User login
What turns wandering thoughts into something worse?
With all the lockdowns and social distancing of the pandemic, millions of people have had a lot of time to themselves. Many may have filled that time with baking, long walks, or video games, but minds wandering during these periods was inevitable. Coincident with these experiences were increases in depression and anxiety, which could be linked to the same brain network that is thought to support a meandering mind, called the default mode network.
Scientists interested in this network wanted to understand how wandering thoughts can lead some people to a state of brooding in which the same negative thoughts resurface repeatedly. To gain some insight into these patterns, they recorded more than 2,000 thoughts spoken aloud by 78 study participants who did nothing but let their minds wander for 10 minutes.
Senior researcher Jessica Andrews-Hanna, PhD, assistant professor of psychology, University of Arizona, Tucson, and colleagues hoped that analyzing these stream-of-consciousness thoughts could yield insights into how people become stuck in negative mental spirals.
They found that most participants thought about the present or future in words that were neither particularly negative nor positive. Almost three-quarters of the thoughts were focused inward on the person or were imaginative.
Negativity breeds negativity
But the investigators found an interesting pattern with regard to negative thoughts. The more negative someone’s thoughts became, the more likely that their next idea would be related to their previous one. In other words, negative thoughts created a chain reaction of more negative thoughts.
, indicating true mental meandering. The pattern suggested that negativity tends to narrow the range of thoughts, whereas positivity tends to expand it during periods in which the mind wanders.
The researchers also found, unsurprisingly, that negative thoughts that were focused on the self and on the past were more likely to result in brooding and that positive thoughts were less likely to arise.
Most study participants were young and educated and may have only said things that they were comfortable allowing the researchers to hear. And because the authors didn’t ask participants about their moods, the investigators could not associate specific patterns of thought with any mental health conditions.
Although the findings, published in Scientific Reports, do not on their own point to solutions for depression or anxiety, they may offer a starting point for future research into how negative trains of thoughts begin – and perhaps how to derail them.
A version of this article first appeared on Medscape.com.
With all the lockdowns and social distancing of the pandemic, millions of people have had a lot of time to themselves. Many may have filled that time with baking, long walks, or video games, but minds wandering during these periods was inevitable. Coincident with these experiences were increases in depression and anxiety, which could be linked to the same brain network that is thought to support a meandering mind, called the default mode network.
Scientists interested in this network wanted to understand how wandering thoughts can lead some people to a state of brooding in which the same negative thoughts resurface repeatedly. To gain some insight into these patterns, they recorded more than 2,000 thoughts spoken aloud by 78 study participants who did nothing but let their minds wander for 10 minutes.
Senior researcher Jessica Andrews-Hanna, PhD, assistant professor of psychology, University of Arizona, Tucson, and colleagues hoped that analyzing these stream-of-consciousness thoughts could yield insights into how people become stuck in negative mental spirals.
They found that most participants thought about the present or future in words that were neither particularly negative nor positive. Almost three-quarters of the thoughts were focused inward on the person or were imaginative.
Negativity breeds negativity
But the investigators found an interesting pattern with regard to negative thoughts. The more negative someone’s thoughts became, the more likely that their next idea would be related to their previous one. In other words, negative thoughts created a chain reaction of more negative thoughts.
, indicating true mental meandering. The pattern suggested that negativity tends to narrow the range of thoughts, whereas positivity tends to expand it during periods in which the mind wanders.
The researchers also found, unsurprisingly, that negative thoughts that were focused on the self and on the past were more likely to result in brooding and that positive thoughts were less likely to arise.
Most study participants were young and educated and may have only said things that they were comfortable allowing the researchers to hear. And because the authors didn’t ask participants about their moods, the investigators could not associate specific patterns of thought with any mental health conditions.
Although the findings, published in Scientific Reports, do not on their own point to solutions for depression or anxiety, they may offer a starting point for future research into how negative trains of thoughts begin – and perhaps how to derail them.
A version of this article first appeared on Medscape.com.
With all the lockdowns and social distancing of the pandemic, millions of people have had a lot of time to themselves. Many may have filled that time with baking, long walks, or video games, but minds wandering during these periods was inevitable. Coincident with these experiences were increases in depression and anxiety, which could be linked to the same brain network that is thought to support a meandering mind, called the default mode network.
Scientists interested in this network wanted to understand how wandering thoughts can lead some people to a state of brooding in which the same negative thoughts resurface repeatedly. To gain some insight into these patterns, they recorded more than 2,000 thoughts spoken aloud by 78 study participants who did nothing but let their minds wander for 10 minutes.
Senior researcher Jessica Andrews-Hanna, PhD, assistant professor of psychology, University of Arizona, Tucson, and colleagues hoped that analyzing these stream-of-consciousness thoughts could yield insights into how people become stuck in negative mental spirals.
They found that most participants thought about the present or future in words that were neither particularly negative nor positive. Almost three-quarters of the thoughts were focused inward on the person or were imaginative.
Negativity breeds negativity
But the investigators found an interesting pattern with regard to negative thoughts. The more negative someone’s thoughts became, the more likely that their next idea would be related to their previous one. In other words, negative thoughts created a chain reaction of more negative thoughts.
, indicating true mental meandering. The pattern suggested that negativity tends to narrow the range of thoughts, whereas positivity tends to expand it during periods in which the mind wanders.
The researchers also found, unsurprisingly, that negative thoughts that were focused on the self and on the past were more likely to result in brooding and that positive thoughts were less likely to arise.
Most study participants were young and educated and may have only said things that they were comfortable allowing the researchers to hear. And because the authors didn’t ask participants about their moods, the investigators could not associate specific patterns of thought with any mental health conditions.
Although the findings, published in Scientific Reports, do not on their own point to solutions for depression or anxiety, they may offer a starting point for future research into how negative trains of thoughts begin – and perhaps how to derail them.
A version of this article first appeared on Medscape.com.
Omega-3s tame inflammation in elderly COVID-19 patients
results of a small randomized controlled trial suggest.
Results of the study, which included 22 patients with multiple comorbidities, were presented at the European Geriatric Medicine Society annual congress, a hybrid live and online meeting.
The patients, who had a median age of 81 years, were randomized to receive an intravenous infusion of an omega-3 polyunsaturated fatty acid (PUFA) emulsion containing 10 g of fish oil per 100 mL or a saline placebo.
Those who received the intravenous infusion had significant decreases from baseline to end of treatment in the neutrophil-to-lymphocyte ratio (NLR), indicating marked reductions in systemic inflammation.
In contrast, patients randomized to a saline placebo had no significant improvements in NLR, Magnus Bäck, MD, PhD, from the Karolinska Institute in Stockholm reported at the meeting.
“Our lipidomic analysis also showed that omega-3 treatment skewed the lipid response, with reduced levels of proinflammatory lipid mediators, and increased levels of proresolving mediators,” according to a late-breaking abstract, which Dr. Bäck presented during the session.
Omega-3 treatment was not significantly associated with reduction in either C-reactive protein (CRP) or the proinflammatory cytokine interleukin-6, however.
‘Eicosanoid storm’
In a review article published in January 2021 in the open-access journal Frontiers in Physiology, Dr. Bäck and colleagues outlined the rationale for their randomized trial.
“Excessive inflammation has been reported in severe cases with respiratory failure and cardiovascular complications,” they wrote. “In addition to the release of cytokines, referred to as cytokine release syndrome or ‘cytokine storm,’ increased proinflammatory lipid mediators derived from the omega-6 polyunsaturated fatty acid (PUFA) arachidonic acid may cause an ‘eicosanoid storm,’ which contributes to the uncontrolled systemic inflammation.”
Omega-3 PUFA contains proresolving mediators that can limit inflammatory reactions, suggesting the possibility of an inflammation-resolving benefit in patients with COVID-19 without concerns about immunosuppression, the authors hypothesized.
Trial details
In the trial, COVID-Omega-F, they enrolled patients with a COVID-19 diagnosis requiring hospitalization. Patients with an allergy to fish oil or who had contraindications to intravenous PUFA administration (for example, risk for bleeding, shock, or emboli) were excluded.
Ten patients were randomly assigned to receive infusions of the omega-3 PUFA and 12 were assigned to receive infusions of the placebo, once daily for 5 days. The primary outcome measure was change in inflammatory biomarkers, including white blood cell counts, CRP, cytokines, and lipid mediators.
Baseline demographic and clinical characteristics were similar between the two study arms, with a median of about 7 days since the onset of symptoms, and 3.5 days since a diagnosis of COVID-19.
All patients had low lymphocyte responses reflected by a high NLR, a prognostic measure for worse outcomes in patients with COVID-19 infections, Dr. Bäck said.
Inflammation was moderate, with a CRP of 65 mg/L in the placebo group and 62 mg/L in the omega-3 group.
Seven patients in each study arm received concomitant corticoid treatment. Two patients in each arm died in hospital, but there were no serious treatment-related adverse events.
Inflammatory markers improve
As noted before, there was a significant decline in NLR from baseline among patients randomized to omega-3 (P = .02) but no corresponding decrease in patients assigned to placebo infusions.
“The significant decrease was largely driven by an increase in the lymphocyte count in the omega-3 treated group (P = .004), whereas lymphocytes did not significantly change,” Dr. Bäck said.
As expected, patients in the omega-3 group had pronounced increases in omega-3 fatty acids, including eicosapentaenoic acid and docosahexaenoic acid.
The metabolism of fatty acids also differed markedly between the groups, with a significant decrease in the omega-3 group but not the placebo group in proinflammatory mediators, and an increase in precursors to proresolving mediators, Dr. Bäck noted.
AFib concerns
In a question-and-answer part of the session, a physician who identified herself as “Senya from Russia” questioned the safety of omega-3 treatment in this population, “because recently there was a meta-analysis which showed that omega-3 fatty acids will increase the risk of atrial fibrillation in older adults especially.”
The systematic review and meta-analysis she referred to, published in Circulation and reported on by this news organization, showed that, among 81,210 patients with a mean age of 65 enrolled in seven randomized controlled trials, omega-3 fatty acid supplementation was associated with a 25% increase in risk for atrial fibrillation. This risk appeared to be higher in trials testing doses greater than 1 g/day, according to the paper.
“This was not monitored in this study,” Dr. Bäck replied. “It is true that the meta-analysis showed an increased incidence of atrial fibrillation, so it would be something to monitor in case this trial would be expanded to a larger population.”
The study was supported by the Karolinska Institute. Dr. Bäck disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
results of a small randomized controlled trial suggest.
Results of the study, which included 22 patients with multiple comorbidities, were presented at the European Geriatric Medicine Society annual congress, a hybrid live and online meeting.
The patients, who had a median age of 81 years, were randomized to receive an intravenous infusion of an omega-3 polyunsaturated fatty acid (PUFA) emulsion containing 10 g of fish oil per 100 mL or a saline placebo.
Those who received the intravenous infusion had significant decreases from baseline to end of treatment in the neutrophil-to-lymphocyte ratio (NLR), indicating marked reductions in systemic inflammation.
In contrast, patients randomized to a saline placebo had no significant improvements in NLR, Magnus Bäck, MD, PhD, from the Karolinska Institute in Stockholm reported at the meeting.
“Our lipidomic analysis also showed that omega-3 treatment skewed the lipid response, with reduced levels of proinflammatory lipid mediators, and increased levels of proresolving mediators,” according to a late-breaking abstract, which Dr. Bäck presented during the session.
Omega-3 treatment was not significantly associated with reduction in either C-reactive protein (CRP) or the proinflammatory cytokine interleukin-6, however.
‘Eicosanoid storm’
In a review article published in January 2021 in the open-access journal Frontiers in Physiology, Dr. Bäck and colleagues outlined the rationale for their randomized trial.
“Excessive inflammation has been reported in severe cases with respiratory failure and cardiovascular complications,” they wrote. “In addition to the release of cytokines, referred to as cytokine release syndrome or ‘cytokine storm,’ increased proinflammatory lipid mediators derived from the omega-6 polyunsaturated fatty acid (PUFA) arachidonic acid may cause an ‘eicosanoid storm,’ which contributes to the uncontrolled systemic inflammation.”
Omega-3 PUFA contains proresolving mediators that can limit inflammatory reactions, suggesting the possibility of an inflammation-resolving benefit in patients with COVID-19 without concerns about immunosuppression, the authors hypothesized.
Trial details
In the trial, COVID-Omega-F, they enrolled patients with a COVID-19 diagnosis requiring hospitalization. Patients with an allergy to fish oil or who had contraindications to intravenous PUFA administration (for example, risk for bleeding, shock, or emboli) were excluded.
Ten patients were randomly assigned to receive infusions of the omega-3 PUFA and 12 were assigned to receive infusions of the placebo, once daily for 5 days. The primary outcome measure was change in inflammatory biomarkers, including white blood cell counts, CRP, cytokines, and lipid mediators.
Baseline demographic and clinical characteristics were similar between the two study arms, with a median of about 7 days since the onset of symptoms, and 3.5 days since a diagnosis of COVID-19.
All patients had low lymphocyte responses reflected by a high NLR, a prognostic measure for worse outcomes in patients with COVID-19 infections, Dr. Bäck said.
Inflammation was moderate, with a CRP of 65 mg/L in the placebo group and 62 mg/L in the omega-3 group.
Seven patients in each study arm received concomitant corticoid treatment. Two patients in each arm died in hospital, but there were no serious treatment-related adverse events.
