 to May 20, 2020. This was during a period when New York City was overwhelmed by COVID: About 35% of hospitalized patients had COVID.
Of those who experienced neurologic events, the most common “by far and away” (51%) was toxic metabolic encephalopathy (TME), said Dr. Wisniewski. Other associations included seizures, hypoxic/anoxic injury, and ischemic stroke.
The most common TMEs were septic and hypoxic ischemia. In most patients (78%), TME had more than one cause.
Researchers followed 196 patients with COVID and neurologic complications (case patients) and 186 matched control patients who had no neurologic complications over a period of 6 months.
“Unfortunately, both groups had poor outcomes,” said Dr. Wisniewski. About 50% had impaired cognition, and 56% experienced limitations in activities of daily living.
However, those patients with COVID-19 who had neurologic sequelae “fared even worse,” said Dr. Wisniewski. Compared with control patients, they had twofold worse Modified Rankin Scale scores and worse scores on activity of daily living, and they were much less likely to return to work.
Mechanisms by which COVID-19 affects longer-term cognitive dysfunction are unclear, but inflammation likely plays a role.
The research team compared a number of Alzheimer’s disease plasma biomarkers in 158 patients with COVID-19 who had neurologic symptoms and 152 COVID patients with COVID but no neurologic symptoms. They found marked elevations of neurofilament light, a marker of neuronal injury, in those with symptoms (P = .0003) as well as increased glial fibrillary acid protein, a marker of neuroinflammation (P = .0098).
Ubiquitin carboxyl-terminal hydrolase L1, another marker of neuronal injury, was also elevated in those with neurologic symptoms. Regarding Alzheimer’s disease pathology, total tau (t-tau) and phosphorylated tau “also tracked with neurological sequelae,” said Dr. Wisniewski.
There was no difference in levels of amyloid beta 40 (A beta 40) between groups. However, A beta 42 plasma levels were significantly lower in those with neurologic effects, suggesting higher levels in the brain. In addition, the ratio of t-tau to A beta 42 “clearly differentiated the two groups,” he said.
“Serum biomarkers of neuroinflammation and neuronal injury and Alzheimer’s disease correlate strongly, perhaps suggesting that folks with COVID infection and neurological sequelae may have an acceleration of Alzheimer’s disease symptoms and pathology,” he said. “That’s something that needs longer follow-up.”
Important differentiation
Commenting on the research, Rebecca Edelmayer, PhD, senior director of scientific engagement, Alzheimer’s Association, said the study provides important information. The inclusion of plasma biomarkers in this research is “really critical to tease out what’s the impact of COVID itself on the brain,” said Dr. Edelmayer.
“We’re in an era of biomarkers when it comes to Alzheimer’s disease and other dementias, and being able to define those changes that are happening in the brain over time is going to be really critical and aid in early detection and accurate diagnoses,” she said.
What is still to be learned is what these biomarkers reveal long term, said Dr. Edelmayer. “Do those biological markers change? Do they go back to normal? A lot of that is still unknown,” she said.
She noted that many diseases that are linked to inflammation produce similar biomarkers in the brain – for example, neurofilament light.
With other viral infections, such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), similar associations between the infection and cognition have been reported, said Dr. Edelmayer.
“But there are still a lot of questions around cause and effect. Is it really a direct effect of the virus on the brain itself? Is it an effect of having an enormous amount of inflammation going on in the body? A lot of that still needs to be teased out,” she commented.
The study was supported by the National Institutes of Health, the Alzheimer’s Association, and the State of New York. Dr. Wisniewski has consulted for Grifols, Amylon Pharmaceuticals, and Alzamed Neuro; 30 NYU patents are related to AD therapeutics.
A version of this article first appeared on Medscape.com.
, a new study shows.
These results suggest that COVID-19 may accelerate Alzheimer’s disease symptoms and pathology, said study investigator Thomas Wisniewski, MD, professor of neurology, pathology, and psychiatry at New York University.
The findings were presented here at the Alzheimer’s Association International Conference (AAIC) 2021.
Strong correlation
There’s a clear association between SARS-CoV-2 infection and Alzheimer’s disease-related dementia. Patients with Alzheimer’s disease are at threefold higher risk for the infection and have a twofold higher risk for death, Dr. Wisniewski told meeting delegates.
He and his colleagues conducted a prospective study of patients who had tested positive for SARS-CoV-2 and who experienced neurologic sequelae and SARS-CoV-2 patients who were without neurologic sequelae. All patients were hospitalized from March 10 to May 20, 2020. This was during a period when New York City was overwhelmed by COVID: About 35% of hospitalized patients had COVID.
Of those who experienced neurologic events, the most common “by far and away” (51%) was toxic metabolic encephalopathy (TME), said Dr. Wisniewski. Other associations included seizures, hypoxic/anoxic injury, and ischemic stroke.
The most common TMEs were septic and hypoxic ischemia. In most patients (78%), TME had more than one cause.
Researchers followed 196 patients with COVID and neurologic complications (case patients) and 186 matched control patients who had no neurologic complications over a period of 6 months.
“Unfortunately, both groups had poor outcomes,” said Dr. Wisniewski. About 50% had impaired cognition, and 56% experienced limitations in activities of daily living.
However, those patients with COVID-19 who had neurologic sequelae “fared even worse,” said Dr. Wisniewski. Compared with control patients, they had twofold worse Modified Rankin Scale scores and worse scores on activity of daily living, and they were much less likely to return to work.
Mechanisms by which COVID-19 affects longer-term cognitive dysfunction are unclear, but inflammation likely plays a role.
The research team compared a number of Alzheimer’s disease plasma biomarkers in 158 patients with COVID-19 who had neurologic symptoms and 152 COVID patients with COVID but no neurologic symptoms. They found marked elevations of neurofilament light, a marker of neuronal injury, in those with symptoms (P = .0003) as well as increased glial fibrillary acid protein, a marker of neuroinflammation (P = .0098).
Ubiquitin carboxyl-terminal hydrolase L1, another marker of neuronal injury, was also elevated in those with neurologic symptoms. Regarding Alzheimer’s disease pathology, total tau (t-tau) and phosphorylated tau “also tracked with neurological sequelae,” said Dr. Wisniewski.
There was no difference in levels of amyloid beta 40 (A beta 40) between groups. However, A beta 42 plasma levels were significantly lower in those with neurologic effects, suggesting higher levels in the brain. In addition, the ratio of t-tau to A beta 42 “clearly differentiated the two groups,” he said.
“Serum biomarkers of neuroinflammation and neuronal injury and Alzheimer’s disease correlate strongly, perhaps suggesting that folks with COVID infection and neurological sequelae may have an acceleration of Alzheimer’s disease symptoms and pathology,” he said. “That’s something that needs longer follow-up.”
Important differentiation
Commenting on the research, Rebecca Edelmayer, PhD, senior director of scientific engagement, Alzheimer’s Association, said the study provides important information. The inclusion of plasma biomarkers in this research is “really critical to tease out what’s the impact of COVID itself on the brain,” said Dr. Edelmayer.
“We’re in an era of biomarkers when it comes to Alzheimer’s disease and other dementias, and being able to define those changes that are happening in the brain over time is going to be really critical and aid in early detection and accurate diagnoses,” she said.
What is still to be learned is what these biomarkers reveal long term, said Dr. Edelmayer. “Do those biological markers change? Do they go back to normal? A lot of that is still unknown,” she said.
She noted that many diseases that are linked to inflammation produce similar biomarkers in the brain – for example, neurofilament light.
With other viral infections, such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), similar associations between the infection and cognition have been reported, said Dr. Edelmayer.
“But there are still a lot of questions around cause and effect. Is it really a direct effect of the virus on the brain itself? Is it an effect of having an enormous amount of inflammation going on in the body? A lot of that still needs to be teased out,” she commented.
The study was supported by the National Institutes of Health, the Alzheimer’s Association, and the State of New York. Dr. Wisniewski has consulted for Grifols, Amylon Pharmaceuticals, and Alzamed Neuro; 30 NYU patents are related to AD therapeutics.
A version of this article first appeared on Medscape.com.
From AAIC 2021
WHO calls for pause on booster doses
The World Health Organization is calling on wealthy nations to wait to give their citizens booster doses of COVID-19 vaccines until at least the end of September to give more people in other countries a chance to get a first dose of these lifesaving shots.
WHO Director-General Tedros Ghebreyesus, PhD, said that more than 80% of the 4 billion vaccine doses given around the world had been distributed to high-income countries, though they represent less than half the world’s population.
“I understand the concern of all governments to protect their people from the Delta variant,” Dr. Ghebreyesus said. “But we cannot accept countries that have already used most of the global supply of vaccines using even more of it, while the world’s most vulnerable people remain unprotected.”
So far, high-income countries have given about 100 vaccine doses for every 100 people, while low-income countries have given just 1.5 doses for every 100 people.
“Which means, in some of the most vulnerable countries in the world with the weakest health systems, health care workers are working without protection. … the older populations remain at high risk,” said Bruce Aylward, MD, the WHO’s senior adviser on organizational change.
But not everyone agrees.
Leana Wen, MD, a visiting professor at the Milken Institute School of Public Health at George Washington University, Washington, said there are doses already in the United States that won’t last long enough to be sent elsewhere.
“Yes, we need to get vaccines to the world (which also includes helping with distribution, not just supply), but there are doses expiring here in the U.S.,” she said on Twitter. “Why not allow those immunosuppressed to receive them?”
