User login
New reports help nail down myocarditis risk with COVID-19 vaccine
Recent literature features new descriptions of myocarditis linked to the two available mRNA vaccines against SARS-CoV-2. They tell a story largely consistent with experience to date, and support what might be its most useful public health message: The associated myocarditis is usually mild and self-limiting, and is far less likely to occur than myocarditis or death in unvaccinated people with COVID-19.
In line with previous research, the new analyses suggest the myocarditis – with onset usually a few days to a week after injection – has an overall incidence that ranges from less than 1 to perhaps 3 per 100,000 people who received at least one of the full mRNA-vaccine regimen’s two injections. Also, as in earlier studies, the incidence climbed higher – sometimes sharply – in certain groups by age and sex, particularly in young men and older male teens.
The new studies “are confirmatory, in terms of the risk being low,” but underscore that clinicians still must be wary of myocarditis as a potential complication of the mRNA vaccines, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, told this news organization.
Dr. Bozkurt, a leading heart failure specialist and researcher, did not contribute to any of the new reports but does study the myocarditis of COVID-19 and was lead author on a recent review of the potential vaccine complication’s features and possible mechanisms.
In the new myocarditis reports, she observed, more than 90% of the cases were mild and “resolved on their own without a major adverse outcome.” Dr. Bozkurt emphasized the need for perspective regarding the risk. For example, the myocarditis associated with SARS-CoV-2 infection is not only more likely than the vaccine-related myocarditis, but it’s also usually far more severe.
Dr. Bozkurt pointed to a recent study in which the mRNA vaccines, compared with no vaccination, appeared to escalate the myocarditis risk by a factor of 3, whereas the risk for myocarditis in SARS-CoV-2 infection was increased 18 times.
In contrast, she observed, the new myocarditis cases reported this week feature a few that are novel or are at least very rare, including the case of a patient who developed cardiogenic shock and another with fulminant myocarditis who died.
The Centers for Disease Control and Prevention in May publicly described the apparent link between myocarditis and the two available mRNA vaccines against SARS-CoV-2: BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). The next month, the Food and Drug Administration added a warning about the risk to the labeling.
Less than 1 case per 100,000
Fifteen confirmed cases of myocarditis were identified among about 2.4 million members of Kaiser Permanente Southern California aged 18 or older who received at least one injection of the Pfizer or Moderna mRNA vaccines between December 2020 and July 2021, in a report published in JAMA Internal Medicine. The study counted cases up to 10 days after the first or second injection, of which there were 2 and 13, respectively.
All eight patients who received the Pfizer BNT162b2 vaccine and the eight given the Moderna mRNA-1273 vaccine were male with a median age of 25 years (interquartile range, 20-32 years).
“The main takeaway messages from our study are that the incidence of myocarditis after COVID-19 mRNA vaccinations is very low, that this condition is primarily observed in young men within a few days after the second dose, and that most patients recover quickly,” senior author Mingsum Lee, MD, PhD, Kaiser Permanente Los Angeles Medical Center, told this news organization.
“The incidence of vaccine-related myocarditis was significantly lower than rates of COVID-19 hospitalization during the same period and population area,” she added.
The group saw a per-million incidence of 0.8 and 5.8 myocarditis cases in the 10 days after first and second injections, respectively. That made for an incidence of 0.58 per 100,000, or 1 case per 172,414 fully vaccinated adults.
The group also considered a cohort of 1,577,741 unvaccinated people with a median age of 39 years (interquartile range, 28-53 years) during the same period. Of the 75 cases of myocarditis, 52% were in men, they reported.
Comparing the vaccinated and unvaccinated cohorts, they saw a 10-day vaccine-associated myocarditis incidence rate ratio of 0.38 (95% confidence interval, 0.05-1.40; P = .15) after the first dose, and 2.7 (95% CI, 1.4-4.8; P = .004) after the second dose.
In a comparison of the vaccinated group with itself using data from a 10-day period in the previous year, the corresponding myocarditis IRRs were 1.0 (P > .99) and 3.3 (P = .03), respectively.
Dr. Lee said none of the 15 patients required admission to an intensive care unit. “All patients with myocarditis responded well to treatment and felt better quickly,” she noted.
Myocarditis after an mRNA vaccine injection is rare and, Dr. Lee said emphatically, and “the benefits of the COVID-19 vaccine greatly outweigh the risks.”
Sex- and age-stratified rates
In a separate analysis of 5,442,696 people given a first dose of the Pfizer BNT162b2 vaccine and 5,125,635 given a second dose, there were 142 cases of myocarditis with onset 21 days after dose 1 and 30 days after dose 2. Of those cases, 136 were documented as “definite or probable” in an Israeli Ministry of Health database that covered up to the end of May 2021.
There were also 40 cases among vaccinated people seen after the 30-day window, which were considered not related to the vaccination, and 101 cases among unvaccinated people; of the latter, 29 had confirmed diagnoses of COVID-19.
Of the 136 people with definite or probable cases, the myocarditis was “generally mild” in 129 and usually resolved on its own, notes the report on the study, published in the New England Journal of Medicine, with lead author Dror Mevorach, MD, Hadassah-Hebrew University Medical Center, Jerusalem.
The estimated myocarditis incidence after a second such vaccine dose across the entire Israeli population, based on the current study, was about one per 26,000 males and one per 218,000 females, the group writes. Those figures compare with one case per 10,857 among “the general unvaccinated population.”
Again, the risk was concentrated among younger men and male adolescents. In an analysis limited to vaccinated people aged 16-19 years, myocarditis in the 21 days after a second mRNA injection was seen in about one of 6,637 males and one of 99,853 females, the group reported.
The standardized incidence ratio of 5.34 (95% CI, 4.48-6.40) after a second injection, across all groups, “was driven mostly by the diagnosis of myocarditis in younger male recipients.” Among that male subgroup, the ratios by age group were 13.60 (95% CI, 9.30-19.20) for 16-19 years, 8.53 (95% CI, 5.57-12.50) for 20-24 years, and 6.96 (95% CI, 4.25-10.75) for 25-29 years.
Among people who received a second injection, compared with unvaccinated people, the 30-day rate ratio was 2.35 (95% CI, 1.10-5.02). Again, the effect was concentrated in males aged 16-19 years. Among them, the myocarditis rate ratios in the 30 days after a second mRNA vaccine injection were 8.96 (95% CI, 4.50-17.83) for the 16-19 years group, 6.13 (95% CI, 3.16-11.88) for the 20-24 group, and 3.58 (95% CI, 1.82-7.01) for 25-29 years.
Most of the patients with myocarditis showed “significant clinical improvement,” with a mean hospitalization time of only 3-4 days, the report notes. Treatment consisted of nonsteroidal anti-inflammatory drugs “with or without colchicine for presumed pericardial inflammation.”
However, seven patients (4.9%) developed important complications, including left-ventricular dysfunction, ventricular arrhythmias, and heart failure. Among them was a 22-year-old patient who died of fulminant myocarditis within 24 hours of diagnosis, the group wrote.
From an Israeli health care organization
Published by the same journal as the study by Dr. Menvorach and associates, an analysis of a separate database showed largely consistent findings among patients in the largest of Israel’s four health care organizations charged by the government to administer health services.
The report, with authors led by Guy Witberg, MD, Rabin Medical Center, Petah Tikva, Israel, focused on members of the health care organization aged 16 years or older who had received at least one Pfizer mRNA vaccine dose by the end of May 2021.
The cohorts from the two separate reports surely overlap substantially, as the Ministry of Health analysis from Dr. Mevorach and colleagues derived from a nationwide database, and – as Dr. Witberg and associates wrote – the health care organization providing their data covers 52% of the Israeli population.
Of 2,558,421 vaccinated people in the analysis, of whom 94% received two doses, 54 developed confirmed myocarditis in the 42 days after the first dose. Their median age was 27 years (interquartile range, 21-35 years) and all but three (94%) were male. Of those 54 cases, 41 were considered mild and 12 intermediate in severity, and one was fulminant with the patient in cardiogenic shock, the group writes. In addition, nonsustained ventricular tachycardia and atrial fibrillation developed in 5% and 3% of cases, respectively.
The estimated myocarditis incidence in the 42 days after administration of at least one mRNA vaccine dose was 2.13 per 100,000 vaccinated people. In that group, Dr. Witberg and colleagues note, the corresponding incidences per 100,000 were 4.12 and 0.23 for males and females, respectively.
Also in the current report, incidences per 100,000 vaccinated people aged 16-29 years, by sex, included 5.49 (95% CI, 3.59-7.39) overall, and 10.69 (95% CI, 6.93-14.46) for males (the highest rate in the report).
There was only one case in a female aged 16-29 years, and two cases in females 30 years or older.
Of note, some authors of the current study are also authors on the high-profile report from Noam Barda, MD, and colleagues published in the New England Journal of Medicine, that used the same database to arrive at an mRNA-vaccine-related incidence of myocarditis of 2.7 per 100,000. Eligibility criteria and follow-up time were different in that report, as were case ascertainment criteria.
The myocarditis risk associated with the two mRNA vaccines is small compared with “the morbidity and mortality of COVID-19 infection, in which up to 28% of hospitalized patients showed signs of myocardial injury,” wrote Vinay Guduguntla, MD, University of California, San Francisco, and Mitchell H. Katz, MD, NYC Health + Hospitals, New York, in an editorial accompanying the report from Dr. Lee and associates.
“Randomized clinical trials show that COVID-19 mRNA vaccines represent a safe and effective method of preventing infection,” they stated. “The identification of rare myocarditis does not change clinical decision-making.”
Dr. Bozkurt, who is immediate past president of the Heart Failure Society of America, has disclosed consulting for Bayer and scPharmaceuticals and serving on a clinical events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. Lee and the report’s other authors had no disclosures. Dr. Mevorach discloses consulting for Enlivex Therapeutics; disclosures for the other authors are available at NEJM.org. Dr. Witberg said he has no interests to disclose; disclosures for the other authors are available at NEJM.org. Dr. Guduguntla is an editorial fellow and Dr. Katz a deputy editor at JAMA Internal Medicine; neither had disclosures.
A version of this article first appeared on Medscape.com.
Recent literature features new descriptions of myocarditis linked to the two available mRNA vaccines against SARS-CoV-2. They tell a story largely consistent with experience to date, and support what might be its most useful public health message: The associated myocarditis is usually mild and self-limiting, and is far less likely to occur than myocarditis or death in unvaccinated people with COVID-19.
In line with previous research, the new analyses suggest the myocarditis – with onset usually a few days to a week after injection – has an overall incidence that ranges from less than 1 to perhaps 3 per 100,000 people who received at least one of the full mRNA-vaccine regimen’s two injections. Also, as in earlier studies, the incidence climbed higher – sometimes sharply – in certain groups by age and sex, particularly in young men and older male teens.
The new studies “are confirmatory, in terms of the risk being low,” but underscore that clinicians still must be wary of myocarditis as a potential complication of the mRNA vaccines, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, told this news organization.
Dr. Bozkurt, a leading heart failure specialist and researcher, did not contribute to any of the new reports but does study the myocarditis of COVID-19 and was lead author on a recent review of the potential vaccine complication’s features and possible mechanisms.
In the new myocarditis reports, she observed, more than 90% of the cases were mild and “resolved on their own without a major adverse outcome.” Dr. Bozkurt emphasized the need for perspective regarding the risk. For example, the myocarditis associated with SARS-CoV-2 infection is not only more likely than the vaccine-related myocarditis, but it’s also usually far more severe.
Dr. Bozkurt pointed to a recent study in which the mRNA vaccines, compared with no vaccination, appeared to escalate the myocarditis risk by a factor of 3, whereas the risk for myocarditis in SARS-CoV-2 infection was increased 18 times.
In contrast, she observed, the new myocarditis cases reported this week feature a few that are novel or are at least very rare, including the case of a patient who developed cardiogenic shock and another with fulminant myocarditis who died.
The Centers for Disease Control and Prevention in May publicly described the apparent link between myocarditis and the two available mRNA vaccines against SARS-CoV-2: BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). The next month, the Food and Drug Administration added a warning about the risk to the labeling.
Less than 1 case per 100,000
Fifteen confirmed cases of myocarditis were identified among about 2.4 million members of Kaiser Permanente Southern California aged 18 or older who received at least one injection of the Pfizer or Moderna mRNA vaccines between December 2020 and July 2021, in a report published in JAMA Internal Medicine. The study counted cases up to 10 days after the first or second injection, of which there were 2 and 13, respectively.
All eight patients who received the Pfizer BNT162b2 vaccine and the eight given the Moderna mRNA-1273 vaccine were male with a median age of 25 years (interquartile range, 20-32 years).
“The main takeaway messages from our study are that the incidence of myocarditis after COVID-19 mRNA vaccinations is very low, that this condition is primarily observed in young men within a few days after the second dose, and that most patients recover quickly,” senior author Mingsum Lee, MD, PhD, Kaiser Permanente Los Angeles Medical Center, told this news organization.
“The incidence of vaccine-related myocarditis was significantly lower than rates of COVID-19 hospitalization during the same period and population area,” she added.
The group saw a per-million incidence of 0.8 and 5.8 myocarditis cases in the 10 days after first and second injections, respectively. That made for an incidence of 0.58 per 100,000, or 1 case per 172,414 fully vaccinated adults.
The group also considered a cohort of 1,577,741 unvaccinated people with a median age of 39 years (interquartile range, 28-53 years) during the same period. Of the 75 cases of myocarditis, 52% were in men, they reported.
Comparing the vaccinated and unvaccinated cohorts, they saw a 10-day vaccine-associated myocarditis incidence rate ratio of 0.38 (95% confidence interval, 0.05-1.40; P = .15) after the first dose, and 2.7 (95% CI, 1.4-4.8; P = .004) after the second dose.
In a comparison of the vaccinated group with itself using data from a 10-day period in the previous year, the corresponding myocarditis IRRs were 1.0 (P > .99) and 3.3 (P = .03), respectively.
Dr. Lee said none of the 15 patients required admission to an intensive care unit. “All patients with myocarditis responded well to treatment and felt better quickly,” she noted.
Myocarditis after an mRNA vaccine injection is rare and, Dr. Lee said emphatically, and “the benefits of the COVID-19 vaccine greatly outweigh the risks.”
Sex- and age-stratified rates
In a separate analysis of 5,442,696 people given a first dose of the Pfizer BNT162b2 vaccine and 5,125,635 given a second dose, there were 142 cases of myocarditis with onset 21 days after dose 1 and 30 days after dose 2. Of those cases, 136 were documented as “definite or probable” in an Israeli Ministry of Health database that covered up to the end of May 2021.
There were also 40 cases among vaccinated people seen after the 30-day window, which were considered not related to the vaccination, and 101 cases among unvaccinated people; of the latter, 29 had confirmed diagnoses of COVID-19.
Of the 136 people with definite or probable cases, the myocarditis was “generally mild” in 129 and usually resolved on its own, notes the report on the study, published in the New England Journal of Medicine, with lead author Dror Mevorach, MD, Hadassah-Hebrew University Medical Center, Jerusalem.
The estimated myocarditis incidence after a second such vaccine dose across the entire Israeli population, based on the current study, was about one per 26,000 males and one per 218,000 females, the group writes. Those figures compare with one case per 10,857 among “the general unvaccinated population.”
Again, the risk was concentrated among younger men and male adolescents. In an analysis limited to vaccinated people aged 16-19 years, myocarditis in the 21 days after a second mRNA injection was seen in about one of 6,637 males and one of 99,853 females, the group reported.
The standardized incidence ratio of 5.34 (95% CI, 4.48-6.40) after a second injection, across all groups, “was driven mostly by the diagnosis of myocarditis in younger male recipients.” Among that male subgroup, the ratios by age group were 13.60 (95% CI, 9.30-19.20) for 16-19 years, 8.53 (95% CI, 5.57-12.50) for 20-24 years, and 6.96 (95% CI, 4.25-10.75) for 25-29 years.
Among people who received a second injection, compared with unvaccinated people, the 30-day rate ratio was 2.35 (95% CI, 1.10-5.02). Again, the effect was concentrated in males aged 16-19 years. Among them, the myocarditis rate ratios in the 30 days after a second mRNA vaccine injection were 8.96 (95% CI, 4.50-17.83) for the 16-19 years group, 6.13 (95% CI, 3.16-11.88) for the 20-24 group, and 3.58 (95% CI, 1.82-7.01) for 25-29 years.
Most of the patients with myocarditis showed “significant clinical improvement,” with a mean hospitalization time of only 3-4 days, the report notes. Treatment consisted of nonsteroidal anti-inflammatory drugs “with or without colchicine for presumed pericardial inflammation.”
However, seven patients (4.9%) developed important complications, including left-ventricular dysfunction, ventricular arrhythmias, and heart failure. Among them was a 22-year-old patient who died of fulminant myocarditis within 24 hours of diagnosis, the group wrote.
From an Israeli health care organization
Published by the same journal as the study by Dr. Menvorach and associates, an analysis of a separate database showed largely consistent findings among patients in the largest of Israel’s four health care organizations charged by the government to administer health services.
The report, with authors led by Guy Witberg, MD, Rabin Medical Center, Petah Tikva, Israel, focused on members of the health care organization aged 16 years or older who had received at least one Pfizer mRNA vaccine dose by the end of May 2021.
The cohorts from the two separate reports surely overlap substantially, as the Ministry of Health analysis from Dr. Mevorach and colleagues derived from a nationwide database, and – as Dr. Witberg and associates wrote – the health care organization providing their data covers 52% of the Israeli population.
Of 2,558,421 vaccinated people in the analysis, of whom 94% received two doses, 54 developed confirmed myocarditis in the 42 days after the first dose. Their median age was 27 years (interquartile range, 21-35 years) and all but three (94%) were male. Of those 54 cases, 41 were considered mild and 12 intermediate in severity, and one was fulminant with the patient in cardiogenic shock, the group writes. In addition, nonsustained ventricular tachycardia and atrial fibrillation developed in 5% and 3% of cases, respectively.
The estimated myocarditis incidence in the 42 days after administration of at least one mRNA vaccine dose was 2.13 per 100,000 vaccinated people. In that group, Dr. Witberg and colleagues note, the corresponding incidences per 100,000 were 4.12 and 0.23 for males and females, respectively.
Also in the current report, incidences per 100,000 vaccinated people aged 16-29 years, by sex, included 5.49 (95% CI, 3.59-7.39) overall, and 10.69 (95% CI, 6.93-14.46) for males (the highest rate in the report).
There was only one case in a female aged 16-29 years, and two cases in females 30 years or older.
Of note, some authors of the current study are also authors on the high-profile report from Noam Barda, MD, and colleagues published in the New England Journal of Medicine, that used the same database to arrive at an mRNA-vaccine-related incidence of myocarditis of 2.7 per 100,000. Eligibility criteria and follow-up time were different in that report, as were case ascertainment criteria.
The myocarditis risk associated with the two mRNA vaccines is small compared with “the morbidity and mortality of COVID-19 infection, in which up to 28% of hospitalized patients showed signs of myocardial injury,” wrote Vinay Guduguntla, MD, University of California, San Francisco, and Mitchell H. Katz, MD, NYC Health + Hospitals, New York, in an editorial accompanying the report from Dr. Lee and associates.
“Randomized clinical trials show that COVID-19 mRNA vaccines represent a safe and effective method of preventing infection,” they stated. “The identification of rare myocarditis does not change clinical decision-making.”
Dr. Bozkurt, who is immediate past president of the Heart Failure Society of America, has disclosed consulting for Bayer and scPharmaceuticals and serving on a clinical events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. Lee and the report’s other authors had no disclosures. Dr. Mevorach discloses consulting for Enlivex Therapeutics; disclosures for the other authors are available at NEJM.org. Dr. Witberg said he has no interests to disclose; disclosures for the other authors are available at NEJM.org. Dr. Guduguntla is an editorial fellow and Dr. Katz a deputy editor at JAMA Internal Medicine; neither had disclosures.
A version of this article first appeared on Medscape.com.
Recent literature features new descriptions of myocarditis linked to the two available mRNA vaccines against SARS-CoV-2. They tell a story largely consistent with experience to date, and support what might be its most useful public health message: The associated myocarditis is usually mild and self-limiting, and is far less likely to occur than myocarditis or death in unvaccinated people with COVID-19.
In line with previous research, the new analyses suggest the myocarditis – with onset usually a few days to a week after injection – has an overall incidence that ranges from less than 1 to perhaps 3 per 100,000 people who received at least one of the full mRNA-vaccine regimen’s two injections. Also, as in earlier studies, the incidence climbed higher – sometimes sharply – in certain groups by age and sex, particularly in young men and older male teens.
The new studies “are confirmatory, in terms of the risk being low,” but underscore that clinicians still must be wary of myocarditis as a potential complication of the mRNA vaccines, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, told this news organization.
