User login
Children and COVID: Decline of summer surge continues
The continuing decline in COVID-19 incidence suggests the latest surge has peaked as new cases in children dropped for the 4th consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
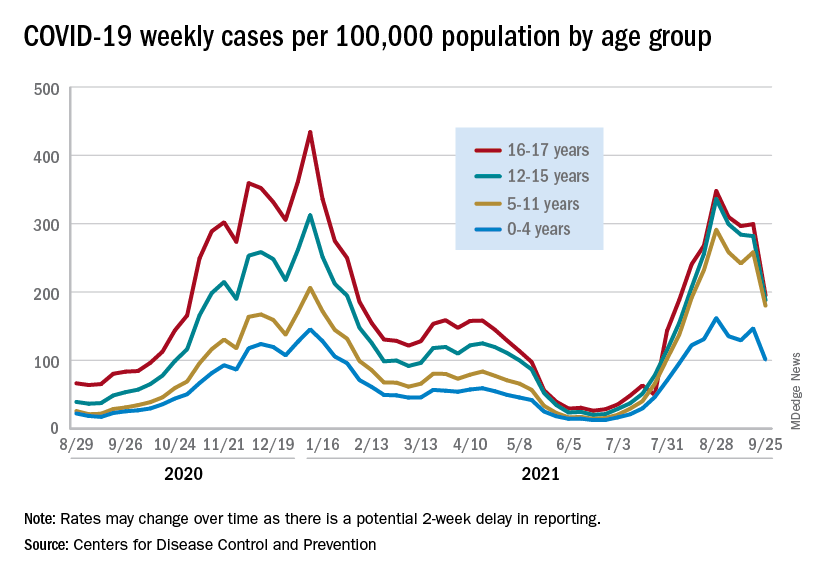
Preliminary data from the Centers for Disease Control and Prevention, however, show an uptick in new cases in late September, largely among younger children, that may indicate otherwise. Those data have a potential 2-week reporting delay, the CDC said on its COVID Data Tracker, so the most recent points on the graph (see above) could still go up.
. Those new cases made up almost 27% of all cases for the week, and the nearly 5.9 million child cases that have been reported since the start of the pandemic represent 16.2% of cases among Americans of all ages, the two groups said in their weekly COVID-19 report.
The CDC data on new cases by age group suggest that younger children have borne a heavier burden in the summer surge of COVID than they did last winter. The rate of new cases was not as high for 16- and 17-year-olds in the summer, but the other age groups all reached higher peaks than in the winter, including the 12- to 15-year-olds, who have been getting vaccinated since May, according to the COVID Data Tracker.
With vaccination approval getting closer for children under age 12 years, initiation in those already eligible continues to slide. Those aged 12-15 made up just 6.9% of new vaccinations during the 2 weeks from Sept. 21 to Oct. 4, and that figure has been dropping since July 13-26, when it was 14.1%. Vaccine initiation among 16- and 17-year-olds over that time has dropped by almost half, from 5.4% to 2.9%, the CDC data show.
All the vaccinations so far add up to this: Almost 55% of those aged 12-15 have gotten at least one dose of COVID vaccine, as have over 62% of those aged 16-17, and 52% of the older group is fully vaccinated, as is 44% of the younger group. Altogether, 10.8 million children were fully vaccinated as of Oct. 4, including those under 12 who may be participating in clinical trials or had a birth date entered incorrectly, the CDC said.
The continuing decline in COVID-19 incidence suggests the latest surge has peaked as new cases in children dropped for the 4th consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.

Preliminary data from the Centers for Disease Control and Prevention, however, show an uptick in new cases in late September, largely among younger children, that may indicate otherwise. Those data have a potential 2-week reporting delay, the CDC said on its COVID Data Tracker, so the most recent points on the graph (see above) could still go up.
. Those new cases made up almost 27% of all cases for the week, and the nearly 5.9 million child cases that have been reported since the start of the pandemic represent 16.2% of cases among Americans of all ages, the two groups said in their weekly COVID-19 report.
The CDC data on new cases by age group suggest that younger children have borne a heavier burden in the summer surge of COVID than they did last winter. The rate of new cases was not as high for 16- and 17-year-olds in the summer, but the other age groups all reached higher peaks than in the winter, including the 12- to 15-year-olds, who have been getting vaccinated since May, according to the COVID Data Tracker.
With vaccination approval getting closer for children under age 12 years, initiation in those already eligible continues to slide. Those aged 12-15 made up just 6.9% of new vaccinations during the 2 weeks from Sept. 21 to Oct. 4, and that figure has been dropping since July 13-26, when it was 14.1%. Vaccine initiation among 16- and 17-year-olds over that time has dropped by almost half, from 5.4% to 2.9%, the CDC data show.
All the vaccinations so far add up to this: Almost 55% of those aged 12-15 have gotten at least one dose of COVID vaccine, as have over 62% of those aged 16-17, and 52% of the older group is fully vaccinated, as is 44% of the younger group. Altogether, 10.8 million children were fully vaccinated as of Oct. 4, including those under 12 who may be participating in clinical trials or had a birth date entered incorrectly, the CDC said.
The continuing decline in COVID-19 incidence suggests the latest surge has peaked as new cases in children dropped for the 4th consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.

Preliminary data from the Centers for Disease Control and Prevention, however, show an uptick in new cases in late September, largely among younger children, that may indicate otherwise. Those data have a potential 2-week reporting delay, the CDC said on its COVID Data Tracker, so the most recent points on the graph (see above) could still go up.
. Those new cases made up almost 27% of all cases for the week, and the nearly 5.9 million child cases that have been reported since the start of the pandemic represent 16.2% of cases among Americans of all ages, the two groups said in their weekly COVID-19 report.
The CDC data on new cases by age group suggest that younger children have borne a heavier burden in the summer surge of COVID than they did last winter. The rate of new cases was not as high for 16- and 17-year-olds in the summer, but the other age groups all reached higher peaks than in the winter, including the 12- to 15-year-olds, who have been getting vaccinated since May, according to the COVID Data Tracker.
With vaccination approval getting closer for children under age 12 years, initiation in those already eligible continues to slide. Those aged 12-15 made up just 6.9% of new vaccinations during the 2 weeks from Sept. 21 to Oct. 4, and that figure has been dropping since July 13-26, when it was 14.1%. Vaccine initiation among 16- and 17-year-olds over that time has dropped by almost half, from 5.4% to 2.9%, the CDC data show.
All the vaccinations so far add up to this: Almost 55% of those aged 12-15 have gotten at least one dose of COVID vaccine, as have over 62% of those aged 16-17, and 52% of the older group is fully vaccinated, as is 44% of the younger group. Altogether, 10.8 million children were fully vaccinated as of Oct. 4, including those under 12 who may be participating in clinical trials or had a birth date entered incorrectly, the CDC said.
Johnson & Johnson requests FDA approval for vaccine booster doses
The company said it filed a request for people ages 18 and older who have received the one-shot vaccine. Johnson & Johnson submitted data for several different booster intervals -- ranging from 2 months to 6 months -- but didn’t formally recommend one to the FDA, The Associated Press reported.
“We’re describing the data to them,” Mathai Mammen, MD, head of global research and development for Janssen, the company’s vaccine division, told CNN.
“The process is not that we asked for a very specific interval -- we’re providing them data and we’re going to be presenting to the committee,” he said. “They’ll take all that into consideration when they ultimately decide on an appropriate interval.”
The FDA’s independent vaccine advisory committee meets next week to review data on booster shots from both Johnson & Johnson and Moderna. It’s the first step in the review process, which then requires approval from leaders at the FDA and Centers for Disease Control and Prevention. If both agencies authorize the extra shots, Americans could receive boosters from Johnson & Johnson and Moderna later this month, the AP reported.
Johnson & Johnson previously released data that showed the vaccine remains highly effective against COVID-19 at least 5 months after vaccination, with 81% efficacy against hospitalizations in the United States.
Two weeks ago, the company reported that a booster dose at 2 months or 6 months further lifted immunity, with a booster at 2 months providing 94% protection against moderate and severe COVID-19. The company said the 6-month booster raised antibodies by 12 times but didn’t release additional data at that time.
In September, the FDA authorized booster shots of the Pfizer vaccine for ages 65 and older, those who live in long-term care facilities, and those with higher risks for contracting COVID-19. The Biden administration is supporting a booster campaign to address potential waning vaccine immunity and remaining surges of the more contagious Delta variant, the AP reported.
A version of this article first appeared on WebMD.com.
The company said it filed a request for people ages 18 and older who have received the one-shot vaccine. Johnson & Johnson submitted data for several different booster intervals -- ranging from 2 months to 6 months -- but didn’t formally recommend one to the FDA, The Associated Press reported.
“We’re describing the data to them,” Mathai Mammen, MD, head of global research and development for Janssen, the company’s vaccine division, told CNN.
“The process is not that we asked for a very specific interval -- we’re providing them data and we’re going to be presenting to the committee,” he said. “They’ll take all that into consideration when they ultimately decide on an appropriate interval.”
The FDA’s independent vaccine advisory committee meets next week to review data on booster shots from both Johnson & Johnson and Moderna. It’s the first step in the review process, which then requires approval from leaders at the FDA and Centers for Disease Control and Prevention. If both agencies authorize the extra shots, Americans could receive boosters from Johnson & Johnson and Moderna later this month, the AP reported.
Johnson & Johnson previously released data that showed the vaccine remains highly effective against COVID-19 at least 5 months after vaccination, with 81% efficacy against hospitalizations in the United States.
Two weeks ago, the company reported that a booster dose at 2 months or 6 months further lifted immunity, with a booster at 2 months providing 94% protection against moderate and severe COVID-19. The company said the 6-month booster raised antibodies by 12 times but didn’t release additional data at that time.
In September, the FDA authorized booster shots of the Pfizer vaccine for ages 65 and older, those who live in long-term care facilities, and those with higher risks for contracting COVID-19. The Biden administration is supporting a booster campaign to address potential waning vaccine immunity and remaining surges of the more contagious Delta variant, the AP reported.
A version of this article first appeared on WebMD.com.
The company said it filed a request for people ages 18 and older who have received the one-shot vaccine. Johnson & Johnson submitted data for several different booster intervals -- ranging from 2 months to 6 months -- but didn’t formally recommend one to the FDA, The Associated Press reported.
“We’re describing the data to them,” Mathai Mammen, MD, head of global research and development for Janssen, the company’s vaccine division, told CNN.
“The process is not that we asked for a very specific interval -- we’re providing them data and we’re going to be presenting to the committee,” he said. “They’ll take all that into consideration when they ultimately decide on an appropriate interval.”
The FDA’s independent vaccine advisory committee meets next week to review data on booster shots from both Johnson & Johnson and Moderna. It’s the first step in the review process, which then requires approval from leaders at the FDA and Centers for Disease Control and Prevention. If both agencies authorize the extra shots, Americans could receive boosters from Johnson & Johnson and Moderna later this month, the AP reported.
Johnson & Johnson previously released data that showed the vaccine remains highly effective against COVID-19 at least 5 months after vaccination, with 81% efficacy against hospitalizations in the United States.
Two weeks ago, the company reported that a booster dose at 2 months or 6 months further lifted immunity, with a booster at 2 months providing 94% protection against moderate and severe COVID-19. The company said the 6-month booster raised antibodies by 12 times but didn’t release additional data at that time.
In September, the FDA authorized booster shots of the Pfizer vaccine for ages 65 and older, those who live in long-term care facilities, and those with higher risks for contracting COVID-19. The Biden administration is supporting a booster campaign to address potential waning vaccine immunity and remaining surges of the more contagious Delta variant, the AP reported.
A version of this article first appeared on WebMD.com.
Vaccine holdouts embrace COVID antibody treatment, mystifying doctors
Houston architect Lanson Jones is one of the nearly 80 million Americans who refuse to get a COVID-19 vaccine, arguing the shots are experimental, were rushed to market, may cause side effects, and aren’t all fully approved by federal officials.
But when he contracted COVID in September, he didn’t hesitate to seek treatment with monoclonal antibodies -- a year-old, laboratory-created therapy no less experimental than the vaccines that is not fully approved by the FDA and can also cause rare side effects.
“I haven’t done the shot because I hear a lot -- a lot -- of information about what are some of the effects of these vaccines and how it’s really not being reported, and I just felt I didn’t want to put something in me that has some question,” says Mr. Jones, 65.
“But with this monoclonal antibody treatment, I didn’t hesitate. I had no doubt in my mind -- not even one ounce of doubt about it. Not one person said, ‘Oh, well some people have had a reaction to it.’”
Mr. Jones, who was treated at Houston Methodist Hospital, is one of more than a million Americans who have received antibody IVs after getting the virus.
Those numbers are growing, with the federal government recently taking over distribution of the supplies of the drugs, which are limited in many states.
The treatment has been effective against COVID, in helping patients recover, stay out of the hospital, or die from the illness.
But what doctors and public health experts say is most surprising is that , as well.
“I think it’s irrational, quite frankly, if you have to boil it down to one word,” says Howard Huang, MD, who heads up Houston Methodist’s infusion program, which is providing up to 900 doses a week. “It really doesn’t make any sense on multiple levels.”
For one thing, he says, the FDA has just granted full approval for the COVID vaccine produced by Pfizer and BioNTech, upgrading its status from its emergency use authorization (EUA). Many experts expect the FDA to grant similar full approvals to the Moderna vaccine and possibly the Johnson and Johnson shot, which currently have EUA designations.
Many vaccine holdouts have cited the EUA status of the COVID vaccines -- one step shy of full approval -- as a reason they don’t trust the shot. But the antibody treatments have also been granted only EUA approval, which hasn’t stopped vaccine-resistant Americans from seeking them.
“So, they’re refusing an FDA-approved and tested [vaccine], and then they’re seeking something that’s still under an FDA EUA,” says Dr. Huang. “I just don’t get it. I really don’t.”
Amesh Adalja, MD, an emerging infectious diseases specialist with the Johns Hopkins University Center for Health Security, calls it “paradoxical” thinking for vaccine holdouts to refuse a shot that boosts your natural antibodies to prevent COVID, but take an antibody drug to treat it after infection.
“I don’t understand it, I can’t,” he says. “But the pandemic has been politicized and … I think consistency is not something to expect from people who are thinking about this irrationally [and] for people engaging in these conspiracies about the vaccine.
“I do think the fact that people like Joe Rogan and Gov. Abbot and Donald Trump received the monoclonal antibodies does probably play a role in some of the thinking in some of these individuals.”
Terry Scoggin, CEO of Titus Regional Medical Center in Mount Pleasant, Tex., says even the hospital’s doctors have been shocked by the demand for the new therapy among unvaccinated Texans.
“It’s mind-blowing that there’s been such resistance to the vaccine, but that demand for the monoclonal antibodies is so high,” he says, noting only 47% of adults in the region have received at least one dose of the shot. That’s far below CDC estimates that say 75.2% of American adults have received one shot, while 64.7% are fully vaccinated.
“But our doctors believe in the monoclonal antibodies, so it’s a trust factor -- they trust our community physicians,” Mr. Scoggin says. “I’ve never put the two and two together about the fear of the vaccine vs. [lack of fear] of the treatment. But it’s really interesting.”
Treatments effective, costly
Like the COVID vaccines given to nearly 214 million Americans, the antibody treatments taken by more than 1 million in the United States are highly effective and cause only rare (and usually minor) side effects.
Federal health officials say the infusions have helped keep the U.S. death toll -- now about 2,000 per day-- from soaring even higher, even as vaccine hesitancy persists, particularly in Southern states.
The FDA first authorized monoclonal antibody drugs in November 2020 -- just weeks before the vaccines were approved. But their popularity has soared as the Delta variant of the virus that causes COVID-19 has surged in recent months.
Clinical trials show that the drugs can cut COVID-related hospitalization or death in high-risk patients by as much as 70%-80%. They also can prevent infection in healthy people who have been exposed to an infected person, according to research published this month in The New England Journal of Medicine.
Monoclonal antibodies have been used for decades to treat cancer, autoimmune disorders, and other diseases, with the FDA approving nearly 100 such treatments since 1994.
The FDA has granted EUA approvals to four antibody treatments for COVID-19.
A two-antibody drug combination from Regeneron -- containing casirivimab and imdevimab -- has been shown to reduce the risk of hospitalization and death by 70% in people infected with COVID. Sotrovimab, made by GlaxoSmithKline and Vir, has had similar results.
The FDA approved a third treatment -- Eli Lilly’s combination of bamlanivimab and etesevimab -- in 2020, but the agency recommended against its use earlier this year after it proved ineffective against the Delta variant. The combination came back on the market in late August, but only in states where fewer than 5% of COVID infections are from strains, such as Delta, that are resistant to the treatment.
In June, the FDA authorized a fourth drug combination, Genentech’s tocilizumab, for people already hospitalized with COVID. But it is only moderately effective against the disease.
Lab-made monoclonal antibodies mimic the antibodies the body makes to fight viruses and illnesses. They work by targeting the spike protein on the surface of the virus. COVID vaccines work by priming the body’s immune system to recognize this very same spike protein and block it from entering your body’s cells, preventing infection.
Antibody treatments are given as an IV to treat an infection but can also be given as shots into the belly for people who have been exposed to the virus but have not yet been sickened by it, Dr. Huang says.
Timing is critical, he says, noting antibodies are most effective when given in the first few days after symptoms emerge.
Demands, concerns on the rise
Orders for monoclonal antibodies have skyrocketed in recent weeks -- to 168,000 doses per week in late August, up from 27,000 in July. The Biden administration, which has been covering the cost of the treatment for most patients, took over its distribution as well this week.
But experts foresee potential problems as patient demand increases.
Federal officials have already warned states of potential shortages ahead. Only about 2.4 million monoclonal antibody doses have been shipped nationally so far, less than half of which have been administered.
More supplies are on the way, with the federal government recently buying another 1.8 million doses for delivery in the months ahead. But for now, some hospitals are uncertain of supplies and are already struggling to meet the demand for the treatments.
Seven Southern states account for 70% of orders: Texas, Alabama, Florida, Mississippi, Tennessee, Georgia, and Louisiana. Those states have among the nation’s lowest vaccine rates and highest infection numbers.
Florida officials said the state’s latest weekly allotment left clinics 41,000 doses short of what they need. Tennessee has begun limiting treatments for unvaccinated patients to give priority to those most at risk of dying from COVID. And in Texas, elective surgeries have been postponed to make room for COVID-19 patients at some hospitals, as operating room nurses have been enlisted to give IVs.
Some strong proponents of monoclonal antibody treatments have been frustrated by Republican governors who are scrambling to push and deliver them, while opposing vaccine and mask mandates.
Raising vaccination rates, scientists say, would make the antibody treatments unnecessary in many cases.
Experts also note the drugs are far more costly than the vaccines -- with a price tag of about $2,100 for each IV, compared to $20-$40 for the shot.
“When you’re talking about just the cost to society as a whole -- turning down a [vaccine] that costs a couple dozen dollars for therapies that cost thousands of dollars -- it just doesn’t make any sense,” says Dr. Huang.
“And the tragedy is that a lot of these infections right now are preventable. It’s not like the pre-vaccine days, when we didn’t have anything better. And for these people, it’s just hard to justify that line of thinking. And so, the challenge is changing people’s minds. And that’s really been the difficult thing.”
In addition, the treatments take 90 minutes to administer, taxing health care workers in hard-hit states that have been slammed by the influx of patients.
Beyond these issues, Dr. Huang cites other public health costs of people choosing treatment over vaccination. The vaccine protects others because it limits transmission of the virus. By contrast, a single antibody IV helps only that patient and does not keep people from infecting others or becoming reinfected, requiring another IV.
“Getting the vaccine helps people beyond yourself; it helps the community, too,” he notes. “There’s just a strong argument for getting the vaccine. I obviously have a very biased opinion, but I would hope I have more of a scientific or expert opinion, but that doesn’t seem to matter these days.”
Vaccine resistance still remains for some
Seth Thurman, an IT technician from Mount Pleasant, Tex., acknowledges he was hesitant to get the vaccine at first because he felt it was fast-tracked, “experimental,” might cause unknown side effects, was developed quickly, and was being pushed by government officials.