Inflammatory markers improve
As noted before, there was a significant decline in NLR from baseline among patients randomized to omega-3 (P = .02) but no corresponding decrease in patients assigned to placebo infusions.
“The significant decrease was largely driven by an increase in the lymphocyte count in the omega-3 treated group (P = .004), whereas lymphocytes did not significantly change,” Dr. Bäck said.
As expected, patients in the omega-3 group had pronounced increases in omega-3 fatty acids, including eicosapentaenoic acid and docosahexaenoic acid.
The metabolism of fatty acids also differed markedly between the groups, with a significant decrease in the omega-3 group but not the placebo group in proinflammatory mediators, and an increase in precursors to proresolving mediators, Dr. Bäck noted.
AFib concerns
In a question-and-answer part of the session, a physician who identified herself as “Senya from Russia” questioned the safety of omega-3 treatment in this population, “because recently there was a meta-analysis which showed that omega-3 fatty acids will increase the risk of atrial fibrillation in older adults especially.”
The systematic review and meta-analysis she referred to, published in Circulation and reported on by this news organization, showed that, among 81,210 patients with a mean age of 65 enrolled in seven randomized controlled trials, omega-3 fatty acid supplementation was associated with a 25% increase in risk for atrial fibrillation. This risk appeared to be higher in trials testing doses greater than 1 g/day, according to the paper.
“This was not monitored in this study,” Dr. Bäck replied. “It is true that the meta-analysis showed an increased incidence of atrial fibrillation, so it would be something to monitor in case this trial would be expanded to a larger population.”
The study was supported by the Karolinska Institute. Dr. Bäck disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
results of a small randomized controlled trial suggest.
Results of the study, which included 22 patients with multiple comorbidities, were presented at the European Geriatric Medicine Society annual congress, a hybrid live and online meeting.
The patients, who had a median age of 81 years, were randomized to receive an intravenous infusion of an omega-3 polyunsaturated fatty acid (PUFA) emulsion containing 10 g of fish oil per 100 mL or a saline placebo.
Those who received the intravenous infusion had significant decreases from baseline to end of treatment in the neutrophil-to-lymphocyte ratio (NLR), indicating marked reductions in systemic inflammation.
In contrast, patients randomized to a saline placebo had no significant improvements in NLR, Magnus Bäck, MD, PhD, from the Karolinska Institute in Stockholm reported at the meeting.
“Our lipidomic analysis also showed that omega-3 treatment skewed the lipid response, with reduced levels of proinflammatory lipid mediators, and increased levels of proresolving mediators,” according to a late-breaking abstract, which Dr. Bäck presented during the session.
Omega-3 treatment was not significantly associated with reduction in either C-reactive protein (CRP) or the proinflammatory cytokine interleukin-6, however.
‘Eicosanoid storm’
In a review article published in January 2021 in the open-access journal Frontiers in Physiology, Dr. Bäck and colleagues outlined the rationale for their randomized trial.
“Excessive inflammation has been reported in severe cases with respiratory failure and cardiovascular complications,” they wrote. “In addition to the release of cytokines, referred to as cytokine release syndrome or ‘cytokine storm,’ increased proinflammatory lipid mediators derived from the omega-6 polyunsaturated fatty acid (PUFA) arachidonic acid may cause an ‘eicosanoid storm,’ which contributes to the uncontrolled systemic inflammation.”
Omega-3 PUFA contains proresolving mediators that can limit inflammatory reactions, suggesting the possibility of an inflammation-resolving benefit in patients with COVID-19 without concerns about immunosuppression, the authors hypothesized.
Trial details
In the trial, COVID-Omega-F, they enrolled patients with a COVID-19 diagnosis requiring hospitalization. Patients with an allergy to fish oil or who had contraindications to intravenous PUFA administration (for example, risk for bleeding, shock, or emboli) were excluded.
Ten patients were randomly assigned to receive infusions of the omega-3 PUFA and 12 were assigned to receive infusions of the placebo, once daily for 5 days. The primary outcome measure was change in inflammatory biomarkers, including white blood cell counts, CRP, cytokines, and lipid mediators.
Baseline demographic and clinical characteristics were similar between the two study arms, with a median of about 7 days since the onset of symptoms, and 3.5 days since a diagnosis of COVID-19.
All patients had low lymphocyte responses reflected by a high NLR, a prognostic measure for worse outcomes in patients with COVID-19 infections, Dr. Bäck said.
Inflammation was moderate, with a CRP of 65 mg/L in the placebo group and 62 mg/L in the omega-3 group.
Seven patients in each study arm received concomitant corticoid treatment. Two patients in each arm died in hospital, but there were no serious treatment-related adverse events.
Inflammatory markers improve
As noted before, there was a significant decline in NLR from baseline among patients randomized to omega-3 (P = .02) but no corresponding decrease in patients assigned to placebo infusions.
“The significant decrease was largely driven by an increase in the lymphocyte count in the omega-3 treated group (P = .004), whereas lymphocytes did not significantly change,” Dr. Bäck said.
As expected, patients in the omega-3 group had pronounced increases in omega-3 fatty acids, including eicosapentaenoic acid and docosahexaenoic acid.
The metabolism of fatty acids also differed markedly between the groups, with a significant decrease in the omega-3 group but not the placebo group in proinflammatory mediators, and an increase in precursors to proresolving mediators, Dr. Bäck noted.
AFib concerns
In a question-and-answer part of the session, a physician who identified herself as “Senya from Russia” questioned the safety of omega-3 treatment in this population, “because recently there was a meta-analysis which showed that omega-3 fatty acids will increase the risk of atrial fibrillation in older adults especially.”
The systematic review and meta-analysis she referred to, published in Circulation and reported on by this news organization, showed that, among 81,210 patients with a mean age of 65 enrolled in seven randomized controlled trials, omega-3 fatty acid supplementation was associated with a 25% increase in risk for atrial fibrillation. This risk appeared to be higher in trials testing doses greater than 1 g/day, according to the paper.
“This was not monitored in this study,” Dr. Bäck replied. “It is true that the meta-analysis showed an increased incidence of atrial fibrillation, so it would be something to monitor in case this trial would be expanded to a larger population.”
The study was supported by the Karolinska Institute. Dr. Bäck disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EUGMS
Pandemic data challenges infection link to Guillain-Barré syndrome
While pediatric cases of various types of infections fell by 45%-95% during the early months of the pandemic, cases of acute inflammatory demyelinating polyneuropathy (AIDP), an inflammatory neuropathy belonging to the clinical spectrum of Guillain-Barré syndrome, only fell by about 32%-37%, a rate that’s similar to the 35.1% decline in overall hospital admissions over that time period, researchers found. There was also no apparent link between the appearance of COVID-19 and the number of reported AIDP cases.
“There was no clear association between respiratory or gastrointestinal infections and rates of AIDP. Further, we found that AIDP did not have the expected dramatic reduction when community-acquired infections decreased during the pandemic,” Children’s Hospital of Philadelphia neurologist Craig A. Press, MD, PhD, said in an interview.
Dr. Press and colleagues presented their findings in a poster at the 50th annual meeting of the Child Neurology Society.
According to Dr. Press, the cause of AIDP in most patients is unclear, although infections and vaccinations are often linked to cases. “However, the data supporting this link is often weak. Infections with Campylobacter jejuni [bacteria that causes food poisoning] are known to be associated with AIDP, while rates of AIDP in the general population and in those with influenza are similar.”
For the new multicenter, cross-sectional study, researchers tracked AIDP data from the 47 pediatric hospitals that provide statistics to the Pediatric Health Information System. They focused on the period from January 2017 to September 2020, which included the first months of the COVID-19 pandemic in the United States.
“Social distancing, masks, and increased hand hygiene decrease community-acquired infectious rates in a dramatic way,” Dr. Press said. “If these infections were causing AIDP, we hypothesized that the cases of AIDP would drop substantially as a result.”
But this didn’t appear to happen. Researchers found that the numbers of various types of infections declined from April to September 2020: Respiratory infections dipped by 73%-78%, gastrointestinal infections fell by 45%-61%, and influenza infections dipped by 88%-95%. But AIDP cases didn’t fall as precipitously. In fact, their levels were about the same as they were in April 2017, a month when rates of gastrointestinal, respiratory disease and influenza infections were at seasonally low – but not abnormal – ebbs.
“While we must be cautious interpreting the results,” Dr. Press said, “this makes the link between infections as the main driver of pediatric AIDP less likely.”
However, he said, “this study does not exclude the possibility that rare infections cause AIDP – the data supporting that some more rare infections like campylobacter have a connection to AIDP are more robust – or that common infections very rarely lead to AIDP. While we look for triggers causing inflammatory disorders, AIDP maybe an autoinflammatory disorder without a clear trigger.”
Going forward, Dr. Press said, “we hope to look at infectious data in a more granular way to identify if specific viral or bacterial infectious may be associated with this or other inflammatory disorders. We believe that the use of data like this and the natural experiment that COVID-19 provided may help us to explore the impact of infections on disorders thought to be postinfectious.”
No study funding is reported, and the authors report no relevant disclosures.
While pediatric cases of various types of infections fell by 45%-95% during the early months of the pandemic, cases of acute inflammatory demyelinating polyneuropathy (AIDP), an inflammatory neuropathy belonging to the clinical spectrum of Guillain-Barré syndrome, only fell by about 32%-37%, a rate that’s similar to the 35.1% decline in overall hospital admissions over that time period, researchers found. There was also no apparent link between the appearance of COVID-19 and the number of reported AIDP cases.
“There was no clear association between respiratory or gastrointestinal infections and rates of AIDP. Further, we found that AIDP did not have the expected dramatic reduction when community-acquired infections decreased during the pandemic,” Children’s Hospital of Philadelphia neurologist Craig A. Press, MD, PhD, said in an interview.
Dr. Press and colleagues presented their findings in a poster at the 50th annual meeting of the Child Neurology Society.
According to Dr. Press, the cause of AIDP in most patients is unclear, although infections and vaccinations are often linked to cases. “However, the data supporting this link is often weak. Infections with Campylobacter jejuni [bacteria that causes food poisoning] are known to be associated with AIDP, while rates of AIDP in the general population and in those with influenza are similar.”
For the new multicenter, cross-sectional study, researchers tracked AIDP data from the 47 pediatric hospitals that provide statistics to the Pediatric Health Information System. They focused on the period from January 2017 to September 2020, which included the first months of the COVID-19 pandemic in the United States.
“Social distancing, masks, and increased hand hygiene decrease community-acquired infectious rates in a dramatic way,” Dr. Press said. “If these infections were causing AIDP, we hypothesized that the cases of AIDP would drop substantially as a result.”
But this didn’t appear to happen. Researchers found that the numbers of various types of infections declined from April to September 2020: Respiratory infections dipped by 73%-78%, gastrointestinal infections fell by 45%-61%, and influenza infections dipped by 88%-95%. But AIDP cases didn’t fall as precipitously. In fact, their levels were about the same as they were in April 2017, a month when rates of gastrointestinal, respiratory disease and influenza infections were at seasonally low – but not abnormal – ebbs.
“While we must be cautious interpreting the results,” Dr. Press said, “this makes the link between infections as the main driver of pediatric AIDP less likely.”
However, he said, “this study does not exclude the possibility that rare infections cause AIDP – the data supporting that some more rare infections like campylobacter have a connection to AIDP are more robust – or that common infections very rarely lead to AIDP. While we look for triggers causing inflammatory disorders, AIDP maybe an autoinflammatory disorder without a clear trigger.”
Going forward, Dr. Press said, “we hope to look at infectious data in a more granular way to identify if specific viral or bacterial infectious may be associated with this or other inflammatory disorders. We believe that the use of data like this and the natural experiment that COVID-19 provided may help us to explore the impact of infections on disorders thought to be postinfectious.”
No study funding is reported, and the authors report no relevant disclosures.
While pediatric cases of various types of infections fell by 45%-95% during the early months of the pandemic, cases of acute inflammatory demyelinating polyneuropathy (AIDP), an inflammatory neuropathy belonging to the clinical spectrum of Guillain-Barré syndrome, only fell by about 32%-37%, a rate that’s similar to the 35.1% decline in overall hospital admissions over that time period, researchers found. There was also no apparent link between the appearance of COVID-19 and the number of reported AIDP cases.
“There was no clear association between respiratory or gastrointestinal infections and rates of AIDP. Further, we found that AIDP did not have the expected dramatic reduction when community-acquired infections decreased during the pandemic,” Children’s Hospital of Philadelphia neurologist Craig A. Press, MD, PhD, said in an interview.
Dr. Press and colleagues presented their findings in a poster at the 50th annual meeting of the Child Neurology Society.
According to Dr. Press, the cause of AIDP in most patients is unclear, although infections and vaccinations are often linked to cases. “However, the data supporting this link is often weak. Infections with Campylobacter jejuni [bacteria that causes food poisoning] are known to be associated with AIDP, while rates of AIDP in the general population and in those with influenza are similar.”
For the new multicenter, cross-sectional study, researchers tracked AIDP data from the 47 pediatric hospitals that provide statistics to the Pediatric Health Information System. They focused on the period from January 2017 to September 2020, which included the first months of the COVID-19 pandemic in the United States.
“Social distancing, masks, and increased hand hygiene decrease community-acquired infectious rates in a dramatic way,” Dr. Press said. “If these infections were causing AIDP, we hypothesized that the cases of AIDP would drop substantially as a result.”