Dr. Bozkurt, a leading heart failure specialist and researcher, did not contribute to any of the new reports but does study the myocarditis of COVID-19 and was lead author on a recent review of the potential vaccine complication’s features and possible mechanisms.
In the new myocarditis reports, she observed, more than 90% of the cases were mild and “resolved on their own without a major adverse outcome.” Dr. Bozkurt emphasized the need for perspective regarding the risk. For example, the myocarditis associated with SARS-CoV-2 infection is not only more likely than the vaccine-related myocarditis, but it’s also usually far more severe.
Dr. Bozkurt pointed to a recent study in which the mRNA vaccines, compared with no vaccination, appeared to escalate the myocarditis risk by a factor of 3, whereas the risk for myocarditis in SARS-CoV-2 infection was increased 18 times.
In contrast, she observed, the new myocarditis cases reported this week feature a few that are novel or are at least very rare, including the case of a patient who developed cardiogenic shock and another with fulminant myocarditis who died.
The Centers for Disease Control and Prevention in May publicly described the apparent link between myocarditis and the two available mRNA vaccines against SARS-CoV-2: BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). The next month, the Food and Drug Administration added a warning about the risk to the labeling.
Less than 1 case per 100,000
Fifteen confirmed cases of myocarditis were identified among about 2.4 million members of Kaiser Permanente Southern California aged 18 or older who received at least one injection of the Pfizer or Moderna mRNA vaccines between December 2020 and July 2021, in a report published in JAMA Internal Medicine. The study counted cases up to 10 days after the first or second injection, of which there were 2 and 13, respectively.
All eight patients who received the Pfizer BNT162b2 vaccine and the eight given the Moderna mRNA-1273 vaccine were male with a median age of 25 years (interquartile range, 20-32 years).
“The main takeaway messages from our study are that the incidence of myocarditis after COVID-19 mRNA vaccinations is very low, that this condition is primarily observed in young men within a few days after the second dose, and that most patients recover quickly,” senior author Mingsum Lee, MD, PhD, Kaiser Permanente Los Angeles Medical Center, told this news organization.
“The incidence of vaccine-related myocarditis was significantly lower than rates of COVID-19 hospitalization during the same period and population area,” she added.
The group saw a per-million incidence of 0.8 and 5.8 myocarditis cases in the 10 days after first and second injections, respectively. That made for an incidence of 0.58 per 100,000, or 1 case per 172,414 fully vaccinated adults.
The group also considered a cohort of 1,577,741 unvaccinated people with a median age of 39 years (interquartile range, 28-53 years) during the same period. Of the 75 cases of myocarditis, 52% were in men, they reported.
Comparing the vaccinated and unvaccinated cohorts, they saw a 10-day vaccine-associated myocarditis incidence rate ratio of 0.38 (95% confidence interval, 0.05-1.40; P = .15) after the first dose, and 2.7 (95% CI, 1.4-4.8; P = .004) after the second dose.
In a comparison of the vaccinated group with itself using data from a 10-day period in the previous year, the corresponding myocarditis IRRs were 1.0 (P > .99) and 3.3 (P = .03), respectively.
Dr. Lee said none of the 15 patients required admission to an intensive care unit. “All patients with myocarditis responded well to treatment and felt better quickly,” she noted.
Myocarditis after an mRNA vaccine injection is rare and, Dr. Lee said emphatically, and “the benefits of the COVID-19 vaccine greatly outweigh the risks.”
Sex- and age-stratified rates
In a separate analysis of 5,442,696 people given a first dose of the Pfizer BNT162b2 vaccine and 5,125,635 given a second dose, there were 142 cases of myocarditis with onset 21 days after dose 1 and 30 days after dose 2. Of those cases, 136 were documented as “definite or probable” in an Israeli Ministry of Health database that covered up to the end of May 2021.
There were also 40 cases among vaccinated people seen after the 30-day window, which were considered not related to the vaccination, and 101 cases among unvaccinated people; of the latter, 29 had confirmed diagnoses of COVID-19.
Of the 136 people with definite or probable cases, the myocarditis was “generally mild” in 129 and usually resolved on its own, notes the report on the study, published in the New England Journal of Medicine, with lead author Dror Mevorach, MD, Hadassah-Hebrew University Medical Center, Jerusalem.
The estimated myocarditis incidence after a second such vaccine dose across the entire Israeli population, based on the current study, was about one per 26,000 males and one per 218,000 females, the group writes. Those figures compare with one case per 10,857 among “the general unvaccinated population.”
Again, the risk was concentrated among younger men and male adolescents. In an analysis limited to vaccinated people aged 16-19 years, myocarditis in the 21 days after a second mRNA injection was seen in about one of 6,637 males and one of 99,853 females, the group reported.
The standardized incidence ratio of 5.34 (95% CI, 4.48-6.40) after a second injection, across all groups, “was driven mostly by the diagnosis of myocarditis in younger male recipients.” Among that male subgroup, the ratios by age group were 13.60 (95% CI, 9.30-19.20) for 16-19 years, 8.53 (95% CI, 5.57-12.50) for 20-24 years, and 6.96 (95% CI, 4.25-10.75) for 25-29 years.
Among people who received a second injection, compared with unvaccinated people, the 30-day rate ratio was 2.35 (95% CI, 1.10-5.02). Again, the effect was concentrated in males aged 16-19 years. Among them, the myocarditis rate ratios in the 30 days after a second mRNA vaccine injection were 8.96 (95% CI, 4.50-17.83) for the 16-19 years group, 6.13 (95% CI, 3.16-11.88) for the 20-24 group, and 3.58 (95% CI, 1.82-7.01) for 25-29 years.
Most of the patients with myocarditis showed “significant clinical improvement,” with a mean hospitalization time of only 3-4 days, the report notes. Treatment consisted of nonsteroidal anti-inflammatory drugs “with or without colchicine for presumed pericardial inflammation.”
However, seven patients (4.9%) developed important complications, including left-ventricular dysfunction, ventricular arrhythmias, and heart failure. Among them was a 22-year-old patient who died of fulminant myocarditis within 24 hours of diagnosis, the group wrote.
From an Israeli health care organization
Published by the same journal as the study by Dr. Menvorach and associates, an analysis of a separate database showed largely consistent findings among patients in the largest of Israel’s four health care organizations charged by the government to administer health services.
The report, with authors led by Guy Witberg, MD, Rabin Medical Center, Petah Tikva, Israel, focused on members of the health care organization aged 16 years or older who had received at least one Pfizer mRNA vaccine dose by the end of May 2021.
The cohorts from the two separate reports surely overlap substantially, as the Ministry of Health analysis from Dr. Mevorach and colleagues derived from a nationwide database, and – as Dr. Witberg and associates wrote – the health care organization providing their data covers 52% of the Israeli population.
Of 2,558,421 vaccinated people in the analysis, of whom 94% received two doses, 54 developed confirmed myocarditis in the 42 days after the first dose. Their median age was 27 years (interquartile range, 21-35 years) and all but three (94%) were male. Of those 54 cases, 41 were considered mild and 12 intermediate in severity, and one was fulminant with the patient in cardiogenic shock, the group writes. In addition, nonsustained ventricular tachycardia and atrial fibrillation developed in 5% and 3% of cases, respectively.
The estimated myocarditis incidence in the 42 days after administration of at least one mRNA vaccine dose was 2.13 per 100,000 vaccinated people. In that group, Dr. Witberg and colleagues note, the corresponding incidences per 100,000 were 4.12 and 0.23 for males and females, respectively.
Also in the current report, incidences per 100,000 vaccinated people aged 16-29 years, by sex, included 5.49 (95% CI, 3.59-7.39) overall, and 10.69 (95% CI, 6.93-14.46) for males (the highest rate in the report).
There was only one case in a female aged 16-29 years, and two cases in females 30 years or older.
Of note, some authors of the current study are also authors on the high-profile report from Noam Barda, MD, and colleagues published in the New England Journal of Medicine, that used the same database to arrive at an mRNA-vaccine-related incidence of myocarditis of 2.7 per 100,000. Eligibility criteria and follow-up time were different in that report, as were case ascertainment criteria.
The myocarditis risk associated with the two mRNA vaccines is small compared with “the morbidity and mortality of COVID-19 infection, in which up to 28% of hospitalized patients showed signs of myocardial injury,” wrote Vinay Guduguntla, MD, University of California, San Francisco, and Mitchell H. Katz, MD, NYC Health + Hospitals, New York, in an editorial accompanying the report from Dr. Lee and associates.
“Randomized clinical trials show that COVID-19 mRNA vaccines represent a safe and effective method of preventing infection,” they stated. “The identification of rare myocarditis does not change clinical decision-making.”
Dr. Bozkurt, who is immediate past president of the Heart Failure Society of America, has disclosed consulting for Bayer and scPharmaceuticals and serving on a clinical events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. Lee and the report’s other authors had no disclosures. Dr. Mevorach discloses consulting for Enlivex Therapeutics; disclosures for the other authors are available at NEJM.org. Dr. Witberg said he has no interests to disclose; disclosures for the other authors are available at NEJM.org. Dr. Guduguntla is an editorial fellow and Dr. Katz a deputy editor at JAMA Internal Medicine; neither had disclosures.
A version of this article first appeared on Medscape.com.
Merck seeks FDA authorization for antiviral COVID-19 pill
, an experimental antiviral COVID-19 treatment.
If the FDA grants authorization, the drug would be the first oral antiviral treatment for COVID-19. The capsule, made by Merck and Ridgeback Biotherapeutics, is intended to treat mild to moderate COVID-19 in adults who are at risk of having severe COVID-19 or hospitalization.
“The extraordinary impact of this pandemic demands that we move with unprecedented urgency, and that is what our teams have done by submitting this application for molnupiravir to the FDA within 10 days of receiving the data,” Robert Davis, CEO and president of Merck, said in a statement. On Oct. 1, Merck and Ridgeback released interim data from its phase III clinical trial, which showed that molnupiravir reduced the risk of hospitalization or death by about 50%. About 7% of patients who received the drug were hospitalized within 30 days in the study, as compared with 14% of patients who took a placebo, the company said.
No deaths were reported in the group that received the drug, as compared with eight deaths in the group that received the placebo. None of the trial participants had been vaccinated.
“Medicines and vaccines are both essential to our collective efforts,” Mr. Davis said. “We look forward to working with the FDA on its review of our application, and to working with other regulatory agencies as we do everything we can to bring molnupiravir to patients around the world as quickly as possible.”
Merck has been producing molnupiravir in anticipation of the clinical trial results and FDA authorization. The company expects to produce 10 million courses of treatment by the end of the year, with more expected for 2022.
In June, Merck signed an agreement with the United States to supply 1.7 million courses of molnupiravir once the FDA authorizes the drug. The company has agreed to advance purchase agreements with other countries as well.
Earlier in the year, Merck also announced voluntary licensing agreements with several generics manufacturers in India to provide molnupiravir to more than 100 low- and middle-income countries after approval from local regulatory agencies.
Data from the company’s late-stage clinical trial has not yet been peer-reviewed or published.
Last week, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said the clinical trial results were “very encouraging” but noted that the FDA should closely scrutinize the drug, CNN reported.
“It is very important that this now must go through the usual process of careful examination of the data by the Food and Drug Administration, both for effectiveness but also for safety, because whenever you introduce a new compound, safety is very important,” Dr. Fauci said, adding that vaccines remain “our best tools against COVID-19.”
A version of this article firsts appeared on WebMD.com.
, an experimental antiviral COVID-19 treatment.
If the FDA grants authorization, the drug would be the first oral antiviral treatment for COVID-19. The capsule, made by Merck and Ridgeback Biotherapeutics, is intended to treat mild to moderate COVID-19 in adults who are at risk of having severe COVID-19 or hospitalization.
“The extraordinary impact of this pandemic demands that we move with unprecedented urgency, and that is what our teams have done by submitting this application for molnupiravir to the FDA within 10 days of receiving the data,” Robert Davis, CEO and president of Merck, said in a statement. On Oct. 1, Merck and Ridgeback released interim data from its phase III clinical trial, which showed that molnupiravir reduced the risk of hospitalization or death by about 50%. About 7% of patients who received the drug were hospitalized within 30 days in the study, as compared with 14% of patients who took a placebo, the company said.
No deaths were reported in the group that received the drug, as compared with eight deaths in the group that received the placebo. None of the trial participants had been vaccinated.
“Medicines and vaccines are both essential to our collective efforts,” Mr. Davis said. “We look forward to working with the FDA on its review of our application, and to working with other regulatory agencies as we do everything we can to bring molnupiravir to patients around the world as quickly as possible.”
Merck has been producing molnupiravir in anticipation of the clinical trial results and FDA authorization. The company expects to produce 10 million courses of treatment by the end of the year, with more expected for 2022.
In June, Merck signed an agreement with the United States to supply 1.7 million courses of molnupiravir once the FDA authorizes the drug. The company has agreed to advance purchase agreements with other countries as well.
Earlier in the year, Merck also announced voluntary licensing agreements with several generics manufacturers in India to provide molnupiravir to more than 100 low- and middle-income countries after approval from local regulatory agencies.
Data from the company’s late-stage clinical trial has not yet been peer-reviewed or published.
Last week, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said the clinical trial results were “very encouraging” but noted that the FDA should closely scrutinize the drug, CNN reported.
“It is very important that this now must go through the usual process of careful examination of the data by the Food and Drug Administration, both for effectiveness but also for safety, because whenever you introduce a new compound, safety is very important,” Dr. Fauci said, adding that vaccines remain “our best tools against COVID-19.”
A version of this article firsts appeared on WebMD.com.
, an experimental antiviral COVID-19 treatment.
If the FDA grants authorization, the drug would be the first oral antiviral treatment for COVID-19. The capsule, made by Merck and Ridgeback Biotherapeutics, is intended to treat mild to moderate COVID-19 in adults who are at risk of having severe COVID-19 or hospitalization.
“The extraordinary impact of this pandemic demands that we move with unprecedented urgency, and that is what our teams have done by submitting this application for molnupiravir to the FDA within 10 days of receiving the data,” Robert Davis, CEO and president of Merck, said in a statement. On Oct. 1, Merck and Ridgeback released interim data from its phase III clinical trial, which showed that molnupiravir reduced the risk of hospitalization or death by about 50%. About 7% of patients who received the drug were hospitalized within 30 days in the study, as compared with 14% of patients who took a placebo, the company said.
No deaths were reported in the group that received the drug, as compared with eight deaths in the group that received the placebo. None of the trial participants had been vaccinated.
“Medicines and vaccines are both essential to our collective efforts,” Mr. Davis said. “We look forward to working with the FDA on its review of our application, and to working with other regulatory agencies as we do everything we can to bring molnupiravir to patients around the world as quickly as possible.”
Merck has been producing molnupiravir in anticipation of the clinical trial results and FDA authorization. The company expects to produce 10 million courses of treatment by the end of the year, with more expected for 2022.
In June, Merck signed an agreement with the United States to supply 1.7 million courses of molnupiravir once the FDA authorizes the drug. The company has agreed to advance purchase agreements with other countries as well.
Earlier in the year, Merck also announced voluntary licensing agreements with several generics manufacturers in India to provide molnupiravir to more than 100 low- and middle-income countries after approval from local regulatory agencies.
Data from the company’s late-stage clinical trial has not yet been peer-reviewed or published.
Last week, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said the clinical trial results were “very encouraging” but noted that the FDA should closely scrutinize the drug, CNN reported.
“It is very important that this now must go through the usual process of careful examination of the data by the Food and Drug Administration, both for effectiveness but also for safety, because whenever you introduce a new compound, safety is very important,” Dr. Fauci said, adding that vaccines remain “our best tools against COVID-19.”
A version of this article firsts appeared on WebMD.com.
HEPA filters may clean SARS-CoV-2 from the air: Study
, researchers report in the preprint server medRxiv.
The journal Nature reported Oct. 6 that the research, which has not been peer-reviewed, suggests the filters may help reduce the risk of hospital-acquired SARS-CoV-2.
Researchers, led by intensivist Andrew Conway-Morris, MBChB, PhD, with the division of anaesthesia in the school of clinical medicine at University of Cambridge, United Kingdom, write that earlier experiments assessed air filters’ ability to remove inactive particles in carefully controlled environments, but it was unknown how they would work in a real-world setting.
Co-author Vilas Navapurkar, MBChB, an ICU physician at Addenbrooke’s Hospital in Cambridge, United Kingdom, said that hospitals have used portable air filters when their isolation facilities are full, but evidence was needed as to whether such filters are effective or whether they provide a false sense of security.
The researchers installed the filters in two fully occupied COVID-19 wards — a general ward and an ICU. They chose HEPA filters because they can catch extremely small particles.
The team collected air samples from the wards during a week when the air filters were on and 2 weeks when they were turned off, then compared results.
According to the study, “airborne SARS-CoV-2 was detected in the ward on all five days before activation of air/UV filtration, but on none of the five days when the air/UV filter was operational; SARS-CoV-2 was again detected on four out of five days when the filter was off.”
Airborne SARS-CoV-2 was not frequently detected in the ICU, even when the filters were off.
Cheap and easy
According to the Nature article, the authors suggest several potential explanations for this, “including slower viral replication at later stages of the disease.” Therefore, the authors say, filtering the virus from the air might be more important in general wards than in ICUs.
The filters significantly reduced the other microbial bioaerosols in both the ward (48 pathogens detected before filtration, 2 after, P = .05) and the ICU (45 pathogens detected before filtration, 5 after P = .05).
National Institute for Occupational Safety and Health (NIOSH) cyclonic aerosol samplers and PCR tests were used to detect airborne SARS-CoV-2 and other microbial bioaerosol.
David Fisman, MD, an epidemiologist at the University of Toronto, who was not involved in the research, said in the Nature article, “This study suggests that HEPA air cleaners, which remain little-used in Canadian hospitals, are a cheap and easy way to reduce risk from airborne pathogens.”This work was supported by a Wellcome senior research fellowship to co-author Stephen Baker. Conway Morris is supported by a Clinician Scientist Fellowship from the Medical Research Council. Dr. Navapurkar is the founder, director, and shareholder of Cambridge Infection Diagnostics Ltd. Dr. Conway-Morris and several co-authors are members of the Scientific Advisory Board of Cambridge Infection Diagnostics Ltd. Co-author Theodore Gouliouris has received a research grant from Shionogi and co-author R. Andres Floto has received research grants and/or consultancy payments from GSK, AstraZeneca, Chiesi, Shionogi, Insmed, and Thirty Technology.
A version of this article first appeared on Medscape.com.
, researchers report in the preprint server medRxiv.
The journal Nature reported Oct. 6 that the research, which has not been peer-reviewed, suggests the filters may help reduce the risk of hospital-acquired SARS-CoV-2.
Researchers, led by intensivist Andrew Conway-Morris, MBChB, PhD, with the division of anaesthesia in the school of clinical medicine at University of Cambridge, United Kingdom, write that earlier experiments assessed air filters’ ability to remove inactive particles in carefully controlled environments, but it was unknown how they would work in a real-world setting.
Co-author Vilas Navapurkar, MBChB, an ICU physician at Addenbrooke’s Hospital in Cambridge, United Kingdom, said that hospitals have used portable air filters when their isolation facilities are full, but evidence was needed as to whether such filters are effective or whether they provide a false sense of security.
The researchers installed the filters in two fully occupied COVID-19 wards — a general ward and an ICU. They chose HEPA filters because they can catch extremely small particles.
The team collected air samples from the wards during a week when the air filters were on and 2 weeks when they were turned off, then compared results.
According to the study, “airborne SARS-CoV-2 was detected in the ward on all five days before activation of air/UV filtration, but on none of the five days when the air/UV filter was operational; SARS-CoV-2 was again detected on four out of five days when the filter was off.”
Airborne SARS-CoV-2 was not frequently detected in the ICU, even when the filters were off.
Cheap and easy
According to the Nature article, the authors suggest several potential explanations for this, “including slower viral replication at later stages of the disease.” Therefore, the authors say, filtering the virus from the air might be more important in general wards than in ICUs.
The filters significantly reduced the other microbial bioaerosols in both the ward (48 pathogens detected before filtration, 2 after, P = .05) and the ICU (45 pathogens detected before filtration, 5 after P = .05).
National Institute for Occupational Safety and Health (NIOSH) cyclonic aerosol samplers and PCR tests were used to detect airborne SARS-CoV-2 and other microbial bioaerosol.
David Fisman, MD, an epidemiologist at the University of Toronto, who was not involved in the research, said in the Nature article, “This study suggests that HEPA air cleaners, which remain little-used in Canadian hospitals, are a cheap and easy way to reduce risk from airborne pathogens.”This work was supported by a Wellcome senior research fellowship to co-author Stephen Baker. Conway Morris is supported by a Clinician Scientist Fellowship from the Medical Research Council. Dr. Navapurkar is the founder, director, and shareholder of Cambridge Infection Diagnostics Ltd. Dr. Conway-Morris and several co-authors are members of the Scientific Advisory Board of Cambridge Infection Diagnostics Ltd. Co-author Theodore Gouliouris has received a research grant from Shionogi and co-author R. Andres Floto has received research grants and/or consultancy payments from GSK, AstraZeneca, Chiesi, Shionogi, Insmed, and Thirty Technology.