Israel became the first country to start giving some residents booster shots on Sunday, offering extra doses to seniors who are more than 5 months past their last vaccinations. On Monday, Germany announced it would also give booster doses to vulnerable patients, such as nursing home residents, beginning in September.
Dr. Aylward said the moratorium was all about “trying to put a hold on those policies until and unless we get the rest of the world caught up.”
He said it’s clear from the emergence of variant after variant that if we don’t stop the transmission of the virus around the world, the pandemic will continue to put pressure on the vaccines, making them less and less effective.
“We cannot get out of it unless the whole world gets out of it together,” Dr. Aylward said.
“We need an urgent reversal, from the majority of vaccines going to high-income countries, to the majority going to low-income countries,” Dr. Ghebreyesus said, asking leaders of high-income countries to wait on distributing booster doses until at least 10% of the world’s population is vaccinated.
“To make that happen, we need everyone’s cooperation, especially the handful of countries and companies that control the global supply of vaccines,” he said.
A version of this article first appeared on WebMD.com.
The World Health Organization is calling on wealthy nations to wait to give their citizens booster doses of COVID-19 vaccines until at least the end of September to give more people in other countries a chance to get a first dose of these lifesaving shots.
WHO Director-General Tedros Ghebreyesus, PhD, said that more than 80% of the 4 billion vaccine doses given around the world had been distributed to high-income countries, though they represent less than half the world’s population.
“I understand the concern of all governments to protect their people from the Delta variant,” Dr. Ghebreyesus said. “But we cannot accept countries that have already used most of the global supply of vaccines using even more of it, while the world’s most vulnerable people remain unprotected.”
So far, high-income countries have given about 100 vaccine doses for every 100 people, while low-income countries have given just 1.5 doses for every 100 people.
“Which means, in some of the most vulnerable countries in the world with the weakest health systems, health care workers are working without protection. … the older populations remain at high risk,” said Bruce Aylward, MD, the WHO’s senior adviser on organizational change.
But not everyone agrees.
Leana Wen, MD, a visiting professor at the Milken Institute School of Public Health at George Washington University, Washington, said there are doses already in the United States that won’t last long enough to be sent elsewhere.
“Yes, we need to get vaccines to the world (which also includes helping with distribution, not just supply), but there are doses expiring here in the U.S.,” she said on Twitter. “Why not allow those immunosuppressed to receive them?”
Israel became the first country to start giving some residents booster shots on Sunday, offering extra doses to seniors who are more than 5 months past their last vaccinations. On Monday, Germany announced it would also give booster doses to vulnerable patients, such as nursing home residents, beginning in September.
Dr. Aylward said the moratorium was all about “trying to put a hold on those policies until and unless we get the rest of the world caught up.”
He said it’s clear from the emergence of variant after variant that if we don’t stop the transmission of the virus around the world, the pandemic will continue to put pressure on the vaccines, making them less and less effective.
“We cannot get out of it unless the whole world gets out of it together,” Dr. Aylward said.
“We need an urgent reversal, from the majority of vaccines going to high-income countries, to the majority going to low-income countries,” Dr. Ghebreyesus said, asking leaders of high-income countries to wait on distributing booster doses until at least 10% of the world’s population is vaccinated.
“To make that happen, we need everyone’s cooperation, especially the handful of countries and companies that control the global supply of vaccines,” he said.
A version of this article first appeared on WebMD.com.
The World Health Organization is calling on wealthy nations to wait to give their citizens booster doses of COVID-19 vaccines until at least the end of September to give more people in other countries a chance to get a first dose of these lifesaving shots.
WHO Director-General Tedros Ghebreyesus, PhD, said that more than 80% of the 4 billion vaccine doses given around the world had been distributed to high-income countries, though they represent less than half the world’s population.
“I understand the concern of all governments to protect their people from the Delta variant,” Dr. Ghebreyesus said. “But we cannot accept countries that have already used most of the global supply of vaccines using even more of it, while the world’s most vulnerable people remain unprotected.”
So far, high-income countries have given about 100 vaccine doses for every 100 people, while low-income countries have given just 1.5 doses for every 100 people.
“Which means, in some of the most vulnerable countries in the world with the weakest health systems, health care workers are working without protection. … the older populations remain at high risk,” said Bruce Aylward, MD, the WHO’s senior adviser on organizational change.
But not everyone agrees.
Leana Wen, MD, a visiting professor at the Milken Institute School of Public Health at George Washington University, Washington, said there are doses already in the United States that won’t last long enough to be sent elsewhere.
“Yes, we need to get vaccines to the world (which also includes helping with distribution, not just supply), but there are doses expiring here in the U.S.,” she said on Twitter. “Why not allow those immunosuppressed to receive them?”
Israel became the first country to start giving some residents booster shots on Sunday, offering extra doses to seniors who are more than 5 months past their last vaccinations. On Monday, Germany announced it would also give booster doses to vulnerable patients, such as nursing home residents, beginning in September.
Dr. Aylward said the moratorium was all about “trying to put a hold on those policies until and unless we get the rest of the world caught up.”
He said it’s clear from the emergence of variant after variant that if we don’t stop the transmission of the virus around the world, the pandemic will continue to put pressure on the vaccines, making them less and less effective.
“We cannot get out of it unless the whole world gets out of it together,” Dr. Aylward said.
“We need an urgent reversal, from the majority of vaccines going to high-income countries, to the majority going to low-income countries,” Dr. Ghebreyesus said, asking leaders of high-income countries to wait on distributing booster doses until at least 10% of the world’s population is vaccinated.
“To make that happen, we need everyone’s cooperation, especially the handful of countries and companies that control the global supply of vaccines,” he said.
A version of this article first appeared on WebMD.com.
Will the Delta variant peak and then burn out?
When the Delta variant of the coronavirus was first identified in India in December 2020, the threat may have seemed too remote to trigger worry in the United States, although the horror of it ripping through the country was soon hard to ignore.
Within months, the Delta variant had spread to more than 98 countries, including Scotland, the United Kingdom, Israel, and now, of course, the United States. The CDC said this week the Delta variant now accounts for 93% of all COVID cases.
Fueled by Delta, COVID-19 cases, hospitalizations, and deaths are increasing in nearly all states, according to the latest CDC data. After the 7-day average number of cases dipped by June 22 to about 11,000, it rose by Aug. 3 to more than 85,000.
Some experts are heartened by the recent decrease in COVID-19 cases in the United Kingdom and India, both hard-hit with the Delta variant. COVID-19 cases in India peaked at more than 400,000 a day in May; by Aug. 2, that had dropped to about 30,500 daily.
Andy Slavitt, former Biden White House senior adviser for COVID-19 response, tweeted July 26 that, if the Delta variant acted the same in the United Kingdom as in India, it would have a quick rise and a quick drop.
The prediction seems to have come true. As of Aug. 3, U.K. cases have dropped to 7,467, compared with more than 46,800 July 19.
So the question of the summer has become: “When will Delta burn out here?”
Like other pandemic predictions, these are all over the board. Here are five predictions about when COVID cases will peak, then fall. They range from less than 2 weeks to more than 2 months:
- Mid-August: Among the most optimistic predictions of when the Delta-driven COVID-19 cases will decline is from Scott Gottlieb, MD, former FDA director. He told CNBC on July 28 that he would expect cases to decline in 2-3 weeks – so by August 11.
- Mid-August to mid-September: Ali Mokdad, PhD, chief strategy officer for population health at the University of Washington, Seattle, said that, “right now for the U.S. as a country, cases will peak mid-August” and then decline. He is citing projections by the university’s Institute for Health Metrics and Evaluation. In its “most likely” scenario, it predicts COVID deaths will peak at about 1,000 daily by mid-September, then decline. (As of Aug. 3, daily deaths averaged 371.)
- September: “I am hoping we get over this Delta hump [by then],” says Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape. “But sometimes, I am too much of an optimist.”
- Mid-October: Experts at the COVID-19 Scenario Modeling Hub, a consortium of researchers from leading institutions who consult with the CDC, said the Delta-fueled pandemic will steadily increase through summer and fall, with a mid-October peak.
- Unclear: Because cases are underestimated, “I think it is unclear when we will see a peak of Delta,” says Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore. He predicts a decline in cases as “more people get infected and develop natural immunity.”
The predictions are based on different scenarios, such as most likely or worst case. Factors such as personal behaviors, public mandates, and vaccination rates could all alter the projections.
What a difference vaccination may make
An uptick in vaccinations could change all the models and predictions, experts agree. As of Aug. 3, almost half (49.7%) of the total U.S. population was fully vaccinated, the CDC said. (And 80.1% of those 65 and over were.)
But that’s a long way from the 70% or 80% figure often cited to reach herd immunity. Recently, Ricardo Franco, MD, of the University of Alabama at Birmingham, said at a briefing by the Infectious Diseases Society of America that the infectiousness of the Delta variant may mean the herd immunity threshold is actually closer to 90%.
Dr. Mokdad estimates that by Nov. 1, based on the current rate of infections, 64% of people in the United States will be immune to a variant like Delta, taking into account those already infected and those vaccinated against COVID-19.
Justin Lessler, PhD, a University of North Carolina at Chapel Hill epidemiologist involved in the modeling hub, says if enough people get vaccinated, it could stop the Delta variant in its tracks. But that percentage is high.
“I am relatively confident that if we could get 90% or more of the eligible population vaccinated that we would see the epidemic begin to recede,” he says.
It’s a huge leap from 50%, or even 64%, to 90%. Could the Delta surge really motivate that many people to head to a vaccination site?