“I shared the same sentiments as a lot of other people [as] some of the reasons why I might have been hesitant in the beginning to get the vaccine, says Mr. Thurman, 47. “A lot of people don’t trust what’s out there, maybe what the government is pushing, so I was taking a wait-and-see approach.”
In August, he relented and received the first of the two-shot Moderna vaccine. But several weeks later, he developed COVID and took his doctor’s advice to receive antibody therapy at Titus Regional Medical Center.
The results were almost immediate.
“I noticed within just a few hours of getting that infusion I was feeling better,” he says. “And by the next day, I was feeling great. No more temperature and no cough and no loss of taste and smell. And today, I’m 100%.”
Having had COVID convinced him of the importance of getting the vaccine, and he plans to get the second dose of the shot after the prescribed 90-day waiting period.
But Mr. Jones, the Houston architect, remains unconvinced, even after suffering what he describes as a “horrible” experience with COVID.
“It’s something I’m still thinking about,” he says of the vaccine. “But I can’t imagine that there wouldn’t be some sort of side effects from something that was developed so fast and had not gone through 4 or 5 years of vetting or trials. So that kind of just leaves doubt in my mind.
“And it’s just so weird that something so personal has become so public -- like people’s medical decisions now are on the front page of The New York Times. When did we think something like that would ever happen?”
The quick results of his treatment were so “remarkable” that he’d recommend it to anyone without hesitation, he says.
“If my story can help people be willing to seek out this infusion and take it early on in their COVID experience, I think it would not only save lives and keep people out of our hospitals and not overwhelm our hospital systems,” he says.
Dr. Huang agrees that the IV therapy is a great “fallback option” for people who’ve been infected, who have weakened immune systems, or can’t receive the vaccine for other health reasons. But for most people, he argues, the vaccine is the best way to go. That’s why Houston Methodist advises the shot for every patient like Mr. Jones, who’s been treated for COVID.
“Getting the vaccine is the way to go for the vast number of people,” he says.
Frederick Thurmond, MD, who oversees COVID-related care at Titus Regional Medical Center, believes it will take more than just doctors’ recommendations to move some patients to get the vaccine. The only thing that will motivate some will be contracting COVID, or knowing someone who does, he says.
“It’s clear that at least here in Texas, I swear man, you tell people they need to do something, and they just say, ‘Well, then I’m NOT going to do it,’” he says. “But once you’ve got COVID, the game becomes a whole lot more serious. And I think most people in the U.S. know someone who’s died from COVID at this point.”
Dr. Thurmond says that for some patients, stubborn resistance to legitimate medical advice persists -- on the vaccine and even treatment -- even after infection.
“We have seen more than one person avoid any medical care whatsoever after they knew they had COVID,” he says. “They languish in private and eventually come to the emergency room extremely sick and doing things with little to no medical value -- such as taking a friend’s hydroxychloroquine, random antibiotics, a horse de-worming dose of ivermectin, and gargling with Betadine and even bleach.”
But most of his patients who have the IV therapy take his advice to get the vaccine afterward.
“The only way to end the pandemic is to vaccinate everybody,” he says.
Dr. Adalja agrees.
“The monoclonal antibodies work, they are great drugs, so I think it is appropriate to praise them,” says Dr. Adalja, who’s given them to his own patients. “But it’s not appropriate to use them as an alternative to vaccination or to think, you know, don’t worry about the getting the vaccine because if you get infected and get the monoclonal antibodies to get through this -- that’s not the way to approach it.
He also worries about what he calls “dark-age mentalities” that have fueled the anti-vaccine movement, which has sought to heighten fears of modern medicine and doctors.
“The anti-vaccine movement has really capitalized on COVID-19, and it’s really a much more virulent form of the anti-vaccine movement than what we’ve seen with measles and other diseases in the past,” he notes. “And I think it’s going to be very difficult to contend with in the future, because no one thought we’d be battling the anti-vaccine movement this late in the pandemic.”
The biggest takeaway?
“When it comes to an infectious disease, prevention is always much better than treatment,” Dr. Adalja says. “If you don’t even need to get to the treatment stage because you prevent people from getting infected, that’s the goal.”
A version of this article first appeared on WebMD.com.
Houston architect Lanson Jones is one of the nearly 80 million Americans who refuse to get a COVID-19 vaccine, arguing the shots are experimental, were rushed to market, may cause side effects, and aren’t all fully approved by federal officials.
But when he contracted COVID in September, he didn’t hesitate to seek treatment with monoclonal antibodies -- a year-old, laboratory-created therapy no less experimental than the vaccines that is not fully approved by the FDA and can also cause rare side effects.
“I haven’t done the shot because I hear a lot -- a lot -- of information about what are some of the effects of these vaccines and how it’s really not being reported, and I just felt I didn’t want to put something in me that has some question,” says Mr. Jones, 65.
“But with this monoclonal antibody treatment, I didn’t hesitate. I had no doubt in my mind -- not even one ounce of doubt about it. Not one person said, ‘Oh, well some people have had a reaction to it.’”
Mr. Jones, who was treated at Houston Methodist Hospital, is one of more than a million Americans who have received antibody IVs after getting the virus.
Those numbers are growing, with the federal government recently taking over distribution of the supplies of the drugs, which are limited in many states.
The treatment has been effective against COVID, in helping patients recover, stay out of the hospital, or die from the illness.
But what doctors and public health experts say is most surprising is that , as well.
“I think it’s irrational, quite frankly, if you have to boil it down to one word,” says Howard Huang, MD, who heads up Houston Methodist’s infusion program, which is providing up to 900 doses a week. “It really doesn’t make any sense on multiple levels.”
For one thing, he says, the FDA has just granted full approval for the COVID vaccine produced by Pfizer and BioNTech, upgrading its status from its emergency use authorization (EUA). Many experts expect the FDA to grant similar full approvals to the Moderna vaccine and possibly the Johnson and Johnson shot, which currently have EUA designations.
Many vaccine holdouts have cited the EUA status of the COVID vaccines -- one step shy of full approval -- as a reason they don’t trust the shot. But the antibody treatments have also been granted only EUA approval, which hasn’t stopped vaccine-resistant Americans from seeking them.
“So, they’re refusing an FDA-approved and tested [vaccine], and then they’re seeking something that’s still under an FDA EUA,” says Dr. Huang. “I just don’t get it. I really don’t.”
Amesh Adalja, MD, an emerging infectious diseases specialist with the Johns Hopkins University Center for Health Security, calls it “paradoxical” thinking for vaccine holdouts to refuse a shot that boosts your natural antibodies to prevent COVID, but take an antibody drug to treat it after infection.
“I don’t understand it, I can’t,” he says. “But the pandemic has been politicized and … I think consistency is not something to expect from people who are thinking about this irrationally [and] for people engaging in these conspiracies about the vaccine.
“I do think the fact that people like Joe Rogan and Gov. Abbot and Donald Trump received the monoclonal antibodies does probably play a role in some of the thinking in some of these individuals.”
Terry Scoggin, CEO of Titus Regional Medical Center in Mount Pleasant, Tex., says even the hospital’s doctors have been shocked by the demand for the new therapy among unvaccinated Texans.
“It’s mind-blowing that there’s been such resistance to the vaccine, but that demand for the monoclonal antibodies is so high,” he says, noting only 47% of adults in the region have received at least one dose of the shot. That’s far below CDC estimates that say 75.2% of American adults have received one shot, while 64.7% are fully vaccinated.
“But our doctors believe in the monoclonal antibodies, so it’s a trust factor -- they trust our community physicians,” Mr. Scoggin says. “I’ve never put the two and two together about the fear of the vaccine vs. [lack of fear] of the treatment. But it’s really interesting.”
Treatments effective, costly
Like the COVID vaccines given to nearly 214 million Americans, the antibody treatments taken by more than 1 million in the United States are highly effective and cause only rare (and usually minor) side effects.
Federal health officials say the infusions have helped keep the U.S. death toll -- now about 2,000 per day-- from soaring even higher, even as vaccine hesitancy persists, particularly in Southern states.
The FDA first authorized monoclonal antibody drugs in November 2020 -- just weeks before the vaccines were approved. But their popularity has soared as the Delta variant of the virus that causes COVID-19 has surged in recent months.
Clinical trials show that the drugs can cut COVID-related hospitalization or death in high-risk patients by as much as 70%-80%. They also can prevent infection in healthy people who have been exposed to an infected person, according to research published this month in The New England Journal of Medicine.
Monoclonal antibodies have been used for decades to treat cancer, autoimmune disorders, and other diseases, with the FDA approving nearly 100 such treatments since 1994.
The FDA has granted EUA approvals to four antibody treatments for COVID-19.
A two-antibody drug combination from Regeneron -- containing casirivimab and imdevimab -- has been shown to reduce the risk of hospitalization and death by 70% in people infected with COVID. Sotrovimab, made by GlaxoSmithKline and Vir, has had similar results.
The FDA approved a third treatment -- Eli Lilly’s combination of bamlanivimab and etesevimab -- in 2020, but the agency recommended against its use earlier this year after it proved ineffective against the Delta variant. The combination came back on the market in late August, but only in states where fewer than 5% of COVID infections are from strains, such as Delta, that are resistant to the treatment.
In June, the FDA authorized a fourth drug combination, Genentech’s tocilizumab, for people already hospitalized with COVID. But it is only moderately effective against the disease.
Lab-made monoclonal antibodies mimic the antibodies the body makes to fight viruses and illnesses. They work by targeting the spike protein on the surface of the virus. COVID vaccines work by priming the body’s immune system to recognize this very same spike protein and block it from entering your body’s cells, preventing infection.
Antibody treatments are given as an IV to treat an infection but can also be given as shots into the belly for people who have been exposed to the virus but have not yet been sickened by it, Dr. Huang says.
Timing is critical, he says, noting antibodies are most effective when given in the first few days after symptoms emerge.
Demands, concerns on the rise
Orders for monoclonal antibodies have skyrocketed in recent weeks -- to 168,000 doses per week in late August, up from 27,000 in July. The Biden administration, which has been covering the cost of the treatment for most patients, took over its distribution as well this week.
But experts foresee potential problems as patient demand increases.
Federal officials have already warned states of potential shortages ahead. Only about 2.4 million monoclonal antibody doses have been shipped nationally so far, less than half of which have been administered.
More supplies are on the way, with the federal government recently buying another 1.8 million doses for delivery in the months ahead. But for now, some hospitals are uncertain of supplies and are already struggling to meet the demand for the treatments.
Seven Southern states account for 70% of orders: Texas, Alabama, Florida, Mississippi, Tennessee, Georgia, and Louisiana. Those states have among the nation’s lowest vaccine rates and highest infection numbers.
Florida officials said the state’s latest weekly allotment left clinics 41,000 doses short of what they need. Tennessee has begun limiting treatments for unvaccinated patients to give priority to those most at risk of dying from COVID. And in Texas, elective surgeries have been postponed to make room for COVID-19 patients at some hospitals, as operating room nurses have been enlisted to give IVs.
Some strong proponents of monoclonal antibody treatments have been frustrated by Republican governors who are scrambling to push and deliver them, while opposing vaccine and mask mandates.
Raising vaccination rates, scientists say, would make the antibody treatments unnecessary in many cases.
Experts also note the drugs are far more costly than the vaccines -- with a price tag of about $2,100 for each IV, compared to $20-$40 for the shot.
“When you’re talking about just the cost to society as a whole -- turning down a [vaccine] that costs a couple dozen dollars for therapies that cost thousands of dollars -- it just doesn’t make any sense,” says Dr. Huang.
“And the tragedy is that a lot of these infections right now are preventable. It’s not like the pre-vaccine days, when we didn’t have anything better. And for these people, it’s just hard to justify that line of thinking. And so, the challenge is changing people’s minds. And that’s really been the difficult thing.”
In addition, the treatments take 90 minutes to administer, taxing health care workers in hard-hit states that have been slammed by the influx of patients.
Beyond these issues, Dr. Huang cites other public health costs of people choosing treatment over vaccination. The vaccine protects others because it limits transmission of the virus. By contrast, a single antibody IV helps only that patient and does not keep people from infecting others or becoming reinfected, requiring another IV.
“Getting the vaccine helps people beyond yourself; it helps the community, too,” he notes. “There’s just a strong argument for getting the vaccine. I obviously have a very biased opinion, but I would hope I have more of a scientific or expert opinion, but that doesn’t seem to matter these days.”
Vaccine resistance still remains for some
Seth Thurman, an IT technician from Mount Pleasant, Tex., acknowledges he was hesitant to get the vaccine at first because he felt it was fast-tracked, “experimental,” might cause unknown side effects, was developed quickly, and was being pushed by government officials.
“I shared the same sentiments as a lot of other people [as] some of the reasons why I might have been hesitant in the beginning to get the vaccine, says Mr. Thurman, 47. “A lot of people don’t trust what’s out there, maybe what the government is pushing, so I was taking a wait-and-see approach.”
In August, he relented and received the first of the two-shot Moderna vaccine. But several weeks later, he developed COVID and took his doctor’s advice to receive antibody therapy at Titus Regional Medical Center.
The results were almost immediate.
“I noticed within just a few hours of getting that infusion I was feeling better,” he says. “And by the next day, I was feeling great. No more temperature and no cough and no loss of taste and smell. And today, I’m 100%.”
Having had COVID convinced him of the importance of getting the vaccine, and he plans to get the second dose of the shot after the prescribed 90-day waiting period.
But Mr. Jones, the Houston architect, remains unconvinced, even after suffering what he describes as a “horrible” experience with COVID.
“It’s something I’m still thinking about,” he says of the vaccine. “But I can’t imagine that there wouldn’t be some sort of side effects from something that was developed so fast and had not gone through 4 or 5 years of vetting or trials. So that kind of just leaves doubt in my mind.
“And it’s just so weird that something so personal has become so public -- like people’s medical decisions now are on the front page of The New York Times. When did we think something like that would ever happen?”
The quick results of his treatment were so “remarkable” that he’d recommend it to anyone without hesitation, he says.
“If my story can help people be willing to seek out this infusion and take it early on in their COVID experience, I think it would not only save lives and keep people out of our hospitals and not overwhelm our hospital systems,” he says.
Dr. Huang agrees that the IV therapy is a great “fallback option” for people who’ve been infected, who have weakened immune systems, or can’t receive the vaccine for other health reasons. But for most people, he argues, the vaccine is the best way to go. That’s why Houston Methodist advises the shot for every patient like Mr. Jones, who’s been treated for COVID.
“Getting the vaccine is the way to go for the vast number of people,” he says.
Frederick Thurmond, MD, who oversees COVID-related care at Titus Regional Medical Center, believes it will take more than just doctors’ recommendations to move some patients to get the vaccine. The only thing that will motivate some will be contracting COVID, or knowing someone who does, he says.
“It’s clear that at least here in Texas, I swear man, you tell people they need to do something, and they just say, ‘Well, then I’m NOT going to do it,’” he says. “But once you’ve got COVID, the game becomes a whole lot more serious. And I think most people in the U.S. know someone who’s died from COVID at this point.”
Dr. Thurmond says that for some patients, stubborn resistance to legitimate medical advice persists -- on the vaccine and even treatment -- even after infection.
“We have seen more than one person avoid any medical care whatsoever after they knew they had COVID,” he says. “They languish in private and eventually come to the emergency room extremely sick and doing things with little to no medical value -- such as taking a friend’s hydroxychloroquine, random antibiotics, a horse de-worming dose of ivermectin, and gargling with Betadine and even bleach.”
But most of his patients who have the IV therapy take his advice to get the vaccine afterward.
“The only way to end the pandemic is to vaccinate everybody,” he says.
Dr. Adalja agrees.
“The monoclonal antibodies work, they are great drugs, so I think it is appropriate to praise them,” says Dr. Adalja, who’s given them to his own patients. “But it’s not appropriate to use them as an alternative to vaccination or to think, you know, don’t worry about the getting the vaccine because if you get infected and get the monoclonal antibodies to get through this -- that’s not the way to approach it.
He also worries about what he calls “dark-age mentalities” that have fueled the anti-vaccine movement, which has sought to heighten fears of modern medicine and doctors.
“The anti-vaccine movement has really capitalized on COVID-19, and it’s really a much more virulent form of the anti-vaccine movement than what we’ve seen with measles and other diseases in the past,” he notes. “And I think it’s going to be very difficult to contend with in the future, because no one thought we’d be battling the anti-vaccine movement this late in the pandemic.”
The biggest takeaway?
“When it comes to an infectious disease, prevention is always much better than treatment,” Dr. Adalja says. “If you don’t even need to get to the treatment stage because you prevent people from getting infected, that’s the goal.”
A version of this article first appeared on WebMD.com.
Houston architect Lanson Jones is one of the nearly 80 million Americans who refuse to get a COVID-19 vaccine, arguing the shots are experimental, were rushed to market, may cause side effects, and aren’t all fully approved by federal officials.
But when he contracted COVID in September, he didn’t hesitate to seek treatment with monoclonal antibodies -- a year-old, laboratory-created therapy no less experimental than the vaccines that is not fully approved by the FDA and can also cause rare side effects.
“I haven’t done the shot because I hear a lot -- a lot -- of information about what are some of the effects of these vaccines and how it’s really not being reported, and I just felt I didn’t want to put something in me that has some question,” says Mr. Jones, 65.
“But with this monoclonal antibody treatment, I didn’t hesitate. I had no doubt in my mind -- not even one ounce of doubt about it. Not one person said, ‘Oh, well some people have had a reaction to it.’”
Mr. Jones, who was treated at Houston Methodist Hospital, is one of more than a million Americans who have received antibody IVs after getting the virus.
Those numbers are growing, with the federal government recently taking over distribution of the supplies of the drugs, which are limited in many states.
The treatment has been effective against COVID, in helping patients recover, stay out of the hospital, or die from the illness.
But what doctors and public health experts say is most surprising is that , as well.
“I think it’s irrational, quite frankly, if you have to boil it down to one word,” says Howard Huang, MD, who heads up Houston Methodist’s infusion program, which is providing up to 900 doses a week. “It really doesn’t make any sense on multiple levels.”
For one thing, he says, the FDA has just granted full approval for the COVID vaccine produced by Pfizer and BioNTech, upgrading its status from its emergency use authorization (EUA). Many experts expect the FDA to grant similar full approvals to the Moderna vaccine and possibly the Johnson and Johnson shot, which currently have EUA designations.
Many vaccine holdouts have cited the EUA status of the COVID vaccines -- one step shy of full approval -- as a reason they don’t trust the shot. But the antibody treatments have also been granted only EUA approval, which hasn’t stopped vaccine-resistant Americans from seeking them.
“So, they’re refusing an FDA-approved and tested [vaccine], and then they’re seeking something that’s still under an FDA EUA,” says Dr. Huang. “I just don’t get it. I really don’t.”
Amesh Adalja, MD, an emerging infectious diseases specialist with the Johns Hopkins University Center for Health Security, calls it “paradoxical” thinking for vaccine holdouts to refuse a shot that boosts your natural antibodies to prevent COVID, but take an antibody drug to treat it after infection.
“I don’t understand it, I can’t,” he says. “But the pandemic has been politicized and … I think consistency is not something to expect from people who are thinking about this irrationally [and] for people engaging in these conspiracies about the vaccine.
“I do think the fact that people like Joe Rogan and Gov. Abbot and Donald Trump received the monoclonal antibodies does probably play a role in some of the thinking in some of these individuals.”
Terry Scoggin, CEO of Titus Regional Medical Center in Mount Pleasant, Tex., says even the hospital’s doctors have been shocked by the demand for the new therapy among unvaccinated Texans.
“It’s mind-blowing that there’s been such resistance to the vaccine, but that demand for the monoclonal antibodies is so high,” he says, noting only 47% of adults in the region have received at least one dose of the shot. That’s far below CDC estimates that say 75.2% of American adults have received one shot, while 64.7% are fully vaccinated.