But this didn’t appear to happen. Researchers found that the numbers of various types of infections declined from April to September 2020: Respiratory infections dipped by 73%-78%, gastrointestinal infections fell by 45%-61%, and influenza infections dipped by 88%-95%. But AIDP cases didn’t fall as precipitously. In fact, their levels were about the same as they were in April 2017, a month when rates of gastrointestinal, respiratory disease and influenza infections were at seasonally low – but not abnormal – ebbs.
“While we must be cautious interpreting the results,” Dr. Press said, “this makes the link between infections as the main driver of pediatric AIDP less likely.”
However, he said, “this study does not exclude the possibility that rare infections cause AIDP – the data supporting that some more rare infections like campylobacter have a connection to AIDP are more robust – or that common infections very rarely lead to AIDP. While we look for triggers causing inflammatory disorders, AIDP maybe an autoinflammatory disorder without a clear trigger.”
Going forward, Dr. Press said, “we hope to look at infectious data in a more granular way to identify if specific viral or bacterial infectious may be associated with this or other inflammatory disorders. We believe that the use of data like this and the natural experiment that COVID-19 provided may help us to explore the impact of infections on disorders thought to be postinfectious.”
No study funding is reported, and the authors report no relevant disclosures.
FROM CNS 2021
Lupus may confer higher risk of death from COVID-19
There is a significantly increased risk for acute respiratory distress syndrome (ARDS)–related death from COVID-19 among people with systemic lupus erythematous (SLE), compared with the general population, according to data collected in Brazil in 2020.
“Special care is therefore necessary for these patients, as well as reinforcement of the importance of preventive measures during a pandemic for this population,” said Eloisa Bonfá, MD, PhD, at the 14th International Congress on Systemic Lupus Erythematosus, which was held together with the 6th International Congress on Controversies in Rheumatology and Autoimmunity.
“We know that lupus patients have an increased susceptibility to infections due to autoimmune dysregulation and use of immunosuppressive therapy,” explained Dr. Bonfá, who is clinical director of the largest tertiary referral center for autoimmune rheumatic diseases in Latin America, the University of São Paulo Faculty of Medicine Hospital Clinics.
“Our study demonstrates for the first time that lupus patients have an increased ARDS severity,” she added.
Prior to the meeting, the study was published in ACR Open Rheumatology.
Collating the evidence
Since the COVID-19 pandemic began, there have been more than 20 million confirmed cases of SARS-CoV-2 infection in Brazil and more than half a million deaths.
Dr. Bonfá presented the results of a cross-sectional study that was part of the country’s national Influenza Epidemiological Reporting Surveillance System. Data from 2020 were used, which included just over 252,000 individuals who had polymerase chain reaction–confirmed SARS-CoV-2 infection. Of these individuals, there were 319 consecutively recruited patients with SLE.
The aim was to look at the effect of being hospitalized for COVID-19–related ARDS on outcomes in people with SLE versus the general population.
ARDS was defined as a positive polymerase chain reaction test and accompanying flu-like symptoms with dyspnea, respiratory discomfort, persistent pressure in the chest, or desaturation less than 95% in room air or having a bluish tinge to the lips or face.
Other telling signs of a serious respiratory infection that were evaluated, but not mandatory for study eligibility, were loss of smell, impaired taste, typical CT findings, or having had contact with a confirmed COVID-19 case in the preceding 2 weeks.
Key findings
The risk for death from COVID-19–related ARDS was “more than double” in patients with SLE, compared with the general population, Dr. Bonfá reported. The relative risk in the fully adjusted, propensity-scored analysis was approximately 2.25.
That analysis did not account for other comorbidities but was fully adjusted for individuals’ age, sex, and region of Brazil where they lived. The latter was important, Dr. Bonfá said, because “we have a high disparity regarding health access and treatment among regions.”
Comorbidities considered as part of the analyses included arterial hypertension, diabetes, malignancies, neurologic disease, and diseases affecting the heart, lung, liver, and kidneys. Researchers also adjusted for smoking, alcohol intake, body weight, pregnancy, and transplantation.
SLE had a greater impact on individuals’ outcomes than all other comorbidities considered.
“We evaluated lupus as one comorbidity compared to all other comorbidities,” Dr. Bonfá explained.
SLE “more than doubled the chances” of dying from ARDS, she said. “This is [a] very impressive finding.”
They found that SLE was associated with an RR for death of 1.73, compared with non-SLE patients, when propensity-score matching without adjustment for comorbidities was used. The RR for death dropped to 1.40 but was still significant when researchers included comorbidities.
Dr. Bonfá and her team also looked at a combined endpoint of death, ICU admission, and need for mechanical ventilation. They found an increased risk in patients with SLE versus the general population in all their analyses, ranging from 1.70 if comorbidities were included in the model to 1.27 if they weren’t to 1.39 if propensity-score matching alone was used.
Got lupus? ‘Get vaccinated’
“The data we have are in nonvaccinated patients,” Dr. Bonfá said. “We didn’t have vaccines in 2020.”
Whether being vaccinated might make a different to the risks found in this study is an “interesting question,” and one that may be examined in the future.
Certainly, other work Dr. Bonfá has been involved in seems to point to a likely benefit of vaccination in patients with autoimmune diseases in terms of reducing mortality from COVID-19, even when rates of infection may be on the rise.
“There’s considerable vaccine hesitancy in SLE patients,” Chi-Chiu Mok, MD, of Tuen Mun Hospital in Hong Kong, observed in a separate presentation at the congress.
This may be for several reasons, such as worry that their disease may flare or the vaccine might compromise their drug treatment or result in uncommon complications.
However, “we should encourage our SLE patients to receive COVID-19 vaccination at a time of clinical remission or low disease activity state,” Dr. Mok advised.
“Physical distancing, protective masks, and personal hygiene [measures]” should also continue.
The bottom line for those with SLE is to get vaccinated, stressed Sandra Navarra, MD, of the University of Santo Tomas Hospital in Manila, the Philippines, during the discussion.
“There’s still so much out there that we do not know about,” she said. “Just get yourself vaccinated.”
The study had no outside funding. Dr. Bonfá, Dr. Mok, and Dr. Navarra reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There is a significantly increased risk for acute respiratory distress syndrome (ARDS)–related death from COVID-19 among people with systemic lupus erythematous (SLE), compared with the general population, according to data collected in Brazil in 2020.
“Special care is therefore necessary for these patients, as well as reinforcement of the importance of preventive measures during a pandemic for this population,” said Eloisa Bonfá, MD, PhD, at the 14th International Congress on Systemic Lupus Erythematosus, which was held together with the 6th International Congress on Controversies in Rheumatology and Autoimmunity.
“We know that lupus patients have an increased susceptibility to infections due to autoimmune dysregulation and use of immunosuppressive therapy,” explained Dr. Bonfá, who is clinical director of the largest tertiary referral center for autoimmune rheumatic diseases in Latin America, the University of São Paulo Faculty of Medicine Hospital Clinics.
“Our study demonstrates for the first time that lupus patients have an increased ARDS severity,” she added.
Prior to the meeting, the study was published in ACR Open Rheumatology.
Collating the evidence
Since the COVID-19 pandemic began, there have been more than 20 million confirmed cases of SARS-CoV-2 infection in Brazil and more than half a million deaths.
Dr. Bonfá presented the results of a cross-sectional study that was part of the country’s national Influenza Epidemiological Reporting Surveillance System. Data from 2020 were used, which included just over 252,000 individuals who had polymerase chain reaction–confirmed SARS-CoV-2 infection. Of these individuals, there were 319 consecutively recruited patients with SLE.
The aim was to look at the effect of being hospitalized for COVID-19–related ARDS on outcomes in people with SLE versus the general population.
ARDS was defined as a positive polymerase chain reaction test and accompanying flu-like symptoms with dyspnea, respiratory discomfort, persistent pressure in the chest, or desaturation less than 95% in room air or having a bluish tinge to the lips or face.
Other telling signs of a serious respiratory infection that were evaluated, but not mandatory for study eligibility, were loss of smell, impaired taste, typical CT findings, or having had contact with a confirmed COVID-19 case in the preceding 2 weeks.
Key findings
The risk for death from COVID-19–related ARDS was “more than double” in patients with SLE, compared with the general population, Dr. Bonfá reported. The relative risk in the fully adjusted, propensity-scored analysis was approximately 2.25.
That analysis did not account for other comorbidities but was fully adjusted for individuals’ age, sex, and region of Brazil where they lived. The latter was important, Dr. Bonfá said, because “we have a high disparity regarding health access and treatment among regions.”
Comorbidities considered as part of the analyses included arterial hypertension, diabetes, malignancies, neurologic disease, and diseases affecting the heart, lung, liver, and kidneys. Researchers also adjusted for smoking, alcohol intake, body weight, pregnancy, and transplantation.
SLE had a greater impact on individuals’ outcomes than all other comorbidities considered.
“We evaluated lupus as one comorbidity compared to all other comorbidities,” Dr. Bonfá explained.
SLE “more than doubled the chances” of dying from ARDS, she said. “This is [a] very impressive finding.”
They found that SLE was associated with an RR for death of 1.73, compared with non-SLE patients, when propensity-score matching without adjustment for comorbidities was used. The RR for death dropped to 1.40 but was still significant when researchers included comorbidities.
Dr. Bonfá and her team also looked at a combined endpoint of death, ICU admission, and need for mechanical ventilation. They found an increased risk in patients with SLE versus the general population in all their analyses, ranging from 1.70 if comorbidities were included in the model to 1.27 if they weren’t to 1.39 if propensity-score matching alone was used.
Got lupus? ‘Get vaccinated’
“The data we have are in nonvaccinated patients,” Dr. Bonfá said. “We didn’t have vaccines in 2020.”
Whether being vaccinated might make a different to the risks found in this study is an “interesting question,” and one that may be examined in the future.
Certainly, other work Dr. Bonfá has been involved in seems to point to a likely benefit of vaccination in patients with autoimmune diseases in terms of reducing mortality from COVID-19, even when rates of infection may be on the rise.
“There’s considerable vaccine hesitancy in SLE patients,” Chi-Chiu Mok, MD, of Tuen Mun Hospital in Hong Kong, observed in a separate presentation at the congress.
This may be for several reasons, such as worry that their disease may flare or the vaccine might compromise their drug treatment or result in uncommon complications.
However, “we should encourage our SLE patients to receive COVID-19 vaccination at a time of clinical remission or low disease activity state,” Dr. Mok advised.
“Physical distancing, protective masks, and personal hygiene [measures]” should also continue.
The bottom line for those with SLE is to get vaccinated, stressed Sandra Navarra, MD, of the University of Santo Tomas Hospital in Manila, the Philippines, during the discussion.
“There’s still so much out there that we do not know about,” she said. “Just get yourself vaccinated.”
The study had no outside funding. Dr. Bonfá, Dr. Mok, and Dr. Navarra reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There is a significantly increased risk for acute respiratory distress syndrome (ARDS)–related death from COVID-19 among people with systemic lupus erythematous (SLE), compared with the general population, according to data collected in Brazil in 2020.
“Special care is therefore necessary for these patients, as well as reinforcement of the importance of preventive measures during a pandemic for this population,” said Eloisa Bonfá, MD, PhD, at the 14th International Congress on Systemic Lupus Erythematosus, which was held together with the 6th International Congress on Controversies in Rheumatology and Autoimmunity.
“We know that lupus patients have an increased susceptibility to infections due to autoimmune dysregulation and use of immunosuppressive therapy,” explained Dr. Bonfá, who is clinical director of the largest tertiary referral center for autoimmune rheumatic diseases in Latin America, the University of São Paulo Faculty of Medicine Hospital Clinics.
“Our study demonstrates for the first time that lupus patients have an increased ARDS severity,” she added.
Prior to the meeting, the study was published in ACR Open Rheumatology.
Collating the evidence
Since the COVID-19 pandemic began, there have been more than 20 million confirmed cases of SARS-CoV-2 infection in Brazil and more than half a million deaths.
Dr. Bonfá presented the results of a cross-sectional study that was part of the country’s national Influenza Epidemiological Reporting Surveillance System. Data from 2020 were used, which included just over 252,000 individuals who had polymerase chain reaction–confirmed SARS-CoV-2 infection. Of these individuals, there were 319 consecutively recruited patients with SLE.
The aim was to look at the effect of being hospitalized for COVID-19–related ARDS on outcomes in people with SLE versus the general population.
ARDS was defined as a positive polymerase chain reaction test and accompanying flu-like symptoms with dyspnea, respiratory discomfort, persistent pressure in the chest, or desaturation less than 95% in room air or having a bluish tinge to the lips or face.
Other telling signs of a serious respiratory infection that were evaluated, but not mandatory for study eligibility, were loss of smell, impaired taste, typical CT findings, or having had contact with a confirmed COVID-19 case in the preceding 2 weeks.
Key findings
The risk for death from COVID-19–related ARDS was “more than double” in patients with SLE, compared with the general population, Dr. Bonfá reported. The relative risk in the fully adjusted, propensity-scored analysis was approximately 2.25.
That analysis did not account for other comorbidities but was fully adjusted for individuals’ age, sex, and region of Brazil where they lived. The latter was important, Dr. Bonfá said, because “we have a high disparity regarding health access and treatment among regions.”