That’s hard to predict, Dr. Topol said. Some unvaccinated people may feel like soldiers in a foxhole, especially if they are in hard-hit states like Louisiana, and rush to get the vaccine as soon as possible. Others, hearing about the “breakthrough” cases in the vaccinated, may dig in their heels and ask: “Why bother?” as they mistakenly conclude that the vaccine has not done its job.
Roles of public policy, individual behavior
Besides an increase in vaccinations, individual behaviors and mandates can change the scenario. Doctors can remind even vaccinated patients that behaviors such as social distancing and masks still matter, experts said.
“Don’t ‘stress test’ your vaccine, “ Dr. Topol said.
The vaccines against COVID are good but not perfect and, he notes, they offer less protection if many months have passed since the vaccines were given.
The best advice now, Dr. Topol said, is: “Don’t be inside without a mask.”
Even if outdoors, depending on how close others are and the level of the conversation, a mask might be wise, he says.
Dr. Mokdad finds that “when cases go up, people put on their best behavior,” such as going back to masks and social distancing.
“Unfortunately, we have two countries,” he said, referring to the way public health measures and mandates vary from state to state.
Once the Delta variant subsides, what’s next?
It’s not a matter of if there is another variant on the heels of Delta, but when, Dr. Topol and other experts said. A new variant, Lambda, was first identified in Peru in August 2020 but now makes up about 90% of the country’s infections.
There’s also Delta-plus, just found in two people in South Korea.
Future variants could be even more transmissible than Delta, “which would be a horror show,” Dr. Topol said. “This [Delta] is by far the worst version. The virus is going to keep evolving. It is not done with us.”
On the horizon: Variant-proof vaccines
What’s needed to tackle the next variant is another approach to vaccine development, according to Dr. Topol and his colleague, Dennis R. Burton, a professor of immunology and microbiology at Scripps Research Institute.
Writing a commentary in Nature published in 2021, the two propose using a special class of protective antibodies, known as broadly neutralizing antibodies, to develop these vaccines. The success of the current COVID-19 vaccines is likely because of the vaccine’s ability to prompt the body to make protective neutralizing antibodies. These proteins bind to the viruses and prevent them from infecting the body’s cells.
The broadly neutralizing antibodies, however, can act against many different strains of related viruses, Dr. Topol and Mr. Burton wrote. Using this approach, which is already under study, scientists could make vaccines that would be effective against a family of viruses. The goal: to stop future outbreaks from becoming epidemics and then pandemics.
A version of this article first appeared on WebMD.com.
When the Delta variant of the coronavirus was first identified in India in December 2020, the threat may have seemed too remote to trigger worry in the United States, although the horror of it ripping through the country was soon hard to ignore.
Within months, the Delta variant had spread to more than 98 countries, including Scotland, the United Kingdom, Israel, and now, of course, the United States. The CDC said this week the Delta variant now accounts for 93% of all COVID cases.
Fueled by Delta, COVID-19 cases, hospitalizations, and deaths are increasing in nearly all states, according to the latest CDC data. After the 7-day average number of cases dipped by June 22 to about 11,000, it rose by Aug. 3 to more than 85,000.
Some experts are heartened by the recent decrease in COVID-19 cases in the United Kingdom and India, both hard-hit with the Delta variant. COVID-19 cases in India peaked at more than 400,000 a day in May; by Aug. 2, that had dropped to about 30,500 daily.
Andy Slavitt, former Biden White House senior adviser for COVID-19 response, tweeted July 26 that, if the Delta variant acted the same in the United Kingdom as in India, it would have a quick rise and a quick drop.
The prediction seems to have come true. As of Aug. 3, U.K. cases have dropped to 7,467, compared with more than 46,800 July 19.
So the question of the summer has become: “When will Delta burn out here?”
Like other pandemic predictions, these are all over the board. Here are five predictions about when COVID cases will peak, then fall. They range from less than 2 weeks to more than 2 months:
- Mid-August: Among the most optimistic predictions of when the Delta-driven COVID-19 cases will decline is from Scott Gottlieb, MD, former FDA director. He told CNBC on July 28 that he would expect cases to decline in 2-3 weeks – so by August 11.
- Mid-August to mid-September: Ali Mokdad, PhD, chief strategy officer for population health at the University of Washington, Seattle, said that, “right now for the U.S. as a country, cases will peak mid-August” and then decline. He is citing projections by the university’s Institute for Health Metrics and Evaluation. In its “most likely” scenario, it predicts COVID deaths will peak at about 1,000 daily by mid-September, then decline. (As of Aug. 3, daily deaths averaged 371.)
- September: “I am hoping we get over this Delta hump [by then],” says Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape. “But sometimes, I am too much of an optimist.”
- Mid-October: Experts at the COVID-19 Scenario Modeling Hub, a consortium of researchers from leading institutions who consult with the CDC, said the Delta-fueled pandemic will steadily increase through summer and fall, with a mid-October peak.
- Unclear: Because cases are underestimated, “I think it is unclear when we will see a peak of Delta,” says Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore. He predicts a decline in cases as “more people get infected and develop natural immunity.”
The predictions are based on different scenarios, such as most likely or worst case. Factors such as personal behaviors, public mandates, and vaccination rates could all alter the projections.
What a difference vaccination may make
An uptick in vaccinations could change all the models and predictions, experts agree. As of Aug. 3, almost half (49.7%) of the total U.S. population was fully vaccinated, the CDC said. (And 80.1% of those 65 and over were.)
But that’s a long way from the 70% or 80% figure often cited to reach herd immunity. Recently, Ricardo Franco, MD, of the University of Alabama at Birmingham, said at a briefing by the Infectious Diseases Society of America that the infectiousness of the Delta variant may mean the herd immunity threshold is actually closer to 90%.
Dr. Mokdad estimates that by Nov. 1, based on the current rate of infections, 64% of people in the United States will be immune to a variant like Delta, taking into account those already infected and those vaccinated against COVID-19.
Justin Lessler, PhD, a University of North Carolina at Chapel Hill epidemiologist involved in the modeling hub, says if enough people get vaccinated, it could stop the Delta variant in its tracks. But that percentage is high.
“I am relatively confident that if we could get 90% or more of the eligible population vaccinated that we would see the epidemic begin to recede,” he says.
It’s a huge leap from 50%, or even 64%, to 90%. Could the Delta surge really motivate that many people to head to a vaccination site?
That’s hard to predict, Dr. Topol said. Some unvaccinated people may feel like soldiers in a foxhole, especially if they are in hard-hit states like Louisiana, and rush to get the vaccine as soon as possible. Others, hearing about the “breakthrough” cases in the vaccinated, may dig in their heels and ask: “Why bother?” as they mistakenly conclude that the vaccine has not done its job.
Roles of public policy, individual behavior
Besides an increase in vaccinations, individual behaviors and mandates can change the scenario. Doctors can remind even vaccinated patients that behaviors such as social distancing and masks still matter, experts said.
“Don’t ‘stress test’ your vaccine, “ Dr. Topol said.
The vaccines against COVID are good but not perfect and, he notes, they offer less protection if many months have passed since the vaccines were given.
The best advice now, Dr. Topol said, is: “Don’t be inside without a mask.”
Even if outdoors, depending on how close others are and the level of the conversation, a mask might be wise, he says.
Dr. Mokdad finds that “when cases go up, people put on their best behavior,” such as going back to masks and social distancing.
“Unfortunately, we have two countries,” he said, referring to the way public health measures and mandates vary from state to state.
Once the Delta variant subsides, what’s next?
It’s not a matter of if there is another variant on the heels of Delta, but when, Dr. Topol and other experts said. A new variant, Lambda, was first identified in Peru in August 2020 but now makes up about 90% of the country’s infections.
There’s also Delta-plus, just found in two people in South Korea.
Future variants could be even more transmissible than Delta, “which would be a horror show,” Dr. Topol said. “This [Delta] is by far the worst version. The virus is going to keep evolving. It is not done with us.”
On the horizon: Variant-proof vaccines
What’s needed to tackle the next variant is another approach to vaccine development, according to Dr. Topol and his colleague, Dennis R. Burton, a professor of immunology and microbiology at Scripps Research Institute.
Writing a commentary in Nature published in 2021, the two propose using a special class of protective antibodies, known as broadly neutralizing antibodies, to develop these vaccines. The success of the current COVID-19 vaccines is likely because of the vaccine’s ability to prompt the body to make protective neutralizing antibodies. These proteins bind to the viruses and prevent them from infecting the body’s cells.
The broadly neutralizing antibodies, however, can act against many different strains of related viruses, Dr. Topol and Mr. Burton wrote. Using this approach, which is already under study, scientists could make vaccines that would be effective against a family of viruses. The goal: to stop future outbreaks from becoming epidemics and then pandemics.
A version of this article first appeared on WebMD.com.
When the Delta variant of the coronavirus was first identified in India in December 2020, the threat may have seemed too remote to trigger worry in the United States, although the horror of it ripping through the country was soon hard to ignore.
Within months, the Delta variant had spread to more than 98 countries, including Scotland, the United Kingdom, Israel, and now, of course, the United States. The CDC said this week the Delta variant now accounts for 93% of all COVID cases.
Fueled by Delta, COVID-19 cases, hospitalizations, and deaths are increasing in nearly all states, according to the latest CDC data. After the 7-day average number of cases dipped by June 22 to about 11,000, it rose by Aug. 3 to more than 85,000.
Some experts are heartened by the recent decrease in COVID-19 cases in the United Kingdom and India, both hard-hit with the Delta variant. COVID-19 cases in India peaked at more than 400,000 a day in May; by Aug. 2, that had dropped to about 30,500 daily.