A version of this article first appeared on Medscape.com.
, researchers report in the preprint server medRxiv.
The journal Nature reported Oct. 6 that the research, which has not been peer-reviewed, suggests the filters may help reduce the risk of hospital-acquired SARS-CoV-2.
Researchers, led by intensivist Andrew Conway-Morris, MBChB, PhD, with the division of anaesthesia in the school of clinical medicine at University of Cambridge, United Kingdom, write that earlier experiments assessed air filters’ ability to remove inactive particles in carefully controlled environments, but it was unknown how they would work in a real-world setting.
Co-author Vilas Navapurkar, MBChB, an ICU physician at Addenbrooke’s Hospital in Cambridge, United Kingdom, said that hospitals have used portable air filters when their isolation facilities are full, but evidence was needed as to whether such filters are effective or whether they provide a false sense of security.
The researchers installed the filters in two fully occupied COVID-19 wards — a general ward and an ICU. They chose HEPA filters because they can catch extremely small particles.
The team collected air samples from the wards during a week when the air filters were on and 2 weeks when they were turned off, then compared results.
According to the study, “airborne SARS-CoV-2 was detected in the ward on all five days before activation of air/UV filtration, but on none of the five days when the air/UV filter was operational; SARS-CoV-2 was again detected on four out of five days when the filter was off.”
Airborne SARS-CoV-2 was not frequently detected in the ICU, even when the filters were off.
Cheap and easy
According to the Nature article, the authors suggest several potential explanations for this, “including slower viral replication at later stages of the disease.” Therefore, the authors say, filtering the virus from the air might be more important in general wards than in ICUs.
The filters significantly reduced the other microbial bioaerosols in both the ward (48 pathogens detected before filtration, 2 after, P = .05) and the ICU (45 pathogens detected before filtration, 5 after P = .05).
National Institute for Occupational Safety and Health (NIOSH) cyclonic aerosol samplers and PCR tests were used to detect airborne SARS-CoV-2 and other microbial bioaerosol.
David Fisman, MD, an epidemiologist at the University of Toronto, who was not involved in the research, said in the Nature article, “This study suggests that HEPA air cleaners, which remain little-used in Canadian hospitals, are a cheap and easy way to reduce risk from airborne pathogens.”This work was supported by a Wellcome senior research fellowship to co-author Stephen Baker. Conway Morris is supported by a Clinician Scientist Fellowship from the Medical Research Council. Dr. Navapurkar is the founder, director, and shareholder of Cambridge Infection Diagnostics Ltd. Dr. Conway-Morris and several co-authors are members of the Scientific Advisory Board of Cambridge Infection Diagnostics Ltd. Co-author Theodore Gouliouris has received a research grant from Shionogi and co-author R. Andres Floto has received research grants and/or consultancy payments from GSK, AstraZeneca, Chiesi, Shionogi, Insmed, and Thirty Technology.
A version of this article first appeared on Medscape.com.
Depression rates up threefold since start of COVID-19
A year into the COVID-19 pandemic, the share of the U.S. adult population reporting symptoms of elevated depression had more than tripled from prepandemic levels and worsened significantly since restrictions went into effect, a study of more than 1,000 adults surveyed at the start of the pandemic and 1 year into it has reported.
The study also found that younger adults, people with lower incomes and savings, unmarried people, and those exposed to multiple stress factors were most vulnerable to elevated levels of depression through the first year of the pandemic.
“The pandemic has been an ongoing exposure,” lead author Catherine K. Ettman, a PhD candidate at Brown University, Providence, R.I., said in an interview. “Mental health is sensitive to economic and social conditions. While living conditions have improved for some people over the last 12 months, the pandemic has been disruptive to life and economic well-being for many,” said Ms. Ettman, who is also chief of staff and director of strategic initiatives in the office of the dean at Boston University. Her study was published in Lancet Regional Health – Americas.
Ms. Ettman and coauthors reported that 32.8% (95% confidence interval, 29.1%-36.8%) of surveyed adults had elevated depressive symptoms in 2021, compared with 27.8% (95% CI, 24.9%-30.9%) in the early months of the pandemic in 2020 (P = .0016). That compares with a rate of 8.5% before the pandemic, a figure based on a prepandemic sample of 5,065 patients from the National Health and Nutrition Examination Survey reported previously by Ms. Ettman and associates.
“The COVID-19 pandemic and its economic consequences have displaced social networks, created ongoing stressors, and reduced access to the resources that protect mental health,” Ms. Ettman said.
Four groups most affected
In this latest research, a longitudinal panel study of a nationally representative group of U.S. adults, the researchers surveyed participants in March and April 2020 (n = 1,414) and the same group again in March and April 2021 (n = 1,161). The participants completed the Patient Health Questionnaire–9 (PHQ-9) and were enrolled in the COVID-19 and Life Stressors Impact on Mental Health and Well-Being study.
The study found that elevated depressive symptoms were most prevalent in four groups:
- Younger patients, with 43.9% of patients aged 18-39 years self-reporting elevated depressive symptoms, compared with 32.4% of those aged 40-59, and 19.1% of patients aged 60 and older.
- People with lower incomes, with 58.1% of people making $19,999 or less reporting elevated symptoms, compared with 41.3% of those making $20,000-$44,999, 31.4% of people making $45,000-$74,999, and 14.1% of those making $75,000 or more.
- People with less than $5,000 in family savings, with a rate of 51.1%, compared with 24.2% of those with more than that.
- People never married, with a rate of 39.8% versus 37.7% of those living with a partner; 31.5% widowed, divorced, or separated; and 18.3% married.
The study also found correlations between the number of self-reported stressors and elevated depression symptoms: a rate of 51.1% in people with four or more stressors; 25.8% in those with two or three stressors; and 17% in people with one or no stressors.
Among the groups reporting the lowest rates of depressive symptoms in 2021 were people making more than $75,000 a year; those with one or no COVID-19 stressors; and non-Hispanic Asian persons.
“Stressors such as difficulties finding childcare, difficulties paying for housing, and job loss were associated with greater depression 12 months into the COVID-19 pandemic,” Ms. Ettman said. “Efforts to address stressors and improve access to childcare, housing, employment, and fair wages can improve mental health.”
The duration of the pandemic is another explanation for the significant rise in depressive symptoms, senior author Sandro Galea, MD, MPH, DrPH, said in an interview. Dr. Galea added. “Unlike acute traumatic events, the COVID-19 pandemic has been ongoing.”
He said clinicians, public health officials, and policy makers need to be aware of the impact COVID-19 has had on mental health. “We can take steps as a society to treat and prevent depression and create conditions that allow all populations to be healthy,” said Dr. Galea, who is dean and a professor of family medicine at Boston University.
Age of sample cited as limitation
The study builds on existing evidence linking depression trends and the COVID-19 pandemic, David Puder, MD, a medical director at Loma Linda (Calif.) University, said in an interview. However, he noted it had some limitations. “The age range is only 18 and older, so we don’t get to see what is happening with a highly impacted group of students who have not been able to go to school and be with their friends during COVID,” said Dr. Puder, who also hosts the podcast “Psychiatry & Psychotherapy.” “Further, the PHQ-9 is often a screening tool for depression and is not best used for changes in mental health over time.”
At the same time, Dr. Puder said, one of the study’s strengths was that it showed how depressive symptoms increased during the COVID lockdown. “It shows certain groups are at higher risk, including those with less financial resources and those with higher amounts of stress,” Dr. Puder said.
Ms. Ettman, Dr. Galea, and Dr. Puder reported no relevant disclosures.
A year into the COVID-19 pandemic, the share of the U.S. adult population reporting symptoms of elevated depression had more than tripled from prepandemic levels and worsened significantly since restrictions went into effect, a study of more than 1,000 adults surveyed at the start of the pandemic and 1 year into it has reported.
The study also found that younger adults, people with lower incomes and savings, unmarried people, and those exposed to multiple stress factors were most vulnerable to elevated levels of depression through the first year of the pandemic.
“The pandemic has been an ongoing exposure,” lead author Catherine K. Ettman, a PhD candidate at Brown University, Providence, R.I., said in an interview. “Mental health is sensitive to economic and social conditions. While living conditions have improved for some people over the last 12 months, the pandemic has been disruptive to life and economic well-being for many,” said Ms. Ettman, who is also chief of staff and director of strategic initiatives in the office of the dean at Boston University. Her study was published in Lancet Regional Health – Americas.
Ms. Ettman and coauthors reported that 32.8% (95% confidence interval, 29.1%-36.8%) of surveyed adults had elevated depressive symptoms in 2021, compared with 27.8% (95% CI, 24.9%-30.9%) in the early months of the pandemic in 2020 (P = .0016). That compares with a rate of 8.5% before the pandemic, a figure based on a prepandemic sample of 5,065 patients from the National Health and Nutrition Examination Survey reported previously by Ms. Ettman and associates.
“The COVID-19 pandemic and its economic consequences have displaced social networks, created ongoing stressors, and reduced access to the resources that protect mental health,” Ms. Ettman said.
Four groups most affected
In this latest research, a longitudinal panel study of a nationally representative group of U.S. adults, the researchers surveyed participants in March and April 2020 (n = 1,414) and the same group again in March and April 2021 (n = 1,161). The participants completed the Patient Health Questionnaire–9 (PHQ-9) and were enrolled in the COVID-19 and Life Stressors Impact on Mental Health and Well-Being study.
The study found that elevated depressive symptoms were most prevalent in four groups:
- Younger patients, with 43.9% of patients aged 18-39 years self-reporting elevated depressive symptoms, compared with 32.4% of those aged 40-59, and 19.1% of patients aged 60 and older.
- People with lower incomes, with 58.1% of people making $19,999 or less reporting elevated symptoms, compared with 41.3% of those making $20,000-$44,999, 31.4% of people making $45,000-$74,999, and 14.1% of those making $75,000 or more.
- People with less than $5,000 in family savings, with a rate of 51.1%, compared with 24.2% of those with more than that.
- People never married, with a rate of 39.8% versus 37.7% of those living with a partner; 31.5% widowed, divorced, or separated; and 18.3% married.
The study also found correlations between the number of self-reported stressors and elevated depression symptoms: a rate of 51.1% in people with four or more stressors; 25.8% in those with two or three stressors; and 17% in people with one or no stressors.
Among the groups reporting the lowest rates of depressive symptoms in 2021 were people making more than $75,000 a year; those with one or no COVID-19 stressors; and non-Hispanic Asian persons.
“Stressors such as difficulties finding childcare, difficulties paying for housing, and job loss were associated with greater depression 12 months into the COVID-19 pandemic,” Ms. Ettman said. “Efforts to address stressors and improve access to childcare, housing, employment, and fair wages can improve mental health.”
The duration of the pandemic is another explanation for the significant rise in depressive symptoms, senior author Sandro Galea, MD, MPH, DrPH, said in an interview. Dr. Galea added. “Unlike acute traumatic events, the COVID-19 pandemic has been ongoing.”
He said clinicians, public health officials, and policy makers need to be aware of the impact COVID-19 has had on mental health. “We can take steps as a society to treat and prevent depression and create conditions that allow all populations to be healthy,” said Dr. Galea, who is dean and a professor of family medicine at Boston University.
Age of sample cited as limitation
The study builds on existing evidence linking depression trends and the COVID-19 pandemic, David Puder, MD, a medical director at Loma Linda (Calif.) University, said in an interview. However, he noted it had some limitations. “The age range is only 18 and older, so we don’t get to see what is happening with a highly impacted group of students who have not been able to go to school and be with their friends during COVID,” said Dr. Puder, who also hosts the podcast “Psychiatry & Psychotherapy.” “Further, the PHQ-9 is often a screening tool for depression and is not best used for changes in mental health over time.”
At the same time, Dr. Puder said, one of the study’s strengths was that it showed how depressive symptoms increased during the COVID lockdown. “It shows certain groups are at higher risk, including those with less financial resources and those with higher amounts of stress,” Dr. Puder said.
Ms. Ettman, Dr. Galea, and Dr. Puder reported no relevant disclosures.
A year into the COVID-19 pandemic, the share of the U.S. adult population reporting symptoms of elevated depression had more than tripled from prepandemic levels and worsened significantly since restrictions went into effect, a study of more than 1,000 adults surveyed at the start of the pandemic and 1 year into it has reported.
The study also found that younger adults, people with lower incomes and savings, unmarried people, and those exposed to multiple stress factors were most vulnerable to elevated levels of depression through the first year of the pandemic.
“The pandemic has been an ongoing exposure,” lead author Catherine K. Ettman, a PhD candidate at Brown University, Providence, R.I., said in an interview. “Mental health is sensitive to economic and social conditions. While living conditions have improved for some people over the last 12 months, the pandemic has been disruptive to life and economic well-being for many,” said Ms. Ettman, who is also chief of staff and director of strategic initiatives in the office of the dean at Boston University. Her study was published in Lancet Regional Health – Americas.
Ms. Ettman and coauthors reported that 32.8% (95% confidence interval, 29.1%-36.8%) of surveyed adults had elevated depressive symptoms in 2021, compared with 27.8% (95% CI, 24.9%-30.9%) in the early months of the pandemic in 2020 (P = .0016). That compares with a rate of 8.5% before the pandemic, a figure based on a prepandemic sample of 5,065 patients from the National Health and Nutrition Examination Survey reported previously by Ms. Ettman and associates.
“The COVID-19 pandemic and its economic consequences have displaced social networks, created ongoing stressors, and reduced access to the resources that protect mental health,” Ms. Ettman said.
Four groups most affected
In this latest research, a longitudinal panel study of a nationally representative group of U.S. adults, the researchers surveyed participants in March and April 2020 (n = 1,414) and the same group again in March and April 2021 (n = 1,161). The participants completed the Patient Health Questionnaire–9 (PHQ-9) and were enrolled in the COVID-19 and Life Stressors Impact on Mental Health and Well-Being study.
The study found that elevated depressive symptoms were most prevalent in four groups:
- Younger patients, with 43.9% of patients aged 18-39 years self-reporting elevated depressive symptoms, compared with 32.4% of those aged 40-59, and 19.1% of patients aged 60 and older.
- People with lower incomes, with 58.1% of people making $19,999 or less reporting elevated symptoms, compared with 41.3% of those making $20,000-$44,999, 31.4% of people making $45,000-$74,999, and 14.1% of those making $75,000 or more.
- People with less than $5,000 in family savings, with a rate of 51.1%, compared with 24.2% of those with more than that.
- People never married, with a rate of 39.8% versus 37.7% of those living with a partner; 31.5% widowed, divorced, or separated; and 18.3% married.
The study also found correlations between the number of self-reported stressors and elevated depression symptoms: a rate of 51.1% in people with four or more stressors; 25.8% in those with two or three stressors; and 17% in people with one or no stressors.
Among the groups reporting the lowest rates of depressive symptoms in 2021 were people making more than $75,000 a year; those with one or no COVID-19 stressors; and non-Hispanic Asian persons.
“Stressors such as difficulties finding childcare, difficulties paying for housing, and job loss were associated with greater depression 12 months into the COVID-19 pandemic,” Ms. Ettman said. “Efforts to address stressors and improve access to childcare, housing, employment, and fair wages can improve mental health.”
The duration of the pandemic is another explanation for the significant rise in depressive symptoms, senior author Sandro Galea, MD, MPH, DrPH, said in an interview. Dr. Galea added. “Unlike acute traumatic events, the COVID-19 pandemic has been ongoing.”
He said clinicians, public health officials, and policy makers need to be aware of the impact COVID-19 has had on mental health. “We can take steps as a society to treat and prevent depression and create conditions that allow all populations to be healthy,” said Dr. Galea, who is dean and a professor of family medicine at Boston University.
Age of sample cited as limitation
The study builds on existing evidence linking depression trends and the COVID-19 pandemic, David Puder, MD, a medical director at Loma Linda (Calif.) University, said in an interview. However, he noted it had some limitations. “The age range is only 18 and older, so we don’t get to see what is happening with a highly impacted group of students who have not been able to go to school and be with their friends during COVID,” said Dr. Puder, who also hosts the podcast “Psychiatry & Psychotherapy.” “Further, the PHQ-9 is often a screening tool for depression and is not best used for changes in mental health over time.”
At the same time, Dr. Puder said, one of the study’s strengths was that it showed how depressive symptoms increased during the COVID lockdown. “It shows certain groups are at higher risk, including those with less financial resources and those with higher amounts of stress,” Dr. Puder said.
Ms. Ettman, Dr. Galea, and Dr. Puder reported no relevant disclosures.
FROM LANCET REGIONAL HEALTH – AMERICAS
Pfizer asks FDA to authorize COVID vaccine for kids 5-11
The request comes after the drugmaker submitted clinical trial data to the FDA on Sept. 28. Pfizer said the study of 2,268 children showed the vaccine was safe and produced a robust immune response.
Participants in the studies received a lower dose of the vaccine, 10 micrograms. Their response 2 weeks after a second dose was reportedly equal to the immune protection in a control group of 16- to 25-year-olds who received the fully approved 30-microgram doses.
Currently, the Pfizer EUA applies to 12- to 15-year-olds and people eligible for a Pfizer booster shot. The drugmaker received full FDA approval for the vaccine for Americans 16 years and older in August.
The filing for authorization in 5- to 11-year-olds comes as overall cases of COVID-19 in the United States continue to decline. The decrease includes a drop in new cases in children for the fourth consecutive week, according to analysis of data from the American Academy of Pediatrics and the Children’s Hospital Association.
The next step is an FDA decision on whether to expand the current emergency use authorization (EUA) for teenagers to the younger age group.
Timing of any official word from the agency is unknown. But possibly in anticipation of today’s filing, the FDA already scheduled a meeting of its Vaccines and Related Biological Products Advisory Committee for Oct. 25.
A version of this article first appeared on WebMD.com.
The request comes after the drugmaker submitted clinical trial data to the FDA on Sept. 28. Pfizer said the study of 2,268 children showed the vaccine was safe and produced a robust immune response.
Participants in the studies received a lower dose of the vaccine, 10 micrograms. Their response 2 weeks after a second dose was reportedly equal to the immune protection in a control group of 16- to 25-year-olds who received the fully approved 30-microgram doses.
Currently, the Pfizer EUA applies to 12- to 15-year-olds and people eligible for a Pfizer booster shot. The drugmaker received full FDA approval for the vaccine for Americans 16 years and older in August.
The filing for authorization in 5- to 11-year-olds comes as overall cases of COVID-19 in the United States continue to decline. The decrease includes a drop in new cases in children for the fourth consecutive week, according to analysis of data from the American Academy of Pediatrics and the Children’s Hospital Association.
The next step is an FDA decision on whether to expand the current emergency use authorization (EUA) for teenagers to the younger age group.
Timing of any official word from the agency is unknown. But possibly in anticipation of today’s filing, the FDA already scheduled a meeting of its Vaccines and Related Biological Products Advisory Committee for Oct. 25.
A version of this article first appeared on WebMD.com.
The request comes after the drugmaker submitted clinical trial data to the FDA on Sept. 28. Pfizer said the study of 2,268 children showed the vaccine was safe and produced a robust immune response.
Participants in the studies received a lower dose of the vaccine, 10 micrograms. Their response 2 weeks after a second dose was reportedly equal to the immune protection in a control group of 16- to 25-year-olds who received the fully approved 30-microgram doses.
Currently, the Pfizer EUA applies to 12- to 15-year-olds and people eligible for a Pfizer booster shot. The drugmaker received full FDA approval for the vaccine for Americans 16 years and older in August.
The filing for authorization in 5- to 11-year-olds comes as overall cases of COVID-19 in the United States continue to decline. The decrease includes a drop in new cases in children for the fourth consecutive week, according to analysis of data from the American Academy of Pediatrics and the Children’s Hospital Association.
The next step is an FDA decision on whether to expand the current emergency use authorization (EUA) for teenagers to the younger age group.
Timing of any official word from the agency is unknown. But possibly in anticipation of today’s filing, the FDA already scheduled a meeting of its Vaccines and Related Biological Products Advisory Committee for Oct. 25.
A version of this article first appeared on WebMD.com.
Case reports underscore risk of cerebral edema, AFCE in children with COVID-19
according to pediatric neurologists who are urging colleagues to watch out for similar cases.
At least one other child in the United States has died after becoming infected with the virus and developing cerebral edema. “The rapid and devastating clinical course in both of these cases highlights the need for early recognition of a cerebral edema and AFCE as potential complications of COVID-19 in pediatric patients,” the neurologists wrote.
The case was highlighted in a poster presented at the annual meeting of the Child Neurology Society and in a report published earlier this year in Child Neurology Open.