Comorbidities considered as part of the analyses included arterial hypertension, diabetes, malignancies, neurologic disease, and diseases affecting the heart, lung, liver, and kidneys. Researchers also adjusted for smoking, alcohol intake, body weight, pregnancy, and transplantation.
SLE had a greater impact on individuals’ outcomes than all other comorbidities considered.
“We evaluated lupus as one comorbidity compared to all other comorbidities,” Dr. Bonfá explained.
SLE “more than doubled the chances” of dying from ARDS, she said. “This is [a] very impressive finding.”
They found that SLE was associated with an RR for death of 1.73, compared with non-SLE patients, when propensity-score matching without adjustment for comorbidities was used. The RR for death dropped to 1.40 but was still significant when researchers included comorbidities.
Dr. Bonfá and her team also looked at a combined endpoint of death, ICU admission, and need for mechanical ventilation. They found an increased risk in patients with SLE versus the general population in all their analyses, ranging from 1.70 if comorbidities were included in the model to 1.27 if they weren’t to 1.39 if propensity-score matching alone was used.
Got lupus? ‘Get vaccinated’
“The data we have are in nonvaccinated patients,” Dr. Bonfá said. “We didn’t have vaccines in 2020.”
Whether being vaccinated might make a different to the risks found in this study is an “interesting question,” and one that may be examined in the future.
Certainly, other work Dr. Bonfá has been involved in seems to point to a likely benefit of vaccination in patients with autoimmune diseases in terms of reducing mortality from COVID-19, even when rates of infection may be on the rise.
“There’s considerable vaccine hesitancy in SLE patients,” Chi-Chiu Mok, MD, of Tuen Mun Hospital in Hong Kong, observed in a separate presentation at the congress.
This may be for several reasons, such as worry that their disease may flare or the vaccine might compromise their drug treatment or result in uncommon complications.
However, “we should encourage our SLE patients to receive COVID-19 vaccination at a time of clinical remission or low disease activity state,” Dr. Mok advised.
“Physical distancing, protective masks, and personal hygiene [measures]” should also continue.
The bottom line for those with SLE is to get vaccinated, stressed Sandra Navarra, MD, of the University of Santo Tomas Hospital in Manila, the Philippines, during the discussion.
“There’s still so much out there that we do not know about,” she said. “Just get yourself vaccinated.”
The study had no outside funding. Dr. Bonfá, Dr. Mok, and Dr. Navarra reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children and COVID-19: U.S. adds latest million cases in record time
The United States just passed the 6-million mark in COVID-19 cases among children, with the last million cases taking less time to record than any of the first five, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.

The five-millionth case was reported during the week of Aug. 27 to Sept. 2, and case number 6 million came during the week of Oct. 1-7, just 5 weeks later, compared with the 6 weeks it took to go from 1 million to 2 million last November and December, the AAP and CHA said in their weekly COVID-19 report.
New cases continued to drop, however, and that weekly count was down by 14.6% from the previous week and by 41.1% from the peak of almost 252,000 reached in early September, the two groups said while also noting limitations to the data, such as three states (Alabama, Nebraska, and Texas) that are no longer updating their COVID-19 dashboards.
Other metrics show similar drops in recent weeks. Among children aged 0-11 years, emergency department visits involving a COVID-19 diagnosis dropped from 4.1% of all ED visits in late August to 1.4% of ED visits on Oct. 6. ED visits with a COVID-19 diagnosis fell from a peak of 8.5% on Aug. 22 to 1.5% on Oct. 6 for 12- to 15-year-olds and from 8.5% to 1.5% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
The rate of new hospital admissions for children aged 0-17 years was down to 0.26 per 100,000 population on Oct. 9 after reaching 0.51 per 100,000 on Sept. 4. Hospitalizations in children totaled just over 64,000 from Aug. 1, 2020, to Oct. 9, 2021, which is just over 2% of all COVID-19–related admissions over that time period, the CDC said on its COVID Data Tracker.
That pattern, unfortunately, also applies to vaccinations. “The number of children receiving their first COVID-19 vaccine this week [Sept. 30 to Oct. 6], about 156,000, was the lowest number since vaccines were available,” the AAP said in a separate report on vaccination trends, adding that “the number of children receiving their first dose has steadily declined from 8 weeks ago when 586,000 children received their initial dose the week ending Aug. 11.”
The United States just passed the 6-million mark in COVID-19 cases among children, with the last million cases taking less time to record than any of the first five, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.

The five-millionth case was reported during the week of Aug. 27 to Sept. 2, and case number 6 million came during the week of Oct. 1-7, just 5 weeks later, compared with the 6 weeks it took to go from 1 million to 2 million last November and December, the AAP and CHA said in their weekly COVID-19 report.
New cases continued to drop, however, and that weekly count was down by 14.6% from the previous week and by 41.1% from the peak of almost 252,000 reached in early September, the two groups said while also noting limitations to the data, such as three states (Alabama, Nebraska, and Texas) that are no longer updating their COVID-19 dashboards.
Other metrics show similar drops in recent weeks. Among children aged 0-11 years, emergency department visits involving a COVID-19 diagnosis dropped from 4.1% of all ED visits in late August to 1.4% of ED visits on Oct. 6. ED visits with a COVID-19 diagnosis fell from a peak of 8.5% on Aug. 22 to 1.5% on Oct. 6 for 12- to 15-year-olds and from 8.5% to 1.5% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
The rate of new hospital admissions for children aged 0-17 years was down to 0.26 per 100,000 population on Oct. 9 after reaching 0.51 per 100,000 on Sept. 4. Hospitalizations in children totaled just over 64,000 from Aug. 1, 2020, to Oct. 9, 2021, which is just over 2% of all COVID-19–related admissions over that time period, the CDC said on its COVID Data Tracker.
That pattern, unfortunately, also applies to vaccinations. “The number of children receiving their first COVID-19 vaccine this week [Sept. 30 to Oct. 6], about 156,000, was the lowest number since vaccines were available,” the AAP said in a separate report on vaccination trends, adding that “the number of children receiving their first dose has steadily declined from 8 weeks ago when 586,000 children received their initial dose the week ending Aug. 11.”
The United States just passed the 6-million mark in COVID-19 cases among children, with the last million cases taking less time to record than any of the first five, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.

The five-millionth case was reported during the week of Aug. 27 to Sept. 2, and case number 6 million came during the week of Oct. 1-7, just 5 weeks later, compared with the 6 weeks it took to go from 1 million to 2 million last November and December, the AAP and CHA said in their weekly COVID-19 report.
New cases continued to drop, however, and that weekly count was down by 14.6% from the previous week and by 41.1% from the peak of almost 252,000 reached in early September, the two groups said while also noting limitations to the data, such as three states (Alabama, Nebraska, and Texas) that are no longer updating their COVID-19 dashboards.
Other metrics show similar drops in recent weeks. Among children aged 0-11 years, emergency department visits involving a COVID-19 diagnosis dropped from 4.1% of all ED visits in late August to 1.4% of ED visits on Oct. 6. ED visits with a COVID-19 diagnosis fell from a peak of 8.5% on Aug. 22 to 1.5% on Oct. 6 for 12- to 15-year-olds and from 8.5% to 1.5% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
The rate of new hospital admissions for children aged 0-17 years was down to 0.26 per 100,000 population on Oct. 9 after reaching 0.51 per 100,000 on Sept. 4. Hospitalizations in children totaled just over 64,000 from Aug. 1, 2020, to Oct. 9, 2021, which is just over 2% of all COVID-19–related admissions over that time period, the CDC said on its COVID Data Tracker.
That pattern, unfortunately, also applies to vaccinations. “The number of children receiving their first COVID-19 vaccine this week [Sept. 30 to Oct. 6], about 156,000, was the lowest number since vaccines were available,” the AAP said in a separate report on vaccination trends, adding that “the number of children receiving their first dose has steadily declined from 8 weeks ago when 586,000 children received their initial dose the week ending Aug. 11.”
Underrepresented Minority Students Applying to Dermatology Residency in the COVID-19 Era: Challenges and Considerations
The COVID-19 pandemic has markedly changed the dermatology residency application process. As medical students head into this application cycle, the impacts of systemic racism and deeply rooted structural barriers continue to be exacerbated for students who identify as an underrepresented minority (URM) in medicine—historically defined as those who self-identify as Hispanic or Latinx; Black or African American; American Indian or Alaska Native; or Native Hawaiian or Pacific Islander. The Association of American Medical Colleges (AAMC) defines URMs as racial and ethnic populations that are underrepresented in medicine relative to their numbers in the general population.1 Although these groups account for approximately 34% of the population of the United States, they constitute only 11% of the country’s physician workforce.2,3
Of the total physician workforce in the United States, Black and African American physicians account for 5% of practicing physicians; Hispanic physicians, 5.8%; American Indian and Alaska Native physicians, 0.3%; and Native Hawaiian and Pacific Islander physicians, 0.1%.2 In competitive medical specialties, the disproportionality of these numbers compared to our current demographics in the United States as shown above is even more staggering. In 2018, for example, 10% of practicing dermatologists identified as female URM physicians; 6%, as male URM physicians.2 In this article, we discuss some of the challenges and considerations for URM students applying to dermatology residency in the era of the COVID-19 pandemic.
Barriers for URM Students in Dermatology
Multiple studies have attempted to identify some of the barriers faced by URM students in medicine that might explain the lack of diversity in competitive specialties. Vasquez and colleagues4 identified 4 major factors that play a role in dermatology: lack of equitable resources, lack of support, financial limitations, and the lack of group identity. More than half of URM students surveyed (1) identified lack of support as a barrier and (2) reported having been encouraged to seek a specialty more reflective of their community.4
Soliman et al5 reported that URM barriers in dermatology extend to include lack of diversity in the field, socioeconomic factors, lack of mentorship, and a negative perception of minority students by residency programs. Dermatology is the second least diverse specialty in medicine after orthopedic surgery, which, in and of itself, might further discourage URM students from applying to dermatology.5
With the minimal exposure that URM students have to the field of dermatology, the lack of pipeline programs, and reports that URMs often are encouraged to pursue primary care, the current diversity deficiency in dermatology comes as no surprise. In addition, the substantial disadvantage for URM students is perpetuated by the traditional highly selective process that favors grades, board scores, and honor society status over holistic assessment of the individual student and their unique experiences and potential for contribution.
Looking Beyond Test Scores
The US Medical Licensing Examination (USMLE) traditionally has been used to select dermatology residency applicants, with high cutoff scores often excluding outstanding URM students. Research has suggested that the use of USMLE examination test scores for residency recruitment lacks validity because it has poor predictability of residency performance.6 Although the USMLE Step 1 examination is transitioning to pass/fail scoring, applicants for the next cycle will still have a 3-digit numerical score.
We strongly recommend that dermatology programs transition from emphasizing scores of residency candidates to reviewing each candidate holistically. The AAMC defines “holistic review” as a “flexible, individualized way of assessing an applicant’s capabilities, by which balanced consideration is given to experiences, attributes, competencies, and academic or scholarly metrics and, when considered in combination, how the individual might contribute value to the institution’s mission.”7 Furthermore, we recommend that dermatology residency programs have multiple faculty members review each application, including a representative of the diversity, inclusion, and equity committee.
Applying to Residency in the COVID-19 Virtual Environment
In the COVID-19 era, dermatology externship opportunities that would have allowed URM students to work directly with potential residency programs, showcase their abilities, and network have been limited. Virtual residency interviews could make it more challenging to evaluate candidates, especially URM students from less prestigious programs or unusual socioeconomic backgrounds, or with lower board scores. In addition, virtual interviews can more easily become one-dimensional, depriving URM students of the opportunity to gauge their personal fit in a specific dermatology residency program and its community. Questions and concerns of URM students might include: Will I be appropriately supported and mentored? Will my cultural preferences, religion, sexual preference, hairstyle, and beliefs be accepted? Can I advocate for minorities and support antiracism and diversity and inclusion initiatives? To that end, we recommend that dermatology programs continue to host virtual meet-and-greet events for potential students to meet faculty and learn more about the program. In addition, programs should consider having current residents interact virtually with candidates to allow students to better understand the culture of the department and residents’ experiences as trainees in such an environment. For URM students, this is highly important because diversity, inclusion, and antiracism policies and initiatives might not be explicitly available on the institution’s website or residency information page.
Organizations Championing Diversity
Recently, multiple dermatology societies and organizations have been emphasizing the need for diversity and inclusion as well as promoting holistic application review. The American Academy of Dermatology pioneered the Diversity Champion Workshop in 2019 and continues to offer the Diversity Mentorship program, connecting URM students to mentors nationally. The Skin of Color Society offers yearly grants and awards to medical students to develop mentorship and research, and recently hosted webinars to guide medical students and residency programs on diversity and inclusion, residency application and review, and COVID-19 virtual interviews. Other national societies, such as the Student National Medical Association and Latino Medical Student Association, have been promoting workshops and interview mentoring for URM students, including dermatology-specific events. Although it is estimated that more than 90% of medical schools in the United States already perform holistic application review and that such review has been adopted by many dermatology programs nationwide, data regarding dermatology residency programs’ implementation of holistic application review are lacking.8
In addition, we encourage continuation of the proposed coordinated interview invite release from the Association of Professors of Dermatology, which was implemented in the 2020-2021 cycle. In light of the recent AAMC letter9 on the maldistribution of interview invitations to highest-tier applicants, coordination of interview release dates and other similar initiatives to prevent programs from offering more invites than their available slots and improve transparency about interview days are needed. Furthermore, continuing to offer optional virtual interviews for applicants in future cycles could make the process less cost-prohibitive for many URM students.4,5
Final Thoughts
Dermatology residency programs must intentionally guard against falling back to traditional standards of assessment as the only means of student evaluation, especially in this virtual era. It is our responsibility to remove artificial barriers that continue to stall progress in diversity, inclusion, equity, and belonging in dermatology.