Andy Slavitt, former Biden White House senior adviser for COVID-19 response, tweeted July 26 that, if the Delta variant acted the same in the United Kingdom as in India, it would have a quick rise and a quick drop.
The prediction seems to have come true. As of Aug. 3, U.K. cases have dropped to 7,467, compared with more than 46,800 July 19.
So the question of the summer has become: “When will Delta burn out here?”
Like other pandemic predictions, these are all over the board. Here are five predictions about when COVID cases will peak, then fall. They range from less than 2 weeks to more than 2 months:
- Mid-August: Among the most optimistic predictions of when the Delta-driven COVID-19 cases will decline is from Scott Gottlieb, MD, former FDA director. He told CNBC on July 28 that he would expect cases to decline in 2-3 weeks – so by August 11.
- Mid-August to mid-September: Ali Mokdad, PhD, chief strategy officer for population health at the University of Washington, Seattle, said that, “right now for the U.S. as a country, cases will peak mid-August” and then decline. He is citing projections by the university’s Institute for Health Metrics and Evaluation. In its “most likely” scenario, it predicts COVID deaths will peak at about 1,000 daily by mid-September, then decline. (As of Aug. 3, daily deaths averaged 371.)
- September: “I am hoping we get over this Delta hump [by then],” says Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape. “But sometimes, I am too much of an optimist.”
- Mid-October: Experts at the COVID-19 Scenario Modeling Hub, a consortium of researchers from leading institutions who consult with the CDC, said the Delta-fueled pandemic will steadily increase through summer and fall, with a mid-October peak.
- Unclear: Because cases are underestimated, “I think it is unclear when we will see a peak of Delta,” says Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore. He predicts a decline in cases as “more people get infected and develop natural immunity.”
The predictions are based on different scenarios, such as most likely or worst case. Factors such as personal behaviors, public mandates, and vaccination rates could all alter the projections.
What a difference vaccination may make
An uptick in vaccinations could change all the models and predictions, experts agree. As of Aug. 3, almost half (49.7%) of the total U.S. population was fully vaccinated, the CDC said. (And 80.1% of those 65 and over were.)
But that’s a long way from the 70% or 80% figure often cited to reach herd immunity. Recently, Ricardo Franco, MD, of the University of Alabama at Birmingham, said at a briefing by the Infectious Diseases Society of America that the infectiousness of the Delta variant may mean the herd immunity threshold is actually closer to 90%.
Dr. Mokdad estimates that by Nov. 1, based on the current rate of infections, 64% of people in the United States will be immune to a variant like Delta, taking into account those already infected and those vaccinated against COVID-19.
Justin Lessler, PhD, a University of North Carolina at Chapel Hill epidemiologist involved in the modeling hub, says if enough people get vaccinated, it could stop the Delta variant in its tracks. But that percentage is high.
“I am relatively confident that if we could get 90% or more of the eligible population vaccinated that we would see the epidemic begin to recede,” he says.
It’s a huge leap from 50%, or even 64%, to 90%. Could the Delta surge really motivate that many people to head to a vaccination site?
That’s hard to predict, Dr. Topol said. Some unvaccinated people may feel like soldiers in a foxhole, especially if they are in hard-hit states like Louisiana, and rush to get the vaccine as soon as possible. Others, hearing about the “breakthrough” cases in the vaccinated, may dig in their heels and ask: “Why bother?” as they mistakenly conclude that the vaccine has not done its job.
Roles of public policy, individual behavior
Besides an increase in vaccinations, individual behaviors and mandates can change the scenario. Doctors can remind even vaccinated patients that behaviors such as social distancing and masks still matter, experts said.
“Don’t ‘stress test’ your vaccine, “ Dr. Topol said.
The vaccines against COVID are good but not perfect and, he notes, they offer less protection if many months have passed since the vaccines were given.
The best advice now, Dr. Topol said, is: “Don’t be inside without a mask.”
Even if outdoors, depending on how close others are and the level of the conversation, a mask might be wise, he says.
Dr. Mokdad finds that “when cases go up, people put on their best behavior,” such as going back to masks and social distancing.
“Unfortunately, we have two countries,” he said, referring to the way public health measures and mandates vary from state to state.
Once the Delta variant subsides, what’s next?
It’s not a matter of if there is another variant on the heels of Delta, but when, Dr. Topol and other experts said. A new variant, Lambda, was first identified in Peru in August 2020 but now makes up about 90% of the country’s infections.
There’s also Delta-plus, just found in two people in South Korea.
Future variants could be even more transmissible than Delta, “which would be a horror show,” Dr. Topol said. “This [Delta] is by far the worst version. The virus is going to keep evolving. It is not done with us.”
On the horizon: Variant-proof vaccines
What’s needed to tackle the next variant is another approach to vaccine development, according to Dr. Topol and his colleague, Dennis R. Burton, a professor of immunology and microbiology at Scripps Research Institute.
Writing a commentary in Nature published in 2021, the two propose using a special class of protective antibodies, known as broadly neutralizing antibodies, to develop these vaccines. The success of the current COVID-19 vaccines is likely because of the vaccine’s ability to prompt the body to make protective neutralizing antibodies. These proteins bind to the viruses and prevent them from infecting the body’s cells.
The broadly neutralizing antibodies, however, can act against many different strains of related viruses, Dr. Topol and Mr. Burton wrote. Using this approach, which is already under study, scientists could make vaccines that would be effective against a family of viruses. The goal: to stop future outbreaks from becoming epidemics and then pandemics.
A version of this article first appeared on WebMD.com.
Analysis: Don’t want a vaccine? Be prepared to pay more for insurance
America’s COVID-19 vaccination rate is around 60% for ages 12 and up. That’s not enough to reach so-called herd immunity, and in states like Missouri – where a number of counties have vaccination rates under 25% – hospitals are overwhelmed by serious outbreaks of the more contagious delta variant.
The vaccine resisters offer all kinds of reasons for refusing the free shots and for ignoring efforts to nudge them to get inoculated. Campaigns urging Americans to get vaccinated for their health, for their grandparents, for their neighbors, or to get free doughnuts or a free joint haven’t done the trick. States have even held lotteries with a chance to win millions or a college scholarship.
And yet there are still huge numbers of unvaccinated people. Federal, state, and municipal governments as well as private businesses continue to largely avoid mandates for their employees out of fears they will provoke a backlash.
So, how about an economic argument? Get a COVID shot to protect your wallet.
Getting hospitalized with COVID in the United States typically generates huge bills. Those submitted by COVID patients to the NPR-Kaiser Health News “Bill of the Month” project include a $17,000 bill for a brief hospital stay in Marietta, Ga., (reduced to about $4,000 for an uninsured patient under a “charity care” policy); a $104,000 bill for a 14-day hospitalization in Miami for an uninsured man; and a bill for possibly hundreds of thousands for a 2-week hospital stay – some of it on a ventilator – for a foreign tourist in Hawaii whose travel health insurance contained a “pandemic exclusion.”
Even though insurance companies negotiate lower prices and cover much of the cost of care, an over-$1,000 out-of-pocket bill for a deductible – plus more for copays and possibly some out-of-network care – should be a pretty scary incentive.
In 2020, before COVID vaccines, most major private insurers waived patient payments – from coinsurance to deductibles – for COVID treatment. But many, if not most, have allowed that policy to lapse. Aetna, for example, ended that policy Feb. 28; UnitedHealthcare began rolling back its waivers late last year and ended them by the end of March.
More than 97% of hospitalized patients last month were unvaccinated. Though the vaccines will not necessarily prevent you from catching the coronavirus, they are highly effective at assuring you will have a milder case and are kept out of the hospital.
For this reason, there’s logic behind insurers’ waiver rollback: Why should patients be kept financially unharmed from what is now a preventable hospitalization, thanks to a vaccine that the government paid for and made available free of charge? It is now in many drugstores, it’s popping up at highway rest stops and bus stops, and it can be delivered and administered at home in parts of the country.
A harsher society might impose tough penalties on people who refuse vaccinations and contract the virus. Recently, the National Football League decreed that teams will forfeit a game canceled because of a COVID outbreak among unvaccinated players – and neither team’s players will be paid.
But insurers could try to do more, like penalizing the unvaccinated. And there is precedent. Already, some policies won’t cover treatment necessitated by what insurance companies deem risky behavior, such as scuba diving and rock climbing.
The Affordable Care Act allows insurers to charge smokers up to 50% more than what nonsmokers pay for some health plans. Four-fifths of states follow that protocol, though most employer-based plans do not do so. In 49 states, people caught driving without auto insurance face fines, confiscation of their car, loss of their license, and even jail. And reckless drivers pay more for insurance.
The logic behind the policies is that the offenders’ behavior can hurt others and costs society a lot of money. If a person decides not to get vaccinated and contracts a bad case of COVID, they are not only exposing others in their workplace or neighborhoods; the tens or hundreds of thousands spent on their care could mean higher premiums for others as well in their insurance plans next year. What’s more, outbreaks in low-vaccination regions could help breed more vaccine-resistant variants that affect everyone.
Yes, we often cover people whose habits may have contributed to their illness – insurance regularly pays for drug and alcohol rehab and cancer treatment for smokers.
That’s one reason, perhaps, that insurers too have so far favored carrots, not sticks, to get people vaccinated. Some private insurers are offering people who get vaccinated a credit toward their medical premiums, or gift cards and sweepstakes prizes, according to America’s Health Insurance Plans, an industry organization.