“But our doctors believe in the monoclonal antibodies, so it’s a trust factor -- they trust our community physicians,” Mr. Scoggin says. “I’ve never put the two and two together about the fear of the vaccine vs. [lack of fear] of the treatment. But it’s really interesting.”
Treatments effective, costly
Like the COVID vaccines given to nearly 214 million Americans, the antibody treatments taken by more than 1 million in the United States are highly effective and cause only rare (and usually minor) side effects.
Federal health officials say the infusions have helped keep the U.S. death toll -- now about 2,000 per day-- from soaring even higher, even as vaccine hesitancy persists, particularly in Southern states.
The FDA first authorized monoclonal antibody drugs in November 2020 -- just weeks before the vaccines were approved. But their popularity has soared as the Delta variant of the virus that causes COVID-19 has surged in recent months.
Clinical trials show that the drugs can cut COVID-related hospitalization or death in high-risk patients by as much as 70%-80%. They also can prevent infection in healthy people who have been exposed to an infected person, according to research published this month in The New England Journal of Medicine.
Monoclonal antibodies have been used for decades to treat cancer, autoimmune disorders, and other diseases, with the FDA approving nearly 100 such treatments since 1994.
The FDA has granted EUA approvals to four antibody treatments for COVID-19.
A two-antibody drug combination from Regeneron -- containing casirivimab and imdevimab -- has been shown to reduce the risk of hospitalization and death by 70% in people infected with COVID. Sotrovimab, made by GlaxoSmithKline and Vir, has had similar results.
The FDA approved a third treatment -- Eli Lilly’s combination of bamlanivimab and etesevimab -- in 2020, but the agency recommended against its use earlier this year after it proved ineffective against the Delta variant. The combination came back on the market in late August, but only in states where fewer than 5% of COVID infections are from strains, such as Delta, that are resistant to the treatment.
In June, the FDA authorized a fourth drug combination, Genentech’s tocilizumab, for people already hospitalized with COVID. But it is only moderately effective against the disease.
Lab-made monoclonal antibodies mimic the antibodies the body makes to fight viruses and illnesses. They work by targeting the spike protein on the surface of the virus. COVID vaccines work by priming the body’s immune system to recognize this very same spike protein and block it from entering your body’s cells, preventing infection.
Antibody treatments are given as an IV to treat an infection but can also be given as shots into the belly for people who have been exposed to the virus but have not yet been sickened by it, Dr. Huang says.
Timing is critical, he says, noting antibodies are most effective when given in the first few days after symptoms emerge.
Demands, concerns on the rise
Orders for monoclonal antibodies have skyrocketed in recent weeks -- to 168,000 doses per week in late August, up from 27,000 in July. The Biden administration, which has been covering the cost of the treatment for most patients, took over its distribution as well this week.
But experts foresee potential problems as patient demand increases.
Federal officials have already warned states of potential shortages ahead. Only about 2.4 million monoclonal antibody doses have been shipped nationally so far, less than half of which have been administered.
More supplies are on the way, with the federal government recently buying another 1.8 million doses for delivery in the months ahead. But for now, some hospitals are uncertain of supplies and are already struggling to meet the demand for the treatments.
Seven Southern states account for 70% of orders: Texas, Alabama, Florida, Mississippi, Tennessee, Georgia, and Louisiana. Those states have among the nation’s lowest vaccine rates and highest infection numbers.
Florida officials said the state’s latest weekly allotment left clinics 41,000 doses short of what they need. Tennessee has begun limiting treatments for unvaccinated patients to give priority to those most at risk of dying from COVID. And in Texas, elective surgeries have been postponed to make room for COVID-19 patients at some hospitals, as operating room nurses have been enlisted to give IVs.
Some strong proponents of monoclonal antibody treatments have been frustrated by Republican governors who are scrambling to push and deliver them, while opposing vaccine and mask mandates.
Raising vaccination rates, scientists say, would make the antibody treatments unnecessary in many cases.
Experts also note the drugs are far more costly than the vaccines -- with a price tag of about $2,100 for each IV, compared to $20-$40 for the shot.
“When you’re talking about just the cost to society as a whole -- turning down a [vaccine] that costs a couple dozen dollars for therapies that cost thousands of dollars -- it just doesn’t make any sense,” says Dr. Huang.
“And the tragedy is that a lot of these infections right now are preventable. It’s not like the pre-vaccine days, when we didn’t have anything better. And for these people, it’s just hard to justify that line of thinking. And so, the challenge is changing people’s minds. And that’s really been the difficult thing.”
In addition, the treatments take 90 minutes to administer, taxing health care workers in hard-hit states that have been slammed by the influx of patients.
Beyond these issues, Dr. Huang cites other public health costs of people choosing treatment over vaccination. The vaccine protects others because it limits transmission of the virus. By contrast, a single antibody IV helps only that patient and does not keep people from infecting others or becoming reinfected, requiring another IV.
“Getting the vaccine helps people beyond yourself; it helps the community, too,” he notes. “There’s just a strong argument for getting the vaccine. I obviously have a very biased opinion, but I would hope I have more of a scientific or expert opinion, but that doesn’t seem to matter these days.”
Vaccine resistance still remains for some
Seth Thurman, an IT technician from Mount Pleasant, Tex., acknowledges he was hesitant to get the vaccine at first because he felt it was fast-tracked, “experimental,” might cause unknown side effects, was developed quickly, and was being pushed by government officials.
“I shared the same sentiments as a lot of other people [as] some of the reasons why I might have been hesitant in the beginning to get the vaccine, says Mr. Thurman, 47. “A lot of people don’t trust what’s out there, maybe what the government is pushing, so I was taking a wait-and-see approach.”
In August, he relented and received the first of the two-shot Moderna vaccine. But several weeks later, he developed COVID and took his doctor’s advice to receive antibody therapy at Titus Regional Medical Center.
The results were almost immediate.
“I noticed within just a few hours of getting that infusion I was feeling better,” he says. “And by the next day, I was feeling great. No more temperature and no cough and no loss of taste and smell. And today, I’m 100%.”
Having had COVID convinced him of the importance of getting the vaccine, and he plans to get the second dose of the shot after the prescribed 90-day waiting period.
But Mr. Jones, the Houston architect, remains unconvinced, even after suffering what he describes as a “horrible” experience with COVID.
“It’s something I’m still thinking about,” he says of the vaccine. “But I can’t imagine that there wouldn’t be some sort of side effects from something that was developed so fast and had not gone through 4 or 5 years of vetting or trials. So that kind of just leaves doubt in my mind.
“And it’s just so weird that something so personal has become so public -- like people’s medical decisions now are on the front page of The New York Times. When did we think something like that would ever happen?”
The quick results of his treatment were so “remarkable” that he’d recommend it to anyone without hesitation, he says.
“If my story can help people be willing to seek out this infusion and take it early on in their COVID experience, I think it would not only save lives and keep people out of our hospitals and not overwhelm our hospital systems,” he says.
Dr. Huang agrees that the IV therapy is a great “fallback option” for people who’ve been infected, who have weakened immune systems, or can’t receive the vaccine for other health reasons. But for most people, he argues, the vaccine is the best way to go. That’s why Houston Methodist advises the shot for every patient like Mr. Jones, who’s been treated for COVID.
“Getting the vaccine is the way to go for the vast number of people,” he says.
Frederick Thurmond, MD, who oversees COVID-related care at Titus Regional Medical Center, believes it will take more than just doctors’ recommendations to move some patients to get the vaccine. The only thing that will motivate some will be contracting COVID, or knowing someone who does, he says.
“It’s clear that at least here in Texas, I swear man, you tell people they need to do something, and they just say, ‘Well, then I’m NOT going to do it,’” he says. “But once you’ve got COVID, the game becomes a whole lot more serious. And I think most people in the U.S. know someone who’s died from COVID at this point.”
Dr. Thurmond says that for some patients, stubborn resistance to legitimate medical advice persists -- on the vaccine and even treatment -- even after infection.
“We have seen more than one person avoid any medical care whatsoever after they knew they had COVID,” he says. “They languish in private and eventually come to the emergency room extremely sick and doing things with little to no medical value -- such as taking a friend’s hydroxychloroquine, random antibiotics, a horse de-worming dose of ivermectin, and gargling with Betadine and even bleach.”
But most of his patients who have the IV therapy take his advice to get the vaccine afterward.
“The only way to end the pandemic is to vaccinate everybody,” he says.
Dr. Adalja agrees.
“The monoclonal antibodies work, they are great drugs, so I think it is appropriate to praise them,” says Dr. Adalja, who’s given them to his own patients. “But it’s not appropriate to use them as an alternative to vaccination or to think, you know, don’t worry about the getting the vaccine because if you get infected and get the monoclonal antibodies to get through this -- that’s not the way to approach it.
He also worries about what he calls “dark-age mentalities” that have fueled the anti-vaccine movement, which has sought to heighten fears of modern medicine and doctors.
“The anti-vaccine movement has really capitalized on COVID-19, and it’s really a much more virulent form of the anti-vaccine movement than what we’ve seen with measles and other diseases in the past,” he notes. “And I think it’s going to be very difficult to contend with in the future, because no one thought we’d be battling the anti-vaccine movement this late in the pandemic.”
The biggest takeaway?
“When it comes to an infectious disease, prevention is always much better than treatment,” Dr. Adalja says. “If you don’t even need to get to the treatment stage because you prevent people from getting infected, that’s the goal.”
A version of this article first appeared on WebMD.com.
COVID-19: Two more cases of mucosal skin ulcers reported in male teens
Irish A similar case in an adolescent, also with ulcers affecting the mouth and penis, was reported earlier in 2021 in the United States.
“Our cases show that a swab for COVID-19 can be added to the list of investigations for mucosal and cutaneous rashes in children and probably adults,” said dermatologist Stephanie Bowe, MD, of South Infirmary-Victoria University Hospital in Cork, Ireland, in an interview. “Our patients seemed to improve with IV steroids, but there is not enough data to recommend them to all patients or for use in the different cutaneous presentations associated with COVID-19.”
The new case reports were presented at the 2021 meeting of the World Congress of Pediatric Dermatology and published in Pediatric Dermatology.
Researchers have noted that skin disorders linked to COVID-19 infection are different than those in adults. In children, the conditions include morbilliform rash, pernio-like acral lesions, urticaria, macular erythema, vesicular eruption, papulosquamous eruption, and retiform purpura. “The pathogenesis of each is not fully understood but likely related to the inflammatory response to COVID-19 and the various pathways within the body, which become activated,” Dr. Bowe said.
The first patient, a 17-year-old boy, presented at clinic 6 days after he’d been confirmed to be infected with COVID-19 and 8 days after developing fever and cough. “He had a 2-day history of conjunctivitis and ulceration of his oral mucosa, erythematous circumferential erosions of the glans penis with no other cutaneous findings,” the authors write in the report.
The boy “was distressed and embarrassed about his genital ulceration and also found eating very painful due to his oral ulceration,” Dr. Bowe said.
The second patient, a 14-year-old boy, was hospitalized 7 days after a positive COVID-19 test and 9 days after developing cough and fever. “He had a 5-day history of ulceration of the oral mucosa with mild conjunctivitis,” the authors wrote. “Ulceration of the glans penis developed on day 2 of admission.”
The 14-year-old was sicker than the 17-year-old boy, Dr. Bowe said. “He was unable to tolerate an oral diet for several days and had exquisite pain and vomiting with his coughing fits.”
This patient had a history of recurrent herpes labialis, but it’s unclear whether herpes simplex virus (HSV) played a role in the COVID-19–related case. “There is a possibility that the patient was more susceptible to viral cutaneous reactions during COVID-19 infection, but we didn’t have any definite history of HSV infection at the time of mucositis,” Dr. Bowe said. “We also didn’t have any swabs positive for HSV even though several were done at the time.”
Both patients received IV steroids – hydrocortisone at 100 mg 3 times daily for 3 days. This treatment was used “because of deterioration in symptoms and COVID-19 infection,” Dr. Bowe said. “IV steroids were used for respiratory symptoms of COVID-19, so we felt these cutaneous symptoms may have also been caused by an inflammatory response and might benefit from steroids. There was very little literature about this specific situation, though.”
She added that intravenous steroids wouldn’t be appropriate for most pediatric patients, and noted that “their use is controversial in the literature for erythema multiforme and RIME.”
In addition, the patients received betamethasone valerate 0.1% ointment once daily, hydrocortisone 2.5 mg buccal tablets 4 times daily, analgesia with acetaminophen and ibuprofen, and intravenous hydration. The first patient also received prednisolone 1% eye drops, while the second patient was given lidocaine hydrochloride mouthwash and total parenteral nutrition for 5 days.
The patients were discharged after 4 and 14 days, respectively.
Dermatologists in Massachusetts reported a similar case earlier in 2021 in a 17-year-old boy who was positive for COVID-19 and presented with “shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities.”
The patient received betamethasone valerate 0.1% ointment for the lips and penis, intraoral dexamethasone solution, viscous lidocaine, acetaminophen, and ibuprofen. He also received oral prednisone at approximately 1 mg/kg daily for 4 consecutive days after worsening oral pain. A recurrence of oral pain 3 months later was resolved with a higher and longer treatment with oral prednisone.
Dermatologists have also reported cases of erythema multiforme lesions of the mucosa in adults with COVID-19. One case was reported in Iran, and the other in France.
The authors report no study funding and disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Irish A similar case in an adolescent, also with ulcers affecting the mouth and penis, was reported earlier in 2021 in the United States.
“Our cases show that a swab for COVID-19 can be added to the list of investigations for mucosal and cutaneous rashes in children and probably adults,” said dermatologist Stephanie Bowe, MD, of South Infirmary-Victoria University Hospital in Cork, Ireland, in an interview. “Our patients seemed to improve with IV steroids, but there is not enough data to recommend them to all patients or for use in the different cutaneous presentations associated with COVID-19.”
The new case reports were presented at the 2021 meeting of the World Congress of Pediatric Dermatology and published in Pediatric Dermatology.
Researchers have noted that skin disorders linked to COVID-19 infection are different than those in adults. In children, the conditions include morbilliform rash, pernio-like acral lesions, urticaria, macular erythema, vesicular eruption, papulosquamous eruption, and retiform purpura. “The pathogenesis of each is not fully understood but likely related to the inflammatory response to COVID-19 and the various pathways within the body, which become activated,” Dr. Bowe said.
The first patient, a 17-year-old boy, presented at clinic 6 days after he’d been confirmed to be infected with COVID-19 and 8 days after developing fever and cough. “He had a 2-day history of conjunctivitis and ulceration of his oral mucosa, erythematous circumferential erosions of the glans penis with no other cutaneous findings,” the authors write in the report.
The boy “was distressed and embarrassed about his genital ulceration and also found eating very painful due to his oral ulceration,” Dr. Bowe said.
The second patient, a 14-year-old boy, was hospitalized 7 days after a positive COVID-19 test and 9 days after developing cough and fever. “He had a 5-day history of ulceration of the oral mucosa with mild conjunctivitis,” the authors wrote. “Ulceration of the glans penis developed on day 2 of admission.”
The 14-year-old was sicker than the 17-year-old boy, Dr. Bowe said. “He was unable to tolerate an oral diet for several days and had exquisite pain and vomiting with his coughing fits.”
This patient had a history of recurrent herpes labialis, but it’s unclear whether herpes simplex virus (HSV) played a role in the COVID-19–related case. “There is a possibility that the patient was more susceptible to viral cutaneous reactions during COVID-19 infection, but we didn’t have any definite history of HSV infection at the time of mucositis,” Dr. Bowe said. “We also didn’t have any swabs positive for HSV even though several were done at the time.”
Both patients received IV steroids – hydrocortisone at 100 mg 3 times daily for 3 days. This treatment was used “because of deterioration in symptoms and COVID-19 infection,” Dr. Bowe said. “IV steroids were used for respiratory symptoms of COVID-19, so we felt these cutaneous symptoms may have also been caused by an inflammatory response and might benefit from steroids. There was very little literature about this specific situation, though.”
She added that intravenous steroids wouldn’t be appropriate for most pediatric patients, and noted that “their use is controversial in the literature for erythema multiforme and RIME.”
In addition, the patients received betamethasone valerate 0.1% ointment once daily, hydrocortisone 2.5 mg buccal tablets 4 times daily, analgesia with acetaminophen and ibuprofen, and intravenous hydration. The first patient also received prednisolone 1% eye drops, while the second patient was given lidocaine hydrochloride mouthwash and total parenteral nutrition for 5 days.
The patients were discharged after 4 and 14 days, respectively.
Dermatologists in Massachusetts reported a similar case earlier in 2021 in a 17-year-old boy who was positive for COVID-19 and presented with “shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities.”
The patient received betamethasone valerate 0.1% ointment for the lips and penis, intraoral dexamethasone solution, viscous lidocaine, acetaminophen, and ibuprofen. He also received oral prednisone at approximately 1 mg/kg daily for 4 consecutive days after worsening oral pain. A recurrence of oral pain 3 months later was resolved with a higher and longer treatment with oral prednisone.
Dermatologists have also reported cases of erythema multiforme lesions of the mucosa in adults with COVID-19. One case was reported in Iran, and the other in France.
The authors report no study funding and disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Irish A similar case in an adolescent, also with ulcers affecting the mouth and penis, was reported earlier in 2021 in the United States.
“Our cases show that a swab for COVID-19 can be added to the list of investigations for mucosal and cutaneous rashes in children and probably adults,” said dermatologist Stephanie Bowe, MD, of South Infirmary-Victoria University Hospital in Cork, Ireland, in an interview. “Our patients seemed to improve with IV steroids, but there is not enough data to recommend them to all patients or for use in the different cutaneous presentations associated with COVID-19.”
The new case reports were presented at the 2021 meeting of the World Congress of Pediatric Dermatology and published in Pediatric Dermatology.
Researchers have noted that skin disorders linked to COVID-19 infection are different than those in adults. In children, the conditions include morbilliform rash, pernio-like acral lesions, urticaria, macular erythema, vesicular eruption, papulosquamous eruption, and retiform purpura. “The pathogenesis of each is not fully understood but likely related to the inflammatory response to COVID-19 and the various pathways within the body, which become activated,” Dr. Bowe said.
The first patient, a 17-year-old boy, presented at clinic 6 days after he’d been confirmed to be infected with COVID-19 and 8 days after developing fever and cough. “He had a 2-day history of conjunctivitis and ulceration of his oral mucosa, erythematous circumferential erosions of the glans penis with no other cutaneous findings,” the authors write in the report.
The boy “was distressed and embarrassed about his genital ulceration and also found eating very painful due to his oral ulceration,” Dr. Bowe said.
The second patient, a 14-year-old boy, was hospitalized 7 days after a positive COVID-19 test and 9 days after developing cough and fever. “He had a 5-day history of ulceration of the oral mucosa with mild conjunctivitis,” the authors wrote. “Ulceration of the glans penis developed on day 2 of admission.”
The 14-year-old was sicker than the 17-year-old boy, Dr. Bowe said. “He was unable to tolerate an oral diet for several days and had exquisite pain and vomiting with his coughing fits.”
This patient had a history of recurrent herpes labialis, but it’s unclear whether herpes simplex virus (HSV) played a role in the COVID-19–related case. “There is a possibility that the patient was more susceptible to viral cutaneous reactions during COVID-19 infection, but we didn’t have any definite history of HSV infection at the time of mucositis,” Dr. Bowe said. “We also didn’t have any swabs positive for HSV even though several were done at the time.”
Both patients received IV steroids – hydrocortisone at 100 mg 3 times daily for 3 days. This treatment was used “because of deterioration in symptoms and COVID-19 infection,” Dr. Bowe said. “IV steroids were used for respiratory symptoms of COVID-19, so we felt these cutaneous symptoms may have also been caused by an inflammatory response and might benefit from steroids. There was very little literature about this specific situation, though.”
She added that intravenous steroids wouldn’t be appropriate for most pediatric patients, and noted that “their use is controversial in the literature for erythema multiforme and RIME.”