According to pediatric neurologist Timothy Gershon, MD, PhD , of the University of North Carolina at Chapel Hill, the child appeared in clinic in July 2020. She had been healthy but was suffering from 1 day of fever, seizure-like activity (generalized convulsions and drooling), anorexia, and lethargy.
The girl, who was subsequently diagnosed with COVID-19, deteriorated in the hospital. “She received IV dexamethasone in attempts to reduce cerebral edema,” the neurologists wrote. “Regarding immunomodulatory therapy, she received intravenous immunoglobulin (2 g/kg), anakinra, and hydrocortisone; despite approval for remdesivir and COVID-19 convalescent plasma, these were ultimately withheld due to poor prognosis.”
Brain death examinations at 24 and 48 hours after cardiac arrest were consistent with brain death, they reported.
Neurologists believe the patient suffered from AFCE, “an often fatal pediatric clinical entity consisting of fever, encephalopathy, and new-onset seizures followed by rapid, diffuse, and medically-refractory cerebral edema.” They add that “AFCE occurs as a rare complication of a variety of common pediatric infections, and a CNS [central nervous system] pathogen is identified in only a minority of cases, suggesting a para-infectious mechanism of edema.”
Neurologists offered a case definition of the “recently recognized” AFCE earlier this year.
“This was an extremely rare rapid progression to cerebral edema. I think it was related to the patient’s COVID infection, but why this patient got it and others don’t is unknown,” Dr. Gershon said in an interview. “The full spectrum of neurological complications of COVID were not yet known [at the time]. We didn’t know, and still don’t know, what the causative links are between COVID and suddenly having seizures and brain swelling.”
He said he’d treat a similar patient differently now and give dexamethasone earlier in the clinical course, although “there is no data to tell us if any therapy could have reversed it.” Specifically, he said, “I’d give dexamethasone at the first sign of brain involvement, using the dosing recommended for cerebral edema, and try to get the MRI earlier in the course.”
Dr. Gershon and colleagues noted another case of fatal cerebral edema in a child, a 7-year-old boy who was treated in New York state. That case “shows that fatal cerebral edema may complicate pediatric multisystem inflammatory syndrome,” they wrote.
Pediatric critical care specialist Preetha Krishnan, MD, of Randall Children’s Hospital at Legacy Emanuel in Portland, Ore., helped develop the new definition of AFCE. In an interview, she said AFCE is difficult to diagnose because the signs/symptoms – such as fever, altered sensorium, and seizures – are found in other conditions such as febrile status epilepticus with a viral illness.
“The key to recognition of AFCE is that unlike other disease processes, these children have rapid neurologic progression,” she said. “In addition, many of our AFCE patients also had vomiting and/or headache, which in retrospect was likely an indication of elevated ICP [intracranial pressure] rather than viral infection.”
She added that “if a child with fever, seizures, and encephalopathy has cerebral edema on imaging and/or has neurologic progression, AFCE should be considered. Most of our cases of AFCE had fulminant progression within the first 3 days of their head imaging noting cerebral edema. There are other neurologic diseases, such as acute necrotizing encephalopathy of childhood, that also have progressive signs/symptoms, but head imaging and lab work should help differentiate many of these etiologies.”
In regard to treatment, she said, “our unit would likely err on the side of providing as much neuroprotective measures as is reasonable, such as maintaining normothermia, consideration of hyperosmolar therapy, maintaining normocarbia and normoxemia, managing seizures, etc. I would recommend getting the entire neurocritical care team involved in the management discussion. This varies by center, but will likely include neurology, ID [infectious disease], possibly neurosurgery, and PICU.”
As for the new case report, Krishnan said COVID-19 has been linked to neurologic complications, “so it does not surprise me that AFCE is part of the neurologic spectrum of disease.”
No funding was reported, and the authors report no relevant disclosures. Dr. Krishnan has no disclosures.
according to pediatric neurologists who are urging colleagues to watch out for similar cases.
At least one other child in the United States has died after becoming infected with the virus and developing cerebral edema. “The rapid and devastating clinical course in both of these cases highlights the need for early recognition of a cerebral edema and AFCE as potential complications of COVID-19 in pediatric patients,” the neurologists wrote.
The case was highlighted in a poster presented at the annual meeting of the Child Neurology Society and in a report published earlier this year in Child Neurology Open.
According to pediatric neurologist Timothy Gershon, MD, PhD , of the University of North Carolina at Chapel Hill, the child appeared in clinic in July 2020. She had been healthy but was suffering from 1 day of fever, seizure-like activity (generalized convulsions and drooling), anorexia, and lethargy.
The girl, who was subsequently diagnosed with COVID-19, deteriorated in the hospital. “She received IV dexamethasone in attempts to reduce cerebral edema,” the neurologists wrote. “Regarding immunomodulatory therapy, she received intravenous immunoglobulin (2 g/kg), anakinra, and hydrocortisone; despite approval for remdesivir and COVID-19 convalescent plasma, these were ultimately withheld due to poor prognosis.”
Brain death examinations at 24 and 48 hours after cardiac arrest were consistent with brain death, they reported.
Neurologists believe the patient suffered from AFCE, “an often fatal pediatric clinical entity consisting of fever, encephalopathy, and new-onset seizures followed by rapid, diffuse, and medically-refractory cerebral edema.” They add that “AFCE occurs as a rare complication of a variety of common pediatric infections, and a CNS [central nervous system] pathogen is identified in only a minority of cases, suggesting a para-infectious mechanism of edema.”
Neurologists offered a case definition of the “recently recognized” AFCE earlier this year.
“This was an extremely rare rapid progression to cerebral edema. I think it was related to the patient’s COVID infection, but why this patient got it and others don’t is unknown,” Dr. Gershon said in an interview. “The full spectrum of neurological complications of COVID were not yet known [at the time]. We didn’t know, and still don’t know, what the causative links are between COVID and suddenly having seizures and brain swelling.”
He said he’d treat a similar patient differently now and give dexamethasone earlier in the clinical course, although “there is no data to tell us if any therapy could have reversed it.” Specifically, he said, “I’d give dexamethasone at the first sign of brain involvement, using the dosing recommended for cerebral edema, and try to get the MRI earlier in the course.”
Dr. Gershon and colleagues noted another case of fatal cerebral edema in a child, a 7-year-old boy who was treated in New York state. That case “shows that fatal cerebral edema may complicate pediatric multisystem inflammatory syndrome,” they wrote.
Pediatric critical care specialist Preetha Krishnan, MD, of Randall Children’s Hospital at Legacy Emanuel in Portland, Ore., helped develop the new definition of AFCE. In an interview, she said AFCE is difficult to diagnose because the signs/symptoms – such as fever, altered sensorium, and seizures – are found in other conditions such as febrile status epilepticus with a viral illness.
“The key to recognition of AFCE is that unlike other disease processes, these children have rapid neurologic progression,” she said. “In addition, many of our AFCE patients also had vomiting and/or headache, which in retrospect was likely an indication of elevated ICP [intracranial pressure] rather than viral infection.”
She added that “if a child with fever, seizures, and encephalopathy has cerebral edema on imaging and/or has neurologic progression, AFCE should be considered. Most of our cases of AFCE had fulminant progression within the first 3 days of their head imaging noting cerebral edema. There are other neurologic diseases, such as acute necrotizing encephalopathy of childhood, that also have progressive signs/symptoms, but head imaging and lab work should help differentiate many of these etiologies.”
In regard to treatment, she said, “our unit would likely err on the side of providing as much neuroprotective measures as is reasonable, such as maintaining normothermia, consideration of hyperosmolar therapy, maintaining normocarbia and normoxemia, managing seizures, etc. I would recommend getting the entire neurocritical care team involved in the management discussion. This varies by center, but will likely include neurology, ID [infectious disease], possibly neurosurgery, and PICU.”
As for the new case report, Krishnan said COVID-19 has been linked to neurologic complications, “so it does not surprise me that AFCE is part of the neurologic spectrum of disease.”
No funding was reported, and the authors report no relevant disclosures. Dr. Krishnan has no disclosures.
according to pediatric neurologists who are urging colleagues to watch out for similar cases.
At least one other child in the United States has died after becoming infected with the virus and developing cerebral edema. “The rapid and devastating clinical course in both of these cases highlights the need for early recognition of a cerebral edema and AFCE as potential complications of COVID-19 in pediatric patients,” the neurologists wrote.
The case was highlighted in a poster presented at the annual meeting of the Child Neurology Society and in a report published earlier this year in Child Neurology Open.
According to pediatric neurologist Timothy Gershon, MD, PhD , of the University of North Carolina at Chapel Hill, the child appeared in clinic in July 2020. She had been healthy but was suffering from 1 day of fever, seizure-like activity (generalized convulsions and drooling), anorexia, and lethargy.
The girl, who was subsequently diagnosed with COVID-19, deteriorated in the hospital. “She received IV dexamethasone in attempts to reduce cerebral edema,” the neurologists wrote. “Regarding immunomodulatory therapy, she received intravenous immunoglobulin (2 g/kg), anakinra, and hydrocortisone; despite approval for remdesivir and COVID-19 convalescent plasma, these were ultimately withheld due to poor prognosis.”
Brain death examinations at 24 and 48 hours after cardiac arrest were consistent with brain death, they reported.
Neurologists believe the patient suffered from AFCE, “an often fatal pediatric clinical entity consisting of fever, encephalopathy, and new-onset seizures followed by rapid, diffuse, and medically-refractory cerebral edema.” They add that “AFCE occurs as a rare complication of a variety of common pediatric infections, and a CNS [central nervous system] pathogen is identified in only a minority of cases, suggesting a para-infectious mechanism of edema.”
Neurologists offered a case definition of the “recently recognized” AFCE earlier this year.
“This was an extremely rare rapid progression to cerebral edema. I think it was related to the patient’s COVID infection, but why this patient got it and others don’t is unknown,” Dr. Gershon said in an interview. “The full spectrum of neurological complications of COVID were not yet known [at the time]. We didn’t know, and still don’t know, what the causative links are between COVID and suddenly having seizures and brain swelling.”
He said he’d treat a similar patient differently now and give dexamethasone earlier in the clinical course, although “there is no data to tell us if any therapy could have reversed it.” Specifically, he said, “I’d give dexamethasone at the first sign of brain involvement, using the dosing recommended for cerebral edema, and try to get the MRI earlier in the course.”
Dr. Gershon and colleagues noted another case of fatal cerebral edema in a child, a 7-year-old boy who was treated in New York state. That case “shows that fatal cerebral edema may complicate pediatric multisystem inflammatory syndrome,” they wrote.
Pediatric critical care specialist Preetha Krishnan, MD, of Randall Children’s Hospital at Legacy Emanuel in Portland, Ore., helped develop the new definition of AFCE. In an interview, she said AFCE is difficult to diagnose because the signs/symptoms – such as fever, altered sensorium, and seizures – are found in other conditions such as febrile status epilepticus with a viral illness.
“The key to recognition of AFCE is that unlike other disease processes, these children have rapid neurologic progression,” she said. “In addition, many of our AFCE patients also had vomiting and/or headache, which in retrospect was likely an indication of elevated ICP [intracranial pressure] rather than viral infection.”
She added that “if a child with fever, seizures, and encephalopathy has cerebral edema on imaging and/or has neurologic progression, AFCE should be considered. Most of our cases of AFCE had fulminant progression within the first 3 days of their head imaging noting cerebral edema. There are other neurologic diseases, such as acute necrotizing encephalopathy of childhood, that also have progressive signs/symptoms, but head imaging and lab work should help differentiate many of these etiologies.”
In regard to treatment, she said, “our unit would likely err on the side of providing as much neuroprotective measures as is reasonable, such as maintaining normothermia, consideration of hyperosmolar therapy, maintaining normocarbia and normoxemia, managing seizures, etc. I would recommend getting the entire neurocritical care team involved in the management discussion. This varies by center, but will likely include neurology, ID [infectious disease], possibly neurosurgery, and PICU.”
As for the new case report, Krishnan said COVID-19 has been linked to neurologic complications, “so it does not surprise me that AFCE is part of the neurologic spectrum of disease.”
No funding was reported, and the authors report no relevant disclosures. Dr. Krishnan has no disclosures.
FROM CNS 2021
Why this round of COVID-19 feels worse
Exhaustion. Defeat. Hopelessness. Physicians, nurses, physician assistants, and nurse practitioners are overwhelmed with burnout.
The recent round of COVID-19 is more frustrating than the first, with scientific evidence supporting ways we can prevent disease and disease progression. The health care team is no longer viewed as heroes but as the enemy, fraudulently proposing a vaccine and painting a fictional story of death, though it’s all true. The daily educational battle with patients and family members creates a challenging environment that cultivates hopelessness.
Clinicians are physically exhausted from the numerous COVID cases. Gone are the medical patients we trained for, who either remain home and risk their health or lack access to medical providers because of excessive wait times. Empathy for COVID patients is being tested even more with this new surge, and without the two-way bond of trust, clinicians are running out of fuel. Anger and distrust regarding vaccination guidance dominate the interaction when patients present demanding urgent intervention, while clinicians know that more than 95% of hospitalized patients are unvaccinated.
The struggle to find the commitment to medicine and serving patients is made worse by the pandemic fog and loss of trust from patients. Every day, health care teams risk their personal well-being to provide medical care and intervention. Not by choice do we gown up, mask up, and glove up. Each time we enter a COVID patient’s room, we expose ourselves and risk our own lives and the lives of our families for the patients who have elected to ignore medical guidance.
This national wave of resistance to vaccination is spurring an exodus from health care. Physicians are retiring early and physician assistants and nurse practitioners are seeking non–patient-facing positions to improve their own wellness and balance. A national nursing shortage is impacting patients seeking care in every medical discipline. The underlying wave of exhaustion and frustration has not completely destroyed their empathy but has depleted their drive.
How can we regain this drive amid exhausting work hours and angry patients?
As much as we have heard it, we need to protect our time to recharge. The demand to pick up extra shifts and support our colleagues has affected our personal health. Setting boundaries and building time for exercise, meditation, and connecting with family is essential for survival. Mental health is key to retaining empathy and finding hope. Education is one path to reigniting the fires of critical thinking and commitment to patient care – consider precepting students to support the growth of health care teams. Memories of patient care before this pandemic give us the hope that there is light at the end of this tunnel.
Dr. Gadalla is a hospitalist at Treasure Coast Hospitalists in Port St. Lucie, Fla. She is a member of the Hospitalist’s editorial advisory board and also serves as a physician assistant program director at South University in West Palm Beach, Fla. She disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Exhaustion. Defeat. Hopelessness. Physicians, nurses, physician assistants, and nurse practitioners are overwhelmed with burnout.
The recent round of COVID-19 is more frustrating than the first, with scientific evidence supporting ways we can prevent disease and disease progression. The health care team is no longer viewed as heroes but as the enemy, fraudulently proposing a vaccine and painting a fictional story of death, though it’s all true. The daily educational battle with patients and family members creates a challenging environment that cultivates hopelessness.
Clinicians are physically exhausted from the numerous COVID cases. Gone are the medical patients we trained for, who either remain home and risk their health or lack access to medical providers because of excessive wait times. Empathy for COVID patients is being tested even more with this new surge, and without the two-way bond of trust, clinicians are running out of fuel. Anger and distrust regarding vaccination guidance dominate the interaction when patients present demanding urgent intervention, while clinicians know that more than 95% of hospitalized patients are unvaccinated.
The struggle to find the commitment to medicine and serving patients is made worse by the pandemic fog and loss of trust from patients. Every day, health care teams risk their personal well-being to provide medical care and intervention. Not by choice do we gown up, mask up, and glove up. Each time we enter a COVID patient’s room, we expose ourselves and risk our own lives and the lives of our families for the patients who have elected to ignore medical guidance.
This national wave of resistance to vaccination is spurring an exodus from health care. Physicians are retiring early and physician assistants and nurse practitioners are seeking non–patient-facing positions to improve their own wellness and balance. A national nursing shortage is impacting patients seeking care in every medical discipline. The underlying wave of exhaustion and frustration has not completely destroyed their empathy but has depleted their drive.
How can we regain this drive amid exhausting work hours and angry patients?
As much as we have heard it, we need to protect our time to recharge. The demand to pick up extra shifts and support our colleagues has affected our personal health. Setting boundaries and building time for exercise, meditation, and connecting with family is essential for survival. Mental health is key to retaining empathy and finding hope. Education is one path to reigniting the fires of critical thinking and commitment to patient care – consider precepting students to support the growth of health care teams. Memories of patient care before this pandemic give us the hope that there is light at the end of this tunnel.
Dr. Gadalla is a hospitalist at Treasure Coast Hospitalists in Port St. Lucie, Fla. She is a member of the Hospitalist’s editorial advisory board and also serves as a physician assistant program director at South University in West Palm Beach, Fla. She disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Exhaustion. Defeat. Hopelessness. Physicians, nurses, physician assistants, and nurse practitioners are overwhelmed with burnout.
The recent round of COVID-19 is more frustrating than the first, with scientific evidence supporting ways we can prevent disease and disease progression. The health care team is no longer viewed as heroes but as the enemy, fraudulently proposing a vaccine and painting a fictional story of death, though it’s all true. The daily educational battle with patients and family members creates a challenging environment that cultivates hopelessness.
Clinicians are physically exhausted from the numerous COVID cases. Gone are the medical patients we trained for, who either remain home and risk their health or lack access to medical providers because of excessive wait times. Empathy for COVID patients is being tested even more with this new surge, and without the two-way bond of trust, clinicians are running out of fuel. Anger and distrust regarding vaccination guidance dominate the interaction when patients present demanding urgent intervention, while clinicians know that more than 95% of hospitalized patients are unvaccinated.
The struggle to find the commitment to medicine and serving patients is made worse by the pandemic fog and loss of trust from patients. Every day, health care teams risk their personal well-being to provide medical care and intervention. Not by choice do we gown up, mask up, and glove up. Each time we enter a COVID patient’s room, we expose ourselves and risk our own lives and the lives of our families for the patients who have elected to ignore medical guidance.
This national wave of resistance to vaccination is spurring an exodus from health care. Physicians are retiring early and physician assistants and nurse practitioners are seeking non–patient-facing positions to improve their own wellness and balance. A national nursing shortage is impacting patients seeking care in every medical discipline. The underlying wave of exhaustion and frustration has not completely destroyed their empathy but has depleted their drive.
How can we regain this drive amid exhausting work hours and angry patients?
As much as we have heard it, we need to protect our time to recharge. The demand to pick up extra shifts and support our colleagues has affected our personal health. Setting boundaries and building time for exercise, meditation, and connecting with family is essential for survival. Mental health is key to retaining empathy and finding hope. Education is one path to reigniting the fires of critical thinking and commitment to patient care – consider precepting students to support the growth of health care teams. Memories of patient care before this pandemic give us the hope that there is light at the end of this tunnel.
Dr. Gadalla is a hospitalist at Treasure Coast Hospitalists in Port St. Lucie, Fla. She is a member of the Hospitalist’s editorial advisory board and also serves as a physician assistant program director at South University in West Palm Beach, Fla. She disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Merck’s new COVID-19 pill: ‘Game changer’ or just one more tool?
Soon after Merck announced on Oct. 1 that it would ask federal regulators for emergency use authorization (EUA) for its auspicious new COVID-19 pill, the accolades began.
Former Food and Drug Administration chief Scott Gottlieb, MD, told CNBC the drug was “a profound game changer.” Top infectious disease expert Anthony S. Fauci, MD, called the early data “impressive.” The World Health Organization termed it “certainly good news,” while saying it awaits more data.
Merck, partnering with Ridgeback Biotherapeutics on the investigational oral antiviral medicine molnupiravir, plans to submit applications to regulatory agencies worldwide, hoping to deliver the first oral antiviral medication for COVID-19.
Interim clinical trial results show that the drug may slash the risk for hospitalization or death by 50% in those with mild to moderate COVID-19.
When the results were found to be so favorable, the study was halted at the recommendation of an independent data-monitoring committee and in consultation with the FDA.
“This anticipated drug has gotten a little more hype than it deserves,” said William Schaffner, MD, professor of preventive medicine and infectious disease specialist at Vanderbilt University Medical Center in Nashville, Tenn. He and others suggest a reality check.
“It’s not exactly a home run, like penicillin for strep throat,” agreed Carl Fichtenbaum, MD, professor of infectious diseases at the University of Cincinnati, who is investigating a similar pill for a rival company, Atea, partnering with Roche.
“But it is encouraging,” he said. “It will probably be an incremental improvement on what we have.” The fact that it can be taken at home is a plus: “Anything we can do to keep people from getting sicker is a good thing.”
“The data show in this higher risk group [those who were studied had at least one risk factor for severe COVID-19, such as age or a medical condition], it reduces the risk of advancing to severe disease by 50%,” Dr. Schaffner said. While that’s a clear benefit for half, it of course leaves the other half without benefit, he said.