Tough love might be easier if the Food and Drug Administration gives vaccines full approval, rather than the current emergency use authorization. Even so, taxpayer-financed plans like Medicaid and Medicare must treat everyone the same and would encounter a lengthy process to secure federal waivers to experiment with incentives, according to Larry Levitt, executive vice president of Kaiser Family Foundation. These programs cannot charge different rates to different patients in a state.
KFF polling shows such incentives are of limited value, anyway. Many holdouts say they will be vaccinated only if required to do so by their employers.
But what if the financial cost of not getting vaccinated were just too high? If patients thought about the price they might need to pay for their own care, maybe they would reconsider remaining unprotected.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
America’s COVID-19 vaccination rate is around 60% for ages 12 and up. That’s not enough to reach so-called herd immunity, and in states like Missouri – where a number of counties have vaccination rates under 25% – hospitals are overwhelmed by serious outbreaks of the more contagious delta variant.
The vaccine resisters offer all kinds of reasons for refusing the free shots and for ignoring efforts to nudge them to get inoculated. Campaigns urging Americans to get vaccinated for their health, for their grandparents, for their neighbors, or to get free doughnuts or a free joint haven’t done the trick. States have even held lotteries with a chance to win millions or a college scholarship.
And yet there are still huge numbers of unvaccinated people. Federal, state, and municipal governments as well as private businesses continue to largely avoid mandates for their employees out of fears they will provoke a backlash.
So, how about an economic argument? Get a COVID shot to protect your wallet.
Getting hospitalized with COVID in the United States typically generates huge bills. Those submitted by COVID patients to the NPR-Kaiser Health News “Bill of the Month” project include a $17,000 bill for a brief hospital stay in Marietta, Ga., (reduced to about $4,000 for an uninsured patient under a “charity care” policy); a $104,000 bill for a 14-day hospitalization in Miami for an uninsured man; and a bill for possibly hundreds of thousands for a 2-week hospital stay – some of it on a ventilator – for a foreign tourist in Hawaii whose travel health insurance contained a “pandemic exclusion.”
Even though insurance companies negotiate lower prices and cover much of the cost of care, an over-$1,000 out-of-pocket bill for a deductible – plus more for copays and possibly some out-of-network care – should be a pretty scary incentive.
In 2020, before COVID vaccines, most major private insurers waived patient payments – from coinsurance to deductibles – for COVID treatment. But many, if not most, have allowed that policy to lapse. Aetna, for example, ended that policy Feb. 28; UnitedHealthcare began rolling back its waivers late last year and ended them by the end of March.
More than 97% of hospitalized patients last month were unvaccinated. Though the vaccines will not necessarily prevent you from catching the coronavirus, they are highly effective at assuring you will have a milder case and are kept out of the hospital.
For this reason, there’s logic behind insurers’ waiver rollback: Why should patients be kept financially unharmed from what is now a preventable hospitalization, thanks to a vaccine that the government paid for and made available free of charge? It is now in many drugstores, it’s popping up at highway rest stops and bus stops, and it can be delivered and administered at home in parts of the country.
A harsher society might impose tough penalties on people who refuse vaccinations and contract the virus. Recently, the National Football League decreed that teams will forfeit a game canceled because of a COVID outbreak among unvaccinated players – and neither team’s players will be paid.
But insurers could try to do more, like penalizing the unvaccinated. And there is precedent. Already, some policies won’t cover treatment necessitated by what insurance companies deem risky behavior, such as scuba diving and rock climbing.
The Affordable Care Act allows insurers to charge smokers up to 50% more than what nonsmokers pay for some health plans. Four-fifths of states follow that protocol, though most employer-based plans do not do so. In 49 states, people caught driving without auto insurance face fines, confiscation of their car, loss of their license, and even jail. And reckless drivers pay more for insurance.
The logic behind the policies is that the offenders’ behavior can hurt others and costs society a lot of money. If a person decides not to get vaccinated and contracts a bad case of COVID, they are not only exposing others in their workplace or neighborhoods; the tens or hundreds of thousands spent on their care could mean higher premiums for others as well in their insurance plans next year. What’s more, outbreaks in low-vaccination regions could help breed more vaccine-resistant variants that affect everyone.
Yes, we often cover people whose habits may have contributed to their illness – insurance regularly pays for drug and alcohol rehab and cancer treatment for smokers.
That’s one reason, perhaps, that insurers too have so far favored carrots, not sticks, to get people vaccinated. Some private insurers are offering people who get vaccinated a credit toward their medical premiums, or gift cards and sweepstakes prizes, according to America’s Health Insurance Plans, an industry organization.
Tough love might be easier if the Food and Drug Administration gives vaccines full approval, rather than the current emergency use authorization. Even so, taxpayer-financed plans like Medicaid and Medicare must treat everyone the same and would encounter a lengthy process to secure federal waivers to experiment with incentives, according to Larry Levitt, executive vice president of Kaiser Family Foundation. These programs cannot charge different rates to different patients in a state.
KFF polling shows such incentives are of limited value, anyway. Many holdouts say they will be vaccinated only if required to do so by their employers.
But what if the financial cost of not getting vaccinated were just too high? If patients thought about the price they might need to pay for their own care, maybe they would reconsider remaining unprotected.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
America’s COVID-19 vaccination rate is around 60% for ages 12 and up. That’s not enough to reach so-called herd immunity, and in states like Missouri – where a number of counties have vaccination rates under 25% – hospitals are overwhelmed by serious outbreaks of the more contagious delta variant.
The vaccine resisters offer all kinds of reasons for refusing the free shots and for ignoring efforts to nudge them to get inoculated. Campaigns urging Americans to get vaccinated for their health, for their grandparents, for their neighbors, or to get free doughnuts or a free joint haven’t done the trick. States have even held lotteries with a chance to win millions or a college scholarship.
And yet there are still huge numbers of unvaccinated people. Federal, state, and municipal governments as well as private businesses continue to largely avoid mandates for their employees out of fears they will provoke a backlash.
So, how about an economic argument? Get a COVID shot to protect your wallet.
Getting hospitalized with COVID in the United States typically generates huge bills. Those submitted by COVID patients to the NPR-Kaiser Health News “Bill of the Month” project include a $17,000 bill for a brief hospital stay in Marietta, Ga., (reduced to about $4,000 for an uninsured patient under a “charity care” policy); a $104,000 bill for a 14-day hospitalization in Miami for an uninsured man; and a bill for possibly hundreds of thousands for a 2-week hospital stay – some of it on a ventilator – for a foreign tourist in Hawaii whose travel health insurance contained a “pandemic exclusion.”
Even though insurance companies negotiate lower prices and cover much of the cost of care, an over-$1,000 out-of-pocket bill for a deductible – plus more for copays and possibly some out-of-network care – should be a pretty scary incentive.
In 2020, before COVID vaccines, most major private insurers waived patient payments – from coinsurance to deductibles – for COVID treatment. But many, if not most, have allowed that policy to lapse. Aetna, for example, ended that policy Feb. 28; UnitedHealthcare began rolling back its waivers late last year and ended them by the end of March.
More than 97% of hospitalized patients last month were unvaccinated. Though the vaccines will not necessarily prevent you from catching the coronavirus, they are highly effective at assuring you will have a milder case and are kept out of the hospital.
For this reason, there’s logic behind insurers’ waiver rollback: Why should patients be kept financially unharmed from what is now a preventable hospitalization, thanks to a vaccine that the government paid for and made available free of charge? It is now in many drugstores, it’s popping up at highway rest stops and bus stops, and it can be delivered and administered at home in parts of the country.
A harsher society might impose tough penalties on people who refuse vaccinations and contract the virus. Recently, the National Football League decreed that teams will forfeit a game canceled because of a COVID outbreak among unvaccinated players – and neither team’s players will be paid.
But insurers could try to do more, like penalizing the unvaccinated. And there is precedent. Already, some policies won’t cover treatment necessitated by what insurance companies deem risky behavior, such as scuba diving and rock climbing.
The Affordable Care Act allows insurers to charge smokers up to 50% more than what nonsmokers pay for some health plans. Four-fifths of states follow that protocol, though most employer-based plans do not do so. In 49 states, people caught driving without auto insurance face fines, confiscation of their car, loss of their license, and even jail. And reckless drivers pay more for insurance.
The logic behind the policies is that the offenders’ behavior can hurt others and costs society a lot of money. If a person decides not to get vaccinated and contracts a bad case of COVID, they are not only exposing others in their workplace or neighborhoods; the tens or hundreds of thousands spent on their care could mean higher premiums for others as well in their insurance plans next year. What’s more, outbreaks in low-vaccination regions could help breed more vaccine-resistant variants that affect everyone.
Yes, we often cover people whose habits may have contributed to their illness – insurance regularly pays for drug and alcohol rehab and cancer treatment for smokers.
That’s one reason, perhaps, that insurers too have so far favored carrots, not sticks, to get people vaccinated. Some private insurers are offering people who get vaccinated a credit toward their medical premiums, or gift cards and sweepstakes prizes, according to America’s Health Insurance Plans, an industry organization.
Tough love might be easier if the Food and Drug Administration gives vaccines full approval, rather than the current emergency use authorization. Even so, taxpayer-financed plans like Medicaid and Medicare must treat everyone the same and would encounter a lengthy process to secure federal waivers to experiment with incentives, according to Larry Levitt, executive vice president of Kaiser Family Foundation. These programs cannot charge different rates to different patients in a state.
KFF polling shows such incentives are of limited value, anyway. Many holdouts say they will be vaccinated only if required to do so by their employers.