In addition, the patients received betamethasone valerate 0.1% ointment once daily, hydrocortisone 2.5 mg buccal tablets 4 times daily, analgesia with acetaminophen and ibuprofen, and intravenous hydration. The first patient also received prednisolone 1% eye drops, while the second patient was given lidocaine hydrochloride mouthwash and total parenteral nutrition for 5 days.
The patients were discharged after 4 and 14 days, respectively.
Dermatologists in Massachusetts reported a similar case earlier in 2021 in a 17-year-old boy who was positive for COVID-19 and presented with “shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities.”
The patient received betamethasone valerate 0.1% ointment for the lips and penis, intraoral dexamethasone solution, viscous lidocaine, acetaminophen, and ibuprofen. He also received oral prednisone at approximately 1 mg/kg daily for 4 consecutive days after worsening oral pain. A recurrence of oral pain 3 months later was resolved with a higher and longer treatment with oral prednisone.
Dermatologists have also reported cases of erythema multiforme lesions of the mucosa in adults with COVID-19. One case was reported in Iran, and the other in France.
The authors report no study funding and disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pfizer COVID vaccine antibodies may disappear in 7 months, study says
, according to a new study published on the bioRxiv preprint server.
In the study, which hasn’t yet been peer-reviewed or formally published in a medical journal, researchers analyzed blood samples from 46 healthy young or middle-aged adults after receiving two doses, and then 6 months after the second dose.
“Our study shows vaccination with the Pfizer-BioNTech vaccine induces high levels of neutralizing antibodies against the original vaccine strain, but these levels drop by nearly 10-fold by 7 months,” the researchers told Reuters.
In about half of the adults, neutralizing antibodies were undetectable at 6 months after the second dose, particularly against coronavirus variants such as Delta, Beta, and Mu.
Neutralizing antibodies only make up part of the body’s immune defense against the virus, Reuters noted, but they are still “critically important” in protecting against coronavirus infections.
“These findings suggest that administering a booster dose at around 6 to 7 months following the initial immunization will likely enhance protection,” the study authors wrote.
BioNTech said a new vaccine formula will likely be needed by mid-2022 to protect against future mutations of the virus, according to the Financial Times.
“This year, [a different vaccine] is completely unneeded, but by mid-next year, it could be a different situation,” Ugur Sahin, MD, cofounder and CEO of BioNTech, told the news outlet.
Current variants, namely the Delta variant, are more contagious than the original coronavirus strain but not different enough to evade current vaccines, he said. But new strains may be able to evade boosters.
“This virus will stay, and the virus will further adapt,” Dr. Sahin said. “This is a continuous evolution, and that evolution has just started.”
A version of this article first appeared on WebMD.com.
, according to a new study published on the bioRxiv preprint server.
In the study, which hasn’t yet been peer-reviewed or formally published in a medical journal, researchers analyzed blood samples from 46 healthy young or middle-aged adults after receiving two doses, and then 6 months after the second dose.
“Our study shows vaccination with the Pfizer-BioNTech vaccine induces high levels of neutralizing antibodies against the original vaccine strain, but these levels drop by nearly 10-fold by 7 months,” the researchers told Reuters.
In about half of the adults, neutralizing antibodies were undetectable at 6 months after the second dose, particularly against coronavirus variants such as Delta, Beta, and Mu.
Neutralizing antibodies only make up part of the body’s immune defense against the virus, Reuters noted, but they are still “critically important” in protecting against coronavirus infections.
“These findings suggest that administering a booster dose at around 6 to 7 months following the initial immunization will likely enhance protection,” the study authors wrote.
BioNTech said a new vaccine formula will likely be needed by mid-2022 to protect against future mutations of the virus, according to the Financial Times.
“This year, [a different vaccine] is completely unneeded, but by mid-next year, it could be a different situation,” Ugur Sahin, MD, cofounder and CEO of BioNTech, told the news outlet.
Current variants, namely the Delta variant, are more contagious than the original coronavirus strain but not different enough to evade current vaccines, he said. But new strains may be able to evade boosters.
“This virus will stay, and the virus will further adapt,” Dr. Sahin said. “This is a continuous evolution, and that evolution has just started.”
A version of this article first appeared on WebMD.com.
, according to a new study published on the bioRxiv preprint server.
In the study, which hasn’t yet been peer-reviewed or formally published in a medical journal, researchers analyzed blood samples from 46 healthy young or middle-aged adults after receiving two doses, and then 6 months after the second dose.
“Our study shows vaccination with the Pfizer-BioNTech vaccine induces high levels of neutralizing antibodies against the original vaccine strain, but these levels drop by nearly 10-fold by 7 months,” the researchers told Reuters.
In about half of the adults, neutralizing antibodies were undetectable at 6 months after the second dose, particularly against coronavirus variants such as Delta, Beta, and Mu.
Neutralizing antibodies only make up part of the body’s immune defense against the virus, Reuters noted, but they are still “critically important” in protecting against coronavirus infections.
“These findings suggest that administering a booster dose at around 6 to 7 months following the initial immunization will likely enhance protection,” the study authors wrote.
BioNTech said a new vaccine formula will likely be needed by mid-2022 to protect against future mutations of the virus, according to the Financial Times.
“This year, [a different vaccine] is completely unneeded, but by mid-next year, it could be a different situation,” Ugur Sahin, MD, cofounder and CEO of BioNTech, told the news outlet.
Current variants, namely the Delta variant, are more contagious than the original coronavirus strain but not different enough to evade current vaccines, he said. But new strains may be able to evade boosters.
“This virus will stay, and the virus will further adapt,” Dr. Sahin said. “This is a continuous evolution, and that evolution has just started.”
A version of this article first appeared on WebMD.com.
Tolerance in medicine
There is a narrative being pushed now about health care professionals being “frustrated” and “tired” in the midst of this current delta COVID wave. This stems from the idea that this current wave was potentially preventable if everyone received the COVID vaccines when they were made available.
I certainly understand this frustration and am tired of dealing with COVID restrictions and wearing masks. Above all I’m tired of talking about it. But frustration and fatigue are nothing new for those in the health care profession. Part of our training is that we should care for everyone, no matter what. Compassion for the ill should not be restricted to patients with a certain financial status, immigration status, race, gender, sexual orientation, or education level. Socially and politically, we are having a reckoning with how we treat people and how we need to do better to create a more just society. A key virtue in all of this is tolerance.
If we are going to have a free society, tolerance is essential. This is because in a free society people are going to, well, be free. In medicine we tolerate people who are morbidly obese, drink alcohol excessively, smoke, refuse to take their medications, won’t exercise, won’t sleep, and do drugs. The overwhelming majority of these people know that what they are doing is bad for their health. Not only do we tolerate them, we are taught to treat them indiscriminately. When someone who is morbidly obese has a heart attack, we treat them, give them medicine, and tell them the importance of losing weight. We do not tell them, “you shouldn’t have eaten so much and gotten so fat,” or “don’t you wish you didn’t get so fat?”
What I am trying to circle back to here is that if you could force people into doing everything they could for their health and eliminate all “preventable” diseases, then the need for health care in this country – including doctors, nurses, hospitals, and pharmaceuticals, just to name a few – would be cut dramatically. While the frustration for the continued COVID surges is understandable, I urge people to remember that in the business of health care we deal with preventable diseases all the time, every day. We are taught to show compassion for everyone, and for good reason. We have no idea what many people’s backstories are, we just know that they are sick and need help.
I urge everyone to put the unvaccinated under the same umbrella you put other people with preventable diseases, which, sadly, is a lot of patients. Continue to educate those about the vaccine as you should about every other aspect of their health. Education is part of our job as health care professionals but judgment is not.
Dr. Matuszak works for Sound Physicians and is a nocturnist at a hospital in the San Francisco Bay Area.
There is a narrative being pushed now about health care professionals being “frustrated” and “tired” in the midst of this current delta COVID wave. This stems from the idea that this current wave was potentially preventable if everyone received the COVID vaccines when they were made available.
I certainly understand this frustration and am tired of dealing with COVID restrictions and wearing masks. Above all I’m tired of talking about it. But frustration and fatigue are nothing new for those in the health care profession. Part of our training is that we should care for everyone, no matter what. Compassion for the ill should not be restricted to patients with a certain financial status, immigration status, race, gender, sexual orientation, or education level. Socially and politically, we are having a reckoning with how we treat people and how we need to do better to create a more just society. A key virtue in all of this is tolerance.
If we are going to have a free society, tolerance is essential. This is because in a free society people are going to, well, be free. In medicine we tolerate people who are morbidly obese, drink alcohol excessively, smoke, refuse to take their medications, won’t exercise, won’t sleep, and do drugs. The overwhelming majority of these people know that what they are doing is bad for their health. Not only do we tolerate them, we are taught to treat them indiscriminately. When someone who is morbidly obese has a heart attack, we treat them, give them medicine, and tell them the importance of losing weight. We do not tell them, “you shouldn’t have eaten so much and gotten so fat,” or “don’t you wish you didn’t get so fat?”
What I am trying to circle back to here is that if you could force people into doing everything they could for their health and eliminate all “preventable” diseases, then the need for health care in this country – including doctors, nurses, hospitals, and pharmaceuticals, just to name a few – would be cut dramatically. While the frustration for the continued COVID surges is understandable, I urge people to remember that in the business of health care we deal with preventable diseases all the time, every day. We are taught to show compassion for everyone, and for good reason. We have no idea what many people’s backstories are, we just know that they are sick and need help.
I urge everyone to put the unvaccinated under the same umbrella you put other people with preventable diseases, which, sadly, is a lot of patients. Continue to educate those about the vaccine as you should about every other aspect of their health. Education is part of our job as health care professionals but judgment is not.
Dr. Matuszak works for Sound Physicians and is a nocturnist at a hospital in the San Francisco Bay Area.
There is a narrative being pushed now about health care professionals being “frustrated” and “tired” in the midst of this current delta COVID wave. This stems from the idea that this current wave was potentially preventable if everyone received the COVID vaccines when they were made available.
I certainly understand this frustration and am tired of dealing with COVID restrictions and wearing masks. Above all I’m tired of talking about it. But frustration and fatigue are nothing new for those in the health care profession. Part of our training is that we should care for everyone, no matter what. Compassion for the ill should not be restricted to patients with a certain financial status, immigration status, race, gender, sexual orientation, or education level. Socially and politically, we are having a reckoning with how we treat people and how we need to do better to create a more just society. A key virtue in all of this is tolerance.
If we are going to have a free society, tolerance is essential. This is because in a free society people are going to, well, be free. In medicine we tolerate people who are morbidly obese, drink alcohol excessively, smoke, refuse to take their medications, won’t exercise, won’t sleep, and do drugs. The overwhelming majority of these people know that what they are doing is bad for their health. Not only do we tolerate them, we are taught to treat them indiscriminately. When someone who is morbidly obese has a heart attack, we treat them, give them medicine, and tell them the importance of losing weight. We do not tell them, “you shouldn’t have eaten so much and gotten so fat,” or “don’t you wish you didn’t get so fat?”
What I am trying to circle back to here is that if you could force people into doing everything they could for their health and eliminate all “preventable” diseases, then the need for health care in this country – including doctors, nurses, hospitals, and pharmaceuticals, just to name a few – would be cut dramatically. While the frustration for the continued COVID surges is understandable, I urge people to remember that in the business of health care we deal with preventable diseases all the time, every day. We are taught to show compassion for everyone, and for good reason. We have no idea what many people’s backstories are, we just know that they are sick and need help.
I urge everyone to put the unvaccinated under the same umbrella you put other people with preventable diseases, which, sadly, is a lot of patients. Continue to educate those about the vaccine as you should about every other aspect of their health. Education is part of our job as health care professionals but judgment is not.
Dr. Matuszak works for Sound Physicians and is a nocturnist at a hospital in the San Francisco Bay Area.
Antibody cocktail reduces chance of developing COVID
A one-time dose of two long-acting monoclonal antibodies reduced the risk of developing symptomatic COVID by 77% in comparison with placebo (P < .001) in a randomized, double-blind, placebo-controlled, phase 3 trial in adults, according to researchers who presented results at IDWeek 2021, an annual scientific meeting on infectious diseases.
The mix of tixagevimab and cilgavimab (AZD7442, Astra Zeneca) in a 300-mg dose is delivered in two intramuscular injections.
“This is the first long-acting combination of monoclonal antibodies that represents a potential new option to augment COVID-19 prevention,” said lead author Myron J. Levin, MD, a professor and pediatric infectious disease specialist at the University of Colorado at Denver, Aurora, who presented the findings of the PROVENT trial.
Both antibodies were taken from B cells donated by patients who had been infected with SARS-CoV-2, and they work synergistically, Dr. Levin said.
“The combination of them is better than adding results of each individually,” he said. “In vitro experiments have already shown that variants of interest and concern, including the Delta variant, are successfully neutralized by this cocktail.”
The trial was conducted in 87 sites in the United States, the United Kingdom, Spain, France, and Belgium. Participants included 5,197 unvaccinated adults who had never been infected with SARS-CoV-2 and either were at higher risk for inadequate response to COVID-19 vaccines because they were immunocompromised or were at high risk for exposure.
“Efficacy was observed through at least 3 months,” Dr. Levin said. “Preliminary pharmacokinetic modeling predicts potential protection for up to 12 months.”
Raymund Razonable, MD, an infectious disease expert with the Mayo Clinic in Rochester, Minn., who was not involved with the trial, told this news organization he was particularly interested in this combination because the developers made use of novel technology that extends the half-life of the antibodies and because of the large number of participants in the study.
Modeling that shows protection could last up to a year is novel and important, he said.
“People won’t need frequent injections,” Dr. Razonable said. With postexposure prophylaxis monoclonal cocktails, people may be given a dose a month, he noted.
Dr. Razonable said, “This is something intended to prevent COVID in people who are unvaccinated. The downside to that is we want people to get vaccinated. The best strategy so far is really vaccination.”
He said AZD7442 could potentially help fill the void for patients who are not able to respond to the COVID vaccines, including some who are immunocompromised or are undergoing chemotherapy.
Dr. Razonable said that, although the 77% reduction for developing symptomatic COVID-19 (95% confidence interval vs. placebo, 46.0-90.0; P < .001) is impressive, it is a reduction in relative risk. Still unknown is how much an individual’s absolute risk is reduced.
He also said it would be helpful to know how many people in the study population were immunocompromised, “because I think that’s where this product will be useful for prevention.”
The primary study endpoints were the first case of SARS-CoV-2 RT-PCR-positive symptomatic illness post dose and prior to day 183 (efficacy) as well as the safety of the product.
The cocktail appeared to be well tolerated. Adverse events occurred in 35% of participants administered AZD7442 and in 34% of the placebo group. Injection-site reactions occurred in 2.4% of the AZD7442 group and in 2.1% of the placebo group. There was one case of severe or critical COVID-19; two COVID-19–related deaths occurred in the placebo group.
AZD7442 is being developed with the help of funding from the U.S. government. Dr. Levin has received support from GlaxoSmithKline companies. Many of the coauthors are employed by AstraZeneca and hold stock in the company. Dr. Razonable has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A one-time dose of two long-acting monoclonal antibodies reduced the risk of developing symptomatic COVID by 77% in comparison with placebo (P < .001) in a randomized, double-blind, placebo-controlled, phase 3 trial in adults, according to researchers who presented results at IDWeek 2021, an annual scientific meeting on infectious diseases.
The mix of tixagevimab and cilgavimab (AZD7442, Astra Zeneca) in a 300-mg dose is delivered in two intramuscular injections.
“This is the first long-acting combination of monoclonal antibodies that represents a potential new option to augment COVID-19 prevention,” said lead author Myron J. Levin, MD, a professor and pediatric infectious disease specialist at the University of Colorado at Denver, Aurora, who presented the findings of the PROVENT trial.
Both antibodies were taken from B cells donated by patients who had been infected with SARS-CoV-2, and they work synergistically, Dr. Levin said.
“The combination of them is better than adding results of each individually,” he said. “In vitro experiments have already shown that variants of interest and concern, including the Delta variant, are successfully neutralized by this cocktail.”
The trial was conducted in 87 sites in the United States, the United Kingdom, Spain, France, and Belgium. Participants included 5,197 unvaccinated adults who had never been infected with SARS-CoV-2 and either were at higher risk for inadequate response to COVID-19 vaccines because they were immunocompromised or were at high risk for exposure.
“Efficacy was observed through at least 3 months,” Dr. Levin said. “Preliminary pharmacokinetic modeling predicts potential protection for up to 12 months.”
Raymund Razonable, MD, an infectious disease expert with the Mayo Clinic in Rochester, Minn., who was not involved with the trial, told this news organization he was particularly interested in this combination because the developers made use of novel technology that extends the half-life of the antibodies and because of the large number of participants in the study.
Modeling that shows protection could last up to a year is novel and important, he said.
“People won’t need frequent injections,” Dr. Razonable said. With postexposure prophylaxis monoclonal cocktails, people may be given a dose a month, he noted.
Dr. Razonable said, “This is something intended to prevent COVID in people who are unvaccinated. The downside to that is we want people to get vaccinated. The best strategy so far is really vaccination.”
He said AZD7442 could potentially help fill the void for patients who are not able to respond to the COVID vaccines, including some who are immunocompromised or are undergoing chemotherapy.
Dr. Razonable said that, although the 77% reduction for developing symptomatic COVID-19 (95% confidence interval vs. placebo, 46.0-90.0; P < .001) is impressive, it is a reduction in relative risk. Still unknown is how much an individual’s absolute risk is reduced.
He also said it would be helpful to know how many people in the study population were immunocompromised, “because I think that’s where this product will be useful for prevention.”
The primary study endpoints were the first case of SARS-CoV-2 RT-PCR-positive symptomatic illness post dose and prior to day 183 (efficacy) as well as the safety of the product.
The cocktail appeared to be well tolerated. Adverse events occurred in 35% of participants administered AZD7442 and in 34% of the placebo group. Injection-site reactions occurred in 2.4% of the AZD7442 group and in 2.1% of the placebo group. There was one case of severe or critical COVID-19; two COVID-19–related deaths occurred in the placebo group.
AZD7442 is being developed with the help of funding from the U.S. government. Dr. Levin has received support from GlaxoSmithKline companies. Many of the coauthors are employed by AstraZeneca and hold stock in the company. Dr. Razonable has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A one-time dose of two long-acting monoclonal antibodies reduced the risk of developing symptomatic COVID by 77% in comparison with placebo (P < .001) in a randomized, double-blind, placebo-controlled, phase 3 trial in adults, according to researchers who presented results at IDWeek 2021, an annual scientific meeting on infectious diseases.
The mix of tixagevimab and cilgavimab (AZD7442, Astra Zeneca) in a 300-mg dose is delivered in two intramuscular injections.
“This is the first long-acting combination of monoclonal antibodies that represents a potential new option to augment COVID-19 prevention,” said lead author Myron J. Levin, MD, a professor and pediatric infectious disease specialist at the University of Colorado at Denver, Aurora, who presented the findings of the PROVENT trial.
Both antibodies were taken from B cells donated by patients who had been infected with SARS-CoV-2, and they work synergistically, Dr. Levin said.
“The combination of them is better than adding results of each individually,” he said. “In vitro experiments have already shown that variants of interest and concern, including the Delta variant, are successfully neutralized by this cocktail.”
The trial was conducted in 87 sites in the United States, the United Kingdom, Spain, France, and Belgium. Participants included 5,197 unvaccinated adults who had never been infected with SARS-CoV-2 and either were at higher risk for inadequate response to COVID-19 vaccines because they were immunocompromised or were at high risk for exposure.
“Efficacy was observed through at least 3 months,” Dr. Levin said. “Preliminary pharmacokinetic modeling predicts potential protection for up to 12 months.”
Raymund Razonable, MD, an infectious disease expert with the Mayo Clinic in Rochester, Minn., who was not involved with the trial, told this news organization he was particularly interested in this combination because the developers made use of novel technology that extends the half-life of the antibodies and because of the large number of participants in the study.
Modeling that shows protection could last up to a year is novel and important, he said.
“People won’t need frequent injections,” Dr. Razonable said. With postexposure prophylaxis monoclonal cocktails, people may be given a dose a month, he noted.