- Underrepresented in medicine definition. Association of American Medical Colleges website. Accessed September 27, 2021. https://www.aamc.org/what-we-do/mission-areas/diversity-inclusion/underrepresented-in-medicine
- Diversity in medicine: facts and figures 2019. table 13. practice specialty, males by race/ethnicity, 2018. Association of American Medical Colleges website. Accessed September 27, 2021. https://www.aamc.org/data-reports/workforce/data/table-13-practice-specialty-males-race/ethnicity-2018 1B
- US Census Bureau. Quick facts: United States. Updated July 1, 2019. Accessed September 20, 2021. https://www.census.gov/quickfacts/fact/table/US/PST045219
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by underrepresented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatology residency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001/jamadermatol.2018.4813
- Williams C, Kwan B, Pereira A, et al. A call to improve conditions for conducting holistic review in graduate medical education recruitment. MedEdPublish. 2019;8:6. https://doi.org/10.15694/mep.2019.000076.1
- Holistic principles in resident selection: an introduction. Association of American Medical Colleges website. Accessed September 27, 2021. https://www.aamc.org/system/files/2020-08/aa-member-capacity-building-holistic-review-transcript-activities-GME-081420.pdf
- Luke J, Cornelius L, Lim H. Dermatology resident selection: shifting toward holistic review? J Am Acad Dermatol. 2020;84:1208-1209. doi:10.1016/j.jaad.2020.11.025
- Open letter on residency interviews from Alison Whelan, MD, AAMC Chief Medical Education Officer. Association of American Medical Colleges website. Published December 18, 2020. Accessed September 27, 2021. https://www.aamc.org/media/50291/download
The COVID-19 pandemic has markedly changed the dermatology residency application process. As medical students head into this application cycle, the impacts of systemic racism and deeply rooted structural barriers continue to be exacerbated for students who identify as an underrepresented minority (URM) in medicine—historically defined as those who self-identify as Hispanic or Latinx; Black or African American; American Indian or Alaska Native; or Native Hawaiian or Pacific Islander. The Association of American Medical Colleges (AAMC) defines URMs as racial and ethnic populations that are underrepresented in medicine relative to their numbers in the general population.1 Although these groups account for approximately 34% of the population of the United States, they constitute only 11% of the country’s physician workforce.2,3
Of the total physician workforce in the United States, Black and African American physicians account for 5% of practicing physicians; Hispanic physicians, 5.8%; American Indian and Alaska Native physicians, 0.3%; and Native Hawaiian and Pacific Islander physicians, 0.1%.2 In competitive medical specialties, the disproportionality of these numbers compared to our current demographics in the United States as shown above is even more staggering. In 2018, for example, 10% of practicing dermatologists identified as female URM physicians; 6%, as male URM physicians.2 In this article, we discuss some of the challenges and considerations for URM students applying to dermatology residency in the era of the COVID-19 pandemic.
Barriers for URM Students in Dermatology
Multiple studies have attempted to identify some of the barriers faced by URM students in medicine that might explain the lack of diversity in competitive specialties. Vasquez and colleagues4 identified 4 major factors that play a role in dermatology: lack of equitable resources, lack of support, financial limitations, and the lack of group identity. More than half of URM students surveyed (1) identified lack of support as a barrier and (2) reported having been encouraged to seek a specialty more reflective of their community.4
Soliman et al5 reported that URM barriers in dermatology extend to include lack of diversity in the field, socioeconomic factors, lack of mentorship, and a negative perception of minority students by residency programs. Dermatology is the second least diverse specialty in medicine after orthopedic surgery, which, in and of itself, might further discourage URM students from applying to dermatology.5
With the minimal exposure that URM students have to the field of dermatology, the lack of pipeline programs, and reports that URMs often are encouraged to pursue primary care, the current diversity deficiency in dermatology comes as no surprise. In addition, the substantial disadvantage for URM students is perpetuated by the traditional highly selective process that favors grades, board scores, and honor society status over holistic assessment of the individual student and their unique experiences and potential for contribution.
Looking Beyond Test Scores
The US Medical Licensing Examination (USMLE) traditionally has been used to select dermatology residency applicants, with high cutoff scores often excluding outstanding URM students. Research has suggested that the use of USMLE examination test scores for residency recruitment lacks validity because it has poor predictability of residency performance.6 Although the USMLE Step 1 examination is transitioning to pass/fail scoring, applicants for the next cycle will still have a 3-digit numerical score.
We strongly recommend that dermatology programs transition from emphasizing scores of residency candidates to reviewing each candidate holistically. The AAMC defines “holistic review” as a “flexible, individualized way of assessing an applicant’s capabilities, by which balanced consideration is given to experiences, attributes, competencies, and academic or scholarly metrics and, when considered in combination, how the individual might contribute value to the institution’s mission.”7 Furthermore, we recommend that dermatology residency programs have multiple faculty members review each application, including a representative of the diversity, inclusion, and equity committee.
Applying to Residency in the COVID-19 Virtual Environment
In the COVID-19 era, dermatology externship opportunities that would have allowed URM students to work directly with potential residency programs, showcase their abilities, and network have been limited. Virtual residency interviews could make it more challenging to evaluate candidates, especially URM students from less prestigious programs or unusual socioeconomic backgrounds, or with lower board scores. In addition, virtual interviews can more easily become one-dimensional, depriving URM students of the opportunity to gauge their personal fit in a specific dermatology residency program and its community. Questions and concerns of URM students might include: Will I be appropriately supported and mentored? Will my cultural preferences, religion, sexual preference, hairstyle, and beliefs be accepted? Can I advocate for minorities and support antiracism and diversity and inclusion initiatives? To that end, we recommend that dermatology programs continue to host virtual meet-and-greet events for potential students to meet faculty and learn more about the program. In addition, programs should consider having current residents interact virtually with candidates to allow students to better understand the culture of the department and residents’ experiences as trainees in such an environment. For URM students, this is highly important because diversity, inclusion, and antiracism policies and initiatives might not be explicitly available on the institution’s website or residency information page.
Organizations Championing Diversity
Recently, multiple dermatology societies and organizations have been emphasizing the need for diversity and inclusion as well as promoting holistic application review. The American Academy of Dermatology pioneered the Diversity Champion Workshop in 2019 and continues to offer the Diversity Mentorship program, connecting URM students to mentors nationally. The Skin of Color Society offers yearly grants and awards to medical students to develop mentorship and research, and recently hosted webinars to guide medical students and residency programs on diversity and inclusion, residency application and review, and COVID-19 virtual interviews. Other national societies, such as the Student National Medical Association and Latino Medical Student Association, have been promoting workshops and interview mentoring for URM students, including dermatology-specific events. Although it is estimated that more than 90% of medical schools in the United States already perform holistic application review and that such review has been adopted by many dermatology programs nationwide, data regarding dermatology residency programs’ implementation of holistic application review are lacking.8
In addition, we encourage continuation of the proposed coordinated interview invite release from the Association of Professors of Dermatology, which was implemented in the 2020-2021 cycle. In light of the recent AAMC letter9 on the maldistribution of interview invitations to highest-tier applicants, coordination of interview release dates and other similar initiatives to prevent programs from offering more invites than their available slots and improve transparency about interview days are needed. Furthermore, continuing to offer optional virtual interviews for applicants in future cycles could make the process less cost-prohibitive for many URM students.4,5
Final Thoughts
Dermatology residency programs must intentionally guard against falling back to traditional standards of assessment as the only means of student evaluation, especially in this virtual era. It is our responsibility to remove artificial barriers that continue to stall progress in diversity, inclusion, equity, and belonging in dermatology.
The COVID-19 pandemic has markedly changed the dermatology residency application process. As medical students head into this application cycle, the impacts of systemic racism and deeply rooted structural barriers continue to be exacerbated for students who identify as an underrepresented minority (URM) in medicine—historically defined as those who self-identify as Hispanic or Latinx; Black or African American; American Indian or Alaska Native; or Native Hawaiian or Pacific Islander. The Association of American Medical Colleges (AAMC) defines URMs as racial and ethnic populations that are underrepresented in medicine relative to their numbers in the general population.1 Although these groups account for approximately 34% of the population of the United States, they constitute only 11% of the country’s physician workforce.2,3
Of the total physician workforce in the United States, Black and African American physicians account for 5% of practicing physicians; Hispanic physicians, 5.8%; American Indian and Alaska Native physicians, 0.3%; and Native Hawaiian and Pacific Islander physicians, 0.1%.2 In competitive medical specialties, the disproportionality of these numbers compared to our current demographics in the United States as shown above is even more staggering. In 2018, for example, 10% of practicing dermatologists identified as female URM physicians; 6%, as male URM physicians.2 In this article, we discuss some of the challenges and considerations for URM students applying to dermatology residency in the era of the COVID-19 pandemic.
Barriers for URM Students in Dermatology
Multiple studies have attempted to identify some of the barriers faced by URM students in medicine that might explain the lack of diversity in competitive specialties. Vasquez and colleagues4 identified 4 major factors that play a role in dermatology: lack of equitable resources, lack of support, financial limitations, and the lack of group identity. More than half of URM students surveyed (1) identified lack of support as a barrier and (2) reported having been encouraged to seek a specialty more reflective of their community.4
Soliman et al5 reported that URM barriers in dermatology extend to include lack of diversity in the field, socioeconomic factors, lack of mentorship, and a negative perception of minority students by residency programs. Dermatology is the second least diverse specialty in medicine after orthopedic surgery, which, in and of itself, might further discourage URM students from applying to dermatology.5
With the minimal exposure that URM students have to the field of dermatology, the lack of pipeline programs, and reports that URMs often are encouraged to pursue primary care, the current diversity deficiency in dermatology comes as no surprise. In addition, the substantial disadvantage for URM students is perpetuated by the traditional highly selective process that favors grades, board scores, and honor society status over holistic assessment of the individual student and their unique experiences and potential for contribution.
Looking Beyond Test Scores
The US Medical Licensing Examination (USMLE) traditionally has been used to select dermatology residency applicants, with high cutoff scores often excluding outstanding URM students. Research has suggested that the use of USMLE examination test scores for residency recruitment lacks validity because it has poor predictability of residency performance.6 Although the USMLE Step 1 examination is transitioning to pass/fail scoring, applicants for the next cycle will still have a 3-digit numerical score.
We strongly recommend that dermatology programs transition from emphasizing scores of residency candidates to reviewing each candidate holistically. The AAMC defines “holistic review” as a “flexible, individualized way of assessing an applicant’s capabilities, by which balanced consideration is given to experiences, attributes, competencies, and academic or scholarly metrics and, when considered in combination, how the individual might contribute value to the institution’s mission.”7 Furthermore, we recommend that dermatology residency programs have multiple faculty members review each application, including a representative of the diversity, inclusion, and equity committee.
Applying to Residency in the COVID-19 Virtual Environment
In the COVID-19 era, dermatology externship opportunities that would have allowed URM students to work directly with potential residency programs, showcase their abilities, and network have been limited. Virtual residency interviews could make it more challenging to evaluate candidates, especially URM students from less prestigious programs or unusual socioeconomic backgrounds, or with lower board scores. In addition, virtual interviews can more easily become one-dimensional, depriving URM students of the opportunity to gauge their personal fit in a specific dermatology residency program and its community. Questions and concerns of URM students might include: Will I be appropriately supported and mentored? Will my cultural preferences, religion, sexual preference, hairstyle, and beliefs be accepted? Can I advocate for minorities and support antiracism and diversity and inclusion initiatives? To that end, we recommend that dermatology programs continue to host virtual meet-and-greet events for potential students to meet faculty and learn more about the program. In addition, programs should consider having current residents interact virtually with candidates to allow students to better understand the culture of the department and residents’ experiences as trainees in such an environment. For URM students, this is highly important because diversity, inclusion, and antiracism policies and initiatives might not be explicitly available on the institution’s website or residency information page.
Organizations Championing Diversity
Recently, multiple dermatology societies and organizations have been emphasizing the need for diversity and inclusion as well as promoting holistic application review. The American Academy of Dermatology pioneered the Diversity Champion Workshop in 2019 and continues to offer the Diversity Mentorship program, connecting URM students to mentors nationally. The Skin of Color Society offers yearly grants and awards to medical students to develop mentorship and research, and recently hosted webinars to guide medical students and residency programs on diversity and inclusion, residency application and review, and COVID-19 virtual interviews. Other national societies, such as the Student National Medical Association and Latino Medical Student Association, have been promoting workshops and interview mentoring for URM students, including dermatology-specific events. Although it is estimated that more than 90% of medical schools in the United States already perform holistic application review and that such review has been adopted by many dermatology programs nationwide, data regarding dermatology residency programs’ implementation of holistic application review are lacking.8
In addition, we encourage continuation of the proposed coordinated interview invite release from the Association of Professors of Dermatology, which was implemented in the 2020-2021 cycle. In light of the recent AAMC letter9 on the maldistribution of interview invitations to highest-tier applicants, coordination of interview release dates and other similar initiatives to prevent programs from offering more invites than their available slots and improve transparency about interview days are needed. Furthermore, continuing to offer optional virtual interviews for applicants in future cycles could make the process less cost-prohibitive for many URM students.4,5
Final Thoughts
Dermatology residency programs must intentionally guard against falling back to traditional standards of assessment as the only means of student evaluation, especially in this virtual era. It is our responsibility to remove artificial barriers that continue to stall progress in diversity, inclusion, equity, and belonging in dermatology.