But what if the financial cost of not getting vaccinated were just too high? If patients thought about the price they might need to pay for their own care, maybe they would reconsider remaining unprotected.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Summer campers spread COVID at home, follow-up finds
In a report published online in The New England Journal of Medicine, researchers found that campers spread COVID to household members after returning home – but transmission was more likely from some than others. Distancing and masking helped reduce the risk.
Victoria T. Chu, MD, MPH, with the Centers for Disease Control and Prevention, Atlanta, and colleagues with the agency and the Georgia Department of Health followed up with 224 camp attendees, aged 7 to 19 years, who had evidence of SARS-CoV-2 infection on laboratory testing.
These index patients – 88% of whom had symptoms – had 526 household contacts, mainly parents and siblings. Of 377 household contacts who underwent testing, 46 (12%) tested positive. Another two cases in household contacts were identified using clinical and epidemiologic criteria.
Family members hospitalized
Of the 41 adult household contacts who were infected, four (about 10%) were hospitalized. Their hospital stays ranged from 5 to 11 days. Of the seven infected household contacts who were younger than 18 years, none were hospitalized.
The four hospitalized adults were parents and grandparents aged 45 to 80 years, Dr. Chu said. Two of the four had underlying conditions. None of the household contacts died.
In an adjusted analysis, campers who had practiced physical distancing were less likely to transmit the virus at home, compared with those who had not practiced physical distancing (adjusted odds ratio, 0.4). Household members who had had close or direct contact with the index patients were more than 5 times more likely to become infected, compared with family members with minimal or no contact, analyses showed.
“This retrospective study showed that the efficient transmission of SARS-CoV-2 from school-age children and adolescents to household members led to the hospitalization of adults with secondary cases of COVID-19,” the researchers write. “In households in which transmission occurred, half the household contacts were infected.”
The secondary attack rates in this report may be an underestimate because testing was voluntary and participants reported the results themselves, the authors note. It is possible that infected household contacts spread the virus further, but this study did not address that question, Dr. Chu said.
For the study, investigators interviewed all camp attendees and their parents or guardians by phone between July 17, 2020 and Aug. 24, 2020, to collect information about demographic and clinical characteristics, SARS-CoV-2 testing, and preventive measures. The researchers’ analysis excluded households in which illness onset in a household contact occurred before or less than 2 days after a camper became sick.
About a third of the index patients began to have symptoms while still at camp. These campers may have been less infectious by the time they got home, compared with those whose symptoms started after they returned.
Two-thirds of the index patients adopted physical distancing at home, which “probably reduced the transmission of SARS-CoV-2 in the household,” Dr. Chu and colleagues wrote.
“Children who have had a known COVID-19 exposure should quarantine and obtain testing if they develop symptoms within the 14 days of returning home,” Dr. Chu advised. “If a child develops COVID-19, the child should be cared for and monitored using the proper combination of physical distancing, isolation when feasible, and mask use to prevent household transmission as much as possible. In addition, any person over the age of 12 is now eligible for vaccination in the United States. If eligible, children attending camp and their family members should get vaccinated to protect themselves and others, as vaccinations are our most effective public health prevention strategy.”
Mitigation can help
Another report regarding four overnight camps in Maine – in which three campers tested positive after they arrived last summer – shows that “aggressive mitigation strategies can be effective” in limiting transmission of the virus, William T. Basco Jr., MD, writes in a commentary for this news organization.
This summer, a range of factors, including vaccination rates at the camp, may influence transmission dynamics, Dr. Chu said in an interview. In July, the Associated Press reported outbreaks tied to summer camps in several states.
“Transmission dynamics will probably vary from summer camp to summer camp depending on many factors, such as vaccination rates of camp attendees, the mitigation measures in place, and the number of individual introductions during camp,” Dr. Chu said. “We would expect that a camp with a low vaccination rate among attendees and no enforcement of mitigation measures” still may experience a large outbreak.
“On the other hand, a large proportion of vaccinated individuals and appropriate implementation of multiple mitigation measures, such as wearing masks, may be quite effective at keeping their transmission rates low,” Dr. Chu added. “For camps with younger children who are not currently eligible for vaccination, implementing layered prevention strategies (e.g., mask use, physical distancing, and encouraging outdoor activities when feasible) is important to prevent transmission.”
Although COVID-19 transmission from children to adults, potentially leading to hospitalization, is not a new phenomenon, “data on the extent of transmission driven by children and adolescents in different settings are still quite sparse,” Dr. Chu said. “A better understanding of their impact on household and community transmission to help guide public health recommendations is particularly important, as most children are still not eligible for vaccination, and in-person schools will be reopening this fall.”
A version of this article first appeared on Medscape.com.
In a report published online in The New England Journal of Medicine, researchers found that campers spread COVID to household members after returning home – but transmission was more likely from some than others. Distancing and masking helped reduce the risk.
Victoria T. Chu, MD, MPH, with the Centers for Disease Control and Prevention, Atlanta, and colleagues with the agency and the Georgia Department of Health followed up with 224 camp attendees, aged 7 to 19 years, who had evidence of SARS-CoV-2 infection on laboratory testing.
These index patients – 88% of whom had symptoms – had 526 household contacts, mainly parents and siblings. Of 377 household contacts who underwent testing, 46 (12%) tested positive. Another two cases in household contacts were identified using clinical and epidemiologic criteria.
Family members hospitalized
Of the 41 adult household contacts who were infected, four (about 10%) were hospitalized. Their hospital stays ranged from 5 to 11 days. Of the seven infected household contacts who were younger than 18 years, none were hospitalized.
The four hospitalized adults were parents and grandparents aged 45 to 80 years, Dr. Chu said. Two of the four had underlying conditions. None of the household contacts died.
In an adjusted analysis, campers who had practiced physical distancing were less likely to transmit the virus at home, compared with those who had not practiced physical distancing (adjusted odds ratio, 0.4). Household members who had had close or direct contact with the index patients were more than 5 times more likely to become infected, compared with family members with minimal or no contact, analyses showed.
“This retrospective study showed that the efficient transmission of SARS-CoV-2 from school-age children and adolescents to household members led to the hospitalization of adults with secondary cases of COVID-19,” the researchers write. “In households in which transmission occurred, half the household contacts were infected.”
The secondary attack rates in this report may be an underestimate because testing was voluntary and participants reported the results themselves, the authors note. It is possible that infected household contacts spread the virus further, but this study did not address that question, Dr. Chu said.
For the study, investigators interviewed all camp attendees and their parents or guardians by phone between July 17, 2020 and Aug. 24, 2020, to collect information about demographic and clinical characteristics, SARS-CoV-2 testing, and preventive measures. The researchers’ analysis excluded households in which illness onset in a household contact occurred before or less than 2 days after a camper became sick.
About a third of the index patients began to have symptoms while still at camp. These campers may have been less infectious by the time they got home, compared with those whose symptoms started after they returned.
Two-thirds of the index patients adopted physical distancing at home, which “probably reduced the transmission of SARS-CoV-2 in the household,” Dr. Chu and colleagues wrote.
“Children who have had a known COVID-19 exposure should quarantine and obtain testing if they develop symptoms within the 14 days of returning home,” Dr. Chu advised. “If a child develops COVID-19, the child should be cared for and monitored using the proper combination of physical distancing, isolation when feasible, and mask use to prevent household transmission as much as possible. In addition, any person over the age of 12 is now eligible for vaccination in the United States. If eligible, children attending camp and their family members should get vaccinated to protect themselves and others, as vaccinations are our most effective public health prevention strategy.”
Mitigation can help
Another report regarding four overnight camps in Maine – in which three campers tested positive after they arrived last summer – shows that “aggressive mitigation strategies can be effective” in limiting transmission of the virus, William T. Basco Jr., MD, writes in a commentary for this news organization.
This summer, a range of factors, including vaccination rates at the camp, may influence transmission dynamics, Dr. Chu said in an interview. In July, the Associated Press reported outbreaks tied to summer camps in several states.
“Transmission dynamics will probably vary from summer camp to summer camp depending on many factors, such as vaccination rates of camp attendees, the mitigation measures in place, and the number of individual introductions during camp,” Dr. Chu said. “We would expect that a camp with a low vaccination rate among attendees and no enforcement of mitigation measures” still may experience a large outbreak.
“On the other hand, a large proportion of vaccinated individuals and appropriate implementation of multiple mitigation measures, such as wearing masks, may be quite effective at keeping their transmission rates low,” Dr. Chu added. “For camps with younger children who are not currently eligible for vaccination, implementing layered prevention strategies (e.g., mask use, physical distancing, and encouraging outdoor activities when feasible) is important to prevent transmission.”
Although COVID-19 transmission from children to adults, potentially leading to hospitalization, is not a new phenomenon, “data on the extent of transmission driven by children and adolescents in different settings are still quite sparse,” Dr. Chu said. “A better understanding of their impact on household and community transmission to help guide public health recommendations is particularly important, as most children are still not eligible for vaccination, and in-person schools will be reopening this fall.”
A version of this article first appeared on Medscape.com.
In a report published online in The New England Journal of Medicine, researchers found that campers spread COVID to household members after returning home – but transmission was more likely from some than others. Distancing and masking helped reduce the risk.
Victoria T. Chu, MD, MPH, with the Centers for Disease Control and Prevention, Atlanta, and colleagues with the agency and the Georgia Department of Health followed up with 224 camp attendees, aged 7 to 19 years, who had evidence of SARS-CoV-2 infection on laboratory testing.
These index patients – 88% of whom had symptoms – had 526 household contacts, mainly parents and siblings. Of 377 household contacts who underwent testing, 46 (12%) tested positive. Another two cases in household contacts were identified using clinical and epidemiologic criteria.