Others critiqued the predicted cost of the drug. The U.S. government has already agreed to pay about $700 per patient, according to a new report from Harvard T. H. Chan School of Public Health, Boston, and King’s College Hospital, London. That analysis concluded that the actual cost of production for the 5-day course is $17.74.
“We fully expect that having an oral treatment that reduces the risk of hospitalizations will be significantly cost effective for society,” Melissa Moody, a Merck spokesperson, told this news organization. “We are optimistic that molnupiravir can become an important medicine as part of the global effort to fight the pandemic.”
Merck expects to produce 10 million courses of treatment by the end of the year, with additional doses expected to be produced in 2022, according to a company press release. Earlier in 2021, Merck finalized its agreement with the U.S. government to supply about 1.7 million courses of the drug at the $700 price, once an EUA or FDA approval is given.
Merck also has supply and purchase agreements with other governments worldwide, pending regulatory approval.
Study details
Details about the study findings came from a Merck press release. In the planned interim analysis, Merck and Ridgeback evaluated data from 775 patients initially enrolled in the phase 3 MOVe-OUT trial.
All adults had lab-confirmed mild to moderate COVID-19, and reported onset of symptoms within 5 days of being randomly assigned to the drug or placebo. All had at least one risk factor linked with poor disease outcome (such as older age or obesity).
The drug is a ribonucleoside and works by creating mutations in the virus’s genome, halting the ability of the virus to replicate.
Through day 29 of the study, the drug reduced the risk or hospitalization or death by about 50%. While 7.3% of those who received the drug either died or were hospitalized by day 29, 14.1% of those on placebo did, a statistically significant difference (P = .0012).
Side effects were similar in both groups, with 35% of the drug-treated and 40% of the placebo group reporting some side effect, Merck reported. Adverse drug-related events were 12% in the drug group and 11% in the placebo group. While 1.3% of the drug-treated group quit the study because of an adverse event, 3.4% of the placebo group quit.
Pros, cons, and unknowns
The ability to take the drug orally, and at home, is a definite plus, Dr. Schaffner said, compared with the monoclonal antibody treatment currently approved that must be given intravenously or subcutaneously and in certain locations.
More people could be reached and helped with the option of an at-home, oral medicine, he and others agreed.
The regimen for molnupiravir is four pills, two times daily, for 5 days, even if symptoms are mild. As with other prescription drugs, “there will always be folks who don’t comply completely” with the prescribed regimen, Dr. Schaffner said. With this pill, that might be especially true if the symptoms are very mild.
The 50% reduction is not as effective as the benefit often quoted for monoclonal antibody treatment. In clinical trials of Regeneron’s monoclonal antibody treatment, the regimen reduced COVID-19–related hospitalization or death in high-risk patients by 70%.
Even so, the new pill could change the pandemic’s course, others say. “I think molnupiravir has the potential to change how we take care of people who have COVID and risk factors for developing severe disease,” Rajesh Tim Gandhi, MD, an infectious disease physician at Massachusetts General Hospital and Harvard Medical School in Boston, told this news organization.
“What we’ll need to do, however, is make sure that people get tested quickly after they develop symptoms and, if they’re confirmed to have COVID, start on the pills within 5 days of developing symptoms,” he said, while warning that more data are needed about the drug and the trial results.
Another concern is that the promise of a pill will stall vaccination rates, with some people figuring why get vaccinated when they can obtain the pill if they do get sick.
Relying on treatment alone won’t work, Dr. Schaffner said. “Let’s [also] focus on prevention, which is the vaccine. We have to keep working both sides of the street.”
Dr. Gandhi added: “It’s important to remember that even though molnupiravir reduced the likelihood of hospitalization and death, a number of people who received the drug still got sick enough to end up in the hospital.”
Also unknown, he said, is how severe their disease was and whether they will develop long COVID.
The Merck study included only unvaccinated people. Might it work for those vaccinated people who get a breakthrough infection? “From a purely scientific perspective, there is no reason to believe molnupiravir would not work in people who are vaccinated, but the overall efficacy on top of the vaccine is likely dependent on how well they were able to mount a protective immune response to the vaccine,” Ms. Moody said. Still, Merck believes the pill could be of benefit for these infections too, she added.
As for the expected cost, Ms. Moody said that the company takes into account a number of factors in setting pricing, “but fundamentally we look at the impact of the disease, the benefits that the drug delivers to patients and to society, and at supporting ongoing drug development.”
On Merck’s heels: Pfizer, Roche, Atea
Pfizer is studying an antiviral pill, PF-07321332, a protease inhibitor that blocks the protease enzymes and halts replication of the virus.
In addition to studying the drug in infected patients at high risk of severe illness and in those at typical risk, Pfizer launched a phase 2-3 study in late September that will enroll people who live in the same household as a person with a confirmed, symptomatic COVID-19 infection to see if the drug can prevent disease in those who have been exposed.
Atea and Roche’s COVID pill, AT527, is in phase 3 trials as well. AT527 is an inhibitor of polymerase, an enzyme many viruses have, to stop replications. Atea is evaluating the drug to reduce disease “burden” and for both pre- and postexposure prevention.
Big picture: Role of COVID-19 pills
It may be necessary to target the coronavirus with more than one antiviral agent, said Dr. Fichtenbaum, a principal investigator for the AT527 trials.
“Sometimes viruses require two or three active agents to control their replication,” he said, citing information gleaned from other viral research, such as HIV. For control of HIV infection, a cocktail or combination of antivirals is often recommended.
That may well be the case for COVID-19, Dr. Fichtenbaum said. The goal would be to attack the virus at more than one pathway.
A version of this article first appeared on Medscape.com.
Soon after Merck announced on Oct. 1 that it would ask federal regulators for emergency use authorization (EUA) for its auspicious new COVID-19 pill, the accolades began.
Former Food and Drug Administration chief Scott Gottlieb, MD, told CNBC the drug was “a profound game changer.” Top infectious disease expert Anthony S. Fauci, MD, called the early data “impressive.” The World Health Organization termed it “certainly good news,” while saying it awaits more data.
Merck, partnering with Ridgeback Biotherapeutics on the investigational oral antiviral medicine molnupiravir, plans to submit applications to regulatory agencies worldwide, hoping to deliver the first oral antiviral medication for COVID-19.
Interim clinical trial results show that the drug may slash the risk for hospitalization or death by 50% in those with mild to moderate COVID-19.
When the results were found to be so favorable, the study was halted at the recommendation of an independent data-monitoring committee and in consultation with the FDA.
“This anticipated drug has gotten a little more hype than it deserves,” said William Schaffner, MD, professor of preventive medicine and infectious disease specialist at Vanderbilt University Medical Center in Nashville, Tenn. He and others suggest a reality check.
“It’s not exactly a home run, like penicillin for strep throat,” agreed Carl Fichtenbaum, MD, professor of infectious diseases at the University of Cincinnati, who is investigating a similar pill for a rival company, Atea, partnering with Roche.
“But it is encouraging,” he said. “It will probably be an incremental improvement on what we have.” The fact that it can be taken at home is a plus: “Anything we can do to keep people from getting sicker is a good thing.”
“The data show in this higher risk group [those who were studied had at least one risk factor for severe COVID-19, such as age or a medical condition], it reduces the risk of advancing to severe disease by 50%,” Dr. Schaffner said. While that’s a clear benefit for half, it of course leaves the other half without benefit, he said.
Others critiqued the predicted cost of the drug. The U.S. government has already agreed to pay about $700 per patient, according to a new report from Harvard T. H. Chan School of Public Health, Boston, and King’s College Hospital, London. That analysis concluded that the actual cost of production for the 5-day course is $17.74.
“We fully expect that having an oral treatment that reduces the risk of hospitalizations will be significantly cost effective for society,” Melissa Moody, a Merck spokesperson, told this news organization. “We are optimistic that molnupiravir can become an important medicine as part of the global effort to fight the pandemic.”
Merck expects to produce 10 million courses of treatment by the end of the year, with additional doses expected to be produced in 2022, according to a company press release. Earlier in 2021, Merck finalized its agreement with the U.S. government to supply about 1.7 million courses of the drug at the $700 price, once an EUA or FDA approval is given.
Merck also has supply and purchase agreements with other governments worldwide, pending regulatory approval.
Study details
Details about the study findings came from a Merck press release. In the planned interim analysis, Merck and Ridgeback evaluated data from 775 patients initially enrolled in the phase 3 MOVe-OUT trial.
All adults had lab-confirmed mild to moderate COVID-19, and reported onset of symptoms within 5 days of being randomly assigned to the drug or placebo. All had at least one risk factor linked with poor disease outcome (such as older age or obesity).
The drug is a ribonucleoside and works by creating mutations in the virus’s genome, halting the ability of the virus to replicate.
Through day 29 of the study, the drug reduced the risk or hospitalization or death by about 50%. While 7.3% of those who received the drug either died or were hospitalized by day 29, 14.1% of those on placebo did, a statistically significant difference (P = .0012).
Side effects were similar in both groups, with 35% of the drug-treated and 40% of the placebo group reporting some side effect, Merck reported. Adverse drug-related events were 12% in the drug group and 11% in the placebo group. While 1.3% of the drug-treated group quit the study because of an adverse event, 3.4% of the placebo group quit.
Pros, cons, and unknowns
The ability to take the drug orally, and at home, is a definite plus, Dr. Schaffner said, compared with the monoclonal antibody treatment currently approved that must be given intravenously or subcutaneously and in certain locations.
More people could be reached and helped with the option of an at-home, oral medicine, he and others agreed.
The regimen for molnupiravir is four pills, two times daily, for 5 days, even if symptoms are mild. As with other prescription drugs, “there will always be folks who don’t comply completely” with the prescribed regimen, Dr. Schaffner said. With this pill, that might be especially true if the symptoms are very mild.
The 50% reduction is not as effective as the benefit often quoted for monoclonal antibody treatment. In clinical trials of Regeneron’s monoclonal antibody treatment, the regimen reduced COVID-19–related hospitalization or death in high-risk patients by 70%.
Even so, the new pill could change the pandemic’s course, others say. “I think molnupiravir has the potential to change how we take care of people who have COVID and risk factors for developing severe disease,” Rajesh Tim Gandhi, MD, an infectious disease physician at Massachusetts General Hospital and Harvard Medical School in Boston, told this news organization.
“What we’ll need to do, however, is make sure that people get tested quickly after they develop symptoms and, if they’re confirmed to have COVID, start on the pills within 5 days of developing symptoms,” he said, while warning that more data are needed about the drug and the trial results.
Another concern is that the promise of a pill will stall vaccination rates, with some people figuring why get vaccinated when they can obtain the pill if they do get sick.
Relying on treatment alone won’t work, Dr. Schaffner said. “Let’s [also] focus on prevention, which is the vaccine. We have to keep working both sides of the street.”
Dr. Gandhi added: “It’s important to remember that even though molnupiravir reduced the likelihood of hospitalization and death, a number of people who received the drug still got sick enough to end up in the hospital.”
Also unknown, he said, is how severe their disease was and whether they will develop long COVID.
The Merck study included only unvaccinated people. Might it work for those vaccinated people who get a breakthrough infection? “From a purely scientific perspective, there is no reason to believe molnupiravir would not work in people who are vaccinated, but the overall efficacy on top of the vaccine is likely dependent on how well they were able to mount a protective immune response to the vaccine,” Ms. Moody said. Still, Merck believes the pill could be of benefit for these infections too, she added.
As for the expected cost, Ms. Moody said that the company takes into account a number of factors in setting pricing, “but fundamentally we look at the impact of the disease, the benefits that the drug delivers to patients and to society, and at supporting ongoing drug development.”
On Merck’s heels: Pfizer, Roche, Atea
Pfizer is studying an antiviral pill, PF-07321332, a protease inhibitor that blocks the protease enzymes and halts replication of the virus.
In addition to studying the drug in infected patients at high risk of severe illness and in those at typical risk, Pfizer launched a phase 2-3 study in late September that will enroll people who live in the same household as a person with a confirmed, symptomatic COVID-19 infection to see if the drug can prevent disease in those who have been exposed.
Atea and Roche’s COVID pill, AT527, is in phase 3 trials as well. AT527 is an inhibitor of polymerase, an enzyme many viruses have, to stop replications. Atea is evaluating the drug to reduce disease “burden” and for both pre- and postexposure prevention.
Big picture: Role of COVID-19 pills
It may be necessary to target the coronavirus with more than one antiviral agent, said Dr. Fichtenbaum, a principal investigator for the AT527 trials.
“Sometimes viruses require two or three active agents to control their replication,” he said, citing information gleaned from other viral research, such as HIV. For control of HIV infection, a cocktail or combination of antivirals is often recommended.
That may well be the case for COVID-19, Dr. Fichtenbaum said. The goal would be to attack the virus at more than one pathway.
A version of this article first appeared on Medscape.com.
Soon after Merck announced on Oct. 1 that it would ask federal regulators for emergency use authorization (EUA) for its auspicious new COVID-19 pill, the accolades began.
Former Food and Drug Administration chief Scott Gottlieb, MD, told CNBC the drug was “a profound game changer.” Top infectious disease expert Anthony S. Fauci, MD, called the early data “impressive.” The World Health Organization termed it “certainly good news,” while saying it awaits more data.
Merck, partnering with Ridgeback Biotherapeutics on the investigational oral antiviral medicine molnupiravir, plans to submit applications to regulatory agencies worldwide, hoping to deliver the first oral antiviral medication for COVID-19.
Interim clinical trial results show that the drug may slash the risk for hospitalization or death by 50% in those with mild to moderate COVID-19.
When the results were found to be so favorable, the study was halted at the recommendation of an independent data-monitoring committee and in consultation with the FDA.
“This anticipated drug has gotten a little more hype than it deserves,” said William Schaffner, MD, professor of preventive medicine and infectious disease specialist at Vanderbilt University Medical Center in Nashville, Tenn. He and others suggest a reality check.
“It’s not exactly a home run, like penicillin for strep throat,” agreed Carl Fichtenbaum, MD, professor of infectious diseases at the University of Cincinnati, who is investigating a similar pill for a rival company, Atea, partnering with Roche.
“But it is encouraging,” he said. “It will probably be an incremental improvement on what we have.” The fact that it can be taken at home is a plus: “Anything we can do to keep people from getting sicker is a good thing.”
“The data show in this higher risk group [those who were studied had at least one risk factor for severe COVID-19, such as age or a medical condition], it reduces the risk of advancing to severe disease by 50%,” Dr. Schaffner said. While that’s a clear benefit for half, it of course leaves the other half without benefit, he said.
Others critiqued the predicted cost of the drug. The U.S. government has already agreed to pay about $700 per patient, according to a new report from Harvard T. H. Chan School of Public Health, Boston, and King’s College Hospital, London. That analysis concluded that the actual cost of production for the 5-day course is $17.74.
“We fully expect that having an oral treatment that reduces the risk of hospitalizations will be significantly cost effective for society,” Melissa Moody, a Merck spokesperson, told this news organization. “We are optimistic that molnupiravir can become an important medicine as part of the global effort to fight the pandemic.”
Merck expects to produce 10 million courses of treatment by the end of the year, with additional doses expected to be produced in 2022, according to a company press release. Earlier in 2021, Merck finalized its agreement with the U.S. government to supply about 1.7 million courses of the drug at the $700 price, once an EUA or FDA approval is given.
Merck also has supply and purchase agreements with other governments worldwide, pending regulatory approval.
Study details
Details about the study findings came from a Merck press release. In the planned interim analysis, Merck and Ridgeback evaluated data from 775 patients initially enrolled in the phase 3 MOVe-OUT trial.
All adults had lab-confirmed mild to moderate COVID-19, and reported onset of symptoms within 5 days of being randomly assigned to the drug or placebo. All had at least one risk factor linked with poor disease outcome (such as older age or obesity).
The drug is a ribonucleoside and works by creating mutations in the virus’s genome, halting the ability of the virus to replicate.
Through day 29 of the study, the drug reduced the risk or hospitalization or death by about 50%. While 7.3% of those who received the drug either died or were hospitalized by day 29, 14.1% of those on placebo did, a statistically significant difference (P = .0012).
Side effects were similar in both groups, with 35% of the drug-treated and 40% of the placebo group reporting some side effect, Merck reported. Adverse drug-related events were 12% in the drug group and 11% in the placebo group. While 1.3% of the drug-treated group quit the study because of an adverse event, 3.4% of the placebo group quit.
Pros, cons, and unknowns
The ability to take the drug orally, and at home, is a definite plus, Dr. Schaffner said, compared with the monoclonal antibody treatment currently approved that must be given intravenously or subcutaneously and in certain locations.
More people could be reached and helped with the option of an at-home, oral medicine, he and others agreed.
The regimen for molnupiravir is four pills, two times daily, for 5 days, even if symptoms are mild. As with other prescription drugs, “there will always be folks who don’t comply completely” with the prescribed regimen, Dr. Schaffner said. With this pill, that might be especially true if the symptoms are very mild.
The 50% reduction is not as effective as the benefit often quoted for monoclonal antibody treatment. In clinical trials of Regeneron’s monoclonal antibody treatment, the regimen reduced COVID-19–related hospitalization or death in high-risk patients by 70%.
Even so, the new pill could change the pandemic’s course, others say. “I think molnupiravir has the potential to change how we take care of people who have COVID and risk factors for developing severe disease,” Rajesh Tim Gandhi, MD, an infectious disease physician at Massachusetts General Hospital and Harvard Medical School in Boston, told this news organization.
“What we’ll need to do, however, is make sure that people get tested quickly after they develop symptoms and, if they’re confirmed to have COVID, start on the pills within 5 days of developing symptoms,” he said, while warning that more data are needed about the drug and the trial results.
Another concern is that the promise of a pill will stall vaccination rates, with some people figuring why get vaccinated when they can obtain the pill if they do get sick.
Relying on treatment alone won’t work, Dr. Schaffner said. “Let’s [also] focus on prevention, which is the vaccine. We have to keep working both sides of the street.”
Dr. Gandhi added: “It’s important to remember that even though molnupiravir reduced the likelihood of hospitalization and death, a number of people who received the drug still got sick enough to end up in the hospital.”
Also unknown, he said, is how severe their disease was and whether they will develop long COVID.
The Merck study included only unvaccinated people. Might it work for those vaccinated people who get a breakthrough infection? “From a purely scientific perspective, there is no reason to believe molnupiravir would not work in people who are vaccinated, but the overall efficacy on top of the vaccine is likely dependent on how well they were able to mount a protective immune response to the vaccine,” Ms. Moody said. Still, Merck believes the pill could be of benefit for these infections too, she added.
As for the expected cost, Ms. Moody said that the company takes into account a number of factors in setting pricing, “but fundamentally we look at the impact of the disease, the benefits that the drug delivers to patients and to society, and at supporting ongoing drug development.”
On Merck’s heels: Pfizer, Roche, Atea
Pfizer is studying an antiviral pill, PF-07321332, a protease inhibitor that blocks the protease enzymes and halts replication of the virus.
In addition to studying the drug in infected patients at high risk of severe illness and in those at typical risk, Pfizer launched a phase 2-3 study in late September that will enroll people who live in the same household as a person with a confirmed, symptomatic COVID-19 infection to see if the drug can prevent disease in those who have been exposed.
Atea and Roche’s COVID pill, AT527, is in phase 3 trials as well. AT527 is an inhibitor of polymerase, an enzyme many viruses have, to stop replications. Atea is evaluating the drug to reduce disease “burden” and for both pre- and postexposure prevention.
Big picture: Role of COVID-19 pills
It may be necessary to target the coronavirus with more than one antiviral agent, said Dr. Fichtenbaum, a principal investigator for the AT527 trials.
“Sometimes viruses require two or three active agents to control their replication,” he said, citing information gleaned from other viral research, such as HIV. For control of HIV infection, a cocktail or combination of antivirals is often recommended.
That may well be the case for COVID-19, Dr. Fichtenbaum said. The goal would be to attack the virus at more than one pathway.
A version of this article first appeared on Medscape.com.
Vaccinations for the ObGyn’s toolbox
CASE 1st prenatal appointment for young, pregnant migrant
A 21-year-old primigravid woman at 12 weeks’ gestation recently immigrated to the United States from an impoverished rural area of Southeast Asia. On the first prenatal appointment, she is noted to have no evidence of immunity to rubella, measles, or varicella. Her hepatitis B surface antigen and hepatitis C antibody tests are negative. She also has negative test results for gonorrhea, chlamydia, syphilis, and HIV infection. Her pap test is negative.
- What vaccinations should this patient receive during her pregnancy?
- What additional vaccinations are indicated postpartum?
Preventive vaccinations: What to know
As ObGyns, we serve as the primary care physician for many women throughout their early and middle decades of life. Accordingly, we have an obligation to be well informed about preventive health services such as vaccinations. The purpose of this article is to review the principal vaccines with which ObGyns should be familiar. I will discuss the vaccines in alphabetical order and then focus on the indications and timing for each vaccine and the relative cost of each immunization. Key points are summarized in the TABLE.