Dr. Razonable said, “This is something intended to prevent COVID in people who are unvaccinated. The downside to that is we want people to get vaccinated. The best strategy so far is really vaccination.”
He said AZD7442 could potentially help fill the void for patients who are not able to respond to the COVID vaccines, including some who are immunocompromised or are undergoing chemotherapy.
Dr. Razonable said that, although the 77% reduction for developing symptomatic COVID-19 (95% confidence interval vs. placebo, 46.0-90.0; P < .001) is impressive, it is a reduction in relative risk. Still unknown is how much an individual’s absolute risk is reduced.
He also said it would be helpful to know how many people in the study population were immunocompromised, “because I think that’s where this product will be useful for prevention.”
The primary study endpoints were the first case of SARS-CoV-2 RT-PCR-positive symptomatic illness post dose and prior to day 183 (efficacy) as well as the safety of the product.
The cocktail appeared to be well tolerated. Adverse events occurred in 35% of participants administered AZD7442 and in 34% of the placebo group. Injection-site reactions occurred in 2.4% of the AZD7442 group and in 2.1% of the placebo group. There was one case of severe or critical COVID-19; two COVID-19–related deaths occurred in the placebo group.
AZD7442 is being developed with the help of funding from the U.S. government. Dr. Levin has received support from GlaxoSmithKline companies. Many of the coauthors are employed by AstraZeneca and hold stock in the company. Dr. Razonable has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 updates dominate IDWeek lineup
Two of the three late-breaking abstract sessions coming up this week at IDWeek 2021, an annual scientific meeting on infectious diseases, are filled with the most recent evidence on COVID-19 prevention and treatment.
Adarsh Bhimraj, MD, a vice chair of the conference, said in an interview that attendees will leave the virtual conference with an up-to-date view of what’s promising in the fight against COVID-19 globally and what questions are as yet unanswered.
Researchers will also present findings on promising new antibiotics in the pipeline, stewardship efforts, health disparities, telemedicine advances, and emerging pathogens, but at least a quarter of the program is devoted to COVID-19.
“It’s hard to ignore the elephant in the room,” Dr. Bhimraj said.
Vaccine distribution will be among the hot topics at the global conference, he said, in light of the recent decisions by the Food and Drug Administration and the Centers for Disease Control and Prevention to reserve boosters for those at greatest risk.
Although the United States and other high-resource countries are deciding who should get boosters, only 10% of the developing world has received even a single dose, he noted.
The conference will also present a worldwide view of scientific collaboration to address the COVID pandemic and pandemics yet to come, Dr. Bhimraj said.
He highlighted a talk on Oct. 2, to be delivered by South African human rights attorney and social justice activist Fatima Hassan, called “Global Vaccines and Preventive Care Inequities: Implications and Solutions Beyond the Pandemic.”
The session looks ahead to building systems to share resources and knowledge to end deadly outbreaks with an equitable approach.
“We live in a global village,” Dr. Bhimraj said. “It isn’t just the right thing to do, it’s the pragmatic thing to do.”
Controversies in non-COVID diseases
Controversies and new treatments are plentiful in other diseases as well.
- At an HIV session, arguments will be presented regarding the sustainability and practicalities of telemedicine in HIV. Speakers will argue for and against telemedicine as a permanent practice changer for the field.
- In a session on Oct. 1, panelists will discuss pros and cons of information published in preprints versus peer-reviewed journals and how to assess when research findings should lead to practice change.
- Also on Oct. 1, panelists in a symposium will discuss advantages and disadvantages of antifungal treatments for children who have received solid organ transplants.
- Antimicrobial stewardship continues to be a primary topic at IDWeek, this year with additional pandemic challenges. Sessions will address trends in use and diagnostic advances to help in prescribing.
- The pipeline for new antibiotics continues to face barriers regarding production and development. No new classes of antibiotics have been discovered since the 1980s. Pew has that there are too few drugs in development to meet current and anticipated need.
- This year’s program offers a symposium on private-public partnerships to help jump-start development.
- One of the most popular sessions returning this year is “Clinical Trials That Will Change Your Practice,” Dr. Bhimraj said. This year, that session will be reserved for non-COVID infectious disease research. Presenters will summarize the findings of top work published in the past year.
Around-the-world COVID view
Again this year, global experts will present a round-the-clock session called “Chasing the Sun” the day before the main sessions. It will include updates on COVID throughout the world. Barney Graham, MD, PhD, deputy director of the National Institutes of Health’s Vaccine Research Center, will kick off the program with an address on the future of vaccinology. This will be followed by updates on the state of the disease in Central and South America, Japan, Asia Pacific, India, and Africa.
Sandra Harwood, IDWeek conference secretariat, who proposed the idea for the first Chasing the Sun session last year, said in an interview that the updates will highlight particular COVID challenges experienced in various countries.
For example, leaders of India’s session will address why a potentially fatal fungal disease struck many COVID-19 patients in that country. Japan’s update will include how Olympic organizers planned for and dealt with the virus’s threat in Tokyo.
Ms. Harwood said that all the COVID sessions in Chasing the Sun and throughout the program will be free to clinicians inside and outside the conference, thanks to a grant from the CDC.
An address by CDC Director Rochelle Walensky, MD, MPH, on Sept. 30 will wrap up Chasing the Sun and launch the main IDWeek program.
A version of this article first appeared on Medscape.com.
Two of the three late-breaking abstract sessions coming up this week at IDWeek 2021, an annual scientific meeting on infectious diseases, are filled with the most recent evidence on COVID-19 prevention and treatment.
Adarsh Bhimraj, MD, a vice chair of the conference, said in an interview that attendees will leave the virtual conference with an up-to-date view of what’s promising in the fight against COVID-19 globally and what questions are as yet unanswered.
Researchers will also present findings on promising new antibiotics in the pipeline, stewardship efforts, health disparities, telemedicine advances, and emerging pathogens, but at least a quarter of the program is devoted to COVID-19.
“It’s hard to ignore the elephant in the room,” Dr. Bhimraj said.
Vaccine distribution will be among the hot topics at the global conference, he said, in light of the recent decisions by the Food and Drug Administration and the Centers for Disease Control and Prevention to reserve boosters for those at greatest risk.
Although the United States and other high-resource countries are deciding who should get boosters, only 10% of the developing world has received even a single dose, he noted.
The conference will also present a worldwide view of scientific collaboration to address the COVID pandemic and pandemics yet to come, Dr. Bhimraj said.
He highlighted a talk on Oct. 2, to be delivered by South African human rights attorney and social justice activist Fatima Hassan, called “Global Vaccines and Preventive Care Inequities: Implications and Solutions Beyond the Pandemic.”
The session looks ahead to building systems to share resources and knowledge to end deadly outbreaks with an equitable approach.
“We live in a global village,” Dr. Bhimraj said. “It isn’t just the right thing to do, it’s the pragmatic thing to do.”
Controversies in non-COVID diseases
Controversies and new treatments are plentiful in other diseases as well.
- At an HIV session, arguments will be presented regarding the sustainability and practicalities of telemedicine in HIV. Speakers will argue for and against telemedicine as a permanent practice changer for the field.
- In a session on Oct. 1, panelists will discuss pros and cons of information published in preprints versus peer-reviewed journals and how to assess when research findings should lead to practice change.
- Also on Oct. 1, panelists in a symposium will discuss advantages and disadvantages of antifungal treatments for children who have received solid organ transplants.
- Antimicrobial stewardship continues to be a primary topic at IDWeek, this year with additional pandemic challenges. Sessions will address trends in use and diagnostic advances to help in prescribing.
- The pipeline for new antibiotics continues to face barriers regarding production and development. No new classes of antibiotics have been discovered since the 1980s. Pew has that there are too few drugs in development to meet current and anticipated need.
- This year’s program offers a symposium on private-public partnerships to help jump-start development.
- One of the most popular sessions returning this year is “Clinical Trials That Will Change Your Practice,” Dr. Bhimraj said. This year, that session will be reserved for non-COVID infectious disease research. Presenters will summarize the findings of top work published in the past year.
Around-the-world COVID view
Again this year, global experts will present a round-the-clock session called “Chasing the Sun” the day before the main sessions. It will include updates on COVID throughout the world. Barney Graham, MD, PhD, deputy director of the National Institutes of Health’s Vaccine Research Center, will kick off the program with an address on the future of vaccinology. This will be followed by updates on the state of the disease in Central and South America, Japan, Asia Pacific, India, and Africa.
Sandra Harwood, IDWeek conference secretariat, who proposed the idea for the first Chasing the Sun session last year, said in an interview that the updates will highlight particular COVID challenges experienced in various countries.
For example, leaders of India’s session will address why a potentially fatal fungal disease struck many COVID-19 patients in that country. Japan’s update will include how Olympic organizers planned for and dealt with the virus’s threat in Tokyo.
Ms. Harwood said that all the COVID sessions in Chasing the Sun and throughout the program will be free to clinicians inside and outside the conference, thanks to a grant from the CDC.
An address by CDC Director Rochelle Walensky, MD, MPH, on Sept. 30 will wrap up Chasing the Sun and launch the main IDWeek program.
A version of this article first appeared on Medscape.com.
Two of the three late-breaking abstract sessions coming up this week at IDWeek 2021, an annual scientific meeting on infectious diseases, are filled with the most recent evidence on COVID-19 prevention and treatment.
Adarsh Bhimraj, MD, a vice chair of the conference, said in an interview that attendees will leave the virtual conference with an up-to-date view of what’s promising in the fight against COVID-19 globally and what questions are as yet unanswered.
Researchers will also present findings on promising new antibiotics in the pipeline, stewardship efforts, health disparities, telemedicine advances, and emerging pathogens, but at least a quarter of the program is devoted to COVID-19.
“It’s hard to ignore the elephant in the room,” Dr. Bhimraj said.
Vaccine distribution will be among the hot topics at the global conference, he said, in light of the recent decisions by the Food and Drug Administration and the Centers for Disease Control and Prevention to reserve boosters for those at greatest risk.
Although the United States and other high-resource countries are deciding who should get boosters, only 10% of the developing world has received even a single dose, he noted.
The conference will also present a worldwide view of scientific collaboration to address the COVID pandemic and pandemics yet to come, Dr. Bhimraj said.
He highlighted a talk on Oct. 2, to be delivered by South African human rights attorney and social justice activist Fatima Hassan, called “Global Vaccines and Preventive Care Inequities: Implications and Solutions Beyond the Pandemic.”
The session looks ahead to building systems to share resources and knowledge to end deadly outbreaks with an equitable approach.
“We live in a global village,” Dr. Bhimraj said. “It isn’t just the right thing to do, it’s the pragmatic thing to do.”
Controversies in non-COVID diseases
Controversies and new treatments are plentiful in other diseases as well.
- At an HIV session, arguments will be presented regarding the sustainability and practicalities of telemedicine in HIV. Speakers will argue for and against telemedicine as a permanent practice changer for the field.
- In a session on Oct. 1, panelists will discuss pros and cons of information published in preprints versus peer-reviewed journals and how to assess when research findings should lead to practice change.
- Also on Oct. 1, panelists in a symposium will discuss advantages and disadvantages of antifungal treatments for children who have received solid organ transplants.
- Antimicrobial stewardship continues to be a primary topic at IDWeek, this year with additional pandemic challenges. Sessions will address trends in use and diagnostic advances to help in prescribing.
- The pipeline for new antibiotics continues to face barriers regarding production and development. No new classes of antibiotics have been discovered since the 1980s. Pew has that there are too few drugs in development to meet current and anticipated need.
- This year’s program offers a symposium on private-public partnerships to help jump-start development.
- One of the most popular sessions returning this year is “Clinical Trials That Will Change Your Practice,” Dr. Bhimraj said. This year, that session will be reserved for non-COVID infectious disease research. Presenters will summarize the findings of top work published in the past year.
Around-the-world COVID view
Again this year, global experts will present a round-the-clock session called “Chasing the Sun” the day before the main sessions. It will include updates on COVID throughout the world. Barney Graham, MD, PhD, deputy director of the National Institutes of Health’s Vaccine Research Center, will kick off the program with an address on the future of vaccinology. This will be followed by updates on the state of the disease in Central and South America, Japan, Asia Pacific, India, and Africa.
Sandra Harwood, IDWeek conference secretariat, who proposed the idea for the first Chasing the Sun session last year, said in an interview that the updates will highlight particular COVID challenges experienced in various countries.
For example, leaders of India’s session will address why a potentially fatal fungal disease struck many COVID-19 patients in that country. Japan’s update will include how Olympic organizers planned for and dealt with the virus’s threat in Tokyo.
Ms. Harwood said that all the COVID sessions in Chasing the Sun and throughout the program will be free to clinicians inside and outside the conference, thanks to a grant from the CDC.
An address by CDC Director Rochelle Walensky, MD, MPH, on Sept. 30 will wrap up Chasing the Sun and launch the main IDWeek program.
A version of this article first appeared on Medscape.com.
Should clinicians recommend vitamin D for psychiatric patients during COVID-19?
Amid a flurry of conflicting reports concerning the efficacy of vitamin D for COVID-19 patients, a sense of consternation has emerged in the health care sector regarding its overall utility.
Vitamin D plays a critical role in the restorative function of mental health. Low vitamin D levels correlate with mood disorders as well as the development of schizophrenia. In light of the rise in mental health dysfunction and the body of evidence examined to develop this article, we recommend that patients continue to incorporate regular vitamin D supplementation during the course of the pandemic with the goal of preventing deterioration of well-being. Recent studies have generally overlooked the role of vitamin D in mental health by primarily focusing on the immediacy of therapeutic management for medical disorders within the context of COVID-19.
What is the role of vitamin D in human physiology?
Vitamins play an integral role in homeostatic metabolism. Vitamin D, in particular, is intimately responsible for regulating the body’s underlying phosphorus and calcium balance, thereby facilitating bone mineralization.1 As an immunomodulatory hormone, vitamin D coordinates activities across innate and adaptive immune systems, providing defense against autoimmune diseases and miscellaneous infections.2
It is uncommon for people to be affected with vitamin D deficiency in equatorial zones, yet an Indonesian study uncovered low vitamin D effects (hypovitaminosis D) in virtually all of the patients in its COVID-19 case series.3
Likewise, a study conducted in Spain indicated that a whopping 82.2% of the COVID-19 patients endorsed clinically deficient levels of vitamin D, often within the context of severe presentation. Those patients also expressed elevated inflammatory markers, namely, D-dimer and ferritin.4
Comparable studies across the globe continue to support a correlative, if not causative, role for hypovitaminosis D and susceptibility to COVID-19. Mental health awareness entails healthy emotional interactions, preservation of well-being, and the ability to govern one’s thoughts and actions in accordance with societal expectations against the backdrop of ongoing psychosocial stressors. Such awareness helps ensure that people can make resourceful choices and meaningful associations, and can handle stress. We know that mental health is pivotal in dictating one’s overall health. This article provides a detailed exploration of the dynamics of mental health, COVID-19, and vitamin D.
The rationale for vitamin D supplementation therapy in COVID-19
When it comes to respiratory tract infections (RTI) such as COVID-19, influenza, and pneumonia, considerable interest has been generated with respect to the therapeutic efficacy of vitamin D in the acute setting. Vitamin D, as an inflammatory modulator, exerts a protective effect in patients with RTI, especially in those with deviations from baseline vitamin D levels.5
What is the rationale for administering vitamin D supplementation therapy for COVID-19? It has been noted that emergent cases of COVID-19 arise during the autumn months for European countries6 and there is also a firmly established connection between the amount of solar radiation/UV exposure (or the lack thereof) and influenza outbreaks,7 further underscoring the relevance of vitamin D levels. Despite those observations, wholesale implementation of vitamin D therapy should not be used in the acute setting for conditions such as COVID-19 or pneumonia as it is not supported by evidence-based practices. Despite the compound’s inherent antimicrobial actions,8 four randomized clinical trials involving pediatric subjects failed to demonstrate a significantly beneficial response (for example, radiographic resolution) to adjunctive supplementation during the course of acute pneumonia symptomatology.9 Likewise, data collected from a randomized controlled trial confirmed the suspicion that high-dose vitamin D therapy has no tangible effect, tied to mortality or otherwise, on moderate or severe presentations of COVID-19.10
Revisiting vitamin D supplementation therapy for mental health patients with COVID-19
It is clear that recent studies have undermined the overall applicability of vitamin D therapy with respect to acute presentations of COVID-19. However, our team would like to underscore the importance of vitamin D supplementation with respect to maintenance of the integrity of underlying mental health processes.
Numerous studies (for example, cross-sectional, cohort, case-control) have uncovered a statistically significant relationship between vitamin D deficiency and depression, including variants such as postpartum and antepartum depression. It should be noted that the pathophysiology for those variables is not entirely known and that the overall clinical utility of supplementation therapy has not previously been recommended because of existing gaps in the literature.11
In another prospective study involving a relatively small sample size, subjects with seasonal affective disorder (SAD) were either exposed to 10,000 IUs of vitamin D or phototherapy, and depression endpoints were evaluated via the Hamilton Rating Scale for Depression, the SIGH-SAD, and the SAD-8 depression scale. Improvements in 25-hydroxyvitamin D (25-OH D) levels correlated with improvements in depression metrics. However, subjects exposed to phototherapy sessions did not exhibit any meaningful improvements in clinical outcome.12
It is also possible that vitamin D deficiency is reflective of an overall poor nutritional status. People with schizophrenia have frequently been observed to have vitamin D deficiency with more than half of all patients also manifesting symptoms of osteoporosis, a condition that often necessitates vitamin D supplementation. The literature shows that the jury is still out regarding the applicability of vitamin D supplementation for schizophrenia patients, with numerous conflicting studies, including one randomized trial indicating an improvement in positive and negative symptoms as well as in the metabolic profile.13
However, in light of the rather large and growing body of evidence suggesting an increased risk of deterioration, psychological distress, and worsened prognosis during the pandemic coupled with the presence of medical and/or mental health morbidities, it would be sensible for psychiatric patients, especially those with preexisting deviations from baseline vitamin D levels, to consider vitamin D supplementation.
Vitamin D supplementation therapy, as a preventive, but not curative measure – one that is also low cost/high benefit – allows for the patient to be in a much better position from the perspective of her/his general health and nutritional status to tackle the ongoing psychosocial challenges of the pandemic and/or COVID-19 exposure.
Dr. Aman is a faculty member in the biology department at City Colleges of Chicago. She is a postdoctoral researcher at the International Maternal and Child Health Foundation (IMCHF) in Montreal; fellow, medical staff development, American Academy of Medical Management; and master online teacher (MOT) at the University of Illinois at Chicago. Dr. Aman disclosed no relevant relationships. Dr. Islam is a medical writer for the IMCHF and is based in New York. He is a postdoctoral fellow, psychopharmacologist, and a board-certified medical specialist. He disclosed no relevant financial relationships. Dr. Dhillon is a staff neurologist at Brigham and Women’s Hospital in Boston and is affiliated with Sturdy Memorial Hospital in Attleboro, Mass. He is on the speakers bureaus/advisory boards of Biogen, Bristol Myers Squibb, Genzyme, and Teva Neuroscience. Mr. Zaid Ulhaq Choudhry is a research assistant at the IMCHF. He has no disclosures. Dr. Zia Choudhry (Mr. Choudhry’s father) is chief scientific officer and head of the department of mental health and clinical research at the IMCHF. Dr. Choudhry has no disclosures.
References
1. van Driel M and van Leeuwen JPTM. Mol Cellular Endocrinol. 2017;453:46-51.