Family members hospitalized
Of the 41 adult household contacts who were infected, four (about 10%) were hospitalized. Their hospital stays ranged from 5 to 11 days. Of the seven infected household contacts who were younger than 18 years, none were hospitalized.
The four hospitalized adults were parents and grandparents aged 45 to 80 years, Dr. Chu said. Two of the four had underlying conditions. None of the household contacts died.
In an adjusted analysis, campers who had practiced physical distancing were less likely to transmit the virus at home, compared with those who had not practiced physical distancing (adjusted odds ratio, 0.4). Household members who had had close or direct contact with the index patients were more than 5 times more likely to become infected, compared with family members with minimal or no contact, analyses showed.
“This retrospective study showed that the efficient transmission of SARS-CoV-2 from school-age children and adolescents to household members led to the hospitalization of adults with secondary cases of COVID-19,” the researchers write. “In households in which transmission occurred, half the household contacts were infected.”
The secondary attack rates in this report may be an underestimate because testing was voluntary and participants reported the results themselves, the authors note. It is possible that infected household contacts spread the virus further, but this study did not address that question, Dr. Chu said.
For the study, investigators interviewed all camp attendees and their parents or guardians by phone between July 17, 2020 and Aug. 24, 2020, to collect information about demographic and clinical characteristics, SARS-CoV-2 testing, and preventive measures. The researchers’ analysis excluded households in which illness onset in a household contact occurred before or less than 2 days after a camper became sick.
About a third of the index patients began to have symptoms while still at camp. These campers may have been less infectious by the time they got home, compared with those whose symptoms started after they returned.
Two-thirds of the index patients adopted physical distancing at home, which “probably reduced the transmission of SARS-CoV-2 in the household,” Dr. Chu and colleagues wrote.
“Children who have had a known COVID-19 exposure should quarantine and obtain testing if they develop symptoms within the 14 days of returning home,” Dr. Chu advised. “If a child develops COVID-19, the child should be cared for and monitored using the proper combination of physical distancing, isolation when feasible, and mask use to prevent household transmission as much as possible. In addition, any person over the age of 12 is now eligible for vaccination in the United States. If eligible, children attending camp and their family members should get vaccinated to protect themselves and others, as vaccinations are our most effective public health prevention strategy.”
Mitigation can help
Another report regarding four overnight camps in Maine – in which three campers tested positive after they arrived last summer – shows that “aggressive mitigation strategies can be effective” in limiting transmission of the virus, William T. Basco Jr., MD, writes in a commentary for this news organization.
This summer, a range of factors, including vaccination rates at the camp, may influence transmission dynamics, Dr. Chu said in an interview. In July, the Associated Press reported outbreaks tied to summer camps in several states.
“Transmission dynamics will probably vary from summer camp to summer camp depending on many factors, such as vaccination rates of camp attendees, the mitigation measures in place, and the number of individual introductions during camp,” Dr. Chu said. “We would expect that a camp with a low vaccination rate among attendees and no enforcement of mitigation measures” still may experience a large outbreak.
“On the other hand, a large proportion of vaccinated individuals and appropriate implementation of multiple mitigation measures, such as wearing masks, may be quite effective at keeping their transmission rates low,” Dr. Chu added. “For camps with younger children who are not currently eligible for vaccination, implementing layered prevention strategies (e.g., mask use, physical distancing, and encouraging outdoor activities when feasible) is important to prevent transmission.”
Although COVID-19 transmission from children to adults, potentially leading to hospitalization, is not a new phenomenon, “data on the extent of transmission driven by children and adolescents in different settings are still quite sparse,” Dr. Chu said. “A better understanding of their impact on household and community transmission to help guide public health recommendations is particularly important, as most children are still not eligible for vaccination, and in-person schools will be reopening this fall.”
A version of this article first appeared on Medscape.com.
Long COVID symptoms rare but real in some kids
School-aged children with SARS-CoV-2 infection had only a few mild symptoms and typically recovered in 6 days, with more than 98% recovering in 8 weeks, a large U.K. study of smartphone data reassuringly reports.
In a small proportion (4.4%), however, COVID-19 symptoms such as fatigue, headache, or loss of smell persisted beyond a month, highlighting the need for ongoing pediatric care, according to Erika Molteni, PhD, a research fellow at King’s College, London, and colleagues.
The results, published online in The Lancet Child & Adolescent Health, also indicated that some children who had non-COVID infections were also susceptible to prolonged symptoms. “Our data highlight that other illnesses, such as colds and flu, can also have prolonged symptoms in children and it is important to consider this when planning for pediatric health services during the pandemic and beyond,” Michael Absoud, PhD, a senior coauthor and a King’s College consultant and senior lecturer, said in a news release. “All children who have persistent symptoms – from any illness – need timely multidisciplinary support linked with education, to enable them to find their individual pathway to recovery.”
Using a “citizen science” approach, the study extracted data from a smartphone app for tracking COVID symptoms in the ZOE COVID Study. The researchers looked at 258,790 children aged 5-17 years whose details were reported by adult proxies such as parents and carers from March 24, 2020, to Feb. 22, 2021. Of these, 75,529 had undergone a valid SARS-CoV-2 test.
The study also assessed symptoms in a randomly selected, age- and sex-matched cohort of 1,734 children in the app database who tested negative for COVID-19 but may have had other illnesses such as colds or flu.
In the 1,734 children testing positive for COVID-19 (approximately 50% each boys and girls), the most common symptoms were headache (62.2%) and fatigue (55.0%). More than 10% of the entire cohort had underlying asthma, but other comorbidities were very rare.
To assess the effect of age, the children were assessed in two groups: 5-11 years (n = 588) and 12-17 years (n = 1,146).
While unable to cross-check app reporting against actual medical records, the study suggested that illness lasted longer in COVID-positive than COVID-negative children, with a median of 6 days (interquartile range, 3-11) versus 3 days (IQR, 2-7). Furthermore, illness duration was positively associated with age: older children (median, 7 days; IQR, 3-12) versus younger children (median, 5 days; IQR, 2-9).
In 77 (4.4%) of the 1,734 COVID-positive children, illness persisted for at least 28 days, again more often in older than younger children: 5.1% of older children versus 3.1% of younger children (P = .046).
In addition, those with COVID-19 were more likely than children with non-COVID illness to be sick for more than 4 weeks: 4.4% versus 0.9%. At 4 weeks, however, the few children with other illnesses tended to have more symptoms, exhibiting a median of five symptoms versus two symptoms in the COVID-positive group.
“I tend to agree with the U.K. findings. COVID-19 in most school-age children is asymptomatic or a brief, self-limiting illness,” Sindhu Mohandas, MD, a pediatric infectious disease specialist at the Children’s Hospital Los Angeles, said in an interview. “The few children who need hospitalization have also mostly fully recovered by the time they are seen for their first outpatient clinic follow-up visit.”
Dr. Mohandas, who was not involved in the U.K. study, added that in her experience a small percentage, particularly adolescents, have some lingering symptoms after infection including fatigue, loss of appetite, and changes in smell and taste. “Identifying children with persistent illness and providing support and multidisciplinary care based on their symptomatology can make a positive impact on patients and their families.”
Recent research has suggested that long symptoms can persist for 3 months in 6% of children with COVID-19. And data from China have indicated that the prevalence of coinfection may be higher than in older patients.
In an accompanying comment, Dana Mahr, PhD, and Bruno J. Strasser, PhD, researchers in the faculty of science at the University of Geneva, said the app-based study “illustrates the potential and challenges of what has been called citizenship science,” in which projects rely on data input from nonscientists.
But while potentially democratizing participation in medical research, this subjective approach has the inherent bias of self-reporting (and in the case of the current study, proxy reporting), and can introduce potential conflicts of interest owing to the politicization of certain diseases.
In the case of the current study, Dr. Mahr and Dr. Strasser argued that, since the COVID-19 test result is known to participants, a pediatrician using objective criteria is better positioned to control for reporting biases than a parent asking a child about symptoms. “Entering data on a smartphone app is not equivalent to discussing with a pediatrician or health care worker who can answer further questions and concerns of participants, an especially important factor for underserved communities,” they wrote. “Citizen science will continue to require a close interaction with professional medical researchers to turn unique illness experiences into research data.”
This study was funded by Zoe Limited, the U.K. Government Department of Health and Social Care, Wellcome Trust, the U.K. Engineering and Physical Sciences Research Council, the U.K. Research and Innovation London Medical Imaging and Artificial Intelligence Centre for Value Based Healthcare, the U.K. National Institute for Health Research, the U.K. Medical Research Council, the British Heart Foundation, and the Alzheimer’s Society. Several study authors have disclosed support from various research-funding agencies and Zoe Limited supported all aspects of building and running the symptom-tracking application. Dr. Mahr and Dr. Strasser declared no competing interests. Dr. Mohandas disclosed no competing interests with regard to her comments.
School-aged children with SARS-CoV-2 infection had only a few mild symptoms and typically recovered in 6 days, with more than 98% recovering in 8 weeks, a large U.K. study of smartphone data reassuringly reports.
In a small proportion (4.4%), however, COVID-19 symptoms such as fatigue, headache, or loss of smell persisted beyond a month, highlighting the need for ongoing pediatric care, according to Erika Molteni, PhD, a research fellow at King’s College, London, and colleagues.