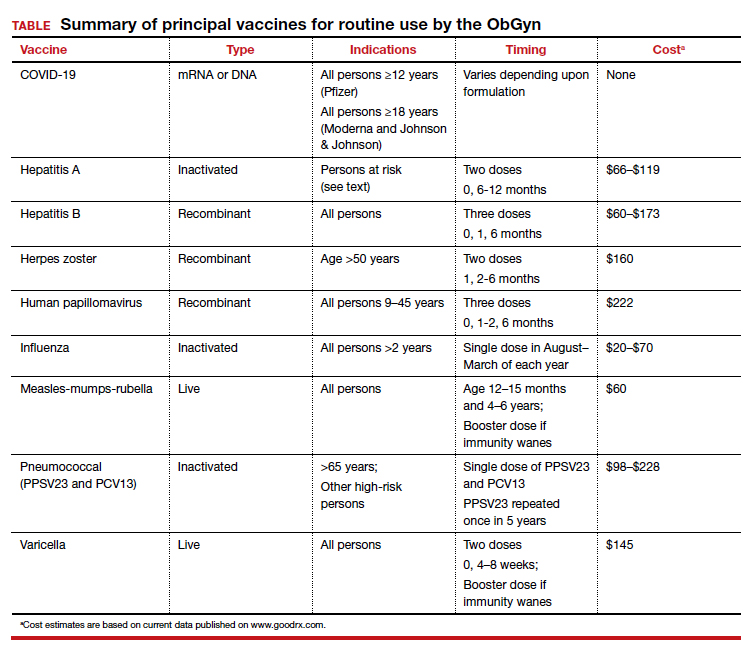
COVID-19 vaccine
In the latter part of 2020 and early part of 2021, three COVID-19 vaccines received emergency use authorization (EUA) from the US Food and Drug Administration (FDA) for individuals 16 years of age and older (Pfizer-BioNTech) and 18 years of age and older (Moderna and Johnson & Johnson).1 The cost of their administration is borne by the federal government. Two of the vaccines are mRNA agents—Moderna and Pfizer-BioNTech. Both are administered in a 2-dose series, separated by 4 and 3 weeks, respectively. The efficacy of these vaccines in preventing serious or critical illness approaches 95%. The Pfizer-BioNTech vaccine has now been fully FDA approved for administration to individuals older than age 16, with EUA for those down to age 12. Full approval of the Moderna vaccine will not be far behind. Because of some evidence suggesting waning immunity over time and because of growing concerns about the increased transmissibility of the delta variant of the virus, the FDA has been strongly considering a recommendation for a third (booster) dose of each of these vaccines, administered 8 months after the second dose for all eligible Americans. On September 17, 2021, the FDA advisory committee recommended a booster for the Pfizer-BioNTech vaccine for people older than age 65 and for those over the age of 16 at high risk for severe COVID-19. Several days later, full FDA approval was granted for this recommendation. Subsequently, the Director of the Centers for Disease Control and Prevention (CDC) included health care workers and pregnant women in the group for whom the booster is recommended.
The third vaccine formulation is the Johnson & Johnson DNA vaccine, which is prepared with a human adenovirus vector. This vaccine is administered in a single intramuscular dose and has a reported efficacy of 66% to 85%, though it may approach 95% in preventing critical illness. The FDA is expected to announce decisions about booster doses for the Johnson & Johnson and Moderna vaccines in the coming weeks.
Although initial trials of the COVID-19vaccines excluded pregnant and lactating women, the vaccines are safe in pregnancy or postpartum. In fact the vaccines do not contain either a killed or attenuated viral particle that is capable of transmitting infection. Therefore, both the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine now support routine immunization during pregnancy.
A recent report by Shimabukuro and colleagues2 demonstrated that the risk of vaccine-related complications in pregnant women receiving the Pfizer-BioNTech or Moderna vaccines was no different than in nonpregnant patients and that there was no evidence of teratogenic effects. The trial included more than 35,000 pregnant women; 2.3% were vaccinated in the periconception period, 28.6% in the first trimester, 43.3% in the second trimester, and 25.7% in the third trimester. Given this, and in light of isolated reports of unusual thromboembolic complications associated with the Johnson & Johnson vaccine, I strongly recommend use of either the Moderna or Pfizer-BioNTech vaccine in our prenatal and postpartum patients.
Continue to: Hepatitis A vaccine...
Hepatitis A vaccine
The hepatitis A vaccine is an inactivated vaccine and is safe for use in pregnancy. It is available in two monovalent preparations—Havrix (GlaxoSmithKline) and Vaqta (Merck & Co.) and is administered in a 2-dose intramuscular injection at time zero and 6 to 12 months later.3 The vaccine is also available in a bivalent form with recombinant hepatitis B vaccine—Twinrix (GlaxoSmithKline). When administered in this form, the vaccine should be given at time zero, 1 month, and 6 months. The wholesale cost of the monovalent vaccine is $66 to $119, depending upon whether the provider uses a multi-dose or a single-dose vial. The cost of Twinrix is $149.
The hepatitis A vaccine is indicated for select pregnant and nonpregnant patients:
- international travelers
- intravenous drug users
- those with occupational exposure (eg, individuals who work in a primate laboratory)
- residents and staff in chronic care facilities
- individuals with chronic liver disease
- individuals with clotting factor disorders
- residents in endemic areas.
Hepatitis B vaccine
The hepatitis B vaccine is a recombinant vaccine that contains an inactivated portion of the hepatitis B surface antigen. It was originally produced in two monovalent formulations: Engerix B (GlaxoSmithKline) and Recombivax-HB (Merck & Co.). These original formulations are given in a 3-dose series at time zero, 1 month, and 6 months. Recently, a new and more potent formulation was introduced into clinical practice. Heplisav-B (Dynavax Technologies Co.) is also a recombinant vaccine that contains a boosting adjuvant. It is programed to be administered in a 2-dose series at time zero and 1 month.4-6
The wholesale cost of the monovalent vaccines varies from $60 to $173, depending upon use of a multi-dose vial versus a single-use vial. The cost of Heplisav-B varies from $146 to $173, depending upon use of a prefilled syringe versus a single-dose vial.
Although the hepatitis B vaccine should be part of the childhood immunization series, it also should be administered to any pregnant woman who has not been vaccinated previously or who does not already have evidence of immunity as a result of natural infection.
Continue to: Herpes zoster vaccine...
Herpes zoster vaccine
Herpes zoster infection (shingles) can be a particularly disabling condition in older patients and results from reactivation of a latent varicella-zoster infection. Shingles can cause extremely painful skin lesions, threaten the patient’s vision, and result in long-lasting postherpetic neuralgia. Both cellular and hormonal immunity are essential to protect against recurrent infection.
The original herpes zoster vaccine (Zoster Vaccine Live; ZVL, Zostavax) is no longer produced in the United States because it is not as effective as the newer vaccine—Recombinant Zoster Vaccine (Shingrix, GlaxoSmithKline).7,8 The antigen in the new vaccine is a component of the surface glycoprotein E, and it is combined with an adjuvant to enhance immunoreactivity. The vaccine is given intramuscularly in two doses at time zero and again at 2 to 6 months and is indicated for all individuals >50 years, including those who may have had an episode of shingles. This newer vaccine is 97% effective in patients >50 years and 90% effective in patients >70. The wholesale cost of each injection is about $160.
Human papillomavirus vaccine
The HPV vaccine (Gardasil-9, Merck & Co.) is a recombinant 9-valent vaccine directed against the human papillomavirus. It induces immunity to serotypes 6 and 11 (which cause 90% of genital warts), 16 and 18 (which cause 80% of genital cancers), and 31, 33, 45, 52, and 58 (viral strains that are responsible for both genital and oropharyngeal cancers). The vaccine is administered intramuscularly in a 3-dose series at time zero, 1-2 months, and 6 months. The principal target groups for the vaccine are males and females, ages 9 to 45 years. Ideally, children of both sexes should receive this vaccine prior to the onset of sexual activity. The wholesale cost of each vaccine injection is approximately $222.9
Influenza vaccine
The inactivated, intramuscular flu vaccine is recommended for anyone over age 2, including pregnant women. Although pregnant women are not more likely to acquire flu compared with those who are not pregnant, if they do become infected, they are likely to become more seriously ill, with higher mortality. Accordingly, all pregnant women should receive, in any trimester, the inactivated flu vaccine beginning in the late summer and early fall of each year and extending through March of the next year.10,11
Multiple formulations of the inactivated vaccine are marketed, all targeting two strains of influenza A and two strains of influenza B. The components of the vaccine vary each year as scientists try to match the new vaccine with the most highly prevalent strains in the previous flu season. The vaccine should be administered in a single intramuscular dose. The cost varies from approximately $20 to $70.
The intranasal influenza vaccine is a live virus vaccine that is intended primarily for children and should not be administered in pregnancy. In addition, there is a higher dose of the inactivated quadrivalent vaccine that is available for administration to patients over age 65. This higher dose is more likely to cause adverse effects and is not indicated in pregnancy.
Continue to: Measles, mumps, rubella vaccine (MMR)...
Measles, mumps, rubella vaccine (MMR)
The MMR is a standard component of the childhood vaccination series. The trivalent preparation is a live, attenuated vaccine that is typically given subcutaneously in a 2-dose series. The first dose is administered at age 12-15 months, and the second dose at age 4-6 years. The vaccine is highly immunogenic, with vaccine-induced immunity usually life-long. In some patients, however, immunity wanes over time. Accordingly, all pregnant women should be screened for immunity to rubella since, of the 3, this infection poses the greatest risk to the fetus. Women who do not have evidence of immunity should be advised to avoid contact with children who may have a viral exanthem. They should then receive a booster dose of the vaccine immediately postpartum and should practice secure contraception for 1 month. The vaccine cost is approximately $60.
Pneumococcal vaccine
The inactivated pneumococcal vaccine is produced in two forms, both of which are safe for administration in pregnancy.12 The original vaccine, introduced in 1983, was PPSV23 (Pneumovax 23, Merck & Co), a 23-serovalent vaccine that was intended primarily for adults. This vaccine is administered in a single subcutaneous or intramuscular dose. The newest vaccine, introduced in 2010, is PCV13 (Prevnar 13, Pfizer Inc), a 13-serovalent vaccine. It was intended primarily for children, in whom it is administered in a 4-dose series beginning at 6 to 8 weeks of age. The cost of the former is approximately $98 to $120; the cost of the latter is $228.
Vaccination against pneumococcal infection is routinely indicated for those older than the age of 65 and for the following at-risk patients, including those who are pregnant11:
- individuals who have had a splenectomy or who have a medical illness that produces functional asplenia (eg, sickle cell anemia)
- individuals with chronic cardiac, pulmonic, hepatic, or renal disease
- individuals with immunosuppressive conditions such as HIV infection or a disseminated malignancy
- individuals who have a cochlear implant
- individuals who have a chronic leak of cerebrospinal fluid.
The recommendations for timing of these 2 vaccines in adults can initially appear confusing. Put most simply, if a high-risk patient first receives the PCV13 vaccine, she should receive the PPSV23 vaccine in about 8 weeks. The PPSV23 vaccine should be repeated in 5 years. If an at-risk patient initially receives the PPSV23 vaccine, the PCV13 vaccine should be given 1 year later.12
Tdap vaccine
The Tdap vaccine contains tetanus toxoid, reduced diptheria toxoid, and an acellular component of the pertussis bacterium. Although it has long been part of the childhood vaccinations series, immunity to each component, particularly pertussis, tends to wane over time.
Pertussis poses a serious risk to the health of the pregnant woman and the newborn infant. Accordingly, the Advisory Committee on Immunization Practices (ACIP), CDC, and the ACOG now advise administration of a booster dose of this vaccine in the early third trimester of each pregnancy.13-15 The vaccine should be administered as a single intramuscular injection. The approximate cost of the vaccine is $64 to $71, depending upon whether the provider uses a single-dose vial or a single-dose prefilled syringe. In nonpregnant patients, the ACIP currently recommends administration of a booster dose of the vaccine every 10 years, primarily to provide durable protection against tetanus.
Continue to: Varicella vaccine...
Varicella vaccine
The varicella vaccine is also one of the main components of the childhood immunization series. This live virus vaccine can be administered subcutaneously as a monovalent agent or as a quadrivalent agent in association with the MMR vaccine.
Pregnant women who do not have a well-documented history of natural infection should be tested for IgG antibody to the varicella-zoster virus at the time of their first prenatal appointment. Interestingly, approximately 70% of patients with an uncertain history actually have immunity when tested. If the patient lacks immunity, she should be vaccinated immediately postpartum.16,17 The vaccine should be administered in a 2-dose series at time zero and then 4 to 8 weeks later. Patients should adhere to secure contraception from the time of the first dose until 1 month after the second dose. The cost of each dose of the vaccine is approximately $145.
Adverse effects of vaccination
All vaccines have many of the same side effects. The most common is simply a reaction at the site of injection, characterized by pain, increased warmth, erythema, swelling, and tenderness. Other common side effects include systemic manifestations, such as low-grade fever, nausea and vomiting, malaise, fatigue, headache, lymphadenopathy, myalgias, and arthralgias. Some vaccines, notably varicella, herpes zoster, measles, and rubella may cause a disseminated rash. Most of these minor side effects are easily managed by rest, hydration, and administration of an analgesic such as acetaminophen or ibuprofen. More serious side effects include rare complications such as anaphylaxis, Bell palsy, Guillain-Barre syndrome, and venous thromboembolism (Johnson & Johnson COVID-19 vaccine). Any of the vaccines discussed above should not be given, or given only with extreme caution, to an individual who has experienced any of these reactions with a previous vaccine.
Barriers to vaccination
Although the vaccines reviewed above are highly effective in preventing serious illness in recipients, the medical profession’s “report card” in ensuring adherence with vaccine protocols is not optimal. In fact, it probably merits a grade no higher than C+, with vaccination rates in the range of 50% to 70%.
One of the major barriers to vaccination is lack of detailed information about vaccine efficacy and safety on the part of both provider and patient. Another is the problem of misinformation (eg, the persistent belief on the part of some individuals that vaccines may cause a serious problem, such as autism).18,19 Another important barrier to widespread vaccination is the logistical problem associated with proper scheduling of multidose regimens (such as those for hepatitis A and B, varicella, and COVID-19). A final barrier, and in my own university-based practice, the most important obstacle is the expense of vaccination. Most, but not all, private insurance companies provide coverage for vaccines approved by the Centers for Disease Control and Prevention and the US Preventive Services Task Force. However, public insurance agencies often provide disappointingly inconsistent coverage for essential vaccines.
By keeping well informed about the most recent public health recommendations for vaccinations for adults and by leading important initiatives within our own practices, we should be able to overcome the first 3 barriers listed above. For example, Morgan and colleagues20 recently achieved a 97% success rate with Tdap administration in pregnancy by placing a best-practice alert in the patients’ electronic medical records. Surmounting the final barrier will require intense effort on the part of individual practitioners and professional organizations to advocate for coverage for essential vaccinations for our patients.
CASE Resolved
This patient was raised in an area of the world where her family did not have easy access to medical care. Accordingly, she did not receive the usual childhood vaccines, such as measles, mumps, rubella, varicella, hepatitis B, and almost certainly, tetanus, diphtheria, and pertussis (Tdap), and the HPV vaccine. The MMR vaccine and the varicella vaccine are live virus vaccines and should not be given during pregnancy. However, these vaccines should be administered postpartum, and the patient should be instructed to practice secure contraception for a minimum of 1 month following vaccination. She also should be offered the HPV vaccine postpartum. During pregnancy, she definitely should receive the COVID-19 vaccine, the 3-dose hepatitis B vaccine series, the influenza vaccine, and Tdap. If her present living conditions place her at risk for hepatitis A, she also should be vaccinated against this illness. ●
- Rasmussen SA, Kelley CF, Horton JP, et al. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy. What obstetricians need to know. Obstet Gynecol. 2021;137:408-414. doi: 10.1097/AOG.0000000000004290.
- Shimabukuro TT, Kim SY, Myers RT, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273-2282. doi: 10.1056/NEJMoa2104983.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471. doi: 10.1016/s0029-7844(97)00669-8.
- Omer SB. Maternal immunization. N Engl J Med. 2017;376:1256-1267. doi: 10.1056/NEJMra1509044.
- Dionne-Odom J, Tita AT, Silverman NS. Society for Maternal-Fetal Medicine Consult Series: #38: hepatitis B in pregnancy screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14. doi: http://dx.doi.org/10.1016/j.ajog.2015.09.100.
- Yawetz S. Immunizations during pregnancy. UpToDate, January 15, 2021.
- Cunningham Al, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016:375:1019-1032. doi: 10.1056/NEJMoa1603800.
- Albrecht MA, Levin MJ. Vaccination for the prevention of shingles (herpes zoster). UpToDate, July 6, 2020.
- ACOG Committee Opinion. Human papillomavirus vaccination. Obstet Gynecol. 2006;108:699-705. doi: 10.1097/00006250-200609000-00047.
- Callaghan WM, Creanga AA, Jamieson DJ. Pregnancy-related mortality resulting from influenza in the United States during the 2009-2010 pandemic. Obstet Gynecol. 2015;126:486-490. doi: 10.1097/AOG.0000000000000996.
- ACOG Committee Opinion. Influenza vaccination during pregnancy. Obstet Gynecol. 2014;124:648-651. doi: 10.1097/01.AOG.0000453599.11566.11.
- Scheller NM, Pasternak B, Molgaard-Nielsen D, et al. Quadrivalent HPV vaccination and the risk of adverse pregnancy outcomes. N Engl J Med. 2017;376:1223-1233. doi: 10.1056/NEJMoa1612296.
- Moumne O, Duff P. Treatment and prevention of pneumococcal infection. Clin Obstet Gynecol. 2019;62:781-789. doi: 10.1097/GRF.0000000000000451.
- ACOG Committee Opinion. Update on immunization and pregnancy: tetanus, diphtheria, and pertussis vaccination. Obstet Gynecol. 2017;130:668-669. doi: 10.1097/AOG.0000000000002293.
- Sukumaran L, McCarthy NL, Kharbanda EO, et al. Safety of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis and influenza vaccinations in pregnancy. Obstet Gynecol. 2015;126:1069-1074. doi: 10.1097/AOG.0000000000001066.
- Duff P. Varicella in pregnancy: five priorities for clinicians. Infect Dis Obstet Gynecol. 1994;1:163-165. doi: 10.1155/S1064744994000013.
- Duff P. Varicella vaccine. Infect Dis Obstet Gynecol. 1996;4:63-65. doi: 10.1155/S1064744996000142.
- Desmond A, Offit PA. On the shoulders of giants--from Jenner's cowpox to mRNA COVID vaccines. N Engl. J Med. 2021;384:1081-1083. doi: 10.1056/NEJMp2034334.
- Poland GA, Jacobson RM. The age-old struggle against the antivaccinationists. N Engl J Med. 2011;364:97-99. doi: 10.1056/NEJMp1010594.
- Morgan JL, Baggari SR, Chung W, et al. Association of a best-practice alert and prenatal administration with tetanus toxoid, reduced diptheria toxoid, and acellular pertussis vaccination rates. Obstet Gynecol. 2015;126:333-337. doi: 10.1097/AOG.0000000000000975.

CASE 1st prenatal appointment for young, pregnant migrant
A 21-year-old primigravid woman at 12 weeks’ gestation recently immigrated to the United States from an impoverished rural area of Southeast Asia. On the first prenatal appointment, she is noted to have no evidence of immunity to rubella, measles, or varicella. Her hepatitis B surface antigen and hepatitis C antibody tests are negative. She also has negative test results for gonorrhea, chlamydia, syphilis, and HIV infection. Her pap test is negative.
- What vaccinations should this patient receive during her pregnancy?
- What additional vaccinations are indicated postpartum?
Preventive vaccinations: What to know
As ObGyns, we serve as the primary care physician for many women throughout their early and middle decades of life. Accordingly, we have an obligation to be well informed about preventive health services such as vaccinations. The purpose of this article is to review the principal vaccines with which ObGyns should be familiar. I will discuss the vaccines in alphabetical order and then focus on the indications and timing for each vaccine and the relative cost of each immunization. Key points are summarized in the TABLE.