2. Charoenngam N and Holick MF. Nutrients. 2020 Jul 15;12(7):2097. doi: 103390/nu12072097.
3. Pinzon RT et al. Trop Med Health. 2020 Dec 20;48:102. doi: 10.1186/S41182-020-00277-w.
4. Hernández JL et al. J Clin Endocrinol Metab. 2021 Mar;106(3)e1343-53.
5. Martineau AR et al. BMJ. 2017;356:i6583. doi: 1136/bmj.i6583.
6. Walrand S. Sci Rep. 2021 Jan 21;11(1981). doi: 10.1038/s41598-021-81419-w.
7. Moan J. et al. Dermatoendocrinol. 2009 Nov-Dec;1(6):307-9.
8. Fabri M et al. Sci Transl Med. 2011 Oct 12;3(104):104ra102. doi: 10.1126/scitranslmed.3003045.
9. Slow S et al. Sci Rep. 2018 Sep 14;8(1):13829. doi: 10.1038/s41598-018-32162-2.
10. Berman R. “Study confirms high doses of vitamin D have no effect on COVID-19.” Medical News Today. 2021 May 4.
11. Menon V et al. Indian J Psychol Med. 2020 Jan-Feb;42(1):11-21.
12. Gloth 3rd FM et al. Nutr Health Aging. 1999;3(1):5-7.
13. Cui X et al. Mol Psychiatry. 2021 Jan 26. doi:10.1038/s41380-021-01025-0.
Amid a flurry of conflicting reports concerning the efficacy of vitamin D for COVID-19 patients, a sense of consternation has emerged in the health care sector regarding its overall utility.
Vitamin D plays a critical role in the restorative function of mental health. Low vitamin D levels correlate with mood disorders as well as the development of schizophrenia. In light of the rise in mental health dysfunction and the body of evidence examined to develop this article, we recommend that patients continue to incorporate regular vitamin D supplementation during the course of the pandemic with the goal of preventing deterioration of well-being. Recent studies have generally overlooked the role of vitamin D in mental health by primarily focusing on the immediacy of therapeutic management for medical disorders within the context of COVID-19.
What is the role of vitamin D in human physiology?
Vitamins play an integral role in homeostatic metabolism. Vitamin D, in particular, is intimately responsible for regulating the body’s underlying phosphorus and calcium balance, thereby facilitating bone mineralization.1 As an immunomodulatory hormone, vitamin D coordinates activities across innate and adaptive immune systems, providing defense against autoimmune diseases and miscellaneous infections.2
It is uncommon for people to be affected with vitamin D deficiency in equatorial zones, yet an Indonesian study uncovered low vitamin D effects (hypovitaminosis D) in virtually all of the patients in its COVID-19 case series.3
Likewise, a study conducted in Spain indicated that a whopping 82.2% of the COVID-19 patients endorsed clinically deficient levels of vitamin D, often within the context of severe presentation. Those patients also expressed elevated inflammatory markers, namely, D-dimer and ferritin.4
Comparable studies across the globe continue to support a correlative, if not causative, role for hypovitaminosis D and susceptibility to COVID-19. Mental health awareness entails healthy emotional interactions, preservation of well-being, and the ability to govern one’s thoughts and actions in accordance with societal expectations against the backdrop of ongoing psychosocial stressors. Such awareness helps ensure that people can make resourceful choices and meaningful associations, and can handle stress. We know that mental health is pivotal in dictating one’s overall health. This article provides a detailed exploration of the dynamics of mental health, COVID-19, and vitamin D.
The rationale for vitamin D supplementation therapy in COVID-19
When it comes to respiratory tract infections (RTI) such as COVID-19, influenza, and pneumonia, considerable interest has been generated with respect to the therapeutic efficacy of vitamin D in the acute setting. Vitamin D, as an inflammatory modulator, exerts a protective effect in patients with RTI, especially in those with deviations from baseline vitamin D levels.5
What is the rationale for administering vitamin D supplementation therapy for COVID-19? It has been noted that emergent cases of COVID-19 arise during the autumn months for European countries6 and there is also a firmly established connection between the amount of solar radiation/UV exposure (or the lack thereof) and influenza outbreaks,7 further underscoring the relevance of vitamin D levels. Despite those observations, wholesale implementation of vitamin D therapy should not be used in the acute setting for conditions such as COVID-19 or pneumonia as it is not supported by evidence-based practices. Despite the compound’s inherent antimicrobial actions,8 four randomized clinical trials involving pediatric subjects failed to demonstrate a significantly beneficial response (for example, radiographic resolution) to adjunctive supplementation during the course of acute pneumonia symptomatology.9 Likewise, data collected from a randomized controlled trial confirmed the suspicion that high-dose vitamin D therapy has no tangible effect, tied to mortality or otherwise, on moderate or severe presentations of COVID-19.10
Revisiting vitamin D supplementation therapy for mental health patients with COVID-19
It is clear that recent studies have undermined the overall applicability of vitamin D therapy with respect to acute presentations of COVID-19. However, our team would like to underscore the importance of vitamin D supplementation with respect to maintenance of the integrity of underlying mental health processes.
Numerous studies (for example, cross-sectional, cohort, case-control) have uncovered a statistically significant relationship between vitamin D deficiency and depression, including variants such as postpartum and antepartum depression. It should be noted that the pathophysiology for those variables is not entirely known and that the overall clinical utility of supplementation therapy has not previously been recommended because of existing gaps in the literature.11
In another prospective study involving a relatively small sample size, subjects with seasonal affective disorder (SAD) were either exposed to 10,000 IUs of vitamin D or phototherapy, and depression endpoints were evaluated via the Hamilton Rating Scale for Depression, the SIGH-SAD, and the SAD-8 depression scale. Improvements in 25-hydroxyvitamin D (25-OH D) levels correlated with improvements in depression metrics. However, subjects exposed to phototherapy sessions did not exhibit any meaningful improvements in clinical outcome.12
It is also possible that vitamin D deficiency is reflective of an overall poor nutritional status. People with schizophrenia have frequently been observed to have vitamin D deficiency with more than half of all patients also manifesting symptoms of osteoporosis, a condition that often necessitates vitamin D supplementation. The literature shows that the jury is still out regarding the applicability of vitamin D supplementation for schizophrenia patients, with numerous conflicting studies, including one randomized trial indicating an improvement in positive and negative symptoms as well as in the metabolic profile.13
However, in light of the rather large and growing body of evidence suggesting an increased risk of deterioration, psychological distress, and worsened prognosis during the pandemic coupled with the presence of medical and/or mental health morbidities, it would be sensible for psychiatric patients, especially those with preexisting deviations from baseline vitamin D levels, to consider vitamin D supplementation.
Vitamin D supplementation therapy, as a preventive, but not curative measure – one that is also low cost/high benefit – allows for the patient to be in a much better position from the perspective of her/his general health and nutritional status to tackle the ongoing psychosocial challenges of the pandemic and/or COVID-19 exposure.
Dr. Aman is a faculty member in the biology department at City Colleges of Chicago. She is a postdoctoral researcher at the International Maternal and Child Health Foundation (IMCHF) in Montreal; fellow, medical staff development, American Academy of Medical Management; and master online teacher (MOT) at the University of Illinois at Chicago. Dr. Aman disclosed no relevant relationships. Dr. Islam is a medical writer for the IMCHF and is based in New York. He is a postdoctoral fellow, psychopharmacologist, and a board-certified medical specialist. He disclosed no relevant financial relationships. Dr. Dhillon is a staff neurologist at Brigham and Women’s Hospital in Boston and is affiliated with Sturdy Memorial Hospital in Attleboro, Mass. He is on the speakers bureaus/advisory boards of Biogen, Bristol Myers Squibb, Genzyme, and Teva Neuroscience. Mr. Zaid Ulhaq Choudhry is a research assistant at the IMCHF. He has no disclosures. Dr. Zia Choudhry (Mr. Choudhry’s father) is chief scientific officer and head of the department of mental health and clinical research at the IMCHF. Dr. Choudhry has no disclosures.
References
1. van Driel M and van Leeuwen JPTM. Mol Cellular Endocrinol. 2017;453:46-51.
2. Charoenngam N and Holick MF. Nutrients. 2020 Jul 15;12(7):2097. doi: 103390/nu12072097.
3. Pinzon RT et al. Trop Med Health. 2020 Dec 20;48:102. doi: 10.1186/S41182-020-00277-w.
4. Hernández JL et al. J Clin Endocrinol Metab. 2021 Mar;106(3)e1343-53.
5. Martineau AR et al. BMJ. 2017;356:i6583. doi: 1136/bmj.i6583.
6. Walrand S. Sci Rep. 2021 Jan 21;11(1981). doi: 10.1038/s41598-021-81419-w.
7. Moan J. et al. Dermatoendocrinol. 2009 Nov-Dec;1(6):307-9.
8. Fabri M et al. Sci Transl Med. 2011 Oct 12;3(104):104ra102. doi: 10.1126/scitranslmed.3003045.
9. Slow S et al. Sci Rep. 2018 Sep 14;8(1):13829. doi: 10.1038/s41598-018-32162-2.
10. Berman R. “Study confirms high doses of vitamin D have no effect on COVID-19.” Medical News Today. 2021 May 4.
11. Menon V et al. Indian J Psychol Med. 2020 Jan-Feb;42(1):11-21.
12. Gloth 3rd FM et al. Nutr Health Aging. 1999;3(1):5-7.
13. Cui X et al. Mol Psychiatry. 2021 Jan 26. doi:10.1038/s41380-021-01025-0.
Amid a flurry of conflicting reports concerning the efficacy of vitamin D for COVID-19 patients, a sense of consternation has emerged in the health care sector regarding its overall utility.
Vitamin D plays a critical role in the restorative function of mental health. Low vitamin D levels correlate with mood disorders as well as the development of schizophrenia. In light of the rise in mental health dysfunction and the body of evidence examined to develop this article, we recommend that patients continue to incorporate regular vitamin D supplementation during the course of the pandemic with the goal of preventing deterioration of well-being. Recent studies have generally overlooked the role of vitamin D in mental health by primarily focusing on the immediacy of therapeutic management for medical disorders within the context of COVID-19.
What is the role of vitamin D in human physiology?
Vitamins play an integral role in homeostatic metabolism. Vitamin D, in particular, is intimately responsible for regulating the body’s underlying phosphorus and calcium balance, thereby facilitating bone mineralization.1 As an immunomodulatory hormone, vitamin D coordinates activities across innate and adaptive immune systems, providing defense against autoimmune diseases and miscellaneous infections.2
It is uncommon for people to be affected with vitamin D deficiency in equatorial zones, yet an Indonesian study uncovered low vitamin D effects (hypovitaminosis D) in virtually all of the patients in its COVID-19 case series.3
Likewise, a study conducted in Spain indicated that a whopping 82.2% of the COVID-19 patients endorsed clinically deficient levels of vitamin D, often within the context of severe presentation. Those patients also expressed elevated inflammatory markers, namely, D-dimer and ferritin.4
Comparable studies across the globe continue to support a correlative, if not causative, role for hypovitaminosis D and susceptibility to COVID-19. Mental health awareness entails healthy emotional interactions, preservation of well-being, and the ability to govern one’s thoughts and actions in accordance with societal expectations against the backdrop of ongoing psychosocial stressors. Such awareness helps ensure that people can make resourceful choices and meaningful associations, and can handle stress. We know that mental health is pivotal in dictating one’s overall health. This article provides a detailed exploration of the dynamics of mental health, COVID-19, and vitamin D.
The rationale for vitamin D supplementation therapy in COVID-19
When it comes to respiratory tract infections (RTI) such as COVID-19, influenza, and pneumonia, considerable interest has been generated with respect to the therapeutic efficacy of vitamin D in the acute setting. Vitamin D, as an inflammatory modulator, exerts a protective effect in patients with RTI, especially in those with deviations from baseline vitamin D levels.5
What is the rationale for administering vitamin D supplementation therapy for COVID-19? It has been noted that emergent cases of COVID-19 arise during the autumn months for European countries6 and there is also a firmly established connection between the amount of solar radiation/UV exposure (or the lack thereof) and influenza outbreaks,7 further underscoring the relevance of vitamin D levels. Despite those observations, wholesale implementation of vitamin D therapy should not be used in the acute setting for conditions such as COVID-19 or pneumonia as it is not supported by evidence-based practices. Despite the compound’s inherent antimicrobial actions,8 four randomized clinical trials involving pediatric subjects failed to demonstrate a significantly beneficial response (for example, radiographic resolution) to adjunctive supplementation during the course of acute pneumonia symptomatology.9 Likewise, data collected from a randomized controlled trial confirmed the suspicion that high-dose vitamin D therapy has no tangible effect, tied to mortality or otherwise, on moderate or severe presentations of COVID-19.10
Revisiting vitamin D supplementation therapy for mental health patients with COVID-19
It is clear that recent studies have undermined the overall applicability of vitamin D therapy with respect to acute presentations of COVID-19. However, our team would like to underscore the importance of vitamin D supplementation with respect to maintenance of the integrity of underlying mental health processes.
Numerous studies (for example, cross-sectional, cohort, case-control) have uncovered a statistically significant relationship between vitamin D deficiency and depression, including variants such as postpartum and antepartum depression. It should be noted that the pathophysiology for those variables is not entirely known and that the overall clinical utility of supplementation therapy has not previously been recommended because of existing gaps in the literature.11
In another prospective study involving a relatively small sample size, subjects with seasonal affective disorder (SAD) were either exposed to 10,000 IUs of vitamin D or phototherapy, and depression endpoints were evaluated via the Hamilton Rating Scale for Depression, the SIGH-SAD, and the SAD-8 depression scale. Improvements in 25-hydroxyvitamin D (25-OH D) levels correlated with improvements in depression metrics. However, subjects exposed to phototherapy sessions did not exhibit any meaningful improvements in clinical outcome.12
It is also possible that vitamin D deficiency is reflective of an overall poor nutritional status. People with schizophrenia have frequently been observed to have vitamin D deficiency with more than half of all patients also manifesting symptoms of osteoporosis, a condition that often necessitates vitamin D supplementation. The literature shows that the jury is still out regarding the applicability of vitamin D supplementation for schizophrenia patients, with numerous conflicting studies, including one randomized trial indicating an improvement in positive and negative symptoms as well as in the metabolic profile.13
However, in light of the rather large and growing body of evidence suggesting an increased risk of deterioration, psychological distress, and worsened prognosis during the pandemic coupled with the presence of medical and/or mental health morbidities, it would be sensible for psychiatric patients, especially those with preexisting deviations from baseline vitamin D levels, to consider vitamin D supplementation.
Vitamin D supplementation therapy, as a preventive, but not curative measure – one that is also low cost/high benefit – allows for the patient to be in a much better position from the perspective of her/his general health and nutritional status to tackle the ongoing psychosocial challenges of the pandemic and/or COVID-19 exposure.
Dr. Aman is a faculty member in the biology department at City Colleges of Chicago. She is a postdoctoral researcher at the International Maternal and Child Health Foundation (IMCHF) in Montreal; fellow, medical staff development, American Academy of Medical Management; and master online teacher (MOT) at the University of Illinois at Chicago. Dr. Aman disclosed no relevant relationships. Dr. Islam is a medical writer for the IMCHF and is based in New York. He is a postdoctoral fellow, psychopharmacologist, and a board-certified medical specialist. He disclosed no relevant financial relationships. Dr. Dhillon is a staff neurologist at Brigham and Women’s Hospital in Boston and is affiliated with Sturdy Memorial Hospital in Attleboro, Mass. He is on the speakers bureaus/advisory boards of Biogen, Bristol Myers Squibb, Genzyme, and Teva Neuroscience. Mr. Zaid Ulhaq Choudhry is a research assistant at the IMCHF. He has no disclosures. Dr. Zia Choudhry (Mr. Choudhry’s father) is chief scientific officer and head of the department of mental health and clinical research at the IMCHF. Dr. Choudhry has no disclosures.
References
1. van Driel M and van Leeuwen JPTM. Mol Cellular Endocrinol. 2017;453:46-51.
2. Charoenngam N and Holick MF. Nutrients. 2020 Jul 15;12(7):2097. doi: 103390/nu12072097.
3. Pinzon RT et al. Trop Med Health. 2020 Dec 20;48:102. doi: 10.1186/S41182-020-00277-w.
4. Hernández JL et al. J Clin Endocrinol Metab. 2021 Mar;106(3)e1343-53.
5. Martineau AR et al. BMJ. 2017;356:i6583. doi: 1136/bmj.i6583.
6. Walrand S. Sci Rep. 2021 Jan 21;11(1981). doi: 10.1038/s41598-021-81419-w.
7. Moan J. et al. Dermatoendocrinol. 2009 Nov-Dec;1(6):307-9.
8. Fabri M et al. Sci Transl Med. 2011 Oct 12;3(104):104ra102. doi: 10.1126/scitranslmed.3003045.
9. Slow S et al. Sci Rep. 2018 Sep 14;8(1):13829. doi: 10.1038/s41598-018-32162-2.
10. Berman R. “Study confirms high doses of vitamin D have no effect on COVID-19.” Medical News Today. 2021 May 4.
11. Menon V et al. Indian J Psychol Med. 2020 Jan-Feb;42(1):11-21.
12. Gloth 3rd FM et al. Nutr Health Aging. 1999;3(1):5-7.
13. Cui X et al. Mol Psychiatry. 2021 Jan 26. doi:10.1038/s41380-021-01025-0.
COVID vaccine controversies: How can hospitalists help?
On April 1, Houston Methodist Hospital in Houston, Texas, announced a new policy that all of its staff would need to be vaccinated against COVID-19 by June 7 in order to hold onto their jobs. Most responded positively but an estimated 150 staff members who did not comply either resigned or were terminated. A lawsuit by employees opposed to the vaccine mandate was dismissed by Federal District Court Judge Lynn Hughes in June, although a subsequent lawsuit was filed Aug. 16.
Vaccines have been shown to dramatically reduce both the incidence and the severity of COVID infections. Vaccinations of health care workers, especially those who have direct contact with patients, are demonstrated to be effective strategies to significantly reduce, although not eliminate, the possibility of viral transmissions to patients – or to health care workers themselves – thus saving lives.
Hospitalists, in their central role in the care of hospitalized patients, and often with primary responsibility for managing their hospital’s COVID-19 caseloads, may find themselves encountering conversations about the vaccine, its safety, effectiveness, and mandates with their peers, other hospital staff, patients, and families, and their communities. They can play key roles in advocating for the vaccine, answering questions, clarifying the science, and dispelling misinformation – for those who are willing to listen.
Becker’s Hospital Review, which has kept an ongoing tally of announced vaccine mandate policies in hospitals, health systems, and health departments nationwide, reported on Aug. 13 that 1,850 or 30% of U.S. hospitals, had announced vaccine mandates.1 Often exceptions can be made, such as for medical or religious reasons, or with other declarations or opt-out provisions. But in many settings, mandating COVID vaccinations won’t be easy.
Amith Skandhan, MD, SFHM, FACP, a hospitalist at Southeast Health Medical Center in Dothan, Ala., and a core faculty member in the internal medicine residency program at Alabama College of Osteopathic Medicine, said that implementing vaccine mandates will be more difficult in smaller health systems, in rural communities, and in states with lower vaccination rates and greater vaccine controversy.
Alabama has the lowest vaccination rates in the country, reflected in the recent rise in COVID cases and hospitalizations, even higher than during the surge of late 2020, Dr. Skandhan said. “In June we had one COVID patient in this hospital.” By late August the number was 119 COVID patients and climbing.
But where he works, in a health system where staffing is already spread thin, a vaccine mandate would be challenging. “What if our staff started leaving? It’s only 10 minutes from here to the Florida or Georgia border,” Dr. Skandhan said. Health care workers opposed to vaccinations would have the option of easily seeking work elsewhere.
When contacted for this article, he had been off work for several days but was mentally preparing himself to go back. “I’m not even following the [COVID-19] numbers but I am prepared for the worst. I know it will be mostly COVID. People just don’t realize what goes into this work.”
Dr. Skandhan, who said he was the third or fourth person in Alabama to receive the COVID vaccine, often finds himself feeling frustrated and angry – in the midst of a surge in cases that could have been prevented – that such a beneficial medical advance for bringing the pandemic under control became so politicized. “It is imperative that we find out why this mistrust exists and work to address it. It has to be done.”