The results, published online in The Lancet Child & Adolescent Health, also indicated that some children who had non-COVID infections were also susceptible to prolonged symptoms. “Our data highlight that other illnesses, such as colds and flu, can also have prolonged symptoms in children and it is important to consider this when planning for pediatric health services during the pandemic and beyond,” Michael Absoud, PhD, a senior coauthor and a King’s College consultant and senior lecturer, said in a news release. “All children who have persistent symptoms – from any illness – need timely multidisciplinary support linked with education, to enable them to find their individual pathway to recovery.”
Using a “citizen science” approach, the study extracted data from a smartphone app for tracking COVID symptoms in the ZOE COVID Study. The researchers looked at 258,790 children aged 5-17 years whose details were reported by adult proxies such as parents and carers from March 24, 2020, to Feb. 22, 2021. Of these, 75,529 had undergone a valid SARS-CoV-2 test.
The study also assessed symptoms in a randomly selected, age- and sex-matched cohort of 1,734 children in the app database who tested negative for COVID-19 but may have had other illnesses such as colds or flu.
In the 1,734 children testing positive for COVID-19 (approximately 50% each boys and girls), the most common symptoms were headache (62.2%) and fatigue (55.0%). More than 10% of the entire cohort had underlying asthma, but other comorbidities were very rare.
To assess the effect of age, the children were assessed in two groups: 5-11 years (n = 588) and 12-17 years (n = 1,146).
While unable to cross-check app reporting against actual medical records, the study suggested that illness lasted longer in COVID-positive than COVID-negative children, with a median of 6 days (interquartile range, 3-11) versus 3 days (IQR, 2-7). Furthermore, illness duration was positively associated with age: older children (median, 7 days; IQR, 3-12) versus younger children (median, 5 days; IQR, 2-9).
In 77 (4.4%) of the 1,734 COVID-positive children, illness persisted for at least 28 days, again more often in older than younger children: 5.1% of older children versus 3.1% of younger children (P = .046).
In addition, those with COVID-19 were more likely than children with non-COVID illness to be sick for more than 4 weeks: 4.4% versus 0.9%. At 4 weeks, however, the few children with other illnesses tended to have more symptoms, exhibiting a median of five symptoms versus two symptoms in the COVID-positive group.
“I tend to agree with the U.K. findings. COVID-19 in most school-age children is asymptomatic or a brief, self-limiting illness,” Sindhu Mohandas, MD, a pediatric infectious disease specialist at the Children’s Hospital Los Angeles, said in an interview. “The few children who need hospitalization have also mostly fully recovered by the time they are seen for their first outpatient clinic follow-up visit.”
Dr. Mohandas, who was not involved in the U.K. study, added that in her experience a small percentage, particularly adolescents, have some lingering symptoms after infection including fatigue, loss of appetite, and changes in smell and taste. “Identifying children with persistent illness and providing support and multidisciplinary care based on their symptomatology can make a positive impact on patients and their families.”
Recent research has suggested that long symptoms can persist for 3 months in 6% of children with COVID-19. And data from China have indicated that the prevalence of coinfection may be higher than in older patients.
In an accompanying comment, Dana Mahr, PhD, and Bruno J. Strasser, PhD, researchers in the faculty of science at the University of Geneva, said the app-based study “illustrates the potential and challenges of what has been called citizenship science,” in which projects rely on data input from nonscientists.
But while potentially democratizing participation in medical research, this subjective approach has the inherent bias of self-reporting (and in the case of the current study, proxy reporting), and can introduce potential conflicts of interest owing to the politicization of certain diseases.
In the case of the current study, Dr. Mahr and Dr. Strasser argued that, since the COVID-19 test result is known to participants, a pediatrician using objective criteria is better positioned to control for reporting biases than a parent asking a child about symptoms. “Entering data on a smartphone app is not equivalent to discussing with a pediatrician or health care worker who can answer further questions and concerns of participants, an especially important factor for underserved communities,” they wrote. “Citizen science will continue to require a close interaction with professional medical researchers to turn unique illness experiences into research data.”
This study was funded by Zoe Limited, the U.K. Government Department of Health and Social Care, Wellcome Trust, the U.K. Engineering and Physical Sciences Research Council, the U.K. Research and Innovation London Medical Imaging and Artificial Intelligence Centre for Value Based Healthcare, the U.K. National Institute for Health Research, the U.K. Medical Research Council, the British Heart Foundation, and the Alzheimer’s Society. Several study authors have disclosed support from various research-funding agencies and Zoe Limited supported all aspects of building and running the symptom-tracking application. Dr. Mahr and Dr. Strasser declared no competing interests. Dr. Mohandas disclosed no competing interests with regard to her comments.
School-aged children with SARS-CoV-2 infection had only a few mild symptoms and typically recovered in 6 days, with more than 98% recovering in 8 weeks, a large U.K. study of smartphone data reassuringly reports.
In a small proportion (4.4%), however, COVID-19 symptoms such as fatigue, headache, or loss of smell persisted beyond a month, highlighting the need for ongoing pediatric care, according to Erika Molteni, PhD, a research fellow at King’s College, London, and colleagues.
The results, published online in The Lancet Child & Adolescent Health, also indicated that some children who had non-COVID infections were also susceptible to prolonged symptoms. “Our data highlight that other illnesses, such as colds and flu, can also have prolonged symptoms in children and it is important to consider this when planning for pediatric health services during the pandemic and beyond,” Michael Absoud, PhD, a senior coauthor and a King’s College consultant and senior lecturer, said in a news release. “All children who have persistent symptoms – from any illness – need timely multidisciplinary support linked with education, to enable them to find their individual pathway to recovery.”
Using a “citizen science” approach, the study extracted data from a smartphone app for tracking COVID symptoms in the ZOE COVID Study. The researchers looked at 258,790 children aged 5-17 years whose details were reported by adult proxies such as parents and carers from March 24, 2020, to Feb. 22, 2021. Of these, 75,529 had undergone a valid SARS-CoV-2 test.
The study also assessed symptoms in a randomly selected, age- and sex-matched cohort of 1,734 children in the app database who tested negative for COVID-19 but may have had other illnesses such as colds or flu.
In the 1,734 children testing positive for COVID-19 (approximately 50% each boys and girls), the most common symptoms were headache (62.2%) and fatigue (55.0%). More than 10% of the entire cohort had underlying asthma, but other comorbidities were very rare.
To assess the effect of age, the children were assessed in two groups: 5-11 years (n = 588) and 12-17 years (n = 1,146).
While unable to cross-check app reporting against actual medical records, the study suggested that illness lasted longer in COVID-positive than COVID-negative children, with a median of 6 days (interquartile range, 3-11) versus 3 days (IQR, 2-7). Furthermore, illness duration was positively associated with age: older children (median, 7 days; IQR, 3-12) versus younger children (median, 5 days; IQR, 2-9).
In 77 (4.4%) of the 1,734 COVID-positive children, illness persisted for at least 28 days, again more often in older than younger children: 5.1% of older children versus 3.1% of younger children (P = .046).
In addition, those with COVID-19 were more likely than children with non-COVID illness to be sick for more than 4 weeks: 4.4% versus 0.9%. At 4 weeks, however, the few children with other illnesses tended to have more symptoms, exhibiting a median of five symptoms versus two symptoms in the COVID-positive group.
“I tend to agree with the U.K. findings. COVID-19 in most school-age children is asymptomatic or a brief, self-limiting illness,” Sindhu Mohandas, MD, a pediatric infectious disease specialist at the Children’s Hospital Los Angeles, said in an interview. “The few children who need hospitalization have also mostly fully recovered by the time they are seen for their first outpatient clinic follow-up visit.”
Dr. Mohandas, who was not involved in the U.K. study, added that in her experience a small percentage, particularly adolescents, have some lingering symptoms after infection including fatigue, loss of appetite, and changes in smell and taste. “Identifying children with persistent illness and providing support and multidisciplinary care based on their symptomatology can make a positive impact on patients and their families.”
Recent research has suggested that long symptoms can persist for 3 months in 6% of children with COVID-19. And data from China have indicated that the prevalence of coinfection may be higher than in older patients.
In an accompanying comment, Dana Mahr, PhD, and Bruno J. Strasser, PhD, researchers in the faculty of science at the University of Geneva, said the app-based study “illustrates the potential and challenges of what has been called citizenship science,” in which projects rely on data input from nonscientists.
But while potentially democratizing participation in medical research, this subjective approach has the inherent bias of self-reporting (and in the case of the current study, proxy reporting), and can introduce potential conflicts of interest owing to the politicization of certain diseases.
In the case of the current study, Dr. Mahr and Dr. Strasser argued that, since the COVID-19 test result is known to participants, a pediatrician using objective criteria is better positioned to control for reporting biases than a parent asking a child about symptoms. “Entering data on a smartphone app is not equivalent to discussing with a pediatrician or health care worker who can answer further questions and concerns of participants, an especially important factor for underserved communities,” they wrote. “Citizen science will continue to require a close interaction with professional medical researchers to turn unique illness experiences into research data.”
This study was funded by Zoe Limited, the U.K. Government Department of Health and Social Care, Wellcome Trust, the U.K. Engineering and Physical Sciences Research Council, the U.K. Research and Innovation London Medical Imaging and Artificial Intelligence Centre for Value Based Healthcare, the U.K. National Institute for Health Research, the U.K. Medical Research Council, the British Heart Foundation, and the Alzheimer’s Society. Several study authors have disclosed support from various research-funding agencies and Zoe Limited supported all aspects of building and running the symptom-tracking application. Dr. Mahr and Dr. Strasser declared no competing interests. Dr. Mohandas disclosed no competing interests with regard to her comments.
FROM THE LANCET CHILD & ADOLESCENT HEALTH