COVID-19 vaccine
In the latter part of 2020 and early part of 2021, three COVID-19 vaccines received emergency use authorization (EUA) from the US Food and Drug Administration (FDA) for individuals 16 years of age and older (Pfizer-BioNTech) and 18 years of age and older (Moderna and Johnson & Johnson).1 The cost of their administration is borne by the federal government. Two of the vaccines are mRNA agents—Moderna and Pfizer-BioNTech. Both are administered in a 2-dose series, separated by 4 and 3 weeks, respectively. The efficacy of these vaccines in preventing serious or critical illness approaches 95%. The Pfizer-BioNTech vaccine has now been fully FDA approved for administration to individuals older than age 16, with EUA for those down to age 12. Full approval of the Moderna vaccine will not be far behind. Because of some evidence suggesting waning immunity over time and because of growing concerns about the increased transmissibility of the delta variant of the virus, the FDA has been strongly considering a recommendation for a third (booster) dose of each of these vaccines, administered 8 months after the second dose for all eligible Americans. On September 17, 2021, the FDA advisory committee recommended a booster for the Pfizer-BioNTech vaccine for people older than age 65 and for those over the age of 16 at high risk for severe COVID-19. Several days later, full FDA approval was granted for this recommendation. Subsequently, the Director of the Centers for Disease Control and Prevention (CDC) included health care workers and pregnant women in the group for whom the booster is recommended.
The third vaccine formulation is the Johnson & Johnson DNA vaccine, which is prepared with a human adenovirus vector. This vaccine is administered in a single intramuscular dose and has a reported efficacy of 66% to 85%, though it may approach 95% in preventing critical illness. The FDA is expected to announce decisions about booster doses for the Johnson & Johnson and Moderna vaccines in the coming weeks.
Although initial trials of the COVID-19vaccines excluded pregnant and lactating women, the vaccines are safe in pregnancy or postpartum. In fact the vaccines do not contain either a killed or attenuated viral particle that is capable of transmitting infection. Therefore, both the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine now support routine immunization during pregnancy.
A recent report by Shimabukuro and colleagues2 demonstrated that the risk of vaccine-related complications in pregnant women receiving the Pfizer-BioNTech or Moderna vaccines was no different than in nonpregnant patients and that there was no evidence of teratogenic effects. The trial included more than 35,000 pregnant women; 2.3% were vaccinated in the periconception period, 28.6% in the first trimester, 43.3% in the second trimester, and 25.7% in the third trimester. Given this, and in light of isolated reports of unusual thromboembolic complications associated with the Johnson & Johnson vaccine, I strongly recommend use of either the Moderna or Pfizer-BioNTech vaccine in our prenatal and postpartum patients.
Continue to: Hepatitis A vaccine...
Hepatitis A vaccine
The hepatitis A vaccine is an inactivated vaccine and is safe for use in pregnancy. It is available in two monovalent preparations—Havrix (GlaxoSmithKline) and Vaqta (Merck & Co.) and is administered in a 2-dose intramuscular injection at time zero and 6 to 12 months later.3 The vaccine is also available in a bivalent form with recombinant hepatitis B vaccine—Twinrix (GlaxoSmithKline). When administered in this form, the vaccine should be given at time zero, 1 month, and 6 months. The wholesale cost of the monovalent vaccine is $66 to $119, depending upon whether the provider uses a multi-dose or a single-dose vial. The cost of Twinrix is $149.
The hepatitis A vaccine is indicated for select pregnant and nonpregnant patients:
- international travelers
- intravenous drug users
- those with occupational exposure (eg, individuals who work in a primate laboratory)
- residents and staff in chronic care facilities
- individuals with chronic liver disease
- individuals with clotting factor disorders
- residents in endemic areas.
Hepatitis B vaccine
The hepatitis B vaccine is a recombinant vaccine that contains an inactivated portion of the hepatitis B surface antigen. It was originally produced in two monovalent formulations: Engerix B (GlaxoSmithKline) and Recombivax-HB (Merck & Co.). These original formulations are given in a 3-dose series at time zero, 1 month, and 6 months. Recently, a new and more potent formulation was introduced into clinical practice. Heplisav-B (Dynavax Technologies Co.) is also a recombinant vaccine that contains a boosting adjuvant. It is programed to be administered in a 2-dose series at time zero and 1 month.4-6
The wholesale cost of the monovalent vaccines varies from $60 to $173, depending upon use of a multi-dose vial versus a single-use vial. The cost of Heplisav-B varies from $146 to $173, depending upon use of a prefilled syringe versus a single-dose vial.
Although the hepatitis B vaccine should be part of the childhood immunization series, it also should be administered to any pregnant woman who has not been vaccinated previously or who does not already have evidence of immunity as a result of natural infection.
Continue to: Herpes zoster vaccine...
Herpes zoster vaccine
Herpes zoster infection (shingles) can be a particularly disabling condition in older patients and results from reactivation of a latent varicella-zoster infection. Shingles can cause extremely painful skin lesions, threaten the patient’s vision, and result in long-lasting postherpetic neuralgia. Both cellular and hormonal immunity are essential to protect against recurrent infection.
The original herpes zoster vaccine (Zoster Vaccine Live; ZVL, Zostavax) is no longer produced in the United States because it is not as effective as the newer vaccine—Recombinant Zoster Vaccine (Shingrix, GlaxoSmithKline).7,8 The antigen in the new vaccine is a component of the surface glycoprotein E, and it is combined with an adjuvant to enhance immunoreactivity. The vaccine is given intramuscularly in two doses at time zero and again at 2 to 6 months and is indicated for all individuals >50 years, including those who may have had an episode of shingles. This newer vaccine is 97% effective in patients >50 years and 90% effective in patients >70. The wholesale cost of each injection is about $160.
Human papillomavirus vaccine
The HPV vaccine (Gardasil-9, Merck & Co.) is a recombinant 9-valent vaccine directed against the human papillomavirus. It induces immunity to serotypes 6 and 11 (which cause 90% of genital warts), 16 and 18 (which cause 80% of genital cancers), and 31, 33, 45, 52, and 58 (viral strains that are responsible for both genital and oropharyngeal cancers). The vaccine is administered intramuscularly in a 3-dose series at time zero, 1-2 months, and 6 months. The principal target groups for the vaccine are males and females, ages 9 to 45 years. Ideally, children of both sexes should receive this vaccine prior to the onset of sexual activity. The wholesale cost of each vaccine injection is approximately $222.9
Influenza vaccine
The inactivated, intramuscular flu vaccine is recommended for anyone over age 2, including pregnant women. Although pregnant women are not more likely to acquire flu compared with those who are not pregnant, if they do become infected, they are likely to become more seriously ill, with higher mortality. Accordingly, all pregnant women should receive, in any trimester, the inactivated flu vaccine beginning in the late summer and early fall of each year and extending through March of the next year.10,11
Multiple formulations of the inactivated vaccine are marketed, all targeting two strains of influenza A and two strains of influenza B. The components of the vaccine vary each year as scientists try to match the new vaccine with the most highly prevalent strains in the previous flu season. The vaccine should be administered in a single intramuscular dose. The cost varies from approximately $20 to $70.
The intranasal influenza vaccine is a live virus vaccine that is intended primarily for children and should not be administered in pregnancy. In addition, there is a higher dose of the inactivated quadrivalent vaccine that is available for administration to patients over age 65. This higher dose is more likely to cause adverse effects and is not indicated in pregnancy.
Continue to: Measles, mumps, rubella vaccine (MMR)...
Measles, mumps, rubella vaccine (MMR)
The MMR is a standard component of the childhood vaccination series. The trivalent preparation is a live, attenuated vaccine that is typically given subcutaneously in a 2-dose series. The first dose is administered at age 12-15 months, and the second dose at age 4-6 years. The vaccine is highly immunogenic, with vaccine-induced immunity usually life-long. In some patients, however, immunity wanes over time. Accordingly, all pregnant women should be screened for immunity to rubella since, of the 3, this infection poses the greatest risk to the fetus. Women who do not have evidence of immunity should be advised to avoid contact with children who may have a viral exanthem. They should then receive a booster dose of the vaccine immediately postpartum and should practice secure contraception for 1 month. The vaccine cost is approximately $60.
Pneumococcal vaccine
The inactivated pneumococcal vaccine is produced in two forms, both of which are safe for administration in pregnancy.12 The original vaccine, introduced in 1983, was PPSV23 (Pneumovax 23, Merck & Co), a 23-serovalent vaccine that was intended primarily for adults. This vaccine is administered in a single subcutaneous or intramuscular dose. The newest vaccine, introduced in 2010, is PCV13 (Prevnar 13, Pfizer Inc), a 13-serovalent vaccine. It was intended primarily for children, in whom it is administered in a 4-dose series beginning at 6 to 8 weeks of age. The cost of the former is approximately $98 to $120; the cost of the latter is $228.
Vaccination against pneumococcal infection is routinely indicated for those older than the age of 65 and for the following at-risk patients, including those who are pregnant11:
- individuals who have had a splenectomy or who have a medical illness that produces functional asplenia (eg, sickle cell anemia)
- individuals with chronic cardiac, pulmonic, hepatic, or renal disease
- individuals with immunosuppressive conditions such as HIV infection or a disseminated malignancy
- individuals who have a cochlear implant
- individuals who have a chronic leak of cerebrospinal fluid.
The recommendations for timing of these 2 vaccines in adults can initially appear confusing. Put most simply, if a high-risk patient first receives the PCV13 vaccine, she should receive the PPSV23 vaccine in about 8 weeks. The PPSV23 vaccine should be repeated in 5 years. If an at-risk patient initially receives the PPSV23 vaccine, the PCV13 vaccine should be given 1 year later.12
Tdap vaccine
The Tdap vaccine contains tetanus toxoid, reduced diptheria toxoid, and an acellular component of the pertussis bacterium. Although it has long been part of the childhood vaccinations series, immunity to each component, particularly pertussis, tends to wane over time.
Pertussis poses a serious risk to the health of the pregnant woman and the newborn infant. Accordingly, the Advisory Committee on Immunization Practices (ACIP), CDC, and the ACOG now advise administration of a booster dose of this vaccine in the early third trimester of each pregnancy.13-15 The vaccine should be administered as a single intramuscular injection. The approximate cost of the vaccine is $64 to $71, depending upon whether the provider uses a single-dose vial or a single-dose prefilled syringe. In nonpregnant patients, the ACIP currently recommends administration of a booster dose of the vaccine every 10 years, primarily to provide durable protection against tetanus.
Continue to: Varicella vaccine...
Varicella vaccine
The varicella vaccine is also one of the main components of the childhood immunization series. This live virus vaccine can be administered subcutaneously as a monovalent agent or as a quadrivalent agent in association with the MMR vaccine.
Pregnant women who do not have a well-documented history of natural infection should be tested for IgG antibody to the varicella-zoster virus at the time of their first prenatal appointment. Interestingly, approximately 70% of patients with an uncertain history actually have immunity when tested. If the patient lacks immunity, she should be vaccinated immediately postpartum.16,17 The vaccine should be administered in a 2-dose series at time zero and then 4 to 8 weeks later. Patients should adhere to secure contraception from the time of the first dose until 1 month after the second dose. The cost of each dose of the vaccine is approximately $145.
Adverse effects of vaccination
All vaccines have many of the same side effects. The most common is simply a reaction at the site of injection, characterized by pain, increased warmth, erythema, swelling, and tenderness. Other common side effects include systemic manifestations, such as low-grade fever, nausea and vomiting, malaise, fatigue, headache, lymphadenopathy, myalgias, and arthralgias. Some vaccines, notably varicella, herpes zoster, measles, and rubella may cause a disseminated rash. Most of these minor side effects are easily managed by rest, hydration, and administration of an analgesic such as acetaminophen or ibuprofen. More serious side effects include rare complications such as anaphylaxis, Bell palsy, Guillain-Barre syndrome, and venous thromboembolism (Johnson & Johnson COVID-19 vaccine). Any of the vaccines discussed above should not be given, or given only with extreme caution, to an individual who has experienced any of these reactions with a previous vaccine.
Barriers to vaccination
Although the vaccines reviewed above are highly effective in preventing serious illness in recipients, the medical profession’s “report card” in ensuring adherence with vaccine protocols is not optimal. In fact, it probably merits a grade no higher than C+, with vaccination rates in the range of 50% to 70%.
One of the major barriers to vaccination is lack of detailed information about vaccine efficacy and safety on the part of both provider and patient. Another is the problem of misinformation (eg, the persistent belief on the part of some individuals that vaccines may cause a serious problem, such as autism).18,19 Another important barrier to widespread vaccination is the logistical problem associated with proper scheduling of multidose regimens (such as those for hepatitis A and B, varicella, and COVID-19). A final barrier, and in my own university-based practice, the most important obstacle is the expense of vaccination. Most, but not all, private insurance companies provide coverage for vaccines approved by the Centers for Disease Control and Prevention and the US Preventive Services Task Force. However, public insurance agencies often provide disappointingly inconsistent coverage for essential vaccines.
By keeping well informed about the most recent public health recommendations for vaccinations for adults and by leading important initiatives within our own practices, we should be able to overcome the first 3 barriers listed above. For example, Morgan and colleagues20 recently achieved a 97% success rate with Tdap administration in pregnancy by placing a best-practice alert in the patients’ electronic medical records. Surmounting the final barrier will require intense effort on the part of individual practitioners and professional organizations to advocate for coverage for essential vaccinations for our patients.
CASE Resolved
This patient was raised in an area of the world where her family did not have easy access to medical care. Accordingly, she did not receive the usual childhood vaccines, such as measles, mumps, rubella, varicella, hepatitis B, and almost certainly, tetanus, diphtheria, and pertussis (Tdap), and the HPV vaccine. The MMR vaccine and the varicella vaccine are live virus vaccines and should not be given during pregnancy. However, these vaccines should be administered postpartum, and the patient should be instructed to practice secure contraception for a minimum of 1 month following vaccination. She also should be offered the HPV vaccine postpartum. During pregnancy, she definitely should receive the COVID-19 vaccine, the 3-dose hepatitis B vaccine series, the influenza vaccine, and Tdap. If her present living conditions place her at risk for hepatitis A, she also should be vaccinated against this illness. ●

CASE 1st prenatal appointment for young, pregnant migrant
A 21-year-old primigravid woman at 12 weeks’ gestation recently immigrated to the United States from an impoverished rural area of Southeast Asia. On the first prenatal appointment, she is noted to have no evidence of immunity to rubella, measles, or varicella. Her hepatitis B surface antigen and hepatitis C antibody tests are negative. She also has negative test results for gonorrhea, chlamydia, syphilis, and HIV infection. Her pap test is negative.
- What vaccinations should this patient receive during her pregnancy?
- What additional vaccinations are indicated postpartum?
Preventive vaccinations: What to know
As ObGyns, we serve as the primary care physician for many women throughout their early and middle decades of life. Accordingly, we have an obligation to be well informed about preventive health services such as vaccinations. The purpose of this article is to review the principal vaccines with which ObGyns should be familiar. I will discuss the vaccines in alphabetical order and then focus on the indications and timing for each vaccine and the relative cost of each immunization. Key points are summarized in the TABLE.

COVID-19 vaccine
In the latter part of 2020 and early part of 2021, three COVID-19 vaccines received emergency use authorization (EUA) from the US Food and Drug Administration (FDA) for individuals 16 years of age and older (Pfizer-BioNTech) and 18 years of age and older (Moderna and Johnson & Johnson).1 The cost of their administration is borne by the federal government. Two of the vaccines are mRNA agents—Moderna and Pfizer-BioNTech. Both are administered in a 2-dose series, separated by 4 and 3 weeks, respectively. The efficacy of these vaccines in preventing serious or critical illness approaches 95%. The Pfizer-BioNTech vaccine has now been fully FDA approved for administration to individuals older than age 16, with EUA for those down to age 12. Full approval of the Moderna vaccine will not be far behind. Because of some evidence suggesting waning immunity over time and because of growing concerns about the increased transmissibility of the delta variant of the virus, the FDA has been strongly considering a recommendation for a third (booster) dose of each of these vaccines, administered 8 months after the second dose for all eligible Americans. On September 17, 2021, the FDA advisory committee recommended a booster for the Pfizer-BioNTech vaccine for people older than age 65 and for those over the age of 16 at high risk for severe COVID-19. Several days later, full FDA approval was granted for this recommendation. Subsequently, the Director of the Centers for Disease Control and Prevention (CDC) included health care workers and pregnant women in the group for whom the booster is recommended.
The third vaccine formulation is the Johnson & Johnson DNA vaccine, which is prepared with a human adenovirus vector. This vaccine is administered in a single intramuscular dose and has a reported efficacy of 66% to 85%, though it may approach 95% in preventing critical illness. The FDA is expected to announce decisions about booster doses for the Johnson & Johnson and Moderna vaccines in the coming weeks.
Although initial trials of the COVID-19vaccines excluded pregnant and lactating women, the vaccines are safe in pregnancy or postpartum. In fact the vaccines do not contain either a killed or attenuated viral particle that is capable of transmitting infection. Therefore, both the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine now support routine immunization during pregnancy.
A recent report by Shimabukuro and colleagues2 demonstrated that the risk of vaccine-related complications in pregnant women receiving the Pfizer-BioNTech or Moderna vaccines was no different than in nonpregnant patients and that there was no evidence of teratogenic effects. The trial included more than 35,000 pregnant women; 2.3% were vaccinated in the periconception period, 28.6% in the first trimester, 43.3% in the second trimester, and 25.7% in the third trimester. Given this, and in light of isolated reports of unusual thromboembolic complications associated with the Johnson & Johnson vaccine, I strongly recommend use of either the Moderna or Pfizer-BioNTech vaccine in our prenatal and postpartum patients.
Continue to: Hepatitis A vaccine...
Hepatitis A vaccine
The hepatitis A vaccine is an inactivated vaccine and is safe for use in pregnancy. It is available in two monovalent preparations—Havrix (GlaxoSmithKline) and Vaqta (Merck & Co.) and is administered in a 2-dose intramuscular injection at time zero and 6 to 12 months later.3 The vaccine is also available in a bivalent form with recombinant hepatitis B vaccine—Twinrix (GlaxoSmithKline). When administered in this form, the vaccine should be given at time zero, 1 month, and 6 months. The wholesale cost of the monovalent vaccine is $66 to $119, depending upon whether the provider uses a multi-dose or a single-dose vial. The cost of Twinrix is $149.
The hepatitis A vaccine is indicated for select pregnant and nonpregnant patients:
- international travelers
- intravenous drug users
- those with occupational exposure (eg, individuals who work in a primate laboratory)
- residents and staff in chronic care facilities
- individuals with chronic liver disease
- individuals with clotting factor disorders
- residents in endemic areas.
Hepatitis B vaccine
The hepatitis B vaccine is a recombinant vaccine that contains an inactivated portion of the hepatitis B surface antigen. It was originally produced in two monovalent formulations: Engerix B (GlaxoSmithKline) and Recombivax-HB (Merck & Co.). These original formulations are given in a 3-dose series at time zero, 1 month, and 6 months. Recently, a new and more potent formulation was introduced into clinical practice. Heplisav-B (Dynavax Technologies Co.) is also a recombinant vaccine that contains a boosting adjuvant. It is programed to be administered in a 2-dose series at time zero and 1 month.4-6
The wholesale cost of the monovalent vaccines varies from $60 to $173, depending upon use of a multi-dose vial versus a single-use vial. The cost of Heplisav-B varies from $146 to $173, depending upon use of a prefilled syringe versus a single-dose vial.
Although the hepatitis B vaccine should be part of the childhood immunization series, it also should be administered to any pregnant woman who has not been vaccinated previously or who does not already have evidence of immunity as a result of natural infection.
Continue to: Herpes zoster vaccine...
Herpes zoster vaccine
Herpes zoster infection (shingles) can be a particularly disabling condition in older patients and results from reactivation of a latent varicella-zoster infection. Shingles can cause extremely painful skin lesions, threaten the patient’s vision, and result in long-lasting postherpetic neuralgia. Both cellular and hormonal immunity are essential to protect against recurrent infection.
The original herpes zoster vaccine (Zoster Vaccine Live; ZVL, Zostavax) is no longer produced in the United States because it is not as effective as the newer vaccine—Recombinant Zoster Vaccine (Shingrix, GlaxoSmithKline).7,8 The antigen in the new vaccine is a component of the surface glycoprotein E, and it is combined with an adjuvant to enhance immunoreactivity. The vaccine is given intramuscularly in two doses at time zero and again at 2 to 6 months and is indicated for all individuals >50 years, including those who may have had an episode of shingles. This newer vaccine is 97% effective in patients >50 years and 90% effective in patients >70. The wholesale cost of each injection is about $160.
Human papillomavirus vaccine
The HPV vaccine (Gardasil-9, Merck & Co.) is a recombinant 9-valent vaccine directed against the human papillomavirus. It induces immunity to serotypes 6 and 11 (which cause 90% of genital warts), 16 and 18 (which cause 80% of genital cancers), and 31, 33, 45, 52, and 58 (viral strains that are responsible for both genital and oropharyngeal cancers). The vaccine is administered intramuscularly in a 3-dose series at time zero, 1-2 months, and 6 months. The principal target groups for the vaccine are males and females, ages 9 to 45 years. Ideally, children of both sexes should receive this vaccine prior to the onset of sexual activity. The wholesale cost of each vaccine injection is approximately $222.9
Influenza vaccine
The inactivated, intramuscular flu vaccine is recommended for anyone over age 2, including pregnant women. Although pregnant women are not more likely to acquire flu compared with those who are not pregnant, if they do become infected, they are likely to become more seriously ill, with higher mortality. Accordingly, all pregnant women should receive, in any trimester, the inactivated flu vaccine beginning in the late summer and early fall of each year and extending through March of the next year.10,11
Multiple formulations of the inactivated vaccine are marketed, all targeting two strains of influenza A and two strains of influenza B. The components of the vaccine vary each year as scientists try to match the new vaccine with the most highly prevalent strains in the previous flu season. The vaccine should be administered in a single intramuscular dose. The cost varies from approximately $20 to $70.