- Underrepresented in medicine definition. Association of American Medical Colleges website. Accessed September 27, 2021. https://www.aamc.org/what-we-do/mission-areas/diversity-inclusion/underrepresented-in-medicine
- Diversity in medicine: facts and figures 2019. table 13. practice specialty, males by race/ethnicity, 2018. Association of American Medical Colleges website. Accessed September 27, 2021. https://www.aamc.org/data-reports/workforce/data/table-13-practice-specialty-males-race/ethnicity-2018 1B
- US Census Bureau. Quick facts: United States. Updated July 1, 2019. Accessed September 20, 2021. https://www.census.gov/quickfacts/fact/table/US/PST045219
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by underrepresented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatology residency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001/jamadermatol.2018.4813
- Williams C, Kwan B, Pereira A, et al. A call to improve conditions for conducting holistic review in graduate medical education recruitment. MedEdPublish. 2019;8:6. https://doi.org/10.15694/mep.2019.000076.1
- Holistic principles in resident selection: an introduction. Association of American Medical Colleges website. Accessed September 27, 2021. https://www.aamc.org/system/files/2020-08/aa-member-capacity-building-holistic-review-transcript-activities-GME-081420.pdf
- Luke J, Cornelius L, Lim H. Dermatology resident selection: shifting toward holistic review? J Am Acad Dermatol. 2020;84:1208-1209. doi:10.1016/j.jaad.2020.11.025
- Open letter on residency interviews from Alison Whelan, MD, AAMC Chief Medical Education Officer. Association of American Medical Colleges website. Published December 18, 2020. Accessed September 27, 2021. https://www.aamc.org/media/50291/download
- Underrepresented in medicine definition. Association of American Medical Colleges website. Accessed September 27, 2021. https://www.aamc.org/what-we-do/mission-areas/diversity-inclusion/underrepresented-in-medicine
- Diversity in medicine: facts and figures 2019. table 13. practice specialty, males by race/ethnicity, 2018. Association of American Medical Colleges website. Accessed September 27, 2021. https://www.aamc.org/data-reports/workforce/data/table-13-practice-specialty-males-race/ethnicity-2018 1B
- US Census Bureau. Quick facts: United States. Updated July 1, 2019. Accessed September 20, 2021. https://www.census.gov/quickfacts/fact/table/US/PST045219
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by underrepresented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatology residency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001/jamadermatol.2018.4813
- Williams C, Kwan B, Pereira A, et al. A call to improve conditions for conducting holistic review in graduate medical education recruitment. MedEdPublish. 2019;8:6. https://doi.org/10.15694/mep.2019.000076.1
- Holistic principles in resident selection: an introduction. Association of American Medical Colleges website. Accessed September 27, 2021. https://www.aamc.org/system/files/2020-08/aa-member-capacity-building-holistic-review-transcript-activities-GME-081420.pdf
- Luke J, Cornelius L, Lim H. Dermatology resident selection: shifting toward holistic review? J Am Acad Dermatol. 2020;84:1208-1209. doi:10.1016/j.jaad.2020.11.025
- Open letter on residency interviews from Alison Whelan, MD, AAMC Chief Medical Education Officer. Association of American Medical Colleges website. Published December 18, 2020. Accessed September 27, 2021. https://www.aamc.org/media/50291/download
Practice Points
- Dermatology remains one of the least diverse medical specialties.
- Underrepresented minority (URM) in medicine residency applicants might be negatively affected by the COVID-19 pandemic.
- The implementation of holistic review, diversity and inclusion initiatives, and virtual opportunities might mitigate some of the barriers faced by URM applicants.
Anxiety, depression symptoms rose and fell with new COVID cases
Anxiety and depression symptoms increased in adults last winter as COVID-19 surged in the United States but declined in the spring as COVID activity approached its nadir, according to an analysis from the Centers for Disease Control and Prevention.
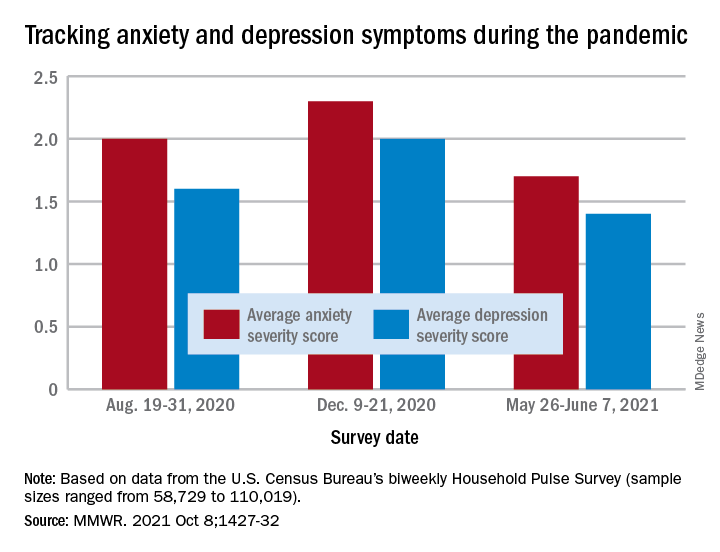
“The relative increases and decreases in frequency of reported symptoms of anxiety and depression at both the national and state levels mirrored the national weekly number of new COVID-19 cases during the same period,” Haomiao Jia, PhD, and associates wrote in the Morbidity and Mortality Weekly Report.
In a national survey conducted Aug. 19-31, 2020, the average anxiety severity score was 2.0 and the average depression score was 1.6 among adults in all 50 states. Those scores rose to 2.3 (+13.0%) and 2.0 (+14.8%), respectively, by Dec. 9-21, but then fell to 1.7 (–26.8%) and 1.4 (–24.8%) during the survey conducted from May 26 to June 7, 2021, the investigators reported.
Despite that decrease in the spring, however, “the frequency of symptoms ... in June 2021 remained elevated compared with estimates from” 2019, said Dr. Jia of Columbia University, New York, and associates. Data from the National Health Interview Survey put the prepandemic severity scores at 0.63 for anxiety and 0.51 for depression.
Weekly symptom frequency in the Household Pulse Survey, which began in April 2020, was assessed with the four-item Patient Health Questionnaire, which includes two questions on anxiety and two on depression. Each answer scored on a scale from 0 (no symptoms at all) to 3 (symptoms nearly every day), making a total of 6 possible for each severity score, they explained. Sample sizes for the biweekly surveys ranged from 58,729 to 110,019.
Among the states, there was something of a pattern involving the drop in scores during the fall and the rise over the winter and spring months. “States with larger increases in severity scores during August–December 2020 also tended to have larger decreases during January–June 2021,” the researchers noted.
That group includes Minnesota, Mississippi, South Dakota, and Utah for anxiety and Idaho, Michigan, Minnesota, and Wisconsin for depression, the survey data show.
Florida and New York had the smallest increases in depression and anxiety scores, respectively, from August to December, and New York had the smallest decrease in both anxiety and depression from January to June, Dr. Jia and associates said.
“ during national emergencies. The observed differences in severity score magnitude and peaks across states in this study indicate that these efforts are important at both the national and state levels,” they wrote.
Anxiety and depression symptoms increased in adults last winter as COVID-19 surged in the United States but declined in the spring as COVID activity approached its nadir, according to an analysis from the Centers for Disease Control and Prevention.

“The relative increases and decreases in frequency of reported symptoms of anxiety and depression at both the national and state levels mirrored the national weekly number of new COVID-19 cases during the same period,” Haomiao Jia, PhD, and associates wrote in the Morbidity and Mortality Weekly Report.
In a national survey conducted Aug. 19-31, 2020, the average anxiety severity score was 2.0 and the average depression score was 1.6 among adults in all 50 states. Those scores rose to 2.3 (+13.0%) and 2.0 (+14.8%), respectively, by Dec. 9-21, but then fell to 1.7 (–26.8%) and 1.4 (–24.8%) during the survey conducted from May 26 to June 7, 2021, the investigators reported.
Despite that decrease in the spring, however, “the frequency of symptoms ... in June 2021 remained elevated compared with estimates from” 2019, said Dr. Jia of Columbia University, New York, and associates. Data from the National Health Interview Survey put the prepandemic severity scores at 0.63 for anxiety and 0.51 for depression.
Weekly symptom frequency in the Household Pulse Survey, which began in April 2020, was assessed with the four-item Patient Health Questionnaire, which includes two questions on anxiety and two on depression. Each answer scored on a scale from 0 (no symptoms at all) to 3 (symptoms nearly every day), making a total of 6 possible for each severity score, they explained. Sample sizes for the biweekly surveys ranged from 58,729 to 110,019.
Among the states, there was something of a pattern involving the drop in scores during the fall and the rise over the winter and spring months. “States with larger increases in severity scores during August–December 2020 also tended to have larger decreases during January–June 2021,” the researchers noted.
That group includes Minnesota, Mississippi, South Dakota, and Utah for anxiety and Idaho, Michigan, Minnesota, and Wisconsin for depression, the survey data show.
Florida and New York had the smallest increases in depression and anxiety scores, respectively, from August to December, and New York had the smallest decrease in both anxiety and depression from January to June, Dr. Jia and associates said.
“ during national emergencies. The observed differences in severity score magnitude and peaks across states in this study indicate that these efforts are important at both the national and state levels,” they wrote.
Anxiety and depression symptoms increased in adults last winter as COVID-19 surged in the United States but declined in the spring as COVID activity approached its nadir, according to an analysis from the Centers for Disease Control and Prevention.

“The relative increases and decreases in frequency of reported symptoms of anxiety and depression at both the national and state levels mirrored the national weekly number of new COVID-19 cases during the same period,” Haomiao Jia, PhD, and associates wrote in the Morbidity and Mortality Weekly Report.
In a national survey conducted Aug. 19-31, 2020, the average anxiety severity score was 2.0 and the average depression score was 1.6 among adults in all 50 states. Those scores rose to 2.3 (+13.0%) and 2.0 (+14.8%), respectively, by Dec. 9-21, but then fell to 1.7 (–26.8%) and 1.4 (–24.8%) during the survey conducted from May 26 to June 7, 2021, the investigators reported.
Despite that decrease in the spring, however, “the frequency of symptoms ... in June 2021 remained elevated compared with estimates from” 2019, said Dr. Jia of Columbia University, New York, and associates. Data from the National Health Interview Survey put the prepandemic severity scores at 0.63 for anxiety and 0.51 for depression.
Weekly symptom frequency in the Household Pulse Survey, which began in April 2020, was assessed with the four-item Patient Health Questionnaire, which includes two questions on anxiety and two on depression. Each answer scored on a scale from 0 (no symptoms at all) to 3 (symptoms nearly every day), making a total of 6 possible for each severity score, they explained. Sample sizes for the biweekly surveys ranged from 58,729 to 110,019.
Among the states, there was something of a pattern involving the drop in scores during the fall and the rise over the winter and spring months. “States with larger increases in severity scores during August–December 2020 also tended to have larger decreases during January–June 2021,” the researchers noted.
That group includes Minnesota, Mississippi, South Dakota, and Utah for anxiety and Idaho, Michigan, Minnesota, and Wisconsin for depression, the survey data show.
Florida and New York had the smallest increases in depression and anxiety scores, respectively, from August to December, and New York had the smallest decrease in both anxiety and depression from January to June, Dr. Jia and associates said.
“ during national emergencies. The observed differences in severity score magnitude and peaks across states in this study indicate that these efforts are important at both the national and state levels,” they wrote.
FROM THE MMWR
Autopsy findings reveal venous thromboembolism in patients with COVID-19
Background: Despite the increased mortality rate of the novel coronavirus compared with influenza, little is understood about its pathogenicity. Prior studies have identified D-dimer levels, high Sequential Organ Failure Assessment score, and older age as markers for more severe disease and mortality. The specific cause of death of COVID-19 remains largely unknown.
Study design: Prospective cohort study.
Setting: Single academic center in Germany.
Synopsis: A complete autopsy was performed on 12 consecutive COVID-19 patient deaths at a single center. Seven had evidence of venous thromboembolism (VTE): three with bilateral lower extremity deep venous thrombosis (DVT) and four with massive pulmonary embolism/associated lower-extremity DVT. Prior to death, VTE was suspected clinically in only a single patient.
This small case series piques interest in the potential underrecognized thromboembolic pathology of COVID-19. While not practice changing, this study highlights the importance of hospitalists staying attuned to further studies regarding VTE prophylaxis in COVID-19.
Bottom line: Autopsies of COVID-19 patients revealed a high incidence of thromboembolic events; COVID-19–induced coagulopathy may play an underrecognized role in pathogenesis.
Citation: Wichmann D et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173(4):268-77.
Dr. Fletcher is a hospitalist at the Lexington (Ky.) VA Health Care System.