Protecting health care professionals
On July 26, the Society of Hospital Medicine joined 50 other health care organizations including the American Medical Association, American Nurses Association, and American Academy of Pediatrics in advocating for all health care employers to require their employees to be vaccinated against COVID, in order to protect the safety of all patients and residents of health care facilities.2
“As an organization, we support vaccinating health care workers, including hospitalists, to help stop the spread of COVID-19 and the increasingly dominant Delta variant,” said SHM’s chief executive officer Eric E. Howell, MD, MHM, in a prepared statement. “We aim to uphold the highest standards among hospitalists and other health care providers to help protect our fellow health care professionals, our patients, and our communities.”
To that end, Dr. Skandhan has started conversations with hospital staff who he knows are not vaccinated. “For some, we’re not able to have a civil conversation, but in most cases I can help to persuade people.” The reasons people give for not getting vaccinated are not based in science, he said. “I am worried about the safety of our hospitalists and staff nurses.” But unvaccinated frontline workers are also putting their patients at risk. “Can we say why they’re hesitating? Can we have an honest discourse? If we can’t do that with our colleagues, how can we blame the patients?”
Dr. Skandhan encourages hospitalists to start simply in their own hospitals, trying to influence their own departments and colleagues. “If you can convince one or two more every week, you can start a chain reaction. Have that conversation. Use your trust.” For some hospitalized patients, the vaccination conversation comes too late, after their infection, but even some of them might consider obtaining it down the road or trying to persuade family members to get vaccinated.
Adult hospitalists, however, may not have received training in how to effectively address vaccine fears and misconceptions among their patients, he said. Because the patients they see in the hospital are already very sick, they don’t get a lot of practice talking about vaccines except, perhaps, for the influenza vaccine.
Pediatric hospitalists have more experience with such conversations involving their patients’ parents, Dr. Skandhan said. “It comes more naturally to them. We need to learn quickly from them about how to talk about vaccines with our patients.”
Pediatric training and experience
Anika Kumar, MD, FHM, FAAP, a pediatric hospitalist at the Cleveland Clinic and the pediatric editor of The Hospitalist, agrees that pediatricians and pediatric hospitalists often have received more training in how to lead vaccination conversations. She often talks about vaccines with the parents of hospitalized children relative to chicken pox, measles, and other diseases of childhood.
Pediatric hospitalists may also ask to administer the hepatitis B vaccine to newborn babies, along with other preventive treatments such as eye drops and vitamin K shots. “I often encourage the influenza vaccine prior to the patient’s hospital discharge, especially for kids with chronic conditions, asthma, diabetes, or premature birth. We talk about how the influenza vaccine isn’t perfect, but it helps to prevent more serious disease,” she said.
“A lot of vaccine hesitancy comes from misunderstandings about the role of vaccines,” she said. People forget that for years children have been getting vaccines before starting school. “Misinformation and opinions about vaccines have existed for decades. What’s new today is the abundance of sources for obtaining these opinions. My job is to inform families of scientific facts and to address their concerns.”
It has become more common recently for parents to say they don’t want their kids to get vaccinated, Dr. Kumar said. Another group is better described as vaccine hesitant and just needs more information. “I may not, by the time they leave the hospital, convince them to allow me to administer the vaccine. But in the discharge summary, I document that I had this conversation. I’ve done my due diligence and tried to start a larger dialogue. I say: ‘I encourage you to continue this discussion with the pediatrician you trust.’ I also communicate with the outpatient team,” she said.
“But it’s our responsibility, because we’re the ones seeing these patients, to do whatever we can to keep our patients from getting sick. A lot of challenging conversations we have with families are just trying to find out where they’re at with the issue – which can lead to productive dialogue.”
Ariel Carpenter, MD, a 4th-year resident in internal medicine and pediatrics at the University of Louisville (Ky.), and a future pediatric hospitalist, agreed that her combined training in med-peds has been helpful preparation for the vaccine conversation. That training has included techniques of motivational interviewing. In pediatrics, she explained, the communication is a little softer. “I try to approach my patients in a family-centered way.”
Dr. Carpenter recently wrote a personal essay for Louisville Medicine magazine from the perspective of growing up homeschooled by a mother who didn’t believe in vaccines.3 As a teenager, she independently obtained the complete childhood vaccine series so that she could do medical shadowing and volunteering. In medical school she became a passionate vaccine advocate, eventually persuading her mother to change her mind on the subject in time for the COVID vaccine.
“There’s not one answer to the vaccination dilemma,” she said. “Different approaches are required because there are so many different reasons for it. Based on my own life experience, I try to approach patients where they are – not from a place of data and science. What worked in my own family, and works with my patients, is first to establish trust. If they trust you, they’re more likely to listen. Simply ask their worries and concerns,” Dr. Carpenter said.
“A lot of them haven’t had the opportunity before to sit down with a physician they trust and have their worries listened to. They don’t feel heard in our medical system. So I remind myself that I need to understand my patients first – before inserting myself into the conversation.”
Many patients she sees are in an information bubble, with a very different understanding of the issue than their doctors. “A lot of well-meaning people feel they are making the safer choice. Very few truly don’t care about protecting others. But they don’t feel the urgency about that and see the vaccine as the scarier option right now.”
Frontline vaccine advocates
Hospitalists are the frontline advocates within their hospital system, in a position to lead, so they need to make vaccines a priority, Dr. Carpenter said. They should also make sure that their hospitals have ready access to the vaccine, so patients who agree to receive it are able to get it quickly. “In our hospital they can get the shot within a few hours if the opportunity arises. We stocked the Johnson & Johnson vaccine so that they wouldn’t have to connect with another health care provider in order to get a second dose.”
Hospitals should also invest in access to vaccine counseling training and personnel. “Fund a nurse clinician who can screen and counsel hospitalized patients for vaccination. If they meet resistance, they can then refer to the dedicated physician of the day to have the conversation,” she said. “But if we don’t mention it, patients will assume we don’t feel strongly about it.”
Because hospitalists are front and center in treating COVID, they need to be the experts and the people offering guidance, said Shyam Odeti, MD, SFHM, FAAFP, section chief for hospital medicine at the Carilion Clinic in Roanoke, Va. “What we’re trying to do is spread awareness. We educated physician groups, learners, and clinical teams during the initial phase, and now mostly patients and their families.” COVID vaccine reluctance is hard to overcome, Dr. Odeti said. People feel the vaccine was developed very quickly. But there are different ways to present it.
“Like most doctors, I thought people would jump on a vaccine to get past the pandemic. I was surprised and then disappointed. Right now, the pandemic is among the unvaccinated. So we face these encounters, and we’re doing our best to overcome the misinformation. My organization is 100% supportive. We talk about these issues every day.”
Carilion, effective Oct. 1, has required unvaccinated employees to get weekly COVID tests and wear an N95 mask while working, and has developed Facebook pages, other social media, and an Internet presence to address these issues. “We’ve gone to the local African-American community with physician leaders active in that community. We had a Spanish language roundtable,” Dr. Odeti said.
Dr. Skandhan reported that the Wiregrass regional chapter of SHM recently organized a successful statewide community educational event aimed at empowering community leaders to address vaccine misinformation and mistrust. “We surveyed religious leaders and pastors regarding the causes of vaccine hesitancy and reached out to physicians active in community awareness.” Based on that input, a presentation by the faith leaders was developed. Legislators from the Alabama State Senate’s Healthcare Policy Committee were also invited to the presentation and discussion.
Trying to stay positive
It’s important to try to stay positive, Dr. Odeti said. “We have to be empathetic with every patient. We have to keep working at this, since there’s no way out of the pandemic except through vaccinations. But it all creates stress for hospitalists. Our job is made significantly more difficult by the vaccine controversy.”
Jennifer Cowart, MD, a hospitalist at Mayo Clinic in Jacksonville, Fla., has been outspoken in her community about vaccination and masking issues, talking to reporters, attending rallies and press conferences, posting on social media, and speaking in favor of mask policies at a local school board meeting. She is part of an informal local group called Doctors Fighting COVID, which meets online to strategize how to share its expertise, including writing a recent letter about masks to Jacksonville’s mayor.
“In July, when we saw the Delta variant surging locally, we held a webinar via local media, taking calls about the vaccine from the community. I’m trying not to make this a political issue, but we are health officials.” Dr. Cowart said she also tries not to raise her voice when speaking with vaccine opponents and tries to remain empathetic. “Even though inwardly I’m screaming, I try to stay calm. The misinformation is real. People are afraid and feeling pressure. I do my best, but I’m human, too.”
Hospitalists need to pull whatever levers they can to help advance understanding of vaccines, Dr. Cowart said. “In the hospital, our biggest issue is time. We often don’t have it, with a long list of patients to see. But every patient encounter is an opportunity to talk to patients, whether they have COVID or something else.” Sometimes, she might go back to a patient’s room after rounds to resume the conversation.
Hospital nurses have been trained and entrusted to do tobacco abatement counseling, she said, so why not mobilize them for vaccine education? “Or respiratory therapists, who do inhaler training, could talk about what it’s like to care for COVID patients. There’s a whole bunch of staff in the hospital who could be mobilized,” she said.
“I feel passionate about vaccines, as a hospitalist, as a medical educator, as a daughter, as a responsible member of society,” said Eileen Barrett, MD, MPH, SFHM, MACP, director of continuing medical education at the University of New Mexico, Albuquerque. “I see this as a personal and societal responsibility. When I speak about the vaccine among groups of doctors, I say we need to stay in our lane regarding our skills at interpreting the science and not undermining it.”
Some health care worker hesitancy is from distrust of pharmaceutical companies, or of federal agencies, she said. “Our research has highlighted to me the widespread inequity issues in our health care system. We should also take a long, hard look at how we teach the scientific method to health professionals. That will be part of a pandemic retrospective.”
Sometimes with people who are vaccine deliberative, whether health care workers or patients, there is a small window of opportunity. “We need to hear people and respond to them as people. Then, if they are willing to get vaccinated, we need to accomplish that as quickly and easily as possible,” Dr. Barrett said. “I see them make a face and say, ‘Well, okay, I’ll do it.’ We need to get the vaccine to them that same day. We should be able to accomplish that.”
References
1. Gamble M. 30% of US hospitals mandate vaccination for employment. Becker’s Hospital Review. 2021 Aug 13. www.beckershospitalreview.com/workforce/covid-19-vaccination-needed-to-work-at-30-of-us-hospitals.html .
2. Society of Hospital Medicine signs on to joint statement in support of health worker COVID-19 vaccine mandates. Press release. 2021 Jul 26. www.hospitalmedicine.org/news-publications/press-releases/society-of-hospital-medicine-signs-on-to-joint-statement-of-support-of-health-worker-covid-19-vaccine-mandates/.
3. Carpenter A. A physician’s lessons from an unvaccinated childhood. Louisville Medicine. 2021 July;69(2):26-7. https://viewer.joomag.com/louisville-medicine-volume-69-issue-2/0045988001624974172?short&.
Lessons for hospitalists from the vaccination controversy
1. Remain up-to-date on information about the COVID infection, its treatment, and vaccination efficacy data.
2. Hospitalists should take advantage of their positions to lead conversations in their facilities about the importance of COVID vaccinations.
3. Other professionals in the hospital, with some additional training and support, could take on the role of providing vaccine education and support – with a physician to back them up on difficult cases.
4. It’s important to listen to people’s concerns, try to build trust, and establish dialogue before starting to convey a lot of information. People need to feel heard.
5. If you are successful in persuading someone to take the vaccine, a shot should be promptly and easily accessible to them.
6. Pediatric hospitalists may have more experience and skill with vaccine discussions, which they should share with their peers who treat adults.
On April 1, Houston Methodist Hospital in Houston, Texas, announced a new policy that all of its staff would need to be vaccinated against COVID-19 by June 7 in order to hold onto their jobs. Most responded positively but an estimated 150 staff members who did not comply either resigned or were terminated. A lawsuit by employees opposed to the vaccine mandate was dismissed by Federal District Court Judge Lynn Hughes in June, although a subsequent lawsuit was filed Aug. 16.
Vaccines have been shown to dramatically reduce both the incidence and the severity of COVID infections. Vaccinations of health care workers, especially those who have direct contact with patients, are demonstrated to be effective strategies to significantly reduce, although not eliminate, the possibility of viral transmissions to patients – or to health care workers themselves – thus saving lives.
Hospitalists, in their central role in the care of hospitalized patients, and often with primary responsibility for managing their hospital’s COVID-19 caseloads, may find themselves encountering conversations about the vaccine, its safety, effectiveness, and mandates with their peers, other hospital staff, patients, and families, and their communities. They can play key roles in advocating for the vaccine, answering questions, clarifying the science, and dispelling misinformation – for those who are willing to listen.
Becker’s Hospital Review, which has kept an ongoing tally of announced vaccine mandate policies in hospitals, health systems, and health departments nationwide, reported on Aug. 13 that 1,850 or 30% of U.S. hospitals, had announced vaccine mandates.1 Often exceptions can be made, such as for medical or religious reasons, or with other declarations or opt-out provisions. But in many settings, mandating COVID vaccinations won’t be easy.
Amith Skandhan, MD, SFHM, FACP, a hospitalist at Southeast Health Medical Center in Dothan, Ala., and a core faculty member in the internal medicine residency program at Alabama College of Osteopathic Medicine, said that implementing vaccine mandates will be more difficult in smaller health systems, in rural communities, and in states with lower vaccination rates and greater vaccine controversy.
Alabama has the lowest vaccination rates in the country, reflected in the recent rise in COVID cases and hospitalizations, even higher than during the surge of late 2020, Dr. Skandhan said. “In June we had one COVID patient in this hospital.” By late August the number was 119 COVID patients and climbing.
But where he works, in a health system where staffing is already spread thin, a vaccine mandate would be challenging. “What if our staff started leaving? It’s only 10 minutes from here to the Florida or Georgia border,” Dr. Skandhan said. Health care workers opposed to vaccinations would have the option of easily seeking work elsewhere.
When contacted for this article, he had been off work for several days but was mentally preparing himself to go back. “I’m not even following the [COVID-19] numbers but I am prepared for the worst. I know it will be mostly COVID. People just don’t realize what goes into this work.”
Dr. Skandhan, who said he was the third or fourth person in Alabama to receive the COVID vaccine, often finds himself feeling frustrated and angry – in the midst of a surge in cases that could have been prevented – that such a beneficial medical advance for bringing the pandemic under control became so politicized. “It is imperative that we find out why this mistrust exists and work to address it. It has to be done.”
Protecting health care professionals
On July 26, the Society of Hospital Medicine joined 50 other health care organizations including the American Medical Association, American Nurses Association, and American Academy of Pediatrics in advocating for all health care employers to require their employees to be vaccinated against COVID, in order to protect the safety of all patients and residents of health care facilities.2
“As an organization, we support vaccinating health care workers, including hospitalists, to help stop the spread of COVID-19 and the increasingly dominant Delta variant,” said SHM’s chief executive officer Eric E. Howell, MD, MHM, in a prepared statement. “We aim to uphold the highest standards among hospitalists and other health care providers to help protect our fellow health care professionals, our patients, and our communities.”
To that end, Dr. Skandhan has started conversations with hospital staff who he knows are not vaccinated. “For some, we’re not able to have a civil conversation, but in most cases I can help to persuade people.” The reasons people give for not getting vaccinated are not based in science, he said. “I am worried about the safety of our hospitalists and staff nurses.” But unvaccinated frontline workers are also putting their patients at risk. “Can we say why they’re hesitating? Can we have an honest discourse? If we can’t do that with our colleagues, how can we blame the patients?”
Dr. Skandhan encourages hospitalists to start simply in their own hospitals, trying to influence their own departments and colleagues. “If you can convince one or two more every week, you can start a chain reaction. Have that conversation. Use your trust.” For some hospitalized patients, the vaccination conversation comes too late, after their infection, but even some of them might consider obtaining it down the road or trying to persuade family members to get vaccinated.
Adult hospitalists, however, may not have received training in how to effectively address vaccine fears and misconceptions among their patients, he said. Because the patients they see in the hospital are already very sick, they don’t get a lot of practice talking about vaccines except, perhaps, for the influenza vaccine.
Pediatric hospitalists have more experience with such conversations involving their patients’ parents, Dr. Skandhan said. “It comes more naturally to them. We need to learn quickly from them about how to talk about vaccines with our patients.”
Pediatric training and experience
Anika Kumar, MD, FHM, FAAP, a pediatric hospitalist at the Cleveland Clinic and the pediatric editor of The Hospitalist, agrees that pediatricians and pediatric hospitalists often have received more training in how to lead vaccination conversations. She often talks about vaccines with the parents of hospitalized children relative to chicken pox, measles, and other diseases of childhood.
Pediatric hospitalists may also ask to administer the hepatitis B vaccine to newborn babies, along with other preventive treatments such as eye drops and vitamin K shots. “I often encourage the influenza vaccine prior to the patient’s hospital discharge, especially for kids with chronic conditions, asthma, diabetes, or premature birth. We talk about how the influenza vaccine isn’t perfect, but it helps to prevent more serious disease,” she said.
“A lot of vaccine hesitancy comes from misunderstandings about the role of vaccines,” she said. People forget that for years children have been getting vaccines before starting school. “Misinformation and opinions about vaccines have existed for decades. What’s new today is the abundance of sources for obtaining these opinions. My job is to inform families of scientific facts and to address their concerns.”
It has become more common recently for parents to say they don’t want their kids to get vaccinated, Dr. Kumar said. Another group is better described as vaccine hesitant and just needs more information. “I may not, by the time they leave the hospital, convince them to allow me to administer the vaccine. But in the discharge summary, I document that I had this conversation. I’ve done my due diligence and tried to start a larger dialogue. I say: ‘I encourage you to continue this discussion with the pediatrician you trust.’ I also communicate with the outpatient team,” she said.
“But it’s our responsibility, because we’re the ones seeing these patients, to do whatever we can to keep our patients from getting sick. A lot of challenging conversations we have with families are just trying to find out where they’re at with the issue – which can lead to productive dialogue.”
Ariel Carpenter, MD, a 4th-year resident in internal medicine and pediatrics at the University of Louisville (Ky.), and a future pediatric hospitalist, agreed that her combined training in med-peds has been helpful preparation for the vaccine conversation. That training has included techniques of motivational interviewing. In pediatrics, she explained, the communication is a little softer. “I try to approach my patients in a family-centered way.”
Dr. Carpenter recently wrote a personal essay for Louisville Medicine magazine from the perspective of growing up homeschooled by a mother who didn’t believe in vaccines.3 As a teenager, she independently obtained the complete childhood vaccine series so that she could do medical shadowing and volunteering. In medical school she became a passionate vaccine advocate, eventually persuading her mother to change her mind on the subject in time for the COVID vaccine.
“There’s not one answer to the vaccination dilemma,” she said. “Different approaches are required because there are so many different reasons for it. Based on my own life experience, I try to approach patients where they are – not from a place of data and science. What worked in my own family, and works with my patients, is first to establish trust. If they trust you, they’re more likely to listen. Simply ask their worries and concerns,” Dr. Carpenter said.
“A lot of them haven’t had the opportunity before to sit down with a physician they trust and have their worries listened to. They don’t feel heard in our medical system. So I remind myself that I need to understand my patients first – before inserting myself into the conversation.”
Many patients she sees are in an information bubble, with a very different understanding of the issue than their doctors. “A lot of well-meaning people feel they are making the safer choice. Very few truly don’t care about protecting others. But they don’t feel the urgency about that and see the vaccine as the scarier option right now.”
Frontline vaccine advocates
Hospitalists are the frontline advocates within their hospital system, in a position to lead, so they need to make vaccines a priority, Dr. Carpenter said. They should also make sure that their hospitals have ready access to the vaccine, so patients who agree to receive it are able to get it quickly. “In our hospital they can get the shot within a few hours if the opportunity arises. We stocked the Johnson & Johnson vaccine so that they wouldn’t have to connect with another health care provider in order to get a second dose.”
Hospitals should also invest in access to vaccine counseling training and personnel. “Fund a nurse clinician who can screen and counsel hospitalized patients for vaccination. If they meet resistance, they can then refer to the dedicated physician of the day to have the conversation,” she said. “But if we don’t mention it, patients will assume we don’t feel strongly about it.”