The intranasal influenza vaccine is a live virus vaccine that is intended primarily for children and should not be administered in pregnancy. In addition, there is a higher dose of the inactivated quadrivalent vaccine that is available for administration to patients over age 65. This higher dose is more likely to cause adverse effects and is not indicated in pregnancy.
Continue to: Measles, mumps, rubella vaccine (MMR)...
Measles, mumps, rubella vaccine (MMR)
The MMR is a standard component of the childhood vaccination series. The trivalent preparation is a live, attenuated vaccine that is typically given subcutaneously in a 2-dose series. The first dose is administered at age 12-15 months, and the second dose at age 4-6 years. The vaccine is highly immunogenic, with vaccine-induced immunity usually life-long. In some patients, however, immunity wanes over time. Accordingly, all pregnant women should be screened for immunity to rubella since, of the 3, this infection poses the greatest risk to the fetus. Women who do not have evidence of immunity should be advised to avoid contact with children who may have a viral exanthem. They should then receive a booster dose of the vaccine immediately postpartum and should practice secure contraception for 1 month. The vaccine cost is approximately $60.
Pneumococcal vaccine
The inactivated pneumococcal vaccine is produced in two forms, both of which are safe for administration in pregnancy.12 The original vaccine, introduced in 1983, was PPSV23 (Pneumovax 23, Merck & Co), a 23-serovalent vaccine that was intended primarily for adults. This vaccine is administered in a single subcutaneous or intramuscular dose. The newest vaccine, introduced in 2010, is PCV13 (Prevnar 13, Pfizer Inc), a 13-serovalent vaccine. It was intended primarily for children, in whom it is administered in a 4-dose series beginning at 6 to 8 weeks of age. The cost of the former is approximately $98 to $120; the cost of the latter is $228.
Vaccination against pneumococcal infection is routinely indicated for those older than the age of 65 and for the following at-risk patients, including those who are pregnant11:
- individuals who have had a splenectomy or who have a medical illness that produces functional asplenia (eg, sickle cell anemia)
- individuals with chronic cardiac, pulmonic, hepatic, or renal disease
- individuals with immunosuppressive conditions such as HIV infection or a disseminated malignancy
- individuals who have a cochlear implant
- individuals who have a chronic leak of cerebrospinal fluid.
The recommendations for timing of these 2 vaccines in adults can initially appear confusing. Put most simply, if a high-risk patient first receives the PCV13 vaccine, she should receive the PPSV23 vaccine in about 8 weeks. The PPSV23 vaccine should be repeated in 5 years. If an at-risk patient initially receives the PPSV23 vaccine, the PCV13 vaccine should be given 1 year later.12
Tdap vaccine
The Tdap vaccine contains tetanus toxoid, reduced diptheria toxoid, and an acellular component of the pertussis bacterium. Although it has long been part of the childhood vaccinations series, immunity to each component, particularly pertussis, tends to wane over time.
Pertussis poses a serious risk to the health of the pregnant woman and the newborn infant. Accordingly, the Advisory Committee on Immunization Practices (ACIP), CDC, and the ACOG now advise administration of a booster dose of this vaccine in the early third trimester of each pregnancy.13-15 The vaccine should be administered as a single intramuscular injection. The approximate cost of the vaccine is $64 to $71, depending upon whether the provider uses a single-dose vial or a single-dose prefilled syringe. In nonpregnant patients, the ACIP currently recommends administration of a booster dose of the vaccine every 10 years, primarily to provide durable protection against tetanus.
Continue to: Varicella vaccine...
Varicella vaccine
The varicella vaccine is also one of the main components of the childhood immunization series. This live virus vaccine can be administered subcutaneously as a monovalent agent or as a quadrivalent agent in association with the MMR vaccine.
Pregnant women who do not have a well-documented history of natural infection should be tested for IgG antibody to the varicella-zoster virus at the time of their first prenatal appointment. Interestingly, approximately 70% of patients with an uncertain history actually have immunity when tested. If the patient lacks immunity, she should be vaccinated immediately postpartum.16,17 The vaccine should be administered in a 2-dose series at time zero and then 4 to 8 weeks later. Patients should adhere to secure contraception from the time of the first dose until 1 month after the second dose. The cost of each dose of the vaccine is approximately $145.
Adverse effects of vaccination
All vaccines have many of the same side effects. The most common is simply a reaction at the site of injection, characterized by pain, increased warmth, erythema, swelling, and tenderness. Other common side effects include systemic manifestations, such as low-grade fever, nausea and vomiting, malaise, fatigue, headache, lymphadenopathy, myalgias, and arthralgias. Some vaccines, notably varicella, herpes zoster, measles, and rubella may cause a disseminated rash. Most of these minor side effects are easily managed by rest, hydration, and administration of an analgesic such as acetaminophen or ibuprofen. More serious side effects include rare complications such as anaphylaxis, Bell palsy, Guillain-Barre syndrome, and venous thromboembolism (Johnson & Johnson COVID-19 vaccine). Any of the vaccines discussed above should not be given, or given only with extreme caution, to an individual who has experienced any of these reactions with a previous vaccine.
Barriers to vaccination
Although the vaccines reviewed above are highly effective in preventing serious illness in recipients, the medical profession’s “report card” in ensuring adherence with vaccine protocols is not optimal. In fact, it probably merits a grade no higher than C+, with vaccination rates in the range of 50% to 70%.
One of the major barriers to vaccination is lack of detailed information about vaccine efficacy and safety on the part of both provider and patient. Another is the problem of misinformation (eg, the persistent belief on the part of some individuals that vaccines may cause a serious problem, such as autism).18,19 Another important barrier to widespread vaccination is the logistical problem associated with proper scheduling of multidose regimens (such as those for hepatitis A and B, varicella, and COVID-19). A final barrier, and in my own university-based practice, the most important obstacle is the expense of vaccination. Most, but not all, private insurance companies provide coverage for vaccines approved by the Centers for Disease Control and Prevention and the US Preventive Services Task Force. However, public insurance agencies often provide disappointingly inconsistent coverage for essential vaccines.
By keeping well informed about the most recent public health recommendations for vaccinations for adults and by leading important initiatives within our own practices, we should be able to overcome the first 3 barriers listed above. For example, Morgan and colleagues20 recently achieved a 97% success rate with Tdap administration in pregnancy by placing a best-practice alert in the patients’ electronic medical records. Surmounting the final barrier will require intense effort on the part of individual practitioners and professional organizations to advocate for coverage for essential vaccinations for our patients.
CASE Resolved
This patient was raised in an area of the world where her family did not have easy access to medical care. Accordingly, she did not receive the usual childhood vaccines, such as measles, mumps, rubella, varicella, hepatitis B, and almost certainly, tetanus, diphtheria, and pertussis (Tdap), and the HPV vaccine. The MMR vaccine and the varicella vaccine are live virus vaccines and should not be given during pregnancy. However, these vaccines should be administered postpartum, and the patient should be instructed to practice secure contraception for a minimum of 1 month following vaccination. She also should be offered the HPV vaccine postpartum. During pregnancy, she definitely should receive the COVID-19 vaccine, the 3-dose hepatitis B vaccine series, the influenza vaccine, and Tdap. If her present living conditions place her at risk for hepatitis A, she also should be vaccinated against this illness. ●
- Rasmussen SA, Kelley CF, Horton JP, et al. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy. What obstetricians need to know. Obstet Gynecol. 2021;137:408-414. doi: 10.1097/AOG.0000000000004290.
- Shimabukuro TT, Kim SY, Myers RT, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273-2282. doi: 10.1056/NEJMoa2104983.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471. doi: 10.1016/s0029-7844(97)00669-8.
- Omer SB. Maternal immunization. N Engl J Med. 2017;376:1256-1267. doi: 10.1056/NEJMra1509044.
- Dionne-Odom J, Tita AT, Silverman NS. Society for Maternal-Fetal Medicine Consult Series: #38: hepatitis B in pregnancy screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14. doi: http://dx.doi.org/10.1016/j.ajog.2015.09.100.
- Yawetz S. Immunizations during pregnancy. UpToDate, January 15, 2021.
- Cunningham Al, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016:375:1019-1032. doi: 10.1056/NEJMoa1603800.
- Albrecht MA, Levin MJ. Vaccination for the prevention of shingles (herpes zoster). UpToDate, July 6, 2020.
- ACOG Committee Opinion. Human papillomavirus vaccination. Obstet Gynecol. 2006;108:699-705. doi: 10.1097/00006250-200609000-00047.
- Callaghan WM, Creanga AA, Jamieson DJ. Pregnancy-related mortality resulting from influenza in the United States during the 2009-2010 pandemic. Obstet Gynecol. 2015;126:486-490. doi: 10.1097/AOG.0000000000000996.
- ACOG Committee Opinion. Influenza vaccination during pregnancy. Obstet Gynecol. 2014;124:648-651. doi: 10.1097/01.AOG.0000453599.11566.11.
- Scheller NM, Pasternak B, Molgaard-Nielsen D, et al. Quadrivalent HPV vaccination and the risk of adverse pregnancy outcomes. N Engl J Med. 2017;376:1223-1233. doi: 10.1056/NEJMoa1612296.
- Moumne O, Duff P. Treatment and prevention of pneumococcal infection. Clin Obstet Gynecol. 2019;62:781-789. doi: 10.1097/GRF.0000000000000451.
- ACOG Committee Opinion. Update on immunization and pregnancy: tetanus, diphtheria, and pertussis vaccination. Obstet Gynecol. 2017;130:668-669. doi: 10.1097/AOG.0000000000002293.
- Sukumaran L, McCarthy NL, Kharbanda EO, et al. Safety of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis and influenza vaccinations in pregnancy. Obstet Gynecol. 2015;126:1069-1074. doi: 10.1097/AOG.0000000000001066.
- Duff P. Varicella in pregnancy: five priorities for clinicians. Infect Dis Obstet Gynecol. 1994;1:163-165. doi: 10.1155/S1064744994000013.
- Duff P. Varicella vaccine. Infect Dis Obstet Gynecol. 1996;4:63-65. doi: 10.1155/S1064744996000142.
- Desmond A, Offit PA. On the shoulders of giants--from Jenner's cowpox to mRNA COVID vaccines. N Engl. J Med. 2021;384:1081-1083. doi: 10.1056/NEJMp2034334.
- Poland GA, Jacobson RM. The age-old struggle against the antivaccinationists. N Engl J Med. 2011;364:97-99. doi: 10.1056/NEJMp1010594.
- Morgan JL, Baggari SR, Chung W, et al. Association of a best-practice alert and prenatal administration with tetanus toxoid, reduced diptheria toxoid, and acellular pertussis vaccination rates. Obstet Gynecol. 2015;126:333-337. doi: 10.1097/AOG.0000000000000975.
- Rasmussen SA, Kelley CF, Horton JP, et al. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy. What obstetricians need to know. Obstet Gynecol. 2021;137:408-414. doi: 10.1097/AOG.0000000000004290.
- Shimabukuro TT, Kim SY, Myers RT, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273-2282. doi: 10.1056/NEJMoa2104983.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471. doi: 10.1016/s0029-7844(97)00669-8.
- Omer SB. Maternal immunization. N Engl J Med. 2017;376:1256-1267. doi: 10.1056/NEJMra1509044.
- Dionne-Odom J, Tita AT, Silverman NS. Society for Maternal-Fetal Medicine Consult Series: #38: hepatitis B in pregnancy screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14. doi: http://dx.doi.org/10.1016/j.ajog.2015.09.100.
- Yawetz S. Immunizations during pregnancy. UpToDate, January 15, 2021.
- Cunningham Al, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016:375:1019-1032. doi: 10.1056/NEJMoa1603800.
- Albrecht MA, Levin MJ. Vaccination for the prevention of shingles (herpes zoster). UpToDate, July 6, 2020.
- ACOG Committee Opinion. Human papillomavirus vaccination. Obstet Gynecol. 2006;108:699-705. doi: 10.1097/00006250-200609000-00047.
- Callaghan WM, Creanga AA, Jamieson DJ. Pregnancy-related mortality resulting from influenza in the United States during the 2009-2010 pandemic. Obstet Gynecol. 2015;126:486-490. doi: 10.1097/AOG.0000000000000996.
- ACOG Committee Opinion. Influenza vaccination during pregnancy. Obstet Gynecol. 2014;124:648-651. doi: 10.1097/01.AOG.0000453599.11566.11.
- Scheller NM, Pasternak B, Molgaard-Nielsen D, et al. Quadrivalent HPV vaccination and the risk of adverse pregnancy outcomes. N Engl J Med. 2017;376:1223-1233. doi: 10.1056/NEJMoa1612296.
- Moumne O, Duff P. Treatment and prevention of pneumococcal infection. Clin Obstet Gynecol. 2019;62:781-789. doi: 10.1097/GRF.0000000000000451.
- ACOG Committee Opinion. Update on immunization and pregnancy: tetanus, diphtheria, and pertussis vaccination. Obstet Gynecol. 2017;130:668-669. doi: 10.1097/AOG.0000000000002293.
- Sukumaran L, McCarthy NL, Kharbanda EO, et al. Safety of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis and influenza vaccinations in pregnancy. Obstet Gynecol. 2015;126:1069-1074. doi: 10.1097/AOG.0000000000001066.
- Duff P. Varicella in pregnancy: five priorities for clinicians. Infect Dis Obstet Gynecol. 1994;1:163-165. doi: 10.1155/S1064744994000013.
- Duff P. Varicella vaccine. Infect Dis Obstet Gynecol. 1996;4:63-65. doi: 10.1155/S1064744996000142.
- Desmond A, Offit PA. On the shoulders of giants--from Jenner's cowpox to mRNA COVID vaccines. N Engl. J Med. 2021;384:1081-1083. doi: 10.1056/NEJMp2034334.
- Poland GA, Jacobson RM. The age-old struggle against the antivaccinationists. N Engl J Med. 2011;364:97-99. doi: 10.1056/NEJMp1010594.
- Morgan JL, Baggari SR, Chung W, et al. Association of a best-practice alert and prenatal administration with tetanus toxoid, reduced diptheria toxoid, and acellular pertussis vaccination rates. Obstet Gynecol. 2015;126:333-337. doi: 10.1097/AOG.0000000000000975.
New York’s largest health care provider fires 1,400 unvaccinated employees
The employees represented less than 2% of Northwell’s 76,000 employees, who are now all fully vaccinated against COVID-19, Joe Kemp, the assistant vice president of public relations for the company, told The Hill.
“Northwell Health is proud to announce that our workforce -- the largest in New York State -- is 100% vaccinated,” the company said in a statement to several news outlets.
“This allows us to continue to provide exceptional care at all of our facilities, without interruption and remain open and fully operational,” Northwell Health said.
Having a fully vaccinated workforce is part of the health system’s duty to protect others, the company said. Northwell Health includes 23 hospitals and more than 830 outpatient facilities, according to ABC News.
“Northwell regrets losing any employee under such circumstances,” the company said. “We owe it to our staff, our patients, and the communities we serve to be 100% vaccinated against COVID-19.”
Former New York Gov. Andrew Cuomo announced in August that the state would require health care workers to receive at least one COVID-19 vaccine shot by Sept. 27. Employees didn’t have the option for weekly testing or religious exemptions, which is being challenged in several lawsuits, according to The New York Times.
The order went into effect last week, prompting tens of thousands of employees to get vaccinated. As of last week, 87% of hospital staff were fully vaccinated, and 92% of hospital and retirement home workers had received at least one dose, according to state health data.
Northwell announced its own vaccine mandate in August as well, which sparked protests among some workers. The order applied to both clinical and non-clinical staff.
A few thousand Northwell employees got vaccinated as the deadline approached, Mr. Kemp told The New York Times. Some who lost their jobs at first were able to return to work, and those who have been terminated can interview for reinstatement for 30 days. The hospital system is also “openly recruiting” for the vacant positions.
“The goal was to get people vaccinated, not to get people terminated,” Mr. Kemp said.
Hospitalized COVID-19 patients in New York hit a low of 350 in mid-July, according to state hospitalization data. Now, about 2,200 people are hospitalized throughout the state, most of whom are unvaccinated.
As of Oct. 3, nearly 72% of New York residents had received at least one vaccine dose, according to the latest state data. About 64% are fully vaccinated.
A version of this article first appeared on WebMD.com.
The employees represented less than 2% of Northwell’s 76,000 employees, who are now all fully vaccinated against COVID-19, Joe Kemp, the assistant vice president of public relations for the company, told The Hill.
“Northwell Health is proud to announce that our workforce -- the largest in New York State -- is 100% vaccinated,” the company said in a statement to several news outlets.
“This allows us to continue to provide exceptional care at all of our facilities, without interruption and remain open and fully operational,” Northwell Health said.
Having a fully vaccinated workforce is part of the health system’s duty to protect others, the company said. Northwell Health includes 23 hospitals and more than 830 outpatient facilities, according to ABC News.
“Northwell regrets losing any employee under such circumstances,” the company said. “We owe it to our staff, our patients, and the communities we serve to be 100% vaccinated against COVID-19.”
Former New York Gov. Andrew Cuomo announced in August that the state would require health care workers to receive at least one COVID-19 vaccine shot by Sept. 27. Employees didn’t have the option for weekly testing or religious exemptions, which is being challenged in several lawsuits, according to The New York Times.
The order went into effect last week, prompting tens of thousands of employees to get vaccinated. As of last week, 87% of hospital staff were fully vaccinated, and 92% of hospital and retirement home workers had received at least one dose, according to state health data.
Northwell announced its own vaccine mandate in August as well, which sparked protests among some workers. The order applied to both clinical and non-clinical staff.
A few thousand Northwell employees got vaccinated as the deadline approached, Mr. Kemp told The New York Times. Some who lost their jobs at first were able to return to work, and those who have been terminated can interview for reinstatement for 30 days. The hospital system is also “openly recruiting” for the vacant positions.
“The goal was to get people vaccinated, not to get people terminated,” Mr. Kemp said.
Hospitalized COVID-19 patients in New York hit a low of 350 in mid-July, according to state hospitalization data. Now, about 2,200 people are hospitalized throughout the state, most of whom are unvaccinated.
As of Oct. 3, nearly 72% of New York residents had received at least one vaccine dose, according to the latest state data. About 64% are fully vaccinated.
A version of this article first appeared on WebMD.com.
The employees represented less than 2% of Northwell’s 76,000 employees, who are now all fully vaccinated against COVID-19, Joe Kemp, the assistant vice president of public relations for the company, told The Hill.
“Northwell Health is proud to announce that our workforce -- the largest in New York State -- is 100% vaccinated,” the company said in a statement to several news outlets.
“This allows us to continue to provide exceptional care at all of our facilities, without interruption and remain open and fully operational,” Northwell Health said.
Having a fully vaccinated workforce is part of the health system’s duty to protect others, the company said. Northwell Health includes 23 hospitals and more than 830 outpatient facilities, according to ABC News.
“Northwell regrets losing any employee under such circumstances,” the company said. “We owe it to our staff, our patients, and the communities we serve to be 100% vaccinated against COVID-19.”
Former New York Gov. Andrew Cuomo announced in August that the state would require health care workers to receive at least one COVID-19 vaccine shot by Sept. 27. Employees didn’t have the option for weekly testing or religious exemptions, which is being challenged in several lawsuits, according to The New York Times.
The order went into effect last week, prompting tens of thousands of employees to get vaccinated. As of last week, 87% of hospital staff were fully vaccinated, and 92% of hospital and retirement home workers had received at least one dose, according to state health data.
Northwell announced its own vaccine mandate in August as well, which sparked protests among some workers. The order applied to both clinical and non-clinical staff.
A few thousand Northwell employees got vaccinated as the deadline approached, Mr. Kemp told The New York Times. Some who lost their jobs at first were able to return to work, and those who have been terminated can interview for reinstatement for 30 days. The hospital system is also “openly recruiting” for the vacant positions.
“The goal was to get people vaccinated, not to get people terminated,” Mr. Kemp said.
Hospitalized COVID-19 patients in New York hit a low of 350 in mid-July, according to state hospitalization data. Now, about 2,200 people are hospitalized throughout the state, most of whom are unvaccinated.
As of Oct. 3, nearly 72% of New York residents had received at least one vaccine dose, according to the latest state data. About 64% are fully vaccinated.
A version of this article first appeared on WebMD.com.