Background: Despite the increased mortality rate of the novel coronavirus compared with influenza, little is understood about its pathogenicity. Prior studies have identified D-dimer levels, high Sequential Organ Failure Assessment score, and older age as markers for more severe disease and mortality. The specific cause of death of COVID-19 remains largely unknown.
Study design: Prospective cohort study.
Setting: Single academic center in Germany.
Synopsis: A complete autopsy was performed on 12 consecutive COVID-19 patient deaths at a single center. Seven had evidence of venous thromboembolism (VTE): three with bilateral lower extremity deep venous thrombosis (DVT) and four with massive pulmonary embolism/associated lower-extremity DVT. Prior to death, VTE was suspected clinically in only a single patient.
This small case series piques interest in the potential underrecognized thromboembolic pathology of COVID-19. While not practice changing, this study highlights the importance of hospitalists staying attuned to further studies regarding VTE prophylaxis in COVID-19.
Bottom line: Autopsies of COVID-19 patients revealed a high incidence of thromboembolic events; COVID-19–induced coagulopathy may play an underrecognized role in pathogenesis.
Citation: Wichmann D et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173(4):268-77.
Dr. Fletcher is a hospitalist at the Lexington (Ky.) VA Health Care System.
Background: Despite the increased mortality rate of the novel coronavirus compared with influenza, little is understood about its pathogenicity. Prior studies have identified D-dimer levels, high Sequential Organ Failure Assessment score, and older age as markers for more severe disease and mortality. The specific cause of death of COVID-19 remains largely unknown.
Study design: Prospective cohort study.
Setting: Single academic center in Germany.
Synopsis: A complete autopsy was performed on 12 consecutive COVID-19 patient deaths at a single center. Seven had evidence of venous thromboembolism (VTE): three with bilateral lower extremity deep venous thrombosis (DVT) and four with massive pulmonary embolism/associated lower-extremity DVT. Prior to death, VTE was suspected clinically in only a single patient.
This small case series piques interest in the potential underrecognized thromboembolic pathology of COVID-19. While not practice changing, this study highlights the importance of hospitalists staying attuned to further studies regarding VTE prophylaxis in COVID-19.
Bottom line: Autopsies of COVID-19 patients revealed a high incidence of thromboembolic events; COVID-19–induced coagulopathy may play an underrecognized role in pathogenesis.
Citation: Wichmann D et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173(4):268-77.
Dr. Fletcher is a hospitalist at the Lexington (Ky.) VA Health Care System.
Hundreds of thousands of U.S. troops remain unvaccinated
, according to data analyzed by The Washington Post.
Overall, the military’s vaccination rate has climbed since August, when the Pentagon announced that COVID-19 immunization would become mandatory for the nation’s 2.1 million troops, the newspaper reported. Acting on a directive from President Joe Biden, leaders said exemptions would be rare and unvaccinated service members would face consequences.
But compliance has varied across the services, the newspaper found. About 90% of the active-duty Navy is fully vaccinated, compared with 76% of the active-duty Marine Corps. Both have a November 28 deadline to show proof of full vaccination.
About 81% of the active-duty Air Force is fully vaccinated, leaving more than 60,000 personnel about 3 weeks to get a shot before a November 2 deadline.
Military officials said the variance in vaccination rates is related to the different deadlines, the newspaper reported. As the dates approach, most troops are expected to meet the order.
At the same time, some deadlines are spaced farther out, with the Army Reserve and National Guard required to be fully vaccinated by next summer. About 40% of the Army Reserve and 38% of the National Guard are fully vaccinated. Combined, they account for a quarter of the U.S. military and 40% of the COVID-19 deaths among service members.
“The Army’s policy is incentivizing inaction until the latest possible date,” Katherine Kuzminski, a military policy expert at the Center for a New American Security, told the Post.“The way we’ve seen the virus evolve tells us looking out to June 30 may need to be reconsidered,” she said.
COVID-19 deaths have surged in some of the services in recent months, the newspaper reported. More military personnel died from COVID-19 in September than in all of 2020. None of those who died were fully vaccinated.
Throughout the pandemic, more than 246,000 COVID-19 cases have been reported among service members, according to the latest data from the Defense Department. More than 2,200 have been hospitalized, and 62 personnel died, including 32 in August and September.
For the Army Reserve and National Guard, the June deadline allows “necessary time to update records and process exemption requests,” Lt. Col. Terence Kelley, an Army spokesman, told the Post.
Lt. Col. Kelley noted that the extended date reflects how large the groups are, compared to the other services and their military reserves, as well as the broad geographic distribution of its members. Still, vaccine mandates will apply.
“We expect all unvaccinated soldiers to receive the vaccine as soon as possible,” Lt. Col. Kelley said. “Individual soldiers are required to receive the vaccine when available.”
A version of this article first appeared on Medscape.com.
, according to data analyzed by The Washington Post.
Overall, the military’s vaccination rate has climbed since August, when the Pentagon announced that COVID-19 immunization would become mandatory for the nation’s 2.1 million troops, the newspaper reported. Acting on a directive from President Joe Biden, leaders said exemptions would be rare and unvaccinated service members would face consequences.
But compliance has varied across the services, the newspaper found. About 90% of the active-duty Navy is fully vaccinated, compared with 76% of the active-duty Marine Corps. Both have a November 28 deadline to show proof of full vaccination.
About 81% of the active-duty Air Force is fully vaccinated, leaving more than 60,000 personnel about 3 weeks to get a shot before a November 2 deadline.
Military officials said the variance in vaccination rates is related to the different deadlines, the newspaper reported. As the dates approach, most troops are expected to meet the order.
At the same time, some deadlines are spaced farther out, with the Army Reserve and National Guard required to be fully vaccinated by next summer. About 40% of the Army Reserve and 38% of the National Guard are fully vaccinated. Combined, they account for a quarter of the U.S. military and 40% of the COVID-19 deaths among service members.
“The Army’s policy is incentivizing inaction until the latest possible date,” Katherine Kuzminski, a military policy expert at the Center for a New American Security, told the Post.“The way we’ve seen the virus evolve tells us looking out to June 30 may need to be reconsidered,” she said.
COVID-19 deaths have surged in some of the services in recent months, the newspaper reported. More military personnel died from COVID-19 in September than in all of 2020. None of those who died were fully vaccinated.
Throughout the pandemic, more than 246,000 COVID-19 cases have been reported among service members, according to the latest data from the Defense Department. More than 2,200 have been hospitalized, and 62 personnel died, including 32 in August and September.
For the Army Reserve and National Guard, the June deadline allows “necessary time to update records and process exemption requests,” Lt. Col. Terence Kelley, an Army spokesman, told the Post.
Lt. Col. Kelley noted that the extended date reflects how large the groups are, compared to the other services and their military reserves, as well as the broad geographic distribution of its members. Still, vaccine mandates will apply.
“We expect all unvaccinated soldiers to receive the vaccine as soon as possible,” Lt. Col. Kelley said. “Individual soldiers are required to receive the vaccine when available.”
A version of this article first appeared on Medscape.com.
, according to data analyzed by The Washington Post.
Overall, the military’s vaccination rate has climbed since August, when the Pentagon announced that COVID-19 immunization would become mandatory for the nation’s 2.1 million troops, the newspaper reported. Acting on a directive from President Joe Biden, leaders said exemptions would be rare and unvaccinated service members would face consequences.
But compliance has varied across the services, the newspaper found. About 90% of the active-duty Navy is fully vaccinated, compared with 76% of the active-duty Marine Corps. Both have a November 28 deadline to show proof of full vaccination.
About 81% of the active-duty Air Force is fully vaccinated, leaving more than 60,000 personnel about 3 weeks to get a shot before a November 2 deadline.
Military officials said the variance in vaccination rates is related to the different deadlines, the newspaper reported. As the dates approach, most troops are expected to meet the order.
At the same time, some deadlines are spaced farther out, with the Army Reserve and National Guard required to be fully vaccinated by next summer. About 40% of the Army Reserve and 38% of the National Guard are fully vaccinated. Combined, they account for a quarter of the U.S. military and 40% of the COVID-19 deaths among service members.
“The Army’s policy is incentivizing inaction until the latest possible date,” Katherine Kuzminski, a military policy expert at the Center for a New American Security, told the Post.“The way we’ve seen the virus evolve tells us looking out to June 30 may need to be reconsidered,” she said.
COVID-19 deaths have surged in some of the services in recent months, the newspaper reported. More military personnel died from COVID-19 in September than in all of 2020. None of those who died were fully vaccinated.
Throughout the pandemic, more than 246,000 COVID-19 cases have been reported among service members, according to the latest data from the Defense Department. More than 2,200 have been hospitalized, and 62 personnel died, including 32 in August and September.
For the Army Reserve and National Guard, the June deadline allows “necessary time to update records and process exemption requests,” Lt. Col. Terence Kelley, an Army spokesman, told the Post.
Lt. Col. Kelley noted that the extended date reflects how large the groups are, compared to the other services and their military reserves, as well as the broad geographic distribution of its members. Still, vaccine mandates will apply.
“We expect all unvaccinated soldiers to receive the vaccine as soon as possible,” Lt. Col. Kelley said. “Individual soldiers are required to receive the vaccine when available.”
A version of this article first appeared on Medscape.com.
Effect of COVID-19 pandemic on respiratory infectious diseases in primary care practice
A secondary consequence of public health measures to prevent the spread of SARS-CoV-2 included a concurrent reduction in risk for children to acquire and spread other respiratory viral infectious diseases. In the Rochester, N.Y., area, we had an ongoing prospective study in primary care pediatric practices that afforded an opportunity to assess the effect of the pandemic control measures on all infectious disease illness visits in young children. Specifically, in children aged 6-36 months old, our study was in place when the pandemic began with a primary objective to evaluate the changing epidemiology of acute otitis media (AOM) and nasopharyngeal colonization by potential bacterial respiratory pathogens in community-based primary care pediatric practices. As the public health measures mandated by New York State Department of Health were implemented, we prospectively quantified their effect on physician-diagnosed infectious disease illness visits. The incidence of infectious disease visits by a cohort of young children during the COVID-19 pandemic period March 15, 2020, through Dec. 31, 2020, was compared with the same time frame in the preceding year, 2019.1
Recommendations of the New York State Department of Health for public health, changes in school and day care attendance, and clinical practice during the study time frame
On March 7, 2020, a state of emergency was declared in New York because of the COVID-19 pandemic. All schools were required to close. A mandated order for public use of masks in adults and children more than 2 years of age was enacted. In the Finger Lakes region of Upstate New York, where the two primary care pediatric practices reside, complete lockdown was partially lifted on May 15, 2020, and further lifted on June 26, 2020. Almost all regional school districts opened to at least hybrid learning models for all students starting Sept. 8, 2020. On March 6, 2020, video telehealth and telephone call visits were introduced as routine practice. Well-child visits were limited to those less than 2 years of age, then gradually expanded to all ages by late May 2020. During the “stay at home” phase of the New York State lockdown, day care services were considered an essential business. Day care child density was limited. All children less than 2 years old were required to wear a mask while in the facility. Upon arrival, children with any respiratory symptoms or fever were excluded. For the school year commencing September 2020, almost all regional school districts opened to virtual, hybrid, or in-person learning models. Exclusion occurred similar to that of the day care facilities.
Incidence of respiratory infectious disease illnesses
Clinical diagnoses and healthy visits of 144 children from March 15 to Dec. 31, 2020 (beginning of the pandemic) were compared to 215 children during the same months in 2019 (prepandemic). Pediatric SARS-CoV-2 positivity rates trended up alongside community spread. Pediatric practice positivity rates rose from 1.9% in October 2020 to 19% in December 2020.
The table shows the incidence of significantly different infectious disease illness visits in the two study cohorts.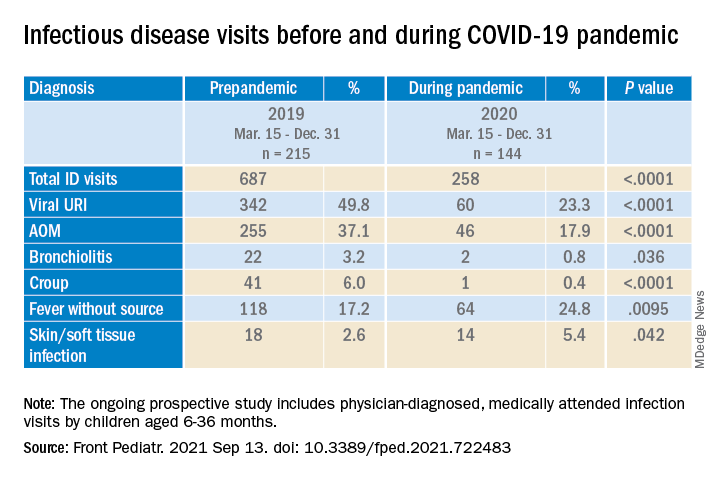
During the pandemic, 258 infection visits occurred among 144 pandemic cohort children, compared with 687 visits among 215 prepandemic cohort children, a 1.8-fold decrease (P < .0001). The proportion of children with visits for AOM (3.7-fold; P < .0001), bronchiolitis (7.4-fold; P = .036), croup (27.5-fold; P < .0001), and viral upper respiratory infection (3.8-fold; P < .0001) decreased significantly. Fever without a source (1.4-fold decrease; P = .009) and skin/soft tissue infection (2.1-fold decrease; P = .042) represented a higher proportion of visits during the pandemic.