Because hospitalists are front and center in treating COVID, they need to be the experts and the people offering guidance, said Shyam Odeti, MD, SFHM, FAAFP, section chief for hospital medicine at the Carilion Clinic in Roanoke, Va. “What we’re trying to do is spread awareness. We educated physician groups, learners, and clinical teams during the initial phase, and now mostly patients and their families.” COVID vaccine reluctance is hard to overcome, Dr. Odeti said. People feel the vaccine was developed very quickly. But there are different ways to present it.
“Like most doctors, I thought people would jump on a vaccine to get past the pandemic. I was surprised and then disappointed. Right now, the pandemic is among the unvaccinated. So we face these encounters, and we’re doing our best to overcome the misinformation. My organization is 100% supportive. We talk about these issues every day.”
Carilion, effective Oct. 1, has required unvaccinated employees to get weekly COVID tests and wear an N95 mask while working, and has developed Facebook pages, other social media, and an Internet presence to address these issues. “We’ve gone to the local African-American community with physician leaders active in that community. We had a Spanish language roundtable,” Dr. Odeti said.
Dr. Skandhan reported that the Wiregrass regional chapter of SHM recently organized a successful statewide community educational event aimed at empowering community leaders to address vaccine misinformation and mistrust. “We surveyed religious leaders and pastors regarding the causes of vaccine hesitancy and reached out to physicians active in community awareness.” Based on that input, a presentation by the faith leaders was developed. Legislators from the Alabama State Senate’s Healthcare Policy Committee were also invited to the presentation and discussion.
Trying to stay positive
It’s important to try to stay positive, Dr. Odeti said. “We have to be empathetic with every patient. We have to keep working at this, since there’s no way out of the pandemic except through vaccinations. But it all creates stress for hospitalists. Our job is made significantly more difficult by the vaccine controversy.”
Jennifer Cowart, MD, a hospitalist at Mayo Clinic in Jacksonville, Fla., has been outspoken in her community about vaccination and masking issues, talking to reporters, attending rallies and press conferences, posting on social media, and speaking in favor of mask policies at a local school board meeting. She is part of an informal local group called Doctors Fighting COVID, which meets online to strategize how to share its expertise, including writing a recent letter about masks to Jacksonville’s mayor.
“In July, when we saw the Delta variant surging locally, we held a webinar via local media, taking calls about the vaccine from the community. I’m trying not to make this a political issue, but we are health officials.” Dr. Cowart said she also tries not to raise her voice when speaking with vaccine opponents and tries to remain empathetic. “Even though inwardly I’m screaming, I try to stay calm. The misinformation is real. People are afraid and feeling pressure. I do my best, but I’m human, too.”
Hospitalists need to pull whatever levers they can to help advance understanding of vaccines, Dr. Cowart said. “In the hospital, our biggest issue is time. We often don’t have it, with a long list of patients to see. But every patient encounter is an opportunity to talk to patients, whether they have COVID or something else.” Sometimes, she might go back to a patient’s room after rounds to resume the conversation.
Hospital nurses have been trained and entrusted to do tobacco abatement counseling, she said, so why not mobilize them for vaccine education? “Or respiratory therapists, who do inhaler training, could talk about what it’s like to care for COVID patients. There’s a whole bunch of staff in the hospital who could be mobilized,” she said.
“I feel passionate about vaccines, as a hospitalist, as a medical educator, as a daughter, as a responsible member of society,” said Eileen Barrett, MD, MPH, SFHM, MACP, director of continuing medical education at the University of New Mexico, Albuquerque. “I see this as a personal and societal responsibility. When I speak about the vaccine among groups of doctors, I say we need to stay in our lane regarding our skills at interpreting the science and not undermining it.”
Some health care worker hesitancy is from distrust of pharmaceutical companies, or of federal agencies, she said. “Our research has highlighted to me the widespread inequity issues in our health care system. We should also take a long, hard look at how we teach the scientific method to health professionals. That will be part of a pandemic retrospective.”
Sometimes with people who are vaccine deliberative, whether health care workers or patients, there is a small window of opportunity. “We need to hear people and respond to them as people. Then, if they are willing to get vaccinated, we need to accomplish that as quickly and easily as possible,” Dr. Barrett said. “I see them make a face and say, ‘Well, okay, I’ll do it.’ We need to get the vaccine to them that same day. We should be able to accomplish that.”
References
1. Gamble M. 30% of US hospitals mandate vaccination for employment. Becker’s Hospital Review. 2021 Aug 13. www.beckershospitalreview.com/workforce/covid-19-vaccination-needed-to-work-at-30-of-us-hospitals.html .
2. Society of Hospital Medicine signs on to joint statement in support of health worker COVID-19 vaccine mandates. Press release. 2021 Jul 26. www.hospitalmedicine.org/news-publications/press-releases/society-of-hospital-medicine-signs-on-to-joint-statement-of-support-of-health-worker-covid-19-vaccine-mandates/.
3. Carpenter A. A physician’s lessons from an unvaccinated childhood. Louisville Medicine. 2021 July;69(2):26-7. https://viewer.joomag.com/louisville-medicine-volume-69-issue-2/0045988001624974172?short&.
Lessons for hospitalists from the vaccination controversy
1. Remain up-to-date on information about the COVID infection, its treatment, and vaccination efficacy data.
2. Hospitalists should take advantage of their positions to lead conversations in their facilities about the importance of COVID vaccinations.
3. Other professionals in the hospital, with some additional training and support, could take on the role of providing vaccine education and support – with a physician to back them up on difficult cases.
4. It’s important to listen to people’s concerns, try to build trust, and establish dialogue before starting to convey a lot of information. People need to feel heard.
5. If you are successful in persuading someone to take the vaccine, a shot should be promptly and easily accessible to them.
6. Pediatric hospitalists may have more experience and skill with vaccine discussions, which they should share with their peers who treat adults.
On April 1, Houston Methodist Hospital in Houston, Texas, announced a new policy that all of its staff would need to be vaccinated against COVID-19 by June 7 in order to hold onto their jobs. Most responded positively but an estimated 150 staff members who did not comply either resigned or were terminated. A lawsuit by employees opposed to the vaccine mandate was dismissed by Federal District Court Judge Lynn Hughes in June, although a subsequent lawsuit was filed Aug. 16.
Vaccines have been shown to dramatically reduce both the incidence and the severity of COVID infections. Vaccinations of health care workers, especially those who have direct contact with patients, are demonstrated to be effective strategies to significantly reduce, although not eliminate, the possibility of viral transmissions to patients – or to health care workers themselves – thus saving lives.
Hospitalists, in their central role in the care of hospitalized patients, and often with primary responsibility for managing their hospital’s COVID-19 caseloads, may find themselves encountering conversations about the vaccine, its safety, effectiveness, and mandates with their peers, other hospital staff, patients, and families, and their communities. They can play key roles in advocating for the vaccine, answering questions, clarifying the science, and dispelling misinformation – for those who are willing to listen.
Becker’s Hospital Review, which has kept an ongoing tally of announced vaccine mandate policies in hospitals, health systems, and health departments nationwide, reported on Aug. 13 that 1,850 or 30% of U.S. hospitals, had announced vaccine mandates.1 Often exceptions can be made, such as for medical or religious reasons, or with other declarations or opt-out provisions. But in many settings, mandating COVID vaccinations won’t be easy.
Amith Skandhan, MD, SFHM, FACP, a hospitalist at Southeast Health Medical Center in Dothan, Ala., and a core faculty member in the internal medicine residency program at Alabama College of Osteopathic Medicine, said that implementing vaccine mandates will be more difficult in smaller health systems, in rural communities, and in states with lower vaccination rates and greater vaccine controversy.
Alabama has the lowest vaccination rates in the country, reflected in the recent rise in COVID cases and hospitalizations, even higher than during the surge of late 2020, Dr. Skandhan said. “In June we had one COVID patient in this hospital.” By late August the number was 119 COVID patients and climbing.
But where he works, in a health system where staffing is already spread thin, a vaccine mandate would be challenging. “What if our staff started leaving? It’s only 10 minutes from here to the Florida or Georgia border,” Dr. Skandhan said. Health care workers opposed to vaccinations would have the option of easily seeking work elsewhere.
When contacted for this article, he had been off work for several days but was mentally preparing himself to go back. “I’m not even following the [COVID-19] numbers but I am prepared for the worst. I know it will be mostly COVID. People just don’t realize what goes into this work.”
Dr. Skandhan, who said he was the third or fourth person in Alabama to receive the COVID vaccine, often finds himself feeling frustrated and angry – in the midst of a surge in cases that could have been prevented – that such a beneficial medical advance for bringing the pandemic under control became so politicized. “It is imperative that we find out why this mistrust exists and work to address it. It has to be done.”
Protecting health care professionals
On July 26, the Society of Hospital Medicine joined 50 other health care organizations including the American Medical Association, American Nurses Association, and American Academy of Pediatrics in advocating for all health care employers to require their employees to be vaccinated against COVID, in order to protect the safety of all patients and residents of health care facilities.2
“As an organization, we support vaccinating health care workers, including hospitalists, to help stop the spread of COVID-19 and the increasingly dominant Delta variant,” said SHM’s chief executive officer Eric E. Howell, MD, MHM, in a prepared statement. “We aim to uphold the highest standards among hospitalists and other health care providers to help protect our fellow health care professionals, our patients, and our communities.”
To that end, Dr. Skandhan has started conversations with hospital staff who he knows are not vaccinated. “For some, we’re not able to have a civil conversation, but in most cases I can help to persuade people.” The reasons people give for not getting vaccinated are not based in science, he said. “I am worried about the safety of our hospitalists and staff nurses.” But unvaccinated frontline workers are also putting their patients at risk. “Can we say why they’re hesitating? Can we have an honest discourse? If we can’t do that with our colleagues, how can we blame the patients?”
Dr. Skandhan encourages hospitalists to start simply in their own hospitals, trying to influence their own departments and colleagues. “If you can convince one or two more every week, you can start a chain reaction. Have that conversation. Use your trust.” For some hospitalized patients, the vaccination conversation comes too late, after their infection, but even some of them might consider obtaining it down the road or trying to persuade family members to get vaccinated.
Adult hospitalists, however, may not have received training in how to effectively address vaccine fears and misconceptions among their patients, he said. Because the patients they see in the hospital are already very sick, they don’t get a lot of practice talking about vaccines except, perhaps, for the influenza vaccine.
Pediatric hospitalists have more experience with such conversations involving their patients’ parents, Dr. Skandhan said. “It comes more naturally to them. We need to learn quickly from them about how to talk about vaccines with our patients.”
Pediatric training and experience
Anika Kumar, MD, FHM, FAAP, a pediatric hospitalist at the Cleveland Clinic and the pediatric editor of The Hospitalist, agrees that pediatricians and pediatric hospitalists often have received more training in how to lead vaccination conversations. She often talks about vaccines with the parents of hospitalized children relative to chicken pox, measles, and other diseases of childhood.
Pediatric hospitalists may also ask to administer the hepatitis B vaccine to newborn babies, along with other preventive treatments such as eye drops and vitamin K shots. “I often encourage the influenza vaccine prior to the patient’s hospital discharge, especially for kids with chronic conditions, asthma, diabetes, or premature birth. We talk about how the influenza vaccine isn’t perfect, but it helps to prevent more serious disease,” she said.
“A lot of vaccine hesitancy comes from misunderstandings about the role of vaccines,” she said. People forget that for years children have been getting vaccines before starting school. “Misinformation and opinions about vaccines have existed for decades. What’s new today is the abundance of sources for obtaining these opinions. My job is to inform families of scientific facts and to address their concerns.”
It has become more common recently for parents to say they don’t want their kids to get vaccinated, Dr. Kumar said. Another group is better described as vaccine hesitant and just needs more information. “I may not, by the time they leave the hospital, convince them to allow me to administer the vaccine. But in the discharge summary, I document that I had this conversation. I’ve done my due diligence and tried to start a larger dialogue. I say: ‘I encourage you to continue this discussion with the pediatrician you trust.’ I also communicate with the outpatient team,” she said.
“But it’s our responsibility, because we’re the ones seeing these patients, to do whatever we can to keep our patients from getting sick. A lot of challenging conversations we have with families are just trying to find out where they’re at with the issue – which can lead to productive dialogue.”
Ariel Carpenter, MD, a 4th-year resident in internal medicine and pediatrics at the University of Louisville (Ky.), and a future pediatric hospitalist, agreed that her combined training in med-peds has been helpful preparation for the vaccine conversation. That training has included techniques of motivational interviewing. In pediatrics, she explained, the communication is a little softer. “I try to approach my patients in a family-centered way.”
Dr. Carpenter recently wrote a personal essay for Louisville Medicine magazine from the perspective of growing up homeschooled by a mother who didn’t believe in vaccines.3 As a teenager, she independently obtained the complete childhood vaccine series so that she could do medical shadowing and volunteering. In medical school she became a passionate vaccine advocate, eventually persuading her mother to change her mind on the subject in time for the COVID vaccine.
“There’s not one answer to the vaccination dilemma,” she said. “Different approaches are required because there are so many different reasons for it. Based on my own life experience, I try to approach patients where they are – not from a place of data and science. What worked in my own family, and works with my patients, is first to establish trust. If they trust you, they’re more likely to listen. Simply ask their worries and concerns,” Dr. Carpenter said.
“A lot of them haven’t had the opportunity before to sit down with a physician they trust and have their worries listened to. They don’t feel heard in our medical system. So I remind myself that I need to understand my patients first – before inserting myself into the conversation.”
Many patients she sees are in an information bubble, with a very different understanding of the issue than their doctors. “A lot of well-meaning people feel they are making the safer choice. Very few truly don’t care about protecting others. But they don’t feel the urgency about that and see the vaccine as the scarier option right now.”
Frontline vaccine advocates
Hospitalists are the frontline advocates within their hospital system, in a position to lead, so they need to make vaccines a priority, Dr. Carpenter said. They should also make sure that their hospitals have ready access to the vaccine, so patients who agree to receive it are able to get it quickly. “In our hospital they can get the shot within a few hours if the opportunity arises. We stocked the Johnson & Johnson vaccine so that they wouldn’t have to connect with another health care provider in order to get a second dose.”
Hospitals should also invest in access to vaccine counseling training and personnel. “Fund a nurse clinician who can screen and counsel hospitalized patients for vaccination. If they meet resistance, they can then refer to the dedicated physician of the day to have the conversation,” she said. “But if we don’t mention it, patients will assume we don’t feel strongly about it.”
Because hospitalists are front and center in treating COVID, they need to be the experts and the people offering guidance, said Shyam Odeti, MD, SFHM, FAAFP, section chief for hospital medicine at the Carilion Clinic in Roanoke, Va. “What we’re trying to do is spread awareness. We educated physician groups, learners, and clinical teams during the initial phase, and now mostly patients and their families.” COVID vaccine reluctance is hard to overcome, Dr. Odeti said. People feel the vaccine was developed very quickly. But there are different ways to present it.
“Like most doctors, I thought people would jump on a vaccine to get past the pandemic. I was surprised and then disappointed. Right now, the pandemic is among the unvaccinated. So we face these encounters, and we’re doing our best to overcome the misinformation. My organization is 100% supportive. We talk about these issues every day.”
Carilion, effective Oct. 1, has required unvaccinated employees to get weekly COVID tests and wear an N95 mask while working, and has developed Facebook pages, other social media, and an Internet presence to address these issues. “We’ve gone to the local African-American community with physician leaders active in that community. We had a Spanish language roundtable,” Dr. Odeti said.
Dr. Skandhan reported that the Wiregrass regional chapter of SHM recently organized a successful statewide community educational event aimed at empowering community leaders to address vaccine misinformation and mistrust. “We surveyed religious leaders and pastors regarding the causes of vaccine hesitancy and reached out to physicians active in community awareness.” Based on that input, a presentation by the faith leaders was developed. Legislators from the Alabama State Senate’s Healthcare Policy Committee were also invited to the presentation and discussion.
Trying to stay positive
It’s important to try to stay positive, Dr. Odeti said. “We have to be empathetic with every patient. We have to keep working at this, since there’s no way out of the pandemic except through vaccinations. But it all creates stress for hospitalists. Our job is made significantly more difficult by the vaccine controversy.”
Jennifer Cowart, MD, a hospitalist at Mayo Clinic in Jacksonville, Fla., has been outspoken in her community about vaccination and masking issues, talking to reporters, attending rallies and press conferences, posting on social media, and speaking in favor of mask policies at a local school board meeting. She is part of an informal local group called Doctors Fighting COVID, which meets online to strategize how to share its expertise, including writing a recent letter about masks to Jacksonville’s mayor.
“In July, when we saw the Delta variant surging locally, we held a webinar via local media, taking calls about the vaccine from the community. I’m trying not to make this a political issue, but we are health officials.” Dr. Cowart said she also tries not to raise her voice when speaking with vaccine opponents and tries to remain empathetic. “Even though inwardly I’m screaming, I try to stay calm. The misinformation is real. People are afraid and feeling pressure. I do my best, but I’m human, too.”
Hospitalists need to pull whatever levers they can to help advance understanding of vaccines, Dr. Cowart said. “In the hospital, our biggest issue is time. We often don’t have it, with a long list of patients to see. But every patient encounter is an opportunity to talk to patients, whether they have COVID or something else.” Sometimes, she might go back to a patient’s room after rounds to resume the conversation.
Hospital nurses have been trained and entrusted to do tobacco abatement counseling, she said, so why not mobilize them for vaccine education? “Or respiratory therapists, who do inhaler training, could talk about what it’s like to care for COVID patients. There’s a whole bunch of staff in the hospital who could be mobilized,” she said.
“I feel passionate about vaccines, as a hospitalist, as a medical educator, as a daughter, as a responsible member of society,” said Eileen Barrett, MD, MPH, SFHM, MACP, director of continuing medical education at the University of New Mexico, Albuquerque. “I see this as a personal and societal responsibility. When I speak about the vaccine among groups of doctors, I say we need to stay in our lane regarding our skills at interpreting the science and not undermining it.”
Some health care worker hesitancy is from distrust of pharmaceutical companies, or of federal agencies, she said. “Our research has highlighted to me the widespread inequity issues in our health care system. We should also take a long, hard look at how we teach the scientific method to health professionals. That will be part of a pandemic retrospective.”
Sometimes with people who are vaccine deliberative, whether health care workers or patients, there is a small window of opportunity. “We need to hear people and respond to them as people. Then, if they are willing to get vaccinated, we need to accomplish that as quickly and easily as possible,” Dr. Barrett said. “I see them make a face and say, ‘Well, okay, I’ll do it.’ We need to get the vaccine to them that same day. We should be able to accomplish that.”
References
1. Gamble M. 30% of US hospitals mandate vaccination for employment. Becker’s Hospital Review. 2021 Aug 13. www.beckershospitalreview.com/workforce/covid-19-vaccination-needed-to-work-at-30-of-us-hospitals.html .
2. Society of Hospital Medicine signs on to joint statement in support of health worker COVID-19 vaccine mandates. Press release. 2021 Jul 26. www.hospitalmedicine.org/news-publications/press-releases/society-of-hospital-medicine-signs-on-to-joint-statement-of-support-of-health-worker-covid-19-vaccine-mandates/.
3. Carpenter A. A physician’s lessons from an unvaccinated childhood. Louisville Medicine. 2021 July;69(2):26-7. https://viewer.joomag.com/louisville-medicine-volume-69-issue-2/0045988001624974172?short&.
Lessons for hospitalists from the vaccination controversy
1. Remain up-to-date on information about the COVID infection, its treatment, and vaccination efficacy data.
2. Hospitalists should take advantage of their positions to lead conversations in their facilities about the importance of COVID vaccinations.
3. Other professionals in the hospital, with some additional training and support, could take on the role of providing vaccine education and support – with a physician to back them up on difficult cases.
4. It’s important to listen to people’s concerns, try to build trust, and establish dialogue before starting to convey a lot of information. People need to feel heard.
5. If you are successful in persuading someone to take the vaccine, a shot should be promptly and easily accessible to them.
6. Pediatric hospitalists may have more experience and skill with vaccine discussions, which they should share with their peers who treat adults.