Prescription of antibiotics significantly decreased (P < .001) during the pandemic.
Change in care practices
In the prepandemic period, virtual visits, leading to a diagnosis and treatment and referring children to an urgent care or hospital emergency department during regular office hours were rare. During the pandemic, this changed. Significantly increased use of telemedicine visits (P < .0001) and significantly decreased office and urgent care visits (P < .0001) occurred during the pandemic. Telehealth visits peaked the week of April 12, 2020, at 45% of all pediatric visits. In-person illness visits gradually returned to year-to-year volumes in August-September 2020 with school opening. Early in the pandemic, both pediatric offices limited patient encounters to well-child visits in the first 2 years of life to not miss opportunities for childhood vaccinations. However, some parents were reluctant to bring their children to those visits. There was no significant change in frequency of healthy child visits during the pandemic.
To our knowledge, this was the first study from primary care pediatric practices in the United States to analyze the effect on infectious diseases during the first 9 months of the pandemic, including the 6-month time period after the reopening from the first 3 months of lockdown. One prior study from a primary care network in Massachusetts reported significant decreases in respiratory infectious diseases for children aged 0-17 years during the first months of the pandemic during lockdown.2 A study in Tennessee that included hospital emergency department, urgent care, primary care, and retail health clinics also reported respiratory infection diagnoses as well as antibiotic prescription were reduced in the early months of the pandemic.3
Our study shows an overall reduction in frequency of respiratory illness visits in children 6-36 months old during the first 9 months of the COVID-19 pandemic. We learned the value of using technology in the form of virtual visits to render care. Perhaps as the pandemic subsides, many of the hand-washing and sanitizing practices will remain in place and lead to less frequent illness in children in the future. However, there may be temporary negative consequences from the “immune debt” that has occurred from a prolonged time span when children were not becoming infected with respiratory pathogens.4 We will see what unfolds in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. Dr. Schulz is pediatric medical director at Rochester (N.Y.) Regional Health. Dr. Pichichero and Dr. Schulz have no conflicts of interest to disclose. This study was funded in part by the Centers for Disease Control and Prevention.
References
1. Kaur R et al. Front Pediatr. 2021;(9)722483:1-8.
2. Hatoun J et al. Pediatrics. 2020;146(4):e2020006460.
3. Katz SE et al. J Pediatric Infect Dis Soc. 2021;10(1):62-4.
4. Cohen R et al. Infect. Dis Now. 2021; 51(5)418-23.
A secondary consequence of public health measures to prevent the spread of SARS-CoV-2 included a concurrent reduction in risk for children to acquire and spread other respiratory viral infectious diseases. In the Rochester, N.Y., area, we had an ongoing prospective study in primary care pediatric practices that afforded an opportunity to assess the effect of the pandemic control measures on all infectious disease illness visits in young children. Specifically, in children aged 6-36 months old, our study was in place when the pandemic began with a primary objective to evaluate the changing epidemiology of acute otitis media (AOM) and nasopharyngeal colonization by potential bacterial respiratory pathogens in community-based primary care pediatric practices. As the public health measures mandated by New York State Department of Health were implemented, we prospectively quantified their effect on physician-diagnosed infectious disease illness visits. The incidence of infectious disease visits by a cohort of young children during the COVID-19 pandemic period March 15, 2020, through Dec. 31, 2020, was compared with the same time frame in the preceding year, 2019.1
Recommendations of the New York State Department of Health for public health, changes in school and day care attendance, and clinical practice during the study time frame
On March 7, 2020, a state of emergency was declared in New York because of the COVID-19 pandemic. All schools were required to close. A mandated order for public use of masks in adults and children more than 2 years of age was enacted. In the Finger Lakes region of Upstate New York, where the two primary care pediatric practices reside, complete lockdown was partially lifted on May 15, 2020, and further lifted on June 26, 2020. Almost all regional school districts opened to at least hybrid learning models for all students starting Sept. 8, 2020. On March 6, 2020, video telehealth and telephone call visits were introduced as routine practice. Well-child visits were limited to those less than 2 years of age, then gradually expanded to all ages by late May 2020. During the “stay at home” phase of the New York State lockdown, day care services were considered an essential business. Day care child density was limited. All children less than 2 years old were required to wear a mask while in the facility. Upon arrival, children with any respiratory symptoms or fever were excluded. For the school year commencing September 2020, almost all regional school districts opened to virtual, hybrid, or in-person learning models. Exclusion occurred similar to that of the day care facilities.
Incidence of respiratory infectious disease illnesses
Clinical diagnoses and healthy visits of 144 children from March 15 to Dec. 31, 2020 (beginning of the pandemic) were compared to 215 children during the same months in 2019 (prepandemic). Pediatric SARS-CoV-2 positivity rates trended up alongside community spread. Pediatric practice positivity rates rose from 1.9% in October 2020 to 19% in December 2020.
The table shows the incidence of significantly different infectious disease illness visits in the two study cohorts.
During the pandemic, 258 infection visits occurred among 144 pandemic cohort children, compared with 687 visits among 215 prepandemic cohort children, a 1.8-fold decrease (P < .0001). The proportion of children with visits for AOM (3.7-fold; P < .0001), bronchiolitis (7.4-fold; P = .036), croup (27.5-fold; P < .0001), and viral upper respiratory infection (3.8-fold; P < .0001) decreased significantly. Fever without a source (1.4-fold decrease; P = .009) and skin/soft tissue infection (2.1-fold decrease; P = .042) represented a higher proportion of visits during the pandemic.
Prescription of antibiotics significantly decreased (P < .001) during the pandemic.
Change in care practices
In the prepandemic period, virtual visits, leading to a diagnosis and treatment and referring children to an urgent care or hospital emergency department during regular office hours were rare. During the pandemic, this changed. Significantly increased use of telemedicine visits (P < .0001) and significantly decreased office and urgent care visits (P < .0001) occurred during the pandemic. Telehealth visits peaked the week of April 12, 2020, at 45% of all pediatric visits. In-person illness visits gradually returned to year-to-year volumes in August-September 2020 with school opening. Early in the pandemic, both pediatric offices limited patient encounters to well-child visits in the first 2 years of life to not miss opportunities for childhood vaccinations. However, some parents were reluctant to bring their children to those visits. There was no significant change in frequency of healthy child visits during the pandemic.
To our knowledge, this was the first study from primary care pediatric practices in the United States to analyze the effect on infectious diseases during the first 9 months of the pandemic, including the 6-month time period after the reopening from the first 3 months of lockdown. One prior study from a primary care network in Massachusetts reported significant decreases in respiratory infectious diseases for children aged 0-17 years during the first months of the pandemic during lockdown.2 A study in Tennessee that included hospital emergency department, urgent care, primary care, and retail health clinics also reported respiratory infection diagnoses as well as antibiotic prescription were reduced in the early months of the pandemic.3
Our study shows an overall reduction in frequency of respiratory illness visits in children 6-36 months old during the first 9 months of the COVID-19 pandemic. We learned the value of using technology in the form of virtual visits to render care. Perhaps as the pandemic subsides, many of the hand-washing and sanitizing practices will remain in place and lead to less frequent illness in children in the future. However, there may be temporary negative consequences from the “immune debt” that has occurred from a prolonged time span when children were not becoming infected with respiratory pathogens.4 We will see what unfolds in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. Dr. Schulz is pediatric medical director at Rochester (N.Y.) Regional Health. Dr. Pichichero and Dr. Schulz have no conflicts of interest to disclose. This study was funded in part by the Centers for Disease Control and Prevention.
References
1. Kaur R et al. Front Pediatr. 2021;(9)722483:1-8.
2. Hatoun J et al. Pediatrics. 2020;146(4):e2020006460.
3. Katz SE et al. J Pediatric Infect Dis Soc. 2021;10(1):62-4.
4. Cohen R et al. Infect. Dis Now. 2021; 51(5)418-23.
A secondary consequence of public health measures to prevent the spread of SARS-CoV-2 included a concurrent reduction in risk for children to acquire and spread other respiratory viral infectious diseases. In the Rochester, N.Y., area, we had an ongoing prospective study in primary care pediatric practices that afforded an opportunity to assess the effect of the pandemic control measures on all infectious disease illness visits in young children. Specifically, in children aged 6-36 months old, our study was in place when the pandemic began with a primary objective to evaluate the changing epidemiology of acute otitis media (AOM) and nasopharyngeal colonization by potential bacterial respiratory pathogens in community-based primary care pediatric practices. As the public health measures mandated by New York State Department of Health were implemented, we prospectively quantified their effect on physician-diagnosed infectious disease illness visits. The incidence of infectious disease visits by a cohort of young children during the COVID-19 pandemic period March 15, 2020, through Dec. 31, 2020, was compared with the same time frame in the preceding year, 2019.1
Recommendations of the New York State Department of Health for public health, changes in school and day care attendance, and clinical practice during the study time frame
On March 7, 2020, a state of emergency was declared in New York because of the COVID-19 pandemic. All schools were required to close. A mandated order for public use of masks in adults and children more than 2 years of age was enacted. In the Finger Lakes region of Upstate New York, where the two primary care pediatric practices reside, complete lockdown was partially lifted on May 15, 2020, and further lifted on June 26, 2020. Almost all regional school districts opened to at least hybrid learning models for all students starting Sept. 8, 2020. On March 6, 2020, video telehealth and telephone call visits were introduced as routine practice. Well-child visits were limited to those less than 2 years of age, then gradually expanded to all ages by late May 2020. During the “stay at home” phase of the New York State lockdown, day care services were considered an essential business. Day care child density was limited. All children less than 2 years old were required to wear a mask while in the facility. Upon arrival, children with any respiratory symptoms or fever were excluded. For the school year commencing September 2020, almost all regional school districts opened to virtual, hybrid, or in-person learning models. Exclusion occurred similar to that of the day care facilities.
Incidence of respiratory infectious disease illnesses
Clinical diagnoses and healthy visits of 144 children from March 15 to Dec. 31, 2020 (beginning of the pandemic) were compared to 215 children during the same months in 2019 (prepandemic). Pediatric SARS-CoV-2 positivity rates trended up alongside community spread. Pediatric practice positivity rates rose from 1.9% in October 2020 to 19% in December 2020.
The table shows the incidence of significantly different infectious disease illness visits in the two study cohorts.
During the pandemic, 258 infection visits occurred among 144 pandemic cohort children, compared with 687 visits among 215 prepandemic cohort children, a 1.8-fold decrease (P < .0001). The proportion of children with visits for AOM (3.7-fold; P < .0001), bronchiolitis (7.4-fold; P = .036), croup (27.5-fold; P < .0001), and viral upper respiratory infection (3.8-fold; P < .0001) decreased significantly. Fever without a source (1.4-fold decrease; P = .009) and skin/soft tissue infection (2.1-fold decrease; P = .042) represented a higher proportion of visits during the pandemic.
Prescription of antibiotics significantly decreased (P < .001) during the pandemic.
Change in care practices
In the prepandemic period, virtual visits, leading to a diagnosis and treatment and referring children to an urgent care or hospital emergency department during regular office hours were rare. During the pandemic, this changed. Significantly increased use of telemedicine visits (P < .0001) and significantly decreased office and urgent care visits (P < .0001) occurred during the pandemic. Telehealth visits peaked the week of April 12, 2020, at 45% of all pediatric visits. In-person illness visits gradually returned to year-to-year volumes in August-September 2020 with school opening. Early in the pandemic, both pediatric offices limited patient encounters to well-child visits in the first 2 years of life to not miss opportunities for childhood vaccinations. However, some parents were reluctant to bring their children to those visits. There was no significant change in frequency of healthy child visits during the pandemic.
To our knowledge, this was the first study from primary care pediatric practices in the United States to analyze the effect on infectious diseases during the first 9 months of the pandemic, including the 6-month time period after the reopening from the first 3 months of lockdown. One prior study from a primary care network in Massachusetts reported significant decreases in respiratory infectious diseases for children aged 0-17 years during the first months of the pandemic during lockdown.2 A study in Tennessee that included hospital emergency department, urgent care, primary care, and retail health clinics also reported respiratory infection diagnoses as well as antibiotic prescription were reduced in the early months of the pandemic.3
Our study shows an overall reduction in frequency of respiratory illness visits in children 6-36 months old during the first 9 months of the COVID-19 pandemic. We learned the value of using technology in the form of virtual visits to render care. Perhaps as the pandemic subsides, many of the hand-washing and sanitizing practices will remain in place and lead to less frequent illness in children in the future. However, there may be temporary negative consequences from the “immune debt” that has occurred from a prolonged time span when children were not becoming infected with respiratory pathogens.4 We will see what unfolds in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. Dr. Schulz is pediatric medical director at Rochester (N.Y.) Regional Health. Dr. Pichichero and Dr. Schulz have no conflicts of interest to disclose. This study was funded in part by the Centers for Disease Control and Prevention.
References
1. Kaur R et al. Front Pediatr. 2021;(9)722483:1-8.
2. Hatoun J et al. Pediatrics. 2020;146(4):e2020006460.
3. Katz SE et al. J Pediatric Infect Dis Soc. 2021;10(1):62-4.
4. Cohen R et al. Infect. Dis Now. 2021; 51(5)418-23.






